Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 11/92/17. The contractual start date was in October 2013. The draft report began editorial review in May 2015 and was accepted for publication in December 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Jonathan AC Sterne was a National Institute for Health Research (NIHR) Health Technology Assessment Clinical Trial Board member from 2010 to 2014 and is a NIHR Senior Investigator (award NF-SI-0611-10168). Chris Salisbury is a NIHR Health Services and Delivery Research Board member, and also reports receipt of a research grant from NIHR. Howard HZ Thom reports personal fees for consultancy work from Novartis Pharma, Eli Lilly and company, and ICON Plc, all outside this work. Sofia Dias reports grants from NIHR, Novartis and Pfizer, all outside this work. Diane Eaton reports other from Boehringer Ingelheim, Pfizer, Bayer, Leo Pharmaceuticals and Bristol-Myers Squib, outside the submitted work, and AntiCoagulation Europe (ACE), a registered charity, the aims of which include raising awareness of the risk and prevention of thrombosis, and providing information, education and support to people who are on anticoagulation therapy for any duration, including long term for those with chronic conditions. Diane Eaton works with ACE in an associate consultant capacity in the role of Project Development Manager. She has over 40 years of personal experience of anticoagulation therapy and represents ACE as a patient expert at the National Institute for Health and Care Excellence (NICE). On behalf of the charity, she has provided the patient perspective for the submissions for the technology appraisals for the novel oral anticoagulants (NOACs) and Diagnostic Guidance for Coagulometers over a 4-year period. Please note that the financial information has been prepared by Eve Knight, Chief Executive of AntiCoagulation Europe, for the purpose of inclusion in this document.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Sterne et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of health problem
Atrial fibrillation, stroke and myocardial infarction
Atrial fibrillation (AF) is the most common cardiac arrhythmia. 1 The prevalence of AF roughly doubles with each decade of age, rising to almost 9% at age 80–90 years. 2 AF substantially increases (by up to five times) the risk of thromboembolic stroke (annual incidence 114 per 100,000) because of blood pooling in the left atrium and systemic embolisation to the brain. More than 20% of the 130,000 annual strokes in England and Wales are attributed to AF. Approximately one-third of stroke patients die in the first 10 days, one-third recover in 1 month and one-third have disabilities necessitating rehabilitation, making stroke the leading cause of adult disability. Patients with thromboembolic stroke from AF have higher mortality and morbidity and longer hospital stay than patients with other stroke subtypes. Warfarin is an effective oral anticoagulant for the prevention of stroke in patients with AF. 3 Although the incidence and mortality of stroke continue to fall in the UK, the underutilisation of anticoagulation in patients with AF at high risk of stroke is a major gap in clinical care. 4 In patients with AF, antiplatelet and anticoagulant therapies are generally considered from the perspective of mitigation of stroke risk. However, the presence of AF is also associated with an approximately twofold higher risk of future acute myocardial infarction (MI),5 whose annual incidence in England (130 and 55.9 per 100,000 for men and women, respectively)6 is higher than that of stroke.
Venous thromboembolic disease
The annual incidence of venous thromboembolic disease from a study7 conducted in Europe is 183 per 100,000. It encompasses clot formation in deep veins of the legs or pelvis [deep-vein thrombosis (DVT); annual incidence 124 per 100,000] and the displacement of clots to the pulmonary arteries [pulmonary embolism (PE); annual incidence 60 per 100,000]. Important risk factors for venous thromboembolism (VTE) include major surgery, particularly lower limb orthopaedic surgery and surgery for cancer, as well as hospitalisation in acutely ill general medical patients (approximate incidence 15%). VTE costs the UK NHS £640M and is responsible for approximately 30,000 deaths each year in hospitals in England. DVT is an important cause of long-term morbidity, being a risk factor for chronic leg ulceration. PE may also lead to long-term morbidity due to pulmonary hypertension. There is an approximately 30% risk of recurrence of VTE within 8 years.
The risk of VTE during hospitalisation for surgical or medical treatment can be reduced by low-molecular-weight heparin (LMWH), fondaparinux (Arixtra®, GlaxoSmithKline, London, UK) or unfractionated heparin. 8 Warfarin is the most frequently prescribed anticoagulant for the initial treatment and for the long-term secondary prevention of VTE in those who are deemed to be at high risk of recurrence.
Current usage and cost of warfarin in the NHS
A 2007 Health Technology Assessment report9 stated that approximately 950,000 people (2% of the general practice population) in the UK are prescribed warfarin, increasing by about 10% per year. Warfarin-related bleeding is one of the top five reasons for hospitalisation for adverse drug effects in England10 because of the narrow therapeutic index and numerous drug/dietary interactions. Although the approximate acquisition cost of warfarin is only £10 per patient per year, the requirement for therapeutic monitoring to ensure optimal efficacy and to reduce the risk of bleeding, through hospital-, primary care- or pharmacist-based anticoagulation clinics, or by home monitoring with anticoagulant clinic support, increases the cost of warfarin treatment. The estimated annual cost of managing patients on warfarin in the NHS in England and Wales is approximately £90M. 11 A 2006 National Institute for Health and Care Excellence (NICE) report11 estimated that 46% of patients who should be on warfarin are not receiving it, and that many receiving anticoagulation are not in the optimal therapeutic range, perhaps because of concern about the risk and inconvenience of warfarin treatment.
Description of interventions under assessment: new oral anticoagulants
The class of novel oral anticoagulants (NOACs) [or non-vitamin K antagonist (VKA)], sometimes called direct-acting oral anticoagulants, includes dabigatran (Pradaxa®, Prazaxa®, Pradax®, Boehringer Ingelheim GmbH, Germany) (a direct inhibitor of clotting factor II) and rivaroxaban (Xarelto®, Bayer HealthCare, Germany), apixaban (Eliquis®, Bristol-Myers Squibb, USA; Pfizer, USA), edoxaban (Lixiana®, Daiichi Sankyo, Japan), otamixaban (Sanofi, Paris, France) and betrixaban (Portola Pharmaceuticals, San Francisco, CA, USA) (which are factor X inhibitors). These agents have a more rapid onset and offset of action than warfarin, and are considered to have more predictable dosing requirements than warfarin, possibly reducing the need for therapeutic drug monitoring, increasing convenience and reducing overall cost. 12 However, the safety and efficacy of at least one of the NOACs (dabigatran) may vary according to achieved plasma concentration, which may differ between individuals receiving the same dose,13 suggesting a potential benefit from therapeutic drug monitoring. If this proved to be the case, the corollary would be an increase in the overall cost of treatment.
These drugs have been evaluated in clinical trials as an alternative to warfarin for the prevention of stroke in patients with AF (in whom warfarin is given lifelong); as an alternative to LMWH for prevention of VTE in high-risk patients who are undergoing major orthopaedic surgery, as well as those hospitalised with acute medical conditions (in whom LMWH is given to cover the high-risk period); as an alternative to a period of LMWH and then warfarin for acute treatment of a first VTE (usually for 6 months); and for secondary prevention after a first episode of VTE, for which there is currently no widely used treatment.
The estimated annual acquisition cost per patient of new anticoagulants is substantially higher than that of warfarin and will remain so until patent expiry (e.g. 2020 for rivaroxaban). However, the higher acquisition cost could be offset by the reduced need for therapeutic monitoring through anticoagulation services, by increased effectiveness, or by improved safety. Potential limitations of NOACs include class- and drug-specific cautions/contraindications, potential for subtherapeutic dosing, reduced adherence owing to lack of regular monitoring, and absence of, or limited experience with, antidotes, as well as the added cost of maintaining stocks of numerous different anticoagulants and the potential for prescribing errors as a result of unfamiliarity.
Rationale for undertaking this evidence review
Limitations of the previous evidence base (and shortfalls in previous attempts at evidence synthesis) make rational selection from the now wide range of available oral anticoagulants difficult for NHS commissioners, doctors and patients. Much of the existing NICE guidance in this area is limited to technology appraisals of the individual agents.
Clinical trials in this area have the following limitations:
-
Few, if any, trials have made direct comparisons of NOAC drugs with one another. This limitation can be addressed through the use of network meta-analysis (NMA) to estimate the comparative efficacy and safety of agents that have been tested against a common comparator, for example warfarin.
-
Different rates of subtherapeutic anticoagulation with warfarin within trials (as measured by the time spent in the therapeutic range) may have artificially inflated the apparent efficacy of newer agents. This limitation can be addressed to some extent by investigating the relation of average time in therapeutic range (TTR) with efficacy, within the NMA framework.
Prior synthesis research in this area has the following limitations:
-
Some meta-analyses preceded recently published, potentially influential trials.
-
Failure to fully incorporate evidence on the adverse effects of oral anticoagulants by including data from all trials, regardless of indication, to maximise power and provide the most robust evidence on the balance between benefit and harm.
-
The lack of cost-effective analyses (CEAs) relevant to England and Wales.
Thus, there is a need for an up-to-date comprehensive evidence synthesis of all competing treatments to inform the rational choice of a minimum set of oral anticoagulants needed by NHS hospitals for the main therapeutic indications to avoid unnecessary overstocking and to reduce the risk of prescription error due to unfamiliarity.
Chapter 2 Research questions
Aim
We set out to determine what is/are the best oral anticoagulant(s) for prevention of stroke in AF, and for primary prevention, treatment and secondary prevention of venous thromboembolic disease.
Objectives of evidence review
Our specific objectives were to:
-
identify the most effective, safe and cost-effective anticoagulant for stroke prevention in AF, and consider whether or not the evidence is consistent across important patient subgroups (e.g. presence of comorbidities, age)
-
identify the most effective, safe and cost-effective oral anticoagulant for primary prevention, treatment and secondary prevention of venous thromboembolic disease, and consider whether or not the evidence is consistent for both prevention and treatment, and across important patient subgroups (e.g. patients undergoing cancer surgery or hip and knee replacement and hospital admission for acute medical illness)
-
identify optimal anticoagulation strategies for use by Trust Drugs and Therapeutics Committees, based on the best drug(s) for each of the main therapeutic indications
-
estimate the value of conducting further research on the cost-effectiveness of these drugs, for example by conducting a head-to-head trial of two or more new anticoagulants.
Chapter 3 Review methods (1): assessment of clinical effectiveness and safety
Introduction
We conducted four systematic reviews, with NMAs, of randomised controlled trials (RCTs) addressing questions relevant to the study objectives.
Effectiveness and safety of oral anticoagulants for:
-
prevention of stroke in non-valvular AF
-
primary prevention of venous thromboembolic disease
-
acute treatment of venous thromboembolic disease
-
secondary prevention of venous thromboembolic disease.
We undertook these reviews in accordance with the Centre for Reviews and Dissemination (CRD) guidelines for undertaking systematic reviews14 and the Cochrane Handbook for Systematic Reviews of Interventions15 (as updated online during 2011: see www.cochrane-handbook.org). We prospectively registered the reviews in the PROSPERO (international prospective register of systematic reviews) database (www.crd.york.ac.uk/prospero), with registration numbers CRD42013005324, CRD42013005331 and CRD42013005330.
Eligibility criteria
Study designs
In all reviews we included Phase II or Phase III RCTs using either a superiority or a non-inferiority design.
Participants
In all reviews we included adults (≥ 18 years) who were eligible for oral anticoagulation or (antithrombotic) treatment. Trials in participants who were eligible for only parenteral (injected) anticoagulation were excluded. Unless otherwise specified, anticoagulation services may have been delivered in hospital-, primary care- and pharmacy-based clinics or through home monitoring and telephone support. The review was not limited to NHS anticoagulation services.
Specific criteria for inclusion in the four reviews were as follows.
-
Stroke prevention in AF Adults with non-valvular AF.
-
Primary prevention of VTE Adults admitted to hospital who were considered to be at high risk of VTE, including those with a medical condition (e.g. cancer, major trauma, stroke) or undergoing a surgical procedure (e.g. total knee or hip arthroplasty, hip fracture surgery) that carries a high risk of VTE.
-
Acute treatment of VTE Adults who have received a new or recurrent objectively confirmed diagnosis of acute symptomatic VTE.
-
Secondary prevention of VTE Adults who have completed a minimum of 3 months of anticoagulant treatment for objectively confirmed first VTE without recurrence (secondary prevention).
Interventions and comparators
Five NOACs were the focus of all reviews: dabigatran, apixaban, edoxaban, betrixaban, rivaroxaban. NOACs not considered were eribaxaban (the current stage of development was unclear), ximelagatran (withdrawn because of liver toxicity), darexaban (YM150) and AZD0837 (both discontinued), LY517717 and letaxaban (TAK442) (no available information on any further clinical development for both); and otamixaban (parenteral administration).
As the reviews were conducted to inform NMAs, we determined the comparator interventions to ensure that they would provide information on the relative effectiveness of the interventions of interest. We constructed preliminary networks of available treatment comparisons from trials included in previously published NMAs (irrespective of the outcome data available from them). Comparators were chosen based on the possibility of informing indirect evidence on the relative effectiveness of oral anticoagulants, and on the ‘distance’ of these comparators from our interventions of interest in the network, which relates to the likely increase in precision in the estimates of relative effectiveness of the oral anticoagulants.
Specific comparators in the four reviews were as follows:
-
Stroke prevention in AF Therapeutic doses of warfarin or other VKA [with optimal international normalised ratio (INR) range 2–4]; aspirin; clopidogrel (Plavix®, Sanofi, USA).
-
Primary prevention of VTE Standard dose LMWH; therapeutic doses of warfarin or other VKA (with optimal INR range 2–4); placebo.
-
Acute treatment of VTE Therapeutic doses of warfarin or other VKA (with optimal INR range 2–4).
-
Secondary prevention of VTE Therapeutic doses of warfarin or other VKA (with optimal INR range 2–4); placebo; no treatment.
Studies evaluating fixed-dose administration of warfarin were excluded. Studies evaluating warfarin with suboptimal target INR compared with UK guidelines were excluded from the main analyses but combined with studies evaluating warfarin with standard target INR in sensitivity analyses. Unfractionated heparin and fondaparinux were excluded from the primary prevention of VTE review, as they would be distant from the NOACs in the network and hence contribute very little information. Non-standard doses of LMWH that were excluded from this review included enoxaparin (Lovenox®, Clexane®, Sanofi-Aventis, France) at 20 mg twice daily (bd), ardeparin (Normiflo, Wyeth-Ayerst, USA) at 25 anti-Xa units/kg bd or 35 anti-Xa units/kg bd and nadroparin (Fraxiparine®, Sanofi-Synthelabo, France) 3800 IU anti-Xa once daily (od).
Outcomes of interest
Prevention of stroke in atrial fibrillation
We sought data on the following outcomes:
-
stroke or systemic embolism (SE)*
-
all stroke
-
ischaemic stroke (major ischaemic stroke or minor ischaemic stroke)*
-
fatal stroke
-
non-fatal stroke
-
haemorrhagic stroke (major haemorrhagic stroke or minor haemorrhagic stroke)
-
any bleeding
-
minor bleeding
-
major bleeding*
-
clinically relevant non-major (CRNM) bleeding
-
clinically relevant bleeding (CRB)* (defined as CRNM bleeding or major bleeding)
-
intracranial bleeding*
-
extracranial major bleeding
-
extracranial minor bleeding
-
fatal bleeding
-
bleeding from surgical site
-
thrombocytopenia
-
MI*
-
transient ischaemic attack (TIA)
-
arterial event
-
quality-of-life outcomes
-
hospital admission
-
death (cardiovascular)
-
all-cause mortality.*
The outcomes addressed in NMAs are marked with an asterisk in the list above. These were chosen based on three considerations: (1) their clinical importance; (2) the consistency of reporting across studies included in the network; and (3) the number of data that were available for inclusion in NMAs.
Venous thromboembolism
For all VTE reviews we sought data on the following outcomes.
Efficacy
-
Symptomatic VTE.*
-
Non-symptomatic VTE.
-
Major VTE (defined as symptomatic or asymptomatic proximal DVT, non-fatal PE and VTE-related death).
-
Fatal VTE.
-
Symptomatic DVT.*
-
Non-symptomatic DVT.
-
Distal DVT.
-
Symptomatic distal DVT.
-
Proximal DVT.
-
Symptomatic proximal DVT.
-
PE.
-
Symptomatic PE.*
-
Non-symptomatic PE.
-
Fatal PE.
-
Non-fatal PE.
-
Symptomatic non-fatal PE.
Safety
-
Any bleeding.
-
Minor bleeding.
-
Major bleeding.*
-
CRNM bleeding.
-
CRB* (defined as CRNM bleeding or major bleeding).
-
Intracranial bleeding.
-
Extracranial major bleeding.
-
Extracranial minor bleeding.
-
Fatal bleeding.
-
Bleeding from surgical site.
-
Thrombocytopenia.
Other
-
MI.*
-
TIA.
-
Arterial event.
-
Quality-of-life outcomes.
-
Hospital admission.
-
Cardiovascular mortality.
-
All-cause mortality.*
The outcomes addressed in NMAs are marked with an asterisk in the list above. These were chosen based on three considerations: (1) their clinical importance; (2) the consistency of reporting across studies included in the network; and (3) the number of data that were available for inclusion in NMAs.
Identification of evidence
Search strategy
Scoping searches that were conducted during protocol development identified some previously published NMAs of oral anticoagulants. We rescreened the studies included in these NMAs against our eligibility criteria, and developed searches to identify any additional studies published beyond the search dates of the most recent NMAs in each population. 8,16–18
We used two separate search strategies, one for the review of stroke prevention in AF (for which the search was run on 12 March 2014 and updated on 15 September 2014, and covered the period 2010 to September 2014) and one for the three reviews in VTE (for all three of which the search was run on 19 March 2014, updated on 15 September 2014, and covered the period 2008 to September 2014). In each search strategy we combined terms for either AF or VTE with terms for the interventions and comparators of interest and added a filter to focus the search on RCTs. We searched MEDLINE and PREMEDLINE In-Process & Other Non-Indexed Citations, EMBASE and The Cochrane Library. The stroke prevention in AF review search was run on the 12 March 2014 and updated on 15 September 2014, and covered the period 2010 to September 2014. The search for the three reviews in VTE was run on the 19 March 2014, updated on the 15 September 2014 and covered the period 2008 to September 2014. We applied no restrictions on language. The principal search strategy is included in Appendix 1. We removed duplicate records, identified by title, authors, journal citation and date published.
We sought information on studies in progress, unpublished research or research reported in the grey literature from www.clinicaltrials.gov (to September 2012). We screened reference lists of retrieved studies and relevant review articles. We also searched the NHS Economic Evaluation Database (NHS EED) and NICE Technology Appraisals.
Assessing relevance and inclusion
Two reviewers independently screened the results of the searches by title and abstract. We resolved disagreements through consensus or referral to a third reviewer where necessary. We obtained full texts of all potentially relevant reports and two reviewers assessed these independently against the eligibility criteria, with disagreements resolved by a third reviewer. We collated multiple reports of the same study and mapped them to unique studies.
Data extraction
We developed data extraction forms and piloted them on a small selection of studies. Data were extracted from the trial reports by one reviewer and checked by a second. Disagreements were resolved through consensus or by referral to a third reviewer where necessary. We extracted data on the following: study details (identifier, study design, location, year, length of follow-up, industry sponsorship); participant details (number of participants, age, gender); intervention details (drug name, dose, timing); comparator details; details relevant to risk-of-bias assessment (including adherence to and withdrawal from randomised allocation); and effect modifiers. Multiple reports from a study informed a single data extraction form. We extracted and managed data using Microsoft Access® 2013 software (Microsoft Corporation, Redmond, WA, USA).
We extracted dichotomous data based on the full randomised samples, as number of events in intervention and control groups and numbers of participants, and we sought details of follow-up time (e.g. participant-years in each treatment group). We also extracted estimates of hazard ratios (HRs) and their confidence intervals (CIs) when available.
Assessment of risk of bias in included trials
We assessed studies using the Cochrane Risk of Bias Tool. 19 This assigns a judgement of high, low or unclear risk of bias for each of the following domains: selection bias (randomisation sequence and allocation concealment), performance bias (blinding of participants and carers), detection bias (blinding of outcome assessment), attrition bias (due to dropouts and exclusions), and reporting bias (selective outcome reporting). Assessments were carried out by one reviewer and checked by a second. We resolved disagreements through consensus or by referral to a third reviewer where necessary.
Selection of data for analysis
Choice of interventions
To perform NMAs we had to allocate each intervention group in each trial to a category, with each intervention category forming a ‘node’ in the network. We kept different doses or frequencies of administration (i.e. od or bd) of oral anticoagulants in separate nodes. We assigned different VKAs to one node (named ‘Warfarin’), but separated intended INR range 2–3 from intended INR range 3–4 and from other ranges. For LMWH interventions in the review of primary prevention of VTE, we separated preoperative (pre-op) LMWH from postoperative (post-op) LMWH. The intervention categories (or network nodes) are labelled throughout the report using drug, frequency and dose, or INR range, as appropriate.
Choice of time points
When outcome data were presented for multiple time points, we took the longest period of follow-up, except for bleeding events in the review of primary prevention of VTE, which we assessed at the end of the treatment period.
Choice of outcomes
When outcome data were not presented directly, we computed or substituted them, using data for other outcomes, making assumptions that we considered to be reasonable. When we could not extract data for the outcome ‘stroke or SE’ in the review of stroke prevention in AF, we used ‘all stroke’. When CRB was not reported but both major bleeding and CRNM bleeding events were, we used the total number of events across these two categories. If symptomatic PE was not reported in any of the three VTE reviews, we used symptomatic non-fatal PE if available, or the sum of fatal PE and non-fatal PE. Additionally, in the review of primary prevention of VTE, when symptomatic VTE was not reported, we added across symptomatic DVT and symptomatic PE, if available.
Quantitative synthesis (including network meta-analysis)
For each analysed outcome in each review (see Choice of outcomes), we undertook both standard meta-analyses of ‘direct evidence’ (evidence based on head-to-head comparisons between interventions made within studies) and a NMA. Results of the individual studies are available in forest plots, arranged within each possible pairwise analysis. The comparisons displayed in the forest plots were computed from the raw data reported in the studies, and we calculated effect estimates using standard frequentist techniques.
Network meta-analysis is a method of synthesising information from a collection of studies by combining evidence from all intervention comparisons that have been made among the studies. The results it produces for each pairwise comparison combine all of the ‘direct evidence’ (evidence based on head-to-head comparisons between interventions made within individual studies) with all of the ‘indirect evidence’ (comparisons between interventions inferred from the network via common comparator interventions). 20,21 For example, indirect evidence comparing the effect of interventions A and B can be inferred from the direct evidence provided by a trial comparing A with C and a trial comparing B with C. NMA thus enables estimation of relative intervention effect estimates for every pair of interventions, regardless of whether or not they have been compared directly in a RCT. It also enables the ranking of treatments according to the probability that each is the best, or worst, for a given outcome.
We plotted the networks to illustrate the data structure for each review and outcome. In these plots, the size of the node for each intervention is proportional to the number of patients randomised to that intervention. When direct evidence comparing two interventions was available, these two interventions are connected by an edge (line) thats thickness is proportional to the number of patients who contributed to the comparison. The intervention labels are formatted as follows:
-
Licensed doses of NOACs are written in bold typeface; these are interventions of primary interest.
-
Interventions that were excluded from the primary analysis labels are presented in square brackets. Such exclusions are because (1) they were not considered to be of interest to inform health decisions in the UK (e.g. warfarin interventions using subtherapeutic INR ranges); or (2) the total number of events was zero, so they are uninformative; or (3) they do not connect with the other trials in the network.
-
Excluded interventions that were included in sensitivity analyses are marked with an asterisk.
We had planned to take a random-effects approach to the meta-analyses, assuming a common heterogeneity variance across all comparisons. 20 In most networks there was insufficient replication of intervention comparisons to allow estimation of the heterogeneity variance. All of our analyses are therefore based on fixed-effects models.
The primary NMAs treat the data as binomial, modelling the number of events out of the total number of participants using a logistic model. When there were no events in either arm of a trial, it was omitted from the analysis. When there were events in at least one arm of a trial, but no events in at least one other arm, we added 0.5 events to all intervention arms in the trial. In supplementary analyses for some outcomes we modelled HRs rather than odds ratios (ORs). For this we used a complementary log–log link to account for differential follow-up times (thereby assuming a constant hazard of the outcome over time), modelled possibly repeated events as rate data or included HRs extracted directly from trial reports. Some of these analyses were used in the economic models (see Chapter 4).
All meta-analyses were performed within a Bayesian framework, using freely available WinBUGS software version 1.4.3 (MRC Biostatistics Unit, Cambridge, UK) and code. 22 We assessed convergence of the Markov chains using the potential scale reduction factor, as well as visual examination of history and autocorrelation plots for each estimated parameter. We assessed goodness of fit by calculating the posterior mean residual deviance. This is defined as the difference between the deviance for the fitted model and the saturated model, whereby the deviance measures the fit of the model using the likelihood function. Comparisons of models were made using the deviance information criterion (DIC), which is equal to the sum of the posterior mean of the residual deviance and the effective number of parameters. 23 The DIC penalises the posterior mean residual deviance (a measure of model fit) by the effective number of parameters in the model (as measure of complexity) and can therefore be viewed as a trade-off between the fit and complexity of the model.
Investigation of heterogeneity
We had planned to use subgroup and meta-regression24 analyses to examine the extent to which patient- and study-level characteristics explain between-study heterogeneity. We prespecified the important characteristics to be age, gender, ethnicity/race, body mass index (BMI) or weight, renal status or creatinine clearance, blood pressure, diabetes mellitus, hypertension, previous thrombotic event, liver disease, chronic heart failure, cancer, pregnancy, intervention dose, average TTR in the warfarin group, and summary assessment of risk of bias for each outcome. Additional factors for AF trials were CHADS2, CHADS2 VASC, HAS-BLED, history of previous stroke or TIA and previous MI. Additional factors for primary prevention of VTE were general surgery compared with orthopaedic surgery, elective emergency surgery compared with non-elective emergency surgery, and medical trials compared with surgical trials. An additional factor for acute treatment or secondary prevention of VTE was the nature of the index event (whether PE or DVT). When available, inferences about subgroup effects would be based on within-trial subgroup analyses (e.g. comparing relative intervention effects in older and younger participants). Investigation of between-study variation using these characteristics could not be studied in most cases because of the lack of multiple trials of the same pairwise comparison, although we conducted some sensitivity analyses for the review of stroke prevention in patients with AF. Specifically, we performed several meta-regressions using the average TTR in the warfarin group as a covariate.
Investigation of inconsistency
The validity of a NMA depends on the assumption that there is no effect modification of the pairwise intervention effects or, that the prevalence of effect modifiers is similar in the different studies. This key assumption has been referred to variously as exchangeability,22 transitivity,25 similarity26 and consistency. 27,28 For a clinical and epidemiological judgement of the plausibility of this assumption we examined whether or not the trials were similar in ways that might modify treatment effect, based on the prespecified list of potential effect modifiers (see Investigation of heterogeneity).
‘Evidence inconsistency’ can be considered an additional layer of heterogeneity that occurs in networks of evidence when there is a discrepancy between the direct and indirect estimates of relative intervention effects. Therefore, inconsistency is a property of ‘closed loops’ of evidence, in which both direct and indirect evidence are available for each comparison. We visually inspected the network diagrams to identify potential for inconsistency (closed loops), and used model fit and selection statistics to informally assess whether or not it was evident. Where there was potential for inconsistency, we compared the residual deviance from the consistency model (providing NMA evidence) with the residual deviance from an ‘inconsistency model’, without consistency constraints (in which only direct evidence is analysed for each comparison). When both direct and indirect evidence were available, and the direct evidence had a standard error that differed (beyond the second decimal place) from the NMA estimate, we used results from these two analyses to back-compute the indirect estimates, on the basis that the NMA estimates (from the consistency model) would be equivalent to a weighted average of the direct estimate (from the inconsistency model) and the indirect estimate. In the results tables we present all three of these estimates. The extent of the disagreement between the direct and indirect estimates can be used as a local measure of inconsistency for that comparison. Note that for the vast majority of comparisons there was either only direct evidence or only indirect evidence, so that the NMA estimates correspond to one of these.
Chapter 4 Review methods (2): cost-effectiveness analysis
Introduction
This chapter describes the structure of the decision analysis models that we developed to assess the cost-effectiveness of NOACs in the primary prevention, treatment and secondary prevention of venous thromboembolic disease, and for prevention of ischaemic stroke in AF. We also provide a brief overview of previous cost-effectiveness models that we identified and used to inform the development of our models.
Our models synthesise evidence on a number of parameters [e.g. incidence of VTE or ischaemic stroke, relative treatment efficacy, adverse events (AEs), costs, etc.] in order to estimate the relative cost-effectiveness of treatment options. The ‘model inputs’ are based on a variety of evidence sources. These include routine data on drug costs and observational studies of the long-term costs and quality of life (i.e. utilities) in AF and VTE. Many of these model inputs are shared between the AF and VTE cost-effectiveness models, and we summarise them in this chapter. However, other model inputs (e.g. on relative treatment efficacy and safety of anticoagulants) are derived from the results of meta-analyses of RCTs that are identified in our systematic review. We summarise these efficacy and safety model inputs in Chapters 6 and 11, which present the results of the cost-effectiveness models for AF and VTE, respectively.
The VTE secondary prevention, acute treatment and primary prevention models were constructed in Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA) and the AF model was constructed in R version 3.02 (The R Foundation for Statistical Computing, Vienna, Austria). 29 All (network) meta-analyses were conducted in WinBUGS. 30
Decision questions
The questions we addressed were:
-
What is the most cost-effective first-line anticoagulant in the prevention of ischaemic stroke for patients with AF?
-
What is the most cost-effective first-line strategy for the secondary prevention of VTE after an initial PE or DVT?
-
What is the most cost-effective first-line anticoagulant for the acute treatment of symptomatic VTE?
-
What is the most cost-effective first-line anticoagulant for primary prevention of VTE following two types of elective surgery [a, total hip replacement (THR) or b, total knee replacement (TKR)]?
In each case, we evaluated cost-effectiveness from a NHS perspective. We modelled costs and outcomes over the expected lifetime of patients. In the next section, we give a brief overview of previous cost-effectiveness models addressing these decision questions. We then describe the patients, interventions, outcomes, model structure and shared model inputs for each of the four decision questions.
Previous economic models
We performed an informal search of the literature, including NICE technology appraisals, for previous model-based CEAs addressing one of the four decision questions. Our literature search was not intended to be exhaustive, but we aimed to identify a representative sample of existing modelling methods and structures to inform our models. We developed the structure of our models from a critical appraisal of these previous models together with discussions with clinical experts and patient group representatives on the project team.
For prevention of ischaemic stroke in AF, we identified 18 previous models, summarised in Table 1 and discussed in detail in Appendix 6. A recently published systematic review49 identified 30 models on prevention of stroke in AF; however, the main model structures identified in that review were covered by the 12 studies we found. For the prevention and treatment of VTE, we identified 16 previous models, two acute treatment models (Table 2) and 14 primary prevention models post orthopaedic surgery (Table 3).
| Author, year | Setting | Model type | Interventions | Events | Health states | Time horizon |
|---|---|---|---|---|---|---|
| Gage 199531 | USA | Markov | Warfarin, aspirin | TIA, stroke, haemorrhage, death | Well, RIND, mild stroke, moderate–severe stroke, second stroke, mild ICH, moderate–severe ICH, RIND and ICH, stroke and ICH, dead | 10 years |
| Lightowlers 199832 | UK | Decision tree | Warfarin (several monitoring strategies), no treatment | Bleed, stroke | NA | 10 years |
| Bayer plc 201133 | UK | Markov | Rivaroxaban, dabigatran, warfarin, aspirin, no treatment | Minor stroke, major stroke, minor bleed, major bleed, MI, ICH, SE, death | On and off treatment for AF stable and post event states for minor stroke, major stroke, minor bleed, major bleed, MI and ICH, dead | Lifetime |
| Shah 201134 | USA | Markov | Dabigatran, warfarin, aspirin | MI, TIA, stroke (four severities), minor bleed, major bleed, dyspepsia, death | Well, TIA, mild stroke, major stroke, second stroke, ICH, stroke and ICH, dead | Lifetime |
| Freeman 201135 | USA | Markov | Dabigatran, warfarin | TIA, stroke, ICH, ECH, MI, death | Well, RIND, mild stroke, moderate–severe stroke, mild ICH, moderate–severe ICH, MI, dead | Lifetime |
| Lee 201236 | USA | Markov | Apixaban, warfarin | Stroke, bleed, MI, ICH, death | Well, RIND, minor ischaemic stroke, major ischaemic stroke, MI, minor ICH, major ICH, ischaemic stroke and ICH, death | Lifetime |
| Lee 201237 | USA | Markov | Rivaroxaban, warfarin | RIND, minor stroke, major stroke, minor ICH, major ICH, stroke and ICH, ECH, MI, death | Well, minor stroke, major stroke, minor ICH, major ICH, MI, dead | Lifetime |
| Harrington 201338 | USA | Markov | Apixaban, dabigatran, rivaroxaban, warfarin | Minor ischaemic stroke, major ischaemic stroke, ICH, MI, death | Well, post minor ischaemic stroke, post major ischaemic stroke, post ICH minor disability, post ICH major disability, post MI, dead | 30 years |
| Kamel 201239 | USA | Markov | Apixaban, warfarin | TIA, ECH, MI, mild ischaemic stroke, moderate–severe ischaemic stroke, mild ICH, moderate–severe ICH, death | AF and history of stroke/TIA, mild ischaemic stroke, moderate–severe ischaemic stroke, mild ICH, moderate–severe ICH, recurrent ischaemic stroke or combined stroke and ICH, dead | 20 years |
| CADTH – Wells 201240 | Canada | Markov | Apixaban, dabigatran, rivaroxaban, warfarin | Minor stroke, major stroke, fatal stroke, non-fatal MI, fatal MI, TIA, non-fatal PE, fatal PE, ICH, major bleed, minor bleed, fatal bleed, no-event death | Well, previous TIA, previous minor stroke, previous major stroke, previous MI | 40 years |
| Wisloff 201341 | Norway | Markov | Apixaban, dabigatran, rivaroxaban, warfarin | Gastrointestinal bleed, ischaemic stroke, ICH, acute MI, heart failure, death | Well, previous bleed, previous stroke, moderate stroke sequelae, severe stroke sequelae, previous MI, death | Lifetime |
| Kansal 201242 | UK | Markov | Dabigatran, warfarin | Ischaemic stroke, haemorrhagic stroke, TIA, SE, MI, minor bleed, ICH, ECH, death | Eight states combining stoke history/no stroke history with no disability and mild, moderate and severe disability, death | Lifetime |
| Canestaro 201343 | USA | Markov | Dabigatran, apixaban, rivaroxaban, warfarin | Ischaemic stroke, MI, SE, ICH, ECH, other cause death | Well, post-MI and death states, as well as three severities of each of post-ischaemic stroke, post-ischaemic stroke and MI, post-ICH, post-ICH and MI, post-ICH and ischaemic stroke, post-ICH ischaemic stroke and MI. 21 states in total | Lifetime |
| Nshimyumukiza 201344 | Canada | Markov | Dabigatran, warfarin | ICH, ECH, stroke, MI, DVT, PE, death | Daily cycles over four states: no event, major bleeding event, major thromboembolism event, mild/severe deficit | 5 years |
| Krejczy 201445 | Germany | Markov | Dabigatran, apixaban, rivaroxaban, warfarin | TIA, ischaemic stroke (fatal, moderate to severe, mild), haemorrhage (fatal, moderate to severe intracranial, mild intracranial, major non-cerebral, minor non-cerebral), MI and death | Healthy with non-valvular AF, TIA, ischaemic stroke (fatal, moderate to severe, mild), haemorrhage (fatal, moderate to severe intracranial, mild intracranial, major non-cerebral, minor non-cerebral), MI and death Combinations of these events were included |
20 years |
| Pink 201146 | UK | Discrete event simulation | Dabigatran, warfarin | Stroke, PE, TIA, congestive heart failure, fatal stroke, fatal PE, other vascular death, ICH, other major bleed, minor bleed, non-bleed AEs, MI, treatment discontinuation | Recorded patient characteristics were hypertension, diabetes mellitus, congestive heart failure, previous stroke, previous TIA, previous MI, previous ICH | Lifetime |
| Lip 201447 | UK | Markov | Dabigatran, apixaban, Rivaroxaban | Ischaemic stroke, ICH, gastrointestinal major bleed, other major bleed, CRNM bleed, MI, SE, other cardiovascular hospitalisation, death | Healthy with non-valvular AF, ischaemic stroke, ICH, gastrointestinal major bleed, other major bleed, CRNM bleed, MI, SE, other cardiovascular hospitalisation, death, non-valvular AF on aspirin | Lifetime |
| Rognoni 201448 | Italy | Markov | Dabigatran, apixaban, rivaroxaban, warfarin | Temporary/mild/moderate–severe ischaemic stroke, temporary/mild/moderate–severe ICH, MI, minor extracranial bleeding, major extracranial bleeding, death | Non-valvular AF only, temporary/mild/moderate–severe ischaemic stroke, temporary/mild/moderate–severe ICH, MI, minor extracranial bleeding, major extracranial bleeding, death | Lifetime |
| Author, year | Setting | Population | Model type | Interventions | Events | Health states | Time horizon |
|---|---|---|---|---|---|---|---|
| Bayer TA261 201250 | UK | Adults receiving acute treatment for DVT | Markov model | Rivaroxaban and dual therapy (LMWH and VKA) | Mortality, VTE recurrence, CTPH, PTS, CRB | On treatment: major bleed – ICH, major bleed – ECH, CRNM bleed, recurrent DVT, recurrent PE, CTPH, post intracranial bleed, long-term chronic thromboembolic pulmonary disease, PTS mild/moderate and severe, off treatment and dead | 40 years |
| Bayer TA287 201351 | UK | Adults who are receiving acute treatment for PE | Markov model | Rivaroxaban, LMWH or fondaparinux with continued therapy as follows VKA or LMW for people for whom a VKA is not considered an appropriate treatment | Mortality, VTE recurrence, CTPH, PTS, CRB | On treatment, major bleed – ICH, major bleed – ECH, CRNM bleed, recurrent DVT, recurrent PE ± DVT, PE post DVT, CTPH, post intracranial bleed, long-term CTPH, severe PTS, off treatment post PE, off treatment post DVT and dead | 40 years |
| Author, year | Setting | Population | Model type | Interventions | Events | Health states | Time horizon |
|---|---|---|---|---|---|---|---|
| Boehringer Ingelheim TA157 200852 | UK | Adults undergoing elective THR or TKR (model parameters and time on treatment differs between populations) | Decision tree and Markov model | Dabigatran, LMWH and fondaparinux | Mortality, incidence of DVT, incidence of PE, post DVT complications including PTS, health-related quality of life, adverse effects of treatment including bleeding events (minor and major) and joint outcomes (medium and long-term) including joint infection | Based on the structure by Botteman et al.53 | 60 years |
| Bayer TA170 201254 | UK | Adults undergoing elective THR or TKR (model parameters and time on treatment differs between populations) | Decision tree and Markov model | Rivaroxaban, and LMWH | VTE, symptomatic VTE, non-fatal PE, fatal PE and prophylaxis-related bleeding | Text and model schematic has been blanked out | Lifetime |
| Bristol-Myers Squibb TA245 201255 | UK | Adults undergoing elective THR or TKR (model parameters and time on treatment differs between populations) | Decision tree and Markov model | Apixaban, LMWH, fondaparinux, rivaroxaban and dabigatran | Mortality, VTE, PTS syndrome and treatment-related bleeding events | Well, untreated VTE, treated VTE, disabled, mild to moderate PTS year 1, mild to moderate PTS year 2+, severe PTS year 1, severe PTS year 2+, DVT, PE, dead | 35 years |
| Botteman 200253 | USA | Adults undergoing elective THR | Decision tree and Markov model | LMWH and warfarin | DVT, PE, PTS and mortality | Surgery, DVT, DVT death, DVT survivor, post DVT, mild/moderate PTS year 1, mild/moderate PTS year 2+, severe PTS year 1, severe PTS year 2+, death | Lifetime |
| Dranitsaris 200956 | Canada | Adults undergoing elective THR, TKR or hip fracture surgery | Decision tree | Dalteparin (Fragmin®, Pfizer, USA) 10 days, dalteparin 35 days and warfarin | Major bleed, symptomatic DVT at discharge, symptomatic DVT by day 35 | NA | 3 months |
| Duran 2012,57 Monreal 201358 | USA, France, Italy and Spain | Adults undergoing elective THR or TKR | Decision tree and Markov model | Rivaroxaban, enoxaparin and dabigatran | Symptomatic VTE, non-fatal PE, fatal PE, prophylaxis-related bleeding | No PTS, PTS, death | 5 years |
| Mahmoudi 201359 | USA | Adults undergoing elective THR or TKR | Decision tree | Xa inhibitors and LMWH | Distal DVT, proximal DVT, fatal PE, non-fatal PE major bleed, stroke | NA | 6 months |
| McCullagh 200960 | Ireland | Adults undergoing elective hip or knee replacement surgery | Decision tree | Rivaroxaban and dabigatran | Distal DVT, proximal DVT, symptomatic PE, fatal PE, major bleed and fatal bleed | NA | 180 days |
| McCullagh 201261 | Ireland | Adults undergoing elective hip replacement | Decision tree and Markov model | Rivaroxaban, dabigatran and enoxaparin sodium | Distal DVT, proximal DVT, symptomatic PE, fatal PE, major bleed and fatal bleed | No VTE, treated VTE, untreated VTE, PTS year 1, PTS maintenance, stroke and dead | Lifetime |
| Lundkvist 200762 | Sweden | Patients following hip fracture surgery | Decision tree | Fondaparinux and enoxaparin | Symptomatic VTE events, fatal and non-fatal recurrent VTE events and PTS | NA (model closely follows the structure of Gordois et al.63 and Sullivan et al.65) | 5 years |
| Gordois 200363 | England and Wales | Adults following major orthopaedic surgery | Decision tree | Fondaparinux and enoxaparin | Clinical VTE and VTE-related deaths | NA | 5 years |
| Pishko 201264 | USA | Ambulatory patients with cancer | Decision tree and Markov model | LMWH and no intervention | Major bleed, minor bleed, post bleed, VTE | Malignancy, major bleed, minor bleed, post bleed, VTE, post VTE | 2 years |
| Sullivan 200465 | USA | Adults following major orthopaedic surgery | Decision tree | Fondaparinux and enoxaparin | Rates of symptomatic thromboembolic events | NA | 5 years |
| Zindel 201266 | Germany | Adults undergoing elective THR or TKR | Decision tree | Rivaroxaban and enoxaparin sodium | DVT, fatal PE, non-fatal PE major bleed | NA | 3 months |
Atrial fibrillation: patients and interventions
Atrial fibrillation: patient population
We considered patients with non-valvular AF who were eligible for anticoagulation. We made no distinction between paroxysmal, persistent and permanent AF. The RCTs identified in the systematic review did not distinguish between AF type, but patients with paroxysmal AF are less likely to be included in RCTs than those with other AF types; therefore, our results are most applicable to patients with persistent and permanent AF. We consider a cohort of patients receiving first-line anticoagulation at the age of 70 years, based on the mean age observed in the RCTs identified in the systematic review [mean age 70 years, standard deviation (SD) 8 years], and consider costs and benefits over a lifetime. We assume a 60 : 40 split in favour of males, similar to that observed in the RCTs.
Atrial fibrillation: interventions
The first-line treatments for AF included in the CEA, alongside their standard or licensed doses, are listed in Table 4. We consider only licensed treatments and doses in our analysis. Although a few small RCTs have compared betrixaban with warfarin in AF, there was not enough evidence to include it in the economic model. Standard care for patients with AF, before the introduction of NOACs, was warfarin. 68
| Intervention | Dose/target INR | Time on treatment |
|---|---|---|
| Apixaban | 2.5 mg bd (elderly) | Lifetime |
| 5 mg bd | ||
| Dabigatran | 110 mg bd (elderly) | |
| 150 mg bd | ||
| Rivaroxaban | 20 mg od | |
| Warfarin | INR 2–3 |
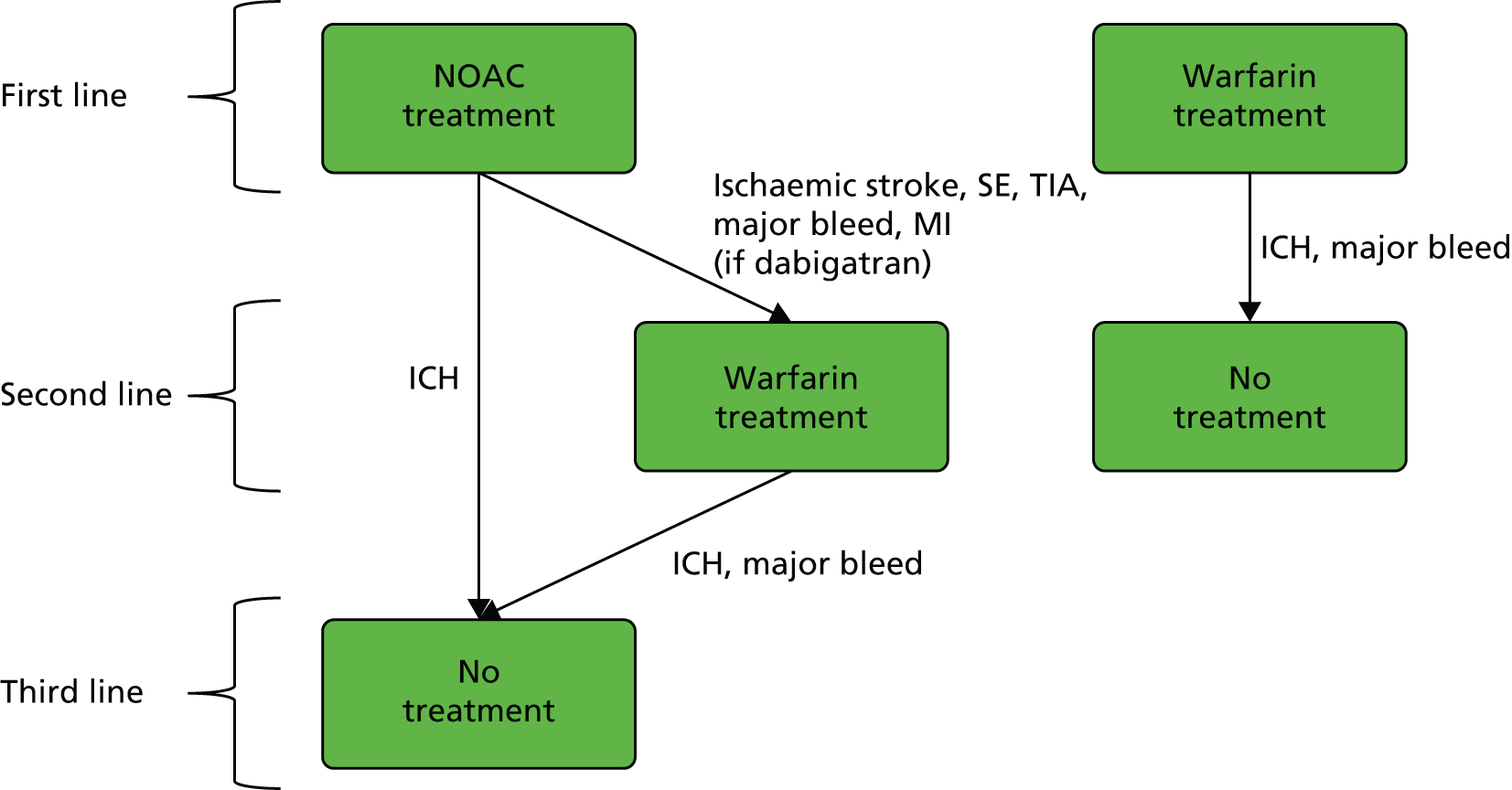
Treatment switching may occur as a result of treatment failure, indicated by ischaemic stroke or serious AEs, such as intracranial haemorrhage (ICH). For patients on warfarin first-line treatment, the only second-line intervention available was assumed to be no treatment. For patients on a NOAC first-line treatment, second-line treatment may be either warfarin or no treatment. No treatment is the only third-line treatment. These rules are illustrated in Figure 1, where the events that may lead to treatment switching are indicated.
FIGURE 1.
Treatment strategies and switching/discontinuation rules. The events that may lead to treatment switching are indicated next to the arrows between treatments.
Venous thromboembolism: patients and interventions
Venous thromboembolism: patient populations
For primary prevention, we estimated cost-effectiveness in two distinct subpopulations: patients undergoing elective THR or TKR. We considered including other populations (e.g. patients who were hospitalised for medical treatment) but there was not enough evidence identified in the literature review to inform a model.
After a confirmed VTE event, patients receive acute treatment. The population of patients in the acute treatment model includes those for whom a non-fatal symptomatic VTE event (DVT or PE) followed a THR or TKR, as well as patients with a symptomatic VTE from other causes. Patients who completed at least 3 months of anticoagulant treatment for symptomatic VTE without recurrence are included in the secondary prevention model.
We assumed an average age of subjects entering the primary prevention model of 68.7 years (SD 11.4 years) and the split between males and females of 40 : 60, based on estimates from the National Joint Registry. 69 The assumed age is in line with the median of the mean age of patients enrolled in the primary prevention RCTs (median 64.6 years). The starting age in the acute and secondary prevention populations was 57.35 years, the median (across RCTs) of the mean age of patients enrolled in the acute treatment and secondary prevention RCTs. We assumed that the index VTE event on entry to the acute treatment and secondary prevention models was split between DVT and based on the proportion of non-fatal PE and DVT in the acute treatment population.
Venous thromboembolism: interventions
For each indication we compared first-line treatments for which we have sufficient evidence to estimate model parameters. There are seven comparators evaluated in the secondary prevention model (Table 5), four in the acute treatment model (Table 6) and four in each of the two primary prevention subpopulations (Table 7). Before the introduction of NOACs, standard practice70 for primary prevention was LMWH, and for acute treatment was LMWH and warfarin for at least 5 days, then continue with warfarin only. In secondary prevention, NICE guidance71 recommends that clinicians, after discussion with patients, consider extending warfarin therapy beyond 3 months if the risk of VTE recurrence is high and there is no additional risk of major bleeding. However, NICE also acknowledged the need for further research to establish the cost-effectiveness of long-term anticoagulation after unprovoked VTE. In clinical practice, patients may be offered long-term anticoagulation after a second VTE event. Owing to this uncertainty about best practice, we compared all anticoagulants to a ‘no pharmacotherapy’ secondary prevention strategy in the base-case model. In a sensitivity analysis, we assumed that patients in this reference group would receive warfarin after a second VTE event. We assumed that all treatment will be stopped for subjects who have an ICH and that no other treatment switching occurs. This assumption differs from the AF population for whom treatment can be stopped or switched for other reasons (see Atrial fibrillation: interventions, above).
| Intervention | Dose/target INR | Time on treatment |
|---|---|---|
| Apixaban | 2.5 mg bd | Lifetime |
| 5 mg bd | Lifetime | |
| Aspirin | 75 mg od | Lifetime |
| Dabigatran | 150 mg bd | Lifetime |
| Rivaroxaban | 20 mg od | Lifetime |
| Warfarin | INR 2–3 | Lifetime |
| No long-term pharmacotherapy | – | – |
| Intervention | Dose | Time on treatment |
|---|---|---|
| Apixaban | 10 mg bd for 7 days then 5 mg bd | 6 months |
| Dabigatran | 150 mg bd | |
| Rivaroxaban | 15 mg bd for 21 days then 20 mg od | |
| Warfarin | INR range 2–3 plus LMWHa for initial 5 days |
| Intervention | Dose | Time on treatment | |
|---|---|---|---|
| THR | TKR | ||
| Apixaban | 2.5 mg bd | 28–35 days | 10–14 days |
| Dabigatran | 220 mg od | ||
| LMWH | a | ||
| Rivaroxaban | 10 mg od | ||
Outcomes of atrial fibrillation and venous thromboembolism models
We present results on total costs and quality-adjusted life-years (QALYs), both discounted at 3.5%. We present a probabilistic analysis, for which model parameters are given probability distributions to reflect uncertainty in their values. 72 We summarised the results with the expected costs, expected QALYs and expected net monetary benefit (NMB) for a range of willingness to pay per additional QALY gained (where expected values are an average over the joint distribution of the model parameters). NICE has a stated willingness-to-pay threshold of £20,000–30,000 per QALY. 73
Uncertainty in the model input parameters is captured using simulation [Monte Carlo simulation for parameters with assumed distributions, and Markov chain Monte Carlo (MCMC) simulation for parameters estimated from the NMA]. We represent decision uncertainty using the cost-effectiveness plane, cost-effectiveness acceptability curves (CEACs), and cost-effectiveness acceptability frontiers (CEAFs). The cost-effectiveness plane plots incremental effects (QALYs) against incremental costs for each simulation sample. The CEAC plots the proportion of the simulation samples where each strategy had the highest net benefit (i.e. was most cost-effective) against willingness-to-pay-per-QALY threshold. These proportions are estimates of the probability that the treatment is the most cost-effective. If this probability is close to one for a particular treatment, this suggests very little uncertainty as to the most cost-effective treatment, whereas if it is low the choice of most cost-effective treatment is uncertain. This allows decision-makers to identify interventions that are unlikely to be cost-effective at any plausible threshold and to judge how sensitive treatment choice is to the amount that the NHS is willing to pay for a QALY. The CEAC is not robust when there is a treatment with a high degree of uncertainty in net benefit, giving high probabilities of being both most cost-effective and least cost-effective. For this reason the CEAF has been proposed. 74 This plots, for each willingness-to-pay threshold, the probability of being most cost-effective only for the treatment with the highest expected net benefit at that willingness-to-pay threshold.
We use value of information (VOI) methods to explore how sensitive the optimal treatment is to uncertainty in the model inputs, and guide research recommendations. We estimate the expected value of perfect information (EVPI) and the expected value of partial perfect information (EVPPI). EVPI and EVPPI measure the expected improvement to our decision-making (in monetary units) if we were to eliminate uncertainty in all (EVPI) or some (EVPPI) of the model input parameters. We present EVPI per person per year and also per population over 10 years discounted at 3.5%, for given annual incidence for each of our populations. EVPPI for subsets of parameters are computed using the Sheffield Accelerated Value of Information (SAVI) version 2.0.9 (University of Sheffield, Sheffield, UK) web application. 75,76 This method gives only approximate results, which can be interpreted as indicative of the relative sensitivity of the decision to different groups of parameters.
Atrial fibrillation model structure
The discrete-time Markov multistate model structure (Figure 2) used a cycle length of 3 months, as in other recent models. 33,40,42 We ran the model for a cohort starting at age 70 years and used a lifetime time horizon with a cut-off at 100 years, thus giving 120 cycles. Patients were initially assigned to first-line treatment, which may be warfarin or a NOAC. There is a probability of switching to another therapy or discontinuing treatment entirely (see Figure 1).
FIGURE 2.
Markov model for AF. Patients can experience transient events (TIA or SE) but stay in same health state, with possibly changed treatment, thereafter. B, other clinically relevant bleed; S, ischaemic stroke.
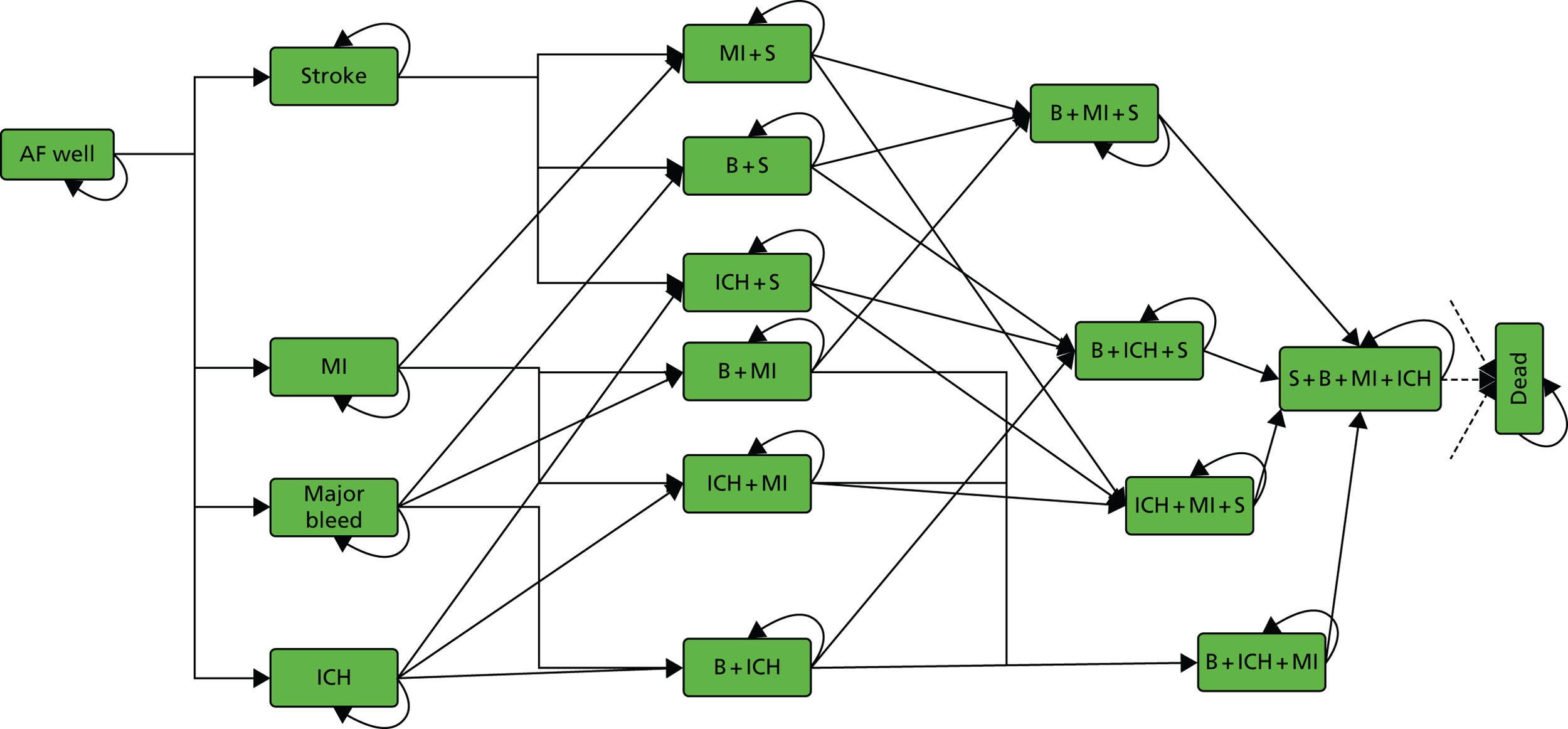
Each of the treatment strategies has the same model structure but with different costs, utilities and event probabilities. From any state, a patient can have a clinically relevant (extracranial) bleed, an ICH, an ischaemic stroke, a MI, a TIA, a SE, can discontinue or switch treatment because of these events or die. These events are similar to those used in earlier models. 34,42 The primary difference is that we do not distinguish between minor and major ischaemic stroke, as there was limited evidence from the RCTs to estimate the relative rates of these events. We also do not include non-clinically relevant minor bleed events, as it is assumed that they will not have a significant impact on costs, quality of life or future risks. As in most previous models, memory states are used to record a history of the most important previous events. The model assumes that SE and TIA have only short-term effects on future risks, costs and utilities, whereas ischaemic stroke, ICH, other CRB and MI have long-term consequences that must be modelled. Up to four major events are therefore recorded and assumed to affect future risks, costs and utilities. For example, patients with MI + ICH will have different risks, costs and utilities to patients with MI or ICH alone. Unlike the Wisloff 2013 model,41 our model does not distinguish between bleed locations, such as gastrointestinal and other types of bleed. Based on advice from clinical project team members, we assumed that the greatest impact on risks, costs and effects is captured by the broad definition of ‘clinically relevant bleeds’, as reported in the RCTs. In total, our model has 17 states, including a well state (‘AF Well’) and death.
At any cycle, patients can switch treatments to second-line or no treatment. All adverse health events increase the probability of treatment switching. An ICH is assumed to always lead to treatment switching. Patients are assumed to always switch treatment from dabigatran to warfarin if they experience a MI as a result of recent findings suggesting a link between dabigatran and MI risk. 77 Whether or not patients switch treatment after an ischaemic stroke depends on whether it was due to treatment failure or non-compliance. We assume it is due to treatment failure but that only a proportion of patients will switch treatment following an ischaemic stroke.
In the Markov model, future state transitions depend only on the current state in which the patient is (and not past history). We assume homogeneous transition probabilities that do not change with time. However, the age of the cohort will increase with each cycle and mortality risk increases accordingly, based on general population life tables. There is no available evidence to suggest treatment effects change with age or that they depend on event history. The model therefore makes the assumption that treatment effects are independent of age and event history.
Venous thromboembolism model structures: overview
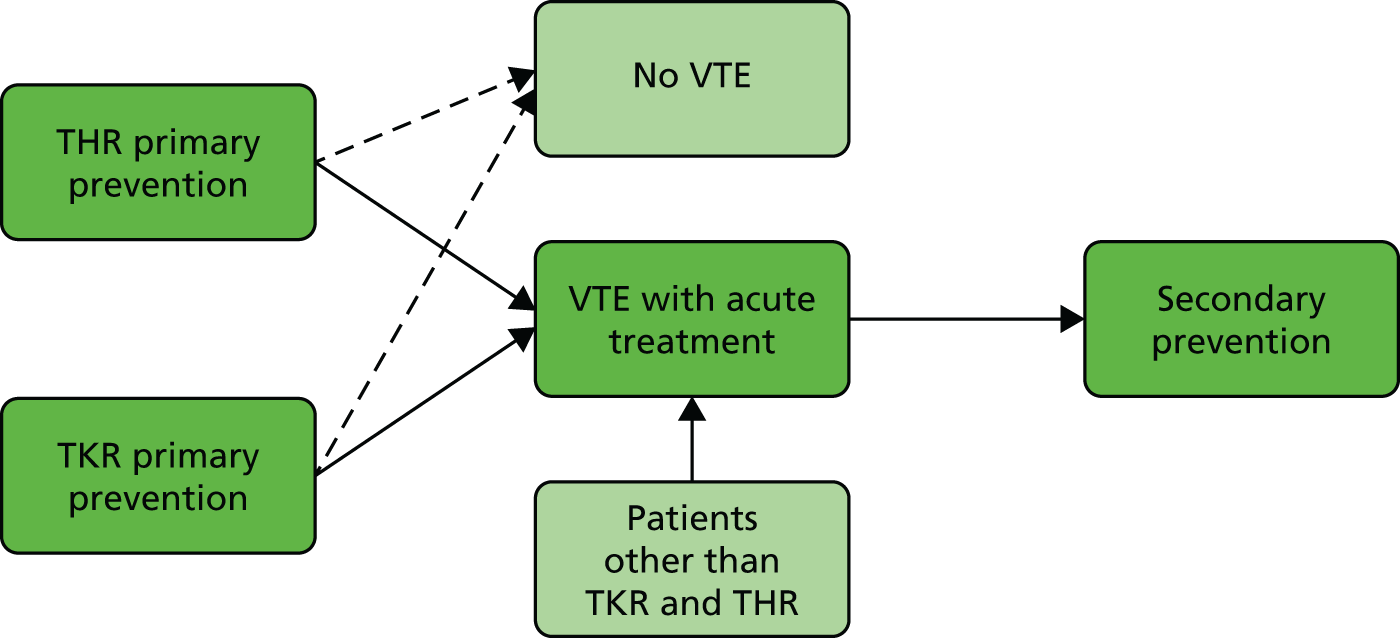
There were three model structures for the primary prevention, acute treatment and secondary prevention decision problems. The structure of the primary prevention model was identical in the two subpopulations (THR and TKR); however, the parameter values differ. Decision trees were used to model the initial costs and outcomes of primary prevention and acute treatment, where anticoagulation is used over short periods of time, and a Markov model evaluated secondary prevention, where anticoagulation may be prescribed over prolonged periods. The models are linked because most patients who have acute treatment for VTE will be considered for extended secondary prevention of recurrence and it is possible that a patient receiving anticoagulation for primary prevention will have a VTE requiring acute treatment and eventually secondary prevention (Figure 3). Therefore, we modelled the decision problems sequentially. We first estimated the most cost-effective method of secondary prevention. We then estimated the most cost-effective method of acute treatment, assuming that all patients who subsequently require secondary prevention are managed using the most cost-effective method from the secondary prevention model. Finally, we estimated the most cost-effective method of primary prevention, with the therapy used for acute treatment and secondary prevention determined based on the results of the first two models. For this reason, we begin our detailed discussion of the three models with the secondary prevention model.
FIGURE 3.
Population pathway. THR primary prevention, TKR primary prevention, VTE with acute treatment and secondary prevention.
Venous thromboembolism model structure: secondary prevention
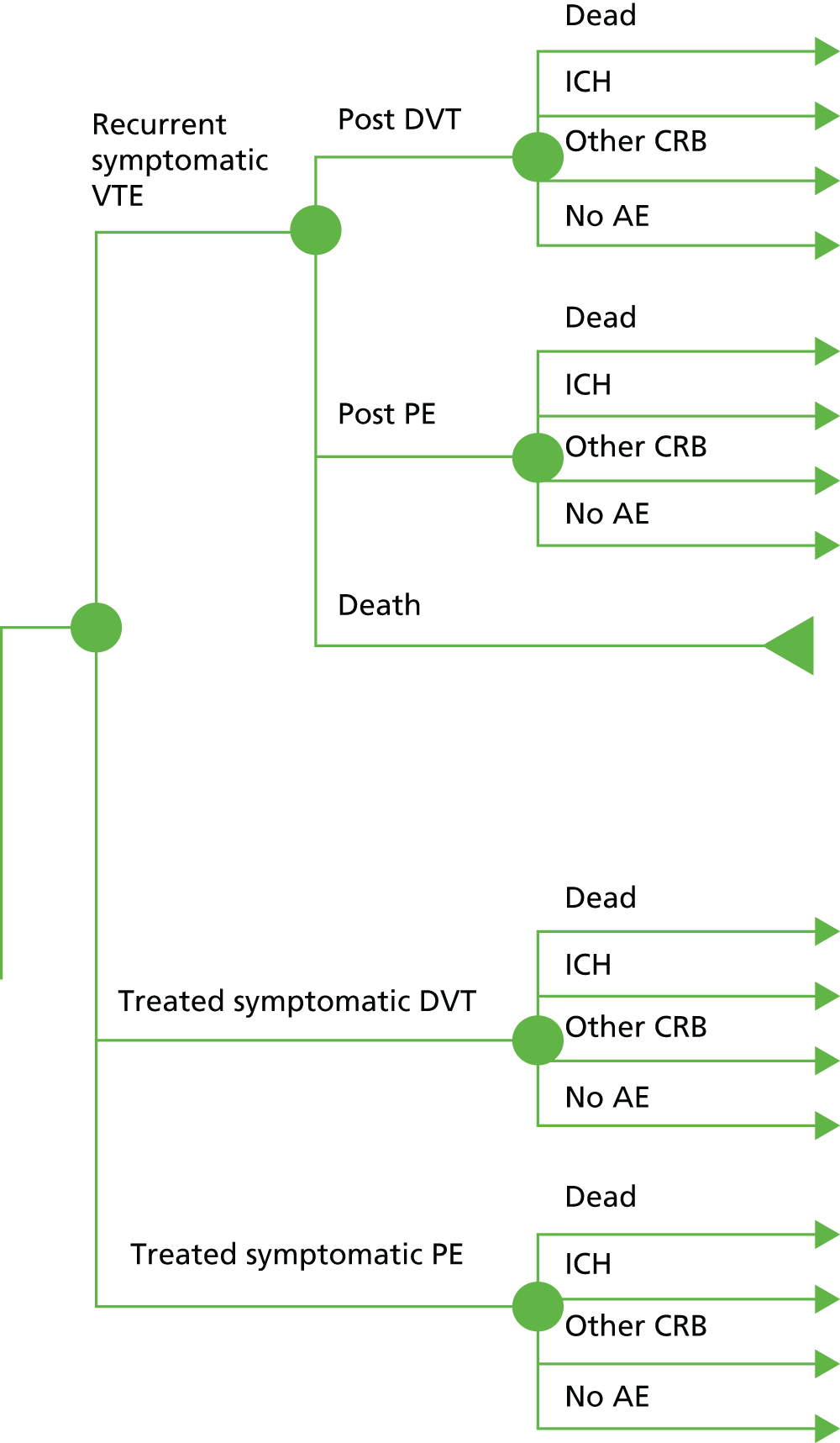
A Markov model with half-cycle correction78 was used to evaluate the cost-effectiveness of prophylaxis in patients who have experienced a previous non-fatal VTE event (Figure 4). The model has a cycle length of 1 year and includes eight health states (Table 8). Subjects enter the model in post PE or post DVT. Subjects in the ‘post DVT’ (or ‘post PE’) state can have an additional non-fatal DVT (or PE) event with a transient utility decrement and cost, but remain in the same health state. Subjects in the ‘post DVT’ state who experience a non-fatal PE and subjects in ‘post PE’ who experience a non-fatal DVT transition to ‘post PE DVT’. Subjects in the ‘post DVT’ and ‘post PE DVT’ states can develop post-thrombotic syndrome (PTS) and transition to either ‘mild/moderate PTS’ or ‘severe PTS’. Subjects who have had a PE may experience chronic thromboembolic pulmonary hypertension (CTPH). Subjects can transition to CTPH from ‘post PE’ and ‘post PE DVT’.
FIGURE 4.
Venous thromboembolism secondary prevention Markov model. Nodes represent the health states; lines between nodes represent possible transitions; all health states can transition to death. ICH, other clinically relevant bleeds, DVT and PE are acute events, which may lead to a change in chronic health state (e.g. post ICH).
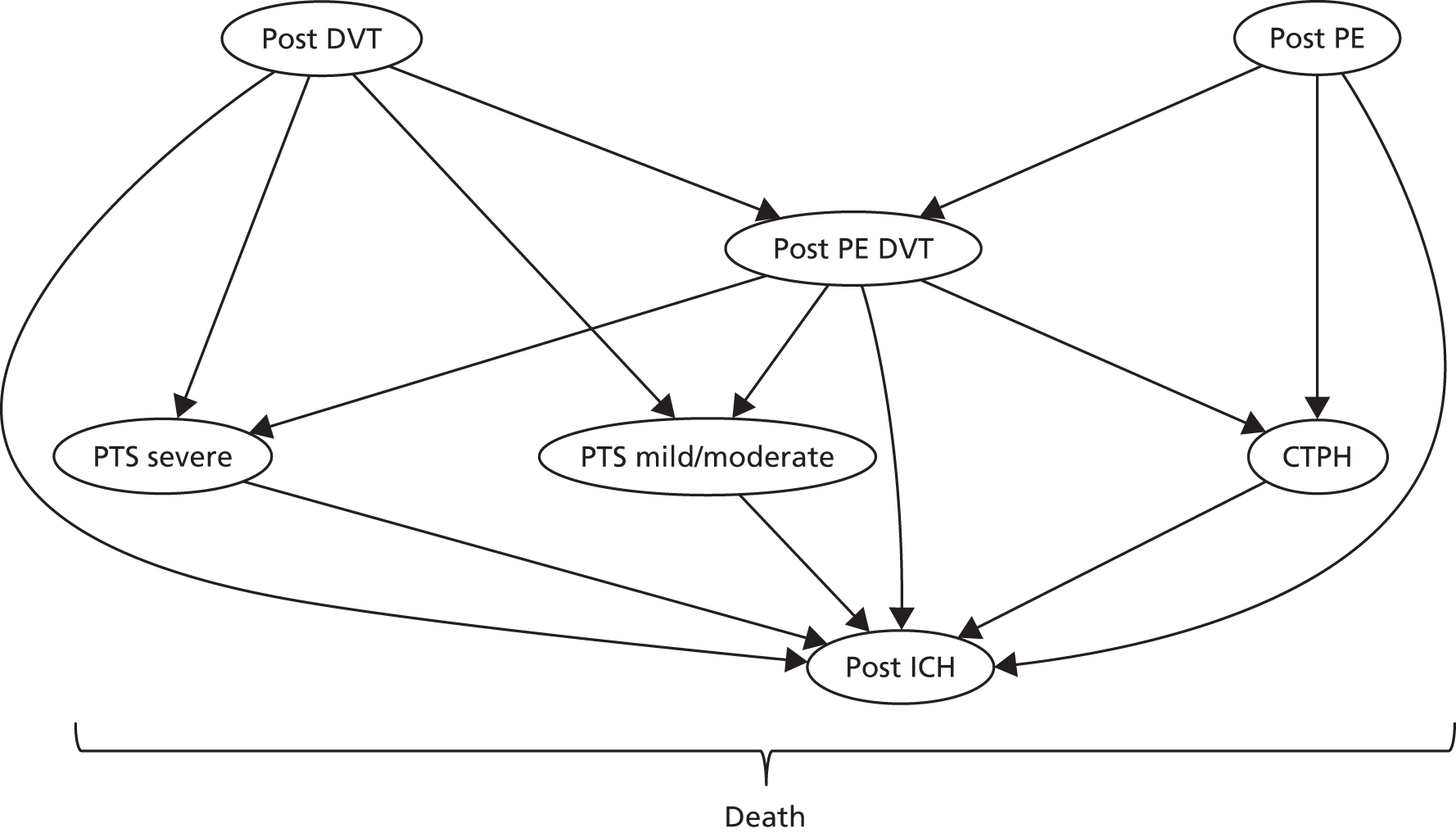
| Health state | Description |
|---|---|
| Post DVT | Experienced at least one DVT event and no PE events |
| Post PE | Experienced at least one PE event and no DVT events |
| Post PE DVT | Experienced at least one DVT event and at least one PE event |
| PTS mild/moderate | Mild/moderate PTS after one or more DVT events |
| PTS severe | Severe PTS after one or more DVT events |
| CTPH | CTPH after PE event |
| Post ICH | Post ICH |
| Death | Dead (any cause) |
All subjects who are receiving treatment can transition to the ICH health state. After entering this state, we assumed that anticoagulation therapy will be stopped and subjects will remain there until death, as this is considered to be the state with the lowest quality of life.
Venous thromboembolism model structure: acute treatment
The acute treatment of symptomatic VTE was modelled using a decision tree covering the first 6 months of therapy, in line with current guidelines for the duration of acute treatment (Figure 5). There is a probability that patients will experience recurrent symptomatic VTE during the acute treatment period and, regardless of VTE recurrence, all patients are at risk of other CRB or ICH. Longer-term costs and outcomes following acute treatment were estimated using the secondary prevention Markov model for patients who are alive at the end of acute treatment.
FIGURE 5.
Venous thromboembolism acute treatment decision tree. At the end of each branch in the decision tree, patients progress to the secondary prevention model. ICH branches enter in ‘post ICH’ state; treated symptomatic DVT (with bleed or no AE) will enter the post DVT state; treated symptomatic PE (with bleed or no AE) will enter the post PE state; recurrent symptomatic VTE post DVT will enter the post DVT or post DVT PE state, depending on the previous event; and recurrent symptomatic VTE post PE will enter the post PE or post DVT PE state, depending on the previous event.
Venous thromboembolism model structure: primary prevention
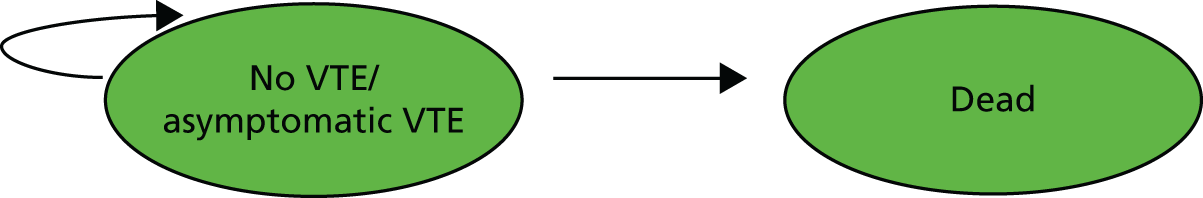
The primary prevention model consists of a decision tree covering the first 180 days of prophylactic anticoagulation (Figure 6). After this initial period, the long-term cost and outcomes of patients who do not have a symptomatic VTE are tracked using a two-state Markov model (Figure 7). This Markov model has two health states: no VTE/asymptomatic VTE and dead. The Markov model has a lifetime time horizon and yearly cycles. The longer-term costs and outcomes of patients who have a symptomatic VTE are tracked in the acute treatment model (see Figure 5) and the secondary prevention model (see Figure 4).
FIGURE 6.
Primary prevention decision tree. At the end of the decision tree, subjects will have experienced a symptomatic VTE or not; if they have then they will enter the acute treatment model. Those who did not experience a symptomatic VTE will enter the two-stage Markov model.

FIGURE 7.
Primary prevention Markov model.
Patients enter the primary prevention model after having elective surgery (TKR or THR). They then experience either a symptomatic VTE event or no VTE/asymptomatic VTE. Patients who experience a symptomatic event have a fatal PE, non-fatal PE or DVT, and are treated. Regardless of VTE incidence, all patients are at risk of another CRB during the initial 90-day period of anticoagulation. Because treatment duration is short for primary prevention, the risk of ICH is very low. and there is no evidence of a relative effect of NOACs compared with LMWH in this patient population. Therefore, we have not incorporated ICH in the primary prevention model.
Inputs partially shared between atrial fibrillation and venous thromboembolism models
Cost of pharmacotherapy
Average drug costs were based on the British National Formulary (BNF) March 2015 update,67 using the most economical pack size (Tables 9–11). Edoxaban does not currently have a list price in the UK. For the base case we assume that the 6-monthly cost is equivalent to dabigatran. We tested this assumption in a sensitivity analysis. As all of the NOACs are taken orally, it was assumed that there are no administration or monitoring costs, following the costing report in AF of Ali et al. 79 Average drug and monitoring cost of warfarin comes from a costing report by NICE68 and is cited in the study by Kansal et al. 42 The cost of LMWH was an average over all of the LMWHs included in the meta-analyses and listed in the BNF.
The unit costs of drugs are assumed to be fixed and known, so that point estimates – rather than distributions – are entered into the models. However, the administration and monitoring cost of warfarin is uncertain, and in the absence of other information we assumed a uniform distribution ranging from 50% to 150% of the estimated cost from the NICE costing report. 68 We performed a sensitivity analysis for the assumed cost of warfarin monitoring.
| Intervention | Dose per day (mg) | mg per tablet | No. in pack | Cost (£) per pack | Cost (£) per day | Administration cost (£) | Cost (£) per 3-month cycle AF model | Cost (£) per annual cycle VTE secondary prevention model |
|---|---|---|---|---|---|---|---|---|
| Apixaban | 10 | 5 | 56 | 61.50 | 2.20 | 0.00 | 200.42 | 802.25 |
| 5 | 2.5 | 60 | 65.90 | 2.20 | 0.00 | 200.44 | 802.33 | |
| Dabigatran | 300 | 150 | 60 | 65.90 | 2.20 | 0.00 | 200.44 | 802.33 |
| 220 | 110 | 60 | 65.90 | 2.20 | 0.00 | 200.44 | NA | |
| Rivaroxaban | 20 | 20 | 100 | 210.00 | 2.10 | 0.00 | 191.63 | 767.03 |
| Warfarin | a105.1368 | 420.52a |
| Intervention | Dose per day (mg) | mg per tablet | No. in pack | Cost (£) per pack | Time (days) | Cost (£) per treatment |
|---|---|---|---|---|---|---|
| Warfarin | 182.5 | 210.26a | ||||
| Dabigatran | 300 | 150 | 60 | 65.90 | 182.5 | 400.89 |
| Edoxaban | 60 | – | – | – | – | 400.89b |
| Rivaroxaban | 30 | 15 | 14 | 29.40 | 21 | 427.35 |
| 20 | 20 | 100 | 210 | 161.5 | ||
| Apixaban | 10 | 5 | 56 | 61.50 | 182.5 | 400.85 |
| Intervention | Dose per day (mg) | mg per tablet | No. in pack | Cost (£) per pack | Cost (£) per day |
|---|---|---|---|---|---|
| Apixaban (2.5 mg bd) | 5 | 2.5 | 60 | 65.90 | 2.20 |
| Dabigatran (220 mg od) | 220 | 110 | 60 | 65.90 | 2.20 |
| Rivaroxaban (10 mg od) | 10 | 10 | 100 | 210.00 | 2.10 |
| LMWH (post-op, standard dose) | – | – | – | – | 4.17a |
Cost of acute venous thromboembolism, atrial fibrillation and anticoagulant-related events
All costs of acute and chronic care were inflated to 2013–14 values using the Office for National Statistics (ONS) Consumer Price Inflation Index for Medical Services (DKC3). 80 Acute management costs for SE, MI, TIA, DVT, PE and CRB come from the 2012–13 NHS reference costs. 81 The reference costs for MI account for only direct hospitalisation; we assumed that total costs would be double this amount to account for follow-up costs. 82 The cost of a sudden fatal PE is assumed to be zero and the patients who have a non-fatal PE are assumed to accrue the full cost of a PE. Acute management costs for ischaemic stroke and ICH come from a study of patients with AF on a UK stroke registry. 83 Normal distributions are assumed for the mean acute costs, with SDs defined by the standard errors of the source data (Table 12).
| Event | Mean event cost (£) | Distribution(mean, standard error) | Source |
|---|---|---|---|
| Ischaemic stroke | 11,626 (SD = 16,868) | Normal(11,626, 1325) | aIschaemic stroke, all strokes83 |
| ICH | 11,453 (SD = 13,815) | Normal(11,453, 3350) | ICH or haemorrhagic stroke, all haemorrhagic strokes83 |
| SE (non-fatal) | 2373 | Uniform(1186.5, 3559.5) | NHS reference costs81 |
| TIA | 1064 | Uniform(532, 1596) | NHS reference costs81 |
| PEb | 1596 | Normal(1596, 159.6)c | NHS reference costs81 |
| DVTd | 712 | Normal(712, 71.2)c | NHS reference costs81 |
| CRBe | 1751.50 | Uniform(875.75, 2627.25) | NHS reference costs81 |
| MI | 4830 | Uniform(2415.24, 7245.72) | Acute MI, NHS reference costs for hospitalisation,81 doubled to include follow-up costs |
Cost of chronic care for venous thromboembolism, atrial fibrillation and anticoagulant-related events
Long-term management costs of stroke (ischaemic stroke or ICH) also come from the UK stroke registry83 (Table 13). This registry83 stratified the severity of ischaemic strokes by disability (non-disabling, moderately disabling, totally disabling) and we averaged their annual costs and SDs, weighted by the number of events. As in the study by Kansal et al. ,42 we assumed the same cost for ICH as for ischaemic stroke. Normal distributions are assumed, with SDs defined by the standard errors of the source data. For states with a history of multiple events, we assumed that the additional post-event management costs were the maximum of the management costs for the constituent events. We divided sampled costs by four to obtain 3-monthly cycle costs.
| Event | Mean cost (£) | Distribution | Source |
|---|---|---|---|
| Non-disabling | 2135 (SD = 3676, n = 66) | Luengo et al.83 | |
| Moderately disabling | 4165 (SD = 7668, n = 58) | ||
| Totally disabling | 6324 (SD = 14,898, n = 6324) | ||
| All (ischaemic stroke and ICH) | 3613 (SD = 4235, n = 136) | Normal (3613, 363) | Weighted average of the mean and SD, inflated to 2013–14 prices |
Costs for mild to moderate and severe PTS have previously been estimated in a NICE technology appraisal,84 which looked at the clinical effectiveness and cost-effectiveness of dabigatran for the prevention of VTE after a TKR or TKR in adults. This study84 converted and inflated costs from a US economic burden study85 of long-term complications of primary prevention of DVT after a THR. This study84 estimated the cost of mild to moderate PTS to be £541 for the first year and £220 for subsequent years, and severe PTS to be £2461 for the first year and £602 for subsequent years. Inflating to 2013–14 values resulted in a cost of £689 for the first year and £280 for subsequent years for mild/moderate PTS, and £3136 for the first year and £767 for subsequent years of severe PTS. NICE guidance for the management of VTE71 estimated a 4-weekly cost of CTPH to be £2173, equivalent to an annual cost of £33,028 in 2013–14 prices.
Utilities
The AF and VTE models used utility weights combined with survival to estimate QALYs. Utility weights are anchored at 1 (best health) and 0 (as bad as death), such that a year spent in an intermediate health state with a utility weight of 0.5 would be considered equivalent to 6 months in the best health state with a utility value of 1. The models have a number of acute health events that affect patients for a short period, followed by a partial or full recovery and a number of chronic health states from which patients do not recover. Several of these health events and health states are shared between the AF and VTE models.
Utilities were identified from a previous NICE technology appraisal submission on rivaroxaban33 and from a rapid literature review to identify quality-of-life studies in VTE. The rivaroxaban technology appraisal submission33 conducted a systematic literature search for evidence on EQ-5D (European Quality of Life-5 Dimensions) utility index in health states related to AF. For VTE events (DVT and PE), Locadia et al. 86 estimated health utilities, using time trade-off methods, from a cohort of 53 patients who had experienced a VTE event.
Utilities of venous thromboembolism, atrial fibrillation and anticoagulant-related acute health events
The acute health event disutilities for AF for other CRB, SE and TIA are reported in Table 14. The remaining acute health event disutilities for AF (acute ICH and acute MI; see Table 14) are obtained by subtracting ‘Stable AF’ from the utility of the event. For example, the disutility for MI would be 0.683 – 0.779 = –0.096.
| Health state | Utility score | Distributiona | Source |
|---|---|---|---|
| Reference group health state | |||
| Stable AF quality of life (for AF model) | 0.779 (SD = 0.253, n = 3045, standard error = 0.0045) | Normal(0.779, 0.0045) | Berg 201087 |
| No VTE quality of life (for VTE model) | 0.96 (SD = 0.046) | Beta(16.52, 0.69) | Locadia 200486 |
| Acute health eventsb | |||
| TIA and SE disutility | –0.131 | Uniform(–0.197, –0.066) | Robinson 200188 |
| Acute ischaemic stroke disutility | –0.59 | Uniform(–0.885, –0.295) | Robinson 200188 |
| DVT (first and subsequent) | 0.84 (SD = 0.087) | Beta(14.17, 2.70) | Locadia 200486 |
| PE (first and subsequent) | 0.63 (SD = 0.128) | Beta(8.40, 4.93) | Locadia 200486 |
| Acute ICH disutility | Median 0.60 (95% CI 0.02 to 1.00) (n = 60) | Normal(0.60, 0.064) – AF well | Lenert 199789 |
| Other CRB disutility | –0.03 (standard error = 0.001531) | Normal(–0.03, 0.001531) | Robinson 200188 |
| Acute MI disutility | 0.683 (SD = 0.233, n = 222, standard error = 0.0156) | Normal(0.683, 0.0156) – AF well | cLacey 200390 |
| Chronic health states | |||
| Post ischaemic stroke quality of life | 0.69 (SD = 0.18, n = 77, standard error = 0.0205) | Normal(0.69, 0.0205) | dHaacke 200691 |
| Mild/moderate PTS | –0.02e | Beta(97.98, 4801.02) | Lenert 199789 |
| Severe PTS | –0.07e | Beta(92.93, 1234.64) | Lenert 199789 |
| CTPH | 0.57 (SD 0.31) | Beta(1.20, 0.94) | Meads 200893 |
| Post ICH quality of life | 0.74 (SD = 0.39, n = 5, standard error = 0.1744) | Beta(3.941, 1.385) | fHaacke 200691 |
| Post MI quality of life | 0.718 (SD = 0.243, n = 222, standard error = 0.0163) | Normal(0.718, 0.0163) | cLacey 200390 |
These disutilities are capped above at 0. Further acute event utility values extracted from the literature for VTE primary prevention, acute treatment and secondary prevention models are reported in Table 15. When uncertainty estimates were reported, we assumed that mean utilities would be normally distributed, as indicated by the central limit theorem. When uncertainty estimates and sample sizes were not available (acute ischaemic stroke, TIA, SE), we assumed mean utilities to follow a uniform distribution ranging from 50% to 150% of the reported mean. The duration of the decrements for DVT and PE was assumed to be 6 months71 and 3 months for ICH, before moving to the post-ICH health state. 51 Duration of decrements was generally not reported for the AF disutilities, so they were assumed to last one cycle.
| Transient event | Utility/decrement | Duration of decrement | Source |
|---|---|---|---|
| DVT (first and subsequent) | 0.84 (0.64 to 0.98) | 6 months | Locadia 200486 |
| PE (first and subsequent) | 0.63 (0.36 to 0.86) | 6 months | Locadia 200486 |
| ICH | 0.60 (0.02 to > 0.99) | 3 months | Lenert 199789 |
| Other clinically relevant bleed | 0.03 (standard error 0.001531)a | Absolute decrement | Robinson 200188 |
In both the AF and VTE model, to account for quality of life decreasing with age, all utility decrements were multiplied by the ratio of the utility for a given age range relative to a reference age (65–75 years), based on general population utilities estimated in Kind et al. 92 (Table 16). Utilities were also adjusted by gender in this way for the VTE models, whereas for the AF models all utilities were weighted averages across gender.
| Age (years) | Male | Female | Source | ||
|---|---|---|---|---|---|
| Mean (SD) | Alpha, beta | Mean (SD) | Alpha, beta | ||
| < 25 | 0.94 (0.12) | 0.94 (0.12) | Kind 199992 | ||
| 25–34 | 0.93 (0.16) | 0.93 (0.15) | |||
| 35–44 | 0.91 (0.17) | 656.7, 65.0 | 0.91 (0.15) | 1006.6, 99.5 | |
| 45–54 | 0.84 (0.27) | 341.4, 65.0 | 0.85 (0.23) | 544.1, 96.0 | |
| 55–64 | 0.78 (0.28) | 330.4, 93.2 | 0.81 (0.26) | 526.6, 123.5 | |
| 65–74 | 0.78 (0.28) | 388.5, 109.6 | 0.78 (0.25) | 551.7, 155.6 | |
| ≥ 75 | 0.75 (0.28) | 191.2, 63.7 | 0.71 (0.27) | 406.4, 166.0 | |
Utilities of venous thromboembolism, atrial fibrillation and anticoagulant-related chronic health states
In the AF model, for which patients can have more than one chronic health condition, utilities for chronic health states are assumed to be multiplicative. For example, the utility of a patient who has experienced both an ischaemic stroke and a MI will be the product of the two utility scores (see Table 14): 0.690 × 0.718 = 0.495.
Utilities are multiplied by 0.25 to get a QALY for 3-month cycle.
For the VTE-related chronic health states, we used estimates from the study by Lenert and Soetikno,89 who elicited preferences in 30 volunteers and 30 medicine physicians with mild/moderate PTS and severe PTS, and the study of Meads et al. ,93 who used the Cambridge Pulmonary Hypertension Outcome Review utility index94 to estimate a utility value for CTPH from 308 patients (see Table 14). Further chronic VTE-related chronic health-state utilities extracted from the literature are reported in Table 17.
| Health state | Utility/decrement | Source |
|---|---|---|
| Reference – no VTE | 0.96 (0.82 to 1.00) | Locadia 200486 |
| Mild/moderate PTS | 0.02 (SD 0.04)a | Lenert 199789 |
| Severe PTS | 0.07 (SD 0.07)a | Lenert 199789 |
| CTPH | 0.57 (SD 0.31) | Meads 200893 |
| Post ICH | 0.74 (standard error 0.1744) | Haacke 200691 |
| Death | 0.00 (0 to 0) | Definition |
Summary
This chapter summarises the decision problems addressed by the cost-effectiveness models; the structure, perspective, and target population of the models; and the interventions and outcomes represented by the models. We developed the structure of the model based on existing cost-effectiveness models that were identified in the literature, and the structure evolved based on feedback from clinical experts in order to reflect current disease knowledge and clinical practice. We used decision trees to reflect the short-term nature of the VTE primary prevention and acute treatment decision problems, and Markov models to address the AF-related ischaemic stroke and VTE secondary prevention decision problems for which longer periods of prophylaxis are required.
This chapter also summarises the cost and utility model inputs shared by the AF and VTE models. Model inputs on the relative treatment efficacy and safety of anticoagulants were derived from the results of meta-analyses of RCTs identified in our systematic review. We summarise these efficacy and safety model inputs in Chapters 6 and 11, which also present the results of the cost-effectiveness models for AF and VTE.
Chapter 5 Clinical results (1): stroke prevention in atrial fibrillation
Included studies
A total of 1852 unique records were identified from various data sources for the review of stroke prevention in AF (Figure 8).
FIGURE 8.
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow chart for review of stroke prevention in AF.

Twenty-three completed eligible RCTs were identified for inclusion in the review, with a total of 41 associated references for these trials. 95–135 No ongoing trials were identified. A summary of the characteristics of the 23 trials is presented in Table 18. Twenty of the trials were multicentre; two trials were each conducted in two centres; and one trial was conducted in one centre. The majority of the multicentre trials were conducted across several countries in North and South America, Europe, Asia, Russia and Israel, Australia and South Africa. The two-centre trials were conducted in one country: one in China and the other in Denmark. The single-centre trial was conducted in Denmark. Sixteen of the trials were Phase III studies and seven were Phase II studies. The number of patients randomised across the 23 trials ranged from 75 to 21,105, with a total of 94,656 patients, of whom 97% (91,333) were from the Phase III studies. Thirteen studies: six Phase III and seven Phase II studies examined a NOAC. Four studies examined edoxaban, three each examined apixaban and dabigatran, two examined rivaroxaban and one examined betrixaban.
| Study (centre type) [countries] | Study type, sponsor (sponsor’s role) | Age eligibility (mean age, years) [% male] | AF type | No. randomised | Interventions compared | Treatment duration (months) | Mean TTR (INR), % | Outcomes | Time of outcome assessment (months) |
|---|---|---|---|---|---|---|---|---|---|
| ACTIVE W100 (Multicentre) [North and South America, Europe, Russia, Israel, Australia, Asia and South Africa] |
Phase III Sanofi-Aventis and Bristol-Myers Squibb (The sponsor contributed to the study design ‘but had no role in data collection, data analysis, data interpretation, or writing of the report’) |
≥ 18 (70.2) [66.1] | Non-valvular ECG diagnosed |
6706 | Antiplatelet: 1. Clopidogrel 75 mg + (aspirin 75–100 mg) od Warfarin: 2. INR 2–3 (some patients may have received other VKAs in use in their country) |
Not given | 63.8 | Efficacy: All stroke, ischaemic stroke, haemorrhagic stroke, MI Safety: All bleeding, major bleeding, minor bleeding, fatal bleeding, death (all causes) |
15.4 |
| AFASAK95 (Two centres) [Denmark] |
Phase III NycoMed AS, Oslo, Norway; Henrik Henriksen’s Foundation; Kathrine and Vigo Skovgaard’s Foundation; and Danish Medical Research Foundation (Not stated) |
≥ 18 (74.2) [53.6] | Chronic non-valvular ECG diagnosed |
1007 | Warfarin: 1. INR 2–3 Antiplatelet (aspirin): 2. 75 mg od 3. Placebo od |
24 | 73 | Efficacy: All stroke, fatal stroke, minor ischaemic stroke, TIA Safety: Bleeding, death (all causes) |
24 |
| AFASAK II97 (Single centre) [Denmark] |
Phase III The Danish Heart Foundation, Copenhagen; Nycomed DAK A/S Roskilde, Denmark; DuPont Pharma, Wilmington, DE, USA; The Danish Foundation for Medical Research for the Region of Copenhagen; and many other non-industry funders (Not stated) |
≥ 18 (74.2) [60] | Chronic non-valvular ECG diagnosed |
677 | Warfarin: 1. 1.25 mg/day fixed dose 2. 1.25 mg/day fixed dose plus aspirin 300 mg/day od 3. INR 2–3 Aspirin: 4. 300 mg od |
42 | 73 | Efficacy: All stroke, ischaemic stroke, haemorrhagic stroke, fatal stroke, stroke or SE, TIA, MI Safety: Major bleeding, minor bleeding, intracranial bleeding, death (all causes) |
42 |
| AF-ASA-VKA-CHINA135 (Two centres) [China] |
Phase III Grant from talent pool subject of Shanghai Shi Dong Hospital (Not applicable) |
≥ 80 (NR) [NR] | Persistent and permanent non-valvular Confirmed by the case history and ECG |
110 | Warfarin: 1. INR 1.6–2.5 Antiplatelet (aspirin): 2. 100 mg od |
24 | NR | Efficacy: Stroke or SE, ischaemic stroke, MI Safety: All bleeding, major bleeding, minor bleeding, fatal bleeding, death (all causes) |
1, 6, 12, 18, 24 |
| AF-DABIG-VKA-JAPAN110 (Multicentre) [Japan] |
Phase II Boehringer Ingelheim (The sponsor was involved in the trial) |
≥ 20 (NR) [NR] | Paroxysmal, persistent or permanent non-valvular ECG diagnosed |
174 | Dabigatran: 1. 110 mg bd 2. 150 mg bd Warfarin: 3. INR 2–3 (INR ≥ 1.6 to ≤ 2.6 in ≥ 70 years) |
3 | NR | Efficacy: Stroke or SE Safety: All bleeding, major bleeding, composite CRB |
3 |
| AF-EDOX-VKA-ASIA115 (Multicentre) [Taiwan, South Korea, Hong Kong and Singapore] |
Phase II Daiichi Sankyo Co., Ltd, Tokyo, Japan (The sponsor had influence on the study design, data management and analysis, and key decisions) |
18–80 (65.1) [65.4] | Non-valvular ECG diagnosed CHADS2 ≥ 1 |
235 | Edoxaban: 1. 30 mg od 2. 60 mg od Warfarin: 3. INR 2–3 |
3 (edoxaban) 6 (warfarin) |
45.1 | Efficacy: Stroke or SE Safety: All bleeding, major bleeding, minor bleeding, CRNM bleeding |
3 |
| AF-EDOX-VKA-JAPAN12 (Multicentre) [Japan] |
Phase II Daiichi Sankyo Co., Ltd, Tokyo, Japan (The funder ‘had input on the study design and data analysis and interpretation of results and wrote the clinical study report’) |
≥ 20 (NR) [NR] | Non-valvular ECG diagnosed CHADS2 ≥ 1 |
536 | Edoxaban: 1. 30 mg od 2. 45 mg od 3. 60 mg od Warfarin: 4. INR 2–3 (INR 1.6–2.6 in ≥ 70 years) |
3 | 83 (≥ 70) 73 (< 70 years) | Efficacy: Stroke or SE Safety: All bleeding, major bleeding, CRNM bleeding, composite CRB |
3 |
| AF-EDOX-VKA-MULTI108 (Multicentre) [North America, Chile, Europe and Russia] |
Phase II Daiichi Sankyo Co., Ltd, Tokyo, Japan (Not clear) |
18–85 (65.1) [62.1] | Persistent non-valvular ECG diagnosed CHADS2 ≤ 2 |
1146 | Edoxaban: 1. 30 mg od 2. 60 mg od 3. 30 mg bd 4. 60 mg bd Warfarin: 5. INR 2–3 |
3 | 49.7 | Efficacy: Stroke or SE, MI, hospital admission Safety: All bleeding, major bleeding, minor bleeding, CRNM bleed, composite CRB, death (cardiovascular) |
3 |
| AF-VKA-ASA-CHINA122 (Multicentre) [China] |
Phase III 10th National Five-year Project of China (Not applicable) |
50–80 (NR) [NR] | Non-valvular Diagnosis based on medical history, ECG and/or Holter recordings |
690 | Warfarin: 1. INR 2.1–2.5 2. INR 1.6–2 Antiplatelet (aspirin): 3. 200 mg od |
24 (mean 15) | NR | Efficacy: All stroke, ischaemic stroke, haemorrhagic stroke, TIA Safety: Major bleeding, minor bleeding, death (all causes) |
24 |
| ARISTOTLE107,114,119,124–127,130,132–134 (Multicentre) [North and South America, Europe, Russia, Israel, Australia, Asia and South Africa] |
Phase III Bristol-Myers Squibb and Pfizer (The trial was designed in conjunction with the sponsors and ‘The primary analyses were performed both at Bristol-Myers Squibb and at the Duke Clinical Research Institute’) |
≥ 18 (median 70) [64.7] | Non-valvular or flutter ECG diagnosed |
18,201 | Apixaban: 1. 5 mg bd (2.5 mg bd in participants with more than one of: ≥ 80 years, ≤ 60 kg body weight, serum creatinine level of 1.5 mg per decilitre or more Warfarin: 2. INR 2–3 |
21.6 (median) | 62.2 | Efficacy: All stroke, ischaemic stroke, haemorrhagic stroke, stroke or SE, MI Safety: All bleeding, major bleeding, composite CRB, intracranial bleeding, death (all causes) |
21.6 (median for intracranial bleeding) |
| ARISTOTLE-J113 (Multicentre) [Japan] |
Phase II Pfizer Inc. and Bristol-Myers Squibb (Not clear) |
≥ 20 (70.3) [82.9] | Non-valvular Diagnosis based on ECG, Holter recording or intracardiac electrogram |
222 | Apixaban: 1. 2.5 mg bd 2. 5 mg bd Warfarin: 3. INR 2–3 (INR 2–2.6 in ≥ 70 years) |
3 | 60 | Efficacy: Stroke or SE, ischaemic stroke, TIA Safety: All bleeding, major bleeding, minor bleeding, CRNM bleeding, composite CRB, death (all causes) |
3 |
| AVERROES105,116,117,121 (Multicentre) [North and South America, Europe, Russia, Israel, Australia, Asia and South Africa] |
Phase III Bristol-Myers Squibb and Pfizer (The sponsor was involved in the design, data collection and analysis) |
≥ 50 (70) [58.5] | Non-valvular ECG diagnosed |
5599 | Apixaban: 1. 5 mg bd (2.5 mg if > 80 years/≤ 60 kg/renal status) Antiplatelet (aspirin): 2. 81–324 mg od |
13.1 (mean) | Efficacy: All stroke, stroke or SE, ischaemic stroke, haemorrhagic stroke, MI Safety: Major bleeding, minor bleeding, CRNM bleeding, intracranial bleeding, fatal bleeding, death (cardiovascular), death (all causes) |
13.1 (mean) | |
| BAFTA103 (Multicentre) [UK] |
Phase III The Medical Research Council UK and supported by MidReC and the Primary Care Research Trust (The sponsor had no direct role in study design, data collection, analysis or interpretation, writing the report the decision to submit for publication) |
≥ 75 (81.5) [54.6] | Non-valvular or atrial flutter ECG diagnosed |
973 | Antiplatelet (aspirin): 1. 75 mg od Warfarin: 2. INR 2–3 |
32.4 (mean) | 67 | Efficacy: All stroke, MI Safety: Major bleeding, death (all causes) |
32.4 (mean) |
| Chinese ATAFS99 (Multicentre) [China] |
Phase III (Not disclosed) |
40–80 (63.3) [59.7] |
Non-valvular | 704 | Antiplatelet (aspirin): 1. 150–160 mg od Warfarin: 2. INR 2–3 (INR 1.6–2.5 in > 75 years) |
NR | NR | Efficacy: All stroke Safety: Death (all causes) |
2–24 (median = 19) |
| ENGAGE AF-TIMI 48111,131 (Multicentre) [North and South America, Europe, Russia, Israel, Australia, Asia and South Africa] |
Phase III Daiichi Sankyo Pharma Development (Not clear) |
≥ 21 (NR) [61.9] | Non-valvular ECG diagnosed CHADS2 ≥ 2 |
21,105 | Edoxaban: 1. 30 mg od 2. 60 mg od (half dose if creatinine clearance is 30–50 ml/minute, ≤ 60 kg body weight, or concomitant use of verapamil or quinidine or dronedarone) Warfarin: 3. INR 2–3 |
29.8 (median) | 64.9 | Efficacy: All stroke, ischaemic stroke, haemorrhagic stroke, fatal stroke, stroke or SE, MI Safety: Major bleeding, minor bleeding, fatal bleeding, intracranial bleeding, CRNM bleeding, composite CRB, death (cardiovascular), death (all causes) |
29.8 (median) |
| EXPLORE-Xa128 (Multicentre) [USA, Canada and Germany] |
Phase II Portola Pharmaceuticals, South San Francisco, CA, USA (Not stated) |
≥ 18 (73) [66.5] | New or existing non-valvular or atrial flutter Diagnosed by Holter, ECG, rhythm strip, pacemaker or other intracardiac recording |
508 | Betrixaban: 1. 40 mg od 2. 60 mg od 3. 80 mg od Warfarin: 4. INR 2–3 |
4.9 (mean) | 63.4 | Efficacy: All stroke Safety: All bleeding, major bleeding, minor bleeding, CRNM bleeding, composite CRB, death (all causes) |
4.9 (mean) |
| J-ROCKET AF120 (Multicentre) [Japan] |
Phase III Bayer Yakuhin Ltd (The funder was ‘responsible for trial design and study data collection’) |
≥ 20 (71.1) [80.6] | Non-valvular ECG diagnosed |
1280 | Rivaroxaban: 1. 15 mg od (10 mg od if creatinine clearance 30–49 ml/minute) Warfarin: 2. INR 2–3 (INR 1.6–2.6 in ≥ 70 years) |
30 | 65% | Efficacy: All stroke, ischaemic stroke, haemorrhagic stroke, stroke or SE, MI Safety: Composite CRB, death (cardiovascular), death (all causes) |
30 |
| PATAF98 (Multicentre) [Netherlands] |
Phase III Prevention fund (grant 002817010), Zorgonder-zoek Nederland; Roche Nicholas BV, Bladel, Holland, donated aspirin (Not stated) |
≥ 60 (74.8) [44.9] | Chronic or intermittent ECG diagnosed |
729 | Warfarin: 1. INR < 2 2. INR 2.5–3.5 (some patients received other coumarins – phenprocoumon or acenocoumarol) Antiplatelet (aspirin): 3. 150 mg od |
32.4 (mean) | NR | Efficacy: All stroke, ischaemic stroke, arterial event Safety: Death (cardiovascular), death (all causes) |
32.4 (mean) |
| PETRO102 (Multicentre) [USA, Denmark, the Netherlands and Sweden] |
Phase II Boehringer Ingelheim Pharmaceuticals, Biberach, Germany (The funder was responsible for the statistical analysis conducted according to a prospectively designed plan approved by the steering committee) |
≥ 18 (69.5) [81.9] | Permanent, persistent and paroxysmal non-valvular with coronary artery disease Diagnosis not explained |
502a | Dabigatran: 1. 50 mg bd 2. 50 mg + (aspirin 81 mg) bd 3. 50 mg + (aspirin 325 mg) bd 4. 150 mg bd 5. 150 mg + (aspirin 81 mg) bd 6. 150 mg + (aspirin 325 mg) bd 7. 300 mg bd 8. 300 mg + (aspirin 81 mg) bd 9. 300 mg + (aspirin 325 mg) bd Warfarin: 10. INR 2–3 |
3 | 57.2 | Efficacy: Stroke or SE Safety: All bleeding, major bleeding, composite CRB |
3 |
| RE-LY104,109 (Multicentre) [North and South America, Europe, Russia, Israel, Australia, Asia and South Africa] |
Phase III Boehringer Ingelheim (The sponsor contributed to the design, conduct, and reporting of the study) |
≥ 18 (71) [63.6] | Non-valvular ECG diagnosed Mean CHADS2 = 2.1 |
18,113 | Dabigatran: 1. 110 mg bd 2. 150 mg bd Warfarin: 3. INR 2–3 |
24 (mean) | 64 | Efficacy: Stroke or SE, ischaemic stroke, haemorrhagic stroke, MI, PE, hospital admission Safety: Major bleeding, minor bleeding, intracranial bleeding, extracranial minor bleeding, death (cardiovascular), death (all causes) |
24 (mean) |
| ROCKET AF106,112,123,129 (Multicentre) [North and South America, Europe, Russia, Israel, Australia, New Zealand, Asia and South Africa] |
Phase III Johnson & Johnson, Bayer (The sponsor was not involved in the coordination of the trial, data management and analyses) |
≥ 18 (median 73) [60.3] | Non-valvular ECG diagnosed CHADS2 ≥ 2 |
14,264 | Rivaroxaban: 1. 20 mg od (15 mg in patients with a creatinine clearance of 30–49 ml/minute) Warfarin: 2. INR 2–3 |
19.4 (median) | 55 | Efficacy: All stroke, stroke or SE, MI Safety: Major bleeding, CRNM bleeding, composite CRB, fatal bleeding, intracranial bleeding, death (all causes) |
19.4 (median) |
| SPAF II96 (Multicentre) [USA] |
Phase III The Division of Stroke and Trauma, National Institute of Neurological Disorders and Stroke (Not clear) |
Not clear (NR) [NR] | Non-valvular | 1100 | Warfarin: 1. INR 2–4.5 in < 75 years 2. INR 2.0–4.5 in > 75 years Antiplatelet (aspirin): 3. 325 mg (in < 75 years) od 4. 325 mg (in > 75 years) od |
37.2 (mean for age < 75 years); 24 (mean for age > 75 years) | NR | Efficacy: Stroke or SE, ischaemic stroke, MI, TIA Safety: Intracranial bleeding, death (all causes) |
27.6 (mean) |
| WASPO101 (Multicentre) [UK] |
Phase III Not declared |
> 80 and < 90 (median 83) [47] | Permanent non-valvular ECG diagnosed |
75 | Warfarin: 1. INR 2–3 Antiplatelet (aspirin): 2. 300 mg od |
12 | 69.2% | Efficacy: All stroke, TIA Safety: Death (all causes) |
12 |
Eligibility criteria for patient participation were similar across studies: all patients having non-valvular AF, whether new or existing, and including paroxysmal, persistent or permanent types. Diagnosis of AF was predominantly by electrocardiography. In a few cases, Holter recording, pacemaker or other intracardiac recording was used. The mean age of included patients was reported in only 61% of the studies and this ranged from 63.3 to 81.5 years. The percentage of male patients was reported in 78% of the studies, and this varied significantly across the studies, ranging from 44.9% to 82.9%. Mean BMI was not often reported and ranged from 24.4 to 30.5 kg/m2. Percentage of patients with previous stroke, hypertension and chronic heart failure varied significantly across the studies, ranging from 5% to 63.8%, from 38% to 93.7%, and from 0% to 100%, respectively. Bleeding risk among patients was assessed predominantly with the CHADS2 scoring system.
Warfarin was examined in all but two of the 23 of the included studies, against a NOAC in 12 studies, and against aspirin in nine studies. Standard intensity warfarin (INR 2–3) was examined by all of the studies, although in a few studies the warfarin arm was a mixture of low intensity (INR < 2) and standard intensity, in unknown proportions. Across all of the studies, mean TTR for warfarin ranged from 45.1% to 83% of the treatment duration. One study98 compared both low intensity warfarin (INR < 2) and standard intensity (INR 2.5–3.5) dicoumarol with aspirin, but the mean TTR was not reported for the standard intensity dicoumarol arm. The doses of NOACs we examined were edoxaban 30 mg, 45 mg, and 60 mg od and 30 mg and 60 mg bd; apixaban 2.5 mg and 5 mg bd; dabigatran 50 mg, 110 mg, 150 mg and 300 mg bd; rivaroxaban 15 mg and 20 mg od; and betrixaban 40 mg, 60 mg and 80 mg od. Examined aspirin dosages ranged from 75 mg to 325 mg od.
Treatment duration in the edoxaban and dabigatran studies was predominantly 3 months, although one study reported mean treatment durations of 24 months and another reported a median treatment duration of 29.8 months. Mean treatment duration for apixaban studies ranged from 13.1 to 21.6 months and one study reported 3 months’ treatment duration. The two studies on rivaroxaban reported 30 months’ treatment duration and a mean treatment duration of 19.4 months, respectively. Mean treatment duration 4.9 months was reported in the betrixaban study. Treatment duration was similar for each comparator in almost all the NOAC studies. Reported efficacy and safety outcome types were similar across studies and these were reported at the end of the treatment periods. All 23 studies reported data on stroke, 15 studies reported data on MI, 18 studies reported data on major bleeding, 12 studies reported data on CRB, and 18 studies reported data on all-cause mortality. Fifteen of the 23 studies, including all the NOAC studies, were sponsored by pharmaceutical companies. Six studies were funded by grants from medical research bodies although two of these grants contained contributions from a pharmaceutical company. Sponsor detail was not reported in two studies. In most of the pharmaceutical company sponsored studies, the sponsor(s) had influence on the study design, data management and analysis.
Time in therapeutic range for warfarin interventions
Table 19 shows the comparator interventions, target INR and (where reported) mean TTR for the 22 studies that included a warfarin intervention arm. Sixteen (73%) of these studies reported mean TTR, which varied substantially (from 45.1% to 83%) between studies.
| Study | Interventions that were compared with warfarin | Warfarin INR | Mean TTR (INR), % |
|---|---|---|---|
| ACTIVE W100 | Antiplatelet (clopidogrel 75 mg + (aspirin 75–100 mg) od | 2–3 (some patients may have received other VKAs) | 63.8 |
| AFASAK95 | Aspirin 75 mg od Placebo od |
2–3 | 73 |
| AFASAK II97 | Aspirin 300 mg od | 2–3 | 73 |
| AF-ASA-VKA-CHINA135 | Aspirin 100 mg od | 1.6–2.5 | NR |
| AF-DABIG-VKA-JAPAN110 | Dabigatran 110 mg, 150 mg bd | 2–3 (≥ 1.6 to ≤ 2.6 in ≥ 70 years) | NR |
| AF-EDOX-VKA-ASIA115 | Edoxaban 30 mg, 60 mg od | 2–3 | 45.1 |
| AF-EDOX-VKA-JAPAN118 | Edoxaban 30 mg, 45 mg, 60 mg od | 2–3 (1.6–2.6 in ≥ 70 years) | 83 (≥ 70) 73 (< 70 years) |
| AF-EDOX-VKA-MULTI108 | Edoxaban 30 mg, 60 mg od, 30 mg, 60 mg bd | 2–3 | 49.7 |
| AF-VKA-ASA-CHINA122 | Aspirin 200 mg od | 2.1–2.5 | NR |
| ARISTOTLE107,114,119,124–127,130,132–134 | Apixaban 5 mg bd | 2–3 | 62.2 |
| ARISTOTLE-J113 | Apixaban 2.5 mg, 5 mg bd | 2–3 (2–2.6 in ≥ 70 years) | 60 |
| BAFTA103 | Aspirin 75 mg od | 2–3 | 67 |
| Chinese ATAFS99 | Aspirin 150–160 mg od | 2–3 (1.6–2.5 in > 75 years) | NR |
| ENGAGE AF-TIMI 48111,131 | Edoxaban 30 mg, 60 mg od | 2–3 | 64.9 |
| EXPLORE-Xa128 | Betrixaban 40 mg, 60 mg, 80 mg od | 2–3 | 63.4 |
| J-ROCKET AF120 | Rivaroxaban 15 mg od | 2–3 (1.6–2.6 in ≥ 70 years) | 65 |
| PATAF98 | Aspirin 150 mg od | 2.5–3.5 (some patients received other coumarins – phenprocoumon or acenocoumarol) | NR |
| PETRO102 | Dabigatran 50 mg, 50 mg + (aspirin 81 mg), 50 mg + (aspirin 325 mg), 150 mg, 150 mg + (aspirin 81 mg), 150 mg + (aspirin 325 mg), 300 mg, 300 mg + (aspirin 81 mg), 300 mg + (aspirin 325 mg) bd | 2–3 | 57.2 |
| RE-LY104,109 | Dabigatran 110 mg, 150 mg bd | 2–3 | 64 |
| ROCKET AF106,112,123,129 | Rivaroxaban 20 mg od | 2–3 | 55 |
| SPAF II96 | Aspirin 325 mg (in < 75 years), 325 mg (in > 75 years) od | 2–4.5 in < 75 years 2–4.5 in > 75 years |
NR |
| WASPO101 | Aspirin 300 mg od | 2–3 | 69.2 |
Risk of bias in included studies
Detailed risk-of-bias assessments for each included study for each domain of the Cochrane assessment tool are provided in Table 20. The assessments ranged from low to high risk of bias, but it was difficult to judge some studies as a result of inaccessibility of study protocols. For most of the outcomes assessed in the studies, all randomised patients were either accounted for in the analysis, or in some cases a small number of patients were unaccounted for with reasons judged likely to be unrelated to the outcome. The majority of the studies were judged to be at low risk of bias for allocation concealment and incomplete outcome data. The majority of the studies were judged to be at a low or unclear risk of bias for sequence generation. Randomisation sequence across the low-risk studies was predominantly computerised. Most studies were also judged to be of low risk of bias for blinding of outcome assessment, with three studies judged to be at high risk of bias in this domain. Fourteen studies were judged to be at high risk of bias for blinding of participants and personnel, mainly because they were open label. Where studies were blinded for different dose groups of a novel anticoagulant, but not in the comparison of these to warfarin, we assigned a high risk of bias because the principal contribution of the study to our analyses would be the comparison of warfarin with the licensed dose of the anticoagulant. Risk-of-bias judgements for studies contributing to analyses of each outcome are presented graphically in the sections that follow.
| Study | Sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcomes assessment | Incomplete outcome data | Selective reporting |
|---|---|---|---|---|---|---|
| ACTIVE W100 | L – ‘Patients were randomised by an automated central interactive voice-response system, in a 1 : 1 ratio, to receive clopidogrel plus aspirin or oral anticoagulation therapy’ | L – By means of a central, interactive, voice response system | H – Treatment was open, with blinded adjudication of outcomes | L – ‘All major outcomes were adjudicated by a blinded committee and all strokes were adjudicated by neurologists’ | L – All patients were included in the analyses | U – Study protocol not found |
| AFASAK95 | L – ‘The patients were randomised to receive warfarin, aspirin 75 mg od, or placebo. They received consecutive numbers, which corresponded to numbered packages containing the study medication, the order of which was determined by computer generated randomisation’ | L – ‘They received consecutive numbers, which corresponded to numbered packages containing the study medication, the order of which was determined by computer-generated randomisation’ | H – ‘Warfarin was given openly, but the aspirin and placebo arms were double blind. The warfarin tablets looked different from the aspirin and placebo tablets, which were indistinguishable’ | U – No information on blinding of outcome assessors | L – All patients were included in the analyses | U – Study protocol not found |
| AFASAK II97 | L – ‘According to a computer-generated sequence, eligible participants were assigned to daily treatment’ | U – No information on whether or not and how treatment allocation was concealed | H – This was an open-label study | L – ‘All end-points were evaluated by an end-point committee unaware of treatment status. The committee consisted of two neurologists and two cardiologists’ | L – All patients were included in the analyses | U – Study protocol not found |
| AF-ASA-VKA-CHINA135 | U – ‘A total of 110 patients met the inclusion criteria and were randomly divided into warfarin study and aspirin control groups’ | H – No information and no indication of treatment allocation concealment | H – No details, but monitoring of INR implies the study was open label | H – No information, and no indication of blinding of outcomes assessors | L – Small numbers of missing data in the two randomised arms and the number missing in each arm seem to be balanced; also with comparable reasons for the missing data. It is unlikely that missing data are related to the outcome | U – Study protocol not found |
| AF-DABIG-VKA-JAPAN110 | U – Available information is from a preliminary report and not a published article. Therefore, no information to enable judgement | U – Available information is from a preliminary report and not a published article. Therefore, no information to enable judgement | U – Available information is from a preliminary report and not a published article. Therefore, no information to enable judgement | U – Available information is from a preliminary report and not a published article. Therefore, no information to enable judgement | U – Available information is from a preliminary report and not a published article. Therefore, no information to enable judgement | U – Available information is from a preliminary report and not a published article. Therefore, no information to enable judgement |
| AF-EDOX-VKA-ASIA115 | L – ‘Via Fisher Automated Clinical Trials System (FACTS)’ | L – ‘Block randomisation was done by FACTS; Cenduit produced the randomisation schedule, which was kept confidential until the end of the study’ | H – ‘The investigator, patients and sponsor were blinded to the dose of edoxaban, but not to the identity of edoxaban and warfarin’ | L – ‘The independent CEC, which was blinded to study treatments, adjudicated all bleeding events and thromboembolic events (stroke, systemic embolic event, MI) during the study’ | L – Only one person with missing outcome data | L – All outcomes are reported as per study protocol |
| AF-EDOX-VKA-JAPAN118 | L – ‘Treatment was assigned using the biased coin method’ | U – ‘Patients were randomised using the specifications of dynamic allocation procedures’ | H – ‘This was a multicentre, randomised, dose-ranging study of edoxaban (double-blind to dose) and open-label warfarin’ | U – ‘Secondary end-points consisted of thromboembolic events including stroke assessed by an independent Event Assessment Committee’ | L – Some missing data with reasons although the number of missing data are quite minimal and unlikely related to the outcome | L – All outcomes are reported as per study protocol |
| AF-EDOX-VKA-MULTI108 | L – ‘The randomisation schedule was generated by an independent biostatistician who was not part of the study team. Using a central, interactive, automated telephone system’ | L – ‘Using a central, interactive, automated telephone system, eligible patients who provided written informed consent were randomly allocated’ | H – ‘The study was double blind with respect to edoxaban dose, but open-label for randomisation between edoxaban and warfarin’ | U – For efficacy outcomes: ‘Stroke confirmed by CT or autopsy; TIA confirmed by a neurologist’ | L – Very minimal missing data in three arms (I patient); otherwise, all patients are accounted for in the analysis | L – All outcomes are reported as per protocol |
| L – For safety outcomes: ‘Suspected bleeding events were assessed by an independent blinded adjudication committee’ | ||||||
| AF-VKA-ASA-CHINA122 | L – ‘Stratified block randomisation’ | U – Not enough information: ‘After giving a signed informed consent, patients who met the inclusion criteria were enrolled and randomly allocated to one of three study groups according to a stratified block randomisation’ | U – ‘In the warfarin groups, an initial dose of 1–3 mg/day of warfarin was prescribed after the baseline INR values were measured’. ‘In the aspirin group, a fixed dose of 200 mg/day of aspirin was used’ | U – Not clearly described ‘Medical records from all potential events were further reviewed by a five-physician clinical outcomes committee’ | U – A total of 96 patients withdrew from the study after randomisation but the remaining 690 patients were all included in the analysis | U – Study protocol not found |
| ARISTOTLE107,114,119,124–127,130,132–134 | L – ‘Randomisation was stratified according to whether patients had received warfarin previously and according to clinical site’ | U – ‘An algorithm was provided to manage temporary discontinuations of the study drug around the time of interventional procedures while maintaining concealment of the group assignments’ | L – ‘An algorithm was provided to manage temporary discontinuations of the study drug around the time of interventional procedures while maintaining concealment of the group assignments’ | L – ‘The primary and secondary efficacy and safety outcomes were adjudicated on the basis of prespecified criteria by a clinical events committee whose members were not aware of study-group assignments’ | L – For efficacy outcomes: No missing outcome data | L – All outcome are reported as per protocol |
| U – For bleeding outcomes: Some missing outcome data with reason which appear to be similar in the groups. However, it is not clear whether or not the reasons for the missing outcome data are related to the outcome | ||||||
| ARISTOTLE-J113 | U – ‘Patients were randomised in a 1 : 1 : 1. The randomisation assignment method (Pocock et al.) incorporated trial site and warfarin status (experienced or naive) as factors’ | U – Not enough information. ‘On the first day of study drug dosing (week 0), patients were randomised in a 1 : 1 : 1 fashion’ | H – ‘This was a randomised, partially blinded study comparing high double-blinded doses of apixaban with open-label warfarin’ | L – ‘An independent blinded end-point committee adjudicated all reported bleeding and efficacy events’ | L – Few outcome missing data with reasons. Reasons almost balance out across groups and it is unlikely that the reasons are related to the outcome | L – All outcomes are reported as per protocol |
| AVERROES105,116,117,121 | L – ‘Randomisation was performed with the use of a 24-hour central, computerised, automated voice-response system’ | L – ‘Randomisation was performed with the use of a 24-hour central, computerised, automated voice-response system’ | L – ‘In keeping with the double-dummy design, patients who were assigned to receive apixaban also received an aspirin placebo, and those assigned to receive aspirin also received an apixaban placebo’ | L – ‘All outcomes were adjudicated by a committee whose members were unaware of the treatment assignments. Cases of stroke and ICH were adjudicated by neurologists’ | L – All participants included in the analyses | L – All outcomes are reported as per protocol |
| BAFTA103 | L – ‘Within each stratum, randomly permuted blocks of eight were generated to produce allocation tables’ | L – ‘Primary care physicians telephoned for the treatment allocation when they had an eligible patient’ | H – ‘BAFTA was a prospective randomised open-label trial’ | L – ‘Clinical details on possible primary events were sent to two independent neurologists who were blind to treatment allocation’ | L – All patients were included in the analyses | L – All outcomes are reported as per protocol |
| Chinese ATAFS99 | U – ‘The randomised study of efficacy and safety of antithrombotic therapy in non-valvular AF: warfarin compared with aspirin’ | U – No information on whether or not and how treatment allocation was concealed | U – No information on whether or not participants and personnel were blinded to treatment | U – No information on blinding of outcome assessors | L – All patients were included in the analyses | U – Study protocol not found |
| ENGAGE AF-TIMI 48111,131 | L – ‘Randomisation was performed with the use of a central, 24-hour, interactive, computerised response system’ | L – ‘Randomisation was performed with the use of a central, 24-hour, interactive, computerised response system’ | L – ‘Each patient received two sets of study drugs: either active edoxaban and a placebo matching warfarin, or a placebo matching edoxaban and active warfarin’ | L – ‘An independent clinical end-point committee, whose members were unaware of the study assignment, adjudicated all deaths and suspected cerebrovascular events, systemic embolic events, MIs, bleeding events, and hepatic events’ | L – All patients were included in the analyses | L – All outcomes are reported as per study protocol |
| EXPLORE-Xa128 | U – ‘Patients were randomly assigned (1 : 1 : 1 : 1 allocation) A dynamic randomisation was used to assign and balance patients by country, concurrent aspirin use, and antecedent warfarin’ | U – Not enough information. ‘Patients were randomly assigned (1 : 1 : 1 : 1 allocation)’ | H – ‘Assignment to betrixaban or warfarin was not blinded, but the betrixaban dose was double-blinded’ | L – ‘An independent adjudicator, blinded to treatment groups, adjudicated all major bleeds, CRNM bleeds, strokes, MI, other SE and deaths’ | L – All participants were included in analyses | L – All outcomes are reported as per study protocol |
| J-ROCKET AF120 | L – No details provided but assumed to follow robust design of the ROCKET AF study | L – No details provided but assumed to follow robust design of the ROCKET AF study | L – ‘As part of the double-dummy design, patients in each group also received a tablet of either titrated warfarin placebo or rivaroxaban placebo, respectively, to preserve the treatment blind’ | U – ‘An independent clinical end-point committee adjudicated all suspected strokes, SEs, MIs, deaths, and bleeding events contributing to the prespecified end-points’ | L – Very few missing data. Unlikely to influence the true outcome | L – All outcomes are reported as per study protocol |
| PATAF98 | L – Randomisation was computer generated | L – ‘Patients eligible for standard anticoagulation were randomly assigned (centrally, by telephone)’ | U – ‘Patients were single blinded for the two intensities of anticoagulant’ | L – ‘End-point ascertainments were blinded for treatment. Events were independently reviewed by two members of the (neurological, cardiological, vascular, ophthalmological and internal medicine) event committees (or three, in case of disagreement’ | U – Some missing data and although with similar reasons across groups, the missing numbers in the groups are not balanced | L – Study protocol not found |
| PETRO102 | U – ‘The PETRO study was a randomised trial of patients with AF at high risk for thromboembolic events’ | U – Not enough information ‘Randomisation was stratified in the ratio 6 : 9 : 9 : 4 (50-, 150- and 300-mg dabigatran, and warfarin, respectively)’ | H – ‘The trial was double-blind with respect to dabigatran dose but open-label for concomitant aspirin treatment’ | U – For efficacy outcomes: No information but the outcomes may have been blinded | L – All patients were included in the analyses | L – All outcomes are reported as per protocol |
| L – For bleeding outcomes: ‘An independent adjudication committee blinded to treatment evaluated all bleeding events’ | ||||||
| RE-LY104,109 | L – ‘After providing written informed consent, all trial participants were randomly assigned to receive one of two doses of dabigatran, or to receive warfarin, by means of a central, interactive, automated telephone system’ | L – By means of a central, interactive, automated telephone system | H – ‘RE-LY was a randomised trial designed to compare two fixed doses of dabigatran, each administered in a blinded manner, with open-label use of warfarin’ | L – ‘Each primary and secondary outcome event was adjudicated by two independent investigators who were unaware of the treatment assignments’ | L – All patients were included in the analyses | L – All outcomes are reported as per protocol |
| ROCKET AF106,112,123,129 | L –’Randomisation was performed with the use of a central 24-hour, computerised, automated voice-response system’ | L – ‘Randomisation was performed with the use of a central 24-hour, computerised, automated voice-response system’ | L – ‘Patients were randomly assigned to receive fixed-dose rivaroxaban or adjusted-dose warfarin. Patients in each group also received a placebo tablet in order to maintain blinding’ | U – ‘An independent clinical end-point committee applied protocol definitions to adjudicate all suspected cases of stroke, SE, MI, death and bleeding events that contributed to the prespecified end-points’ | L – Very few missing outcome data but with reasons which appear to balance across groups. Unlikely that the missing data are related to the true outcome | L – All outcomes are reported as per protocol |
| SPAF II96 | L – Randomisation was done separately at each clinical site by computer | U – Not enough information: ‘The randomisation sequence could not be pre-reviewed’ | H – Both patient and investigator were aware of therapy assignment | L – For neurological efficacy outcomes: ‘All suspected neurological events were evaluated by an on-site study neurologist and verified by an events committee; evaluation was based on review of original medical records, from which information about therapy assignment had been removed’ | L – All patients were included in the analyses | U – Study protocol not found |
| H – For safety outcomes: No details on blinding of outcome assessment | ||||||
| WASPO101 | L – ‘Randomisation was prepared from a computer-generated random numbers programme’ | L – ‘Randomisation was performed by opening sealed envelopes in numbered sequence’ | H – This was an open-label study | H – No information and no indication of blinding | L – All patients were included in the analyses | U – Study protocol not found |
Results on clinical effectiveness and safety
The 27 different interventions considered in the 23 trials are listed in Tables 21–23, which show the number of patients analysed and the number of outcome events for each outcome reported in each trial. We performed NMAs for seven outcomes: stroke or SE, ischaemic stroke, MI, major bleeding, CRB, intracranial bleeding and all-cause mortality. Arms that were considered not to provide any evidence of interest to inform health decisions in the UK were excluded from the analyses. Specifically, we excluded the warfarin arm with INR range 1.6–2 from the AF-VKA-ASA-CHINA trial,122 the warfarin arm with INR range of < 2 from PATAF,98 the placebo arm from AFASAK,95 and the two warfarin arms with a fixed daily dose from AFASAK II. 97
| No. | Intervention |
|---|---|
| 1 | Warfarin (INR 2–3) |
| 2 | Warfarin (INR 1.6–3) |
| 3 | Warfarin (INR 3–4 od) |
| 4 | Antiplatelet (< 150 mg od) |
| 5 | Antiplatelet (≥ 150 mg od) |
| 6 | Dabigatran (50 mg bd) + aspirin (81 mg bd) |
| 7 | Dabigatran (50 mg bd) + aspirin (325 mg bd) |
| 8 | Dabigatran (150 mg bd) + aspirin (81 mg bd) |
| 9 | Dabigatran (150 mg bd) + aspirin (325 mg bd) |
| 10 | Dabigatran (300 mg bd) + aspirin (81 mg bd) |
| 11 | Dabigatran (300 mg bd) + aspirin (325 mg bd) |
| 12 | Apixaban (2.5 mg bd) |
| 13 | Apixaban (5 mg bd) |
| 14 | Dabigatran (50 mg bd) |
| 15 | Dabigatran (110 mg bd) |
| 16 | Dabigatran (150 mg bd) |
| 17 | Dabigatran (300 mg bd) |
| 18 | Betrixaban (40 mg od) |
| 19 | Betrixaban (60 mg od) |
| 20 | Betrixaban (80 mg od) |
| 21 | Edoxaban (30 mg od) |
| 22 | Edoxaban (45 mg od) |
| 23 | Edoxaban (60 mg od) |
| 24 | Edoxaban (30 mg bd) |
| 25 | Edoxaban (60 mg bd) |
| 26 | Rivaroxaban (15 mg od) |
| 27 | Rivaroxaban (20 mg od) |
| Study | Study size | TIA | All stroke | Stroke or SE | Ischaemic stroke | Minor ischaemic stroke | Major ischaemic stroke | Haemorrhagic stroke | Fatal stroke | PE | MI | Hospital admission |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACTIVE W100 | 6706 | 159 | 132 | 20 | 59 | |||||||
| AF-ASA-VKA-CHINA135 | 101 | 18 | 14 | 5 | ||||||||
| AF-DABIG-VKA-JAPAN110 | 166 | 1 | ||||||||||
| AF-EDOX-VKA-ASIA115 | 234 | 0 | ||||||||||
| AF-EDOX-VKA-JAPAN118 | 519 | 1 | ||||||||||
| AF-EDOX-VKA-MULTI108 | 1143 | 11 | 5 | 12 | ||||||||
| AF-VKA-ASA-CHINA122 | 440 | 13 | 10 | 9 | 1 | |||||||
| AFASAK95 | 671 | 2 | 20 | 1 | 4 | |||||||
| AFASAK II97 | 339 | 3 | 19 | 22 | 8 | 2 | 2 | 8 | ||||
| ARISTOTLE107,114,119,124–127,130,132–134 | 18,140 | 449 | 477 | 337 | 118 | 192 | ||||||
| ARISTOTLE-J113 | 218 | 1 | 3 | 1 | 0 | |||||||
| AVERROES105,116,117,121 | 5599 | 154 | 164 | 128 | 15 | 52 | ||||||
| BAFTA103 | 973 | 94 | 30 | |||||||||
| Chinese ATAFS99 | 704 | 23 | ||||||||||
| ENGAGE AF-TIMI 48111,131 | 21,026 | 958 | 1016 | 804 | 169 | 239 | 443 | |||||
| EXPLORE-Xa128 | 508 | 2 | 2 | 0 | ||||||||
| J-ROCKET AF120 | 1278 | 31 | 33 | 24 | 7 | 4 | ||||||
| PATAF98 | 272 | 7 | 7 | 2 | 5 | 5 | ||||||
| PETRO102 | 515 | 2 | ||||||||||
| RE-LY104,109 | 18,113 | 519 | 389 | 71 | 43 | 270 | 7199 | |||||
| ROCKET AF106,112,123,129 | 14,236 | 405 | 575 | 310 | 227 | |||||||
| SPAF II96 | 1100 | 25 | 67 | 63 | 34 | |||||||
| WASPO101 | 75 | 1 | 0 |
| Study | Study size | All bleeding | Minor bleeding | Major bleeding | Fatal bleeding | Extracranial minor bleeding | Intracranial bleeding | Arterial event | CRNM bleeding | CRB | Cardiovascular deaths | All-cause mortality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACTIVE W100 | 6706 | 1199 | 1049 | 194 | 18 | 317 | ||||||
| AF-ASA-VKA-CHINA135 | 101 | 14 | 9 | 3 | 2 | 4 | ||||||
| AF-DABIG-VKA-JAPAN110 | 166 | 45 | 3 | 14 | ||||||||
| AF-EDOX-VKA-ASIA115 | 234 | 57 | 48 | 2 | 9 | 11 | ||||||
| AF-EDOX-VKA-JAPAN118 | 519 | 115 | 5 | 15 | 20 | |||||||
| AF-EDOX-VKA-MULTI108 | 1143 | 114 | 52 | 13 | 49 | 62 | 8 | |||||
| AF-VKA-ASA-CHINA122 | 440 | 25 | 8 | 11 | ||||||||
| AFASAK95 | 671 | 23 | 15 | |||||||||
| AFASAK II97 | 339 | 68 | 9 | 3 | 31 | |||||||
| ARISTOTLE107,114,119,124–127,130,132–134 | 18,140 | 5416 | 789 | 174 | 1490 | 1272 | ||||||
| ARISTOTLE-J113 | 218 | 41 | 36 | 1 | 5 | 6 | 0 | |||||
| AVERROES105,116,117,121 | 5599 | 341 | 83 | 10 | 24 | 180 | 263 | 180 | 251 | |||
| BAFTA103 | 973 | 50 | 215 | |||||||||
| Chinese ATAFS99 | 704 | 12 | ||||||||||
| ENGAGE AF-TIMI 48111,131 | 21,026 | 1851 | 1196 | 112 | 234 | 3579 | 4450 | 1668 | 2349 | |||
| EXPLORE-Xa128 | 508 | 118 | 109 | 8 | 12 | 18 | 2 | |||||
| J-ROCKET AF120 | 1278 | 15 | 262 | 8 | 12 | |||||||
| PATAF98 | 272 | 8 | 18 | 29 | ||||||||
| PETRO102 | 515 | 88 | 4 | 36 | ||||||||
| RE-LY104,109 | 18,113 | 5284 | 1162 | 956 | 150 | 880 | 1371 | |||||
| ROCKET AF106,112,123,129 | 14,236 | 781 | 82 | 139 | 2336 | 2924 | 458 | |||||
| SPAF II96 | 1100 | 18 | 127 | |||||||||
| WASPO101 | 75 | 10 | 3 | 3 |
We defined two independent nodes for warfarin interventions, labelled as ‘warfarin (INR 2–3)’ and ‘warfarin (INR 3–4)’, respectively. The first of these formed the reference treatment across all networks in the AF review. We included in ‘warfarin (INR 2–3)’ the trials with a therapeutic INR range of 2–3 (e.g. ACTIVE W,100 AFASAK95), as well as some interventions with an INR range of 2.5–3.5 (AF-EDOX-VKA-ASIA115 and PATAF98) or 2.0–4.5 (SPAF II96). In some trials the INR range for some of patients in the warfarin arm was subtherapeutic (< 2.0), so that the total INR range was 1.6–3.0. These interventions were excluded from the main analysis, but merged with the INR 2–3 node in a sensitivity analysis. As a consequence, there were three two-arm trials (J-ROCKET AF,120 Chinese ATAFS99 and AF-ASA-VKA-CHINA135) that were included only in sensitivity analyses.
We also defined two independent nodes for antiplatelet interventions (‘aspirin’ or ‘aspirin plus clopidogrel’), using the cut-off point of 150 mg, with the understanding that daily doses above that were appropriate for stroke prevention in AF, whereas lower doses were appropriate for secondary prevention of cardiovascular events. The dose range considered in the AVERROES trial105,116,117,121 (81–324 mg od) was much wider than in any other trial, and we included this intervention in the lower-dose node (< 150 mg od) because some patients from that study had received a low daily dose. As a sensitivity analysis, we excluded the AVERROES trial105,116,117,121 from the network. Finally, our main analysis used a binomial model, assuming equal follow-up times across arms within trials and ignoring some variations in how results were reported. We undertook a separate analysis for all outcomes taking into account the differences in duration of follow-up within and between trials, and the differences in the definition of event used across trials (e.g. total number of events vs. first events only).
Results are presented as follows for each of the six outcomes. First, we provide network plots to illustrate the comparisons of interventions made in the different trials. Second, we illustrate the risk-of-bias assessments specific to the outcome for each trial included in the network. Third, we present results tables for each intervention compared with the reference treatment (warfarin with a target INR range of 2–3). Fourth, we present results tables for pairwise comparisons among licensed doses of the NOACs. For both sets of results tables, posterior median ORs and 95% credible intervals from Bayesian fixed-effects analyses are shown, although we refer to the latter as CIs for convenience. In these tables we present results separately for any available direct evidence, for any indirect comparisons that can be made (excluding the direct evidence) and for the NMA (which combines the direct and the indirect evidence). Comparisons from the NMA with a ratio between interval limits of > 9 were considered ‘imprecisely estimated’ and are presented at the bottom of each table (note that calculation of indirect evidence was not undertaken for imprecisely estimated comparisons). A summary of results across outcomes is provided at the end, in the form of a ‘rankogram’, which illustrates the probability that each treatment is best, second best, and so on, for each outcome. Last, forest plots of all contributing data, with ORs calculated using standard frequentist methods, are included in Appendix 2.
Stroke or systemic embolism
Sixteen studies reported the number of stroke or SE events, and the other seven trials reported the number of stroke events, so that the resulting network was based on data from all 23 trials, comparing a total of 26 interventions (Figure 9). There were 3217 stroke or SE events. Twenty studies were included in the main analysis, with the remaining three included only in sensitivity analyses. The thicker lines joining interventions, which mainly correspond with comparisons between licensed doses of NOACs and warfarin (INR 2–3) represent the larger (mainly Phase III) trials. Similarly, the larger green circles represent the interventions to which the largest number of patients were randomised. Importantly, there were no direct comparisons between different NOACs, although there were numerous comparisons between different doses of the same NOAC in mainly Phase II trials, and some such comparisons in larger trials. Therefore, comparisons between the effects of different NOACs need to be inferred from the network (indirect evidence).
FIGURE 9.
Network plot for stroke or SE (stroke prevention in AF). a, Excluded interventions that were included in sensitivity analyses.
Table 24 shows risk-of-bias judgements for studies reporting stroke or SE. The studies were at mixed risks of bias: there were concerns about lack of blinding of participants for most trials, and about lack of allocation concealment and blinding of outcome assessment in some.
| Study | Interventions compared | SG | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| ACTIVE W98 | 1, 4 | + | + | – | + | + | ? |
| AFASAK95 | 1, 4 | + | + | – | ? | + | ? |
| AFASAK II97 | 1, 5 | + | ? | – | + | + | ? |
| AF-ASA-VKA-CHINA135 | 2, 4 | ? | – | – | – | + | ? |
| AF-DABIG-VKA-JAPAN110 | 2, 15, 16 | ? | ? | ? | ? | ? | ? |
| AF-EDOX-VKA-ASIA115 | 1, 21, 23 | + | + | – | + | + | + |
| AF-EDOX-VKA-JAPAN118 | 2, 21, 22, 23 | + | ? | – | ? | + | + |
| AF-EDOX-VKA-MULTI108 | 1, 21, 23, 24, 25 | + | + | – | ? | + | + |
| AF-VKA-ASA-CHINA122 | 1, 5 | + | ? | ? | ? | ? | ? |
| ARISTOTLE107,114,119,124–127,130,132–134 | 1, 13 | + | ? | + | + | + | + |
| ARISTOTLE-J113 | 1, 12, 13 | ? | ? | – | + | + | + |
| AVERROES103,114,115,119 | 4, 13 | + | + | + | + | + | + |
| BAFTA103 | 1, 4 | + | + | – | + | + | + |
| Chinese ATAFS99 | 2, 5 | ? | ? | ? | ? | + | ? |
| ENGAGE AF-TIMI 48111,131 | 1, 21, 23 | + | + | + | + | + | + |
| EXPLORE-Xa126 | 1, 18, 19, 20 | ? | ? | – | + | + | + |
| J-ROCKET AF120 | 2, 26 | + | + | + | ? | + | + |
| PATAF98 | 1, 5 | + | + | ? | + | ? | + |
| PETRO102 | 1, 6, 7, 8, 9 10, 11, 14, 16, 17 | ? | ? | – | ? | + | + |
| RE-LY104,109 | 1, 15, 16 | + | + | – | + | + | + |
| ROCKET AF106,112,123,129 | 1, 27 | + | + | + | ? | + | + |
| SPAF II96 | 1, 5 | + | ? | – | + | + | ? |
| WASPO101 | 1, 5 | + | + | – | – | + | ? |
Table 25, which shows comparisons of licensed doses with warfarin (INR 2–3), suggests that both low- and high-dose antiplatelet drugs increase the risk of stroke or SE compared with warfarin (INR 2–3). Among NOACs, there was some evidence that apixaban [5 mg bd (bd)], dabigatran (150 mg bd), edoxaban (60 mg od) and rivaroxaban (20 mg od) reduce the risk of stroke or SE compared with warfarin (INR 2–3). Most other comparisons were imprecisely estimated. Comparisons among licensed doses of NOACs were almost all based on indirect evidence (Table 26). Among the comparisons that were not classified as imprecisely estimated, there was some evidence that edoxaban (60 mg od) and rivaroxaban (20 mg od) increase the risk of stroke or SE compared with dabigatran (150 mg bd).
| Comparisons with warfarin (INR 2–3) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Antiplatelet (< 150 mg od) | 1.99 (1.28 to 3.15) | 1.80 (1.22 to 2.65) | 1.88 (1.40 to 2.51) |
| Antiplatelet (≥ 150 mg od) | 1.61 (1.25 to 2.07) | – | 1.61 (1.25 to 2.07) |
| Apixaban (5 mg bd) | 0.79 (0.66 to 0.94) | – | 0.79 (0.66 to 0.94) |
| Dabigatran (110 mg bd) | 0.90 (0.74 to 1.10) | – | 0.90 (0.74 to 1.10) |
| Dabigatran (150 mg bd) | 0.65 (0.52 to 0.81) | – | 0.65 (0.52 to 0.81) |
| Edoxaban (30 mg od) | 1.13 (0.97 to 1.32) | – | 1.13 (0.97 to 1.32) |
| Edoxaban (60 mg od) | 0.86 (0.74 to 1.01) | – | 0.86 (0.74 to 1.01) |
| Rivaroxaban (20 mg od) | 0.88 (0.74 to 1.03) | – | 0.88 (0.74 to 1.03) |
| Imprecisely estimated comparisons | |||
| Warfarin (INR 3–4) | – | 0.58 (0.17 to 1.62) | 0.58 (0.17 to 1.62) |
| Dabigatran (50 mg bd) + aspirin (81 mg bd) | 11.4 (0.63 to 402) | – | 11.4 (0.63 to 402) |
| Dabigatran (50 mg bd) + aspirin (325 mg bd) | 1.62 (0 to 94.3) | – | 1.62 (0 to 94.3) |
| Dabigatran (150 mg bd) + aspirin (81 mg bd) | 1.23 (0 to 75.3) | – | 1.23 (0 to 75.3) |
| Dabigatran (150 mg bd) + aspirin (325 mg bd) | 1.35 (0 to 81.1) | – | 1.35 (0 to 81.1) |
| Dabigatran (300 mg bd) + aspirin (81 mg bd) | 1.32 (0 to 77.1) | – | 1.32 (0 to 77.1) |
| Dabigatran (300 mg bd) + aspirin (325 mg bd) | 1.50 (0 to 89.1) | – | 1.50 (0 to 89.1) |
| Apixaban (2.5 mg bd) | 0.11 (0 to 1.69) | – | 0.11 (0 to 1.69) |
| Dabigatran (50 mg bd) | 3.90 (0.21 to 137) | – | 3.90 (0.21 to 137) |
| Dabigatran (300 mg bd) | 0.42 (0 to 24) | – | 0.42 (0 to 24) |
| Betrixaban (40 mg od) | 1.01 (0 to 977) | – | 1.01 (0 to 977) |
| Betrixaban (60 mg od) | 5.14 (0.17 to 3780) | – | 5.14 (0.17 to 3780) |
| Betrixaban (80 mg od) | 5.18 (0.17 to 3920) | – | 5.18 (0.17 to 3920) |
| Edoxaban (45 mg od) | 3.36 (0.18 to 121) | – | 3.36 (0.18 to 121) |
| Edoxaban (30 mg bd) | 1.39 (0.27 to 5.61) | – | 1.39 (0.27 to 5.61) |
| Edoxaban (60 mg bd) | 1.19 (0.15 to 5.56) | – | 1.19 (0.15 to 5.56) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | – | 0.82 (0.62 to 1.08) | 0.82 (0.62 to 1.08) |
| Edoxaban (60 mg od) vs. apixaban (5 mg bd) | – | 1.09 (0.87 to 1.39) | 1.09 (0.87 to 1.39) |
| Rivaroxaban (20 mg od) vs. apixaban (5 mg bd) | – | 1.11 (0.87 to 1.41) | 1.11 (0.87 to 1.41) |
| Edoxaban (60 mg od) vs. dabigatran (150 mg bd) | – | 1.33 (1.02 to 1.75) | 1.33 (1.02 to 1.75) |
| Rivaroxaban (20 mg od) vs. dabigatran (150 mg bd) | – | 1.35 (1.03 to 1.78) | 1.35 (1.03 to 1.78) |
| Rivaroxaban (20 mg od) vs. edoxaban (60 mg od) | – | 1.01 (0.80 to 1.27) | 1.01 (0.80 to 1.27) |
| Imprecisely estimated comparisons | |||
| Apixaban (5 mg bd) vs. apixaban (2.5 mg bd) | 7.01 (0.50 to 3450) | – | 7.01 (0.47 to 3450) |
| Dabigatran (150 mg bd) vs. apixaban (2.5 mg bd) | – | 5.77 (0.38 to 2850) | 5.77 (0.38 to 2850) |
| Betrixaban (40 mg od) vs. apixaban (2.5 mg bd) | – | 12.1 (0.01 to 70300) | 12.1 (0.01 to 70300) |
| Edoxaban (60 mg od) vs. apixaban (2.5 mg bd) | – | 7.67 (0.51 to 3730) | 7.67 (0.51 to 3730) |
| Rivaroxaban (20 mg od) vs. apixaban (2.5 mg bd) | – | 7.78 (0.52 to 3820) | 7.78 (0.52 to 3820) |
| Betrixaban (40 mg od) vs. apixaban (5 mg bd) | – | 1.28 (0 to 1210) | 1.28 (0 to 1210) |
| Betrixaban (40 mg od) vs. dabigatran (150 mg bd) | – | 1.56 (0 to 1490) | 1.56 (0 to 1490) |
| Edoxaban (60 mg od) vs. betrixaban (40 mg od) | – | 0.85 (0 to 566) | 0.85 (0 to 566) |
| Rivaroxaban (20 mg od) vs. betrixaban (40 mg od) | – | 0.86 (0 to 575) | 0.86 (0 to 575) |
Results from a supplementary analysis taking into account the differences in duration of follow-up within and between trials, and the differences in the definition of event used across trials (e.g. total number of events vs. first events only), are presented in Tables 27 and 28. They are very similar to those for ORs.
| Comparisons with warfarin (INR 2–3) | HR (95% CI) |
|---|---|
| Warfarin (INR 3–4) | 0.58 (0.18 to 1.58) |
| Antiplatelet (< 150 mg od) | 1.82 (1.39 to 2.41) |
| Antiplatelet (≥ 150 mg od) | 1.58 (1.23 to 2.02) |
| Apixaban (5 mg bd) | 0.79 (0.67 to 0.94) |
| Dabigatran (110 mg bd) | 0.91 (0.75 to 1.11) |
| Dabigatran (150 mg bd) | 0.66 (0.53 to 0.82) |
| Edoxaban (30 mg od) | 1.13 (0.98 to 1.31) |
| Edoxaban (60 mg od) | 0.87 (0.74 to 1.01) |
| Rivaroxaban (20 mg od) | 0.88 (0.75 to 1.03) |
| Imprecisely estimated comparisons | |
| Dabigatran (50 mg bd) + aspirin (81 mg bd) | 11.0 (0.66 to 366) |
| Dabigatran (50 mg bd) + aspirin (325 mg bd) | 1.73 (0 to 94.9) |
| Dabigatran (150 mg bd) + aspirin (81 mg bd) | 1.33 (0 to 63.4) |
| Dabigatran (150 mg bd) + aspirin (325 mg bd) | 1.41 (0 to 72.6) |
| Dabigatran (300 mg bd) + aspirin (81 mg bd) | 1.33 (0 to 75.9) |
| Dabigatran (300 mg bd) + aspirin (325 mg bd) | 1.48 (0 to 86.3) |
| Apixaban (2.5 mg bd) | 0.11 (0 to 1.66) |
| Dabigatran (50 mg bd) | 3.96 (0.18 to 121) |
| Dabigatran (300 mg bd) | 0.44 (0 to 23.9) |
| Betrixaban (40 mg od) | 0.82 (0 to 313) |
| Betrixaban (60 mg od) | 4.98 (0.17 to 1420) |
| Betrixaban (80 mg od) | 4.87 (0.16 to 1340) |
| Edoxaban (45 mg od) | 3.54 (0.19 to 159) |
| Edoxaban (30 mg bd) | 1.40 (0.28 to 5.57) |
| Edoxaban (60 mg bd) | 1.20 (0.15 to 5.39) |
| Licensed NOACs only | HR (95% CI) |
|---|---|
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | 0.83 (0.63 to 1.10) |
| Edoxaban (60 mg od) vs. apixaban (5 mg bd) | 1.10 (0.87 to 1.38) |
| Rivaroxaban (20 mg od) vs. apixaban (5 mg bd) | 1.11 (0.88 to 1.40) |
| Edoxaban (60 mg od) vs. dabigatran (150 mg bd) | 1.32 (1.01 to 1.73) |
| Rivaroxaban (20 mg od) vs. dabigatran (150 mg bd) | 1.34 (1.02 to 1.76) |
| Rivaroxaban (20 mg od) vs. edoxaban (60 mg od) | 1.01 (0.81 to 1.27) |
| Imprecisely estimated comparisons | |
| Apixaban (5 mg bd) vs. apixaban (2.5 mg bd) | 7.39 (0.48 to 1990) |
| Dabigatran (150 mg bd) vs. apixaban (2.5 mg bd) | 6.16 (0.38 to 1650) |
| Betrixaban (40 mg od) vs. apixaban (2.5 mg bd) | 10.1 (0 to 22900) |
| Edoxaban (60 mg od) vs. apixaban (2.5 mg bd) | 8.11 (0.51 to 2190) |
| Rivaroxaban (20 mg od) vs. apixaban (2.5 mg bd) | 8.29 (0.53 to 2230) |
| Betrixaban (40 mg od) vs. apixaban (5 mg bd) | 1.05 (0 to 401) |
| Betrixaban (40 mg od) vs. dabigatran (150 mg bd) | 1.26 (0 to 466) |
| Edoxaban (60 mg od) vs. betrixaban (40 mg od) | 1.05 (0 to 2320) |
| Rivaroxaban (20 mg od) vs. betrixaban (40 mg od) | 1.07 (0 to 2270) |
As a post hoc sensitivity analysis, we fitted a fixed-effects meta-regression model using the mean TTR for warfarin patients (see Table 19) as a covariate and the mean log-odds ratio (log-OR) from each pairwise comparison (with warfarin as the reference category) as the response variable. There was little evidence of effect modification due to mean TTR (estimated coefficient 0.0021 with 95% CI −0.07 to 0.08 per 1% increase). The model fit indices were very similar with and without the covariate.
Ischaemic stroke
Fourteen studies reported on 2228 ischaemic stroke events, leading to a connected network comparing a total of 15 interventions (Figure 10). Twelve studies were included in the main analysis, with the remaining two included only in sensitivity analyses. The studies were at mixed risks of bias (Table 29). There were concerns about lack of blinding of participants for most trials, and about lack of allocation concealment and blinding of outcome assessment in one trial (AF-ASA-VKA-CHINA,135 only included in sensitivity analyses due to implementation of warfarin within non-standard INR range).
FIGURE 10.
Network plot for ischaemic stroke (stroke prevention in AF). a, Excluded interventions that were included in sensitivity analyses.
| Study | Interventions compared | SG | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| ACTIVE W98 | 1, 4 | + | + | – | + | + | ? |
| AFASAK II97 | 1, 5 | + | ? | – | + | + | ? |
| AF-ASA-VKA-CHINA135 | 2, 4 | ? | – | – | – | + | ? |
| AF-VKA-ASA-CHINA122 | 1, 5 | + | ? | ? | ? | ? | ? |
| ARISTOTLE107,114,119,124–127,130,132–134 | 1, 13 | + | ? | + | + | + | + |
| ARISTOTLE-J113 | 1, 12, 13 | ? | ? | – | + | + | + |
| AVERROES103,114,115,119 | 4, 13 | + | + | + | + | + | + |
| ENGAGE AF-TIMI 48111,131 | 1, 21, 23 | + | + | + | + | + | + |
| EXPLORE-Xa126 | 1, 18, 19, 20 | ? | ? | – | + | + | + |
| J-ROCKET AF120 | 2, 26 | + | + | + | ? | + | + |
| PATAF98 | 1, 5 | + | + | ? | + | ? | + |
| RE-LY104,109 | 1, 15, 16 | + | + | – | + | + | + |
| ROCKET AF106,112,123,129 | 1, 27 | + | + | + | ? | + | + |
| SPAF II96 | 1, 5 | + | ? | – | + | + | ? |
Table 30, which shows comparisons of all interventions with warfarin (INR 2–3), suggests that both low- and high-dose antiplatelets increase the risk of ischaemic stroke compared with warfarin (INR 2–3). Among NOACs, there was some evidence that dabigatran (150 mg bd) reduces the risk of ischaemic stroke compared with warfarin, whereas edoxaban (30 mg od) increases that risk. There was little evidence that the risk of ischaemic stroke differed between licensed doses of NOACs (Table 31).
| Comparisons with warfarin (INR 2–3) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Antiplatelet (< 150 mg od) | – | 2.52 (1.62 to 3.99) | 2.52 (1.62 to 3.99) |
| Antiplatelet (≥ 150 mg od) | 2.00 (1.51 to 2.67) | – | 2.00 (1.51 to 2.67) |
| Apixaban (5 mg bd) | 0.92 (0.74 to 1.14) | – | 0.92 (0.74 to 1.14) |
| Dabigatran (110 mg bd) | 1.14 (0.90 to 1.44) | – | 1.14 (0.90 to 1.44) |
| Dabigatran (150 mg bd) | 0.76 (0.58 to 0.98) | – | 0.76 (0.58 to 0.98) |
| Edoxaban (30 mg od) | 1.44 (1.21 to 1.71) | – | 1.44 (1.21 to 1.71) |
| Edoxaban (60 mg od) | 1.01 (0.84 to 1.21) | – | 1.01 (0.84 to 1.21) |
| Rivaroxaban (20 mg od) | 0.93 (0.74 to 1.16) | – | 0.93 (0.74 to 1.16) |
| Imprecisely estimated comparisons | |||
| Apixaban (2.5 mg bd) | 0.26 (0 to 5.89) | – | 0.26 (0 to 5.89) |
| Betrixaban (40 mg od) | 1.05 (0 to 751) | – | 1.05 (0 to 751) |
| Betrixaban (60 mg od) | 5.41 (0.18 to 3290) | – | 5.41 (0.18 to 3290) |
| Betrixaban (80 mg od) | 5.43 (0.17 to 3230) | – | 5.43 (0.17 to 3230) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | – | 0.83 (0.59 to 1.16) | 0.83 (0.59 to 1.16) |
| Edoxaban (60 mg od) vs. apixaban (5 mg bd) | – | 1.10 (0.83 to 1.46) | 1.10 (0.83 to 1.46) |
| Rivaroxaban (20 mg od) vs. apixaban (5 mg bd) | – | 1.01 (0.74 to 1.38) | 1.01 (0.74 to 1.38) |
| Edoxaban (60 mg od) vs. dabigatran (150 mg bd) | – | 1.33 (0.97 to 1.83) | 1.33 (0.97 to 1.83) |
| Rivaroxaban (20 mg od) vs. dabigatran (150 mg bd) | – | 1.22 (0.87 to 1.73) | 1.22 (0.87 to 1.73) |
| Rivaroxaban (20 mg od) vs. edoxaban (60 mg od) | – | 0.92 (0.69 to 1.23) | 0.92 (0.69 to 1.23) |
| Imprecisely estimated comparisons | |||
| Apixaban (5 mg bd) vs. apixaban (2.5 mg bd) | 3.47 (0.16 to 1730) | – | 3.47 (0.16 to 1730) |
| Dabigatran (150 mg bd) vs. apixaban (2.5 mg bd) | – | 2.88 (0.13 to 1430) | 2.88 (0.13 to 1430) |
| Betrixaban (40 mg od) vs. apixaban (2.5 mg bd) | – | 5.02 (0 to 25,800) | 5.02 (0 to 25,800) |
| Edoxaban (60 mg od) vs. apixaban (2.5 mg bd) | – | 3.82 (0.17 to 1920) | 3.82 (0.17 to 1920) |
| Rivaroxaban (20 mg od) vs. apixaban (2.5 mg bd) | – | 3.52 (0.16 to 1740) | 3.52 (0.16 to 1740) |
| Betrixaban (40 mg od) vs. apixaban (5 mg bd) | – | 1.15 (0 to 847) | 1.15 (0 to 847) |
| Betrixaban (40 mg od) vs. dabigatran (150 mg bd) | – | 1.39 (0 to 1010) | 1.39 (0 to 1010) |
| Edoxaban (60 mg od) vs. betrixaban (40 mg od) | – | 0.96 (0 to 633) | 0.96 (0 to 633) |
| Rivaroxaban (20 mg od) vs. betrixaban (40 mg od) | – | 0.88 (0 to 578) | 0.88 (0 to 578) |
In a sensitivity analysis to take into account the differences in duration of follow-up, NMA results were as presented in Tables 32 and 33, and show very similar results.
| Comparisons with warfarin (INR 2–3) | HR (95% CI) |
|---|---|
| Antiplatelet (< 150 mg od) | 2.46 (1.59 to 3.92) |
| Antiplatelet (≥ 150 mg od) | 1.94 (1.47 to 2.59) |
| Apixaban (5 mg bd) | 0.92 (0.75 to 1.15) |
| Dabigatran (110 mg bd) | 1.12 (0.89 to 1.42) |
| Dabigatran (150 mg bd) | 0.76 (0.59 to 0.99) |
| Edoxaban (30 mg od) | 1.43 (1.22 to 1.69) |
| Edoxaban (60 mg od) | 1.01 (0.84 to 1.20) |
| Rivaroxaban (20 mg od) | 0.92 (0.74 to 1.15) |
| Imprecisely estimated comparisons | |
| Apixaban (2.5 mg bd) | 0.26 (0 to 5.77) |
| Betrixaban (40 mg od) | 0.90 (0 to 233) |
| Betrixaban (60 mg od) | 4.72 (0.18 to 787) |
| Betrixaban (80 mg od) | 4.67 (0.18 to 838) |
| Licensed NOACs only | HR (95% CI) |
|---|---|
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | 0.83 (0.59 to 1.15) |
| Edoxaban (60 mg od) vs. apixaban (5 mg bd) | 1.09 (0.83 to 1.44) |
| Rivaroxaban (20 mg od) vs. apixaban (5 mg bd) | 1.00 (0.73 to 1.35) |
| Edoxaban (60 mg od) vs. dabigatran (150 mg bd) | 1.32 (0.96 to 1.80) |
| Rivaroxaban (20 mg od) vs. dabigatran (150 mg bd) | 1.21 (0.86 to 1.70) |
| Rivaroxaban (20 mg od) vs. edoxaban (60 mg od) | 0.92 (0.69 to 1.22) |
| Imprecisely estimated comparisons | |
| Apixaban (5 mg bd) vs. apixaban (2.5 mg bd) | 3.54 (0.16 to 1750) |
| Dabigatran (150 mg bd) vs. apixaban (2.5 mg bd) | 2.90 (0.13 to 1480) |
| Betrixaban (40 mg od) vs. apixaban (2.5 mg bd) | 4.05 (0 to 9940) |
| Edoxaban (60 mg od) vs. apixaban (2.5 mg bd) | 3.81 (0.18 to 1960) |
| Rivaroxaban (20 mg od) vs. apixaban (2.5 mg bd) | 3.50 (0.16 to 1780) |
| Betrixaban (40 mg od) vs. apixaban (5 mg bd) | 0.96 (0 to 241) |
| Betrixaban (40 mg od) vs. dabigatran (150 mg bd) | 1.18 (0 to 307) |
| Edoxaban (60 mg od) vs. betrixaban (40 mg od) | 1.11 (0 to 723) |
| Rivaroxaban (20 mg od) vs. betrixaban (40 mg od) | 1.03 (0 to 660) |
Myocardial infarction
A total of 15 studies reported 1334 MI events, leading to a network of 16 interventions (Figure 11). Thirteen studies were included in the main analysis, with the other two included only in sensitivity analyses. The studies were at mixed risks of bias (Table 34). There were concerns about lack of blinding of participants for most trials, and about lack of allocation concealment and blinding of outcome assessment in some.
FIGURE 11.
Network plot for MI (stroke prevention in AF). a, Excluded interventions that were included in sensitivity analyses.
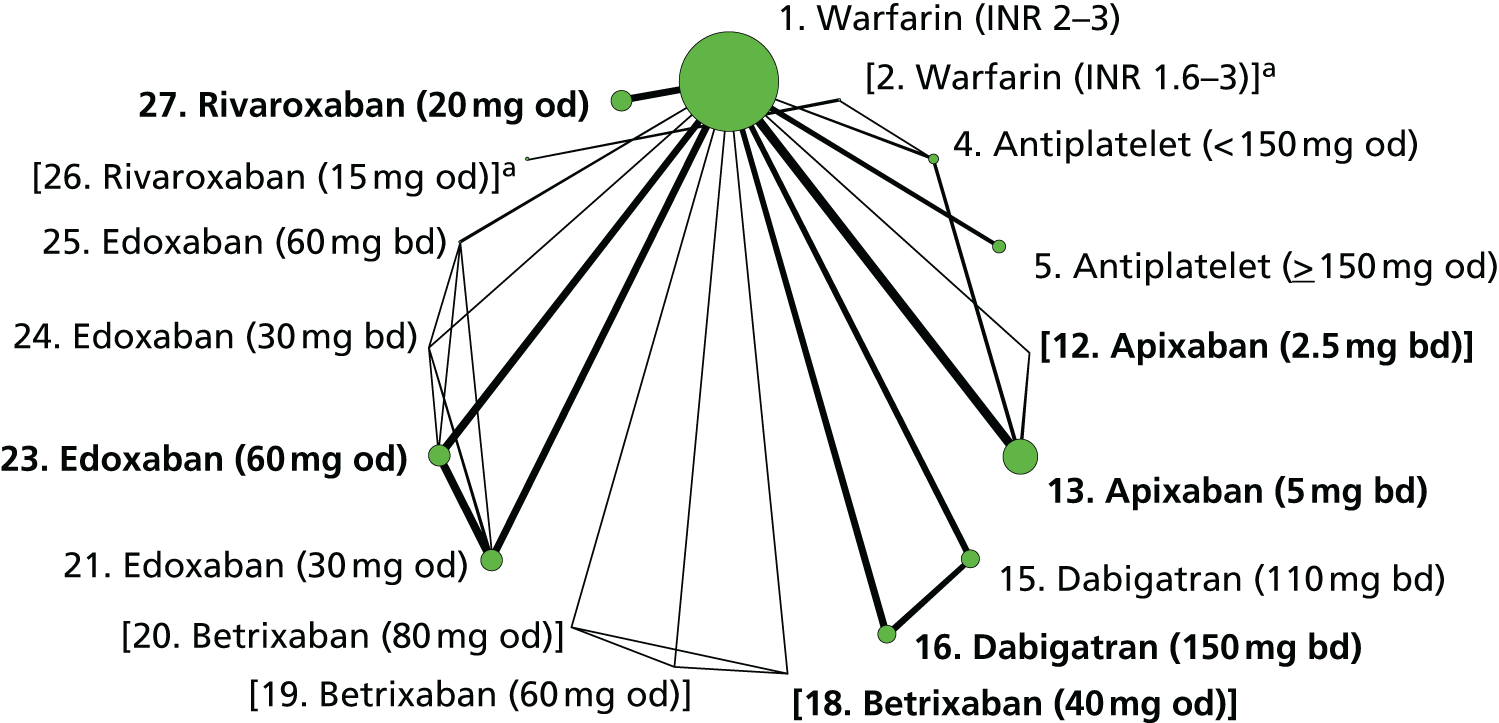
| Study | Interventions compared | SG | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| ACTIVE W98 | 1, 4 | + | + | – | + | + | ? |
| AFASAK II97 | 1, 5 | + | ? | – | + | + | ? |
| AF-ASA-VKA-CHINA135 | 2, 4 | ? | – | – | – | + | ? |
| AF-EDOX-VKA-MULTI108 | 1, 21, 23, 24, 25 | + | + | – | ? | + | + |
| ARISTOTLE107,114,119,124–127,130,132–134 | 1, 13 | + | ? | + | + | + | + |
| ARISTOTLE-J113 | 1, 12, 13 | ? | ? | – | + | + | + |
| AVERROES103,114,115,119 | 4, 13 | + | + | + | + | + | + |
| BAFTA103 | 1, 4 | + | + | – | + | + | + |
| ENGAGE AF-TIMI 48111,131 | 1, 21, 23 | + | + | + | + | + | + |
| EXPLORE-Xa126 | 1, 18, 19, 20 | ? | ? | – | + | + | + |
| J-ROCKET AF120 | 2, 26 | + | + | + | ? | + | + |
| PATAF98 | 1, 5 | + | + | ? | + | ? | + |
| RE-LY104,109 | 1, 15, 16 | + | + | – | + | + | + |
| ROCKET AF106,112,123,129 | 1, 27 | + | + | + | ? | + | + |
| SPAF II96 | 1, 5 | + | ? | – | – | + | ? |
Table 35 shows weak evidence that dabigatran (110 mg bd), dabigatran (150 mg bd) and edoxaban (30 mg od) increase the risk of MI compared with warfarin (INR 2–3), and weak evidence that rivaroxaban (20 mg od) decreases risk of MI compared with warfarin (INR 2–3). None of the interventions was superior or inferior to warfarin (INR 2–3). The pairwise comparisons of licensed NOACs, presented in Table 36, show weak evidence that dabigatran (150 mg bd) increases the risk of MI compared with apixaban (5 mg bd), and evidence that rivaroxaban (20 mg od) reduces the risk of MI compared with dabigatran (150 mg bd). Results were similar in a sensitivity analysis, taking into account the differences in duration of follow-up within and between trials, and the differences in the definition of event used across trials (e.g. total number of events vs. first events only) (Tables 37 and 38).
| Comparisons with warfarin (INR 2–3) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Antiplatelet (< 150 mg od) | 1.00 (0.47 to 2.10) | 1.02 (0.55 to 1.87) | 1.01 (0.64 to 1.61) |
| Antiplatelet (≥ 150 mg od) | 1.38 (0.94 to 2.03) | – | 1.38 (0.94 to 2.03) |
| Apixaban (5 mg bd) | 0.87 (0.66 to 1.15) | – | 0.87 (0.66 to 1.15) |
| Dabigatran (110 mg bd) | 1.32 (0.97 to 1.79) | – | 1.32 (0.97 to 1.79) |
| Dabigatran (150 mg bd) | 1.29 (0.96 to 1.75) | – | 1.29 (0.96 to 1.75) |
| Edoxaban (30 mg od) | 1.22 (0.97 to 1.53) | – | 1.22 (0.97 to 1.53) |
| Edoxaban (60 mg od) | 0.96 (0.75 to 1.22) | – | 0.96 (0.75 to 1.22) |
| Rivaroxaban (20 mg od) | 0.80 (0.61 to 1.04) | – | 0.80 (0.61 to 1.04) |
| Imprecisely estimated comparisons | |||
| Edoxaban (30 mg bd) | 0.71 (0.06 to 3.97) | – | 0.71 (0.06 to 3.97) |
| Edoxaban (60 mg bd) | 0.19 (0 to 2.60) | – | 0.19 (0 to 2.60) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | – | 1.48 (0.98 to 2.22) | 1.48 (0.98 to 2.22) |
| Edoxaban (60 mg od) vs. apixaban (5 mg bd) | – | 1.10 (0.76 to 1.58) | 1.10 (0.76 to 1.58) |
| Rivaroxaban (20 mg od) vs. apixaban (5 mg bd) | – | 0.92 (0.63 to 1.34) | 0.92 (0.63 to 1.34) |
| Edoxaban (60 mg od) vs. dabigatran (150 mg bd) | – | 0.74 (0.50 to 1.09) | 0.74 (0.50 to 1.09) |
| Rivaroxaban (20 mg od) vs. dabigatran (150 mg bd) | – | 0.62 (0.41 to 0.93) | 0.62 (0.41 to 0.93) |
| Rivaroxaban (20 mg od) vs. edoxaban (60 mg od) | – | 0.84 (0.59 to 1.20) | 0.84 (0.59 to 1.20) |
| Comparisons with warfarin (INR 2–3) | HR (95% CI) |
|---|---|
| Antiplatelet (< 150 mg od) | 1.01 (0.64 to 1.61) |
| Antiplatelet (≥ 150 mg od) | 1.36 (0.93 to 2.01) |
| Apixaban (5 mg bd) | 0.88 (0.67 to 1.16) |
| Dabigatran (110 mg bd) | 1.31 (0.96 to 1.77) |
| Dabigatran (150 mg bd) | 1.30 (0.96 to 1.77) |
| Edoxaban (30 mg od) | 1.22 (0.97 to 1.52) |
| Edoxaban (60 mg od) | 0.96 (0.76 to 1.22) |
| Rivaroxaban (20 mg od) | 0.80 (0.62 to 1.04) |
| Imprecisely estimated comparisons | |
| Edoxaban (30 mg bd) | 0.97 (0.09 to 5.40) |
| Edoxaban (60 mg bd) | 0.13 (0 to 1.81) |
| Licensed NOACs only | HR (95% CI) |
|---|---|
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | 1.48 (0.98 to 2.23) |
| Edoxaban (60 mg od) vs. apixaban (5 mg bd) | 1.09 (0.76 to 1.57) |
| Rivaroxaban (20 mg od) vs. apixaban (5 mg bd) | 0.91 (0.62 to 1.33) |
| Edoxaban (60 mg od) vs. dabigatran (150 mg bd) | 0.74 (0.49 to 1.08) |
| Rivaroxaban (20 mg od) vs. dabigatran (150 mg bd) | 0.62 (0.41 to 0.92) |
| Rivaroxaban (20 mg od) vs. edoxaban (60 mg od) | 0.84 (0.59 to 1.19) |
Major bleeding
Eighteen studies reported 4314 major bleeding events, leading to a network of 24 interventions (Figure 12). Seventeen studies were included in the main analysis, with the remaining study included only in sensitivity analyses. These studies were at mixed risks of bias (Table 39). There were concerns about lack of blinding of participants for most trials, and about lack of allocation concealment and blinding of outcome assessment in some.
FIGURE 12.
Network plot for major bleeding (stroke prevention in AF). a, Excluded interventions that were included in sensitivity analyses.
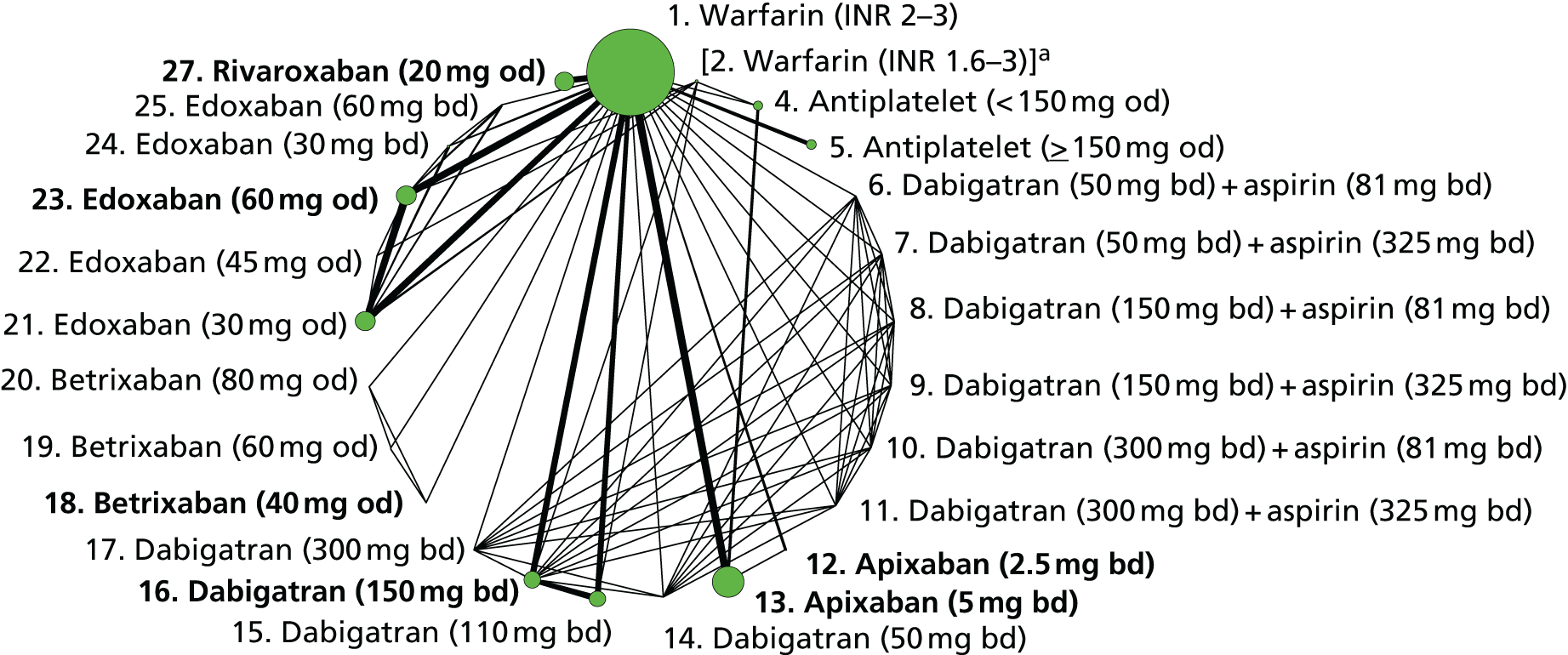
| Study | Interventions compared | SC | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| ACTIVE W98 | 1, 4 | + | + | – | + | + | ? |
| AFASAK II97 | 1, 5 | + | ? | – | + | + | ? |
| AF-ASA-VKA-CHINA135 | 2, 4 | ? | – | – | – | + | ? |
| AF-DABIG-VKA-JAPAN110 | 2, 15, 16 | ? | ? | ? | ? | ? | ? |
| AF-EDOX-VKA-ASIA115 | 1, 21, 23 | + | + | – | + | + | + |
| AF-EDOX-VKA-JAPAN118 | 2, 21, 22, 23 | + | ? | – | ? | + | + |
| AF-EDOX-VKA-MULTI108 | 1, 21, 23, 24, 25 | + | + | – | + | + | + |
| AF-VKA-ASA-CHINA122 | 1, 5 | + | ? | – | ? | ? | ? |
| ARISTOTLE107,114,119,124–127,130,132–134 | 1, 13 | + | ? | + | + | ? | + |
| ARISTOTLE-J113 | 1, 12, 13 | ? | ? | – | + | + | + |
| AVERROES103,114,115,119 | 4, 13 | + | + | + | + | + | + |
| BAFTA103 | 1, 4 | + | + | – | + | + | + |
| ENGAGE AF-TIMI 48111,131 | 1, 21, 23 | + | + | + | + | + | + |
| EXPLORE-Xa126 | 1, 18, 19, 20 | ? | ? | – | + | + | + |
| PETRO102 | 1, 6, 7, 8, 9, 10, 11, 14, 16, 17 | ? | ? | – | + | + | + |
| RE-LY104,109 | 1, 15, 16 | + | + | – | + | + | + |
| ROCKET AF106,112,123,129 | 1, 27 | + | + | + | ? | + | + |
| WASPO101 | 1, 5 | + | + | – | – | + | ? |
There was weak evidence that antiplatelet therapy (< 150 mg od) reduced major bleeding compared with warfarin (INR 2–3). There was evidence that apixaban (5 mg bd), dabigatran (110 mg bd), edoxaban (30 mg od) and edoxaban (60 mg od) reduced major bleeding risk compared with warfarin (INR 2–3) (Table 40). Comparisons among licensed doses of NOACs, presented in Table 41, suggest that dabigatran (150 mg bd) increases risk of major bleeding compared with apixaban (5 mg bd), whereas rivaroxaban (20 mg od) increases risk of major bleeding compared with apixaban (5 mg bd) and edoxaban (60 mg od).
| Comparisons with warfarin (INR 2–3) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Antiplatelet (< 150 mg od) | 1.00 (0.56 to 1.77) | 0.63 (0.40 to 0.98) | 0.75 (0.52 to 1.06) |
| Antiplatelet (≥ 150 mg od) | 1.07 (0.82 to 1.42) | – | 1.07 (0.82 to 1.42) |
| Apixaban (5 mg bd) | 0.71 (0.61 to 0.81) | – | 0.71 (0.61 to 0.81) |
| Dabigatran (110 mg bd) | 0.80 (0.69 to 0.93) | – | 0.80 (0.69 to 0.93) |
| Dabigatran (150 mg bd) | 0.94 (0.81 to 1.08) | – | 0.94 (0.81 to 1.08) |
| Edoxaban (30 mg od) | 0.46 (0.40 to 0.54) | – | 0.46 (0.40 to 0.54) |
| Edoxaban (60 mg od) | 0.78 (0.69 to 0.90) | – | 0.78 (0.69 to 0.90) |
| Rivaroxaban (20 mg od) | 1.03 (0.89 to 1.18) | – | 1.03 (0.89 to 1.18) |
| Imprecisely estimated comparisons | |||
| Dabigatran (50 mg bd) + aspirin (81 mg bd) | 2.54 (0 to 146) | – | 2.54 (0 to 146) |
| Dabigatran (50 mg bd) + aspirin (325 mg bd) | 1.99 (0 to 112) | – | 1.99 (0 to 112) |
| Dabigatran (150 mg bd) + aspirin (81 mg bd) | 1.52 (0 to 82.0) | – | 1.52 (0 to 82.0) |
| Dabigatran (150 mg bd) + aspirin (325 mg bd) | 1.63 (0 to 90.4) | – | 1.63 (0 to 90.4) |
| Dabigatran (300 mg bd) + aspirin (81 mg bd) | 8.38 (0.45 to 266) | – | 8.38 (0.45 to 266) |
| Dabigatran (300 mg bd) + aspirin (325 mg bd) | 27.6 (3.05 to 749) | – | 27.6 (3.05 to 749) |
| Apixaban (2.5 mg bd) | 0.24 (0 to 5.48) | – | 0.24 (0 to 5.48) |
| Dabigatran (50 mg bd) | 0.89 (0 to 52.4) | – | 0.89 (0 to 52.4) |
| Dabigatran (300 mg bd) | 0.50 (0 to 28.6) | – | 0.50 (0 to 28.6) |
| Betrixaban (40 mg od) | 0.04 (0 to 0.58) | – | 0.04 (0 to 0.58) |
| Betrixaban (60 mg od) | 0.04 (0 to 0.59) | – | 0.04 (0 to 0.59) |
| Betrixaban (80 mg od) | 0.60 (0.13 to 2.40) | – | 0.60 (0.13 to 2.40) |
| Edoxaban (45 mg od) | 1.45 (0.27 to 8.29) | – | 1.45 (0.27 to 8.29) |
| Edoxaban (30 mg bd) | 3.68 (0.94 to 16.9) | – | 3.68 (0.94 to 16.9) |
| Edoxaban (60 mg bd) | 6.01 (1.64 to 27.0) | – | 6.01 (1.64 to 27.0) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | – | 1.33 (1.09 to 1.62) | 1.33 (1.09 to 1.62) |
| Edoxaban (60 mg od) vs. apixaban (5 mg bd) | – | 1.11 (0.92 to 1.35) | 1.11 (0.92 to 1.35) |
| Rivaroxaban (20 mg od) vs. apixaban (5 mg bd) | – | 1.45 (1.19 to 1.78) | 1.45 (1.19 to 1.78) |
| Edoxaban (60 mg od) vs. dabigatran (150 mg bd) | – | 0.84 (0.69 to 1.02) | 0.84 (0.69 to 1.02) |
| Rivaroxaban (20 mg od) vs. dabigatran (150 mg bd) | – | 1.10 (0.90 to 1.34) | 1.10 (0.90 to 1.34) |
| Rivaroxaban (20 mg od) vs. edoxaban (60 mg od) | – | 1.31 (1.07 to 1.59) | 1.31 (1.07 to 1.59) |
| Imprecisely estimated comparisons | |||
| Apixaban (5 mg bd) vs. apixaban (2.5 mg bd) | 2.93 (0.13 to 1320) | – | 2.93 (0.13 to 1320) |
| Dabigatran (150 mg bd) vs. apixaban (2.5 mg bd) | – | 3.88 (0.17 to 1740) | 3.88 (0.17 to 1740) |
| Betrixaban (40 mg od) vs. apixaban (2.5 mg bd) | – | 0.17 (0 to 124) | 0.17 (0 to 124) |
| Edoxaban (60 mg od) vs. apixaban (2.5 mg bd) | – | 3.25 (0.14 to 1460) | 3.25 (0.14 to 1460) |
| Rivaroxaban (20 mg od) vs. apixaban (2.5 mg bd) | – | 4.27 (0.19 to 1910) | 4.27 (0.19 to 1910) |
| Betrixaban (40 mg od) vs. apixaban (5 mg bd) | – | 0.06 (0 to 0.84) | 0.06 (0 to 0.84) |
| Betrixaban (40 mg od) vs. dabigatran (150 mg bd) | – | 0.04 (0 to 0.63) | 0.04 (0 to 0.63) |
| Edoxaban (60 mg od) vs. betrixaban (40 mg od) | – | 18.7 (1.34 to 9160) | 18.7 (1.34 to 9160) |
| Rivaroxaban (20 mg od) vs. betrixaban (40 mg od) | – | 24.5 (1.76 to 12,000) | 24.5 (1.76 to 12,000) |
In a sensitivity analysis to take into account the differences in duration of follow-up, NMA results were as presented in Tables 42 and 43, and show very similar results. Another sensitivity analysis involved fitting a fixed-effects meta-regression model using the mean TTR for warfarin patients (see Table 19) as a covariate and the mean log-OR from each pairwise comparison (with warfarin as the reference category) as the response variable. We found no evidence of an effect modification according to mean TTR (estimated coefficient 0.04 with 95% CI −0.03 to 0.12 per 1% increase). The model fit indices yielded almost identical values for the models with and without the covariate.
| Comparisons with warfarin (INR 2–3) | HR (95% CI) |
|---|---|
| Antiplatelet (< 150 mg od) | 0.76 (0.53 to 1.08) |
| Antiplatelet (≥ 150 mg od) | 1.07 (0.82 to 1.41) |
| Apixaban (5 mg bd) | 0.72 (0.62 to 0.82) |
| Dabigatran (110 mg bd) | 0.81 (0.70 to 0.93) |
| Dabigatran (150 mg bd) | 0.94 (0.82 to 1.07) |
| Edoxaban (30 mg od) | 0.47 (0.41 to 0.55) |
| Edoxaban (60 mg od) | 0.79 (0.70 to 0.90) |
| Rivaroxaban (20 mg od) | 1.02 (0.89 to 1.18) |
| Imprecisely estimated comparisons | |
| Dabigatran (50 mg bd) + aspirin (81 mg bd) | 2.58 (0 to 151) |
| Dabigatran (50 mg bd) + aspirin (325 mg bd) | 2.07 (0 to 114) |
| Dabigatran (150 mg bd) + aspirin (81 mg bd) | 1.51 (0 to 78.3) |
| Dabigatran (150 mg bd) + aspirin (325 mg bd) | 1.62 (0 to 94.8) |
| Dabigatran (300 mg bd) + aspirin (81 mg bd) | 8.36 (0.50 to 281) |
| Dabigatran (300 mg bd) + aspirin (325 mg bd) | 26.3 (3.08 to 697) |
| Apixaban (2.5 mg bd) | 0.25 (0 to 5.49) |
| Dabigatran (50 mg bd) | 0.93 (0 to 53.2) |
| Dabigatran (300 mg bd) | 0.52 (0 to 29.7) |
| Betrixaban (40 mg od) | 0.05 (0 to 0.55) |
| Betrixaban (60 mg od) | 0.04 (0 to 0.60) |
| Betrixaban (80 mg od) | 0.60 (0.13 to 2.38) |
| Edoxaban (45 mg od) | 1.49 (0.28 to 8.31) |
| Edoxaban (30 mg bd) | 3.64 (0.95 to 17.1) |
| Edoxaban (60 mg bd) | 6.00 (1.66 to 27.5) |
| Licensed NOACs only | HR (95% CI) |
|---|---|
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | 1.31 (1.08 to 1.59) |
| Edoxaban (60 mg od) vs. apixaban (5 mg bd) | 1.10 (0.91 to 1.33) |
| Rivaroxaban (20 mg od) vs. apixaban (5 mg bd) | 1.43 (1.17 to 1.75) |
| Edoxaban (60 mg od) vs. dabigatran (150 mg bd) | 0.84 (0.70 to 1.02) |
| Rivaroxaban (20 mg od) vs. dabigatran (150 mg bd) | 1.09 (0.90 to 1.33) |
| Rivaroxaban (20 mg od) vs. edoxaban (60 mg od) | 1.30 (1.07 to 1.57) |
| Imprecisely estimated comparisons | |
| Apixaban (5 mg bd) vs. apixaban (2.5 mg bd) | 2.89 (0.13 to 519) |
| Dabigatran (150 mg bd) vs. apixaban (2.5 mg bd) | 3.80 (0.17 to 683) |
| Betrixaban (40 mg od) vs. apixaban (2.5 mg bd) | 0.18 (0 to 61.9) |
| Edoxaban (60 mg od) vs. apixaban (2.5 mg bd) | 3.19 (0.14 to 579) |
| Rivaroxaban (20 mg od) vs. apixaban (2.5 mg bd) | 4.13 (0.19 to 751) |
| Betrixaban (40 mg od) vs. apixaban (5 mg bd) | 0.06 (0 to 0.77) |
| Betrixaban (40 mg od) vs. dabigatran (150 mg bd) | 0.05 (0 to 0.58) |
| Edoxaban (60 mg od) vs. betrixaban (40 mg od) | 17.1 (1.44 to 2160) |
| Rivaroxaban (20 mg od) vs. betrixaban (40 mg od) | 22.1 (1.89 to 2770) |
Clinically relevant bleeding
Twelve studies reported 9556 CRB events, leading to a network of 23 interventions (Figure 13). Eleven studies were included in the main analysis, with the remaining study included only in sensitivity analyses. These studies were at mixed risks of bias (Table 44), the concerns being due to lack of blinding of participants for most trials.
FIGURE 13.
Network plot for CRB (stroke prevention in AF). a, Excluded interventions that were included in sensitivity analyses.
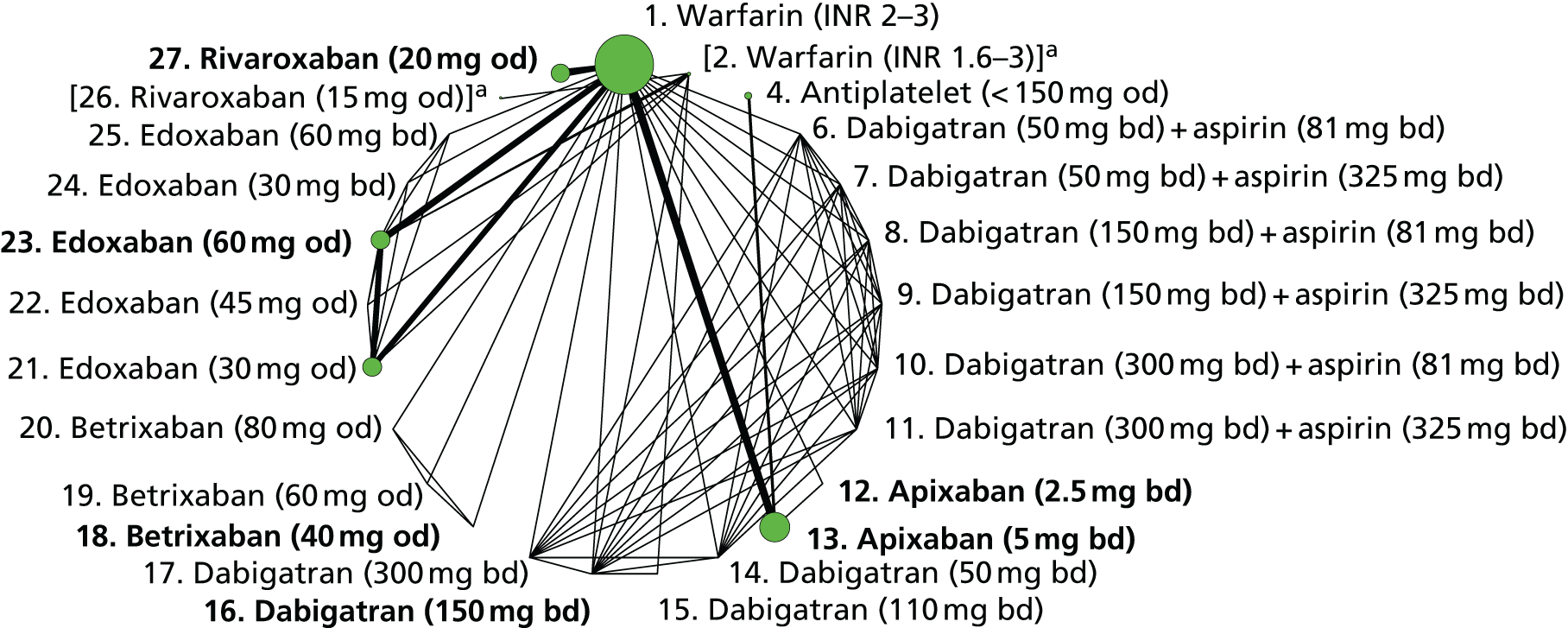
| Study | Interventions compared | SG | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| AF-DABIG-VKA-JAPAN110 | 2, 15, 16 | ? | ? | ? | ? | ? | ? |
| AF-EDOX-VKA-ASIA115 | 1, 21, 23 | + | + | – | + | + | + |
| AF-EDOX-VKA-JAPAN118 | 2, 21, 22, 23 | + | ? | – | ? | + | + |
| AF-EDOX-VKA-MULTI108 | 1, 21, 23, 24, 25 | + | + | – | + | + | + |
| ARISTOTLE107,114,119,124–127,130,132–134 | 1, 13 | + | ? | + | + | ? | + |
| ARISTOTLE-J113 | 1, 12, 13 | ? | ? | – | + | + | + |
| AVERROES103,114,115,119 | 4, 13 | + | + | + | + | + | + |
| ENGAGE AF-TIMI 48111,131 | 1, 21, 23 | + | + | + | + | + | + |
| EXPLORE-Xa126 | 1, 18, 19, 20 | ? | ? | – | + | + | + |
| J-ROCKET AF120 | 2, 26 | + | + | + | ? | + | + |
| PETRO102 | 1, 6, 7, 8, 9, 10, 11, 14, 16, 17 | ? | ? | – | + | + | + |
| ROCKET AF106,112,123,129 | 1, 27 | + | + | + | ? | + | + |
Results presented in Table 45 suggest that antiplatelet therapy (< 150 mg od) reduces CRB compared with warfarin (INR 2–3). Note that the licensed dose for of antiplatelet therapy for AF is ≥ 150 mg od: no studies provided data for that dose for CRB. Among NOACs, there was evidence that apixaban (5 mg bd), edoxaban (30 mg od) and edoxaban (60 mg od) reduce CRB compared with warfarin (INR 2–3). However, edoxaban (30 mg bd) and edoxaban (60 mg bd) increased CRB compared with warfarin (INR 2–3). Among licensed NOACs (Table 46), there was evidence that edoxaban (60 mg od) and rivaroxaban (20 mg od) increase CRB compared with apixaban (5 mg bd) and that rivaroxaban (20 mg od) increased CRB compared with edoxaban (60 mg od).
| Comparisons with warfarin (INR 2–3) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Antiplatelet (< 150 mg od) | – | 0.59 (0.45 to 0.77) | 0.59 (0.45 to 0.77) |
| Apixaban (5 mg bd) | 0.67 (0.60 to 0.75) | – | 0.67 (0.60 to 0.75) |
| Edoxaban (30 mg od) | 0.59 (0.54 to 0.64) | – | 0.59 (0.54 to 0.64) |
| Edoxaban (45 mg od) | 1.09 (0.37 to 3.04) | – | 1.09 (0.37 to 3.04) |
| Edoxaban (60 mg od) | 0.84 (0.77 to 0.90) | – | 0.84 (0.77 to 0.90) |
| Edoxaban (30 mg bd) | 1.97 (1.04 to 3.67) | – | 1.97 (1.04 to 3.67) |
| Edoxaban (60 mg bd) | 2.76 (1.46 to 5.17) | – | 2.76 (1.46 to 5.17) |
| Rivaroxaban (20 mg od) | 1.03 (0.95 to 1.11) | – | 1.03 (0.95 to 1.11) |
| Imprecisely estimated comparisons | |||
| Dabigatran (50 mg bd) + aspirin (81 mg bd) | 0.91 (0.07 to 5.87) | – | 0.91 (0.07 to 5.87) |
| Dabigatran (50 mg bd) + aspirin (325 mg bd) | 0.70 (0.06 to 4.50) | – | 0.70 (0.06 to 4.50) |
| Dabigatran (150 mg bd) + aspirin (81 mg bd) | 0.99 (0.15 to 4.99) | – | 0.99 (0.15 to 4.99) |
| Dabigatran (150 mg bd) + aspirin (325 mg bd) | 1.08 (0.17 to 5.58) | – | 1.08 (0.17 to 5.58) |
| Dabigatran (300 mg bd) + aspirin (81 mg bd) | 2.76 (0.71 to 11.7) | – | 2.76 (0.71 to 11.7) |
| Dabigatran (300 mg bd) + aspirin (325 mg bd) | 3.98 (1.10 to 16.3) | – | 3.98 (1.10 to 16.3) |
| Apixaban (2.5 mg bd) | 0.25 (0.01 to 1.88) | – | 0.25 (0.01 to 1.88) |
| Dabigatran (50 mg bd) | 0.06 (0 to 0.91) | – | 0.06 (0 to 0.91) |
| Dabigatran (110 mg bd) | 0.67 (0.06 to 5.47) | – | 0.67 (0.06 to 5.47) |
| Dabigatran (150 mg bd) | 1.56 (0.50 to 5.74) | – | 1.56 (0.50 to 5.74) |
| Dabigatran (300 mg bd) | 0.96 (0.27 to 3.78) | – | 0.96 (0.27 to 3.78) |
| Betrixaban (40 mg od) | 0.10 (0 to 0.67) | – | 0.10 (0 to 0.67) |
| Betrixaban (60 mg od) | 0.69 (0.19 to 2.27) | – | 0.69 (0.19 to 2.27) |
| Betrixaban (80 mg od) | 0.69 (0.19 to 2.22) | – | 0.69 (0.19 to 2.22) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Edoxaban (60 mg od) vs. apixaban (5 mg bd) | – | 1.24 (1.09 to 1.42) | 1.24 (1.09 to 1.42) |
| Rivaroxaban (20 mg od) vs. apixaban (5 mg bd) | – | 1.53 (1.33 to 1.75) | 1.53 (1.33 to 1.75) |
| Rivaroxaban (20 mg od) vs. edoxaban (60 mg od) | – | 1.23 (1.10 to 1.37) | 1.23 (1.10 to 1.37) |
| Imprecisely estimated comparisons | |||
| Apixaban (5 mg bd) vs. apixaban (2.5 mg bd) | 2.69 (0.35 to 79.9) | – | 2.69 (0.35 to 79.9) |
| Dabigatran (150 mg bd) vs. apixaban (2.5 mg bd) | – | 6.59 (0.60 to 220) | 6.59 (0.60 to 220) |
| Betrixaban (40 mg od) vs. apixaban (2.5 mg bd) | – | 0.39 (0.01 to 18.7) | 0.39 (0.01 to 18.7) |
| Edoxaban (60 mg od) vs. apixaban (2.5 mg bd) | – | 3.35 (0.44 to 99.4) | 3.35 (0.44 to 99.4) |
| Rivaroxaban (20 mg od) vs. apixaban (2.5 mg bd) | – | 4.12 (0.54 to 123) | 4.12 (0.54 to 123) |
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | – | 2.32 (0.74 to 8.63) | 2.32 (0.74 to 8.63) |
| Betrixaban (40 mg od) vs. apixaban (5 mg bd) | – | 0.15 (0 to 1.00) | 0.15 (0 to 1.00) |
| Betrixaban (40 mg od) vs. dabigatran (150 mg bd) | – | 0.06 (0 to 0.60) | 0.06 (0 to 0.60) |
| Edoxaban (60 mg od) vs. dabigatran (150 mg bd) | – | 0.54 (0.14 to 1.68) | 0.54 (0.14 to 1.68) |
| Rivaroxaban (20 mg od) vs. dabigatran (150 mg bd) | – | 0.66 (0.18 to 2.07) | 0.66 (0.18 to 2.07) |
| Edoxaban (60 mg od) vs. betrixaban (40 mg od) | – | 8.50 (1.25 to 251) | 8.50 (1.25 to 251) |
| Rivaroxaban (20 mg od) vs. betrixaban (40 mg od) | – | 10.4 (1.53 to 309) | 10.4 (1.53 to 309) |
Supplementary NMAs of HRs rather than ORs show very similar results (Tables 47 and 48).
| Comparisons with warfarin (INR 2–3) | HR (95% CI) |
|---|---|
| Antiplatelet (< 150 mg od) | 0.59 (0.46 to 0.76) |
| Apixaban (5 mg bd) | 0.67 (0.60 to 0.75) |
| Edoxaban (30 mg od) | 0.59 (0.55 to 0.64) |
| Edoxaban (45 mg od) | 1.09 (0.37 to 3.01) |
| Edoxaban (60 mg od) | 0.83 (0.77 to 0.90) |
| Edoxaban (30 mg bd) | 1.98 (1.05 to 3.71) |
| Edoxaban (60 mg bd) | 2.78 (1.46 to 5.20) |
| Rivaroxaban (20 mg od) | 1.03 (0.95 to 1.11) |
| Imprecisely estimated comparisons | |
| Dabigatran (50 mg bd) + aspirin (81 mg bd) | 0.93 (0.07 to 5.79) |
| Dabigatran (50 mg bd) + aspirin (325 mg bd) | 0.72 (0.06 to 4.54) |
| Dabigatran (150 mg bd) + aspirin (81 mg bd) | 1.01 (0.15 to 4.99) |
| Dabigatran (150 mg bd) + aspirin (325 mg bd) | 1.10 (0.17 to 5.53) |
| Dabigatran (300 mg bd) + aspirin (81 mg bd) | 2.84 (0.72 to 11.4) |
| Dabigatran (300 mg bd) + aspirin (325 mg bd) | 4.06 (1.10 to 16.1) |
| Apixaban (2.5 mg bd) | 0.25 (0.01 to 1.87) |
| Dabigatran (50 mg bd) | 0.06 (0 to 0.89) |
| Dabigatran (110 mg bd) | 0.68 (0.06 to 5.65) |
| Dabigatran (150 mg bd) | 1.60 (0.51 to 5.72) |
| Dabigatran (300 mg bd) | 0.99 (0.28 to 3.71) |
| Betrixaban (40 mg od) | 0.10 (0 to 0.66) |
| Betrixaban (60 mg od) | 0.69 (0.19 to 2.26) |
| Betrixaban (80 mg od) | 0.69 (0.19 to 2.30) |
| Licensed NOACs only | HR (95% CI) |
|---|---|
| Edoxaban (60 mg od) vs. apixaban (5 mg bd) | 1.24 (1.09 to 1.42) |
| Rivaroxaban (20 mg od) vs. apixaban (5 mg bd) | 1.53 (1.33 to 1.74) |
| Rivaroxaban (20 mg od) vs. edoxaban (60 mg od) | 1.23 (1.10 to 1.37) |
| Imprecisely estimated comparisons | |
| Apixaban (5 mg bd) vs. apixaban (2.5 mg bd) | 2.67 (0.36 to 81.0) |
| Dabigatran (150 mg bd) vs. apixaban (2.5 mg bd) | 6.69 (0.61 to 235) |
| Betrixaban (40 mg od) vs. apixaban (2.5 mg bd) | 0.39 (0.01 to 19.0) |
| Edoxaban (60 mg od) vs. apixaban (2.5 mg bd) | 3.32 (0.44 to 100) |
| Rivaroxaban (20 mg od) vs. apixaban (2.5 mg bd) | 4.08 (0.55 to 124) |
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | 2.38 (0.75 to 8.56) |
| Betrixaban (40 mg od) vs. apixaban (5 mg bd) | 0.15 (0.01 to 0.99) |
| Betrixaban (40 mg od) vs. dabigatran (150 mg bd) | 0.06 (0 to 0.58) |
| Edoxaban (60 mg od) vs. dabigatran (150 mg bd) | 0.52 (0.15 to 1.64) |
| Rivaroxaban (20 mg od) vs. dabigatran (150 mg bd) | 0.64 (0.18 to 2.03) |
| Edoxaban (60 mg od) vs. betrixaban (40 mg od) | 8.45 (1.25 to 247) |
| Rivaroxaban (20 mg od) vs. betrixaban (40 mg od) | 10.4 (1.55 to 305) |
Intracranial bleeding
Eight studies reported a total of 757 intracranial bleeds, leading to a network of 10 interventions (Figure 14). Seven trials were included in the primary analysis, with the remaining study included only in sensitivity analyses. These studies were at mixed risks of bias (Table 49), the concerns being due to lack of blinding of participants and, in one study, lack of blinding of outcome assessment.
FIGURE 14.
Network plot for intracranial bleeding (stroke prevention in AF). a, Excluded interventions that were included in sensitivity analyses.
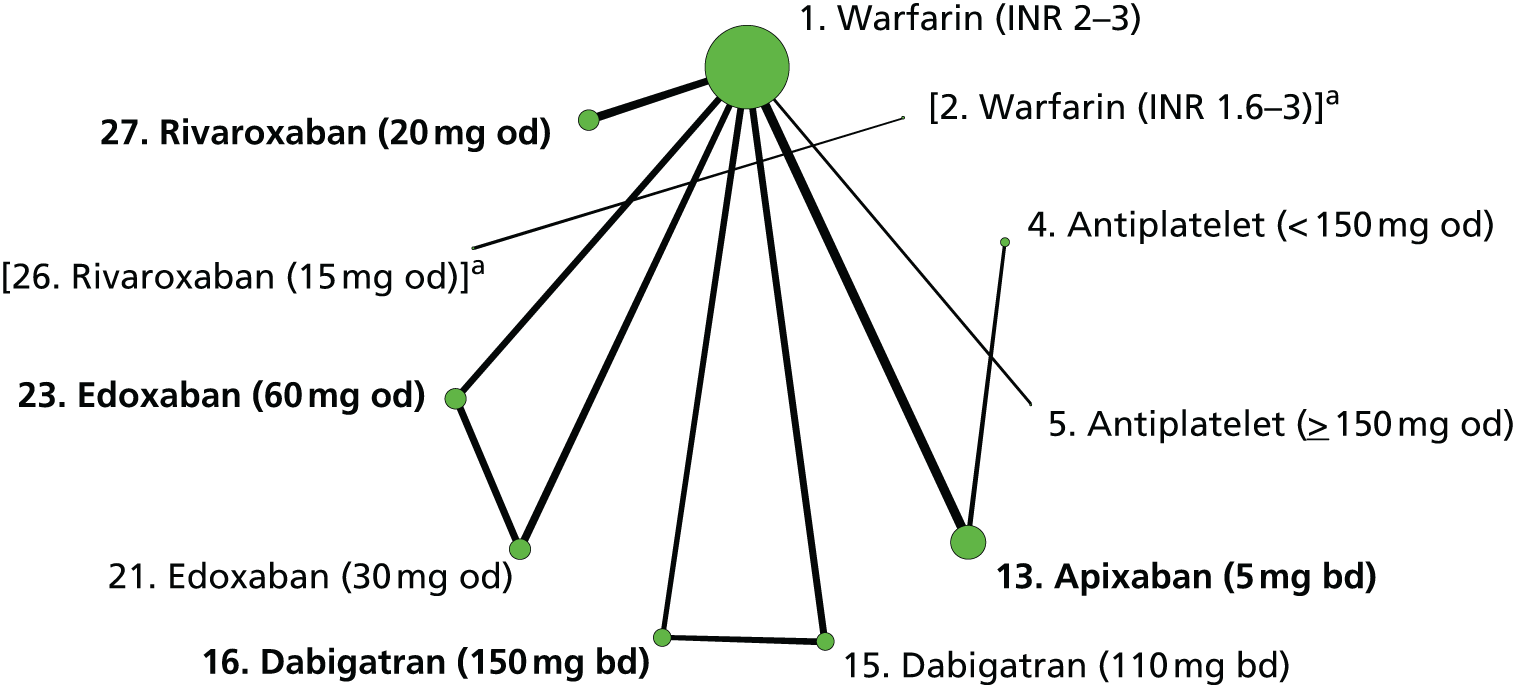
| Study | Interventions compared | SG | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| AFASAK II97 | 1, 5 | + | ? | – | + | + | ? |
| ARISTOTLE107,114,119,124–127,130,132–134 | 1, 13 | + | ? | + | + | ? | + |
| AVERROES103,114,115,119 | 4, 13 | + | + | + | + | + | + |
| ENGAGE AF-TIMI 48111,131 | 1, 21, 23 | + | + | + | + | + | + |
| RE-LY104,109 | 1, 15, 16 | + | + | – | + | + | + |
| J-ROCKET AF120 | 2, 26 | + | + | + | ? | + | + |
| ROCKET AF106,112,123,129 | 1, 27 | + | + | + | ? | + | + |
| SPAF II96 | 1, 5 | + | ? | – | – | + | ? |
There was strong evidence that apixaban (5 mg bd), dabigatran (110 mg bd), dabigatran (150 mg bd), edoxaban (30 mg od), edoxaban (60 mg od) and rivaroxaban (20 mg od) reduced risk of intracranial bleeding compared with warfarin (INR 2–3) (Table 50). For each of these NOAC doses except rivaroxaban (20 mg od), the estimated reduction in risk was > 50%. There was weak evidence that risk of intracranial bleeding was increased for rivaroxaban (20 mg od) compared with apixaban (5 mg bd), dabigatran (150 mg bd) and edoxaban (60 mg od) (Table 51). Analysing HRs rather than ORs led to similar results (Tables 52 and 53).
| Comparisons with warfarin (INR 2–3) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Antiplatelet (< 150 mg od) | – | 0.50 (0.21 to 1.23) | 0.50 (0.21 to 1.23) |
| Antiplatelet (≥ 150 mg od) | 0.39 (0.13 to 0.98) | – | 0.39 (0.13 to 0.98) |
| Apixaban (5 mg bd) | 0.42 (0.30 to 0.58) | – | 0.42 (0.30 to 0.58) |
| Dabigatran (110 mg bd) | 0.31 (0.19 to 0.47) | – | 0.31 (0.19 to 0.47) |
| Dabigatran (150 mg bd) | 0.40 (0.27 to 0.59) | – | 0.40 (0.27 to 0.59) |
| Edoxaban (30 mg od) | 0.31 (0.21 to 0.43) | – | 0.31 (0.21 to 0.43) |
| Edoxaban (60 mg od) | 0.46 (0.33 to 0.62) | – | 0.46 (0.33 to 0.62) |
| Rivaroxaban (20 mg od) | 0.65 (0.46 to 0.91) | – | 0.65 (0.46 to 0.91) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | – | 0.96 (0.58 to 1.60) | 0.96 (0.58 to 1.60) |
| Edoxaban (60 mg od) vs. apixaban (5 mg bd) | – | 1.09 (0.69 to 1.70) | 1.09 (0.69 to 1.70) |
| Rivaroxaban (20 mg od) vs. apixaban (5 mg bd) | – | 1.55 (0.97 to 2.49) | 1.55 (0.97 to 2.49) |
| Edoxaban (60 mg od) vs. dabigatran (150 mg bd) | – | 1.13 (0.69 to 1.87) | 1.13 (0.69 to 1.87) |
| Rivaroxaban (20 mg od) vs. dabigatran (150 mg bd) | – | 1.61 (0.96 to 2.72) | 1.61 (0.96 to 2.72) |
| Rivaroxaban (20 mg od) vs. edoxaban (60 mg od) | – | 1.43 (0.90 to 2.26) | 1.43 (0.90 to 2.26) |
| Comparisons with warfarin (INR 2–3) | HR (95% CI) |
|---|---|
| Antiplatelet (< 150 mg od) | 0.50 (0.21 to 1.20) |
| Antiplatelet (≥ 150 mg od) | 0.39 (0.14 to 0.97) |
| Apixaban (5 mg bd) | 0.42 (0.30 to 0.58) |
| Dabigatran (110 mg bd) | 0.31 (0.19 to 0.46) |
| Dabigatran (150 mg bd) | 0.41 (0.27 to 0.59) |
| Edoxaban (30 mg od) | 0.31 (0.21 to 0.43) |
| Edoxaban (60 mg od) | 0.46 (0.34 to 0.62) |
| Rivaroxaban (20 mg od) | 0.66 (0.47 to 0.91) |
| Licensed NOACs only | HR (95% CI) |
|---|---|
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | 0.97 (0.57 to 1.58) |
| Edoxaban (60 mg od) vs. apixaban (5 mg bd) | 1.09 (0.70 to 1.71) |
| Rivaroxaban (20 mg od) vs. apixaban (5 mg bd) | 1.55 (0.97 to 2.48) |
| Edoxaban (60 mg od) vs. dabigatran (150 mg bd) | 1.13 (0.70 to 1.87) |
| Rivaroxaban (20 mg od) vs. dabigatran (150 mg bd) | 1.62 (0.96 to 2.74) |
| Rivaroxaban (20 mg od) vs. edoxaban (60 mg od) | 1.43 (0.91 to 2.25) |
All-cause mortality
Eighteen studies reported 6479 all-cause mortality events, leading to a network of fifteen interventions (Figure 15). Fifteen studies were included in the primary analysis, with the remaining three studies included in sensitivity analyses. These studies were at mixed risks of bias (Table 54). There were concerns about lack of blinding of participants for most trials, and about lack of allocation concealment and blinding of outcome assessment in some studies.
FIGURE 15.
Network plot for all-cause mortality (stroke prevention in AF). a, Excluded interventions that were included in sensitivity analyses.
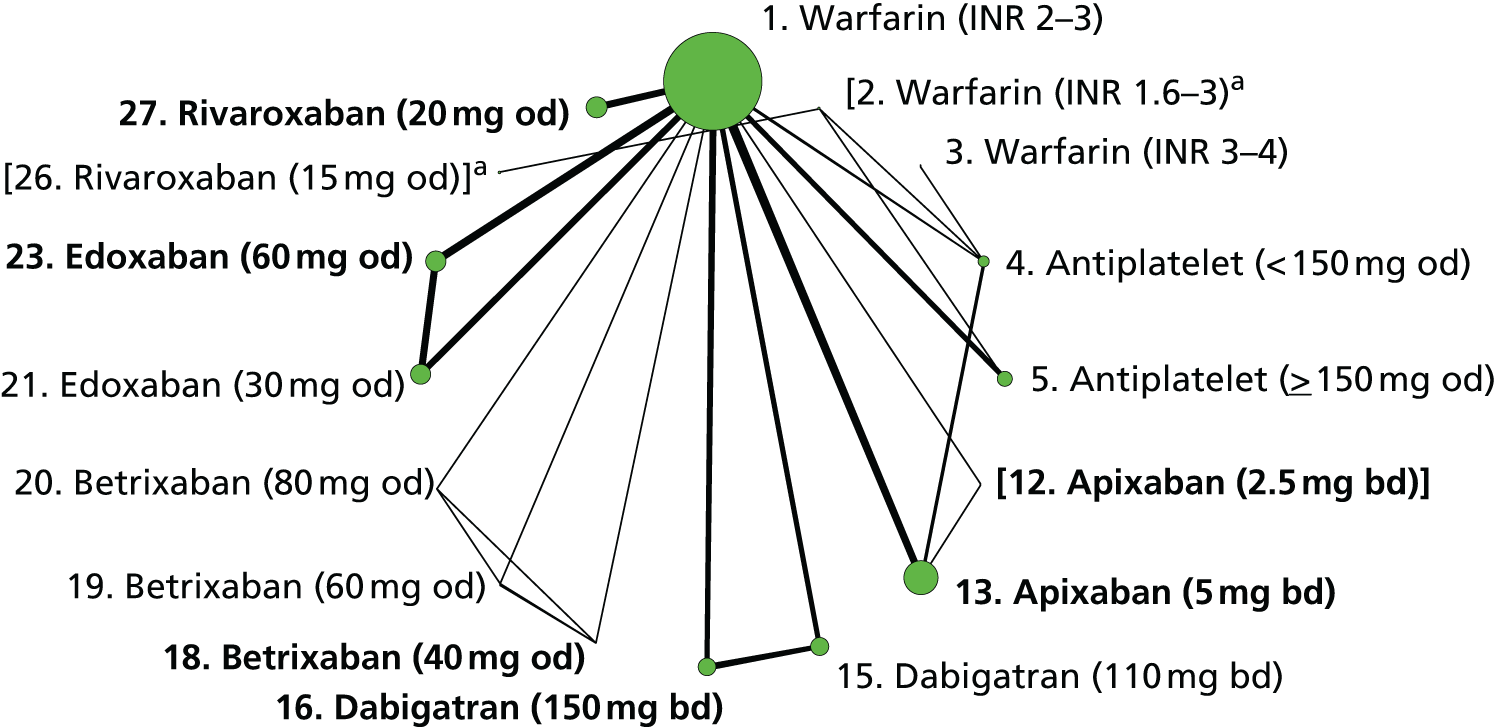
| Study | Interventions compared | SG | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| ACTIVE W98 | 1, 4 | + | + | – | + | + | ? |
| AFASAK95 | 1, 4 | + | + | – | ? | + | ? |
| AFASAK II97 | 1, 5 | + | ? | – | + | + | ? |
| AF-ASA-VKA-CHINA135 | 2, 4 | ? | – | – | – | + | ? |
| AF-VKA-ASA-CHINA122 | 1, 5 | + | ? | – | ? | ? | ? |
| ARISTOTLE107,114,119,124–127,130,132–134 | 1, 13 | + | ? | + | + | + | + |
| ARISTOTLE-J113 | 1, 12, 13 | ? | ? | – | + | + | + |
| AVERROES103,114,115,119 | 4, 13 | + | + | + | + | + | + |
| BAFTA103 | 1, 4 | + | + | – | + | + | + |
| Chinese ATAFS99 | 2, 5 | ? | ? | ? | ? | + | ? |
| ENGAGE AF-TIMI 48111,131 | 1, 21, 23 | + | + | + | + | + | + |
| EXPLORE-Xa126 | 1, 18, 19, 20 | ? | ? | – | + | + | + |
| J-ROCKET AF120 | 2, 26 | + | + | + | ? | + | + |
| PATAF98 | 1, 5 | + | + | ? | + | ? | + |
| RE-LY104,109 | 1, 15, 16 | + | + | – | + | + | + |
| ROCKET AF106,112,123,129 | 1, 27 | + | + | + | + | + | + |
| SPAF II96 | 1, 5 | + | ? | – | – | + | ? |
| WASPO101 | 1, 5 | + | + | – | – | + | ? |
Table 55 suggests that all NOAC doses with comparisons that were not imprecisely estimated [apixaban (5 mg bd), dabigatran (110 mg bd), dabigatran (150 mg bd), edoxaban (30 mg od), edoxaban (60 mg od) and rivaroxaban (20 mg od)] were associated with a reduced risk of all-cause mortality compared with warfarin (INR 2–3). There was little evidence that the risk of all-cause mortality differed between licensed doses of NOACs (Table 56). Analysing HRs rather than ORs produced similar results (Tables 57 and 58).
| Comparisons with warfarin (INR 2–3) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Antiplatelet (< 150 mg od) | 1.02 (0.75 to 1.38) | 1.13 (0.87 to 1.47) | 1.08 (0.88 to 1.33) |
| Antiplatelet (≥ 150 mg od) | 1.04 (0.87 to 1.25) | – | 1.04 (0.87 to 1.25) |
| Apixaban (5 mg bd) | 0.88 (0.79 to 0.98) | – | 0.88 (0.79 to 0.98) |
| Dabigatran (110 mg bd) | 0.91 (0.80 to 1.04) | – | 0.91 (0.80 to 1.04) |
| Dabigatran (150 mg bd) | 0.88 (0.77 to 1.01) | – | 0.88 (0.77 to 1.01) |
| Edoxaban (30 mg od) | 0.86 (0.78 to 0.96) | – | 0.86 (0.78 to 0.96) |
| Edoxaban (60 mg od) | 0.91 (0.82 to 1.01) | – | 0.91 (0.82 to 1.01) |
| Rivaroxaban (20 mg od) | 0.83 (0.69 to 1.00) | – | 0.83 (0.69 to 1.00) |
| Imprecisely estimated comparisons | |||
| Warfarin (INR 3–4) | – | 0.24 (0.05 to 0.81) | 0.24 (0.05 to 0.81) |
| Betrixaban (40 mg od) | 0.99 (0.06 to 15.5) | – | 0.99 (0.06 to 15.5) |
| Betrixaban (60 mg od) | 0.19 (0 to 5.70) | – | 0.19 (0 to 5.70) |
| Betrixaban (80 mg od) | 0.19 (0 to 5.88) | – | 0.19 (0 to 5.88) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | – | 1.00 (0.84 to 1.19) | 1.00 (0.84 to 1.19) |
| Edoxaban (60 mg od) vs. apixaban (5 mg bd) | – | 1.03 (0.89 to 1.20) | 1.03 (0.89 to 1.20) |
| Rivaroxaban (20 mg od) vs. apixaban (5 mg bd) | – | 0.94 (0.76 to 1.17) | 0.94 (0.76 to 1.17) |
| Edoxaban (60 mg od) vs. dabigatran (150 mg bd) | – | 1.03 (0.87 to 1.22) | 1.03 (0.87 to 1.22) |
| Rivaroxaban (20 mg od) vs. dabigatran (150 mg bd) | – | 0.94 (0.74 to 1.18) | 0.94 (0.74 to 1.18) |
| Rivaroxaban (20 mg od) vs. edoxaban (60 mg od) | – | 0.91 (0.73 to 1.13) | 0.91 (0.73 to 1.13) |
| Imprecisely estimated comparisons | |||
| Betrixaban (40 mg od) vs. apixaban (5 mg bd) | – | 1.13 (0.07 to 17.7) | 1.13 (0.07 to 17.7) |
| Betrixaban (40 mg od) vs. dabigatran (150 mg bd) | – | 1.12 (0.07 to 17.6) | 1.12 (0.07 to 17.6) |
| Edoxaban (60 mg od) vs. betrixaban (40 mg od) | – | 0.92 (0.06 to 14.1) | 0.92 (0.06 to 14.1) |
| Rivaroxaban (20 mg od) vs. betrixaban (40 mg od) | – | 0.83 (0.05 to 13.0) | 0.83 (0.05 to 13.0) |
| Comparisons with warfarin (INR 2–3) | HR (95% CI) |
|---|---|
| Antiplatelet (< 150 mg od) | 1.07 (0.88 to 1.30) |
| Antiplatelet (≥ 150 mg od) | 1.04 (0.87 to 1.24) |
| Apixaban (5 mg bd) | 0.89 (0.80 to 0.99) |
| Dabigatran (110 mg bd) | 0.91 (0.80 to 1.04) |
| Dabigatran (150 mg bd) | 0.89 (0.78 to 1.01) |
| Edoxaban (30 mg od) | 0.88 (0.80 to 0.97) |
| Edoxaban (60 mg od) | 0.92 (0.83 to 1.02) |
| Rivaroxaban (20 mg od) | 0.83 (0.69 to 1.00) |
| Imprecisely estimated comparisons | |
| Warfarin (INR 3–4) | 0.24 (0.05 to 0.81) |
| Betrixaban (40 mg od) | 1.01 (0.06 to 15.7) |
| Betrixaban (60 mg od) | a |
| Betrixaban (80 mg od) | a |
| Licensed NOACs only | HR (95% CI) |
|---|---|
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | 1.00 (0.85 to 1.18) |
| Edoxaban (60 mg od) vs. apixaban (5 mg bd) | 1.03 (0.90 to 1.20) |
| Rivaroxaban (20 mg od) vs. apixaban (5 mg bd) | 0.94 (0.76 to 1.15) |
| Edoxaban (60 mg od) vs. dabigatran (150 mg bd) | 1.03 (0.88 to 1.22) |
| Rivaroxaban (20 mg od) vs. dabigatran (150 mg bd) | 0.93 (0.75 to 1.17) |
| Rivaroxaban (20 mg od) vs. edoxaban (60 mg od) | 0.90 (0.73 to 1.11) |
| Imprecisely estimated comparisons | |
| Betrixaban (40 mg od) vs. apixaban (5 mg bd) | 1.13 (0.07 to 17.6) |
| Betrixaban (40 mg od) vs. dabigatran (150 mg bd) | 1.14 (0.07 to 17.6) |
| Edoxaban (60 mg od) vs. betrixaban (40 mg od) | 0.91 (0.06 to 14.7) |
| Rivaroxaban (20 mg od) vs. betrixaban (40 mg od) | 0.82 (0.05 to 13.2) |
Summary of results and ranking of interventions
Results from NMAs suggest that a number of the licensed doses of NOACs reduce the risk of the outcomes stroke or SE, major bleeding, CRB, intracranial bleeding and all-cause mortality compared with the reference treatment, warfarin (INR 2–3). There was evidence that edoxaban increased CRB compared with warfarin (INR 2–3). Risk of MI appeared higher for some NOACs than for warfarin (INR 2–3). Comparisons for some licensed NOAC doses, such as apixaban (2.5 mg bd) and betrixaban (40 mg od), could not be estimated precisely.
Several studies conducted in Asian countries considered a lower INR range for warfarin interventions in elderly patients. We excluded these from the main analysis but included them (merged with the reference treatment, warfarin INR 2–3) as a second sensitivity analysis for each outcome. This allowed us to incorporate a non-licensed dose of rivaroxaban (15 mg od) that was included in the J-ROCKET AF trial,106,112,123,129 showing a reduced risk of stroke compared with warfarin (INR 1.6–3), with a median OR of 0.49 (95% CI 0.24 to 0.99). Apart from this, results (available on request) showed the same trends as described above.
The dose range for the antiplatelet arm in the AVERROES trial105,116,117,121 was unusually wide (81–324 mg od). Because some of the patients had received a dose that was below standard, it was decided to merge it with the antiplatelets (< 150 mg od) node for the primary analysis. In a further sensitivity analysis for each outcome, this trial105,116,117,121 was excluded. Again, the results (available from the authors) were not substantially different from those presented above. With regard to model appraisal, we did not identify any instance of lack of convergence among the Markov chains, poor model fit or inconsistency. Few of the comparisons were replicated across studies; when there were multiple estimates we did not find evidence of statistical heterogeneity.
Rankograms plotting the probability that each of the licensed interventions for AF is ranked best, second best, and so on, for preventing each outcome, are displayed in Figure 16. The non-NOAC interventions (warfarin, INR 2–3) and antiplatelet therapy (aspirin/clopidogrel, ≥ 150 mg od) were ranked worst for stroke or SE and ischaemic stroke and were not among the best three interventions for any of the outcomes. Warfarin (INR 2–3) was also ranked as the worst intervention to reduce the risk of intracranial bleeding. Among the licensed NOACs, apixaban (5 mg bd) was ranked as among the best interventions for major bleeding, intracranial bleeding, all-cause mortality, stroke or SE, ischaemic stroke and MI. Edoxaban (60 mg od) was ranked second for major bleeding and all-cause mortality. Except for all-cause mortality and MI, outcomes for rivaroxaban (20 mg od) were ranked less highly than those for apixaban (5 mg bd), dabigatran (150 mg bd) and edoxaban (60 mg od).
FIGURE 16.
Rankogram for licensed interventions examined in stroke prevention in AF. IC, intracranial.
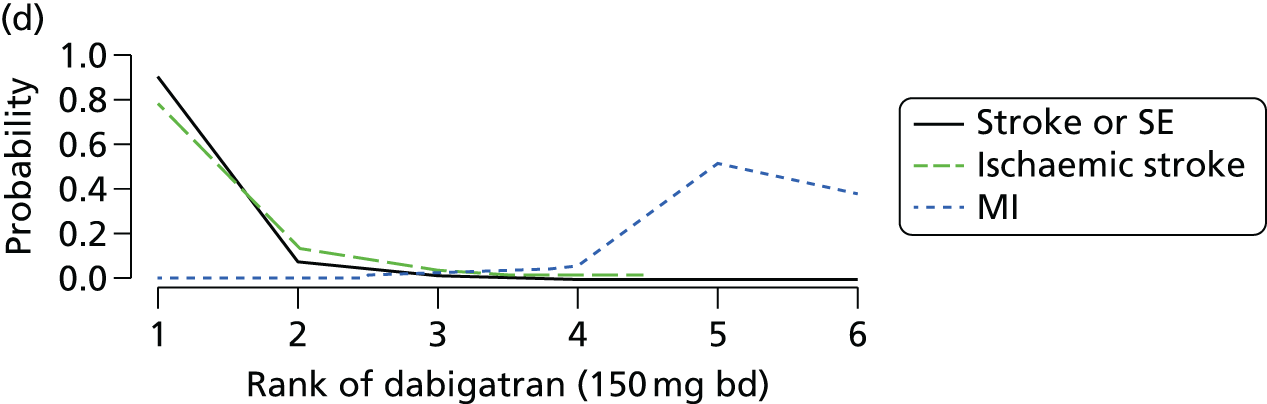
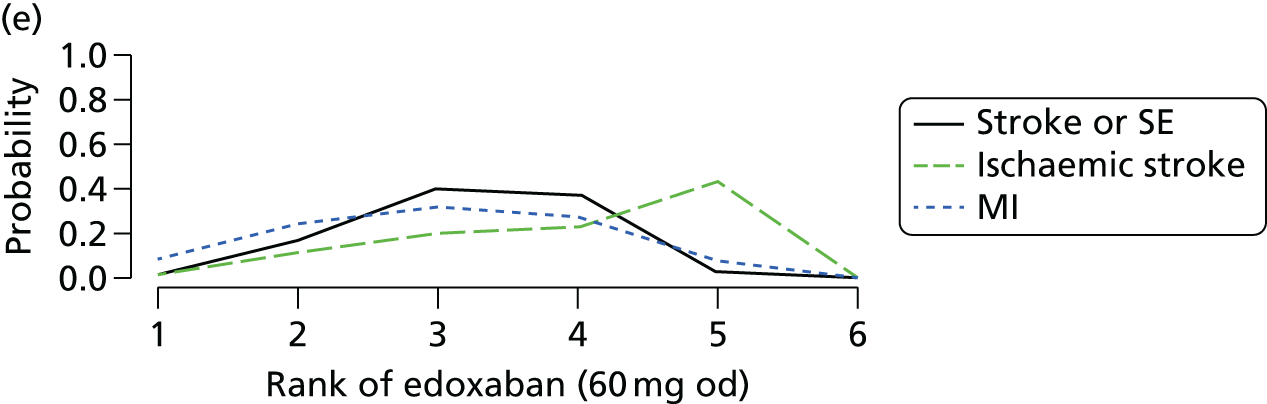
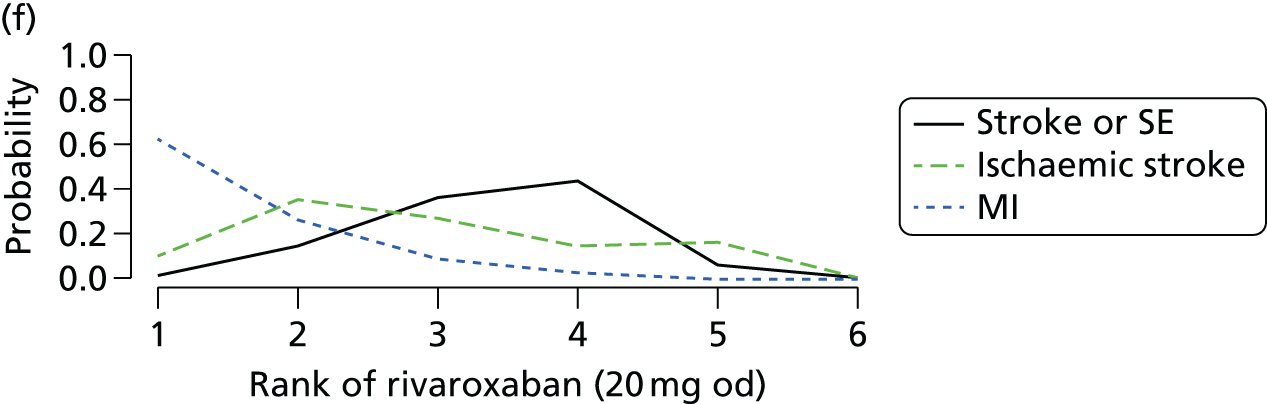
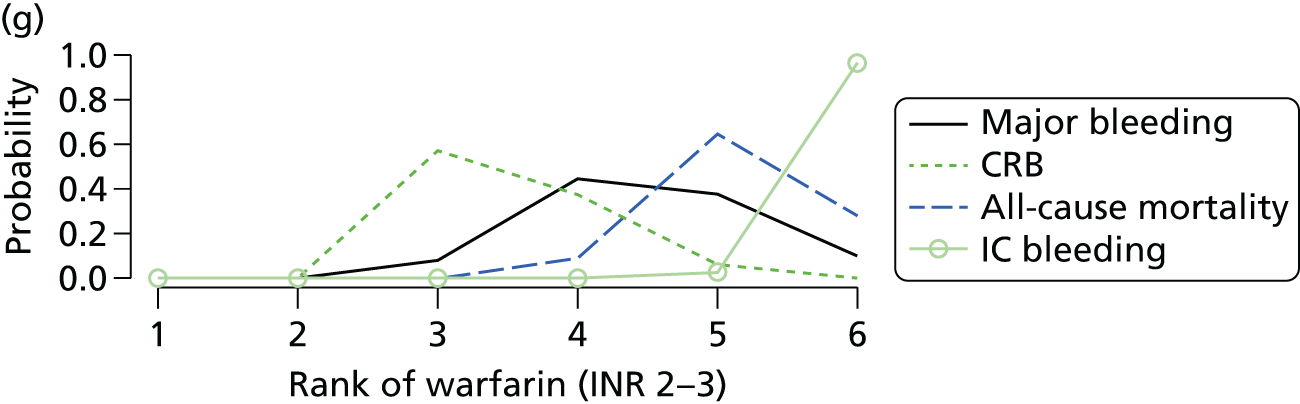
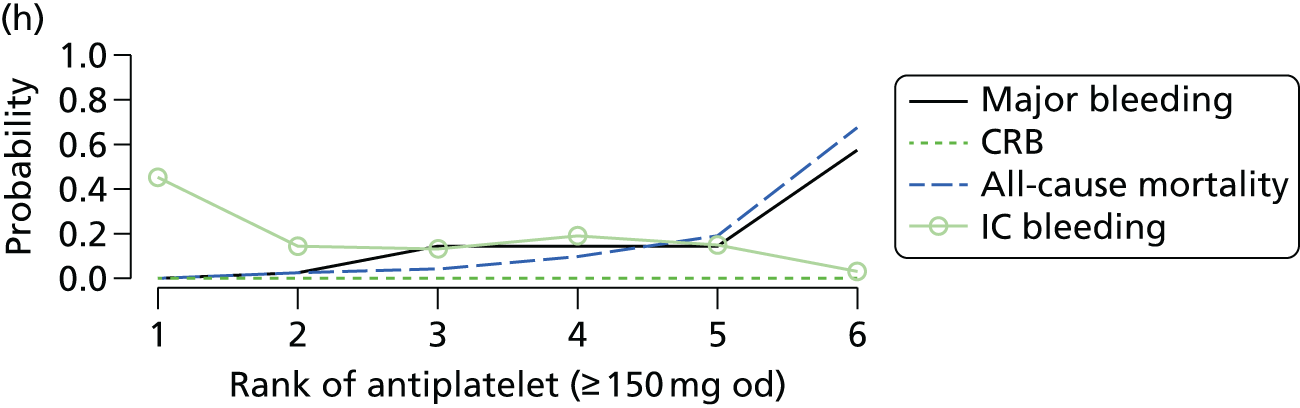
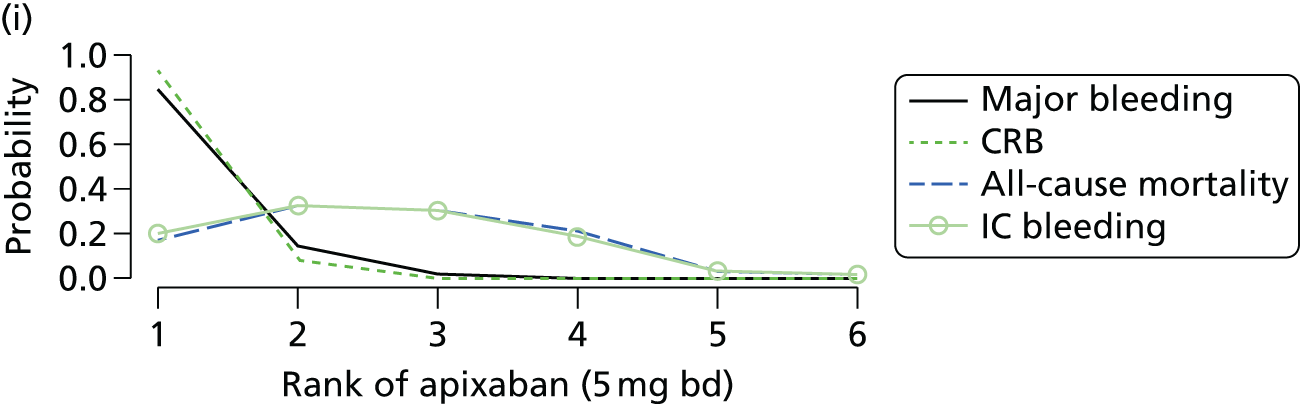
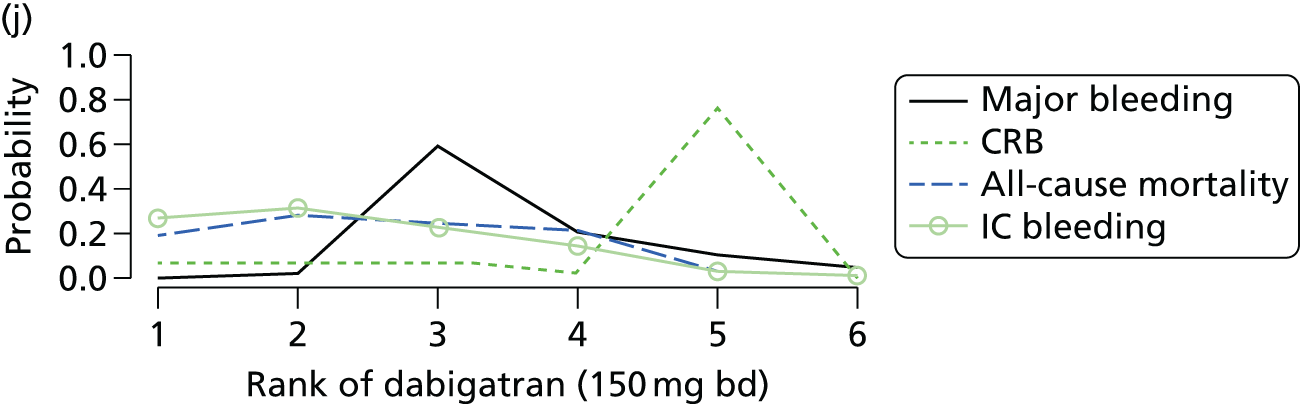
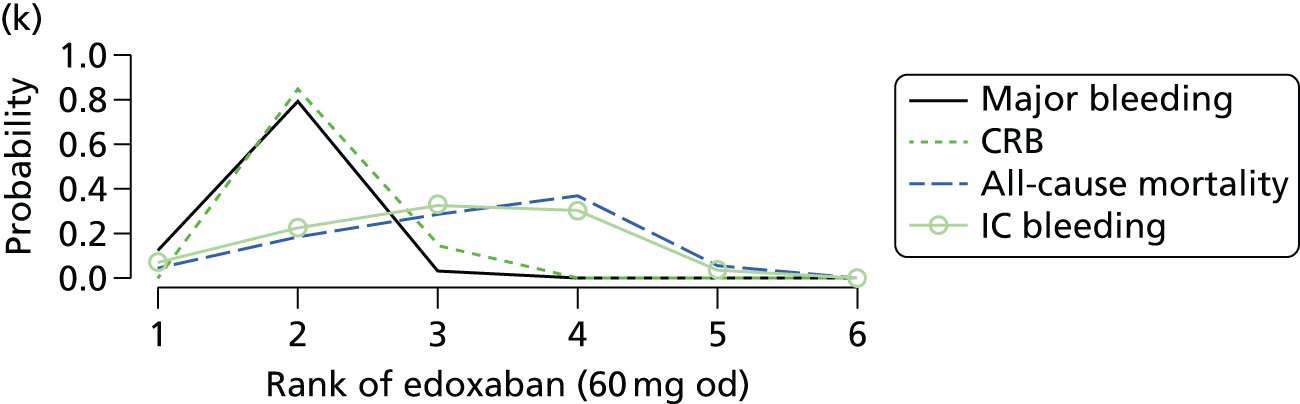

Chapter 6 Cost-effectiveness results (1) stroke prevention in atrial fibrillation
Introduction
In this chapter we present the results of the CEA for first-line treatments for patients with AF. The decision question, population, interventions, outcomes, model structure, cost and utility inputs have been previously described in Chapter 4. In this chapter we begin by describing clinical effectiveness inputs to the model, including relative treatment effects based on the evidence identified in the systematic review (see Chapter 5), other state-transition probabilities based on evidence from longitudinal studies, transition probabilities on the reference treatment (warfarin) on which relative effects are applied, mortality, and treatment switching parameters. We then present the results from our cost-effectiveness model, together with sensitivity analyses to key assumptions made. Results are presented from Bayesian analyses with 95% credible intervals, although we refer to these as confidence intervals for convenience.
Model inputs
Relative treatment efficacy
The NMA results presented in Chapter 5 consider each outcome separately and independently. However, for our economic model we need to consider the different outcomes jointly. We use a competing risks NMA model to jointly estimate the log-HRs for the different possible events needed in the economic model. The analysis uses data from the RCTs identified in our systematic review; however, results were reported in three different ways in the RCTs: number of first events, number of patients experiencing at least one event, and total number of events. The analysis needs to account for the way the results are reported. For example, if a patient’s first event was CRB, they cannot also have ischaemic stroke as their first event. Joint estimation leads to correlated estimates that need to be reflected in the economic model. In Appendix 7 we provide details on the competing risks NMA, and HRs relative to warfarin (INR 2–3) are given in Table 59. Note that it was possible to include studies with zero events in this analysis. Lower doses for apixaban and dabigatran are included, as they were evaluated in a sensitivity analysis. MI and all-cause mortality are common to both the NMA of Chapter 5 and the competing risks analysis, and their estimated HRs are similar. The competing risks model is restricted to ischaemic stroke and excludes both haemorrhagic stroke and SE, and so it is not precisely comparable to the stroke outcome of Chapter 5.
| NOAC | Ischaemic stroke | TIA | SEa | ICH | Other CRB | MI | Death (all causes) |
|---|---|---|---|---|---|---|---|
| Apixaban (5 mg bd) | 0.90 (0.72 to 1.11) | 0.74 (0.041 to 3.26) | 0.65 (0.33 to 1.18) | 0.46 (0.36 to 0.58) | 0.82 (0.70 to 0.94) | 0.86 (0.65 to 1.1) | 0.89 (0.8 to 0.99) |
| Dabigatran (150 mg bd) | 0.75 (0.58 to 0.97) | 2.68 (0.062 to 16.1) | 0.65 (0.52 to 0.80) | 0.36 (0.26 to 0.49) | 1.07 (0.92 to 1.24) | 1.27 (0.93 to 1.68) | 0.88 (0.77 to 1) |
| Edoxaban (60 mg od) | 1.00 (0.83 to 1.2) | 2.76 (0.06 to 15.8) | 0.58 (0.30 to 0.97) | 0.49 (0.39 to 0.61) | 0.88 (0.82 to 0.94) | 0.95 (0.74 to 1.19) | 0.92 (0.83 to 1.01) |
| Rivaroxaban (20 mg od) | 0.92 (0.73 to 1.13) | 2.68 (0.063 to 15.9) | 0.95 (0.79 to 1.13) | 0.65 (0.46 to 0.89) | 1.05 (0.98 to 1.13) | 0.79 (0.61 to 1.01) | 0.83 (0.69 to 0.99) |
| Apixaban (2.5 mg bd) | 0.74 (0.042 to 3.37) | 0.76 (0.041 to 3.51) | 0.48 (0.031 to 1.97) | 2.78 (0.06 to 16.2) | 0.63 (0.080 to 2.06) | 1.01 (0.049 to 4.67) | 1.03 (0.050 to 5.03) |
| Dabigatran (110 mg bd) | 1.13 (0.89 to 1.42) | 2.82 (0.062 to 16.4) | 0.90 (0.73 to 1.1) | 0.31 (0.22 to 0.43) | 0.94 (0.81 to 1.09) | 1.29 (0.94 to 1.71) | 0.91 (0.80 to 1.03) |
Patients may discontinue NOACs and warfarin, and so we also need estimates of the relative efficacy of warfarin compared with no treatment. Warfarin has been the established standard of care for patients with AF for at least 20 years and we therefore relied on previous meta-analyses to estimate the relative effect of warfarin compared with no treatment. We chose the meta-analysis by Hart et al. ,136 as it is the most recent and comprehensive. Hart et al. 136 identified six studies137–142 comparing warfarin to either ‘control’ or placebo, from which we extracted evidence on stroke, bleeds, ICH, death, SE and TIA, summarised in Table 60. The Boston Area Anticoagulation Trial for Atrial Fibrillation (BAATAF) study139 used patients on no treatment but with the option of aspirin as the control; this study was omitted in a sensitivity analysis. The INR ranges for warfarin were frequently outside the 2–3 range chosen for our NMA. Under clinical advice, we did not exclude on the basis of INR range; however, we note that the results from the only study with INR 2–3 [the Canadian Atrial Fibrillation Anticoagulation (CAFA) study140] were in line with the results from the other studies, providing support for the inclusion of all six studies. For each outcome, we separately conducted a random-effects meta-analysis using a Poisson likelihood, as described in Appendix 7, but without accounting for competing risks as a result of insufficient detail available from the trials. Random-effects models were used, as we expected some heterogeneity because of differences in INR range; however, on the basis of the Deviance Information Criteria23 there was not any evidence in favour of or against a fixed-effects model, and results were similar. We excluded studies with no events in any arm and added a continuity correction of 0.5 to arms with zero events if other arms in the trial had an event. The results of this analysis are presented in Table 60. Owing to insufficient evidence for ICH, we assumed the treatment effect was the same as that for bleeds, as these are clinically similar AEs. However, the estimated HRs for NOACs presented in Table 59 do not support this assumption of similarity. We therefore conducted a sensitivity analysis that sets the HR of ‘no treatment’ against warfarin for ICH to 1.
| Treatment | AFASAK95 | SPAF I138 | BAATAF139 | CAFA140 | SPINAF137 | EAFT141 | HR, mean (SD) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Warfarin | Placebo | Warfarin | Controla | Warfarin | Placebo | Warfarin | Placebo | Warfarin | Placebo | Warfarin | ||
| Patients | 336 | 335 | 211 | 210 | 208 | 212 | 191 | 187 | 290 | 281 | 214 | 225 | – |
| Patient-years at risk | 398 | 413 | 245 | 263 | 435 | 487 | 241 | 237 | 483 | 489 | 405 | 507 | – |
| Warfarin INR | 2.8–4.2 | 2.0–4.5 | 1.5–2.7 | 2.0–3.0 | 1.4–2.8 | 2.5–4.0 | – | ||||||
| Strokes | 19 | 9 | 19 | 8 | 13 | 2 | 9 | 6 | 23 | 7 | 50 | 20 | 0.359 (0.213) |
| Bleeds | NR | NR | 1 | 5 | 21 | 38 | 18 | 35 | 50 | 72 | 14 | 60 | 2.3 (3.53) |
| Deaths | NR | NR | NR | NR | 25 | 11 | 8 | 10 | 26 | 20 | 44 | 41 | 0.849 (3) |
| TIA | 3 | 1 | NR | NR | NR | NR | 2 | 2 | 7 | 4 | 0 | 1 | 4.86 (369) |
| SEb | NR | NR | NR | NR | NR | NR | 2 | 1 | 1 | 2 | 4 | 1 | 3.18 (63) |
| ICH | NR | NR | NR | NR | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | –c |
Effect of past health events and states on future event rates
The primary source of evidence for the effect of prior events on stroke, SE, TIA and bleed risk is a study in 182,678 Swedish patients by Friberg et al. 143 Reported HRs (Table 61) are for male patients aged < 65 years. We make the assumption that these HRs can be generalised to a population of 70-year-olds with 60 : 40 split of males–females.
| Risk factor | Future ischaemic stroke (95% CI) | Future TIA/SE (95% CI) | Future intracranial bleeding (ICH) (95% CI) | Future bleed (95% CI) |
|---|---|---|---|---|
| Ischaemic stroke | 4.00 (3.78 to 4.22) | 3.61 (3.44 to 3.78) | 1.64 (1.39 to 1.94) | 1.39 (1.27 to 1.52) |
| ICH | 1.78 (1.56 to 2.03) | 1.82 (1.62 to 2.04) | 10.2 (8.59 to 12.2) | 2.95 (2.57 to 3.39) |
| Any significant bleeding (major bleed) | 1.32 (1.21 to 1.44) | 1.36 (1.26 to 1.46) | 3.54 (3.02 to 4.17) | 3.32 (3.06 to 3.60) |
| MI | 1.24 (1.17 to 1.33) | 1.29 (1.22 to 1.36) | 0.94 (0.78 to 1.12) | 1.24 (1.15 to 1.35) |
We also estimated the effect of previous events on mortality. Andersen and Olsen144 provided estimates of the HRs for the effect of prior stroke or MI in patients with AF. These are reproduced in Table 62, and normal distributions representing uncertainty in the estimated log-HRs are summarised in Table 63. No evidence was available for the effect of prior bleeds or ICH on mortality. We made the assumption that bleeds and ICH would have the same effect as stroke on future risk of death. We conducted a sensitivity analysis in which we assumed bleeds and ICH to have no effect on future risk of death. The effects of prior events on future risks are assumed to be multiplicative, so a history of both stroke and MI will give a HR for mortality of 1/0.758 × 1/0.972 = 1.03 × 1.32 = 1.36.
| Event history | Effect on mortality (HR with 95% CI) |
|---|---|
| No MI | 0.972 (0.687 to 1.378) |
| No stroke | 0.758 (0.565 to 1.017) |
| Risk factor | Future ischaemic stroke | Future TIA/SE | Future ICH | Future bleed | Future death |
|---|---|---|---|---|---|
| Stroke | 1.39 (0.03) | 1.28 (0.02) | 0.49 (0.09) | 0.33 (0.05) | 0.28 (0.15) |
| ICH | 0.58 (0.07) | 0.60 (0.06) | 2.32 (0.09) | 1.08 (0.07) | 0.28 (0.15) |
| Bleed | 0.28 (0.04) | 0.31 (0.04) | 1.26 (0.08) | 1.20 (0.04) | 0.28 (0.15) |
| MI | 0.22 (0.03) | 0.25 (0.03) | –0.06 (0.09) | 0.22 (0.04) | 0.03 (0.18) |
We reflect uncertainty in the mean estimates by assuming normal distributions for the logs of these HRs (see Table 63).
Transition probabilities with usual care (warfarin)
We estimated transition probabilities for the usual care (first-line warfarin) treatment strategy using the trials identified in our systematic review that included a warfarin arm. The model includes the following correlated outcomes: (1) ischaemic stroke; (2) ICH; (3) other clinically relevant bleed; (4) TIA; (5) SE; (6) MI; and (7) death.
Previous economic models have used evidence from single trials, such as RE-LY104,109 in Kansal et al. ,42 to estimate the risk of events with warfarin treatment. However, this disregards the evidence available from other published trials. QRISK2145 provides long-term information on MI in patients with AF. However, this estimates only a joint risk of stroke and MI, rather than for each event individually. Another possible source of evidence for the rate of MI in AF is Soliman et al. ,5 but this provides a HR for MI only in AF relative to the non-AF population, which is not what is needed for our model. Therefore, we used evidence from the warfarin arms in the trials that were identified in our systematic review because it is based on patients with AF, has similar demographics to our target population and represented the risk for patients specifically on warfarin treatment.
We estimated the hazard of events on warfarin, taking into account the competing risks nature of the outcomes and the format in which results are reported, in the same way as we did for the relative effects (see Appendix 7). Details of the model are given in Appendix 8 and estimated hazards are shown in Table 64.
| Event | Mean hazard (95% CI) |
|---|---|
| MI | 0.0079 (0.0064 to 0.01) |
| Ischaemic stroke | 0.012 (0.01 to 0.013) |
| Death (all causes) | 0.038 (0.028 to 0.052) |
| TIA | 0.025 (0.006 to 0.089) |
| CRB | 0.066 (0.031 to 0.13) |
| SE | 0.017 (0.0059 to 0.041) |
| ICH | 0.0094 (0.0057 to 0.017) |
Mortality
The risk of death in a 70-year-old AF population on warfarin with a 60 : 40 male–female split is obtained from the usual care hazard described above. This is adjusted for each age group aged > 70 years using the 2011–13 life tables for England and Wales,146 which provide the probability that an individual from the general population and at a specific age will die within 1 year. The hazard of death (λ) in each age group is: λ = –log(1 – (0.6 × PDmale + 0.4 × PDfemale)), where PDmale and PDfemale are the annual probability of death for males and females, respectively. We use the ratio of this hazard for each age group to the hazard for 70-year-olds to adjust the usual care (warfarin) hazard of death for each age group in the model.
Treatment switching probabilities
Post-event treatment switching rules and probabilities were based on clinical opinion. Clinicians advised ‘definite’ switching in the event of ICH for all treatments and also in the event of MI for dabigatran; a ‘chance’ of switching in the case of CRB and ischaemic stroke; and a ‘slight chance’ of switching following SE or TIA, due to concern about treatment failure. We assume a probability of switching of 0.3 for ‘chance’ and 0.1 for ‘slight chance’, but reflect our high degree of uncertainty in these switching probabilities with beta distributions, summarised in Table 65. We subject these assumed switching probabilities to sensitivity analysis.
| Event leading to switching | Probability of switching, mean (95% CI) | Distribution for probability of switching | Rule for switching |
|---|---|---|---|
| ICH | 1.00 | – | Always switch to no treatment |
| MI | 1.00 | – | If on dabigatran, switch to warfarin; no switching otherwise |
| CRB | 0.30 (0.00 to 1.00) | Beta(0.3,0.7) | Switch to next line treatment |
| Ischaemic stroke | 0.30 (0.00 to 1.00) | Beta(0.3,0.7) | Switch to next line treatment |
| TIA | 0.10 (0.00 to 1.00) | Beta(0.1,0.9) | Switch to next line treatment |
| SE | 0.10 (0.00 to 1.00) | Beta(0.1,0.9) | Switch to next line treatment |
Sensitivity analyses
We explored the robustness of our results to various assumptions through sensitivity analyses.
Warfarin monitoring costs In this sensitivity analysis, we assumed that there is no drug or monitoring cost associated with warfarin. This explores whether or not warfarin is cost-effective, even in the absence of monitoring costs. We also considered running sensitivity analyses to fixed warfarin monitoring costs at £70.75 and £106.13 per 3-month cycle (mean and upper limit of assumed distribution for warfarin monitoring costs). Note, however, it is worth doing these sensitivity analyses only if warfarin is found to be cost-effective with no monitoring costs (otherwise it clearly will not be cost-effective for positive monitoring costs).
Mortality risk following bleeds/ICH In this sensitivity analysis we assumed that there is no effect of previous bleeds and ICH on future risk of death. This was motivated by the lack of evidence on this effect and the assumption of the base case that the effect of previous bleeds and ICH on mortality risk was the same as that of stroke.
Probabilities of treatment switching We ran three sensitivity analyses to the assumptions around treatment switching: in the first, we assumed that no patients switch treatment following ischaemic stroke, bleed, SE or TIA; in the second, we assumed that all patients switch after a ischaemic stroke or bleed, but none switches after a SE or TIA; and, in the third, we assumed that all patients switch treatments following these four events. In all sensitivity analyses, it is assumed that all patients discontinue treatment following an ICH, and patients on dabigatran switch to warfarin following a MI, as in the base case.
Excluding ‘no treatment control’ study from meta-analysis of warfarin vs. placebo trials The meta-analysis estimating the effect of warfarin compared with ‘no treatment’ included five studies comparing warfarin with placebo and one study, BAATAF,139 comparing warfarin with ‘control’. This control arm consisted of patients on no treatment who had the option of starting aspirin. When the BAATAF study139 is removed from the meta-analysis comparing warfarin with no treatment (Table 66), the effect of no treatment, compared with warfarin, on bleeds and deaths is decreased, although the uncertainty is greatly increased. This sensitivity analysis uses a meta-analysis that excludes the BAATAF study. 139
| Event | Mean HR | |
|---|---|---|
| Including BAATAF139 (SD) | Excluding BAATAF139 (SD) | |
| Strokes | 0.359 (0.213) | 0.391 (0.246) |
| Bleeds | 2.3 (3.53) | 3.23 (18.9) |
| Deaths | 0.849 (3) | 1.37 (13.6) |
| TIA | 4.86 (369) | 4.86 (369) |
| SE | 3.18 (63) | 3.18 (63) |
| ICH | NA | NA |
Sensitivity to initial age of cohort We ran two sensitivity analyses to the initial age of the cohort, ages 60 and 80 years, respectively, rather than 70 years in the base case. These ages are roughly one SD from the mean age of patients included in the trials.
Apixaban 2.5 mg bd and dabigatran 110 mg bd This sensitivity analysis uses different doses (apixaban 2.5 mg bd and dabigatran 110 mg bd) than those used in the base-case analysis (5 mg and 150 mg, respectively). This is motivated by the licensing of these drugs by the European Medicines Agency, which specifies that the lower dose should be prescribed for older patients (> 75 years).
No difference in hazard of ICH between ‘no treatment’ and warfarin As our meta-analysis comparing warfarin and ‘no treatment’ had insufficient evidence to estimate the HR for ICH, we assumed it to be the same as for bleeds. In this sensitivity analysis we assumed that the hazard of ICH is the same in warfarin patients and ‘no treatment’ patients.
Results of the cost-effectiveness model: atrial fibrillation
Results of base-case analyses
We ran 10,000 iterations of our model for 120 cycles (each iteration representing a simulation from the joint distribution of our model parameters). We estimated expected total costs and QALYs for each first-line anticoagulation strategy (Table 67). Expected incremental costs and QALYs for each first-line strategy compared with warfarin (INR 2–3) are also given.
| Estimated costs and outcomes | Warfarin (INR 2–3): mean (95% CI) | Apixaban (5 mg bd): mean (95% CI) | Dabigatran (150 mg bd): mean (95% CI) | Edoxaban (60 mg od): mean (95% CI) | Rivaroxaban (20 mg od): mean (95% CI) |
|---|---|---|---|---|---|
| Expected total costs (£) | 24,418 (12,189 to 50,365) | 23,340 (12,842 to 45,753) | 23,064 (12,674 to 46,075) | 23,985 (13,098 to 46,319) | 24,841 (13,198 to 47,603) |
| Expected QALYs | 5.166 (3.629 to 6.541) | 5.488 (3.841 to 6.795) | 5.416 (3.817 to 6.701) | 5.405 (3.819 to 6.678) | 5.451 (3.824 to 6.797) |
| Expected incremental total costs (£) | (– to –) | –1078 (–7626 to 2568) | –1354 (–8049 to 2273) | –433.4 (–6430 to 3619) | 422.5 (–4730 to 5104) |
| Incremental expected QALYs | (– to –) | 0.3227 (–0.01486 to 0.8142) | 0.2505 (–0.08034 to 0.7025) | 0.2389 (–0.1122 to 0.6841) | 0.2851 (–0.06816 to 0.8096) |
| Incremental expected net benefit (£20,000) | (– to –) | 7533 (489.9 to 18,228) | 6365 (–167.7 to 17,039) | 5212 (–893.8 to 14,826) | 5279 (–1097 to 15,180) |
| Incremental expected net benefit (£30,000) | (– to –) | 10,760 (576.2 to 25,861) | 8871 (–597.3 to 23,402) | 7601 (–1556 to 20,987) | 8130 (–1399 to 22,819) |
Dabigatran (150 mg bd) has the lowest expected total cost (£23,064), followed by apixaban (5 mg bd), edoxaban (60 mg od), warfarin (INR 2–3) and rivaroxaban (20 mg od) which has the highest expected total cost (£24,841). Expected costs are similar across all treatments, and there is a high degree of uncertainty around the costs for all treatments.
Apixaban (5 mg bd) has the highest expected QALYs (5.49), followed by rivaroxaban (20 mg od) (5.45), dabigatran (150 mg bd) and edoxaban (60 mg od) (both with 5.41), and warfarin (INR 2–3) (5.16). The NOACs have similar expected QALYs, all of which are higher than for warfarin (INR 2–3). There is a high degree of uncertainty around the QALY estimates.
At a willingness-to-pay threshold of £20,000 per QALY, all NOACs have positive expected incremental net benefit (INB) compared with warfarin (INR 2–3), suggesting that they may be a cost-effective use of NHS resources. Apixaban (5 mg bd) has the highest expected INB (£7533), followed by dabigatran (150 mg bd; £6365), rivaroxaban (20 mg od; £5279) and edoxaban (60 mg od; £5212). Apixaban (5 mg bd) is the only NOAC for which the 95% CI around INB is positive, suggesting that apixaban is cost-effective compared with warfarin. These conclusions also hold at the higher threshold of £30,000.
The key drivers of the results are the lower rates of MI, ICH and other CRB for apixaban (see Table 59), as found in the NMA of Chapter 5. The high cost and disutility of ICH has a great influence on total costs, total QALYs and net benefits. Apixaban also has a low rate of TIA, but the uncertainty surrounding the other treatment effects, and the minimal impact of this event, means that it is not a driving factor in the results. Dabigatran also has a low rate of ICH but the higher rate of MI offsets this benefit.
The uncertainty in the estimated total costs and QALYs is illustrated in the cost-effectiveness plane (Figure 17). The CEAC (Figure 18) plots the probability of each intervention having the highest net benefit against a willingness to pay per QALY. It indicates that apixaban (5 mg bd) has the highest probability of being the most cost-effective first-line therapy for AF – close to 60% in the £20,000–30,000 range of willingness-to-pay thresholds generally considered by NICE. Dabigatran (150 mg bd) has the highest probability of being cost-effective if the willingness-to-pay threshold is very low, as a result of having the lowest expected total costs. Warfarin (INR 2–3) and edoxaban (60 mg od) are unlikely to be cost-effective. These results are further highlighted by the CEAF (Figure 19), which plots the probability of having the highest net benefit against a willingness to pay per QALY for the intervention with the highest expected net benefit. Apixaban (5 mg bd) has the highest expected net benefit at a wide range of willingness-to-pay thresholds. Apixaban (5 mg bd) is likely to be the most cost-effective first-line therapy for AF, under the assumptions of our model.
FIGURE 17.
Incremental cost-effectiveness plane, warfarin (INR 2–3) is reference. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
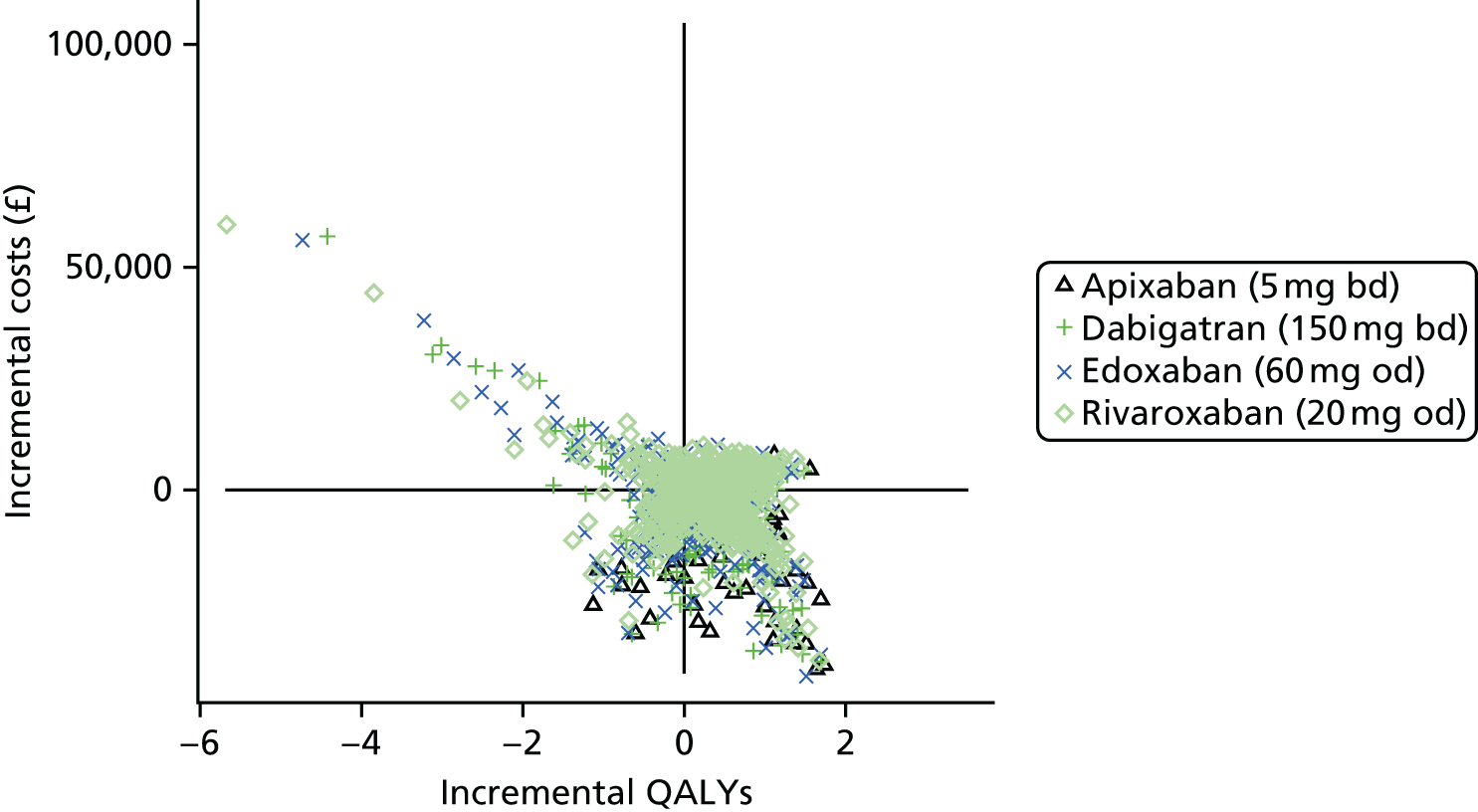
FIGURE 18.
Cost-effectiveness acceptability curves. The probability each first-line treatment is most cost-effective against willingness-to-pay-per-QALY threshold. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
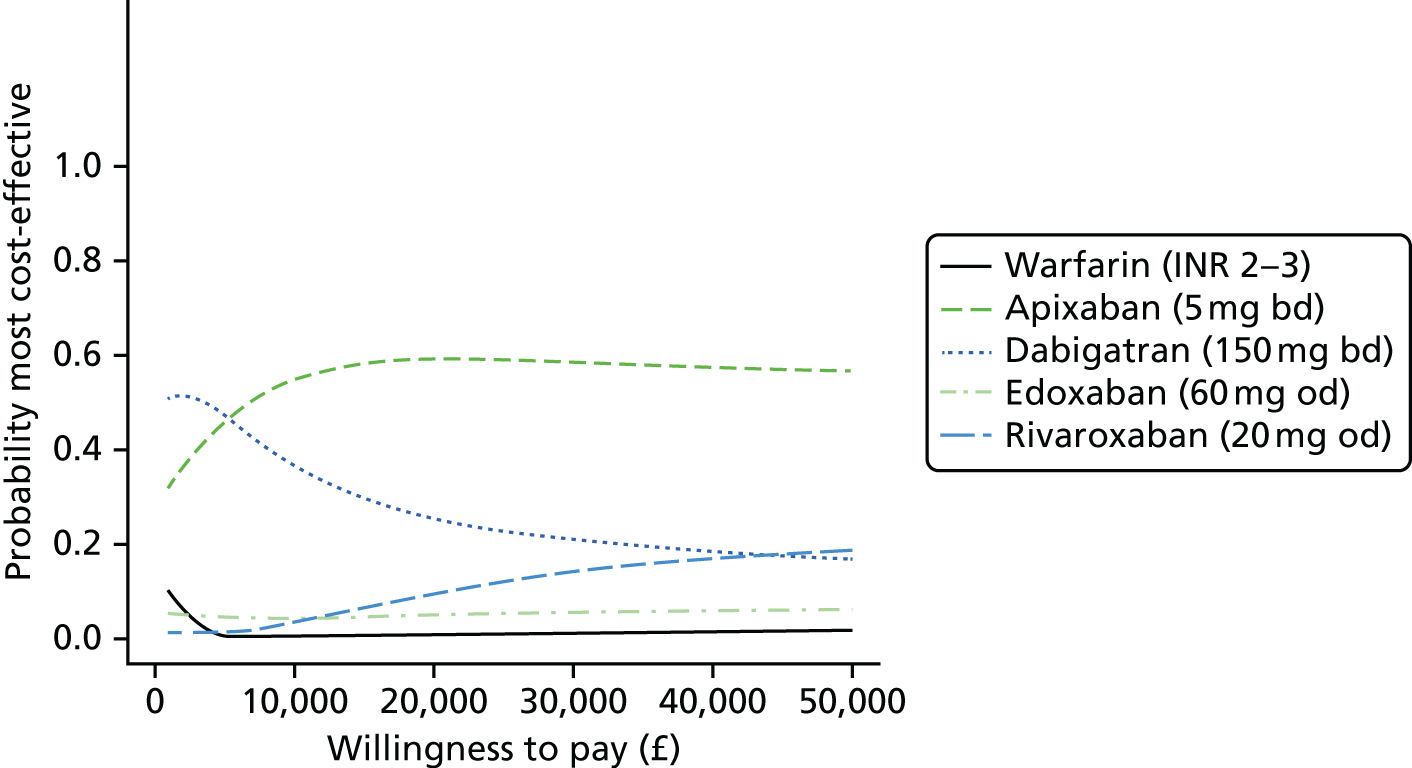
FIGURE 19.
Cost-effectiveness acceptability frontier. For each willingness-to-pay-per-QALY threshold, the probability of being most cost-effective is plotted for the treatment that has the highest expected net benefit at that willingness-to-pay threshold. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
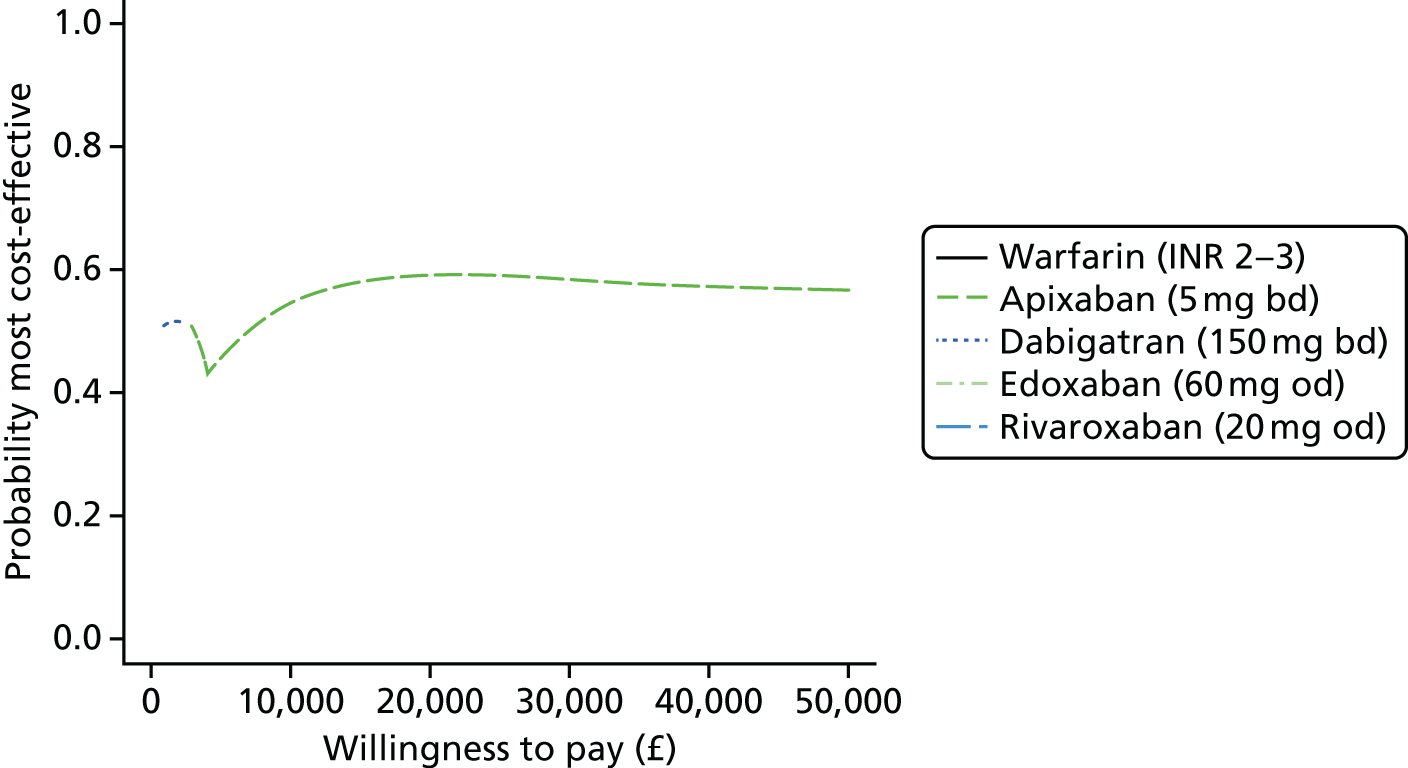
The per-person EVPI (Figure 20) estimated was £608 at a willingness-to-pay of £20,000 and £938 at £30,000. Assuming an incidence of 1%,147 and that there are 500,000 70-year-olds in England and Wales,148 there are 5000 new cases of AF every year. Extrapolating the EVPI over 10 years for 5000 patients, and discounting at 3.5%, gives a population EVPI of approximately £26M at a willingness to pay of £20,000, and £40M at £30,000. This suggests that there may be value in conducting further research to inform this decision.
FIGURE 20.
Per-person EVPI over range of willingness-to-pay thresholds.
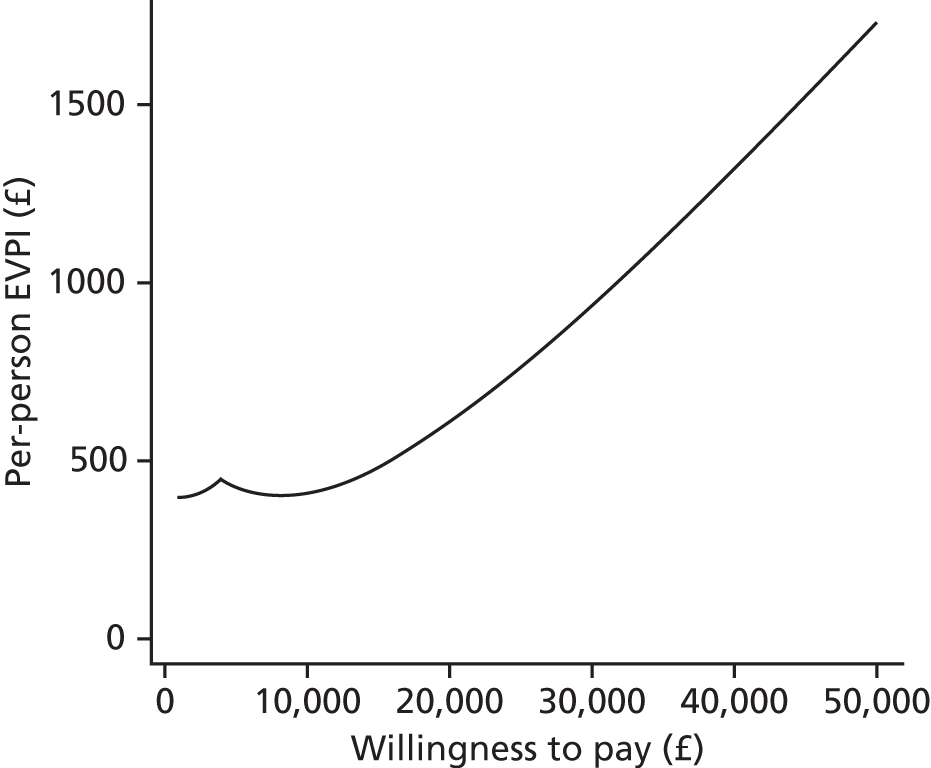
Expected value of partial perfect information (Figure 21) was estimated for various groups of parameters using the SAVI tool. 76 Note that this is an approximation only, with large standard errors. We therefore interpret the results cautiously but as indicative of the relative impact of the different groups of parameters on decision uncertainty. The optimal decision is most sensitive to the HRs for the NOACs, suggesting that a head-to-head trial may be of value. The decision is also sensitive to costs, the effect of past events on future HRs and probabilities of treatment switching. The decision is less sensitive to utilities and event rates on the reference treatment (warfarin). The estimated EVPPI for a trial comparing apixaban and dabigatran (the two NOACs with highest probability of being most cost-effective at willingness to pay per QALY of £20,000), with a warfarin control arm, indicated that such a trial could potentially be of value, particularly if it were designed to inform baseline event rates on warfarin, costs and switching probabilities. However, a study powered to measure all of these outcomes with sufficient precision would require a very large sample size, which may be prohibitively expensive.
FIGURE 21.
Expected value of partial perfect information for subsets of parameters. SAVI-estimated EVPPI scaled by EVPPI of all parameters as estimated by SAVI. 95% intervals are ± 1.96 × standard error and are truncated above at 1 and below at 0. ‘A vs. D’ is apixaban vs. dabigatran vs. warfarin trial.
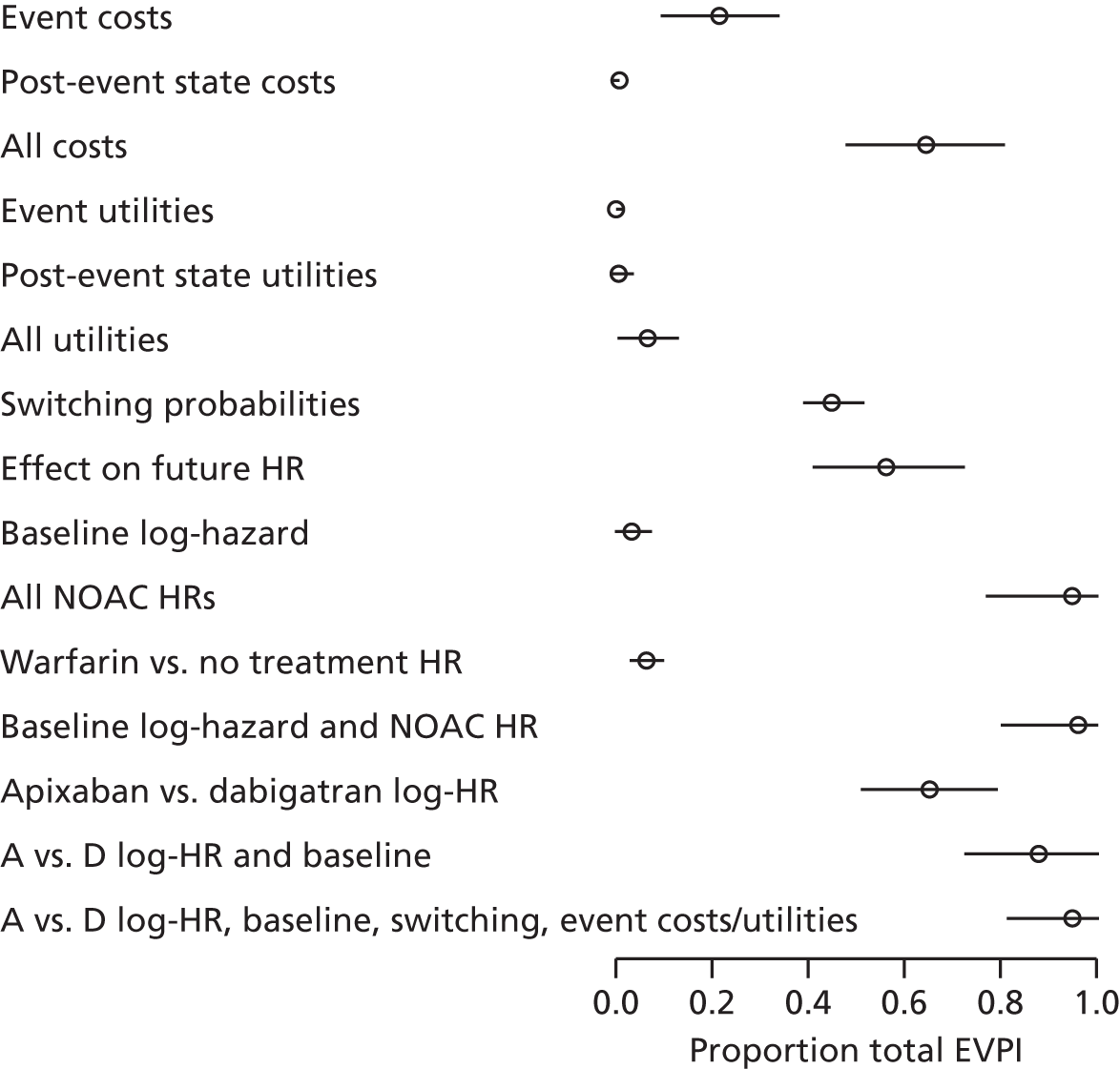
Expected (mean) values are reported with 95% CIs. Incremental values are relative to warfarin (INR 2–3). INB is the difference in QALYs and costs for willingness-to-pay thresholds of £20,000 and £30,000 per QALY.
Results of sensitivity analyses
We used 1000 simulations of the model for each sensitivity analysis. To explore whether or not results were sensitive to the assumed costs of warfarin, we began with the extreme case in which there is no administration or monitoring costs for warfarin. We found that this had little effect on the conclusion that apixaban 5 mg bd is the most cost-effective strategy (Figure 22). Clearly, if warfarin is not cost-effective with zero monitoring costs then it will not be cost-effective with monitoring costs greater than this. We therefore omit the sensitivity analyses with higher monitoring costs. Similarly, the assumption that ICH and other CRBs have no effect on future mortality risk did not alter the conclusion that apixaban 5 mg bd is most likely to be cost-effective (Figure 23).
FIGURE 22.
Cost-effectiveness acceptability curves for sensitivity analysis assuming that the cost of warfarin treatment is zero. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.

FIGURE 23.
Cost-effectiveness acceptability curves for sensitivity analysis assuming no effect of bleed or ICH on mortality risk. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
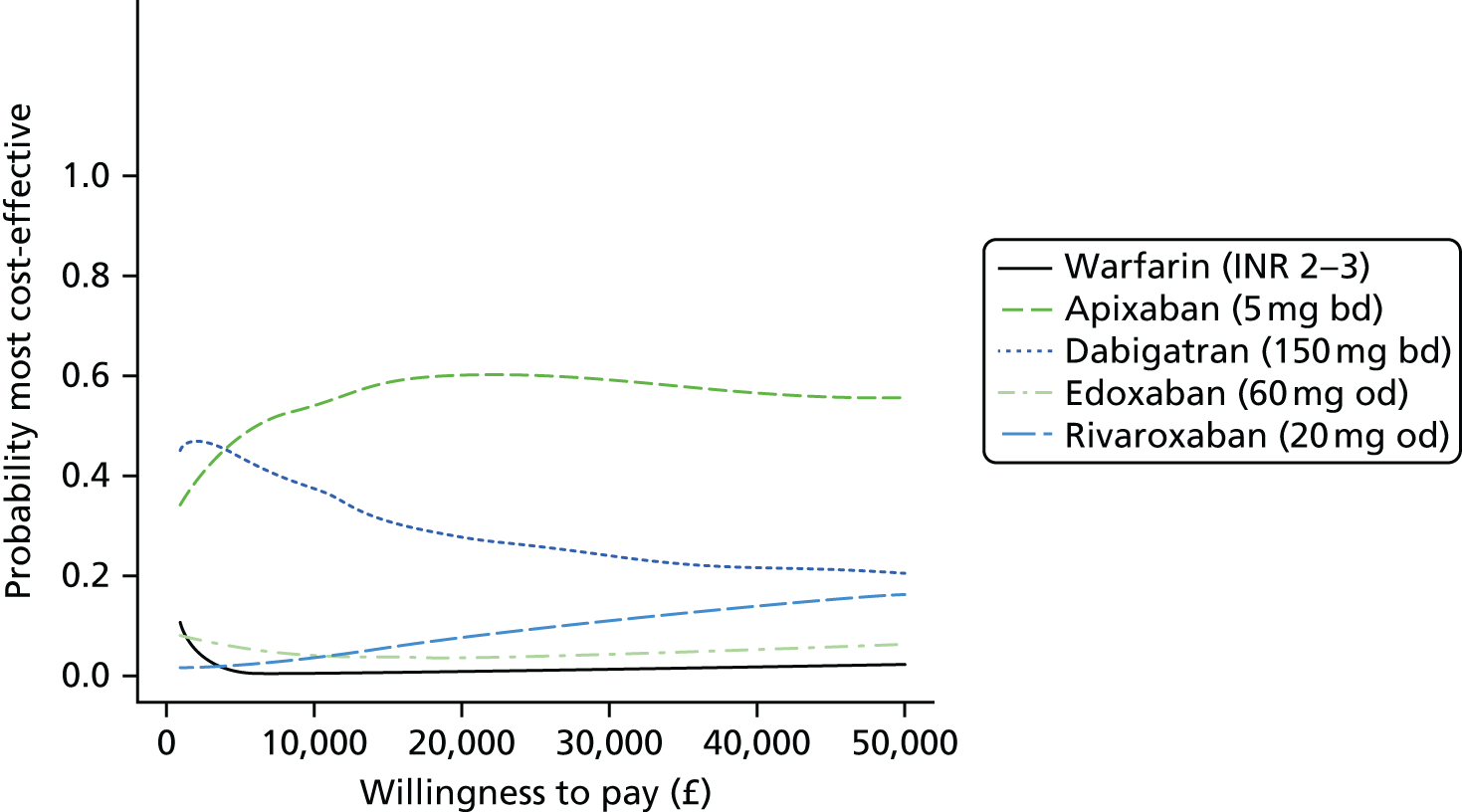
Different treatment switching strategies were also explored. If patients switch to no treatment only when they experience an ICH or a MI (if on dabigatran), the results are similar to our primary analysis (Figure 24). If all patients switch treatments after ischaemic stroke, bleed, SE and TIA, in addition to the switching after ICH and MI (for dabigatran) then patients spend only a short time on a NOAC before switching to warfarin. In this scenario, it is perhaps unsurprising that warfarin is the most cost-effective strategy (Figure 25). We also considered a switching strategy by which all patients switch after an ischaemic stroke or clinically relevant bleed, and none switches after a TIA or SE, and found that the results are similar to our primary analysis (Figure 26). Excluding the BAATAF study139 has no effect on the conclusion that apixaban 5 mg bd is likely to be the most cost-effective treatment (Figure 27).
FIGURE 24.
Cost-effectiveness acceptability curves for sensitivity analysis assuming that no patients switch treatment following stroke, bleed, SE or TIA. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
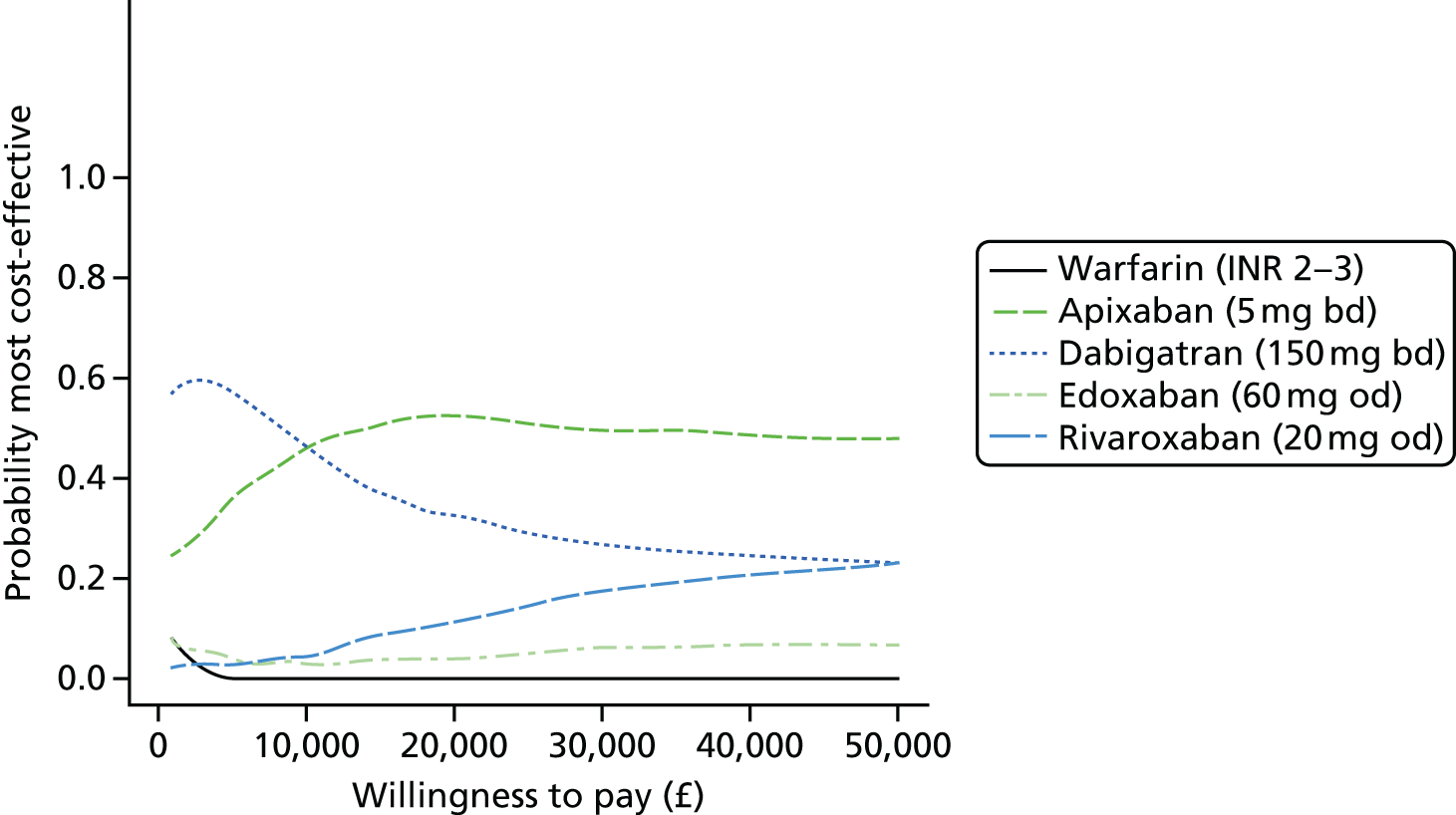
FIGURE 25.
Cost-effectiveness acceptability curves for sensitivity analysis assuming that all patients switch treatment following stroke, bleed, SE or TIA. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.

FIGURE 26.
Cost-effectiveness acceptability curves for sensitivity analysis assuming that all patients switch treatment following stroke or bleed, and none switches following SE or TIA. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
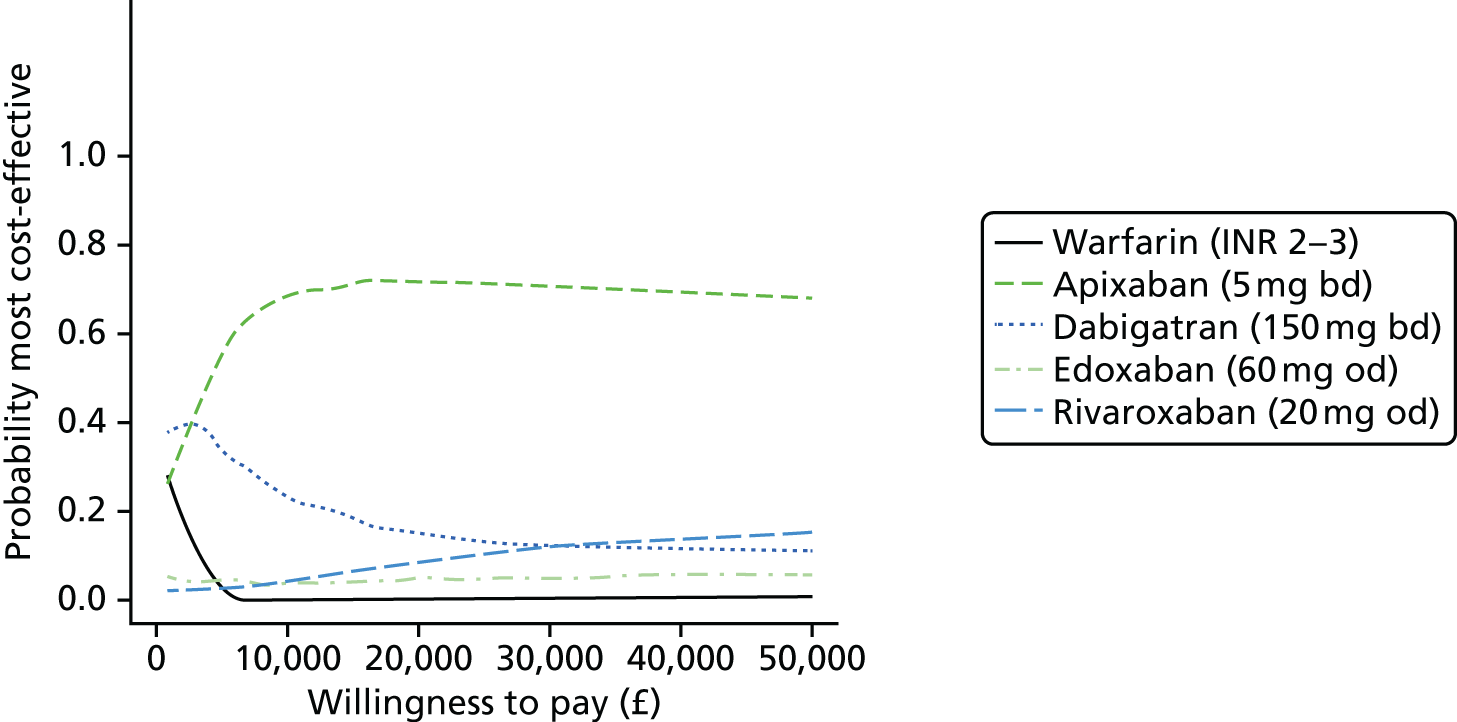
FIGURE 27.
Cost-effectiveness acceptability curves for sensitivity analysis excluding the BAATAF study139 from meta-analysis of the treatment effect of warfarin compared with no treatment. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
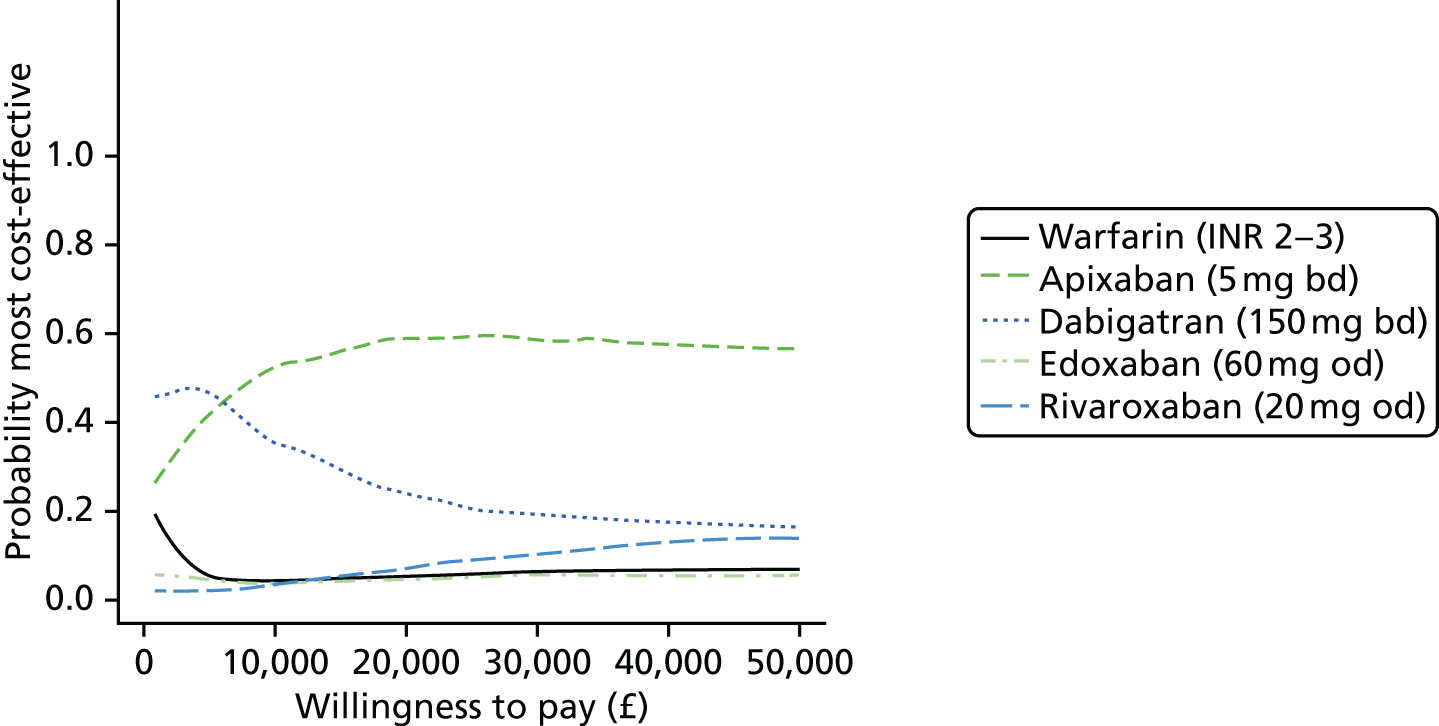
Different initial ages for the cohort were also explored. Apixaban 5 mg bd is the strategy that is most likely to be cost-effective, assuming an initial cohort age of 60 years (Figure 28) or 80 years (Figure 29). Lower doses of apixaban (2.5 mg bd) and dabigatran (110 mg bd) are recommended for elderly patients, and were compared in a sensitivity analysis (Figure 30). The uncertainty is much greater in this comparison, but apixaban (2.5 mg bd) is most likely to be the most cost-effective first-line therapy for the prevention of stroke in AF. Results were robust to assuming that the hazard of ICH is the same for no treatment as for warfarin (Figure 31).
FIGURE 28.
Cost-effectiveness acceptability curves for sensitivity analysis assuming that the cohort starts at age 60 years, rather than 70 years. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
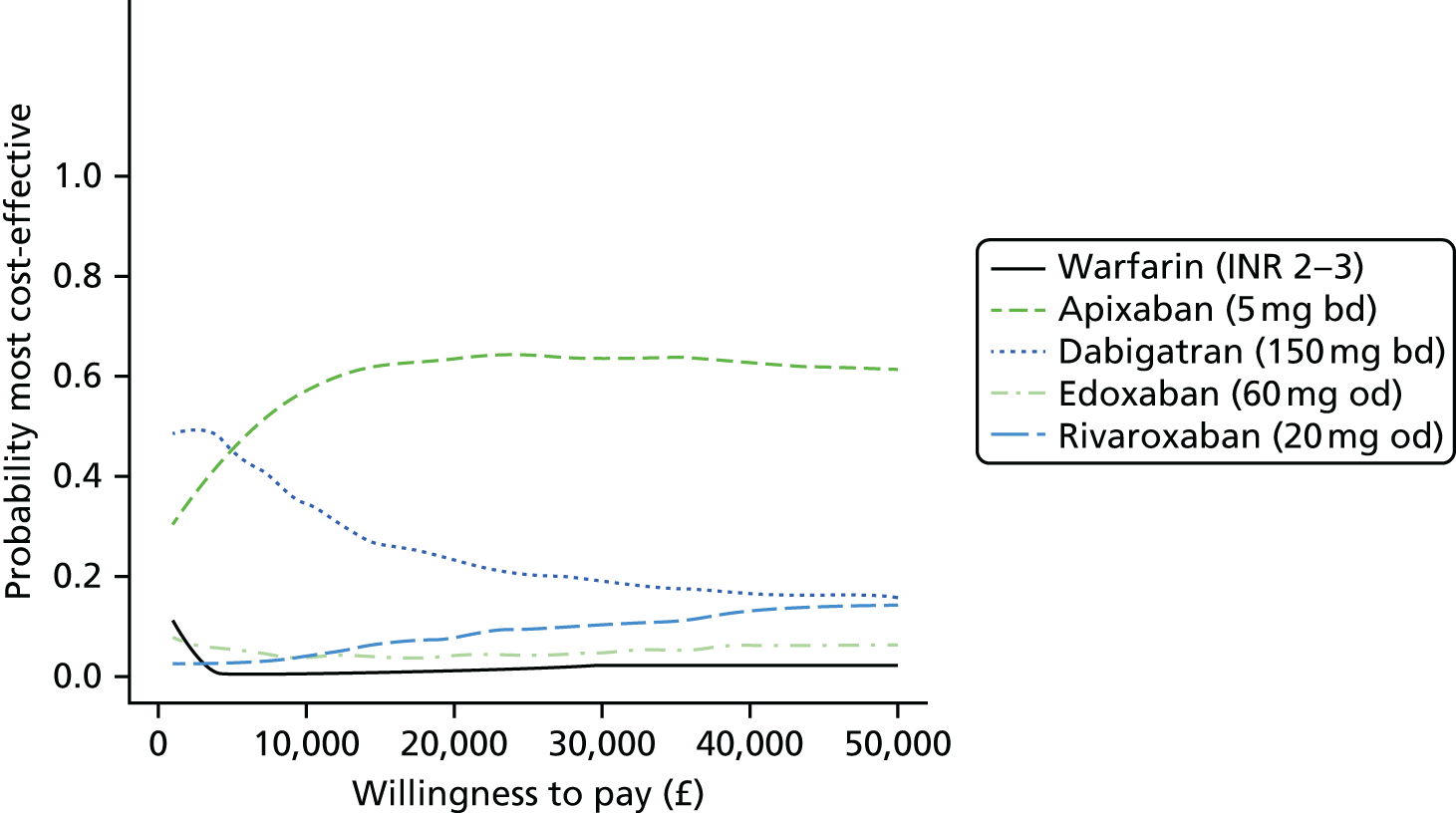
FIGURE 29.
Cost-effectiveness acceptability curves for sensitivity analysis assuming that the cohort starts at age 80 years, rather than 70 years. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
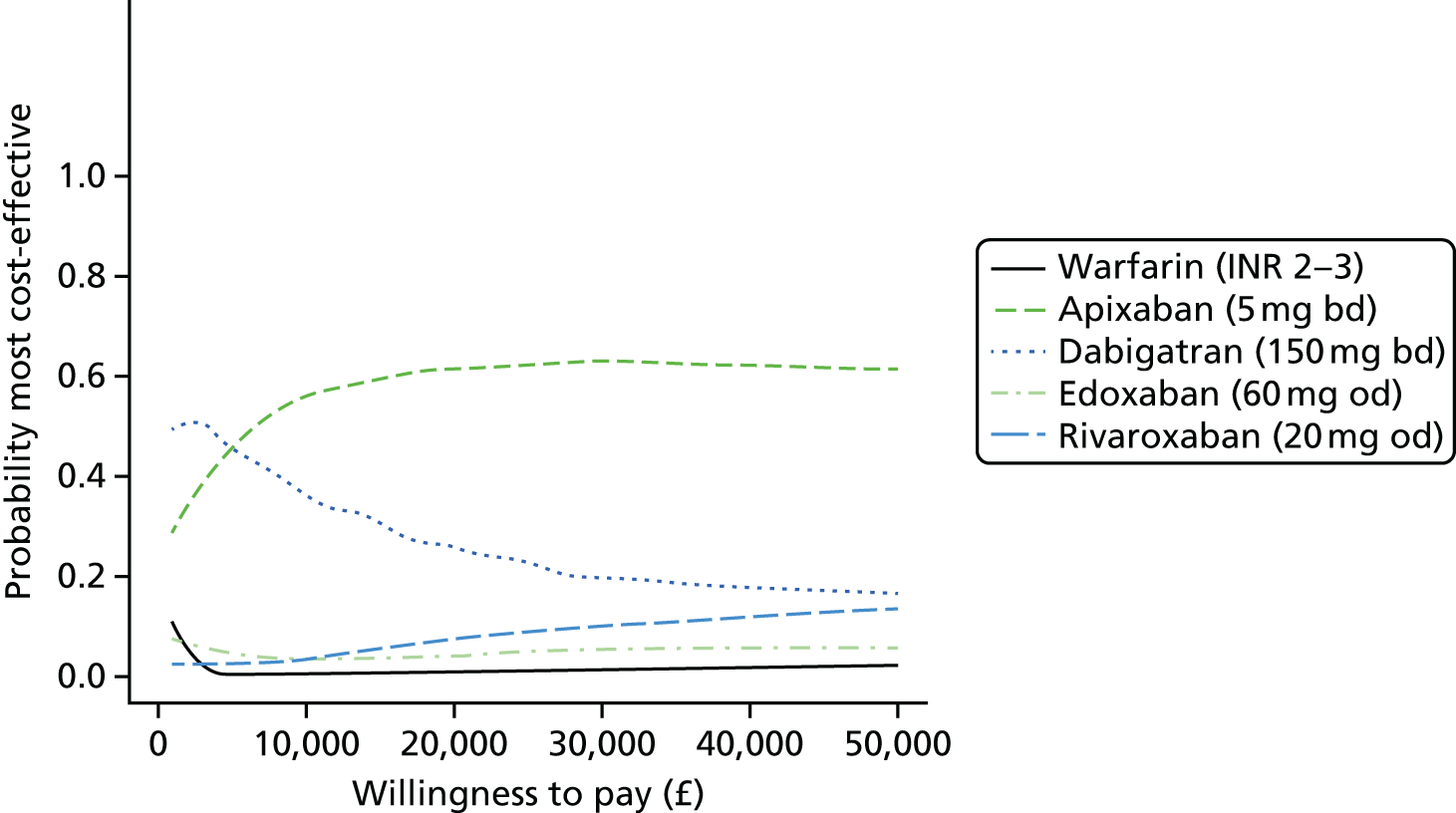
FIGURE 30.
Cost-effectiveness acceptability curves for sensitivity analysis comparing lower doses of apixaban and dabigatran, as would be administered in older patients with AF. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
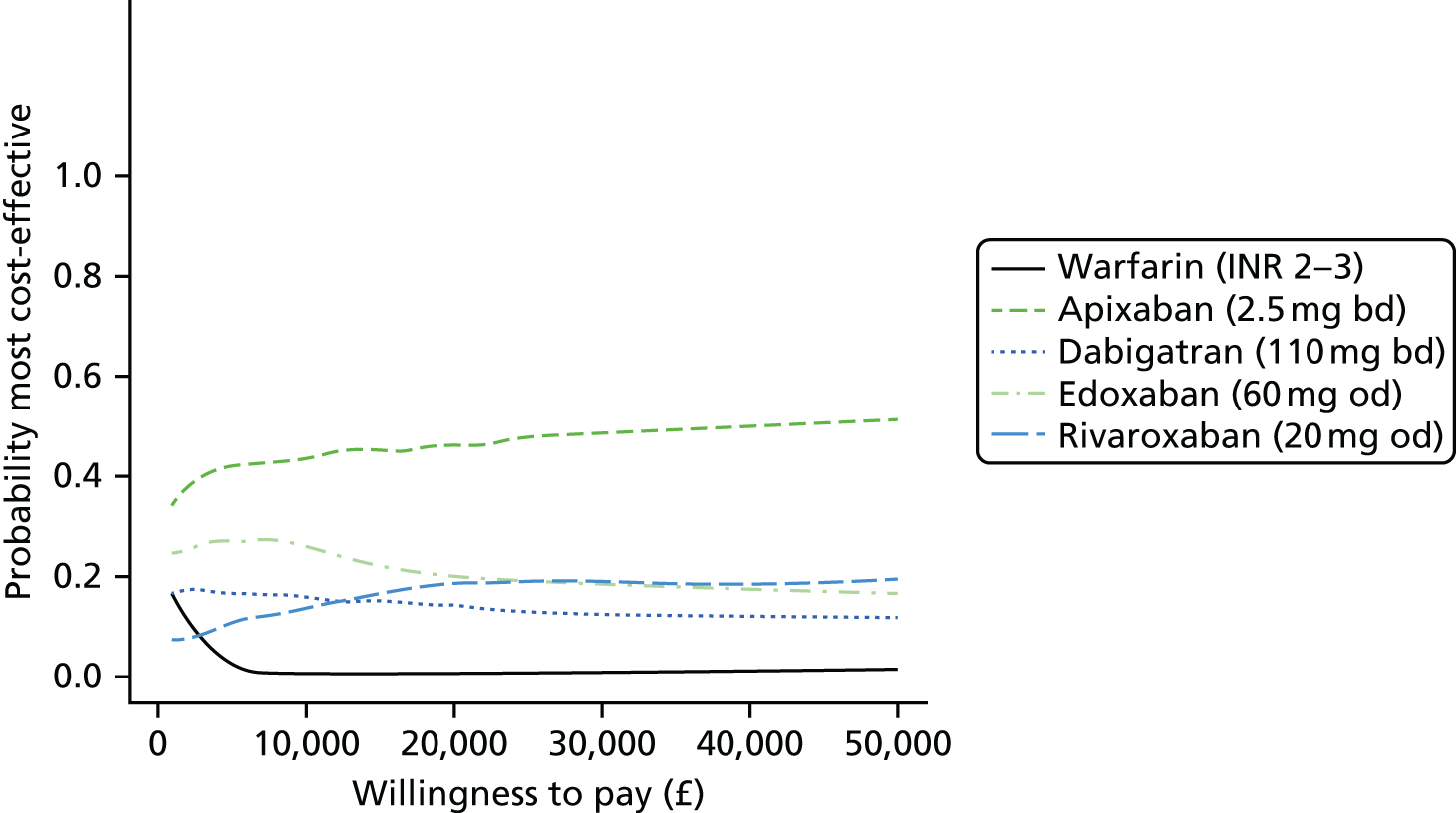
FIGURE 31.
Cost-effectiveness acceptability curves for sensitivity analysis assuming that the hazard of ICH is the same on warfarin and no treatment.
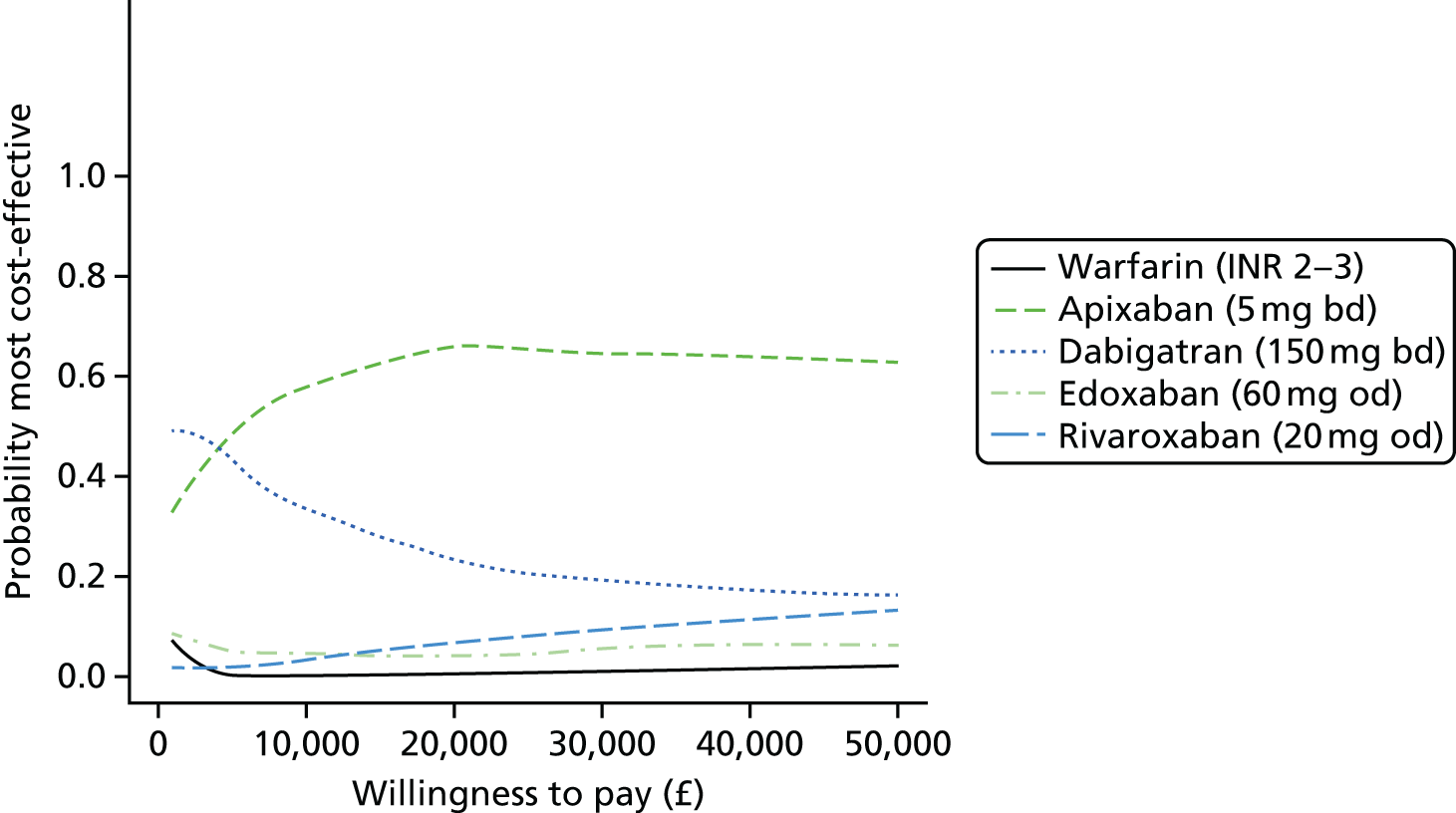
Summary of cost-effectiveness findings
We found that although there was a high degree of uncertainty in the inputs to our model, apixaban (5 mg bd) was identified with the highest probability of being the most cost-effective first-line treatment over a range of willingness-to-pay-per-QALY thresholds. The driver of this result is the generally lower rates of MI, ICH and other CRB on apixaban (5 mg bd) than the other NOACs.
Our model makes several assumptions (summarised in Table 68). However, the conclusion was robust to a wide range of sensitivity analyses. The only sensitivity analysis found to affect the conclusion was the assumption about treatment switching strategy; if treatment switching is assumed to always occur after stroke, bleed, SE or TIA then warfarin was identified as most cost-effective treatment. However, our clinical advice was that this extreme switching strategy was not considered realistic in practice. We have taken the costs of warfarin from the NICE costing report68 but there is uncertainty in this estimate, which is difficult to quantify. We therefore conducted an extreme case scenario analysis in which we assumed zero cost for warfarin treatment and monitoring. Apixaban 5 mg bd was still the most cost-effective treatment under this assumption. Apixaban and dabigatran may be given in lower doses to the elderly. We assumed that all patients would receive the higher dose, and remain on it, even as they age. However, results were robust to a sensitivity analysis assuming only the lower doses of apixaban (2.5 mg bd) and dabigatran (110 mg bd) were administered.
| 1. | Does not include minor non-clinically relevant bleeds as transient events |
| 2. | No distinction between severity of ischaemic strokes SE assumed to be a transient event without long-term consequences |
| 3. | Dose of apixaban and dabigatran given does not reduce as patients age |
| 4. | Bleeds and ICH (and with it, haemorrhagic stroke) have same effect on future risk of death as stroke |
| 5. | Patients on dabigatran who experience a MI will always switch to warfarin |
| 6. | Patients switch to no treatment after ICH/haemorrhagic stroke |
| 7. | Patients may switch (with an assumed probability) from NOAC to warfarin or warfarin to no treatment after ischaemic stroke, bleed, SE or TIA |
| 8. | Patients may (with an assumed probability) discontinue warfarin treatment or switch from a NOAC to warfarin, even if they do not experience an event (due to lack of compliance) |
| 9. | Warfarin arms from the RCTs identified in our systematic review are representative of the AF population in England and Wales |
| 10. | Events rate and relative treatment effects are assumed not to vary with age |
| 11. | Relative mortality rate in patients with AF relative to the general population does not vary with age |
| 12. | Warfarin treatment costs over 3 months are taken from the NICE costing report. Uncertainty in this is represented using a uniform distribution from 50% to 150% of the NICE costing report estimate |
| 14. | Assumes no monitoring or administration costs for NOACs |
| 15. | Assumes post-ICH management costs to be similar to post-ischaemic stroke management costs |
| 16. | Combined management costs for post-multiple event states (e.g. MI + stroke) to be the maximum of management costs for constituent events |
| 17. | Assumed quality of life for patients with a history of multiple events to be multiplicative combination of quality of life for constituent events |
We were unable to include betrixaban due to lack of evidence, and are therefore unable to draw any conclusions about the relative cost-effectiveness of betrixaban or other unlicensed treatments. We have assumed that age determines mortality rate, but that other event rates and relative treatment effects do not depend on age. We have not distinguished between minor and major stroke in our model. Some previous models have done so34,37,149 but we found that there was insufficient evidence to be able to estimate rates differently. We have assumed that SE is a transient event with no long-term consequences. Although there can be long-term consequences, such as limb loss, these are very rare, and we would not expect inclusion of these to affect the results.
One notable limitation of our model is that we have not distinguished between different types of AF. There is emerging evidence that there may be a ‘dose–response’ relationship in stroke risk with increasing ‘persistence’ of AF,150 although others have suggested that risk of stroke is as high in paroxysmal patients with AF as with persistent or permanent AF. 151 The RCTs included in our review are likely to have recruited mostly persistent or permanent patients with AF, and so our conclusions may not extend to patients with paroxysmal AF.
There have been few CEAs of NOACs for the prevention of stroke in AF in the UK population. Kansal et al. 42 found dabigatran to be cost-effective compared with warfarin and aspirin in the UK setting, as in our model. However, they did not include any other NOACs. The Bayer submission to NICE on rivaroxaban33 found it be cost-effective compared with warfarin. This submission also found rivaroxaban and dabigatran to have equivalent effects but dabigatran to have higher costs, thus concluding that rivaroxaban is the most cost-effective. Their CEACs compared only rivaroxaban with warfarin but found close to a 60% probability that rivaroxaban was cost-effective in the £20,000–30,000 threshold range, similar to our probability that a NOAC (apixaban) was most cost-effective. The Harrington et al. 38 model in the US setting compared apixaban (5 mg bd), dabigatran (110 mg bd), rivaroxaban (20 mg od), and warfarin, and found that apixaban had the highest expected QALYs, followed by dabigatran, rivaroxaban and warfarin. Our model also found apixaban to have the highest expected QALYs and that dabigatran and rivaroxaban would have higher expected QALYs than warfarin, although the high degree of uncertainty in our results renders them compatible with the order found by Harrington et al. 38 Harrington et al. 38 also found apixaban and dabigatran to be cost-effective compared with warfarin, and other US studies found apixaban,36 rivaroxaban37 and dabigatran34 to be cost-effective compared with warfarin. Although costs in the USA are not strictly comparable with those in the UK setting, our results are in line with these earlier findings.
Chapter 7 Clinical results (2): primary prevention of venous thromboembolism
Included studies
A total of 2727 unique records were identified from various data sources for the three VTE reviews (Figure 32).
FIGURE 32.
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow chart for reviews of primary prevention, acute treatment and secondary prevention of VTE.
Forty-three completed eligible RCTs were identified for inclusion in the review of primary prevention of VTE, with a total of 46 associated references. 152–197 One further trial198 contained insufficient detail to include in the quantitative synthesis. Three additional ongoing trials199–201 were also identified; two trials199,200 in knee surgery patient population and one trial201 in medical patient population. A summary of the characteristics of the completed trials included in the analyses is presented in Table 69. There were 18 trials in hip surgery patient population (with 20 associated references153–155,158–160,164–166,174,176,177,179,181,183,186,189,192–194), 17 trials in knee surgery patient population (with 18 associated references152,156,157,161–163,167–169,171–173,175,178,180,182,187,196), seven trials in medical patient population (with seven associated references170,184,185,188,190,191,197) and one trial (with one associated reference195) involving both hip and knee surgery patients. Thirty-nine of the trials were multicentre and four were single-centre trials. Most of the multicentre trials were conducted across several countries mainly in North and South America, Europe, Asia, Russia and Israel, Australia and South Africa. Three of the single-centre studies were conducted in Japan, Brazil and China, and one study did not report the country where it was conducted. Thirty-one of the trials were Phase III studies and 12 were Phase II studies. The number of patients randomised ranged from 67 to 5407 patients across the 18 trials on hip surgery; 160 to 3195 patients across the 17 trials on knee surgery (one trial was below-knee fracture patient population); 125 to 8823 patients across the seven trials on medical cases; 1973 patients in the trial involving both hip and knee surgery patients; and 67 to 8323 patients across the whole trials, with a total of 77,563 patients of whom 88.9% (68,953 patients) were from Phase III studies. Thirty-one studies (19 Phase III and 12 Phase II) examined a NOAC. Overall, 11 studies examined rivaroxaban, seven studies examined dabigatran, six studies each examined apixaban and edoxaban, and one study examined betrixaban. Apart from two studies without sponsor information, all studies on NOACs were sponsored by one or more pharmaceutical companies. The role of sponsor was not declared in some of the studies, but, where the sponsor role was declared, the sponsor was commonly involved in the study design, data management and analysis.
| Study (centre type) [countries] | Study type, sponsor (sponsor’s role) | Age eligibility, years (mean age), [% male] | Clinical condition | No. randomised | Interventions compared | Treatment duration (days) | Outcomes | Time of outcome assessment (days) |
|---|---|---|---|---|---|---|---|---|
| ADOPT188 (Multicentre) [North and South America, Europe, Russia, Ukraine, Israel, Australia, Asia and South Africa] |
Phase III Bristol-Myers Squibb and Pfizer (NR) |
≥ 40 (66.8) [49.1] | Acute medical conditions | 6528 | Apixaban 1. 2.5 mg bd LMWH 2. Enoxaparin 40 mg od |
14.9–34.9 Apixaban 3.3–11.3 LMWH |
Efficacy: Major VTE, symptomatic DVT, proximal DVT, symptomatic proximal DVT, symptomatic distal DVT, symptomatic non-fatal PE Safety: All bleeding, major bleeding, composite CRB, intracranial bleeding |
30 (for efficacy outcomes) 2–30 (for safety outcomes) |
| ADVANCE-1171 (Multicentre) [North and South America, Europe, Russia, Israel and Australia] |
Phase III Bristol-Myers Squibb and Pfizer (Data were collected and analysed by the study sponsors) |
≥ 18 (65.8) [37.9] | TKR surgery for one or both knees, including revision of a previously inserted artificial joint | 3195 | Apixaban 1. 2.5 mg bd LMWH 2. Enoxaparin 30 mg bd |
10–14 | Efficacy: DVT, Symptomatic DVT, proximal DVT, symptomatic PE, fatal PE, all stroke Safety: All bleeding, major bleeding, minor bleeding, fatal bleeding, CRNM bleeding, composite CRB, fatal bleeding, thrombocytopenia, MI, death (all causes) |
10–14 (for the efficacy outcomes) 16 (for the safety outcomes) |
| ADVANCE-2178 (Multicentre) [South America, Europe, Russia, Ukraine, Israel, Australia, Asia and South Africa] |
Phase III Bristol-Myers Squibb and Pfizer (NR) |
≥ 18 (67) [27.5] | Either elective unilateral or same-day bilateral TKR surgery or a revision of at least one component of a TKR | 3057 | Apixaban 1. 2.5 mg bd LMWH 2. Enoxaparin 40 mg od |
10–14 | Efficacy: Major VTE, DVT, symptomatic proximal DVT, symptomatic PE, fatal PE, all stroke Safety: All bleeding, major bleeding, minor bleeding, CRNM bleeding, composite CRB, bleeding from surgical site, thrombocytopenia, MI, death (all causes) |
2–14 |
| ADVANCE-3176 (Multicentre) [North and South America, Europe, Russia, Ukraine, Israel, Australia and Asia] |
Phase III Bristol-Myers Squibb and Pfizer (The sponsor was involved in data collection and analyse) |
≥ 18 (60.8) [46.7] | Elective unilateral THR or a revision of at least one component of a THR | 5407 | Apixaban 1. 2.5 mg bd LMWH 2. Enoxaparin 40 mg od |
32–38 | Efficacy: Major VTE, DVT, proximal DVT, symptomatic DVT, fatal PE, symptomatic PE, symptomatic non-fatal PE, all stroke Safety: All bleeding, major bleeding, minor bleeding, CRNM bleeding, bleeding from surgical site, thrombocytopenia, MI, death (all causes) |
32–38 (for efficacy outcomes and thrombocytopenia) 38 (for other safety outcomes and all stroke) |
| APROPOS162 (Multicentre) [North America, Argentina, Denmark, Poland, Israel and Australia] |
Phase II Bristol-Myers Squibb (NR) |
18–90 (66.7) [36.7] | Elective unilateral TKR surgery and who are willing and able to undergo bilateral ascending contrast venography | 1238 | Apixaban 1. 5 mg od 2. 10 mg od 3. 20 mg od 4. 2.5 mg bd 5. 5 mg bd 6. 10 mg bd LMWH 7. Enoxaparin 30 mg bd Warfarin 8. INR 1.8–3 |
10–14 | Efficacy: VTE, symptomatic DVT, symptomatic proximal DVT, symptomatic PE, fatal PE Safety: All bleeding, major bleeding, minor bleeding, fatal bleeding, MI, all stroke, death (all causes) |
10–14 [42 for major bleeding and death (all cause)] |
| ARDEPARIN ARTHROPLASTY STUDY196 (Multicentre) [USA] |
Phase II Supported by a grant from Wyeth-Ayerst Research, Philadelphia, PA, USA (NR) |
≥ 18 (68.6) [42.1] | Primary unilateral, simultaneous bilateral or unilateral revision TKR surgery | 860 | LMWH 1. Ardeparin 25 anti-Xa units/kg bd 2. Ardeparin 35 anti-Xa units/kg bd 3. Ardeparin 50 anti-Xa units/kg bd Warfarin 4. INR 2–3 |
14 Or at discharge post-op |
Efficacy: VTE, DVT, proximal DVT, symptomatic PE Safety: Major bleeding, bleeding from surgical site, thrombocytopenia, death (all causes) |
5–14 (for efficacy outcomes except symptomatic PE, which was prior to discharge) Unclear for safety outcomes |
| BISTRO II195 (Multicentre) [Europe and South Africa] |
Phase II Boehringer Ingelheim (The sponsor was responsible for the overall planning and conduct of the study, and statistical analyses) |
≥ 18 (66) [39] | THR or TKR surgery | 1973 | Dabigatran 1. 50 mg bd 2. 150 mg bd 3. 300 mg od 4. 225 mg bd LMWH 5. Enoxaparin 40 mg od |
6–10 | Efficacy: VTE, symptomatic VTE, DVT, symptomatic DVT, proximal DVT, distal DVT, symptomatic PE Safety: Major bleeding, minor bleeding, CRNM bleeding, composite CRB |
6–10 |
| EXPERT168 (Multicentre) [USA and Canada] |
Phase II Portola Pharmaceuticals Inc., South San Francisco, CA, USA (NR) |
18–75 (63.3) [39.7] | Elective primary unilateral total knee arthroplasty | 215 | Betrixaban 1. 15 mg bd 2. 40 mg bd LMWH 3. Enoxaparin 30 mg bd |
10–14 | Efficacy: VTE, symptomatic VTE, symptomatic distal DVT, symptomatic proximal DVT, symptomatic DVT, non-symptomatic DVT, symptomatic PE Safety: All bleeding, major bleeding, CRNM bleeding, composite CRB |
10–14 |
| LIFENOX185 (Multicentre) [Asia, Mexico and Tunisia] |
Phase III Sanofi (The data were gathered by the sponsor) |
≥ 40 (65.5) [62.7] | Acute medical conditions | 8323 | LMWH 1. Enoxaparin 40 mg od 2. Placebo od |
6 –14 | Safety: All bleeding, major bleeding, minor bleeding, CRNM bleeding, death (cardiovascular), death (all causes) | 14 (for bleeding outcomes) 14, 30, 90 (for death outcomes) |
| MAGELLAN184,191 (Multicentre) [North and South America, Europe, Israel, Australia, New Zealand and Asia] |
Phase III Bayer HealthCare Pharmaceuticals and Janssen Research & Development (The data were collected and analysed by the sponsors) |
≥ 40 (71.1) [54.2] | Acute medical conditions | 8101 | Rivaroxaban 1. 10 mg od LMWH 2. Enoxaparin 40 mg od |
31–39 Rivaroxaban 6–14 LMWH |
Efficacy: Major VTE, symptomatic DVT, symptomatic non-fatal PE Safety: Major bleeding, composite CRB, fatal bleeding, death (all causes) |
10 and 35 |
| ODiXa-HIP194 (Multicentre) [Europe and Israel] |
Phase II Bayer (The sponsor was involved in the study but the exact contributions are NR) |
≥ 18 (65.1) [40.9] | THR surgery | 641 | Rivaroxaban 1. 2.5 mg bd 2. 5 mg bd 3. 10 mg bd 4. 30 mg od 5. 20 mg bd 6. 30 mg bd LMWH 7. Enoxaparin 40 mg od |
5–9 Mean – rivaroxaban 7.5 ± 1.0 LMWH 7.6 ± 1.5 |
Efficacy: Major VTE, DVT, symptomatic DVT, proximal DVT, symptomatic PE, symptomatic non-fatal PE, fatal PE Safety: Major bleeding, minor bleeding, CRNM bleeding, composite CRB, fatal bleeding, death (all causes) |
5–9 |
| ODiXa-HIP2159 (Multicentre) [Europe and Israel] |
Phase II Bayer HealthCare AG (NR) |
≥ 18 (65.3) [40.3] | Elective primary THR | 722 | Rivaroxaban 1. 2.5 mg bd 2. 5 mg bd 3. 10 mg bd 4. 20 mg bd 5. 30 mg bd LMWH 6. Enoxaparin 40 mg od |
5–9 | Efficacy: Major VTE, DVT, proximal DVT, symptomatic PE Safety: Major bleeding, minor bleeding, CRNM bleeding, bleeding from surgical site |
5–9 |
| ODiXa-KNEE157 (Multicentre) [USA and Canada] |
Phase II Bayer HealthCare AG, Germany (NR) |
≥ 18 (66.5) [38.5] | Elective TKR | 621 | Rivaroxaban 1. 2.5 mg bd 2. 5 mg bd 3. 10 mg bd 4. 20 mg bd 5. 30 mg bd LMWH 6. Enoxaparin 30 mg bd |
5–9 | Efficacy: Major VTE, DVT, distal DVT, proximal DVT, symptomatic DVT, symptomatic PE Safety: Major bleeding, minor bleeding, CRNM bleeding, composite CRB, death (all causes) |
5–9 (for efficacy outcomes) 11 (for safety outcomes) |
| ODiXa-OD.HIP158 (Multicentre) [Europe and Israel according to study report, but protocol says Japan] |
Phase II Bayer HealthCare (NR) |
≥ 18 (64.9) [41.1] | Primary THR surgery | 873 | Rivaroxaban 1. 5 mg od 2. 10 mg od 3. 20 mg od 4. 30 mg od 5. 40 mg od LMWH 6. Enoxaparin 40 mg od |
5–9 | Efficacy: Major VTE, DVT, distal DVT, proximal DVT, symptomatic distal DVT, symptomatic PE Safety: Major bleeding, minor bleeding, CRNM bleeding, death (all causes) |
10 |
| PROTECHT170 (Multicentre) [Japan] |
Phase III Italfarmaco SpA, Milan, Italy (NR) |
≥ 18 (62.9) [51.7] | Metastatic or locally advanced cancer | 1166 | LMWH 1. Nadroparin 3800 IU anti-Xa od 2. Placebo od |
110–130 | Efficacy: Symptomatic VTE, symptomatic DVT, symptomatic PE Safety: All bleeding, major bleeding, minor bleeding, death (all causes) |
111–113 (median) |
| RECORD 1165 (Multicentre) [North and South America, Europe, Israel, Australia and South Africa] |
Phase III Bayer HealthCare and Johnson & Johnson (The data were collected and analysed by the sponsors) |
≥ 18 (63.2) [44.5] | Elective total hip arthroplasty | 4541 | Rivaroxaban 1. 10 mg od LMWH 2. Enoxaparin 40 mg od |
35 (31–39) | Efficacy: Major VTE, symptomatic VTE, DVT, distal DVT, proximal DVT, symptomatic non-fatal PE, ischaemic stroke Safety: All bleeding, major bleeding, minor bleeding, CRNM bleeding, composite CRB, MI, death (cardiovascular), death (all causes) |
36 (30–42) for all efficacy outcomes 37 (for all safety outcomes) |
| RECORD 2166 (Multicentre) [North and South America, Europe, Australia, New Zealand, Asia and South Africa] |
Phase III Bayer HealthCare AG, Johnson & Johnson Pharmaceutical Research and Development LLC (The study sponsors were involved in the study design, data collection and analysed) |
≥ 18 (61.5) [46.4] | Elective total hip arthroplasty | 2509 | Rivaroxaban 1. 10 mg od LMWH 2. Enoxaparin 40 mg od |
31–39 Rivaroxaban 10–14 LMWH |
Efficacy: Symptomatic VTE, major VTE, DVT, distal DVT, proximal DVT, symptomatic non-fatal PE, fatal PE, ischaemic stroke Safety: All bleeding, major bleeding, CRNM bleeding, composite CRB, fatal bleeding, MI, death (cardiovascular), death (all causes) |
30–42 (32–42 for major VTE, DVT, symptomatic non-fatal PE and composite CRB) |
| RECORD 3163 (Multicentre) [North and South America, Europe, Israel, China and South Africa] |
Phase III Bayer HealthCare and Johnson & Johnson Pharmaceutical Research & Development (Data were collected and analysed by the study sponsors) |
≥ 18 (67.6) [31.8] | Total knee arthroplasty | 2531 | Rivaroxaban 1. 10 mg od LMWH 2. Enoxaparin 40 mg od |
10–14 | Efficacy: Major VTE, symptomatic VTE, DVT, distal DVT, proximal DVT, symptomatic non-fatal PE, ischaemic stroke Safety: All bleeding, major bleeding, CRNM bleeding, fatal bleeding, MI, death (cardiovascular), death (all causes) |
17 (for all efficacy outcomes excluding ischaemic stroke) 16 (for bleeding outcomes) 15 (for MI, ischaemic stroke and death outcomes) |
| RECORD 4173 (Multicentre) [North America, Europe, Israel, India and Sri Lanka] |
Phase III Bayer Schering Pharma AG, Johnson & Johnson Pharmaceutical Research & Development (The study sponsors were involved in the design of the trial and collected and analysed the data) |
≥ 18 (64.6) [34.9] | Total knee arthroplasty | 3148 | Rivaroxaban 1. 10 mg od LMWH 2. Enoxaparin 30 mg bd |
10–14 | Efficacy: Major VTE, symptomatic VTE, DVT, symptomatic DVT, non-symptomatic DVT, symptomatic PE, symptomatic non-fatal PE, fatal PE, ischaemic stroke Safety: Major bleeding, fatal bleeding, CRNM bleeding, composite CRB, fatal bleeding, MI, death (cardiovascular), death (all causes) |
17 (for all efficacy outcomes excluding ischaemic stroke) 16 (for all safety outcomes and ischaemic stroke) |
| RE-MOBILISE167 (Multicentre) [North America and UK] |
Phase III Boehringer Ingelheim (The sponsor was responsible for data collection and statistical analysis) |
≥ 18 (66.1) [42.3] | Primary elective unilateral total knee arthroplasty | 2615 | Dabigatran 1. 150 mg od 2. 220 mg od LMWH 3. Enoxaparin 30 mg bd |
12–15, median 14 | Efficacy: VTE, major VTE, distal DVT, proximal DVT, symptomatic DVT, symptomatic PE, symptomatic non-fatal PE Safety: All bleeding, major bleeding, death (all causes) |
12–15 |
| RE-MODEL161 (Multicentre) [Europe, Australia and South Africa] |
Phase III Boehringer Ingelheim, Copenhagen, Denmark (The sponsor was responsible for data collection and statistical analysis) |
≥ 18 (67.7) [34] | Primary elective unilateral TKR | 2101 | Dabigatran 1. 150 mg od 2. 220 mg od LMWH 3. Enoxaparin 40 mg od |
6–10 | Efficacy: VTE, symptomatic DVT, symptomatic PE Safety: All bleeding, major bleeding, minor bleeding, CRNM bleeding, composite CRB, death (all causes) |
6–10 |
| RE-NOVATE160 (Multicentre) [Europe, Australia and South Africa] |
Phase III Boehringer Ingelheim, Alkmaar, The Netherlands (Data collection and analysis were done by the sponsor) |
≥ 18 (64) [43.5] | Primary elective unilateral THR | 3494 | Dabigatran 1. 150 mg od 2. 220 mg od LMWH 3. Enoxaparin 40 mg od |
28–35 | Efficacy: Symptomatic DVT, symptomatic PE, fatal PE Safety: All bleeding, major bleeding, minor bleeding, CRNM bleeding, composite CRB |
31–38 |
| RE-NOVATE II183,189 (Multicentre) [North America, Europe, Australia, New Zealand, India and South Africa] |
Phase III Boehringer Ingelheim, Sweden (NR) |
≥ 18 (62) [48.2] | Unilateral, elective total hip arthroplasty | 2055 | Dabigatran 1. 220 mg od LMWH 2. Enoxaparin 40 mg od |
28–35 | Efficacy: Major VTE, symptomatic VTE, DVT, distal DVT, proximal DVT, symptomatic DVT, symptomatic non-fatal PE, ischaemic stroke Safety: All bleeding, major bleeding, minor bleeding, CRNM bleeding, composite CRB, MI, death (all causes) |
28–35 |
| STARS E-3182 (Multicentre) [Japan and Taiwan] |
Phase III Daiichi Sankyo Inc. (NR) |
20–84 (NR) [NR] | Unilateral total knee arthroplasty | 716 | Edoxaban 1. 30 mg od LMWH 2. Enoxaparin 2000 IU (20 mg) bd |
11–14 | Efficacy: VTE, DVT, symptomatic PE Safety: Major bleeding, composite CRB |
14 |
| STARS J-1172,180 (Multicentre) [Japan] |
Phase II Daiichi Sankyo Co., Ltd, Tokyo, Japan (NR) |
20–84 (71.1) [21.2] | Unilateral total knee arthroplasty | 523 | Edoxaban 1. 5 mg od 2. 15 mg od 3. 30 mg od 4. 60 mg od 5. Placebo od |
11–14 | Efficacy: VTE, DVT, distal DVT, proximal DVT, symptomatic DVT, symptomatic PE Safety: All bleeding, major bleeding, CRNM bleeding, composite CRB |
14 |
| STARS J-2174 (Multicentre) [Japan and Taiwan] |
Phase II Daiichi Sankyo Inc. (NR) |
20–84 (NR) [NR] | Unilateral total hip arthroplasty | 264 | Edoxaban 1. 15 mg od 2. 30 mg od LMWH 3. Enoxaparin 20 mg bd |
11–14 | Efficacy: VTE, distal DVT Safety: Composite CRB |
14 |
| STARS J-4181,193 (Multicentre) [Japan] |
Phase III Daiichi Sankyo Co., Ltd. Tokyo, Japan (NR) |
≥ 20 (76) [20.5] | Hip surgery – for inner or outer femoral neck (trochanteric or subtrochanteric) fracture | 92 | Edoxaban 1. 30 mg od LMWH 2. Enoxaparin 2000 IU (20 mg) bd |
11–14 | Efficacy: VTE, major VTE, symptomatic DVT, non-symptomatic DVT, symptomatic PE Safety: All bleeding, major bleeding, minor bleeding, CRNM bleeding, composite CRB, death (all causes) |
14 |
| STARS J-V179 (Multicentre) [Japan] |
Phase III Daiichi Sankyo Ltd. Tokyo, Japan (NR) |
20–84 (62.8) [NR] | Unilateral total hip arthroplasty | 610 | Edoxaban 1. 30 mg od LMWH 2. Enoxaparin 20 mg bd |
11–14 | Efficacy: VTE, symptomatic DVT, non-symptomatic DVT, symptomatic PE Safety: Major bleeding, composite CRB |
14 |
| TOPIC-1197 (Multicentre) [Germany, Czech Republic, Ukraine, Romania and Belarus] |
Phase III Novartis Pharma GmbH Germany (NR) |
Adults (55.6) [NR] | Metastatic breast cancer | 353 | LMWH 1. Certoparin 3000 IU od 2. Placebo od |
182.6 | Efficacy: VTE, DVT, symptomatic VTE, symptomatic DVT, non-symptomatic DVT, symptomatic PE Safety: All bleeding, major bleeding, minor bleeding, thrombocytopenia, death (all causes) |
182.6 |
| TOPIC-2197 (Multicentre) [Germany, Czech Republic, Ukraine, Romania and Belarus] |
Phase III Novartis Pharma GmbH Germany (NR) |
Adults (60.6) [NR] | Inoperable disseminated primary non-small cell lung carcinoma | 547 | LMWH 1. Certoparin 3000 IU od 2. Placebo od |
182.6 | Efficacy: VTE, DVT, symptomatic VTE, symptomatic DVT, non-symptomatic DVT, symptomatic PE Safety: All bleeding, major bleeding, minor bleeding, thrombocytopenia, death (all causes) |
182.6 |
| VTE-APIX-PLACEBO-USACAN190 (Multicentre) [USA and Canada] |
Phase II Bristol-Myers Squibb and Pfizer Inc. (NR) |
≥ 18 (60) [50.4] | Receiving either first- or second-line chemotherapy for advanced or metastatic cancer | 125 | Apixaban 1. 5 mg od 2. 10 mg od 3. 20 mg od 4. Placebo od |
84 (16–90) | Efficacy: Symptomatic VTE Safety: Major bleeding, CRNM bleeding, composite CRB |
114–121 |
| VTE-DABIG-LMWH-GREECE187 (Single centre) [NR] |
Phase III Not declared (NR) |
Adults (NR) [13.1] | Total knee arthroplasty | 160 | LMWH 1. Dalteparin 2.5 mg od 2. Enoxaparin 40 mg od 3. Tinzaparin 0.45 ml od Dabigatran 4. 110 mg od |
Not given | Efficacy: VTE, DVT, PE, ischaemic stroke Safety: All bleeding |
Not given |
| VTE-DABIG-PLAC-JAPAN175 (Multicentre) [Japan] |
Phase III Boehringer Ingelheim Co, Ltd Kawanishi, Japan (NR) |
≥ 20 (71.6) [17] | Primary, unilateral, elective total knee arthroplasty | 512 | Dabigatran 1. 110 mg od 2. 150 mg od 3. 220 mg od 4. Placebo od |
11–14 | Efficacy: Major VTE, DVT, proximal DVT, symptomatic DVT, symptomatic PE Safety: All bleeding, major bleeding, minor bleeding, CRNM bleeding, composite CRB, fatal bleeding, bleeding from surgical site, death (all causes) |
14 |
| VTE-EDOX-LMWH-MULTI177 (Multicentre) [North and South America, Europe, Russian and Ukraine] |
Phase II Daiichi Sankyo Pharma Development (NR) |
≥ 18 (57.8) [39.9] | Primary, unilateral THR surgery | 903 | Edoxaban 1. 15 mg od 2. 30 mg od 3. 60 mg od 4. 90 mg od LMWH 5. Dalteparin 5000 IU od |
7–10 | Efficacy: VTE, major VTE, proximal DVT Safety: All bleeding, major bleeding, CRNM bleeding, composite CRB, death (all causes) |
7–10 (for efficacy outcomes) 10 (for safety outcomes) |
| VTE-LMWH-PLAC-CAN169 (Multicentre) [Canada] |
Phase III Fragmin, Pharmacia, Pfizer Global Pharmaceuticals, Kirkland, QC, Canada (NR) |
18–75 (41) [62] | Unilateral isolated fractures below the knee, which required operative fixation (patients with minor simultaneous injuries were also included if they were able to mobilise) |
305 | LMWH 1. Dalteparin 5000 IU od 2. Placebo od |
14 | Efficacy: Non-symptomatic DVT Safety: All bleeding, major bleeding, minor bleeding, thrombocytopenia, death (all causes) |
14 |
| VTE-LMWH-PLAC-JAPAN186 (Single centre) [Japan] |
Phase III None declared (NR) |
≥ 20 (NR) [18.4] | Unilateral THR surgery | 255 | LMWH 1. Fondaparinux 2.5 mg od 2. Enoxaparin 20 mg bd 3. Placebo od |
10 | Efficacy: VTE, symptomatic VTE, DVT, distal DVT, proximal DVT, symptomatic DVT, symptomatic PE Safety: All bleeding, major bleeding, minor bleeding |
11 |
| VTE-RIVAROX-LMWH-BRAZIL164 (Single centre) [Brazil] |
Phase III Bayer HealthCare (NR) |
≥ 18 (57.9) [55.4] | Elective total hip arthroplasty | 67 | Rivaroxaban 1. 10 mg od LMWH 2. Enoxaparin 40 mg od |
32–36 | Efficacy: DVT, symptomatic PE | 32–36 |
| VTE-RIVAROX-LMWH-CHINA192 (Single centre) [China] |
Phase III Not declared (NR) |
> 50 (64.6) [56.6] | Unilateral hip arthroplasty | 106 | Rivaroxaban 1. 10 mg od LMWH 2. 4100 IU od (type NR) |
35 | Efficacy: DVT | 182.6 |
| VTE-VKA-LMWH-CANADA152 (Multicentre) [NR] |
Phase III Rhône-Poulenc Rorer Canada (NR) |
Adults (68.8) [36.9] | Knee arthroplasty | 670 | Warfarin 1. INR 2–3 LMWH 2. Enoxaparin 30 mg bd |
5.9–11.5 (mean) | Efficacy: Symptomatic VTE, DVT, proximal DVT, symptomatic PE Safety: All bleeding, major bleeding, minor bleeding, thrombocytopenia, death (all causes) |
14 |
| VTE-VKA-LMWH-US153 (Multicentre) [USA] |
Phase III National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD, USA, and a grant from Pharmacia & Upjohn, Kalamazoo, MI, USA (NR) |
≥ 18 (63) [47.1] | Unilateral primary or revision total hip arthroplasty | 580 | Warfarin 1. INR 2.0–3.0 LMWH 2. Dalteparin 5000 IU od |
5–9 | Efficacy: DVT, distal DVT, proximal DVT Safety: Major bleeding |
5–9 Unclear for major bleeding |
| VTE-VKA-LMWH-US-2154 (Multicentre) [NR] |
Phase III Rhône-Poulenc Rorer Pharmaceuticals (NR) |
≥ 18 (64) [44.4] | Elective unilateral primary hip arthroplasty | 3011 | Warfarin 1. INR 2–3 LMWH 2. Enoxaparin 30 mg bd |
14 | Efficacy: Symptomatic VTE, symptomatic DVT, symptomatic PE, fatal PE Safety: All bleeding, major bleeding, minor bleeding, bleeding from surgical site, thrombocytopenia, death (all causes) |
14 |
| VTE-VKA-LMWH-US-3155 (Multicentre) [USA and Canada] |
Phase III Grant-in-aid by Pharmacia & Upjohn to the University of Calgary (NR) |
≥ 18 (63.3) [48.2] | Elective unilateral total hip arthroplasty (primary or revision) | 1501 | Warfarin 1. INR 2–3 LMWH 2. Dalteparin 5000 IU – started pre-operatively and then od 3. Dalteparin 5000 IU – started post-operatively and then od |
4–8 | Efficacy: DVT, symptomatic DVT, proximal DVT, symptomatic PE Safety: Major bleeding, minor bleeding, death (all causes) |
5–9 (for efficacy outcomes) 8 (for safety outcomes) |
| VTE-VKA-LMWH-US-4156 (Multicentre) [USA] |
Phase III Aventis Pharmaceuticals, Inc., Bridgewater, NJ, USA (NR) |
≥ 38 (NR) [44] | Unilateral total knee arthroplasty | 349 | Warfarin 1. INR 2–3 LMWH 2. Enoxaparin 30 mg bd |
4–14 | Efficacy: VTE, DVT, distal DVT, proximal DVT, symptomatic PE Safety: All bleeding, major bleeding, minor bleeding, death (all causes) |
5–15 |
Eligibility criteria for patient participation were similar across surgical studies of the same type, with all patients in hip surgery studies having elective unilateral hip arthroplasty, and all patients in knee surgery studies having elective unilateral knee arthroplasty. Patients in medical studies were selected based on specific clinical conditions, either having a metastatic cancer or one or more acute medical conditions, so the criteria varied slightly across the medical studies. The minimum age for inclusion in a majority of the studies was 18 years, the mean age across studies (where reported) ranged from 41 years to 76 years. The percentage of male patients, reported in 88% of the studies, ranged from 13.1% to 62.7%. Mean BMI and mean weight ranged from 23 to 32.4 kg/m2 and from 52.3 to 90.9 kg, respectively, across studies, when reported. Proportions of comorbidities were poorly reported across studies. When reported, the proportion of patients with a previous thromboembolic event, chronic heart failure and cancer ranged from 0.1% to 10.2%, 0.6% to 34.8% (higher of the range from medical patient population studies), and 6% to 100% (100% in cancer patient studies), respectively.
Of the 31 studies that examined NOACs, a NOAC was compared with a LMWH in 27 studies, with placebo in three studies, and with both a LMWH and warfarin in one study. Fourteen of the 31 studies were on hip surgery patients, 12 on knee surgery patients, one on below knee fracture patients, one on both hip and knee surgery patients, and three on medical patients. The doses of NOACs examined were apixaban 5 mg, 10 mg and 20 mg od, and 2.5 mg, 5 mg and 10 mg bd; edoxaban 5 mg, 15 mg, 30 mg, 60 mg and 90 mg od; rivaroxaban 5 mg, 10 mg, 20 mg, 30 mg and 40 mg od, and 2.5 mg, 5 mg, 10 mg, 20 mg and 30 mg bd; betrixaban 15 mg and 40 mg bd; and dabigatran 110 mg, 150 mg, 220 mg and 300 mg od, and 50 mg, 150 mg and 225 mg bd. Among the studies that did not examine a NOAC, six studies each compared LMWH with warfarin, and with placebo. Standard intensity warfarin (INR 2–3) was examined in all studies involving a warfarin arm, although in one study the lower end of the INR range was 1.8. None of these studies that examined warfarin reported mean TTR. LMWHs varied in type and dose across studies. Start of treatment with LMWH varied across surgical patient studies with pre-op treatment start in 11 studies in hip surgery, four studies in knee surgery, and one study involving both hip and knee surgery patients, and post-op treatment start in eight studies in hip surgery and 11 studies in knee surgery. In one (hip surgery) study, pre- and post-op LMWH treatment start were compared.
Treatment duration varied greatly across hip surgery, knee surgery and medical patient studies, from 4 to 130 days. There is less variation in treatment duration within the knee and hip surgery studies, with treatment duration ranging from 10 to 14 days in most of the knee surgery studies, and from 5 to 14 days and 28 to 35 days in most of the hip surgery studies. Treatment duration was the same for the interventions compared with studies, except in three studies where the LMWH comparator was given for a shorter duration than the NOAC (rivaroxaban in two studies and apixaban in one study). However, time to outcome assessment was the same in all studies including those with different treatment durations for the interventions compared.
Reported efficacy and safety outcome types were similar across studies irrespective of the patient group, and were reported at the end of the treatment periods. Two rivaroxaban studies reported only efficacy outcomes: in both cases few outcomes were reported. One study reported only safety outcomes. Overall, 29 studies reported data on symptomatic VTE; 25 on symptomatic DVT, 35 on symptomatic PE, nine on MI, 39 on major bleeding, 27 on CRB, and 28 on all-cause mortality. Diagnosis of VTE was predominantly by compression ultrasonography or venography for DVT, and by spiral computerised tomography scan or ventilation/perfusion lung scan for PE.
Time in therapeutic range for warfarin interventions
Seven studies of primary prevention of VTE included a warfarin intervention arm, but none of these reported mean TTR.
Risk of bias in included studies
Detailed risk-of-bias assessments for each included study for each domain of the Cochrane assessment tool are provided in Table 70. Overall, the studies were judged to be at low risk of bias. Assessment of a few studies was based on abstract information only, in which case risk of bias for most domains was judged to be unclear. The majority of the studies were judged to be at low risk of bias for blinding of outcome assessment and incomplete outcome data. The risk of bias in these two domains differed slightly in a few studies because of differences in blinding of outcome assessment and the number of patients included in analysis according to outcome type, mainly whether an outcome is for efficacy or for safety. Most studies were judged to be at low risk of bias for selective outcome reporting. Among those not judged to be at low risk, the main reason for the judgement was either unavailability of the study protocol or insufficient information to enable a judgement of low risk. Randomisation sequence generation and allocation concealment were predominantly by computer generation and central allocation, respectively. In some studies, randomisation was used a standard permuted block and some of the studies were stratified according to study centre. A few studies,153,154,156,168,174,193,194 predominantly of open-label design, were judged to be at high risk of bias for blinding of participants. The risk-of-bias judgements for studies contributing to analyses of each outcome are presented graphically in the sections that follow.
| Study | Sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting |
|---|---|---|---|---|---|---|
| ADOPT188 | L: ‘Randomization was performed through a central telephone system with the use of a computer-generated randomization list’ | L: ‘Randomization was performed through a central telephone system with the use of a computer-generated randomization list’ | L: ‘The study medications were packaged in identical-appearing dispensing kits. Patients who were randomly assigned to apixaban received daily injections of an enoxaparin placebo’ ‘Patients who were randomly assigned to enoxaparin received tablets containing an apixaban placebo’ | L: ‘All components of the primary efficacy outcome were adjudicated by the independent central adjudication committee. All compression ultrasound examinations were recorded for submission to an independent central adjudication committee whose members were unaware of the treatment assignments’ | L: For efficacy outcomes: All patients were included in the analyses | L: All outcomes are reported as per protocol |
| L: ‘Each of these events (bleeding) was reviewed and adjudicated by the independent central adjudication committee (whose members were unaware of the treatment assignments)’ | L: For safety outcomes: Some missing data; reasons given; reasons for missing data unlikely to be related to true outcome | |||||
| ADVANCE-1171 | U: ‘The randomization was stratified according to study site and whether a patient was undergoing replacement of one or both knees, with a block size of 4’ | U: No information to enable judgement | L: One group of patients received 2.5 mg of apixaban orally bd as well as an injection of placebo that mimicked injection with enoxaparin. The other group received 30 mg of enoxaparin subcutaneously every 12 hours along with placebo tablets that were identical in appearance to apixaban tablets | L: ‘All venograms and all episodes of suspected symptomatic VTE, bleeding, MI, stroke, thrombocytopenia, or death were adjudicated, without knowledge of the patient’s assigned treatment, by an independent central adjudication committee’ | L: No missing outcome data except for DVT outcomes | L: All outcomes are reported as per protocol |
| U: For DVT outcomes: Some missing outcome data; not entirely balanced in numbers across intervention groups Not clear if missing data are unlikely to be related to true outcome |
||||||
| ADVANCE-2178 | L: ‘The randomisation schedule was generated by the Bristol-Myers Squibb randomisation centre with SAS and was stratified by study site and by unilateral or bilateral surgery with a block size of four’ | L: ‘The randomisation schedule was generated by the Bristol-Myers Squibb randomisation centre with SAS and was stratified by study site and by unilateral or bilateral surgery with a block size of four’ | L: ‘Investigators, patients, statisticians, adjudicators, and the steering committee were masked to treatment allocation’ | L: ‘Investigators, patients, statisticians, adjudicators, and the steering committee were masked to treatment allocation’ | U: For safety outcomes: High proportion of missing outcome data; reasons for missing data given and reasons are similar across intervention groups and appear to be balanced in number across intervention groups However, it is not clear whether the reasons are related to true outcome or not |
L: All outcomes are reported as per protocol |
| L: For safety outcomes: Missing outcome data, however few reasons for missing data given and reasons balance in number across intervention groups; it is unlikely that that reason for missing data are related to true outcome | ||||||
| L: For death outcome: All patients were included in the analyses | ||||||
| ADVANCE-3176 | L: ‘The randomization schedule was generated at the randomisation centre of Bristol-Myers Squibb with the use of SAS software and was stratified according to study site, with a block size of four’ | L: ‘Potentially eligible patients were identified during a screening period of up to 14 days before surgery and were randomly assigned, with the use of an interactive telephone system’ | L: ‘The study was a randomized, double-blind, double-dummy clinical trial’ Patients were assigned ‘to receive apixaban at a dose of 2.5 mg orally bd plus placebo injections od or enoxaparin at a dose of 40 mg subcutaneously od plus placebo tablets bd’ | L: ‘All venograms and all episodes of suspected symptomatic VTE, bleeding, MI, stroke, thrombocytopenia, and death were adjudicated by an independent central adjudication committee whose members were unaware of the treatment assignments’ | L: For symptomatic DVT and symptomatic PE: All patients were included in the analyses | L: All outcomes are reported as per protocol |
| U: For other efficacy outcomes and safety outcomes: Some missing outcome data; similar reasons for missing data across groups but not balanced in numbers. Reasons for missing outcome data may be related to true outcome | ||||||
| APROPOS162 | L: ‘Randomization was done by computer generated allocation’ | U: Not enough information to enable judgement. ‘Patients were randomly assigned to one of the following eight treatment groups’ | U: ‘The study was conducted in a blinded fashion with regards to apixaban dosing and enoxaparin; the warfarin arm was open-label. In order to maintain blinding, apixaban and enoxaparin were administered in a double-dummy fashion’ | L: ‘Efficacy, bleeding events and cause of death were adjudicated by an independent central committee whose members were unaware of treatment assignments’ | L: For efficacy outcomes: Some missing data but reasonable reasons for the missing data were provided and it is unlikely that missing data could influence the result | L: All outcomes are reported as per protocol |
| L: For safety outcomes: Minimal missing data unlikely to affect result Also, reasons were provided for the missing data |
||||||
| ARDEPARIN ARTHROPLASTY STUDY196 | U: ‘The study utilized a randomized, multicenter, stratified, parallel, double blind design’ ‘Eligible patients were randomly assigned to one of three ardeparin doses or oral warfarin prophylaxis in a 1 : 1 : 2 : 2 ratio’ | U: Not enough information to enable judgement. ‘Eligible patients were randomly assigned to one of three ardeparin doses or oral warfarin prophylaxis in a 1 : 1 : 2 : 2 ratio’ | L: ‘To maintain blinding of prophylaxis assignment, all patients received bd injections (either ardeparin or placebo), daily tablets (either placebo or warfarin) and daily prothrombin time measurement’ | L: ‘The efficacy endpoint measures (mandatory venography of the operated leg, or lung scan or pulmonary angiogram for clinically suspected PE) were determined by objective testing and were interpreted by experts blinded to treatment assignment’ ‘All other members of the clinical team, the patient, the pharmacist, and the sponsor, were blinded to prophylaxis treatment’ | L: For efficacy outcomes: ‘Twenty one percent of randomized patients failed to complete the study. The number of patients who did not completed the study was evenly distributed among the four prophylaxis groups in proportion to the randomization ratio’ | L: All outcomes are reported as per protocol |
| L: For safety outcomes: ‘All patients who received at least one dose of the study drug were included in the analysis’ | ||||||
| BISTRO II195 | L: ‘On the day before surgery, patients were assigned randomly to five treatment groups, stratified by the study center and surgical procedure (hip or knee replacement), using a computer-generated scheme’ | U: ‘On the day before surgery, patients were assigned randomly to five treatment groups, stratified by the study center and surgical procedure (hip or knee replacement), using a computer-generated scheme. Separate medication kits for hip and knee replacement were provided to each site in blocks of 10’ | L: ‘Patients were assigned to either oral dabigatran etexilate with doses of 50 and 150 mg bd, 300 mg od and 225 mg bd, or 40 mg of enoxaparin (Aventis Pharma, Bridgewater, NJ, USA) subcutaneously, od. Both study groups received active or matching placebo medications’ | L: For efficacy outcomes: ‘All tests for VTE during the treatment period were first evaluated locally and subsequently by an independent central adjudication committee blinded to the treatment allocation. The results of the central adjudication were used in the primary analysis’ L: For safety outcomes: ‘A centralized independent committee classified all bleeding events’ |
L: For efficacy outcomes: Of 1973 randomised patients, only 1464 were included in efficacy outcome analysis However, missing outcome data are balanced in numbers across the trial arms and the reasons for missing data in the dabigatran arms have a similar spread to those of the enoxaparin arm L: For safety outcomes: ‘1973 were randomized to either dabigatran etexilate (1576) or enoxaparin (397). Of these, 24 were not treated The safety population comprised 1949 patients who received at least one dose of study drug’ |
L: All outcomes are reported as per protocol |
| EXPERT168 | L: ‘The computer-generated randomization code provided assignments in a 2 : 2 : 1 ratio to either betrixaban 15 mg, betrixaban 40 mg, or enoxaparin 30 mg, respectively’ | U: Not enough information to judge allocation concealment. ‘The computer-generated randomization code provided assignments in a 2 : 2 : 1 ratio to either betrixaban 15 mg, betrixaban 40 mg, or enoxaparin 30 mg, respectively’ | H: ‘Randomization was either to enoxaparin or one of two dose levels (15 or 40 mg bid) of betrixaban; patients and physicians were blinded to the betrixaban dose level, but unblinded to enoxaparin versus betrixaban’ | L: ‘All primary efficacy data and suspected bleeding events were evaluated centrally by an Independent Central Adjudication Committee (ICAC) blinded to treatment allocation’ | U: For efficacy outcomes: There are missing data and although reasons for the missing data are provided, they do not balance in numbers across intervention groups and may be related to true outcome | L: All outcomes are reported as per protocol |
| L: For safety outcomes: All patients were included in the analyses | ||||||
| LIFENOX185 | L: ‘The treatment-code list of random permuted blocks was generated by an independent contract research organization and was stratified according to center’ | U: ‘The investigators assigned the patients to a group in the sequential order of the treatment numbers available at the site’ | L: ‘The investigators, patients, and research personnel, as well as the members of the steering committee and of the data and safety monitoring committee, were unaware of the group assignments’ | L: ‘The investigators, patients, and research personnel, as well as the members of the steering committee and of the data and safety monitoring committee, were unaware of the group assignments’ | L: A negligible number of participants did not receive study drug and had no follow-up data This reason is the same in both arms. All patients who received study drug were included in the analyses |
L: All outcomes are reported as per protocol |
| MAGELLAN184,191 | L: ‘Randomization was performed in permuted blocks with the use of an interactive voice response system, with stratification according to centre’ | L: ‘Randomization was performed in permuted blocks with the use of an interactive voice response system, with stratification according to centre’ | L: ‘Eligible patients were randomly assigned to receive subcutaneous enoxaparin, 40 mg od, for 10 ± 4 days and oral placebo, od, for 35 ± 4 days or to receive subcutaneous placebo, od, for 10 ± 4 days and oral rivaroxaban, 10 mg od, for 35 ± 4 days’ | L: ‘All outcomes were assessed by an independent, central adjudication committee whose members were unaware of the study assignments’ | L: Missing data; reasons provided with similarity between the treatment groups; reasons also balance in number in the treatment groups | L: All outcomes are reported as per protocol |
| ODiXa-HIP194 | U: ‘In this dose-escalation study, patients were randomized to receive rivaroxaban (Bayer HealthCare AG) or enoxaparin (Clexane®/Lovenox®, Sanofi-Aventis), in a 3 : 1 ratio’ | U: ‘In this dose-escalation study, patients were randomized to receive rivaroxaban (Bayer HealthCare AG) or enoxaparin (Clexane®/Lovenox®, Sanofi-Aventis), in a 3 : 1 ratio’ | H: ‘This was a randomized, open-label, active-comparator-controlled, European, multinational, dose-escalation study’ | L: ‘All symptomatic events, including deaths, were assessed centrally by the VTE Adjudication Committee. Study drug allocation was not revealed to the adjudication committees, who performed their assessments in a blinded manner’ | H: For efficacy outcomes: Analysis was per protocol, n = 466; 14, 21, 13, 18, 20, 34, and 55 patients were excluded from the randomised numbers in arms 1 to 7, respectively, of which 16 patients did not receive allocated drug treatment ‘A patient was valid for the per-protocol (PP) analysis if they were valid for the ITT analysis, had no major protocol deviations and had adequate assessment of VTE no more than 1 day after stopping study medication’ U: For safety outcomes: Analysis was based on n = 625; 1, 4, 3, 2 and 6 patients did not receive allocated drug treatment in arms 1, 2, 4, 5 and 6, respectively ‘16 patients did not receive allocated drug treatment’ |
L: All outcomes are reported as per protocol |
| ODiXa-HIP2159 | U: Not enough information to enable judgement ‘In this double-blind, double-dummy, dose-ranging study, patients were randomized to oral BAY 59–7939 (2.5, 5, 10, 20, or 30 mg b.i.d.), starting 6–8 h after surgery, or s.c. enoxaparin 40 mg od, starting on the evening before surgery’ |
U: Not enough information to enable judgement ‘This was a prospective, randomized, double-blind, double-dummy, active-comparator-controlled, multicentre, multinational study. All patients received matching placebo injections or tablets’ |
L: ‘This was a prospective, randomized, double-blind, double-dummy, active-comparator-controlled, multicentre, multinational study. All patients received matching placebo injections or tablets’ | L: ‘All adjudication committees were independent and blinded to treatment allocation’ | U: For efficacy outcomes: Large numbers of outcome missing data The number of missing data seem to be balanced in the treatment groups, with similar reasons for missing data However, it is not clear whether or not missing data could be related to the true outcome |
L: All outcomes are reported as per protocol |
| L: For safety outcomes: Very few numbers of missing data; however, number is balanced in the treatment groups Reason for missing data are unlikely to be related to the true outcome |
||||||
| ODiXa-KNEE157 | L: ‘Patients were randomly assigned to six treatment groups, using a computer-generated randomization list’ | L: ‘Patients were randomly assigned to six treatment groups, using a computer-generated randomization list and interactive voice response system’ | L: ‘This was a randomized, double-blind, double-dummy, active comparator controlled, parallel-group, dose-ranging study. All patients received matching placebo injections or tablets’ | L: For efficacy outcomes: ‘The assessment of the efficacy endpoints was based solely on the analysis made by two independent central adjudication committees (Venography and VTE) blinded to the treatment allocation’ | U: For efficacy outcomes: Some missing outcome data and reasons for missing data are provided but missing outcome data do not balance in numbers across intervention groups Not clear whether or not reason for missing data are unrelated to true outcome |
L: All outcomes are reported as per protocol |
| L: For safety outcomes: ‘All bleeding events were assessed centrally by a blinded independent bleeding event committee’ | L: For safety outcomes: All patients were included in the analyses | |||||
| ODiXa-OD.HIP158 | U: Not enough information to enable judgement ‘The ODIXa-OD-HIP study was a randomized, double-blind, double-dummy, active-comparator– controlled, multinational, dose-ranging study’ |
U: Not enough information to enable judgement ‘The ODIXa-OD-HIP study was a randomized, double-blind, double-dummy, active-comparator– controlled, multinational, dose-ranging study. |
L: ‘The ODIXa-OD-HIP study was a randomized, double-blind, double-dummy, active-comparator– controlled, multinational, dose-ranging study. Patients received matching placebo tablets or injections, so that each patient received 2 tablets and an injection every evening’ | L: ‘All venograms were assessed centrally by the Venography Adjudication Committee. All adjudication committees were independent and blinded to treatment allocation’ | U: For efficacy outcomes: Fairly large proportions of missing data; reasons for missing data are given but the reasons and numbers do not balance across the groups Reasons for missing outcome data may be related to the true outcome |
U: Symptomatic VTE was not reported |
| L: ‘All bleeding events were assessed centrally by the Bleeding Event Adjudication Committee. All adjudication committees were independent and blinded to treatment allocation’ | L: For safety outcomes: Small number of missing data with similar reasons Unlikely that reasons are related to the true outcome |
|||||
| PROTECHT170 | L: ‘The randomisation list was generated by an independent statistician who used a standard permuted block of six without stratification. The list was generated with SAS version 8.2’ | L: ‘The allocation sequence was available online to the investigators using the Hypernet web-based system’ | L: ‘Treatment assignments were masked from all study personnel and participants for the duration of the study’ | L: ‘All study outcomes were assessed by a central independent adjudication committee whose members were unaware of patients’ study-group allocation’ | L: Almost 99% of the randomised patients were included in the efficacy and safety analysis Reason for the minimal loss unlikely related to the outcome |
L: All outcomes are reported as per protocol |
| RECORD 1165 | L: ‘Before surgery, patients were randomly assigned to a study group with the use of permuted blocks and stratification according to center by means of a central telephone system with a computer-generated randomization list’ | L: ‘Before surgery, patients were randomly assigned to a study group with the use of permuted blocks and stratification according to center by means of a central telephone system with a computer-generated randomization list’ | U: ‘In a double-blind fashion, patients were assigned to receive either once-daily oral rivaroxaban in 10-mg tablets (Xarelto, Bayer HealthCare) or 40 mg of enoxaparin sodium administered by subcutaneous injection (Clexane/Lovenox, Sanofi-Aventis)’ | L: ‘All outcomes were assessed by central independent adjudication committees whose members were unaware of the patients’ study-group assignments’ | L: There is a substantial amount of missing data with reasons However, missing data appear to be balanced in numbers across intervention groups |
L: All outcomes are reported as per protocol |
| RECORD 2166 | L: ‘Patients were randomly assigned to study medication before surgery, using permutated blocks (size four) with stratification according to centre, via a central telephone system using a computer-generated randomisation code’ | L: ‘Patients were randomly assigned to study medication before surgery, using permutated blocks (size four) with stratification according to centre, via a central telephone system using a computer-generated randomisation code’ | U: ‘Patients were randomly assigned to receive double-blind, oral rivaroxaban 10 mg tablets od (Xarelto, Bayer HealthCare AG, Wuppertal, Germany) or subcutaneous injections of enoxaparin sodium 40 mg od (Clexane/Lovenox, Sanofi-Aventis, Frankfurt am Main, Germany)’ | L: ‘All outcomes were assessed by independent, central adjudication committees blinded to treatment allocation’ | U: For efficacy outcomes: Some missing data (about 31%); missing data seem balanced in number across intervention groups with similar reasons for missing data across groups but not sure if missing data are related to true outcome or not | L: All outcomes are reported as per protocol |
| L: For safety outcomes: Few missing data; missing data seem balanced in number across intervention groups with similar reasons for missing data across groups and missing data are unlikely related to true outcome | ||||||
| RECORD 3163 | U: ‘On a double-blind and double-dummy basis, before surgery, patients were randomly assigned through a central telephone system’ | L: ‘On a double-blind and double-dummy basis, before surgery, patients were randomly assigned through a central telephone system’ | U: ‘On a double-blind and double-dummy basis, before surgery, patients were randomly assigned through a central telephone system to receive once-daily oral rivaroxaban (Bayer HealthCare), in a 10-mg tablet, or a once-daily injection of enoxaparin sodium (Clexane or Lovenox, Sanofi-Aventis), in a 40-mg dose’ | L: ‘All outcomes were assessed by central, independent adjudication committees who were unaware of the treatment assignments’ | U: For efficacy outcomes: Some missing outcome data; reasons for missing data provided However, it is not clear whether or not missing data are unlikely to be related to true outcome |
L: All outcomes are reported as per protocol |
| L: For safety outcomes: Few missing outcomes data; reasons for missing data provided and appears to be the same in both intervention arms; it is unlikely that missing data are related to true outcome | ||||||
| RECORD 4173 | L: ‘Before surgery, participants were randomly assigned to study drug through a central telephone system, stratified by centre with permuted blocks of four patients, on a double-blind and double-dummy basis’ | L: ‘Before surgery, participants were randomly assigned to study drug through a central telephone system, stratified by centre with permuted blocks of four patients, on a double-blind and double-dummy basis’ | U: ‘Before surgery, participants were randomly assigned to study drug through a central telephone system, stratified by centre with permuted blocks of four patients, on a double-blind and double-dummy basis’ | L: ‘Central independent adjudication committees masked to allocation assessed all outcomes’ | L: For efficacy outcomes: ‘Proportions of patients with venograms adequate for assessment for the primary efficacy analysis were lower than anticipated but similar (including the underlying reasons) in the two treatment groups (965 [60.9%] of 1584 patients in the rivaroxaban group and 959 [61.3%] of 1564 patients in the enoxaparin group). The groups were well balanced in terms of baseline demographic and surgery characteristics’ | L: All outcomes are reported as per protocol |
| L: For safety outcomes: All patients were included in the analyses | ||||||
| RE-MOBILISE167 | L: ‘An Interactive Voice Response System was used for randomization in blocks of 6 and was based on an independently generated scheme’ | L: ‘This was a double-blind, centrally randomized trial’ ‘An Interactive Voice Response System was used for randomization’ | L: ‘This was a randomized, double-blind, active controlled, noninferiority study’. ‘All 3 groups received one active and one placebo treatment (i.e., double-dummy blinding)’ | L: For efficacy outcomes: ‘Diagnostic tests for VTE events were initially evaluated locally and subsequently reviewed by an independent central adjudication committee blinded to treatment allocation’ | L: Missing data were accounted for and similar across study groups It is unlikely that missing data and reasons are related to the outcomes |
L: All outcomes are reported as per protocol |
| L: For safety outcomes: ‘An independent expert adjudication committee blinded to treatment allocation classified and reviewed all bleeding events’ | ||||||
| RE-MODEL161 | L: ‘Patients were randomly assigned to one of three treatment groups, using a computer-generated central scheme stratified by study centre’ | L: ‘Patients were randomly assigned to one of three treatment groups, using a computer-generated central scheme stratified by study centre’ | U: ‘This was a randomized, double-blind, active controlled, noninferiority study conducted at 105 centers in Europe, Australia, and South Africa’ | L: For efficacy outcomes: ‘Diagnostic tests for VTE events were initially evaluated locally, and subsequently reviewed by an independent central adjudication committee blinded to treatment allocation’ | L: Missing data almost balanced across intervention groups and clear reasons given as to why data was missing | L: All outcomes are reported as per protocol |
| U: For safety outcomes: ‘An independent expert adjudication committee classified all bleeding events’ | ||||||
| RE-NOVATE160 | L: ‘Patients were randomly assigned to one of three treatment groups, stratified by study centre with a central computer generated scheme’ | L: ‘Patients were randomly assigned to one of three treatment groups, stratified by study centre with a central computer generated scheme’ | L: ‘All three groups received one active and one placebo medication identical in appearance to the other active treatment’ | L: For efficacy outcomes: ‘Diagnostic tests for venous thromboembolic events were initially assessed locally, then by an independent central adjudication committee blinded to treatment allocation. The results of the independent committee were used in the primary analysis’. Other outcomes were also reviewed by Independent committees, masked to treatment allocation | U: For efficacy outcomes: Data for a substantial number of participants in the three groups – missing! Reasons for the missing data were provided. Proportion of missing data are not the same for the groups |
L: All outcomes are reported as per protocol |
| L: For safety outcomes: All patients were included in the analyses | ||||||
| RE-NOVATE II183,189 | L: ‘Up to three days before surgery, eligible patients were randomised in accordance with a computer-generated scheme using a central telephone randomisation procedure’ | L: ‘Up to three days before surgery, eligible patients were randomised in accordance with a computer-generated scheme using a central telephone randomisation procedure’ | L: ‘Treatment-group assignment was concealed from the investigators and their staff and the clinical monitors’ ‘Patients were assigned to either once-daily oral dabigatran 220 mg (2 × 110 mg capsules) or enoxaparin 40 mg subcutaneous injection, together with a placebo of the other study drug (double-dummy design). Active and placebo medications were identical in appearance’ | L: For efficacy outcomes: ‘Diagnostic tests for thromboembolic events were initially evaluated locally, and subsequently by an independent central adjudication committee who were blinded to treatment allocation’ | L: For symptomatic DVT, symptomatic non-fatal PE and all safety outcomes, no missing outcome data However, missing data for other efficacy outcomes but with reasons Reasons for missing data are balanced in number across intervention groups; unlikely that reasons are related to true outcome |
L: All outcomes are reported as per protocol |
| U: For safety outcomes: Not clear: ‘Perioperative and post-operative blood loss that was considered normal by the investigator was not recorded as a bleeding event’ | ||||||
| STARS E-3182 | U: Abstract; not enough information ‘This was a double-blind, double-dummy, centrally randomized trial’ |
L: ‘This was a double-blind, double-dummy, centrally randomized trial’ | U: Abstract; not enough information ‘This was a double-blind, double-dummy, centrally randomized trial’ |
U: Abstract; not enough information ‘This was a double-blind, double-dummy, centrally randomized trial’ |
U: Abstract; not enough information ‘This was a double-blind, double-dummy, centrally randomized trial’ |
U: Study protocol not found |
| STARS J-1172,180 | L: ‘Patients were randomized via an allocation table containing random numbers according to the Excel Visual Basic program using the permuted block method, and a pre-treatment examination was then performed’ | U: Not enough information to enable judgement. ‘Patients were randomized via an allocation table containing random numbers according to the Excel Visual Basic program using the permuted block method, and a pre-treatment examination was then performed’ | U: ‘This was a multicenter, randomized, double-blind, placebo controlled, dose-ranging study’ | L: ‘All venograms were assessed centrally by The Venous Thromboembolic Event Adjudication Committee under blinded conditions’ | L: For efficacy outcomes: Some missing outcome data Reasons for missing outcome was provided and it is unlikely that missing outcome is related to true outcome |
L: All outcomes are reported as per protocol |
| L: For safety outcomes: All patients were included in the analyses | ||||||
| STARS J-2174 | U: Abstract information; not enough information to enable judge ‘This was a randomized, enoxaparin-controlled, multicenter, parallel group study’ |
U: Abstract information; not enough information to enable judge ‘This was a randomized, enoxaparin-controlled, multicenter, parallel group study’ |
H: ‘Double-blind edoxaban 15 mg or 30 mg od or open-label, subcutaneous enoxaparin 20 mg BID was administered for 11 to 14 days’ | U: ‘Outcome assessors were blinded to treatment allocation but not for enoxaparin allocation which was open-blinded’ | U: Some missing outcome data (substantial proportion) Reasons for missing outcome data not available Unclear if reasons for missing data are related to true outcome |
L: All outcomes are reported as per protocol |
| STARS J-4181,193 | U: Not enough information to enable judgement ‘Japanese patients were randomized 2:1 to receive an oral dose of edoxaban 30 mg od or the active control, enoxaparin 2000 IU sc every 12 hours (BID), which is the approved dosing regimen in Japan’ |
U: Not enough information to enable judgement ‘Japanese patients were randomized 2:1 to receive an oral dose of edoxaban 30 mg od or the active control, enoxaparin 2000 IU sc every 12 hours (BID), which is the approved dosing regimen in Japan’ |
H: ‘This was a multicenter, open-label, active-comparator, Phase 3 trial’ | L: ‘To ensure objectivity, independent committees assessed bleeding events and thromboembolic events under blinded conditions’ | U: For efficacy outcomes: Some missing outcome data, although with reasons; the number of missing data is not balanced between the arms The reasons for missing data may be related to the true outcome |
L: All outcomes are reported as per protocol |
| L: For safety outcomes: Small number of missing outcome data with reasons; the number of missing data is balanced between the arms Unlikely that the reasons for missing data are related to the true outcome |
||||||
| STARS J-V179 | U: Abstract; not enough information to enable judgement ‘This was a randomized, double-blind, double-dummy, enoxaparin-controlled, multicentre trial’ |
U: Abstract; not enough information to enable judgement ‘This was a randomized, double-blind, double-dummy, enoxaparin-controlled, multicentre trial’ |
U: Abstract; not enough information to enable judgement ‘This was a randomized, double-blind, double-dummy, enoxaparin-controlled, multicentre trial’ |
U: Abstract; not enough information to enable judgement ‘This was a randomized, double-blind, double-dummy, enoxaparin-controlled, multicentre trial’ |
U: For efficacy outcomes: Some missing outcome data, although with reasons; not enough information to judge whether or not the number of missing data are balanced between the arms The reasons for missing data may be related to the true outcome |
L: All outcomes are reported as per protocol |
| L: For safety outcomes: Minimal number of missing outcome data and, although not enough information to judge the balance between the groups, it is unlikely that the reasons for missing data are related to the true outcome | ||||||
| TOPIC-1197 | L: ‘Patients were randomly assigned to placebo or certoparin sodium (Mono Embolex, Novartis GmbH, Nürnberg, Germany) using a computer-generated randomization list’ | L: ‘Randomization numbers were allocated sequentially as patients were enrolled at each centre. Only the external statistician from the Safety Committee had access to the randomization codes’ | U: ‘These were randomized, double-blind, adaptive group sequential, placebo controlled trials’ | L: For efficacy outcomes: ‘Validated by a blinded, independent Central Thrombosis Evaluation Team’ | L: Only one and two patients were not included in the efficacy and safety analyses, respectively | U: Study protocol not found |
| L: For safety outcomes: ‘Validated by a Data Safety Monitoring Committee consisting of 2 clinicians (blinded to treatment) and an independent statistician with access to the treatment assignments’ | ||||||
| TOPIC-2197 | L: ‘Patients were randomly assigned to placebo or certoparin sodium (Mono Embolex, Novartis GmbH, Nurnberg, Germany) using a computer-generated randomization list’ | L: ‘Randomization numbers were allocated sequentially as patients were enrolled at each centre. Only the external statistician from the Safety Committee had access to the randomization codes’ | U: ‘These were randomized, double-blind, adaptive group sequential, placebo controlled trials’ | L: For efficacy outcomes: ‘Validated by a blinded, independent Central Thrombosis Evaluation Team’ | L: Some missing outcome data; reasons not given and missing data are not exactly balanced in both arms However, the numbers are quite small and reasons unlikely to be related to the outcome |
U: Study protocol not found |
| L: For safety outcomes: ‘Validated by a Data Safety Monitoring Committee consisting of 2 clinicians (blinded to treatment) and an independent statistician with access to the treatment assignments’ | ||||||
| VTE-APIX-PLACEBO-USACAN190 | L: ‘Randomization was performed centrally by contacting a computerized telephone voice response system provided by Bristol-Myers Squibb (BMS) (Lawrenceville, NJ, USA). Treatment assignments were implemented with a randomization schedule with blocks of size four; blocks were stratified by the presence (or not) of metastatic liver disease and clinical center’ | L: ‘Randomization was performed centrally by contacting a computerized telephone voice response system provided by Bristol-Myers Squibb (BMS) (Lawrenceville, NJ, USA). Treatment assignments were implemented with a randomization schedule with blocks of size four; blocks were stratified by the presence (or not) of metastatic liver disease and clinical center’ | L: ‘All subjects took four tablets orally od; these consisted of a combination of apixaban and matching placebo tablets for the apixaban treatment groups, or all placebo tablets for the placebo treatment group, such that the study supplies for subjects in all treatment groups were identical in appearance’ | L: Outcome assessors were blinded to treatment allocation | L: Missing data are of the same quantity (minimal) in all groups and reasons unlikely to be related to the true outcome | L: All outcomes are reported as per protocol |
| VTE-DABIG-LMWH-GREECE187 | U: Abstract; not enough information to enable judgement ‘The patients were randomly assigned in the first group, that fondaparinux 2.5 mg were used for thromboprophylaxis, in the enoxaparin 40 mg group, in the Tinzaparin 0.45 group and in the forth Dabigatran 110 mg group (75 mg over 75 years old)’ |
U: Abstract; not enough information to enable judgement ‘The patients were randomly assigned in the first group, that fondaparinux 2.5 mg were used for thomboprophylaxis, in the enoxaparin 40 mg group, in the Tinzaparin 0.45 group and in the forth Dabigatran 110 mg group (75 mg over 75 years old)’ |
U: Abstract; there is no information on blinding of participants and personnel | U: Abstract; there is no information on blinding of outcome assessment | U: Abstract; there is no information on the number of participants included in the analyses, for comparison with the number randomised to treatments | U: Study protocol not found |
| VTE-DABIG-PLAC-JAPAN175 | L: ‘Patients were randomly assigned to 1 of 4 treatment groups using a computer-generated scheme stratified by study center’ | U: Not enough information to enable judgement ‘Randomization was performed in blocks of 4’ |
U: ‘This was a double-blind, multicenter, randomized, parallel-group, placebo-controlled study conducted at 38 centers in Japan’ | L: For efficacy outcomes: ‘Diagnostic tests for VTE were evaluated centrally by an independent adjudication committee blinded to treatment allocation’ | L: There were missing data but missing data appear to balance in numbers across intervention groups, with similar reasons for missing data across groups | L: All outcomes are reported as per protocol |
| U: For safety outcomes: ‘Two medical experts reviewed all cases of bleeding’ | ||||||
| VTE-EDOX-LMWH-MULTI177 | L: ‘The study was a multicentre study that used a randomized, parallel- group, multi-dose, active-controlled, double-blind, and double-dummy design’ | L: ‘randomly allocated, using an interactive voice recognition system’ | L: ‘Eligible patients who provided written informed consent were randomly allocated, using an interactive voice recognition system to receive either oral edoxaban and subcutaneous injections of placebo, or subcutaneous dalteparin and oral placebo’ | L: ‘All venograms were interpreted by a central independent adjudication committee blinded to treatment allocation and were categorised as proximal DVT (with or without associated distal thrombosis), distal DVT only, normal, or non-evaluable. All episodes of suspected bleeding, suspected symptomatic DVT or PE, and all deaths were reviewed by a blinded central independent clinical events committee and classified according to the definitions provided’ | U: For efficacy outcomes: Some missing outcome data and reasons for missing data are provided but missing outcome data do not balance in numbers across intervention groups Not clear whether or not reason for missing data are unrelated to true outcome |
L: All outcomes are reported as per protocol |
| L: For safety outcomes: Very few missing outcome data; reasons for missing outcome data are provided and it is likely that reasons are unrelated to true outcome | ||||||
| VTE-LMWH-PLAC-CAN169 | L: ‘A statistician and pharmacist at the co-ordinating centre randomised a total of 305 patients via computer generation in a ratio of 1:1 to receive either LMWH or a placebo for 14 days’ | U: ‘A statistician and pharmacist at the co-ordinating centre randomised a total of 305 patients via computer generation in a ratio of 1:1 to receive either LMWH or a placebo for 14 days’ | U: ‘Owing to the double-blind nature of the study, all patients received a general anaesthetic for surgical fixation to avoid any potential adverse reaction to spinal anaesthesia in those patients receiving Fragmin’ | L: For efficacy outcomes: ‘Three senior interventional radiologists reviewed the venograms, with any difference of opinion resolved by consensus. All the radiologists were blinded to the study group’ | L: The number randomised is not the number analysed However, participants removed from the analyses are those who did not meet baseline venography eligibility after randomisation even though they met the study inclusion criteria prior to randomisation All finally included participants were accounted for in the analysis |
U: Study protocol not found |
| U: For safety outcomes: Not enough information to enable judgement ‘All adverse events were monitored and recorded with clinical examination and regular haematological, biochemical and urinary investigations during the routine management of the patients while in hospital’ |
||||||
| VTE-LMWH-PLAC-JAPAN186 | U: Not enough information to enable judgement ‘A randomised controlled trial was performed to evaluate whether the incidence of postoperative VTE was reduced by using pharmacological anticoagulation with either fondaparinux or enoxaparin in addition to our prophylactic mechanical regimen’ |
U: Not enough information to enable judgement ‘The 255 patients were randomly assigned into three Groups’ |
U: Not enough information to enable judgement ‘The 255 patients were randomly assigned into three groups (each of 85) to receive postoperative subcutaneous injections of fondaparinux (Arixtra; GlaxoSmithKline, London, United Kingdom: 2.5 mg od), enoxaparin (Clexane; Sanofi-Aventis, Paris, France: 40 mg, 20 mg twice daily) or placebo (0.5 ml of isotonic saline) for ten consecutive days’ |
L: All of the scans were performed by experienced vascular technicians and were read by experienced radiologists who were blinded to the patient’s randomisation | L: For efficacy outcomes: Very few missing data, < 1%; missing data unlikely to be related to the outcome | U: Study protocol not found |
| L: For safety outcomes: All patients were included in the analyses | ||||||
| VTE-RIVAROX-LMWH-BRAZIL164 | U: Not enough information to enable judgement ‘From September 2006 to April 2007, at the Orthopedics and Traumatology Clinic of the Hospital Complex of the Santa Casa of Porto Alegre, State of Rio Grande do Sul, a randomized, double-blind clinical trial was carried out’ |
U: Not enough information to enable judgement ‘From September 2006 to April 2007, at the Orthopedics and Traumatology Clinic of the Hospital Complex of the Santa Casa of Porto Alegre, State of Rio Grande do Sul, a randomized, double-blind clinical trial was carried out’ |
L: One of the groups was given subcutaneous 40 mg enoxaparin 6 hours to 8 hours before surgery, and after surgery a placebo pill was added, for once a day oral intake, during the first 32 to 36 days The other group was given oral 10 mg rivaroxaban, once a day, during the first 32–36 post-op days In order to have the double-blind feature of the study, a subcutaneous placebo injection was given 6–8 hours before surgery and on the 32–36 days following surgery |
U: There is no specific information on blinding of outcome assessors This may have been done but not stated |
L: All patients were included in the analyses | U: Study protocol not found |
| VTE-RIVAROX-LMWH-CHINA192 | U: ‘The patients were randomly divided into rivaroxaban group and low-molecular-weight heparin group’ | H: No information and no indication of concealment of treatment allocation | U: Not enough information ‘The patients in two groups were given drugs at 6 hours after replacement, the patients in the rivaroxaban group were given rivaroxaban 10 mg/d with the course of 5 weeks; the patients in the LMWH group were given LMWH 4 100 U/d with the course of 2 weeks’ |
H: No information and no indication of blinding of outcome assessors | L: All patients were included in the analyses | U: Study protocol not found |
| VTE-VKA-LMWH-CANADA152 | L: ‘The 670 eligible and consenting patients were randomly allocated after surgery to receive either warfarin sodium (334 patients) or enoxaparin (336 patients) in a 1:1 ratio in blocks of four. A computer generated the randomization schedule’ | U: Not enough information to enable judgement. ‘We stratified randomization by study center, history of VTE, and use of a cemented or uncemented prosthesis’ | L: ‘Patients in the warfarin group also received subcutaneous saline placebo every 12 hours. Patients in the enoxaparin group received 30 mg of enoxaparin subcutaneously every 12 hours and warfarin placebo od’ | L: ‘All diagnostic tests and bleeding episodes were adjudicated by a central committee that was unaware of treatment allocation or clinical findings’ | L: Missing outcome data with reasons which are balance between the treatment arms Unlikely to be related to the true outcome |
U: Study protocol not found |
| VTE-VKA-LMWH-US153 | U: Not enough information to enable judgement ‘The effectiveness and safety of warfarin were compared with those of a low-molecular-weight heparin (dalteparin) for the prevention of DVT after total hip arthroplasty in a prospective, randomized, multi-institutional trial’ |
U: Not enough information to enable judgement ‘The patients were randomly assigned to receive prophylaxis with either warfarin or LMWH’ |
H: The study used an open-label design | L: For efficacy outcomes: ‘All venograms were evaluated by a radiologist who had no knowledge of the treatment-group assignment’ | L: ‘Thirty patients (seventeen who were randomized to treatment with dalteparin and thirteen who were randomized to treatment with warfarin) were excluded from the intent-to-treat population because they had never received the drug (twenty-seven patients) or they had received the drug but the operation had been cancelled (three patients). All patients in the intent-to-treat population were included in the per-protocol analysis if they had at least one evaluable venogram’ | U: Study protocol not found |
| U: For safety outcomes: No information is reported about who assessed major bleeding and if the assessor was blinded | ||||||
| VTE-VKA-LMWH-US-2154 | U: ‘The study was a randomized, open-label, parallel group clinical trial conducted in 156 centres and divided into two phases’ | U: Not enough information to enable judgement ‘The study was a randomized, open-label, parallel group clinical trial conducted in 156 centres and divided into two phases’ |
H: The study was a randomized, open-label, parallel group clinical trial conducted in 156 centres and divided into two phases | U: Not enough information to enable judgement. ‘Each patient was examined for clinical signs and symptoms of DVT (pain, inflammation, swelling, and redness of the lower extremity) and PE (chest pain and difficulty breathing)’ | L: ‘As already stated, the results and conclusions are based on the intent-to-treat analysis, including all patients who received at least one dose of a study medication’ | U: Study protocol not found |
| VTE-VKA-LMWH-US-3155 | L: ‘We used a randomized, computer-derived treatment schedule to assign treatment regimens. To obtain continuing balance of treatments, the randomization list was divided into consecutive blocks’ | U: Not enough information to enable judgement. Allocation sequence was generated by computer but not clear if allocation was done centrally | L: ‘Patients randomized to receive warfarin also received subcutaneous placebo injections. Patients randomized to receive dalteparin also received placebo capsules (warfarin and its placebo were encapsulated to maintain blinding)’ | L: ‘Venograms were interpreted by the local radiologist and an independent, blinded central reader. Disagreements between the local radiologist and the central reader were resolved by a second blinded independent central interpretation; this second reading was decisive’ ‘Thus the use of placebo capsules and injections and the assignment of an independent anticoagulant monitor to adjust INR values maintained double blinding throughout the study’ | L: ‘Twenty nine patients were randomized but did not received study medication; this occurred because of traumatic spinal tap (1,3, and 3 patients per group respectively), cancelled operation (0,2 and 1 patients), presence of exclusion criteria (1,1, and 1 patient), withdrawn consent (2,1, and 3 patients), or miscellaneous reasons making the patient ineligible (4,2, and 4 patients)’ | U: Study protocol not found |
| VTE-VKA-LMWH-US-4156 | U: ‘Randomization numbers generated by the study sponsor were affixed to the exterior of each kit; randomization was performed by the investigator allocating the kits in ascending order’ | L: ‘Each center was provided with sealed medication kits containing either syringes filled with enoxaparin or warfarin tablets’ ‘Randomization numbers generated by the study sponsor were affixed to the exterior of each kit; randomization was performed by the investigator allocating the kits in ascending order’ | H: ‘We report the results of a prospective, randomized, multicenter, open-label, inpatient, parallel-group study’ | L: ‘In addition to the assessment by the investigator, a blinded, independent review of all venograms and ultrasonograms was carried out by a panel of vascular imaging specialists’ | L: All patients were included in the analyses | U: Study protocol not found |
Results of clinical effectiveness and safety
Three trials (TOPIC-1,197 TOPIC-2197 and ARDEPARIN ARTHROPLASTY STUDY196) were not included in any of the networks. They used non-standard variants of heparin that could not be assumed to be comparable with standard heparin, so these studies do not contribute information on the comparisons of interest.
The 38 trials included in these analyses implemented a total of 35 interventions, listed in Table 71. The interventions labelled as ‘standard dose’ for LMWH included tinzaparin (0.45 ml od), enoxaparin (40 mg od or 30 mg bd) and dalteparin (5000 IU). The ‘warfarin variable’ node included interventions in which a subtherapeutic INR range had been considered for some patients, and for that reason this node was included only in sensitivity analyses in which it was merged with the warfarin (INR 2–3 node). Tables 72 and 73 show the numbers of events for each outcome reported in each trial. We performed NMAs for seven outcomes: symptomatic VTE, symptomatic DVT, symptomatic PE, MI, major bleeding, CRB and all-cause mortality. For the first three outcomes, hip surgery, knee surgery and non-surgical patients were analysed separately, whereas for each of the four remaining outcomes all patients were combined in a single network.
| 1 | LMWH | Post-op (standard dose) |
| 2 | Pre-op (standard dose) | |
| 3 | Standard dose | |
| 4 | Noxaparin 20 mg bd | |
| 5 | (100 IU od) | |
| 6 | (ardeparin 3800 IU anti-Xa od | |
| 7 | Warfarin | (INR 2–3) |
| 8 | Variable | |
| 9 | Placebo | |
| 10 | Apixaban | 2.5 mg bd |
| 11 | 5 mg od | |
| 12 | 5 mg bd | |
| 13 | 10 mg od | |
| 14 | 10 mg bd | |
| 15 | 20 mg od | |
| 16 | Betrixaban | 15 mg bd |
| 17 | 40 mg bd | |
| 18 | Dabigatran | 110 mg od |
| 19 | 150 mg od | |
| 20 | 220 mg od | |
| 21 | Edoxaban | 5 mg od |
| 22 | 15 mg od | |
| 23 | 30 mg od | |
| 24 | 60 mg od | |
| 25 | 90 mg od | |
| 26 | Rivaroxaban | 2.5 mg bd |
| 27 | 5 mg od | |
| 28 | 5 mg bd | |
| 29 | 10 mg od | |
| 30 | 10 mg bd | |
| 31 | 20 mg od | |
| 32 | 30 mg od | |
| 33 | 20 mg bd | |
| 34 | 40 mg od | |
| 35 | 30 mg bd |
| Study | Study size | DVT | Symp. DVT | Non-symp. DVT | Proximal DVT | Distal DVT | Symp. proximal DVT | Symp. distal DVT | PE | Symp. PE | Fatal PE | Symp. non-fatal PE | VTE | Symp. VTE | Major VTE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADOPT188 | 6401 | 21 | 110 | 17 | 5 | 15 | 130 | ||||||||
| ADVANCE-1171 | 3184 | 181 | 10 | 20 | 23 | 4 | |||||||||
| ADVANCE-2178 | 3009 | 385 | 10 | 35 | 4 | 1 | 39 | ||||||||
| ADVANCE-3176 | 4394 | 90 | 6 | 27 | 8 | 1 | 7 | 35 | |||||||
| APROPOS162 | 856 | 5 | 13 | 4 | 1 | 100 | |||||||||
| EXPERT168 | 215 | 2 | 24 | 1 | 1 | 2 | 28 | 4 | |||||||
| LIFENOX185 | 8307 | ||||||||||||||
| MAGELLAN184,191 | 7998 | 28 | 24 | 160 | |||||||||||
| ODiXa-HIP2159 | 548 | 81 | 14 | 0 | 14 | ||||||||||
| ODiXa-KNEE157 | 613 | 121 | 4 | 12 | 109 | 2 | 14 | ||||||||
| ODiXa-OD.HIP158 | 618 | 82 | 18 | 64 | 1 | 0 | 18 | ||||||||
| PROTECHT170 | 1150 | 16 | 6 | 22 | |||||||||||
| RE-MOBILISE167 | 1896 | 26 | 44 | 513 | 15 | 11 | 569 | 56 | |||||||
| RE-MODEL161 | 2076 | 12 | 2 | 587 | |||||||||||
| RE-NOVATE160 | 3463 | 16 | 9 | 1 | |||||||||||
| RE-NOVATE II183,189 | 2013 | 127 | 4 | 48 | 78 | 3 | 7 | 51 | |||||||
| RECORD 1165 | 4433 | 65 | 32 | 33 | 5 | 22 | 37 | ||||||||
| RECORD 2166 | 2457 | 85 | 1 | 5 | 21 | 55 | |||||||||
| RECORD 3163 | 1833 | 239 | 29 | 210 | 4 | 40 | 33 | ||||||||
| RECORD 4173 | 3034 | 147 | 16 | 131 | 13 | 1 | 12 | 35 | 35 | ||||||
| STARS E-3182 | 706 | 63 | 0 | 63 | |||||||||||
| STARS J-1172,180 | 520 | 111 | 1 | 6 | 110 | 0 | 112 | ||||||||
| STARS J-2174 | 261 | 8 | 8 | ||||||||||||
| STARS J-4181,193 | 88 | 0 | 4 | 0 | 4 | 0 | |||||||||
| STARS J-V179 | 604 | 0 | 23 | 0 | 23 | ||||||||||
| VTE-APIX-PLACEBO-USACAN190 | 122 | 3 | |||||||||||||
| VTE-DABIG-LMWH-GREECE187 | 120 | 0 | 0 | 0 | |||||||||||
| VTE-DABIG-PLAC-JAPAN175 | 512 | 156 | 6 | 10 | 0 | 10 | |||||||||
| VTE-EDOX-LMWH-MULTI177 | 896 | 40 | 183 | 41 | |||||||||||
| VTE-LMWH-PLAC-CAN169 | 237 | 25 | |||||||||||||
| VTE-LMWH-PLAC-JAPAN186 | 170 | 11 | 0 | 0 | 11 | 0 | 11 | 0 | |||||||
| VTE-RIVAROX-LMWH-BRAZIL164 | 65 | 5 | 0 | ||||||||||||
| VTE-RIVAROX-LMWH-CHINA192 | 106 | 7 | |||||||||||||
| VTE-VKA-LMWH-CANADA152 | 670 | 185 | 46 | 4 | 4 | ||||||||||
| VTE-VKA-LMWH-US153 | 550 | 77 | 26 | 64 | |||||||||||
| VTE-VKA-LMWH-US-2154 | 3011 | 96 | 27 | 1 | 111 | ||||||||||
| VTE-VKA-LMWH-US-3155 | 1472 | 161 | 30 | 17 | 0 | ||||||||||
| VTE-VKA-LMWH-US-4156 | 349 | 123 | 23 | 100 | 1 | 124 |
| Study | Study size | MI | TCP | All bleeds | Minor bleeds | Major bleeds | Fatal bleeds | IC bleeds | Bleeds from surgical site | CRNM bleeding | CRB | CV death | All-cause mortality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ADOPT188 | 6401 | 465 | 21 | 2 | 152 | ||||||||
| ADVANCE-1171 | 3184 | 5 | 2 | 193 | 79 | 33 | 1 | 1 | 82 | 115 | 9 | ||
| ADVANCE-2178 | 3009 | 2 | 1 | 230 | 105 | 23 | 0 | 95 | 102 | 125 | 2 | ||
| ADVANCE-3176 | 4394 | 8 | 5 | 647 | 384 | 40 | 0 | 201 | 229 | 269 | 4 | ||
| APROPOS162 | 856 | 4 | 78 | 57 | 18 | 0 | 1 | ||||||
| EXPERT168 | 215 | 5 | 1 | 4 | 5 | ||||||||
| LIFENOX185 | 8307 | 151 | 120 | 27 | 32 | 59 | 425 | 703 | |||||
| MAGELLAN184,191 | 7998 | 58 | 8 | 2 | 231 | 312 | |||||||
| ODiXa-HIP2159 | 548 | 42 | 17 | 0 | 15 | 20 | 37 | ||||||
| ODiXa-KNEE157 | 613 | 43 | 16 | 0 | 21 | 37 | 0 | ||||||
| ODiXa-OD.HIP158 | 618 | 44 | 27 | 0 | 18 | 45 | 0 | ||||||
| PROTECHT170 | 1150 | 92 | 87 | 5 | 1 | 49 | |||||||
| RE-MOBILISE167 | 1896 | 88 | 22 | 7 | |||||||||
| RE-MODEL161 | 2076 | 341 | 188 | 28 | 125 | 153 | 3 | ||||||
| RE-NOVATE160 | 3463 | 415 | 216 | 56 | 143 | 199 | |||||||
| RE-NOVATE II183,189 | 2013 | 2 | 181 | 115 | 23 | 43 | 66 | 1 | |||||
| RECORD 1165 | 4433 | 22 | 264 | 148 | 8 | 119 | 127 | 5 | 9 | ||||
| RECORD 2166 | 2457 | 7 | 149 | 2 | 0 | 72 | 10 | ||||||
| RECORD 3163 | 1833 | 3 | 120 | 13 | 0 | 61 | 74 | 1 | 6 | ||||
| RECORD 4173 | 3034 | 10 | 14 | 1 | 2 | 69 | 83 | 8 | 12 | ||||
| STARS E-3182 | 706 | 5 | 0 | 35 | |||||||||
| STARS J-1172,180 | 520 | 53 | 1 | 18 | 19 | ||||||||
| STARS J-2174 | 261 | 5 | |||||||||||
| STARS J-4181,193 | 88 | 20 | 16 | 2 | 0 | 2 | 4 | 0 | |||||
| STARS J-V179 | 604 | 8 | 19 | ||||||||||
| VTE-APIX-PLACEBO-USACAN190 | 122 | 3 | 4 | 7 | |||||||||
| VTE-DABIG-LMWH-GREECE187 | 120 | 0 | |||||||||||
| VTE-DABIG-PLAC-JAPAN175 | 512 | 50 | 39 | 5 | 0 | 3 | 6 | 11 | 0 | ||||
| VTE-EDOX-LMWH-MULTI177 | 896 | 24 | 5 | 10 | 14 | 4 | |||||||
| VTE-LMWH-PLAC-CAN169 | 237 | 0 | 0 | 0 | 0 | 0 | |||||||
| VTE-LMWH-PLAC-JAPAN186 | 170 | 8 | 8 | 0 | |||||||||
| VTE-RIVAROX-LMWH-BRAZIL164 | 65 | ||||||||||||
| VTE-RIVAROX-LMWH-CHINA192 | 106 | ||||||||||||
| VTE-VKA-LMWH-CANADA152 | 670 | 2 | 190 | 177 | 13 | 2 | |||||||
| VTE-VKA-LMWH-US153 | 550 | 10 | |||||||||||
| VTE-VKA-LMWH-US-2154 | 3011 | 0 | 262 | 249 | 26 | 0 | 19 | 19 | |||||
| VTE-VKA-LMWH-US-3155 | 1472 | 60 | 98 | 4 | |||||||||
| VTE-VKA-LMWH-US-4156 | 349 | 99 | 86 | 13 | 4 |
Results are presented as follows for each of the seven outcomes. First, we provide network plots to illustrate the comparisons of interventions made in the different trials. Second, we illustrate the risk-of-bias assessments that were specific to the outcome for each trial included in the network. Third, we present results tables for each intervention compared with the reference treatment (standard dose of LMWH administered before surgery for hip surgery patients, after surgery for knee surgery patients, or at start of treatment for other patients). Fourth, we present results tables for pairwise comparisons among licensed doses of the NOACs. For both sets of results tables, posterior median ORs and 95% credible intervals from Bayesian fixed-effects analyses are shown, although we refer to the latter as CIs for convenience. In these tables we present results separately for any available direct evidence, for any indirect comparisons that can be made (excluding the direct evidence) and for the NMA (which combines the direct and the indirect evidence). Comparisons from the NMA with a ratio between interval limits exceeding nine were considered ‘imprecisely estimated’ and are presented at the bottom of each table (note that calculation of indirect evidence was not undertaken for imprecisely estimated comparisons). A summary of results across outcomes is provided at the end in the form of a ‘rankogram’, which illustrates the probability that each treatment is best, second best, and so on, for each outcome. Last, forest plots of all contributing data, with ORs calculated using standard frequentist methods, are included in Appendix 3.
Symptomatic venous thromboembolism
Of 28 studies that contributed data to analyses of symptomatic VTE, 11 reported direct data on symptomatic VTE events (see Table 72). Table 74 shows risk-of-bias judgements for these studies. They were generally judged to be at low risk of bias, although with some concerns about allocation concealment and blinding of participants and personnel.
| Study | Interventions compared | SG | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| ADOPT188 | 3, 10 | + | + | + | + | + | + |
| ADVANCE-1171 | 1, 10 | ? | ? | + | + | + | + |
| ADVANCE-3176 | 2, 10 | + | + | + | + | + | + |
| APROPOS162 | 1, 7, 10, 11, 12, 13, 14, 15 | + | ? | ? | + | + | + |
| EXPERT168 | 1, 16, 17 | + | ? | – | + | ? | + |
| MAGELLAN184,191 | 3, 29 | + | + | + | + | + | + |
| ODiXa-KNEE157 | 1, 26, 28, 30, 33, 35 | + | + | + | + | ? | + |
| ODiXa-OD.HIP158 | 2, 27, 29, 31, 32, 34 | ? | ? | + | + | ? | ? |
| PROTECHT170 | 6, 9 | + | + | + | + | + | + |
| RECORD 1165 | 2, 29 | + | + | ? | + | + | + |
| RECORD 2166 | 2, 29 | + | + | ? | + | ? | + |
| RECORD 3163 | 2, 29 | ? | + | ? | + | ? | + |
| RECORD 4173 | 1, 29 | + | + | ? | + | + | + |
| RE-MOBILISE167 | 1, 19, 20 | + | + | + | + | + | + |
| RE-MODEL161 | 2, 19, 20 | + | + | ? | + | + | + |
| RE-NOVATE160 | 2, 19, 20 | + | + | + | + | ? | + |
| RE-NOVATE II183,189 | 2, 20 | + | + | + | + | + | + |
| VTE-APIX-PLACEBO-USACAN190 | 9, 11, 13, 15 | + | + | + | + | + | + |
| VTE-DABIG-PLAC-JAPAN175 | 9, 18, 19, 20 | + | ? | ? | + | + | + |
| VTE-LMWH-PLAC-JAPAN186 | 1, 4, 9 | ? | ? | ? | + | + | ? |
| VTE-VKA-LMWH-CANADA152 | 1, 7 | + | ? | + | + | + | ? |
| VTE-VKA-LMWH-US-2154 | 1, 7 | ? | ? | – | ? | + | ? |
| VTE-VKA-LMWH-US-3153 | 1, 2, 7 | + | ? | + | + | + | ? |
Nine studies of hip surgery patients reported 231 symptomatic VTE events, leading to a network of 13 interventions (Figure 33). This network was disconnected so that two interventions could not be included in the analysis. Most comparisons were imprecisely estimated, but there was evidence that risk of symptomatic VTE is lower with rivaroxaban (10 mg od) than LMWH (pre-op, standard dose) but higher with LMWH (post-op, standard dose) and warfarin (INR 2–3) than LMWH (pre-op, standard dose) (Table 75). Indirect evidence about warfarin (INR 2–3) versus LMWH (pre-op, standard dose) pointed in the opposite direction to the direct evidence, but was extremely imprecisely estimated.
FIGURE 33.
Network plot for symptomatic VTE in hip surgery patients (primary prevention of VTE).
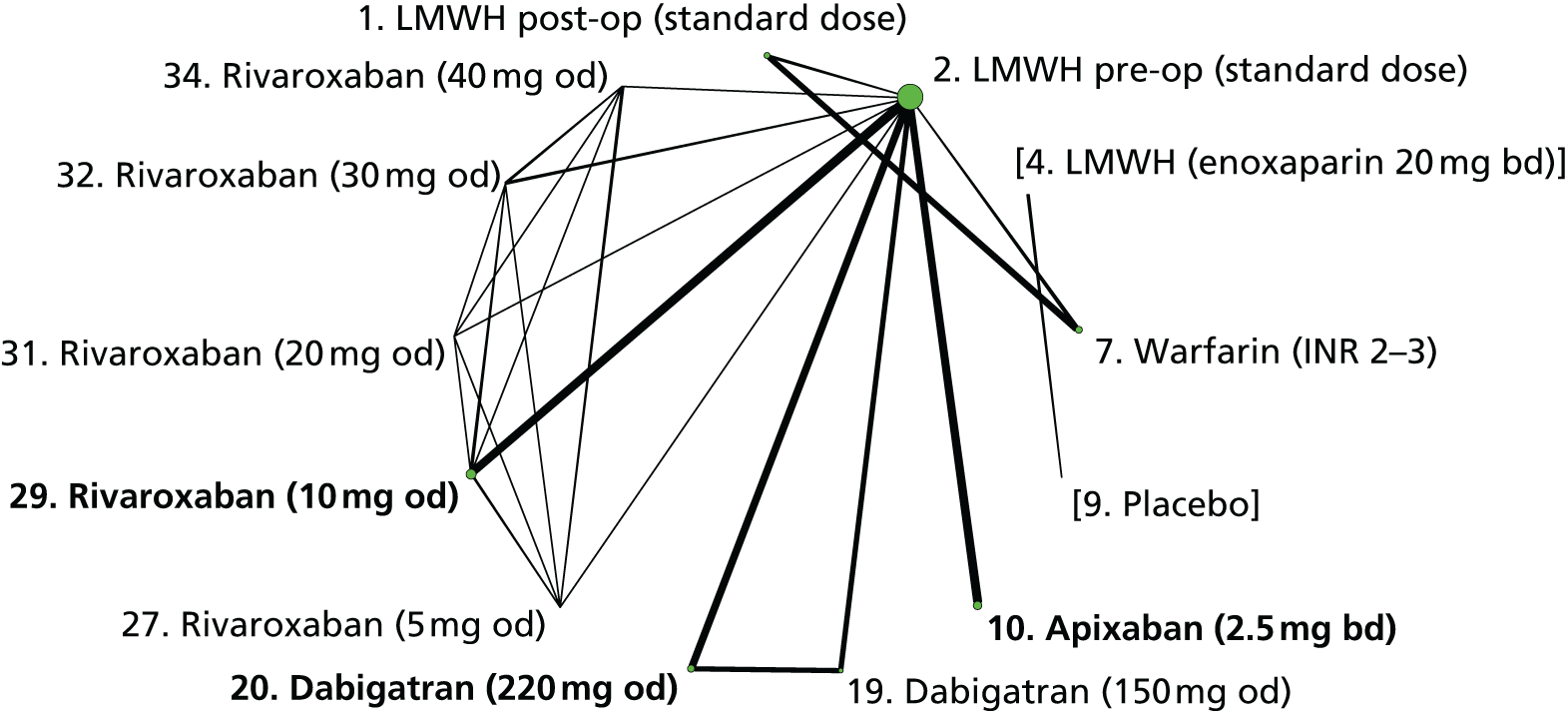
| Comparisons with LMWH (pre-op, standard dose) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| LMWH post-op (standard dose) | 2.16 (0.73 to 7.03) | 6.49 (0.50 to 83.8) | 2.59 (1.03 to 8.36) |
| Warfarin (INR 2–3) | 3.33 (1.21 to 10.4) | 0.29 (0 to 19.5) | 2.87 (1.14 to 9.25) |
| Dabigatran (150 mg od) | 1.46 (0.57 to 3.75) | – | 1.46 (0.57 to 3.75) |
| Dabigatran (220 mg od) | 1.20 (0.51 to 2.86) | – | 1.20 (0.51 to 2.86) |
| Rivaroxaban (10 mg od) | 0.33 (0.16 to 0.64) | – | 0.33 (0.16 to 0.64) |
| Imprecisely estimated comparisons | |||
| Apixaban (2.5 mg bd) | 0.38 (0.10 to 1.16) | – | 0.38 (0.10 to 1.16) |
| Rivaroxaban (5 mg od) | 0.22 (0 to 4.76) | – | 0.22 (0 to 4.76) |
| Rivaroxaban (20 mg od) | 0.19 (0 to 4.01) | – | 0.19 (0 to 4.01) |
| Rivaroxaban (30 mg od) | 0.19 (0 to 4.19) | – | 0.19 (0 to 4.19) |
| Rivaroxaban (40 mg od) | 0.21 (0 to 4.62) | – | 0.21 (0 to 4.62) |
Comparisons between licensed doses of NOACs were imprecisely estimated (Table 76). In addition, there was some heterogeneity in the direction of effects among studies of dabigatran (150 mg od) compared with post-op LMWH (standard dose) and of dabigatran (220 mg od) compared with post-op LMWH (standard dose) (see Appendix 3).
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Rivaroxaban (10 mg od) vs. dabigatran (220 mg od) | – | 0.28 (0.09 to 0.81) | 0.28 (0.09 to 0.81) |
| Imprecisely estimated comparisons | |||
| Dabigatran (220 mg od) vs. apixaban (2.5 mg bd) | – | 3.21 (0.77 to 15.5) | 3.21 (0.77 to 15.5) |
| Rivaroxaban (10 mg od) vs. apixaban (2.5 mg bd) | – | 0.89 (0.23 to 3.90) | 0.89 (0.23 to 3.90) |
Ten trials including knee surgery patients reported 186 symptomatic VTE events, leading to a network of 21 interventions (Figure 34). There was little evidence that risk of symptomatic VTE differed between apixaban (2.5 mg bd), dabigatran (220 mg od) or rivaroxaban (10 mg od) compared with LMWH (post-op, standard dose) (Table 77). Comparisons between licensed doses of NOACs were imprecisely estimated (Table 78).
FIGURE 34.
Network plot for symptomatic VTE in knee surgery patients (primary prevention of VTE).
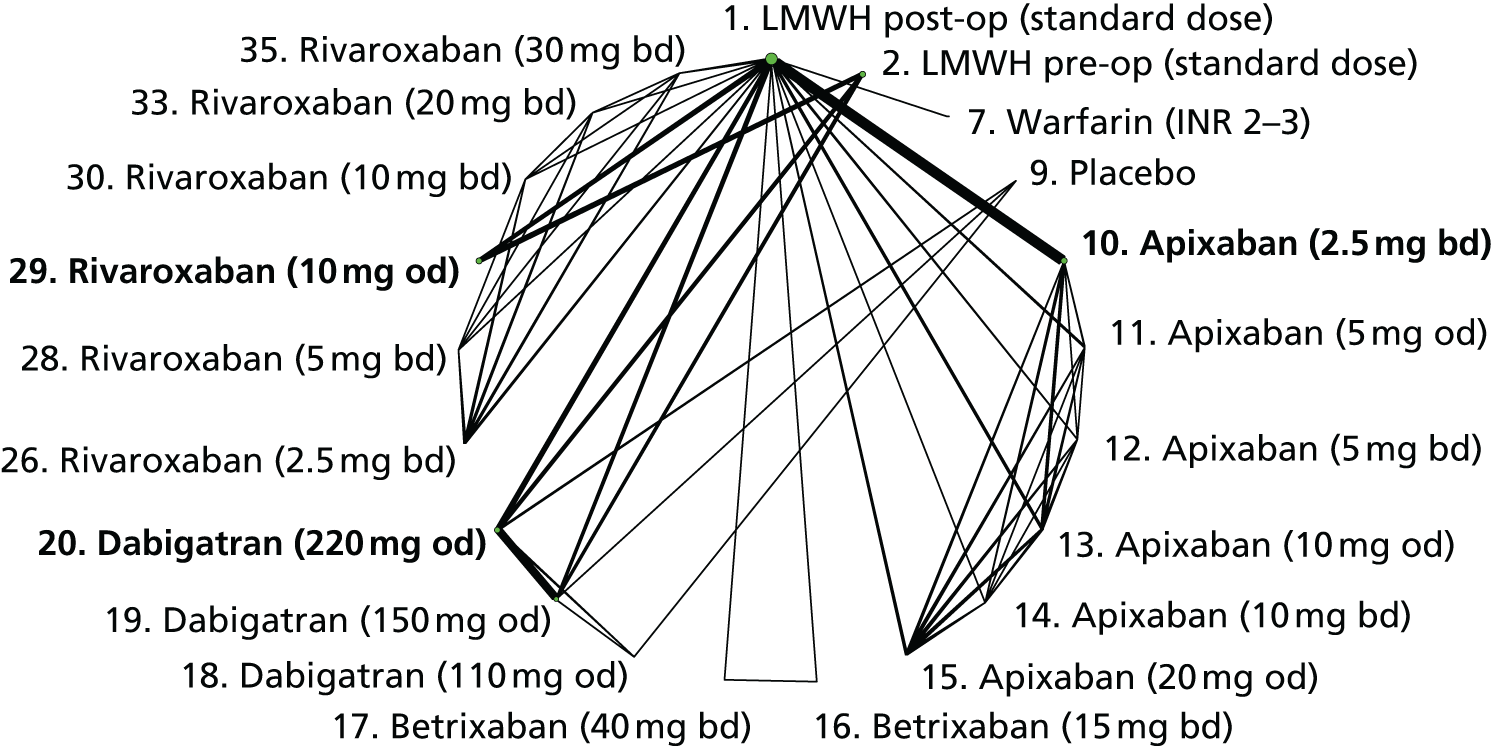
| Comparisons with LMWH (post-op, standard dose) | Direct evidence | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| LMWH pre-op (standard dose) | – | 1.96 (0.91 to 4.27) | 1.96 (0.91 to 4.27) |
| Apixaban (2.5 mg bd) | 1.24 (0.64 to 2.43) | – | 1.24 (0.64 to 2.43) |
| Dabigatran (150 mg od) | 0.82 (0.40 to 1.67) | – | 0.82 (0.40 to 1.67) |
| Dabigatran (220 mg od) | 0.92 (0.45 to 1.86) | – | 0.92 (0.45 to 1.86) |
| Rivaroxaban (10 mg od) | 0.80 (0.43 to 1.46) | – | 0.80 (0.43 to 1.46) |
| Imprecisely estimated comparisons | |||
| Warfarin (INR 2–3) | 0.25 (0.01 to 2.34) | – | 0.25 (0.01 to 2.34) |
| Placebo | – | 1.14 (0.12 to 8.36) | 1.14 (0.12 to 8.36) |
| Apixaban (5 mg od) | 0.12 (0 to 1.84) | – | 0.12 (0 to 1.84) |
| Apixaban (5 mg bd) | 0.11 (0 to 1.66) | – | 0.11 (0 to 1.66) |
| Apixaban (10 mg od) | 1.11 (0.17 to 5.41) | – | 1.11 (0.17 to 5.41) |
| Apixaban (10 mg bd) | 0.57 (0.05 to 3.43) | – | 0.57 (0.05 to 3.43) |
| Apixaban (20 mg od) | 0.57 (0.04 to 3.45) | – | 0.57 (0.04 to 3.45) |
| Betrixaban (15 mg bd) | 1.34 (0.10 to 44.6) | – | 1.34 (0.10 to 44.6) |
| Betrixaban (40 mg bd) | 0.59 (0.01 to 22.8) | – | 0.59 (0.01 to 22.8) |
| Dabigatran (110 mg od) | – | 0.43 (0.01 to 4.41) | 0.43 (0.01 to 4.41) |
| Rivaroxaban (2.5 mg bd) | 0.59 (0.04 to 5.21) | – | 0.59 (0.04 to 5.21) |
| Rivaroxaban (5 mg bd) | 1.24 (0.17 to 9.07) | – | 1.24 (0.17 to 9.07) |
| Rivaroxaban (10 mg bd) | 0.12 (0 to 2.36) | – | 0.12 (0 to 2.36) |
| Rivaroxaban (20 mg bd) | 0.66 (0.05 to 5.93) | – | 0.66 (0.05 to 5.93) |
| Rivaroxaban (30 mg bd) | 0.12 (0 to 2.3) | – | 0.12 (0 to 2.33) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Dabigatran (220 mg od) vs. apixaban (2.5 mg bd) | – | 0.74 (0.28 to 1.95) | 0.74 (0.28 to 1.95) |
| Rivaroxaban (10 mg od) vs. apixaban (2.5 mg bd) | – | 0.64 (0.26 to 1.56) | 0.64 (0.26 to 1.56) |
| Rivaroxaban (10 mg od) vs. dabigatran (220 mg od) | – | 0.87 (0.37 to 2.01) | 0.87 (0.37 to 2.01) |
Four trials in non-surgical patients reported 45 symptomatic VTE events, leading to a network of eight interventions (Figure 35). Because the network was disconnected we excluded two Phase II trials (PROTECHT170 and VTE-APIX-PLACEBO-USACAN190) so that analyses were of the connected network. This enabled us to compare two licensed doses of NOACs. There was weak evidence that risk of symptomatic VTE is lower with apixaban (2.5 mg bd) than LMWH (standard dose) (Table 79), and also when compared with rivaroxaban (10 mg od) (Table 80), although these comparisons were imprecisely estimated.
FIGURE 35.
Network plot for symptomatic VTE in medical patients (primary prevention of VTE).
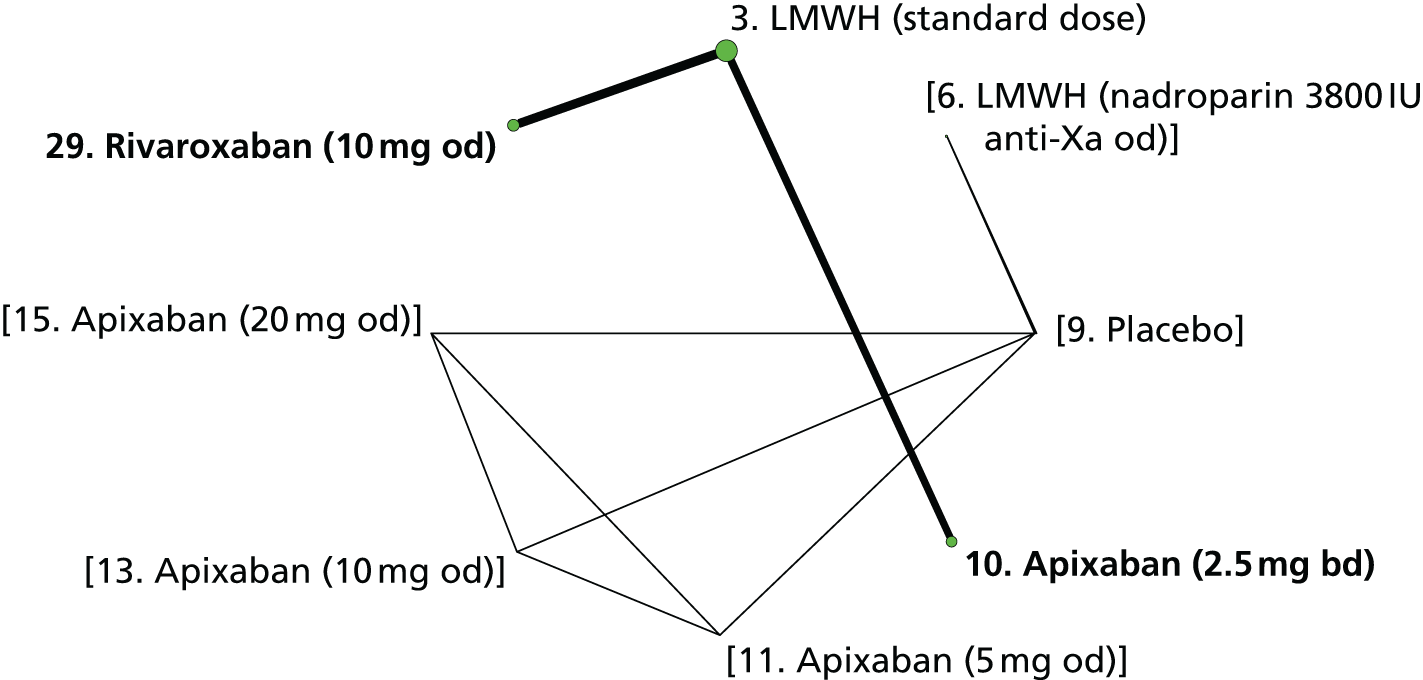
| Comparisons with LMWH (standard dose) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Apixaban (2.5 mg bd) | 0.50 (0.24 to 0.97) | – | 0.50 (0.24 to 0.97) |
| Rivaroxaban (10 mg od) | 1.53 (0.73 to 3.28) | – | 1.53 (0.73 to 3.28) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Rivaroxaban (10 mg od) vs. apixaban (2.5 mg bd) | – | 3.09 (1.13 to 8.87) | 3.09 (1.13 to 8.87) |
Symptomatic deep-vein thrombosis
Twenty studies contributed data to analyses of symptomatic DVT. Table 81 shows risk-of-bias judgements for these studies. Most were judged to be at low risk of bias, although with a few concerns about blinding of participants and personnel.
| Study | Interventions compared | SG | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| ADOPT188 | 3, 10 | + | + | + | + | + | + |
| ADVANCE-1171 | 1, 10 | ? | ? | + | + | + | + |
| ADVANCE-3176 | 2, 10 | + | + | + | + | + | + |
| APROPOS162 | 1, 7, 10, 11, 12, 13, 14, 15 | + | ? | ? | + | + | + |
| EXPERT168 | 1, 16, 17 | + | ? | – | + | ? | + |
| MAGELLAN184,191 | 3, 29 | + | + | + | + | + | + |
| ODiXa-KNEE157 | 1, 26, 28, 30, 33, 35 | + | + | + | + | ? | + |
| PROTECHT170 | 6, 9 | + | + | + | + | + | + |
| RECORD 4173 | 1, 29 | + | + | ? | + | + | + |
| RE-MOBILISE167 | 1, 19, 20 | + | + | + | + | + | + |
| RE-MODEL161 | 2, 19, 20 | + | + | ? | + | + | + |
| RE-NOVATE160 | 2, 19, 20 | + | + | + | + | ? | + |
| RE-NOVATE II183,189 | 2, 20 | + | + | + | + | + | + |
| STARS J-1172,180 | 9, 21, 22, 23, 24 | + | ? | ? | + | + | + |
| STARS J-4181,193 | 4, 23 | ? | ? | – | + | ? | + |
| STARS J-V179 | 4, 23 | ? | ? | ? | ? | ? | + |
| VTE-DABIG-PLAC-JAPAN175 | 9, 18, 19, 20 | + | ? | ? | + | + | + |
| VTE-LMWH-PLAC-JAPAN186 | 1, 4, 9 | ? | ? | ? | + | + | ? |
| VTE-VKA-LMWH-US-2154 | 1, 7 | ? | ? | – | ? | + | ? |
| VTE-VKA-LMWH-US-3153 | 1, 2, 7 | + | ? | + | + | + | ? |
Eight studies of hip surgery patients provided data on 157 symptomatic DVT events, leading to a network of nine interventions (Figure 36). Because the resulting network was disconnected, we excluded several interventions from the analysis. All comparisons were imprecisely estimated (Tables 82 and 83), but there was evidence that risk of symptomatic DVT is higher for that LMWH (post-op, standard dose) and warfarin (INR 2–3) than LMWH (pre-op, standard dose).
FIGURE 36.
Network plot for symptomatic DVT in hip surgery patients (primary prevention of VTE).
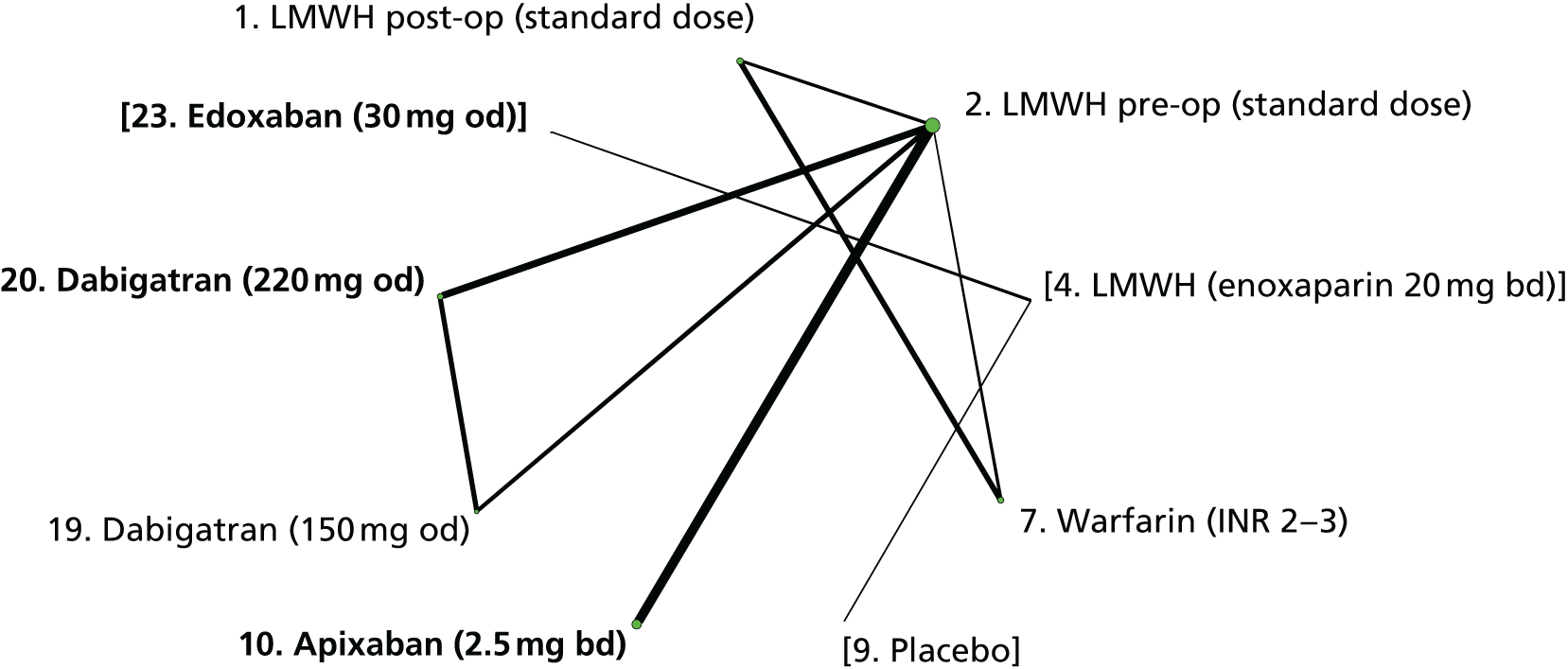
| Comparisons with LMWH (pre-op, standard dose) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| LMWH post-op (standard dose) | 2.14 (0.72 to 7.34) | 4.95 (0.57 to 42.8) | 2.58 (1.03 to 7.94) |
| Warfarin (INR 2–3) | 3.31 (1.21 to 10.8) | 0.84 (0.05 to 13.1) | 2.74 (1.10 to 8.39) |
| Imprecisely estimated comparisons | |||
| Apixaban (2.5 mg bd) | 0.15 (0.01 to 1.09) | – | 0.15 (0.01 to 1.09) |
| Dabigatran (150 mg od) | 2.90 (0.93 to 10.5) | – | 2.90 (0.93 to 10.5) |
| Dabigatran (220 mg od) | 1.19 (0.37 to 4.05) | – | 1.19 (0.37 to 4.05) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Imprecisely estimated comparisons | |||
| Dabigatran (220 mg od) vs. apixaban (2.5 mg bd) | – | 8.37 (0.79 to 286) | 8.37 (0.79 to 286) |
Nine studies of knee surgery patients reported 81 symptomatic DVT events, leading to a network of 24 interventions (Figure 37). All comparisons were imprecisely estimated (Tables 84 and 85). Indirect evidence about warfarin (INR 2–3) compared with LMWH (pre-op, standard dose) pointed in the opposite direction to the direct evidence, but was very imprecisely estimated.
FIGURE 37.
Network plot for symptomatic DVT in knee surgery patients (primary prevention of VTE).
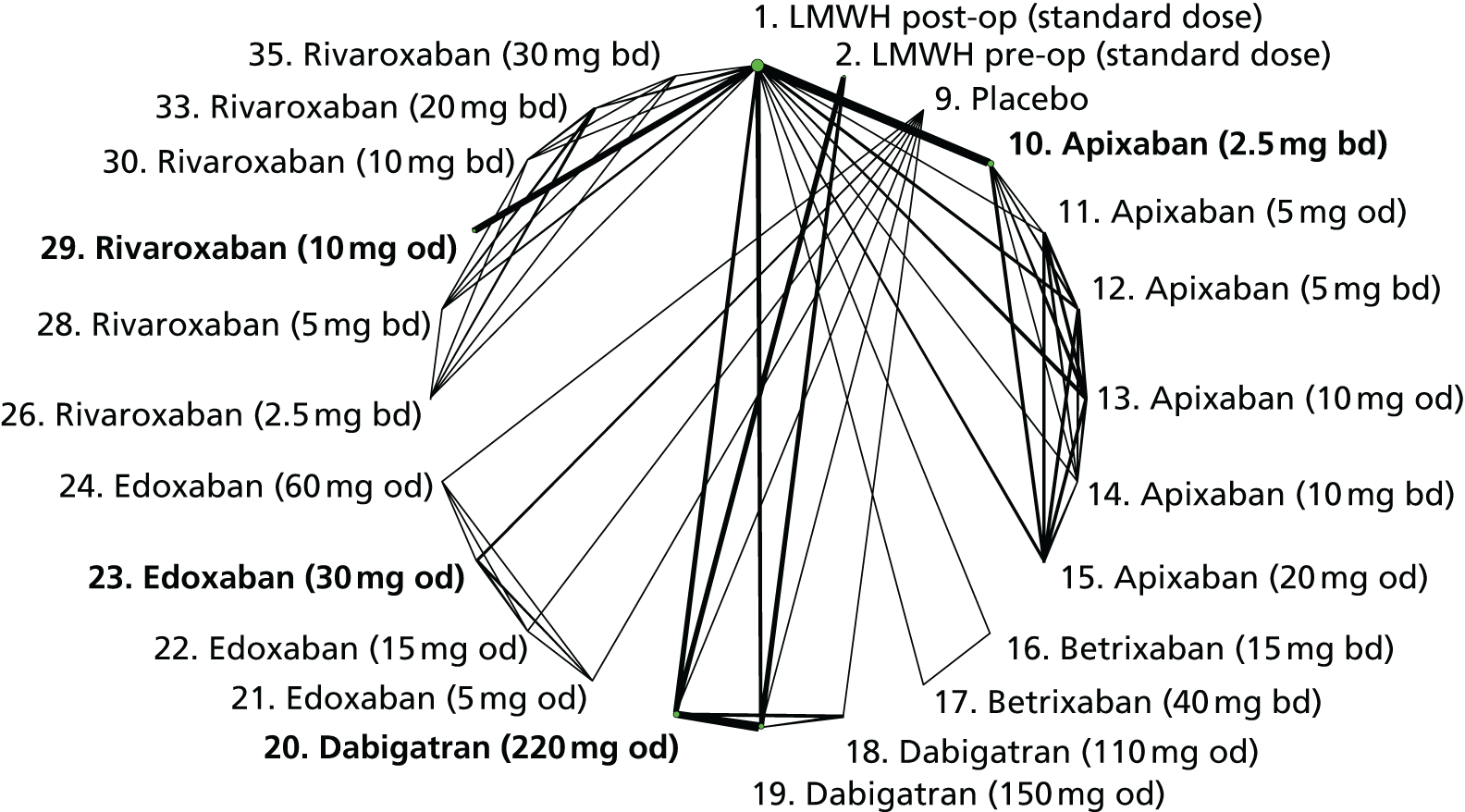
| Comparisons with LMWH (post-op, standard dose) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Dabigatran (150 mg od) | 1.58 (0.64 to 4.31) | – | 1.58 (0.64 to 4.31) |
| Dabigatran (220 mg od) | 1.21 (0.46 to 3.43) | – | 1.21 (0.46 to 3.43) |
| Rivaroxaban (10 mg od) | 0.58 (0.19 to 1.59) | – | 0.58 (0.19 to 1.59) |
| Imprecisely estimated comparisons | |||
| LMWH pre-op (standard dose) | – | 6.06 (1.38 to 31.0) | 6.06 (1.38 to 31.0) |
| Placebo | – | 1.82 (0.18 to 15.1) | 1.82 (0.18 to 15.1) |
| Apixaban (2.5 mg bd) | 0.50 (0.14 to 1.55) | – | 0.50 (0.14 to 1.55) |
| Apixaban (5 mg od) | 0.15 (0 to 2.64) | – | 0.15 (0 to 2.64) |
| Apixaban (5 mg bd) | 0.13 (0 to 2.48) | – | 0.13 (0 to 2.48) |
| Apixaban (10 mg od) | 1.32 (0.18 to 8.59) | – | 1.32 (0.18 to 8.59) |
| Apixaban (10 mg bd) | 0.13 (0 to 2.37) | – | 0.13 (0 to 2.37) |
| Apixaban (20 mg od) | 0.13 (0 to 2.37) | – | 0.13 (0 to 2.37) |
| Betrixaban (15 mg bd) | 0.57 (0.04 to 8.47) | – | 0.57 (0.04 to 8.47) |
| Betrixaban (40 mg bd) | 0.12 (0 to 3.43) | – | 0.12 (0 to 3.43) |
| Dabigatran (110 mg od) | – | 0.69 (0.02 to 8.04) | 0.69 (0.02 to 8.04) |
| Edoxaban (5 mg od) | 9.54 (0.15 to 3760) | – | 9.54 (0.15 to 3760) |
| Edoxaban (15 mg od) | 1.60 (0 to 894) | – | 1.60 (0 to 894) |
| Edoxaban (30 mg od) | 1.72 (0 to 978) | – | 1.72 (0 to 978) |
| Edoxaban (60 mg od) | 1.69 (0 to 1010) | – | 1.69 (0 to 1010) |
| Rivaroxaban (2.5 mg bd) | 0.60 (0.04 to 5.56) | – | 0.60 (0.04 to 5.56) |
| Rivaroxaban (5 mg bd) | 0.12 (0 to 2.52) | – | 0.12 (0 to 2.52) |
| Rivaroxaban (10 mg bd) | 0.12 (0 to 2.36) | – | 0.12 (0 to 2.36) |
| Rivaroxaban (20 mg bd) | 0.66 (0.05 to 5.99) | – | 0.66 (0.05 to 5.99) |
| Rivaroxaban (30 mg bd) | 0.12 (0 to 2.41) | – | 0.12 (0 to 2.41) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Imprecisely estimated comparisons | |||
| Dabigatran (220 mg od) vs. apixaban (2.5 mg bd) | – | 2.43 (0.54 to 12.6) | 2.43 (0.54 to 12.6) |
| Edoxaban (30 mg od) vs. apixaban (2.5 mg bd) | – | 3.47 (0 to 2150) | 3.47 (0 to 2150) |
| Rivaroxaban (10 mg od) vs. apixaban (2.5 mg bd) | – | 1.16 (0.24 to 5.84) | 1.16 (0.24 to 5.84) |
| Edoxaban (30 mg od) vs. dabigatran (220 mg od) | – | 1.41 (0 to 779) | 1.41 (0 to 779) |
| Rivaroxaban (10 mg od) vs. dabigatran (220 mg od) | – | 0.47 (0.11 to 1.97) | 0.47 (0.11 to 1.97) |
| Rivaroxaban (10 mg od) vs. edoxaban (30 mg od) | – | 0.33 (0 to 295) | 0.33 (0 to 295) |
Three studies of medical patients provided data on 65 symptomatic DVT events, leading to a network of five interventions. Because the resulting network was disconnected (Figure 38), we excluded the PROTECHT trial,170 which allowed us to make an indirect comparison between two licensed NOAC doses. All comparisons were imprecisely estimated, although there was evidence that risk of symptomatic DVT is lower for apixaban (2.5 mg bd) than LMWH (standard dose) (Table 86). The comparison between apixaban (2.5 mg bd) and rivaroxaban (10 mg od) was imprecisely estimated (Table 87).
FIGURE 38.
Network plot for symptomatic DVT in medical patients (primary prevention of VTE).
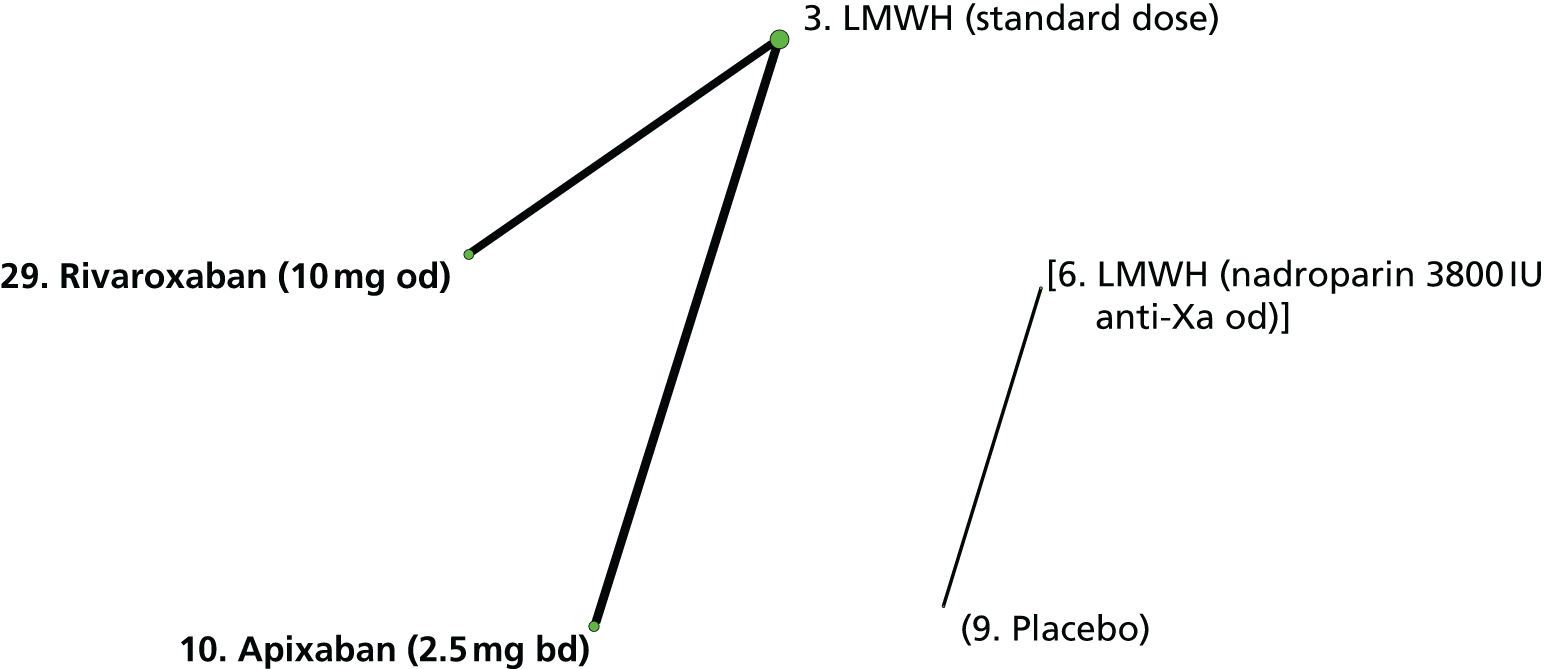
| Comparisons with LMWH (standard dose) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Apixaban (2.5 mg bd) | 0.30 (0.10 to 0.78) | – | 0.30 (0.10 to 0.78) |
| Rivaroxaban (10 mg od) | 0.89 (0.41 to 1.89) | – | 0.89 (0.41 to 1.89) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Imprecisely estimated comparisons | |||
| Rivaroxaban (10 mg od) vs. apixaban (2.5 mg bd) | – | 3.01 (0.87 to 11.6) | 3.01 (0.87 to 11.6) |
Symptomatic pulmonary embolism
Thirty studies contributed data to analyses of symptomatic PE: few reported directly on symptomatic PE events (see Table 72) so we inferred these by summing symptomatic non-fatal and fatal PE events if that information was available. Most studies were judged to be at low risk of bias (Table 88), although there were some concerns about sequence generation, lack of allocation concealment, blinding of participants and personnel, and incomplete outcome data.
| Study | Interventions compared | SG | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| ADOPT188 | 3, 10 | + | + | + | + | + | + |
| ADVANCE-1171 | 1, 10 | ? | ? | + | + | + | + |
| ADVANCE-2178 | 2, 10 | + | + | + | + | ? | + |
| ADVANCE-3176 | 2, 10 | + | + | + | + | + | + |
| APROPOS162 | 1, 7, 10, 11, 12, 13, 14, 15 | + | ? | ? | + | + | + |
| EXPERT168 | 1, 16, 17 | + | ? | – | + | ? | + |
| MAGELLAN184,191 | 3, 29 | + | + | + | + | + | + |
| ODiXa-HIP2159 | 2, 26, 28, 30, 33, 35 | ? | ? | + | + | ? | + |
| ODiXa-KNEE157 | 1, 26, 28, 30, 33, 35 | + | + | + | + | ? | + |
| ODiXa-OD.HIP158 | 2, 27, 29, 31, 32, 34 | ? | ? | + | + | ? | ? |
| PROTECHT170 | 6, 9 | + | + | + | + | + | + |
| RECORD 1165 | 2, 29 | + | + | ? | + | + | + |
| RECORD 2166 | 2, 29 | + | + | ? | + | ? | + |
| RECORD 3163 | 2, 29 | ? | + | ? | + | ? | + |
| RECORD 4173 | 1, 29 | + | + | ? | + | + | + |
| RE-MOBILISE167 | 1, 19, 20 | + | + | + | + | + | + |
| RE-MODEL161 | 2, 19, 20 | + | + | ? | + | + | + |
| RE-NOVATE160 | 2, 19, 20 | + | + | + | + | ? | + |
| RE-NOVATE II183,189 | 2, 20 | + | + | + | + | + | + |
| STARS E-3182 | 1, 23 | ? | + | ? | ? | ? | ? |
| STARS J-1172,180 | 9, 21, 22, 23, 24 | + | ? | ? | + | + | + |
| STARS J-4181,193 | 4, 23 | ? | ? | – | + | ? | + |
| STARS J-V179 | 4, 23 | ? | ? | ? | ? | ? | + |
| VTE-DABIG-PLAC-JAPAN175 | 9, 18, 19, 20 | + | ? | ? | + | + | + |
| VTE-LMWH-PLAC-JAPAN186 | 1, 4, 9 | ? | ? | ? | + | + | ? |
| VTE-RIVAROX-LMWH-BRAZIL162 | 2, 29 | ? | ? | + | ? | + | ? |
| VTE-VKA-LMWH-CANADA152 | 1, 7 | + | ? | + | + | + | ? |
| VTE-VKA-LMWH-US-2154 | 1, 7 | ? | ? | – | ? | + | ? |
| VTE-VKA-LMWH-US-3153 | 1, 2, 7 | + | ? | + | + | + | ? |
| VTE-VKA-LMWH-US-4154 | 1, 7 | ? | + | – | + | + | ? |
Thirteen studies in hip surgery patients provided data on 58 symptomatic PE events, leading to a network of 19 interventions (Figure 39). However, most interventions were either disconnected from the network or considered only in trials in which there were no events in any arm, so that only five interventions were included in the analysis. All comparisons were imprecisely estimated (Tables 89 and 90).
FIGURE 39.
Network plot for symptomatic PE in hip surgery patients (primary prevention of VTE).
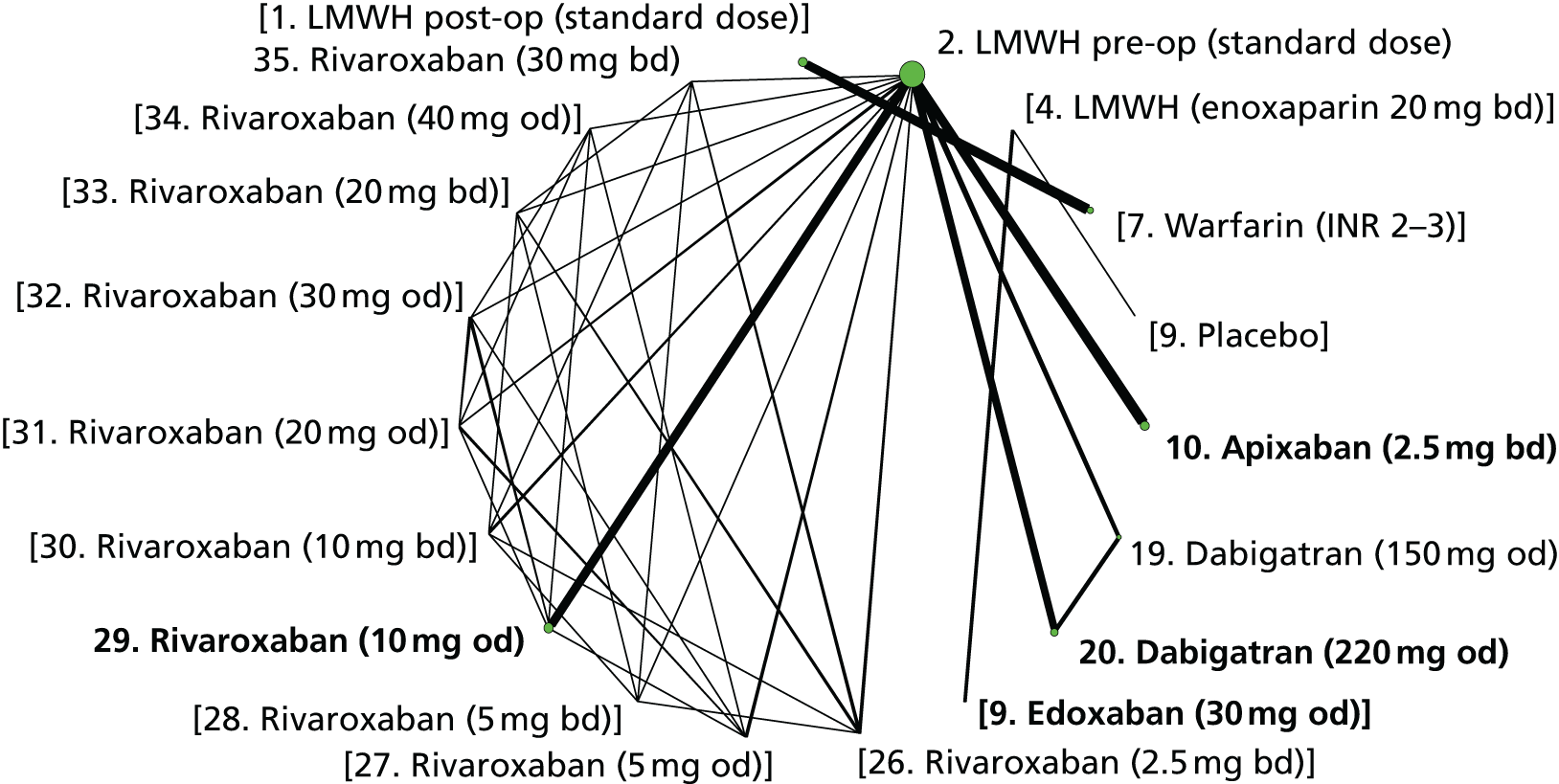
| Comparisons with LMWH (pre-op, standard dose) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Imprecisely estimated comparisons | |||
| Apixaban (2.5 mg bd) | 0.57 (0.11 to 2.40) | – | 0.57 (0.11 to 2.40) |
| Dabigatran (150 mg od) | 0.20 (0.01 to 1.56) | – | 0.20 (0.01 to 1.56) |
| Dabigatran (220 mg od) | 1.22 (0.35 to 4.31) | – | 1.22 (0.35 to 4.31) |
| Rivaroxaban (10 mg od) | 0.82 (0.22 to 2.84) | – | 0.82 (0.22 to 2.84) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Imprecisely estimated comparisons | |||
| Dabigatran (220 mg od) vs. apixaban (2.5 mg bd) | – | 2.16 (0.32 to 16.7) | 2.16 (0.32 to 16.7) |
| Rivaroxaban (10 mg od) vs. apixaban (2.5 mg bd) | – | 1.46 (0.21 to 11.1) | 1.46 (0.21 to 11.1) |
| Rivaroxaban (10 mg od) vs. dabigatran (220 mg od) | – | 0.67 (0.11 to 3.95) | 0.67 (0.11 to 3.95) |
Fourteen studies in knee surgery patients reported 74 symptomatic PE events, leading to a network of 26 interventions (Figure 40). We excluded three trials with zero events in each arm, hence some interventions were not part of the analysis. All comparisons were imprecisely estimated (Table 91) but there was some evidence that risk of symptomatic PE is lower with dabigatran (150 mg od) and higher with apixaban (2.5 mg bd) than LMWH (post-op, standard dose). Among licensed doses of NOACs the risk of symptomatic PE may be lower for rivaroxaban (10 mg od) than apixaban (2.5 mg bd) (Table 92).
FIGURE 40.
Network plot for symptomatic PE in knee surgery patients (primary prevention of VTEs).
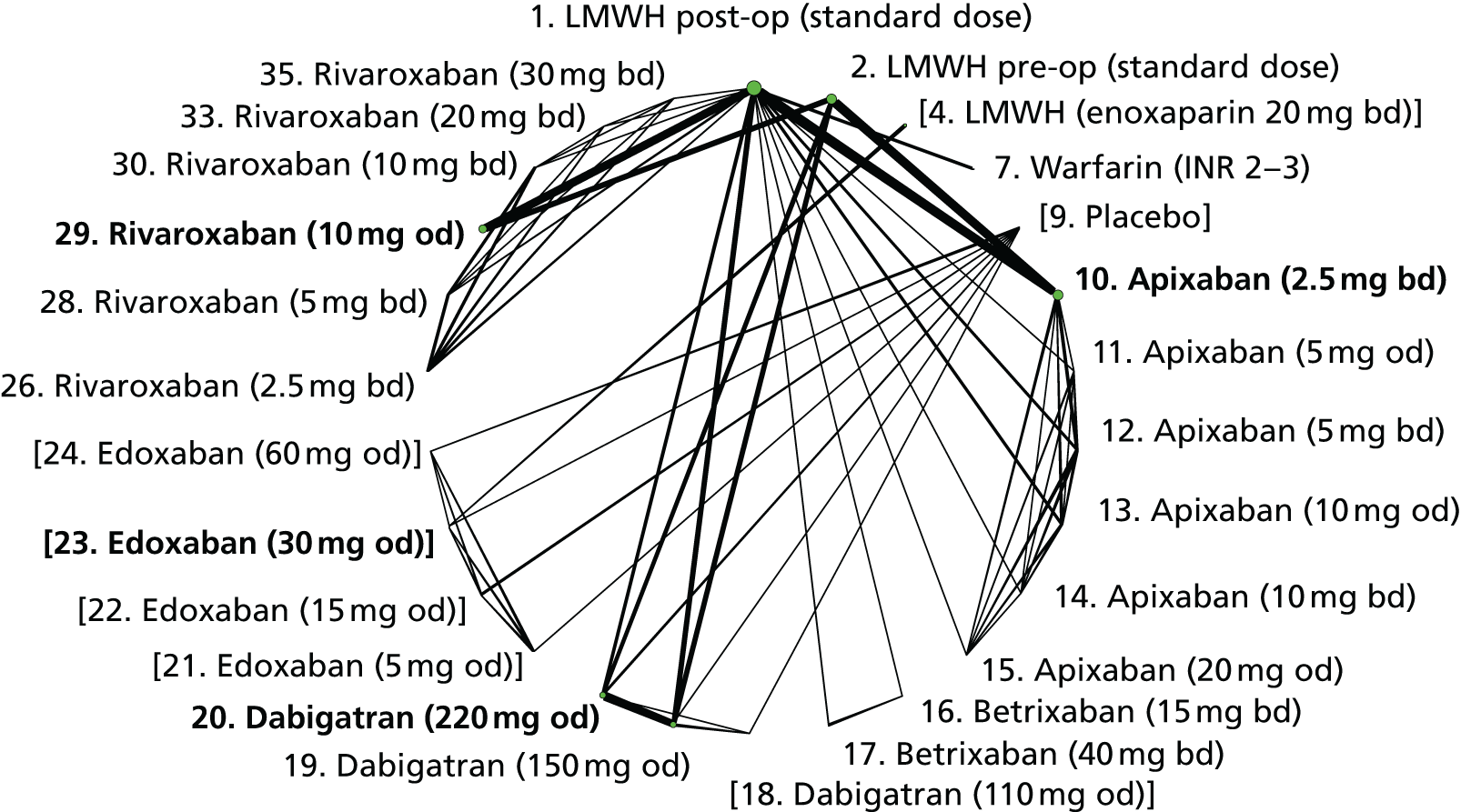
| Comparisons with LMWH (post-op, standard dose) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Apixaban (2.5 mg bd) | 2.14 (1.00 to 4.94) | – | 2.14 (1.00 to 4.94) |
| Dabigatran (220 mg od) | 1.05 (0.39 to 2.85) | – | 1.05 (0.39 to 2.85) |
| Imprecisely estimated comparisons | |||
| LMWH pre-op (standard dose) | – | 0.90 (0.23 to 3.39) | 0.90 (0.23 to 3.39) |
| Warfarin (INR 2–3) | 3.44 (0.58 to 44.0) | – | 3.44 (0.58 to 44.0) |
| Apixaban (5 mg od) | 0.31 (0 to 5.87) | – | 0.31 (0 to 5.87) |
| Apixaban (5 mg bd) | 0.28 (0 to 5.31) | – | 0.28 (0 to 5.31) |
| Apixaban (10 mg od) | 0.29 (0 to 5.32) | – | 0.29 (0 to 5.32) |
| Apixaban (10 mg bd) | 1.43 (0.10 to 11.6) | – | 1.43 (0.10 to 11.6) |
| Apixaban (20 mg od) | 1.42 (0.11 to 11.8) | – | 1.42 (0.11 to 11.8) |
| Betrixaban (15 mg bd) | 2.99 (0.10 to 1930) | – | 2.99 (0.10 to 1930) |
| Betrixaban (40 mg bd) | 3.23 (0.11 to 2070) | – | 3.23 (0.11 to 2070) |
| Dabigatran (150 mg od) | 0.19 (0.02 to 0.80) | – | 0.19 (0.02 to 0.80) |
| Rivaroxaban (2.5 mg bd) | 1.03 (0 to 759) | – | 1.03 (0 to 759) |
| Rivaroxaban (5 mg bd) | 11.0 (0.61 to 6860) | – | 11.0 (0.61 to 6860) |
| Rivaroxaban (10 mg od) | 0.41 (0.12 to 1.17) | – | 0.41 (0.12 to 1.17) |
| Rivaroxaban (10 mg bd) | 1.08 (0 to 769) | – | 1.08 (0 to 769) |
| Rivaroxaban (20 mg bd) | 1.13 (0 to 887) | – | 1.13 (0 to 887) |
| Rivaroxaban (30 mg bd) | 1.10 (0 to 781) | – | 1.10 (0 to 781) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Imprecisely estimated comparisons | |||
| Dabigatran (220 mg od) vs. apixaban (2.5 mg bd) | – | 0.49 (0.14 to 1.66) | 0.49 (0.14 to 1.66) |
| Rivaroxaban (10 mg od) vs. apixaban (2.5 mg bd) | – | 0.19 (0.05 to 0.67) | 0.19 (0.05 to 0.67) |
| Rivaroxaban (10 mg od) vs. dabigatran (220 mg od) | – | 0.39 (0.09 to 1.58) | 0.39 (0.09 to 1.58) |
Three studies in medical patients reported 45 symptomatic PE events. Because the resulting network was disconnected (Figure 41), we excluded the PROTECHT trial. 170 This led to a connected network that enabled an indirect comparison among two licensed NOACs. All comparisons were imprecisely estimated (Tables 93 and 94).
FIGURE 41.
Network plot for symptomatic PE in medical patients (primary prevention of VTE).

| Comparisons with LMWH (post-op, standard dose) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Apixaban (2.5 mg bd) | 0.88 (0.30 to 2.48) | – | 0.88 (0.30 to 2.48) |
| Rivaroxaban (10 mg od) | 0.73 (0.31 to 1.64) | – | 0.73 (0.31 to 1.64) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Imprecisely estimated comparisons | |||
| Rivaroxaban (10 mg od) vs. apixaban (2.5 mg bd) | – | 0.83 (0.22 to 3.18) | 0.83 (0.22 to 3.18) |
Myocardial infarction
Nine studies provided data on 63 MI events, leading to a network of 11 interventions (Figure 42). The included studies were mainly judged to be at low risk of bias (Table 95), although there were some concerns about blinding of participants and personnel.
FIGURE 42.
Network plot for MI (primary prevention of VTE). a, Excluded interventions that were included in sensitivity analyses.
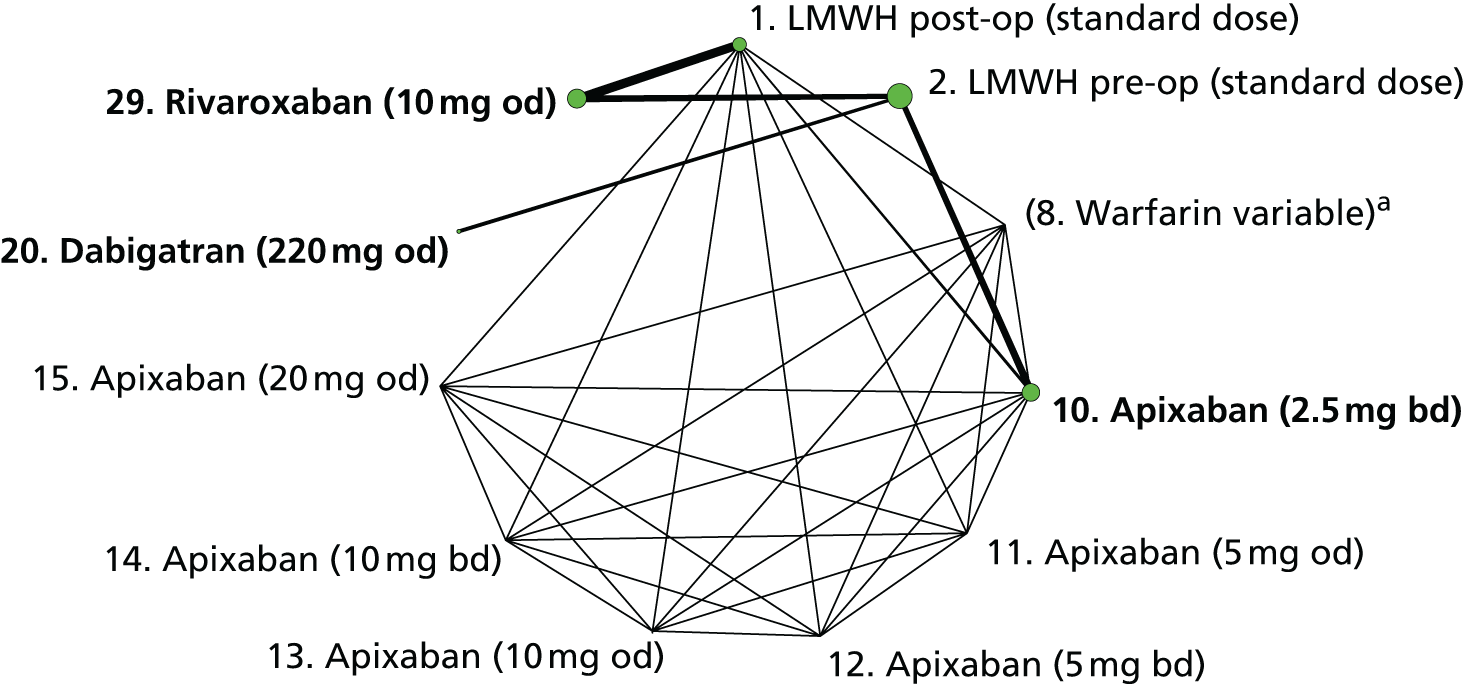
| Study | Interventions compared | SG | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| ADVANCE-1171 | 1, 10 | ? | ? | + | + | + | + |
| ADVANCE-2178 | 2, 10 | + | + | + | + | + | + |
| ADVANCE-3176 | 2, 10 | + | + | + | + | ? | + |
| APROPOS162 | 1, 7, 10, 11, 12, 13, 14, 15 | + | ? | ? | + | + | + |
| RECORD 1165 | 2, 29 | + | + | ? | + | + | + |
| RECORD 2166 | 2, 29 | + | + | ? | + | + | + |
| RECORD 3163 | 2, 29 | ? | + | ? | + | + | + |
| RECORD 4173 | 1, 29 | + | + | ? | + | + | + |
| RE-NOVATE II183,189 | 2, 20 | + | + | + | ? | + | + |
All comparisons were imprecisely estimated (Tables 96 and 97), although there was some evidence that rivaroxaban (10 mg od) may reduce the risk of MI compared with LMWH (post-op, standard dose).
| Comparisons with LMWH (post-op, standard dose) | Direct evidence | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Imprecisely estimated comparisons | |||
| LMWH pre-op (standard dose) | – | 0.37 (0.09 to 1.25) | 0.37 (0.09 to 1.25) |
| Apixaban (2.5 mg bd) | 0.65 (0.18 to 2.11) | – | 0.65 (0.18 to 2.11) |
| Apixaban (5 mg od) | 0.75 (0.05 to 6.23) | – | 0.75 (0.05 to 6.23) |
| Apixaban (5 mg bd) | 0.14 (0 to 2.63) | – | 0.14 (0 to 2.63) |
| Apixaban (10 mg od) | 0.14 (0 to 2.61) | – | 0.14 (0 to 2.61) |
| Apixaban (10 mg bd) | 0.14 (0 to 2.64) | – | 0.14 (0 to 2.64) |
| Apixaban (20 mg od) | 0.14 (0 to 2.69) | – | 0.14 (0 to 2.69) |
| Dabigatran (220 mg od) | 0.37 (0.01 to 17.5) | – | 0.37 (0.01 to 17.5) |
| Rivaroxaban (10 mg od) | 0.27 (0.07 to 0.88) | – | 0.27 (0.07 to 0.88) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Imprecisely estimated comparisons | |||
| Dabigatran (220 mg od) vs. apixaban (2.5 mg bd) | – | 0.57 (0.01 to 26.4) | 0.57 (0.01 to 26.4) |
| Rivaroxaban (10 mg od) vs. apixaban (2.5 mg bd) | – | 0.42 (0.12 to 1.44) | 0.42 (0.12 to 1.44) |
| Rivaroxaban (10 mg od) vs. dabigatran (220 mg od) | – | 0.74 (0.02 to 31.0) | 0.74 (0.02 to 31.0) |
Major bleeding
Thirty-four studies reported 706 major bleeding events, leading to a network of 32 interventions (Figure 43). The studies were mainly judged to be at low risk of bias (Table 98), although there were some concerns about sequence generation and blinding of participants and personnel.
FIGURE 43.
Network plot for major bleeding (primary prevention of VTE). a, Excluded interventions that were included in sensitivity analyses.
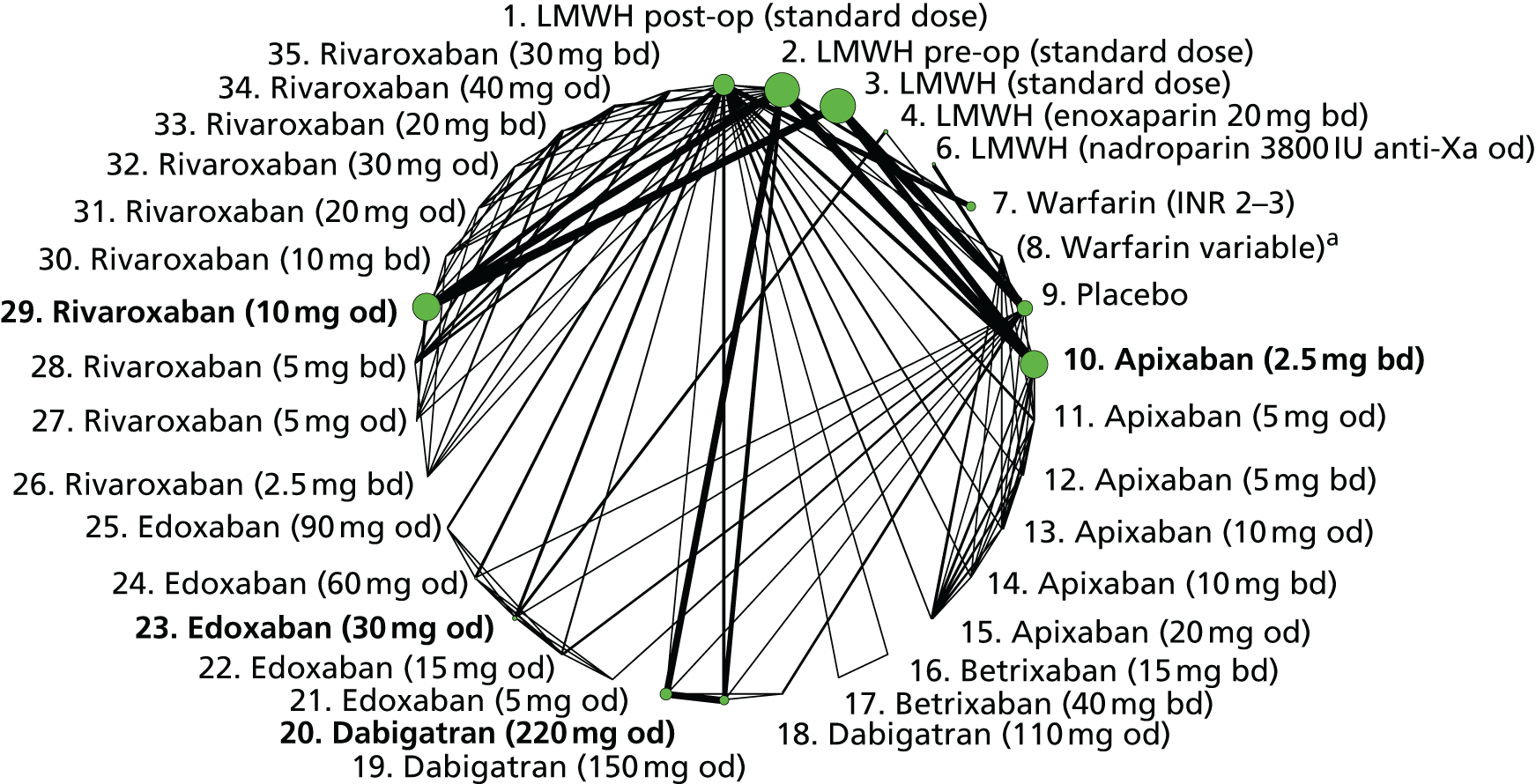
| Study | Interventions compared | SG | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| ADOPT188 | 3, 10 | + | + | + | + | + | + |
| ADVANCE-1171 | 1, 10 | ? | ? | + | + | + | + |
| ADVANCE-2178 | 2, 10 | + | + | + | + | + | + |
| ADVANCE-3176 | 2, 10 | + | + | + | + | ? | + |
| APROPOS162 | 1, 7, 10, 11, 12, 13, 14, 15 | + | ? | ? | + | + | + |
| EXPERT168 | 1, 16, 17 | + | ? | – | + | + | + |
| LIFENOX185 | 3, 9 | + | ? | + | + | + | + |
| MAGELLAN184,191 | 3, 29 | + | + | + | + | + | + |
| ODiXa-HIP2159 | 2, 26, 28, 30, 33, 35 | ? | ? | + | + | + | + |
| ODiXa-KNEE157 | 1, 26, 28, 30, 33, 35 | + | + | + | + | + | + |
| ODiXa-OD.HIP158 | 2, 27, 29, 31, 32, 34 | ? | ? | + | + | + | ? |
| PROTECHT170 | 6, 9 | + | + | + | + | + | + |
| RECORD 1165 | 2, 29 | + | + | ? | + | + | + |
| RECORD 2166 | 2, 29 | + | + | ? | + | + | + |
| RECORD 3163 | 2, 29 | ? | + | ? | + | + | + |
| RECORD 4173 | 1, 29 | + | + | ? | + | + | + |
| RE-MOBILISE167 | 1, 19, 20 | + | + | + | + | + | + |
| RE-MODEL161 | 2, 19, 20 | + | + | ? | ? | + | + |
| RE-NOVATE160 | 2, 19, 20 | + | + | + | + | + | + |
| RE-NOVATE II183,189 | 2, 20 | + | + | + | ? | + | + |
| STARS E-3182 | 4, 23 | ? | + | ? | ? | ? | ? |
| STARS J-1172,180 | 9, 21, 22, 23, 24 | + | ? | ? | + | + | + |
| STARS J-4181,193 | 4, 23 | ? | ? | – | + | + | + |
| STARS J-V179 | 4, 23 | ? | ? | ? | ? | + | + |
| VTE-APIX-PLACEBO-USACAN190 | 9, 11, 13, 15 | + | + | + | + | + | + |
| VTE-DABIG-PLAC-JAPAN175 | 9, 18, 19, 20 | + | ? | ? | ? | + | + |
| VTE-EDOX-LMWH-MULTI177 | 1, 22, 23, 24, 25 | + | + | + | + | + | + |
| VTE-LMWH-PLAC-CAN169 | 2, 9 | + | ? | ? | ? | + | ? |
| VTE-LMWH-PLAC-JAPAN186 | 1, 4, 9 | ? | ? | ? | + | + | ? |
| VTE-VKA-LMWH-CANADA152 | 1, 7 | + | ? | + | + | + | ? |
| VTE-VKA-LMWH-US153 | 2, 7 | ? | ? | – | + | + | ? |
| VTE-VKA-LMWH-US-2154 | 1, 7 | ? | ? | – | ? | + | ? |
| VTE-VKA-LMWH-US-3153 | 1, 2, 7 | + | ? | + | + | + | ? |
| VTE-VKA-LMWH-US-4154 | 1, 7 | ? | + | – | + | + | ? |
There was little evidence that risk of major bleeding differs between pre-op and post-op LMWH (standard dose). There was evidence that risk of major bleeding is lower with warfarin (INR 2–3) and higher with rivaroxaban (10 mg od) than LMWH (post-op, standard dose) (Table 99). We observed statistical inconsistency between the direct and indirect estimates comparing dabigatran (220 mg od) with post-op LMWH (standard dose). The direct evidence indicated a reduction in bleeding with dabigatran and the indirect evidence indicated an increase. The estimated OR from the NMA was 1.20 (95% CI 0.75 to 1.92). All three of these results had CIs compatible with increases and decreases in risk. There was evidence that risk of major bleeding is higher with rivaroxaban (10 mg od) than with LMWH (post-op, standard dose) and than apixaban (2.5 mg bd) and dabigatran (220 mg od) (Table 100).
| Comparisons with LMWH (post-op, standard dose) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| LMWH pre-op (standard dose) | 1.32 (0.85 to 2.06) | 0.90 (0.58 to 1.40) | 1.09 (0.79 to 1.49) |
| Warfarin (INR 2–3) | 0.59 (0.39 to 0.88) | 0.47 (0.18 to 1.23) | 0.57 (0.39 to 0.82) |
| Placebo | 0.68 (0.31 to 1.50) | 1.75 (0.36 to 8.54) | 0.82 (0.41 to 1.64) |
| Apixaban (2.5 mg bd) | 0.93 (0.55 to 1.58) | 1.02 (0.57 to 1.82) | 0.97 (0.65 to 1.45) |
| Dabigatran (150 mg od) | 0.39 (0.13 to 1.16) | 1.00 (0.53 to 1.89) | 0.79 (0.465 to 1.35) |
| Dabigatran (220 mg od) | 0.39 (0.13 to 1.17) | 1.55 (0.92 to 2.60) | 1.20 (0.755 to 1.92) |
| Rivaroxaban (10 mg od) | 2.86 (1.67 to 4.88) | 1.41 (0.61 to 3.26) | 2.33 (1.515 to 3.68) |
| Imprecisely estimated comparisons | |||
| LMWH (enoxaparin 20 mg bd) | – | 2.98 (0.18 to 93.9) | 2.98 (0.185 to 93.9) |
| LMWH (nadroparin 3800 IU anti-Xa od) | – | 9.42 (0.61 to 4420) | 9.42 (0.615 to 4420) |
| Apixaban (5 mg od) | 3.53 (0.75 to 23.1) | – | 3.53 (0.755 to 23.1) |
| Apixaban (5 mg bd) | 4.66 (0.93 to 31.3) | – | 4.66 (0.935 to 31.3) |
| Apixaban (10 mg od) | 1.25 (0.14 to 10.0) | – | 1.25 (0.14 to 10.0) |
| Apixaban (10 mg bd) | 4.65 (0.95 to 30.9) | – | 4.65 (0.95 to 30.9) |
| Apixaban (20 mg od) | 5.94 (1.49 to 37.4) | – | 5.94 (1.49 to 37.4) |
| Betrixaban (15 mg bd) | 0.09 (0 to 2.90) | – | 0.09 (0 to 2.90) |
| Betrixaban (40 mg bd) | 0.10 (0 to 3.02) | – | 0.10 (0 to 3.02) |
| Dabigatran (110 mg od) | – | 0.63 (0.05 to 3.72) | 0.63 (0.05 to 3.72) |
| Edoxaban (5 mg od) | – | 0.85 (0 to 51.4) | 0.85 (0 to 51.4) |
| Edoxaban (15 mg od) | – | 2.03 (0.16 to 55.4) | 2.03 (0.165 to 55.4) |
| Edoxaban (30 mg od) | – | 2.24 (0.17 to 61.1) | 2.24 (0.17 to 61.1) |
| Edoxaban (60 mg od) | – | 3.32 (0.36 to 87.5) | 3.32 (0.36 to 87.5) |
| Edoxaban (90 mg od) | – | 4.80 (0.42 to 135) | 4.80 (0.42 to 135) |
| Rivaroxaban (2.5 mg bd) | – | 0.56 (0.09 to 2.78) | 0.56 (0.09 to 2.78) |
| Rivaroxaban (5 mg od) | – | 2.90 (0.52 to 14.2) | 2.90 (0.52 to 14.2) |
| Rivaroxaban (5 mg bd) | 0.79 (0.16 to 3.53) | – | 0.79 (0.16 to 3.53) |
| Rivaroxaban (10 mg bd) | 1.31 (0.36 to 5.32) | – | 1.31 (0.36 to 5.32) |
| Rivaroxaban (20 mg od) | – | 5.77 (1.53 to 24.4) | 5.77 (1.53 to 24.4) |
| Rivaroxaban (30 mg od) | – | 6.69 (1.87 to 27.7) | 6.69 (1.87 to 27.7) |
| Rivaroxaban (20 mg bd) | 2.41 (0.77 to 9.05) | – | 2.41 (0.77 to 9.05) |
| Rivaroxaban (40 mg od) | – | 6.98 (1.92 to 28.6) | 6.98 (1.92 to 28.6) |
| Rivaroxaban (30 mg bd) | 4.46 (1.43 to 16.9) | – | 4.46 (1.43 to 16.9) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Dabigatran (220 mg od) vs. apixaban (2.5 mg bd) | – | 1.23 (0.72 to 2.12) | 1.23 (0.72 to 2.12) |
| Rivaroxaban (10 mg od) vs. apixaban (2.5 mg bd) | – | 2.40 (1.37 to 4.29) | 2.40 (1.37 to 4.29) |
| Rivaroxaban (10 mg od) vs. dabigatran (220 mg od) | – | 1.95 (1.06 to 3.61) | 1.95 (1.06 to 3.61) |
| Imprecisely estimated comparisons | |||
| Edoxaban (30 mg od) vs. apixaban (2.5 mg bd) | – | 2.31 (0.16 to 64.3) | 2.31 (0.16 to 64.3) |
| Edoxaban (30 mg od) vs. dabigatran (220 mg od) | – | 1.87 (0.13 to 52.5) | 1.87 (0.13 to 52.5) |
| Rivaroxaban (10 mg od) vs. edoxaban (30 mg od) | – | 1.04 (0.04 to 14.5) | 1.04 (0.04 to 14.5) |
Clinically relevant bleeding
Twenty-five studies reported 1973 CRB events, leading to a network of 29 interventions (Figure 44). The studies were mostly judged to be at low risk of bias (Table 101), although there were some concerns about lack of blinding of participants and personnel.
FIGURE 44.
Network plot for CRB (primary prevention of VTE).
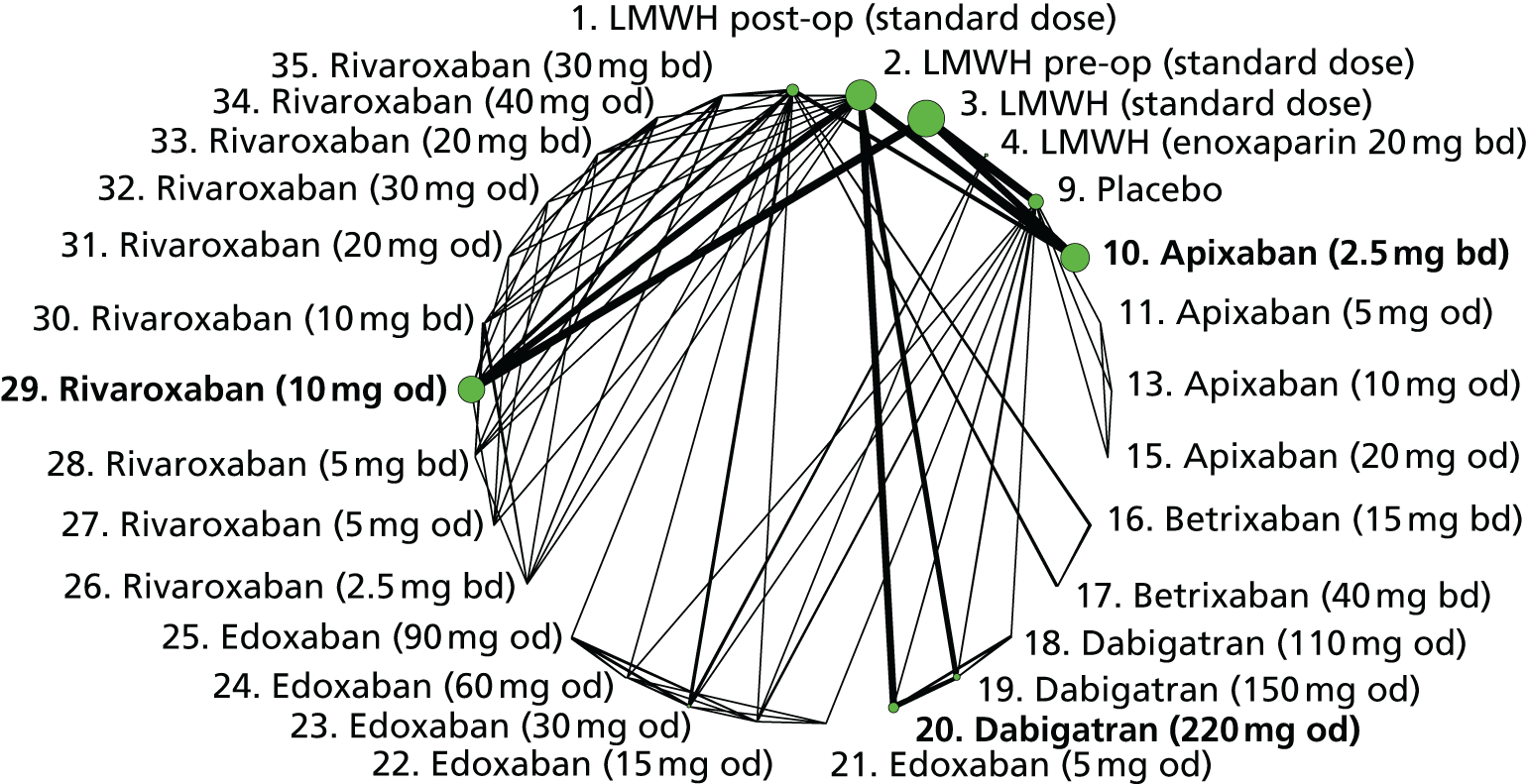
| Study | Interventions compared | SG | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| ADOPT188 | 3, 10 | + | + | + | + | + | + |
| ADVANCE-1171 | 1, 10 | ? | ? | + | + | + | + |
| ADVANCE-2178 | 2, 10 | + | + | + | + | + | + |
| ADVANCE-3176 | 2, 10 | + | + | + | + | + | + |
| EXPERT168 | 1, 16, 17 | + | ? | – | + | + | + |
| LIFENOX185 | 3, 9 | + | ? | + | + | + | + |
| MAGELLAN184,191 | 3, 29 | + | + | + | + | + | + |
| ODiXa-HIP2159 | 2, 26, 28, 30, 33, 35 | ? | ? | + | + | + | + |
| ODiXa-KNEE157 | 1, 26, 28, 30, 33, 35 | + | + | + | + | + | + |
| ODiXa-OD.HIP158 | 2, 27, 29, 31, 32, 34 | ? | ? | + | + | + | ? |
| RECORD 1165 | 2, 29 | + | + | ? | + | + | + |
| RECORD 2166 | 2, 29 | + | + | ? | + | + | + |
| RECORD 3163 | 2, 29 | ? | + | ? | + | + | + |
| RECORD 4173 | 1, 29 | + | + | ? | + | + | + |
| RE-MODEL161 | 2, 19, 20 | + | + | ? | ? | + | + |
| RE-NOVATE160 | 2, 19, 20 | + | + | + | + | + | + |
| RE-NOVATE II183,189 | 2, 20 | + | + | + | ? | + | + |
| STARS E-3182 | 4, 23 | ? | + | ? | ? | ? | ? |
| STARS J-1172,180 | 9, 21, 22, 23, 24 | + | ? | ? | + | + | + |
| STARS J-2174 | 4, 22, 23 | ? | ? | – | + | + | + |
| STARS J-4181,193 | 4, 23 | ? | ? | – | + | + | + |
| STARS J-V179 | 4, 23 | ? | ? | ? | ? | ? | + |
| VTE-APIX-PLACEBO-USACAN190 | 9, 11, 13, 15 | + | + | + | + | + | + |
| VTE-DABIG-PLAC-JAPAN175 | 9, 18, 19, 20 | + | ? | ? | ? | + | + |
| VTE-EDOX-LMWH-MULTI177 | 1, 22, 23, 24, 25 | + | + | + | + | + | + |
There was evidence that risk of CRB is higher for pre-op LMWH (standard dose) than post-op LMWH (standard dose), and higher for dabigatran (150 mg or 220 mg od) and rivaroxaban (10 mg od) than LMWH (post-op, standard dose) (Table 102). We observed statistical inconsistency between direct and indirect estimates comparing rivaroxaban with post-op LMWH (standard dose). In particular, the direct evidence for rivaroxaban (5 mg bd) indicated a reduction in bleeding with rivaroxaban, whereas the indirect evidence indicated an increase. The combined estimate for this comparison from the NMA suggested a small increase with OR 1.53 (95% CI 0.54 to 4.47); all three of these results had CIs compatible with increases and decreases in risk. There was evidence that risk of CRB is higher for dabigatran (220 mg od) and rivaroxaban (10 mg od) than apixaban (2.5 mg bd) (Table 103).
| Comparisons with LMWH (post-op, standard dose) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| LMWH pre-op (standard dose) | – | 1.30 (1.03 to 1.62) | 1.30 (1.03 to 1.62) |
| Placebo | 0.71 (0.45 to 1.12) | – | 0.71 (0.45 to 1.12) |
| Apixaban (2.5 mg bd) | 0.97 (0.76 to 1.24) | 1.16 (0.74 to 1.82) | 1.06 (0.86 to 1.30) |
| Dabigatran (150 mg od) | – | 1.53 (1.09 to 2.15) | 1.53 (1.09 to 2.15) |
| Dabigatran (220 mg od) | – | 1.55 (1.12 to 2.15) | 1.55 (1.12 to 2.15) |
| Rivaroxaban (10 mg od) | 1.85 (1.52 to 2.26) | 1.30 (0.91 to 1.85) | 1.85 (1.52 to 2.26) |
| Rivaroxaban (5 mg bd) | 0.56 (0.11 to 2.54) | 5.94 (1.76 to 20.0) | 2.45 (0.97 to 6.73) |
| Rivaroxaban (10 mg bd) | 0.55 (0.11 to 2.49) | 3.55 (0.85 to 14.9) | 1.53 (0.54 to 4.47) |
| Rivaroxaban (20 mg od) | 1.93 (0.68 to 5.07) | – | 1.93 (0.68 to 5.07) |
| Rivaroxaban (30 mg od) | 2.81 (1.13 to 6.88) | – | 2.81 (1.13 to 6.88) |
| Rivaroxaban (20 mg bd) | 1.84 (0.57 to 6.44) | 10.5 (2.47 to 44.4) | 3.73 (1.57 to 9.98) |
| Rivaroxaban (40 mg od) | 3.26 (1.34 to 7.89) | – | 3.26 (1.34 to 7.89) |
| Rivaroxaban (30 mg bd) | 3.53 (1.25 to 11.1) | 32.5 (4.47 to 236) | 5.94 (2.39 to 16.4) |
| Imprecisely estimated comparisons | |||
| LMWH (enoxaparin 20 mg bd) | – | 1.25 (0.35 to 4.95) | 1.25 (0.35 to 4.95) |
| Apixaban (5 mg od) | – | 0.64 (0.02 to 32.0) | 0.64 (0.02 to 32.0) |
| Apixaban (10 mg od) | – | 0.71 (0.02 to 36.0) | 0.71 (0.02 to 36.0) |
| Apixaban (20 mg od) | – | 3.78 (0.41 to 150) | 3.78 (0.41 to 150) |
| Betrixaban (15 mg bd) | 0.03 (0 to 0.54) | – | 0.03 (0 to 0.54) |
| Betrixaban (40 mg bd) | 0.33 (0.05 to 1.88) | – | 0.33 (0.05 to 1.88) |
| Dabigatran (110 mg od) | – | 0.25 (0.01 to 1.63) | 0.25 (0.01 to 1.63) |
| Edoxaban (5 mg od) | 0.54 (0.06 to 3.04) | – | 0.54 (0.06 to 3.04) |
| Edoxaban (15 mg od) | 1.40 (0.43 to 5.03) | – | 1.40 (0.43 to 5.03) |
| Edoxaban (30 mg od) | 1.42 (0.44 to 5.17) | – | 1.42 (0.44 to 5.17) |
| Edoxaban (60 mg od) | 1.77 (0.56 to 6.33) | – | 1.77 (0.56 to 6.33) |
| Edoxaban (90 mg od) | 2.13 (0.49 to 9.24) | – | 2.13 (0.49 to 9.24) |
| Rivaroxaban (2.5 mg bd) | 1.01 (0.31 to 3.18) | – | 1.01 (0.31 to 3.18) |
| Rivaroxaban (5 mg od) | 1.46 (0.43 to 4.16) | – | 1.46 (0.43 to 4.16) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Dabigatran (220 mg od) vs. apixaban (2.5 mg bd) | – | 1.47 (1.09 to 1.98) | 1.47 (1.09 to 1.98) |
| Rivaroxaban (10 mg od) vs. apixaban (2.5 mg bd) | – | 1.75 (1.40 to 2.20) | 1.75 (1.40 to 2.20) |
| Rivaroxaban (10 mg od) vs. dabigatran (220 mg od) | – | 1.19 (0.88 to 1.63) | 1.19 (0.88 to 1.63) |
| Imprecisely estimated comparisons | |||
| Edoxaban (30 mg od) vs. apixaban (2.5 mg bd) | – | 1.34 (0.41 to 5.00) | 1.34 (0.41 to 5.00) |
| Edoxaban (30 mg od) vs. dabigatran (220 mg od) | – | 0.92 (0.27 to 3.44) | 0.92 (0.27 to 3.44) |
| Rivaroxaban (10 mg od) vs. edoxaban (30 mg od) | – | 1.30 (0.35 to 4.32) | 1.30 (0.35 to 4.32) |
All-cause mortality
Twenty-four studies reported 1161 all-cause mortality events, leading to a network of 29 interventions (Figure 45). The studies were mostly judged to be at low risk of bias (Table 104), with some concerns about lack of blinding of participants and personnel.
FIGURE 45.
Network plot for all-cause mortality (primary prevention of VTE). a, Excluded interventions that were included in sensitivity analyses.
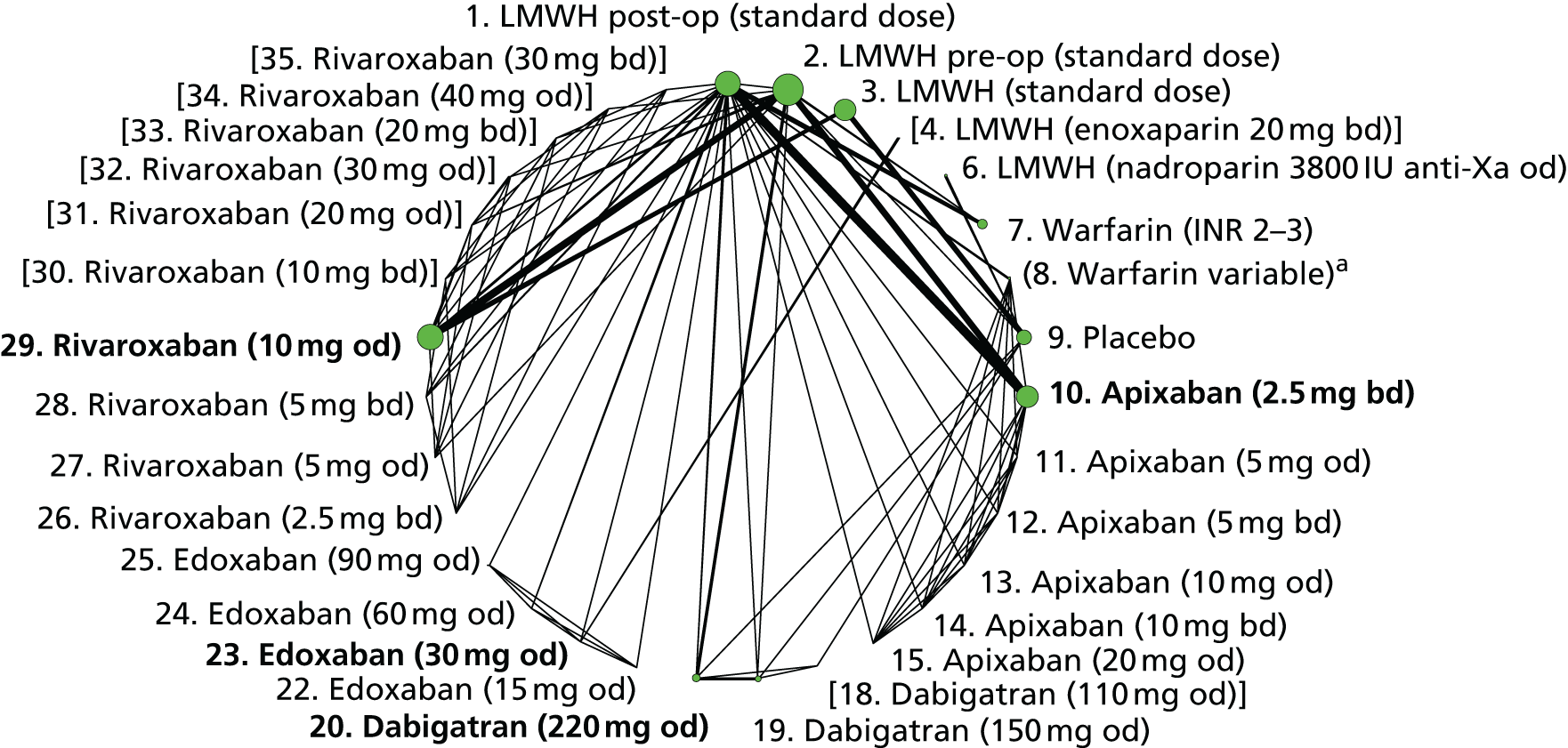
| Study | Interventions compared | SG | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| ADVANCE-1171 | 1, 10 | ? | ? | + | + | + | + |
| ADVANCE-2178 | 2, 10 | + | + | + | + | + | + |
| ADVANCE-3176 | 2, 10 | + | + | + | + | ? | + |
| APROPOS162 | 1, 7, 10, 11, 12, 13, 14, 15 | + | ? | ? | + | + | + |
| LIFENOX185 | 3, 9 | + | ? | + | + | + | + |
| MAGELLAN184,191 | 3, 29 | + | + | + | + | + | + |
| ODiXa-KNEE157 | 1, 26, 28, 30, 33, 35 | + | + | + | + | + | + |
| ODiXa-OD.HIP158 | 2, 27, 29, 31, 32, 34 | ? | ? | + | + | + | ? |
| PROTECHT170 | 6, 9 | + | + | + | + | + | + |
| RECORD 1165 | 2, 29 | + | + | ? | + | + | + |
| RECORD 2166 | 2, 29 | + | + | ? | + | + | + |
| RECORD 3163 | 2, 29 | ? | + | ? | + | + | + |
| RECORD 4173 | 1, 29 | + | + | ? | + | + | + |
| RE-MOBILISE167 | 1, 19, 20 | + | + | + | + | + | + |
| RE-MODEL161 | 2, 19, 20 | + | + | ? | ? | + | + |
| RE-NOVATE II183,189 | 2, 20 | + | + | + | ? | + | + |
| STARS J-4181,193 | 4, 23 | ? | ? | – | + | + | + |
| VTE-DABIG-PLAC-JAPAN175 | 9, 18, 19, 20 | + | ? | ? | ? | + | + |
| VTE-EDOX-LMWH-MULTI177 | 1, 22, 23, 24, 25 | + | + | + | + | + | + |
| VTE-LMWH-PLAC-CAN169 | 2, 9 | + | ? | ? | ? | + | ? |
| VTE-VKA-LMWH-CANADA152 | 1, 7 | + | ? | + | + | + | ? |
| VTE-VKA-LMWH-US-2154 | 1, 7 | ? | ? | – | ? | + | ? |
| VTE-VKA-LMWH-US-3153 | 1, 2, 7 | + | ? | + | + | + | ? |
| VTE-VKA-LMWH-US-4154 | 1, 7 | ? | + | – | + | + | ? |
Rates of all-cause mortality were substantially higher in studies of patients with cancer than in studies of surgical patients (see Table 73). There was little evidence that risk of all-cause mortality differed for any intervention compared with LMWH (post-op, standard dose) (Table 105). We observed statistical inconsistency between the direct and indirect estimates comparing apixaban (2.5 mg bd) with post-op LMWH (standard dose). The direct evidence indicated a reduction in bleeding with apixaban and the indirect evidence showed an increase. The combined estimate from the NMA suggested a small increase with OR 1.57 (95% CI 0.6 to 4.37). Comparisons between licensed doses of NOACs were imprecisely estimated (Table 106).
| Comparisons | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| LMWH pre-op (standard dose) | 2.00 (0.30 to 13.47) | 1.79 (0.86 to 3.74) | 1.82 (0.93 to 3.62) |
| LMWH (nadroparin 3800 IU anti-Xa od) | – | 1.06 (0.57 to 2.05) | 1.06 (0.57 to 2.05) |
| Warfarin (INR 2–3) | 1.44 (0.69 to 3.06) | – | 1.44 (0.69 to 3.06) |
| Placebo | 1.03 (0.88 to 1.20) | – | 1.03 (0.88 to 1.20) |
| Apixaban (2.5 mg bd) | 0.66 (0.18 to 2.29) | 6.29 (1.25 to 31.5) | 1.57 (0.6 to 4.37) |
| Rivaroxaban (10 mg od) | 1.06 (0.85 to 1.33) | 0.80 (0.35 to 1.83) | 1.04 (0.83 to 1.29) |
| Imprecisely estimated comparisons | |||
| Apixaban (5 mg od) | 0.41 (0 to 9.80) | – | 0.41 (0 to 9.80) |
| Apixaban (5 mg bd) | 0.37 (0 to 9.27) | – | 0.37 (0 to 9.27) |
| Apixaban (10 mg od) | 0.38 (0 to 9.42) | – | 0.38 (0 to 9.42) |
| Apixaban (10 mg bd) | 0.36 (0 to 8.91) | – | 0.36 (0 to 8.91) |
| Apixaban (20 mg od) | 0.36 (0 to 8.71) | – | 0.36 (0 to 8.71) |
| Dabigatran (150 mg od) | 1.49 (0.31 to 7.13) | – | 1.49 (0.31 to 7.13) |
| Dabigatran (220 mg od) | 1.04 (0.21 to 4.86) | – | 1.04 (0.21 to 4.86) |
| Edoxaban (15 mg od) | 4.37 (0.15 to 1610) | – | 4.37 (0.15 to 1610) |
| Edoxaban (30 mg od) | 13.6 (0.87 to 4510) | – | 13.6 (0.87 to 4510) |
| Edoxaban (60 mg od) | 0.88 (0 to 421) | – | 0.88 (0 to 421) |
| Edoxaban (90 mg od) | 0.93 (0 to 423) | – | 0.93 (0 to 423) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Rivaroxaban (10 mg od) vs. apixaban (2.5 mg bd) | – | 0.66 (0.23 to 1.76) | 0.66 (0.23 to 1.76) |
| Imprecisely estimated comparisons | |||
| Dabigatran (220 mg od) vs. apixaban (2.5 mg bd) | – | 0.66 (0.10 to 3.85) | 0.66 (0.10 to 3.85) |
| Edoxaban (30 mg od) vs. apixaban (2.5 mg bd) | – | 8.79 (0.44 to 3220) | 8.79 (0.44 to 3220) |
| Edoxaban (30 mg od) vs. dabigatran (220 mg od) | – | 13.8 (0.53 to 5360) | 13.8 (0.53 to 5360) |
| Rivaroxaban (10 mg od) vs. dabigatran (220 mg od) | – | 0.99 (0.21 to 4.95) | 0.99 (0.21 to 4.95) |
| Rivaroxaban (10 mg od) vs. edoxaban (30 mg od) | – | 0.08 (0 to 1.22) | 0.08 (0 to 1.22) |
Summary of results and ranking of interventions
Despite the substantial number of patients randomised to trials of primary prevention of VTE, low numbers of clinically relevant outcome events meant that most comparisons were imprecisely estimated. Conclusions can mainly be drawn from analyses of symptomatic VTE, major bleeding and CRB. There was evidence that risk of symptomatic VTE is lower with rivaroxaban (10 mg od) than LMWH (pre-op, standard dose) in hip surgery patients, but that risk of major bleeding and CRB is higher with rivaroxaban (10 mg od) than LMWH (post-op, standard dose).
We conducted sensitivity analyses merging warfarin interventions with variable INR range with those with INR range 2–3. Results, which are available from the authors up request, were similar to those presented above. With regard to model appraisal, we did not identify any instance of lack of convergence among the Markov chains or poor model fit. There were some instances of inconsistency between direct and indirect estimates of the same effect, although in most instances these results were accompanied by wide CIs. Few of the comparisons were replicated across studies; when there were multiple estimates we did not find evidence of statistical heterogeneity.
Because of the substantial imprecision in comparisons of efficacy outcomes, we present only one rankogram containing the bleeding and death outcomes for which all patients were jointly analysed (Figure 46). Warfarin was ranked with high probability as the best intervention for major bleeding events, and LMWH (post-op, standard dose) was ranked with high probability as best, or second-best, intervention for CRB. Rivaroxaban (10 mg od) was ranked among the worst interventions for bleeding outcomes.
FIGURE 46.
Rankogram for licensed interventions examined in primary prevention of VTE.
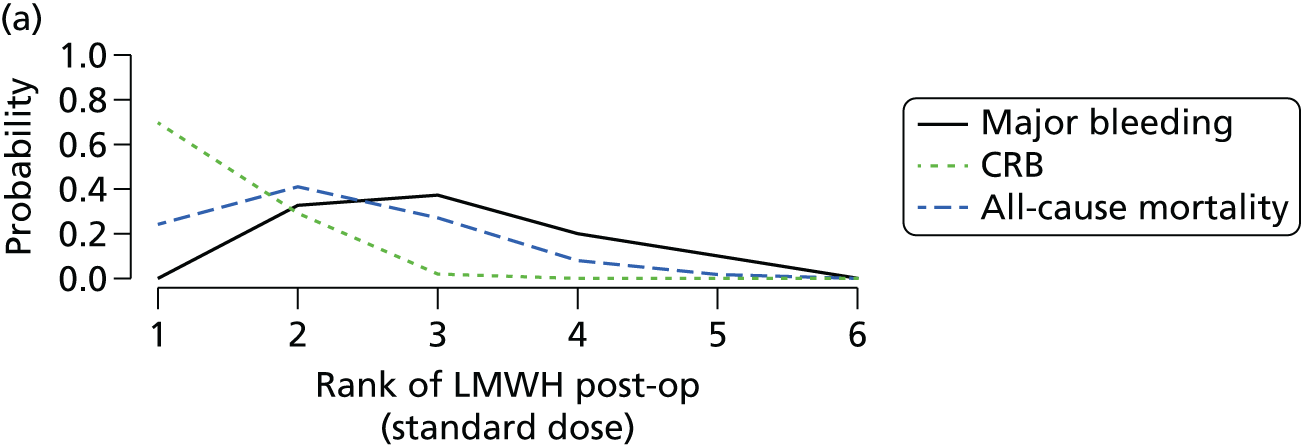



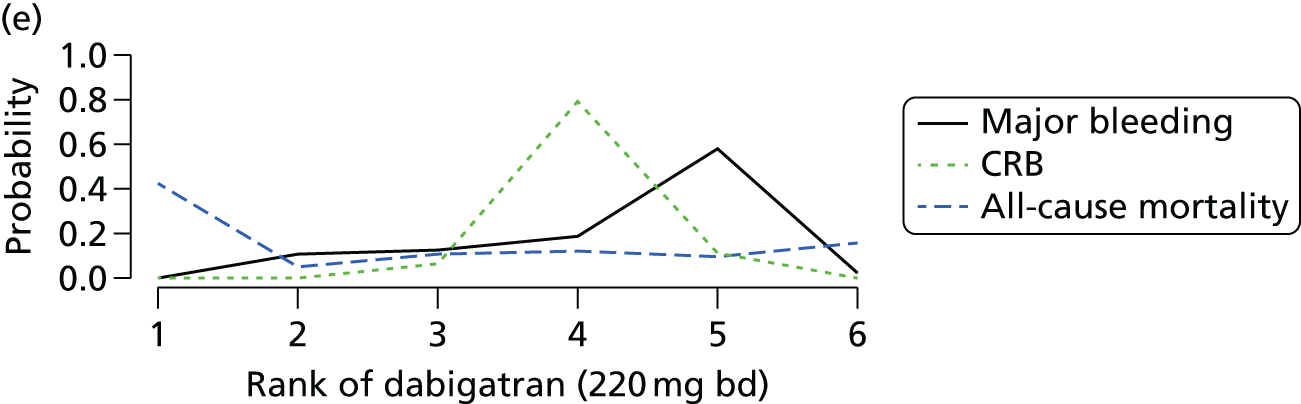

Chapter 8 Clinical results (3): acute treatment of venous thromboembolism
Included studies
Nine completed RCTs with 10 references202–211 were identified for inclusion in the review of acute treatment of VTE (see Figure 32), as well as one ongoing trial. 212 A summary of the characteristics of the nine included studies202–211 is presented in Table 107. All studies were multicentre and many were conducted across countries in North and South America, Europe, Asia, Australia, New Zealand, South Africa, Russia and Israel. Six were Phase III205–211 studies and three were Phase II202–204 studies. The number of patients randomised ranged from 520 to 8292, with a total of 28,803 patients across the nine studies. The Phase III studies examined edoxaban, apixaban, dabigatran and rivaroxaban, and these studies randomised 27,127 patients (94% of the total). The Phase II studies,202–204 which examined apixaban and rivaroxaban, contributed 1676 patients (6%).
| Study (centre type) [countries] | Study type, sponsor (sponsor’s role) | Age eligibility (mean age, years) [% male] | Clinical condition | No. randomised | Interventions compared | Treatment duration (weeks) | Outcomes | Time of outcome assessment (weeks) |
|---|---|---|---|---|---|---|---|---|
| AMPLIFY210 (Multicentre) [North and South America, Europe, Russia, Israel, Australia, Asia and South Africa] |
Phase III Pfizer and Bristol-Myers Squibb(The sponsors collected and maintained the data; the academic authors had full access to the data through the sponsors) |
≥ 18 (57) [58.7] | Acute objectively confirmed, symptomatic proximal DVT or PE (with or without DVT) | 5400 | Apixaban 1.5 mg bd Warfarin 2. INR 2–3 (Mean TTR: 61%) |
24 | Efficacy: Symptomatic VTE, symptomatic DVT, symptomatic non-fatal PE, fatal PE Safety: Major bleeding, CRNM bleeding, composite CRB, fatal bleeding, MI, death (all causes) |
24 |
| BOTTICELLI DVT204 (Multicentre) [USA, European, Israel, Australia and South Africa] |
Phase II Bristol-Myers Squibb (Not declared) |
≥ 18 (58.5) [62.1] | Acute symptomatic and objectively confirmed proximal DVT or extensive calf-vein thrombosis involving at least the upper third of the deep calf veins | 520 | Apixaban 1. 5 mg bd 2. 10 mg bd 3. 20 mg bd Warfarin 4. INR 2–3 (Mean TTR: 57%) |
12–13 | Efficacy: Symptomatic DVT, symptomatic PE Safety: Major bleeding, minor bleeding, CRNM bleeding, composite CRB, death (all causes) |
12–13 |
| EINSTEIN DVT206 (Multicentre) [North and South America, Europe, Israel, Australia, New Zealand, Asia and South Africa] |
Phase III Bayer Schering Pharma and Ortho-McNeil(The data were collected and maintained by the sponsor) |
≥ 18 (56.1) [56.8] | Acute, objectively confirmed proximal DVT without symptomatic PE | 3449 | Rivaroxaban 1. 15 mg bd (then 20 mg od) Warfarin 2. INR 2–3 (Mean TTR: 57.7%) |
12–48 | Efficacy: Symptomatic VTE, symptomatic DVT, fatal PE, symptomatic non-fatal PE Safety: Major bleeding, CRNM bleeding, composite CRB, MI, death (cardiovascular), death (all causes) |
12–48 |
| EINSTEIN DVT dose-ranging study203 (Multicentre) [North and South America, Europe, Israel, Australia and South Africa] |
Phase II Bayer HealthCare(The data were gathered and maintained by the sponsor) |
≥ 18 (58) [51.1] | Acute symptomatic and objectively confirmed DVT (proximal or isolated extensive calf vein thrombosis involving at least the upper one-third of the calf veins) | 543 | Rivaroxaban 1. 20 mg od 2. 30 mg od 3. 40 mg od Warfarin 4. INR 2–3 (Mean TTR: NR) |
12 | Efficacy: Symptomatic DVT, symptomatic non-fatal PE, symptomatic VTE Safety: All bleeding, major bleeding, CRNM bleeding, CRB, death (all causes) |
12 |
| EINSTEIN PE207 (Multicentre) [North and South America, Europe, Israel, Australia, New Zealand, Asia and South Africa] |
Phase III Bayer HealthCare and Janssen Pharmaceuticals(The data were collected and maintained by the sponsor) |
≥ 18 (57.7) [52.9] | Acute symptomatic PE, objectively confirmed, with or without DVT | 4833 | Rivaroxaban 1. 15 mg bd (then 20 mg od) Warfarin 2. INR 2–3 (Mean TTR: 62.7%) |
31 (mean) | Efficacy: Symptomatic VTE, Symptomatic DVT, fatal PE, symptomatic non-fatal PE Safety: Major bleeding, CRNM bleeding, composite CRB, death (all causes) |
12–48 |
| HOKUSAI-VTE208,209 (Multicentre) [North and South America, Europe, Russia, Israel, Australia, Asia and South Africa] |
Phase III Daiichi Sankyo (The sponsor was responsible for the collection and maintenance of the data) |
≥ 18 (55.8) [57.2] | Acute objectively confirmed, symptomatic DVT involving the popliteal, femoral, or iliac veins, or acute, symptomatic PE (with or without DVT) | 8292 | Edoxaban 1. 60 mg oda Warfarin 2. INR 2–3 (Mean TTR: 63.5%) |
12–48 | Efficacy: Symptomatic VTE, symptomatic DVT, symptomatic non-fatal PE, fatal PE Safety: All bleeding, major bleeding, fatal bleeding, CRNM bleeding, composite CRB, MI, death (all causes) |
48 |
| ODiXa-DVT202 (Multicentre) [Canada, South America, Europe, Israel, Australia, New Zealand and South Africa] |
Phase II Bayer HealthCare AG (The statistical analysis was performed by the sponsor) |
≥ 18 (59.1) [60.9] | Acute symptomatic and objectively confirmed thrombosis of the popliteal or more proximal veins, who have no symptoms of PE | 613 | Rivaroxaban 1. 10 mg bd 2. 20 mg bd 3. 30 mg bd 4. 40 mg od Warfarin 5. INR 2–3 (Mean TTR: 60%) |
12 | Efficacy: Symptomatic VTE, symptomatic DVT, symptomatic non-fatal PE Safety: All bleeding, major bleeding, minor bleeding |
12 |
| RE-COVER205 (Multicentre) [North and South America, Europe, Russia, Israel, Australia, New Zealand, India and South Africa] |
Phase III Boehringer Ingelheim (The study was funded, designed, conducted, and the data analysed by the sponsor in conjunction with the steering committee) |
≥ 18 (54.7) [58.4] | Acute, symptomatic, objectively confirmed proximal DVT of the legs or PE, and for whom 6 months of anticoagulant therapy was considered to be an appropriate treatment | 2564 | Dabigatran 1. 150 mg bd Warfarin 2. INR 2–3 (Mean TTR: 59.9%) |
24 | Efficacy: Symptomatic VTE, symptomatic DVT, symptomatic non-fatal PE Safety: All bleeding, major bleeding, composite CRB, MI, death (all causes) |
24 |
| RE-COVER II211 (Multicentre) [North and South America, Europe, Russia, Israel, Australia, New Zealand, Asia and South Africa] |
Phase III Boehringer Ingelheim (The study was funded, designed, conducted, and the data analysed, by the sponsor in conjunction with the steering committee) |
≥ 18 (54.9) [60.6] | Acute symptomatic unilateral or bilateral DVT of the leg involving proximal veins, and/or PE | 2589 | Dabigatran 1. 150 mg bd Warfarin 2. INR 2–3 (Mean TTR: 56.9%) |
24 | Efficacy: Symptomatic VTE, symptomatic DVT, symptomatic non-fatal PE Safety: All bleeding, major bleeding, CRNM bleeding, composite CRB, fatal bleeding, MI, death (all causes) |
24 |
Eligibility criteria were similar across studies: all patients had acute symptomatic and objectively confirmed DVT and/or PE. The mean ages of included patients were similar, ranging from 54.7 to 59.1 years. The percentage of males across studies ranged from 51% to 62%. Mean BMI was reported by four studies,202,203,205,211 ranged from 27 to 28.9 kg/m2, and was comparable between study arms. Five studies203,204,209–211 reported percentages of cancer cases, which were comparable between study arms and ranged from 2% to 12%.
All of the studies compared a NOAC with standard intensity warfarin (INR 2–3): mean TTR ranged from 50.3% to 62.7%. Of the studies that examined rivaroxaban, two Phase III studies administered 15 mg bd and two Phase II studies examined six dosing strategies. Two studies examined apixaban: one Phase III study administered 5 mg bd and one Phase II study compared this with two alternative dosing strategies. Two Phase III studies examined dabigatran 150 mg bd; and one Phase III study examined edoxaban 60 mg od.
Treatment duration ranged from 12 to 48 weeks in the rivaroxaban studies, from 12 to 24 weeks in the apixaban studies, from 12 to 48 weeks in the edoxaban study and was 24 weeks in the dabigatran studies. Reported efficacy and safety outcome types were similar across studies and reported at the end of the treatment periods. All nine studies202–211 reported symptomatic DVT, symptomatic PE and major bleeding. Eight studies reported all-cause mortality and CRB, seven reported symptomatic VTE and five reported MI. Each of the studies was sponsored by one or more pharmaceutical companies. In almost all studies the sponsor(s) was responsible for the study design and data collection, and in some cases data analysis.
Time in therapeutic range for warfarin interventions
Table 108 shows the comparator interventions, target INR and (where reported) mean TTR for the nine studies202–211 that included a warfarin intervention arm. Eight202,204–211 (89%) of these studies reported mean TTR, which varied between 56.9% and 63.5%.
| Study | Interventions that were compared with warfarin | Warfarin INR | Mean time (%) in therapeutic range (INR) |
|---|---|---|---|
| AMPLIFY210 | Apixaban 5 mg bd | 2–3 | 61 |
| BOTTICELLI DVT204 | Apixaban 5 mg, 10 mg, 20 mg bd | 2–3 | 57 |
| EINSTEIN DVT206 | Rivaroxaban 15 mg bd (then 20 mg od) | 2–3 | 57.7 |
| EINSTEIN DVT dose-ranging study203 | Rivaroxaban 20 mg, 30 mg, 40 mg od | 2–3 | NR |
| EINSTEIN PE207 | Rivaroxaban 15 mg bd (then 20 mg od) | 2–3 | 62.7 |
| HOKUSAI-VTE208,209 | Edoxaban 60 mg od | 2–3 | 63.5 |
| ODiXa-DVT202 | Rivaroxaban 10 mg, 20 mg, 30 mg bd, 40 mg od | 2–3 | 60 |
| RE-COVER205 | Dabigatran 150 mg bd | 2–3 | 59.9 |
| RE-COVER II211 | Dabigatran 150 mg bd | 2–3 | 56.9 |
Risk of bias in included studies
Table 109 shows the detailed risk-of-bias assessments for each included study for each domain. Generally, the studies were judged to be at low risk of bias. The randomisation sequence was predominantly computer generated. The studies were judged to be at low risk of bias for sequence generation, blinding of outcome assessment and selective reporting, although one study did not explain how randomisation was performed, stating only that a veiled randomisation process was carried out. In all studies, concealed allocation to intervention arms was achieved through central allocation, either an interactive voice or a web-based system. Five studies were of open-label design and, as such, were judged to be of high risk of bias for blinding of participants and personnel. Completeness of the data analysed depended – in a few studies – on whether the outcome was for efficacy or safety. For the majority of outcomes all patients were accounted for in the analysis or, in some situations, a small number of patients were not included in the analysis but reasons were provided and judged to be similar across intervention arms and unlikely to be related to the outcome. These studies were therefore judged to be at low risk of bias as a result of incomplete outcome data. In one study the reasons for not including some patients in the efficacy analyses were judged to be similar across study arms but were judged to be potentially related to the outcome, and the study was therefore considered at high risk of bias for the domain. Outcomes were reported as stated in the protocols in all studies, which were therefore judged to be at low risk of bias because of selective reporting. Risk-of-bias judgements for studies contributing to analyses of each outcome are presented graphically in the sections that follow.
| Study | Sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting |
|---|---|---|---|---|---|---|
| AMPLIFY 210 | L: ‘Randomisation was performed with the use of an interactive voice-response system’ | L: ‘Randomisation was performed with the use of an interactive voice-response system’ | L: ‘Patients were assigned to receive apixaban tablets plus placebo enoxaparin injections and placebo warfarin tablets or conventional therapy with enoxaparin injections and warfarin tablets plus placebo apixaban tablets’ | L: ‘An independent committee, whose members were unaware of the study-group assignments, adjudicated the qualifying diagnosis, the anatomical extent of the initial DVT or PE, and all suspected outcomes’ | U: For efficacy and safety outcomes except symptomatic DVT: There are missing outcome data with reasons. Although missing outcome data seem to be balanced in numbers across intervention groups, it is not quite clear whether or not the reasons could be related to true outcome | L: Outcomes reported as stated in the study protocol |
| L: For symptomatic DVT: All patients were included in the analyses | ||||||
| BOTTICELLI DVT 204 | U: ‘The Botticelli study was a veiled randomised, parallel group dose-ranging study’ | L: ‘An interactive voice response system was used for randomisation. The study was conducted according to current methodological standards; that is, consecutive patients were centrally randomised’ | H: ‘The Botticelli study was a veiled randomised, parallel group dose-ranging study that was double-blind for the different doses of apixaban and open-label for the LMWH/VKA comparator’ | L: ‘All potential study outcomes were assessed by an independent committee, whose members were unaware of treatment assignment’ | L: There are missing data; however numbers missing in each arm are almost the same; also reasons for missing data unlikely to be related to the outcomes | L: Outcomes reported according to study protocol |
| EINSTEIN DVT 206 | L: ‘Patients were randomly assigned to a study group with the use of a computerised voice–response system, with stratification by country’ | L: ‘Patients were randomly assigned to a study group with the use of a computerised voice–response system’ | H: ‘The Acute DVT Study was a randomised, open-label study’ | L: ‘All suspected outcome events were classified by a central adjudication committee whose members were unaware of the treatment assignments’ | L: Few missing data with reasons and number of missing data similar in the two groups; reasons for missing data unlikely to be related to true outcome. Analysis by intension to treat | L: Outcomes reported according to study protocol |
| EINSTEIN DVT dose-ranging study 203 | L: ‘Patients were randomised, via an interactive voice response system’ | L: ‘Patients were randomised, via an interactive voice response system’ – a central allocation system | H: ‘The Einstein–DVT study was a randomised, dose-ranging study that was double-blind for rivaroxaban doses and open-label for the LMWH/VKA’ | L: ‘An independent adjudication committee, unaware of treatment allocation, evaluated all suspected thromboembolic complications, deaths, baseline and repeat ultrasound and perfusion lung scans, as well as all episodes of suspected bleeding’ | H: For efficacy outcomes: Missing data but with reasons. Reasons are similar across all rivaroxaban arms. Numbers are similar across rivaroxaban arm but differ significantly when each is compared with the comparator arm. Reasons for missing data may be related to the outcome | L: Outcomes reported according to study protocol |
| L: For safety outcomes: All patients were included in the analyses | ||||||
| EINSTEIN PE 207 | L: ‘Randomisation was performed with the use of a computerised voice-response system’ | L: ‘Randomisation was performed with the use of a computerised voice-response system’ | H: ‘The EINSTEIN–PE study was a randomised, open-label trial’ | L: ‘All events were adjudicated and confirmed by a central independent adjudication committee blinded to treatment’ | L: Few missing data; missing data are balanced in numbers across groups. Reasons for missing data given, unlikely to be related to true outcome | L: Outcomes reported according to study protocol |
| HOKUSAI-VTE 208 , 209 | L: ‘Randomisation was performed with the use of an interactive Web-based system’ | L: ‘Randomisation was performed with the use of an interactive Web-based system’ – a central allocation | L: ‘Edoxaban or warfarin was administered in a double-blind, double-dummy fashion’ | L: ‘An independent committee, whose members were unaware of the study-group assignments, adjudicated all suspected outcomes’ | L: Small numbers of missing data; however, balanced in the treatment groups. Reason for missing data are unlikely to be related to the true outcome. Data analysis was by intention to treat | L: Outcomes reported as stated in the study protocol |
| ODiXa-DVT 202 | L: ‘The ODIXa-DVT study was a multinational, multicentre, partially blinded, parallel-group study in which patients were randomised by central computer’ | L: ‘The ODIXa-DVT study was a multinational, multicentre, partially blinded, parallel-group study in which patients were randomised by central computer’ – a Central allocation system | H: ‘The ODIXa-DVT study was a multinational, multicentre, partially blinded, parallel-group study’ ‘Patients in the oral rivaroxaban treatment groups received double blinded doses of 10, 20, or 30 mg bd (BID) or 40 mg od, with food, for 12 weeks. Patients in the open-label, standard-anticoagulant group received enoxaparin 1 mg/kg BID by subcutaneous injection and a VKA’ | L: ‘All clinically suspected VTE, bleeding events, deaths, and paired perfusion lung scans were adjudicated, without knowledge of the treatment group, by an independent central adjudication committee’ | L: All patients were included in the analyses | L: Outcomes reported according to study protocol |
| RE-COVER 205 | L: ‘We used a computer generated randomisation scheme with variable block sizes, stratified according to presentation’ | L: ‘Staff members at the clinical centres called an interactive voice-response system that randomly assigned subjects to one of the supplied medication kits’ – a central allocation system | L: ‘Active dabigatran and warfarin- like placebo or active warfarin and dabigatran- like placebo were then given for 6 months (‘double-dummy phase’)’ | L: ‘All suspected outcome events and deaths were classified by central adjudication committees, whose members were unaware of the treatment assignments’ | L: All patients were included in the analyses | L: Outcomes reported according to study protocol |
| RE-COVER II 211 | L: ‘Patients were randomised using an interactive voice response system and a computer generated randomisation scheme in blocks of 4’ | L: ‘Patients were randomised using an interactive voice response system’ | L: ‘Patients were assigned in a 1:1 ratio to receive active fixed dose dabigatran 150 mg bd and warfarin-like placebo, or active warfarin and dabigatran-like placebo’ | L: ‘All suspected outcome events and deaths were classified by central adjudication committees, whose members were unaware of the treatment assignments’ | L: All patients were included in the analyses | L: Outcomes reported as stated in the study protocol |
Results of clinical effectiveness and safety
The nine trials of acute treatment for VTE examined 13 distinct interventions (Table 110). Tables 111 and 112 show the number of outcome events for each outcome as reported in each trial. We performed NMAs for seven outcomes: symptomatic DVT, symptomatic PE, symptomatic VTE, MI, major bleeding, CRB and all-cause mortality.
| 1. | Warfarin (INR 2–3) |
| 2. | Apixaban (5 mg bd) |
| 3. | Apixaban (10 mg bd) |
| 4. | Apixaban (20 mg od) |
| 5. | Dabigatran (150 mg bd) |
| 6. | Edoxaban [60 or 30 (17.6%) mg od]a |
| 7. | Rivaroxaban (10 mg bd) |
| 8. | Rivaroxaban (20 mg od) |
| 9. | Rivaroxaban (15 mg bd then 20 mg od) |
| 10. | Rivaroxaban (30 mg od) |
| 11. | Rivaroxaban (20 mg bd) |
| 12. | Rivaroxaban (40 mg od) |
| 13. | Rivaroxaban (30 mg bd) |
| Study | Study size | Symptomatic DVT | Symptomatic proximal DVT | Symptomatic PE | Fatal PE | Symptomatic non-fatal PE | Symptomatic VTE | Cardiovascular deaths | All-cause mortality |
|---|---|---|---|---|---|---|---|---|---|
| AMPLIFY210 | 5365 | 53 | 3 | 50 | 130 | 10 | 93 | ||
| BOTTICELLI DVT204 | 511 | 10 | 1 | 5 | |||||
| EINSTEIN DVT206 | 3429 | 42 | 1 | 38 | 87 | 6 | 87 | ||
| EINSTEIN DVT dose-ranging study203 | 542 | 10 | 0 | 3 | 16 | 19 | |||
| EINSTEIN PE207 | 4817 | 35 | 3 | 41 | 94 | 108 | |||
| HOKUSAI-VTE208,209 | 8240 | 120 | 7 | 108 | 276 | 27 | 258 | ||
| ODiXa-DVT202 | 543 | 5 | 6 | 2 | 3 | 10 | |||
| RE-COVER205 | 2539 | 34 | 20 | 57 | 42 | ||||
| RE-COVER II211 | 2568 | 42 | 20 | 58 | 50 |
| Study | Study size | MI | All bleeding | Minor bleeding | Major bleeding | Fatal bleeding | Intracranial bleeding | CRNM bleeding | CRB |
|---|---|---|---|---|---|---|---|---|---|
| AMPLIFY210 | 5365 | 6 | 1110 | 64 | 3 | 9 | 318 | 376 | |
| BOTTICELLI DVT204 | 511 | 29 | 3 | 0 | 35 | 38 | |||
| EINSTEIN DVT206 | 3429 | 6 | 34 | 4 | 245 | 277 | |||
| EINSTEIN DVT dose-ranging study203 | 542 | 127 | 5 | 1 | 26 | 31 | |||
| EINSTEIN PE207 | 4817 | 78 | 14 | 463 | 523 | ||||
| HOKUSAI-VTE208,209 | 8240 | 33 | 23 | ||||||
| ODiXa-DVT202 | 543 | 52 | 44 | 10 | 0 | ||||
| RE-COVER205 | 2539 | 6 | 482 | 44 | 2 | 3 | 182 | ||
| RE-COVER II211 | 2568 | 6 | 485 | 37 | 1 | 4 | 129 | 166 |
Results are presented as follows for each of the seven outcomes. First, we provide network plots to illustrate the comparisons of interventions made in the different trials. Second, we illustrate the risk-of-bias assessments specific to the outcome for each trial included in the network. Third, we present results tables for each intervention compared with the reference treatment (warfarin with a target INR range of 2–3). Fourth, we present results tables for pairwise comparisons among licensed doses of the NOACs. For both sets of results tables, posterior median ORs and 95% credible intervals from Bayesian fixed-effects analyses are shown, although we refer to the latter as CIs for convenience. In these tables we present results separately for any available direct evidence, for any indirect comparisons that can be made (excluding the direct evidence) and for the NMA (which combines the direct and the indirect evidence). Comparisons from the NMA with a ratio between interval limits exceeding nine were considered ‘imprecisely estimated’ and are presented at the bottom of each table (note that calculation of indirect evidence was not undertaken for imprecisely estimated comparisons). A summary of results across outcomes is provided at the end in the form of a ‘rankogram’, which illustrates the probability that each treatment is best, second best, and so on, for each outcome. Last, forest plots of all contributing data, with ORs calculated using standard frequentist methods, are included in Appendix 4.
Symptomatic venous thromboembolism
Eight studies reported 728 symptomatic VTE events, leading to a network of 11 interventions (Figure 47). Table 113 shows risk-of-bias judgements for these studies. They were mostly judged to be at low risk of bias, although there were some concerns about lack of blinding of participants and personnel. There was little evidence that risk of symptomatic VTE differed for any of the NOAC interventions compared with warfarin (INR 2–3) (Table 114). Neither was there evidence that risk of symptomatic VTE differed between licensed doses of NOACs (Table 115).
FIGURE 47.
Network plot for symptomatic VTE (acute treatment of VTE).

| Study | Interventions compared | SG | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| AMPLIFY210 | 1, 2 | + | + | + | + | ? | + |
| EINSTEIN DVT206 | 1, 9 | + | + | − | + | + | + |
| EINSTEIN DVT dose-ranging study203 | 1, 8, 10, 12 | + | + | − | + | − | + |
| EINSTEIN PE207 | 1, 9 | + | + | − | + | + | + |
| HOKUSAI-VTE208,209 | 1, 2 | + | + | + | + | + | + |
| ODiXa-DVT202 | 1, 7, 11, 12, 13 | + | + | ? | + | + | + |
| RE-COVER205 | 1, 5 | + | + | + | + | + | + |
| RE-COVER II211 | 1, 5 | + | + | + | + | + | + |
| Comparisons | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| With warfarin (INR 2–3) | |||
| Apixaban (5 mg bd) | 0.83 (0.58 to 1.18) | – | 0.83 (0.58 to 1.18) |
| Dabigatran (150 mg bd) | 1.09 (0.75 to 1.58) | – | 1.09 (0.75 to 1.58) |
| Edoxaban [60 or 30 (17.6%) mg od] | 0.89 (0.70 to 1.13) | – | 0.89 (0.70 to 1.13) |
| Rivaroxaban (15 mg bd then 20 mg od) | 0.90 (0.67 to 1.20) | – | 0.90 (0.67 to 1.20) |
| Imprecisely estimated comparisons | |||
| Rivaroxaban (10 mg bd) | 0.77 (0.09 to 4.53) | – | 0.77 (0.09 to 4.53) |
| Rivaroxaban (20 mg od) | 0.44 (0.09 to 1.76) | – | 0.44 (0.09 to 1.76) |
| Rivaroxaban (30 mg od) | 0.63 (0.15 to 2.29) | – | 0.63 (0.15 to 2.29) |
| Rivaroxaban (20 mg bd) | 0.81 (0.09 to 4.81) | – | 0.81 (0.09 to 4.81) |
| Rivaroxaban (40 mg od) | 0.52 (0.15 to 1.65) | – | 0.52 (0.15 to 1.65) |
| Rivaroxaban (30 mg bd) | 0.73 (0.09 to 4.42) | – | 0.73 (0.09 to 4.42) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | – | 1.31 (0.79 to 2.19) | 1.31 (0.79 to 2.19) |
| Edoxaban [60 or 30 (17.6%) mg od] vs. apixaban (5 mg bd) | – | 1.06 (0.70 to 1.63) | 1.06 (0.70 to 1.63) |
| Rivaroxaban (15 mg bd then 20 mg od) vs. apixaban (5 mg bd) | – | 1.08 (0.68 to 1.71) | 1.08 (0.68 to 1.71) |
| Edoxaban [60 or 30 (17.6%) mg od] vs. dabigatran (150 mg bd) | – | 0.81 (0.52 to 1.27) | 0.81 (0.52 to 1.27) |
| Rivaroxaban (15 mg bd then 20 mg od) vs. dabigatran (150 mg bd) | – | 0.82 (0.51 to 1.33) | 0.82 (0.51 to 1.33) |
| Rivaroxaban (15 mg bd then 20 mg od) vs. edoxaban [60 or 30 (17.6%) mg od] | – | 1.01 (0.69 to 1.48) | 1.01 (0.69 to 1.48) |
Symptomatic deep-vein thrombosis
Nine studies reported 351 symptomatic DVT events, leading to a network of 13 interventions (Figure 48). The studies were mostly judged to be at low risk of bias (Table 116), with some concerns about lack of blinding of participants and personnel. There was little evidence that risk of symptomatic DVT differed for any of the NOAC interventions compared with warfarin (INR 2–3) (Table 117). Neither was there evidence that risk of symptomatic VTE differed between licensed doses of NOACs (Table 118).
FIGURE 48.
Network plot for symptomatic DVT (acute treatment of VTE).
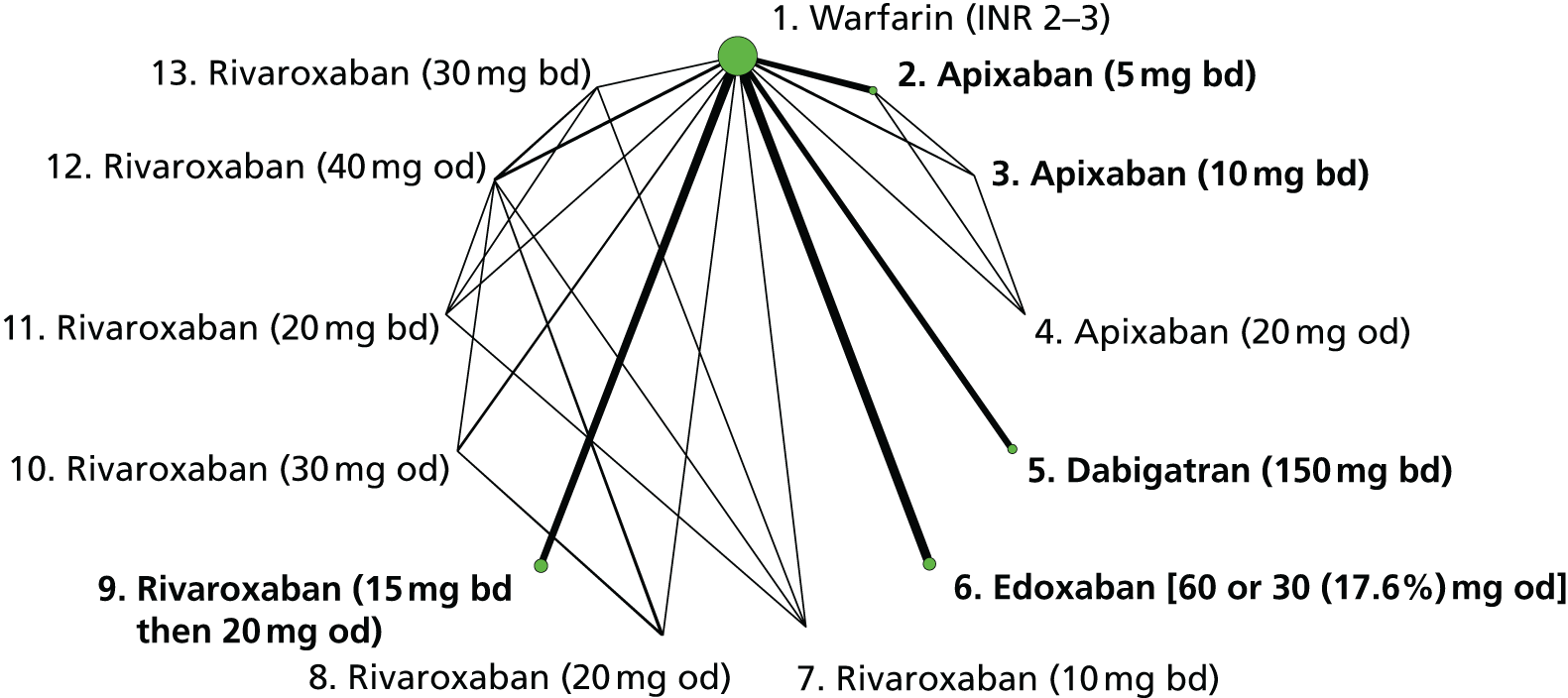
| Study | Interventions compared | SG | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| AMPLIFY210 | 1, 2 | + | + | + | + | + | + |
| BOTTICELLI DVT204 | 1, 2, 3, 4 | ? | + | – | + | + | + |
| EINSTEIN DVT206 | 1, 9 | + | + | – | + | + | + |
| EINSTEIN DVT dose-ranging study203 | 1, 8, 10, 12 | + | + | – | + | – | + |
| EINSTEIN PE207 | 1, 9 | + | + | – | + | + | + |
| HOKUSAI-VTE206,207 | 1, 2 | + | + | + | + | + | + |
| ODiXa-DVT202 | 1, 7, 11, 12, 13 | + | + | ? | + | + | + |
| RE-COVER205 | 1, 5 | + | + | + | + | + | + |
| RE-COVER II211 | 1, 5 | + | + | + | + | + | + |
| Comparisons with warfarin (INR 2–3) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Apixaban (5 mg bd) | 0.66 (0.38 to 1.11) | – | 0.66 (0.38 to 1.11) |
| Dabigatran (150 mg bd) | 1.18 (0.75 to 1.86) | – | 1.18 (0.75 to 1.86) |
| Edoxaban [60 or 30 (17.6%) mg od] | 0.91 (0.63 to 1.30) | – | 0.91 (0.63 to 1.30) |
| Rivaroxaban (15 mg bd then 20 mg od) | 0.70 (0.44 to 1.10) | – | 0.70 (0.44 to 1.10) |
| Imprecisely estimated comparisons | |||
| Apixaban (10 mg bd) | 1.27 (0.29 to 5.11) | – | 1.27 (0.29 to 5.11) |
| Apixaban (20 mg od) | 0.25 (0.01 to 1.87) | – | 0.25 (0.01 to 1.87) |
| Rivaroxaban (10 mg bd) | 0.56 (0.02 to 7.51) | – | 0.56 (0.02 to 7.51) |
| Rivaroxaban (20 mg od) | 0.28 (0.03 to 1.33) | – | 0.28 (0.03 to 1.33) |
| Rivaroxaban (30 mg od) | 0.12 (0 to 0.86) | – | 0.12 (0 to 0.86) |
| Rivaroxaban (20 mg bd) | 0.59 (0.02 to 8.08) | – | 0.59 (0.02 to 8.08) |
| Rivaroxaban (40 mg od) | 0.21 (0.03 to 0.94) | – | 0.21 (0.03 to 0.94) |
| Rivaroxaban (30 mg bd) | 0.53 (0.02 to 7.27) | – | 0.53 (0.02 to 7.27) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | – | 1.80 (0.90 to 3.64) | 1.80 (0.90 to 3.64) |
| Edoxaban [60 or 30 (17.6%) mg od] vs. apixaban (5 mg bd) | – | 1.38 (0.73 to 2.65) | 1.38 (0.73 to 2.65) |
| Rivaroxaban (15 mg bd then 20 mg od) vs. apixaban (5 mg bd) | – | 1.07 (0.53 to 2.18) | 1.07 (0.53 to 2.18) |
| Edoxaban [60 or 30 (17.6%) mg od] vs. dabigatran (150 mg bd) | – | 0.77 (0.43 to 1.38) | 0.77 (0.43 to 1.38) |
| Rivaroxaban (15 mg bd then 20 mg od) vs. dabigatran (150 mg bd) | – | 0.60 (0.31 to 1.13) | 0.60 (0.31 to 1.13) |
| Rivaroxaban (15 mg bd then 20 mg od) vs. edoxaban [60 or 30 (17.6%) mg od] | – | 0.77 (0.43 to 1.39) | 0.77 (0.43 to 1.39) |
| Imprecisely estimated comparisons | |||
| Apixaban (10 mg bd) vs. apixaban (5 mg bd) | 1.94 (0.44 to 7.95) | – | 1.94 (0.44 to 7.95) |
| Dabigatran (150 mg bd) vs. apixaban (10 mg bd) | – | 0.93 (0.21 to 4.36) | 0.93 (0.21 to 4.36) |
| Edoxaban [60 or 30 (17.6%) mg od] vs. apixaban (10 mg bd) | – | 0.71 (0.17 to 3.27) | 0.71 (0.17 to 3.27) |
| Rivaroxaban (15 mg bd then 20 mg od) vs. apixaban (10 mg bd) | – | 0.55 (0.13 to 2.60) | 0.55 (0.13 to 2.60) |
Symptomatic pulmonary embolism
One study reported direct data on symptomatic PE events (see Table 111), whereas for the remaining eight studies, we derived symptomatic PE events by adding fatal PE and symptomatic non-fatal PE events, leading to a total of 300 symptomatic PE events across the network, which is displayed in Figure 49. The studies were mostly judged to be at low risk of bias (Table 119), with some concerns about lack of blinding of participants and personnel. There was little evidence that risk of symptomatic PE differed for any of the NOAC interventions compared with warfarin (INR 2–3) (Table 120). Neither was there evidence that risk of symptomatic PE differed between licensed doses of NOACs (Table 121).
FIGURE 49.
Network plot for symptomatic PE (acute treatment of VTE).
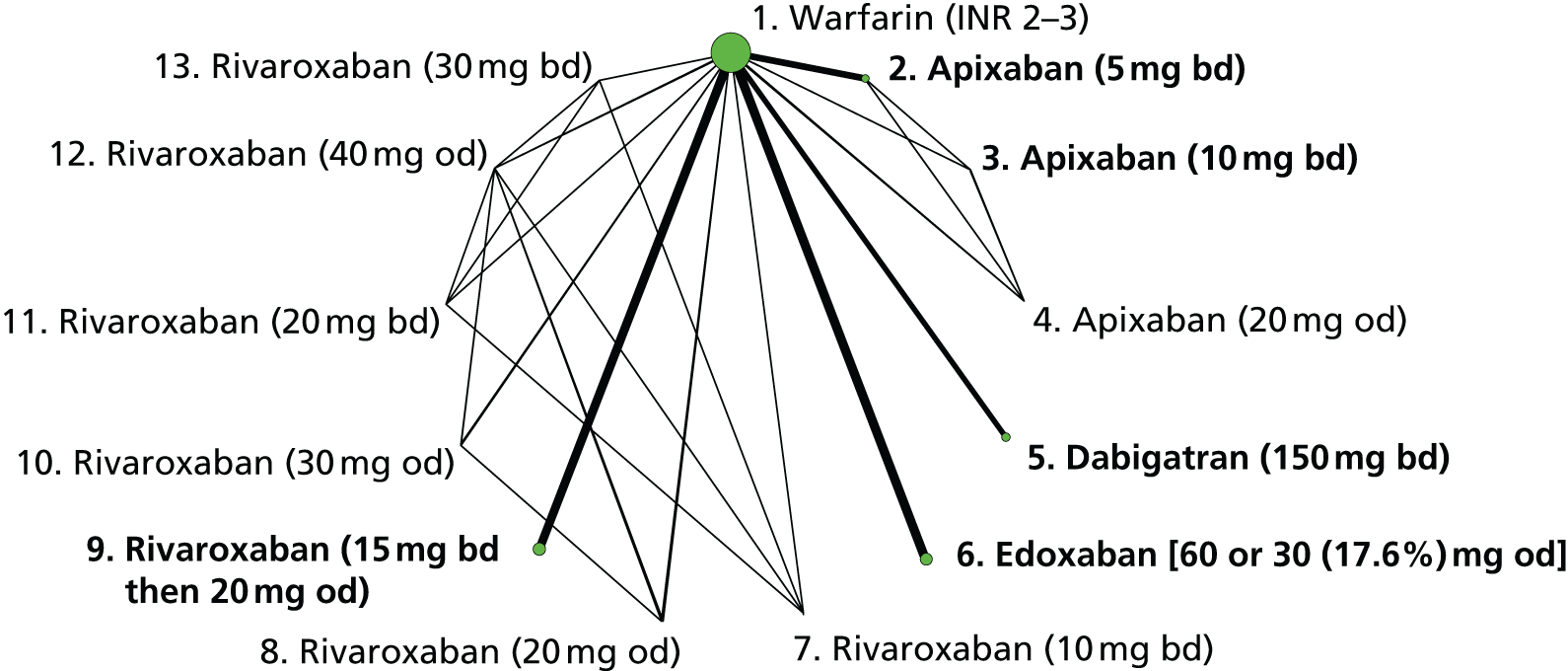
| Study | Interventions compared | SG | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| AMPLIFY210 | 1, 2 | + | + | + | + | ? | + |
| BOTTICELLI DVT204 | 1, 2, 3, 4 | ? | + | – | + | + | + |
| EINSTEIN DVT206 | 1, 9 | + | + | – | + | + | + |
| EINSTEIN DVT dose-ranging study203 | 1, 8, 10, 12 | + | + | – | + | – | + |
| EINSTEIN PE207 | 1, 9 | + | + | – | + | + | + |
| HOKUSAI-VTE206,207 | 1, 2 | + | + | + | + | + | + |
| ODiXa-DVT202 | 1, 7, 11, 12, 13 | + | + | ? | + | + | + |
| RE-COVER205 | 1, 5 | + | + | + | + | + | + |
| RE-COVER II211 | 1, 5 | + | + | + | + | + | + |
| Comparisons with warfarin (INR 2–3) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Apixaban (5 mg bd) | 1.09 (0.64 to 1.87) | – | 1.09 (0.64 to 1.87) |
| Dabigatran (150 mg bd) | 1.00 (0.53 to 1.89) | – | 1.00 (0.53 to 1.89) |
| Edoxaban [60 or 30 (17.6%) mg od] | 0.85 (0.59 to 1.23) | – | 0.85 (0.59 to 1.23) |
| Rivaroxaban (15 mg bd then 20 mg od) | 1.18 (0.77 to 1.83) | – | 1.18 (0.77 to 1.83) |
| Imprecisely estimated comparisons | |||
| Apixaban (10 mg bd) | 0.28 (0 to 6.40) | – | 0.28 (0 to 6.40) |
| Apixaban (20 mg od) | 0.29 (0 to 6.53) | – | 0.29 (0 to 6.53) |
| Rivaroxaban (10 mg bd) | 0.73 (0.02 to 11.6) | – | 0.73 (0.02 to 11.6) |
| Rivaroxaban (20 mg od) | 1.10 (0.07 to 14.9) | – | 1.10 (0.07 to 14.9) |
| Rivaroxaban (30 mg od) | 1.12 (0.07 to 15.6) | – | 1.12 (0.07 to 15.6) |
| Rivaroxaban (20 mg bd) | 0.78 (0.02 to 12.2) | – | 0.78 (0.02 to 12.2) |
| Rivaroxaban (40 mg od) | 0.49 (0.04 to 4.19) | – | 0.49 (0.04 to 4.19) |
| Rivaroxaban (30 mg bd) | 0.69 (0.02 to 11.3) | – | 0.69 (0.02 to 11.3) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | – | 0.92 (0.40 to 2.09) | 0.92 (0.40 to 2.09) |
| Edoxaban [60 or 30 (17.6%) mg od] vs. apixaban (5 mg bd) | – | 0.78 (0.41 to 1.49) | 0.78 (0.41 to 1.49) |
| Edoxaban [60 or 30 (17.6%) mg od] vs. dabigatran (150 mg bd) | – | 0.85 (0.41 to 1.77) | 0.85 (0.41 to 1.77) |
| Rivaroxaban (15 mg bd then 20 mg od) vs. apixaban (5 mg bd) | – | 1.09 (0.54 to 2.16) | 1.09 (0.54 to 2.16) |
| Rivaroxaban (15 mg bd then 20 mg od) vs. dabigatran (150 mg bd) | – | 1.18 (0.55 to 2.54) | 1.18 (0.55 to 2.54) |
| Rivaroxaban (15 mg bd then 20 mg od) vs. edoxaban [60 or 30 (17.6%) mg od] | – | 1.39 (0.79 to 2.46) | 1.39 (0.79 to 2.46) |
| Imprecisely estimated comparisons | |||
| Apixaban (10 mg bd) vs. apixaban (5 mg bd) | 0.25 (0 to 5.86) | – | 0.25 (0 to 5.86) |
| Dabigatran (150 mg bd) vs. apixaban (10 mg bd) | – | 3.66 (0.15 to 1860) | 3.66 (0.15 to 1860) |
| Edoxaban [60 or 30 (17.6%) mg od] vs. apixaban (10 mg bd) | – | 3.10 (0.13 to 1530) | 3.10 (0.13 to 1530) |
| Rivaroxaban (15 mg bd then 20 mg od) vs. apixaban (10 mg bd) | – | 4.32 (0.18 to 2160) | 4.32 (0.18 to 2160) |
Myocardial infarction
Five studies reported 57 MI events, leading to a network of five interventions (Figure 50). These studies were judged to be at low risk of bias (Table 122). All comparisons were imprecisely estimated (Tables 123 and 124).
FIGURE 50.
Network plot for MI (acute treatment of VTE).
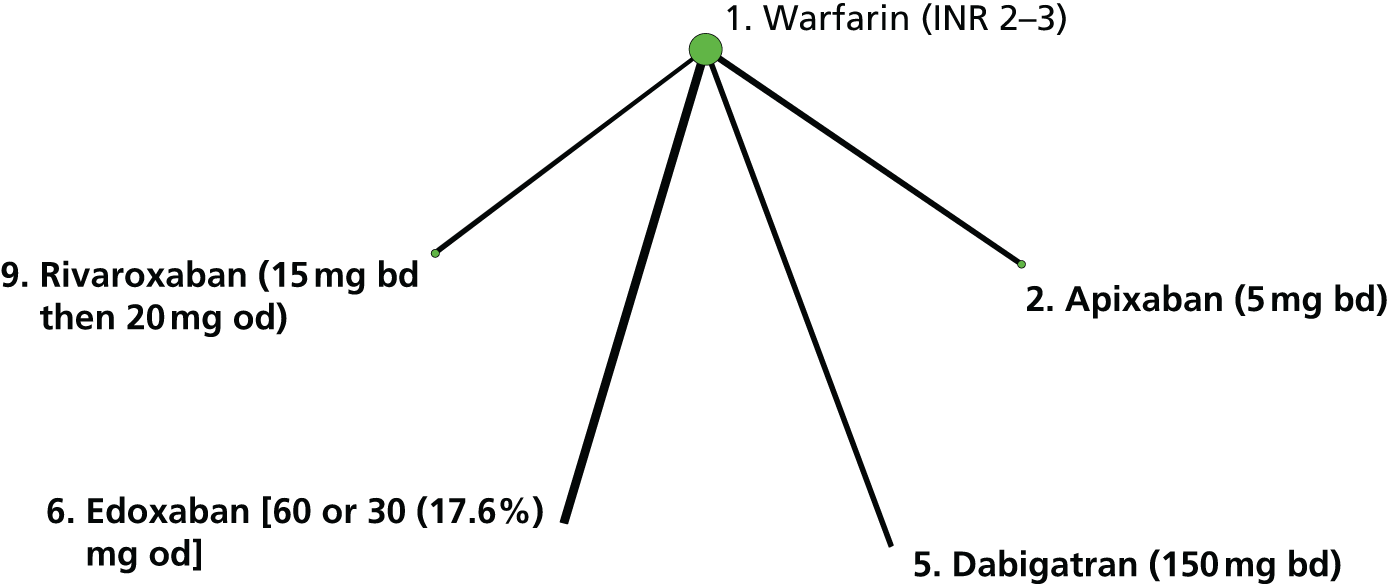
| Study | Interventions compared | SG | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| AMPLIFY210 | 1, 2 | + | + | + | + | ? | + |
| EINSTEIN DVT206 | 1, 9 | + | + | – | + | + | + |
| HOKUSAI-VTE206,207 | 1, 2 | + | + | + | + | + | + |
| RE-COVER205 | 1, 5 | + | + | + | + | + | + |
| RE-COVER II211 | 1, 5 | + | + | + | + | + | + |
| Comparisons with warfarin (INR 2–3) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Edoxaban [60 or 30 (17.6%) mg od] | 1.56 (0.78 to 3.24) | – | 1.56 (0.78 to 3.24) |
| Imprecisely estimated comparisons | |||
| Apixaban (5 mg bd) | 2.18 (0.40 to 17.9) | – | 2.18 (0.40 to 17.9) |
| Dabigatran (150 mg bd) | 2.11 (0.64 to 8.12) | – | 2.11 (0.64 to 8.12) |
| Rivaroxaban (15 mg bd then 20 mg od) | 6.81 (0.90 to 219) | – | 6.81 (0.90 to 219) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Imprecisely estimated comparisons | |||
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | – | 0.96 (0.09 to 8.47) | 0.96 (0.09 to 8.47) |
| Edoxaban [60 or 30 (17.6%) mg od] vs. apixaban (5 mg bd) | – | 0.71 (0.08 to 4.49) | 0.71 (0.08 to 4.49) |
| Rivaroxaban (15 mg bd then 20 mg od) vs. apixaban (5 mg bd) | – | 3.17 (0.17 to 145) | 3.17 (0.17 to 145) |
| Edoxaban [60 or 30 (17.6%) mg od] vs. dabigatran (150 mg bd) | – | 0.74 (0.16 to 3.03) | 0.74 (0.16 to 3.03) |
| Rivaroxaban (15 mg bd then 20 mg od) vs. dabigatran (150 mg bd) | – | 3.27 (0.29 to 124) | 3.27 (0.29 to 124) |
| Rivaroxaban (15 mg bd then 20 mg od) vs. edoxaban [60 or 30 (17.6%) mg od] | – | 4.44 (0.50 to 143) | 4.44 (0.50 to 143) |
Major bleeding
The nine trials200–209 reported 228 major bleeding events, leading to a network of 13 interventions (Figure 51). These studies200–209 were judged to be at low risk of bias (Table 125). There was strong evidence that apixaban (5 mg bd) and rivaroxaban (15 mg bd then 20 mg od) reduce risk of major bleeding compared with warfarin (INR 2–3) (Table 126). There was evidence that risk of major bleeding was higher for edoxaban [60 or 30 (17.6%) mg od] and dabigatran (150 mg bd) than apixaban (5 mg bd) (Table 127).
FIGURE 51.
Network plot for major bleeding (acute treatment of VTE).
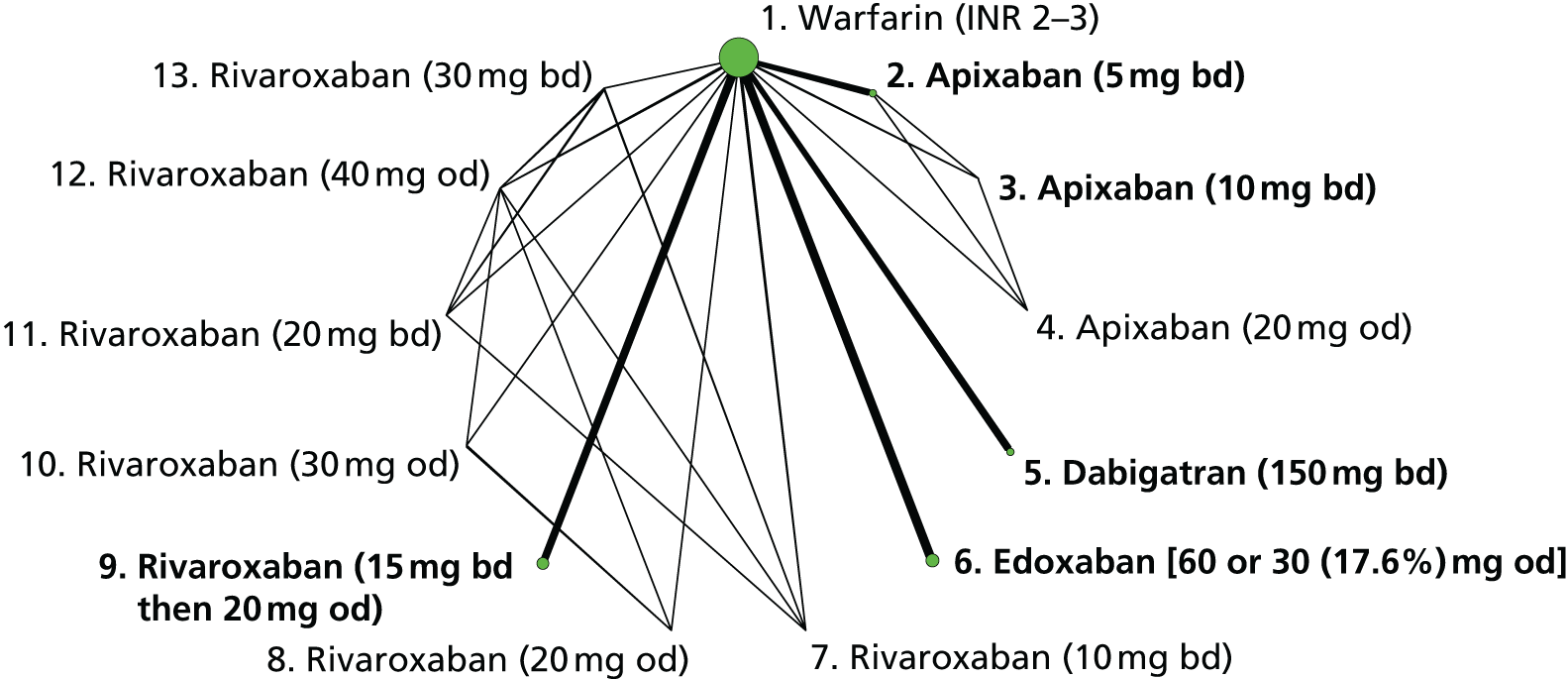
| Study | Interventions compared | SG | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| AMPLIFY210 | 1, 2 | + | + | + | + | ? | + |
| BOTTICELLI DVT204 | 1, 2, 3, 4 | ? | + | – | + | + | + |
| EINSTEIN DVT206 | 1, 9 | + | + | – | + | + | + |
| EINSTEIN DVT dose-ranging study203 | 1, 8, 10, 12 | + | + | – | + | + | + |
| EINSTEIN PE207 | 1, 9 | + | + | – | + | + | + |
| HOKUSAI-VTE206,207 | 1, 2 | + | + | + | + | + | + |
| ODiXa-DVT202 | 1, 7, 11, 12, 13 | + | + | ? | + | + | + |
| RE-COVER205 | 1, 5 | + | + | + | + | + | + |
| RE-COVER II211 | 1, 5 | + | + | + | + | + | + |
| Comparisons with warfarin (INR 2–3) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Apixaban (5 mg bd) | 0.33 (0.18 to 0.56) | – | 0.33 (0.18 to 0.56) |
| Dabigatran (150 mg bd) | 0.76 (0.48 to 1.18) | – | 0.76 (0.48 to 1.18) |
| Edoxaban [60 or 30 (17.6%) mg od] | 0.85 (0.59 to 1.22) | – | 0.85 (0.59 to 1.22) |
| Rivaroxaban (15 mg bd then 20 mg od) | 0.55 (0.37 to 0.80) | – | 0.55 (0.37 to 0.80) |
| Imprecisely estimated comparisons | |||
| Apixaban (10 mg bd) | 0.18 (0 to 3.84) | – | 0.18 (0 to 3.84) |
| Apixaban (20 mg od) | 1.79 (0.23 to 15.8) | – | 1.79 (0.23 to 15.8) |
| Rivaroxaban (10 mg bd) | 1.86 (0.23 to 16) | – | 1.86 (0.23 to 16) |
| Rivaroxaban (20 mg od) | 0.97 (0.07 to 9.40) | – | 0.97 (0.07 to 9.40) |
| Rivaroxaban (30 mg od) | 1.81 (0.24 to 14.8) | – | 1.81 (0.24 to 14.8) |
| Rivaroxaban (20 mg bd) | 1.90 (0.24 to 15.4) | – | 1.90 (0.24 to 15.4) |
| Rivaroxaban (40 mg od) | 1.03 (0.18 to 6.02) | – | 1.03 (0.18 to 6.02) |
| Rivaroxaban (30 mg bd) | 3.58 (0.65 to 26.6) | – | 3.58 (0.65 to 26.6) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Rivaroxaban (15 mg bd then 20 mg od) vs. apixaban (5 mg bd) | – | 1.68 (0.85 to 3.40) | 1.68 (0.85 to 3.40) |
| Edoxaban [60 or 30 (17.6%) mg od] vs. apixaban (5 mg bd) | – | 2.60 (1.35 to 5.21) | 2.60 (1.35 to 5.21) |
| Edoxaban [60 or 30 (17.6%) mg od] vs. dabigatran (150 mg bd) | – | 1.12 (0.63 to 1.98) | 1.12 (0.63 to 1.98) |
| Rivaroxaban (15 mg bd then 20 mg od) vs. dabigatran (150 mg bd) | – | 0.72 (0.40 to 1.30) | 0.72 (0.40 to 1.30) |
| Rivaroxaban (15 mg bd then 20 mg od) vs. edoxaban [60 or 30 (17.6%) mg od] | – | 0.64 (0.38 to 1.10) | 0.64 (0.38 to 1.10) |
| Imprecisely estimated comparisons | |||
| Apixaban (10 mg bd) vs. apixaban (5 mg bd) | 0.54 (0 to 12.1) | – | 0.54 (0 to 12.1) |
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | – | 2.32 (1.15 to 4.86) | 2.32 (1.15 to 4.86) |
| Dabigatran (150 mg bd) vs. apixaban (10 mg bd) | – | 4.31 (0.19 to 2090) | 4.31 (0.19 to 2090) |
| Edoxaban [60 or 30 (17.6%) mg od] vs. apixaban (10 mg bd) | – | 4.84 (0.22 to 2300) | 4.84 (0.22 to 2300) |
| Rivaroxaban (15 mg bd then 20 mg od) vs. apixaban (10 mg bd) | – | 3.12 (0.14 to 1470) | 3.12 (0.14 to 1470) |
Clinically relevant bleeding
Eight studies201–209 reported 2365 CRB events, leading to a network of 10 interventions (Figure 52). These studies were mostly judged to be at low risk of bias (Table 128), with some concerns about lack of blinding of participants and personnel. There was evidence that apixaban (5 mg bd), dabigatran (150 mg bd) and edoxaban [60 or 30 (17.6%) mg od] reduce risk of CRB compared with warfarin (INR 2–3) (Table 129). There was some evidence that rivaroxaban (15 mg bd then 20 mg od) reduces risk of CRB compared with warfarin (INR 2–3). There was evidence that risk of CRB is higher with dabigatran (150 mg bd), edoxaban [60 or 30 (17.6%) mg od] and rivaroxaban (15 mg bd then 20 mg od) than apixaban (5 mg bd) (Table 130). There was evidence that risk of CRB is higher with edoxaban [60 or 30 (17.6%) mg od] and rivaroxaban (15 mg bd then 20 mg od) than dabigatran (150 mg bd).
FIGURE 52.
Network plot for CRB (acute treatment of VTE).

| Study | Interventions compared | SG | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| AMPLIFY210 | 1, 2 | + | + | + | + | ? | + |
| BOTTICELLI DVT204 | 1, 2, 3, 4 | ? | + | – | + | + | + |
| EINSTEIN DVT206 | 1, 9 | + | + | – | + | + | + |
| EINSTEIN DVT dose-ranging study203 | 1, 8, 10, 12 | + | + | – | + | + | + |
| EINSTEIN PE207 | 1, 9 | + | + | – | + | + | + |
| HOKUSAI-VTE206,207 | 1, 2 | + | + | + | + | + | + |
| RE-COVER205 | 1, 5 | + | + | + | + | + | + |
| RE-COVER II211 | 1, 5 | + | + | + | + | + | + |
| Comparisons with warfarin (INR 2–3) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Apixaban (5 mg bd) | 0.44 (0.35 to 0.55) | – | 0.44 (0.35 to 0.55) |
| Apixaban (10 mg bd) | 0.36 (0.12 to 0.87) | – | 0.36 (0.12 to 0.87) |
| Apixaban (20 mg od) | 0.76 (0.34 to 1.61) | – | 0.76 (0.34 to 1.61) |
| Dabigatran (150 mg bd) | 0.61 (0.49 to 0.76) | – | 0.61 (0.49 to 0.76) |
| Edoxaban [60 or 30 (17.6%) mg od] | 0.81 (0.70 to 0.94) | – | 0.81 (0.70 to 0.94) |
| Rivaroxaban (15 mg bd then 20 mg od) | 0.93 (0.80 to 1.08) | – | 0.93 (0.80 to 1.08) |
| Rivaroxaban (20 mg od) | 0.54 (0.20 to 1.39) | – | 0.54 (0.20 to 1.39) |
| Rivaroxaban (30 mg od) | 0.56 (0.21 to 1.43) | – | 0.56 (0.21 to 1.43) |
| Imprecisely estimated comparisons | |||
| Rivaroxaban (40 mg od) | 0.17 (0.04 to 0.58) | – | 0.17 (0.04 to 0.58) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Apixaban (10 mg bd) vs. apixaban (5 mg bd) | 0.81 (0.28 to 2.00) | – | 0.81 (0.28 to 2.00) |
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | – | 1.39 (1.02 to 1.90) | 1.39 (1.02 to 1.90) |
| Edoxaban [60 or 30 (17.6%) mg od] vs. apixaban (5 mg bd) | – | 1.84 (1.41 to 2.40) | 1.84 (1.41 to 2.40) |
| Rivaroxaban (15 mg bd then 20 mg od) vs. apixaban (5 mg bd) | – | 2.12 (1.63 to 2.76) | 2.12 (1.63 to 2.76) |
| Dabigatran (150 mg bd) vs. apixaban (10 mg bd) | – | 1.72 (0.68 to 5.02) | 1.72 (0.68 to 5.02) |
| Edoxaban [60 or 30 (17.6%) mg od] vs. apixaban (10 mg bd) | – | 2.27 (0.91 to 6.56) | 2.27 (0.91 to 6.56) |
| Rivaroxaban (15 mg bd then 20 mg od) vs. apixaban (10 mg bd) | – | 2.62 (1.05 to 7.55) | 2.62 (1.05 to 7.55) |
| Edoxaban [60 or 30 (17.6%) mg od] vs. dabigatran (150 mg bd) | – | 1.32 (1.01 to 1.73) | 1.32 (1.01 to 1.73) |
| Rivaroxaban (15 mg bd then 20 mg od) vs. dabigatran (150 mg bd) | – | 1.52 (1.17 to 1.99) | 1.52 (1.17 to 1.99) |
| Rivaroxaban (15 mg bd then 20 mg od) vs. edoxaban [60 or 30 (17.6%) mg od] | – | 1.15 (0.93 to 1.42) | 1.15 (0.93 to 1.42) |
All-cause mortality
Eight studies201–209 reported 662 all-cause mortality events, leading to a network of 10 interventions (Figure 53). These studies201–209 were mostly judged to be at low risk of bias (Table 131), with some concerns about lack of blinding of participants and personnel. There was little evidence that risk of all-cause mortality differed for any of the NOAC interventions compared with warfarin (INR 2–3) (Table 132). Neither was there evidence that risk of all-cause mortality differed between licensed doses of NOACs (Table 133).
FIGURE 53.
Network plot for all-cause mortality (acute treatment of VTE).
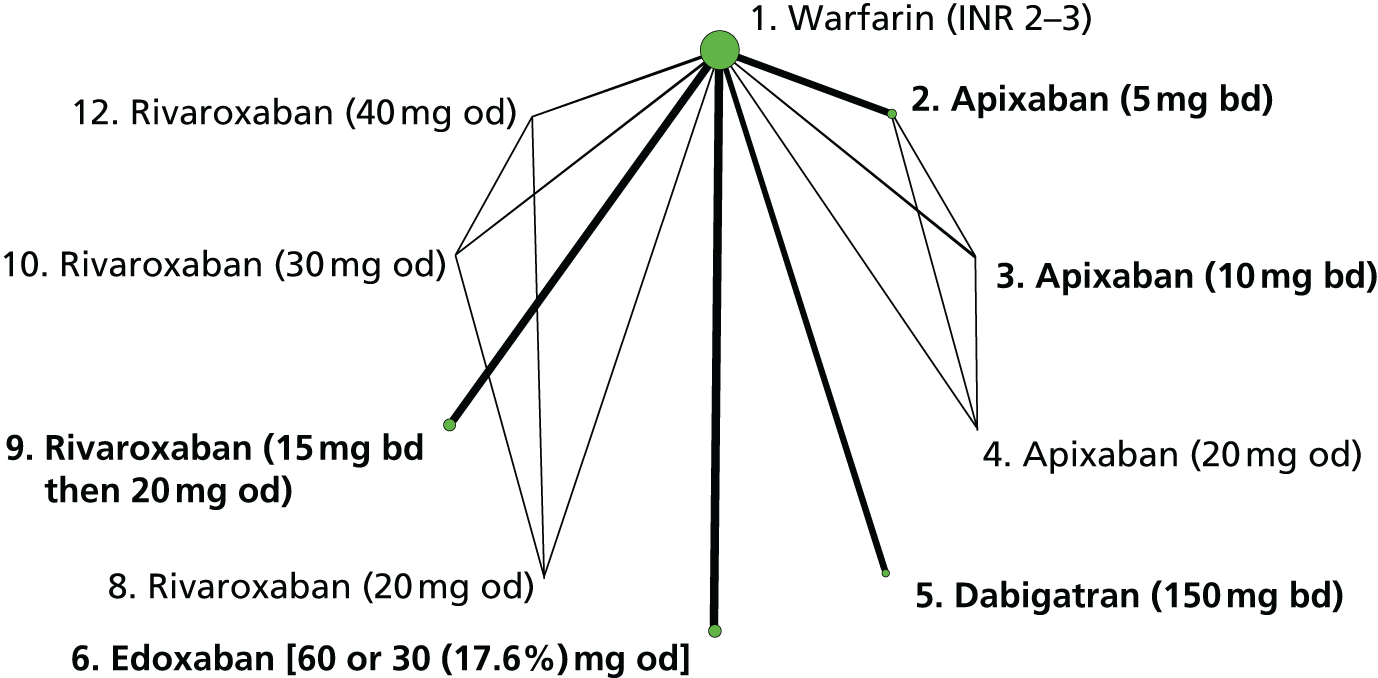
| Study | Interventions compared | SG | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| AMPLIFY210 | 1, 2 | + | + | + | + | ? | + |
| BOTTICELLI DVT204 | 1, 2, 3, 4 | ? | + | – | + | + | + |
| EINSTEIN DVT206 | 1, 9 | + | + | – | + | + | + |
| EINSTEIN DVT dose-ranging study203 | 1, 8, 10, 12 | + | + | – | + | + | + |
| EINSTEIN PE207 | 1, 9 | + | + | – | + | + | + |
| HOKUSAI-VTE206,207 | 1, 2 | + | + | + | + | + | + |
| RE-COVER205 | 1, 5 | + | + | + | + | + | + |
| RE-COVER II211 | 1, 5 | + | + | + | + | + | + |
| Comparisons with warfarin (INR 2–3) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Apixaban (5 mg bd) | 0.85 (0.57 to 1.27) | – | 0.85 (0.57 to 1.27) |
| Dabigatran (150 mg bd) | 1.00 (0.66 to 1.52) | – | 1.00 (0.66 to 1.52) |
| Edoxaban [60 or 30 (17.6%) mg od] | 1.05 (0.82 to 1.35) | – | 1.05 (0.82 to 1.35) |
| Rivaroxaban (15 mg bd then 20 mg od) | 0.96 (0.73 to 1.29) | – | 0.96 (0.73 to 1.29) |
| Imprecisely estimated comparisons | |||
| Apixaban (10 mg bd) | 0.58 (0.05 to 3.74) | – | 0.58 (0.05 to 3.74) |
| Apixaban (20 mg od) | 0.61 (0.05 to 3.87) | – | 0.61 (0.05 to 3.87) |
| Rivaroxaban (20 mg od) | 0.80 (0.18 to 3.16) | – | 0.80 (0.18 to 3.16) |
| Rivaroxaban (30 mg od) | 1.73 (0.55 to 5.88) | – | 1.73 (0.55 to 5.88) |
| Rivaroxaban (40 mg od) | 0.35 (0.04 to 1.82) | – | 0.35 (0.04 to 1.82) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | – | 1.18 (0.66 to 2.12) | 1.18 (0.66 to 2.12) |
| Edoxaban [60 or 30 (17.6%) mg od] vs. apixaban (5 mg bd) | – | 1.24 (0.77 to 1.99) | 1.24 (0.77 to 1.99) |
| Rivaroxaban (15 mg bd then 20 mg od) vs. apixaban (5 mg bd) | – | 1.14 (0.70 to 1.87) | 1.14 (0.70 to 1.87) |
| Edoxaban [60 or 30 (17.6%) mg od] vs. dabigatran (150 mg bd) | – | 1.05 (0.65 to 1.70) | 1.05 (0.65 to 1.70) |
| Rivaroxaban (15 mg bd then 20 mg od) vs. dabigatran (150 mg bd) | – | 0.97 (0.58 to 1.59) | 0.97 (0.58 to 1.59) |
| Rivaroxaban (15 mg bd then 20 mg od) vs. edoxaban [60 or 30 (17.6%) mg od] | – | 0.92 (0.63 to 1.34) | 0.92 (0.63 to 1.34) |
| Imprecisely estimated comparisons | |||
| Apixaban (10 mg bd) vs. apixaban (5 mg bd) | 0.68 (0.05 to 4.47) | – | 0.68 (0.05 to 4.47) |
| Dabigatran (150 mg bd) vs. apixaban (10 mg bd) | – | 1.73 (0.25 to 22.6) | 1.73 (0.25 to 22.6) |
| Edoxaban [60 or 30 (17.6%) mg od] vs. apixaban (10 mg bd) | – | 1.82 (0.27 to 23.2) | 1.82 (0.27 to 23.2) |
| Rivaroxaban (15 mg bd then 20 mg od) vs. apixaban (10 mg bd) | – | 1.67 (0.25 to 21.4) | 1.67 (0.25 to 21.4) |
Summary of results and ranking of interventions
There was little evidence that risk of symptomatic VTE, symptomatic DVT or symptomatic PE differed for any of the NOAC interventions compared with warfarin (INR 2–3). Neither was there evidence that risk of these outcomes differed between licensed doses of NOACs. However, there was evidence of substantial reductions in risk of both major bleeding and CRB for apixaban (5 mg bd) compared with warfarin (INR 2–3). There was also evidence that other NOACs reduced bleeding compared with warfarin (INR 2–3). In comparisons between licensed doses of NOACs, there was evidence that apixaban (5 mg bd) reduced major bleeding risk compared with some other NOACs. With regard to model appraisal, we did not identify any instance of lack of convergence among the Markov chains, poor model fit or inconsistency.
Figure 54 presents the rankogram for all licensed interventions and all seven outcomes examined in this review. There was a high probability that warfarin (INR 2–3) is ranked worst for major bleeding and CRB. There was a high probability that apixaban 5 mg bd is ranked best for major bleeding and CRB, and this intervention also had a high probability of being ranked best or second best for symptomatic DVT, symptomatic VTE and all-cause mortality.
FIGURE 54.
Rankogram for licensed interventions examined in acute treatment of VTE. CR, clinically relevant.


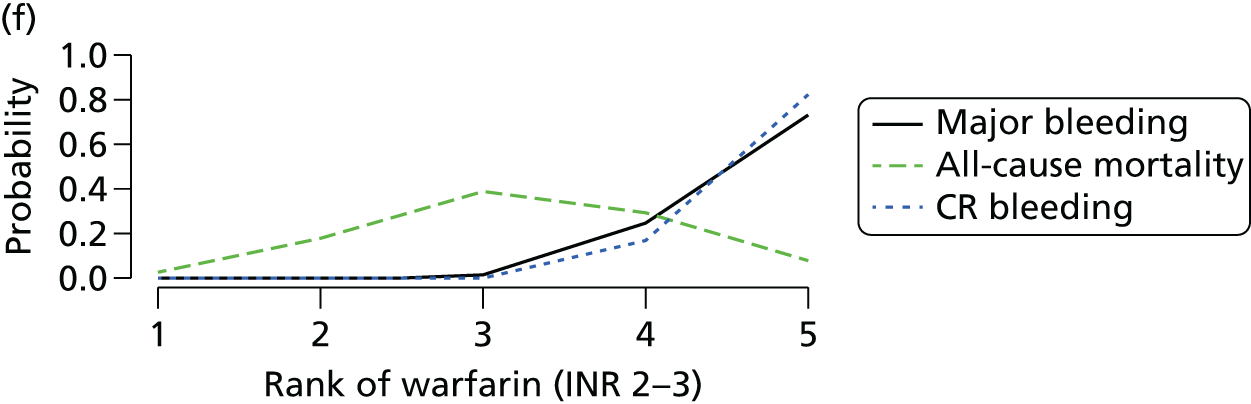
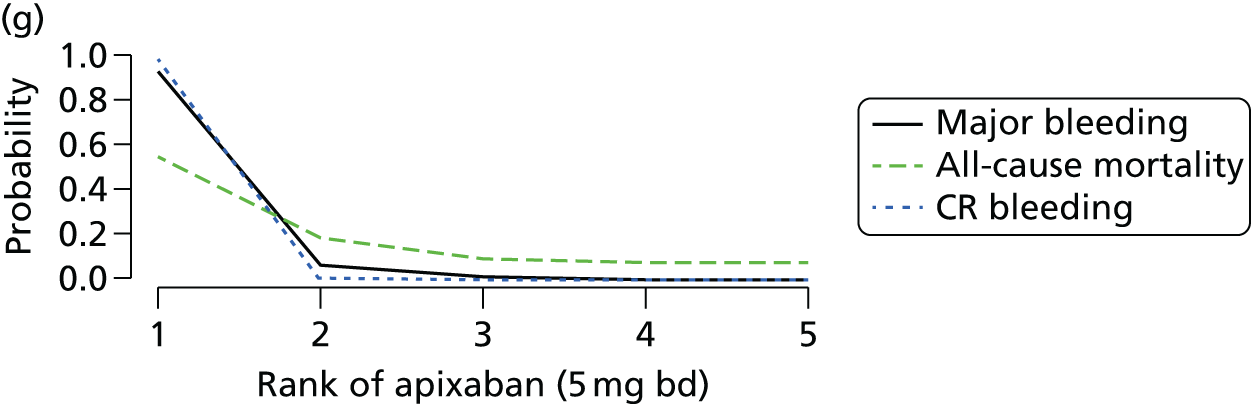
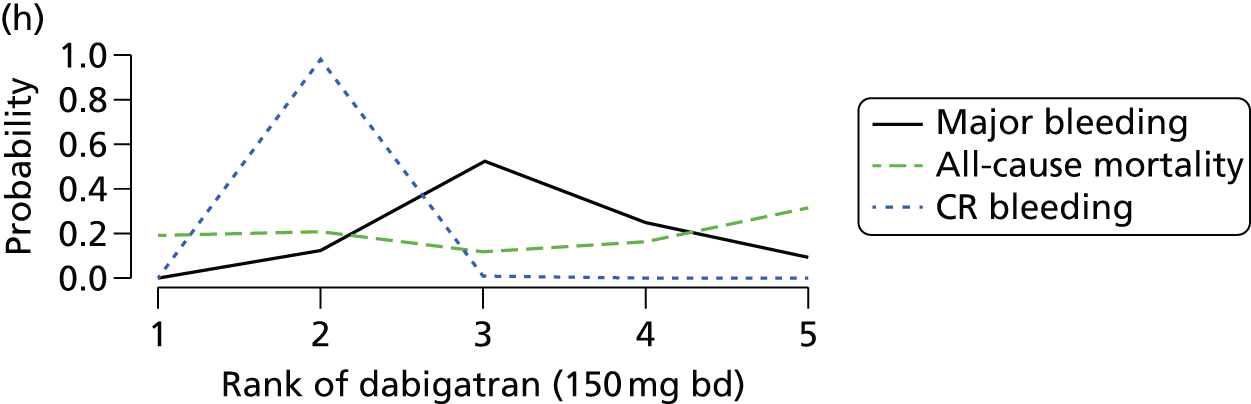

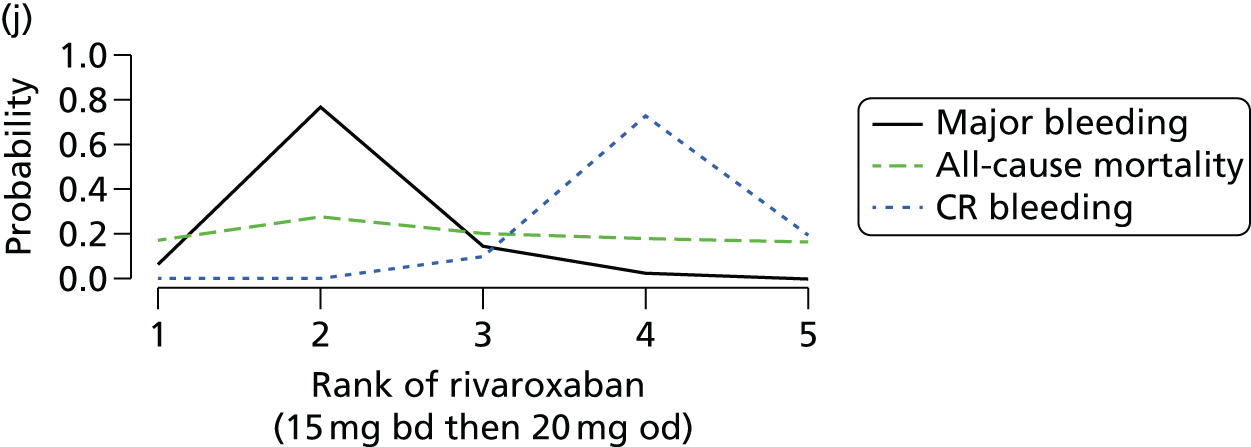
Chapter 9 Clinical results (4): secondary prevention of venous thromboembolism
Included studies
Ten completed RCTs with 11 references,206,213–222 one ongoing trial223 and one trial224 reported in insufficient detail to include in the quantitative synthesis met the eligibility criteria for the review (see Figure 32). A summary of the characteristics of the 10 studies included in the analyses is presented in Table 134. All were multicentre and many were conducted across countries in North and South America, Europe, Asia, and Australia, New Zealand, South Africa, Russia and Israel. All were Phase III trials. A total of 10,390 patients were included; the number of patients randomised ranged from 162 to 2866. Four studies, with a randomised total of 7902 patients, examined a NOAC (against placebo in three studies and against warfarin in one study). Four studies, with a randomised total of 1263 patients, examined warfarin (against placebo in two studies and against no treatment in two studies). Two studies, with a randomised total of 1225 patients, examined aspirin against placebo.
| Study (centre type) [countries] | Sponsor (sponsor’s role) | Age eligibility (mean age, years) [% male] | Clinical condition | No. randomised | Interventions compared | Treatment duration (months) | Outcomes | Time of outcome assessment (months) |
|---|---|---|---|---|---|---|---|---|
| AMPLIFY-EXT221 (Multicentre) [North and South America, Europe, Russia, Israel, Australia, Asia and South Africa] |
Pfizer and Bristol-Myers Squibb(The sponsors collected and maintained the data; the academic authors had access to the data at all times, through the sponsors) | ≥ 18 (56.7) [57.4] | Already treated for a first-ever objectively confirmed, symptomatic DVT or PE (with or without DVT) | 2486 | Apixaban 1. 2.5 mg bd 2. 5 mg bd 3. Placebo bd |
12 | Efficacy: Symptomatic VTE, symptomatic DVT, symptomatic non-fatal PE, fatal PE Safety: Major bleeding, CRNM bleeding, composite CRB, fatal bleeding, MI, death (cardiovascular), death (all causes) |
12 |
| ASPIRE220 (Multicentre) [Argentina, Australia, New Zealand and Asia] |
National Health and Medical Research Council Australia and others (not specified)(The funder was responsible for the collection, maintenance, integrity, and confidentiality of all data) | ≥ 18 (54.5) [54.4] | Already treated for a first-ever unprovoked episode of objectively diagnosed symptomatic DVT or an acute PE | 822 | Aspirin 1. 100 mg od 2. Placebo od |
Up to 48 | Efficacy: Symptomatic VTE, symptomatic DVT, symptomatic distal DVT, symptomatic proximal DVT, symptomatic PE, fatal PE Safety: All bleeding, major bleeding, CRNM bleeding, composite CRB, fatal bleeding, MI, all stroke, death (cardiovascular), death (all causes) |
37.2 (median) |
| EINSTEIN-EXTENSION206,217,218 (Multicentre) [North and South America, Europe, Israel, Australia, New Zealand, Asia and South Africa] |
Bayer Healthcare(The data were collected and maintained by the sponsor) | ≥ 18 (58.3) [58] | Already treated for mixed (first ever and ≥ 1 previous VTE) confirmed symptomatic PE or DVT | 1197 | Rivaroxaban 1. 20 mg od 2. Placebo od |
6–12 | Efficacy: Symptomatic VTE, symptomatic DVT, symptomatic non-fatal PE, fatal PE Safety: Major bleeding, CRNM bleeding, composite CRB, fatal bleeding, death (all causes) |
6.2 (mean) |
| LAFIT213 (Multicentre) [Canada and USA] |
Supported by a grant from DuPont Pharma, Wilmington, DE, USA,and by the Medical Research Council of Canada, the Heart and Stroke Foundation of Canada and the Ministry of Health of Ontario (Not declared) |
Adults (59) [60] | Already treated for a first-ever episode of idiopathic VTE | 162 | Warfarin 1. INR 2–3 (mean TTR: 64%) 2. Placebo od |
24 | Efficacy: Symptomatic VTE, symptomatic DVT, symptomatic non-fatal PE, fatal PE Safety: All bleeding, major bleeding, minor bleeding, death (all causes) |
24 |
| PREVENT216 (Multicentre) [USA] |
National Heart, Lung, and Blood Institute, USA (Note: Study drug and placebo were supplied without fee by Bristol-Myers Squibb)(The funder appointed an independent data and safety monitoring committee that monitored the primary end point of recurrent venous thromboembolism) |
≥ 30 (median 53) [52.8] | Already treated for idiopathic VTE. VTE episode is not clearly reported but texts suggest this may be a first-ever event | 508 | Warfarin 1. INR 1.5–2 (mean TTR: NR) 2. Placebo od |
51.6 (mean 25.2) | Efficacy: Symptomatic VTE, fatal PE Safety: Major bleeding, minor bleeding, MI, death (all causes) |
51.6 (mean 25.2) |
| RE-MEDY222 (Multicentre) [North and South America, Europe, Russia, Israel, Australia, New Zealand, Asia, New Zealand and South Africa] |
Boehringer Ingelheim(Study was designed, conducted, and data analysed by the funder in conjunction with the steering committee) | ≥ 18 (54.7) [61] | Already treated for mixed (first-ever and ≥ 1 previous) objectively confirmed, symptomatic, proximal DVT or PE | 2866 | Dabigatran 1. 150 mg bd Warfarin 2. INR 2–3 (median TTR: 65.3%) |
6–36 | Efficacy: Symptomatic VTE, symptomatic DVT, symptomatic non-fatal PE Safety: All bleeding, major bleeding, composite CRB, intracranial bleeding, MI, death (all causes) |
36 |
| RE-SONATE222 (Multicentre) [North America, Europe, Russia, Australia, New Zealand, Asia and South Africa] |
Boehringer Ingelheim(Study was designed, conducted, and data analysed by the funder in conjunction with the steering committee) | ≥ 18 (55.8) [55.5] | Already treated for mixed (first-ever and ≥ 1 previous) objectively confirmed, symptomatic, proximal DVT or PE. A small proportion (< 1%) had ≥ 1 previous | 1353 | Dabigatran 1. 150 mg bd 2. Placebo bd |
6 | Efficacy: Symptomatic VTE, symptomatic DVT, symptomatic non-fatal PE Safety: All bleeding, major bleeding, CRNM bleeding, composite CRB, MI |
6 |
| WARFASA219 (Multicentre) [Austria and Italy] |
University of Perugia, Italy, and others (not specified)(Data were collected, maintained, and analysed by the Clinical Research Unit of the University of Perugia) | ≥ 18 (62) [63.9] | Already treated for a first-ever, objectively confirmed, symptomatic, unprovoked, proximal DVT, PE, or both | 403 | Aspirin 1. 100 mg od 2. Placebo od |
24 | Efficacy: Symptomatic VTE, symptomatic DVT, symptomatic PE, fatal PE, arterial event Safety: Major bleeding, CRNM bleeding, composite CRB, death (all causes) |
24 |
| WODIT-DVT214 (Multicentre) [Italy] |
Not declared | 15–85 (67.3) [57.9] | Already treated for a first-ever episode of symptomatic objectively confirmed idiopathic proximal DVT | 267 | Warfarin 1. INR 2–3 (mean TTR: 81%) 2. No treatment |
9 | Efficacy: Symptomatic VTE, symptomatic DVT, symptomatic non-fatal PE, fatal PE Safety: Major bleeding, fatal bleeding, death (cardiovascular), death (all causes) |
33 |
| WODIT-PE215 (Multicentre) [Italy] |
Not declared | 15–85 (62) [59.5] | Already treated for a first-ever episode of symptomatic, objectively confirmed PE | 326 | Warfarin 1. INR 2–3 (mean TTR: NR) 2. No treatment |
3 | Efficacy: Symptomatic VTE, symptomatic PE, symptomatic non-fatal PE, symptomatic DVT, fatal PE Safety: All bleeding, major bleeding, fatal bleeding, death (cardiovascular), death (all causes) |
3 |
Eligibility criteria were similar across the studies, all patients having already been treated for first-ever objectively confirmed symptomatic DVT and/or PE. The mean age of patients was similar across studies that compared NOACs, ranging from 54.7 to 58 years. The mean age of patients across all the 10 included studies206,213–222 ranged from 53 to 67.3 years. The percentage of male patients was similar across studies that compared NOACs, ranging from 55.5% to 61%. The percentage of males across the 10 studies206,213–222 ranged from 52.8% to 63.9%. Mean BMI was reported in only three studies216,219,221 and ranged from 27.1 to 29.9 kg/m2 across study arms. Mean body weight ranged from 83.7 to 86.1 kg across study arms when data were reported. The proportion of patients with comorbidities was not well reported. Three studies216,222 reported the proportion of patients who were diabetic, which ranged from 6.7% to 10.5%. Two studies221,222 reported proportions with hypertension and cancer, which ranged from 36.3% to 41.3% and from 1% to 4%, respectively. Half of the studies that reported each comorbidity examined a NOAC.
Two studies examined dabigatran 150 mg bd: against standard intensity warfarin (INR 2–3) in one study and against placebo in the other. One study examined each of apixaban 2.5 mg and 5 mg bd, and rivaroxaban 20 mg od, against placebo in both studies. Two studies examined aspirin 100 mg od against placebo. Four studies examined warfarin: against placebo in two studies and against no treatment in two studies. Three of these four studies examined standard intensity warfarin and one study examined low intensity warfarin (INR 1.5–2). Mean TTR for standard intensity warfarin arms was reported in only one study215 and was 83%.
The duration of treatment varied across studies, ranging from 6 to 36 months in the NOAC studies, from 24 to 48 months in the aspirin studies and from 3 to 51.6 months in the warfarin studies. Efficacy and safety outcomes reported across studies were similar irrespective of the intervention examined, and were reported at the end of the treatment periods. All 10 studies206,213–222 reported data on symptomatic VTE and major bleeding. Nine studies each reported data on symptomatic DVT, symptomatic PE and all-cause mortality. Six studies reported data on CRB, and five studies reported data on MI. Only the four NOACs studies were sponsored by a pharmaceutical company. Four other studies were conducted with funding from more than one source: mainly medical research councils or institutes. In all sponsored studies, the sponsors were responsible for study design and data collection, and in the majority of cases data analysis (particularly the pharmaceutical company funded studies). Funding source was not declared in two studies.
Time in therapeutic range for warfarin interventions
Table 135 shows the comparator interventions, target INR and (where reported) mean TTR for the five studies213–216,222 that included a warfarin intervention arm. Three (60%) of these studies213,214,222 reported mean TTR, which was 64% in LAFIT,213 65.3% in RE-MEDY222 and 81% in WODIT-DVT. 214
Risk of bias in included studies
Table 136 shows detailed risk-of-bias assessments for each included study for each domain of the Cochrane assessment tool. Generally, the studies were judged to be at low risk of bias for sequence generation, blinding of outcome assessment and incomplete outcome data. However, one study did not describe how the randomisation sequence was generated. Eight studies described how treatment allocation was concealed: these studies were judged to be at low risk of bias for this domain. One study provided insufficient information to enable a judgement on allocation concealment, and one study provided no information on this domain: these studies were judged to be at unclear and high risk of bias, respectively. Overall, the risk of bias due to selective reporting was judged to be low. Three studies were open-label and so were judged to be at a high risk of bias for blinding of participants and personnel.
| Study | Sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting |
|---|---|---|---|---|---|---|
| AMPLIFY-EXT 221 | L: ‘Randomization was performed with the use of an interactive voice-response system and was stratified according to the initial diagnosis (DVT or PE) and participation or no participation in the AMPLIFY trial’ | L: ‘Randomization was performed with the use of an interactive voice-response system AMPLIFY trial’ | U: ‘We conducted a randomized, double-blind study.’ ‘Patients were assigned, in a 1:1:1 ratio, to receive 2.5 mg of apixaban, 5 mg of apixaban, or placebo, all given bd’ | L: ‘An independent committee, whose members were unaware of the study-group assignments, adjudicated the qualifying initial diagnosis (DVT or PE) and all suspected outcomes’ | L: All patients were included in the analyses | L: All outcomes reported as per study protocol |
| ASPIRE 220 | L: ‘Randomization was performed through a central Web-based randomization system, with stratification according to centre and duration of initial oral anticoagulation therapy (≤ 26 weeks or > 26 weeks)’ | L: ‘Randomization was performed through a central web-based randomization system’ | L: ‘Enteric-coated aspirin, in L00-mg tablets, and matching placebo were provided without charge by Bayer Health-Care Pharmaceuticals’ | L: ‘All primary and secondary events were adjudicated by an independent event adjudication committee whose members were unaware of the group assignments’ | L: Very few missing outcome data but these almost balance out across intervention groups, with similar reasons for missing data. However, analysis was by intension to treat | L: All outcomes reported as per study protocol |
| EINSTEIN-EXTENSION 206 , 217 , 218 | L: ‘This was a randomized, double-blind, placebo-controlled superiority study in which patients who completed the first 6–12 months of oral anticoagulant treatment with VKA or with rivaroxaban (if previously enrolled in the EINSTEIN-DVT or EINSTEIN-PE studies)’ As this study is related to EINSTEIN DVT206 and PE studies, for which randomisation was by use of computerised voice-response system, it is assumed that randomisation was done |
L: As this study is related to EINSTEIN DVT206 and PE studies, it is assumed that there was central allocation of treatment | H: As this study is related to EINSTEIN DVT206 and PE studies, both of open-label type, it is assumed that participants and personnel may not have been blinded | L: ‘All suspected outcome events were classified by a central adjudication committee whose members were unaware of the treatment assignments’ | L: For all outcomes (except bleeding outcomes): No missing outcome data – analysis was by intention to treat | L: All outcomes reported as per study protocol |
| L: For bleeding outcomes: Very minimal missing data – unlikely to influence outcome | ||||||
| LAFIT 213 | L: ‘A computer algorithm, with a randomly determined block size of two or four within each stratum, had previously determined whether the patient received warfarin or placebo’ | L: ‘Patients were provided with consecutively numbered supplies of study drug’ | L: ‘We performed a double-blind, randomized trial. Patients were provided with consecutively numbered supplies of study drug either tablets containing 5 mg of warfarin or identical-appearing placebo’ | L: ‘Information on all suspected outcome events and deaths was reviewed and classified by a central adjudication committee whose members were unaware of the treatment assignments’ | L: All patients were included in the analyses | U: Study protocol not found |
| PREVENT 216 | L: ‘Randomization was stratified according to clinical site, time since the index event (≤ 6 months or > 6 months) and whether or not the index event was the patient’s first venous thromboembolism’ Randomisation to low-intensity warfarin (coumadin, provided without charge by Bristol-Myers Squibb; target INR, 1.5 to 2.0) or to matching placebo was performed centrally |
L: Randomization to low-intensity warfarin (coumadin, provided without charge by Bristol-Myers Squibb; target INR, 1.5 to 2.0) or to matching placebo was performed centrally | L: ‘To ensure blinding, sham dose adjustments were made in the placebo group. These devices were altered electronically to provide a coded INR value that was transmitted in a double-blind fashion to the data coordinating centre’ | L: ‘All end points were reviewed by a committee of physicians who were unaware of treatment-group assignments’ | L: All patients were included in the analyses | L: All outcomes reported as per study protocol |
| RE-MEDY 222 | L: ‘Patients underwent randomization by means of an interactive voice-response system The true or sham INR was then obtained by means of an interactive voice-response system with a central computer that had been programmed with the randomization schedule’ |
L: ‘Patients underwent randomization by means of an interactive voice-response system’ | L: ‘A randomized, double-blind design. Patients were assigned in a 1:1 ratio to receive active dabigatran (at a fixed dose of 150 mg bd) and a warfarin-like placebo or active warfarin and a dabigatran-like placebo’ | L: ‘Central committees, whose members were not aware of the treatment assignments, adjudicated suspected cases of recurrent VTE, bleeding, death, acute coronary events, and liver function abnormalities’ | L: All patients were included in the analyses | L: All outcomes reported as per study protocol |
| RE-SONATE 222 | L: ‘Patients underwent randomization by means of an interactive voice-response system. Randomization was stratified according to the presence or absence of active cancer’ and according to study centre in the placebo-control study | L: ‘Patients underwent randomization by means of an interactive voice-response system’ | U: ‘A randomized, double-blind design’ ‘Patients were assigned in a 1:1 ratio to receive dabigatran (at a fixed dose of 150 mg bd) or a matching placebo’ |
L: ‘Central committees, whose members were not aware of the treatment assignments, adjudicated suspected cases of recurrent VTE, bleeding, death, acute coronary, and liver function abnormalities’ | L: All patients were included in the analyses | L: All outcomes reported as per study protocol |
| WARFASA 219 | L: ‘WARFASA was a multicenter, investigator-initiated, randomized, double-blind clinical trial Eligible patients were randomly assigned to aspirin, 100 mg od, or placebo for 2 years, with the option of extending the study treatment Randomization occurred within 2 weeks after VKAs had been withdrawn’ |
U: Not enough information on whether or not treatment allocation was concealed. ‘Eligible patients were randomly assigned to aspirin, 100 mg od, or placebo for 2 years, with the option of extending the study treatment’ | U: ‘WARFASA was a multicentre, investigator-initiated, randomized, double-blind clinical trial’ | L: ‘All suspected study outcome events were assessed by a central, independent adjudication committee whose members were unaware of the group assignments and who reviewed the imaging results’ | L: All patients were included in the analyses | L: All outcomes reported as per study protocol |
| WODIT-DVT 214 | U: ‘The Warfarin Optimal Duration Italian Trial was a randomized, multicentre, open trial’ | H: No information on allocation concealment | H: ‘The Warfarin Optimal Duration Italian Trial was a randomized, multicentre, open trial’ | L: ‘All suspected outcome events and all deaths were reviewed centrally, for both the interim and final analyses, by an independent, external adjudication committee whose members were unaware of the treatment group assignments’ | L: All patients were included in the analyses | U: Study protocol not found |
| WODIT-PE 215 | L: ‘Randomization was performed centrally in permuted blocks of six’ | L: ‘Randomization was performed centrally in permuted blocks of six’ | H: ‘Our study, like other studies with oral anticoagulant therapy, was not a placebo-controlled, double-blind trial’ | L: ‘All suspected outcome events and all deaths were reviewed centrally by an independent, external adjudication committee whose members were unaware of the treatment group assignments’ | L: All patients were included in the analyses | U: Study protocol not found |
Results of clinical effectiveness and safety
This review included 10 trials comparing a total number of nine interventions (Table 137). The outcomes reported in the 10 studies, along with the number of events per outcome, are displayed in Tables 138 and 139. We performed NMAs for seven outcomes: symptomatic DVT, symptomatic PE, symptomatic VTE, MI, major bleeding, CRB and all-cause mortality.
| No. | Intervention |
|---|---|
| 1 | Placebo |
| 2 | No treatment |
| 3 | Aspirin (100 mg od) |
| 4 | Warfarin (INR 1.5–2) |
| 5 | Warfarin (INR 2–3) |
| 6 | Apixaban (2.5 mg bd) |
| 7 | Apixaban (5 mg bd) |
| 8 | Dabigatran (150 mg bd) |
| 9 | Rivaroxaban (20 mg od) |
| Study | Study size | Symptomatic DVT | Symptomatic proximal DVT | Symptomatic distal DVT | Symptomatic PE | Fatal PE | Symptomatic non-fatal PE | Symptomatic VTE | Cardiovascular deaths | All-cause mortality |
|---|---|---|---|---|---|---|---|---|---|---|
| AMPLIFY-EXT221 | 2482 | 67 | 0 | 27 | 101 | 15 | 25 | |||
| ASPIRE220 | 822 | 82 | 68 | 25 | 48 | 2 | 130 | 12 | 34 | |
| EINSTEIN-EXTENSION206,217,218 | 1188 | 36 | 1 | 15 | 50 | 3 | ||||
| LAFIT213 | 162 | 11 | 1 | 6 | 18 | 4 | ||||
| PREVENT216 | 508 | 2 | 51 | 12 | ||||||
| RE-MEDY222 | 2856 | 30 | 15 | 44 | 36 | |||||
| RE-SONATE222 | 1343 | 24 | 15 | 38 | ||||||
| WARFASA219 | 402 | 44 | 25 | 2 | 71 | 11 | ||||
| WODIT-DVT214 | 267 | 34 | 0 | 8 | 42 | 6 | 14 | |||
| WODIT-PE215 | 326 | 14 | 12 | 2 | 10 | 33 | 3 | 19 |
| Study | Study size | MI | Arterial event | All bleeding | Minor bleeding | Major bleeding | Fatal bleeding | Intracranial bleeding | CRNM bleeding | CRB |
|---|---|---|---|---|---|---|---|---|---|---|
| AMPLIFY-EXT221 | 2482 | 9 | 7 | 0 | 78 | 84 | ||||
| ASPIRE220 | 822 | 8 | 22 | 14 | 2 | 8 | 22 | |||
| EINSTEIN-EXTENSION206,217,218 | 1188 | 4 | 0 | 39 | 43 | |||||
| LAFIT213 | 162 | 10 | 7 | 3 | ||||||
| PREVENT216 | 508 | 5 | 94 | 7 | ||||||
| RE-MEDY222 | 2856 | 11 | 650 | 38 | 6 | 225 | ||||
| RE-SONATE222 | 1343 | 2 | 111 | 2 | 46 | 48 | ||||
| WARFASA219 | 402 | 13 | 2 | 6 | 8 | |||||
| WODIT-DVT214 | 267 | 6 | 2 | |||||||
| WODIT-PE215 | 326 | 11 | 4 | 0 |
Results are presented as follows for each of the six outcomes. First, we provide network plots to illustrate the comparisons of interventions made in the different trials. Second, we illustrate the risk-of-bias assessments that were specific to the outcome for each trial included in the network. Third, we present results tables for each intervention compared with the reference treatment (placebo). Fourth, we present for each NOAC intervention compared with aspirin and warfarin. Fifth, we present results tables for pairwise comparisons among licensed doses of the NOACs. For all sets of results tables, posterior median ORs and 95% credible intervals from Bayesian fixed-effects analyses are shown, although we refer to the latter as CIs for convenience. In these tables, we present results separately for any available direct evidence, for any indirect comparisons that can be made (excluding the direct evidence) and for the NMA (which combines the direct and the indirect evidence). Comparisons from the NMA with a ratio between interval limits exceeding nine were considered ‘imprecisely estimated’ and are presented at the bottom of each table (note that calculation of indirect evidence was not undertaken for imprecisely estimated comparisons). A summary of results across outcomes is provided at the end, in the form of a ‘rankogram’, which illustrates the probability that each treatment is best, second best, and so on, for each outcome. Lastly, forest plots of all contributing data, with ORs calculated using standard frequentist methods, are included in Appendix 5.
Symptomatic venous thromboembolism
All 10 studies206,213–222 reported on symptomatic VTE (578 events), leading to a network of all nine interventions (Figure 55). The included studies were judged to be at mostly low risk of bias, with concerns about only lack of blinding of participants and personnel in some studies (Table 140). There was evidence that aspirin (100 mg od) decreased the risk of symptomatic VTE compared with placebo (Table 141). Both warfarin (INR 1.5–2) and warfarin (INR 2–3) substantially reduced risk of symptomatic VTE compared with placebo. All NOACs at the doses included in the network substantially reduced risk of symptomatic VTE compared with placebo. Risk of symptomatic VTE was lower for all NOACs at doses included in the network compared with aspirin (Table 142). However, there was no clear evidence that risk of symptomatic VTE differed between these NOAC interventions and warfarin (INR 2–3), although most comparisons were imprecisely estimated (Table 143). There was no clear evidence that risk of symptomatic VTE differed between licensed doses of NOACs (Table 144), although all comparisons were imprecisely estimated.
FIGURE 55.
Network plot for symptomatic VTE (secondary prevention of VTE).
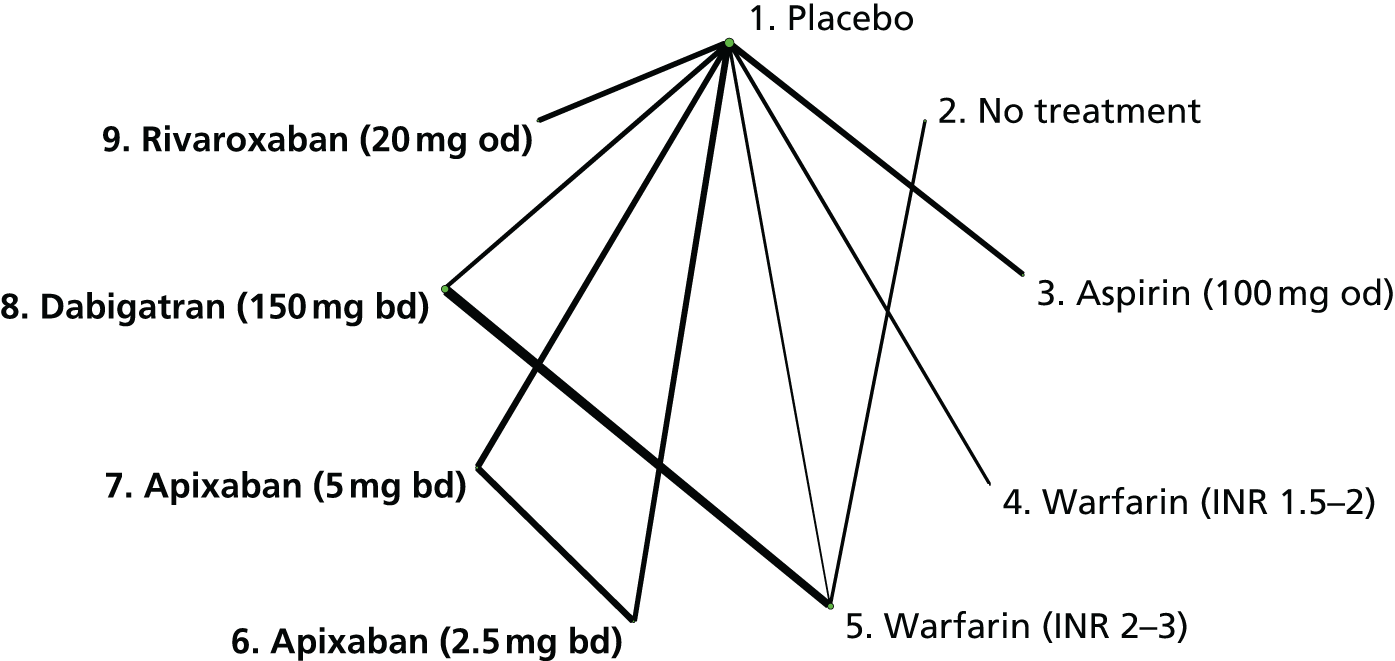
| Study | Interventions compared | SG | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| AMPLIFY-EXT221 | 1, 6, 7 | + | + | ? | + | + | + |
| ASPIRE220 | 1, 3 | + | + | + | + | + | + |
| EINSTEIN-EXTENSION206,217,218 | 1, 9 | + | + | – | + | + | + |
| LAFIT211 | 1, 5 | + | + | + | + | + | ? |
| PREVENT216 | 1, 4 | + | + | + | + | + | + |
| RE-MEDY222 | 5, 8 | + | + | + | + | + | + |
| RE-SONATE222 | 1, 8 | + | + | ? | + | + | + |
| WARFASA219 | 1, 3 | + | ? | ? | + | + | + |
| WODIT-DVT214 | 2, 5 | ? | – | – | + | + | ? |
| WODIT-PE215 | 2, 5 | + | + | – | + | + | ? |
| Comparisons with placebo | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Aspirin (100 mg od) | 0.68 (0.50 to 0.92) | – | 0.68 (0.50 to 0.92) |
| Warfarin (INR 1.5–2) | 0.33 (0.17 to 0.63) | – | 0.33 (0.17 to 0.63) |
| Apixaban (2.5 mg bd) | 0.17 (0.09 to 0.30) | – | 0.17 (0.09 to 0.30) |
| Apixaban (5 mg bd) | 0.18 (0.09 to 0.31) | – | 0.18 (0.09 to 0.31) |
| Dabigatran (150 mg bd) | 0.07 (0.02 to 0.18) | – | 0.07 (0.02 to 0.18) |
| Rivaroxaban (20 mg od) | 0.17 (0.07 to 0.35) | 0.17 (0.07 to 0.35) | |
| Imprecisely estimated comparisons | |||
| No treatment | – | 0.05 (0.01 to 0.17) | 0.05 (0.01 to 0.17) |
| Warfarin (INR 2–3) | 0.05 (0.01 to 0.14) | – | 0.05 (0.01 to 0.14) |
| Comparisons with aspirin | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Apixaban (2.5 mg bd) | – | 0.25 (0.13 to 0.48) | 0.25 (0.13 to 0.48) |
| Apixaban (5 mg bd) | – | 0.26 (0.13 to 0.50) | 0.26 (0.13 to 0.50) |
| Rivaroxaban (20 mg od) | – | 0.25 (0.10 to 0.55) | 0.25 (0.10 to 0.55) |
| Imprecisely estimated comparisons | |||
| Dabigatran (150 mg bd) | – | 0.10 (0.03 to 0.28) | 0.10 (0.03 to 0.28) |
| Comparisons with warfarin (INR 2–3) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Dabigatran (150 mg bd) | 1.36 (0.67 to 2.80) | – | 1.36 (0.67 to 2.80) |
| Imprecisely estimated comparisons | |||
| Apixaban (2.5 mg bd) | – | 2.10 (0.42 to 14.0) | 2.10 (0.42 to 14.0) |
| Apixaban (5 mg bd) | – | 2.96 (0.64 to 19.1) | 2.96 (0.64 to 19.1) |
| Rivaroxaban (20 mg od) | – | 3.01 (0.55 to 20.4) | 3.01 (0.55 to 20.4) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Apixaban (5 mg bd) vs. apixaban (2.5 mg bd) | 1.04 (0.48 to 2.22) | – | 1.04 (0.48 to 2.22) |
| Rivaroxaban (20 mg od) vs. apixaban (2.5 mg bd) | – | 0.99 (0.36 to 2.6) | 0.99 (0.36 to 2.6) |
| Rivaroxaban (20 mg od) vs. apixaban (5 mg bd) | – | 0.96 (0.35 to 2.48) | 0.96 (0.35 to 2.48) |
| Imprecisely estimated comparisons | |||
| Dabigatran (150 mg bd) vs. apixaban (2.5 mg bd) | – | 0.41 (0.11 to 1.29) | 0.41 (0.11 to 1.29) |
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | – | 0.40 (0.11 to 1.25) | 0.40 (0.11 to 1.25) |
| Rivaroxaban (20 mg od) vs. dabigatran (150 mg bd) | – | 2.41 (0.67 to 9.93) | 2.41 (0.67 to 9.93) |
We conducted a supplementary analysis using HRs for symptomatic recurrent VTE. The structure of the network was exactly the same as that presented in Figure 55. Results, presented in Tables 145–148, were similar to those based on ORs.
| Comparisons with placebo | HR (95% CI) |
|---|---|
| Aspirin (100 mg od) | 0.68 (0.51 to 0.90) |
| Warfarin (INR 1.5–2) | 0.36 (0.19 to 0.68) |
| Warfarin (INR 2–3) | 0.05 (0.02 to 0.16) |
| Apixaban (2.5 mg bd) | 0.17 (0.10 to 0.31) |
| Apixaban (5 mg bd) | 0.18 (0.10 to 0.32) |
| Dabigatran (150 mg bd) | 0.08 (0.03 to 0.22) |
| Rivaroxaban (20 mg od) | 0.18 (0.09 to 0.37) |
| Imprecisely estimated comparisons | |
| No treatment | 0.06 (0.02 to 0.23) |
| Comparisons with aspirin | HR (95% CI) |
|---|---|
| Apixaban (2.5 mg bd) | 0.25 (0.13 to 0.49) |
| Apixaban (5 mg bd) | 0.26 (0.14 to 0.51) |
| Dabigatran (150 mg bd) | 0.11 (0.04 to 0.34) |
| Rivaroxaban (20 mg od) | 0.27 (0.12 to 0.58) |
| Comparisons with warfarin (INR 2–3) | HR (95% CI) |
|---|---|
| Dabigatran (150 mg bd) | 1.45 (0.80 to 2.60) |
| Imprecisely estimated comparisons | |
| Apixaban (2.5 mg bd) | 3.24 (0.92 to 11.4) |
| Apixaban (5 mg bd) | 3.36 (0.95 to 11.7) |
| Rivaroxaban (20 mg od) | 3.41 (0.88 to 12.6) |
| Licensed NOACs only | HR (95% CI) |
|---|---|
| Imprecisely estimated comparisons | |
| Apixaban (5 mg bd) vs. apixaban (2.5 mg bd) | 0.57 (0.14 to 1.94) |
| Dabigatran (150 mg bd) vs. apixaban (2.5 mg bd) | 0.48 (0.01 to 6.79) |
| Rivaroxaban (20 mg od) vs. apixaban (2.5 mg bd) | 0.86 (0.02 to 13.0) |
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | 0.85 (0.02 to 13.4) |
| Rivaroxaban (20 mg od) vs. apixaban (5 mg bd) | 1.54 (0.04 to 25.7) |
| Rivaroxaban (20 mg od) vs. dabigatran (150 mg bd) | 1.79 (0.03 to 121) |
Symptomatic deep-vein thrombosis
Nine studies204,211–213,215–220 reported 342 symptomatic DVT events, leading to a network of eight interventions (Figure 56). These studies were mostly judged to be at low risk of bias (Table 149), with some concerns about lack of blinding of participants and personnel. There was no clear evidence that aspirin (100 mg od) reduced risk of symptomatic DVT compared with placebo (Table 150). There was evidence that warfarin (INR 2–3) and all NOACs at doses included in the network substantially reduced risk of symptomatic DVT compared with placebo. These NOAC interventions substantially reduced risk of symptomatic DVT compared with aspirin (Table 151). By contrast, there was no clear evidence that risk of symptomatic DVT differed between these NOACs and warfarin (INR 2–3), although comparisons were imprecisely estimated (Table 152). There was no clear evidence that risk of symptomatic DVT differed between NOACs at licensed doses, although all comparisons were imprecisely estimated (Table 153).
FIGURE 56.
Network plot for symptomatic DVT (secondary prevention of VTE).

| Study | Interventions compared | SG | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| AMPLIFY-EXT221 | 1, 6, 7 | + | + | ? | + | + | + |
| ASPIRE220 | 1, 3 | + | + | + | + | + | + |
| EINSTEIN-EXTENSION206,217,218 | 1, 9 | + | + | – | + | + | + |
| LAFIT211 | 1, 5 | + | + | + | + | + | ? |
| RE-MEDY222 | 5, 8 | + | + | + | + | + | + |
| RE-SONATE222 | 1, 8 | + | + | ? | + | + | + |
| WARFASA219 | 1, 3 | + | ? | ? | + | + | + |
| WODIT-DVT214 | 2, 5 | ? | – | – | + | + | ? |
| WODIT-PE215 | 2, 5 | + | + | – | + | + | ? |
| Comparisons with placebo | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Aspirin (100 mg od) | 0.74 (0.51 to 1.07) | – | 0.74 (0.51 to 1.07) |
| Apixaban (2.5 mg bd) | 0.1 (0.04 to 0.22) | – | 0.1 (0.04 to 0.22) |
| Apixaban (5 mg bd) | 0.14 (0.06 to 0.28) | – | 0.14 (0.06 to 0.28) |
| Rivaroxaban (20 mg od) | 0.14 (0.05 to 0.34) | – | 0.14 (0.05 to 0.34) |
| Imprecisely estimated comparisons | |||
| No treatment | – | 0.05 (0.01 to 0.22) | 0.05 (0.01 to 0.22) |
| Warfarin (INR 2–3) | 0.05 (0.01 to 0.17) | – | 0.05 (0.01 to 0.17) |
| Dabigatran (150 mg bd) | 0.07 (0.01 to 0.21) | – | 0.07 (0.01 to 0.21) |
| Comparisons with aspirin | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Apixaban (2.5 mg bd) | – | 0.14 (0.05 to 0.32) | 0.14 (0.05 to 0.32) |
| Apixaban (5 mg bd) | – | 0.19 (0.08 to 0.42) | 0.19 (0.08 to 0.42) |
| Rivaroxaban (20 mg od) | – | 0.19 (0.06 to 0.51) | 0.19 (0.06 to 0.51) |
| Imprecisely estimated comparisons | |||
| Dabigatran (150 mg bd) | – | 0.09 (0.02 to 0.30) | 0.09 (0.02 to 0.30) |
| Comparisons with warfarin (INR 2–3) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Imprecisely estimated comparisons | |||
| Apixaban (2.5 mg bd) | – | 2.10 (0.42 to 14.0) | 2.10 (0.42 to 14.0) |
| Apixaban (5 mg bd) | – | 2.96 (0.64 to 19.1) | 2.96 (0.64 to 19.1) |
| Dabigatran (150 mg bd) | 1.36 (0.67 to 2.80) | – | 1.36 (0.67 to 2.80) |
| Rivaroxaban (20 mg od) | – | 3.01 (0.55 to 20.4) | 3.01 (0.55 to 20.4) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Imprecisely estimated comparisons | |||
| Apixaban (5 mg bd) vs. apixaban (2.5 mg bd) | 1.40 (0.48 to 4.37) | – | 1.40 (0.48 to 4.37) |
| Dabigatran (150 mg bd) vs. apixaban (2.5 mg bd) | – | 0.65 (0.10 to 3.04) | 0.65 (0.10 to 3.04) |
| Rivaroxaban (20 mg od) vs. apixaban (2.5 mg bd) | – | 1.44 (0.37 to 5.36) | 1.44 (0.37 to 5.36) |
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | – | 0.46 (0.07 to 1.98) | 0.46 (0.07 to 1.98) |
| Rivaroxaban (20 mg od) vs. apixaban (5 mg bd) | – | 1.02 (0.28 to 3.46) | 1.02 (0.28 to 3.46) |
| Rivaroxaban (20 mg od) vs. dabigatran (150 mg bd) | – | 2.21 (0.43 to 14.2) | 2.21 (0.43 to 14.2) |
Symptomatic pulmonary embolism
Three studies reported symptomatic PE events, and a further six studies reported symptomatic non-fatal and fatal PE events, which were added together. The studies reported a total 173 symptomatic PE events, leading to a network comparing eight interventions (Figure 57). The included studies were mostly judged to be at low risk of bias (Table 154), with some concerns about lack of blinding of participants and personnel. There was evidence that warfarin (INR 2–3), apixaban (5 mg bd), dabigatran (150 mg bd) and rivaroxaban (20 mg od) substantially reduce the risk of symptomatic PE compared with placebo (Table 155). There was evidence that dabigatran (150 mg bd) and rivaroxaban (20 mg od) reduce the risk of symptomatic PE compared with aspirin (Table 156). There was evidence that risk of symptomatic PE was higher for apixaban (2.5 mg bd) than warfarin (INR 2–3) (Table 157). There was weak evidence that risk of symptomatic PE was lower for dabigatran (150 mg bd) and rivaroxaban (20 mg od) than apixaban (2.5 mg bd) (Table 158).
FIGURE 57.
Network plot for symptomatic PE (secondary prevention of VTE).

| Study | Interventions compared | SG | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| AMPLIFY-EXT221 | 1, 6, 7 | + | + | ? | + | + | + |
| ASPIRE220 | 1, 3 | + | + | + | + | + | + |
| EINSTEIN-EXTENSION206,217,218 | 1, 9 | + | + | – | + | + | + |
| LAFIT211 | 1, 5 | + | + | + | + | + | ? |
| RE-MEDY222 | 5, 8 | + | + | + | + | + | + |
| RE-SONATE222 | 1, 8 | + | + | ? | + | + | + |
| WARFASA219 | 1, 3 | + | ? | ? | + | + | + |
| WODIT-DVT214 | 2, 5 | ? | – | – | + | + | ? |
| WODIT-PE215 | 2, 5 | + | + | – | + | + | ? |
| Comparisons with placebo | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Aspirin (100 mg od) | 0.63 (0.38 to 1.02) | – | 0.63 (0.38 to 1.02) |
| Imprecisely estimated comparisons | |||
| No treatment | – | 0.05 (0.01 to 0.32) | 0.05 (0.01 to 0.32) |
| Warfarin (INR 2–3) | 0.05 (0.01 to 0.24) | – | 0.05 (0.01 to 0.24) |
| Apixaban (2.5 mg bd) | 0.51 (0.20 to 1.21) | – | 0.51 (0.20 to 1.21) |
| Apixaban (5 mg bd) | 0.25 (0.07 to 0.71) | – | 0.25 (0.07 to 0.71) |
| Dabigatran (150 mg bd) | 0.09 (0.01 to 0.35) | – | 0.09 (0.01 to 0.35) |
| Rivaroxaban (20 mg od) | 0.12 (0.02 to 0.45) | – | 0.12 (0.02 to 0.45) |
| Comparisons with aspirin | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Apixaban (2.5 mg bd) | – | 0.81 (0.29 to 2.19) | 0.81 (0.29 to 2.19) |
| Imprecisely estimated comparisons | |||
| Apixaban (5 mg bd) | – | 0.40 (0.10 to 1.28) | 0.40 (0.10 to 1.28) |
| Dabigatran (150 mg bd) | – | 0.14 (0.02 to 0.61) | 0.14 (0.02 to 0.61) |
| Rivaroxaban (20 mg od) | – | 0.19 (0.03 to 0.78) | 0.19 (0.03 to 0.78) |
| Comparisons with warfarin (INR 2–3) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Dabigatran (150 mg bd) | 1.76 (0.64 to 5.24) | – | 1.76 (0.64 to 5.24) |
| Imprecisely estimated comparisons | |||
| Apixaban (2.5 mg bd) | – | 10.1 (1.66 to 102) | 10.1 (1.66 to 102) |
| Apixaban (5 mg bd) | – | 4.94 (0.66 to 53.6) | 4.94 (0.66 to 53.6) |
| Rivaroxaban (20 mg od) | – | 2.29 (0.19 to 28.4) | 2.29 (0.19 to 28.4) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Imprecisely estimated comparisons | |||
| Apixaban (5 mg bd) vs. apixaban (2.5 mg bd) | 0.49 (0.13 to 1.62) | – | 0.49 (0.13 to 1.62) |
| Dabigatran (150 mg bd) vs. apixaban (2.5 mg bd) | – | 0.18 (0.02 to 0.92) | 0.18 (0.02 to 0.92) |
| Rivaroxaban (20 mg od) vs. apixaban (2.5 mg bd) | – | 0.23 (0.03 to 1.18) | 0.23 (0.03 to 1.18) |
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | – | 0.36 (0.04 to 2.38) | 0.36 (0.04 to 2.38) |
| Rivaroxaban (20 mg od) vs. apixaban (5 mg bd) | – | 0.47 (0.05 to 3.04) | 0.47 (0.05 to 3.04) |
| Rivaroxaban (20 mg od) vs. dabigatran (150 mg bd) | – | 1.31 (0.12 to 14.0) | 1.31 (0.12 to 14.0) |
Myocardial infarction
Five studies214,218–220 reported 35 MI events, leading to a network of seven interventions (Figure 58). These studies were judged to be at low risk of bias (Table 159). All comparisons were imprecisely estimated (Tables 160–163).
FIGURE 58.
Network plot for MI (secondary prevention of VTE).
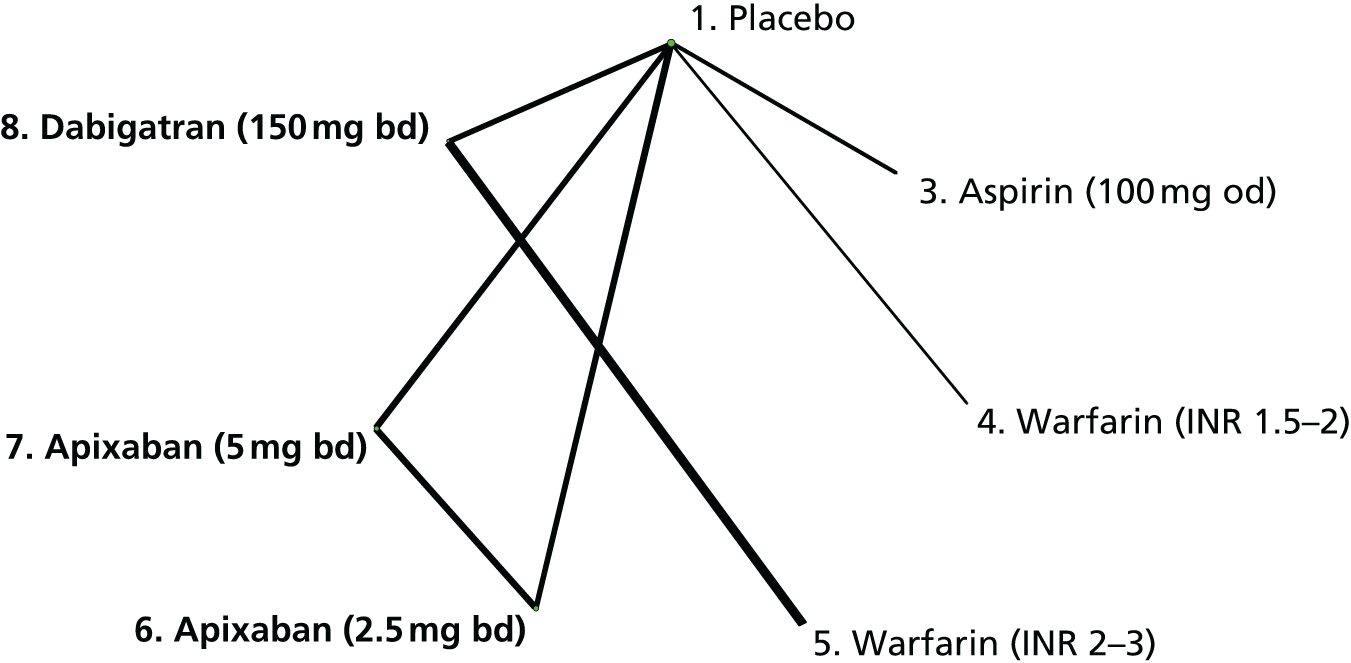
| Study | Interventions compared | SG | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| AMPLIFY-EXT221 | 1, 6, 7 | + | + | ? | + | + | + |
| ASPIRE220 | 1, 3 | + | + | + | + | + | + |
| PREVENT216 | 1, 4 | + | + | + | + | + | + |
| RE-MEDY222 | 5, 8 | + | + | + | + | + | + |
| RE-SONATE222 | 1, 8 | + | + | ? | + | + | + |
| Comparisons with placebo | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Imprecisely estimated comparisons | |||
| Aspirin (100 mg od) | 0.29 (0.04 to 1.37) | – | 0.29 (0.04 to 1.37) |
| Warfarin (INR 1.5–2) | 1.57 (0.24 to 14.0) | – | 1.57 (0.24 to 14.0) |
| Warfarin (INR 2–3) | 0.06 (0 to 3.26) | – | 0.06 (0 to 3.26) |
| Apixaban (2.5 mg bd) | 0.45 (0.06 to 2.51) | – | 0.45 (0.06 to 2.51) |
| Apixaban (5 mg bd) | 0.74 (0.13 to 3.59) | – | 0.74 (0.13 to 3.59) |
| Dabigatran (150 mg bd) | 0.90 (0.02 to 29.8) | – | 0.90 (0.02 to 29.8) |
| Comparisons with aspirin | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Imprecisely estimated comparisons | |||
| Apixaban (2.5 mg bd) | – | 1.57 (0.12 to 21.7) | 1.57 (0.12 to 21.7) |
| Apixaban (5 mg bd) | – | 2.60 (0.26 to 33.1) | 2.60 (0.26 to 33.1) |
| Dabigatran (150 mg bd) | – | 3.19 (0.05 to 174) | 3.19 (0.05 to 174) |
| Comparisons with warfarin (INR 2–3) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Imprecisely estimated comparisons | |||
| Apixaban (2.5 mg bd) | – | 7.48 (0.08 to 1220) | 7.48 (0.08 to 1220) |
| Apixaban (5 mg bd) | – | 12.6 (0.15 to 2000) | 12.6 (0.15 to 2000) |
| Dabigatran (150 mg bd) | 13.6 (2.26 to 409) | – | 13.6 (2.26 to 409) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Imprecisely estimated comparisons | |||
| Apixaban (5 mg bd) vs. apixaban (2.5 mg bd) | 1.65 (0.25 to 14.2) | – | 1.65 (0.25 to 14.2) |
| Dabigatran (150 mg bd) vs. apixaban (2.5 mg bd) | – | 2.06 (0.03 to 117) | 2.06 (0.03 to 117) |
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | – | 1.22 (0.02 to 57.5) | 1.22 (0.02 to 57.5) |
Major bleeding
All 10 studies204,212–220 reported on major bleeding (87 events), leading to a network of nine interventions (Figure 59). These studies204,212–220 were mostly judged to be at low risk of bias (Table 164), with some concerns about lack of blinding of participants and personnel. There was evidence that risk of major bleeding is higher for warfarin (INR 2–3) and rivaroxaban (20 mg od) than placebo, although these comparisons were imprecisely estimated (Table 165). Comparisons of the risk of major bleeding for NOACs compared with aspirin were imprecisely estimated (Table 166). There was evidence that risk of major bleeding is lower with dabigatran (150 mg bd), apixaban (2.5 mg bd) and apixaban (5 mg bd) than warfarin (INR 2–3) (Table 167). There was evidence that risk of major bleeding is higher with dabigatran (150 mg bd) and rivaroxaban (20 mg od) than apixaban (2.5 mg bd and 5 mg bd) (Table 168).
FIGURE 59.
Network plot for major bleeding (secondary prevention of VTE).
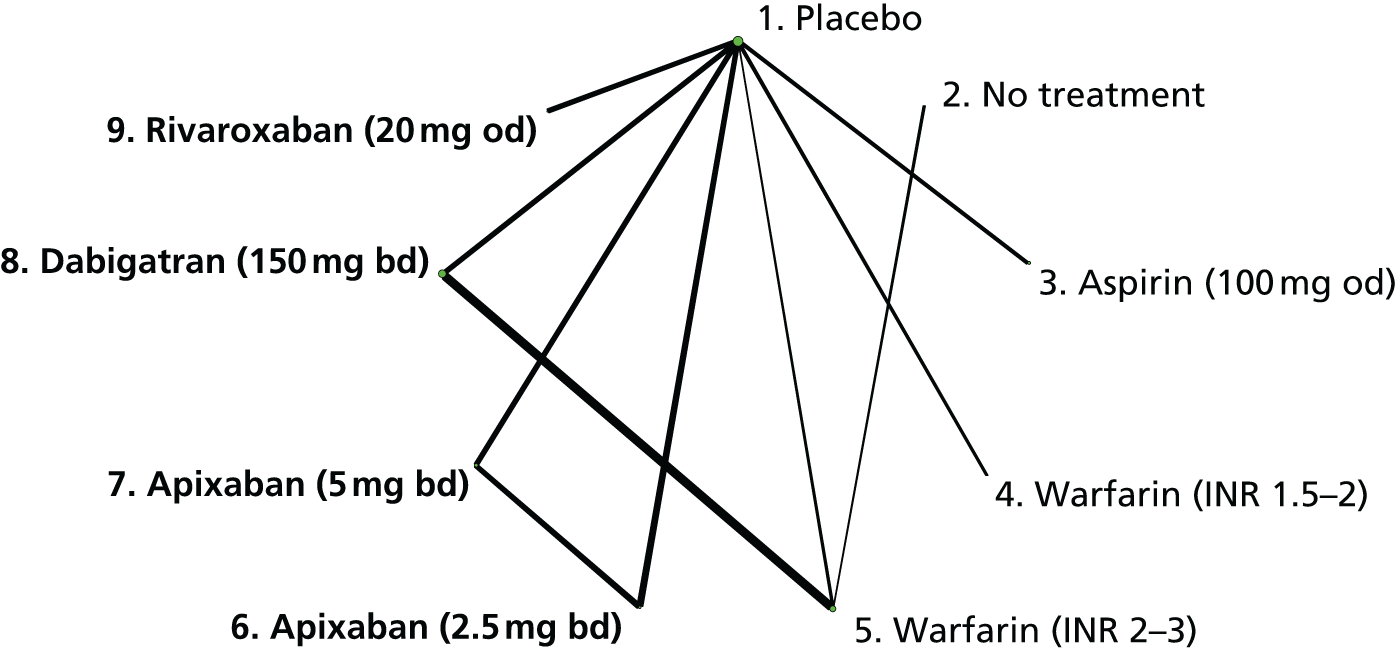
| Study | Interventions compared | SG | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| AMPLIFY-EXT221 | 1, 6, 7 | + | + | ? | + | + | + |
| ASPIRE220 | 1, 3 | + | + | + | + | + | + |
| EINSTEIN-EXTENSION206,217,218 | 1, 9 | + | + | – | + | + | + |
| LAFIT211 | 1, 5 | + | + | + | + | + | ? |
| PREVENT216 | 1, 4 | + | + | + | + | + | + |
| RE-MEDY222 | 5, 8 | + | + | + | + | + | + |
| RE-SONATE222 | 1, 8 | + | + | ? | + | + | + |
| WARFASA219 | 1, 3 | + | ? | ? | + | + | + |
| WODIT-DVT214 | 2, 5 | ? | – | – | + | + | ? |
| WODIT-PE215 | 2, 5 | + | + | – | + | + | ? |
| Comparisons with placebo | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Aspirin (100 mg od) | 1.3 (0.47 to 3.76) | – | 1.3 (0.47 to 3.76) |
| Imprecisely estimated comparisons | |||
| No treatment | – | 4.93 (0.36 to 142) | 4.93 (0.36 to 142) |
| Warfarin (INR 1.5–2) | 2.78 (0.55 to 22.2) | – | 2.78 (0.55 to 22.2) |
| Warfarin (INR 2–3) | 12.0 (1.66 to 279) | – | 12.0 (1.66 to 279) |
| Apixaban (2.5 mg bd) | 0.45 (0.06 to 2.57) | – | 0.45 (0.06 to 2.57) |
| Apixaban (5 mg bd) | 0.19 (0.01 to 1.56) | – | 0.19 (0.01 to 1.56) |
| Dabigatran (150 mg bd) | 6.11 (0.83 to 145) | – | 6.11 (0.83 to 145) |
| Rivaroxaban (20 mg od) | 17.8 (1.25 to 8340) | – | 17.8 (1.25 to 8340) |
| Comparisons with aspirin | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Imprecisely estimated comparisons | |||
| Apixaban (2.5 mg bd) | – | 0.34 (0.03 to 2.60) | 0.34 (0.03 to 2.60) |
| Apixaban (5 mg bd) | – | 0.14 (0 to 1.54) | 0.14 (0 to 1.54) |
| Dabigatran (150 mg bd) | – | 4.81 (0.50 to 126) | 4.81 (0.50 to 126) |
| Rivaroxaban (20 mg od) | – | 13.9 (0.78 to 6690) | 13.9 (0.78 to 6690) |
| Comparisons with warfarin | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Dabigatran (150 mg bd) | 0.51 (0.25 to 0.98) | – | 0.51 (0.25 to 0.98) |
| Imprecisely estimated comparisons | |||
| Apixaban (2.5 mg bd) | – | 0.03 (0 to 0.53) | 0.03 (0 to 0.53) |
| Apixaban (5 mg bd) | – | 0.01 (0 to 0.29) | 0.01 (0 to 0.29) |
| Rivaroxaban (20 mg od) | – | 1.52 (0.03 to 712) | 1.52 (0.03 to 712) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Imprecisely estimated comparisons | |||
| Apixaban (5 mg bd) vs. apixaban (2.5 mg bd) | 0.43 (0.01 to 5.42) | – | 0.43 (0.01 to 5.42) |
| Dabigatran (150 mg bd) vs. apixaban (2.5 mg bd) | – | 14.7 (0.96 to 582) | 14.7 (0.96 to 582) |
| Rivaroxaban (20 mg od) vs. apixaban (2.5 mg bd) | – | 44.8 (1.60 to 24,100) | 44.8 (1.60 to 24,100) |
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | – | 37.1 (1.70 to 2980) | 37.1 (1.70 to 2980) |
| Rivaroxaban (20 mg od) vs. apixaban (5 mg bd) | – | 116 (2.87 to 92,100) | 116 (2.87 to 92,100) |
| Rivaroxaban (20 mg od) vs. dabigatran (150 mg bd) | – | 3.01 (0.05 to 1390) | 3.01 (0.05 to 1390) |
Clinically relevant bleeding
Six studies204,215–220 reported 430 CRB events across trials, leading to a network of seven interventions (Figure 60). These studies204,215–220 were mostly judged to be at low risk of bias (Table 169) with some concerns about lack of blinding of participants and personnel. There was evidence that risk of CRB is substantially higher with warfarin (INR 2–3), dabigatran (150 mg od) and rivaroxaban (20 mg od) than placebo (Table 170) and that risk of CRB is higher with rivaroxaban (20 mg od) than aspirin (Table 171). There was evidence that risk of CRB is lower with apixaban (2.5 mg or 5 mg bd) and dabigatran (150 mg bd) than warfarin (INR 2–3) (Table 172). All comparisons between NOACs at licensed doses were imprecisely estimated, but there was evidence that risk of CRB is higher with dabigatran (150 mg bd) and rivaroxaban (20 mg od) than apixaban (2.5 mg bd and 5 mg bd) (Table 173).
FIGURE 60.
Network plot for CRB (secondary prevention of VTE).
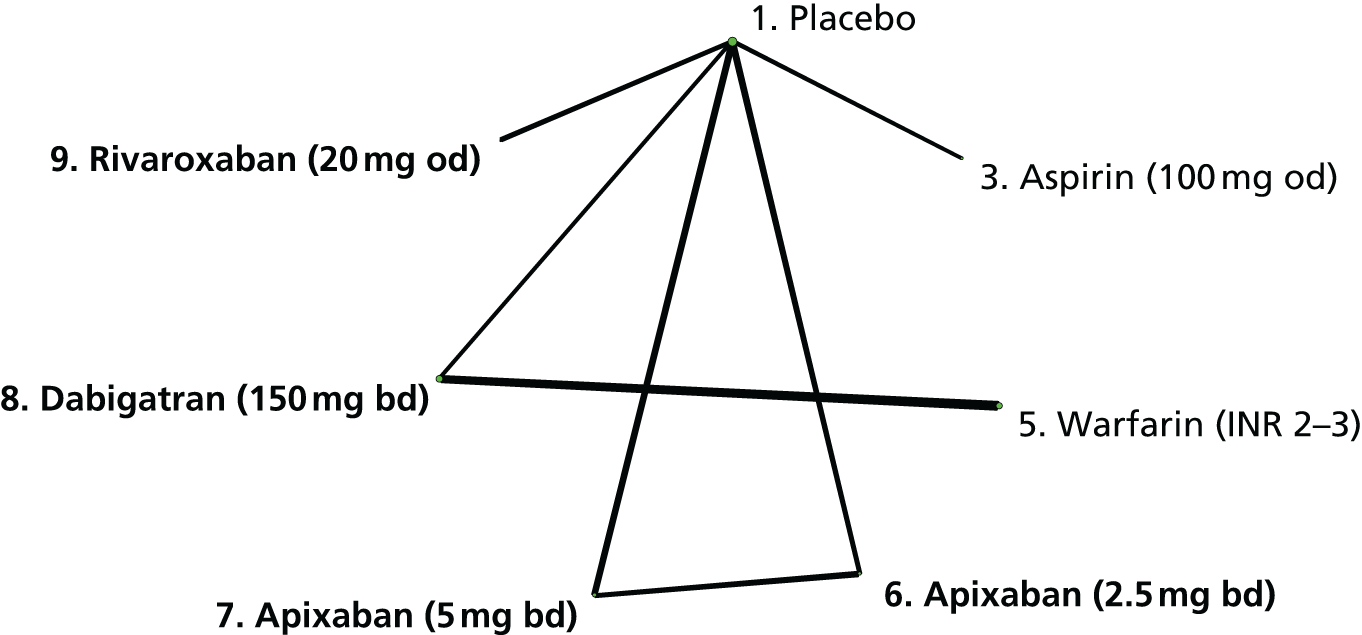
| Study | Interventions compared | SG | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| AMPLIFY-EXT221 | 1, 6, 7 | + | + | ? | + | + | + |
| ASPIRE220 | 1, 3 | + | + | + | + | + | + |
| EINSTEIN-EXTENSION206,217,218 | 1, 9 | + | + | – | + | + | + |
| RE-MEDY222 | 5, 8 | + | + | + | + | + | + |
| RE-SONATE222 | 1, 8 | + | + | ? | + | + | + |
| WARFASA219 | 1, 3 | + | ? | ? | + | + | + |
| Comparisons with placebo | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Aspirin (100 mg od) | 1.51 (0.72 to 3.27) | – | 1.51 (0.72 to 3.27) |
| Warfarin (INR 2–3) | 5.85 (2.93 to 12.6) | – | 5.85 (2.93 to 12.6) |
| Apixaban (2.5 mg bd) | 1.22 (0.69 to 2.19) | – | 1.22 (0.69 to 2.19) |
| Apixaban (5 mg bd) | 1.66 (0.96 to 2.89) | – | 1.66 (0.96 to 2.89) |
| Dabigatran (150 mg bd) | 3.05 (1.62 to 6.25) | – | 3.05 (1.62 to 6.25) |
| Rivaroxaban (20 mg od) | 5.56 (2.58 to 14.0) | – | 5.56 (2.58 to 14.0) |
| Comparisons with aspirin | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Apixaban (2.5 mg bd) | – | 0.81 (0.31 to 2.08) | 0.81 (0.31 to 2.08) |
| Apixaban (5 mg bd) | – | 1.10 (0.43 to 2.78) | 1.10 (0.43 to 2.78) |
| Dabigatran (150 mg bd) | – | 2.03 (0.75 to 5.66) | 2.03 (0.75 to 5.66) |
| Rivaroxaban (20 mg od) | – | 3.70 (1.25 to 12.0) | 3.70 (1.25 to 12.0) |
| Comparisons with warfarin (INR 2–3) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Apixaban (2.5 mg bd) | – | 0.21 (0.08 to 0.52) | 0.21 (0.08 to 0.52) |
| Apixaban (5 mg bd) | – | 0.28 (0.11 to 0.69) | 0.28 (0.11 to 0.69) |
| Dabigatran (150 mg bd) | 0.52 (0.39 to 0.69) | – | 0.52 (0.39 to 0.69) |
| Rivaroxaban (20 mg od) | – | 0.95 (0.32 to 3.01) | 0.95 (0.32 to 3.01) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Imprecisely estimated comparisons | |||
| Apixaban (5 mg bd) vs. apixaban (2.5 mg bd) | 0.43 (0.01 to 5.42) | – | 0.43 (0.01 to 5.42) |
| Dabigatran (150 mg bd) vs. apixaban (2.5 mg bd) | – | 14.7 (0.96 to 582) | 14.7 (0.96 to 582) |
| Rivaroxaban (20 mg od) vs. apixaban (2.5 mg bd) | – | 44.8 (1.60 to 24,100) | 44.8 (1.60 to 24,100) |
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | – | 37.1 (1.70 to 2980) | 37.1 (1.70 to 2980) |
| Rivaroxaban (20 mg od) vs. apixaban (5 mg bd) | – | 116 (2.87 to 92,100) | 116 (2.87 to 92,100) |
| Rivaroxaban (20 mg od) vs. dabigatran (150 mg bd) | – | 3.01 (0.05 to 1390) | 3.01 (0.05 to 1390) |
Bleeding (sensitivity analysis)
We conducted a supplementary analysis based on HRs for bleeding events reported in some studies. We extracted HRs for CRB, or for major bleeding if that was the only information available. The structure of this resulting network is presented in Figure 61. Results are similar to those for CRB (Tables 174–177).
FIGURE 61.
Network plot for bleeding (secondary prevention of VTE).
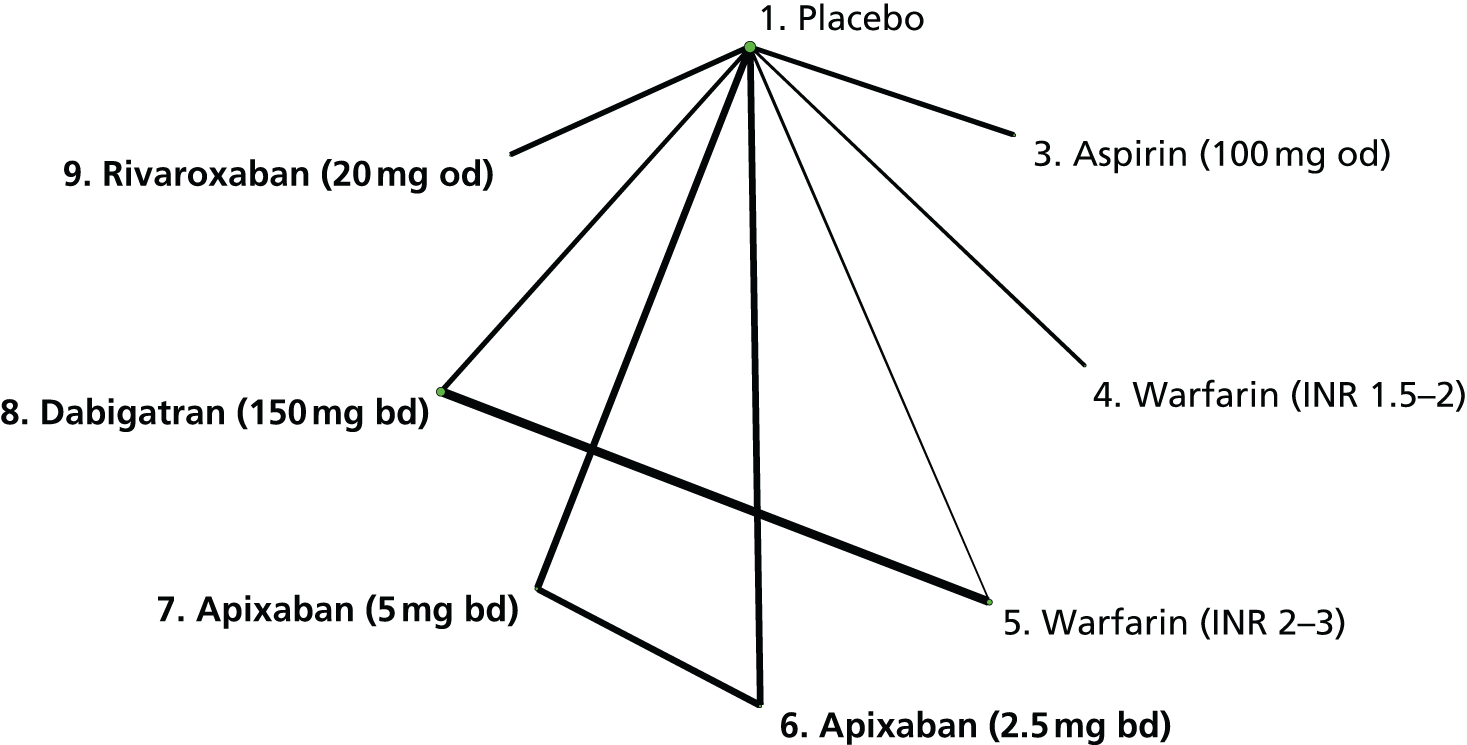
| Comparisons with placebo | NMA |
|---|---|
| HR (95% CI) | |
| Aspirin (100 mg od) | 1.48 (0.70 to 3.09) |
| Warfarin (INR 2–3) | 5.39 (2.64 to 10.8) |
| Apixaban (2.5 mg bd) | 1.29 (0.72 to 2.33) |
| Apixaban (5 mg bd) | 1.82 (1.05 to 3.17) |
| Dabigatran (150 mg bd) | 2.91 (1.51 to 5.54) |
| Rivaroxaban (20 mg od) | 5.19 (2.28 to 11.6) |
| Imprecisely estimated comparisons | |
| Warfarin (INR 1.5–2) | 2.54 (0.48 to 13.1) |
| Comparisons with aspirin | NMA |
|---|---|
| HR (95% CI) | |
| Apixaban (2.5 mg bd) | 0.87 (0.34 to 2.25) |
| Apixaban (5 mg bd) | 1.23 (0.48 to 3.14) |
| Dabigatran (150 mg bd) | 1.97 (0.73 to 5.25) |
| Rivaroxaban (20 mg od) | 3.51 (1.17 to 10.5) |
| Comparisons with warfarin (INR 2–3) | NMA |
|---|---|
| HR (95% CI) | |
| Apixaban (2.5 mg bd) | 0.24 (0.09 to 0.61) |
| Apixaban (5 mg bd) | 0.34 (0.14 to 0.84) |
| Dabigatran (150 mg bd) | 0.54 (0.41 to 0.71) |
| Rivaroxaban (20 mg od) | 0.96 (0.33 to 2.82) |
| Licensed NOACs only | NMA |
|---|---|
| HR (95% CI) | |
| Apixaban (5 mg bd) vs. apixaban (2.5 mg bd) | 1.65 (0.25 to 14.2) |
| Dabigatran (150 mg bd) vs. apixaban (2.5 mg bd) | 2.06 (0.03 to 117) |
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | 1.22 (0.02 to 57.5) |
All-cause mortality
Nine studies204,211–220 reported 158 all-cause mortality events, leading to a network of nine interventions (Figure 62). These studies204,211–220 were mostly judged to be at low risk of bias (Table 178), with some concerns about lack of blinding of participants and personnel. All comparisons of risk of all-cause mortality with placebo, except that for aspirin (100 mg od), were imprecisely estimated (Table 179). However, there was evidence that risk of all-cause mortality was lower for apixaban (5 mg bd) than placebo. Comparisons of NOACs with aspirin were imprecisely estimated, although there was weak evidence that risk of all-cause mortality is lower with apixaban (5 mg bd) than aspirin (Table 180). There was no evidence that risk of all-cause mortality differed for NOACs compared with warfarin (INR 2–3), although all comparisons – except that with dabigatran (150 mg bd) – were imprecisely estimated (Table 181). Comparisons of risk of all-cause mortality between NOACs at licensed doses were imprecisely estimated (Table 182).
FIGURE 62.
Network plot for all-cause mortality (secondary prevention of VTE).
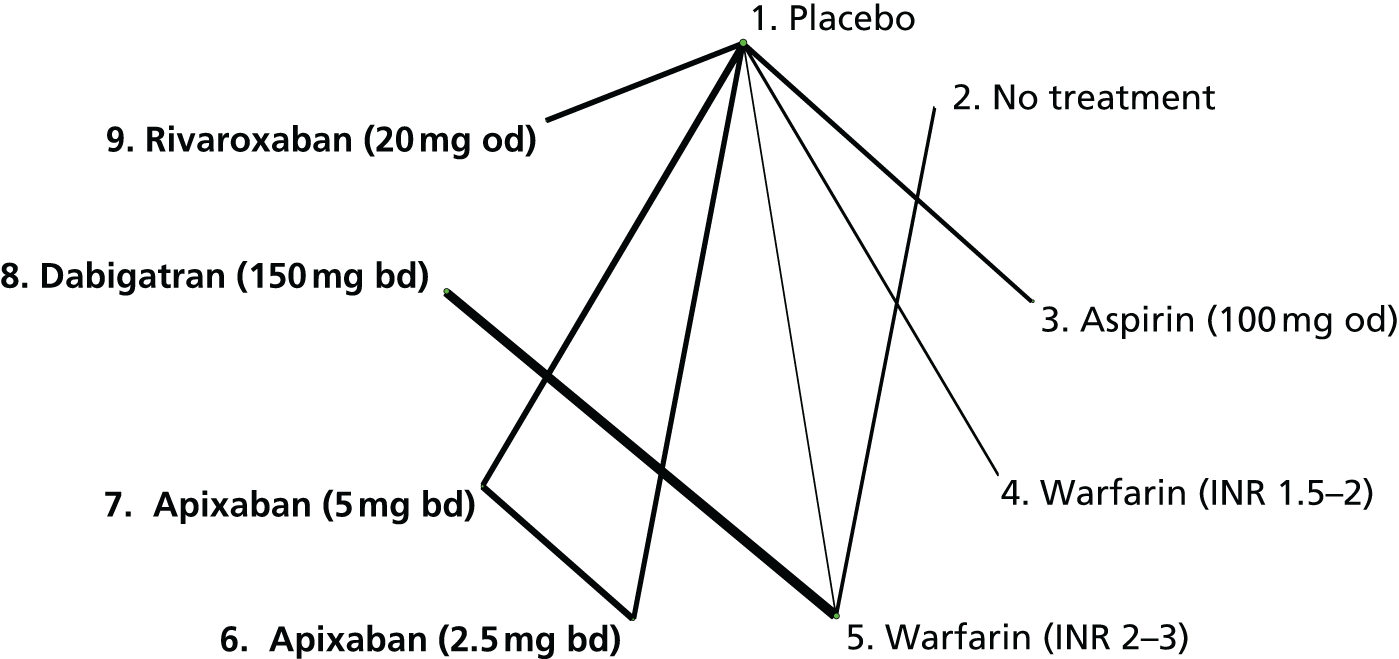
| Study | Interventions compared | SG | AC | BPP | BOA | IOD | SR |
|---|---|---|---|---|---|---|---|
| AMPLIFY-EXT221 | 1, 6, 7 | + | + | ? | + | + | + |
| ASPIRE220 | 1, 3 | + | + | + | + | + | + |
| EINSTEIN-EXTENSION206,217,218 | 1, 9 | + | + | – | + | + | + |
| LAFIT211 | 1, 5 | + | + | + | + | + | ? |
| PREVENT216 | 1, 4 | + | + | + | + | + | + |
| RE-MEDY222 | 5, 8 | + | + | + | + | + | + |
| WARFASA219 | 1, 3 | + | ? | ? | + | + | + |
| WODIT-DVT214 | 2, 5 | ? | – | – | + | + | ? |
| WODIT-PE215 | 2, 5 | + | + | – | + | + | ? |
| Comparisons with placebo | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Aspirin (100 mg od) | 0.94 (0.52 to 1.73) | – | 0.94 (0.52 to 1.73) |
| Apixaban (2.5 mg bd) | 0.48 (0.18 to 1.17) | – | 0.48 (0.18 to 1.17) |
| Imprecisely estimated comparisons | |||
| No treatment | – | 0.20 (0.01 to 2.03) | 0.20 (0.01 to 2.03) |
| Warfarin (INR 1.5–2) | 0.47 (0.12 to 1.54) | – | 0.47 (0.12 to 1.54) |
| Warfarin (INR 2–3) | 0.28 (0.01 to 2.47) | – | 0.28 (0.01 to 2.47) |
| Apixaban (5 mg bd) | 0.27 (0.07 to 0.78) | – | 0.27 (0.07 to 0.78) |
| Dabigatran (150 mg bd) | 0.25 (0.01 to 2.50) | – | 0.25 (0.01 to 2.50) |
| Rivaroxaban (20 mg od) | 0.41 (0.01 to 5.21) | – | 0.41 (0.01 to 5.21) |
| Comparisons with aspirin | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Imprecisely estimated comparisons | |||
| Apixaban (2.5 mg bd) | – | 0.50 (0.16 to 1.49) | 0.50 (0.16 to 1.49) |
| Apixaban (5 mg bd) | – | 0.29 (0.07 to 0.98) | 0.29 (0.07 to 0.98) |
| Dabigatran (150 mg bd) | – | 0.26 (0.01 to 2.87) | 0.26 (0.01 to 2.87) |
| Rivaroxaban (20 mg od) | – | 0.43 (0.01 to 5.90) | 0.43 (0.01 to 5.90) |
| Comparisons with warfarin (INR 2–3) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Dabigatran (150 mg bd) | 0.89 (0.45 to 1.73) | – | 0.89 (0.45 to 1.73) |
| Imprecisely estimated comparisons | |||
| Apixaban (2.5 mg bd) | – | 1.71 (0.15 to 60.6) | 1.71 (0.15 to 60.6) |
| Apixaban (5 mg bd) | – | 0.97 (0.08 to 35.1) | 0.97 (0.08 to 35.1) |
| Rivaroxaban (20 mg od) | – | 1.52 (0.03 to 98.3) | 1.52 (0.03 to 98.3) |
| Licensed NOACs only | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Imprecisely estimated comparisons | |||
| Apixaban (5 mg bd) vs. apixaban (2.5 mg bd) | – | 0.57 (0.14 to 1.94) | 0.57 (0.14 to 1.94) |
| Dabigatran (150 mg bd) vs. apixaban (2.5 mg bd) | – | 0.51 (0.01 to 6.39) | 0.51 (0.01 to 6.39) |
| Rivaroxaban (20 mg od) vs. apixaban (2.5 mg bd) | – | 0.85 (0.02 to 12.9) | 0.85 (0.02 to 12.9) |
| Dabigatran (150 mg bd) vs. apixaban (5 mg bd) | – | 0.91 (0.02 to 13.0) | 0.91 (0.02 to 13.0) |
| Rivaroxaban (20 mg od) vs. apixaban (5 mg bd) | – | 1.52 (0.04 to 26.3) | 1.52 (0.04 to 26.3) |
| Rivaroxaban (20 mg od) vs. dabigatran (150 mg bd) | – | 1.79 (0.03 to 121) | 1.79 (0.03 to 121) |
Summary of results
Our analyses of a network of 10 RCTs found evidence that warfarin (INR 2–3), apixaban (2.5 mg bd), apixaban (5 mg bd), dabigatran (150 mg bd) and rivaroxaban (20 mg od) reduce the risk of recurrent VTE, symptomatic DVT and symptomatic PE compared with placebo. Some of these reductions were substantial. We also found evidence that aspirin (100 mg od) and warfarin (INR 1.5–2) reduce the risk of recurrent VTE. The risk of recurrent VTE and symptomatic DVT is generally lower for NOACs at doses included in the network than for aspirin (100 mg od). However, there was little evidence that risks of recurrent VTE and symptomatic DVT differ comparing NOACs with warfarin (INR 2–3) or that the risk of these outcomes differs between licensed doses of NOACs. There was evidence that risk of symptomatic PE is higher with apixaban (2.5 mg bd) than warfarin (INR 2–3), and lower with dabigatran (150 mg bd) and rivaroxaban (20 mg od) than apixaban (2.5 mg bd).
By contrast, the risk of major bleeding and CRB is higher with warfarin (INR 2–3), dabigatran (150 mg od) and rivaroxaban (20 mg od) than placebo. However, the risk of these outcomes is lower for dabigatran (150 mg bd), apixaban (2.5 mg bd) and apixaban (5 mg bd) than warfarin (INR 2–3). There was evidence that the risk of major bleeding and CRB is higher with dabigatran (150 mg bd) and rivaroxaban (20 mg od) than apixaban (2.5 mg bd and 5 mg bd). However, results should be interpreted with caution because many comparisons were imprecisely estimated: for this reason it was not possible to derive a rankogram for this network.
For some outcomes there was evidence that patients who remained untreated had lower outcome risks than those on active interventions. This counterintuitive finding is based on the from WODIT-DVT214 and WODIT-PE215 trials. With regard to model appraisal, we did not identify any instance of lack of convergence among the Markov chains, poor model fit or inconsistency.
Chapter 10 Clinical results (5): combined safety analyses
In this chapter, we present network plots and pairwise comparisons from NMAs using the information from all four reviews. These should not be regarded as main results, but as a set of supplementary analyses in which we aimed to gain power by combining all databases in a single network for each of the following outcomes: MI, major bleeding, CRB and all-cause mortality.
A number of decisions were made in order to define the list of relevant nodes (e.g. interventions). We excluded the TOPIC-1,197 TOPIC-2197 and ARDEPARIN ARTHROPLASTY STUDY196 trials, as for the analyses of primary prevention of VTE. We also excluded several individual interventions that were not considered to provide relevant information and were not necessary to keep our networks connected. These were warfarin arms with a subtherapeutic INR range, arms combined dabigatran and aspirin (considered only in PETRO102), no treatment arms (only found in WODIT-DVT214 and WODIT-PE215 and compared with warfarin), LMWH (nadroparin 3800 IU anti-Xa od, implemented only in PROTECHT170 and compared with placebo), and warfarin with INR range 3–4 (considered only in AFASAK95 and compared with aspirin). If the intervention had been implemented in a two-arm trial then the trial was excluded from these analyses.
We also made several decisions in order to reduce the number of intervention arms compared. The reference treatment in our networks was warfarin (INR 2–3), which may include other VKA interventions, as was described for the analyses of AF. The antiplatelet interventions were defined as in the AF review (e.g. < 150 mg od and ≥ 150 mg od). The standard dose of LMWH was as in the review of primary prevention of VTE, and LMWH administered to non-surgical patients was combined with post-op LMWH. We merged some NOAC intervention doses and labelled these according to total daily dose. The edoxaban (60 mg) intervention included one arm from the review of acute VTE treatment in which 17% of patients received 30 mg instead. The list of interventions included in the networks is presented in Table 183.
| Warfarin | INR 2–3 |
|---|---|
| LMWH post-op | Standard dose |
| LMWH pre-op | Standard dose |
| LMWH | Enoxaparin 20 mg bd |
| Antiplatelet | < 150 mg od |
| ≥ 150 mg od | |
| Placebo | |
| Apixaban | 5 mg |
| 10 mg | |
| 20 mg | |
| Betrixaban | 30–60 mg |
| 80 mg | |
| Dabigatran | 100–150 mg |
| 220 mg | |
| 300–600 mg | |
| Edoxaban | 5–15 mg |
| 30–45 mg | |
| 60 mg | |
| 90–120 mg | |
| Rivaroxaban | 5 mg |
| 10 mg | |
| 20–30 mg |
Results are presented as follows for each outcome. First, we provide network plots. Second, we present results tables for each intervention compared with the reference treatment [warfarin (INR range 2–3)]. These tables show posterior median ORs and 95% credible intervals from Bayesian fixed-effects analyses are shown, but we refer to the latter as CIs for convenience. We present results separately for any available direct evidence, for any indirect comparisons that can be made and for the NMA (which combines the direct and the indirect evidence). Comparisons from the NMA with a ratio between interval limits exceeding nine were considered to be imprecisely estimated and are presented at the bottom of each table (back-calculation of indirect evidence was not done for imprecisely estimated comparisons).
Myocardial infarction
A total of 34 trials reported on MI across the four reviews, leading to a network of 18 interventions (Figure 63). The total number of events was 1489. Comparisons with the reference interventions [warfarin (INR 2–3)], presented in Table 184, suggest that the risk of MI is higher with dabigatran (220 mg daily), dabigatran (300–600 mg daily) and edoxaban (30–45 mg daily) than warfarin (INR 2–3).
FIGURE 63.
Network plot for MI (combined analysis).
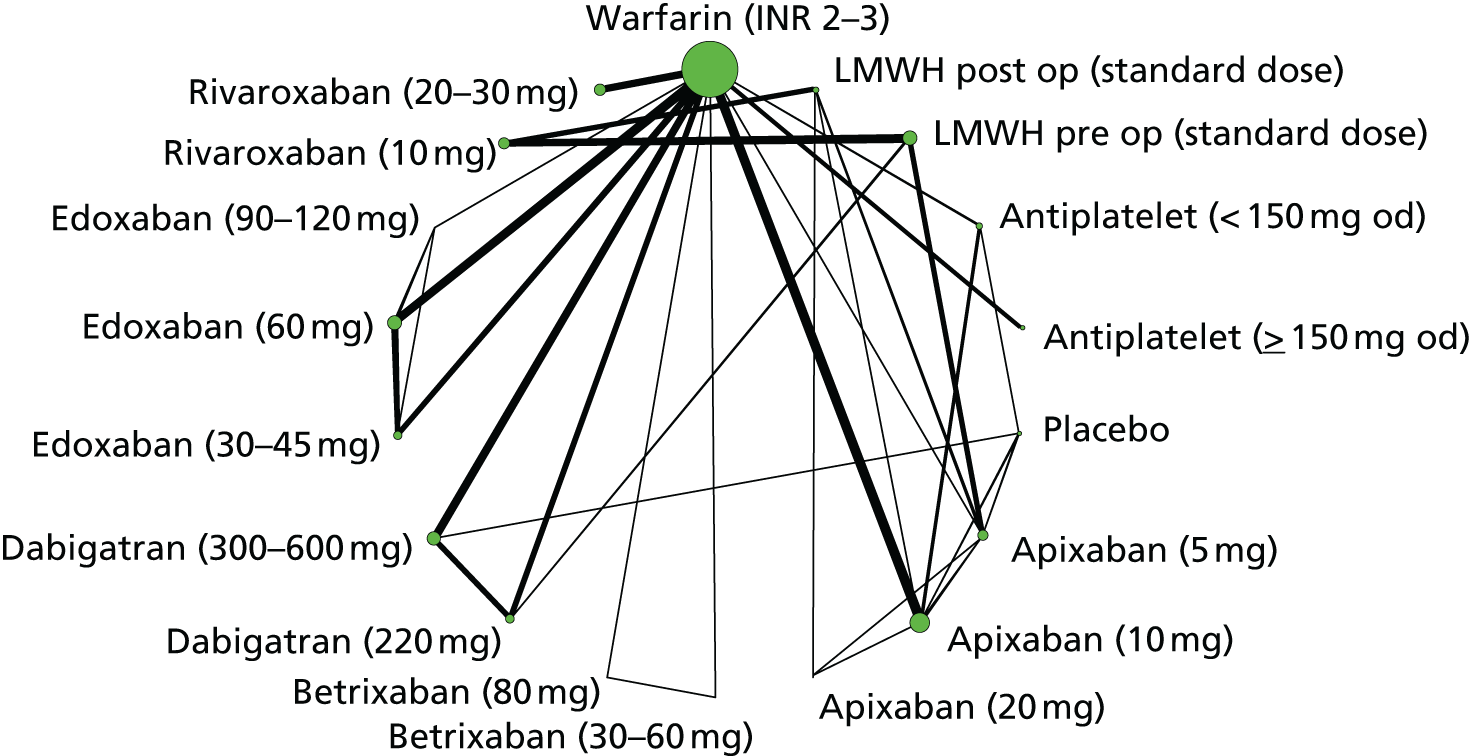
| Comparisons with warfarin (INR 2–3) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| Antiplatelet (< 150 mg) | 1.01 (0.63 to 1.60) | – | 1.01 (0.63 to 1.60) |
| Antiplatelet (≥ 150 mg) | 1.36 (0.88 to 2.13) | – | 1.36 (0.88 to 2.13) |
| Placebo | – | 2.23 (0.79 to 6.72) | 2.23 (0.79 to 6.72) |
| Apixaban (10 mg) | 0.90 (0.69 to 1.18) | – | 0.90 (0.69 to 1.18) |
| Dabigatran (220 mg) | 1.39 (1.03 to 1.89) | – | 1.39 (1.03 to 1.89) |
| Dabigatran (300–600 mg) | 1.44 (1.08 to 1.91) | – | 1.44 (1.08 to 1.91) |
| Edoxaban (30–45 mg) | 1.25 (1.00 to 1.56) | – | 1.25 (1.00 to 1.56) |
| Edoxaban (60 mg) | 1.01 (0.81 to 1.26) | – | 1.01 (0.81 to 1.26) |
| Rivaroxaban (20–30 mg) | 0.84 (0.64 to 1.09) | – | 0.84 (0.64 to 1.09) |
| Imprecisely estimated comparisons | |||
| LMWH post-op (standard dose) | – | 2.17 (0.50 to 9.72) | 2.17 (0.50 to 9.72) |
| LMWH pre-op (standard dose) | – | 0.82 (0.19 to 3.42) | 0.82 (0.19 to 3.42) |
| Apixaban (5 mg) | – | 1.33 (0.40 to 4.46) | 1.33 (0.40 to 4.46) |
| Apixaban (20 mg) | – | 0.46 (0.01 to 4.23) | 0.46 (0.01 to 4.23) |
| Edoxaban (90–120 mg) | 0.19 (0 to 2.61) | – | 0.19 (0 to 2.61) |
| Rivaroxaban (10 mg) | – | 0.60 (0.13 to 2.80) | 0.60 (0.13 to 2.80) |
Major bleeding
A total of 71 trials reported on major bleeding across the four reviews, leading to a network of 23 interventions (Figure 64). In total there were 5335 major bleeding events. The pairwise comparisons with warfarin, shown in Table 185, suggest that the risk of major bleeding is similar for both pre-op and post-op LMWH than warfarin (INR 2–3). However, there was notable inconsistency between the directly and indirectly estimated ORs. There was evidence that risk of major bleeding is lower for NOAC interventions than warfarin (INR 2–3), in agreement with the results from the AF review. This applies to the apixaban (10 mg daily), dabigatran (100–150 mg and 220 mg daily) and edoxaban interventions. Risk of major bleeding appeared higher with rivaroxaban (10 mg and 30–40 mg daily) than warfarin (INR 2–3), a finding that might stem from the evidence on primary prevention of VTE.
FIGURE 64.
Network plot for major bleeding (combined analysis).
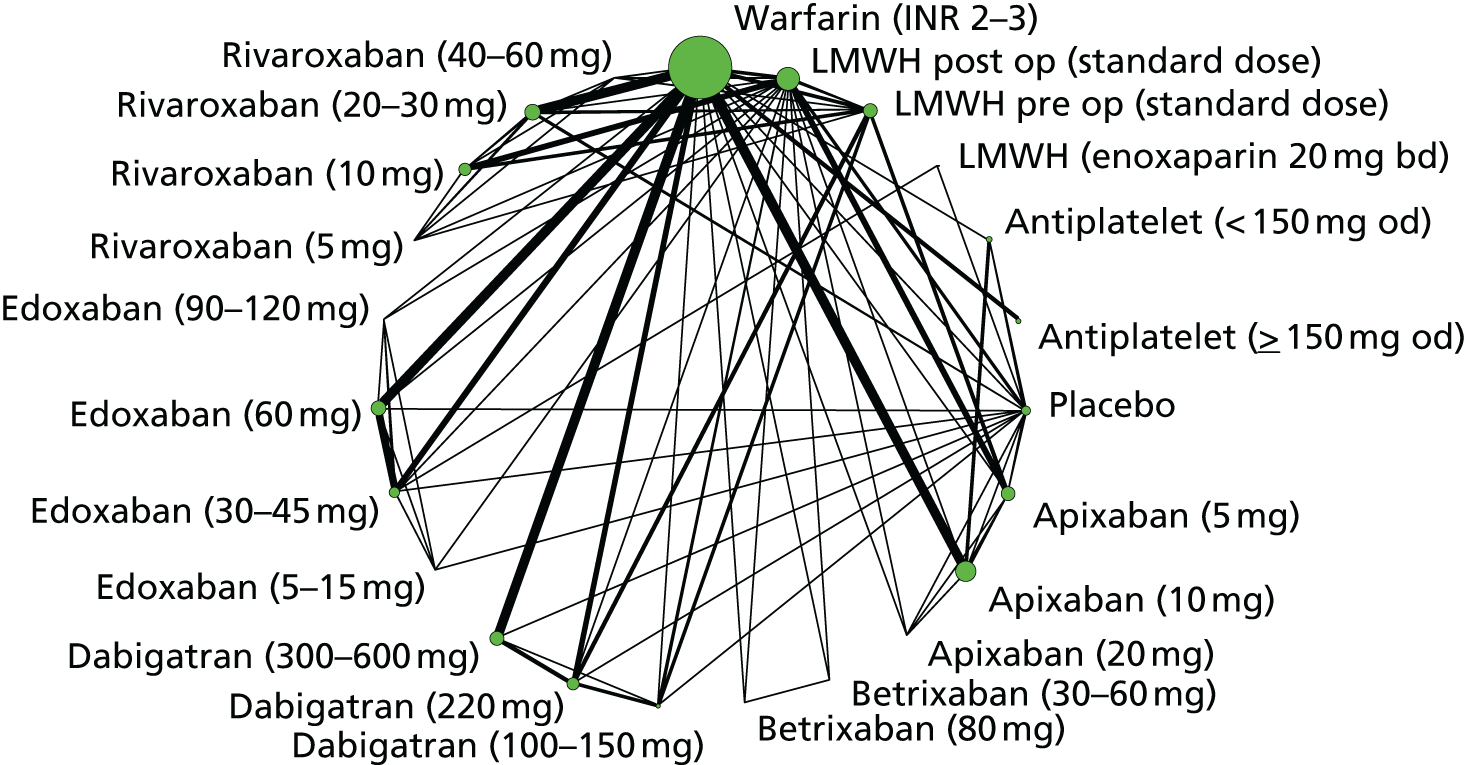
| Comparisons with warfarin (INR 2–3) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| LMWH post-op (standard dose) | 1.65 (1.11 to 2.44) | 0.61 (0.41 to 0.89) | 0.99 (0.75 to 1.29) |
| LMWH pre-op (standard dose) | 2.14 (1.36 to 3.36) | 0.62 (0.45 to 0.89) | 0.99 (0.75 to 1.3) |
| LMWH (enoxaparin 40 mg) | – | 0.61 (0.21 to 1.84) | 0.61 (0.21 to 1.84) |
| Antiplatelet (< 150 mg) | 1.01 (0.57 to 1.80) | 0.62 (0.41 to 0.93) | 0.73 (0.52 to 1.03) |
| Antiplatelet (≥ 150 mg) | 1.07 (0.82 to 1.41) | – | 1.07 (0.82 to 1.41) |
| Placebo | 0.60 (0.36 to 0.99) | – | 0.60 (0.36 to 0.99) |
| Apixaban (5 mg) | – | 0.89 (0.60 to 1.31) | 0.89 (0.60 to 1.31) |
| Apixaban (10 mg) | 0.67 (0.59 to 0.77) | – | 0.67 (0.59 to 0.77) |
| Apixaban (20 mg) | 1.77 (0.84 to 3.76) | – | 1.77 (0.84 to 3.76) |
| Dabigatran (100–150 mg) | – | 0.62 (0.39 to 0.97) | 0.62 (0.39 to 0.97) |
| Dabigatran (220 mg) | 0.82 (0.71 to 0.94) | – | 0.82 (0.71 to 0.94) |
| Dabigatran (300–600 mg) | 0.91 (0.80 to 1.04) | – | 0.91 (0.80 to 1.04) |
| Edoxaban (30–45 mg) | 0.47 (0.40 to 0.54) | – | 0.47 (0.40 to 0.54) |
| Edoxaban (60 mg) | 0.80 (0.70 to 0.90) | – | 0.80 (0.70 to 0.90) |
| Edoxaban (90–120 mg) | 2.43 (0.97 to 5.76) | – | 2.43 (0.97 to 5.76) |
| Rivaroxaban (5 mg) | – | 0.65 (0.22 to 1.55) | 0.65 (0.22 to 1.55) |
| Rivaroxaban (10 mg) | – | 1.71 (1.14 to 2.57) | 1.71 (1.14 to 2.57) |
| Rivaroxaban (20–30 mg) | 1.01 (0.88 to 1.15) | – | 1.01 (0.88 to 1.15) |
| Rivaroxaban (40–60 mg) | 1.18 (0.45 to 3.12) | 3.53 (2.00 to 6.22) | 2.67 (1.63 to 4.36) |
| Imprecisely estimated comparisons | |||
| Betrixaban (30–60 mg) | – | 0.09 (0 to 2.87) | 0.09 (0 to 2.87) |
| Betrixaban (80 mg) | – | 0.10 (0 to 3.07) | 0.10 (0 to 3.07) |
| Edoxaban (5–15 mg) | – | 0.63 (0.10 to 2.64) | 0.63 (0.10 to 2.64) |
Clinically relevant bleeding
A total of 51 trials reported on CRB, leading to a network of 22 interventions (Figure 65). These trials reported a total of 14,324 CRB events. Comparisons with the reference intervention [warfarin (INR 2–3)], presented in Table 186, suggest that risk of CRB was lower with LMWH than warfarin (INR 2–3). The risk of CRB was also lower for antiplatelets and placebo than warfarin (INR 2–3), as found in the AF and VTE secondary prevention reviews. Among the NOAC interventions, risk of CRB was lower with apixaban (5 mg and 10 mg daily), betrixaban (30–60 mg daily), dabigatran, edoxaban (30–45 mg and 60 mg daily) and rivaroxaban (5 mg and 10 mg daily) than warfarin (INR 2–3) but higher with edoxaban (90 mg daily). These findings are generally in agreement with those from the AF and VTE treatment reviews.
FIGURE 65.
Network plot for CRB (combined analysis).
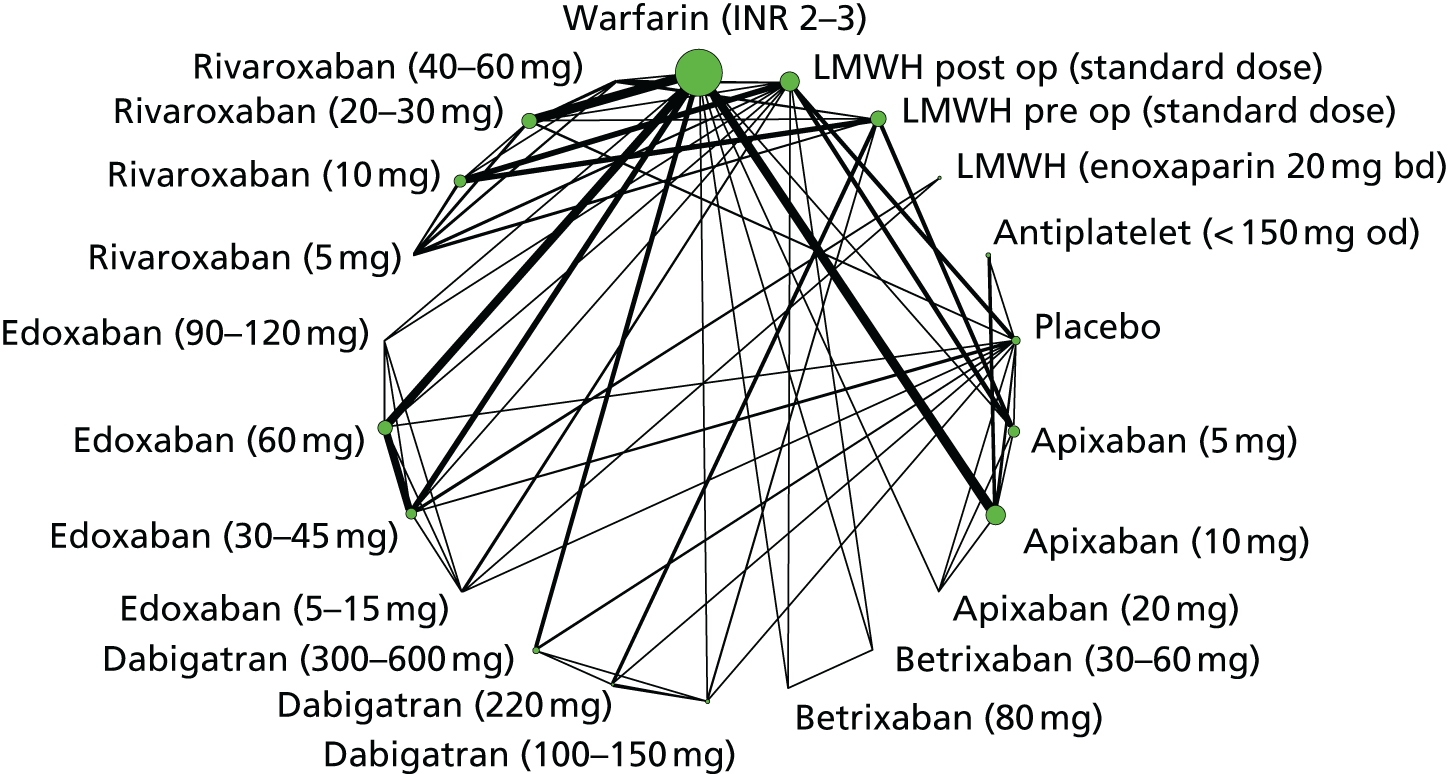
| Comparisons with warfarin (INR 2–3) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| LMWH post-op (standard dose) | – | 0.39 (0.29 to 0.53) | 0.39 (0.29 to 0.53) |
| LMWH pre-op (standard dose) | – | 0.48 (0.36 to 0.66) | 0.48 (0.36 to 0.66) |
| LMWH (enoxaparin 40 mg) | – | 0.52 (0.31 to 0.86) | 0.52 (0.31 to 0.86) |
| Antiplatelet (< 150 mg) | – | 0.52 (0.40 to 0.67) | 0.52 (0.40 to 0.67) |
| Placebo | 0.28 (0.21 to 0.37) | – | 0.28 (0.21 to 0.37) |
| Apixaban (5 mg) | 0.40 (0.30 to 0.54) | – | 0.40 (0.30 to 0.54) |
| Apixaban (10 mg) | 0.61 (0.55 to 0.67) | – | 0.61 (0.55 to 0.67) |
| Apixaban (20 mg) | 0.74 (0.40 to 1.38) | – | 0.74 (0.40 to 1.38) |
| Betrixaban (30–60 mg) | 0.24 (0.08 to 0.64) | – | 0.24 (0.08 to 0.64) |
| Betrixaban (80 mg) | 0.45 (0.16 to 1.21) | – | 0.45 (0.16 to 1.21) |
| Dabigatran (100–150 mg) | 0.54 (0.37 to 0.78) | – | 0.54 (0.37 to 0.78) |
| Dabigatran (220 mg) | – | 0.57 (0.39 to 0.82) | 0.57 (0.39 to 0.82) |
| Dabigatran (300–600 mg) | 0.62 (0.52 to 0.73) | – | 0.62 (0.52 to 0.73) |
| Edoxaban (5–15 mg) | – | 0.53 (0.25 to 1.08) | 0.53 (0.25 to 1.08) |
| Edoxaban (30–45 mg) | 0.59 (0.54 to 0.64) | – | 0.59 (0.54 to 0.64) |
| Edoxaban (60 mg) | 0.83 (0.78 to 0.89) | – | 0.83 (0.78 to 0.89) |
| Edoxaban (90–120 mg) | 2.04 (1.15 to 3.62) | 0.82 (0.27 to 2.52) | 1.69 (1.00 to 2.80) |
| Rivaroxaban (5 mg) | – | 0.42 (0.21 to 0.80) | 0.42 (0.21 to 0.80) |
| Rivaroxaban (10 mg) | – | 0.72 (0.53 to 0.98) | 0.72 (0.53 to 0.98) |
| Rivaroxaban (20–30 mg) | 1.00 (0.93 to 1.07) | – | 1.00 (0.93 to 1.07) |
| Rivaroxaban (40–60 mg) | 0.23 (0.06 to 0.85) | 1.46 (0.97 to 2.21) | 1.24 (0.84 to 1.83) |
All-cause mortality
In total 59 trials reported on all-cause mortality, leading to a network of 23 interventions (Figure 66). The total number of deaths was 8508. Comparisons with the reference intervention [warfarin (INR 2–3)], shown in Table 187, suggest that risk of all-cause mortality was higher with antiplatelet therapy (≥ 150 mg daily) than warfarin (INR 2–3). Risk of all-cause mortality was generally lower among the NOAC interventions [estimated ORs compared with warfarin (INR 2–3) were between 0.87 and 0.93].
FIGURE 66.
Network plot for all-cause mortality (combined analysis).
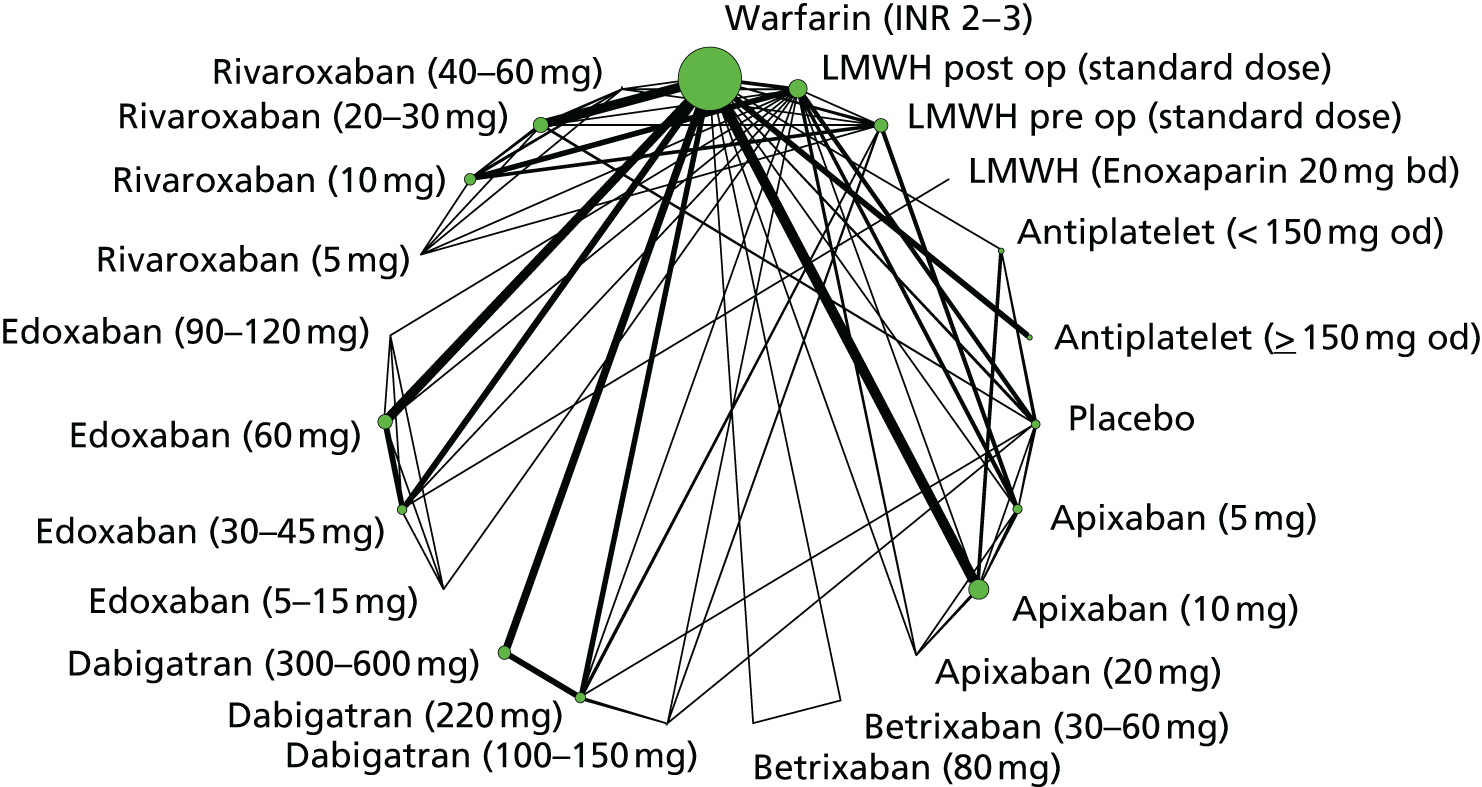
| Comparisons with warfarin (INR 2–3) | Direct evidence, OR (95% CI) | Indirect evidence, OR (95% CI) | NMA, OR (95% CI) |
|---|---|---|---|
| LMWH pre-op (standard dose) | – | 1.70 (0.84 to 3.50) | 1.70 (0.84 to 3.50) |
| LMWH post-op (standard dose) | 0.68 (0.32 to 1.47) | 1.26 (0.80 to 1.98) | 1.07 (0.72 to 1.6) |
| Antiplatelet (< 150 mg) | 1.02 (0.76 to 1.37) | 1.13 (0.87 to 1.47) | 1.08 (0.88 to 1.32) |
| Antiplatelet (≥ 150 mg) | 1.23 (1.02 to 1.48) | – | 1.23 (1.02 to 1.48) |
| Placebo | 1.15 (0.77 to 1.71) | – | 1.15 (0.77 to 1.71) |
| Apixaban (5 mg) | – | 1.07 (0.54 to 2.08) | 1.07 (0.54 to 2.08) |
| Apixaban (10 mg) | 0.87 (0.78 to 0.97) | – | 0.87 (0.78 to 0.97) |
| Dabigatran (220 mg) | 0.92 (0.81 to 1.04) | – | 0.92 (0.81 to 1.04) |
| Dabigatran (300–600 mg) | 0.89 (0.79 to 1.01) | – | 0.89 (0.79 to 1.01) |
| Edoxaban (30–45 mg) | 0.88 (0.79 to 0.97) | – | 0.88 (0.79 to 0.97) |
| Edoxaban (60 mg) | 0.93 (0.85 to 1.02) | – | 0.93 (0.85 to 1.02) |
| Rivaroxaban (10 mg) | – | 1.10 (0.70 to 1.72) | 1.10 (0.70 to 1.72) |
| Rivaroxaban (20–30 mg) | 0.87 (0.75 to 1.02) | – | 0.87 (0.75 to 1.02) |
| Imprecisely estimated comparisons | |||
| Apixaban (20 mg) | 0.67 (0.17 to 2.34) | – | 0.67 (0.17 to 2.34) |
| Betrixaban (30–60 mg) | 0.71 (0.07 to 10.2) | – | 0.71 (0.07 to 10.2) |
| Betrixaban (80 mg) | 0.19 (0 to 5.78) | – | 0.19 (0 to 5.78) |
| Dabigatran (100–150 mg) | – | 1.36 (0.32 to 5.00) | 1.36 (0.32 to 5.00) |
| Edoxaban (5–15 mg) | – | 0.76 (0.06 to 4.54) | 0.76 (0.06 to 4.54) |
| Edoxaban (90–120 mg) | – | 0.16 (0 to 2.30) | 0.16 (0 to 2.30) |
| Rivaroxaban (40–60 mg) | 0.27 (0.04 to 1.03) | – | 0.27 (0.04 to 1.03) |
Chapter 11 Cost-effectiveness results (2): venous thromboembolism
Introduction
In this chapter we present the results of the CEA for first-line secondary prevention, acute treatment and primary prevention of venous thromboembolic disease. The decision questions, populations, interventions, outcomes, model structures, cost and utility inputs have been previously described in Chapter 4. In this chapter we begin by describing clinical effectiveness inputs to the models, including relative treatment effects based on the evidence identified in the systematic reviews (see Chapters 7–9), transition probabilities on the reference treatment on which relative effects are applied, other state-transition probabilities based on evidence from longitudinal studies, and mortality. We then present the results from our cost-effectiveness model, together with sensitivity analyses to key assumptions made. Results are presented from Bayesian analyses with 95% credible intervals, although we refer to these as confidence intervals for convenience.
Model inputs: venous thromboembolism secondary prevention
Overview
The state-transition parameters that inform the secondary prevention model have two components. The relative effects of the different treatments come from the NMAs of the studies that were identified in the systematic review (see Chapter 9). The transition parameters under standard care (i.e. no pharmacological treatment) are taken from longitudinal studies that provide information on the natural history of VTE.
Relative treatment efficacy
Hazard ratios for the relative treatment effects of aspirin, warfarin and three NOACs (apixaban, dabigatran and rivaroxaban) compared with placebo are derived from the NMA (see Table 145). These HRs were applied to the risk of symptomatic VTE on the reference treatment (no pharmacotherapy) to estimate the efficacy of each intervention. The NMA revealed inconsistent results between the WODIT trials,212,213 which used a no-treatment control arm and other trials that used a placebo control arm. The estimated hazard for no treatment lacked face validity, as it was much lower than placebo and aspirin, and was similar to the NOACs. Therefore, we decided that aspirin, warfarin and NOAC efficacy relative to placebo was the more reliable estimate for the cost-effectiveness model.
Given a recurrent VTE event, we estimated the probability that it is a DVT, which can be subtracted from 1 to give the probability that it is a PE. If the recurrent VTE is a PE, we estimated the probability that is a non-fatal PE. Owing to very small numbers of events in the secondary prevention RCTs, we are unable to estimate relative treatment effects for these conditional probabilities and assumed that they are treatment independent. We therefore treat each arm of each trial as an independent source of information on the probability of (1) a DVT only, given a recurrent VTE event, and (2) a non-fatal PE, given a PE event. Eight out of the 10 studies in the systematic review were included in this analysis; two studies did not record counts of DVT, non-fatal PE and fatal PE. The counts of VTE, DVT, non-fatal PE and fatal PE are in Table 138. Both fixed- and random-effects single-arm meta-analyses were explored (including study arms with zero events). The random-effects models did not show evidence of a better fit than the fixed-effects models. We therefore used the results from the fixed-effects meta-analysis to estimate conditional probabilities and uncertainty using beta distributions (Table 188).
| Event | Proportion | Alpha | Beta | Distribution |
|---|---|---|---|---|
| DVT given recurrent VTE | 0.626 | 268 | 160 | Beta |
| Non-fatal PE, given PE | 0.919 | 147 | 13 | Beta |
Relative treatment safety
The criteria for and classification of bleeding events is not uniform across RCTs and is the subject of wider debate. 225 Our model distinguishes between fatal bleeds, non-fatal ICH and other clinically relevant bleeds (those which require an intervention or hospital admission). Minor bleeds, identified through close monitoring in RCTs, which do not require intervention, are not considered clinically relevant and have not been included in the model because of the minimal impact on quality of life and costs.
The incidence of ICH was not commonly reported in the secondary prevention VTE RCTs. Therefore, the relative treatment effects of the NOACs compared with warfarin for ICH were derived from RCTs conducted in the AF population (see Table 59). These trials included all NOAC and dose combinations compared in the secondary prevention of VTE. We assumed that the relative treatment effects of no pharmacotherapy and aspirin compared with warfarin for ICH are similar to those estimated for CRB (see Table 174).
The VTE secondary prevention RCTs did not provide sufficient information to determine what proportion of ICHs are fatal. Therefore, the proportion of non-fatal ICHs was estimated from a study226 that investigated ICHs in patients with AF using data from the RE-LY104 trial. In total there were 56 fatal and 98 non-fatal ICHs. The relative treatment effects for other CRB, compared with the reference group (no pharmacotherapy), for the interventions, were estimated in a NMA (see Table 174).
Transition probabilities with usual care (no pharmacotherapy)
A rapid literature review was conducted to identify long-term follow-up studies in a patient population with VTE to inform the natural history of VTE with usual care (no pharmacotherapy). The initial search identified 3915 abstracts. After abstract selection and full-paper review of the most relevant subset of papers, the following three studies,227–229 based in the same region of Italy, were selected as most relevant to parameterise the secondary prevention model.
Prandoni et al. 227 recruited 528 patients with a first episode of venography proven DVT in a prospective cohort study conducted in a single centre in Italy. Patients were treated initially with unfractioned heparin or LMWH and then warfarin (INR target 2–3) for at least 3 months. Patients were advised to wear compression stockings for at least 2 years and followed up every 6 months for up to 8 years. The aim of the study was to assess VTE recurrence, PTS incidence and mortality. The results of this study were used to parameterise the rate of mild/moderate and severe PTS.
Prandoni et al. 228 broadened their previous work and reported on a prospective cohort of 1626 patients, recruited in three centres in Italy. Patients with a previous, imaging confirmed, symptomatic proximal DVT or a PE after discontinuation of anticoagulation (warfarin for, on average, 3 months) treatment were eligible. Patients were followed up in clinic or by telephone at least once every 6 months for a maximum of 10 years (median 50 months). The study228 estimated the cumulative incidence of symptomatic recurrent VTE, confirmed by imaging, and we used these results to estimate the risk of recurrent VTE with no anticoagulation in our model.
Pengo et al. 229 estimated the incidence of CTPH in a prospective cohort of 223 patients with a first episode of acute PE in one Italian centre. Patients initially received heparin and then oral anticoagulation for at least 6 months (target INR 2–3). Follow-up was performed at least every 6 months during the first 2 years and then annually for up to 10 years; mean follow-up was 94.3 months. CTPH was diagnosed in patients with unexplained persistent dyspnoea, with supportive evidence on pulmonary angiography and mean pulmonary artery pressures. We used these findings to estimate transition from ‘post PE’ and ‘post PE DVT’ to CTPH in our model.
Parameters informing the risk of recurrent VTE in the usual care group (no long-term pharmacotherapy) were derived from Prandoni et al. 228 Individual patient data were reconstructed from the cumulative risk plot, and exponential and Weibull parametric distributions were fitted to these data. The Akaike information criterion (AIC)230 was used to determine the best-fitting curve for the within-study period (preferring models with lower AIC) and a visual examination determined the validity of the extrapolation. The best-fitting curve for the within-study period was the Weibull distribution (Figure 67 and Table 189).
FIGURE 67.
Parametric distributions for recurrent VTE baseline risk fitted to results reported in Prandoni et al. 228
| Distribution | Scale | Shape | AIC |
|---|---|---|---|
| Exponential | 0.005487 | – | 2758.604 |
| Weibull | 0.016565 | 0.721213 | 2702.246 |
The risk of a clinically relevant bleed in the reference group was estimated based on PREVENT. 216 The PREVENT trial216 had a follow-up of up to 4.3 years, with a mean of 2.1 years. The observed rate of major bleeding – requiring hospitalisation or transfusion – in the placebo arm was 4 per 1000 person-years. Patients not receiving pharmacotherapy are assumed to be at equal risk of bleeds as placebo.
Future venous thromboembolism-related events
A proportion of patients develop PTS after a DVT. The incidence of PTS, stratified by mild/moderate or severe, was derived from Prandoni et al. ,227 which provides a plot of the cumulative incidence of all PTS and severe PTS. Data from this plot were extracted using WebPlotDigitizer (http://arohatgi.info/WebPlotDigitizer/) to estimate the yearly incidence of severe PTS and mild/moderate PTS (Table 190). The cumulative incidence of PTS levels off 2 years after the index VTE event, and we assume that patients have no additional risk of PTS after that time. The rate of CTPH given a PE was taken from Pengo et al. 229 In total, 7 of 223 patients developed symptoms of CTPH, and all seven events occurred in the initial 2 years (Table 191).
| Year | Cumulative incidence | Source | |
|---|---|---|---|
| PTS (95% CI) | Severe PTS (95% CI) | ||
| 1 | 0.172 (0.135 to 0.215) | 0.029 (0.009 to 0.044) | Prandoni227 |
| 2 | 0.231 (0.180 to 0.277) | 0.062 (0.032 to 0.090) | |
| Month | Cumulative incidence of CTPH | 95% CI | Source |
|---|---|---|---|
| 12 | 0.031 | 0.007 to 0.055 | Pengo229 |
| 24 | 0.038 | 0.011 to 0.065 |
Mortality
In the model, patients can die from a fatal PE, an ICH or other, all-cause, mortality. The rates of recurrent VTE and ICH including fatal PE and ICH events are described previously. We assumed that ICH was the cause of all of the fatal bleeding events. Seven trials213–216,218,220,222 reported on fatal bleeds; all had low counts and four had zero events. We assumed that sudden fatal PEs do not accrue a cost and non-sudden fatal PEs accrue the full cost of treating a PE. This proportion of sudden fatal PEs was assumed to be 74.4% as recorded in Prandoni et al. 228
All-cause mortality rates are applied to every health state in the model to incorporate other causes of death. These were obtained from the ONS,10 stratified by gender and age to match our population (see Appendix 10).
Model inputs: venous thromboembolism acute treatment
Overview
Relative treatment effects for the probabilities in the first-line acute treatment decision tree model have been derived from three NMAs and a pairwise meta-analysis (described below) for the following four events: (1) recurrent VTE; 2) non-fatal ICH (pairwise meta-analysis); (3) other CRB; and (4) non-VTE-related mortality.
Relative treatment efficacy
The ORs of four NOACs (apixaban, dabigatran, edoxaban, rivaroxaban) compared with warfarin were derived from the NMA (Table 192, and see Table 114). The probabilities of DVT given recurrent VTE and non-fatal PE given PE were, as with secondary prevention, assumed to be treatment independent and derived from single arms from all nine studies in the acute treatment review using a fixed-effects meta-analysis (Table 193 and see Table 111).
| Intervention | OR | 95% CI | Distribution |
|---|---|---|---|
| Dabigatran | 1.09 | 0.75 to 1.59 | MCMC posterior simulations |
| Rivaroxaban | 0.90 | 0.67 to 1.21 | |
| Apixaban | 0.83 | 0.58 to 1.18 |
| Event | Proportion | Alpha | Beta | Distribution |
|---|---|---|---|---|
| DVT given recurrent VTE | 0.47 | 341 | 387 | Beta |
| Non-fatal PE given a PE event | 0.73 | 283 | 104 |
Relative treatment safety
Four out of the nine studies identified in the literature review reported non-fatal ICH. The incidence was low: 38 patients out of 21,916 experienced an event, and there were not enough data to perform a NMA. Instead, we assumed that all NOACs have a similar risk, and performed a pairwise meta-analysis for all NOACs combined compared with warfarin. Fixed- and random-effects pairwise meta-analyses were explored resulting in DIC23 values of 40.31 and 39.54, respectively. We preferred models with lower DIC, for which differences of at least ‘3’ are considered to be meaningful. On this basis, the fixed-effects model was used to estimate the OR and uncertainty (Table 194). This assumption was explored in a sensitivity analysis, for which we assumed that the risk of non-fatal ICH for NOACs is the same as the risk for warfarin. The relative treatment effects for individual NOACs compared with warfarin for other clinically relevant bleeds were estimated in Table 129 and are provided below (Table 195).
| Intervention | OR | 95% CI | Distribution |
|---|---|---|---|
| NOACs | 0.395 | 0.189 to 0.790 | MCMC posterior simulations |
| Intervention | OR | 95% CI | Distribution |
|---|---|---|---|
| Dabigatran | 0.61 | 0.49 to 0.76 | MCMC posterior simulations |
| Rivaroxaban | 0.93 | 0.81 to 1.08 | |
| Edoxaban | 0.81 | 0.70 to 0.94 | |
| Apixaban | 0.44 | 0.35 to 0.55 |
Mortality
To derive the mortality in the 6 months of acute treatment a NMA was performed. The counts are the reported all-cause mortality with VTE-related mortality deducted. Eight out of the nine studies identified in the literature review reported all-cause mortality and VTE-related mortality separately (see Table 111). The data used in the NMA are provided in Appendix 9. The results relative to warfarin are in Table 196.
| Intervention | OR | 95% CI | Distribution |
|---|---|---|---|
| Dabigatran | 0.98 | 0.64 to 4.84 | MCMC posterior simulations |
| Rivaroxaban | 0.96 | 0.71 to 1.30 | |
| Edoxaban | 1.06 | 0.81 to 1.39 | |
| Apixaban | 0.87 | 0.54 to 1.39 |
Transition probabilities with usual care (warfarin)
The risks of experiencing recurrent VTE, non-fatal ICH, clinically relevant bleed and non-VTE-related mortality on usual care (warfarin) have been estimated from a single-arm, fixed-effects meta-analysis model for each outcome using all of the warfarin arms that were identified in the systematic review (including study arms with zero counts). The fixed-effects model was chosen over the random-effects model on the basis of lower DIC. Each of the outcomes is considered to be independent and so each is modelled separately to estimate parameters and beta distributions representing uncertainty (Table 197).
| Event | Proportion | Alpha | Beta | Distribution |
|---|---|---|---|---|
| Recurrent VTE | 0.027 | 378 | 13,474 | Beta |
| Non-fatal ICH | 0.002 | 27 | 10,930 | |
| Clinically relevant bleed | 0.097 | 1319 | 12,288 | |
| Non-VTE-related mortality | 0.018 | 244 | 13,496 |
Model inputs: venous thromboembolism primary prevention
Overview
Absolute probabilities of VTE, clinically relevant bleeds and mortality on reference treatment (LMWH) are estimated from the LMWH arms of the primary prevention trials identified in our systematic review, and these probabilities differ between THR and TKR populations (due to different length of time on treatment). All of the relative effects of NOACs have been derived from NMAs, and the MCMC simulations are used directly as inputs to our probabilistic model, retaining all correlations between parameter estimates. We stratified relative effects of NOACs compared with LMWH by THR and TKR populations. However, as a result of sparse data for AEs, and for consistency with the clinical effectiveness results, we assumed that relative effects are common across THR and TKR populations for clinically relevant bleeds and all-cause mortality.
Relative treatment efficacy
The proportion of patients who experience a symptomatic VTE event was derived from NMAs stratified by post THR and TKR, reported in Tables 75 and Table 77, respectively. The reference comparator for these two populations is post-op LMWH. We pooled relative treatment effects over the THR and TKR populations in a sensitivity analysis.
Relative treatment safety
Intracranial haemorrhage was reported in only 12 primary prevention studies. Within these studies, the total count of ICH is 6 out of 32,879 patients (it may also be the case that studies that did not report ICH did not observe any events, which would mean that the risk is even lower). Patients who are receiving primary prevention (for up to 35 days) are at much lower risk of ICH than patients who are receiving acute treatment (up to 6 months) or long-term secondary prevention. Owing to extremely low incidence, this outcome has not been incorporated into the primary prevention model. The relative treatment effects for CRB compared with post-op LMWH are reported in Table 102.
Mortality
Relative treatment effects for all-cause mortality have been derived from the NMA. This includes fatal VTE events, so to avoid double counting VTE-related mortality, only the rates from all-cause mortality informed the transition to death in the model. The results relative to post-op LMWH are given in Table 105. The mortality rates for patients who do not experience a symptomatic VTE and enter the two-stage Markov model were taken from the ONS all-cause mortality (see Appendix 10).
Transition probabilities with usual care (low-molecular-weight heparin)
Usual care in the primary prevention model is post-op LMWH. 8 The risk of experiencing each event (VTE, clinically relevant bleed and mortality) on the reference treatment was estimated from single-arm, fixed-effects meta-analyses for each outcome and population (THR or TKR), using reference treatment arms that were identified in the systematic review (including studies with zero events). The outcomes are considered to be independent and so are modelled separately. The absolute risk of recurrent VTE and all-cause mortality on LMWH was estimated separately for the THR and TKR populations, as THR patients remain on treatment for longer. There was not enough information on other CRB in the THR population to estimate an absolute risk; we therefore pooled over TKR and THR populations. We fitted a random-effects model over the pooled TKR and THR population for clinically relevant bleeds as a result of the substantial heterogeneity, with the random-effects model giving a DIC of 22.7 compared with 43.0 for a fixed-effects model. The resulting parameter estimates and beta distributions that represent the uncertainty in these estimates are given in Table 198.
| Event | Proportion | Alpha | Beta | Distribution |
|---|---|---|---|---|
| Recurrent VTE THR | 0.035 | 65 | 1,787 | Beta |
| Recurrent VTE TKR | 0.023 | 36 | 1,527 | |
| Mortality THR | 0.019 | 53 | 2,619 | |
| Mortality TKR | 0.004 | 16 | 4,342 | |
| Event | Proportion | LB | UB | Distribution |
| Clinically relevant bleed | 0.029 | 0.005 | 0.121 | MCMC simulations |
Sensitivity analyses
We tested the robustness of the models’ results to some of the model parameters in one-way sensitivity analyses, listed below.
Proportion of venous thromboembolism events that are fatal and non-fatal pulmonary embolism (secondary prevention)
We varied the proportion of DVT, non-fatal PE and fatal PE when a patient experienced a recurrent VTE event in the secondary prevention model. Having a large proportion of non-fatal recurrent VTE events has a small effect on quality of life compared with having a large proportion of fatal events. We used a beta distribution with proportions estimated in Prandoni et al. :227 101 recurrent events consisting of 80 DVTs, 10 non-fatal PEs and 11 fatal PEs.
Risk of clinically relevant bleed on warfarin (secondary prevention)
The rate used in the base case was the observed rate for major bleeds in a secondary prevention RCT (PREVENT216). This could be an underestimate as a result of including only major and not clinically relevant minor bleeds. In sensitivity analysis we use, instead, the odds of experience of a CRB taken from the AF population [see Chapter 6, Transition probabilities with usual care (warfarin)].
Rate of non-fatal intracranial haemorrhage (acute treatment)
We did not have enough data to perform a NMA on this outcome. In the base case we assumed that all NOACs have the same relative treatment effect compared with warfarin. We tested this assumption by instead assuming that the rate of non-fatal ICH is equal among NOACs and warfarin in this sensitivity analysis.
Cost of edoxaban (acute treatment)
Edoxaban does not currently have a list price in the UK. For the base case we assume a cost similar to the list price of other NOACs, and test this assumption in a sensitivity analysis. We do this through a threshold analyses to see what the cost of edoxaban would have to be for it to be considered cost-effective at a willingness-to-pay threshold of £20,000 per QALY. We begin by assuming a zero drug cost for edoxaban, noting that if it is not found to be cost-effective at a zero cost then increasing the cost will not change our results.
Changing the time on treatment (acute treatment)
In the majority of trials in our review, patients receive acute treatment for 6 months; however, NICE guidance recommends 3 months in acute treatment, with an additional 3 months treatment if necessary. We assume 6 months’ treatment in our base case, and reduce this to 3 months in a sensitivity analysis. Note that due to a lack of evidence, we assume that relative treatment efficacy is unchanged if given for 3 months rather than 6 months; however; absolute event rates for AEs decrease with time on treatment, and treatment costs are reduced.
Pooling post total hip replacement and post total knee replacement populations for relative treatment effect of venous thromboembolism (primary prevention)
We pooled THR and TKR populations to estimate relative treatment effects of VTE in primary prevention in this sensitivity analysis. The relative treatment effects are provide in Appendix 11.
Dabigatran dose for elderly patients (primary prevention)
In this sensitivity analysis we costed dabigatran at a lower dose in the primary prevention models to match the dose recommended in the BNF for elderly patients: 150 mg od.
Cost of treatment-related adverse events (all models)
We varied the cost of treatment-related AEs by ± 50%. These included clinically relevant bleeds, ICH and post ICH.
Cost of venous thromboembolism events (all models)
We varied the cost of VTE events by ± 50%. These included DVT, PE, mild moderate PTS, severe PTS and CTPH.
Utility decrements of treatment-related adverse events (all models)
We varied the utility decrement of treatment-related AEs by ± 50%. These included clinically relevant bleeds, ICH and post ICH.
Utility decrements of venous thromboembolism events (all models)
We varied the utility decrement of VTE events by ± 50%. These included DVT, PE, mild moderate PTS, severe PTS and CTPH.
Cost of warfarin (all models)
We assessed sensitivity of our results to administration and monitoring cost of warfarin through a threshold analysis to see what the cost of warfarin would have to be for it to be considered cost-effective at a willingness-to-pay threshold of £20,000 per QALY. We begin by assuming a zero cost for warfarin, noting that if it is not found to be cost-effective at a zero cost then increasing the cost will not change our results.
Results of the cost-effectiveness model: venous thromboembolism secondary prevention
We estimated expected costs, QALYs, incremental costs, incremental QALYs and incremental NMB at willingness to pay of £20,000 and £30,000 for first-line prevention therapy (Table 199). The cheapest comparator is aspirin (total expected cost £20,671). No pharmacotherapy is the next cheapest treatment, with benefits similar to aspirin. Warfarin and the NOACs all have substantially higher costs than aspirin and no pharmacotherapy, and the NOACs are more expensive than warfarin. Dabigatran and apixaban (5 mg) have marginally higher expected QALYs than no pharmacotherapy. Apixaban (2.5 mg) has the lowest expected QALYs, followed by warfarin. Apixaban (2.5 mg) has the highest HR for the risk of ICH, albeit estimated imprecisely. Although the NOACs and warfarin prevent more recurrent VTEs than no pharmacotherapy or aspirin, the rate of recurrent VTE is low and the rate of AEs (ICH and clinically relevant bleeds), which can have a long-term impact on quality of life, are generally higher for the NOACs than aspirin or no pharmacotherapy.
Aspirin has the highest expected net benefit at willingness-to-pay thresholds of £20,000 and £30,000 per QALY (see Table 199). However, the CI for the INB of aspirin includes zero, indicating uncertainty about whether or not it is more cost-effective than no pharmacotherapy. All NOACs have negative expected INBs at the £20,000 and £30,000 thresholds, and all CIs are negative at the £20,000 threshold, indicating that they are not cost-effective compared with no pharmacotherapy. Dabigatran, which had the lowest estimated HR for recurrent VTE and ICH of all the NOACs, also has the highest expected net benefit of any NOAC. However, dabigatran is not cost-effective relative to no pharmacotherapy, even at the £30,000 threshold, as the incremental NMB is negative (–£3402; 95% CI –£12,338 to £5424). Figure 68 shows that although there is uncertainty in the estimated costs and QALYs, it is clear that aspirin has lower costs and similar benefits in the majority of the samples. Over a wide range of willingness-to-pay-per-QALY thresholds, aspirin has the highest expected net benefit (Figure 69), and also the highest probability of being the most cost-effective (Figure 70), although there is a non-negligible probability that no pharmacotherapy is the most cost-effective intervention for secondary prevention of VTE at a threshold of £20,000–30,000. These results suggest that it is not cost-effective to prescribe NOACs or warfarin for secondary prevention of VTE over the range of willingness-to-pay thresholds that we explored (up to £40,000 per QALY).
FIGURE 68.
Incremental cost-effectiveness plane for secondary prevention (no pharmacotherapy: reference). See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
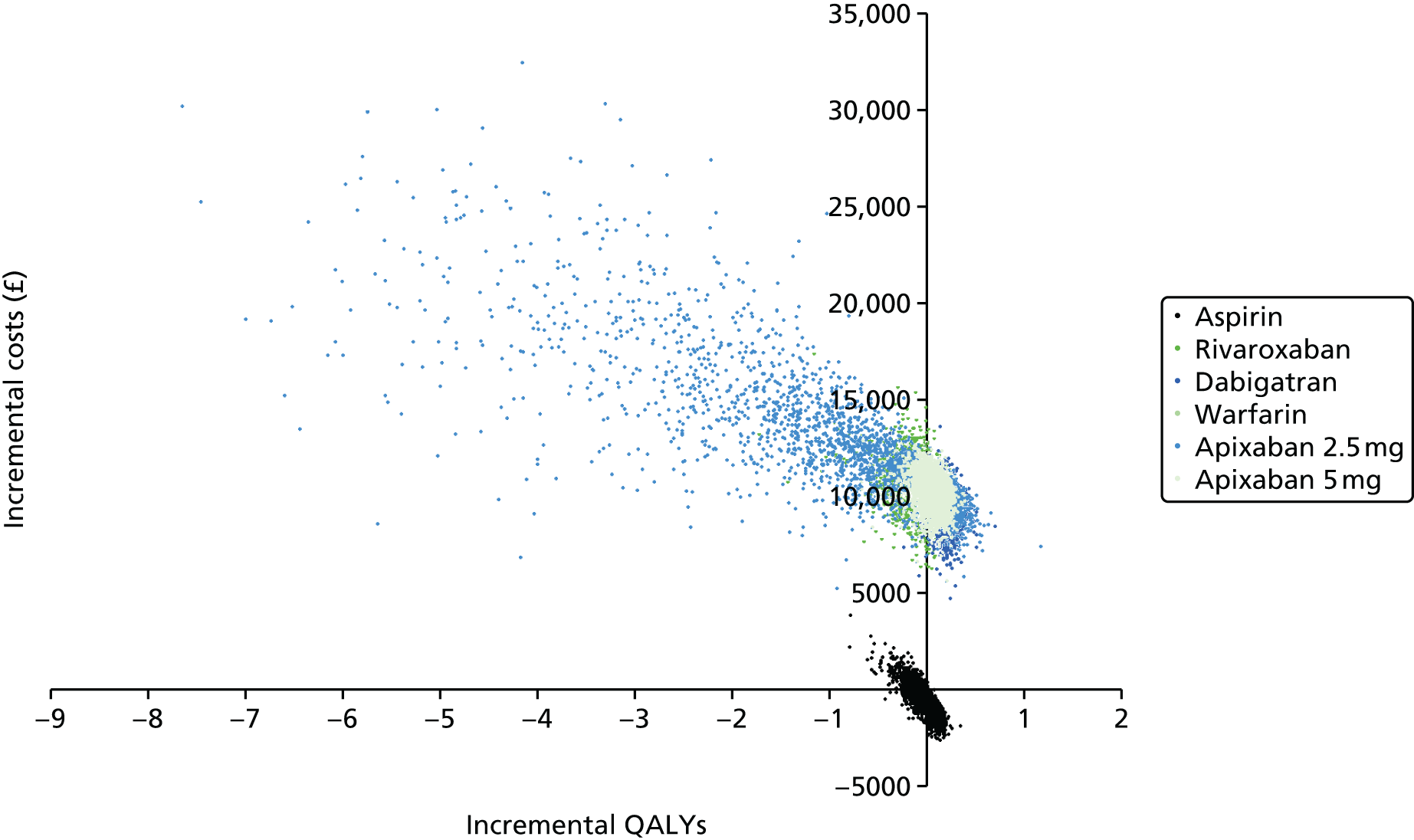
FIGURE 69.
Cost-effectiveness acceptability frontier for secondary prevention. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
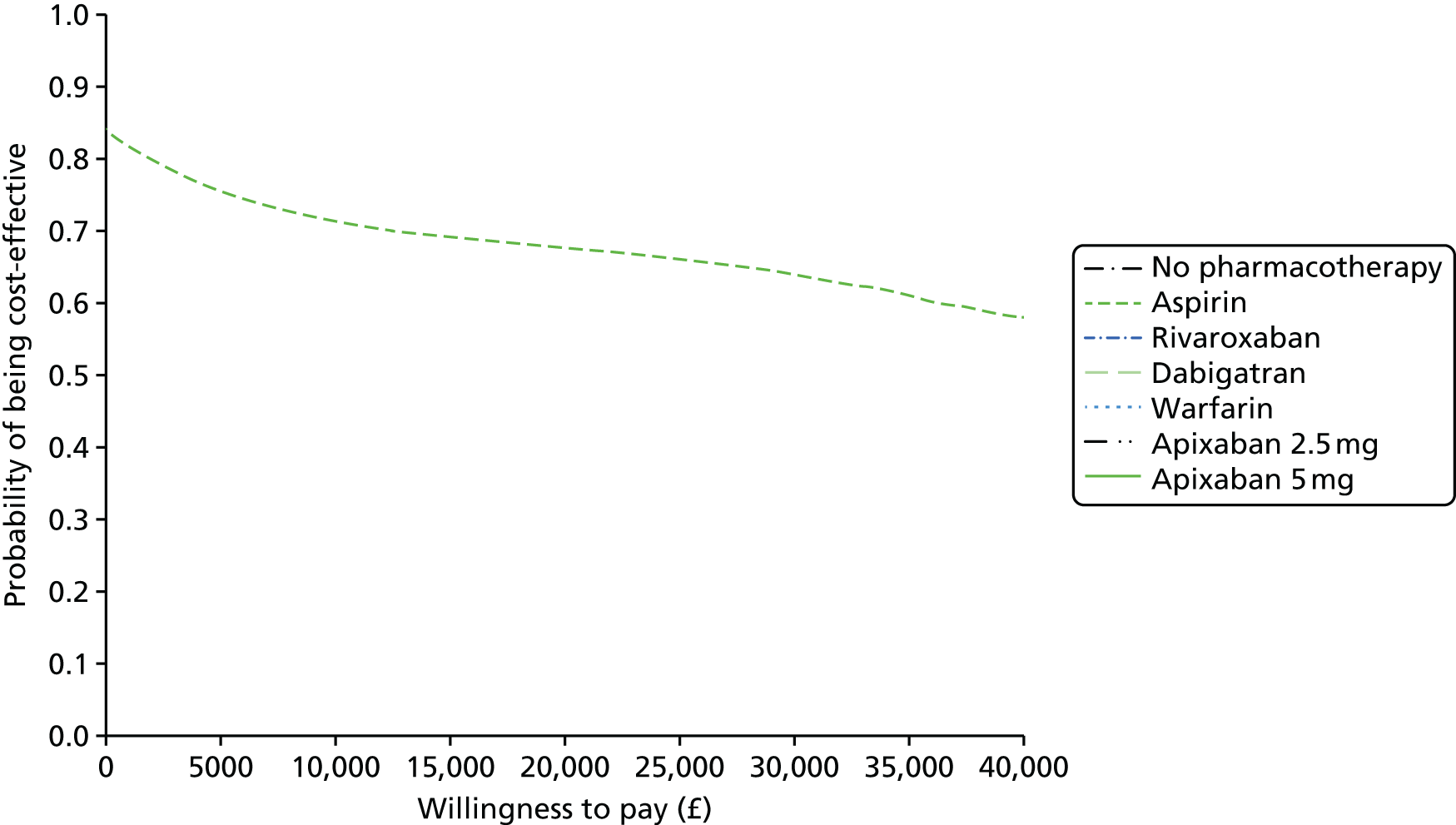
FIGURE 70.
Cost-effectiveness acceptability curve for secondary prevention. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
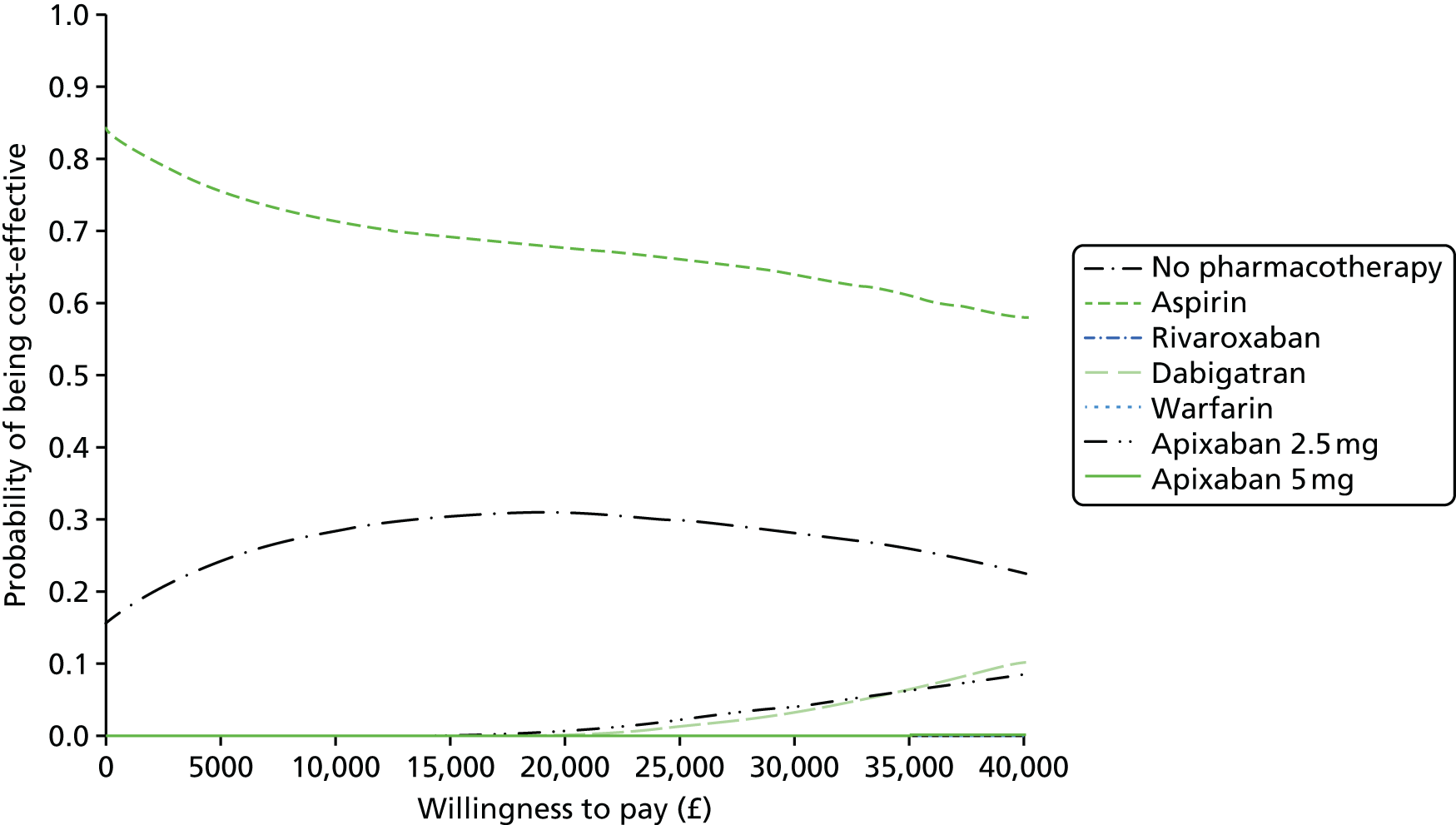
The estimated per-person EVPI was £757 at a willingness to pay of £20,000, and £1291 at £30,000. Assuming a VTE incidence of 183 per 100,000 in a European population,7 and a population of 65- to 70-year-olds in England and Wales of approximately 3 million (2011 Census), gives an estimated VTE incidence rate per year of 5490. Population EVPI over a 10-year time horizon, discounting at 3.5%, is approximately £36M and £61M at willingness-to-pay thresholds of £20,000 and £30,000, respectively.
Figure 71 shows the proportion of the EVPI that is attributable to different groups of parameters. The optimal decision is most sensitive to the relative treatment effects, suggesting that there may be value in running a large trial comparing a NOAC with aspirin and no pharmacotherapy. Note, however, that, as a result of low event rates, a study that is powered to capture VTE events may be prohibitive.
| Estimated costs and outcomes | No pharmacotherapy | Aspirin | Rivaroxaban | Dabigatran | Warfarin | Apixaban 2.5 mg | Apixaban 5 mg |
|---|---|---|---|---|---|---|---|
| Costs | £21,282 (£14,619 to £30,388) | £20,671 (£14,342 to £29,346) | £31,781 (£26,270 to £39,317) | £30,952 (£25,613 to £38,396) | £26,379 (£21,103 to £33,550) | £33,496 (£26,672 to £43,389) | £31,557 (£26,061 to £39,312) |
| QALYs | 12.58 (12.16 to 12.94) | 12.58 (12.05 to 12.99) | 12.50 (11.97 to 12.91) | 12.74 (12.32 to 13.09) | 12.32 (11.67 to 12.78) | 11.83 (8.1 to 13.07) | 12.63 (12.17 to 13.00) |
| Incremental costs | – | –£611 (–£1834 to £939) | £10,498 (£8197 to £12,640) | £9670 (£7545 to £11,406) | £5097 (£2337 to £7829) | £12,213 (£8365 to £22,029) | £10,275 (£8429 to £11,810) |
| Incremental QALYs | – | 0.00 (–0.27 to 0.15) | –0.08 (–0.42 to 0.16) | 0.16 (–0.05 to 0.36) | –0.26 (–0.71 to 0.03) | –0.75 (–4.40 to 0.39) | 0.06 (–0.18 to 0.25) |
| Incremental NMB (at £20,000) | – | £623 (–£6404 to £4602) | –£12,119 (–£19,983 to –£6238) | –£6536 (–£116,711 to £ –1513) | –£10,351 (–£20,582 to –£3256) | –£27,180 (–£109,197 to –£1272) | –£9171 (–£14,548 to –£4565) |
| Incremental NMB (at £30,000) | – | £629 (–£9176 to £6085) | –£13,740 (–£28,266 to –£3216) | –£3402 (–£12,388 to £5424) | –£15,606 (–£34,467 to –£2776) | –£42,146 (–£197,897 to £6442) | –£8067 (–£18,012 to £294) |
FIGURE 71.
Expected value of partial perfect information for subsets of model input parameters in the VTE secondary prevention model, presented as a proportion of the total EVPI. SAVI-estimated EVPPI scaled by EVPPI of all parameters as estimated by SAVI. 95% intervals are ± 1.96 × standard error and are truncated above at 1 and below at 0.
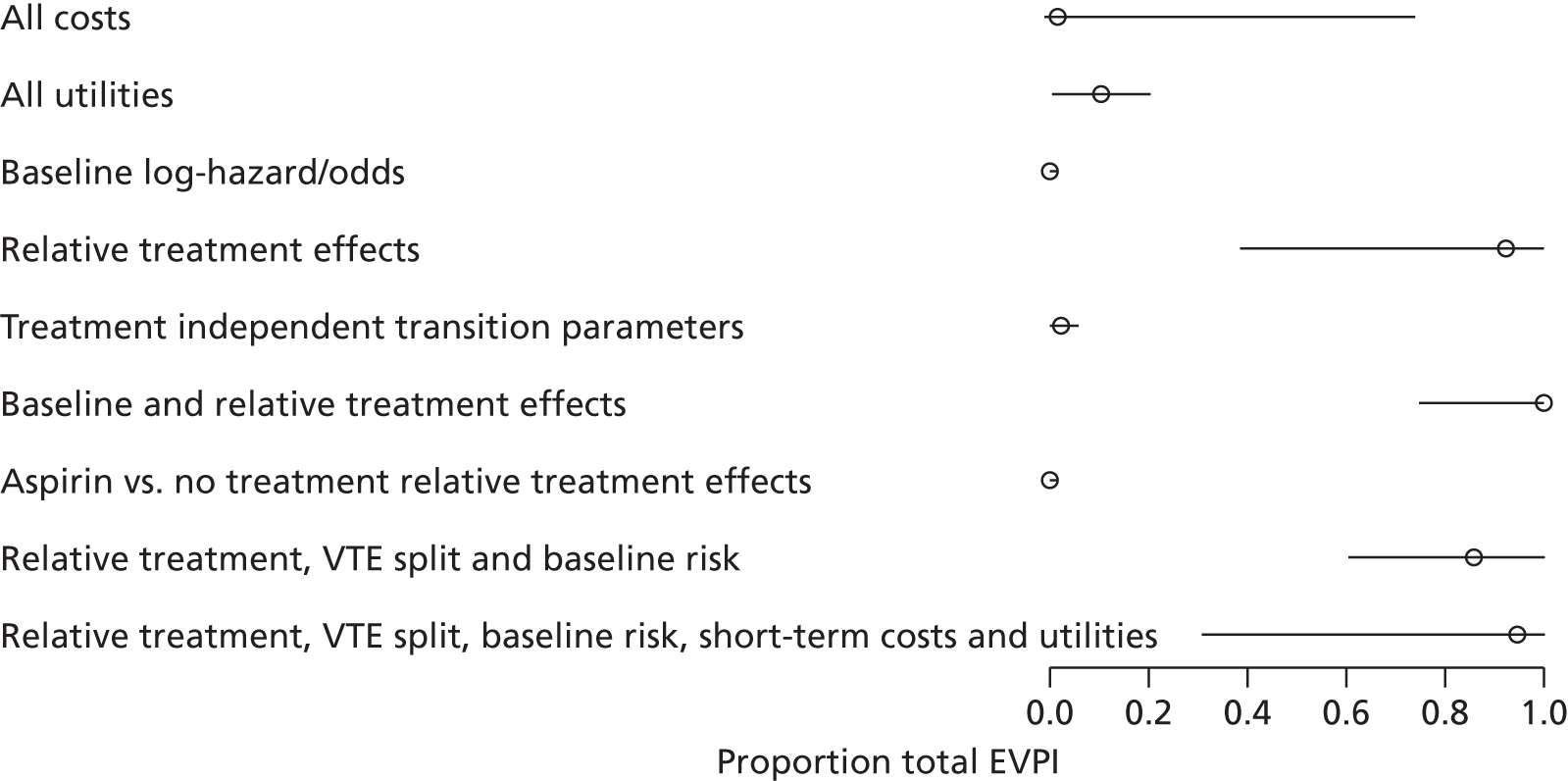
Results of the cost-effectiveness model: low-molecular-weight heparin acute treatment
We estimated expected costs, QALYs, incremental costs, incremental QALYs and incremental NMB at a willingness to pay of £20,000 and £30,000 for first-line therapy (Table 200). Expected costs and benefits are similar across all treatments because of the short (6-month) treatment duration and the small, and imprecisely estimated, effects of NOACs on VTE recurrence and AEs compared with warfarin. Warfarin has the lowest expected cost (£19,651), followed by dabigatran, edoxaban and apixaban, and rivaroxaban is the most expensive (£19,753). Apixaban had the highest expected QALYs (12.02) but this is only 0.04 QALYs greater than the interventions with the lowest expected QALYs (edoxaban, warfarin and dabigatran).
The expected net benefit is highest for apixaban at willingness-to-pay thresholds of £20,000 and £30,000 per QALY. This is due to the marginally lower risk of recurrent VTE, CRB and non-VTE-related mortality with apixaban relative to other NOACs. However, there is substantial uncertainty around this estimate. Rivaroxaban also has a positive INB compared with warfarin. CIs for INB are wide for all treatments, reflecting substantial uncertainty that is also seen in the incremental cost-effectiveness plane (Figure 72).
FIGURE 72.
Incremental cost-effectiveness plane for acute treatment (warfarin: reference). See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
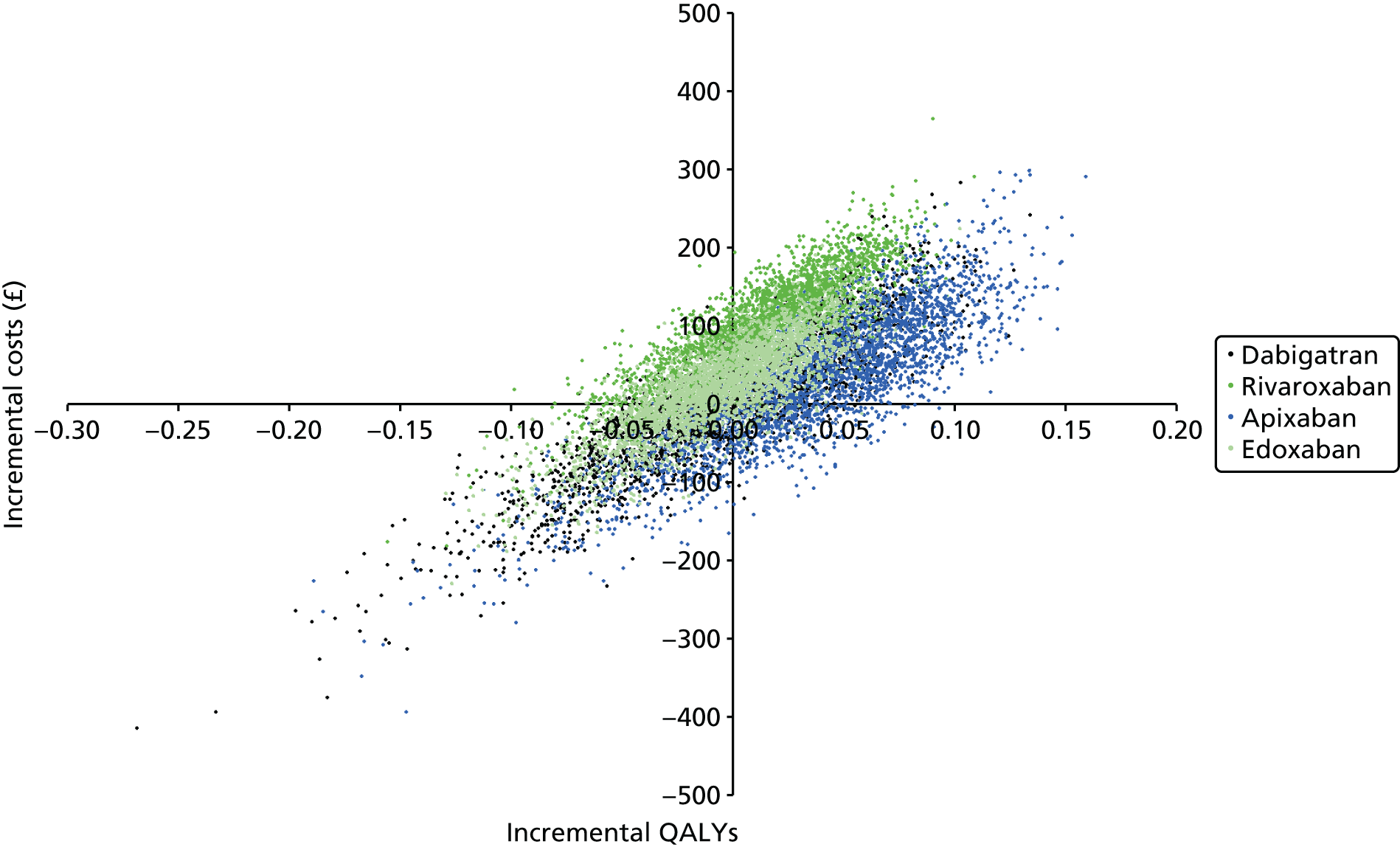
The CEACs (Figure 73) show that for very low willingness to pay per QALY, warfarin is the most cost-effective treatment (because it has lowest expected costs). For willingness-to-pay thresholds of > £1000, apixaban (5 mg) has the highest expected net benefit (Figure 74), with a probability of being most cost-effective at £20,000–30,000 per QALY thresholds of approximately 0.54. However, it is possible that rivaroxaban or dabigatran are the most cost-effective interventions, even at high willingness-to-pay thresholds.
FIGURE 73.
Cost-effectiveness acceptability curve for acute treatment. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
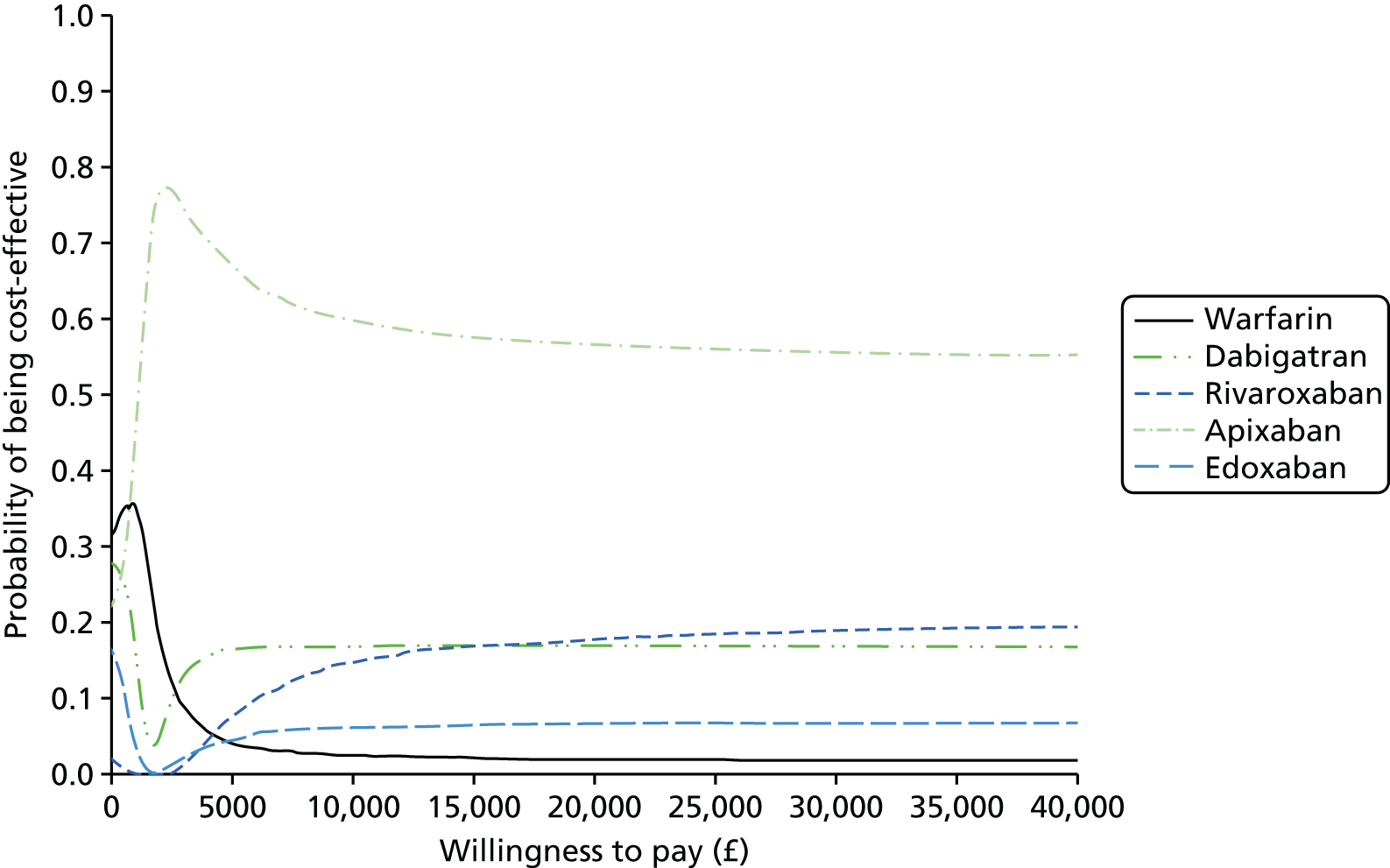
FIGURE 74.
Cost-effectiveness acceptability frontier for acute treatment. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
The per-person EVPI was £365 at willingness-to-pay thresholds of £20,000 and £579 at £30,000. Assuming a VTE incidence rate per year of 5490 (as for secondary prevention). Population EVPI over a 10-year time horizon, discounting at 3.5%, is approximately £17M and £27M at willingness-to-pay thresholds of £20,000 and £30,000, respectively.
Figure 75 shows the proportion of the EVPI that is attributable to different groups of parameters. The optimal decision is most sensitive to uncertainty in the cost and utility model inputs. This suggests there may be value in conducting a study to estimate the utilities associated with VTE events and treatment-related events. As such a study is likely to be relatively inexpensive to conduct (compared with a RCT), and given the magnitude of likely benefits, this should be considered a research priority. The optimal decision is not very sensitive to event rates on the reference comparator (baseline risk), relative treatment effects for all comparators, relative treatment effects of apixaban compared with warfarin (the two comparators with the highest probability of being cost-effective at a willingness to pay of £20,000 per QALY) and treatment-independent transition parameters.
| Estimated costs and outcomes | Warfarin (95% CI) | Dabigatran (95% CI) | Rivaroxaban (95% CI) | Apixaban (95% CI) | Edoxaban (95% CI) |
|---|---|---|---|---|---|
| Costs | £19,651 (£13,543 to £27,667) | £19,663 (£13,522 to £27,695) | £19,753 (£13,579 to £27,819) | £19,683 (£13,543 to £27,801) | £19,675 (£13,557 to £27,732) |
| QALYs | 11.98 (11.46 to 12.36) | 11.98 (11.46 to 12.37) | 11.99 (11.48 to 12.38) | 12.02 (11.49 to 12.41) | 11.98 (11.46 to 12.36) |
| Incremental costs | £12 (–£168 to £152) | £102 (–£22 to £211) | £31 (–£149 to £180) | £24 (–£99 to £133) | |
| Incremental QALYs | 0.00 (–0.10 to 0.08) | 0.01 (–0.06 to 0.07) | 0.04 (–0.07 to 0.12) | –0.01 (–0.07 to 0.05) | |
| Incremental NMB (at £20,000) | £21 (–£1885 to £1498) | £196 (–£1123 to £1281) | £710 (–£1322 to £2185) | –£132 (–£1369 to £920) | |
| Incremental NMB (at £30,000) | £38 (–£2903 to £2324) | £344 (–£1686 to £2018) | £1080 (–£2059 to £3351) | –£186 (–£2084 to £1434) |
FIGURE 75.
Expected value of partial perfect information subsets of parameters in the VTE acute treatment model, as a proportion of the total EVPI. SAVI-estimated EVPPI scaled by EVPPI of all parameters as estimated by SAVI. 95% intervals are ± 1.96 × standard error and are truncated above at 1 and below at 0.
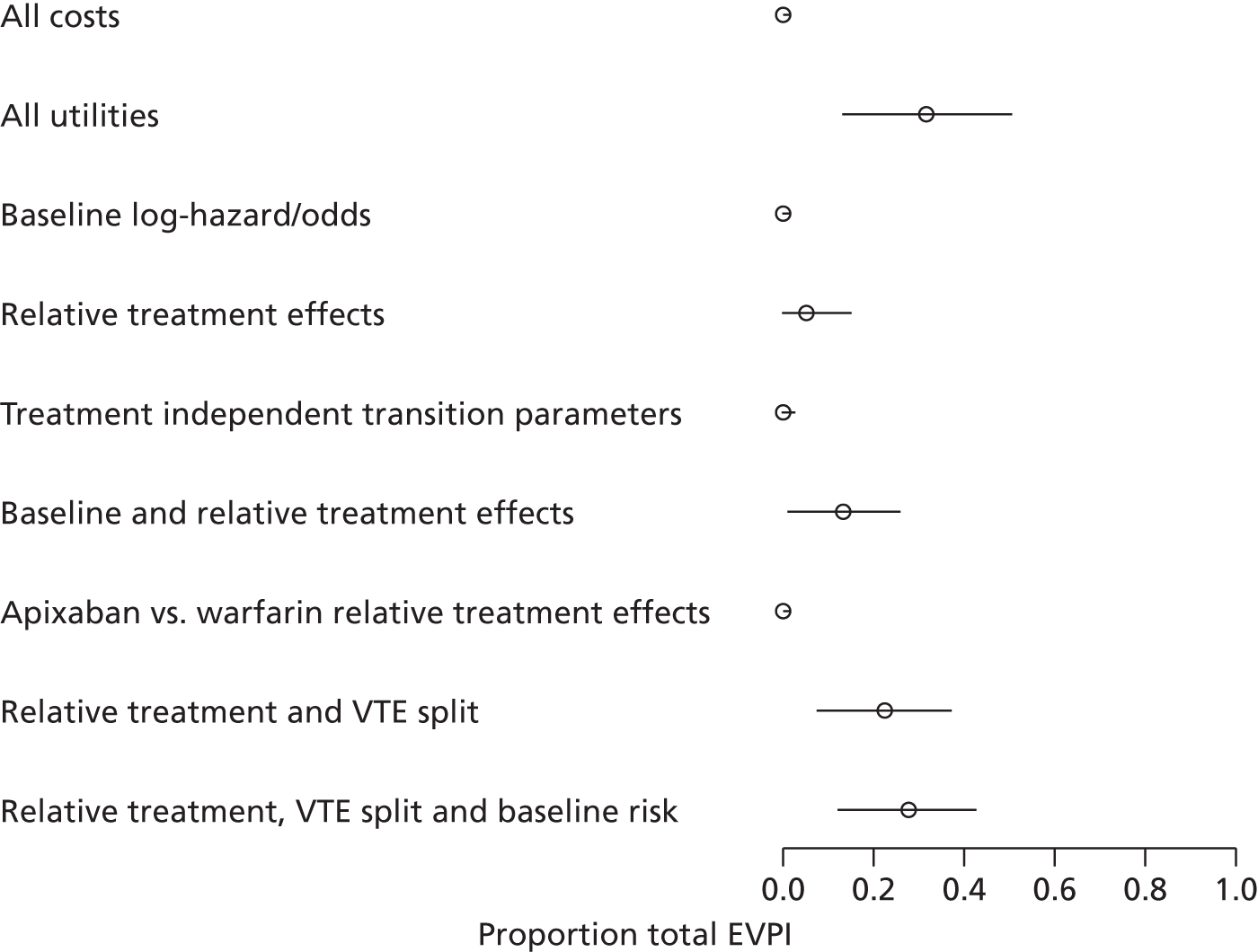
Results of the cost-effectiveness model: low-molecular-weight heparin primary prevention
Total hip replacement
The expected total costs, QALYs, incremental costs, incremental QALYs and incremental NMB at willingness-to-pay thresholds of £20,000 and £30,000 per QALY for first-line prevention therapy are reported in Table 201 and illustrated in Figure 76). The lowest expected total costs are for apixaban (£702) followed by rivaroxaban (£718) and then dabigatran (£893). LMWH has the highest expected cost (£1062). Expected benefits are highest for rivaroxaban and LMWH (9.10 QALYs), followed by dabigatran (9.04 QALYs) then apixaban (8.96 QALYs). At both willingness-to-pay thresholds of £20,000 and £30,000 per QALY, rivaroxaban has the highest expected INB, although CIs around net benefit are wide (particularly for dabigatran) and also skewed (apixaban).
FIGURE 76.
Incremental cost-effectiveness plane for THR primary prevention (LMWH reference). See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
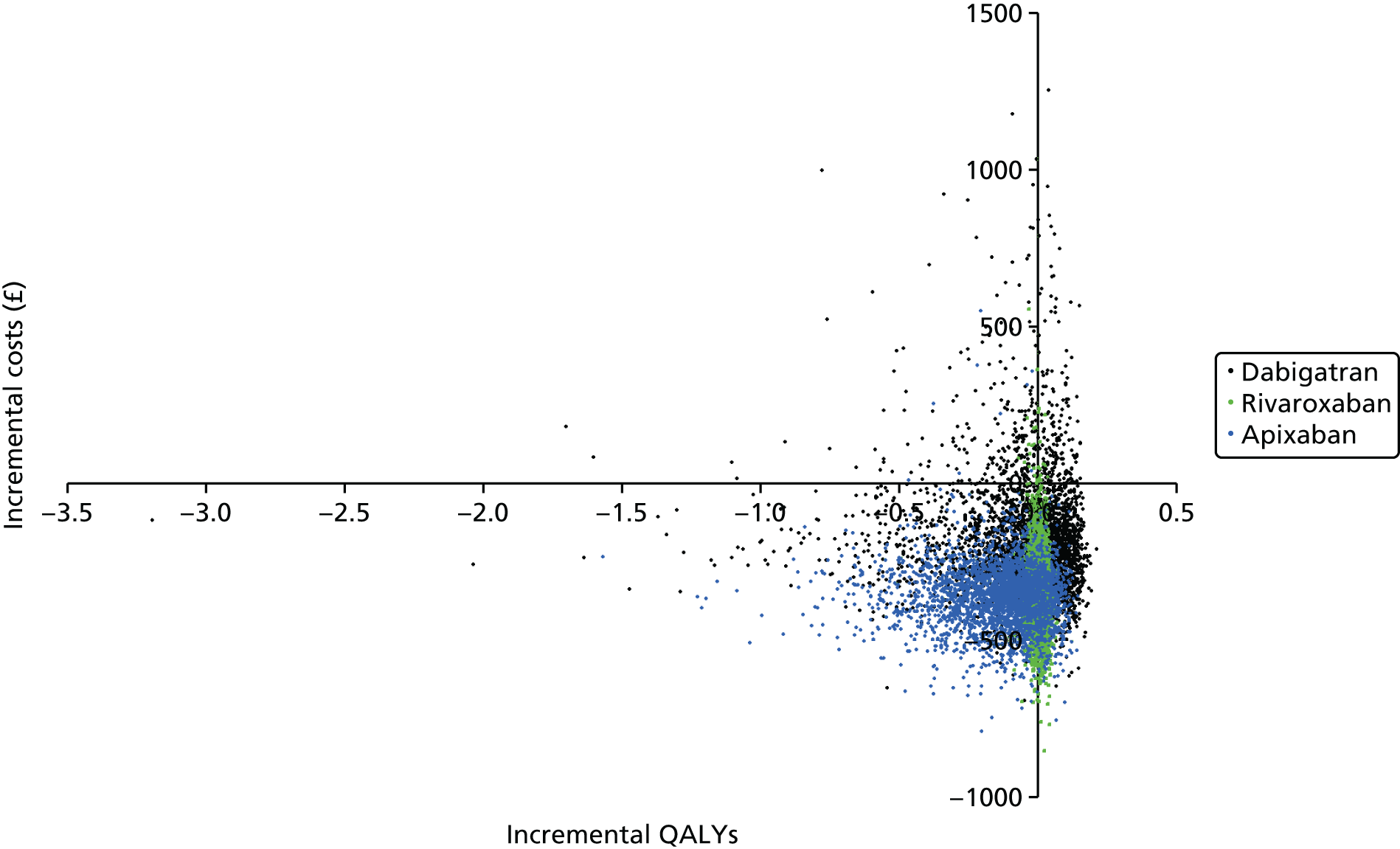
Rivaroxaban has the highest expected net benefit over the range of willingness-to-pay thresholds that we explored (Figure 77) but with substantial uncertainty: its probability of being the most cost-effective was 0.35 for willingness-to-pay threshold of £30,000 per QALY (Figure 78). Because of the very wide confidence limits for dabigatran, there is an apparently contradictory finding that it has the highest probability of being the most cost-effective NOAC (see Figure 77) for thresholds of > £14,000 but does not have the highest expected net benefit (see Figure 78 and Table 201). This phenomenon is documented in the literature, and in these circumstances the CEAF (see Figure 78) is a better summary than the CEAC (see Figure 77). 231 Note the general high degree of uncertainty as to which treatment is the most cost-effective.
FIGURE 77.
Cost-effectiveness acceptability curve for THR primary prevention. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
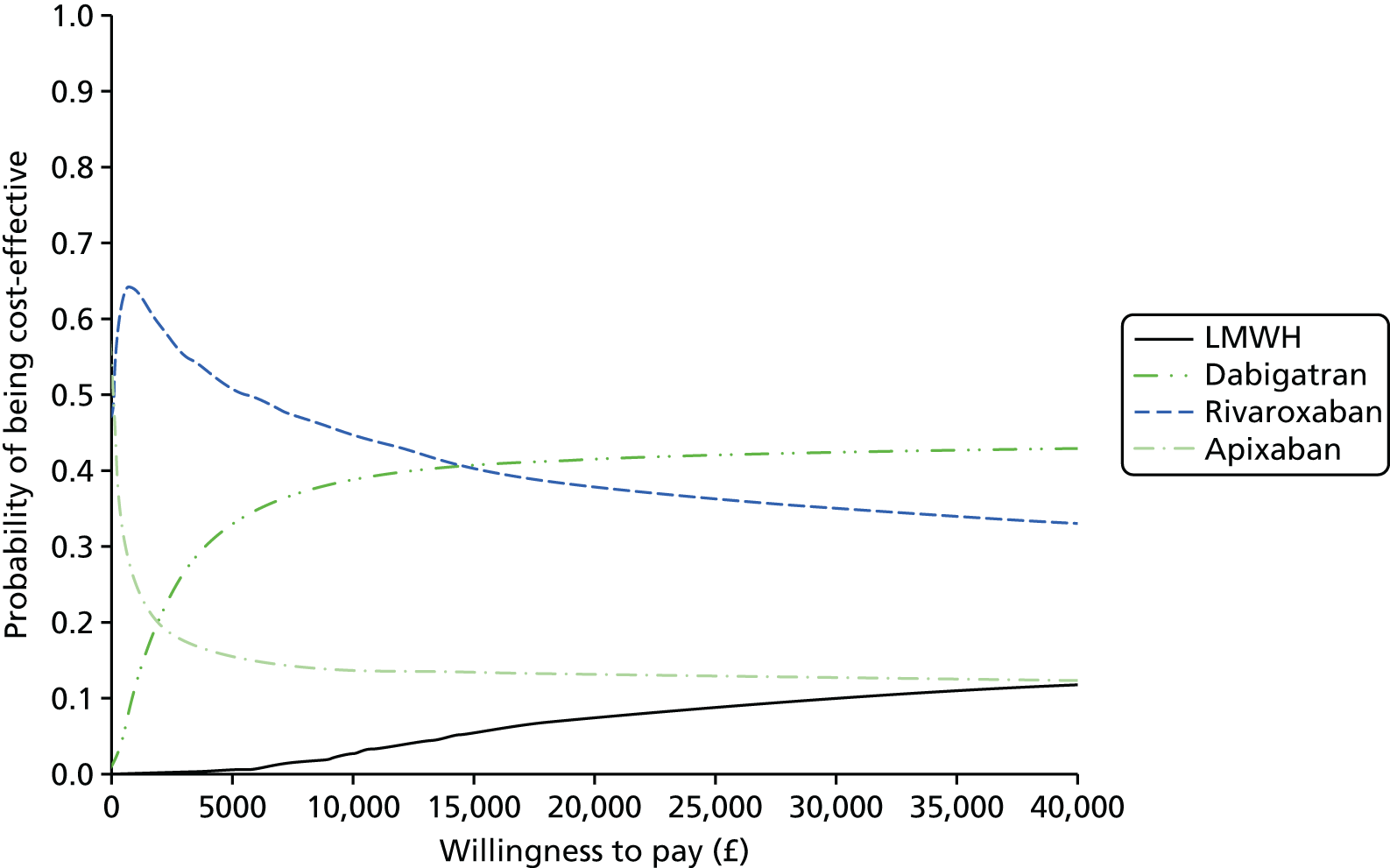
FIGURE 78.
Cost-effectiveness acceptability frontier for THR primary prevention. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.

The per-person EVPI estimated was £730 at a willingness-to-pay threshold of £20,000, and £1138 at £30,000. Assuming an annual incidence of primary THR operations per year69 of 76,000, population EVPI over a 10-year time horizon, discounting at 3.5%, is approximately £475M and £741M at willingness-to-pay thresholds of £20,000 and £30,000, respectively. These very high figures reflect the high per-person EVPI (driven by the uncertainty in the available evidence) and also the large volume of primary THR operations that are conducted.
Figure 79 shows the proportion of the EVPI that is attributable to different groups of parameters. The optimal decision is most sensitive to uncertainty in the treatment-independent transition parameters, and also sensitive to uncertainty in the cost parameters. The decision is not very sensitive to uncertainty in utility values, event rates on the reference comparator (baseline risk), relative treatment effects for all comparators, relative treatment effects of rivaroxaban compared with LMWH (the two comparators with the highest probability of being cost-effective at a willingness-to-pay threshold of £20,000 per QALY), and the proportion of VTE events. This suggests that there may be value in running a longitudinal study examining the treatment-independent transition parameters: rates of mild/moderate PTS, severe PTS, CTPH and the proportion split of VTE events.
| Estimated costs and outcomes | LMWH | Dabigatran | Rivaroxaban | Apixaban |
|---|---|---|---|---|
| Costs | £1062 (£888 to £1311) | £893 (£635 to £1495) | £718 (£571 to £1045) | £702 (£573 to £953) |
| QALYs | 9.1 (8.85 to 9.35) | 9.04 (8.44 to 9.40) | 9.10 (8.84 to 9.36) | 8.96 (8.47 to 9.31) |
| Incremental costs | –£169 (–£430 to £345) | –£344 (–£558 to –£99) | –£360 (–£559 to –£156) | |
| Incremental QALYs | –0.06 (–0.61 to 0.15) | 0.01 (–0.04 to 0.04) | –0.13 (–0.57 to 0.09) | |
| Incremental NMB (at £20,000) | –£1066 (–£12,127 to £3191) | £453 (–£485 to £1312) | –£2284 (–£11,017 to £2085) | |
| Incremental NMB (at £30,000) | –£1684 (–£18,241 to £4649) | £507 (–£883 to £1739) | –£3606 (–£16,704 to £2917) |
FIGURE 79.
Expected value of partial perfect information for subsets of model input parameters in the VTE primary prevention THR model, presented as a proportion of the total EVPI. SAVI-estimated EVPPI scaled by EVPPI of all parameters as estimated by SAVI. 95% intervals are ± 1.96 × standard error and are truncated above at 1 and below at 0.

Total knee replacement
The expected total costs, QALYs, incremental costs, incremental QALYs and incremental NMB at a willingness to pay of £20,000 and £30,000 for first-line prevention therapy are reported in Table 202. Both benefits and uncertainty in the benefits are similar across interventions. Rivaroxaban has the lowest expected total costs (£834), followed by post-op LMWH (£855) and dabigatran (£871), whereas apixaban has the highest expected total costs of £932. Rivaroxaban and LMH had similar INB at willingness-to-pay thresholds of £20,000 and £30,000 per QALY. Dabigatran and apixaban have negative INB compared with post-op LMWH.
The cost-effectiveness plane (Figure 80) and the CEACs (Figure 81) show substantial uncertainty around the relative costs and benefits of these interventions. Rivaroxaban has the highest expected net benefit over the range of willingness-to-pay thresholds that we explored (Figure 82), and the highest probability of being the most cost-effective treatment for willingness-to-pay thresholds of up to approximately £20,000 per QALY (see Figure 81). Beyond that, dabigatran has the highest probability of being the most cost-effective but not the highest expected net benefit because of the high level of uncertainty around the cost-effectiveness of dabigatran (as seen also in the THR population). As previously noted we prefer the CEAF summary (see Figure 82) in this situation. Note that there is a non-negligible chance that each of the treatments may be the most cost-effective, and this decision uncertainty increases as we increase our willingness to pay per QALY.
FIGURE 80.
Incremental cost-effectiveness plane for TKR primary prevention (LMWH: reference). See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
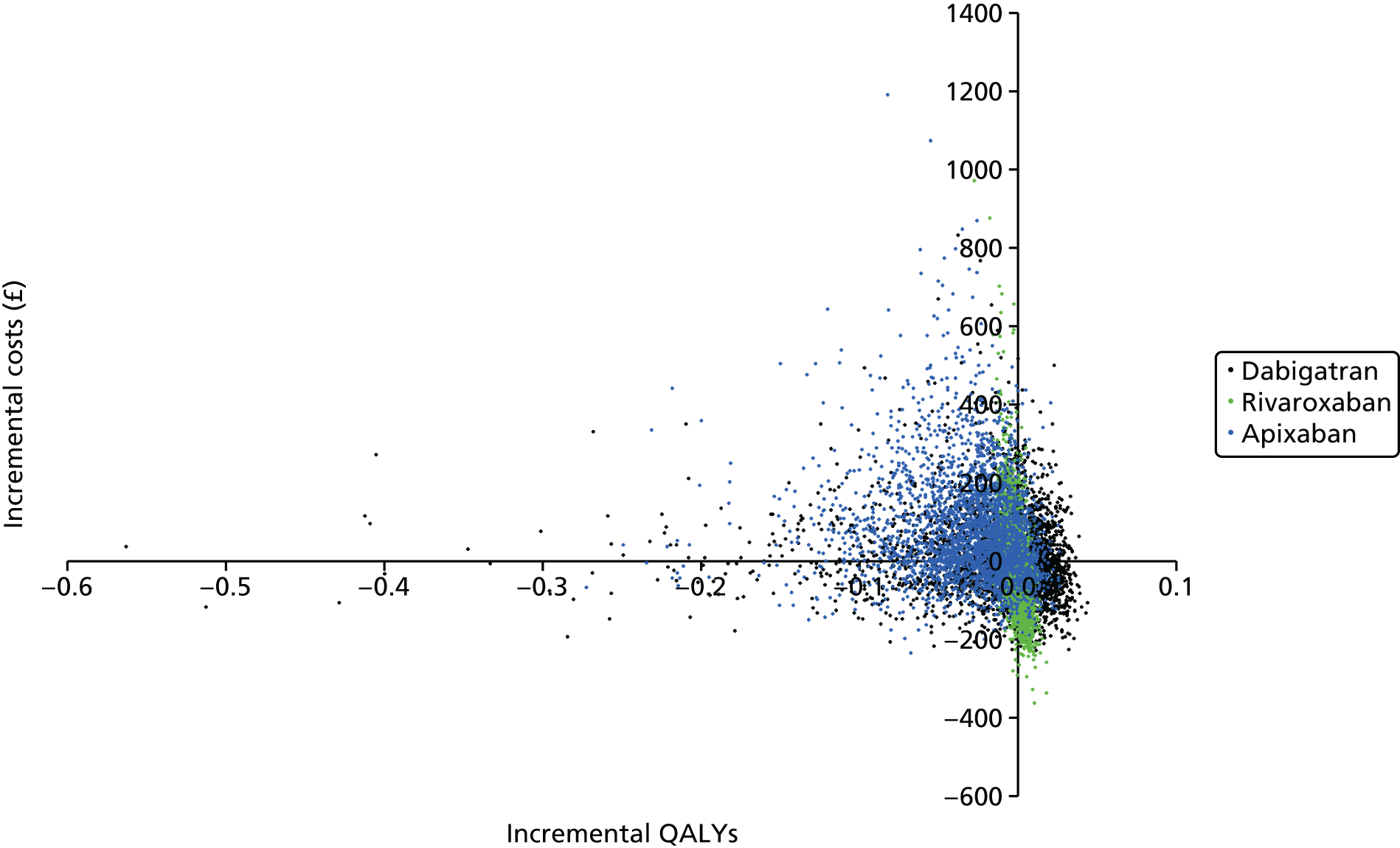
FIGURE 81.
Cost-effectiveness acceptability curve for TKR primary prevention. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
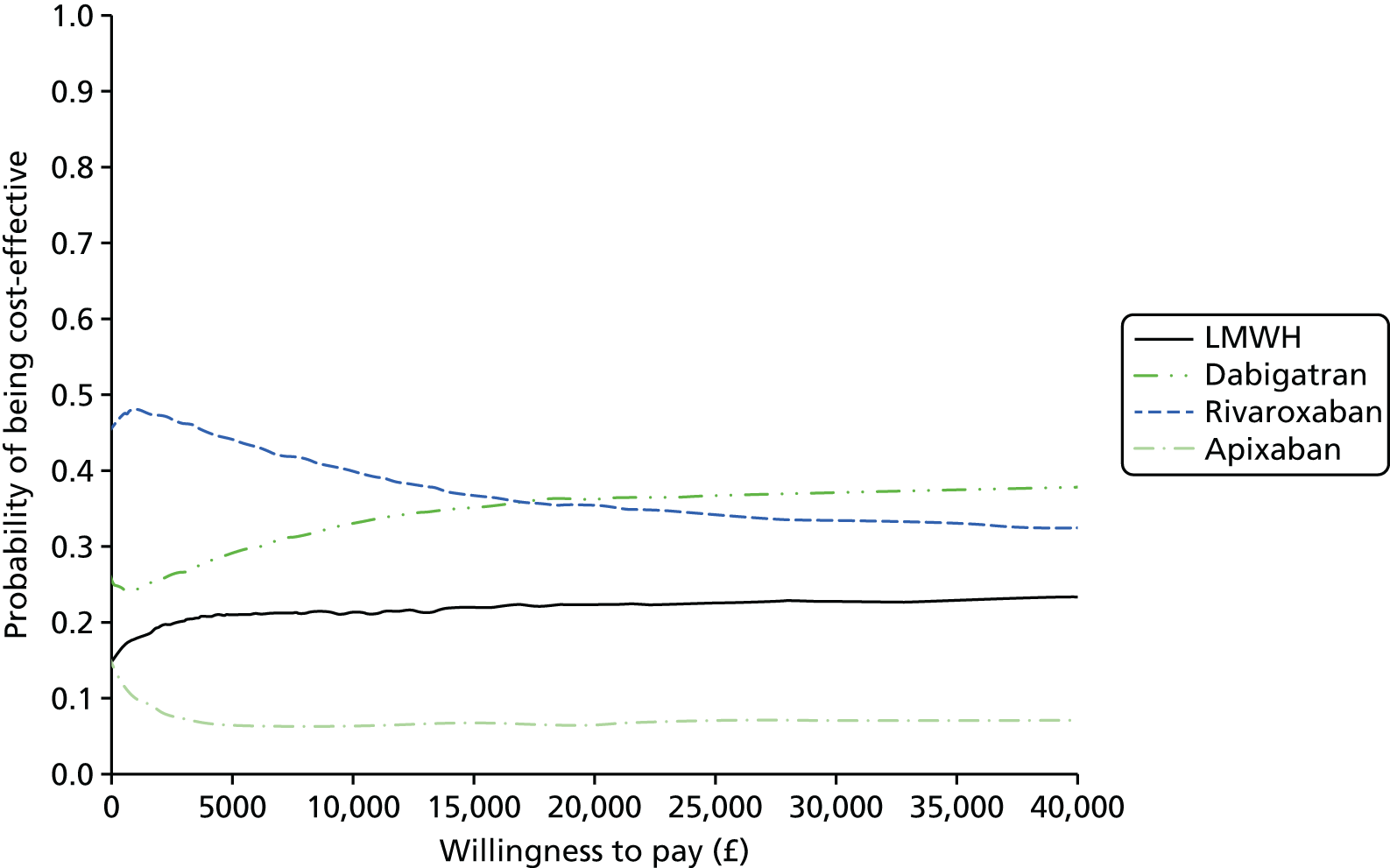
FIGURE 82.
Cost-effectiveness acceptability frontier for TKR primary prevention. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
The per-person EVPI was £171 at a willingness-to-pay threshold of £20,000, and £249 at £30,000, which is lower than that seen in other populations, reflecting the larger number of studies on this population. Assuming an annual incidence of primary TKR operations per year69 of 76,000, population EVPI over a 10-year time horizon, discounting at 3.5%, is approximately £111M and £161M at willingness-to-pay thresholds of £20,000 and £30,000, respectively. These high figures reflect the large volume of primary TKR operations that are conducted.
Figure 83 shows the proportion of the EVPI that is attributable to different groups of parameters. The optimal decision is most sensitive to uncertainty in the utilities, relative treatment effects and treatment-independent transition parameters, and also sensitive to uncertainty in the cost parameters, but not to uncertainty in the risk on the reference comparator. This suggests that there may be value in running a large trial comparing NOACs and warfarin to reduce the uncertainty in the relative treatment effects. There may also be value in conducting a study to estimate the utility values associated with VTE events and treatment-related events, and a longitudinal study examining the treatment-independent transition parameters: rates of mild/moderate PTS, severe PTS, CTPH and the proportion split of VTE events.
| Estimated costs and outcomes | LMWH | Dabigatran | Rivaroxaban | Apixaban |
|---|---|---|---|---|
| Costs | £855 (£706 to £1078) | £871 (£646 to £1252) | £834 (£632 to £1183) | £932 (£688 to £1388) |
| QALYs | 9.25 (9.00 to 9.49) | 9.24 (8.96 to 9.48) | 9.25 (9.00 to 9.49) | 9.22 (8.96 to 9.46) |
| Incremental costs | £16 (–£149 to £284) | –£20 (–£187 to £223) | £77 (–£113 to £417) | |
| Incremental QALYs | –0.02 (–0.14 to 0.03) | 0.00 (–0.01 to 0.01) | –0.03 (–0.12 to 0.01) | |
| Incremental NMB (at £20,000) | –£320 (–£2844 to £638) | £16 (–£406 to £329) | –£686 (–£2458 to £266) | |
| Incremental NMB (at £30,000) | –£472 (–£4214 to £919) | £13 (–£509 to £414) | –£991 (–£3658 to £375) |
FIGURE 83.
Expected value of partial perfect information for subsets of parameters in the VTE primary prevention TKR model, as a proportion of the total EVPI. SAVI-estimated EVPPI scaled by EVPPI of all parameters as estimated by SAVI. 95% intervals are ± 1.96 × standard error and are truncated above at 1 and below at 0.
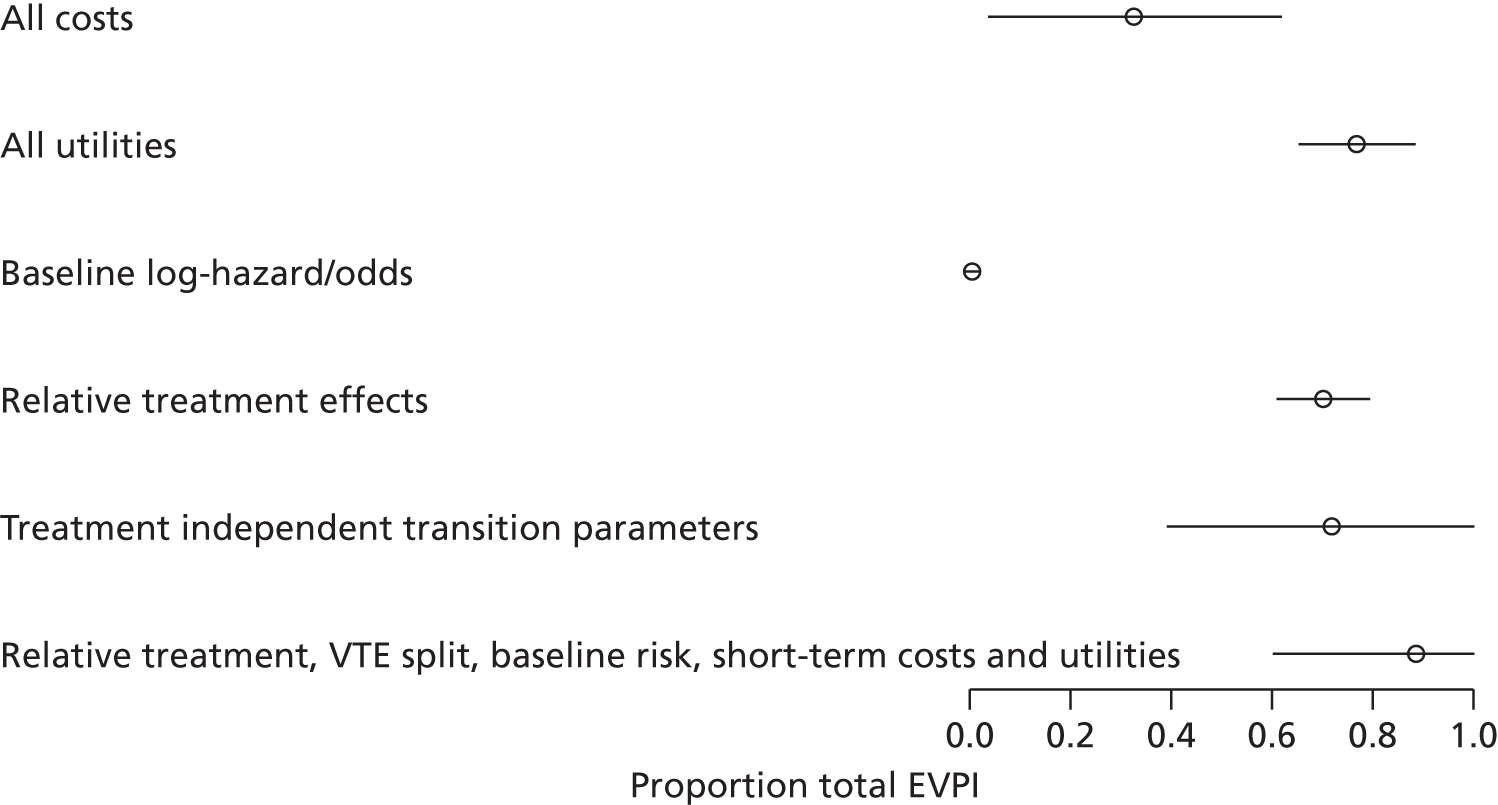
Results of sensitivity analyses for secondary prevention model
We varied the proportion of recurrent VTEs that are DVT, non-fatal PE and fatal PE using the proportions estimated in the study by Prandoni et al. 227 (79% DVT, 10% non-fatal PE and 11% fatal PE) rather than the proportions estimated from the RCTs included in the systematic review. At a willingness-to-pay threshold of > £25,000 per QALY dabigatran becomes the most cost-effective treatment (Figure 84). This indicates that NOACs are more likely to be cost-effective in secondary prevention if the risk of fatal VTE is higher than we assumed in our base-case analysis.
FIGURE 84.
Cost-effectiveness acceptability frontier for secondary prevention sensitivity analysis: vary proportion of DVT, non-fatal PE and fatal PE of recurrent VTE. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.

We varied the clinically relevant bleed rate to match that assumed in the AF model. The results were robust to this assumption, with aspirin having the highest expected net benefit over all willingness-to-pay thresholds that we explored. We explored sensitivity of results to a policy of switching patients to warfarin after a second VTE event, and the sensitivity to the cost of warfarin by reducing the cost to £0 in one-way sensitivity analyses. The results were robust to these assumptions (see Appendix 12).
The results were robust to the seven sensitivity analyses for which we varied the utilities of VTE and AEs by ± 50%, the AE costs by ± 50% and the VTE costs by +50% (see Appendix 12). When we reduced the cost of VTE events by 50%, no pharmacotherapy has the highest expected net benefit over the willingness-to-pay thresholds that we explored (Figure 85).
FIGURE 85.
Cost-effectiveness acceptability frontier for secondary prevention sensitivity analyses: reduction in VTE costs by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.

When the rate of ICH for no pharmacotherapy was assumed to be zero, no pharmacotherapy then had the highest probability of being cost-effective and the highest net benefit over a willingness-to-pay range of £0–40,000 (Figure 86). In this analysis the risk of having an ICH while on aspirin and NOACs outweighed the benefit gained from reduced recurrent VTE.
FIGURE 86.
Cost-effectiveness acceptability frontier secondary prevention: risk of ICH for no pharmacotherapy is set to zero. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.

Results of sensitivity analyses for acute treatment model
Changing the time on treatment from 6 months to 3 months and varying the cost and utilities by ± 50% over VTE events and AEs did not alter the conclusion that apixaban was most likely to be cost-effective over a threshold of £1000 (see Appendix 12). The assumption that NOACs have the same non-fatal ICH rate as warfarin had little effect on the conclusion that apixaban has the highest expected net benefit at a willingness-to-pay thresholds of £20,000 and £30,000 per QALY (see Appendix 12).
Assuming a zero cost for edoxaban, we find that edoxaban has the highest expected net benefit and highest probability of being cost-effective at a willingness-to-pay threshold of < £10,000 per QALY. However, as willingness to pay per QALY increases to > £10,000, apixaban is the most cost-effective treatment because of the higher benefits (Figure 87, and see Table 200).
FIGURE 87.
Cost-effectiveness acceptability frontier acute treatment model: assuming a zero cost for edoxaban. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.

Results of sensitivity analyses for primary prevention model
Total knee replacement
When we increased the AEs utilities by 50%, LMWH became the most cost-effective treatment at a willingness-to-pay threshold of > £27,000 per QALY (Figure 88). Increasing the AE costs by 50% changed the comparators with the highest average net benefit from rivaroxaban to LMWH over willingness-to-pay thresholds that we explored (Figure 89).
FIGURE 88.
Cost-effectiveness acceptability frontier for TKR primary prevention sensitivity analysis: increasing AE costs by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
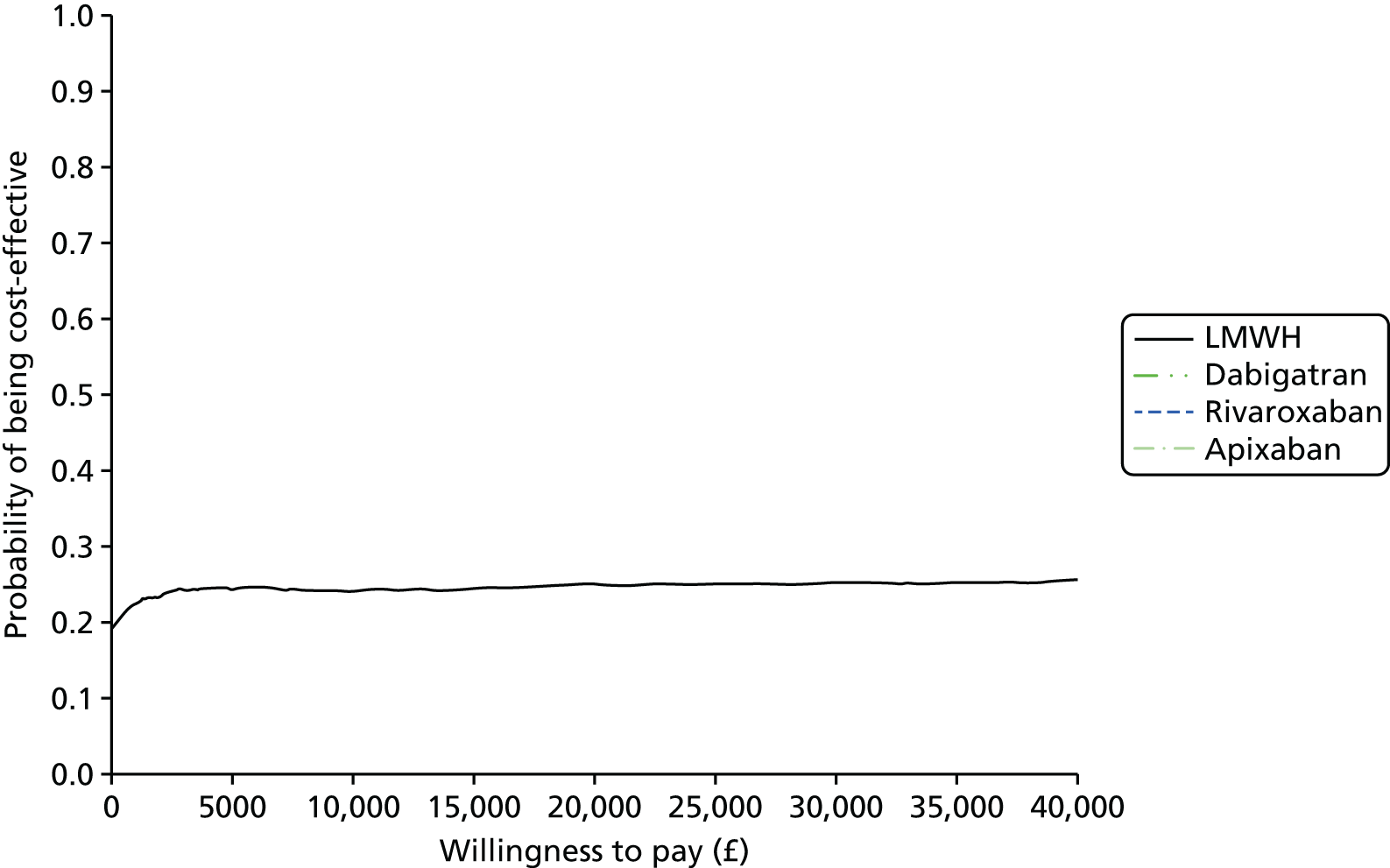
FIGURE 89.
Cost-effectiveness acceptability frontier for TKR primary prevention sensitivity analysis: increasing AE utilities by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
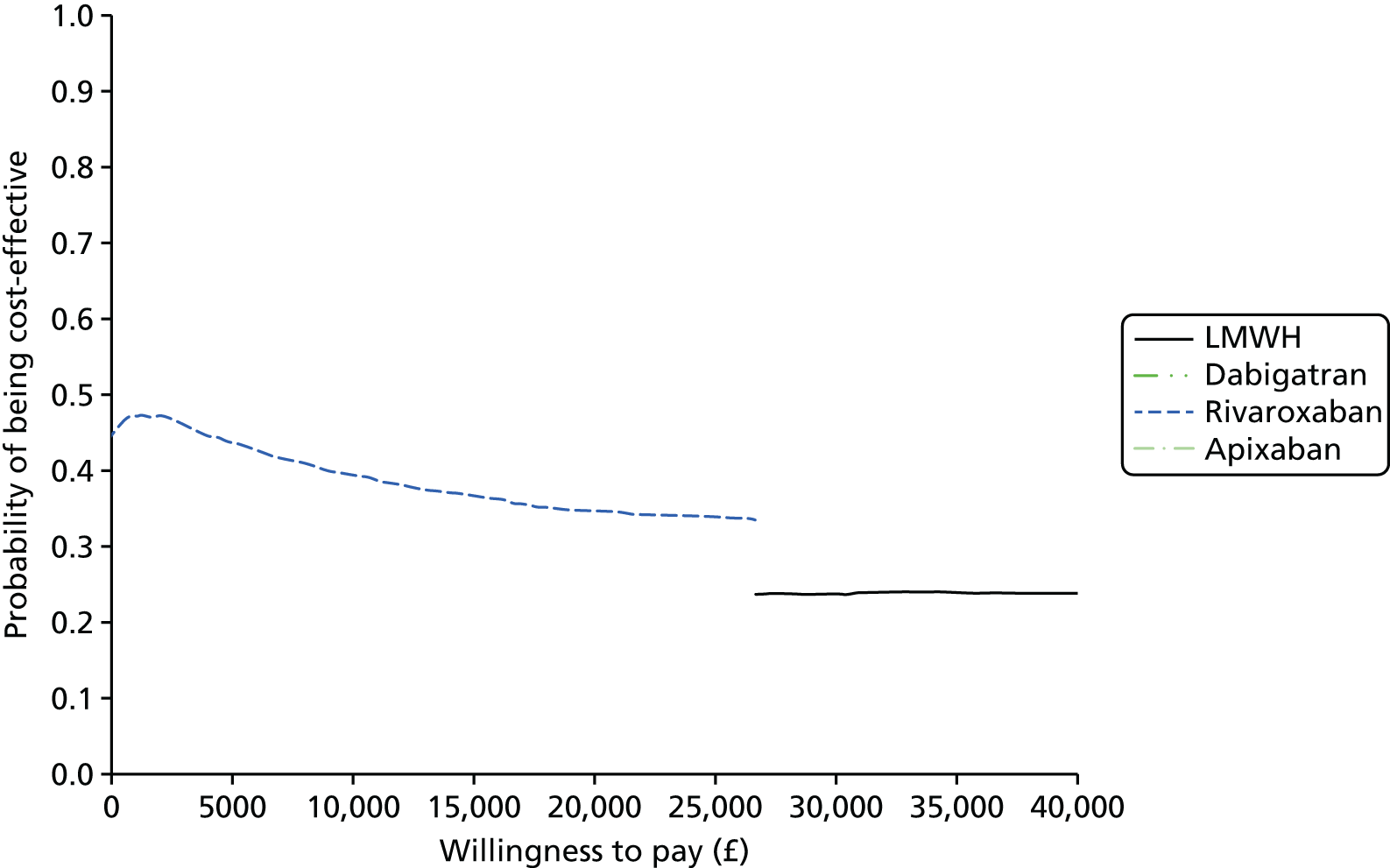
Decreasing the VTE event costs by 50% changed the comparators with the highest average net benefit from rivaroxaban to LMWH over willingness-to-pay thresholds that we explored (Figure 90). When we decreased the VTE utilities by 50%, LMWH became the most cost-effective comparator above a willingness-to-pay threshold of £20,000 (Figure 91).
FIGURE 90.
Cost-effectiveness acceptability frontier for TKR primary prevention sensitivity analysis: decreasing VTE costs by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
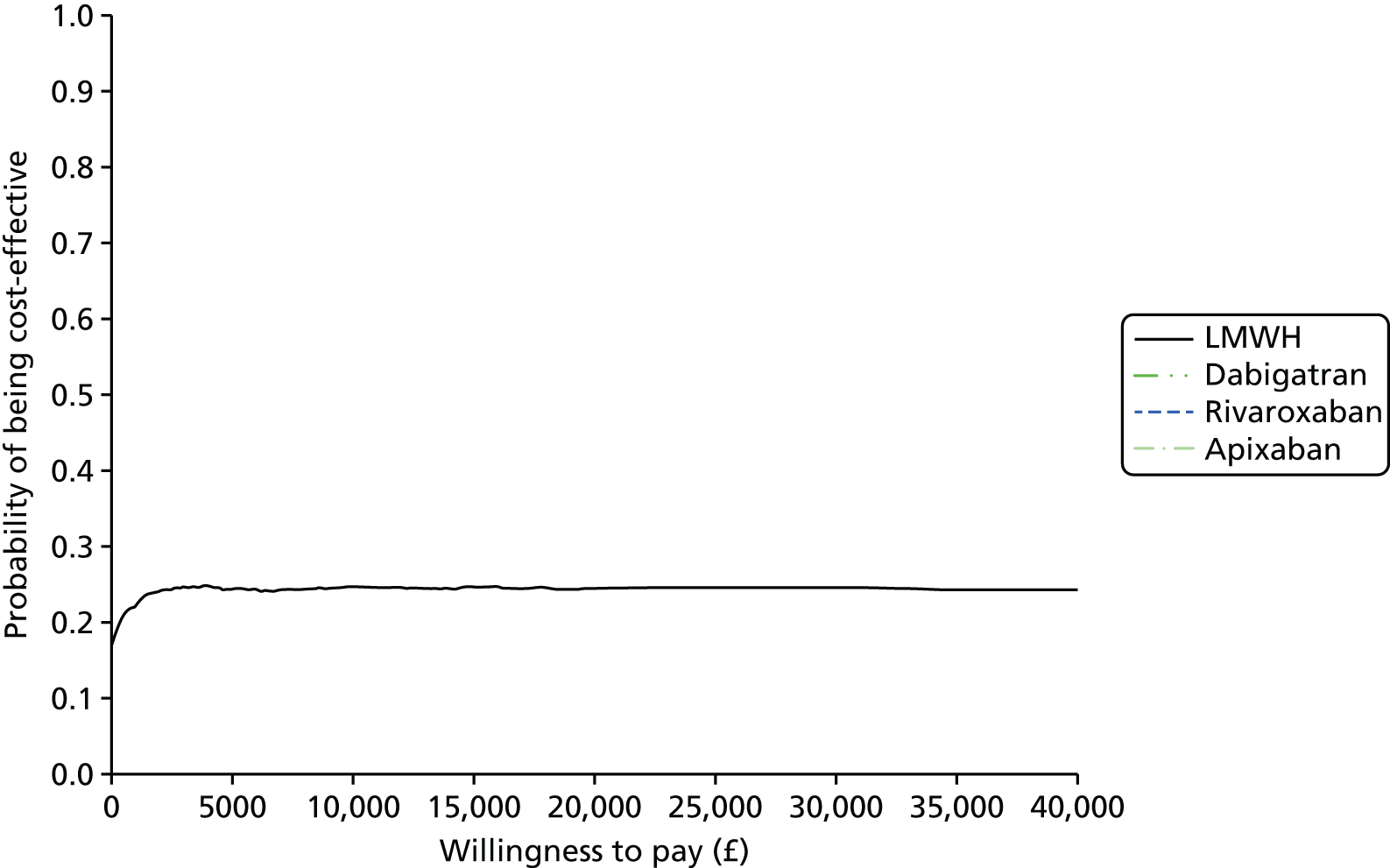
FIGURE 91.
Cost-effectiveness acceptability frontier for TKR primary prevention sensitivity analysis: decreasing VTE utilities by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
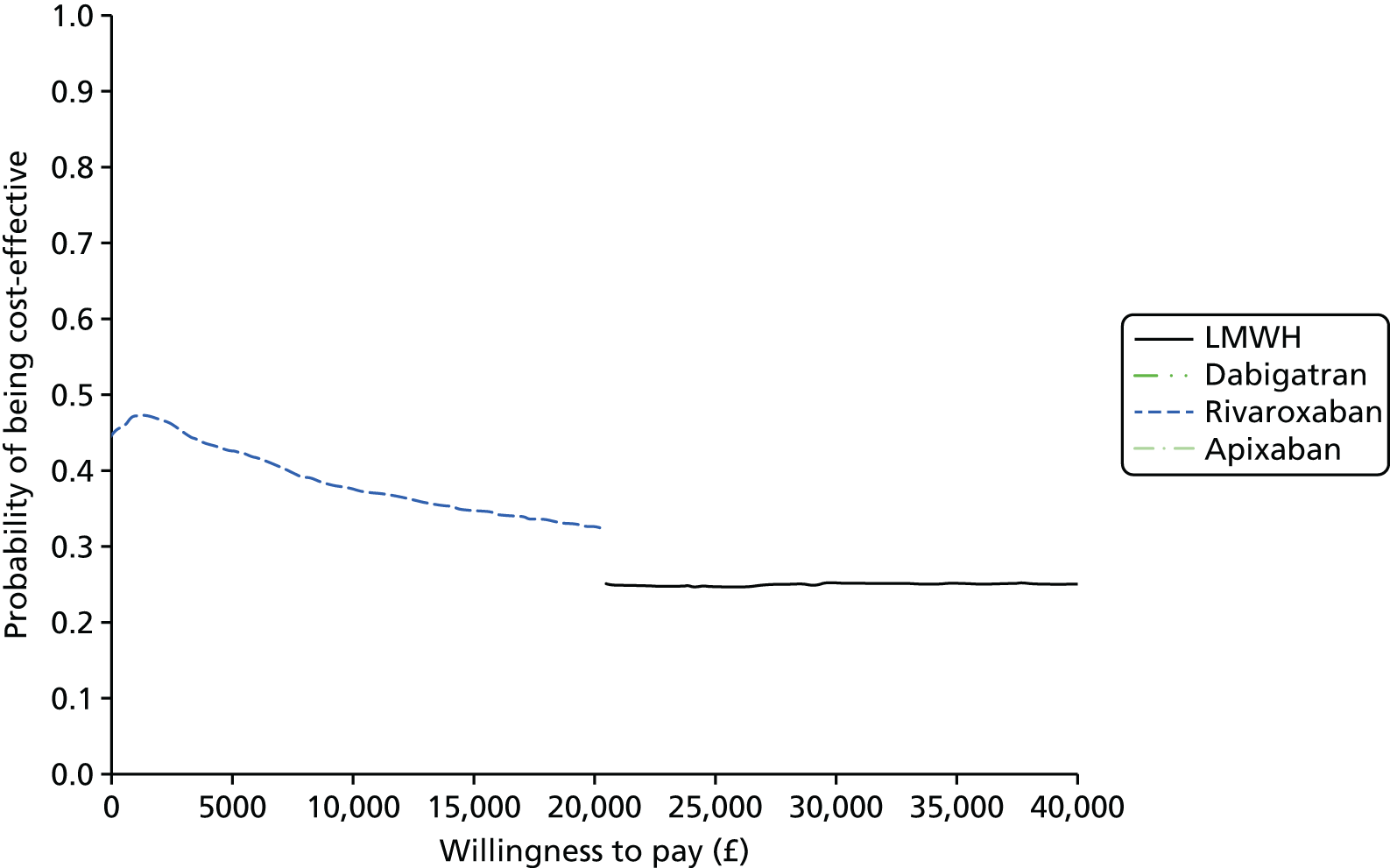
Our results were robust to all other sensitivity analyses that were conducted on the primary prevention populations: pooling post-THR and post-TKR populations for relative treatment effect of VTE; costing dabigatran at a lower dose to match the licensed dose for an elderly population; decreasing the costs and utilities for AEs; and increasing the costs and utilities for VTE events by 50% (see Appendix 12).
Total hip replacement
Our results were robust to all of the sensitivity analyses conducted on the primary prevention populations: pooling post-THR and post-TKR populations for relative treatment effect of VTE, costing dabigatran at a lower dose to match the licensed dose for an elderly population; and varying the costs and utilities for VTE and AEs by ± 50% (see Appendix 12).
Summary of cost-effectiveness findings
The economic analyses of the use of NOACs in the prevention and treatment of VTE attempt to balance the costs of pharmacotherapy against the benefits of reducing VTE-related events and the risks of anti-coagulant-related AEs. To a large extent the findings of the economic analyses reflect the evidence and uncertainty identified by the NMAs in previous chapters.
In secondary prevention, we found no strong evidence that NOACs (apixaban, dabigatran and rivaroxaban) were more cost-effective than no pharmacotherapy or aspirin. The RCT evidence that NOACs reduce the risk of VTE was counterbalanced by the relatively low underlying risk of VTE, the low proportion of fatal VTE events and the potentially elevated risk of AEs as a result of bleeding. Our base-case analysis indicated that the relatively small benefits of NOACs compared with no pharmacotherapy or aspirin did not justify the high costs of long-term NOAC treatment. This finding was sensitive to assumptions about the incidence of fatal PE. We found that aspirin was most likely to be cost-effective for secondary prevention, although there was uncertainty whether or not no pharmacotherapy was more cost-effective, and choice between aspirin and no pharmacotherapy was particularly sensitive to assumptions around AEs (ICH) under no pharmacotherapy, and costs associated with VTEs. Further research on the relative cost-effectiveness of aspirin and no pharmacotherapy would be of value.
In acute treatment, we found that NOACs, particularly apixaban, are likely to be cost-effective compared with warfarin at conventional NICE willingness-to-pay thresholds of £20,000–30,000 per QALY. Although there was little evidence that NOACs substantially reduced the risk of VTE compared with warfarin, the reduced risk of ICH and CRB contributed to our finding that there was a relatively high probability (> 0.5) that apixaban is the most cost-effective intervention in this setting. This finding was robust to sensitivity analyses on the model assumptions, although further research on the relative efficacy and safety of apixaban compared with other NOACs would be valuable to increase the strength of evidence.
For primary prevention of VTE following hip surgery, expected clinical benefits were similar for rivaroxaban and LMWH, whereas the lower costs of intervention with rivaroxaban meant that it was the most cost-effective intervention at the usual NICE thresholds. For primary prevention of VTE following knee surgery there was little difference in clinical benefit between the interventions, with rivaroxaban and LMWH being similarly cost-effective. There is a substantial potential value of further research in both THR and TKR populations, partly due to the large volume of these operations, meaning that a large population of patients may be given these treatments, but also because of the high levels of uncertainty in the relative treatment effects. This arises partly because of the fact that events are rare, and so very large studies are required to provide sufficient power to detect treatment differences when they exist, especially for AEs, which can have long-term consequences.
Our models make several assumptions (summarised in Box 1). In order to make the models tractable for each decision problem, we assumed that the most cost-effective comparator in secondary prevention would be used after acute treatment and that the most cost-effective comparator in acute treatment would be used after the failure of primary prevention. This assumes independence between treatments (i.e. the efficacy of secondary prevention does not depend on the therapy used for acute treatment). It also assumes that evidence from the wider acute/secondary prevention population (e.g. including medical patients) provides valid evidence for those primary prevention (i.e. surgical) patients who require acute treatment and secondary prevention.
In our base-case secondary prevention model, we assumed that patients would stop treatment only after ICH. In reality, patients may discontinue or switch treatment for various reasons. A proportion of patients will not comply with treatment because of side-effects or difficulty achieving a stable INR (on warfarin). Patients may also switch treatment after a recurrent symptomatic VTE event, which may be interpreted as ‘treatment failure’. The secondary prevention RCTs, which have relatively short follow periods, provide very little evidence on long-term treatment compliance. Our finding that NOACs were not more cost-effective than aspirin or ‘no pharmacotherapy’ was robust to a sensitivity analysis showing that patients switched to warfarin after a recurrent VTE, but may be sensitive to other treatment switching and non-compliance.
There is evidence that dabigatran is associated with MI in the AF population. The VTE RCTs typically did not report MI as an outcome, and we did not include it in the VTE models. It is likely to be most influential in the secondary prevention of VTE, when patients may be on therapy for prolonged periods. However, including the risk of MI in the secondary prevention model would not change our conclusion that none of the NOACs (including dabigatran) was cost-effective.
Edoxaban for the acute treatment of VTE is under review by NICE, but has not yet been approved and does not have BNF list cost in the UK. We assumed that the cost would be similar to other NOACs, and we performed a threshold analysis on cost to see how price influenced cost-effectiveness in acute treatment. Because edoxaban had very similar efficacy to warfarin, with lower benefits to apixaban, we found that it was not cost-effective at willingness-to-pay-threshold values of £20,000 or £30,000 per QALY, even at zero cost. When willingness to pay per QALY was low then it became cost-effective as the price decreased below that of warfarin, but such low threshold values are not used in practice.
Our systematic literature review identified evidence to inform model parameters for two primary prevention models (post THR and post TKR). We did not identify enough data to parameterise a model to estimate the cost-effectiveness of NOACs for patients who were hospitalised for medical treatment. These findings may not generalise to these patients and other patient groups.
Patients with asymptomatic VTE have no greater risk of symptomatic recurrent VTE than patients with no VTE event.
VTE and bleeding events are independent.
Patients cannot move out of the ‘PTS’ or ‘CTPH’ states, with the exception to the death state.
All anticoagulation will be stopped for patients who have an ICH.
Proportion of VTE that is DVT vs. non-fatal PE vs. fatal PE is treatment independent.
ICH relative safety from AF population.
Quality of life and costsMinor bleeds do not impact on quality of life and costs.
Clinically relevant bleeds, DVT and non-fatal PE do not have a long-term impact on quality of life.
Comparisons with the literature
There have been relatively few previous CEAs of NOACs for the prevention or treatment of VTE in the peer-reviewed literature. Most of the published studies focus on primary prevention after surgery and few compare more than one NOAC to LMWH. 59–61,232 The published comparisons of rivaroxaban, dabigatran and LMWH are based on direct trial evidence and conclude that, although rivaroxaban, in particular, may be cost-effective, there is great uncertainty about which strategy is the most cost-effective. 60,61,232 One industry-sponsored cost-effectiveness model comparing rivaroxaban to LMWH and a VKA, based on the EINSTEIN trial,204,215,216 concluded that there was a high probability that rivaroxaban was cost-effective. 233 We also found that rivaroxaban was likely to be cost-effective for primary prevention after TKR and THR. However, despite including a larger number of trials in a NMA than previous cost-effectiveness models, our interpretation is tentative because of imprecise estimates about effect and safety.
Chapter 12 Discussion and conclusions
Main findings
In the following sections, we summarise the main findings for each therapeutic area, first summarising efficacy and safety comparisons of NOACs with established treatments and then comparing individual NOACs with one another. We also summarise the results of the CEAs.
Atrial fibrillation: results of clinical effectiveness analyses
There was evidence that apixaban (5 mg bd), dabigatran (150 mg bd), edoxaban (60 mg od) and rivaroxaban (20 mg od) all reduce the risk of stroke or SE compared with warfarin (INR 2–3). Among the NOACs, there was evidence of a higher risk of stroke or SE with edoxaban (60 mg od) and rivaroxaban (20 mg od) than dabigatran (150 mg bd).
There was evidence that dabigatran (150 mg bd) reduces the risk of ischaemic stroke compared with warfarin, whereas edoxaban (30 mg od) increases that risk. There was little evidence that the risk of ischaemic stroke differed between licensed doses of NOACs.
There was weak evidence that the risk of MI is higher with dabigatran (110 mg bd), dabigatran (150 mg bd) and edoxaban (30 mg od) than warfarin (INR 2–3), and weak evidence that the risk of MI is lower with rivaroxaban (20 mg od) than warfarin (INR 2–3). Among the NOACs, there was weak evidence that MI risk is higher with dabigatran (150 mg bd) than apixaban (5 mg bd), and lower with rivaroxaban (20 mg od) than dabigatran (150 mg bd).
There was evidence that apixaban (5 mg bd), dabigatran (110 mg bd), edoxaban (30 mg od) and edoxaban (60 mg od) all reduced risk of major bleeding compared with warfarin (INR 2–3). Among the NOACs, there was evidence that risk of major bleeding is higher with dabigatran (150 mg bd) than apixaban (5 mg bd), and with rivaroxaban (20 mg od) than apixaban (5 mg bd) and edoxaban (60 mg od).
There was evidence that the risk of CRB during antiplatelet therapy (aspirin < 150 mg od) is lower than with warfarin (INR 2–3). There was evidence that the risk of CRB with apixaban (5 mg bd), edoxaban (30 mg od) and edoxaban (60 mg od) is also lower than with warfarin (INR 2–3). However, edoxaban (30 mg bd) and edoxaban (60 mg bd) increased CRB compared with warfarin (INR 2–3). In comparisons among NOACs, there was evidence that CRB with edoxaban (60 mg od) and rivaroxaban (20 mg od) is higher than with apixaban (5 mg bd), and that rivaroxaban (20 mg od) increases CRB compared with edoxaban (60 mg od).
There was strong evidence that risk of intracranial bleeding was lower with apixaban (5 mg bd), dabigatran (110 mg bd), dabigatran (150 mg bd), edoxaban (30 mg od), edoxaban (60 mg od) and rivaroxaban (20 mg od) than warfarin (INR 2–3). For each of these NOACs and doses, except for rivaroxaban (20 mg od), the estimated relative risk reduction for intracranial bleeding was > 50%. There was weak evidence that risk of intracranial bleeding is higher with rivaroxaban (20 mg od) than apixaban (5 mg bd), dabigatran (150 mg bd) and edoxaban (60 mg od).
Risk of all-cause mortality was lower with apixaban (5 mg bd), dabigatran (110 mg bd), dabigatran (150 mg bd), edoxaban (30 mg od), edoxaban (60 mg od) and rivaroxaban (20 mg od) than warfarin (INR 2–3), but there was little evidence of a difference between the licensed doses of NOACs for this outcome.
Apixaban (5 mg bd) was ranked as being among the best interventions for a wide range of the outcomes that were evaluated, including stroke or SE, MI, major bleeding and all-cause mortality. Edoxaban (60 mg od) was ranked second for major bleeding and all-cause mortality. Except for all-cause mortality, outcomes for rivaroxaban (20 mg od) were ranked less highly than several other NOACs. The non-NOAC interventions [warfarin (INR 2–3) and antiplatelet therapy (aspirin/clopidogrel ≥ 150 mg od)] were ranked worst for stroke or SE and were not among the best three interventions for any of the outcomes. We did not include apixaban (2.5 mg bd) or betrixaban (40 mg od) because comparisons involving these interventions were imprecisely estimated.
In our sensitivity analyses, results were similar when using HRs instead of ORs. Moreover, we found no evidence of effect modification according to mean TTR for patients on warfarin. However, our meta-regression models assumed a common interaction effect across treatments: that assumption could not be empirically tested because of a lack of replication for most comparisons. An important limitation is that primary studies did not report the mean time above or below therapeutic range for warfarin arms. Therefore, we were unable to address some clinically relevant questions regarding the impact of treatment settings for warfarin on stroke prevention, as well as on bleeding and other AEs.
Atrial fibrillation: results of cost-effectiveness analyses
Dabigatran (150 mg bd) has the lowest expected total cost (£23,064), followed by apixaban (5 mg bd), edoxaban (60 mg od), warfarin (INR 2–3) and rivaroxaban (20 mg od), which had the highest expected total cost (£24,841). Expected costs are similar across all treatments, and there is a high degree of uncertainty around the costs for all treatments.
Apixaban (5 mg bd) has the highest expected QALYs (5.49), followed by rivaroxaban (20 mg od) (5.45), dabigatran (150 mg bd) (5.42), edoxaban (60 mg od) (5.41) and warfarin (INR 2–3) (5.17). The NOACs have similar expected QALYs, all of which are higher than for warfarin (INR 2–3). There is a high degree of uncertainty around the QALY estimates.
At a willingness-to-pay threshold of £20,000 per QALY, all NOACs have positive expected INB compared with warfarin (INR 2–3), suggesting that they may be a cost-effective use of NHS resources. Apixaban (5 mg bd) has the highest expected INB (£7533), followed by dabigatran (150 mg bd) (£6365), rivaroxaban (20 mg od) (£5279) and edoxaban (60 mg od) (£5212). Apixaban (5 mg bd) is the only NOAC for which the 95% CI around INB is positive, suggesting that apixaban is cost-effective compared with warfarin. These conclusions also hold at the higher threshold of £30,000. The key drivers of these results are the lower rates of MI, ICH and other CRB for apixaban (5 mg bd).
The CEAC indicates that apixaban (5 mg bd) has the highest probability of being the most cost-effective first-line therapy for AF, close to 60% in the £20,000–30,000 range of willingness-to-pay thresholds generally considered by NICE. Dabigatran (150 mg bd) has the highest probability of being cost-effective if the willingness-to-pay threshold is very low as a result of having the lowest expected total costs. Warfarin (INR 2–3) and edoxaban (60 mg od) are unlikely to be cost-effective. These results are further highlighted by the CEAF. Apixaban (5 mg bd) has the highest expected net benefit at a wide range of willingness-to-pay thresholds. Apixaban (5 mg bd) is likely to be the most cost-effective first-line therapy for AF, under the assumptions of our model.
Primary prevention of venous thromboembolism: results of clinical effectiveness analyses
In hip surgery patients most treatment comparisons were imprecisely estimated, but there was evidence that risk of symptomatic VTE is lower with rivaroxaban (10 mg od) than LMWH (pre-op, standard dose) but higher with LMWH (post-op, standard dose) and warfarin (INR 2–3) than LMWH (pre-op, standard dose). Comparisons between the licensed doses of NOACs were imprecisely estimated. For knee surgery patients, there was little evidence that risk of symptomatic VTE differed between apixaban (2.5 mg bd), dabigatran (220 mg od) or rivaroxaban (10 mg od) compared with LMWH (post-op, standard dose). Comparisons between licensed doses of NOACs were also imprecisely estimated. For medical patients there was weak evidence that the risk of symptomatic VTE is lower with apixaban (2.5 mg bd) than LMWH (standard dose), and also compared with rivaroxaban (10 mg od), although these comparisons were imprecisely estimated.
For symptomatic DVT, all comparisons for hip surgery patients were imprecisely estimated, but there was evidence that risk of symptomatic DVT is higher for LMWH (post-op, standard dose) and warfarin (INR 2–3) than LMWH (pre-op, standard dose). All comparisons for knee surgery patients were imprecisely estimated but there was evidence that risk of symptomatic DVT was higher for LMWH pre-op (standard dose) than LMWH (post-op, standard dose). For medical patients, all comparisons were imprecisely estimated, but there was evidence that risk of symptomatic DVT is lower for apixaban (2.5 mg bd) than LMWH (standard dose).
For symptomatic PE, all comparisons for trials in hip surgery, knee surgery and medical patients were imprecisely estimated. For knee surgery patients, there was some evidence that the risk of symptomatic PE is lower with dabigatran (150 mg od) and higher with apixaban (2.5 mg bd) than LMWH (post-op, standard dose). Among licensed doses of NOACs the risk of symptomatic PE may be lower for rivaroxaban (10 mg od) than apixaban (2.5 mg bd).
For MI, all comparisons were imprecisely estimated, although there was some evidence that risk of MI is lower for rivaroxaban (10 mg od) than LMWH (post-op, standard dose).
There was little evidence that risk of major bleeding differs between pre-op and post-op LMWH (standard dose). There was evidence that risk of major bleeding is lower with warfarin (INR 2–3) and higher with rivaroxaban (10 mg od) than LMWH (post-op, standard dose). There was evidence that risk of major bleeding is higher with rivaroxaban (10 mg od) than apixaban (2.5 mg bd) and dabigatran (220 mg od).
There was evidence that risk of CRB is higher for pre-op LMWH (standard dose) than post-op LMWH (standard dose), and higher for dabigatran (150 mg or 220 mg od) and rivaroxaban (10 mg od) than LMWH (post-op, standard dose). There was evidence that risk of CRB is higher for dabigatran (220 mg od) and rivaroxaban (10 mg od) than apixaban (2.5 mg bd).
There was little evidence that risk of all-cause mortality differed for any intervention compared with LMWH (post-op, standard dose). Comparisons between licensed doses of NOACs were imprecisely estimated.
Warfarin was ranked with high probability as the best intervention for major bleeding events, and LMWH (post-op, standard dose) was ranked with high probability as best or second-best intervention for CRB. Rivaroxaban (10 mg od) was ranked among the worst interventions for bleeding outcomes.
Primary prevention of venous thromboembolism following hip and knee surgery: results of cost-effectiveness analyses
Total hip replacement
The lowest expected total costs are for apixaban (£702), followed by rivaroxaban (£718) then dabigatran (£893). LMWH has the highest expected cost (£1062). Expected benefits are highest for rivaroxaban and LMWH (9.10 QALYs), followed by dabigatran (9.04 QALYs) then apixaban (8.96 QALYs). At both £20,000 and £30,000 willingness-to-pay QALY thresholds per QALY, rivaroxaban has the highest expected INB, although CIs around net benefit are wide (particularly for dabigatran) and also skewed (apixaban). Rivaroxaban has the highest expected net benefit over the range of willingness-to-pay thresholds we explored, but with substantial uncertainty: its probability of being the most cost-effective was 0.35 for willingness-to-pay threshold of £30,000 per QALY.
Total knee replacement
Rivaroxaban has the lowest expected total costs (£834), followed by post-op LMWH (£855) and dabigatran (£871), whereas apixaban has the highest expected total costs of £932. Rivaroxaban and LMWH had similar INB at willingness-to-pay thresholds of £20,000 and £30,000 per QALY. Dabigatran and apixaban have negative INB compared with post-op LMWH. The cost-effectiveness plane and the CEACs show substantial uncertainty around the relative costs and benefits of these interventions. Rivaroxaban has the highest expected net benefit over the range of willingness-to-pay thresholds that we explored, and the highest probability of being the most cost-effective treatment for willingness-to-pay thresholds of up to approximately £20,000 per QALY.
Acute treatment of venous thromboembolic disease: results of clinical effectiveness analyses
The planned edoxaban dose in the HOKUSAI-VTE study206,207 was 60 mg od but 17.6% of the patients in that intervention arm received a lower dose of 30 mg od. This intervention is denoted ‘Edoxaban [60 or 30 (17.6%) mg od]’.
Compared with warfarin (INR 2–3), none of the NOACs reduced the risk of symptomatic VTE, symptomatic DVT or symptomatic PE on follow-up, nor did the risk of any of these outcomes differ between licensed doses of NOACs.
For risk of MI, all comparisons were imprecisely estimated.
There was strong evidence that apixaban (5 mg bd) and rivaroxaban (15 mg bd then 20 mg od) reduce risk of major bleeding compared with warfarin (INR 2–3). There was evidence that risk of major bleeding was higher for edoxaban [60 or 30 (17.6%) mg od] and dabigatran (150 mg bd) compared with apixaban (5 mg bd).
There was evidence that apixaban (5 mg bd), dabigatran (150 mg bd) and edoxaban [60 or 30 (17.6%) mg od] reduce risk of CRB compared with warfarin (INR 2–3). There was some evidence that rivaroxaban (15 mg bd then 20 mg od) reduces risk of CRB compared with warfarin (INR 2–3). There was evidence that risk of CRB is higher with dabigatran (150 mg bd), edoxaban [60 or 30 (17.6%) mg od] and rivaroxaban (15 mg bd then 20 mg od) than apixaban (5 mg bd). There was evidence that risk of CRB is higher with edoxaban [60 or 30 (17.6%) mg od] and rivaroxaban (15 mg bd then 20 mg od) than dabigatran (150 mg bd).
There was little evidence that risk of all-cause mortality differed for any of the NOAC interventions compared with warfarin (INR 2–3). Neither was there evidence that risk of all-cause mortality differed between licensed doses of NOACs.
There was a high probability that warfarin (INR 2–3) is ranked worst for major bleeding and CRB. There was a high probability that apixaban (5 mg bd) is ranked best for major bleeding and CRB, and this intervention also had a high probability of being ranked best or second best for symptomatic DVT, symptomatic VTE and all-cause mortality.
Acute treatment of venous thromboembolic disease: results of cost-effectiveness analyses
We estimated expected costs, QALYs, incremental costs, incremental QALYs and incremental NMB at willingness-to-pay thresholds of £20,000 and £30,000. Expected costs and benefits are similar across all treatments because of the short (6-month) treatment duration, and the small and imprecisely estimated effects of NOACs on VTE recurrence and AEs compared with warfarin. Warfarin has the lowest expected cost (£19,651), followed by dabigatran, edoxaban and apixaban, with rivaroxaban the most expensive (£19,753). Apixaban had the highest expected QALYs (12.02) but this is only 0.04 QALYs greater than the interventions with the lowest expected QALYs (edoxaban, warfarin and dabigatran).
The expected net benefit is highest for apixaban at willingness-to-pay thresholds of £20,000 and £30,000 per QALY. This is due to the marginally lower risk of recurrent VTE, CRB and non-VTE-related mortality with apixaban relative to other NOACs. However, there is substantial uncertainty around this estimate. Rivaroxaban also has a positive INB compared with warfarin. CIs for INB are wide for all treatments, reflecting substantial uncertainty that is also seen in the incremental cost-effectiveness plane.
The CEACs show that for very low willingness to pay per QALY, warfarin is the most cost-effective treatment (because it has lowest expected costs). For willingness-to-pay thresholds of > £1000, apixaban (5 mg) has the highest expected net benefit, with a probability of being most cost-effective at £20,000–30,000 per QALY thresholds of approximately 0.54. However, it is possible that rivaroxaban or dabigatran are the most cost-effective interventions, even at high willingness-to-pay thresholds.
Secondary prevention of venous thromboembolism: results of clinical effectiveness analyses
There was evidence that aspirin (100 mg od), warfarin (INR 1.5–2) and warfarin (INR 2–3) substantially reduced risk of symptomatic VTE compared with placebo. All NOACS at the doses included in the network also substantially reduced risk of symptomatic VTE compared with placebo. Risk of symptomatic VTE was lower for all NOACs at doses included in the network than aspirin. However, there was no clear evidence that risk of symptomatic VTE differed between these NOAC interventions and warfarin, although most comparisons were imprecisely estimated. There was no clear evidence that risk of symptomatic VTE differed between licensed doses of NOACs, although these comparisons were imprecisely estimated.
There was no clear evidence that aspirin (100 mg od) reduced risk of symptomatic DVT considered as an individual end point compared with placebo. There was evidence that warfarin (INR 2–3) and all NOACs at doses included in the network substantially reduced risk of symptomatic DVT compared with placebo. These NOAC interventions substantially reduced risk of symptomatic DVT compared with aspirin. By contrast, there was no clear evidence that risk of symptomatic DVT differed between these NOACs and warfarin (INR 2–3), although comparisons were imprecisely estimated. There was no clear evidence that risk of symptomatic DVT differed between NOACs at licensed doses, although all comparisons were imprecisely estimated.
There was evidence that warfarin (INR 2–3), apixaban (5 mg bd), dabigatran (150 mg bd) and rivaroxaban (20 mg od) substantially reduce risk of symptomatic PE compared with placebo. There was evidence that dabigatran (150 mg bd) and rivaroxaban (20 mg od) reduce risk of symptomatic PE compared with aspirin. There was evidence that risk of symptomatic PE was higher for apixaban (2.5 mg bd) than warfarin (INR 2–3). There was weak evidence that risk of symptomatic PE was lower for dabigatran (150 mg bd) and rivaroxaban (20 mg od) than apixaban (2.5 mg bd).
All comparisons of risk of MI were imprecisely estimated.
There was evidence that risk of major bleeding is higher for warfarin (INR 2–3) and rivaroxaban (20 mg od) than placebo, although these comparisons were imprecisely estimated. Comparisons of the risk of major bleeding for NOACs compared with aspirin were imprecisely estimated. There was evidence that risk of major bleeding is lower for dabigatran (150 mg bd), apixaban (2.5 mg bd) and apixaban (5 mg bd) than warfarin (INR 2–3). There was evidence that risk of major bleeding is higher with dabigatran (150 mg bd) and rivaroxaban (20 mg od) than apixaban (2.5 mg bd and 5 mg bd).
There was evidence that risk of CRB is substantially higher with warfarin (INR 2–3), dabigatran (150 mg od) and rivaroxaban (20 mg od) than placebo, and that risk of CRB is higher with rivaroxaban (20 mg od) than aspirin. There was evidence that risk of CRB is lower with apixaban (2.5 mg or 5 mg bd) and dabigatran (150 mg bd) than warfarin (INR 2–3). All comparisons between NOACs at licensed doses were imprecisely estimated, but there was evidence that risk of CRB is higher with dabigatran (150 mg bd) and rivaroxaban (20 mg od) than apixaban (2.5 mg bd and 5 mg bd).
All comparisons of risk of all-cause mortality with placebo, except that for aspirin (100 mg od), were imprecisely estimated. However, there was evidence that risk of all-cause mortality was lower for apixaban (5 mg bd) than placebo. Comparisons of NOACs with aspirin were imprecisely estimated, although there was weak evidence that risk of all-cause mortality is lower with apixaban (5 mg bd) than aspirin. There was no evidence that risk of all-cause mortality differed for NOACs than warfarin (INR 2–3), although all comparisons except that with dabigatran (150 mg bd) were imprecisely estimated. Comparisons of risk of all-cause mortality between NOACs at licensed doses were imprecisely estimated.
Secondary prevention of venous thromboembolism: results of cost-effectiveness analyses
We estimated expected costs, QALYs, incremental costs, incremental QALYs and incremental NMB at willingness-to-pay thresholds of £20,000 and £30,000. The cheapest comparator is aspirin (total expected cost £20,671). No pharmacotherapy is the next cheapest treatment with benefits similar to aspirin. Warfarin and the NOACs all have substantially higher costs than aspirin and no pharmacotherapy, and the NOACs are more expensive than warfarin. Dabigatran and apixaban (5 mg) have marginally higher expected QALYs than no pharmacotherapy. Apixaban (2.5 mg) has the lowest expected QALYs, followed by warfarin. Apixaban (2.5 mg) has the highest HR for the risk of ICH, albeit estimated imprecisely. Although the NOACs and warfarin prevent more recurrent VTEs than no pharmacotherapy or aspirin, the rate of recurrent VTE is low, and the rate of AEs (ICH and clinically relevant bleeds), which can have a long-term impact on quality of life, are generally higher for the NOACs than aspirin or no pharmacotherapy.
Aspirin has the highest expected net benefit at willingness-to-pay thresholds of £20,000 and £30,000 per QALY. However, the CI for the INB of aspirin includes zero, indicating uncertainty about whether or not it is more cost-effective than no pharmacotherapy. All NOACs have negative expected INBs at the £20,000 and £30,000 thresholds, and all CIs are negative at the £20,000 threshold, indicating that they are not cost-effective compared with no pharmacotherapy. Dabigatran, which had the lowest estimated HR for recurrent VTE and ICH of all the NOACs, also has the highest expected net benefit of any NOAC. However, dabigatran is not cost-effective relative to no pharmacotherapy, even at the £30,000 threshold, as the incremental NMB is negative (–£3402; 95% CI –£12,388 to £5424). Although there is uncertainty in the estimated costs and QALYs, it is clear that aspirin has lower costs and similar benefits in the majority of the samples. Over a wide range of willingness-to-pay-per-QALY thresholds, aspirin has the highest expected net benefit, and also the highest probability of being the most cost-effective, although there is a non-negligible probability that no pharmacotherapy is the most cost-effective intervention for secondary prevention of VTE at a threshold of £20,000–30,000. These results suggest that it is not cost-effective to prescribe NOACs or warfarin for secondary prevention of VTE over the range of willingness-to-pay thresholds that we explored (up to £40,000 per QALY).
Analyses of the value of information from future research
Value of information analyses exploit the cost-effectiveness models to quantify and summarise the value (in cost terms) of evidence that could potentially be generated from future research studies.
For AF, the optimal decision regarding the most cost-effective NOAC is most sensitive to the HRs comparing the NOACs, suggesting that a head-to-head trial comparing NOACs may be of value. The decision is also sensitive to costs, the effect of past events on future HRs, and probabilities of treatment switching. A head-to-head trial could also provide information about baseline event rates, costs and switching probabilities. However, a study powered to measure all of these outcomes with sufficient precision would require a very large sample size, which may be prohibitively expensive.
For VTE primary prevention in the THR population, the optimal decision is most sensitive to uncertainty in the treatment-independent transition parameters, and also the cost parameters. This suggests that there may be value in running a longitudinal study examining the treatment-independent transition parameters: rates of mild/moderate PTS, severe PTS, CTPH and the proportion split of VTE events.
For VTE primary prevention in the TKR population, the optimal decision is most sensitive to uncertainty in the utilities, relative treatment effects and treatment-independent transition parameters, and is also sensitive to the cost parameters. This suggests that there may be value in running a large trial comparing NOACs and warfarin, which would reduce the uncertainty in the relative treatment effects. There may also be value in conducting a study to estimate the utility values associated with VTE events and treatment-related events, and a longitudinal study examining the treatment-independent transition parameters: rates of mild/moderate PTS, severe PTS, CTPH and the proportion split of VTE events.
For VTE acute treatment, the optimal decision is most sensitive to uncertainty in the cost and utility model inputs. This suggests there may be value in conducting a study to estimate the utilities and costs associated with VTE events and treatment-related events. As such, a study is likely to be relatively inexpensive to conduct (compared with a RCT), and given the magnitude of likely benefits, this should be considered a research priority.
For VTE secondary prevention, the optimal decision is most sensitive to the relative treatment effects, suggesting that there may be value in running a large trial comparing one or more NOACs with aspirin and no pharmacotherapy. However, a study powered to capture VTE events may be prohibitively expensive, because event rates are low.
Strengths and limitations
Strengths
The strengths of this technology appraisal include its comprehensive coverage of all the therapeutic areas in which NOACS have been evaluated to date, using the same methodology. Previous analyses of comparative effectiveness have focused on individual therapeutic areas, making it more difficult to judge if one of the four licensed NOACs might emerge as a frontrunner in more than one therapeutic area. Additional strengths include careful appraisal of study quality; focus on clinically relevant end points; an evaluation of safety that considers evidence spanning all therapeutic areas together, to maximise power; the development of a possible treatment hierarchy for the different anticoagulant indications, where the data allowed it; and a CEA that is relevant to the NHS.
Limitations
The limitations of this technology appraisal relate mainly to shortfalls in the primary data, on which the overview is based. In particular:
-
There were no direct head-to-head comparisons between different NOAC drugs – all such comparisons were therefore based on indirect evidence derived from the networks.
-
Economic analyses for conditions such as AF and VTE necessarily make long-term projections on the basis of short-term trial evidence, observational data and clinically informed assumptions about plausible treatment pathways and health-state transitions. These assumptions and evidence limitations are discussed in previous sections [see Chapter 6 (Summary of cost-effectiveness findings) and Chapter 11 (Summary of cost-effectiveness findings)].
-
The profile of patients entering trials may not be the same as those treated in practice, who may be older and have more comorbidities. Treatment benefits in such patients may be smaller, and rates of harm higher, than estimated by trials.
-
As for all new drugs, adverse effects that remained undetected during development may come to light with high-volume use post licensing.
-
It is possible that patients treated with warfarin in practice are at higher risk of bleeding complications than those in trials because of a greater number of comorbidities and less stringent control of anticoagulation. However, concerns have also been raised previously that the time spent in the therapeutic range was suboptimal among patients in clinical trials who were assigned to warfarin. Thus, clinical trials could have underestimated both the benefits and the risks of warfarin treatment.
For these reasons, guideline developers, prescribers and patients may wish to exercise caution when considering the prescription of new therapies over older, more established ones.
Several factors led to imprecision in the estimation of certain treatment effects. These included low rates of occurrence of certain end points, particularly in trials evaluating the safety and efficacy of NOACs in the primary and secondary prevention of VTE; widespread use of composite end points, with low rates of occurrence of certain (more clinically relevant) components of the composite; as well as substantial inconsistency in the reporting of end points in different trials in the same therapeutic area, leading to a substantial number of missing end point data (see Tables 22, 23, 72, 73, 111, 112, 138 and 139). Owing to the low event rates and lack of substantial replication of specific comparisons across studies, we used fixed-effects models for the NMA. This does not account for heterogeneity in treatment effects. Under fixed-effects models, our Bayesian analyses with vague priors will produce results very similar to frequentist analyses.
The evidence base for established antiplatelet and anticoagulant treatments in primary prevention of VTE among hospitalised patients extends to groups of patients beyond those who were evaluated in this report, for which comparisons were focused on patients undergoing hip and knee surgery. No trials of NOACS were identified among patients who were undergoing neurosurgery, gastroenterological surgery or gynaecological surgery. For this reason, conclusions about the comparative effectiveness of NOACs compared with established antiplatelet and anticoagulant medications for primary prevention of VTE should be limited to hip and knee surgery patients.
The apparent efficacy of NOACs when compared with warfarin could be inflated if control of the INR was suboptimal among patients who were randomised to warfarin. For this reason, many of the studies reported time spent in the therapeutic range (TTR), as an index of anticoagulant control. This is a potentially important issue for the studies of stroke prevention in AF, for which 16 (73%) of the 22 studies that included a warfarin intervention arm reported mean TTR. There was substantial variation in TTR (from 45.1% to 83%) between these studies. For acute treatment of VTE, eight (89%) of the nine studies that included a warfarin intervention arm reported mean TTR, but variation between them was less marked than for the AF studies (from 56.9% to 63.5%). For secondary prevention of VTE, mean TTR was reported in three (60%) of the five studies that included a warfarin intervention arm. The prespecified protocol for this health technology appraisal specified TTR as a potential modifier of NOAC treatment effect in trials in which warfarin was the comparator. We plan future analyses that address this issue.
The clinical effectiveness analyses reported are based on relative rather than absolute risk differences. However, event rates for different safety and efficacy end points were estimated within and contributed to the CEAs.
Factors beyond those considered in this technology appraisal could influence the choice of the optimal anticoagulant in each of the therapeutic areas evaluated.
In some situations, the need for anticoagulation monitoring with warfarin treatment may be viewed as a useful means to confirm adherence to anticoagulant therapy rather than as an inconvenience.
Recent studies have suggested that the efficacy and safety of dabigatran could be improved by monitoring of achieved drug levels, because these exhibit wide inter-individual variation. This may reduce the convenience of this NOAC and increase its cost compared with warfarin or other NOACs but we did not model this in the current report. Only one of the studies included in our reviews considered whether or not monitoring improves the efficacy and safety of NOACs: in a subsample of 9183 patients in the RE-LY trial,13 ischaemic stroke and major bleeding both correlated with dabigatran plasma concentrations. Specific tests to measure the anticoagulation effects of NOACs are being developed but are not yet widely available234 and routine coagulation tests such as prothrombin time and activated partial thromboplastin time are of limited use. 235,236 It is therefore currently unclear whether or not the efficacy and safety profiles of NOACs can be improved by monitoring and dose adjustment. Monitoring may be particularly helpful in certain clinical situations (e.g. emergency surgery or patients presenting with bleeding236) and patient groups (advanced age, renal impairment).
Finally, therapeutic decision making may be influenced by recognition that effective treatments for reversal of anticoagulation with NOACs are still in the developmental phase. For example:
-
Aripazine (PER-977; PER 977; ciraparantag) is a synthetic cationic molecule that binds unfractionated and LMWH, the factor Xa inhibitors edoxaban, rivaroxaban and apixaban, and the factor II inhibitor dabigatran, but not to warfarin. 237 In a Phase I trial involving 80 healthy volunteers, intravenous PER977 reversed the prolongation of whole blood clotting time induced by a single oral dose of edoxaban 60 mg in a dose-dependent fashion, within 10–30 minutes of administration. 238 Phase II clinical studies of this agent are in progress.
-
Andexanet alpha (PRT4445; PRT064445) is a recombinant modified factor Xa molecule that acts as an antidote to factor Xa inhibitors through a decoy mechanism. A number of Phase III studies of this agent are under way. 239,240
-
Idarucizumab (BI 655075) is a humanised monoclonal antibody fragment that binds dabigatran to reverse its anticoagulant activity. 241,242 Phase I/II studies of this agent have been completed. A Phase III study investigating reversal of anticoagulation in patients receiving dabigatran who have uncontrolled bleeding or who require emergency surgery or invasive procedures is under way.
Research needs
Evidence on the comparative efficacy of NOACs in this review has come exclusively from indirect comparisons, because of the lack of head-to-head trials. Among patients with AF, a long-term condition, the trials have also been of relatively short duration. A different manufacturer has developed each of the agents evaluated in this review and it is therefore unlikely that any head-to-head trials will be initiated by industry.
Reliable estimation of the cost-effectiveness of NOACs in different clinical scenarios requires high-quality data on absolute event rates for the various efficacy and safety outcomes. NHS health record data could provide a rich source for information, but so far health record data have been insufficiently utilised for this purpose.
Although NOACs were developed in part to supersede warfarin by obviating the need for therapeutic monitoring of anticoagulation, to improve convenience, recent studies13 have suggested that monitoring of drug levels may improve safety and efficacy of dabigatran treatment. Whether or not this is also the case for other NOACs is not known.
The requirement for therapeutic drug monitoring with warfarin also serves as a means to assess adherence. Thus far, long-term adherence rates for NOACs (e.g. among patients with AF who may require anticoagulation for many years have not been evaluated).
For secondary prevention of VTE, use of NOACs in high-risk patients is a potential area for further study. Further research is needed to clarify whether aspirin or no treatment should be the standard of care in this setting.
The research needs identified by this review are therefore as follows:
-
To complete calculations of the expected value of sample information, in order to clarify whether it is justifiable to conduct one or more trials making direct comparisons between the most promising NOACs and NOAC doses, in situations typical of NHS clinical practice.
-
To consider the merits of conducting cohort studies that reduce uncertainties in costs, utilities and transition probabilities in order to improve estimates of relative cost-effectiveness, in particular in the context of primary prevention of VTE in THR and TKR, and acute treatment of VTE.
-
Information on long-term rates of the main efficacy and safety outcomes among patients receiving anticoagulants for AF (e.g. from registries or health record data).
-
Information on the role (if any) of therapeutic monitoring to enhance the safety and efficacy of NOACs.
-
Information on long-term adherence rates in patients receiving NOACs for AF.
-
Development of tools to stratify risk of recurrent VTE.
-
Further research is also needed to establish whether the secondary prevention of VTE with aspirin or other agents is cost-effective, with an adequate safety margin, in patients identified as being at particularly high risk of recurrence by validated risk stratification tools.
Implications for practice
This health technology appraisal was conducted to help guideline developers, doctors and patients decide when a NOAC might be preferred to an established anticoagulant and, when a NOAC is preferred to warfarin, if there is sufficient evidence to support the use of one particular NOAC over another. The evidence provided by this health technology appraisal indicates:
-
NOACs have advantages over warfarin in patients with AF and, of the available NOACs, apixaban 5 mg bd offers the best balance between efficacy and safety, and has the highest probability of being most cost-effective.
-
NOACs offer no efficacy advantage over warfarin in the acute treatment of VTE, but have a lower rate of bleeding complications albeit at a higher cost. For a willingness-to-pay threshold of > £5000, apixaban 5 mg bd emerges as the most cost-effective alternative to warfarin.
-
Neither the clinical nor CEA provided strong evidence that NOACs replace post-op LMWH in primary prevention of VTE in patients who are undergoing hip or knee surgery.
-
If secondary prevention after 3–6 months of anticoagulation for a first episode of VTE is to be considered (this is not currently established practice), NOACs provide no advantage over aspirin 100 mg od.
Chapter 13 Patient perspective
Anticoagulation Europe is a charity that provides education, information and support to patients requiring anticoagulation therapy in the UK.
Patients requiring anticoagulants for the treatment and prevention of VTE are given VKAs warfarin, heparin and LMWH. These treatments are effective and are now joined by newer technologies that work differently to warfarin.
The NOACs have become available to be used in the prevention of stroke in non-valvular AF and the treatment and secondary prevention of DVT and PE, complementing existing treatments.
In the UK, NICE and the Scottish Medicines Consortium (SMC) have produced guidelines that recommend the new agents. The benefits to patients and clinicians are that there is now a broader range of treatment options available to treat and prevent blood clots.
AntiCoagulation Europe has welcomed the opportunity to participate in this project, and has contributed the patient perspective of current anticoagulation practice, as captured by the experiences and feedback derived from their patient databases.
In our role as a dedicated anticoagulation charity, we have highlighted the need for equality of access to all of the anticoagulation therapies as recommended by NICE and the SMC. We advocate that patients should be adequately informed of the benefits and risks of all anticoagulation treatments in order that they can make an informed choice around their therapy options with the appropriate health-care professionals.
AntiCoagulation Europe anticipates that this comprehensive study will provide a helpful and informative reference resource for clinicians when considering and presenting the most effective and safe anticoagulation treatment options to patients for their condition.
Acknowledgements
We are grateful to Alexandra McAleenan for help with data extraction, Juan-Pablo Casas and Penny Whiting for help in preparing the original grant submission, and Alison Richards and Margaret Burke for help with designing and running search strategies.
Contributions of authors
PNB, PAB, PAD, JAL, GNO and HHT worked for substantial periods of time on the project, and are listed alphabetically. Other authors (DMC, SD, DE, JPTH, WH, CS, JS, RS, AS and NJW) apart from the first author (JACS) and last author (ADH) are then listed alphabetically.
Professor Jonathan AC Sterne (Professor of Medical Statistics and Epidemiology) co-conceived the project, led the grant application, led the project, contributed to statistical analyses and finalised the report.
Dr Pritesh N Bodalia (Principal Pharmacist) contributed to the grant application, extracted data and assessed risk of bias for the AF review, checked these aspects for the VTE reviews and provided pharmaceutical expertise.
Peter A Bryden (Research Associate in Health Economics Modelling) developed and analysed the economic models for VTE reviews and drafted relevant parts of the report.
Dr Philippa A Davies (Research Associate in Evidence Synthesis/Systematic Reviewing) extracted and checked data, assessed risk of bias and contributed to writing the report.
Dr Jose A López-López (Research Associate in Medical Statistics) undertook the statistical analyses of clinical effectiveness and safety and drafted relevant parts of the report.
Dr George N Okoli (Research Associate in Systematic Reviews) extracted data and assessed risk of bias for the VTE reviews, checked these aspects for the AF review, and drafted relevant parts of the report.
Dr Howard HZ Thom (Research Associate in Health Economic Modelling) developed and analysed the economic models for the AF review, and drafted relevant parts of the report.
Dr Deborah M Caldwell (Lecturer in Public Health Research) contributed to the grant application, contributed to the systematic review and planning of NMAs.
Dr Sofia Dias (Research Fellow) contributed to the statistical analyses.
Diane Eaton (Project Manager, AntiCoagulation Europe) provided a patient perspective.
Professor Julian PT Higgins (Professor of Evidence Synthesis) contributed to the grant application, contributed to the NMAs of clinical effectiveness and safety, contributed to management of the project and contributed to writing the report.
Professor Will Hollingworth (Professor of Health Economics) contributed to the grant application, contributed to the CEAs and contributed to writing the report.
Professor Chris Salisbury (Professor in Primary Health Care) contributed to the grant application and provided clinical expertise.
Dr Jelena Savović (Research Fellow) contributed to the grant application and contributed to the systematic review.
Dr Reecha Sofat (Senior Lecturer) provided clinical expertise and contributed to writing the report.
Annya Stephens-Boal (Executive Officer, Thrombosis UK) provided a patient perspective.
Dr Nicky J Welton (Reader in Statistical and Health Economic Modelling) contributed to the grant application, contributed to the statistical analyses and CEAs and contributed to writing the report.
Professor Aroon D Hingorani (Professor of Genetic Epidemiology) co-conceived the project, provided clinical expertise and contributed to writing the report.
Data sharing statement
All study data will be available from the corresponding author on request, once papers reporting the study findings have been published. Corresponding author’s contact details: School of Social and Community Medicine, Canynge Hall, University of Bristol, Bristol, UK.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Atrial Fibrillation: National Clinical Guideline for Management in Primary and Secondary Care. London: Royal College of Physicians; 2006.
- Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol 1998;82:2-9N. http://dx.doi.org/10.1016/S0002-9149(98)00583-9.
- Hart RG, Benavente O, McBride R, Pearce LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation: a meta-analysis. Ann Intern Med 1999;131:492-501. http://dx.doi.org/10.7326/0003-4819-131-7-199910050-00003.
- Lee S, Shafe AC, Cowie MR. UK stroke incidence, mortality and cardiovascular risk management 1999–2008: time-trend analysis from the General Practice Research Database. BMJ Open 2011;1. http://dx.doi.org/10.1136/bmjopen-2011-000269.
- Soliman EZ, Safford MM, Muntner P, Khodneva Y, Dawood FZ, Zakai NA, et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med 2014;174:107-14. http://dx.doi.org/10.1001/jamainternmed.2013.11912.
- Smolina K, Wright FL, Rayner M, Goldacre MJ. Incidence and 30-day case fatality for acute myocardial infarction in England in 2010: national-linked database study. Eur J Public Health 2012;22:848-53. http://dx.doi.org/10.1093/eurpub/ckr196.
- Oger E. Incidence of venous thromboembolism: a community-based study in Western France. EPI-GETBP Study Group. Groupe d’Etude de la Thrombose de Bretagne Occidentale. Thromb Haemost 2000;83:657-60.
- Venous Thromboembolism: Reducing the Risk of Venous Thromboembolism (Deep Vein Thrombosis and Pulmonary Embolism) in Patients Admitted to Hospital. London: Royal College of Physicians; 2010.
- Connock M, Stevens C, Fry-Smith A, Jowett S, Fitzmaurice D, Moore D, et al. Clinical effectiveness and cost-effectiveness of different models of managing long-term oral anticoagulation therapy: a systematic review and economic modelling. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11380.
- Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18820 patients. BMJ 2004;329:15-9. http://dx.doi.org/10.1136/bmj.329.7456.15.
- Clinical Guideline 36. National Cost Impact Report to accompany ‘Atrial Fibrillation: the Management of Atrial Fibrillation’. London: NICE; 2006.
- Garcia D, Libby E, Crowther MA. The new oral anticoagulants. Blood 2010;115:15-20. http://dx.doi.org/10.1182/blood-2009-09-241851.
- Reilly PA, Lehr T, Haertter S, Connolly SJ, Yusuf S, Eikelboom JW, et al. The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulation Therapy). J Am Coll Cardiol 2014;63:321-8. http://dx.doi.org/10.1016/j.jacc.2013.07.104.
- Systematic Reviews. CRD’s Guidance for Undertaking Reviews in Health Care. York: CRD, University of York; 2009.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: Wiley; 2008.
- Assiri A, Al-Majzoub O, Kanaan AO, Donovan JL, Silva M. Mixed treatment comparison meta-analysis of aspirin, warfarin, and new anticoagulants for stroke prevention in patients with nonvalvular atrial fibrillation. Clin Ther 2013;35:967-84.e2. http://dx.doi.org/10.1016/j.clinthera.2013.05.011.
- Castellucci LA, Cameron C, Le Gal G, Rodger MA, Coyle D, Wells PS, et al. Efficacy and safety outcomes of oral anticoagulants and antiplatelet drugs in the secondary prevention of venous thromboembolism: systematic review and network meta-analysis. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f5133.
- Fox BD, Kahn SR, Langleben D, Eisenberg MJ, Shimony A. Efficacy and safety of novel oral anticoagulants for treatment of acute venous thromboembolism: direct and adjusted indirect meta-analysis of randomised controlled trials. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e7498.
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d5928.
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105-24. http://dx.doi.org/10.1002/sim.1875.
- Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897-900. http://dx.doi.org/10.1136/bmj.331.7521.897.
- Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making 2013;33:607-17. http://dx.doi.org/10.1177/0272989X12458724.
- Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A. Bayesian measures of model complexity and fit. J R Statist Soc B 2002;64:583-616. http://dx.doi.org/10.1111/1467-9868.00353.
- Berkey CS, Hoaglin DC, Mosteller F, Colditz GA. A random-effects regression model for meta-analysis. Stat Med 1995;14:395-411. http://dx.doi.org/10.1002/sim.4780140406.
- Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Stat Methods Med Res 2008;17:279-301. http://dx.doi.org/10.1177/0962280207080643.
- Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ 2003;326:472-5. http://dx.doi.org/10.1136/bmj.326.7387.472.
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010;29:932-44. http://dx.doi.org/10.1002/sim.3767.
- Dias S, Welton NJ, Sutton AJ, Ades AE. A Generalised Linear Modelling Framework for Pairwise and Network Meta-analysis of Randomised Controlled Trials. London: NICE; 2011.
- The R Foundation . The R Project for Statistical Computing n.d. www.r-project.org/ (accessed 12 August 2016).
- Lunn DJ, Thomas A, Best N, Spiegelhalter DJ. WinBUGS – a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 2000;10:325-37. http://dx.doi.org/10.1023/A:1008929526011.
- Gage BF, Cardinalli AB, Albers GW, Owens DK. Cost-effectiveness of warfarin and aspirin for prophylaxis of stroke in patients with nonvalvular atrial fibrillation. JAMA 1995;274:1839-45. http://dx.doi.org/10.1001/jama.1995.03530230025025.
- Lightowlers S, McGuire A. Cost-effectiveness of anticoagulation in nonrheumatic atrial fibrillation in the primary prevention of ischemic stroke. Stroke 1998;29:1827-32. http://dx.doi.org/10.1161/01.STR.29.9.1827.
- Single Technology Appraisal (STA) of Rivaroxaban (Xarelto). Newbury: Bayer plc; 2011.
- Shah SV, Gage BF. Cost-effectiveness of dabigatran for stroke prophylaxis in atrial fibrillation. Circulation 2011;123:2562-70. http://dx.doi.org/10.1161/CIRCULATIONAHA.110.985655.
- Freeman JV, Zhu RP, Owens DK, Garber AM, Hutton DW, Go AS, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med 2011;154:1-11. http://dx.doi.org/10.7326/0003-4819-154-1-201101040-00289.
- Lee S, Mullin R, Blazawski J, Coleman CI. Cost-effectiveness of apixaban compared with warfarin for stroke prevention in atrial fibrillation. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0047473.
- Lee S, Anglade MW, Pham D, Pisacane R, Kluger J, Coleman CI. Cost-effectiveness of rivaroxaban compared to warfarin for stroke prevention in atrial fibrillation. Am J Cardiol 2012;110:845-51. http://dx.doi.org/10.1016/j.amjcard.2012.05.011.
- Harrington AR, Armstrong EP, Nolan PE, Malone DC. Cost-effectiveness of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in atrial fibrillation. Stroke 2013;44:1676-81. http://dx.doi.org/10.1161/STROKEAHA.111.000402.
- Kamel H, Easton JD, Johnston SC, Kim AS. Cost-effectiveness of apixaban vs warfarin for secondary stroke prevention in atrial fibrillation. Neurology 2012;79:1428-34. http://dx.doi.org/10.1212/WNL.0b013e31826d5fe8.
- Wells G, Coyle D, Cameron C, Steiner S, Coyle K, Kelly S, et al. Safety, Effectiveness, and Cost-Effectiveness of New Oral Anticoagulants Compared with Warfarin in Preventing Stroke and Other Cardiovascular Events in Patients with Atrial Fibrillation. Ottawa, ON: Canadian Collaborative for Drug Safety, Effectiveness and Network Meta-Analysis; 2012.
- Wisloff T, Tove R, Gunhild H, Asmund R, Marianne K. Efficacy and Cost-effectiveness of New Oral Anticoagulants Compared to Warfarin for the Prevention of Stroke in Patients with Atrial Fibrillation. Oslo: Kunnskapssenteret; 2013.
- Kansal AR, Sorensen SV, Gani R, Robinson P, Pan F, Plumb JM, et al. Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in UK patients with atrial fibrillation. Heart 2012;98:573-8. http://dx.doi.org/10.1136/heartjnl-2011-300646.
- Canestaro WJ, Patrick AR, Avorn J, Ito K, Matlin OS, Brennan TA, et al. Cost-effectiveness of oral anticoagulants for treatment of atrial fibrillation. Circ Cardiovasc Qual Outcomes 2013;6:724-31. http://dx.doi.org/10.1161/CIRCOUTCOMES.113.000661.
- Nshimyumukiza L, Duplantie J, Gagnon M, Douville X, Fournier D, Lindsay C, et al. Dabigatran versus warfarin under standard or pharmacogenetic-guided management for the prevention of stroke and systemic thromboembolism in patients with atrial fibrillation: a cost/utility analysis using an analytic decision model. Thromb J 2013;11. http://dx.doi.org/10.1186/1477-9560-11-14.
- Krejczy M, Harenberg J, Marx S, Obermann K, Frölich L, Wehling M. Comparison of cost-effectiveness of anticoagulation with dabigatran, rivaroxaban and apixaban in patients with non-valvular atrial fibrillation across countries. J Thromb Thrombolysis 2014;37:507-23. http://dx.doi.org/10.1007/s11239-013-0989-6.
- Pink J, Lane S, Pirmohamed M, Hughes DA. Dabigatran etexilate versus warfarin in management of non-valvular atrial fibrillation in UK context: quantitative benefit-harm and economic analyses. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d6333.
- Lip GY, Kongnakorn T, Phatak H, Kuznik A, Lanitis T, Liu LZ, et al. Cost-effectiveness of apixaban versus other new oral anticoagulants for stroke prevention in atrial fibrillation. Clin Ther 2014;36:192-210.e20. http://dx.doi.org/10.1016/j.clinthera.2013.12.011.
- Rognoni C, Marchetti M, Quaglini S, Liberato NL. Apixaban, dabigatran, and rivaroxaban versus warfarin for stroke prevention in non-valvular atrial fibrillation: a cost-effectiveness analysis. Clin Drug Investig 2014;34:9-17. http://dx.doi.org/10.1007/s40261-013-0144-3.
- Limone BL, Baker WL, Mearns ES, White CM, Kluger J, Coleman CI. Common flaws exist in published cost-effectiveness models of pharmacologic stroke prevention in atrial fibrillation. J Clin Epidemiol 2014;67:1093-102. http://dx.doi.org/10.1016/j.jclinepi.2014.05.013.
- Bayer . Rivaroxaban for the Treatment of Deep Vein Thrombosis and Prevention of Recurrent Deep Vein Thrombosis and Pulmonary Embolism 2012.
- Bayer . Rivaroxaban for Treating Pulmonary Embolism and Preventing Recurrent Venous Thromboembolism 2013.
- Boehringer Ingelheim . Dabigatran Etexilate for the Prevention of Venous Thromboembolism After Hip or Knee Replacement Surgery in Adults 2008.
- Botteman MF, Caprini J, Stephens JM, Nadipelli V, Bell CF, Pashos CL, et al. Results of an economic model to assess the cost-effectiveness of enoxaparin, a low-molecular-weight heparin, versus warfarin for the prophylaxis of deep vein thrombosis and associated long-term complications in total hip replacement surgery in the United States. Clin Ther 2002;24:1960-86. http://dx.doi.org/10.1016/S0149-2918(02)80091-1.
- Bayer . Rivaroxaban for the Prevention of Venous Thromboembolism After Total Hip or Total Knee Replacement in Adults 2009.
- Bristol-Myers Squibb . Apixaban for the Prevention of Venous Thromboembolism After Total Hip or Knee Replacement in Adults 2012.
- Dranitsaris G, Stumpo C, Smith R, Bartle W. Extended dalteparin prophylaxis for venous thromboembolic events: cost-utility analysis in patients undergoing major orthopedic surgery. Am J Cardiovasc Drugs 2009;9:45-58. http://dx.doi.org/10.1007/BF03256594.
- Duran A, Sengupta N, Diamantopoulos A, Forster F, Kwong L, Lees M. Cost effectiveness of rivaroxaban versus enoxaparin for prevention of post-surgical venous thromboembolism from a U.S. payer’s perspective. Pharmacoeconomics 2012;30:87-101. http://dx.doi.org/10.2165/11599370-000000000-00000.
- Monreal M, Folkerts K, Diamantopoulos A, Imberti D, Brosa M. Cost-effectiveness impact of rivaroxaban versus new and existing prophylaxis for the prevention of venous thromboembolism after total hip or knee replacement surgery in France, Italy and Spain. Thromb Haemost 2013;110:987-94. http://dx.doi.org/10.1160/TH12-12-0919.
- Mahmoudi M, Sobieraj DM. The cost-effectiveness of oral direct factor Xa inhibitors compared with low-molecular-weight heparin for the prevention of venous thromboembolism prophylaxis in total hip or knee replacement surgery. Pharmacotherapy 2013;33:1333-40. http://dx.doi.org/10.1002/phar.1269.
- McCullagh L, Tilson L, Walsh C, Barry M. A cost-effectiveness model comparing rivaroxaban and dabigatran etexilate with enoxaparin sodium as thromboprophylaxis after total hip and total knee replacement in the Irish healthcare setting. PharmacoEconomics 2009;27:829-46. http://dx.doi.org/10.2165/11313800-000000000-00000.
- McCullagh L, Walsh C, Barry M. Value-of-information analysis to reduce decision uncertainty associated with the choice of thromboprophylaxis after total hip replacement in the Irish healthcare setting. PharmacoEconomics 2012;30:941-59. http://dx.doi.org/10.2165/11591510-000000000-00000.
- Lundkvist J, Bergqvist D, Jönsson B. Cost-effectiveness of extended prophylaxis with fondaparinux compared with low molecular weight heparin against venous thromboembolism in patients undergoing hip fracture surgery. Eur J Health Econ 2007;8:313-23. http://dx.doi.org/10.1007/s10198-006-0017-2.
- Gordois A, Posnett J, Borris L, Bossuyt P, Jönsson B, Levy E, et al. The cost-effectiveness of fondaparinux compared with enoxaparin as prophylaxis against thromboembolism following major orthopedic surgery. J Thromb Haemost 2003;1:2167-74. http://dx.doi.org/10.1046/j.1538-7836.2003.00396.x.
- Pishko AM, Smith KJ, Ragni MV. Anticoagulation in ambulatory cancer patients with no indication for prophylactic or therapeutic anticoagulation: a cost-effectiveness analysis from a U.S. perspective. Thromb Haemost 2012;108:303-10. http://dx.doi.org/10.1160/TH12-03-0185.
- Sullivan SD, Kwong L, Nutescu E. Cost-effectiveness of fondaparinux compared with enoxaparin as prophylaxis against venous thromboembolism in patients undergoing hip fracture surgery. Value Health 2006;9:68-76.
- Zindel S, Stock S, Müller D, Stollenwerk B. A multi-perspective cost-effectiveness analysis comparing rivaroxaban with enoxaparin sodium for thromboprophylaxis after total hip and knee replacement in the German healthcare setting. BMC Health Serv Res 2012;12. http://dx.doi.org/10.1186/1472-6963-12-192.
- Gómez-Outes A, Terleira-Fernández AI, Suárez-Gea ML, Vargas-Castrillón E. Dabigatran, rivaroxaban, or apixaban versus enoxaparin for thromboprophylaxis after total hip or knee replacement: systematic review, meta-analysis, and indirect treatment comparisons. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e3675.
- Atrial Fibrillation: The Management of Atrial Fibrillation. London: NICE; 2006.
- National Joint Registry Editorial Board . 10th Annual Report 2013. www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/10th_annual_report/NJR%2010th%20Annual%20Report%202013%20B.pdf (accessed 4 April 2015).
- National Institute for Health and Care Excellence (NICE) . National Clinical Guideline Centre – Acute and Chronic Conditions. Venous Thromboembolism: Reducing the Risk of Venous Thromboembolism (Deep Vein Thrombosis and Pulmonary Embolism) in Patients Admitted to Hospital. Methods, Evidence and Guidance 2010. www.nice.org.uk/guidance/cg92/evidence/full-guideline-243920125 (accessed 29 September 2016).
- Venous Thromboembolism: Reducing the Risk of Venous Thromboembolism (Deep Vein Thrombosis and Pulmonary Embolism) in Patients Admitted to Hospital. London: NICE; 2010.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. http://dx.doi.org/10.1002/hec.635.
- Strong M BP, Thomas C, Brennan A. Sheffield Accelerated Value of Information 2015.
- Strong M, Oakley JE, Brennan A. Estimating multiparameter partial expected value of perfect information from a probabilistic sensitivity analysis sample: a nonparametric regression approach. Med Decis Making 2014;34:311-26. http://dx.doi.org/10.1177/0272989X13505910.
- Hori M, Connolly SJ, Zhu J, Liu LS, Lau CP, Pais P, et al. Dabigatran versus warfarin: effects on ischemic and hemorrhagic strokes and bleeding in Asians and non-Asians with atrial fibrillation. Stroke 2013;44:1891-6. http://dx.doi.org/10.1161/STROKEAHA.113.000990.
- Naimark DM, Bott M, Krahn M. The half-cycle correction explained: two alternative pedagogical approaches. Med Decis Making 2008;28:706-12. http://dx.doi.org/10.1177/0272989X08315241.
- Ali A, Bailey C, Abdelhafiz AH. Stroke prophylaxis with warfarin or dabigatran for patients with non-valvular atrial fibrillation-cost analysis. Age Ageing 2012;41:681-4. http://dx.doi.org/10.1093/ageing/afs017.
- ONS Consumer Price Inflation Index for Medical Services (DKC3) for 2013/14. London: ONS; 2013.
- NHS Reference Costs 2012–13. London: DH; 2014.
- Ioannides-Demos LL, Makarounas-Kirchmann K, Ashton E, Stoelwinder J, McNeil JJ. Cost of myocardial infarction to the Australian community: a prospective, multicentre survey. Clin Drug Investig 2010;30:533-43. http://dx.doi.org/10.2165/11536350-000000000-00000.
- Luengo-Fernandez R, Yiin GS, Gray AM, Rothwell PM. Population-based study of acute- and long-term care costs after stroke in patients with AF. Int J Stroke 2013;8:308-14. http://dx.doi.org/10.1111/j.1747-4949.2012.00812.x.
- Dabigatran Etexilate for the Prevention of Venous Thromboembolism After Hip or Knee Replacement Surgery in Adults. London: NICE; 2008.
- Caprini JA, Botteman MF, Stephens JM, Nadipelli V, Ewing MM, Brandt S, et al. Economic burden of long-term complications of deep vein thrombosis after total hip replacement surgery in the United States. Value Health 2003;6:59-64. http://dx.doi.org/10.1046/j.1524-4733.2003.00204.x.
- Locadia M, Bossuyt PM, Stalmeier PF, Sprangers MA, van Dongen CJ, Middeldorp S, et al. Treatment of venous thromboembolism with vitamin K antagonists: patients’ health state valuations and treatment preferences. Thromb Haemost 2004;92:1336-41. http://dx.doi.org/10.1160/th04-02-0075.
- Berg J, Lindgren P, Nieuwlaat R, Bouin O, Crijns H. Factors determining utility measured with the EQ-5D in patients with atrial fibrillation. Qual Life Res 2010;19:381-90. http://dx.doi.org/10.1007/s11136-010-9591-y.
- Robinson A, Thomson R, Parkin D, Sudlow M, Eccles M. How patients with atrial fibrillation value different health outcomes: a standard gamble study. J Health Serv Res Policy 2001;6:92-8. http://dx.doi.org/10.1258/1355819011927288.
- Lenert L, Soetikno RM. Automated computer interviews to elicit utilities: potential applications in the treatment of deep venous thrombosis. JAMA 1997;4:49-56. http://dx.doi.org/10.1136/jamia.1997.0040049.
- Lacey EA, Walters SJ. Continuing inequality: gender and social class influences on self perceived health after a heart attack. J Epidemiol Community Health 2003;57:622-7. http://dx.doi.org/10.1136/jech.57.8.622.
- Haacke C, Althaus A, Spottke A, Siebert U, Back T, Dodel R. Long-term outcome after stroke: evaluating health-related quality of life using utility measurements. Stroke 2006;37:193-8. http://dx.doi.org/10.1161/01.STR.0000196990.69412.fb.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York: Centre for Health Economics, University of York; 1999.
- Meads DM, McKenna SP, Doughty N, Das C, Gin-Sing W, Langley J, et al. The responsiveness and validity of the CAMPHOR Utility Index. Eur Respir J 2008;32:1513-19. http://dx.doi.org/10.1183/09031936.00069708.
- McKenna SP, Ratcliffe J, Meads DM, Brazier JE. Development and validation of a preference based measure derived from the Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR) for use in cost utility analyses. Health Qual Life Outcomes 2008;6. http://dx.doi.org/10.1186/1477-7525-6-65.
- Petersen P, Boysen G, Godtfredsen J, Andersen ED, Andersen B. Placebo-controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. The Copenhagen AFASAK study. Lancet 1989;1:175-9. http://dx.doi.org/10.1016/S0140-6736(89)91200-2.
- Stroke Prevention in Atrial Fibrillation Investigators . Warfarin versus aspirin for prevention of thromboembolism in atrial fibrillation: Stroke Prevention in Atrial Fibrillation II Study. Lancet 1994;343:687-91.
- Gulløv AL, Koefoed BG, Petersen P, Pedersen TS, Andersen ED, Godtfredsen J, et al. Fixed minidose warfarin and aspirin alone and in combination vs adjusted-dose warfarin for stroke prevention in atrial fibrillation: Second Copenhagen Atrial Fibrillation, Aspirin, and Anticoagulation Study. Arch Intern Med 1998;158:1513-21. http://dx.doi.org/10.1001/archinte.158.14.1513.
- Hellemons BS, Langenberg M, Lodder J, Vermeer F, Schouten HJ, Lemmens T, et al. Primary prevention of arterial thromboembolism in non-rheumatic atrial fibrillation in primary care: randomised controlled trial comparing two intensities of coumarin with aspirin. BMJ 1999;319:958-64. http://dx.doi.org/10.1136/bmj.319.7215.958.
- Hu DY, Zhang HP, Sun YH, Jiang LQ. Antithrombotic Therapy in Atrial Fibrillation Study Group . The randomized study of efficiency and safety of antithrombotic therapy in nonvalvular atrial fibrillation: warfarin compared with aspirin. Zhonghua Xin Xue Guan Bing Za Zhi 2006;34:295-8.
- Connolly S, Pogue J, Hart R, Pfeffer M, Hohnloser S, Chrolavicius S, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 2006;367:1903-12. http://dx.doi.org/10.1016/S0140-6736(06)68845-4.
- Rash A, Downes T, Portner R, Yeo WW, Morgan N, Channer KS. A randomised controlled trial of warfarin versus aspirin for stroke prevention in octogenarians with atrial fibrillation (WASPO). Age Ageing 2007;36:151-6. http://dx.doi.org/10.1093/ageing/afl129.
- Ezekowitz MD, Reilly PA, Nehmiz G, Simmers TA, Nagarakanti R, Parcham-Azad K, et al. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO Study). Am J Cardiol 2007;100:1419-26. http://dx.doi.org/10.1016/j.amjcard.2007.06.034.
- Mant J, Hobbs FD, Fletcher K, Roalfe A, Fitzmauricie D, Lip GY, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007;370:493-50. http://dx.doi.org/10.1016/S0140-6736(07)61233-1.
- Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51. http://dx.doi.org/10.1056/NEJMoa0905561.
- Eikelboom JW, O’Donnell M, Yusuf S, Diaz R, Flaker G, Hart R, et al. Rationale and design of AVERROES: apixaban versus acetylsalicylic acid to prevent stroke in atrial fibrillation patients who have failed or are unsuitable for vitamin K antagonist treatment. Am Heart J 2010;159:348-53.e1. http://dx.doi.org/10.1016/j.ahj.2009.08.026.
- ROCKET AF. Study Investigators . Rivaroxaban-once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation: Rationale and Design of the ROCKET AF study. Am Heart J 2010;159:340-7.e1. http://dx.doi.org/10.1016/j.ahj.2009.11.025.
- Lopes RD, Alexander JH, Al-Khatib SM, Ansell J, Diaz R, Easton JD, et al. Apixaban for reduction in stroke and other Thromboembolic events in atrial fibrillation (ARISTOTLE) trial: design and rationale. Am Heart J 2010;159:331-9. http://dx.doi.org/10.1016/j.ahj.2009.07.035.
- Weitz JI, Connolly SJ, Patel I, Salazar D, Rohatagi S, Mendell J, et al. Randomised, parallel-group, multicentre, multinational phase 2 study comparing edoxaban, an oral factor Xa inhibitor, with warfarin for stroke prevention in patients with atrial fibrillation. Thromb Haemost 2010;104:633-41. http://dx.doi.org/10.1160/TH10-01-0066.
- Connolly SJ, Ezekowitz MD, Yusuf S, Reilly PA, Wallentin L. Randomized Evaluation of Long-Term Anticoagulation Therapy Investigators . Newly identified events in the RE-LY trial. N Engl J Med 2010;363:1875-6. http://dx.doi.org/10.1056/NEJMc1007378.
- Yamaguchi T. A Dose Response Study of Dabigatran Etexilate (BIBR 1048) in Pharmacodynamics and Safety in Patients With Non-Valvular Atrial Fibrillation in Comparison to Warfarin 2010.
- Ruff CT, Giugliano RP, Antman EM, Crugnale SE, Bocanegra T, Mercuri M, et al. Evaluation of the novel factor Xa inhibitor edoxaban compared with warfarin in patients with atrial fibrillation: design and rationale for the Effective aNticoaGulation with factor xA next GEneration in Atrial Fibrillation-Thrombolysis In Myocardial Infarction study 48 (ENGAGE AF-TIMI 48). Am Heart J 2010;160:635-41. http://dx.doi.org/10.1016/j.ahj.2010.06.042.
- Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883-91. http://dx.doi.org/10.1056/NEJMoa1009638.
- Ogawa S, Shinohara Y, Kanmuri K. Safety and efficacy of the oral direct factor Xa inhibitor apixaban in Japanese patients with non-valvular atrial fibrillation – The ARISTOTLE-J study. Circ J 2011;75:1852-9. http://dx.doi.org/10.1253/circj.CJ-10-1183.
- Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92. http://dx.doi.org/10.1056/NEJMoa1107039.
- Chung N, Jeon HK, Lien LM, Lai WT, Tse HF, Chung WS, et al. Safety of edoxaban, an oral factor Xa inhibitor, in Asian patients with non-valvular atrial fibrillation. Thromb Haemost 2011;105:535-44. http://dx.doi.org/10.1160/TH10-07-0451.
- Hohnloser S, Yusuf S, Eikelboom J, Steg G, Atar D, Budaj A, et al. Apixaban in patients with atrial fibrillation and their risk for cardiovascular hospitalization: insights from the AVERROES trial. Eur Heart J 2011;32.
- Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806-17. http://dx.doi.org/10.1056/NEJMoa1007432.
- Yamashita T, Koretsune Y, Yasaka M, Inoue H, Kawai Y, Yamaguchi T, et al. Randomized, multicenter, warfarin-controlled phase II study of edoxaban in Japanese patients with non-valvular atrial fibrillation. Circ J 2012;76:1840-7. http://dx.doi.org/10.1253/circj.CJ-11-1140.
- Hohnloser SH, Hijazi Z, Thomas L, Alexander JH, Amerena J, Hanna M, et al. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J 2012;33:2821-30. http://dx.doi.org/10.1093/eurheartj/ehs274.
- Hori M, Matsumoto M, Tanahashi N, Momomura S, Uchiyama S, Goto S, et al. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation – the J-ROCKET AF study –. Circ J 2012;76:2104-11. http://dx.doi.org/10.1253/circj.CJ-12-0454.
- Diener HC, Eikelboom J, Connolly SJ, Joyner CD, Hart RG, Lip GY, et al. Apixaban versus aspirin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a predefined subgroup analysis from AVERROES, a randomised trial. Lancet Neurol 2012;11:225-31. http://dx.doi.org/10.1016/S1474-4422(12)70017-0.
- Chen KP, Huang CX, Huang DJ, Cao KJ, Ma CS, Wang FZ, et al. Anticoagulation therapy in Chinese patients with non-valvular atrial fibrillation: a prospective, multi-center, randomized, controlled study. Chin Med J 2012;125:4355-60.
- Hankey GJ, Patel MR, Stevens SR, Becker RC, Breithardt G, Carolei A, et al. Rivaroxaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of ROCKET AF. Lancet Neurol 2012;11:315-22. http://dx.doi.org/10.1016/S1474-4422(12)70042-X.
- Easton JD, Lopes RD, Bahit MC, Wojdyla DM, Granger CB, Wallentin L, et al. Apixaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of the ARISTOTLE trial. Lancet Neurol 2012;11:503-11. http://dx.doi.org/10.1016/S1474-4422(12)70092-3.
- Flaker GC, Hohnloser S, Wojdyla D, Hylek E, Garcia D, Sullivan R, et al. Apixaban is efficacious and safe in patients with atrial fibrillation using concomitant amiodarone: an analysis from the ARISTOTLE trial. J Am Coll Cardio 2013;1. http://dx.doi.org/10.1016/S0735-1097(13)60317-4.
- Al-Khatib SM, Thomas L, Wallentin L, Lopes RD, Gersh B, Garcia D, et al. Outcomes of apixaban vs. warfarin by type and duration of atrial fibrillation: results from the ARISTOTLE trial. Eur Heart J 2013;34:2464-71. http://dx.doi.org/10.1093/eurheartj/eht135.
- Bahit MC, Lopes RD, Wojdyla DM, Hohnloser SH, Alexander JH, Lewis BS, et al. Apixaban in patients with atrial fibrillation and prior coronary artery disease: insights from the ARISTOTLE trial. Int J Cardiol 2013;170:215-20. http://dx.doi.org/10.1016/j.ijcard.2013.10.062.
- Connolly SJ, Eikelboom J, Dorian P, Hohnloser SH, Gretler DD, Sinha U, et al. Betrixaban compared with warfarin in patients with atrial fibrillation: results of a phase 2, randomized, dose-ranging study (Explore-Xa). Eur Heart J 2013;34:1498-505. http://dx.doi.org/10.1093/eurheartj/eht039.
- Mahaffey KW, Wojdyla D, Hankey GJ, White HD, Nessel CC, Piccini JP, et al. Clinical outcomes with rivaroxaban in patients transitioned from vitamin K antagonist therapy: a subgroup analysis of a randomized trial. Ann Intern Med 2013;158:861-8. http://dx.doi.org/10.7326/0003-4819-158-12-201306180-00003.
- Garcia DA, Wallentin L, Lopes RD, Thomas L, Alexander JH, Hylek EM, et al. Apixaban versus warfarin in patients with atrial fibrillation according to prior warfarin use: results from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation trial. Am Heart J 2013;166:549-58. http://dx.doi.org/10.1016/j.ahj.2013.05.016.
- Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093-104. http://dx.doi.org/10.1056/NEJMoa1310907.
- McMurray JJ, Ezekowitz JA, Lewis BS, Gersh BJ, van Diepen S, Amerena J, et al. Left ventricular systolic dysfunction, heart failure, and the risk of stroke and systemic embolism in patients with atrial fibrillation: insights from the ARISTOTLE trial. Circ Heart Fail 2013;6:451-60. http://dx.doi.org/10.1161/CIRCHEARTFAILURE.112.000143.
- Alexander JH, Lopes RD, Thomas L, Alings M, Atar D, Aylward P, et al. Apixaban vs. warfarin with concomitant aspirin in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J 2014;35:224-32. http://dx.doi.org/10.1093/eurheartj/eht445.
- Hylek EM, Held C, Alexander JH, Lopes RD, De Caterina R, Wojdyla DM, et al. Major bleeding in patients with atrial fibrillation receiving apixaban or warfarin: The ARISTOTLE Trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation): Predictors, Characteristics, and Clinical Outcomes. J Am Coll Cardiol 2014;63:2141-7. http://dx.doi.org/10.1016/j.jacc.2014.02.549.
- Liu X, Huang H, Yu J, Cao G, Feng L, Xu Q, et al. Warfarin compared with aspirin for older Chinese patients with stable coronary heart diseases and atrial fibrillation complications. Int J Clin Pharmacol Ther 2014;52:454-9. http://dx.doi.org/10.5414/CP201996.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857-67. http://dx.doi.org/10.7326/0003-4819-146-12-200706190-00007.
- Ezekowitz MD, Bridgers SL, James KE, Carliner NH, Colling CL, Gornick CC, et al. Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation. Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation Investigators. N Engl J Med 1992;327:1406-12. http://dx.doi.org/10.1056/NEJM199211123272002.
- Cairns JA. Stroke prevention in atrial fibrillation study. Final results. Circulation 1991;84:527-39. http://dx.doi.org/10.1111/j.1524-4733.2006.00085.x.
- The Boston Area Anticoagulation Trial for Atrial Fibrillation Investigators . The effect of low-dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. N Engl J Med 1990;323:1505-11. http://dx.doi.org/10.1056/NEJM199011293232201.
- Connolly SJ, Laupacis A, Gent M, Roberts RS, Cairns JA, Joyner C. Canadian Atrial Fibrillation Anticoagulation (CAFA) Study. J Am Coll Cardiol 1991;18:349-55. http://dx.doi.org/10.1016/0735-1097(91)90585-W.
- EAFT (European Atrial Fibrillation Trial) Study Group . Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. Lancet 1993;342:1255-62.
- Chimowitz MI, Furlan AJ. Prevention of stroke in atrial fibrillation. N Engl J Med 1990;323:481-4. http://dx.doi.org/10.1056/NEJM199008163230712.
- Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J 2012;33:1500-10. http://dx.doi.org/10.1093/eurheartj/ehr488.
- Andersen KK, Olsen TS. Reduced poststroke mortality in patients with stroke and atrial fibrillation treated with anticoagulants: results from a Danish quality-control registry of 22,179 patients with ischemic stroke. Stroke 2007;38:259-63. http://dx.doi.org/10.1161/01.STR.0000254622.52483.03.
- Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, Minhas R, Sheikh A, et al. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ 2008;336:1475-82. http://dx.doi.org/10.1136/bmj.39609.449676.25.
- Office for National Statistics (ONS) . National Life Tables, United Kingdom: 2011–13. Stat Bull 2014.
- Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J 2006;27:949-53. http://dx.doi.org/10.1093/eurheartj/ehi825.
- 2011 Census, Population and Household Estimates for England and Wales. London: ONS; 2012.
- Kamel H, Johnston SC, Easton JD, Kim AS. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in patients with atrial fibrillation and prior stroke or transient ischemic attack. Stroke 2012;43:881-3. http://dx.doi.org/10.1161/STROKEAHA.111.641027.
- Vanassche T, Lauw MN, Eikelboom JW, Healey JS, Hart RG, Alings M, et al. Risk of ischaemic stroke according to pattern of atrial fibrillation: analysis of 6563 aspirin-treated patients in ACTIVE-A and AVERROES. Eur Heart J 2015;36:281-7a. http://dx.doi.org/10.1093/eurheartj/ehu307.
- Hohnloser SH, Pajitnev D, Pogue J, Healey JS, Pfeffer MA, Yusuf S, et al. ACTIVE W Investigators . Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: an ACTIVE W Substudy. J Am Coll Cardiol 2007;50:2156-61. http://dx.doi.org/10.1016/j.jacc.2007.07.076.
- Leclerc JR, Geerts WH, Desjardins L, Laflamme GH, L’Espérance B, Demers C, et al. Prevention of venous thromboembolism after knee arthroplasty. A randomized, double-blind trial comparing enoxaparin with warfarin. Ann Intern Med 1996;124:619-26. http://dx.doi.org/10.7326/0003-4819-124-7-199604010-00001.
- Francis CW, Pellegrini VD, Totterman S, Boyd AD, Marder VJ, Liebert KM, et al. Prevention of deep-vein thrombosis after total hip arthroplasty. Comparison of warfarin and dalteparin. J Bone Joint Surg Am 1997;79:1365-72.
- Colwell CW, Collis DK, Paulson R, McCutchen JW, Bigler GT, Lutz S, et al. Comparison of enoxaparin and warfarin for the prevention of venous thromboembolic disease after total hip arthroplasty. Evaluation during hospitalization and three months after discharge. J Bone Joint Surg Am 1999;81:932-40.
- Hull RD, Pineo GF, Francis C, Bergqvist D, Fellenius C, Soderberg K, et al. Low-molecular-weight heparin prophylaxis using dalteparin in close proximity to surgery vs warfarin in hip arthroplasty patients: a double-blind, randomized comparison. The North American Fragmin Trial Investigators. Arch Intern Med 2000;160:2199-207. http://dx.doi.org/10.1001/archinte.160.14.2199.
- Fitzgerald RH, Spiro TE, Trowbridge AA, Gardner JA, Whitsett TL, O’Connell MB, et al. Prevention of venous thromboembolic disease following primary total knee arthroplasty. A randomized, multicenter, open-label, parallel-group comparison of enoxaparin and warfarin. J Bone Joint Surg Am 2001;83A:900-6.
- Turpie AG, Fisher WD, Bauer KA, Kwong LM, Irwin MW, Kälebo P, et al. BAY 59–7939: an oral, direct factor Xa inhibitor for the prevention of venous thromboembolism in patients after total knee replacement. A phase II dose-ranging study. J Thromb Haemost 2005;3:2479-86. http://dx.doi.org/10.1111/j.1538-7836.2005.01602.x.
- Eriksson BI, Borris LC, Dahl OE, Haas S, Huisman MV, Kakkar AK, et al. A once-daily, oral, direct Factor Xa inhibitor, rivaroxaban (BAY 59–7939), for thromboprophylaxis after total hip replacement. Circulation 2006;114:2374-81. http://dx.doi.org/10.1161/CIRCULATIONAHA.106.642074.
- Eriksson BI, Borris L, Dahl OE, Haas S, Huisman MV, Kakkar AK, et al. Oral, direct Factor Xa inhibition with BAY 59–7939 for the prevention of venous thromboembolism after total hip replacement. J Thromb Haemost 2006;4:121-8. http://dx.doi.org/10.1111/j.1538-7836.2005.01657.x.
- Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007;370:949-56. http://dx.doi.org/10.1016/S0140-6736(07)61445-7.
- Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007;5:2178-85. http://dx.doi.org/10.1111/j.1538-7836.2007.02748.x.
- Lassen MR, Davidson BL, Gallus A, Pineo G, Ansell J, Deitchman D. The efficacy and safety of apixaban, an oral, direct factor Xa inhibitor, as thromboprophylaxis in patients following total knee replacement. J Thromb Haemost 2007;5:2368-75. http://dx.doi.org/10.1111/j.1538-7836.2007.02764.x.
- Lassen MR, Ageno W, Borris LC, Lieberman JR, Rosencher N, Bandel TJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008;358:2776-86. http://dx.doi.org/10.1056/NEJMoa076016.
- Kanan PS, Schwartsmann CR, Boschin LC, Conrad S, Silva MF. Comparative study between rivaroxaban and enoxaparin in deep venous thromboembolism prophylaxis in patients submitted to total hip arthroplasty. Rev Bras Ortop 2008;43:319-28. http://dx.doi.org/10.1590/S0102-36162008000800002.
- Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008;358:2765-75. http://dx.doi.org/10.1056/NEJMoa0800374.
- Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Muntz J, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 2008;372:31-9. http://dx.doi.org/10.1016/S0140-6736(08)60880-6.
- Ginsberg JS, Davidson BL, Comp PC, Francis CW, Friedman RJ, Huo MH, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty 2009;24:1-9. http://dx.doi.org/10.1016/j.arth.2008.01.132.
- Turpie AG, Bauer KA, Davidson BL, Fisher WD, Gent M, Huo MH, et al. A randomized evaluation of betrixaban, an oral factor Xa inhibitor, for prevention of thromboembolic events after total knee replacement (EXPERT). Thromb Haemost 2009;101:68-76.
- Goel DP, Buckley R, deVries G, Abelseth G, Ni A, Gray R. Prophylaxis of deep-vein thrombosis in fractures below the knee: a prospective randomised controlled trial. J Bone Joint Surg Br 2009;91:388-94. http://dx.doi.org/10.1302/0301-620X.91B3.20820.
- Agnelli G, Gussoni G, Bianchini C, Verso M, Mandalà M, Cavanna L, et al. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncol 2009;10:943-9. http://dx.doi.org/10.1016/S1470-2045(09)70232-3.
- Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med 2009;361:594-60. http://dx.doi.org/10.1056/NEJMoa0810773.
- Fuji T, Fujita S, Tachibana S, Kawai Y. Randomized, double-blind, multi-dose efficacy, safety and biomarker study of the oral factor Xa inhibitor DU-176b compared with placebo for prevention of venous thromboembolism in patients after total knee arthroplasty [abstract no. 35]. Blood 2009;112.
- Turpie AG, Lassen MR, Davidson BL, Bauer KA, Gent M, Kwong LM, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet 2009;373:1673-80. http://dx.doi.org/10.1016/S0140-6736(09)60734-0.
- Fuji T, Wang CJ, Fujita S, Tachibana S, Kawai Y. Edoxaban in patients undergoing total hip arthroplasty: A phase IIb dose-finding study. Blood 2009;114.
- Fuji T, Fuijita S, Ujihira T, Sato T. Dabigatran etexilate prevents venous thromboembolism after total knee arthroplasty in Japanese patients with a safety profile comparable to placebo. J Arthroplasty 2010;25:1267-74. http://dx.doi.org/10.1016/j.arth.2009.08.010.
- Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM. ADVANCE-3 Investigators . Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med 2010;363:2487-98. http://dx.doi.org/10.1056/NEJMoa1006885.
- Raskob G, Cohen AT, Eriksson BI, Puskas D, Shi M, Bocanegra T, et al. Oral direct factor Xa inhibition with edoxaban for thromboprophylaxis after elective total hip replacement. A randomised double-blind dose-response study. Thromb Haemost 2010;104:642-9. http://dx.doi.org/10.1160/TH10-02-0142.
- Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P. ADVANCE-2 investigators . Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet 2010;375:807-15. http://dx.doi.org/10.1016/S0140-6736(09)62125-5.
- Fuji T, Fujita S, Tachibana S, Kawai Y, Koretsune Y, Yamashita T. Efficacy and safety of edoxaban versus enoxaparin for the prevention of venous thromboembolism following total hip arthroplasty: Stars J-V trial [abstract no. 3320]. Blood 2010;116.
- Fuji T, Fujita S, Tachibana S, Kawai Y. A dose-ranging study evaluating the oral factor Xa inhibitor edoxaban for the prevention of venous thromboembolism in patients undergoing total knee arthroplasty. J Thromb Haemost 2010;8:2458-68. http://dx.doi.org/10.1111/j.1538-7836.2010.04021.x.
- Fujita S, Fuji T, Tachibana S, Nakamura M, Kawai Y. Safety and efficacy of edoxaban in patients undergoing hip fracture surgery. Pathophysiol Haemost Thromb 2010;37.
- Fuji T, Wang CJ, Fujita S, Tachibana S, Kawai Y, Koretsune Y, et al. Edoxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty: the STARS E-3 trial. Pathophysiol Haemost Thromb 2010;37.
- Eriksson B, Dahl OE, Kurth AA, Hantel S, Huo MH, Hermansson K, et al. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty: the RE-NOVATE II randomised trial. Pathophysiol Haemost Thromb 2010;37.
- Cohen AT, Spiro TE, Büller HR, . Rivaroxaban compared with enoxaparin for the prevention of venous thromboembolism in acutely ill medical patients: MAGELLAN study methodology. Blood 2010;116.
- Kakkar AK, Cimminiello C, Goldhaber SZ, Parakh R, Wang C, Bergmann JF. LIFENOX Investigators . Low-molecular-weight heparin and mortality in acutely ill medical patients. N Engl J Med 2011;365:2463-72. http://dx.doi.org/10.1056/NEJMoa1111288.
- Yokote R, Matsubara M, Hirasawa N, Hagio S, Ishii K, Takata C. Is routine chemical thromboprophylaxis after total hip replacement really necessary in a Japanese population?. J Bone Joint Surg Br 2011;93:251-6. http://dx.doi.org/10.1302/0301-620X.93B2.25795.
- Iliopoulos E, Fotiadis E, Kravas A, Vittis F, Nenopoulos A, Ntovas T, et al. Thromboprophylaxis management in total knee arthroplasty. Osteoporos Int 2011;22:S120-1.
- Goldhaber SZ, Leizorovicz A, Kakkar AK, Haas SK, Merli G, Knabb RM, et al. ADOPT Trial Investigators . Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med 2011;365:2167-77. http://dx.doi.org/10.1056/NEJMoa1110899.
- Eriksson BI, Dahl OE, Huo MH, Kurth AA, Hantel S, Hermansson K, et al. Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*). A randomised, double-blind, non-inferiority trial. Thromb Haemost 2011;105:721-9. http://dx.doi.org/10.1160/TH10-10-0679.
- Levine MN, Gu C, Liebman HA, Escalante CP, Solymoss S, Deitchman D, et al. A randomized phase II trial of apixaban for the prevention of thromboembolism in patients with metastatic cancer. J Thromb Haemost 2012;10:807-14. http://dx.doi.org/10.1111/j.1538-7836.2012.04693.x.
- Cohen AT, Spiro TE, Büller HR, Haskell L, Hu D, Hull R, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 2013;368:513-23. http://dx.doi.org/10.1056/NEJMoa1111096.
- Zhang H, Wang D, Sun HY, Li SW, Liu L. Efficacy and safety of rivaroxaban in the prevention of deep vein thrombosis after hip arthroplasty. Chin J Tissue Eng Res 2013;17:5440-5.
- Fuji T, Fujita S, Kawai Y, Nakamura M, Kimura T, Kiuchi Y, et al. Safety and efficacy of edoxaban in patients undergoing hip fracture surgery. Thromb Res 2014;133:1016-22. http://dx.doi.org/10.1016/j.thromres.2014.03.009.
- Eriksson BI, Borris LC, Dahl OE, Haas S, Huisman MV, Kakkar AK, et al. Dose-escalation study of rivaroxaban (BAY 59–7939) – an oral, direct Factor Xa inhibitor – for the prevention of venous thromboembolism in patients undergoing total hip replacement. Thromb Res 2007;120:685-93. http://dx.doi.org/10.1016/j.thromres.2006.12.025.
- Eriksson BI, Dahl OE, Büller HR, Hettiarachchi R, Rosencher N, Bravo ML, et al. A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: the BISTRO II randomized trial. J Thromb Haemost 2005;3:103-11. http://dx.doi.org/10.1111/j.1538-7836.2004.01100.x.
- Heit JA, Berkowitz SD, Bona R, Cabanas V, Corson JD, Elliott CG, et al. Efficacy and safety of low molecular weight heparin (ardeparin sodium) compared to warfarin for the prevention of venous thromboembolism after total knee replacement surgery: a double-blind, dose-ranging study. Ardeparin Arthroplasty Study Group. Thromb Haemost 1997;77:32-8.
- Haas SK, Freund M, Heigener D, Heilmann L, Kemkes-Matthes B, von Tempelhoff GF, et al. Low-molecular-weight heparin versus placebo for the prevention of venous thromboembolism in metastatic breast cancer or stage III/IV lung cancer. Clin Appl Thromb Hemost 2012;18:159-65. http://dx.doi.org/10.1177/1076029611433769.
- Moise AI, Dragulin E, Casabalian D, Guran C, Mincu N, Stelea G. Dabigatran etexilate Is effective and safe in patients undergoing total hip arthoplasty. Eur J Anaesthesiol 2011;28. http://dx.doi.org/10.1097/00003643-201106001-00277.
- Camporese G, Bernardi E, Noventa F. Efficacy of rivaroxaban for prevention of venous thromboembolism after knee arthroscopy: a randomized double-blind trial (Erika study). J Thromb Haemost 2011;9:156-7.
- van der Veen L, van Raay JJ, Gerritsma-Bleeker CL, Veeger NJ, van Hulst M. Direct treatment comparison of DAbigatran and RIvaroxaban versus NAdroparin in the prevention of venous thromboembolism after total knee arthroplasty surgery: design of a randomised pilot study (DARINA). BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2012-002218.
- Cohen AT, Harrington R, Goldhaber SZ, Hull R, Gibson CM, Hernandez AF, et al. The design and rationale for the Acute Medically Ill Venous Thromboembolism Prevention with Extended Duration Betrixaban (APEX) study. Am Heart J 2014;167:335-41. http://dx.doi.org/10.1016/j.ahj.2013.11.006.
- Agnelli G, Gallus A, Goldhaber SZ, Haas S, Huisman MV, Hull RD, et al. Treatment of proximal deep-vein thrombosis with the oral direct factor Xa inhibitor rivaroxaban (BAY 59–7939): the ODIXa-DVT (Oral Direct Factor Xa Inhibitor BAY 59–7939 in Patients With Acute Symptomatic Deep-Vein Thrombosis) study. Circulation 2007;116:180-7. http://dx.doi.org/10.1161/CIRCULATIONAHA.106.668020.
- Büller HR, Lensing AW, Prins MH, Agnelli G, Cohen A, Gallus AS, et al. A dose-ranging study evaluating once-daily oral administration of the factor Xa inhibitor rivaroxaban in the treatment of patients with acute symptomatic deep vein thrombosis: the Einstein-DVT Dose-Ranging Study. Blood 2008;112:2242-7. http://dx.doi.org/10.1182/blood-2008-05-160143.
- Büller H, Deitchman D, Prins M, Segers A. Writing Committee . Efficacy and safety of the oral direct factor Xa inhibitor apixaban for symptomatic deep vein thrombosis. The Botticelli DVT dose-ranging study. J Thromb Haemost 2008;6:1313-18. http://dx.doi.org/10.1111/j.1538-7836.2008.03054.x.
- Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;361:2342-52. http://dx.doi.org/10.1056/NEJMoa0906598.
- Bauersachs R, Berkowitz SD, Brenner B, Büller HR, Decousus H, Gallus AS, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499-510. http://dx.doi.org/10.1056/NEJMoa1007903.
- Büller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar E, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287-97. http://dx.doi.org/10.1056/NEJMoa1113572.
- Raskob GE, Büller H, Angchaisuksiri P, Oh Boda Z, Lyons RM, . Edoxaban for long-term treatment of venous thromboembolism in cancer patients. Blood 2013;122.
- Büller HR, Décousus H, Grosso MA, Mercuri M, Middeldorp S, Prins MH, et al. Hokusai-VTE Investigators . Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013;369:1406-15. http://dx.doi.org/10.1056/NEJMoa1306638.
- Agnelli G, Büller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013;369:799-808. http://dx.doi.org/10.1056/NEJMoa1302507.
- Schulman S, Kakkar AK, Goldhaber SZ, Schellong S, Eriksson H, Mismetti P, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation 2014;129:764-72. http://dx.doi.org/10.1161/CIRCULATIONAHA.113.004450.
- Young A, Dunn J, Chapman O, Grumett J, Marshall A, Phillips A, et al. SELECT-D: Anticoagulation therapy in selected cancer patients at risk of recurrence of venous thromboembolism. J Clin Oncol 2014;32.
- Kearon C, Gent M, Hirsh J, Weitz J, Kovacs MJ, Anderson DR, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999;340:901-7. http://dx.doi.org/10.1056/NEJM199903253401201.
- Agnelli G, Prandoni P, Santamaria MG, Bagatella P, Iorio A, Bazzan M, et al. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin Optimal Duration Italian Trial Investigators. N Engl J Med 2001;345:165-9. http://dx.doi.org/10.1056/NEJM200107193450302.
- Agnelli G, Prandoni P, Becattini C, Silingardi M, Taliani MR, Miccio M, et al. Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann Intern Med 2003;139:19-25. http://dx.doi.org/10.7326/0003-4819-139-1-200307010-00008.
- Ridker PM, Goldhaber SZ, Danielson E, Rosenberg Y, Eby CS, Deitcher SR, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med 2003;348:1425-34. http://dx.doi.org/10.1056/NEJMoa035029.
- Büller HR. Once-daily oral rivaroxaban versus placebo in the long-term prevention of recurrent symptomatic venous thromboembolism. The EINSTEIN-extension study. Blood 2009;114.
- Romualdi E, Donadini MP, Ageno W. Oral rivaroxaban after symptomatic venous thromboembolism: the continued treatment study (EINSTEIN-extension study). Expert Rev Cardiovasc Ther 2011;9:841-4. http://dx.doi.org/10.1586/erc.11.62.
- Becattini C, Agnelli G, Schenone A, Eichinger S, Bucherini E, Silingardi M, et al. Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med 2012;366:1959-67. http://dx.doi.org/10.1056/NEJMoa1114238.
- Brighton TA, Eikelboom JW, Mann K, Mister R, Gallus A, Ockelford P, et al. Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med 2012;367:1979-87. http://dx.doi.org/10.1056/NEJMoa1210384.
- Agnelli G, Büller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013;368:699-708. http://dx.doi.org/10.1056/NEJMoa1207541.
- Schulman S, Kearon C, Kakkar AK, Schellong S, Eriksson H, Baanstra D, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013;368:709-18. http://dx.doi.org/10.1056/NEJMoa1113697.
- Tullett J, Murray E, Nichols L, Holder R, Lester W, Rose P, et al. Trial Protocol: a randomised controlled trial of extended anticoagulation treatment versus routine anticoagulation treatment for the prevention of recurrent VTE and post thrombotic syndrome in patients being treated for a first episode of unprovoked VTE (The ExACT Study). BMC Cardiovasc Disord 2013;13. http://dx.doi.org/10.1186/1471-2261-13-16.
- Mazilu L, Parepa IR, Suceveanu AI, Suceveanu A, Baz R, Catrinoiu D. Venous thromboembolism: secondary prevention with dabigatran vs. acenocumarolin patients with paraneoplastic deep vein thrombosis. Results from a small prospective study in Romania. Cardiovasc Res 2014;103. http://dx.doi.org/10.1093/cvr/cvu082.154.
- White HD. Editorial: do we need another bleeding definition? What does the Bleeding Academic Research Consortium definition have to offer?. Curr Opin Cardiol 2011;26:275-8. http://dx.doi.org/10.1097/HCO.0b013e32834706a9.
- Hart RG, Diener HC, Yang S, Connolly SJ, Wallentin L, Reilly PA, et al. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran: the RE-LY trial. Stroke 2012;43:1511-17. http://dx.doi.org/10.1161/STROKEAHA.112.650614.
- Prandoni P, Villalta S, Bagatella P, Rossi L, Marchiori A, Piccioli A, et al. The clinical course of deep-vein thrombosis. Prospective long-term follow-up of 528 symptomatic patients. Haematologica 1997;82:423-8.
- Prandoni P, Noventa F, Ghirarduzzi A, Pengo V, Bernardi E, Pesavento R, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica 2007;92:199-205. http://dx.doi.org/10.3324/haematol.10516.
- Pengo V, Lensing AW, Prins MH, Marchiori A, Davidson BL, Tiozzo F, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004;350:2257-64. http://dx.doi.org/10.1056/NEJMoa032274.
- Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr 1974;19:716-23. http://dx.doi.org/10.1109/TAC.1974.1100705.
- Fenwick E, Byford S. A guide to cost-effectiveness acceptability curves. Br J Psychiatry 2005;187:106-8. http://dx.doi.org/10.1192/bjp.187.2.106.
- Hamidi V, Ringerike T, Hagen G, Reikvam A, Klemp M. New anticoagulants as thromboprophylaxis after total hip or knee replacement. Int J Technol Assess Health Care 2013;29:234-43. http://dx.doi.org/10.1017/S0266462313000251.
- Lefebvre P, Coleman CI, Bookhart BK, Wang ST, Mody SH, Tran KN, et al. Cost-effectiveness of rivaroxaban compared with enoxaparin plus a vitamin K antagonist for the treatment of venous thromboembolism. J Med Econ 2014;17:52-64. http://dx.doi.org/10.3111/13696998.2013.858634.
- Liew A, Eikelboom JW, O’Donnell M, Hart RG. Assessment of anticoagulation intensity and management of bleeding with old and new oral anticoagulants. Can J Cardiol 2013;29:34-4. http://dx.doi.org/10.1016/j.cjca.2013.04.013.
- Yang E. A clinician’s perspective: novel oral anticoagulants to reduce the risk of stroke in nonvalvular atrial fibrillation – full speed ahead or proceed with caution?. Vasc Health Risk Manag 2014;10:507-22. http://dx.doi.org/10.2147/VHRM.S68117.
- Fenger-Eriksen C, Münster AM, Grove EL. New oral anticoagulants: clinical indications, monitoring and treatment of acute bleeding complications. Acta Anaesthesiol Scand 2014;58:651-9. http://dx.doi.org/10.1111/aas.12319.
- UKMi New Drugs Online Database . New Drugs Online Report for Ciraparantag n.d. www.ukmi.nhs.uk/applications/ndo/record_view_open.asp?newDrugID=6443 (accessed 18 May 2015).
- Ansell JE, Bakhru SH, Laulicht BE, Steiner SS, Grosso M, Brown K, et al. Use of PER977 to reverse the anticoagulant effect of edoxaban. N Engl J Med 2014;371:2141-2. http://dx.doi.org/10.1056/NEJMc1411800.
- Lu G, DeGuzman FR, Hollenbach SJ, Karbarz MJ, Abe K, Lee G, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med 2013;19:446-51. http://dx.doi.org/10.1038/nm.3102.
- UKMi New Drugs Online Database . New Drugs Online Report for Andexanet Alfa n.d. www.ukmi.nhs.uk/applications/ndo/record_view_open.asp?newDrugID=6169 (accessed 18 May 2015).
- Schiele F, van Ryn J, Canada K, Newsome C, Sepulveda E, Park J, et al. A specific antidote for dabigatran: functional and structural characterization. Blood 2013;121:3554-62. http://dx.doi.org/10.1182/blood-2012-11-468207.
- UKMi New Drugs Online Database . New Drugs Online Report for Idarucizumab n.d. www.ukmi.nhs.uk/applications/ndo/record_view_open.asp?newDrugID=6275 (accessed 18 May 2015).
- Framingham Heart Study n.d. www.framinghamstudy.org (accessed 22 August 2016).
- Sorensen SV, Dewilde S, Singer DE, Goldhaber SZ, Monz BU, Plumb JM. Cost-effectiveness of warfarin: trial versus “real-world” stroke prevention in atrial fibrillation. Am Heart J 2009;157:1064-73. http://dx.doi.org/10.1016/j.ahj.2009.03.022.
- Roskell NS, Lip GY, Noack H, Clemens A, Plumb JM. Treatments for stroke prevention in atrial fibrillation: a network meta-analysis and indirect comparisons versus dabigatran etexilate. Thromb Haemost 2010;104:1106-15. http://dx.doi.org/10.1160/TH10-10-0642.
Appendix 1 MEDLINE search strategy
Venous thromboembolism
Database: MEDLINE 1950 to present (search date: 20 March 2014).
Search strategy
-
exp Venous Thrombosis/ (43,921)
-
exp Pulmonary Embolism/ (30,904)
-
thromboembolism/ or venous thromboembolism/ (24,405)
-
((venous or vein$) adj3 (thrombus$ or thrombo$)).ti,ab. (45,827)
-
(DVT or VTE).ti,ab. (9537)
-
(thrombophlebitis or thromboprophylaxis or thrombo-prophylaxis or thrombophlebitides).ti,ab. (7132)
-
((pulmonary or lung or lungs) adj3 embol$).ti,ab. (27,169)
-
((leg or legs) adj3 (embol$ or thrombo$ or thrombus$)).ti,ab. (1141)
-
or/1-8 (111,695)
-
exp *Anticoagulants/ (94,334)
-
exp *Coumarins/ (24,282)
-
Warfarin/ (14,323)
-
exp Vitamin K/ai [Antagonists & Inhibitors] (1537)
-
Thrombin/ai [Antagonists & Inhibitors] (3372)
-
Factor Xa/ai [Antagonists & Inhibitors] (2203)
-
Aspirin/ (37,741)
-
(anticoagula$ or anti-coagula$).ti. (20,512)
-
(oral anticoagula$ or oral anti-coagula$).ti,ab. (7048)
-
(coumarin$ or coumadin$ or warfarin or marevan or dicoumarol or dicoumarin or dicumarin or dicumarol or acenocoumarol or phenindione or aldocumar).ti,ab. (24,194)
-
(factor Xa adj2 (antagonist$ or inhibitor$)).ti,ab. (1356)
-
(factor 10a adj2 (antagonist$ or inhibitor$)).ti,ab. (2)
-
(factor IIa adj2 (antagonist$ or inhibitor$)).ti,ab. (25)
-
((vitamin K or vitamin-k) adj2 (antagonist$ or inhibitor$)).ti,ab. (1830)
-
(dabigatram or pradaxa or BIBR1048 or Apixaban or Eliquis or BMS-562247-01 or Edoxaban or Lixiana or savaysa or DU-176b or betrixaban or PRT-054021 or PRT0504021 or rivaroxaban or xarelto or BAY-59739 or Erixaban or D0913).ti,ab. (1015)
-
(NOAC or NOACS).ti,ab. (86)
-
(aspirin or acetyl-salicylic acid or acetylsalicylic acid).ti,ab. (40,463)
-
or/10-26 (170,042)
-
*heparin/ or exp heparin, low-molecular-weight/ or heparinoids/ (33,841)
-
(Dalteparin or fragmin$ or enoxaparin or clexane or lovenox or tinzaparin or innohep or bemiparin or badyket or hepadren or hibor or ivor or ivorat or zibor or certoparin or mono-embolex or sandoparin$ or nadroparin$ or fraxiparin$ or parnaparin or fluxum or reviparin or clivarine or lowmorin).ti,ab. (4597)
-
(LMWH$ or heparinoid$ or danaparoid or orgaran).ti,ab. (4469)
-
(low$ molecular adj2 heparin$).ti,ab. (9114)
-
or/28-31 (37,314)
-
27 or 32 (173,069)
-
9 and 33 (22,835)
-
letter/ (803,375)
-
editorial/ (333,336)
-
news/ (151,695)
-
exp historical article/ (318,208)
-
Anecdotes as topic/ (4506)
-
comment/ (528,857)
-
case report/ (1,665,228)
-
(letter or comment$).ti. (84,259)
-
or/35-42 (3,214,189)
-
randomized controlled trial/ or Randomized Controlled Trials as Topic/ or random$.ti,ab. (774,175)
-
43 not 44 (3,185,399)
-
animals/ not humans/ (3,810,079)
-
exp Animals, Laboratory/ (714,848)
-
exp Animal Experimentation/ (6196)
-
exp Models, Animal/ (407,481)
-
exp rodentia/ (2,630,754)
-
(rat or rats or mouse or mice).ti. (1,065,119)
-
or/45-51 (7,547,456)
-
34 not 52 (16,525)
-
meta-analysis/ (45,670)
-
meta-analysis as topic/ (13,522)
-
(meta analy$ or metaanaly$ or metanaly$ or meta regression).ti,ab. (54,350)
-
((systematic$ or evidence$) adj2 (review$ or overview$)).ti,ab. (61,810)
-
(reference list$ or bibliograph$ or hand search$ or manual search$ or relevant journals).ab. (22,477)
-
(search strategy or search criteria or systematic search or study selection or data extraction).ab. (24,122)
-
(search$ adj4 literature).ab. (23,275)
-
(medline or pubmed or cochrane or embase or psychlit or psyclit or psychinfo or cinahl or science citation index or bids or cancerlit).ab. (71,805)
-
cochrane.jw. (9850)
-
((multiple treatment$ or indirect or mixed) adj2 comparison).ti,ab. (813)
-
or/54-63 (169,922)
-
randomized controlled trial.pt. or randomized controlled trial/ or Randomized Controlled Trials as Topic/ (452,316)
-
controlled clinical trial.pt. (87,802)
-
randomi#ed.ab. (318,385)
-
placebo.ab. (143,748)
-
drug therapy.fs. (1,675,613)
-
randomly.ab. (189,528)
-
trial.ab. (275,251)
-
groups.ab. (1,220,973)
-
or/65-72 (3,169,503)
-
clinical trials as topic.sh. (168,638)
-
trial.ti. (114,737)
-
or/65-68,70,74-75 (899,851)
-
64 or 76 (1,015,181)
-
53 and 77 (4596)
-
limit 78 to yr=“2008-Current” (1408)
-
atrial fibrillation.ti. (19,641)
-
*atrial fibrillation/ (25,973)
-
80 or 81 (26,290)
-
79 not 82 (1281)
Atrial fibrillation
Database: MEDLINE In-Process & Other Non-Indexed Citations – Current week, MEDLINE 1950 to present.
Search date: 20 March 2014.
Search strategy
-
tachycardia, supraventricular/ or tachycardia, ectopic atrial/ (5440)
-
atrial fibrillation/ (33,510)
-
((atrial or atrium or auricular) adj3 fibrillat$).ti,ab. (38,980)
-
heart fibrillat$.ti,ab. (42)
-
(supraventricul$ adj3 (arrhythmi$ or tachycardia$)).ti,ab. (7547)
-
((atrial or atrium) adj3 (tachycardia$ or arrhythmi$)).ti,ab. (6888)
-
(atrial adj3 tachyarrhythmi$).ti,ab. (1210)
-
Atrial Flutter/ (4944)
-
((atrial or auricular) adj3 flutter$).ti,ab. (5382)
-
or/1-9 (59,756)
-
exp *Anticoagulants/ (94,278)
-
exp *Coumarins/ (24,265)
-
Warfarin/ (14,307)
-
exp Vitamin K/ai [Antagonists & Inhibitors] (1534)
-
Thrombin/ai [Antagonists & Inhibitors] (3370)
-
Factor Xa/ai [Antagonists & Inhibitors] (2197)
-
Aspirin/ (37,712)
-
(anticoagula$ or anti-coagula$).ti. (21,584)
-
(oral anticoagula$ or oral anti-coagula$).ti,ab. (7768)
-
(coumarin$ or coumadin$ or warfarin or marevan or dicoumarol or dicoumarin or dicumarin or dicumarol or enocoumarol or phenindione or aldocumar).ti,ab. (26,479)
-
(factor Xa adj2 (antagonist$ or inhibitor$)).ti,ab. (1502)
-
(factor 10a adj2 (antagonist$ or inhibitor$)).ti,ab. (2)
-
(factor IIa adj2 (antagonist$ or inhibitor$)).ti,ab. (29)
-
((vitamin K or vitamin-k) adj2 (antagonist$ or inhibitor$)).ti,ab. (2080)
-
(dabigatram or pradaxa or BIBR1048 or Apixaban or Eliquis or BMS-562247-01 or Edoxaban or Lixiana or savaysa or DU-176b or betrixaban or PRT-054021 or PRT0504021 or rivaroxaban or xarelto or BAY-59739 or Erixaban or D0913).ti,ab. (1330)
-
(NOAC or NOACS).ti,ab. (152)
-
(aspirin or acetyl-salicylic acid or acetylsalicylic acid).ti,ab. (42,763)
-
or/11-27 (175,520)
-
10 and 28 (6721)
-
letter/ (829,317)
-
editorial/ (348,841)
-
news/ (159,814)
-
exp historical article/ (318,220)
-
Anecdotes as topic/ (4506)
-
comment/ (572,414)
-
case report/ (1,665,104)
-
(letter or comment$).ti. (94,907)
-
or/30-37 (3,300,100)
-
randomized controlled trial/ or Randomized Controlled Trials as Topic/ or random$.ti,ab. (835,720)
-
38 not 39 (3,270,043)
-
animals/ not humans/ (3,807,926)
-
exp Animals, Laboratory/ (714,413)
-
exp Animal Experimentation/ (6188)
-
exp Models, Animal/ (407,073)
-
exp rodentia/ (2,629,200)
-
(rat or rats or mouse or mice).ti. (1,097,935)
-
or/40-46 (7,662,407)
-
29 not 47 (5201)
-
systematic review/ (0)
-
meta analysis/ (45,623)
-
(meta analy$ or metaanaly$ or metanaly$ or meta regression).ti,ab. (61,909)
-
((systematic$ or evidence$) adj2 (review$ or overview$)).ti,ab. (71,965)
-
(reference list$ or bibliograph$ or hand search$ or manual search$ or relevant journals).ab. (24,936)
-
(search strategy or search criteria or systematic search or study selection or data extraction).ab. (26,492)
-
(search$ adj4 literature).ab. (26,789)
-
(medline or pubmed or cochrane or embase or psychlit or psyclit or psychinfo or cinahl or science citation dex or bids or cancerlit).ab. (82,698)
-
cochrane.jw. (10,337)
-
((multiple treatment$ or indirect or mixed) adj2 comparison).ti,ab. (901)
-
or/49-58 (186,127)
-
randomized controlled trial.pt. or randomized controlled trial/ or Randomized Controlled Trials as Topic/ (452,445)
-
controlled clinical trial.pt. (87,837)
-
randomi#ed.ab. (343,274)
-
placebo.ab. (151,447)
-
drug therapy.fs. (1,674,296)
-
randomly.ab. (208,182)
-
trial.ab. (297,177)
-
groups.ab. (1,328,911)
-
or/60-67 (3,312,451)
-
clinical trials as topic.sh. (168,554)
-
trial.ti. (123,158)
-
or/60-63,65,69-70 (946,554)
-
59 or 71 (1,075,719)
-
48 and 72 (1764)
-
74 limit 73 to yr=“2010-Current” (728)
Appendix 2 Forest plots: stroke prevention in atrial fibrillation
FIGURE 92.
Stroke or SE [1/4] (stroke prevention in AF).
FIGURE 93.
Stroke or SE [2/4] (stroke prevention in AF).
FIGURE 94.
Stroke or SE [3/4] (stroke prevention in AF).

FIGURE 95.
Stroke or SE [4/4] (stroke prevention in AF).
FIGURE 96.
Ischaemic stroke (stroke prevention in AF).
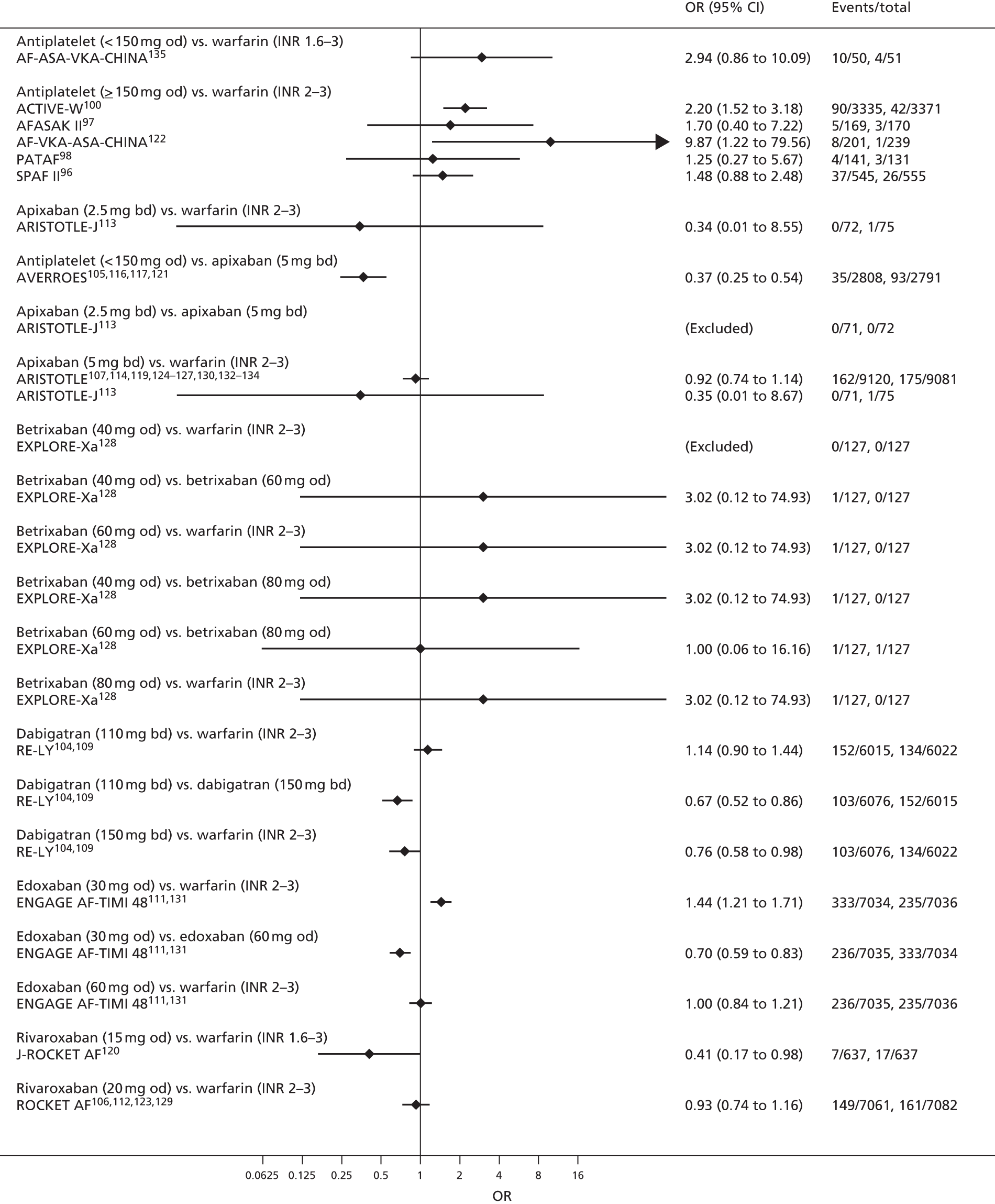
FIGURE 97.
Myocardial infarction (stroke prevention in AF).
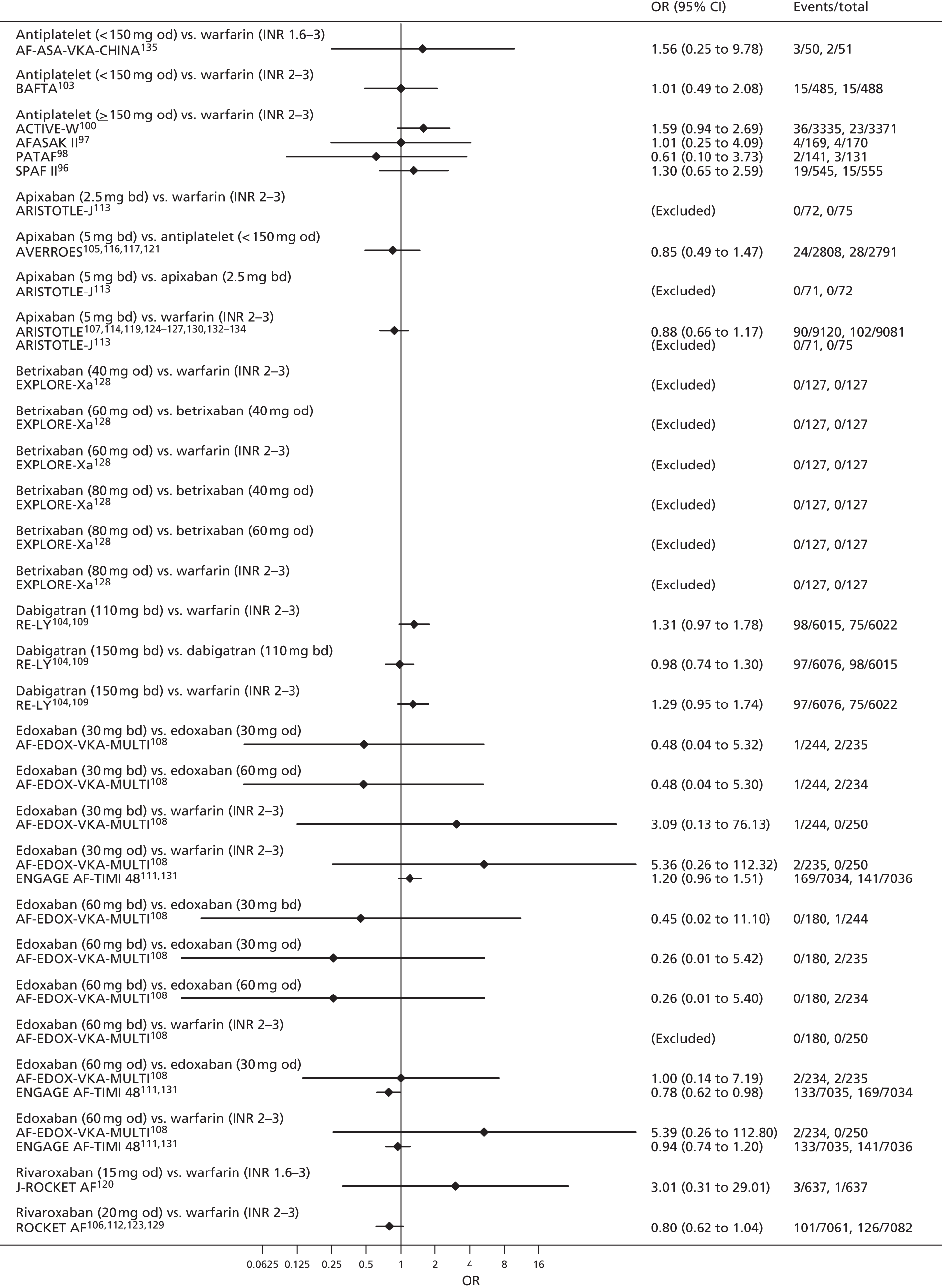
FIGURE 98.
Major bleeding [1/4] (stroke prevention in AF).

FIGURE 99.
Major bleeding [2/4] (stroke prevention in AF).
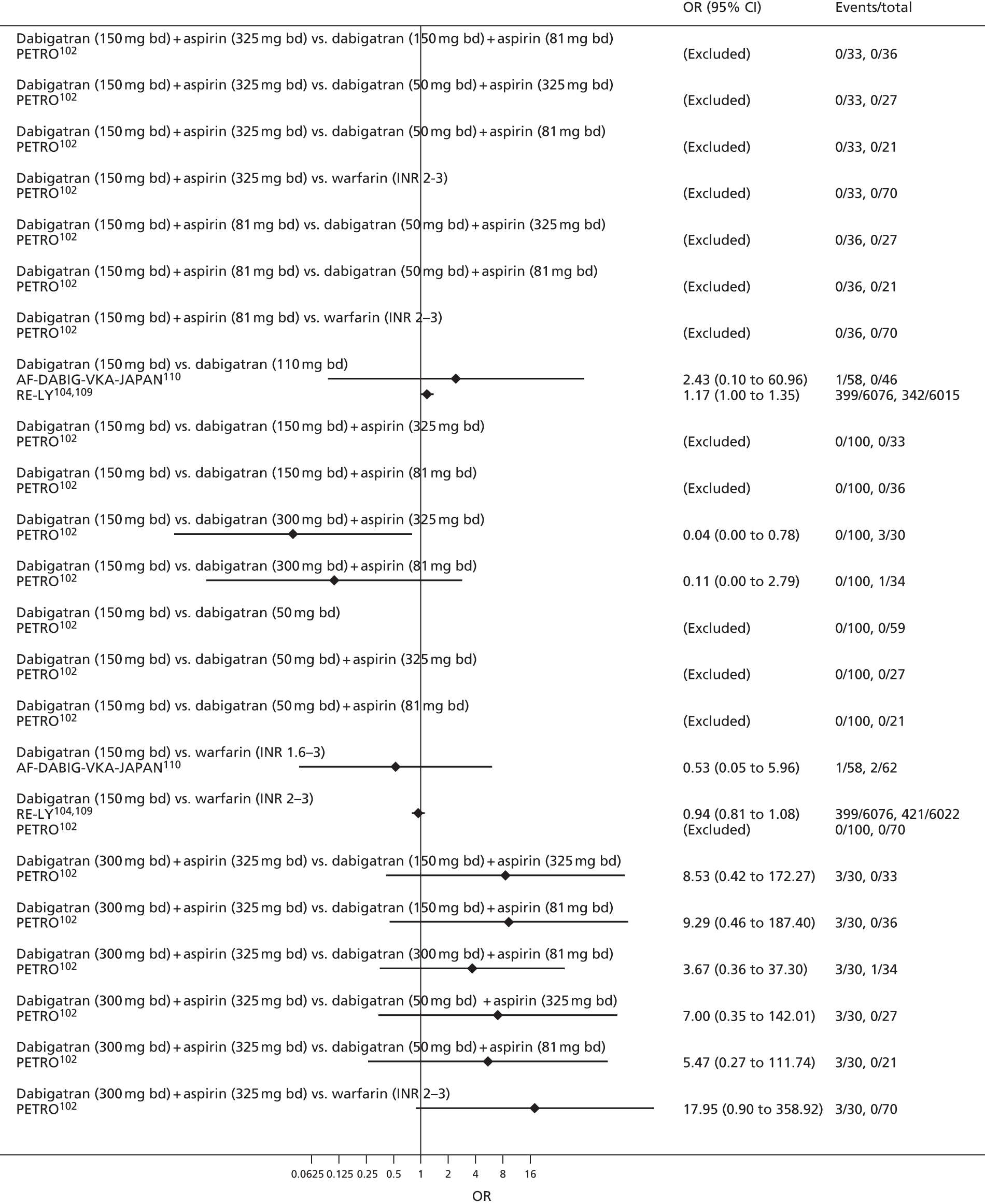
FIGURE 100.
Major bleeding [3/4] (stroke prevention in AF).
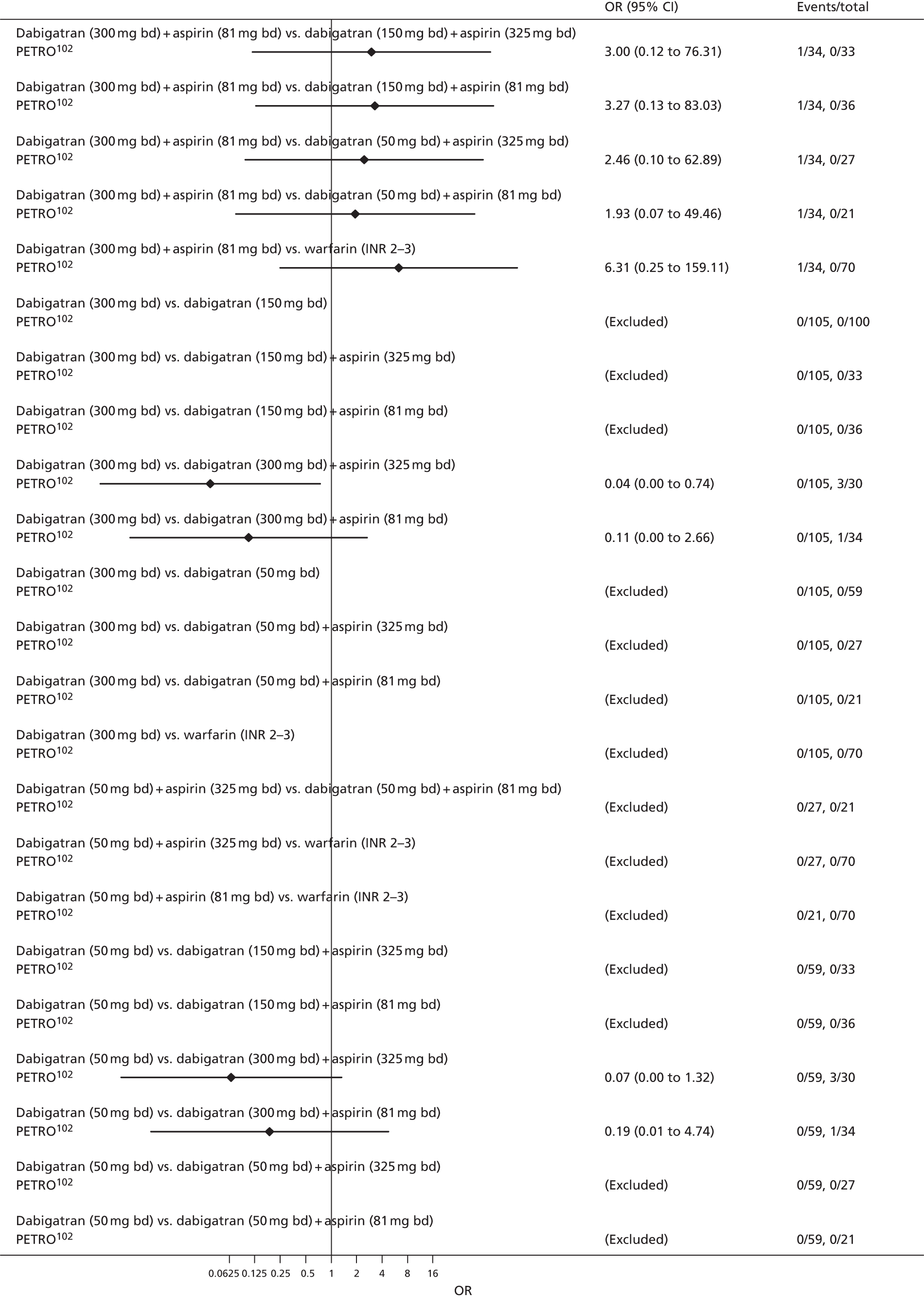
FIGURE 101.
Major bleeding [4/4] (stroke prevention in AF).

FIGURE 102.
Clinically relevant bleeding [1/3] (stroke prevention in AF).

FIGURE 103.
Clinically relevant bleeding [2/3] (stroke prevention in AF).

FIGURE 104.
Clinically relevant bleeding [3/3] (stroke prevention in AF).
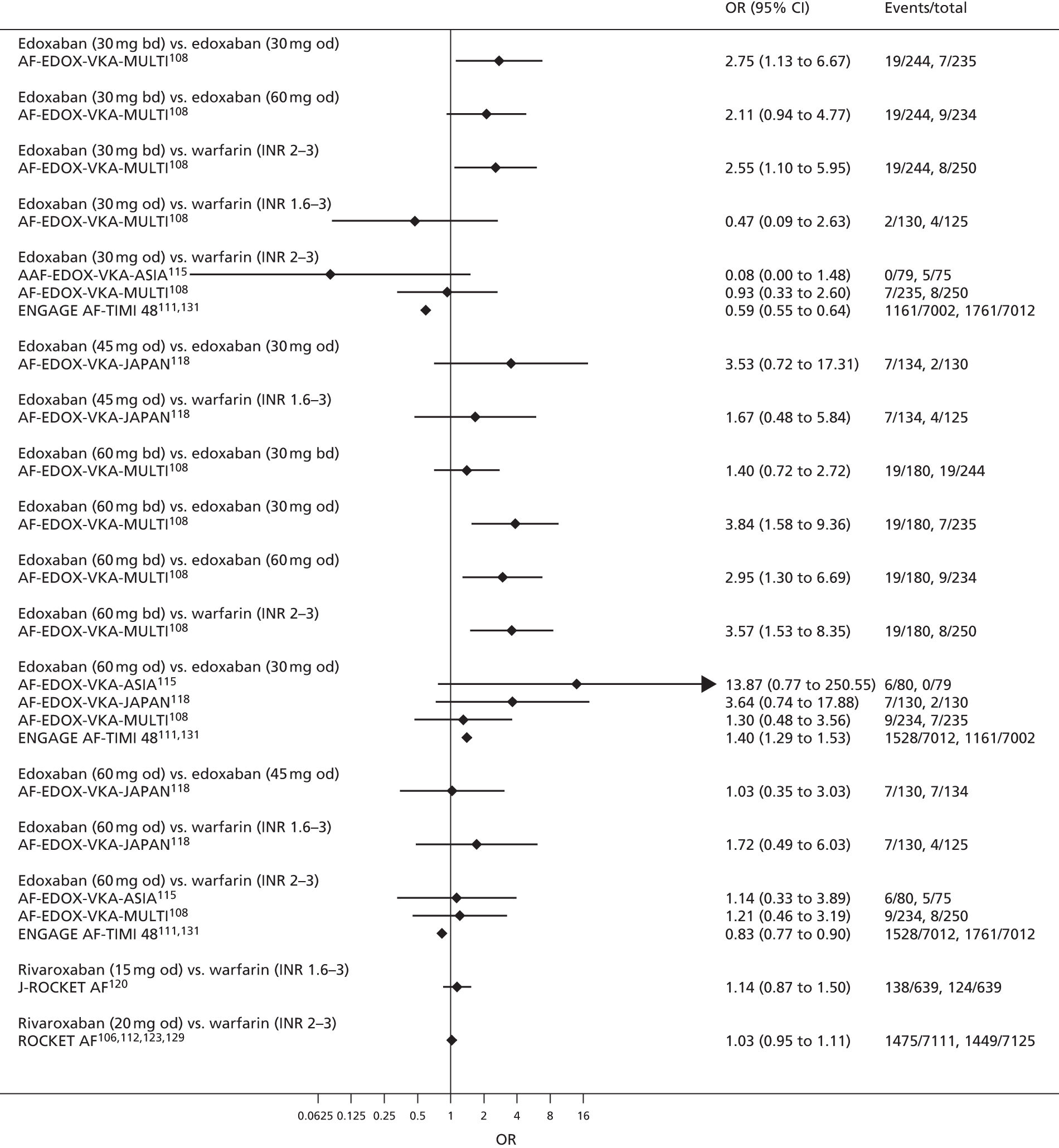
FIGURE 105.
Intracranial bleeding (stroke prevention in AF).
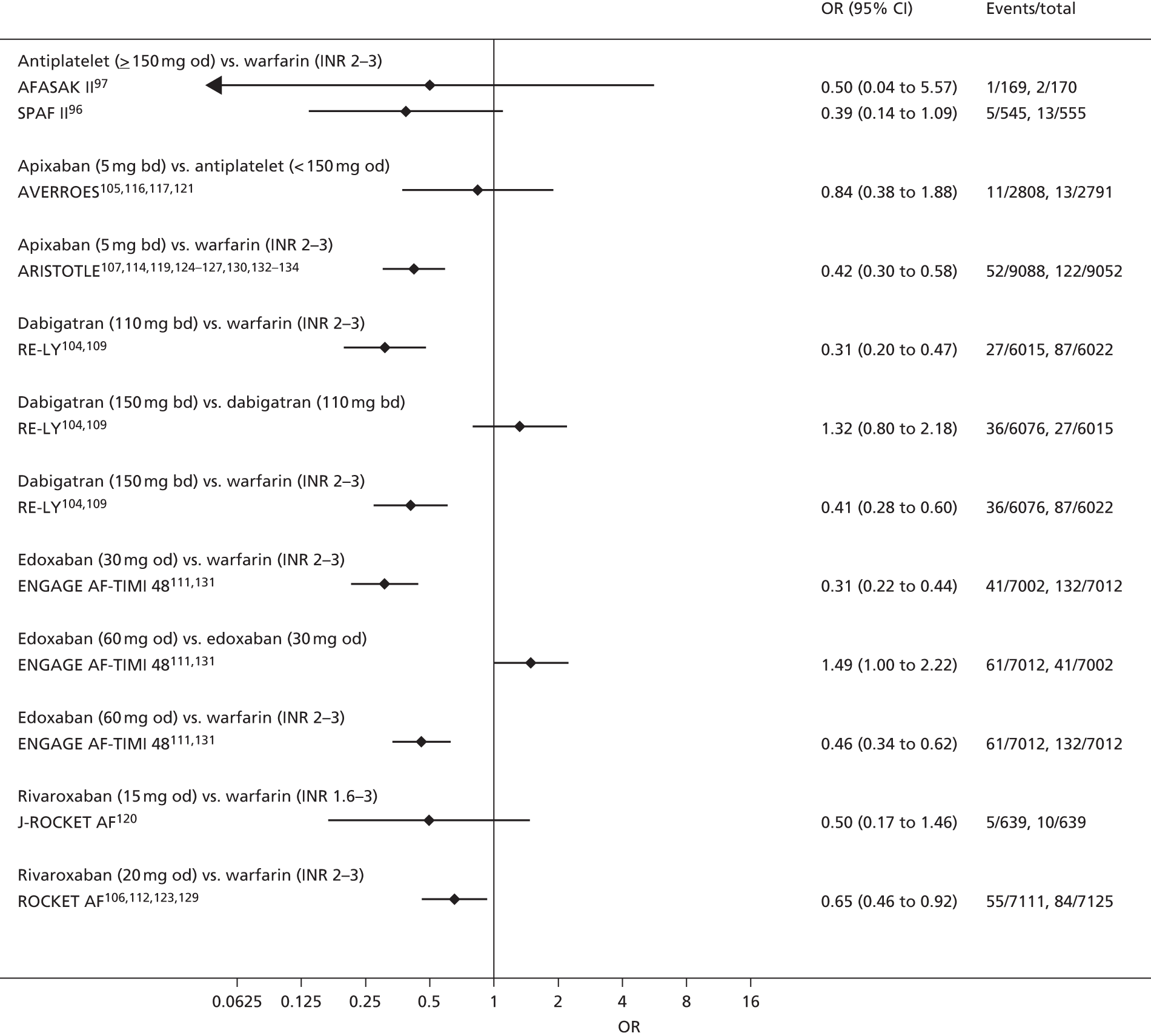
FIGURE 106.
All-cause mortality (stroke prevention in AF).
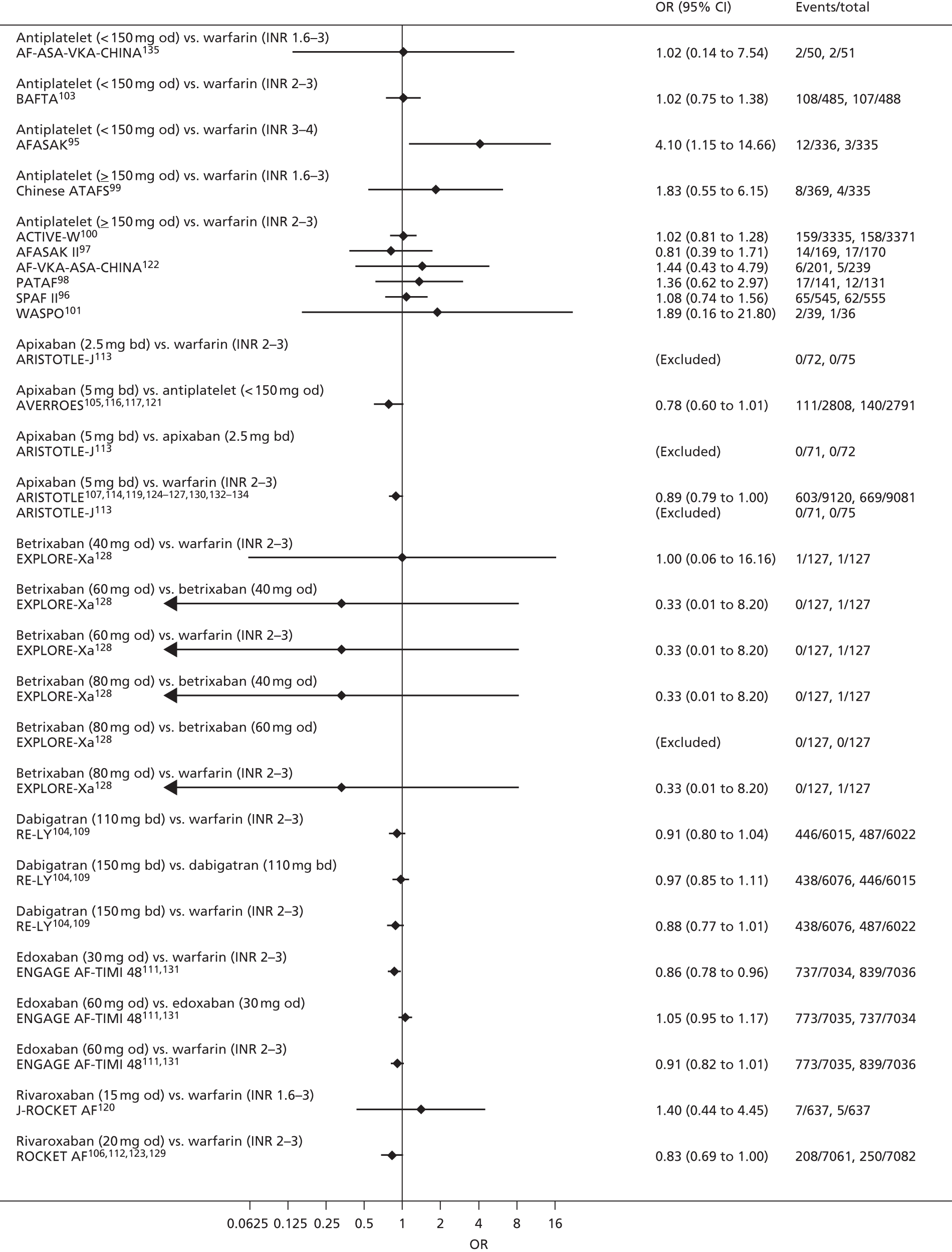
Appendix 3 Forest plots: primary prevention of venous thromboembolism
FIGURE 107.
Symptomatic DVT [1/3] (primary prevention of VTE).
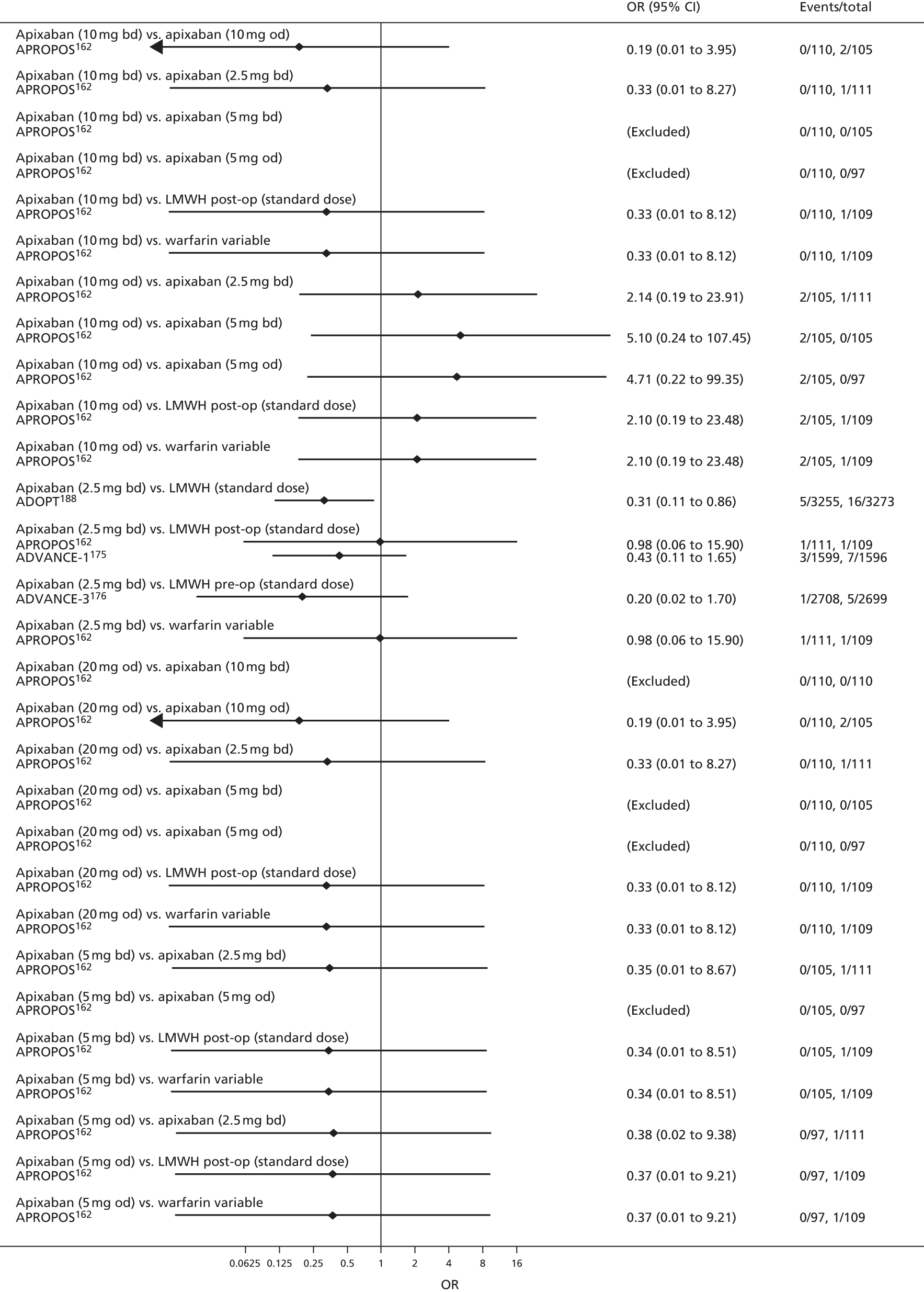
FIGURE 108.
Symptomatic DVT [2/3] (primary prevention of VTE).
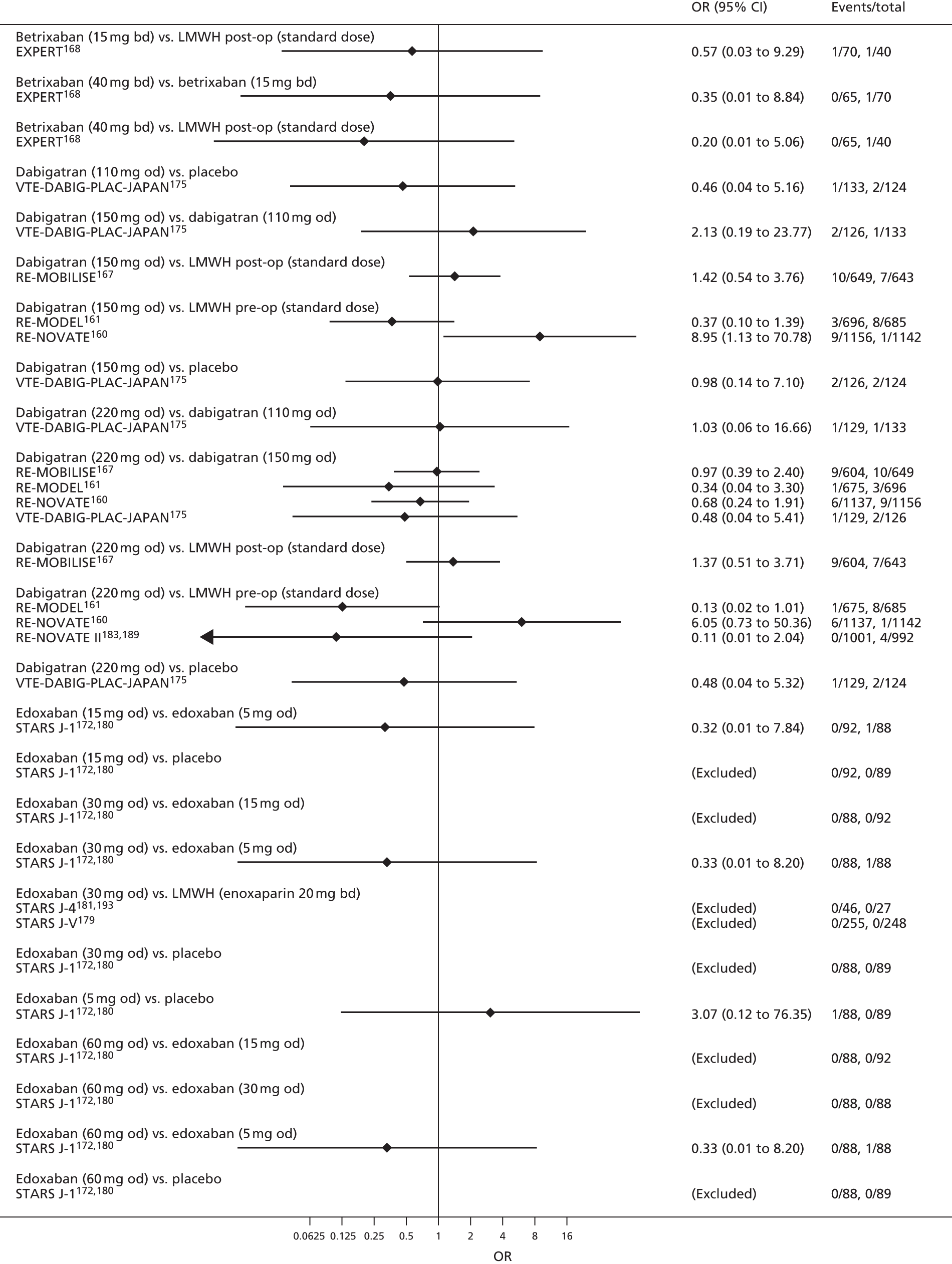
FIGURE 109.
Symptomatic DVT [3/3] (primary prevention of VTE).
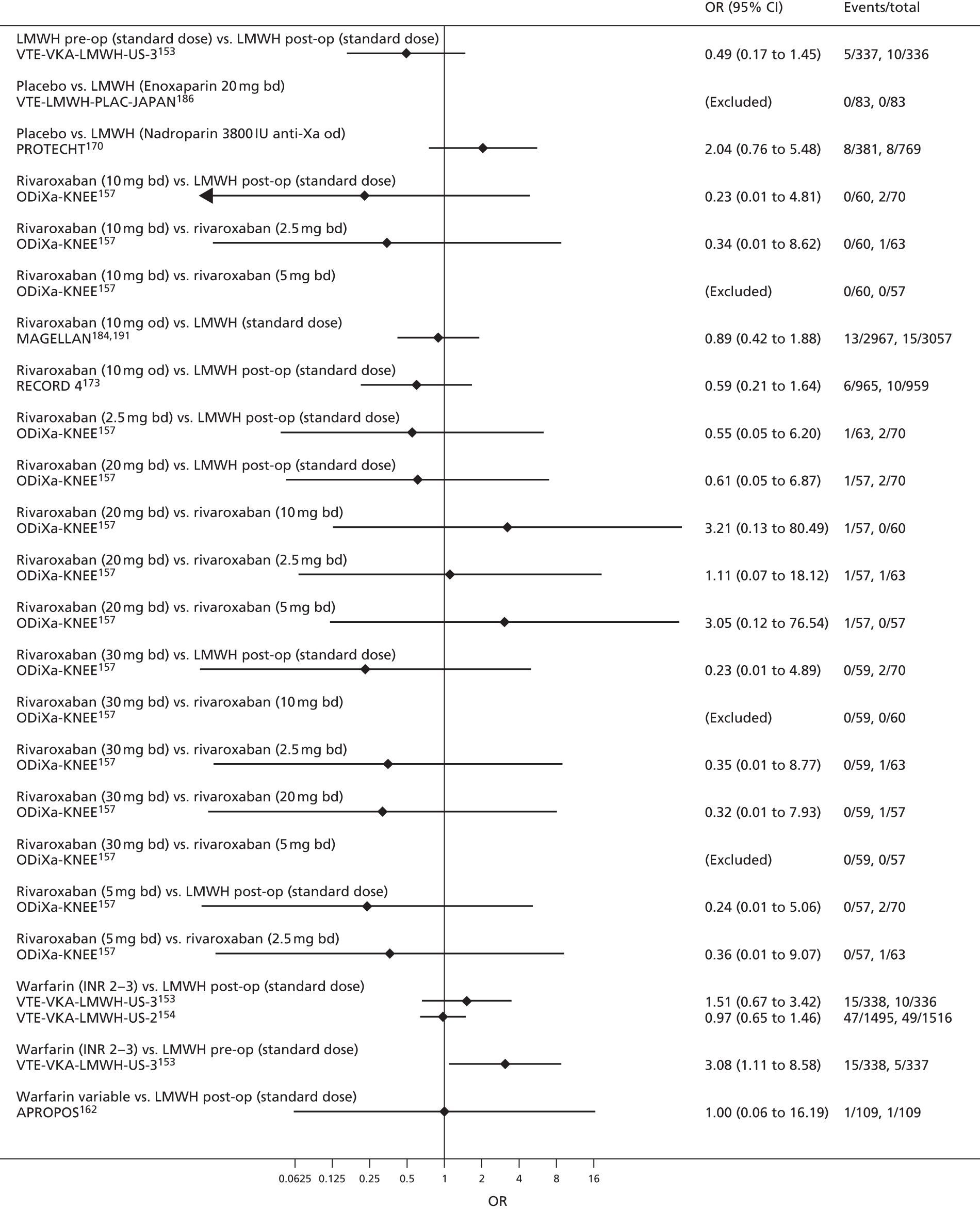
FIGURE 110.
Symptomatic PE [1/4] (primary prevention of VTE).

FIGURE 111.
Symptomatic PE [2/4] (primary prevention of VTE).

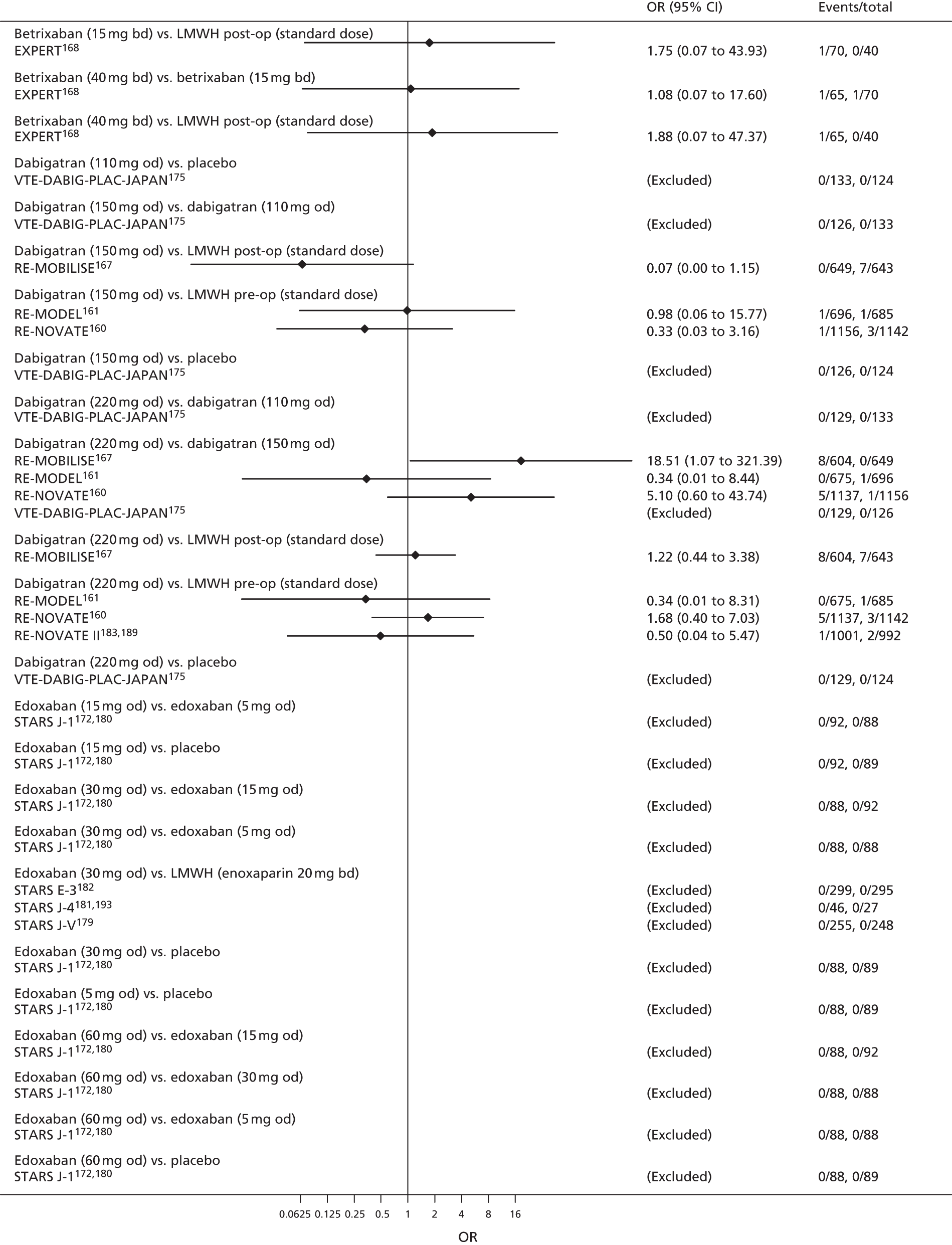
FIGURE 112.
Symptomatic PE [3/4] (primary prevention of VTE).
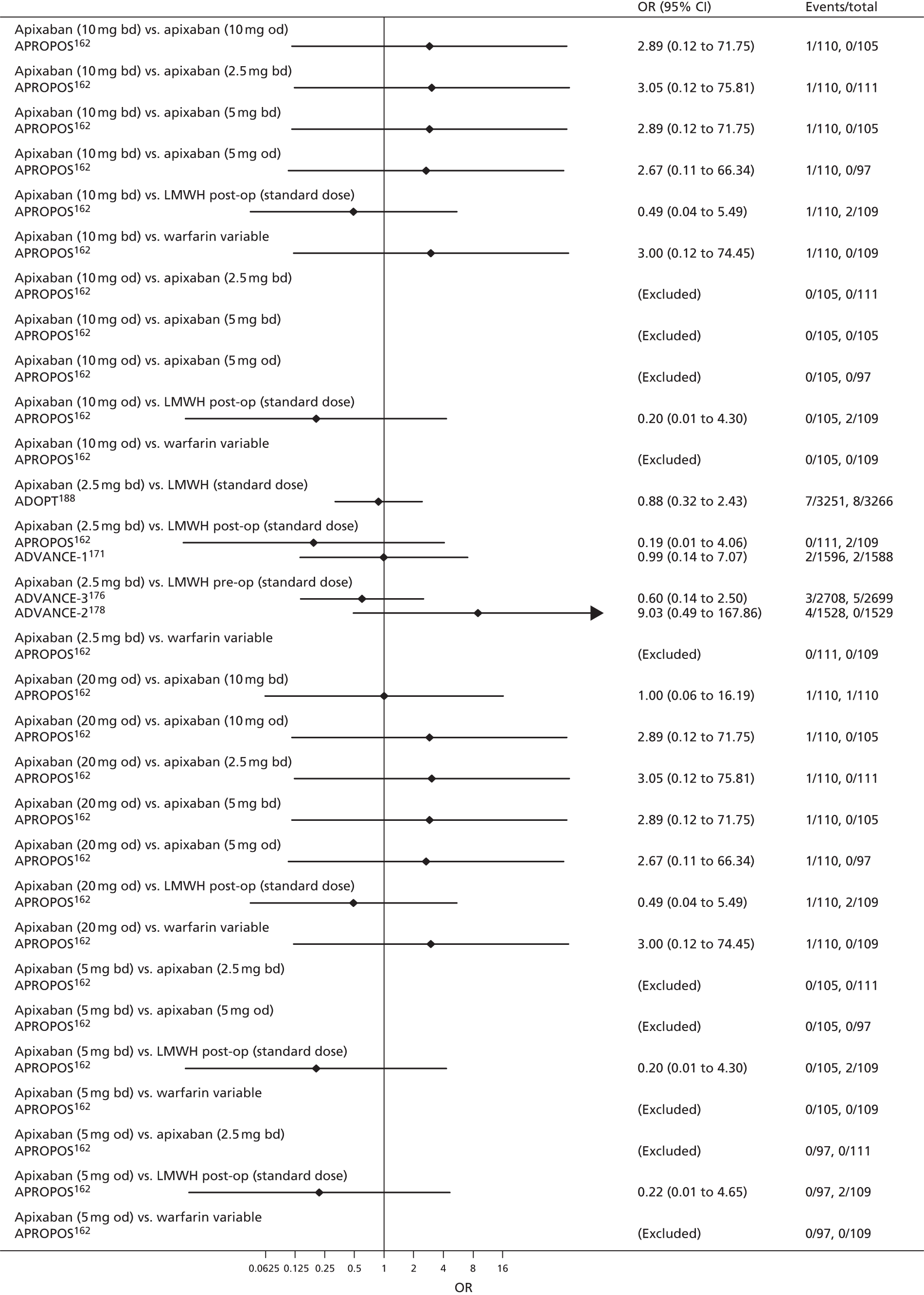
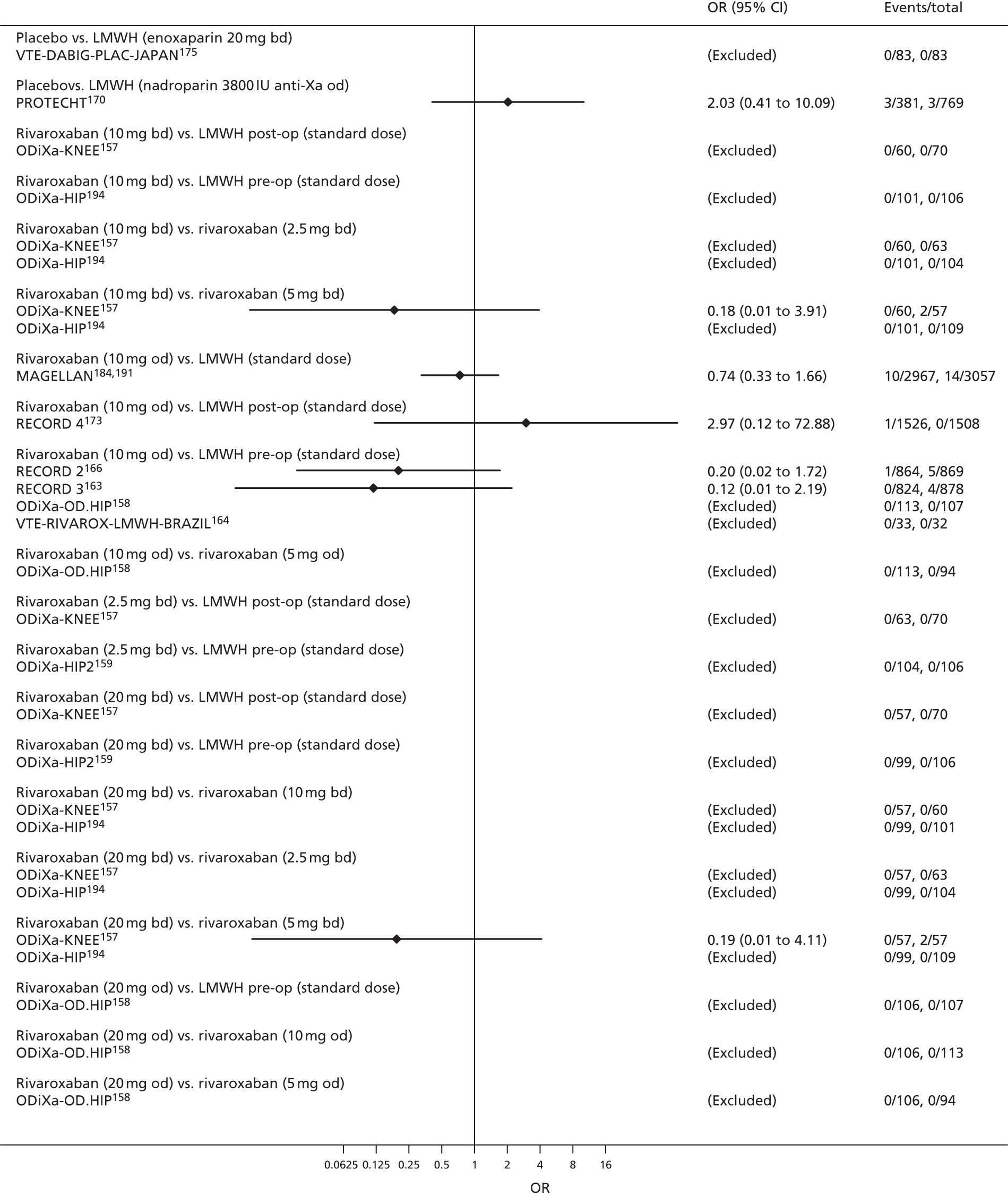
FIGURE 113.
Symptomatic PE [4/4] (primary prevention of VTE).

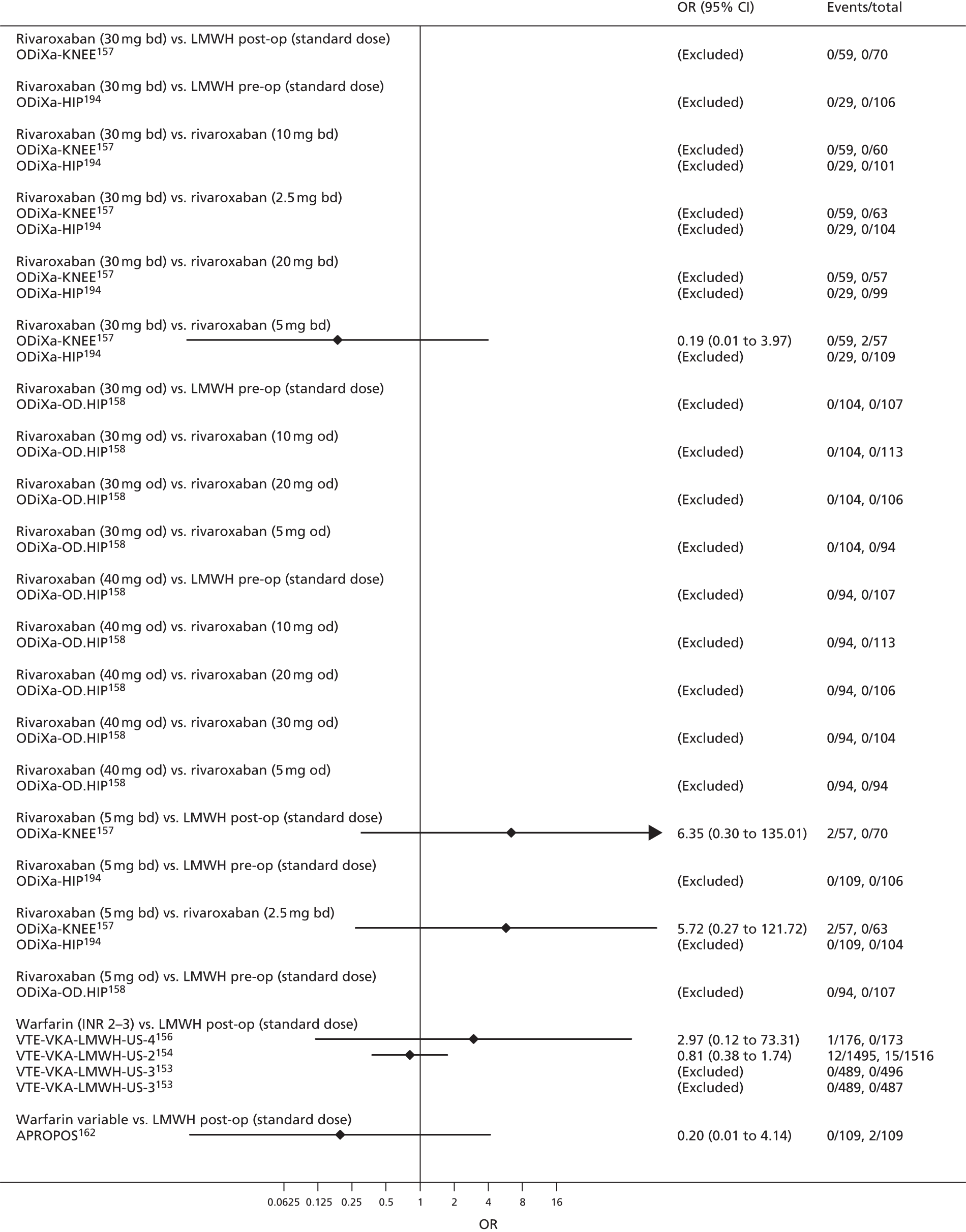
FIGURE 114.
Symptomatic VTE [1/3] (primary prevention of VTE).
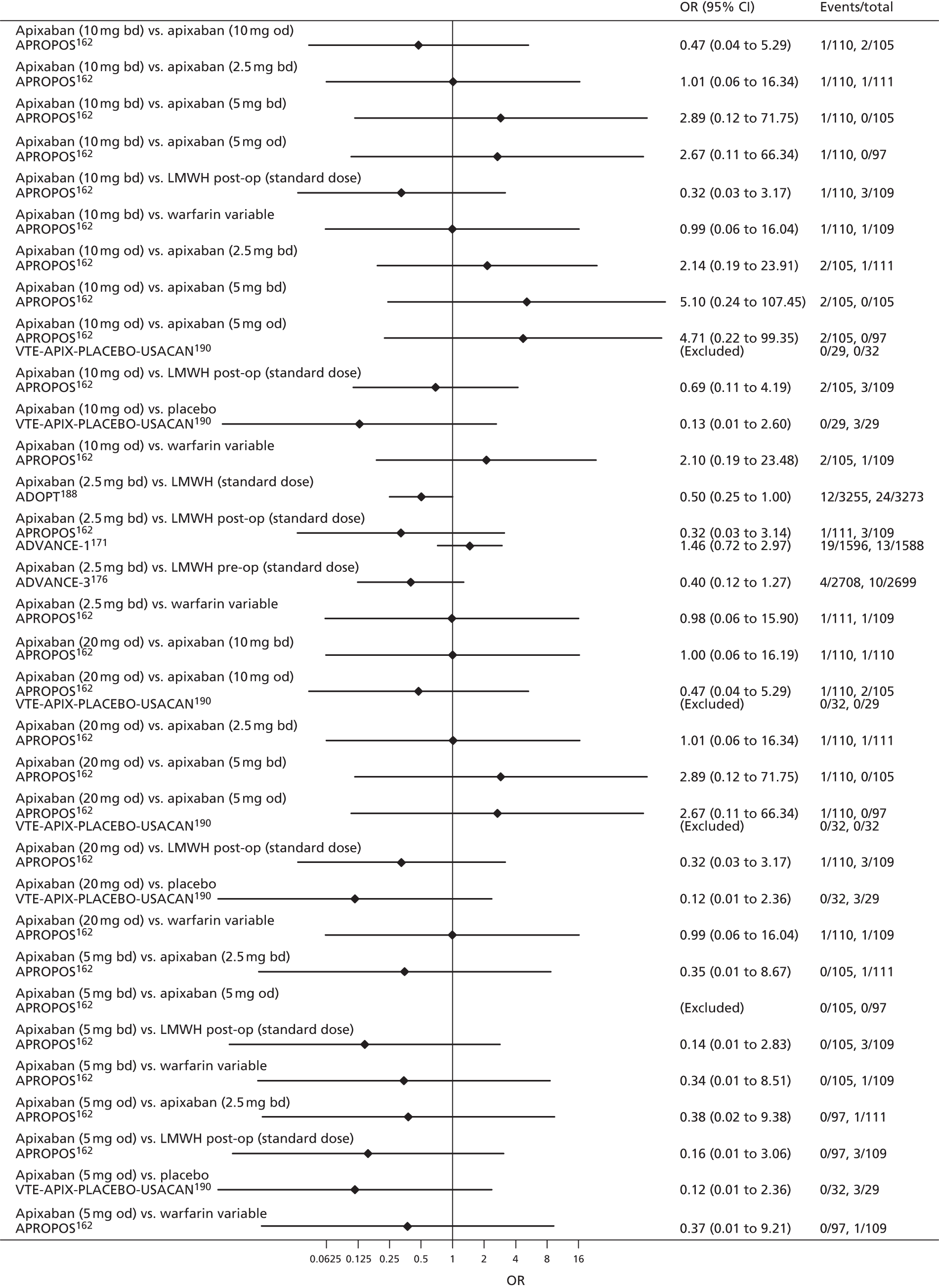
FIGURE 115.
Symptomatic VTE [2/3] (primary prevention of VTE).
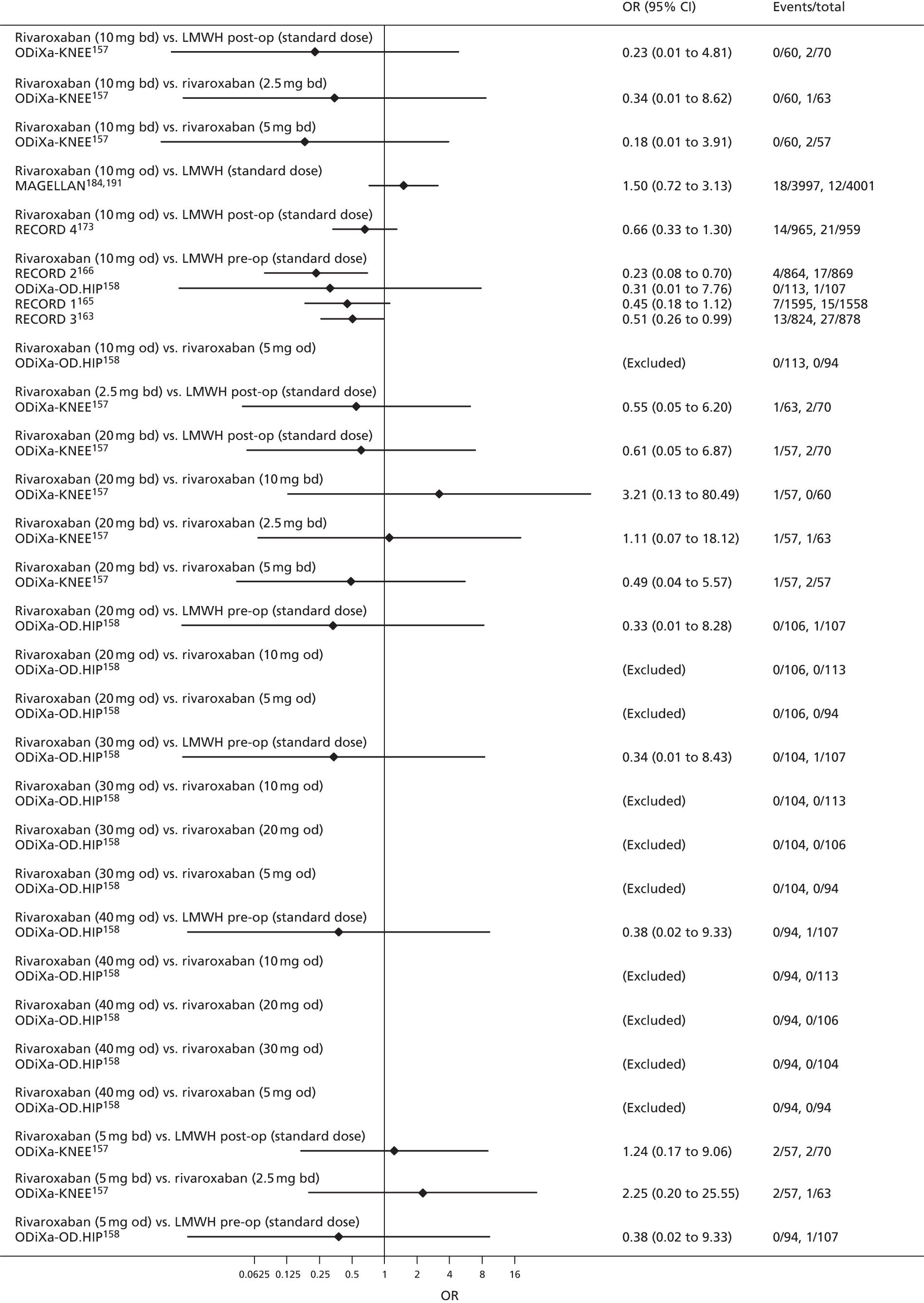
FIGURE 116.
Symptomatic VTE [3/3] (primary prevention of VTE).

FIGURE 117.
Myocardial infarction (primary prevention of VTE).
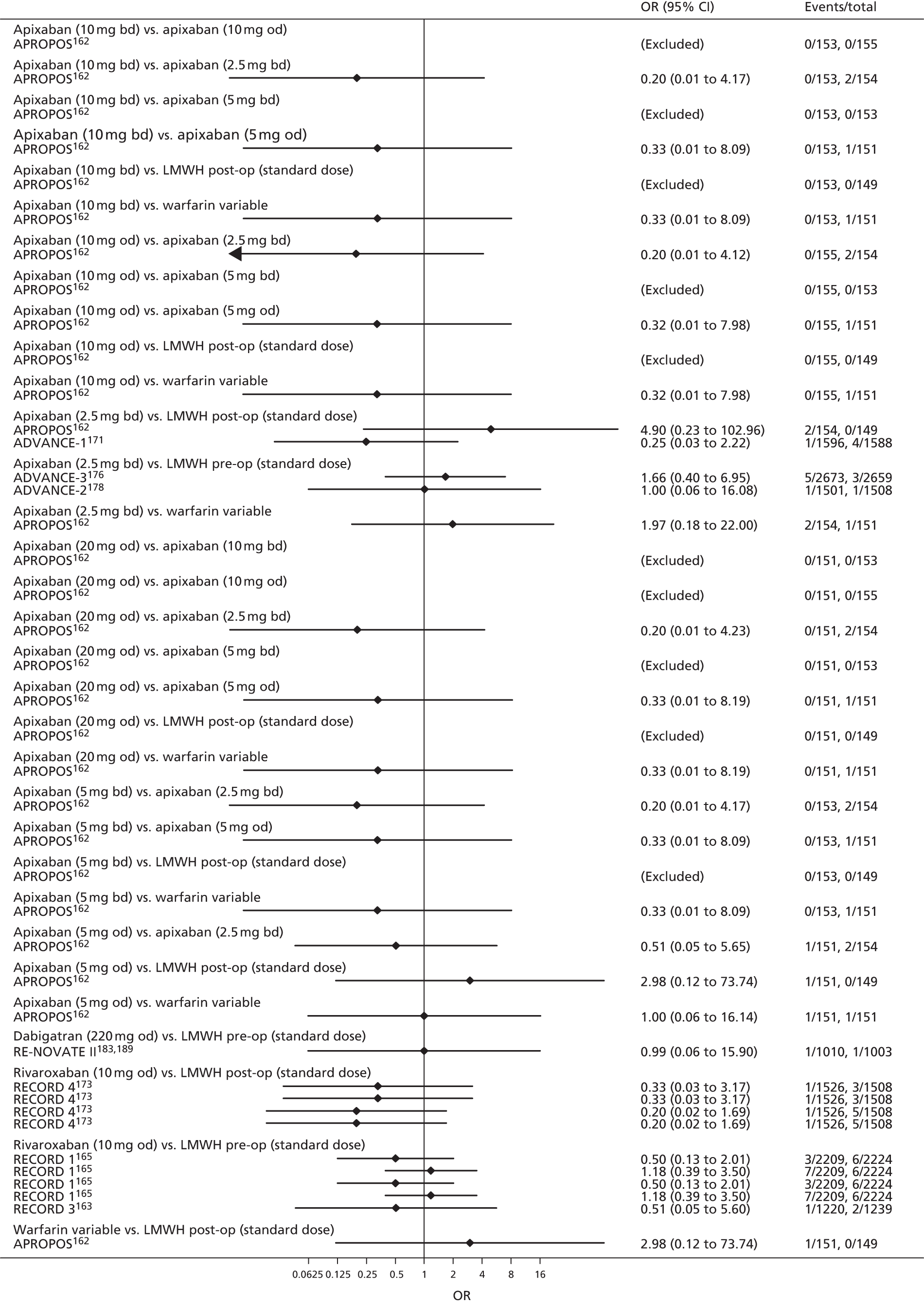
FIGURE 118.
Major bleeding [1/4] (primary prevention of VTE).

FIGURE 119.
Major bleeding [2/4] (primary prevention of VTE).
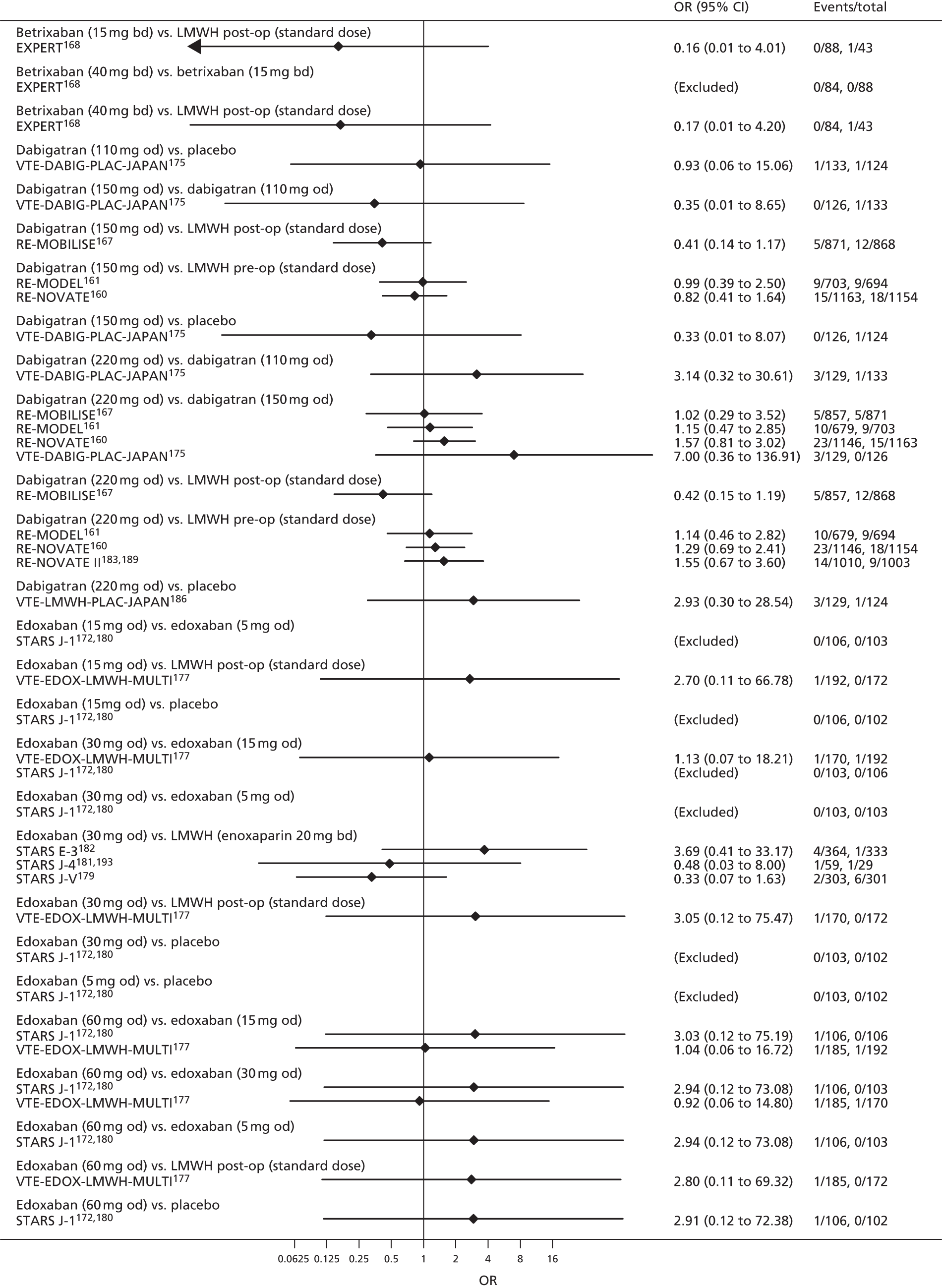
FIGURE 120.
Major bleeding [3/4] (primary prevention of VTE).
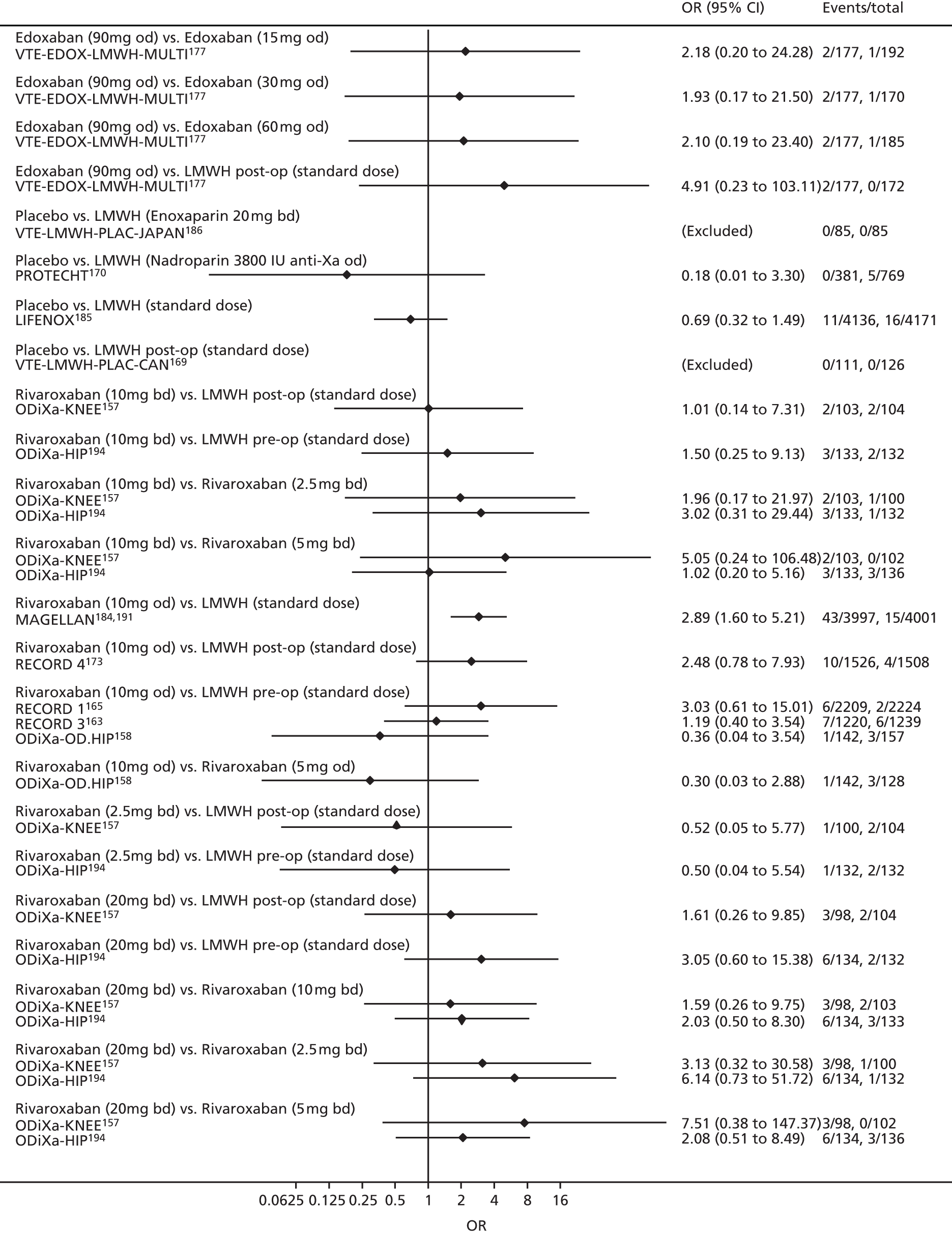
FIGURE 121.
Major bleeding [4/4] (primary prevention of VTE).

FIGURE 122.
Clinically relevant bleeding [1/2] (primary prevention of VTE).
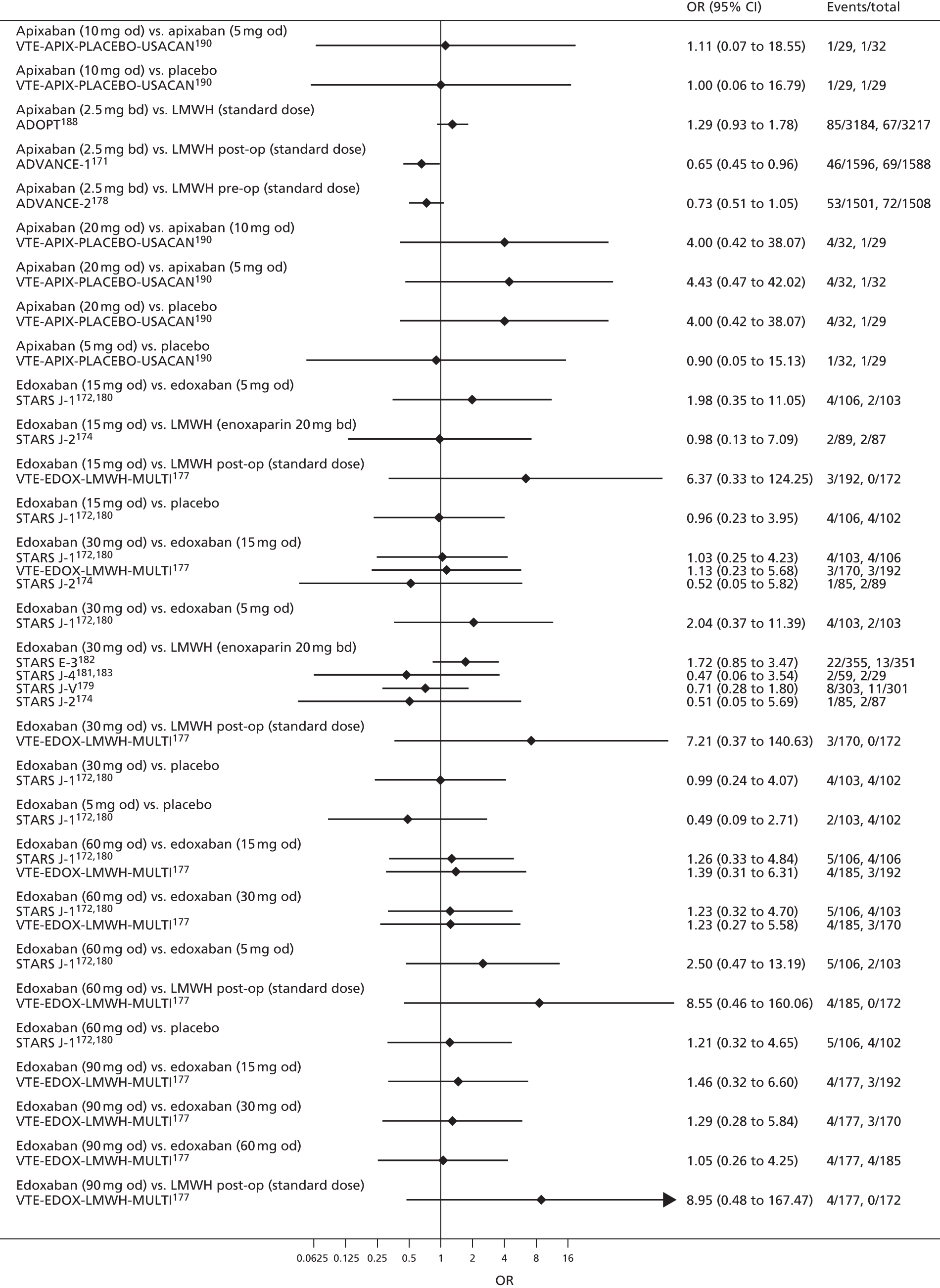
FIGURE 123.
Clinically relevant bleeding [2/2] (primary prevention of VTE).
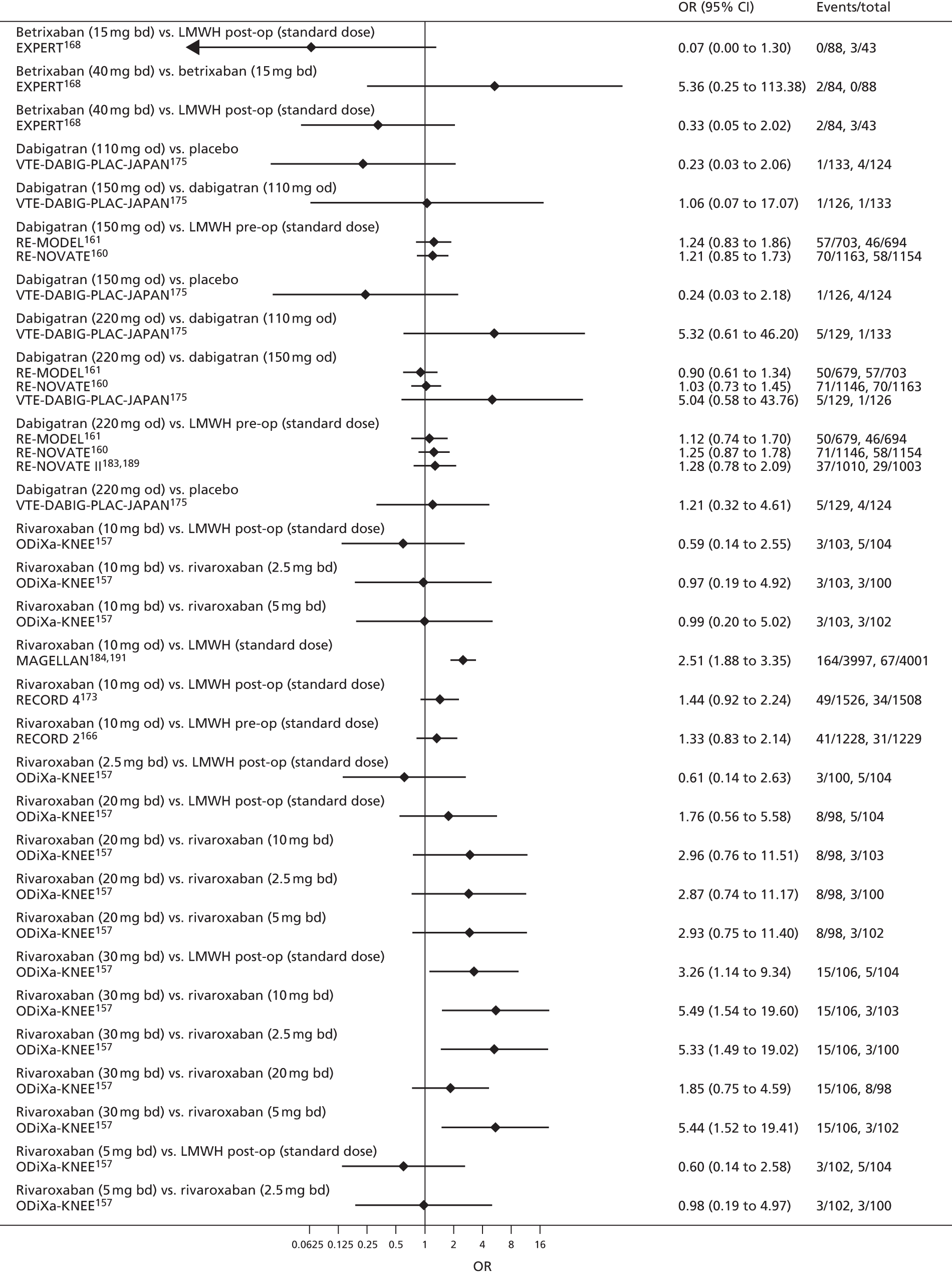
FIGURE 124.
All-cause mortality [1/3] (primary prevention of VTE).
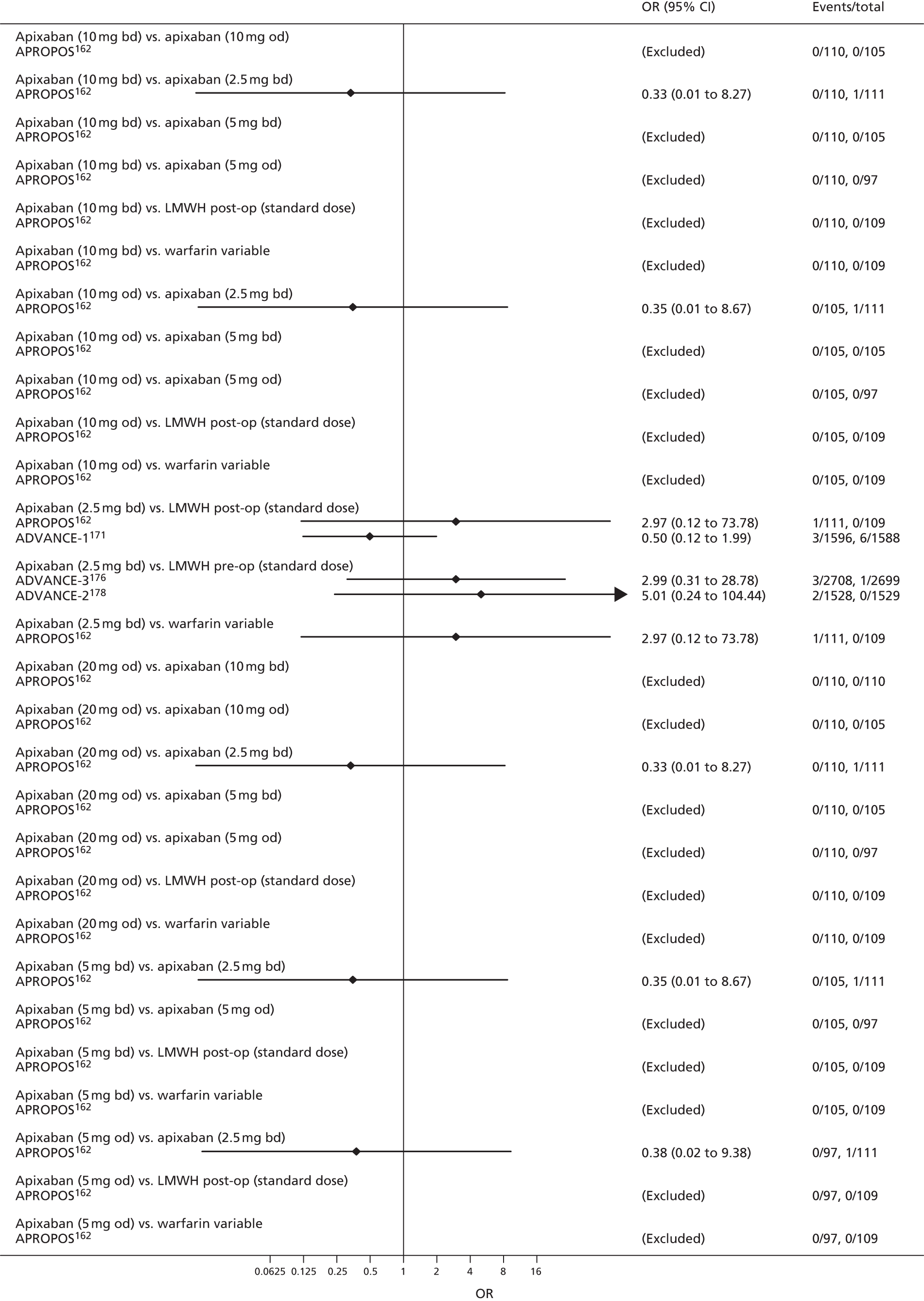
FIGURE 125.
All-cause mortality [2/3] (primary prevention of VTE).
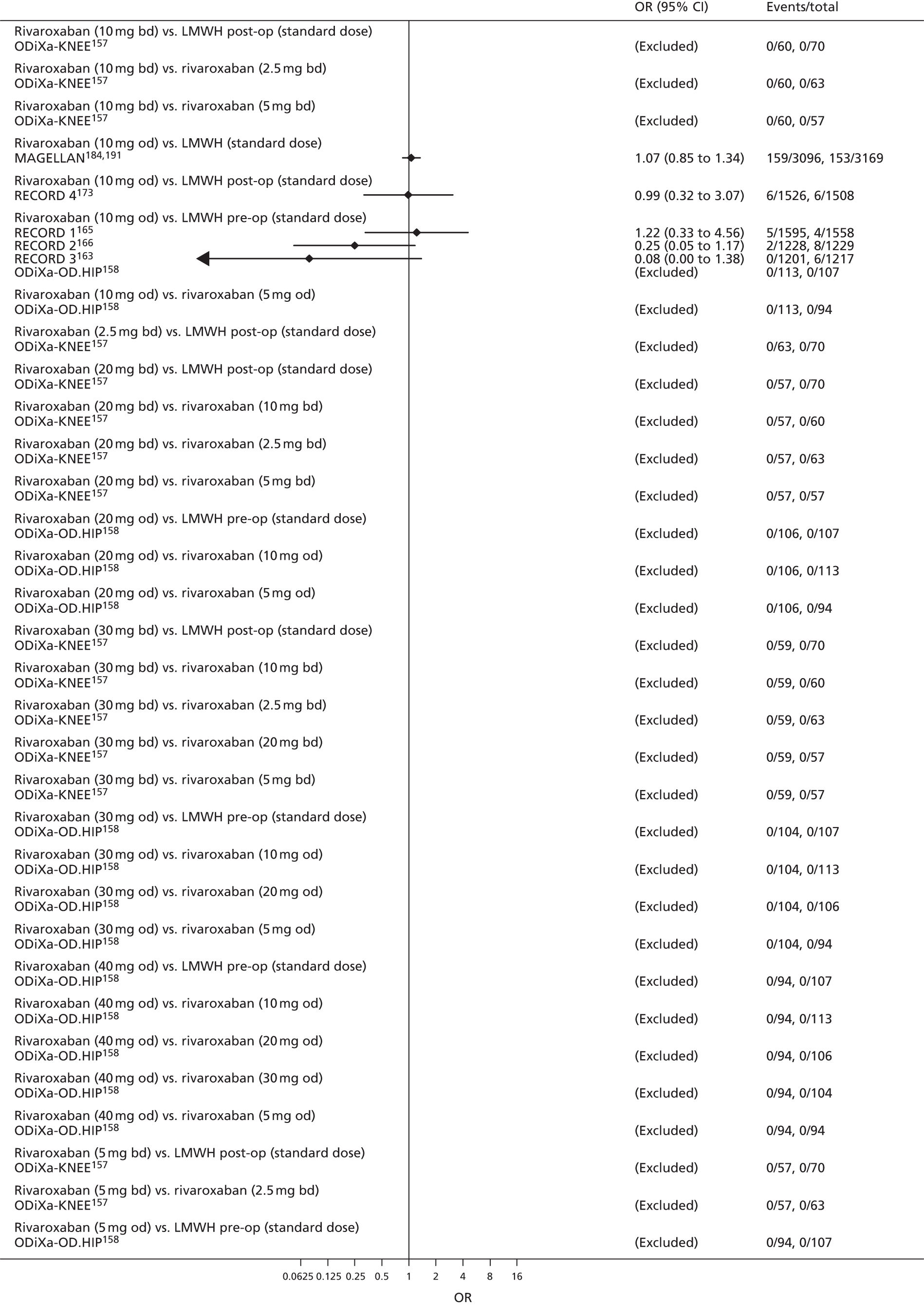
FIGURE 126.
All-cause mortality [3/3] (primary prevention of VTE).

Appendix 4 Forest plots: acute treatment of venous thromboembolism
FIGURE 127.
Symptomatic DVT (acute treatment of VTE).

FIGURE 128.
Symptomatic PE (acute treatment of VTE).
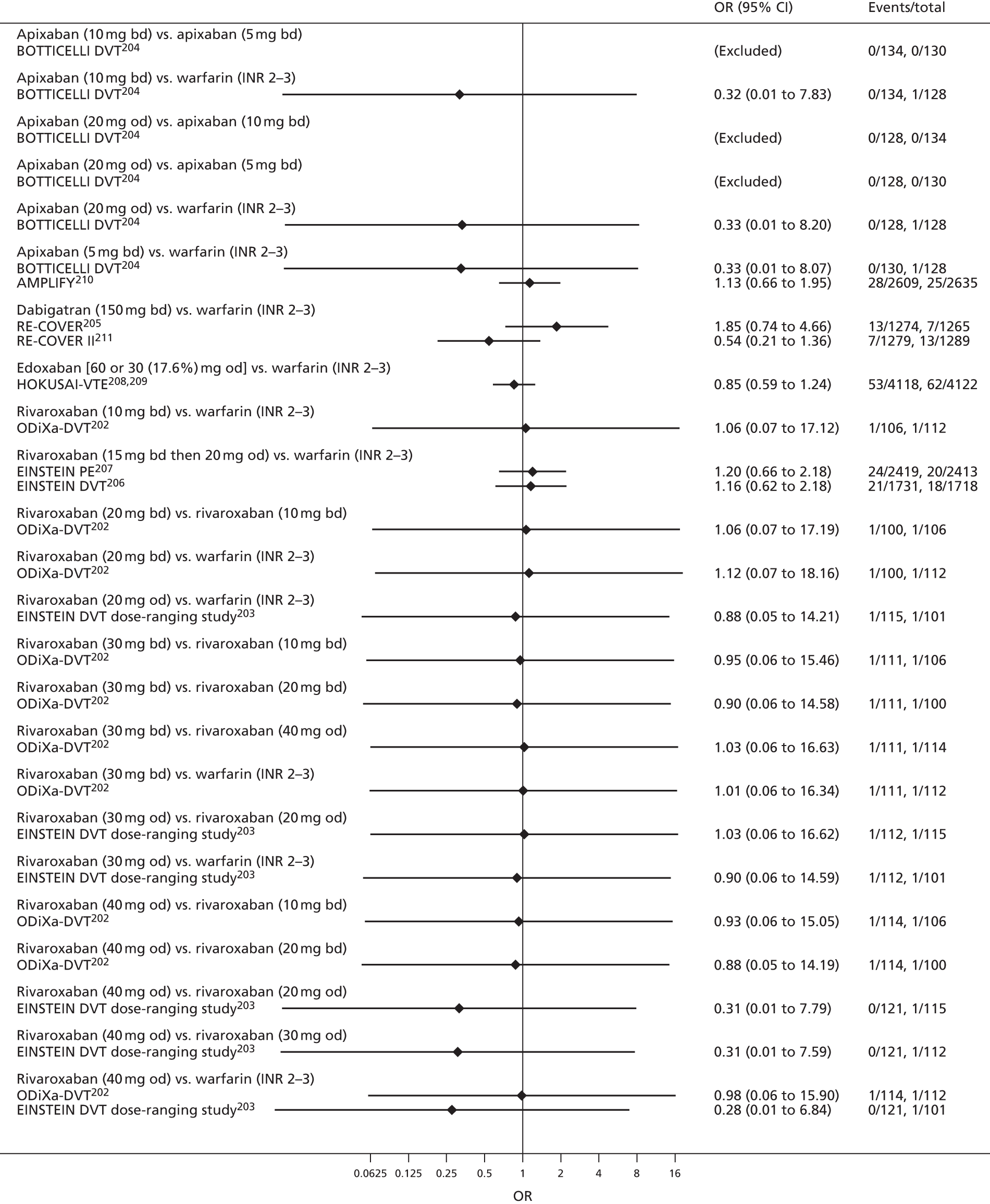
FIGURE 129.
Symptomatic VTE (acute treatment of VTE).
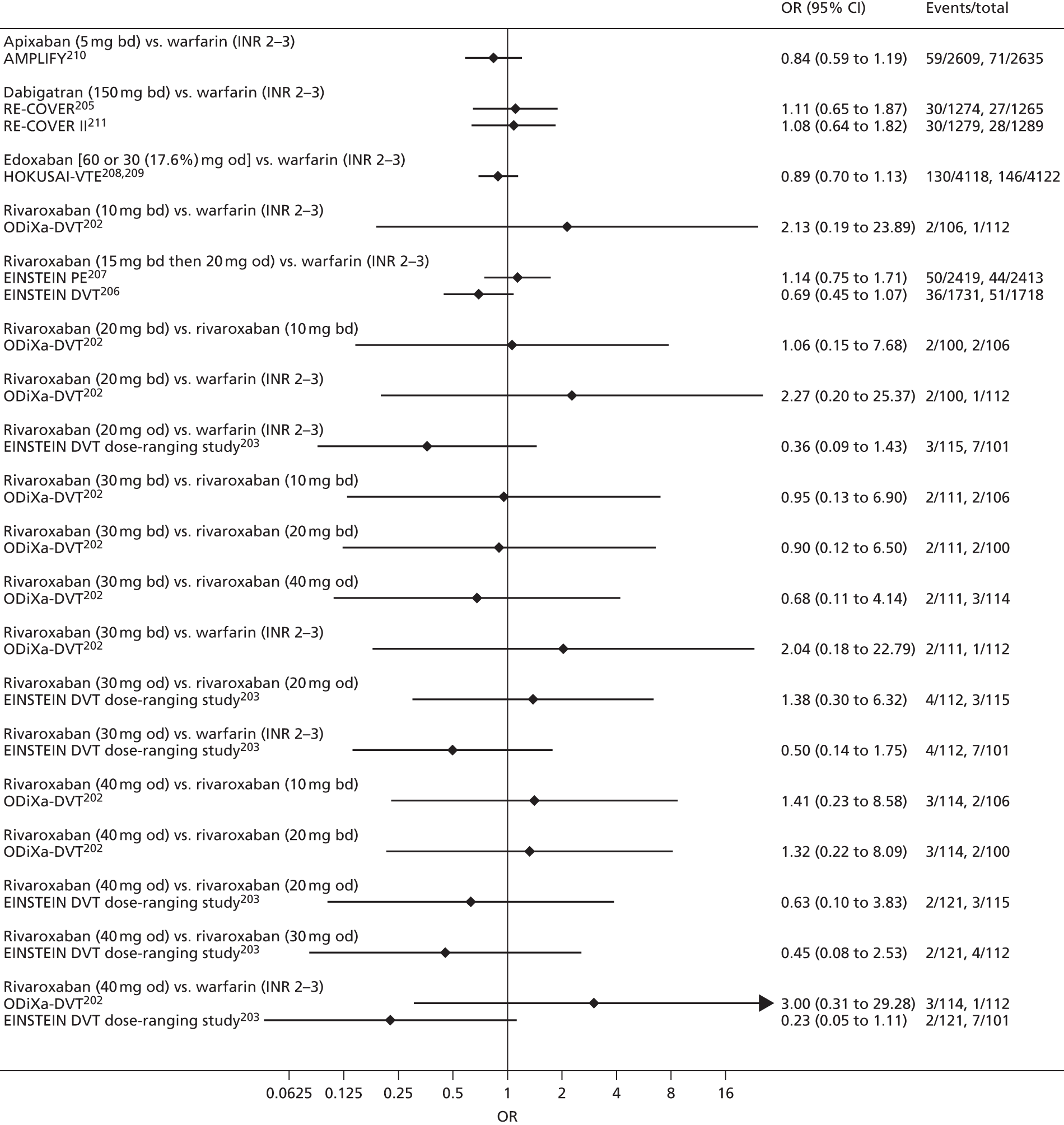
FIGURE 130.
Myocardial infarction (acute treatment of VTE).
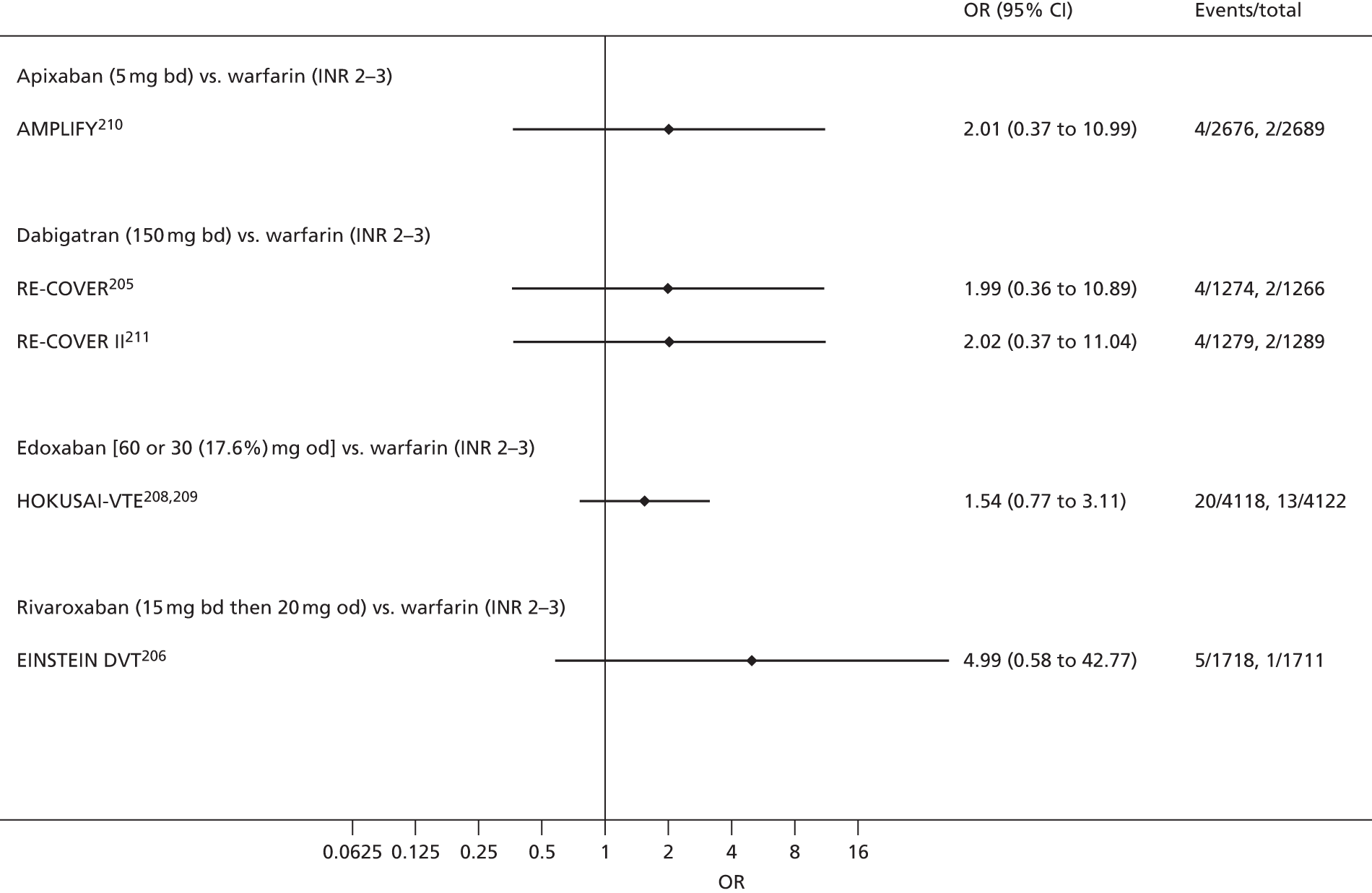
FIGURE 131.
Major bleeding (acute treatment of VTE).
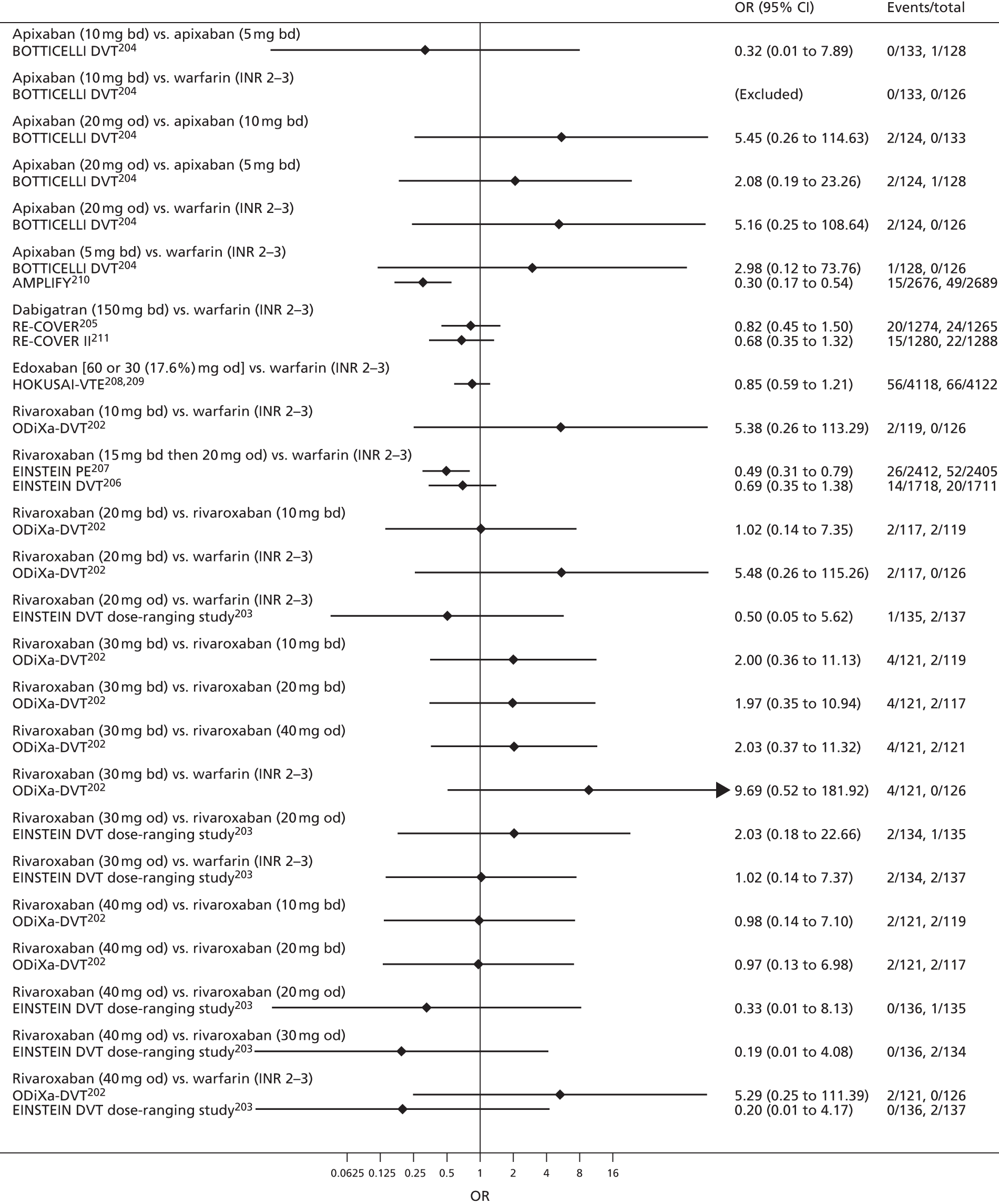
FIGURE 132.
Clinically relevant bleeding (acute treatment of VTE).
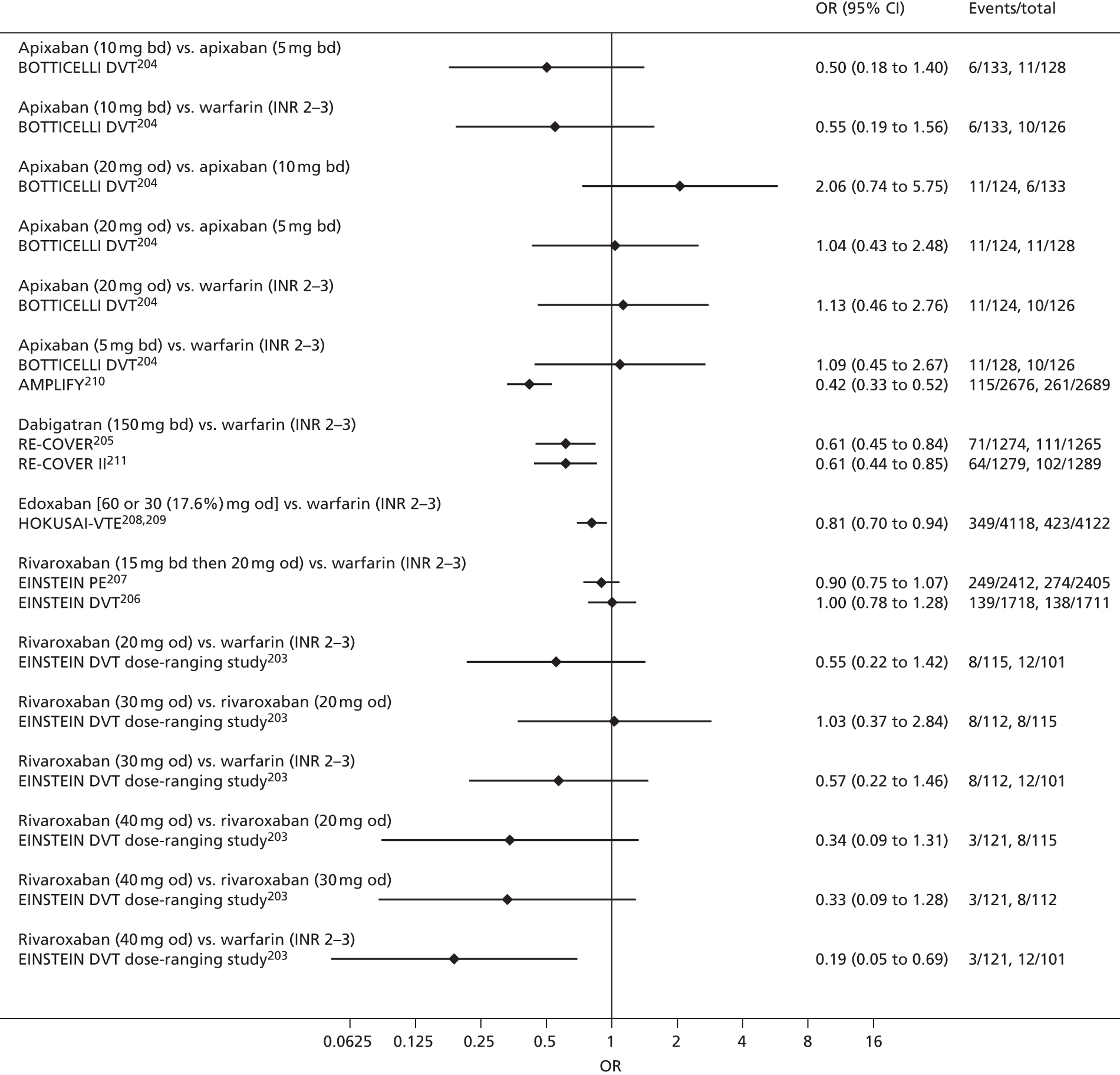
FIGURE 133.
All-cause mortality (acute treatment of VTE).

Appendix 5 Forest plots: secondary prevention of venous thromboembolism
FIGURE 134.
Symptomatic DVT (secondary prevention of VTE).
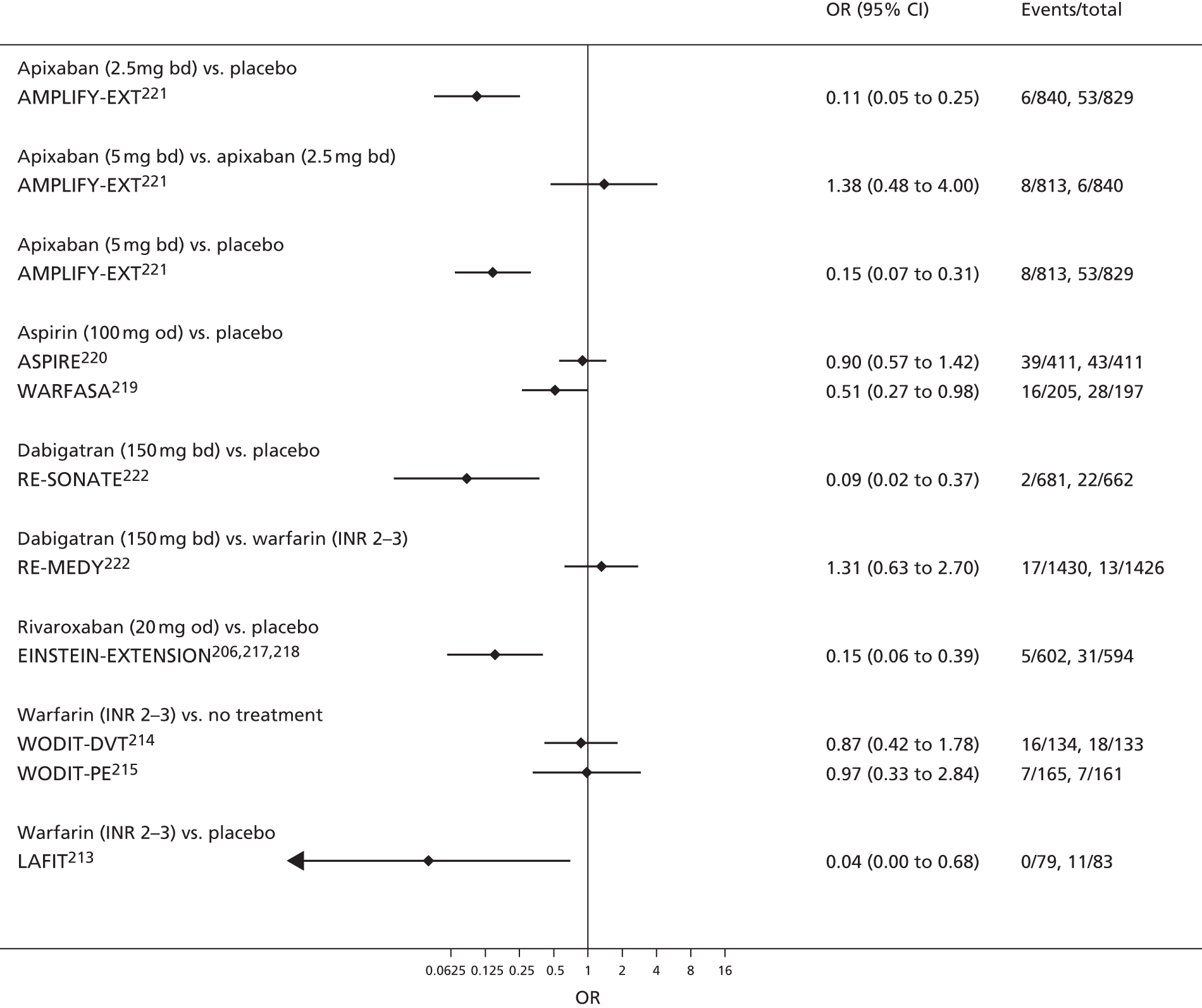
FIGURE 135.
Symptomatic PE (secondary prevention of VTE).
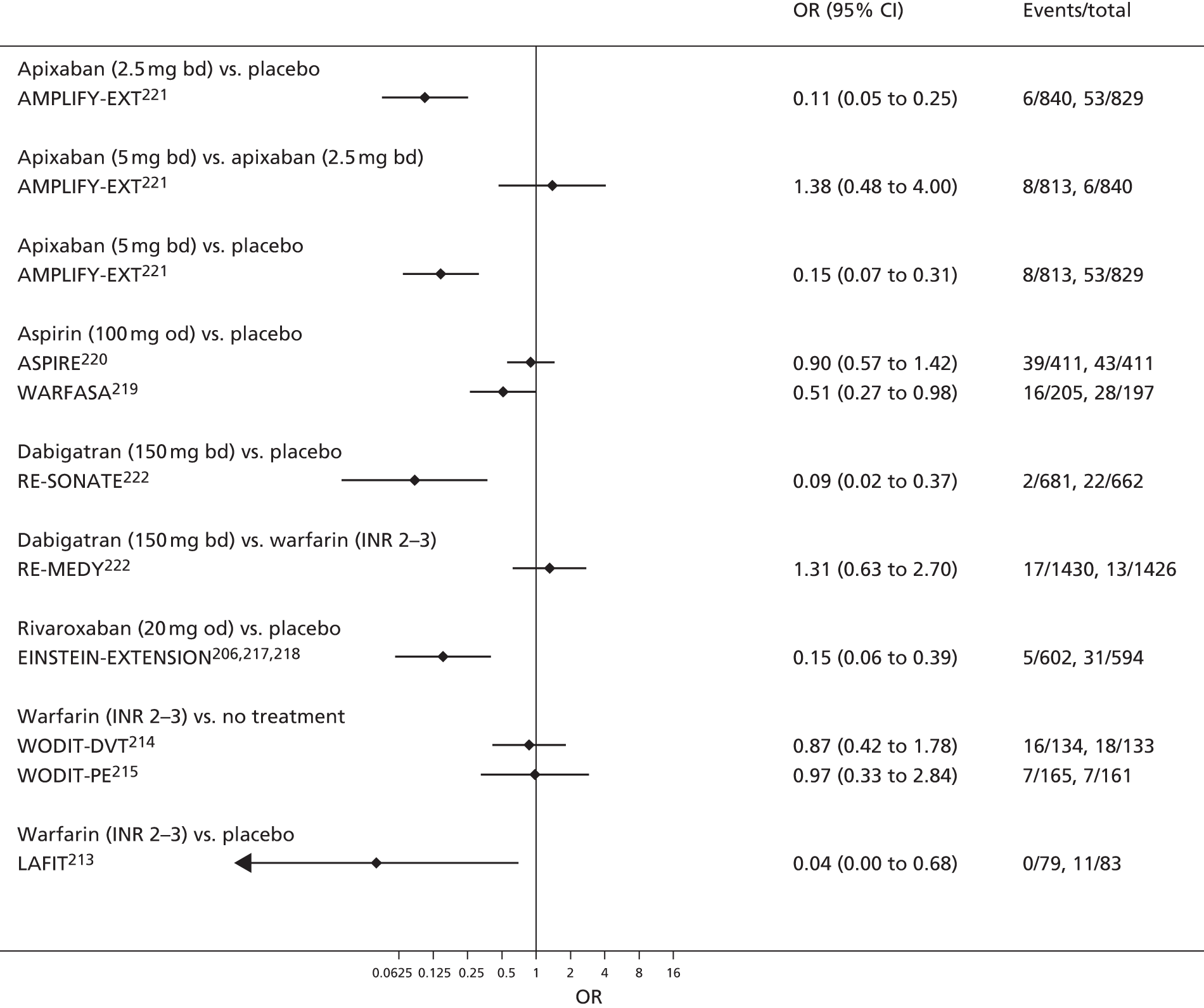
FIGURE 136.
Symptomatic VTE (secondary prevention of VTE).
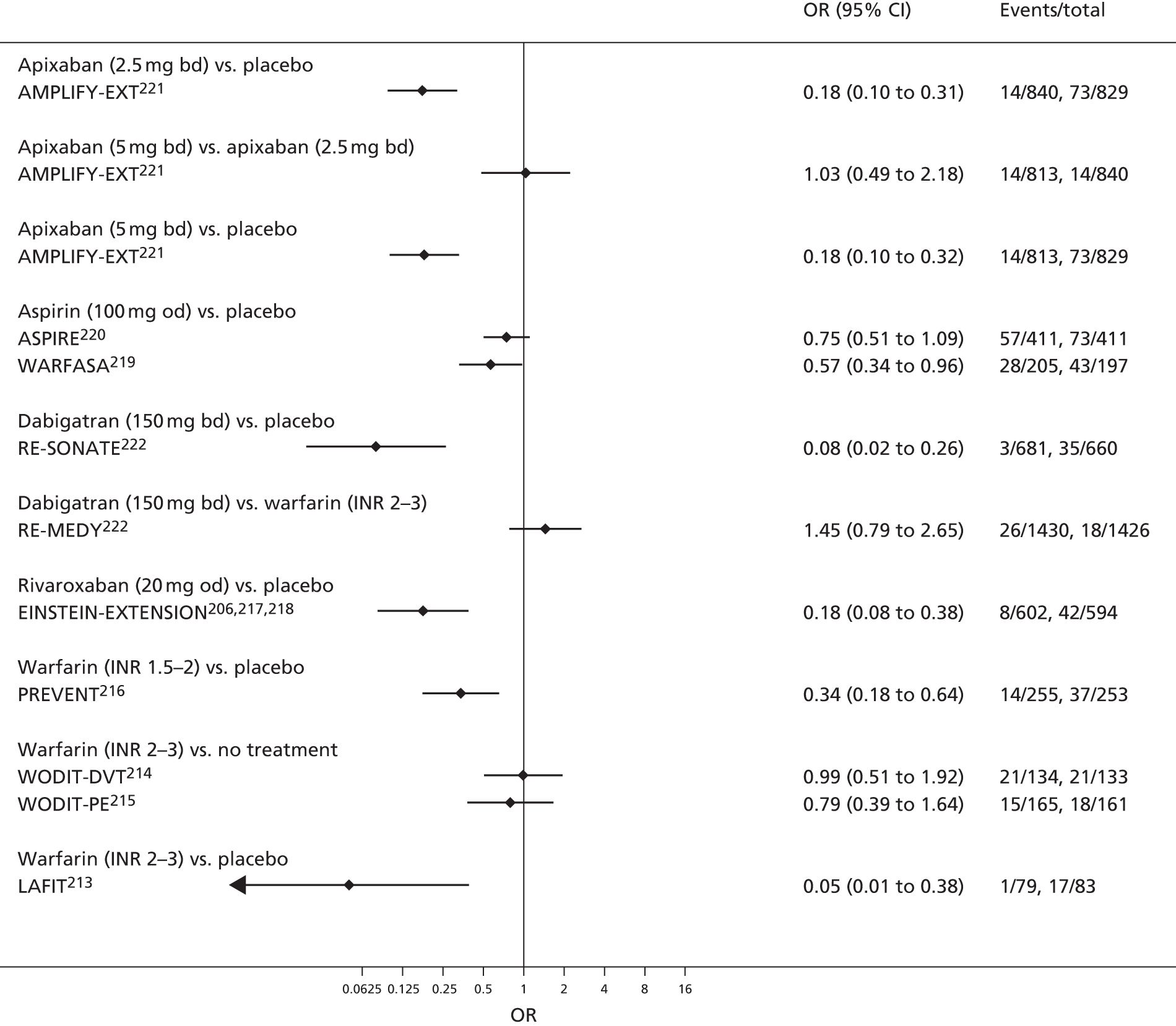
FIGURE 137.
Myocardial infarction (secondary prevention of VTE).

FIGURE 138.
Major bleeding (secondary prevention of VTE).
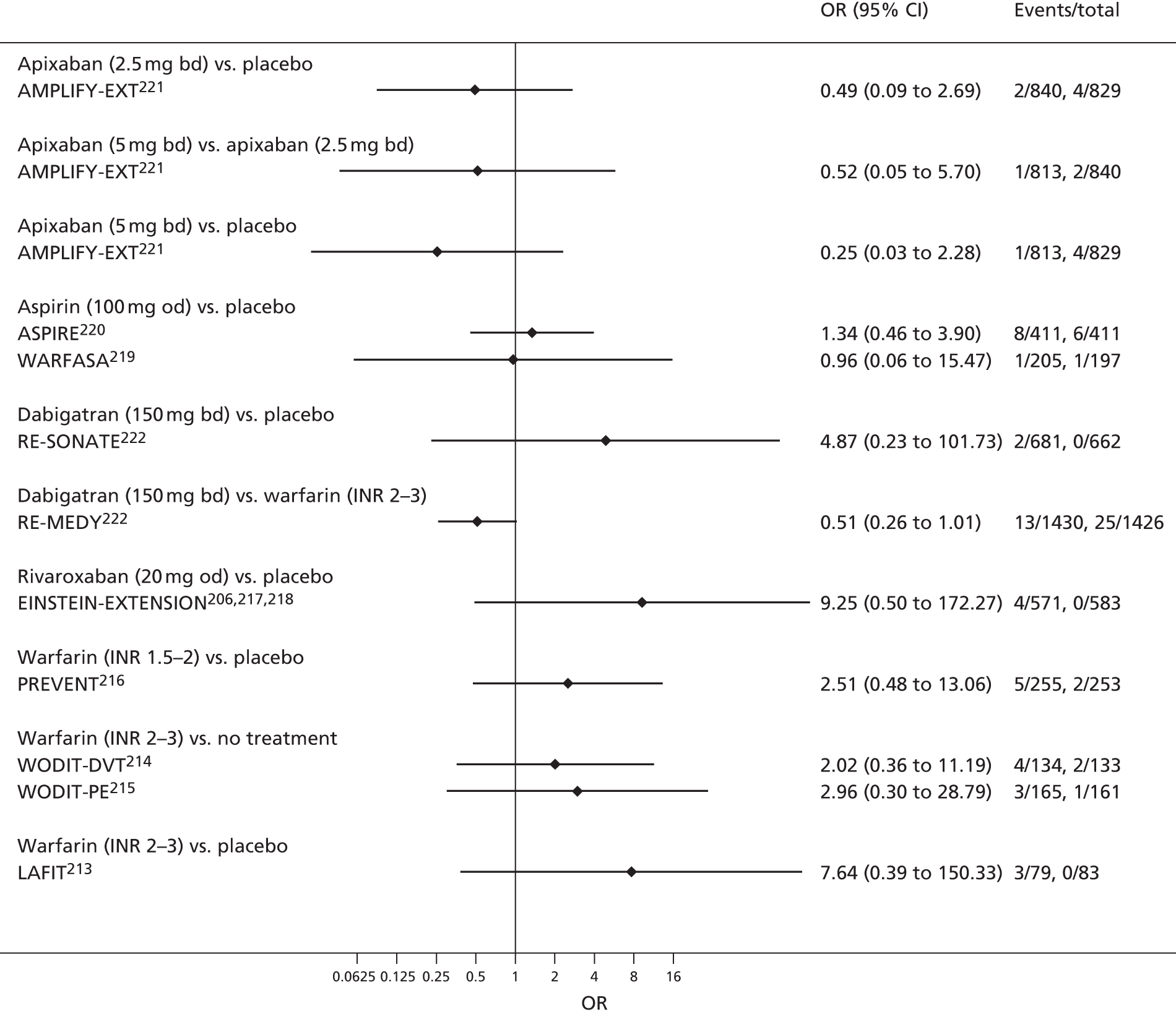
FIGURE 139.
Clinically relevant bleeding (secondary prevention of VTE).
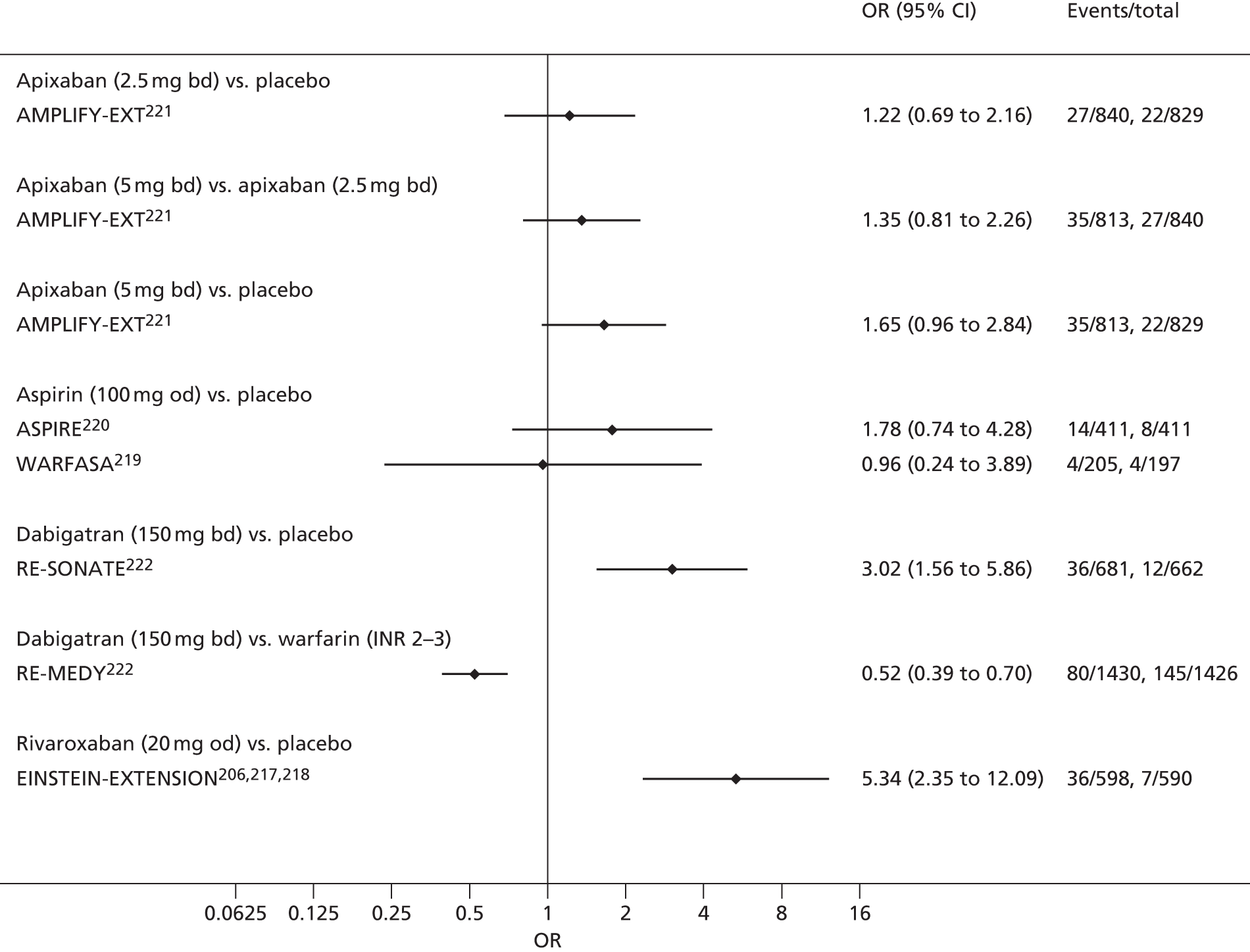
FIGURE 140.
For all-cause mortality (secondary prevention of VTE).

Appendix 6 Discussion of previous economic models
The earliest model we identified was developed by Gage et al. 31 in 1995. This used a Markov model comparing warfarin, aspirin and no therapy. States, such as ‘second stroke’, were used to record the event history of patients, and this history had an effect on risks of future events, costs and utilities. Strokes, ICH and TIA were included, whereas MI and extracranial bleeds were not. The time horizon was 10 years and the cycle length was 1 month. The RIND state [reversible ischaemic neurological deficit (RIND)] was used to represent recovery from a temporary stroke or TIA. Patients were assumed to switch treatments from warfarin to aspirin if they experienced a bleed, and from aspirin to warfarin if they experienced a stroke. In common with this model, we will adopt a Markov modelling framework with states that record event histories to account for their effect on risks, costs and utilities. In our model, we include all of the events in this model, although we rename haemorrhage as a bleed and model it in more detail, categorised by severity. We also account for the possibility of treatment switching. We also model the general RIND state in more detail, recording specific event histories, such as history of both MI and stroke.
One of the first published models in the UK setting was that by Lightowlers and McGuire32 in 1998. They used a very simple decision tree, with a 10-year time horizon, to assess the cost-effectiveness of different monitoring strategies for warfarin, compared with each other and ‘no treatment’ for over 75-year-olds. They included bleeding as an AE but made the simplifying assumption that it was roughly twice as likely in the warfarin group as in the no-treatment group. Our model will be more sophisticated than this decision tree approach. Our Markov model structure will allow us to evaluate lifetime cost-effectiveness as we can account for recurring events and long-term treatment effects, costs and utilities.
Recently, some more complicated model structures have been explored. The Bayer submission to NICE on rivaroxaban in 2011 used a 22-state Markov model to compare treatments for non-valvular AF in the 73-year-old population in the UK,33 similar to our target population. The cycle length for the Markov model was 3 months and, as in our model, they used a life-time time horizon. The model accounted for treatment switching and for discontinuation of treatment, both of which be accounted for in our model. In a similar fashion to our model and that of Gage et al. 199531 patient history was accounted for by memory states, such as the ‘post minor stroke’ states. Unlike in Gage et al. 199531 ICH and minor/major bleed were distinguished and, under clinical advisement, this is a distinction we will also make. The model also separated SE and stroke, a distinction that we will adopt. The evidence used to inform the model was a mixture of trial data (ROCKET AF106,112,123,129) and the results of a Bayesian NMA comparing rivaroxaban 20 mg od, dabigatran 110 mg, dabigatran 150 mg, warfarin, aspirin and placebo. We do not have access to individual patient trial data but will use a Bayesian NMA of aggregate data from RCTs to inform a majority of our model’s transition probabilities.
Several recent publications have been largely based on the template set down by Gage et al. 31 The models by Shah and Gage in 2011,34 Freeman et al. 2011,35 two by Lee et al. 201236,37 separately looking at rivaroxaban and apixaban, and by Harrington et al. 201338 all used a similar structure to Gage et al. 31 but with updated input evidence, extra states and different treatments. As in our model, these models used longer time horizons (up to 35 years). Owing to the availability of superior data, some of these models used shorter cycle lengths (2 weeks). They additionally used TIA itself rather than RIND to represent a non-disabling minor stroke, a choice that we will adopt in accordance with clinician advice, and some of the models included a MI event, using evidence of adverse treatment effect on MI rate of dabigatran 110 mg and 150 mg compared with warfarin from the RE-LY trial104,109 and the Framingham study. 243 As in the study by Gage et al. ,31 memory states were used to record event histories but these models also included a history of both stroke and ICH, a choice we will extend by including states with a history of up to four events (stroke, bleed, ICH and MI). Kamel et al. 39 used a similar structure to the Lee et al. models,36,37 with evidence from the ARISTOTLE trial,107,114,119,124–127,130,132–134 but investigated apixaban and warfarin for the prevention of only secondary stroke. Our model will be interested in primary, secondary, and any subsequent stroke, so this is not a model of particular interest. The Harrington et al. study,38 in the USA setting, is the latest of this series of models, and its parameters are based on the results of the ARISTOTLE,107,114,119,124–127,130,132–134 RE-LY104,109 and ROCKET AF106,112,123,129 studies of the NOACs.
A highly complex model was published by the Canadian Agency for Drugs and Technologies in Health (CADTH) comparing rivaroxaban, dabigatran and apixaban with each other and with warfarin in the Canadian setting. As in our model and previous AF models, this was a Markov model. 40 The model used a cycle length of 3 months and base-case time horizon of 40 years. CADTH analysed populations that were stratified by risk of stroke, assessed by CHADS2 and by age (< 75 and ≥ 75 years), and allowed event rates to vary with the age of the cohort: an important feature that we will adopt for our model. A difference from our model is that CADTH included fatal and non-fatal PE, an event that we will not include as clinical advice was that PE was not an AF treatment. The CADTH model was informed by a broad evidence base, combining results from RE-LY,104,109 ARISTOTLE107,114,119,124–127,130,132–134 and ROCKET AF106,112,123,129 via a NMA conducted in both the Bayesian and frequentist setting.
Wisloff et al. 41 used a decision tree followed by an eight-state Markov model to compare dabigatran, apixaban and rivaroxaban with warfarin for populations with a range of ages in the Norwegian setting. The model used eight health states, notably including gastrointestinal bleeding as the only possible bleed type. We grouped all CRB events as clinical advice was that they would have similar sequelae and effects on risks of future bleeds and other events. The cycle length of the Wisloff model41 was 12 months, as shorter cycle lengths of only 1 month led to spurious results, most likely due to limited data, although the model was based on the results of ROCKET AF, RE-LY and ARISOTLE. A lifetime time horizon was used but a cut-off at 105 years was imposed. Our model will adopt a similar cut-off at 100 years. The Wisloff study41 is significant, as it was one of the few to conduct a VOI analysis.
Discrete event simulation was used by Pink et al. 46 in 2011 as an alternative to Markov modelling. This modelled similar events to our model, including stroke, MI, ICH, TIA and major bleeding, and simulated 50,000 individuals over a lifetime time horizon in the UK to compare dabigatran and warfarin. The model primarily used the RE-LY trial104,109 to inform its parameters. Although discrete event simulation has the advantage over Markov models of modelling events in continuous time and modelling individual patients, we decided that this extra level of detail was unnecessary and that the available data were, in any case, insufficient.
A recent model of dabigatran for stroke prevention in AF in the UK setting was published by Kansal et al. 42 in 2012. This was a Markov model, which built on a previous model by Sorensen et al. 244 This model used a 3-month cycle length and lifetime time horizon, with a cut-off at 100 years, as in our model. The model used a NMA of Roskell et al. 245 to inform its clinical parameters. Although we will use a separate NMA and other long-term sources for clinical parameters, the costs and utilities in our model will largely follow those used in this Kansal et al. model,42 although we will update or inflate to today’s prices where possible. Kansal et al. 42 found that dabigatran was both more effective and less costly than warfarin for the prevention of stroke in AF, although they assumed that dabigatran did not require monitoring and the results may be very sensitivity to this assumption.
Appendix 7 Competing risks network meta-analysis for hazard ratios of events
All event types reported in the systematic literature review must be included to account for correlation and competing risks, giving a total of 17 types of events, although all trials report only a subset of these events:
-
ischaemic stroke
-
bleeding
-
minor bleeding
-
fatal bleeding
-
MI
-
death (all causes)
-
TIA
-
fatal stroke
-
composite CRB
-
hospital admission
-
death (cardiovascular)
-
arterial event
-
PE
-
extracranial minor bleeding
-
SE (obtained by subtracting ‘All stroke’ from ‘Stroke or SE’ in trials that report both)
-
intracranial bleeding (ICH) (to which we added haemorrhagic stroke, under clinical advice)
-
CRB (a combination of major bleeding and CRNM bleeding).
Events of interest to our model are death (‘All causes’), MI, TIA, CRB, ischaemic stroke, SE and ICH.
In all of the following models, λi is the rate of events of type i, which is modelled on the log-scale. The data are reported in three different ways, which we describe, in turn, below. The interpretation of the λi’s is the same across different data types and can be estimated in a shared parameter model.
For each study j, arm k, and outcome i, the log of the hazard λjki is related to the study-specific baseline hazard µji and log-HR of the treatment in arm k (tjk) relative to the treatment in arm 1 (tj1): log(λjki)=µji+dtjki−dtj1i.
The baseline hazards µji are treated as nuisance parameters and vague priors are placed on them: µjiN(0,0.0001).
Vague priors are also placed on the log-HRs for all outcomes i and treatments t: dti ∼ N(0,0.0001)
Number of first events
Here, only the first event is recorded for each individual, and they are assumed censored at the point at which the first event occurs. The outcomes are therefore competing risks, and need to be modelled jointly.
Let r1, r2, . . ., rm be the number of individuals with first event being of type i, for i = 1, . . ., m, and R=∑i=1mri.
Let E be the observed person-years at risk. Then the likelihood is:
R∼Po(E∑i=1mλi),
and conditional on R:
(r1,r2,…,rm)∼Multinomial((λ1∑i=1mλ1,…,λm∑i=1mλ1);R).
There are five studies97,98,100,103,105,116,117,121 that report in this format, of which four report the mean follow-up time. The observed person-years at risk can be obtained from the mean follow-up time by multiplying by the number of individuals randomised. In one study,100 median follow-up and also study duration are reported, but not mean follow-up. Median follow-up is just over half that of study duration, owing to censoring. If we assume that mean follow-up is approximately equal to median follow-up then we can obtain the person-years at risk as if mean follow-up were reported.
Number of individuals experiencing at least one event of a given type
Here, the number of individuals experiencing at least one event of a given type are recorded. Each individual may count towards more than one event type, but only once for each event type. We need to consider mortality slightly differently from other event types because this event can happen only once.
Now let ri be the number of individuals with at least one event of type i and rm the number of mortalities.
The likelihood for the number of mortalities is: rm ∼ Po(Eλm).
The likelihood for other events is approximately (assuming an average follow-up time, ri∼Bin(pi,n), for each individual, and number randomised n): ri ∼ Bin(pi,n), where pi is the probability that an individual has one or more event of type i over the follow-up period t¯, giving cloglog(pi) = log(t¯) + log (λi), where cloglog(pi) = log(–log(1 – pi)).
There are 14 studies12,95,99,101,102,106,108,110–113,115,120,122,123,128,129,131 reporting outcomes in this format. Of these, only three studies111,122,128,131 report mean follow-up time, t¯, which can be used in the likelihood as described above. Two studies96,106,112,123,129 report median follow-up time, which we can use if we assume that the mean follow-up time is approximately equal to the median follow-up. One study120 does not report any information on follow-up, and so has to be excluded from the analysis.
The remaining eight studies12,95,101,102,108,110,113,115 report only the study duration, which, we know from those studies reporting both study duration and mean or median follow-up, greatly overestimates mean follow-up time. In studies that report both, the mean follow-up time, as a proportion of the study duration, ranges from 36% to 69%. We used a prior for this proportion, π, then set t¯ = π t (where t = study duration). This allowed us to include these studies but reflected our uncertainty in the mean follow-up time.
Total number of events
Here we have total number of events of type i for given person-years at risk E.
Now let ri be the number events of type i, including repeat events within individuals.
The likelihood is ri ∼ Po(Eλi).
There are three studies96,104,107,109,114,119,124–127,130,132–134 reporting results in this format. Of these, one study96 reports mean follow-up time, from which we can derive E. The other two studies104,107,109,114,119,124–127,130,132–134 report median follow-up time, which we can use if we assume mean follow-up time is approximately equal to median follow-up time.
Estimating mean follow-up time from median follow-up time
If censoring follows an exponential distribution then mean = median/log(2) giving mean > median. However, in the only study11 that reports both, they are very similar. This is probably because of the various different censoring mechanisms (mortality, lost to follow-up). We will therefore make the assumption that our analyses can use the median follow-up when the mean follow-up is not available.
Appendix 8 Competing risks model for hazard in warfarin arms of trials
The natural history model on standard care (warfarin, INR 2–3) requires estimates of the baseline log-hazard, rather than HRs, of events of interest. As in the treatment effects NMA, there are three types of outcomes data to be incorporated into the model. The main difference is that a common, random effect, baseline log-hazard for the warfarin arm (labelled 1) is assumed across studies with mi and precision ωi.
For each study j with a warfarin arm and outcome i, the log of the hazard λji is log(λji) = µji.
The trial-specific baseline hazards are related to the across trial baseline hazard: µji ∼ N(mi,ωi).
A vague prior is placed on the mean of baseline hazard: mi ∼ N(0,0.0001).
A vague prior is placed on the precision of the baseline hazard, on the SD scale: 1√ωi∼Uniform(0,5).
The rest of the model is identical to that presented in Appendix 7.
Appendix 9 All-cause mortality minus venous thromboembolism-related mortality data (acute treatment of venous thromboembolism)
| Study | Comparator | n | All-cause mortality minus VTE-related mortality |
|---|---|---|---|
| AMPLIFY210 | Apixaban 2 × 5 mg | 2691 | 0 |
| Warfarin | 2704 | 37 | |
| BOTTICELLI DVT204 | Apixaban 2 × 5 mg | 130 | 3 |
| Apixaban 2 × 10 mg | 134 | 1 | |
| Apixaban 1 × 20 mg | 128 | 1 | |
| Warfarin | 128 | 0 | |
| EINSTEIN DVT206 | Rivaroxaban 2×15 mg (first 21 days), then 1 × 20 mg | 1731 | 36 |
| Warfarin | 1718 | 43 | |
| EINSTEIN DVT dose-ranging study203 | Rivaroxaban 1 × 20 mg | 115 | 4 |
| Rivaroxaban 1 × 30 mg | 112 | 6 | |
| Rivaroxaban 1 × 40 mg | 121 | 1 | |
| Warfarin | 101 | 0 | |
| EINSTEIN PE207 | Rivaroxaban 2 × 15 mg (first 21 days) then 1 × 20 mg | 2419 | 48 |
| Warfarin | 2413 | 44 | |
| HOKUSAI-VTE208,209 | Edoxaban 60 or 30 (17.6%) mg | 4118 | 108 |
| Warfarin | 4122 | 102 | |
| RE-COVER205 | Dabigatran 2 × 150 mg | 1274 | 20 |
| Warfarin | 1265 | 18 | |
| RE-COVER II211 | Dabigatran 2 × 50 mg | 1279 | 22 |
| Warfarin | 1289 | 25 |
Appendix 10 Office for National Statistics life tables stratified by age and gender
| Age (years) | Males | Females |
|---|---|---|
| 55 | 0.0052 | 0.0034 |
| 56 | 0.0059 | 0.0038 |
| 57 | 0.0062 | 0.0042 |
| 58 | 0.0069 | 0.0045 |
| 59 | 0.0074 | 0.0050 |
| 60 | 0.0082 | 0.0054 |
| 61 | 0.0090 | 0.0059 |
| 62 | 0.0098 | 0.0064 |
| 63 | 0.0105 | 0.0068 |
| 64 | 0.0115 | 0.0075 |
| 65 | 0.0124 | 0.0081 |
| 66 | 0.0142 | 0.0092 |
| 67 | 0.0155 | 0.0101 |
| 68 | 0.0167 | 0.0109 |
| 69 | 0.0190 | 0.0123 |
| 70 | 0.0213 | 0.0139 |
| 71 | 0.0235 | 0.0150 |
| 72 | 0.0257 | 0.0169 |
| 73 | 0.0279 | 0.0183 |
| 74 | 0.0311 | 0.0206 |
| 75 | 0.0340 | 0.0228 |
| 76 | 0.0380 | 0.0257 |
| 77 | 0.0420 | 0.0290 |
| 78 | 0.0470 | 0.0327 |
| 79 | 0.0516 | 0.0368 |
| 80 | 0.0581 | 0.0416 |
| 81 | 0.0657 | 0.0471 |
| 82 | 0.0735 | 0.0531 |
| 83 | 0.0817 | 0.0608 |
| 84 | 0.0915 | 0.0685 |
| 85 | 0.1019 | 0.0766 |
| 86 | 0.1130 | 0.0866 |
| 87 | 0.1263 | 0.0959 |
| 88 | 0.1386 | 0.1082 |
| 89 | 0.1570 | 0.1217 |
| 90 | 0.1694 | 0.1392 |
| 91 | 0.1840 | 0.1507 |
| 92 | 0.1974 | 0.1672 |
| 93 | 0.2147 | 0.1792 |
| 94 | 0.2382 | 0.2028 |
| 95 | 0.2594 | 0.2240 |
| 96 | 0.2830 | 0.2455 |
| 97 | 0.3041 | 0.2631 |
| 98 | 0.3240 | 0.2828 |
| 99 | 0.3424 | 0.3056 |
| 100 | 0.3654 | 0.3252 |
Appendix 11 Log-odds ratios relative to low-molecular-weight heparin
| Treatment | Log-OR | 95% CI | Distribution |
|---|---|---|---|
| Apixaban (2.5 mg bd) | –0.05 | –0.63 to 0.52 | MCMC posterior simulations |
| Dabigatran (220 mg od) | –0.02 | –0.60 to 0.56 | |
| Rivaroxaban (10 mg od) | –0.67 | –1.18 to –0.18 |
Appendix 12 Venous thromboembolism sensitivity analyses
Primary prevention of venous thromboembolism: total knee replacement
FIGURE 141.
Cost-effectiveness acceptability frontier for TKR primary prevention sensitivity analysis: pooling post THR and post TKR populations for relative treatment effect of VTE. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
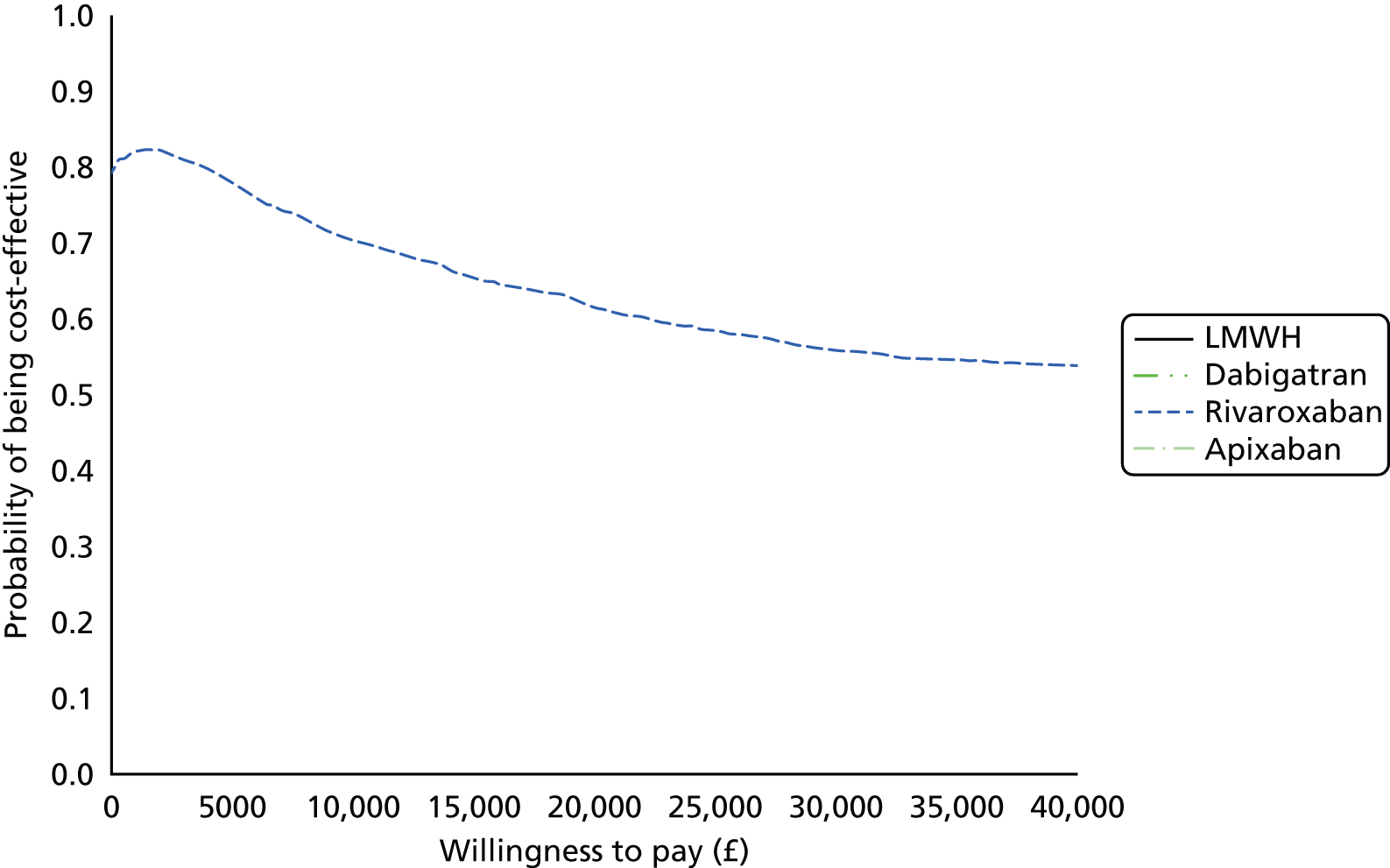
FIGURE 142.
Cost-effectiveness acceptability frontier for TKR primary prevention sensitivity analysis: setting the cost of dabigatran to 150 mg od. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.

FIGURE 143.
Cost-effectiveness acceptability frontier for TKR primary prevention sensitivity analysis: pooling over surgical population for VTE relative treatment effects. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.

FIGURE 144.
Cost-effectiveness acceptability frontier for TKR primary prevention sensitivity analysis: decreasing AE costs by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
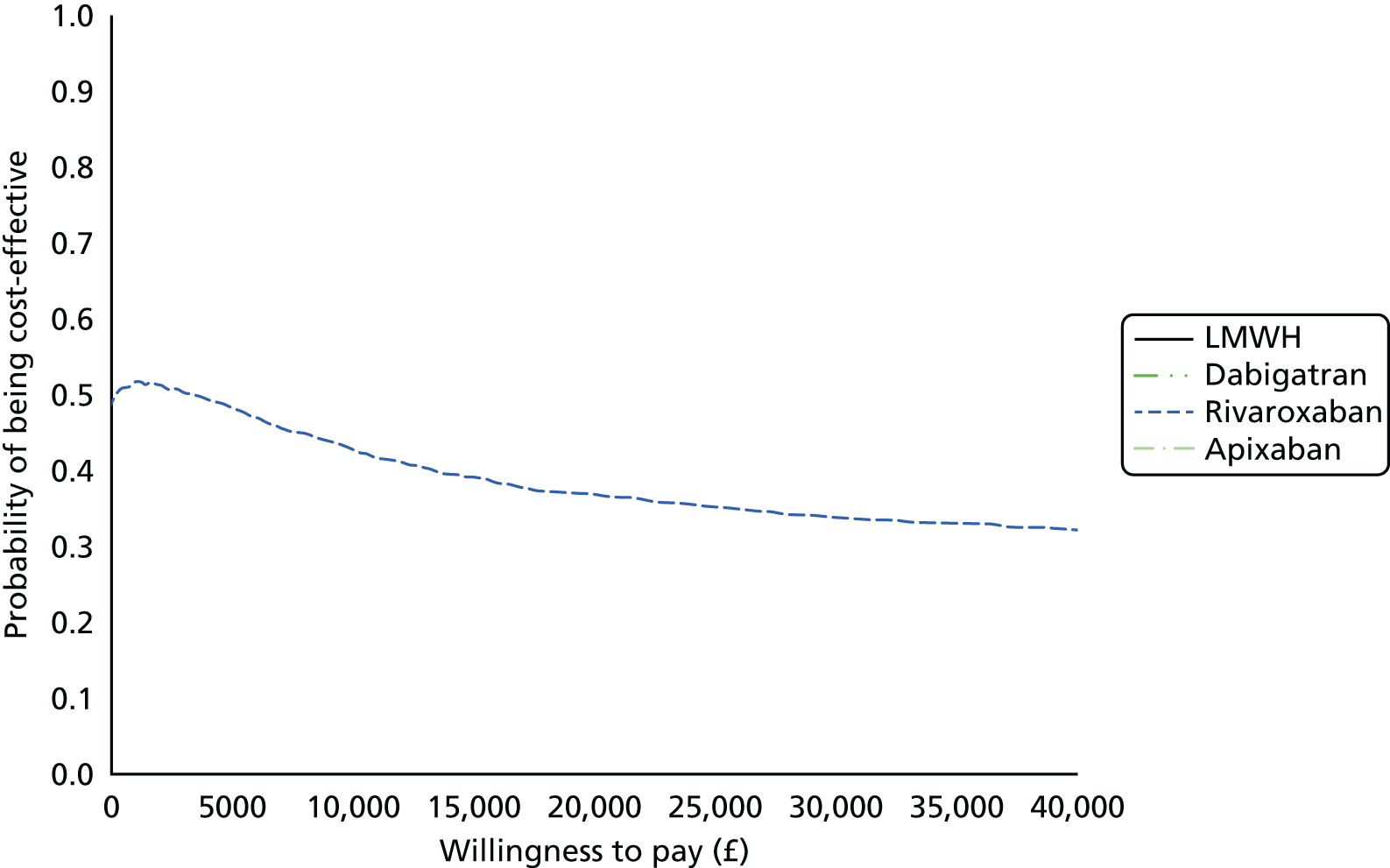
FIGURE 145.
Cost-effectiveness acceptability frontier for TKR primary prevention sensitivity analysis: decreasing AE utilities by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
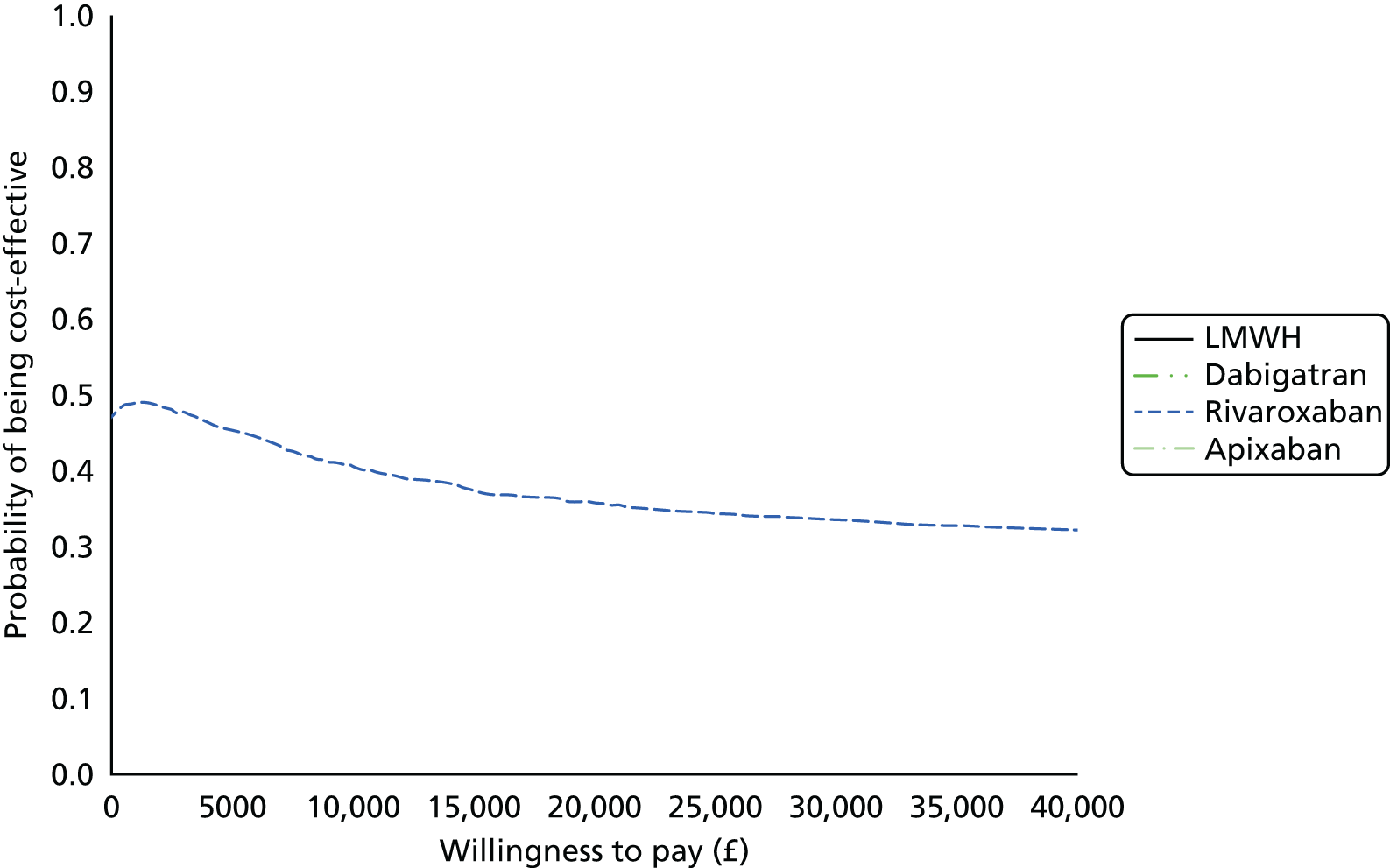
FIGURE 146.
Cost-effectiveness acceptability frontier for TKR primary prevention sensitivity analysis: increasing VTE costs by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
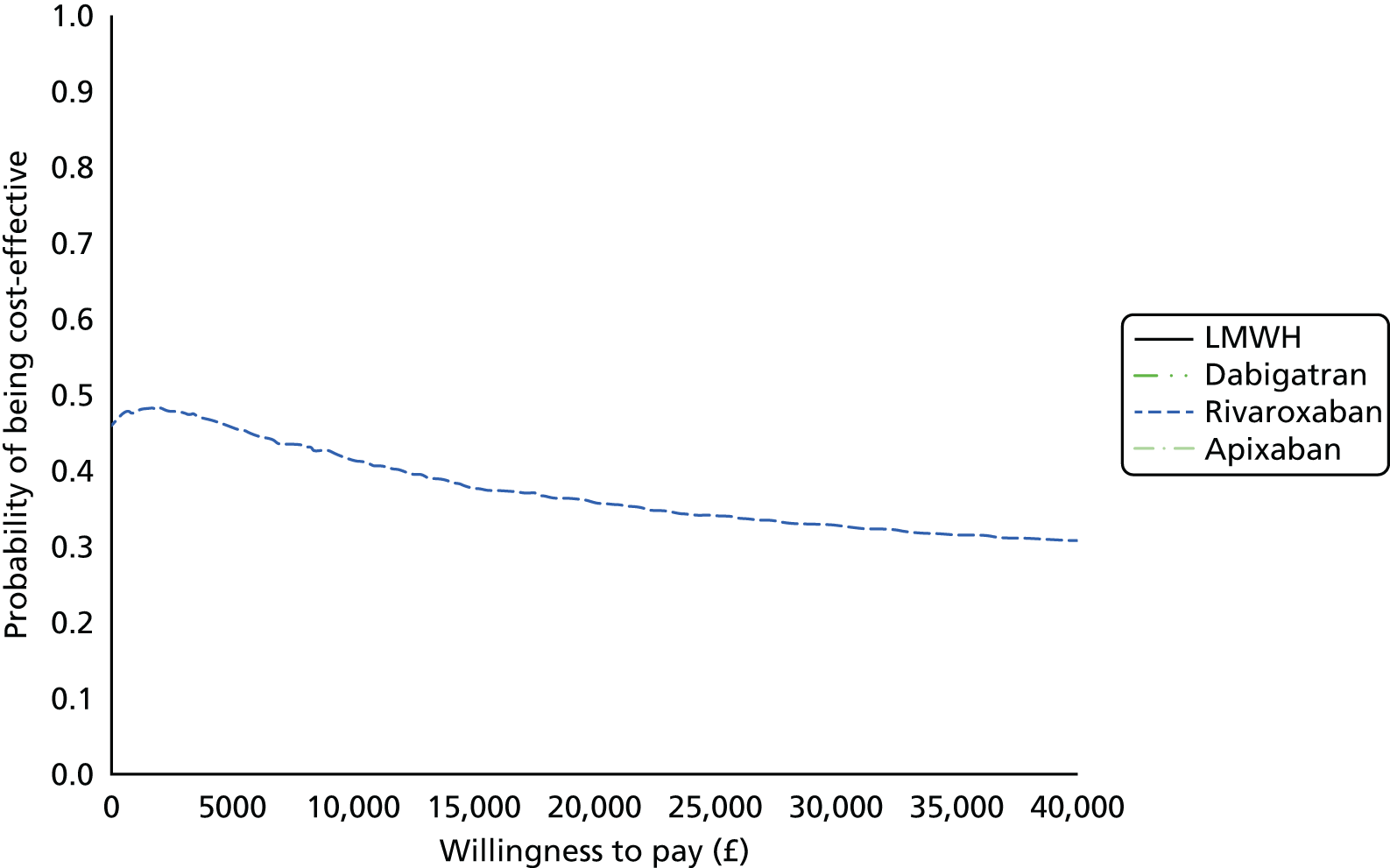
FIGURE 147.
Cost-effectiveness acceptability frontier for TKR primary prevention sensitivity analysis: increasing VTE utilities by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.

Primary prevention of venous thromboembolism: post hip replacement surgery
FIGURE 148.
Cost-effectiveness acceptability frontier for THR primary prevention sensitivity analysis: pooling post THR and post TKR populations for relative treatment effect of VTE. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.

FIGURE 149.
Cost-effectiveness acceptability frontier for THR primary prevention sensitivity analysis: setting the cost of dabigatran to 150 mg od. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
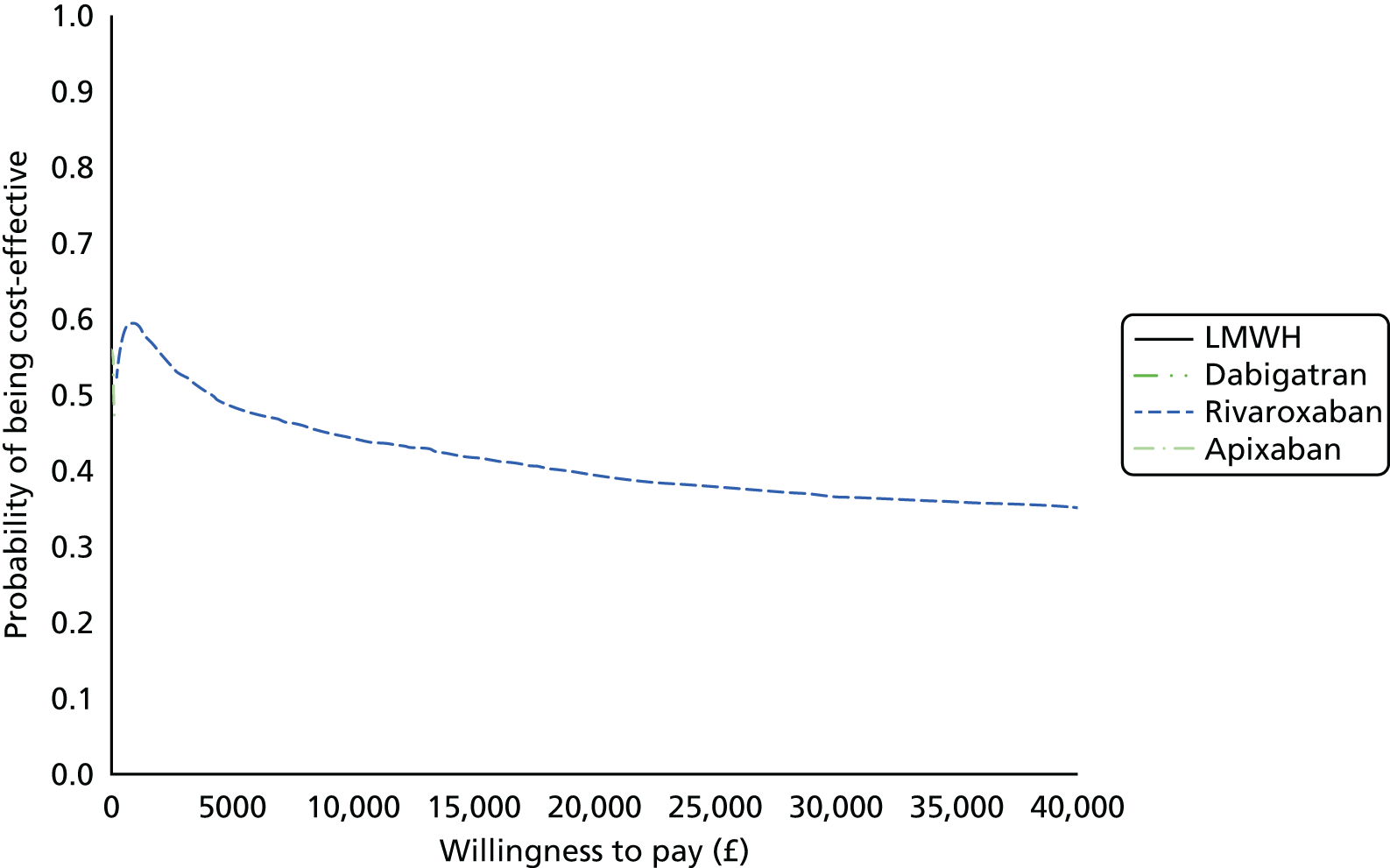
FIGURE 150.
Cost-effectiveness acceptability frontier for THR primary prevention sensitivity analysis: decreasing AE costs by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.

FIGURE 151.
Cost-effectiveness acceptability frontier for THR primary prevention sensitivity analysis: increasing AE costs by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
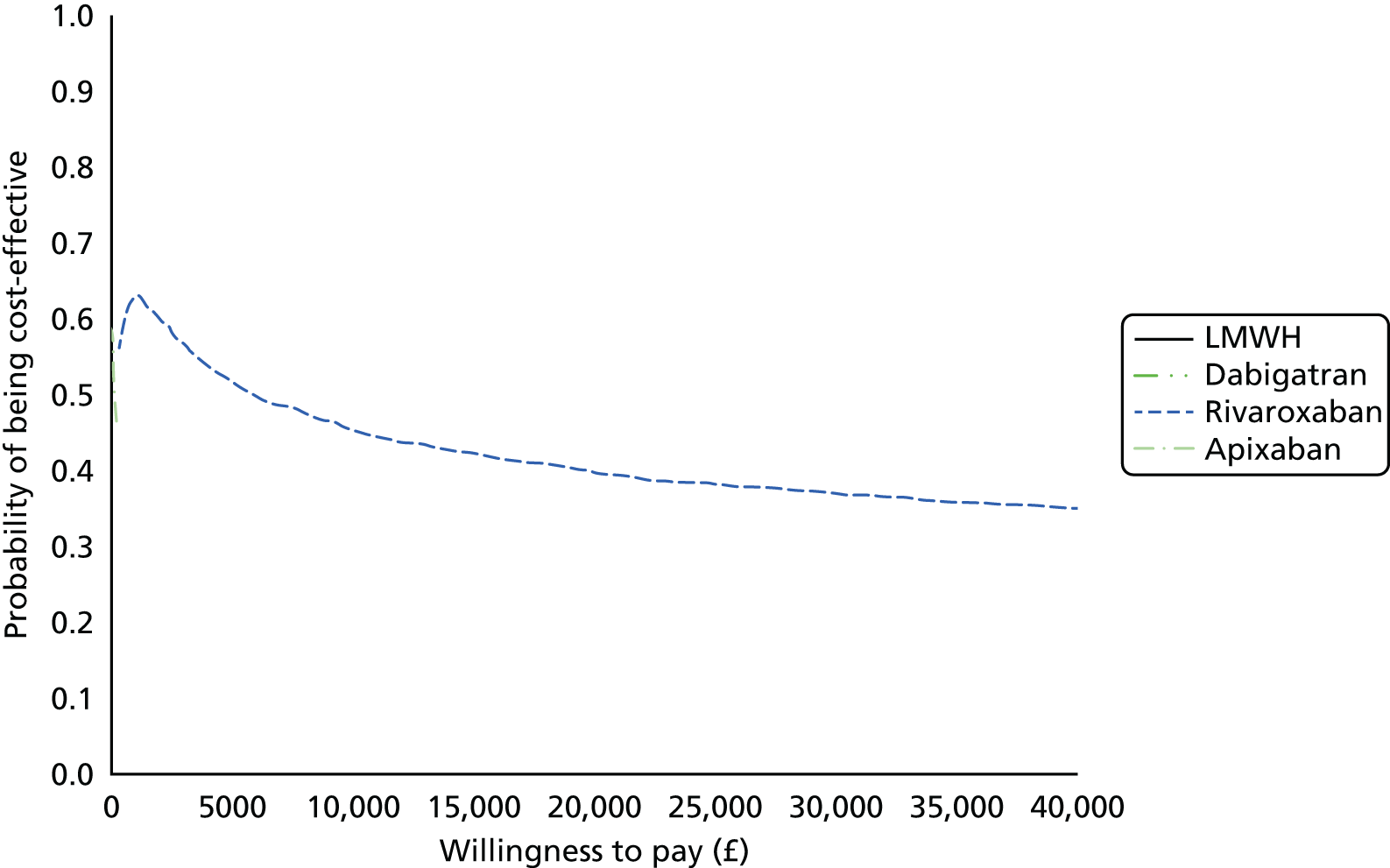
FIGURE 152.
Cost-effectiveness acceptability frontier for THR primary prevention sensitivity analysis: decreasing AE utilities by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
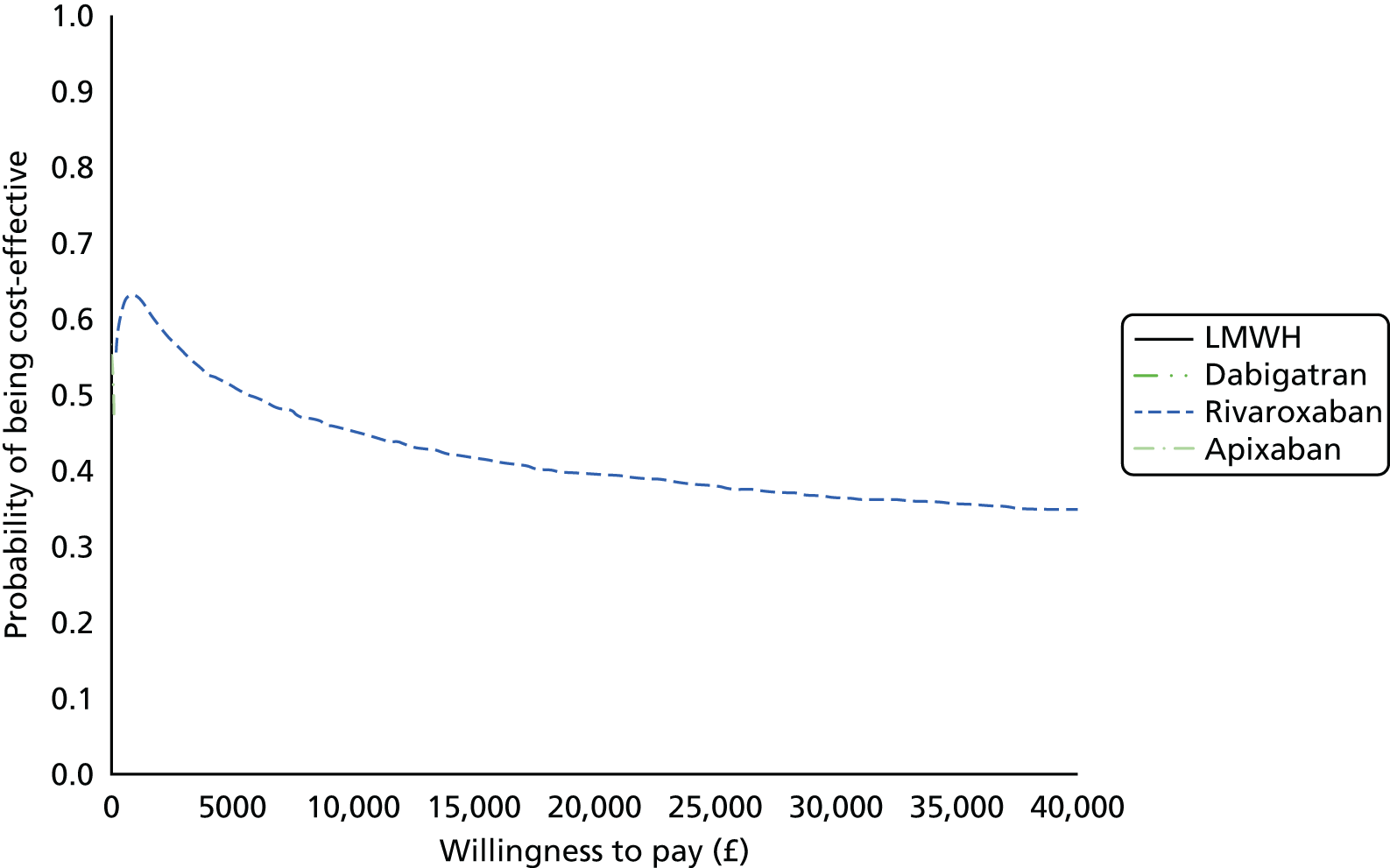
FIGURE 153.
Cost-effectiveness acceptability frontier for THR primary prevention sensitivity analysis: increasing AE utilities by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.

FIGURE 154.
Cost-effectiveness acceptability frontier for THR primary prevention sensitivity analysis: decreasing VTE costs by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
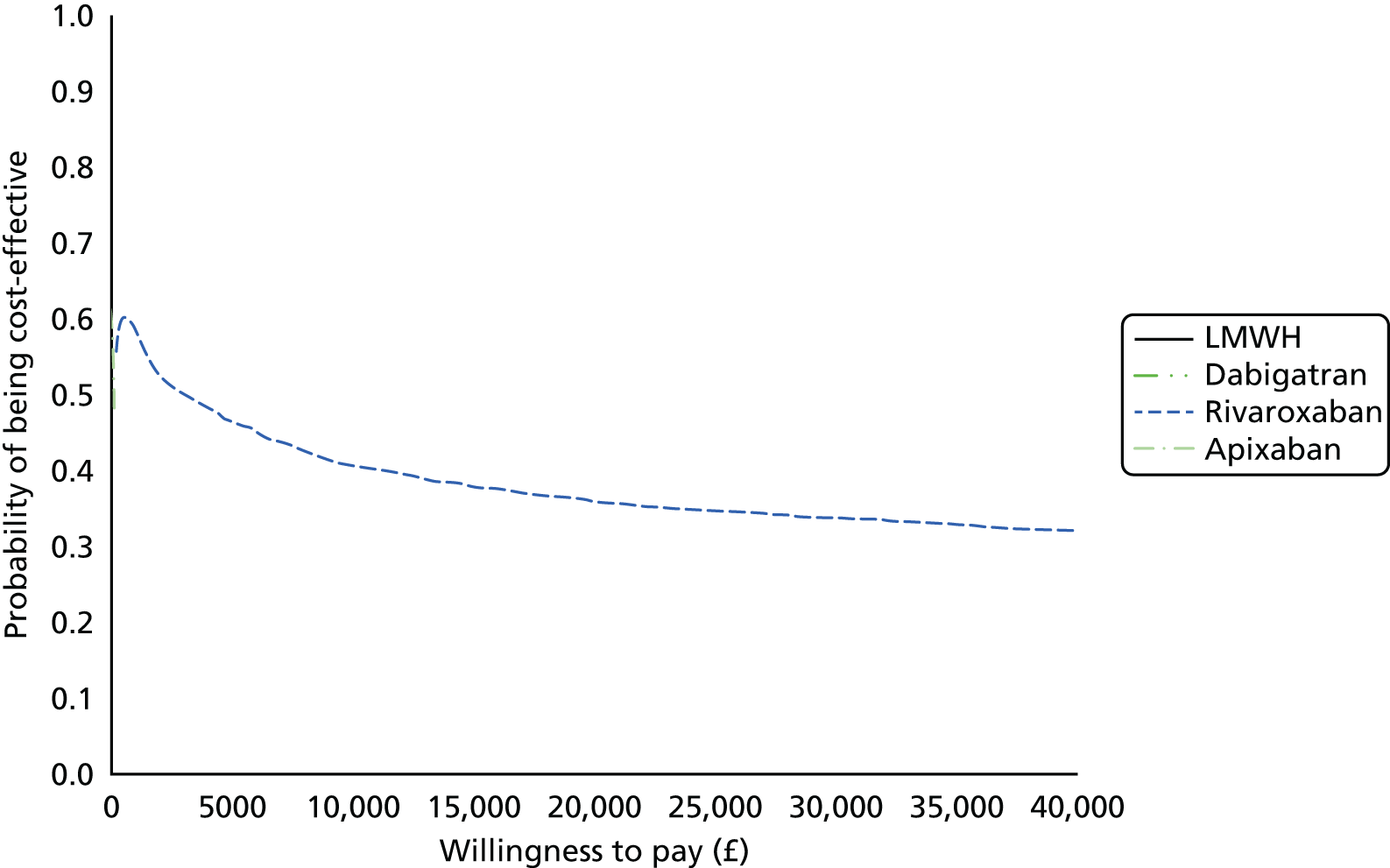
FIGURE 155.
Cost-effectiveness acceptability frontier for THR primary prevention sensitivity analysis: increasing VTE costs by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
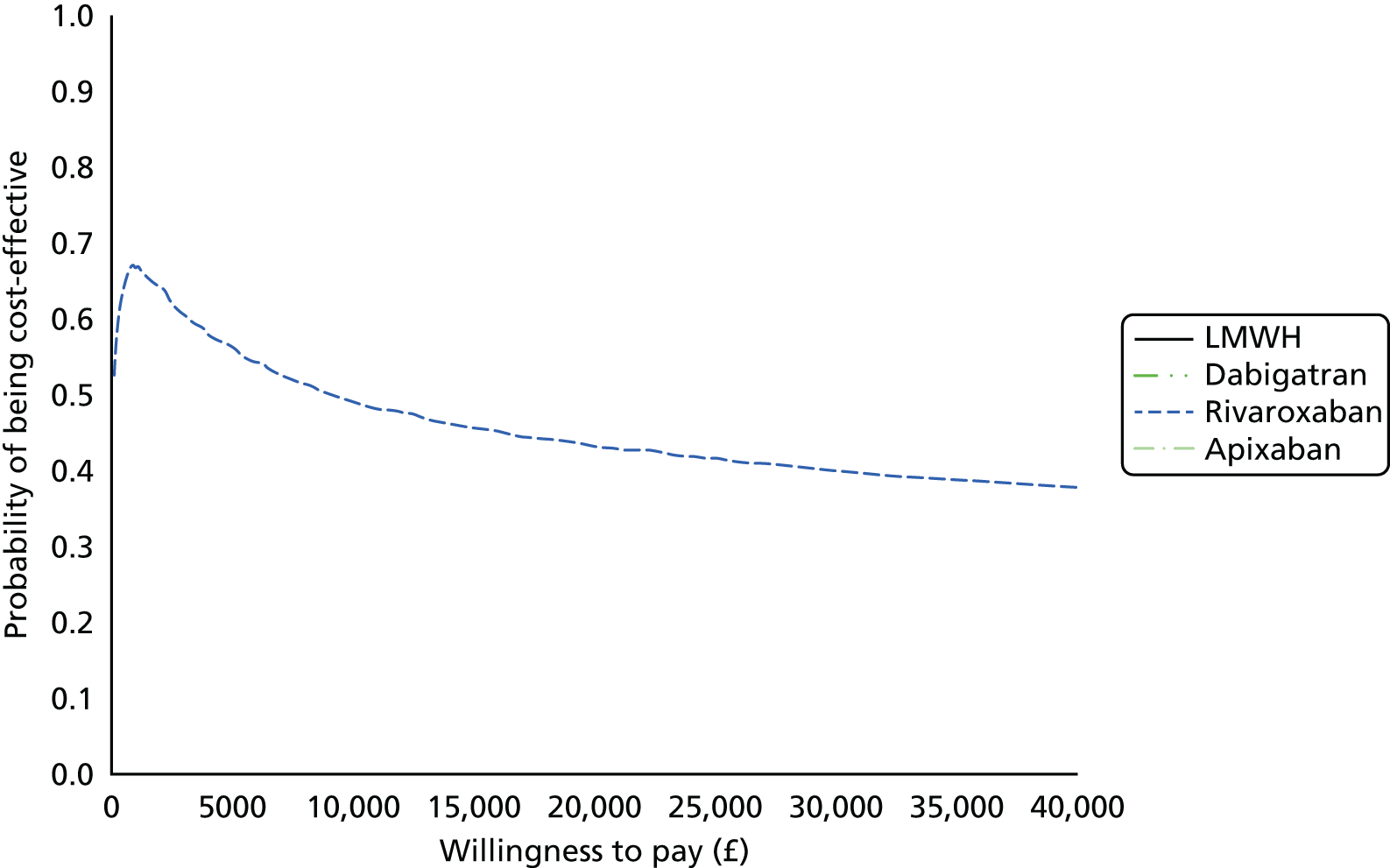
FIGURE 156.
Cost-effectiveness acceptability frontier for THR primary prevention sensitivity analysis: decreasing VTE utilities by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
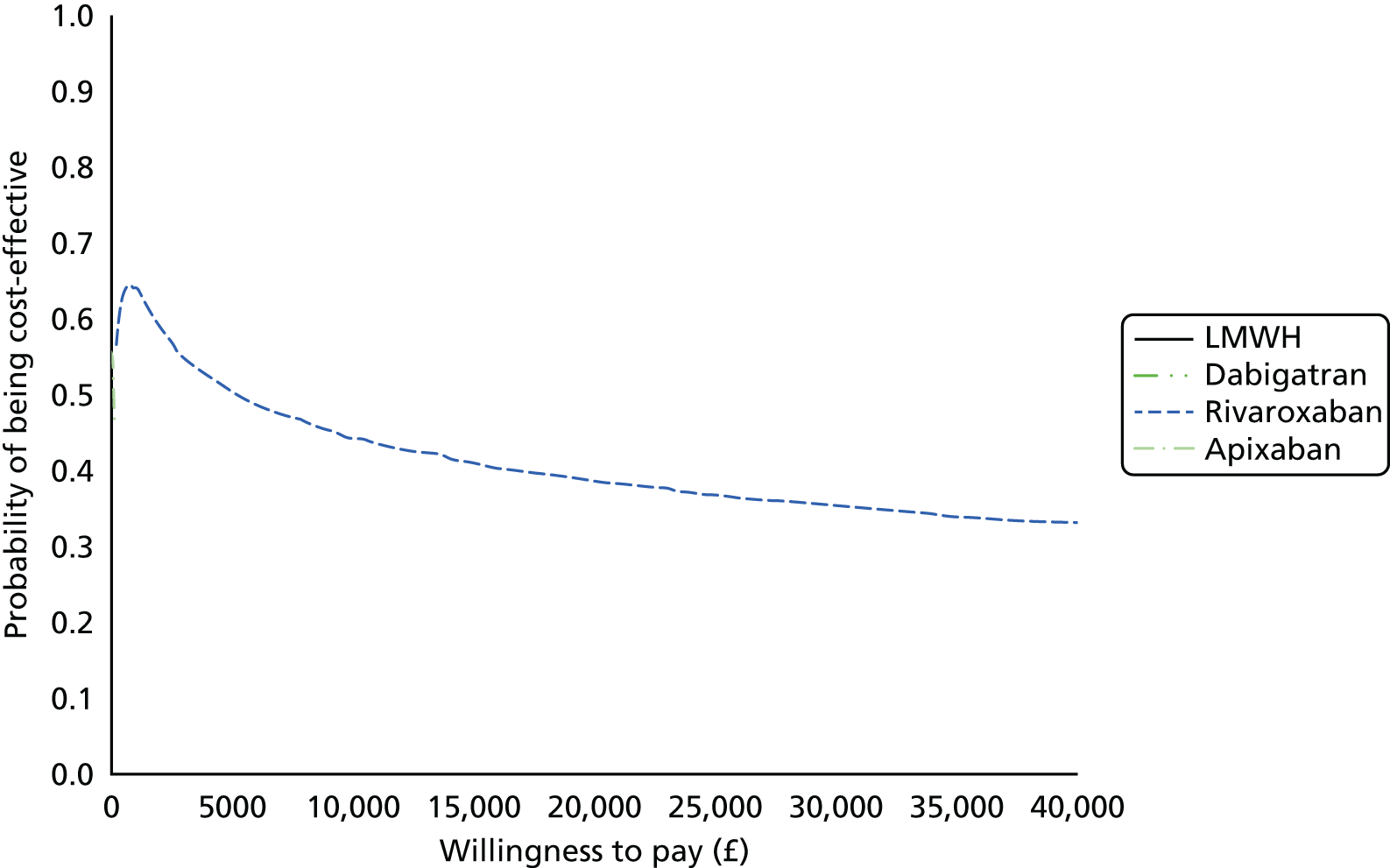
FIGURE 157.
Cost-effectiveness acceptability frontier for THR primary prevention sensitivity analysis: increasing VTE utilities by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
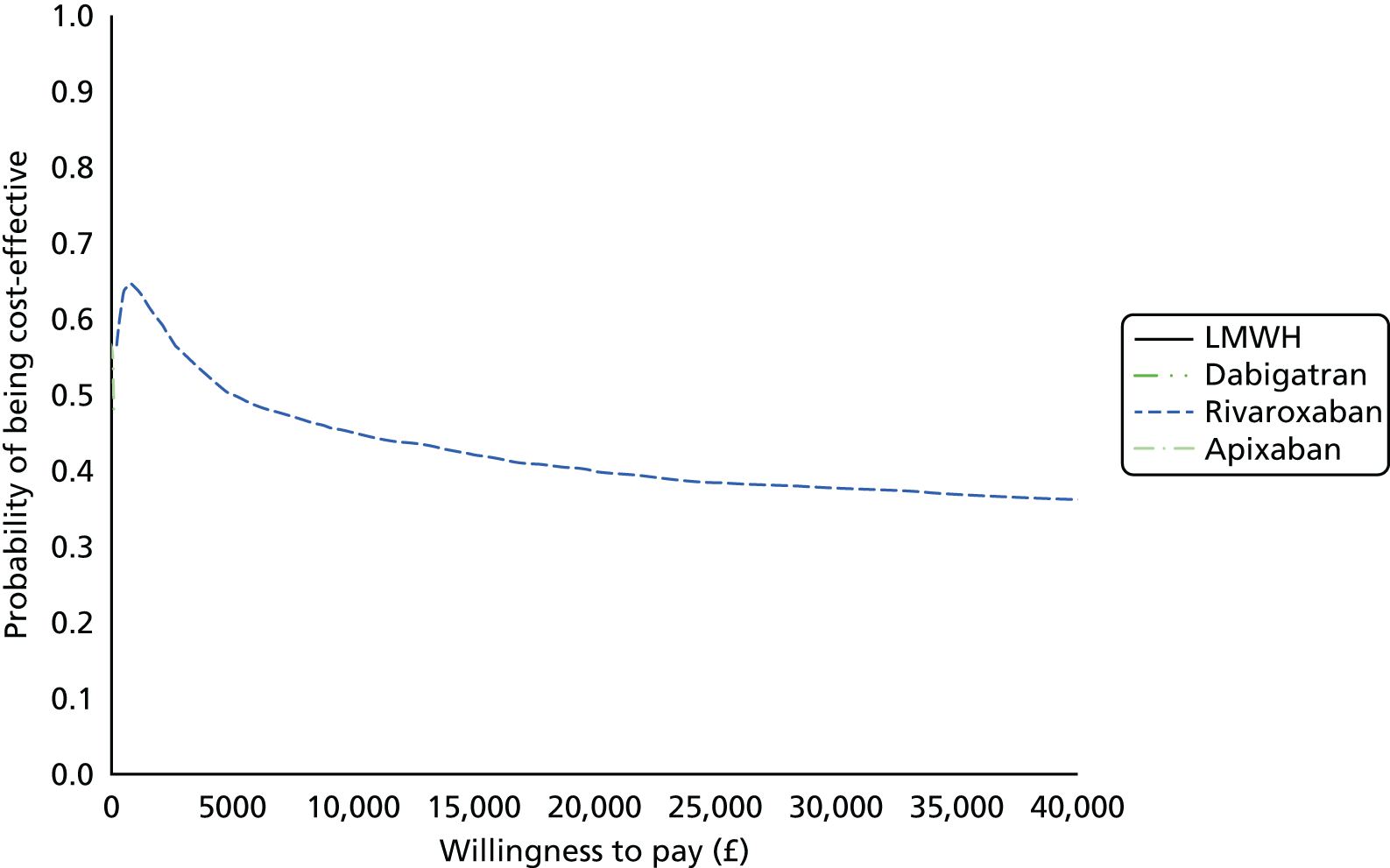
Acute treatment of venous thromboembolism
FIGURE 158.
Cost-effectiveness acceptability frontier for acute treatment sensitivity analysis: decreasing AE cost by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
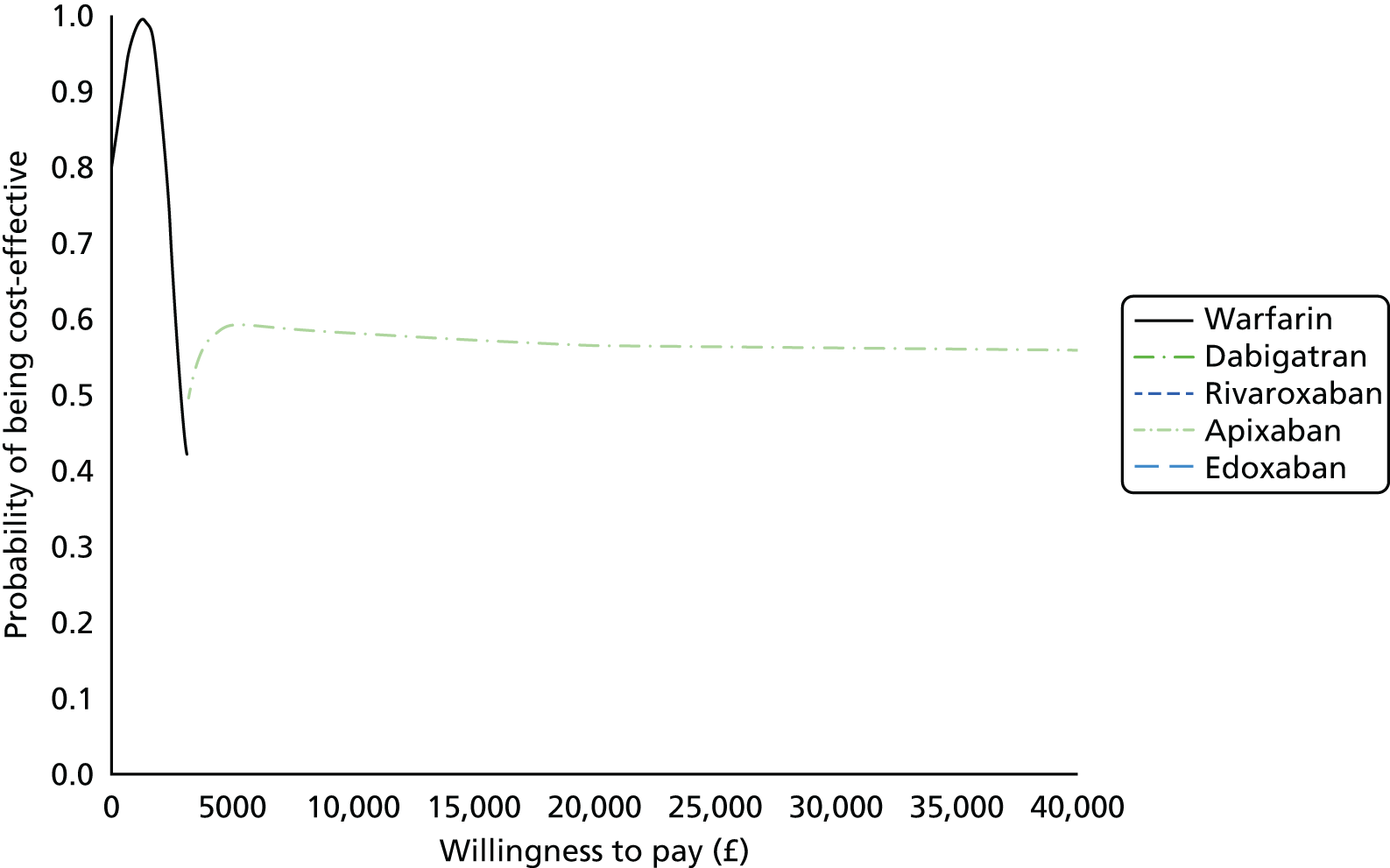
FIGURE 159.
Cost-effectiveness acceptability frontier acute treatment model: increasing AE costs by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
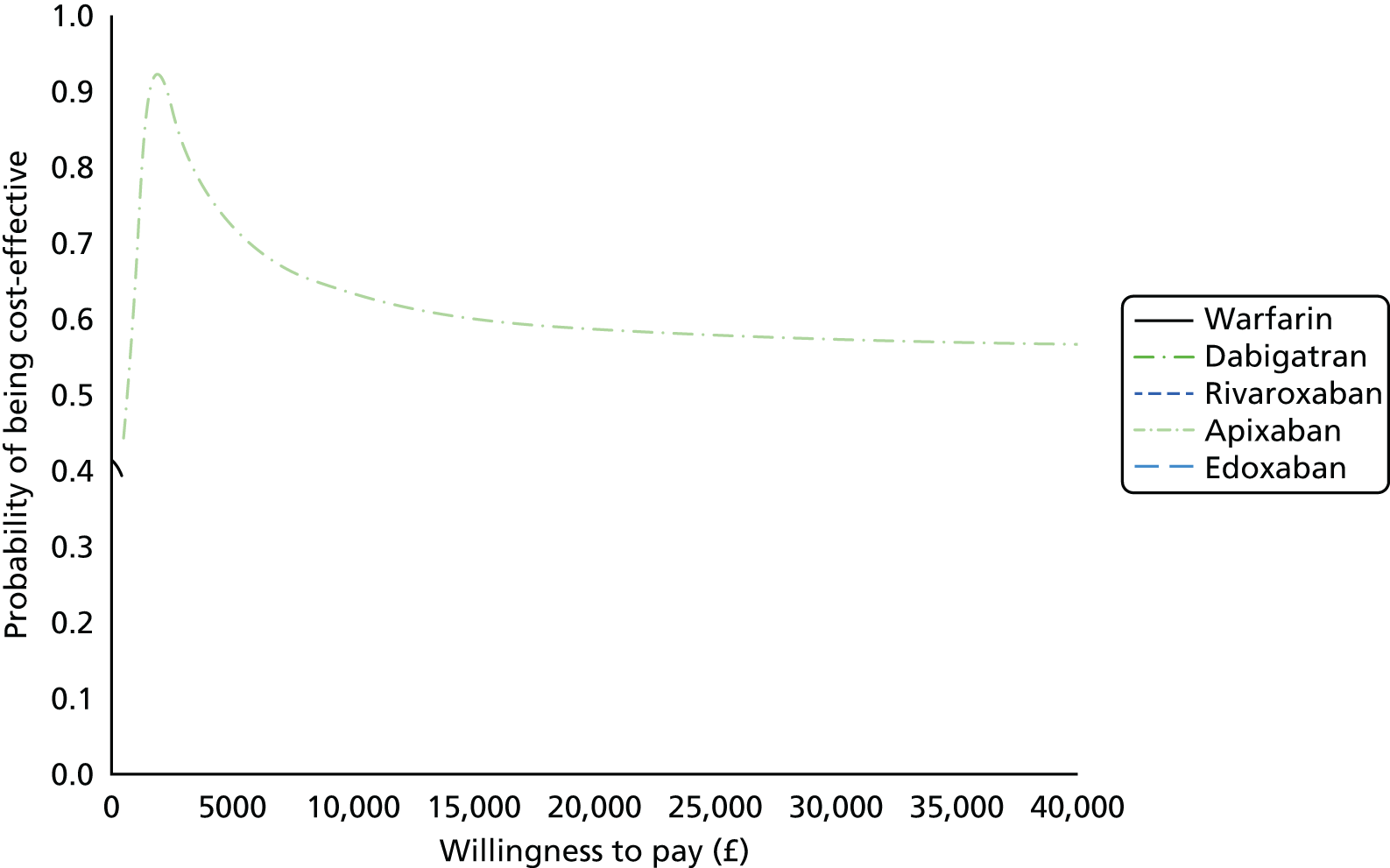
FIGURE 160.
Cost-effectiveness acceptability frontier for acute treatment sensitivity analysis: decreasing AE utility by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
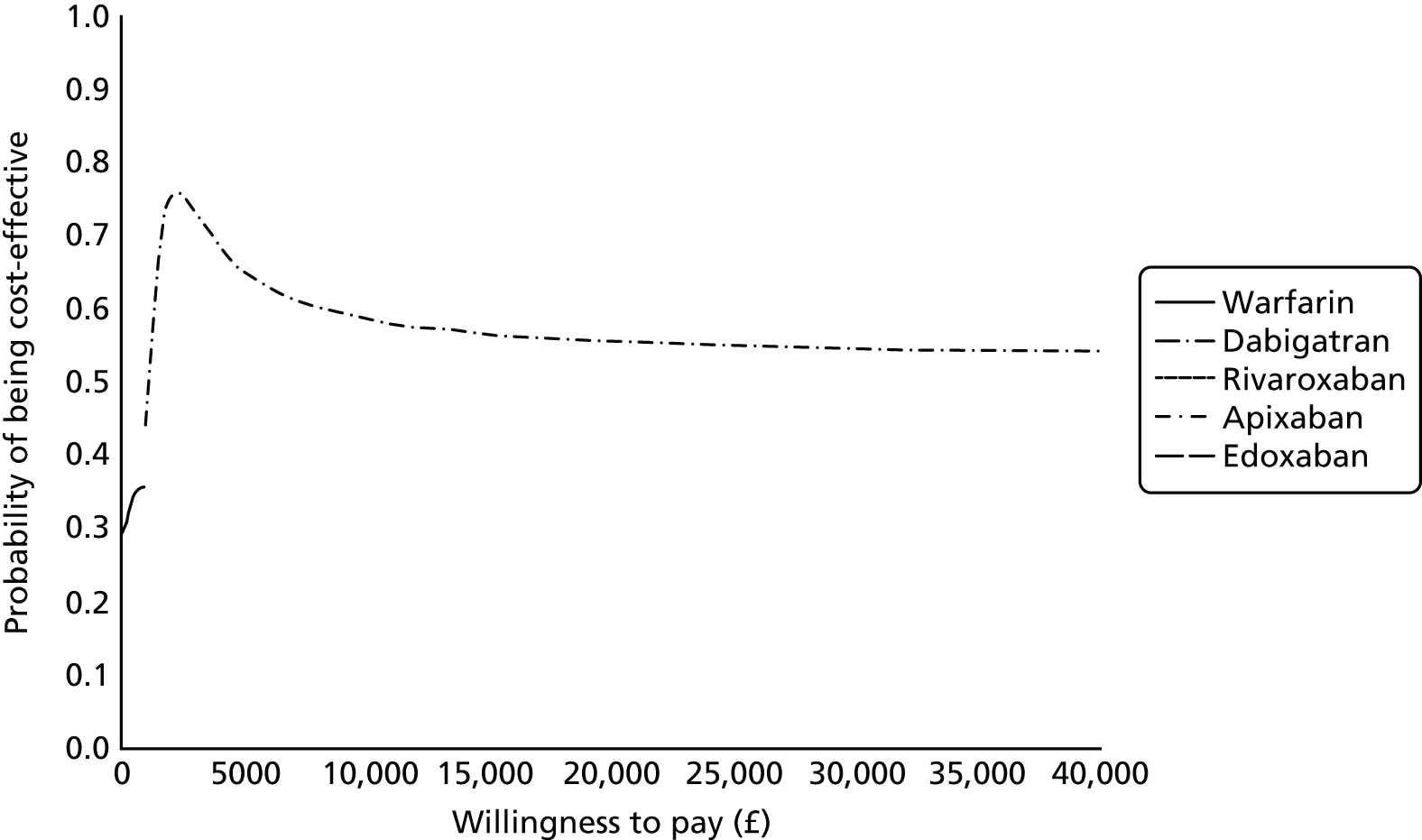
FIGURE 161.
Cost-effectiveness acceptability frontier for acute treatment sensitivity analysis: increasing AE utility by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
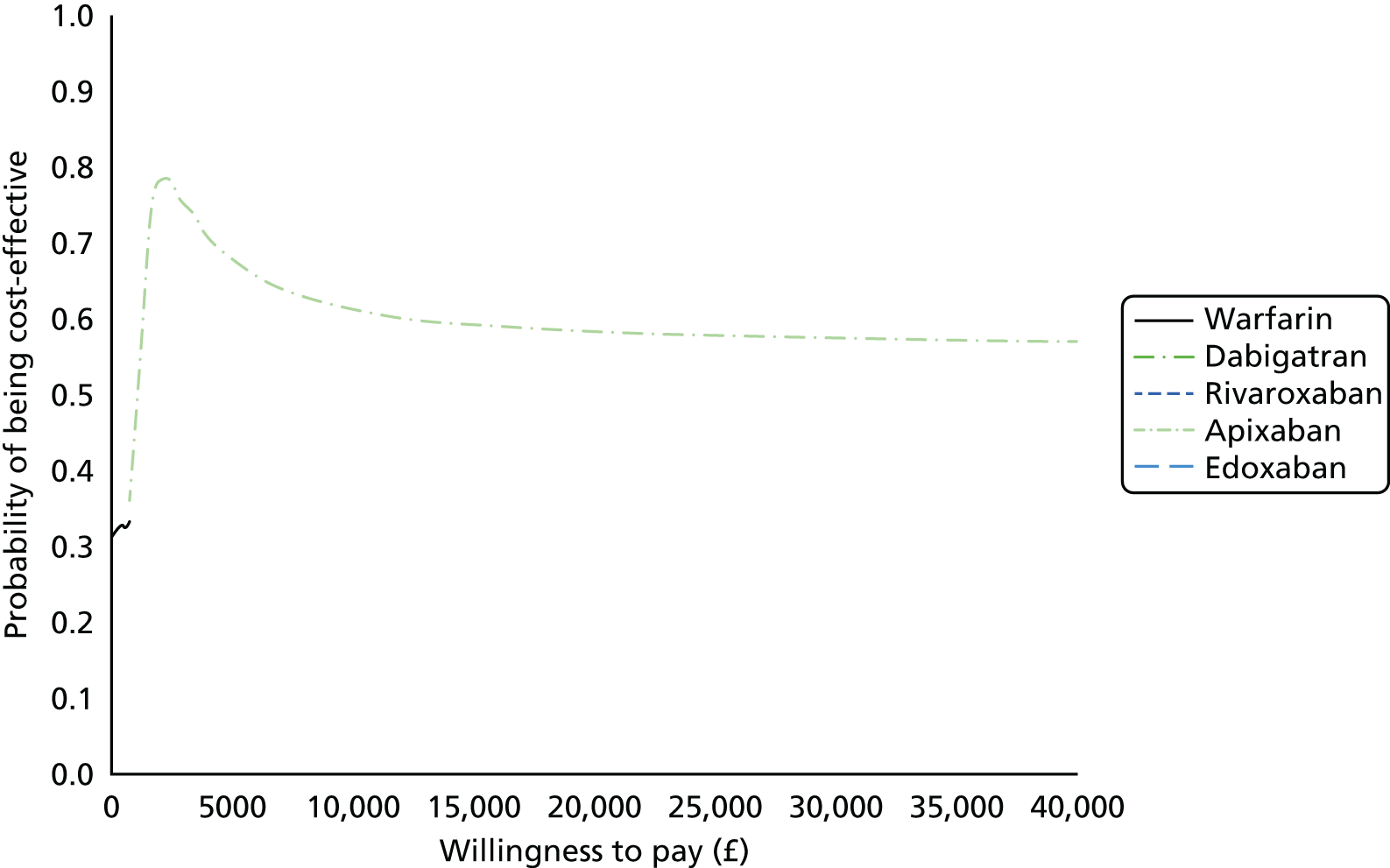
FIGURE 162.
Cost-effectiveness acceptability frontier for acute treatment sensitivity analysis: decreasing VTE cost by 50%.
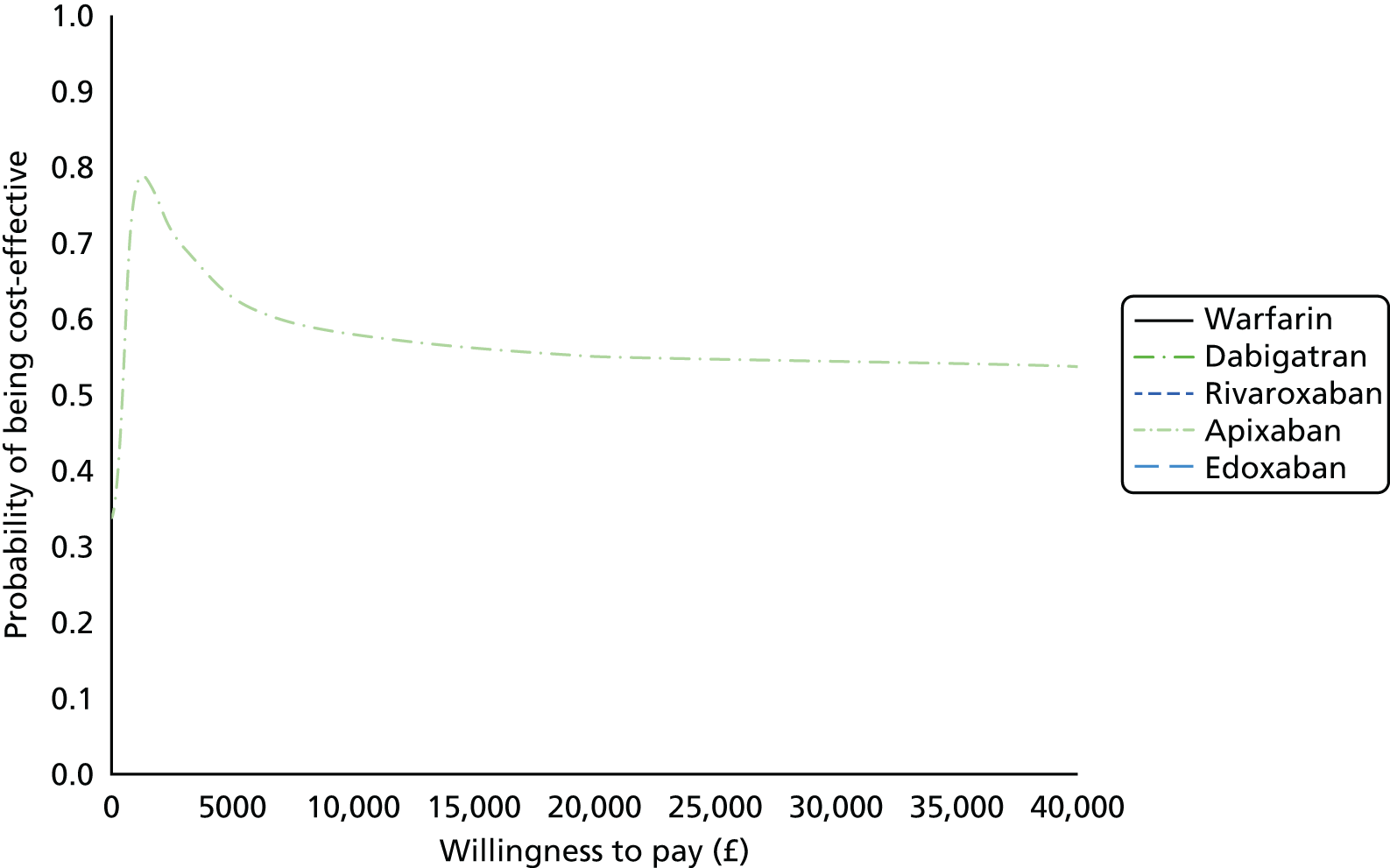
FIGURE 163.
Cost-effectiveness acceptability frontier for acute treatment sensitivity analysis: increasing VTE cost by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
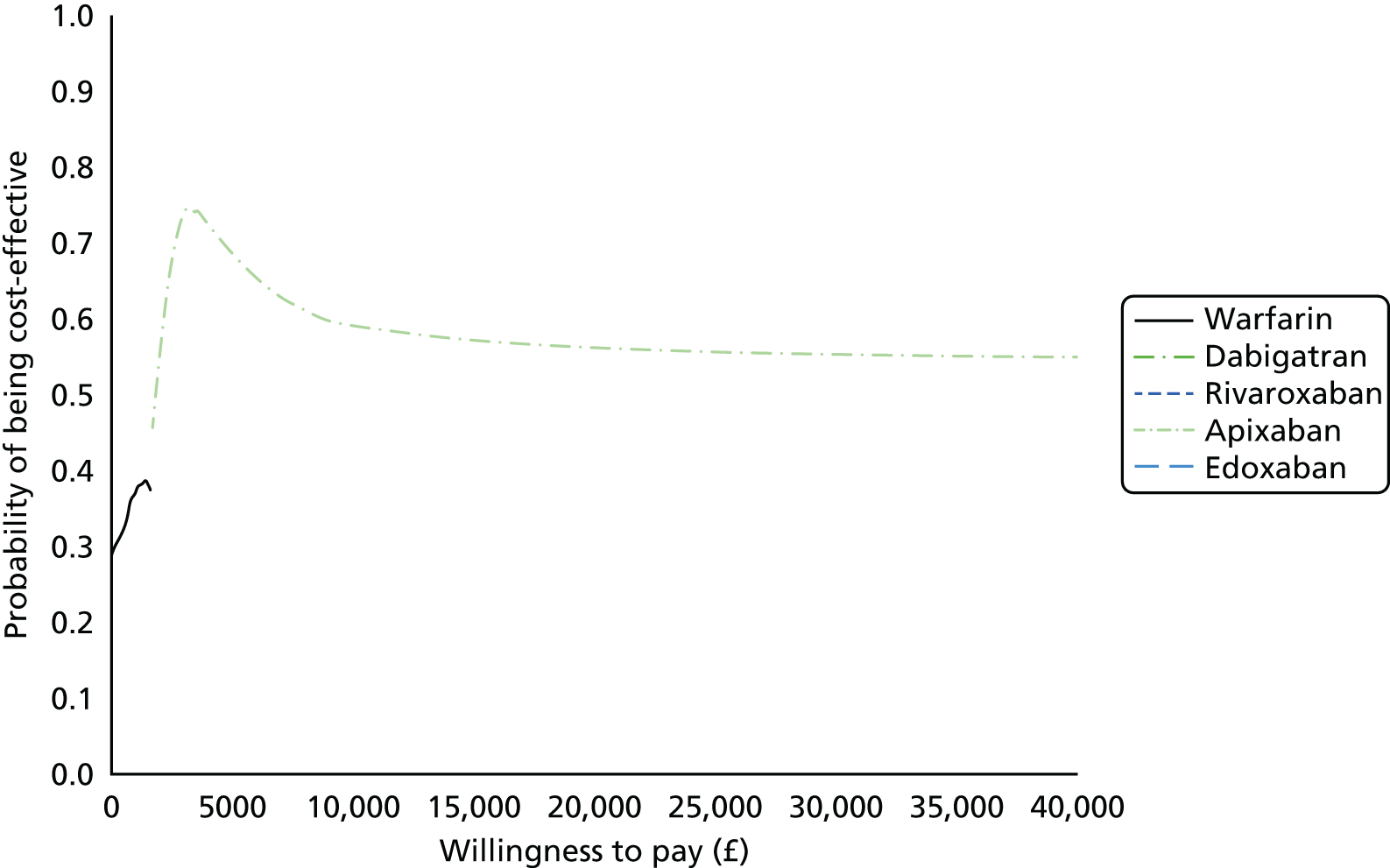
FIGURE 164.
Cost-effectiveness acceptability frontier for acute treatment sensitivity analysis: decreasing VTE utility by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
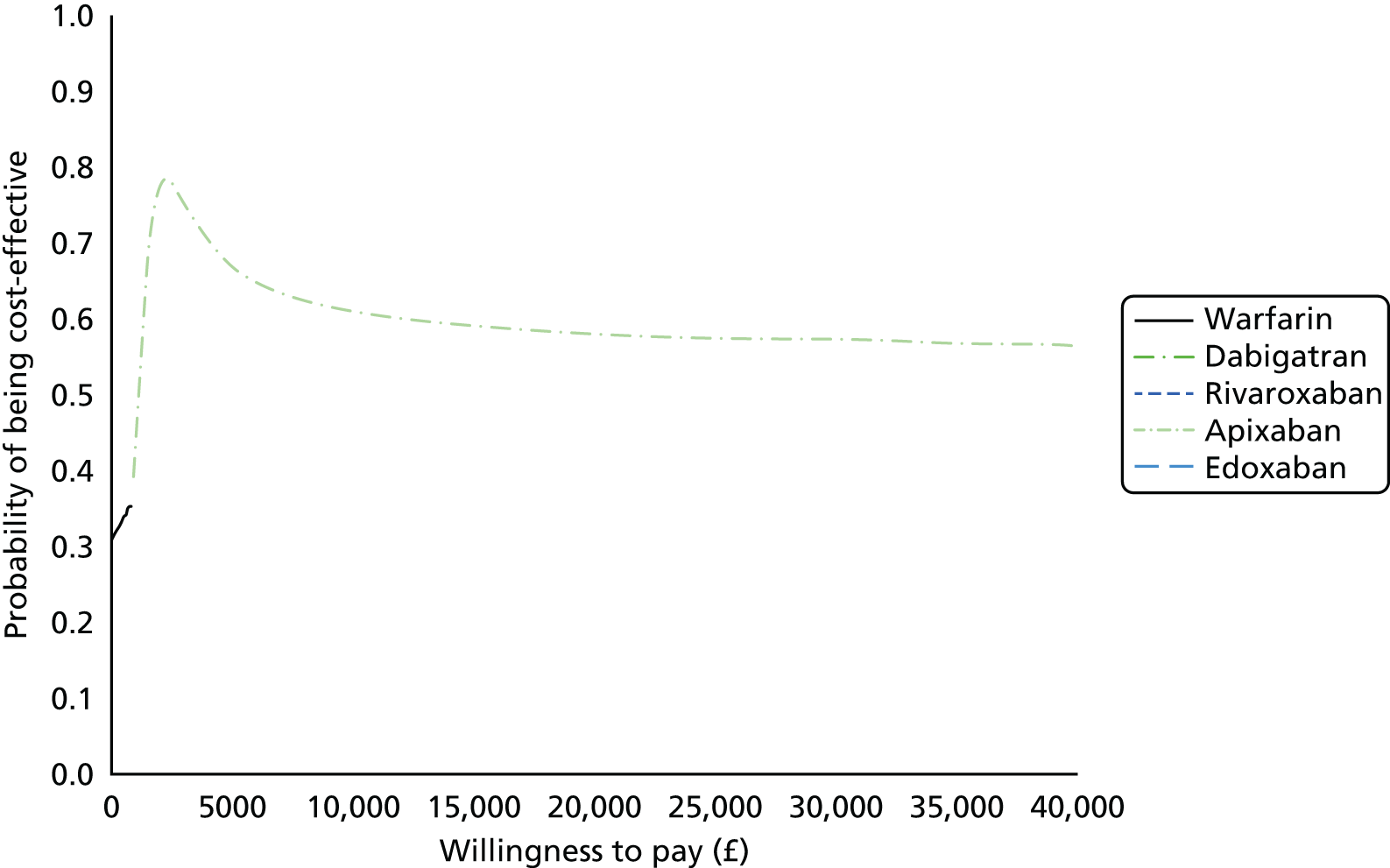
FIGURE 165.
Cost-effectiveness acceptability frontier for acute treatment sensitivity analysis: increasing VTE utility by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
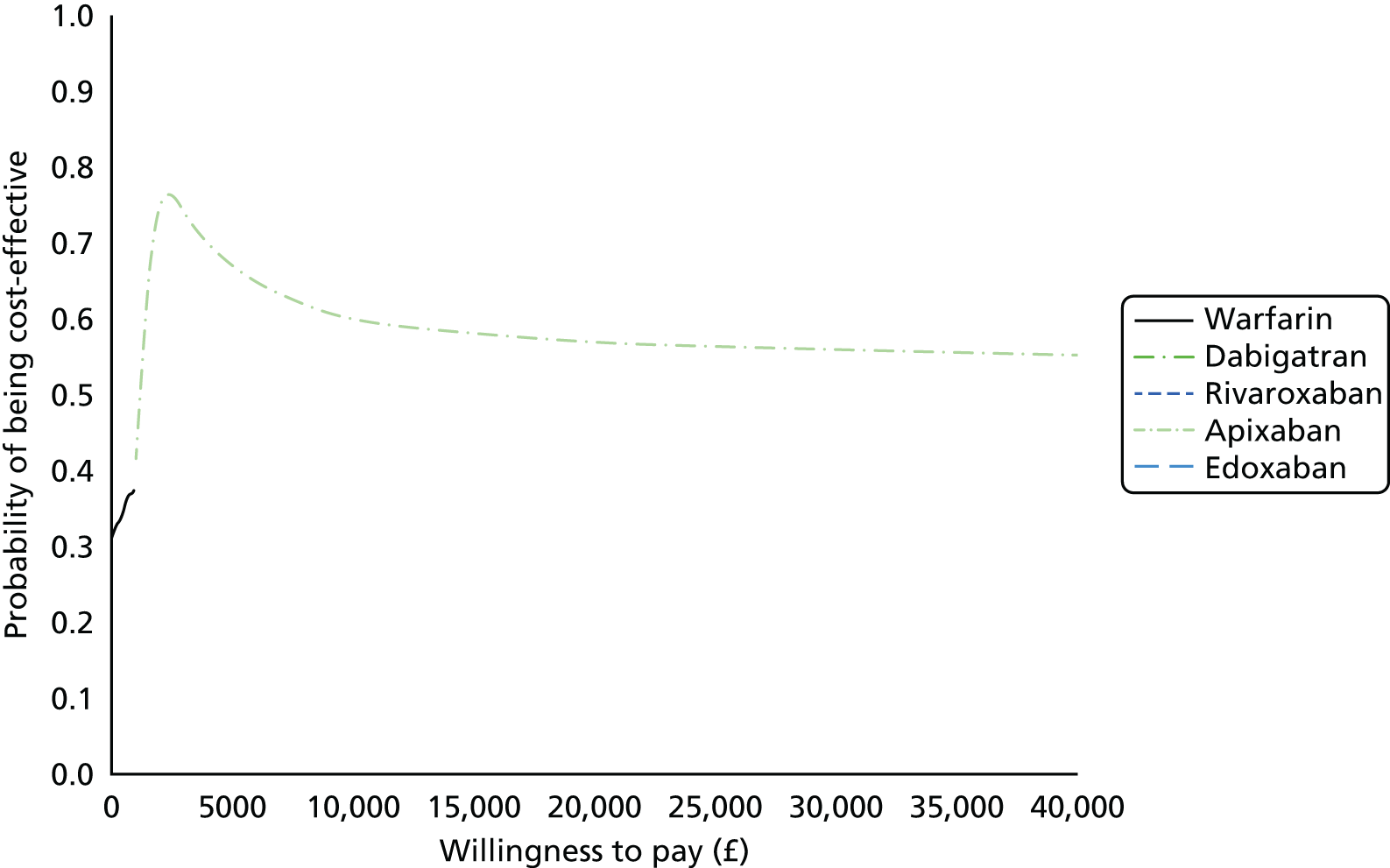
FIGURE 166.
Cost-effectiveness acceptability frontier for acute treatment sensitivity analysis: NOACs rate of non-fatal ICH rate equal to warfarin. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
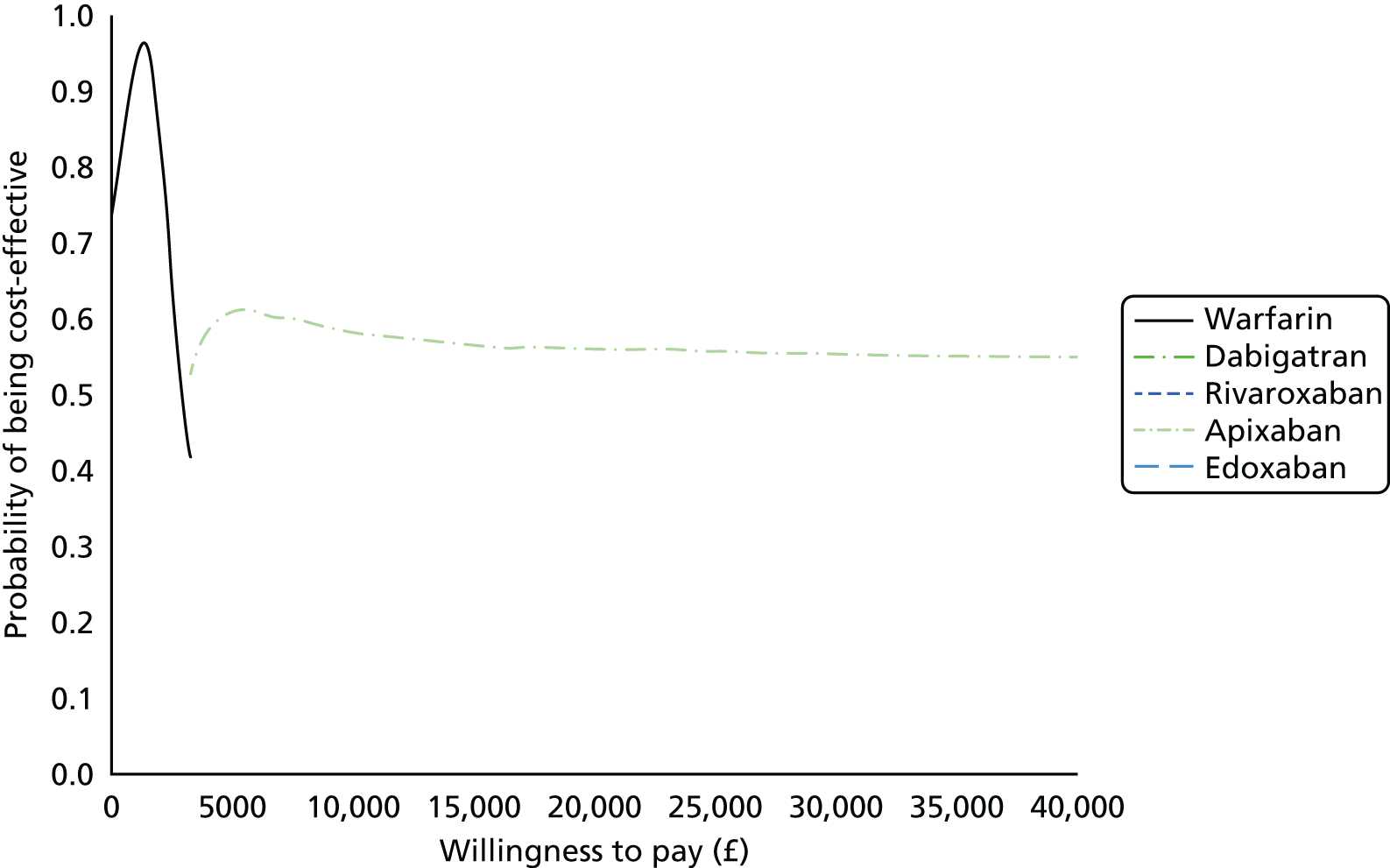
FIGURE 167.
Cost-effectiveness acceptability frontier for acute treatment sensitivity analysis: changing time on treatment from 6 months to 3 months. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.

Secondary prevention of venous thromboembolism
FIGURE 168.
Cost-effectiveness acceptability frontier for secondary prevention sensitivity analysis: patients on no pharmacotherapy and aspirin receive warfarin after a second VTE event. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
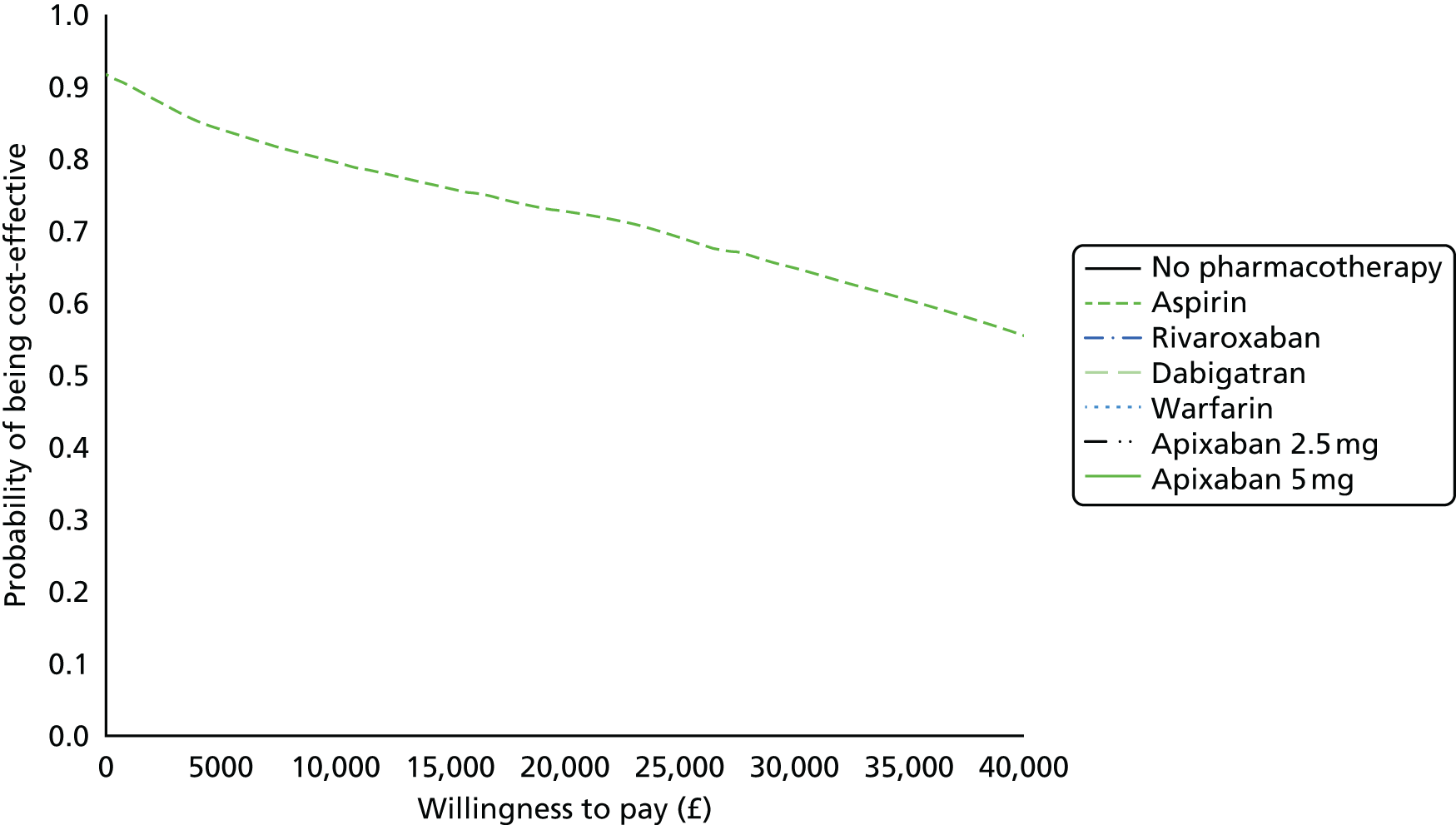
FIGURE 169.
Cost-effectiveness acceptability frontier for secondary prevention sensitivity analysis: change bleed base rate. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
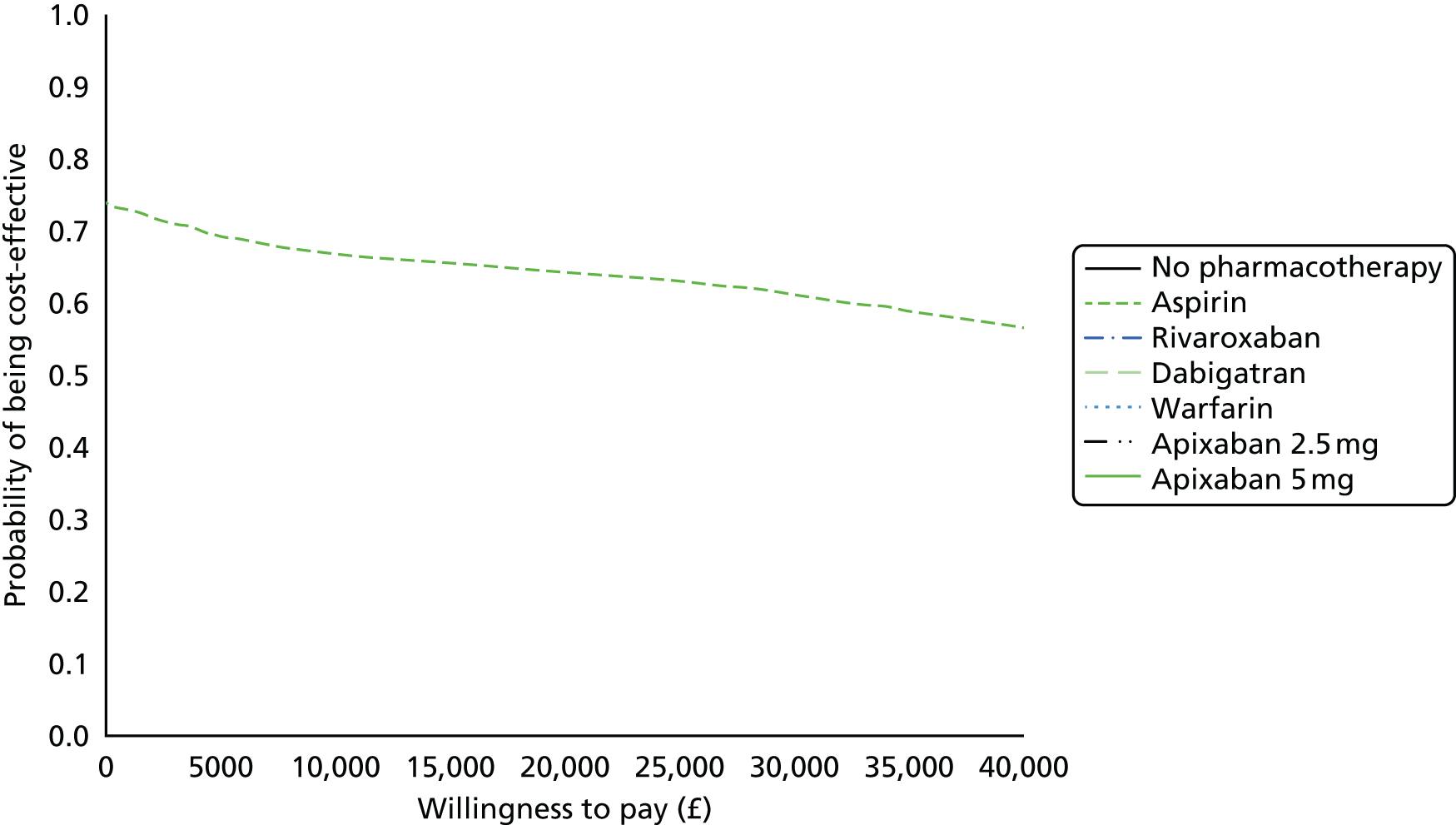
FIGURE 170.
Cost-effectiveness acceptability frontier for secondary prevention sensitivity analysis: setting the cost of warfarin to £0. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
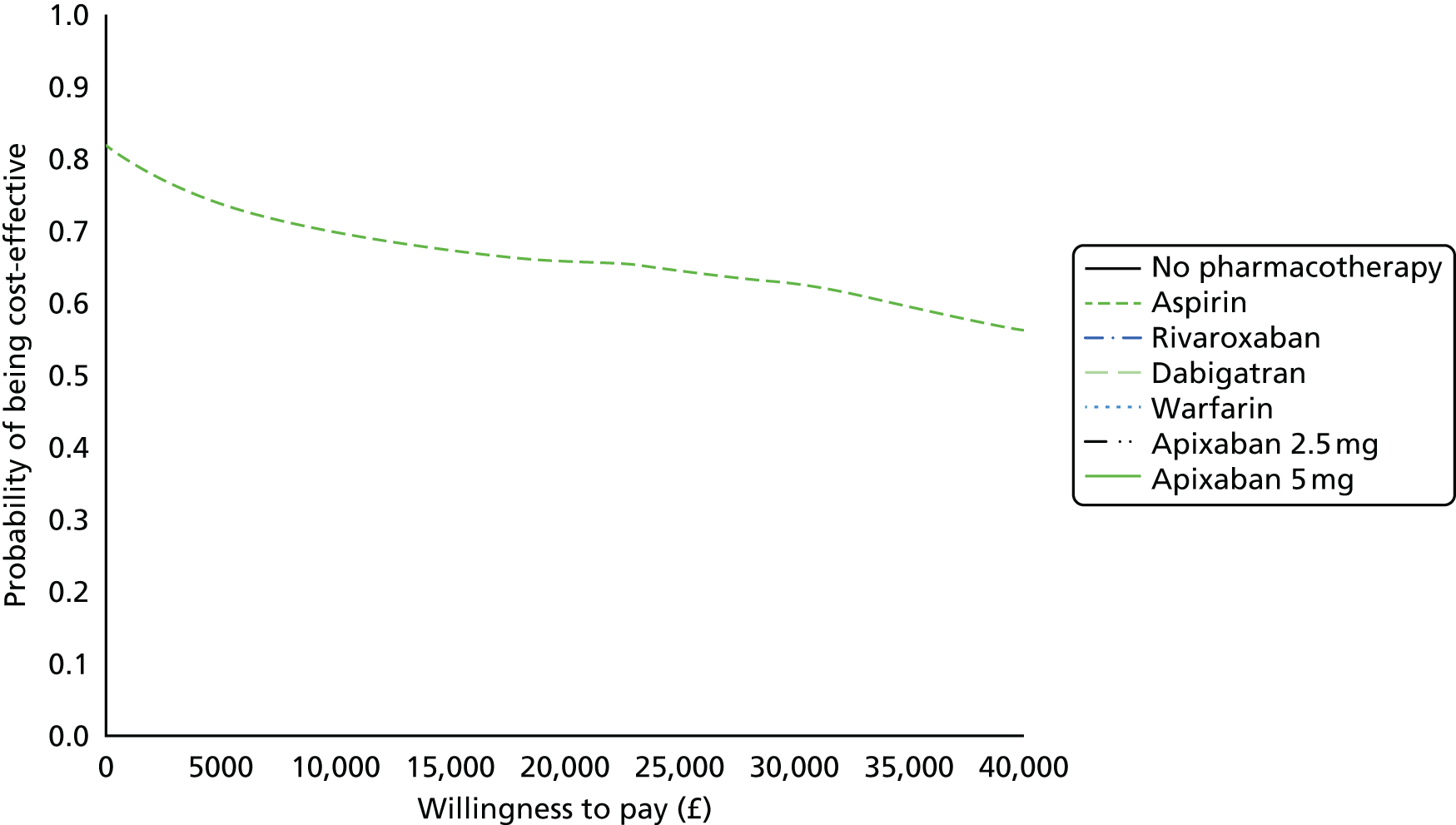
FIGURE 171.
Cost-effectiveness acceptability frontier for secondary prevention sensitivity analysis: decreasing the AE cost by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.

FIGURE 172.
Cost-effectiveness acceptability frontier for secondary prevention sensitivity analysis: increasing the AE cost by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
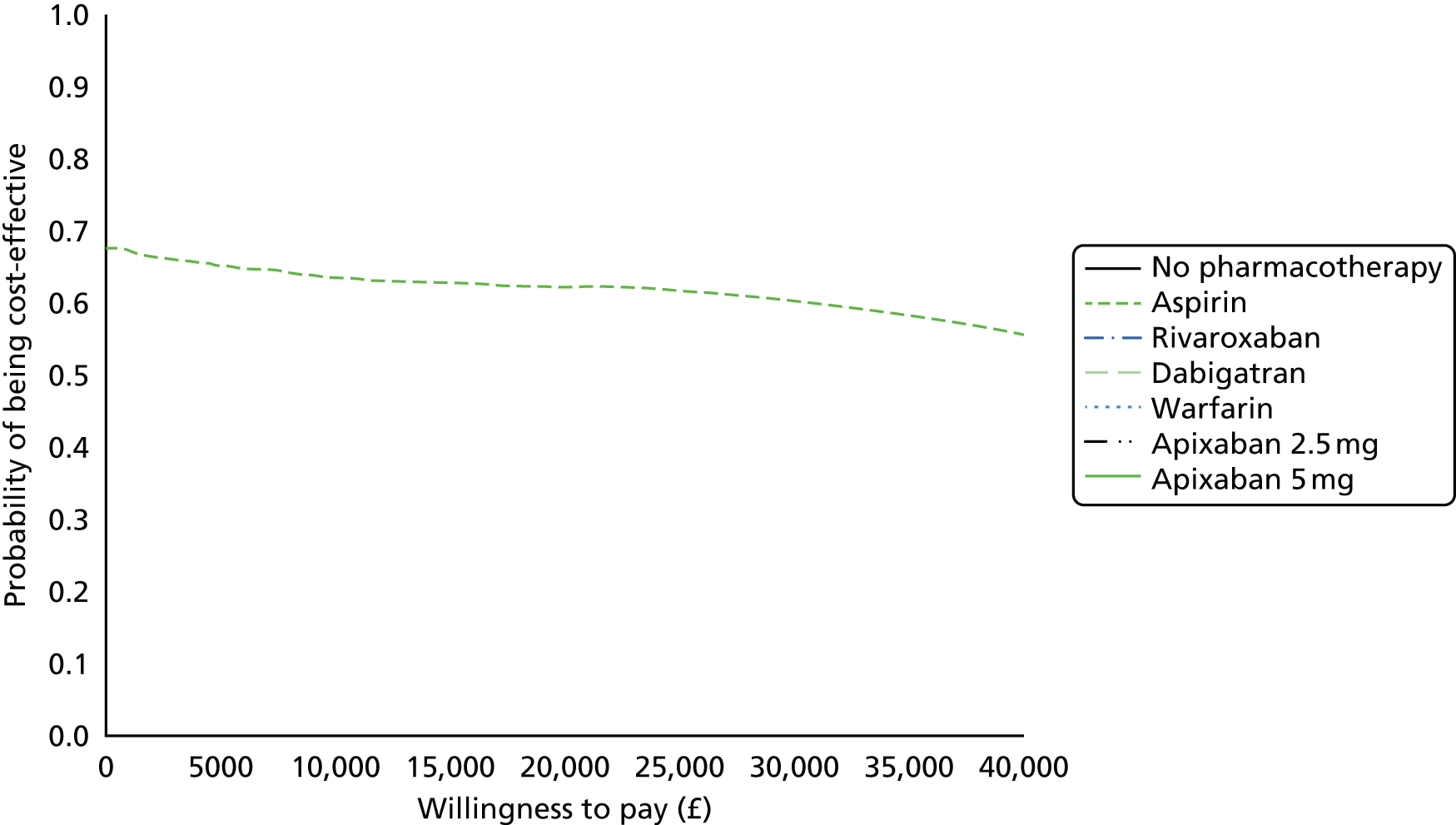
FIGURE 173.
Cost-effectiveness acceptability frontier for secondary prevention sensitivity analysis: decreasing the AE utility by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
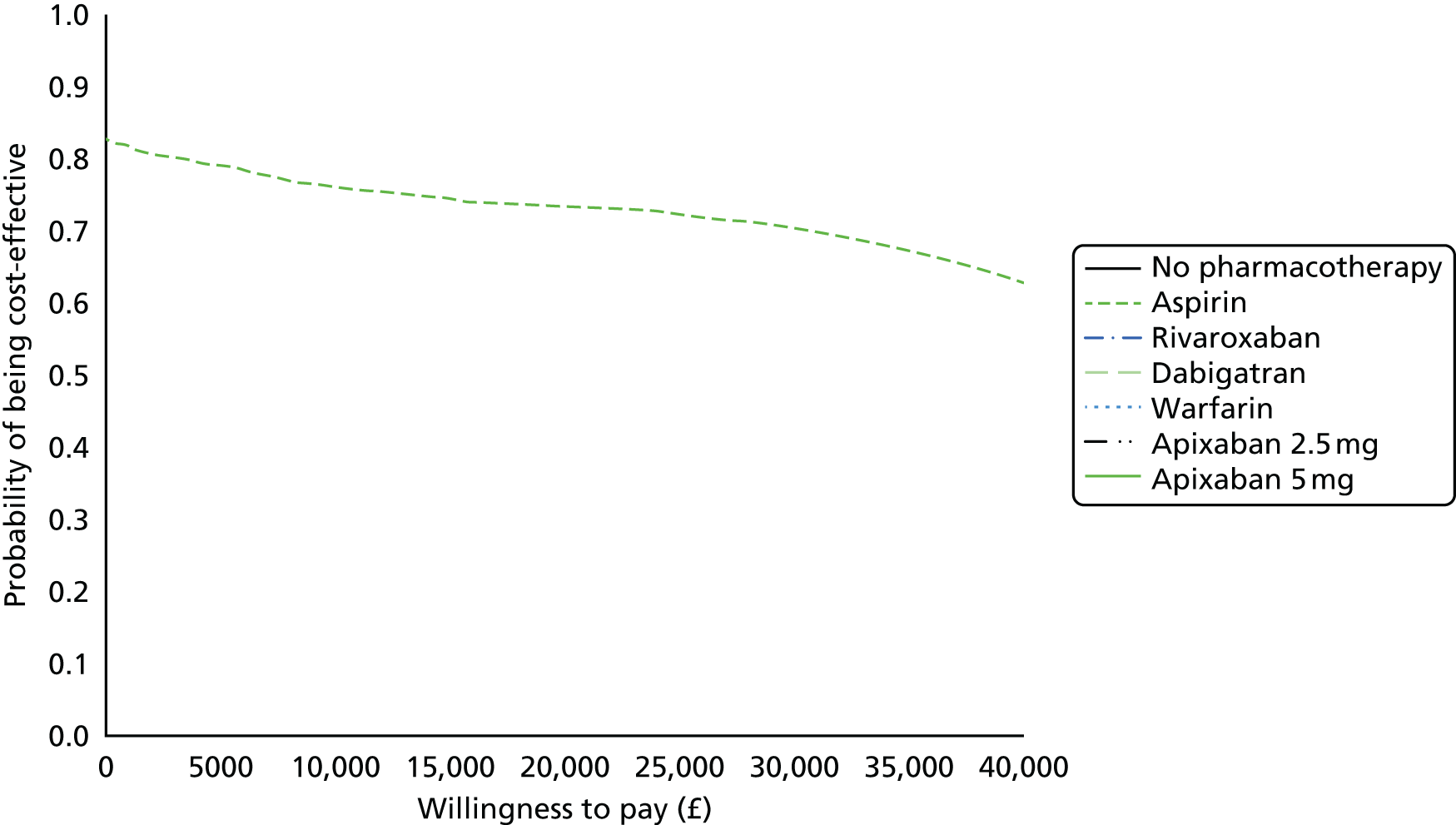
FIGURE 174.
Cost-effectiveness acceptability frontier for secondary prevention sensitivity analysis: increasing the AE utility by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
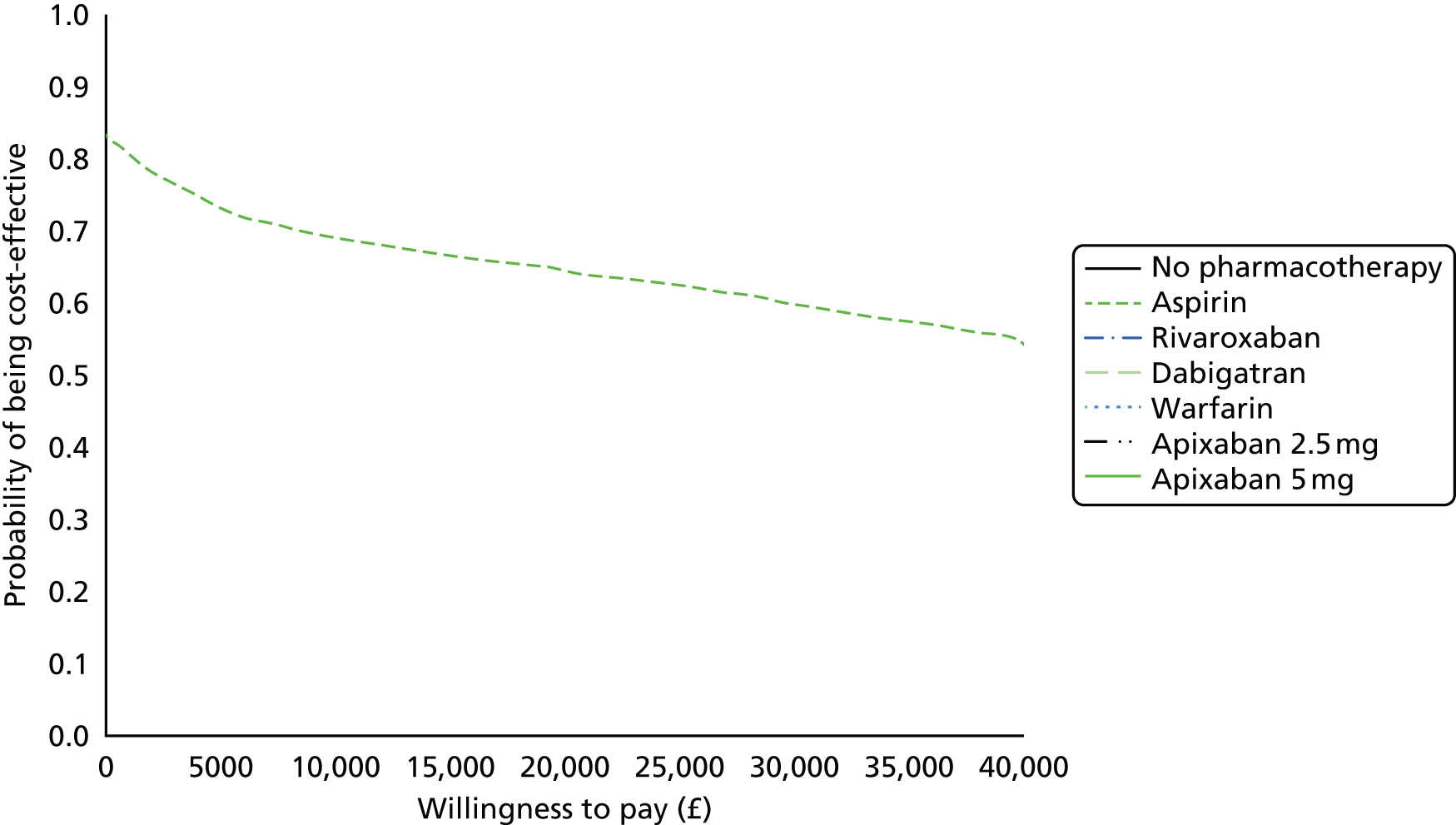
FIGURE 175.
Cost-effectiveness acceptability frontier for secondary prevention sensitivity analysis: increasing the cost of VTE events by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
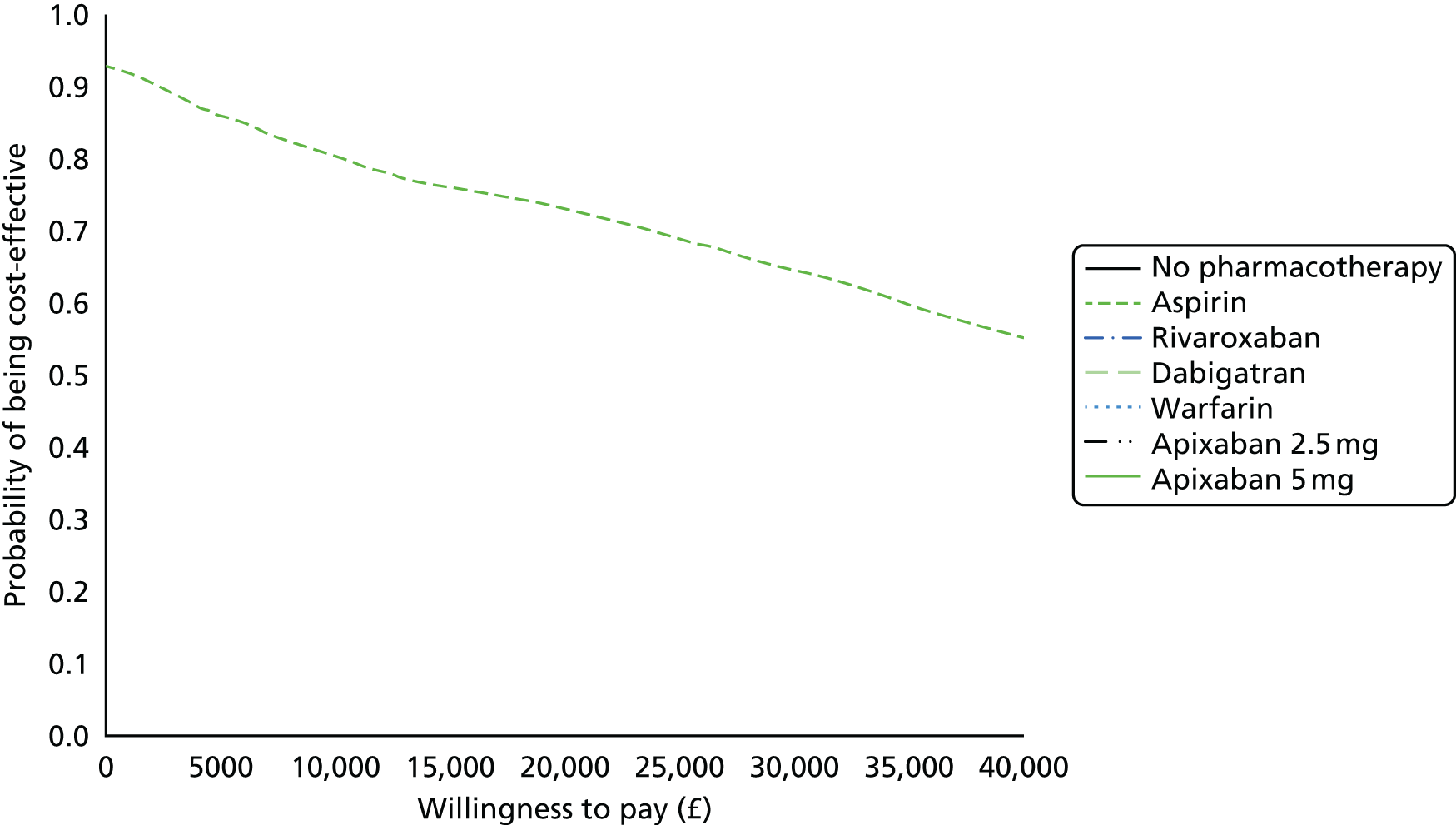
FIGURE 176.
Cost-effectiveness acceptability frontier for secondary prevention sensitivity analysis: decreasing VTE utility by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.

FIGURE 177.
Cost-effectiveness acceptability frontier for secondary prevention sensitivity analysis: increasing VTE utility by 50%. See Chapter 4 (Outcomes of atrial fibrillation and venous thromboembolism models) for further details.
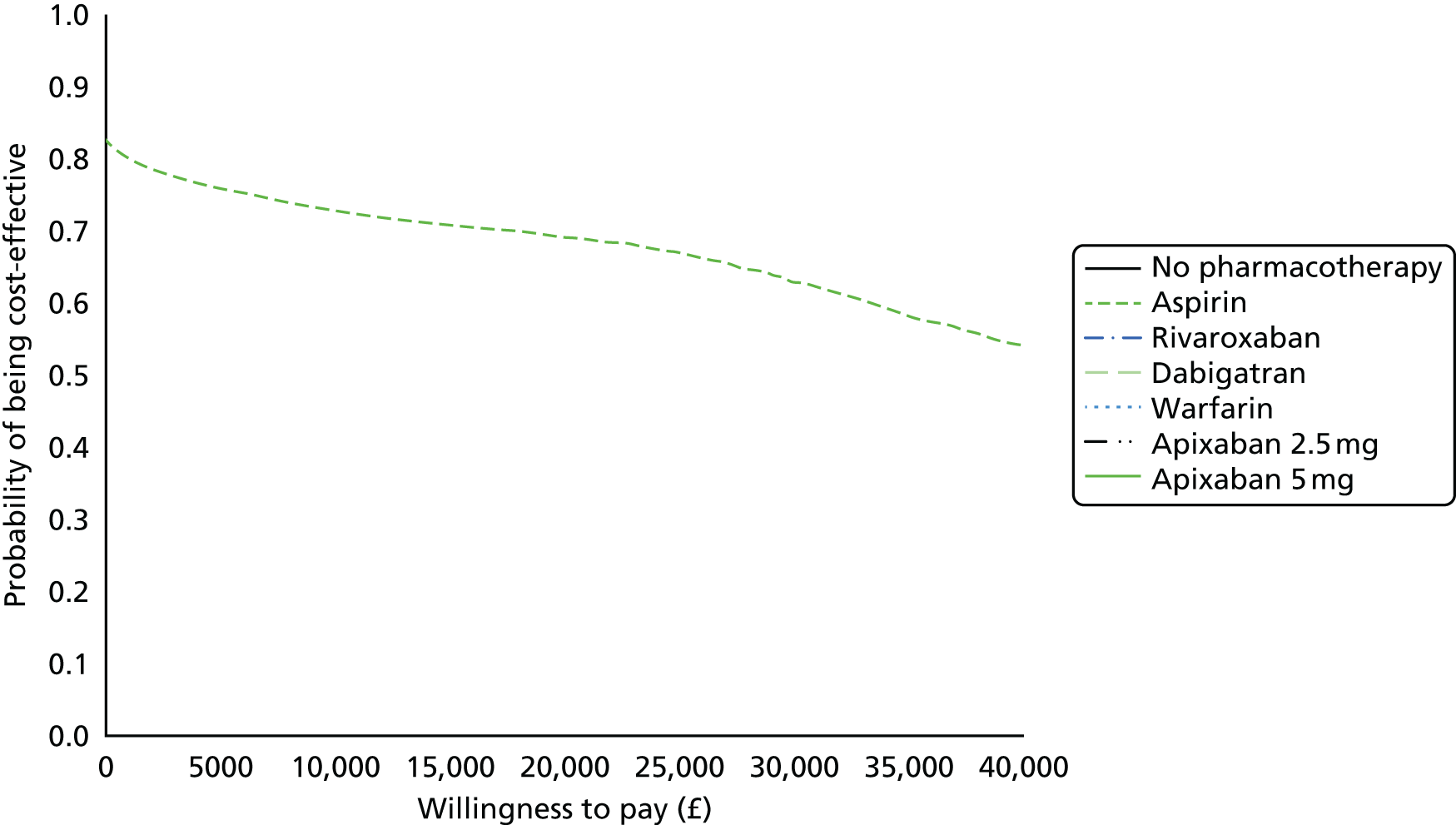
Glossary
- CHADS2
- A clinical prediction rule for estimating the risk of stroke in patients with non-rheumatic atrial fibrillation.
- CHADS2 VASC
- A modified clinical prediction rule for estimating the risk of stroke in patients with non-rheumatic atrial fibrillation.
- HAS-BLED
- Hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalised ratio, elderly (aged > 65 years), drugs/alcohol concomitantly: this estimates risk of major bleeding for patients on anticoagulation for atrial fibrillation.
List of abbreviations
- AE
- adverse event
- AF
- atrial fibrillation
- AIC
- Akaike information criterion
- BAATAF
- Boston Area Anticoagulation Trial for Atrial Fibrillation
- bd
- twice daily
- BMI
- body mass index
- BNF
- British National Formulary
- CADTH
- Canadian Agency for Drugs and Technologies in Health
- CAFA
- Canadian Atrial Fibrillation Anticoagulation
- CEA
- cost-effectiveness analysis
- CEAC
- cost-effectiveness acceptability curve
- CEAF
- cost-effectiveness acceptability frontier
- CI
- confidence interval
- CRB
- clinically relevant bleeding
- CRD
- Centre for Reviews and Dissemination
- CRNM
- clinically relevant non-major
- CTPH
- chronic thromboembolic pulmonary hypertension
- DIC
- deviance information criterion
- DVT
- deep-vein thrombosis
- EQ-5D
- European Quality of life-5 Dimensions
- EVPI
- expected value of perfect information
- EVPPI
- expected value of perfect partial information
- HR
- hazard ratio
- ICH
- intracranial haemorrhage
- INB
- incremental net benefit
- INR
- international normalised ratio
- LMWH
- low-molecular-weight heparin
- log-OR
- log-odds ratio
- MCMC
- Markov chain Monte Carlo
- MI
- myocardial infarction
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMA
- network meta-analysis
- NMB
- net monetary benefit
- NOAC
- novel oral anticoagulant
- od
- once daily
- ONS
- Office for National Statistics
- OR
- odds ratio
- PE
- pulmonary embolism
- post-op
- postoperative
- pre-op
- preoperative
- PROSPERO
- international prospective register of systematic reviews
- PTS
- post-thrombotic syndrome
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RIND
- reversible ischaemic neurological deficit
- SAVI
- Sheffield Accelerated Value of Information
- SD
- standard deviation
- SE
- systemic embolism
- SMC
- Scottish Medicines Consortium
- THR
- total hip replacement
- TIA
- transient ischaemic attack
- TKR
- total knee replacement
- TTR
- time in therapeutic range
- VKA
- vitamin K antagonist
- VOI
- value of information
- VTE
- venous thromboembolism