Notes
Article history
The contractual start date for this research was in March 2016. This article began editorial review in July 2022 and was accepted for publication in January 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The Health Technology Assessment editors and publisher have tried to ensure the accuracy of the authors’ article and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article. This article was published based on current knowledge at the time and date of publication. NIHR is committed to being inclusive and will continually monitor best practice and guidance in relation to terminology and language to ensure that we remain relevant to our stakeholders.
Permissions
Copyright statement
Copyright © 2023 Agus et al. This work was produced by Agus et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Agus et al.
Introduction
Acute hypoxaemic respiratory failure (AHRF) requiring mechanical ventilation is a major cause of morbidity and mortality and has significant resource implications in terms of intensive care unit (ICU) and hospital stay. 1–3 The average cost per ICU bed-day exceeds £18004 and delivery of critical care to patients with AHRF accounts for a significant proportion of ICU capacity with an average length of stay of approximately 15 days. 5 In addition, survivors often have long-term physical and cognitive impairment requiring support in the community after hospital discharge. The high incidence, mortality, long-term consequences and high economic costs mean that AHRF is an extremely important problem. 1–3
The clinical findings from the pRotective vEntilation with veno-venouS lung assisT in respiratory failure (REST) trial6 reported that extracorporeal carbon dioxide removal (ECCO2R) did not significantly reduce 90-day mortality or ventilator-free days compared to ventilation alone (standard care) and more serious adverse events occurred in the intervention group. However, the National Institute for Health and Care Excellence (NICE) recommends that the effects of an intervention on health-related quality of life (HRQoL) should also be quantified to allow the calculation of quality-adjusted life-years (QALYs) and the evaluation of cost-effectiveness. 7 Evidence on the cost-effectiveness of ECCO2R is lacking. 8 A recent preliminary model-based analysis9 reported ECCO2R may be cost-effective in the treatment of acute respiratory distress syndrome, but further data from clinical trials and observational studies are required to support this finding. The aim of this paper is to report on the findings of a cost-utility analysis to assess the cost-effectiveness of ECCO2R compared to ventilation alone in patients with AHRF.
Methods
A within-trial cost-utility analysis was embedded within the REST trial6 to determine whether ECCO2R and lower tidal volume mechanical ventilation are cost-effective at 12 months post randomisation compared to standard care with conventional lung protective mechanical ventilation alone in patients with acute hypoxaemic respiratory failure in the critical care setting. The incremental cost-effectiveness ratio (ICER) was the cost per QALY. Initially, the aim of the REST economic analysis was to assess cost-effectiveness at both 12 months and over the lifetime of the patients using a de novo decision model. However, owing to the limitations of available data resulting from the trial being stopped early, the economic analysis was changed from those described in the original protocol and a decision model was not undertaken. Current guidelines for conducting7,10,11 and reporting12 economic evaluations were followed. The analysis was performed from the perspective of the National Health Service (NHS) and personal social services (PSS). 7 Discounting was not required for the analysis as the time horizon for analysis did not exceed one year.
Measurement of health resource use and costs
Hospital resource use data were collected prospectively using the case report form during the participants’ primary admission. Length of stay for the primary critical care admission was calculated from the date of randomisation to the date of critical care discharge or date of death if this occurred within critical care. General hospital ward length of stay was calculated from the date of critical care discharge to the date of hospital discharge or date of death if this occurred on the ward. For ICUs that participate in the case mix programme (CMP), additional information was obtained from the Intensive Care National Audit and Research Centre (ICNARC) on any readmissions to critical care. For the four Scottish, non-CMP ICUs the intention had been to obtain this data from the Scottish Intensive Care Society Audit Group (SICSAG) via the electronic Data Research and Innovation Service (eDRIS) but unfortunately the application to obtain this data was delayed and data linkage could not be obtained prior to the early closure of the study. This meant data on readmission to critical care was unavailable for some participants.
To facilitate the costing of the critical care admissions the Healthcare Resource Group (HRG) codes corresponding to each critical care admission and any readmission during the primary hospital admission were provided by ICNARC for the CMP sites. The HRG codes represented the highest level of complexity, based on the total number of organs supported during the admission. Scotland has not fully adopted the HRG methodology, so for a consistent costing approach we applied the modal HRG code observed for the critical care admissions of participants at the CMP sites to the critical care admissions for the Scottish participants. Critical care costs were calculated by multiplying the unit cost associated with the HRG by the length of stay for that admission. The unit costs associated with the HRGs were obtained from the National Schedule of NHS Costs 2018/194 (see Appendix 1, Table 5). Ward stay costs were calculated by multiplying the number of ward days during the primary hospital admission by the unit cost associated with rehabilitation for respiratory disorders.
Participants’ use of health and social care services from hospital discharge to 12 months was captured via a postal questionnaire completed at 6 and 12 months post randomisation. Telephone completion was also used for non-responders. Participants were provided with a health service log booklet at hospital discharge and again at 6 months to encourage them to keep track of their health resource use and facilitate questionnaire completion. Mortality status was confirmed prior to participant contact by contacting general practitioners (GPs).
Individual-level resource use was combined with unit costs to estimate costs for each participant. Unit costs were obtained from publicly available sources; National Schedule of NHS Costs4 and Unit Cost of Health and Social Care from PSS Research Unit. 13 The cost of supplying oxygen at home was provided by the Northern Ireland Health and Social Care Board and costs for intervention consumables were obtained directly from Alung, the device provider. The last follow-up data was collected in 2019 and the price year was set at 2018/2019 (see Appendix 1, Table 5).
Measurement of health outcomes
The outcome of interest in the cost-effectiveness analysis was the QALY, a generic HRQoL measure. Utilities for the calculation of the QALYs were obtained using the EuroQol-5 Dimensions, five-level version (EQ-5D-5L)14 administered at 6 and 12 months post randomisation via a postal questionnaire. Telephone completion was also used for non-responders. The EQ-5D-5L is a generic preference-based measure of HRQoL, which provides a description of health using five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) each with five levels of severity. Responses were converted into utility scores using the Crosswalk Value Set15 for the UK population. This tariff maps the EQ-5D-5L responses on to the EuroQol-5 Dimensions, three-level version (EQ-5D-3L) and is currently the approach recommended by NICE. 16 QALYs were calculated using the area under the curve method. As patients were critically ill at baseline, an EQ-5D-5L utility score of zero was assumed, in keeping with other studies in the critical care setting. 5,17,18
Analysis of health resource use, costs and outcomes
The descriptive statistics were used to summarise (by treatment arm) the resource use (during primary hospital admission and after discharge until 12 months), the associated costs, EQ-5D-5L scores and QALYs. Death was not treated as a censoring event and periods after death were counted as observations with known outcome19 an approach used previously in similar patient populations5,18 This meant that for participants who had died in hospital, resource use and EQ-5D-5L scores after hospital discharge until 12-month follow-up were considered to be zero. For patients who died between hospital discharge and 6 months resource use and EQ-5D-5L scores from 6 to 12 months were considered to be zero. Total costs and QALYs were analysed using linear regression. Significance (p < 0.05) was judged where the confidence intervals (CIs) excluded zero. Analyses were undertaken using Stata 15/IC for Windows® (StataCorp LP, College Station, TX, USA).
Cost-effectiveness analysis
Trial-based economic evaluations tend to measure participants’ health resource use and health outcomes at multiple time over the duration of the study using self-complete questionnaires. As a result, missing data is a common problem due to reasons such as non-returns or loss to follow up. This has the potential to introduce bias into the results as participants with incomplete health economic data may be systematically different from those with complete data. 20 Therefore for the cost-effectiveness analysis we imputed missing total cost and QALY data using multiple imputation with chained equations and predictive mean matching using the ‘mi impute chained command’ in Stata. This assumes that data are missing at random (MAR). This involved a regression model being specified to predict the missing total cost and QALY data: treatment group, baseline acute physiology and chronic health evaluation II (APACHE II) score, age, mortality at 28 days and primary hospital admission costs were entered into the model as predictors. Forty imputed data sets were generated, which was similar to the maximum percentage of incomplete cases observed (40%) in the data as recommended by White et al. 21 The ‘mi estimate’ Stata command was used to facilitate the analysis of each of the imputed data sets and then combine the results. Linear regression was used to estimate the incremental (differential) mean costs and QALYs. The ICER was calculated by dividing the incremental mean costs by the incremental mean QALYs to estimate the cost per QALY. As negative ICERs are not meaningful, if this occurred we stated whether the intervention was dominant (i.e. more effective and less costly than standard care) or was dominated (less effective and more costly than standard care).
Uncertainty in the cost and QALY data was explored by the non-parametric bootstrapping of the linear regression cost and QALY models simultaneously, drawing 1000 samples of the same size as the original sample with replacement. 22 The resulting 1000 ICER replicates were plotted on the cost-effectiveness plane,23 and used to construct a cost-effectiveness acceptability curve (CEAC). 24 This showed the probability of ECCO2R being cost-effective compared to ventilation alone at various willingness to pay (WTP) per QALY thresholds. In general NICE considers interventions with an ICER of ˂£20,000 to be cost-effective. 7
The incremental net monetary benefit (INMB) was also used to aid interpretation. The INMB is a summary statistic representing the value of an intervention in monetary terms when a WTP threshold for a unit of benefit is known. This was calculated by multiplying the incremental mean QALY by NICE’s threshold of £20,000 and then subtracting the incremental mean costs. A positive INMB indicates the intervention is cost-effective. 10
Sensitivity analysis for the cost-effectiveness analysis
The robustness of the results from the cost-effectiveness analysis was explored via the following sensitivity analyses: adjusting for baseline age and APACHE II score via multiple regression; reducing the time horizon of the cost-effectiveness analysis to 6 months, and scenarios of plausible departures from the MAR assumption. The latter was done via pattern-mixture models implemented using multiple imputation. 25 The impact of the following scenarios was explored: participants with missing QALY data were assumed to have worse HRQoL (10%) than those with observed QALY data; participants with missing cost data were assumed to have higher costs (10%) than those with complete cost data; and those with missing QALY and cost data were assumed to have both lower QALYs and higher costs.
Results
In total 412 participants were randomised; 202 to receive ECCO2R and 210 to receive ventilation alone. Levels of missing health economic data by type and treatment group are in Table 1. These were similar between groups for all data types.
| Data type | ECCO2R (n = 202) | Ventilation alone (n = 210) | ||
|---|---|---|---|---|
| Complete (%) | Incomplete (%) | Complete (%) | Incomplete (%) | |
| Health resource | ||||
| Primary hospital admission (randomisation to hospital discharge) | 193 (95.5) | 9 (4.5) | 202 (96.2) | 8 (3.8) |
| Discharge to 6 months | 149 (73.8) | 53 (26.2) | 150 (71.4)) | 60 (28.6) |
| 6–12 months | 154 (76.2) | 48 (23.8) | 154 (73.3) | 56 (26.7) |
| Randomisation to 12 months | 125 (61.9) | 77 (38.1) | 126 (60.0) | 84 (40.0) |
| EQ-5D-5L | ||||
| 6 months | 162 (80.2) | 40 (19.8) | 161 (76.7) | 49 (23.3) |
| 12 months | 150 (74.3) | 52 (25.7) | 155 (73.8) | 55 (26.2) |
| QALYs at 12 months | 140 (69.3) | 62 (30.7) | 143 (68.1) | 67 (30.9) |
Health resource use and costs
Resource use during the primary admission is presented in Appendix 1, Table 6 and self-reported health service use from hospital discharge to 12 months is presented in Appendix 1, Tables 7 and 8. Data is presented for all patients with available data without imputation of missing cases, by treatment arm. The costs (Great British pounds) associated with resource use are presented in Table 2. There was a trend for patients receiving ECCO2R to require marginally longer primary ICU stay, more ICU readmission days and more ward days than those receiving ventilation alone; however, none of these differences were statistically significantly different and the overall difference in primary admission costs (excluding intervention costs) was also not significantly different (mean difference £2666.97; 95% CI –2886.42 to 8220.35). The cost difference for the period from discharge to 6 months was relatively small (mean difference £172.70; 95% CI –871.81 to 1217.21), but a larger and statistically significant difference for the 6- to 12-month period (mean difference £858.52; 95% CI 125.83 to 1591.22) was observed. The resource use and costs associated with ECCO2R were also considered. These consisted of a cartridge for the Alung device (£3000 per patient) and a catheter (£650 per patient) and this total cost of £3650 was added to the total for each patient in the ECCO2R arm. Total health service use costs (including intervention costs) over the full 12 months were calculated for those patients with complete cost data (125 ECCO2R patients and 126 ventilation alone patients). The difference was large and statistically significantly different (£7668.76, 95% CI 159.75 to 15,177.77).
| Health resource costs | ECCO2R (n = 202) | Ventilation alone (n = 210) | Mean difference (95% CI) ECCO2R – ventilation alone | ||
|---|---|---|---|---|---|
| Obs | Mean (95% CI) | Obs | Mean (95% CI) | ||
| Primary ICU stay | 202 | 30,846.73 (26,200.92 to 354,92.55) | 210 | 28,404.31 (25,354.45 to 31,454.17) | 2442.42 (–3057.37 to 7942.21) |
| Other ICU readmission days | 202 | 2584.77 (1073.83 to 4095.71) | 210 | 822.21 (184.03 to 1460.40) | 1762.56 (149.09 to 3376.03) |
| Wards days | 193 | 4310.21 (3049.48 to 5570.94) | 202 | 3843.62 (2841.85 to 4845.40) | 466.58 (–1131.20 to 2064.37) |
| Total primary admission costs | 193 | 35,242.26 (30,989.03 to 39,495.50) | 202 | 32,575.30 (28,950.86 to 36,199.73) | 2666.97 (–2886.42 to 8220.35) |
| Intervention | 202 | 3650 (0) | 210 | 0 (0) | 3650 (0) |
| Health service use discharge to 6 months | 149 | 2070.65 (1376.05 to 2765.25) | 150 | 1897.95 (1112.72 to 2683.18) | 172.70 (–871.81 to 1217.21) |
| Health service use 6–12 months | 154 | 1531.92 (850.77 to 2213.07) | 154 | 673.40 (395.60 to 951.20) | 858.52 (125.83 to 1591.22) |
| Total health-care costs over 12 months | 125 | 40,292.58 (34,390.4 to 46,194.70) | 126 | 32,623.82 (27,911.58 to 37,336.06) | 7668.76 (159.75 to 15,177.77) |
Health utility scores and QALYs
Mean EQ-5D-5L utilities and QALYs over the study period are presented in Table 3 for patients with available data. Overall mean utility scores were low and similar for each group at both 6 and 12 months with no discernible difference in QALYs (mean difference –0.01; 95% CI –0.06 to 0.05).
| Time point | ECCO2R (n = 202) | Ventilation alone (n = 210) | Difference (95% CI) ECCO2R – ventilation alone |
||
|---|---|---|---|---|---|
| Obs | Mean (95% CI) | Obs | Mean (95% CI) | ||
| 6-month utility | 162 | 0.23 (0.18 to 0.28) | 161 | 0.23 (0.18 to 0.29) | –0.00 (–0.08 to 0.07) |
| 12-month utility | 150 | 0.24 (0.18 to 0.29) | 155 | 0.24 (0.19 to 0.30) | –0.01 (–0.09 to 0.08) |
| QALYs at 12 monthsa | 140 | 0.15 (0.11 to 0.19) | 143 | 0.16 (0.12 to 0.20) | –0.01 (–0.06 to 0.05) |
Cost-effectiveness analysis
The results of the cost-effectiveness analysis for the base-case analysis and the sensitivity analyses are presented in Table 4. For all scenarios ECCO2R was associated with lower QALYs compared to ventilation alone, but the differences were small and not statistically significant indicating broad equivalence in terms of HRQoL impact. However, statistically significantly higher health-care costs were observed with ECCO2R compared to ventilation alone and so ECCO2R can be described as being dominated by ventilation alone and not cost-effective given the data. Uncertainty in the cost and QALYs estimates was explored by displaying the results of the non-parametric bootstrapping on the cost-effectiveness plane for the base-case analysis (see Figure 1). It can be seen that ICER replicates straddle the north-west and north-east quadrants reflecting the consistently higher costs associated with ECCO2R in the data, and similarity in QALYs. The CEAC (see Figure 2) is in fact a line running along the x-axis indicating that there was 0% probability of ECCO2R being cost-effective compared to ventilation alone for any of the WTP threshold per QALY we considered (£0–50,000) in the analysis. The negative INMBs reflect that the intervention is not cost-effective compared to standard care at a WTP threshold of £20,000.
| Analyses | Total costs (£) (mean, 95% CI) | QALY (mean, 95% CI) | Mean incremental costs (95% CI) | Mean incremental QALYs (95% CI) | ICERa | INMBb (£) (mean, 95% CI) | ||
|---|---|---|---|---|---|---|---|---|
| ECCO2R (n = 202) | Ventilation alone (n = 210) | ECCO2R (n = 202) | Ventilation alone (n = 210) | |||||
| Base-case analysis | 42,755.11 (38,541.62 to 46,968.59) | 34,855.02 (30,751.14 to 38,958.91) | 0.211 (0.169 to 0.253) | 0.220 (0.174 to 0.265) | 7900.08 (2008.48 to 13,791.68) | –0.008 (–0.052 to 0.035) | Dominated | –8069.25 (–13,741 to –2396.73) |
| Sensitivity analyses | ||||||||
| Adjusting for baseline age and APACHE II score | 42,579.54 (38,352.34 to 46,806.73) | 35,022.77 (30,905.08 to 39,140.46) | 0.210 (0.168 to 0.252) | 0.221 (0.176 to 0.266) | 7556.77 (1695.29 to 13,418.25) | –0.011 (–0.053 to 0.032) | Dominated | –7770.74 (–13,418.74 to –2122.73) |
| 6 months time horizon | 40,972.04 (36,846.58 to 45,097.51) | 34,585.23 (30,543.72 to 38,626.74) | 0.069 (0.056 to 0.082) | 0.072 (0.058 to 0.086) | 6386.81 (538.76 to 12,234.87) | –0.003 (–0.018 to 0.012) | Dominated | –6445.44 (–12,229.49 to –661.40) |
| Missing not at random (MNAR), –10% QALYs | 42755.11 (38,541.62, 46968.59) | 34,855.02 (30,751.14 to 38,958.91) | 0.201 (0.161 to 0.241) | 0.209 (0.166 to 0.251) | 7900.08 (2008.48 to 13,791.68) | –0.008 (–0.050 to 0.035) | Dominated | –8054.00 (–13,731.66 to –2376.35) |
| +10% Costs | 44,420.99 (40,021.53 to 48,820.46) | 36,305.57 (32,022.24 to 40,588.91) | 0.211 (0.169 to 0.253) | 0.220 (0.174 to 0.265) | 8115.42 (1980.32 to 14,250.52) | –0.008 (–0.052 to 0.035) | Dominated | –8284.59 (–14,199.13 to –2370.05) |
| –10% QALYs and +10% Costs | 44,420.99 (40,021.53 to 48,820.46) | 36,305.57 (32,022.24 to 40,588.91) | 0.201 (0.161 to 0.241) | 0.209 (0.166 to 0.251) | 8115.42 (1980.32 to 14,250.52) | –0.008 (–0.050 to 0.035) | Dominated | –8269.34 (–14,192.10 to –2346.59) |
FIGURE 1.
Cost-effectiveness plane for the base-case cost-utility analysis showing 1000 bootstrapped replications of incremental mean costs and QALYs and the WTP threshold of £20,000/QALY.
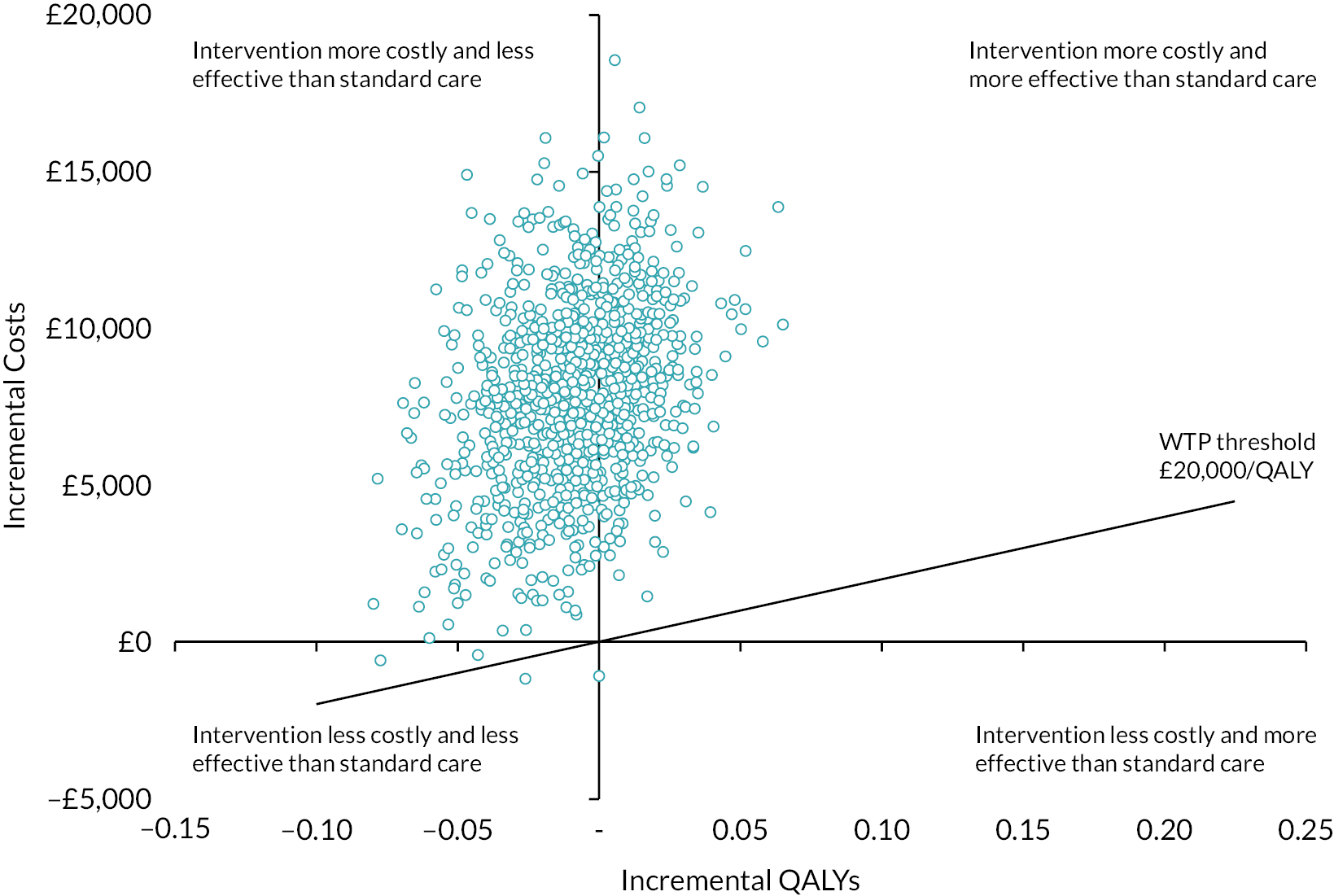
FIGURE 2.
Cost-effectiveness acceptability curve showing the probability of intervention being cost-effective compared to standard care (base-case analysis).

Discussion
The results showed that ECCO2R was associated with statistically significantly higher health-care costs compared to ventilation alone, with the intervention itself (£3650 per patient) contributing to approximately half of the incremental costs. The additional costs were not offset by any benefits since no difference in QALYs occurred between groups, indicating that the intervention had no impact on the HRQoL participants. The CEAC showed that there was 0% probability of ECCO2R being cost-effective compared to ventilation alone for any of the WTP thresholds per QALY considered (£0–50,000), given the data, and this finding was robust to sensitivity analyses.
Strengths of the analysis included the measures we took to handle missing data. As anticipated, there was varying degrees of missingness in the economic data collected from baseline to 12 months. We assigned zero utility scores and zero costs to participants who had died and employed multiple imputation for the cost-effectiveness analysis, therefore maximising the use of the available data. There was ˂5% missing data observed in the resource use collected during the participants’ primary admission, thus our cost estimates are a meaningful addition to the cost of illness literature in the critical care population.
There were a number of limitations to the analysis. Since participants were critically ill and ventilated at baseline we did not measure their HRQoL with the EQ-5D-5L. Instead we assigned all participants’ baseline utility score zero in keeping with previous studies in the critical care setting. Since HRQoL was only measured at 6 and 12 months post randomisation, any short-term impact of the intervention on health participants’ health may have gone undetected. Future studies should consider measuring HRQoL as soon as possible after the participants have regained capacity. We were unable to obtain data from SICSAG via eDRIS on Scottish participants. This meant we did not have information on any readmission to ICU they may have had during their primary admission. This would probably only have led to minor data loss since it only applied to 35 participants and only 17 participants from the remaining sample were readmitted to ICU. We did not include the additional staff time associated with the application of ECCO2R, therefore the cost of ECCO2R is likely to be underestimated. Participants who did not return their follow-up questionnaires by post were given the opportunity to complete via telephone which may have introduced response bias. Finally, the economic evaluation had originally intended to estimate long-term cost-effectiveness of ECCO2R via a de novo decision model. Unfortunately the study closed early, limiting the data available to inform the model. Despite these limitations, the findings from this economic evaluation make an important contribution to the existing evidence base on ECCO2R therapy.
Conclusion
Extracorporeal carbon dioxide removal was associated with significantly higher costs, but no benefit in HRQoL. Given the data, ECCO2R is not considered to be a cost-effective approach to treating patients with acute hypoxaemic respiratory failure.
Acknowledgements
We thank all the patients who participated in the trial; all of the medical and nursing staff in participating centres who cared for patients and collected data; Maria O’Kane, Kelly Green, Mark Bonar, Jeanette Mills and Loren McGinley-Keag for endeavouring to maximise patient follow-up and the whole REST study team at the Northern Ireland Clinical Trials Unit (NICTU), Belfast Health and Social Care Trust (BHSCT) for conducting the trial. We are grateful for the support of the Intensive Care National Audit and Research Centre (ICNARC) for providing level of care data and health resource group information for sites that participate in ICNARC’s Case Mix Programme. The authors acknowledge the support of the Northern Ireland Clinical Research Network and the National Institute for Health and Care Research Clinical Research Network. A full list of the REST investigators is provided in Appendix 1, Table 9.
Contributions of authors
Ashley Agus (https://orcid.org/0000-0001-9839-6282) (Senior Health Economist) designed and performed the economic evaluation, interpreted the analysis and drafted the manuscript.
James J. McNamee (https://orcid.org/0000-0002-2564-8511) (Consultant in Intensive Care Medicine) conceived the main study, interpreted the analysis and helped to revise the manuscript.
Colette Jackson (https://orcid.org/0000-0001-7814-0749) (Trial Manager) coordinated the acquisition of the data and helped to revise the manuscript.
Danny F. McAuley (https://orcid.org/0000-0002-3283-1947) (Professor and Consultant in Intensive Care Medicine) conceived the main study, interpreted the analysis and helped to revise the manuscript. All authors approved the final version of the manuscript. All authors vouch for the integrity, accuracy and completeness of the data.
Ethics statement
South Berkshire REC 16/SC/0089 (England, Wales and Northern Ireland); Scotland A REC 16/SS/0048 (Scotland).
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data or trial materials may be granted following review.
Funding
This article presents independent research funded by the National Institute for Health and Care Research (NIHR) Health Technology Assessment programme as award number 13/143/02. The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
This article
The contractual start date for this research was in March 2016. This article began editorial review in July 2022 and was accepted for publication in January 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The Health Technology Assessment editors and publisher have tried to ensure the accuracy of the authors’ article and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article. This article was published based on current knowledge at the time and date of publication. NIHR is committed to being inclusive and will continually monitor best practice and guidance in relation to terminology and language to ensure that we remain relevant to our stakeholders.
Disclaimer
The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute for Health and Care Research, or the Department of Health. BHSCT is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679. Under the Data Protection legislation, BHSCT is the Data Controller, and you can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer here (https://belfasttrust.hscni.net/about/access-to-information/data-protection/).
This article reports on one component of the research award Extracorporeal carbon dioxide removal compared to ventilation alone in patients with acute hypoxaemic respiratory failure: cost-utility analysis of the REST RCT. For more information about this research please view the award page [https://www.fundingawards.nihr.ac.uk/award/13/143/02]
References
Appendix 1
| Resource item/HRG code | Unit cost (£) | Details | Source |
|---|---|---|---|
| XC01Z | 2281 | Adult Critical Care, 6 or more Organs Supported | National Schedule of NHS Costs 2018/194 |
| XC02Z | 2097 | Adult Critical Care, 5 Organs Supported | National Schedule of NHS Costs 2018/194 |
| XC03Z | 1967 | Adult Critical Care, 4 Organs Supported | National Schedule of NHS Costs 2018/194 |
| XC04Z | 1764 | Adult Critical Care, 3 Organs Supported | National Schedule of NHS Costs 2018/194 |
| XC05Z | 1575 | Adult Critical Care, 2 Organs Supported | National Schedule of NHS Costs 2018/194 |
| XC06Z | 1152 | Adult Critical Care, 1 Organ Supported | National Schedule of NHS Costs 2018/194 |
| XC07Z | 933 | Adult Critical Care, 0 Organs Supported | National Schedule of NHS Costs 2018/194 |
| Post-ICU ward day | 351 | VC40Z Rehabilitation for Respiratory Disorders | National Schedule of NHS Costs 2018/194 |
| GP surgery consultation | 39 | Based on 9.22 minute consultation | National Schedule of NHS Costs 2018/194 |
| GP phone consultation | 15.52 | Based on a 4 minute call | Unit Costs of Health and Social Care7 |
| GP home consultation | 99.45 | 11.4 minute consultation and 12 minutes travel time | Unit Costs of Health and Social Care7 |
| GP out-of-hours consultation | 99.45 | Assumed the same cost as home consultation | Unit Costs of Health and Social Care7 |
| GP nurse surgery consultation | 10.85 | Based on 15.5 minute appointment | Unit Costs of Health and Social Care7 |
| GP nurse phone consultation | 7.80 | Based on 6.56 minute call | Unit Costs of Health and Social Care7 |
| District nurse visit | 46 | Band 6 | Unit Costs of Health and Social Care7 |
| Specialist nurse visit | 55 | Band 7 | Unit Costs of Health and Social Care7 |
| Social worker visit | 51 | Unit Costs of Health and Social Care7 | |
| Physiotherapist visit | 45 | Band 6 | Unit Costs of Health and Social Care7 |
| Occupational therapist visit | 48 | Unit Costs of Health and Social Care7 | |
| Dietitian visit | 46 | Band 6 | Unit Costs of Health and Social Care7 |
| Counsellor visit | 45 | Band 6 | Unit Costs of Health and Social Care7 |
| Home help/carer visit | 23 | Unit Costs of Health and Social Care7 | |
| Emergency department attendance | |||
| Attendance, not admitted | 171 | Weighted average non-admitted (excluding dead on arrival) | National Schedule of NHS Costs 2018/194 |
| Attendance, admitted | 247 | Weighted average admitted (excluding dead on arrival) | National Schedule of NHS Costs 2018/194 |
| Ambulance | 257 | National Schedule of NHS Costs 2018/194 | |
| Outpatient visit | 135 | Weighted average of all outpatient attendances | National Schedule of NHS Costs 2018/194 |
| Hospital bed day | 413 | Weighed mean of non-elective admissions, divided by weighted average length of stay of non-elective admissions. | National Schedule of NHS Costs 2018/194 (Length of stay obtained through freedom of information request to NHS; FOI – 2104-1442254 NHSE:0426102) |
| Oxygen | 1239 | Per annum, includes installation, high flow concentrator, ambulatory cylinders and electricity. | Northern Ireland Health and Social Care Board personal communication. |
| ECCO2R intervention | |||
| Cartridge | 3000 | Per patient | Alung communication |
| Catheter | 650 | Per patient | Alung communication |
| ECCO2R (n = 202) | Ventilation alone (n = 210) | Mean difference (95% CI) | |||
|---|---|---|---|---|---|
| Obs | Mean (95% CI) | Obs | Mean (95% CI) | ||
| Primary ICU stay days | 202 | 18.21 (15.72 to 20.69) | 210 | 16.31 (14.64 to 17.97) | 1.90 (–1.06 to 4.86) |
| Other ICU readmission days | 202 | 0.35 (0.11 to 0.60) | 210 | 0.16 (0.00 to 0.32) | 0.19 (–0.10 to 0.48) |
| Ward days | 193 | 12.28 (8.69 to 15.57) | 202 | 10.95 (8.10 to 13.80) | 1.33 (–3.22 to 5.88) |
| Hospital length of stay | 193 | 29.59 (25.05 to 34.14) | 202 | 27.16 (23.31 to 31.00) | 2.43 (–3.48 to 8.35) |
| Service | Discharge – 6 months | ||||||
|---|---|---|---|---|---|---|---|
| ECCO2R (n = 202) | Ventilation alone (n = 210) | Mean difference (95% CI) | |||||
| Obs | N (%) | Mean (95% CI) | Obs | N (%) | Mean (95% CI) | ||
| GP contact | |||||||
| Face-to-face | 149 | 64 (43.0) | 1.44 (1.07 to 1.80) | 150 | 63 (42.0) | 1.66 (0.98 to 2.33) | –0.22 (–0.99 to 0.55) |
| Telephone | 149 | 39 (26.2) | 0.67 (0.44 to 0.91) | 150 | 37 (24.7) | 0.73 (0.46 to 1.01) | –0.06 (–0.42 to 0.30) |
| Home | 149 | 15 (10.1) | 0.18 (0.07 to 0.30) | 150 | 21 (14.0) | 0.45 (0.22 to 0.67) | –0.27 (–0.52 to –0.01) |
| Out of hours | 149 | 6 (4.0) | 0.05 (0.00 to 0.10) | 150 | 9 (6.0) | 0.09 (0.02 to 0.17) | –0.04 (–0.13 to 0.05) |
| GP nurse contact | |||||||
| Face to face | 149 | 49 (32.9) | 1.00 (0.49 to 1.51) | 150 | 33 (22.0) | 1.13 (0.40 to 1.85) | –0.13 (–1.01 to 0.75) |
| Telephone | 149 | 9 (6.0) | 0.20 (–0.02 to 0.43) | 150 | 11 (7.3) | 0.19 (0.06 to 0.31) | 0.01 (–0.24 to 0.27) |
| District nurse | 149 | 25 (16.8) | 1.68 (0.77 to 2.60) | 150 | 31 (20.7) | 3.17 (0.98 to 5.36) | –1.49 (–3.86 to 0.89) |
| Specialist nurse | 149 | 18 (12.1) | 0.60 (0.08 to 1.12) | 150 | 21 (14.0) | 0.48 (0.21 to 0.75) | 0.12 (–0.46 to 0.70) |
| Social worker | 149 | 9 (6.1) | 0.25 (–0.03 to 0.53) | 150 | 6 (4.0) | 0.25 (–0.07 to 0.57) | 0.00 (–0.42 to 0.42) |
| NHS physiotherapist | 149 | 30 (20.1) | 1.21 (0.52 to 1.91) | 150 | 30 (20.0) | 0.85 (0.47 to 1.22) | 0.37 (–0.41 to 1.15) |
| Occupational therapist | 149 | 23 (15.4) | 0.52 (0.21 to 0.84) | 150 | 25 (16.7) | 1.28 (–0.53 to 3.10) | –0.76 (–2.60 to 1.08) |
| Dietitian | 149 | 15 (10.1) | 0.18 (0.08 to 0.28) | 150 | 14 (9.3) | 0.28 (0.02 to 0.54) | –0.10 (–0.38 to 0.18) |
| Counselling/therapy | 149 | 11 (7.4) | 0.36 (0.03 to 0.68) | 150 | 10 (6.7) | 1.22 (–0.60 to 3.05) | –0.87 (–2.72 to 0.98) |
| Speech and language therapy | 149 | 3 (2.0) | 0.03 (–0.00 to 0.07) | 150 | 0 (0) | 0 (0) | 0.03 (–0.01 to 0.07) |
| NHS carer | 149 | 11 (7.4) | 4.39 (0.34 to 8.44) | 150 | 7 (4.7) | 7.04 (0.90 to 13.18) | –2.65 (–9.98 to 4.68) |
| Emergency department attendance | |||||||
| Attendance, not admitted | 149 | 11 (7.4) | 0.13 (0.06 to 0.21) | 150 | 13 (8.7) | 0.13 (0.06 to 0.20) | 0.01 (–0.09 to 0.11) |
| Attendance, admitted | 149 | 16 (10.7) | 0.15 (0.08 to 0.22) | 150 | 11 (7.3) | 0.13 (0.05 to 0.22) | 0.01 (–0.10 to 0.13) |
| Ambulance | 149 | 27 (18.1) | 0.28 (0.17 to 0.39) | 150 | 24 (16.0) | 0.26 (0.15 to 0.37) | 0.02 (-0.14, 0.18) |
| Hospital outpatient appointment | 149 | 65 (43.6) | 2.31 (1.53 to 3.10) | 150 | 60 (40.0) | 1.66 (1.11 to 2.21) | 0.65 (–0.30 to 1.61) |
| Hospital admission | 149 | 26 (17.5) | 0.60 (0.50 to 0.71) | 150 | 17 (11.3) | 0.57 (0.47 to 0.66) | 0.04 (–0.11 to 0.18) |
| Hospital days | 149 | – | 2.87 (1.45 to 4.30) | 150 | 2.14 (0.47 to 0.66) | 0.73 (–1.26 to 2.71) | |
| Oxygen therapy | 149 | 2 (1.3) | – | 150 | 2 (1.3) | – | |
| Service | 6–12 months | ||||||
|---|---|---|---|---|---|---|---|
| ECCO2R (n = 202) | Ventilation alone (n = 210) | Mean difference (95% CI) | |||||
| Obs | N (%) | Mean (95% CI) | Obs | N (%) | Mean (95% CI) | ||
| GP contact | |||||||
| Face-to-face | 154 | 48 (31.2) | 1.00 (0.65 to 1.35) | 154 | 52 (33.8) | 1.27 (0.88 to 1.66) | –0.27 (–0.79 to 0.25) |
| Telephone | 154 | 29 (18.8) | 0.73 (0.39 to 1.08) | 154 | 34 (22.1) | 0.72 (0.34 to 1.10) | 0.01 (–0.50 to 0.53) |
| Home | 154 | 8 (5.2) | 0.14 (0.01 to 0.26) | 154 | 10 (6.5) | 0.26 (–0.01 to 0.53) | –0.12 (–0.42 to 0.17) |
| Out of hours | 154 | 6 (3.9) | 0.06 (0.01 to 0.11) | 154 | 4 (2.6) | 0.03 (–0.00 to 0.07) | 0.03 (–0.04 to 0.09) |
| GP nurse contact | |||||||
| Face to face | 154 | 42 (27.3) | 0.79 (0.41 to 1.16) | 154 | 36 (23.4) | 1.10 (0.50 to 1.70) | –0.31 (–1.01 to 0.40) |
| Telephone | 154 | 8 (5.2) | 0.10 (0.02 to 0.19) | 154 | 12 (7.8) | 0.17 (0.06 to 0.28) | –0.06 (–0.21 to 0.08) |
| District nurse | 154 | 8 (5.2) | 0.26 (–0.03 to 0.55) | 154 | 6 (3.9) | 0.19 (–0.00 to 0.38) | 0.07 (–0.27 to 0.42) |
| Specialist nurse | 154 | 17 (11.0) | 0.47 (0.18 to 0.77) | 154 | 15 (9.7) | 0.22 (0.09 to 0.35) | 0.25 (–0.07 to 0.57) |
| Social worker | 154 | 5 (3.3) | 0.09 (–0.00 to 0.19) | 154 | 2 (1.3) | 0.02 (–0.01 to 0.05) | 0.07 (–0.03 to 0.17) |
| NHS Physiotherapist | 154 | 20 (13.0) | 0.82 (0.34 to 1.30) | 154 | 14 (9.01) | 0.52 (0.12 to 0.92) | 0.30 (–0.32 to 0.92) |
| Occupational therapist | 154 | 12 (7.8) | 0.22 (0.09 to 0.36) | 154 | 10 (6.5) | 0.16 (0.04 to 0.29) | 0.06 (–0.13 to 0.24) |
| Dietitian | 154 | 14 (9.1) | 0.19 (0.08 to 0.31) | 154 | 8 (5.2) | 0.15 (0.01 to 0.29) | 0.05 (–0.14 to 0.23) |
| Counselling/therapy | 154 | 10 (6.5) | 0.33 (0.10 to 0.56) | 154 | 6 (3.9) | 0.18 (–0.01 to 0.36) | 0.16 (–0.14 to 0.45) |
| Speech and language therapy | 154 | 0 (0) | 0 (0) | 154 | 0 (0) | 0 (0) | 0 (0) |
| NHS carer | 154 | 3 (2.0) | 2.97 (–2.08 to 8.01) | 154 | 2 (1.3) | 1.61 (–1.56 to 4.77) | 1.36 (–4.56 to 7.27) |
| Emergency department attendance | |||||||
| Attendance, not admitted | 154 | 10 (6.5) | 0.09 (0.03 to 0.15) | 154 | 7 (4.6) | 0.05 (0.01 to 0.09) | 0.04 (–0.03 to 0.10) |
| Attendance, admitted | 154 | 15 (9.8) | 0.16 (0.07 to 0.25) | 154 | 9 (5.8) | 0.10 (0.03 to 0.16) | 0.06 (–0.05 to 0.17) |
| Ambulance | 154 | 25 (16.2) | 0.25 (0.15 to 0.36) | 154 | 16 (10.4) | 0.15 (0.07 to 0.23) | 0.10 (–0.03 to 0.24) |
| Hospital outpatient appointment | 154 | 54 (35.0) | 2.44 (0.95 to 3.93) | 154 | 46 (29.9) | 1.37 (0.86 to 1.88) | 1.07 (–0.50 to 2.64) |
| Hospital admission | 154 | 20 (13.0) | 0.53 (0.41 to 0.64) | 154 | 11 (7.1) | 0.47 (0.39 to 0.56) | 0.05 (–0.09 to 0.20) |
| Hospital days | 154 | – | 1.96 (0.62 to 3.30) | 154 | – | 0.49 (0.06 to 0.92) | 1.47 (0.07 to 2.88) |
| Oxygen therapy | 154 | 3 (2.0) | – | 154 | 2 (1.3) | – | – |
| First name and middle initial(s) | Last name | Institution | Role or contribution, for example, chair, principal investigator |
|---|---|---|---|
| Temi | Adedoyin | Northwick Park Hospital | Research Co-ordinator |
| Kayode | Adeniji | Queen Alexandra Hospital | Principal Investigator |
| Caroline | Aherne | Royal Blackburn Hospital | Research Nurse |
| Gopal | Anand Iyer | Royal Liverpool Hospital | Coinvestigator |
| Prematie | Andreou | Leicester Royal Infirmary | Research Nurse |
| Gillian | Andrew | Edinburgh Royal Infirmary | Research Nurse |
| Ian | Angus | Royal Oldham Hospital | Research Nurse |
| Gill | Arbane | St Thomas’s Hospital | Research Service |
| Manager | |||
| Pauline | Austin | Ninewells Hospital | Coinvestigator |
| Karen | Austin | Worcester Hospital | Research Nurse |
| Georg | Auzinger | King’s College London | Principal Investigator |
| Jonathan | Ball | St George’s Hospital | Coinvestigator |
| Dorota | Banach | Charing Cross and Hammersmith | Research Nurse |
| Jonathan | Bannard-Smith | Manchester Royal Infirmary | Principal Investigator |
| Leona | Bannon | Royal Hospitals | Research Nurse |
| Lucy | Barclay | Edinburgh Royal Infirmary | Research Nurse |
| Helena | Barcraft-Barnes | Poole Hospital | Research Nurse |
| Richard | Beale | St Thomas’s Hospital | Coinvestigator |
| Sarah | Bean | Royal Cornwall Hospital | Research Nurse |
| Andrew | Bentley | Wythenshawe Hospital | Principal Investigator |
| Georgia | Bercades | University College Hospital | Research Nurse |
| Colin | Bergin | Birmingham Queen Elizabeth Hospital | Research Nurse |
| Sian | Bhardwaj | Worcester Hospital | Principal Investigator |
| Colin | Bigham | Derriford Hospital | Principal Investigator |
| Isobel | Birkinshaw | York Teaching Hospital | Research Nurse |
| Aneta | Bociek | St Thomas’s Hospital | Research Nurse |
| Andrew | Bodenham | Leeds General Hospital | Coinvestigator |
| Malcolm G | Booth | Glasgow Royal Infirmary | Coinvestigator |
| Christine | Bowyer | Wythenshawe Hospital | Research Nurse |
| David A | Brealey | University College Hospital | Coinvestigator |
| Stephen | Brett | Charing Cross and Hammersmith | Principal Investigator |
| Jennifer | Brooks | University of Wales Hospital | Research Nurse |
| Karen | Burt | Royal Cornwall Hospital | Research Nurse |
| Louise | Cabrelli | Ninewells Hospital | Research Nurse |
| Leilani | Cabreros | Charing Cross and Hammersmith | Research Nurse |
| Hazel | Cahill | York Teaching Hospital | Research Nurse |
| Aidan | Campbell | Altnagelvin Hospital | Coinvestigator |
| Luigi | Camporota | St Thomas’s Hospital | Principal Investigator |
| Sara | Campos | St Thomas’s Hospital | Research Nurse |
| Julie | Camsooksai | Poole Hospital | Senior Research Nurse |
| Ronald | Carrera | Birmingham Queen Elizabeth Hospital | Research Nurse |
| Joseph | Carter | York Teaching Hospital | Principal Investigator |
| Jaime | Carungcong | Chelsea and Westminster | Research Nurse |
| Anelise | Catelan-Zborowski | Royal Brompton Hospital | Research Nurse |
| Susanne | Cathcart | Glasgow Royal Infirmary | Research Nurse |
| Shreekant | Champanerkar | Royal Gwent Hospital | Coinvestigator |
| Matthew | Charlton | Leicester Royal Infirmary | Coinvestigator |
| Shiney | Cherian | Royal Gwent Hospital | Research Nurse |
| Linsey | Christie | Chelsea and Westminster | Coinvestigator |
| Srikanth | Chukkambotla | Royal Blackburn Hospital | Principal Investigator |
| Amy | Clark | Birmingham Queen Elizabeth Hospital | Research Nurse |
| Sarah | Clark | Edinburgh Royal Infirmary | Research Nurse |
| Richard | Clark | Manchester Royal Infirmary | Research Nurse |
| Ian | Clement | Royal Victoria Infirmary | Principal Investigator |
| Eve | Cocks | University of Wales Hospital | Research Nurse |
| Stephen | Cole | Ninewells Hospital | Principal Investigator |
| Sonia | Cole | Sandwell General Hospital | Research Nurse |
| Jade | Cole | University of Wales Hospital | Research Nurse |
| Nick | Coleman | Royal Stoke Hospital | Coinvestigator |
| Emma | Connaughton | Manchester Royal Infirmary | Research Nurse |
| Andrew | Conway Morris | Addenbrookes Hospital | Coinvestigator |
| Lauren | Cooper | Birmingham Queen Elizabeth Hospital | Trial Co-ordinator |
| Ian | Cooper | Royal Oldham Hospital | Research Nurse |
| Carolyn | Corbett | Royal Oldham Hospital | Research Nurse |
| Sarah | Cornell | Sunderland Royal Hospital | Research Nurse |
| Carmen | Correia | Royal London Hospital | Research Nurse |
| Victoria | Cottam | New Cross Hospital | Research Nurse |
| Keith | Couper | Birmingham Heartlands Hospital | Research Nurse |
| Laura | Creighton | Royal Hospitals | Research Nurse |
| Maryam | Crews | Royal Liverpool Hospital | Coinvestigator |
| Neil | Crooks | Birmingham Heartlands Hospital | Coinvestigator |
| Jacqueline | Curtin | University of Wales Hospital | Research Nurse |
| Zoe | Daly | Queen Alexandra Hospital | Research Nurse |
| Alan | Davidson | Glasgow Queen Elizabeth | Coinvestigator |
| Rhys | Davies | University of Wales Hospital | Research Nurse |
| Michelle | Davies | University of Wales Hospital | Research Nurse |
| Christopher | Day | Royal Devon and Exeter Hospital | Principal Investigator |
| Mike | Dean | Northwick Park Hospital | Principal Investigator |
| Ged | Dempsey | Aintree Hospital | Principal Investigator |
| Anna | Dennis | Birmingham Heartlands Hospital | Coinvestigator |
| Susan | Dermody | Royal Oldham Hospital | Research Nurse |
| Liesl | Despy | Birmingham Queen Elizabeth Hospital | Research Nurse |
| Murugesh | Devaramani | Manchester Royal Infirmary | Research Nurse |
| Patricia | Doble | Musgrove Park Hospital | Research Nurse |
| Robert | Docking | Glasgow Queen Elizabeth | Coinvestigator |
| Adrian | Donnelly | Altnagelvin Hospital | Coinvestigator |
| Natalie | Dooley | Birmingham Queen Elizabeth Hospital | Research Nurse |
| Natalie | Dormand | Royal Brompton Hospital | Research Manager |
| Andrew | Drummond | Royal Oldham Hospital | Coinvestigator |
| Mark JG | Dunn | Edinburgh Royal Infirmary | Coinvestigator |
| Leigh | Dunn | Royal Victoria Infirmary | Research Nurse |
| Christine | Eastgate | Royal Free Hospital | Research Nurse |
| Karen | Ellis | Birmingham Queen Elizabeth Hospital | Research Nurse |
| Sarah | Farnell | St George’s Hospital | Research Nurse |
| Helen | Farrah | St George’s Hospital | Research Nurse |
| Emma | Fellows | Birmingham Queen Elizabeth Hospital | Research Nurse |
| Timothy | Felton | Wythenshawe Hospital | Coinvestigator |
| Helder | Filipe | Royal Free Hospital | Research Nurse |
| Clare | Finney | King’s College London | Research Nurse |
| Simon | Finney | Royal Brompton Hospital | Coinvestigator |
| Jillian | Fitchett | Royal Blackburn Hospital | Research Nurse |
| Brian | Gammon | Sandwell General Hospital | Research Nurse |
| Saibal | Ganguly | New Cross Hospital | Coinvestigator |
| Minerva | Gellamucho | Royal Stoke Hospital | Research Nurse |
| Susan | Gibson | Ninewells Hospital | Research Nurse |
| Charles | Gibson | Royal Devon and Exeter Hospital | Coinvestigator |
| Lynn | Gilfeather | Altnagelvin Hospital | Principal Investigator |
| Michael A | Gillies | Edinburgh Royal Infirmary | Principal Investigator |
| Stuart | Gillon | Glasgow Queen Elizabeth | Coinvestigator |
| Shameer | Gopal | New Cross Hospital | Principal Investigator |
| Anthony | Gordon | Imperial College | Coinvestigator |
| Stephanie | Goundry | Birmingham Queen Elizabeth Hospital | Research Nurse |
| Lia | Grainger | York Teaching Hospital | Research Nurse |
| Neus | Grau Novellas | St Thomas’s Hospital | Research Nurse |
| Joanne | Gresty | Birmingham Heartlands Hospital | Research Nurse |
| Mark | Griffiths | St Bartholomews | Coinvestigator |
| Jamie | Gross | Northwick Park Hospital | Coinvestigator |
| Una | Gunter | Royal Gwent Hospital | Research Nurse |
| Karen | Hallett | Royal Oldham Hospital | Research Nurse |
| Samantha | Harkett | Birmingham Queen Elizabeth Hospital | Research Nurse |
| Donna | Harrison-Briggs | Royal Blackburn Hospital | Research Nurse |
| Louise | Hartley | Glasgow Queen Elizabeth | Coinvestigator |
| Ingrid | Hass | University College Hospital | Research Nurse |
| Noel | Hemmings | Altnagelvin Hospital | Coinvestigator |
| Steven | Henderson | Glasgow Queen Elizabeth | Research Nurse |
| Helen | Hill | University of Wales Hospital | Research Nurse |
| Gemma | Hodkinson | Royal Gwent Hospital | Research Nurse |
| Kate | Howard | York Teaching Hospital | Research Nurse |
| Clare | Howcroft | St James Hospital | Research Nurse |
| Ying | Hu | Royal London Hospital | Research Nurse |
| Jonathan | Hulme | Sandwell General Hospital | Principal Investigator |
| Tariq | Husain | Northwick Park Hospital | Coinvestigator |
| Joanne | Hutter | Musgrove Park Hospital | Research Nurse |
| Dorothy | Ilano | University College Hospital | Staff Nurse |
| Richard | Innes | Musgrove Park Hospital | Principal Investigator |
| Nicola | Jacques | Royal Berkshire Hospital | Research Lead Nurse |
| Sarah | James | Royal Free Hospital | Research Nurse |
| Sarah | Jenkins | Poole Hospital | Research Nurse |
| Paul | Johnston | Antrim Area Hospital | Principal Investigator |
| Brian | Johnston | Royal Liverpool Hospital | Coinvestigator |
| Colette | Jones-Criddle | Aintree Hospital | Research Sister |
| Santhana | Kannan | Sandwell General Hospital | Coinvestigator |
| Parminder | Kaur Bhuie | Royal Berkshire Hospital | Research Nurse |
| Andrea | Kelly | St Thomas’s Hospital | Research Nurse |
| Sophie | Kennedy-Hay | Glasgow Queen Elizabeth | Research Nurse |
| Liana | Lankester | Derriford Hospital | Research Nurse |
| Susannah | Leaver | St George’s Hospital | Principal Investigator |
| Stephane | Ledot | Royal Brompton Hospital | Principal Investigator |
| Rosario | Lim | St Thomas’s Hospital | Research Nurse |
| Lucie | Linhartova | Birmingham Heartlands Hospital | Coinvestigator |
| Fei | Long | Northwick Park Hospital | Research Nurse |
| Niall S | MacCallum | University College Hospital | Principal Investigator |
| Sarah | MacGill | Royal Berkshire Hospital | Research Nurse |
| Andrew | Mackay | Glasgow Queen Elizabeth | Coinvestigator |
| Sarah | Maclean | Ninewells Hospital | Coinvestigator |
| Amber | Markham | Sandwell General Hospital | Matron |
| Daniel | Martin | Royal Free Hospital | Principal Investigator |
| Tim | Martin | Royal London Hospital | Research Nurse |
| Tracy | Mason | Birmingham Queen Elizabeth Hospital | Research Nurse |
| Nick | Mason | Royal Gwent Hospital | Coinvestigator |
| Justine | McCann | Royal Hospitals | Research Nurse |
| Corrienne | McCulloch | Edinburgh Royal Infirmary | Research Nurse |
| Christopher | McGhee | Birmingham Queen Elizabeth Hospital | Research Nurse |
| Loren | McGinley-Keag | Royal Hospitals | Research Nurse |
| Michael | McLaughlin | Glasgow Royal Infirmary | Coinvestigator |
| Lia | McNamee | Royal Hospitals | Research Physician Associate |
| Margaret | McNeil | Royal Free Hospital | Research Nurse |
| Laura | Mee | Birmingham Queen Elizabeth Hospital | Research Administrator |
| Claire | Mellis | King’s College London | Research Nurse |
| Teresa | Melody | Birmingham Heartlands Hospital | Research Manager |
| Jeanette | Mills | Royal Hospitals | Research Nurse |
| Esther | Molina | Leicester Royal Infirmary | Research Nurse |
| Matt PG | Morgan | University of Wales Hospital | Principal Investigator |
| Mushiya | Mpelembue | Northwick Park Hospital | Research Co-ordinator |
| Stephanie | Muldoon | Birmingham Queen Elizabeth Hospital | Research Nurse |
| Sheila | Munt | Royal Oldham Hospital | Research Nurse |
| Alistair | Nichol | University College Dublin | Collaborator |
| Nazril | Nordin | Watford General Hospital | Coinvestigator |
| Christopher | Nutt | Royal Hospitals | Coinvestigator |
| Sinead | O’Kane | Altnagelvin Hospital | Research Nurse |
| Aisling | O’Neill | Royal Hospitals | Research Nurse |
| Valerie | Page | Watford General Hospital | Principal Investigator |
| Elankumaran | Paramasivam | St James Hospital | Principal Investigator |
| Dhruv | Parekh | Birmingham Queen Elizabeth Hospital | Coinvestigator |
| Sarah | Patch | Poole Hospital | Research Nurse |
| Sameer | Patel | King’s College London | Coinvestigator |
| Lia | Paton | Glasgow Royal Infirmary | Coinvestigator |
| Gavin | Perkins | Birmingham Heartlands Hospital | Principal Investigator |
| Manuel | Pinto | Royal Free Hospital | Research Nurse |
| David | Pogson | Queen Alexandra Hospital | Coinvestigator |
| Petra | Polgarova | Addenbrookes Hospital | Research Nurse |
| Jagtar | Pooni | New Cross Hospital | Coinvestigator |
| Martin | Pope | Birmingham Queen Elizabeth Hospital | Clinical Trials Assistant |
| Grant C | Price | Edinburgh Royal Infirmary | Coinvestigator |
| Jashmin | Priya Maria | Manchester Royal Infirmary | Research Nurse |
| Lynda | Purdy | Royal Hospitals | Research Nurse |
| Alex | Puxty | Glasgow Royal Infirmary | Principal Investigator |
| John | Rae | Ninewells Hospital | Coinvestigator |
| Mark | Raper | University of Wales Hospital | Coinvestigator |
| Henrik | Reschreiter | Poole Hospital | Principal Investigator |
| Steve | Rose | Queen Alexandra Hospital | Research Nurse |
| Anthony | Rostron | Sunderland Royal Hospital | Coinvestigator |
| Alistair | Roy | Sunderland Royal Hospital | Principal Investigator |
| Christine | Ryan | St George’s Hospital | Research Nurse |
| Jung | Ryu | University College Hospital | Study Co-ordinator |
| Kiran | Salaunkey | Papworth Hospital | Principal Investigator |
| Julia | Sampson | Birmingham Heartlands Hospital | Research Nurse |
| Vivian | Sathianathan | Northwick Park Hospital | Coinvestigator |
| Lorraine | Scaife | Royal Devon and Exeter Hospital | Senior Research Nurse |
| Simon WM | Scott | Leicester Royal Infirmary | Principal Investigator |
| Timothy E | Scott | Royal Stoke Hospital | Principal Investigator |
| Sumant | Shanbhag | Manor Hospital | Principal Investigator |
| David | Shaw | Royal Liverpool Hospital | Research Nurse |
| Malcolm | Sim | Glasgow Queen Elizabeth | Principal Investigator |
| Suveer | Singh | Chelsea and Westminster | Principal Investigator |
| Andrew | Smallwood | New Cross Hospital | Research Nurse |
| Hazel | Smith | Birmingham Queen Elizabeth Hospital | Research Paramedic |
| John | Smith | King’s College London | Senior Research Nurse |
| Jayne | Smith | Poole Hospital | Senior Research |
| Facilitator | |||
| Deborah | Smyth | University College Hospital | Senior Nurse |
| Catherine | Snelson | Birmingham Queen Elizabeth Hospital | Coinvestigator |
| Michael | Spivey | Royal Cornwall Hospital | Principal Investigator |
| Elaine | Spruce | Birmingham Queen Elizabeth Hospital | Research Nurse |
| Charlotte | Summers | Addenbrookes Hospital | Principal Investigator |
| Peter | Sutton | Birmingham Heartlands Hospital | Research Nurse |
| Tamas | Szakmany | Royal Gwent Hospital | Principal Investigator |
| Nicholas | Talbot | Birmingham Queen Elizabeth Hospital | Coinvestigator |
| Maie | Templeton | Charing Cross and Hammersmith | Research Nurse |
| Jessica | Thrush | Worcester Hospital | Research Nurse |
| Redmond | Tully | Royal Oldham Hospital | Principal Investigator |
| William | Tunnicliffe | Birmingham Queen Elizabeth Hospital | Principal Investigator |
| Ian | Turner-Bone | Aintree Hospital | Research Nurse |
| Tonny | Veenith | Birmingham Queen Elizabeth Hospital | Coinvestigator |
| Alan | Vuylsteke | Papworth Hospital | Coinvestigator |
| Andrew | Walden | Royal Berkshire Hospital | Principal Investigator |
| Jonathan | Walker | Royal Liverpool Hospital | Coinvestigator |
| Kathryn | Ward | Royal Hospitals | Research Nurse |
| Tim | Walsh | Edinburgh Royal Infirmary | Coinvestigator |
| Victoria | Waugh | Royal Liverpool Hospital | Research Nurse |
| Colin | Wells | Derriford Hospital | Research Nurse |
| Ingeborg | Welters | Royal Liverpool Hospital | Principal Investigator |
| Tony | Whitehouse | Birmingham Queen Elizabeth Hospital | Coinvestigator |
| Arlo | Whitehouse | Birmingham Queen Elizabeth Hospital | Research Nurse |
| Christopher | Whitton | University of Wales Hospital | Research Nurse |
| Elizabeth | Wilby | St James Hospital | Research Nurse |
| Danielle | Wilcox | York Teaching Hospital | Research Nurse |
| Laura | Wilding | Aintree Hospital | Research Nurse |
| James | Williams | Royal Gwent Hospital | Coinvestigator |
| Karen | Williams | Royal Liverpool Hospital | Research Nurse |
| Sarah | Winnard | Royal Oldham Hospital | Research Nurse |
| Lindsey | Woods | Sunderland Royal Hospital | Research Nurse |
| Chris | Wright | Glasgow Queen Elizabeth | Coinvestigator |
| Neil H | Young | Edinburgh Royal Infirmary | Coinvestigator |
| Xiaobei | Zhao | Watford General Hospital | Research Nurse |
| Parjam | Zolfaghari | Royal London Hospital | Principal Investigator |
List of abbreviations
- AHRF
- acute hypoxaemic respiratory failure
- APACHE II
- acute physiology and chronic health evaluation II
- CEAC
- cost-effectiveness acceptability curve
- CMP
- case mix programme
- eDRIS
- electronic Data Research and Innovation Service
- EQ-5D-3L
- EuroQol-5 Dimensions, three-level version
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- ECCO2R
- extracorporeal carbon dioxide removal
- GP
- general practitioner
- HRQoL
- health-related quality of life
- HRG
- Healthcare Resource Group
- ICER
- incremental cost-effectiveness ratio
- INMB
- incremental net monetary benefit
- ICNARC
- Intensive Care National Audit and Research Centre
- ICU
- intensive care unit
- NHS
- National Health Service
- NICE
- National Institute for Health and Care Excellence
- PSS
- personal social services
- QALY
- quality-adjusted life-year
- REST
- pRotective vEntilation with veno-venouS lung assisT in respiratory failure
- SICSAG
- Scottish Intensive Care Society Audit Group
- WTP
- willingness to pay
