Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number PTC-RP-PG-0213-20002. The contractual start date was in April 2015. The final report began editorial review in April 2022 and was accepted for publication in March 2023. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Williamson et al. This work was produced by Williamson et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Williamson et al.
Synopsis
Research summary
Low back pain (BP) is the most common cause of disability in the world. 1 The prevalence of severe BP increases with age,2 and it is associated with immobility, disability, frailty and falls. 3–7 In the UK, estimates suggest that one in four older adults suffer from BP. 2,8 However, many older people either do not consult, or health professionals do not prioritise BP treatment, presuming nothing can be done. 2,9,10 Many clinical trials exclude older people. 11 Little attention has been paid to understanding the presentation of BP in older people and developing effective treatments.
Older adults may also experience leg pain referred from the lumbar spine. A common clinical presentation of spinal-related leg pain in older adults is neurogenic claudication (NC). 12 It presents as an easily recognised clinical pattern of symptoms radiating from the spine into the buttocks and legs which are provoked by walking or standing and relieved by sitting or lumbar flexion. 12 BP is often present, but not always. NC is particularly problematic for older people, as not only can it cause severe pain and discomfort but it substantially reduces an individual’s confidence and ability to walk and their quality of life. 13 It is estimated that 11% of community-dwelling older adults report symptoms of NC, although there were no estimates from community-dwelling UK populations. 14 Symptoms of NC are thought to arise from pressure on nerves and blood vessels in the spinal canal caused by degenerative changes narrowing the volume of the spinal canal (referred to as spinal stenosis). NC is the most common reason that older adults undergo spinal surgery,15 and physiotherapy is recommended prior to surgical intervention. However, there was a lack of high-quality evidence to guide conservative care of people with NC, including primary care management or physiotherapy. 16,17 We identified a need to better understand the presentation of BP in older people and to improve the care they receive for their BP, including conditions like NC.
Aims and objectives
The overall aim of the Better Outcomes for Older people with Spinal Trouble (BOOST) programme was to conduct a series of linked studies to understand and reduce the burden of BP on older people by increasing knowledge and developing evidence-based treatment strategies.
We set out the following objectives:
-
to describe the presentation and impact of BP in community-dwelling older adults (work packages 2 and 3)
-
to refine a physiotherapy intervention for older people with NC to be tested in a randomised controlled trial (RCT) (work package 1)
-
to undertake a definitive RCT evaluating the clinical and cost-effectiveness of physiotherapy interventions for NC (work packages 2, 4 and 5)
-
to undertake a longitudinal qualitative study embedded within the BOOST RCT to understand the participants’ experiences of taking part in the trial, including acceptability and impact of the interventions, and issues related to implementation (work package 4)
-
to develop a prognostic tool using the Oxford Pain Activity and Lifestyle (OPAL) cohort study data to predict when older people are at risk of mobility decline and to understand the role of BP (work packages 2 and 3)
-
to integrate the findings into an implementation package (work package 6).
Work packages
We have undertaken:
-
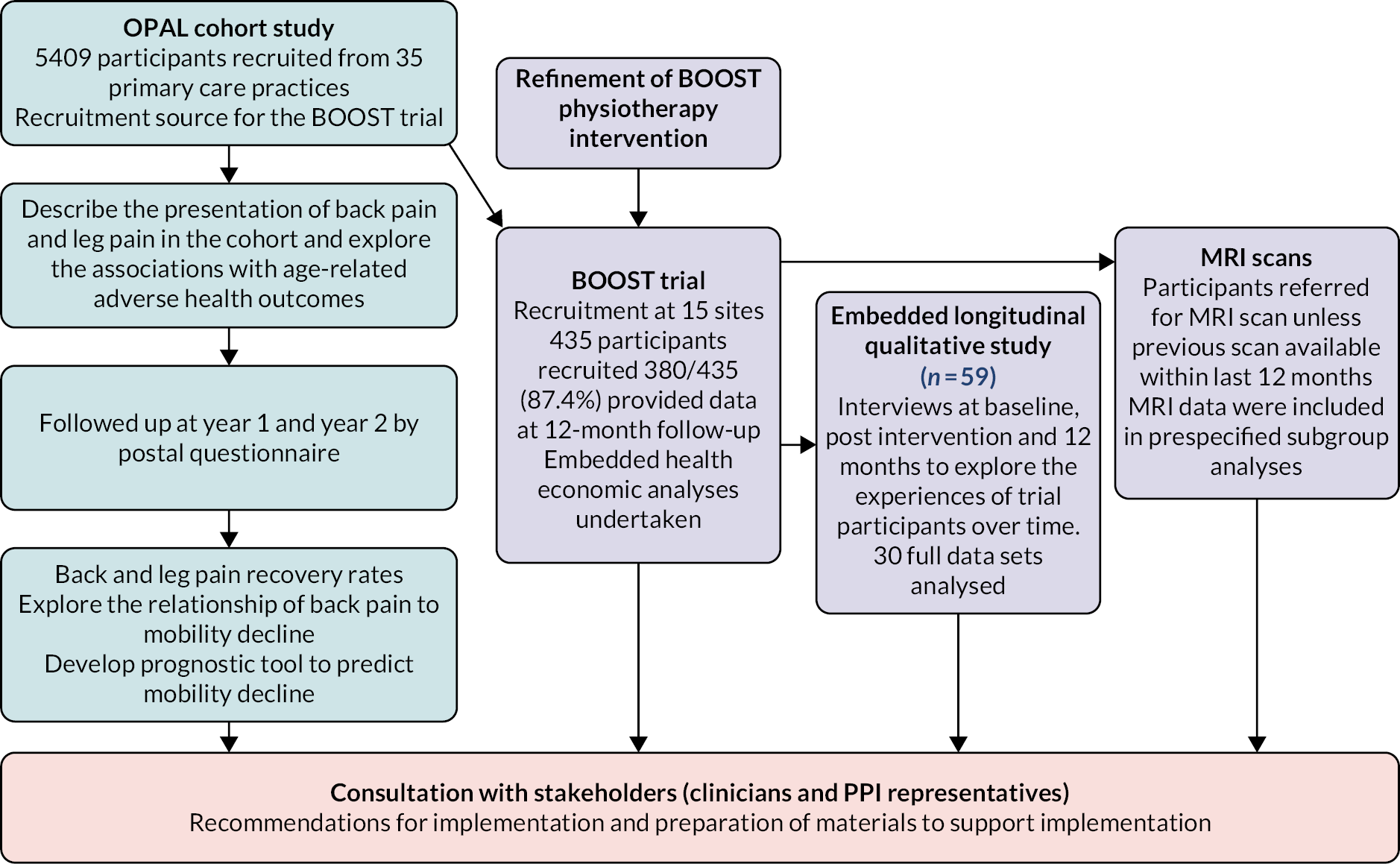
a large population-based cohort study of community-dwelling older people recruited from primary care practices (the OPAL cohort study)
-
An RCT of physiotherapy interventions for NC, including an economic evaluation (the BOOST RCT) and prespecified subgroup analyses
-
a longitudinal qualitative study embedded within the BOOST RCT.
This research was planned in six work packages. There was considerable overlap between work packages, and they were not undertaken chronologically. The original work packages as described in the funding application are described below. Overall, this proceeded as planned, with some changes to work package 3 which we describe.
Work package 1: Refinement of a physiotherapy intervention for neurogenic claudication
We planned to integrate knowledge of exercise, behavioural and pain management strategies, alongside practical issues to develop a physiotherapy-delivered group physical and psychological intervention for older people with NC.
Work package 2: Feasibility
There was a feasibility phase for the two main studies (OPAL cohort study and BOOST RCT) to establish feasibility related to site set-up, recruitment, intervention delivery and data completion.
Work package 3: Develop a prognostic tool
We planned to collect a range of factors related to participants’ BP, general health and mobility that would be used to develop a prognostic tool to identify the BP presentation likely to result in a poor outcome. We set a target of 1000 people retained in the OPAL cohort study with complete data at 2 years’ follow-up.
Work package 4: A pragmatic randomised controlled trial of physiotherapy for neurogenic claudication and nested longitudinal qualitative study
We aimed to recruit a minimum of 402 patients with NC from primary care and musculoskeletal hubs to test the physiotherapy intervention developed in work package 1 with a longitudinal qualitative study, process and economic evaluation running alongside.
Work package 5: Predictors of the participants’ response to treatment – enhancing stratified approaches
We planned to investigate the role of magnetic resonance imaging (MRI) parameters and a small selection of other subgrouping variables to determine who will respond best to physiotherapy.
Work package 6: Project integration and preparation for implementation
We proposed to gain the perspectives of health professionals to help inform the final integration of the project outputs and, if appropriate, ongoing implementation studies and dissemination.
Changes to the programme
During the first year of this programme of research, we made refinements to the OPAL cohort study.
We originally planned to enrol 1000 older adults with BP in the cohort study. To identify the 1000 older adults with BP, we needed to screen a minimum of 4000 older adults who responded to the invitation to participate in the cohort study. This was based on the most recent estimates of disabling BP in the population aged 70–90 years, with a prevalence of 25–30% for these age groups, respectively. 8
We decided to enrol all invitation respondents in the cohort regardless of their BP status. In addition to the original research objective (to recognise when and what types of BP presentation were important treatment targets for older people), we would be better able to evaluate the contribution of BP to mobility decline within a broader context. This change to the original proposal was reflected in the study protocol approved by the London–Brent Research Ethics Committee (16/LO/0348) on 10 March 2016. Changes were supported by the Programme Steering Committee and reviewed and accepted by the Programme Grants Manager on 30 March 2016.
Work package 6 could not be delivered as planned due to the challenges arising from the COVID-19 pandemic. We had initially planned to run focus groups with clinicians to discuss the study results and implications. However, delays to results meant this was not completed. The OPAL cohort study was nearing completion of the 2-year follow-up mail-outs when the COVID-19 lockdown started in March 2020. Although the team managed to send out all year 2 questionnaires just prior to the lockdown, this had a major impact on the study team’s ability to process the large volume of data collected from home, which was very time-consuming using remote access. Data were available 6 months late, delaying the analysis needed to develop the OPAL prognostic tool. The BOOST trial health economic analysis was delayed by 6 months as our health economist was working at home with young children throughout lockdown. This work was essential to interpretation of the trial findings and significantly delayed our plans to publish the results. Despite these challenges, we had strong engagement with clinical collaborators throughout this research and we worked as best we could to engage with clinical staff to form our dissemination plan.
Research pathway diagram
Objective 1: To describe the presentation and impact of back pain in community-dwelling older adults
Background
We lacked a good understanding of the prevalence and impact of back and leg pain within the community-dwelling population in the UK. There were estimates for the prevalence of BP in the UK2,8 but not for back-related leg pain. A single UK-based study (n = 30) estimated the prevalence of NC among patients (50 years and over) presenting with back and leg pain to primary care to be 87%, suggesting it was the predominant cause of spinal-related leg pain when patients sought care. 18 However, we wanted to understand the extent of the problem among community-dwelling older adults and not just those seeking care.
In addition to understanding the presentation of back and leg pain, we wanted to understand the broader impact of having back and leg pain in later life. We were interested in understanding the relationship between different presentations of back and leg pain with quality of life and age-related adverse health states including frailty, mobility decline, falls, poor sleep quality and incontinence. These health states are common in later life and associated with substantial morbidity and poor outcomes. 19 Little was known about the relationship between BP and different patterns of leg pain and these age-related adverse health states. 5–7,20,21 Back and leg pain had been associated with falls and functional difficulties, but studies were limited. 22 Different patterns of back and leg pain had not been taken into consideration, and it was unknown whether certain presentations were more strongly associated with adverse health states.
There was also little information about the natural history of symptoms among community-dwelling older adults reporting back and leg pain within the UK. Cohorts of older people with BP have reported that around 50–60% of participants will continue to experience pain and disability at 12 months’ follow-up. 23,24 There were some data on the natural history of symptoms due to spinal stenosis, with the majority of people (around 55%) reporting no change in pain over a 3-year period while around one-third improved and 10–13% reported a worsening of symptoms. 25 It was unclear how recovery may differ among those with different back and leg pain presentations.
In this section, we will examine:
-
the presentation of back and leg pain symptoms among OPAL cohort participants at baseline and the association with adverse health outcomes
-
for those with back and leg pain at baseline, the persistence of symptoms at 2 years’ follow-up.
Methods
The OPAL protocol and a description of the participants at baseline26 can be accessed here: http://doi.org/10.1136/bmjopen-2020-037516
A more detailed description of the baseline cross-sectional analyses can be accessed here: https://ora.ox.ac.uk/objects/uuid:911cf725-135f-4951-a1b2-28eb2032d9ab
Briefly, there are 5409 community-dwelling older adults enrolled in the OPAL cohort study. Participants are over 65 years of age, registered with one of 35 participating primary care practices, and were recruited using electronic record searches. Older adults living in residential care or nursing homes were ineligible. Potential participants received an information leaflet, consent form and baseline questionnaire. Those who returned a completed consent form and questionnaire to the study office were enrolled in the study. Of the individuals invited, 42% (5409/12,839) were enrolled in the study. Follow-up was by yearly postal questionnaire. A summary of the data collected is available in Appendix 1, Table 5. Participants were included in these analyses if they completed the questions about back and leg pain.
Back and leg pain presentation at baseline
Firstly, participants reported whether they had been troubled by BP and related symptoms in the last 6 weeks and, if so, supplied further information about frequency (every day, most days, some days, few days, rarely), troublesomeness (intensity) (extremely, very, moderately, slightly, not at all)27 and spread into the legs over the last 6 weeks. They were asked about the age when they were first troubled by BP (onset) and its pattern since onset (getting better, getting worse, fairly constant, comes and goes over time).
We categorised participants into four mutually exclusive back and leg pain presentations.
-
no BP (reference category)
-
BP only
-
BP and NC
-
BP and leg pain that is not NC (non-NC).
We used questions commonly used in clinical practice with high sensitivity and specificity when identifying people with symptomatic spinal stenosis. 28 NC was defined as the presence of BP or other symptoms that travel from the back into the buttocks or legs and are worse when standing and/or walking and better when sitting and/or bending. 28
Baseline adverse health states
We collected the following:
-
mobility decline over the last year
-
confidence to walk half a mile using a question from the Modified Gait Self-efficacy Scale31
-
falls in the last year32
-
incontinence, reported using the urinary incontinence item from the Barthel Index33,34
-
sleep quality during the past month, using the sleep quality overall rating from the Pittsburgh Sleep Quality Index. 35
We also measured health-related quality of life (HRQoL) measured by the EQ-5D-5L36 and the clock drawing test to screen for cognitive impairment. 37
Back pain and leg pain at 2 years’ follow-up
To examine recovery rates among participants who reported BP and related symptoms at baseline, we used data from the year 2 questionnaire to see how many had been troubled by BP and related symptoms in the last 6 weeks; those who had were asked about frequency and troublesomeness.
Participants rated how much their BP interfered with their daily activities (0 = no interference, 10 = unable to carry out the activities). This question was based on the Von Korff Pain Scale. 38 Severely interfering BP was defined as a report of ≥ 7/10 based on the cut point used in previous cohorts. 39,40 If a participant was no longer reporting BP at 2 years, then they were allocated a score of 0/10.
Analysis
Descriptive statistics were used to summarise the characteristics of participants at baseline. Modified Poisson and ordered logistic regression analyses were conducted to assess the relationship between BP groups (independent variable) and adverse health states (dependent variables) at baseline. Models were adjusted for age, sex, education, body mass index (BMI), socioeconomic status [Index of Multiple Deprivation (IMD)],41 smoking, physical demands of their main occupation, comorbidities and presence of multisite pain based on the Nordic Pain Questionnaire. 42,43 We used multiple imputation by chained equations to handle missing data. 44,45
Descriptive statistics were used to summarise the BP outcomes at 2 years’ follow-up, and we stratified by baseline BP presentation.
Results
Baseline presentation
The mean age of participants included in these analyses was 75 ± 6.8 years. Over half of the participants reported BP, with 33.7% (1786/5304) reporting BP only, 11.2% (594/5304) reporting BP and NC, and 8.3% (441/5304) reporting BP and non-NC leg pain. Only the prevalence of BP and NC increased with age. BP was more common in females and those reporting more comorbidities. Those with leg pain reported poorer health behaviours (smoked more, higher BMI), lived in more deprived areas and reported lower education levels. Participants with BP had lower quality of life compared to participants without BP. Quality of life was poorest in those with NC. The age of onset was similar across BP groups. Around 30% of participants reported that their BP started 25 years ago or more. Those with leg pain reported more frequent BP. A report of pain on most days/every day was most common among those with NC. Participants with NC reported the highest ratings of pain troublesomeness. Very few reported improving symptoms since onset (3.7%) and, most commonly, symptoms fluctuated over time (75%). The proportion of participants reporting persistent pain or pain getting worse since onset was highest in those with NC (75%). See Appendix 1, Table 6 for a full description.
Just over half of the participants (55%; 2937/5304) reported at least one adverse health state. The most common adverse health states reported were a fall in the last year (29%; 1534/5304) and being frail (27%; 433/5304) (Figure 1). Confidence to walk half a mile was generally high among the cohort except for those reporting NC (see Appendix 1, Table 6). Across the three BP groups, there was a greater prevalence of all adverse health states compared to those with no BP; this increased further among those who reported leg pain, and it was highest among those with NC.
FIGURE 1.
Prevalence of adverse health outcomes by BP groups. Notes: Poor sleep quality = fairly bad or very bad sleep quality on Pittsburgh Sleep Quality Index. Urinary incontinence = never or less than once per week was considered continent and the remaining responses were considered incontinent. Mobility decline: ‘Compared to one year ago, how would you rate your walking in general?’ scored on a five-point scale constructed for the study. Participants reporting worsening of walking were classified as having mobility decline. Participants who rated their confidence to walk at 9–10/10 were categorised as having low walking confidence (reverse scored). Frail = ≥ 5 on Tilburg Frailty Index.
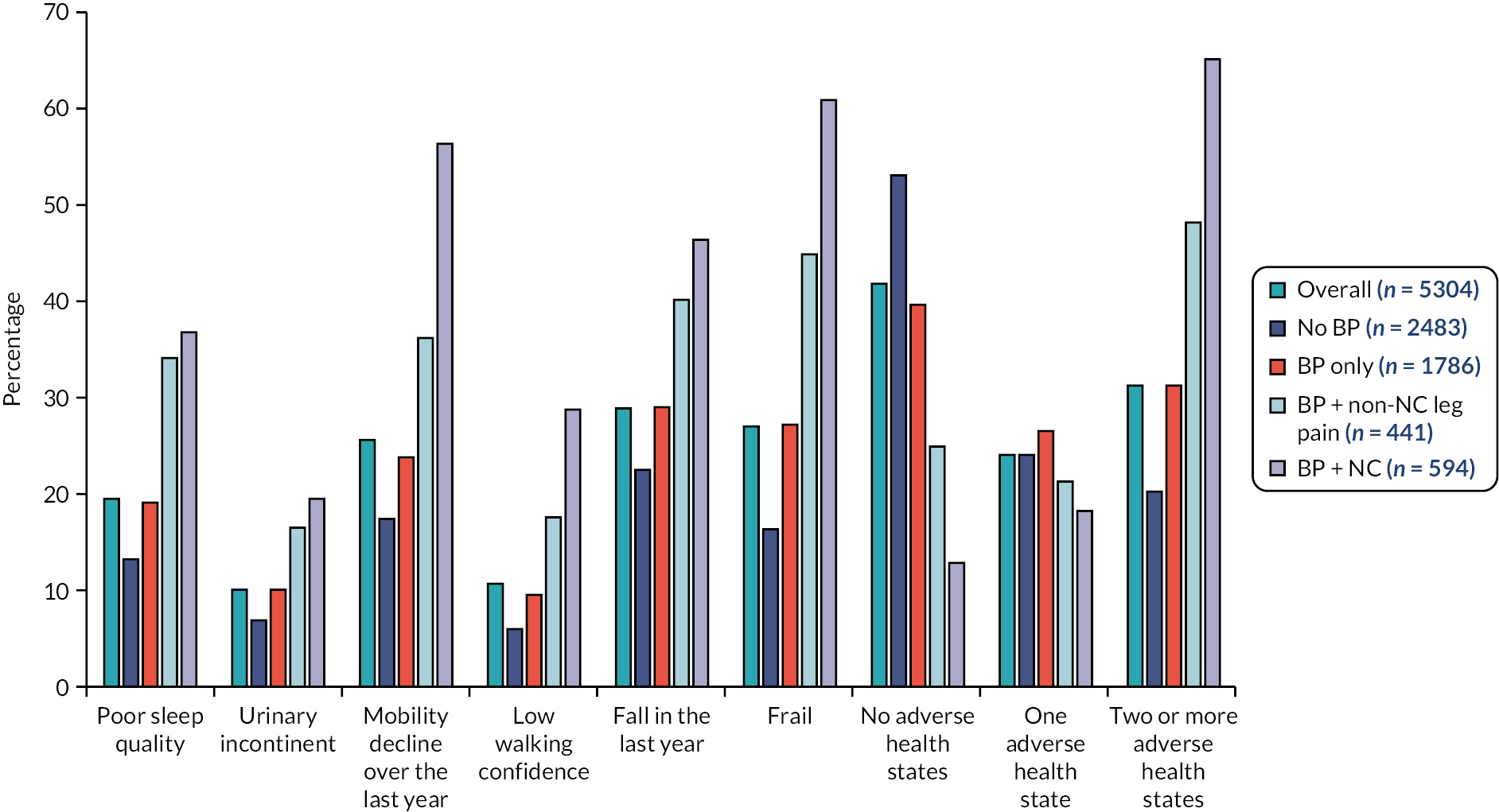
After adjusting for demographics, lifestyle factors, comorbidities and multisite pain, all BP groups were associated with the adverse health states studied (Table 1). For all the adverse health states, there was an increase in the strength of association with the addition of leg pain. This was particularly noticeable in participants with NC, where we observed the strongest associations with frailty, mobility decline, low walking confidence and falls compared to no BP.
| Age-related adverse health outcomes | BP only | BP + non-NC leg pain | BP + NC |
|---|---|---|---|
| Confidence to walka | 1.23 (1.06 to 1.43) | 1.89 (1.52 to 2.36) | 3.11 (2.56 to 3.78) |
| Mobility decline | 1.13 (1.00 to 1.26) | 1.42 (1.22 to 1.64) | 1.74 (1.54 to 1.97) |
| Falls in previous year | 1.14 (1.03 to 1.26) | 1.40 (1.22 to 1.61) | 1.42 (1.25 to 1.61) |
| Frail | 1.38 (1.24 to 1.54) | 1.77 (1.55 to 2.02) | 1.88 (1.67 to 2.11) |
| Poor sleep quality | 1.17 (1.02 to 1.35) | 1.68 (1.41 to 1.99) | 1.67 (1.42 to 1.97) |
| Urinary incontinence | 1.17 (0.95 to 1.43) | 1.59 (1.22 to 2.07) | 1.43 (1.12 to 1.83) |
Back pain presentation at 2 years’ follow-up
Of the participants reporting BP and related symptoms at baseline, 2139/2859 (74.8%) returned the 2-year follow-up questionnaire and completed at least some of the BP variables.
Overall, 23% of respondents (489/2100) no longer reported back or leg pain symptoms (see Appendix 1, Table 7). Recovery was highest among those that reported only BP at baseline (27.4%) and lowest among those with back and NC leg pain at baseline (10.9%). Participants reporting BP only at baseline were least affected at follow-up, and participants reporting NC leg pain at baseline were the most affected. Those with NC leg pain at baseline reported BP more frequently, and this group had the highest proportion reporting very or extremely troublesome BP. Very few participants from all groups reported that their BP was improving (3%), and those with back and NC leg pain at baseline had the highest proportion reporting that it was getting worse (13.2%). Those with BP only at baseline reported the lowest amount of pain interference with daily activities (6.4%). Those with NC leg pain reported the highest pain interference ratings at follow-up and had the greatest proportion reporting severe interference with activity (26%).
Limitations
Data were collected using postal questions, so we were reliant on self-reporting of symptoms. Some participants may have been misclassified or symptoms misdiagnosed as spinal-related leg pain (e.g. vascular claudication can have similar symptoms).
Discussion
Back and leg pain is a common problem for community-dwelling older adults, and for many it is a long-standing problem, with few reporting an improving clinical picture. At 2 years’ follow-up, only 23% of participants no longer reported symptoms. Symptom persistence was highest among those with NC at baseline, with 90% still reporting symptoms.
At baseline, older adults with back and leg pain reported lower HRQoL, with those with NC most affected. The report of back and leg pain was associated with adverse health states, including frailty, falls and mobility decline, compared to no report of BP. Participants reporting symptoms of NC were most affected and the strongest associations were with mobility-related adverse outcomes. At follow-up, those with NC at baseline remained the most severely affected, with a quarter of this group reporting severe activity limitations due to their symptoms. These findings confirmed our hypothesis that NC is particularly problematic for older people and highlighted the need to develop effective interventions to reduce the burden of this condition. However, BP alone is also a widespread persistent problem associated with age-related adverse health states, so ways to reduce the burden of BP are needed.
Objective 2: To refine a physiotherapy intervention for older people with neurogenic claudication to be tested in a randomised controlled trial
Starting point
As we have described, NC is a common, debilitating spinal condition affecting older people. 28 At the start of this programme of work, we had identified a strong theoretic underpinning and limited evidence from clinical practice, cohort studies and small RCTs for a physiotherapy intervention for NC, providing us with a firm starting point to refine and test an intervention. Experts hypothesised that stretching and mobilising the spine relieved pressure on spinal nerves and blood vessels and that aerobic exercises improve circulation, alleviating ischaemic changes. 46
However, recommendations were not substantiated by high-quality evidence46 despite being used commonly in clinical practice. 47,48 Systematic literature reviews reported that the current evidence from non-operative care was of very low to low quality, thus prohibiting recommendations to guide clinical practice. 16,17,49–51 Trials to date were of small sample sizes and often included only short-term follow-up. Interventions focused on the mechanics of spinal stenosis with little regard to the psychological impact of pain or ageing. Notably, interventions did not include strengthening exercises to counter the loss of muscle mass associated with ageing that contributes to loss of mobility in older people;52 nor had the authors considered addressing negative beliefs about pain or ageing that were potential barriers to engaging with and adhering to rehabilitation. 53,54
We explored the rehabilitation needs of older people with NC during a preparatory qualitative study. 55 Participants sought rehabilitation in the hope of re-engaging with meaningful activities and reducing pain. A combination of one-to-one and group sessions was preferred to deliver rehabilitation. One-to-one time with a physiotherapist was considered an important prerequisite for treatment to allow the therapist to understand the participant’s condition and circumstances. Participants endorsed exercising in a group for peer-to-peer support and socialising. Discourses around ageing influenced their experiences – especially regarding the use of walking aids, which they considered stigmatising. These views and preferences informed the refinement of the proposed intervention to ensure it would meet the needs of people with NC.
A full description of the BOOST intervention56 can be accessed here: https://doi.org/10.1016/j.physio.2019.01.019
The preparatory qualitative study55 can be accessed here: https://ora.ox.ac.uk/objects/uuid:92c9ee3e-30eb-4fc0-95fd-08db3bc4707b
Methods
We followed Medical Research Council guidance on developing a complex intervention. 57
Step 1: identify existing evidence
We consulted recent systematic literature reviews and interrogated the individual studies for intervention components that warranted consideration16,17,49–51 as well as National Institute for Health and Care Excellence (NICE) guidelines. 58 In parallel, we considered the literature related to pain, frailty, falls and disability in older adults,3,5–7,20,21,59–63 clinical guidelines for exercise in older adults,64,65 behaviour change interventions66 and psychological models of pain and disability. 67,68 We drew on the findings from the preparatory interview study. 55
Step 2: identify and develop theory
From existing evidence, we identified treatment targets and matched possible intervention elements to them to discuss with stakeholders. Twelve clinicians, two patient representatives and nine researchers attended an intervention refinement day. The aim of this day was to gain consensus about the key elements of an intervention that would be acceptable to users and deliverable within the NHS.
Discussions firstly centred on the overall approach to take, including mode of delivery (individual vs. group), then we focused on the individual components. A physiotherapy-delivered group physical and psychological intervention was agreed utilising two parallel and synergistic approaches: (1) exercises targeting strength, balance, flexibility and endurance, and (2) education and peer support to counter negative beliefs about pain or ageing that may impede exercise engagement and adherence, underpinned by behaviour change techniques and pain management principles.
Step 3: modelling processes and outcomes
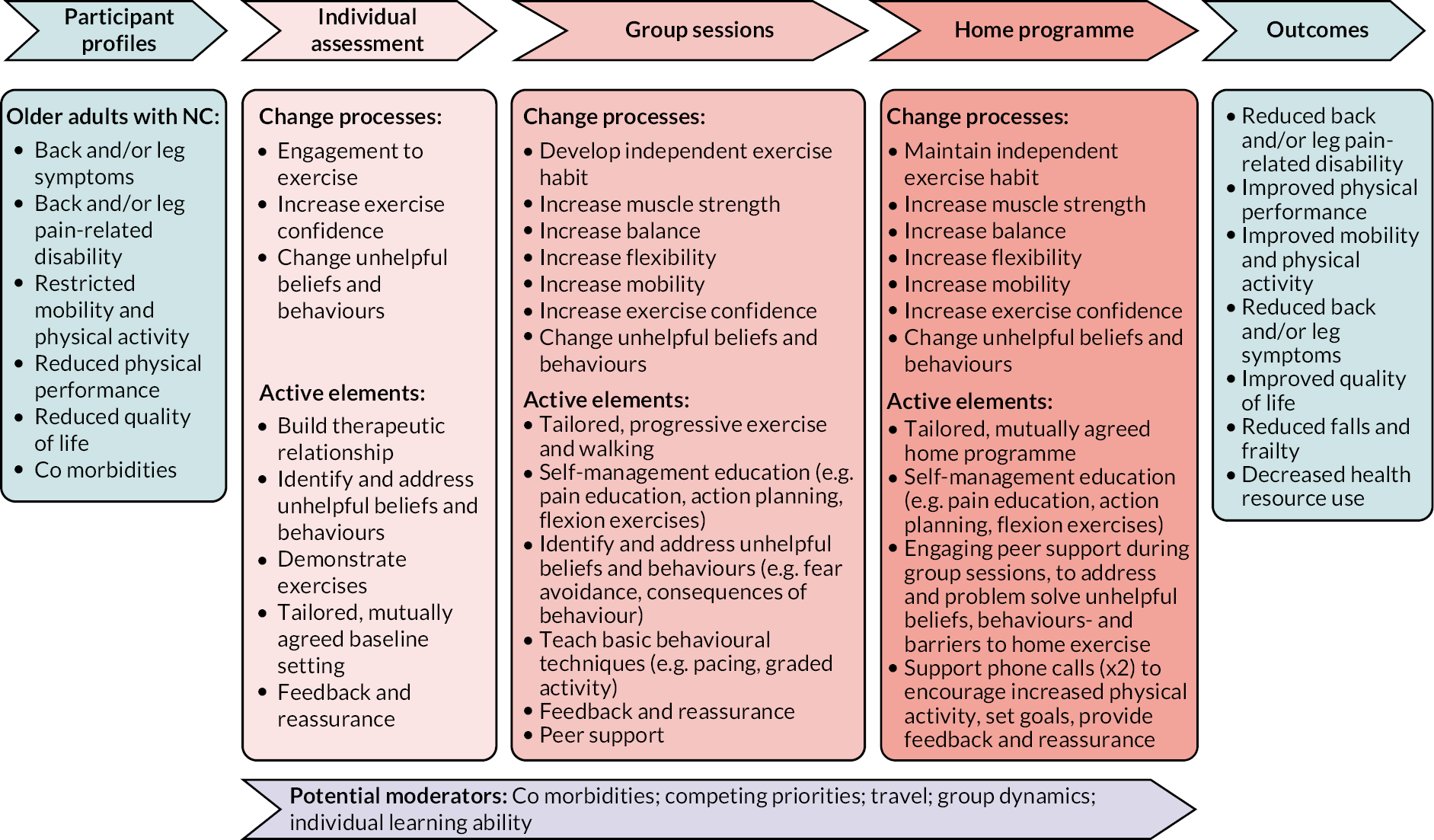
A conceptual model of the change processes, active intervention elements and potential moderators of the BOOST intervention was produced (Figure 2).
The BOOST programme
The programme consisted of an individual assessment, 12 group sessions and two support telephone calls.
Prior to attending the group programme, each participant attended an individual appointment (1 hour) for an assessment and to set their individualised exercise and walking circuit targets for the group sessions.
Participants attended twelve 90-minute group sessions over a 12-week period. Each session followed the same format. Participants took part in an education and discussion session (30 minutes) which incorporated behavioural change strategies to encourage adherence with home exercises. The topics of discussion are included in Appendix 3, Table 14. This was followed by the exercise programme, lasting approximately 1 hour.
There was a short warm-up of seated exercises, which included arm raises, trunk rotation, pelvic tilting and knee lifts. Then participants undertook a circuit of strengthening which included the following exercises:
-
sitting knee extension
-
sit to stand
-
standing hip abduction
-
standing hip extension
-
a combined hip flexor and calf stretch
-
a progressive balance sequence (feet together static balance, semi-tandem stance static balance, full tandem stance static balance, forward tandem heel-to-toe walking, backward tandem heel-to-toe walking).
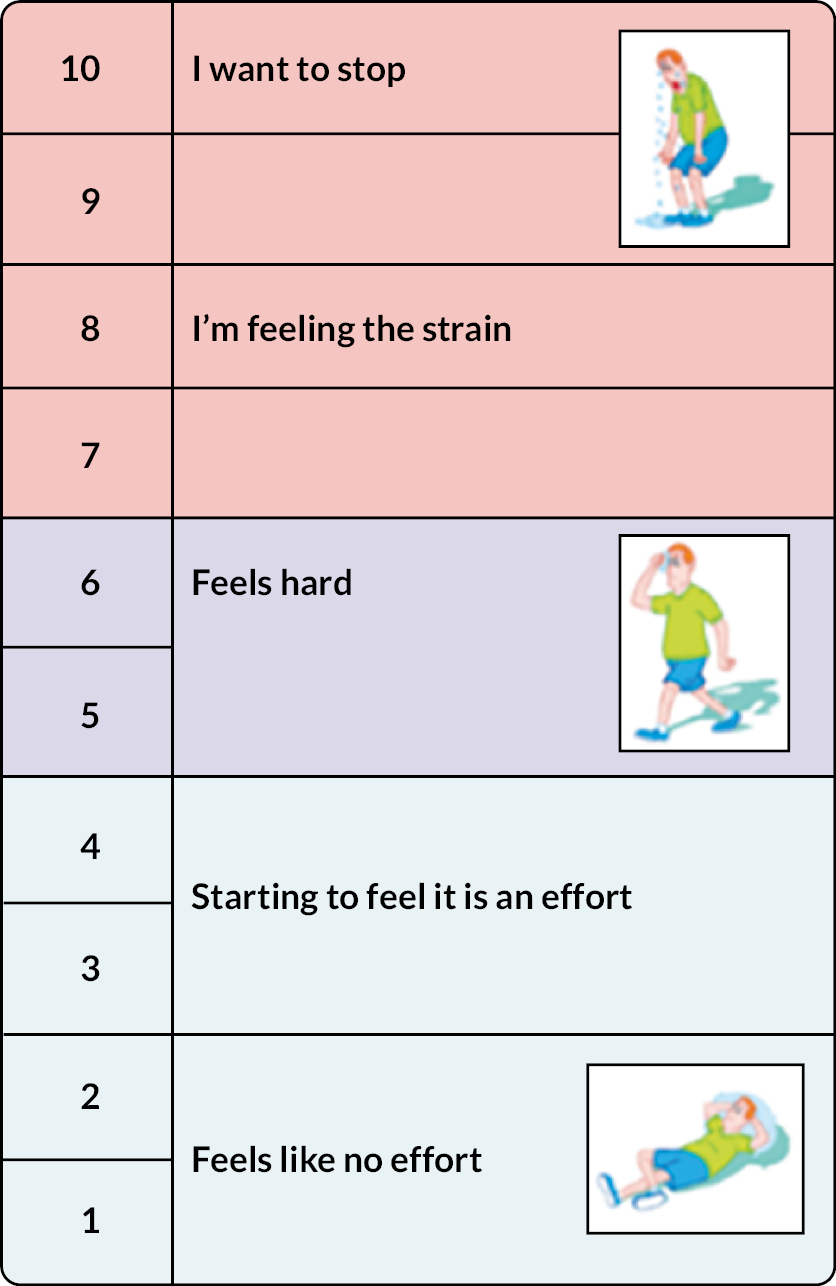
Each participant completed their individually tailored programme. Exercises were progressed over the 12 weeks. We used the Borg Rating Scale of Perceived Exertion to ensure participants achieved an adequate stimulus to promote strength gains and encouraged them to work at level 5–6 on this scale (the exercise feels hard) during the strengthening exercises (Figure 3).
FIGURE 3.
Borg scale.
Participants then undertook a supervised walking circuit, designed to improve walking ability and fitness, which also progressed over the 12 weeks by increasing the distance walked, increasing walking speed, adding balance challenges such as stairs or obstacles, or adding weights. The exercises carried out during the supervised sessions made up the home exercise programme (warm-up, exercise circuit and walking).
On completion of the 12 group sessions, participants were asked to carry out their home exercise programme at least twice per week. The physiotherapist reviewed each participant by telephone 1 and 2 months after they completed the supervised sessions, to promote long-term adherence with home exercises.
Control intervention
The control intervention was best practice advice (BPA) delivered in individual physiotherapy appointments, which reflected what we considered a ‘good’ standard of usual care physiotherapy. This intervention was informed by a survey of current physiotherapy practice47 and a recent trial,56 and through consultation with clinicians and patient representatives. We recommended that participants receive one session of advice and education. However, the clinicians highlighted situations where a review would be necessary (e.g. to review walking aids) so this was permissible.
Participants attended a 1-hour appointment consisting of an assessment followed by the provision of tailored advice and education and written information. Participants were prescribed up to four home exercises. Flexion and trunk stabilisation were recommended. The physiotherapist could prescribe a walking aid if indicated. A maximum of two half-hour review appointments were permitted. During review sessions, physiotherapists reinforced the verbal advice given, and reviewed walking aids or exercises.
Intervention delivery training
We developed a clinician manual and training programme. Physiotherapists delivering the BOOST programme attended a full day of training with the research team. They were also asked to complete some online training prior to attending. Those delivering the control intervention attended a separate half-day of training.
Pilot work
We piloted the training, which was refined following feedback. We conducted the first BOOST programme with two participants, and feedback indicated satisfaction with the programme content, duration and timing of sessions. Minor modifications were made. A researcher observed the control interventions, which were delivered as intended.
Summary of work undertaken
We focused on refining a complex intervention that was underpinned by existing knowledge, would meet the needs of older people with NC, and would be acceptable to clinicians and deliverable in the NHS. By combining group delivery with an individual assessment and follow-up calls, we ensured each participant underwent a thorough assessment so they were confident in the programme but could benefit from peer-to-peer support and socialising. This was also a way to deliver a longer-duration intervention for participants, which would increase the likelihood of achieving meaningful gains in muscle strength and mobility without placing excessive burden on physiotherapy departments.
Objective 3: To undertake a randomised controlled trial evaluating the clinical and cost-effectiveness of physiotherapy interventions for neurogenic claudication
Methods
The full protocol69 is available here: https://doi.org/10.1136/bmjopen-2020-037121
The statistical analysis plan70 is available here: https://doi.org/10.1186/s13063-020-04590-x
The paper reporting clinical effectiveness71 is available here: https://doi.org/10.1093/gerona/glac063
The paper reporting the cost-effectiveness72 is available here: https://doi.org/10.1186/s12962-022-00410-y
Design
This study was a pragmatic, multicentre, superiority RCT.
Participants
Community-dwelling adults, aged 65 years and over, who reported symptoms consistent with NC, were eligible. Exclusion criteria included nursing home residents, inability to walk 3 metres independently, awaiting surgery, cauda equina syndrome or signs of serious pathology, cognitive impairment, registered blind, or unable to follow instructions in a group setting. Potential participants were identified through community-based physiotherapy clinics and secondary care spinal clinics in 15 NHS Trusts in England.
Participants were also identified through the OPAL cohort study. 26 Potential participants were identified from questions asking about symptoms suggestive of NC in their most recent questionnaire and invited for eligibility screening.
Randomisation
Participants were randomised to the BOOST programme or BPA in a 2 : 1 ratio (intervention : control) to ensure that we could fill BOOST groups without participants experiencing long waiting times.
Intervention fidelity
The research team monitored intervention fidelity by observing treatment sessions (see Appendix 3 and Report Supplementary Material 1) and checking physiotherapy treatment logs.
Data collection
Participants completed a questionnaire, and the blinded researcher conducted physical testing at baseline and 6 and 12 months after randomisation. If participants did not attend the follow-up appointment, then the physical tests were not completed, and participants were sent a postal questionnaire.
The variables collected at outcomes are presented in Table 2. The primary outcome was the Oswestry Disability Index (ODI v2.1a)73 at 12 months after randomisation. This participant-reported measure of pain-related disability is scored 0–100, with a higher score indicating greater disability. We collected adverse events. At baseline, we also collected demographic information and factors related to health and mobility, which are listed in Appendix 2, Table 10.
We used data from lumbar spine MRI scans. Pre-existing scans, taken in the 12 months preceding randomisation, were used if available to reduce the need for scanning. Otherwise, participants were referred for a scan. Information about the assessment of the MRI scans is available in the trial protocol69 and in the thesis by Gagen (http://wrap.warwick.ac.uk/161673). 84
| Outcomes (0, 6 and 12 months unless indicated) |
BP and leg symptoms | ODI Troublesomeness of back and leg problems27 Swiss Spinal Stenosis Scale (symptom subscale)75 Ability to manage back and leg pain (0–10: 0 = not managing at all; 10 = extremely well) |
| Mobility | Walking item from the ODI 6MWT76,a |
|
| Physical performance | SPPB77,a | |
| Balance and falls | Self-reported falls and fall-related injuries32 | |
| Physical activity | Two items from Rapid Assessment Disuse Index78 [(1) time moving; (2) time sitting] | |
| Frailty | Tilburg Frailty Index29 hand grip strength79,a |
|
| Global rating of change | Change in back and leg problems80,b | |
| Satisfaction | Changes in back and leg problem; treatment (0–4; 0 = very dissatisfied; 4 = very satisfied)b | |
| Exercise adherence | Self-reported adherence to home exercisesb | |
| Quality of life | EQ-5D-5L81,82 | |
| Health resource use | Client Service Receipt Inventory83,b |
Sample size
At 80% power and 5% two-sided significance levels, a sample size of 321 participants (214 in the intervention group and 107 in the BPA group) was required. With an inflation for potential loss to follow-up (20%), this led to an overall target of 402 (268 intervention, 134 control). The sample size assumed a between-group difference of five points in the ODI to be clinically significant, with a baseline standard deviation (SD) of 15. 74
Statistical analysis
The primary outcome of ODI at 12 months’ follow-up was analysed in an intention-to-treat (ITT) population and effect estimates with their 95% confidence intervals (CIs) were reported at a 0.05 significance level. The ODI difference between the two treatment groups was estimated using a repeated measures linear mixed-effects regression multilevel model with fixed effects for participant age, gender and baseline ODI, and random effects for recruiting centre and observations within participant (6 and 12 months). A model additionally accounting for potential heterogeneity due to the treating physiotherapist was assessed in a sensitivity analysis. Similarly, we assessed whether there was a group effect. We assessed the impact of intervention compliance using a complier-average causal effect (CACE) analysis. 85 Compliance with the BOOST programme was defined as attending at least 9 out of the 12 sessions (75%).
Secondary outcomes were analysed in the ITT population, using similar methods and adjusting for the relevant baseline covariate where applicable. All analyses were completed using Stata® version 15.1 (StataCorp, LLC, College Station, TX, USA).
We undertook a series of prespecified subgroup analyses to see whether we could identify subgroups of participants who responded better or worse to the interventions tested (see Appendix 2, Table 8). Subgroup effects were analysed using interaction with treatment tests. 86
Economic evaluation
The economic analyses involved evaluation of economic costs, HRQoL outcomes and cost-effectiveness of the BOOST programme, where cost-effectiveness was expressed in terms of incremental cost per quality-adjusted life-year (QALY) gained. The base-case economic evaluation took the form of an ITT, imputed analysis conducted from a UK NHS and personal social services (PSS) perspective in line with the NICE reference case,87 and separately from a societal perspective. A 12-month time horizon for the economic evaluation was used, mirroring the trial follow-up period, and therefore no discounting was required.
Three broad resource use and costs categories were estimated: (1) intervention costs; (2) broader health and PSS use during the 12 months’ follow-up; and (3) broader societal resource use and costs encompassing economic values of lost productivity (e.g. income lost by participants and carers). All costs were expressed in GBP and valued in 2018–19 prices. Participants completed the Client Service Receipt Inventory83 at 6 and 12 months. HRQoL was assessed using the EQ-5D-5L instrument88 completed at baseline and at 6 and 12 months post randomisation.
A bivariate regression of costs and QALYs, with multiple imputation of missing data, was conducted to estimate the incremental cost per QALY gained and the incremental net monetary benefit (INMB) of BOOST in comparison to BPA.
Results
Recruitment and follow-up
Recruitment was undertaken between 1 August 2016 and 29 August 2018 at 15 sites. We selected sites in different areas across England to maximise recruitment of participants from different ethnic and socioeconomic backgrounds. A list of trial sites and description of the local population is provided in Appendix 2, Table 9.
In total, 438 participants were found to be eligible, provided informed consent and were randomised (spinal clinics n = 394, OPAL n = 44). Three participants withdrew post randomisation and removed consent to use their data, so the final sample size was 435 participants (BPA n = 143, BOOST programme n = 292) (Figure 4).
FIGURE 4.
CONSORT diagram. a Not all participants who were eligible to be screened for BOOST were invited. b Numbers included in analysis are all participants with at least one follow-up ODI outcome and the baseline variables used in the model. This was due to lack of staff capacity at sites to accept additional referrals for screening or due to lack of a referral pathways between primary care centres and study sites.
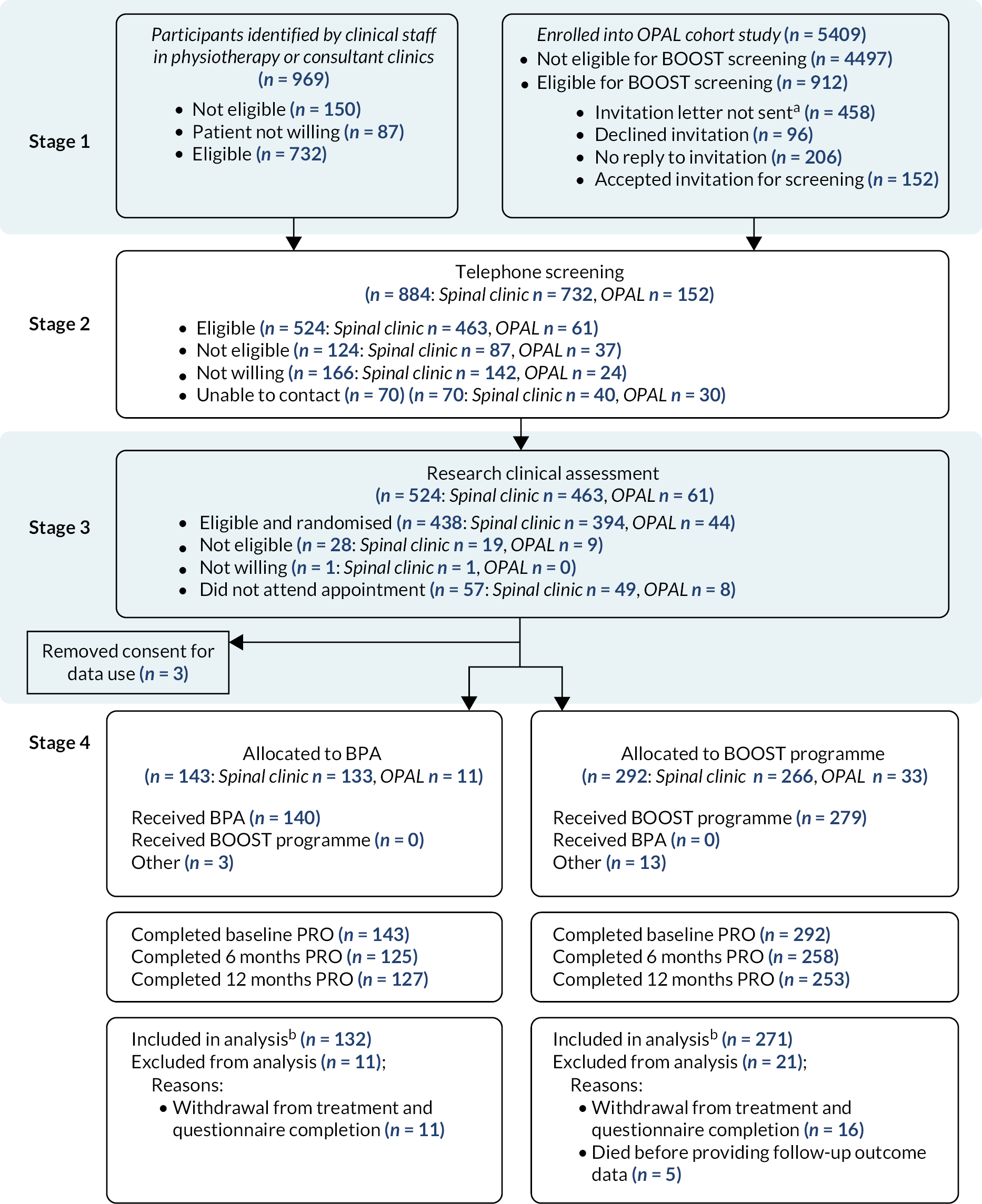
The primary outcome was obtained for 88.0% (383/435) and 87.4% (380/435) of participants at 6 months and 12 months, respectively, with 93.0% (403/435) contributing data to the primary analysis.
Baseline characteristics
Participants had a mean age of 74.9 years (SD 6.0) and were predominantly white (400/435; 91.9%). The randomised groups were well matched on baseline characteristics (see Appendix 2, Tables 10 and 12). In the BPA group, a larger proportion of participants were classified as frail (55.9% vs. 44.5%) using the Tilburg Frailty Index but other markers of frailty (6MWT, SPPB and hand grip strength) were similar. Four-fifths of participants (351/435; 81%) reported more than one health condition. The most frequently reported conditions were arthritis (272/435; 62.5%) and high blood pressure (252/435; 57.9%).
The proportion of BOOST RCT participants also taking part in OPAL was 10.1% (44/435). Twenty-five participants were identified from their baseline questionnaire and 18 from their year 1 questionnaire. We were interested in understanding how similar the OPAL–BOOST participants, who had NC confirmed by clinical assessment, were to OPAL participants categorised as having NC based on self-report. This was to help us understand the generalisability of the trial findings to the general population reporting symptoms of NC. In Appendix 2, Table 11 we present a description of participants at baseline using variables available in both data sets. At baseline, there were 594/5304 (11.2%) OPAL participants who were categorised as having symptoms of NC. 28 OPAL–BOOST participants and OPAL participants were broadly similar in age, sex, BMI, number of comorbidities, BP troublesomeness scores, multisite pain, change in walking over the last year and falls. However, compared to OPAL–BOOST participants, OPAL participants reported lower levels of education, lived in areas of higher deprivation, and reported lower quality of life and walking self-efficacy, and a greater proportion were classified as frail.
Intervention delivery
Sixty-nine physiotherapists delivered the interventions. Thirty physiotherapists delivered BPA, 34 physiotherapists delivered the BOOST programme, and five physiotherapists delivered both. In total, 24/143 (16.8%) participants allocated to BPA were treated by physiotherapists who were also trained in the BOOST intervention.
Treatment attendance was good for both interventions. Of the 143 participants allocated to BPA, 140 (98%) received the intervention. Most commonly, participants attended two BPA appointments (59/143; 41.3%). Of the 292 participants allocated to the BOOST programme, 279 (96.0%) attended the individual physiotherapy assessment. Thirteen participants (4.5%) did not attend this assessment. After the individual assessment, participants joined the next available group. In total, 203/292 (69.5%) attended at least 9 of the 12 sessions, indicating compliance. Having attended the individual assessment, 13 participants (4.5%) subsequently did not attend any group sessions.
We conducted 48 fidelity assessments. Interventions were delivered to a high standard. Eighteen fidelity assessments were undertaken of BPA sessions and 97.2% of checklist items were fully achieved. Thirty fidelity assessments of the BOOST programme group sessions were conducted, with 97.4% of checklist items fully achieved. Monitoring of treatment logs showed that exercises were progressed regularly across the key parameters, including increased repetitions and load and the addition of speed to the strengthening exercises. During the walking circuit, increasingly difficult elements were added. More information about delivery of the BOOST programme is contained in Appendix 3.
Primary outcome
Participants randomised to BPA showed a small increase in ODI scores at 6 months with very little subsequent change at 12 months. BOOST programme participants showed a reduction in ODI scores at 6 months which increased again at 12 months but remained lower than baseline scores. At the 12-month primary end point, there was no statistically significant difference in ODI scores between the two treatment groups (adjusted mean difference −1.4, 95% CI −4.03 to 1.17). There was a statistically significant difference in ODI in favour of the BOOST programme group (adjusted mean difference −3.7, 95% CI −6.27 to −1.06) at 6 months. There was no evidence of a therapist or group effect.
In the CACE analysis, the difference favouring the BOOST programme was larger, reaching the predefined clinically significant threshold (five points on the ODI) when group attendance was taken into consideration (−5.0, 95% CI −8.02 to −1.88) at 6 months. At 12 months, this difference was reduced (−2.4, 95% CI −6.02 to 1.32).
Key secondary outcomes
See Williamson et al. 71 for a full report of the secondary outcomes (see also Appendix 2, Tables 12 and 13).
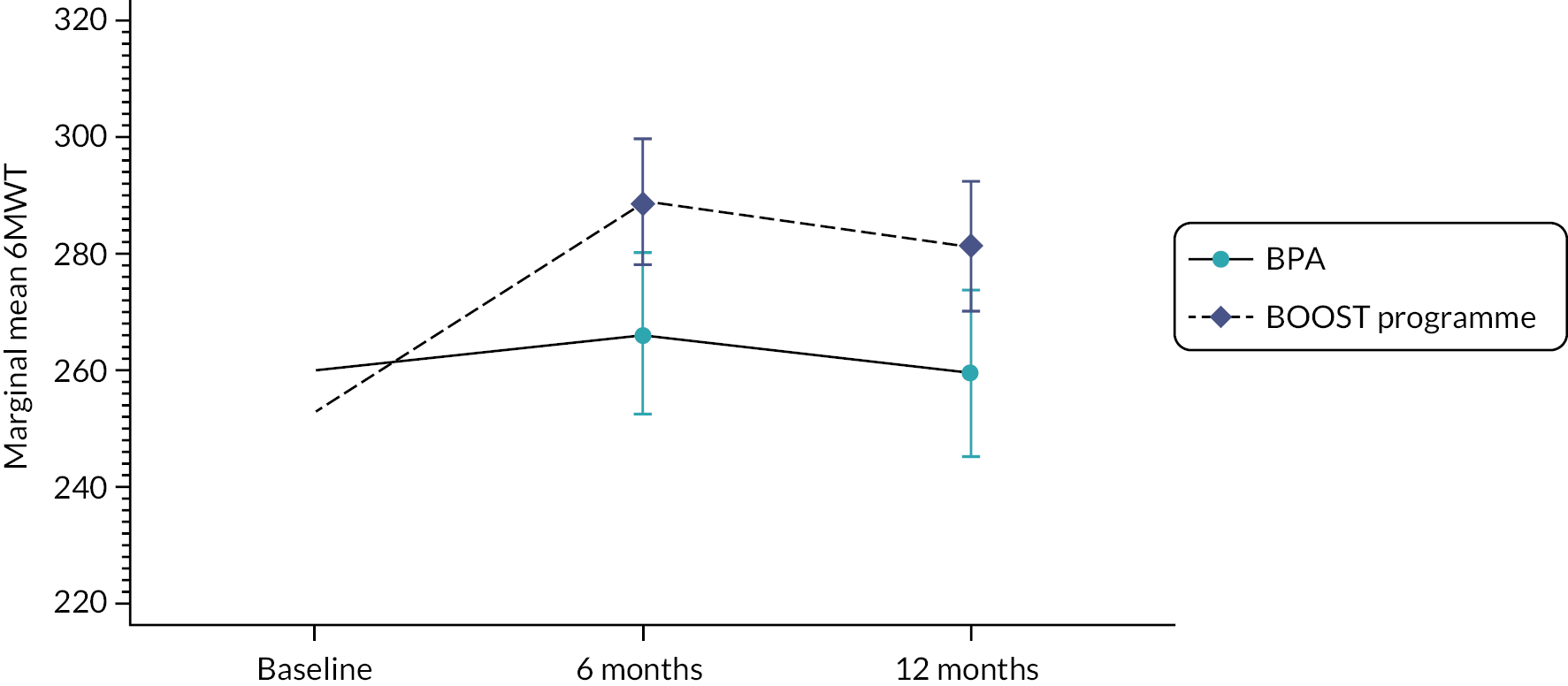
Walking and physical performance
The BOOST programme had a lasting impact on walking capacity (6MWT) (Figure 5) at 6 and 12 months’ follow-up, favouring the BOOST programme. The ODI walking item also reflected this finding. BPA participants showed very little change across the two follow-up time points. A similar response was observed for physical performance (SPPB) (see Appendix 2, Table 13).
Falls
Participants in the BOOST programme had a substantially reduced risk (by 40%) of reporting a fall over the 12-month period. The proportion of participants sustaining a fall-related fracture was very small but similar between groups.
Pain and other symptoms
Both groups reported a small reduction in Swiss Spinal Stenosis Scale (SSSS) symptoms subscale scores at 6 months, and these were larger for the BOOST programme. Small reductions were maintained at 12 months, and there was no longer a difference between the groups at 12 months. Similar findings were observed for troublesomeness, global rating of change and satisfaction with changes in back and leg problems.
Participant satisfaction
Participants in the BOOST programme were more likely to be satisfied with their treatment at 6 and 12 months compared to the control group.
Exercise adherence
Participants were asked how often they performed their home exercises. At 6 months, 190/257 (73.9%) BOOST programme participants reported performing their exercises at least twice per week, which reduced to 143/250 (57.2%) at 12 months. At 6 months, 102/125 (81.6%) BPA participants reported doing their exercises at least twice a week, which reduced to 89/125 (71.2%) at 12 months.
Adverse events
One serious adverse event (cardiac symptoms) occurred during a BOOST session which was deemed unrelated to the intervention. There were no serious adverse events reported for BPA. There were 12 adverse events reported for the BOOST programme. Four were assessed as definitely related to the programme, including aggravation of joint pains (n = 2), a fall during the walking circuit (no injuries) and skin irritation by an ankle weight. Two adverse events were reported for BPA, and neither was definitely related to the intervention.
Prespecified subgroup analyses
Overall, 354 MRI scans from 435 participants (80.8%) were available: 120 MRI scans from 143 (83.4%) BPA participants and 234 MRI scans from 292 (80.1%) BOOST programme participants. There were no statistically significant subgroup effects identified at 12 months’ follow-up.
Economic analyses
Complete QALY profiles were available for 357 (82%) participants based on the EQ-5D-5L. Completion of health resource use data for the base-case economic evaluation was similar at each time point between the BOOST and BPA groups. All data needed to calculate the cost components were available for 75–88% of health resource categories apart from hospital outpatient services, which ranged from 57% to 75%.
Intervention costs and resource utilisation
Mean total intervention costs varied between £242 and £911. The average cost per group session per participant varied from £11.80 to £67.00 depending on the number of participants per session. The mean cost per participant was generally lower across all sites if the target number of participants (n = 6) was achieved.
For health and PSS use, there were non-significant differences between the two groups in utilisation of hospital inpatient and outpatient care, community-based health care and social services, and days off work.
For the primary analysis, mean NHS and PSS costs, inclusive of intervention costs, over 12 months were £1974.06 for the BOOST programme versus £1826.64 for the BPA group. There was a non-significant cost difference in favour of the BPA group of £147.42 (95% CI £419 to £714). Mean total societal costs, inclusive of the intervention cost, were £2176.01 in the intervention group compared with £2140.54 in the BPA group. This generated a mean cost difference of £35.47 (95% CI £469.57 to £540.51) in favour of the BPA group. Societal costs (excluding NHS and PSS costs) were higher in the BPA group and primarily driven by economic valuation of time taken off work by a small number of patients and carers in the BPA group.
Health-related quality-of-life outcomes
The adjusted mean participant-reported QALY estimate for the base-case analysis over 12 months favoured the BOOST programme [0.621 (standard error 0.009) vs. 0.599 (standard error 0.006); between-group difference 0.021 (95% CI 0 to 0.044)].
Cost-effectiveness results: base-case analysis
NHS and PSS perspective
The baseline economic evaluation indicated that the BOOST programme was associated with marginally higher NHS and PSS costs (£147, 95% CI −419 to 714) and an increase in QALYs (0.020, 95% CI −0.003 to 0.045). The mean incremental cost-effectiveness ratio (ICER) for the BOOST programme was estimated at £7211 per QALY gained; that is, on average, the BOOST programme was associated with a higher cost and an increase in QALYs. The associated mean INMBs at cost-effectiveness thresholds of £15,000, £20,000 and £30,000 per QALY were £145, £244 and £464, respectively. The base-case mean INMB was >0, suggesting that the BOOST programme would result in an average net economic gain of approximately £244 (INMB = £244, 95% CI −£570 to £1058). The probability of cost-effectiveness for the BOOST programme was estimated as 67%, 78% and 83% at cost-effectiveness thresholds of £15,000, £20,000 and £30,000 per QALY, respectively (Figure 6). The joint distribution of costs and outcomes for the base-case analysis is presented graphically in Figure 7.
FIGURE 6.
Cost-effectiveness acceptability curve for the base-case imputed (covariate-adjusted) analysis. (From Maredza et al. 72)

FIGURE 7.
Cost-effectiveness plane for the base-case imputed (covariate-adjusted) analysis. (From Maredza et al. 72)
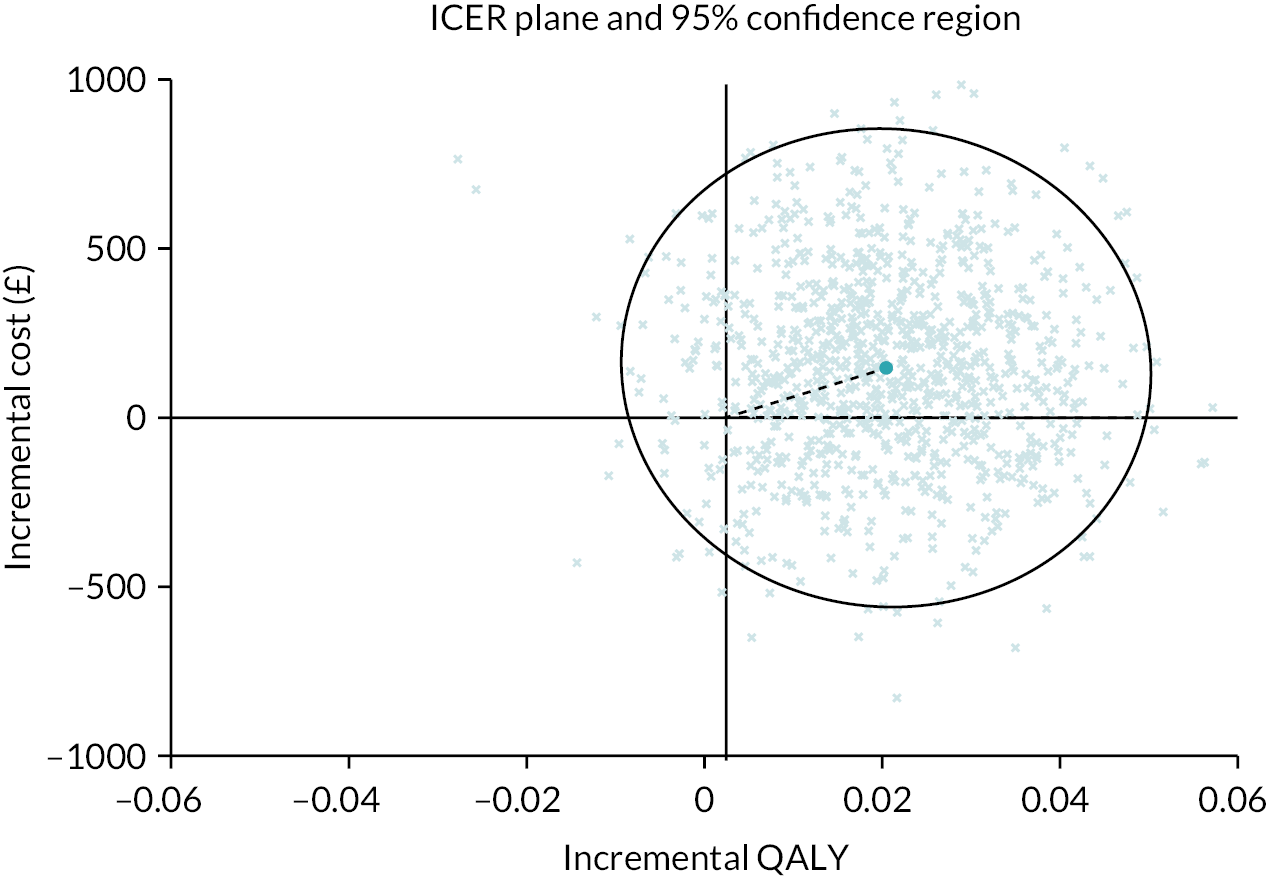
Societal perspective
The probability that the BOOST programme is cost-effective was higher from a societal perspective, ranging between 79% and 89% across cost-effectiveness thresholds.
Limitations
Five physiotherapists trained in delivery of the BOOST programme also treated 24/143 participants (16.8%) allocated to BPA due to physiotherapist availability. There was a risk that these physiotherapists could integrate elements of the BOOST programme such as behaviour change principles into their treatment sessions and provide treatment beyond the BPA protocol. However, the proportion of participants exposed to potential contamination is well below the 30% threshold considered a serious threat. 89 We carefully monitored intervention delivery, including fidelity assessments, and are confident that the risk of contamination between arms was minimised.
Resource use data for the health economic analyses were retrospectively recalled by participants, which may mean they were affected by recall bias. However, we would expect any bias to be similar in each randomised group. Also, our approaches to collecting resource use data did not disentangle resource use associated with NC from use associated with broader health factors.
Discussion
The BOOST programme improved walking capacity and physical performance and reduced walking disability and falls risk compared to a control intervention of BPA for older adults with NC at 12 months’ follow-up. There were also larger short-term improvements in symptoms and pain-related disability. We plan to optimise the programme to better target pain-related disability and symptoms for longer-term impact as part of the planned implementation. Exercise adherence decreased over time, and we will also focus on improving long-term adherence during optimisation.
The economic analyses suggest that the BOOST programme is a good use of NHS resources.
There were broadly similar clinical presentations between OPAL participants with NC and BOOST–OPAL participants (BP troublesomeness, comorbidities, multisite pain, change in walking and falls), meaning that we should be able to generalise findings from the BOOST RCT to the OPAL participants reporting NC. However, there were differences in socioeconomic factors and frailty, which may have been barriers to OPAL participants seeking treatment through the trial. This has implications for our implementation strategy to ensure the programme is available to all older people with NC.
With limited treatment options available to older people with NC, based on improvement in mobility and value for money, implementation of the BOOST programme should be considered.
Objective 4: To undertake a longitudinal qualitative study embedded within the randomised controlled trial to understand the participants’ experiences of taking part in the trial
Methods
A full description of methods90 is available here: https://doi.org/10.1136/bmjopen-2021-060128
We used a longitudinal qualitative design with semistructured interviews and open-ended questions. All BOOST RCT participants were eligible to take part. Consecutive participants were invited to take part. From those who agreed, we sampled participants to ensure that a range of age, gender, ethnicity, treatment allocation and recruitment sites were represented.
We conducted face-to-face interviews at participants’ homes after randomisation (T1), and telephone interviews (or face-to-face if requested) 1 month after the interventions (T2) and 12 months post randomisation (T3). Interviews were audio-recorded.
We aimed to recruit 60 participants, as we expected 50% attrition. We planned to analyse up to 30 participants interviewed at each time point. 91 Retention was better than expected, and 49/59 (82%) participants completed all three interviews (one withdrawal).
Analysis
We sorted the T1, T2 and T3 interviews of 49 participants into five batches based on the richness of data. The narratives provided by participants varied, and some participants provided more detailed and thorough descriptions of their experiences (richer data) compared to others. As per the original plan, we analysed 30 data sets. These were the interviews with the richest data (30 participants, 90 interviews), which were split into three batches of 10. The T1, T2 and T3 interviews of the first batch and T1 interviews of the second and third batches were transcribed verbatim. For the T2 and T3 which were not transcribed verbatim, we took notes from the audio recordings. This was to limit costs related to the study, and the lead qualitative researcher was experienced in gathering data in this way.
To facilitate analyses of data in a time-efficient way, firstly, we read the transcripts of the first batch of interviews and developed data summaries called ‘pen portraits’. 92 These portraits became the source documents to develop a coding template. From the pen portraits, we wrote mini-statements to describe participants’ narratives. The mini-statements, accompanied by supporting participant quotes from T1, T2 and T3, were added to the coding template to produce individual frameworks (see Report Supplementary Material 2 for an example). For the second and third batches, the individual frameworks included mini-statements and supporting quotes at T1, but only mini-statements at T2 and T3 taken from listening to the interview recordings. Finally, we combined individual frameworks in a master Excel® (Microsoft Corporation, Redmond, WA, USA) sheet for analysis.
We undertook an inductive thematic analysis of T1 interviews using the information in the frameworks to understand participants’ experiences of living with NC before taking part in the BOOST trial. We then comparatively analysed T1, T2 and T3 interviews to explore their experiences with the trial interventions, including the impact on their symptoms and their experiences of continuing with the home exercises independently.
Through comparative analysis, we also identified and explored participant trajectories over time for the domains of pain, mobility and activities of daily living, psychological impact, and social and recreational participation. We explored the interactions between domain trajectories to understand the pathway to improving social and recreational participation, which we considered essential to well-being in older age. This analysis is published here: https://doi.org/10.1136/bmjopen-2021-060128
Results
Interviews from 16 men and 14 women were included in this analysis; 14 participants were allocated to BPA and 16 were allocated to the BOOST programme. The demographics of interview participants are included in Appendix 4, Table 19 but they were broadly similar with regard to age and gender. We interviewed a greater proportion of non-white participants compared to white participants in the trial to ensure we heard from participants from different backgrounds.
Experience of living with neurogenic claudication
At T1, we identified four categories to describe how NC impacted on participants’ lives (see Appendix 4, Table 20).
Pain and its psychological impact
Pain was experienced by all participants. However, the onset, nature, intensity and location varied. Participants also reported a wide range of psychological responses such as depression, frustration, low mood, worry and lack of motivation in carrying out day-to-day or leisure activities.
Activity limitation
Most participants had difficulty in everyday activities due to the NC. This included self-care, walking, doing household chores, accessing public transport and using stairs.
Restricted participation
In two-thirds of the participants, pain and mobility issues negatively impacted their ability to participate in social, leisure and recreational activities like gardening, socialising in clubs and voluntary organisations, and going on holidays.
Coping strategies
Participants adopted a variety of coping strategies. Examples included sitting/resting between walks (activity pacing), limiting shopping trips or choosing online shopping (activity planning), using a stairlift (home adaptations), driving to local shops (activity modification) and using walking sticks (mobility aids).
Experiences of trial participation and Better Outcomes for Older people with Spinal Trouble interventions
Expectations and preferences
Participants participated in the trial expecting pain relief and improved walking, independence and general well-being. They also felt participation would help healthcare professionals to provide better treatments for other people with NC.
… whether through part of a study it gives a wider understanding to professionals about what they can do to help people of my age deal with the problems in a more proactive way.
08, T1
Most participants had no treatment preference on entry to the trial. Some preferred BPA due to the time commitment, while others preferred additional appointments and opportunities to interact with other people in the group sessions.
… the 12 sessions may be an embarrassment to me if I’m trying to run my business, and all the other things that are going on, and it does mean I presume an hour and a half plus, because I’ve got to get there and get back again, of my time.
37, T1
Acceptability of BOOST trial interventions
Satisfaction with BPA was mixed. Just over half the participants in the BPA arm (8/14) were satisfied with their sessions and felt the number of sessions was adequate. However, others were less satisfied (6/14) and a few mentioned that there was neither adequate feedback nor a follow-up.
But as far as going there, I only had a couple of sessions with them and that’s about it really; it wasn’t any proper sort of treatment or guidance or anything like that.
02, T2
In contrast, most participants (14/16) in the BOOST programme arm found it enjoyable and satisfactory.
I thank everybody for putting me on to the course because that’s what helped me … If they (other people) were in a similar condition, I would say, go for it!
57, T3
However, one participant was dissatisfied as he saw no clear benefit from the BOOST programme. Another participant joined the trial to get an MRI scan. This participant felt that the BOOST programme did not provide any new information and the exercises did not help.
Intervention components and perceptions about impact
Best practice advice participants were provided with an information booklet containing self-management advice and home exercises. Most BPA participants felt the booklet was useful and informative. The majority of participants (10/14) felt that the home exercises were appropriate and benefited them.
I felt it was enough information for me ... I think it is nicely produced, easy to read leaflet. Certainly in my case, going on the fear factor, it reassured right away.
15, T2
I get it [back pain] a bit first thing of a morning. But if I do the standing exercise, where you bend forward to touch your toes, that seems to put things right in a way.
31, T2
However, some participants did not perceive the exercises as helpful:
the exercises I was given, or any exercises I do, do not improve the problem.
03, T3
When assessing the overall impact of BPA on participants’ symptoms, 9/14 participants experienced some improvements in pain over the course of their involvement in the study, although the timing of this varied (five improved in a progressive manner; four reported being improved at T1 compared to entry into trial, or immediately after T2 that was maintained at T3, or only at T3). Pain did not change in four participants and worsened in one participant.
Participants in the BOOST programme liked the information folder containing session notes, photos and instructions for BOOST exercises and a home exercise planner. Participants reported benefits from the discussion and education element of the group sessions. They appreciated the peer support and interactions and the opportunity for group problem-solving. They were encouraged by their peers, enjoyed being part of the group and learnt skills to manage their condition, such as managing a flare-up or understanding the need to exercise for the long term (see Appendix 4, Table 21).
All participants except two described benefits from the exercise, which included reducing pain and improving posture, muscle strength and self-confidence.
And I think the exercises that I did has strengthened the muscles that I wanted strengthening – I think.
30, T2
One participant felt that exercise and physical activity would aggravate the condition.
Personally, I don’t think it [exercise and physical activity] would help. Full stop. I’ve had it so long now, and the wear and tear on my spine, overdoing it I think it would worsen the situation and that would be catastrophic … So the more you wear it, it’s going to get worse, in my eyes it is.
11, T2
Another participant had a mixed response to the programme, finding the BOOST walking regime and exercises with weights difficult but the hip mobility and balance exercises helpful.
In the BOOST programme arm (n = 16), we see similar variability in changes in pain. Pain improved in seven participants [four progressively improved; three improved at T1 (compared to entry into trial) or T2 that was maintained at T3, or only at T3]; one participant had initial improvements between T1 and T2 that got reversed to the baseline level of pain at T3. Pain did not change in seven participants and worsened in one participant.
Self-reported adherence to home exercises
Participants in both arms felt that the exercises prescribed by their physiotherapists could be fitted into their daily routine, but how successfully they did this varied.
At T2, most BPA participants (12/14) reported continuing exercises prescribed by their physiotherapist at least once a day. Low motivation and competing priorities such as work were reasons for stopping.
At T3, only 50% of BPA participants reported continuing the home exercises.
I mean I don’t ever miss at home, you know, and I said to [research clinician], I’ve done my exercises every day.
31, T3
Some reported still doing them at least once a day, but others had reduced the exercise frequency or stopped doing particular exercises that caused pain. Reasons for stopping the exercises were low self-motivation, recent surgery and impact on other pain problems (e.g. irritated hip pain).
Don’t ask me about them … No … Well, last time because it was really starting to hurt me on some of the exercises … and I do have a problem with motivation about that sort of thing on my own, as you will know. If I was with a group of people doing this, it would have been a lot much better for me.
33, T3
In contrast to the main trial results, self-reported adherence to the exercises prescribed by the physiotherapist was better with the BOOST programme than with BPA. At T2, all BOOST programme participants (16/16) reported doing their home exercises between one and three times every week or daily.
At T3, all but two participants continued their home exercises. Those who exercised independently tended to perceive the exercises as beneficial. At both time points, participants who exercised regularly described how they integrated the BOOST exercises into their daily activities; for example, exercising while watching television. One participant felt they were easily transferable to the daily routine and home environment because no special equipment was needed.
Some had made adaptations to allow them to continue exercising. This included one participant avoiding the weighted exercises that caused pain, and another avoiding specific exercises which aggravated a knee problem. Another participant had stopped the walking element as she felt she got enough walking in her everyday activities.
Two participants reported ceasing the BOOST exercises completely. One found the walking element overwhelming. Another participant found his usual activities such as walking the dog, going to the gym and swimming were adequate exercise.
For more information, see Appendix 4, Table 22.
Limitations
We analysed data sets from a selected sample of 30 participants who provided a rich description of their experiences. The BOOST trial participants who did not take part in the interviews may have had different experiences from those described here. However, the strength of the study is that we used in-depth repeated interviews that focused on the participants’ current experience, so we were not reliant on their recollection of past experiences and can be confident that the data available reflected their experiences of the trial.
Discussion
Our interview findings resonate with the previous qualitative studies in NC,93,94 as pain was the main complaint impacting on their activities and leading to negative psychological responses. Expectations of trial participants were aligned well with the treatment targets of the BOOST programme. BOOST programme participants appeared more satisfied with their intervention compared to BPA. Dissatisfaction among BPA participants seemed to arise from lack of feedback and follow-up. BOOST participants benefited from peer support and discussions, which may have added to their satisfaction with the programme. Most participants felt the exercises were appropriate and helpful, although this did not necessarily translate to improvements in pain. Dissatisfaction with the BOOST programme was mostly related to lack of pain relief. Pain remained a substantial problem for some participants, and this highlights the importance of trying to better target pain. The BOOST programme participants talked about other improvements from the exercises, including improved posture, strength and confidence, highlighting the broader benefits of the BOOST programme. These types of changes were less evident in the narratives of BPA participants. BOOST participants also reported benefit from the cognitive–behavioural component of the programme, highlighting how the programme helped them to find their own solutions, manage flare-ups and understand the importance of long-term exercise.
Reasons for stopping the home exercises were similar between treatment arms. These included competing priorities, lack of motivation and aggravation of pain. Adaptations allowed some participants to continue at least some of the exercises. Some participants did not make adaptations when finding the exercises difficult or unhelpful, and stopped altogether. One participant had stopped the BOOST exercises to focus on participating in activities they enjoyed. These types of physical activity or exercise were not captured in the main trial follow-up questions, so it is not possible to know how many other participants may have gone on to other activities in lieu of the BOOST programme.
The barriers to long-term exercise identified in this study were not unique95,96 but highlight areas where we can optimise the BOOST programme. These include additional support to provide motivation for ongoing exercises, either by the physiotherapist or by linking to exercise opportunities in the community; to provide greater guidance on how to adapt exercises, rather than stopping altogether, if they are painful or if patients have a flare-up of symptoms; and to help plan integration into everyday life or transition to activities they enjoy for long-term adherence.
Objective 5: To develop a prognostic tool using the Oxford Pain Activity and Lifestyle cohort study data to predict when older people are at risk of mobility decline
Methods
The full description of this study97 is available here: https://doi.org/10.1016/j.jclinepi.2022.09.002
The aim was to develop a tool to identify when older people were at risk of mobility decline. In particular, we wanted to understand whether BP was important, alongside other health conditions and symptoms, physical ability, psychological factors, and lifestyle and sociodemographic factors.
Baseline candidate predictors
We completed a systematic literature review to inform the selection of baseline predictors (searches for this review were conducted on 26 June 2019, with an update run on 2 June 2020),98 held discussions with our patient and public involvement (PPI) group, and utilised the expertise within the study team (including, but not limited to, primary care, geriatrics, mobility decline, falls, pain, and prognostic tool design and statistical analysis). Thirty-one candidate variables (Table 3) were preselected from the OPAL baseline variables (see Appendix 1, Table 5).
| Domain | Variables | Domain | Variables |
|---|---|---|---|
| Demographics | Age | Socioeconomic | Receive enough support Yes/no |
| Sex | Yes/no | ||
| Living arrangements | Miss other people around | ||
| Education | No/sometimes/yes | ||
| Higher education | No of organisations/clubs/societies | ||
| Secondary education | Adequacy of income | ||
| None or primary | Quite comfortably off | ||
| Manage without much difficulty | |||
| Careful with money | |||
| Mobility-related factors | Mobility problems | Occupational physical demands | |
| None | Very light | ||
| Slight | Light | ||
| Moderate | Moderate | ||
| Severe | Strenuous | ||
| Unable to walk | Very strenuous | ||
| Usual walking pace | Physical activity | Hours/day moving around | |
| Fast/fairly brisk | 7 hours/day or more | ||
| Normal | 5–7 hours/day | ||
| Stroll at any easy pace | 3–5 hours/day | ||
| Very slow/unable to walk | < 3 hours/day | ||
| Difficulties maintaining balance | Falls | Falls last year | |
| Confidence to walk (0–10) | None | ||
| Use of walking aid inside | One | ||
| Use of walking aid outside | More than one | ||
| Change in walking ability compared to last year | Fractures last year | ||
| General health | BMI | ||
| Better | Number of health conditions | ||
| About the same | Physical tiredness | ||
| Worse | Yes/no | ||
| Anxiety/depression | |||
| Pain | BP and leg symptoms | No | |
| No BP | Slightly | ||
| BP only | Moderately | ||
| BP with leg symptoms | Severely/extremely | ||
| Pain distribution | Poor hearing Yes/no | ||
| No pain | Poor vision Yes/no | ||
| One site pain | Problems in daily life due to lack of hand strength Yes/no | ||
| Multisite pain | |||
| Widespread pain | Unintentional weight loss Yes/no | ||
| Lower limb pain in the last 6 weeks Yes/no |
Self-reported general health (0–100) |
||
| Current pain/discomfort severity (0–4) |
Outcomes
The outcome was mobility decline at 2 years’ follow-up, defined using the EQ-5D-5L Mobility Question (no problems with mobility, mild problems, moderate problems, severe problems, unable to walk).
Among participants who could walk at baseline, mobility decline was considered present if a participant reported a worsening change in mobility status at year 2; that is, mobility progressed from one level to another or an individual had no problems at baseline and reported any problems at follow-up.
Sample size
The prevalence of mobility decline among the OPAL cohort at 2 years was 18.6%. Based on a conservative Nagelkerke’s R2 of 0.15 and a prespecified maximum number of predictor parameters of 50, the minimum sample size required to develop the model to minimise overfitting was estimated to be 4585, with 853 events. 99
Analysis
We present the baseline factors stratified by the 2-year outcome and estimated the univariate associations between baseline variable and the outcome using logistic regression.
Least absolute shrinkage and selection operator (LASSO) regression was used to select potential predictors for the multivariable model to predict mobility decline. 100 Model performance was assessed by calculating the c-statistic and the Brier score. 101 Models were internally validated using bootstrapping. 102 Missing data were imputed using the ‘multiple imputation by chained equation’ technique. 103,104
As a sensitivity analysis, we repeated the analyses, including participants who died during 2-year follow-up, who were added to those with mobility decline.
To facilitate application into clinical practice, we simplified the presentation of the model to a simple scoring system based on the procedures described by Sullivan et al. 105
Results
There were 5358 participants who could walk at baseline and were eligible for this analysis. However, 174 (3.2%) died before the 2-year follow-up. Therefore, 5184 participants were included in the main model. Two-year follow-up data were provided by 4115/5184 (79.4%) participants. The mean age of participants was 74.7 years (SD 6.6), and 51.8% (n = 2683/5184) were women. At baseline, the majority of participants reported no mobility problems (3146/5184; 60.7%), and 5.4% (280/5184) reported severe mobility problems.
Among responders at 2 years, 18.6% (n = 765/4115) reported mobility decline (Figure 8). There were 375 (9.1%), 42 (1.0%) and 10 (0.2%) individuals who improved their mobility status by one, two and three points at 2 years, respectively. Participants who reported slight mobility problems (see lower panel, plot A) at baseline had the biggest decline over the 2 years of follow-up (35.6%).
FIGURE 8.
Absolute change in mobility status between baseline and year 2. Note: Participants who could walk at baseline (upper panel). Stratified by mobility status at baseline (lower panel): (A) no mobility problems; (B) slight mobility problems and (C) moderate or severe mobility problems. (From Sanchez et al. 97)
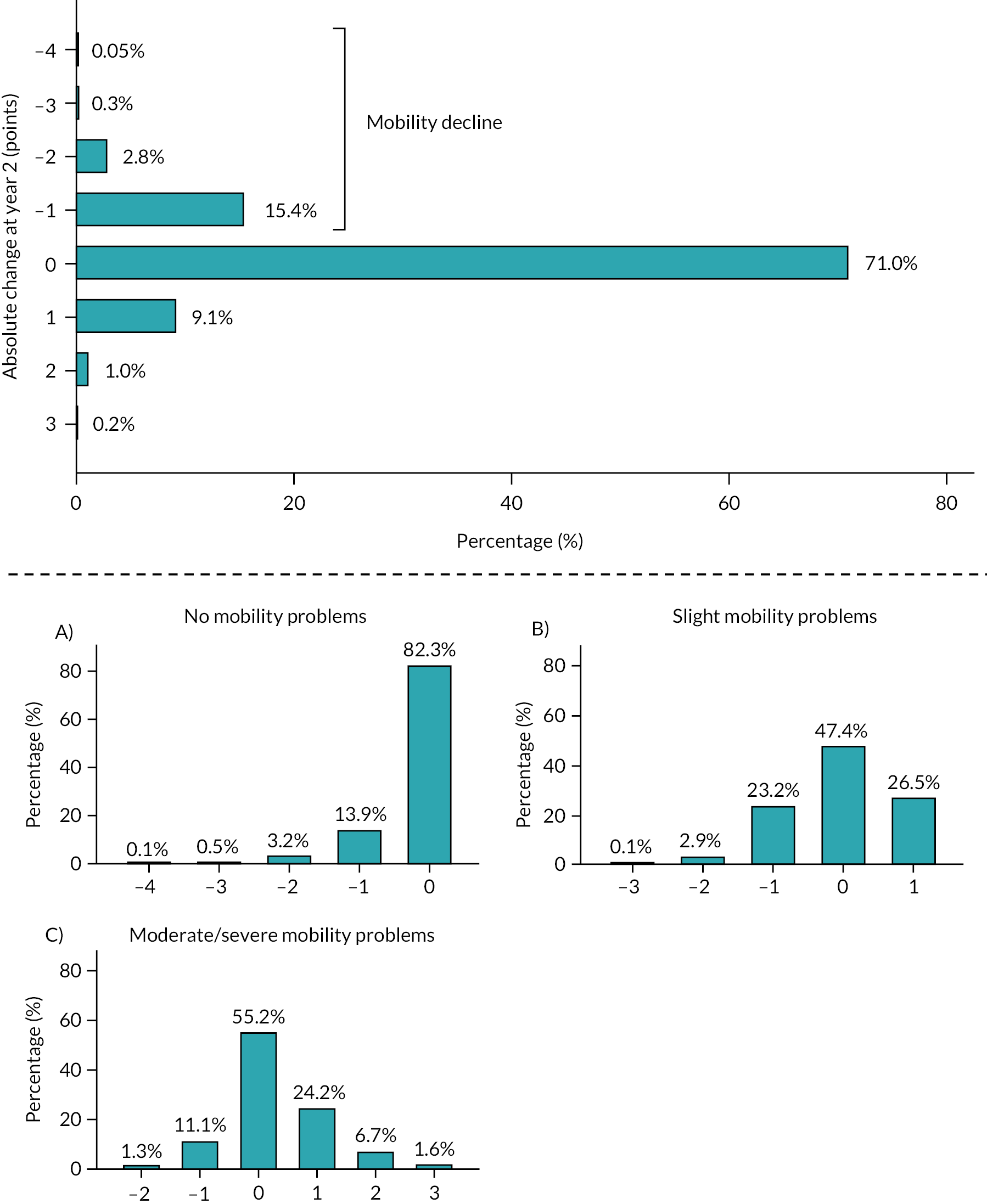
We examined the univariate relationship between the baseline variables and the outcome at 2 years (see Appendix 5, Table 23). Nearly all the selected baseline factors had a univariate relationship with the outcome. Among those with mobility decline at 2 years, there were more reports of back and leg pain and greater numbers reporting multisite pain and widespread pain at baseline. The biggest difference in pain presentation was lower limb pain at baseline, which was reported by 68% of participants with mobility decline at follow-up compared to 56% without. Baseline pain severity was also associated with mobility decline at follow-up.
The multivariable analyses found that in addition to mobility status (no problems or mild problems) at baseline, 13 variables were identified as predictors of mobility decline at 2 years in ≥ 80% of the multiple imputed data sets (Table 4). These included demographic and socioeconomic factors, multiple factors related to mobility, and general health-related factors. BP with and without leg pain was not associated with poor mobility in this multivariable model. However, lower limb pain and the presence of severe general pain were predictors of mobility decline.
| Predictor variables | LASSO regression (% times selected) |
Average penalised coefficient |
|---|---|---|
| Intercept | −6.757 | |
| Age (65–100 years) | 100 | 0.035 |
| Adequacy of income | ||
| Careful with money | 100 | 0.239 |
| BMI (15–70 kg/m2) | 100 | 0.025 |
| Usual walking pace | ||
| Stroll at any easy pace | 100 | 0.494 |
| Very slow/slow | 100 | 0.573 |
| Difficulties maintaining balance | 96 | 0.166 |
| Confidence to walk | 100 | 0.056 |
| Use of walking aid outside | ||
| Sometimes | 96 | 0.158 |
| Change in walking ability compared to last year | ||
| About the same | 80 | −0.087 |
| Worse | 96 | 0.141 |
| Lower limb pain (last 6 weeks) | 100 | 0.226 |
| Current pain/discomfort severity | 98 | 0.070 |
| Number of health conditions | 100 | 0.117 |
| Physical tiredness | 98 | 0.092 |
| Self-reported general health | 100 | −0.011 |
| Predictors forced to be included in the final model | ||
| Mobility problems at baseline | ||
| I have slight problems | 100 | 1.963 |
| I have no problems | 100 | 2.387 |
The multivariable model revealed a median c-statistic of 0.740 (range 0.737–0.743), indicating moderately good discrimination. The median Brier score was 0.136 (range 0.135–0.137).
We developed a point scoring system, which produced the risk of mobility decline at 2 years (see Appendix 5, Table 24). The total score has a possible range from 0 to 39, with predicted risks ranging from 0.9% (0 points) to 90.1% (39 points). The median c-statistic of this scoring system was 0.734 (range 0.730–0.735), similar to that of the regression model, indicating no noticeable loss in performance. A hypothetical example is shown in Appendix 5, Tables 24–26.
After including participants who died during the 2 years’ follow-up, the model showed similar performance measures, but some differences in predictors (see Appendix 5, Table 27). Back and leg pain now featured in this model. Other factors included sex, occupation (very strenuous physical demand), hours moving (< 3 hours/day), fractures and unintentional weight loss as additional predictors. Pain severity and use of walking aid outside were no longer part of the model.
Limitations
The results are based on self-reported predictors, which may be prone to recall bias or not be as accurate as clinical assessment. Self-reported assessment of mobility using the walking item from the EQ-5D may not reflect the participants’ actual mobility status, but it was an indication of how they perceived their mobility. EQ-5D scores were consistent with other self-reported questions related to mobility in the OPAL cohort study, and its validity and reproducibility in older adults has been reported in several studies. 106,107 Self-reported predictors are particularly useful in clinical settings or in research when a detailed clinical assessment is impractical.
We combined participants with back and non-NC leg pain and those with NC leg pain into a single group to ensure we had adequate numbers in each of the BP groups for the analyses and to avoid including too many variables. This means we were unable to separate out the potential contribution of the different leg pain presentations in the analyses. We did not externally validate the models.
Discussion
We set out to understand the role of back and leg pain in mobility decline when considered alongside the broader perspective of health-related factors. We have identified a set of questions with a scoring system that could easily be applied in clinical practice to identify older people at risk of mobility decline.
Back and leg pain was associated with mobility decline in the univariable association but not in the multivariable model. Many of the factors associated with mobility decline were related to mobility. We saw in our previous analyses (see Objective 1) that back and leg pain was associated with mobility-related adverse outcomes, including confidence to walk and change in walking over the last year. The strongest association was with NC. Pain is likely to be a contributor to this mobility-related factor, and lower limb pain was identified as an independent predictor of mobility decline. General pain severity was also predictive, suggesting that the amount of pain experienced (regardless of the type of pain or area of pain) is important.
When considering possible interventions to reduce the risk of older people experiencing mobility decline, these findings suggest that pain needs to be addressed. While back and leg pain was not an independent predictor of mobility decline, it is important that it is not completely disregarded, as it may contribute to low walking confidence and mobility decline. We know from our earlier analyses of OPAL data that back and leg pain tend to be persistent and that pain management is often not prioritised by older people108 or by health professionals. Lower limb pain, such as that arising from hip and knee osteoarthritis, can be effectively managed through rehabilitation incorporating pain self-management, coping strategies and exercise. 109 The mobility-related factors identified can be addressed through rehabilitation. We have demonstrated in the BOOST RCT that walking ability can be improved through progressive tailored rehabilitation. These types of programmes should be made available to older people at risk of mobility decline.
Back and leg pain did feature in the sensitivity analyses, which included participants who had died. A possible explanation for this may be that the spine is a common site for metastatic cancer and so back and leg pain may be symptomatic of underlying disease. 110
Finally, a socioeconomic factor – a report of needing to be careful with money – was also identified in the risk assessment tool. The systematic literature review completed for this study found consistent evidence that low annual income and low number of financial assets were risk factors for mobility decline. 98 Our findings add to evidence of the impact of health inequalities due to socioeconomic factors on the health of older people. Income influences the choices that people make regarding their health, such as their diet or participation in physical activity and exercise, as well as their ability to access health care. 111 Addressing health inequalities has been identified as a key priority for the NHS,112 and ensuring adequate access to rehabilitation for older people needs to be part of this.
Objective 6: To integrate the findings of the linked studies into an implementation package for use in primary care and other settings
Implementation plan
We have developed a package of implementation for the BOOST programme which has been funded by the NIHR and is underway.
We consider the BOOST programme is worthwhile in its current format. We have presented the findings at a national conference (BritSpine) and held a seminar for 30 clinicians involved in the trial. Nearly all clinicians agreed it was worth implementing due to the associated clinical improvements and cost-effectiveness. Concerns about implementation were logistical as opposed to clinical; for example, colleagues working in small departments found it difficult to assemble sufficient patients to run the groups.
Implementation is guided by the i-PARIHS (Integrated Promoting Action on Research Implementation in Health Services) framework, a Knowledge Mobilisation theory (Figure 9). 113
FIGURE 9.
i-PARIHS framework.
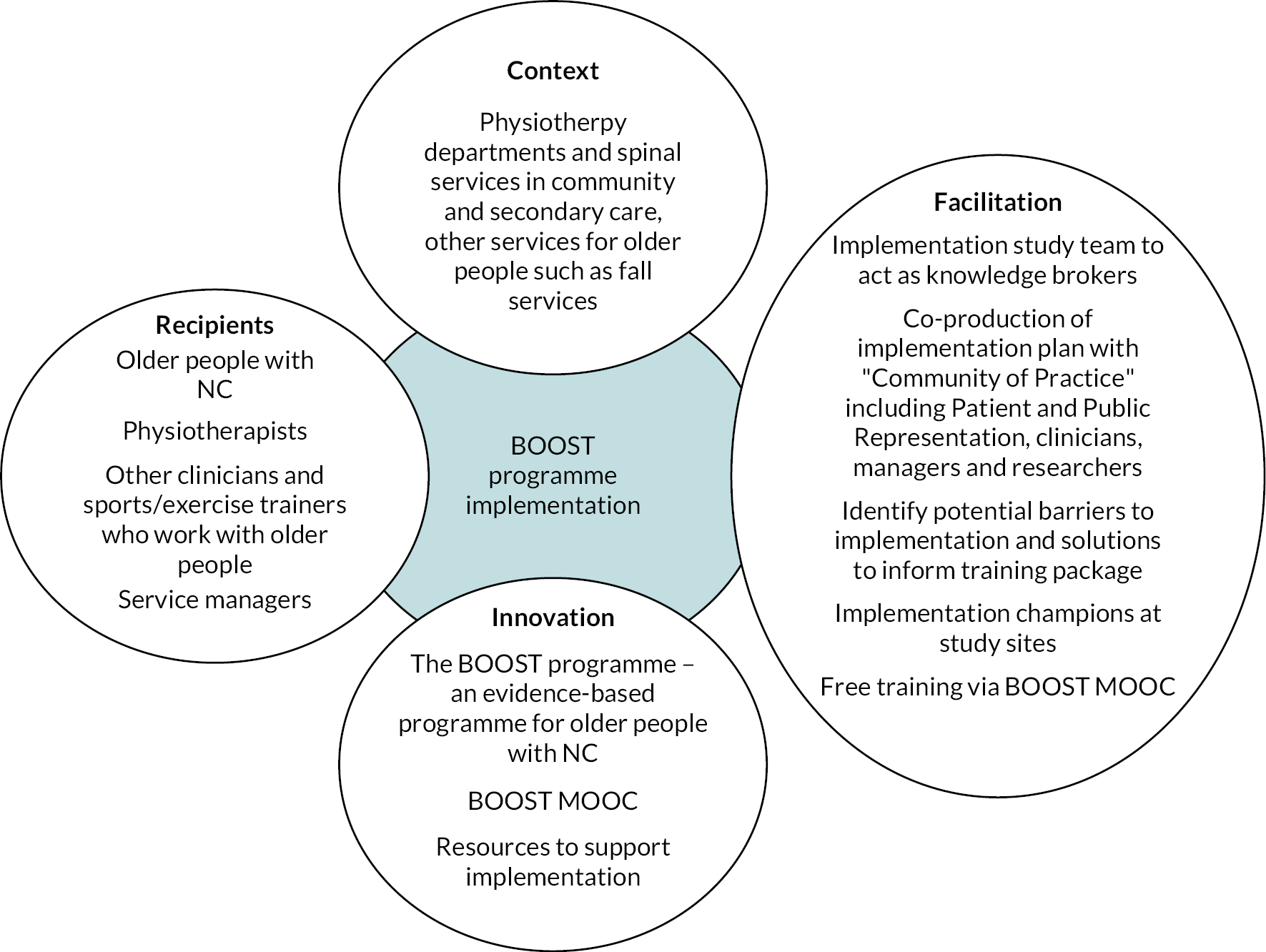
Central to our implementation strategy is the development of a Massive Open Online Course (MOOC). On completion of the implementation study, the online course will be freely available here: https://learn.exeter.ac.uk/. We have used this approach previously. 114,115 The online training addresses implementation barriers as well as providing the content needed for successful implementation.
We are undertaking a two-stage implementation study. First will be an optimisation stage to improve the programme and launch a MOOC. The second stage will evaluate the MOOC learning outcomes and clinical outcomes of the BOOST programme delivered in routine NHS care.
The aims of the implementation study are to:
-
Optimise the content of the BOOST programme.
-
Design the online course and evaluate learning and clinical outcomes to ensure outcomes are the same or better than the original programme.
-
Establish the framework for a longer-term evaluation and implementation strategy.
Stage 1: optimisation
We propose two areas of optimisation: (1) improve pain-related disability by enhancing the cognitive–behavioural elements of the programme and education about pain medications; (2) increase long-term exercise adherence using behavioural strategies and support. Consultation with stakeholders (PPI representatives, clinicians, managers and other researchers) will inform this work.
We will test the optimised programme in an identical way to the original trial delivered by therapists who participated in the BOOST trial (up to 24 participants). Outcomes will be collected at baseline and 6 months and will include the ODI (from which the walking and pain items will be the primary outcomes), pain self-management, EQ-5D-5L and the 6MWT. We will interview the clinicians delivering the optimised programme and observe group sessions to ensure the new elements are being delivered as intended and are worthwhile.
We will use a synthetic control method to evaluate the probability that the optimisation has improved the intervention. 116 Data from the BOOST trial will allow us to make comparisons with the original BOOST programme and the control arm.
Stage 2: implementation using the Better Outcomes for Older people with Spinal Trouble Massive Open Online Course
We will develop the online course in parallel with optimisation. We will invite up to 30 physiotherapists to complete the course. Learners will be asked to complete a post-course questionnaire and we will follow up 6 months later to find out whether they went on to implement the BOOST programme.
Learners will be invited to take part in an evaluation. We will conduct this evaluation with at least 10 physiotherapists from different centres and measure outcomes for 60 patients at baseline and 6 months (as for stage 1). We will assess the fidelity of intervention delivery through treatment logs and session observations and interview participating physiotherapists.
We will then carry out an analysis as described for stage 1 to ensure that the estimates of changes in pain and walking are similar to or better than those in the original trial before beginning widespread implementation.
The involvement of patients and/or the public
During the application process for this programme of work we assembled a PPI group, and we have continued to work closely with them. We advertised for PPI representatives via the INVOLVE website (https://involve.org.uk/) and approached organisations including the Rotary Club, the British Legion, Age UK, Men’s Sheds, BackCare and the British Orthopaedic Associations Patient Liaison Group. PPI representatives were also identified from participants in the preparatory interview study. 55 Ms Judith Fitch is the lead PPI representative and a co-applicant. We appointed a PPI representative to be an independent member of the Programme Steering Committee (Dr James Watson). Ms Fitch attended the co-applicants’ 6-monthly to yearly meetings to provide input into the progress of the programme of research. Dr Watson attended yearly Programme Steering Committee meetings.
Patient and public involvement engagement has been undertaken in face-to-face meetings, online meetings and via e-mails and phone calls. PPI representatives assisted with the development of the physiotherapy interventions, including attending the intervention refinement day, testing out the home exercises, assisting with development of the patient materials for the intervention, and posing as models for the exercise sheets.
Patient and public involvement representatives helped to develop the patient-facing materials. They provided feedback on layout and wording to make materials user-friendly. We piloted questionnaires with our PPI group, including completion of the OPAL baseline questionnaire by 20 older adults who spoke English as a second language to ensure suitability to participants from ethnic minorities. PPI representatives helped to develop the interview schedules and took part in practice interviews.
Patient and public involvement representatives helped to develop the prognostic tool. To widen the input we received, we advertised via the Oxford Biomedical Research Centre (BRC) and Oxford Collaborations in Leadership and Research in Health Care (CLARHC) PPI networks, the Patients Active in Research website (now known as ‘involve’: www.involve.org.uk/), and social media for more PPI representatives. We held a meeting in Oxford with seven PPI representatives and a meeting in Leeds with nine PPI representatives (aged 70–92 years). These discussions informed the final selection of the outcome (mobility question from EQ-5D-5L) and how we defined mobility decline. PPI representatives felt strongly that any decline (i.e. a single-step change downwards) needed to be considered important and included in the definition of decline. This is reflected in the analysis undertaken.
We met with our PPI contributors, who were very supportive of implementation of the BOOST programme, highlighting the importance of improving walking. Two PPI representatives are still involved in the BOOST implementation study. PPI representatives helped to write the plain English summary to disseminate findings.
Successes and challenges
Successes
At the outset of this programme of research, we outlined five success criteria:
-
Continued excellent engagement of PPI.
-
Predicted participant recruitment within the proposed time frame.
-
Necessary follow-up rates to ensure that each study is adequately powered, allowing us to have confidence in the findings.
-
Trial interventions have been delivered as intended through the quality control and process evaluation elements.
-
Achieve anticipated outputs, which were:
-
provide new knowledge interactions between health conditions, and attributable risk of disability and immobility associated with BP that will improve clinical and health decision-making
-
refine prognostic algorithms for primary care
-
potentially an evidence-based treatment package for NC
-
information on cost and effectiveness to inform cost-effectiveness judgements, and provide high-quality evidence applicable and generalisable to the NHS to inform clinical guidelines and practice in primary care
-
qualitative studies to support implementation
-
a strong dissemination package.
-
All five criteria have been achieved. We have worked closely with our PPI group and will continue to do so during BOOST programme implementation. The BOOST RCT and OPAL cohort study achieved their recruitment targets with high levels of participant follow-up. The interventions delivered in the BOOST RCT were delivered as intended with a high level of fidelity.
We have achieved a substantial number of outputs. As well as the outputs presented in this report, we have done additional OPAL cohort data analyses to increase knowledge and understanding of different health conditions, including knee and hip osteoarthritis, and how they contribute to health outcomes for older people. 117,118 We have produced a manualised physiotherapy intervention for older people with NC. The planned implementation work will make this available to clinical staff.
In addition, we have established a long-term cohort study which can be used to recruit participants to other intervention trials [e.g. Tailored Exercise Management for People aged 80 years or older with hip/knee Osteoarthritis: a feasibility study (www.isrctn.com/ISRCTN75983430); a study of the impact of the COVID pandemic on older people] and study longer-term outcomes (up to 5 years’ follow-up). This programme of research has allowed us to establish new collaborations with a wide network of primary care practices and clinicians to deliver the BOOST RCT.
Challenges
We hoped to recruit a substantial number of participants via the OPAL cohort study into the BOOST RCT. Around 10% of trial participants were from OPAL. Barriers to recruitment via OPAL included existing referral mechanisms between primary care practices and physiotherapy providers, and lack of capacity within physiotherapy departments to deliver the BOOST intervention. Some departments would not accept OPAL participants because the participating primary care practice did not have a referral pathway/agreement with the participating physiotherapy department. At other sites, capacity was an issue, with departments being constrained in the number of participants they could accommodate for treatment. This was capped at some departments at the start of the trial, with no flexibility to increase despite recruiting well. These barriers need to be overcome to make best use of cohorts like OPAL and deliver efficient research.
There were some differences between OPAL–BOOST participants and OPAL participants reporting symptoms of NC. Those who took part in BOOST were better educated and from areas of less deprivation. We need to consider socioeconomic barriers to trial participation and tailor recruitment strategies to achieve good representation across different socioeconomic groups.
We were very fortunate to have completed the elements of this research that relied on face-to-face contact with participants prior to the COVID pandemic. Our team were able to complete the year 2 OPAL follow-up while working at home, and data analyses were undertaken; however, this did lead to delays in producing the final study results. The pandemic made it difficult to engage with clinical staff due to their overwhelming clinical demands, but we have done our best to take advantage of opportunities to engage with the clinical community, such as during conference presentations and results meetings with clinical collaborators.
Key findings
Back pain is a substantial and persistent problem for older people, associated with lower quality of life and age-related adverse health states including falls, frailty, low walking confidence and mobility decline. The impact is greatest when leg pain, especially NC, is reported. Although NC had the biggest impact, other presentations should not be ignored, due to the high proportion of participants who reported persistent symptoms.
The BOOST programme improved mobility and physical capacity, reduced falls in older people with NC and provided good value to the NHS. We have produced a manualised intervention that is ready for implementation. However, we only achieved short-term reduction in pain and pain-related disability. For a proportion of participants, pain remained problematic. On average, there were modest improvements in pain-related disability while participants were engaged with the programme and supported by the physiotherapist, but quantitative and qualitative data demonstrated the difficulty in maintaining long-term exercise adherence, which may have contributed to the reduced impact over time.
We have developed a prognostic tool to identify when older people are at risk of mobility decline. Back and leg pain had a univariable association with mobility decline but was not an independent predictor of mobility decline in the multivariable model. There were a variety of factors predictive of mobility decline in this model, including a range of domains (demographic and socioeconomic, mobility, general health and pain). We developed a scoring system for this tool so that it could be easily implemented in clinical practice, but it requires external validation.
Implications for practice and any lessons learnt
We have focused on NC, but there is also a need to consider how to better manage BP more generally in older adults. The majority of OPAL participants who reported BP at baseline had persistent symptoms. We are confident that the OPAL cohort is generalisable to community-dwelling older adults in England, so this represents a large proportion of the older population who are living with BP and not necessarily seeking health care. OPAL participants with symptoms suggestive of NC who did not take part in the BOOST trial reported lower educational attainment and lived in more deprived areas, but their clinical presentation was similar to those who did participate, suggesting they would have benefited from the BOOST programme. There is a need to develop interventions that could be delivered to the large number of older adults affected by BP, especially for those with more severe symptoms, and to understand how we can promote the uptake of interventions by people from lower socioeconomic groups to address health inequalities.
The improvement in clinical outcomes and cost-effectiveness of the BOOST programme indicates that implementation should be considered by clinicians and commissioners of services. However, we hope that we can better target pain during the planned implementation work. Treatment options are limited for this population, but there remains a need to help patients successfully manage their pain and to promote function and participation as part of healthy ageing. More consideration also needs to be given to how we support patients to transition from formal rehabilitation into lifelong habits of exercise and physical activity. There is a need for better linkage between rehabilitation services and community-based exercise provision and physical activity programmes.
We have focused on the development of a prognostic tool to identify mobility decline as this is a priority for older adults and key to maintaining independence in older age. This work highlighted a broader need to manage pain better in older people regardless of the type or presentation of pain. There were several mobility-related factors that were identified as predictors of mobility decline. These were present in some people who considered their mobility to be unaffected at baseline, so could be considered precursors to loss of mobility; they included slower walking speed, sometimes using a walking stick, low walking confidence and reduced balance. These are all modifiable factors that could be targeted through rehabilitation. The prognostic tool could be used not only by clinicians but also by older people and their families to spot signs of mobility decline and prompt them to seek rehabilitation rather than accepting it as part of old age. Matching treatments to risk factors may potentially slow mobility decline, and this requires evaluation.
Recommendations for future research
-
Develop and evaluate interventions that can be delivered at scale to the large number of older people who experience low BP, including consideration of health inequalities and how they impact on care-seeking by older people with BP.
-
Identify ways to achieve long-term improvements in pain and pain-related disability in older people with NC.
-
Externally validate the OPAL predictive tool.
-
Evaluate the clinical and cost-effectiveness of implementation of the OPAL predictive tool to reduce mobility decline among those at risk of mobility decline.
Conclusions
We have successfully undertaken a cohort study, a RCT and an embedded qualitative study. The assembled cohort and associated data remain a valuable asset for future trial recruitment and data analyses to further our knowledge about the role of pain, activity and comorbidities in the development of disability, frailty, falls and immobility in older adults. The prognostic tool will enable clinicians, their patients and families to identify when an older person is at risk of mobility decline. We have demonstrated the substantial impact that back and leg pain has on older people’s lives. We have produced an intervention for older adults with NC that improves mobility and physical capacity and reduces falls as well as being cost-effective, although we plan to further optimise the programme during implementation. Findings from this programme of research will inform future research focused on improving the lives of older people and the care they receive.
Acknowledgements
We would like to thank all the individuals who agreed to participate in this programme of research. We would also like to thank the research and physiotherapy teams at the following hospitals for their support and participation in delivering the BOOST programme: Birmingham Community NHS Trust, Cambridgeshire Community Services NHS Trust, Croydon Health Services NHS Trust, Dorset Healthcare University NHS Foundation Trust, East Cheshire NHS Trust, Gloucestershire Care Services NHS Trust, Leeds Community NHS Healthcare Trust, Leeds Teaching Hospitals NHS Trust, Northwest Boroughs Partnership NHS Trust, Oxford University Hospitals NHS Foundation Trust, Royal Liverpool NHS Trust, Salisbury NHS Foundation Trust, Sandwell and West Birmingham NHS Trust, The Royal Orthopaedic Hospital NHS Foundation Trust, and Wirral University Teaching Hospital NHS Trust.
We are grateful to the general practitioners and their staff who assisted with the identification and invitation of eligible individuals for the OPAL study from the following general practices: Grange Hill Surgery, Birmingham; Gosford Hill Medical Centre, Oxford; River Brook Medical Centre, Birmingham; The Key Medical Practice, Oxford; Summertown Health Centre, Oxford; Alconbury and Brampton Surgeries, Cambridgeshire; Old Exchange Surgery, Cambridgeshire; The Wand Medical Centre, Birmingham; Kingsfield Medical Centre, Birmingham; Buckden and Little Paxton Surgery, Cambridgeshire; Temple Cowley Medical Group, Oxford; Keynell Covert Surgery, Birmingham; Burbury Medical Centre, Birmingham; Hollow Way Medical Centre, Oxford; Priory View Medical Centre, Leeds; Newton Surgery, Leeds; Priory Fields Surgery, Cambridgeshire; Cromwell Place Surgery, Cambridgeshire; Craven Road Medical Centre, Leeds; Queslett Medical Practice, Birmingham; Hall Street Medical Centre, St Helens; Vauxhall Health Centre, Liverpool; Ireland Wood Surgery, Leeds; Civic Medical Centre, Wirral; Brownlow Group Practice, Liverpool; Wareham Surgery, Dorset; The Adam Practice, Poole; The Harvey Practice, Dorset; Three Chequers Medical Practice, Salisbury; Gate Medical Centre, Birmingham; Rendcomb Surgery, Cirencester; Cotswold Medical Practice, Cheltenham; Brigstock and South Norwood Partnership, Croydon; Portland Practice, Gloucestershire; Eversley Medical Centre, Croydon. We would also like to thank the following NIHR Clinical Research Networks for their support with the OPAL study: Thames Valley and South Midlands; East of England; Yorkshire and the Humber; North West Coast; Wessex; West of England; West Midlands; South London.
We thank our PPI representatives Judith Fitch, Eileen Turner and John Arden for their assistance in designing and running the studies.
We are grateful to the members of the Data Monitoring and Ethics Committee and Trial Programme Steering Committee who have guided and supported this programme of work. The members were: Data Monitoring and Ethics Committee: David Torgerson (Chair), Catherine Sackley, Candy McCabe. Programme Steering Committee: Stephanie Taylor (Chair), Catherine Hewitt, Anne Forster, Lindsey Bearne, Jim Watson (PPI representative).
Other members of the BOOST and OPAL study teams that we would like to thank for their contribution to this project are Oliver Conway, Frances Darton, Varsha Gandhi, Daryl Hagan, Damian Haywood, Aimee Hewitt, Laura Nevay, Phillipa Nicolson, Mandy Slark and Karan Vadher. We would like to thank Professor Nigel Arden and the late Professor Douglas Altman for helping with the funding application.
Contributions of authors
Esther Williamson (https://orcid.org/0000-0003-0638-0406) was a co-applicant and the programme lead for this project, oversaw its design and delivery and is lead author of this report.
Maria T Sanchez-Santos (https://orcid.org/0000-0003-1908-8623) undertook the analysis of the OPAL cohort data.
Ioana R Marian (https://orcid.org/0000-0002-0692-8112) conducted the statistical analysis of the BOOST RCT data.
Mandy Maredza (https://orcid.org/0000-0002-2030-3338) conducted the analysis of the health economic data.
Cynthia Srikesavan (https://orcid.org/0000-0002-3540-8052) conducted the analysis of the qualitative research data.
Angela Garrett (https://orcid.org/0000-0002-7271-6345) was the trial manager for the project and participated in the design, data collection and analysis of the study.
Alana Morris (https://orcid.org/0000-0002-3001-7888) was instrumental in the conduct of the OPAL cohort study including co-ordinating database searches and mail-outs and liaising with primary care practice staff.
Graham Boniface (https://orcid.org/0000-0003-1253-0060) was the lead research physical therapist who worked closely with BOOST study sites to ensure the BOOST interventions were correctly delivered, and supported the analysis of the qualitative research data.
Susan J Dutton (https://orcid.org/0000-0003-4573-5257) provided statistical oversight for the BOOST RCT.
Frances Griffiths (https://orcid.org/0000-0002-4173-1438) was a co-applicant and lead qualitative researcher on the study.
Gary S Collins (https://orcid.org/0000-0002-2772-2316) was a co-applicant who aided the statistical design and analysis of the study.
Stavros Petrou (https://orcid.org/0000-0003-3121-6050) was a co-applicant and lead health economist on the study.
Julie Bruce (https://orcid.org/0000-0002-8462-7999) was a co-applicant who aided the design and analysis of the study.
Jeremy Fairbank (https://orcid.org/0000-0002-0050-874X) was a co-applicant, who provided expertise on NC that aided the design and conduct of the study.
Zara Hansen (https://orcid.org/0000-0001-8915-7765) was a co-applicant, who aided the design and delivery of the study.
Karen Barker (https://orcid.org/0000-0001-9363-0383) was a co-applicant, who aided the design and delivery of the study.
Charles Hutchinson (https://orcid.org/0000-0003-3387-9229) was a co-applicant and lead radiologist on the study.
Christian Mallen (https://orcid.org/0000-0002-2677-1028) was a co-applicant who aided the design and analysis of the study.
Lesley Ward (https://orcid.org/0000-0001-7285-2032) contributed to developing the BOOST interventions and undertook the participant interviews for the qualitative study.
Richard Gagen (https://orcid.org/0000-0002-2750-1011) was instrumental in the collection and analysis of the BOOST MRI data.
Judith Fitch (https://orcid.org/0000-0002-6687-5200) was a co-applicant who aided the design and implementation of the study from the patients’ perspective.
David P French (https://orcid.org/0000-0002-7663-7804) was a co-applicant who aided the design and analysis of the study.
Sallie E Lamb (https://orcid.org/0000-0003-4349-7195) was the chief investigator. She led the team that secured funding, developed interventions and undertook the studies reported. She is guarantor for the data and approved the final version of the manuscript.
The BOOST programme was conducted as part of the portfolio of trials in the registered UKCRC OCTRU at the University of Oxford. It has followed their standard operating procedures, ensuring compliance with the principles of good clinical practice and the Declaration of Helsinki and any applicable regulatory requirements. We would therefore like to thank them for their support and guidance with this work.
Publications
| Published papers | Related BOOST programme of research objective (if applicable) | Related work package (if applicable) |
|---|---|---|
| BOOST trial and related work | ||
| Williamson E, Boniface G, Marian IR, Dutton SJ, Garrett A, Morris A, Hansen Z, Ward L, Nicolson PJA, Rogers D, Barker KL, Fairbank J, Fitch J, French DP, Comer C, Mallen CD, Lamb SE; BOOST Research Group. The clinical effectiveness of a physiotherapy delivered physical and psychological group intervention for older adults with neurogenic claudication: the BOOST randomised controlled trial. The Journals of Gerontology: Series A, glac063. https://doi.org/10.1093/gerona/glac063 |
3 | 1,2,4 |
| Griffiths F, Srikesavan C, Ward L, Boniface G, Williamson E, Lamb SE. Longitudinal qualitative study of living with neurogenic claudication. BMJ Open 2022;12(9):e060128. https://doi.org/10.1136/bmjopen-2021-060128 | 4 | 4 |
| Maredza M, Khan K, Marian IR, Dutton SJ, Williamson E, Lamb SE, Petrou S. Economic costs, health-related quality of life outcomes and cost-utility of a physical and psychological group intervention targeted at older adults with neurogenic claudication. 2023;21:14. https://doi.org/10.1186/s12962-022-00410-y | 4 | 4 |
| Williamson E, Ward L, Vadher K, Dutton SJ, Parker B, Petrou S, Hutchinson CE, Gagen R, Arden NK, Barker K, Boniface G, Bruce J, Collins G, Fairbank J, Fitch J, French DP, Garrett A, Gandhi V, Griffiths F, Hansen Z, Mallen C, Morris A, Lamb SE. Better Outcomes for Older people with Spinal Trouble (BOOST) Trial: a randomised controlled trial of a combined physical and psychological intervention for older adults with neurogenic claudication, a protocol. BMJ Open 2018;8(10):e022205. https://doi.org/10.1136/bmjopen-2018-022205 | 3 | 1,4 |
| Marian IR, Williamson E, Garrett A, Lamb SE, Dutton SJ. Better Outcomes for Older people with Spinal Trouble (BOOST) trial: statistical analysis plan for a randomised controlled trial of a combined physical and psychological intervention for older adults with neurogenic claudication. Trials 2020;21:667. https://doi.org/10.1186/s13063-020-04590-x | 3 | 4 |
| Ward L, Williamson E, Hansen Z, French DP, Boniface G, Rogers D, Lamb SE; Better Outcomes for Older Adults with Spinal Trouble BOOST Group. Development and delivery of the BOOST (Better Outcomes for Older adults with Spinal Trouble) intervention for older adults with neurogenic claudication. Physiotherapy 2019;105(2):262–74. https://doi.org/10.1016/j.physio.2019.01.019 | 2 | 1 |
| Lyle S, Williamson E, Darton F, Griffiths FE, Lamb SE. A qualitative study of older people’s experience of living with neurogenic claudication to inform the development of a physiotherapy intervention. Disability and Rehabilitation 2017;10:1009–17. URL: https://ora.ox.ac.uk/objects/uuid:92c9ee3e-30eb-4fc0-95fd-08db3bc4707b | 2 | 1 |
| Gagen R. The value of magnetic resonance imaging in the assessment of degenerative lumbar spinal stenosis. PhD thesis: 2021 University of Warwick. URL: http://webcat.warwick.ac.uk/record=b3719133 | 3 | 4,5 |
| Comer C, Lee H, Williamson E, Lamb SE. Understanding the mechanisms of a combined physical and psychological intervention for people with neurogenic claudication: protocol for a causal mediation analysis of the BOOST trial. BMJ Open 2020:037121 https://doi.org/10.1136/bmjopen-2020-037121 | 3 | 4 |
| OPAL cohort study and related work | ||
| Sanchez-Santos MT, Williamson E, Bruce J, Ward L, Mallen CD, Garrett A, Morris A, Lamb SE on behalf of the OPAL study team. Cohort profile: Oxford Pain, Activity and Lifestyle (OPAL) Study, a prospective cohort study of older adults in England. BMJ Open 2020:037516. http://doi.org/10.1136/bmjopen-2020-037516 | 1,5 | 2,3 |
| Nicolson PJA, Sanchez-Santos MT, Bruce J, Kirtley S, Ward L, Williamson E, Lamb SE. Risk factors for mobility decline in community-dwelling older adults: a systematic literature review. Journal of Aging and Physical Activity 2021;29:1053–66. https://doi.org/10.1123/japa.2020-0482 | 5 | 3 |
| Sanchez-Santos MT, Williamson E, Nicolson PJA, Bruce J, Collins GS, Mallen CD, Griffiths F, Garret A, Morris A, Slark M, Lamb SE; OPAL study team. Development and validation of a prediction model for self-reported mobility decline in community-dwelling older adults. Journal of Clinical Epidemiology 2022;152:70–9. https://doi.org/10.1016/j.jclinepi.2022.09.002 | 5 | 3 |
| Williamson E, Sanchez-Santos MT, Morris A, Garrett A, Conway O, Boniface G, Fairbank J, Lamb SE. The prevalence of back and leg pain and the cross-sectional association with adverse health outcomes in community dwelling older adults in England. Spine 2021;46:54–61. URL: https://ora.ox.ac.uk/objects/uuid:911cf725-135f-4951-a1b2-28eb2032d9ab | 1 | 3 |
| Nicolson PJA, Williamson E, Morris A, Sanchez-Santos MT, Bruce J, Silman A, Lamb SE. Musculoskeletal pain and loneliness, social support and social engagement among older adults: analysis of the Oxford Pain, Activity and Lifestyle cohort. Musculoskeletal Care 2021;19(3):269–77. https://doi.org/10.1002/msc.1526 | ||
| Nicolson PJA, Williamson E, Lee H, Morris A, Garrett A, Sanchez-Santos MT, Lamb SE. Synergistic effects of hip/knee osteoarthritis and comorbidities on mobility and self-care limitations among older adults: cross-sectional analysis of the Oxford Pain, Activity and Lifestyle study. Journal of Comorbidity 2020;10:2235042X20974529. https://doi.org/10.1177/2235042x20974529 | ||
| Other | ||
| Gandhi V, Brown D, Williamson E. Rehabilitation for Older Adults with Low Back Pain. Lumbar Spine Textbook Section 10: Non-operative Spine Care, Chapter 11. URL: www.wheelessonline.com/ISSLS/section-10-chapter-11-rehabilitation-for-older-adults-with-low-back-pain (accessed 18 August 2023). | ||
Ethical statement
Ethical approval for the OPAL cohort study and the BOOST trial was provided by the London–Brent Research Ethics Committee (16/LO/0348) on 10 March 2016.
Data-sharing statement
All data requests should be submitted to the Chief Investigator of this programme of research for consideration. Access to anonymised data may be granted following review. Requests can also be made to Professor Sallie Lamb via e-mail s.e.lamb@exeter.ac.uk.
Presentations
Lyle SA, Williamson E, Darton FC, Griffiths FE, Lamb SE. From Data Collection to Intervention development: A qualitative study of older people’s experiences of living with neurogenic claudication. Oral Presentation: 14th International Forum for Back and Neck Pain Research in Primary Care. 2016.
Boniface G, Williamson E, Ward L, Hansen Z, French D, Rogers D, Lamb SE. Development of the BOOST Programme: a physiotherapy intervention addressing the physical and psychological impact of neurogenic claudication in older adults. Oral Presentation: 16th International Forum for Back and Neck Pain Research in Primary Care. July 2019.
Boniface G, Williamson E, Ward L, Hansen Z, Gandhi V, Nicolson P, Garrett A, Lamb SE. Ensuring treatment fidelity to a group based physical and psychological intervention for older adults with neurogenic claudication. Poster Presentation: 16th International Forum for Back and Neck Pain Research in Primary Care. July 2019.
Ward L, Griffiths F, Williamson E, Robinson R, Boniface G, Lamb SE. Developing a framework for the analysis of longitudinal qualitative data in clinical trials. Oral Presentation: 16th International Forum for Back and Neck Pain Research in Primary Care. July 2019.
Ward L, Griffiths F, Williamson E, Boniface G, Lamb SE. The physical and psychological impact of neurogenic claudication on older adults’ responses to physiotherapy. Oral Presentation: 16th International Forum for Back and Neck Pain Research in Primary Care. July 2019.
Ward L, Lamb SE, Williamson E, Robinson R, Griffiths FE. Drowning in data! Designing a novel approach to longitudinal qualitative analysis. Oral Presentation: 4th Qualitative Health Research Network (QHRN) Conference. March 2019.
Williamson E, Boniface G, Marian IR, Dutton SJ, Maredza M, Petrou S, Garrett A, Morris A, Hansen Z, Ward L, Nicolson P, Barker K, Fairbank J, Fitch J, Rogers D, Comer C, French D, Mallen C, Lamb SE. The clinical and cost-effectiveness of a physiotherapy delivered physical and psychological group intervention for older adults with neurogenic claudication: the BOOST randomised controlled trial. Oral Presentation: 17th International Forum for Back and Neck Pain Research in Primary Care. Nov 2021.
Williamson E, Garrett A, Marian IR, Dutton SJ, Maredza M, Petrou S, Boniface G, Hansen Z, Nicolson P, Barker K, Lamb SE. The prevalence of back pain with and without associated leg pain in older adults in England and the association with immobility, falls and frailty. Oral Presentation: Chartered Society of Physiotherapy (CSP) Conference. Nov 2021.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, PGfAR or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the PGfAR programme or the Department of Health and Social Care.
References
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163-96.
- Macfarlane GJ, Beasley M, Jones EA, Prescott GJ, Docking R, Keeley P, et al. MUSICIAN Study Team . The prevalence and management of low back pain across adulthood: results from a population-based cross-sectional study (the MUSICIAN study). Pain 2012;153:27-32.
- Eggermont LH, Penninx BW, Jones RN, Leveille SG. Depressive symptoms, chronic pain, and falls in older community-dwelling adults: the MOBILIZE Boston Study. J Am Geriatr Soc 2012;60:230-7.
- Koponen MP, Bell JS, Karttunen NM, Nykänen IA, Desplenter FA, Hartikainen SA. Analgesic use and frailty among community-dwelling older people: a population-based study. Drugs Aging 2013;30:129-36.
- Leveille SG, Bean J, Ngo L, McMullen W, Guralnik JM. The pathway from musculoskeletal pain to mobility difficulty in older disabled women. Pain 2007;128:69-77.
- Makris UE, Fraenkel L, Han L, Leo-Summers L, Gill TM. Restricting back pain and subsequent mobility disability in community-living older persons. J Am Geriatr Soc 2014;62:2142-7.
- Shega JW, Dale W, Andrew M, Paice J, Rockwood K, Weiner DK. Persistent pain and frailty: a case for homeostenosis. J Am Geriatr Soc 2012;60:113-7.
- Docking RE, Fleming J, Brayne C, Zhao J, Macfarlane GJ, Jones GT. Cambridge City over-75s Cohort Study collaboration . Epidemiology of back pain in older adults: prevalence and risk factors for back pain onset. Rheumatology 2011;50:1645-53.
- Scheele J, Enthoven WT, Bierma-Zeinstra SM, Peul WC, van Tulder MW, Bohnen AM, et al. Characteristics of older patients with back pain in general practice: BACE cohort study. Eur J Pain 2013;18:279-87.
- Scheele J, Enthoven WT, Bierma-Zeinstra SM, Peul WC, van Tulder MW, Bohnen AM, et al. Course and prognosis of older back pain patients in general practice: a prospective cohort study. Pain 2013;154:951-7.
- Paeck T, Ferreira ML, Sun C, Lin CW, Tiedemann A, Maher CG. Are older adults missing from low back pain clinical trials? A systematic review and meta-analysis. Arthritis Care Res 2013;66:1220-6.
- Suri P, Rainville J, Kalichman L, Katz JN. Does this older adult with lower extremity pain have the clinical syndrome of lumbar spinal stenosis?. JAMA 2010;304:2628-36.
- Battié MC, Jones CA, Schopflocher DP, Hu RW. Health-related quality of life and comorbidities associated with lumbar spinal stenosis. Spine J 2012;12:189-95.
- Jensen RK, Jensen TS, Koes B, Hartvigsen J. Prevalence of lumbar spinal stenosis in general and clinical populations: a systematic review and meta-analysis. Eur Spine J 2020;29:2143-63.
- Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA 2010;303:1259-65.
- Jarrett MS, Orlando JF, Grimmer-Somers K. The effectiveness of land based exercise compared to decompressive surgery in the management of lumbar spinal-canal stenosis: a systematic review. BMC Musculoskelet Disord 2012;13.
- Macedo LG, Hum A, Kuleba L, Mo J, Truong L, Yeung M, et al. Physical therapy interventions for degenerative lumbar spinal stenosis: a systematic review. Phys Ther 2013;93:1646-60.
- Dobbs R, May S, Hope P. The validity of a clinical test for the diagnosis of lumbar spinal stenosis. Manual Therapy 2016;25:27-34.
- Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc 2007;55:780-91.
- Leveille SG, Jones RN, Kiely DK, Hausdorff JM, Shmerling RH, Guralnik JM, et al. Chronic musculoskeletal pain and the occurrence of falls in an older population. JAMA 2009;302:2214-21.
- Eggermont LH, Shmerling RH, Leveille SG. Tender point count, pain, and mobility in the older population: the mobilize Boston study. J Pain 2010;11:62-70.
- Hicks GE, Gaines JM, Shardell M, Simonsick EM. Associations of back and leg pain with health status and functional capacity of older adults: findings from the retirement community back pain study. Arthritis Rheum 2008;59:1306-13.
- van der Gaag WH, Chiarotto A, Heymans MW, Enthoven WTM, van Rijckevorsel-Scheele J, Bierma-Zeinstra SMA, et al. Developing clinical prediction models for nonrecovery in older patients seeking care for back pain: the back complaints in the elders prospective cohort study. Pain 2021;162:1632-40.
- Rundell SD, Sherman KJ, Heagerty PJ, Mock CN, Dettori NJ, Comstock BA, et al. Predictors of persistent disability and back pain in older adults with a new episode of care for back pain. Pain Med 2017;18:1049-62.
- Wessberg P, Frennered K. Central lumbar spinal stenosis: natural history of non-surgical patients. Eur Spine J 2017;26:2536-42.
- Sanchez Santos MT, Williamson E, Bruce J, Ward L, Mallen CD, Garrett A, et al. OPAL Study Team . Cohort profile: Oxford Pain, Activity and Lifestyle (OPAL) Study, a prospective cohort study of older adults in England. BMJ Open 2020;10.
- Parsons S, Carnes D, Pincus T, Foster N, Breen A, Vogel S, et al. Measuring troublesomeness of chronic pain by location. BMC Musculoskelet Disord 2006;7.
- de Schepper EI, Overdevest GM, Suri P, Peul WC, Oei EH, Koes BW, et al. Diagnosis of lumbar spinal stenosis: an updated systematic review of the accuracy of diagnostic tests. Spine 2013;38:E469-81.
- Gobbens RJ, van Assen MA, Luijkx KG, Wijnen-Sponselee MT, Schols JM. The Tilburg Frailty Indicator: psychometric properties. J Am Med Dir Assoc 2010;11:344-55.
- Gobbens RJ, van Assen MA, Luijkx KG, Wijnen-Sponselee MT, Schols JM. Determinants of frailty. J Am Med Dir Assoc 2010;11:356-64.
- Newell AM, VanSwearingen JM, Hile E, Brach JS. The modified Gait Efficacy Scale: establishing the psychometric properties in older adults. Phys Ther 2012;92:318-28.
- Lamb SE, Jorstad-Stein EC, Hauer K, Becker C. Prevention of Falls Network Europe and Outcomes Consensus Group . Development of a common outcome data set for fall injury prevention trials: the Prevention of Falls Network Europe consensus. J Am Geriatr Soc 2005;53:1618-22.
- Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud 1988;10:61-3.
- Gompertz P, Pound P, Ebrahim S. A postal version of the Barthel Index. Clin Rehabil 1994;8:233-9.
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193-21.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36.
- Pinto E, Peters R. Literature review of the Clock Drawing Test as a tool for cognitive screening. Dement Geriatr Cogn Disord 2009;27:201-13.
- Von Korff M, Ormel J, Keefe F, Dworkin S. Grading the severity of chronic pain. Pain 1992;50:133-49.
- Bifulco L, Anderson DR, Blankson ML, Channamsetty V, Blaz JW, Nguyen-Louie TT, et al. Evaluation of a chronic pain screening program implemented in primary care. JAMA Network Open 2021;4:e2118495-95.
- Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain 1995;61:277-84.
- Department for Communities and Local Government . The English Indices of Deprivation 2015 2015.
- Kuorinka I, Jonsson B, Kilbom A, Vinterberg H, Biering-Sorensen F, Andersson G, et al. Standardised Nordic questionnaires for the analysis of musculoskeletal symptoms. Appl Ergon 1987;18:233-7.
- Parsons S, Breen A, Foster NE, Letley L, Pincus T, Vogel S, et al. Prevalence and comparative troublesomeness by age of musculoskeletal pain in different body locations. Fam Pract 2007;24:308-16.
- Janssen KJ, Donders AR, Harrell FE, Vergouwe Y, Chen Q, Grobbee DE, et al. Missing covariate data in medical research: to impute is better than to ignore. J Clin Epidemiol 2010;63:721-7.
- Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338.
- Backstrom KM, Whitman JM, Flynn TW. Lumbar spinal stenosis-diagnosis and management of the aging spine. Man Ther 2011;16:308-17.
- Comer CM, Redmond AC, Bird HA, Conaghan PG. Assessment and management of neurogenic claudication associated with lumbar spinal stenosis in a UK primary care musculoskeletal service: a survey of current practice among physiotherapists. BMC Musculoskelet Disord 2009;10.
- Tomkins CC, Dimoff KH, Forman HS, Gordon ES, McPhail J, Wong JR, et al. Physical therapy treatment options for lumbar spinal stenosis. J Back Musculoskelet Rehabil 2010;23:31-7.
- Ammendolia C, Stuber KJ, Rok E, Rampersaud R, Kennedy CA, Pennick V, et al. Nonoperative treatment for lumbar spinal stenosis with neurogenic claudication. Cochrane Database Syst Rev 2013;8.
- May S, Comer C. Is surgery more effective than non-surgical treatment for spinal stenosis, and which non-surgical treatment is more effective? A systematic review. Physiotherapy 2013;99:12-20.
- Iversen M, Choudhary V, Patel S. Therapeutic exercise and manual therapy for person with lumbar spinal stenosis. Int J Clin Rheumatol 2010;5:425-37.
- Venturelli M, Schena F, Richardson RS. The role of exercise capacity in the health and longevity of centenarians. Maturitas 2012;73:115-20.
- Bandura A. Self-efficacy: The Exercise of Control. New York: Freeman; 1997.
- Self-Regulation and Behavior Change: Disentangling Behavioral Initiation and Behavioral Maintenance. New York: Guilford Press; 2011.
- Lyle S, Williamson E, Darton F, Griffiths F, Lamb SE. A qualitative study of older people’s experience of living with neurogenic claudication to inform the development of a physiotherapy intervention. Disabil Rehabil 2017;39:1009-17.
- Ward L, Williamson E, Hansen Z, French DP, Boniface G, Rogers D, et al. Better Outcomes for Older Adults with Spinal Trouble BOOST Group . Development and delivery of the BOOST (Better Outcomes for Older adults with Spinal Trouble) intervention for older adults with neurogenic claudication. Physiotherapy 2019;105:262-74.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337.
- NICE . Low Back Pain and Sciatica in Over 16s: Assessment and Management 2016. https://www.nice.org.uk/guidance/ng59 (accessed 9 February 2023).
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Cardiovascular Health Study Collaborative Research Group . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146-56.
- Eggermont LH, Leveille SG, Shi L, Kiely DK, Shmerling RH, Jones RN, et al. Pain characteristics associated with the onset of disability in older adults: the maintenance of balance, independent living, intellect, and zest in the Elderly Boston Study. J Am Geriatr Soc 2014;62:1007-16.
- Byrne C, Faure C, Keene DJ, Lamb SE. Ageing, muscle power and physical function: a systematic review and implications for pragmatic training interventions. Sports Med 2016;46:1311-32.
- Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev 2012;40:4-12.
- Reid MC, Eccleston C, Pillemer K. Management of chronic pain in older adults. BMJ 2015;350.
- Chodzko-Zajko W. ACSM’s Exercise for Older Adults. Lippincott Williams & Wilkins; 2013.
- NICE . NICE CG161: Falls: Assessment and Prevention of Falls in Older People 2013. www.nice.org.uk/guidance/cg161 (accessed 9 February 2023).
- NICE . Behavioural Change: Individual Approaches 2014. www.nice.org.uk/guidance/ph49 (accessed 9 February 2023).
- Jette AM. Toward a common language for function, disability, and health. Phys Ther 2006;86:726-34.
- Jette AM, Keysor JJ. Disability models: implications for arthritis exercise and physical activity interventions. Arthritis Care Res 2003;49:114-20.
- Williamson E, Ward L, Vadher K, Dutton SJ, Parker B, Petrou S, et al. Better Outcomes for Older people with Spinal Trouble (BOOST) Trial: a randomised controlled trial of a combined physical and psychological intervention for older adults with neurogenic claudication, a protocol. BMJ Open 2018;8.
- Marian IR, Williamson E, Garrett A, Lamb SE, Dutton SJ. Better Outcomes for Older people with Spinal Trouble (BOOST) trial: statistical analysis plan for a randomised controlled trial of a combined physical and psychological intervention for older adults with neurogenic claudication. Trials 2020;21.
- Williamson E, Boniface G, Marian IR, Dutton SJ, Garrett A, Morris A, et al. The clinical effectiveness of a physiotherapy delivered physical and psychological group intervention for older adults with neurogenic claudication: the BOOST randomised controlled trial. J Gerontol A Biol Sci Med Sci 2022;77:1654-64.
- Mandy M, Kamran K, Marian IR, Dutton SJ, Esther W, Lamb SE, et al. Economic costs, health-related quality of life outcomes and cost–utility of a physical and psychological group intervention targeted at older adults with neurogenic claudication. Cost Eff Resour Alloc 2023;21.
- Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine 2000;25:2940-52.
- Pua YH, Cai CC, Lim KC. Treadmill walking with body weight support is no more effective than cycling when added to an exercise program for lumbar spinal stenosis: a randomised controlled trial. Aust J Physiother 2007;53:83-9.
- Cleland JA, Whitman JM, Houser JL, Wainner RS, Childs JD. Psychometric properties of selected tests in patients with lumbar spinal stenosis. Spine J 2012;12:921-31.
- Bennell K, Dobson F, Hinman R. Measures of physical performance assessments. Arthritis Care Res 2011;63:S350-70.
- Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 2000;55:M221-31.
- Shuval K, Kohl HW, Bernstein I, Cheng D, Pettee Gabriel K, Barlow CE, et al. Sedentary behaviour and physical inactivity assessment in primary care: the Rapid Assessment Disuse Index (RADI) study. Br J Sports Med 2014;48:250-5.
- Roberts HC, Syddall HE, Cooper C, Aihie Sayer A. Is grip strength associated with length of stay in hospitalised older patients admitted for rehabilitation? Findings from the Southampton grip strength study. Age Ageing 2012;41:641-6.
- Kamper SJ, Maher CG, Mackay G. Global rating of change scales: a review of strengths and weaknesses and considerations for design. J Man Manip Ther 2009;17:163-70.
- Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res 2013;22:1717-27.
- Agborsangaya CB, Lahtinen M, Cooke T, Johnson JA. Comparing the EQ-5D 3L and 5L: measurement properties and association with chronic conditions and multimorbidity in the general population. Health Qual Life Outcomes 2014;12.
- Beecham J, Knapp M. Costing Psychiatric Interventions. London: Gaskell; 2001.
- Gagen R. The Value of Magnetic Resonance Imaging in the Assessment of Degenerative Lumbar Spinal Stenosis: University of Warwick, United Kingdom 2021. http://wrap.warwick.ac.uk/161673 (accessed 9 February 2023).
- Dunn G, Maracy M, Tomenson B. Estimating treatment effects from randomized clinical trials with noncompliance and loss to follow-up: the role of instrumental variable methods. Stat Methods Med Res 2005;14:369-95.
- Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey Smith G. Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives. Health Technol Assess 2001;5:1-56.
- NICE 2013. www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 (accessed 9 February 2023).
- EuroQol Research Foundation 2019.
- Torgerson DJ. Contamination in trials: is cluster randomisation the answer?. BMJ 2001;322:355-7.
- Griffiths F, Srikesavan C, Ward L, Boniface G, Williamson E, Lamb SE. Longitudinal qualitative study of living with neurogenic claudication. BMJ Open 2022;12.
- Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res 2016;26:1753-60.
- Sheard LMC, Marsh C. How to analyse longitudinal data from multiple sources in qualitative health research: the pen portrait analytic technique. BMC Med Res Methodol 2019;19:1-10.
- Ammendolia C, Schneider M, Williams K, Zickmund S, Hamm M, Stuber K, et al. The physical and psychological impact of neurogenic claudication: the patients’ perspectives. J Can Chiropr Assoc 2017;61:18-31.
- Bussieres A, Cancelliere C, Ammendolia C, Comer CM, Zoubi FA, Chatillon CE, et al. Non-surgical interventions for lumbar spinal stenosis leading to neurogenic claudication: a clinical practice guideline. J Pain 2021;22:1015-39.
- Collado-Mateo D, Lavin-Perez AM, Penacoba C, Del Coso J, Leyton-Roman M, Luque-Casado A, et al. Key factors associated with adherence to physical exercise in patients with chronic diseases and older adults: an umbrella review. Int J Environ Res Public Health 2021;18.
- Beinart NA, Goodchild CE, Weinman JA, Ayis S, Godfrey EL. Individual and intervention-related factors associated with adherence to home exercise in chronic low back pain: a systematic review. Spine J 2013;13:1940-50.
- Sanchez-Santos MT, Williamson E, Nicolson PJA, Bruce J, Collins GS, Mallen CD, et al. OPAL Study Team . Development and validation of a prediction model for self-reported mobility decline in community-dwelling older adults. J Clin Epidemiol 2022;152:70-9.
- Nicolson PJA, Sanchez-Santos MT, Bruce J, Kirtley S, Ward L, Williamson E, et al. Risk factors for mobility decline in community-dwelling older adults: a systematic literature review. J Aging Phys Act 2021;1.
- Riley RD, Snell KI, Ensor J, Burke DL, Harrell FE, Moons KG, et al. Minimum sample size for developing a multivariable prediction model: PART II – binary and time-to-event outcomes. Stat Med 2019;38:1276-96.
- Tibshirani R. Regression shrinkage and selection via the LASSO. J R Stat Soc Series B 1996;58:267-88.
- Marshall A, Altman DG, Holder RL, Royston P. Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol 2009;9.
- Steyerberg EW. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating. Springer; 2019.
- Royston P, White IR. Multiple Imputation by Chained Equations (MICE): implementation in stata. J Stat Soft 2011;45.
- Vergouwe Y, Royston P, Moons KG, Altman DG. Development and validation of a prediction model with missing predictor data: a practical approach. J Clin Epidemiol 2010;63:205-14.
- Sullivan LM, Massaro JM, D’Agostino RB. Presentation of multivariate data for clinical use: the Framingham Study risk score functions. Stat Med 2004;23:1631-60.
- Hoeymans N, Feskens EJ, van den Bos GA, Kromhout D. Measuring functional status: cross-sectional and longitudinal associations between performance and self-report (Zutphen Elderly Study 1990–1993). J Clin Epidemiol 1996;49:1103-10.
- Mänty M, Heinonen A, Leinonen R, Törmäkangas T, Sakari-Rantala R, Hirvensalo M, et al. Construct and predictive validity of a self-reported measure of preclinical mobility limitation. Arch Phys Med Rehabil 2007;88:1108-13.
- Makris UE, Higashi RT, Marks EG, Fraenkel L, Sale JE, Gill TM, et al. Ageism, negative attitudes, and competing co-morbidities – why older adults may not seek care for restricting back pain: a qualitative study. BMC Geriatr 2015;15.
- Hurley MV, Walsh NE, Mitchell HL, Pimm TJ, Patel A, Williamson E, et al. Clinical effectiveness of a rehabilitation program integrating exercise, self-management, and active coping strategies for chronic knee pain: a cluster randomized trial. Arthritis Rheum 2007;57:1211-9.
- Ziu E, Viswanathan VK, Mesfin FB. Spinal Metastasis. StatPearls; 2022.
- Williams E, Buck D, Babalola G, Maquire D. The Kings Fund: What Are Health Inequalities? 2020. www.kingsfund.org.uk/publications/what-are-health-inequalities#what (accessed 8 February 2023).
- NHS England . 2021/22/Priorities/and/Operational/Planning/Guidance 2021. www.england.nhs.uk/publication/2021-22-priorities-and-operational-planning-guidance/ (accessed 8 February 2023).
- Swaithes L, Dziedzic K, Finney A, Cottrell E, Jinks C, Mallen C, et al. Understanding the uptake of a clinical innovation for osteoarthritis in primary care: a qualitative study of knowledge mobilisation using the i-PARIHS framework. Implement Sci 2020;15.
- Sugavanam T, Williamson E, Fordham B, Hansen Z, Richmond H, Hall A, et al. Evaluation of the implementation of the Back Skills Training (BeST) programme using online training: a cohort implementation study. Physiotherapy 2020;109:4-12.
- Williamson E, Srikesavan C, Thompson J, Tonga E, Eldridge L, Adams J, et al. Translating the Strengthening and Stretching for Rheumatoid Arthritis of the Hand Programme from clinical trial to clinical practice: an effectiveness–implementation study. Hand Therapy 2020;25:87-9.
- Thorlund K, Dron L, Park JJH, Mills EJ. Synthetic and external controls in clinical trials: a primer for researchers. Clin Epidemiol 2020;12:457-67.
- Nicolson PJ, Williamson E, Lee H, Morris A, Garrett A, Sanchez-Santos MT, et al. Synergistic effects of hip/knee osteoarthritis and comorbidities on mobility and self-care limitations among older adults: cross-sectional analysis of the Oxford pain, Activity and Lifestyle study. J Comorb 2020;10.
- Nicolson PJA, Williamson E, Morris A, Sanchez-Santos MT, Bruce J, Silman A, et al. Musculoskeletal pain and loneliness, social support and social engagement among older adults: analysis of the Oxford Pain, Activity and Lifestyle cohort. Musculoskeletal Care 2021;19:269-77.
- Guralnik JM, Fried LP, Simonsick EM, Kasper JD, Lafferty ME. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda, MD: National Institute of Aging; 1995.
- Driscoll T, Jacklyn G, Orchard J, Passmore E, Vos T, Freedman G, et al. The global burden of occupationally related low back pain: estimates from the Global Burden of Disease 2010 study. Ann Rheum Dis 2014;73:975-81.
- Mottram S, Peat G, Thomas E, Wilkie R, Croft P. Patterns of pain and mobility limitation in older people: cross-sectional findings from a population survey of 18,497 adults aged 50 years and over. Qual Life Res 2008;17:529-39.
- Lamb SE, Lall R, Hansen Z, Castelnuovo E, Withers EJ, Nichols V, et al. A multicentred randomised controlled trial of a primary care-based cognitive behavioural programme for low back pain. The Back Skills Training (BeST) trial. Health Technol Assess 2010;14:1iii-253iv.
- Rose SB, Elley CR, Lawton BA, Dowell AC. A single question reliably identifies physically inactive women in primary care. N Z Med J 2008;121.
- Evidence from the English Longitudinal Study of Ageing 2002–2012 (Wave 6). London: The Institute for Fiscal Studies; 2014.
- Syddall HE, Westbury LD, Cooper C, Sayer AA. Self-reported walking speed: a useful marker of physical performance among community-dwelling older people?. J Am Med Dir Assoc 2015;16:323-8.
- Peel C, Sawyer Baker P, Roth DL, Brown CJ, Brodner EV, Allman RM. Assessing mobility in older adults: the UAB Study of Aging Life-Space Assessment. Phys Ther 2005;85:1008-119.
- Laidlaw K, Power MJ, Schmidt S. WHOQOL-OLD Group . The Attitudes to Ageing Questionnaire (AAQ): development and psychometric properties. Int J Geriatr Psychiatry 2007;22:367-79.
- Hill JC, Dunn KM, Lewis M, Mullis R, Main CJ, Foster NE, et al. A primary care back pain screening tool: identifying patient subgroups for initial treatment. Arthritis Rheum 2008;59:632-41.
- Fishbein M, Ajzen I. Predicting and Changing Behavior: The Reasoned Action Approach. New York: Psychology Press; 2010.
- Resnick B, Jenkins LS. Testing the reliability and validity of the self-efficacy for exercise scale. Nurs Res 2000;49:154-9.
Appendix 1 Additional information about the OPAL study
| Measure | |
|---|---|
| Demographic | Age, sex, ethnicity, relationship status, weight and height, number of children. Alcohol and smoking behaviour.119 Postcode, current occupation, main occupation during lifetime,120 self-rating of strenuousness of occupation, type of housing, education, requires a carer or not, adequacy of income,121 internet access |
| Comorbidity | Self-report of current health conditions Self-reported fractures |
| Incontinence | Two items from Barthel Index33,34 |
| Medication | Self-reported medication use |
| Sleep and fatigue | Fatigue – Tilburg Frailty Index question29 Sleep – Sleep quality overall rating from Pittsburgh Sleep Quality Index35 and average number of hours’ sleep each night |
| BP | Report of BP in last 6 weeks, troublesomeness, onset of BP and nature of BP (comes and goes, fairly constant; better, worse)122 Report of leg pain and symptoms Screening questions for neurogenic claudication28 |
| Other pain | Nordic pain questionnaire42,43 |
| Disability and HRQoL | EQ-5D-5L81,82 |
| Physical activity | Rapid Assessment Disuse Index (modified)78 Lifetime physical activity123 Self-report of physical activity over lifespan124 Participation in clubs and groups124 |
| Mobility | Change in mobility in the last year Self-rated walking speed125 Use of walking aids Mobility concerns Life-space assessment126 Access to transport124 |
| Frailty | Tilburg Frailty Index29 |
| Balance and falls | Balance question in Tilburg Frailty Index Self-reported falls in the last 12 months32 |
| Cognition | Clock Drawing Test37 |
| Quality of life | EQ-5D-5L81,82 |
| Self-efficacy | Single item from the Modified Gait Self-Efficacy Scale (10-item)31 |
| Depression and anxiety | Questions in the EQ-5D, self-report of current health conditions and Tilburg Frailty Index |
| Beliefs about ageing | Attitude to Ageing questionnaire – physical changes subscale127 |
| Health resource use | Consent to collect HES data included on OPAL cohort study consent form |
| Characteristics | Overall (n = 5304) |
BP groups | |||
|---|---|---|---|---|---|
| No BP (n = 2483) | BP only (n = 1786) | BP + non-NC leg pain (n = 441) | BP + NC (n = 594) |
||
| Age (years), mean (SD) | 74.9 (6.8) | 74.8 (6.8) | 74.7 (6.6) | 74.4 (6.6) | 76.2 (7.2) |
| Age categories, n (%) 65–74 years | 2972 (56.0) | 1415 (57.0) | 1016 (56.9) | 259 (58.7) | 282 (47.5) |
| 75–84 years | 1823 (34.4) | 833 (33.5) | 615 (34.4) | 144 (32.6) | 231 (38.9) |
| ≥ 85 years | 509 (9.6) | 235 (9.5) | 155 (8.6) | 38 (8.6) | 81 (13.6) |
| Female, n (%) | 2720 (51.3) | 1154 (46.5) | 970 (54.3) | 256 (58.1) | 340 (57.2) |
| Education, n (%) Higher education |
1889 (35.6) | 922 (37.1) | 657 (36.8) | 130 (29.5) | 180 (30.3) |
| Secondary | 2990 (56.4) | 1392 (56.1) | 1004 (56.2) | 258 (58.5) | 336 (56.6) |
| None or primary | 393 (7.4) | 159 (6.4) | 117 (6.6) | 44 (10.0) | 73 (12.3) |
| IMD, n (%) C1 – most affluent | 2980 (56.2) | 1417 (57.1) | 1053 (59.0) | 217 (49.2) | 293 (49.3) |
| C2 | 1512 (28.5) | 712 (28.7) | 491 (27.5) | 139 (31.5) | 170 (28.6) |
| C3 | 436 (8.2) | 217 (8.7) | 121 (6.8) | 40 (9.1) | 58 (9.8) |
| C4 – most deprived | 376 (7.1) | 137 (5.5) | 121 (6.8) | 45 (10.2) | 73 (12.3) |
| BMI, mean (SD) | 26.6 (4.9) | 26.2 (4.6) | 26.4 (4.7) | 27.2 (5.1) | 28.5 (5.8) |
| Smoking, n (%), never | 2640 (49.8) | 1288 (51.9) | 880 (49.3) | 199 (45.1) | 273 (46.0) |
| Ex/current | 2649 (49.9) | 1193 (48.1) | 900 (50.4) | 239 (54.2) | 317 (53.4) |
| Multisite pain (0–7), median (IQR) | 2 (1–3) | 1 (0–2) | 2 (1–3) | 3 (1–4) | 3 (2–5) |
| Comorbidities (0–11), median (IQR) | 2 (1–2) | 1 (1–2) | 2 (1–2) | 2 (1–3) | 2 (1–3) |
| Number of adverse health states (0–6), median (IQR)a | 1 (0–2) | 0 (0–1) | 1 (0–2) | 2 (0–3) | 2 (1–4) |
| Confidence to walk (1–10)b, median (IQR) | 1 (1–3) | 1 (1–1) | 1 (1–3) | 1 (1–6) | 5 (1–9) |
| Reduced cognitive function, n (%) | 760 (14.3) | 349 (14.1) | 224 (12.5) | 88 (20.0) | 99 (16.7) |
| EQ-5D crosswalk index value, mean (SD) | 0.77 (0.20) | 0.85 (0.16) | 0.76 (0.18) | 0.65 (0.25) | 0.59 (0.23) |
| Age at onset of BP, n (%) | |||||
| ≤ 40 years | 571 (32.0) | 144 (32.7) | 171 (28.8) | ||
| 41–64 years | 644 (36.1) | 163 (37.0) | 228 (38.4) | ||
| 65–74 years | 360 (20.2) | 90 (20.4) | 117 (19.7) | ||
| ≥ 75 years | 191 (10.7) | 40 (9.1) | 69 (11.6) | ||
| BP frequency, n (%) | |||||
| Rarely/few days | 702 (39.3) | 69 (15.7) | 50 (8.4) | ||
| Some days | 494 (27.7) | 140 (31.8) | 114 (19.2) | ||
| Most days/every day | 567 (31.8) | 227 (51.5) | 422 (71.0) | ||
| BP troublesomeness (intensity), n (%) | |||||
| Not at all/slightly | 1140 (63.8) | 180 (40.8) | 136 (22.9) | ||
| Moderate | 493 (27.6) | 160 (36.3) | 244 (41.1) | ||
| Very or extreme | 145 (8.1) | 97 (22.0) | 207 (34.9) | ||
| BP pattern since onset, n (%) | |||||
| Getting better | 66 (3.7) | 11 (2.5) | 9 (1.5) | ||
| Getting worse | 70 (3.9) | 50 (11.3) | 135 (22.7) | ||
| Fairly constant | 293 (16.4) | 126 (28.6) | 200 (33.7) | ||
| Comes and goes over time | 1338 (74.9) | 249 (56.5) | 245 (41.3) | ||
| Two-year follow-up | All participants with BP with and without leg pain at baseline (n = 2139) | Baseline back and leg pain presentationa | ||
|---|---|---|---|---|
| BP only (n = 1411) | BP + non-NC leg pain (n = 316) | BP + NC leg pain (n = 394) | ||
| BP presentation at 2 years | ||||
| No BP | 489 (22.9) | 386 (27.4) | 55 (17.4) | 43 (10.9) |
| BP only | 1056 (49.4) | 816 (57.8) | 119 (37.7) | 115 (29.2) |
| BP + non-NC leg pain | 216 (10.1) | 82 (5.8) | 79 (25.0) | 53 (13.5) |
| BP + NC leg pain | 339 (15.9) | 105 (7.4) | 56 (17.7) | 175 (44.4) |
| BP frequency, n (%) | ||||
| Rarely/few days | 376 (17.6) | 289 (20.5) | 52 (16.5) | 34 (8.6) |
| Some days | 441 (20.6) | 309 (21.9) | 57 (18.0) | 72 (18.3) |
| Most days/every day | 795 (37.2) | 403 (28.6) | 148 (46.8) | 236 (59.9) |
| BP troublesomeness (intensity), n (%) | ||||
| Not at all/slightly | 805 (37.6) | 598 (42.4) | 104 (32.9) | 100 (25.4) |
| Moderate | 554 (25.9) | 317 (22.5) | 88 (27.9) | 143 (36.3) |
| Very or extreme | 262 (12.3) | 93 (6.6) | 65 (20.6) | 101 (25.6) |
| BP pattern, n (%) | ||||
| Getting better | 61 (2.9) | 36 (2.6) | 11 (3.5) | 13 (3.3) |
| Getting worse | 125 (5.8) | 45 (3.2) | 27 (8.5) | 52 (13.2) |
| Fairly constant | 434 (20.3) | 207 (14.7) | 86 (27.2) | 134 (34.0) |
| Comes and goes over time | 988 (46.2) | 715 (50.7) | 131 (41.5) | 139 (35.3) |
| Pain interference with daily activities, mean (SD) | 3.4 (2.6) | 2.8 (2.4) | 3.7 (2.6) | 4.8 (2.5) |
| Proportion reporting severely interfering BP,a n (%) | 233 (10.8) | 90 (6.4) | 39 (12.3) | 103 (26.1) |
Appendix 2 Additional information about the BOOST trial
| Measures | Subgroups |
|---|---|
| MRI parameters | |
| Central canal stenosis present | Minimum dural sac cross-sectional area (DS-CSA) < 100 mm2 |
| Lateral recess stenosis present | Minimum diameter < 3 mm |
| Single/multiple level stenosis | No spinal level with DS-CSA ≤ 100 mm2 / a single DS-CSA ≤ 100 mm2 / more than one level with DS-CSA ≤ 100 mm2 |
| Qualitative grading of central canal stenosis | Grades A and B / grades C and D of central canal stenosis based on the degree of stenosis based on the amount of cerebrospinal fluid space around the nerve roots of the cauda equina |
| Qualitative grading of entrapment in the lateral recess | No entrapment (grades 0 and 1) / entrapment (grades 2 and 3) |
| Qualitative grading of nerve root entrapment in the neural exit foramen | No foraminal nerve root entrapment (grade 0) / foraminal nerve root entrapment (grades 1, 2 or 3) |
| Other factors | |
| Age | 65–74 years / ≥ 75 years |
| Gender | Male/female |
| Tilburg Frailty Index | Scores 0–4 / 5 + |
| Fear Avoidance Beliefs Questionnaire | Scores 0–14 / 15 + |
| STartBack Risk Stratification | Low/medium/high risks |
| Hand grip strength | Men: < 30 / 30 + Women: < 20 / 20 + |
| SPPB scores | Scores: 0–6 low performance / 7–9 intermediate performance / 10–12 high performance |
| BOOST trial site | Number of participants | Region name | Area name | Asian or Asian British | Black, African, Caribbean or Black British | Mixed or multiple ethnic groups | White | Other ethnic group | Deprivation index | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | N | % | n | % | |||||
| 1. Royal Orthopaedic Hospital Birmingham | 50 | West Midlands | Birmingham | 285,640 | 26.6 | 96,360 | 9.0 | 47,605 | 4.4 | 621,636 | 57.9 | 21,804 | 2.0 | 38.10 |
| 2. Sandwell and West Birmingham | 30 | West Midlands | Sandwell | 59,258 | 19.2 | 18,357 | 6.0 | 10,199 | 3.3 | 215,471 | 69.9 | 4778 | 1.6 | 34.90 |
| 3. Oxford University Hospitals | 54 | South East | Oxford | 18,827 | 12.4 | 7028 | 4.6 | 6035 | 4.0 | 117,957 | 77.7 | 2059 | 1.4 | 16.7 |
| South East | Cherwell | 6039 | 4.3 | 1961 | 1.4 | 2560 | 1.8 | 130,761 | 92.2 | 547 | 0.4 | 14.4 | ||
| South East | South Oxfordshire | 2405 | 1.8 | 768 | 0.6 | 1801 | 1.3 | 128,993 | 96.1 | 290 | 0.2 | 8.5 | ||
| South East | Vale of White Horse | 2962 | 2.4 | 1230 | 1.0 | 1574 | 1.3 | 114,824 | 94.9 | 398 | 0.3 | 8.4 | ||
| South East | West Oxfordshire | 1424 | 1.4 | 437 | 0.4 | 1263 | 1.2 | 101,469 | 96.8 | 186 | 0.2 | 8.7 | ||
| 4. North West Boroughs NHS Trust | 34 | North West | Knowsley | 1403 | 1.0 | 505 | 0.3 | 1913 | 1.3 | 141,858 | 97.2 | 214 | 0.1 | 43.00 |
| North West | St. Helens | 1764 | 1.0 | 248 | 0.1 | 1179 | 0.7 | 171,877 | 98.0 | 240 | 0.1 | 31.50 | ||
| 5. East Cheshire NHS Trust | 23 | North West | Cheshire East | 6060 | 1.6 | 1402 | 0.4 | 3873 | 1.0 | 357,940 | 96.7 | 852 | 0.2 | 14.50 |
| 6. Cambridgeshire Community Services | 39 | East | Huntingdonshire | 4190 | 2.5 | 1642 | 1.0 | 2530 | 1.5 | 160,691 | 94.8 | 455 | 0.3 | 12.60 |
| 7. Wirral University Teaching Hospital | 35 | North West | Wirral | 5116 | 1.6 | 695 | 0.2 | 3286 | 1.0 | 310,156 | 97.0 | 530 | 0.2 | 29.60 |
| 8. Leeds Teaching Hospitals | 2 | Yorkshire and The Humber | Leeds | 58,243 | 7.8 | 25,893 | 3.4 | 19,632 | 2.6 | 639,487 | 85.1 | 8230 | 1.1 | 27.30 |
| 9. Birmingham Community Trust | 35 | West Midlands | Birmingham | 285,640 | 26.6 | 96,360 | 9.0 | 47,605 | 4.4 | 621,636 | 57.9 | 21,804 | 2.0 | 38.10 |
| 10. Leeds Community Healthcare | 30 | Yorkshire and The Humber | Leeds | 58,243 | 7.8 | 25,893 | 3.4 | 19,632 | 2.6 | 639,487 | 85.1 | 8230 | 1.1 | 27.30 |
| 11. Royal Liverpool and Broadgreen University Hospital | 25 | North West | Liverpool | 19,403 | 4.2 | 12,308 | 2.6 | 11,756 | 2.5 | 414,671 | 88.9 | 8277 | 1.8 | 42.40 |
| 12. Dorset University Hospitals NHS Trust | 26 | South West | Dorset | 3338 | 0.9 | 841 | 0.2 | 2895 | 0.8 | 357,726 | 98.0 | 353 | 0.1 | 15.70 |
| 13. Croydon University Hospital | 14 | London | Croydon | 59,627 | 16.4 | 73,256 | 20.2 | 23,895 | 6.6 | 200,195 | 55.1 | 6405 | 1.8 | 22.50 |
| 14. Gloucestershire Community Care Services NHS Trust | 21 | South West | Cotswold | 794 | 1.0 | 229 | 0.3 | 698 | 0.8 | 81,075 | 97.8 | 85 | 0.1 | 11.10 |
| 15. Wiltshire Health and Care | 20 | South West | Wiltshire | 6178 | 1.3 | 3228 | 0.7 | 5568 | 1.2 | 454,971 | 96.6 | 1036 | 0.2 | 13.40 |
| Variablesa | BPA (n = 143) |
BOOST programme (n = 292) |
Overall (n = 435) |
|---|---|---|---|
| Age (years) at baseline | 75.0 (5.6) | 74.8 (6.2) | 74.9 (6.0) |
| Female | 83 (58.0%) | 163 (55.8%) | 246 (56.6%) |
| White ethnicity | 132 (92.3%) | 268 (91.8%) | 400 (91.9%) |
| Relationship status: Married/civil union/cohabiting | 97 (67.8%) | 194 (66.40%) | 291 (66.9%) |
| Unmarried/separated/divorced | 16 (11.2%) | 31 (10.7%) | 47 (10.8%) |
| Widow/widower | 30 (21.0%) | 67 (22.9%) | 97 (22.3%) |
| Care requirements: Has an unpaid carer | 31 (21.7%) | 54 (18.5%) | 85 (19.5%) |
| Has a paid carer | 6 (4.2%) | 10 (3.4%) | 16 (3.7%) |
| Work status: Retired | 125 (87.4%) | 263 (90.1%) | 388 (89.2%) |
| Working (full- or part-time) | 10 (6.9%) | 24 (8.2%) | 34 (7.8%) |
| Education: None or primary education | 4 (2.8%) | 18 (6.2%) | 22 (5.1%) |
| Secondary education | 80 (55.9%) | 170 (58.2%) | 250 (57.5%) |
| Higher professional/university education | 59 (41.3%) | 104 (35.6%) | 163 (37.5%) |
| Smoking status: Never smoked | 61 (42.7%) | 136 (46.6%) | 197 (45.3%) |
| Former smoker | 75 (52.4%) | 140 (47.9%) | 215 (49.4%) |
| Current smoker | 7 (4.9%) | 16 (5.5%) | 23 (5.3%) |
| BMI | 30.0 (5.4) | 29.9 (4.8) | 29.9 (5.0) |
| Number of comorbidities reported, median (IQR) | 3 (2–4) | 2 (2–4) | 2 (2–4) |
| Nordic pain questionnaire:42,43 Single-site pain | 14 (9.8%) | 16 (5.5%) | 30 (6.9%) |
| Multisite pain | 129 (90.2%) | 276 (94.5%) | 405 (93.1%) |
| STarTBack:128 Low risk | 48 (33.8%) | 109 (37.6%) | 157 (36.3%) |
| Medium risk | 67 (47.2%) | 138 (47.6%) | 205 (47.5%) |
| High risk | 27 (19.0%) | 43 (14.8%) | 70 (16.2%) |
| Classified as frail,b n (%) | 80 (55.9%) | 130 (44.5%) | 210 (48.3%) |
| Self-rated outdoor walking speed,125 median (IQR) | 4 (3–4) | 4 (3–4) | 4 (3–4) |
| Change in mobility: better now than 1 year ago | 9 (6.3%) | 15 (5.2%) | 24 (5.5%) |
| About the same | 30 (21.0%) | 86 (29.5%) | 116 (26.7%) |
| Worse now than 1 year ago | 104 (72.7%) | 191 (65.4.8%) | 295 (67.8%) |
| Use of walking aids outside: Yes | 40 (28.0%) | 75 (25.7%) | 115 (26.4%) |
| Sometimes | 28 (19.6%) | 55 (18.8%) | 83 (19.1%) |
| Use of walking aids inside: Yes | 9 (6.3%) | 16 (5.5%) | 25 (5.7%) |
| Sometimes | 15 (10.5%) | 35 (12.0%) | 50 (11.5%) |
| Attitudes to Ageing Questionnaire127,c | 28.7 (6.6) | 29.0 (5.9) | 28.9 (6.1) |
| Intention to exercise,129 median (IQR)d | 6 (6–7) | 6 (6–7) | 6 (6–7) |
| Exercise self-efficacy scale,130,e median (IQR) | 68 (54–80) | 70 (52–81) | 69 (53–80) |
| Walking self-efficacyf | 5.3 (3.3) | 5.7 (3.3) | 5.6 (3.3) |
| Confidence in ability to self-manage symptomsg | 6.1 (1.78) | 6.1 (1.81) | 6.1 (1.80) |
| Fear avoidance beliefsh | 12.7 (5.4) | 13.0 (6.1) | 12.9 (5.9) |
| BOOST–OPAL participants (n = 44a) |
OPAL participants reporting NC symptoms and not in BOOST (n = 568b) | |
|---|---|---|
| Sociodemographic | ||
| Age (years) at baseline | 73.6 (1.0) | 76.3 (0.3) |
| Female | 24 (54.6) | 329 (57.9) |
| Education: None/primary education | 4 (9.1) | 70 (12.3) |
| Secondary education | 17 (38.6) | 325 (57.2) |
| Higher professional/university education | 23 (52.3) | 168 (29.6) |
| IMD quintiles, n (%): C1 – most affluent | 12 (27.3) | 176 (31.0) |
| C2 | 12 (27.3) | 104 (18.3) |
| C3 | 14 (31.8) | 108 (19.0) |
| C4 | 6 (13.6) | 77 (13.6) |
| C5 – most deprived | 0 (0.0) | 103 (18.1) |
| General health | ||
| EQ-5D Utility Score | 0.647 (0.181) | 0.584 (0.010) |
| BMI | 29.7 (1.07) | 28.44 (0.24) |
| Number of comorbidities reported, median (IQR) | 2 (1–3) | 2 (1–3) |
| Pain | ||
| BP troublesomeness | ||
| Not at all/slightly | 13 (29.5) | 129 (22.7) |
| Moderate | 15 (34.1) | 235 (41.4) |
| Very or extreme | 16 (36.4) | 197 (34.7) |
| Nordic Pain Questionnaire | ||
| Pain-free | 0 (0.0) | 7 (1.2) |
| Single-site pain | 0 (0.0) | 46 (8.1) |
| Multisite musculoskeletal pain | 44 (100.0) | 515 (90.7) |
| Mobility | ||
| Change in mobility | ||
| Much better now than 1 year ago | 1 (2.3) | 9 (1.6) |
| Somewhat better than 1 year ago | 3 (6.8) | 19 (3.4) |
| About the same | 19 (43.2) | 214 (37.7) |
| Somewhat worse than 1 year ago | 17 (38.6) | 239 (42.1) |
| Much worse now than 1 year ago | 4 (9.1) | 84 (14.8) |
| Walking self-efficacy,c median (IQR) | 2 (1–6) | 5 (1–9) |
| Frailty and falls | ||
| Classified as frail,d n (%) | 20 (45.5) | 349 (61.4) |
| Fall in the last year | 21 (47.7) | 262 (46.1) |
| Outcome | BPA | BOOST programme | Between-group differencea (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|
| n | Unadjusted mean (SD)a | n | Unadjusted mean (SD)a | ||||
| ODIb | Baseline | 143 | 32.3 (14.2) | 292 | 33.2 (13.7) | n/a | |
| 6 months | 125 | 33.2 (15.9) | 258 | 30.2 (16.5) | −3.7 (−6.27 to –1.06) | 0.006 | |
| 12 months | 127 | 33.0 (17.4) | 253 | 31.7 (18) | −1.4 (−4.03 to 1.17) | 0.281 | |
| ODI walking itemb | Baseline | 143 | 1.8 (1.2) | 292 | 1.8 (1.2) | n/a | |
| 6 months | 125 | 1.8 (1.3) | 258 | 1.6 (1.3) | −0.2 (−0.44 to −0.02) | 0.033 | |
| 12 months | 126 | 1.9 (1.4) | 253 | 1.6 (1.4) | −0.2 (−0.45 to –0.01) | 0.041 | |
| One or more falls,c n (%) | Baseline | 143 | 50 (35%) | 292 | 115 (39.4%) | n/a | |
| Over 12 months | 125 | 59 (41.3%) | 257 | 96 (32.9%) | 0.6 (0.40 to 0.98)d | 0.041 | |
| Broken bones from a fall,e n (%) | Baseline | 143 | 4 (2.8%) | 292 | 8 (2.7%) | n/a | |
| Over 12 months | 127 | 9 (7.1%) | 253 | 17 (6.7%) | n/a | ||
| SSSS symptom scalef | Baseline | 143 | 3.0 (0.60) | 292 | 3.0 (0.60) | n/a | |
| 6 months | 119 | 2.8 (0.80) | 247 | 2.7 (0.80) | −0.2 (−0.28 to –0.02) | 0.025 | |
| 12 months | 113 | 2.8 (0.80) | 229 | 2.7 (0.80) | −0.1 (−0.19 to 0.08) | 0.428 | |
| Troublesomeness,c median (IQR) | Baseline | 125 | 4.0 (3.0–4.0) | 258 | 4.0 (3.0–4.0) | n/a | |
| 6 months | 125 | 3.0 (3.0–4.0) | 258 | 3.0 (2.0–4.0) | 0.5 (0.27 to 0.87)d | 0.014 | |
| 12 months | 127 | 3.0 (2.0–4.0) | 253 | 3.0 (2.0–4.0) | 0.8 (0.45 to 1.43)d | 0.454 | |
| Global rating of perceived changeg | Baseline | n/a | n/a | n/a | |||
| 6 months | 125 | 4.0 (3.0–5.0) | 257 | 3.0 (2.0, 5.0) | −0.4 (−0.75 to −0.11) | 0.009 | |
| 12 months | 127 | 4.0 (3.0–5.0) | 252 | 4.0 (3.0, 5.0) | 0.0 (−0.30 to 0.34) | 0.902 | |
| Satisfaction: treatment,h median (IQR) | Baseline | n/a | n/a | n/a | |||
| 6 months | 125 | 3.0 (2.0–4.0) | 256 | 3.0 (2.0–4.0) | 2.5 (1.41 to 4.44)d | 0.002 | |
| 12 months | 126 | 2.0 (2.0–4.0) | 248 | 3.0 (2.0–4.0) | 2.7 (1.54 to 4.83)d | 0.001 | |
| Outcome | BPA | BOOST programme | Between-group difference (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|
| n | Unadjusted mean (SD)a | n | Unadjusted mean (SD)a | ||||
| 6MWTb | Baseline | 143 | 260.4 (101.30) | 292 | 252.9 (98.10) | n/a | |
| 6 months | 118 | 266.3 (103.40) | 240 | 283.5 (99.40) | 22.5 (7.11 to 37.82) | 0.004 | |
| 12 months | 111 | 263.2 (106.70) | 216 | 284.7 (105.40) | 21.7 (5.96 to 37.38) | 0.007 | |
| SPPB,b median (IQR) | Baseline | 143 | 9.0 (8.00, 11.00) | 291 | 9.0 (7.00, 11.00) | n/a | |
| 6 months | 118 | 9.0 (7.00, 11.00) | 245 | 10.0 (8.00, 11.00) | 0.6 (0.19 to 0.97) | 0.003 | |
| 12 months | 112 | 9.5 (7.00, 11.00) | 218 | 10.5 (8.00, 12.00) | 0.4 (0.00 to 0.80) | 0.052 | |
Appendix 3 More information about the BOOST programme
| Week/session | Theme | Topics covered | Key behaviour change15 |
|---|---|---|---|
| 1/1 | Introduction to BOOST | Overview of NC; role of activity in managing NC; lumbar flexion for pain relief; exercising guidelines | Consequences of behaviour (individual); performance feedback |
| 1/2 | Increasing mobility – the role of pain | Pain ≠ damage; sensitivity and deconditioning; pain memory; medication use | As above; set graded tasks |
| 2/3 | Increasing mobility – improving strength and fitness | Age- and activity-related muscle changes; benefits of exercise; overcoming barriers to exercise; fear avoidance behaviour | |
| 2/4 | Increasing mobility – modifying activities | Under-/over-activity cycle; pacing; baseline setting; graded activity; managing symptom aggravation | |
| 3/5 | Increasing mobility – home programme phase 1 | Exercising safely at home; integrating exercise into daily routines; using exercise planners; introduce phase 1 home exercises | As above; goal setting; action planning; teach cues; prompt practice |
| 3/6 | Increasing mobility – home programme phase 2 | Peer discussion of phase 1 home exercise experiences; exercise barriers and facilitators; overview of phase 2; concerns/ideas for managing home exercises | As above; barrier identification/problem-solving |
| 4/7 | Increasing mobility – building confidence | Peer discussion of phase 2 home exercise experiences; exercise barriers and facilitators; fear and activity cycle; falls risk factors; walking aids | |
| 5/8 | Increasing mobility – home programme phase 3 | Peer discussion of phase 2 home exercise experiences; exercise barriers and facilitators; overview of phase 3 programme; use of walking planners | |
| 6/9 | Increasing mobility – increasing independence | Peer discussion of phase 3 home exercise experiences; integrating exercise into daily routines; planning a long-term independent home exercise programme | |
| 7/– | No group this week – independent exercise | ||
| 8/10 | Improving mood | Peer discussion on home programme; exercise confidence and routines; exploring links between pain, mood and activity; noted benefits of exercise | |
| 9/– | No group this week – independent exercise | ||
| 10/11 | Maintaining an active lifestyle 1 | Peer discussion on home programme; exercise confidence and motivation; positive differences in activities or mood; information sharing on groups and activities available in the local community | |
| 11/– | No group this week – independent exercise | ||
| 12/12 | Maintaining an active lifestyle 2 | Peer discussion on home programme; review of education and behavioural concepts; coping with flare-ups | |
The delivery of the BOOST programme
We randomised 438 participants, with 295 allocated to the BOOST programme. There were three complete study withdrawals (including use of any data) from the BOOST programme arm. Two were due to health problems and one because the individual would be unable to attend any treatment sessions. Therefore, 292 participants allocated to the BOOST programme are reported on here. Physiotherapists completed an intervention log for each participant. Each log acted as a structured record of the intervention and was used to monitor fidelity and adherence with the interventions.
We also carried out observation of intervention sessions. A structured checklist (see Report Supplementary Material 1) was used to assess the delivery of the core elements of interventions, which was scored as: not completed, partially completed or fully completed. Initial observations were used to provide feedback and support physiotherapists to deliver the interventions. Later in the trial, these visits were fidelity assessments to understand how the intervention would be implemented in a real-world clinical setting with no feedback to the physiotherapists.
Individual assessment
Among those randomised to the BOOST programme, 279/292 (95.5%) attended the individual assessment with the physiotherapist prior to attending the BOOST group sessions, while 16/435 (3.7%) did not attend their initial appointment. Reasons for non-attendance are listed in Appendix 3, Table 15.
| Reasons | BOOST programme (n = 13) |
|---|---|
| Work commitments | 2 |
| Allocated to the BOOST programme and did not want to attend (wanted BPA)a | 3 |
| Lack of time | 1 |
| Carer responsibilities | 1 |
| Decided to have spinal surgery | 4 |
| Lack of time for the study | 1 |
| Health problems | 1 |
Sites aimed to see participants within 6 weeks of randomisation (and referral to physiotherapy) to complete the individual assessment. This target was not always achieved: 63 (22.6%) participants across 13 (86.7%) of the 15 sites waited longer than the 6-week target.
BOOST programme participants waited a mean of 31.2 days (SD 27.3 days, range 0–299 days) for their individual assessment. Reasons for waiting longer than 6 weeks included limited capacity of BOOST programme-trained physiotherapists, participants putting treatment on hold due to other medical problems (e.g. fractured foot, operation, radiotherapy for prostate cancer), and participants being unavailable (e.g. on holiday, therefore entered a later group).
Baseline setting
Baseline setting of the four strength exercises (sit to stand, knee extension, hip abduction, hip extension) was determined using the modified Borg scale (scored 5–6/10: ‘the exercise feels hard’), using weights to achieve the required intensity if indicated.
The most common prescription at baseline was two sets of 10 repetitions for each exercise.
For the sit to stand exercise, 165/279 (59.1%) participants had weight prescribed. The most common starting weight was 4.5 kg.
For the knee extension exercise, 222/279 (79.1%) participants had weights added. The most common starting weight was 1.5 kg.
Just under three-quarters of participants (196/279; 71.0%) had weight added for both hip abduction and hip extension. The most common starting weight was 1.5 kg.
In a very small number of participants (4/279; 1.4%) one or more of the strengthening exercises was not prescribed. One participant was deemed unsuitable for the groups so was not prescribed any of the exercises. This participant was withdrawn from the intervention by their physiotherapist due to health problems. They were experiencing dizziness and ‘funny turns’ and referred back to their general practitioner.
In the other three participants, the following were not given: sit to stand, n = 2; knee extension, n = 1; hip abduction, n = 3 and hip extension, n = 1. Reasons for not prescribing the exercises included recent surgery and pain.
The most frequently prescribed baseline balance exercise was the full tandem stance (n = 115.0; 41.4%). This was followed by semi-tandem stance (n = 83.0; 29.9%), parallel stance (n = 25.0; 9.0%) and forward tandem walking (n = 47.0; 16.9%). See Appendix 3, Figure 10.
FIGURE 10.
Progressive balance exercises.
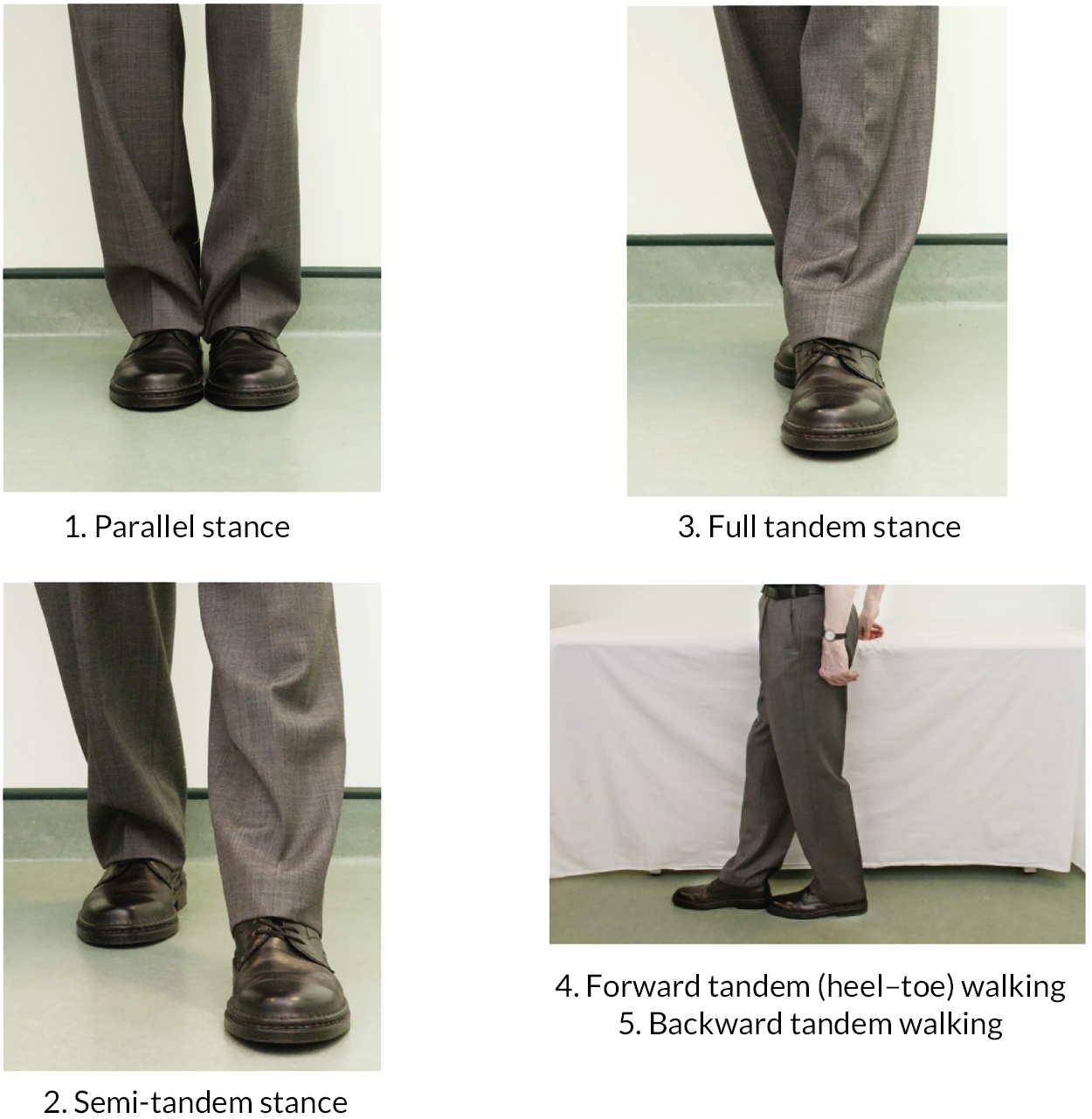
The flexibility exercise (hip flexor stretch) had a recommended default baseline setting of three 10-second holds per leg. All participants were given this at baseline.
Baseline walking levels for the programme were based on participant-rated ability and confidence and the physiotherapist’s observations during the appointment. Tailoring of the walking component of the programme included walking duration (including rest stops), use of obstacles, walking speed, carrying of hand weights, or a combination of these. The majority of participants (214/276; 77%) were prescribed 20 minutes’ walking only to start the group programme (see Appendix 3, Figure 16). Two participants had the baseline walking missing from their treatment log, and one was withdrawn from the programme.
At the baseline appointment, walking aids were prescribed if indicated. Nine participants (3.2%) were prescribed a walking aid at the baseline appointment. Six participants (2.2%) were prescribed with a standard walking stick, two participants (0.7%) were prescribed with an outdoor four-wheeled rollator frame, and one participant (0.3%) was prescribed with two anatomical-fit (Fischer-type) walking sticks.
Group sessions
After attending their individual assessment, participants joined the next available group. Overall, participants allocated to the BOOST programme waited a mean time of 27.1 days (SD 26.4 days, range 0–153 days) from their individual appointment to starting the group sessions. Longer wait times were related to study recruitment. Some sites found it difficult to recruit sufficient participants and delayed groups starting until they had enough participants (we aimed for six participants in each group). Other reasons included participants delaying entry to upcoming groups due to family commitments, medical appointments, site administration error (misplaced referral) and recent (unrelated) surgery.
Fifty-three groups were delivered across 14 sites. One site closed early after recruiting only two participants (both randomised to the BOOST programme) due to changes in clinical pathways for BP affecting recruitment. We were able to transfer the two participants to another nearby BOOST site for their intervention. Groups were a mean size of 5.3 (SD 1.8) participants. Attendance at the group sessions is shown in Appendix 3, Table 16. Compliance with the BOOST programme group was defined as attendance at group sessions of at least 9 out of the 12 sessions (75%), which was achieved by 69.52% of participants. The most common reasons for not attending a group session were holidays or sickness.
| Number of group sessions attended | Number of participants | % | Number of group sessions attended | Number of participants | % |
|---|---|---|---|---|---|
| Did not attend individual appointment | 13 | 4.5 | Six sessions | 11 | 3.8 |
| Seven sessions | 16 | 5.5 | |||
| No group sessions | 13 | 4.5 | Eight sessions | 20 | 6.8 |
| One session | 4 | 1.4 | Nine sessions | 37 | 12.7 |
| Two sessions | 2 | 0.7 | Ten sessions | 49 | 16.8 |
| Three sessions | 3 | 1.0 | Eleven sessions | 60 | 20.5 |
| Four sessions | 4 | 1.4 | Twelve sessions | 57 | 19.5 |
| Five sessions | 3 | 1.0 | Attended at least 9 sessions | 203 | 69.5 |
Exercise progression
Strengthening exercises
Over the duration of the group sessions (12 sessions over 12 weeks), participants’ exercises were progressed by manipulating sets, repetitions, and load. Physiotherapists also introduced power (perform the exercise faster) to some participants to make the exercises more challenging.
We calculated the dose completed at each session by multiplying sets × repetitions × load (kg). If the participant completed an exercise without load (e.g. two sets, 10 repetitions), an arbitrary load value of 0.1 kg was used. Where an exercise was performed on the right leg and then the left leg, if the load differed then we took the lowest number for this calculation.
This allowed us to plot the progressions over time. We have plotted the median dose (IQR represented by the line) in Appendix 3, Figures 11–14 for each of the strengthening exercises. The number of participants contributing data is displayed above each data point. We have also plotted participants classified as compliers (nine sessions or more) or non-compliers.
FIGURE 11.
Strengthening exercise: sit to stand prescription.
FIGURE 12.
Strengthening exercise: knee extension prescription.
FIGURE 13.
Strengthening exercise: hip abduction prescription.

FIGURE 14.
Strengthening exercise: hip extension prescription.
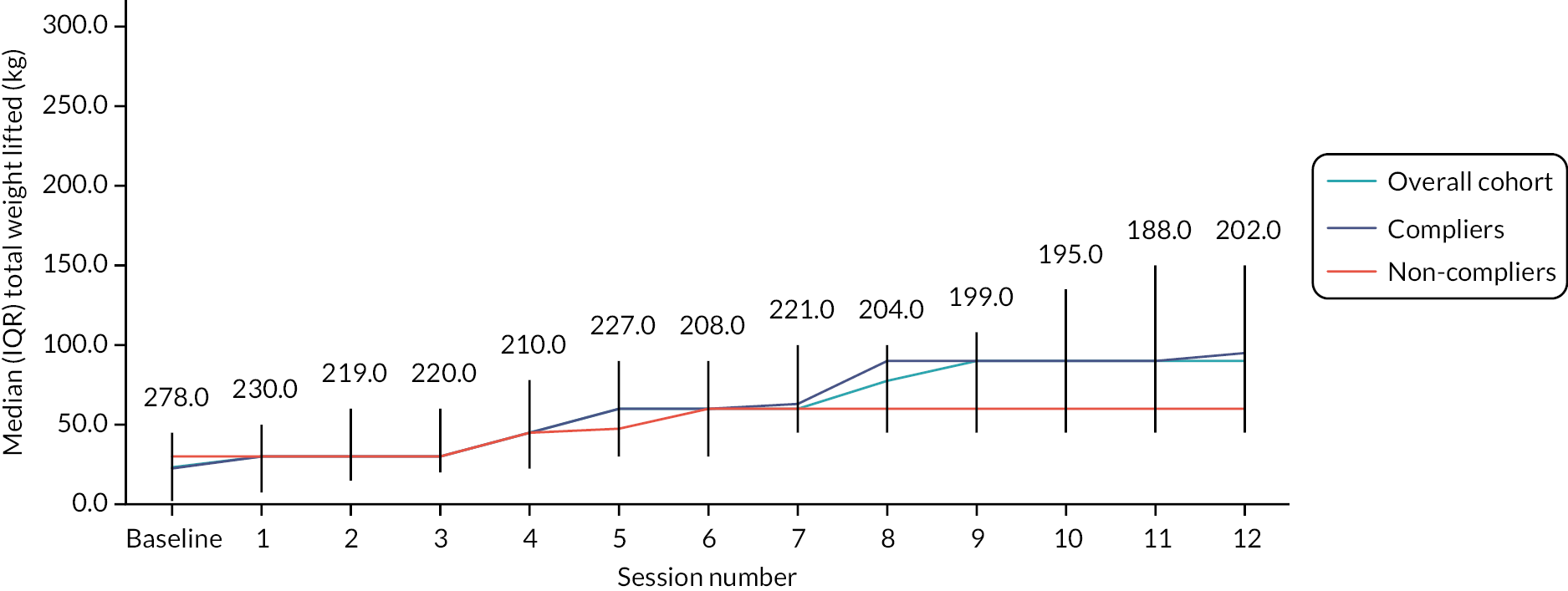
Overall, the BOOST programme participants were progressed across the 12 group sessions. Across the four exercises, compliers were prescribed a greater dose than non-compliers. Non-compliers still showed progression over time, but it was more variable than for those classified as compliers.
Speed (power) was also used to progress the exercises. Very few participants were asked to add speed to their movements at baseline; this tended to be added towards the later stages of the programme. At session 12, around a quarter of participants were adding speed to knee extension, hip abduction and hip extension exercises and one-fifth to sit to stand (see Appendix 3, Table 17).
| Exercise | Baseline | Session 1 | Session 2 | Session 3 | Session 4 | Session 5 | Session 6 |
|---|---|---|---|---|---|---|---|
| Exercise | Baseline | Session 7 | Session 8 | Session 9 | Session 10 | Session 11 | Session 12 |
| Sit to stand | 3.0 (1.1) | 3.0 (1.1) | 5.0 (1.8) | 10.0 (4.5) | 11.0 (5.3) | 23.0 (10.1) | 16.0 (7.7) |
| Knee extension | 6.0 (2.2) | 1.0 (0.4) | 4.0 (1.8) | 18.0 (8.2) | 12.0 (5.7) | 25.0 (11.0) | 16.0 (7.7) |
| Hip abduction | 3.0 (1.1) | 5.0 (2.2) | 6.0 (2.7) | 13.0 (5.9) | 13.0 (6.2) | 23.0 (10.1) | 9.0 (4.3) |
| Hip extension | 5.0 (1.8) | 2.0 (0.9) | 5.0 (2.3) | 13.0 (5.9) | 10.0 (4.8) | 22.0 (9.7) | 6.0 (2.9) |
| Sit to stand | 20.0 (7.2) | 16.0 (5.7) | 35 (17.7) | 25.0 (12.9) | 39.0 (20.7) | 40.0 (19.8) | |
| Knee extension | 21.0 (9.5) | 17.0 (8.3) | 35.0 (17.6) | 27.0 (13.8) | 47.0 (25.0) | 54.0 (26.7) | |
| Hip abduction | 15.0 (6.8) | 13.0 (6.4) | 28.0 (14.1) | 27.0 (9.7) | 37.0 (19.7) | 51.0 (25.4) | |
| Hip extension | 15.0 (6.8) | 13.0 (6.4) | 28.0 (14.1) | 23.0 (11.8) | 40.0 (21.2) | 52.0 (25.7) |
Balance and flexibility exercises
Over the 12 group sessions, the balance exercise was progressed with participants, with the majority of participants moving from static balance exercises to dynamic balance exercises (see Appendix 3, Figure 15).
FIGURE 15.
Balance exercise prescription.

Participants were also holding their stretches for longer towards the end of the 12 group sessions. At baseline, the median total stretch time was 30 seconds (IQR 30–30); at session 12, participants were holding the stretch for a median total of 90 seconds (IQR 60–90).
Walking programme
Over the 12 group sessions, participants progressed their walking during the supervised circuit and were undertaking a variety of added challenges by session 12 compared to baseline, where most were only walking (see Appendix 3, Figure 16).
FIGURE 16.
Walking programme prescription.
Walking aids prescribed in the group sessions
During the group sessions, seven participants (2.5%) were prescribed a walking aid (session 1: n = 3; session 8: n = 1; session 9: n = 1; session 11: n = 2). One participant (0.4%) who was prescribed a walking stick at their baseline appointment was also prescribed an outdoor three-wheeled rollator frame during the group sessions to use. Of the remaining six participants, three (1.1%) were prescribed standard walking sticks, one (0.3%) with an anatomical-fit (Fischer-type) walking stick and two (0.7%) with rollator frames (indoor use: n = 1; outdoor use: n = 1). One participant (0.3%) started to use their mother-in-law’s rollator frame from session 11 onwards.
Non-completion of exercises
There were occasions when participants attended a group but did not complete some or all of the exercises during the group session (see Appendix 3, Table 18). The most common reason for non-completion overall was participants staying only for the education component or having to leave early due to another commitment. We delivered 53 groups over a total of 636 sessions, so the proportion of sessions where participants did not complete exercises was very small.
| Sit to stand | Knee ext | Hip abduction | Hip extension | Balance | Stretch | Walking circuit | |
|---|---|---|---|---|---|---|---|
| Reason missing | 4 (5) | 3 (4) | 5 (7) | 2 (2) | 4 (4) | 8 (9) | 12 (12) |
| Attended education session only or needed to leave early – had another appointment or commitment | 5 (8) | 5 (8) | 7 (10) | 8 (11) | 6 (9) | 6 (9) | 14 (17) |
| Attended education session only – recent cataract surgery | 1 (4) | 1 (4) | 1 (4) | 1 (4) | 1 (4) | 1(4) | 1 (4) |
| Discomfort from recent unrelated surgery/procedure | 1 (1) | 2(2) | |||||
| Attended education session only due to feeling unwell | 4 (5) | 4 (5) | 4 (5) | 4 (5) | 4 (5) | 4 (5) | 4 (5) |
| Pain | 2 (2) | 2 (2) | 5 (6) | 4 (4) | 2 (2) | 1 (1) | 2(2) |
| Tired | 1 (1) | 1 (1) | 1 (1) | 1 (1) | 2 (2) | 2 (2) | |
| Heart condition and shortness of breath | 1(1) | ||||||
| Planned to do it at home | 1 (1) | ||||||
| Could not manage it | 1 (1) | ||||||
| Arrived late so did not do it | 2 (2) | ||||||
| Dizzy | 1 (1) | ||||||
| Had walked before class or planning to walk later | 5 (8) | ||||||
| Too hot | 4 (4) | ||||||
| Total | 18 (26) | 16 (24) | 23 (33) | 21 (28) | 20 (27) | 49 (59) |
The sit to stand exercise was not completed by 18 participants on a total of 26 occasions. Knee extension was not completed by 16 participants on a total of 24 occasions. Hip abduction was not completed by 23 participants on a total of 33 occasions. Pain was not commonly reported as a reason for non-completion, but it was reported more often for hip abduction than for the other exercises. Hip extension was not completed by 21 participants on a total of 28 occasions. The balance exercises were not completed by 20 participants on a total of 27 occasions.
The walking circuit was not completed most frequently out of the exercises. It was not done by 49 participants on a total of 59 occasions. The most common reason was participants staying only for the education component or having to leave early due to another commitment. This probably affected the walking circuit the most as it was the final part of the programme. Some participants also preferred to do their walking before or after the groups (e.g. to walk their dog).
Appendix 4 More information about the Better Outcomes for Older people with Spinal Trouble interview study
| Demographics | BOOST trial cohort | Interview cohort | Interview analysis cohort |
|---|---|---|---|
| n (%) Mean (SD) |
n (%) Mean (SD) |
n (%) Mean (SD) |
|
| Total participants | 435 | 59 | 30a |
| Age (years at baseline) | 74.9 (6) | 75.1 (6.2) | 74.8 (5.6) |
| Gender | |||
| Female | 246 (56.6) | 32 (53) | 14 (47) |
| Male | 189 (43.4) | 27 (45) | 16 (53) |
| Ethnicity | |||
| White British/White Other | 400 (92) | 52 (87) | 25 (86) |
| Black British | 12 (2.8) | 3 (5) | 2 (7) |
| Indian | 9 (2.1) | 3 (5) | 2 (7) |
| Pakistani | 6 (1.4) | 1 (2) | 0 (0) |
| Treatment allocationa | |||
| BOOST programme | 292 (67.1) | 32 (53) | 16 (53.3) |
| BPA | 143 (32.9) | 27 (45) | 14 (46.7) |
| Complete sets of three interviews | 49 | 30 | |
| Themes | Participant quotes |
|---|---|
| Psychological impact | I’ve got that pain, varying degrees, all the time really. (05, T1) ‘It does depress you. And when I went out I used to feel like dressing up and took pride in that, and I think, well, what’s the use, sometimes I think.’ (24, T1) ‘… I’mjust existing aren’t I, you know? Everything I do I’m worrying about, which I don’t, I’m not a worrying person, never have been …’ (31, T1) |
| Activity limitations | ‘it’s affected me in everyday life sort of thing. Where I used to be able to do all my cleaning and shopping and everything all in a day, where now I have to do a bit of one room each day, and ironing one day, washing another day.’ (32, T1) |
| Restricted participation | ‘I used to belong to a walking group which was a social outlet but, so I’ve lost that.’ (03, T1) |
| Adopting coping strategies | Using online delivery: ‘I can’t be carrying milk and all the bulky things, your loo rolls and all that, I can’t cope with that. And Sainsbury’s deliver.’ (34, T1) |
| Intervention component | Participant quotes |
|---|---|
| Increased engagement and enjoyment | ‘I have really enjoyed going and seeing others … It certainly helped me and encouraged me, apart from the exercises it encouraged me to do it. I feel, yes it has done me good.’ (25, T3) |
| Opportunity for discussion | ‘Most certainly the discussions really helped.’ (43, T2). ‘And the way it went, the first part of the day was that we’d go and discuss things in the office, in the room, and then go out and then start on the exercises. And I think that was good, because we bounced off each other what we were doing, and what we were feeling, and I think we put it over to the two girls that were doing the physio at the time.’ (30, T2) |
| Peer support and interaction | ‘Actually in a way it was good to be with a group because ... There was one lady, she must have been eighties or over, she carried on really, put her mind to it … she is a bit slow walking … but she did it … there was one man and he was determined to be top of the class and he really worked hard and walking very faster ... so talking to other people is good as well to see what they were going through.’ (39, T2) |
| Group problem solving and support | ‘Because we had turns of talking of how we got through the week and what we’d been doing. So you learn things from other people … if you’ve got lots of people, six people there, they will discuss things between us and find easier ways to get round things.’ (14, T2) ‘Not so much the exercise because I knew how I had to do, but I think just being a part of something. Like having a team like that and they’re all for you, there to help you, to explain things to you, and knowing how to help yourself, if you like, and see how different we all are … I think that’s what I really do like, is the, being with other people and talking about the subject. I think that helped.’ (14, T2) |
| Self-management skills | ‘But the BOOST has explained things to me, has made me realise that I’ve got to do the exercises to keep supple. And by doing that I know what I’m in for and try to keep it up.’ (14, T2) ‘… as I said, [physiotherapist], they give us all that information for when we get flare-ups and things like that you know and what to do. So all that was really helpful.’ (04, T2) |
| Experiences of independent exercise | Participant quotes |
|---|---|
| A regular routine | ‘And then usually what I do is, when I go out on my walks, I get to a certain spot … and then I do my exercises in the fresh air … If I can’t do it through the day on my walk, I do it in the kitchen before I go to bed at night time’ (14, T2) ‘A couple of times a week, which is what I was told. As long as I kept it to a couple of times a week.’ (04, T2) |
| Seeing the benefits | ‘I do all the, mainly the warming up exercises, while I’m sitting down of an evening at the box and that. I get behind the settee and do my movements there, or when I’m sitting down do my leg movements, lifting and that, and rising and sitting down from the chair. So I try to keep them in like that, and I think that’s what keeps me a little bit fitter.’ (30, T3) |
| Fitting them into everyday life | ‘This is why you can build them [exercises] into your everyday … you don’t have to go anywhere special to do it. You don’t have to go out and buy all this equipment, you don’t need this equipment. Use your everyday things you’ve got there. As I say, like I use the edge of my sink to lean on, so why need a bar at the gym for that? Why do I need a bench to sit on when I can sit in an armchair?’ (24, T3) |
| Making adaptations allowed them to continue | ‘I do all of them, yes. I haven’t touched the weights for a while. Again, because that’s the extra, I know what that’ll do.’ (04, T3) ‘I think if I didn’t have that knee problem I could have done more exercises. I’m still doing the exercises with the knee, sort of your legs out the side, and the back ones. But obviously I can’t do the bending of the knee or that.’ (14, T3) |
| No need for the BOOST exercises – active enough | Well, no, they gone to the board actually, the actual exercises at home … With the swimming and gym and walking with the dog and walking around shops and theatre, I don’t think I need the BOOST exercises’. (57, T3) |
Appendix 5 More information about the Oxford Pain Activity and Lifestyle risk assessment tool
| Potential risk factors | Mobility decline at year 2 | ||
|---|---|---|---|
| No (n = 3350) | Yes (n = 765) | p-valuea | |
| Demographics | |||
| Age, mean (SD) | 73.8 (6.1) | 76.1 (6.8) | < 0.001 |
| Sex: Male (ref.) | 1637 (48.9%) | 367 (48.0%) | – |
| Female | 1713 (51.1%) | 398 (52.0%) | 0.656 |
| Living arrangements: Living alone | 870 (26.0%) | 249 (32.6%) | < 0.001 |
| Education: Higher education (ref.) | 1335 (39.9%) | 272 (35.6%) | – |
| Secondary education | 1820 (54.3%) | 427 (55.8%) | 0.099 |
| None or primary | 181 (5.4%) | 62 (8.1%) | 0.001 |
| Socioeconomic | |||
| Receive enough support (No) | 277 (8.3%) | 69 (9.0%) | 0.549 |
| Miss other people around: No (ref.) | 2230 (66.6%) | 454 (59.4%) | – |
| Sometimes | 607 (18.1%) | 165 (21.6%) | 0.005 |
| Yes | 493 (14.7%) | 140 (18.3%) | 0.002 |
| Number of organisations/clubs/societies, median (IQR) | 1 (0–2) | 1 (0–2) | 0.017 |
| Adequacy of income | |||
| Quite comfortably off (ref.) | 1289 (38.5%) | 224 (29.3%) | – |
| Able to manage without much difficulty | 1237 (36.9%) | 284 (37.1%) | 0.004 |
| To be careful with money | 607 (18.1%) | 208 (27.2%) | < 0.001 |
| Occupational physical demands | |||
| Very light (ref.) | 341 (10.2%) | 46 (6.0%) | – |
| Light | 655 (19.6%) | 146 (19.1%) | 0.006 |
| Moderate | 1573 (47.0%) | 347 (45.4%) | 0.003 |
| Strenuous | 578 (17.3%) | 165 (21.6%) | < 0.001 |
| Very strenuous | 183 (5.5%) | 59 (7.7%) | < 0.001 |
| Pain-related factors | |||
| BP and leg symptoms | |||
| No BP (ref.) | 1643 (49.0%) | 316 (41.3%) | – |
| BP without leg symptoms | 1135 (33.9%) | 265 (34.6%) | 0.035 |
| BP with leg symptoms | 536 (16.0%) | 163 (21.3%) | < 0.001 |
| Pain distribution: No pain (ref.) | 582 (17.4%) | 76 (9.9%) | – |
| One site pain | 858 (25.6%) | 165 (21.6%) | 0.009 |
| Multisite pain | 1123 (33.5%) | 288 (37.7%) | < 0.001 |
| Widespread pain | 787 (23.5%) | 236 (30.9%) | < 0.001 |
| Lower limb pain (Yes) | 1877 (56.0%) | 522 (68.2%) | < 0.001 |
| Severity of pain/discomfort, median (IQR) | 2 (1–2) | 2 (2-3) | < 0.001 |
| Mobility-related factors | |||
| Mobility problems: No problems (ref.) | 2178 (65.0%) | 468 (61.2%) | – |
| I have slight problems | 619 (18.5%) | 219 (28.6%) | < 0.001 |
| I have moderate problems | 378 (11.3%) | 75 (9.8%) | 0.559 |
| I have severe problems | 175 (5.2%) | 3 (0.4%) | < 0.001 |
| Usual walking pace: Fast/fairly brisk (ref.) | 1006 (30.0%) | 84 (11.0%) | – |
| Normal | 1306 (39.0%) | 296 (38.7%) | < 0.001 |
| Stroll at any easy pace | 702 (21.0%) | 282 (36.9%) | < 0.001 |
| Very slow | 322 (9.6%) | 102 (13.3%) | < 0.001 |
| Difficulties maintaining balance (Yes) | 393 (11.7%) | 144 (18.8%) | < 0.001 |
| Confidence to walk, median (IQR) | 1 (1–1) | 1 (1–4) | < 0.001 |
| Use of walking aid inside: No (ref.) | 3131 (93.5%) | 709 (92.7%) | – |
| Sometimes | 93 (2.8%) | 32 (4.2%) | 0.045 |
| Yes | 106 (3.2%) | 24 (3.1%) | 1.000 |
| Use of walking aid outside: No (ref.) | 2778 (82.9%) | 574 (75.0%) | – |
| Sometimes | 221 (6.6%) | 88 (11.5%) | < 0.001 |
| Yes | 333 (9.9%) | 100 (13.1%) | 0.002 |
| Change in walking ability compared to last year | |||
| Better (ref.) | 225 (6.7%) | 55 (7.2%) | – |
| About the same | 2429 (72.5%) | 473 (61.8%) | 0.152 |
| Worse | 681 (20.3%) | 237 (31.0%) | 0.036 |
| Falls | |||
| Falls in the last year: None (ref.) | 2434 (72.7%) | 519 (67.8%) | – |
| Once | 654 (19.5%) | 175 (22.9%) | 0.020 |
| More than one | 245 (7.3%) | 70 (9.2%) | 0.042 |
| Fractures last year (Yes) | 88 (2.6%) | 19 (2.5%) | 0.814 |
| Physical activity | |||
| Hours/day moving around | |||
| 7 or more (ref.) | 828 (24.7%) | 131 (17.1%) | – |
| 5–7 | 904 (27.0%) | 228 (29.8%) | < 0.001 |
| 3–5 | 1022 (30.5%) | 242 (31.6%) | 0.001 |
| < 3 | 577 (17.2%) | 162 (21.2%) | < 0.001 |
| General health | |||
| BMI, mean (SD) | 26.4 (4.7) | 27.4 (5.0) | < 0.001 |
| Number of health conditions, median (IQR) | 1 (0–2) | 2 (1–2) | < 0.001 |
| Physical tiredness (Yes) | 770 (23.0%) | 259 (33.9%) | < 0.001 |
| Anxiety/depression, median (IQR) | 1 (1–2) | 1 (1–2) | 0.002 |
| Poor hearing (Yes) | 710 (21.2%) | 220 (28.8%) | < 0.001 |
| Poor vision (Yes) | 338 (10.1%) | 114 (14.9%) | < 0.001 |
| Problems in daily life due to lack of strength in hands (Yes) | 628 (18.8%) | 204 (26.7%) | < 0.001 |
| Loss of weight (Yes) | 87 (2.6%) | 33 (4.3%) | 0.011 |
| Self-reported general health, mean (SD) | 81.2 (15.6) | 76.6 (15.6) | < 0.001 |
| Number of health conditions, median (IQR) | 1 (0–2) | 2 (1–2) | < 0.001 |
| Physical tiredness (Yes) | 770 (23.0%) | 259 (33.9%) | < 0.001 |
| Anxiety/depression, median (IQR) | 1 (1–2) | 1 (1–2) | 0.002 |
| Poor hearing (Yes) | 710 (21.2%) | 220 (28.8%) | < 0.001 |
| Poor vision (Yes) | 338 (10.1%) | 114 (14.9%) | < 0.001 |
| Problems in daily life due to lack of strength in hands (Yes) | 628 (18.8%) | 204 (26.7%) | < 0.001 |
| Loss of weight (Yes) | 87 (2.6%) | 33 (4.3%) | 0.011 |
| Self-reported general health, mean (SD) | 81.2 (15.6) | 76.6 (15.6) | < 0.001 |
| Baseline predictor | βi | Reference value (Wij) | βi(Wij− WiREF) | Pointij= βi(Wij− WiREF)/Ba |
|---|---|---|---|---|
| Intercept | −6.757 | |||
| Age, years | 0.0353 | |||
| < 75 | 69.5 (W1REF) | 0 | 0 | |
| 75–84 | 79.5 | 0.353 | 2 | |
| ≥ 85 | 92.5 | 0.812 | 5 | |
| To be careful with money | 0.239 | |||
| No | 0 (W2REF) | 0 | 0 | |
| Yes | 1 | 1.962985 | 1 | |
| BMI (kg/m2) | 0.025 | |||
| < 25 – Normal weight | 21.5 (W3REF) | 0 | 0 | |
| 25–30 – Overweight | 27.5 | 0.151 | 1 | |
| ≥ 30 – Obese | 35.5 | 0.351 | 2 | |
| Usual walking pace – Stroll at any easy pace | 0.494 | |||
| No | 0 (W4REF) | 0 | 0 | |
| Yes | 1 | 0.494 | 3 | |
| Usual walking pace – Slow | 0.573 | |||
| No | 0 (W5REF) | 0 | 0 | |
| Yes | 1 | 0.573 | 3 | |
| Difficulties maintaining balance | 0.166 | |||
| No | 0 (W6REF) | 0 | 0 | |
| Yes | 1 | 0.166 | 1 | |
| Confidence to walk | 0.056 | |||
| 1–2 (high confidence) | 1.5 (W7REF) | 0 | 0 | |
| 3–5 | 4 | 0.139 | 1 | |
| 6–8 | 7 | 0.306 | 2 | |
| 9–10 (low confidence) | 9.5 | 0.446 | 3 | |
| Use sometimes walking aid outside | 0.158 | |||
| No | 0 (W8REF) | 0 | 0 | |
| Yes | 1 | 0.158 | 1 | |
| No change in walking ability compared to last year | −0.087 | |||
| No | 0 (W9REF) | 0 | 0 | |
| Yes | 1 | −0.087 | 0 | |
| Worse walking ability than last year | 0.141 | |||
| No | 0 (W10REF) | 0 | 0 | |
| Yes | 1 | 0.141 | 1 | |
| Lower limb pain in the last 6 weeks | 0.226 | |||
| No | 0 (W11REF) | 0 | 0 | |
| Yes | 1 | 0.226 | 1 | |
| Current pain/discomfort severity | 0.070 | |||
| 1 (no pain) | 1 (W12REF) | 0 | 0 | |
| 2–3 (slight/moderate pain) | 2.5 | 0.105 | 1 | |
| 4–5 (severe/extreme pain) | 4.5 | 0.245 | 1 | |
| Number of health conditions | 0.117 | |||
| 0–1 | 0.5 (W13REF) | 0 | 0 | |
| 1–2 | 1.5 | 0.176 | 1 | |
| 3–4 | 3.5 | 0.410 | 2 | |
| 5–7 | 6 | 0.702 | 4 | |
| Physical tiredness | 0.092 | |||
| No | 0 (W14REF) | 0 | 0 | |
| Yes | 1 | 0.092 | 1 | |
| Self-reported general health | −0.011 | |||
| < 71 (poor general health) | 50 | 0.520 | 3 | |
| 72–80 | 76 | 0.223 | 1 | |
| 81–90 | 85.5 | 0.114 | 1 | |
| 91–100 (good general health) | 95.5 (W15REF) | 0 | 0 | |
| Slight mobility problems | 1.963 | |||
| No | 0 (W16REF) | 0 | 0 | |
| Yes | 1 | 1.963 | 11 | |
| No mobility problems | 2.387 | |||
| No | 0 (W17REF) | 0 | 0 | |
| Yes | 1 | 2.387 | 13 |
| Point total | Risk estimate | Point total | Risk estimate | Point total | Risk estimate |
|---|---|---|---|---|---|
| 0 | 0.00956 | 14 | 0.10313 | 28 | 0.57813 |
| 1 | 0.01139 | 15 | 0.12069 | 29 | 0.62060 |
| 2 | 0.01356 | 16 | 0.14077 | 30 | 0.66130 |
| 3 | 0.01614 | 17 | 0.16357 | 31 | 0.69975 |
| 4 | 0.01921 | 18 | 0.18925 | 32 | 0.73558 |
| 5 | 0.02285 | 19 | 0.21791 | 33 | 0.76854 |
| 6 | 0.02715 | 20 | 0.24957 | 34 | 0.79853 |
| 7 | 0.03224 | 21 | 0.28416 | 35 | 0.82551 |
| 8 | 0.03824 | 22 | 0.32150 | 36 | 0.84955 |
| 9 | 0.04531 | 23 | 0.36126 | 37 | 0.87081 |
| 10 | 0.05361 | 24 | 0.40302 | 38 | 0.88945 |
| 11 | 0.06333 | 25 | 0.44623 | 39 | 0.90569 |
| 12 | 0.07468 | 26 | 0.49028 | ||
| 13 | 0.08787 | 27 | 0.53447 |
| Baseline predictor | Mobility decline at year 2 (Model 1) | |
|---|---|---|
| Value | Point totals | |
| Age | 65 | 0 |
| Inadequacy of income | Yes | 1 |
| BMI (kg/m2) | 32 | 2 |
| Usual walking pace | Normal | 0 |
| Difficulties maintaining balance | No | 0 |
| Confidence to walk (1–10 less confident) | 1 | 0 |
| Use sometimes walking aid outside | No | 0 |
| Walking ability compared with last year | The same | 0 |
| Lower limb pain | Yes | 1 |
| Pain/discomfort severity | 3 | 1 |
| Number of health conditions | 1 | 1 |
| Physical tiredness | Yes | 1 |
| Self-reported general health | 80 | 1 |
| Problems with mobility | No problem | 13 |
| Point total | 21 | |
| Estimate of risk | 0.284 (28.4%) | |
| Selected predictors at baseline | LASSO regression (% of times selected) |
Average penalised coefficient |
|---|---|---|
| Age | 100% | 0.050 |
| Sex | 80% | −0.036 |
| Adequacy of income: careful with money | 100% | 0.228 |
| Occupation physical demands: very strenuous | 100% | 0.189 |
| Moving around < 3 hours/day | 88% | 0.063 |
| BMI | 100% | 0.015 |
| Usual walking pace | ||
| Stroll at an easy pace | 100% | 0.564 |
| Very slow | 100% | 0.780 |
| Difficulties maintaining balance | 100% | 0.165 |
| Confidence walking | 100% | 0.062 |
| Change in walking ability compared to last year: worse | 100% | 0.208 |
| Fractures last year | 94% | –0.197 |
| BP and leg symptoms | 82% | 0.057 |
| Lower limb pain | 100% | 0.150 |
| Number of health conditions | 100% | 0.123 |
| Physical tiredness | 94% | 0.062 |
| Unintentional weight loss | 92% | 0.280 |
| Self-reported general health | 100% | –0.013 |
| Predictors forced to be included in the final model | ||
| Mobility problems at baseline | ||
| I have slight problems | 100% | 1.556 |
| I have no problems | 100% | 1.972 |
| Area under the curve (range) | 0.746 (0.744–0.749) | |
| Brier score (range) | 0.151 (0.150–0.152) | |
List of abbreviations
- 6MWT
- 6-minute walk test
- BMI
- body mass index
- BOOST
- Better Outcomes for Older people with Spinal Trouble (study)
- BP
- back pain
- BPA
- best practice advice
- CACE
- complier-average causal effect
- CI
- confidence interval
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- IMD
- Index of Multiple Deprivation
- INMB
- incremental net monetary benefit
- IQR
- interquartile range
- ITT
- intention to treat
- LASSO
- least absolute shrinkage and selection operator
- MOOC
- Massive Open Online Course
- MRI
- magnetic resonance imaging
- NC
- neurogenic claudication
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- ODI
- Oswestry Disability Index
- OPAL
- Oxford Pain Activity and Lifestyle (study)
- PPI
- patient and public involvement
- PSS
- personal social services
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SD
- standard deviation
- SPPB
- Short Physical Performance Battery
- SSSS
- Swiss Spinal Stenosis Scale
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/LKWX3424).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.