Notes
Article history
The contractual start date for this research was in January 2017. This article began editorial review in January 2022 and was accepted for publication in August 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The Health Technology Assessment editors and publisher have tried to ensure the accuracy of the authors’ article and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2023 Menon et al. This work was produced by Menon et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – Journals Library, and the DOI of the publication must be cited.
2023 Menon et al.
Background and introduction
Ovarian and tubal cancers remain the most fatal of all gynaecological cancers. In the UK, 1 in 52 women will develop the cancer, with a third of the cases diagnosed in women aged over 75. Every day in the UK 20 women are diagnosed and 11 die of the disease. 1 While overall ovarian and tubal cancer mortality rates have decreased since 1970, they remain high in women aged 70–79 and in those over 80. 2 Advanced stage at diagnosis and non-specific symptoms are key contributors to the high mortality. The majority (58%) of women are diagnosed with Stage III and IV, which is associated with poor survival (5-year survival 27% Stage III, 13% Stage IV). 2 The over 90% 5-year survival rates in women detected at Stage I underpin the worldwide early detection efforts, now spanning over four decades.
In the UK, earlier diagnosis of ovarian and tubal cancer has been central to the government’s efforts to improve cancer outcomes. It is part of all major Department of Health cancer initiatives which include earlier diagnosis such as the International Cancer Benchmarking Partnership,3,4 The National Awareness and Early Diagnosis Initiative,5 Be Clear on Cancer6 and more recently the NHS-Galleri Trial. 7 In parallel, the ovarian cancer charities have made significant efforts to raise ovarian cancer symptom awareness and support earlier diagnosis in primary care. However, there is little evidence that symptom awareness efforts on their own8–11 can significantly reduce deaths due to the disease. A cost-effective ovarian and tubal cancer screening strategy therefore remains highly relevant and important to the future needs of the NHS, especially in view of the ageing population.
The initial screening efforts in the 1980s followed the discovery of the tumour marker CA125 in the USA12 and the refinement of ultrasound to assess ovaries in the UK. 13 This led to multiple prospective interventional studies in the general population. The first to report early-stage screen-detected ovarian cancers was the single-arm Stockholm study of 5500 women aged over 40 who were screened with CA125 and ultrasound. 14 Improved survival was reported for women diagnosed with ovarian cancer during the screening phase (100 months) compared to after screening had ended (20 months). 15 In parallel in the UK, a study of 5479 women showed that transabdominal ultrasound could detect the disease at an early stage. 16 A trial evaluating a multimodal approach which combined annual serum CA125 (>30 U/ml) with ultrasound as a second-line test in postmenopausal women (over 45 years) was also initiated. 17 Following a baseline screen,18 21,935 participants were randomised to three rounds of annual screening between 1989 and 1993. At a median follow-up of 7 years, this pilot randomised controlled trial (RCT) showed encouraging survival in women in the screened arm (72.9 months) compared to the control arm (41.8 months). 19 Findings from the Japanese Shizuoka Cohort Study on Ovarian Cancer Screening (SCSOCS) RCT of 82,487 postmenopausal women using CA125 (>35 U/ml) and transvaginal ultrasound (TVS) as a combined annual screen for 3 years also showed a trend to earlier detection of ovarian cancer. 20 By 2001, the US Prostate Lung Colorectal Ovarian (PLCO) Cancer Screening Trial had randomised 68,557 eligible women (over 55 years) to annual screening with CA125 (>35 U/ml) and TVS for 4 years followed by serum CA125 alone for a further 2 years and a no-screening control group. 21 All these trials used the biomarker CA125 interpreted by a single cut-off value and/or pelvic imaging using ultrasound scanning of the ovaries. In addition to these RCTs, the University of Kentucky Ovarian Cancer Screening Project, a single-arm ultrasound study, was initiated in 1987. 22 By 2007 it had recruited 25,327 predominantly older asymptomatic women aged >50 years as well as women >25 with a family history of ovarian cancer. 23
In the early-1990s, data from the initial pilot RCT24 were used to refine the interpretation of serum CA125 by incorporating longitudinal change and age-specific incidence of ovarian cancer into a risk of ovarian cancer algorithm (ROCA). 25 The feasibility of this refined multimodal approach was evaluated in the mid-90s in a further pilot RCT. 26 The growing evidence of the possibility of earlier detection of ovarian cancer led to the set-up of the United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) to provide a definitive answer.
Aims and objectives
The overall goal of UKCTOCS was to assess whether ovarian cancer screening of the general population could save lives and be introduced as a cancer screening programme by the UK National Health Service (NHS) and more widely. The primary objective was to evaluate the impact on ovarian and tubal cancer mortality of annual screening using two available strategies compared to no screening. The two strategies were multimodal (MMS – CA125 interpreted by ROCA with repeat testing and TVS as a second-line test) and ultrasound (USS – TVS as the first- and second-line test). Secondary objectives included evaluating performance characteristics of the two screening strategies, adherence to annual screening, physical and psychological morbidity resulting from screening, and cost-effectiveness of a national ovarian cancer screening programme in the context of the UK NHS. An additional objective was to create a bioresource of data and serum samples that could be used in future research studies, where a key focus would be early detection and treatment of disease. The aim was to ensure a wide access to researchers working both in academia and in industry.
Methods
UKCTOCS was a randomised controlled trial comparing two screening strategies with no screening. The psychosocial cohort study that ran in parallel had a prospective longitudinal questionnaire design. All women provided separate written consent to participate in the two studies.
Setting
The trial was set up within 27 participating primary care trusts (PCTs) (including local health boards in Wales) in the catchment area of 13 NHS Trusts in England, Wales and Northern Ireland between 2001 and 2005. Pre-identified Scottish centres were unable to participate due to a variety of logistical reasons (lack of space, retirement of potential trial-centre leads, unwillingness of NHS Trust management to commit to a 10-year trial, involvement in other ovarian cancer screening trials). The co-ordinating centre (CC) for the main trial was located at Barts and the London Medical School, Queen Mary University London, between January 2001 and March 2004 and moved to University College London (UCL) in April 2004. In UCL, from April 2004 to July 2018, the CC was based in the Department of Women’s Cancer, Elizabeth Garrett Anderson (EGA) UCL Institute for Women’s Health. It then relocated (August 2018 to January 2021) to the Medical Research Council Clinical Trials Unit (MRC CTU at UCL) at the Institute of Clinical Trials & Methodology, where all the trial data are archived. The bioresource created during the trial, UKCTOCS Longitudinal Women’s Cohort (UKLWC), is hosted and managed by the MRC CTU at UCL27 with biological samples located at the NIHR National Biosample Centre (UK Biocentre). 28 The co-ordinating centre for the psychosocial study was located at Sussex Health Outcomes Research and Education in Cancer (SHORE-C), Brighton and Sussex Medical School, University of Sussex.
Participants
Between April 2001 and October 2005, women aged 50–74 were randomly invited from age-sex registers of the 27 participating PCTs. Women were eligible if they were aged between 50 and 74 and were postmenopausal (defined as either >12 months amenorrhoea following a natural menopause or hysterectomy, or >12 months of hormone replacement therapy commenced for menopausal symptoms). Exclusion criteria were bilateral oophorectomy, previous ovarian malignancy, increased risk of familial ovarian cancer, active non-ovarian malignancy, and participation in other ovarian cancer screening trials (Project documentation A).
Approval was obtained from the Caldicott guardians (data controllers) of the 27 PCTs to access contact details of eligible women aged 50–74 resident in their jurisdiction (Figure 1). Specialised software was commissioned from the NHS which allowed 2000 to 10,000 women aged 50–74 to be randomly selected on a regular (usually 3-monthly) basis from the age/sex registers held by the participating PCTs. Their contact details, date of birth, NHS number and general practitioner (GP) details were forwarded as an electronic file to the CC. The software ensured that the women selected were flagged on the PCT register so that their details were not included in future downloads. These files were uploaded into a bespoke online trial management system (TMS) specifically developed for UKCTOCS. Women were then sent personal invitations (Project documentation B) to join the trial along with a brochure outlining the objectives, design and inclusion/exclusion criteria (Project documentation C). Some PCTs were unwilling to transfer contact details. For them, we negotiated an alternative method – the PCTs were sent standard invitation letters without a recipient’s name in sealed franked envelopes which they forwarded after pasting address labels. All women who wished to participate returned a tear-away slip in a Freepost envelope to the CC. If they fulfilled eligibility criteria, they were sent a detailed trial information sheet (Project documentation D) and an appointment to attend for recruitment at their local regional centre (RC). A dedicated telephone reception at the CC ensured that women were able to reschedule appointments easily.
FIGURE 1.
Invitation, recruitment and randomisation. (a) Recruitment in which primary care trusts (PCTs) provided electronic details of women; (b) recruitment in which PCTs did not allow access to contact details; TMS, trial management system; CC, co-ordinating centre.
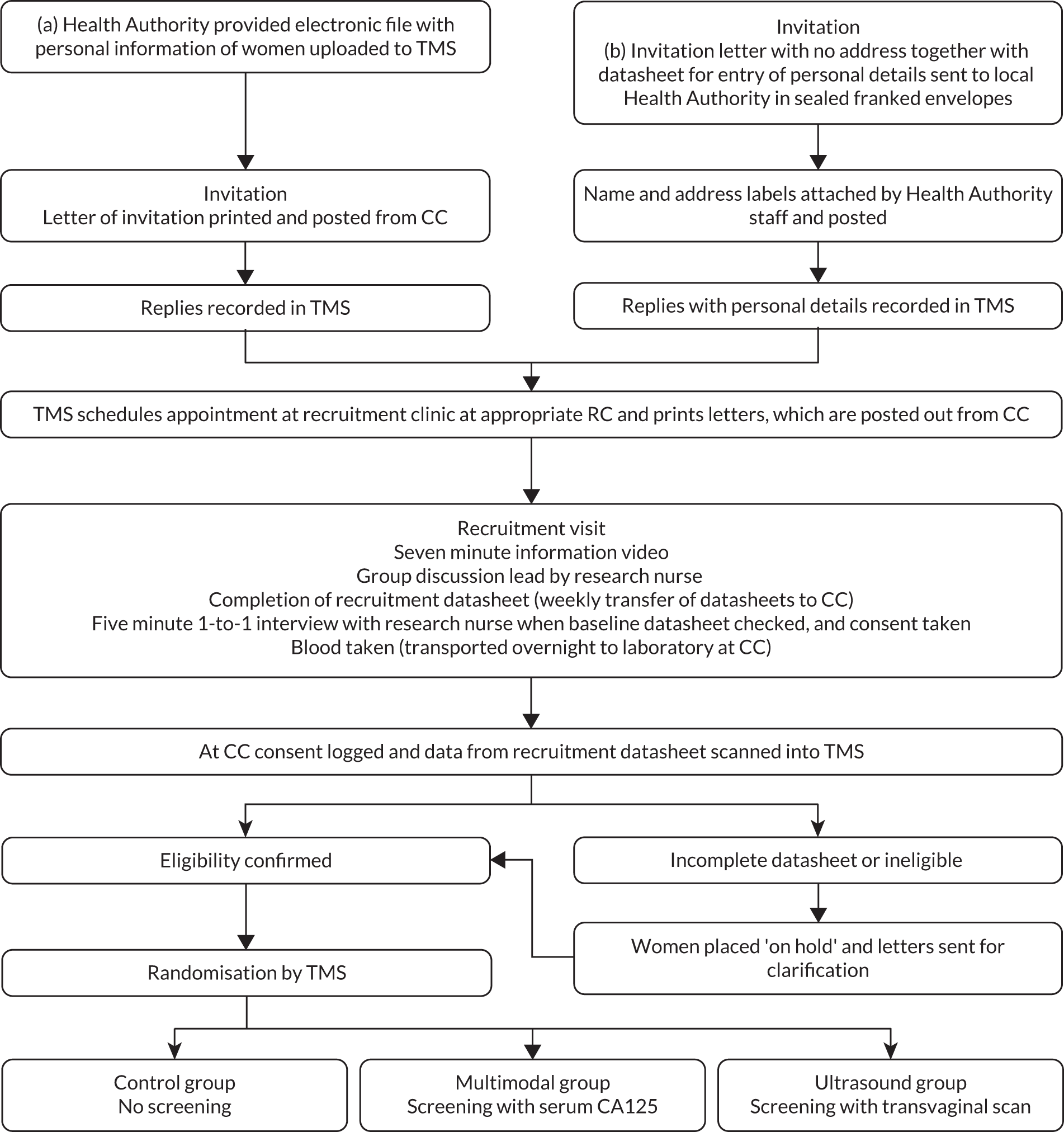
At the recruitment appointment, women viewed an information video and participated in a group discussion. They completed an 18-item recruitment questionnaire (Project documentation E) which included information regarding eligibility. They then had an individual appointment with the study nurse, who went through the patient information and ensured that their questions were answered prior to signing consent. The nurse also checked through their completed recruitment questionnaire to ensure that the information was complete and accurate. 29 Women who were willing to participate in the psychosocial study were given a set of baseline quality-of-life questionnaires to complete and return to the researchers in a pre-paid envelope. 30 All volunteers provided a baseline serum sample.
The GPs of individual women were kept informed at all stages of recruitment and randomisation of their patients into the trial.
Consent
Separate written consent was obtained for the main trial, serum bank (Project documentation F) and the psychosocial study (Project documentation G). Each individual woman was also asked to sign a data protection form (Project documentation H). A copy of the consent form was sent to the CC, where it was checked and any exceptions to future use of data or samples noted on the TMS.
Processing and biobanking of blood samples
Blood was collected in two 8-ml gel separation serum tubes (VACUETTE®; Greiner Bio-One, Stonehouse, UK) at the trial centres at recruitment. Additionally, in women randomised to the multimodal group, annual and repeat blood samples as per protocol were collected between 2001 and 2011. The barcoded blood tubes were scanned into the data management system at venepuncture and transported daily at ambient temperature to the central laboratory (Tumour Marker Laboratory) at the CC. Samples were scanned when they were received at the central laboratory, centrifuged at 1500×g for 10 minutes. Exact times for interval to spin (median 24 hours) were recorded for each sample. If it exceeded 56 hours, the sample was discarded, and the TMS sent women an appointment for a repeat blood draw. The separated serum was aliquoted into 10 500-μl straws (company – IMV) using a semi-automated MAPI platform (IMV). The straws were stored in liquid-nitrogen tanks at the central laboratory for 2001–2004. The tanks once full were transferred to a HTA licensed commercial cryofacility (Fisher Bioservices, UK) from 2004 onwards. The biobank was relocated to the UK Biocentre in March 2018.
Confirmation of eligibility
The completed questionnaires were sent to the CC, where they were scanned electronically using computerised intelligent character and optical mark reading software (Cardiff Software Inc, Teleform Elite version 8.1.1) which allowed rapid and accurate data entry. Any inconsistency or information not recognised by the data-capture software was verified manually by trained data-entry staff, who validated the computer-interpreted data. The TMS checked for completeness of eligibility data. When data were incomplete, women were placed ‘on hold’ and letters requesting further information were automatically sent. If the volunteer was placed ‘on hold’ because 12 months had not elapsed since her last menstrual period or start of HRT, she was informed and included in the randomisation process when 12 months had elapsed from the relevant date. The TMS generated lists of women who had a family history suggestive of ‘increased risk of ovarian cancer’. Such women were individually contacted and eligibility was confirmed. If they fulfilled criteria which put them at ‘increased risk’ of familial ovarian cancer, their GP was sent a letter requesting that the individual be referred to the Clinical Genetics department for risk assessment.
Randomisation and blinding
Following confirmation of eligibility, the TMS randomised women to no screening (control or C group) or to annual screening with serum CA125 (Multimodal or MMS group) or TVS (Ultrasound or USS group) in a 2:1:1 ratio using a computer-generated random-number algorithm. Randomisation was carried out by the TMS allocating a set of 32 random numbers to each RC with the lowest 8 allocated to the MMS group, the next 8 to the USS group and the remaining 16 to the control group. Each successive volunteer within the RC was randomly allocated one of the random numbers and subsequently randomised into a group. When all 32 random numbers had been used up a further set of 32 was generated. The randomisation was accomplished by using the Visual Basic randomisation statement and the SQL Rnd function.
Blinding of participants and trial staff was not possible given the nature of the interventions. However, the members of the outcome review committee were blinded to randomisation group.
The trial design, including details of recruitment, randomisation and screening, is summarised below and has been detailed in the protocol (Project documentation A).
Intervention – screening, clinical assessment, trial surgery
The women were invited for annual screening from randomisation (2001–2005) to 31 December 2011.
Screening
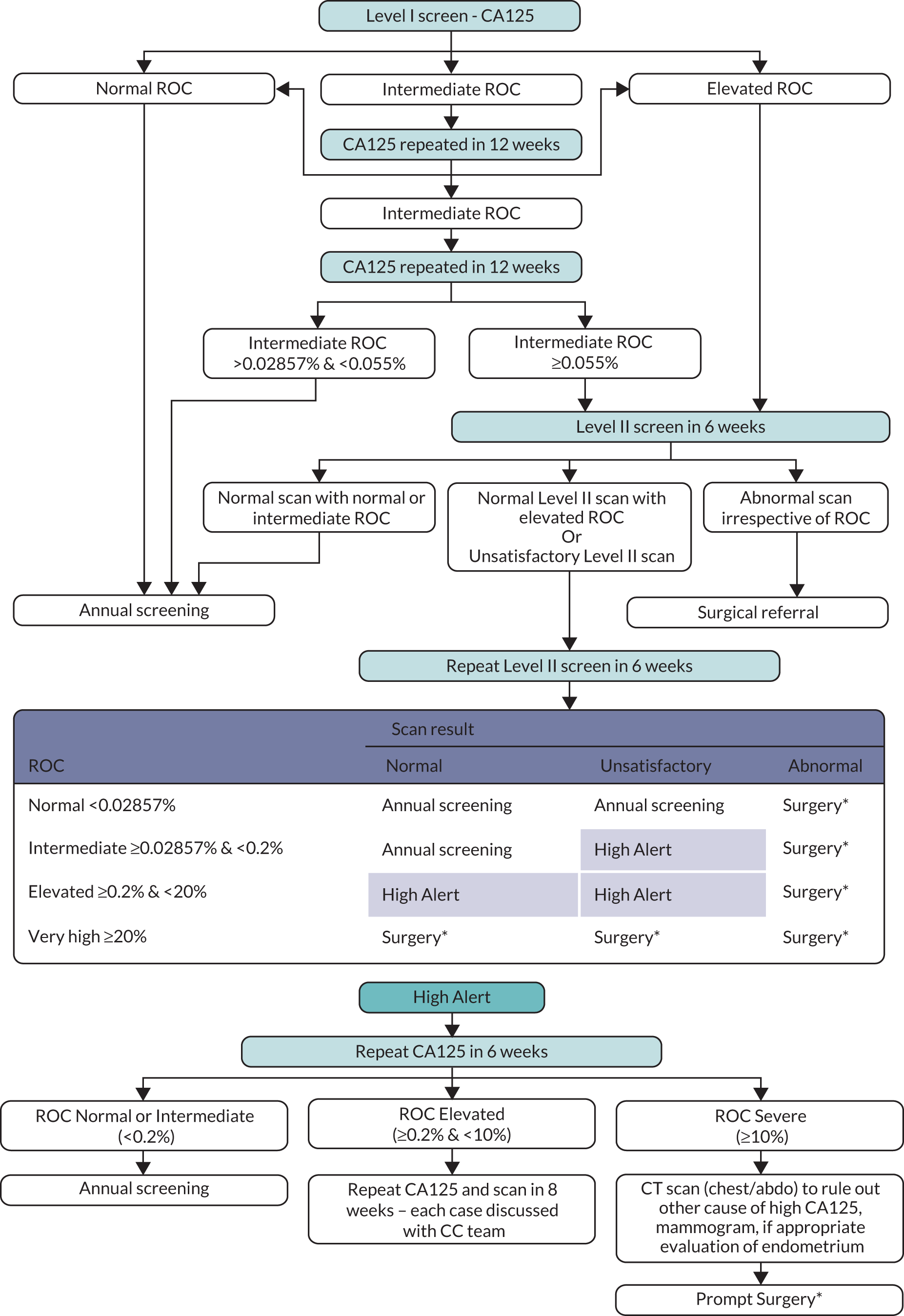
In the MMS group, annual screening was undertaken using a blood test to measure serum CA125 (Level I screen). The analysis was performed centrally at the CC laboratory throughout the trial. Serum CA125 concentrations were determined by electro-chemiluminescence sandwich immunoassay on an Elecsys 2010 (Roche Diagnostics, Burgess Hill, UK) using two monoclonal antibodies (OC125 and M11; Fujirebio Diagnostics AB, Göteborg, Sweden). The pattern of CA125 over time was interpreted using the ROCA,25 which identifies significant rises in CA125 concentration above baseline. For each woman, the ROCA calculated the risk of having had a CA125 change-point from one or more serial CA125 values using her own CA125 results as control. The risk was re-calculated for each additional CA125 measurement. Based on risk, women were triaged to normal (annual screening), intermediate (repeat CA125 ROCA test in 3 months, repeat Level I), and elevated (repeat CA125 ROCA test and transvaginal USS as a second-line test in 6 weeks, Level II) risk. If risk remained elevated, women were referred for clinical assessment. Women who had normal or unsatisfactory scans at Level II were triaged based on risk categories to further testing including repeat Level II screen in 6 weeks (Figure 2). The complete screening protocol involving further repeat testing and triage is detailed in the protocol (Project documentation A).
FIGURE 2.
Multimodal screening algorithm. ROC, risk of ovarian cancer.
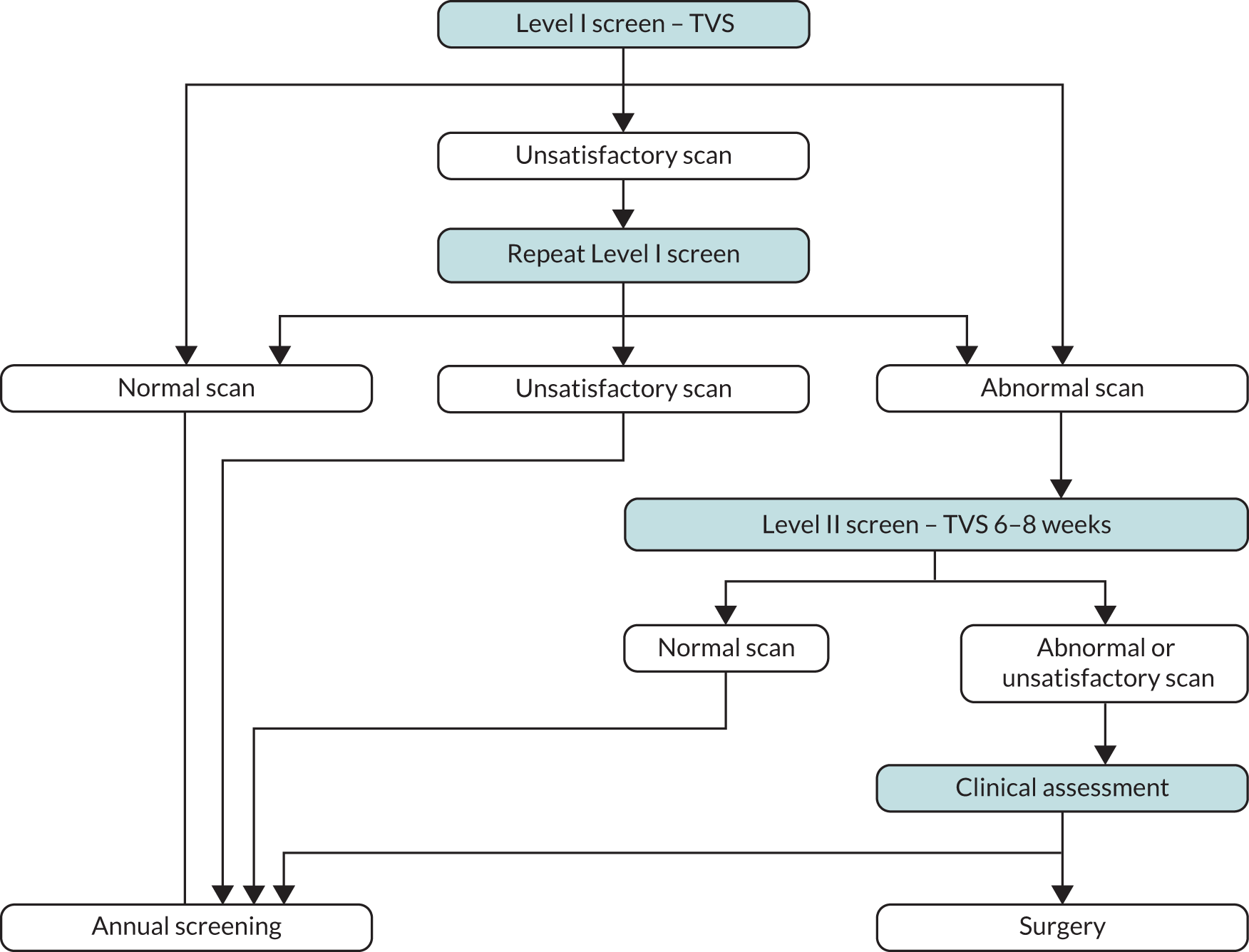
In the USS group, annual screening was undertaken using TVS (Level I scan). 31 Scans were classified as
-
Normal if (a) both ovaries had normal morphology (uniform hypoechogenicity and smooth outline with or without spots of calcifications or a single <10 mm inclusion cyst not distorting the outline) or (b) one or both ovaries had a single simple cyst <60 cm3 or (c) ovaries were not visualised but a good view of the iliac vessels was obtained. They were returned to annual screening.
-
Unsatisfactory (one or both ovaries were not visualised due to a poor view), with women recalled for a repeat Level I scan in 3 months.
-
Abnormal (one or both ovaries/adnexa had complex morphology – non-uniform ovarian echogenicity excluding single simple cyst, simple cysts >60 cm3 or fluid in Pouch of Douglas >10 mm), with women returning for a Level II scan within 6 weeks.
-
Complex unchanged (abnormal adnexal masses that had been previously managed conservatively and remained unchanged in morphology or volume), with the option on subsequent annual screens for clinical review of results and return to annual screening without undergoing Level II.
Women with abnormal scans on Level II underwent clinical assessment (Figure 3).
FIGURE 3.
Screening strategy used in the ultrasound group. TVS, trans-vaginal scan.
During the trial, a quality assurance programme for TVS in USS and MMS groups was implemented by the UKCTOCS ultrasound sub-committee with the oversight of the process by the national lead sonographer. This included induction and training of sonographers, standardisation of data collection, weekly ‘fail-safe’ checks, regular monitoring of quality measures and accreditation in postmenopausal pelvic scanning. 32
Both screening protocols were implemented centrally from the CC. The web-based trial management system with automated classification of results, implementation of screening algorithms, scheduling of further screening appointments as appropriate, letter printing and ‘fail-safe’ data queries ensured that protocol deviations were kept to a minimum.
Communication of test results was done in a carefully monitored manner, using standard letters that had been vetted by the study psychologists. Patients considered to be at increased risk were told they had an increased risk of ovarian or tubal cancer compared to the general population based on their test results. They were provided with telephone contact numbers both for their RC and the CC. Any concerns were clarified by the senior trial nurse or clinical team at the CC. This was facilitated by the fact that all information was online and accessible by both RC and CC teams.
To ensure that normal results did not cause harm by providing a false sense of security, women were counselled on the need to consult their GP in the case of any symptoms. This recommendation was provided at several stages: recruitment, in all normal/low-risk results letters and at the end of the trial.
Clinical assessment
This was undertaken at the RC by a designated trial clinician working within the UK NHS. It included detailed history, clinical examination, assessment of comorbidity and further investigations as appropriate. The latter included serum CA125 , repeat transvaginal scans and Doppler studies, CT/MRI of the abdomen and pelvis, and occasionally assessment of other tumour markers. A decision was made either to offer surgery or to manage conservatively, taking into account the views of the woman, any significant comorbidity, the morphological features of the ultrasound lesion, and previous hysterectomy or major pelvic surgery that could contribute to false-positive ultrasound findings. If there was a high index of suspicion, women were referred for surgery within the NHS. If the risk of ovarian or tubal cancer was considered low or if the patient declined surgery, then conservative management involved a TVS and serum CA125 at 3 months with a possible repeat at 6 months and return to annual screening if the findings were unchanged.
Trial surgery
The primary aim was to remove both ovaries and tubes for histopathological examination, with the preferred approach operative laparoscopy. An exception were women with dense pelvic adhesions which increased the risk of complications. In such situations, the clinician could opt to only remove the abnormal ovary and not dissect the contralateral ‘normal-looking’ ovary. Hysterectomy was not recommended unless there were clear clinical indications. If pre-operative findings were strongly suggestive of ovarian or tubal cancer, or if laparoscopy was inappropriate for technical difficulties, a laparotomy was undertaken.
Follow-up
All participants were linked using their UK NHS number (surname and date of birth used where NHS number was incorrect) initially to the Office of National Statistics and then to NHS Digital (formerly Health and Social Care Information Centre, HSCIC) in England and Wales and Business Services Organisation (BSO) (formerly Central Services Agency) and Northern Ireland Cancer Registry in Northern Ireland for data on deaths and cancer registrations. In addition, women residing in England and Wales were also linked to the hospital administrative databases – Hospital Episodes Statistics (HES) and Patient Episode Database for Wales (PEDW) respectively. Data on cancers for 2001–2010 were also received from the National Disease Registration Service (NCRAS, formerly NCIN) for English participants. The dates when data were last received from the various agencies are detailed in Table 1.
| Sources and type of notification | Date last update received |
|---|---|
| Death registration | |
| England | 18/09/2020 |
| Wales | 18/09/2020 |
| Northern Ireland | 14/09/2020 |
| Cancer registration | |
| England | 07/10/2020 |
| Walesa | 31/12/2016 |
| Northern Ireland | 23/03/2020 |
| Hospital episode statistics | |
| England | 05/06/2020 |
| Wales | 14/07/2020 |
| Registry flagging (members and posting) | |
| England | 18/09/2020 |
| Wales | 18/09/2020 |
| NCIN cancer data | |
| England | 13/02/2015 |
| UKCTOCS health questionnaires | |
| FUQ1 | 2005–2010 |
| FUQ2 | 04/04/2014 |
| FUQ3b | 16/06/2020 |
In addition, all participants were sent two postal questionnaires (Project documentation I and J); the first 3–5 years post randomisation and the second in April 2014. A third questionnaire (Project documentation K) was sent to a subset of participants in June 2020 who had either exited the national registries or for whom it was not possible from HES data to ascertain if both ovaries had been removed. We also received ad hoc direct communication from trial participants, their families and clinicians.
Cancer site and cause of death review
Nineteen International Classification of Diseases (ICD-10) codes (Table 2) were identified at start of trial to require independent review to confirm or rule out a diagnosis of ovarian or tubal cancer. Throughout the trial, all the data sources were interrogated to identify women reported to be diagnosed with any of these 19 codes post randomisation. 33 Copies of medical notes were retrieved from hospitals where the women were treated. 33,34 The only exception was women with malignant neoplasm of uncertain origin (ICD-10 C80) who also had another non-ovarian cancer registration. Notes, with any reference to randomisation group redacted, were reviewed by the outcomes review (OR) committee consisting of gynaecological pathologists and oncologists. The OR committee assigned cancer site (using a previously audited pre-specified algorithm; Project documentation L),33 International Federation of Gynaecology and Obstetrics (FIGO) 2014 stage, grade, histotype, type of ovarian cancer and cause of death (Project documentation M). Ovarian and tubal cancer was defined using the revised WHO 2014 classification. 35,36 Death due to ovarian and tubal cancer was based on disease progression as evidenced by appearance of new lesions or increase in size of previously documented lesions with imaging, clinical worsening, or rising biomarker concentrations.
| ICD-10 code | Description |
|---|---|
| C56 | Malignant neoplasm of ovary |
| C57.0 | Malignant neoplasm of fallopian tube |
| C57.4 | Uterine adnexa, unspecified |
| C57.7 | Other specified female genital organs |
| C57.8 | Malignant neoplasm of overlapping lesion of female genital organs |
| C57.9 | Malignant neoplasm of female genital organ, unspecified |
| C48.0 | Retroperitoneum |
| C48.1 | Specified parts of peritoneum |
| C48.2 | Malignant neoplasm of peritoneum, unspecified |
| C48.8 | Overlapping lesions of retroperitoneum and peritoneum |
| C76.2 | Malignant neoplasm of abdomen |
| C76.3 | Malignant neoplasm of pelvis |
| C80 | Malignant neoplasm without specification of site |
| D07.3 | Carcinoma in situ of other/unspecified female genital organ |
| D28.2 | Benign neoplasm of fallopian tube |
| D28.9 | Benign neoplasm of female genital organ, unspecified |
| D36.9 | Benign neoplasm of unspecified site |
| D39.1 | Neoplasm of uncertain or unknown behaviour of ovary |
| D39.9 | Neoplasm of uncertain or unknown behaviour of female genital organ, unspecified |
Additional data for secondary outcomes
For analyses related to ovarian and tubal cancer incidence and compliance with screening, it was essential to identify women who had bilateral (or unilateral if the other adnexa had been previously removed) salpingo-oophorectomy outside the trial. We did this by identifying all women who post randomisation had undergone any ovarian/adnexal surgery outside the trial by searching for the relevant procedure codes in the HES records of women resident in England and self-reported text in the questionnaires. Where possible and particularly if this was based on self-reporting, the procedure was confirmed by contacting the relevant hospital for copies of surgery and histology notes.
To collect data on any ovarian cancer screening undergone by the women in the C group (contamination), a series of questions related to screening for ovarian cancer outside UKCTOCS and reasons (symptoms, screening, at the woman’s own request or for other reason) for any CA125 or ovarian ultrasound performed after trial recruitment were included in the second postal follow-up questionnaire (Project documentation J) sent in 2014, three years after end of screening.
To ensure risks related to screening were properly recorded, RC teams were asked to report all complications that resulted from screening as well as trial surgery resulting in benign pathology findings. In addition, they were asked to report potential suspected unexpected serious adverse reactions (SUSAR) to a designated safety officer at the CC. The safety officer also reviewed operative and other relevant pages of the hospital notes of all women who underwent trial surgery and had false-positive benign pathology for any unreported complications. A designated senior gynaecological oncologist blinded to the randomisation group reviewed the medical notes and confirmed all surgical complications. This individual classified the complications as major or minor using a standard review form.
To assess whether the trial resulted in a cost-effective reduction in ovarian and tubal cancer deaths in the screen groups:
-
For each trial participant in the MMS and USS groups, all NHS resource usage relative to screening was captured. For those referred for clinical assessment, data related to hospital clinic visits, additional imaging, blood tests and trial surgery were captured through review of medical notes. The majority of the participants had treatment in the NHS, with a small minority treated privately, with the private health-care resource usage also captured.
-
Between February 2017 and March 2020 changes in quality of life were captured through the NICE preferred quality-of-life instrument, the EuroQoL EQ-5D-5L37 instrument (www.nice.org.uk/process/pmg9/chapter/foreword), and the disease-specific FACT-O questionnaires. These were sent to all the women diagnosed with ovarian and tubal cancer whose diagnosis was confirmed by the OR Committee, after ensuring that they were still alive. Those for whom the current address was not known to the team or who had previously requested ‘no further contact’ were excluded. The EuroQol instrument included EQ-5D-5L (five-question instrument using five domains) as well as the EQ-5D-5L Visual Analogue Scale (a numerical representation of QoL).
-
Additional EQ-5D-3L data on ovarian cancer patients – In view of the limited number of questionnaires received by October 2019, a decision was made to supplement the UKCTOCS QoL data with those collected in the course of the International Collaboration on Ovarian Neoplasms (ICON8) trial. The ICON8 trial had a population with considerable overlap with the UKCTOCS population with respect to treatment for ovarian cancer. The ICON8 EQ-5D-3L data on participants recruited from England to the standard-care 3-weekly carboplatin and paclitaxel arm of ICON8 were therefore requested as they map to the treatment the women in UKCTOCS received. Having these additional data from women with all Stages (I–IV) from the ICON8 cohort (collected at baseline, 9 months, 18 months, 5 years) would have enabled greater accuracy in the adjustment for overall quality of life and subsequent quality-of-life tariffs used in the QALY calculation for our cost-effectiveness/utility analysis.
Psychosocial study
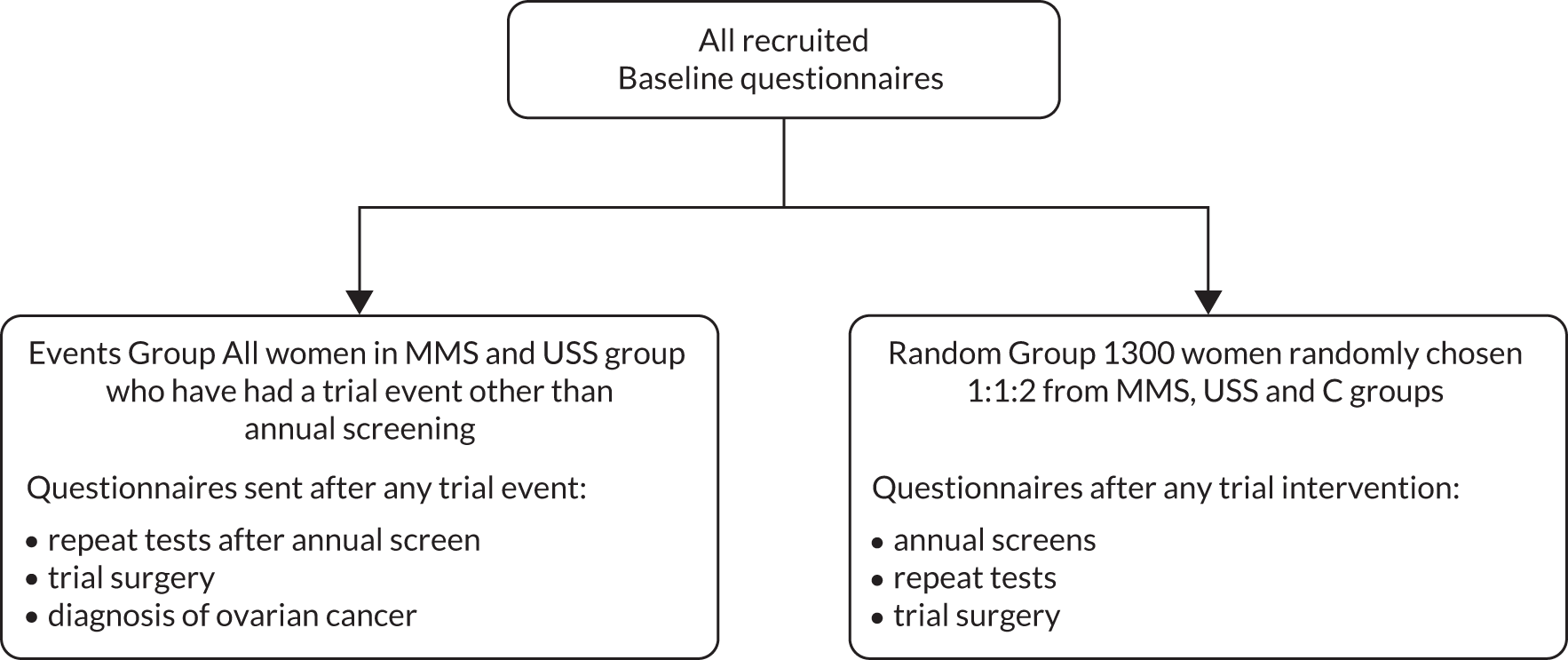
All consented women completed psychosocial baseline questionnaires at randomisation. These include an examination of their knowledge, beliefs and attitudes to ovarian cancer screening and standardised questionnaires including the General Health Questionnaire 12 (GHQ-12), Speilberger Trait Anxiety Inventory (STAI) and Fallowfield’s Sexual Activity Questionnaire (SAQ). Additionally, as detailed in Figure 4, a longitudinal study was undertaken in two groups:
-
Random group About 1300 women were randomly chosen from the C, MMS and USS groups. Baseline data were entered into a database and all women received annual questionnaires plus additional assessments if results from annual screens indicated a need for repeat testing. The women from the C group, who did not receive any form of screening, received annual questionnaires only.
-
Events group This consisted of all participants in the MMS and USS groups who were recalled for repeat tests following their annual screen or underwent trial surgery. They were sent the GHQ-12, STAI and SAQ questionnaires for completion and return within 2 weeks of the repeat tests in the screening episode. If they were diagnosed with ovarian cancer, they were followed longitudinally at 6 months post treatment start. 30
FIGURE 4.
Design of psychosocial study.
Outcomes
All outcome data were kept confidential until unblinding. For most outcomes, subgroup analysis was undertaken for invasive epithelial ovarian and tubal cancer.
Primary outcomes
The primary outcome was death due to ovarian (ICD-10 C56) or tubal (ICD-10 C57.0) cancer as ascertained by the OR committee. Ovarian cancer includes primary non-epithelial ovarian cancer, borderline epithelial ovarian cancer and invasive epithelial ovarian cancer (WHO 2014 classification). 35 Ovarian cancer was defined using the revised WHO 2014 definition.
Secondary outcomes
There were a number of secondary outcomes:
-
ovarian and tubal cancer incidence
-
stage at diagnosis of ovarian and tubal cancer
-
compliance with screening in the MMS and USS groups
-
performance characteristics of screening in the MMS and the USS groups
-
complications due to screening
-
complications in women found to have benign pathology at trial surgery in the MMS and USS groups
-
psychological impact of screening
-
contamination in the control group
-
cost-effectiveness of screening
-
bioresource for future research with a focus on early detection and treatment of disease
Changes to trial design
The trial spanned 20 years (April 2001–June 2021). During this period, there were a number of changes, with the most significant ones related to external growing evidence on the origins and natural history of ovarian cancer and on cancer screening. All are detailed below.
Extension of screening in screen groups
In the original trial protocol, women in the MMS and USS groups were to receive six annual screens. 38 In 2008, an analysis of both overall and cause-specific standardised mortality and incidence ratios at a mean of 5.55 years from randomisation in the C group compared to the national mortality statistics demonstrated a ‘healthy volunteer effect’. Trial participants were less likely to have died or had a cancer diagnosis compared to the general population. 39 As the event rate for the ovarian and tubal cancer death (primary outcome) in the C group was lower than expected, the trial protocol was amended. Screening was extended in the USS and MMS groups to 31 December 2011, resulting in women being offered seven to 11 screens depending on the year of randomisation, compared to six as originally planned.
Change of ROCC cut-offs used in the screening algorithm in the MMS group
At the start of the trial, in the MMS group, the ROCC cut-offs (<1 in 1818 and <1 in 500) at annual screen were set to allow approximately 15% and 2% of women to be triaged to intermediate and elevated risk categories respectively. Based on test performances within UKCTOCS, in April 2005 and after discussion with the Data Monitoring and Ethics (DMEC) and Trial Steering (TSC) committees, the cut-offs were decreased to <1 in 3500 and <1 in 1000 respectively to maintain the initially specified target proportions for triage. This has been detailed in previous publications. 40,41
Length of follow-up
The initial design for the primary outcome measure (death due to ovarian cancer) involved follow-up of all participants for 7 years from date of individual randomisation. In 2008, as a result of the low event rate42 (see above), follow-up was extended for all trial participants, irrespective of randomisation date, to 31 December 2014 for the initial analysis of the trial.
At censorship on 31 December 2014, compared to C group, there was a significant increase in women with Stage I and II (early stage) invasive epithelial ovarian/tubal/peritoneal cancers in the MMS but not the USS group. The reduction in ovarian and tubal deaths compared to the C arm was not conventionally significant, with ‘average’ estimated relative mortality reductions of 15% (p = 0.10) in the MMS and 11% (p = 0.21) in the USS groups on the primary Cox analysis. However, there was evidence of an increasing mortality reduction over time, as noted in other screening trials. 43–46 The reductions in the MMS arm were significant38 in a pre-specified analysis excluding prevalent cases and a post hoc weighted log-rank analysis.
Additionally, the ovarian and tubal cancer mortality rate in the C group was continuing to rise linearly at censorship, whereas it appeared to be plateauing in the MMS and USS groups. Also extrapolated cost-effectiveness estimates to 25 years showed much improved value for money in the MMS group. 47 As the effect of screening appeared much later than anticipated, it was agreed following scientific peer review to extend follow-up till 591 primary outcomes events (deaths due to ovarian and tubal cancer) had occurred following randomisation in the C group. This was initially estimated to occur by December 2018. However, due to a lower event rate than anticipated in the C group, it was extended to 30 June 2020.
Ovarian and tubal cancer definition
The pre-specified primary outcome was death due to ovarian or tubal cancer as confirmed by the OR committee. No change was made to the primary outcome. However, till 2014, the committee used the 2003 World Health Organisation (WHO) criteria36 to define ovarian and tubal cancers. It included (1) malignant neoplasms of the ovary (ICD-10 C56), which included borderline and non-epithelial ovarian cancers in addition to invasive epithelial ovarian cancer, (2) malignant neoplasms of the fallopian tube (ICD-10 C57.0) and (3) undesignated malignancies where it is not possible to assign primary site definitively to ovary, tube or peritoneum. 48 It did not include primary peritoneal carcinoma which was diagnosed on the basis of the following WHO 2003 criteria: (i) both ovaries must be normal in size or enlarged by a benign process; (ii) involvement in extra-ovarian sites must be greater than that on the surface of either ovary; (iii) ovarian tumour involvement must be either non-existent, confined to surface epithelium without stromal invasion, or involving cortical stroma with tumour size less than 5 × 5 mm. In view of the growing understanding of the biology of the disease, the WHO updated their definitions in the 2014 classification to include as ovarian and tubal cancer most cancers that were previously classified as primary peritoneal cancers. In the trial, the primary site definition was therefore revised to that specified by the WHO 2014 classification. The OR committee Chair reviewed all 41 cancers previously classified as primary peritoneal as per the WHO 2003 classification. 36
Ovarian and tubal cancer staging criteria
The cancers diagnosed between 2001 and 2014 were initially staged as per FIGO 2003 staging criteria. The latter criteria were revised by FIGO in 2014. 48 Cancers diagnosed from 2015 were staged as per FIGO 2014 criteria. The OR committee also re-staged using FIGO 2014 criteria all ovarian and tubal cancers diagnosed in 2001–2014.
Primary analysis
Screen group comparison
The primary analysis was initially planned as a comparison of the combined primary outcome results from the MMS and USS groups versus the C group, followed by comparisons of MMS versus C and USS versus C. However, this assumed that the sensitivity of the two screening strategies would be similar. During the course of the trial, the DMEC indicated that this was not the case and released the prevalence screen data for publication. Based on the difference in performance characteristics for detection of ovarian and tubal cancer,40 the final statistical analysis plan promoted the MMS versus C and USS versus C comparisons to be the primary analyses. This was done independently before the DMEC had seen any accumulating results on the primary outcome measure (ovarian and tubal cancer death) comparison.
Approach
In the primary outcome analysis following censorship on 31 December 2014,38 we used a Cox version of the log-rank test that is most powerful under proportional hazards. In the years that followed, there was extensive discussion within Trial Management and Trial Steering Committees on the best approach to analyse the data, given the accumulating external evidence from other screening trials of delayed mortality effects. We finally consulted 12 independent international statistical, trial and screening experts. The majority of experts supported a change in primary analysis to a test sensitive to delayed effects. The details and rationale underpinning this important change were reported separately prior to unblinding of the final data. 49 We chose the Versatile test that was agnostic to the specific form of the screening effect. The Versatile test, described in 2016,50 is a combination test of three log-rank test statistics (Z1, Z2, Z3), covering early, constant and late effects respectively.
Stopping guidelines and interim analysis
There were stopping guidelines for safety specified in terms of excess morbidity resulting from screening or ‘false positive’ trial surgery that resulted in a diagnosis of benign pathology. This was monitored annually by the independent DMEC, as were the operating characteristics of each screening strategy. We undertook an interim analysis of the primary outcome when approximately half the number of initially planned ovarian and tubal cancer deaths had occurred in C group. There were no stopping guidelines for futility and the critical significance level guideline for a stopping for benefit for either screening strategy was very small (α = 0.001).
Sample size and power calculation
In 2000, the sample size was set at 200,000 women to be randomised 2:1:1 (C, MMS, USS groups), with six annual screens and follow-up of seven years. A 4% annual attrition rate was assumed. This was estimated to provide 80% power at a two-sided 5% significance level for a difference in ovarian and tubal cancer mortality of 25% (and over 90% power for a difference of 30%) between the combined screening groups and the C group and 70% power to detect a reduction of 30% in MMS versus C or USS versus C comparisons.
Power was recalculated following the extension of trial duration to 31 December 2014. It was estimated to be 80% at a two-sided 5% significance level to detect a reduction of 30% in MMS versus C or USS versus C comparisons.
At initial censorship on 31 December 2014, there were 358 ovarian and tubal cancer deaths in the C group. Compared to the C group, the ‘average’ estimated relative reduction in deaths was 11% (Cox model p = 0.240) MMS and 9% (Cox model p = 0.32) USS. Any mortality reduction was only apparent about seven years after randomisation. 45% (162/358) of the deaths in the C group during 2001–2014 had occurred before seven years. In 2015, for the no screening versus MMS or USS comparisons, we estimated that an additional 233 C group events would give 80% power at a two-sided 5% significance level for a difference in relative mortality of 25% during long-term (2015–2020) follow-up conditional on the observed mortality reduction of 11%. This translated to a target sample size of 591 overall events in the C group. All 233 new and 73% (431/591) of total C group events would occur beyond seven years.
Statistical analysis
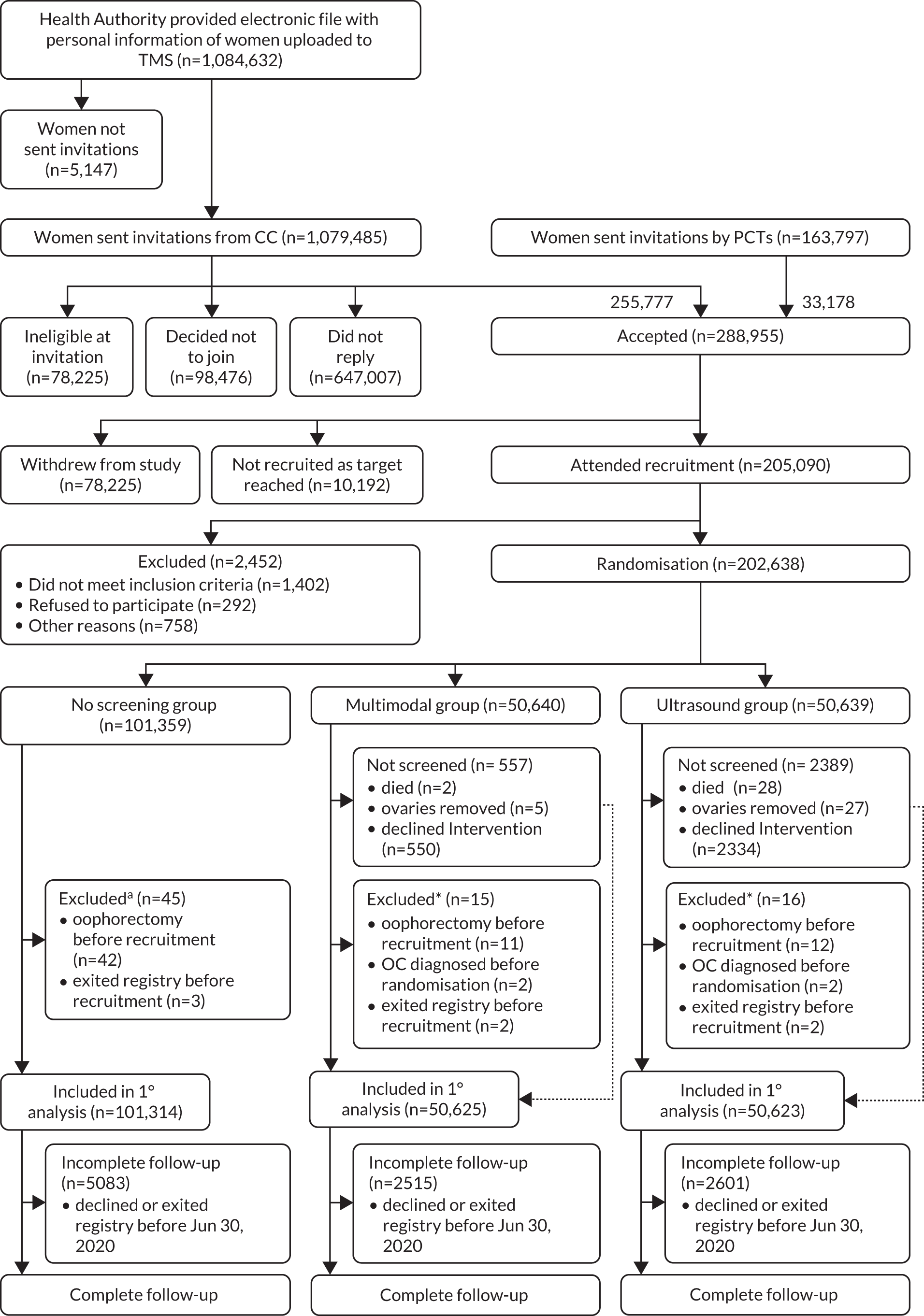
Uptake and recruitment rates were described using descriptive statistics. A Consolidated Standards of Reporting Trials (CONSORT) diagram was constructed for the analysis of primary outcome (Figure 5). Descriptive statistics by randomisation group were calculated for baseline characteristics of all eligible participants. The trial consisted of three phases:
FIGURE 5.
CONSORT diagram. a, Events occurred before recruitment, but were discovered after randomisation.
-
Recruitment and randomisation phase between 2001 and 2005.
-
Screening phase, which ran concurrent with recruitment and continued till 2012, one year after the end of screening in 2011. For individual trial participants, it was defined as the period from randomisation to the anniversary of the randomisation date in 2012.
-
Follow-up with no screening from individual randomisation dates in 2012 to 30 June 2020 (final censorship date).
All ovarian and tubal cancers confirmed by the OR committee were classified by randomisation group as appropriate into:
-
screen detected (screen positives)
-
those not detected by screening (clinically diagnosed); the latter consisted of:
-
screen negatives diagnosed within one year of last test in a screen episode (the false negatives used for calculating performance characteristics of screening strategies)
-
screen negatives diagnosed more than one year after last test of screen episode during the screening phase
-
diagnosed greater than one year after screening phase had ended
-
diagnosed in women who never attended screening.
-
Details of stage, histotype, morphology and type of ovarian and tubal cancers were tabulated as were deaths due to the disease.
Primary outcome
All analyses were by intention to screen. The primary mortality analysis was a MMS versus C and USS versus C analysis of the deaths due to ovarian and tubal cancer (primary outcome). We defined survival time from date of randomisation (t0 = 0) to date of death due to ovarian cancer or censorship, or sooner if the volunteer died of another cause or was lost to follow-up. In those who were lost to follow-up through exiting the national registries and where we had no evidence that they were dead, we censored using the last date where we had evidence that they were alive through completing a follow-up questionnaire, attending a screening appointment, directly contacting the trial to withdraw or attending hospital as per HES and PEDW records. No allowances were made for non-compliance to screening or contamination (ovarian cancer screening) in the C group.
Initial censorship (31 December 2014)
A Cox proportional hazards model was used. To allow for the fact that we were comparing two intervention groups against a control group, we made a Dunnett correction for multiple testing against a control to the critical α (α = 0.0258). Mortality reduction estimates were 1 – hazard ratio (HR) estimates. Additional analyses of ovarian and tubal deaths as well as methods and results of sensitivity analyses are detailed elsewhere. 38
Final censorship (30 June 2020)
The Versatile test50 was used for the MMS versus C and USS versus C comparison with a Royston–Parmar (RP) model51 to estimate survival differences. No formal adjustment was made to the test for (a) having previously analysed the 2001–2014 data or (b) making two screen group comparisons. Instead, it was decided to openly describe the multiplicity issues and acknowledge the unadjusted p-values.
The potential time-dependent features of the screening effect were explored by estimating the hazard ratio and the absolute survival difference at the pre-specified time-points of t = 5, 10, 15 and 18 years (maximum follow-up was 19.3 years) using a flexible parametric RP model. 51,52 A subgroup analysis of invasive epithelial ovarian and tubal cancer death where other ovarian cancers were censored at death was performed. Secondary analyses of the primary outcome as well as methods and results of sensitivity analyses are detailed in a previous publication. 53
Survival from diagnosis in women with ovarian and tubal cancer in the no-screening group was also compared to national age and period-adjusted 1, 5 and 10-year survival rates.
Secondary outcomes
Ovarian and tubal cancer incidence
Cumulative incidence of ovarian and tubal cancer was graphically presented using standard Kaplan–Meier (KM) methods, based on time from randomisation to diagnosis. Death from other causes and bilateral oophorectomy were censoring events. Administrative censorship was the same as for the mortality analysis (30 June 2020). The underlying incidence (hazard) rates for each group were also investigated with RP models which have been published previously. 53 Bilateral salpingo-oophorectomy undertaken in women both within and outside the trial was treated as a competing (risk) event.
Stage at diagnosis of ovarian and tubal cancer
Both for ovarian and tubal cancer and the subgroup of invasive epithelial ovarian and tubal cancers, incidence rate ratios (IRRs) with 95% CI were used to compare C versus MMS and USS groups separately. As during extended follow-up between 2015 and 2020 there were no screen-detected cases, this analysis was done for both censorship dates (31 December 2014 and 30 June 2020). Stage-specific ovarian and tubal cancer case fatality rates were also calculated.
Ovarian cancer survival in C arm
It was expected that with time, the ‘healthy volunteer effect’ initially observed would decrease. Survival from date of diagnosis for ovarian cancers in the C arm was calculated to explore external validity of UKCTOCS by comparison to UK ovarian cancer survival statistics, in both the 2015 and 2021 analysis.
Adherence to screening in the MMS and USS groups
All women were eligible for screening unless they had undergone bilateral (or unilateral if the other adnexa had been previously removed) salpingo-oophorectomy, been diagnosed with ovarian or tubal cancer or died prior to the next screening date. Compliance was calculated for annual screening episodes as the proportion of women who attended all tests that comprised the screening episode out of the total who were eligible for that screening episode. A woman who only attended the annual screen but did not attend all repeat tests as per protocol was deemed non-compliant for that screening episode.
Performance characteristics of screening in the MMS and the USS groups
The annual screen episode was defined as a single or series of repeat tests (serum CA125 or transvaginal ultrasound scans) as outlined in the respective MMS and USS screening protocols (see Figures 2 and 3) that culminated in surgery or a return to annual screening. For this analysis, all women in the MMS and USS groups were censored one year after the last test of the last annual screening episode they attended. The primary outcome measure for this analysis was OR committee confirmed ovarian or tubal cancer.
The screen was considered positive (screen positive) if the woman was referred for surgery or biopsy for suspected ovarian or tubal cancer following clinical evaluation. Included in this category were some women who, while awaiting repeat testing on the trial, underwent imaging followed by surgery for ovarian masses privately. The screen was considered negative (screen-negative) if the woman was returned to annual screening. A screen-detected cancer was one that resulted from screen-positive surgery and/or biopsy. A screen-negative (interval) cancer was an ovarian or tubal cancer diagnosed clinically within 12 months of the last test of the screen episode, in women who were returned to annual screening.
The analysis was done for all screens combined and for the initial (prevalence) and remaining (incidence) annual screens separately. Sensitivity (proportion detected of all ovarian and tubal cancers diagnosed within one year of last attended screen), specificity (proportion of those without ovarian or tubal cancer within one year of last attended screen who had a negative screen) and positive predictive value (proportion with a screen-positive result who had ovarian or tubal cancer at trial surgery/biopsy) were calculated. Subgroup analysis of invasive epithelial cancers (borderline epithelial and non-epithelial ovarian cancers were excluded) was undertaken.
Complications
All complications resulting from screening and all complications related to trial surgery in women who were found to have benign pathology were detailed using descriptive statistics.
Contamination in the control group
Of the C group women who completed the follow-up questionnaire in 2014, the proportion who reported ovarian cancer screening was documented.
The bulk of the statistical analyses were done using Stata® (version 16; StataCorp LP, College Station, TX, USA) and R (version 4.0.2; The R Foundation for Statistical Computing, Vienna, Austria).
Psychological impact of screening
The two (random and events) groups were followed for seven years from randomisation. Scores from the STAI state 20-item questionnaire54 ranging from 20 to 80 were treated as continuous variables. GHQ-12 scores were dichotomised, with ≥4, signifying probable psychological morbidity. 55 Anxiety scores were compared between participants using data from annual screens. Repeat screen data were included in subsequent analyses to examine within-participant effects of time-varying explanatory variables. Analyses were adjusted for centre, age at randomisation, year of screening, baseline STAI trait score and screening group. P-values were calculated using Wald tests. Separate analyses were carried out for the random sample and the event sample. 30,47
Cost-effectiveness of screening
This was undertaken at initial censorship and planned for the whole follow-up period (i.e. following final censorship). The resource use for screening and the relevant hospital episodes (in-patient, day case and outpatient), procedures, blood tests and clinics were mapped. The associated NHS tariff prices were attached to these visits. The unit costs arising from treatment of ovarian cancer with chemotherapy agents were supplemented from a number of secondary sources, primarily reports from NICE (UK) and the British National Formulary prices. The only exception to the use of published unit costs was the unit cost of the CA125 -ROCA test, which was not available in the NHS. The unit cost was estimated at £20 and subjected to extensive sensitivity analysis to account for the gross uncertainty surrounding this estimated NHS value. 47
Following initial censorship (31 December 2014), an incremental cost-effectiveness ratio (ICER) analysis was undertaken of the MMS and USS screening programmes separately comparing them to the C arm over the period of the trial. It was based on individual patient-level data collected during the trial between 2001 and 2014. It was assessed from the perspective of a national NHS screening programme. Hence only direct health service costs covering the programme costs of the MMS and USS screening and the subsequent treatment were included. In addition, lifetime extrapolation of no screening (C group) was compared to a MMS programme using both a predictive and a Markov model.
A similar analysis was planned for the whole follow-up period based on individual patient-level data collected during 2001–2014 and the follow-up period to 2020.
Results
Between March 2001 and August 2005, invitations to participate were sent to women aged 50–74 from the age-sex register of 27 PCTs. This included 3266 general practices. Twenty-four (89%) of the PCTs provided contact details of all women, and we sent invitations as outlined above. Three (11%) PCTs (adjoining the Liverpool, Belfast and initially Gateshead RCs) attached address labels to blank envelopes containing invitations as they refused to release contact details directly to the CC.
Overall, 1,243,282 women were invited, 1,079,485 directly by the CC and 163,797 by the PCTs. 78,225 reported they were ineligible. Of those remaining, 288,955 (24.8%) women accepted the invitation to participate in the trial. The acceptance rate varied between centres from 19% (East London) to 33% (Bristol). Between 18 April 2001 and 29 September 2005, 205,090 women (73.6% of those sent appointments) attended the recruitment appointment, and by 21 October 2005, 202,638 women were randomised (Figure 5).
The final cohort eligible for analysis at censorship on 30 June 2020 (Figure 5) consisted of 202,562 (>99.9%) of the 202,638 women: 50,625 (>99.9%) of the MMS group, 50,623 (>99.9%) of the USS group, and 101,314 (>99.9%) of the C group. Seventy-six (<0.1%) women (15 [<0.1%] in the MMS group; 16 [<0.1%] in the USS group; and 45 [<0.1%] in the no-screening group) were excluded as they had bilateral salpingo-oophorectomy, ovarian cancer before joining the trial, or had exited the national death registry before randomisation.
Baseline characteristics of eligible women by group and overall
The median age of participants at recruitment was 60.6 years. The majority were white, parous with two children, and more than half had used the oral contraceptive pill. About 40% of the oldest birth cohort (1925–9) reported no formal educational qualification, in contrast to 19% of women in the youngest birth cohort (1950–5). 56 Six per cent had a personal history of cancer and 1.6% reported maternal history of ovarian cancer. The baseline characteristics were balanced between the study groups (Table 3).
| Variable | MMS | USS | No Screening | Total |
|---|---|---|---|---|
| (n = 50,625) | (n = 50,623) | (n = 101,314)a | (n = 202,562)b | |
| Age at randomisation (years) | 60.61 | 60.61 | 60.58 | 60.59 |
| (56.03–66.15) | (55.99–66.16) | (55.97–66.15) | (55.99–66.15) | |
| Time since last period at randomisation (years) | 11.36 | 11.34 | 11.3 | 11.3 |
| (5.26–18.49) | (5.25–18.47) | (5.22–18.46) | (5.23–18.47) | |
| Duration of HRT use in those who had used it (years) | 8.1 | 8.15 | 8.17 | 8.15 |
| (4.56–11.99) | (4.555–12.11) | (4.5–12.1) | (4.54–12.07) | |
| Duration of OCP use in those who had used it (years) | 5 (2–10) | 5 (2–10) | 5 (2–10) | 5 (2–10) |
| Pregnancies <6 months | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) |
| Children (pregnancies >6 months) | 2 (2–3) | 2 (2–3) | 2 (2–3) | 2 (2–3) |
| Height (cm) | 162.6 | 162.6 | 162.6 | 162.6 |
| (157.5–165.1) | (157.5–165.1) | (157.5–165.1) | (157.5–165.1) | |
| Weight (kg) | 67.6 | 67.6 | 67.6 | 67.6 |
| (60.3–76.2) | (60.3–76.2) | (60.3–76.2) | (60.3–76.2) | |
| Ethnic origin | ||||
| White | 48,846 (96.5%) | 48,749 (96.3%) | 97,612 (96.3%) | 19,5207 (96.4%) |
| Black | 670 (0.7%) | 717 (0.7%) | 1378 (1.4%) | 2765 (1.4%) |
| Asian | 442 (0.4%) | 477 (0.5%) | 936 (0.9%) | 1855 (0.9%) |
| Other | 428 (0.4%) | 424 (0.4%) | 839 (0.8%) | 1691 (0.8%) |
| Missing | 239 (0.2%) | 256 (0.3%) | 549 (0.5%) | 1044 (0.5%) |
| Hysterectomy | 9680 (19.1%) | 9495 (18.8%) | 18,992 (18.7%) | 38167 (18.8%) |
| Ever use of OCP | 30,099 (59.5%) | 30,308 (59.9%) | 60,291 (59.5%) | 120,698 (59.6%) |
| Use of HRT at recruitment | 9457 (18.7%) | 9383 (18.5%) | 19,151 (18.9%) | 37,991 (18.8%) |
| Personal history of cancerb | 2973 (5.9%) | 2972 (5.9%) | 6104 (6%) | 12,049 (5.9%) |
| Personal history of breast cancer | 1848 (3.7%) | 1891 (3.7%) | 3912 (3.9%) | 7651 (3.8%) |
| Maternal history of ovarian cancer | 802 (1.6%) | 778 (1.5%) | 1579 (1.6%) | 3159 (1.6%) |
| Maternal history of breast cancer | 3159 (6.2%) | 3206 (6.3%) | 6621 (6.5%) | 12,986 (6.4%) |
Follow-up information was available until death or censorship (30 June 2020) in 192,478 (95.0%) women (48,110 [95.0%] in the MMS group, 48,022 [94.9%] in the USS group and 96,276 [95.0%] in the C group). Overall, this amounted to 3.16 million women-years. Median follow-up of a participant was 16.3 years (IQR 15.1–17.3).
At censorship (30 June 2020), the OR committee reviewed 4482 women identified to have one of the 19 prespecified ICD-10 codes for possible ovarian or tubal cancer. Of them, 2055 (45.9%) women were confirmed to have ovarian or tubal cancer. The numbers diagnosed by years from randomisation are detailed in Table 4. This translated to 522 (11.6%) in the MMS group, 517 (11.5%) cancers in the USS group and 1016 (22.7%) in the C group.
| Time from randomisation | Ovarian and tubal cancer cases | Ovarian and tubal cancer deaths | ||||||
|---|---|---|---|---|---|---|---|---|
| Group | Group | |||||||
| No screening | MMS | USS | All | No screening | MMS | USS | All | |
| 0 ≤ years <1 | 47 | 46 | 56 | 149 | 4 | 2 | 4 | 10 |
| 1 ≤ years <2 | 52 | 36 | 37 | 125 | 14 | 8 | 6 | 28 |
| 2 ≤ years <3 | 62 | 41 | 25 | 128 | 19 | 13 | 8 | 40 |
| 3 ≤ years <4 | 60 | 22 | 26 | 108 | 28 | 10 | 14 | 52 |
| 4 ≤ years <5 | 59 | 31 | 32 | 122 | 28 | 16 | 17 | 61 |
| 5 ≤ years <6 | 67 | 27 | 27 | 121 | 43 | 15 | 13 | 71 |
| 6 ≤ years <7 | 66 | 36 | 38 | 140 | 26 | 16 | 15 | 57 |
| 7 ≤ years <8 | 69 | 34 | 28 | 131 | 40 | 15 | 23 | 78 |
| 8 ≤ years <9 | 60 | 39 | 25 | 124 | 45 | 23 | 24 | 92 |
| 9 ≤ years <10 | 69 | 28 | 24 | 121 | 55 | 27 | 24 | 106 |
| 10 ≤ years <11 | 62 | 26 | 23 | 111 | 45 | 16 | 18 | 79 |
| 11 ≤ years <12 | 67 | 34 | 35 | 136 | 47 | 17 | 12 | 76 |
| 12 ≤ years <13 | 58 | 41 | 32 | 131 | 44 | 31 | 36 | 111 |
| 13 ≤ years <14 | 67 | 27 | 39 | 133 | 42 | 28 | 13 | 83 |
| 14 ≤ years <15 | 65 | 17 | 28 | 110 | 47 | 23 | 26 | 96 |
| 15 ≤ years <16 | 45 | 22 | 21 | 88 | 50 | 27 | 20 | 97 |
| 16 ≤ years <17 | 25 | 10 | 13 | 48 | 27 | 6 | 10 | 43 |
| 17 ≤ years <18 | 14 | 5 | 7 | 26 | 13 | 2 | 6 | 21 |
| 18 ≤ years <19 | 1 | 0 | 1 | 2 | 2 | 1 | 2 | 5 |
| 19 ≤ years <20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 1015a | 522 | 517 | 2054 | 619 | 296 | 291 | 1206 |
Overall, 1805 (87.8%) of 2055 women had invasive epithelial ovarian or tubal cancers: 452 (0.9%) in the MMS group, 445 (0.9%) in the USS group and 905 (0.9%) in the no-screening group (Table 5).
| Cancer type | Total | Screen detected (screen positive) | Clinically diagnosed (not detected by screening) | |||
|---|---|---|---|---|---|---|
| Screen negatives – less than 1 year from last test of screening | Screen negatives > 1 year after last test of screening episode | Never attended screening | Diagnosed >1 year after end of screening | |||
| MMS (50,625 women, 789,129 women-years) | ||||||
| Ovarian and tubal cancer | 522 (100%) | 212 (41%) | 41 (8%) | 41 (8%) | 3 (1%) | 225 (43%) |
| Non-epithelial ovarian cancer | 16 (100%) | 7 (44%) | 2 (13%) | 2 (13%) | 0 | 5 (31%) |
| Borderline epithelial ovarian cancer | 54 (100%) | 24 (44%) | 10 (19%) | 5 (9%) | 0 | 15 (28%) |
| Invasive epithelial ovarian and tubal cancer | 452 (100%) | 181 (40%) | 29 (6%) | 34 (8%) | 3 (1%) | 205 (45%) |
| USS (50,623 women, 790,231 women-years) | ||||||
| Ovarian and tubal cancer | 517 (100%) | 164 (32%) | 63 (12%) | 50 (10%) | 19 (4%) | 221 (43%) |
| Non-epithelial ovarian cancer | 13 (100%) | 11 (85%) | 0 | 1 (8%) | 0 | 1 (8%) |
| Borderline epithelial ovarian cancer | 59 (100%) | 48 (81%) | 2 (3%) | 1 (2%) | 3 (5%) | 5 (8%) |
| Invasive epithelial ovarian and tubal cancer | 445 (100%) | 105 (24%) | 61 (14%) | 48 (11%) | 16 (4%) | 215 (48%) |
| No screening (101,314 women, 1,577,517 women-years) | ||||||
| Ovarian and tubal cancer | 1016a (100%) | .. | .. | 514 (51%) | .. | 499 (49%) |
| Non-epithelial ovarian cancer | 17 (100%) | .. | .. | 7 (41%) | .. | 10 (59%) |
| Borderline epithelial ovarian cancer | 91 (100%) | .. | .. | 50 (55%) | .. | 41 (45%) |
| Invasive epithelial ovarian and tubal cancer | 905 (100%) | .. | .. | 457 (50%) | .. | 448 (50%) |
| Group | Screen-detected status | Stage | Proportion detected in Stage I/II by screening status | |||||
|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | Unable to stage | Total | |||
| Serous low-grade (n = 49) | ||||||||
| No screening | Clinically diagnosed | 8 (38%) | 3 (14%) | 8 (38%) | 2 (10%) | 0 (0%) | 21 | 52% |
| MMS | Clinically diagnosed | 2 (40%) | 0 (0%) | 3 (60%) | 0 (0%) | 0 (0%) | 5 | 40% |
| Screen detected | 8 (73%) | 0 (0%) | 3 (27%) | 0 (0%) | 0 (0%) | 11 | 73% | |
| USS | Clinically diagnosed | 1 (14%) | 1 (14%) | 3 (43%) | 2 (29%) | 0 (0%) | 7 | 29% |
| Screen detected | 1 (20%) | 0 (0%) | 4 (80%) | 0 (0%) | 0 (0%) | 5 | 20% | |
| Endometrioid (n = 59) | ||||||||
| No screening | Clinically diagnosed | 25 (81%) | 4 (13%) | 1 (3%) | 1 (3%) | 0 (0%) | 31 | 94% |
| MMS | Clinically diagnosed | 8 (80%) | 0 (0%) | 2 (20%) | 0 (0%) | 0 (0%) | 10 | 80% |
| Screen detected | 8 (80%) | 1 (10%) | 0 (0%) | 1 (10%) | 0 (0%) | 10 | 90% | |
| USS | Clinically diagnosed | 3 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 | 100% |
| Screen detected | 4 (80%) | 1 (20%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 | 100% | |
| Mucinous (n = 52) | ||||||||
| No screening | Clinically diagnosed | 26 (79%) | 5 (15%) | 1 (3%) | 1 (3%) | 0 (0%) | 33 | 94% |
| MMS | Clinically diagnosed | 7 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 7 | 100% |
| Screen detected | 1 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 | 100% | |
| USS | Clinically diagnosed | 6 (75%) | 0 (0%) | 1 (13%) | 1 (13%) | 0 (0%) | 8 | 75% |
| Screen detected | 2 (67%) | 1 (33%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 | 100% | |
| Clear cells (n = 58) | ||||||||
| No screening | Clinically diagnosed | 14 (56%) | 4 (16%) | 6 (24%) | 1 (4%) | 0 (0%) | 25 | 72% |
| MMS | Clinically diagnosed | 10 (71%) | 2 (14%) | 2 (14%) | 0 (0%) | 0 (0%) | 14 | 86% |
| Screen detected | 3 (60%) | 2 (40%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 | 100% | |
| USS | Clinically diagnosed | 0 (0%) | 1 (33%) | 1 (33%) | 1 (33%) | 0 (0%) | 3 | 33% |
| Screen detected | 10 (91%) | 1 (9%) | 0 (0%) | 0 (0%) | 0 (0%) | 11 | 100% | |
| Serous high-grade (n = 1240) | ||||||||
| No screening | Clinically diagnosed | 37 (6%) | 43 (7%) | 379 (63%) | 143 (24%) | 1 (0%) | 603 | 13% |
| MMS | Clinically diagnosed | 16 (9%) | 14 (8%) | 102 (55%) | 52 (28%) | 1 (1%) | 185 | 16% |
| Screen detected | 22 (16%) | 18 (13%) | 84 (63%) | 10 (7%) | 0 (0%) | 134 | 30% | |
| USS | Clinically diagnosed | 15 (6%) | 14 (6%) | 151 (61%) | 66 (27%) | 1 (0%) | 247 | 12% |
| Screen detected | 10 (14%) | 10 (14%) | 46 (65%) | 5 (7%) | 0 (0%) | 71 | 28% | |
| Carcinosarcoma (n = 67) | ||||||||
| No screening | Clinically diagnosed | 1 (3%) | 5 (13%) | 28 (72%) | 4 (10%) | 1 (3%) | 39 | 15% |
| MMS | Clinically diagnosed | 0 (0%) | 2 (22%) | 5 (56%) | 2 (22%) | 0 (0%) | 9 | 22% |
| Screen detected | 1 (33%) | 0 (0%) | 2 (67%) | 0 (0%) | 0 (0%) | 3 | 33% | |
| USS | Clinically diagnosed | 0 (0%) | 3 (23%) | 8 (62%) | 2 (15%) | 0 (0%) | 13 | 23% |
| Screen detected | 0 (0%) | 0 (0%) | 3 (100%) | 0 (0%) | 0 (0%) | 3 | 0% | |
| Carcinoma, NOS (n = 241) | ||||||||
| No screening | Clinically diagnosed | 3 (2%) | 5 (4%) | 67 (50%) | 48 (36%) | 10 (8%) | 133 | 6% |
| MMS | Clinically diagnosed | 1 (3%) | 1 (3%) | 20 (59%) | 8 (24%) | 4 (12%) | 34 | 6% |
| Screen detected | 4 (27%) | 1 (7%) | 8 (53%) | 2 (13%) | 0 (0%) | 15 | 33% | |
| USS | Clinically diagnosed | 1 (2%) | 3 (6%) | 27 (52%) | 20 (38%) | 1 (2%) | 52 | 8% |
| Screen detected | 1 (14%) | 0 (0%) | 4 (57%) | 2 (29%) | 0 (0%) | 7 | 14% | |
| Serous grade unknown (n = 32) | ||||||||
| No screening | Clinically diagnosed | 0 (0%) | 0 (0%) | 11 (65%) | 6 (35%) | 0 (0%) | 17 | 0% |
| MMS | Clinically diagnosed | 0 (0%) | 0 (0%) | 5 (71%) | 2 (29%) | 0 (0%) | 7 | 0% |
| Screen detected | 0 (0%) | 0 (0%) | 1 (100%) | 0 (0%) | 0 (0%) | 1 | 0% | |
| USS | Clinically diagnosed | 1 (14%) | 0 (0%) | 1 (14%) | 5 (71%) | 0 (0%) | 7 | 14% |
| Endometrioid grade unknown (n = 2) | ||||||||
| No screening | Screen detected | 0 (0%) | 0 (0%) | 0 (0%) | 2 (100%) | 0 (0%) | 2 | 0% |
| Other (n = 3) | ||||||||
| No screening | Clinically diagnosed | 2 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 | 100% |
| MMS | Screen detected | 0 (0%) | 0 (0%) | 0 (0%) | 1 (100%) | 0 (0%) | 1 | 0% |
Primary outcome – ovarian and tubal cancer deaths
Initial censorship (31 December 2014)
By 31 December 2014, 649 (0.32%) women had died of ovarian cancer: 347 (0.34%) in the C group, 148 (0.29%) in the MMS group and 154 (0.30%) in the USS group. The reduction in ovarian cancer mortality over years 0–14 using the Cox model was 15% (95% CI –3 to 30; p = 0.10) in the MMS and 11% (95% CI –7 to 27; p = 0.21) in the USS group. 38
Final censorship (30 June 2020)
There was no evidence of a reduction in ovarian and tubal cancer deaths in either the MMS (p = 0.58) or USS (p = 0.36) group compared with the C group at final censorship, using the Versatile test (primary analysis). The divergence between the screen and C groups of the Kaplan–Meier cumulative death rates was minimal. At 18 years after randomisation, compared to the C group, the Royston-Parmar model estimates of survival differences per 100,000 women were 36.7 (95% CI –65.3 to 138.8) for MMS and 52.9 (–48.2 to 153.9) for USS groups respectively (Table 7).
| MMS vs. no screening | USS vs. no screening | |||||
|---|---|---|---|---|---|---|
| Time-point | Estimate | L95% CI | U95% CI | Estimate | L95% CI | U95% CI |
| Absolute survival difference per 100,000 women | ||||||
| at 5 years | –0.9 | –28.2 | 26.5 | –3 | –30.7 | 24.6 |
| at 10 years | 6.6 | –43 | 56.1 | 7.1 | –42.4 | 56.6 |
| at 15 years | 22.8 | –53.1 | 98.8 | 31.6 | –44 | 107.1 |
| at 18 years | 36.7 | –65.3 | 138.8 | 52.9 | –48.2 | 153.9 |
| Hazard ratio | ||||||
| at 5 years | 0.98 | 0.82 | 1.18 | 0.98 | 0.82 | 1.17 |
| at 10 years | 0.95 | 0.82 | 1.09 | 0.93 | 0.8 | 1.07 |
| at 15 years | 0.93 | 0.78 | 1.12 | 0.9 | 0.75 | 1.08 |
| at 18 years | 0.92 | 0.75 | 1.14 | 0.88 | 0.72 | 1.09 |
Compared to national one- (68%) and five- (37%) year age- and period-adjusted survival rates from diagnosis in women with ovarian and tubal cancer, the rates in the C group were 77% and 40% respectively. 53
Secondary outcomes
Ovarian and tubal cancer incidence
The incidence of ovarian and tubal cancer per 100,000 women-years was 67.7 (95% CI 61.9 to 73.5; 522 cancers; 770,967 women-years) in the MMS group, 68.2 (95% CI 62.4 to 74.1; 517 cancers; 755,677 women-years) in the USS group, and 65.4 (95% CI 61.4 to 69.4; 1016 cancers; 1,552,703 women-years) in the C group. 53
Stage at diagnosis of ovarian and tubal cancer
At censorship (30 June 2020), 9.5 years after the end of screening, in the MMS compared with the C group, there was a 47.2% (95% CI 19.7 to 81.1) higher incidence of Stage I and a 24.5% (–41.8 to –2.0) lower incidence of Stage IV disease. Overall, in the MMS group compared with the C group, the incidence of Stage I or II was 39.2% (95% CI 16.1 to 66.9) higher and Stage III or IV disease 10.2% (–21.3 to 2.4) lower. 53 The changes in stage distribution in the MMS compared with the C group persisted in the subgroup analysis of invasive epithelial ovarian and tubal cancers. 53 Further analysis revealed that the Stage I incidence rate was significantly increased in Type II
(high-grade serous and carcinosarcoma) cancers in the MMS versus C comparison (Table 8). There was no change in incidence of any stage in the USS compared with the C group.
| Screening group | Variable | Stage | sAll | ||||
|---|---|---|---|---|---|---|---|
| I | II | III | IV | Unable to stage | |||
| All | |||||||
| No screening | Cases – no (%a) | 116 (12.8%) | 69 (7.6%) | 501 (55.3%) | 208 (23.0%) | 12 (1.3%) | 906 |
| Deaths – no (%b) | 34 (29.3%) | 27 (39.1%) | 396 (79.0%) | 174 (83.7%) | 11 (91.7%) | 642 (70.9%) | |
| MMS | Cases – no (%a) | 91 (20.1%) | 41 (9.1%) | 237 (52.4%) | 78 (17.3%) | 5 (1.1%) | 452 |
| Deaths – no (%b) | 32 (35.2%) | 18 (43.9%) | 195 (82.3%) | 62 (79.5%) | 4 (80.0%) | 311 (68.8%) | |
| USS | Cases – no (%a) | 55 (12.4%) | 35 (7.9%) | 249 (56.0%) | 104 (23.4%) | 2 (0.4%) | 445 |
| Deaths – no (%b) | 13 (23.6%) | 8 (22.9%) | 189 (75.9%) | 86 (82.7%) | 2 (100%) | 298 (67%) | |
| Type II (high-grade serous and carcinosarcoma) | |||||||
| No screening | Cases – no (%a) | 40 (4.4%) | 51 (5.6%) | 435 (48.0%) | 164 (18.1%) | 2 (0.2%) | 692 |
| Deaths – no (%b) | 12 (30%) | 21 (41.2%) | 332 (76.3%) | 132 (80.5%) | 2 (100%) | 499 (72.1%) | |
| MMS | Cases – no (%a) | 43 (9.5%) | 36 (8.0%) | 208 (46.0%) | 70 (15.5%) | 1 (0.2%) | 358 |
| Deaths – no (%b) | 15 (34.9%) | 13 (36.1%) | 168 (80.8%) | 55 (78.6%) | 1 (100%) | 252 (70.4%) | |
| USS | Cases – no (%a) | 27 (6.1%) | 29 (6.5%) | 225 (50.6%) | 84 (18.9%) | 1 (0.2%) | 366 |
| Deaths – no (%b) | 2 (7.4%) | 4 (13.8%) | 169 (75.1%) | 68 (81.0%) | 1 (100%) | 244 (66.7%) | |
| MMS versus no screening | Between group differencesc | 116.5 (40.8, 233) | 42.2 (–7.2, 117.8) | –3.7 (–18.4, 13.6) | –14 (–35, 13.7) | NA | NA |
| USS versus no screening | Between group differencesc | 38.3 (–15.1, 125.4) | 16.5 (–26.1, 83.8) | 6 (–9.8, 24.5) | 5 (–19.3, 36.5) | NA | NA |
| Type I (mucinous, clear cell, endometrioid, low-grade serous) | |||||||
| No screening | Cases – no (%a) | 76 (8.4%) | 16 (1.8%) | 18 (2.0%) | 5 (0.6%) | 0 (0%) | 115 |
| Deaths – no (%b) | 6 (7.9%) | 2 (12.5%) | 11 (61.1%) | 4 (80.0%) | 0 (0%) | 23 (14.8%) | |
| MMS | Cases – no (%a) | 47 (10.4%) | 5 (1.1%) | 10 (2.2%) | 2 (0.4%) | 0 (0%) | 64 |
| Deaths – no (%b) | 6 (12.8%) | 3 (60.0%) | 4 (40.0%) | 2 (100%) | 0 (0%) | 15 (23.4%) | |
| USS | Cases – no (%a) | 27 (6.1%) | 5 (1.1%) | 9 (2.0%) | 4 (0.9%) | 0 (0%) | 45 |
| Deaths – no (%b) | 5 (18.5%) | 1 (20.0%) | 3 (33.3%) | 3 (75.0%) | 0 (0%) | 12 (26.7%) | |
| MMS versus no screening | Between group differencesc | 24.5 (–13.4, 79.2) | –37.1 (–76.9, 71.8) | 11.9 (–48.4, 142.4) | –19.4 (–84.4, 315.2) | NA | NA |
| USS versus no screening | Between group differencesc | –27.2 (–53.1, 12.9) | –36 (–76.5, 74.8) | 2.5 (–54, 128.1) | 63.9 (–56, 510.5) | NA | NA |
| Type uncertain | |||||||
| No screening | Cases – no (%a) | 0 (0%) | 2 (0.2%) | 48 (5.3%) | 39 (4.3%) | 10 (1.1%) | 99 |
| Deaths – no (%b) | 0 (0%) | 1 (50.0%) | 48 (100%) | 38 (97.4%) | 8 (80.0%) | 95 (96.0%) | |
| MMS | Cases – no (%a) | 1 (0.2%) | 0 (0%) | 19 (4.2%) | 6 (1.3%) | 4 (0.9%) | 30 |
| Deaths – no (%b) | 1 (100%) | 0 (0%) | 18 (94.7%) | 5 (83.3%) | 3 (75.0%) | 27 (90.0%) | |
| USS | Cases – no (%a) | 1 (0.2%) | 1 (0.2%) | 15 (3.4%) | 16 (3.6%) | 1 (0.2%) | 34 |
| Deaths – no (%b) | 0 (0%) | 1 (100%) | 14 (93.3%) | 16 (100%) | 1 (100%) | 32 (94.1%) | |
Adherence to annual screening
In the MMS group, of 427,448 eligible annual screening episodes, 80.8% (345, 570) women attended all tests that comprised the screening episode (Table 9). If restricted to compliance with annual screen alone, then it was 81.2% (346,989/427,448). In the USS group, of 420,047 eligible screening episodes, compliance with annual screening episode was 78.0% (327,775/420,047) and 78.3% (328,764/420,047) with annual screen alone. The observed compliance was 99% of predicted compliance in the MMS and 98% in the USS group.
| Variable | Group | Annual screen | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |||
| Women due screena | MMS | 50,624 | 50,624 | 50,624 | 50,624 | 50,624 | 50,624 | 50,622 | 40,430 | 28,235 | 13,864 | 2132 | 439,027 |
| USS | 50,623 | 50,623 | 50,623 | 50,623 | 50,623 | 50,623 | 50,615 | 40,406 | 28,227 | 13,849 | 2115 | 438,950 | |
| Women ineligibleb for screen | MMS | 10 | 265 | 569 | 898 | 1275 | 1660 | 2064 | 2043 | 1666 | 954 | 175 | 11,579 |
| USS | 67 | 1019 | 1458 | 1862 | 2239 | 2624 | 3021 | 2842 | 2240 | 1278 | 253 | 18,903 | |
| Women eligible for screen | MMS | 50,614 | 50,359 | 50,055 | 49,726 | 49,349 | 48,964 | 48,558 | 38,387 | 26,569 | 12,910 | 1957 | 427,448 |
| USS | 50,556 | 49,604 | 49,165 | 48,761 | 48,384 | 47,999 | 47,594 | 37,564 | 25,987 | 12,571 | 1862 | 420,047 | |
| Women who attended screen | MMS | 49,822 | 45,893 | 43,588 | 41,669 | 39,925 | 38,283 | 35,170 | 26,091 | 16,878 | 7328 | 923 | 345,570 |
| USS | 47,955 | 44,106 | 41,951 | 40,025 | 38,286 | 36,345 | 32,969 | 23,949 | 15,185 | 6334 | 668 | 327,773 | |
| Compliancec | MMS | 96.6% | 90.0% | 86.2% | 82.9% | 80.0% | 77.0% | 70.9% | 65.9% | 61.0% | 53.6% | 41.7% | 79.5% |
| USS | 98.4% | 91.1% | 87.1% | 83.8% | 80.9% | 78.2% | 72.4% | 68.0% | 63.5% | 56.8% | 47.2% | 80.8% | |
| Predicted complianced | MMS | 100.0% | 94.5% | 87.5% | 83.6% | 80.4% | 77.6% | 75.0% | 69.5% | 65.2% | 61.0% | 54.5% | 82.0% |
| USS | 100.0% | 91.0% | 85.4% | 81.9% | 78.8% | 76.0% | 72.7% | 66.5% | 61.2% | 56.1% | 48.3% | 80.0% | |
| Observed/predicted compliance | MMS | 0.98 | 0.96 | 1.00 | 1.00 | 1.01 | 1.01 | 0.96 | 0.98 | 0.97 | 0.93 | 0.87 | 0.98 |
| USS | 0.95 | 0.98 | 1.00 | 1.00 | 1.00 | 1.00 | 0.95 | 0.96 | 0.95 | 0.90 | 0.74 | 0.98 | |
Performance characteristics of screening in the MMS and the USS groups
Women in the screened groups underwent a median of eight (IQR 6–9) annual screens till 31 December 2011. Overall, 345,570 annual screens were performed in the MMS group and 327,775 in the USS group, with 737 and 1805 women, respectively, undergoing trial surgery (screen positives).
MMS screening
In the MMS group, 212 women of the 737 who underwent trial surgery were found to have ovarian or tubal cancer. Within one year of the last test in a screening episode, a further 41 women were clinically diagnosed with ovarian or tubal cancer (screen negatives). The stage and histology of these cancers are detailed in Table 10. Overall, 45.8% of the screen-detected ovarian and tubal cancers and 51.2% of the screen-negative cancers were Stage I or II (early stage). In the subgroup analysis of invasive epithelial ovarian and tubal cancer, 38.1% of the screen-detected ovarian and tubal cancers and 31.0% of the screen-negative cancers were Stage I or II (early stage).
| Screen-detected status | Stage | ||||
|---|---|---|---|---|---|
| I | II | III | IV | All | |
| Serous low-grade (n = 11) | |||||
| Screen detected | 8 (73%) | 0 (0%) | 3 (27%) | 0 (0%) | 11 |
| Endometrioid (n = 12) | |||||
| Clinically diagnosed | 1 (50%) | 0 (0%) | 1 (50%) | 0 (0%) | 2 |
| Screen detected | 8 (80%) | 1 (10%) | 0 (0%) | 1 (10%) | 10 |
| Mucinous (n = 2) | |||||
| Clinically diagnosed | 1 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| Screen detected | 1 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| Clear cell (n = 10) | |||||
| Clinically diagnosed | 3 (60%) | 2 (40%) | 0 (0%) | 0 (0%) | 5 |
| Screen detected | 3 (60%) | 2 (40%) | 0 (0%) | 0 (0%) | 5 |
| Serous high-grade (n = 146) | |||||
| Clinically diagnosed | 2 (17%) | 0 (0%) | 9 (75%) | 1 (8%) | 12 |
| Screen detected | 22 (16%) | 18 (13%) | 84 (63%) | 10 (7%) | 134 |
| Carcinosarcoma (n = 4) | |||||
| Clinically diagnosed | 0 (0%) | 0 (0%) | 1 (100%) | 0 (0%) | 1 |
| Screen detected | 1 (33%) | 0 (0%) | 2 (67%) | 0 (0%) | 3 |
| Carcimona, NOS (n = 21) | |||||
| Clinically diagnosed | 0 (0%) | 0 (0%) | 3 (50%) | 3 (50%) | 6 |
| Screen detected | 4 (27%) | 1 (7%) | 8 (53%) | 2 (13%) | 15 |
| Serous unknown type (n = 3) | |||||
| Clinically diagnosed | 0 (0%) | 0 (0%) | 2 (100%) | 0 (0%) | 2 |
| Screen detected | 0 (0%) | 0 (0%) | 1 (100%) | 0 (0%) | 1 |
| Small cell (n = 1) | |||||
| Screen detected | 0 (0%) | 0 (0%) | 0 (0%) | 1 (100%) | 1 |
For all ovarian and tubal cancers diagnosed within one year of the last test in a screening episode, in the MMS group, the sensitivity, specificity and positive predictive values were 83.8% (95% CI 78.7 to 88.1), 99.8% (95% CI 99.8 to 99.9) and 28.8% (95% CI 25.5 to 32.2) for all ovarian and tubal cancers, with 3.5 operations per case detected. When the analysis was restricted to invasive epithelial ovarian and tubal cancers, sensitivity, specificity and positive predictive values were 86.2% (95% CI 80.8 to 90.6), 99.8% (95% CI 99.8 to 99.9) and 24.6% (95% CI 21.5 to 27.8), with 4.1 surgeries per true positive (Table 11).
| Characteristics | All malignant ovarian and tubal cancer | Invasive epithelial ovarian and tubal cancer |
|---|---|---|
| Prevalence (first) screen | ||
| Number of women screen years | 50,078 | 50,078 |
| Number of surgeries | 97 | 97 |
| Primary ovarian and tubal malignancies (ICD-10 codes C56 and C57) within one year of screen | ||
| Screen positives | 43 | |
| Screen negatives | 5 | 4 |
| Sensitivity | 89.6 (77.3, 96.5) | 89.7 (75.8, 97.1) |
| Specificity | 99.9 (99.9, 99.9) | 99.9 (99.8, 99.9) |
| Positive predictive value | 44.3 (34.2, 54.8) | 36.1 (26.6, 46.5) |
| No. of operations per true positive | 2.3 | 2.8 |
| Incidence (all other than first annual) screens | ||
| Number of women screen years | 296,911 | 296,911 |
| Number of surgeries | 640 | 640 |
| Primary ovarian and tubal malignancies (ICD-10 codes C56 and C57) within one year of screen | ||
| Screen positives | 166 | 145 |
| Screen negatives | 35 | 25 |
| Sensitivity | 82.6 (76.6, 87.6) | 85.3 (79.1, 90.3) |
| Specificity | 99.8 (99.8, 99.9) | 99.8 (99.8, 99.8) |
| Positive predictive value | 25.9 (22.6, 29.5) | 22.7 (19.5, 26.1) |
| No. of operations per true positive | 3.9 | 4.4 |
| All screens | ||
| Number of women screen years | 346,989 | 346,989 |
| Number of surgeries | 737 | 737 |
| Primary ovarian and tubal malignancies (ICD-10 codes C56 and C57) within one year of screen | ||
| Screen positives (screen detected) | 212 | 181 |
| Screen negatives (clinically diagnosed) | 41 | 29 |
| Sensitivity | 83.8 (78.7, 88.1) | 86.2 (80.8, 90.6) |
| Specificity | 99.8 (99.8, 99.9) | 99.8 (99.8, 99.9) |
| Positive predictive value | 28.8 (25.5, 32.2) | 24.6 (21.5, 27.8) |
| No. of operations per true positive | 3.5 | 4.1 |
In the MMS group, of those who underwent trial surgery, 489 were found to have benign adnexal pathology or normal adnexa. This finding translates to 14 (489/345,572 annual screens) unnecessary false-positive surgeries per 10,000 screens in the MMS group. For each ovarian or tubal cancer detected by screening, an additional two (489 false positives; 212 ovarian and tubal cancers) women in the MMS group had unnecessary false-positive surgery.
USS screening
In the USS group, 164 women of the 1805 who underwent trial surgery were found to have ovarian or tubal cancer. Within one year of the last test in a screening episode, a further 63 women were clinically diagnosed with ovarian or tubal cancer (screen negatives). The stage and histology of these cancers are detailed in Table 12. Overall, 59.1% of the screen-detected ovarian and tubal cancers and 6.3% of the screen-negative cancers were Stage I or II (early stage). In the subgroup analysis of invasive epithelial ovarian and tubal cancer, 39.0% of the screen-detected ovarian and tubal cancers and 4.9% of the screen-negative cancers were Stage I or II (early stage).
| Screen-detected status | Stage | ||||
|---|---|---|---|---|---|
| I | II | III | IV | All | |
| Serous low-grade (n = 5) | |||||
| Screen detected | 1 (20%) | 0 (0%) | 4 (80%) | 0 (0%) | 5 |
| Endometrioid (n = 5) | |||||
| Screen detected | 4 (80%) | 1 (20%) | 0 (0%) | 0 (0%) | 5 |
| Mucinous (n = 4) | |||||
| Clinically diagnosed | 1 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| Screen detected | 2 (67%) | 1 (33%) | 0 (0%) | 0 (0%) | 3 |
| Clear cell (n = 11) | |||||
| Screen detected | 10 (91%) | 1 (9%) | 0 (0%) | 0 (0%) | 11 |
| Serous high-grade (n = 115) | |||||
| Clinically diagnosed | 1 (2%) | 1 (2%) | 27 (61%) | 15 (34%) | 44 |
| Screen detected | 10 (14%) | 10 (14%) | 46 (65%) | 5 (7%) | 71 |
| Carcinosarcoma (n = 4) | |||||
| Clinically diagnosed | 0 (0%) | 0 (0%) | 1 (100%) | 0 (0%) | 1 |
| Screen detected | 0 (0%) | 0 (0%) | 3 (100%) | 0 (0%) | 3 |
| Carcinoma, NOS (n = 21) | |||||
| Clinically diagnosed | 0 (0%) | 0 (0%) | 6 (43%) | 8 (57%) | 14 |
| Screen detected | 1 (14%) | 0 (0%) | 4 (57%) | 2 (29%) | 7 |
| Serous unknown type (n = 1) | |||||
| Clinically diagnosed | 0 (0%) | 0 (0%) | 0 (0%) | 1 (100%) | 1 |
For USS screening, the sensitivity, specificity, and positive-predictive value for all ovarian and tubal cancers diagnosed within one year of the last test in a screening episode were 72.2% (95% CI 65.9 to 78.0), 99.5% (95% CI 99.5 to 99.5), and 9.1% (95% CI 7.8 to 10.5), with 11 operations per case detected. In the subgroup analysis of invasive epithelial ovarian and tubal cancers, sensitivity, specificity, and positive predictive value were 63.3% (95% CI 55.4 to 70.6), 99.5% (95% CI 99.5 to 99.5), and 5.8% (95% CI 4.8 to 7.0), with 17.2 surgeries per true positive (Table 13).
| Characteristics | All malignant ovarian and tubal cancer | Invasive epithelial ovarian and tubal cancer |
|---|---|---|
| Prevalence (first) screen | ||
| Number of women screen years | 48,230 | 48,230 |
| Number of surgeries | 845 | 845 |
| Primary ovarian and tubal malignancies (ICD-10 codes C56 and C57) within one year of screen | ||
| Screen positives | 51 | 25 |
| Screen negatives | 11 | 11 |
| Sensitivity | 82.3 (70.5, 90.8) | 69.4 (51.9, 83.7) |
| Specificity | 98.4 (98.2, 98.5) | 98.3 (98.2, 98.4) |
| Positive predictive value | 6.0 (4.5, 7.9) | 3.0 (1.9, 4.3) |
| No. of operations per true positive | 16.6 | 33.8 |
| Incidence (all other than first annual) screens | ||
| Number of women screen years | 280,534 | 280,534 |
| Number of surgeries | 960 | 960 |
| Primary ovarian and tubal malignancies (ICD-10 codes C56 and C57) within one year of screen | ||
| Screen positives | 113 | 80 |
| Screen negatives | 52 | 50 |
| Sensitivity | 68.5 (60.8, 75.5) | 61.5 (52.6, 69.9) |
| Specificity | 99.7 (99.7, 99.7) | 99.7 (99.7, 99.7) |
| Positive predictive value | 11.8 (9.8, 14) | 8.3 (6.7, 10.3) |
| No. of operations per true positive | 8.5 | 12 |
| All screens | ||
| Number of women screen years | 328,764 | 328,764 |
| Number of surgeries | 1805 | 1805 |
| Primary ovarian and tubal malignancies (ICD-10 codes C56 and C57) within one year of screen | ||
| Screen positives | 164 | 105 |
| Screen negatives | 63 | 61 |
| Sensitivity | 72.2 (65.9, 78) | 63.3 (55.4, 70.6) |
| Specificity | 99.5 (99.5, 99.5) | 99.5 (99.5, 99.5) |
| Positive predictive value | 9.1 (7.8, 10.5) | 5.8 (4.8, 7) |
| No. of operations per true positive | 11 | 17.2 |
In the USS group, of those who underwent trial surgery, 1630 were found to have benign adnexal pathology or normal adnexa. An additional 10 (1630 false positives; 164 ovarian and tubal cancers) women in the USS group had unnecessary false-positive surgery for each ovarian or tubal cancer detected by screening. This finding translates to 50 (1630/327,775 annual screens) false-positive surgeries per 10,000 screens in the USS group.
Complications related to screening tests
Less than 1% of women reported screening complications – 30 (<1%) in the MMS group and 61 (<1%) in the USS group. The screening-related complication rate was 8.6 per 100,000 in the MMS group and 18.6 per 100,000 in the USS group. The most common complication reported in the MMS group was bruising (13 of the 30 women), and pain (20/61) and cystitis (11/61) in the USS group (Table 14).
| MMS | USS | ||
|---|---|---|---|
| Complication type | No. of women | Complication type | No. of women |
| Complications related to screening | |||
| Bruising | 13 | Pain | 20 |
| Pain | 8 | Cystitis/infection | 11 |
| Haematoma | 3 | Discomfort | 5 |
| Fainting | 1 | Bruising | 2 |
| Cystitis/infection | 1 | Fainting | 1 |
| Other | 4 | Other | 22 |
| Total | 30 | Total | 61 |
| Rate | 8.6/100,000 | Rate | 18.6/100,000 |
| Complications related to screen-positive surgery | |||
| Anaesthetic | 1 | Injury to hollow viscus (4 GI, 3 bladder, 4 ureter) | 11 |
| Injury to hollow viscus (2 GI, 1 bladder) | 3 | Haemorrhage | 11 |
| Haemorrhage | 2 | Anaesthetic/myocardial Infarction | 3 |
| Deep vein thrombosis | 1 | Hernia | 6 |
| Bowel obstruction | 4 | Deep vein thrombosis/pulmonary embolism | 3 |
| Wound breakdown - total dehiscence | 1 | Wound breakdown | 6 |
| Significant ileus | 1 | Bowel obstruction | 4 |
| Uterine perforation | 1 | Wound/supravaginal haematoma | 4 |
| Infection | 1 | Infection | 6 |
| Pain – ward readmission/further operation | 3 | ||
| Total | 15 | Total | 57 |
| Rate | 3.1% (15/488) | 3.5% (57/1634) | |
Among women who were screen-positive and had trial surgery, 488 (1.0% of all randomised to the group) in the MMS group and 1634 (3.2% of those randomised to the group) in the USS group were found to have benign adnexal pathology or normal adnexa. In these women, there was a complication rate of 3.1% (95% CI 1.7 to 5.0; 15 of 488) in the MMS group and 3.5% (95% CI 2.7 to 4.5; 57 of 1634) in the USS group. 38
Contamination
In the C group women (38,238) who completed the 2014 follow-up questionnaire, ovarian cancer screening outside of UKCTOCS was documented in 1660 (4.3% [95% CI 4.1 to 4.5]).
Psychosocial study
Compared to the C group, there was no evidence that the state anxiety levels of women in the MMS and USS groups differed in the random group (n = 1339). The estimated differences were –0.52 (95% CI –1.88 to 0.85) in the MMS and 1.27 (95% CI –2.67 to 0.13) in the USS. A comparable analysis for the events group (with no control group) gave an estimated difference between USS and MMS groups of 0.33 (95% CI 0.01 to 0.65). Of note, screening itself did not cause anxiety unless more intense repeat testing was required following abnormal screens.
With every year from randomisation, anxiety decreased significantly; the estimated mean decrease over 5 years was 1.35 (5 × 0.27) (95% CI 0.70 to 1.95; p < 0.01) in the random group and 1.1 (95% CI 0.75 to 1.45; p < 0.01) in the events group. In the random group, anxiety level, after adjustment for the mean difference between anxiety scores after repeat screening and those following the annual screen, was 0.4 (95% CI 0.46 to 1.27), and in the events group it was 0.37 (95% CI 0.23 to 0.51). Women requiring Level 2 screens in the events group had an increased risk (OR 1.28, 95% CI 1.18 to 1.39) of psychological morbidity (a GHQ-12 score ≥ 4). In women with ovarian cancer, the risk of psychological morbidity was higher at both 6 weeks (OR 16.2, 95% CI 9.19 to 28.54) and 6 months (OR 3.32, 95% CI 1.91 to 5.77) following surgery. 30
Cost-effectiveness
Initial censorship 31 December 2014
The cost-effectiveness of an NHS ovarian cancer screening programme was calculated, on the assumption that there was a mortality reduction on extended follow-up to 2020. The within-trial to 2014 results, using a CA125 -ROCA cost of £20, showed USS to be strictly dominated by MMS. The ICER for MMS was £91,452 per life year gained in the MMS versus C comparison. Predictive extrapolation over the expected lifetime of the UKCTOCS participants returned an ICER of £30,033 per life year gained, while Markov modelling produced an ICER of £46,922 per quality-adjusted life year (QALY). 47
Final censorship 30 June 2020
Although data were collected and prepared for beyond the trial cost-effectiveness analysis using the follow-up data, given that there was no mortality reduction in either screening group compared to C, the follow-up data were not analysed.
Bioresource
Of the recruited women, 202,365 (99.9%) consented to use of data and samples in secondary ethically approved research. Of them, only 85 (0.004%) excluded use of samples in research collaborations involving industry. Due to the evolution of time since the initial consent in 2001–2005, for women resident in England and Wales in 2014 additional Section 251 approval was obtained from the Confidentiality Advisory Group (CAG; www.hra.nhs.uk/planning-and-improving-research/application-summaries/confidentiality-advisory-group-registers/). This is renewed on an annual basis. For Northern Ireland, similar approval was granted by the Chair of the Privacy Advisory Committee N Ireland in April 2020.
The large bioresource of serum samples and linked data built through the generosity of the participants has been designated the UKCTOCS Longitudinal Women’s Cohort (UKLWC). 27 There were 544,808 serum samples (10 × 500-μl aliquots in straws), composed of 189,642 baseline samples from 189,452 women and a unique longitudinal set of 355,166 annual serial samples (median 8) from 50,262 women in the MMS group. All donated serum samples are stored in liquid-nitrogen tanks at a HTA-licensed commercial cryofacility (Fisher Bioservices, UK, until March 2018 and NIHR UK Biocentre since then). A transparent governance and access system has been set up to ensure researchers, both academic and from industry, can avail themselves of the samples in line with the conditions of donation. Several nested case–control collaborative research projects have been undertaken using a variety of multi-omics analysis such as genotyping, proteomics including SWATH technology, DNA methylation, NMR metabolomics, autoantibody profiling, ELISA-based assays, lipidomics, miRNA and exosomes.
Discussion
Principal findings
UKCTOCS, the largest ovarian cancer screening trial to date, did not find any evidence of a reduction in deaths from ovarian and tubal cancer compared to no screening at a median follow-up of 16.3 years from randomisation, with either the multimodal or the ultrasound screening strategy investigated in the trial. The suggestion following the 2014 censorship of a trend to delayed mortality reduction38 was not borne out. This was despite a stage shift with an increased incidence of Stage I (47%) and decreased incidence of Stage IV disease (24.5%) in the MMS compared to the C group that persisted even at nine and a half years after the end of screening. However, it must be noted that the overall decrease in incidence of advanced (Stage III and IV) disease was only 10%. There was no difference in stage between the USS group and the C group. With both strategies, there was no increase in incidence of ovarian and tubal cancer, suggesting that overdiagnosis was not a major issue. The trial findings reinforce the current guidance that women in the general population should not be offered ovarian cancer screening outside trials.
The UKCTOCS result should not be directly extrapolated to ovarian and tubal cancer screening of women at increased risk as they were specifically excluded from UKCTOCS. Previous single-arm screening studies in this population exploring three or four monthly screens using a MMS strategy alongside encouraging risk-reducing surgery found a reduction in number of women diagnosed with advanced disease. 27 One also needs to consider that women with BRCA gene mutations respond better to current treatments compared to the general population.
The UKCTOCS participants were predominantly white. Given that there is evidence of ethnic differences in histotype, with an increased incidence of clear-cell cancer in Asian populations, the mortality impact of population screening may be different in these populations. However, it is important to note that the major contributor to mortality in all populations is high-grade serous ovarian cancer. A future publication is in draft where we explore in detail the stage shift and mortality impact by histotype.
In the MMS group, earlier diagnosis of invasive epithelial ovarian and tubal cancer in a proportion of mainly asymptomatic women did not translate into lives saved. This suggests that it is likely that earlier detection in the symptomatic population may not translate to reduced mortality. However, this should not be considered as a reason to abandon the significant symptom-awareness efforts that are in place. As detailed above, there has been significant progress in the treatment of advanced ovarian cancer since 2011 when screening ended. These advances together with earlier diagnosis could contribute to better quality of life and improved outcomes. In addition, once symptomatic, a rapid diagnosis is invaluable to women and their families, as is avoiding emergency presentation to secondary care.
The detailed implications are discussed below using the UK National Screening Committee (NSC) criteria. 57
The condition
Ovarian cancer remains an important health problem because of the poor outcome (10-year survival of 35%), which has not changed dramatically over the past four decades. At the start of the trial, there was little understanding of the natural history of the disease. For many decades, the prevailing thought was that majority of the ovarian cancers arose from the surface epithelium of the ovary. It has only become widely accepted in the last decade (after screening had ended in UKCTOCS) that the majority of high-grade serous ovarian cancers arise from serous tubal intraepithelial carcinoma (STIC) lesions at the fimbrial end of the fallopian tube and spread when the adnexal lesion is very small. 58 Work is still under way to understand how the cancer develops from this latent stage to clinical presentation.
There are no primary prevention strategies available on a population basis. However, based on the above understanding there is increasing adoption of removal of tubes during hysterectomy for benign disease58,59 and as a family planning measure (in place of tubal ligation). 60 In women at increased risk of ovarian cancer, removal of tubes and ovaries is recommended after completion of family to reduce the risk of developing ovarian cancer. In this population, trials (PROTECTOR UK,61,62 TUBA Netherlands,63 Fimbriectomy USA64) are under way to explore the possibility of risk-reducing salpingectomy with delayed oophorectomy to mitigate the effect of early menopause.
The test
In the trial, two screening strategies were evaluated.
MMS strategy: this involved an annual CA125 blood test. Venepuncture is widely used in the clinical setting. Its safety in UKCTOCS is highlighted by the fact that complications were reported in only 1 per 10,000 annual screens. Serum CA125 has been used in the clinical setting since its discovery in 1981 for diagnosing and monitoring ovarian cancer. It is routinely performed in NHS laboratories, on multiple platforms with an established national quality assurance programme (NEQAS). 65 In UKCTOCS, all CA125 measurements were done centrally in a single laboratory that was enrolled in the NEQAS programme. The UKCTOCS laboratory at UCL was accredited for clinical work by the United Kingdom Accreditation Service (UKAS,66 formerly known as Clinical Pathology Accreditation Ltd).
The CA125 results were automatically uploaded from the Laboratory Information Management System (LIMS) into the UKCTOCS Trial Management System. Instead of using a cut-off, as in the clinical setting, in UKCTOCS serial CA125 was interpreted using a bespoke risk of ovarian cancer algorithm (ROCA) to triage women based on risk. This had already been piloted in a prospective trial of over 13,000 women with three rounds of annual screening to define risk cut-off levels for triage. 26 While the detailed MMS screening protocol was complex, it was built into the Trial Management System with automated classification of risk, follow-up actions and appointment scheduling. As a result, despite performing over 345,000 annual MMS screens over 11 years there were no instances of misclassification or errors in triaging, which suggests that it should be possible to run such a complex intervention as part of a screening programme. The only resource-intensive aspects were manual checking of all letters to women with elevated risk and regular monitoring of multiple steps of the blood test to results process.
USS strategy: women underwent a transvaginal ultrasound annually. The TVS was similar to that routinely performed in the NHS. However, it differed in that the majority of women had normal tubes and ovaries with no adnexal pathology. In addition, the subjective element of ultrasound scanning and age-related atrophy of adnexal structures made their visualisation more difficult. To address this, a training and accreditation programme in ultrasound screening of postmenopausal ovaries was developed for trial ultrasonographers by the Ultrasound Sub-Committee. 32 Alongside this, a quality assurance programme involving regular monitoring of individual ultrasonographers was developed and implemented with day-to-day oversight by a senior trial lead ultrasonographer. Despite this, a retrospective analysis by a single expert of static images of normal ovaries suggested a significant discrepancy between sonographer and expert. 67
Both screening procedures, venepuncture and TVS were only associated with minor complications with low complication rates. The psychosocial study found that screening generally did not increase anxiety. However, attending more intense screening for elevated risk (Level II) was associated with some general psychological morbidity (a mixture of depressive thoughts and worry, reflected by change in GHQ-12 scores) compared with repeat CA125 or TVS for intermediate risk. A meta-analysis of 12 randomised trials of screening (cancer and other diseases) that used similar measures to evaluate anxiety and psychological morbidity found comparable findings. In those receiving positive test results compared with those receiving negative results, there was no evidence of short (<1 month) or longer-term (beyond 1 month) anxiety. 68
UKCTOCS involved 7–11 annual screens with half the women undergoing >8 annual screens. Compliance was high, with almost 80% of women attending eligible screening appointments. The only other trials with six or more years of screening are the UK Age trial69 (seven annual screens) and the PLCO ovarian70 (six annual screens) and prostate cancer71 (six annual screens) screening trials. In the UK Age trial, participation in screening was 68.1% at the first screen and 69.1% subsequently. 69 During the initial four rounds of screening in the ovarian arm of PLCO, the compliance for combined screening with both CA125 and TVS decreased slightly from the 83% at the first to 78% at the fourth screen. When analysed separately, for CA125 alone, compliance in PLCO was 84% and 79%, and for TVS alone it was 83% and 78% for the first and fourth screen, respectively. 70 Equivalent data from UKCTOCS for MMS were 97% and 83% and for USS 98% and 84% for the first and fourth screen, respectively. In the prostate arm of PLCO, the compliance with routine PSA testing was much lower at 33% in year 1, increasing to 46% in year 5. 71 In UKCTOCS, despite the length of the trial, adherence with annual screening remained high, with the observed versus predicted compliance ratios 0.99 for the MMS and 0.98 for the USS group for the eleventh annual screen.
There was a clear diagnostic pathway for women found to have positive results on screening. They were assessed by the trial clinician in dedicated trial clinics (see methods). Options included conservative management if risk was equivocal or referral for trial surgery. All decisions were made in discussion with participants. Where appropriate, the women were referred to the NHS gynaecological oncology team using the two-week urgent cancer referral pathway. The diagnostic work-up undertaken in the trial is now part of the 2011 NICE 122 Guidance Ovarian cancer: recognition and initial management. 72
The sensitivity of the MMS strategy, following over 340,000 women-years of screening, was similar for detection of all (84%) and the subgroup of invasive epithelial (86%) ovarian and tubal cancers diagnosed within one year of the last test in a screening episode. Of the screen-detected invasive epithelial cancers, 38% were early Stage (I–II). Sensitivity of over 80% for ovarian cancer (83%; 6 of 7 detected) has been reported in the recent feasibility study, DETECT-A (Detecting cancers Earlier Through Elective mutation-based blood Collection and Testing) of 10,000 women aged 65–75 using a multimodal multi-cancer strategy incorporating the early version of the CancerSEEK blood test as first-line and diagnostic PET-CT as the second-line test. Of note, only 16% (1 of 6) of the screen-detected ovarian cancers in DETECT-A were early Stage (I–II), which suggests that significant improvements in test performance are necessary before the test can be used for ovarian cancer screening. 73 Encouraging performance has been reported in clinical sample sets using the targeted methylation of plasma cell-free DNA (cfDNA) test74 with the multi-cancer early detection (MCED) test in the Circulating Cell-free Genome Atlas study and using a micro-RNA signature in the OCaMIR test. 75 It is noteworthy that at the annual screen, half the women with screen-detected invasive epithelial ovarian and tubal cancer had CA125 below the normal cut-off (35 IU/ml)26 which was used in PLCO21 and SCSOCS76 trials. The results provide evidence of the significant improvement that longitudinal biomarker algorithms bring to early detection. This was further confirmed by use of the trial CA125 data to build and compare the performance of the available longitudinal algorithms. 77
For each woman detected with ovarian or tubal cancer, 2.5 additional women underwent surgery. This translated to 14 unnecessary (benign pathology or normal adnexa) false-positive operations per 10,000 screens in the MMS group. This was considerably fewer than the additional women who underwent surgery per cancer detected in the PLCO (18.5)70 and the SCSOCS (32)76 RCTs. However, they are still significant. While women participating in the trial consented to even higher false-positive rates (10 operations per cancer detected), it needs to be noted that they represented only 16.3% of the invited population. Additionally, the burden on the NHS would be considerable. Based on the projected mid-2020 UK population of 10.23 million women aged 50–75 and an 80% uptake of MMS annual screening, this would mean 11,455 false-positive operations per year. Surgical morbidity in these women is an additional major concern, especially with increasing comorbidity with age. The serious-complications rate (3.1%) in women who had benign pathology or normal adnexa, most of whom underwent laparoscopic bilateral salpingo-oophorectomy, was again significantly lower than that reported in the PLCO21 trial (15%). Rates were similar to that reported in a contemporary UK78 series of risk-reducing salpingo-oophorectomy (3.9%) in high-risk women.
The longitudinal algorithm used to interpret CA125 levels was innovative and forward-thinking. Such algorithms are now being explored for early-detection strategies in other cancers and are being improved through use of machine learning and Artificial Intelligence (AI). The latest UK health research programme ‘Our Future Health’,79 aimed to prevent, detect and treat disease, incorporates longitudinal collection of biospecimens to facilitate such approaches.
The sensitivity (73%) of USS screening was significantly higher than the sensitivity of TVS alone (44.6%; 33/74) noted during four rounds of ovarian cancer screening in the PLCO trial. 70 It must be noted that a combined strategy with first-line CA125 and TVS was used in the PLCO trial, so overall sensitivity was higher. Despite the improved sensitivity, performance characteristics of USS screening in UKCTOCS suggest that TVS is not an effective first-line test for population screening. The sensitivity for invasive epithelial ovarian cancer (63%) was low and 16 additional operations were undertaken per invasive cancer detected. It needs to be noted that when all (including non-epithelial and borderline) ovarian and tubal cancers were considered, in the USS group 10 additional operations were undertaken per cancer detected. This was below the 15 operations per cancer detected that was stated in the patient information leaflet (Project documentation D) based on estimates at the start of the trial in 2001. Despite a similar proportion (39%) of screen-detected invasive cancers detected at early stage in the USS group, nearly all (95%) missed cancers (clinically diagnosed) were advanced (III/IV) unlike in the MMS (69%) group. This together with the lower sensitivity of USS resulted in no evidence of a difference in incidence of early-stage (I–II) cancer between USS and C groups on the final intention-to-screen analysis.
A major limitation was the length of the trial – it spanned two decades. During this period, knowledge about this cancer evolved massively. By the end of the screening phase, it was widely accepted that a significant number of ‘ovarian’ cancers arose in the tube. 80,81 In 2009, modelling based on occult cancers found in risk-reducing surgery in BRCA gene mutation carriers suggested that the majority of Stage I high-grade serous cancers are 0.4–1.3 cm in diameter and therefore difficult to reliably image. 82 If this had been known prior to trial design, an ultrasound strategy using TVS as a first-line test might not have been included in the design. There would also have been less reliance on visualising an abnormal adnexal mass on TVS prior to recommending trial surgery in the MMS group. The MMS screening strategy did include trial surgery for the small minority of women for whom the ROCA indicated ‘severe’ risk due to rising biomarker levels but repeated imaging was normal. 83 However, it was challenging to convince clinicians to operate soon when faced with this scenario given the lack of understanding of disease evolution. In retrospect, second-line tests in the MMS strategy should have been further optimised so that the interval from annual intermediate risk screens to cancer diagnosis was reduced. 41 If we were planning a new trial, we would consider a second-line test such as circulating tumour DNA (ctDNA)84 or microRNA (miRNA)75 alongside TVS.
The intervention
The worldwide accepted standard of care for most women diagnosed with ovarian and tubal cancer (Stage IC–IV) is complete cytoreductive surgery and platinum-based chemotherapy. The two available options are (1) primary surgery followed by adjuvant chemotherapy and (2) neo-adjuvant chemotherapy with interval debulking surgery (three cycles, surgery, further three cycles). The standards are based on evidence from randomised controlled trials and are enshrined in numerous evidence-based recommendations. Those spanning the trial include ESMO 2001,85 200886 and 2010. 87 There are also nationally agreed treatment protocols for the United Kingdom (NICE, 201164). All women detected with ovarian cancer in the course of UKCTOCS, with few exceptions, were treated using these standard protocols at cancer centres within the NHS, ensuring that there was equity and balance between the groups.
However, the length of follow-up does mean that majority of screen-detected women were diagnosed and treated more than a decade ago (2001–2011). Since then, there have been further advances in clinical management (better staging, widespread use of ultraradical surgery, earlier treatment modulation based on better prognostic indicators, targeted therapies). The latter, if available during screening, may have impacted on trial outcomes.
Since end of screening on the trial, genetic testing for mutations in BRCA1/2 genes has been introduced for all women diagnosed with invasive epithelial ovarian and tubal cancer. It is likely that about 15% will be found to have germline mutations. 88 This would magnify the impact of any future ovarian cancer screening strategy through cascade testing in families of individuals who are mutation positive.
The screening programme
UKCTOCS did not find any evidence of a reduction in deaths from ovarian and tubal cancer compared to no screening at a median follow-up of 16.3 years from randomisation, with either the multimodal or the ultrasound screening strategy investigated in the trial. The only other RCT to report on the mortality impact of ovarian cancer screening was the PLCO cancer screening trial. Similar to UKCTOCS, there was no evidence of a reduction in ovarian cancer deaths between the screen and no-screen arms, either at initial (median follow-up of 12.5 years)21 or extended (14.8 years)89 follow-up. However, unlike in the UKCTOCS MMS group, there was no evidence of a difference in stage distribution between the screened and non-screened arms in the PLCO trial. In both trials there were varying rates of harm due to unnecessary surgery with its attendant complications. The trial findings reinforce the current guidance that women in the general population should not be offered ovarian cancer screening outside trials.
There is the possibility of dilution of the screening effect during the 9.5 years interval from end of screening (end 2011) to censorship (mid 2020). However, in most screening trials, long-term follow-up is the norm. In the European Randomized Study of Screening for Prostate Cancer,90 this did not impact on mortality reduction. Also, the ovarian cancer mortality hazard ratio (HR) was similar at 15 and 18 years from randomisation in our trial. In UKCTOCS, in line with other screening trials, disease-specific rather than all-cause mortality is the primary outcome.
Finally, the late effect of screening was not anticipated in the statistical design. It was only after the initial analysis38 that growing evidence from other screening trials of delayed mortality reductions led to significant concerns that the test chosen (proportional hazards Cox-model) for comparing the primary outcome may not be the best approach. Based on consultations with experts in the field of trials and statistics, the constant-effect primary analysis approach was changed to the Versatile test, which allows for a delayed effect. A transparent process with publication49 of all details was adopted. Our experience strongly suggests the need for discussion and consensus-building on how best to design and analyse large-scale screening trials.
Over 1.24 million women were invited to the trial and 202,638 took part, which equated to 1 in 6 eligible women aged 50–74 being invited to UKCTOCS and 1 in 6 of them taking part. Of the women invited, 23% accepted the invitation and finally 16% were randomised. As a result, the women who accepted the invitation were a self-selected motivated cohort who were more health-aware than most in the general population. This is a well-acknowledged aspect of participants of population screening trials and also part of real-world screening. We have explored these issues of uptake and its impact in previous publications. 29,39
The acceptance rate varied between different parts of the country from 19% in East London to 33% in Bristol. It was similar to that seen in contemporaneous UK trials of colorectal cancer screening and HRT use after the menopause (WISDOM) trial. We had not explored recruitment via invitation from the NHS registers in our feasibility pilot RCT of 13,500 women. 26 We instead estimated uptake from the sparse literature that was available in 1999. Parkes et al. 91 reported an 82% uptake in their RCT of ovarian cancer screening of 8678 women aged 50–64, where women attending a breast cancer screening centre in the UK were invited. Based on this, we estimated a somewhat lower uptake rate of 66% given that we were inviting all women rather than those attending screening. This turned out to be an overestimate. It highlights the importance of piloting all aspects prior to undertaking the main trial. Since then, there have been large studies in the UK that have used NHS registers to recruit participants from the general population. Even in non-interventional studies using this mode of invitation acceptance rates are low. For example, in UK Biobank, the uptake of invitations was 5.45%. 92 Acceptance rates also vary widely for population screening programmes, despite established evidence of benefit. In London, breast screening uptake is 63%. 64 For the more recent colorectal cancer screening programmes, uptake ranges from 68% in the Netherlands, 52% in England to 16% in Canada. 93 There are now many efforts focused on interventions (messaging, community engagement, education, improving test acceptability) to increase screening uptake. 94
Women underwent up to 11 years of annual screening involving over 670,000 annual screening episodes with high compliance. Women consented at trial recruitment to false-positive surgery rates of 9 in 10. In the MMS group, this was lower, with three false-positive operations per ovarian cancer detected. Diagnostic work-up and treatment was undertaken in 13 NHS Trusts located in England, Northern Ireland and Wales. There were very few protocol deviations or complaints from the wider group of health professionals in the NHS or the trial participants. Throughout the trial there was huge support from the ovarian cancer charities both in the UK and in the USA. There was also interest from politicians, especially the All Party Parliamentary Group on Ovarian Cancer. This suggests that such strategies (test, diagnostic procedures, treatment/intervention), had they been effective, might have been clinically, socially and ethically acceptable to the wider public.
The cost-effectiveness analyses based on data accrued till December 2014 suggested that an NHS ovarian cancer screening programme using the MMS strategy extrapolated out to lifetime and after accounting for lead time could approach the NICE thresholds for cost-effectiveness. This was highly dependent on a 20% mortality reduction being confirmed on continued follow-up. 47 Other groups also modelled the UKCTOCS data (2001–2014) and found that lifetime cost-effectiveness of MMS-based screening was promising, subject to long-term effectiveness. 52 However, as there was no reduction in deaths in the MMS arm on follow-up, this is no longer applicable.
Implementation criteria
If future screening trials show benefit, then it would be important to ensure that clinical management of ovarian cancer is further standardised across the UK. Recent national audit of the disease has shown that in England there is significant variability in the use of surgery and chemotherapy standard protocols. 95 Significant variation in the utilisation of both have also been reported previously. 96–98 Meanwhile other options being investigated to improve outcomes in this disease include improved risk prediction through use of algorithms incorporating genetic and epidemiological data99,100 and prevention options based mainly on risk-reducing surgery after completion of family in high-risk women.
For the trial, a bespoke commercially built web-based trial management system was developed by the team in 2000 with automation of key processes, remote data entry and concurrent central monitoring to ensure strict adherence to protocol. Use of electronic health records data to invite women ensured timely recruitment despite the huge sample size; appropriate consent and linkage from the start of the trial to national registries and administrative databases ensured completeness of follow-up; an independent outcomes review committee masked to randomisation group and following a strict algorithm for site assignment and cause of death ensured accuracy and reproducibility over time. Furthermore, keeping with new insights, cancer site assignment was revised to reflect the WHO 2014 classification and cancers were re-staged using the FIGO 2014 criteria. Many of the above approaches that were part of UKCTOCS from the start of the trial in 2001 are now considered best practice for trial conduct. Much could have been adapted to use in a screening programme had the trial been successful.
However, an ultrasound-based strategy would have been challenging to implement given the need to train a larger number of sonographers nationally to deliver first-line screening as well as the constant need for monitoring quality. The latter would require a set-up similar to those used for cervical cytological screening. A blood-based longitudinal CA125 strategy using NHS laboratories that routinely perform CA125 measurements, additional quality assurance measures developed in the trial and a digital platform would have been easier to implement. The MMS strategy too would have required an expanded NHS ultrasound workforce, but smaller as less than 1% of women undergoing MMS screening required a transvaginal ultrasound. An equally challenging issue would be the need for timely surgery of screen positives. This would be required for any ovarian cancer screening programme irrespective of the strategy. In this respect it is significantly different from cervical, breast, prostate and bowel cancer screening where it is possible to diagnose cancer safely and accurately using a directed biopsy. In ovarian cancer screening, women with abnormal screening results would be required to undergo surgery under general anaesthesia with complete removal of the tubes and ovaries, preferably laparoscopically.
The trial used age 50 and above and lack of high-risk family history of ovarian cancer to establish eligibility for general population screening. It is likely that in the future, risk will be further personalised using the new risk-prediction algorithms, but this would only serve to move women into the high-risk-group category. There is little scope in widening the eligibility criteria based on age as the risk of ovarian cancer in women under the age of 50 is low. A screening interval shorter than a year on a population scale would not have been possible to implement.
Conclusions
The group has worked to reduce deaths from ovarian and tubal cancer since the 1980s. 16,17 UKCTOCS, which has spanned 20 years, followed on from a pilot randomised controlled trial in the mid-1990s. 26 The trial owes its successful completion to the over 200,000 women who gave generously of their time, unstinting support from many UK funding agencies, efforts of numerous NHS staff over many years both at the RC and more widely, and the backing of multiple UK and international cancer charities, societies and expert groups. The trial has delivered a clear and definitive answer and addressed comprehensively all the questions posed. Currently, general population screening for ovarian and tubal cancer cannot be recommended. The findings of our trial are applicable to women from the general population and may not apply to those at high risk.
Key insights include the lack of impact on mortality despite detecting the disease at an early stage. This highlights the need to detect the cancer in asymptomatic women even earlier during its natural history and in a larger proportion of women than was possible in UKCTOCS. It reinforces the importance of having deaths due to the disease as opposed to stage at diagnosis as the primary outcome in ovarian cancer screening trials in the general population. It also sets benchmarks for surrogate clinical utility outcomes such as performance characteristics, unnecessary surgery rates, and compliance that need to be surpassed.
The women were exemplary in their support of the trial. In 2012, following the end of screening, we individually thanked all 202,638 participants who were alive and had not had ovarian cancer. Artwork, a painting of an ovarian follicle that was then digitised, was commissioned. Each dot on the digital image represented eight women who took part in the trial. The image was used in the card that was sent to each participant (Figure 6). 64 Further details of our engagement with the participants are detailed in the ‘Dissemination to participants and related patient and public communities’ section.
FIGURE 6.
Thank You card.

The generosity of the women has enabled collaboration with researchers around the world to explore new biomarkers for early detection and risk prediction in ovarian as well as other common cancers and in cardiovascular disease. It has spurred the development and validation of longitudinal algorithms. 77,101 The screening data provide a unique opportunity to study the natural history of ovarian and tubal cancer. The team are committed to ensuring wide access and sharing of samples and data with researchers worldwide.
What this study adds/key learning points
To our knowledge, this is the first RCT to show that ovarian and tubal cancer screening in the general population can lead to earlier detection with increased incidence of Stage I–II and decreased incidence of Stage III–IV in the screen compared to control (no screen) arms. This is key to future efforts to impact on disease mortality through early detection.
It provides definitive new evidence that neither screening approaches based on longitudinal CA125 or TVS as used in UKCTOCS reduce deaths from ovarian and tubal cancer, compared with no screening. Additionally, it is associated with some harm.
It reinforces the guidance that women in the general population should not currently be offered ovarian cancer screening outside trials.
It provides substantial evidence on
-
feasibility and safety of conducting large challenging RCTs in the NHS
-
effectiveness of electronic health records for trial recruitment and follow-up
-
high compliance of trial participants with annual screening
-
low complications and low anxiety associated with an annual screening strategy involving blood tests and imaging including repeat tests
-
the improvement longitudinal algorithms can make to performance characteristics of early detection biomarkers
-
benchmarks for surrogate clinical utility outcomes such as performance characteristics, unnecessary surgery rates, and compliance that need to be surpassed.
It has generated a biorepository with over half a million serum samples and linked data with longitudinal samples that provides a unique opportunity to advance early detection biomarker research.
Acknowledgements
We thank the women who volunteered for their courage, generosity and time – without them there would not have been a trial. We thank all UKCTOCS investigators and staff for helping us conduct a trial where participants were central to all our efforts. We are very grateful to the independent members of the UKCTOCS Trial Steering Committee: Henry Kitchener (Chair 2015–2020), David Luesley (Chair 2001–2014), Julietta Patnick, Jack Cuzick, Louise Bayne (2001–2014) and Annwen Jones (2015–2020) and the Data Monitoring and Ethics Committee (Peter Boyle (chair), Susanne Kjaer, Edward Trimble, and Peter Heintz). We are indebted to the administrative support provided by Anna Widdup. We are very grateful to the funding agencies for working together to support this large trial, and for their sustained support over many years.
Contributions of authors
Lesley Fallowfield (https://orcid.org/0000-0003-0577-4518), Alistair J McGuire (https://orcid.org/0000-0002-5367-9841), Stuart Campbell (https://orcid.org/0000-0003-4943-155x) and Steven J Skates (https://orcid.org/0000-0001-9249-4316) were co-investigators.
Mahesh Parmar (https://orcid.org/0000-0003-0166-1700) was the trial statistician.
Ian J Jacobs (https://orcid.org/0000-0002-5005-2672) was CI from 2000 to 2014 and co-investigator from 2015 to 2020.
All contributed to trial concept and, together with Aleksandra Gentry-Maharaj, Matthew Burnell, Andy Ryan and Naveena Singh, to trial design. Usha Menon, Andy Ryan and Aleksandra Gentry-Maharaj drafted the manuscript. Andy Ryan, Matthew Burnell and Usha Menon prepared the figures and tables. All contributed to review and revision of the manuscript. All authors approved the report before submission.
Ethics approval
UKCTOCS was approved in June 2000 by the UK North West Multicentre Research Ethics Committees (Ref: North West MREC 00/8/34), currently NRES Committee North West – Haydock, with site-specific approval from the local regional ethics committees and the Caldicott guardians (data controllers) of the 27 participating PCTs. All women provided written consent. Approval for follow-up of the entire cohort is until 31 December 2027.
The psychosocial study was approved by the North West research ethics committee (MREC 00/8/34), and separate written consent was obtained from all women.
Trial registration: UKCTOCS was registered with ISRCTN number 22488978; ClinicalTrials.gov. number NCT00058032.
Ethics statement
UKCTOCS was approved by the UK North West Multicentre Research Ethics Committee, currently Haydock NRES (Ref: 00/8/34) on 21 June 2000 with site-specific approval from the local regional ethics committees and the Caldicott guardians (data controllers) of the PCTs.
Information governance statement
UCL is both the data controller and processor of all personal data handled throughout the UKCTOCS trial. As per the EU General Data Protection Regulation and the UK Data Protection Act 2018, all trial/study participants were provided with additional details regarding the legal basis that allows us to hold and process their personal data (http://ukctocs.mrcctu.ucl.ac.uk/participant-info/).
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives. You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Funding statement
The long-term follow-up UKCTOCS (2015–2020) was supported by National Institute for Health and Care Research (NIHR HTA grant 16/46/01), Cancer Research UK, and The Eve Appeal. UKCTOCS (2001–2014) was funded by the Medical Research Council (G9901012 and G0801228), Cancer Research UK (C1479/A2884), and the UK Department of Health, with additional support from The Eve Appeal. Researchers at UCL were supported by the NIHR UCL Hospitals Biomedical Research Centre and by Medical Research Council Clinical Trials Unit at UCL core funding (MR_UU_12023). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
This article
The contractual start date for this research was in January 2017. This article began editorial review in January 2022 and was accepted for publication in August 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The Health Technology Assessment editors and publisher have tried to ensure the accuracy of the authors’ article and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Disclaimer
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Dissemination to participants and related patient and public communities
At the end of recruitment where the trial target of 200,000 was exceeded, there was a visit involving two researchers (AGM and CJ) funded by The Eve Appeal to each RC to congratulate individual members for their contribution to successful recruitment at their centre. This was accompanied by a press release marking this milestone from individual NHS Trusts.
In 2007, a newsletter was produced updating the participants on the progress of UKCTOCS, including a section regarding completion of recruitment, an update on the psychosocial study, details about the secondary studies under way and a write-up on the staff at the RC. The newsletter was sent with the appointment letter for the women in the multimodal and ultrasound arm, provided at the screening clinics or sent directly to the women in the control arm.
All of the 202,638 participants who were alive at the end of screening (excluding those who had an ovarian cancer not detected through screening, to avoid unnecessary upset to the individual woman) were personally thanked for taking part in the trial. Following the end of screening, artwork was commissioned – an ovarian follicle was painted and then digitised so that each dot represented eight women who took part in the trial. a ‘Thank You’ card was send to each participant in 2012 (Figure 6). This was sent to all the women at the end of screening in 2012. 64
On 17 December 2015, when the initial results were published, there was a scientific meeting102 broadcasted live from the Royal College of Obstetrics and Gynaecology, London, which received significant media attention. The same afternoon a meeting was organised to update the patient groups with the findings of the initial mortality results. The Eve Appeal, Target Ovarian Cancer, Ovarian Cancer Action and Ovacome were all represented with an opening word by an ovarian cancer patient and an overview from Professor Anne Mackie, the Director of Screening for Public Health England.
For the final mortality results, the draft scientific publication was shared with NIHR prior to submission to the journal. The UKCTOCS Chief Investigator and Trial Management Committee worked with the MRC CTU at UCL research impact group and UCL press office to develop the first draft of the press release and key messages. These were then shared and further developed with input from the press office of key organisations such as the NIHR, CRUK, MRC press office, The Eve Appeal and Target Ovarian Cancer.
The key messages summarising the findings of the trial both in 2015 and in 2021 were shared with the funding agencies (NIHR, CRUK, The Eve Appeal, UKRI), the policymakers (NHS Screening Committee, Department of Health/NHS in England, Wales, Scotland and Northern Ireland), the NHS England Gynaecological Oncology Clinical Reference Group and the international policymakers, such as the US Preventive Services Task Force (USPSTF).
In parallel, these messages were shared with the patient groups / charities, namely The Eve Appeal, Ovacome, Target Ovarian Cancer, Ovarian Cancer Action and smaller charities. Senior investigators continue to speak at various patient organisation meetings to ensure the results are clarified and the future directions are explored. Lay audiences were also informed though TV and radio interviews and newspaper websites.
The clinical audiences in the UK and globally were informed of the final results. In the UK, the trial team contacted the British Gynaecological Cancer Society (BGCS) and the Royal College of Obstetricians and Gynaecologists (RCOG). The results were relayed to the European Society of Gynaecological Oncology (ESGO) and the International Gynaecologic Cancer Society (IGCS). In addition, through various presentations, the scientific audiences, such as statisticians, triallists, and researchers working on early-detection cancer biomarkers/ovarian cancer, were appraised of the final results. An animated abstract/video was prepared and is available on the trial website. Twitter was used to share the publication of the results in The Lancet in mid May 2021.
Throughout the trial, the patient charities The Eve Appeal and Target Ovarian Cancer were involved in drafting of messages, identifying participants who might be interviewed by media, organising meetings to share results with participants, women with ovarian cancer and their families, and organising meetings/events in Parliament.
Despite wishing to write to all 202,638 women thanking them and sharing the results, due to resource constraints in 2021 the results could only be shared on the trial website and through the media and the ovarian cancer charities. We hope that given the wide coverage including national news that many of the participants who selflessly devoted more than a decade of their life to this effort were made aware of the results.
This article reports on one component of the research award Mortality impact, risks, and benefits of general population screening for ovarian cancer: the UKCTOCS randomised controlled trial. For more information about this research please view the award page [https://www.fundingawards.nihr.ac.uk/award/16/46/01]
References
- CRUK 2018. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/ovarian-cancer#heading-One.
- CRUK 2018. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/ovarian-cancer/survival#heading-Three.
- Coleman MP, Forman D, Bryant H, Butler J, Rachet B, Maringe C, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995-2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet 2011;377:127-38.
- CRUK 2021. www.cancerresearchuk.org/health-professional/data-and-statistics/international-cancer-benchmarking-partnership-icbp.
- Richards MA. The National Awareness and Early Diagnosis Initiative in England: assembling the evidence. Br J Cancer 2009;101:S1-4.
- CRUK 2021. www.cancerresearchuk.org/health-professional/awareness-and-prevention/be-clear-on-cancer.
- NHSx 2021. www.england.nhs.uk/2020/11/nhs-to-pilot-potentially-revolutionary-blood-test/.
- TOC 2021. https://targetovariancancer.org.uk/about-ovarian-cancer/symptoms.
- TEA 2021. https://eveappeal.org.uk/gynaecological-cancers/ovarian-cancer/.
- OCA 2021. https://ovarian.org.uk/ovarian-cancer/ovarian-cancer-symptoms/.
- WOCC 2021. https://worldovariancancercoalition.org/about-ovarian-cancer/symptoms-risk-factors/signs-symptoms/.
- Bast RC, Feeney M, Lazarus H, Nadler LM, Colvin RB, Knapp RC. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest 1981;68:1331-7.
- Campbell S, Goessens L, Goswamy R, Whitehead M. Real-time ultrasonography for determination of ovarian morphology and volume. A possible early screening test for ovarian cancer?. Lancet 1982;1:425-6.
- Einhorn N, Sjovall K, Knapp RC, Hall P, Scully RE, Bast RC, et al. Prospective evaluation of serum CA 125 levels for early detection of ovarian cancer. Obstet Gynecol 1992;80:14-8.
- Einhorn N, Bast R, Knapp R, Nilsson B, Zurawski V, Sjovall K. Long-term follow-up of the Stockholm screening study on ovarian cancer. Gynecol Oncol 2000;79:466-70.
- Campbell S, Bhan V, Royston P, Whitehead MI, Collins WP. Transabdominal ultrasound screening for early ovarian cancer. BMJ 1989;299:1363-7.
- Jacobs I, Stabile I, Bridges J, Kemsley P, Reynolds C, Grudzinskas J, et al. Multimodal approach to screening for ovarian cancer. Lancet 1988;1:268-71.
- Jacobs I, Davies AP, Bridges J, Stabile I, Fay T, Lower A, et al. Prevalence screening for ovarian cancer in postmenopausal women by CA 125 measurement and ultrasonography. BMJ 1993;306:1030-4.
- Jacobs IJ, Skates SJ, MacDonald N, Menon U, Rosenthal AN, Davies AP, et al. Screening for ovarian cancer: a pilot randomised controlled trial. Lancet 1999;353:1207-10.
- Kobayashi H, Ooi H, Yamada Y, Sakata M, Kawaguchi R, Kanayama S, et al. Serum CA125 level before the development of ovarian cancer. Int J Gynaecol Obstet 2007;99:95-9.
- Buys SS, Partridge E, Black A, Johnson CC, Lamerato L, Isaacs C, et al. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA 2011;305:2295-303.
- UK-OCSP 2021. https://ukhealthcare.uky.edu/markey-cancer-center/patient-care/cancer-screening-program/ovarian.
- van Nagell JR, DePriest PD, Ueland FR, DeSimone CP, Cooper AL, McDonald JM, et al. Ovarian cancer screening with annual transvaginal sonography: findings of 25,000 women screened. Cancer 2007;109:1887-96.
- Jacobs IJ, Skates S, Davies AP, Woolas RP, Jeyerajah A, Weidemann P, et al. Risk of diagnosis of ovarian cancer after raised serum CA 125 concentration: a prospective cohort study. BMJ 1996;313:1355-8.
- Skates SJ, Pauler DK, Jacobs IJ. Screening based on the risk of cancer calculation from bayesian hierarchical changepoint and mixture models of longitudinal markers. J Am Stat Assoc 2001;96:429-39.
- Menon U, Skates SJ, Lewis S, Rosenthal AN, Rufford B, Sibley K, et al. Prospective study using the risk of ovarian cancer algorithm to screen for ovarian cancer. J Clin Oncol 2005;23:7919-26.
- UKLWC n.d. http://uklwc.mrcctu.ucl.ac.uk/.
- Biocentre U n.d. www.ukbiocentre.com/.
- Menon U, Gentry-Maharaj A, Ryan A, Sharma A, Burnell M, Hallett R, et al. Recruitment to multicentre trials – lessons from UKCTOCS: descriptive study. BMJ 2008;337.
- Barrett J, Jenkins V, Farewell V, Menon U, Jacobs I, Kilkerr J, et al. Psychological morbidity associated with ovarian cancer screening: results from more than 23,000 women in the randomised trial of ovarian cancer screening (UKCTOCS). BJOG 2014;121:1071-9.
- Kalsi J, Gentry-Maharaj A, Ryan A, Singh N, Burnell M, Massingham S, et al. Performance characteristics of the ultrasound strategy during incidence screening in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Cancers (Basel) 2021;13.
- Sharma A, Burnell M, Gentry-Maharaj A, Campbell S, Amso NN, Seif MW, et al. Quality assurance and its impact on ovarian visualization rates in the multicenter United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Ultrasound Obstet Gynecol 2016;47:228-35.
- Kalsi JK, Ryan A, Gentry-Maharaj A, Margolin-Crump D, Singh N, Burnell M, et al. Completeness and accuracy of national cancer and death registration for outcome ascertainment in trials-an ovarian cancer exemplar. Trials 2021;22.
- UKCTOCS 2022. http://ukctocs.mrcctu.ucl.ac.uk/media/1066/ukctocs-protocol_v90_19feb2020.pdf.
- Daya D, Cheung AN, Khunamornpong S. WHO Classification of Tumors of Female Reproductive Organs. Lyon: International Agency for Research on Cancer; 2014.
- Tavassoli FA, Devilee P. Tumors of the Breast and Female Genital Organs. World Health Organization Classification of Tumours: Pathology and Genetics. Lyon: World Health Organization; 2003.
- EuroQol n.d. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/.
- Jacobs IJ, Menon U, Ryan A, Gentry-Maharaj A, Burnell M, Kalsi JK, et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet 2016;387:945-56.
- Burnell M, Gentry-Maharaj A, Ryan A, Apostolidou S, Habib M, Kalsi J, et al. Impact on mortality and cancer incidence rates of using random invitation from population registers for recruitment to trials. Trials 2011;12.
- Menon U, Gentry-Maharaj A, Hallett R, Ryan A, Burnell M, Sharma A, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Lancet Oncol 2009;10:327-40.
- Menon U, Ryan A, Kalsi J, Gentry-Maharaj A, Dawnay A, Habib M, et al. Risk algorithm using serial biomarker measurements doubles the number of screen-detected cancers compared with a single-threshold rule in the United Kingdom Collaborative Trial of Ovarian Cancer Screening. J Clin Oncol 2015;33:2062-71.
- Burnell M, Gentry-Maharaj A, Ryan A, Apostolidou S, Habib M, Kalsi J, . Impact on mortality and cancer incidence rates of using random invitation from population registers for recruitment to trials. Trials 2011;12.
- Tabar L, Vitak B, Chen TH, Yen AM, Cohen A, Tot T, et al. Swedish two-county trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology 2011;260:658-63.
- Atkin W, Wooldrage K, Parkin DM, Kralj-Hans I, MacRae E, Shah U, et al. Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: the UK Flexible Sigmoidoscopy Screening randomised controlled trial. Lancet 2017;389:1299-311.
- Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Zappa M, Nelen V, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 2014;384:2027-35.
- Holme O, Loberg M, Kalager M, Bretthauer M, Hernan MA, Aas E, et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. JAMA 2014;312:606-15.
- Menon U, McGuire AJ, Raikou M, Ryan A, Davies SK, Burnell M, et al. The cost-effectiveness of screening for ovarian cancer: results from the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Br J Cancer 2017;117:619-27.
- Prat J. Oncology FCoG . Staging classification for cancer of the ovary, fallopian tube, and peritoneum: abridged republication of guidelines from the International Federation of Gynecology and Obstetrics (FIGO). Obstet Gynecol 2015;126:171-4.
- Burnell M, Gentry-Maharaj A, Skates SJ, Ryan A, Karpinskyj C, Kalsi J, et al. UKCTOCS update: applying insights of delayed effects in cancer screening trials to the long-term follow-up mortality analysis. Trials 2021;22.
- Karrison TG. Versatile tests for comparing survival curves based on weighted log-rank statistics. Stata J 2016;16:678-90.
- Royston P, Parmar MK. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med 2002;21:2175-97.
- Kearns B, Chilcott J, Whyte S, Preston L, Sadler S. Cost-effectiveness of screening for ovarian cancer amongst postmenopausal women: a model-based economic evaluation. BMC Med 2016;14.
- Menon U, Gentry-Maharaj A, Burnell M, Singh N, Ryan A, Karpinskyj C, et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet 2021;397:2182-93.
- Spielberger C, Gorsuch R, Lushene RE, Vagg PR, Jacobs GA. Manual for the Stait-Trait Anxiety Inventory (Form Y1–Y2). Palo Alto, CA: Consulting Psychology Press; 1983.
- Goldberg D. Manual of the General Health Questionnaire. Windsor: NFER-Nelson; 1978.
- Gentry-Maharaj A, Glazer C, Burnell M, Ryan A, Berry H, Kalsi J, et al. Changing trends in reproductive/lifestyle factors in UK women: descriptive study within the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). BMJ Open 2017;7.
- NCS . UK National Screening Committee: criteria for appraising the viability, effectiveness and appropriateness of a screening programme 2015.
- ACOG . Summary: opportunistic salpingectomy as a strategy for epithelial ovarian cancer prevention. Am Coll Obstet Gynecol 2019;133.
- Hanley GE, Rozenberg NMK, McAlpine JN. Risk-reducing surgery in women at low lifetime risk of developing ovarian carcinoma: opportunistic salpingectomy. Clin Obstet Gynecol 2017;60:758-70.
- Powell CB, Alabaster A, Simmons S, Garcia C, Martin M, McBride-Allen S, et al. Salpingectomy for sterilization: change in practice in a large integrated health care system, 2011–2016. Obstet Gynecol 2017;130:961-7.
- Gaba F, Robbani S, Singh N, McCluggage WG, Wilkinson N, Ganesan R, et al. Preventing Ovarian Cancer through early Excision of Tubes and late Ovarian Removal (PROTECTOR): protocol for a prospective non-randomised multi-center trial. Int J Gynecol Cancer 2021;31:286-91.
- Gaba F, Goyal S, Marks D, Chandrasekaran D, Evans O, Robbani S, et al. Surgical decision making in premenopausal BRCA carriers considering risk-reducing early salpingectomy or salpingo-oophorectomy: a qualitative study. J Med Genet 2022;59:122-32.
- Steenbeek MP, Harmsen MG, Hoogerbrugge N, de Jong MA, Maas A, Prins JB, et al. Association of salpingectomy with delayed oophorectomy versus salpingo-oophorectomy with quality of life in BRCA1/2 pathogenic variant carriers: a nonrandomized controlled trial. JAMA Oncol 2021;7:1203-12.
- UKCTOCS I 2012. www.ucl.ac.uk/womens-health/.
- UKNEQAS 2021. https://ukneqas.org.uk/programmes/result/?programme=tumour-markers-%28ca-series%29.
- UKAS . What is accreditation? 2021.
- Stott W, Gentry-Maharaj A, Ryan A, Amso N, Seif M, Jones C, et al. Audit of transvaginal sonography of normal postmenopausal ovaries by sonographers from the United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). F1000Res 2018;7.
- Collins RE, Lopez LM, Marteau TM. Emotional impact of screening: a systematic review and meta-analysis. BMC Public Health 2011;11.
- Duffy SW, Vulkan D, Cuckle H, Parmar D, Sheikh S, Smith RA, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol 2020;21:1165-72.
- Partridge E, Kreimer AR, Greenlee RT, Williams C, Xu JL, Church TR, et al. Results from four rounds of ovarian cancer screening in a randomized trial. Obstet Gynecol 2009;113:775-82.
- Pinsky PF, Blacka A, Kramer BS, Miller A, Prorok PC, Berg C. Assessing contamination and compliance in the prostate component of the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial. Clin Trials 2010;7:303-11.
- NICE 2011. www.nice.org.uk/guidance/cg122.
- Lennon AM, Buchanan AH, Kinde I, Warren A, Honushefsky A, Cohain AT, et al. Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science 2020;369.
- Klein EA, Richards D, Cohn A, Tummala M, Lapham R, Cosgrove D, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncol 2021;32:1167-77.
- Kandimalla R, Wang W, Yu F, Zhou N, Gao F, Spillman M, et al. OCaMIR-A noninvasive, diagnostic signature for early-stage ovarian cancer: a multi-cohort retrospective and prospective study. Clin Cancer Res 2021;27:4277-86.
- Kobayashi H, Yamada Y, Sado T, Sakata M, Yoshida S, Kawaguchi R, et al. A randomized study of screening for ovarian cancer: a multicenter study in Japan. Int J Gynecol Cancer 2008;18:414-20.
- Blyuss O, Burnell M, Ryan A, Gentry-Maharaj A, Marino IP, Kalsi J, et al. Comparison of longitudinal CA125 algorithms as a first-line screen for ovarian cancer in the general population. Clin Cancer Res 2018;24:4726-33.
- Manchanda R, Abdelraheim A, Johnson M, Rosenthal AN, Benjamin E, Brunell C, et al. Outcome of risk-reducing salpingo-oophorectomy in BRCA carriers and women of unknown mutation status. BJOG 2011;118:814-24.
- NHSOFH 2021. https://ourfuturehealth.org.uk/.
- Levanon K, Crum C, Drapkin R. New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol 2008;26:5284-93.
- Kurman RJ, Shih Ie M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer–shifting the paradigm. Hum Pathol 2011;42:918-31.
- Brown PO, Palmer C. The preclinical natural history of serous ovarian cancer: defining the target for early detection. PLOS Med 2009;6.
- Menon U, Gentry-Maharaj A, Ryan A, Burnell M, Dawnay A, Habib M, et al. Serial CA125 Interpreted Using the Risk of Ovarian Cancer Algorithm Can Detect Ovarian Cancer in Absence of Ultrasound Abnormalities London n.d.
- Widschwendter M, Zikan M, Wahl B, Lempiainen H, Paprotka T, Evans I, et al. The potential of circulating tumor DNA methylation analysis for the early detection and management of ovarian cancer. Genome Med 2017;9.
- ESMO minimum clinical recommendations for diagnosis, treatment and follow-up of ovarian cancer. Ann Oncol 2001;12:1205-7.
- Aebi S, Castiglione M, Group EGW. Epithelial ovarian carcinoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2008;19:ii14-6.
- Colombo N, Peiretti M, Parma G, Lapresa M, Mancari R, Carinelli S, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21:v23-30.
- Chandrasekaran D, Sobocan M, Blyuss O, Miller RE, Evans O, Crusz SM, et al. Implementation of multigene germline and parallel somatic genetic testing in epithelial ovarian cancer: SIGNPOST Study. Cancers (Basel) 2021;13.
- Pinsky PF, Yu K, Kramer BS, Black A, Buys SS, Partridge E, et al. Extended mortality results for ovarian cancer screening in the PLCO trial with median 15 years follow-up. Gynecol Oncol 2016;143:270-5.
- Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med 2012;366:981-90.
- Parkes CA, Smith D, Wald NJ, Bourne TH. Feasibility study of a randomised trial of ovarian cancer screening among the general population. J Med Screen 1994;1:209-14. 10.1177/096914139400100404.
- Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, et al. Comparison of sociodemographic and health-related characteristics of UK biobank participants with those of the general population. Am J Epidemiol 2017;186:1026-34.
- Navarro M, Nicolas A, Ferrandez A, Lanas A. Colorectal cancer population screening programs worldwide in 2016: an update. World J Gastroenterol 2017;23:3632-42.
- Agide FD, Garmaroudi G, Sadeghi R, Shakibazadeh E, Yaseri M, Koricha ZB, et al. A systematic review of the effectiveness of health education interventions to increase cervical cancer screening uptake. Eur J Public Health 2018;28:1156-62.
- NCRAS 2020. www.ncin.org.uk/cancer_type_and_topic_specific_work/cancer_type_specific_work/gynaecological_cancer/gynaecological_cancer_hub/ovarian_cancer_audit_feasibility_pilot_outputs.
- Timmermans M, Sonke GS, Slangen BFM, Baalbergen A, Bekkers RLM, Fons G, et al. Outcome of surgery in advanced ovarian cancer varies between geographical regions; opportunities for improvement in The Netherlands. Eur J Surg Oncol 2019;45:1425-31.
- Narasimhulu DM, Kumar A, Weaver AL, McGree ME, Langstraat CL, Cliby WA. Using an evidence-based triage algorithm to reduce 90-day mortality after primary debulking surgery for advanced epithelial ovarian cancer. Gynecol Oncol 2019;155:58-62.
- Warren JL, Harlan LC, Trimble EL, Stevens J, Grimes M, Cronin KA. Trends in the receipt of guideline care and survival for women with ovarian cancer: a population-based study. Gynecol Oncol 2017;145:486-92.
- Lee A, Yang X, Tyrer J, Gentry-Maharaj A, Ryan A, Mavaddat N, et al. Comprehensive epithelial tubo-ovarian cancer risk prediction model incorporating genetic and epidemiological risk factors. J Med Genet 2021;59:632-43.
- Blackford AL, Childs EJ, Porter N, Petersen GM, Rabe KG, Gallinger S, et al. A risk prediction tool for individuals with a family history of breast, ovarian, or pancreatic cancer: BRCAPANCPRO. Br J Cancer 2021;125:1712-17.
- Russell MR, D’Amato A, Graham C, Crosbie EJ, Gentry-Maharaj A, Ryan A, et al. Novel risk models for early detection and screening of ovarian cancer. Oncotarget 2017;8:785-97.
- UKCTOCS . UKCTOCS Ovarian Cancer Mortality Results Meeting n.d.
Linked articles/related specialty collections, presentations, and other media
Media
2021
BBC News
www.bbc.co.uk/news/health-57087477
BBC Radio 4 The Today Programme (skip to 2:08:03)
www.bbc.co.uk/sounds/play/m000vyn2
BBC Radio 2 News (skip to 1:32:38)
www.bbc.co.uk/sounds/play/m000vyg8
BBC World Service’s ‘The Newsroom’ (skip to 16:55)
www.bbc.co.uk/sounds/play/w172xyxfw79rfxh
Sky News
Yahoo News
https://uk.news.yahoo.com/ovarian-cancer-screening-doesnt-save-lives-223016448.html
PA via Yahoo
https://uk.news.yahoo.com/ovarian-cancer-population-screening-did-223000849.html
Telegraph
www.telegraph.co.uk/news/2021/05/13/screening-women-ovarian-cancer-does-not-reduce-deaths-study/
Guardian
Independent
www.independent.co.uk/news/health/ovarian-cancer-screening-deaths-women-b1846182.html
iNews
Sun
www.thesun.co.uk/fabulous/14942639/7-ovarian-cancer-signs-screening-plans-delayed-decade/
BMJ
CBC Radio (Canada)
Irish News
ABC (Spain)
2019
The Adelaide Advertiser
On behalf of Ovarian Cancer Australia for Ovarian Cancer Awareness on 4 February 2019.
Body and Soul publication
On behalf of Ovarian Cancer Australia about Ovarian Cancer Awareness month published on 16 January 2019.
2014–2015
BBC News
Blood test “boost” in ovarian cancer fight (Tuesday 5 May 2015)
The Guardian
New ovarian cancer test twice as effective as existing methods (Monday 4 May 2015)
BBC News
Skirt size increase linked to breast cancer risk, says study (Thursday 25 September 2014)
NHS News
Skirt size increase ups breast cancer risk (Thursday, 25 September 2014)
2001–2010
NHS News
Ovarian cancer screening (11 March 2009)
BBC News
Trials offer ovarian cancer hope (Wednesday 9 March 2009)
The Guardian
Ovarian cancer screening on trial (Wednesday 22 March 2000; p. 7)
BBC News
Cancer screening trials to begin (Tuesday 21 March 2000)
Websites
MRC CTU website
Cancer Research UK website
Invited presentations
| Date | Details | Presenter |
|---|---|---|
| 12 December 2021 | Management of women at increased risk for ovarian cancer - current status and future strategies, RCOG International Representative Committee India North Annual Conference, India | Prof Menon |
| 10 December 2021 | The outcome of UKCTOCS: Where to now for Ovarian Cancer Screening?, EGA IfWH ’16th’ Annual Conference, London, UK | Prof Menon |
| 16 November 2021 | The outcome of UKCTOCS: Where to now for Ovarian Cancer Screening?, NY Obstetrical Society, New York, USA | Prof Menon |
| 16 November 2021 | What’s next for research into ovarian cancer screening? Reflections on UKCTOCS, Target Ovarian Cancer Digital Conference Moving Forwards Together, London, UK | Prof Menon |
| 27 October 2021 | Ovarian and endometrial cancer screening, Division of Cancer Prevention Cancer Screening Trial Network Workshop, DCP Cancer Screening Trial Network Workshop, National Cancer Institute, Maryland, USA | Prof Menon |
| 25 October 2021 | Should we screen for ovarian cancer in high risk women? – current status and future strategies, ESGO Annual Meeting, Prague, Czech Republic | Prof Menon |
| 8 October 2021 | ‘What do we do once we detect early?’, Panel discussion, Early Detection of Cancer Conference, CRUK, London, UK | Prof Menon |
| 28 September 2021 | The outcome of UKCTOCS: where to now for ovarian cancer screening?, Ovarian Cancer National Conference, Ovarian Cancer Research Alliance, New York, USA | Prof Menon |
| 21 September 2021 | Evidence from the Ovarian Cancer Screening trials, Cancer Screening Expert Workshop, SAPEA (Science Advice for Policy by European Academies) | Prof Menon |
| 2 March 2020 | Ovarian Cancer Screening, Advancing Progress in the Development and Implementation of Effective, High-Quality Cancer Screening: A Workshop by National Cancer Policy Forum, Washington, USA | Prof Menon |
| 14 December 2019 | Progress in screening for Ovarian Cancer, Manipal University, India | Prof Jacobs |
| 11 December 2019 | Early detection of ovarian cancer, University of New South Wales Academy for Women’s wellbeing | Prof Jacobs |
| 24 October 2019 | Early Detection and Surgical Prevention, IARC/NCI Seminar: Ovarian Cancer, Lyon, France | Prof Menon |
| 16 October 2019 | How can clinical trial units and networks help deliver high quality cancer trials, Choosing Treatments Wisely Summit, Delhi, India | Prof Menon |
| 10 October 2019 | Research on detecting ovarian cancer, Annual Meeting, Academy of Health and Medical Sciences, Perth, Australia | Prof Jacobs |
| 31 August 2019 | Accelerating progress, identifying where a collective voice for change is needed, and what women want from a national action plan, Ovarian Cancer National Action Plan summit, Sydney, Australia | Prof Jacobs |
| 19 June 2019 | Association of hysterectomy and primary invasive ovarian cancer risk in postmenopausal women: a cohort study within the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS), Top Abstracts plenary lecture, Royal College of Obstetricians and Gynaecology (RCOG) World Congress | Dr Gentry-Maharaj |
| 13 June 2019 | Generating evidence for population screening - the UKCTOCS experience, Indian Alliance 9th Annual Fellows’ Meeting, Bangalore, India | Prof Menon |
| 11 January 2019 | Advances in the management of ovarian cancer risk, All India Congress of Obstetrics and Gynaecology (AICOG 2019), Bangalore, India | Prof Menon |
| 4 December 2018 | Screening for gynaecological cancers - what is the evidence?, Women’s Health Concern 28th Annual Symposium, Royal College of Obstetricians & Gynaecologists, London, UK | Prof Menon |
| 31 May 2018 | Biomarkers for screening and differential diagnosis of ovarian cancer, Frontiers of Science Seminar, Biocity Turku, Finland | Prof Menon |
| 1 December 2017 | The 8th Tropé-Kolstad Lecture: Ovarian cancer screening: Past, present and future, 8th Tropé-Kolstad meeting, Institute for Cancer Research, Norwegian Radium Hospital, Oslo, Norway | Prof Menon |
| 19 May 2017 | Lessons from the UK Ovarian Cancer screening trials, Ovarian Cancer – Molecular Aspects in Modern Diagnostics and Therapy, 19–20 May 2017, Radisson Blu Sobieski Hotel, Warsaw, Poland | Prof Menon |
| 6 May 2017 | Early detection of ovarian cancer: What does the evidence show, how can we better our efforts?, 9th International Charité-Mayo-Conference, 3–6 May 2017, Langenbeck-Virchow-Haus, Berlin, Germany | Prof Menon |
| 31 March 2017 | Ovarian Cancer Screening - lessons from the UK trials, Multidisciplinary Approach to Gynecological Cancers conference, Krakow, Poland | Dr Gentry-Maharaj |
| 13 January 2017 | Sustaining a biobank long-term – The UKCTOCS experience, Biobank Workshop, 12–13 January 2017, Tata Medical Center, Kolkata, India | Prof Menon |
| 12 January 2017 | Data collection and IT solutions, Biobank Worshop, 12–13 January 2017, Tata Medical Center, Kolkata, India | Prof Menon |
| 25 November 2016 | Ovarian Cancer Screening and Risk Stratification, L’ovaire normal et pathologique: de la réalité au fantasme, Cliniquies Universitaires, Saint-Luc UCL Brussels, Belgium | Dr Gentry-Maharaj |
| 29 October 2016 | Plenary lecture: Are we able to effectively screen for ovarian cancer?, 16th Biennial Meeting of the International Gynecologic Cancer Society (IGCS 2016), 29–31 October 2016, Lisbon Congress Centre, Lisbon, Portugal | Prof Menon |
| 6 October 2016 | Ovarian cancer screening, New Cancer Screening Programmes, Swedish Medical Society, Stockholm, Sweden | Prof Menon |
| 6 October 2016 | Ovarian Cancer Screening - UKCTOCS, JRO UCLH | Dr Gentry-Maharaj |
| 1 October 2016 | Screening for Ovarian Cancer, 15th IMS World Congress on Menopause, 28 September – 1 October 2016, Prague Congress Centre, Prague, Czech Republic | Prof Menon |
| 28 September 2016 | The UK Collaborative Trial of Ovarian Cancer Screening – Outcomes, UCL Frontiers of Oncology: Women’s Health, Royal College of Physicians, London, UK | Prof Menon |
| 12 September 2016 | Plenary lecture: UKCTOCS – exploring the results, 11th Biennial Ovarian Cancer Research Symposium, 12–13 September 2016, University of Washington, Seattle, Washington DC, USA | Prof Menon |
| 10 September 2016 | Risk stratification and screening of ovarian cancer, 2nd ESGO State of Art Conference: Prevention in Gynaecological Malignancies, 8–10 September 2016, Susesi Hotel, Antalya, Turkey | Prof Menon |
| 20 June 2016 | Screening for Ovarian Cancer – update from UK trials, RCOG World Congress 2016, 20–22 Jun 2016, The ICC, Birmingham, UK | Prof Menon |
| 5 June 2016 | Performance characteristics and stage distribution of invasive epithelial ovarian/tubal/peritoneal cancers in UKCTOCS, 2016 ASCO Annual Meeting, 3–7 Jun 2016, McCormick Place Convention Center, Chicago, Illinois, USA | Prof Menon |
| 18 May 2016 | Advances in ovarian cancer screening, Primary Care & Public Health 2016, 18–19 May 2016, The NEC, Birmingham, UK | Prof Menon |
| 12 May 2016 | Ovarian cancer screening – what do we know now?, British Gynaecological Cancer Society Annual Scientific Meeting (BGCS 2016), 12–13 May 2016, The ICC, Birmingham, UK | Prof Menon |
| 27 April 2016 | UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS), Ovarian Cancer Association Consortium (OCAC) Meeting, Buckinghamshire, UK | Prof Menon |
| 16 April 2016 | Mortality outcomes of the UK Ovarian Cancer Screening programme (UKCTOCS), British Society for Gynaecological Imaging (BSGI) Annual Scientific Meeting, RCOG, London, UK | Prof Menon |
| 3 March 2016 | UKCTOCS – the benefits and harms of ovarian cancer screening, Annual Academic Meeting – Joint RCOG/BBRS Meeting RCOG, London, UK | Prof Menon |
| 29 February 2016 | Current role of symptom awareness, OvSTAT Event: Ovarian Cancer Tool Seminar, Park Plaza Cardiff, Wales, UK | Prof Menon |
| 25 February 2016 | Plenary lecture: Ovarian cancer screening, Northern Sweden Gynecologic Society, Umeå University, Sweden | Prof Menon |
| 8 February 2016 | Update on the UKCTOCS trial: Compliance, performance of MMS & USS arms, stage distribution, After UKCTOCS: Public Messaging on Screening and Early Detection of Ovarian Cancer, Banbury Center, Cold Spring Harbor Laboratory, USA | Prof Menon |
| 27 November 2015 | Screening for ovarian cancer using CA125 interpreted with the Risk of Ovarian Cancer algorithm, UCLH Clinical Biochemistry seminar series | Dr Gentry-Maharaj |
| 25 November 2015 | UKCTOCS update, RCOG Annual Professional Development Conference, RCOG, UK | Prof Menon |
| 13 November 2015 | Population Screening and Early Detection of Ovarian Cancer Using CA125 , 2nd Sino-Euro Experts Conference on Immune Biomarkers for Personalized Medicine in Oncology, Fudan University Shanghai Cancer Center, Shanghai, China | Dr Gentry-Maharaj |
| 4 November 2015 | Ovarian Cancer screening – update from the UK trials, 2015 NCRI Cancer Conference, Liverpool, UK | Prof Menon |
| 27 October 2015 | Management of Ovarian Cancer risk, 19th International Meeting of the European Society of Gynaecological Oncology, Nice, France | Prof Menon |
| 19 October 2015 | Plenary lecture: Ovarian cancer screening, AACR Advances in Ovarian Cancer Research: Exploiting Vulnerabilities, 17–20 October 2015, Orlando, Florida, USA | Prof Menon |
| 8 October 2015 | Ovarian cancer screening – role of biomarkers, 4th Biomarkers in Diagnoscs, Berlin, Germany | Prof Menon |
| 5 October 2015 | Ovarian cancer screening: Hype or hope, Ovarian Cancer Academy, Cluj-Napoca, Romania | Dr Gentry-Maharaj |
| 21 July 2015 | Population screening for ovarian cancer using CA125 interpreted by the risk of ovarian cancer algorithm, Mucins in Health and Disease (13th International Workshop on Carcinoma-associated Mucins), Cambridge, UK | Dr Gentry-Maharaj |
| 31 May 2015 | Early Ovarian Cancer: Can We Find It, Can We Stop It, Can We Afford It?, 2015 American Society of Clinical Oncology Annual Meeting, Chicago, USA | Prof Menon |
| 17 April 2015 | The UKCTOCS and UKFOCSS trials – what have we learnt so far?, 2nd International Ovarian Tumor Analysis Congress (IOTA 2015), Leuven, Belgium | Prof Menon |
| 17 April 2015 | Ovarian cancer screening: UKCTOCS Incidence Screening results update British Society for Gynaecological Imaging - Annual Scientific Meeting | Dr Gentry-Maharaj |
| 27 February 2015 | Ovarian Cancer screening – any progress?, Oncology in obstetrics and gynaecology – “State of the art”, Royal Society of Medicine, London, UK | Prof Menon |
| 8 September 2014 | Keynote Lecture: Screening for Ovarian Cancer: The Way Forward, 10th Biennial Ovarian Cancer Research Symposium, Seattle, USA | Prof Menon |
| 13 July 2014 | Ovarian cancer screening, AGOI Annual meeting, Bangalore, India | Prof Menon |
| 27 June 2014 | Screening for Ovarian Cancer, Biennial Ovarian Cancer Symposium, National University of Singapore, Singapore | Prof Menon |
| 8 May 2014 | Ovarian and endometrial ultrasound cancer screening, 23rd European Congress of Obstetrics and Gynaecology (EBCOG 2014), Glasgow, Scotland | Prof Menon |
| 25 April 2014 | The evolution of invasive epithelial ovarian cancer – Insights from UKCTOCS, BSGI, Annual Scientific Meeting, RCOG, London, UK | Prof Menon |
| 29 March 2014 | Singapore Lecture: Managing Ovarian Cancer Risk, RCOG World Congress 2014, Hyderabad, India | Prof Menon |
| 18 January 2014 | Ovarian cancer symptoms - where to set the bar?, 39th Annual Conference of The Bengal Obstetric & Gynaecological Society, Kolkata, India | Prof Menon |
| 30 November 2013 | Genes, lifestyle and risk of ovarian cancer, Ranbaxy Science Foundation’s 30th Round Table Conference on ‘Lifestyle and Cancer with focus on Cancer Prevention’, All India Institute of Medical Sciences, New Delhi, India | Prof Menon |
| 29 November 2013 | Keynote lecture: Ovarian cancer screening, 46th Dr Subodh Mitra Memorial Oration, Bengal Obstetric & Gynaecological Society, Chittaranjan Seva Sadan College of Obstetrics, Kolkata, India | Prof Menon |
| 22 November 2013 | Ovarian cancer screening, Indian Cancer Congress Annual Meeting, New Delhi, India | Prof Menon |
| 24 October 2013 | Time series algorithms – what can we learn from UKCTOCS?, Ovarian Cancer: Developing Research-Based Public Messaging on Early Detection and Screening, Cold Spring Harbour, New York, USA | Prof Menon |
| 21 October 2013 | Clinical Trials – management and implementation, 18th International Meeting ESGO, Liverpool, UK | Prof Menon |
| 3 October 2013 | Screening for ovarian cancer – New insights, future directions, Gynaecological Visiting Society (GVS), London, UK | Prof Menon |
| 25 September 2013 | Screening for ovarian cancer, Annual General Meeting, The Royal Society of Medicine, London, UK | Prof Menon |
| 7 September 2013 | Screening for ovarian cancer – new insights, future direction, Indo-UK Oncology Summit, Chennai, India | Prof Menon |
| 11 April 2013 | Rethinking screening strategies for ovarian cancer, Nordic Society of Gynecologic Oncology (NSGO) Annual Meeting 2013, Stockholm, Sweden | Prof Menon |
| 15 March 2013 | Volunteer acceptance of transvaginal scanning: UKCTOCS experience, British Society of Gynaecological Imaging, London, UK | Dr Gentry-Maharaj |
| 15 March 2013 | Update from the UKCTOCS Trial, Annual Scientific Meeting – Joint RCOG/BSGI, RCOG, London, UK | Prof Menon |
| 5 November 2012 | Ovarian Cancer Screening – the future, 8th NCRI Cancer Conference, Liverpool, UK | Prof Menon |
| 18 May 2012 | Does transvaginal ultrasound have any role in the screening for endometrial cancer in asymptomatic postmenopausal women?, Annual Scientific Meeting and Workshops, Joint RCOG/BSGI Meeting, Royal College of Obstetricians and Gynaecologists, London, UK | Dr Gentry-Maharaj |
| 30 November 2011 | Quo Vadis? – Biomarker discovery for Ovarian Cancer Screening Consensus Workshop and Changing the clinical paradigm for ovarian cancer – insights from UKCTOCS, Ovarian Cancer Screening Conference, The Royal College of Physicians, London, UK | Prof Menon |
| 29 November 2011 | General population screening – current status and insights from the trials: UKCTOCS, International Conference on Ovarian Cancer Screening, London, UK | Prof Menon |
| 18 November 2011 | Morbidity in Gynaecological Oncology – United Kingdom Gynaecological Oncology Surgical Outcomes and Complications (UKGOSOC), BGCS Autumn Scientific Meeting, Birmingham, UK | Prof Menon |
| 10 September 2011 | Screening for ovarian and endometrial cancer, 9th Congress of the European Society of Gynecology, Copenhagen, Denmark | Prof Menon |
| 3 June 2011 | Surgery, Co-morbidity and Complications; Preliminary data from United Kingdom Gynaecological Oncology Surgical Outcomes and Complications, BGCS meeting, Cardiff, UK | Prof Menon |
| 19 May 2011 | Ultrasound scanning accuracy in UKCTOCS, BSGI, Royal College of Obstetricians and Gynaecologists, London, UK | Prof Menon |
| 16 January 2011 | Serial CA125 can detect ovarian cancer in the absence of ultrasound abnormalities, Helene Harris Memorial Trust 12th International Forum on Ovarian Cancer, Hallandale, Florida, USA | Prof Menon |
| 23 September 2010 | UKCTOCS – an update, 13 Biennial IGCS Meeting, Prague, Czech Republic | Prof Menon |
| 6 September 2010 | Biomarkers for Screening and Early Detection of Ovarian Cancer, 38th International Society for Oncodevelopmental Biology Meeting, Munich, Germany | Prof Menon |
| 6 June 2010 | High Risk Ovarian cancer Screening: Is It Useful?, ASGO Annual Meeting, Chicago, USA | Prof Menon |
| 15 April 2010 | Screening for Ovarian Cancer – Biochemical Markers or Ultrasound, Joint RCOG/BSGI Meeting and Workshop, London, UK | Prof Menon |
| 27 February 2010 | Familial Ovarian Cancer – Risk Management, AICC RCOG 25th Annual Conference, Kolkata, India | Prof Menon |
| 25 February 2010 | Interactive case discussions with cytology, colposcopy & histology co-relation, and unedited video presentation, AICC RCOG 25th Annual Conference, Kolkata, India | Prof Menon |
| 11 February 2010 | Ovarian Cancer Screening Current Trials and Unanswered Questions, Ovarian Cancer Research Forum, RCOG, London, UK | Prof Menon |
| 14 January 2010 | Better outcomes for Ovarian Cancer Generating the evidence, 8th CMC Winter Symposium Evidence for Better Health, Christian Medical College, Vellore, India | Prof Menon |
| 8 December 2009 | Prevalence Screen in the United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS), British Medical Ultrasound Society Meeting, Edinburgh, UK | Dr Gentry-Maharaj |
| 8 December 2009 | Volunteer Satisfaction Survey for UKCTOCS. British Medical Ultrasound Society Meeting, Edinburgh, UK | Dr Gentry-Maharaj |
| 26 November 2009 | Preliminary screening information for UKCTOCS, Senior Staff Conference, Royal College of Obstetricians and Gynaecologists (RCOG), London, UK | Dr Gentry-Maharaj |
| 23 July 2009 | Does serum CA125 hold the key to decreasing deaths from ovarian cancer?, 10th International Mucin Meeting, Cambridge, UK | Prof Menon |
| 8 June 2009 | The prevalence screen in UKCTOCS, Early Origins of Ovarian Cancer Symposium, Norris Cancer Centre, Los Angeles, USA | Prof Menon |
| 19 March 2009 | The Prevalence Screen in the United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS), British Society for Gynaecological Imaging, London, UK | Dr Gentry-Maharaj |
| 8 January 2009 | Prevalence Screen in the United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS), Controversies of Ovarian Cancer Screening, Geneva, Switzerland | Dr Gentry-Maharaj |
| 29 November 2008 | Ovarian Cancer Symptoms – where to set the bar, Annual meeting of Sexual Health, London, UK | Prof Menon |
| 4 September 2008 | The prevalence screen in the United Kingdom Collaborative Trial of Ovarian Cancer Screening, 7th Biennial Ovarian Cancer Research Symposium, Seattle, USA | Prof Menon |
| 9 July 2008 | The United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS), Beckman Coulter Symposium, London, UK | Dr Gentry-Maharaj |
| 20 May 2008 | Clinical aspects of ovarian cancer and current markers, FOCUS 2008 Annual National Meeting of the Association of Clinical Biochemistry, Birmingham, UK | Prof Menon |
| 12 April 2008 | Screening for Ovarian Cancer, 2nd World Congress, International Society for Mild Approaches to Assisted Reproduction, London, UK | Prof Menon |
| 7 April 2008 | The State of Art in Ovarian Cancer Screening, Innovations and progress in Healthcare for Women, 1st International Conference of the UCL Institute for Women’s Health, London, UK | Prof Menon |
| 5 October 2007 | The relevance of screening clinical trials and their potential effect on the participants, Clinical Research Nurses Association Annual Conference, Canterbury, UK | Prof Menon |
| 17 September 2007 | Managing Familial Ovarian Cancer Risk, British Human Genetics Society Annual Conference, York, UK | Prof Menon |
| 22 June 2007 | Ovarian Cancer Screening Can we Save Lives?, Christian Medical College Alumni Association Annual Meeting, Iowa, USA | Prof Menon |
| 2 June 2007 | Lead Discussant: Screening and Management of Early Stage Ovarian Cancer, ASCO Annual meeting, Chicago, USA | Prof Menon |
| 17 May 2007 | Update on UKCTOCS Trial, Female Malignancies – Imaging, Screening, Managing Treatment Meeting, Institute of Physics, London, UK | Prof Menon |
| 18 April 2007 | Ovarian Cancer Screening, Royal College of Radiologists Screening Day, London, UK | Prof Menon |
| 14 December 2006 | Ultrasound screening for ovarian cancer, 38th Annual Scientific Meeting British Medical Ultrasound Society, RCOG, London, UK | Prof Menon |
| 24 October 2005 | UKCTOCS: Design And Characteristics of the Study Population, Ovarian Cancer Symposium, Pittsburgh, USA | Prof Menon |
| 20 May 2004 | Will ovarian cancer screening save lives?, 25th Anniversary Meeting BGCS, London, UK | Prof Menon |
| 5 December 2003 | Screening for Sporadic Ovarian Cancer, 13th Annual Meeting in Honour of Professor Per Kolstad, Norwegian Radium Hospital, Oslo, Norway | Prof Menon |
| 15 October 2003 | Screening for Ovarian Cancer – Current Trials, Annual Joint Gynaecology Oncology Meeting, Institute of Molecular Medicine, Oxford, UK | Prof Menon |
| 8 September 2003 | Screening Studies – UKCTOCS / UKFOCSS, Joint National Conference of the National Cancer Research Network and British Oncology Data Manager’s Association, University of Nottingham, UK | Prof Menon |
| 7 April 2003 | Prevalence screening for ovarian cancer using Risk of Ovarian Cancer Algorithm, ESGO Meeting, Brussels, Belgium | Prof Menon |
| 27 March 2003 | Current Ovarian Cancer Screening Trials, Helene Harris Memorial Trust Meeting, Stratford on Avon, UK | Prof Menon |
| 8 January 2003 | Progress in Screening for Ovarian Cancer, All India Congress of Obstetrics and Gynaecology, Bangalore, India | Prof Menon |
| 19 September 2002 | Ovarian cancer screening, 4th Ovarian Cancer Research Symposium, Swedish Medical Centre, Seattle, USA | Prof Menon |
| 17 July 2002 | Screening for Ovarian Cancer, Key Advances in the Effective Management of Ovarian Cancer, Royal College of Physicians, London, UK | Prof Menon |
| 17 June 2002 | Screening for Ovarian Cancer, Annual Meeting of the European Society of Urogenital Radiology, Genoa, Italy | Prof Menon |
Review articles, chapters, and All-Parliamentary Groups
Review articles
Alexander-Sefre F, Menon U, Jacobs IJ. Ovarian cancer screening. Hosp Med 2002;63(4):210–13.
Campbell S, Gentry-Maharaj A. The role of transvaginal ultrasound in screening for ovarian cancer. Climacteric 2018;21(3):221–6. https://doi.org/10.1080/13697137.2018.1433656. Epub 2018 March 1. PMID: 29490504.
Crosbie E, Menon U. Epithelial ovarian cancer and induction of ovulation. Rev Gynaecol Pract 2005;5:131–8.
Gentry-Maharaj A, Menon U. Screening for ovarian cancer in the general population. Best Pract Res Clin Obstet Gynaecol 2012;26(2):243–56. https://doi.org/10.1016/j.bpobgyn.2011.11.006. Epub 2011 December 17. PMID: 22182415.
Gentry-Maharaj A, Sharma A, Menon U. Ovarian cancer (review). Geriatr Med 2007;37(11):8–12.
Hamilton W, Menon U. Ovarian cancer. BMJ 2009;339:b4650. https://doi.org/10.1136/bmj.b4650.
Jacobs I, Menon U. Can ovarian cancer screening save lives? The question remains unanswered. Obstet Gynecol 2011;118(6):1209–11.
Jacobs I, Menon U. The sine qua non of discovering novel biomarkers for early detection of ovarian cancer: carefully selected preclinical samples. Cancer Prev Res (Phila) 2011;4(3):299–302.
Jacobs IJ, Menon U. Progress challenges in screening for early detection of ovarian cancer. Mol Cell Proteomics 2004;3(4):355-66.
Kalsi J, Manchanda R, Menon U. Screening for gynecologic cancers. Exp Rev Obstet Gynecol 2013;8(2):143–60.
Lewis S, Menon U. Screening for ovarian cancer. Exp Rev Anticancer Ther 2003;3(1):55–62.
Menon U, Gentry-Maharaj A, Jacobs I. Ovarian cancer screening and mortality. JAMA 2011;306(14):1544.
Menon U, Griffin M, Gentry-Maharaj A. Ovarian cancer screening – current status, future directions. Gynecol Oncol. 2014;132(2):490–5. https://doi.org/10.1016/j.ygyno.2013.11.030. Epub 3 December 2013. PMID: 24316306; PMCID: PMC3991859.
Menon U, Jacobs IJ. Ovarian cancer screening in the general population – an update. Curr Opin Obstet Gynaecol 2001;13(1):61–4.
Menon U, Jacobs IJ. Ovarian cancer screening in the general population – current status. Int J Gynecol Cancer 2001;11(S1):3–6.
Menon U, Karpinskyj C, Gentry-Maharaj A. Ovarian cancer prevention and screening. Obstet Gynecol 2018;131(5):909–27. https://doi.org/10.1097/AOG.0000000000002580. PMID: 29630008.
Menon U. Ovarian cancer screening has no effect on disease-specific mortality. Evid Based Med 2012;17(2):47–8.
Menon U. Ovarian cancer screening. CMAJ 2004;171(4):323–4.
Menon U. Ovarian cancer: challenges of early detection. Nat Clin Pract Oncol 2007;4(9):498–9.
Menon U. Steps towards effective gynaecological cancer screening. Nat Rev Clin Oncol 2018;15(9):538–40. https://doi.org/10.1038/s41571-018-0049-4. PMID: 29855609.
Nash Z, Menon U. Ovarian cancer screening: current status and future directions. Best Pract Res Clin Obstet Gynaecol 2020;65:32–45. https://doi.org/10.1016/j.bpobgyn.2020.02.010. Epub 2020 Mar 3. PMID: 32273169.
Rosenthal AN, Menon U, Jacobs IJ. Screening for ovarian cancer. Clin Obstet Gynecol 2006;49(3):433–47.
Rufford B, Menon U, Jacobs IJ. Ovarian Cancer Screening. Proceedings of the 2nd World Congress on Controversies in Obstetrics, Gynaecology and Infertility, September 2001, Paris, France.
Sharma A, Menon U. Screening for gynaecological cancers. Eur J Surg Oncol 2006;32(8):818–24, ISSN: 0748-7983.
Sharma A, Menon U. The value of ovarian cancer screening. Br J Hosp Med 2006;67(6):314–17.
Widschwendter M, Menon U. Circulating methylated DNA: a new generation of tumor markers. Clin Cancer Res 2006;12(24):7205–8.
Chapters
Alexander Sefre F, Menon U, Jacobs IJ. Ovarian cancer screening. In Farquharson R, editor. Vignettes for the MRCOG. London: Quay Books; 2005. pp. 57–62.
Fourkala E-O, Gentry-Maharaj A, Menon U. MUC16, alias CA125 and ovarian cancer. In Taylor-Papadimitriou J, Burchell JM, editors. Mucins and Cancer. London, UK: Future Medicine; 2013. pp. 82–93.
Gentry-Maharaj A, Griffin M, Menon U. Ovarian cancer prevention and screening. In Eeles R, Berg C, Tobias J, editors. Cancer Prevention and Screening: Concepts, Principles and Controversies. Oxford: Wiley-Blackwell; 2018. pp. 331–48.
Gentry-Maharaj A, Jacobs I, Menon U. Development and identification of tumor serum markers. In Barakat RR, Berchuck A, Markman M, Randall ME, editors. Principles and Practice of Gynecologic Oncology. 6th edn. Baltimore: Lippincott Williams & Wilkins; 2013. pp. 89–114.
Gentry-Maharaj A, Jacobs I, Menon U. Development and identification of tumour markers. In Barakat RR, Markman M, Randall ME, editors. Principles and Practice of Gynecologic Oncology. 5th edn. USA: Wolter Kluwer Health; Lippincott, Williams & Wilkins; 2009. pp. 145–62.
Gentry-Maharaj A, Jacobs I, Menon U. Ovarian cancer tumor markers and screening. In Berek JS, Hacker NF, editors. Berek and Hacker’s Gynecologic Oncology. 6th edn. Lippincott Williams & Wilkins; 2014. pp. 443–63.
Gentry-Maharaj A, Kalsi J, Menon U. Screening for gynaecological cancers. In Patel HRH, Mould T, Joseph JV and Delaney CP, editors. Pelvic Cancer Surgery: Modern Breakthroughs and Future Advances. Chap 26, Springer; ISBN 978-1-4471-4257-7; 2015. pp. 267–82.
Gentry-Maharaj A, Kalsi J, Menon U. Screening for gynecologic cancers. In Patel H, Mould T, Joseph J, Delaney C, editors. Pelvic Cancer Surgery: Modern Breakthroughs & Future Advances. Berlin/Heidelberg, Germany: Springer Science; 2014. pp. 267–81.
Gentry-Maharaj A, Menon U. Development and identification of tumour markers. In Chi D, Berchuck A, Dizon DS, Yashar CM, editors. Principles and Practice of Gynecologic Oncology. 7th edn. Wolters Kluwer; 2017. pp.79–99.
Gentry-Maharaj A, Menon U. Early detection of hereditary gynaecological cancers. In Jacobs C, Robinson L, Webb P, editors. Genetics for Health Professionals in Cancer Care: from Principles to Practice. 1st edn. Oxford, UK: Oxford University Press; 2014. pp. 173–9.
Gentry-Maharaj A, Menon U. Screening for ovarian cancer in the general population. Best Pract Res Clin Obstet Gynaecol 2012;26(2):243–56.
Manchanda R, Jacobs IJ, Menon U. Tumour markers and screening. In Berek J, Hacker N, editors. Practical Gynecologic Oncology. 5th edn. Philadelphia: Lippincott, Williams & Wilkin; 2010. pp. 233–66.
Menon U, Brinkman DA, Skates SJ, Jacobs IJ. Screening: ovarian cancer. In Cuzick J, editor. Evidence Based Oncology Part 3. Amsterdam, Netherlands: Elsevier; 2003.
Menon U, Dilley J. Tumour markers. In Richard Smith J, Del Priore G, Coleman RL, Monaghan JM, editors. An Atlas of Gynecologic Oncology: Investigation and Surgery. 4th edn. Chap 7, CRC Press; ISBN 135114166X- 9781351141666; 2018.
Menon U, Jacobs IJ. CA125 and other tumour markers in screening and monitoring of ovarian cancer. In Altchek A, Deligdisch L, editors. Diagnosis and Management of Ovarian Disorders. New York: Academic Press; 2002.
Menon U, Jacobs IJ. Screening for ovarian cancer. In Arulkumaran S, editor. Baillière’s Best Practice & Research Clinical Obstetrics & Gynaecology. London; 2002. pp. 469–82.
Menon U, Jacobs IJ. Serum tumour markers. In Smith JR, Priore GD, Curtin J, Monaghan J, editors. An Atlas of Gynaecological Oncology. London: Martin Dunitz; 2005. pp. 21–43.
Menon U, Jacobs IJ. Serum tumour markers. In Smith JR, Priore GD, Curtin J, Monaghan J, editors. An Atlas of Gynaecological Oncology. London: Martin Dunitz; 2001. pp. 19–34.
Menon U, Jacobs IJ. The current status of screening for ovarian cancer. In Jacobs IJ, Shepherd JH, Oram DH, Blackett AD, Luesley D, Berchuck A, Hudson CN, editors. Ovarian Cancer. London: Oxford University Press; 2002. pp. 171–8.
Menon U, Jacobs IJ. Tumour markers and screening. In Berek J, Hacker N, editors. Practical Gynecologic Oncology. 4th edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. pp. 43–66.
Reynolds K, Menon U, Jacobs IJ. Development and identification of tumour markers. In Hoskins WJ, Perez CA, Young RC, editors. Principles and Practice of Gynecologic Oncology. 4th edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2005, pp. 157–78.
Rosenthal A, Menon U, Jacobs IJ. Ovarian cancer screening. In Reznick R, editor. Contemporary Issues in Cancer Imaging: A Multidisciplinary Approach. Cambridge: Cambridge University Press; 2006.
Rufford B, Menon U, Jacobs IJ. Ovarian cancer screening. Cancer Imaging 2001;1(2):14–16.
Rufford B, Menon U, Jacobs IJ. Screening for familial ovarian cancer. In Morrison PK, Hodgson SV, Haites NE, editors. Familial Breast and Ovarian Cancer Genetics, Screening and Management. Cambridge: Cambridge University Press; 2002. pp. 220–33.
Sharma A, Menon U, Jacobs IJ. Symptoms in the diagnosis of ovarian cancer. In Hillard T, editor. The Yearbook of Obstetrics and Gynaecology. London: RCOG Press; 2007. pp. 159–70.
Sharma A, Menon U, Ledermann J. Advanced Ovarian Cancer (Published online 3/10/2006). BMJ Clinical Evidence handbook.
Presentation to All-Parliamentary Group
Prof Menon gave evidence to the panel of the All-Party Parliamentary Group (APPG) on Ovarian Cancer in 2010 and answered questions regarding ovarian cancer screening which included two UK studies – UKCTOCS and UKFOCSS.
Appendix 1
UKCTOCS committees and teams
Trial investigators
Prof U Menon (CI 2015–2021, Co-CI 2001–2015), Prof I Jacobs (CI 2015–2021), Prof M Parmar OBE, Dr S Skates, Prof S Campbell, Prof A McGuire, Prof Dame L Fallowfield.
Trial Steering Committee (2001–2021)
Prof D Luesley (Independent Chair; 2001–2015), Prof H Kitchener (Independent Chair; 2016–2021), Prof J Patnick CBE (independent member), Prof J Cuzick (independent member), Ms L Bayne (independent member; 2001–2014), Ms Annwen Jones OBE (independent member; 2017–2021), Prof U Menon, Prof I Jacobs, Prof M Parmar OBE, Prof Dame L Fallowfield.
Data Monitoring and Ethics Committee (2001–2014)
Prof P Boyle (Chair), Prof APM Heintz, Prof S Kjaer, Dr Edward L Trimble.
Trial Management Committee (2001–2021)
Prof U Menon (Chair 2015–2021), Prof I Jacobs (Chair 2001–2014), Prof S Campbell, Prof Dame L Fallowfield, Prof A McGuire, Prof M Parmar OBE, Dr S Skates, Mr R Woolas, Mr T Mould, Dr N Singh, Dr A Dawnay, Dr A Gentry-Maharaj, Dr J Kalsi, Dr A Ryan, Dr M Burnell.
Outcome Review Committee (2001–2021)
Dr N Singh (Chair), Dr E Benjamin (2001–2014), Ms K Reynolds (2001–2005), Prof M Widschwendter (2009–2014), Mr Rob Woolas, Prof Ranjit Manchandra (2017–2021), Mr Tim Mould (2001–2014), Ms Karin Williamson (2018–2021), Ms Aarti Sharma (2018–2021), Dr Rupali Arora (2019–2021), Dr Laura Casey (2020–2021).
Ultrasound Sub-Committee (2001–2014)
Prof U Menon (Chair), Prof N Amso, Dr C Brunell, Prof S Campbell, Mrs G Fletcher, Ms K Ford, Dr A Gentry-Maharaj, Dr J Kalsi, Ms R Rangar, Dr A Ryan, Mr M Seif, Dr G Turner.
Coordinating Centre Team
Prof U Menon (Lead), M Ahmad, T Akbar, N Alves, Dr S Apostolidou, M Bacon, Dr C Brunell, Dr M Burnell, G Carlino, J Chapman, D Crump, J Cunningham, L Danquah, S Davies, Dr A Dawnay, A Dyer, J Ford, Dr A Gentry-Maharaj (Trial Manager 2017–2021), A Gibson, T Goodall, S Grant, R Gunu, M Habib, L Hadcocks, Dr R Hallett, N Hinkey, Dr J Kalsi (Trial Manager 2009–2017), C Karpinskyj, J Kerkhoff, Z Khan, S Lewis, W Liston, S Mohamed, L Odunlami, M Pamboris, S Philpott, T Roberts, Dr A Ryan, Dr A Sharma, J Sheals, K Sibley, C Spicer, S Spicer, L Sterry, C Stubbs, K Tamm, J Taylor, Dr F Warburton, Y Wold.
Regional Trial Centre Teams
Gateshead (Northern Gynaecological Oncology Centre, Queen Elizabeth Hospital)
K Godfrey (Lead), A Lopes (Lead), J Callaghan, G Dorman, J Gibson, C Green, A Guest, A Harvey, P Kilbourn, A Kucukmetin, M Meirovitz, J Monaghan, R Rangar, N Rashid, A Richardson, B Sarker, M Sihkanyisiwe, A Tailor, G Thompson, G Wilson, B Wright, C Youlton, J Youlton.
Barts (Department of Gynaecological Oncology, St. Bartholomew’s Hospital, London)
D Oram (Lead), U Menon (Lead), J Ademi, C Amarasinghe, CM Baque-Juxton, S Bhola, J Bramble, J Chapman, J Charalambous, A Clough, L Cole, L Crosby, J Cunningham, E d’Tisi, E Ferrier, E Forde, P Goulding, B Heyer, J Jonsson, A Knowles, E Liu-Koo, AM Mackinson, V Medic, U Menon, A Relf, K Reynolds, B Rufford, E Ryan, S Sheik, C Stubbs, L Walters, D Warrington, J Webb.
Liverpool (Department of Gynaecology, Liverpool Women’s Hospital)
J Herod (Lead), C Atherton, T Aust, L Bailey, S Bassi, L Baty, M Brown, H Burgin, J Carter, J Chapman, B Cheetham, JP Conway, H Crocker, B Daniels, L Diment, A Drought, C Finnegan, K Ford, L Greenfield, S Hailward, J Hazelton, M Herod, S Inwood, S Jones, V Jones, L Korb, H Lee, L Limbert, K Lord, J Maloney, M Maraj, J McCarthy, L McGlynn, D Ndlela, J Newman, A Nicolson, K Pearson, S Pennington, D Petter, P Stewart, A Tannock, B Thomson, A Webster, J Webster, S West, H Wright, G Zabroski.
Nottingham (Department of Gynaecological Oncology, Nottingham City Hospital)
K Williamson (Lead), E Bailey, V Barker, J Barkes, C Bower-Smith, A Bowley, C Bown, S Chowdhary, C Church, V Clements, S Colbeck, F Dack, B Gibbs, M Gill, V Hessom, C Hewitt, R Hutchinson, C Hynes, J Kythreotis, L Lacy, M Mahal, K Manderson, E Mercer, C Norris, D Nunns, C Oakley, T Parkes, C Reynolds, R Rock, H Rushbrook, C Sampson, K Sihra, S Sinclair, Z Thomas, S Thompson, S Vimplis, H Ward, N Ward, K Warner, G Wilson.
Manchester (Academic Unit of Obstetrics and Gynaecology, St. Mary’s Hospital)
MW Seif (Lead), K Reynolds (Lead), G Atanga, S Atkinson, L Bailey, C Barber, P Bhakar, N Bhandari, A Blackman, K Bowden, S Briggs, J Brown, D Bushell, K Butler, S Charles, J Collins, M Condon, M Dale, M Doyle, J Dunscombe, R Elfin, R Elven, M Faheem-Siddiqui, MR Green, Z Griffiths, J Harris, N Harwood, J Hawnaur, H Haydock, S Heywood, P Hughes, L Ivers, S Kaye, C Kilkelly, J Lees, M Maheem, G Martin, S Mawn, S McDonald, M Moore, T Morgan, J Nelson, E Oughton, A Panteli, V Parker, J Peacock, C Philipps, J Prior, V Purnell, S Renshaw, L Roberts, S Robin, J Robinson, R Simpson, T Speakman, F Storton, S Subin, J Taylor, X Vanakara, A Webb, C Webb, C Wilde, M Williams, V Williams, A Wood, C Wood, H Wright.
Derby (Department of Gynaecological Oncology, Derby City Hospital)
H Jenkins (Lead), I Scott (Lead), A Bali, J Barke, C Benson, C Bower-Smith, H Bullock, J Caborn, S Crockett, A Ferguson, G Forbes, J Gomes, R Harrison, C Hollins, M Jones, A North, R Rock, M Scott, H Stanton, S Thompson, M Tudge, G Turner, J Weston, C Williams.
Royal Free (Department of Gynaecological Oncology, Royal Free Hospital, London)
T Mould (Lead), I Aitken, S Amin, I Beal, S Bhola, S Blackmoore, K Borroughs, H Brown, S Burke, D Colia, L Crosby, G Desai, C Efueye, H Evans, E Ferrier, K Fitzgerald, G Fletcher, C Fox, G Gaston, K Harvey, B Heyer, T Hitchen, K Isherwood, E Izard, E Koo, M Lagos, K Lakhani, AM Mackinson, L McKenzie, V Medic, S Mohamed, S More, K Muir, W Myburg, N Nayak, O Ojo, N Old, A Oldham, S Porcherot, E Rawstron, M Sharma, T Stevens, C Sundstrom, E Sweeney, J Terwin, D Townshend, F Turner, S Wellington, D White, J Wickes, L Young, E Zard.
Portsmouth (Department of Gynaecological Oncology, St. Mary’s Hospital)
R Woolas (Lead), S Aldcock, M Anderson, E Barclay, S Bell, R Bonner, E Bowes, D Brinkmann, J Burns, K Chorley, C Dhar, K Fairley,F Gardner, B Gibbs, Y Griffiths, R Harrison, L Hayward, C Ihezue, C Isaac, D James, F Jones, D Mason, E Merritt, R Morris, M Oakey, J Skinner, S Tilbury, J Turpitt, A Webb, C West, J Woolas.
Bristol (Department of Gynaecological Oncology, St. Michael’s Hospital)
J Murdoch (Lead), B Anderson, F Anderson, H Andrews, E Barrow, R Brown, J Chippett, A D’Angelo, K Gale, N Hammadieh, K Henson, A Hobbs, K Horton-Fawkes, K Hunstman, T James, N Jeal, S King, E Langdon, M Lord, J Marsden-Williams, K McMillan, V Mitchell, K Nicholls, D Park, R Phillips, C Pretsell, RC Sanders, B Schaefer, C Shahin, S Shahin, S Sizer, P Taylor, M Tovey, N Vickers, S Wilmot.
Belfast (Department of Gynaecological Oncology, Belfast City Hospital)
S Dobbs (Lead), M Alkalbani, M Carey, J Caruth, M Clarke, J Forbes, M Gallagher, B Gibbs, M Hendron, R McClelland, M McComskey, D Morgan, M Murray, A O’Donnell, C Poh, J Price, H Sheldon, M Yoong, A Zawislak.
Cardiff (Department of Obstetrics and Gynaecology, University of Wales College of Medicine)
N Amso (Lead), S Basu, D Bell, H Clarke, A Evans, J Evans, T Griffiths, P Henderson, R Howells, R Jones, G Jose, G Looker, C Morgan, G Rieck, A Rogers, A Sharma, A Sims, S Underwood, D Williams, G Williams.
North Wales (Department of Gynaecological Oncology, Llandudno Hospital, Llandudno)
S Leeson (Lead), A Baker, V Byrne, C Chapman, B Davies, S Edwards, J Galley, L Griffiths, S Hogg, JY Houghton-Wright, M Howarth, K Hughes, M Hughes, H Jones, D Longley, A Roberts, L Sharpe, S Thomas, BJ Turner, B Warmington, B Waterson.
Middlesbrough (Department of Gynaecological Oncology, James Cook University Hospital)
D Cruickshank (Lead), A Bullen, V Chadwick, K Chapman, J Francis, R Goldie, C Ikwan-McCabe, K Jan, D Khan, L Lewis, J Nevin, L Prentis, J Proll, R Shanbhag, G Tarry.
Appendix 2
Project documentation
-
Protocol Version 9.0
-
Invitation letter
-
Brochure
-
Patient Information Sheet
-
Recruitment Questionnaire
-
Consent for the Main Trial
-
Consent for the Psychosocial Study
-
Data Protection form
-
Follow Up Questionnaire 1
-
Follow Up Questionnaire 2
-
Follow Up Questionnaire 3
-
Outcomes Review Form – Cancer site
-
Outcomes Review Form – Cause of death
List of abbreviations
- BGCS
- British Gynaecological Cancer Society
- BRCA
- breast cancer gene
- BSO
- Business Services Organisation
- C
- control group
- CA125
- cancer antigen 125
- CAG
- Confidentiality Advisory Group
- CC
- co-ordinating centre
- cfDNA
- cell-free DNA (deoxyribonucleic acid)
- CI / Co-CI
- chief investigator, co-chief investigator
- CONSORT
- Consolidated Standards of Reporting Trials
- CRUK
- Cancer Research UK
- CT
- computerised tomography
- DETECT-A
- Detecting cancers Earlier Through Elective mutation-based blood Collection and Testing
- DMEC
- Data Monitoring and Ethics Committee
- DNA
- deoxyribonucleic acid
- EGA
- Elizabeth Garrett Anderson
- ELISA
- enzyme-linked immunosorbent assay
- ESGO
- European Society of Gynaecological Oncology
- FACT-O
- functional assessment of cancer therapy – ovarian
- FIGO
- International Federation of Gynaecology and Obstetrics
- GDPR
- General Data Protection Regulations
- GHQ-12
- General Health Questionnaire 12
- GP
- general practitioner
- HES
- Hospital Episode Statistics
- HR
- hazard ratio
- HRT
- hormone replacement therapy
- HSCIC
- Health and Social Care Information Centre
- HTA
- Health Technology Assessment
- ICD
- International Statistical Classification of Diseases and Related Health Problems
- ICER
- incremental cost-effectiveness ratio
- ICON8
- International Collaboration on Ovarian Neoplasms
- IGCS
- International Gynaecologic Cancer Society
- IQR
- interquartile range
- IRR
- incidence rate ratio
- KM
- Kaplan–Meier
- MCED
- multi-cancer early detection
- miRNA
- microRNA
- MMS
- multimodal screening
- MRC
- Medical Research Council
- MRC CTU
- Medical Research Council Clinical Trials Unit
- MREC
- Multicentre Research Ethics Committee
- MRI
- magnetic resonance imaging
- NCIN
- National Cancer Intelligence Network
- NCRAS
- National Disease Registration Service
- NHS
- National Health Service
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- NMR
- nuclear magnetic resonance
- NEQAS
- national quality assurance programme
- NRES
- National Research Ethics Service
- OC
- ovarian cancer
- OCP
- oral contraceptive pill
- PET-CT
- positron emission tomography computerised tomography
- PCT
- primary care trust
- PEDW
- Patient Episode Database for Wales
- PLCO
- Prostate Lung Colorectal Ovarian Cancer Screening Trial
- QALY
- quality-adjusted life year
- QoL
- quality of life
- RC
- regional centre
- RCOG
- Royal College of Obstetricians and Gynaecologists
- RCT
- randomised controlled trial
- ROC
- risk of ovarian cancer
- ROCA
- risk of ovarian cancer algorithm
- ROCC
- risk of ovarian cancer calculation
- RP
- Royston–Parmar
- SAQ
- Sexual Activity Questionnaire
- SCSOCS
- Shizuoka Cohort Study on Ovarian Cancer Screening
- SHORE-C
- Sussex Health Outcomes Research and Education in Cancer
- SQL
- structured query language
- STAI
- Speilberger Trait Anxiety Inventory
- SUSAR
- suspected unexpected serious adverse reactions
- SWATH
- sequential window acquisition of all theoretical fragment ion spectra
- TMS
- trial management system
- TSC
- Trial Steering committee
- TVS
- transvaginal ultrasound
- UCL
- University College London
- UKCTOCS
- United Kingdom Collaborative Trial of Ovarian Cancer Screening
- UKRI
- UK Research and Innovation
- UKLWC
- UKCTOCS Longitudinal Women’s Cohort
- USPSTF
- US Preventive Services Task Force
- USS
- ultrasound screening
- WHO
- World Health Organisation
