Notes
Article history
The contractual start date for this research was in June 2016. This article began editorial review in August 2023 and was accepted for publication in March 2024. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The Health Technology Assessment editors and publisher have tried to ensure the accuracy of the authors’ article and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Zanganeh et al. This work was produced by Zanganeh et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library and the DOI of the publication must be cited.
2024 Zanganeh et al.
Background
Maintenance of residual kidney function (RKF) in patients commencing dialysis has been linked to notable benefits, including improved survival, better quality of life and lower risk of intradialytic hypotension, cardiac stunning and death due to removal of high fluid volumes. 1–4 However, there is little evidence on the effectiveness of interventions to maintain RKF in patients undergoing haemodialysis; where trials have been undertaken, they have been limited by their small sample sizes (< 50 participants). 5–7 This issue is also confounded by inconsistency in the design and application of dialysis unit protocols to guide fluid management, which was evident in the findings of a recent UK-wide survey of practices. 8
Of the approximately 68,000 people in the UK treated with kidney replacement therapies for end-stage kidney disease in 2019, about one-third (24,000) received centre-based haemodialysis at an annual cost of 0.5% of the NHS budget, excluding additional costs, such as travel, drugs, access procedures and inpatient episodes. 9,10
A key objective of kidney replacement therapies is to regulate the body’s fluid or ‘volume’ status. Getting this wrong leads to either volume excess or depletion, and both can be very harmful. In this high-cost setting, bioimpedance spectroscopy devices have been developed and are frequently used in haemodialysis units, where they have the potential to enhance the productivity of care by helping clinicians make appropriate and safe treatment decisions. For example, improved accuracy in the assessment of a patient’s fluid status when compared to clinical judgement alone may reduce the risk of volume depletion which may, in turn, help to preserve RKF. The anticipated benefit to patients would be a change in clinical practice in which a more balanced approach to the bidirectional risks of volume status is taken that is associated with improved well-being, fewer dialysis-related symptoms, possibly less dialysis in those commencing treatment in an incremental fashion, and potentially better survival. 11–15 Through these benefits, bioimpedance also has potential to address several of the NHS Outcomes Framework domains. These include prevention of premature death, improving outcomes by addressing a number of National Institute for Health and Care Excellence (NICE) chronic kidney disease standards, such as cardiovascular risk, blood pressure and avoidance of acute illness episodes16,17 and enhancing the quality of life for people on dialysis, and contributing through improved engagement and activation to a more positive patient experience. 18,19
In making recommendations for the use of bioimpedance, NICE considered that there was insufficient evidence to recommend its routine application20 and called for rigorous evidence on its clinical effectiveness and cost-effectiveness. In response, the National Institute for Health and Care Research (NIHR) funded the BioImpedance Spectroscopy to maintain Renal Output (BISTRO) randomised controlled trial (RCT), through its Health Technology Assessment (HTA) programme. The trial demonstrated that bioimpedance added to a standardised fluid management protocol does not significantly improve preservation of RKF in patients on incident haemodialysis. 21 Recognising that the correct fluid status of a person with kidney failure will have an influence on a broader range of outcomes than just residual kidney function, it is appropriate that the cost-effectiveness of bioimpedance incorporating these outcomes is undertaken. To date, the cost-effectiveness of bioimpedance-informed haemodialysis is unknown. For example, it is unclear whether the extra cost of bioimpedance would be balanced out by reduced use of resources (e.g. because of a possible reduction in serious adverse events, hospital admissions) and/or improved outcomes in terms of quality-adjusted life-years (QALYs).
Aim of the bioimpedance spectroscopy to maintain renal output economic evaluation
The overarching aim of the BISTRO economic evaluation was to determine the costs, outcomes and overall cost-effectiveness of bioimpedance-guided fluid management (BGFM), compared with current fluid management (CFM). The primary (base-case) analysis was conducted from the perspective of the NHS and Personal Social Services (PSS). For each of the comparators, costs included use of healthcare resources associated with the intervention and care received in the primary care and hospital settings. Outcomes were expressed in terms of QALYs. Sensitivity analyses were carried out to present results based on different scenarios and sources of data.
Bioimpedance spectroscopy to maintain renal output trial methods
Trial design and participants
The economic evaluation was embedded into the BISTRO RCT. The trial’s protocol and clinical effectiveness results are reported in detail elsewhere. 21,22 In brief, BISTRO was an open-label, pragmatic, randomised, multicentre UK wide trial of patients having incident haemodialysis, comparing current best practice in setting the post-dialytic target weight with the same assessment guided by serial bioimpedance measurements. In terms of inclusion criteria, potential participants were adult patients undergoing haemodialysis, aged > 18 years, within 3 months of commencing centre-based maintenance haemodialysis due to advanced kidney disease (CKD stage 5). Patients required evidence of RKF > 500 ml urine volume/day or residual glomerular filtration rate > 3 ml/minute/1.73 m2. Participants should have entered the study on outpatient treatment. Exclusion criteria were the inability or unwillingness to give informal consent, or inability to comply with trial procedures, and either high risk of death or expected transplantation within 6 months.
Intervention
The study intervention was the incorporation of bioimpedance-derived information about body composition into the clinical assessment of fluid status of dialysis patients. In essence, the intervention was the availability of this additional information, specifically the normally hydrated weight reported by the device, which could then be used in conjunction with clinical judgement. Clinicians whose usual role was to assess fluid status were trained in the use of the fluid assessment proforma and asked to set the post-dialysis target weight to avoid excessive volume depletion, where possible. For patients in the control arm, the target weight was set using clinical judgement only. To achieve blinding, bioimpedance measurements were taken in both study groups but the results were concealed from the clinical teams and trial participants in the control group. To minimise performance bias and information bias, the bioimpedance measurements were taken independently of the fluid assessments by trained nurses. In an independent selection process, overseen by Kidney Research UK, the Fresenius body composition monitor (BCM) was selected and used for measuring bioimpedance. 22 All participating centres received bespoke training at the site during visits prior to enrolling patients, which included a standardised approach to taking bioimpedance measurements by the research nurses. Full bioimpedance data sets were downloaded on to the computers at participating renal centres and, throughout the trial, regular blinded quality control assessments of submitted readings were undertaken by the study team.
Randomisation and follow-up
Randomisation was one to one for the bioimpedance intervention and control groups, with random permuted blocks stratified by centre. All participants were followed up until the point when the first of the following events occurred: the participant became anuric, died, had a kidney transplantation, or withdrew from the study due to stopping dialysis (e.g. recovery of function), patient choice or investigator exclusion (e.g. medical condition) or reached the end of the trial with a maximum study follow-up period of 24 months. In those patients who reached the end point before 24 months, data on quality of life and health-related costs were collected while they remained in the study.
Bioimpedance spectroscopy to maintain renal output economic evaluation methods
The economic evaluation took the form of a cost–utility analysis using, as a primary outcome, incremental costs (or cost savings) per QALY gained. The perspective of the base-case analysis was that of the UK NHS and PSS as recommended by NICE reference case for appraising health technologies. 23 Additional analyses were undertaken to explore different sources of data for particular cost categories, including broader costs, different health-related quality of life (HRQoL) instruments and value sets. The time horizon of this within-trial analysis matched the length of the BISTRO follow-up, that is, 24 months following randomisation. In line with recommendations,23 costs and QALYs accruing beyond the first year were discounted at 3.5% per annum.
Analyses were carried out in accordance with the aims and methods specified in the BISTRO trial protocol and the health economics analysis plan (HEAP). The latter detailed the objectives to be pursued and the methods to be followed for the BISTRO economic evaluation. Deviations from the protocol were minor and served the purposes of meeting the study’s objectives when obstacles were presented. The most noteworthy deviation was the additional use of Hospital Episode Statistics (HES) data when available data on resource use collected through case report forms were limited. The trial protocol and the HEAP were reviewed and approved by the BISTRO independent trial steering committee.
As specified in the study’s HEAP, a decision model would be considered to extend the time horizon beyond 2 years, if findings suggested significant differential costs and outcomes as a result of the intervention accruing beyond the trial follow-up period. Analysis of the study’s effectiveness carried out subsequently found the interventions to be equivalent (small, non-statistically significant effect of BGFM compared with CFM in terms of risk of anuria and rate of loss of RKF),21 which was not anticipated to change beyond the 2-year period. This, and the paucity of reliable evidence linking BGFM-related effects to long-term outcomes,24 suggested limited value (and caution) in extrapolating beyond the trial follow-up period.
Health outcomes
The main measure of health outcome in the BISTRO economic evaluation was QALYs. The QALY is a widely used and recommended23 metric that combines quantity (length) of life and preference-based HRQoL into a single value. HRQoL was obtained through participants’ responses to the EuroQol EQ-5 dimension-5 level (EQ-5D-5L)25 and Short-Form 12 (SF-12) instruments26 at baseline and 3-monthly thereafter, until month 24. The EQ-5D-5L consists of two parts: the EQ-5D-5L descriptive system and the EQ visual analogue scale (EQ VAS). The descriptive system asks respondents to indicate their health state by ticking boxes next to the statement that represents the level of health (no problems, slight problems, moderate problems, severe problems and extreme problems) across five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression). The EQ VAS asks respondents to rate their health on a vertical visual analogue scale, where the end points are labelled ‘The best health you can imagine’ and ‘The worst health you can imagine’. 25 The SF-12 status description instrument asks respondents to answer 12 questions about their perceived status in relation to seven dimensions (physical activities, social activities, usual activities, pain, mental health, energy and fatigue, and general health). 26 Each participant’s responses to the EQ-5D-5L and SF-12 status description instruments can be translated into a single, preference-based HRQoL index score (utility value) using appropriate value sets. Although a new UK specific EQ-5D-5L value set exists,27 it has been a subject of controversy28 and, currently, NICE recommends29 the use of the Hernandez Alava et al. algorithm. 30 Therefore, for the base-case analysis, each participant’s responses to the instrument’s health status classification system were translated into a single, preference-based (utility) index score using the Hernandez Alava et al. value set for the EQ-5D-5L. 30 SF-12 was converted to the Short-Form 6 Dimensions (SF-6D) quality of life instrument and utility values31 (presented in sensitivity analysis). QALYs were calculated as the area under the curve connecting utility scores reported at different time points. 32 Deceased patients were assigned a utility of zero from the date of death.
Resource use and costs
Key healthcare resource use and costs for both comparators were obtained from two primary resources: (1) patient-level data collected within the BISTRO trial and (2) routinely collected data for care received within a hospital through HES. As per the BISTRO protocol, the latter source was used to provide more accurate information on episodes of care provided in hospital (i.e. scheduled and unscheduled inpatient admissions, critical care admissions and hospital outpatient visits). Data collected as part of the BISTRO trial were captured through case report forms (CRFs) and a client services receipt inventory for chronic kidney disease questionnaire. 33 CRFs were completed at baseline and 3-monthly thereafter until month 24 (bioimpedance and haemodialysis sessions were also completed at months 1 and 2). A table listing information about the type of data collected from CRFs and the schedule of data collection can be found in Appendix 1, Table 12. Administrative hospitalisation data (HES) for BISTRO participating sites were obtained from NHS Digital in England, Public Health in Scotland, NHS Informatics Service in Wales and by the site research teams in Northern Ireland. HES data were cleaned (e.g. cancellations and duplicates removed) and checked against randomisation date, analysis date, event type, date of 2-year follow-up and trial end date. Table 1 gives the source of data for each key resource use category in the base-case analysis.
| Resource use or cost category | Base-case analysis |
|---|---|
| Bioimpedance sessions | CRF |
| Haemodialysis sessions | CRF |
| Inpatient admissions | |
| Scheduled | HES (incl. EL, DC, RP) |
| Unscheduled | HES (incl. NES, NEL) |
| Nursing home | CRF |
| Critical care admissions | HES |
| Outpatient appointments | |
| Hospital outpatient visits | HES |
| Nursing home | CRF |
| Primary and community care services | CRF |
Healthcare resources were translated into costs using unit cost values taken from up-to-date national sources, including the Unit Costs of Health and Social Care 2020 [Personal Social Services Research Unit (PSSRU)]34 and the National Schedule of NHS Costs 2019–20. 35 Monetary values throughout the study were expressed in Great British pounds using 2019–20 as the base year. Service use over the 24-month period was multiplied by unit costs to arrive at total cost for each patient in each of the alternative comparators.
Bioimpedance sessions
The cost of a bioimpedance device was obtained from the manufacturer Fresenius Medical Care (UK) Ltd (www.freseniusmedicalcare.co.uk), and the additional, bioimpedance-related cost per session over the duration of the study was calculated taking into account the device cost, necessary disposables, maintenance costs, device depreciation, personnel training and staff cost associated with measurements and interpretation (see Appendix 2, Tables 13 and 14).
Haemodialysis sessions
Haemodialysis sessions took place in outpatient haemodialysis centres, within main or satellite units and in their associated inpatient renal units. 22 For each participating patient, the number of haemodialysis sessions was recorded in CRFs and, for the period between CRFs, this number was assumed to be fixed. The unit cost of a haemodialysis session was taken from the National Schedule of NHS Costs 2019–2035 (Table 2).
| Service | Unit cost (£) | Source |
|---|---|---|
| Bioimpedance session (CRF) | 25.10a | Fresenius Medical Care (UK) Ltd, Unit Costs of Health and Social Care 202034 |
| Haemodialysis sessions (CRF) | ||
| Catheter or line for hospital/satellite | 165b | National Schedule of NHS Costs 2019–202035 |
| Fistula or graft for hospital/satellite | 163.50b | |
| Inpatient admissions | ||
| Scheduled (HES) | See Appendix 3, Table 15 | National Schedule of NHS Costs 2019–202035 |
| Unscheduled (HES) | See Appendix 3, Table 16 | |
| Scheduled (CRF) | 4168c | Unit Costs of Health and Social Care 202034 |
| Unscheduled (CRF) | See Appendix 4, Table 17 | National Schedule of NHS Costs 2019–202035 |
| Nursing home (CRF) | 184d | Unit Costs of Health and Social Care 202034 |
| Critical care admissions (HES) | See Appendix 5, Table 18 | National Schedule of NHS Costs 2019–202035 |
| Outpatient appointments | ||
| Hospital outpatient visits (HES) | See Appendix 6, Table 19 | National Schedule of NHS Costs 2019–202035 |
| Hospital outpatient visits (CRF) | 135e | Unit Costs of Health and Social Care 202034 |
| Daycare centre (nursing home) (CRF) | 64e | |
| Primary and community care services (CRF) | ||
| General practitioner, NHS | 184f | Unit Costs of Health and Social Care 202034 |
| Dietitian, NHS | 36f,g | |
| Social worker, PSS | 45f,g | |
| Home care worker, PSS | 24f,g | |
| Palliative care nurse, NHS | 89f | |
| Dialysis nurse specialist, NHS | 89f | |
| District nurse, NHS | 89f | |
| Counsellor, NHS | 48f,g | |
| Other | ||
| Nurse (e.g. diabetic), NHS | 89f | |
| Occupational therapist, NHS | 36f,g | |
| Physiotherapist, NHS | 36f,g | |
| Optician, NHS | 36f,g | |
| Chiropodist, NHS | 36f,g | |
| Podiatrist, NHS | 36f,g | |
| Clinical support worker nursing higher level, NHS | 52f | |
| Consultant medical, NHS | 119f,g | |
| Consultant surgical, NHS | 114f,g | |
| Clinical psychologist consultant, NHS | 114f,g | |
Inpatient admissions
Data on scheduled and unscheduled inpatient admissions were available from HES. Data sets were cleaned, checked and converted to the format accepted by HRG4 + 2020 National Costs Grouper36 for admitted patient care. This was to check the healthcare resource group (HRG) codes attached to any of the inpatient admissions. A total of 17 different body system categories including 171 different HRG code types were defined for the scheduled admissions. Each of the scheduled HRG codes was then assigned a relevant elective, daycase or regular day/night average unit cost obtained from the NHS Reference Costs Guide35 (see Appendix 3, Table 15). For the unscheduled admissions, 17 different body system categories including 236 different HRG code types were defined. According to the NHS Data Model and Dictionary 2021 and the Reference Costs Guidance 2015–16, non-elective short stay is defined as any length of inpatient stay ˂ 2 days and non-elective long stay defined as any length of inpatient stay of 2 days or more. 37,38 Based on these definitions, each of the unscheduled HRG codes was assigned a relevant non-elective short- or long-stay average unit cost obtained from the NHS Reference Costs Guide35 (see Appendix 3, Table 16).
A limited amount of information regarding number and dates of admissions was made available through CRFs, and these were used in sensitivity analyses. There was no information available regarding the reason of scheduled admission to be able to provide exact unit cost for hospital inpatient ward. Therefore, an average unit cost per episode of an average elective HRG was attached to scheduled admissions34 (Table 2). A unit cost per day was attached to nursing home admissions34 (see Table 2). Unscheduled inpatient admissions due to serious adverse events that led to hospital admission were captured in CRFs and unscheduled admissions, whether or not due to serious adverse events, were available from HES. The former were costed using unit costs from NHS Reference Cost Schedules. The latter were costed as part of HES data costing. 35 Sixteen different body system categories including different Common Terminology Criteria for Adverse Events (CTCAE) Dictionary term types and their grades were defined within the serious adverse events collected through CRF. The CTCAE terms and their descriptions were linked to 44 best-possible HRG code types and currency description; they were assigned a non-elective short- or long-stay average unit cost35 (see Appendix 4, Table 17).
Critical care admissions
Critical care admissions were obtained from HES. Files were cleaned, checked and converted to the format accepted by National Costs Grouper36 for adult critical care, to attach HRG codes to each critical care admission episode. Six different critical care/HRG code types were defined and each of the adult critical care HRG codes was then assigned a relevant unit cost considering the number of supported organs (0, 1, 2, 3 or 4)35 (see Appendix 5, Table 18).
Outpatient appointments
Outpatient appointments are captured by HES; thus, data on such appointments were available from this source. All files were cleaned, checked and converted to the format accepted by National Costs Grouper36 for non-admitted consultation (i.e. hospital outpatient visits). This was to attach HRG codes to any of the outpatient appointments. I39 different outpatient services/HRG code types were defined. Each of the outpatient HRG codes was then assigned a relevant face-to-face, first or follow-up attendance or telephone/telemedicine, first or follow-up, consultation unit cost35 (see Appendix 6, Table 19).
Limited information about outpatient hospital appointments was collected through CRF. This information was used in a sensitivity analysis. There was no information available regarding the reason for outpatient visits, so an average unit cost34 was assigned (see Table 2). In addition, a question about nursing home visits in the CRF made available nursing home (daycare centre) attendances, which were assigned a unit cost from the PSSRU Unit Costs of Health and Social Care report34 (see Table 2).
Primary and community care services
Primary and community care services, including appointments with general practitioners and nurses, were collected through CRFs. Unit costs for costing of different professions were taken from the PSSRU Unit Costs of Health and Social Care report34 (see Table 2).
Time devoted to caring by unpaid carers
Data were also collected on time devoted to caring by unpaid carers through a relevant question in the Client Services Receipt Inventory. These data were used to calculate costs incurred by patients’ family to be included in a sensitivity analysis. These included help patients had received from friends or family members as a result of their illness, including personal care (e.g. bathing), help with medical procedures (e.g. taking medication), help inside the home (e.g. cooking), help outside the home (e.g. shopping), and time spent ‘on call’. Cost of these forms of unpaid carer work were calculated according to the replacement cost method39 (see Table 2).
Missing data
Missing data are a common occurrence in studies collecting patient-level data. The choice of analytic method to be used for accounting for missing data depends on the extent of missing information and the likely underlying mechanism through which data were missing. Descriptive analyses of missing data were carried out to investigate the patterns of missing data (through graphs) and the likely mechanism of missingness. Utility values and cost values for deceased participants were imputed by zero from the time of their death. Additionally, a cost of zero was attached to bioimpedance sessions (in the intervention group) and haemodialysis (in both groups) after transplantation or recovery of sufficient kidney function to allow the patient to stop dialysis. The remaining missing utility and costs data were imputed under the missing at random assumption, at each time point for utility and for year 1 and 2 for different cost components, using fully conditional multiple imputation by chained equations implemented through the MICE package in STATA® version 17 (StataCorp LP, College Station, TX, USA). 40 The appropriateness of using missing at random was evaluated by investigating the missing data patterns and comparing attributes with and without missing utility data at each follow-up time point; and cost components data at years 1 and 2. The multiple imputation model used covariates and utility scores collected at baseline. The imputation was conducted separately by trial arm. 41
Statistical and cost–utility analyses
In line with recommended practice for the analysis of RCT data, the intention-to-treat (ITT) principle was adopted. 42 The ITT data set comprised all randomised patients, analysed according to randomised groups, and including those deviating from protocol, switching treatment, withdrawn or lost to follow-up. The unadjusted calculated mean total per-patient values were given for the intervention and control arms alongside mean difference and 95% confidence intervals (CIs) obtained through non-parametric bootstrap (bias corrected and accelerated) methods using 1000 replications. 43 The distributions of the calculated costs and QALYs were interrogated using graphs and skewness statistics and generalised linear regression models (GLM) were used. 44 Adjustment for baseline utility and for the same covariates (Table 5) as for the primary outcome was made. Cost–utility is presented as an incremental cost-effectiveness ratio (ICER), calculated as difference in costs between comparators over difference in QALYs between comparators. 45 The use of bioimpedance was selected as the intervention and current management without bioimpedance as control. Measures of uncertainty were calculated for the mean difference estimates through bootstrap methods using 1000 replications. 43 For the base-case analysis, to account for the inherent uncertainty in the results due to sampling variation, the joint distribution of differences in cost and QALYs was derived using 5000 of non-parametric bootstrap simulations/iterations. 46 The simulated cost and QALYs pairs were depicted on a cost-effectiveness plane and were plotted as cost-effectiveness acceptability curves (CEACs). 47 A CEAC shows the probability of the bioimpedance-guided and standard fluid management options being cost-effective across a range of possible values of willingness to pay (WTP) for an additional QALY. Net monetary benefits (NMB) for each compared option, as well as incremental net monetary benefits (BGFM vs. CFM), were estimated for a range of different WTP thresholds, where a positive incremental NMB would indicate that BGFMI is cost-effective compared with CFM. Data management tasks and statistical analyses were carried out in STATA version 17. Findings of this economic evaluation were reported in accordance with the Consolidated Health Economic Evaluation Reporting Standards statement. 48
Base-case and sensitivity analyses
The primary analysis was complemented by a series of additional sensitivity analyses carried out to explore the impact of different sources of data (i.e. BISTRO CRFs, HES), specifications and assumptions in the cost-effectiveness analysis. The base-case and sensitivity analyses are described in Table 3.
| Analysis | Description |
|---|---|
| Base-case analysis (ITT) | Data: missing data imputed using multiple imputation40 Data source: HES for scheduled and unscheduled inpatient admissions, critical care admissions, and hospital outpatient visits; CRF for other resource use (see Table 1) QALY derivation: through EQ-5D-5L using a value set by Hernandez Alava et al.30 Statistical model specification: GLM – costs adjusted for relevant covariates; QALYs adjusted for both relevant covariates and baseline utility |
| Sensitivity analysis 1 (available complete data) | Data: available complete data, non-imputed49 Data source: HES for scheduled and unscheduled inpatient admissions, critical care admissions, and hospital outpatient visits; CRF for other resource use (see Table 4) QALY derivation: through EQ-5D-5L using a value set by Hernandez Alava et al.30 Statistical model specification: unadjusted |
| Sensitivity analysis 2 (CRF rather than HES for resource use) | Data: missing data imputed using multiple imputation40 Data source: HES for critical care admissions; CRF for other resource use (see Table 4) QALY derivation: through EQ-5D-5L using a value set by Hernandez Alava et al.30 Statistical model specification: GLM – costs adjusted for relevant covariates; QALYs adjusted for both relevant covariates and baseline utility |
| Sensitivity analysis 3 (unadjusted ITT) |
Data: missing data imputed using multiple imputation40 Data source: HES for scheduled and unscheduled inpatient admissions, critical care admissions, and hospital outpatient visits; CRF for other resource use (see Table 4) QALY derivation: through EQ-5D-5L using a value set by Hernandez Alava et al.30 Statistical model specification: unadjusted ITT |
| Sensitivity analysis 4 (EQ-5D using Devlin) | Data: missing data imputed using multiple imputation40 Data source: HES for scheduled and unscheduled inpatient admissions, critical care admissions, and hospital outpatient visits; CRF for other resource use (see Table 4) QALY derivation: through EQ-5D-5L using a value set by Devlin et al.27 Statistical model specification: GLM – costs adjusted for relevant covariates; QALYs adjusted for both relevant covariates and baseline utility |
| Sensitivity analysis 5 (SF-6D) | Data: missing data imputed using multiple imputation40 Data source: HES for scheduled and unscheduled inpatient admissions, critical care admissions, and hospital outpatient visits; CRF for other resource use (see Table 4) QALY derivation: through SF-12 (converted to SF-6D) using Brazier and Roberts algorithm31 Statistical model specification: GLM – costs adjusted for relevant covariates; QALYs adjusted for both relevant covariates and baseline utility |
| Sensitivity analysis 6 (excluded nursing home and primary/community care) | Data: missing data imputed using multiple imputation40 Data source: HES for scheduled and unscheduled inpatient admissions, critical care admissions, and hospital outpatient visits; CRF for other resource use, excluding nursing home, and primary and community care services (see Table 4) QALY derivation: through EQ-5D-5L using a value set by Hernandez Alava et al.30 Statistical model specification: GLM – costs adjusted for relevant covariates; QALYs adjusted for both relevant covariates and baseline utility |
| Sensitivity analysis 7 (included patients’ family incurred costs) | Data: missing data imputed using multiple imputation40 Data source: HES for scheduled and unscheduled inpatient admissions, critical care admissions, and hospital outpatient visits; CRF for other resource use, including patients’ family incurred costs to adopt a broader perspective than NHS than PSS (see Table 4) QALY derivation: through EQ-5D-5L using a value set by Hernandez Alava et al.30 Statistical model specification: GLM – costs adjusted for relevant covariates; QALYs adjusted for both relevant covariates and baseline utility |
Detail information regarding resource use data sources used in sensitivity analyses are given in Table 4.
| Resource use or cost category | Sensitivity analysis no. 2 | All other sensitivity analysesa |
|---|---|---|
| Bioimpedance sessions | CRF | CRF |
| Haemodialysis sessions | CRF | CRF |
| Inpatient admissions | ||
| Scheduled | CRF (incl. EL) | HES (incl. EL, DC, RP) |
| Unscheduled | CRF (incl. SAEs: NES, NEL) | HES (incl. NES, NEL) |
| Nursing home | CRF | CRF |
| Critical care admissions | HES | HES |
| Outpatient appointments | ||
| Hospital outpatient visits | CRF | HES |
| Nursing home | CRF | CRF |
| Primary and community care services | CRF | CRF |
Results
Participants characteristics
The study recruitment process is summarised in the Consolidated Standards of Reporting Trials diagram, which has been published elsewhere. 21 Overall, 439 patients were initially recruited from 34 centres, for a maximum follow-up period of 24 months. Randomisation led to well-balanced study arms according to prespecified baseline patient characteristics (Table 5).
Data completeness
The availability of key economic data (healthcare resource use, EQ-5D-5L and SF-12) by comparator is provided in Appendix 7, Table 20. At each point in time, EQ-5D-5L data were considered incomplete if the health status classification part of the questionnaire was not completed or it was partially completed (i.e. fewer than its five domains were completed), which precluded the calculation of utility values. Similarly, resource use and costs were considered missing if an item or answer was not available (i.e. it was not recorded in the CRF or it was unavailable through HES).
As anticipated, there was a high level of completeness of HES data (only 14 missing data from HES England). As expected, the amount of missing data increased with time in trial and the level of completeness was markedly low for particular resource use data collected via CRFs (e.g. completeness of bioimpedance sessions, haemodialysis sessions, and primary and community care services dropped to 22%, 22% and 13%, respectively, at year 2: 12–24 months). In a similar fashion, completion of EQ-5D dropped to levels below 60% from month 6 onwards, with less than one-third of the expected completed questionnaires returned at the 18-month follow-up point. Overall, there were entirely complete data for all resource use categories for only 40 participants (9.15% of all participants; 22 in BGFM group, 18 in CFM group) and only for 48 participants (10.98% of all participants; 28 in BGFM group, 20 in CFM group) for all EQ-5D-5L measurements. The low availability of data over the 24 months made imputation of missing data highly necessary. Further information on data completion is given in Appendix 7.
| BGFM (n = 222) | CFM (n = 213)a | ||
|---|---|---|---|
| Sex; male/female (% male) | 157/65 (70.7) | 149/63 (69.3) | |
| Age, mean (SD) | 60.06 (14.3) | 62.7 (13.7) | |
| Ethnicities, n (%) | White | 174 (78.4) | 173 (81.2) |
| Black/Black British | 6 (0.3) | 0 (0) | |
| Asian/Asian British | 7 (0.3) | 2 (0.4) | |
| Other | 35 (15.8) | 38 (17.8) | |
| Planned/unplanned start, n (%) | 180 (81.1)/42 (18.9) | 184 (86.4)/29 (13.6) | |
| Comorbidities, n (%) | Malignancy | 14 (6.3) | 14 (6.6) |
| Ischaemic heart disease | 41 (18.4) | 47 (22.1) | |
| Peripheral vascular disease | 19 (8.5) | 33 (15.3) | |
| Left ventricular dysfunction | 31 (14.0) | 25 (11.2) | |
| Diabetes mellitus | 107 (48.2) | 91 (42.3) | |
| Systemic collagen vascular disease | 6 (2.7) | 7 (3.3) | |
| Comorbidity score, median (IQR) | 1 (0, 2) | 1 (0, 2) | |
| Patients on diuretics, n (%) | 115 (51.8) | 111 (51.6) | |
| Patients on RAAS inhibition, n (%) | 61 (27.4) | 49 (22.7) | |
| Patients on calcium antagonists, n (%) | 103 (46.4) | 117 (52) | |
Costs
Imputed costs for each resource use category and assessed comparator for the base-case analysis are given in Table 6. Findings show BGFM to be associated with greater costs for the cost categories of primary and community care (£201.22), bioimpedance (£185.86), critical care (£71.92), outpatient consultations (£41.32) and unscheduled inpatient admissions (£9.93). Conversely, BGFM was associated with cost savings for the cost categories of haemodialysis (−£494.48), inpatient nursing home admissions (−£54.39), outpatient nursing home visits (−£11.92) and scheduled inpatient admissions (−£4.10). Differences in costs for bioimpedance, inpatient stay and outpatient visits in nursing homes, and care received in primary and community settings were statistically significant (p < 0.00).
| Resource use category | BGFM (n = 222) £, mean (SD) | CFM (n = 215) £, mean (SD) | BGFM–CFM £,a mean difference (95% CI) | p-valuea |
|---|---|---|---|---|
| CRF haemodialysis | 38,338.58 (9963.56) | 38,833.06 (9379.89) | −494.48 (−2199.08 to 1210.11) | 0.57 |
| CRF bioimpedance | 185.86 (78.63) | 0 (0) | 185.86 (175.46 to 196.27) | 0.00 |
| Inpatient | 10,320.87 (744.32) | 10,369.42 (756.34) | −48.55 (−2079.66 to 1982.554) | 0.96 |
| HES inpatient (scheduled) | 4451.29 (5522.02) | 4455.39 (5869.24) | −4.10 (−1103.56 to 1095.36) | 0.99 |
| HES inpatient (unscheduled) | 5869.57 (7438.17) | 5859.64 (9204.54) | 9.93 (−1503.85 to 1523.72) | 0.99 |
| CRF inpatient, nursing home | 0 (0) | 54.39 (87.58) | −54.39 (−66.02 to −42.75) | 0.00 |
| HES adult critical care | 306.23 (702.78) | 234.31 (754.52) | 71.92 (−65.78 to 209.62) | 0.31 |
| Outpatient | 1946.53 (2200.46) | 1917.13 (1869.96) | 29.40 (−350.2878 to 409.09) | 0.88 |
| HES outpatient consultation | 1946.53 (2200.47) | 1905.20 (1867.28) | 41.32 (−347.16 to 429.81) | 0.83 |
| CRF outpatient, nursing home | 0 (0) | 11.92 (12.79) | −11.92 (−13.69 to −10.16) | 0.00 |
| CRF primary, community care | 711.68 (526.99) | 510.45 (301.74) | 201.22 (120.84 to 281.61) | 0.00 |
The total costs and the difference between the assessed options for the base-case analysis are given in Table 7. Overall, over 24 months, the mean per-patient cost with BGFM was £382 lower (non-significant, 95% CI −3319 to 2556) than that with CFM.
| Comparators | Total cost | Differencea | 95% CIa | ||
|---|---|---|---|---|---|
| Mean | SD | Lower CI | Upper CI | ||
| CFM | 52,030.51 | 17,041.81 | −381.65 | −3318.97 | 2555.67 |
| BGFM | 51,648.86 | 13,668.27 | |||
Health outcomes
Table 8 summarises the imputed health outcomes (EQ-5D-5L utility scores) and between group mean differences at each time point and across the follow-up period for the base-case analysis. EQ-5D utility scores from follow-up time point 12 suggest a small non-significant gain for the BGFM group.
| EQ-5D-5La | BGFM (n = 222), mean (SD) | CFM (n = 215), mean (SD) | BGFM–CFM, mean differenceb (95% CI) | p-valueb |
|---|---|---|---|---|
| Baseline | 0.554 (0.278) | 0.600 (0.276) | −0.046 (−0.099 to 0.006) | 0.09 |
| Month 3 | 0.541 (0.277) | 0.549 (0.270) | −0.008 (−0.059 to 0.043) | 0.75 |
| Month 6 | 0.538 (0.266) | 0.566 (0.248) | −0.028 (−0.075 to 0.019) | 0.25 |
| Month 9 | 0.513 (0.260) | 0.529 (0.243) | −0.016 (−0.062 to 0.031) | 0.50 |
| Month 12 | 0.504 (0.256) | 0.486 (0.265) | 0.018 (−0.031 to 0.067) | 0.47 |
| Month 15 | 0.506 (0.247) | 0.485 (0.239) | 0.021 (−0.023 to 0.065) | 0.35 |
| Month 18 | 0.485 (0.246) | 0.466 (0.252) | 0.019 (−0.028 to 0.067) | 0.42 |
| Month 21 | 0.449 (0.276) | 0.412 (0.262) | 0.037 (−0.013 to 0.087) | 0.15 |
| Month 24 | 0.444 (0.263) | 0.415 (0.255) | 0.029 (−0.019 to 0.076) | 0.25 |
The total QALYs and the difference between the assessed options for the base-case analysis are given in Table 9. Overall, over 24 months, there was a small, non-significant QALY gain for the BGFM group 0.043 (95% CI −0.019 to 0.105).
| Comparators | Total QALYsa | Differenceb | 95% CIb | ||
|---|---|---|---|---|---|
| Mean | SD | Lower CI | Upper CI | ||
| CFM | 0.966 | 0.438 | 0.043 | −0.019 | 0.105 |
| BGFM | 1.009 | 0.443 | |||
Cost–utility results
Results for the base-case analysis are presented in Table 10. Over 24 months BGFM was associated with a slightly lower total per-patient cost, giving an estimated total per-patient cost-saving of £382 (non-statistically significant, 95% CI −£3319 to £2556). In terms of QALYs gained, BGFM appeared to be slightly more effective than CFM, resulting in a gain of 0.043 QALYs (non-statistically significant, 95% CI −0.019 to 0.105). On average, BGFM was found to be less costly and more effective than CFM.
| Parameter | BGFM | CFM | Incremental cost (£) (95% CI) | Incremental QALY (95% CI) | ICER (BGFM vs. CFM) (£ per QALY) | ||
|---|---|---|---|---|---|---|---|
| Total costs (£) | Total QALYs | Total costs (£) | Total QALYs | ||||
| Base-case analysis | 51,648.86 | 1.009 | 52,030.51 | 0.966 | −381.65 (−3318.97 to 2555.67) | 0.043 (−0.019 to 0.105) | BGFM less costly and more effective |
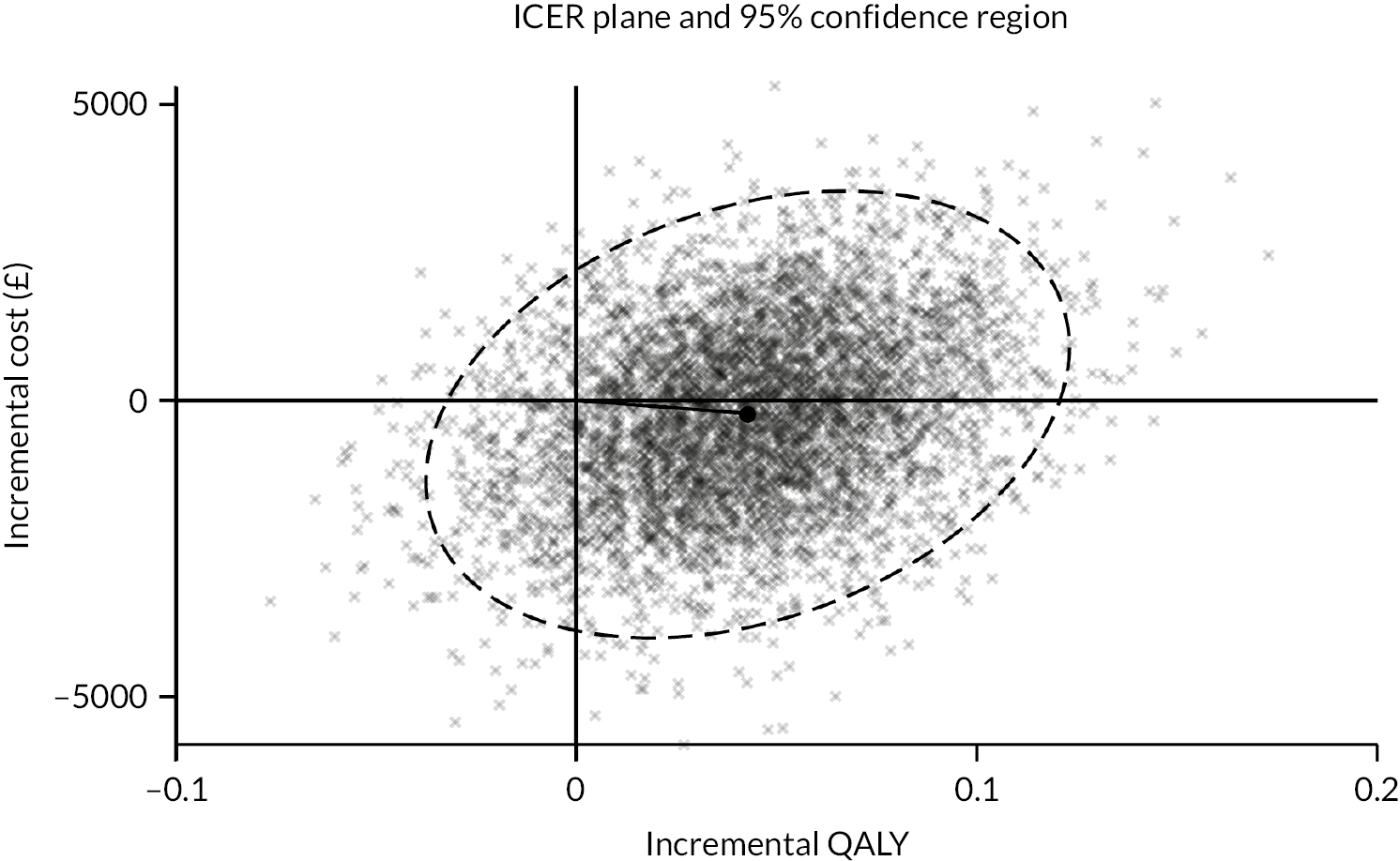
Figure 1 depicts the results of 5000 bootstrap replications plotted on the cost-effectiveness plane. Each point represents a pair of incremental cost and incremental effectiveness estimates for the comparison between BGFM and CFM. Approximately 90% of the simulated pairs are located in the east half of the plane, indicating that BGFM is likely to be more effective than CFM. Simulated estimates are fairly split between the north and south halves of the plane, with 44% being located in the north half and 56% in the south. Overall, 48% of the simulated estimates are located in the south-east quadrant, indicating that BGFM is less costly and more effective than CFM. Of the remaining simulated estimates, 42% are in the north-east quadrant, 8% in the south-west and 2% in the north-west.
FIGURE 1.
Cost-effectiveness plane depicting the distribution of simulated cost and QALY pairs.
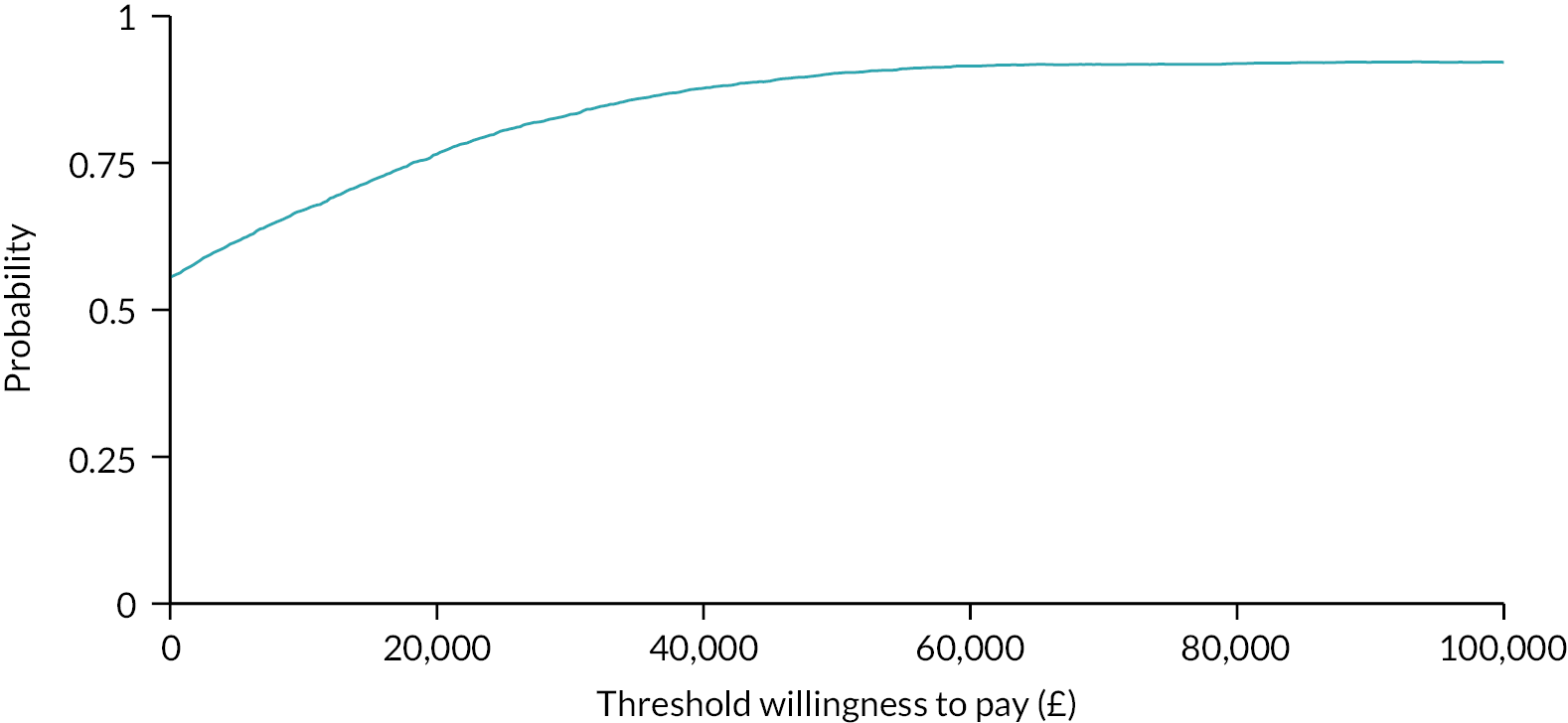
The probability that BGFM is cost-effective at different WTP thresholds, representing the (hypothetical) amount decision-makers may be willing to pay for an additional QALY, is shown in Figure 2. At £0, the probability that BGFM is cost-effective is 57%. At £20,000 per QALY, this rises to 76% and, at £30,000 per QALY, it further increases to 83% (see Figure 2).
FIGURE 2.
Cost-effectiveness acceptability curve showing the probability of BGFM being cost-effective at different values of WTP for a QALY.
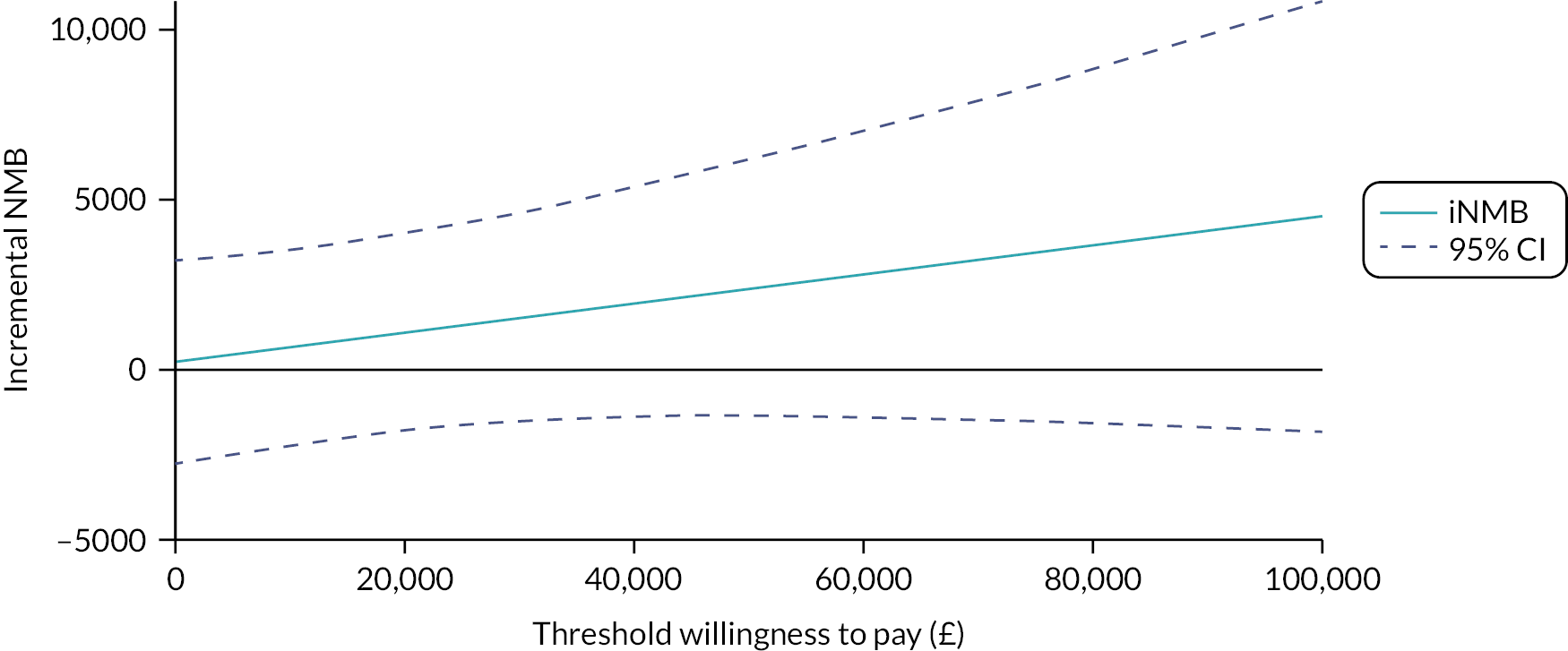
Incremental NMBs, which measure the difference in NMB between alternative interventions, are plotted in Figure 3. At the WTP threshold of £20,000 per additional QALY the incremental NMB is £1091, and it increases to £1519 at the WTP threshold of £30,000 per additional QALY. A positive incremental NMB indicates that, at a particular WTP threshold, BGFM is cost-effective.
FIGURE 3.
Incremental net monetary benefit (NMB) across different WTP values for a QALY. iNMB, incremental net monetary benefit.
Overall, the findings suggest that the BGFM is likely to be cost-effective, although the incremental cost savings and QALY gains were marginal.
Additional sensitivity analyses
Findings of sensitivity analyses can be found in Table 11, with more detailed information given in Appendix 8 (Tables 21 and 22) and Appendix 9 (Tables 23 and 24). Findings were largely similar to the base-case analysis, showing that, under different assumptions, BGFM is likely to be slightly less costly and modestly more effective than CFM. As an exception, a sensitivity analysis that took into account only 40 patients (9.15% of the sample) with complete data across all resource use categories and EQ-5D-5L measurements, showed an ICER of £16,780 per QALY, suggesting that the BGFM is more costly and more effective than CFM. Complete case analysis (with 90.85% missing data) was carried out for completeness. In this analysis, all cost categories apart from total outpatient visits cost and outpatient nursing home visits presented a small, non-significant increase of cost with BGFM group at 24 months (see Appendix 8, Table 21). However, findings of this analysis should be considered with caution, as they are calculated on the basis of a small number of complete data. Overall, all sensitivity analyses showed that use of bioimpedance is likely to be cost-effective at a cost-effectiveness threshold of £20,000–30,000 per QALY in the population studied.
| Analysesb | BGFM | CFM | Incremental cost (£) (95% CI) | Incremental QALY (95% CI) | ICER (BGFM vs. CFM) (£ per QALY) | ||
|---|---|---|---|---|---|---|---|
| Total costs (£) | Total QALYs | Total costs (£) | Total QALYs | ||||
| Sensitivity analysis 1 | 43,305.38 | 0.972 | 41,392.45 | 0.858 | 1912.93 (−14,298.08 to 18,123.95) | 0.114 (−0.230 to 0.458) | 16,780 |
| Sensitivity analysis 2 | 48,341.43 | 1.009 | 48,410.83 | 0.966 | −69.40 (−2373.58 to 2234.77) | 0.043 (−0.019 to 0.105) | BGFM less costly and more effective |
| Sensitivity analysis 3 | 51,809.74 | 0.993 | 51,864.37 | 0.985 | −54.63 (−2850.65 to 2741.40) | 0.008 (−0.072 to 0.091) | BGFM less costly and more effective |
| Sensitivity analysis 4 | 51,648.86 | 1.174 | 52,030.51 | 1.118 | −381.65 (−3318.97 to 2555.67) | 0.056 (−0.007 to 0.119) | BGFM less costly and more effective |
| Sensitivity analysis 5 | 51,648.86 | 1.139 | 52,030.51 | 1.101 | −381.65 (−3318.97 to 2555.67) | 0.038 (−0.011 to 0.088) | BGFM less costly and more effective |
| Sensitivity analysis 6 | 50,943 | 1.009 | 51,447.73 | 0.966 | −504.73 (−3444.15 to 2434.69) | 0.043 (−0.019 to 0.105) | BGFM less costly and more effective |
| Sensitivity analysis 7 | 52,355.80 | 1.009 | 52,794.28 | 0.966 | −438.48 (−3405.78 to 2528.82) | 0.043 (−0.019 to 0.105) | BGFM less costly and more effective |
Discussion
Patient and public involvement
The BISTRO trial was supported by patient and public involvement (PPI) from inception and design to delivery and dissemination of the results. It was co-led by a patient with lived experience of dialysis and expertise in research on devices who was a funded co-applicant, employed by NIHR Devices for Dignity. This co-applicant was a full member of the trial management group and led the patient advisory group that supported trial design, delivery and dissemination. He designed all the patient-facing communications. Deciding the optimal amount of fluid removal on dialysis is a complex clinical decision and measuring the primary outcome, RKF is a complex procedure. PPI was key in ensuring that the patient perspective was considered in all aspects of the trial, which resulted in excellent adherence to trial procedures.
Equality, diversity and inclusion
BioImpedance Spectroscopy to maintain Renal Output was an inclusive trial and the proportions of patients from minority groups are reported in the report of the primary findings21 where they are compared with contemporary data reported to the UK Renal Registry.
Principal findings
Compared with CFM, the addition of bioimpedance measurements was associated with slightly lower costs and slightly higher QALYs. Apart from an additional analysis that used only the small number of complete data sets available, which found costs to be marginally higher in BGFM, sensitivity analyses also lent support to this option being slightly less costly and more effective than CFM. It is observed that the 95% CIs for incremental costs and incremental QALYs were wide both in base-case and sensitivity analyses. This indicated uncertainty around the estimate of the incremental costs and QALYs. Although the CIs for the ICERs were wide, BGFM remains likely to be cost-effective, as the simulated estimates were largely in the south-east (48%) and north-east (42%) quadrants and the probability of BGFM being cost-effective at the WTP threshold of £20,000 per QALY is 76%.
Bioimpedance spectroscopy to maintain renal output effectiveness findings versus cost-effectiveness findings
The results need to be considered jointly with findings of the BISTRO effectiveness analysis. The latter found that the primary outcome (time to anuria) despite a hazard ratio of 0.74, did not differ significantly between the BGFM and the CFM (control) groups, with a 95% CI that spanned one. The primary outcome event rate was half that predicted prior to trial design and the difference between the normally hydrated weight and the target weight set by clinicians did not differ by group. Findings of the BISTRO economic evaluation, on the other hand, suggest that the intervention is likely to be cost-effective, being slightly less costly and more effective than CFM. In interpreting these results, it is useful to keep a number of considerations in mind. First, the economic evaluation seeks to answer a question about BGFM’s cost and effectiveness, which has a broader evaluative space. Given this, the primary outcome of the economic evaluation differs from that of the clinical study: it is a composite measure of differences in costs per difference in QALYs (with the latter being a broader measure, combining time in a health state and generic HRQoL), and hence there is always a possibility that findings about effectiveness will differ in direction from findings about cost-effectiveness. Second, the BISTRO RCT was not powered to estimate costs, QALYs or cost per QALY ratios with a desired precision. With this in mind, the question of interest is whether, despite appearing to be cost-effective, an intervention should be rejected for not reaching significance levels using common rules of inference. Such inference, it has been argued, is irrelevant for decision-making: if the aim is to maximise health benefits from a given budget, one should select the alternative that is shown to be the most cost-effective. 50 In this particular case, failing to do so would mean that an average cost-saving of approximately £380 per patient (which equates to as much as £9 million in total) and a slight increase of 0.04 QALYs would be forfeited.
Key strengths and limitations
This is the first study evaluating the cost-effectiveness of BGFM using patient-level data collected within a pragmatic, RCT. Strengths of the BISTRO trial, in which this economic evaluation is embedded, include a larger sample size compared with studies on the topic,5–7 a 24-month follow-up period and frequent collection of data useful for the economic analysis (resource use and HRQoL). Additionally, data collected as part of the BISTRO trial were complemented by detailed data on care provided in hospital from HES (e.g. inpatient admission, critical care admissions, outpatient appointments), which offered more granular and complete information. Given this, BISTRO provides detailed new evidence on a range of relevant cost components. A number of sensitivity analyses were carried out to generate results on the basis of different sources of data (i.e. CRFs, HES), cost categories and HRQoL instruments and value sets. Finally, methods used throughout the economic analysis, including the base-case and additional analyses, were in line with ‘good practice’ recommendations and requirements for the allocation of healthcare resources in the UK. 23,45,51
Despite these strengths, this study has certain limitations. First, BISTRO was not able to recruit to the initial target within the funding period, despite a funded extension and our attempt to compensate for this by extending the follow-up period. This would have resulted in a larger sample size for both the effectiveness and cost-effectiveness analyses. Second, the level of missing data was high for some resource use categories collected via CRFs, largely as a result of COVID disruptions and a significant dropout rate during the trial. Third, limited data on use of medications were collected through CRFs (at baseline only, to facilitate statistical adjustment), which precluded the inclusion of costs of medications (chiefly antihypertensives) provided outside the hospital setting (medications costs are incorporated in inpatient admission recorded in HES data). Additionally, costs beyond the NHS and PSS were limited to the opportunity cost of time devoted by unpaid carers. However, given no differential effectiveness of BGFM and CFM, it is not anticipated that medication use and broader costs accruing outside the NHS (e.g. time off work) attributable to BGFM and CFM would differ in a systematic way across the compared options. It is also worth noting that data completeness for the EQ-5D data was markedly reduced at 6 months and, as a result, the value of total QALYs over the 24-month period is influenced, at least to some extent, by the results of multiple imputation. Reassuringly, the direction and pattern of EQ-5D results generated by multiple imputation were in agreement with those of the complete case analysis based on available observations only (see Appendix 9, Table 23). It must be also noted that the results of this analysis are applicable to the patient population of interest in BISTRO (see Trial design and participants) and may not be generalisable to other populations.
Future research
Ongoing analysis as part of the trial is exploring the association between primary outcomes and longer-term survival. Should an important link be established, it would be informative to determine whether and how this might affect longer-term costs and benefits associated with BGFM.
Conclusions
The results of this economic evaluation demonstrate that BGFM results in modestly lower per-patient costs and a slightly higher number of QALYs than current fluid management. We anticipate that these findings offer further insights and useful evidence to support a decision about the wider use of bioimpedance spectroscopy in the particular patient population and setting.
Additional information
CRediT contribution statement
Mandana Zanganeh (https://orcid.org/0000-0003-3934-6044): Conceptualisation, Data curation (lead), Formal analysis, Investigation, Methodology, Project administration, Validation, Writing – original draft, Writing – reviewing and editing (supporting). John Belcher (https://orcid.org/0000-0001-6844-0932): Data curation (supporting), Investigation, Project administration, Writing – reviewing and editing (supporting). James Fotheringham (https://orcid.org/0000-0002-8980-2223): Investigation, Methodology, Writing – reviewing and editing (supporting). David Coyle (https://orcid.org/0000-0003-0441-2996): Funding acquisition, Investigation, Project administration, Writing – reviewing and editing (supporting). Elizabeth J Lindley (https://orcid.org/0000-0003-4957-4489): Funding acquisition, Investigation, Writing – reviewing and editing (supporting). David F Keane (https://orcid.org/0000-0002-4744-4106): Investigation, Writing – reviewing and editing (supporting). Fergus J Caskey (https://orcid.org/0000-0002-5199-3925): Funding acquisition, Investigation, Writing – reviewing and editing (supporting). Indranil Dasgupta (https://orcid.org/0000-0002-7448-2677): Funding acquisition, Investigation, Writing – reviewing and editing (supporting). Andrew Davenport (https://orcid.org/0000-0002-4467-6833): Funding acquisition, Investigation, Writing – reviewing and editing (supporting). Ken Farrington (https://orcid.org/0000-0001-8862-6056): Funding acquisition, Investigation, Writing – reviewing and editing (supporting). Sandip Mitra (https://orcid.org/0000-0003-0389-636X): Funding acquisition, Investigation, Writing – reviewing and editing (supporting). Paula Ormandy (https://orcid.org/0000-0002-6951-972X): Funding acquisition, Investigation, Writing – reviewing and editing (supporting). Martin Wilkie (https://orcid.org/0000-0003-1059-6453): Funding acquisition, Investigation, Project administration, Writing – reviewing and editing (supporting). Jamie H Macdonald (https://orcid.org/0000-0002-2375-146X): Funding acquisition, Investigation, Writing – reviewing and editing (supporting). Ivonne Solis-Trapala (https://orcid.org/0000-0002-1264-1396): Funding acquisition, Investigation, Visualisation, Writing – reviewing and editing (supporting). Julius Sim (https://orcid.org/0000-0002-1816-1676): Funding acquisition, Investigation, Project administration, Visualisation, Writing – reviewing and editing (supporting). Simon J Davies (https://orcid.org/0000-0001-5127-4755): Conceptualisation, Funding acquisition, Investigation, Project administration, Supervision, Visualisation, Writing – reviewing and editing (supporting). Lazaros Andronis (https://orcid.org/0000-0001-7998-7431): Conceptualisation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – reviewing and editing (lead).
Acknowledgements
Trial management
Keele Clinical Trials Unit – especially Nancy Fernandes Da Silva, Vicki Harper, Andrea Cherrington, Sarah Bathers and Louise Phillips-Darby
Patient advisory group
David Coyle (chair), Liam Howorth, Cassie Brzoza, Pauline Obi, Michael Winfrow
Trial steering committee
Richard Fluck (chair), James Wason, Elaine Davies, James Mason, Katherine Crompton, Nicola Heron, Melina Dritsaki, Brett Dowds, Liz Pryde
Independent data monitoring committee
Richard Haynes (chair), Simon Bond, Russell Roberts
Participating sites (principal investigators)
Julie Wessels, University Hospitals of North Midlands, Stoke-on-Trent, UK
Arfi Khwaja, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, UK
Indranil Dasgupta, University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK
Andrew Davenport, Royal Free Hampstead NHS Trust, London, UK
Enric Vilar, East and North Hertfordshire NHS Trust, Stevenage, UK
Sandip Mitra, Manchester University NHS Foundation Trust, Manchester, UK
Albert Power, North Bristol NHS Trust, Bristol, UK
David Meredith, Royal Devon and Exeter NHS Foundation Trust, Exeter, UK
Michael Schulz, Royal Liverpool and Broadgreen Hospitals NHS Trust, Liverpool, UK
Michael Bedford, East Kent Hospitals University NHS Trust, Canterbury, UK
Kieran McCafferty, Barts Health NHS Trust, London, UK
Punit Yadav, University Hospitals Birmingham NHS Foundation Trust, Birmingham, UK
Sally Pugh, Gloucestershire Hospital NHS Foundation Trust, Gloucester, UK
Stewart Lambie, NHS Highland, Raigmore, Scotland, UK
Beng So, Lancashire Teaching Hospitals, Preston, UK
James Burton, University Hospitals of Leicester NHS Trust, Leicester, UK
Janet Hegarty, Salford Royal NHS Foundation Trust, Salford, UK
Timothy Shipley, South Tees NHS Trust, Middlesbrough, UK
Neal Morgan, Southern Health and Social Care Trust, Newry, Northern Ireland, UK
Linda Bisset, Nottingham University Hospitals NHS Trust, Nottingham, UK
Thomas Connor, Oxford University Hospital NHS Trust, Oxford, UK
James Chess, Abertawe Bro Morgannwg University Health Board, Swansea, Wales, UK
Colin Jones, York Teaching Hospital NHS Foundation Trust, York, UK
Azra Nache, Aintree University Hospital NHS Foundation Trust, Aintree, UK
Rajan Patel, NHS Greater Glasgow and Clyde, Glasgow, Scotland, UK
Neill Duncan, Imperial College Healthcare NHS Trust, London, UK
Johann Nicholas, Shrewsbury and Telford Hospital NHS Trust, Shrewsbury, UK
Sharlene Greenwood, King’s College Hospital NHS Foundation Trust, London, UK
Kanwaljit Sandhu, The Royal Wolverhampton NHS Trust, Wolverhampton, UK
Subash Somolanka, Epsom and St Helier University Hospitals NHS Trust, UK
Kate Shiell, NHS Fife, Kirkaldy, Scotland, UK
Stephanie Bolton, Northern Health and Social Care Trust, Antrim, Northern Ireland, UK
Abdulfattah Alejmi, Betsi Cadwalader University Health Board, Bangor, Wales, UK
Data-sharing statement
The trial data are available to investigators under the conditions of a data-sharing agreement. This will include group- and individual-level fully anonymised data. Applications should be made to the corresponding author.
Ethics statement
The study had UK Integrated Research Ethics approval: 206213 (date of approval: 18 August 2016) and all participants gave their written consent.
Information governance statement
Keele University is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679. Under the Data Protection legislation, Keele University is the Data Controller, and you can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer here: https://www.keele.ac.uk/legalgovernancecompliance/legalandinformationcompliance/informationgovernance/ or by e-mail dpo@keele.ac.uk.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org10.3310/JYPR4287.
Primary conflicts of interest: None of the authors report conflicts of interest related to bioimpedance research, nor do they receive research funding from or have financial interests in Fresenius MC, manufacturers of the BCM device (which was selected for this trial via an independent panel overseen by Kidney Research UK). James Fotheringham is a member and vice-chair of the NICE Technology Assessment Board.
Patient data statement
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Department of Health and Social Care disclaimer
This publication presents independent research commissioned by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, MRC, NIHR Coordinating Centre, the Health Technology Assessment programme or the Department of Health and Social Care.
This article was published based on current knowledge at the time and date of publication. NIHR is committed to being inclusive and will continually monitor best practice and guidance in relation to terminology and language to ensure that we remain relevant to our stakeholders.
Trial registration
This trial is registered as ISCCTN Number: 11342007.
Funding
This article presents independent research funded by the National Institute for Health and Care Research (NIHR) Health Technology Assessment programme as award number 14/216/01 (NIHR136142).
This article reports on one component of the research award Cost-effectiveness of bioimpedance guided fluid management in patients undergoing haemodialysis: the BISTRO RCT. For more information about this research please view the award page (https://fundingawards.nihr.ac.uk/award/14/216/01)
About this article
The contractual start date for this research was in June 2016. This article began editorial review in August 2023 and was accepted for publication in March 2024. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The Health Technology Assessment editors and publisher have tried to ensure the accuracy of the authors’ article and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
This article was published based on current knowledge at the time and date of publication. NIHR is committed to being inclusive and will continually monitor best practice and guidance in relation to terminology and language to ensure that we remain relevant to our stakeholders.
Copyright
Copyright © 2024 Zanganeh et al. This work was produced by Zanganeh et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0. For attribution the title, original author(s), the publication source – NIHR Journals Library and the DOI of the publication must be cited.
List of abbreviations
- BCM
- body composition monitor
- BGFM
- bioimpedance-guided fluid management
- BISTRO
- BioImpedance Spectroscopy to maintain Renal Output
- CC
- Charlson comorbidity
- CEAC
- cost-effectiveness acceptability curve
- CFM
- current fluid management
- CRF
- case report form
- EQ-5D-5L
- EuroQol EQ-5 dimension-5 level
- EQ VAS
- EQ visual analogue scale
- GLM
- generalised linear regression models
- HEAP
- health economic analysis plan
- HES
- Hospital Episode Statistics
- HRG
- healthcare resource group
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ITT
- intention to treat
- NEL
- non-elective long stay
- NES
- non-elective short stay
- NHW
- normally hydrated weight
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- NMB
- net monetary benefits
- PPI
- patient and public involvement
- PSS
- Personal Social Services
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RKF
- residual kidney function
- SF-12
- Short-Form 12 Dimensions
- SF-6D
- Short-Form 6 Dimensions
- WTP
- willingness to pay
References
- Bargman JM, Thorpe KE, Churchill DN. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol: JASN 2001;12:2158-62.
- Termorshuizen F, Dekker FW, van Manen JG, Korevaar JC, Boeschoten EW, Krediet RT. NECOSAD Study Group . Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol: JASN 2004;15:1061-70.
- Obi Y, Rhee CM, Mathew AT, Shah G, Streja E, Brunelli SM, et al. Residual kidney function decline and mortality in incident hemodialysis patients. J Am Soc Nephrol: JASN 2016;27:3758-68.
- Merkus MP, Jager KJ, Dekker FW, Boeschoten EW, Stevens P, Krediet RT. Quality of life in patients on chronic dialysis: self-assessment 3 months after the start of treatment. Am J Kidney Dis: Off J Nat Kidney Found 1997;29:584-92.
- Schiffl H, Lang SM, Fischer R. Ultrapure dialysis fluid slows loss of residual renal function in new dialysis patients. Nephrol Dial Transp: Off Eur Dial Transp Assoc: Eur Renal Assoc 2002;17:1814-8.
- Lu W, Ren C, Han X, Yang X, Cao Y, Huang B. The protective effect of different dialysis types on residual renal function in patients with maintenance hemodialysis: a systematic review and meta-analysis. Medicine 2018;97.
- Lang SM, Bergner A, Töpfer M, Schiffl H. Preservation of residual renal function in dialysis patients: effects of dialysis-technique–related factors. Perit Dial Int: J Int Soc Perit Dial 2001;21:52-7.
- Dasgupta I, Farrington K, Davies SJ, Davenport A, Mitra S. UK National Survey of practice patterns of fluid volume management in haemodialysis patients: a need for evidence. Blood Purif 2016;41:324-31.
- Renal Registry . Chapter 3: Adults on Renal Replacement Therapy (RRT) in the UK at the End of 2019. UK Kidney Association. UK Renal Registry 23rd Annual Report 2020. https://ukkidney.org/sites/renal.org/files/23rd_UKRR_ANNUAL_REPORT_PREV_Ch3.pdf (accessed 3 June 2023).
- Kidney Research UK . Kidney Disease: A UK Public Health Emergency. The Health Economics of Kidney Disease to 2033 2023. www.kidneyresearchuk.org/wp-content/uploads/2023/06/Economics-of-Kidney-Disease-full-report_accessible.pdf (accessed 12 February 2023).
- Tabinor M, Elphick E, Dudson M, Kwok CS, Lambie M, Davies SJ. Bioimpedance-defined overhydration predicts survival in end stage kidney failure (ESKF): systematic review and subgroup meta-analysis. Sci Rep 2018;8:1-14.
- Dekker M, Marcelli D, Canaud B, Konings C, Leunissen K, Levin N, et al. Unraveling the relationship between mortality, hyponatremia, inflammation and malnutrition in hemodialysis patients: results from the international MONDO initiative. Eur J Clin Nutr 2016;70:779-84.
- Hecking M, Moissl U, Genser B, Rayner H, Dasgupta I, Stuard S, et al. Greater fluid overload and lower interdialytic weight gain are independently associated with mortality in a large international hemodialysis population. Nephrol Dial Transp: Off Eur Dial Transp Assoc: Eur Renal Assoc 2018;33:1832-42.
- Zoccali C, Moissl U, Chazot C, Mallamaci F, Tripepi G, Arkossy O, et al. Chronic fluid overload and mortality in ESRD. J Am Soc Nephrol: JASN 2017;28:2491-7.
- National Institute for Health and Care Research . BioImpedance Spectroscopy to Maintain Renal Output: The BISTRO Trial 2021. https://fundingawards.nihr.ac.uk/award/14/216/01 (accessed 12 March 2024).
- National Institute for Health and Care Excellence . Quality Standard QS5. Chronic Kidney Disease in Adults 2017. www.nice.org.uk/guidance/qs5 (accessed 16 January 2023).
- National Institute for Health and Care Excellence . Renal Replacement Therapy Services for Adults. NICE Quality Standard 72 2018. www.nice.org.uk/guidance/qs72 (accessed 25 January 2023).
- Greene J, Hibbard JH. Why does patient activation matter? An examination of the relationships between patient activation and health-related outcomes. J Gen Intern Med 2012;27:520-6.
- Hibbard J, Gilburt H. Supporting People to Manage Their Health: An Introduction to Patient Activation. London: King’s Fund; 2014.
- National Institute for Health and Care Excellence . Diagnostics Guidance DG29. Multiple Frequency Bioimpedance Devices to Guide Fluid Management in People With Chronic Kidney Disease Having Dialysis 2017. www.nice.org.uk/guidance/dg29 (accessed 23 May 2024).
- Davies SJ, Coyle D, Lindley EJ, Keane D, Belcher J, Caskey FJ, et al. Bio-impedance spectroscopy added to a fluid management protocol does not improve preservation of residual kidney function in incident hemodialysis patients in a randomized controlled trial. Kidney Int 2023;104:587-98.
- Davies SJ, Caskey FJ, Coyle D, Lindley E, Macdonald J, Mitra S, et al. Rationale and design of BISTRO: a randomized controlled trial to determine whether bioimpedance spectroscopy-guided fluid management maintains residual kidney function in incident haemodialysis patients. BMC Nephrol 2017;18:1-11.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013 2013. www.nice.org.uk/process/pmg9/chapter/foreword (accessed 5 December 2022).
- Scotland G, Cruickshank M, Jacobsen E, Cooper D, Fraser C, Shimonovich M, et al. Multiple-frequency bioimpedance devices for fluid management in people with chronic kidney disease receiving dialysis: a systematic review and economic evaluation. Health Technol Assess 2018;22:1-138.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36.
- Ware JE Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;220.
- Devlin NJ, Shah KK, Feng Y, Mulhern B, van Hout B. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ 2018;27:7-22.
- Sampson C. Bad reasons not to use the EQ-5D-5L. Aheblog: The Academic Health Economists’ Blog 2018. https://aheblog.com/2018/03/23/bad-reasons-not-to-use-the-eq-5d-5l (accessed 11 October 2022).
- NICE . Position Statement on Use of the EQ-5D-5L Value Set for England (updated October 2019) n.d. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed 23 May 2022).
- Hernandez Alava M, Pudney S, Wailoo AJP. Estimating the relationship between EQ-5D-5L and EQ-5D-3L: results from a UK population study. PharmacoEcon 2023;41:199-207.
- Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care 2004;42:851-9.
- Billingham L, Abrams KR, Jones DR. Methods for the analysis of quality-of-life and survival data in health technology assessment. Health Technol Assess 1999;3:1-152.
- Personal Social Services Research Unit . Client Service Receipt Inventory (CSRI) 2019. www.pssru.ac.uk/csri/client-service-receipt-inventory (accessed July 2022).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2020, Personal Social Services Research Unit (PSSRU). Canterbury: University of Kent; 2020.
- NHS England . National Schedule of NHS Costs: All NHS Trusts and NHS Foundation Trusts: HRG Data 2019 20 n.d. www.england.nhs.uk/publication/2019-20-national-cost-collection-data-publication (accessed 27 March 2022).
- NHS England . HRG4 + 2020/21/National/Costs/Grouper n.d. https://digital.nhs.uk/services/national-casemix-office/downloads-groupers-and-tools/hrg4-2020-21-national-costs-grouper (accessed 21 April 2022).
- NHS England . NHS Data Model and Dictionary: Point of Delivery Code (Patient Level Information Costing) 2021 n.d. https://datadictionary.nhs.uk/data_elements/point_of_delivery_code__patient_level_information_costing_.html (accessed 5 February 2023).
- Department of Health . Reference Costs Guidance 2015–16 2016. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/497127/Reference_costs_guidance_2015-16.pdf (accessed 8 January 2023).
- Posnett J, Jan S. Indirect cost in economic evaluation: the opportunity cost of unpaid inputs. Health Econ 1996;5:13-2.
- Rubin DB. Statistical matching using file concatenation with adjusted weights and multiple imputations. J Bus Econ Stat 1986;4:87-94.
- Faria R, Gomes M, Epstein D, White I. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEcon 2014;32:1157-70.
- Ramsey S, Willke R, Briggs A, Brown R, Buxton M, Chawla A, et al. Good research practices for cost-effectiveness analysis alongside clinical trials: the ISPOR RCT-CEA task force report. Value Health: J Int Soc Pharmacoecon Outc Res 2005;8:521-33.
- Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med 2000;19:3219-36.
- Lee Y, Nelder JA. Hierarchical generalised linear models: a synthesis of generalised linear models, random‐effect models and structured dispersions. Biometrika 2001;88:987-1006.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Glick HA, Jalpa DA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2007.
- Barton GR, Briggs AH, Fenwick EA. Optimal cost-effectiveness decisions: the role of the cost-effectiveness acceptability curve (CEAC), the cost-effectiveness acceptability frontier (CEAF), and the expected value of perfection information (EVPI). Value Health 2008;11:886-97.
- Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. ISPOR Health Economic Evaluation Publication Guidelines – CHEERS Good Reporting Practices Task Force . Consolidated Health Economic Evaluation Reporting Standards (CHEERS): explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health 2013;16:231-50.
- Wright C, Sim J. Intention-to-treat approach to data from randomized controlled trials: a sensitivity analysis. J Clin Epidemiol 2003;56:833-42.
- Claxton KJJ. The irrelevance of inference: a decision-making approach to the stochastic evaluation of health care technologies. J Health Econ 1999;18:341-64.
- Ramsey SD, Willke RJ, Glick H, Reed SD, Augustovski F, Jonsson B, et al. Cost-effectiveness analysis alongside clinical trials II – an ISPOR Good Research Practices Task Force report. Value Health 2015;18:161-72.
- UK Kidney Association . Renal Units n.d. https://units.renal.org/index.pl?qp=1 (accessed 22 May 2022).
Appendix 1
| Category | Baseline | M 1 | M 2 | M 3 | M 6 | M 9 | M12 | M 15 | M 18 | M 21 | M 24 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HDa | x | x | x | x | x | x | x | x | x | x | x |
| BIb | x | x | x | x | x | x | x | x | x | x | x |
| Hospital and nursing home inpatient electivec | x | x | x | x | x | x | x | x | |||
| Inpatient SAEsd | xe | ||||||||||
| Hospital and nursing home outpatient appointmentsf | x | x | x | x | x | x | x | x | |||
| Primary and community careg | x | x | x | x | x | x | x | x | |||
| Patients’ family costsh | x | x | x | x | x | x | x | x | |||
| Quality of life | x | x | x | x | x | x | x | x | x | ||
Appendix 2
Calculation of bioimpedance spectroscopy cost. Detail information of cost of BCM machine, related accessories, training and staff time.
For the purposes of this economic evaluation, it is necessary to determine the per-session cost of bioimpedance. The key steps in calculating this are explained below.
-
Average number of bioimpedance sessions.
The majority of patients had one measurement every 3 months (four sessions per year).
-
Determine the average number of patients served by bioimpedance device.
Also, for calculating bioimpedance costs per session, it was necessary to estimate the average number of patients per haemodialysis unit on haemodialysis. Looking at the latest UK renal annual report,9 the total adult patients receiving kidney replacement therapy for end-stage kidney disease was 68,111. Of these patients, 35.8% (n = 24,383) were on haemodialysis. 9 Therefore, dividing the total number of patients on haemodialysis in the UK (24,383) by total number of haemodialysis units in the UK (274) gives an average of 89 patients per unit were on haemodialysis. 52 This number, which was considered in our calculations, was very similar to the average 70–80 patients per unit of the BISTRO trial which we received from Leeds Teaching Hospital NHS Trust.
-
Costs of bioimpedance device and related equipment.
The costs of the bioimpedance device (BCM) and related accessories (2020), which were available through Akeso NHS Supply Chain, were received from the Fresenius Medical Care (UK) Ltd.
The cost of the BCM devices (the BCM machine plus first electrode cable, card reader and carry case was £5177.36). This cost was annuitised over effective lifetime of BCM of 10 years (£622.53) using an annual depreciation rate of 3.5%. 23,24 The effective lifetime of BCM was decided by consulting with Leeds Teaching Hospital NHS Trust. Estimated costs of BCM equipment maintenance were provided at £666.36 for annual maintenance, including spare electrode cable and batteries by Fresenius Medical Care (UK) Ltd. In terms of consumables, four electrodes were used per session and the price of each electrode was £0.75; each card was usable for 5 years and the price of each card was £6.28.
-
Cost of training.
Training resources were provided free of charge by the manufacturer, but the cost of NHS personnel’s time is incurred by the NHS. The unit costs of staff involved in bioimpedance training (as well as measurement and interpretation) were taken from the Unit Costs of Health and Social Care 2020. 34 Total training costs (opportunity cost of time) for a typical haemodialysis centre were estimated based on the number of staff (two hospital nurses, band 6, and two clinical support workers, band 4) trained, multiplied by their costs per contract hour (£50 and £31, respectively) and the number of hours of training attended. They attended training 2 hours initially and then 15 minutes every year over 10 years. The total training investment was spread over 10 years, and the equivalent annual cost was divided by the number of patients in the centre (n = 89) to give a cost per patient per year. For each centre the total training costs were estimated to be £672.30. Divided by 10 years, this comes to £67.23 and £0.76 per patient per year.
-
Cost of bioimpedance testing and interpretation.
Staff costs associated with the time required to conduct each test were estimated based on 10 minutes of direct patient contact with a band 6 hospital nurse or band 4 clinical support worker. Therefore, an average of these two was considered (£11.75). This was further multiplied by four to estimate the staff costs per patient per year (£47). The added hospital nurse (band 6) and consultant time required to interpret the findings of each bioimpedance test as part of clinical management were assumed to be 5 minutes and 1 minute, respectively, giving £6.14 per session and £24.56 per year.
| Description | Unit cost (£) | Source |
|---|---|---|
| Body composition monitora | 4500 | Fresenius Medical Care (UK) Ltd |
| BCM skin electrodes – pack of 40b | 30.00 | |
| BCM patient card – pack of 10c | 62.80 | |
| BCM electrode cabled | 156.98 | |
| BCM card readere | 40.38 | |
| BCM carry casef | 480.00 | |
| Purchase price of BCM + first electrode cable + card reader + carry case | 5177.36 | Fresenius Medical Care (UK) Ltd |
| Annual maintenance cost of BCM | 666.36 | Fresenius Medical Care (UK) Ltd |
| Cost per electrode | 0.75 | Fresenius Medical Care (UK) Ltd |
| Cost per card | 6.28 | Fresenius Medical Care (UK) Ltd |
| Description | Unit cost per patient year (£) | Unit cost per session (£) |
|---|---|---|
| BCM equivalent annual cost | 6.99 | 1.75 |
| Annual maintenance cost | 7.49 | 1.87 |
| Cost of electrodes | 12.00 | 3.00 |
| Card estimated annual cost of purchase | 1.25 | 0.31 |
| Training cost (opportunity cost of time) | 0.76 | 0.19 |
| Staff costs associated with the time required to conduct/measure | 47.00 | 11.75 |
| Staff costs associated with the time required to interpret | 24.56 | 6.14 |
| Total | 100.05 | 25.10 |
Appendix 3
| Body system | HRG code and currency description | Scheduled admissions unit cost (£)35 | ||
|---|---|---|---|---|
| Elective | Day case | Regular day/night | ||
| Blood and lymphatic disorders | Sickle-cell anaemia with crisis, with CC score 2–5 | 3243 | 383 | |
| Sickle-cell anaemia with crisis, with CC score 6 + | 5301 | 321 | ||
| Plasma cell disorders with CC score 2–4 | 341 | |||
| Plasma cell disorders with CC score 5–7 | 343 | |||
| Other red blood cell disorders with CC score 2–5 | 369 | |||
| Other red blood cell disorders with CC score 6–9 | 384 | |||
| Other red blood cell disorders with CC score 10–13 | 356 | |||
| Single plasma exchange or other intravenous blood transfusion, 19 years and over | 584 | |||
| Cardiac disorders | Arrhythmia or conduction disorders, with CC score 4–6 | 1173 | ||
| Arrhythmia or conduction disorders, with CC score 13 + | 1087 | |||
| Actual or suspected myocardial infarction, with CC score 4–6 | 1625 | |||
| Actual or suspected myocardial infarction, with CC score 7–9 | 1760 | |||
| Cardiac valve disorders with CC score 5–8 | 2962 | |||
| Explanation or attention to, cardiac pacemaker or cardioverter defibrillator, with CC score 6 + | 1906 | |||
| Implantation of dual-chamber pacemaker with CC score 6–8 | 2097 | |||
| Implantation of electrocardiography loop recorder with CC score 0–2 | 1508 | |||
| Implantation of electrocardiography loop recorder with CC score 3 + | 1646 | |||
| Other acquired cardiac conditions with CC score 0–2 | 540 | 207 | ||
| Other acquired cardiac conditions with CC score 3–5 | 2100 | 523 | 234 | |
| Other acquired cardiac conditions with CC score 6–8 | 2539 | 576 | ||
| Other acquired cardiac conditions with CC score 9–12 | 3023 | 673 | ||
| Other acquired cardiac conditions with CC score 13 + | 4805 | 802 | ||
| Complex cardiac catheterisation with CC score 4–6 | 1525 | |||
| Transient ischaemic attack with CC score 0–4 | 328 | |||
| Complex cardiac catheterisation with CC score 7 + | 5325 | 1362 | ||
| Complex percutaneous transluminal coronary angioplasty with CC score 4–7 | 4101 | |||
| Standard cardiac catheterisation with CC score 2–3 | 1174 | |||
| Standard cardiac catheterisation with CC score 4–6 | 1227 | |||
| Standard cardiac catheterisation with CC score 7–9 | 1292 | |||
| Standard cardiac catheterisation with CC score 13 + | 10,074 | |||
| Standard, other operations on heart or pericardium, with CC score 10 + | 7196 | |||
| Standard percutaneous transluminal ablation of heart with CC score 3 + | 2138 | |||
| Standard, coronary artery bypass graft with single heart valve replacement or repair, with CC score 11 + | 18,309 | |||
| Complex echocardiogram | 652 | |||
| Percutaneous transluminal angioplasty of single blood vessel with CC score 0–2 | 1768 | |||
| Percutaneous transluminal angioplasty of single blood vessel with CC score 3–5 | 2280 | 1280 | ||
| Percutaneous transluminal angioplasty with insertion of single metal stent into peripheral blood vessel, with CC score 6 + | 4682 | |||
| Complex repair of aortic root with CC score 7 + | 21,785 | |||
| Dermatology | Skin disorders without interventions, with CC score 0–1 | 1009 | 360 | 279 |
| Minor skin procedures 19 years and over | 672 | |||
| Gastrointestinal disorders | Diagnostic colonoscopy with biopsy, 19 years and over | 692 | ||
| Diagnostic flexible sigmoidoscopy, 19 years and over | 417 | |||
| Diagnostic endoscopic upper gastrointestinal tract procedures with biopsy, 19 years and over | 551 | 561 | ||
| Non-malignant gastrointestinal tract disorders without interventions, with CC score 3–5 | 1809 | |||
| Non-malignant gastrointestinal tract disorders with single intervention, with CC score 5–8 | 5295 | |||
| Malignant gastrointestinal tract disorders with multiple interventions, with CC score 3–6 | 6595 | |||
| Malignant gastrointestinal tract disorders without interventions, with CC score 3–4 | 342 | |||
| Diagnostic flexible sigmoidoscopy 19 years and over | 417 | |||
| Very major small intestine procedures, 19 years and over, with CC score 5–7 | 10,436 | |||
| Major therapeutic endoscopic, upper or lower gastrointestinal tract procedures, 19 years and over, with CC score 3 + | 4073 | |||
| Therapeutic colonoscopy, 19 years and over | 703 | |||
| Complex therapeutic endoscopic, upper or lower gastrointestinal tract procedures | 848 | |||
| Radiological insertion of gastrostomy tube, 19 years and over | 700 | |||
| Complex, oesophageal, stomach or duodenum procedures, 19 years and over, with CC score 4 + | 13,539 | |||
| Intermediate anal procedures, 19 years and over, with CC score 3 + | 1503 | |||
| Inflammatory bowel disease without interventions, with CC score 5 + | 3793 | |||
| Gastrointestinal bleed with multiple interventions, with CC score 0–4 | 2811 | |||
| General disorders | Special screening, examinations or other genetic disorders | 533 | ||
| Abnormal findings without diagnosis without interventions with CC score 1 + | 223 | |||
| Diagnostic flexible cystoscopy, 19 years and over | 488 | |||
| Major general abdominal procedures, 19 years and over, with CC score 6–9 | 1520 | |||
| Major general abdominal procedures, 19 years and over, with CC score 10 + | 10,580 | |||
| Open operations, on other or unspecified blood vessels, with CC score 2 + | 7139 | |||
| Abdominal hernia procedures, 19 years and over, with CC score 4 + | 6367 | |||
| Inguinal, umbilical or femoral hernia procedures, 19 years and over, with CC score 3–5 | 3304 | |||
| Unspecified chest pain with CC score 5–10 | 397 | |||
| Intermediate therapeutic general abdominal procedures, 19 years and over, with CC score 1–2 | 4241 | |||
| Gynaecology | Diagnostic hysteroscopy | 1083 | ||
| Diagnostic hysteroscopy with biopsy and implantation of intrauterine device | 1208 | |||
| Hepatobiliary | Appendicectomy procedures, 19 years and over, with CC score 3–4 | 5133 | ||
| Endoscopic ultrasound examination of hepatobiliary or pancreatic duct | 1379 | |||
| Percutaneous punch biopsy of lesion of liver, 19 years and over | 713 | |||
| Major therapeutic endoscopic retrograde cholangiopancreatography with CC score 5 + | 1178 | |||
| Non-malignant, hepatobiliary or pancreatic disorders, without interventions, with CC score 0–1 | 1165 | |||
| Non-malignant, hepatobiliary or pancreatic disorders, without interventions, with CC score 8 + | 3037 | |||
| Injury/poison/ procedure (vascular access) |
Attention to arteriovenous fistula, graft or shunt | 2053 | 1217 | 916 |
| Attention to central venous catheter, 19 years and over | 283 | |||
| Coagulation defect with CC score 5 + | 637 | |||
| Open arteriovenous fistula, graft or shunt procedures | 3290 | 2300 | 1152 | |
| Procedure not carried out, for other or unspecified reasons | 640 | 345 | 428 | |
| Procedure not carried out, for medical or patient reasons | 735 | |||
| Removal of central venous catheter, 19 years and over | 423 | |||
| Major endoscopic, kidney or ureter procedures, 19 years and over, with CC score 0–2 | 3523 | |||
| Intermediate endoscopic ureter procedures 19 years and over | 1246 | |||
| Insertion of tunnelled central venous catheter, 19 years and over | 1405 | 889 | ||
| Insertion of non-tunnelled central venous catheter, 19 years and over | 1533 | 800 | ||
| Peripheral insertion of central venous catheter, 19 years and over | 524 | |||
| Thrombocytopenia with CC score 5–7 | 325 | |||
| Thrombocytopenia with CC score 8 + | 351 | |||
| Infections and infestations | Infections of bones or joints, with CC score 0–1 | 3704 | ||
| Lobar, atypical or viral pneumonia, without interventions, with CC score 4–6 | 2206 | |||
| Sepsis with single intervention, with CC score 0–4 | 5878 | |||
| Sepsis with multiple interventions, with CC score 9 + | 16,817 | |||
| Infections or other complications of procedures, without interventions, with CC score 0–1 | 1588 | |||
| Infections or other complications of procedures, with single intervention, with CC score 0–1 | 3848 | |||
| Metabolism and nutrition disorders | Nutritional disorders without interventions, with CC score 2–5 | 2317 | ||
| Parathyroid procedures with CC score 2 + | 4035 | |||
| Musculoskeletal and connective tissue disorders or fractures | Diagnostic bone marrow extraction | 423 | ||
| Single, amputation stump or partial foot amputation procedure, for diabetes or arterial disease, with CC score 0–4 | 3772 | |||
| Major shoulder procedures for non-trauma with CC score 2–3 | 2937 | |||
| Minimal foot procedures | 768 | |||
| Denervation or injection around spinal facet, for pain management | 905 | |||
| Degenerative spinal conditions without interventions, with CC score 3–5 | 1533 | |||
| Single, amputation stump or partial foot amputation procedure, for diabetes or arterial disease, with CC score 5–7 | 1674 | |||
| Single, amputation stump or partial foot amputation procedure, for diabetes or arterial disease, with CC score 8 + | 5448 | |||
| Single, amputation stump or partial foot amputation procedure, for diabetes or arterial disease, with imaging intervention, with CC score 8 + | 11,413 | |||
| Epidural under image control for pain management | 839 | |||
| Extraction of multiple teeth, 19 years and over | 979 | |||
| Multiple trauma with diagnosis score 24–32, with intervention score 0 | 4849 | |||
| Very major hip procedures for non-trauma with CC score 6–7 | 8250 | |||
| Very major knee procedures for non-trauma with CC score 6–7 | 7921 | |||
| Neoplasms (other) |
Other or unspecified neoplasm, without interventions, with CC score 2 + | 3659 | ||
| Nervous system disorders | Muscular balance cranial or peripheral nerve disorders epilepsy or head injury with CC score 9–11 | 4076 | ||
| Cerebrovascular accident, nervous system infections or encephalopathy, with CC score 5–7 | 3066 | |||
| Stroke with CC score 0–3 | 2475 | |||
| Syncope or collapse, with CC score 0–3 | 508 | |||
| Syncope or collapse, with CC score 4–6 | 940 | |||
| Syncope or collapse, with CC score 7–9 | 1190 | |||
| Ophthalmology | Non-surgical ophthalmology without interventions, with CC score 0–1 | 433 | ||
| Intermediate, cataract or lens procedures, with CC score 0–1 | 1056 | |||
| Intermediate vitreous retinal procedures, 19 years and over, with CC score 0–1 | 267 | |||
| Intermediate vitreous retinal procedures, 19 years and over, with CC score 2 + | 275 | |||
| Phacoemulsification cataract extraction and lens implant, with CC score 0–1 | 967 | |||
| Phacoemulsification cataract extraction and lens implant, with CC score 2–3 | 1008 | |||
| Phacoemulsification cataract extraction and lens implant, with CC score 4 + | 1048 | |||
| Complex vitreous retinal procedures, 19 years and over, with CC score 2 + | 3082 | 1606 | ||
| Complex, cataract or lens procedures, with CC score 0–1 | 1583 | |||
| Very complex vitreous retinal procedures, 19 years and over, with CC score 0–1 | 2385 | |||
| Very complex vitreous retinal procedures, 19 years and over, with CC score 2 + | 3894 | |||
| Very major, cataract or lens procedures, CC score 2 + | 1192 | |||
| Very major, glaucoma or iris procedures, CC score 0–1 | 2134 | |||
| Very major vitreous retinal procedures, 19 years and over, with CC score 2 + | 1766 | |||
| Renal and urinary disorders | Fluid or electrolyte disorders, without interventions, with CC score 0–1 | 778 | ||
| Fluid or electrolyte disorders, without interventions, with CC score 2–3 | 859 | |||
| Fluid or electrolyte disorders, without interventions, with CC score 10 + | 2713 | |||
| Kidney transplant 19 years and over from cadaver heart-beating donor | 14,411 | |||
| Kidney transplant, 19 years and over, from cadaver non-heart-beating donor | 13,461 | |||
| Kidney transplant, 19 years and over, from live donor | 12,787 | |||
| General renal disorders without interventions, with CC score 3–5 | 1600 | |||
| Minor or intermediate, urethra procedures, 19 years and over | 1064 | |||
| Intermediate endoscopic ureter procedures, 19 years and over | 2112 | 1246 | ||
| Ureteric or bladder disorders, without interventions, with CC score 0–1 | 478 | |||
| Chronic kidney disease without interventions CC score 0–2 | 1679 | 476 | 357 | |
| Chronic kidney disease without interventions, CC score 3–4 | 2083 | |||
| Chronic kidney disease without interventions, CC score 5–7 | 2129 | 565 | ||
| Chronic kidney disease without interventions, CC score 8–10 | 2813 | |||
| Chronic kidney disease with interventions, CC score 0–2 | 4210 | |||
| Chronic kidney disease with interventions CC score 3–5 | 4922 | 1793 | ||
| Chronic kidney disease with interventions, CC score 6 + | 7466 | |||
| Renal replacement peritoneal dialysis associated procedures | 2112 | 1057 | ||
| Transurethral prostate resection procedures with CC score 0–2 | 3439 | |||
| Transurethral prostate resection procedures with CC score 6 + | 4030 | |||
| Unspecified haematuria with interventions, with CC score 3–6 | 2766 | |||
| Major, open or percutaneous, kidney or ureter procedures, 19 years and over, with CC score 2–3 | 7891 | |||
| Major, open or percutaneous, kidney or ureter procedures, 19 years and over, with CC score 4–6 | 8777 | |||
| Major endoscopic bladder procedures with CC score 7 + | 3885 | |||
| Respiratory, thoracic and mediastinal disorders | Cystic fibrosis with CC | 600 | 352 | |
| Fibrosis or pneumoconiosis, without interventions, with CC score 0–3 | 2034 | |||
| Respiratory sleep study | 809 | |||
| Pulmonary oedema without interventions, with CC score 0–5 | 675 | 140 | ||
| Pleural effusion without interventions, CC score 0–5 | 297 | |||
| Pleural effusion with multiple interventions, score 6–10 | 5120 | |||
| Intermediate thoracic procedures, 19 years and over, with CC score 3–5 | 3921 | 1135 | ||
| Intermediate thoracic procedures, 19 years and over, with CC score 6 + | 1126 | |||
| Endobronchial ultrasound examination of mediastinum | 928 | |||
| Diagnostic bronchoscopy, 19 years and over | 847 | |||
| Vascular disorders | Hypertension | 341 | ||
| Peripheral vascular disorders with CC score 2–4 | 2105 | 523 | ||
| Peripheral vascular disorders with CC score 8–10 | 2619 | 572 | ||
| Single open procedure, on blood vessel or upper limb with CC score 0–4 | 2022 | |||
| Percutaneous transluminal arteriography, of intracranial or extracranial blood vessel | 1535 | |||
| Body system | HRG code and currency description | Unscheduled admissions unit cost (£)35 | |
|---|---|---|---|
| Non-elective short stay | Non-elective long stay | ||
| Blood and lymphatic disorders | Other red blood cell disorders with CC score 6–9 | 637 | – |
| Sickle-cell anaemia with crisis, with CC score 6 + | 1451 | 4831 | |
| Sickle-cell anaemia without crisis | 810 | 4958 | |
| Cardiac disorders | Cardiac arrest with CC score 0–4 | – | 1986 |
| Cardiac arrest with CC score 9 + | – | 3225 | |
| Cardiac valve disorders with CC score 9–12 | 1122 | – | |
| Complex percutaneous transluminal coronary angioplasty with CC score 4–7 | 2780 | – | |
| Complex percutaneous transluminal coronary angioplasty with CC score 8–11 | 3186 | – | |
| Chest pain with CC score 5–10 | 402 | 1420 | |
| Chest pain with CC score 11 + | 509 | – | |
| Heart failure or shock, with CC score 4–7 | 605 | – | |
| Heart failure or shock, with CC score 8–10 | 509 | – | |
| Heart failure or shock with CC score 11–13 | – | 3146 | |
| Actual or suspected myocardial infarction, with CC score 4–6 | – | 1967 | |
| Actual or suspected myocardial infarction, with CC score 10–12 | 831 | – | |
| Actual or suspected myocardial infarction, with CC score 13 + | 1159 | 3450 | |
| Angina with CC score 8–11 | 508 | 1739 | |
| Angina with CC score 12 + | 619 | 2223 | |
| Arrhythmia or conduction disorders, with CC score 4–6 | 509 | 1808 | |
| Arrhythmia or conduction disorders, with CC score 7–9 | 583 | 2076 | |
| Arrhythmia or conduction disorders, with CC score 10–12 | 679 | 2538 | |
| Arrhythmia or conduction disorders, with CC score 13 + | 1051 | 3387 | |
| Other acquired cardiac conditions with CC score 0–2 | – | 1982 | |
| Other acquired cardiac conditions with CC score 3–5 | 733 | 2501 | |
| Other acquired cardiac conditions with CC score 6–8 | 858 | 2949 | |
| Other acquired cardiac conditions with CC score 9–12 | 982 | 3309 | |
| Other acquired cardiac conditions with CC score 13 + | 1523 | 4445 | |
| Percutaneous transluminal angioplasty of single blood vessel, CC score 0–2 | – | 3970 | |
| Percutaneous transluminal angioplasty of single blood vessel, CC score 6–8 | – | 2763 | |
| Percutaneous transluminal angioplasty of single blood vessel, CC score 9 + | – | 9184 | |
| Percutaneous transluminal angioplasty, including stenting, of intracranial or extracranial blood vessel | – | 9371 | |
| Implantation of single-chamber pacemaker with CC score 6–8 | 2644 | – | |
| Implantation of single-chamber pacemaker with CC score 9–11 | – | 5626 | |
| Implantation of dual-chamber pacemaker with CC score 6–8 | – | 4707 | |
| Implantation of biventricular pacemaker with CC score 6 + | – | 8368 | |
| Standard cardiac catheterisation with CC score 4–6 | 1673 | 3747 | |
| Standard cardiac catheterisation with CC score 13 + | – | 7959 | |
| Standard, single heart valve replacement or repair, with CC score 0–5 | – | 12,424 | |
| Standard percutaneous transluminal coronary angioplasty with CC score 12 + | – | 6520 | |
| Transient ischaemic attack with CC score 5–7 | 565 | – | |
| Transient ischaemic attack with CC score 11 + | 732 | 2913 | |
| Very complex percutaneous transluminal coronary angioplasty, CC score 4–7 | – | 6045 | |
| Dermatology | Minor skin procedures 19 years and over | 632 | – |
| Skin disorders without interventions, with CC score 2–5 | 474 | 2005 | |
| Skin disorders without interventions, with CC score 6–9 | 634 | – | |
| Skin disorders without interventions, with CC score 14–18 | 1016 | 3707 | |
| Skin disorders without interventions, with CC score 19 + | – | 4656 | |
| Skin disorders with interventions, with CC score 4–7 | – | 4058 | |
| Skin disorders with interventions, with CC score 8–11 | – | 5260 | |
| ENT | minor treatment of epistaxis, 19 years and over | – | 1141 |
| Non-malignant, ear, nose, mouth, throat or neck disorders, without interventions, with CC score 5 + | 556 | 2342 | |
| Non-malignant, ear, nose, mouth, throat or neck disorders, with interventions, with CC score 5 + | 2190 | – | |
| Gastrointestinal disorders | Abdominal pain without interventions | 415 | – |
| Diagnostic colonoscopy, 19 years and over | 622 | – | |
| Endoscopic insertion of luminal stent into gastrointestinal tract, CC score 4–6 | – | 5560 | |
| Intermediate therapeutic endoscopic retrograde cholangiopancreatography with CC score 6 + | – | 5902 | |
| Major therapeutic endoscopic retrograde cholangiopancreatography CC score 5 + | 6775 | – | |
| Gastrointestinal bleed without interventions, with CC score 5–8 | 662 | – | |
| Gastrointestinal bleed with single intervention, with CC score 5–7 | – | 2953 | |
| Inflammatory bowel disease without interventions with CC score 5 + | – | 3135 | |
| Non-malignant gastrointestinal tract disorders without interventions, CC score 3–5 | 633 | 2425 | |
| Non-malignant gastrointestinal tract disorders without interventions, CC score 6–10 | – | 3132 | |
| Non-malignant gastrointestinal tract disorders with single intervention, CC score 9 + | – | 5730 | |
| Non-malignant gastrointestinal tract disorders with multiple interventions, CC score 3–4 | – | 5768 | |
| Malignant gastrointestinal tract disorders without interventions, CC score 5–8 | 1010 | 2732 | |
| Gastrointestinal bleed with multiple interventions, with CC score 5 + | – | 5500 | |
| General disorders | Admission related to social factors without interventions, with CC score 0 | 513 | – |
| Abnormal findings without diagnosis, without interventions, with CC score 0 | 358 | – | |
| Abnormal findings without diagnosis, without interventions, with CC score 1 + | – | 2043 | |
| Major general abdominal procedures 19 years and over with CC score 6–9 | 4623 | – | |
| Special screening, examinations or other genetic disorders | – | 2244 | |
| Unspecified chest pain with CC score 0–4 | 304 | – | |
| Hepatobiliary | Laparoscopic cholecystectomy, 19 years and over, with CC score 4 + | – | 6912 |
| Non-malignant, hepatobiliary or pancreatic disorders, with single intervention, with CC score 4–8 | – | 3897 | |
| Injury/poison/procedure (vascular access) | Attention to arteriovenous fistula, graft or shunt | 1846 | 3050 |
| Attention to central venous catheter, 19 years and over | 541 | – | |
| Attention to suprapubic bladder catheter | – | 1042 | |
| Insertion of non-tunnelled central venous catheter, 19 years and over | 1131 | 1938 | |
| Insertion of tunnelled central venous catheter 19 years and over | 1351 | 1832 | |
| Removal of central venous catheter, 19 years and over | 763 | – | |
| Open arteriovenous fistula, graft or shunt procedures | 2504 | 3412 | |
| Major, open or percutaneous, kidney or ureter procedures, 19 years and over, with CC score 2–3 | – | 5146 | |
| Poisoning diagnosis without interventions, with CC score 0–1 | – | 1366 | |
| Procedure not carried out, for medical or patient reasons | 577 | – | |
| Procedure not carried out, for other or unspecified reasons | 581 | – | |
| Percutaneous transluminal, embolectomy or thrombolysis, of blood vessel, with CC score 0–4 | 2493 | – | |
| Percutaneous transluminal embolization of peripheral blood vessel, CC score 6 + | – | 9003 | |
| Infections and infestations | Fever of unknown origin without interventions, with CC score 4 + | 634 | 2306 |
| Sepsis without interventions, with CC score 0–4 | 694 | 2273 | |
| Sepsis without interventions, with CC score 5–8 | – | 2940 | |
| Sepsis without interventions, with CC score 9 + | – | 3694 | |
| Sepsis with single intervention with CC score 0–4 | – | 3677 | |
| Sepsis with single intervention, with CC score 9 + | – | 5816 | |
| Sepsis with multiple interventions, with CC score 5–8 | – | 7236 | |
| Sepsis with multiple interventions, with CC score 9 + | – | 9660 | |
| Spinal infection without interventions, with CC score 3–5 | – | 4543 | |
| Spinal infection without interventions, with CC score 10 + | – | 5774 | |
| Spinal infection with interventions, with CC Score 0–5 | – | 8093 | |
| Gastrointestinal infections without interventions, with CC score 2–4 | – | 2061 | |
| Gastrointestinal infections without interventions, with CC score 5–7 | 685 | – | |
| gastrointestinal infections without interventions, with CC score 8 + | – | 3342 | |
| Infections of bones or joints, with CC score 0–1 | – | 3500 | |
| Infections of bones or joints, with CC score 2–4 | – | 3949 | |
| Infections of bones or joints, with CC score 9–12 | – | 5094 | |
| Infections of bones or joints, with CC score 13 + | – | 6092 | |
| Lobar, atypical or viral pneumonia, with single intervention, with CC score 8–12 | – | 3910 | |
| Lobar, atypical or viral pneumonia, with single intervention, with CC score 13 + | – | 5118 | |
| Lobar, atypical or viral pneumonia, without interventions, with CC score 0–3 | 518 | 1612 | |
| Lobar, atypical or viral pneumonia, without interventions, with CC score 4–6 | 615 | 1964 | |
| Lobar, atypical or viral pneumonia, without interventions, with CC score 7–9 | – | 2330 | |
| Lobar, atypical or viral pneumonia, without interventions, with CC score 10–13 | 587 | 2984 | |
| Lobar, atypical or viral pneumonia, without interventions, with CC score 14 + | 1380 | 3872 | |
| Lobar, atypical or viral pneumonia, with multiple interventions, CC score 9–13 | – | 6324 | |
| Chronic obstructive pulmonary disease or bronchitis, without interventions, with CC score 5–8 | – | 2027 | |
| Chronic obstructive pulmonary disease or bronchitis, without interventions, with CC score 9–12 | – | 2461 | |
| Chronic obstructive pulmonary disease or bronchitis, without interventions, with CC score 13 + | – | 3158 | |
| Infections or other complications of procedures without interventions with CC score 0–1 | 516 | 2183 | |
| Unspecified acute lower respiratory infection without interventions, with CC score 0–4 | 417 | – | |
| Unspecified acute lower respiratory infection without interventions, with CC score 5–8 | – | 1978 | |
| Unspecified acute lower respiratory infection without interventions with CC score 9–12 | – | 2492 | |
| Standard infectious diseases without interventions with CC score 4–6 | 712 | 2465 | |
| Kidney or urinary tract infections, without interventions, with CC score 0–1 | – | 1587 | |
| Kidney or urinary tract infections, without interventions, with CC score 2–3 | 561 | 1951 | |
| Kidney or urinary tract infections, without interventions, with CC score 4–7 | – | 2535 | |
| Kidney or urinary tract infections, with interventions, with CC score 3–5 | – | 3049 | |
| Kidney or urinary tract infections, with interventions, with CC score 6–8 | – | 4058 | |
| Metabolism and nutrition disorders | Diabetes with hyperglycaemic disorders, with CC score 0–1 | – | 1309 |
| Diabetes with hyperglycaemic disorders, with CC score 8 + | – | 3029 | |
| Diabetes with lower limb complications, with CC score 5–8 | – | 3131 | |
| Diabetes with lower limb complications, with CC score 9 + | – | 4088 | |
| Musculoskeletal and connective tissue disorders or fractures | Low back pain without interventions, with CC score 6 + | 697 | – |
| Inflammatory, spine, joint or connective tissue disorders, with CC score 3–4 | – | 2238 | |
| Inflammatory, spine, joint or connective tissue disorders, with CC score 9–11 | 669 | – | |
| Non-inflammatory, bone or joint disorders, with CC score 5–7 | – | 3174 | |
| Complex, hip or knee procedures for trauma, with CC score 12 + | – | 17,071 | |
| Foot fracture without interventions, with CC score 8 + | – | 4868 | |
| Intermediate knee procedures for non-trauma, 19 years and over, CC score 4 + | – | 10,699 | |
| Musculoskeletal signs or symptoms, with CC score 4–7 | – | 2017 | |
| Multiple trauma with diagnosis score ≥ 51, with intervention score 0 | 1756 | – | |
| Multiple trauma with diagnosis score ≥ 51, with intervention score 9–18 | – | 10,826 | |
| Musculoskeletal signs or symptoms, with CC score 12 + | 883 | 3188 | |
| Major foot procedures for trauma, 19 years and over, with CC score 4 + | – | 10,540 | |
| Very major foot procedures for non-trauma with CC score 4 + | – | 15,762 | |
| Major hip procedures for trauma with CC score 3–5 | – | 7972 | |
| Major hip procedures for non-trauma, 19 years and over, with CC score 6–9 | – | 12,828 | |
| Soft-tissue disorders with CC score 3–5 | 364 | – | |
| Soft-tissue disorders with CC score 6–8 | 438 | – | |
| Soft-tissue disorders with CC score 9–11 | – | 2949 | |
| Soft-tissue disorders with CC score 12 + | – | 4126 | |
| Vertebral column injury without interventions, with CC score 6 + | – | 4984 | |
| Other injury, of rib or chest, without interventions, with CC score 2–3 | 423 | – | |
| Rib or chest fracture, without interventions, with CC score 3–5 | – | 2743 | |
| Tendency to fall, senility or other conditions affecting cognitive functions, without interventions, with CC score 0–1 | – | 2215 | |
| Tendency to fall, senility or other conditions affecting cognitive functions, without interventions, with CC score 2–3 | – | 2421 | |
| Vertebral column injury without interventions, with CC score 6 + | – | 4984 | |
| Neoplasms (other) | Other or unspecified neoplasm, without interventions, with CC score 0–1 | – | 2407 |
| Malignant breast disorders with interventions, with CC score 7 + | – | 6933 | |
| Malignant gynaecological disorders without interventions, with CC score 7–9 | – | 4221 | |
| Nervous system disorders | Headache, migraine or cerebrospinal fluid leak, with CC score 0–6 | 383 | – |
| Headache, migraine or cerebrospinal fluid leak, with CC score 11 + | 641 | 2424 | |
| Syncope or collapse, with CC score 4–6 | – | 1638 | |
| Syncope or collapse, with CC score 7–9 | 515 | 1963 | |
| Syncope or collapse, with CC score 10–12 | 608 | – | |
| Syncope or collapse, with CC score 13 + | – | 3273 | |
| Cerebrovascular accident, nervous system infections or encephalopathy, CC score 11–13 | – | 5419 | |
| Cerebrovascular accident, nervous system infections or encephalopathy, CC score 14 + | – | 6823 | |
| Muscular, balance, cranial or peripheral nerve disorders, epilepsy or head injury, with CC score 3–5 | 506 | 2056 | |
| Muscular, balance, cranial or peripheral nerve disorders, epilepsy or head injury, with CC score 9–11 | – | 2895 | |
| Muscular, balance, cranial or peripheral nerve disorders, epilepsy or head injury, with CC score 12–14 | – | 3660 | |
| Major intracranial procedures, 19 years and over, with CC score 12 + | 8003 | – | |
| Very major intracranial procedures 19 years and over with CC score 8–11 | – | 10,757 | |
| Very complex intracranial procedures, 19 years and over, with CC score 12 + | 18,492 | – | |
| Stroke with CC score 4–6 | 857 | – | |
| Stroke with CC score 7–9 | 980 | 3754 | |
| Stroke with CC score 13–15 | 1475 | 5908 | |
| Stroke with CC score 16 + | – | 8104 | |
| Muscular, balance, cranial or peripheral nerve disorders, epilepsy or head injury, with CC score 0–2 | 404 | – | |
| Muscular, balance, cranial or peripheral nerve disorders, epilepsy or head injury, with CC score 6–8 | – | 2388 | |
| Ophthalmology | Non-surgical ophthalmology without interventions, with CC score 0–1 | 450 | – |
| Renal and urinary disorders | Acute kidney injury without interventions, with CC score 4–7 | – | 2390 |
| Acute kidney injury without interventions, with CC score 8–11 | – | 2946 | |
| Acute kidney injury without interventions, with CC score 12 + | 1354 | 3728 | |
| Unspecified haematuria without interventions, with CC score 0–3 | 380 | – | |
| Unspecified haematuria without interventions, with CC score 8 + | – | 2647 | |
| Unspecified haematuria with interventions, with CC score 3–6 | – | 2389 | |
| Unspecified haematuria with interventions, with CC score 7 + | – | 3802 | |
| General renal disorders without interventions, with CC score 0–2 | 500 | 1577 | |
| General renal disorders with interventions, with CC score 0–2 | – | 3295 | |
| General renal disorders without interventions, with CC score 3–5 | – | 2172 | |
| Kidney transplant, 19 years and over, from cadaver heart-beating donor | – | 13,402 | |
| Kidney transplant 19 years and over from cadaver non-heart-beating donor | – | 15,572 | |
| Major, open or percutaneous, kidney or ureter procedures, 19 years and over, with CC score 4–6 | – | 5359 | |
| Major, open or percutaneous, kidney or ureter procedures, 19 years and over, with CC score 10 + | – | 7538 | |
| Transplant failure and rejection, without interventions, with CC score 0–1 | – | 2810 | |
| Fluid or electrolyte disorders, without interventions, with CC score 0–1 | 361 | – | |
| Fluid or electrolyte disorders, without interventions, with CC score 2–3 | 454 | – | |
| Fluid or electrolyte disorders, without interventions, with CC score 4–6 | 550 | 1940 | |
| Fluid or electrolyte disorders, without interventions, with CC score 7–9 | 644 | 2370 | |
| Fluid or electrolyte disorders, without interventions, with CC score 10 + | 866 | – | |
| Fluid or electrolyte disorders, with interventions, with CC score 5 + | 3843 | – | |
| Chronic kidney disease without interventions, with CC score 0–2 | 967 | 2455 | |
| Chronic kidney disease without interventions, with CC score 3–4 | 966 | 2691 | |
| Chronic kidney disease without interventions, with CC score 5–7 | 1151 | 3075 | |
| Chronic kidney disease without interventions, with CC score 8–10 | 1533 | 3776 | |
| Chronic kidney disease without interventions, with CC score 11 + | – | 4661 | |
| Chronic kidney disease with interventions, with CC score 3–5 | 5532 | 5126 | |
| Chronic kidney disease with interventions, with CC score 6 + | – | 7483 | |
| Ureteric or bladder disorders, with interventions, with CC score 4 + | – | 3861 | |
| Complex endoscopic bladder procedures with CC score 3 + | – | 8401 | |
| Respiratory, thoracic and mediastinal disorders | Asthma without interventions, with CC score 3–5 | 514 | 1662 |
| Asthma without interventions, with CC score 6–8 | 586 | – | |
| Allergy or adverse allergic reaction | 356 | – | |
| Cystic fibrosis with CC | – | 3887 | |
| Fibrosis or pneumoconiosis, with interventions, with CC score 0–6 | 2162 | – | |
| Inhalation, lung injury or foreign body, with single intervention, CC score 10 + | – | 6041 | |
| Other respiratory disorders without interventions, with CC score 0–4 | 370 | – | |
| Other respiratory disorders without interventions, with CC score 5–10 | 474 | – | |
| Pulmonary embolus without interventions, with CC score 9–11 | 829 | – | |
| Pulmonary oedema without interventions, with CC score 0–5 | 509 | 1800 | |
| Pulmonary oedema without interventions, with CC score 6 + | 718 | – | |
| Pleural effusion without interventions, with CC score 6–10 | – | 2168 | |
| Pleural effusion without interventions, with CC score 11 + | – | 2946 | |
| Pleural effusion with single intervention, with CC score 11 + | – | 4093 | |
| Pleural effusion with multiple interventions, with CC score 11 + | – | 6353 | |
| Respiratory failure without interventions, with CC score 0–5 | 552 | – | |
| Respiratory failure without interventions, with CC score 6–10 | – | 2430 | |
| Respiratory failure with single intervention, with CC score 11 + | – | 3948 | |
| Unspecified acute lower respiratory infection without interventions CC score 5–8 | 587 | – | |
| Unspecified acute lower respiratory infection without interventions CC score 13 + | – | 3295 | |
| Peripheral vascular disorders with CC score 5–7 | 801 | 3044 | |
| Peripheral vascular disorders with CC score 8–10 | – | 3454 | |
| Peripheral vascular disorders with CC score 15 + | – | 5297 | |
| Hypertension | 394 | 1941 | |
Appendix 4
| Body system | CTCAE term/grade | HRG code and currency description35 | Non-elective short-stay unit cost (£)35 | Non-elective long-stay unit cost (£)35 |
|---|---|---|---|---|
| Blood and lymphatic disorders | Haemolysis/G3 | Sickle-cell anaemia with crisis, with CC score 6 + | 1451 | 4831 |
| Cardiac disorders | Atrial fibrillation because of cardiac valve disorders/G3 | Cardiac valve disorders with CC sore 5–8 | 933 | 2989 |
| Chest pain: cardiac/G3–4 | Chest pain with CC score 5–10 | 402 | 1420 | |
| Atrioventricular block first degree/G3 | Heart failure or shock, with CC score 8–10 | 714 | 2558 | |
| Myocardial infarction/G3–4 | Actual or suspected myocardial infarction, with CC score 7–9 | 762 | 2231 | |
| Cardiac arrest/G4–5 | Cardiac arrest with CC score 9 + | 950 | 3225 | |
| Bradycardia or ventricular arrhythmia/G3–4 | Arrhythmia or conduction disorders, with CC score 7–9 | 583 | 2076 | |
| Pericarditis/G3 | Standard, other operations on heart or pericardium, with CC score 5–9 | 2478 | 4747 | |
| ENT | Vertigo/G3 | Non-malignant, ear, nose, mouth, throat or neck disorders, without interventions, with CC score 1–4 | 423 | 1554 |
| Gastrointestinal disorders | Visceral arterial ischaemic of bowel/G5 | Non-malignant gastrointestinal tract disorders with multiple interventions, with CC score 8 + | 8726 | 9099 |
| Vomiting, pain, nausea or diarrhoea/G3 | Non-malignant gastrointestinal tract disorders with single intervention, with CC score 5–8 | 3691 | 4054 | |
| Upper or lower gastrointestinal haemorrhage or bleed/G3 | Gastrointestinal bleed with single intervention, with CC score 5–7 | 2821 | 2953 | |
| General disorders | Oedema limbs/G3 | Oedema with CC score 2 + | 488 | 2714 |
| Hepatobiliary | Abdominal pain, cholecystitis/G3 | Intermediate, hepatobiliary or pancreatic procedures, with CC sore 3 + | 5342 | 8381 |
| Injury/poison/procedure (vascular access) | 33 Vascular access complications/thrombosis, 14 other procedures/G3 | Other complications of, internal devices, implants or grafts, with CC score 6 | 1244 | 4138 |
| Infections and Infestations | Sepsis/G3 | Sepsis with single intervention, with CC score 5–8 | 2901 | 4687 |
| Enterocolitis infectious/G3 | Gastrointestinal infections with single intervention, with CC score 5–7 | 4895 | 5011 | |
| Bone infection/G3 | Infections of bones or joints, with CC score 5–8 | 1158 | 4332 | |
| Viremia because of diabetes/G3 | Diabetes with lower limb complications, with CC score 5–8 | 753 | 3131 | |
| Pleural infection/effusion/G3 | Pulmonary, pleural or other tuberculosis, with interventions, with CC score 5–7 | 4988 | 6138 | |
| Bacteraemia/G3 | Bacteraemia with CC score 5–9 | 1469 | 4949 | |
| Respiratory/lung infection (pneumonia)/G3–4 | Lobar, atypical or viral pneumonia, with single intervention, with CC score 0–8 | 1511 | 2243 | |
| Respiratory/lung infection (COPD)/G3 | Chronic obstructive pulmonary disease or bronchitis, with single intervention, with CC score 5–8 | 1308 | 2680 | |
| Wound infection/G4 | Infection or inflammatory reaction, due to, internal devices, implants or grafts, with CC score 6 + | 1768 | 7627 | |
| Urinary tract infection/G3–4 | Kidney or urinary tract infections, with interventions, with CC score 6–8 | 3868 | 4058 | |
| Metabolism and nutrition disorders | Diabetes (hyperglycaemia, hyperkalaemia)/G3 | Diabetes with hyperglycaemic disorders, with CC score 5–7 | 599 | 2074 |
| Musculoskeletal and connective tissue disorders or fractures | Arthralgia, trismus, chest-wall pain (non-cardiac), cramp, myalgia/G3 | Musculoskeletal signs or symptoms, with CC score 4–8 | 487 | 2017 |
| Tendency to fall and/or bone fracture/G3 | Tendency to fall, senility or other conditions affecting cognitive functions, with single intervention, with CC score 3 + | 3936 | 4551 | |
| Neoplasms | Neoplasms benign, malignant or unspecified/G3 | Other or unspecified neoplasm, with interventions, CC score 2 + | 3885 | 6594 |
| Renal or prostate neoplasm/G3 | Kidney, urinary tract or prostate neoplasms, with interventions, with CC score 6–8 | 4588 | 4747 | |
| Nervous system disorders | Headache/G3 | Headache, migraine or cerebrospinal fluid leak, with CC score 7–10 | 497 | 1873 |
| Syncope/G3 | Syncope or collapse, with CC score 7–9 | 515 | 1963 | |
| Cognitive disturbance/G5 | Conditions affecting cognitive functions, with multiple interventions, with CC score 6 + | 6204 | 8362 | |
| Ischaemia cerebrovascular/G3–4 | Cerebrovascular accident/ischaemia, with CC score 8–10 | 991 | 3824 | |
| Seizure/G3 | Muscular, balance, cranial or peripheral nerve disorders, epilepsy/seizure or head injury, with CC score 6–8 | 601 | 2388 | |
| Intracranial haemorrhage/G5 | Haemorrhagic cerebrovascular disorders with CC score 6–9 | 1040 | 4029 | |
| Psychiatric disorders | Delirium/G3 | Delirium, treated by a non-specialist mental health provider | 848 | 3777 |
| Renal and urinary disorders | Haematuria/G3 | Unspecified haematuria with interventions, with CC score 4–7 | 2043 | 2389 |
| Urinary tract pain/G3 | Pain with CC score 1 + | 696 | 3219 | |
| Respiratory, thoracic and mediastinal disorders | Pulmonary oedema/G3 | Pulmonary oedema with interventions, with CC score 6–8 | 886 | 4307 |
| Respiratory failure/G3 | Respiratory failure with single intervention, with CC score 6–10 | 1074 | 2866 | |
| Vascular disorders | Hypotension or peripheral vascular disorders/G3–4 | Peripheral vascular disorders with CC score 5–7 | 801 | 3044 |
| Hypertension/G3 | Hypertension | 394 | 1941 | |
| Abdominal aortic aneurysm, critical ischaemia/G3 | Standard endovascular repair of abdominal aortic aneurysm, with CC score 6 + | 6988 | 11,946 |
Appendix 5
| Adult critical care, organs supported (n) | |||||
|---|---|---|---|---|---|
| Service description for HRG | 0 | 1 | 2 | 3 | 4 |
| Unit cost of HRG code (£) | |||||
| Cardiac surgical adult patients predominate | – | 1215 | 1520 | – | 2251 |
| Non-specific, general adult critical care patients predominate | 1185 | 1355 | 1862 | 2055 | 2242 |
| Neurosciences adult patients predominate | – | – | – | – | 1832 |
| Renal adult patients predominate | 733 | 1421 | 1398 | – | – |
| Surgical adult patients (unspecified specialty) | – | 1266 | 1632 | – | – |
| Thoracic surgical adult patients predominate | – | – | – | 1514 | – |
Appendix 6
| Service description | NAC_HRG code | First attendance face to face (WF01B) | Follow-up attendance face to face (WF01A) | First telephone or telemedicine consultation (WF01D) | Follow-up telephone or telemedicine consultation (WF01C) |
|---|---|---|---|---|---|
| Unit cost of HRG code (£) | |||||
| Accident and emergency | 180 | 140 | 163 | 85 | 49 |
| Adult mental illness | 710 | 352 | 333 | 192 | 340 |
| Anaesthetics | 190 | 148 | 126 | 113 | 72 |
| Cardiology | 320 | 174 | 139 | 89 | 101 |
| Cardiothoracic surgery | 170 | 272 | 208 | 164 | 166 |
| Chemical pathology | 822 | 146 | 107 | 130 | 78 |
| Clinical genetics | 311 | 565 | 372 | 219 | 257 |
| Clinical haematology | 303 | 235 | 172 | 98 | 96 |
| Clinical neurophysiology | 401 | 251 | 354 | 215 | 43 |
| Clinical oncology | 800 | 166 | 151 | 53 | 95 |
| Clinical physiology | 304 | 129 | 54 | 135 | 82 |
| Critical care medicine | 192 | 236 | 269 | 195 | 80 |
| Dental medicine specialties | 450 | 151 | 187 | 76 | 58 |
| Dermatology | 330 | 131 | 123 | 56 | 72 |
| Endocrinology | 302 | 219 | 155 | 85 | 106 |
| Ear, nose and throat | 120 | 118 | 136 | 79 | 101 |
| Gastroenterology | 301 | 183 | 148 | 85 | 97 |
| General medicine | 300 | 233 | 201 | 47 | 109 |
| General surgery | 100 | 162 | 145 | 123 | 88 |
| Geriatric medicine | 430 | 341 | 253 | 148 | 151 |
| Gynaecology | 502 | 172 | 145 | 84 | 79 |
| Infectious diseases | 350 | 299 | 245 | 126 | 251 |
| Medical oncology | 370 | 253 | 200 | 396 | 136 |
| Medical ophthalmology | 460 | 126 | 108 | 62 | 58 |
| Nephrology | 361 | 221 | 171 | 86 | 113 |
| Neurology | 400 | 239 | 188 | 126 | 105 |
| Neurosurgery | 150 | 224 | 175 | 182 | 107 |
| Nuclear medicine | 371 | 177 | 99 | 68 | 71 |
| Obstetrics | 501 | 196 | 136 | 120 | 87 |
| Ophthalmology | 130 | 130 | 102 | 71 | 85 |
| Oral surgery | 140 | 149 | 120 | 138 | 118 |
| Palliative medicine | 315 | 376 | 241 | 378 | 167 |
| Plastic surgery | 160 | 138 | 110 | 98 | 83 |
| Psychotherapy | 713 | 469 | 168 | 580 | 252 |
| Radiology | 811 | 111 | 155 | 35 | 36 |
| Respiratory medicine | 340 | 198 | 154 | 109 | 86 |
| Rheumatology | 410 | 247 | 155 | 128 | 85 |
| Trauma and orthopaedics | 110 | 142 | 122 | 85 | 105 |
| Urology | 101 | 129 | 111 | 89 | 77 |
Appendix 7
| Parameter (months) | BGFM | CFM | Total |
|---|---|---|---|
| n = 222 | n = 215 | n = 437 | |
| N (%) | N (%) | N (%) | |
| Data obtained through CRFs | |||
| Haemodialysis sessions | |||
| 0–12 | 97 (44) | 86 (40) | 183 (42) |
| 12–24 | 55 (25) | 41 (19) | 96 (22) |
| Bioimpedance sessions | |||
| 0–12 | 93 (42) | 82 (38) | 175 (40) |
| 12–24 | 53 (24) | 44 (20) | 97 (22) |
| Inpatient admissions (scheduled) | |||
| 0–12 | 67 (30) | 57 (27) | 124 (28) |
| 12–24 | 44 (20) | 34 (16) | 78 (18) |
| Inpatient (unscheduled: SAEs) | |||
| 0–12 | 222 (100) | 215 (100) | 437 (100) |
| 12–24 | 222 (100) | 215 (100) | 437 (100) |
| Inpatient nursing home admissions | |||
| 0–12 | 60 (27) | 54 (25) | 114 (26) |
| 12–24 | 41 (18) | 32 (15) | 73 (17) |
| Outpatient (non-admitted consultation) | |||
| 0–12 | 51 (23) | 42 (20) | 93 (21) |
| 12–24 | 38 (17) | 30 (14) | 68 (16) |
| Outpatient nursing home appointments | |||
| 0–12 | 58 (26) | 54 (25) | 112 (25) |
| 12–24 | 38 (17) | 34 (16) | 72 (16) |
| Primary and community care | |||
| 0–12 | 51 (23) | 40 (19) | 91 (21) |
| 12–24 | 30 (13) | 28 (13) | 58 (13) |
| Caring by unpaid carers (non-NHS cost) | |||
| 0–12 | 41 (18) | 36 (17) | 77 (18) |
| 12–24 | 34 (15) | 25 (12) | 59 (14) |
| Data obtained through HES | |||
| Inpatient admissions (scheduled, unscheduled) a | |||
| 0–12 | 215 (97) | 208 (97) | 423 (97) |
| 12–24 | 215 (97) | 208 (97) | 423 (97) |
| Adult critical care (NHS) a | |||
| 0–12 | 215 (97) | 208 (97) | 423 (97) |
| 12–24 | 215 (97) | 208 (97) | 423 (97) |
| Outpatient (non-admitted consultation) (NHS) a | |||
| 0–12 | 215 (97) | 208 (97) | 423 (97) |
| 12–24 | 215 (97) | 208 (97) | 423 (97) |
| EQ-5D-5L | |||
| Baseline | 193 (87) | 192 (89) | 385 (88) |
| 3 | 157 (71) | 158 (73) | 315 (72) |
| 6 | 133 (60) | 127 (59) | 260 (59) |
| 9 | 118 (53) | 96 (45) | 214 (49) |
| 12 | 114 (51) | 96 (45) | 210 (48) |
| 15 | 87 (39) | 77 (36) | 164 (37) |
| 18 | 84 (38) | 65 (30) | 149 (34) |
| 21 | 77 (35) | 59 (27) | 136 (31) |
| 24 | 87 (39) | 71 (33) | 158 (36) |
| SF-12 (SF-6D) | |||
| Baseline | 189 (85) | 183 (85) | 372 (85) |
| 3 | 154 (69) | 156 (72) | 310 (71) |
| 6 | 128 (58) | 127 (59) | 255 (58) |
| 9 | 114 (51) | 94 (44) | 208 (47) |
| 12 | 114 (51) | 96 (45) | 210 (48) |
| 15 | 87 (39) | 77 (36) | 164 (37) |
| 18 | 84 (38) | 61 (28) | 145 (33) |
| 21 | 77 (35) | 57 (27) | 134 (31) |
| 24 | 83 (37) | 70 (33) | 153 (35) |
Appendix 8
| Resource use category | n | BGFM, £, mean (SD) | n | CFM, £, mean (SD) | BGFM–CFM, £,a mean difference (95% CI) | p-valuea |
|---|---|---|---|---|---|---|
| CRF haemodialysis | 43 | 39,255.33 (15,626.66) | 34 | 37,726.66 (17,062.8) | 1528.67 (−5796.57 to 8853.91) | 0.68 |
| CRF bioimpedance | 41 | 237.37 (70.15) | 31 | 0 (0) | 237.37 (217.15 to 257.59) | 0.00 |
| Inpatient | 26 | 9322.61 (11,219.52) | 19 | 5877.07 (7096.00) | 3445.54 (−1653.64 to 8544.74) | 0.18 |
| HES inpatient (scheduled) | 215 | 4455.31 (5609.89) | 208 | 4442.08 (5954.61) | 13.22 (−1087.52 to 1113.98) | 0.98 |
| HES inpatient (unscheduled) | 215 | 5859.07 (7536.29) | 208 | 5697.28 (8949.95) | 161.78 (−1468.33 to 1791.89) | 0.85 |
| CRF inpatient, nursing home | 29 | 0 (0) | 19 | 0 (0) | 0 (0 to 0) | 0.00 |
| HES adult critical care | 215 | 300.61 (708.95) | 208 | 219.27 (716.06) | 81.34 (−47.65 to 210.33) | 0.22 |
| Outpatient | 25 | 1690.43 (1247.58) | 25 | 2564.34 (2333.64) | −873.90 (−1869.42 to 121.62) | 0.08 |
| HES outpatient consultation | 215 | 1950.98 (2235.58) | 208 | 1900.12 (1889.61) | 50.86 (−346.94 to 448.67) | 0.80 |
| CRF outpatient, nursing home | 27 | 0 (0) | 25 | 4.04 (13.56) | −4.04 (−9.06 to 0.97) | 0.11 |
| CRF primary, community care | 24 | 513.86 (574.56) | 20 | 379.87 (298.90) | 133.98 (−125.86 to 393.84) | 0.31 |
| Resource use category | BGFM (n = 222) £, mean (SD) | CFM (n = 215) £, mean (SD) | BGFM–CFM £,a mean difference (95% CI) | p-valuea |
|---|---|---|---|---|
| CRF haemodialysis | 38,338.58 (9963.56) | 38,833.06 (9379.89) | −494.48 (−2199.08 to 1210.11) | 0.57 |
| CRF bioimpedance | 185.86 (78.63) | 0 (0) | 185.86 (175.46 to 196.27) | 0.00 |
| Inpatient | 8105.52 (7634.56) | 7875.60 (6380.60) | 229.92 (−1047.94 to 1507.78) | 0.72 |
| CRF inpatient (scheduled) | 5045.86 (4076.06) | 4826.91 (2727.16) | 218.94 (−429.95 to 867.84) | 0.51 |
| CRF inpatient (unscheduled) | 3059.66 (5948.09) | 2994.30 (5385.88) | 65.36 (−956.78 to 1087.50) | 0.90 |
| CRF inpatient, nursing home | 0 (0) | 54.39 (87.58) | −54.39 (−66.02 to −42.75) | 0.00 |
| HES adult critical care | 306.23 (702.78) | 234.31 (754.52) | 71.92 (−65.78 to 209.62) | 0.31 |
| Outpatient | 644.22 (401.66) | 1008.35 (552.95) | −364.14 (−454.50 to −273.77) | 0.00 |
| CRF outpatient consultation | 644.22 (401.66) | 996.43 (553.00) | −352.21 (−442.59 to −261.84) | 0.00 |
| CRF outpatient, nursing home | 0 (0) | 11.92 (12.79) | −11.92 (−13.69 to −10.16) | 0.00 |
| CRF primary, community care | 711.68 (526.99) | 510.45 (301.74) | 201.22 (120.84 to 281.61) | 0.00 |
| Additional (non-NHS) cost | 690.65 (799.08) | 780.61 (717.47) | −89.96 (−233.48 to 53.55) | 0.21 |
Appendix 9
| EQ-5D-5L (months)a | n | BGFM, mean (SD) | n | CFM, mean (SD) | BGFM–CFM, mean differenceb (95% CI) | p-valueb |
|---|---|---|---|---|---|---|
| Baseline | 193 | 0.554 (0.295) | 192 | 0.601 (0.287) | −0.047 (−0.105 to 0.012) | 0.12 |
| 3 | 157 | 0.553 (0.306) | 158 | 0.563 (0.294) | −0.010 (−0.078 to 0.058) | 0.78 |
| 6 | 133 | 0.538 (0.306) | 127 | 0.568 (0.285) | −0.030 (−0.101 to 0.041) | 0.41 |
| 9 | 118 | 0.532 (0.297) | 96 | 0.525 (0.301) | 0.007 (−0.073 to 0.087) | 0.86 |
| 12 | 114 | 0.521 (0.296) | 96 | 0.478 (0.316) | 0.043 (−0.036 to 0.122) | 0.29 |
| 15 | 87 | 0.513 (0.316) | 77 | 0.453 (0.317) | 0.060 (−0.034 to 0.154) | 0.21 |
| 18 | 84 | 0.463 (0.308) | 65 | 0.425 (0.325) | 0.038 (−0.064 to 0.141) | 0.46 |
| 21 | 77 | 0.411 (0.359) | 59 | 0.372 (0.353) | 0.039 (−0.079 to 0.157) | 0.52 |
| 24 | 87 | 0.417 (0.347) | 71 | 0.347 (0.349) | 0.070 (−0.037 to 0.177) | 0.20 |
| Health statusa | BGFM (n = 222) mean (SD) | CFM (n = 215) mean (SD) | BGFM–CFM,b mean difference (95% CI) | p-valueb |
|---|---|---|---|---|
| EQ-5D-5L (months)a | ||||
| Baseline | 0.639 (0.253) | 0.681 (0.250) | −0.042 (−0.088 to 0.006) | 0.08 |
| 3 | 0.623 (0.265) | 0.637 (0.252) | −0.014 (−0.062 to 0.033) | 0.55 |
| 6 | 0.626 (0.257) | 0.650 (0.235) | −0.024 (−0.068 to 0.021) | 0.31 |
| 9 | 0.604 (0.252) | 0.596 (0.238) | 0.008 (−0.038 to 0.053) | 0.74 |
| 12 | 0.585 (0.250) | 0.564 (0.260) | 0.021 (−0.026 to 0.068) | 0.38 |
| 15 | 0.591 (0.248) | 0.552 (0.242) | 0.039 (−0.004 to 0.084) | 0.08 |
| 18 | 0.567 (0.243) | 0.539 (0.253) | 0.028 (−0.018 to 0.074) | 0.24 |
| 21 | 0.528 (0.277) | 0.486 (0.271) | 0.042 (−0.009 to 0.093) | 0.11 |
| 24 | 0.519 (0.266) | 0.498 (0.264) | 0.021 (−0.028 to 0.070) | 0.40 |
| SF-6D (months)a | ||||
| Baseline | 0.627 (0.128) | 0.636 (0.119) | −0.009 (−0.031 to 0.014) | 0.46 |
| 3 | 0.618 (0.161) | 0.619 (0.137) | −0.001 (−0.029 to 0.026) | 0.93 |
| 6 | 0.617 (0.150) | 0.603 (0.156) | 0.014 (−0.015 to 0.043) | 0.34 |
| 9 | 0.597 (0.164) | 0.588 (0.175) | 0.009 (−0.024 to 0.041) | 0.61 |
| 12 | 0.584 (0.175) | 0.551 (0.178) | 0.033 (0.001 to 0.066) | 0.05 |
| 15 | 0.564 (0.174) | 0.534 (0.182) | 0.030 (−0.002 to 0.062) | 0.07 |
| 18 | 0.555 (0.185) | 0.523 (0.173) | 0.032 (−0.001 to 0.066) | 0.06 |
| 21 | 0.522 (0.188) | 0.498 (0.187) | 0.024 (−0.012 to 0.060) | 0.20 |
| 24 | 0.519 (0.189) | 0.496 (0.194) | 0.023 (−0.013 to 0.058) | 0.21 |
