Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0613-20007. The contractual start date was in May 2015. The final report began editorial review in July 2021 and was accepted for publication in October 2022. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Wright et al. This work was produced by Wright et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Wright et al.
SYNOPSIS
Summary of programme alterations
There were no substantive changes to the original study aims and objectives or changes to the investigators or grant holders.
Programme delivery
There have been a few changes in the programme to improve procedures or optimise effectiveness.
Qualitative evaluation
A focus group and an online survey were planned to capture the PIP’s experience at the end of the feasibility study; this was changed to a face-to-face workshop to facilitate a fuller understanding of the intervention delivery and research procedures.
Recruitment of triads
The unit of recruitment was refined to triads consisting of a PIP/general practitioner (GP)/care home(s). To manage workload, triad recruitment was divided into three time phases to ensure that peak activity points did not overlap.
Recruitment of resident participants
The target of residents recruited in each triad was changed from ‘20’ to a ‘mean of 20’.
Health economic model beyond trial
This was not undertaken because the intervention was estimated to increase costs and reduce quality-adjusted life-years (QALYs), such that the extrapolation would not change the outcome.
Validation of methods for capturing quality of life
This was not undertaken as EuroQol Five Dimensions (EQ-5D) was the only utility measure used in the final trial. Previously, a validation work for use in care homes (by proxy) has already been undertaken.
Coronavirus
The trial was delayed in Scotland for the triads recruited at the end of Phase III. Final data collection was undertaken remotely immediately after the first lockdown was eased and with appropriate approval. The dissemination events were conducted on Zoom.
Background
The idea for Care Home Independent Pharmacist Prescriber Study (CHIPPS) resulted from three publications originating from the core research team. The 2009 Care Homes' Use of Medicines Study (Alldred) reported that prescribing, monitoring and administration of medicines could be significantly improved1 and resulted in a Department of Health alert requiring significant overhaul in the ways in which medication was managed within this environment. 2 A Cochrane review (Hughes, updated in 2018) suggested no improvement in clinical outcomes from interventions to improve the appropriate use of polypharmacy in care homes. 3 An exploratory trial (Bond, Holland, Wright) to determine the potential effectiveness of PIPs providing care for individuals with chronic pain demonstrated significant improvements in clinical outcomes. 4
The hypothesis proposed in 2012 was that improvements in clinical outcomes could be realised by allowing PIPs to assume responsibility for pharmaceutical care provision for residents within care homes. Furthermore, PIPs would be able to support all medication-related activities, thereby reducing medication errors resulting from prescription, ordering, storage, administering and recording.
The team deliberately chose not to use ‘errors’ as an outcome measure within an open-label trial owing to previous experience of a significant control arm reactivity bias in response to being planned to be observed for medication errors. 5
Whilst we were confident that with the ability to support and monitor all medication-related practices in the care home, a PIP would be able to demonstrate a positive clinical effect; we were unsure as to which clinical outcome measure would be most appropriate and created WP2 to explore this.
Recognising that the use of PIPs in care homes was a step change from current models of care within this environment, we planned to listen to all stakeholders to understand what would be appropriate to be included in such an intervention and how it should be best implemented (WP1). WP3 resulted from the need for cost-effectiveness to be captured.
With a desire to enhance intervention fidelity and recognition that pharmacist prescribers are required to be competent within their defined area of practice, we developed a training package (WP4).
Following Medical Research Council (MRC) guidance regarding the development and evaluation of complex interventions,6 we included feasibility (WP5), pilot and definitive trial stages (WP6) (see Figure 1). Latterly, we used the MRC guidance for process evaluation7 informed by the logic model, which was refined as the project progressed.
FIGURE 1.
Research pathway diagram.
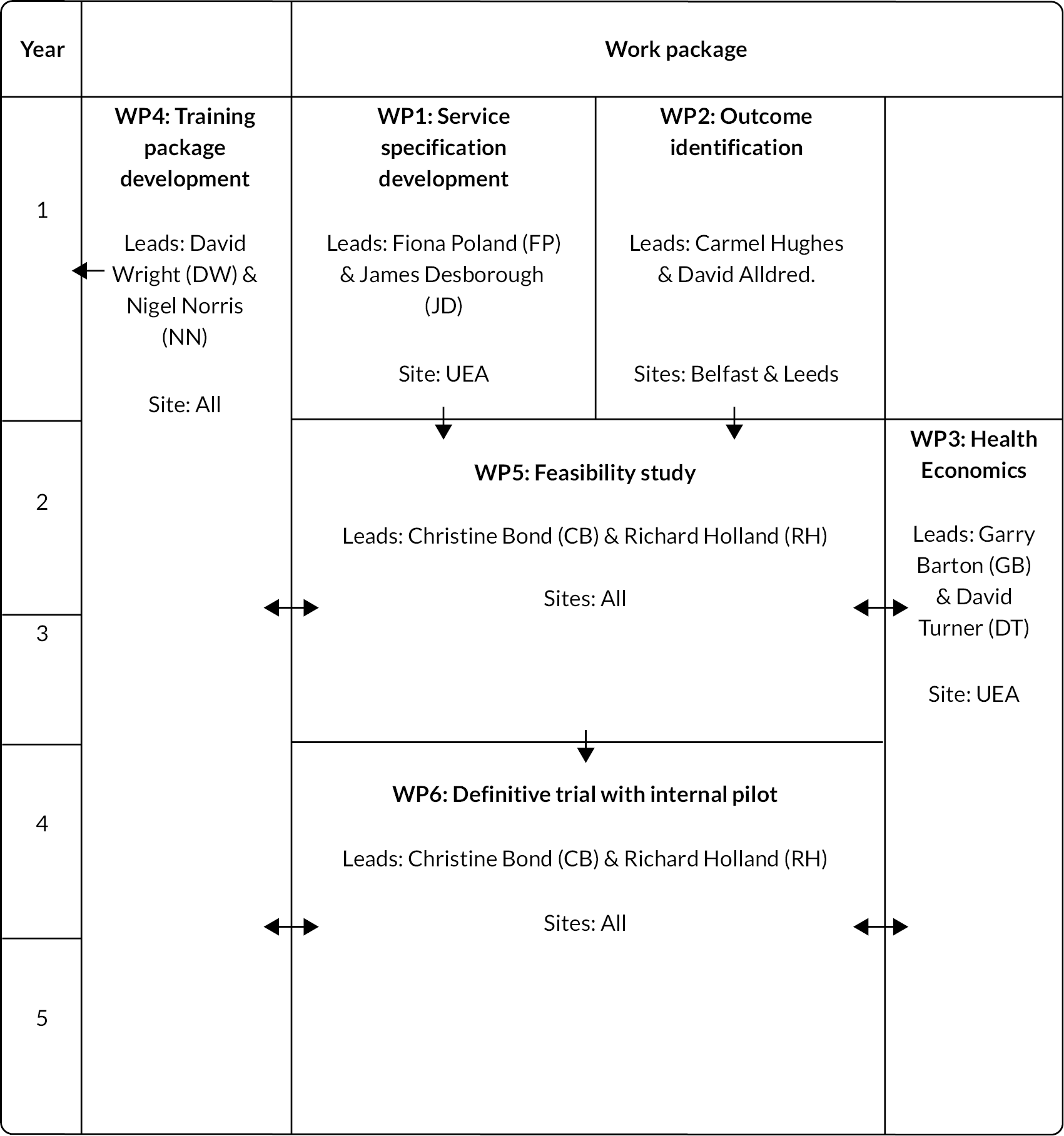
The programme of work was carried out in four locations: Norfolk (England), Yorkshire (England), Grampian (Scotland) and Belfast (Northern Ireland).
Work package 1: development of service specification
Approvals
Research ethics approved by the NRES Committee Yorkshire and the Humber Sheffield REC Ref.: 15/YH/0172.
NHS Research and Development approved by NHS Grampian R&D Ref.: IRAS: 173232.
Aim
To qualitatively explore stakeholders’ expectations and understanding of introducing a new service to inform ways to introduce an acceptable service, anticipating and mitigating potential barriers.
Research question
What components stakeholders would specify in a feasible and acceptable PIP service and what did they consider were barriers and enablers to implementing it?
Objectives
To access stakeholders in four demographically and culturally diverse study sites [England (two), Scotland and Northern Ireland (one in each)] to collect their views through focus group discussions and interviews and validate analytic themes through consensus discussions.
Method
A purposive sampling approach gained stakeholders’ views with experience of living or working in, or with, care homes, to maximise the range of data relevant to the research goals. 8 Stakeholders were GPs, pharmacists (Ps), care home managers (CHMs), care home staff (CHS), care home residents and residents’ relatives.
Our semi-structured topic guide was informed by the Theoretical Domains Framework (TDF) for behaviour change9 to identify expected characteristics, barriers for and benefits of the proposed PIP role (see Appendix 1, Table 1).
We drew on the interdisciplinary research team’s broad experience to identify theory- and practice-related data collection topics relevant to
-
working in current medication management
-
stakeholders’ knowledge of scientific, procedural or environmental factors shaping care home practices
-
how a PIP service and GP-PIP partnership might work
-
potential benefits and risks
-
social and professional roles and identity
-
topics for PIP training (reported in WP4).
We contacted GPs, pharmacists and care homes – through our own and local professional networks. Additional care homes were recruited through national and site-specific regulatory bodies, local primary care and care home networks. Our maximum variation sample targeted: differently managed care homes, varying resident needs, nursing and care residents and diverse funding arrangements.
Transcripts were iteratively analysed using the TDF to identify key components, initial codes and themes for our study design.
Results
Thirteen stakeholder group-specific focus groups (n = 72 participants) and interviews (n = 13 participants) were held with GPs, pharmacists (P), one pharmacy technician, care home managers, care home staff, residents and residents’ relatives in four study sites (see Appendix 1, Table 2). Fifteen pharmacists described themselves as being employed in primary care, 11 within community pharmacy and one as split across the two (see Appendix 1, Table 3).
All focus group, interview and workshop stakeholders largely welcomed introducing the PIP service as offering benefits for residents, care homes and doctors. Reasons included viewing this new role as relevant for improving medication management, benefiting residents, overcoming communication lapses between care home, GP practice and pharmacy, residents and their relatives. Nonetheless, stakeholders identified specific potential contextual and implementation barriers and facilitators specifically in relation to
-
chronic disease management (contextual)
-
knowledge of older people’s medication and care homes (contextual)
-
clarity of PIPs’ role and responsibilities (implementation)
-
integrated social and professional team-working (implementation).
GPs’ working patterns, together with multiple care home staff involved in medication, were seen to constrain effective chronic disease management and effective communication around residents’ medication needs.
All stakeholders prioritised regular, responsive medication reviews by PIPs to address gaps in managing chronic disease and enhance the safety of residents living with comorbidities.
GPs’ onerous workload limited their capacity for ‘time-consuming’ procedures and ‘complexities’ in reviewing and managing medication (GP8-FG), and pharmacists argued that PIPs could do ‘more proactive work’ (P10-FG).
Contextual barriers and facilitators
Knowledge of older people’s medication, chronic disease management and care homes was seen as underpinning an effective PIP service.
Chronic disease management
All stakeholders emphatically identified chronic disease management as core in managing medication in care homes. Any viable PIP service must successfully address ‘many points in the circuit of prescribing where it can go wrong’ (GP15-FG), including working patterns that disrupted continuity of residents’ care, infrequent medication reviews and communication shortcomings in ordering and overseeing medications. GPs admitted that they found it ‘difficult managing all the complexity and the comorbidity’ (GP6-FG). If a PIP oversaw and bridged gaps in these processes, much communication ‘mayhem’ between care homes, pharmacies and GP practices could be eliminated (GP7-FG).
Knowledge of older people’s medication and care homes
Knowledge of older people’s conditions and their life in a care home was considered essential for the PIP, taking into account the whole person, how care homes operate and care homes’ practices and cultures. Stakeholders prioritised well-developed communication skills to interact and share knowledge with residents, ‘particularly [those] with cognitive impairment’ (P1-FG).
Implementation barriers and facilitators
Stakeholders questioned how PIPs’ specific responsibilities and roles should be understood and incorporated into care home environments. They highlighted two implementation issues: (1) clarity of a PIP’s roles and responsibilities, and (2) integrated team-working with the PIP.
-
clarity of a PIP’s roles and responsibilities
Stakeholders advocated clarity about the PIP’s role. GPs, who hold ultimate responsibility for patients’ healthcare, argued for monitoring information with the PIP, based on effective, regular mutual communication, eliminating duplicate orders and preventing omissions of medication responsibilities. For care home staff, residents and relatives, shared understanding of the PIP’s role would help improve communication in medication management.
-
integrated social and professional team working
Stakeholders believed that to embed the new service, PIPs must take time to establish communicative relationships with GP practices and care homes, to promote shared understanding of roles, working co-operatively, developing trust, providing service continuity and gaining contextual knowledge of older residents’ health.
Many participants reflected on their previous positive experiences of multi-professional working for integrating PIPs into teams, including ‘effective working relationships’ between GPs and pharmacists (GP6-FG) (P5-FG). Some GPs and care home managers envisaged PIPs educating care home staff to raise their medications’ awareness, which resonated with residents’ wishes to know about their medications as: … nobody [is there] to ask things about your medication, (RR4-FG) with staff and residents able to see PIPs as part of a resident’s ‘care package’ team, rather than ‘checking up on [staff]’ (P6-FG).
Stakeholders emphasised clarity in team-working to integrate PIPs to strengthen, not complicate, their working collaborations in care homes. Welcoming a PIP was therefore conditional on a clearly defined PIP role communicated to stakeholders; collaborating across doctors, PIPs and care home staff; dialogue with residents and relatives on developing the service and trustful and effective communication.
Discussion
Work package 1 explored stakeholders’ expectations of the components and context for a feasible PIP service, with a TDF-informed approach identifying components, stakeholders and contextual practices as relevant to mitigate implementation barriers. This identified care home environments as complex, with diverse participants, organisational processes, systems and resources providing care to frail older people, posing chronic disease management and review challenges.
A PIP model was envisaged to offer means to address this, but only if well informed about older people’s medication and care homes. Stakeholders believed that an acceptable service required the PIP to offer means to strengthen mechanisms to ensure efficient, effective ‘whole team’ approach to prescribing in care homes. 1 All stakeholders gave priority to a PIP conducting medication reviews to save GPs’ time/work. They welcomed PIP-related relationships as enhancing trustful communication around medication issues, mutually recognising remits and competencies.
We gained comprehensive understanding from stakeholders about processes to maximise chances of the impact of the PIP intervention on practice. 10 Central here was for everyone involved to ‘understand each other’s systems’ by recognising established organisational and cultural practices in care homes and primary care during implementation and improving communication around related changes. Care home managers, residents and relatives saw PIPs spending time with residents to explain and reassure around medication, to be more fully partners in their own care. 11
Integrated team-working was another key component: stakeholders were more likely to consider the PIP’s role as acceptable and viable if effective team-working were embedded in implementing such healthcare change. 12 GPs and pharmacists strongly preferred PIPs to be integrated into their practice teams.
Clearly defined PIP roles were considered crucial at micro-level experiences of individual actions and at macro-level experiences in care home and GP practice organisation. Residents and relatives were more likely to accept the new service if its purpose was carefully explained to them. GPs and pharmacists required explicit agreement on areas for PIP prescribing.
Contextual factors framed how stakeholders envisaged implementation issues. For example, effective team-working with the PIP, in GP practices and care homes (an implementation concern), depended upon the PIP acquiring appropriate knowledge of older people’s medication and frailties (a contextual issue). Guidelines and existing research underscore that specifically addressing both context and implementation barriers can better guarantee improved outcomes for older people in residential settings. 13 Multiple stakeholders shared the belief that for any proposed PIP innovation to be acceptable and viable, it would be dependent on all stakeholders understanding each other’s systems. 14
Work package 2: outcomes identification
Aim
This WP aimed to identify the most appropriate outcomes to measure the impact of the CHIPPS intervention through the development of a core outcome set (COS).
Objectives
The purpose of determining the effectiveness of the CHIPPS service was to
-
identify potentially appropriate outcome measures
-
rate and select outcome measures based on validity, reliability, utility and proximity to the intervention.
Method
In WP2, we followed methodology for the development of a COS (standardised set of outcomes that should be measured and reported at minimum in all clinical trials)15 for CHIPPS. The full study has been published. 16
The methodology used was informed by published guidance17 and conducted in two phases. Phase I encompassed the first three steps recommended by Williamson et al. : (1) to explicitly describe the scope of the COS, (2) to identify existing outcomes, and (3) to identify outcomes that are important to key stakeholders. This resulted in a long list of outcomes that fed into Phase II, corresponding to step (4) of the aforementioned guidance – prioritising the most important outcomes using a consensus method.
Phase I: Generating and refining a long list of outcomes
Step 1: Scope of COS
-
Health condition and population: older adults with any type/number of health conditions.
-
Intervention: those aiming to optimise prescribing.
-
Setting: care homes, defined as nursing and/or residential homes, skilled nursing, assisted living and aged-care facilities.
Step 2: Literature review
A relevant Cochrane review, assessing interventions to optimise prescribing in care homes, was used, as it reported outcomes pertinent to COS development. 18 Briefly, 12 randomised controlled trials (RCTs) involving over 10,000 older adults residing in 355 care homes were included.
Step 3: Stakeholder involvement
Focus groups and semi-structured interviews were conducted with GPs, pharmacists, care home managers/staff and care home residents/relatives from the four CHIPPS study sites to determine outcomes for inclusion. These stakeholder consultations were conducted primarily for the development of the CHIPPS intervention and are described elsewhere in this report (WP1: Service Specification Development). 14 Transcripts of the focus groups and interviews were independently analysed by two researchers who recorded verbatim all outcomes proposed by stakeholders. The stakeholder study was approved by the National Research Ethics Service (NRES) Committee Yorkshire and the Humber, Sheffield; reference: 15/YH/0172.
The resulting long list of outcomes was reviewed, with removal of duplicate items and process measures. Four members of the CHIPPS WP2 team then independently assessed the remaining outcomes and voted anonymously to determine whether each outcome should progress to Stage 2. All four team members involved in this process had either practised clinically or undertaken clinical trials in care homes previously.
Phase II: Delphi consensus exercise
We used the Delphi technique to achieve consensus on a final COS.
The Delphi Panel
The Delphi panel comprised the 19 members of the wider CHIPPS management team (excluding the four aforementioned team members). The group included academic pharmacists (n = 3), geriatricians (n = 2), patient-public involvement (PPI) representatives (n = 2), health economists (n = 2), senior CHIPPS research fellows (n = 2), a prescribing advisor pharmacist (n = 1), an academic sociologist (n = 1), a research governance manager (n = 1), a care home quality director (n = 1), an educationalist (n = 1), an academic doctor (n = 1), a GP (n = 1) and an academic nurse (n = 1).
The questionnaires
The first questionnaire was structured around the Phase I long list of outcomes. Each outcome formed a single questionnaire item, accompanied by a brief explanation, to prevent misinterpretation. For example, ‘Duplicate drugs’ was described as a situation where two medicines of the same pharmacological class were prescribed, such as the prescribing of two concurrent opiates. 19
Questionnaires were distributed using a web-based survey tool (Survey Gizmo®; Alchemer, Louisville, CO, USA). Panellists were emailed a link to each questionnaire and asked to complete within 4 weeks. Reminder emails were sent as needed. Panellists responding to the first questionnaire were invited to participate in the second round.
Panellists rated the importance of including each outcome in the COS, using a scoring system derived from the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group. 20 Scoring was based on a Likert scale, ranging from 1 to 9. Scores of 1–3 indicated an outcome of ‘limited importance’, 4–6 indicated ‘important but not critical’ and 7–9 indicated ‘critical’. Panellists were also given the option to select ‘unable to score’. During the first round, panellists were invited to suggest additional outcomes for inclusion, which were reviewed and discussed by the WP2 study team.
The second questionnaire (with the same rating method) included a revised list of outcomes, inclusive of ‘non-consensus’ outcomes (see below for a definition of consensus) and any new outcomes generated from the first round. A summary of first-round scores for each outcome (including individual score, group mean score and group median score) accompanied the link to the second questionnaire. Panellists were encouraged to consider this information whilst re-scoring outcomes.
Definition of consensus
Consensus for inclusion of an outcome in the COS (consensus ‘in’) was defined as ≥70% of respondents rating an outcome 7–9, that is, ‘critical’ and fewer than 15% of respondents rating it 1–3, that is, ‘of limited importance’. Similar thresholds have been previously reported. 21,22 Consensus for exclusion (consensus ‘out’) from the COS was defined as fewer than 15% of respondents rating an outcome 7–9, that is, ‘critical’ and ≥70% of respondents rating it 1–3, that is, ‘of limited importance’. All other score distributions were categorised as ‘no’ consensus.
Data analysis
Responses were analysed using Statistical Package for the Social Sciences Statistics version 22 (IBM Corporation, Armonk, NY, USA). The proportion of respondents rating the outcome as ‘critical’, ‘important but not critical’ or ‘not important’ was calculated for each outcome to determine whether consensus had been reached, as described above.
After the first Delphi round, outcomes that reached consensus ‘in’ were included in the COS, and outcomes that reached consensus ‘out’ were excluded. ‘No’ consensus outcomes proceeded to the second round. This approach was also used after the second round; however, ‘no’ consensus outcomes were excluded from the final COS.
Key findings
An overview of the COS development process is provided in Appendix 1, Figure 2.
Phase I
Sixty-three outcomes for potential inclusion in the COS were identified in Phase I (22 from 12 studies included in the Cochrane review and 41 from stakeholder focus groups and interviews). Sixteen duplicates were removed. Further 16 were classified as process outcomes (e.g. ‘satisfaction with PIP service’) and excluded. Two outcomes (‘pain’ and ‘accidents’) were also excluded unanimously by the CHIPPS review panel. A total of 29 outcomes (see Appendix 1, Figure 2 and Table 1) were considered by the Delphi panel in the consensus exercise.
Phase II
The first round of the Delphi consensus exercise was completed by all 19 Delphi panellists (100% response rate). Twelve outcomes met the consensus criteria for inclusion in the COS. No outcomes met the exclusion criteria, and no consensus was achieved for 17 outcomes.
Outcomes (n = 6) suggested by the panel during the first Delphi round were discussed by three members of the research team (AM, CH and DA). The outcomes were ‘patient mobility’; ‘making sure drug charts are kept up to date’; ‘anticholinergic burden’; ‘nutritional status’, for example, Malnutrition Universal Screening Tool score or ‘use of nutrition supplements’; and ‘appropriate use of covert medication.’ ‘Anticholinergic burden’ was added to the second Delphi round. ‘Patient mobility’ was not added because it was considered a subset of ‘physical functioning’. The other suggestions were considered beyond the COS scope. Another outcome, ‘number of medicines (and associated costs)’, was reformulated into two separate outcomes: ‘number of medicines’ and ‘costs of medicines’.
The 17 outcomes for which consensus had not been achieved, together with the three outcomes added/reformulated based on panel feedback, resulted in a total of 20 outcomes being included in the second Delphi round (see Appendix 1, Table 4).
The second round was completed by 18 of the 19 respondents (94.7% response). Two outcomes (‘number of medicines’ and ‘anticholinergic burden’) met the criteria for inclusion in the COS, with the remainder (n = 18) being excluded (see Appendix 1, Table 5).
Therefore, a total of 13 individual outcomes met the inclusion criteria in the COS (see Appendix 1, Table 6).
Discussion
This WP was informed, in part, by the stakeholder engagement activities organised in WP1. Stakeholder engagement is a crucial part of COS development methodology to ensure that outcomes represent those that are considered of importance to service users and/or their representatives.
The final COS provided the basis for planning the next phases of the trial, notably the feasibility study (WP5), internal pilot and main trial (WP6).
Work package 3: health economics
A summary of the health economic work undertaken is given below. More details on the Methods and Results for the health economics component of the main trial/WP6 are given in the associated health economics report (see Appendix 8).
Background
Medication administration errors are common within care homes. 1 The PIP intervention therefore has the potential to improve outcomes. However, not all treatments can be provided. We therefore undertook an economic evaluation of the PIP intervention to assess whether it represented a good use of scarce resources. This was deemed feasible for the full trial (outlined in WP6) based on, amongst other things, the high response rate achieved for proxy EQ-5D scores in the feasibility study (see WP5). The methods of resource use data collection were also informed in a previous work in the care home setting. 23
Objective
To estimate the cost-effectiveness of the PIP intervention.
Methods
Study overview
The economic evaluation was conducted alongside the CHIPPS cluster randomised trial (see WP6 of this report).
Costs
The methods used to estimate costs are described in the associated health economics report (see Appendix 8). In brief, costs were estimated in Great British pounds (£) at 2017/2018 financial year levels, from the perspective of the NHS and PSS, over the 6-month trial (no discounting was undertaken).
To estimate the cost of the PIP intervention applied in WP6, each PIP was asked to complete a training log and a log of all activity associated with the intervention, including contacts with others, for example, care home residents, care home staff, GP or any other professionals (geriatricians, community pharmacists, district nurses, etc.). Subsequently, unit costs24 (see Appendix 1, Table 22) were assigned to estimated times for all PIPs and other staff time.
It was envisaged that the PIP intervention would enable GPs to spend less time undertaking prescription management for intervention arm participants. Accordingly, based on previously published work,24 the estimated GP time (cost) saving per resident was also estimated.
The total PIP intervention cost was subsequently estimated by summing the PIP training and activity costs and deducting the estimated GP time/cost saving.
Other costs
As informed in previous research,24,25 GP and practice nurse visit data were extracted from primary care records, along with medication data, outpatient attendances, inpatient stays, tests and investigations. All other health professional contacts (phone calls, visits and their location) were extracted from care home records.
Data for the previous 3 months were collected at baseline and those for the previous 6 months were collected at 6-month follow-up. Unit costs24,26 were assigned to each contact/admission in line with Underwood et al. ;27 medication costs were extracted from the prescription cost analysis (PCA). 28,29
Outcomes
Quality of life was measured using the EuroQol Five Dimensions and Five Level Rating Scale (EQ-5D-5L). 30 The EQ-5D-5L (proxy version) was chosen as it is deemed the preferred measure of utility in the National Institute for Health and Clinical Excellence (NICE) methods guide31 and in the light of work undertaken in the CHIPPS feasibility study32 (see WP5). The latter included an assessment of outcome measures used in the CHIPPS feasibility study where the EQ-5D-5L was chosen to be used in the full RCT, as it had been previously validated,33 and had a relatively good completion rate and low time taken to collect. It is recommended that QALYs are used to measure and value health effects, as QALY is a generic measure of health benefit that considers both mortality and health-related quality of life. 34
For all participants, proxy respondents were asked to report participants’ level of problems (none to extreme/unable) in five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) at baseline, 3- and 6-month follow-up. In line with the NICE position statement,33 the crosswalk mapping function was used to convert these responses into utility scores. 35 Those who were known to have died by the particular follow-up points were assigned a utility score of zero. QALY scores were subsequently estimated based on the total area under the curve method and the assumption of linear interpolation. 36
Analyses
Base-case results were based on those with complete cost data at both baseline and 6-month follow-up and those with complete EQ-5D data at baseline, 3- and 6-month follow-up. Bivariate regression37 was used to analyse the cost and QALY data, based on the intention-to-treat approach, enabling the mean incremental cost (mean difference in cost between the two arms) and the mean incremental effect (the mean difference in QALYs) to be estimated. The cost-effectiveness acceptability curve (CEAC)38 was also used to estimate the probability of the PIP intervention being cost-effective at the λ value of £20,000/QALY compared with standard care. In addition, three key sensitivity analyses were undertaken: using multiple imputation to account for missing values, removing training costs (as they are one-off cost) and undertaking a threshold analysis to establish at what threshold the intervention would be effective (in terms of cost or effect).
Results
PIP intervention costs
The training logs were returned by 23/25 PIPs, and the activity logs by 22/25 PIPs. Training costs were assigned to the two non-responding PIPs, but activity costs were not assigned to three non-responding PIPs, as they did not implement the PIP intervention. Overall, the mean reported activity time for each PIP was equivalent to an average of just over 3 hours per resident (see Appendix 1, Table 23). The total PIP intervention cost was subsequently estimated to be £323 per resident by summing the PIP training and activity costs and deducting the estimated GP time/cost saving.
Other costs
It is notable that the mean cost associated with the intervention was lower than that associated with overall medication costs, GP/practice nurse contacts, other professional contacts and in-patient stays. In terms of total (NHS and PSS) costs, the (unadjusted) mean difference between arms was £246. This might suggest that some of the aforementioned PIP intervention costs were partially offset, for example, by lower medication costs (see Appendix 1, Table 24).
Outcomes
EQ-5D scores are shown in Appendix 1, Table 25, where it can be seen that both arms had lower mean scores at the 3- and 6-month follow-up points. Although the mean QALY score was higher for the intervention arm, the mean baseline EQ-5D score was also higher for these participants. This means we cannot infer that the intervention was more effective based on these results and that there is a need to adjust for, amongst other things, the baseline difference in EQ-5D scores between arms.
Analyses
A total of 609 participants (70%) had complete cost and EQ-5D data. Bivariate regression estimated that the mean (95% confidence interval) incremental cost of the intervention, compared with standard care, after adjusting for baseline costs, age and gender was £279.86 (£19.39–540.33). The estimated mean incremental effect was −0.004 (−0.016 to 0.009) QALYs. The PIP intervention was therefore estimated to be dominated, as it was associated with higher costs and lower effect, and the CEAC estimated that it had a 3.8% probability of being cost-effective at the £20,000/QALY value. These results suggest that there would be no added benefit of a long run model as (given lower effect and higher cost) as the results of a long-term model would be unchanged/in line with the within trial analysis, that is, the PIP intervention would not be estimated to be cost-effective. All sensitivity analyses were not found to change conclusions from the above findings.
Discussion
In terms of overall costs, the estimated mean incremental cost of £280 was lower than the estimated cost of the PIP intervention, suggesting that intervention costs were partially offset by certain lower costs, for example, medication. However, as the PIP intervention had higher mean costs and was not estimated to be associated with an improved QALY score, it is not estimated to be cost-effective. These conclusions are based on the 6-month within trial analysis for those with complete data, but the conclusions were the same when multiple imputations or other sensitivity analyses were carried out (see Appendix 1, Table 26).
Work package 4: development of PIP training package
Approvals
Research ethics approved by the Faculty of Medicine and Health Sciences Research Ethics Committee Ref.: 20142015-77.
NHS Research and Development approved by NHS Grampian Research and Development Ref.: NRS15/PH18.
Aim
Develop a training package to ensure that pharmacist independent prescribers are appropriately prepared to deliver the service.
Method
WP4 consisted of six phases:
-
systematic review and narrative synthesis
-
initial stakeholder engagement
-
training specific interviews and focus groups
-
stakeholder engagement and consensus
-
feasibility testing
-
validation.
Systematic review
The systematic review was registered with PROSPERO (CRD42015026693) and adheres to PRISMA. 39 Papers and abstracts were selected for review to inform both content and design of any future pharmacist training package.
Synonyms for care home (population), pharmacist (intervention), education and training (outcome) and pharmaceutical care (intervention) were used. This review included articles published until 30 June 2015.
Inclusion criteria:1
-
Description of education and training of pharmacists before service/intervention delivery in a care home, OR
-
Description of expertise of the pharmacist, for example, title denoting additional expertise or training to perform role, OR
-
Training provided by pharmacists to care home staff for which they would need to have sufficient knowledge to deliver, OR
-
Materials provided to support the pharmacist in service delivery in care homes, AND
-
English language.
Exclusion criteria:2
-
Studies not primarily focused on provision of services to older people residing in care homes
-
Studies where the primary focus was to determine the effectiveness of an individual drug, OR
-
Papers without empirical data for example, editorials, opinion pieces commentaries, OR
-
Abstracts, OR systematic reviews and narrative syntheses.
Databases searched (July 2015) were Academic Search Complete, EBSCOH, Ovid MEDLINE® and EMBASE, OvidSP, ASSIA (Applied Social Sciences Index and Abstracts), CSA, ProQuest XML, Cochrane Database of Systematic Reviews, Cochrane Reviews (Issue 6 of 12, June 2015), E-theses online service (EThOS), Ingenta Connect (Ingenta), Wiley Online Science, EPOC Group Specialised Register, Reference Manger, Ageline (EbscoH), CINAHL (Cumulative Index to Nursing and Allied Health Literature), EBSCOH, International Pharmaceutical Abstract (OvidSP) and PsycINFO (EbsoH).
Titles, abstracts and full-text papers were screened for eligibility, independently by two authors. Differences were resolved by consensus. A PRISMA diagram39 was populated, and Kappa coefficients40 calculated.
As the narrative synthesis focused on learning from the content of published care home interventions, the quality of the included papers was not appraised.
In line with Cochrane guidance, the following information was extracted from papers and abstracts by two independent researchers (DW and VM):
-
year, design, location, setting
-
main findings
-
pharmacist expertise
-
education and training provided
-
service delivery support tools provided
-
training of care staff provided by pharmacist
-
clinical and therapeutic area(s) of intervention focus (three most commonly reported)
-
intervention description.
The results were compared and again agreed by consensus by two independent reviewers.
Analytical approach
Data were themed and collated to inform the development of a care home pharmacist training package. All training methods outlined within selected papers were extracted.
Initial stakeholder engagement
As part of the main programme of CHIPPS work, focus groups and interviews were undertaken primarily to define the PIP service specification. 14 Incorporated into the topic guides was a question regarding the pharmacist-training package.
The elements from the content analysis were combined with those from the previously reported literature review41 by NN and DW to create a training package including a personal development framework (PDF) consisting of domains (i.e. our grouping name for a selection of similar competencies), competencies and behaviours. This was presented to the External Advisory Panel (EAP) to review and amend.
Training-specific focus groups and interviews
Focus groups with different healthcare professional groups were organised and located across four locations as follows:
-
primary care pharmacists (Yorkshire and Humber)
-
general practitioners (Aberdeen)
-
community pharmacists (Belfast)
-
care home staff (Norwich).
Within each location, an appropriate healthcare professional with significant local care home experience regarding medication management was interviewed to enable identification of local environmental and contextual factors.
The current draft of the training package was provided before focus groups and interviews. The topic guide consisted of the following:
-
initial views on the draft training package
-
therapeutic and clinical areas to be included
-
care home-specific processes which pharmacists would need to be aware of
-
knowledge required to be effective
-
inter-professional related knowledge required
-
advice relating to pharmacist preparation.
Where possible, consensus on how best to amend and enhance the training package and framework was identified within the focus groups. All the focus groups/interviews were recorded digitally and transcribed verbatim. Interviews and focus groups were content analysed by DW and validated by NN.
To create the next draft of the training package, where consensus was not clear, a final decision was sought from the EAP.
Expert consensus
A consensus day held at each study site (outlined in WP1), included a session to obtain feedback on the draft training package regarding:
-
training content
-
PDF
-
assessment processes
-
Points of dissonance identified within the stakeholder focus groups and interviews.
Detailed notes were taken from the consensus panels and used by NN and DW to create a final draft training package for feasibility testing.
Feasibility testing
Four PIPs and four care homes, each with 10 consented residents, were recruited and PIPs trained to deliver the intervention over 3 months. 42 At the end of the feasibility phase, a face-to-face focus group with the PIPs was convened to obtain feedback regarding
-
personal development planning and support process
-
PDF
-
assessment processes
-
impact of the training
-
elements that worked well and those that worked less well.
Focus groups were recorded, transcribed verbatim and content analysed to create the final draft training package for use within the main trial.
Validation
All intervention PIPs undertook the final training plan. The PIPs’ experience of the training days and the aligned professional development and assessment of competence were evaluated through training day evaluation forms (PIP n = 21), online survey (PIP n = 17) and semi-structured interviews (PIP n = 14). Using a mixed-methods approach, each dataset was analysed separately and then triangulated to provide a detailed evaluation of the process. The evaluation forms and online survey were described quantitatively and tabulated. Qualitative interview data were coded by two researchers, and descriptive categories and interpretative themes identified through thematic analysis were agreed upon among the researchers.
Results
Systematic review
Paper selection and description
Fifty-two papers were selected for the review (see Appendix 1, Figure 3). Characteristics of the included papers are provided in Appendix 1, Table 7. All studies reported that their intervention was effective.
Pharmacist, education and training characteristics
Descriptions regarding qualifications and training provided before pharmacist role in care homes were generally vague. Six papers reported the pharmacists being provided with a tool to support the service.
Two papers described the pharmacists being trained in inter-professional relationship development. Ten papers described some form of training in a limited manner.
Codified and practical knowledge
A summary of the main clinical and therapeutic areas identified and the most commonly cited activities is provided in Appendix 1, Table 8.
Care home–specific cultural knowledge
Care home staff training was seen as important for developing relationships and changing care home medication-related cultures, for example, requests for medication such as antibiotics, antipsychotics, analgesia and laxatives43 and willingness to implement changes in therapy. Care home culture was cited in one paper as a reason for medication changes not being implemented. 43,3
Stakeholder engagement
Thirteen interviews and 13 focus groups with 72 participants were undertaken. The main results have been reported elsewhere. 44 The different types of knowledge identified as important through the interviews and focus groups are summarised in Appendix 1, Box 1. Appendix 1, Figure 3: Draft 1 provides a copy of the first draft of the PDF used to underpin the training package.
A variety of activities were added to enable the development of identified cultural knowledge requirements, for example, spending time with different medical practice and care home staff to identify expectations and preferences and learning to use local systems.
The practical knowledge identified as important was how to provide pharmaceutical care for older people with frailty.
The EAP identified the need for ‘context’ to be included as a domain within the PDF and to change the ‘chronic disease management’ domain to ‘managing complexity in late life’ (see Appendix 1, Figure 4: Draft 2).
Training-specific focus groups and interviews
Six primary care pharmacists, six GPs, five community pharmacists, six care home staff and four local experts participated. Additional changes to the training package were identified as being required, including the addition of activities to enable the PIP to understand local cultures and to integrate into the teams.
Expert consensus
Four consensus panels were held (Grampian n = 12, Yorkshire and Humber n = 12, Norfolk n = 15, Belfast n = 14). The content of the face-to-face training days was agreed along with the expectation that a geriatrician should be involved in delivery.
The consensus panel emphasised the need for a significant amount of time to be allocated to the development of relationships and that training care home staff was an important element within this.
A number of further changes to competencies and behaviours were recommended.
A large number of topics about which PIPs should be knowledgeable were identified (see Appendix 1, Box 2). It was agreed to provide this information through a knowledge pack, consisting of relevant links.
It was proposed that the PIPs would use the PDF for self-assessment purposes and they should be allocated a mentor (senior care home pharmacist) to support them through the process.
Completion of these competencies would be signed off both by their mentor and by a medical practitioner with expertise in providing medical care to care home residents (independent assessor). Sign-off by both parties would provide the ‘accreditation’ for the PIPs.
A copy of the training package and PDF created following this exercise and before feasibility testing is provided in Appendix 1 and Figure 5.
Feasibility testing
The PIPs reported that the process of personal development planning, being supported by a mentor and assessed on their final competence through oral viva, was appropriate and effective. Greater guidance on evidence collection for assessment purposes was requested at the outset.
The elements of the face-to-face training that were perceived as particularly effective were the case studies surrounding the management of complexity, legal issues and covert administration and the session on the management of psychotropic medication.
The training was viewed positively and reported to be motivational, helping to enhance confidence. This resulted in no major changes to the training package.
Evaluation
All PIPs found the training useful, specifically the input from the geriatric specialists who produced case studies on medication management in older people: ‘probably about the best CPD I’ve done for a long time’. Overwhelmingly, the PIPs strongly agreed that the training content was appropriate.
-
Comprehensive training, mentorship, competency assessment and explanation of research processes amply equipped these experienced PIPs to carry out their role.
-
Input from older people psychiatric specialists and specific training on antipsychotics was reported to be of huge value to the majority of PIPs, increasing their confidence and understanding around this area of medication management.
Post-training mentorship by a qualified pharmacist with extensive care home experience was reported as being extremely helpful in guiding their CPD and in preparing a portfolio of evidence for the assessment of competence by a GP. All PIPs achieved competency at first attempt and within expected timelines. Only one PIP stated that mentorship was not very useful at this stage. The PIPs reported that they had less contact with the mentor as the intervention progressed.
The training days and mentoring appeared to increase confidence, especially in PIPs with limited experience in older people medicine. Even for those with extensive experience in older people medicine, the training provided a chance to consolidate knowledge. Alongside consolidating clinical knowledge, the PIPs needed to be able to successfully develop relationships with care home staff and primary care staff if they were joining a new practice. PIPs reported arranging peer support through the cloud-based messaging app ‘Telegram’. Mentorship and peer support was reported as useful in the early stages of the intervention but use tailed off as individuals’ confidence grew.
The need to demonstrate competency through completion of the competency framework and professional discussion with a GP was appreciated by the PIPs. Evidence from the PCP reviews suggested all were competent in the role. PIPs’ comments on their practice during the trial, evidence the increased competency and confidence they had due to the training programme:
There were a few people that we’d got off a medication, antipsychotics particularly, because that’s something I probably wouldn’t have touched, but after having the training session, and the group discussions, and more of an awareness, I felt more comfortable
Pharmacist independent prescribers suggested that individualised country-specific sessions could run in tandem during main training days without affecting the length of the training programme. An additional refresher event mid-intervention was recommended as was advice on building relationships in CHs where work cultures may be new to pharmacists.
Discussion
The results from the feasibility study suggest that the iteratively designed training package is likely to ensure that PIPs are competent to undertake their envisaged role. Evaluation within WP6 will enable the researchers to determine whether it is generalisable to a broader range of PIPs.
Work package 5: feasibility study4
Approvals
Research ethics approved by NRES East of England – Essex REC Ref: 16/EE/0284 and Scotland A REC Ref.: 16/SS/0125.
Health Research Authority approved Ref: IRAS 206970.
Aims
To test and refine the service specification and proposed study processes to inform the cRCT (WP6).
Objectives
To
-
test processes for participant identification, recruitment and consent and assess retention rates
-
determine suitability of outcome measures and data collection processes from care homes and GP practices
-
assess service and research acceptability
-
test and refine the service specification.
Method
Design
This was a single-arm, open-feasibility study conducted in care homes for older people in all four locations across the UK from August 2016 to April 2017.
The recruitment target was one eligible general practice, one PIP and up to three care homes associated with each participating practice in each location. Each GP/PIP/care home(s) triad had a target of recruiting 10 residents.
Inclusion criteria
GP practice
General practitioner practice managing sufficient care home residents to recruit a minimum of 10 residents in up to three (in case one or more care homes did not have sufficient eligible participants) care homes. An existing arrangement with a PIP was preferred, but not mandatory.
PIPs
Pharmacists registered with the General Pharmaceutical Council or Pharmaceutical Society of Northern Ireland as independent prescribers.
Care homes
Care home primarily caring for residents aged ≥65 years, registered as caring for adults aged ≥5 years.
Residents
Permanent residents under the care of the participating GP practice, prescribed at least one regular medication, aged ≥65 years; they should be able to provide informed consent/assent, or for this to be provided by a nominated representative.
Residents on an end-of-life care pathway were excluded.
Patient identification and recruitment
PIPs and GP practices
Each location used locally defined strategies and networks (see Appendix 2) to obtain expressions of interest (EOIs) from GP practices and PIPs, for either the feasibility study or the planned main RCT.
Care homes
The consenting GP practice in each study location approached up to three care homes. Care home managers expressing interest were sent a formal invitation pack by the local researchers. A second or third care home was contacted only if there were insufficient residents in one home.
Residents
General practitioners identified eligible care home residents from their computerised records. An invitation pack [letter from the GP, participant information sheet (spoken version, if necessary) and consent form] was distributed to each resident by the care home manager. After a minimum of 24 hours, the care home manager visited each resident and obtained verbal consent from residents willing to discuss the study with the study research associate (RA) who then visited the care home, met with interested residents and assessed resident capacity to give consent. Where a resident was identified by the care home manager or RA as lacking capacity, a legally appropriate third party [e.g. relative/friend known as consultee (England and Northern Ireland)] or welfare power of attorney (WPOA; Scotland), was contacted by mailed invitation pack, through the care home. Reminder letters were issued after 2 weeks as needed. In England and Northern Ireland, a member of the care home staff could be nominated as the consultee, but in Scotland, a member of staff could not be the WPOA. The recruitment process is outlined in Appendix 1, Figure 5.
Sample size
No formal power calculation was conducted. The recruitment target was 10 participants per site (a total of 40), judged as sufficient to assess feasibility. 45
Intervention
The intervention included medication review; prescribing and deprescribing; care home staff training, medication-related support and communication (see Appendix 3). It spanned for 90 days (4 hours per week per PIP per 10 patients). Before the intervention, PIPs attended training (described in WP4). Pharmaceutical care plans (see Appendix 4) were used by the PIPs to document each intervention. A random sample of eight PCPs (two per location) was selected for review of appropriateness (based on professional judgement) by one of the two specialists in ‘care-of-the-elderly’ grant holders.
Estimating the participating proportion of the eligible population
The proportion of general practices, pharmacists, care homes and residents approached and consented was recorded along with the proportion of residents followed up at 3 months to assess recruitment and retention.
Suitability of outcome measures
Potential outcome measures identified in WP2 (see Appendix 1, Box 3) were collected at baseline and 3-month follow-up for each participant. To determine their suitability for inclusion in the RCT, each outcome measure was assessed against the following criteria: availability of the data source, potential for bias, potential for missing data, resident centeredness, sensitivity to the intervention, reliability, whether validated, potential for third party completion, ability to blind and time taken to collect per patient and quantity of data. At minimum, the measure had to be judged objective, discriminating and efficient to collect.
Assessment of service acceptability and trial feasibility
Participant views
After the intervention, face-to-face semi-structured interviews were held with stakeholders at each location and a focus group held with the PIPs. Proceedings were digitally recorded and transcribed verbatim.
Serious adverse drug events
All admissions to hospital and deaths were recorded as serious adverse events (SAEs) and assessed for causality by a medical doctor in the study management team, using professional judgement.
Data analysis
Descriptive statistics were used for the quantitative outcome measures at baseline and follow-up. No statistical comparisons were conducted. Interview and focus group transcripts were thematically analysed.
Approvals and registration information
A favourable ethical opinion was received from East of England – Essex Research Ethics Committee (5 September 2016: 16/EE/0284) and Scotland A Research Ethics Committee (8 September 2016:rec ref. number 206970) with subsequent approval from the Health Research Authority/NHS Research and Development.
The trial was registered on the ISRCTN registry Registration number ISRCTN10663852.
Results
Recruitment and retention
In total, 4 PIPs, 4 GPs, 6 care homes and 40 residents were recruited (see Appendix 1, Table 9 and Figure 6). The four recruited PIPs were employed directly either by the practice (1) or by the NHS (one with no existing relationship with a GP) (2) or were self-employed (1). The GP or PIP identified 122 residents from GP records and invited 86. Thirty-six (30%) residents were excluded on screening and 33 (27%) of those invited declined the invitation or did not reply. Forty were recruited and retained for 3 months.
Suitability of outcome measures
There were data for all outcome measures (see Appendix 1, Table 10) for all or most residents, other than the MMSE, which could be completed only by 40% and 35% of residents, respectively, at baseline and follow-up. The direction of change between baseline and follow-up suggested that the intervention could improve care.
Appendix 5 provides a review of the suitability of outcome measures following feasibility testing. The outcome ‘falls per patient’ met most criteria and was selected as the primary outcome measure with Drug Burden Index (DBI),46 hospitalisations, mortality, Barthel (proxy)47 and ED-5Q-5L (face-to-face and proxy). 30,33 Whilst hospitalisations and mortality were also considered as the potential clinical primary outcome, when considering the sample size necessary to detect a clinically important difference, falls was the only feasible option. STOPP/START was not used due to perceived subjectivity. 19 The QUALIDEM48 and MMSE49 were excluded as too time-consuming to complete, a better measure was available (to replace the QUALIDEM) or a high potential for bias existed and/or data were missing.
Quality of pharmaceutical care plans and adverse events
Eight pharmaceutical care plans (PCPs) were reviewed. Six PCPs were considered appropriate. Two included insufficient detail to fully judge appropriateness. Just over 10% of residents [5/40 (12.5%)] were admitted to hospital (11 hospital admissions), and two residents (5%) died. None of these events were related to the intervention.
Participants’ views of service and research acceptability
All 28 participants invited to interviews agreed (6 care home managers, 6 GPs, 12 care home staff, 12 residents, 3 relatives and 1 dietician). Lack of capacity restricted the number of residents interviewed.
Perceived benefits of the service
All participants expressed positive views about the service. The main themes are summarised below and illustrated with exemplar quotes detailed in Appendix 1, Table 11 and indicated in the text.
Improved patient care and safety
Regular medication review led to improved patient care and quality of life (quote 1). The key pharmacist skills were their knowledge, ability to prescribe, professionalism, autonomy and ability to provide training and communication (quote 2). Care home managers highlighted that medication practice had become safer (quote 3) and efficiency was increased. The PIP facilitated prompt implementation of medication changes and acute prescriptions (quote 4) and saved care home manager time (quote 5). A nurse highlighted the value of having pharmacists who could prescribe (quote 6). The PIP had time to communicate with relatives and care home staff (quote 7) and had more time than GPs to complete detailed medication reviews (quote 8).
The PIP service freed up GP time and was the most ‘efficient’ way of conducting medication reviews (quote 9), with the ability of the PIP to work autonomously being valued (quote 10). Conversely, some care home managers did not feel it freed up any time for them (quote 12), but neither did it impede care home processes (quote 11). However, it did reduce stress levels and improve communication (quote 13).
Potential disadvantages of the service
Few disadvantages were mentioned. The main one was that the PIP was not as familiar with the patients as the GP (quote 14). GPs also expressed concern that they would become less familiar with the residents and the care home staff if they were less involved (quote 15).
Refinements to service specification
Only two of four PIPs attended the focus group. Two were unwell, and views were obtained by later telephone interviews. Few changes were proposed. Delivering the service took more than the indicative 4 hours per week, not having an existing relationship with the GP was a disadvantage, and PCPs needed simplifying.
Discussion
The acceptability of the service and feasibility of proceeding to the main trial were confirmed. The processes to identify and recruit trial participants (GPs, PIPs, care homes and residents) were successful and scalable, including those for participants without capacity. Participants were retained for 3 months. The primary outcome measure for the main trial was confirmed as the fall rate per person. In contrast to other potential outcomes, a clinically important difference in falls would be detectable with acceptable power within a realistic sample size. Minor areas of refinement to the service specification and the research process were identified. For example, using the professional judgement of a study team member to assess causality between reported adverse event(s) (AEs) and the intervention was subject to bias, and an alternative approach was developed, that is, using independent GPs for WP6. Similarly, a review of the PCPs, conducted subjectively by the study team members, could have been biased, and WP6 includes details of the standardised protocol and reporting templates used in the main trial.
The participant demographics were similar to those of the UK care home population. 1 Participation rates were high, suggesting there had not been selective recruitment. The PIPs participating in this study included pharmacists employed by either primary care or the GP practice, providing evidence that this service specification is adaptable to either model; PIPs with a pre-existing relationship with the GP found it easier to arrange meetings.
The level of EOI confirmed that there would be sufficient participants for the main trial, and the consent rate of residents informed the target number of care home patients required to be registered with the participating GP.
Work package 6: definitive trial with internal pilot
Approvals
Research ethics approved by NRES East of England Central Cambridge REC Ref.: 17/EE/0360 and Scotland A REC Ref.: 17/SS/0118.
Health Research Authority approved, Ref.: IRAS 233964.
Aim
To estimate the effectiveness, cost-effectiveness and safety of a PIP assuming responsibility for providing pharmaceutical care to residents in care homes.
Methods
This was a cRCT conducted in primary care involving triads of a GP practice, PIP and a sufficient number of care homes to provide 20 care home residents per triad. 50
Recruitment
Inclusion and exclusion criteria
Pharmacist Independent Prescribers were excluded if they were already providing an intensive service to the care home or could have a conflict of interest by holding employment with the community pharmacy supplying the home(s).
General practitioner practices were included where they managed sufficient care home residents to support recruitment of our target of approximately 20 eligible participants.
Care homes needed to be primarily caring for adults aged ≥65 years and associated with a participating general practice. Homes were excluded if they already received a regular medication-focused review service (monthly or more) or were under formal investigation by a regulator [e.g. Care Quality Commission (CQC) for England].
Care home residents needed to be under the care of the participating GP practice if they were ≥65 years old, a permanent resident in a participating care home, prescribed one or more medications and able to provide (directly or through an appropriate representative) informed consent or advice. They were excluded if they were receiving end-of-life care or additional instructions on their residence (e.g. held securely) or were participating in another study.
Participant identification and recruitment
Pharmacist Independent Prescribers were identified using local networks and related GPs (if links already established) were recruited concurrently, followed by their relevant care home(s) who were approached by participating GPs. Where one single linked home had too few potential resident participants, up to two further homes were recruited.
Resident recruitment
General practitioners identified residents in participating care homes taking one or more medications and screened them against the study inclusion and exclusion criteria. Care home managers handed out invitation packs to potential residents, re-visited each resident after at least 24 hours and obtained verbal consent for the local researcher to approach them to discuss study participation. For residents who were considered to lack capacity, packs were posted to the resident’s next of kin. The process is summarised in Figure 7.
Randomisation and blinding
Randomisation was at triad level to minimise contamination occurring between two homes in the same practice where one received the intervention but the other did not. Residents were not individually randomised, as part of the intervention was for PIPs to help care homes improve their overall medication management processes. Randomisation was stratified by the four geographical areas, using a web-based electronic system integrated into the study database, in an allocation ratio of one intervention triad to one control triad. Because of the nature of the intervention, PIPs, care homes and GPs were not blinded to their allocation arm once the intervention began. Researchers, however, were blinded to arm allocation during the recruitment phase.
Six weeks were provided for PIP training to be undertaken and completed. Consequently, time zero for data collection purposes was standardised at 6 weeks after randomisation.
Intervention
This was delivered by trained PIPs for a period of 6 months and involved the PIP, in collaboration with the care home resident’s GP and care home staff, assuming responsibility for managing the medication of each resident, including
-
reviewing the resident’s medication and developing and implementing a PCP
-
assuming prescribing/deprescribing responsibilities
-
supporting systematic ordering, prescribing and administration processes with each care home, general practice and supplying pharmacy where needed
-
providing care home staff training
-
liaising with GP practice, care home and supplying community pharmacy.
We anticipated each PIP providing approximately 4 hours of intervention per week per 20 residents for 6 months.
Control: Triads allocated to the control arm received usual GP-led care, which could include pharmacist review/services to care homes where routinely provided, excluding those of an intensity equivalent to the study intervention.
Outcome measures
The primary outcome was fall rate per person over 6 months after time zero, as documented in care home falls record for those residents recruited into the trial only.
Secondary outcomes, all at 6 months after time zero unless stated otherwise:
-
Resident (i.e. self-report) or proxy resident quality of life (EQ-5D-5L) at 3 months and 6 months: utility scale where a score of 0 indicates equivalent to death and that of 1 indicates full health. 30
-
Proxy modified physical functioning score (Barthel): a score of 0 is most dependent on 20, which indicates least dependent. 47
-
DBI: a measure of anti-cholinergic and sedative drug exposure, which was collected through GP-recorded medication data: higher scores indicate greater anticholinergic potential and increased risk of drug-related morbidity. 46
-
Health service utilisation (and associated costs); notably unplanned hospital admissions in the past 6 months, at 6-month follow-up, collected from care home and GP records (see WP3 for health economics results of WP6).
-
Mortality.
Sample size
A sample size calculation indicated that 880 participants (440 in each arm) would detect a 21% decrease in fall rate from 1.50 per resident over 6 months with 80% power, at the 5% significance level and an assumed intraclass correlation coefficient not more than 0.05. With approximately 20 patients per triad, this equated to 44 triads. The relative reduction of 21% was half of that detected within a UK-based, pharmacist-led medication review service provided to care homes. 51 Furthermore, we assumed a loss to follow-up of 20% based on mortality and loss observed in the CAREMED study. 23
Statistical methods
All analyses were by intention-to-treat, and it was anticipated that the primary outcome (‘falls per resident’) would follow a Poisson distribution; hence, the between-arm comparison of falls was to be made using a Poisson Regression model. Data subsequently demonstrated that the best fit was in fact a negative binomial model and parameters were estimated using a generalised estimating equation approach adjusted for the clustered design. The final model included baseline fall rate, prognostic variables (specifically DBI, Barthel Index and Charlson scores) and home status (nursing/residential) with arm as a fixed factor.
Safety
Processes were developed for recording sudden unexpected serious adverse reactions (SUSARs), SAEs and AEs. SAEs were defined as inpatient hospitalisation and death requiring immediate reporting. If SAEs were believed to be related to the study intervention, then they were reported as SUSARs. We used a mixture of prospective and retrospective SUSAR notification by asking GPs to report SUSARs immediately, and retrospectively, and by our trial manager proactively contacting care homes each month to ask about SAEs. The resident’s GP was then assessed for causality of the SAE, and whether it was linked, or not, to the PIP intervention.
Concerns could also be confidentially raised by any member of care home staff using a dedicated email address.
A random (computerised number generation) 20% sample of the PCPs and associated resident documents were assessed by a study geriatrician, to ensure clinical appropriateness and safety.
Process evaluation
Complementary to the main RCT, a process evaluation was conducted,52 following MRC guidance. 7 This evaluation used a mixed methods approach to inform interpretation of trial findings and subsequent implementation, should the intervention be effective. Objectives were as follows: to provide description of the intervention in terms of quality, quantity and variability in delivery; to explore the effect of individual components on the primary outcome; to investigate the mechanisms of action; to describe views of the intervention (including training of PIPs and care home staff) from GP, care home, PIP, resident and relative perspectives; to describe the characteristics of each arm and to estimate how ‘normalised’ the intervention became.
A mix of quantitative (surveys of care home staff, GPs and PIPs, PIP activity logs, PCP review and the trial outcomes) and qualitative (interviews with care home staff, residents, GPs and PIPs) approaches were used. Data were collected relating to delivery of detailed tasks required to implement the new service, to collect data to confirm the mechanism of action as hypothesised in the logic model (see Appendix 6), to collect explanatory process data and data on contextual factors that could have facilitated/hindered effective and efficient delivery of the service. Detailed analysis of PCPs additionally involved determining which medication-related changes would be associated with the risk of falls guided by recent comprehensive systematic reviews. 53–55
All data were collected, from intervention arm participants only, after the study period for each home had finished. The tasks, aims, data and data sources are summarised in Appendix 1, Tables 18–21.
Interviews schedules were informed by normalisation process theory (NPT),56,57 and questionnaires included a set of NoMaD questions that translates NPT domains for survey use. 58 Interviews were conducted face-to-face or by telephone; all were audio recorded and transcribed verbatim. Thematic analysis of qualitative data was based on the NPT framework, but a complementary inductive approach enabled recognition of unexpected emergent themes. All data sets (qualitative and quantitative) were integrated to identify relationships, explain findings and identify optimal intervention contexts. 52 Full details are published elsewhere. 50
Ethics
Ethics approval was provided by East of England Central Cambridge Research Ethics Committee (for England and Northen Ireland) – Ref.: 17/EE/0360; and by Scotland A REC – Ref.: 17/SS/0118.
Protocol
This is published and is available at: https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-019-3827-0.
Results
As shown in Appendix 1, Figure 8 (CONSORT diagram), we recruited 49 triads (49 general practices, 49 PIPs and 72 care homes) between December 2017 and May 2019. Of these 49 triads, 25 were randomly allocated to the intervention arm and 24 to the control arm. There were 454 residents in the intervention arm and 428 residents in the control arm. Almost all losses were due to resident deaths (134/166 losses = 81%). Three intervention PIPs (12%) failed to deliver the service due to personal reasons.
Baseline comparison between arms is provided in Appendix 1 and Table 12. Whilst most variables including age, medications, admissions, DBI, Charlson comorbidity, and EQ-5D-5L proxy scores were similar between arms, the control arm had a slightly greater proportion of male residents (33% vs. 28%), and a considerably greater proportion in nursing home care (59% vs. 42%). In line with those findings, the intervention arm had a higher Barthel score (8.34 vs. 7.07 of 20, where higher scores imply greater independence) and those self-reporting EQ-5D (11% of participants) reported better health 0.50 versus 0.35 (scale 0–1, 1 = perfect health). Residents in the intervention arm had a mean number of falls of 0.78 in the previous 90 days compared with that of 0.57 in residents of the control arm. Follow-up data collection commenced in September 2018 and concluded in July 2020 (this was delayed by 4 months for the final triads due to COVID-19).
Primary outcome analysis
Although there were a greater number of falls recorded in the intervention arm in the 6-month follow-up period (697 vs. 538) and a higher crude rate of falling, when adjusted for baseline falls, no difference was observed between arms (RR = 1.00, 95% CI 0.73 to 1.36; p = 0.99). Further adjustment for key potential confounders reduced the RR, in favour of the intervention, to 0.91, although this too was not statistically significant (95% CI 0.66 to 1.26; p = 0.58; see Appendix 1, Table 13).
Similarly, there was no evidence of an effect on fall rate at 3 months. The RR favoured the intervention arm (RR = 0.86, 95% CI 0.63 to 1.19; p = 0.36), but this was not statistically significant.
Mortality
There were 66 (14.7%) deaths in the intervention arm compared with 71 (16.6%) deaths in the control arm, with a mean time to death of 109 versus 103 days. However, a Cox’s proportional hazards model indicated no evidence of a beneficial effect on the death rate (hazard ratio = 0.93, 95% CI 0.64 to 1.35; p = 0.68). Appendix 1 and Figure 9 illustrate the Kaplan–Meier survival curves.
Other secondary outcomes
The remaining secondary outcomes included DBI, hospitalisation, Barthel score (see Appendix 1, Table 14), and quality of life as measured using the EQ-5D (see Appendix 1, Table 15). Of these outcomes, the intervention improved (decreased) residents’ DBI by 8% from 0.72 at baseline to 0.66 at 6 months. By contrast, the DBI among control arm residents increased from 0.70 to 0.73. When analysed using a natural log transform, the rate ratio of DBI scores at 6 months between the intervention and control arms was 0.83 (95% CI 0.75 to 0.92; p < 0.001), suggesting that more effective deprescribing occurred in the intervention arm. No other secondary outcome showed a statistically significant difference. No evidence of a decrease in hospital admissions between arms was found (RR = 0.98, 95% CI 0.66 to 1.46; p = 0.93), nor was there any evidence of any difference between arms with respect to the Barthel Index at 6 months, although scores favoured the intervention arm (ratio of means = 1.20, 95% CI 0.96 to 1.49; p = 0.11).
Quality of life scores were collected as self-reported or by proxy, as shown in Table 15 (see Appendix 1). Few residents were able to report their own scores (only 6.5% at 6 months) and for those who could report, scores favoured the intervention arm at baseline by 0.14 points (>40% higher). There was no evidence of any effect of the intervention on self-reported EQ-5D scores at either 3 months (mean difference = 0.079, 95% CI: −0.028 to 0.186; p = 0.146) or 6 months (mean difference = 0.010, 95% CI: −0.115 to 0.135; p = 0.873). Proxy reported EQ-5D scores were available from almost 70% of residents. These scores were very similar at baseline between arms and changed very little through follow-up, with a small, non-significant difference observed at 3 months (mean difference = –0.017, 95% CI −0.073 to 0.039; p = 0.556), and 6 months (mean difference = 0.030, 95% CI −0.021 to 0.080; p = 0.249).
Exploratory/additional analyses
Several additional post hoc analyses were performed on the primary outcome to explore our findings further. First, those lost to follow-up, or died within 10 days or 28 days of baseline, were excluded on the basis that those residents would have been unlikely to benefit from any intervention. Excluding these individuals left 811 and 809 residents, respectively (i.e. over 92% of the study sample). The RR of falls between arms was almost identical in both analyses at 0.91 (95% CI 0.66 to 1.26), and neither was statistically significant (p = 0.58). These estimates did not differ substantially from those including the full study sample.
Some residents fell very often, with one resident falling 59 times during follow-up. This led to a highly skewed distribution. Excluding those with the very highest rate of falls (the top 5%) also made little difference to the estimated RR (0.96, 95% CI 0.73 to 1.26; p = 0.78). The analysis was also conducted removing residents who were immobile, on the basis that they were not able to fall and, therefore, could not contribute to the primary outcome. Removing these individuals left 60% of the sample in the model (n = 526), but again little difference was observed (RR = 1.07, 95% CI 0.79 to 1.45; p = 0.66).
Finally, dividing residents into those who fell before baseline and those who did not fall found quite different rates of falling during follow-up, that is, a rate of 1.3 falls per annum amongst ‘non-fallers’ and 7.3 falls per annum amongst ‘fallers’. However, there was no evidence of a differential effect between the intervention and control arms with respect to these two arms: 1.27 and 1.33 falls per annum for non-fallers in the intervention versus control arms; and 7.41 and 6.12 falls per annum amongst fallers in each arm, respectively. An interaction term (faller status*arm) was not statistically significant (p = 0.16).
Process evaluation
Overall, the PIPs adhered to the service specification and delivered all aspects of the role, although to varying degrees in different homes according to need. Some care home managers had said there was no need for any training to be delivered to staff. Across all PIPs, 24% of their time was spent face-to-face with residents, 43% of their time spent on resident-related desk activities; 24% of their time spent on other general activities in the care home and 10% of the time spent on travelling. In questionnaire responses, eight reported that they personally visited the care home monthly and eight visited weekly. Regarding sufficiency of time, five stated that 4 hours was not enough, eight found it sufficient and three stated it was too much time. Resident-related prescribing changes are summarised in Appendix 1, Table 16, and the data are based on the analysis of 368 completed PCPs with a total of 668 interventions. British National Formulary therapeutic categories are reported in Appendix 1, Table 17. Of the 566 clinical interventions, 202 (35%) were related to medication likely to cause falls, of which medication discontinuation (94) and dose reduction (54) would reduce fall risk (148/566; 26.1%). By contrast, there were dose increases (17), medication changes (9) and new medication initiation (20), which increased the risk of falls (46/566; 8.1%). A detailed PCP review also suggested that the PIPs identified the majority of medication changes that could have reduced a resident’s fall risk.
No safety concerns were identified from the review of PCPs or independent assessment of SAEs. No reports of safety concerns were received through the bespoke email address.
When interviewed, the GPs were very positive about the pharmacists’ interventions (saved them time, or reassured them about medication appropriateness and safety) as were the care home managers who sometimes specifically commented how they had observed the benefit of the reduced medications on the patient (see example quotes in Appendix 1, Box 4) as well as increased efficiencies.
For optimum implementation, all stakeholders need to believe in the idea of the service and actively support it. A detailed analysis of the different triads against this framework illustrates how this worked in practice, as shown in Appendix 1, Box 5.
Finally, there were 26 questionnaires returned (PIP × 16, GP × 8, CHM × 2) and 38 interviews conducted (PIP × 14, GP × 8, CHM/staff 15, resident 1). Both questionnaires and interviews asked about satisfaction with specific aspects of the role and overall satisfaction. Very high overall satisfaction was reported (see Appendix 1, Figure 10) reflecting the vast majority of quotes (see Appendix 1, Box 6).
Discussion
This large, rigorously conducted, cRCT did not demonstrate that PIPs who took responsibility for medication management for older patients in care homes could reduce falls significantly. Moreover, health economic analysis showed an overall cost per resident of £280, with no effect on quality of life.
However, the PIPs did contribute to a decrease in the residents’ mean DBI compared with that of the controls, suggesting the occurrence of effective deprescribing, and the data suggest there were savings in medication costs. In the longer term, a reduced DBI would have theoretical health benefits. All outcomes’ point estimates, after adjustment for baseline differences, including Barthel index, mortality, hospital admission, proxy quality of life and falls favoured the intervention arm although not reaching statistical significance.
Drug burden is associated with increased mortality,59 falls,60 hip fractures,61 frailty62 and reduced quality of life. 62 Consequently, the significant reduction in DBI is predictive of improved resident outcomes. With data on DBI and risk based on a minimum 12 months of observation,60–63 the 6-month follow-up in this study may again have been unlikely to fully realise clinical improvements.
Whilst we should be cautious in overinterpreting such findings, the data combined with the generally very positive views from the stakeholders and confirmed safety suggest that this intervention merits further refinement and investigation. In particular, the facilitating contextual factors need to be further explored in light of the ongoing NHS roll out of this and similar pharmacist-led medication-related services.
Involvement of patients and the public
We worked with our local public and patient in research (PPIRES) arm from the outset with feedback received on the original idea from PPIRES members and subsequent involvement in the grant application as it developed. At that early stage, with support from our care home expert (HH), we visited a care home to undertake a focus group of residents to listen to their views regarding the idea underpinning the project and what we needed to consider from a resident’s perspective. They were clear that whilst they had no concerns regarding a pharmacist prescriber looking after their medicines, they wanted residents to be involved in all decisions. Training regarding this was incorporated into the final model.
On receipt of the grant, we recruited four patient and public involvement members, with an interest in care homes, through PPIRES: two members for the management group and to support the process as it developed [Kate Massey (KM) and Christine Handford (CHD)] and two members for the independent steering committee (Joyce Groves and Elaine Bounds). KM and CHD were invited to, and actively involved in, all project management meetings, with pre-meetings organised with team members beforehand to inform them of the main issues. Their views were sought at all points in the process to ensure that they were an integral part of the research process.
Whilst preparing our protocols for submission for ethical approval, we actively involved KM and CM in reviewing participant information leaflets and consent forms. KM and CHD reviewed qualitative data, which had been generated within WP4 to ensure our interpretations matched theirs. The insights of both KM and CHD increased our confidence in what we believed was being said but, at times, provided a different perspective.
Kate Massey and CHD provided excellent feedback on the training materials that we had created for the pharmacists to train care home staff, enabling us to be confident that the information was pitched at an appropriate level. We also routinely invited KM and CHD to review abstracts and papers before publication.
Kate Massey wrote and published an editorial on her positive experiences of working within CHIPPS, promoting this to the pharmacy research community. 45 Very sadly and unexpectedly, Kate passed away before the article was published. Three years into the project, we therefore recruited Janet Gray (JG) as her replacement. CHD and JG were actively involved in preparing our dissemination strategy and identifying the areas within which they could most effectively participate. As a result, we engaged with the Patients’ Association to seek their support in the grant’s final year with respect to dissemination of the findings, allowing Frances Hollwey to join the project team. CHD, JG and FH were all active members of the small team responsible for organising our final dissemination event and contributed to the session with pre-recorded videos where they expressed their views and opinions on the process.
In summary, our PPI members have been an integral part of the CHIPPS process, engaging effectively and helping the research team throughout all stages.
Reflections
Within this report, we present the results of 5 years of work where we have carefully considered each element of the research process and assiduously followed MRC guidance. 6 The model of care, which we developed, was well received by all stakeholders. We developed a COS, which can be used internationally for all interventions of a similar nature, and a unique training programme, which supported competent service delivery. Our feasibility study found that our proposed service specification was likely acceptable and the research design was appropriate. We recruited to target, had sufficient power at the end of the trial to test our hypothesis and, excepting COVID-19, we would have completed the programme of work to time.
We originally requested a 6-month no-cost extension recognising that, given the complexity of the data, we would not have completed the final report within the required 2 weeks of project completion. Fortuitously, this extension enabled final data collection from two Scottish sites, recruited at the end of Phase III, whose 6-month follow-up was scheduled during the first COVID-19 lockdown. R&D permission was sought and gained to collect the data remotely in July 2020, and following data cleaning, analysis started in August 2020.
With the delivery of a successful project, the focus of the reflection is therefore on the final outcome, as the intervention did not have a significant effect on our primary outcome measure. We note however that, with the exception of the QALY scores, once baseline data and confounding factors were considered, the differences identified in all outcomes favoured the intervention arm, although only the change in DBI was statistically significant.
Given what we learned from the listening exercise in WP1, it was unsurprising that the predominant messages from the earlier WPs were the need for the PIP to integrate into these teams, as opposed to being ‘an external body coming in to improve practices’ and that effective communication with all stakeholders would be the key to their success.
In retrospect, a 12-month intervention, rather than a 6-month one, would have allowed the PIPs to have more time to not only integrate but also to ensure that all medication-related systems were appropriate within the home alongside their work to manage patients’ medication. Furthermore, the PIPs would have had more time to prioritise their activities and interventions.
The selection of a suitable primary outcome measure for a generic medicine–related intervention is difficult, and this had been identified as a potential failure point within the application process by one of the original reviewers. WP2 identified three possible primary measures (falls, hospitalisation and quality of life), but within the project budget, ‘falls’ was the only practical choice. Our decision was, however, bolstered by recent systematic reviews, suggesting that ‘falls’ had been significantly reduced by pharmacist interventions in care homes. 64,65
Through our process evaluation, we found that the PIPs made appropriate medication changes for the majority of residents: more than a quarter of these medication changes had the potential to reduce falls, and approximately 5% had the potential to increase them. Consequently, as an indicator of intervention effectiveness, it was proximal to the primary outcome for only one third of the PIP interventions. Furthermore, when considering the relationship between medication and falls, the two are not co-located chronologically, that is, by stopping a medicine known to be associated with falls, a reduction in falls is not immediately seen. It is the probability of falling, which is reduced, and this effect may not be seen, if at all, until sometime in the future. Consequently, again the 6-month follow-up may have been too short.
The introduction of the Medicines Optimisation in Care Homes (MOCH) initiative by NHS England mid-way through CHIPPS, where national funding was found to employ pharmacists to work within care homes, had the potential to contaminate our results. By raising standards in homes across the board, it could theoretically have created a ceiling effect, thereby minimising our opportunity to enhance care within the intervention arm, but we have no evidence to suggest this occurred.
The number of interventions per resident was slightly lower than that reported in the SHINE Project, where a single medication review was performed. 66 This may reflect the fact that homes were already receiving pharmacist visits and that this was not precluded from the CHIPPS control homes. We perhaps should have captured the nature of this activity in the control arms more carefully at the end of the study as control arm care may have been better than expected.
Limitations
Work package 1
Minimal representation of residents and relatives in intervention-development workshops, held on university premises outside their usual residence. Participant self-selection may bias them toward favourable expectations for the PIP role.
Work package 2
Delphi panel participants, drawn from the research team, were located in the UK, which limited generalisability. We included just two PPI representatives in our panel where other COS developers have recruited PPI representatives and professionals to their Delphi panels in a 2:1 ratio. 67,68 Outcomes that had not received consensus following the second round were excluded from the COS. Further rounds may have resulted in consensus for more outcomes. For pragmatic reasons, a final face-to-face ‘consensus meeting’ did not occur, potentially limiting further consideration of some outcomes. This approach has been used in other COS research. 67–69
Work package 3
We were unable to collect data in relation to all items of resource use. Assumptions were made about the GP time saved due to no longer approving prescriptions, but there could have been other differences, for example, shorter GP medication reviews and length of home visits. The analysis also did not include any time implications for care home staff. Estimated PIP intervention costs were also based on data reported by PIPs in the activity logs, which suggested under-reporting as mean reported time tended to be lower than the time remunerated for.
Work package 4
The training package was tested for final validation purposes only on 25 PIPs.
Work package 5
Research processes and service specification tested in one triad only in each location, that is, four PIPs, four general practices and six care homes in total.
Work package 6
Whilst GP selection of care homes may have introduced an element of selection bias, the number of homes available to them was frequently limited and there was no evidence of obvious selection bias in the final cohort of randomised homes.
Three PIPs (12%) failed to deliver the intervention.
Follow-up was carried out for 6 months only, and this may have been an insufficient time period. Some residents’ PCPs had agreed only 3 months into the intervention period. Furthermore, where medication changes reduced the likelihood of falls, this is rarely an immediate effect.
The primary outcome of falls was proximal to only a third of the PIP interventions, and therefore, more focused inclusion criteria based on the risk of falls due to medication may have been more appropriate.
The concurrent national roll out of MOCH in England may have reduced intervention opportunities.
Conclusions
WP1 service specification development
To effectively embed this new pharmaceutical care service in care homes, stakeholders highlighted that securing PIP role acceptability and viability would require time and steps to ensure that all involved could ‘understand each other’s systems’. This should address contextual and implementation barriers and identify what practices were feasible for addressing residents’ medication-related safety issues. GP and care homes required reassurance that the model was likely safe.
WP2 outcome identification and selection
Using the evidence-based and stakeholder views, we conducted a Delphi consensus exercise to identify outcomes that should be measured to evaluate the service and published this as a COS for trials testing prescribing interventions in care homes. This was registered on the COMET database. The outcomes meeting our criteria were subsequently feasibility tested in WP5.
WP3 health economics
The methods used to estimate cost-effectiveness in WP6 were developed through WP1 to WP4 and tested in WP5. The mean incremental cost of the PIP intervention, compared with that in control participants, was estimated to be £280. This suggests that the cost of the PIP intervention might be partially offset by, for example, lower medication costs. However, no improvement in QALYs was identified, and consequently, based on the results presented, the PIP intervention was not estimated to be cost-effective.
WP4 training package
The CHIPPS training package consisted of 2-day face-to-face training, a PDF, personal development planning with supported mentorship, time to ‘understand each other’s systems’ and independent formative assessment by oral viva, which enabled the PIPs to practice safely.
WP5 feasibility study
Feasibility testing demonstrated that the PIP service was acceptable and practical. Trial processes for recruitment, retention, data collection and choice of outcome measures were confirmed. A robust process for monitoring safety of the PIP medication changes was developed, which involved independent assessment of all deaths and hospitalisations, independent review of PIP-created PCPs through sampling and provision of an independent email address to enable reporting of safety concerns.
WP6 definitive trial with internal pilot
No differences were found in the primary outcome of falls. This can be explained by the relatively small proportion of interventions that were identified as potentially affecting this outcome. The intervention did significantly reduce residents’ drug burden. No differences in the other secondary outcomes were identified, although all outcomes favoured the intervention arm. Proactive monitoring found the model of care to be safe.
Frequent discontinuation of medication used within the central nervous system explained the reduction in drug burden seen. Most, but not all, PIPs delivered the intervention as intended. The view of stakeholders was generally very positive. Care home staff valued the pharmacist’s advice and prompt resolution of queries. GPs reported reduced workload, improved patient safety and less inappropriate prescribing. To be successful in integrating the new systems of working, PIPs need to become fully integrated into GP and care home teams.
Recommendations for future research
Future research should
-
determine the optimal model, processes and time for integrating pharmacists effectively into care home and GP practice teams
-
determine the optimum follow-up time for interventions focused on fall reduction
-
identify mechanisms of action leading to proactive deprescribing to enhance current practice
-
develop an outcome measure that is meaningful to patients and practical for researchers, which accurately captures the impact of pharmacist interventions.
Implications for practice and decision makers
The challenge of optimising medication regimens in care homes is well recognised, and the CQC identified the proper and safe use of medicines as one area of care that requires regular review and that continues to fall below the expected standards (see Appendix 1, Box 7). 70 Pharmacists have been identified as health care professionals with the skills and knowledge to improve care home medication use (see Appendix 1, Box 8). 71
As a result, pharmacists increasingly work in the care home environment ranging from 12-monthly visits to assuming full responsibility for medication within the home. However, these service changes have been introduced without robust evidence. In the SHINE project66 on which the Medicines Optimisation in Care Homes project in England71 was based, there was an estimated savings of £100 per resident per annum but no evidence of clinical effectiveness. Savings were based on medication costs only under the assumption that residents lived for 1 year on average.
The CHIPPS RCT demonstrated a significant reduction in individual DBI, thus lowering the likelihood of long-term negative drug effects on morbidity and mortality. Although the PIPs saved some of the GP time, our model of a session per week per 20 residents did not save money once the cost of the pharmacist time and additional monitoring costs were considered. Given that the PIPs visited each home every week over 6 months, it was relatively inexpensive and a reduction in DBI could be used to justify the importance of integrating a prescribing pharmacist into the care home setting, providing the amount of resource required was believed to justify this outcome.
Feedback from GPs and care home staff provided evidence that this pharmacist service is valued by, and acceptable to, the key stakeholders. GPs felt reassured about the safety and appropriateness of a resident’s medication regimen and reported time saved, with independent prescribing authority core to this. Care home managers and staff reported that residents received better care, many improved visibly and systems became more efficient. Thus, CHIPPS has provided (across the programme) a holistic account of the more intangible benefits of this new pharmacy service. This is particularly relevant to jurisdictions where there is not yet a formal programme for involving pharmacists in care homes, such as Scotland (whose care home policy does not mention a pharmacy role) and Northern Ireland.
A second important policy and practice relevant finding is a theoretically informed understanding of the optimum way to implement the service. Mutual trust and a strong and established relationship between the PIP and the responsible GP are key; the care home staff also need time to establish that mutual trust with the pharmacist too. All stakeholders need a shared understanding of the service and a will to make it work.
The PIP service was, however, not found to be cost-effective based on standard UK government assessment criteria. Therefore, the CHIPPS model, as tested in the trial, cannot be recommended for adoption and implementation at this time. However, the reduction in DBI, a finding that all other measures favoured the intervention, suggests the merit in assessing this intervention for longer (c. 12 months) and with a stronger primary outcome.
Acknowledgements
This research was supported by the National Institute for Health and Care Research (NIHR) Yorkshire and Humber Patient Safety Translational Research Centre (NIHR YH PSTRC). The views expressed in this article are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
This report is dedicated to Kate Massey, an active and enthusiastic member of the CHIPPS patient and public involvement team who sadly passed away during the delivery of this study.
Contributions of authors (see Appendix 7)
David Wright (https://orcid.org/0000-0003-3690-9593) (Professor of Pharmacy Practice) co-led WP4 to develop the training package for pharmacist independent prescribers and was Co-Chief Investigator for the CHIPPS Programme.
Richard Holland (https://orcid.org/0000-0002-4663-6923) (Professor of Public Health Medicine) co-led WP5 to test and refine the service specification and proposed study processes and co-led WP6 to perform a definitive trial and was Co-Chief Investigator for the CHIPPS Programme.
David Phillip Alldred (https://orcid.org/0000-0002-2525-4854) (Professor of Medicines Use and Safety) co-led WP2 to identify the outcomes of the trial and was Principal Investigator for Yorkshire and Humber.
Christine Bond (https://orcid.org/0000-0003-0429-5208) (Professor of Primary Care, Pharmacy) co-led WP5 to test and refine the service specification, proposed study processes and co-led WP6 to perform a definitive trial, led the WP6 process evaluation and was Principal Investigator for Scotland.
Carmel Hughes (https://orcid.org/0000-0002-4656-6021) (Professor of Primary Care, Pharmacy) co-led WP2 to identify the outcomes of the trial and was Principal Investigator for Northern Ireland.
Garry Barton (https://orcid.org/0000-0001-9040-011X) (Professor of Heath Economics) led WP3 to estimate the cost-effectiveness of the intervention and conducted analysis of the economic data.
Fiona Poland (https://orcid.org/0000-0003-0003-6911) (Professor of Social Research Methodology) co-led WP1 to develop the service specification and supported qualitative analysis in WP6.
Lee Shepstone (https://orcid.org/0000-0001-5524-7818) (Professor of Medical Statistics) conducted statistical analysis and prepared the results for publication.
Antony Arthur (https://orcid.org/0000-0001-8617-5714) (Professor of Nursing Science) provided nursing practice expertise throughout the programme.
Linda Birt (https://orcid.org/0000-0002-4527-4414) (Senior Research Associate) conducted process evaluation and qualitative analysis.
Jeanette Blacklock (https://orcid.org/0000-0001-5845-3182) (Senior Research Associate) conducted local recruitment of Norwich sites and participants and collected study data.
Annie Blyth (https://orcid.org/0000-0001-5380-5353) (Senior Programme Coordinator & Research Fellow) co-coordinated the CHIPPS programme.
Stamatina Cheilari (https://orcid.org/0000-0003-2701-2020) (Research Associate in Health Economics) contributed to analysis of the economic data
Amrit Daffu-O’Reilly (https://orcid.org/0000-0002-3022-4596) (Senior Research Fellow) conducted local recruitment of Leeds sites and participants and collected, analysed study data and contributed to writing WP2.
Lindsay Dalgarno (https://orcid.org/0000-0001-9994-5861) (Research Fellow) conducted local recruitment of Scotland sites and participants, collected study data and contributed to the WP6 process evaluation analysing and interpreting qualitative datasets.
James Desborough (https://orcid.org/0000-0001-5807-1731) (Senior Lecturer in Pharmacy) co-led WP1, contributed to the delivery of training in WP4 and aided in the delivery of WP5 and WP6 in Norwich.
Joanna Ford (https://orcid.org/0000-0002-8480-393X) (Consultant Geriatrician) contributed to the training, provided specialist advice on medication reviews and working with primary care–based pharmacists and reviewed pharmaceutical care plans and related records to ensure the safety of the participants.
Kelly Grant (https://orcid.org/0000-0001-5319-8127) (Trial Statistician) conducted statistical analysis.
Janet Gray (Public and Patient Representative) provided service user perspectives throughout the programme and developed plain language summary.
Christine Handford (Public and Patient Representative) provided service user perspectives throughout the programme and developed plain language summary.
Bronwen Harry (https://orcid.org/0000-0002-6938-567X) (Junior Clinical Trial Manager) supported the delivery of the definitive trial.
Helen Hill (https://orcid.org/0000-0002-8036-1935) (Independent Consultant) provided expertise in the development of medication management policies and shared experiences of management within the adult social care and care of older people settings.
Jacqueline Inch (https://orcid.org/0000-0003-3805-2060) (Research Fellow) conducted local recruitment of Scotland sites and participants, collected study data and contributed to the process evaluation.
Phyo Kyaw Myint (https://orcid.org/0000-0003-3852-6158) (Professor of Medicine of Old Age) (co-applicant) provided local Scotland support and reviewed pharmaceutical care plans and related records to ensure the safety of the participants.
Nigel Norris (https://orcid.org/0000-0002-9214-2692) (Professor of Education) co-led WP4 to provide qualitative analysis support throughout the work package.
Maureen Spargo (Research Fellow) conducted local recruitment of Northern Ireland sites and participants, collected study data and contributed to process evaluation.
Vivienne Maskrey (Retired Senior Programme Coordinator & Research Fellow) co-coordinated the CHIPPS programme until 31 October 2018.
David Turner (https://orcid.org/0000-0002-1689-4147) (Senior Research Fellow in Health Economics) conducted analysis of economic data.
Laura Watts (https://orcid.org/0000-0002-1816-966X) (Senior Programme Coordinator & Research Fellow) co-coordinated the CHIPPS programme.
Arnold Zermansky (General Practitioner, Research Fellow) provided general practice expertise and supported local recruitment of general practices.
Ethics statement
Ethics approval was provided by East of England Central Cambridge Research Ethics Committee (for England and Nothern Ireland) – Ref.: 17/EE/0360; and by Scotland A REC – Ref.: 17/SS/0118.
Data-sharing statement
We will make data available to the scientific community with as few restrictions as feasible, while retaining exclusive use until the publication of major outputs. All data requests will be submitted to the corresponding author for consideration.
Data from the randomised controlled trial described in WP6 have been added to the Virtual International Care Homes Trials Archive (VICHTA). Access to anonymised data may be granted following review and appropriate agreements being in place. It is envisaged VICHTA data will be available at www.virtualtrialsarchive.org from December 2023 [see Irvine L, Burton JK, Ali M, Quinn TJ, Goodman C. Protocol for the development of a repository of individual participant data from randomised controlled trials conducted in UK adult care homes (The Virtual International Care Homes Trials Archive, VICHTA)]. Submitted to BMC Trials, August 2020 www.dachastudy.com).
The authors will be happy to discuss potential collaborations regarding other data and will make anonymised data available on that basis.
Publications
| WP | Date | Venue | Title | Output | URL (where available), *Also listed as a reference in References in the report | Citation |
|---|---|---|---|---|---|---|
| Prog | 4 December 2015 | RCN RPS Joint Summit | CHIPPS: Care Homes Independent Pharmacist Prescribing Service: Development and delivery of a cluster randomised controlled trial to determine both its effectiveness and cost-effectiveness. | Poster | Publications and Conferences – Groups and Centres – UEA Scroll down to download the document | Wright D on behalf of the CHIPPS team. CHIPPS: Care Homes Independent Pharmacist Prescribing Service: Development and delivery of a cluster randomised controlled trial to determine both its effectiveness and cost-effectiveness. |
| WP1 | 22 July 2016 | Health Services Research in Pharmacy Practice conference (HSRPP) | GP views on the potential role for pharmacist independent prescribers within care homes: Care Homes Independent Pharmacist Prescribing Study (CHIPPS) | Presentation | Abstracts of the 19th International Social Pharmacy Workshop, 19–22 July 2016, Aberdeen, UK: International Journal of Pharmacy Practice: Vol 24, No. S2 https://onlinelibrary.wiley.com/toc/20427174/2016/24/S2 Select Oral Abstracts/PDF and scroll down to page 6 |
Bond C, Lane K, Poland F, Maskrey V, Blyth A, Desborough J, Barton G, Alldred D, Hughes C, Arthur A, Myint P, Massey K, Holland R, Wright D. GP Views on the potential role for pharmacist independent prescribers within care homes: Care Homes Independent Pharmacist Prescribing Study (CHIPPS). Journal of Pharmacy and Practice, 2016, 24:S2 |
| WP1 | 6–8 July 2016 | British Society of Gerontology (BSG) conference | Envisioning pharmacists in care homes for older people: inter-disciplinary research to develop a new role | Poster | https://research-portal.uea.ac.uk/en/publications/envisioning-pharmacists-in-care-homes-for-older-people-inter-disc | Lane K, Alldred D, Blyth A, Bond C, Desborough J, Holland R, Hughes C, Maskrey V, Millar A, Myint P, Wright D, Poland F. Envisioning pharmacists in care homes for older people: Inter-disciplinary research to develop a new role. BSG; 2016, Communities in Later Life: Engaging with Diversity Conference Handbook |
| WP1 | 29 August 2016 | International Social Pharmacy Workshop Conference – SP16 | Care Homes Independent Pharmacist Prescribing Study (CHIPPS): GP views on the potential role for pharmacist independent prescribers within care homes | Presentation | https://onlinelibrary.wiley.com/doi/epdf/10.1111/ijpp.12278 Scroll down to page 6 | Bond C.M, Maskrey V, Alldred DP, Blyth A, Daffu-O’Reilly A, Inch J, Millar A, Notman F, Hughes C, Holland R, Wright D. Care Homes Independent Pharmacist Prescribing Study (CHIPPS): GP views on the potential role for pharmacist independent prescribers within care homes. International Journal of Pharmacy and Practice 2016: S2, page 6 |
| WP5 | 12–14 July 2017 | SAPC | Care Homes Independent Pharmacist Prescribing Study (CHIPPS). Feasibility study early experiences | Presentation | https://sapc.ac.uk/conference/2017/abstract/care-homes-independent-pharmacist-prescribing-study-chipps-feasibility | Bond C.M, Maskrey V, Alldred DP, Blyth A, Daffu-O’Reilly A, Inch J, Millar A, Notman F, Hughes C, H0olland R, Wright D. Care Homes Independent Pharmacist Prescribing Study (CHIPPS). Feasibility Study early experiences. SAPC ASM 2017, 3A.5 |
| WP4 | 29 August 2016 | RPS Royal Pharmaceutical Society | Development of a training plan for pharmacists to assume responsibility for medicines management in care homes: Results from a rapid review of the literature | Poster | https://onlinelibrary.wiley.com/toc/20427174/2016/24/S3 Select ‘Poster/PDF’ and scroll down to page 60, No. 0083 |
Wright D, Norris N, Maskrey V, Blyth A, Lane K, Arthur A, Desborough J, Holland R, Poland F, Bond C, Myint P, Hughes C, Millar A, Alldred D, Massey K. Development of a training plan for pharmacists to assume responsibility for medicines management in care homes: Results from a rapid review of the literature. International Journal of Pharmacy and Practice, 2016, Supplement 3, no. 0083 |
| WP2 | 5 September 2016 | RPS Royal Pharmaceutical Society | Development of a core outcome set (COS) for studies relating to prescribing in care homes: The Care Homes Independent Pharmacist Prescriber Study (CHIPPS) | Poster | https://onlinelibrary.wiley.com/toc/20427174/2016/24/S3 Select ‘Medicines Optimisation/PDF’ and scroll down to page 17, No. 0161 |
Millar A, Hughes C, Alldred D, Wright D, Holland R, Maskrey V, Blyth A, Desborough J, Norris N, Arthur A, Barton G, Lane K, Poland F, Shepstone L, Bond C, Myint P, Massey K, Ford J. Development of a core outcome set (COS) for studies relating to prescribing in care homes: The Care Homes Independent Pharmacist Prescriber Study (CHIPPS). International Journal of Pharmacy and Practice, 2016, Supplement 3, no. 0161 |
| WP2 | 28 September 2016 | International Day of the Older Person: Medicines Optimisation Showcase | CHIPPS: Care Home Independent Pharmacist Prescribing Study | Poster | Publications and Conferences – Groups and Centres – UEA Scroll down to download the document | Wright D, Holland R, Alldred D, Arthur A, Barton G, Blyth A, Bond C, Daffu-O’Reilly A, Desborough J, Handford C, Hill H, Inch J, Lane K, Maskrey V, Massey K, Myint PK, Norris N, Notman F, Poland F, Shepstone L, Small I, Swart AM, Symms C, Turner D, Zermansky A. CHIPPS: Care Home Independent Pharmacist Prescribing Study |
| WP1 | 5–7 October 2016 | European Union Geriatric Medicine Society (EUGMS) | Integrating independent pharmacist prescribers into care homes: Messages from a year of listening and learning | Presentation | Publications and Conferences – Groups and Centres – UEA Scroll down to download the document | Desborough J. Integrating independent pharmacist prescribers into care homes: Messages from a year of listening and learning |
| WP1, WP2, WP4 | 6 November 2016 | RPS Royal Pharmaceutical Society | Development and delivery of a cluster randomised controlled trial to determine both its effectiveness and cost-effectiveness | Presentation | Publications and Conferences – Groups and Centres – UEA Scroll down to download the document | Wright D on behalf of the CHIPPS team. Development and delivery of a cluster randomised controlled trial to determine both its effectiveness and cost-effectiveness |
| WP1, WP2, WP4 | 29 November 2016 | National Care Homes Research and Development Forum | Development and delivery of a cluster randomised controlled trial to determine both its effectiveness and cost-effectiveness | Presentation | Publications and Conferences – Groups and Centres – UEA Scroll down to download the document | Wright D on behalf of the CHIPPS team. Development and delivery of a cluster randomised controlled trial to determine both its effectiveness and cost-effectiveness |
| WP1, WP2, WP4 | 17 January 2017 | Primary Care Pharmacy Association (PCPA) Care Homes Group Symposium 2017 | Development and delivery of a cluster randomised controlled trial to determine both its effectiveness and cost-effectiveness | Presentation | Publications and Conferences – Groups and Centres – UEA Scroll down to download the document | Wright D on behalf of the CHIPPS team. Development and delivery of a cluster randomised controlled trial to determine both its effectiveness and cost-effectiveness. |
| WP1 | 26 January 2017 | National Conference of Scottish Departments of General Practice (NADEGS) | GP views on the potential role for pharmacist independent prescribers within care homes: Care Homes Independent Pharmacist Prescribing Study (CHIPPS): ‘There has to be something in it for me’ | Presentation | n/a | n/a |
| WP2 | 30 January 2017 | The Pharmaceutical Care Network Europe | Developing core outcome sets for pharmaceutical care | Workshop | n/a | n/a |
| WP2 | 12 April 2017 | Trials | Development of a core outcome set for effectiveness trials aimed at optimising prescribing in older adults in care homes | Paper | https://doi.org/10.1186/s13063-017-1915-6 * Reference number16 |
Millar A, Daffu-O’Reilly A, Hughes CM, Alldred DP, Barton G, Bond CM, et al. Development of a core outcome set for effectiveness trials aimed at optimising prescribing in older adults in care homes. Trials. 2017;18(1):175 |
| WP5 | 4 September 2017 | RPS Royal Pharmaceutical Society | Care Homes Independent Pharmacist Prescribing Study (CHIPPS): Early experiences | Presentation | Publications and Conferences – Groups and Centres – UEA Scroll down to download the document | Bond C on behalf of the CHIPPS team. Care Homes Independent Pharmacist Prescribing Study (CHIPPS): Early experiences |
| WP5 | 14 September 2017 | Community Pharmacy Scotland (CPS) – University Research Day | Care Homes Independent Pharmacist Prescribing Study (CHIPPS): Feasibility Study – Early experiences | Poster | Publications and Conferences – Groups and Centres – UEA Scroll down to download the document | Bond C, Notman F, Inch J on behalf of the CHIPPS team. Care Homes Independent Pharmacist Prescribing Study (CHIPPS): Feasibility study – Early experiences |
| WP5 | 1 October 2017 | RPS Royal Pharmaceutical Society Workshop at the Scottish National Seminar | Care Homes Independent Pharmacist Prescribing Study (CHIPPS): Feasibility study | Presentation | Publications and Conferences – Groups and Centres – UEA Scroll down to download the document | Frances Notman on behalf of CHIPPS team. Care Homes Independent Pharmacist Prescribing Study (CHIPPS): Feasibility Study |
| WP5 | 5 December 2017 | RPS Winter Summit | Care Homes Independent Pharmacist Prescribing Study (CHIPPS): experiences from a non-randomised feasibility study | Presentation | Publications and Conferences – Groups and Centres – UEA Scroll down to download the document | Bond C on behalf of CHIPPS team. Care homes Independent Pharmacist Prescribing Study (CHIPPS): experiences from a non-randomised feasibility study |
| WP1, WP2, WP4 | 20 September 2017 | CRN Eastern | Development and delivery of a cluster randomised controlled trial to determine both its effectiveness and cost-effectiveness | Presentation | Publications and Conferences – Groups and Centres – UEA Scroll down to download the document | David Wright on behalf of CHIPPS team. Development and delivery of a cluster randomised controlled trial to determine both its effectiveness and cost-effectiveness |
| WP5 | 31 January 2018 | Primary care Pharmacy Association (PCPA) Care Homes Group Symposium 2018 | Care Homes Independent Pharmacist Prescribing Study (CHIPPS): Early experiences | Presentation | Publications and Conferences – Groups and Centres – UEA Scroll down to download the document | Wright D on behalf of the CHIPPS team. Care Homes Independent Pharmacist Prescribing Study (CHIPPS): Early experiences |
| WP5 | 18 March 2018 | NHS Excellence in Pharmaceutical Care in Grampian One day Conference | Care Homes Independent Pharmacist Prescribing Study (CHIPPS). Feasibility study | Poster | n/a | n/a |
| WP5 | 23 July 2018 | ISPW – International Social Pharmacy Workshop | Care Homes Independent Pharmacist Prescribing Study (CHIPPS): experiences from a non-randomised feasibility study | Presentation | Publications and Conferences – Groups and Centres – UEA Scroll down to download the document | Bond C on behalf of CHIPPS team. Care-homes Independent Pharmacist Prescribing Study (CHIPPS): experiences from a non-randomised feasibility study. |
| WP5 | 6 September 2018 | FIP World Congress of Pharmacy and Pharmaceutical Sciences | Care Homes Independent Pharmacist Prescribing Study (CHIPPS): experiences from a feasibility study | Poster | Publications and Conferences – Groups and Centres – UEA Scroll down to download the document | Bond C, Notman F, Inch J on behalf of the CHIPPS team. Care Homes Independent Pharmacist Prescribing Study (CHIPPS): Feasibility study- Early experiences |
| WP5 | 2 November 2018 | Pharmacy Together – Care Homes – The Next Steps | Care Homes Independent Pharmacist Prescribing Study (CHIPPS): Feasibility study- Early experiences | Poster | Publications and Conferences – Groups and Centres – UEA Scroll down to download the document | Bond C, Notman F, Inch J on behalf of the CHIPPS team. Care Homes Independent Pharmacist Prescribing Study (CHIPPS): Feasibility study- Early experiences |
| WP5 | 11 July 2019 | Pilot & Feasibility Studies Journal | The Care Home Independent Prescribing Pharmacist Study (CHIPPS)-A non-randomised feasibility study of independent pharmacist prescribing in care homes | Paper | https://doi.org/10.1186/s40814-019-0465-y * Reference number32 |
Inch J, Notman F, Bond C, Alldred D, Arthur A, Blyth A, Daffu-O’Reilly A, Ford J, Hughes C, Maskrey V, Millar A, Myint P, Poland F, Shepstone L, Zermansky A, Holland R, Wright D & On behalf of the CHIPPS Team. The Care Home Independent Prescribing Pharmacist Study (CHIPPS)-A non-randomised feasibility study of independent pharmacist prescribing in care homes. Pilot & Feasibility Studies, 2019;5(89) |
| Prog | 4 May 2018 | International Journal of Pharmacy Practice | Not just a ‘tick box exercise’ – meaningful public involvement in research | Paper | https://doi.org/10.1111/ijpp.12450 * Reference number72 |
Massey K. Not just a ‘tick box exercise’ – meaningful public involvement in research. The International Journal of Pharmacy Practice. 2018;26(3):197–8. |
| WP4 | 12 November 2019 | International Journal of Pharmacy Practice | Systematic review and narrative synthesis of pharmacist provided medicines optimisation services in care homes for older people to inform the development of a generic training or accreditation process | Paper | https://doi.org/10.1111/ijpp.12591 * Reference number41 |
Wright DJ, Maskrey V, Blyth A, Norris N, Alldred DP, Bond CM, Desborough J, Hughes C, Holland R. Systematic review and narrative synthesis of pharmacist provided medicines optimisation services in care homes for older people to inform the development of a generic training or accreditation process. The International Journal of Pharmacy Practice. 2020;28(3):207–19. |
| WP5 | 9 December 2019 | ASHP Midyear Clinical Meeting | Care Homes Independent Pharmacist Prescriber Study (CHIPPS): Results from an open-label, non-randomised feasibility study | Presentation | n/a | Wright D on behalf of the CHIPPS team. Care Homes Independent Pharmacist Prescribing Study (CHIPPS): Feasibility test results |
| WP5 | 19 August 2019 | Dementia Forum, UEA | Care Homes Independent Pharmacist Prescribing Study (CHIPPS): Feasibility test results | Presentation | Publications and Conferences – Groups and Centres – UEA Scroll down to download the document | Wright D on behalf of the CHIPPS team. Care Homes Independent Pharmacist Prescribing Study (CHIPPS): Feasibility test results |
| WP6 | 20 May 2020 | Trials | Protocol for the process evaluation of a cluster randomised controlled trial to determine the effectiveness and cost-effectiveness of independent pharmacist prescribing in care home: the CHIPPS study | Paper | https://doi.org/10.1186/s13063-020-04264-8 * Reference number52 |
Bond CM, Holland R, Alldred DP, Arthur A, Barton G, Birt L, Blyth A, Desborough J, Ford J, Handford C, Hill H, Hughes C, Maskrey V, Massey K, Myint P, Poland F, Shepstone L, Zermansky A, Wright D & On behalf of CHIPPS Team. Protocol for the process evaluation of a cluster randomised controlled trial to determine the effectiveness and cost-effectiveness of independent pharmacist prescribing in care home: the CHIPPS study. Trials. 2020;21(1):439. |
| WP1 | 2 March 2020 | Health and Social Care in the Community | Everyone needs to understand each other’s systems: Stakeholder views on the acceptability and viability of a Pharmacist Independent Prescriber role in care homes for older people in the UK | Paper | https://doi.org/10.1111/hsc.1297014 * Reference number14 |
Lane K, Bond C, Wright D, Alldred DP, Desborough J, Holland R, Hughes C, Poland F. ‘Everyone needs to understand each other’s systems’: Stakeholder views on the acceptability and viability of a Pharmacist Independent Prescriber role in care homes for older people in the UK. Health & Social Care in the Community. 2020;28(5):1479–87 |
| WP6 | 21 January 2020 | Trials | Protocol for a cluster randomised controlled trial to determine the effectiveness and cost-effectiveness of independent pharmacist prescribing in care home: the CHIPPS study | Paper | https://doi.org/10.1186/s13063-019-3827-0 * Reference number52 |
Bond CM, Holland R, Alldred DP, Arthur A, Barton G, Blyth A, et al. Protocol for a cluster randomised controlled trial to determine the effectiveness and cost-effectiveness of independent pharmacist prescribing in care homes: the CHIPPS study. Trials. 2020;21(1):103. |
| WP6 | 1–3 July 2020 | British Society of Gerontology (BSG) conference | A pharmacist calls: exploring the role of pharmacist independent prescribers in care homes across the UK | Abstract Cancelled due to the COVID-19 pandemic |
n/a | Birt L, Delgarno L, Bond C, Poland F, Wright D. A pharmacist calls: exploring the role of pharmacist independent prescribers in care homes across the UK |
| WP6 | 9–11 September 2020 | SSM Society for Social Medicine and Population Health, Scientific Meeting 2020 | The CHIPPS study: a cluster randomised controlled trial to determine the effectiveness and cost-effectiveness of independent pharmacist prescribing in care homes | Abstract Virtual conference |
n/a | Holland R on behalf of CHIPPS team. The CHIPPS study: a cluster randomised controlled trial to determine the effectiveness and cost-effectiveness of independent pharmacist prescribing in care homes |
| WP6 | 19 June 2020 | RPS Science & Research Summit 2020 | Care Homes Independent Pharmacist Prescriber Study (CHIPPS): Using MRC guidance on complex interventions to effectively deliver a UK wide trial | Abstract Cancelled due to the COVID-19 pandemic |
Abstracts – 2020 – International Journal of Pharmacy Practice – https://onlinelibrary.wiley.com/doi/full/10.1111/ijpp.12633 Scroll down to abstract number 4 |
Wright D., Bond C, Hughes C, Alldred A. Care Homes Independent Pharmacist Prescriber Study (CHIPPS): Using MRC guidance on complex interventions to effectively deliver a UK wide trial. The International Journal of Pharmacy Practice. 2020; Abstracts, 4 |
| WP6 | 16–17 April 2020 | Health Services Research in Pharmacy Practice (HSRPP) | Development and testing of an effective model for monitoring patient safety within the Care Homes Independent Pharmacist Prescribing Study (CHIPPS) | Abstract Cancelled due to the COVID-19 pandemic |
Publications and Conferences – Groups and Centres – UEA Scroll down to download the document | Wright D, Bond C, Hughes C, Alldred D, Holland R on behalf of the CHIPPS team. Development and testing of an effective model for monitoring patient safety within the Care Homes Independent Pharmacist Prescribing Study (CHIPPS) |
| WP6 | 15 July 2020 | Society of Academic Primary Care (SAPC) | The CHIPPS study: a cluster randomised controlled trial to determine the effectiveness and cost-effectiveness of independent pharmacist prescribing in care homes | Abstract Cancelled due to the COVID-19 pandemic |
https://sapc.ac.uk/conference/2020/abstract/chipps-study-cluster-randomised-controlled-trial-determine-effectiveness | SAPC | Bond C, Wright D, Holland R, Alldred D, Hughes C, Poland F on behalf of the CHIPPS Team. The CHIPPS study: a cluster randomised controlled trial to determine the effectiveness and cost-effectiveness of independent pharmacist prescribing in care homes. SAPC ASM 2020, U.1 |
| WP5 | 11 February 2020 | SAGE Research Methods Cases | Lessons learned from CHIPPS (Care Homes Independent Pharmacist Prescribing Study): how feasibility studies informed ultimate randomised controlled trial design | Paper | https://research-portal.uea.ac.uk/en/publications/lessons-learned-from-chipps-care-homes-independent-pharmacist-pre | Bond C, Alldred D, Hughes C, Holland R, Poland F, Wright D. Lessons learned from CHIPPS (Care Homes Independent Pharmacist Prescribing Study): how feasibility studies informed ultimate randomised controlled trial design. 2020; SAGE Research Methods Cases Part 2 |
| WP4 | 21 May 2021 | International Journal of Pharmacy Practice | Development and feasibility testing of an evidence-based training programme for pharmacist independent prescribers responsible for the medicines-related activities within care homes | Paper | https://doi.org/10.1093/ijpp/riab025 | Wright, DJ., Blyth, A., Maskrey, V., Norris, N., Bond, CM., Hughes, CM., Alldred, DP., Holland, RC., CHIPPS Team. International Journal of Pharmacy Practice. 29(4):376–84 |
| WP6 | 2 October 2021 | BMC Health Services Research | Process evaluation for the Care Homes Independent Pharmacist Prescriber Study (CHIPPS) | Paper | https://doi.org/10.1186/s12913-021-07062-3 | Linda Birt, Lindsay Dalgarno, David J Wright, Mohammed Alharthi, Jackie Inch, Maureen Spargo, Jeanette Blacklock, Fiona Poland, Richard C Holland, David P. Alldred, Carmel M. Hughes & Christine M. Bond on behalf of the CHIPPS study team |
| WP4 | 15 July 2022 | BMC Medical Education | Evaluation of a training programme for Pharmacist Independent Prescribers in a care home medicine management intervention | Paper | https://doi.org/10.1186/s12909-022-03575-5 | Linda Birt, Lindsay Dalgarno, Christine M. Bond, Richard C Holland, David P. Alldred, Carmel M. Hughes, Annie Blyth, Laura Watts & David J. Wright, on behalf of the CHIPPS study team BMC Medical Education 22; 551 |
Planned remaining publications
| Short title | Title |
|---|---|
| Main trial | Effectiveness and cost-effectiveness of integrating a pharmacist independent prescriber into care homes for older people. A cluster randomised controlled trial. |
| The qualitative benefits | Pharmacist Independent Prescriber a linchpin in multidisciplinary primary care: a qualitative investigation of the impact of a pharmacy-intervention in UK care homes. |
| Researcher reflection | From study protocol to sitting in a cupboard: account of trial researchers experience of set-up and data collection during an RCT sited in care homes |
| The NOMAD results | The ‘work’ of normalising the place of prescribing pharmacists within UK care homes: understanding supportive and disruptive practices using Normalization Process Theory |
| The logical model methodology | Logic models a tool to enhance understanding of the relationships between resources, activities, outputs and the outcomes and impact of a programme of practice |
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the PGfAR programme or the Department of Health and Social Care.
References
- Barber ND, Alldred DP, Raynor DK, Dickinson R, Garfield S, Jesson B, et al. Care homes’ use of medicines study: prevalence, causes and potential harm of medication errors in care homes for older people. Qual Saf Health Care 2009;18:341-6. https://doi.org./10.1136/qshc.2009.034231.
- Department of Health . The Use of Medicines in Care Homes for Older People 2010.
- Rankin A, Cadogan CA, Patterson SM, Kerse N, Cardwell CR, Bradley MC, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev 2018;9. https://doi.org/10.1002/14651858.CD008165.pub4.
- Bruhn H, Bond CM, Elliott AM, Hannaford PC, Lee AJ, McNamee P, et al. Pharmacist-led management of chronic pain in primary care: results from a randomised controlled exploratory trial. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-002361.
- Serrano Santos JM, Poland F, Wright D, Longmore T. Medicines administration for residents with dysphagia in care homes: a small scale observational study to improve practice. Int J Pharm 2016;512:416-21. https://doi.org/10.1016/j.ijpharm.2016.02.036.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and Evaluating Complex Interventions. London: Medical Research Council; 2006.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Bryman A. Social Research Methods. Oxford, UK: Oxford University Press; 2012.
- Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci 2012;7. https://doi.org/10.1186/1748-5908-7-37.
- Davidoff F, Dixon-Woods M, Leviton L, Michie S. Demystifying theory and its use in improvement. BMJ Qual Saf 2015;24:228-38. https://doi.org/10.1136/bmjqs-2014-003627.
- Social Care Institute for Excellence . GP Services for Older People: A Guide for Care Home Managers 2013.
- Scott T, Mannion R, Davies HT, Marshall MN. Implementing culture change in health care: theory and practice. Int J Qual Health Care 2003;15:111-8. https://doi.org/10.1093/intqhc/mzg021.
- Chaplin S. The expanding role of pharmacists in care homes. Prescriber 2016;27:44-6.
- Lane K, Bond C, Wright D, Alldred DP, Desborough J, Holland R, et al. ‘Everyone needs to understand each other’s systems’: stakeholder views on the acceptability and viability of a Pharmacist Independent Prescriber role in care homes for older people in the UK. Health Soc Care Community 2020;28:1479-87. https://doi.org/10.1111/hsc.12970.
- Williamson P, Altman D, Blazeby J, Clarke M, Gargon E. Driving up the quality and relevance of research through the use of agreed core outcomes. J Health Serv Res Policy 2012;17:1-2. https://doi.org/10.1258/jhsrp.2011.011131.
- Millar AN, Daffu-O’Reilly A, Hughes CM, Alldred DP, Barton G, Bond CM, et al. Development of a core outcome set for effectiveness trials aimed at optimising prescribing in older adults in care homes. Trials 2017;18. https://doi.org/10.1186/s13063-017-1915-6.
- Williamson PR, Altman DG, Blazeby JM, Clarke M, Devane D, Gargon E, et al. Developing core outcome sets for clinical trials: issues to consider. Trials 2012;13. https://doi.org/10.1186/1745-6215-13-132.
- Alldred DP, Kennedy MC, Hughes C, Chen TF, Miller P. Interventions to optimise prescribing for older people in care homes. Cochrane Database Syst Rev 2016;2. https://doi.org/10.1002/14651858.CD009095.pub3.
- O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2014;44:213-8. https://doi.org/10.1093/ageing/afu145.
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2 – framing the question and deciding on important outcomes. J Clin Epidemiol 2011;64:395-400. https://doi.org/10.1016/j.jclinepi.2010.09.012.
- Harman NL, Bruce IA, Callery P, Tierney S, Sharif MO, O’Brien K, et al. MOMENT – Management of Otitis Media with Effusion in Cleft Palate: protocol for a systematic review of the literature and identification of a core outcome set using a Delphi survey. Trials 2013;14. https://doi.org/10.1186/1745-6215-14-70.
- Waters AMI, Tudur Smith C, Young B, Jones TM. The CONSENSUS study: protocol for a mixed methods study to establish which outcomes should be included in a core outcome set for oropharyngeal cancer. Trials 2014;15. https://doi.org/10.1186/1745-6215-15-168.
- Desborough JA, Clark A, Houghton J, Sach T, Shaw V, Kirthisingha V, et al. Clinical and cost effectiveness of a multi-professional medication reviews in care homes (CAREMED). Int J Pharm Pract 2020;28:626-34. https://doi.org/10.1111/ijpp.12656.
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2018. Kent, England: Personal Social Services Research Unit, University of Kent; 2018.
- Sach T, Desborough J, Houghton J, Holland R. Unit Costs of Health and Social Care. Kent, England: Personal Social Services Research Unit, University of Kent; 2018.
- Health and Social Care Information Centre, Department of Health . LHS Schedule of Reference Costs 2017 18 2018.
- Underwood M, Lamb SE, Eldridge S, Sheehan B, Slowther A, Spencer A, et al. Exercise for depression in care home residents: a randomised controlled trial with cost-effectiveness analysis (OPERA). Health Technol Assess 2013;17:1-281. https://doi.org/10.3310/hta17180.
- Health and Social Care Information Centre . Prescription Cost Analysis: England 2015 2016.
- NHS Digital . Prescription Cost Analysis, England 2017: Health and Social Care Information Centre 2018.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- National Institute of Health and Clinical Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013: National Institute of Health and Clinical Excellence (NICE) Publications 2013.
- Inch J, Notman F, Bond CM, Alldred DP, Arthu A, Blyth A, et al. The Care Home Independent Prescribing Pharmacist Study (CHIPPS): a non-randomised feasibility study of independent pharmacist prescribing in care homes. Pilot Feasibil Stud 2019;5. https://doi.org/10.1186/s40814-019-0465-y.
- National Institute for Health and Care Excellence (NICE) . Position Statement on Use of the EQ-5D-5L Valuation Set (Updated November 2018) 2018.
- National Institute for Health and Care Excellence (NICE) . NICE Health Technology Evaluations: The Manual 2022 n.d. www.nice.org.uk/process/pmg36 (accessed 21 June 2022).
- van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.008.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;14:461-75. https://doi.org/10.1002/hec.843.
- Fenwick E, Claxton K, Sculpher MJ. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. https://doi.org/10.1002/hec.635.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med 2009;3:e123-30.
- Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bullet 1968;70:213-20. https://doi.org/10.1037/h0026256.
- Wright DJ, Maskrey V, Blyth A, Norris N, Alldred DP, Bond CM, et al. Systematic review and narrative synthesis of pharmacist provided medicines optimisation services in care homes for older people to inform the development of a generic training or accreditation process. Int J Pharm Pract 2020;28:207-19. https://doi.org/10.1111/ijpp.12591.
- Inch J, Notman F, Bond CM, Alldred DP, Arthur A, Blyth A, et al. CHIPPS Team . The Care Home Independent Prescribing Pharmacist Study (CHIPPS)-a non-randomised feasibility study of independent pharmacist prescribing in care homes. Pilot Feasibility Stud 2019;5. https://doi.org/10.1186/s40814-019-0465-y.
- Roberts MS, Stokes JA, King MA, Lynne TA, Purdie DM, Glasziou PP, et al. Outcomes of a randomized controlled trial of a clinical pharmacy intervention in 52 nursing homes. Br J Clin Pharmacol 2001;51:257-65. https://doi.org/10.1046/j.1365-2125.2001.00347.x.
- Lane K, Bond C, Wright D, Alldred DP, Desborough J, Holland R, et al. ‘Everyone needs to understand each other’s systems’: stakeholder views on the acceptability and viability of a pharmacist independent prescriber role in care homes for older people in the UK. Health Soc Care Community 2020;28:1479-87. https://doi.org/10.1111/hsc.12970.
- Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004;10:307-12. https://doi.org/10.1111/j.2002.384.doc.x.
- Hilmer SN, Mager DE, Simonsick EM, Cao Y, Ling SM, Windham BG, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med 2007;167:781-7. https://doi.org/10.1001/archinte.167.8.781.
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J 1965;14:61-5.
- Ettema TP, Dröes RM, de Lange J, Mellenbergh GJ, Ribbe MW. QUALIDEM: development and evaluation of a dementia specific quality of life instrument--validation. Int J Geriatr Psychiatry 2007;22:424-30. https://doi.org/10.1002/gps.1692.
- Folstein MF, Robins LN, Helzer JE. The mini-mental state examination. Arch Gen Psychiatry 1983;40. https://doi.org/10.1001/archpsyc.1983.01790060110016.
- Bond CM, Holland R, Alldred DP, Arthur A, Barton G, Blyth A, et al. Protocol for a cluster randomised controlled trial to determine the effectiveness and cost-effectiveness of independent pharmacist prescribing in care homes: the CHIPPS study. Trials 2020;21. https://doi.org/10.1186/s13063-019-3827-0.
- Zermansky AG, Alldred DP, Petty DR, Raynor DK, Freemantle N, Eastaugh J, et al. Clinical medication review by a pharmacist of elderly people living in care homes: randomised controlled trial. Age Ageing 2006;35:586-91. https://doi.org/10.1093/ageing/afl075.
- Bond CM, Holland R, Alldred DP, Arthur A, Barton G, Birt L, et al. Protocol for the process evaluation of a cluster randomised controlled trial to determine the effectiveness and cost-effectiveness of independent pharmacist prescribing in care home: the CHIPPS study. Trials 2020;21. https://doi.org/10.1186/s13063-020-04264-8.
- de Vries M, Seppala LJ, Daams JG, van de Glind EMM, Masud T, van der Velde N. EUGMS Task and Finish Group on Fall-Risk-Increasing Drugs . Fall-risk-increasing drugs: a systematic review and meta-analysis: I – cardiovascular drugs. J Am Med Dir Assoc 2018;19:371.e1-9. https://doi.org/10.1016/j.jamda.2017.12.013.
- Seppala LJ, van de Glind EMM, Daams JG, Ploegmakers KJ, de Vries M, Wermelink A, et al. Fall-risk-increasing drugs: a systematic review and meta-analysis: III – others. J Am Med Dir Assoc 2018;19:372.e1-72.e8. https://doi.org/10.1016/j.jamda.2017.12.099.
- Seppala LJ, Wermelink A, de Vries M, Ploegmakers KJ, van de Glind EMM, Daams JG, et al. Fall-risk-increasing drugs: a systematic review and meta-analysis: II – psychotropics. J Am Med Dir Assoc 2018;19:371.e11-71.e17. https://doi.org/10.1016/j.jamda.2017.12.098.
- May CR, Mair F, Finch T, MacFarlane A, Dowrick C, Treweek S, et al. Development of a theory of implementation and integration: normalization process theory. Implement Sci 2009;4. https://doi.org/10.1186/1748-5908-4-29.
- May C, Finch T, Mair F, Ballini L, Dowrick C, Eccles M, et al. Understanding the implementation of complex interventions in health care: the normalization process model. BMC Health Serv Res 2007;7. https://doi.org/10.1186/1472-6963-7-148.
- NoMaD Survey n.d. www.implementall.eu/9-outcomes-and-resources.html (accessed 16 November 2020).
- Ali S, Peterson GM, Bereznicki LR, Salahudeen MS. Association between anticholinergic drug burden and mortality in older people: a systematic review. Eur J Clin Pharmacol 2020;76:319-35. https://doi.org/10.1007/s00228-019-02795-x.
- Wilson NM, Hilmer SN, March LM, Cameron ID, Lord SR, Seibel MJ, et al. Associations between drug burden index and falls in older people in residential aged care. J Am Geriatr Soc 2011;59:875-80. https://doi.org/10.1111/j.1532-5415.2011.03386.x.
- Jamieson HA, Nishtala PS, Scrase R, Deely JM, Abey-Nesbit R, Hilmer SN, et al. Drug burden index and its association with hip fracture among older adults: a national population-based study. J Gerontol A Biol Sci Med Sci 2019;74:1127-33. https://doi.org/10.1093/gerona/gly176.
- Byrne C, Walsh C, Cahir C, Bennett K. Impact of drug burden index on adverse health outcomes in Irish community-dwelling older people: a cohort study. BMC Geriatr 2019;19. https://doi.org/10.1186/s12877-019-1138-7.
- Wouters H, Hilmer SN, Twisk J, Teichert M, Van Der Meer HG, Van Hout HPJ, et al. Drug burden index and cognitive and physical function in aged care residents: a longitudinal study. J Am Med Dir Assoc 2020;21:1086.e-92.e1. https://doi.org/10.1016/j.jamda.2020.05.037.
- Kua CH, Mak VSL, Huey Lee SW. Health outcomes of deprescribing interventions among older residents in nursing homes: a systematic review and meta-analysis. J Am Med Dir Assoc 2019;20:362.e-372.e11. https://doi.org/10.1016/j.jamda.2018.10.026.
- Lee SWH, Mak VSL, Tang YW. Pharmacist services in nursing homes: a systematic review and meta-analysis. Br J Clin Pharmacol 2019;85:2668-88. https://doi.org/10.1111/bcp.14101.
- Centre for Public Impact . Shine 2012: Optimising Medicine Use for Care Home Residents in Northumbria 2012. www.centreforpublicimpact.org/case-study/shine-project-optimising-medicine-use-care-home-residents-northumbria-healthcare-nhs-foundation-trust/ (accessed 17 November 2020).
- Potter S, Holcombe C, Ward JA, Blazeby JM, Group BS. Development of a core outcome set for research and audit studies in reconstructive breast surgery. Br J Surg 2015;102:1360-71. https://doi.org/10.1002/bjs.9883.
- Wylde V, MacKichan F, Bruce J, Gooberman-Hill R. Assessment of chronic post-surgical pain after knee replacement: development of a core outcome set. Eur J Pain 2015;19:611-20. https://doi.org/10.1002/ejp.582.
- van ‘t Hooft J, Duffy JMN, Daly M, Williamson PR, Meher S, Thom E, et al. A core outcome set for evaluation of interventions to prevent preterm birth. Obstet Gynecol 2016;127:49-58. https://doi.org/10.1097/aog.0000000000001195.
- Care Quality Commission . Medicines in Health and Social Care 2019.
- NHS England . Medicines Optimisation in Care Homes 2016 n.d. www.england.nhs.uk/primary-care/pharmacy/medicines-optimisation-in-care-homes/ (accessed 4 December 2019).
- Massey K. Not just a ‘tick box exercise’: meaningful public involvement in research. Int J Pharm Pract 2018;26:197-8. https://doi.org/10.1111/ijpp.12450.
- Gallagher P, Barry P, O’Mahony D. Inappropriate prescribing in the elderly. J Clin Pharm Ther 2007;32:113-21. https://doi.org/10.1111/j.1365-2710.2007.00793.x.
- Joint Formulary Committee . British National Formulary 2016.
- Mallet L, Spinewine A, Huang A. The challenge of managing drug interactions in elderly people. Lancet 2007;370:185-91. https://doi.org/10.1016/S0140-6736(07)61092-7.
- Ruxton K, Woodman RJ, Mangoni AA. Drugs with anticholinergic effects and cognitive impairment, falls and all-cause mortality in older adults: a systematic review and meta-analysis. Br J Clin Pharmacol 2015;80:209-20. https://doi.org/10.1111/bcp.12617.
- Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, et al. Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA 1995;274:29-34. https://doi.org/10.1001/jama.1995.03530010043033.
- Dean B, Barber N, Schachter M. What is a prescribing error?. Qual Health Care 2000;9:232-7. https://doi.org/10.1136/qhc.9.4.232.
- World Health Organization . Falls: Fact Sheet No 344.2012 2012.
- Fallowfield L. The Quality of Life: The Missing Measurement in Health Care. London, England: Souvenir; 1990.
- King MA, Roberts MS. Multidisciplinary case conference reviews: improving outcomes for nursing home residents, carers and health professionals. Pharm World Sci 2001;23:41-5. https://doi.org/10.1023/a:1011215008000.
- Smith MA, Simpson JM, Benrimoj SI. General practitioner acceptance of medication review in Sydney nursing homes. J Pharm Pract Res 2002;32:227-31. https://doi.org/10.1002/jppr2002323227.
- Crotty M, Halbert J, Rowett D, Giles L, Birks R, Williams H, et al. An outreach geriatric medication advisory service in residential aged care: a randomised controlled trial of case conferencing. Age Ageing 2004;33:612-7. https://doi.org/10.1093/ageing/afh213.
- Beer C, Loh P-k, Peng YG, Potter K, Millar A. A pilot randomized controlled trial of deprescribing. Ther Adv Drug Saf 2011;2:37-43. https://doi.org/10.1177/2042098611400332.
- Khalil H. A review of pharmacist recommendations in an aged care facility. Aust J Prim Health 2011;17:35-9. https://doi.org/10.1071/PY10044.
- Verrue CL, Mehuys E, Somers A, Van Maele G, Remon JP, Petrovic M. Medication administration in nursing homes: pharmacists’ contribution to error prevention. J Am Med Dir Assoc 2010;11:275-83. https://doi.org/10.1016/j.jamda.2009.10.013.
- Verrue C, Mehuys E, Boussery K, Adriaens E, Remon JP, Petrovic M. A pharmacist-conducted medication review in nursing home residents: impact on the appropriateness of prescribing. Acta Clin Belg 2012;67:423-9. https://doi.org/10.2143/ACB.67.6.2062707.
- Soon J. Assessment of an adverse drug reaction monitoring program in nursing homes. Can J Hosp Pharm 1985;38:120-5.
- Kroger E, Wilchesky M, Voyer P, Morin M, Champoux N, Monette J, et al. OptimaMed: an intervention to reduce inappropriate medication use among nursing home residents with advanced dementia: C148. J Am Geriatr Soc 2015;63:S211-2. https://doi.org/10.1186/s12877-018-0895-z.
- Norgaard LS, Petrovics M. Medication reconciliation and review at sector transition from a psychiatric centre to residential care. Int J Clin Pharm 2015;37.
- Leguelinel G, Di Trapani L, Rolain J, Kinowski JM, Richard H. Impact of prescriptions review on the quality and cost of nursing home residents’ therapeutic management. Int J Clin Pharm 2013;35.
- Frankenthal DM, Lerman YMD, Kalendaryev EMD, Lerman YMD. Intervention with the screening tool of older persons potentially inappropriate prescriptions/screening tool to alert doctors to right treatment criteria in elderly residents of a chronic geriatric facility: a randomized clinical trial. J Am Geriatr Soc 2014;62:1658-65. https://doi.org/10.1111/jgs.12993.
- Finkers F, Maring JG, Boersma F, Taxis K. A study of medication reviews to identify drug-related problems of polypharmacy patients in the Dutch nursing home setting. J Clin Pharm Ther 2007;32:469-76. https://doi.org/10.1111/j.1365-2710.2007.00849.x.
- Stuijt CCM, Franssen EJF, Egberts ACG, Hudson SA. Appropriateness of prescribing among elderly patients in a Dutch residential home: observational study of outcomes after a pharmacist-led medication review. Drugs Aging 2008;25:947-54. https://doi.org/10.2165/0002512-200825110-00005.
- Stuijt CCM, Klopotowska JE, van Driel CK, Le N, Binnekade J, van der Kleij B, et al. Improving medication administration in nursing home residents with swallowing difficulties: sustainability of the effect of a multifaceted medication safety programme+. Pharmacoepidemiol Drug Saf 2013;22:423-9. https://doi.org/10.1002/pds.3373.
- Connolly MJ, Broad JB, Boyd M, Kerse N, Foster S, Lumley T, et al. Cluster-randomised controlled trial (RCT) of a multidisciplinary intervention package for reducing disease-specific hospitalisations from Long Term Care (LTC). Age Ageing 2014;43. https://doi.org/10.1093/ageing/afu131.4.
- Ruths S, Straand J, Nygaard HA. Multidisciplinary medication review in nursing home residents: what are the most significant drug-related problems? The Bergen District Nursing Home (BEDNURS) study. Qual Saf Health Care 2003;12:176-80. https://doi.org/10.1136/qhc.12.3.176.
- Bellingan M, Wiseman IC. Pharmacist intervention in an elderly care facility. Int J Pharm Pract 1996;4:25-9. https://doi.org/10.1111/j.2042-7174.1996.tb00835.x.
- Jodar-Sanchez FM, Martin JJP, Lopez del Amo PMP, Garcia LM, Araujo-Santos JMP, Epstein DP. Cost-utility analysis of a pharmacotherapy follow-up for elderly nursing home residents in Spain. J Am Geriatr Soc 2014;62:1272-80. https://doi.org/10.1111/jgs.12890.
- Bergman A, Olsson J, Carlsten A, Waern M, Fastbom J. Evaluation of the quality of drug therapy among elderly patients in nursing homes. Scand J Prim Health Care 2007;25:9-14. https://doi.org/10.1080/02813430600991980.
- Kuo CN, Lin YM, Wu MT, Kuo LN, Lee LW, Chen HY. Pharmacist-directed reconciliation for reducing medication discrepancies: a pilot study in a nursing home setting in Taiwan. J Food Drug Anal 2013;21:160-4. https://doi.org/10.1016/j.jfda.2013.05.005.
- Newman G. A mid glamorgan system. Pharm J 1982;229:201-2.
- Narula N, Shulman S, Sheridan J. Medication related problems in residential homes for the elderly. Pharm J 1992;248:623-5.
- Rees JK, Livingstone DJ, Livingstone CR, Clarke CD. Community clinical pharmacy service for elderly people in residential homes. Pharm J 1995;255:54-6.
- Furniss L, Burns A, Craig SK, Scobie S, Cooke J, Faragher B. Effects of a pharmacist’s medication review in nursing homes: randomised controlled trial. Br J Psychiatry 2000;176:563-7. https://doi.org/10.1192/bjp.176.6.563.
- Alldred DP, Zermansky AG, Petty DR, Raynor DK, Freemantle N, Eastaugh J, et al. Clinical medication review by a pharmacist of elderly people living in care homes: pharmacist interventions. Int J Pharm Pract 2007;15:93-9. https://doi.org/10.1211/ijpp.15.2.0003.
- Saeed M, Stretch G. Introduction of a nursing home-based intensive pharmaceutical interventions programme: audit of initial outcomes: 55. Int J Pharm Pract 2010;18:54-5.
- Patterson SMP, Hughes CMP, Cardwell CP, Lapane KLP, Murray AMP, Crealey GEP. A cluster randomized controlled trial of an adapted U.S. model of pharmaceutical care for nursing home residents in Northern Ireland (Fleetwood Northern Ireland study): a cost-effectiveness analysis. J Am Geriatr Soc 2011;59:586-93. https://doi.org/10.1111/j.1532-5415.2011.03354.x.
- Nabc Hampson . Evaluation of a care home clinical medication review service by a primary care pharmacist: 0115. Int J Pharm Pract 2012;20:86-7.
- Cooper J, Bagwell CG. Contribution of the consultant pharmacist to rational drug usage in the long-term care facility. J Am Geriatr Soc 1978;26:513-20. https://doi.org/10.1111/j.1532-5415.1978.tb03336.x.
- Cooper J. Effect of initiation, termination, and reinitiation of consultant clinical pharmacist services in a geriatric long-term care facility. Med Care 1985;23:84-8. https://doi.org/10.1097/00005650-198501000-00009.
- Andolsek KM, Hanlon JT, Lyons RK, Proffitt VS. Drug therapy team review in a long-term care facility. Drug Intell Clin Pharm 1987;21. https://doi.org/10.1177/1060028087021007-821.
- Pucino F, Baumgart PJ, Strommen GL, Silbergleit IL, Forbes D, Hoag SG, et al. Evaluation of therapeutic drug monitoring in a long-term care facility: a pilot project. Drug Intell Clin Pharm 1988;22:594-6. https://doi.org/10.1177/106002808802200717.
- Cooper J. Consultant pharmacist contribution to diabetes mellitus patient outcomes in two nursing facilities. Consult Pharm 1995;10.
- Cooper JW. Consultant pharmacist drug therapy intervention recommendations in a geriatric nursing facility: a one-year study. J Geriatr Drug Ther 1997;11:51-63.
- Jeffrey S, Ruby CM, Twesky J, Hanlon JT. Effect of an interdisciplinary team on sub-optimal prescribing in a long term care facility. Consult Pharm 1999;14:1386-91.
- Elliott AR, Thomson AT. Assessment of a nursing home medication review service provided by hospital-based clinical pharmacists. Aust J Pharm 1999;29:255-60. https://doi.org/10.1002/JPPR1999295255.
- Lai L, Alfred L. Impact of consultant pharmacist intervention on antibiotic therapy for nursing facility residents aged 85 and older. Consult Pharm 2001;16:146-51.
- Christensen D, Trygstad T, Sullivan R, Garmise J, Wegner SE. A pharmacy management intervention for optimizing drug therapy for nursing home patients. Am J Geriatr Pharmacother 2004;2:248-56. https://doi.org/10.1016/j.amjopharm.2004.12.002.
- Crotty M, Rowett D, Spurling L, Giles LC, Phillips PA. Does the addition of a pharmacist transition coordinator improve evidence-based medication management and health outcomes in older adults moving from the hospital to a long-term care facility? Results of a randomized, controlled trial. Am J Geriatr Pharmacother 2004;2:257-64. https://doi.org/10.1016/j.amjopharm.2005.01.001.
- Buhr GT, White HK. Quality improvement initiative for chronic pain assessment in the nursing home: a pilot study. JAMDA 2006;7:246-53. https://doi.org/10.1016/j.jamda.2005.11.002.
- Cooper JW, Wade WE, Cook CL, Burfield AH. Consultant pharmacist drug therapy recommendations acceptance and rejection from monthly drug regimen reviews in a geriatric nursing facility: fourth year results and cost analysis. Hosp Pharm 2007;42:729-36. https://doi.org/10.1310/hpj4208-729.
- Hursh DK, Caldwell E, Maines CR. Reduction of antipsychotic medication use in a nursing facility: thinking outside of the black box. J Am Med Dir Assoc 2010;11:B21-2.
- Gunning K, Saffel-Shrier S, Lehmann W, Miniclier N, Farrell T. Development of a novel medical home: an interprofessional teaching clinic in an assisted living facility. Pharmacotherapy 2010;30:435e-6e.
- Lapane KL, Hughes CM, Daiello LA, Cameron KA, Feinberg J. Effect of a pharmacist-led multicomponent intervention focusing on the medication monitoring phase to prevent potential adverse drug events in nursing homes. J Am Geriatr Soc 2011;59:1238-45. https://doi.org/10.1111/j.1532-5415.2011.03418.x.
- Motycka C, Kesgen C, Smith SM, Alvarez E, Jones K. Potential benefits of warfarin monitoring by a clinical pharmacist in a long term care facility. J Thromb Thrombolysis 2012;33:173-7. https://doi.org/10.1007/s11239-011-0642-1.
- Zarowitz BJ, Erwin WG, Ferris M, Losben N, Proud T. Methotrexate safety improvement in nursing home residents. J Am Med Dir Assoc 2012;13:69-74. https://doi.org/10.1016/j.jamda.2010.08.006.
- Nye A. Interventions of a pharmacist on a teaching nursing home team. Consult Pharm 2012;27.
- Phillippe HM. Results of a pharmacist-managed anticoagulation service at a long-term care facility. Pharmacotherapy 2012;32:e204-5.
- Lemay CA, Mazor KM, Field TS, Donovan J, Kanaan A, Briesacher BA, et al. Knowledge of and perceived need for evidence-based education about antipsychotic medications among nursing home leadership and staff. J Am Med Dir Assoc 2013;14:895-900. https://doi.org/10.1016/j.jamda.2013.08.009.
- Skills for Care . GO Online: Inspection Toolkit: Medicines Administration n.d. www.skillsforcare.org.uk/Support-for-leaders-and-managers/Good-and-outstanding-care/inspect/Topic-focus.aspx?services=residential-nursing-care&kloe=safe&topic=medicines (accessed 6 July 2022).
- Briggs A, Clark T, Wolstenholme J, Clarke P. Missing. presumed at random: cost-analysis of incomplete data. Health Econ 2003;12:377-92. https://doi.org/10.1002/hec.766.
- Royston P. Multiple imputation of missing values. Stata J 2004;4:227-41.
- Little R, Rubin DB. Statistical Analysis with Missing Data. Hoboken, NJ: John Wiley & Sons; 2002.
Appendix 1 Synopsis tables, figures and boxes
Work package 1 tables
| Question | |
|---|---|
| What is your role? | |
| Knowledge Professional role Skills Environmental context |
How are medicines managed at the moment in your experience for care home residents? |
| Knowledge Beliefs about capabilities of a PIP service? Beliefs about consequences Skills |
What might be the key ingredients? |
| Beliefs about capabilities Environmental context and resources |
What organisational barriers could affect putting this into practice? |
| Social professional role and identity Social influences |
What professional barriers could affect putting this into practice? |
| Intention Goals |
What might be the solutions to these barriers |
| Skills Behavioural regulation |
What could we include in training pharmacists undertaking this role? |
| Is there anything else important about this proposed service you want to mention? |
| Stakeholder group | Study sites involved | No. of focus groups (groups) | Interviews | Total |
|---|---|---|---|---|
| Pharmacists | All 4 sites | 25 (4) | 2 | 27 |
| General Practitioners | All 4 sites | 24 (4) | 5 | 29 |
| Care home managers | All 4 sites | 3 (1) | 3 | 6 |
| Care home staff | England (2) and Northern Ireland | 6 (2) | 3 | 9 |
| Residents and relatives | England and Scotland | 7 residents, 7 relatives | 0 | 14 |
| Total | 72 | 13 | 85 |
| Sector | Prescribing pharmacists | Non-prescribing pharmacists | Pharmacy technician | Total |
|---|---|---|---|---|
| Primary care | 2 | 13 | 15 | |
| Community | 1 | 9 | 1 | 11 |
| Portfolioa | 1 | — | 1 | |
| Total | 4 | 22 | 1 | 27 |
Work package 2 tables
| Outcome | Mean Delphi score | Median Delphi score | Respondents scoring 7–9 ‘critically important’ (%) | Respondents scoring 1–3 ‘not important’ (%) | Result (in, out or no consensus) |
|---|---|---|---|---|---|
| Number of medications (and associated costs) | 7.7 | 8.5 | 83.3 | 0 | In |
| Medication wastage (and associated costs) | 6.6 | 7 | 68.4 | 10.5 | No consensus |
| Polypharmacy (≥4 medicines) | 6.5 | 7 | 57.9 | 10.5 | No consensus |
| Medication appropriateness (potentially inappropriate prescribing) | 8.2 | 9 | 84.2 | 0 | In |
| Duplicate drugs | 7.2 | 7.5 | 72.2 | 5.6 | In |
| Use of antipsychotics | 7.4 | 8 | 73.7 | 0 | In |
| Medication changes made (by anyone) | 6.9 | 8 | 63.2 | 10.5 | No consensus |
| Number of medication reviews conducted (by anyone) | 6.7 | 7 | 63.2 | 10.5 | No consensus |
| Admissions to hospital (and associated costs) | 8.2 | 8 | 100 | 0 | In |
| Accident & emergency visits (and associated costs) | 7.8 | 8 | 83.3 | 0 | In |
| Visits to outpatients (and associated costs) | 5.3 | 5 | 26.3 | 31.6 | No consensus |
| Visits to/from GP (and associated cost) | 7.1 | 7 | 63.2 | 5.3 | No consensus |
| Visits to/from nurse (and associated cost) | 6.1 | 6 | 42.1 | 5.3 | No consensus |
| Adverse drug events | 8.4 | 9 | 94.7 | 0 | In |
| Falls | 7.4 | 7 | 84.2 | 0 | In |
| Acute kidney injury | 6.7 | 6 | 46.7 | 0 | No consensus |
| Prescribing errors | 7.9 | 8 | 89.5 | 5.3 | In |
| Harmful interactions | 7.7 | 8 | 84.2 | 5.3 | In |
| All-cause mortality | 7.5 | 9 | 78.9 | 5.3 | In |
| Physical functioning | 6.5 | 7 | 57.9 | 15.8 | No consensus |
| Behaviour | 6.6 | 7 | 63.2 | 5.3 | No consensus |
| Cognitive functioning | 6.6 | 7 | 57.9 | 5.3 | No consensus |
| Depression | 6.3 | 7 | 55.6 | 5.6 | No consensus |
| Quality of life | 7.7 | 8 | 83.3 | 0 | In |
| Compliance with NICE guidelines | 6.3 | 7 | 52.6 | 10.5 | No consensus |
| Compliance with medicines | 6.7 | 7 | 68.4 | 5.3 | No consensus |
| Care home staff job satisfaction | 5 | 5 | 26.3 | 36.8 | No consensus |
| Efficiency of medication administration by care home staff | 6.3 | 6 | 42.1 | 5.3 | No consensus |
| Accuracy of administration of medications by care home staff | 6.9 | 7 | 57.9 | 5.3 | No consensus |
| Outcome | Mean | Med | Respondents scoring 7–9 ‘critically important’ (%) | Respondents scoring 1–3 ‘not important’ (%) | Consensus result (in, out or no consensus) |
|---|---|---|---|---|---|
| Number of medications | 7.3 | 8.0 | 83.3 | 11.1 | In |
| Costs of prescribed medication | 6.3 | 7.0 | 61.1 | 11.1 | No consensus |
| Medication wastage (and associated costs) | 6.6 | 7.0 | 66.7 | 5.6 | No consensus |
| Polypharmacy (≥4 medicines) | 6.6 | 7.0 | 66.7 | 5.6 | No consensus |
| Medication changes made (by anyone | 6.5 | 7.0 | 55.6 | 5.6 | No consensus |
| Number of medication reviews conducted (by anyone) | 6.6 | 7.0 | 66.7 | 5.6 | No consensus |
| Visits to outpatients (and associated costs) | 5.6 | 5.0 | 33.3 | 5.6 | No consensus |
| Visits to/from GP (and associated cost) | 6.6 | 6.5 | 50.0 | 0 | No consensus |
| Visits to/from nurse (and associated cost) | 6.1 | 6.5 | 50.0 | 0 | No consensus |
| Acute kidney injury | 6.8 | 7.0 | 53.3 | 0 | No consensus |
| Physical functioning | 6.5 | 7.0 | 61.1 | 5.6 | No consensus |
| Behaviour | 6.9 | 7.0 | 61.1 | 5.6 | No consensus |
| Cognitive functioning | 6.8 | 7.0 | 61.1 | 0 | No consensus |
| Depression | 6.7 | 7.0 | 61.1 | 0 | No consensus |
| Compliance with NICE guidelines | 6.4 | 7.0 | 55.6 | 16.7 | No consensus |
| Compliance with medicines | 6.9 | 7.5 | 61.1 | 5.6 | No consensus |
| Care home staff job satisfaction | 5.1 | 5.0 | 22.2 | 5.6 | No consensus |
| Efficiency of medication administration by care home staff | 6.4 | 6.0 | 38.9 | 0 | No consensus |
| Accuracy of administration of medications by care home staff | 7.3 | 7.0 | 55.6 | 0 | No consensus |
| Anticholinergic burden | 7.3 | 7.0 | 75.0 | 0 | In |
| Outcome | Explanation from Delphi questionnaire |
|---|---|
| Medication appropriateness (potentially inappropriate prescribing) | Potentially inappropriate prescribing ‘encompasses the use of medicines that introduce a significant risk of an adverse drug-related event where there is evidence for an equally or more effective but lower-risk alternative therapy available for treating the same condition … also includes the use of medicines at a higher frequency and for longer than clinically indicated, the use of multiple medicines that have recognised drug-drug interactions and drug-disease interactions, and importantly, the under-use of beneficial medicines that are clinically indicated but not prescribed for ageist or irrational reasons’ (Gallagher et al., 2007)73 |
| Number of prescribed medicines | Number of medications prescribed for a care home resident |
| Duplicate drugs | ‘Duplicate drugs’ described a situation where an individual is prescribed two medicines of the same pharmacological class, e.g. the prescribing of two concurrent opiates (O’Mahony et al.)47 |
| Use of antipsychotics | The prescription of antipsychotic medicines in care homes residents. ‘Antipsychotic drugs are also known as “neuroleptics” and (misleadingly) as “major tranquillisers”. In the short term, they are used to calm disturbed patients whatever the underlying psychopathology … The balance of risks and benefits should be considered before prescribing antipsychotic drugs for elderly patients’ (Joint Formulary Committee, 2016)74 |
| Harmful interactions | A ‘harmful interaction’ in a care home resident may describe the prescription of a medication that causes or has the potential to cause a clinically significant drug-drug or drug-disease interaction. A drug-drug interaction is when a medicine affects the pharmacological effect of another medicine. A drug-disease interaction is when a medicine, which may be used to treat or prevent a disease, can have a detrimental effect on another existing disease/condition in the individual (Mallet et al.)75 |
| Anticholinergic burden | This refers to the anticholinergic burden associated with care home residents’ medication regimens. Medicines with anticholinergic effects are commonly prescribed for various conditions; however, increased overall exposure to anticholinergics has been associated with an increased risk of cognitive impairment, falls and all-cause mortality in older adults (Ruxton et al.)76 |
| Adverse drug events | Adverse drug events experienced by care home residents. ‘An adverse drug event is any undesirable event experienced by a patient whilst taking a medicine, including physical harm, mental harm or loss of function’ (Bates et al.)77 |
| Prescribing errors | Prescribing errors in care home residents’ medication regimens. A prescribing error is ‘a prescribing decision that results in an unintentional, significant reduction in the probability of treatment being timely and effective or a significant increase in the risk of harm, when compared with that in generally accepted practice’ (Dean et al.)78 |
| Falls | Falls occurring amongst care home residents. A fall is ‘an event that results in a person coming to rest inadvertently on the ground or floor or other lower level’ (World Health Organization)79 |
| Quality of life | A measure of care home residents’ quality of life. Quality of life is ‘a ubiquitous concept that has different philosophical, political and health-related definitions. Health-related quality of life includes the physical, functional, social and emotional well-being of an individual’ (Fallowfield)80 |
| All-cause mortality | All deaths of care home residents |
| Admissions to hospital (and associated costs) | The number of care home residents having a hospital admission/number of hospital admissions per resident (and the associated cost) |
| Accident and Emergency visits to hospital (and associated costs) | The number of care home residents attending Accident and Emergency departments/number of Accident and Emergency visits per resident (and the associated cost) |
Work package 4 tables
| Author | Location | Year | Setting | Study type | Service | Main outcome |
|---|---|---|---|---|---|---|
| Roberts, MS, et al.43 | Australia | 2001 | Nursing home | Randomised controlled trial | Medication review | Reduction in the number of medicines |
| King, MA, et al.81 | Australia | 2001 | Nursing home | Service evaluation | Medication review | Non-significant reductions in medication orders, cost and mortality |
| Smith, MA, et al.82 | Australia | 2002 | Nursing home | Service evaluation | Medication review | Significant reduction in the number of doses of regular medicine prescribed |
| Crotty, M, et al.83 | Australia | 2004 | Residential aged care | Randomised controlled trial | Pharmacist supported patient transit from hospital | Improved pain control and hospital usage in the intervention arm |
| Beer, C, et al.84 | Australia | 2011 | Residential aged care | Randomised controlled trial | Withdrawal of one target medicine | Trial acceptable to patients |
| Khalil, H85 | Australia | 2011 | Aged care facility | Service evaluation | Medication review | Clinical recommendations implemented |
| Verrue, CL, et al.86 | Belgium | 2010 | Nursing home | Service evaluation | Training on medication administration | Reduction in medication administration errors |
| Verrue, C, et al.87 | Belgium | 2012 | Nursing home | Controlled study | Medication review service | Modest improvement in medication appropriateness |
| Soon, JA88 | Canada | 1985 | Nursing home | Service evaluation | Adverse drug reaction monitoring program | Earlier detection and resolution of adverse drug reactions |
| Kroger, E, et al.89 | Canada | 2015 | Nursing home | Service evaluation | Medication review | Service to optimise medicine use is feasible |
| Norgaard, LS, et al.90 | Denmark | 2015 | Residential care setting | Service evaluation | Medication review and medicine reconciliation | Reduction in drug-related problems |
| Legueline, G, et al.91 | France | 2013 | Nursing home | Controlled study | Medication review | Significant reduction in adverse drug event risk |
| Frankenthal, D, et al.92 | Israel | 2014 | Chronic geriatric facility | Randomised controlled trial | Medication review with STOPP/START | Significant reduction in the number of medicines |
| Finkers, F, et al.93 | The Netherlands | 2007 | Nursing home | Service evaluation | Medication review | Significant reduction in drug-related problems |
| Stujit, CCM, et al.94 | The Netherlands | 2008 | Residential home | Service evaluation | Medication review | Significant improvement in medication appropriateness |
| Stujit, CCM, et al.95 | The Netherlands | 2013 | Nursing home | Service evaluation | Training in medication administration for patients with dysphagia | Significant reduction in medication administration errors |
| Connolly, MJ, et al.96 | New Zealand | 2014 | Long-term care facility | Cluster randomised controlled trial | Nurse-led staff education Multi-professional review |
No effect on hospitalisations and mortality; fewer acute admissions |
| Ruths, S, et al.97 | Norway | 2003 | Nursing home | Service evaluation | ||
| Bellingan, M, et al.98 | South Africa | 1996 | Elderly care facility | Service evaluation | Medication review | Significant reduction in drug-related problems and polypharmacy |
| Jodar-Sanchez, F, et al.99 | Spain | 2014 | Nursing home | Controlled study | Medication review | Number of medicines significantly reduced |
| Bergman, A, et al.100 | Sweden | 2006 | Nursing home | Service evaluation | Medication appropriateness review | 70% of prescriptions potentially inappropriate |
| Kuo, CN, et al.101 | Taiwan | 2013 | Nursing home | Service evaluation | Medicine reconciliation | Reduction in medication discrepancies |
| Newman, GR102 | UK | 1982 | Residential aged care | Service evaluation | Medication handling intervention | Positive feedback on scheme from participants |
| Narula, N, et al.103 | UK | 1992 | Residential home | Service evaluation | Review of medication administration problems | Problem report form developed |
| Rees, JK, et al.104 | UK | 1995 | Residential home | Service evaluation | Medication review | Clinical recommendations implemented |
| Furniss, L, et al.105 | UK | 2000 | Nursing home | Randomised controlled trial | Significant reduction in medicines; no effect on mortality | |
| Zermansky, A, et al.51 | UK | 2006 | Care home for elderly people | Randomised controlled trial | Medication review | Significant difference in the number of drug changes per patient |
| Alldred, DP, et al.106 | UK | 2007 | Care home for elderly people | Randomised controlled trial | Medication review | Clinical recommendations implemented |
| Saaed, M, et al.107 | UK | 2010 | Nursing home | Service evaluation | Audit medication administration records | Reduction in medication errors |
| Patterson, SM, et al.108 | UK | 2011 | Nursing home | Randomised controlled trial | Medication review | Significant improvement in antipsychotic medication appropriateness |
| Hampson, N109 | UK | 2012 | Care home | Service evaluation | Medication review | Reduction in medication costs |
| Cooper, JW, et al.110 | USA | 1978 | Long-term care facility | Service evaluation | Medication review | Number of medicines reduced |
| Cooper, JW111 | USA | 1985 | Long-term care facility | Service evaluation | Medication review | Reduction in drug use |
| Andolesk, K112 | USA | 1987 | Long-term care facility | Service evaluation | Medication review | Number of medicines reduced |
| Pucino, F113 | USA | 1988 | Long-term care facility | Service evaluation | Therapeutic drug monitoring service | Anecdotal improvements in patient control |
| Cooper, JW114 | USA | 1995 | Nursing facility | Controlled study | Medication review | Significant reduction in hypoglycaemic and hyperglycaemic events in home where the pharmacist is authorised to make changes independently |
| Cooper, JW115 | USA | 1997 | Geriatric nursing facility | Service evaluation | Medication review | Clinical recommendations implemented |
| Jeffrey, S, et al.116 | USA | 1999 | Long-term care facility | Service evaluation | Medication review | Reduction in the number of unnecessary medicines |
| Elliott, RA, et al.117 | USA | 1999 | Nursing home | Service evaluation | Medication review | Reduction in medication costs |
| Lai, LL, et al.118 | USA | 2001 | Nursing home | Service evaluation | Antibiotic medication review | Clinical recommendations implemented |
| Christensen, D, et al.119 | USA | 2004 | Nursing home | Service evaluation | Medication review | Reduction in polypharmacy |
| Crotty, M, et al.120 | USA | 2004 | Long-term care facility | Randomised controlled trial | Medication review | Significant improvement in medication appropriateness |
| Buhr, GT, et al.121 | USA | 2006 | Nursing home | Service evaluation | Quality improvement project to improve pain management | Staff pain management knowledge improved |
| Cooper, JW, et al.122 | USA | 2007 | Geriatric nursing facility | Service evaluation | Medication review | Clinical recommendations implemented |
| Hursh, D, et al.123 | USA | 2010 | Nursing facility | Service evaluation | Improving antipsychotic medication use | Reduced antipsychotic medication use |
| Gunning, K, et al.124 | USA | 2010 | Assisted living facility | Service evaluation | Integration of pharmacists into the team | Students valued the experience, felt valued as medication experts |
| Lapane, KL, et al.125 | USA | 2011 | Nursing home | Randomised controlled trial | Implementation of Geriatric Risk Assessment MedGuide | Significantly lower rate of potential delirium onset |
| Motycka, C, et al.126 | USA | 2012 | Long-term care facility | Service evaluation | Warfarin medication review | Significantly greater proportion of patients in the therapeutic range |
| Zarowitz, BJ, et al.127 | USA | 2012 | Nursing home | Service evaluation | Quality improvement project to improve methotrexate administration | Reduction in methotrexate administration errors |
| Nye, A128 | USA | 2012 | Nursing home | Service evaluation | Medication review | Clinical recommendations implemented |
| Phillippe, HM129 | USA | 2012 | Long-term care facility | Service evaluation | Anticoagulation service | Improved time in therapeutic range |
| Lemay, CA, et al.130 | USA | 2013 | Nursing home | Survey | Education needs for staff regarding antipsychotic medication | Multifaceted intervention to improve care home staff knowledge regarding the antipsychotics required |
| Codified knowledge | Practical knowledge | |
|---|---|---|
| Therapeutic area (n) | Clinical area (n) | Activity (n) |
| Psychotropic (15) | Dementia (10) | Medication review (40) |
| Cardiovascular (11) | Pain (5) | Medicine discontinuation (24) |
| Gastrointestinal (6) | Diabetes (4) | Medicine change (23) |
| Benzodiazepines (4) | Cardiovascular disease (4) | Monitoring recommendations (20) |
| Analgesia (4) | Stroke (2) | Multidisciplinary intervention (20) |
| Nutrition and blood (3) | Dysphagia (2) | Medicine initiation (12) |
| Anticoagulants (2) | Infection (1) | Care home staff training (8) |
| Antimicrobials (2) | Behavioural problems (1) | Error management (6) |
| Urinary tract (1) | Pulmonary disease (1) | Medicine reconciliation (4) |
| Fall prevention (1) | Use of the STOPP/START tool (2) | |
| Medicine administration (1) | ||
Work package 5 tables
| Total number invited | Total number of EOIs (%) | Number participating at baseline | Number participating at follow-up (%) | |
|---|---|---|---|---|
| GP practicesa | 346 | 33 (9.5) | 4 | 4 (100) |
| PIPs | 22 | 14 (57.1) | 4 | 4 (100) |
| Care homes | 6 | n/a | 6 | 6 (100) |
| Residents | 86 | 53 (62.2) | 40 | 40 (100); 2 died |
| Measure | Baseline | Follow-up |
|---|---|---|
| STOPP | ||
| Mean (per patient) (range) SD | 3.27 (0–7) 1.96 | 2.54 (0–5) 1.24 |
| Median (IQR) | 3.0 (2.0–4.25) | 2.0 (2.0–3.25) |
| START | ||
| Mean (per patient) (range) SD | 2.35 (0–6) 1.42 | 2.05 (0–6) 1.55 |
| Median (IQR) | 2 (1–3) | 2.0 (1.0–3.0) |
| Falls in the past 3 months (total number)a | 12 | 10 |
| Falls in the past 3 months (number of patients falling) | 9/40 (20%) | 7/40 (17.5%) |
| Falls per person | ||
| Mean (range) SD | 0.33 (0–3) 0.694 | 0.25 (0–2) 0.588 |
| Median (IQR) | 0.0 (0–0) | 0.0 (0–0) |
| (n = 40) | (n = 38) | |
| Barthel Index | ||
| Mean (range) SD | 6.53 (0–17) 5.50 | 6.38 (0–19) 5.51 |
| Median (IQR) | 6.5 (1–9) | 6.0 (1.0–10.0) |
| MMSE | ||
| Mean (range) SD | 20.13 (9–30) 7.03 | 20.79 (11–29) 5.90 |
| Median (IQR) | 21.0 (14.25–25.5) | 20.5 (15–26) |
| Number | (n = 16) | (n = 14) |
| Drug Burden Index | ||
| Score 0 (n (%)) | 14 (35%) | 14 (35%) |
| Drug Burden Indexb | ||
| N Score > 0 (n (%) | 26 (65%) | 26 (65%) |
| Mean (range) SD | 1.11 (0.14–3.37) 0.74 | 0.93 (0.14–3.34) 0.67 |
| Median (IQR) | 1.035 (0.5–1.5) | 0.76 (0.5–1.17) |
| Number of medicines per patientc | ||
| Mean (range) SD) | 9.3 (1–26) 5.9 | 8.7 (0–31) 6.0 |
| Median (IQR) | 8.5 (6–12) | 8 (5–10) |
| Total QUALIDEM | ||
| Mean (range) SD | 93 (67–130) 15.55 | 77.85 (31–109) 18.32 |
| Median (IQR) | 91.0 (79.25–101.5) | 79.0 (71.0–92.5) |
| Care relationship | ||
| Mean (range) SD | 15.33 (10–23) 3.53 | 15.83 (4–21) 5.00 |
| Median (IQR) | 14.0 (12.25–18.0) | 16.0 (13.0–20.0) |
| Positive affect | ||
| Mean (range) SD | 14.33 (6–24) 5.50 | 12.43 (2–18) 4.33 |
| Median (IQR) | 14.5 (9.0–18.75) | 14.0 (8.5–16.0) |
| Negative affect | ||
| Mean (range) SD | 8.25 (3–12) 2.25 | 5.35 (0–9) 2.28 |
| Median (IQR) | 9.0 (6.0–10.0) | 5.0 (4.0–7.0) |
| Restless/tense behaviour | ||
| Mean (range) SD | 8.48 (3–12) 2.59 | 5.13 (0–9) 2.41 |
| Median (IQR) | 9.0 (6.25–10.0) | 5.0 (4.0–7.0) |
| Positive self-image | ||
| Mean (range) SD | 7.88 (3–11) 1.88 | 6.23 (0–9) 2.24 |
| Median (IQR) | 8.0 (6.0–9.0) | 6.0 (4.25–8.75) |
| Social relations | ||
| Mean (range) SD | 12.03 (7–22) 3.68 | 11.13 (1–18) 4.79 |
| Median (IQR) | 11.0 (10.0–13.0) | 10.5 (8.25–15.75) |
| Social isolation | ||
| Mean (range) SD | 6.00 (3–11) 1.45 | 4.60 (10–9) 1.99 |
| Median (IQR) | 6.0 (5.0–7.0) | 5.0 (4.0–6.0) |
| Feeling at home | ||
| Mean (range) SD | 8.88 (6–14) 2.22 | 9.68 (1–12) 2.74 |
| Median (IQR) | 8.0 (7.0–11.0) | 11.0 (8.0–12.0) |
| EQ-5D-5L (self)d | ||
| Mean (range) SD | 0.449 (−0.281 to −0.951) 0.335 | 0.572 (−0.027 to −1.000) 0.327 |
| Median (IQR) | 0.379 (0.279–0.719) | 0.585 (0.34–0.77) |
| N | 16 | 13 |
| EQ-5D-5L (proxy)d | ||
| Mean (range) SD | 0.434 (−0.027 to −0.896) 0.229 | 0.406 (−0.019 to −0.887) 0.236 |
| Median (IQR) | 0.356 (0.267–0.664) | 0.341 (0.206–0.581) |
| N | 39 | 38 |
| EQ-5D-5L baseline VAS (self)d | ||
| Mean (range) SD | 57.5 (0–100) 31.9 | 62.9 (10–99) 30.2 |
| Median (IQR) | 67.5 (32.5–75) | 75 (30–85) |
| N | 14 | 11 |
| EQ-5D-5L baseline VAS (proxy)d | ||
| Mean (range) SD | 51.6 (2–99) 23.9 | 50.2 (2–90) 27.3 |
| Median (IQR) | 57.5 (38.75–66.25) | 50 (30–70) |
| N | 38 | 37 |
| Adverse drug events related to the intervention in the past 3 months | 0/40 (0%) | 0/40 (0%) |
| Theme | Sub theme | Quote no., quote, interviewee type |
|---|---|---|
| Perceived benefits of the service | Improved patient care | 1. ‘I think you know overall it just had led to better patient care, better medicines management you know for those patients and nursing homes’. (GP) |
| 2. ‘She’s very professional in the fact that you know that she knows what she’s talking about so there’s no question about it, XXX (PIP) gonna know the answer for us and she doesn’t take time up to do anything’. (Care home manager) | ||
| Improved patient safety | 3. ‘Swallowing issues, what medicines are suitable for crushing, different formulations especially when we’re looking at medications where people can no longer take, the refusals, all due to their cognitive behaviours, you know, looking at whether we should, well you know we know about crushing and then changing it to liquid form, having the pharmacist part of the care home makes it safer again for that reason because GPs will just automatically say oh well crush because we can’t afford to give you liquid’. (Care home manager) | |
| Saving staff time and effort – more efficient | 4. ‘Sometimes I find when you go through GPs it takes much longer if, you know, if you ask them to reduce something, … then they pass it on. I found with XXX (PIP) after her phone call, it’s implemented straight away, you know, there’s no hanging around, which is good, I like that’. (Care home manager) | |
| 5. ‘I also know there’s a professional behind me that’s doing something that I don’t have to double check at all. I don’t have to turn round and double check what she’s doing as this day of audits and auditing everything that happens, with somebody like that around I have got somebody else to share the job’ (Care home manager) | ||
| 6. ‘Well I really welcomed it because I think all the care homes could do with an independent practitioner …… having to wait 48 hours for an urgent prescription and it’s just been horrendous before you came in regards to trying to get what we need from the surgery so having XXX (PIP) here was wonderful’. (Dementia nurse) | ||
| 7. ‘I have said to her, you know, could you explain to the relatives about this for me. It takes another 20 minutes out of my day’. (Care home manager) | ||
| Perceived benefits of the service | Saving staff time and effort – more efficient | 8. ‘I think the pharmacist was able to spend more time with us and the resident looking at the medications that they were on, speaking to the staff who knew the residents really well and getting a detailed history which unfortunately we know the GPs haven’t got the time to do that so we thought it was really …… really helpful, yeah’. (Care home manager) |
| 9. The most worthwhile thing is that it’s not a GP that’s doing it, that it’s someone qualified and the right person to do it … it’s good that that’s done with a back-up from us and it’s done in the most efficient time-responsible way which is I guess having a pharmacist to do it’. (GP) | ||
| 10. ‘It was very good. XXX (PIP) did most of the work. I had some involvement with looking at the plans and reviewing them and also kind of any bits of advice, but she did most of the work herself and led it herself so I wasn’t hugely involved’. (GP) | ||
| 11. ‘It didn’t really impede on the day to day running of the home, it wasn’t really intrusive to the residents’ day to day lives’. (Deputy care home manager) | ||
| 12. ‘There was some increased workload for the staff because of the time that we needed to give to the pharmacist to discuss the resident in more detail and sought of you know providing the relevant care plans … but generally any changes were done at the beginning of the monthly cycle so there wasn’t that much extra work involved’. (Care home manager) | ||
| 13. ‘Absolutely, if we could have a XXX (PIP) in every single practice, and I know that’s hopefully what’s gonna happen, it would make my job so much easier …. I can see it could make my job a lot less stressful if we had that service right across the board’. (Care home manager) | ||
| Perceived disadvantages of the service | Knowledge of the patient | 14. ‘With the lady in question she was saying but the pharmacist doesn’t know her, the pharmacist doesn’t know her history’. (Care home manager) |
| 15. ‘because XXX (PIP) is going in and dealing with maybe some of the issues that we would have dealt with in the past, that there’s the potential that you see your patients less and you have less of a close relationship with some patients in the nursing homes so that would be a potential negative going forward …. you would have less contact with the nursing home staff cos quite a number of our contacts with nursing homes are you know medication issues so if a pharmacist was picking up them, yeah, we may have less contact, but I don’t think there would be any major negative impact from such a scheme’. (GP) |
Work package 6 tables
| Intervention, N = 449 residents | Control, N = 427 residents | Overall, N = 876 residents | |
|---|---|---|---|
| Age at consent in years, mean (SD) | 85.1 (7.7) | 85.4 (7.6) | 85.3 (7.7) |
| Gender, n (%) | |||
| Male | 125 (27.8%) | 141 (33.0%) | 266 (30.4%) |
| Female | 324 (72.2%) | 286 (67.0%) | 610 (69.6%) |
| Consent, n (%) | |||
| Participant | 59 (13.1%) | 51 (11.9%) | 110 (12.6%) |
| Consultee | 390 (86.9%) | 376 (88.1%) | 766 (87.4%) |
| Resident care home status, n (%) | |||
| With nursing | 188 (42.3%) | 250 (59.0%) | 438 (50.5%) |
| Residential only | 256 (57.7%) | 174 (41.0%) | 430 (49.5%) |
| Missing | 5 | 3 | 8 |
| Number of medications: | |||
| Median (q0.25, q0.75) | 6 (4, 9) | 6 (4, 9) | 6 (4, 9) |
| Minimum, maximum | 1, 19 | 1, 19 | 1, 19 |
| Missing | 2 | 4 | 6 |
| Falls in previous 90 days | |||
| Median (q0.25, q0.75) | 0 (0, 1) | 0 (0, 1) | 0 (0, 1) |
| Minimum, maximum | 0, 30 | 0, 18 | 0, 30 |
| Mean (SD) | 0.78 (2.30) | 0.57 (1.43) | 0.68 (1.93) |
| Hospital admissions in previous 90 days | |||
| Median (q0.25, q0.75) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) |
| Minimum, maximum | 0, 2 | 0, 3 | 0, 3 |
| Mean (SD) | 0.07 (0.26) | 0.08 (0.30) | 0.09 (0.33) |
| Barthel Index, mean (SD) | 8.34 (5.78) | 7.07 (5.77) | 7.74 (5.81) |
| Missing | 10 | 35 | 45 |
| Drug Burden Index, mean (SD) | 0.72 (0.75) | 0.70 (0.69) | 0.71 (0.72) |
| Missing | 5 | 2 | 7 |
| Charlson Comorbidity Index: mean (SD) | 5.94 (1.84) | 5.98 (1.52) | 5.96 (1.69) |
| Missing | 5 | 6 | 11 |
| EQ-5D Self-Utility Score, mean (SD) | 0.49 (0.37) | 0.33 (0.36) | 0.41 (0.37) |
| Missing | 396 | 377 | 773 |
| EQ-5D Proxy Utility score, mean (SD) | 0.31 (0.35) | 0.29 (0.37) | 0.30 (0.36) |
| Missing | 33 | 53 | 86 |
| Intervention, N = 449 | Control, N = 427 | Rate ratioa (model 1) | Rate ratiob (model 2) | |
|---|---|---|---|---|
| Total falls | 697 | 538 | ||
| Follow-up (person-days) | 79 803 | 76 904 | ||
| Crude fall rate/year and rate ratio | 4.78 | 3.71 | 1.00 | 0.91 |
| Confidence interval | 0.73–1.36 | 0.66–1.26 | ||
| p-value | 0.580 | 0.992 | ||
| Minimum, maximum | 0, 59 | 0, 27 | ||
| Q25, Q75 | 0, 2 | 0, 1 | ||
| Median | 0 | 0 |
| Intervention, N = 449 | Control, N = 427 | Comparisona | Fully adjusted comparisonb | |
|---|---|---|---|---|
| Hospitalisations per person | ||||
| Median (q0.25,q0.75) | 0 (0, 0) | 0 (0, 0) | ||
| Minimum, maximum | 0, 4 | 0, 3 | ||
| Mean (SD) or RR | 0.19 (0.50) | 0.18 (0.47) | 0.98 | 0.90 |
| 95% CI | 0.66–1.46 | 0.60–1.31 | ||
| p-value | 0.932 | 0.573 | ||
| Barthel Index | ||||
| Mean (SD) or RR | 8.12 (5.84) | 6.46 (5.66) | 1.19 | 1.20 |
| 95% CI | 0.96–1.49 | 0.96–1.49 | ||
| p-value | 0.116 | 0.107 | ||
| Missing | 113 | 110 | ||
| Drug Burden Index | ||||
| Mean (SD) or RR | 0.66 (0.74) | 0.73 (0.69) | 0.83 | 0.83 |
| 95% CI | 0.75 to 0.93 | 0.74–0.92 | ||
| p-value | <0.001 | <0.001 | ||
| Missing | 10 | 9 | ||
| Intervention, N = 449 | Control, N = 427 | Absolute difference between intervention and controla | Absolute difference fully adjusted comparisonb | |
|---|---|---|---|---|
| Three months | ||||
| EQ-5D Self-Utility score | ||||
| Mean (SD) | 0.32 (0.37) | 0.18 (0.33) | 0.079 (−0.028 to 0.186) | 0.047 (−0.021 to 0.114) |
| Missing | 372 | 352 | p = 0.146 | p = 0.175 |
| EQ-5D Proxy Utility score | ||||
| Mean (SD) | 0.28 (0.35) | 0.28 (0.35) | −0.017 (−0.073 to 0.039) | −0.043 (−0.092 to 0.006) |
| Missing | 77 | 47 | p = 0.556 | p = 0.082 |
| Six months | ||||
| EQ-5D self-utility score | ||||
| Mean (SD) | 0.18 (0.33) | 0.14 (0.29) | 0.010 (−0.115 to 0.135) | 0.012 (−0.114 to 0.139) |
| Missing | 353 | 326 | p = 0.873 | p = 0.849 |
| EQ-5D proxy utility score | ||||
| Mean (SD) | 0.26 (0.35) | 0.21 (0.33) | 0.030 (−0.021 to 0.080) | 0.042 (−0.043 to 0.052) |
| Missing | 53 | 47 | p = 0.249 | p = 0.862 |
| Category | ||
|---|---|---|
| Interventions per resident (average) | 1.8 | |
| Technical interventions [n (%)] | 99 (11.2) | |
| Educational intervention [n (%)] | 3 (0.4) | |
| Clinical interventions [n (%)] | 566 (85) | |
| Type of clinical intervention | Medicine discontinuation/dose reduction [n (%)] | 379 (67) |
| Start new medication [n (%)] | 60 (10.6) | |
| Change medication [n (%)] | 49 (8.6) | |
| Dose increase [n (%)] | 26 (4.6) | |
| Monitoring [n (%)] | 52 (9.2) |
| BNF therapeutic area | Pharmacist independent prescriber medication intervention | Total, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Dose increase | Medication discontinuation | Changing the medication | Dose reduction | Starting new medication | Drug monitoring recommendation | |||
| 1 | Gastrointestinal system | 3 | 43 | 2 | 20 | 21 | 0 | 89 (15.8) |
| 2 | Cardiovascular system | 6 | 53 | 11 | 18 | 8 | 7 | 103 (18.2) |
| 3 | Respiratory system | 1 | 15 | 2 | 0 | 6 | 0 | 24 (4.3) |
| 4 | Nervous system | 10 | 81 | 15 | 51 | 3 | 29 | 189 (33.5) |
| 6 | Endocrine system | 4 | 17 | 4 | 5 | 3 | 8 | 41 (7.3) |
| 9 | Blood and nutrition | 2 | 43 | 5 | 1 | 13 | 5 | 69 (12.2) |
| 13 | Skin | 0 | 10 | 10 | 0 | 0 | 3 | 23 (4) |
| Others | 0 | 22 | 0 | 0 | 6 | 0 | 28 (4.6) | |
| Total n (%) | 26 (4.6) | 284 (50.3) | 49 (8.6) | 95 (16.6) | 60 (10.6) | 52 (9) | 566 (100) | |
| Contextual factor | Data collected | Data source | |
|---|---|---|---|
| Barriers to delivering the intervention | Feedback from stakeholders | Care home staff interview | |
| GP interview | |||
| PIP interview | |||
| NoMAD16 survey to GPs/PIPs and care home staff | |||
| Other anecdotal feedback | |||
| Facilitators to delivering the intervention | Feedback from stakeholders | Care home staff interviews | |
| GP interview | |||
| PIP interview | |||
| NoMAD16 survey to GPs/PIPs and care home staff | |||
| Other anecdotal feedback | |||
| Site and participant factors | Inter-PIP variation | Competency | Variation in outcomes |
| Review of PCPs for both safety and missed opportunity | |||
| GP interview | |||
| Care home interviews | |||
| Employment status | Baseline PIP questionnaire | ||
| Qualifications | Baseline PIP questionnaire | ||
| Inter-site variation | Care home factors | Baseline CH survey | |
| Resident factors | Baseline resident data | ||
| Inter-location variation | Views of researchers | Meeting minutes | |
| Normalisation of the intervention into routine practice | Actions taken by participants to ensure the intervention works | Coherence (making sense of the service) | NoMAD survey16 to PIPs, CH staff, GPs |
| GP Interview CH staff Interviews |
|||
| PIP interview | |||
| Cognitive participation (engaging with the service) | NoMAD16 survey to PIP, CH staff, GPs | ||
| Interviews (GP and CH staff) | |||
| PIP interview | |||
| Collective action (delivering the service/responding to the service) | NoMAD16 survey to PIP, CH staff, GPs | ||
| GP interview CH staff interviews |
|||
| PIP interview | |||
| Reflexive monitoring (appraising and reviewing the service) | NoMAD16 survey to PIP, CH staff, GPs | ||
| GP interview CH staff interviews |
|||
| PIP interview |
| Task assessed | Data collected | Data source |
|---|---|---|
| Effectiveness of training | PIPs’ views on training | Post-training feedback forms |
| PIP Interview | ||
| PIP questionnaire | ||
| Online survey | ||
| Competency | Competency assessments | |
| Appropriateness of PCPs (20% of the sample) | ||
| Missed opportunities (50% of the sample) | ||
| Views of stakeholders (interviews) | ||
| Intervention fidelity | Services provided and frequency with which provided | PIP activity logs |
| No. of pharmaceutical care plans | ||
| PIP questionnaire | ||
| Quality of medication review | Review of 20% of PCPs |
| Impact | Mechanism of impact | Data collected | Data source |
|---|---|---|---|
| Medication changes identified | PIP medication review | Recommendations for change and rationale | Pharmaceutical care plans |
| PIP interview PIP questionnaire |
|||
| Medication changes made | PIP prescribing | Total no. of medications per patient at baseline and 6 months | Pharmaceutical care plans |
| GP records | |||
| No. of medications stopped per patient at 6 months | Pharmaceutical care plans | ||
| GP records | |||
| No. of medications started per patient at 6 months | Pharmaceutical care plans | ||
| GP records | |||
| No. of medications amended, e.g. dose change, formulation change | Pharmaceutical care plans | ||
| GP records | |||
| No. of antipsychotics/psychotropics prescribed at baseline and 6 months | Pharmaceutical care plans | ||
| GP records | |||
| Categorised description of drugs changed, stopped and started | Resident medical records | ||
| Biochemical monitoring | PIP medication review | Recommendations made for biochemical monitoring | Pharmaceutical care plans |
| Medication errors | PIP medication review | No. of prescribing, dispensing and administration errors | Pharmaceutical care plans |
| GP records | |||
| Non-patient facing activities improved, e.g. medication storage advice | PIP support for care home | Services provided and frequency | PIP activity log |
| Views on the usefulness of services | Care home staff interviews | ||
| PIP interview PIP questionnaire |
|||
| Better/tailored training for staff | PIP training for care home staff | Training provided and frequency | PIP activity log |
| Views on the usefulness of training | Care home staff interviews | ||
| PIP interview PIP questionnaire |
|||
| Quality of communication between care home, GP and community pharmacy | PIP input into communication | Views of care home staff | Care home staff interviews |
| Views of GPs | GP interview | ||
| Views of PIPs | PIP interview PIP questionnaire |
| Aim | Outcome | Data collected | Data source |
|---|---|---|---|
| To improve quality of care for those over 65 years old resident in care homes | Falls | Fall rate per person at 3 months | Care home fall record |
| Fall rate per person at 6 months | Care home fall record | ||
| Quality of life | Self-reported quality of life | Face-to-face self-reported EQ-5D-5L (applicable only for participants with capacity) at baseline, 3 months and 6 months | |
| Carer-assessed quality of life | Proxy EQ-5D-5L (quality of life) at baseline, 3 months and 6 months | ||
| Physical functioning | Carer-assessed physical functioning | Proxy Barthel Index (physical functioning) at baseline and 6 months | |
| Health service utilisation and associated costs | Costs of care (medication, health care team contacts, monitoring and tests) | GP records at baseline and 6 months | |
| Drug Burden Index | Calculate Drug Burden Index based on medications | GP records at baseline and 6 months | |
| To assess intervention safety | Mortality | Information on the number of residents dying | Monthly call to care homes |
| Hospitalisations (not always a negative marker of safety) | Information on the number of residents hospitalised | Monthly call to care homes | |
| Global viewa | Perceptions of GPs | GP interview | |
| Perceptions of care home staff | Care home staff interviews | ||
| Perception of residents/consultee/WPOA | Resident/consultee/WPOA interviews | ||
| Perceptions of PIPs | PIP interview | ||
| Adverse eventsa | New drug-related symptoms | Stakeholder feedback using the standard template | |
| Serious adverse eventsa | See hospitalisations/deaths | Monthly call to care homes | |
| Sudden unexpected serious adverse eventsa | See hospitalisations/deaths | Feedback from GPs/independent medical assessor on the causal link with the PIP intervention |
| Unit cost (£) | ||||
|---|---|---|---|---|
| Resource item | Per hour of employment | Care home visit | Hospital/community visit | Telephone call |
| PIP (band 7) | 5324 | |||
| PIP mentor/trainer (8a) | 6324 | |||
| GP | 11024 | 8524 | 2824 | 2124 |
| Practice nurse | 2724 | 1624 | 624 | |
| Ambulance call out | 19224 | 25224 | ||
| A&E visit | 13824 | |||
| Pharmacist (not CHIPPS PIP) | 2924 | 2924 | 924 | |
| District nurse | 3324 | 3324 | 824 | |
| Nurse specialist | 4324 | 4324 | 1124 | |
| Dietitian | 8624 | 8624 | 724 | |
| Podiatrist | 4324 | 4324 | 1124 | |
| Physiotherapist | 5724 | 5724 | 1424 | |
| Occupational therapist | 8124 | 8124 | 2024 | |
| Speech therapist | 9624 | 9624 | 2424 | |
| Community care assistant | 1124 | 1124 | ||
| MRI | 14624 | |||
| ECG | 13624 | |||
| CT scan | 10424 | |||
| DEXA scan | 7724 | |||
| Ultrasound | 5524 | |||
| Radiography | 3124 | |||
| Directly accessed pathology services | 224 | |||
| Resource use item | Time period | PIP intervention (n = 449) | Control (n = 427) |
|---|---|---|---|
| Training: PIP time (hours per PIP) | 6-month FU | 32.30 (n = 449) | - |
| Total PIP activity time (minutes) | 6-month FU | 191.33 (n = 449) | |
| Medication (number of different items prescribed/packs ordered) | Baselineb | 26.95 (n = 448)) | 27.74 (n = 425) |
| 6-month FU | 34.85 (n = 448) | 33.66 (n = 425) | |
| Outpatient attendances | Baselineb | 0.10 (n = 448) | 0.10 (n = 426) |
| 6-month FU | 0.18 (n = 443) | 0.20 (n = 420) | |
| Inpatient admissions | Baselineb | 0.08 (n = 449) | 0.07 (n = 427) |
| 6-month FU | 0.16 (n = 448) | 0.15 (n = 426) | |
| Tests/investigations | Baselineb | 1.36 (n = 44) | 1.75 (n = 427) |
| 6-month FU | 3.10 (n = 443) | 2.57 (n = 420) | |
| GP/practice nurse visits/telephone calls | Baselineb | 3.24 (n = 448) | 3.32 (n = 426) |
| 6-month FU | 5.26 (n = 443) | 5.95 (n = 420) | |
| Other healthcare professional visits/telephone calls | Baselineb | 3.55 (n = 446) | 3.66 (n = 422) |
| 6-month FU | 6.91 (n = 398) | 6.36 (n = 402) |
| Resource use, mean (SD) (n) | Time period | PIP intervention (n = 449) | Control (n = 427) |
|---|---|---|---|
| PIP training costa | 6-month FU | £137.90 (£0.00) (n = 449) | |
| PIP activity costa | 6-month FU | £205.29 (£170.31) (n = 449) | - |
| GP time saving | 6-month FU | −£20.60 (£0.00) (n = 449) | - |
| Total PIP intervention cost | 6-month FU | £322.59 (£170.31) (n = 449) | - |
| Overall medication costs | Baseline | £248.28 (£289.39) (n = 448) | £279.25 (£300.58) (n = 425) |
| 6-month FU | £443.53 (£577.36) (n = 448) | £504.92 (£618.02) (n = 425) | |
| Outpatient attendances | Baselineb | £12.57 (£46.07) (n = 448) | £12.38 (£47.03) (n = 426) |
| 6-month FU | £21.91 (£78.20) (n = 443) | £23.40 (£76.33) (n = 420) | |
| Inpatient stays | Baselineb | £229.28 (£911.06) (n = 449) | £160.42 (£669.52) (n = 427) |
| 6-month FU | £518.08 (£1338.60) (n = 448) | £509.79 (£1303.44) (n = 426) | |
| Tests/investigations | Baselineb | £3.83 (£9.43) (n = 448) | £5.19 (£16.38) (n = 427) |
| 6-month FU | £11.26 (£26.24) (n = 444) | £8.38 (£19.51) (n = 421) | |
| GP/practice nurse visits/telephone calls | Baselineb | £182.16 (£222.58) (n = 448) | £183.01 (£185.22) (n = 426) |
| 6-month FU | £302.30 (£330.63) (n = 443] | £340.53 (£282.36) (n = 420) | |
| Other healthcare professional visits/telephone calls | Baselineb | £160.52 (£229.49) (n = 446) | £170.23 (£253.22) (n = 422) |
| 6-month FU | £337.20 (£400.25) (n = 398) | £321.01 (£398.46) (n = 402) | |
| Total other (non-medication) costs | Baselineb | £590.56 (£1050.61) (n = 445) | £532.82 (£820.47) (n = 422) |
| 6-month FU | £1189.44 (£1544.28) (n = 398) | £1213.31 (£1584.07) (n = 401) | |
| Total costs | Baselineb | £840.27 (£1122.87) (n = 445) | £811.83 (£881.58) (n = 422) |
| 6-month FU | £1970.42 (£1690.20) (n = 398) | £1724.82 (£1746.38) (n = 401) |
| Item, mean (SD) (n) (% response rate) | Intervention (n = 449) | Control (n = 427) |
|---|---|---|
| Baseline EQ-5D-5L score | 0.313 (0.350) [416] (92.7%) | 0.287 (0.369) [374] (87.6%) |
| Three-month EQ-5D-5L score | 0.284 (0.349) [372] (82.9%) | 0.278 (0.349) [380] (89.0%) |
| Three-month change in the EQ-5D-5L score | −0.056 (0.256) [353] (82.7%) | −0.018 (0.271) [347] (81.3%) |
| Six-month EQ-5D-5L score | 0.263 (0.348) [396] (88.2%) | 0.209 (0.330) [380] (89.0%) |
| Six-month change in EQ-5D-5L score | −0.061 (0.279) [369] (82.2%) | −0.071 (0.262) [344] (80.9%) |
| QALY score | 0.150 (0.160) [316] (70.4%) | 0.136 (0.161) [322] (75.4%) |
| Analysis (Nc, Ni) | Incremental cost (95% CI) | Incremental effect (95% CI) | ICER | CEAC (%)a |
|---|---|---|---|---|
| QALYs | ||||
| Base-case: complete case (303, 306) | £279.86 (£19.39 to £540.33) | −0.004 (−0.016 to 0.009) | Dominated | 3.8 |
| SA1: imputed (449, 427) | £239.32 (£26.26 to £452.39) | −0.003 (−0.049 to 0.042) | Dominated | 26.6 |
| SA2: complete case, no training costs (303, 306) | £141.96 (−£118.51 to £402.43) | −0.004 (−0.016 to 0.009) | Dominated | 14.1 |
Work package 2 figures
FIGURE 2.
Overview of core outcome set development.
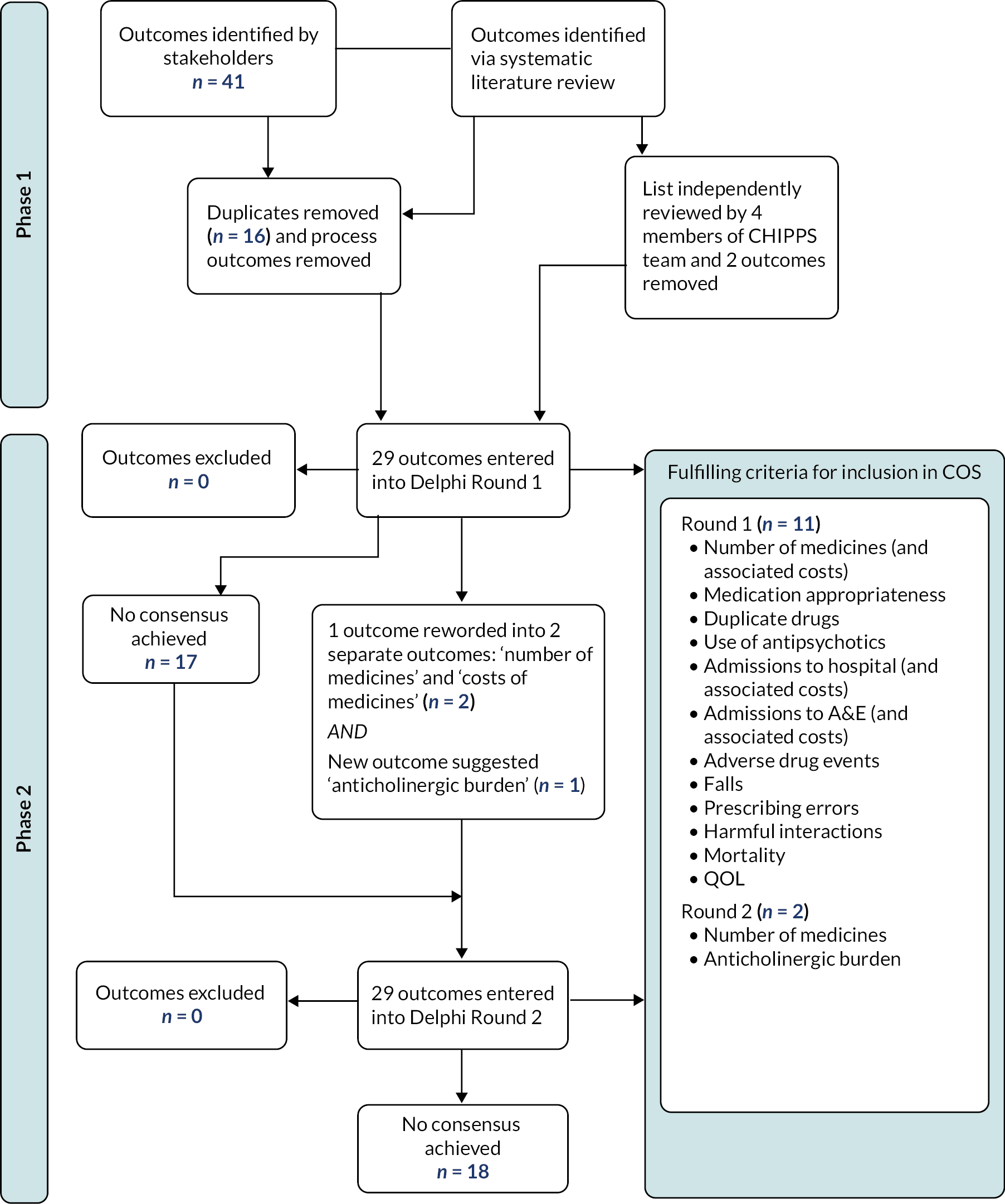
Work package 4 figures
FIGURE 3.
PRISMA diagram showing the literature review process.
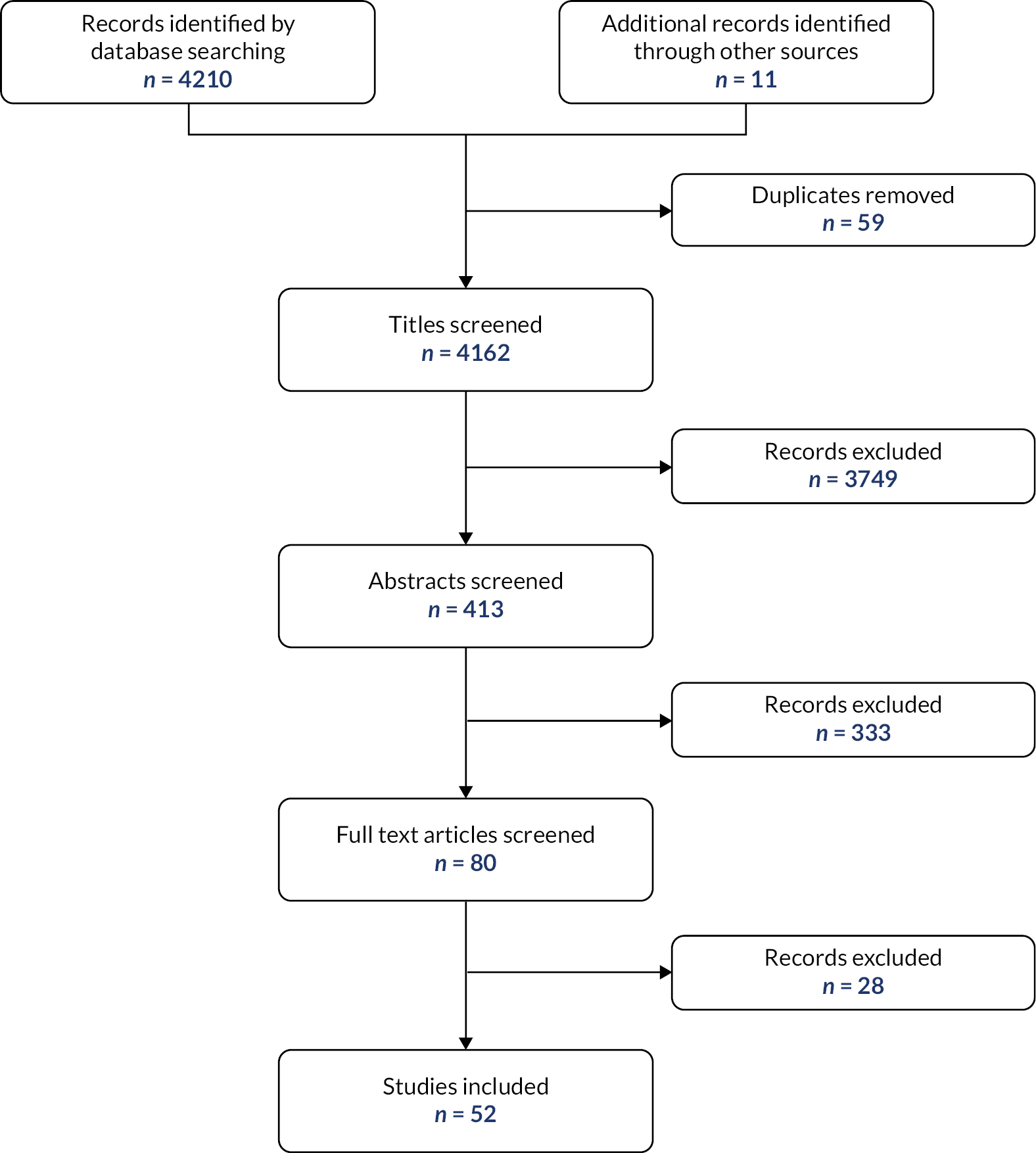
FIGURE 4.
First two personal development framework iterations.
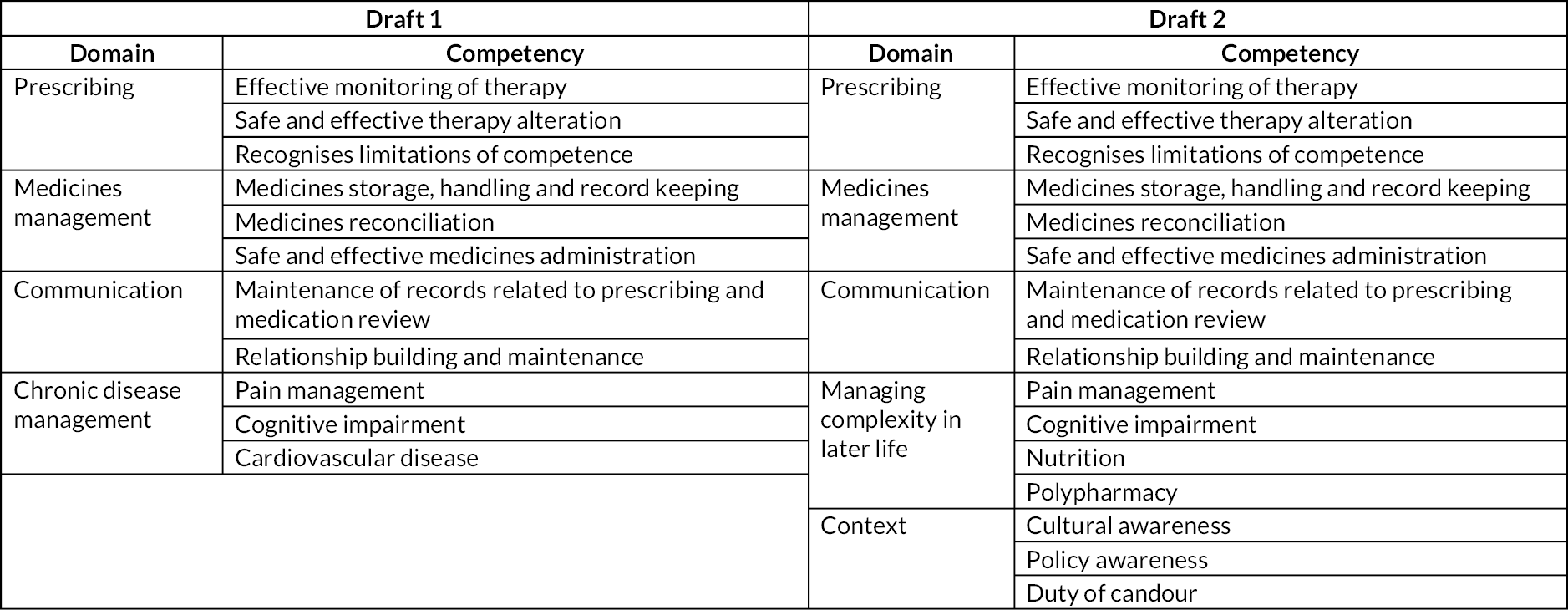
FIGURE 5.
Final training package.

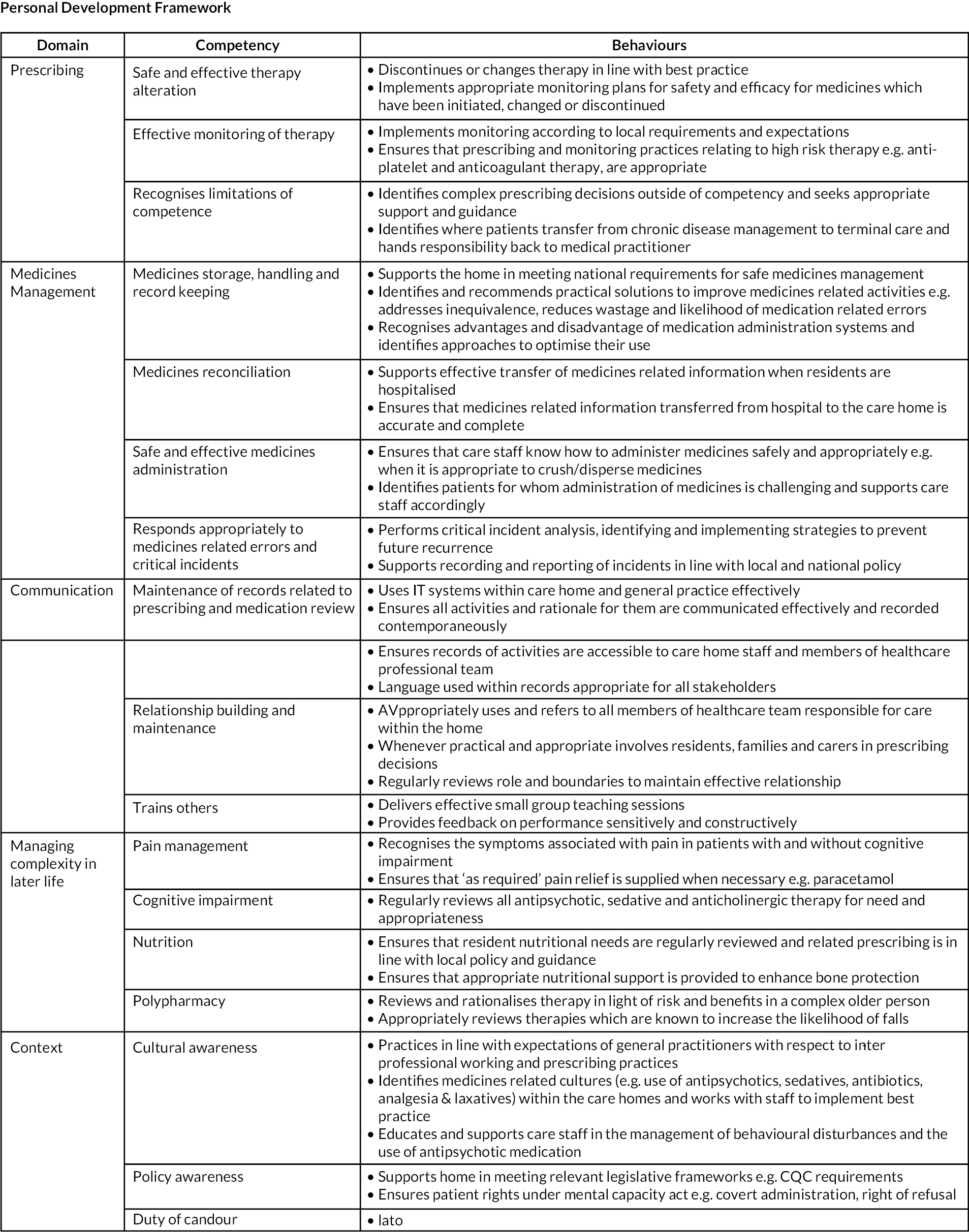
Work package 5 figures
FIGURE 6.
Care Home Independent Pharmacist Prescriber Study work package 5 feasibility study CONSORT diagram.
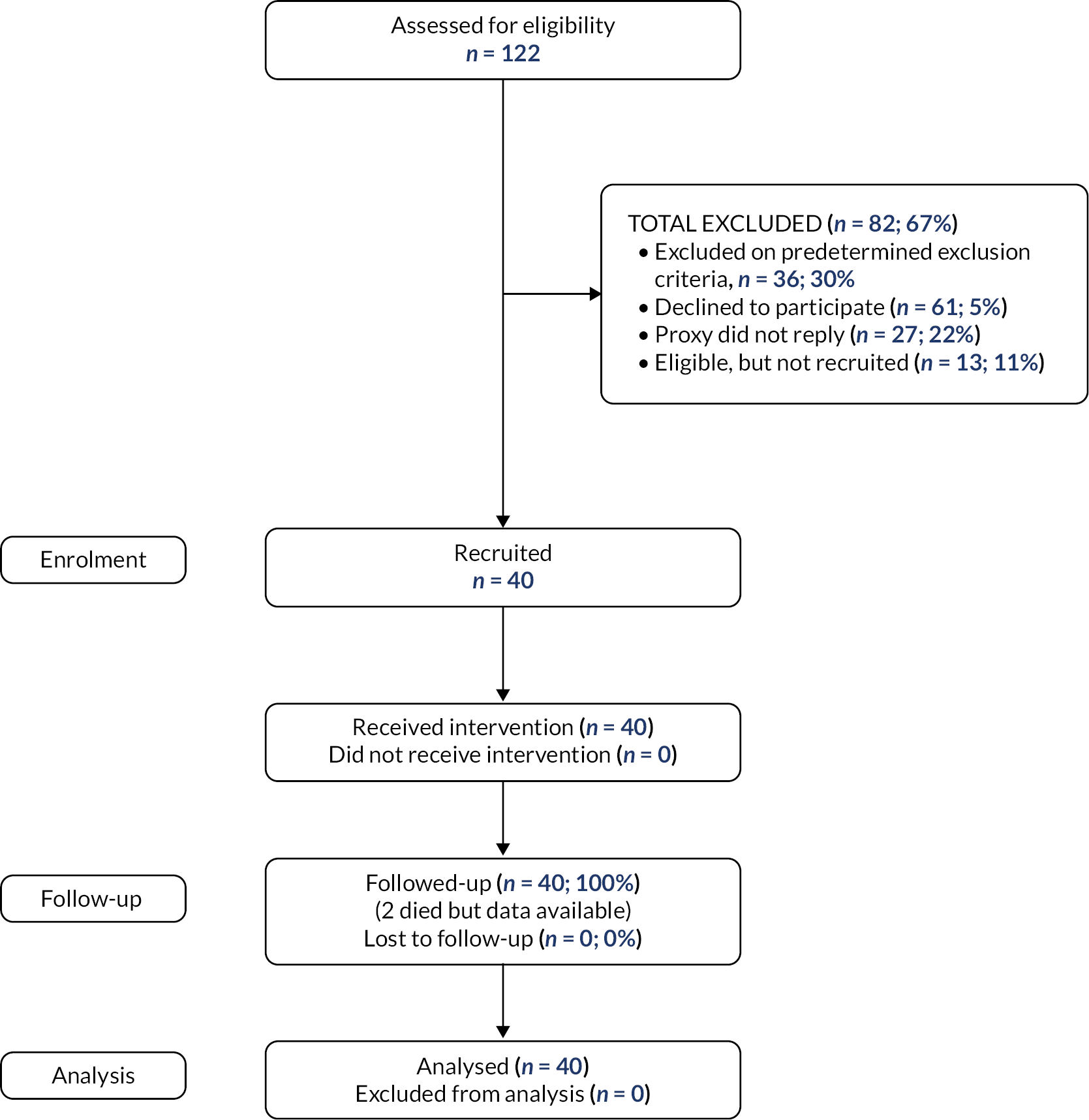
Work package 6 figures
FIGURE 7.
Flowchart of care home resident recruitment.
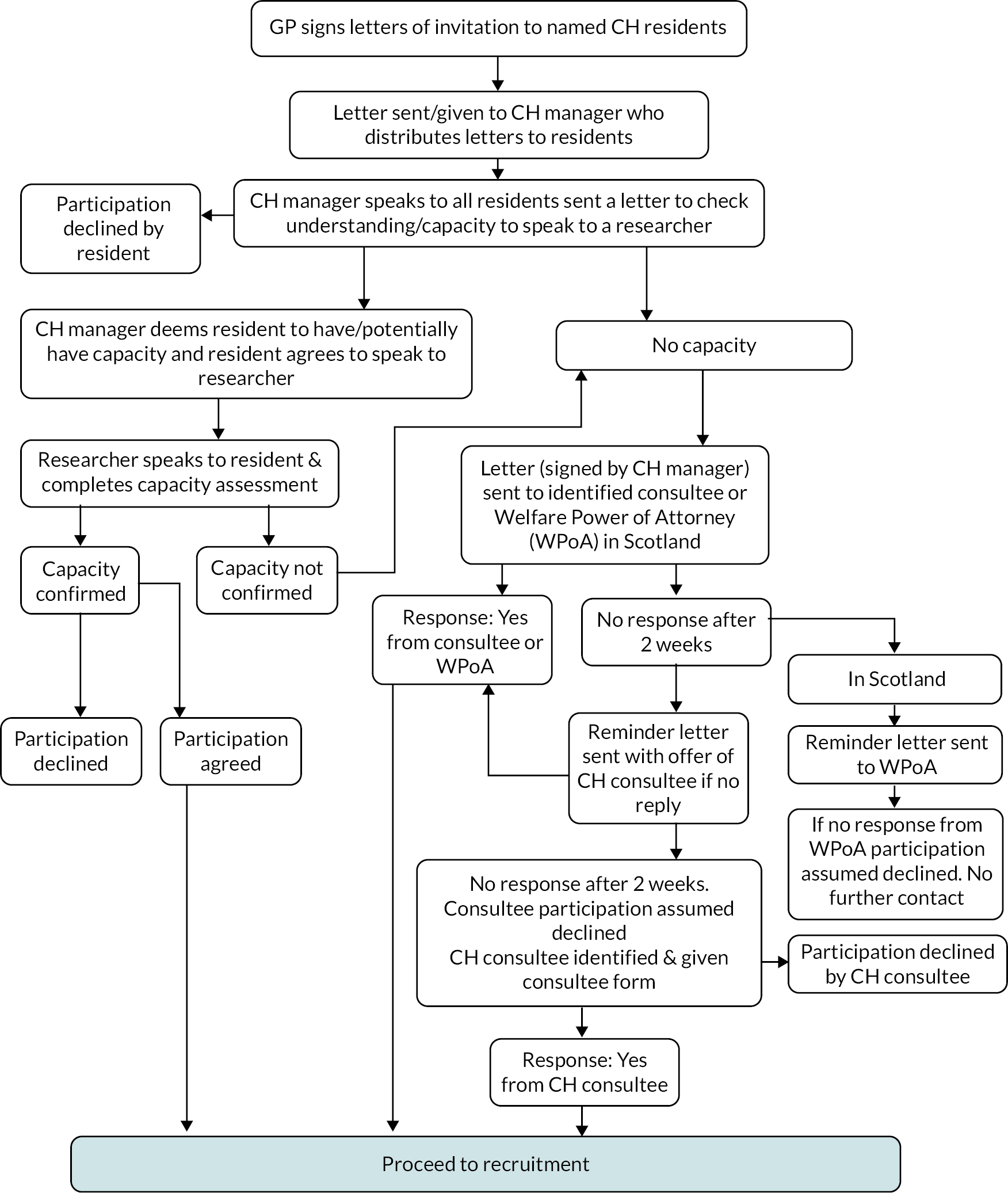
FIGURE 8.
Consort diagram of a cluster randomised controlled trial.
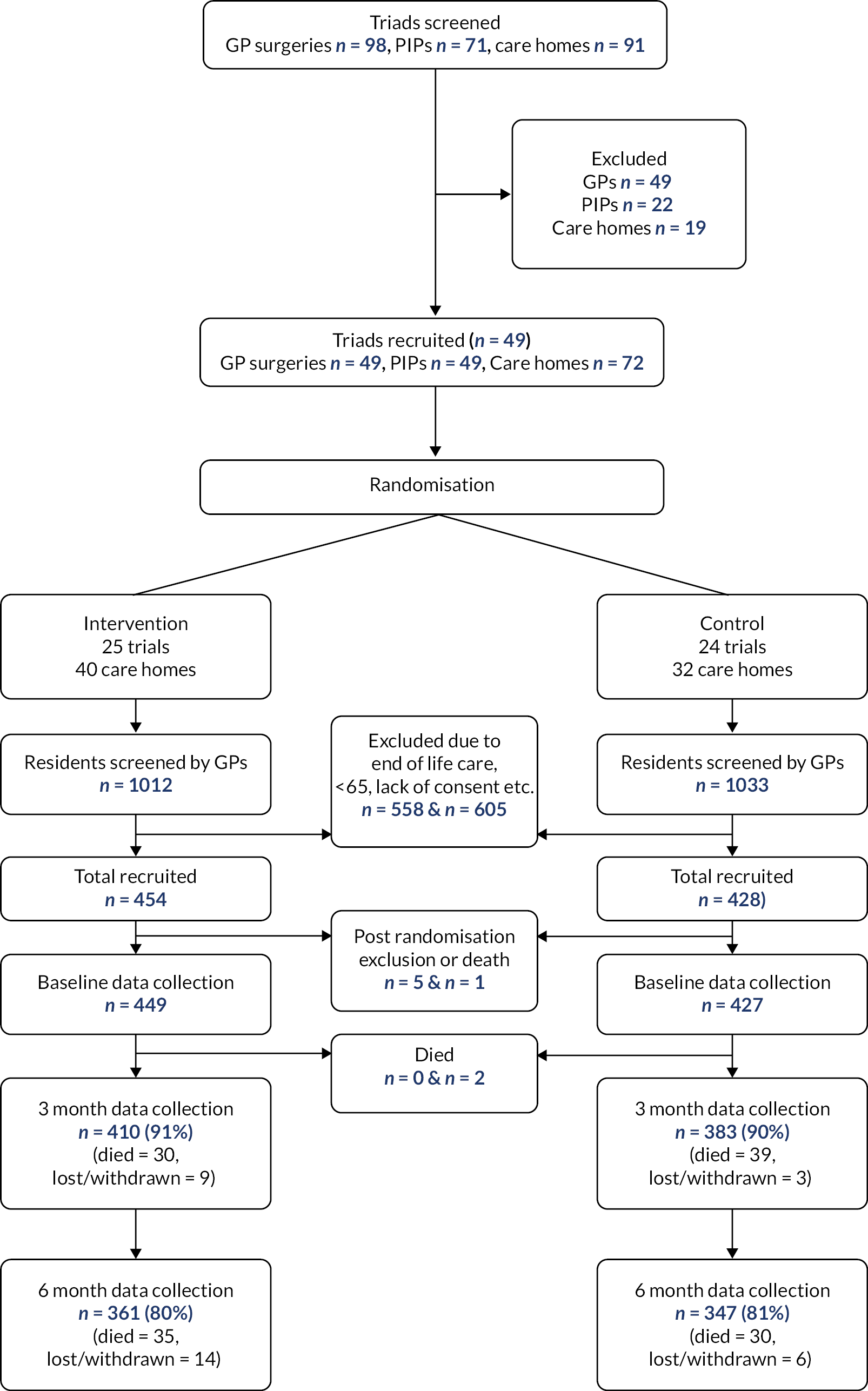
FIGURE 9.
Survival analysis comparing arm 1 (intervention arm) with arm 2 (control arm).
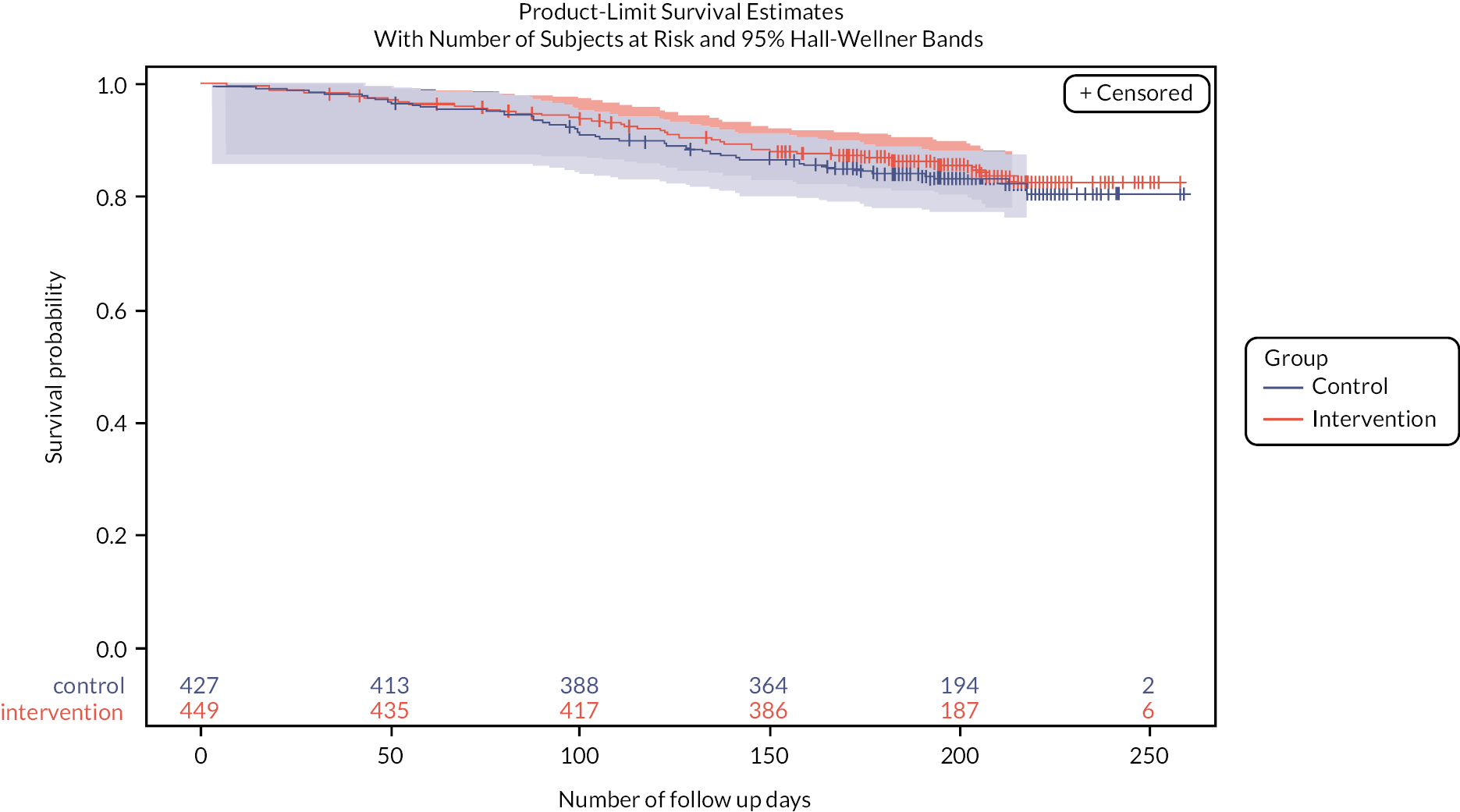
FIGURE 10.
Responses to the satisfaction statement by stakeholder group.
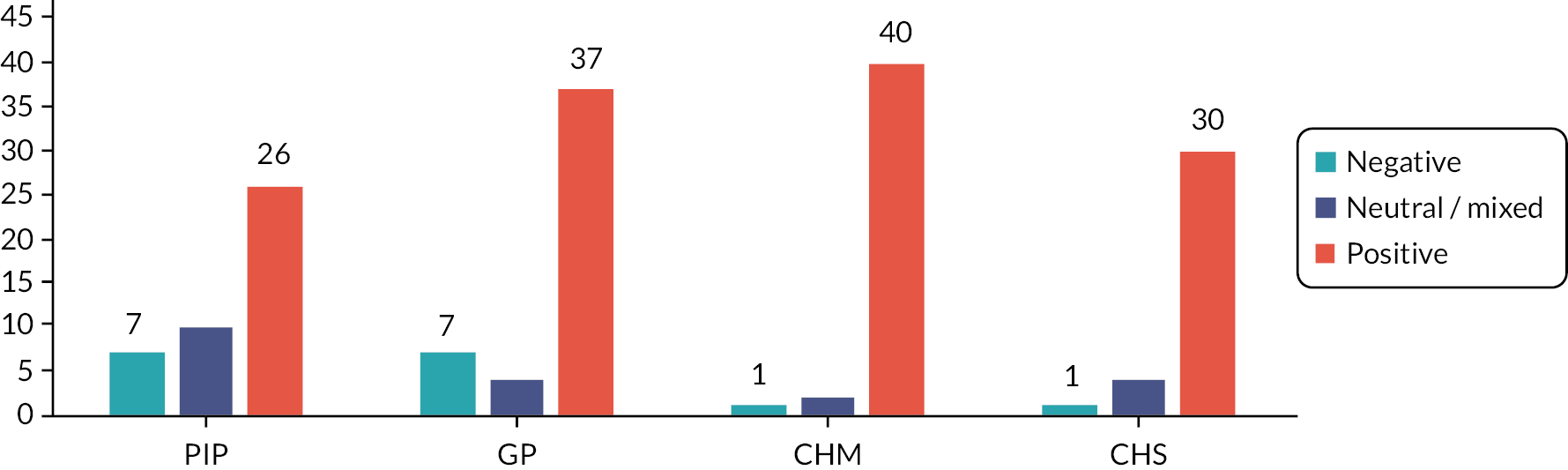
Work package 4 boxes
-
Frailty.
-
Harmful drugs for older people.
-
Capacity and how to support residents without it.
-
End-of-life care.
-
Role and boundaries of self and others.
-
Management of geriatric conditions.
-
Medicines regulations in care homes.
-
Importance of involving residents and relatives in decision making.
-
Know limitations and to work within them.
-
How to integrate into a team.
-
Good communication with the team, with residents and relatives.
-
Need for the use of IT systems at home and in medical practice.
-
Develop relationships with everyone involved in team.
-
How medical practice servicing the home operates.
-
Care home culture with respect to medicines.
-
Impact of medicines within the care home.
-
Medicine ordering and supply processes to enable effective access to medicines.
-
To support integration into the team.
-
Ensure includes effective communication about the role of a PIP to the home and wider team members.
-
Mentoring and/or shadowing, as part of training, doctors and care workers.
-
PIPs to communicate to staff regarding the importance of managing medicines effectively.
-
PIPs to understand and support good medicine administration practices.
-
Parkinson’s disease.
-
Cognitive impairment and behavioural disturbances.
-
Delirium.
-
Common skin conditions seen in care homes.
-
Dysphagia.
-
Wound management and catheter prescribing guidelines. a
-
Nutrition guidelines. a
-
Pain.
-
Dose optimisation based on renal function.
-
Cardiovascular (hypertension, secondary prevention, heart failure).
-
Asthma and chronic obstructive pulmonary disease.
-
Anticoagulant.
-
Anticholinergics and burden.
-
Antipsychotic.
-
Sedatives.
-
Antidepressants.
-
Gastrointestinal (laxatives, proton pump inhibitors).
-
Diabetes.
-
Mental Capacity Act or local equivalent and gaining consent.
-
Covert administration.
-
Controlled drugs.
a Locally derived
Work package 5 boxes
-
Adverse drug events as would normally be reported by a care home.
-
STOPP/START (medication appropriateness tool).
-
Mortality.
-
Fall rate per patient as per standard care home safety data collection.
-
Barthel Index (physical functioning).
-
Mini-Mental State Examination (MMSE).
-
Drug Burden Index.
-
Number of medicines.
-
QUALIDEM.
-
EuroQoL EQ-5D-5L (Proxy version 1). The proxy (care home staff, key worker) was asked to rate how they (i.e., the proxy), would rate the subject’s health (quality of life).
-
EuroQol EQ-5D-5L face-to-face with the resident.
-
Adverse drug events as would normally be reported by a care home.
Work package 6 boxes
-
‘Having a pharmacist who had good knowledge of all kinds of medications, going through polypharmacy, with a fine-tooth comb and picking up on any errors or things’. GP 2
-
‘It has reduced the time taken to see patients as …. their meds are all up to date, tests required for routine monitoring have been flagged up and I have been able to action these. From a safety and medicine waste point of view things have much improved’ GP 52
-
‘A resident was on a huge amount of anti-psychotic drugs … seeing PIP on a weekly basis meant we were able to make some huge reductions; I don’t think I have had any falls from her for a couple of months ….’. CHM 32.
| Characteristics of triads where CHIPPS service well | Characteristics of triads where CHIPPS service is less well embedded |
|---|---|
| Resonance between PIPs’ activities and GP and CH needs | Dissonance within relationships |
| GPs welcomed second clinical professional input and resident safety improvements | Lack of understanding of roles and responsibilities |
| PIP and GP have established working relationships GP and CH trust in the PIP’s clinical competency Stable CH management |
No established GP-PIP working relationship ‘I found it very difficult that I was not an employee of the GP practice … having to start from scratch building relationships … I was not a known entity’. PIP 41 |
| Communication channels enabling discussion of residents’ clinical care | New ways of working did not become embedded |
| Regular PIP access to the CH, PIP readily available, including as the main contact for medication queries | PIP was not seen as key contact for medication queries |
| PIP service seen to continue post-intervention | PIP service did not continue post-intervention |
‘I want it back! It was very helpful … it took the workload off … and reduced medications that we tend to leave the patients on’ GP 54
‘It made ordering easier, a little bit simpler, put the MAR charts into place a bit better … things … that were no longer needed were taken off’ CH staff 11
‘Her husband is much more positive about his experiences when he visits her … that’s really positive’ CHM 32
It was just pushing it a bit, looking at the patient as a whole, and being able to do a little bit more and involved the families …… it made me more thorough as a prescriber and a pharmacist’ PIP 8
Standard 4 How does the provider ensure the proper and safe use of medicines?
-
S4.1 Is the service’s role in relation to medicines clearly defined and described in relevant policies, procedures and training? Is current and relevant professional guidance about the management of medicines followed?
-
S4.2 How does the service make sure that people receive their medicines (both prescribed and non-prescribed) as intended (including controlled drugs and ‘as required’ medicines), and that this is recorded appropriately?
-
S4.3 How are medicines ordered, transported, stored and disposed of safely and securely in ways that meet current and relevant legislation and guidance?
-
S4.4 Are there clear procedures for giving medicines covertly, in line with the Mental Capacity Act 2005?
-
S4.5 How does the service make sure that people’s behaviour is not controlled by excessive or inappropriate use of medicines?
-
S4.6 How do staff assess the level of support a person needs to take their medicines safely, particularly where there are difficulties in communicating, when medicines are being administered covertly, and when undertaking risk enablement assessments designed to promote self-administration?
-
S4.7 How does the service engage with healthcare professionals in relation to reviews of medicines at appropriate intervals?
-
S4.8 How do staff make sure that accurate, up-to-date information about people’s medicines is available when people move between care settings? How do medicines remain available to people when they do so?
-
NHS England.
-
The Medicines Optimisation in Care Homes (MOCH) 2016: funding for 200 pharmacists and pharmacy technicians to be employed across the country.
-
Primary Care Networks funded to employ pharmacists with one target to enhance health in care homes in 2020.
-
National review of overprescribing with a particular focus on problematic polypharmacy.
-
-
NHS Scotland.
-
Strategy for pharmaceutical care 2015 noted the need for high-quality pharmaceutical care in care homes.
-
Health and Social Care Delivery Plan—every GP practice to have access to a pharmacist with advanced clinical skills by 2021.
-
Appendix 2 Recruitment strategies for GP and PIPs
| Locations | GP practice | PIP |
|---|---|---|
| Aberdeen | Letters of invitation and EOI forms were sent through the Scottish Clinical Research Network co-ordinator to 25 GP practices identified as providing services to care homes. Seven GP practices returned the EOI forms stating they had a PIP working at their practice | The seven PIP pharmacists identified through the GP invitations were sent invitation letters and EOI forms regarding the CHIPPS feasibility study. The third PIP pharmacist contacted was happy to take part in the feasibility study and fulfilled all our inclusion criteria. No other PIPs were contacted |
| Belfast | EOI forms were sent out to a random selection of 100 GP practices identified using the Business Services Organisation website. Twelve GP practices expressed an interest to take part but none employed a PIP at that time The GP practice that was eventually recruited resulted from a suggestion made by the recruited PIP who had previous experience working with the practice |
EOIs were sent to eight PIPs in Northern Ireland identified using local networks. Four PIPs expressed an interest. The first PIP contacted who could attend the training was recruited |
| Leeds | The GP practice was approached through the CCG pharmacist who was recruited for CHIPPS. The PIP made initial contact on our behalf | Found locally through the Principal Investigator |
| Norwich | The Clinical Research Network Eastern Primary Care Locality Manager for Norfolk Great Yarmouth and Waveney invited GP practices on the Research Active list to express an interest in the study. Ultimately, however, the GP practice selected for the feasibility study was approached directly by a PIP based on the PIP’s familiarity with the practice and travel logistics (to ensure that the shortest time possible of the PIP’s 16 hours/month would be used for travel during the intervention) |
Norfolk PIPs received invitation emails either from the Medicines Management lead for Norfolk CCGs or from the Local Clinical Research Network Pharmacist lead for the East of England. They were asked to express an interest in the study to the CHIPPS research team |
Appendix 3 Service specification used in the feasibility study
Service outline
CHIPPS is a National Institute for Health and Care Research (NIHR) programme grant to develop and deliver a cluster randomised controlled trial to determine the effectiveness and cost-effectiveness of making pharmacist prescribers part of a team working alongside care home staff and general practitioners (GPs) in care homes for older people. CHIPPS will provide a pharmacist independent prescriber (PIP) to review and optimise prescribing in recruited residents and facilitate and support cost effective evidence-based prescribing and medicine management in care homes for older people.
Aims and objectives
The aim of the service is to improve health outcomes and well-being of care home residents and ensure medicines are prescribed and managed in a safe, effective and cost-effective manner.
To meet the stated aims, recruited GP practices and care homes will work with a PIP who has demonstrated competency in care home medicine management and prescribing in older people. The PIP will be based at the GP practice, for the duration of the study, and will have developed an excellent working relationship with the GP practice and care home before commencing the service delivery. The service will run for a period of 3 months.
Inclusion/exclusion criteria for the service
Pharmacist independent prescriber
Inclusion criteria
-
Registered as a PIP.
-
Following training can demonstrate competence to deliver the service (see Service requirements).
-
Ability to work flexibly and commit a minimum of 16 hours a month to deliver the service for 3 months.
Exclusion criteria
-
Substantive employment with the community pharmacy (branch/store) that supplies medicines to the care home with which the PIP would work.
Care home
Inclusion criteria
-
CQC registered specialism as caring for adults aged ≥65 years.
-
Primarily caring for residents over 65 years.
Exclusion criteria
-
Care homes that receive additional medication-focused services with a visit frequency of monthly or more.
-
Care homes that provide only carer or support remotely (they do not have carers onsite 24 hours a day).
-
Care homes that are currently under formal investigation with the CQC or an equivalent body.
Residents
Inclusion criteria
-
Resident under the care of the participating GP practice.
-
Residents currently prescribed at least one medicine.
-
Residents or their appropriate representative who are/is able to provide informed consent/assent.
-
Permanent resident in a care home (not registered for respite care/temporary resident).
-
Residents must be aged ≥65 years.
Exclusion criteria
-
Residents who are currently receiving end-of-life care [equivalent to yellow (stage C) of the Gold Standards Framework prognostic indicator].
-
Resident with additional limitations on their residence (e.g. held securely).
-
Participating in another research study.
Service requirements
Recruitment and employment of the pharmacist independent prescriber
Initial identification and recruitment of the PIP will be conducted by the CHIPPS management committee. The PIP will require
-
excellent interpersonal, communication and IT skills
-
familiarity with relevant GP software systems
-
experience of providing prescribing and medicine management advice and support
-
previous experience of working in a GP practice environment
-
be able to travel to site locations
-
a mobile phone to be contactable for the purposes of delivering this service
-
appropriate indemnity insurance for prescribing.
The PIP will be employed according to local arrangements and seconded to the relevant GP practice for the study duration and during training and competency assessments (see Training and competency assessment of PIP).
Training and competency assessment of the PIP
See text in main paper.
PIP roles and responsibilities (NB: categorised as essential or not)
The PIP will, where appropriate:
Review each resident’s medication and develop and implement a pharmaceutical care plan5 (essential)
-
Optimise prescribing ensuring clear indication and evidence base for each medication (taking into consideration national and local pathways, guidelines and formularies), informed by tools such as STOPP/START.
-
Minimise the potential for adverse effects.
-
Optimise the dose of all medications.
-
Co-ordinate appropriate monitoring and associated tests for all medicines and conditions.
-
Agree the initial care plan with GP, care staff and resident (where appropriate).
-
Document and maintain records relating to the review and care plan in GP and care home records as appropriate.
Prescribing (essential)
-
Authorise repeat prescriptions.
-
Co-ordinate appropriate monitoring and associated tests for all medicines and conditions.
-
Deprescribe medicines according to the agreed pharmaceutical care plan.
-
Document medication changes in GP and care home records and notify supplying pharmacy of all changes to medication within 24 hours.
-
Initiate only new medicines for existing diagnoses or for common ailments that can be managed with medicines classified by the Medicines and Healthcare products Regulatory Agency (MHRA) as Pharmacy (P) or General Sales List (GSL).
-
Any additional areas of prescribing must be agreed and documented with the GP practice before prescribing (e.g. antibiotics for simple urinary tract infections).
Communication (essential)
-
Agree local protocols for communication with GP practice and care home before commencing service. This should include the following aspects.
-
Process of communication and messaging when the PIP is not available.
-
Documentation location and level of detail for all interventions made by the PIP.
-
Process and communication of referrals for activities outside the competence of the PIP.
-
Inform supplying community pharmacy about the service and role (before the start of the service).
-
Communicate all changes in medication to supplying pharmacy.
-
Complete all documentation and recording of activities as required by the study team.
Support systematic ordering, prescribing and administration processes with each care home, GP practice and supplying pharmacy where needed: (undertaken at the PIP’s discretion)
-
Provide instructions on how to administer each drug.
-
Synchronise residents prescription quantities for monthly cycles.
-
Add or clarify directions for all medications where it is currently not clear.
-
Provide advice on repeat prescription ordering processes to minimise missed items and optimise quantities.
-
Optimise the use of homely remedies within the care home.
-
Reconcile resident medication following transfer of care.
Training provision (undertaken at the PIP’s discretion)
-
Review training needs of care home and GP practice and draft proposed training package.
-
Provide training to care home staff on training needs basis from the agreed list of potential topics/areas.
-
Provide guidance to relevant GP practice on training needs basis from the agreed list of potential topics/areas.
Safe and effective service provision
-
The PIP will be contactable and respond to messages within 24 hours (Monday–Friday).
-
The GP practice will triage all medicine-related contacts for CHIPPS participants following a locally agreed protocol for referral to the PIP [see Training provision (undertaken at PIP’s discretion)].
-
PIP will have full (read/write) access to the GP record system to issues prescriptions and update records.
-
Where possible, the PIP will use remote access to update records when changes are made to GP-held records.
-
Where remote access is not feasible, the PIP must update records within 24 hours of making a change.
-
PIP will have full (read/write) access to care home records to update records during all visits using appropriate local reporting systems.
-
The PIP will visit/contact the care home at least once a week.
-
The PIP will visit/contact the GP practice at least once a week.
-
All annual leaves must be agreed at least 4 weeks before the leave and clear system for the transfer of responsibility communicated to GP, care home and supplying pharmacy.
-
The PIP will work within the local prescribing formularies of GP practice and primary care organisation.
-
The PIP will report and document all significant clinical events or near misses using local reporting procedures and study documentation.
-
The PIP will ensure that all records are aligned.
Outcomes from the service
As part of the feasibility study, we will measure levels of resource use associated with the PIP intervention, which will be estimated using the PIP log, which the PIP will complete every day.
End-of-service transitional arrangements
The duration of service will be clearly documented in study documentation and signed agreement to service provision completed by Care Home and GP practice before commencing the study.
-
All original policy and procedure documentation will be kept before the amendments are made during service provision.
-
Transfer meeting with PIPs, GP practice and care home at least 3 weeks before the end of service.
-
Agree transfer of responsibilities from the PIP.
-
Agree named contact point for medication-related issues at GP practice.
-
Communicate current plans for each resident.
-
Transfer of care plan and set review date.
-
Agree changes in policy and procedures.
Appendix 4 Pharmaceutical care plan
Appendix 5 Assessment of outcome measures based on data from Cochrane review and experience of the feasibility study
| Outcome | Data source | Potential for bias | Potential for missing data | Resident centred | Sensitivity to intervention | Reliability | Validated? | Third party completion | Ability to blind? | Time taken to collect | Quantity of data | Inclusion in RCT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EQ-5D (Quality of Life) | Direct interview with resident | High | High | ++ | None of included studies used this measure of QOL | ++ | ✓ | x | - | 10–15 min | Poor | Secondary outcome measure |
| EQ-5D proxy (Quality of life) | Care home staff | Low (if completed by the same person) | Low | + | None of the included studies used this measure of QOL | + | ✓ | ✓ | - | 3–5 min | Good; 100% | Secondary outcome measure |
| MMSE (cognitive function) | Direct interview with resident | High | High | + | No significant difference reported | ++ | ✓ | x | - | 10–20 min | Poor | Not included |
| Barthel Index (proxy) | Care home staff | Medium | Low | + | No significant difference reported | ++ | ✓ | ✓ | - | 2–10 min | Good; 100% | Secondary outcome measure |
| QUALIDEM (proxy) | Care home staff | Low | Low | + | Recommend QOL tool for those with dementia | + | Dementia Care Home population only | ✓ | - | 5–10 min | Good; 100% | Not included |
| Falls | Care home register | Low | Low | + | Three studies found no significant difference, two reported a significant difference | ++ | N/A | ✓ | + | 1–30 min | Good | Primary outcome measure |
| Adverse drug events | Care home record | High | High | + | Verification between the medicine and the event is difficult. No significant difference reported | - | N/A | ✓ | - | 2–30 min | None recorded | Monitor for patient safety only |
| STOPP/START | GP medical records | High | Medium | - | Significant improvement in two studies | -Concerns about inter-rater reliability- not designed as a research tool | ✓ clinical tool only | ✓ | + | 10–20 min | Good | Secondary outcome measure – reduced list |
| No. of medicines | GP records | Low | Low | - | Three studies no significant effect Two studies significant decrease |
++ | N/A | ✓ | + | None | Good | Process measure |
| Drug Burden Index | GP record | Low | Low | - | Not previously reported- only relates to anticholinergic medicines | + | ✓ | ✓ | + | 5 min | Good | Secondary outcome measure |
| Hospitalisations | Care home | Low | Low | + | No effect found in three studies Positive in two |
+ | N/A | ✓ | - | 5 min | Good | Secondary outcome measures |
| Mortality | Care home | Low | Low | - | No effect reported in five studies | ++ | N/A | ✓ | + | 5 min | Good | Secondary outcome measure |
Appendix 6 Final Logic Model v9 FINAL, 6 February 2019 (vs. 1–8 in Supplementary file)
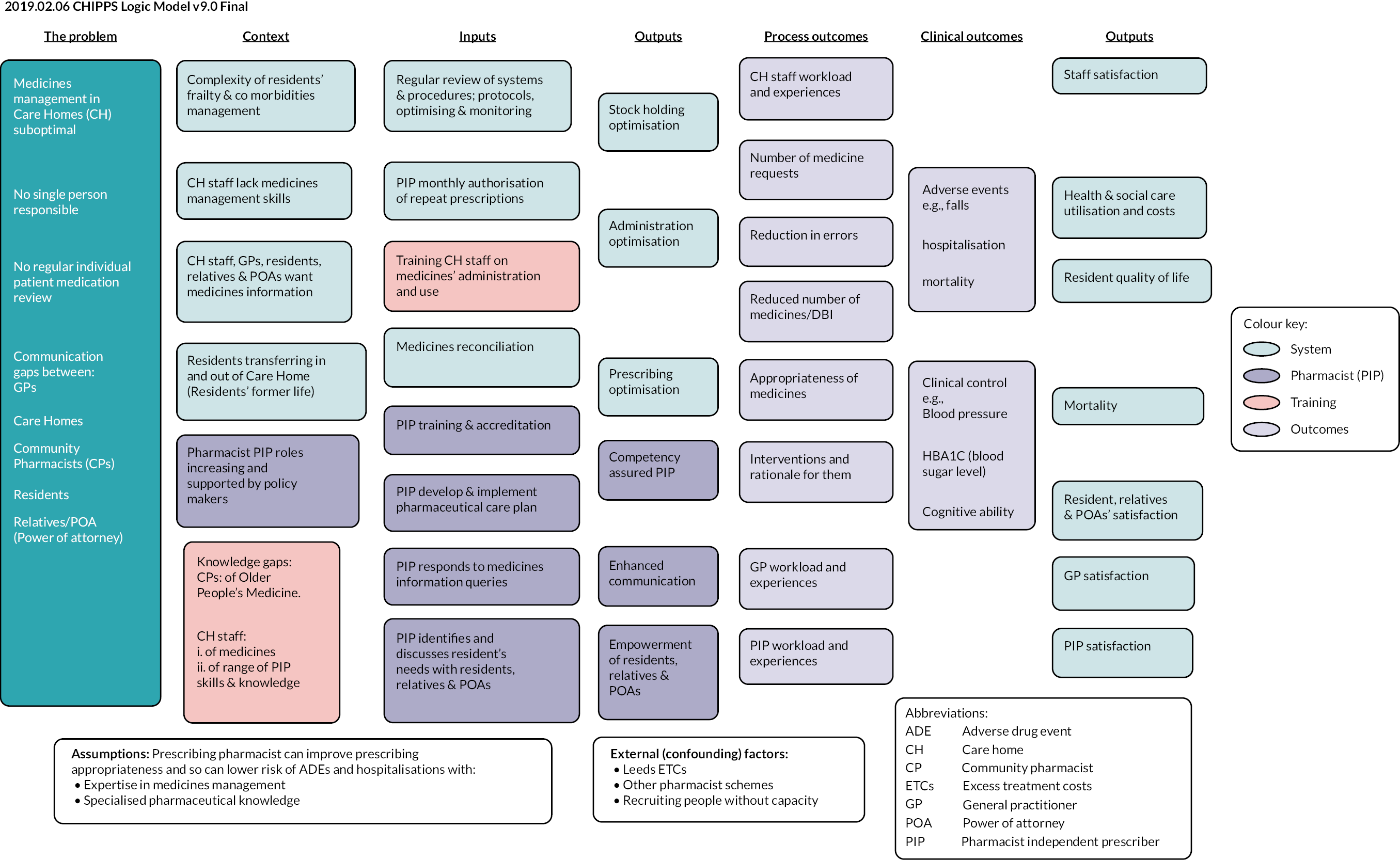
Appendix 7 Contributors
Norwich Clinical Trials Unit (NCTU)
-
Professor Ann Marie Swart, Director NCTU
-
Mr Antony Colles, Senior Data Programmer
-
Mr Tony Dyer, Data Manager
-
Mr John Gibson, Trials Assistant
-
Ms Cecile Guillard, Data Assistant
-
Ms Bronwen Harry, Clinical Trial Manager
-
Miss Juliet High, Senior Trials Manager
-
Ms Suzanne Lockyer, Trials Assistant
-
Mr Martin Pond, Head of Data Management
-
Dr Erika Sims, Operations Manager
-
Ms Joanna Williams, Clinical Trial Manager
University of East Anglia, Norwich
-
Professor David Wright, Professor of Pharmacy Practice, CI
-
Mr Mohammed Alharthi, Postgraduate Researcher
-
Professor Antony Arthur, Professor of Nursing Science
-
Professor Garry Barton, Professor of Heath Economics
-
Dr Linda Birt, Qualitative Research Associate
-
Ms Jeanette Blacklock, Senior Research Associate
-
Ms Annie Blyth, Research Fellow, Senior Programme Co-ordinator
-
Dr Stamatina Chelari, Health Economics Research Associate
-
Ms Heather Cutting, Transcribing Administrator
-
Dr James Desborough, Senior Lecturer in Pharmacy
-
Ms Kelly Grant, Statistics Research Associate
-
Ms Caroline Hill, Administrator
-
Ms Lisa Irvine, Health Economics Research Associate
-
Ms Frances Johnston, Administrator
-
Dr Kathleen Lane, Qualitative Research Associate
-
Ms Vivienne Maskrey, Research Fellow, Senior Programme Co-ordinator
-
Professor Nigel Norris, Professor of Education
-
Professor Fiona Poland, Professor of Social Research Methodology
-
Professor Lee Shepstone, Professor of Medical Statistics
-
Mr David Turner, Health Economics Senior Research Fellow
-
Ms Laura Watts, Research Fellow, Senior Programme Co-ordinator
-
Ms Susan Westgate, Data Entry Administrator
University of Aberdeen
-
Professor Christine Bond, Professor of Primary Care, Pharmacy, PI
-
Ms Jacqueline Inch, Research Fellow
-
Ms Frances Johnston, Administrator
-
Professor Phyo Kyaw Myint, Professor of Medicine of Old Age
-
Dr Frances Notman, Research Fellow
-
Ms Hazel Riley, Administrator
-
Ms Vladimira Vladimirova
Queens’s University Belfast
-
Professor Carmel Hughes, Professor of Primary Care, Pharmacy, PI
-
Dr Anna Millar, Senior Research Associate
-
Ms Mairead McGrattan, Senior Research Associate
-
Dr Maureen Spargo, Senior Research Associate
University of Leeds
-
Professor David Alldred, Professor of Medicines Use and Safety, PI
-
Ms Suzanne Banks, Financial Advisor
-
Dr Amrit Daffu-O’Reilly, Senior Research Associate
-
Ms Hannah Hartley, Administrator
-
Ms Elizabeth Lavendar, Administrator
-
page
University of Leicester
-
Professor Richard Holland, Professor of Public Health Medicine, CI.
-
Ms Jaede Todner, Administrator
Norfolk and Waveney CCG
-
Mr Ian Small, Deputy Head of Prescribing
-
Ms Clare Symms, Sponsor’s Representative
-
Dr Judy Henwood, Head of Research Design and Evaluation
Intervention development, training and delivery
-
Ms Amy Benterman, Prescribing Pharmacist and Clinical Lecturer, Intervention Trainer
-
Ms Jenny Desborough, Senior Clinical Pharmacist, Intervention Trainer
-
Dr Joanna Ford, Consultant Geriatrician
-
Dr Ian Gibson, Lecturer in Medical Education, GP Assessor
-
Mrs. Helen Hill, Independent Consultant
-
Ms Barbara Jesson, Community Pharmacy Adviser, Principal Pharmacist, Mentor
-
Ms Valerie Sillito Practice Pharmacist and Independent Prescriber, Mentor
-
Dr Martyn Patel, Consultant in Older People’s Medicine, Intervention Trainer
-
Ms Helen Whiteside, Pharmacist and Independent Prescriber, Mentor
-
Dr Arnold Zermansky, General Practitioner, Research Fellow
-
Mr Ian Small, Retired Deputy Head of Prescribing, NHS Commissioning Board
Public and patient involvement in research (PPI)
-
Ms Elaine Bounds, Programme Steering Committee
-
Ms Janet Gray, Programme Management Group
-
Ms Joyce Groves, Programme Steering Committee
-
Ms Christine Handford, Programme Management Group
-
Ms Frances Hollwey, The Patients Association
-
Ms Kate Massey, Programme Management Group
-
Ms Jacqueline Romero, PPIRes Co-ordinator
Research participants
-
Care homes and their staff
-
GP practices and their staff
-
PIPs
Appendix 8 Health economics report
Methods
Costs
Costs were estimated in Great British pounds (£) at 2017/2018 financial year levels from the perspective of the NHS and personal social services (PSS). No discounting was undertaken due to the time frame of the study (costs were estimated over a 3-month period before the baseline/intervention and a 6-month follow-up period).
Estimating the cost of the PIP intervention
To estimate the PIP costs, each PIP was asked to complete two particular items over the 6-month post-randomisation period: a training log and an activity log. The training log enabled the PIP to record the time spent with others/courses undertaken as part of their training/professional development, for example, in-person training, contacts with a mentor, GP, community pharmacist, as well as any self-directed learning. The PIPs were also asked to record any activities associated with the PIP role within the PIP activity log, including the duration of (1) any contacts with others, for example, care home residents (participants), care home staff, GP, or any other professionals (e.g. geriatricians, community pharmacists, and district nurses), and (2) any other non-contact activity, for example, resident prescription management, care home medication storage, staff training and travel.
For both training and activity, log estimates of the cost per hour of employment were assigned to estimated times for the PIP and other professionals (including care home staff), where it was assumed that the PIP would have band 7 costs and their mentors/trainers band 8a costs. 24 Overheads were included as part of these unit costs, and these were deemed to cover any additional costs associated with the intervention.
As in the intervention arm, the PIP’s role enabled the undertaking of prescription management for the individual care home residents, and it was envisaged that this would enable GPs to spend less time undertaking prescription management for intervention arm participants. We estimated the GP time saving that would have occurred, for each individual participant, because of no longer undertaking prescription time management, would have been as follows. It has been estimated that the mean time spent per prescription-related event is 56.0 seconds (28). Based on this figure, if each participant were to have two prescriptions issued each month (each of which contain up to four items), which no longer required GP approval, then this would equate to a total GP time saving of 11.2 minutes (per resident across the 6-month study period). At a cost of £110 per hour for a GP24, this equates to a total cost saving, for GP time, of £20.60 per resident/participant.
Subsequently, the total PIP intervention cost was estimated by summing the per-participant PIP training and PIP activity costs and deducting the estimated GP time/cost saving.
Other costs
As it was considered that there was the potential for the introduction of the PIP role to improve outcomes/impact the use of other NHS and PSS resource-items, the following items were extracted from medical records.
Medication details were extracted from primary care records for both the 3-month period before baseline and the 6-month study follow-up period. In terms of unit cost, when extracting the medication data (directly into an electronic format), researchers were able to select medication details from the 2015 version of the Prescription Cost Analysis (PCA),28,29 This was undertaken with a view to reduce the number of misspelt medications to which it would not be possible to attach cost at the end of the study (it was still possible to enter a medication name if the drug was not listed in the 2015 PCA). Unit costs were automatically selected from the 2015 PCA as part of the data collection process, and no adjustment was made to these costs, as the average cost per prescription item has not increased in recent years. 28,29 Medications that were not automatically assigned a 2015 PCA price at the time of data entry were assigned a unit cost from the 2018 PCA. 28,29 The above enabled overall medication costs to be estimated.
Details of outpatient attendances, inpatient stays, tests and investigations and GP and practice nurse visits/phone calls were also extracted from primary care records. Conversely, informed by previous research,23 the details of all other health professional contacts were extracted from care home records, where professional data of the number of visits (and location: care home/community) and number of phone calls were extracted. For all items, data in relation to the previous 3 months were collected at baseline and those for the previous 6 months were collected at the 6-month follow-up. Unit costs were subsequently assigned to each of these resource items (see Appendix 1, Table 1) and totalled to estimate the total other (non-medication) costs.
Finally, the total PIP intervention cost, overall medication costs and other (non-medication) costs were summed to estimate the total (NHS and PSS) cost.
Outcomes
In line with the National Institute for Health and Clinical Excellence (NICE) methods guidelines,31 quality of life was measured using the EQ-5D-5L. 30 For all participants, proxy respondents were asked to report the participants’ level of problems (on a range from none to extreme/unable) on five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) at baseline, 3-month follow-up and 6-month follow-up. As recommended in the NICE position statement,33 the crosswalk mapping function35 was used to convert these responses into utility scores, where a score of zero corresponds to death and one to full health. Participants who were known to have died were assigned a utility score of zero at the time points where this was known. Quality-adjusted life-year (QALY) scores were subsequently estimated for each individual based on the total area under the curve method and the assumption of linear interpolation. 36
Analyses
The base-case analysis was based on the complete case approach (24) and included only those with complete total (NHS and PSS) cost data at baseline and 6-month follow-up and complete QALY scores. Because of the potential for skewed data/correlation between costs and effects, bivariate regression37 was used to analyse the cost and QALY data, based on the intention-to-treat principle, that is, according to allocation arm (regardless of, e.g., whether the PIP intervention was received). Each regression included trial arm, age and gender as covariates, with the cost regression also including baseline costs and the QALY regression including the baseline EQ-5D score. Together, this enabled the mean incremental cost and the mean incremental effect (mean difference in QALYs) associated with the PIP intervention to be estimated.
If the PIP intervention were found to be more costly and less effective, then it would be dominated (by the other intervention), and not be recommended for implementation (money could be better spent elsewhere). Alternatively, the incremental cost-effectiveness ratio (ICER) (mean incremental cost/mean incremental effect) would be estimated, where an ICER (incremental cost per QALY) below the cost-effectiveness threshold (λ) value of £20,000–30,000 per QALY can be inferred to be cost-effective. 31
Analyses of uncertainty
To estimate the level of uncertainty associated with the decision regarding cost-effectiveness, we depict results on the cost-effectiveness acceptability curve,38 where we report the probability of the PIP intervention being cost-effective at the λ value of £20,000/QALY compared with that with standard care.
Additionally, to assess the robustness of the results to assumptions made in the above base-case analysis, the following sensitivity analyses were undertaken. First, as missing data can lead to bias,132 multiple imputation (MI) was undertaken. 133 Specifically, the Stata mi impute command was used to create multiple datasets, which were then pooled using Rubin’s rules. 134 The MI model included the trial arm, age, gender and the aforementioned component costs and EQ-5D scores at baseline and at 3- and 6-month follow-ups (cost components were included as they had different levels of missing data, whereas levels of missing data did not tend to differ across the dimensions of the EQ-5D). Total costs and QALY scores were then estimated based on the component costs and EQ-5D scores, respectively. In the second sensitivity analysis, the PIP training costs were excluded from the total costs. This (best case scenario) was justified on the basis that, as training has now been undertaken, in certain circumstances, no further training would be required if the intervention were continued. In both these sensitivity analyses, the analysis was otherwise as reported above, that is, bivariate regression was undertaken to estimate mean incremental cost and mean incremental effect, and the probability of the PIP intervention being cost-effective at the λ value of £20,000/QALY was also estimated.
Finally, threshold analysis was undertaken in order to identify when the decision as to whether an intervention is cost-effective switches, for example, from being estimated to be not cost-effective, to being estimated to be cost-effective. 34 In particular, we sought to identify the estimated cost (effect), holding the effect (cost) as in the base-case, at which the decision as to cost-effectiveness would change. Thus, showing the range of costs (effects) where the PIP intervention would be estimated to be cost-effective.
Results
A total of 25 (449) triads (participants) were allocated to the intervention, and 24 (427) to the control. Aside from the intervention group having a lower proportion of participants in nursing home care (42% vs. 59%), a higher mean Barthel score (8.34 vs. 7.07, i.e. greater independence), and a greater number of falls (0.78 vs. 0.57 mean falls in the previous 90 days), the groups were otherwise broadly similar.
Cost of the PIP intervention
The training logs were returned by 23/25 PIPs, and the activity logs by 22/25 PIPs. Certain assumptions were therefore necessary to deal with this and other types of missing data, in order that mean PIP costs be estimated for all PIPs/participants. By way of example, in cases where a particular training/activity was reported, but the associated time was not, the mean time listed for that particular training/activity by other PIPs was estimated and assigned to the described activity. This meant, for example, that the mean cost for the 23 PIPs who returned the training logs was applied to the two PIPs who did not as it was known that these two PIPs had completed their training.
The mean training time per PIP was 4.3 days (see Table 2), where this included a 2-day course, approximately a further day of contact time with other professionals, for example, mentor/GP along and a further day of self-directed learning. The mean total training cost (including the costs for contacts with other professionals) was estimated to be £61,916.07, which was equivalent to £2476.64 per PIP or £137.90 per study participant. Though these costs are based on actual study data, they may overestimate future training costs as in practice training could be delivered to more individuals at once, and the costs spread across more residents, reducing the cost per resident (this is assessed as part of the aforementioned second sensitivity analysis).
No PIP activity costs were assigned to intervention participants in the care homes where the three PIPs did not return their activity logs (these PIPs did not deliver the intervention and zero PIP time/costs were therefore assigned to the residents under their care). Conversely, one PIP reported activities that amounted to a time of 19.4 days (assuming a 7.5-hour working day) per resident. As such, there was wide variation around the mean PIP reported activity time of 7.6 days (this equates to 191 minutes per participant, see Appendix 1, Table 23).
In terms of costs, the mean PIP activity cost was £205.29 (range: £0–1168.67) per participant, where the mean PIP cost was £169.68 per participant, compared to £35.61 per participant for other professionals’ time. When added to the aforementioned training cost, and the assumed GP cost saving due to them undertaking fewer prescriptions, this gives an estimated mean PIP intervention cost of £322.59 (see Appendix 1, Table 24).
Other costs
In terms of the other NHS and PSS resource-items that it was considered could change due to the introduction of the PIP role (medication, out-patient attendances, in-patient stays, tests and investigations, GP and practice nurse visits/phone calls and other health professional visits/phone calls), the associated mean estimated levels of resource use and costs are reported in Tables 2 and 3, respectively, where it can be seen that overall medication costs and other (non-medication) costs are broadly similar between groups. Accordingly, the mean total (NHS and PSS) cost was estimated to be higher in the PIP intervention group (see Appendix 1, Table 24), where this difference between groups was largely due to the costs associated with the PIP intervention.
Outcomes
The response rates for both groups and mean utility scores at the baseline, 3- and 6-month follow-up points, along with mean QALY scores, are shown in Appendix 1, Table 25. It can be seen that the mean utility scores tended to fall over time in both groups (i.e. somewhat explained by zero scores being assigned to those who died). The baseline imbalance in EQ-5D scores between groups also demonstrates why this variable should be adjusted for when estimating the incremental QALY scores.
Analyses
A total of 609 participants (70%) had complete cost and EQ-5D data. The base-case estimated mean incremental cost and effect (mean difference in QALYs) are provided in Appendix 1, Table 26. It can be seen that the PIP intervention is estimated to be both more costly, and less effective, with only a 4% probability of being cost-effective at the effective at the λ value of £20,000/QALY. Within the two sensitivity analyses the PIP intervention was again estimated to be more costly and less effective.
In terms of threshold analysis, the aforementioned base-case analysis estimated that the PIP intervention was associated with higher costs (£279.86) and a (marginally) lower effect (−0.004), and thus was estimated not to be cost-effective. When considering a cost-effectiveness threshold of £20,000/QALY, assuming the same base-case cost (£279.86) the PIP intervention would be estimated to switch to being cost-effective if the QALY gain was estimated to be ≥0.14. Additionally, for the same base-case effect (−0.004) a cost-saving of ≥£72.02 would be necessary for the PIP intervention to switch to being estimated to be cost-effective (have a cost/QALY <£20,000/QALY). Both of the above threshold values are outside the 95% CI surrounding both the base-case mean cost and the mean effect, which would suggest that one would consider it unlikely that these thresholds would be reached based on the data collected, and associated assumptions, in this analysis.
List of abbreviations
- AE
- adverse event
- CEAC
- cost-effectiveness acceptability curve
- CH
- care home
- CI
- confidence interval
- CHIPPS
- Care Homes Independent Pharmacist Prescriber Study
- CHM
- care home manager
- CHS
- care home staff
- COS
- core outcome set
- CPD
- continuing professional development
- CQC
- Care Quality Commission
- cRCT
- cluster randomised controlled trial
- DBI
- Drug Burden Index
- EAP
- expert advisory panel
- EOI
- expression of interest
- EQ-5D-5L
- EuroQol Five Dimensions and Five Levels Rating Scale
- GP
- general practitioner
- ICER
- incremental cost-effectiveness ratio
- MI
- multiple imputation
- MMSE
- Mini-Mental State Examination
- MOCH
- Medicines Optimisation in Care Homes
- MRC
- Medical Research Council
- NICE
- National Institute for Health and Care Excellence
- NPT
- normalisation process theory
- NRES
- National Research Ethics Service
- PCA
- prescription cost analysis
- PCP
- pharmaceutical care plan
- PIP
- pharmacist independent prescriber
- PPI
- patient-public involvement
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-analyses
- PSS
- personal social services
- QALY
- quality-adjusted life-year
- RA
- research associate
- RCT
- randomised controlled trial
- RR
- rate ratio
- SAE
- serious adverse event
- SUSAR
- suspected unexpected serious adverse reaction
- TDF
- theoretical domains framework
- WP(n)
- work package (number)
- WPOA
- welfare power of attorney
