Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number PTC-RP-PG-0213-20001. The contractual start date was in April 2015. The final report began editorial review in April 2021 and was accepted for publication in October 2022. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2024 Mullis et al. This work was produced by Mullis et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Mullis et al.
Synopsis
Introduction
The last decade has seen important advances in the acute treatment and prevention of stroke, and this has been associated with falls in both stroke incidence and mortality. 1,2 This improved survival coupled with projected ageing of the population means that the number of people living with stroke in the UK is anticipated to rise despite the decline in incidence. 2 Thus, stroke is likely to remain the commonest cause of adult disability in the UK for the foreseeable future. 1
There is evidence that stroke survivors and their carers have longer-term needs that are not well addressed by current services. 3,4 However, there is a dearth of evidence-based interventions to address these needs. Indeed, the review of evidence to inform the 2013 National Institute for Health and Care Excellence (NICE) stroke rehabilitation guideline addressing community participation and long-term recovery noted that: ‘no evidence (was) identified’, and NICE based its recommendations on modified Delphi consensus statements. 5 An updated systematic search performed for NICE in 2019, despite identifying two randomised controlled trials (RCTs) that did address longer-term stroke care (LoTS)6,7 published since the 2013 guideline, concluded that the new evidence was unlikely to affect guideline recommendations. 8
There are two broad approaches to service delivery that have been developed to address this shortfall in meeting the longer-term needs of people living with stroke in the community: to extend contact with specialist stroke services or to enhance generalist primary care services.
One way of extending contact with specialist stroke services is to expand use of the early supported discharge services. There is good evidence from RCTs that early supported discharge services that offer people in hospitals an early discharge with rehabilitation at home are effective at reducing long-term dependency for selected patients. 9 Therefore, it was hypothesised that extending contact with such services up to 18 months post stroke might improve longer-term outcomes for people with stroke. This approach was tested in an evaluation of an Extended Stroke Rehabilitation Service (EXTRAS). 10 While this RCT did not demonstrate any evidence of effect on its primary outcome (the Nottingham Extended Activities of Daily Living Scale), it did suggest that the intervention was cost-saving and improved quality of life (QoL) and so was likely to be cost-effective.
A second way to extend contact with specialist stroke services is through community stroke services. A national policy introduced in 2001 as part of the National Service Framework for Older People was that patients and carers should have access to a named stroke care co-ordinator who would be available to provide advice, discuss changing needs and facilitate access to rehabilitation services as appropriate. 11 Uptake of this policy has been patchy. The most recent national audit of stroke care in England, Wales and Northern Ireland reported that 40% of stroke patients received a 6-month review in 2019/20 and only 20% in 2013. 12 There was an expectation in the National Service Framework that stroke care co-ordinators would be performing this role, though more recent guidance does not specify this. 13 Evidence for the effectiveness of the stroke care co-ordinator approach is lacking. A cluster randomised trial of a specific approach to stroke care co-ordination – LoTS – found no improvement in psychological well-being of patients or carers in the intervention arm compared to usual care. 6 There was also no effect on QoL or social activities or overall costs of care provision. Given that the unit of allocation in this trial was the stroke care co-ordinator service, this does not provide any direct evidence that using stroke care co-ordinators is ineffective, since patients in the usual care arm will have received such care. Building on the negative findings of the LoTS care intervention, the New Start intervention was developed. 14 The New Start intervention is a programme of self-management facilitated by a healthcare professional (HCP) working in a stroke service. It builds on the stroke care co-ordinator model, in that the intervention involves an initial assessment of needs. The intervention encourages participants, through a series of one-on-one meetings, to explore how their own social networks might help them address these needs.
Both the approaches described above offer interventions to patients in the first few months after their stroke and provide a prolonged period of support (compared to usual care). Implicitly, such interventions would meet the national clinical guideline recommendation that people with stroke should be offered a structured review at 6 months after their stroke. 15 A complementary approach to extension of specialist services is to enhance support in primary care for people with stroke. There are several arguments that can be made for such an approach, particularly as time passes after the acute stroke event. Primary care is set up to maintain long-term contact with its patients and deal with problems over time. It is a common first point of contact for someone with stroke who has a health problem and is the only (non-research) structure in the NHS that has a population-based register of all people with stroke, so it can ensure that people do not ‘fall through the net’. 16 Many people with stroke have multiple comorbidities and so may receive more holistic care from a generalist rather than a specialist. 17 Primary care is in a better position to carry out the guideline recommendation that people with stroke should be offered annual reviews15 than specialist services.
Aim
The aim of the Improving Primary Care After Stroke (IPCAS) programme was to develop and evaluate interventions to enable primary care to meet the needs of stroke survivors living in the community.
There were three workstreams:
-
develop a primary care-based model to optimise postdischarge longer-term care
-
develop a ‘Managing Life After Stroke’ (MLAS) programme (including self-management) for people with stroke
-
evaluate the effectiveness and cost effectiveness of these interventions.
Overview of programme
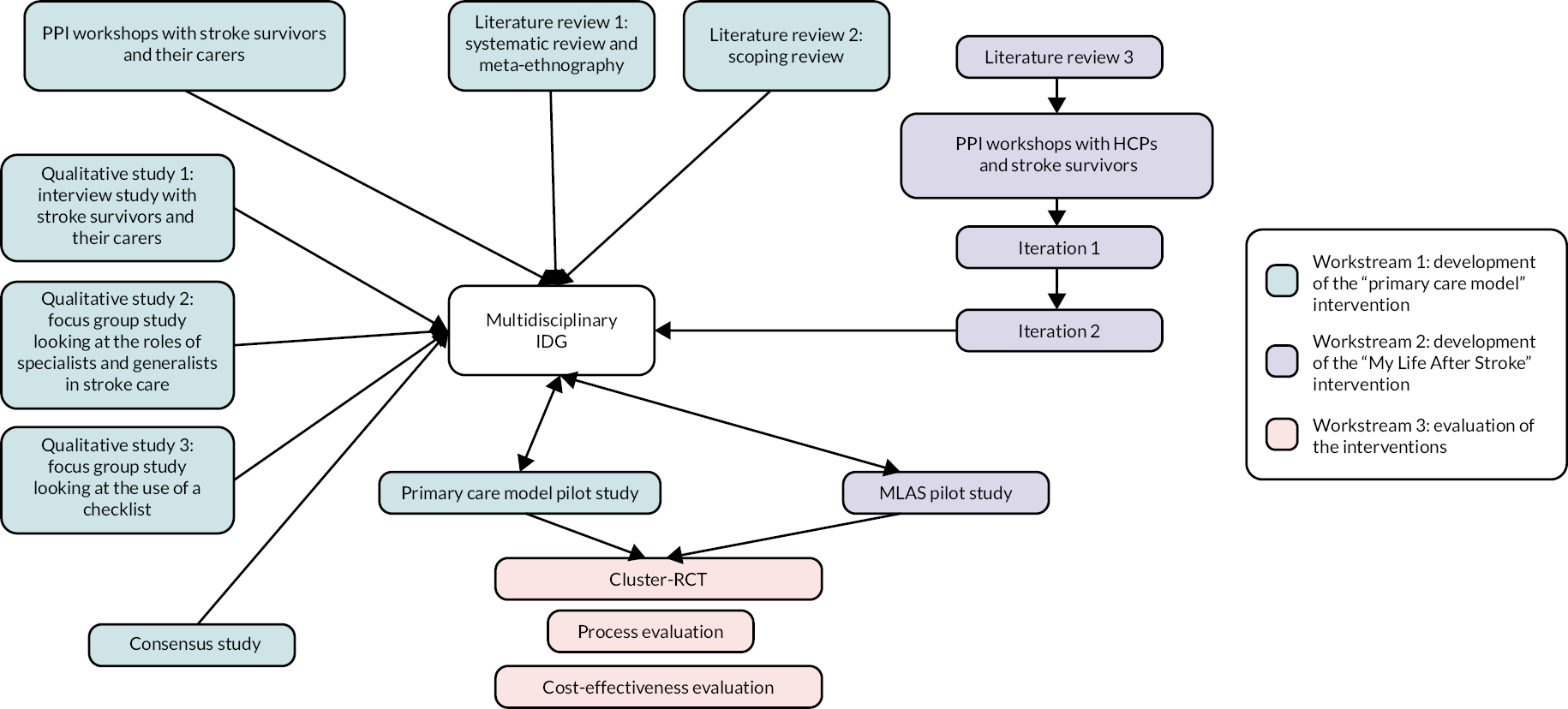
The IPCAS research pathway diagram (see Figure 1) summarises the constituent parts of the programme and how they interlink. The development of the primary care model (workstream 1) involved information gathering in the form of literature reviews, patient and public involvement (PPI) workshops, qualitative studies (interviews and focus groups), a consensus study and a pilot study, all feeding into a multidisciplinary intervention development group (IDG) that approved what the final primary care model would comprise. In parallel, a further literature review, consultation workshops with HCPs and PPI fed into the iterative development of the MLAS programme (workstream 2), which then passed through two iterative development phases before being reviewed by the multidisciplinary IDG and going on to a feasibility phase. In the final phase of the programme (workstream 3), the two interventions were evaluated in a cluster RCT which included a process evaluation and a cost-effectiveness analysis.
FIGURE 1.
Improving Primary Care After Stroke research pathway diagram.
Changes to the programme’s original design
In the initial programme application, it was envisaged to run two trials: one cluster RCT to evaluate the IPCAS primary care model and one individual RCT to evaluate the MLAS self-management programme. We did explore nesting the evaluation of MLAS within the cluster RCT, whereby participants in the intervention arm would be individually randomised to be offered MLAS at the time of their review. After discussion with our programme steering committee, we agreed that this would be complex to prosecute and interpret. Furthermore, as the IPCAS research programme evolved during the development stages, it became evident that the two interventions were complementary to each other. Therefore, we decided to run a single trial with both components, IPCAS and MLAS, included in the intervention. We would carry out a non-randomised analysis to explore the effect of MLAS itself by comparing outcomes in stroke survivors in the intervention arm of the trial who did and did not take part in MLAS. A second change to the original programme, made in response to feedback from the funding panel, was to enlarge the process evaluation of the trial.
Multidisciplinary Intervention Development Group
The multidisciplinary IDG was convened at the outset of the IPCAS programme. The aim was to provide researcher, clinician and stroke survivor input to guide the development process and to agree the components of the primary care model. This involved reviewing results from the earlier studies and using these to inform both the content of the later studies and ultimately the design of the intervention.
The members (n = 25) included specialist stroke consultants, stroke nurses, general practitioners (GPs), practice nurses, a stroke survivor, a Clinical Commissioning Group (CCG) representative, representatives from the Stroke Association, community neurorehabilitation team members, clinical psychologists, a rehabilitation specialist, a return-to-work specialist and a behavioural science specialist.
We conducted five meetings, between September 2015 and November 2016, plus an additional meeting with representatives from primary care only (three GPs, two practice nurses) to discuss the implementation of model components in general practice.
During the meetings, we discussed the proposed components of the model and their iterations; results from qualitative work (literature reviews, interviews and focus groups) to inform model development and plans for model implementation in general practice. In the final meeting, the components of the primary care model were agreed.
Development of a primary care-based model to optimise postdischarge longer-term care
There were six objectives in this work stream:
-
Understand perspectives of stroke survivors and carers on experiences of health and care services since discharge from specialist services.
-
Explore roles of specialist and primary care services for stroke aftercare and avenues of communication between specialist and primary care.
-
Consider poststroke checklists for primary care.
-
Establish criteria for rereferral from primary care to specialist care.
-
Agree the components of the primary care model.
-
Pilot the primary care model.
1. Understand perspectives of stroke survivors and carers on experiences of health and care services since discharge from specialist services
Two studies informed our understanding of stroke survivor and carer perspectives: a systematic review and meta-ethnography of the literature and a qualitative interview study with stroke survivors and their carers.
Systematic review and meta-ethnography
The protocol for this review (including the search strategy) and its results have both been published (http://doi.org/10.1136/bmjopen-2015-009244; https://doi.org/10.1371/journal.pone.0192533). 18,19
This was the first systematic review in the literature to focus specifically on patient and caregiver experiences of primary care and community healthcare services. We chose meta-ethnography as an analytical approach as we sought to generate a conceptual framework that went beyond simple aggregation of the findings of the individual studies.
We used the population, interest, context (PICo) mnemonic to structure our search. 20 Our population included stroke survivors and family caregivers. Our interest was in experiences, perspective, satisfaction and needs. Our context was primary care, community health services and general practice. Our approach to meta-ethnography was to record themes identified by the authors of the included studies (second-order constructs). These themes were grouped into four categories reflecting different aspects of postdischarge care: continuity of care, access to services, information and quality of communication. From these themes, we developed our own explanation of why stroke survivors and caregivers had the experiences that they did (i.e. third-order constructs).
We identified 51 studies which included 566 patients and 593 caregivers. The key theme under continuity of care related to dissatisfaction with a lack of proactive follow-up, be it from primary care, the hospital or allied healthcare professionals. With regard to access to services, a major theme was what was perceived as premature withdrawal of services. A second theme was lack of support in areas such as rehousing, transport and psychological and interpersonal difficulties, which appeared to be outside the remit of the services that they did receive. Caregivers also felt they had a lack of support, with healthcare professionals making the assumption that they would provide the care that was required. The issues with information included lack of detail about local services and how to access them and lack of specific information about the consequences of stroke and realistic timescales for recovery at the right time. Concerning quality of communication, the key issues related to both communication between survivors, caregivers and healthcare services and between the different healthcare professionals. From these themes, we generated three third-order constructs: marginalisation of stroke survivors and caregivers by the healthcare system; passivity and activity in the relationship with healthcare services and fluidity of needs for both patients and caregivers.
This review gave us important insights into how to shape the primary care model. From our third-order constructs, we hypothesised that addressing passivity of both services and patients and caregivers would reduce the feelings of marginalisation and enable patients and caregivers to be better able to address their changing information needs. In practical terms, this translates into offering active follow-up in primary care and enhancing patient and carer self-management. Both aspects were taken up in the primary care model that we developed.
Interview study with stroke survivors and caregivers
This study has been published in abstract form,21,22 and further details are given in Appendix 1.
Building on the findings of the systematic review that patient needs were fluid, our aim was to explore the changing care needs of stroke survivors over time and identify how these needs, and the needs of carers, could be addressed in primary care.
Twenty-two stroke survivors were recruited from the stroke registers of five general practices in the East of England. Semistructured interviews were carried out in the patients’ homes. Patients were asked to identify which family member or friend gave them the most help and support, from whom 14 caregivers were interviewed. All interviews were audio-recorded, transcribed and analysed using a framework approach.
The 22 patients had a mean age of 73, with a range from 49 to 93. The median time since their stroke was 3 years, with a range from 3 months to 22 years. Eleven of the 14 caregivers were female. From the patient perspective, physical needs that were highlighted included fatigue and loss of sexual function. Psychological needs included fear of another stroke; anxiety and depression; loss of confidence and feeling abandoned. Information needs that were highlighted included lack of knowledge of what services were available, feeling unprepared and having a lack of information about prognosis and what to expect. With regard to health services, patients noted a lack of continuity and lack of advance planning. There were differences in the phases of recovery. In the stroke crisis phase, there was surprise at the lack of contact after discharge. In the postdischarge phase, the psychological needs of anxiety and feelings of abandonment were most pronounced. In the longer term, physical needs were more to the forefront, but in the context of other comorbidities. Difficulty of access to services was also raised.
From a caregiver perspective, the needs were divided into those relating to the stroke survivor and those relating to the carer’s own needs, but sometimes the boundaries were blurred. General practice was the first point of contact to address these needs. The caregivers particularly stressed fear of another stroke, lack of information and lack of follow-up as their key concerns for the person they were caring for. For themselves, there was a loss of the physical and the emotional side of a relationship; loss of confidence; anxiety and depression; and a feeling of being trapped in the caregiving role.
These findings added granularity and depth to the findings of our systematic review. In particular, they emphasised the importance of provision of information about what services are available locally, the unmet needs of caregivers, the importance of health services maintaining active contact with stroke survivors and the changing nature of the needs. In particular, it was notable that in the longer term, the needs were couched more in terms of multiple comorbidities and less in the context of the stroke.
2. Explore roles of specialist and primary care services for stroke aftercare and avenues of communication between specialist and primary care
As the programme developed, we realised that the roles of specialist and primary care services for stroke aftercare would in fact be better met by a consensus study, which is described under objective 4 of this workstream. We performed a focus group study to explore communication between specialist and primary care in the context of stroke.
Focus group study
This study has been published (http://doi.org/10.5334/ijic.5465). 23
While there has been some investigation of communication between generalists and specialists, there has been only limited assessment of the transfer arrangements between hospital and the community, and to our knowledge, there have been no studies of the communication of generalists and specialists in the context of postdischarge stroke care. 24,25 Therefore, in this study, we aimed to understand the communication processes between generalists and specialists with regard to stroke care after discharge from hospital and what are the barriers and enablers to communication between these two groups.
Six focus groups were carried out in 2016. The participants were recruited from six NHS acute trusts in the East of England and East Midlands and via the NIHR Clinical Research Network (for primary care representatives). Each focus group was run by two facilitators and lasted between an hour and 90 minutes. The focus groups followed a topic guide, and two clinical vignettes were used to explore indications for referral and how guidelines were applied in practice. The focus groups were recorded, and content was transcribed. Two researchers analysed the data iteratively using framework analysis, with initial coding done independently. Themes that were identified were cross-checked with the group moderators for validity.
Forty-eight HCPs took part. Fifteen were from primary care (9 GPs; 6 nurses), and there were 33 specialists. These included 23 from secondary care (5 occupational therapists; 5 physiotherapists; 3 speech and language therapists; 4 doctors; 3 nurses; 1 psychologist; 1 assistant practitioner and 1 stroke review officer) and 10 from specialist community teams (4 occupational therapists; 3 physiotherapists; 3 speech and language therapists). Four themes were identified. Firstly, that the roles of the generalist and the specialist overlapped, but each tended to work in a silo. Secondly, the referral decision-making process was closely related to quality of communication between generalist and specialist. From the generalist perspective, a lack of information from the specialist hampered onward referrals. Thirdly, communication between generalist and specialist was characterised as variable and utilised a mixture of formal and informal methods. Barriers to communication included different information technology systems, different languages (e.g. use of abbreviations) and lack of knowledge of generalists regarding services to which they could refer and what are the role boundaries of such services. Conversely, communication was facilitated where there were established relationships and modes of communication (e.g. dedicated phone number or hospital bleeps). Fourthly, ways to improve dialogue between generalists and specialists were identified, including the ability to contact people directly, to have shared templates for information and shared information systems.
Developing ways to improve communication between generalists and specialists will facilitate better support of stroke survivors. Improving the ability of information technology systems to communicate with each other is important, but it is outside the ability of this programme of research to influence this. The key aspects that appear amenable to change in the context of this programme include increasing contact between primary and secondary care, providing clear pathways for communication and providing information to both sectors as to what the other has to offer. As a result of this work, the implementation of enhanced communication pathways between primary and secondary care became one of the components of the primary care model that we were developing.
3. Consider poststroke checklists for primary care
Traditionally, primary care responds to issues raised by patients rather than by using checklists. However, checklists have underpinned other initiatives aiming to address longer-term issues facing stroke survivors, including LoTS care6 and the Greater Manchester Stroke Assessment tool (GM-SAT, updated in 2018 to GM-SAT2). 26,27 Furthermore, the use of a standardised tool is recommended in implementation guidance for poststroke reviews. 13 While there is evidence that the GM-SAT is feasible to administer in the community using third-sector co-ordinators and that it is acceptable to patients and carers,26 the number of items on the tool (35) and the length of time it takes to complete (a mean of 74 minutes) do not make it a practical tool for primary care facing mounting workload pressures. 28 Recognising the need for a parsimonious checklist, an international expert panel developed an 11-item checklist for the identification of long-term problems after stroke in a primary care setting. 29 However, primary care clinicians, stroke survivors and carers were not represented in the panel that produced the checklist. Therefore, we ran focus groups to explore the potential role of a checklist in the setting of UK primary care.
Focus group study
This study has been published (https://doi.org/10.1186/s12875-018-0894-3). 30
The aim was to explore the feasibility and acceptability of using a checklist to support management of the healthcare needs of people with stroke in primary care and to gauge opinion on an existing 11-item checklist. 29
There were two participant groups: healthcare providers (n = 19), including GPs, practice nurses, allied health professionals and third-sector staff, and stroke survivors (n = 12) and their caregivers (n = 7). The former were recruited through the NIHR Clinical Research Network and community contacts of the study investigators. The latter were recruited from general practices in diverse areas in the East of England: Cambridge, Peterborough and Bedford. Three focus groups were held with HCPs and two with patients and carers. Each was facilitated by two researchers using a topic guide. The sessions were recorded and transcribed. Analysis was done by a single researcher using thematic analysis.
Both participant groups liked the concept of a checklist, feeling that it would help structure a consultation, reduce variation, avoid problems being missed and facilitate awareness of issues that patients might be reluctant to raise. Stroke survivors and their carers wanted appointments specifically related to their stroke, but they did not feel that primary care automatically offered this. With regard to content, some important areas were felt to have been missed, and the wording of some items was challenged. Participants suggested that the checklist might be completed in advance of the review and issues prioritised. Healthcare providers emphasised the importance of having a pathway to address the needs that would be identified.
In conclusion, the concept of a checklist to facilitate reviews of stroke survivors in primary care settings was broadly welcomed by both HCPs and patients. The modified checklist that we developed subsequently informed the consensus study and was piloted for use in the primary care model.
4. Establish criteria for rereferral from primary care to specialist care
Guidelines in England and Wales for rehabilitation after stroke do not specify where these interventions should happen or when referrals to specialist services should occur. 5 Critical to understanding the potential for a primary care stroke model is whether unmet needs of stroke survivors are best met by developing primary care services or by improving access to specialist services. To inform this debate, a consensus study was performed.
Modified Research And Development Corporation-appropriateness consensus study
This study has been published (https://doi.org/10.1186/s12875-020-01139-4). 31
The aim was to understand from a HCP perspective when it is appropriate to refer from primary care to specialist services in the longer-term management of people with stroke.
A 10-person panel was recruited, representing both generalists (4 GPs; 1 practice nurse) and specialists (3 stroke physicians; 1 physiotherapist; 1 occupational therapist). Fictional poststroke scenarios were composed to cover each of the problem areas on the 11-item checklist that had been modified following our own focus group work. 29,30 Seventeen scenarios were devised, each requiring between three and five referral decisions. For each scenario, as it developed, the severity or impact of the problem increased. In the first round of the study, panellists were sent a link to an online survey and asked to rate each referral decision for all the scenarios on a 9-point scale. The two questions that they were asked to rate were: should the patient be referred to a specialist? And if so, should this be to a member of the stroke specialist team? In the second round of the study, the panellists met face to face where each referral decision was discussed before voting again.
After round two, consensus was not reached in 10 (14%) out of 69 referral decisions. In 44 (64%), there was consensus that referral was needed, and in 15 (22%), consensus that it was not needed. With regard to the 44 where referral was deemed appropriate, in 18 of these there was consensus that this should be to a stroke specialist and for 14, that referral to a stroke specialist was not necessary. For 12, there was no consensus reached as to whether or not the referral should be to a stroke specialist. Features that were common among scenarios where referral was required were if specific resources were needed or if the intervention was complex and out of the usual scope of primary care. Conversely, topics within the agreed scope of primary care did not require referral unless the scenario was complex or the severity was too great for primary care. Lack of consensus tended to be in scenarios where it was questioned whether specialist input would lead to patient benefit. Such disagreement was not related to professional background of the panellists.
This study suggests that the longer-term care for people with stroke should involve primary care, stroke services and non-stroke specialist services. This reinforces the importance of better co-ordination and communication between primary and secondary care. The scenarios devised in this study were subsequently used in the training of HCPs in delivery of the primary care model.
5. Agree the components of the primary care model
In addition to the studies described above that related to specific objectives in workstream 1, two other pieces of work were carried out to inform the primary care model, a scoping review of the literature and PPI workshops.
Scoping review
The protocol for this review (including search strategy) has been published (http://doi.org/10.1136/bmjopen-2016-012840). 32 A summary is provided in Appendix 2.
The aim was to provide an overview of interventions delivered in primary care and community healthcare services to address long-term outcomes after stroke and to describe the characteristics of effective interventions.
We conducted a systematic search to identify reviews of RCTs, reviews of observational studies and single trials published in the last 5 years (2011–6).
We identified 19 systematic reviews and 15 RCTs (covering topics where there were no systematic reviews). Rehabilitation interventions to address activities of daily living were most common in the literature, followed by physiotherapy interventions to improve mobility and information focused interventions to improve secondary prevention. Cognitive problems, fatigue and specific mental health outcomes were not covered.
We identified key gaps in primary care and community-based interventions to address long-term problems after stroke. Interventions to address long-term care after stroke have primarily been developed and evaluated in the context of further access to rehabilitation. There is a lack of primary care interventions to target key unmet needs around cognitive problems, fatigue and mental health, and primary care and community-based interventions to address these issues are under-researched. If these gaps remain, then there are likely to be issues of effectiveness over a primary care model that identifies needs for which there are no evidence-based interventions. However, research in these areas continues. For example, a recent review of non-pharmacological interventions for poststroke fatigue identified 10 RCTs, with some promising interventions. 33
Patient and public involvement workshops
Patient and public involvement in the programme is detailed in a separate section. These workshops influenced how the results from the studies described above were interpreted and applied to the primary care model. Preprogramme workshops also identified potential components of the primary care model, such as a single point of contact for stroke survivors and their carers to approach practices, the importance of handover of care from secondary to primary care and the need for primary care to be more proactive.
Components of the primary care model
The IDG (see above) reviewed all the evidence discussed in this section, including the literature reviews, individual research studies and patient and public workshops. The final model is described in the published protocol of the IPCAS trial. 34 It involved five components: a structured review, a direct point of contact (DPoC), improving communication, local service mapping and training of primary care professionals.
A structured review would be performed by a practice nurse as part of the regular annual review recommended by current guidelines. 15 In advance of the review, a 15-item checklist of poststroke needs30 would be sent by the general practice to the stroke survivor, who would be asked to identify the needs which are relevant to them, choose three key needs to address during the structured review and grade them in order of importance. The review should last approximately 20 minutes and include a routine physical check-up based on Quality and Outcomes Framework recommendations (e.g. blood pressure, records of immunisation and medication review), followed by the discussion of the needs as identified by the stroke survivor on the checklist. The expected outcome would be an action plan agreed with the stroke survivor on how to address each need.
The DPoC would provide signposting to further specialist or community services, offer advice for stroke-specific issues, give brief support over the phone and arrange follow-up appointments and, if appropriate, case management. A single or several practice nurses would assume the role. If unavailable at the time of a call, a designated member of the care team would return the call later.
To improve communication between primary and secondary care, a meeting would be arranged for general practice staff, representatives from hospital stroke services and the community neurorehabilitation team. The aims of the meeting would be to inform the practice staff of options available in the community or hospital for stroke care (referrals); facilitate further contact; discuss referral criteria, and routes into the services; and discuss case vignettes derived for the consensus study.
To address the information need regarding local services for stroke-related problems, we catalogued stroke (and other relevant) services in participating localities and included information on how to access them. An Information Scientist identified services available in the locality to address the problem areas listed in the poststroke checklist, how to access them and which individuals and organisations act as gatekeepers. Set-up of the resource in the optimal format for users was done in collaboration with administrative staff at the practice. This compendium of stroke services was also made available for participants of the MLAS programme (see below).
Training for practice staff involved in structured stroke reviews would be provided: an overview of stroke and stroke-related long-term needs, followed by discussion of three to four vignettes based on items from the stroke review checklist. Practice staff would discuss the most suitable course of action in each situation and receive feedback from the research team. The list of key health and social services available in the local area would be provided. We would discuss with the practice how best to embed the DPoC role.
6. Pilot the primary care model
Aspects of the pilot study, which is more correctly described as a feasibility study,35 were published in our paper on using a checklist to facilitate management of long-term care needs after stroke. 30
This was a 6-month non-randomised, uncontrolled study in a single practice in Cambridgeshire. The aim was to assess feasibility of delivering the new model of primary care for stroke. Specific objectives were to assess the uptake of the new service by patients, the workload implications for the practice and acceptability of the model. We also used the results to inform the subsequent trial design (recruitment rate and completion rate of questionnaires intended as outcome measures).
A training session for practice staff to deliver the IPCAS intervention was set up, and a one-off meeting was organised for general practice staff, representatives from hospital stroke services and the community neuro-rehabilitation team. The study population was adult stroke survivors identified from the general practice stroke register. Patients were excluded if they were on the palliative care register or resident in a nursing home. The practice generated a list of potentially eligible people from their clinical computer system which was reviewed by the GP. Potential participants were sent an invitation letter to take part in the study, with a consent form to return to the researchers. A single reminder was sent if there was no response. On receipt of the consent form, the stroke survivor was invited for a review and sent the checklist to complete. At the end of the 6-month period, a debrief meeting was held with practice staff. Patients completed a feedback questionnaire immediately after the review and after 3 months.
Forty-eight stroke survivors were sent an invite from the general practice, and 13 participants were recruited. All attended a stroke review with a practice nurse. Nine had completed the checklist prior to the review. The four who had not did not feel they had any stroke-related problems. While participants did identify their needs from the checklist, none of them ranked what were their top three. Clear action plans were agreed in 10 reviews. Twelve participants completed the first feedback questionnaire, and 11 the second one. Most found the checklist easy to complete and useful, and most were quite or very satisfied with the review. The mean review length was 44 (range 25–55) minutes. Part of this time was taken dealing with questions about the research. The other components of the model (DPoC; service mapping resource) were found to be acceptable to practice staff.
The results from this feasibility study were used to set realistic target recruitment for practices that would be engaged in the main trial. We also modified the intervention by adapting the staff training, simplifying the checklist and reducing the paperwork needed to be completed by the practice nurse.
Develop a ‘Managing Life After Stroke’ programme (including self-management) for people with stroke
Self-management programmes have been demonstrated to be effective for other long-term conditions, such as type 2 diabetes. 36 A variety of self-management programmes have been developed for stroke, and there is some evidence of benefit in terms of QoL. 37 However, it is not clear what implementation criteria should be for a self-management programme for stroke. The MLAS programme was developed in three phases: a literature review, a consultation phase with HCPs and stroke survivors and an iterative phase. The development work has been published (https://www.tandfonline.com/doi/full/10.1080/09638288.2022.2029959). 38 A feasibility study was then carried out, which has also been published (https://www.tandfonline.com/doi/full/10.1080/09638288.2022.2029960). 39
Literature review
A literature search was carried out (see publication for search strategy)38 to identify pilot studies, RCTs and systematic reviews that had been published between 2005 and 2015 on self-management interventions for stroke.
The literature review identified 25 RCTs and 6 systematic reviews relevant to the aims of this study. Interventions were delivered individually face to face (n = 11), group-based (n = 7), via telephone (n = 3), online (n = 2) or as a workbook (n = 2). Topics across the identified interventions included: understanding stroke (recovery, recurrence, medication, prevention); practical advice; managing emotions and behaviours; health behaviour change (promotion of health lifestyle); dealing with stress and fatigue and future focus. Resources used within the interventions included personalised manual/workbook, written tip sheets, card tasks (to identify goals), prevention package, risk factor profiles, keeping well plan, fatigue diary and problem rating sheets.
Consultation phase
Building on the findings from the literature review, we undertook consultation with a multidisciplinary healthcare professional group and stroke survivor groups. Workshops were held with HCPs and stroke survivors separately to identify the different perspectives on stroke survivor needs, the timing and format of a stroke self-management programme as well as priorities for content. HCPs included physiotherapists, occupational therapists, nurses, speech and language therapists and stroke consultants. Three separate stroke survivor groups (including carers) were consulted across Leicester and Cambridge.
Key areas that were highlighted by HCPs included emotional needs and practical strategies to manage low mood; prevention strategies to reduce the chance of further strokes; follow-on support and education to aid stroke survivors’ recovery, adjustment and adaptation; and an introductory individual appointment prior to attending group sessions.
It was acknowledged that challenges may exist in offering a programme suitable for all stroke survivors at different stages of recovery with varying disabilities and effects (e.g. catering for physical disabilities as well as cognitive and speech problems).
Stroke survivors and carers also supported the idea of an individual appointment at the outset. They stressed that the programme should be held in an accessible venue and should focus on including management of emotions and confidence building. Stroke survivors felt the programme should consist of four to five group sessions, preferably held in the morning due to fatigue experienced later in the daytime.
Iterative phase
Based on the literature review and consultation, a prototype self-management programme was developed. The philosophy underpinning MLAS was that it should be patient-centred, enable empowerment and assist people who have had a stroke to maximise their well-being and QoL. The aim is to achieve this across four areas: social well-being and integration; acquiring a level of understanding of, and capacity to manage, emotional responses to living with a stroke; maximise own physical potential (including cognitive and sensory abilities) and minimise risk of future stroke. MLAS was grounded in a narrative approach (i.e. participants would become more aware of their journey with stroke) and utilised Social Learning Theory, in particular self-efficacy. 40 The course also utilised aspects of cognitive behavioural theory and the capability, opportunity, motivation and behaviour (COM-B) model. 41 In terms of format, MLAS consists of one 30- to 45-minute one-to-one individual appointment, four weekly group sessions of 2.5 hours each delivered by two facilitators and a final 30-minute one-to-one individual appointment 4 weeks after the final group session.
MLAS was tested in two iterative cycles. The first involved volunteers (10 stroke survivors and 4 carers) from an existing stroke support group in the community. The second was delivered to six stroke survivors and three carers who volunteered from contacts within the research development team or wider support groups. After each iterative session, feedback and observation notes were discussed within the development team, and further amendments were made to the draft curriculum plans. A feedback PPI group was held by a researcher not involved in the delivery at the end of each iteration programme, for participants to share their thoughts and insights on the programme.
Overall, MLAS was well received. Minor amendments were made to content, resources and delivery. Some written activities were changed to a verbal format to accommodate communication needs and physical abilities of participants. Written summaries of key points were added to aid those with memory problems. The narrative journey metaphor was incorporated and referred to more often throughout MLAS to enhance participant engagement. Feedback led to changes to the structure and content of some sessions. For example, some group sessions were made interchangeable based on participants’ preferences. An additional section about relationships was also introduced.
Following feedback from the iterative phase, we developed a final curriculum detailing key outcomes, content, facilitator behaviours, participant activities and resources for each session. We also provided a session outline, example open questions and guidance. We provided resources, including a road map, action plan and photographic images of ways to manage health and well-being. Handbooks for participants for each group session were developed. These included worksheets and opportunities for participants to write down their own notes. A stroke directory of useful local and national services, developed as part of the IPCAS primary care model, was made available.
Feasibility study
The aim of this study was to test the acceptability and feasibility of MLAS as well as to explore what outcome measures to include as part of further testing.
First, six facilitators were trained on a 3-day training course incorporating both the underlying principles of MLAS and its content and stroke-specific knowledge. Second, invitation letters were sent to people on the stroke registers of four general practices (three in Leicestershire; one in Cambridgeshire). People who expressed interest were offered an appointment at a community venue. At this visit, consent was obtained, and a number of baseline questionnaires were completed. The stroke survivors, some with their carers, then attended the MLAS courses. A feedback session was held at the end of the last session, facilitated by an independent researcher, and a separate feedback session was held with facilitators. Further questionnaires were sent out to participants after the final session.
Seventeen stroke survivors and seven carers participated in three MLAS courses. Fifteen (88%) patients and five (71%) carers completed the course. Fourteen out of 15 (93%) participants who completed a follow-up questionnaire said they would recommend the course to someone else. Participants approved of using a community venue (rather than a hospital), felt that the one-to-one with a facilitator prior to the group sessions was important and valued the sense of not being alone that came from the groups. Some participants would have liked to have attended the course earlier after their stroke – the average time lapse was 7 years. With regard to the questionnaires that were used as possible outcome measures, change was observed in the Southampton Stroke Self-Management Questionnaire (SSSMQ),42 but not in the others. Facilitators wanted more time in training and a chance to practice their skills. Suggestions were made for how to improve the training (e.g. showing videos of facilitators delivering sessions; including delivery ‘tips’). In parallel to the participant feedback, facilitators speculated as to whether the course should be offered earlier in the stroke pathway, as some participants had already largely adapted to their stroke.
This study has shown that My Life After Stroke was an acceptable and feasible self-management programme to deliver to stroke survivors in the community. Feedback from MLAS was positive, although completion of all the questionnaires was difficult for some. Attendance rates and retention to MLAS itself were high, suggesting it is a feasible programme to deliver. Retention and feedback from those who attended MLAS were positive, suggesting that attenders found it beneficial.
Evaluate the effectiveness and cost effectiveness of these interventions
The final evaluation phase of the programme consisted of three elements: a cluster RCT, a process evaluation and an economic evaluation.
Cluster randomised controlled trial
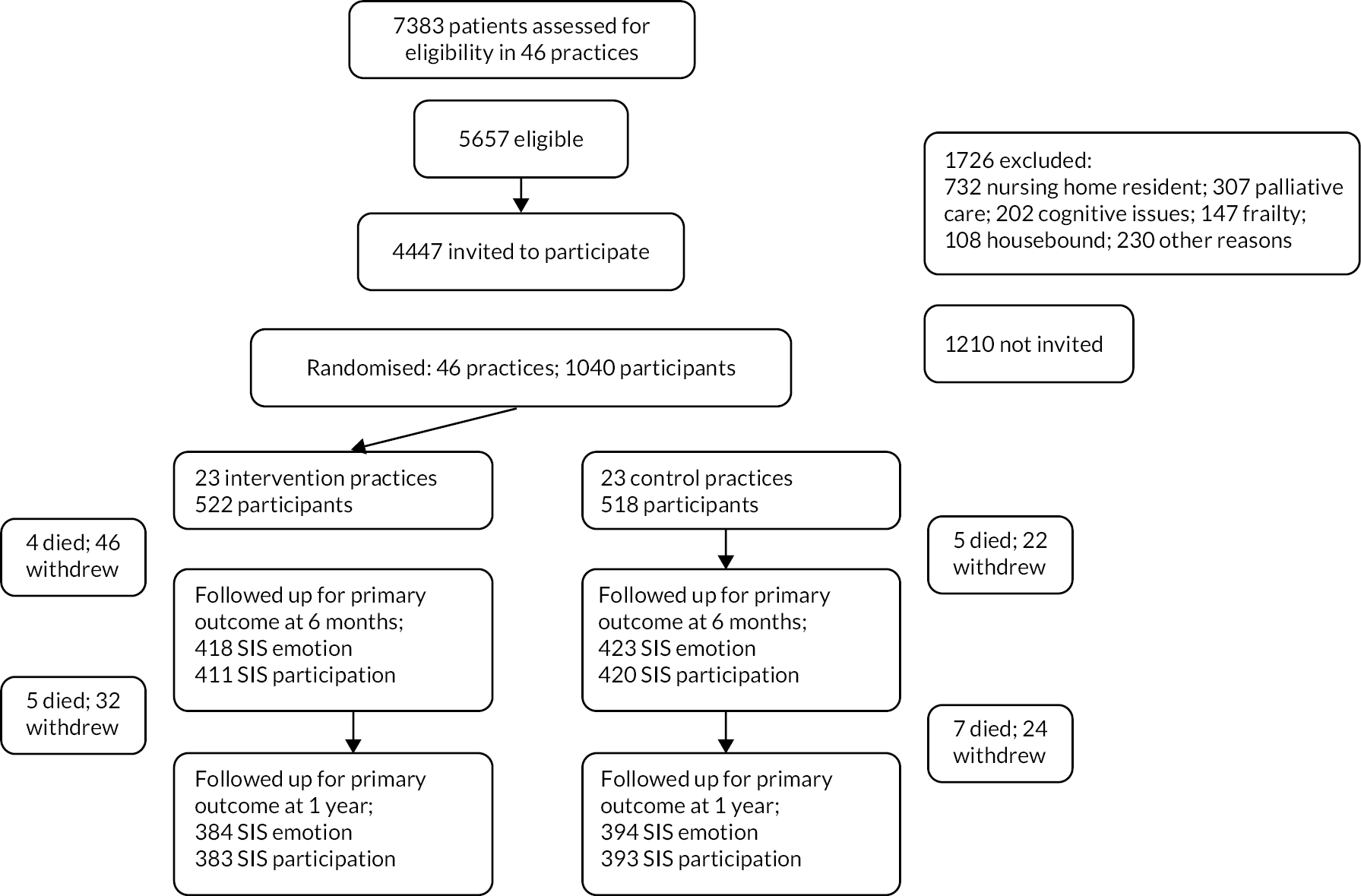
The protocol for the trial has been published (http://doi.org/10.1136/bmjopen-2019-030285). 34 Since the intervention was to be delivered at the level of a general practice, a cluster design was adopted. See Figure 2 for the trial profile.
FIGURE 2.
Trial profile.
The aim was to evaluate the effectiveness of the IPCAS model in improving emotional health and increasing participation of people with stroke living in the community compared to standard care. The design was a two-arm cluster RCT, with the unit of randomisation being a general practice. The coprimary outcomes for the trial were two subscales (emotion and participation) of the Stroke Impact Scale (SIS) v3.0 12 months after randomisation (adjusted for baseline). 43 Analysis set significance at 2.5% [and confidence intervals (CIs) correspondingly at 97.5%] to take account of the two coprimaries in the analysis.
We recruited practices with at least 100 patients on their stroke register from the East of England and East Midlands. We aimed to recruit an average of 20 patients per cluster from 46 general practices (i.e. 920 participants). We estimated this would give us 90% power to detect an effect size of 0.33. We recruited patients by mail. Once we had sent all invitation letters and reminders for a practice, it was randomised centrally using a stratified random permuted block design. Practices in the intervention arm would then send out invitations for a stroke review and our 15-item checklist. 30 At the review, the patient was given information about the MLAS programme, including information about how to access it. It was intended that the research team would arrange meetings between the primary care staff of intervention practices and specialist staff from the hospital and the community in order to enhance primary/secondary care communication. In the event, this proved logistically difficult, so in most cases, the primary care staff were provided videos of the specialist staff explaining their service and how the practice could contact them. Training was provided for all intervention practices which included discussion of the mapping of local services that was provided and how the single point of contact would work. In the control arm, we asked general practices to continue to provide ‘usual care’. Outcome data were collected by postal questionnaire at baseline, 6 and 12 months after randomisation. If data were missing for the coprimary outcomes, then patients were followed up by telephone. The general practice records were reviewed after 12 months to ascertain health service use of trial participants.
More details on the results are provided in Appendices 3–5.
Recruitment took place between May 2018 and April 2019. We sent invitations to 4432 patients, of whom 23.5%, 1040 patients (113% of target), were recruited from 46 practices. The number of patients recruited per practice varied from 8 to 36. The mean age of participants was 70.6 years, and 63.1% were male. Twenty-three participants (2.2%) were of non-white ethnicity. The median time since last stroke of the trial population was 5 years. There were no important differences in baseline characteristics between intervention and control participants (see Table 1). Over the course of a 12-month follow-up, there were 21 deaths (9 in the intervention arm; 12 in the control arm), and 124 patients withdrew (78 in the intervention arm; 46 in the control arm).
| Intervention (n = 522) | Control (n = 518) | |
|---|---|---|
| Men | 331 (63.4) | 325 (62.7) |
| Age | 70.3 (12.1) | 70.9 (12.0) |
| White ethnicity | 508 (97.3) | 509 (98.2) |
| IMD score | 17.0 (11.6) | 18.0 (12.5) |
| Time since last stroke, months | 65.7 (25.0–128.4) | 51.4 (18.4–113.8) |
| SIS emotion | 69.6 (19.0) | 69.1 (19.2) |
| SIS participation | 69.4 (25.2) | 70.1 (23.3) |
Primary outcome data at 12 months were available for 778 (emotion) and 776 (participation) patients (75% of those randomised, 76% of those still alive). The primary analysis found that at 12 months, the intervention was not associated with any significant change in either of the coprimary outcomes: 0.08 (97.5% CI −2.3 to +2.5) in the emotion outcome and 1.3 (97.5% CI −2.3 to + 4.9) in the participation outcome (see Table 2). An effect size of 0.33 for which the trial was powered is the equivalent of a 6-point change in the emotion outcome and a 9.1-point change in the participation outcome. The 95% CIs of the observed differences between the change in outcome in intervention and control do not include these prior assessments of a clinically important effect. Therefore, it can be concluded that the intervention did not have a clinically important impact on emotion or participation of participants.
| Baseline | n | 6 months | n | 1 year | n | Modelled effect 1 year | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SE) | p-value | 97.5% CI | ||||
| SIS emotion | |||||||||
| Control | 69.1 (19.2) | 513 | 71.2 (18.9) | 423 | 70.9 (19.0) | 394 | |||
| Intervention | 69.6 (19.0) | 511 | 71.7 (17.9) | 418 | 71.6 (18.9) | 384 | 0.08 (1.1) | 0.94 | −2.3 to 2.5 |
| SIS participation | |||||||||
| Control | 70.2 (23.3) | 511 | 71.4 (23.0) | 420 | 71.5 (23.0) | 393 | |||
| Intervention | 69.5 (25.2) | 509 | 72.3 (24.0) | 411 | 72.4 (24.0) | 383 | 1.3 (1.7) | 0.46 | −2.3 to 4.9 |
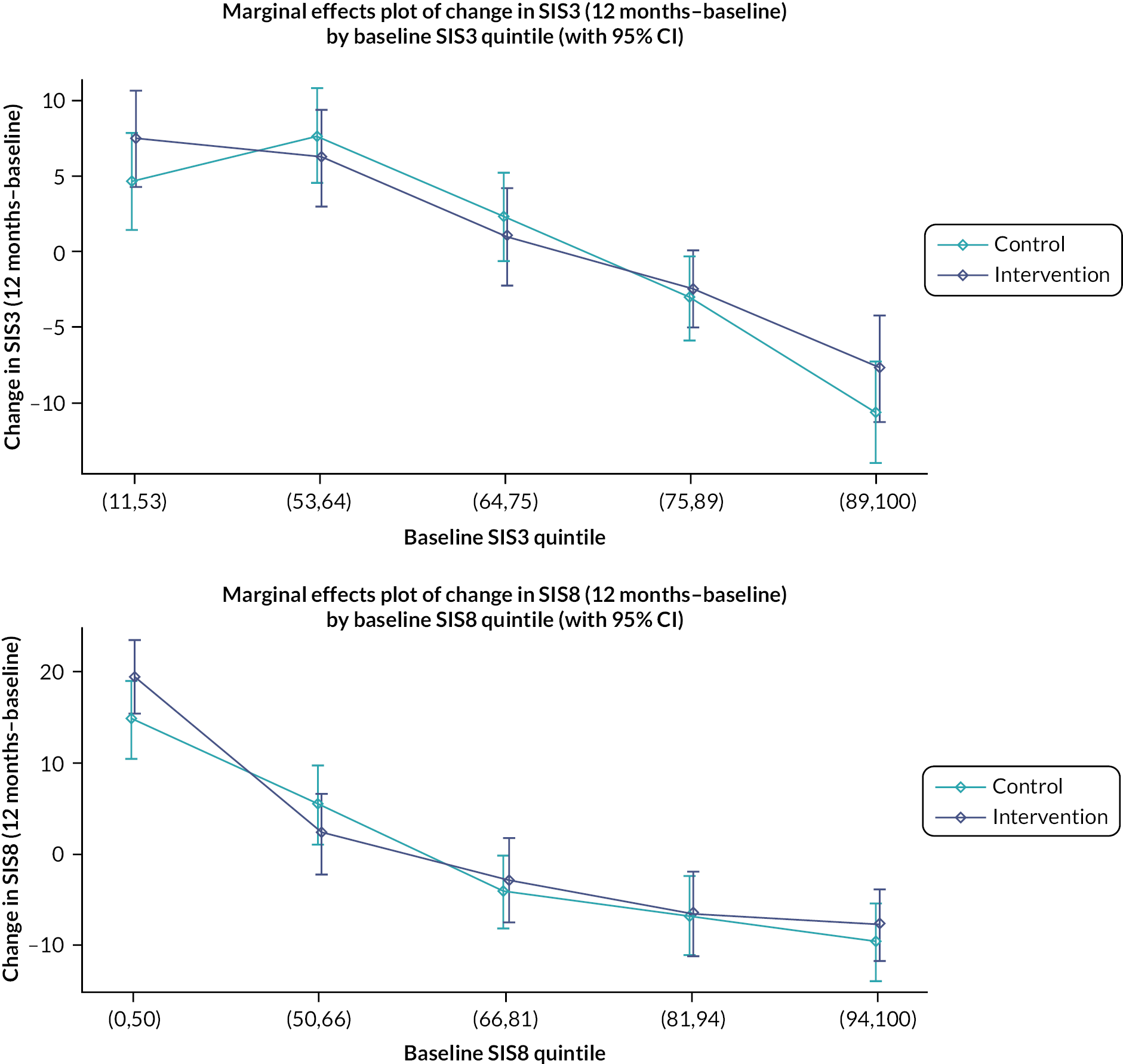
It was noted that there were ceiling effects, in that some participants had maximum scores on the SIS at baseline and so could not undergo a clinically important improvement. Therefore, in a secondary analysis, the study population was split into quintiles by baseline scores for emotion and participation (see Figure 3). For patients in the lowest quintile of emotional health at baseline, there was a non-significant 4-point increase in score in the intervention arm relative to the control arm. A similar effect is seen for participation, where people in the lowest quintile in the intervention arm gained a non-significant 3.4 points more than the control arm. However, even in these quintiles, the effect size was not what had been deemed clinically important.
FIGURE 3.
Change in emotional health (SIS3) and participation (SIS8) in intervention and control from baseline to 1 year by baseline SIS score.
With regard to secondary outcomes, there was also no evidence of effect of the intervention on short form SIS, QoL [EuroQol-5 Dimension, five level questionnaire (EQ-5D-5L)], well-being ICEpop CAPability measure for Adults (ICECAP-A), Southampton Stroke Self-Management questionnaire or Health Literacy Questionnaire (HLQ).
Four hundred and twenty-one (81%) of 522 patients in the intervention arm received a structured stroke review. Three hundred and ninety-three patients completed the checklist. One hundred and seventy patients (43%) identified fatigue as a need. Mobility (153, 39%), mood (130, 33%) and cognition (128, 33%) were the next most commonly identified needs. This high prevalence of fatigue, mood and cognition problems was also noted in the feasibility testing of the Greater Manchester checklist. 26 Two hundred and thirty-seven (56%) of the 421 who had a review had an action plan produced. The principal actions included practical advice given (n = 189), follow-up appointments arranged in primary care (n = 127) and referral to specialist care (n = 104). With regard to both the emotion and participation subscales, patients who attended the review had higher (i.e. better) scores at baseline compared to those who did not, but over time the difference narrowed. Among those who attended a review, people with an action plan had worse emotional health at baseline than people who did not have an action plan, and this difference persisted over time. Thus, there was no evidence that attending a review or having an action plan had any impact on emotional health or participation. Four hundred and twenty participants were invited to an MLAS course, but only 139 participants took part, of whom 102 completed it (73%). Given this variable uptake of the intervention components in the trial, a non-randomised analysis was performed to compare the outcomes in people in the intervention arm who had different levels of intervention. People who attended the MLAS course had lower scores on the emotion and participation scales at baseline, and these differences also persisted. Thus, there was no evidence of an effect of attending the MLAS course on emotional health or participation. We also looked at the effect on the Southampton Stroke Self-Management questionnaire, since this had been specifically selected for its relevance to MLAS, but again, there was no evidence of effect.
In conclusion, the trial showed no evidence of any effect of the IPCAS intervention or the MLAS component on any measured aspect of health status of participants. The study was of sufficient size to rule out a positive effect, and secondary analysis suggested that it was not an artefact due to ceiling effects. There was no association in the intervention arm of receipt of intervention with outcome providing further strength to the conclusion that the intervention and its components do not have an important effect on stroke survivors in the population studied. It is possible that the lack of effect relates to intensity of intervention. We successfully developed an approach to a review of longer-term stroke needs that could be delivered in primary care (i.e. delivered within a 30-minute practice nurse appointment). This contrasts with the 74 minutes to administer the GM-SAT, the impact of which has not been subject to trial evaluation. 26 The EXTRAS trial involved five reviews over 18 months, as opposed to the single review in this study. 10 The study was not powered to explore effect in different subgroups. Thus, we cannot exclude the possibility that the intervention or some of its components might be effective, for example, if offered to stroke survivors earlier in their stroke journey. Nevertheless, this trial strongly suggests that a primary care model targeted at all people on stroke registers is unlikely to be effective.
Process evaluation
The approach taken for the process evaluation has been published (http://bmjopen.bmj.com/cgi/content/full/bmjopen-2020-036879). 44 More detail is provided in Appendix 6.
The specific objectives of the evaluation were to describe how the intervention was delivered, how participants engaged with it and the impact of context on intervention delivery.
A mixed-methods design was used. We were interested in fidelity of design, fidelity of training, fidelity of delivery and fidelity of engagement. Methods included questionnaires sent to practices (to ascertain ‘usual care’); video-recording and audio-recording of training sessions of staff; direct observations; and interviews with staff and participants.
Both IPCAS and MLAS components were coded by two raters against the underpinning theoretical framework (Normalisation Process Theory for IPCAS; Social Learning Theory and Narrative Approach for MLAS). Both interventions were found to align with the appropriate framework(s). As noted above, 81% of intervention patients in the intervention arm received a stroke review. On average, this review lasted 28 minutes (within the 30-minute target). Nineteen of the training sessions of intervention practices were recorded. Each session lasted an average of 90 minutes with three members of staff present (typically a GP, a practice nurse and a practice administrator). The fidelity of training was high, with 96% of planned items covered. Two MLAS training sessions were observed, each involving 10 trainees. Again, the fidelity was high, with 87.5% of planned components covered. With regard to delivery of information at the stroke review, this was assessed both by self-report (high fidelity reported) and by audio-recording and review by a researcher (moderate fidelity observed). The biggest discrepancy was for explanation about the DPoC, which the audio-recording found was only done with moderate fidelity. In terms of the structured review, three-quarters of patients completed the 15-item checklist. Actions (n = 431) reported by staff included follow-up appointments (29%), referrals (25%) and provision of advice (45%). Only 139 patients attended an MLAS course. The main reasons for not attending were that patients felt that they had made a good recovery from their stroke, that they had other health issues, that it was not relevant to them or that they would find it difficult to attend. Six MLAS sessions were observed. Overall fidelity of delivery was high, ranging from 78% to 92%. A sample of patients completing post-MLAS questionnaires gave positive feedback that the objectives of the course had been achieved.
Twenty-seven stroke survivors in the intervention arm were interviewed. Some valued the stroke review, while others felt it would have been useful earlier post stroke. Some felt that their healthcare problems were not related to the stroke. Several participants did not remember being told about the DPoC but said that such a service would be useful. Those that did remember it did not use it but said that was because they did not need to. People liked the checklist, and they valued the service directory (given to them in MLAS). People who had attended MLAS reported benefits to participation, including greater self-awareness, more positive mindset, improving their knowledge and providing them with social support. Control patients that were interviewed did not discuss any stroke-specific needs with their practice during the study.
Eleven HCPs were interviewed. Generally, they found the checklist useful, though time-consuming, as often they needed to help the patient complete it during the appointment (i.e. it had not been filled in advance). Those that used the service mapping found it helpful, but others thought it had too much information. Only one healthcare professional reported being contacted as a result of the DPoC service, but they felt that the practice had gone to considerable effort to set this up. The option of the MLAS course was inconsistently conveyed to patients. The reviews were delivered either by practice nurses or by research nurses. The latter found the reviews more difficult as they were not an integral part of the practice team and did not know the patients.
In summary, with regard to the different components of IPCAS, delivery of the structured review had the most uptake, with over 80% of participants receiving a review, with the checklist being used in three quarters, and action plans being generated. Only a minority of patients attended the MLAS course, but those that did appeared to value it. Practice staff perceived that a lot of effort was put into setting up the DPoC service, but this was hardly used. The local directory of services had variable take-up by HCPs. It did not prove possible to support improved communication between primary and secondary care as originally intended. Instead, we needed to rely on videos of specialist staff explaining what they did and how to access their service. Training fidelity was high.
Health economic evaluation
Though the trial did not demonstrate that the intervention had an effect, it is still important to consider the cost consequences of the intervention. For example, in the EXTRAS trial of extended contact with the early discharge support team after stroke, although the intervention had no impact on the primary outcome measure (extended activities of daily living), it did reduce costs and so was found to be a cost-effective intervention. 10
Costs were measured from the perspective of the UK NHS. Resources used to implement the intervention were costed using a micro-costing approach which took into account the time spent by healthcare staff on training and delivery of the intervention. At 12 months, we sent patients a questionnaire on their contacts with secondary care services and with social services over the course of the trial. We obtained data on contact with primary care from the general practice electronic health records. Quality-adjusted life-years (QALYs) over the course of the trial were estimated from the EQ-5D-5L questionnaire at baseline, 6 and 12 months. Missing data were assumed to be missing at random and were imputed using multiple imputations. We calculated incremental cost-effectiveness ratios (ICERs) to represent the incremental cost required to obtain one additional QALY when moving from usual care to the new intervention. Prespecified analyses included use of multiple imputed data and unadjusted estimates. We also performed ad hoc analyses to determine the impact of baseline QALY scores and performed a complete case analysis.
Detailed results are provided in Appendix 7. The average cost of the intervention was £68 per person. In addition, there was significantly greater use of practice nurses, healthcare assistants and respite care services. There was no significant difference in use of secondary care services. At 1 year, the mean EQ-5D-5L was 0.734 in the intervention arm and 0.721 in the control arm. There was a non-significant mean QALY difference of 0.013 (95% CI −0.024 to 0.048) in favour of the intervention. The intervention was associated with an incremental cost to the NHS of £267.07 per person. This equated to an incremental cost per QALY of £20,863. Using a threshold of £20,000 per QALY, there is a 48.4% chance that the intervention is cost-effective. If we adjust for differences in baseline QALYs, then the incremental cost per QALY rises to £45,489. Using a complete case analysis, the incremental cost per QALY is £39,532.
In conclusion, it is unlikely that the primary care model that we tested is cost-effective at a threshold of £20,000 per QALY. The intervention increased primary care workload, and while it was associated with a non-significant, higher QALY at 12 months, the size of this difference diminished when adjusted for baseline QALY scores.
Patient and public involvement activities across the Improving Primary Care After Stroke programme
Our aim for PPI was to ensure that all aspects of intervention development and trial design were informed by input from stroke survivors and their carers. We actively engaged PPI across the two developmental streams of the programme (Primary Care Model and MLAS) through several routes. These include lay membership on our Programme Steering Committee and our IDG, a lay representative as a research team grant co-applicant, active engagement with The Stroke Association and in several meetings with stroke survivors and carer support groups in the community.
The independent Programme Steering Committee
The independent Programme Steering Committee which includes Marney Williams as a lay member, retains oversight of the research programme. This group met eight times between May 2015 and September 2020, through both the developmental and evaluation stages.
The Intervention Development Group
The IDG met on six occasions between September 2015 and November 2016. The group included two representatives from The Stroke Association and Bundy Mackintosh (a research grant co-applicant and stroke survivor).
Consultation with a stroke survivors support group in Cambridge facilitated by The Stroke Association
Around 20 stroke survivors and carers attended on three separate occasions between April 2015 and March 2017. Participants were informed about the programme of work and invited to comment and provide feedback on specific aspects of our research plan. We gained valuable feedback on study materials and outcome measures for both developmental streams of the programme.
Consultation with a stroke survivors support group in Bedford facilitated by The Stroke Association
Two stroke survivors and one carer attended in April 2016. They were informed about the programme of work and invited to comment and provide feedback on specific aspects of our research plan. Specifically, they provided feedback on the findings from the focus group and materials planned to be used as part of the primary care model for stroke (checklist).
Meeting with a stroke survivor and caregiver consumer research advisory group in Leeds
A research associate from the programme presented the aims and objectives of the research to a group of stroke survivors and caregivers at one of their regular meetings (September 2016). We received patient and caregiver feedback on the results of the qualitative studies, validation of the findings, and informed patients of the progress with the research they had advised on previously. This stimulated a group discussion, and multiple suggestions were incorporated into the study materials following the group’s feedback.
Stroke survivors and carers have been involved throughout the development of the Managing Life After Stroke course
Three consultation meetings with stroke survivor groups took place in April 2016 (2 in Leicester, 1 in Cambridge – 20 people per group) to seek their experience of the impact of stroke, support they received, opinions on content of a self-management intervention, its format, as well as on initial drafts of the programme. Once we had a draft intervention, we tried it out with interested patients and carers and refined the intervention based on feedback from this PPI, as well as the facilitators who ran the sessions.
Secondment of a staff member from The Stroke Association
Between April 2016 and April 2017, a staff member from the charity organisation was seconded to support development of the new primary care model. Specifically, they worked on the service mapping exercise relating to the provision of information about health, social and community care availability within the local area.
Piloting of the stroke survivor topic guides used for intervention fidelity assessment of the randomised controlled trial
The topic guides were initially developed from semistructured interviews completed during intervention development work. The topic guides were then pilot tested, involving six e-mails, three telephone calls and a mock interview with a member of our PPI group, and refinements were made following feedback.
Reflections
Key findings
What does a primary model for longer term stroke care need to address?
-
Our systematic review and meta-ethnography identified that stroke survivors and caregivers feel abandoned because they have become marginalised by services, and they do not have the knowledge or skills to re-engage.
-
Our own qualitative interview studies complemented these findings. Over time, the healthcare needs of patients tend to be less dominated by their stroke and more by factors associated with ageing and multimorbidity. We highlighted the complex needs of caregivers, in particular the impact of the stroke on the relationship with the patient.
-
Our scoping review found that community-based research evidence is largely focused on improving access to rehabilitation, and there is a lack of evidence for how to address problems such as poststroke fatigue, cognitive problems and mental health in primary care.
-
This lack of evidence is problematic since three of the four most frequent needs reported by people with stroke in our trial were fatigue, mood and cognition.
-
Our focus groups with HCPs found that while the need for better communication between primary and secondary care is recognised, the reality is that current care is characterised by silo-based working.
-
Our consensus study on management of longer-term needs after stroke identified that there was a role for primary care, stroke specialists and other specialists. This reinforces the importance of better communication between primary and secondary care.
-
We identified from literature reviews and PPI workshops that the key issues to address in a primary care-based self-management programme to address the longer-term needs of stroke patients were: social well-being and integration, emotional responses to living with a stroke, maximising physical potential and secondary prevention.
Results from our evaluations
-
While we demonstrated that all components of our intervention (use of a checklist to form the basis of a structured review, a group self-management course, a single point of contact in primary care, a local mapping of available services, videos to facilitate contact between primary and secondary care, training of primary care staff) were perceived to be useful by HCPs and patients, we found no evidence of benefit of any of these components, either individually or collectively. Indeed, the CIs around our results were sufficiently narrow to exclude a clinically important effect.
-
We found good evidence from our process evaluation that the intervention was largely implemented as intended.
-
Our cost-effectiveness analysis rules out any plausible wider economic value of the intervention (it increased costs and was not associated with better outcomes).
Impact of patient and public involvement
As summarised previously, PPI had a major impact on the design of the interventions and implicitly provided validation that the interventions would be helpful. We obtained a different perspective from some stroke survivors from our qualitative studies, with feedback that some aspects were not relevant or might have been more helpful earlier in the stroke pathway. Our PPI was composed of longer term stroke survivors, so why the discrepancy? The key point is that it is not the role of PPI members to provide representative views but their own perspectives. It is likely that people who are still engaged with stroke charities and stroke clubs (our sources of PPI members) continue to identify stroke as a major part of their lives and do not reflect the attitudes of people who feel that stroke is no longer something that defines them. This is not to diminish the value of our PPI input but simply to emphasise that other approaches to gaining the patient's voice (such as qualitative research) remain important complementary methods.
Successes
With over 1000 participants, this is the largest completed RCT of an intervention to address longer-term needs of people with stroke. We exceeded our recruitment targets and achieved acceptable follow-up rates (76% of those still alive at 12 months) for our primary outcome measures. In parallel with the trial was an in-depth process evaluation that enabled us to conclude that the lack of impact of the intervention was not due to flawed implementation.
Despite the start of the coronavirus disease 2019 (COVID-19) pandemic during the final year of the trial, we were able to rapidly adapt our process and complete the programme within the original budget and with only a 6-month extension (which was related to the complexities of earlier phases of the programme, rather than to COVID-19).
While the overall result of the trial was to demonstrate that the intervention was not effective, we were able to draw important conclusions for clinical practice and research, as discussed below.
Challenges and lessons learnt
We underestimated the difficulties of incorporating improved primary–secondary care communication into our model. Our original plan, which was prosecuted in the feasibility study, was to set up face-to-face meetings between the specialist stroke team and general practice staff. As we moved into the trial phase, this proved very difficult to arrange due to availability of personnel to attend such meetings in both settings. Therefore, we moved to a more pragmatic, but perhaps less effective approach, of obtaining videos to circulate to the practices of the relevant secondary care staff, which covered what their roles were and how to contact them.
A second challenge was achieving our objective of establishing criteria for re-referral to specialist care through a consensus study. When we originally conceived this study, we anticipated that its output would be a set of consensus-based recommendations to guide referral decisions. However, as we developed this study, we recognised that, in the absence of evidence and in the face of subtle nuances that would influence decisions, such guidelines would have limited face validity. Therefore, we used this study to better understand the types of issues that come into play when rereferral is being considered. Thus, rather than have a set of guidelines to distribute to primary care, we had case scenarios as an outcome that we explored in the training of the primary care professionals.
A third challenge was our choice of primary outcome measure for the trial. We deferred specifying this in advance of developing the primary care model, as we wanted the primary outcome to reflect what the model was specifically aiming to address. We wanted the primary outcome to be sensitive to change, yet not too burdensome to complete. Trials of related interventions addressing long-term issues facing people with stroke had used the Nottingham Extended Activities of Daily Living Scale10 and the General-Health Questionnaire-12. 6 Both these trials had not demonstrated any effects on these outcomes. We selected two outcomes of the SIS – participation and emotion. 43 In the event, we did not demonstrate any difference in outcome between intervention and control in these measures, and ceiling effects were observed. Unlike the EXTRAS trial,10 we did not observe significant differences in any secondary outcomes either, so we have no evidence that had we chosen different primary outcomes, we would have obtained a different result.
Our intervention was delivered within the trial before the COVID-19 pandemic struck. The impact of this pandemic on longer-term support for people with chronic conditions such as stroke has yet to be fully understood. Patterns of care are likely to change permanently with greater use of remote support. This creates some challenges with regard to considering the implications of our findings. It may be, for example, that remote delivery might address some of the practical difficulties of access for some patients and therefore make the intervention more attractive. Similarly, the needs of people with stroke might have changed as a result of the increased isolation imposed by the pandemic.
Finally, we faced the challenge of how to evaluate a multicomponent intervention where there is value in knowing the impact of the individual components as well as the impact of the whole package. While initially we had considered evaluating one of the components (the self-management component, MLAS) using an individually randomised trial nested within the cluster-randomised trial, we recognised that this was going to be impractical. Instead, we decided to do a cluster RCT of the whole model with an embedded observational analysis of the impact of MLAS and a process and qualitative evaluation to explore what was likely to have had the greatest effect. In the event, the overall trial did not detect any benefit, and the observational analysis of MLAS did not suggest any effect. However, given the relatively small numbers of people (n = 139) who attended an MLAS course, we cannot exclude an effect of that component, particularly in an appropriate subgroup (e.g. patients in the first 6 months after their stroke).
Limitations
Although we exceeded our recruitment target per practice, the age, sex and ethnicity distribution of our trial population was not typical of people on a primary care stroke register, being younger with a higher proportion of men and low representation of minority ethnic groups. 45 Given our qualitative findings that some older people identified their health problems as related to ageing rather than to their stroke, the age distribution of our trial population does not have a major impact on the applicability of our findings, since such people would be less likely to benefit from the intervention.
Some components of the intervention had only limited take-up. There was over 80% uptake of the structured stroke review, but only a third of people who were invited to take part in the self-management training programme took up the offer. While available, the single point of contact at the practice was hardly used. We had not been able to implement our improved communication between primary and secondary care in the way that we had planned.
Our primary outcome measures did have ceiling effects in that some participants had maximum scores on the SIS at baseline. Nevertheless, clinically important differences between intervention and control groups were not observed in the subgroups where changes in scores were possible.
A key component of the intervention was a structured review of needs. An implicit assumption in this is that once a need has been identified it can be addressed. This gives two possible explanations for why the intervention did not work. Firstly, there is a lack of evidence as to how to address the most commonly identified needs. Secondly, even if there is an evidence base, there may be a lack of service availability. As we showed, psychological needs post stroke are common, but access to specialist psychological services is limited, even when patients are still under specialist care. 46
Our follow-up was limited to 1 year. It is conceivable that the effect of the intervention might have increased over time. While the observed differences in the coprimary outcomes were larger at 12 months rather than 6 months, they were still well short of what we had prespecified as a clinically important threshold. The intervention was not repeated at 1 year, so it is unlikely that the differences would have crossed this threshold had we followed up for a longer period of time.
Conclusions
This programme of research has reaffirmed the importance of addressing longer-term needs of people after stroke in the community. Unfortunately, the primary care model that we developed was not effective at addressing these needs in terms of improving participation and emotional health of participants. It is conceivable on the basis of our qualitative findings that an intervention focused on patients earlier after their stroke or one which is more intensive might be effective, though we have no quantitative evidence to support these possibilities. There was a mismatch between the needs reported by stroke survivors and evidence for how to address these needs. It might be that the primary care model would be of value if there were effective interventions to address the identified needs.
Implications for practice
-
We found no evidence that structured stroke reviews offered in primary care to all stroke survivors in the community are effective at addressing stroke-related needs. Such reviews should perhaps therefore be time-limited after stroke and only continued in selected patients.
-
The 15-item checklist that we developed is practicable to be used in primary care for patients where it is perceived to be useful.
-
Given that stroke survivors in this study report high prevalence of fatigue, low mood and cognitive issues, greater attention needs to be paid to services that can address these needs, informed by evidence on interventions, described below under research recommendations.
-
From the evidence in the wider literature, self-management programmes have a role in improving QoL in stroke survivors in the community. 37 Our findings suggest that such programmes are likely to be of greatest value if offered early after discharge from hospital.
Implementation issues
The process evaluation found that the intervention was largely implemented as intended, though we did not implement improved communication between primary and secondary care in the way we intended. The new Integrated Care Systems that came into operation in the NHS in July 2022 are an explicit acknowledgement of the need to better integrate health and social care. The Integrated Care Partnerships that will be set up under these may facilitate better integration between primary and secondary care. New efforts to integrate primary and secondary postacute stroke care might fall within the remit of the Integrated Community Stroke Service (ICSS). 47 It is envisaged that ICSS provision will be for 6 months post discharge from hospital or 6 months for community referrals. However, the model does not specify how the ICSS will engage with primary care, which will be particularly relevant for referrals from the community.
In the post-July 2022 NHS, it would be appropriate for the ICSS to consider referral of patients to self-management programmes, since they are supporting people in the time period when our qualitative feedback suggested they might be most beneficial. The checklist that we developed could be appropriate for use by the ICSS, since the intention is that it will be a needs-led service.
The ability of primary care to undertake routine reviews is likely to be more limited than it was prepandemic. 48 We found no benefit of needs-based reviews applied to the whole population of stroke survivors, so any implementation of needs-based reviews in primary care should be focused only on people where there might be benefit.
Recommendations for future research
-
Research to inform who should be offered poststroke assessment of needs in the community in the longer term (after 6 months).
-
Development and evaluation of interventions to address fatigue, low mood and cognitive problems of people with stroke in the community.
The first recommendation is consistent with the observation of the National Stroke Programme research demand signalling which acknowledges that stroke survivors and carers may need support long after hospital discharge and that what is needed may not be obvious during initial follow-up. 49 The second recommendation is consistent with one of the research questions that demand signalling identified:
-
- what are the optimal ways to manage and treat the non-apparent (hidden) effects of stroke, including incontinence, fatigue, emotional ability, cognitive deficits, memory problems, dysphasia and secondary complications?
In terms of prevalence (not the only consideration), our findings support the inclusion of fatigue, mood and cognition as priorities on the NHS Demand Signalling list.
Acknowledgements
We acknowledge the input of:
The IPCAS Investigators: Melanie Davies, Marian Carey, Yvonne Doherty, Kamlesh Khunti, Bundy Mackintosh, Adrian Mander, Christopher McKevitt, Martin Roland, Stephen Sutton, Marion Walker, Elizabeth Warburton, Sue Jowett.
The MLAS development group involved a number of team members, including Rosie Horne, Clare Makepeace, Lorraine Martin Stacey, Yvonne Doherty, Jayna Mistry, as well as facilitators and PPI members who contributed to various aspects of study set-up, trial management, intervention development, refinement and delivery.
Other research staff working on the programme: James Brimicombe (database management); Sarah Dawson (statistician); Lisa Lim (clinical research associate); Caroline Moore (research associate); Alex Newbrook (study administrator); Dominika Pindus (research associate); Peter Scott-Reid (NIHR research methods fellow); Victoria Theobald (study administrator); Grace Turner (NIHR post-doctoral fellow); Julie Wych (statistician).
Other senior research staff in Cambridge who contributed to supervision: Robbie Duschinsky (university senior lecturer, social science); Steve Morris (RAND professor of health services research).
The IPCAS Independent Programme Steering Committee: Professor Alastair Hay (Chair); Professor Anne Forster; Professor Richard Stevens; Ms Marney Williams.
Professor Noor Azah Abd Aziz, Department of Family Medicine, National University of Malaysia.
Contributions of authors
Ricky Mullis (https://orcid.org/0000-0002-5129-290X) (Senior Research Associate) Programme Manager and wrote the first draft of this report.
Maria Raisa Jessica Aquino (https://orcid.org/0000-0002-3989-1221) (Research Associate) led the process evaluation.
Elizabeth Kreit (https://orcid.org/0000-0001-9623-0553) (Trial Manager) ran the IPCAS trial.
Vicki Johnson (https://orcid.org/0000-0001-6709-7634) (Research Assistant) wrote up the MLAS development.
Julie Grant (https://orcid.org/0000-0002-6547-5221) (Research Assistant) carried out interviews, trial fidelity observations and 2-researcher coding and agreements for the process evaluation.
Emily Blatchford (https://orcid.org/0000-0003-3429-402X) (Research Administrator) analysed the process evaluation of the MLAS programme.
Mark Pilling (https://orcid.org/0000-0002-7446-6597) (Senior Research Associate, Statistician) analysed the IPCAS trial.
Francesco Fusco (https://orcid.org/0000-0001-5515-3977) (Research Associate, Health Economics) carried out the economic analysis of IPCAS.
Jonathan Mant (https://orcid.org/0000-0002-9531-0268) (Professor of Primary Care Research) Chief Investigator and led on second and final drafts of this report.
Publications
Full publications
Aziz NA, Pindus DM, Muillis R, Walter FM, Mant J. Understanding stroke survivors’ and informal carers’ experiences of and need for primary care and community health services – a systematic review of the qualitative literature: protocol. BMJ Open 2016:e009244. http://doi.org/10.1136/bmjopen-2015-009244
Pindus DM, Lim L, Rundell AV, Hobbs V, Aziz NA, Mullis R, Mant J. Primary care interventions and current service innovations in modifying long term outcomes after stroke: a protocol for a scoping review. BMJ Open 2016;6:e012840. http://doi.org/10.1136/bmjopen-2016-012840
Pindus DM, Mullis R, Lim L, Wellwood I, Rundell AV, Aziz NAA, Mant J. Stroke survivors’ and informal caregivers’ experiences of primary care and community health services – a systematic review and meta-ethnography. PLOS ONE 2018;13(2):e0192533. https://doi.org/10.1371/journal.pone.0192533
Mullis R, Aquino MRJ, Dawson SN, Johnson V, Jowett S, Kreit E, Mant J; on behalf of the IPCAS Investigator Team. Improving Primary Care After Stroke (IPCAS) trial: protocol of a randomised controlled trial to evaluated a novel model of care for stroke survivors living in the community. BMJ Open 2019;9:e030285. http://doi.org/10.1136/bmjopen-2019-030285
Turner GM, Mullis R, Lim L, Kreit L, Mant J. Using a checklist to facilitate management of long-term care needs after stroke: insights from focus groups and a feasibility study. BMC Family Practice 2019;20:2. https://doi.org/10.1186/s12875-018-0894-3
Aquino MR, Mullis R, Kreit E, Johnson V, Grant J, Lim L, et al. Improving Primary Care After Stroke (IPCAS) randomised controlled trial: protocol for a multidimensional process evaluation. BMJ Open 2020;10:e036879. URL: http://bmjopen.bmj.com/cgi/content/full/bmjopen-2020-036879
Aquino MRJ, Mullis R, Moore C, Kreit E, Lim L, McKevitt C, et al. ‘It’s difficult, there’s no formula’: qualitative study of stroke related communication between primary and secondary healthcare professionals. Int J Integr Care 2020;20:11:1–10. http://doi.org/10.5334/ijic.5465
Lim L, Mant J, Mullis R, Roland M. When is referral from primary care to specialist services appropriate for survivors of stroke? A modified RAND-appropriateness consensus study. Family Practice 2020;21:66. https://doi.org/10.1186/s12875-020-01139-4
Johnson VL, Apps L, Kreit E, Mullis R, Mant J, Davies MJ; on behalf of the MLAS Development Group. The feasibility of a self-management programme (My Life After Stroke; MLAS) for stroke survivors. Disability and Rehabilitation, published online 1 February 2022. URL: https://www.tandfonline.com/doi/full/10.1080/09638288.2022.2029960
Johnson V, Apps L, Carey ME, Kreit E, Mullis R, Hadjiconstantinou M, et al.; on behalf of the MLAS Development Group. The development of a self-management interventions for stroke survivors – My Life After Stroke (MLAS). Disability and Rehabilitation, published online 3 February 2022. https://www.tandfonline.com/doi/full/10.1080/09638288.2022.2029959
Scott Reid P, Neville E, Cater C, Mullis R, Mant J, Duschinsky R. Accounts of preventative coping: an interview study of stroke survivors on General Practice registers. BMJ Open 2022;12:e058441. https://doi.org/10.1136/bmjopen-2021-058441
Blatchford EG, Aquino MRJ, Grant J, Johnson V, Mullis R, Lim L, Mant J. Patients’ experience of and participation in a stroke self-management programme, My Life After Stroke (MLAS): a multimethod study. BMJ Open 2022;12(11):e062700. https://doi.org/10.1136/bmjopen-2022-062700
Conference abstracts
Pindus DM, Mullis R, Lim L, Wellwood I, Rundell AV, Aziz NA, et al. The experiences of and need for primary care and community health services in informal carers of stroke survivors – a systematic qualitative review. Eur Stroke J 2016;1(1_suppl):570.
Moore C, Pindus DM, Kreit E, Lim L, Mullis R, Mant J. Changing care needs of stroke survivors in primary care. Eur Stroke J 2017;2(1_suppl):369.
Pindus DM, Mullis R, Moore C, Kreit E, Lim L, Mant J. Improving communication between generalists and specialists for patient benefit: insights from focus groups on long-term care after stroke. Eur Stroke J 2017;2(1_suppl):369.
Lim L, Pindus DM, Mullis R, Moore C, Kreit E, Mant J. Factors influencing re-referral decisions to specialist care in stroke survivors: insights from focus groups on long-term care after stroke. Eur Stroke J 2017;2(1_suppl):370.
Moore C, Pindus DM, Kreit E, Lim L, Mullis R, Mant JM. Identifying the long-term needs of carers of stroke survivors living in the community to inform a new primary care model. Eur Stroke J 2017;2(1_suppl):371.
Kreit E, Pindus DM, Mullis R, Chowienczyk S, Mant J. Clinical practice guidelines for stroke rehabilitation and long-term management: similarities and differences in recommendations for mood, aphasia and cognitive deficits. Eur Stroke J 2017;2(1_suppl):372.
Chowienczyk S, Nguyen Q, Pindus DM, Kreit E, Mant J, Mullis R. A comparison of national guidelines for secondary prevention of ischaemic stroke. European Stroke Journal 2017;2(1_suppl):347.
Pindus DM, Mullis R, Lim L, Wellwood I, Kreit E, Lim L, Mant J. Primary care interventions for long-term outcomes after stroke: a scoping review of reviews and recent trials. Eur Stroke J 2017;2(1_suppl):453.
Moore C, Pindus DM, Kreit E, Lim L, Mullis R, Mant J. Exploring stroke survivor and informal carer need: informing a new primary care model. Int J Stroke 2017;12(S3):14.
Johnson V, Schreder S, Kreit E, Mullis R, Mant J. A feasibility study of My Life After Stroke (MLAS) – a structured self-management programme for stroke survivors. Int J Stroke 2018;13(S3):25.
Lim L, Mullis R, Roland M, Mant J. Care of post-stroke patients: identifying circumstances in which specialist referral may be beneficial. Int J Stroke 2018;13(S3):30.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Ethics statement
The focus groups and interview studies to inform intervention development were approved by the East of England Cambridge South Research Ethics Committee (REC) (REC Reference: 15/EE/0374). The Consensus study was approved by the University of Cambridge Psychology Research Ethics Committee (REC) – REC Reference PRE.2017.059. The MLAS feasibility work was approved by the West Midlands, South Birmingham Research Ethics Committee (17/WM/0036). The IPCAS feasibility study was approved by the West Midlands Edgbaston REC (17/WM/0104). The process evaluation and RCT were approved by the Yorkshire & Humber-Bradford Leeds NHS Research Ethics Committee (17/YH/0441).
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the PGfAR programme or the Department of Health and Social Care.
References
- The NHS Long term Plan n.d. https://www.longtermplan.nhs.uk/ (accessed 27 January 2021).
- Li L, Scott CA, Rothwell PM. Oxford Vascular Study . Trends in stroke incidence in high-income countries in the 21st Century: population based study and systematic review. Stroke 2020;51:1372-80.
- McKevitt C, Fudge N, Redfern J, Sheldenkar A, Crichton S, Rudd AR, et al. Self-reported long term needs after stroke. Stroke 2011;42:1398-403.
- Chen T, Zhang B, Deng Y, Fan J-C, Zhang L, Song F. Long-term unmet needs after stroke: systematic review of evidence from survey studies. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-028137.
- National Clinical Guideline Centre . Stroke Rehabilitation: Long Term Rehabilitation After Stroke. Clinical Guideline 162: Methods, Evidence and Recommendations 2013. www.nice.org.uk/guidance/cg162/evidence (accessed 27 January 2021).
- Forster A, Young J, Chapman K, Nixon J, Patel A, Holloway I, et al. Cluster randomized controlled trial: clinical and cost-effectiveness of a system of longer term stroke care. Stroke 2015;46:2212-9.
- Mayo NE, Anderson S, Barclay R, Cameron JI, Desrosiers J, Eng JJ, et al. Getting on with the rest of your life following stroke: a randomized trial of a complex intervention aimed at enhancing life participation post stroke. Clin Rehabil 2015;29:1198-211.
- National Institute for Health and Care Excellence . 2019 Surveillance of Stroke Rehabilitation in Adults (NICE Guideline CG162) 2013. www.nice.org.uk/guidance/cg162/evidence (accessed 27 January 2021).
- Langhorne P, Baylan S. Early Supported Discharge Trialists . Early supported discharge services for people with acute stroke. Cochrane Database Sys Rev 2017;2017. https://doi.org/10.1002/14651858.CD000443.pub4.
- Rodgers H, Howel D, Bhattarai N, Cant R, Drummond A, Ford GA, et al. Evaluation of an Extended Stroke Rehabilitation Service (EXTRAS). A randomized controlled trial and economic analysis. Stroke 2019;50:3561-8.
- Department of Health . National Service Framework for Older People 2001.
- Sentinel Stroke National Audit Programme . Springboard for progress: the seventh SSNAP annual report: stroke care received for patients admitted to hospital between April 2019 to March 2020. Sentinel Stroke National Audit Programme 2020. www.strokeaudit.org/Home.aspx (accessed 28 January 2021).
- Health Improvement Scotland, Royal College of Physicians, Royal College of Physicians of Ireland . National Clinical Guideline for Stroke for the UK and Ireland 2023:169 n.d. www.strokeguideline.org (accessed 22 November 2023).
- Forster A, Hartley S, Barnard L, Ozer S, Hardicre N, Crocker T, et al. LoTS2Care Programme Management Group . An intervention to support stroke survivors and their carers in the longer term (LoTS2Care): study protocol for a cluster randomized controlled feasibility trial. Trials 2018;19.
- National Clinical Guideline for Stroke. London: Royal College of Physicians; 2016.
- Hare R, Rogers H, Lester H, McManus RJ, Mant J. What do stroke patients and their carers want from community services?. Fam Pract 2006;23:131-6.
- Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross sectional study. Lancet 2012;380:37-43.
- Aziz NA, Pindus DM, Muillis R, Walter FM, Mant J. Understanding stroke survivors’ and informal carers’ experiences of and need for primary care and community health services – a systematic review of the qualitative literature: protocol. BMJ Open 2015;6. http://doi.org/10.1136/bmjopen-2015-009244.
- Pindus DM, Mullis R, Lim L, Wellwood I, Rundell AV, Aziz NAA, et al. Stroke survivors’ and informal caregivers’ experiences of primary care and community health services – a systematic review and meta-ethnography. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0192533.
- The Joanna Briggs Institute . Joanna Briggs Reviewers’ Manual: 2011 Edition 2011.
- Moore C, Pindus DM, Kreit E, Lim L, Mullis R, Mant J. Changing care needs of stroke survivors in primary care. Eur Stroke J 2017;2.
- Moore C, Pindus DM, Kreit E, Lim L, Mullis R, Mant JM. Identifying the long-term needs of carers of stroke survivors living in the community to inform a new primary care model. Eur Stroke J 2017;2.
- Aquino MRJ, Mullis R, Moore C, Kreit E, Lim L, McKevitt C, et al. ‘It’s difficult, there’s no formula’: qualitative study of stroke related communication between primary and secondary healthcare professionals. Int J Integr Care 2020;20:1-10. http://doi.org/10.5334/ijic.5465.
- Verbakel NJ, Van Melle M, Langelaan M, Verheij TJ, Wagner C, Zwart DL. Exploring patient safety culture in primary care. Int J Qual Health Care 2014;26:585-91. https://doi.org/10.1093/intqhc/mzu074.
- Waring J, Marshall F, Bishop S, Sahota O, Walker MF, Currie G, et al. An ethnographic study of knowledge sharing across the boundaries between care processes, services and organisations: the contributions to ‘safe’ hospital discharge. Health Serv Deliv Res 2014;2:1-160. https://doi.org/10.3310/hsdr02290.
- Rothwell K, Boaden R, Bamford D, Tyrell PJ. Feasibility of assessing the needs of stroke patients after six months using the GM-SAT. Clin Rehabil 2012;27:264-71.
- NIHR Applied Research Collaboration Greater Manchester . The Greater Manchester Stroke Assessment Tool – Version 2 (GM-SAT2) n.d. https://www.arc-gm.nihr.ac.uk/projects/gm-sat-2 (accessed 16 February 2021).
- Hobbs FDR, Bankhead C, Mukhtar T, Stevens S, Perea-Salazar R, Holt T, et al. on behalf of the NIHR School for Primary Care Research . Clinical workload in primary care: a retrospective analysis of 100 million consultations in England, 2007–14. Lancet 2016;387:2323-30.
- Philp I, Brainin M, Walker MF, Ward AB, Gillard P, Shields AL, et al. Global Stroke Community Advisory Panel . Development of a post-stroke checklist to standardize follow-up care for stroke survivors. J Stroke Cerebrovasc Dis 2013;22:e173-80.
- Turner GM, Mullis R, Lim L, Kreit L, Mant J. Using a checklist to facilitate management of long-term care needs after stroke: insights from focus groups and a feasibility study. BMC Fam Pract 2019;20.
- Lim L, Mant J, Mullis R, Roland M. When is referral from primary care to specialist services appropriate for survivors of stroke? A modified RAND-appropriateness consensus study. Fam Pract 2020;21.
- Pindus DM, Lim L, Rundell AV, Hobbs V, Aziz NA, Mullis R, et al. Primary care interventions and current service innovations in modifying long term outcomes after stroke: a protocol for a scoping review. BMJ Open 2016;6.
- Su Y, Yuki M, Otsuki M. Non-pharmacological interventions for post-stroke fatigue: systematic review and network meta-analysis. J Clin Med 2020;9.
- Mullis R, Aquino MRJ, Dawson SN, Johnson V, Jowett S, Kreit E, et al. on behalf of the IPCAS investigator team . Improving Primary Care After Stroke (IPCAS) trial: protocol of a randomised controlled trial to evaluated a novel model of care for stroke survivors living in the community. BMJ Open 2019;9.
- Eldridge SM, Lancaster GA, Campbell MJ, Thabane L, Hopewell S, Coleman CL, et al. Defining feasibility and pilot studies in preparation for randomised controlled trials: development of a conceptual framework. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0150205.
- Davies MJ, Heller S, Skinner TC, Campbell MJ, Carey ME, Cradock S, et al. Diabetes Education and Self Management for Ongoing and Newly Diagnosed Collaborative . Effectiveness of the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cluster randomised controlled trial. BMJ 2008;336:491-5.
- Fryer CE, Luker JA, McDonnell MN, Hillier SL. Self management programmes for quality of life in people with stroke. Cochrane Database Sys Rev 2016;2019.
- Johnson V, Apps L, Carey ME, Kreit E, Mullis R, Hadjiconstantinou M, et al. on behalf of the MLAS Development Group . The development of a self-management interventions for stroke survivors – My Life After Stroke (MLAS). Disabil Rehabil 2023;45:226-34. https://www.tandfonline.com/doi/full/10.1080/09638288.2022.2029959.
- Johnson VL, Apps L, Kreit E, Mullis R, Mant J, Davies MJ. on behalf of the MLAS development group . The feasibility of a self-management programme (My Life After Stroke; MLAS) for stroke survivors. Disabil Rehabil 2023;45:235-43. https://www.tandfonline.com/doi/full/10.1080/09638288.2022.2029960.
- Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977;84:191-215.
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6.
- Boger EJ, Hankins M, Demain SH, Latter SM. Development and psychometric evaluation of a new patient -reported outcome measure for stroke self -management: The Southampton Stroke Self-Management Questionnaire (SSSMQ). Health Qual Life Outcomes 2015;13.
- Jenkinson C, Fitzpatrick R, Crocker H, Peters M. The Stroke Impact Scale: validation in a UK setting and development of a SIS short form and SIS index. Stroke 2013;44:2532-5.
- Aquino MR, Mullis R, Kreit E, Johnson V, Grant J, Lim L, et al. Improving Prinary Care After Stroke (IPCAS) randomised controlled trial: protocol for a multidimensional process evaluation. BMJ Open 2020;10.
- Mant J, McManus RJ, Hare R. Applicability to primary care of national clinical guidelines on blood pressure lowering for people with stroke: cross sectional study. BMJ 2006;332:635-7.
- Harrison M, Ryan T, Gardiner C, Jones A. Psychological and emotional needs, assessment, and support post-stroke: a multi-perspective qualitative study. Top Stroke Rehabil 2017;24:119-25.
- National Health Service . National Service Model for an Integrated Community Stroke Service 2022. www.england.nhs.uk/wp-content/uploads/2022/02/stroke-integrated-community-service-february-2022.pdf (accessed 22 November 2023).
- REAL Centre . Projections: general practice workforce in England. Summary of findings. The Health Foundation 2022:1-22. www.health.org.uk/publications/reports/projections-general-practice-workforce-in-england (accessed 22 November 2023).
- National Health Service . Research Demand Signalling: National Stroke Programme 2022. https://www.england.nhs.uk/aac/wp-content/uploads/sites/50/2022/03/B0687-research-demand-signalling-national-stroke-progeamme.pdf (accessed 22 November 2023).
Appendix 1 Interview study with stroke survivors and caregivers
Caroline Moore, Dominika Pindus, Elizabeth Kreit, Lisa Lim, Ricky Mullis, Jonathan Mant
Introduction
After discharge from hospital and from specialist rehabilitation services, stroke survivors are managed in primary care. There is evidence from surveys, audits and interview studies that their longer-term needs in this setting are often not adequately addressed. Research to date has tended to focus on improving access to acute stroke services rather than considering the role of primary care in stroke management.
Over a third of long-term stroke survivors are functionally dependent, and 1 in 15 is cared for by family and friends. These informal carers have their own particular needs. The majority are affected by the emotional impact of the stroke, and many experience anger and frustration. Many report relationship difficulties, and three-quarters feel ill-prepared for this role. Despite the importance of informal carers in the process of rehabilitation and longer-term care for stroke survivors and increasing recognition of the problems that carers face, there is no formal long-term model of stroke care to support them in primary care.
The aim of this study was twofold:
-
To explore the changing care needs across stroke survivors’ trajectories and identify how these needs could be addressed in primary care.
-
To identify how the needs of carers of stroke survivors are being met in primary care.
Methods
Potentially eligible stroke survivors were identified from stroke registers of five participating general practices in the East of England. A GP in each practice reviewed a random sample of patients to confirm eligibility. Inclusion criteria were confirmed diagnosis of stroke, good understanding of English and capacity to provide written informed consent. People with terminal illness, severe depression or other severe comorbidities were excluded. In order to identify carers to take part, we asked stroke survivors who had agreed to participate the question: ‘Which family member or friend gives you the most help and support following your stroke?’
Twenty-two stroke survivors agreed to take part, and they identified 15 informal carers, of whom 14 were also interviewed.
Interviews were conducted in people’s homes. We used a topic guide with questions dealing with life after stroke. These included family and social relations, sense of self, emotions, interactions with healthcare professionals and reflections on experiences. We were interested both in the nature of specific practical adjustments made and in a wider perspective on overall experience after stroke.
All semistructured interviews were recorded and transcribed verbatim. A framework approach was used to analyse the data.
Results
The characteristics of participants are shown in Table 3. Equal numbers of each sex were represented among stroke survivors, with a mean age of 73 and a median time since their last stroke of 3 years. Over half had no limitation in daily living using the Barthel Index. Carers were predominately female and had been in this role for a median of 3 years.
| Stroke survivor characteristic (n = 22) | Number (%) |
|---|---|
| Male | 11 (50) |
| Age: mean, median and range | 73, 70, 49–93 |
| Time since stroke: mean, median, range (years) | 7, 3, 0.25–22 |
| Barthel Index: median, range (0–100) | 100, 50–100 |
| Index of Multiple Deprivation (in deciles): median and range | 7, 3–10 |
| Carer characteristics (n = 14) | |
| Male | 3 (21) |
| Age range | 43–91 |
| Time as carer: mean, median, range (years) | 12, 3, 2–53 |
| Caregiver Strain Index: median, range (0–13) | 3, 0–13 |
| Index of Multiple Deprivation (in deciles): median and range | 8, 2–10 |
| Hours per week as carer: range | 7 hours – full time |
Changing needs of stroke survivors in primary care
Needs were categorised into four key areas: physical, psychological, information and health services. Examples of specific needs within these areas are shown in Table 4. The needs changed in importance over the time course since the stroke (see Figure 4). Psychological needs, particularly anxiety and feelings of abandonment, were most apparent in the postdischarge phase. Abandonment is illustrated by the following quote:
Well I thought someone would come in and see if I was alright or if there was anything they could do but we got nothing at all. Did I need this, did I need that ….
| Physical needs | Survivor expectations of recovery; fatigue and muscle weakness; loss of sexual function |
| Psychological needs | Fear of secondary stroke; anxiety and depression; loss of confidence; feeling abandoned |
| Information needs | Unaware of available services; feeling unprepared; lacking information about prognosis and what to expect |
| Health service needs | Lack of advance care planning; delays in receiving medication; lack of continuity of care |
FIGURE 4.
Three phases of poststroke recovery.
Physical needs were identified in the longer-term phase by some stroke survivors. It was notable that those survivors who talked about their longer-term physical needs referred to them in the context of their other comorbidities and in comparison to other stroke survivors. Need for information and for health services was apparent at all stages in the recovery process. Illustrative quotes include:
To say I’m getting better, that’s what you want to hear, you know, or why can’t I lift my leg? Well it has affected so and so, so and so, and you can think: oh well, that’s alright, you know.
If you want to see him you’ll have to ring up first thing of a morning … after you’ve done that for about a week and there’s no appointments you say oh forget it. It’s just really difficult to get an appointment.
Needs of carers
These related both to those of the cared-for stroke survivor and to the carer’s own needs, but sometimes the boundaries were blurred between what was a carer need and what was a patient need. An example of a need that related to the stroke survivor:
I had to fight to get my mum physio. I went to the doctor’s to fight for physio because my mums’ right side, literally it was nothing. She would literally just slump to one side.
An example of a carer need, slightly blurred:
He’s never been my husband anymore. I’ve never had any help since then … I was 54 (crying) and I’ve never had a husband anymore. So we just muddle through really.
The key issues from a carer perspective in terms of the stroke survivor were fear of a further stroke, lack of stroke-related information and lack of follow-up. The consequences for the carers themselves were loss of physical and emotional relationships, loss of confidence, anxiety and depression and feelings of abandonment and being trapped in the caregiver role. Indeed, psychological needs were the ones that were most forcibly expressed. In terms of seeking support, the general practice was regarded as the first point of contact.
Discussion
These data provide insights into the longer-term needs of a prevalent stroke survivor population and their carers in a primary care setting. These findings added granularity and depth to the findings of our systematic review. In particular, they emphasised the importance of provision of information about what services are available locally, the unmet needs of caregivers, the importance of health services maintaining active contact with stroke survivors and the changing nature of these needs. In particular, it was notable that in the longer term, the needs were couched more in terms of multiple comorbidities and less in the context of the stroke.
Strengths include gaining the perspective of both patients and carers and gaining insights from people several years after their stroke (who make up the bulk of people on primary care stroke registers). Limitations include a lack of insight of experiences of people from diverse ethnic backgrounds and under-representation of people with cognitive impairment (a common sequela of stroke).
The findings are consistent with other qualitative studies of experiences after stroke, which also emphasise lack of follow-up, lack of information, emotional distress and abandonment. They provide actionable findings that can be incorporated into a primary care model of poststroke aftercare.
Appendix 2 Primary care interventions for long-term outcomes after stroke: a scoping review of reviews and recent trials
Dominika M Pindus, Ricky Mullis, Ian Wellwood, Elisabeth Kreit, Lisa Lim, Jonathan Mant
Background
-
Stroke is the third most important cause of disability burden (Feign et al. , 2014, Lozano et al. , 2012).
-
In the UK 78% of stroke survivors are discharged home and live in the community (Intercollegiate Stroke Working Party, 2016).
-
Stroke survivors report multiple long-term unmet needs (McKevitt et al. , 2011; Pindus et al. , submitted).
-
An integrative account of primary care-based interventions for long-term care of stroke is lacking.
-
Key characteristics of successful interventions could help target future research efforts and inform care.
Methods
-
See Pindus et al. http://doi.org/10.1136/bmjopen-2016-012840
-
Established scoping review methodology (Arksey and O’ Malley, 2005).
-
Systematic search of six databases: MEDLINE, EMBASE, PsycINFO, CINAHL and either COCHRANE Reviews searched up to 06/2015.
-
Inclusion criteria: (1) peer-reviewed systematic reviews and meta-analyses of RCTs and/or controlled trials, supplemented with the most recent (2011–5) trials, (2) interventions delivered in primary care and/or community, (3) by generalist healthcare professionals and (4) to adult stroke survivors and/or informal caregivers.
-
Exclusion criteria: (1) drug efficacy reviews/trials and (2) not published in English.
Results
-
Two thousand and forty-one reviews and 1839 RCTs and controlled trials were identified.
-
Full texts of 94 reviews and 40 trials were assessed for eligibility.
-
Twenty-one reviews and 10 trials were analysed.
Key findings
-
Thirteen (62%) reviews and 5 (50%) trials reported positive small- to medium-sized effects of interventions on long-term outcomes after stroke.
-
Only 19% (n = 4) of reviews and 10% (n = 1) of trials included caregivers.
-
Forty-three per cent (n = 9) of reviews and 60% (n = 6) of trials focused on physical function (e.g. mobility, balance, physical fitness).
-
Increased interest in psycho-social outcomes was observed in recent trials: 33% of trials compared to 19% of reviews addressed caregiver burden or QoL in survivors.
-
Two reviews reported positive effects of information provision on depression in survivors (Smith et al. , 2010) and education and support interventions on QoL in caregivers (Lee et al. , 2007).
-
Sixty per cent of positive review and trial findings related to physical (e.g. mobility, fitness, balance) and functional (activities of daily living, ADLs or disability) outcomes.
Conclusions
Although stroke survivors report many unmet long-term psychological needs, primary care interventions focus on their physical health and global function. Since psychological outcomes are related to functional recovery after stroke, interventions aimed at improving survivors’ and caregivers’ long-term mental health are also needed.
Appendix 3 IPCAS RCT descriptive results
FIGURE 5.
Monthly cumulative practice recruitment (N = 46).
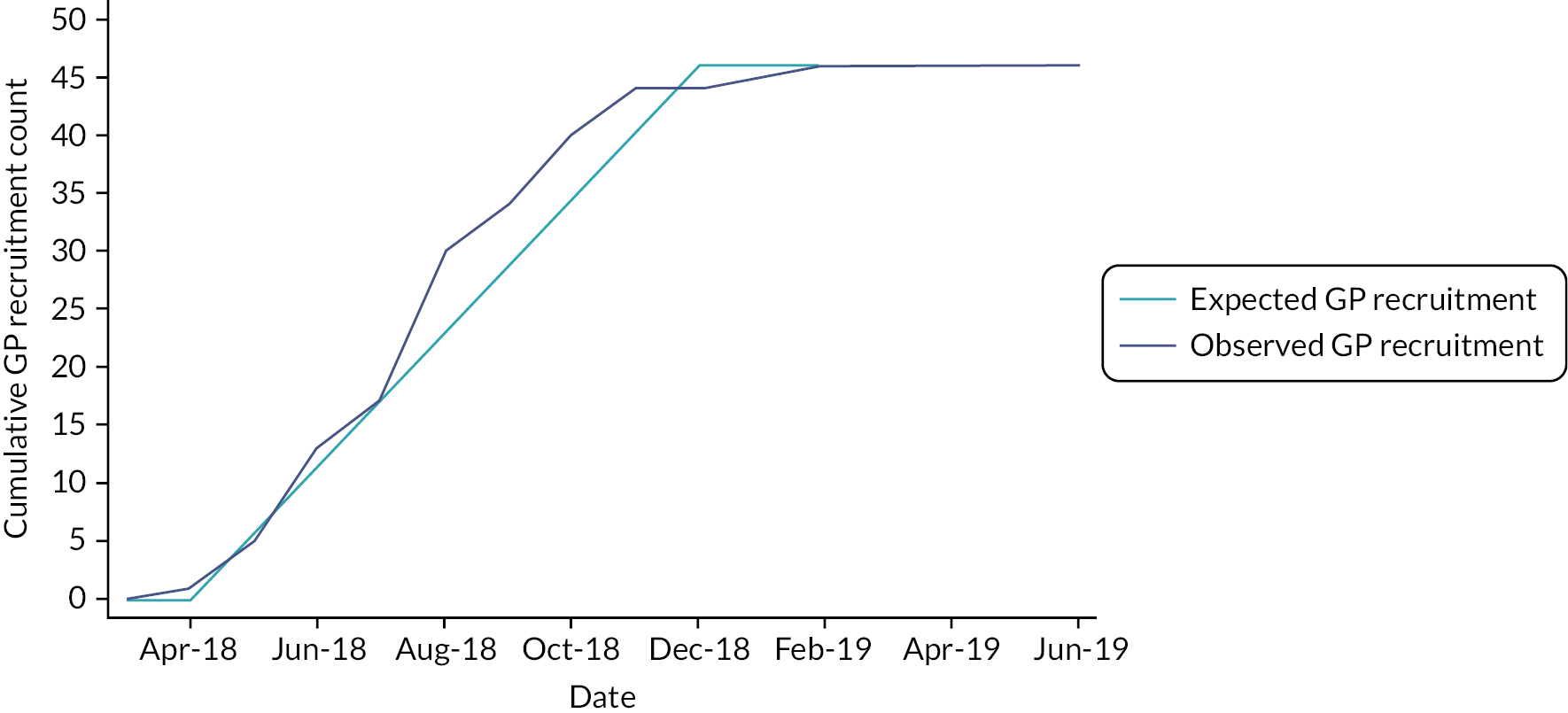
FIGURE 6.
Monthly cumulative patient recruitment (N = 1040).
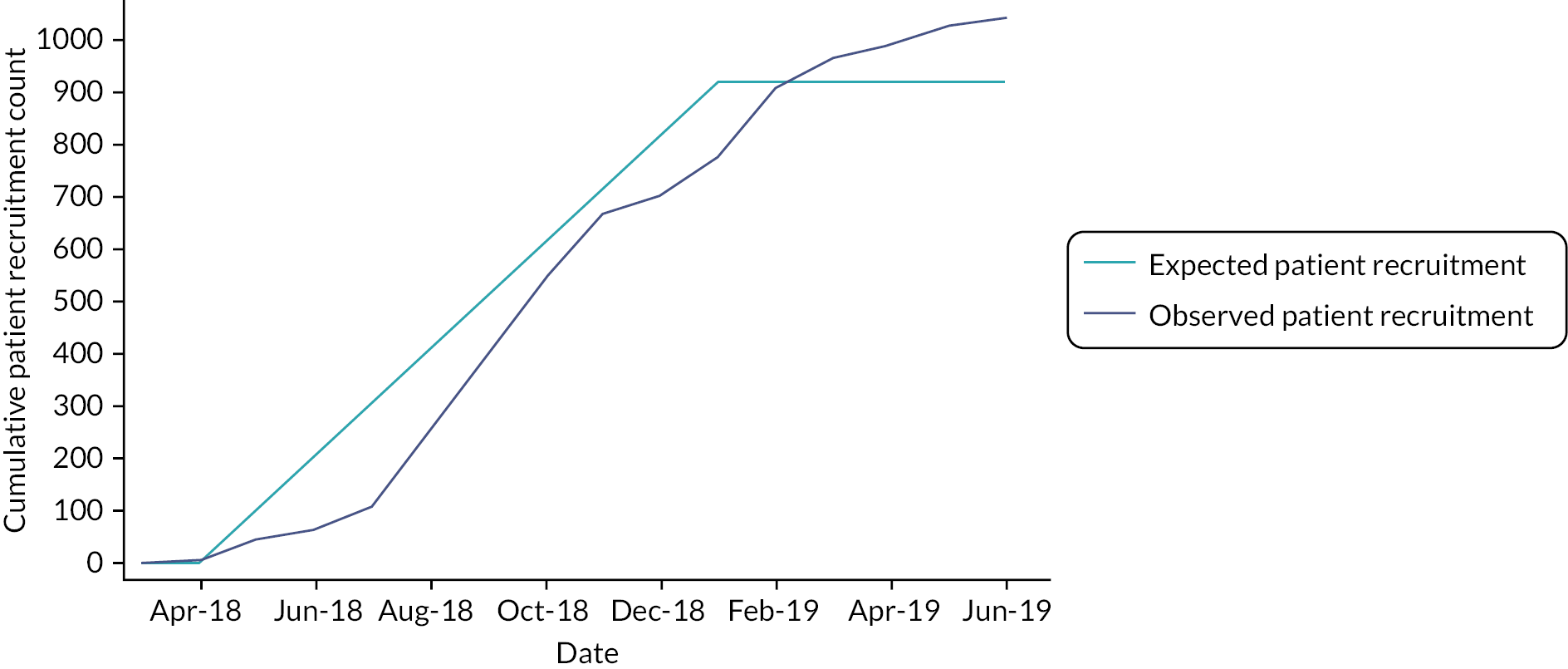
| Arm | |||
|---|---|---|---|
| Control | Intervention | ||
| Participant withdrawn | N | 58 | 87 |
| Female | N | 22 | 37 |
| Male | N | 36 | 50 |
| Female (%) | Mean | 37.9 | 42.5 |
| Male (%) | Mean | 62.1 | 57.5 |
| Age at baseline | Mean | 74.1 | 74.0 |
| SD | 10.9 | 10.6 | |
| Months since last stroke at consent | Mean | 86.0 | 96.1 |
| SD | 113.0 | 97.4 | |
| Maximum | 624.8 | 523.4 | |
| Minimum | 2.5 | 0.9 | |
| Number of days to withdrawal | Mean | 248.8 | 218.1 |
| SD | 139.0 | 144.6 | |
| Maximum | 525.0 | 538.0 | |
| Minimum | 4.0 | 18.0 | |
| Arm | ||||
|---|---|---|---|---|
| Control | Intervention | Total | ||
| Reason code | Death | 12 20.69% |
9 10.34% |
21 |
| Health deterioration | 9 15.52% |
10 11.49% |
19 | |
| Taking part is too burdensome | 6 10.34% |
18 20.69% |
24 | |
| Second questionnaire not relevant | 6 10.34% |
6 6.90% |
12 | |
| 6 months questionnaire not relevant | 11 18.97% |
9 10.34% |
20 | |
| Left the practice | 4 6.90% |
8 9.20% |
12 | |
| No reason given | 2 3.45% |
14 16.09% |
16 | |
| Intervention is not relevant | 0 0% |
2 2.30% |
2 | |
| Will not complete 12 months questionnaire | 7 12.07% |
8 9.20% |
15 | |
| Not a stroke | 1 1.72% |
3 3.45% |
4 | |
| Total | 58 | 87 | ||
Baseline demographics
Tables summarise patient baseline demographics. The intention-to-treat (ITT) population includes every participant who is randomised ignoring non-compliance, protocol deviations and withdrawal.
| Arm | |||
|---|---|---|---|
| Control | Intervention | ||
| Participant randomised | N | 518 | 522 |
| Female | N | 193 | 191 |
| Male | N | 325 | 331 |
| Female (%) | Mean | 37.3 | 36.6 |
| Male (%) | Mean | 62.7 | 63.4 |
| Age at baseline | Mean | 70.9 | 70.3 |
| SD | 12.0 | 12.1 | |
| Median | 72.0 | 72.0 | |
| Interquartile range | 64.0–79.0 | 64.0–79.0 | |
| Arm | ||||
|---|---|---|---|---|
| Control | Intervention | Total | ||
| Self-defined ethnicity | White | 509 | 508 | 1017 |
| 98.26% | 97.32% | |||
| 50.05% | 49.95% | |||
| Black | 1 | 1 | 2 | |
| 0.19% | 0.19% | |||
| 50.00% | 50.00% | |||
| Asian | 5 | 6 | 11 | |
| 0.97% | 1.15% | |||
| 45.45% | 54.55% | |||
| Chinese | 2 | 3 | 5 | |
| 0.39% | 0.57% | |||
| 40.00% | 60.00% | |||
| Aboriginal | 1 | 4 | 5 | |
| 0.19% | 0.77% | |||
| 20.00% | 80.00% | |||
| Total | 518 | 522 | ||
| Arm | |||
|---|---|---|---|
| Control | Intervention | ||
| Last stroke date available | N | 513 | 521 |
| Days since last stroke at consent | Mean | 2524.87 | 2869.33 |
| SD | 2752.83 | 3046.07 | |
| Maximum | 19,005.00 | 20,685.00 | |
| Minimum | 4.00 | 26.00 | |
| Median | 1565.00 | 1998.00 | |
| p25 | 561.00 | 760.00 | |
| p75 | 3461.00 | 3907.00 | |
| Months since last stroke at consent | Mean | 83.01 | 94.33 |
| SD | 90.50 | 100.14 | |
| Maximum | 624.82 | 680.05 | |
| Minimum | 0.13 | 0.85 | |
| Median | 51.45 | 65.69 | |
| p25 | 18.44 | 24.99 | |
| p75 | 113.79 | 128.45 | |
| Arm | Total | |||
|---|---|---|---|---|
| Control | Intervention | |||
| Time since last stroke at consent | 0–6 months | 48 | 33 | 81 |
| 9.27% | 6.32% | |||
| 59.26% | 40.74% | |||
| 6 months–1 year | 37 | 32 | 69 | |
| 7.14% | 6.13% | |||
| 53.62% | 46.38% | |||
| 1–2 years | 73 | 62 | 135 | |
| 14.09% | 11.88% | |||
| 54.07% | 45.93% | |||
| 2–5 years | 121 | 115 | 236 | |
| 23.36% | 22.03% | |||
| 51.27% | 48.73% | |||
| 5–10 years | 113 | 132 | 245 | |
| 21.81% | 25.29% | |||
| 46.12% | 53.88% | |||
| 10 years+ | 121 | 147 | 268 | |
| 23.36% | 28.16% | |||
| 45.15% | 54.85% | |||
| 5 | 1 | 6 | ||
| 0.97% | 0.19% | |||
| 83.33% | 16.67% | |||
| Total | 518 | 522 | ||
Baseline questionnaire scores
In all cases, summary scores have been calculated and are presented for participants with complete baseline question responses by trial arm and sex. The IPCAS baseline questionnaire comprises questions from four questionnaires:
-
SIS Q3 (emotional domain) and Q8 (handicap domain). The emotional domain has nine components, and the handicap domain has eight components; all components are scored on a scale from 1 (all of the time) to 5 (none of the time). For each domain, a standardised score is derived that can be compared against a normative score of 50.
-
Stroke Impact Scale – Short Form (SIS-SF) Q1–8 are used to calculate a standardised score. Questions 1–8 are scored on a scale from 1 (could not do at all) to 5 (not difficult at all). The SIS-SF index is calculated by summing the eight items and standardising on a scale from 0 to 100. Q9 is a visual analogue scale (VAS) which asks participants to indicate their overall health post stroke, ranging from 0 (no recovery) to 100 (full recovery).
-
EQ-5D-5L Q1–5 are used to calculate a standardised score and Q6 is a VAS score of perceived health on the day the questionnaire is completed. This measure covers five health states: mobility, self-care, usual activities, pain/discomfort and anxiety/depression, scored on a scale from 1 (no problems) to 5 (extreme problems). The VAS score ranges from 0 (worst health) to 100 (best health imaginable).
-
ICECAP-A Q1–5 are used to calculate a tariff value for overall well-being. ICECAP-A comprises five attributes: Attachment (an ability to have love, friendship and support), Stability (an ability to feel settled and secure), Achievement (an ability to achieve and progress in life), Enjoyment (an ability to experience enjoyment and pleasure) and Autonomy (an ability to be independent). Values for each question are summed to give an overall tariff from 1 (maximum capacity) to −0.001 (minimum capacity).
| Arm | |||
|---|---|---|---|
| Control | Intervention | ||
| Number of responses – SIS emotion | N | 513 | 511 |
| % response of ITT pop – SIS emotion | Mean | 99.0 | 97.9 |
| SIS summary score – emotion domain | Mean | 69.09 | 69.60 |
| SD | 19.21 | 19.01 | |
| Minimum | 11.11 | 13.89 | |
| Maximum | 100.00 | 100.00 | |
| Number of responses – SIS handicap | N | 511 | 509 |
| % response of ITT pop – SIS handicap | Mean | 98.6 | 97.5 |
| SIS summary score – handicap domain | Mean | 70.11 | 69.39 |
| SD | 23.31 | 25.20 | |
| Minimum | 0.00 | 0.00 | |
| Maximum | 100.00 | 100.00 | |
| Arm | |||
|---|---|---|---|
| Control | Intervention | ||
| Number of responses SIS-SF (Q1–8) | N | 412 | 427 |
| % response of ITT pop SIS-SF (Q1–8) | Mean | 79.5 | 81.8 |
| SIS-SF summary score | Mean | 73.62 | 73.37 |
| SD | 22.35 | 21.70 | |
| Minimum | 0.00 | 3.13 | |
| Maximum | 100.00 | 100.00 | |
| Number of responses SIS-SF (Q9) | N | 477 | 484 |
| % response of ITT pop SIS-SF (Q9) | Mean | 92.1 | 92.7 |
| Recovery after stroke (0 = none, 100 = full) | Mean | 75.89 | 74.91 |
| SD | 24.10 | 24.10 | |
| Minimum | 0 | 0 | |
| Maximum | 100.00 | 100.00 | |
| Arm | |||
|---|---|---|---|
| Control | Intervention | ||
| Number of responses EQ-5D | N | 457 | 465 |
| % response of ITT pop EQ-5D | Mean | 88.2 | 89.1 |
| Number of responses EQ-5D VAS | N | 481 | 484 |
| % response of ITT pop EQ-5D VAS | Mean | 92.9 | 92.7 |
| Perceived health (0 = worst, 100 = best) | Mean | 72.82 | 74.33 |
| SD | 21.84 | 20.93 | |
| Minimum | 0 | 0 | |
| Maximum | 100.00 | 100.00 | |
| Arm | |||
|---|---|---|---|
| Control | Intervention | ||
| Number of responses ICECAP-A | N | 463 | 479 |
| % response of ITT pop ICECAP-A | Mean | 89.4 | 91.8 |
| Tariff | Mean | 0.82 | 0.82 |
| SD | 0.18 | 0.18 | |
| Minimum | 0.19 | 0.08 | |
| Maximum | 1.00 | 1.00 | |
Six-month questionnaire scores
| Arm | |||
|---|---|---|---|
| Control | Intervention | ||
| Number of responses – SIS emotion | N | 423 | 418 |
| % response of ITT pop – SIS emotion | Mean | 81.7 | 80.1 |
| SIS summary score – emotion domain | Mean | 71.22 | 71.66 |
| SD | 18.89 | 17.94 | |
| Minimum | 0.00 | 5.56 | |
| Maximum | 100.00 | 100.00 | |
| Number of responses – SIS handicap | N | 420 | 411 |
| % response of ITT pop – SIS handicap | Mean | 81.1 | 78.7 |
| SIS summary score – handicap domain | Mean | 71.52 | 70.93 |
| SD | 23.95 | 24.39 | |
| Minimum | 0.00 | 3.13 | |
| Maximum | 100.00 | 100.00 | |
| Arm | |||
|---|---|---|---|
| Control | Intervention | ||
| Number of responses SIS-SF (Q1–8) | N | 352 | 350 |
| % response of ITT pop SIS-SF (Q1–8) | Mean | 68.0 | 67.0 |
| SIS-SF summary score | Mean | 72.94 | 72.57 |
| SD | 21.84 | 21.59 | |
| Minimum | 3.13 | 6.25 | |
| Maximum | 100.00 | 100.00 | |
| Number of responses SIS-SF (Q9) | N | 422 | 414 |
| % response of ITT pop SIS-SF (Q9) | Mean | 81.5 | 79.3 |
| Recovery after stroke (0 = none, 100 = full) | Mean | 77.65 | 75.43 |
| SD | 22.62 | 24.11 | |
| Minimum | 0 | 0 | |
| Maximum | 100.00 | 100.00 | |
| Arm | |||
|---|---|---|---|
| Control | Intervention | ||
| Number of responses EQ-5D | N | 413 | 401 |
| % response of ITT pop EQ-5D | Mean | 79.7 | 76.8 |
| Number of responses EQ-5D VAS | N | 426 | 416 |
| % response of ITT pop EQ-5D VAS | Mean | 82.2 | 79.7 |
| Perceived health (0 = worst, 100 = best) | Mean | 73.43 | 74.30 |
| SD | 22.11 | 20.75 | |
| Minimum | 5 | 10 | |
| Maximum | 100.00 | 100.00 | |
| Arm | |||
|---|---|---|---|
| Control | Intervention | ||
| Number of responses ICECAP-A | N | 419 | 413 |
| % response of ITT pop ICECAP-A | Mean | 80.9 | 79.1 |
| Tariff | Mean | 0.82 | 0.82 |
| SD | 0.19 | 0.18 | |
| Minimum | −0.00 | 0.19 | |
| Maximum | 1.00 | 1.00 | |
End-of-study questionnaire scores
| Arm | |||
|---|---|---|---|
| Control | Intervention | ||
| Number of responses – SIS emotion | N | 394 | 384 |
| % response of ITT pop – SIS emotion | Mean | 76.1 | 73.6 |
| SIS summary score – emotion domain | Mean | 70.90 | 71.61 |
| SD | 19.03 | 18.96 | |
| Minimum | 13.89 | 0.00 | |
| Maximum | 100.00 | 100.00 | |
| Number of responses – SIS handicap | N | 393 | 383 |
| % response of ITT pop – SIS handicap | Mean | 75.9 | 73.4 |
| SIS summary score – handicap domain | Mean | 71.45 | 72.29 |
| SD | 22.96 | 24.03 | |
| Minimum | 6.25 | 0.00 | |
| Maximum | 100.00 | 100.00 | |
| Arm | |||
|---|---|---|---|
| Control | Intervention | ||
| Number of responses SIS-SF (Q1–8) | N | 303 | 287 |
| % response of ITT pop SIS-SF (Q1–8) | Mean | 58.5 | 55.0 |
| SIS-SF summary score | Mean | 72.73 | 71.80 |
| SD | 19.76 | 20.72 | |
| Minimum | 18.75 | 6.25 | |
| Maximum | 100.00 | 100.00 | |
| Number of responses SIS-SF (Q9) | N | 360 | 359 |
| % response of ITT pop SIS-SF (Q9) | Mean | 69.5 | 68.8 |
| Recovery after stroke (0 = none, 100 = full) | Mean | 78.45 | 76.50 |
| SD | 21.35 | 24.04 | |
| Minimum | 0.00 | 0.00 | |
| Maximum | 100.00 | 100.00 | |
| Arm | |||
|---|---|---|---|
| Control | Intervention | ||
| Number of responses EQ-5D | N | 350 | 353 |
| % response of ITT pop EQ-5D | Mean | 67.6 | 67.6 |
| Number of responses EQ-5D VAS | N | 362 | 358 |
| % response of ITT pop EQ-5D VAS | Mean | 69.9 | 68.6 |
| Perceived health (0 = worst, 100 = best) | Mean | 73.27 | 77.56 |
| SD | 21.37 | 53.17 | |
| Minimum | 10.00 | 5.00 | |
| Maximum | 100.00 | 999.00 | |
| Arm | |||
|---|---|---|---|
| Control | Intervention | ||
| Number of responses ICECAP-A | N | 348 | 347 |
| % response of ITT pop ICECAP-A | Mean | 67.2 | 66.5 |
| Tariff | Mean | 0.82 | 0.83 |
| SD | 0.18 | 0.18 | |
| Minimum | 0.26 | 0.12 | |
| Maximum | 1.00 | 1.00 | |
| Arm | |||
|---|---|---|---|
| Control | Intervention | ||
| Number of responses SSSMQ (Q1–28) | N | 265 | 252 |
| % response of ITT pop SSSMQ (Q1–28) | Mean | 51.2 | 48.3 |
| eos_sssmq_SCORE | Mean | 112.76 | 112.02 |
| SD | 13.49 | 15.96 | |
| Minimum | 62.00 | 60.00 | |
| Maximum | 146.00 | 148.00 | |
Appendix 4 Randomised controlled trial: analysis of primary outcome
Sample size statement from protocol:
The coprimary end points were SIS3 (emotion) and SIS8 (handicap) (two domains of the SIS) at 12 months. SIS3 added together 9 Likert 1–5 questions, and SIS8 added together 8 Likert 1–5 questions. Higher scores are ‘better’. These are normalised to a 0–100 scale for the full range of possible responses, before regression modelling.
-
Descriptive summary
There were 1040 subjects randomised (participants 19064Y and 44020J withdrew prior to site randomisation) at 46 sites, averaging 22.6 participants/site. This is greater than the planned 920 randomised participants.
From Figures 7 and 8, these scores are bounded below 0 and above 100 and are negatively skewed (especially the SIS8 scores), suggesting ceiling effects where participants tended to answer at the top of the scale.
| SIS3 | Baseline | 6 months | 12 months |
|---|---|---|---|
| SIS8 | Baseline | 6 months | 12 months |
| n | 1024 | 841 | 778 |
| Missing | 16 | 199 | 262 |
| Total | 1040 | 1040 | 1040 |
| % missing | 2 | 19 | 25 |
| n | 1020 | 831 | 776 |
| Missing | 20 | 209 | 264 |
| Total | 1040 | 1040 | 1040 |
| % missing | 2 | 20 | 25 |
FIGURE 7.
Distribution of SIS3 (emotion) and SIS8 (participation) by arm and time.
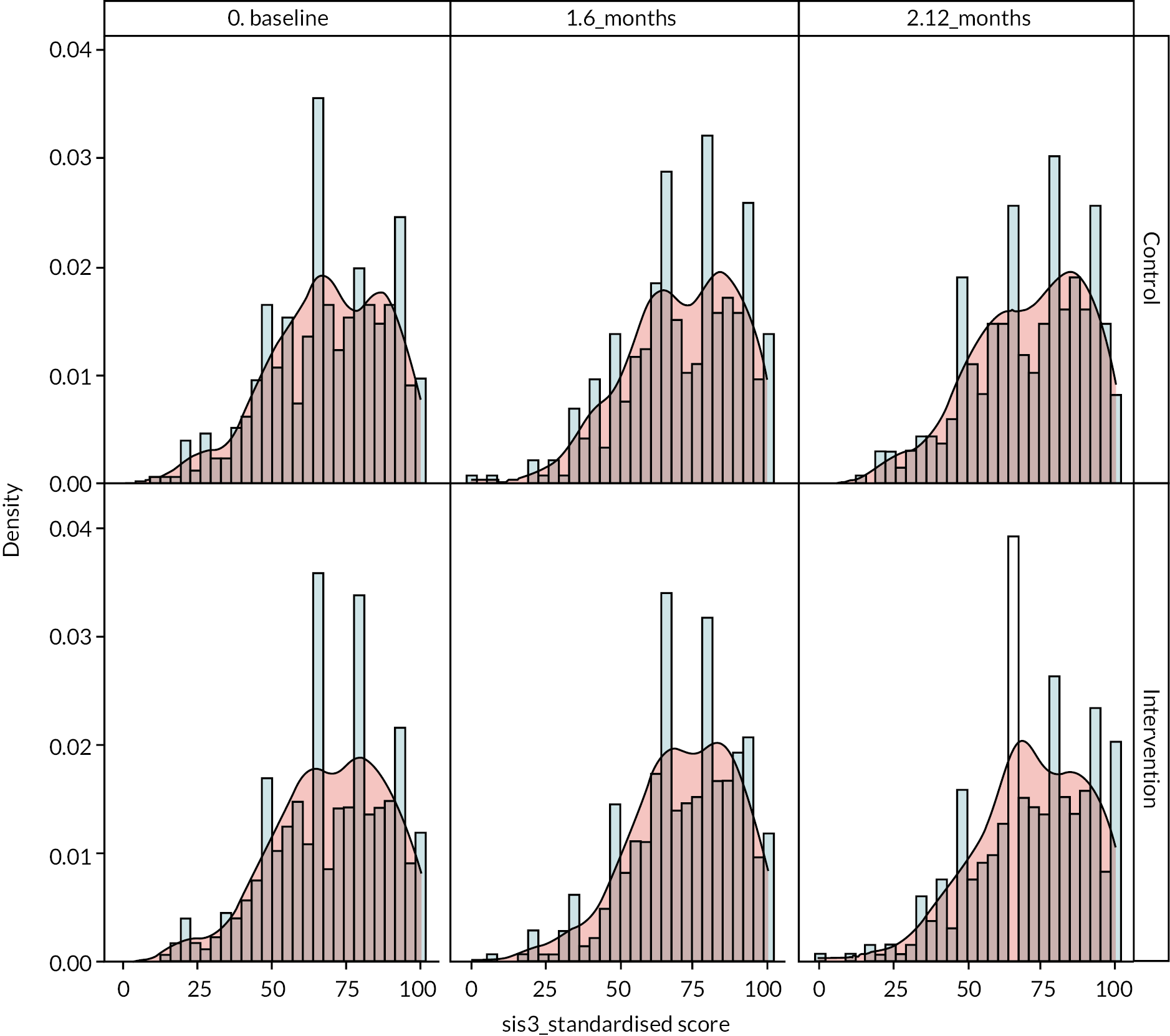
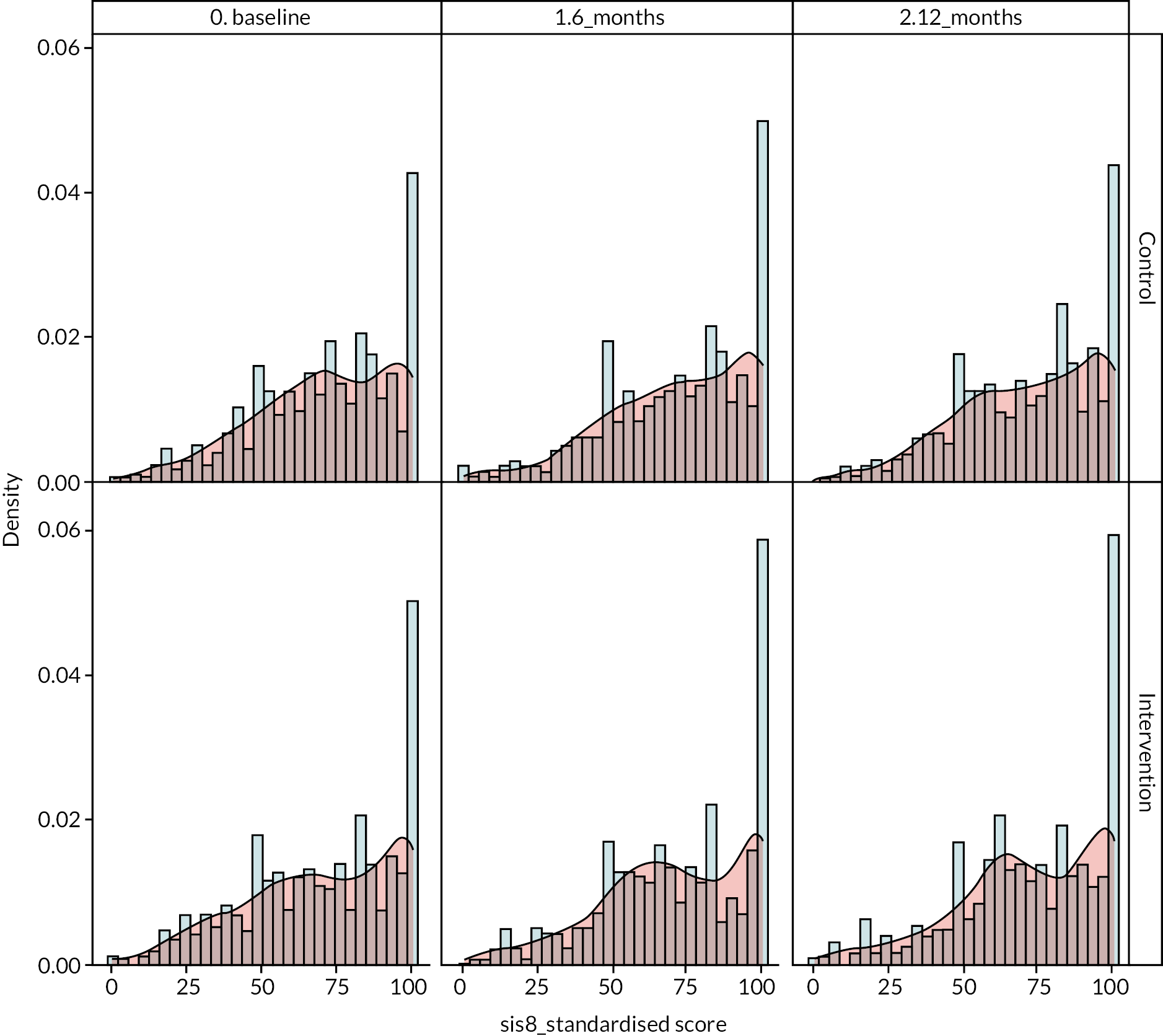
| End point | Arm | Time | n | Mean | SD | 97.5% CI | |
|---|---|---|---|---|---|---|---|
| SIS3 | Control | Baseline | 513 | 69.092 | 19.2077 | 67.186 | 70.999 |
| Intervention | Baseline | 511 | 69.5967 | 19.0127 | 67.706 | 71.487 | |
| Control | 6 months | 423 | 71.2241 | 18.8938 | 69.158 | 73.29 | |
| Intervention | 6 months | 418 | 71.6574 | 17.9381 | 69.684 | 73.631 | |
| Control | 12 months | 394 | 70.8968 | 19.0287 | 68.74 | 73.054 | |
| Intervention | 12 months | 384 | 71.6074 | 18.9561 | 69.431 | 73.784 | |
| SIS8 | Control | Baseline | 511 | 70.1076 | 23.313 | 67.789 | 72.426 |
| Intervention | Baseline | 509 | 69.3885 | 25.1979 | 66.878 | 71.899 | |
| Control | 6 months | 420 | 71.518 | 23.9528 | 68.889 | 74.147 | |
| Intervention | 6 months | 411 | 70.932 | 24.3898 | 68.226 | 73.639 | |
| Control | 12 months | 393 | 71.4536 | 22.95521 | 68.848 | 74.059 | |
| Intervention | 12 months | 383 | 72.2911 | 24.03137 | 69.528 | 75.054 |
FIGURE 8.
Change in SIS3 (emotion) and SIS8 (participation) over time.
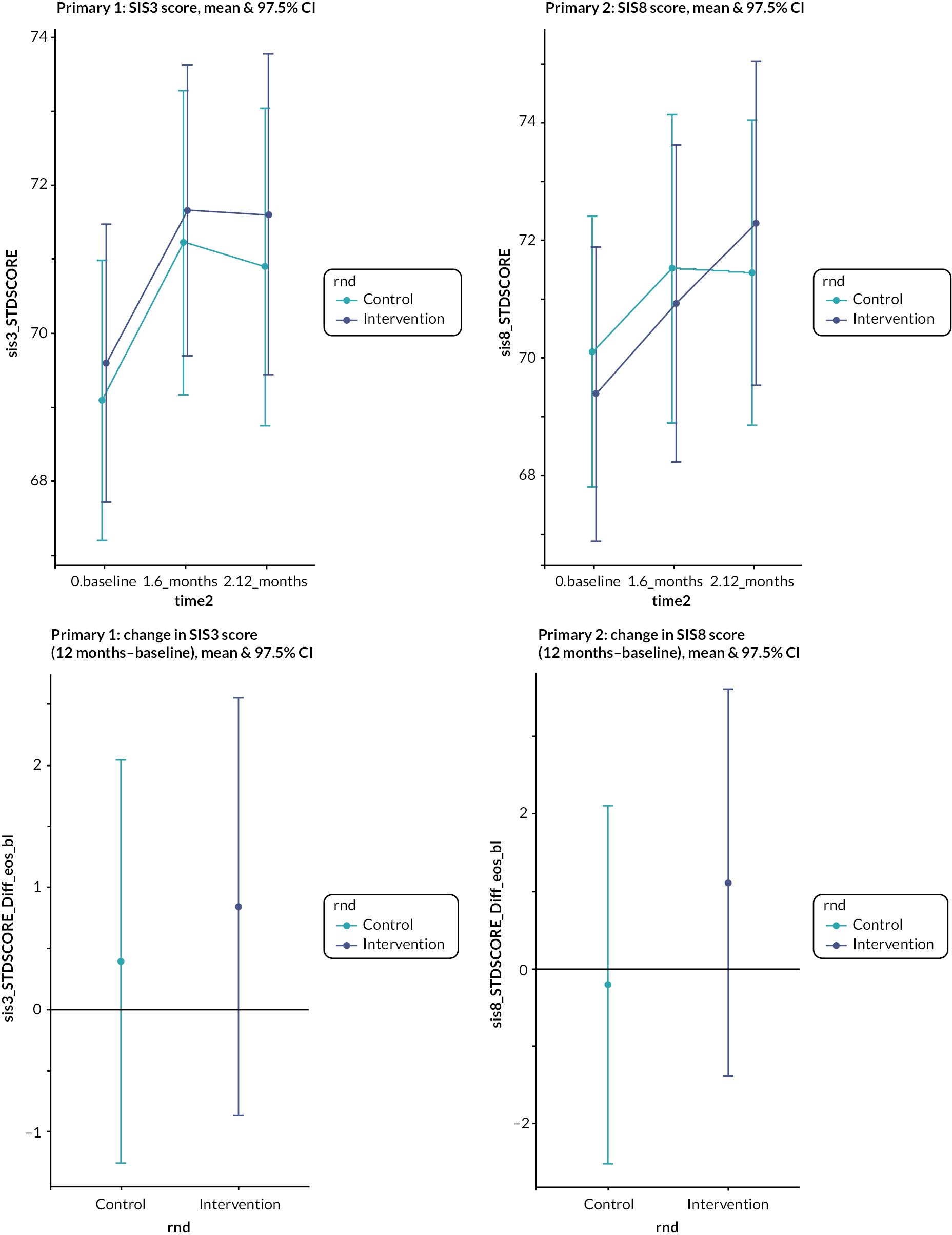
Missing data
Although there is evidence that attrition is not at random and more likely to occur to those with lower (worse) baseline SIS3 and 8 scores (t-test, p < 0.001), the randomisation of clusters balanced the baseline scores between the two treatment arms. There was no evidence of differential attrition between treatment arms (chi-squared test, ps ≥ 0.39). Therefore, attrition did not cause bias in the comparison of treatment arms.
-
Model estimates
Linear mixed-effect models using participants nested within practices as random terms used all available data. The data were structured as three rows (time points) per subject and the participant deprivation decile as well as practice deprivation decile (or the median if the practice was split-site) were included as continuous variables (lowest decile = most deprived).
Issues:
-
There was some evidence that perhaps participant deprivation and practice deprivation were causing multicollinearity (causing convergence errors), but the model estimates with and without practice deprivation were robust.
-
The bounded distribution of the data and the preponderance of scores on a boundary cause some concern that the assumptions of normal regression (unbounded, symmetric) might not be met. However, modelling using beta regression (bounded, non-symmetric) generated very similar conclusions. Such modelling was allowed for in the wording of the SAP. Beta regression required a variable transformation and can be briefly mentioned as a sensitivity analysis and not reported in full.
-
There was a small difference in the baseline scores between groups.
-
In our primary modelled analysis (see Table 26 ), we use a model that ignores the -month data and uses the baseline values of the primary outcome as a covariate.
-
Additional modelling using repeated measures gave the same conclusions while also taking into account the baseline data.
-
Additional modelling using a different formula where baseline was used as a covariate and the outcome was the change (12 months–baseline) reached very similar conclusions.
-
Due to small numbers in the age category 20–29 (3 participants), the 90 + age category (29 participants) was used as the reference. Stroke category 0–6 months was the reference.
-
Results are significant at the 2.5% level.
| SIS3 Model estimate (SE) | p-value | 97.5% CI | SIS8 Model estimate (SE) | p-value | 97.5% CI | |
|---|---|---|---|---|---|---|
| Intervention | 0.08 (1.14) | 0.943 | −2.3 to 2.47 | 1.29 (1.72) | 0.456 | −2.3 to 4.86 |
| SIS baseline | 0.7 (0.03) | < 0.001 | 0.64 to 0.76 | 0.58 (0.03) | < 0.001 | 0.52 to 0.64 |
| Sex: male | 0.84 (1.03) | 0.417 | −1.51 to 3.07 | −0.11 (1.42) | 0.936 | −3.28 to 3 |
| Age (ref: 90 +) | ||||||
| 20–29 | 16.23 (14.19) | 0.253 | −14.98 to 47.75 | 25.85 (19.43) | 0.184 | −16.09 to 70.08 |
| 30–39 | 4.52 (5.6) | 0.420 | −7.94 to 16.82 | 11.36 (7.76) | 0.144 | −5.29 to 29.2 |
| 40–49 | 2.01 (4.53) | 0.658 | −8.08 to 11.97 | 7.83 (6.3) | 0.215 | −5.98 to 21.91 |
| 50–59 | −0.73 (3.81) | 0.849 | −9.23 to 7.62 | 13.04 (5.36) | 0.015 | 1.41 to 25.17 |
| 60–69 | −0.64 (3.67) | 0.862 | −8.79 to 7.43 | 14.41 (5.17) | 0.005 | 3.15 to 26.07 |
| 70–79 | 1.19 (3.6) | 0.741 | −6.85 to 9.08 | 14.14 (5.08) | 0.006 | 3.1 to 25.63 |
| 80–89 | −0.12 (3.68) | 0.975 | −8.24 to 8.05 | 10.83 (5.2) | 0.037 | −0.38 to 22.7 |
| Stroke category (ref: 10 + years) | ||||||
| 0–6 months | 4.01 (2.03) | 0.048 | −0.47 to 8.5 | 2.65 (2.74) | 0.333 | −3.3 to 8.83 |
| 6 months–1 year | −0.23 (2.1) | 0.913 | −4.8 to 4.51 | 0.42 (2.94) | 0.887 | −5.97 to 7.09 |
| 1–2 years | 2.71 (1.69) | 0.111 | −1.08 to 6.43 | 0.24 (2.32) | 0.916 | −4.8 to 5.45 |
| 2–5 years | 0.78 (1.41) | 0.580 | −2.42 to 3.83 | −2.05 (1.94) | 0.290 | −6.29 to 2.29 |
| 5–10 years | 2.18 (1.42) | 0.123 | −0.98 to 5.28 | −0.74 (1.95) | 0.705 | −4.93 to 3.72 |
| Practice size: small | −0.96 (1.19) | 0.423 | −3.45 to 1.5 | 2.54 (1.79) | 0.163 | −1.22 to 6.24 |
| Practice median IMD deciles | ||||||
| 3–4 | 1.47 (2.37) | 0.538 | −3.55 to 6.41 | 0.19 (3.49) | 0.958 | −7.17 to 7.5 |
| 5–6 | 2.73 (2.35) | 0.249 | −2.24 to 7.66 | −0.95 (3.43) | 0.782 | −8.17 to 6.29 |
| 7–8 | 2.26 (2.4) | 0.350 | −2.75 to 7.36 | 0.58 (3.53) | 0.870 | −6.78 to 8.06 |
| 9–10 | 1.01 (2.54) | 0.691 | −4.38 to 6.32 | 0.31 (3.72) | 0.935 | −7.53 to 8.11 |
| Patient IMD deciles | ||||||
| 3–4 | 6.3 (2.16) | 0.004 | 1.41 to 10.96 | −0.37 (2.99) | 0.902 | −7.16 to 6.06 |
| 5–6 | 5.53 (2.11) | 0.009 | 0.87 to 10.11 | 0.85 (2.91) | 0.769 | −5.7 to 7.1 |
| 7–8 | 7.16 (2.12) | 0.001 | 2.43 to 11.75 | 0.9 (2.95) | 0.761 | −5.65 to 7.3 |
| 9–10 | 5.38 (2.11) | 0.011 | 0.74 to 10 | 0.85 (2.92) | 0.770 | −5.61 to 7.19 |
The intervention was not associated with any significant improvements in the emotion outcome, 0.08 (97.5% CI −2.30 to 2.47) representing an effect size of 0.02, with an adjusted ICC of 0.016. Similarly, the intervention was not associated with any significant improvements in the participation outcome, 1.29 (97.5% CI −2.30 to 4.86) representing an effect size of 0.25, with an adjusted ICC of 0.032. These are strongly associated with the baseline values. In our original power calculation, we had assumed an ICC of 0.03 for each. The impact of both the correlation of within-person measurements and the lower ICC (for emotion at least) is to increase the study power.
-
Extra analysis
From the 4 September 2020 TSC meeting, we were asked to consider if any effect was different for subpopulations. One issue has been the ceiling effect of SIS3 and 8 values. Given ~1000 subjects, I have created five quintiles based on the baseline score and used this in various models. These conclusions seem robust across all the models I have investigated.
One of the simplest to explain is for predicting change at 12 months from baseline. The main issue is that lower baseline SIS3/8 scores tend to increase at 12 months, and higher baseline SIS3/8 scores tend to decrease at 12 months.
The results are presented in the synopsis (p14)
Being in the lowest SIS3 quintile at baseline means the intervention has an additional 4.056-point increase compared to control (i.e. a greater increase, p = 0.2). Also, being in the highest SIS3 quintile at baseline SIS3 quintile at baseline means the intervention has an additional 4.167-point increase compared to control (i.e. a smaller decrease, p = 0.2).
Appendix 5 Randomised controlled trial: analysis of secondary outcomes
-
Modelled estimates
| Model estimate (SE) | p-value | 95% CI | |
|---|---|---|---|
| SIS short form | −0.32 (1.18) | 0.784 | −2.34 to 1.74 |
| SIS recovery | −1.71 (1.33) | 0.207 | −4.10 to 0.70 |
| EQ-5D-5L index | 0.02 (0.02) | 0.288 | −0.02 to 0.06 |
| EQ-5D-5L VAS | 2.03 (2.98) | 0.499 | −3.63 to 7.77 |
| ICEpop CAPability | 0.00 (0.02) | 0.903 | −0.04 to 0.03 |
-
Unadjusted estimates
| Baseline | 12 months | Change | p-value | ||||
|---|---|---|---|---|---|---|---|
| Control | Intervention | Control | Intervention | Control | Intervention | ||
| SIS | |||||||
| Short form | 412 73.7 (22.4) |
427 73.5 (21.7) |
303 72.8 (19.8) |
287 71.9 (20.7) |
267 −2.2 (13.3) |
266 −2.5 (12.2) |
0.796 |
| Recovery | 477 75.9 (24.1) |
484 74.9 (24.1) |
360 78.4 (21.4) |
359 76.5 (24.0) |
348 1.4 (15.7) |
356 −0.1 (15.0) |
0.183 |
| EQ-5D-5L | |||||||
| Index | 457 0.85 (0.36) |
465 0.86 (0.37) |
350 0.84 (0.37) |
353 0.88 (0.33) |
328 −0.02 (0.29) |
334 0.00 (0.27) |
0.487 |
| VAS | 481 72.8 (21.8) |
484 74.3 (20.9) |
361 73.4 (21.3) |
358 77.6 (53.2) |
350 −0.66 (14.3) |
352 1.04 (51.5) |
0.552 |
| ICEpop CAPability | 463 0.91 (0.29) |
479 0.93 (0.25) |
348 0.94 (0.24) |
347 0.94 (0.24) |
329 0.01 (0.28) |
341 −0.01 (0.25) |
0.385 |
| SSSMQ | 265 112.8 (13.5) |
252 112.0 (16.0) |
N/A | N/A | N/A | ||
| Health Literacy Questionnaire | |||||||
| 1. Feeling understood and supported by healthcare providers | 342 2.8 (0.8) |
323 2.8 (0.8) |
N/A | N/A | N/A | ||
| 2. Having sufficient information to manage my health | 351 3.0 (0.6) |
345 3.0 (0.6) |
N/A | N/A | N/A | ||
| 3. Actively managing my health | 344 3.0 (0.5) |
339 3.0 (0.6) |
N/A | N/A | N/A | ||
| 4. Social support for health | 348 3.0 (0.7) |
335 3.0 (0.7) |
N/A | N/A | N/A | ||
| 5. Appraisal of health information | 337 2.6 (0.7) |
325 2.6 (0.7) |
N/A | N/A | N/A | ||
| 6. Ability to actively engage with healthcare providers | 338 3.6 (0.9) |
326 3.6 (1.0) |
N/A | N/A | N/A | ||
| 7. Navigating the healthcare system | 338 3.6 (0.9) |
329 3.5 (1.0) |
N/A | N/A | N/A | ||
| 8. Ability to find good health information | 339 3.6 (0.8) |
330 3.6 (0.9) |
N/A | N/A | N/A | ||
| 9. Understand health information well enough to know what to do | 337 3.8 (0.8) |
333 3.8 (0.9) |
N/A | N/A | N/A | ||
-
Cohort analysis of intervention arm examining association of receipt of intervention with outcome
FIGURE 9.
Association of receipt of intervention with SIS3 (emotion) over time. Left: by whether a structured review was carried out; Right: by whether a structured review was carried out with or without an action plan.
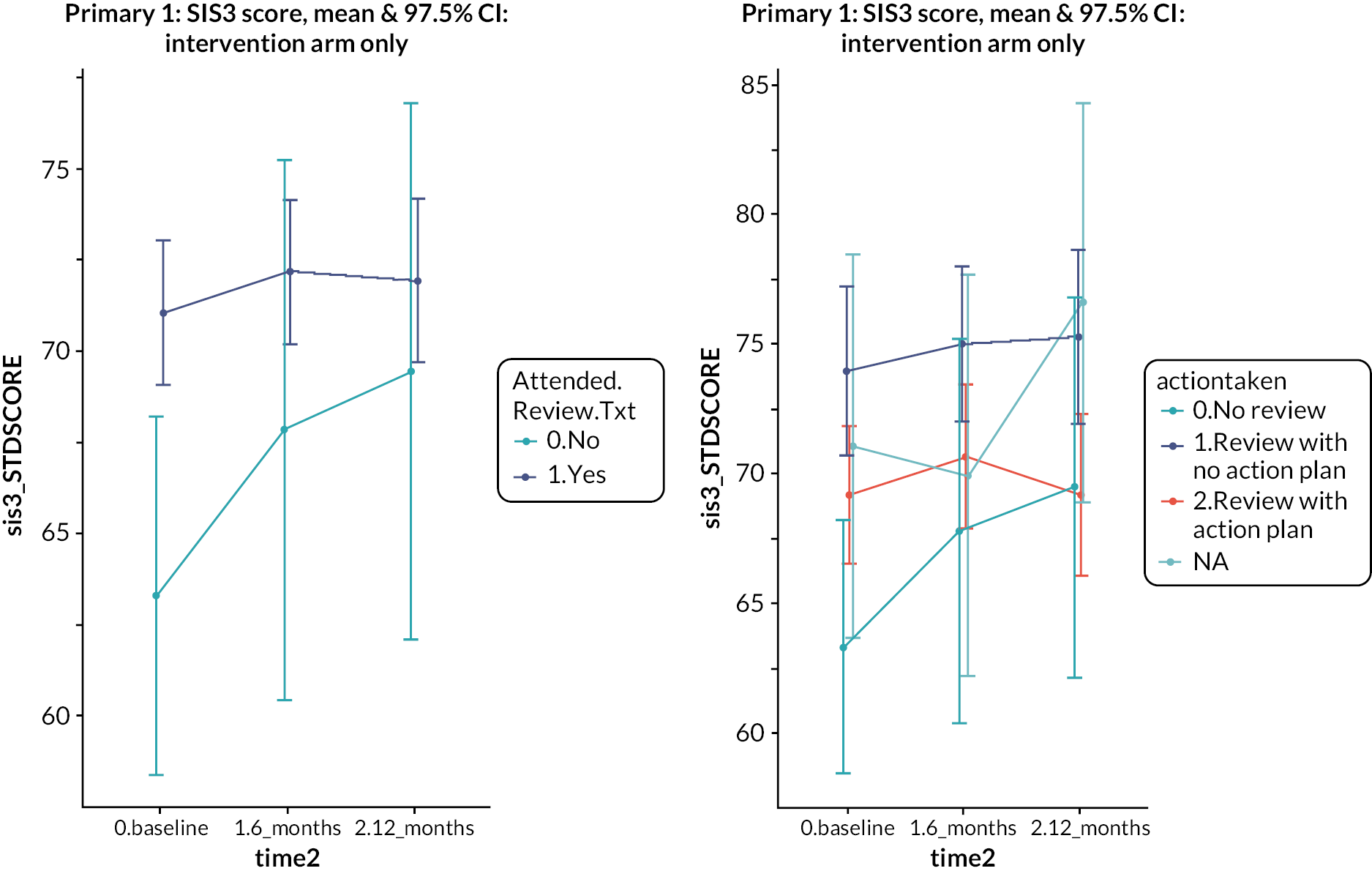
FIGURE 10.
Association of receipt of intervention with change in SIS3 (emotion) between baseline and 12 months. Left: by whether a structured review was carried out; Right: by whether a structured review was carried out with or without an action plan.
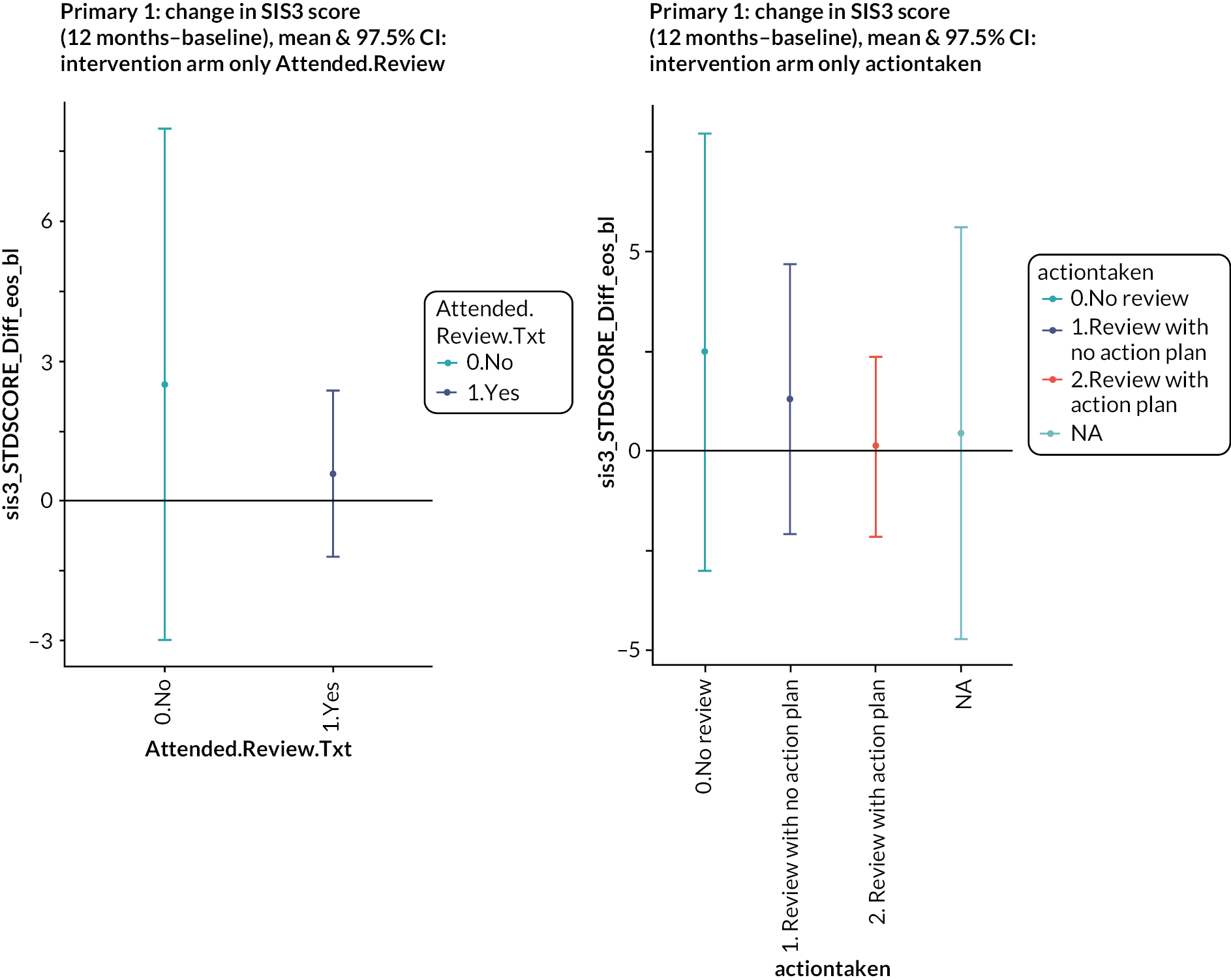
FIGURE 11.
Association of receipt of intervention with SIS8 (participation) over time. Left: by whether a structured review was carried out; Right: by whether a structured review was carried out with or without an action plan.
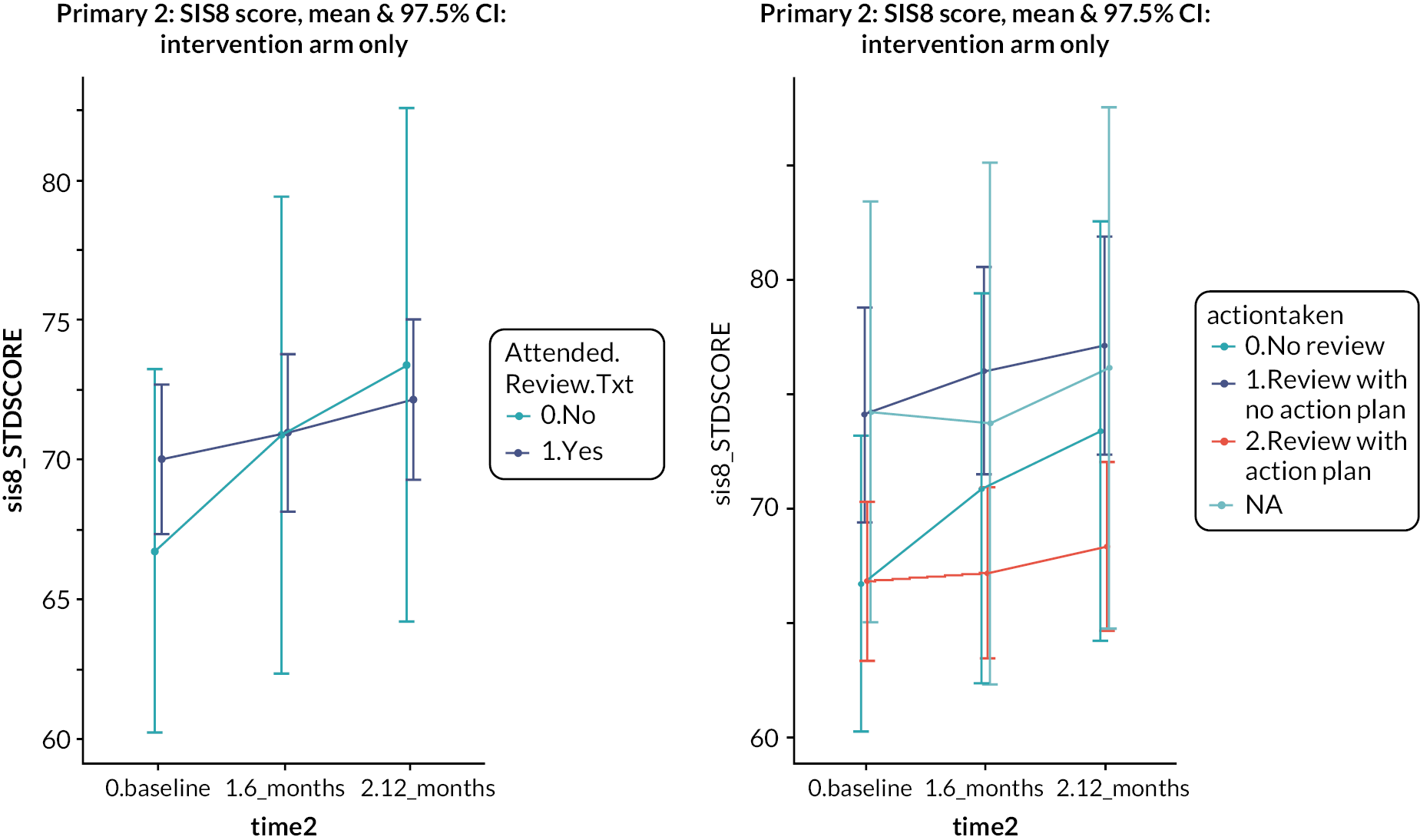
FIGURE 12.
Association of receipt of intervention with change in SIS8 (participation) between baseline and 12 months. Left: by whether a structured review was carried out; Right: by whether a structured review was carried out with or without an action plan.
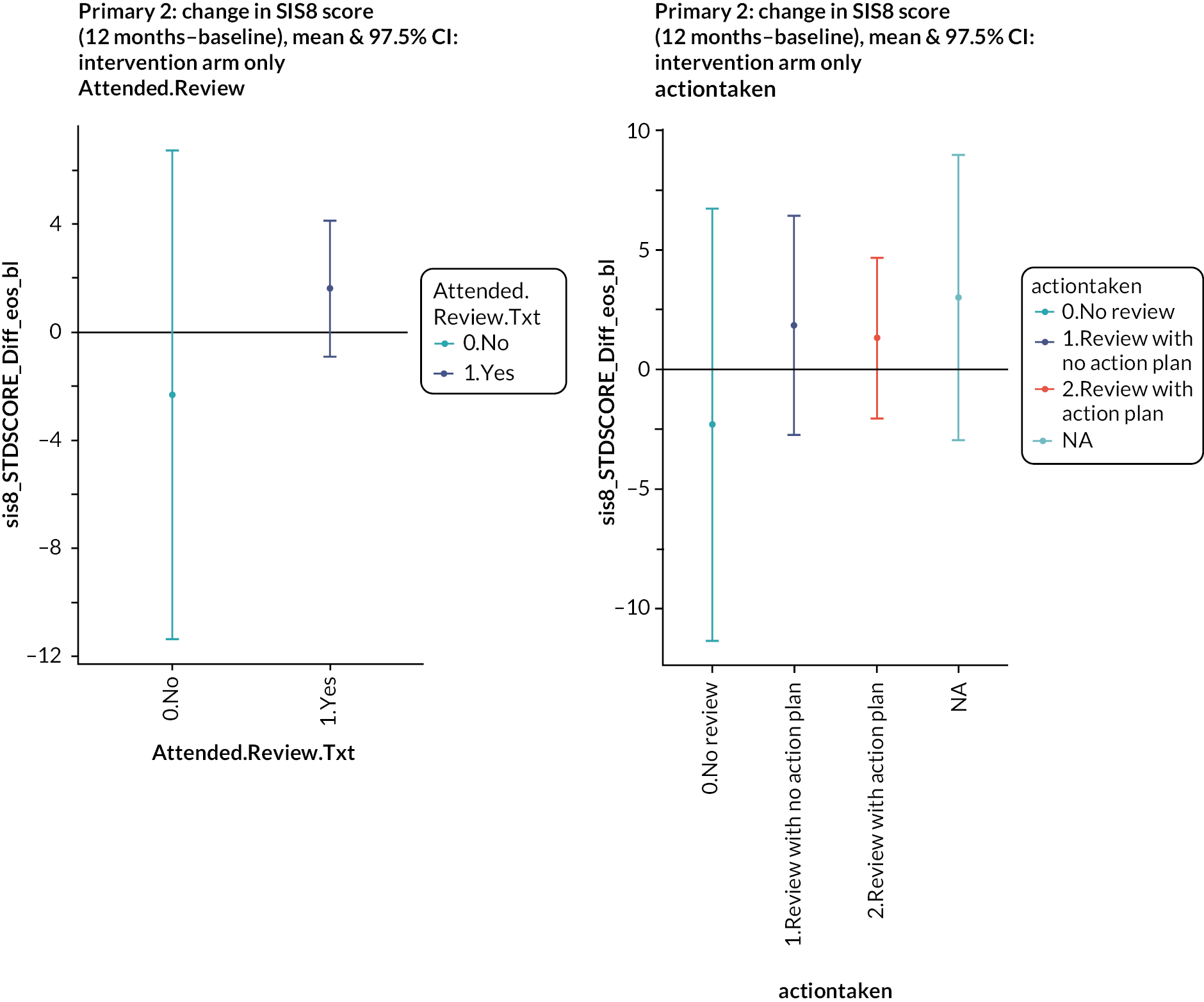
-
Cohort analysis of intervention arm examining association of receipt of MLAS with outcome
FIGURE 13.
Association of receipt of MLAS with SIS3 (emotion) and SIS8 (participation). Top left: SIS3 over time; top right: SIS8 over time; bottom left: SIS3 change from baseline to 1 year; bottom right: SIS8 change from baseline to 1 year.
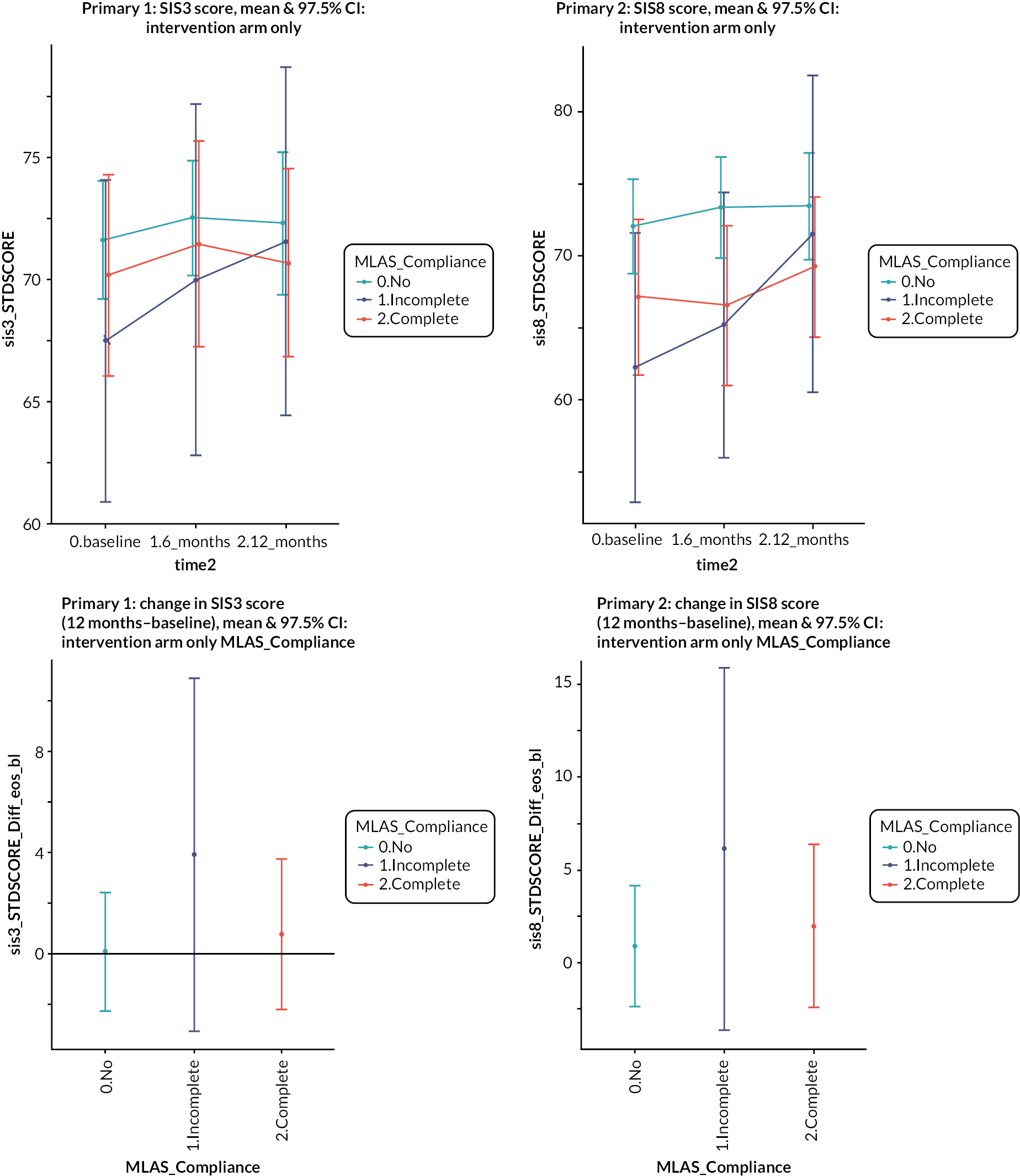
| No MLAS | Partial MLAS | Complete MLAS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | CI | n | Mean | CI | n | Mean | 95% CI | |
| SSSMQ | 136 | 113 | 110 to 115 | 19 | 109 | 102 to 117 | 69 | 111 | 108 to 114 |
| HLQ | |||||||||
| 1. Feeling understood and supported by healthcare providers | 174 | 2.85 | 2.73 to 2.97 | 25 | 2.56 | 2.28 to 2.84 | 85 | 2.75 | 2.59 to 2.92 |
| 2. Having sufficient information to manage my health | 189 | 3.07 | 2.98 to 3.16 | 26 | 2.85 | 2.70 to 2.99 | 90 | 3.02 | 2.89 to 3.15 |
| 3. Actively managing my health | 183 | 2.94 | 2.85 to 3.03 | 26 | 2.77 | 2.57 to 2.97 | 90 | 3.03 | 2.92 to 3.15 |
| 4. Social support for health | 181 | 3.09 | 2.99 to 3.18 | 26 | 2.92 | 2.68 to 3.16 | 87 | 3.03 | 2.91 to 3.16 |
| 5. Appraisal of health information | 170 | 2.58 | 2.47 to 2.69 | 25 | 2.28 | 2.04 to 2.52 | 89 | 2.60 | 2.46 to 2.73 |
| 6. Ability to actively engage with healthcare providers | 181 | 3.69 | 3.54 to 3.83 | 25 | 3.44 | 2.99 to 3.89 | 82 | 3.50 | 3.32 to 3.68 |
| 7. Navigating the healthcare system | 180 | 3.65 | 3.50 to 3.80 | 25 | 3.64 | 2.97 to 3.75 | 86 | 3.34 | 3.15 to 3.52 |
| 8. Ability to find good health information | 179 | 3.70 | 3.57 to 3.83 | 25 | 3.44 | 3.05 to 3.83 | 88 | 3.40 | 3.21 to 3.58 |
| 9. Understand health information well enough to know what to do | 182 | 3.93 | 3.80 to 4.05 | 25 | 3.68 | 3.29 to 4.07 | 88 | 3.76 | 3.59 to 3.93 |
Appendix 6 IPCAS process evaluation analysis
The analytical approach for this mixed-methods process evaluation is reported elsewhere (Aquino et al. , 2020). A brief summary is provided below.
Methods: quantitative data
Descriptive statistics (mean scores, mean %, weighted mean % across GP practices where applicable) were calculated for intervention fidelity assessments (i.e. audio- and video-recorded training, self-reported receipt questionnaires, audio-recorded structured reviews, self-reported delivery questionnaires) and study process data (i.e. participant and practice characteristics, GP practice notes from structured reviews) using MS Excel. Data were analysed by GP practice. Each component was scored either as ‘done’ (score: 2/2), ‘partially done’ (score: 1/2) or ‘not done’ (score: 0/2). Total scores and percentages of these were calculated and then averaged for each practice. A weighted mean was calculated across practices. Fidelity scores between 80% and 100% represented ‘high fidelity’, scores between 51% and 79% represented ‘moderate fidelity’ and < 50% represented ‘low fidelity’.
Methods: qualitative data
Qualitative data were analysed using thematic analysis on NVivo 12. Analysis was conducted iteratively by two researchers experienced in qualitative methods, following a six-phase approach: (1) data familiarisation or reading and rereading transcripts and listening to interviews; (2) generating initial codes or identifying and naming data excerpts that are relevant to the research question; (3) searching for themes or identifying overlapping areas between generated codes and exploring the relationship between these; (4) reviewing potential themes or checking the generated themes against data excerpts and vice versa; (5) defining and naming themes or summarising the focus/scope of the themes; and (6) producing the report or writing the themes in a coherent and logical way which answers the research question. Analysis by two researchers from different backgrounds facilitated reflexivity, where the analysts were able to reflect on and discuss their assumptions and interpretations of the data.
Results: quantitative data
Intervention process data
This section reports on GP practice, participant characteristics and data on participation at the structured stroke review. Each will be discussed in turn.
GP practice characteristics
There were 46 participating GP practices, of which 23 were randomised to the intervention arm and 23 to the control arm.
Participation at structured stroke review
Participants in the intervention arm (n = 522; 23 GP practices) were invited to attend a 30-minute structured stroke review at their GP practice. Across 23 intervention practices, on average, 82.00% (SD = 9.00; range = 59.09–100.00; CI = 78.33 to 85.58) of participants attended a structured stroke review. These reviews lasted approximately 27.91 minutes on average (range = 16.88–39.38), in line with the planned duration of 30 minutes.
Fidelity of design
-
Fidelity of IPCAS training
GP practice and healthcare professional characteristics
Intervention sites (n = 23) were trained to deliver the IPCAS model of care by members of the research team (LK, RM) between June 2018 and July 2019. A total of 19 training sessions were conducted; sessions lasted between 1 and 2 hours (mean = 1 : 33 : 41 hours). On average, three people were in attendance in each of the training sessions. Trainees (n = 63) consisted of GPs (n = 18; 28.57%), nurses (n = 24; 38.10%), healthcare assistants (HCAs; n = 3; 4.76%) and practice administrators (n = 18; 28.57%).
Audio-recorded fidelity of training scores
Four of the 19 training sessions (21%) were audio-recorded and assessed for fidelity of training by two independent raters (RA, JG). Overall, 96.09% (SD = 9.75; range = 96.88; CI = 82.58 to 109.61) of planned training components (n = 16) were delivered, achieving high fidelity. The raters achieved 86% Cohen’s kappa agreement for the audio-recordings [Prevalence and Bias Adjusted Kappa (PABAK) = 0.72].
All of the fidelity of training items (n = 16) achieved high fidelity, which suggests training sessions provided to GP practices were aligned with planned IPCAS training content.
-
Fidelity of MLAS training
Participant characteristics
Two 3-day training sessions were conducted for the self-management programme, My Life After Stroke (MLAS), in May 2018 and January 2019. The first course had a total of 13 trainees. The second training course had a total of 10 new trainees and 10 from the first training session. Over the intervention period, three of the facilitators dropped out before running any MLAS courses, and three facilitators dropped out after having run an MLAS course.
Video-recorded fidelity of training scores
MLAS fidelity of training items was grouped into two: planned materials and planned content. Training sessions were video-recorded, coded and scored by one rater. Of 217 fidelity of training items, 16 items (8 planned content; 8 materials – all from Day 3) were excluded from the analysis because video-recorded data were not obtained. Overall, MLAS trainers delivered 87.50% of all planned training components (88.89% of planned materials; 86.88% of planned content), achieving high fidelity.
-
Fidelity of receipt of structured review
Fidelity of receipt self-report scores
Of 421 participants attending their structured review (80.65% of participants randomised to intervention), 71 (16.86%) participants from 22 practices were contacted and asked to complete a fidelity of receipt questionnaire by telephone. Of these, 67 (94.37%) had complete data that could be scored. Overall, a mean of 63.18% (SD = 7.89, range = 27.78–85.19%, 95% CI = 59.89 to 66.48) of nine structured review components were reported to be received by participants. This shows that the IPCAS structured reviews were received with moderate fidelity.
Of the nine receipt questionnaire items, two were of low fidelity, five were of moderate fidelity and two were of high fidelity. Items of low fidelity concerned being given information on the DPoC service and ways to access this. Items of medium fidelity concerned discussing stroke-related needs, discussing an action plan to address identified needs and reviewing/noting these and being given information regarding the self-management course My Life After Stroke and how to access this. Finally, items of high fidelity concerned attendance at the structured stroke review and completing the 15-item checklist of needs.
Fifteen-item checklist of needs
Data on the 15-item checklist of needs were collected through participating GP practices. All but one practice (95.65%; 22 of 23) provided these data.
There were data available for 393 of 421 (93.35%) participants attending structured stroke reviews. Across 22 practices, on average, 74.05% of participants completed the 15-item checklist (SD = 8.83; range = 30.43–100.00; CI = 70.44 to 77.65).
The frequency of reporting of each of the 15 stroke-related needs specified on the checklist was summarised. Across 393 participants in 22 practices, 1456 needs were reported (mean = 97.07; median = 119). The most frequently reported need was fatigue (12.16%), and the least reported need was work (1.85%).
Action planning at structured review
This concerns any onward action plans discussed and agreed with stroke survivors during the structured stroke reviews, which are categorised into: (1) follow-up appointments, (2) referrals and (3) advice. More than half of participants (n = 237; 60.31) had at least one action plan recorded, while 39.69% (n = 156) did not have any. Across 22 practices, there were 431 recorded action plans, split into 29.47% (n = 127) follow-up appointments, 25.29% (n = 109) referrals and 45.24% (n = 195) advice. Follow-up appointments were recorded across the practices 7.16 times on average (SD = 2.48; range = 0–13; CI = 6.12 to 8.19), referrals were recorded 6.73 times on average (SD = 2.39; range = 0–19; CI = 5.73 to 7.73) and advice was recorded given 12.48 times on average (SD = 3.39; range = 0–24; CI = 11.07 to 13.90).
-
Fidelity of delivery of structured review
In all, 24 healthcare professionals (19 nurses, 1 GP, 3 healthcare assistants, 1 research administrator) delivered IPCAS structured reviews, and all were contacted and asked to complete a fidelity of delivery questionnaire by telephone. There were no missing data, and all completed self-report questionnaires were entered into this analysis. Overall, a mean of 83.05% (SD = 9.06; range = 41.67–100.00; 95% CI = 79.27 to 41.67) of 9 structured review components were reported to be delivered by healthcare professionals across 22 practices. This shows that the IPCAS structured reviews, from healthcare professionals’ self-reports, were delivered with high fidelity.
Of the nine fidelity of delivery questionnaire items, none were of low fidelity. There were 4 items of moderate fidelity, which concerned stroke survivors completing the 15-item checklist of needs, discussing action plans with them and logging/reviewing any actions with stroke survivors and healthcare professionals using the service mapping tool (SMT). There were 5 items of high fidelity, and these related to the discussion of up to three needs from the 15-item checklist: providing information about MLAS, providing instructions for accessing MLAS, explaining the DPoC, providing instructions for accessing the DPoC and using the SMT.
A subsample of all structured reviews (n = 34) were also audio-recorded, coded and scored to obtain fidelity of delivery scores. Overall, 68.63% (SD = 8.22; range = 13.89–100; 95% CI = 64.72 to 72.54) of 9 structured review components were found to be delivered by healthcare professionals across 17 practices. This shows that the IPCAS structured reviews, from independently scored audio-recorded structured reviews, were delivered with moderate fidelity.
Results: qualitative data
This section summarises the qualitative data gathered from interviews with healthcare professionals delivering the IPCAS intervention/model of care and trial participants.
Interviews with healthcare professionals
Interviews with HCPs from both intervention arms were conducted by telephone. There were 14 participants (11 intervention, 3 control, Table 30).
| Study arm | Role | Site ID | Gender | Years of experience | Interview duration |
|---|---|---|---|---|---|
| Intervention HCP | GP | 20 | M | 25 | 19 : 11 |
| Intervention HCP | HCA | 13 | F | 19 | 37 : 31 |
| Intervention HCP | PN | 14 | F | 32 | 38 : 47 |
| Intervention HCP | PN | 04 | F | 36 | 39 : 00 |
| Intervention HCP | PN | 19 | F | 40 | 39 : 36 |
| Intervention HCP | PN | 29 | F | 35 | 48 : 41 |
| Intervention HCP | PN | 31 | F | 26 | 39 : 03 |
| Intervention HCP | RN | 46 | F | 33 | 44 : 59 |
| Intervention HCP | PN | 37–43 | F | 11 | 44 : 39 |
| Intervention HCP | PN | 11 | F | 10 | 25 : 48 |
| Intervention HCP | PN | 33 | F | 17 | 31 : 02 |
| Control HCP | PN | 22 | F | 14 | 33 : 44 |
| Control HCP | PN | 06 | F | 21 | 30 : 43 |
| Control HCP | PN | 07 | F | 3 | 34 : 10 |
| Theme | Subtheme |
|---|---|
| 1. HCP experience of delivering IPCAS | Checklist SMT DPoC Communicating follow-up services (MLAS and Action Planning) |
| 2. Factors influencing intervention delivery | Healthcare professional role Healthcare professional’s previous experience enabling responsive delivery |
| 3. Experience of IPCAS training | Perceived benefits of training Descriptions of participating in training Increased knowledge of poststroke needs |
| 4. Post-IPCAS involvement | Increased awareness of stroke survivors Did not adopt IPCAS New (IPCAS) practice normalised |
Theme 1: healthcare professional experience of delivering IPCAS
This theme concerns healthcare professionals’ experiences of delivering the components that comprised the enhanced stroke review: the 15-item checklist of stroke survivor needs, the SMT, DPoC and communicating follow-up services (MLAS and action planning).
The enhanced stroke review was designed to take 30 minutes. HCPs in the control arm of the study described that stroke needs were included in an annual review after initial stroke care, which includes anthropomorphic measurements. The reported duration of these reviews was around 20 minutes (and could often overrun):
The HCPs who were treating patients allocated to the control arm of the study confirmed that usual care was carried out during their participation in IPCAS.
I don’t think anything has altered because of the study, because everything is as it was prior to us beginning the study.
[HCP1_22]
Checklist
Overall, healthcare professionals found the 15-item checklist of needs useful, albeit with some reservations that there were a lot of items listed. Some HCPs reported that this tool facilitated a focused conversation with stroke survivors and carers, thereby optimising available consultation time:
… with them coming in with that pre- filled in it really did focus one’s mind on areas that were obviously important to the patient. So yeah, it was good.
[GP1_20]
If the patient had ticked items of concern on the checklist, the HCP would decide with the patient whether a referral was required. Depending on the nature of the referral, concerns could be actioned by a clinician within the practice. For example, clinical referrals were made in the usual practice way (see also theme 2), and self-referrals were suggested using the SMT.
Some HCPs also reported that using the checklist encouraged stroke survivors to openly discuss their needs and allowed them to identify the issues that were important to them, making it a patient-centred consultation:
… I found that in a lot of cases it was the first time in ages that they’d actually had time to actually talk about how their stroke had affected them and what it does affect. […] I think the checklist was really good because it opened up the conversation.
[PN1_29]
Differences were observed between stroke survivors’ use of the checklist, with some ticking most items and others hardly any at all, demonstrating the varying levels of patient needs.
I found it good, yeah, how it was planned out in different sections it was quite easy and people understood which bit they had to tick and, if not, they used to ask when they get here and we used to sort them out and that was fine. Yeah, I found it really easy and good.
[HCA1_13]
And that was really interesting because some patients just ticked everything …
[PN1_29]
Those HCPs who found that the checklist was lengthy recalled having to support some stroke survivors with completing the checklist during the enhanced stroke reviews, making time management challenging.
And at the end of the day, you’ve got a patient in front of you. You want to do what’s right for the patient. I understand you have to tick these things. I do understand why. I do get that. But at the same time you think, hang on, there’s a … if you squeeze your time too much you can’t actually give what patients often really, really need.
[RN1_46]
It’s really difficult to steer a consultation in that kind of forum with a list that long, because even the well … it’s like I always say, in practice, when you have the ‘worried well’, they’ve still got a story to tell you …
[PN1_11]
And a lot of patients, they would tick a lot, because they’re obviously issues but they weren’t always prioritised, they’d go through the checklist and they’d say, yes, I’m having trouble here, I’m having … so it was quite difficult to get … to keep pertinent for that top three to four when some patients found that they had a lot of problems, so that made it more difficult for people to prioritise what their main problem was …
[PN1_04]
… if they didn’t bring their checklist, we would go through it at the appointment.
[PN1_31]
HCPs also suggested improvements for the future. These included reminding patients to complete the checklist before the review appointment, reducing the checklist to one page and making clear on the checklist instructions to select patients’ three main concerns, in line with the design of the structured review.
the way that we did that is that [name] sent out the checklist and things with the letter and everything for them to complete, then she also rang them all before they came in, one, to make sure they were going to attend because obviously I was given a half an hour appointment so to make sure that they were actually attending, but also to remind them to fill that in before they came. So the fact that she did that, I think that’s what really helped.
[PN1_19]
But […] even though they don’t need your help now, they want to tell you their story […] they would tick several of the boxes, and then it was a case of saying to them, right prioritise these for me, which ones do you feel are … and trying to just keep them on … you could have spent two hours, sat discussing some of the lists.
[PN1_11]
Using the service mapping tool/use of service mapping tool
Participants had mixed views about the usefulness of the SMT, which was available for healthcare professionals to use during the structured reviews and any other contact with stroke survivors during the course of the study. Some HCPs found it too lengthy and complex:
Yes, that was as clear as mud! That was a very long list of … that definitely could be improved […]Yeah, and it was very small print, and it was very, kind of, you really were having to, you know, it … yeah […]The print was small, there was too much on one … was it a piece of paper or was it a screen?
[PN1_11]
Yeah, I mean there’s quite … it’s quite a big document isn’t it? And it’s unpicking what would be appropriate for that patient. I mean we had the template for different areas.
[RN1_37]
However, on familiarisation with the SMT, other HCPs reported that it was not only helpful but, on familiarisation, became easier to use:
Actually I found them very useful and, off the top of my head, to be honest, I can’t find anything that I’d like to add to it because on the mapping side and information I seemed to have found it [ … ] and if I didn’t then I must have just gone somewhere and got hold of the information and just posted it to the patient anyway.
[HCA1_13]
Direct point of contact
All but one HCP reported that they had not knowingly received any communications from stroke survivors using the DPoC, despite there being a clearly understood process for the practice staff on how to direct and deal with any queries. At some practices, it was expected to generate a heavy workload of patient enquiries:
A GP that was also involved in it didn’t want his point of contact to be put on there, ‘cause, I think, he thought it was more workload. I think, he was … so he said that the nurse would be the point of contact and then I would then go to him with anything that I felt relevant when … but other than once patient, I didn’t have anybody else call.
[PN1_11]
HCPs reported that they had received clear instructions on how to deliver DPoC, for example, using the template and a named contact. Some even provided DPoC information in print to stroke survivors.
None of the patients had made any contact with us on the direct point of contact, because we have a special template on for that, and all the staff were educated as to what to do if a patient who is on the IPCAS trial did phone in and needed to speak to either myself or the nurse who was involved, and we’ve not had one phone call about that. So that was interesting.
[GP1_20]
[…] we did, we printed out our name and our manager put a dedicated line to the stroke … but, yeah, we didn’t get any calls … I always gave them the number and said, if there was anything that you wanted to bring up that you haven’t brought up then just drop us a line, ask for myself or [other HCA], who was the other person, and we’ll be happy to return your call any time [...]. But we never got any so I assume everyone was happy, I don’t know.
[HCA1_13]
R: No, no, I’ve had no messages because I gave my contact and also the practice partner, so if I wasn’t around then she would take the messages and then either deal with them or come back to me, yeah.
I: And she had none to pass on to you..?
R: No, no. Interesting, isn’t it?
[PN1_04]
We had some little cards made up in the practice, that we gave out at the consultation … And, the contact was for me and for the other nurse but then, I think, hers got taken off ‘cause she was off on long term sick.[…]
One HCP felt that IPCAS as a whole intervention was a success because they confirmed they were the DPoC with every study participant, yet received no further patient queries going forward, which to them indicated having met stroke survivors’ needs:
I know it must have been successful because although I religiously told every single person that I was the main point of contact and they knew how they could contact me and request a telephone call back, I haven’t had anybody ring me. […] All I’m saying is they must have got all their relevant needs met, because otherwise they would have been in contact, wouldn’t they? […] I again dutifully told them, every single one of them that all they needed to do, as I was the main point of contact for any queries, if they rang in and left a message, I would ring them back, and I have protected telephone slots every day in the morning and in the afternoon, but not one.
[PN1_19]
Communicating follow-up services (My Life After Stroke and Action Planning)
My Life After Stroke
There were varied reports of how the self-management course (MLAS) was introduced to stroke survivors. All HCPs recalled offering the course to every participating stroke survivor, regardless of whether they thought the stroke survivor may or may not benefit from the course:
R: Everybody I saw got offered the My Life After Stroke.
I: So there were no patient characteristics ….
R: No. Everybody I saw I offered it to them.
I: And that was including people with aphasia or comorbidities or …..?
R: Yeah, absolutely.
[GP_20]
Some HCPs offered the descriptive leaflets to the stroke survivor and left the decision with each individual:
I grinned at them and said, well, take it home, think about it, no pressure, no pressure (laughs), but read it in your own time, and if you want to, you can, and if you don’t want to, that’s fine, but please read through it and see what you think!
[PN_04]
Whereas some healthcare professionals explained the course in detail, one HCP described how she ‘really sold it’:
I kept it into my … how would you explain it, like an introduction to what I was actually going to do and then I said, I’ll explain it a little bit better, and as I started going through it they […] let me explain what it was all about, what it actually involved and that they had the choice. And, yeah, they let me talk all the way through it and sell it to them.
[HCA_13]
One HCP described how she would have liked to have attended MLAS herself, explaining that some experiential understanding of the course would have been beneficial. It might also give a positive affirmation to the participating stroke survivors:
I would have loved to been able to actually attend that course myself, I think that that would have been really beneficial because not only would it have been good for me to actually get the knowledge of what was happening during those sessions, but also it would have given a positive affirmation to all those ones that I’d encouraged to go, oh, look, [‘my name’] is here, you know …
[PN_19]
Action planning, whether it was providing further information/advice or making onward referrals to health and social care services, appeared to make little or no difference to how HCPs referred their patients. HCPs recalled referring patients in their normal way, that is not enhanced as a result of the checklist or SMT:
I think I did a normal routine referral for somebody to a physiotherapist, and I think I did one to occupational therapy to check the house, but it didn’t change the way I would normally do it. Certainly didn’t make any difference at all. I would have done it if I’d seen the patient ordinarily, but it just came up at the IPCAS meeting that they needed that so I did the referral.
[GP_20]
I did try and contact the stroke referral mobility type sort of team, but they didn’t accept it because she wasn’t just past a stroke.
[PN_14]
HCPs reported mixed experiences of making onward referrals, with some practices having established electronic referral systems already in place.
Depending on what it is that you want to refer to, you either refer them straight away on a pre-designed referral form, which is already in place, or you pass on to the secretaries.
[PN_31]
Whereas some reported using the SMT extensively:
Some of the questions that they were asking me were quite interesting, stuff that they didn’t know how to get involved in. So I’ve referred quite a few people to [physical activity group] through the stroke trial […] It’s only through the study that I found out about [physical activity group].
[HCA_13]
Onward referral sources such as these were reported lacking by HCPs in the control arm of the study, suggesting that the SMT would have benefited:
I feel that I don’t really know where to refer my patients who have suffered a stroke. Other than to the GP, I don’t really know where else that I can send them for, if they need physio or that sort of thing […] because I don’t really know what services are available that I can physically send my patient to.
[PN1_07]
Yeah, I think, there is a bit of an unknown, I have to be honest, there isn’t … apart from the links that we have on the template for Slimming World and smoking cessation. All the other things are just, sort of, if you, kind of, if anything that we become aware of as a team of nurses, we will bring up and let the others know. But that’s just really word of mouth, there’s not really and objective way it’s not really set out in any way at the moment.
[HCP1_22]
Control arm HCPs also made some suggestions aligned with the SMT:
Well, that would be almost like a little bit of, probably, an online directory […] Yeah, like, just something that’s held online that you can access just to see, within your area, what is available.
[HCP_22]
Theme 2: factors influencing intervention delivery
Healthcare professional role
This theme concerns factors identified by HCPs that made a difference to how the intervention was delivered.
A distinction in IPCAS delivery emerged between the roles of the practice nurse and research nurse. Where a practice nurse conducting reviews was an integral member of staff within the surgery, there was often an already-established rapport with the patient. Having some familiarisation with patients and prior knowledge of the patients’ needs as a whole led to a smoother delivery.
I think it’s good to have a person that you know, most of the patients I would say know that if they’ve got anything to do with their heart circulatory stuff they’ll give me a shout …
[PN_04]
I think, because I’ve seen these patients or I had seen them for the last five, six years, a lot of information that we were trying to find out, we had already discussed in previous appointments, not maybe as much in depth …
[PN_31]
Research nurses, on the other hand, reported experiencing some challenges to delivering IPCAS. These included not knowing the patient beforehand, taking time from the structured review to build a rapport and not having prior familiarisation of IT hardware and administrational processes.
I’m just there for this brief period of time and that I don’t get any of the follow up.
[PN_04]
And this is where probably … this is where I think it’s a … a little shame, if I had been a practice nurse at any of these different surgeries, then maybe I would have, the likelihood of me seeing these patients again would have occurred.
[RN_37]
(Or this quote – same RN)
But I did think it would have been kinder, maybe more direct, if I’d have just done that referral myself to them. So, whether it may have been just a … a patient could just have gone to see that person direct, say if it was a physio or a speech therapist. But then, you know, I … as not working in that practice regularly, I had to ensure that it was an appropriate referral too. So, it kind of made sense to go either via the GP or the practice nurse, yeah.
[RN_37]
It wasn’t all negative though; one HCP felt that it was a good thing to be a research nurse as she had more time for the patients:
… as the research nurse doing the research is our, actually our job and our priority […] I mean, fortunately, as a research nurse, we can just concentrate solely on that research, yeah.
[RN_37]
Having the clinical autonomy to make referrals also appeared to make a difference to how an action plan could be executed. For example, hospital-based referrals appeared to be accepted mainly from clinicians, suggesting other staff were restricted to what they could achieve within the consultation:
… it has got to be through a GP, because a lot of the hospital-based clinics don’t like nurse referrals, so, you know.
[PN_04]
HCPs in the control arm reported a similar role limitation of being reliant upon GPs to refer patients to other services:
I guess I do rely a little bit heavily on a GP, so because of the limitations of my role, if I have a patient that comes to see me, and they have these problems that I cannot fix, then I refer them to the GP, and I then assume that the GP is going to potentially sort it out, or signpost them in the right direction.
[PN1_07]
Healthcare professional experience
HCPs with prior clinical experience said that it helped in delivering the IPCAS review:
This is a new study that they wanted me to take on, previously I worked in the (hospital) for 19 years in cardiology. […] I enjoyed doing the study, I learnt a lot and I found it very interesting and very helpful for the patients that I actually saw.
[HCA_13]
Inevitably you do find whatever experience you’ve had in your personal career, you bring it in anyway don’t you …
[RN_46]
Experience of technology use between colleagues appeared to have influenced the smooth delivery of the intervention. For example, HCPs with limited IT experience reported that they struggled with the templates, whereas others said that they found them straightforward and easy to use.
… what we did is we put it on the computer. One of my colleagues is a computer geek – he sets up templates on our computer system, so we had an IPCAS template, we’ve actually still got it on the system.
[GP1_20]
Theme 3: experience of IPCAS training
This theme addresses the descriptions of participating in training and the perceived benefits of training.
Descriptions of participating in training
All HCPs who attended the training sessions described how training met their needs adequately, giving them confidence to deliver the structured review.
… after a couple I found that all the stuff that I had been taught and I’ve learnt actually came in useful and it all came flooding back after a couple […] I was impressed with the training that I did get and how informative it was.
[HCA_13]
Those HCPs who missed some or all of the training felt they ‘caught up’ adequately with what was required:
I was a late attender, I did go to the IPCAS meeting, but due to the pressures in primary care, I think, I got there about 45 minutes in, so missed a big chunk and then had patients booked. So, kind of, had the catchup brief and picked up on the, I think, there was a PowerPoint presentation, if I remember correctly.
[PN_11]
One HCP had little recollection of the training and misremembered some aspects. This was reflected in some miscommunication within the consultation:
I knew there were going to be some, like, ‘clinics’, or like an outpatients thing, [...] I wasn’t really very sure who was running them or what it would entail in the groups that they were being invited to … so I don’t know if that just hadn’t been filtered down to my level, or … I can’t remember. They probably did say; they wanted to be doing the presentation just before I had my lunch! [laughs].
[PN_33]
Perceived benefits of training
HCPs reported that having ongoing support and knowing the research team was always on hand helped build and maintain confidence.
But I know that when I started doing them, I felt well-prepared, and I think I’d spoken to somebody beforehand and I definitely got a lot of support during, so if I had any queries, there was always someone there that I could get support from.
[PN_14]
as soon as [researcher’s name] came in and just explained stuff […] I felt a lot more confident. And I felt confident in myself that actually I can go back and I can actually do this and feel like I’ve done it properly.
[HCA_13]
Benefits also included having a clear understanding of the research methodology.
Well, I think for me personally, it was a nice in depth look at the post stroke pathways and really how when patients are discharged from secondary care, they are very much dumped in primary care and there’s not too much happens in between.
[PN_11]
HCPs reported that being part of the review process enhanced their knowledge of the heterogeneity of stroke survivors:
It was quite an eye-opener for me […] to see how patients’, you know, experiences were very different … Yeah, yeah […] their journey post stroke had been very different.
[RN_37]
Theme 4: post-IPCAS involvement
Being part of IPCAS allowed HCPs more insight into the experiences of stroke survivors, giving them increased awareness of poststroke pathways:
I do think I’m a little bit more comprehensive when I see a patient who’s had a stroke. Having spoken to patients about their intimate relationships, that sort of thing, that now comes to mind when I see them just to make sure that all is okay.
[GP_20]
[….] I was caring anyway but I think that’s opened my eyes a little bit more of the needs of other people and not just take everything [at] face value. Some people are struggling, aren’t they, they just don’t ask or they might not realise?
[HCA_13]
Did not adopt IPCAS
Some HCPs reported that they did not adopt IPCAS and reverted to their usual practice model of care post study. Those HCPs that did this, however, were able to use their knowledge to enhance their practice:
… going forward we haven’t continued with the template that we were given, we reverted back to just a QOF one […] No, but it’s still good, in that it’s probably nudges me to ask … to delve a little bit deeper to what is just basically on the QOF, kind of, checklist. And, I will now ask about personal relationships and things that I, perhaps, wouldn’t have done prior.
[PN_11]
New (IPCAS) practice normalised
Aspects of the intervention have been kept and are still being used within normal practice.
As I said, we’ve still got the IPCAS template on the computer, so I’ve still got that as an aide memoir, so I will be using that when I next see stroke patients just to keep my mind on what we went through. So yeah, it has helped from that point of view. […] Just an aide memoir to go through everything it was brilliant.
[GP_20]
Interviews with stroke survivors
Interviews with stroke survivors from both intervention arms were conducted. There were 27 participants (19 intervention, 8 control). Four themes, with eight subthemes, were identified.
| Theme | Subtheme |
|---|---|
| Views and experiences of structured stroke reviews | N/A |
| Perceptions of eligibility for stroke care or support influential to engagement | Time since stroke |
| Perception of health status or recovery in relation to age | |
| Engagement with other intervention components, materials and resources | Awareness, use and perceived importance of DPoC |
| Using the 15-item checklist of needs | |
| Receipt and use of tailored service directory | |
| Benefits gained from participation in IPCAS | Knowledge |
| Skills | |
| Emotional well-being |
Theme 1: views and experiences of structured stroke reviews
Stroke survivors across trial arms had mixed opinions of the value of having structured stroke reviews at their GP practice. Those randomised to intervention and attended structured stroke reviews reported that they felt cared for and brought to the fore issues that they might not have associated with their stroke (e.g. daily living activities, exercise), resulting in these being addressed:
[…] the main consideration was what I found most difficult, having had a stroke, and one was that I’ve put on a lot of weight since then and exercising was difficult because I get out of breath quite easily, especially going up hills and lots of stairs and things, but no, he gave me … he helped for that.
(20040K – Female, 74 years old, 4 years 6 months post-stroke, Ipswich)
Intervention arm participants found it useful to be able to talk to someone about their stroke and have someone become familiar with their history, medication regime and other related needs: ‘Well, as far as I’m concerned, with having the stroke, it’s nice that somebody still cares about it, you know, that I’ve had one and how do I feel about it?’ (37092Y – Female, 68 years old, 43 years 10 months since stroke, East Midlands).
Meanwhile, participants in the control arm reported having no involvement with their GP practice to discuss their stroke and stroke-specific needs for years, which continued throughout their involvement in the IPCAS study: ‘I have never called my GP Surgery about a stroke-related issue. [ … ] I have contacted my GP Surgery whenever needed, just not needed to with any stroke-related issues’ (21061P – Male, 59 years old, 10 years 1 months post stroke, Norfolk). They also reported observing no changes in how their GP surgery provides services, care or support since taking part in IPCAS:
It [my stroke] doesn’t come up at all. I’ve had a stroke and that’s registered there and they know it was in 2009 and that’s all they know. But then we’ve got all these other problems that they are attending to, which they see on a day to day basis.
(24006Y – 79 years old, 9 years 4 months post-stroke, East Midlands)
A number of control group participants (n = 3) shared their experiences of attending their GP surgery for drugs/medication and diabetes reviews, which for many took place annually. Such reviews involved checking stroke survivors’ medication regimes, blood tests and blood pressure measurements:
Well, I used to get a review every year with Dr [name] and he signed me off, he said, there’s nothing else I can do for you. So, okay, fine, I’m happy with that. Every year I’ve got a blood pressure, test my bloods and just a general chat about things.
(01061Q – Male, 74 years old, 6 years 5 months post-stroke, Eastern)
Control arm participants without lasting stroke impacts were sceptical about the value of a structured stroke review and whether it would have a positive impact on their health or recovery (Theme 2):
Not really. I mean … […] … what were they going to do? […] I don’t think it would have changed anything. I can only obviously say from my point of view and I consider myself very lucky, my particular situation, no I don’t think it would have made any difference or helped.
(01050H – Female, 55 years old, Eastern [RA52])
Indeed, intervention arm participants also highlighted that the structured stroke reviews could be more beneficial at earlier stages post stroke. Barriers to attendance at the structured stroke review were work commitments and identifying as well-recovered from their stroke (Theme 2):
I was invited to go to the GPs, but the days that they … because they wanted to do an interview there, and I’d had to say, I’m more than willing to do the interview, but I work fulltime, I physically can’t get there to do that at the moment.
(30136T – Female, 35 years old, 10 years 11 months post-stroke, East Midlands)
Findings from this theme suggest there was no contamination across trial arms.
Several stroke survivors could not distinguish the IPCAS structured stroke review from other contacts with the GP practice, owing to high volume of attendance in primary care for other health issues or impaired memory.
Theme 2: ‘feeling a fraud’ – perceptions of eligibility for stroke care or support influential to engagement
This concerns stroke survivors’ perceptions of their personal eligibility for GP care or support. Two subthemes were identified: time since stroke and perception of health status or recovery relative to age, which were found influential to participants’ level of engagement with IPCAS.
Time since stroke
Stroke survivors who were years post stroke found the IPCAS model of care less relevant or useful to them. However, they acknowledged that if the model of care had been in place in the acute/subacute phases of stroke, then they likely could have benefited from the support and resources offered: ‘What most people need, I would imagine, is support when they first come out and then to review it as they go along’ (08062S – Male, 84 years old, 5 years 4 months post stroke, Norfolk).
‘I would have wanted to know (12 years ago), at my current rate of improvement, how, and this maybe a question that everybody asks, how likely is it that I will get back to being the person I was’.
(30136T – Female, 35 years old, 10 years 11 months post stroke, East Midlands)
This was echoed by participants in the control group when asked to consider the value of a structured stroke review. Some participants thought extra GP support or input would have been useful to monitor their poststroke health and symptoms, including emotional/mental health needs, especially in the earlier stages post stroke: ‘I think seeing a GP a little while, say, 6 months or 12 months after a stroke would be handy but later on, now in this time, 13, 14 years later, it doesn’t do a lot of good’ (03031V – Male, 79 years old, 12 years 4 months post stroke, Eastern).
Perceptions of health status or recovery relative to age
Participants emphasised the importance of providing support that is individualised or tailored to stroke survivors’ needs. Reflecting on the 15-item checklist, some participants reported the questions were not matched to their age or experience of stroke (i.e. not severely affected/recovered fully): ‘I think when I was filling in those forms [ … ] it was very applicable to the older person who’s very disabled as a result’ (11053Z – Female, 53 years old, 18 years 10 months post stroke, Norfolk).
Participants also reported the importance of healthcare professionals listening to patient needs and reported symptoms and goals post stroke. One reported barrier to attendance/participation in IPCAS activities was participants’ views that they were fit and well, and therefore, any support or care offered to them should be allocated to those experiencing severe poststroke impacts: ‘I didn’t think I was serious enough. I thought I was so fit I thought I’d be taking up space for somebody else’ (30123R – Male, 77 years old, 1 year 6 months post stroke).
Theme 3: engagement with other intervention components, materials and resources
Participants also discussed how they used or engaged with other intervention components and shared their opinions of these. This formed three subthemes: awareness, use and perceived importance of DPoC, using the 15-item checklist of needs and receipt and use of a tailored service directory.
Awareness, use and perceived importance of direct point of contact
Although many participants did not recall receiving information about the DPoC, many reported it was a good idea in principle to have a nominated person to call on for support at their GP practice as it provides an opportunity to ask questions specific to one’s stroke: ‘I think this [direct point of contact] is an essential and long overdue aid for wellbeing of patient and all concerned’ (46047W – Female, 81 years old, 6 years 8 months since stroke, Ipswich).
Yeah, I think, that is very useful, very useful indeed […] so you don’t necessarily have to start from scratch saying, this is my diagnosis, this is when I found it, etcetera, etcetera. You can just ask that question and they go, oh yes not a problem we do know the answer to that, or I’ll find out and I’ll give you a ring back.
(30136T – Female, 35 years old, 10 years 11 months post-stroke, East Midlands)
Of those who did have an awareness of the DPoC, none have accessed it throughout the study period as they did not feel it was needed:
Now I had an appointment and it was discussing the effect the stroke has had, what’s support I’m getting, any problems I’ve felt weren’t being covered by anybody, and it also gave me a point of contact […] Any worries, go straight to them.
(11111Z – 56 years old, 7 months post-stroke, Norwich)
Some also reported that the service is superfluous given the availability of other services, such as 111 or participants having an established relationship with their GP surgery. Some also questioned the value of the point of contact if they do not speak to or see the same healthcare professional or had someone without an interest in or knowledge of stroke. However, participants noted that the DPoC could be beneficial to those who have been severely impacted by their stroke or those who have had strokes more recently.
Using the 15-item checklist of needs
Participants who completed the checklist reported that this was useful for identifying concerns or needs and found it an opportunity to be able to share their experience or symptoms to a healthcare professional, which they have not had the opportunity to do so in the past:
I was just glad that somebody was sort of taking enough interest in me, really, although that had been a lot of years, you know, and I’d been left to get on with it. […] But because I didn’t really have a problem, because I managed sort of quite well on my own.
(08076E – Female, 65 years old, 4 years 2 months post-stroke, Lowestoft)
Although most could not recall specific items they ticked in the checklist, participants reported that it enhanced their understanding of their experiences post stroke and addressed concerns where needed: ‘Yes, it [15-item checklist] was useful, because it focused me on what has happened to me since or what I’ve found problems, and I think exercise was one of the main things’ (20040K – Female, 74 years old, 4 years 6 months post stroke, Ipswich).
Some suggested points for improving the checklist, such as including open text boxes to allow participants to articulate, in their own words, their experiences and needs, as well as taking into consideration stroke survivors’ age in the questionnaire items:
I remember even writing on the side [of the checklist] ‘I had a minor stroke’, you know, i.e. adapt this to fit me and if it’s just an ‘other’ box at the end saying, ‘if your symptoms are different to the above, please describe them...’ or something like that?
(11053Z – 53 years old, Norwich)
Receipt and use of tailored service directory
A number of participants found the tailored service directory (‘service mapping tool’) provided during MLAS useful, specifically information on services that were local to them that they would not have heard about otherwise: ‘I’ve been looking through it and I’m sure I will [use the service mapping tool]. I mean, there’s, for instance, OneLife [Area] the health walks which I’d like to do …’ (20040K – Female, 74 years old, 4 years 6 months post stroke, Ipswich).
‘It [service mapping tool] was very helpful, especially when they gave [ … ] us a sheet of different organisations to try and help. One was to do with transport’ (31111Y – Male, 61 years old, 6 years 4 months post stroke, Norfolk).
Theme 4: benefits gained from participation in IPCAS
Stroke survivors identified a range of benefits that they gained from participation in IPCAS, which formed three subthemes: knowledge, skills and emotional well-being.
Knowledge
Participants found that through engaging with intervention components such as the 15-item checklist of needs and MLAS, they deepened their knowledge and understanding of stroke impacts, increasing their confidence to seek support where needed: ‘I feel that now, albeit it is a while afterwards, if there was a problem and I thought it was connected, I feel a lot more confident in ringing the doctors and not having to explain myself’ (08076E – Female, 65 years old, 4 years 2 months post stroke, Norfolk).
I went through it first before I ticked anything to make sure I understood what was on the sheet, I was looking down and thinking, flipping heck, its … I’m jolly lucky looking a half of these […] I can do this, I can do that, shopping and one or two other bits and pieces that I think were on there, walking quite a distance, working my way round the system and that […] I would have said it was more of an eye opener.
(31111Y – Male, 61 years old, 6 years 4 months post-stroke, Norfolk)
Finally, participation in the MLAS component of IPCAS was found to be empowering. As a result, it motivated stroke survivors to increase their activities and work on their newfound or rekindled goals (Theme 4, subtheme Skills).
Skills
They also reported adopting new skills they learnt such as problem-solving and action planning, which has allowed them to identify the barriers to some of their goals (e.g. physical activity), and developing a plan or identifying ways to overcome such barriers:
I wasn’t actually doing some of the things that I should be doing, even if it’s not to do with the stroke, but to do with my age. Like, I play the clarinet and I hadn’t touched it for a month. […] I came straight back [after attending MLAS] and started playing again. […] And the other thing is, I’ve got an exercise bike, I hadn’t used it for about four years, and it made me come back and start using that again.
(19029F – Male, 89 years old, 10 years 2 months post-stroke, East Midlands)
Concerning action planning, some participants reported being offered and accepting extra support or referrals (e.g. memory assessment, physiotherapy) to services at the stroke review: ‘One thing … yes, he did suggest [at the stroke review] that I had a memory test, and he wasn’t available to do it, he said I could see one of his colleagues and I did go for that’ (20040K – Female, 74 years old, 4 years 6 months post stroke, Ipswich).
Meanwhile, those attending MLAS reported that an outcome of attendance was adopting new behaviours to maintain health and well-being such as walking, healthy eating habits and crafts.
Emotional well-being
Several stroke survivors expressed that participating in MLAS made them feel like they were a part of something, and this was helpful to them: ‘I thought it was very helpful, yes, I did. [ … ] I looked forward to going, and to having a bit of a say, you know’ (37092Y – Female, 68 years old, 43 years 10 months post stroke, East Midlands).
Meeting stroke survivors and carers in MLAS courses highlighted people’s varying experiences and health status to interviewees. This made them feel supported and empathetic to one another: ‘People were there, the same as me – not as bad as me, but there were different views from different people, so that was good’ (37063P – 58 years old, East Midlands).
Appendix 7 IPCAS: health economics results report
Summary of methods
A cost-effectiveness analysis was performed using the 12-month data of the IPCAS trial, a two-arm cluster RCT with general practice as the unit of randomisation. One arm received a new model of care for stroke survivors, and the other arm received usual care. Volume of resource use was recorded adopting the UK NHS perspective and costed using national sources. Quality of life was captured using the EQ-5D-5L questionnaire and used to estimate QALYs to 12 months. Missing data were handled using multiple imputations, and mean costs and QALYs for each trial arm were compared. The differences in mean costs and QALYs per patient were combined to obtain an ICER, which was compared to the recommended UK cost-effectiveness threshold.
Summary of results
Twenty-three out of 46 practices (522 individuals) were randomised to the new model of care. The remaining practices received usual care (518 individuals). On average, the new model of care was more expensive (mean incremental cost per patient £267.07; 95% CI −156.913 to 713.687) and more effective (mean QALYs gained per patient 0.013; 95% CI −0.024 to 0.048) – though these differences were not statistically significant. The ICER was £20,863, which is above the recommended threshold (£20,000); the probability the intervention is cost-effective was 0.48.
Methods
Measuring costs
A UK NHS perspective was adopted for the costing component of the analyses, with costs expressed using 2018–9 Great British pounds.
Intervention costs
The resources used to implement the intervention were costed using a micro-costing approach that considered the time needed by medical personnel for their training and for reviewing the patients’ needs, alongside the attendance of participants to the MLAS self-management programme.
Costs of health service use
At 12 months, participants were asked to complete a questionnaire to retrospectively record their contacts with the secondary care services of the NHS over the course of the trial. Each patient was asked to provide data on the frequency of visits to outpatient clinics and their hospitalisations due to any reason related to their stroke, including the length of stay. The hospitalisations with a length of stay ≤ 1 day were treated as day cases. The questionnaire collected information on contacts with social services, including home care, private home help, day centre, meals on wheels, laundry service and respite care. Data on the access to primary care were obtained from the medical records provided by the practices involved in the study via SystmOne. These were clinic visits, home visits and phone calls with GPs, practice nurses, healthcare assistants, psychologists and community services. Practices also collected information on prescribed drugs to patients at 12 months, and it was assumed that these medications were prescribed for 6 months and the dosage followed the defined daily dose. Drug use was costed using the unit costs publicly available from the British National Formulary.
Measuring outcomes
Individuals completed the five-level version of the EQ-5D-5L at baseline, 6 and 12 months. The EQ-5D-5L is a generic health-related quality-of-life instrument which contains questions on five domains: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each domain has five levels: no problems, slight problems, moderate problems, severe problems and extreme problems. Individuals identify which level they are in for each of the five domains, placing them in one of 3127 unique health states (55 plus unconscious or dead), which can then be converted into a single score by using a tariff estimated using data from a sample of the UK population and a crosswalk algorithm. The resulting utility score is anchored at zero and one, which reflect death and perfect health, respectively. Negative scores represent health states considered to be worse than death. A linear trend was assumed between a patient’s scores at each time point, and the area under the resulting curve gave the number of QALYs experienced by that patient to 12 months.
Statistical analysis
It was assumed that the probability of data being missing depended on observed data and not on unobserved data, namely, the data were missing at random and could be predicted by using the information collected during the trial. Missing data were imputed using multiple imputation, a regression-based approach to predict m values for each missing data cell. This accounts for the uncertainty generated in the process and allows both complete and incomplete variables to be used to predict the values of missing data cells. Fifty imputed data sets were used in our analyses, and Rubin’s rules were used to summarise their data. Thus, this approach accounts for the variance between and within imputed data sets.
Resource use, costs, EQ-5D scores and QALYs were summarised using mean, SD and 95% CIs for each study group. Ninety-five per cent CIs in the complete case analysis were obtained using 5000 non-parametric bootstrap replications; in the multiple imputed data, these were obtained evoking Rubin’s rules and assuming normality. Mixed-effect models were used to model each of the dependent variables with a random intercept for each centre and fixed effects for the intervention. In an ad hoc analysis, we controlled for EQ-5D-5L scores at baseline. To account for the skewness of data, the CIs of costs and QALYs were obtained by sampling 5000 non-parametric bootstrap replicates, accounting for the clustered nature of the trial. We therefore calculated 5000 sets of costs and QALYs for the intervention and control and used these to calculate 5000 ICER values, representing the incremental cost required to obtain one additional QALY when moving from usual care to a new intervention. The ICERs were plotted as single points on the cost-effectiveness plane, a two-dimensional figure with four quadrants representing all possible outcome combinations of cost and QALYs differences.
A study’s ICER is commonly compared to the maximum reimbursable ICER (i.e. cost-effectiveness threshold), which for the UK ranges from £20,000 to £30,000 per QALY. Calculating the proportion of the bootstrapped simulations with a corresponding ICER below £20,000 per QALY, we estimated the probability that the new model of care will be cost-effective when compared with usual care (i.e. the cost-effectiveness probability). Varying the cost-effectiveness threshold and re-estimating the cost-effectiveness probability, enabled the construction of a cost-effectiveness acceptability curve (CEAC). This represents the probability that the new model of care will be accepted based on different values of the cost-effectiveness threshold.
The prespecified analyses included the cost-effectiveness assessment using multiple imputed data and unadjusted estimates. A set of ad hoc analyses was performed to determine the impact of (1) adjusting for EQ-5D-5L scores at baseline and (2) using only complete cases.
All statistical analyses were performed using Stata SE v14.0 (StataCorp, College Station, TX, USA).
| Unit cost (£) | Source | |
|---|---|---|
| Primary care | ||
| Clinic visit | ||
| Healthcare assistant | £6.26 | PSSRU 2019 |
| Psychologist | £68.71 | PSSRU 2019 |
| Nurse | £9.88 | PSSRU 2019 |
| GP | £33.50 | PSSRU 2019 |
| Community Services | £19.88 | PSSRU 2019 |
| Home visit | ||
| Healthcare assistant | - | |
| Psychologist | £80.17 | PSSRU 2019 |
| Nurse | £16.46 | PSSRU 2019 |
| GP | £91.56 | PSSRU 2019 |
| Community Services | £33.13 | PSSRU 2019 |
| Phone call | ||
| Healthcare assistant | - | |
| Psychologist | £7.51 | PSSRU 2019 |
| Nurse | £4.32 | PSSRU 2019 |
| GP | £14.65 | PSSRU 2019 |
| Community Services | £8.69 | PSSRU 2019 |
| Social services | ||
| Home care | £14.14 | PSSRU 2019 |
| Private home help | £14.14 | PSSRU 2019 |
| Day centre | £97.00 | PSSRU 2019 |
| Meals on Wheels | £3.60 | NACC 2018 |
| Laundry service | £17.00 | Oxfordshire county council |
| Respite care | £107.14 | NHS |
| Other | £42.17 | |
| Hospitalisation per admission | £2073.88a | NHS reference cost |
| Day case | £733.10a | NHS reference cost |
| Time (minutes) | Cost per minute | Personnel | Unit cost per patient | Source | |
|---|---|---|---|---|---|
| Structured review of patient needs and training for general practice staff | |||||
| Healthcare assistant | - | £0.42 | NA | NA | PSSRU |
| Nurse | - | £0.66 | NA | NA | PSSRU |
| GP | - | £3.66 | NA | NA | PSSRU |
| Administrator | - | £0.42 | NA | NA | PSSRU |
| Other | - | £1.58 | NA | NA | NA |
| Training staff | £0.45 | Two research associates | |||
| Self-management programme (MLAS) for stroke survivors and their carers | |||||
| Individual appointments (introductory and final follow-up appointment) | 37.5 | £0.42 | 1. HCPs | £15.65 | PSSRU |
| Group sessions per individual | 150 | £0.42 | 2. HCPs per group of seven individuals | £17.89 | PSSRU |
Complete cases
Resource use – intervention
Training
| Variable | Mean (SD; 95% CI) [N] |
|---|---|
| Healthcare assistant | 103.33 (11.55; 92.7 to 113.97) [3] |
| Nurse | 106.29 (24.29; 97.87 to 114.71) [31] |
| GP | 112.41 (28.87; 101.61 to 123.21) [27] |
| Administrator | 99.21 (25.29; 88.22 to 110.21) [19] |
| Total | 106.56 (25.93; 100.93 to 112.2) [80] |
Patients’ review
| Variable | Mean (SD; 95% CI) [N] |
|---|---|
| Healthcare assistant | 26.72 (6.14; 24.98 to 28.45) [48] |
| Nurse | 27.16 (8.28; 26.29 to 28.03) [347] |
| GP | 17.63 (6.06; 14.71 to 20.54) [16] |
| Other | 34.5 (6.27; 30.4 to 38.6) [8] |
| Total | 21.58 (13; 20.46 to 22.7) [522] |
Self-management meeting
| Variable | Mean (SD; 95% CI) [N] |
|---|---|
| Initial meeting | 26.63 (44.24; 22.81 to 30.45) [522] |
| First group’s session attendance (%) | 22.61 (41.87; 18.99 to 26.22) [522] |
| Second group’s session attendance (%) | 21.84 (41.36; 18.25 to 25.43) [522] |
| Third group’s session attendance (%) | 19.92 (39.98; 16.46 to 23.39) [522] |
| Fourth group’s session attendance (%) | 20.88 (40.68; 17.33 to 24.43) [522] |
| Follow-up meeting | 23.37 (42.36; 19.71 to 27.04) [522] |
Resource use – healthcare services
| Variable | Control mean (SD; 95% CI) [N] | Intervention mean (SD; 95% CI) [N] |
|---|---|---|
| GP visit | 2.92 (4.3; 2.55 to 3.29) [518] | 3.57 (4.88; 3.15 to 4) [522] |
| Nurse visit | 1.82 (2.86; 1.57 to 2.07) [518] | 2.69 (5.72; 2.2 to 3.18) [522] |
| Healthcare assistant visit | 0.28 (0.81; 0.21 to 0.34) [518] | 0.63 (2.49; 0.41 to 0.84) [522] |
| Psychologist visit | 0 (-) [518] | 0 (-) [522] |
| Community Services visit | 0.61 (3.86; 0.28 to 0.94) [518] | 0.59 (2.88; 0.35 to 0.84) [522] |
| GP phone call | 0.39 (1.25; 0.29 to 0.5) [518] | 0.22 (0.94; 0.14 to 0.3) [522] |
| Nurse phone call | 0.41 (1.37; 0.3 to 0.53) [518] | 0.36 (1.28; 0.25 to 0.47) [522] |
| Psychologist phone call | 0 (-) [518] | 0 (-) [522] |
| Community Services phone call | 0.09 (0.67; 0.04 to 0.15) [518] | 0.12 (0.73; 0.05 to 0.18) [522] |
| GP home visit | 0.18 (1.04; 0.09 to 0.27) [518] | 0.22 (1.16; 0.12 to 0.32) [522] |
| Nurse home visit | 1.19 (11.23; 0.21 to 2.16) [518] | 2.51 (20.88; 0.73 to 4.28) [522] |
| Community Services home visit | 0.64 (4.71; 0.24 to 1.04) [518] | 0.54 (3.94; 0.21 to 0.88) [522] |
| At least one drug (%) | 75.1 (43.29; 71.42 to 78.78) [518] | 76.44 (42.48; 72.78 to 80.09) [522] |
| At least one inpatient access (%) | 2.14 (14.47; 0.89 to 3.39) [515] | 2.89 (16.77; 1.46 to 4.32) [519] |
| At least one day case access (%) | 1.36 (11.59; 0.35 to 2.37) [515] | 0.96 (9.78; 0.13 to 1.8) [519] |
| At least one outpatient access (%) | 45.17 (49.81; 40.96 to 49.39) [518] | 41.38 (49.3; 37.08 to 45.68) [522] |
| Home care | 5.68 (42.93; 1.97 to 9.39) [515] | 10.04 (59.16; 4.97 to 15.1) [520] |
| Private home help | 3.58 (29.59; 1.08 to 6.08) [516] | 5.3 (38.01; 2.01 to 8.59) [517] |
| Day centre | 0.93 (9.96; 0.08 to 1.77) [518] | 0.7 (8.74; −0.04 to 1.44) [520] |
| Meals on Wheels | 0.5 (8.24; −0.21 to 1.22) [518] | 0 (0.09; 0 to 0.01) [520] |
| Laundry service | 0.58 (5.25; 0.14 to 1.02) [518] | 0.42 (4.76; 0.01 to 0.82) [521] |
| Respite care | 0.02 (0.34; −0.01 to 0.05) [518] | 0.08 (1.84; −0.07 to 0.24) [521] |
| Other | 0.34 (5.15; −0.11 to 0.79) [516] | 0.2 (3.41; −0.09 to 0.48) [519] |
Cost
| Variable | Control mean (SD; 95% CI) [N] | Intervention mean (SD; 95% CI) [N] |
|---|---|---|
| GP visit | 97.85 (144.11; 85.46 to 110.23) [518] | 119.75 (163.61; 105.67 to 133.84) [522] |
| Nurse visit | 17.99 (28.25; 15.54 to 20.43) [518] | 26.61 (56.51; 21.76 to 31.46) [522] |
| Healthcare assistant visit | 1.73 (5.09; 1.3 to 2.16) [518] | 3.92 (15.6; 2.58 to 5.27) [522] |
| Psychologist visit | 0 (-) [518] | 0 (-) [522] |
| Community Services visit | 12.12 (76.68; 5.51 to 18.73) [518] | 11.77 (57.17; 6.92 to 16.61) [522] |
| GP phone call | 5.77 (18.24; 4.18 to 7.36) [518] | 3.23 (13.76; 2.07 to 4.39) [522] |
| Nurse phone call | 1.78 (5.9; 1.28 to 2.29) [518] | 1.56 (5.54; 1.09 to 2.02) [522] |
| Psychologist phone call | 0 (-) [518] | 0 (-) [522] |
| Community Services phone call | 0.82 (5.8; 0.33 to 1.32) [518] | 1.02 (6.32; 0.48 to 1.56) [522] |
| GP home visit | 16.62 (95.03; 8.43 to 24.8) [518] | 19.82 (106.17; 10.78 to 28.86) [522] |
| Nurse home visit | 19.54 (184.91; 3.47 to 35.61) [518] | 41.24 (343.66; 11.98 to 70.51) [522] |
| Community Services home visit | 21.23 (155.91; 7.96 to 34.5) [518] | 17.96 (130.58; 6.83 to 29.08) [522] |
| Primary care total cost | 195.45 (461.8; 155.56 to 235.35) [518] | 246.87 (571.1; 198.21 to 295.54) [522] |
| Self-management programme cost | 0 (-) [518] | 23.08 (40.2; 19.59 to 26.56) [522] |
| Review of patient needs | 0 (-) [518] | 15.76 (13.64; 14.57 to 16.95) [522] |
| Training cost | 0 (-) [518] | 29.26 (14.33; 28.03 to 30.49) [522] |
| Intervention overall cost | 0 (-) [518] | 68.1 (47.71; 63.96 to 72.23) [522] |
| Drug total cost | 258.18 (1520.18; 129.83 to 386.53) [518] | 236.16 (1278.02; 127.1 to 345.22) [522] |
| Inpatient cost | 44.3 (300.13; 18.38 to 70.21) [515] | 59.94 (347.77; 30.36 to 89.52) [519] |
| Day case cost | 9.96 (84.97; 2.59 to 17.34) [515] | 7.06 (71.68; 0.96 to 13.17) [519] |
| Outpatient cost | 306.51 (851.38; 229.89 to 383.12) [470] | 411.39 (2313.55; 204.44 to 618.35) [477] |
| Secondary care total cost | 357.86 (959.86; 271.03 to 444.7) [467] | 473.52 (2368.05; 260.91 to 686.13) [476] |
| Home care | 80.35 (607.04; 27.88 to 132.83) [515] | 141.92 (836.45; 70.32 to 213.51) [520] |
| Private home help | 50.64 (418.34; 15.28 to 85.99) [516] | 74.94 (537.42; 28.36 to 121.53) [517] |
| Day centre | 89.75 (965.87; 8.18 to 171.32) [518] | 68.03 (848.02; −4.01 to 140.07) [520] |
| Meals on Wheels | 1.81 (29.65; −0.77 to 4.39) [518] | 0.01 (0.32; −0.01 to 0.04) [520] |
| Laundry service | 9.81 (89.24; 2.32 to 17.29) [518] | 7.09 (80.87; 0.22 to 13.96) [521] |
| Respite care | 2.28 (36.12; −0.88 to 5.43) [518] | 9.05 (197.35; −7.78 to 25.88) [521] |
| Other | 14.26 (217.24; −4.7 to 33.21) [516] | 8.37 (143.77; −3.71 to 20.44) [519] |
| Social services total cost | 249.83 (1461.02; 124.3 to 375.37) [511] | 304.34 (1564.68; 169.04 to 439.64) [511] |
| Total cost | 1054.8 (2570.66; 820.8 to 1288.81) [465] | 1235.11 (3283.2; 939.65 to 1530.57) [469] |
Quality-adjusted life-years
| Variable | Control mean (SD; 95% CI) [N] | Intervention mean (SD; 95% CI) [N] |
|---|---|---|
| Baseline EQ-5D-5L utility | 0.743 (0.254; 0.72 to 0.767) [457] | 0.749 (0.243; 0.727 to 0.771) [465] |
| Six months EQ-5D-5L utility | 0.737 (0.254; 0.712 to 0.762) [413] | 0.75 (0.233; 0.727 to 0.773) [401] |
| 12 months EQ-5D-5L utility | 0.746 (0.248; 0.72 to 0.772) [350] | 0.754 (0.231; 0.73 to 0.778) [353] |
| 12 months QALY | 0.759 (0.223; 0.734 to 0.783) [326] | 0.769 (0.203; 0.746 to 0.791) [322] |
Multiple imputation
Resource use
| Variable | Control mean (SD; 95% CI) [N] | Intervention mean (SD; 95% CI) [N] |
|---|---|---|
| GP visit | 2.92 (4.3; 2.55 to 3.29) [518] | 3.57 (4.88; 3.15 to 3.99) [522] |
| Nurse visit | 1.82 (2.86; 1.57 to 2.07) [518] | 2.69 (5.72; 2.2 to 3.19) [522] |
| Healthcare assistant visit | 0.28 (0.81; 0.21 to 0.35) [518] | 0.63 (2.49; 0.41 to 0.84) [522] |
| Psychologist visit | 0 (-) [518] | 0 (-) [522] |
| Community Services visit | 0.61 (3.86; 0.28 to 0.94) [518] | 0.59 (2.88; 0.34 to 0.84) [522] |
| GP phone call | 0.39 (1.25; 0.29 to 0.5) [518] | 0.22 (0.94; 0.14 to 0.3) [522] |
| Nurse phone call | 0.41 (1.37; 0.3 to 0.53) [518] | 0.36 (1.28; 0.25 to 0.47) [522] |
| Psychologist phone call | 0 (-) [518] | 0 (-) [522] |
| Community Services phone call | 0.09 (0.67; 0.04 to 0.15) [518] | 0.12 (0.73; 0.05 to 0.18) [522] |
| GP home visit | 0.18 (1.04; 0.09 to 0.27) [518] | 0.22 (1.16; 0.12 to 0.32) [522] |
| Nurse home visit | 1.19 (11.23; 0.22 to 2.16) [518] | 2.51 (20.88; 0.71 to 4.3) [522] |
| Community Services home visit | 0.64 (4.71; 0.23 to 1.05) [518] | 0.54 (3.94; 0.2 to 0.88) [522] |
| At least one drug (%) | 75.1 (43.29; 71.36 to 78.83) [518] | 76.44 (42.48; 72.78 to 80.09) [522] |
| At least one inpatient access (%) | 1.36 (11.59; 0.35 to 2.36) [518] | 0.96 (9.75; 0.12 to 1.8) [522] |
| At least one day case access (%) | 2.12 (14.43;0.88 to 3.37) [518] | 2.89 (16.81; 1.44 to 4.33) [522] |
| At least one outpatient access (%) | 45.17 (49.81; 40.87 to 49.47) [518] | 41.38 (49.3; 37.14 to 45.62) [522] |
| Home care | 5.67 (42.94; 1.96 to 9.38) [518] | 10.51 (61.02; 5.26 to 15.75) [522] |
| Private home help | 3.59 (29.71; 1.03 to 6.16) [518] | 5.29 (38.1; 2.01 to 8.56) [522] |
| Day centre | 0.93 (9.96; 0.07 to 1.78) [518] | 0.7 (8.73; −0.05 to 1.45) [522] |
| Meals on Wheels | 0.5 (8.24; −0.21 to 1.21) [518] | 0 (0.09; 0 to 0.01) [522] |
| Laundry service | 0.58 (5.25; 0.12 to 1.03) [518] | 0.42 (4.75; 0.01 to 0.82) [522] |
| Respite care | 0.02(0.34; −0.01 to 0.05) [518] | 0.08 (1.84; −0.07 to 0.24) [522] |
| Other | 0.34 (5.22; −0.11 to 0.79) [518] | 0.2 (3.4; −0.09 to 0.49) [522] |
Cost
| Variable | Control mean (SD; 95% CI) [N] | Intervention mean (SD; 95% CI) [N] |
|---|---|---|
| GP visit | 97.85 (144.11; 85.41 to 110.29) [518] | 119.75 (163.61; 105.68 to 133.82) [522] |
| Nurse visit | 17.99 (28.25; 15.55 to 20.43) [518] | 26.61 (56.51; 21.75 to 31.47) [522] |
| Healthcare assistant visit | 1.73 (5.09; 1.29 to 2.17) [518] | 3.92 (15.6; 2.58 to 5.26) [522] |
| Psychologist visit | 0 (-) [518] | 0 (-) [522] |
| Community Services visit | 12.12 (76.68; 5.51 to 18.74) [518] | 11.77 (57.17; 6.85 to 16.68) [522] |
| GP phone call | 5.77 (18.24; 4.19 to 7.34) [518] | 3.23 (13.76; 2.04 to 4.41) [522] |
| Nurse phone call | 1.78 (5.9; 1.28 to 2.29) [518] | 1.56 (5.54; 1.08 to 2.03) [522] |
| Psychologist phone call | 0 (-) [518] | 0 (-) [522] |
| Community Services phone call | 0.82 (5.8; 0.32 to 1.32) [518] | 1.02 (6.32; 0.47 to 1.56) [522] |
| GP home visit | 16.62 (95.03; 8.41 to 24.82) [518] | 19.82 (106.17; 10.69 to 28.95) [522] |
| Nurse home visit | 19.54 (184.91; 3.58 to 35.5) [518] | 41.24 (343.66; 11.69 to 70.79) [522] |
| Community Services home visit | 21.23 (155.91; 7.77 to 34.69) [518] | 17.96 (130.58; 6.73 to 29.19) [522] |
| Primary care total cost | 195.45 (461.8; 155.59 to 235.31) [518] | 246.87 (571.1; 197.77 to 295.98) [522] |
| Self-management programme cost | 0 (-) [518] | 23.08 (40.2; 19.62 to 26.53) [522] |
| Review of patient needs | 0 (-) [518] | 15.76 (13.64; 14.59 to 16.93) [522] |
| Training cost | 0 (-) [518] | 29.26 (14.33; 28.03 to 30.49) [522] |
| Intervention overall cost | 0 (-) [518] | 68.1 (47.71; 63.99 to 72.2) [522] |
| Drug total cost | 258.18 (1520.18; 126.96 to 389.4) [518] | 236.16 (1278.02; 126.27 to 346.05) [522] |
| Inpatient cost | 44.04 (299.28; 18.21 to 69.87) [518] | 59.91 (348.6; 29.94 to 89.89) [522] |
| Day case cost | 9.94 (84.97; 2.6 to 17.27) [518] | 7.02 (71.47; 0.88 to 13.17) [522] |
| Outpatient cost | 336.08 (1011.7; 248.72 to 423.44) [518] | 426.12 (2400.13; 219.69 to 632.55) [522] |
| Secondary care total cost | 390.05 (1104.88; 294.66 to 485.45) [518] | 493.05 (2448.23; 282.49 to 703.62) [522] |
| Home care | 80.19 (607.19; 27.78 to 132.61) [518] | 148.54 (862.78; 74.35 to 222.73) [522] |
| Private home help | 50.77 (420.16; 14.5 to 87.04) [518] | 74.76 (538.74; 28.44 to 121.09) [522] |
| Day centre | 89.75 (965.87; 6.38 to 173.12) [518] | 67.77 (846.4; −5.01 to 140.55) [522] |
| Meals on Wheels | 1.81 (29.65; −0.75 to 4.37) [518] | 0.01 (0.32; −0.01 to 0.04) [522] |
| Laundry service | 9.81 (89.24; 2.1 to 17.51) [518] | 7.08 (80.79; 0.13 to 14.02) [522] |
| Respite care | 2.28 (36.12; −0.84 to 5.39) [518] | 9.04 (197.17; −7.91 to 25.99) [522] |
| Other | 14.38 (220.26; −4.64 to 33.39) [518] | 8.34 (143.37; −3.98 to 20.67) [522] |
| Social services total cost | 248.98 (1453.59; 123.51 to 374.45) [518] | 315.55 (1578.67; 179.8 to 451.29) [522] |
| Total cost | 1092.66 (2532.81; 874.03 to 1311.3) [518] | 1359.73 (3438.34; 1064.05 to 1655.41) [522] |
Quality-adjusted life-years
| Variable | Control mean (SD; 95% CI) [N] | Intervention mean (SD; 95% CI) [N] |
|---|---|---|
| Baseline EQ-5D-5L utility | 0.742 (0.24; 0.721 to 0.763) [518] | 0.749 (0.23; 0.73 to 0.769) [522] |
| Six months EQ-5D-5L utility | 0.725 (0.263; 0.703 to 0.748) [518] | 0.741 (0.243; 0.72 to 0.762) [522] |
| 12 months EQ-5D-5L utility | 0.721 (0.279; 0.697 to 0.745) [518] | 0.734 (0.247; 0.713 to 0.755) [522] |
| 12 months QALY | 0.728 (0.244; 0.707 to 0.75) [518] | 0.741 (0.224; 0.722 to 0.761) [522] |
Results
Complete cases: unadjusted
| Control | Intervention | Difference | ICER | |
|---|---|---|---|---|
| Cost | 1054.803 (813.836 to 1350.528) |
1235.112 (900.996 to 1649.039) |
180.31 (−262.975 to 661.643) | |
| QALY | 0.759 (0.732 to 0.786) | 0.769 (0.743 to 0.794) | 0.01 (−0.027 to 0.047) | 18,013.232 |
FIGURE 14.
Cost-effectiveness plane (a) and cost-effectiveness acceptability curve (b): complete cases – unadjusted.
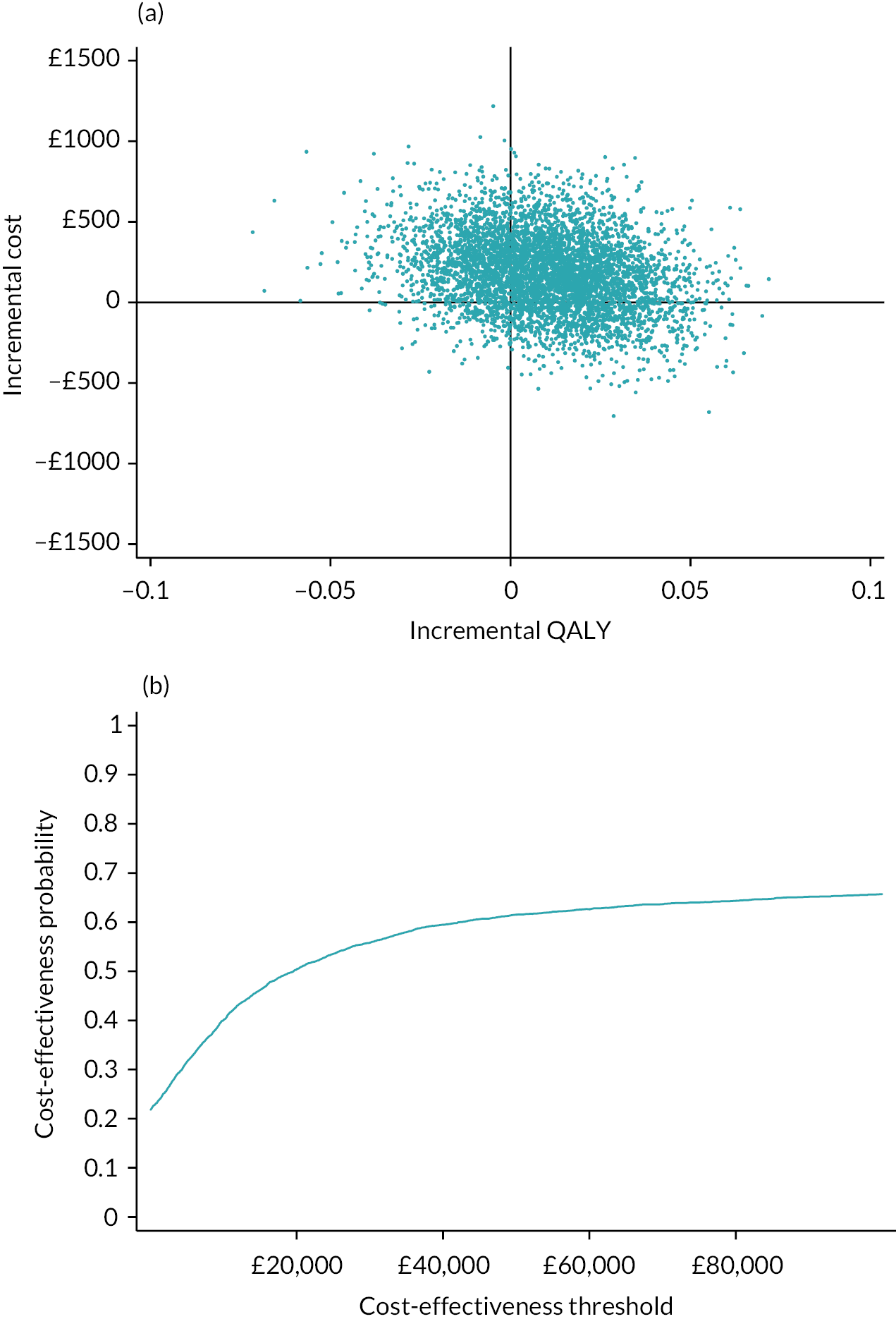
Complete cases: adjusted
| Control | Intervention | Difference | ICER | |
|---|---|---|---|---|
| Cost | 1086.246 (831.326 to 1402.219) |
1302.617 (918.993 to 1764.421) |
216.371 (−272.306 to 741.538) | |
| QALY | 0.74 (0.72 to 0.76) | 0.746 (0.726 to 0.766) | 0.005 (−0.007 to 0.018) | 39,532.387 |
FIGURE 15.
Cost-effectiveness plane (a) and cost-effectiveness acceptability curve (b): adjusted for baseline EQ-5D-5L scores.
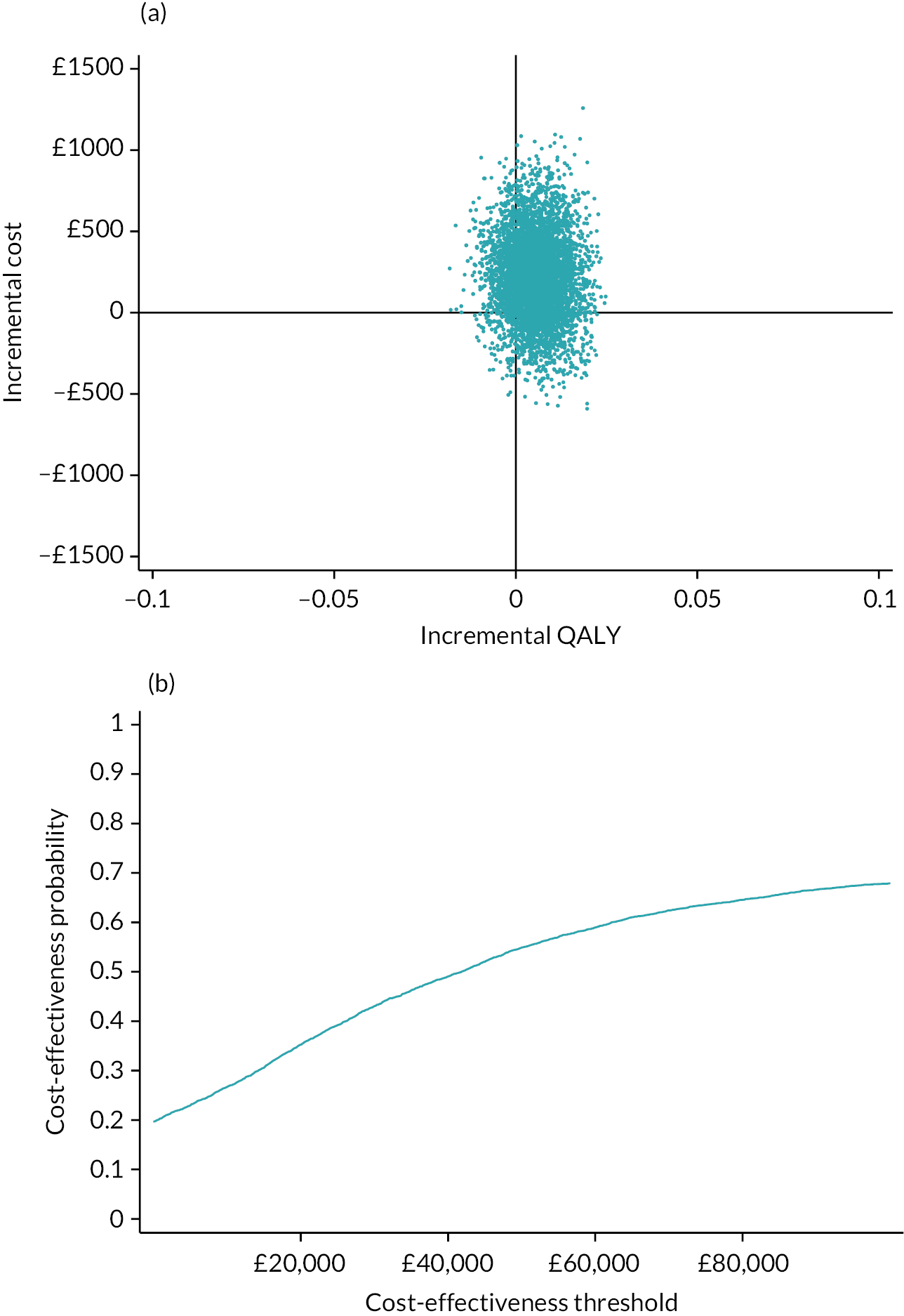
Multiple imputation: unadjusted
| Control | Intervention | Difference | ICER | |
|---|---|---|---|---|
| Cost | 1092.664 (883.886 to 1346.126) |
1359.733 (1017.138 to 1741.553) |
267.069 (−156.913 to 713.687) | |
| QALY | 0.728 (0.702 to 0.756) | 0.741 (0.718 to 0.763) | 0.013 (−0.024 to 0.048) | 20,862.736 |
FIGURE 16.
Cost-effectiveness plane (a) and cost-effectiveness acceptability curve (b), multiple imputation, unadjusted.
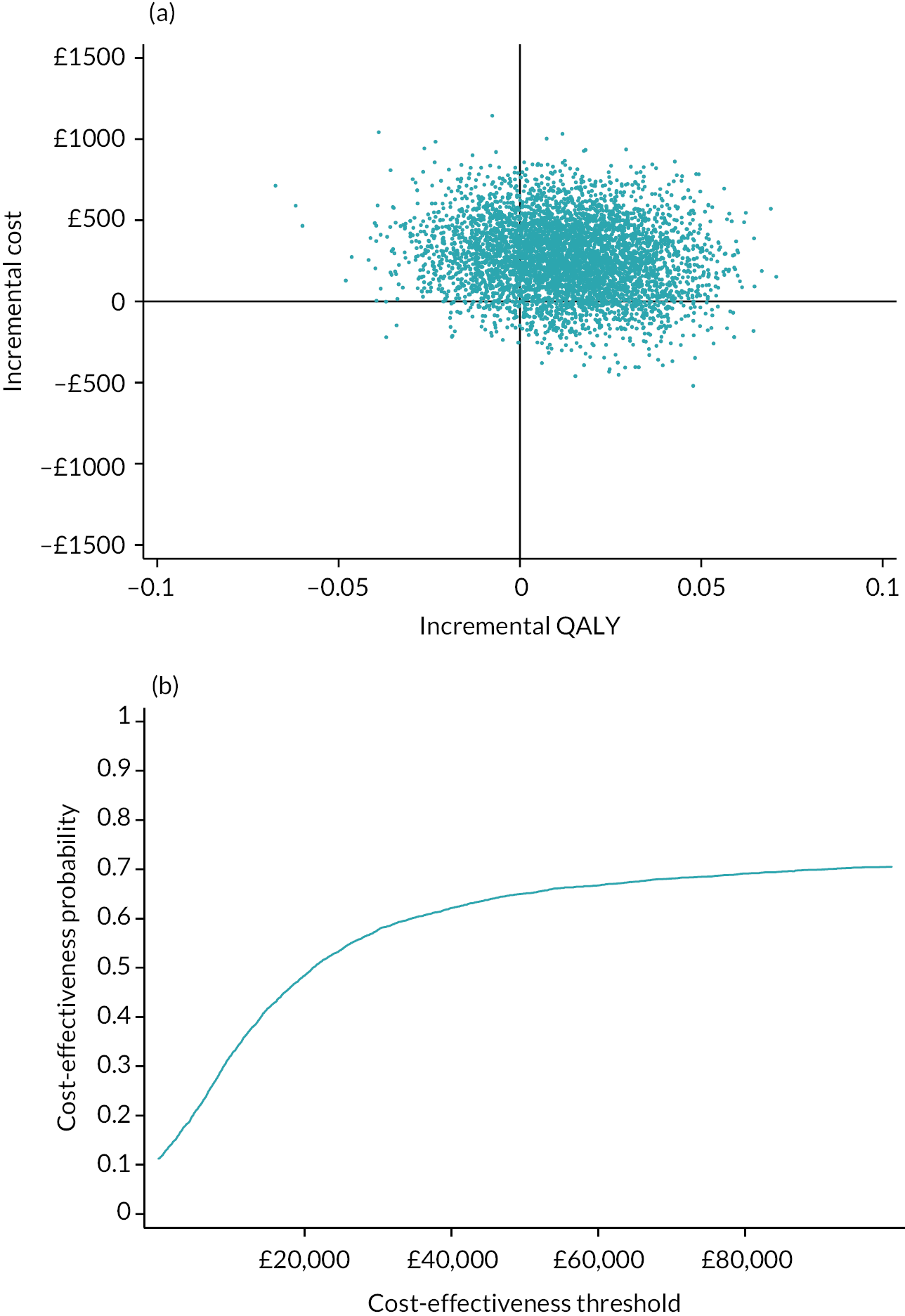
Multiple imputation: adjusted
| Control | Intervention | Difference | ICER | |
|---|---|---|---|---|
| Cost | 1084.198 (874.35 to 1335.426) |
1368.134 (1024.4 to 1754.325) |
283.936 (−126.117 to 728.025) | |
| QALY | 0.732 (0.713 to 0.75) | 0.738 (0.72 to 0.756) | 0.006 (−0.003 to 0.015) | 45,488.621 |
FIGURE 17.
Cost-effectiveness plane (a) and cost-effectiveness acceptability curve (b), multiple imputed data, adjusted for baseline EQ-5D-5L.
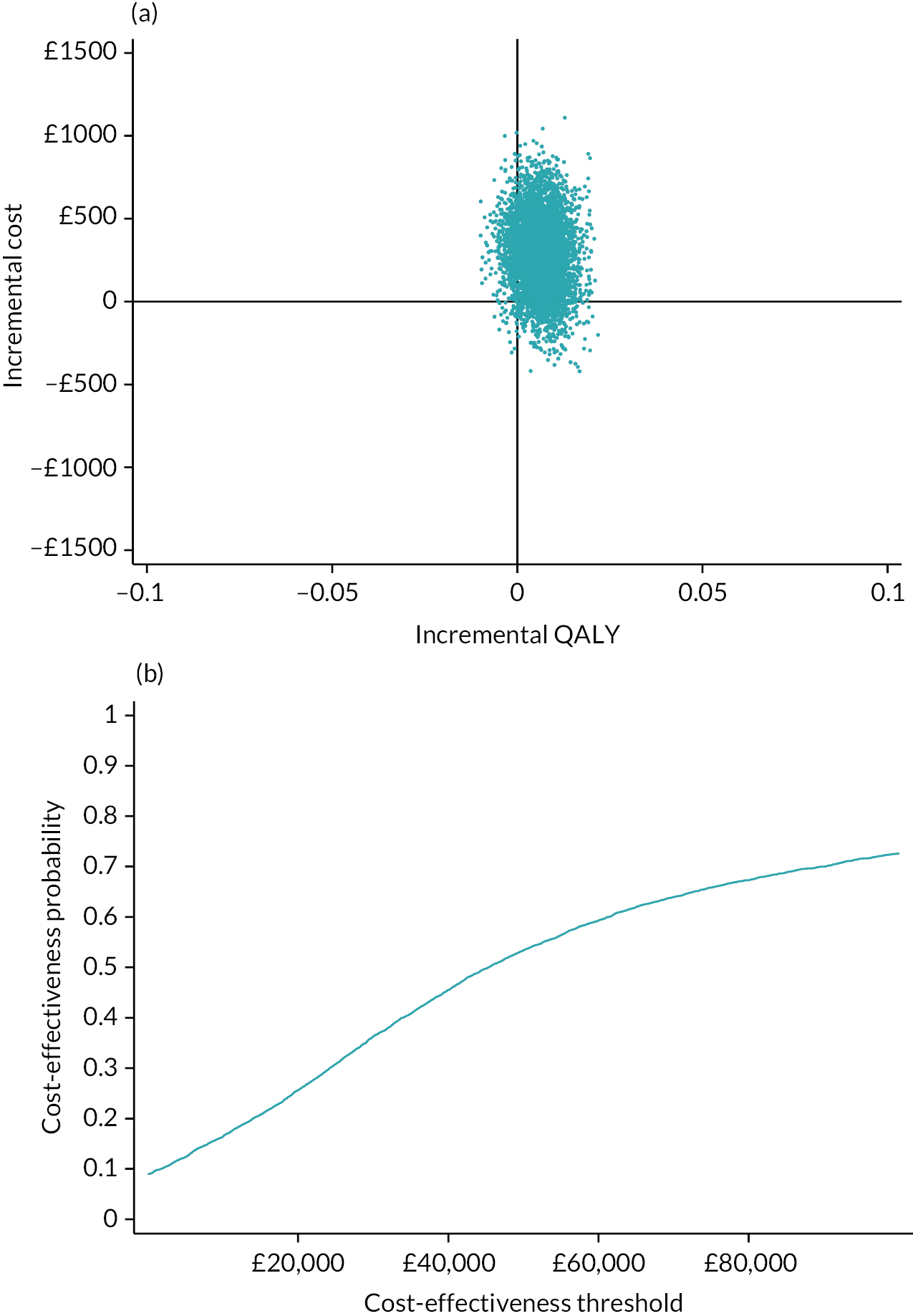
List of abbreviations
- CI
- confidence interval
- COVID-19
- coronavirus disease 2019
- DPoC
- direct point of contact
- EQ-5D-5L
- EuroQol-5 Dimension, five level questionnaire
- EXTRAS
- evaluation of an Extended Stroke Rehabilitation Service
- GM-SAT
- Greater Manchester Stroke Assessment tool
- GP
- general practitioner
- HCP
- healthcare professional
- HLQ
- Health Literacy Questionnaire
- ICECAP-A
- ICEpop CAPability measure for Adults
- ICER
- incremental cost-effectiveness ratio
- ICSS
- Integrated Community Stroke Service
- IDG
- intervention development group
- IPCAS
- Improving Primary Care After Stroke
- ITT
- intention to treat
- LoTS
- longer-term stroke care
- MLAS
- Managing Life After Stroke (self-management training programme)
- NICE
- National Institute for Health and Care Excellence
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- SD
- standard deviation
- SIS
- Stroke Impact Scale
- SIS-SF
- Stroke Impact Scale – Short Form
- SMT
- service mapping tool
- SSSMQ
- Southampton Stroke Self-Management Questionnaire
- VAS
- visual analogue scale
