Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0606-1170. The contractual start date was in August 2007. The final report began editorial review in May 2013 and was accepted for publication in January 2014. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Rosalind L Smyth has been a member of the Commission on Human Medicines and Chairperson of the Paediatric Medicines Advisory Group of the Medicines and Healthcare products Regulatory Agency (MHRA) until December 2013. Munir Pirmohamed is currently Chairperson of the Pharmacovigilance Expert Advisory Group for the MHRA and is a Commissioner on Human Medicines. Anthony J Nunn is a member of MHRA Paediatric Medicines Expert Advisory Group, a member of the European Medicines Agency Paediatric Committee (PDCO) and a temporary advisor to the World Health Organization.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Smyth et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Drug safety is an important issue in all disciplines. The World Health Organization’s (WHO) stated definition of an adverse drug reaction (ADR) is ‘A response to a drug which is noxious, and unintended, and which occurs at doses normally used in man for prophylaxis, diagnosis or therapy of disease, or for the modification of physiological function’. 1
The problem of drug safety in paediatrics is compounded by the fact that medicines are often not tested in children, and therefore at the time of licensing there is no indication for use in children. For example, in 2006 around 75% of all 317 centrally licensed medicines were relevant for children but only half (34%) had a paediatric indication. 2 This leads to off-label and/or unlicensed (OLUL) prescribing: this has been estimated to occur in 25%3 of paediatric inpatient prescriptions and 65% of neonatal prescriptions. 4 It is clear that extrapolation of efficacy, dosing regimens and ADRs from adult data are inappropriate owing to developmental changes in physiology and drug handling. 5 Taken together with the fact that the pattern of diseases in children is different from that in adults, this puts them at high risk of serious and unpredictable ADRs from the use of medicines. As with any population, distinguishing between an ADR and non-drug-induced pathology is difficult but is further compounded in this age group. Important examples of ADRs in children include deaths associated with propofol used for paediatric intensive care unit (PICU) sedation,6 acute adrenal crisis associated with inhaled corticosteroids,7 grey baby syndrome in neonates associated with chloramphenicol,8 the threefold increase in Stevens–Johnson syndrome with lamotrigine9 and colonic strictures in cystic fibrosis owing to high-strength pancreatic enzymes. 10 The last of these examples was first reported at Alder Hey Children’s NHS Foundation Trust (Alder Hey) in Liverpool; the primary author led the study confirming the association with pancreatic enzyme therapy and because of this, and subsequent regulatory measures, this problem is no longer reported in the UK. A trend towards more ADRs when OLUL medicines are prescribed in children and young people was first reported from this research group. 11 However, the recognition of serious new ADRs generally depends on a cluster of individuals presenting with a similar pattern of unexplained clinical features and there is no systematic, pre-emptive approach to this problem in children, largely because of the dearth of good-quality scientific evidence on this topic. The burden of ADRs in children has not been systematically assessed and the knowledge base of the impact of ADRs in children on morbidity, overall health economy and societal consequences is poorly understood. In addition, methods to detect and assess causality and avoidability of suspected ADRs have not been validated in children. Evidence suggests that patients are generally poorly informed about medicines and the systems to ensure drug safety. 12 There is a need to consider communication about ADRs as an integral component of medicine adherence as well as an important transaction in its own right. Particular concerns surround children’s medicines; although health-care professionals have access to mechanisms to report and manage suspected ADRs, little is known about the understanding and experiences of families who experience a suspected ADR. Given the lack of robust evidence in the broad spectrum of ADR burden and characterisation in the population of children and young people, and the lack of knowledge and understanding of the interactions between health-care professionals and families whose child experiences a suspected ADR, Adverse Drug Reactions In Children (ADRIC) was designed to address this knowledge gap.
The regulatory and policy perspective of pharmacovigilance in children and young people
The Department of Health recognised the importance of the development of medicines and drug safety science in children by establishing the National Institute for Health Research (NIHR) Medicines for Children Research Network (MCRN) in 2006. Assessment of the harms of medicines is as important as assessment of their benefits and is integral to the proposals within the European regulation on medicines for children13 and the guidance on pharmacovigilance in children. In July 2012, new pharmacovigilance legislation came into effect across the European Union (EU),14 including centralised reporting by industry of ADRs to the EudraVigilance database at the European Medicines Agency (EMA) and the inclusion of reports from patients as valid, reportable ADRs. The Children and Young People’s Outcomes Strategy (commissioned by the Secretary of State for Health) identified the need to optimise the safe use of medicines. 15 In 2008, there were 33,000 safety incidents in children reported to the National Reporting and Learning System by health-care professionals, and of these 19% were for a medication problem. This led to a recommendation report that ‘the Medicines and Healthcare Products Regulatory Agency (MHRA), with immediate effect, prioritises pharmacovigilance of children’s medicines, including medication errors and off-label use, in line with the new EU legislation effective in July 2012’. 16
The MHRA is responsible for monitoring the safety of medicines in the UK. 17 The collection and analysis of reports of ADRs is critical to the MHRA’s responsibility to monitor the safety of medicines in practice: this is achieved through the submission of spontaneous reports of suspected ADRs by health-care professionals and the public through the Yellow Card Scheme. 18 The Yellow Card Scheme is designed to detect signals that may indicate a potential hazard with a medicine, leading to further investigations that may result in withdrawal of the medicine or changes in prescribing recommendations and restrictions in its use. Signal detection from spontaneous reports of ADRs and subsequent guidance in risk–benefit decisions is highly dependent on the availability of reliable instruments to assess ADR causality, which presents difficulties in paediatric pharmacovigilance. There is considerable variation in reporting of ADRs by practitioners and the potential for under-reporting and, partly in response to these concerns, the Yellow Card Scheme was extended to patients and families in 2005. The detail of individual ADR reports from patients is generally superior to those of health-care professionals, contributing to the EU pharmacovigilance legislation. 16 There is a clear statement through legislation, regulation and policy recommendations that the framework for pharmacovigilance in children is suboptimal and that a broader understanding of the assessment and improvement in the systems and quality of reporting of ADRs in this age group is urgently needed.
Burden of adverse drug reactions in children and young people
Given the stated importance of medicines for children by the Department of Health and the EMA, as well as other international authorities, it is important that we perform robust studies to fill knowledge gaps in the burden of ADRs in children and young people. Drugs are the mainstay of treatment in paediatric practice, yet a high proportion of drugs have not been tested in children. This leads to OLUL prescribing, the use of inappropriate doses, the use of age-inappropriate formulations, which may result in underdosing or overdosing, and drug development without due regard for the processes that are vital for normal development of a child into an adult. There are data showing that the current practice of drug development and drug use in paediatrics leads to avoidable adverse effects, which lead to morbidity and mortality. There is a need to identify the burden of ADRs in children; this has been emphasised by recent documents from the EMA and the US Food and Drug Administration (FDA), and is one of the important aims of the MCRN.
A number of studies have been performed in children to determine the incidence of ADRs; however, there are deficiencies in the evidence available at present. There is a lack of reliable and contemporary data estimating how frequently ADRs are causing admissions, how frequently they occur in general paediatric wards, how frequently they are life-threatening or cause death, and how often they could have been avoidable by better prescribing, better information in summary of product characteristics (SmPC) or better monitoring. Additionally, we do not have the tools to prevent these reactions. A number of studies have attempted to estimate the incidence of ADRs in children and have reported data on ADR rates causing admission to hospital, within inpatients and in the outpatient setting. The summary data confirmed that ADRs in children are a considerable burden. However, studies to date have varied considerably in their methodological rigour,19 including the definition of an ADR used, the age range of the study population and the clinical settings for data collection. Similarly, systematic reviews and meta-analyses of studies of ADRs in children also demonstrate methodological limitations, including the source bibliographical databases, definitions of ADRs to include adverse events (AEs) and exclusion of paediatric data from studies that included both adults and children. For example, a previous systematic review pointed out that there was substantial heterogeneity in the incidence estimates in the different studies reflecting differing, and often inadequate, methodologies that were used. Often the severity of the ADR was not reported, and patient age, diagnosis and drug prescription patterns were often not reported, and thus could not be considered in determining factors associated with ADRs in children. The need to conduct rigorous, prospective studies of ADRs in children, both which cause hospital admission20,21 and within the inpatient setting,22 was clearly needed. In addition, a methodologically rigorous systematic review incorporating the findings of these novel prospective studies was required. 19
Assessment of causality and avoidability of adverse drug reactions in children and young people
In addition to the overall burden of ADRs, the characterisation of individual ADRs provides essential information in the context of drug safety science: two important factors are assessment of causality and avoidability. Causality assessment estimates the strength of the relationship between drug exposure and the occurrence of an ADR. Assessment of ADR avoidability, or preventability, does not have a universally accepted definition, but there are two conventionally recognised principles: whether in the absence of error an event is preventable, and, if so, whether the event can be prevented. The concepts of causality and avoidability are relevant to health-care professionals, regulators, the pharmaceutical sector and the academic community, although the context may vary among these constituencies. Regardless of the reason or motivation to undertake causality or avoidability assessments, the availability of reliable and valid instruments to generate meaningful data is essential. Given the difficulties in distinguishing between ADRs and non-drug-induced pathology in children, these aspects are of particular importance in this population.
The Naranjo ADR probability scale23 is most widely used and reported causality assessment tool (CAT). This instrument contains 10 weighted items that generate a construct to produce a total score resulting in categorisation of the event as either unlikely, possible, probable or definite. Each item is based on concepts including temporal relationships, biological plausibility and rechallenge/previous exposure. The instrument was developed by adult physicians and psychiatrists using published case reports to validate instrument reliability. The validity and reliability of the Naranjo tool has been subject to challenge and, in addition, the use of the instrument to assess causality of ADRs in children is questionable given that individual items were developed and validated using adult case reports. 24
There is currently no standardised method for determining ADR avoidability and many of the established tools are not suitable for use in paediatric practice. A number of instruments have been developed for assessment of ADR avoidability and a systematic review found that several definitions exist for the preventability of drug-related harm as a consequence of the variability in methodological approaches to assessment of avoidability, and none fits all circumstances. 25 The authors of the systematic review proposed an approach to preventability, based on analysis of the mechanisms of ADRs and their clinical manifestations. Some authors have proposed a methodological framework for future studies of ADR avoidability and the development of valid instruments. 26 This includes reliability and validity testing, standardisation of the measurement processes, description of assessor training and experience in assessing preventability, details of independent or consensus assessments and rationalisation of assessor disagreement. These authors recommend that there is a need to modify existing instruments or develop novel instruments for use in different settings and populations.
The available instruments for assessment of causality and avoidability of ADRs vary in reliability and validity. 19 In particular, the assessment of avoidability is compromised by a lack of consensus on the definition of avoidability and associated heterogeneity in underpinning methodology for instrument development. No instruments are available specifically for characterisation of ADRs in children and young people, and there is a requirement to develop such instruments.
Communication about drug safety in children and young people
Previous literature on communication concerning medicines highlights the benefits of open discussion between health-care professionals and patients, at the time of prescribing, on their potential risks and the importance of supplementary written information in conveying key messages about drug safety. 12,27 Despite the movement towards patient reporting of ADRs and changes in EU legislation on pharmacovigilance in children,16 patients and their families are generally poorly informed about ADRs and pharmacovigilance systems to optimise safety of medicines. Other than in the context of understanding parental beliefs and attitudes to childhood vaccination, very little is known about the experiences of parents whose child has experienced a suspected ADR. 28 As a consequence, health-care professionals have no approriate evidence base from which to inform either generic or individualised communication strategies with the family when a child experiences a suspected ADR. In addition, there is little knowledge and documentary evidence of the experience of health-care professionals in the same circumstances. The perspectives of health-care professionals regarding what information parents require during episodes of suspected ADRs, have not been described and the mechanisms for decision analysis and motivations which underpin the timing, content and narrative of communication by clinicians has not been explored. In addition, there has been no attempt to identify if there are barriers to effective communication with families from the perspective of clinicians following a suspected ADR. More fundamentally, we did not know if the nature of the communication by health-care professionals about ADRs meets the needs and expectations of parents. Despite the beneficial impact of written supplementary information on the understanding of drug safety at the point of prescribing, this paradigm has not extended into circumstances when there is a suspected ADR in children. There are no customised written materials, generated with the involvement of relevant stakeholders, which provide a framework within which communication between health-care professionals and parents can be guided, and, in particular, documentation intended for parents, which allows enquiry and dialogue to be initiated and led from their perspective. Beyond this, it is important that further materials to guide communication pay adequate attention to key principles of transaction and process, including what and when to communicate to families, as opposed to developing and modifying the communication skills of clinicians. 29
The Adverse Drug Reactions In Children programme
Most studies of pharmacovigilance in children and young people to date have focused on individual aspects of ADRs, for example ADRs causing hospital admission and ADRs occurring within small specialised units. However, no programme of work has investigated a broad spectrum incorporating when and where ADRs occur, characterising the nature of ADRs in this age group, developing instruments customised for assessment of casuality and avoidability of ADRs in paediatric practice, and understanding the narratives and communications between families and health-care professionals during episodes of suspected ADRs. ADRIC was designed to undertake a comprehensive and coherent suite of studies aiming to add significantly to the existing evidence base and to generate outputs that can be adopted into both clinical practice and further research to improve methodologies for our understanding and management of pharmacovigilance in this vulnerable age group.
The ADRIC research strategy comprised the following component parts, which logically follow on from each other:
-
Quantification To estimate the incidence of ADRs in children, causing admission to hospital, and to estimate the burden to the health-care economy; to estimate the incidence of ADRs in hospitalised children.
-
Evaluation To identify risk factors for ADRs in causing admission to hospital and for causing ADRs in hospitalised children, and to characterise these ADRs in terms of type, drug aetiology, causality, avoidability and severity. To identify the unmet communication needs of parents whose child has experienced a suspected ADR.
-
Instrument development To develop and validate instruments to improve the assessment of causality and avoidability of ADRs in children.
-
Intervention To develop written materials that will guide the communication between parents and health-care professionals following an episode of a suspected ADR(s).
The methodologies used to undertake the above aims included two large and comprehensive single-centre prospective observational studies; a systematic review; reliability and validity testing of novel ADR assessment instruments; qualitative enquiry; structured interviews and evaluation of intervention implementation.
The ADRIC team was assembled with the necessary expertise to achieve the aims set out above. The Senior Investigator Team comprised paediatricians and neonatologists, a clinical pharmacologist with extensive experience in leadership and design of studies of assessment of ADRs in the adult population, experienced secondary researchers, a senior paediatric pharmacist, a senior academic psychologist with experience in qualitative methodologies for understanding the experience of children and families, and a NIHR Paediatric Clinical Research Network Director. Members of the team also hold executive positions within the NIHR MCRN and membership of expert committees, including the EMA and the UK Commission on Human Medicines.
The ADRIC study was supported by a steering group to provide an independent strategic overview of the programme. The steering group was overseen by an independent chairperson (Professor Sir Alisdair Breckenridge, Chairman, MHRA) and included senior representation from the MHRA (Director of Vigilance and Risk Management), US FDA, international academic paediatric pharmacovigilance expertise, and the chairperson of the NIHR Research Methods programme. A management group, comprising ADRIC senior investigators and members of the research team, was responsible for the design, implementation, analysis and reporting of each study within the overall of the programme.
A multidisciplinary research team comprised paediatric research nurses, research pharmacists, paediatric medical research fellows and research associates in qualitative methodologies. The setting for the ADRIC study was Alder Hey, widely recognised as the largest specialist children’s health-care provider in Western Europe, serving a population of children and young people in excess of two million and acting as a tertiary referral centre for much of the north-west of England and north Wales. Alder Hey provides general and all specialist paediatric services at local, regional and national levels. Community child health services are provided alongside Child and Adolescent Mental Health Services (CAMHS) over a large geographical footprint. The full range of paediatric services is provided at a single site with 325 beds and typically there are annually 120,000 outpatient episodes, 26,000 inpatient admissions including day case episodes, 70,000 accident and emergency (A&E) attendances, 1000 critical care admissions and 13,000 CAMHS episodes at Alder Hey (with 14,400 CAMHS outpatient episodes in community teams). ADRIC was conducted over a 5-year period, between May 2008 and April 2013.
Chapter 2 Adverse drug reactions causing admission to a paediatric hospital
This chapter contains information reproduced from Gallagher RM, Mason JR, Bird KA, Kirkham JJ, Peak M, Williamson PR, et al. Adverse drug reactions causing admission to a paediatric hospital. PLOS ONE 2012;7:e50127,21 © 2012 Gallagher et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution and reproduction in any medium, provided that the original author and source are credited; and information reproduced with permission from Bellis JR, Kirkham JJ, Nunn AJ, Pirmohamed M. Adverse drug reactions and off-label and unlicensed medicines in children: a prospective cohort study of unplanned admissions to a paediatric hospital. Br J Clin Pharmacol 2013;77:545–53,30 with permission from the British Pharmacological Society and Blackwell Publishing; and information reproduced with permission from Bellis JR. Adverse Drug Reactions in Children – The Contribution of Off-label and Unlicenced Prescribing. PhD thesis. Liverpool: University of Liverpool, 2013. 31
Abstract
Objective(s)
To determine the incidence of ADRs, which were associated with admission to hospital in children, describe their causality, severity, avoidability and nature, and identify which children were particularly at risk of this complication. To identify potential areas where intervention may reduce the burden of ill health.
Design
Prospective observational study.
Setting
A large children’s hospital providing general and specialty care in the UK.
Participants
All acute paediatric admissions over a 1-year period.
Main exposure
Any medication taken in the 2 weeks prior to admission.
Outcome measures
Occurrence of ADR.
Results
In total, 240 out of 8345 admissions in 178 out of 6821 patients who were admitted acutely to a paediatric hospital were thought to be related to an ADR, giving an estimated incidence of 2.9% [95% confidence interval (CI) 2.5% to 3.3%], with the reaction directly causing, or contributing to the cause of, admission in 97.1% of cases. No deaths were attributable to an ADR. Overall, 22.1% (95% CI 17% to 28%) of the reactions were either definitely or possibly avoidable. Prescriptions originating in the community accounted for 44 out of 249 (17.7%) of ADRs, the remainder originating from hospital. Of 16,551 prescription medicine courses, 11,511 (69.5%) were authorised, 4080 (24.7%) were off-label and 960 (5.8%) were unlicensed. A total of 120 out of 249 (48.2%) reactions resulted from treatment for malignancies. The drugs most commonly implicated in causing admissions were cytotoxic agents, corticosteroids, non-steroidal anti-inflammatory drugs (NSAIDs), vaccines and immunosuppressants. OLUL medicines were more likely to be implicated in an ADR than an authorised medicines [relative risk (RR) 1.67, 95% CI 1.38 to 2.02; p < 0.001]. When medicines used for the treatment of malignancies were excluded, OLUL medicines were not more likely to be implicated in an ADR than authorised medicines (RR 1.03, 95% CI 0.72 to 1.48; p = 0.830). The most common reactions were neutropenia, immunosuppression and thrombocytopenia.
Conclusions
Adverse drug reactions in children are an important public health problem. Most of those serious enough to require hospital admission are due to hospital-based prescribing, of which just over one-fifth may be avoidable. Strategies are needed to reduce the burden of ill health from ADRs causing admission.
Introduction
Children are vulnerable to ADRs. 32–37 A recent retrospective study by Hawcutt et al. 38 identified 31,726 of 222,755 (14.2%) ADR reports received by the UK MHRA through the Yellow Card Scheme, from 2000 to 2009, concerned children of < 17 years of age. 38 However, it is well recognised that spontaneous reporting systems, such as the Yellow Card Scheme in the UK,39 are subject to under-reporting of ADRs, even those that are severe. 40 Thus, it is likely that the number of paediatric ADR reports received each year by the MHRA is a considerable underestimate of the magnitude of the problem in the UK.
Hospital-based ADRs can be identified by retrospective studies using case note review; such studies, however, are likely to be less reliable than prospective studies in estimating the frequency with which ADRs occur owing to the inadequacy of recorded information. To obtain reliable information about the incidence of ADRs, prospective studies are needed.
A systematic review of observational studies of ADRs causing paediatric hospital admissions, between 1976 and 1996, estimated the overall rate of paediatric hospital admissions due to ADRs in children to be 2.1% (95% CI 1.0% to 3.8%). 34 This review included five prospective observational studies investigating ADRs causing admission in children. 32,33,41–43 Three of the studies were large, including > 1000 admissions each. 32,33,41 Three of the studies used published measures to assess causality of ADRs,41–43 whereas the two largest studies in the review used self-derived definitions for assessing causality. 32,33 Only one of the studies,41 with a low comparative reported ADR incidence of 10 out of 1682 admissions (0.6%), reported on avoidability of the cases using an adapted version of a published method44 but did not detail which ADRs were deemed avoidable or the reasons for assessing ADRs as being avoidable. The other studies did not report avoidability of the admissions associated with ADRs.
A further systematic review of prospective studies published between 2001 and 2007 included four studies37,45–47 but did not identify any large significant studies detailing the incidence and nature of ADRs causing admission of children to hospital. 35 Three studies37,45,46 included < 1000 admissions and the remaining study47 included a study population of 39,625 admissions but resulted in an ADR admission rate of only 0.16%. All four studies37,45–47 assessed causality using a published algorithm. However, only one study reported the avoidability of the ADR cases. 46 This study46 did not give detail of the method used for assessing avoidability, nor did the investigators detail the reasons for assigning cases as avoidable.
There have been no large paediatric studies that have looked at ADRs leading to hospital admission and then gone on to consider the influence of OLUL medicine use. There is one small pilot study48 that included ADRs to medicines administered before admission and recorded whether or not the medicines implicated were off-label. Of the 41 ADRs detected in 41 out of 1619 patients, 12 were attributed to medicines administered before admission and 29 were attributed to medicines administered in the hospital. In 16 out of the 41 patients experiencing an ADR, an off-label medicine was implicated; five of these were patients who experienced an ADR due to medicines administered before admission.
The aim of this study was to prospectively identify ADRs in children causing admission to hospital during a 1-year period in order to quantify and characterise the burden of ADRs. One important aspect of the study was to determine the avoidability of the ADRs identified and detail the reasons for categorising the reactions as ‘possibly’ or ‘definitely’ avoidable. In addition, the impact of OLUL medicine use on ADR risk in this context was examined.
Methods
The study hospital had an induction programme that was delivered to new members of staff to educate them about the hospital and some aspects of specific practice within the setting. This provided training to clinicians regarding medication prescribing and drug safety for children but did not specifically address ADRs, their diagnosis or how to report them. Therefore, before the start of this observational study, a comprehensive educational programme was undertaken within the hospital among clinicians of all grades. The study team attended hospital induction for new clinicians (and continued to do so through the entirety of the study period) to give formal presentations about the study and ADRs in children. The study team gave a formal presentation to an audience at the main weekly educational hospital meeting (for clinicians and staff from all specialties), as well as presenting at individual specialty team meetings occurring within the hospital.
The goal of this educational programme was to raise awareness about the aims of the study and to increase clinicians’ understanding of their role in information recording. First, clinicians were made aware of the primary aim of the study, which was to identify prospectively ADRs causing admission to the hospital. Clinicians were reminded of the importance of good record-keeping with regard to descriptions of symptoms and signs to allow for more accurate assessment of causality by the study team. Second, the study team aimed to raise awareness of taking detailed medication histories in relation to identifying ADRs accurately and assigning causality. A structured medication history was added to acute general paediatric medical admission documentation with the aim of ensuring all families were asked for details about medication taken in the preceding 2 weeks. A 2-week medication history was chosen as the time when reactions causing admission were most likely to have occurred following exposure to a drug. A 2-week pilot study to develop and refine the methodology for this larger study was conducted prior to the commencement of this study. 20
The study team prospectively screened all unplanned admissions to a large paediatric centre (which provides local and specialist regional and national services) for ADRs over a 1-year period, including weekends and public holidays, from 1 July 2008 to 30 June 2009. Weekends were included in routine daily data collection to eliminate any bias that may occur in trends of possible ADR admissions. Admissions were excluded if they were planned or occurred as a result of accidental or intentional overdose. The definition of ADR used was that of Edwards and Aronson,49 which is ‘an appreciably harmful or unpleasant reaction, resulting from an intervention related to the use of a medicinal product, which predicts hazard from future administration and warrants prevention or specific treatment, or alteration of the dosage regimen, or withdrawal of the product’.
Hospital information systems at the study hospital routinely recorded demographic data about admitted patients. These data, with assistance from the hospital information technology department, were automatically downloaded each morning at 06.00, for the patients coded as having an emergency admission, from the hospital computer system to a password-protected Microsoft Access 2007 database (Microsoft Corporation, Redmond, WA, USA) stored on a secure hospital hard drive. Only the study team had access to the database and the patient information recorded within.
Members of the study team, consisting of a paediatric registrar (RMG), a research pharmacist (JRB) and a research nurse (KAB) collected the following information from the case notes of each patient: presenting complaint, summary of clinical history and diagnosis (if available at the time of admission). The details of any medication taken at any time during the 2 weeks before admission were recorded, specifically drug name, route, dose, frequency, duration, indication (if this required clarification) and whether it was a prescription or non-prescription medicine. The data on prescription medicine use were scrutinised in order to define each medicine course as either authorised, off-label, unlicensed or unknown. Authorised use was defined as the use of a medicine with a UK marketing authorisation (MA), within the terms of that MA. The terms of the MA were found in the SmPC available online from the Electronic Medicines Compendium (EMC). 50 If no SmPC was available, the British National Formulary for Children (BNF-C)51 was consulted for details of the product MA. If neither reference source provided adequate clarity of information, the manufacturer of the medicine was contacted. Off-label use was defined as the use of a medicine with a UK MA, outside the terms of that MA. According to the definitions described by Turner et al. ,52 unlicensed medicines were defined as those without a UK MA, and an ‘unknown’ category was reserved for medicine courses for which inadequate detail was available to decide whether use was authorised or OLUL. If any information was unclear, study team members interviewed the family, patient or carers as appropriate to clarify the history (i.e. medication history), symptoms and timing of events.
The study team cross-referenced the presenting symptoms/signs against medication history for each patient using the ADR profile for relevant drugs from the SmPC50 in the EMC or, if not available, the BNF-C. 51 Possible ADRs were identified using this information combined with the clinical history and temporal relationships of the medication(s) taken. All possible ADRs were reported by the study team to the responsible clinicians during the study. All possible ADRs were reported to the MHRA using the electronic Yellow Card Scheme at the end of the study period. Reporting to the MHRA occurred after internal causality assessment of the possible ADR cases. The origin of prescription, for drugs thought to be associated with ADRs, was classified using the following criteria:
-
Community Drugs where prescriptions originated in community settings, for example general practice, or where administration took place prior to hospital admission (e.g. paramedic administered).
-
Hospital Drugs where the prescription originated, or administration took place, in hospital and then may or may not have been continued, for example by repeat prescription, in community or outpatient settings.
-
Oncology All drugs administered, or prescribed, from the oncology ward. These drugs may or may not be cytotoxic in nature.
We performed assessment of causality for all cases using the Liverpool Causality Assessment Tool (LCAT). 53 Three investigators (RMG, JRB, KAB) independently assessed causality for all possible ADR cases. Agreement on causality category between all three investigators was taken as accepted consensus. In cases when the three investigators did not achieve consensus, a fourth investigator assessed cases to decide on causality (MPir).
Avoidability of the ADR cases was assessed by consensus meeting between the investigators, using the definitions developed by Hallas et al. 54 Cases were assessed as definitely avoidable, possibly avoidable or unavoidable. In addition, the type of ADR for each case identified was determined according to the classification of Rawlins and Thompson55 as either Type A (predictable from the known pharmacology) or Type B (not predictable). Severity was determined using an adapted Hartwig scale. 56 This adapted scale is shown in Table 1 . Grades 3 and 4 are adapted from the original schema, as not all ADR admissions necessitate cessation of the causative drug(s).
| Severity score | Description |
|---|---|
| 6 | Directly or indirectly resulted in patient death |
| 5 | Caused permanent harm or significant haemodynamic instability |
| 4 | Resulted in patient transfer to higher level of care |
| 3 | Required treatment (admission) or drug discontinued |
| 2 | Drug dosing or frequency changed, without treatment |
| 1 | No change in treatment with suspected drug |
We chose these assessment tools to describe the nature of the ADRs in our study as they have been used previously in ADR studies by other investigators and can be completed quickly. Three investigators independently assessed 217 out of 4514 (4.8%) reports of admissions exposed to medication, but deemed not to have had an ADR, to assess for occurrence of possible ADR cases wrongly classified by the study team (AJN, MPir, MAT).
Statistical analysis
Analyses of the rates of ADRs were based on the number of admissions with the rate expressed as ADR per 100 admissions, together with 95% CIs. Other results are presented either as medians and interquartile ranges (IQRs) or percentage frequencies and 95% CI, as appropriate. The formal statistical analysis was based on the data obtained at the first admission for patients exposed to a medication. Univariate statistical analyses were performed using the Mann–Whitney U-test except for frequency data, which were analysed using a chi-squared test. Multivariate logistic regression analysis was undertaken to calculate odds ratios (ORs) for possible risk factors for ADR. The RR (with 95% CI) for OLUL medicines being implicated in an ADR was calculated for prescription medicines. A p-value of < 0.05 was regarded as being significant.
Ethics
This study used routinely collected clinical data in an anonymised format. The chairperson of Liverpool Paediatric Local Research Ethics Committee informed us that this study did not require individual patient consent or review by an ethics committee.
Results
Over the study period, there were 6821 patients admitted acutely to the study hospital, accounting for 8345 unplanned admissions. Boys accounted for 3961 out of 6821 (58.1%) patients and 4793 out of 8345 (57.4%) admissions. The median number of admissions per patient was one, with 932 patients having more than one acute admission, up to a maximum of 15. A total of 178 patients experienced 240 admissions with an ADR. This gives an incidence of 2.9 ADRs per 100 admissions (95% CI 2.5 to 3.3); 233 of the 240 (97.1%) admissions were deemed to have been directly caused, or contributed to, by at least one ADR. There were 249 ADRs in 240 admissions, with nine admissions having two separate ADRs; 35 out of 178 (19.7%) patients had more than one admission with an ADR, up to a maximum of seven.
There were 4656 patients exposed to a medication in the 2 weeks prior to acute admission to the hospital. Of these patients, 142 (3%) had a suspected ADR on their first hospital admission within the study period. There was no significant difference between the proportion of boys (76/2677, 2.8%) and girls (66/1979, 3.3%) experiencing an ADR on their first admission, for the group as a whole or oncology patients studied separately ( Table 2 ). For non-oncology patients, there was a slightly higher proportion of girls admitted with an ADR [boys 48/2627 (1.8%), girls 53/1955 (2.7%); p = 0.044], although overall more boys than girls were admitted to the hospital.
| Gender | All | No ADR | ADR | Chi-squared test | p-value | |
|---|---|---|---|---|---|---|
| All boys | 2677 | 2601 (97.2%) | 76 (2.8%) | 0.947 | 0.331 | |
| All girls | 1979 | 1913 (96.7%) | 66 (3.3%) | |||
| Oncology | Boys | 50 | 22 (44.0%) | 28 (56.0%) | 0.022 | 0.882 |
| Girls | 24 | 11 (45.8%) | 13 (54.2%) | |||
| Non-oncology | Boys | 2627 | 2579 (98.2%) | 48 (1.8%) | 4.062 | 0.044 |
| Girls | 1955 | 1902 (97.3%) | 53 (2.7%) | |||
The median age of the 4656 patients who had been exposed to a drug on their first admission was 3 years 1 month (IQR 9 months to 9 years). Patients with an ADR (6 years, IQR 2 years 4 months to 11 years) were significantly older (p < 0.001) than those without (3 years, IQR 9 months to 9 years) ( Table 3 ). There was no age difference between the 41 oncology patients admitted with an ADR (6 years, IQR 3–10 years) and the 33 oncology patients admitted without an ADR (6 years, IQR 3 years 6 months to 13 years). There was a significant age difference (p < 0.001) between 101 non-oncology patients admitted with ADR (6 years, IQR 1 year 7 months to 11 years) and 4481 admitted without ADR (2 years 11 months, IQR 9 months to 9 years).
| Age (years, months); median: quartile 1, quartile 3 | All | No ADR | ADR | Mann–Whitney U-test | p-value |
|---|---|---|---|---|---|
| All | 3 years 1 month; 9 months, 9 years (n = 4656) | 3 years 0 months; 9 months, 9 years (n = 4514) | 6 years 0 months; 2 years 4 months, 11 years (n = 142) | 244,161 | < 0.001 |
| Oncology | 6 years; 3 years 6 months, 12 years (n = 74) | 6 years; 3 years 6 months, 13 years (n = 33) | 6 years; 3 years 0 months, 10 years (n = 41) | 580.5 | 0.296 |
| Non-oncology | 3 years; 9 months, 9 years (n = 4582) | 2 years 11 months; 9 months, 9 years (n = 4481) | 6 years; 1 year 7 months, 11 years (n = 101) | 178,319.5 | < 0.001 |
Patients admitted with an ADR had taken a greater number of drugs than those admitted for other reasons ( Table 4 ). For patients admitted with an ADR (n = 142), the number of medicines taken was higher (6, IQR 3–9; p < 0.001) than those for other reasons (n = 4514) (2, IQR 1–3). The number of medicines taken by oncology patients admitted with an ADR (8, IQR 5–10) was higher than those admitted without an ADR (4, IQR 3–7) and this difference was also found for non-oncology patients (with ADR 5, IQR 3–9; without ADR 2, IQR 1–3).
| Drug count | All (median; IQR) | No ADR | ADR | Mann–Whitney U-test | p-value |
|---|---|---|---|---|---|
| All | 2 (1–3) (n = 4656) | 2 (1–3) (n = 4514) | 6 (3–9) (n = 142) | 115,391.5 | < 0.001 |
| Oncology | 6 (4–9) (n = 74) | 4 (3–7) (n = 33) | 8 (5–10) (n = 41) | 380.5 | 0.001 |
| Non-oncology | 2 (1–3) (n = 4582) | 2 (1–3) (n = 4481) | 5 (3–9) (n = 101) | 100,371.5 | < 0.001 |
Logistic regression analysis showed a trend towards boys being less likely to experience an ADR than girls, with an OR of 0.77 (95% CI 0.52 to 1.12; p = 0.17) ( Table 5 ). There was an increased likelihood of ADRs with increasing age (OR 1.04, 95% CI 1.003 to 1.08; p = 0.03). No children were admitted with an ADR in the first month of life. Oncology patients were much more likely to have an ADR causing admission (OR 29.71, 95% CI 17.35 to 50.88; p < 0.001). The likelihood of a child being admitted with an ADR increased with the number of medicines taken (OR 1.24, 95% CI 1.19 to 1.29; p < 0.001). Therefore, for each additional medicine taken by a patient the risk of an ADR occurring increases by almost 25%.
| Parametera | OR | 95% CI for OR | p-value |
|---|---|---|---|
| Gender (male) | 0.77 | 0.52 to 1.12 | 0.17 |
| Age | 1.04 | 1 to 1.08 | 0.03 |
| Oncology | 29.71 | 17.35 to 50.88 | < 0.01 |
| No. of medicines | 1.24 | 1.19 to 1.29 | < 0.01 |
A further univariate analysis was carried out which included only patients on their first admission who had received at least one prescription medicine in the 2 weeks prior to admission (n = 3869). This analysis compared each continuous variable in the group of patients who had experienced at least one ADR with those who had not in three subpopulations: all patients, patients who had been exposed to at least one OLUL medicine and patients who had received only authorised medicines. There was no significant difference in the proportion of each gender in any of the subpopulations. The median age and median number of medicines was greater in patients who had experienced at least one ADR; however, within the population of patients exposed to authorised medicines only there was no difference. The median number of medicines was significantly greater in children who experienced an ADR for all subpopulations. Oncology patients and patients exposed to OLUL medicines were significantly more likely to experience an ADR ( Table 6 ). Multivariate analysis indicated oncology patients were more likely to have experienced an ADR: OR 25.70 (95% CI 14.56 to 45.38; p < 0.001). The number of authorised medicines courses administered in the 2 weeks before admission was a significant ADR risk factor (OR 1.25, 95% CI 1.16 to 1.35; p < 0.001) but so was the number of OLUL medicines administered (OR 1.23, 95% CI 1.10 to 1.36; p < 0.001). In addition, increasing age was associated with an increased risk of ADR; OR 1.04 (95% CI 1.00 to 1.08; p = 0.045). There was a trend towards females being more likely to experience an ADR: OR 0.74 (95% CI 0.51 to 1.09; p = 0.130) ( Table 7 ).
| Variable | All | No ADR | ADR | p-value | |
|---|---|---|---|---|---|
| Gender | |||||
| All boys | 2247 | 2172 (96.7%) | 75 (3.3%) | 0.271a | |
| All girls | 1622 | 1557 (96.0%) | 65 (4.0%) | ||
| OLUL exposed | Boys | 869 | 812 (93.4%) | 57 (6.6%) | 0.982a |
| Girls | 627 | 585 (93.3%) | 42 (6.7%) | ||
| Authorised only | Boys | 1321 | 1303 (98.6%) | 18 (1.4%) | 0.063a |
| Girls | 953 | 930 (97.6%) | 23 (2.4%) | ||
| Age (years, months): median; quartile 1, quartile 3 | |||||
| All | 3 years 1 month; 8 months, 9 years (n = 3869) | 3 years; 8 months, 9 years (n = 3729) | 6 years; 2 years 4 months, 11 years (n = 140) | < 0.001b | |
| OLUL exposed | 2 years 5 months; 3 months, 8 years (n = 1595) | 2 years 1 month; 3 months, 7 years (n = 1496) | 7 years; 3 years 7 months, 12 years (n = 99) | < 0.001b | |
| Authorised only exposed | 3 years 8 months; 1 year, 10 years (n = 2274) | 3 years 8 months; 1 year, 10 years (n = 2233) | 3 years 9 months; 8 months; 8 years 6 months (n = 41) | 0.968b | |
| No. of medicines: median; quartile 1, quartile 3 | |||||
| All | 2; 1, 4 (n = 3869) | 2; 1, 4 (n = 3729) | 6; 3, 9 (n = 140) | < 0.001b | |
| OLUL exposed | 3; 2, 6 (n = 1565) | 3; 2, 5 (n = 1496) | 8; 5, 10 (n = 99) | < 0.001b | |
| Authorised only exposed | 2; 1, 3 (n = 2274) | 2; 1, 3 (n = 2233) | 3; 2, 6 (n = 41) | 0.003b | |
| Specialty | |||||
| Oncology | 73 | 32 (43.8%) | 41 (56.2%) | < 0.001a | |
| Non-oncology | 3796 | 3697 (97.4%) | 99 (2.6%) | ||
| OLUL exposure | |||||
| OLUL exposed | 1595 | 1496 (93.8%) | 99 (6.2%) | < 0.001a | |
| Authorised only exposed | 2274 | 2233 (98.2%) | 41 (1.8%) | ||
| Variable | OR (95% CI) | p-value |
|---|---|---|
| Gender (male) | 0.74 (0.51 to 1.09) | 0.130 |
| Specialty (oncology) | 25.70 (14.56 to 45.38) | < 0.001 |
| No. of authorised medicines | 1.25 (1.16 to 1.35) | < 0.001 |
| No. of OLUL medicines | 1.23 (1.10 to 1.36) | < 0.001 |
| No. of unknown medicines | 0.84 (0.59 to 1.18) | 0.303 |
| Age in years | 1.04 (1.00 to 1.08) | 0.045 |
Drug classes and drugs
The main class of drugs contributing to ADR-related admissions (n = 110; 44.2%) was cytotoxic drugs ( Table 8 ). Corticosteroids (n = 102, 41%), NSAIDs (n = 31, 12.4%), vaccines (n = 22, 8.8%) and immunosuppressant drugs (n = 18, 7.2%) were the next most commonly implicated drug classes causing ADR-related hospital admissions.
| Drug class (no. of cases) | No. of drugs | Drugs | ADRs |
|---|---|---|---|
| Cytotoxics (110) | 275 | Vincristine, 51; doxorubicin, 38; methotrexate, 35; etoposide, 30; mercaptopurine, 27; cytarabine, 24; ifosfamide, 18; cyclophosphamide, 15; carboplatin, 7; vinblastine, 5; peg asparaginase, 5; dactinomycin, 5; daunorubicin, 4; cisplatin, 3; irinotecan, 3; temozolomide, 2; fludarabine, 1; amsacrine, 1; imatinib, 1 | Neutropenia, 89; thrombocytopenia, 55; anaemia, 38; vomiting, 8; mucositis, 8; deranged liver function tests, 7; immunosuppression, 7; diarrhoea, 5; nausea, 4; constipation, 3; headache, 2; abdominal pain, 1; back pain, 1; haematuria, 1; leucoencephalopathy, 1; deranged renal function, 1 |
| Corticosteroids (102) | 107 | Dexamethasone, 68; prednisolone, 33; hydrocortisone, 2; betamethasone, 1; mometasone, 1; methylprednisolone, 1; fluticasone, 1 | Immunosuppression, 71; postoperative bleeding, 23; hyperglycaemia, 3; hypertension, 1; gastritis, 1; increased appetite, 1; impaired healing, 1; adrenal suppression, 1 |
| NSAIDs (31) | 43 | Ibuprofen, 28; diclofenac, 15 | Postoperative bleeding, 27; haematemesis, 2; constipation, 1; abdominal pain, 1 |
| Vaccines (22) | 37 | Diphtheria, tetanus, pertussis, inactivated polio Haemophilus influenzae vaccine, 11; pneumococcal conjugate, 9; meningococcal C, 8; measles; mumps and rubella (MMR), 7; Haemophilus influenzae type b, 1; influenza, 1 | Fever, 8; rash 5; irritability, 4; seizure, 4; vomiting, 3; pallor, 1; apnoea, 1; limb swelling, 1; lethargy, 1; thrombocytopenia, 1; diarrhoea, 1; abdominal pain, 1; respiratory distress, 1; Kawasaki disease, 1 |
| Drugs affecting the immune response (18) | 26 | Tacrolimus, 15; mycophenolate, 7; azathioprine, 2; methotrexate, 1; infliximab, 1 | Immunosuppression, 18 |
| Antibacterial drugs (16) | 17 | Co-amoxiclav, 4; penicillin v, 3; amoxicillin, 3; flucloxacillin, 2; cefaclor, 1; cefalexin, 1; cefotaxime, 1; teicoplanin, 1; erythromycin, 1 | Diarrhoea, 7; rash, 4; vomiting, 4; lip swelling, 1; deranged LFTs, 1; thrush, 1 |
| Drugs used in diabetes (9) | 13 | Insulin detemir, 4; insulin aspart, 3; isophane insulin, 2; biphasic isophane, 2; human insulin, 2 | Hypoglycaemia, 9 |
| Drugs used in status epilepticus (8) | 12 | Lorazepam, 5; diazepam, 5; midazolam, 2 | Respiratory depression, 8 |
| Opioid analgesia (6) | 7 | Dihydrocodeine, 3; codeine phosphate, 3; fentanyl, 1 | Constipation, 4; ileus, 1; decreased conscious level, 1 |
| Drugs used in nausea (4) | 4 | Ondansetron, 4 | Constipation, 4 |
| Antiepileptic drugs (2) | 2 | Carbamazepine, 1; nitrazepam, 1 | Constipation, 1; respiratory depression, 1 |
| Drugs that suppress rheumatic disease (2) | 2 | Methotrexate, 1; anakinra, 1 | Immunosuppression, 2 |
| Other (16) | 4 | Calcium carbonate and amlodipine, 1; oxybutynin, 1; baclofen, 1 | Constipation, 3 |
| 2 | Dimethicone, 1; carbocysteine, 1 | Rash, 2 | |
| 2 | Desmopressin acetate, 1; alimemazine, 1 | Seizure, 2 | |
| 10 | Glucose and dextrose, 1; propanolol, 1; acetazolomide, 1; spironolactone, 1; loperamide, 1; macrogols, 1; captopril, 1; alfacalcidol, 1; ethinylestradiol, 1 | Hyperglycaemia, 1; wheeze/difficulty in breathing, 1; headache, 1; hyperkalaemia, 1; intestinal obstruction, 1; diarrhoea, 1; renal dysfunction, 1; hypercalcaemia, 1; intermenstrual bleed, 1 |
A total of 551 courses of medicines contributed to the 249 ADRs causing 240 admissions. The median number of drugs causing an ADR admission was two (n = 79), with a maximum of six (three admissions). Seven admissions were caused by five drugs, 25 by four drugs and 57 by three drugs. A total of 69 admissions were caused by one drug only. None of the ADRs, caused by more than one drug, occurred as a result of a pharmacokinetic drug–drug interaction. All of the ADRs caused by more than one drug were a result of pharmacodynamic interactions.
There were 17,758 prescription medicine courses given to 3869 patients in the 2 weeks prior to admission. Of these, 1207 (6.8%) could not be categorised, 11,511 (64.8%) were authorised, 4080 (23.0%) were off-label and 960 (5.4%) were unlicensed. OLUL medicines were more likely to be implicated in an ADR than authorised medicines [RR 1.67 (95% CI 1.38 to 2.02)]. In total, 14,923 out of 16,106 medicine courses administered to non-oncology patients could be categorised. Of these, 71% were authorised, 24% off-label and 5% unlicensed and OLUL medicines were not more likely to be implicated in an ADR than authorised medicines [RR 1.03 (95% CI 0.72 to 1.48)]. In comparison, among the 1652 medicine courses administered to oncology patients, 1628 could be classified and 57% were approved, 34% were off-label and 9% were unlicensed, and OLUL medicines were more likely to be implicated in an ADR than authorised medicines [RR 1.39 (95% CI 1.12 to 1.71)].
Nature of the adverse drug reactions
The most common ADRs were oncology related, including neutropenia (n = 89), thrombocytopenia (n = 55) and anaemia (n = 38). The next most common ADR was immunosuppression (n = 74), occurring in both oncology and non-oncology patients. Overall, 84 cases of neutropenia, thrombocytopenia, anaemia and/or immunosuppression among oncology patients involved at least one OLUL medicine, and 12 cases of immunosuppression among non-oncology patients involved at least one OLUL medicine. Postoperative bleeding, linked to perioperative corticosteroid administration and/or NSAIDs, caused 28 admissions (26 post tonsillectomy), and 21 of the post-tonsillectomy bleeds were attributed to at least one OLUL medicine. Vomiting (n = 15), diarrhoea (n = 14), rash (n = 11) and constipation (n = 9) were all common ADRs causing admission. Hypoglycaemia in diabetic patients treated with regular insulin caused nine admissions and none of the insulin prescriptions were off-label. Respiratory depression following treatment for status epilepticus caused eight admissions to the hospital’s PICU: unlicensed buccal midazolam liquid was implicated in two of these.
Origin of adverse drug reaction drug prescriptions
Prescriptions originating from community settings accounted for 44 out of 249 (17.7%) of the ADRs, and 85 out of 249 (34.1%) ADRs arose from prescriptions originating in hospital for the treatment of conditions other than oncology. Prescriptions originating from oncology accounted for 120 out of 249 (48.2%) of ADRs. Of the patients with one ADR (n = 140) in the study period, 39 (27.9%) occurred with community-originated prescriptions, 71 (50.7%) with hospital-originated prescriptions and 30 (21.4%) with oncology-originated prescriptions. Of patients with two ADRs (n = 22) in the study period, two (9.1%) occurred with community prescriptions, six (27.3%) with hospital prescriptions and 14 (63.6%) with oncology prescriptions. Prescriptions originating from oncology accounted for 15 out of 16 patients with three or more ADRs. One patient, with three ADRs in the study period, had two ADRs to hospital-originated prescriptions and one ADR to a community prescription.
Adverse drug reaction assessments (reaction type, causality, severity, avoidability)
A total of 238 out of 249 (95.6%) ADRs were classified as type A (predictable from the known pharmacology), with 11 out of 249 (4.4%) being type B (not predictable). Assessment of causality using the LCAT showed the highest proportion of cases (94/249, 37.8%) to be in the ‘definite’ category. Oncology cases accounted for 80 of these 94 definite causality cases ( Table 9 ). In total, 41 out of 55 (74.5%) of possibly or definitely avoidable cases were classified as ‘definite’ or ‘probable’, 92 out of 238 (39.1%) type A reactions were assessed to be of definite causality, and 8 out of 11 (72.7%) type B reactions were assessed to be ‘possible’. The majority (16/17, 94.1%) of the more severe reactions (adapted Hartwig severity score of grade 4 or more) were assessed to have definite or probable causality.
| Origin of prescription | Type of reaction | Severity score | Avoidability | Causality | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | 1 | 2 | 3 | 4 | 5 | Unavoidable | Possibly | Definitely | Possible | Probable | Definite | |
| Oncology (120) | 119 | 1 | 5 | 0 | 111 | 2 | 2 | 112 | 6 | 2 | 9 | 31 | 80 |
| Hospital (85) | 85 | 0 | 1 | 2 | 74 | 8 | 0 | 57 | 25 | 3 | 51 | 24 | 10 |
| Community (44) | 34 | 10 | 1 | 0 | 38 | 4 | 1 | 25 | 14 | 5 | 23 | 17 | 4 |
A total of 223 out of 249 (89.6%) of the ADRs were classified as grade 3 (‘required treatment or drug administration discontinued’) according to the Hartwig severity scale, as we defined anyone requiring admission to hospital as ‘needing treatment’. Fourteen (5.6%) were classified as grade 4 (‘resulted in patient transfer to higher level of care’), including respiratory depression (n = 8), immunosuppression (n = 4), neutropenia (n = 1), fever/seizure (n = 1) and leukoencephalopathy (n = 1). Three ADRs were classified as grade 5 (‘caused permanent harm or significant haemodynamic instability’). Two of these most severe ADRs occurred in oncology patients with febrile neutropenia and septicaemia, and the remaining case was a child who required bowel resection for ileus, with impacted faecal matter, following treatment with loperamide. No ADRs contributed to death. Two ADRs were classified as grade 2 (‘drug dosing or frequency changed, without treatment’) and seven were classified as grade 1 with (‘no change in treatment with the suspected drug’).
Of the ADRs, 194 out of 249 (78%) were assessed as ‘unavoidable’, whereas 45 (18%) were classified as ‘possibly avoidable’ and 10 (4%) as ‘definitely avoidable’. Five of the cases deemed to be definitely avoidable were associated with hospital-prescribed drugs and five with community-prescribed drugs; 31 possibly avoidable cases were associated with hospital-prescribed drugs and 14 with community-prescribed drugs. A total of 114 (47.5%) of the ADR admissions occurred in oncology patients, accounting for 120 ADRs. Of the ADRs due to oncology drugs, 112 out of 120 (93.3%) were unavoidable, with a further six being possibly avoidable and two definitely avoidable. These ‘definitely avoidable’ cases were oncology patients with constipation following treatment with vincristine and ondansetron (with one also having dihydrocodeine) without laxative prophylaxis.
Of the ADR admissions not associated with oncology patients (n = 126 admissions and 129 ADRs), 82 out of 129 ADRs (63.6%) were classified as unavoidable, 39 (30.2%) as possibly avoidable and eight (7.6%) as definitely avoidable. The eight ‘definitely avoidable’ cases comprised four patients who were prescribed antibiotics, for whom the antibiotic choice or indication was deemed to be inconsistent with good practice: one patient with intestinal obstruction being treated with loperamide, who had not passed stool for 2 days prior to admission; one patient who had a seizure after alimemazine, having had two previous occurrences of seizure after the antihistamine; one patient with deranged renal function, which improved after cessation of captopril, for whom the ADR may have been avoided through improved renal function monitoring; and one patient who presented with adrenal suppression following 2 years of continuous treatment with intranasal corticosteroids. The possibly and definitely avoidable cases and the reasons for their allocation are summarised in Table 10 .
| Avoidable? | Frequency | ADR(s) | Drug classes | Reason for potential avoidability |
|---|---|---|---|---|
| Definitely | 3 | Diarrhoea and/or vomiting | Antibacterial drugs | Inappropriate indication, signs/symptoms of viral illness |
| 2 | Constipation | Cytotoxic drugs, drugs used in nausea, opioid analgesia | Appropriate prophylaxis not used | |
| 1 | Lip swelling, rash | Antibacterial drugs | Same ADR previously to same medication | |
| 1 | Seizure | Antihistamine | Same ADR previously to similar medication | |
| 1 | Adrenal suppression | Corticosteroids | Avoidable with more rational prescribing (prolonged use of drugs) and improved monitoring | |
| 1 | Intestinal obstruction | Antimotility drugs | Could be prevented by improved parent/patient education | |
| 1 | Deranged renal function | Drugs affecting the renin–angiotensin system | Avoidable with improved monitoring | |
| Possibly | 9 | Hypoglycaemia | Drugs used in diabetes | Avoidable with improved patient education (e.g. insulin use when unwell) and more rational prescribing |
| 8 | Respiratory depression | Drugs used in status epilepticus, hypnotic drugs | Alternative medicine available, multiple doses given; avoidable with more rational prescribing | |
| 6 | Diarrhoea/vomiting | Antibacterial | Inappropriate indication, symptoms suggested viral infection | |
| 5 | Constipation | Antiepileptic drugs, opioid analgesia, drugs used in nausea, NSAIDs, cytotoxic drugs, calcium channel blockers, calcium supplements | Prophylaxis not used | |
| 4 | Immunosuppression | Drugs affecting the immune response, corticosteroids | Possibly avoidable with improved monitoring of drug levels; avoidable with more rational prescribing | |
| 2 | Haematemesis | NSAIDs | Avoidable with improved patient education/more rational prescribing (less NSAID use) | |
| 1 | Neutropenia | Cytotoxic drugs | Same ADR previously at same dose of medication | |
| 1 | Neutropenia, thrombocytopenia, anaemia | Cytotoxic drugs | Superficial infection after recent admission with febrile neutropenia; possibly avoidable by prolonging antibiotic use or commencing granulocyte colony-stimulating factor | |
| 1 | Hyperglycaemia | Corticosteroids | Avoidable with more rational prescribing (prolonged course steroids used) | |
| 1 | Hyperglycaemia | Parenteral preparations | Avoidable with more rational prescribing (more judicial use) or improved monitoring | |
| 1 | Seizure | Posterior pituitary hormones | Possibly inappropriate medication used for a patient with seizures | |
| 1 | Diarrhoea | Laxatives | Avoidable with improved patient education | |
| 1 | Ileus | Opioid analgesia | Avoidable with more rational prescribing (possibly use alternative analgesia) | |
| 1 | CNS depression | Opioid analgesia | Avoidable with improved patient education | |
| 1 | Vomiting | Cytotoxic drugs | Possibly avoidable with more appropriate antiemetic prophylaxis | |
| 1 | Gastritis | Corticosteroids | Previous gastritis; possibly avoidable with improved prophylaxis | |
| 1 | Hypercalcaemia | Vitamins | Avoidable with improved monitoring |
Drug exposure prior to acute admission
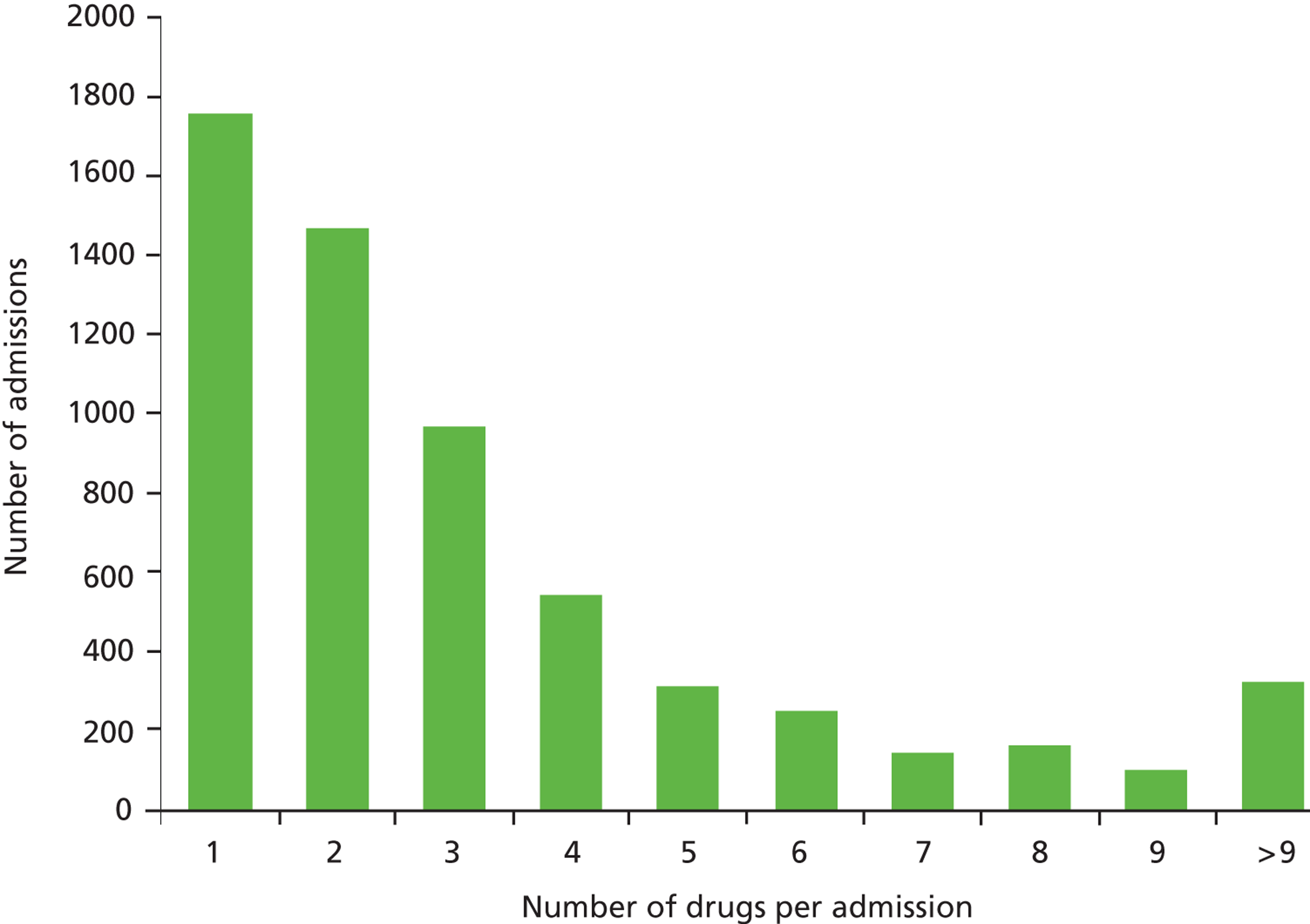
Of 8345 admissions, 6020 (72.1%) were exposed to medication in the 2 weeks prior to admission; 3417 (56.8%) of these were male and 2603 were female (43.2%). The median number of drugs taken was 2 (IQR 1–4), with one child exposed to 34 courses of medication owing to an admission for cardiothoracic surgery in the 2 weeks prior to readmission. Figure 1 shows the distribution of drugs per admission.
FIGURE 1.
Number of drugs per admission.
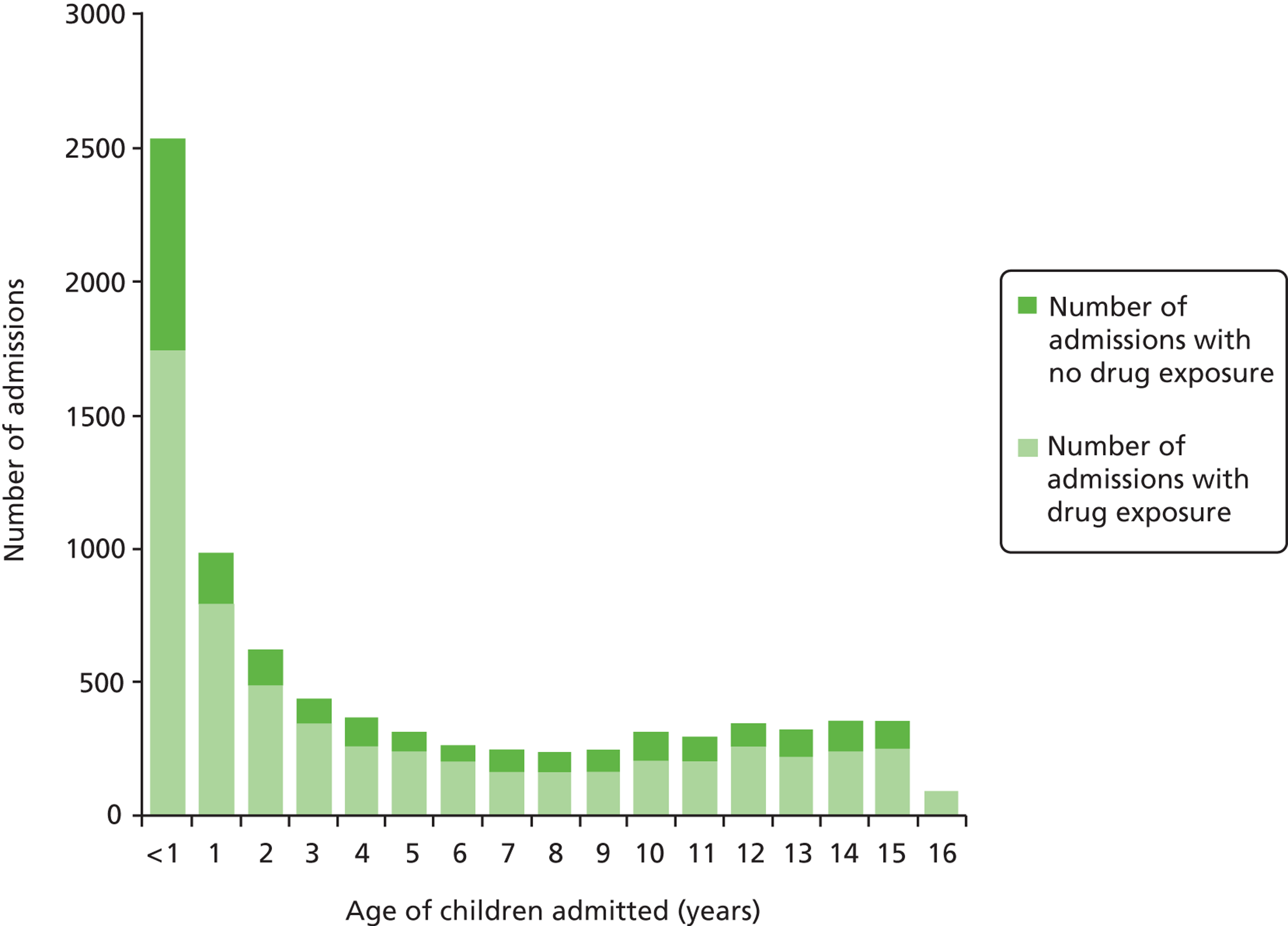
Children of < 1 year of age accounted for the most number of admissions: 1737 out of 2539 (68.4%) of < 1-year-olds had been exposed to medication prior to admission ( Figure 2 ). Of the other children admitted, the age group most frequently exposed to medication was the 16-year-old group (95/99 admissions, 96%). Children aged 7 years were the least exposed to medication (163/245, 66.5%) prior to admission.
FIGURE 2.
Age (1-year intervals) and number of children exposed to medication prior to admission.
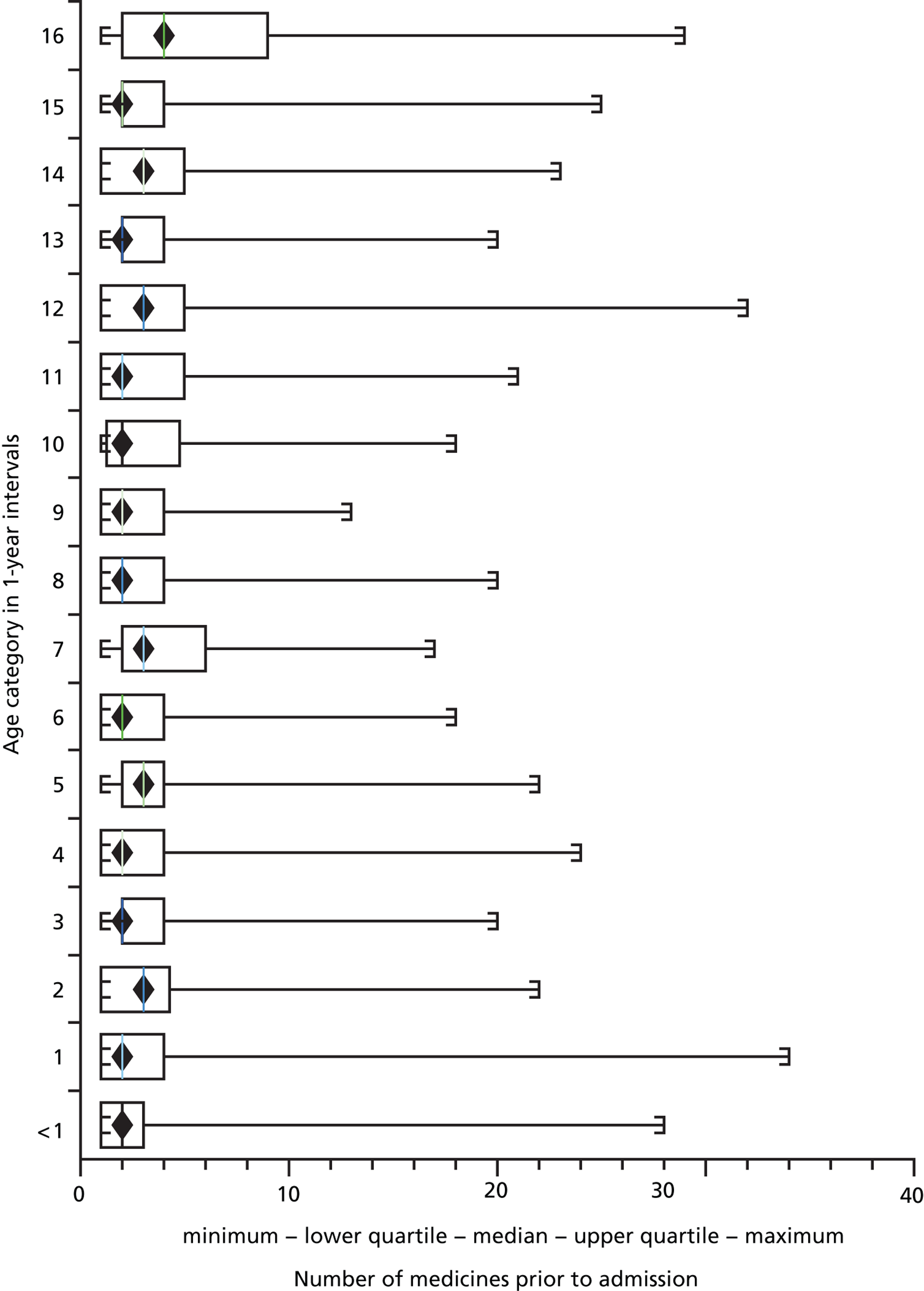
Of 6020 children exposed to at least one medicine prior to admission, those aged 16 years were exposed to the greatest number of drugs per admission, with a mean of 5.93 (95% CI 4.92 to 6.93) drugs. Children aged < 1 year were exposed to fewer medicines on average, with a mean of 2.82 (95% CI 2.71 to 2.93) drugs per admission ( Figures 3 and 4 ).
FIGURE 3.
Mean number of drugs taken by each age group (1-year intervals).
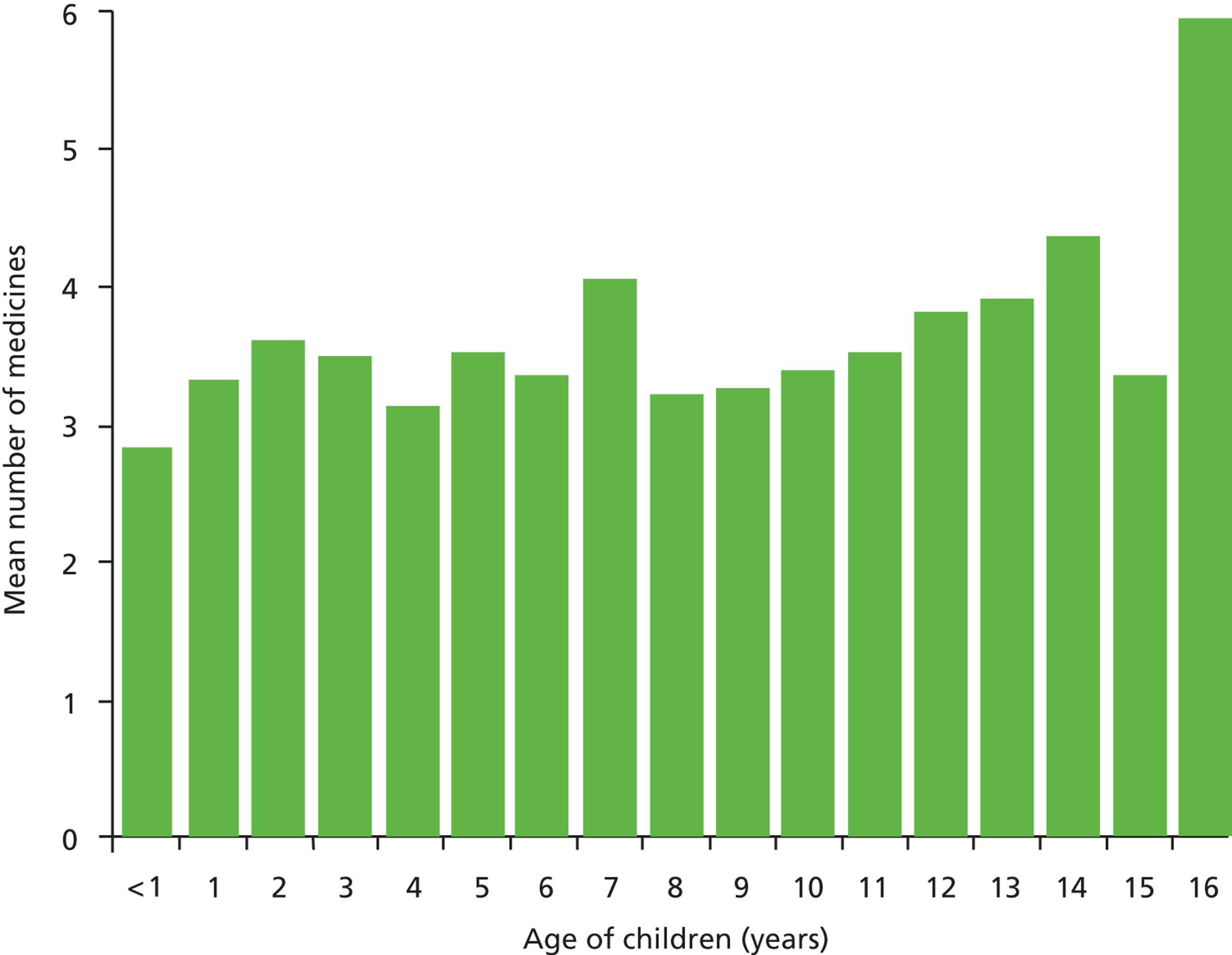
FIGURE 4.
Box and whisker plot of number of medicines taken by age group.
Cost of adverse drug reactions and length of stay
The mean cost of 238 out of 240 ADR admissions to the study hospital, using information provided by the finance department, was calculated to be £4753 per admission (95% CI £3439 to £6066). Cost data were missing for two ADR admissions: one oncology admission and one non-oncology patient admission. The mean cost of 113 oncology ADR admissions to the study hospital was £5428.91 (95% CI £4041.24 to £6816.58). The mean cost of 125 non-oncology admissions was £4141.40 (95% CI £1963.84 to £6318.95). The mean length of stay (LOS) of all 240 ADR admissions was 5.67 (95% CI 3.28 to 8.06) days. The mean LOS for the oncology admissions was 5.45 (95% CI 4.35 to 6.55) days, and 5.87 (95% CI 1.4 to 10.34) days for the non-oncology admissions.
Data from the Health and Social Care Information Centre57 showed that in 1 year, between 2009 and 2010, the total number of paediatric emergency admissions in England was approximately 597,800 (includes paediatrics and paediatric surgery, cardiology and neurology). We estimate the annual mean cost of paediatric ADR admissions to the NHS in England to be £82.4M using the mean cost of all ADR admissions to the study hospital. Using the upper and lower CIs for both our estimate of ADR incidence and study hospital costs we estimate the cost to the NHS in England of paediatric ADR admissions to be between £51.4M and £119M.
Discussion
This prospective observational study is the largest of its kind in children and the only one to comprehensively assess causality, type of reaction (predictable or not), severity, origin of drug prescription and avoidability. This is the first large study in children to investigate risk factors for the occurrence of an ADR-related admission inclusive of the use of OLUL medicines. The majority of admissions associated with ADRs in children occurred as a result of prescriptions originating in hospital. Potential preventative strategies for ADRs causing admission in children should therefore be targeted at hospital prescribing. Analysis of the ‘definitely avoidable’ ADRs in this study suggests that more careful attention to practical aspects of care – such as improved monitoring, following prescribing guidelines, improved patient education and heightened suspicion about potential adverse reactions – could lead to a reduction in the frequency of ADRs causing admission.
The incidence of ADRs causing admission in this study (2.9%, 95% CI 2.5% to 3.3%) was similar to the incidence in two systematic reviews: 2.09% (95% CI 1.02% to 3.77%) and 1.8% (95% CI 0.4% to 3.2%) but was significantly less than that of a large US study published in 1988. 33 In that study, the top three drugs causing ADRs were phenobarbital, aspirin and phenytoin, all of which are used in children much less now than in 1988. As these medicines were hardly used in our population, it is possible that the discrepancy in incidence rates relates in part to the reduction in use of these medicines.
This prospective observational study is the first to attempt the identification of possible risk factors for ADRs causing hospital admission in children. Older children, those exposed to more medicines in the 2 weeks prior to admission and oncology patients were shown to have an increased risk of ADR in this study. Girls showed a trend towards being more likely to experience an ADR than boys but this result was not statistically significant. An increased risk of ADRs occurring in the female gender has been described in studies in adult populations. 58,59 The number of authorised medicines and the number of OLUL medicines administered in the 2 weeks before admission were both significant predictors of ADR risk in this study, which supports the finding that the administration of multiple medicines increases ADR risk.
Causality was determined, of the ADR cases, using a novel CAT, the LCAT. The largest proportion of ADR causality classifications were ‘definite’ and most of these occurred in oncology patients. In order for a case report to achieve a score of ‘definite’ it would have to include a positive rechallenge or a previous history of the ADR to the same medication, a condition which these oncology-related ADRs satisfied. Type A reactions were more likely to be assigned a definite or possible causality, and type B reactions were more likely to be deemed possible. This may be due to assessors being less confident with type B ADRs, which are unpredictable and less frequent. The more severe reactions in our study were more often assessed to have definite or probable causality. This may reflect a confidence in assessing severe ADRs, which are more likely to be described in the drug safety literature.
The majority of the ADRs seen during the study were oncology related. These were mainly children with a febrile illness who developed neutropenia 1–2 weeks after intravenous chemotherapy. Clearly, patients with malignancy are often exposed to medications that cause ADRs,60 such as neutropenia (with fever), nausea, vomiting, diarrhoea, anaemia and bleeding secondary to thrombocytopenia, all of which may require hospital admission. ADRs to cytotoxic chemotherapy drugs are expected and, for the most part, may be unavoidable given the nature of the underlying illness and the treatment options currently available. Although several studies have evaluated a potential preventative strategy for neutropenia,61 no definitive evidence exists regarding the routine prophylactic use of granulocyte colony-stimulating factors to prevent ADRs due to myelosuppression. 62
Steroids, along with other immunosuppressant drugs, increase the risk of infection. 63 Immunosuppressant drugs featured frequently in our study as causative agents for ADRs. The nature of ADRs associated with immunosuppressive therapy included proven bacterial infections and viral infections (e.g. shingles). Although we recognise that infections may also occur in healthy children, the role of immunosuppressive therapy in predisposing patients to infections is well recognised. 64–66
Another frequently recorded ADR in our study was postoperative bleeding, in particular secondary haemorrhage following elective tonsillectomy. The majority (23 out of 28 admissions) of these occurred in patients exposed to intravenous dexamethasone (as prophylaxis for postoperative nausea and vomiting) and NSAIDs, with ibuprofen being used commonly in the postoperative period. A few patients received either dexamethasone or NSAIDs. Dexamethasone has been linked to post-tonsillectomy bleeding67 but its role, and the role of NSAIDs, in causing secondary haemorrhage in these children needs further study. 68,69 However, intraoperative steroid has played a major role in improving outcomes for postoperative nausea and vomiting (PONV) in children undergoing operations68,70 and has enabled day-case surgery for many conditions, thereby reducing the LOS in hospital.
Respiratory depression following treatment of seizures with benzodiazepines – a well-recognised and potentially serious event71 – was the cause of eight admissions to PICU for ventilation until recovery. Some of these cases were transfers from other regional district general hospitals to the study hospital tertiary PICU. Some, in fact, occurred as a result of rectal diazepam being used by paramedics in out-of-hospital care of seizures. Drugs used to treat status epilepticus have been widely studied and their efficacy and adverse reactions compared. 72,73 There may be drugs, other than diazepam, which have an improved benefit–risk ratio when used to treat seizures in children. 74 Further research is therefore warranted to optimise strategies for treating seizures, for both in-hospital and out-of-hospital care.
In terms of OLUL medicine use, the results described here cannot be compared easily with those of other studies, as this is the first large admissions study of this type. Previous inpatient studies report 27–45% of prescriptions being OLUL,3,75,76 and two previous community-based studies report 7% and 43% of prescriptions being off-label;77,78 compare this to 28% of prescriptions in this study. With the exception of Neubert et al. 75 these studies all found an increased ADR risk associated with OLUL medicine use. In this study, OLUL medicines were more likely to be implicated in an ADR than medicines used within the terms of their MA; however, it is important to highlight that 87.2% of ADRs that involved at least one OLUL also involved at least one other medicine; in some cases the OLUL medicine may not have caused the ADR in the absence of an approved medicine. Previous studies have examined exposure to OLUL medicines as an explanatory variable in their multivariate analysis. 3,75,76 Unlike our analysis, this approach to analysis does not take into account the relative contribution of authorised medicines. We have been able to demonstrate that, although the number of OLUL medicines contributes to ADR risk, it does so to a similar extent as the number of authorised medicines. Different OLUL medicines have different propensities to cause ADRs, it is not appropriate to consider them to be a homogeneous group. There are various types of OLUL medicines used, some of which may carry a greater risk of being implicated in an ADR than others. A key consideration is whether these medicines would be any less likely to be implicated in an ADR if they were used within the terms of their MA or if licensed preparations were used. A more detailed examination of the characteristics of the OLUL medicines that are implicated in ADRs may improve our understanding of why these medicines increase ADR risk and inform potential interventions to reduce that risk.
The design of the cohort study had limitations. The detection of suspected ADRs by the study team relied on two things: (1) signs and symptoms associated with the ADR being recorded by the clinical team looking after the patient and (2) the study team suspecting a link between signs and symptoms recorded and the medicines administered before admission. When signs and symptoms were not recorded or the study team missed the link, the ADR will not have been highlighted or evaluated. Although this was a single-centre study, it was carried out in a large centre providing a comprehensive range of paediatric services to a diverse population. The ADRs reported in this study highlight some of the adverse consequences of drugs in children. A limitation of this study is that we have not taken into account the benefits of these medications. Furthermore, we cannot be certain of the aetiological fraction (the risk of an event occurring in the presence of a risk factor) for some of the drugs in our study (e.g. immunosuppressant drugs) in their contribution to the stated reactions. For these drugs, more research is needed to accurately assess their contribution to ADRs and the ill health of children, to allow for more detailed risk–benefit evaluation. In this study, we have not considered ADRs caused by medications during inpatient stay in hospital. This aspect of drug reactions is likely to add greatly to the burden of ill health to children, and requires investigation of paediatric inpatient ADRs using a similar prospective study design to accurately identify the epidemiology of the problem.
The cost of ADRs to the NHS in England was calculated using knowledge of the cost of admissions to the study hospital, our estimate of the incidence of ADRs causing admission and an estimate of total paediatric admissions annually to hospitals in England. Information regarding total annual admissions does not include emergency paediatric admissions from other specialties, thereby underestimating the total number of emergency paediatric admissions to hospitals in England. Although the ADR admission incidence from this study includes oncology cases, which is not included in the total annual admissions number used for our cost calculation, our estimate of costs of paediatric ADR admissions may be an underestimation.
The results of this study will be used to inform paediatric pharmacovigilance practice. We have demonstrated that ADRs cause admissions to a paediatric hospital and some of these are serious and potentially avoidable. Strategies to reduce the burden of ill health from these ADRs are needed. Prevention will depend on whether an ADR is avoidable or not, ADRs that are avoidable by applying existing knowledge require efforts to implement good prescribing practice. The vast majority of ADRs identified were type A (predictable or dose related). We have shown that OLUL prescribing is a risk factor for ADRs and identified some drugs/classes for which further work is needed. This finding must be put in context of the fact that the number of medicines per se, irrespective of the licensing status, is also a risk factor for ADRs. Better dosing schedules for medicines, particularly those with a narrow therapeutic index, are likely to be key in reducing the burden of ADRs in children. Other ADRs that are currently unavoidable may be ameliorated by comedication, for example concomitant use of laxatives to prevent constipation. As many ADRs are unavoidable in the light of current knowledge, there is likely to be a continuing burden of ADRs in paediatric hospitals and further research is needed. Consideration should also be given to how suspected ADRs are handled in hospitals to improve identification of, and communication about, ADRs. Clinicians prescribing for children should be vigilant for the occurrence of ADRs, and should prescribe the minimum number of drugs at the lowest possible dose and shortest duration of time, with continual monitoring to stop drugs when relevant and to ensure that ADRs are detected as early as possible.
Chapter 3 Adverse drug reactions in hospitalised children
This chapter contains information reproduced from Thiesen S, Conroy EJ, Bellis JR, Bracken LE, Mannix HL, Bird KA, et al. Incidence, characteristics and risk factors of Adverse Drug Reactions (ADRs) in hospitalised children: a prospective observational cohort study of 6601 admissions. BMC Med 2013;11:237,22 © 2013 Thiesen et al. ; licensee BioMed Central Ltd, an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided that the original work is properly cited; from Bellis J, Kirkham J, Thiesen S, Conroy E, Bracken L, Mannix H, et al. Adverse drug reactions and off-label and unlicensed medicines in children: a nested case-control study of inpatients in a pediatric hospital. BMC Med 2013;11:238,79 © 2013 Bellis et al. , licensee BioMed Central Ltd, an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided that the original work is properly cited; and reproduced with permission from Bellis JR. Adverse Drug Reactions in Children – The Contribution of Off-label and Unlicenced Prescribing. PhD thesis. Liverpool: University of Liverpool; 2013. 31
Abstract
Background
Adverse drug reactions are an important cause of iatrogenic harm in children. We aimed to determine the incidence of ADRs, identify risk factors for ADRs in hospitalised children, and characterise these ADRs in terms of type, drug aetiology, causality and severity.
Methods
We undertook a prospective observational cohort study in admissions to a single UK paediatric hospital. A nested case–control study within the cohort examined the impact of OLUL drug use on ADR risk. Participants were aged between 0 and 16 years 11 months, who were admitted for > 48 hours between 1 October 2009 and 30 September 2010.
Results
In total, 5118 children participated and 17.7% of all children experienced at least one ADR. Opiate analgesic drugs and drugs used in general anaesthesia (GA) accounted for > 50% of all drugs implicated in ADRs. Our nested case–control study included 1388 patients. The OR of an OLUL drug being implicated in an ADR compared with an authorised drug was 2.25 (95% CI 1.95 to 2.59; p < 0.001). Risk factors identified were exposure to a GA, age, oncology treatment and number of medicines.
Conclusions
The incidence of ADRs is higher in hospitalised children than in hospitalised adults, with GA agents and opiate analgesic drugs being the chief causes. OLUL drugs are more likely to be implicated in an ADR than approved drugs. It is important to develop strategies to reduce the burden of ADRs occurring in hospitalised children.
Introduction
Adverse drug reactions are an important cause of iatrogenic morbidity and mortality in patients of all ages. 59,80–83 ADRs in children may differ from those in adults owing to age-dependent physiological characteristics that affect the pharmacokinetics and pharmacodynamics of drugs. 80,81,84,85
Off-label and/or unlicensed drug use has been identified in previous studies as an ADR risk factor. However, this has not been demonstrated consistently by all studies, many of which were small, used different methodologies and used inexact and varying definitions. 86 Off-label drug use is the use of a drug outside of the terms of its MA, and unlicensed drugs are those without a MA in the country in which they are prescribed. The reported incidences of OLUL use of medicines in children ranges from 36% to 100%. 87 OLUL prescribing has been prevalent in paediatric practice because of the lack of assessment of the use of drugs in children during the drug development process. 88 Although the recently introduced paediatric regulation in Europe13 and the updated regulation89 in the USA are likely to improve the situation, it is going to take time.
The aim of this study was to determine the incidence of ADRs in paediatric medical and surgical inpatients; characterise those ADRs identified in terms of type, drugs implicated, involvement of OLUL drugs, causality and severity; and also identify factors that increase the risk of ADRs. Reducing the impact of paediatric ADRs needs precise estimates of the incidence and nature of ADRs. Given the discordances in the extant literature, we designed a study that was large enough and of robust design to overcome the problems identified in the previous literature.
Methods
Study design and setting
The study was a prospective observational cohort study conducted over 1 year in a single paediatric referral centre providing a local and also specialist regional and national services in the north-west of England (Alder Hey).
The study population comprised children aged between 0 and 16 years and 11 months, who were inpatients between 1 October 2009 and 30 September 2010. Extensive pilot work was conducted prior to the beginning of the study. This pilot work established that the study team did not have the resources to carry out a detailed review of every inpatient every day. Three alternative inclusion criteria were considered: all inpatients, children admitted for > 24 hours and children admitted for > 48 hours. ‘Patients admitted for > 48 hours’ was the inclusion criterion selected, as this allowed study procedures to be optimised for the full observational study (frequency of follow-up visits, amount of prescription and clinical data to be recorded, source data to be considered, use of electronic database). Admissions included elective and emergency admissions to all paediatric medical and paediatric surgical specialties. Observations were carried out on 17 wards, including oncology wards and the high-dependency unit (HDU). Patients were not observed while admitted to PICU, transitional care unit (TCU), theatre, recovery or the department of radiology. Patients who spent their entire admission on PICU were excluded. The study methodology did not cover all aspects of the clinical complexity and the study team did not have the tools or expertise required to identify and assess ADRs in an intensive care environment. Patients who spent their entire admission on TCU were excluded. These patients have complex medical and nursing needs but are clinically stable. In general, they are awaiting transfer home or to a placement in the community. If they became acutely unwell during the study, they would have been admitted to the hospital and become eligible for inclusion. Children meeting the inclusion criteria were identified twice daily by means of an automated computer download. Each child was followed up every 48 hours or 72 hours on weekdays and weekends, respectively, by one member of a multidisciplinary team (MDT) of researchers comprising two research pharmacists, one research nurse and a paediatrician (LEB, JRB, KB, HM, ST). For each child, details were recorded of all drugs administered on the wards, occurrence of new symptoms or those that had worsened, and abnormal results that may indicate the occurrence of an ADR, taking into account the case history, the ADR profiles of medication and the temporal relationship between drug exposure and reaction. We aimed to include all potential reactions to any medication administered in hospital (including those started prior to admission) and present after admission to a ward; each suspected reaction was followed up with a detailed assessment by one research team member.
As highlighted by the pilot study, it was not practical to follow all admissions in the prospective cohort study in sufficient detail; therefore, a subset study of nested case–control design was undertaken to assess the involvement of OLUL drugs that were implicated in observed ADRs. Cases were those who had experienced at least one probable or definite ADR on their first admission and were matched 1 : 1 to control subjects who had not experienced any probable or definite ADRs. Matching was based on the closest date and time of admission.
Adverse drug reaction definition
In our study we used the ADR definition of Edwards and Aronson:49 ‘an appreciably harmful or unpleasant reaction, resulting from an intervention related to the use of a drug product, which predicts hazard from future administration and warrants prevention or specific treatment, or alteration of the dose regimen, or withdrawal of the product’. Prescribing errors, administration errors and accidental overdoses were thus not considered ADRs in this study. ADR cases were defined as suspected reactions to any systemic or topical drug product administered in hospital and presenting after admission to the ward or in A&E prior to admission to the ward. This included reactions to drug products administered in PICU, theatre, recovery, the department of radiology or TCU, providing that the reaction became apparent after transfer to a ward. Reactions to a drug product started prior to admission were included if it was continued in hospital and the reaction was not apparent on admission. Suspected reactions to certain drug products (including some blood products, total parenteral nutrition and intravenous hydration fluids) were excluded from this study. For details of included/excluded drug products see Table 11 .
Drug categories
For each patient in the nested case–control study, the record of drugs administered was updated to include a detailed OLUL category for every drug. There were 30 off-label drug categories and five unlicensed drug categories (see Table 11 ). Off-label categories were defined according to the reason(s) why their use was deemed off-label when compared with the terms of the MA. The terms of the MA were found in the SmPC available online from the EMC. 50 With regard to age, if the SmPC mentioned children, the definition of this was assumed to be 28 days to 18 years. If no specific information pertaining to use in neonates was provided, the use of that drug in neonates was considered to be off-label. Although it was certainly not the case, it was assumed that all neonates were born at term because gestational age was not recorded in this study. Owing to the complex nature of the regimens used to treat malignant disease, the classification of cytotoxic drugs was simplified by consulting the BNF-C90 for cytotoxic drugs with a UK MA. If the BNF-C monograph stated the relevant indication, it was assumed that the use was authorised. If the BNF-C monograph stated ‘not licensed in children’ then the use was considered to be off-label. The implications of dosage from manipulation by parents or nursing staff, such as the crushing of tablets or the addition of licensed drugs to food or drinks for ease of administration, was considered to be outside the scope of this study.
| Category | Definition | ||
|---|---|---|---|
| Off-label drugs | Drugs licensed for use in children | 1 | Authorised – drug used within the terms of its marketing authorisation |
| 2 | Contraindication exists | ||
| 3 | Dose greater than recommended | ||
| 4 | Dose greater than recommended and contraindication exists | ||
| 5 | Not licensed in child of this age (or child below minimum weight stated) | ||
| 6 | Not licensed in child of this age and contraindication exists | ||
| 7 | Not licensed by this route | ||
| 8 | Not licensed by this route and contraindication exists | ||
| 9 | Not licensed by this route or in a child of this age | ||
| 10 | Not licensed by this route or in a child of this age and contraindication exists | ||
| 11 | Not licensed for this indication | ||
| 12 | Not licensed for this indication and contraindication exists | ||
| 13 | Not licensed for this indication or at this dose | ||
| 14 | Not licensed for this indication or at this dose and contraindication exists | ||
| 15 | Not licensed for this indication or at this age | ||
| 16 | Not licensed for this indication or at this age and a contraindication exists | ||
| 17 | Not licensed for this indication or by this route | ||
| 18 | Not licensed for this indication or by this route and a contraindication exists | ||
| 19 | Not licensed for this indication or by this route or at this age | ||
| 20 | Not licensed for this indication or by this route or at this age and a contraindication exists | ||
| Drugs not licensed for use in children | 21 | Not licensed for use in children | |
| 22 | Not licensed for use in children and a contraindication exists | ||
| 23 | Not licensed for use in children or in adults by this route | ||
| 24 | Not licensed for use in children or in adults by this route and a contraindication exists | ||
| 25 | Not licensed for use in children or in adults for this indication | ||
| 26 | Not licensed for use in children or in adults for this indication and a contraindication exists | ||
| 27 | Not licensed for use in children or in adults for this indication or in adults by this route | ||
| 28 | Not licensed for use in children or in adults for this indication or in adults by this route and a contraindication exists | ||
| Medicines excluded from analysis | 29 | Category cannot be assigned | |
| 30 | Theatre drug | ||
| Unlicensed drugs | 31 | Prepared extemporaneously | |
| 32 | Manufactured under a specials manufacturing licence | ||
| 33 | Chemical | ||
| 34 | Import | ||
| 35 | Awaiting a MA (e.g. previous trial drug) | ||
Causality and severity assessment of adverse drug reactions
Each suspected ADR was followed up with a detailed assessment by one research team member. The ADR case report was then assessed independently by a research nurse, a research pharmacist and a paediatrician using the LCAT53 as unlikely, possible, probable or definite. Outcome reporting was based on consensus agreement between the three assessors; if agreement could not be achieved the case was referred to a panel of two senior investigators (MAT, RLS, AJN and MPir); each panel reached consensus about causality. For ADRs with a high or uncertain probability that the reaction is due to an underlying disease, the causality outcome is ‘possible’ unless objective evidence of the causal ADR mechanism is available. 53 Our estimate for the overall incidence was based on the sum of probable and definite ADRs only, as these ADRs are deemed to have a low probability of the underlying disease being responsible for the reaction. Severity of ADRs was assessed by the researcher compiling the case report using the Hartwig scale. 56 In addition, all ADRs that occurred prior to a patient’s admission to PICU or HDU were also assessed by a paediatrician and, if required, reviewed by a panel of two senior investigators in order to evaluate their contribution to the patient being transferred to a higher level of care (Hartwig scale level 4). Reactions classified as level 4 and above were considered severe.
Incidence
Incidence was calculated in two ways: (1) the number of admissions in which at least one ADR occurred divided by the total number of admissions regardless of drug exposure and (2) the number of children with at least one ADR divided by the total number of children regardless of drug exposure.
Odds ratios
For analysis of the nested case–control study, the OR with 95% CI of a drug being implicated in a probable or definite ADR was calculated for each category of OLUL drugs administered compared with a baseline risk for authorised drugs.
Risk factor analysis
Time from admission to first ADR was calculated in days. For patients admitted to PICU, this was time to first ADR prior to PICU admission. If no ADR occurred prior to PICU admission, time from admission to first ADR was censored at the time of admission to PICU. ADRs occurring after PICU were included in the overall incidence calculation. For the analysis of risk factors, data collected for each patient during only their first admission were included.
Within the main study, we assessed age, gender, number of drugs, receipt of a GA and oncology patient status as risk factors. Oncology patients were those requiring ongoing medical treatment for a malignancy of solid organ or haematopoietic system. The number of drugs refers to the daily number of drugs administered to the patient on the ward. This risk factor was treated as a continuous, time-varying covariate in the multivariate model.
The factor ‘received a GA’ was considered to be present from the first day the patient received a GA until discharge from hospital. This risk factor was treated, as a binary, time-varying variable in the multivariate model that takes the value ‘0’ on days up to the GA and ‘1’ thereafter for the remaining days of a patient’s admission.
The same risk factors were assessed in our nested case–control study. However, to avoid issues caused by including dependent variables, we replaced the ‘daily number of drugs’ variable in the model with the two variables ‘daily number of OLUL drugs’ and ‘daily number of authorised drugs’. These were treated as continuous, time-varying covariates in the model.
Statistical methods
Time to first ADR was compared between groups using a log-rank test (extending to a log-rank test for trend when appropriate) and Kaplan–Meier curves estimated.
A Cox proportional hazards regression model for an ADR was fitted to the data. Results are given in terms of the hazard ratio (HR) and associated 95% CI. Owing to their clinical importance, all of the risk factor variables were included in the multivariate models fit in both the main analysis and the subset analysis.
The assumptions of the model were assessed as follows. The proportional hazards assumption for each covariate was investigated using log-cumulative hazard plots and Schoenfeld residual plots. The assumption was also tested by including a time-dependent covariate effect. Deviance residuals were plotted against the linear predictor to look for mismodelling of the data and empirical validation for the model was carried out using a data-splitting technique to assess model accuracy. Patients with missing prescription details for the entire duration of the admission were excluded from the analysis. The inclusion of patients with partially missing prescription details (e.g. prescription details for day of discharge) was assessed on a case-by-case basis.
All statistical analysis was carried out using the statistical software package R version 2.13.2 (The R Foundation for Statistical Computing, Vienna, Austria) using a two-sided significance level of 0.05 (5%) throughout.
Reporting
This study was reported according to Strengthening The Reporting of OBservational studies in Epidemiology (STROBE) guidelines. 91
Ethics
This study used routinely collected clinical data in an anonymised format. The chairperson of Liverpool Paediatric Local Research Ethics Committee informed us that this study did not require individual patient consent or review by an ethics committee.
Results
Participants and descriptive data
Prospective cohort study – 6825 eligible admissions were identified. A total of 181 (2.7%) admissions could not be included owing to missing data. Forty-three patients spent their entire admission on PICU and were thus excluded. The median length of follow-up time across admission was 5 days (IQR 3–8 days, range 2–280 days). The median age on admission was 3.4 years (IQR 0.6–10.7). Overall, 2297 (44.8%) were female, 4284 (83.7%) of children had one admission, 834 children had more than one admission, 2856 children (55.8%) underwent at least one GA during 3265 admissions, and 114 children (2.2%) were oncology patients.
In total, 2934 suspected ADRs were assessed. After causality assessment, 213 (7.3%) of suspected ADRs were deemed definite, 1233 (42.0%) probable, 896 (30.5%) possible and 592 (20.2%) unlikely. Consensus was reached by independent agreement in 1805 cases (61.5%) and by panel decision in 1128 cases (38.5%). All definite and probable ADRs were included in the further analysis (total number 1446; Figure 5 ).
FIGURE 5.
Flow chart outlining number of first admissions included in the cohort study univariate and multivariate analysis.
The overall incidence of definite and probable ADRs based on all admissions was 15.9% (95% CI 15.0% to 16.8%) and 17.7% per patient (95% CI 16.7% to 18.8%). The ADR incidence for patients with only one admission was 14.8% (95% CI 13.8% to 15.9%). For patients with more than one admission, the incidence per admission was 18.0% (95% CI 13.8% to 15.9%) and 32.7% per patient (95% CI 29.6% to 35.9%). Of the ADRs 0.9% were severe and required patient transfer to a higher level of care. One patient sustained permanent harm (peripheral neuropathy due to vincristine). No ADR resulted in patient death (see Table 12 ). Details of all severe reactions by reaction type and associated drugs are listed ( Tables 12 and 13 ).
| Severity level | Description | No. of ADRs at each severity levela | |
|---|---|---|---|
| n | % | ||
| 1 | Required no change in treatment | 322 | 22.3 |
| 2 | Drug dosing or frequency changed | 66 | 4.6 |
| 3 | Required treatment, or drug administration discontinued | 1046 | 72.3 |
| 4 | Result in patient transfer to higher level of care | 12 | 0.8 |
| 5 | Caused permanent harm to patient or significant haemodynamic instability | 1 | 0.1 |
| 6 | Directly or indirectly resulted in patient death | 0 | 0 |
| Severity level | ADR type (count) | Medication implicated (count) | Admission to PICU/HDU (if more than once) |
|---|---|---|---|
| 4 | Cardiac failure (1) | Bisoprolol (1), carvedilol (1) | HDU |
| Sedation withdrawal (1) | Fentanyl (1), midazolam (1), promethazine (1), chloral hydrate (1) | PICU | |
| Raised international normalised ratio and haemorrhage (1) | Warfarin (1) | HDU | |
| Pulmonary oedema (1) | Diazoxide (1) | HDU | |
| Respiratory depression (5) | Fentanyl (4), ketamine (2), midazolam (1) | PICU (3a), HDU (2) | |
| Respiratory arrest (2) | Fentanyl (2), sevoflurane (1), isoflurane (1), ketamine (1) | PICU, HDU | |
| 5 | Peripheral neuropathy (1) | Vincristine (1) | N/A |
Nested case–control study
A total of 1388 patients were analysed throughout their first admission: 694 (50%) were cases; 634 (45.6%) were female; 294 (21.2%) were < 1 year old; 341 (24.6%) were aged 1–4 years; 384 (27.7%) were 5–11 years and 369 (26.6%) were teenagers (> 12 years). The median age was 5.9 years (IQR 1.4–12.4 years). A total of 10,699 drug courses were administered in this study.
Within this nested cohort, there were 785 suspected ADRs deemed definite or probable in 694 patients during the first admission. Of the suspected ADRs, 62 (7.9%) were deemed definite and 723 (92.1%) probable. Of these, 505 (64.3%) involved one drug course, 172 (21.9%) involved two drug courses, 77 (9.8%) three and 31 (3.9%) four or more. Of the total drug courses, 10,145 (94.8%) could be categorised using Table 11 . The remaining 554 (5.2%) courses could not be categorised, as the prescription record did not provide the required information, for example, missing dose information or insufficient detail about the exact preparation used. Of the 785 definite and probable ADRs, 301 (38%) involved only OLUL medicines, 290 (37%) involved only authorised medicines, 160 (20.4%) involved a combination of OLUL and authorised medicines, and the remaining 4.3% involved at least one medicine that could not be categorised.
Reaction types, drug classes and ‘off-label and/or unlicensed’ categories implicated in adverse drug reactions
Prospective cohort study
The 10 most common reaction types were vomiting and/or nausea, pruritus, constipation, diarrhoea, somnolence without cardiorespiratory symptoms, respiratory depression or arrest, candidiasis, urinary retention, rash and hypokalaemia, which, together, accounted for 76.6% of all ADRs. Pruritus, respiratory depression and urinary retention occurred almost exclusively in the post-anaesthetic setting. In over two-thirds of patients with nausea/vomiting, constipation or somnolence, drugs given during the anaesthetic and/or used in postoperative pain management were implicated ( Table 14 ).
| Reaction type | All reactions | Following GAa | ||
|---|---|---|---|---|
| n | % of all reactionsb | n | % of reaction type where reaction followed GA | |
| Nausea and/or vomiting | 400 | 27.5 | 295 | 73.8 |
| Pruritus | 243 | 16.7 | 232 | 95.5 |
| Constipation | 155 | 10.6 | 107 | 69.0 |
| Diarrhoea (nine with vomiting) | 88 | 6.0 | 0 | 0.0 |
| Somnolence (without cardiorespiratory symptoms) | 50 | 3.4 | 34 | 68.0 |
| Respiratory depression/arrest (41/3) | 44 | 3.0 | 43 | 97.7 |
| Candidiasis | 41 | 2.8 | 0 | 0.0 |
| Urinary retention | 40 | 2.7 | 37 | 92.5 |
| Rash | 31 | 2.1 | 3 | 9.7 |
| Hypokalaemia | 25 | 1.7 | 0 | 0.0 |
| Hypotension | 22 | 1.5 | 9 | 40.9 |
| Hepatotoxicity (12 transaminases increased only) | 18 | 1.2 | 1 | 5.6 |
| Stomatitis | 16 | 1.1 | 0 | 0.0 |
| Myoclonus | 15 | 1.0 | 14 | 93.3 |
| Pancytopenia | 13 | < 1 | 0 | 0.0 |
| Hyperglycaemia | 12 | < 1 | 0 | 0.0 |
| Hypertension | 11 | < 1 | 2 | 18.2 |
| Allergic reactions | 10 | < 1 | 3 | 30.0 |
| Pain (four with pain in jaw, two with back pain) | 10 | < 1 | 0 | 0.0 |
| Other reactions (occurred < 10 times) | 213 | 14.6 | 65 | 30.5 |
| Total | 1457 | 845 | 58.0 | |
Drugs implicated in ADRs and associated reactions are listed in Table 15 . Opioid analgesic drugs and anaesthetic agents were the most commonly implicated drug groups and accounted for 54% of all drugs associated with ADRs. Cytotoxic drugs accounted for 13% and antibiotics for 11% of medication implicated. Drugs used in postoperative pain management accounted for 6%. Each other drug group accounted for ≤ 2%.
| Drug group (n of ADR cases) | Total no. of drugs (% of total) | Drugs (n) | ADR typea (n) |
|---|---|---|---|
| Opioid analgesic drugs (688) | 844 (27.9) | Morphine (426), fentanyl (267), codeine (144), dihydrocodeine (4), diamorphine (2), tramadol (1) | Pruritus (198), nausea or vomiting (186), constipation (143), respiratory arrest/depression (3/37), somnolence without cardiorespiratory symptoms (37), urinary retention (28), myoclonus (13), hallucination (8), rash (4), bradycardia (3), dizziness (3), drug withdrawal syndrome (3), ileus (3), agitation (2), delayed recovery from anaesthesia (2), flushing (2), visual disturbance (2), otherb (11) |
| Drugs used in GA (excluding opiate analgesic drugs other than remifentanil) (322) | 779 (25.8) | Sevoflurane (253), propofol (200), nitrous oxide (131), remifentanil (83), desflurane (54), isoflurane (38), ketamine (6), atracurium (4), rocuronium (4), thiopental (4), atropine (1), vecuronium (1) | Nausea or vomiting (266), urinary retention (21), respiratory arrest or depression (2/6), delayed recovery from anaesthesia (5), flushing (4), bradycardia (3), allergic reaction (3), hypotension (3), pruritus (2), otherb (7) |
| Cytotoxic drugs and drugs used for cytotoxic-induced side effects (179) | 405 (13.4) | Vincristine (70), etoposide (56), cyclophosphamide (46), cytarabine (41), methotrexate (31), doxorubicin (22), ifosfamide (21), mesna (15), daunorubicin (13), carboplatin (12), cisplatin (12), melphalan (11), busulfan (7), asparaginase (6), fludarabine (6), clofarabine (5), actinomycin d (5), allopurinol (4), mitoxantrone (4), rasburicase (4), idarubicin (3), thiotepa (3), amsacrine (2), temozolomide (2), cladribine (1), gemcitabine (1), irinotecan (1), tretinoin (1) | Nausea or vomiting (81), stomatitis (16), pancytopenia (13), diarrhoea and vomiting (9), diarrhoea without vomiting (9), hepatotoxicity (11; 8 increased transaminases only), febrile neutropenia (6), rash (6), pain in jaw (3), constipation (3), pain other than jaw (2), headache (2), hyperglycaemia (3), oral candidiasis (3), otherb (14) |
| Antibiotic drugs (162) | 319 (10.6) | Cefotaxime (56), metronidazole (29), gentamicin (29), piperacillin and tazobactam (28), cefuroxime (18), teicoplanin (19), cefalexin (17), ciprofloxacin (16), flucloxacillin (15), co-amoxiclav (16), ceftazidime (13), rifampicin (10), amoxicillin (8), clarithromycin (7), vancomycin (7), penicillin V (5), benzylpenicillin (4), meropenem (4), amikacin (3), co-trimoxazole (3), tobramycin (3), trimethoprim (3), clindamycin (2), cefradine (1), ceftriaxone (1) | Diarrhoea (66), candidiasis (38), rash (16), nausea or vomiting (8), Clostridium difficile colitis (7), colonisation with candida (4), transaminases increased (4), anaphylactic reaction (2), angioedema (2), flushing (2), hepatotoxicity (3), pruritus (2), otherb (8) |
| Drugs used in epidurals, regional anaesthetics and intravenous drugs used in postoperative pain management other than opioid drugs (188) | 195 (6.4) | Fentanyl and levobupivacaine (116), ketamine (36), clonidine and levobupivacaine (25), levobupivacaine (11), clonidine (7) | Pruritus (52), nausea and/or vomiting (35), constipation (24), urinary retention (15), somnolence without cardiorespiratory symptoms (11), respiratory depression/arrest (8/1), hypotension (7), paraesthesia (6), bradycardia (4), myoclonus (3), hypoaesthesia (2), visual disturbance (2), hallucination (2), hypertension (2), urinary incontinence (2), otherb (12) |
| Corticosteroids (51) | 62 (2.05) | Dexamethasone (24), methylprednisolone (14), prednisolone (14), hydrocortisone (8), beclomethasone (1), fludrocortisone (1) | Hyperglycaemia (13), hypertension (8), candidiasis (9), fluid retention (2), gastritis (2), otherb (17) |
| Bronchodilators (31) | 58 (1.92) | Salbutamol (35), aminophylline (21), ipratropium (2) | Hypokalaemia (15), nausea and/or vomiting (7), tremor (4), tachycardia (2), otherb (3) |
| Antiemetic drugs (50) | 55 (1.82) | Ondansetron (51), levomepromazine (3), cyclizine (1) | Constipation (45), disorientation (2), otherb (4) |
| Antiepileptic drugs (45) | 49 (1.62) | Midazolam (35), pregabalin (4), carbamazepine (3), diazepam (3), gabapentin (2), lorazepam (1), valproate (1) | Nausea and/or vomiting (24), somnolence without cardiorespiratory symptoms (6), abnormal behaviour (2), constipation (2), delayed recovery from anaesthesia (2), respiratory depression (2), otherb (7) |
| Diuretic drugs (28) | 41 (1.36) | Furosemide (30), spironolactone (8), metolazone (2), chlorothiazide (1) | Hyponatraemia (9), hypokalaemia (8), hypotension (3), hypomagnesaemia (4), otherb (5) |
| Drugs affecting the immune responses (suppression and modulation) + cytokine modulators (31) | 34 (1.12) | Alemtuzumab (11), ciclosporin (7), aldesleukin (5), rabbit antihuman thymocyte immunoglobulin (3), tacrolimus (3), rituximab (2), azathioprine (1), mycophenolate (1), tocilizumab (1) | Pyrexia (4), candidiasis (4), infusion associated reaction (3), stomatitis (3), oedema (2), pruritus (2), vomiting (2), otherb (11) |
| Drugs affecting the cardiovascular system (23) | 27 (0.89) | Captopril (10), lisinopril (4), amlodipine (4), milrinone (3), bisoprolol (1), dinoprostone (1), enalapril (1), hydralazine (1), isoprenaline (1), carvedilol (1) | Hypotension (11), hyperglycaemia and glycosuria (3), otherb (9) |
| NSAIDS (+ aspirin) (24) | 24 (0.79) | Diclofenac (15), ibuprofen (5), naproxen (2), aspirin (2) | Nausea and/or vomiting (11), haematemesis (3), other gastrointestinal bleed (2), constipation (2), otherb (5) |
| Laxatives (20) | 22 (0.73) | Lactulose (12), macrogol (6), docusate (3), sennoside (1) | Diarrhoea (17), abdominal pain (2), vomiting (1) |
| Antifungal and antiviral drugs (20) | 21 (0.69) | Amphotericin (7), aciclovir (5), fluconazole (4), voriconazole (2), itraconazole (1), miconazole (1), ribivarin (1) | Diarrhoea (8), hepatotoxicity (3), hypokalaemia (3), otherb (5) |
| Drugs used in diabetes and hypoglycaemia (13) | 16 (0.53) | Insulin (4), insulin aspart (4), insulin detemir (4), diazoxide (3), glucagon (1) | Hypoglycaemia (7), fluid overload (2), hypokalaemia (2), otherb (2) |
| Other (69) | 73 (2.41) | – | – |
Nested case–control study – of the 10,145 categorised drug courses, 6980 (68.8%) were authorised, 2407 (23.7%) off-label and 758 (7.5%) unlicensed; 435 (6.2%) of authorised, 298 (12.4%) of off-label, and 113 (14.9%) of unlicensed drug courses were implicated in at least one probable or definite ADR. The OR of an OLUL drug being implicated in an ADR when compared with an authorised drug course was 2.25 (95% CI 1.95 to 2.59; p < 0.001). In total, 19 (54.3%) of the OLUL categories (see Table 11 ) were utilised. Table 16 shows the number of drug courses in each of these categories. Category 11 (‘drug licensed for children but given for a different indication’) is the most common category of off-label drug use (n = 764; 31.7%). Categories 3, 5 and 11 together represented 2050 (85.2%) of off-label drug courses. Category 32 (‘manufactured under a specials manufacturing licence’) is the most common category of unlicensed drug use (n = 577; 76%). Table 16 shows the proportion of drug courses from each category implicated in at least one probable or definite ADR in comparison with the proportion of authorised drug courses implicated (n = 6980; 6.2%). Further analysis was undertaken on six of these categories, which contained > 100 drug courses. Results showed that category 3 (‘drugs licensed for use in children but given at a dose greater than recommended’) had a lower risk of being implicated in an ADR than category 1 – authorised drugs (OR 0.42, 95% CI 0.26 to 0.67). Category 5 (‘drugs licensed in children but given to a child below the minimum age or weight’) had the greatest risk of being implicated in an ADR (OR 3.54, 95% CI 2.82 to 4.44).
| Categorya | No. of drug courses | % of courses implicated in at least one possible and definite ADR | OR of ADR vs. authorised | 95% CI | ||
|---|---|---|---|---|---|---|
| Off-label drugs | Drugs licensed for use in children | 1 | 6980 | 6.2 | 1.00 | – |
| 2 | 1 | 0 | – | – | ||
| 3 | 698 | 2.7 | 0.42 | 0.26 to 0.67 | ||
| 5 | 588 | 19.0 | 3.54 | 2.82 to 4.44 | ||
| 6 | 1 | 0 | – | – | ||
| 7 | 61 | 9.8 | 1.64 | 0.70 to 3.83 | ||
| 11 | 764 | 14.3 | 2.50 | 2.00 to 3.13 | ||
| 13 | 8 | 0 | – | – | ||
| 15 | 35 | 25.7 | 5.21 | 2.43 to 11.18 | ||
| 17 | 21 | 0 | – | – | ||
| 19 | 2 | 0 | – | – | ||
| Drugs not licensed for use in children | 21 | 215 | 18.6 | 3.44 | 2.41 to 4.91 | |
| 22 | 1 | 100.0 | – | – | ||
| 23 | 1 | 0 | – | – | ||
| 25 | 11 | 18.2 | 3.34 | 0.72 to 15.52 | ||
| Unlicensed drugs | 31 | 143 | 14.7 | 2.59 | 1.61 to 4.16 | |
| 32 | 577 | 14.9 | 2.64 | 2.05 to 3.38 | ||
| 33 | 1 | 0 | – | – | ||
| 34 | 37 | 16.2 | 2.91 | 1.21 to 7.02 | ||
Table 17 shows the proportion of drug courses implicated in a probable or definite ADR specifically for drugs, with > 100 courses administered, and the proportion of courses that were categorised as OLUL. Fentanyl via any route excluding epidural had the greatest proportion of courses implicated in an ADR, 48.0% of courses were implicated with 99.3% of courses categorised as off-label. Fentanyl via the epidural route had 44.3% of courses implicated with 100% of courses categorised as unlicensed. Morphine via any route had 35.0% of courses implicated, of which 39.6% were OLUL. Table 18 shows the four most frequently implicated drugs by OLUL category. The majority of fentanyl courses were category 11 – given for a different indication; 91.7% of implicated fentanyl courses fell into this category. A total of 60.4% of morphine courses were authorised and 49.1% of implicated morphine courses fell into this category. Over one-third of morphine courses were category 5 (‘not licensed in child of this age or child below minimum weight stated’) and 50.3% of implicated morphine courses fell into this category.
| Category of drug use | Drug (no. of courses) | % of courses off-label or unlicenseda | % of courses implicated in at least one ADR | No. of courses unknown |
|---|---|---|---|---|
| Drugs with only authorised courses | Cefuroxime (245) | 0 | 4.5 | 1 |
| Cefotaxime (388) | 0 | 9.0 | 0 | |
| Drugs with off-label courses | Chlorphenamine (339) | 0.3 | 0.3 | 1 |
| Diazepam (107) | 1.9 | 1.9 | 0 | |
| Ibuprofen (545) | 4.8 | 0.7 | 0 | |
| Lactulose (272) | 4.8 | 2.2 | 0 | |
| Cefalexin (148) | 6.1 | 7.4 | 0 | |
| Metronidazole (257) | 8.2 | 7.8 | 0 | |
| Furosemide (123) | 11.8 | 9.8 | 0 | |
| Paracetamol (1786) | 33.4 | 0.1 | 2 | |
| Ondansetron (550) | 52.7 | 5.8 | 48 | |
| Salbutamol (146) | 56.8 | 8.9 | 0 | |
| Ranitidine (109) | 59.6 | 0.9 | 0 | |
| Dexamethasone (166) | 64.5 | 6.6 | 7 | |
| Fentanyl (150) | 99.3 | 48.0 | 0 | |
| Drugs with unlicensed courses | Fentanyl and levobupivicaine epidural (106) | 100 | 44.3 | 0 |
| Drugs with off-label and unlicensed courses | Codeine phosphate (752) | 1.6 | 13.2 | 257 |
| Morphine (500) | 39.6 | 35.0 | 0 | |
| Diclofenac (331) | 45.0 | 1.5 | 159 |
| Categorya | Fentanyl (implicated) | Fentanyl + levobupivicaine epidural (implicated) | Morphine (implicated) | Codeine (implicated) |
|---|---|---|---|---|
| 1 | 1 (0) | – | 302 (86) | 483 (67) |
| 3 | – | – | 2 (0) | – |
| 5 | 1 (0) | – | 189 (88) | 9 (0) |
| 11 | 136 (66) | – | 6 (1) | – |
| 15 | 12 (6) | – | – | – |
| 29 | – | – | – | 257 (32) |
| 32 | – | 106 (47) | 1 (0) | 3 (0) |
| Total | 150 (72) | 106 (47) | 500 (175) | 752 (99) |
Risk factor analysis
Prospective cohort study
In the univariate analysis, age was a significant predictor of ADR risk and oncology patients were more likely to experience an ADR than non-oncology patients ( Table 19 and Figure 6 ). Multivariate risk factor analysis of first admissions (n = 4724, see Figure 5 ) showed that at any time, the hazard of an ADR in a child following a GA was six times greater than the hazard of an ADR in a child who had not had a GA; the hazard of an ADR increased by 25% with each additional drug given (median daily number of drugs administered 3, IQR 1–5); the hazard of an ADR in oncology patients is nearly twice that of non-oncology patients; and the hazard of an ADR in children increased by 6% for each year of age.
| Covariate | Total patients | No. of patients with ADR | Log-rank statistic (p-value) | |
|---|---|---|---|---|
| Gender | Male | 2602 | 382 | 0.900 |
| Female | 2122 | 312 | ||
| Age | Infant (< 1 year) | 1369 | 78 | < 0.001 |
| Pre-school (1–5 years) | 1259 | 155 | ||
| School aged (5–11 years) | 1105 | 231 | ||
| Teenage (> 11 years) | 991 | 230 | ||
| Oncology | Yes | 106 | 45 | < 0.001 |
| No | 4625 | 649 | ||
FIGURE 6.
Cumulative incidence curves by categorical time invariant risk factor. (a) Gender; (b) age (by category); and (c) oncology status.
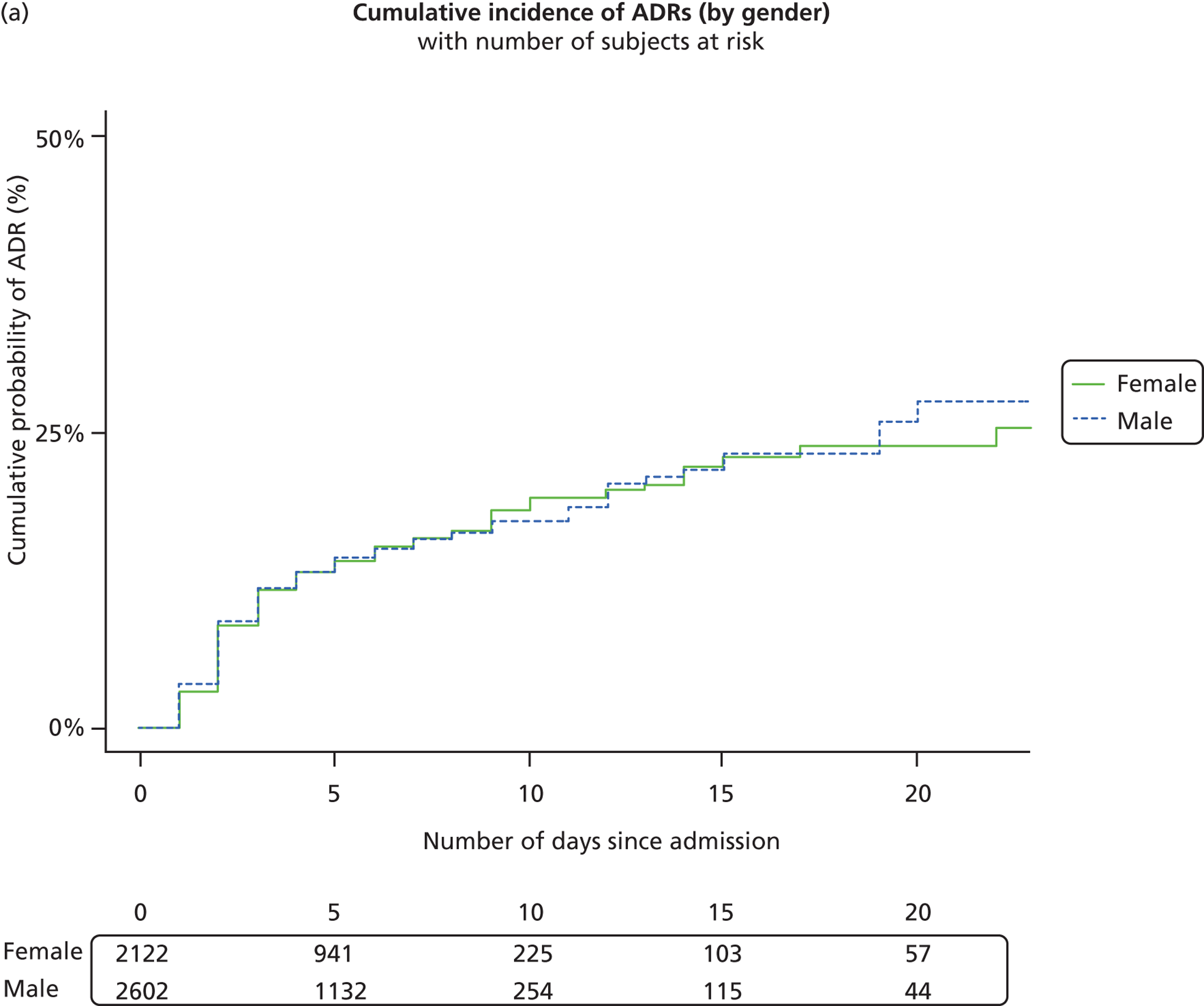
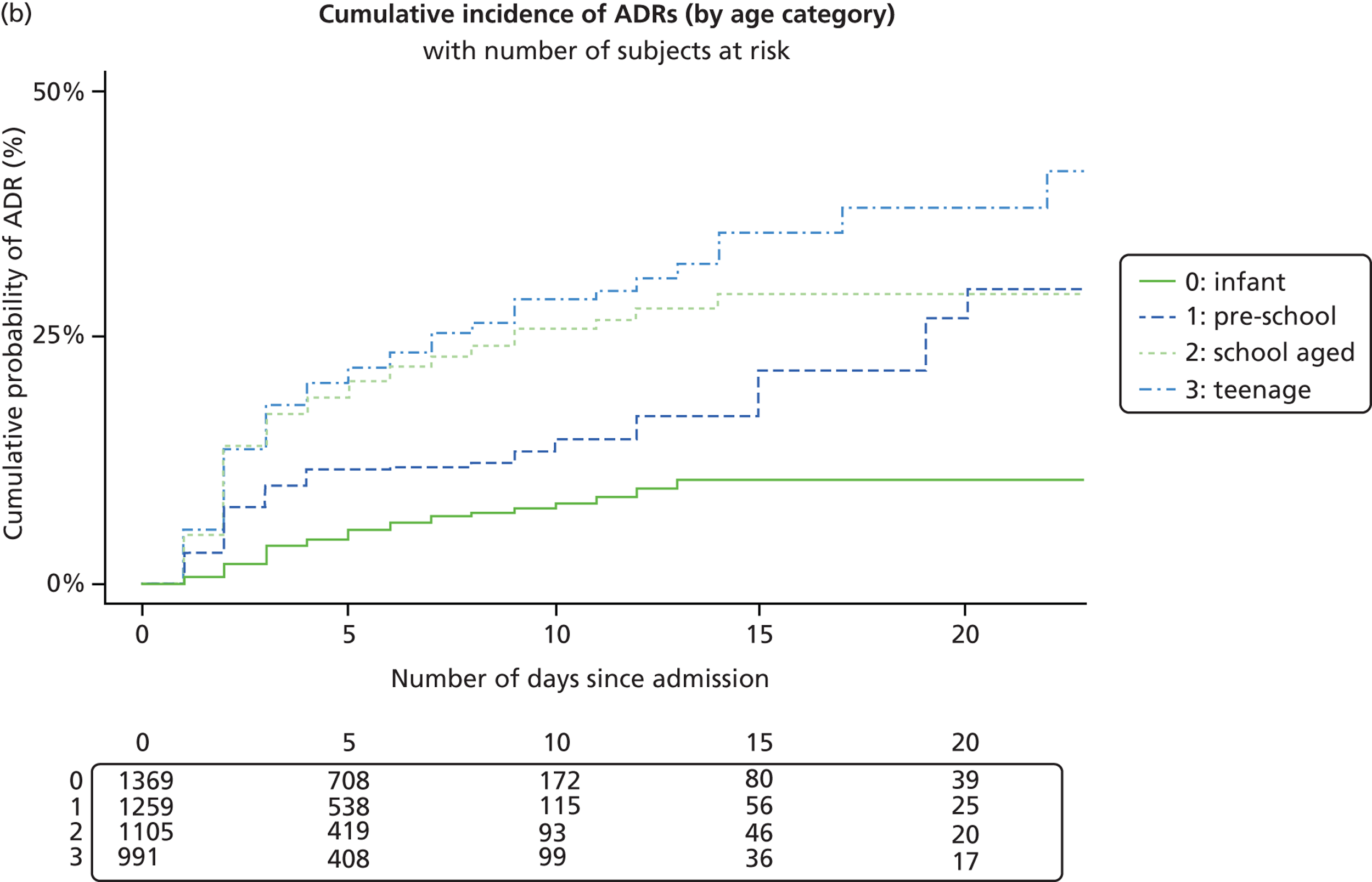
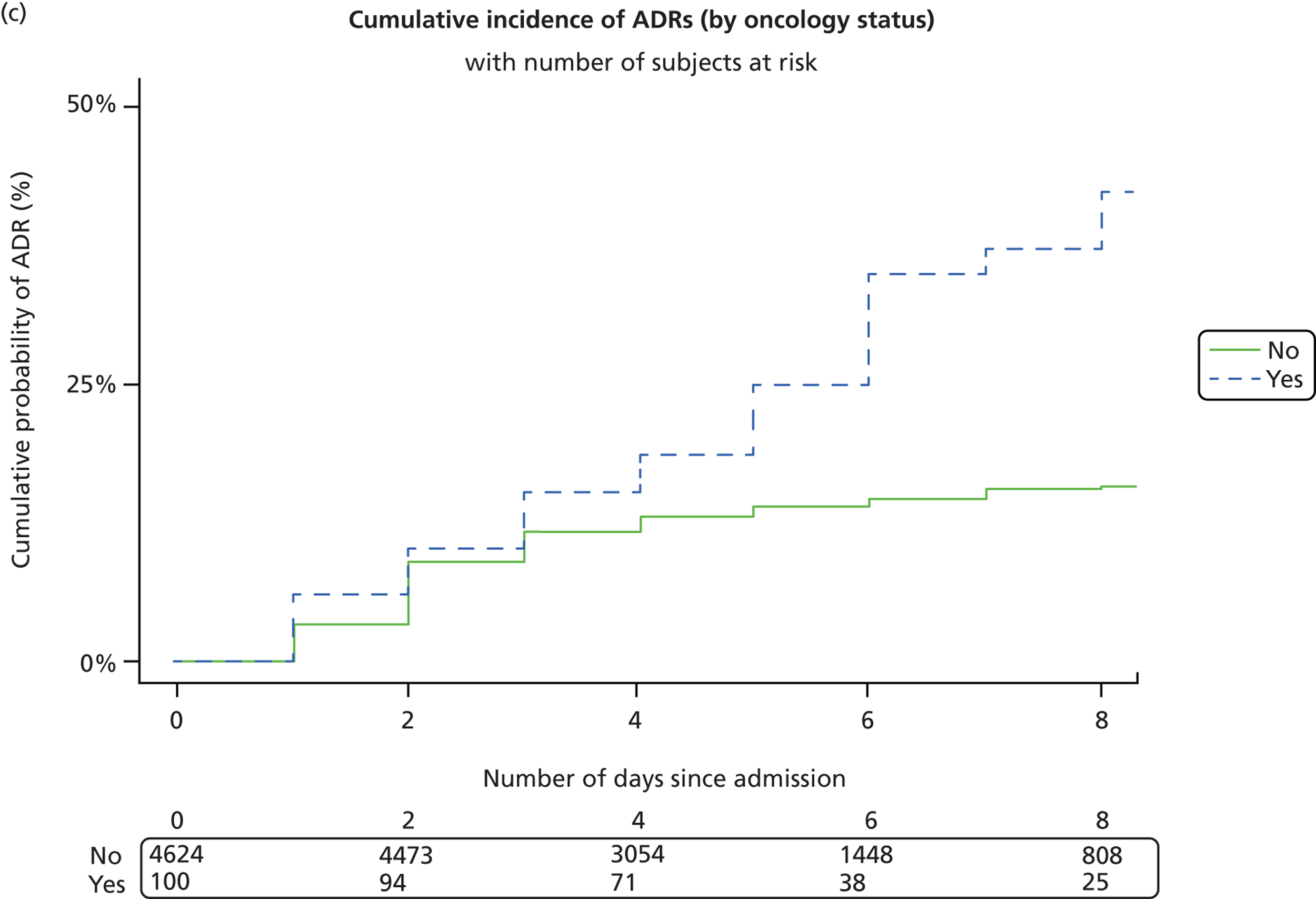
Nested case–control study
Multivariate risk factor analysis of the nested cohort showed that age on admission and receipt of a GA both had a significant effect on ADR risk. Gender and oncology patient status did not have a significant effect on the hazard of an ADR. The hazard of an ADR increased by 30% with each additional OLUL drug given (median daily number of OLUL drugs administered 1, IQR 0–2). Similarly, the hazard of an ADR increased by 20% with each additional authorised drug (median daily number of authorised drugs administered 2, IQR 1–3) ( Table 20 ).
| Covariate | Prospective cohort study | Nested case–control study | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age on admission (in years) | 1.06 (1.04 to 1.07) | < 0.001 | 1.04 (1.02 to 1.05) | < 0.001 | |
| Gender | Female | 1 | 0.301 | 1 | 0.152 |
| Male | 0.93 (0.80 to 1.08) | 0.90 (0.77 to 1.04) | |||
| No. of drugs | 1.25 (1.22 to 1.28) | < 0.001 | N/A | ||
| No. of authorised drugs | N/A | 1.22 (1.17 to 1.26) | < 0.001 | ||
| No. of OLUL drugs | N/A | 1.27 (1.20 to 1.34) | < 0.001 | ||
| Received a GA | No | 1 | 1 | ||
| Yes | 6.38 (5.30 to 7.68) | < 0.001 | 5.30 (4.42 to 6.35) | < 0.001 | |
| Oncology | No | 1 | 1 | ||
| Yes | 1.89 (1.36 to 2.63) | < 0.001 | 0.93 (0.66 to 1.30) | 0.655 | |
Discussion
Our data show that 17.7% of all children who spent > 48 hours as an inpatient experienced at least one ADR, 58% of which occurred after a GA. Opiate analgesic drugs and drugs used in GA were the most commonly implicated drugs. The risk of experiencing an ADR in patients undergoing a procedure under GA has not been assessed previously, including age, oncology treatment and the use of multiple drugs. OLUL medicines were significantly more likely to be implicated in an ADR than medicines used within the terms of their MA (OR 2.25, 95% CI 1.95 to 2.59). Multivariate analysis in our case–control study indicated that risk factors for ADRs were the administration of a GA and the number of medicines administered per day, consistent with the findings of the cohort study.
Strengths and weaknesses of the study
This is the largest paediatric in-hospital study investigating ADRs. Although this was a single-centre study, the study population represents a wide range of paediatric medical and surgical specialties, as the hospital serves as a paediatric centre for the local catchment area, and is the regional paediatric referral centre. Our methodology included causality and severity assessments using validated tools. Denominator data are available for all medication administered on the wards, whereas details on medication given during GA were recorded only in patients if there was a suspected ADR to those drugs. As a result, the effect that increasing the number of drugs given during GA has on the risk of an ADR remains unknown.
The observational approach depends on documentation by the clinical team regarding signs and symptoms. Despite this intense surveillance, it is possible that some ADRs will be missed. Most symptoms that are dependent on patient communication (e.g. nausea, pain, hallucinations) are under-represented in younger or mentally disabled children. This could explain why the risk of developing an ADR increased with age. In addition, some of the most common reaction types observed in our study, such as vomiting and diarrhoea, are perhaps more likely to be manifestations of underlying illness among hospitalised infants and toddlers. The possibility that an underlying illness is an alternative cause may mean that these events are less likely to be assessed as probable or definite ADRs.
We recorded ADRs observed between 1 October 2009 and 30 September 2010. Patients who experienced an ADR before 1 October 2009 or after 30 September 2010, respectively, were counted as admissions without an ADR in the analysis. Consequently, there are 180 admissions that lie outside the observation period where an ADR may have occurred that has not been recorded.
In terms of assigning drug courses an OLUL category, our nested case–control study also had its limitations. First, we required a minimum amount of information to be available about the use of a drug before it could be categorised as off-label. The absence of this information was a result of how drugs were recorded on prescription charts; in general they were prescribed by the name of the active ingredient, and details such as the exact preparation administered were not recorded. Hence the prescription chart records were adequate for their primary purpose but not for our study. Second, there were assumptions outlined in our methodology pertaining to the SmPC definitions of age, gestational age and the classification of cytotoxic drugs.
Strengths and weaknesses in relation to other studies
Our study confirmed risk factors previously identified: increasing age, oncology treatment and multiple drug therapy. The risk of experiencing an ADR in patients undergoing a procedure under GA has not been assessed previously. In our study it increased the risk by more than six times. Most previous paediatric inpatient studies were carried out in general paediatric settings in which only a small number of patients will have undergone GAs. Rashed et al. 81 conducted a paediatric study on general medical wards and reported that anaesthetic drugs, which accounted for only 1% of all prescriptions, were among the drugs most commonly implicated in ADRs. 81 In the two previous inpatient studies investigating paediatric surgical patients and providing medication details, opiate analgesic drugs were among the two most commonly implicated drugs. However, GAs were not included, perhaps because they were not specifically investigated. 3,92 The differences of our study population are also reflected in the spectrum and severity of common reaction types observed. Some reaction types, such as urinary retention and respiratory depression/arrest, occurred almost exclusively in the postoperative period. In addition to the differences in study populations, it is likely that reactions to drugs used in postoperative pain management were well documented in our study population, as patients on these medications are specifically monitored for side effects and are followed up by a specialist pain management team.
A recent review of preventability assessment (called avoidability assessment by some authors) of ADRs by Ferner and Aronson25 concluded that no universal definition of preventability exists and that the reliability of existing definitions is imperfect. The most frequently used avoidability assessment tools (AATs) were Schumock and Thornton44 and Hallas,54 which are based on appropriateness of prescribing or treatment choice. Although these tools might be used successfully to improve prescribing practice in specific clinical circumstances, they become problematic wherever treatment is guided by tertiary paediatric specialist advice, as it would be desirable to use the same expert input when assessing the prescribed treatment. The same applies to areas outside paediatric specialties in which expert input would be required, for example to assess the choice of GA drugs used. It is noticeable that studies that previously reported avoidability of ADRs in hospitalised children had much smaller event numbers compared with 1446 ADRs in our study (0–41 ADRs/study);19 one multicentre study detected only 408 ADRs between five centres,81 which would make literature searches and gathering expert advice more feasible.
The prevalence of OLUL prescriptions in paediatric inpatients ranges from 18% to 60%, and 3.4% to 36%, respectively. 87 The corresponding figures in our case–control study were 23.7% and 7.5%, respectively. These figures collectively are similar to a previous study from our centre3 in 1999, which showed that 35% of prescriptions were OLUL. Our data show that OLUL were significantly more likely to be implicated in an ADR than drugs used within the terms of their MA (OR 2.25, 95% CI 1.95 to 2.59). The risk estimate is higher than that found previously,3,75 which may be a reflection of the fact that the previous studies were smaller (OR 1.5 and OR 1.08),3,75 looked at different ward types (e.g. included paediatric intensive care)3 and used different definitions of OLUL drugs. 75 We also categorised ADR risk according to the type of OLUL use. By focusing on six categories that all had > 100 drug courses, we found that (1) drugs licensed for use in children, but given at a dose greater than recommended, had a lower risk of being implicated in an ADR than authorised drugs and (2) drugs licensed in children, but given to a child below the minimum age or weight, had the greatest risk of being implicated in an ADR. These two findings seem counterintuitive but can be explained by the fact that 69% of the drug courses given at a higher dose than recommended were paracetamol. This reflects the widespread use of 15–20-mg/kg doses for ‘severe symptoms’, as recommended in the BNF-C. 90 Paracetamol at these doses is relatively safe particularly in inpatient settings, and indeed, paracetamol was rarely implicated in ADRs throughout the entire study (see Table 17 ). We removed all paracetamol courses from our data set and reanalysed; 15.4% of OLUL courses were implicated in at least one ADR and the OR of an OLUL medicine being implicated in an ADR was 2.24 (95% CI 1.94 to 2.59). Category 5 (‘drugs licensed in children but given to a child below the minimum age or weight’) had a diminished RR of being implicated in an ADR (OR 2.97, 95% CI 2.36 to 3.73) but were still the most likely category to be implicated in an ADR. Category 3 (‘drugs licensed for use in children, but given at a dose greater than recommended’) had an increased (rather than a reduced) risk of being implicated in an ADR compared with category 1 (‘authorised medicines’) (OR 1.20, 95% CI 0.74 to 1.94).
Multivariate analysis of both the full cohort data and the nested case–control data indicates that risk factors for ADRs are the administration of a GA and the number of drugs administered per day. Furthermore, these findings are consistent with those of Santos et al. 76 who found that off-label drug use was significantly associated with ADR risk (RR 2.44, 95% CI 2.12 to 2.89). However, in our study, we have dissected drug use, and show that the number of OLUL drugs administered per day had a similar influence on ADR risk to the number of authorised drugs administered per day. Most studies, including those in adults, have shown that ADR risk increases with the number of drugs used by patients,3,59,75,93,94 which reflects the complex interaction that occurs between drugs targeting different biological systems within the body, the interaction with disease (i.e. sicker patients are more likely to require a higher number of drugs) and the occurrence of drug–drug interactions.
Implications of study findings
Our study used the same incidence calculations as a comparable prospective adult study by Davies et al. ,59 who reported incidence figures of 14.7% on episode (admission) level and 15.8% on patient level. This compares to our incidence figures of 15.9% and 17.7%, respectively. However, Davies et al. 59 used the Naranjo CAT and included definite, probable and possible ADRs, whereas we excluded ‘possible’ and ‘unlikely’ ADRs. It is therefore likely that our figures underestimate the true incidence of untoward events that should be attributed to drugs. The assessment of symptoms due to the underlying condition and differentiating these from those caused by drugs (e.g. tachycardia in patient treatment for acute asthma) remains a challenge.
Although < 1% of reactions in our study were classified as severe, this does not take into account what impact an ADR might have on the child and/or parent. The most common reaction in our study was vomiting, which was mainly observed in postoperative patients. Vomiting is a common and non-specific symptom in children. Such episodes are unlikely to be regarded as particularly significant by clinicians. However, parents and children often have very different views. For instance, Diez95 reported that parents placed a very high value on the distress caused by PONV. A teenage patient is likely to feel very distressed about having to be catheterised because of urinary retention or having to receive an enema to treat constipation. Parents of children included in this study reported that suspected ADRs cause them concern, irrespective of the ‘medical’ severity of the suspected reaction. Parents valued the proactive explanations of ADRs given by oncologists and we suggest that a detailed discussion of ADRs should form part of the preoperative assessment. 96
In terms of OLUL drug use, our findings highlight the impact of the use of multiple drugs (whether OLUL or authorised) and thus the need for good prescribing practice in reducing ADRs. The minimum number of drugs should be given for the treatment of a disease process, at the lowest possible dose for the shortest possible time. Our data implicate OLUL drugs as risk factors for ADRs in paediatric inpatients. Off-label use is complicated and in some cases can be justified by the fact that evidence which may not necessarily have led to a change in the SmPC is available in the scientific literature as a result of academic investigations. 97 For instance, some of the most commonly implicated drugs in our study were frequently used off-label (e.g. dexamethasone). However, we have no evidence that if these products were used in accordance with a MA, they would be implicated in any fewer ADRs. An area of concern identified in our data is the use of fentanyl, commonly OLUL, where 48% of courses were implicated in ADRs. A key issue with fentanyl may be the dose used in children, suggesting a need for further evaluation of dosing strategies.
With all drugs, irrespective of their licensing status, the dose administered and thus the systemic exposure to that drug, is an important determinant of the likelihood of an ADR. The importance of this is highlighted by our finding that drugs licensed in children, but given to a child below the minimum age or weight had the greatest risk of being implicated in an ADR, reflecting the lack of pharmacokinetic data in children of different ages and/or weights. Advances in the development, and application, of paediatric pharmacokinetic models will be important in the defining, and implementation, of age- and weight (or body surface area)-specific dosing regimens. 98 Although such approaches are now being incorporated in paediatric investigation plans for new drugs, the challenge for all stakeholders will be how to improve this knowledge for drugs already on the market, most of which are not only generic off-patent compounds but are also the most widely used.
In conclusion, our data show that ADRs in hospitalised children are as common as those observed in hospitalised adults. 59 The high proportion of ADRs occurring in the postoperative period and the indication that OLUL drugs are more likely to be implicated in ADRs are of particular concern.
Chapter 4 Systematic review of paediatric adverse drug reactions
This chapter contains information reproduced from Smyth RMD, Gargon E, Kirkham J, Cresswell L, Golder S, Smyth R, et al. Adverse drug reactions in children: a systematic review. PLOS ONE 2012;7:e24061,19 © 2012 Smyth et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided that the original author and source are credited.
Abstract
Background
Adverse drug reactions in children are an important public health problem. We have undertaken a systematic review of observational studies in children in three settings: causing admission to hospital, occurring during hospital stay, and occurring in the community. We were particularly interested in understanding how ADRs might be better detected, assessed and avoided.
Methods
We searched 19 electronic databases using a comprehensive search strategy. In total, 102 studies were included. The primary outcome was any clinical event described as an ADR to one or more drugs. Additional information relating to the ADR was collected: associated drug classification; clinical presentation; associated risk factors; and methods used for assessing causality, severity and avoidability.
Results
Seventy-one per cent (72/102) of studies assessed causality and 33% (34/102) performed a severity assessment. Only 19 studies (19%) assessed avoidability. Incidence rates for ADRs causing hospital admission ranged from 0.4% to 10.3% of all children [pooled estimate of 2.9% (95% CI 2.6% to 3.1%)] and from 0.6% to 16.8% of all children exposed to a drug during hospital stay. Anti-infective drugs and antiepileptic drugs were the most frequently reported therapeutic class associated with ADRs in children admitted to hospital (17 studies and 12 studies, respectively) and children in hospital (24 studies and 14 studies, respectively), whereas anti-infective drugs and NSAIDs were frequently reported as associated with ADRs in outpatient children (13 studies and six studies, respectively). Fourteen studies reported rates ranging from 7% to 98% of ADRs being either definitely or possibly avoidable.
Conclusions
There is extensive literature that investigates ADRs in children. Although these studies provide estimates of incidence in different settings and some indication of the therapeutic classes most frequently associated with ADRs, further work is needed to address how such ADRs may be prevented.
Introduction
Adverse drug reactions are a major health problem to the individual as well as for society. 99 The WHO’s definition of an ADR is ‘a response to a drug which is noxious, and unintended, and which occurs at doses normally used in man for prophylaxis, diagnosis or therapy of disease, or for the modification of physiological function’. 100 The high incidence of ADRs in children has been reported in three previous systematic reviews of observational studies covering the period from 1966 to 2010. 34,35,101 The reviews provided estimates of ADR rates causing hospital admission, in hospitalised children and in outpatient children, and demonstrated that ADRs in hospitalised children are a considerable problem. Two of the reviews35,101 provide data on the clinical presentation of the ADR and the drugs involved. In addition, the more recent review101 provides information on the methods and persons involved in identifying ADRs.
However, there are a number of limitations to the previous reviews. Each review34,35,101 applied a search strategy, using a limited number of keywords to just two electronic bibliographic databases – MEDLINE and EMBASE. Importantly, as a consequence, relevant studies may have been excluded. In addition, the reviews excluded studies that included adults as well as children, thus reducing the number of eligible studies, and the more recent reviews excluded studies that evaluated adverse drug events (ADEs).
These reviews do not provide information about the drugs involved in ADRs or about which methods were used for detecting, or assessing, the causality of an ADR. 23 Establishing the relationship between the drug and suspected reaction is fundamental to drug safety and being able to determine the avoidability44 of an ADR in order to try to prevent its future occurrence is crucial to reducing the burden of ADRs.
We therefore undertook this systematic review to provide a more comprehensive assessment of all relevant studies and to understanding how ADRs might be better detected, assessed and avoided.
Methods
Study selection
Criteria for considering studies for this review
Included studies Observational studies that estimate the incidence of ADRs including retrospective and prospective cohort studies of children.
Excluded studies Studies that focus on ADRs in relation to a specific drug (e.g. antibiotic drugs or carbamazepine), clinical condition (e.g. epilepsy, asthma) or specific clinical presentations of ADRs (anaphylaxis); case–control studies; those carried out exclusively on a neonatal intensive care unit; and studies reporting medication errors, therapeutic failures, non-compliance, accidental and intentional poisoning, and drug abuse.
Participants
Children as defined by the researchers. Studies included three defined populations:
-
children admitted to hospital
-
children in hospital
-
children within the community.
Interventions
Exposure to any systemic or topical medicinal product, including herbals and aromatherapy, as defined by researchers.
Types of outcome measure
Any clinical event described as an ADR or non-avoidable ADE to an individual or group of drugs.
Search methods for identification of studies
A range of electronic bibliographic databases were searched (see Appendix 1 ) using a search strategy of text words and medical subject headings (MeSH) terms (see Appendix 2 ). In addition, we examined references in relevant studies and those cited by previous systematic reviews. Contact with experts was made to identify other potentially relevant published and unpublished studies. We did not apply language restrictions to the search.
Selection of studies
Screening on title, abstract and full publication stage
Duplicate citations were removed. A study eligibility screening pro forma based on prespecified inclusion criteria was used. Two reviewers (RMDS, EG) independently screened each title and categorised as ‘include’, ‘exclude’ or ‘unsure’. The two independent categorisations for all titles were compared and the title was categorised again following discussion if two reviewers disagreed. When there was agreement to exclude, the citation was excluded at this stage. All other citations were reviewed at abstract level. This process was repeated and when there was disagreement, discussion took place between reviewers and citations were recategorised. Those with agreement to include or considered ‘unsure’ were reviewed at full publication level. The process was repeated at full publication stage. Studies considered as unsure or included at full publication stage were reviewed by a third reviewer (JJK). Reasons for exclusion were documented at the abstract and full paper stage of the screening process.
Checking for correct exclusion at each stage
Two reviewers (RMDS, EG) independently viewed the abstracts for a proportion (2%) of studies excluded at title screening stage. Independent categorisations were compared (as above). This process was repeated at abstract stage where a third reviewer (JJK) reviewed 10% of full papers for studies excluded based on abstract. This was repeated at full publication stage, when the same reviewer (JJK) reviewed 20% of excluded full papers. If any studies were excluded incorrectly at any stage then additional checking was performed.
Data extraction
We extracted the following data from each study:
-
Study characteristics Country; year completed; duration; number of sites; design (prospective or retrospective); clinical setting; number of children.
-
Identification of ADR Definition of ADR, including definition of drug exposure; incidence definition and calculation (numerator and denominator, either at patient or episode level); assessment of causal relationship to drug; person who assessed and categorised ADRs; any method (e.g. case record review) or reporting system used (e.g. Yellow Card scheme).
-
Information relating to the ADR Clinical presentation; associated drug(s)/drug classification; associated risk factors (including age, gender, polypharmacy); ADR considered avoidable.
Assessment of methodological quality of included studies
As we were unable to find a validated assessment tool for critically appraising observational studies of ADRs, we developed a quality assessment form specifically for the review. The following aspects were deemed important when assessing study quality: study design; methods for identifying ADRs; methods used to establish the causal relationship between drug and effect; tools for assessing avoidability of the ADR; and tools for assessing severity of the ADR. Criteria were graded as ‘yes’, ‘no’, ‘unclear’ or ‘not reported’. Two reviewers (RMDS, EG) independently assessed methodological quality of each study ( Table 21 ).
| Aspect of study | Criteria | Grade |
|---|---|---|
| Study design | Was the study design clear (prospective, retrospective or combined)? | Yes/no/unclear/not reported |
| Methods for identifying ADRs | Were the methods used to identify ADRs described in sufficient detail? | Yes/no/unclear/not reported |
| Were data collection methods (case record review, drug chart review and laboratory data) clearly described? | Yes/no/unclear/not reported | |
| Were the individuals (clinicians, self-reported, researchers) who identified ADRs clearly described? | Yes/no/unclear/not reported | |
| Methods for determining causality | Was the process of establishing the causal relationship described in detail? | Yes/no/unclear/not reported |
| Were standard methods (validated tool) used in the assessment? | Yes/no/unclear/not reported | |
| Methods for determining avoidability | Was the assessment process of establishing avoidability described in detail? | Yes/no/unclear/not reported |
| Were standard methods (validated tool) used in the assessment? | Yes/no/unclear/not reported | |
| Methods for determining predictability | Was the assessment process of establishing predictability described in detail? | Yes/no/unclear/not reported |
| Were standard methods (validated tool) used in the assessment? | Yes/no/unclear/not reported |
Statistical analysis and data synthesis
For each of the three defined populations; children admitted to hospital, children in hospital and children within the community, a forest plot was produced to present the ADR incidence rate and 95% CI for each relevant study. Studies were subgrouped according to whether the incidence rate was reported at the patient and/or episode level, and whether or not all patients had been exposed to a drug. Further, for rates reported at the patient level a distinction was made between studies that had included one admission per patient and those that had included multiple admissions per patient. All results provided per study were included. Pooled estimates were calculated if the variability in incidence rates was not considered too large.
Univariate meta-regression was used to determine if study level characteristics (setting, gender, age, oncology and number of drugs used) are associated with ADR incidence. Incidence rates for ADRs causing admission and occurring in hospital, calculated at the patient level for a single episode were included. Multivariate meta-regression was not undertaken owing to the paucity of covariate data. Risk factor analyses reported by any study were collated.
Results
The search was originally undertaken in November 2009 and retrieved 20,906 potentially relevant citations. An update search was subsequently performed in October 2010 and retrieved an additional 3234 citations. Combining both searches we identified 24,140 potentially relevant citations, of which 5039 duplicate citations were removed. Screening at title and abstract stage excluded a further 18,592 and 251 citations, respectively. Full papers were reviewed and 95 citations met the inclusion criteria. Agreement between reviewers at each stage of the review is described in Figure 7 . Additional citations were identified through checking for correct exclusion at each stage (n = 3), reference checking (n = 13) and personal communication with authors (n = 5). In total, 116 citations relating to 101 studies were included in the review (see Figure 7 ).
FIGURE 7.
Agreement of reviewers.
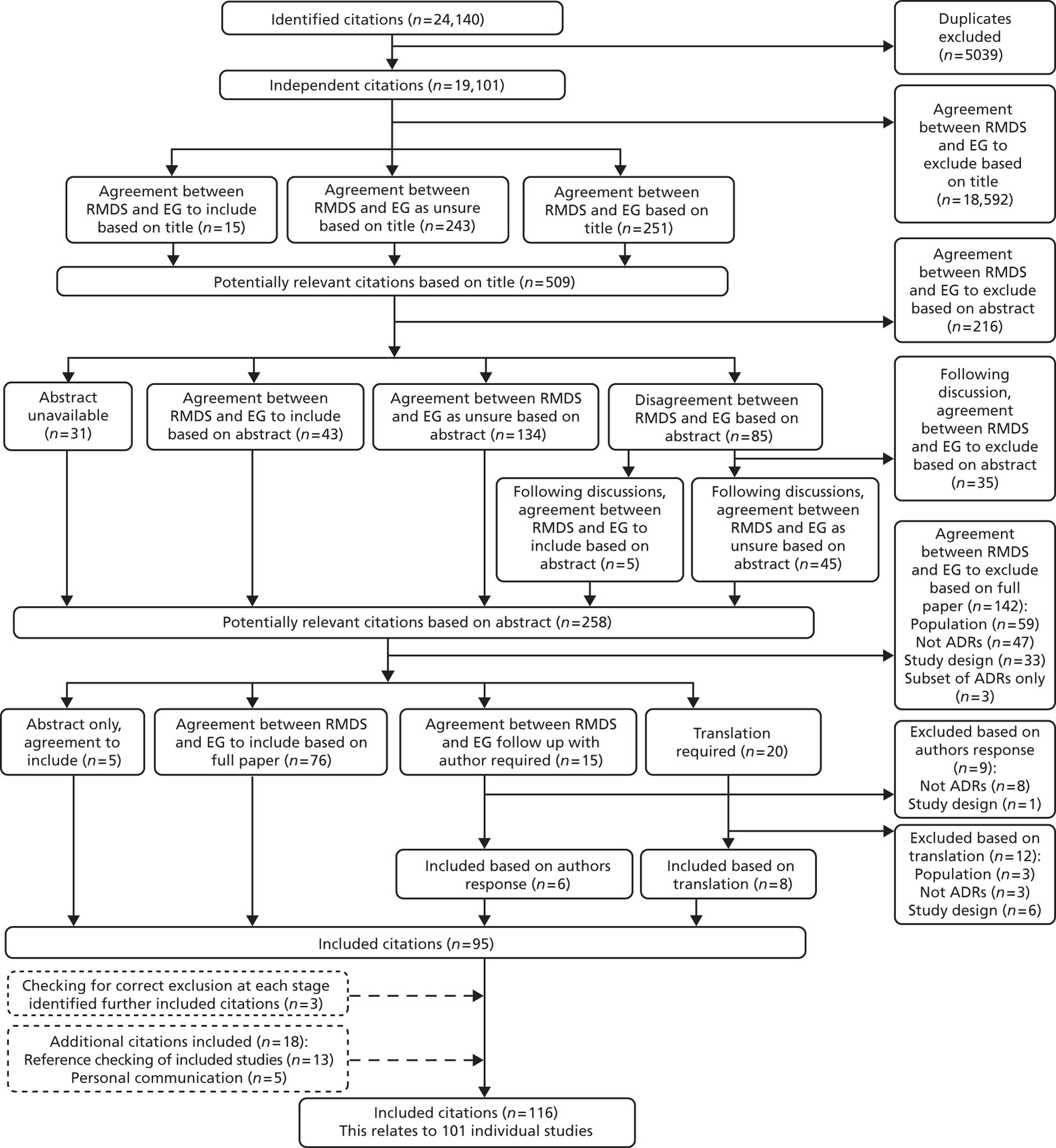
Included studies
A total of 101 studies (116 citations), were included in the review. Eighty (80/101) studies described the clinical event as an ADR. In 10 of these studies, ADR was a category within ‘drug-related’ problems/admissions; three studies described ADRs as drug-induced disease/illness. Sixteen studies described an ADE, in which the non-preventable ADE was the same as our definition, and two studies used the term ‘iatrogenic disease’ to describe an ADR. Some studies included multiple settings: 42 studies investigated ADRs as the cause of admission to hospital, 53 studies investigated ADRs in the hospital setting, and 35 studies investigated ADRs in the community setting. Characteristics for each individual study are provided in Appendix 3 .
Assessment of methodological quality of included studies
All studies, including those that evaluated ADEs, explicitly stated that they had used either the WHO ADR definition1 or a similar one, and that they excluded drug errors. Methodological features of each individual study are provided in Tables 22–27 .
| Drug class | Study | Population of study | No. of ADRs | No. of ADRs due to drug class (%) | Clinical presentation |
|---|---|---|---|---|---|
| Anti-infective drugs (n = 17) | Easton (1998)41 | 1682 admissions | 10 | 1 (10) | Colitis, ileus |
| Impicciatore (2002)48 | 116 children | 12 | 4 (33.3) | Urticaria, periorbital oedema, neutropenia | |
| Lamababusuriya (2003)47 | 39,625 admissions | 63 | 38 (60.3) | Erythema multiforme, Stevens–Johnson syndrome, rash, raised intracranial pressure | |
| Oshikoya (2007)46 | 3821 children | 17 | 7 (41.1) | Provided for deaths only × 1 | |
| Easton (2004)102 | 2933 admissions | 29 | Not reported | Not reported | |
| Mitchell (1988)33 | 7271 children | 288 | 10 (3.5) | Diarrhoea, fever, erythema multiforme death × 2 | |
| Major (1998)103 | 457 children | 26 | 6 (23) | Not reported | |
| Santos (2000)104 | 624 children | 14 | 6 (42.8) | Not reported | |
| Gallagher (2011)20 | 462 children | 18 | 3 (16.6) | Diarrhoea | |
| Duczmal (2006)105 | 4996 admissions | 58 | Not reported | Not reported | |
| Ganeva (2007)106 | 73 children | 6 | 4 (66.6) | Not reported | |
| Fattahi (2005)107 | 404 children | 9 | 4 (44.4) | Not reported | |
| Martinez-Mir (1996)42 | 490 children | 21 | 10 (47.6) | Not reported | |
| Yosselson-Superstine (1982)43 | 906 children | 29 | Not reported | Not reported | |
| McKenzie (1976)32 | 3556 admissions | 72 | Not reported | Provided for deaths only × 2 | |
| ADRIC 1 | 6821 children | 249 | 16 (6.4) | Diarrhoea, rash, vomiting, lip swelling, deranged LFTs, thrush | |
| Antiepileptic drugs (n = 12) | Easton (1998)41 | 1682 admissions | 10 | 3 (30) | Increased fitting, rash, aphasia/motor regression |
| Impicciatore (2002)48 | 116 children | 12 | 2 (16.6) | Coma | |
| Lamababusuriya (2003)47 | 39,625 admissions | 63 | 4 (6.3) | Ataxia and cerebellar signs, liver failure, Stevens–Johnson syndrome | |
| Oshikoya (2007)46 | 3821 children | 17 | 1 (5.8) | Not reported | |
| Mitchell (1988)33 | 7271 children | 288 | 23 (7.9) | Lethargy, ataxia, rash, erythema | |
| Le (2006)36 | 64,403 admissions | 35 | Not reported | Not reported | |
| Santos (2000)104 | 624 children | 14 | 1 (7.1) | Not reported | |
| Yosselson-Superstine (1982)43 | 906 children | 29 | Not reported | Not reported | |
| McKenzie (1976)32 | 3556 admissions | 72 | Not reported | Not reported | |
| Fattahi (2005)106 | 404 children | 9 | 1 (11.1) | Not reported | |
| Jonville-Bera (2002)37 | 260 children | 4 | 1 (25) | Convulsion | |
| ADRIC 1 | 6821 children | 249 | 2 (0.8) | Constipation, respiratory depression | |
| NSAIDS (n = 9) | Duczmal (2006)105 | 4996 admissions | 58 | Not reported | Not reported |
| Impicciatore (2002)48 | 116 children | 12 | 1 (8.3) | Coma | |
| Lamababusuriya (2003)47 | 39,625 admissions | 63 | 3 (4.7) | Rectal bleeding, aspirin – Reye's syndrome | |
| Major (1998)103 | 457 children | 26 | 2 (7.6) | Not reported | |
| Gill (1995)108 | 909 admissions | 10 | 1 (10) | Not reported | |
| ADRIC 1 | 6821 children | 249 | 31 (12.4) | Postoperative bleeding, haematemesis, constipation, abdominal pain | |
| Gallagher (2011)20 | 462 children | 18 | 1 (5.5) | Haematemesis | |
| Mitchell (1988)33 | 7271 children | 288 | 12 (4.1) | Gastritis | |
| Jonville-Bera (2002)37 | 260 children | 4 | 1 (25%) | Melaena | |
| Cytotoxic drugs (n = 8) | Mitchell (1988)33 | 7271 children | 288 | Not reported | Deaths × 2 |
| Major (1998)103 | 457 children | 26 | 10 (38.4) | Not reported | |
| Santos (2000)104 | 624 children | 14 | 2 (14.2) | Not reported | |
| Yosselson-Superstine (1982)43 | 906 children | 29 | Not reported | Death × 1 | |
| McKenzie (1976)32 | 3556 admissions | 72 | Not reported | Provided for deaths only × 3 | |
| Fattahi (2005)107 | 404 children | 9 | 2 (22.2) | Not reported | |
| ADRIC 1 | 6821 children | 249 | 110 (44.2) | Thrombocytopenia, anaemia, vomiting, mucositis, deranged liver function tests, immunosuppression, diarrhoea, nausea, constipation, headache, abdominal pain, back pain, haematuria, leukoencephalopathy, deranged renal function | |
| Gallagher (2011)20 | 462 children | 18 | 9 (50%) | Pyrexia, neutropenia, lethargy, decreased responsiveness, vomiting | |
| Corticosteroids (n = 7) | Easton (1998)41 | 1682 admissions | 10 | 1 (10%) | Unstable diabetes |
| Santos (2000)104 | 624 children | 14 | 1 (7.1%) | Upper gastrointestinal bleed | |
| Yosselson-Superstine (1982)43 | 906 children | 29 | Not reported | Not reported | |
| McKenzie (1976)32 | 3556 admissions | 72 | Not reported | Not reported | |
| Ganeva (2007)106 | 73 children | 6 | 2 (33.3%) | Not reported | |
| ADRIC 1 | 6821 children | 249 | 102 (41.0%) | Immunosuppression, postoperative bleeding, hyperglycaemia, hypertension, gastritis, increased appetite, impaired healing, adrenal suppression | |
| Gallagher (2011)20 | 462 children | 18 | 1 (5.5%) | Vomiting | |
| Vaccines (n = 7) | Easton (1998)41 | 1682 admissions | 10 | 1 (10%) | Hypotonic–hyporesponsive episode |
| Lamababusuriya (2003)47 | 39,625 admissions | 63 | 9 (14.2%) | Rash, encephalopathy, fits, head lag | |
| Easton (2004)102 | 2933 admissions | 29 | Not reported | Not reported | |
| Mitchell (1988)33 | 7271 children | 288 | 5 (1.7%) | Not reported | |
| Santos (2000)104 | 624 children | 14 | 1 (7.1%) | Not reported | |
| Gill (1995)108 | 909 admissions | 10 | 2 (20%) | Seizures, fever | |
| ADRIC 1 | 6821 children | 142 | Fever, rash, irritability, seizure, vomiting, pallor, apnoea, limb swelling, lethargy, thrombocytopenia, diarrhoea, abdominal pain, respiratory distress, Kawasaki’s disease |
| Drug class | Study | Population of study | No. of ADRs | No. of ADRs due to drug class (%) | Clinical presentation |
|---|---|---|---|---|---|
| Anti-infective drugs (n = 24) | Al-Tajir (2005)109 | 2351 episodes | 2 | 2 (100) | Not reported |
| Baniasadi (2008)110 | 693 children | 27 | Not reported | Not reported | |
| Choonara (1984)111 | 268 children | 15 | 5 (33.3) | Vomiting, oral monilia, diarrhoea | |
| Dharnidharka (1993)112 | 703 children | 7 | 1 (14.2) | Skin rash | |
| dos Santos (2006)113 | 265 children | 47 | 18 (38.2) | Not reported | |
| dos Santos (2009)114 | 3726 episodes | 302 | 57 (18.8) | Not reported | |
| Easton-Carter (2003)115 | 17,432 episodes | 41 | Not reported | Not reported | |
| Farrokhi (2006)92 | 81 children | 3 | 1(33.3) | Not reported | |
| Fattahi (2005)107 | 380 children | 40 | 35 (87.5) | Not reported | |
| Gill (1995)108 | 899 episodes | 76 | 15 (19.7) | Not reported | |
| Gonzalez-Martin (1998)116 | 219 children | 46 | 4 (8.6) | Not reported | |
| Jha (2007)117 | 943 children | 13 | 12 (92.3) | Macupapular rashes, vomiting, diarrhoea, drug fever | |
| Jonville-Bera (2002)37 | 227 children | 6 | 2 (33.3) | Diarrhoea, rash | |
| Impicciatore (2002)48 | 1619 children | 29 | 9 (31.0) | Urticaria, increased transaminase levels, vomiting, diarrhoea | |
| Le (2006)36 | 64,403 admissions | 1060 | Not reported | Not reported | |
| Leach (1998)118 | 499 episodes | 58 | 23 (39.6) | Vomiting, rash, diarrhoea, arthropathy, neutropenia, nausea, fits | |
| Mitchell (1979)119 | 1669 children | 280 | Not reported | Not reported | |
| Maistrello (1999)120 | 1103 children | 59 | 24 (40.6) | Gastointestinal disorders | |
| Martinez-Mir (1999)121 | 490 children | 68 | Not reported | Not reported | |
| Neubert (2004)75 | 156 children | 31 | Not reported | Not reported | |
| Oshikoya (2007)46 | 3821 children | 27 | 12 (44.4) | Red man syndrome, pustular rash, Stevens–Johnson syndrome, erythema, jaundice, anaphylaxis, urticaria, fever | |
| Shockrollah (2009)122 | 230 children | 5 | 2 (40) | Not reported | |
| Turner (1999)3 | 936 episodes | 157 | 34 (21.6) | Not reported | |
| Vazquez de la Villa (1989)123 | 597 children | 26 | 9 (34.6) | Diarrhoea, vomiting, rash | |
| Antiepileptic drugs (n = 14) | Choonara (1984)111 | 268 children | 15 | 7 (46.6) | Drowsiness, hyperactivity, ataxia |
| Dharnidharka (1993)112 | 703 children | 7 | 1 (14.2) | Skin rash | |
| dos Santos (2009)114 | 3726 episodes | 302 | 26 (8.6) | Not reported | |
| Easton-Carter (2003)115 | 17432 episodes | 41 | Not reported | Not reported | |
| Gill (1995)108 | 899 episodes | 76 | 3 (3.9) | Not reported | |
| Gonzalez-Martin (1998)116 | 219 children | 46 | 5 (10.8) | Not reported | |
| Le (2006)36 | 64,403 admissions | 1060 | Not reported | Not reported | |
| Leach (1998)118 | 499 episodes | 58 | 1 | Apnoea | |
| Mitchell (1979)119 | 1669 children | 280 | Not reported | Not reported | |
| Martinez-Mir (1999)121 | 490 children | 68 | Not reported | Not reported | |
| Neubert (2004)75 | 156 children | 31 | Not reported | Not reported | |
| Oshikoya (2007)46 | 3821 children | 27 | 2 (7.4) | Erythema | |
| Telechea (2010)a | 123 children | 46 | 15 (32.6) | Not reported | |
| Vazquez de la Villa (1989)123 | 597 children | 26 | 4 (15.3) | Sedation, paradoxical reaction | |
| Corticosteroids (n = 10) | dos Santos (2006)113 | 265 children | 47 | 11 (23.4) | Not reported |
| Gill (1995)108 | 899 episodes | 76 | 6 (7.8) | Not reported | |
| Gonzalez-Martin (1998)116 | 219 children | 46 | 3 (6.5) | Not reported | |
| Impicciatore (2002)48 | 1619 children | 29 | 1 (3.4) | Rash | |
| Leach (1998)118 | 499 episodes | 58 | 1 (1.7) | Gastric irritation | |
| Mitchell (1979)119 | 1669 children | 280 | Not reported | Not reported | |
| Neubert (2004)75 | 156 children | 31 | Not reported | Not reported | |
| Telechea (2010)a | 123 children | 46 | 4 (8.6) | Not reported | |
| Turner (1999)3 | 936 episodes | 157 | 10 (6.3) | Not reported | |
| Vazquez de la Villa (1989)123 | 597 children | 26 | 1 (3.8) | Cushing syndrome | |
| Bronchodilators (n = 9) | Choonara (1984)111 | 268 children | 15 | 3 (20) | Tachycardia |
| Easton-Carter (2003)115 | 17,432 episodes | 41 | Not reported | Not reported | |
| Gill (1995)108 | 899 episodes | 76 | 8 (10.5) | Not reported | |
| Gonzalez-Martin (1998)116 | 219 children | 46 | 8 (17.3) | Not reported | |
| Impicciatore (2002)48 | 1619 children | 29 | 5 (17.2) | Tremor, tachycardia | |
| Neubert (2004)75 | 156 children | 31 | Not reported | Not reported | |
| Telechea (2010)a | 123 children | 46 | 8 (17.3) | Not reported | |
| Turner (1999)3 | 936 episodes | 157 | 8 (5.0) | Not reported | |
| Vazquez de la Villa (1989)123 | 597 children | 26 | 11 (42.3) | Tachycardia, nervousness, vomiting | |
| Cytotoxic drugs (n = 7) | dos Santos (2009)114 | 3726 episodes | 302 | 10 (3.3) | Not reported |
| Gonzalez-Martin (1998)116 | 219 children | 46 | 7 (15.2) | Not reported | |
| Jonville-Bera (2002)37 | 227 children | 6 | 4 (66.6) | Vomiting | |
| Le (2006)36 | 64,403 admissions | 1060 | Not reported | Not reported | |
| Leach (1998)118 | 499 episodes | 58 | 1 (1.7) | Thrombocytopenia | |
| Mitchell (1979)119 | 1669 children | 280 | Not reported | Not reported | |
| Telechea (2010)a | 123 children | 46 | 1 (2.1) | Not reported | |
| Diuretic drugs (n = 6) | Easton-Carter (2003)115 | 17,432 episodes | 41 | Not reported | Not reported |
| Leach (1998)118 | 499 episodes | 58 | 1 (1.7) | Overdiuresis | |
| Mitchell (1979)119 | 1669 children | 280 | Not reported | Not reported | |
| Neubert (2004)75 | 156 children | 31 | Not reported | Not reported | |
| Telechea (2010)a | 123 children | 46 | 9 (19.5) | Not reported | |
| Turner (1999)3 | 936 episodes | 157 | 31 (19.7) | Not reported |
| Drug class | Study | Population of study | No. of ADRs | No. of ADRs due to drug class (%) | Clinical presentation |
|---|---|---|---|---|---|
| Anti-infective drugs (n = 13) | Cirko-Begovic (1989)124 | 2459 children | 63 | 49 (78) | Not reported |
| Easton-Carter (2003)125 | 8601 consultations | 118 | Not reported | Not reported | |
| Horen (2002)77 | 1419 consultations | 20 | 9 (45) | Not reported | |
| Juntti-Patinen (2006)126 | Not reported for children only | 4 | Not reported for children only | Not reported for children only | |
| Kaushal (2007)127 | 1689 children | 226 | 158 (70) | Nausea, vomiting and diarrhoea | |
| Kramer (1985)78 | 4244 courses of therapy | 200 | Not reported | Diarrhoea, other gastrointestinal complaints and skin rashes | |
| Menniti-Ippolito (2000)128 | 7890 children | 119 | 79 (66) | Cutaneous, gastrointestinal, eosinophilia, neurological, angioedema, fever | |
| Planchamp (2009)129 | 12,995 consultations | 43 | Not reported | Not reported | |
| Sanz and Boada (1987)130 | 1327 children | 10 | 4 (40) | Cutaneous reaction and diarrhoea | |
| Munoz (1998)131 | 47,107 consultations | 447 | 49.5% | Included skin reactions | |
| Jonville-Bera (2002)37 | A&E, 428 children; private paediatricians, 1192 children | A&E, 4; private paediatricians, 8 | A&E, 2 (50); private paediatricians, 6 (75) | Diarrhoea, rash, vomiting | |
| Woods (1987)132 | 1590 children | 235 | 40 (17) | Diarrhoea, drowsiness, rash, headache, hyperactivity, anorexia, abdominal pain, vomiting, sleep disturbance | |
| Zahraoui (2010)133 | Not reported | 24 | Not reported | Not reported | |
| NSAIDs (n = 6) | Kaushal (2007)127 | 1689 children | 226 | 2 (1) | Not reported |
| Menniti-Ippolito (2000)128 | 7890 children | 119 | 3 (3) | Cutaneous, haematuria, hypertranspiration | |
| Munoz (1998)131 | 47107 consultations | 447 | Not reported | Not reported | |
| Planchamp (2009)129 | 12,995 consultations | 43 | Not reported | Not reported | |
| Sanz and Boada (1987)130 | 1327 children | 10 | 1 (10) | Not reported | |
| Woods (1987)132 | 1590 children | 235 | 9 (4) | Drowsiness, abdominal pain, aggressiveness, vomiting | |
| Analgesic drugs (n = 5) | Kaushal (2007)127 | 1689 children | 226 | 1 (0.4) | Not reported |
| Munoz (1998)131 | 47,107 consultations | 447 | Not reported | Not reported | |
| Planchamp (2009)129 | 12,995 consultations | 43 | Not reported | Not reported | |
| Woods (1987)132 | 1590 children | 235 | 11 (5) | Drowsiness, irritability, aggressiveness | |
| Zahraoui (2010)133 | Not reported | 24 | Not reported | Not reported | |
| Vaccines (n = 5) | Horen (2002)77 | 1419 consultations | 20 | 5 (25) | Not reported |
| Jonville-Bera (2002)37 | A&E, 428; private, 1192 (children) | A&E, 4; private 8 | A&E, 1 (25); private, 2 (25) | A&E rash; private, fever | |
| Menniti-Ippolito (2000)128 | 7890 children | 119 | 14 (12) | Not reported | |
| Munoz (1998)131 | 47,107 consultations | 447 | ? 9.2% | Not reported | |
| Planchamp (2009)129 | 12,995 consultations | 43 | Not reported | Not reported | |
| Antihistamine drugs (n = 4) | Cirko-Begovic (1989)124 | 2459 children | 63 | 2 (3) | Not reported |
| Kaushal (2007)127 | 1689 children | 226 | 2 (1) | Not reported | |
| Menniti-Ippolito (2000)128 | 7890 children | 119 | 2 (2) | Not reported | |
| Woods (1987)132 | 1590 children | 235 | 46 (20) | Drowsiness, aggressiveness, dry mouth, headache, irritability, diarrhoea | |
| Bronchodilators (n = 3) | Kaushal (2007)127 | 1689 children | 226 | 16 (7) | Not reported |
| Kramer (1985)78 | 4244 courses of therapy | 200 | Not reported | Various manifestations of central nervous stimulation | |
| Woods (1987)132 | 1590 children | 235 | 6 (3) | Hyperactivity, shakiness, dizziness, irritability, sleep disturbance | |
| Steroids (n = 3) | Horen (2002)77 | 1419 consultations | 20 | 1 (0.05) | Not reported |
| Kaushal (2007)127 | 1689 children | 226 | 12 (5) | Not reported | |
| Woods (1987)132 | 1590 children | 235 | 5 (2) | Abdominal pain, diarrhoea |
| Drug class | Study | Population of study | No. of ADRs | No. of ADRs due to drug class (%) | Clinical presentation |
|---|---|---|---|---|---|
| Anti-infective drugs (n = 2) | Haffner (2005)134 | 703 admissions | 101 | Not reported | Not reported |
| Speranza (2008)135 | 173 children | 24 | 10 (41.6) | Not reported | |
| Bronchodilators (n = 1) | Haffner (2005)134 | 703 admissions | 101 | Not reported | Not reported |
| Antiepileptic drugs (n = 2) | Haffner (2005)134 | 703 admissions | 101 | Not reported | Not reported |
| Speranza (2008)135 | 173 children | 24 | 4 (16.6) | Not reported | |
| Cardiovascular drugs (n = 1) | Haffner (2005)134 | 703 admissions | 101 | Not reported | Not reported |
| Analgesic drugs (n = 1) | Speranza (2008)135 | 173 children | 24 | 2 (8.3) | Not reported |
| Antiulcer drugs (n = 1) | Speranza (2008)135 | 173 children | 24 | 2 (8.3) | Not reported |
| Psychotropic drugs (n = 1) | Speranza (2008)135 | 173 children | 24 | 2 (8.3) | Not reported |
| Drug class | Study | Population of study | No. of ADRs | No. of ADRs due to drug class (%) | Clinical presentation |
|---|---|---|---|---|---|
| Anti-infective drugs (n = 1) | Kushwaha (1994)136 | 20,310 admissions | 267 | Not reported | Erythematous maculopapular rash, thrombophlebitis, erythema multiforme, fixed drug reaction, urticaria, jaundice, aplastic anaemia, thrombocytopenia purpura |
| Vaccines (n = 1) | Kushwaha (1994)136 | 20,310 admissions | 267 | Not reported | Nodular cyst in gluteal region, injection abscess |
| NSAIDs (n = 1) | Kushwaha (1994)136 | 20,310 admissions | 267 | Not reported | Erythematous maculopapular rash |
| Analgesic drugs (n = 1) | Kushwaha (1994)136 | 20,310 admissions | 267 | Not reported | Erythematous maculopapular rash, urticaria |
| Steroids (n = 1) | Kushwaha (1994)136 | 20,310 admissions | 267 | Not reported | Injection abscess |
| Drug class | Study | Population of study | No. of ADRs | No. of ADRs due to drug class (%) | Clinical presentation |
|---|---|---|---|---|---|
| Steroid (n = 1) | McKenzie (1973)137 | 658 children | 175 | Not reported | Psychotic reaction, Cushing syndrome, cataracts, hypertension |
| Anti-infective drugs (n = 1) | McKenzie (1973)137 | 658 children | 175 | Not reported | Rash, diarrhoea, facial flush, monilia, pain in injection site |
| Cytotoxics (n = 1) | McKenzie (1973)137 | 658 children | 175 | Not reported | Alopecia, peripheral neuritis, mouth ulcer, injection site inflammation, leukopenia, secondary infection |
Meta-regression
Study design
The majority of studies were carried out prospectively (n = 84; 83%), which included 13 in those causing admission, 26 studies with the ADR occurring in hospital, 23 in the community, 16 in hospital and causing admission, and six in mixed hospital and community settings. Fourteen studies were carried out retrospectively, which included six causing hospital admission, two in hospital studies, and four in the community, one causing admission and in the hospital setting, and one the study that considered ADRs that resulted in any medical care contact. Two studies (one in hospital and one in hospital and causing admission) used both study designs. For the remaining study we were unable to determine the study design (see Tables 22–27 ).
Persons involved in identifying adverse drug reactions
Sixty-three studies reported that a clinician – medical doctor, nurse or pharmacist – was involved in the identification of ADRs. Thirty studies reported also involving either the child or parent. Eight studies did not provide information about who identified the ADRs.
Methods for identifying adverse drug reactions
Several methods were used to detect ADRs. Multiple ADR detection methods were used in 58/101 studies; these consisted of a combination of case record review, drug chart review, laboratory data, computerised ADR reporting system, attendance at ward rounds, and interviewing patients/parents or clinicians. In 31 studies, case record review alone was undertaken. The remaining 11 studies used parental interviews/questionnaires (five studies), clinical assessments (three studies), clinician questionnaires (one study), ward round (one study) and a nationwide computer database (one study). The remaining study report did not refer to the methods used.
Studies estimating the proportion of paediatric hospital admissions related to adverse drug reactions
Description of studies
There were 42 studies in which ADRs had been investigated as the cause of admission to hospital. The period under study varied widely, and ranged from 1 week to 11 years. The majority of studies were described as being performed in a general paediatric unit or ward (n = 22). 20,21,32,33,43,45,46,48,102,104,105,109,135,137–145 Four studies41,47,103,117,146 included general medicine, one study147 in a hospital emergency department. Two studies37,148 covered general medicine and a hospital emergency department, and one study149 an integrated primary care information database. Two studies were performed in the PICU,108 one134 in combination with general paediatrics. Seven studies110,150–155 covered a combination of clinical settings. The three remaining studies were performed in dermatology and venereology,106 infectious diseases107 and an isolation ward. 42
Adverse drug reaction incidence
We do not have ADR incidence rates for 12 out of 42 of these studies, as the child-only data were not available (n = 4), data were not split by clinical setting (n = 5), data were provided for ADRs in hospital but not causing admission (n = 2), and data were provided for the total number of ADRs but not the ADR frequency at the patient or episode level (n = 1). Figure 8 presents data from all of the studies that provide incidence rates for ADRs causing admission to hospital (n = 30). These rates range from 0.4% to 10.3% of children (single admission). One study was an extreme outlier48 and if this was excluded we found a reduction in the upper limit of this range to 4%, and a pooled incidence estimate of 2.9% (95% CI 2.6% to 3.1%).
FIGURE 8.
What proportion of all paediatric hospital admissions are ADR related? ICU, intensive care unit.
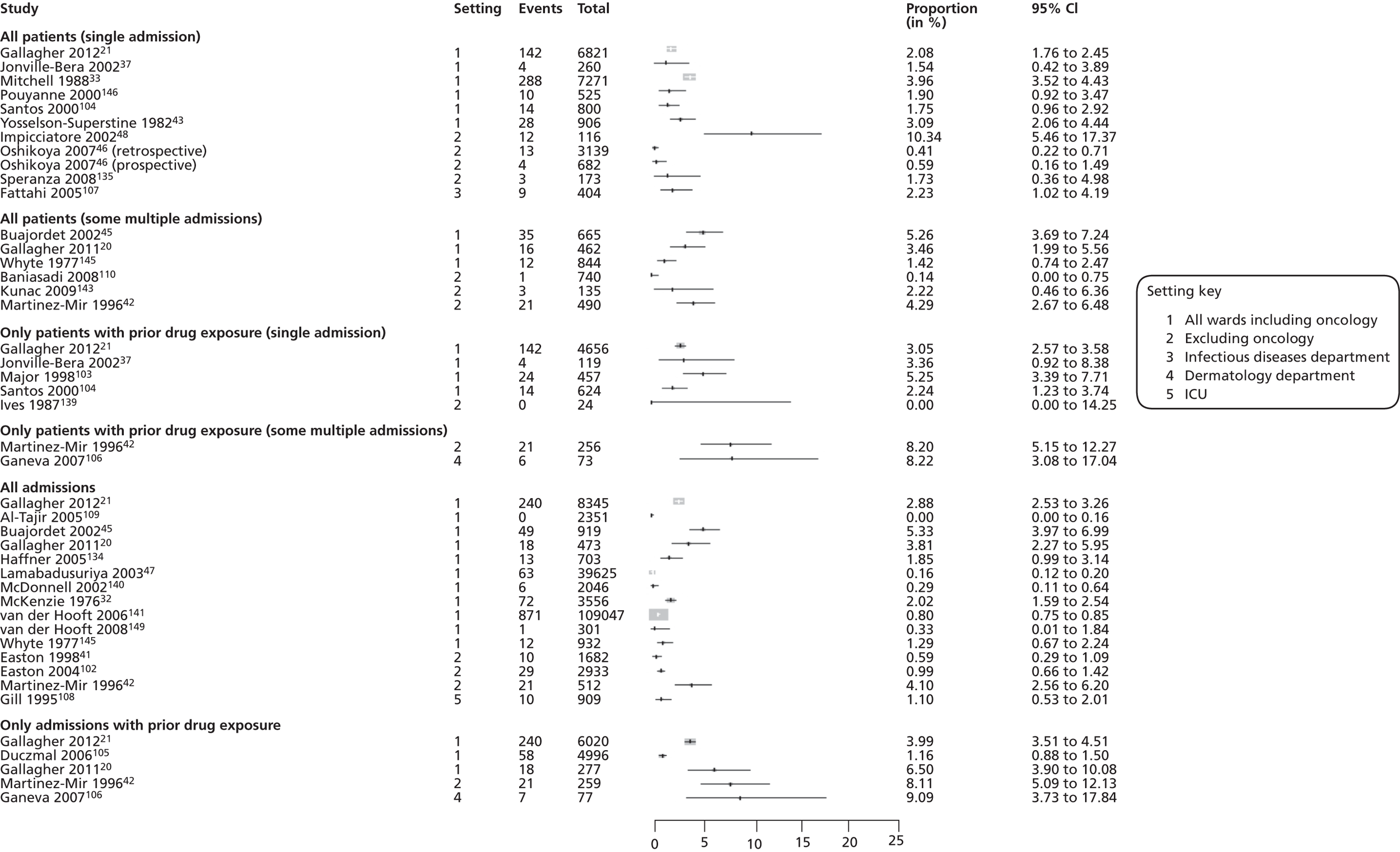
Studies estimating the proportion of children experiencing an adverse drug reaction during their admission
Description of studies
We have included 51 studies in which ADRs have been investigated in the hospital setting. The period under study varied widely and ranged from 1 day to 10 years. The majority of studies were described as being performed in a general paediatric unit or ward (n = 24),32,37,45,46,48,109,111–117,123,135,137,142,144,145,156–160 two134,161 of which included intensive care also. Six studies108,122,124,162–164 were performed solely in the intensive care setting, one118 of which included general medicine. Three studies75,165,166 included children on an isolation ward. One study was performed using an integrated primary care information database. 149 The remaining 13 studies covered a combination of clinical settings. 3,92,107,110,119,120,150,152–155,167,168
Adverse drug reaction incidence
We do not have ADR incidence rates for 18 out of 54 of these studies, as the child-only data were not available (n = 3), the data were not split by clinical setting (n = 7), data were provided for the total number of ADRs but not the ADR frequency at the patient or episode level (n = 5), data were provided for ADRs and ADEs combined (n = 2), and data provided for ADRs causing admission but not in hospital (n = 1). Figure 9 presents data from all of the studies that provide incidence rates for ADRs in hospital (n = 36). These estimates range from 0.6% to 16.8% of patients (at a single episode and with prior drug exposure). A pooled estimate has not been calculated, as the rates are considered too varied.
FIGURE 9.
What proportion of children in hospital experience an ADR during their admission? ICU, intensive care unit; IHS, intensive hospital surveillance; IVS, voluntary hospital surveillance.
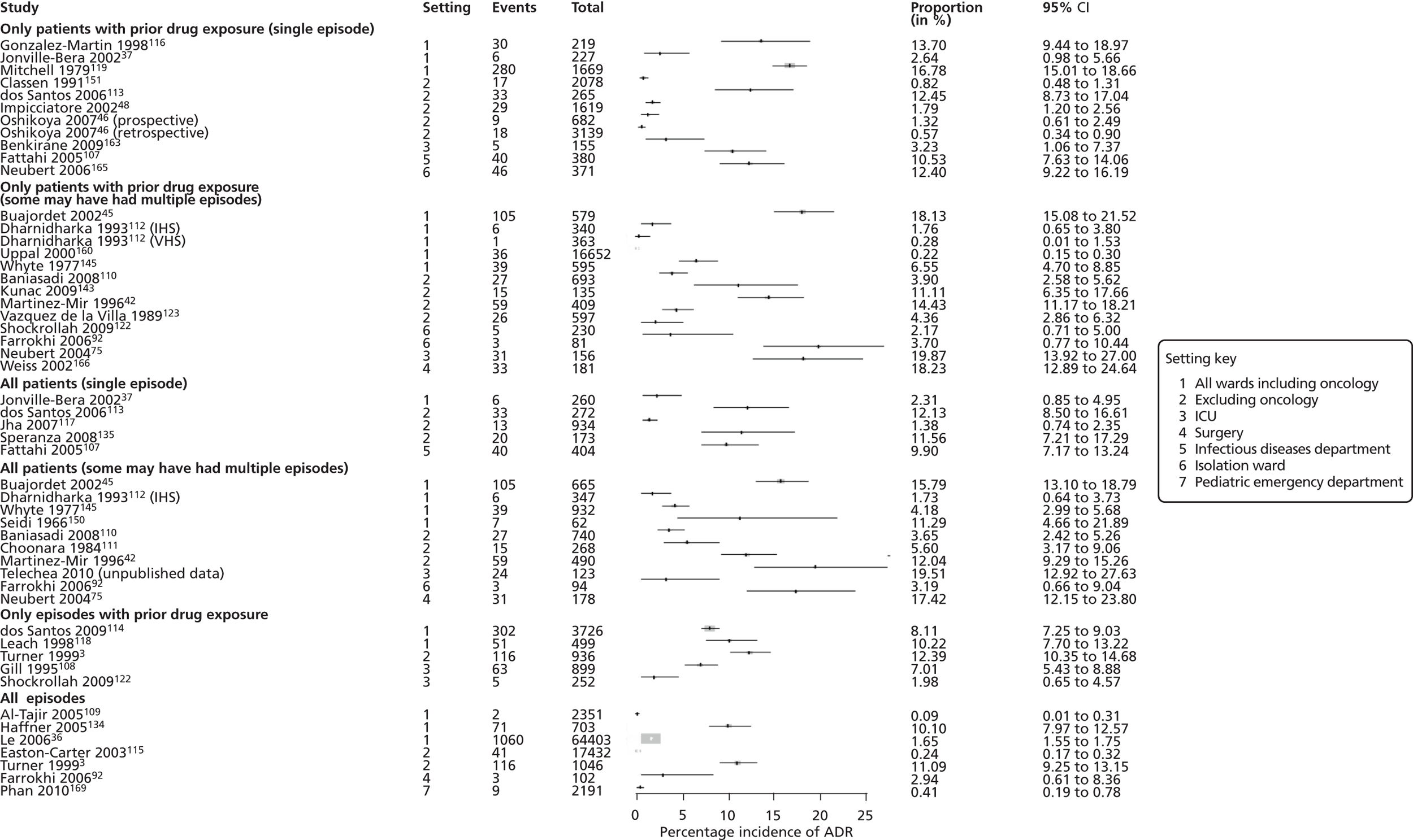
Studies estimating the incidence of adverse drug reactions in outpatient children
Description of studies
We have included 36 studies, where ADRs have been investigated in the community setting. The period under study varied widely and ranged from 1 week to 11 years. The majority of studies were described as being performed in a hospital outpatient or A&E department (n = 21). 109,124–126,129,131,133,155,168–180 Nine studies were performed in general practice. 77,78,127,128,130,181–184 The remaining six studies were performed in an infant care and educational establishment,133 local community setting,185,186 general practice and emergency department,37 outpatient population seeking medical care187 and after discharge from hospital. 45
Adverse drug reaction incidence
We do not have ADR incidence rates for 18 (18/35) of these studies, as the child-only data were not available (n = 10), the data were not split by clinical setting (n = 3), data were not available for the total number of children/visits (n = 3), data were provided for the total number of ADRs but not the ADR frequency at the patient or visit level (n = 1), and data were provided for errors only (n = 1). Figure 10 presents data from studies that provide incidence rates for ADRs in the community (n = 15). Two studies were not included in this figure owing to their method of ADR ascertainment.
FIGURE 10.
What proportion of outpatient children experience ADRs? GP, general practice.
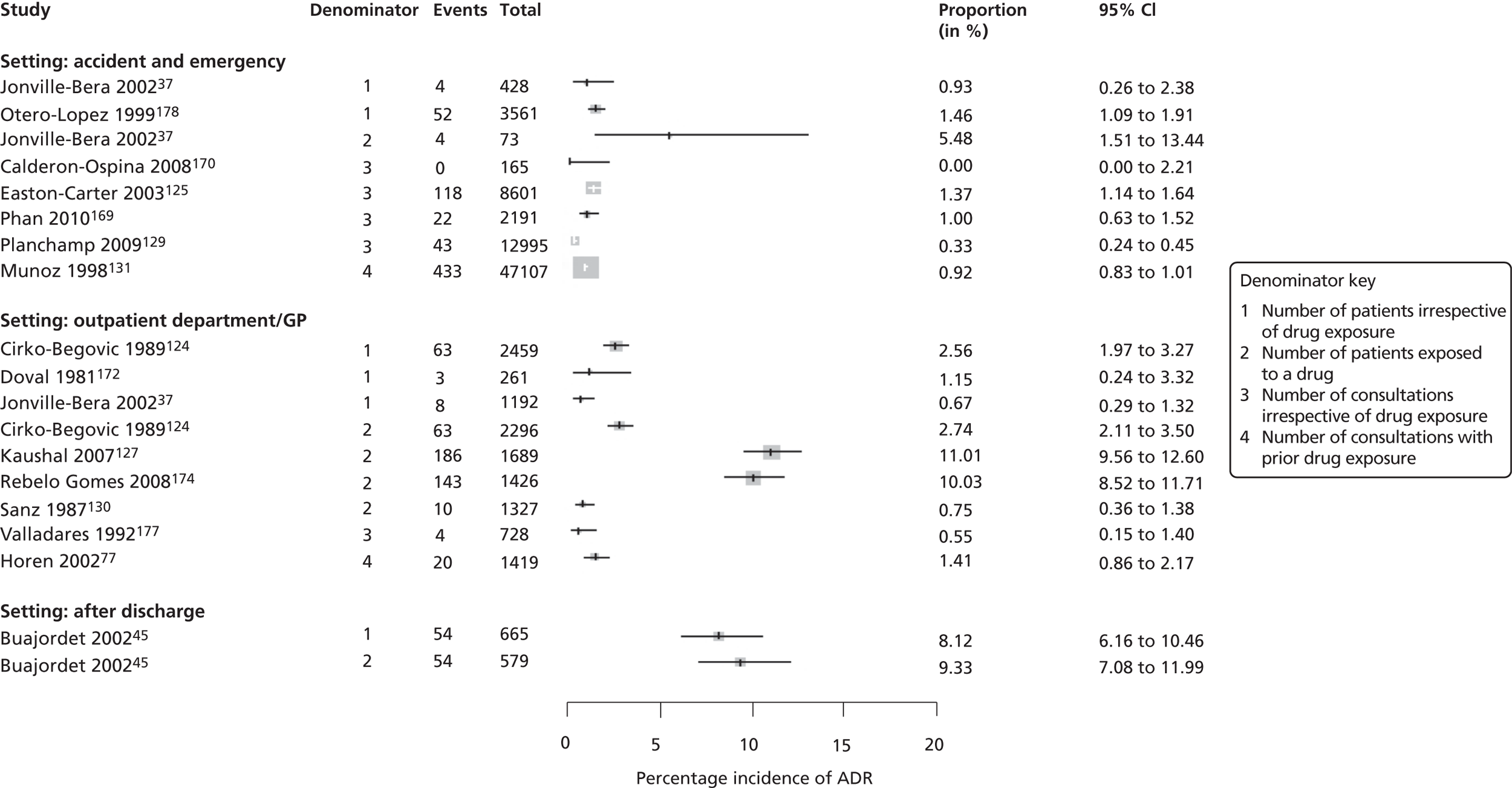
All settings
Drugs and clinical presentation associated with adverse drug reaction
We do not have information on the drugs involved in ADRs for 49 out of 101 studies, as the child-only data were not available (37 studies), ADRs were a subset of events looked at and ADR-specific data were not reported (10 studies), and drug data were not available in the publication (two studies). For studies that provided data (52/101); anti-infective drugs were the drug class most commonly reported across the three settings. Proportions ranged from 3.5% to 66.6% for causing admission studies (17 studies); 8.6% to 100% for in-hospital studies (24 studies); and 17% to 78% for community studies (13 studies). The most common associated clinical presentations reported were nausea, vomiting, diarrhoea and skin rash. Antiepileptic drugs were the second most common reported drug class in both the causing admission and in-hospital studies; proportions ranging from 0.8% to 30% (12 studies) and 3.9% to 46.6% (14 studies), respectively. Reported clinical presentations were ataxia, skin rash, increased fitting and drowsiness. NSAIDs were frequently reported as being associated with ADRs in studies in children in outpatients, with proportions ranging from 1% to 10% (six studies). Reported clinical presentations were cutaneous reactions, haematuria, hypertranspiration, drowsiness, abdominal pain, aggressiveness and vomiting.
In addition, corticosteroids were commonly reported across the three settings. Proportions ranging from 5.5% to 41.0% for causing admission studies (seven studies); 1.7% to 23.4% for in-hospital studies (10 studies); and 0.05% to 5% for community studies (three studies). The most common associated clinical presentations reported were immunosuppression, postoperative bleeding, gastric irritation and diarrhoea.
The distribution of drugs implicated in ADRs reflect the prescribing practices for the individual settings. For example, vaccines were commonly reported in causing admission studies (seven studies) and community studies (five studies). Proportions ranged from 1.7% to 41.0% and 9.2% to 25% respectively, with rash and fever being the most commonly associated clinical presentations. Cytotoxic drugs were reported in both causing admission (eight studies) and in hospital studies (seven studies), and proportions ranged from 14.2% to 50% and 1.7% to 66.6%, respectively. The remaining studies reported a variety of drugs implicated in ADRs, and for some more than one drug was the cause of a single ADR (see Tables 22–27 ).
Univariate meta-regression results ( Table 28 ) suggest that the incidence rate for ADRs occurring in hospital is higher than for ADRs causing admission (OR = 2.73, 95% CI 0.93 to 8.03). In addition, the results suggest that the incidence rate is higher for studies with a relatively high mean/median number of drugs per patient (OR = 1.49, 95% CI 1.14 to 1.94), a high percentage of females (OR = 1.13, 95% CI 0.91 to 1.40), a high percentage of oncology patients (OR = 1.15, 95% CI 0.89 to 1.50) and low mean age of patients (OR = 0.71, 95% CI 0.39 to 1.27). However, only the variable representing the mean/median number of drugs per patient achieves statistical significance.
| Covariate | OR (95% CI) | p-value | |
|---|---|---|---|
| Setting | Admission | 1 (0.93 to 8.03) | 0.07 |
| Hospital | 2.73 (0.93 to 8.03) | ||
| % female patients | 1.13 (0.91 to 1.40) | 0.23 | |
| Mean age (years) | 0.71 (0.39 to 1.27) | 0.21 | |
| Mean/median number of drugs | 1.49 (1.14 to 1.94) | 0.01 | |
| % oncology patients | 1.15 (0.89 to 1.50) | 0.25 | |
Risk factors
Risk factor analyses reported by all studies were collated. Consistent with the meta-regression results, evidence is provided – from 10 out of 19 studies that consider gender as a risk factor – that boys are less likely to have an ADR and, from 16/17 studies, that risk increases with the number of drugs taken. In addition, three out of three studies suggest that the risk of ADRs is greater with off-label use. Only two studies considered oncology as a risk factor. The results for the age analyses do not follow a clear pattern and are difficult to interpret owing to the variety of age categorisations used.
Tools for assessing causality
Nearly three-quarters of the studies (71/101) mentioned a causality assessment, of which the Naranjo algorithm was the most frequently used tool (29/71). Of the 71 studies, six used a self-assessment method rather than a published CAT. Despite the majority of studies mentioning a causality assessment, only half of these studies (36/71) reported causality data that were complete for all identified ADRs, specific to the paediatric population and did not include errors as part of the assessment.
Tools for assessing severity
Thirty-four (34/101) studies performed an ADR severity assessment. Rates ranged from 0% to 66.7% of reported ADRs considered to be severe. By setting, the proportion of ADRs occurring in hospital assessed as severe ranged from 0% to 66.7%, compared with 0–45.5% of ADRs causing admission, and 0–32.6% of ADRs occurring in the community. Twenty studies provided a reference to indicate the severity tools used; however, tools differed widely. Examples of ADRs assessed as severe were those that caused death or were directly life-threatening, caused hospital admission, prolonged hospitalisation or caused transfer to higher level of clinical care.
Assessment of avoidability
Nineteen (19/101) studies performed an avoidability assessment; however, data were available for only 14 out of 19 studies, as child-only data were not available in 4 out of 19 and ADR-specific data were not provided in 1 out of 19 studies. For these 14 studies, 7–98% of ADRs were designated as either definitely or possibly avoidable. Three studies provided the rationale for 62 avoidable ADRS: inappropriate selection or indication for use of drug (n = 14), inadequate patient education (n = 14), prescribing not rational (n = 11), lack of appropriate prophylaxis for known ADR (n = 9), lack of appropriate monitoring of drugs (n = 5), previous known ADR to medication (n = 3), dose prescribed was too high (n = 3), inappropriate duration of treatment (n = 1), drug was not prescribed per treatment protocol (n = 1), inappropriate duration of drug and monitoring of treatment (n = 1). Ten studies used a recognised AAT, of which half used that of Schumock and Thornton. 44
Discussion
This is the largest systematic review of ADRs in children to date and shows clearly that ADRs are an important clinical problem for children and have been the subject of a large number of studies. Unlike other systematic reviews,34,35,101 our review searched for studies using a comprehensive search strategy of a large number of databases, including those specific to toxicology and pharmacology. We included studies in which ADEs had been evaluated, and that included both adults and children. When compared with the previous reviews this resulted in an additional 69 studies being included in our review, of which we were able to extract data from 24. In addition, we contacted authors of studies to obtain unpublished information. As a result, we were able to obtain unreported ADR incidence data for an additional 24 out of 101 studies. This allowed us to make a more informed judgement regarding ADR incidence estimates.
In agreement with previous studies, this review found that ADR incidence rates were generally higher in hospitalised children than ADR rates causing hospital admission or in an outpatient setting. One of the main difficulties with comparing ADR incidence rates, particularly from observational studies, is the different numbers and denominators used, leading to high levels of variability between studies in the calculation and reporting of incidence rates. Owing to this, a pooled estimate has been provided for ADRs causing admission only.
Concerning risk factors associated with ADRs, we found evidence – from both univariate meta-regression and the collation of risk factor analyses from individual studies – that the use of multiple drugs is an important predictor of ADRs. This may be due to the additive risk of an ADR when receiving several drugs or drug–drug interactions.
We examined the methods used for detecting, and assessing, the causality, severity and avoidability of an ADR. The assessment of causality in individual cases of ADRs is required to establish whether or not there is an association between the untoward clinical event and the suspected drug. 23 The detection of ADRs depends on the validity and reliability of the tests used and if sensitive methods are performed, in theory, all ADRs should be detected. We found that one-third (30/101) of studies did not report which CAT they used, with an additional six not using a recognised algorithm. As a consequence there may be either an underestimation or overestimation of ADRs in these studies. Over one-third of studies (34/101) assessed ADRs for the severity of the reactions, just eight of which did not report any severe ADRs. The ability to classify ADRs by severity provides a mechanism for clinicians to identify problem areas and implement interventions to inform paediatric pharmacovigilance practice.
The absence of avoidability data was most noticeable in this review, with only 14 studies (14/101; 14%) providing avoidability data. Therefore, it is not possible to consider this important aspect of drug safety in order to prevent future ADRs. 44 Further studies are clearly required to determine which ADRs are potentially avoidable. These studies could provide the necessary data in order to enable clinicians to administer medications in the safest and appropriate way.
The reporting quality of some of the included studies was poor, which may have affected the results. Not all provided a clear definition of the term ‘ADR’, and often insufficient information was given in the publication in order to determine whether ADRs included medication or prescribing errors. ADR incidence data were not always clearly described in the publications; in many studies (n = 48/101) reporting was unclear regarding whether the incidence rate was reported at the patient and/or episode level, and whether or not all children had been exposed to a drug.
It is disappointing, given the large number of studies that we identified that addressed this problem, that most did not include these important methodological aspects, which means that few lessons have been learnt about how to prevent ADRs in children.
Conclusions
This review confirms previous studies that have shown ADRs to be an important problem in children and has highlighted therapeutic classes of drugs most commonly associated with them. Further work to address prescribing practices in different settings and avoidability of ADRs is needed to indicate how such ADRs may be prevented.
Chapter 5 Causality assessment of adverse drug reactions
This chapter contains information reproduced from Gallagher RM, Kirkham JJ, Mason JR, Bird KA, Williamson PR, Nunn AJ, et al. Development and Inter-Rater Reliability of the Liverpool Adverse Drug Reaction Causality Assessment Tool. PLOS ONE 2011;6:e28096,51 © 2011 Gallagher et al. , an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided that the original author and source are credited.
Abstract
Aim
To develop and test a new ADR CAT.
Methods
A comparison between seven assessors of a new CAT (formulated by an expert focus group) with the Naranjo CAT in 80 cases from a prospective observational study and 37 published ADR case reports (819 causality assessments in total).
Main outcome measures
Utilisation of causality categories, measure of disagreements, inter-rater reliability (IRR).
Results
The LCAT, using 40 cases from an observational study, showed causality categories of one unlikely, 62 possible, 92 probable and 125 definite (1, 62, 92, 125) and ‘moderate’ IRR [kappa (κ) = 0.48] compared with Naranjo (0, 100, 172, 8) with ‘moderate’ IRR (κ = 0.45). In a further 40 cases, the LCAT (0, 66, 81, 133) showed ‘good’ IRR (κ = 0.6), whereas Naranjo (1, 90, 185, 4) remained ‘moderate’.
Conclusions
The LCAT assigns the full range of causality categories and shows good IRR. Further assessment by different investigators in different settings is needed to fully assess the utility of this tool.
Introduction
Causality assessment of ADRs is a method used for estimating the strength of relationship between drug(s) exposure and occurrence of adverse reaction(s). Causality assessment of ADRs may be undertaken by clinicians, academics, the pharmaceutical industry and regulators, and in different settings, including clinical trials. 188–191 At an individual level, health-care providers assess causality informally when dealing with ADRs in patients to make decisions regarding future therapy. Many regulatory authorities assess spontaneous ADR reports,189,191 where causality assessment can help in signal detection and risk–benefit decisions regarding medicines,192,193 using formal CATs to aid in this process.
An early paper by Sir Austin Bradford Hill194 describing minimum criteria for establishing causality of AEs, pre-dates the earliest attempts to formulate ADR CATs. Bradford Hill set out criteria for establishing causality, which included assessment of strength of the association, consistency of the association, specificity, temporal relationship, biological gradient (dose response), biological plausibility, coherence, experimental evidence and reasoning by analogy. These elements of assessing strength of relationship between exposure (drugs) and outcome (adverse reaction) are used widely in ADR CATs. Attempts to formalise causality assessment of ADRs into structured CATs have been ongoing for > 30 years. 23,195 It is known that assessing ADR likelihood without structure can lead to wide disagreements between assessors. 196 Disagreements may mean that opportunities to avoid or ameliorate harm are missed during clinical care or that cases are misclassified in epidemiological studies. These disagreements may be the result of differing clinical backgrounds, specialties and experience between assessors. A large number of CATs have been developed ranging from the simple to the complex. These tools aim to limit disagreement between assessors of ADR cases as to the likelihood that a reaction is related to a particular medication taken by the patient. None has gained universal acceptance. 197
One of the most widely used CATs is the Naranjo ADR probability scale. 23 This is a simple 10-item questionnaire that classifies the likelihood that a reaction is related to a drug using concepts such as timing, plausibility/evidence, de-challenge and rechallenge/previous exposure. Each element of the questionnaire is weighted and the total score is used to categorise the event into unlikely, possible, probable and definite. The tool was developed 30 years ago by adult pharmacologists/physicians and psychiatrists. Published case reports were used to validate the reliability of the tool in assessing causality. It has been widely used, including recently by investigators in two large prospective observational studies of ADRs causing hospital admission and occurring in hospital inpatients. 59,198 However, the reliability and validity of the Naranjo scale has been questioned by a number of investigators. 24,188,193,199,200
While undertaking a prospective observational pilot study of ADRs in children, we found several difficulties with using the Naranjo scale, and aimed to address those difficulties. Our original aim was to use the Naranjo ADR Probability Scale for the larger observational study; we planned to assess the causality of the ADRs prospectively rather than at the end of the study period. When beginning to assess this heterogeneous mix of potential ADR cases during the pilot study with the Naranjo scale, the investigators found that some questions were not appropriate in this clinical context. This led to many elements of the Naranjo scale being categorised as ‘unknown’. In particular, question six (‘Did the reaction reappear when a placebo was given?’) and question seven [‘Was the drug detected in the blood (or other fluids) in concentrations known to be toxic?’] were very often answered as ‘unknown’. Administration of a placebo and assessment of drug concentrations are not part of practice when assessing potential causality of ADRs in this clinical setting. An answer assigned as ‘unknown’ gives a zero score for that element in the Naranjo scale. This will lower the total achievable score on an individual case basis. This meant that the thresholds for recognising ADRs were not achieved, which, in turn, underestimated the likelihood of an ADR. This led to a lack of sensitivity for many of the early cases assessed in our study, as the overall score obtained for each causality assessment was artificially lowered. The investigators encountered several cases that were unanimously thought to be definite ADRs (e.g. repeated episodes of febrile neutropenia during oncological chemotherapy) but which did not reach the threshold for ‘definite’ causality using the published Naranjo scale. Accordingly, the Naranjo score did not have face validity when applied to our patient population. Moreover, the weighting for each question and the ADR classification scoring boundaries used in the Naranjo scale were not justified in the original publication, or subsequently. Therefore, we developed a CAT that would overcome some of these issues, while at the same time (1) making it as easy, or easier, to use than the Naranjo scale (a feature that holds a distinct advantage for large observational studies of ADRs among other situations) and (2) ensuring that the basic principles of assessing causality were maintained. The specific aim of this study was to develop a CAT with good face validity and acceptable inter-rater reproducibility.
Methods
The pilot study team (RG, JB, KB) noted concerns with using the Naranjo scale. This triggered a process in which each of seven investigators (RG, JB, KB, MPir, TN, RS, MT) independently assessed the first 40 consecutive case reports from an observational study of suspected ADRs causing hospital admission using the Naranjo scale. In summary, there were eight cases in which problems with assessments were found. There was one case in which major discrepancies occurred between at least two out of seven raters, i.e. where the range of causality probability differed by more than one category (e.g. possible and definite), and seven cases in which close to half of the raters differed from the others by one causality category. The questions (within the Naranjo scale) that caused the discrepancies in these cases were identified and reviewed. This exercise led to the recognition that a new CAT was required.
The team made several choices at the start of the development of the new CAT. In order to relate to the existing literature, it was agreed that the output of the new tool would take the same form as the Naranjo scale. That is, categorical scores from both the Naranjo scale and the new tool would take the same four-point ordinal scale (unlikely, possible, probable and definite). In order to fit with clinicians’ experiences, the format of the new tool was an algorithm, with dichotomous responses to each decision followed by routing to further, specific questions, rather than the weighted responses used in the Naranjo scale. The study team decided to develop the new tool in two stages: first, use the extensive clinical and pharmacovigilance expertise in the group to develop a tool that had face validity to the team, and, second, iteratively assess the tool to optimise interobserver agreement within the study team. In the first step of the process, each question in the Naranjo scale was reviewed by the investigators at a consensus meeting to assess whether it was appropriate to (1) retain it (with or without modification); (2) reject it; or (3) combine it with another question(s). The aim was to create a new, more appropriate CAT ( Table 29 ).
| No. | Naranjo scale questions | Yes | No | Do not know | Outcome for LCAT |
|---|---|---|---|---|---|
| Q1 | Are there previous conclusive reports on this reaction? | +1 | 0 | 0 | Retained Knowledge of previous reports can be important when assessing if an AE is due to drug or disease |
| Q2 | Did the AE appear after the suspected drug was administered? | +2 | –1 | 0 | Modified Timing of event in relation to drug exposure is important when determining causality |
| Q3 | Did the adverse reaction improve when the drug was discontinued or a specific antagonist was administered? | +1 | 0 | 0 | Modified Knowledge of de-challenge, if available, may provide further evidence as to causality of an event. However, an event may have long-lasting sequelae. A new question was added to the Liverpool tool to cover this possibility |
| Q4 | Did the adverse reaction reappear after the drug was re-administered? | +2 | –1 | 0 | Combined Knowledge of rechallenge, if available, may add to the level of certainty regarding causality assessment. This question is combined with Naranjo Q8 regarding dose–response relationship to increasing dose. This can also provide evidence to support or refute causality |
| Q5 | Are there alternative causes (other than the drug) that could on their own have caused the reaction? | –1 | +2 | 0 | Modified This question is replaced within the Liverpool tool by a question involving likelihood of alternative cause, with an option to answer ‘unsure’ (which prompts the user to seek further evidence of the reaction). Naranjo Q5 is worded such that it is difficult to answer ‘no’ |
| Q6 | Did the reaction reappear when a placebo was given? | –1 | +1 | 0 | Rejected With the exception of clinical trials, placebo use is not common practice and this question is no longer relevant |
| Q7 | Was the drug detected in the blood (or other fluids) in concentrations known to be toxic? | +1 | 0 | 0 | Modified Objective evidence of the ADR occurrence will already be taken in to account when the user is deciding whether the event is likely to be drug or disease related. A question in the Liverpool tool asks for objective evidence of likely ADR mechanism. If apparent, this may provide evidence of causality to an assessor |
| Q8 | Was the reaction more severe when the dose was increased, or less severe when the dose was decreased? | +1 | 0 | 0 | Combined This question is combined with one addressing de-challenge in the Liverpool tool. The answer to this question may be important in establishing if there is a dose–response relationship between drug and AE |
| Q9 | Did the patient have a similar reaction to the same or similar drugs in any previous exposure? | +1 | 0 | 0 | Modified This is included in the Liverpool algorithm, in relation to the same drug(s) only, and given the same weighting as a positive rechallenge. This may provide evidence of susceptibility, and likelihood, of the event being related to a drug |
| Q10 | Was the AE confirmed by any objective evidence? | +1 | 0 | 0 | Modified See Q7 |
The new LCAT was then used to assess 20 new suspected ADR case reports from our observational study. The collated causality categories for all seven assessors showed 1 (0.7%) unlikely, 18 (12.9%) possible, 2 (1.4%) probable and 119 (85%) definite. The assessors achieved moderate agreement with a kappa score of 0.51 (95% CI 0.19 to 0.82). The team considered that there was an inappropriate bias towards the category of definite. Accordingly, the CAT was reviewed. Major discrepancies between scorers were identified and each question within the algorithm was reviewed to assess face validity and likelihood of inter-rater disagreement. Questions that caused the major discrepancies were then modified. The new CAT was then tested on a further 20 case reports: 10 from the ADRIC study and 10 from an observational study of inpatient ADRs in an adult hospital. Collated causality categories for the 10 ADRIC 1 cases showed 0 (0%) unlikely, 24 (34%) possible, 39 (56%) probable and 7 (10%) definite with a kappa score of 0.27 (95% CI 0.11 to 0.44). Collated causality categories for the 10 adult cases showed 0 (0%) unlikely, 13 (19%) possible, 48 (69%) probable and 9 (13%) definite, with a kappa score of 0.13 (95% CI –0.14 to 0.38). The results of these assessments prompted another review of the appropriateness of the tool and questions. A third iteration was used so that the development and evaluation of tool prototypes was based on discussions in which 80 cases were used ( Figure 11 ).
FIGURE 11.
Flow chart of the development of the Liverpool ADR Causality Assessment Tool.
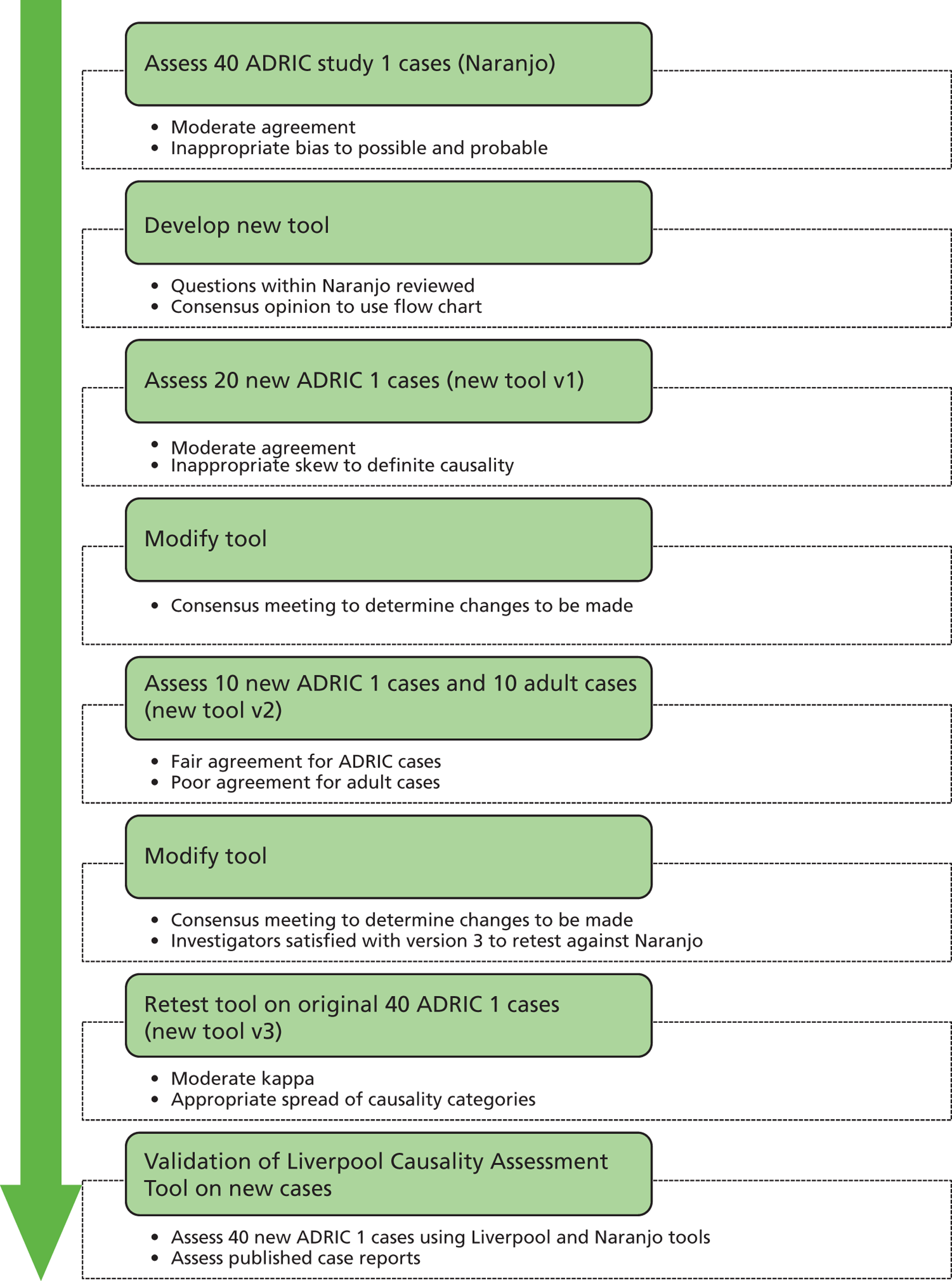
After the third iteration, the investigators were satisfied with the final version of the new tool ( Figure 12 ) in terms of ease of use, lack of ambiguity and appropriateness of the causality assignment. This was judged by expert opinion and consensus within the group.
FIGURE 12.
Liverpool Causality Assessment Tool (LCAT). a, unassessable refers to situations where the medicine is administered on one occasion (e.g. vaccine), the patient receives intermittent therapy (e.g. chemotherapy), or is on medication which cannot be stopped (e.g. immunosuppressant drugs); and b, examples of objective evidence: positive laboratory investigations of the causal ADR mechanism (not those merely confirming the adverse reaction), supratherapeutic drug levels and good evidence of a dose-dependent relationship with toxicity in the patient. An independent panel with extensive expertise in pharmacovigilance and statistics (the ADRIC Steering Group) was asked to review the tool upon completion of the internal evaluation.
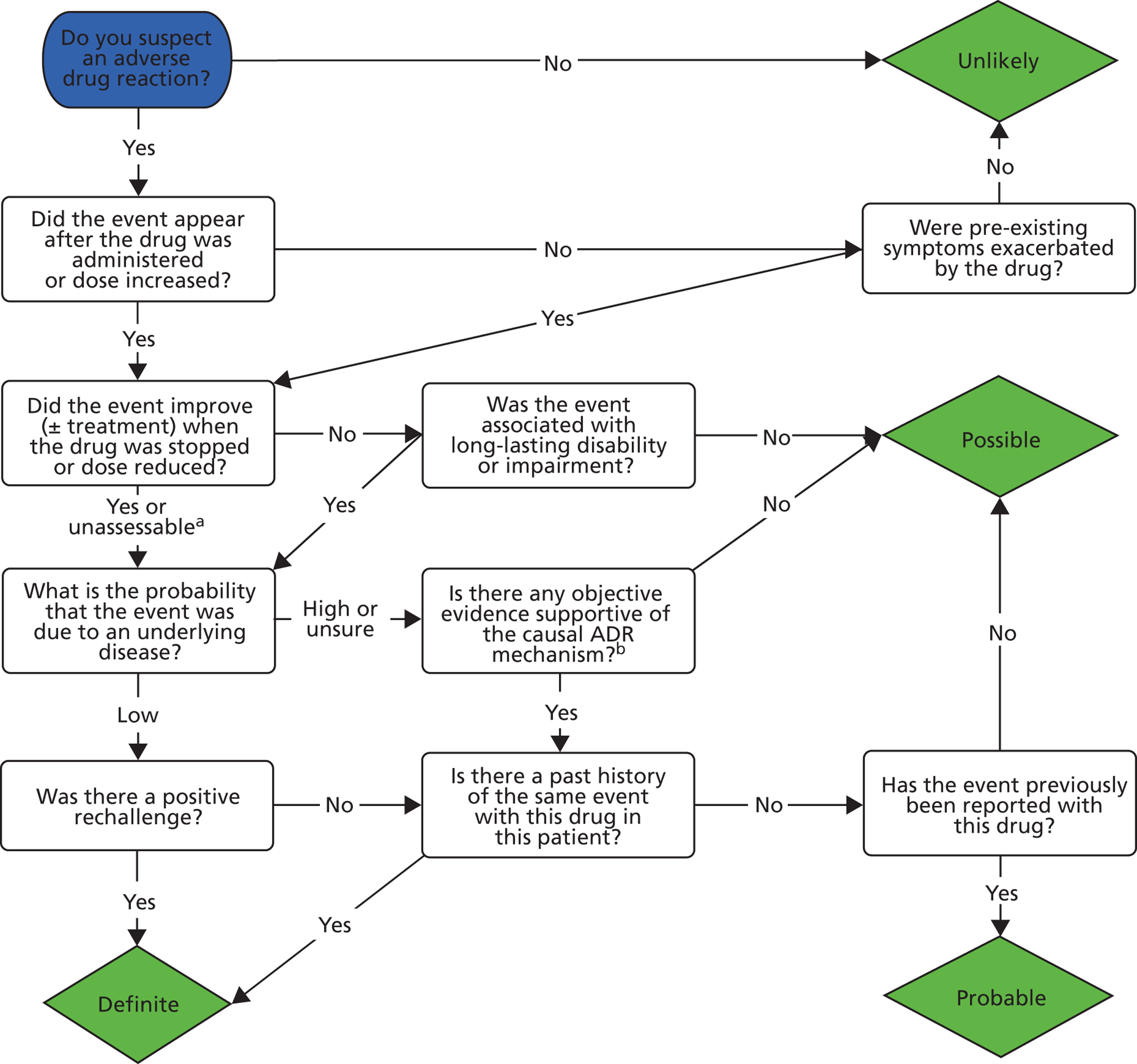
The assessment of IRR within the study team for the LCAT followed a stepwise procedure:
-
The original 40 case reports (case reports of raw clinical data from an observational study) initially assessed using the Naranjo scale were assessed by each of the seven investigators using the new CAT to compare the outcomes of the methods and the IRR between the two tools.
-
In order to examine the tool using cases other than those collected in our observational study, 37 cases of ADRs were randomly selected from the Annals of Pharmacotherapy and independently evaluated by the seven assessors using only the new tool.
-
As the original 40 cases from our observational study had been used in the design of the new tool, a further new set of 40 ADR case reports from our study was then used to assess IRR using both the Naranjo scale and the LCAT.
Analysis
The inter-rater agreements at each stage of the assessment process were assessed using a linear weighted kappa with 95% CI for ordered categories. Exact agreement percentages (%EA) were computed to measure the absolute concordances between assessor scores. Percentage of extreme disagreement (%ED), where the causality scores between two raters of the same case are wider than one causality interval apart (e.g. definite for one rater and possible for the other), were also computed to measure extreme disagreements between pairwise rater assessments. To supplement the pairwise kappa scores, a global kappa score measuring nominal scale agreement across multiple assessors was calculated with 95% CI. 201 The global kappa score provides a single statistic to quantify assessor agreement for each set of cases. Kappa values were interpreted according to the guidance from Altman:202 poor agreement < 0.2; fair 0.21–0.40; moderate 0.41–0.60; good 0.61–0.80; and very good 0.81–1.00.
Results
Assessment of the original 40 consecutive ADR cases by the seven investigators using the Naranjo scale showed collated categorisation of causality scores for all assessors (n = 280 assessments) of 0 (0%) unlikely, 100 (36%) possible, 172 (61%) probable and 8 (3%) definite ( Table 30 ). %EA for the pairwise comparisons between raters ranged from 43% to 93%. The %ED was 2.5% for four of the 21 pairwise comparisons. There were no extreme disagreements in 17 out of 21 pairwise comparisons. Pairwise kappa scores ranged from 0.27 to 0.86 and the assessors achieved moderate IRR with a global kappa score of 0.45 (95% CI 0.35 to 0.54) ( Table 31 ). The same cases assessed using the new LCAT showed collated causality categories of 1 (0.4%) unlikely, 62 (22%) possible, 92 (33%) probable and 125 (45%) definite. %EA ranged from 43% to 93%. All 21 pairwise comparisons displayed with %ED ranging from 5–20%. Pairwise kappa scores ranged from 0.27 to 0.84, and the assessors achieved moderate IRR with a global kappa score of 0.48 (95% CI 0.42 to 0.54) (see Table 31 ).
| Assessor | Tool | ADRIC original (N = 40) | |||
|---|---|---|---|---|---|
| Unlikely: n (%) | Possible: n (%) | Probable: n (%) | Definite: n (%) | ||
| RG | Naranjo | 0 (0.0) | 18 (45.0) | 22 (55.0) | 0 (0.0) |
| Liverpool | 0 (0.0) | 7 (17.5) | 23 (57.5) | 10 (25.0) | |
| JB | Naranjo | 0 (0.0) | 17 (42.5) | 22 (55.0) | 1 (2.5) |
| Liverpool | 0 (0.0) | 15 (37.5) | 8 (20.0) | 17 (42.5) | |
| KB | Naranjo | 0 (0.0) | 18 (45.0) | 21 (52.5) | 1 (2.5) |
| Liverpool | 0 (0.0) | 18 (45.0) | 4 (10.0) | 18 (45.0) | |
| MT | Naranjo | 0 (0.0) | 14 (35.0) | 24 (60.0) | 2 (5.0) |
| Liverpool | 1 (2.5) | 5 (12.5) | 17 (42.5) | 17 (42.5) | |
| TN | Naranjo | 0 (0.0) | 10 (25.0) | 29 (72.5) | 1 (2.5) |
| Liverpool | 0 (0.0) | 3 (7.5) | 15 (37.5) | 22 (55.0) | |
| MPir | Naranjo | 0 (0.0) | 12 (30.0) | 27 (67.5) | 1 (2.5) |
| Liverpool | 0 (0.0) | 7 (17.5) | 12 (30.0) | 21 (52.5) | |
| RS | Naranjo | 0 (0.0) | 11 (27.5) | 27 (67.5) | 2 (5.0) |
| Liverpool | 0 (0.0) | 7 (17.5) | 13 (32.5) | 20 (50.0) | |
| Totals | Naranjo | 0 (0.0) | 100 (35.7) | 172 (61.4) | 8 (2.9) |
| Liverpool | 1 (0.36) | 62 (22.1) | 92 (32.9) | 125 (44.6) | |
| Assessor 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| RG | JB | KB | MT | TN | MPir | RS | |||
| Assessor 1 | RG | %EA/ED | 57.5/0 | 42.5/0 | 55.0/0 | 52.5/0 | 62.5/0 | 55.5/0 | |
| Kappa (95% CI) | 0.52 (0.27 to 0.77) | 0.47 (0.21 to 0.73) | 0.44 (0.19 to 0.69) | 0.45 (0.21 to 0.69) | 0.36 (0.09 to 0.62) | 0.29 (0.04 to 0.54) | |||
| JB | %EA/ED | 57.5/5 | 92.5/0 | 70.0/0 | 77.5/0 | 72.5/0 | 70.0/2.5 | ||
| Kappa (95% CI) | 0.46 (0.26 to 0.67) | 0.86 (0.71 to 1.00) | 0.46 (0.22 to 0.69) | 0.56 (0.34 to 0.78) | 0.47 (0.19 to 0.75) | 0.40 (0.15 to 0.65) | |||
| KB | %EA/ED | 42.5/10 | 75.0/5 | 77.5/0 | 70.0/0 | 70.0/0 | 77.5/2.5 | ||
| Kappa (95% CI) | 0.28 (0.08 to 0.49) | 0.69 (0.52 to 0.87) | 0.60 (0.39 to 0.81) | 0.43 (0.19 to 0.66) | 0.43 (0.15 to 0.71) | 0.55 (0.32 to 0.77) | |||
| MT | %EA/ED | 55.0/7.5 | 70.0/5 | 57.5/7.5 | 72.5/0 | 62.5/0 | 70.0/2.5 | ||
| Kappa (95% CI) | 0.31 (0.06 to 0.56) | 0.62 (0.45 to 0.80) | 0.49 (0.31 to 0.67) | 0.45 (0.20 to 0.70) | 0.37 (0.11 to 0.62) | 0.48 (0.23 to 0.73) | |||
| TN | %EA/ED | 52.5/7.5 | 62.5/15 | 52.5/20 | 70.0/7.5 | 70.0/0 | 72.5/2.5 | ||
| Kappa (95% CI) | 0.27 (0.07 to 0.46) | 0.42 (0.21 to 0.62) | 0.30 (0.10 to 0.50) | 0.49 (0.26 to 0.72) | 0.33 (0.05 to 0.62) | 0.35 (0.06 to 0.63) | |||
| MPir | %EA/ED | 62.5/5 | 77.5/7.5 | 67.5/12.5 | 80.0/5 | 80.0/7.5 | 70.0/0 | ||
| Kappa (95% CI) | 0.47 (0.25 to 0.69) | 0.68 (0.49 to 0.86) | 0.54 (0.33 to 0.74) | 0.69 (0.49 to 0.89) | 0.62 (0.39 to 0.84) | 0.38 (0.11 to 0.65) | |||
| RS | %EA/ED | 55.5/10 | 70.0/12.5 | 62.5/15 | 80.0/7.5 | 75.0/10 | 92.5/5 | ||
| Kappa (95% CI) | 0.30 (0.05 to 0.55) | 0.54 (0.32 to 0.76) | 0.46 (0.24 to 0.67) | 0.66 (0.44 to 0.87) | 0.52 (0.27 to 0.76) | 0.84 (0.66 to 1.00) | |||
The 37 randomly selected ADR case reports from the Annals of Pharmacotherapy assessed by the seven investigators using the LCAT showed collated categorisation of causality scores (n = 259 assessments) of 1 (0.4%) unlikely, 67 (26%) possible, 136 (53%) probable and 55 (21%) definite ( Table 32 ). %EA ranged from 57% to 97%. Pairwise comparisons between raters showed some extreme disagreement (18/21), with the %ED ranging from 5% to 11%, whereas three showed no extreme disagreements. Pairwise kappa scores ranged from 0.31 to 0.96 and the assessors achieved moderate IRR with a global kappa of 0.43 (95% CI 0.34 to 0.51) ( Table 33 ).
| Assessor | Tool | Annals of Pharmacotherapy (N = 37) | |||
|---|---|---|---|---|---|
| Unlikely: n (%) | Possible: n (%) | Probable: n (%) | Definite: n (%) | ||
| RG | Liverpool | 0 (0.0) | 11 (29.7) | 18 (48.7) | 8 (21.6) |
| JB | Liverpool | 0 (0.0) | 11 (29.7) | 20 (54.1) | 6 (16.2) |
| KB | Liverpool | 0 (0.0) | 12 (32.4) | 19 (51.4) | 6 (16.2) |
| MT | Liverpool | 0 (0.0) | 10 (27.0) | 18 (48.7) | 9 (24.3) |
| TN | Liverpool | 1 (2.7) | 10 (27.0) | 20 (54.1) | 6 (16.2) |
| MPir | Liverpool | 0 (0.0) | 10 (27.0) | 17 (46.0) | 10 (27.0) |
| RS | Liverpool | 0 (0.0) | 3 (8.1) | 24 (64.9) | 10 (27.0) |
| Totals | Naranjo | 0a (0) | 5a (13.5) | 29a (78.4) | 3a (8.1) |
| Liverpool | 1 (0.39) | 67 (25.9) | 136 (52.5) | 55 (21.2) | |
| Assessor 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| RG | JB | KB | MT | TN | MPir | RS | |||
| Assessor 1 | RG | %EA/ED | 62.2/10.8 | 64.9/10.8 | 73.0/0 | 56.8/8.1 | 59.5/5.4 | 67.6/5.4 | |
| Kappa (95% CI) | 0.307 (0.03 to 0.58) | 0.38 (0.10 to 0.65) | 0.65 (0.44 to 0.85) | 0.32 (0.05 to 0.59) | 0.41 (0.16 to 0.66) | 0.46 (0.22 to 0.69) | |||
| JB | %EA/ED | 97.3/0 | 62.2/10.8 | 64.9/8.1 | 56.8/8.1 | 64.9/8.1 | |||
| Kappa (95% CI) | 0.93 (0.82 to 1.00) | 0.31 (0.04 to 0.59) | 0.34 (0.06 to 0.61) | 0.29 (0.02 to 0.57) | 0.33 (0.09 to 0.57) | ||||
| KB | %EA/ED | 59.5/10.8 | 67.6/8.1 | 59.5/8.1 | 62.2/8.1 | ||||
| Kappa (95% CI) | 0.31 (0.03 to 0.59) | 0.41 (0.13 to 0.68) | 0.36 (0.10 to 0.63) | 0.34 (0.10 to 0.58) | |||||
| MT | %EA/ED | 64.9/8.1 | 64.9/5.4 | 78.4/5.4 | |||||
| Kappa (95% CI) | 0.40 (0.13 to 0.66) | 0.48 (0.23 to 0.72) | 0.61 (0.38 to 0.84) | ||||||
| TN | %EA/ED | 62.2/8.1 | 67.6/5.4 | ||||||
| Kappa (95% CI) | 0.38 (0.11 to 0.64) | 0.42 (0.19 to 0.65) | |||||||
| MPir | %EA/ED | 70.3/0 | |||||||
| Kappa (95% CI) | 0.58 (0.38 to 0.77) | ||||||||
| RS | |||||||||
These case reports were not assessed by the investigators using the Naranjo scale. The Annals of Pharmacotherapy require authors to apply a Naranjo assessment prior to publication of each case report in the journal. The collated categorisation of the case report author assessments for the 37 cases showed 0 unlikely, 5 (14%) possible, 29 (78%) probable and 3 (8%) definite (see Table 32 ).
The 40 newly selected ADR cases assessed by the seven investigators using the Naranjo scale showed collated categorisation of causality scores (n = 280 assessments) of 1 (0.4%) unlikely, 90 (32%) possible, 185 (66%) probable and 4 (1%) definite ( Table 34 ). %EA ranged from 63% to 90%. %ED was 2.5% for four pairwise comparisons. There were no extreme disagreements in 17 out of 21 comparisons. The pairwise kappa scores ranged from 0.19 to 0.81, with moderate IRR and a global kappa score of 0.44 (95% CI 0.33 to 0.55) ( Table 35 ). The same cases assessed using the LCAT showed collated causality categories of 0 (0%) unlikely, 66 (24%) possible, 81 (29%) probable and 133 (48%) definite. %EA ranged from 65% to 88%. %ED ranged from 2.5% to 7.5% for 14 pairwise comparisons. There were no extreme disagreements in 7 out of 21 comparisons. Pairwise kappa scores ranged from 0.51 to 0.85 and the assessors achieved good IRR with a global kappa of 0.60 (95% CI 0.54 to 0.67) (see Table 35 ).
| Assessor | Tool | ADRIC new (N = 40) | |||
|---|---|---|---|---|---|
| Unlikely: n (%) | Possible: n (%) | Probable: n (%) | Definite: n (%) | ||
| RG | Naranjo | 0 (0.0) | 18 (45.0) | 21 (52.5) | 1 (2.5) |
| Liverpool | 0 (0.0) | 11 (27.5) | 12 (30.0) | 17 (42.5) | |
| JB | Naranjo | 0 (0.0) | 19 (47.5) | 21 (52.5) | 0 (0.0) |
| Liverpool | 0 (0.0) | 14 (35.0) | 8 (20.0) | 18 (45.0) | |
| KB | Naranjo | 0 (0.0) | 15 (37.5) | 25 (62.5) | 0 (0.0) |
| Liverpool | 0 (0.0) | 13 (32.5) | 10 (25.0) | 17 (42.5) | |
| MT | Naranjo | 1 (2.5) | 9 (22.5) | 27 (67.5) | 3 (7.5) |
| Liverpool | 0 (0.0) | 8 (20.0) | 9 (22.5) | 23 (57.5) | |
| TN | Naranjo | 0 (0.0) | 13 (32.5) | 27 (67.5) | 0 (0.0) |
| Liverpool | 0 (0.0) | 8 (20.0) | 12 (30.0) | 20 (50.0) | |
| MPir | Naranjo | 0 (0.0) | 12 (30.0) | 28 (70.0) | 0 (0.0) |
| Liverpool | 0 (0.0) | 9 (22.5) | 13 (32.5) | 18 (45.0) | |
| RS | Naranjo | 0 (0.0) | 4 (10.0) | 36 (90.0) | 0 (0.0) |
| Liverpool | 0 (0.0) | 3 (7.5) | 17 (42.5) | 20 (50.0) | |
| Totals | Naranjo | 1 (0.36) | 90 (32.1) | 185 (66.1) | 4 (1.4) |
| Liverpool | 0 (0.0) | 66 (23.6) | 81 (28.9) | 133 (47.5) | |
| Assessor 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| RG | JB | KB | MT | TN | MPir | RS | |||
| Assessor 1 | RG | %EA/ED | 90.0/0% | 80.0/0% | 70.0/2.5% | 75.0/0% | 72.5/0% | 62.5/0% | |
| Kappa (95% CI) | 0.81 (0.64 to 0.98) | 0.61 (0.38 to 0.84) | 0.46 (0.25 to 0.66) | 0.51 (0.26 to 0.75) | 0.46 (0.20 to 0.71) | 0.23 (0.03 to 0.42) | |||
| JB | %EA/ED | 70.0/5% | 75.0/0% | 67.5/0% | 80.0/0% | 77.5/0% | 62.5/0% | ||
| Kappa (95% CI) | 0.62 (0.43 to 0.81) | 0.49 (0.23 to 0.76) | 0.45 (0.25 to 0.64) | 0.59 (0.35 to 0.83) | 0.54 (0.29 to 0.79) | 0.22 (0.02 to 0.41) | |||
| KB | %EA/ED | 65.0/0% | 77.5/2.5% | 70.0/2.5% | 80.0/0% | 77.5/0% | 67.5/0% | ||
| Kappa (95% CI) | 0.62 (0.44 to 0.79) | 0.73 (0.57 to 0.90) | 0.40 (0.16 to 0.63) | 0.56 (0.29 to 0.83) | 0.50 (0.22 to 0.78) | 0.19 (–0.06 to 0.44) | |||
| MT | %EA/ED | 70.0/2.5% | 75.0/5% | 75.0/7.5% | 70.0/2.5% | 70.0/2.5% | 72.5/0% | ||
| Kappa (95% CI) | 0.63 (0.45 to 0.81) | 0.70 (0.52 to 0.88) | 0.64 (0.45 to 0.84) | 0.367 (0.12 to 0.62) | 0.40 (0.15 to 0.65) | 0.25 (0.003 to 0.50) | |||
| TN | %EA/ED | 82.5/2.5% | 77.5/2.5% | 70.0/2.5% | 82.5/0% | 77.5/0% | 77.5/0% | ||
| Kappa (95% CI) | 0.77 (0.61 to 0.93) | 0.73 (0.57 to 0.88) | 0.61 (0.43 to 0.79) | 0.79 (0.64 to 0.93) | 0.48 (0.18 to 0.77) | 0.38 (0.09 to 0.66) | |||
| MPir | %EA/ED | 70.0/2.5% | 80.0/2.5% | 72.5/2.5% | 80.0/0% | 87.5/0% | 80.0/0% | ||
| Kappa (95% CI) | 0.63 (0.44 to 0.81) | 0.75 (0.59 to 0.91) | 0.64 (0.46 to 0.82) | 0.76 (0.61 to 0.91) | 0.85 (0.73 to 0.97) | 0.41 (0.12 to 0.71) | |||
| RS | %EA/ED | 70.0/2.5% | 70.0/5% | 65.0/5% | 80.0/0% | 82.5/0% | 75.0/0% | ||
| Kappa (95% CI) | 0.60 (0.42 to 0.78) | 0.57 (0.40 to 0.74) | 0.50 (0.31 to 0.69) | 0.73 (0.58 to 0.88) | 0.77 (0.62 to 0.91) | 0.67 (0.51 to 0.84) | |||
Discussion
A recent systematic review of studies assessing the reliability of causality assessments concluded that ‘no causality assessment method has shown consistent and reproducible measure of causality’. 188 In order to do this, we planned to have assessments conducted independently by seven assessors. Initial assessments revealed some significant issues with the Naranjo scale, which led us to develop the LCAT.
In assessing the original 40 possible ADR cases with the Naranjo tool, several difficulties were found with some of the questions in the tool. Some of the questions were frequently, or always, answered as ‘unknown’. There were two questions that caused discrepancies between raters in eight cases when scoring with the Naranjo tool. The first question that caused difficulty was question 5 (see Table 30 ) [‘Are there alternative causes (other than the drug) that could on their own have caused the reaction?’]. Individual raters interpreted this question in two different ways: some raters took a literal approach and interpreted the question to mean any ‘alternative cause’, almost always answering with a ‘yes’, whereas other raters took a more practical approach and interpreted the question as ‘Was there an alternative plausible cause?’, and, in doing so, these raters gave variable answers to the question. Question 10 (‘Was the AE confirmed by any objective evidence?’) was the second that caused discrepancies in Naranjo scoring. This caused problems for assessors in two very different ways: first, assessors had difficulty in deciding, on an individual case basis, what constitutes objective evidence and, second, assessors had difficulty defining whether the objective evidence related to evidence that the ADR had occurred or evidence of the mechanism. For example, a patient taking an opioid for analgesia might develop abdominal pain secondary to constipation and need admission to hospital for treatment and symptom control. In this case, raters may differ in their interpretation regarding question 5 and whether or not there may be alternative causes to explain the constipation (some of this may have to with the level of detail in the case report). Raters may also have difficulty in answering question 10. Some raters may suggest that a physical examination of a palpable faecal mass constitutes objective evidence, whereas others may suggest that it is not objective and might argue that an abdominal radiograph showing faecal loading is more objective. Others might use either of these two findings to aid in their assessment of ‘alternative causes’. If so, these raters might score question 5 in a positive manner because of the available evidence and then score question 10 positively because of the evidence, in effect scoring positively for the same information twice. It seems counterintuitive to take account of positive evidence and score it twice when assessing a possible ADR report. Even so, there were still very few discrepancies between the scores overall with most assessments resulting in a ‘possible’ or ‘probable’ causality being assigned.
We designed a new method, the LCAT, using an algorithm in the form of a flow chart. This new tool was assessed to have face validity by a multidisciplinary investigating group. Seven assessors used both the LCAT and Naranjo tool to initially assess 40 possible ADR cases from the large observational study. The LCAT performed just as well as Naranjo in terms of IRR but gave a broader range of causality outcomes, which was deemed more appropriate by the investigating group. When the seven investigators assessed a second different set of 40 cases the LCAT outperformed Naranjo, showing a ‘good’ IRR.
We believe that the LCAT has several advantages over the Naranjo scale. First, it performed as well as the Naranjo scale with the first set of cases that were assessed. More importantly, the IRR improved over time with the new tool, whereas the IRR when using Naranjo remained similar, despite the fact that there was as much exposure to this tool within the assessing group. The improved IRR with the new tool may be explained by increasing experience of its use. The proportion of exact agreements between assessors was similar between the two tools for both sets of cases despite the improvement in the global kappa score for the new tool. This is because it is difficult to achieve a ‘definite’ category using the Naranjo scale, and assessors mainly scored cases as ‘possible’ or ‘probable’. Therefore, the chances of exact agreement between two assessors of the same case using the Naranjo scale are likely to be falsely elevated compared with the kappa scores that adjust for chance agreement. This paradox has been discussed previously in the literature. 203–205 The percentage of extreme disagreement between raters was higher for the LCAT than the Naranjo tool. Owing to the difficulty in achieving a ‘definite’ score with the Naranjo tool, the chances of finding extreme disagreement, when comparing pairwise assessments, is likely to be falsely low. The observed %ED decreased when using the LCAT, from the first set of 40 cases to the last set. This may also be explained by increasing experience of its use. The implication of this explanation would be that there is a learning curve associated with using the LCAT. An e-learning package is under evaluation.
Second, the IRR on assessing published case reports with the new tool was similar to that when we assessed our observational study cases with the Naranjo scale. Five of the seven assessors work in paediatric practice and the published case reports were adult cases. This perhaps provides an indication, albeit indirectly, of the robustness of the tool, even when used for cases from unfamiliar clinical settings.
Third, in the Naranjo scale, almost all cases were categorised as possible or probable. With the new tool, the range of categorisations was broader with some cases judged as being definite. A novel aspect of the tool which makes this possible is that prior exposure that led to the same ADR, for example during a previous course of chemotherapy, was judged as being equivalent to a prospective rechallenge. It is also important to note that the cases were extracted from an observational study of suspected ADRs in children, and thus some case selection had occurred, making it improbable to record a score of ‘unlikely’ when assessing with either tool.
Fourth, a flow diagram rather than scoring system was used in the new CAT and was felt by assessors to be easy to follow and quick to complete. We used a classification approach based on binary decisions (taking account of ‘don’t know’ responses). In this case we need to ensure that the binary decisions are robust. Once this has been done then the instrument should be relatively context independent. A weighted scoring system, such as the Naranjo scale, will give more influence to some variables than others. A weighting scheme involves the validation of the items in the tool and the weightings. Ideally, the weightings need to be developed and validated in a context that is similar to the context in which it is applied. Thus a weighting scheme is more likely to be sensitive and specific within a defined context (as long as you have a gold standard) but is more likely to be context dependent. We feel it is more important to develop a tool that is context independent, as we need to compare different settings when assessing causality of ADRs.
In summary, we present a new CAT, developed by a MDT, which we believe to be at least equivalent to, if not better than, the Naranjo scale. We believe the new tool to be practicable and likely to be acceptable for use by health-care staff in assessing ADRs. We have undertaken an extensive validation of the tool, with a total of 819 causality assessments by seven investigators, using investigators within ADRIC. Although this validation is equivalent to, if not better than, that undertaken for many other tools,23,206,207 one limitation is that the increase in IRR for the second set of 40 case reports using the new tool remains unexplained. A second limitation is that the study has been undertaken internally and not yet assessed independently by other investigators.
Chapter 6 Development of the Liverpool Adverse Drug Reaction Avoidability Tool
Abstract
Background
A recent systematic review of ADRs in children19 highlighted that few studies performed an avoidability assessment (19/102). There was wide variation in the results with rates ranging from 7% to 98% of ADRs being classed as either possibly or definitely avoidable. 19 There is currently no standardised method for determining avoidability and many of the established tools are not suitable for use in paediatrics. We have used an adapted version of the Hallas scale54 as a basis for the development of a new AAT.
Objectives
-
To develop and test a new AAT that is more suitable for use in paediatrics but which is also generalisable and applicable to a variety of other settings.
-
To compare individual to group assessments of avoidability.
Setting
A large children’s hospital providing a local and also specialist regional and national services: Alder Hey.
Main outcome measures
Inter-rater reliability, measure of disagreement, utilisation of avoidability categories.
Methods
The study involved multiple phases. Phase 1 consisted of three parts (defining the tool, modifying the tool and refining the tool), all of which involved a MDT approach. Phase 2 involved the independent assessment of 50 ADR cases from the ADRIC inpatient study by six reviewers and a comparison of the results. Phase 3 will involve consensus meetings and group testing.
Results
Phase 1 The assessment of 20 ADR cases was undertaken by two different MDT groups. Group members commented that a mixture of professions was needed to give a full assessment of avoidability. Changes to the tool were made as a result of the findings with two of the questions being amended to include ‘known preventative strategies’.
Phase 2 The assessment of 50 ADR case reports by six individual reviewers, where pairwise kappa scores ranged from poor to good. Stronger agreement was found within professions than between professions.
Discussion
To date, the ADRIC avoidability work stream has defined a tool to assess avoidability of suspected ADRs. We have conducted preliminary testing. Following the completion of phase 2, further discussion of the tool and methodology suggested that additional testing was needed and that this should be carried out in a group setting using consensus methods.
Conclusion
Avoidability assessment is feasible but needs careful attention to methods. Further testing in a group setting is required to develop and validate the tool. The next step in the development process will be to investigate how to optimise group assessment.
Introduction
Preventability, or avoidability as it is sometimes referred to, is an important concept in the study of ADRs. 25 Preventing avoidable harm due to ADRs is a prime clinical motivation for studying drug safety. According to the WHO, ADRs rank among the top 10 leading causes of mortality in some countries. ADRs are common yet often preventable. 208 Patient and medication safety is high on the agenda of the EMA,209 the Council of Europe210 and the WHO. 208 The WHO has identified some key areas including measuring harm, understanding causes, identifying solutions, evaluating impact and translating evidence into safer care. Hakkarainen et al. 211 conducted a meta-analysis of preventable ADR studies and they concluded that preventable ADRs are a significant burden to the health-care system and a cause of morbidity among outpatients, and that roughly half of all ADRs in adults both inpatients (45%) and outpatients (52%) may be preventable.
The importance of examining avoidability of ADRs became clear from two sources: the ADRIC systematic review indicated the few previous studies that had examined avoidability and those that had used inconsistent methods;19 difficulties were encountered during the assessment of avoidability using existing tools (see Chapter 3 ).
The study of avoidability is complex. A key factor causing this complexity is that there is no universally accepted definition for preventability. 25 Ferner and Aronson25 stated that there are two aspects to preventability: whether or not in principle an event is preventable, in the absence of error and, if it is, whether or not we can, in fact, prevent it. They gave the example of penicillin hypersensitivity reactions, which, in principle, can be avoided in patients who are known to be susceptible, by not giving the drug; however, in practice these reactions can still occur owing to lack of information available to the prescriber. 25 They also stated that harm is never absolutely preventable, but any intervention that reduces the probability of harm can be said to have made a contribution to prevention. 25 Ferner and Aronson25 concluded in their systematic review that several definitions exist and none fits all circumstances. In a follow-up paper, they outlined a novel method for determining preventability. 212 This novel method involves classifying ADRs by mechanism and clinical manifestation to inform judgement about theoretical preventability. 212 According to Ferner and Aronson,25 complete analysis requires consideration of pharmacodynamic and pharmacokinetic mechanisms of the ADR, its time course, its dose-responsiveness and individual susceptibility factors. 212
Despite the importance of avoiding ADRs, this area remains under-researched. This may be attributable to the methodological problems in the area, which Hakkarainen et al. 26 have summarised in a systematic review on methods for assessing the preventability of ADEs. 26 They listed inconsistent terminology as one of the problems; there is wide variation in the terms and definitions used (ADRs, ADEs, etc.) and this hinders the interpretation and comparison of studies. 26 In their review they used the term ADE, which included ADRs and other AEs related to medications. The definition of an ADR used in this study is that of Edwards and Aronson:49 ‘an appreciably harmful or unpleasant reaction, resulting from an intervention related to the use of a medicinal product, which predicts hazard from future administration and warrants prevention or specific treatment, or alteration of the dosage regimen or withdrawal of the product’.
The need for developing a new AAT arose during the ADRIC inpatient study when we tried to use the Hallas scale54 to determine avoidability but had difficulties with some of the language used particularly ‘present-day knowledge of good medical practice’ and that the event could have been avoided by ‘an effort exceeding obligatory demands’. In the ADRIC admissions study Gallagher et al. 21 used the Hallas scale54 to determine avoidability and found that 78% of ADRs were unavoidable, and 22% were either possibly or definitely avoidable. Gallagher et al. 21 suggested some potential prevention strategies for ADRs based on their assessment of the ADRs they classed as ‘definitely avoidable’ – that more careful attention to practical aspects of care, such as improved monitoring, following prescribing guidelines and improved patient education, could lead to a reduction in the frequency of ADRs causing admission. 21 The Hallas scale54 was used for the ADRIC admissions study but appeared unsuitable for the ADRIC inpatient study owing to difficulties mentioned before around the terminology used. As a result of this it was decided by the study group that we would design a new AAT that would be more suitable for use in paediatrics but could also be used in other settings. Ideally, the newly developed AAT should be generalisable to a variety of different patient groups, reproducible and easy to use.
Aim and objectives of this work
The aim of the ADRIC avoidability work stream was to assess the avoidability of ADRs reported in ADRIC and to identify strategies for clinical practice that might reduce the incidence of ADRs. A preliminary step was to develop a new avoidability tool that met all of the criteria of a good tool as described by Hakkarainen et al. 26 and was also generalisable. The objectives were to develop an algorithm with dichotomous responses based on Hallas et al. 54 and to conduct reliability/validity testing on the new tool as per Hakkarainen et al. 26 recommendations.
Methods
Preliminary work
A modified version of the Hallas scale54 was used as the starting point for the development of a new AAT but the focus was on the available information sources. We wanted to ascertain if the relevant information was available in sources that prescribers would be expected to use, and, if so, whether the recommended advice was followed. The intention was to keep the tool as generalisable as possible by asking if accessible management or treatment plans were available. These could be local, national or international. We recommended that only high-quality guidelines were considered, for example Scottish Intercollegiate Guidelines Network (SIGN), the National Institute for Health and Care Excellence (NICE), Royal College of Paediatrics and Child Health (RCPCH) or other peer-reviewed guidelines. 213 Because guidelines are not always available, or contain no information on prevention of ADRs, we added other information sources, for example BNF-C, SmPC (see Appendix 4 ).
A pilot study was carried out in November 2011, when three reviewers independently assessed 50 cases using a modified version of the Hallas scale and 50 cases using the original Hallas scale. 54 The results were compared and IRR testing was carried out on both groups. The kappa scores for both groups were low – the modified Hallas54 group scores were poor and it was decided that the AAT should be converted to a flow diagram in an attempt to make it easier and more consistent to use. It was also decided that some questions needed reviewing and that this should be done by a consensus approach. 25 We achieved consensus by agreement among peers without pre-set criteria and the consensus group was a MDT (research nurse, doctor and pharmacist).
Phase 1a: define the tool
It was agreed that the best way to develop a new tool was to take a consensus approach in reviewing cases. The format of the new tool was a flow diagram, with dichotomous responses to each question followed by a routing to the next relevant question; it was decided this would differ from the specific criteria Hallas54 has for each avoidability category. Initially, 20 cases were reviewed to define the tool. This was carried out by a MDT [MAT, AJN and HLM (LEB as an observer)] working together to discuss clinical practice and avoidability outcome. Each question in the newly modified avoidability flow diagram was reviewed by the investigators during the consensus meetings and any necessary changes were made. Any cases that were classified as ‘unassessable’ had the rationale recorded as either lack of information about the case or of available guidance. The MDT initially looked at 20 cases (randomly selected) from the ADRIC inpatient study and carried out an avoidability assessment. It was felt that it was not appropriate to distinguish between guidelines, and, for the purpose of ADRIC inpatient study cases, we accepted any available guidance based on an acceptable body of opinion, for example SIGN, Alder Hey guidance or NICE guidance. A glossary was prepared to further explain this and other terms. Any areas of disagreement or discrepancies were reviewed by MPir, who also reviewed the iterations as they moved through the various stages of development.
Phase 1b: modify the tool
The flow diagram was modified using 20 randomly selected cases from the ADRIC inpatient study, with rephrasing of questions and adding information. Consensus of opinion was reached and this version of the tool ( Figure 13 ) was carried forward to the next phase.
FIGURE 13.
Version of the AAT used in phase 1c.
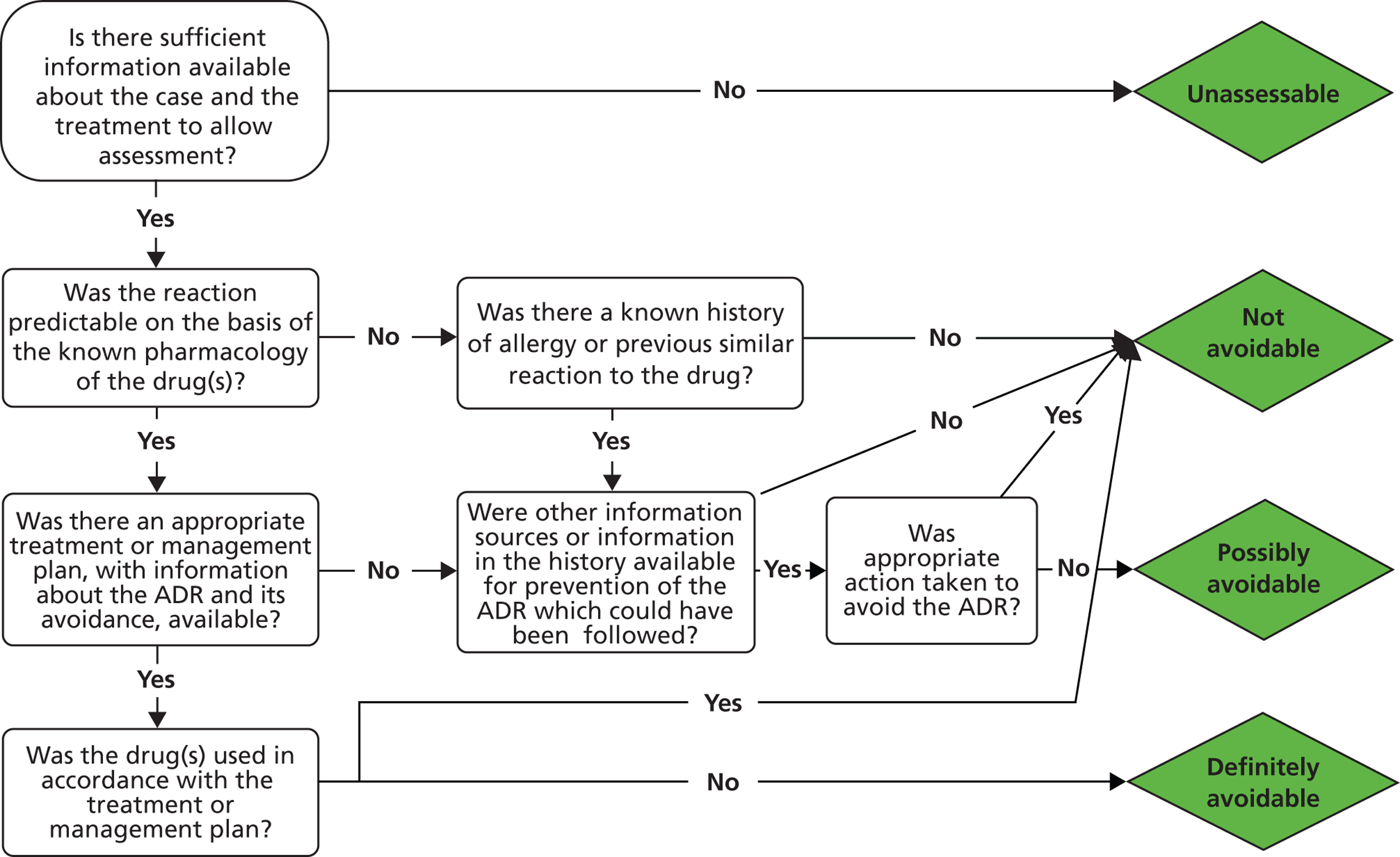
Phase 1c: refine the tool
Two MDT groups (the original plus a new group: nurse, pharmacist and paediatrician) reached consensus about a second set of 20 cases from the ADRIC inpatient study, which were a randomly selected stratified sample (probable and definite cases: 11 surgical, 4 oncology, 2 medical and 3 cardiology). Both groups reviewed the same 20 cases. The results were compared, kappa scores were calculated and the concordance of routes and the final avoidability categories were assigned. Both MDT meetings were observed with assumptions and approaches of the teams recorded. Changes to the tool were made as a result of the findings with two of the questions being amended to include ‘known preventative strategies’.
Phase 2: testing and validation of the tool
The refined tool ( Figure 14 ) was then tested on a further set of ADRIC inpatient study cases with the aim being to improve IRR. This phase involved the assessment of a further 50 cases by six individual reviewers using the newly refined tool. See the accompanying glossary and guide (see Appendix 4 ) to the questions in the tool for further details on completing an avoidability assessment. These 50 cases were a stratified sample (possible, probable and definite cases: 26 surgical, nine oncology, nine medical and six cardiology). The reviewers included two nurses, two pharmacists and two doctors. These cases were assessed in terms of pairwise agreements between the investigators. Cases where extreme disagreement occurred, i.e. where the avoidability assessment differed by more than one category, for example ‘not avoidable’ and ‘definitely avoidable’, and any cases for which half of the raters differed in assigning a category were identified and the questions that caused the discrepancies were reviewed.
FIGURE 14.
The Liverpool ADR Avoidability Assessment Tool.
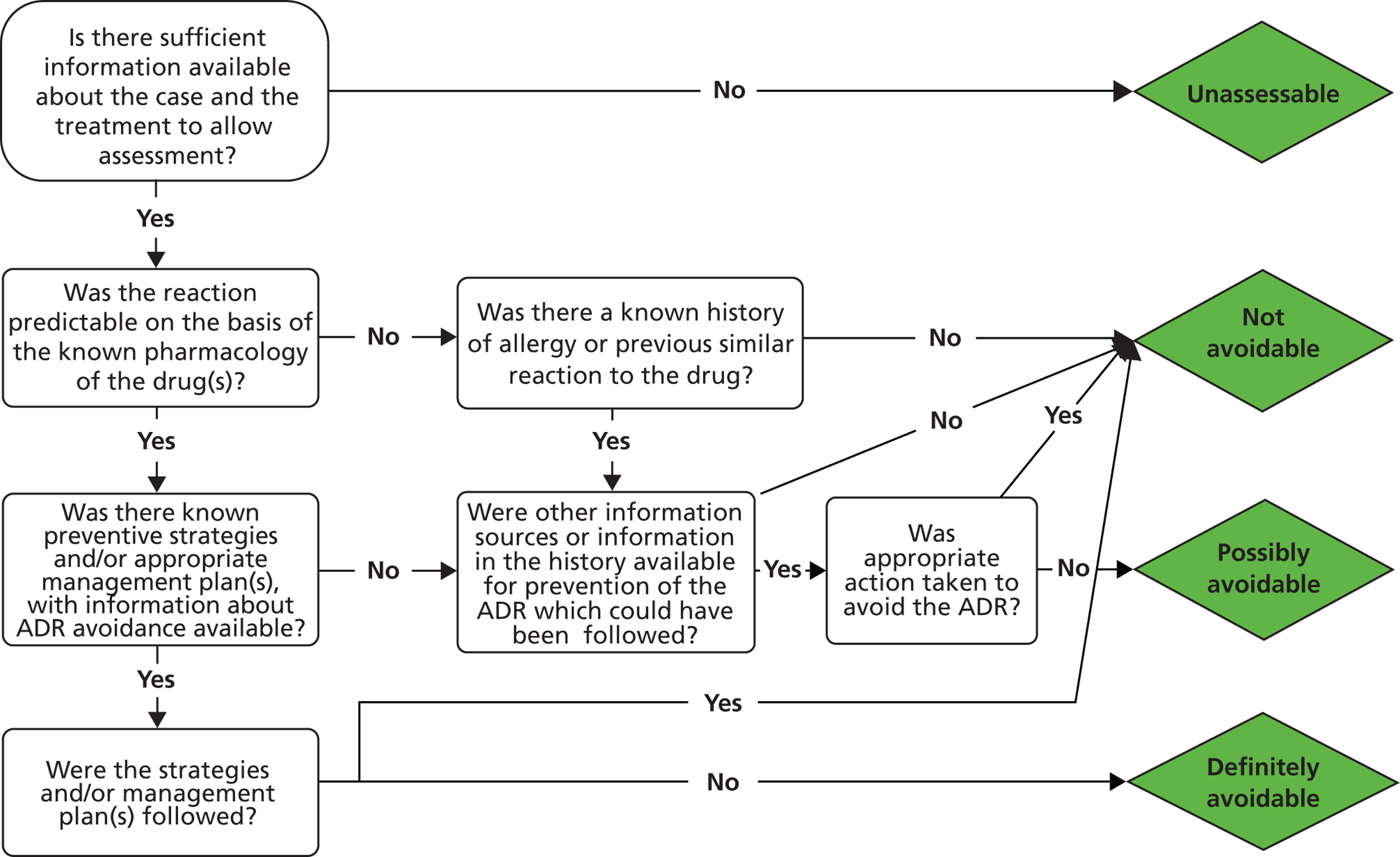
The results were presented as categorical scores from the newly developed tool and inter-rater agreements were calculated using kappa scores with 95% CI, and pairwise kappa scores were compared with global kappa scores. The %ED where the avoidability scores between two raters of the same case are wider than one interval apart were calculated to measure extreme disagreement between pairwise kappa scores. Pairwise kappa scores were also calculated by specialty to investigate the differences between surgical, medical, oncology and cardiology cases.
Phase 3: consensus meetings and individual testing
The next step in the development process ( Figure 15 ) will be to investigate if group avoidability assessments are superior to individual avoidability assessments.
FIGURE 15.
The development process.
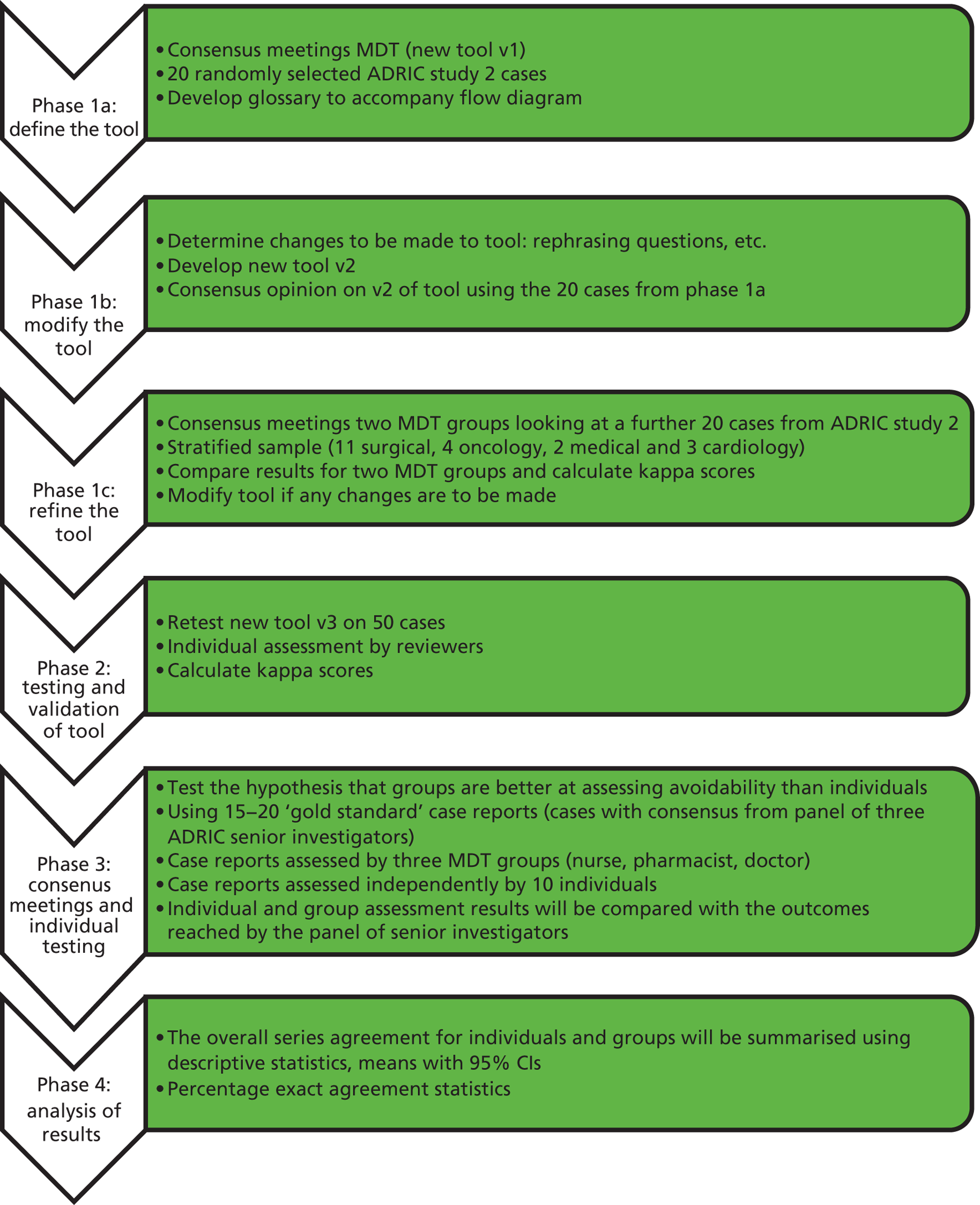
Following the completion of phase 2, it was decided that further testing was needed and that perhaps the best way to assess avoidability is in a group setting. Agreement in phase 2 ranged from poor to good; possible reasons for this may be due to lack of experience in certain specialty areas or a possible training effect. The next step in the development process will be to carry out group assessments of additional cases and compare these with assessments made by individual reviewers. This further testing in a group setting is required to develop and validate the tool.
Results
Phase 1 The assessment of 20 ADR cases was undertaken by two different MDT groups. Group members commented that a mixture of professions was needed to give a full assessment of avoidability. Changes to the tool were made as a result of the findings, with two of the questions being amended to include ‘known preventative strategies’.
Phase 2 The assessment of 50 ADR case reports by six individual reviewers, where pairwise kappa scores ranged from poor to good. Stronger agreement was found within professions than between professions.
Discussion
To date, the ADRIC avoidability work stream has defined a tool to assess avoidability of suspected ADRs. We have conducted preliminary testing. The tool has face validity and is easy to use. However, a number of issues were raised. These include the dependence on guidelines and variations in clinical practice. It may not be possible to define a generalisable tool. It may be possible to define a tool that individuals can use consistently. However, the tool in itself may not be sufficient to develop consistent results between individuals or across settings. Consistent results may require a standard body of guidelines, or gold standards for acceptable care. Consistent results may require clinical experience relevant to the suspected ADR. Nevertheless, the tool may provide useful insights within an individual setting. The next step in the development process will be to investigate if group assessment improves agreement and reliability.
There have been many attempts to devise tools or scales to help determine avoidability. Commonly used scales include Hallas et al. ,54 Schumock and Thornton,44 Dormann et al. ,214 Ducharme et al. 215 and Olivier et al. 216 The Ferner and Aronson25 systematic review identified eight different approaches to assessing avoidability. They suggested an approach to preventability based on analysis of the mechanisms of ADRs and their clinical manifestations. 25,212 Hakkarainen et al. 26 identified 18 unique instruments for assessing preventability of ADEs, which ranged from implicit instruments to explicit algorithms in which criteria for preventability were clearly expressed. 26 They also reported that although there was wide variation in the methods used they all shared a common theme; the basis for defining preventability was whether an error or substandard care had resulted in an ADE. 26
Hakkarainen et al. 26 have made some useful suggestions for future research. They recommend that future studies include reliability and validity testing; take action to standardise the measurement process; provide information on the assessors in terms of training and experience in assessing preventability; and describe how the assessments took place (i.e. whether cases were assessed independently or via consensus and how any disagreement is dealt with). They also stated that owing to the limitations and diversity of assessments it remains unknown if variation in preventability rates in different settings and populations is due to the methodology used or actual differences in preventability rates. 26 They suggested that there is a need for modifying previous instruments or developing new ones for use in different settings, and that a starting point for developing a new instrument could be to begin with a clear definition for the preventability of different types of ADEs. 26 They also recommended that any newly developed instruments should be compared with existing ones and that if one or more instrument gained rigorous evidence and became a gold standard it would facilitate comparisons of different studies.
Future work in adverse drug reactions in children
-
The assessment of ADRIC admissions study cases using the newly developed avoidability tool, comparing the results with the Hallas (14) assessments carried out in Chapter 2 .
-
Identifying reasons for avoidable ADRs.
-
Using suggestions about possible strategies to avoid ADRs from ADRIC to see if there are generalisable steps to take that will promote avoidability.
Conclusions
Avoidability assessment is feasible but needs careful attention to methods. The Liverpool ADR AAT showed mixed IRR in the individual assessment phase; therefore, further testing in a group setting is required to develop and validate the tool.
Chapter 7 Families’ experiences of suspected adverse drug reactions: implications for communication and pharmacovigilance
This chapter contains information reproduced from Arnott J, Hesselgreaves H, Nunn AJ, Peak M, Pirmohamed M, Smyth RL, et al. Enhancing communication about paediatric medicines: lessons from a qualitative study of parents’ experiences of their child’s suspected adverse drug reaction. PLOS ONE 2012;7:e46022,29 © Arnott et al. , an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided that the original author and source are credited; and information reproduced with permission from Arnott J, Hesselgreaves H, Nunn AJ, Peak M, Pirmohamed M, Smyth RL, et al. What can we learn from parents about enhancing participation in pharmacovigilance? Br J Clin Pharmacol 2012;75:1109–17,96 with permission from the British Pharmacological Society and Blackwell Publishing.
Abstract
Background
There is little research on families’ experiences of suspected ADRs in children and little evidence to guide clinicians when communicating with families about problems with medicines.
Aims
To identify any unmet information and communication needs described by families following a suspected ADR in a child.
Methods
Semistructured qualitative interviews with 20 children and young people and the parents of 44 children and young people who had experienced a suspected ADR. Interviews were conducted face to face or by telephone; most were audio recorded and transcribed. Analysis was informed by the principles of the constant comparative method.
Results
Many parents described being dissatisfied with clinicians’ communication about ADRs. In contrast, the accounts of parents of children with cancer emphasised confidence in clinicians’ management of ADRs and the way clinicians communicated about medicines. The accounts of children and young people largely reflected parents’ accounts. Families were positive about the Yellow Card Scheme and felt that recording and reporting ADRs was important. Parents, children and young people linked symptoms to medicines using a similar reasoning as clinicians use to evaluate the possibility of an ADR.
Conclusions
Most parents felt that clinicians’ communication about ADRs was poor, suggesting that improvements are needed. The accounts of parents of children with cancer indicate that prospective explanation about ADRs can be effective. Convergence between parents and clinicians in their reasoning for linking children’s symptoms to medicines could be a starting point for improved communication.
Introduction
To inform communication with families about ADRs and to guide strategies for actively involving families in pharmacovigilance, we conducted qualitative interviews with children who had experienced a suspected ADR and their parents – the ADRIC-QUAL study.
Clinical communication with families about adverse drug reactions
The literature on communicating about medicines indicates the advantages of involving patients in open discussions about the benefits and risks of medicines in order to support informed consent and decision-making. However, patients have generally been found to be poorly informed about medicines. 12,27 A great deal of work on communication about medicines has been motivated by a concern to promote treatment adherence, rather than on enhancing communication about medicines as an important goal in its own right. 217 Although optimising adherence is an important objective, concern with promoting treatment adherence has, arguably, meant that little attention has been given to examining the experiences of patients following a suspected ADR. 28
Even less attention has been given to investigating the particular experiences and needs of child patients and their parents following a suspected ADR. Their situation is likely to be complicated by the frequent prescribing of OLUL drugs in paediatrics218–220 and by parents’ distinctive role in caring for their children. 221 Evidence that members of the public are particularly concerned about the risks of medicines to children comes from a study comparing laypeople’s responses to hypothetical scenarios involving medicines for child or adult patients. Respondents perceived the risks of ADRs to be more severe and reported that they would be less likely to take (or give) a medicine when the recipient was a child rather than an adult. 222
Families’ involvement in pharmacovigilance
The MHRA is responsible for monitoring medicines in the UK. One way they do this is by collecting spontaneous reports of suspected ADRs submitted via the Yellow Card Scheme. 223 Given the frequent use of OLUL medicines in paediatrics,218 health practitioners are strongly recommended to submit Yellow Cards for suspected ADRs in children. 17 However, there is considerable concern about under-reporting of ADRs,40,224,225 and partly in response to such concerns, the Yellow Card Scheme was extended to patients and their families in 2005. 223
Adult patients who use the Yellow Card Scheme or its international equivalents have been found to provide more detailed reports of ADRs than clinical practitioners and to value the opportunity to contribute to pharmacovigilance. 12,226–232 These patients have spoken of having altruistic motives for reporting ADRs, as do clinicians,226,233 and being motivated by the severity of the ADR and a concern that certain ADRs were not listed on the medicine patient information leaflet. 233 However, public awareness and participation in the Yellow Card Scheme is low12,27,28 and studies of patients who have managed to access the Yellow Card Scheme are, therefore, likely to be of limited use in identifying strategies to promote wider participation. Research with patients who have experienced an ADR but have not used the Yellow Card Scheme is limited. A recent study of adult patients who had been hospitalised because of a suspected ADR but had not used the Yellow Card Scheme indicated that they considered the scheme to be remote and impersonal, and they felt that it was not a patient’s responsibility to report ADRs to the MHRA. 28
Exploring the particular motives of parents for using the Yellow Card Scheme and the barriers they encounter is important for several reasons. Previous research has focused primarily on adult patients, yet the need for parental confidence in pharmacovigilance is particularly pressing owing to the widespread use of OLUL medicines in children218,234 and public concern about the safety of children’s medicines. 219,235–240 Also, the perspectives of parents may differ from those of other lay users of spontaneous reporting pharmacovigilance schemes because of parents’ distinctive caring and protective role. 221,241 Moreover, previous research has largely focused on the experiences of people who have used the Yellow Card Scheme, which as we note above, is likely to be of limited use in enhancing participation. It is important to test those assumptions by examining the views of parents who have witnessed ADRs in their children but have not previously used the Yellow Card Scheme, as well as those who have used the Yellow Card Scheme.
Aims
We designed our qualitative study to explore all aspects of participants’ experiences and views, from their accounts of communication at the point at which medicines were prescribed to their views about the implications of ADRs for future health, and their views and experiences of reporting ADRs using the Yellow Card Scheme.
Methods
Sampling, setting and recruitment
The methods used have been detailed elsewhere,29,96 so only a brief outline is provided here. As recommended in qualitative research when there has been little previous research on a topic, we sampled for maximum variation242,243 using three sampling routes to ensure participant diversity, particularly in terms of clinical specialty and the nature of the suspected ADRs. Route 1 comprised the two cohort studies (see Chapters 2 and 3 ) that were part of the ADRIC programme. ‘ADRIC families’ were eligible for the study if they could be approached before discharge. Treating clinicians initially introduced the study to families. The interviewers subsequently provided the parents, children and young people who expressed an interest in participating with more detailed information and then arranged the interview. We used route 2, the Yellow Card Scheme,223 to access parents with experience of reporting ADRs to the MHRA. 242,243 The MHRA sent invitation letters to all parents who had submitted a Yellow Card on behalf of a child of < 17 years of age, outlining the study and inviting parents to return a reply slip to the study team if they wished to participate. Qualitative interviewers telephoned parents to further explain the study and arrange an interview.
As we recruited few children via route 1 and none via route 2, we used a third sampling route to extend the sample of children in the study. For this extended sample, clinical teams identified children who had experienced a suspected ADR while receiving inpatient care at Alder Hey. ADRIC researchers facilitated this process by publicising the study, regularly visiting wards and clinics to prompt staff to identify eligible participants, and checking nursing notes via the hospital computerised records system.
Sampling to all routes ran in parallel with data analysis and was discontinued when saturation on the main analytical categories was reached. 244 A UK NHS research ethics committee approved the study (Northwest 3 Research Ethics Committee 08/H1002/7). All participants gave written informed consent or assent.
Interviews
Interviewers (JA, HH and ES) explained their independence from clinical teams and the MHRA before all interviews. Face-to-face interviews were conducted with participants, with the exception of Yellow Card parents, who we interviewed by telephone as they resided across all parts of the UK. Interviews were semistructured and informed by a topic guide that contained prompts about families’ experiences of children’s signs and symptoms and how they linked these to a medicine; awareness of suspected ADRs; written and verbal communication with clinicians and views about the implications of ADRs for children, and views and experiences of the Yellow Card Scheme. Interviewers tailored their approach and questions to ensure that interviews were conversational and suited to the needs of both parent and child participants. Interviews were audio-recorded and transcribed. Transcripts were checked by the interviewer, who removed all identifying details before analysis.
Analysis
The analysis drew on the constant comparative approach244–250 and was broadly interpretive. JA led the analysis reading transcripts several times to develop analytic categories. BY and MT supported this process by reading a sample of the transcripts and by ‘testing’ and developing the analysis through periodic discussion with JA. All three analysts compared within and between transcripts, and iterated between developing analytical categories and new data. 245,247–253 We used a number of methods that are recommended to help ensure rigour in the analysis of qualitative data including respondent validation,245,252 attending to exceptional or ‘outlier’ cases247–250,252,253 and scrutinising the quality of the developing analysis for its coherence and potential to influence practice. The latter was also assisted by discussion among the wider ADRIC team252,254 to support multidisciplinary investigator triangulation. 255,256 Excerpts from interviews are presented to evidence the analysis; in these omitted speech is indicated by [. . .], explanatory text by [text] and excerpts are coded ‘AP’ (ADRIC parents), ‘AC’ (ADRIC children) ‘YCP’ (Yellow Card parents) or ‘EC’ (extended child sample).
Results
Participants
We conducted audio-recorded interviews with a total of 45 parents (41 mothers, 4 fathers) and 19 children. Of these, 27 parents and 11 children were recruited via the ADRIC cohort studies (route 1), 17 parents were recruited via the Yellow Card Scheme (route 2) and eight children were recruited via route 3 (the extended children’s sample).
Interviews lasted approximately 60 minutes (range 17 to 138 minutes) and were conducted between 1 and 56 weeks after the suspected ADR. Four participants were interviewed in a private setting in the hospital. The remainder were interviewed in their homes. Appendix 5 shows the characteristics of participants, including child age range, gender, ranked Index of Multiple Deprivation (IMD), type of drug associated with the suspected ADR and the body system affected by the ADR.
The findings from the interviews are presented in two parts: Part 1 focuses on participants’ perspectives on communication about ADRs; Part 2 focuses on participants’ perspectives on contributing to pharmacovigilance through the UK’s spontaneous reporting scheme.
Part 1: participants’ perspectives on communication about adverse drug reactions
Little explanation of the risks of medicines at the time they were prescribed
Although some of the children reported receiving general advice about potential ADRs before a medicine was given, most children and parents indicated that clinicians did not explain the risks of medicines when the medicines were prescribed: ‘No side-effects were made known to me’ (YCP5); ‘I didn’t know codeine would make me constipated’ (AC07); ‘They didn’t really tell me about anything about being sick or being itchy. They never really said anything about that’ (EC13). Parents explained how clinicians focused on other issues, such as explaining their child’s condition and the importance of medicines or surgery in treating the condition: ‘They [the surgeons] don’t discuss the drugs; they discuss the surgery itself’ (AP23). If the risks of medicines were discussed, it was often at a time when parents struggled to absorb information, such as shortly before a child was due to be anaesthetised: ‘On the day your child is being operated on or when the anaesthetist comes up you are not thinking of anything other than [. . .] what’s going to happen in the operation’ (AP16). Participants also reported difficulties with written information about medicines and potential ADRs. They either did not receive these documents or found them hard to engage with: ‘I did a carefree glance [at the patient information leaflet] and chucked it’ (YCP13).
A key exception to these accounts was the parents of children with cancer, who described how clinicians provided comprehensive information about the types of reactions that medicines could cause and emphasised how clinicians carefully timed and paced their explanations so that parents could absorb the information: ‘They explained things in little bits so it sinks in [. . .] they did say he would become neutropenic’ (AP6).
How participants become aware of adverse drug reactions
Parents usually described an initial period in which they began to suspect something was wrong based on a wide collection of physical symptoms and changes in their child’s behaviour that were ‘out of the ordinary’. With the exception of patients whose suspected ADR had first been identified by clinicians or those who had cancer, participants initially tended to attribute symptoms to trivial causes, such as minor illness, injury, or changes in lifestyle or environment. It was only when symptoms worsened that participants became concerned: ‘His colour dropped and his breathing went a bit funny and he started to panic, that worried me’ (AP25) and they started to consider possible links to medicines.
Participants reported how they started to link symptoms to a medicine when they noticed patterns, such as temporal associations between a medicine being given and the onset of symptoms: ‘It just seems strange to me that she had it [the medicine] and then straight away like she got that temperature’ (AP10); ‘When I went on the ketamine I wasn’t being sick at all. Then when I went on the morphine I was being sick. So, it was quite obvious that it was the morphine that was making me sick’ (AC09). Some also noticed how symptoms receded between doses and then returned following another dose: ‘I noticed a difference [. . .] when she was having it [the medicine] and when she wasn’t having it [. . .] she started on it again and then we noticed the symptoms within a few days again of having it’ (YCP7). The absence of an alternative explanation for the symptoms also influenced participants’ attributions: ‘[The medicine] is the only thing she’s had and she hadn’t had a cold or been ill before it’ (YCP10); ‘I was like, well, the only reason this would have happened would probably have been the medication’ (AC08).
With few exceptions, parents were critical about adverse drug reaction management and communication
Parents indicated that clinicians’ communication about suspected ADRs was often poorly matched to their needs. They described receiving contradictory information and a lack of communication that might help them understand what was happening to their child while his/her symptoms were being assessed: ‘No-one actually ever said why it [the hallucination] was happening, the nurses thought it was a bit funny, they all kept coming over to see him and laughing with him sort of thing’ (AP14). The way in which clinicians managed and communicated uncertainty surrounding an ADR’s identification did little to reassure parents ‘I was saying “well, when she goes home, can I give her paracetamol? Can she never have paracetamol or can she never have a drug that might affect her liver?” And they were going “well [. . .] it should be fine” but no-one was saying “well you can, I’ll write it down and you can have it” ’ (AP12). Parents also described receiving detailed information at times when they were anxious (e.g. when a child was critically unwell or immediately prior to surgery). At these times parents found it hard to absorb information. They reported receiving little or no information at times when they were less anxious and better able to absorb information. Children voiced fewer concerns in communicating with practitioners than parents, perhaps because most children relied on their parents for information about medicines ‘Sometimes I can’t understand them [the doctors] so I just ask my dad’ (AC01); ‘My mum deals, is the information box and if I have any questions I just ask her’ (AC10).
Some parents were intensely critical of how practitioners communicated about ADRs. One parent was frustrated during a visit to outpatients when clinicians could not explain what was happening to his/her child and spoke of feeling that he/she was being lied to by clinicians: ‘They were fobbing me off [. . .] I felt like they were lying to us’ (AP5). More commonly, parents emphasized how their concerns had been ignored or dismissed by clinicians: ‘Dismissive and wasn’t taking me very seriously’ (AP10). Yellow Card and ADRIC parents both voiced criticisms of clinicians’ communication, although Yellow Card parents were particularly emphatic in their criticisms. This was prominent when they felt clinicians had ruled out the possibility that a child’s symptoms could be related to a medicine with seemingly little exploration of parents’ concerns or explanation of the reasons for ruling out an ADR: ‘She [general practitioner] literally said word for word “what would you like me to do?” And I just felt that was really dismissive’ (YCP14). Parents who felt clinicians had ignored or dismissed their concerns described a sense of abandonment: ‘I just, just felt like nobody cared, nobody was interested and they just wanted me to go away’ (YCP5).
A striking exception to the highly critical accounts of these parents came from the parents of children with cancer. These parents were almost uniformly highly positive in their accounts of how clinicians communicated about ADRs.
Parents of children with cancer were positive about adverse drug reaction communication
Despite the life-threatening nature of the illness and the risks of cancer treatment, parents of children with cancer felt well supported by how clinicians communicated with them about medicines. There was a sense from these parents that clinicians took ADRs seriously, were adept in communicating about them and had well-developed systems in place for the management of ADRs: ‘It’s quite scary when you first go home with this big bag of drugs [. . .] they said [. . .] you can ring any time, and I rang nearly every day’ (AP7). Parents pointed to how clinicians discussed possible ADR symptoms and how to respond before an ADR happened, so that parents were clear about what to look out for and what action to take in the event of a suspected ADR. Consequently, parents felt that clinicians communicated about medicines and ADRs in a way that was ordered, timely and reassuring.
Implications of poor communication about suspected adverse drug reactions
Parents who were dissatisfied with how practitioners had communicated reflected on the implications. They commented on how a lack of information about potential ADRs at the time of prescription had prevented them from being involved in decisions about their child’s care. In one case, a lack of information at the time of prescription had resulted in a parent continuing to give morphine to alleviate their child’s agitation, only to subsequently discover that agitation could be a result of itchiness caused by morphine: ‘As she kept getting more and more agitated we kept boosting it [the morphine] [. . .] and the more we pressed the booster [. . .] the itchier she got’ (AP16). A few parents remarked on how they blamed themselves for what had happened because they felt ‘responsible for what goes into’ their child (YCP10) and pointed to the distress this had caused: ‘I was devastated [. . .] I just felt like crying all the time’ (AP8). Parents also spoke of fearing a repetition of the ADR: ‘Will it happen again? [. . .] could it happen to him, to the baby?’ (AP8) and of their uncertainty about the implications of ADRs for their child’s future health and use of medicines. Parents were also confused about whose responsibility it was to prevent a recurrence of the ADR: ‘I don’t know if it would be down to me to turn round and say something or whether they have actually put something in their notes’ (AP14). Some assumed that the responsibility was theirs alone: ‘It’s something that I [. . .] have to ask to make sure he never gets given that again’ (AP18). By contrast, children tended to focus on their experience of the symptoms of the suspected ADR. One child emphasised how he had not been perturbed because the hallucinations that he experienced had ‘distracted’ (AC04) him from the pain that he was feeling. However, most children described the experience as unpleasant or frightening: ‘It was really scary. I wasn’t bothered about pain [. . .] I just felt so scared’ (AC10).
In the context of poor communication, the experience of a suspected ADR sometimes coloured parents’ views about medicines and some expressed reluctance to give certain medicines to their child in the future. For example, one parent became convinced that her child’s ADR was a reaction to morphine and that her child could never have morphine again: ‘She’s due for this big operation and she can’t have morphine’ (AP11). However, clinical review of this particular case suggested that the suspected ADR was linked to an avoidable over-dosage and that, rather than avoiding morphine altogether in future, it might be in the child’s best interests to personalise the dose. Another parent refused to allow her child to have the final course of her vaccine: ‘I will categorically say that [. . .] I will definitely not let her have the third [human papilloma virus] vaccine’ (YCP3). One child said he would refuse a medicine again: ‘I don’t want to have that medicine ever again because [. . .] it just makes you go all angry’ (AC09), although most children reported they would take the medicine again if it was likely to help them.
How participants thought communication about suspected adverse drug reactions should be handled
Reflecting their accounts of poor communication about ADRs and the resulting implications as described above, parents wanted clinicians to help them to understand what had happened to their child. Children similarly described a need to understand their experience ‘I would have liked to have known that the floppiness wasn’t just me and I would have liked to have known that I would have felt sick after’ [. . .] all I want to know is what is going to happen, when is it going to happen, how is it going to happen and am I going to be in pain’ (EC17). As one parent explained, the need to understand the ADR seemed to be intrinsic rather than motivated by ulterior considerations: ‘[It’s] not necessarily the case that everyone’s going to jump and say, “Right, I’m going to sue the drug company” and all of these sorts of things. I think parents genuinely, who are concerned about their child’s health, want to know what it was’ (YCP8). Participants wanted discussions about ADRs to be paced and timed in a way that would help them to absorb the information: ‘You just don’t think straight when you’re there [. . .] doctors have got to understand that [. . .] and maybe spend a little more time to try and explain a little bit more than they do’ (AP11); ‘Because when you are in hospital and they ask have you got any questions, you can’t really think of it because you are drugged up [. . .] and your mind goes blank. And when you get home and then you do a bit of revising and stuff like you think “oh, I should have asked that question” and stuff like that’ (EC12).
Parents particularly wanted to understand what the suspected ADR meant for their child’s future health care, to know what steps would be taken to help prevent their child suffering further ADRs and to ensure he/she would receive appropriate medicines in the future. Without exception, parents accepted that a certain level of risk came with medicines and most appreciated that clinicians faced uncertainty in identifying ADRs: ‘I think it was the antibiotics. The doctors think it is that but they can never say it is that, because there is a possibility that it’s not that’ (AP1); ‘It’s just something that, you know, just happens [. . .] I’m sort of accepting about it’ (YCP13). Many parents were critical of how clinicians communicated about ADRs and some of them got the impression that clinicians were unwilling to discuss ADRs. However, none of the parents blamed clinicians for their child’s ADR or said they intended to formally complain. Only one parent expressed a slight ‘loss of trust’ (YCP8) in clinicians. However, as we note above, a few participants explained that their trust in medicines had diminished. Alongside their wish for dialogue with clinicians about ADRs, several participants also wanted accessible and reliable written information about ADRs: ‘They should give you a little pamphlet or something to say [. . .] look this is what she’s got’ (AP12).
Part 2: participants’ perspectives on pharmacovigilance
Awareness of the Yellow Card Scheme
Most Yellow Card parents remarked that they had found out about the Yellow Card Scheme through their training or work as a health practitioner: ‘The only reason I knew about it was because of the course that I’d done’ (YCP7), or through friends or relatives who were health practitioners. In contrast, only two ADRIC parents had heard of the Yellow Card Scheme before we interviewed them and both were nurses. None of the children had previously heard of the Yellow Card Scheme. None of the ADRIC or Yellow Card participants knew for certain whether or not the practitioners had submitted a Yellow Card reporting the suspected ADR: ‘I don’t know if one was filled in or not’ (AP20), but some remarked that they would appreciate being informed if a practitioner had done so: ‘Yeah, I think they should tell you’ (AC02).
Motivations, views and experiences of parents who submitted Yellow Cards
Most Yellow Card parents emphasised how they had submitted a Yellow Card because they wanted to help prevent other children experiencing the sorts of ADRs that their child had suffered. They also hoped that their report would contribute to the review of certain medicines ‘if they look into things, and [. . .] if there is too many incidents, they might have to relook at the tablet or relabel the information leaflet’ (YCP7). Parents did not usually think their reports would directly help their own child: ‘I didn’t think it would help me at all. I didn’t have any expectation for us’ (YCP10) and none of them wanted a medicine to be withdrawn from the market solely because of the difficulties their child had experienced. Linked to their altruistic motivations, Yellow Card parents also described a sense, albeit nebulous, of ‘achieving something positive from that experience rather than just sort of happening’ (YCP14). Those Yellow Card parents who had professional knowledge of the Yellow Card Scheme added that they were motivated to submit a report by a sense of professional obligation. Those who were not health practitioners expressed a preference for reports about suspected ADRs to come from health practitioners rather than themselves: ‘I wished it [Yellow Card] had come from the doctor first’ (YCP10).
Some Yellow Card parents seemed to understand that a certain number of reports would be needed in order to trigger action by the MHRA: ‘if enough people say something about this then something should and probably will get done’ (YCP16). Others were unsure about what happened to the data after they had submitted it. Most parents did not report expecting to receive feedback from the MHRA in response to their Yellow Card but those that had received a response were pleased: ‘What I’m delighted about is the response it makes you feel very pleased, glad that I followed it up’ (YCP3). As noted in Part 1, many Yellow Card parents emphasised how the health practitioners they consulted had not taken their concerns about their child’s ADRs seriously. In this context, the opportunity the Yellow Card Scheme offered a welcome opportunity for parents to voice their concerns about medicines in a way that was not filtered or influenced by practitioners: ‘I felt very pleased that I could [. . .] take control of it really and let someone know regardless of whether the doctor thought’ (YCP8). Other parents spoke of how submitting a Yellow Card provided a form of redress: ‘It’s kind of restorative justice in a way’ (YCP6) or helped to resolve their feelings of guilt about what had happened to their child: ‘It felt that I might have failed [my child] so that’s what I am doing it all for, really, to try and offload that information’ (YCP10).
Views and experiences of participants who had not submitted Yellow Cards
As we note above, the children we interviewed and most ADRIC parents knew nothing of the Yellow Card Scheme prior to participating in this study. When we explained the Yellow Card Scheme to them during the interviews, like the Yellow Card parents, most of the children and ADRIC parents were generally positive about the Scheme ‘I think that is a good idea as patients might think [an ADR] is important and doctors don’t’ (EC16) or spoke of the need for more to be done to publicise the Yellow Card Scheme: ‘We should be told about things like this [the Yellow Card Scheme] [. . .] if anyone has a reaction to a drug then they need to know that something is going to happen about it. It should be recorded’ (AC10). All but one parent said they would consider using the Yellow Card Scheme in future: ‘Now that I know about it, yeah, I would do. I’ll tell my friends about this actually’ (AP23). Despite this positivity, none of the ADRIC parents said that they would like to complete a Yellow Card for the particular ADR that we had discussed during the interview. Parents’ reluctance may be linked to their experiences of their child’s ADR. As described above, both ADRIC and Yellow Card parents had been dissatisfied with how health practitioners had communicated about ADRs, but ADRIC parents also described confusion and uncertainty about roles and responsibilities for recording and reporting a suspected ADR. Some assumed this was a practitioner’s role: ‘[I] just assume the doctor would sort it out’ (AP14), or expected that practitioners would submit Yellow Cards as a matter of course: ‘I would more than likely think that the doctors would do it [. . .] if the child has had a reaction they would automatically’ (AP13). Others implied that practitioners might disapprove of parents who submitted Yellow Cards and regard such parents as stepping beyond their role: ‘they might think that you are trying to do their job for them’ (AP20).
Some ADRIC parents were also reluctant to submit a Yellow Card on this occasion because they were uncertain about whether an ADR had occurred: ‘I don’t think they linked it to an adverse reaction at the time’ (AP25) or they felt that they or other members of the public were not equipped to decide if an ADR had occurred: ‘I’m not medical so I wouldn’t know what a reaction would be’ (AP18); it [the side effect] may not be from the drug, and [a parent] might think it is and go onto the internet and say that on a Yellow Card’ (AP22).
Discussion
Perspectives on communication about adverse drug reactions
Parents were generally disappointed with how clinicians communicated about suspected ADRs. Although children focused on the concern or distress the ADR had caused them, they voiced fewer problems with clinicians’ communication. Children pointed to how their parents acted as intermediaries or conduits for communication about medicines and ADRs and this parental role may explain why children voiced fewer problems in communication compared with parents. The majority of parents reported receiving little or no advance explanation about the problems that might be associated with medicines. When information was provided, it was in ways that parents found hard to absorb. As a result, parents were taken by surprise when their child experienced a suspected ADR. This turned into frustration and confusion when clinicians were unresponsive to parents’ concerns and some parents felt dismissed or abandoned as a result. In the absence of explanation about what steps could be taken to prevent further ADRs, a few parents were reluctant to give their children medicines in the future. The key exception to these negative parental accounts was parents of children with cancer, who, despite their intense fears about the illness and treatment, were generally highly satisfied with how clinicians communicated about ADRs.
As well as being a source of avoidable distress, poor clinician–parent communication about suspected ADRs will impact on what parents communicate to their children about the ADR, challenge parents’ and children’s confidence in medicine, and contribute to negative perceptions and misunderstandings of medicines. 257,258 This could lead to poor adherence in the future. We found considerable convergence among participants about the nature of helpful communication. Their suggestions, which are similar to those reported elsewhere, included the importance of the timing and pacing of information, as well as the need for clinicians to explicitly acknowledge what had happened and help families to understand events that they perceived to be significant, even if the event is not significant from the perspective of clinicians. 258–260 The accounts of parents of children being treated for cancer indicated that, despite the complexities involved in prospectively explaining about ADRs while not raising undue alarm about medicines, communication about ADRs can be conducted in ways that parents find informative, understandable and reassuring.
One important challenge facing clinicians who communicate about ADRs is the uncertainty involved in attributing symptoms to medicines. We found that families’ accounts of how they linked symptoms to a medicine resembled the logic that underpins tools for assessing ADRs53,188 in research and clinical practice. Participants noted temporal associations between a medicine’s administration and the onset of symptoms, the receding of symptoms between doses and the absence of alternative explanations for symptoms. This common ground could be a starting point for improving communication about ADRs. Alongside our other findings – parents accepted that all medicines come with risks, appreciated the uncertainty in attributing symptoms to medicines and did not blame clinicians for suspected ADRs – we think there is reason to be optimistic about the potential to improve clinician–family communication about medicines. However, this needs to be confirmed by investigating clinicians’ perspectives on communicating with parents about suspected ADRs.
Perspectives on spontaneous reporting of suspected adverse drug reactions
To our knowledge, this is the first study to specifically investigate how parents and children view the opportunity to report suspected ADRs directly to the MHRA. All participants saw value in direct reporting and those who had submitted Yellow Cards were satisfied with the Yellow Card Scheme. However, our key findings come from the ADRIC parents, none of whom had previously submitted a Yellow Card. These parents were generally supportive of the aims of the Yellow Card Scheme after it had been explained. Although they were positive about using the Scheme in the future, they were reluctant to use the Scheme to report the ADR discussed in their interviews. Comparing the settings, roles and perceptions of the Yellow Card and ADRIC parents helps to shed light on these findings. The Yellow Card parents generally reported events that had happened in the community, and linked to their professional roles, many were confident about using the Yellow Card Scheme. In contrast, the children of ADRIC parents had received hospital care for their ADR or were hospital inpatients at the time the ADR occurred. As such, these parents either expected that it was the responsibility of the practitioners looking after their child to submit a Yellow Card, or they were uncertain about whether it was legitimate for parents to report the ADR. Moreover, only a few ADRIC parents had personal links to health practitioners or were themselves health practitioners.
Parents who submitted a Yellow Card reported multiple motivations. Altruistic motivations, such as a desire to contribute to the improving the safety of medicines at a population level, were particularly prominent in their accounts. This is similar to findings on other patient groups who have reported ADRs, to clinicians’ motivations for submitting Yellow Cards12,261,262 and it is also consistent with the goals of the MHRA. 223 Linked to their dissatisfaction with practitioners for not taking their concerns about suspected ADRs seriously, some parents experienced reporting as providing a form of redress or felt reassured, as others have also described,12,261,262 by the availability of an independent vehicle for ‘officially’ recording ADRs. A few parents pointed to how submitting a Yellow Card had helped to resolve feelings of guilt (about the medicines they had given or allowed their child to take), a motivation that has not been previously described and may be unique to parents and others who care for vulnerable patients. In this way, the Yellow Card Scheme seemed to enable parents to take action that seemed psychologically important following their child’s ADR, even if it would not directly benefit their child.
Consistent with previous research on adult patient reporters,12,27,28 our findings indicate that awareness of the Yellow Card Scheme is limited and that further work is needed to promote the Yellow Card Scheme. Our study provides insight into the perspectives of parents and children who had not used the Yellow Card Scheme but were ‘eligible’ to do so. As we note above, participants supported the aims of the Yellow Card Scheme but they were reluctant to use it to report the ADR that we interviewed them about. The reasons for their reluctance may help inform strategies to widen participation in pharmacovigilance. The parents we interviewed were concerned that, because they lacked medical knowledge, their reports would be inaccurate or of little value. Emphasising that reports from members of the public can make a valuable contribution to drug safety would help to overcome such barriers, as would emphasising that people do not need to be certain that a medicine definitely caused a reaction in order to submit a report. Some parents expected that their child’s practitioners would report the ADR, yet practitioner participation in spontaneous reporting and other forms of pharmacovigilance is poor. 40 Informing the public that their reports are an adjunct to practitioner reporting may help to motivate them to participate in pharmacovigilance. Parents also worried that their reports might be perceived as undermining practitioners. These concerns could be addressed by emphasising that the Yellow Card Scheme is confidential and that information will not be shared with practitioners without a reporter’s consent.
Limitations
Our study had some limitations. First, we relied on clinical teams for access to children and ADRIC families. Clinical teams may have filtered out participants with whom their relationships were strained. To address this we sampled Yellow Card parents, as we could access them without consulting with clinicians. However, many Yellow Card parents were health professionals themselves, or had contacts who were, and their views on communication about ADRs and pharmacovigilance may be distinctive. Previous studies of patients’ perspectives on spontaneous reporting pharmacovigilance schemes share similar limitations. This arises from the limited public awareness of the Yellow Card Scheme. In this context, our sampling of participants with experience of a suspected ADR but who had not used the Yellow Card Scheme is particularly important. The views of such groups have rarely been investigated, yet they are crucial in identifying how public participation in pharmacovigilance may be promoted. Moreover, the accounts of both ADRIC and Yellow Card parents triangulate in pointing to the difficulties parents experience in communication about ADRs. Finally, the interviews were conducted sometime after the suspected ADR, which may have shaped participants’ accounts in certain ways. However, understanding the meanings that parents and children take away from their experiences of ADRs is crucial in learning how to enhance their experiences and it is these meanings that were the focus of our study.
Conclusions
Poor communication about children’s ADRs was a source of significant difficulty for parents and our findings will help to guide clinicians regarding what topics to cover in their discussions about medicines and ADRs. At the time of prescription, parents wanted to know the potential risks associated with medicines. In the aftermath of a suspected ADR, both parents and children wanted to understand what had happened and in some cases this might include explicit acknowledgement that an ADR had possibly occurred. Parents also wanted know the potential future implications of the suspected ADR for their child. Parents and children linked symptoms to medicines in ways that resembled the reasoning used clinically for identifying ADRs. Clinicians could possibly use this common ground as a starting point for communicating with families when an ADR is suspected. However, our study’s most important contributions may lie in providing insight for clinicians into how valuable discussions of ADRs can be for parents and the important role that parents have as a conduit for communicating with children about medicines and ADRs.
Parents who had used the Yellow Card Scheme found it straightforward and were satisfied with its aims. Participants who had not used the Yellow Card Scheme were also satisfied with its aims but parents were uncertain about their role in reporting ADRs and many assumed that submitting a Yellow Card was the responsibility of practitioners. Therefore, although raising public awareness of reporting schemes is important, our findings indicate that this will not improve public participation by itself and that pharmacovigilance agencies will need to present their schemes in ways that empower and support lay reporters. Based on our findings, we recommend that agencies emphasise the following points when publicising their schemes: (1) the value of laypeople’s reports in promoting drug safety; (2) that reports will not be shared with practitioners without the reporter’s permission; and (3) that reports can be submitted even when there is uncertainty about whether or not a medicine caused a reaction.
Chapter 8 Developing a communication strategy about suspected adverse drug reactions affecting children
Abstract
Background
Families have unmet information needs following a suspected ADR in a child or young person.
Aims
To develop a strategy to support communication about suspected ADRs between families and clinicians by:
-
identifying any barriers to effective communication with families from the perspective of clinicians following a suspected ADR
-
developing information leaflets about ADRs for parents, children and young people to support their communication with clinicians.
Methods
Semistructured qualitative interviews with 42 clinicians about their experiences of ADRs in children. Face-to-face interviews were audio-recorded and transcribed. Analysis was informed by the principles of the constant comparative method. A parental leaflet on ADRs was developed, based on feedback from a range of stakeholders, including parents and clinicians. The usefulness of the leaflet was examined by conducting structured interviews with 17 clinicians after they had used the leaflet during routine parent–clinician discussions about suspected ADRs.
Results
Clinicians described using all the features of communication that parents wanted to see. However, clinicians made active decisions about when and what to communicate to families about suspected ADRs. These decisions mean that communication may not always match families’ needs and expectations. Clinicians describe a number of complexities with effective communication, some of which are unique to paediatric settings. The complexities perceived by clinicians may explain, at least in part, the discordance between clinician and family perspectives. Clinicians found the leaflet useful in supporting discussions with parents about a suspected ADR in their child.
Conclusions
The parent leaflet was useful in supporting discussions between parents and clinicians about suspected ADRs. Further strategies to improve communication between families and clinicians should focus on aligning clinicians’ decision-making about what and when to communicate with families following a suspected ADR rather than focusing on developing clinicians’ communication skills.
Introduction
The ADRIC-QUAL interviews (see Chapter 7 ) elucidated the communication difficulties that many families experienced following a suspected ADR. In their interviews parents also indicated that written information about ADRs in the form of a leaflet may help to address their communication needs by supporting discussions between families and clinicians about suspected ADRs. In response to these findings, we developed information leaflets that could be given to parents and children following a suspected ADR and used to support them in communicating with clinicians about ADRs. As well as being the catalyst for developing the leaflets, the findings from the families’ interviews also informed the content of the leaflets.
The findings described in Chapter 7 suggest that from the perspective of parents, there is a need to enhance communication about ADRs between clinicians and parents. However, as communication is a two-way process, it is important to also examine communication about ADRs from clinicians’ perspectives, particularly to identify which strategies are likely to be feasible for use in everyday practice to enhance communication with families. Accordingly, this study aimed to describe clinicians’ views of communicating with families about ADRs and to relate the findings to the accounts given by parents.
Methods: development of a leaflet for parents
Process
The parent leaflet was developed iteratively through numerous cycles of drafting, comment and redrafting, culminating in a pilot study to test the suitability of the leaflet for supporting communication about ADRs in clinical practice.
External review
The text and format of the parents’ leaflet was reviewed externally through three routes:
-
During interviews with clinicians about their experiences of ADRs, clinicians were shown a copy of the leaflet and asked to comment on the content and the potential usability of the leaflet.
-
The leaflet was reviewed by parent members of the MCRN at Alder Hey and by parents from the Research User Group at Liverpool Women’s Hospital.
-
The leaflet was reviewed by the Paediatric Medicines Expert Advisory Group at the MHRA.
Two prototype text-only versions of information leaflets for children were reviewed internally and revised within the ADRIC team. The children’s leaflets were further reviewed and revised as follows:
-
Children’s views of the leaflets were sought during some of the qualitative interviews that we conducted (see Chapter 7 ). In response to comments during these interviews we developed a third leaflet aimed at young people aged ≥ 16 years. This leaflet matched the level of information provided on the parents’ leaflet.
-
We engaged a professional designer to enhance the age-appropriateness of the design of the leaflets to ensure their suitability for the target age groups.
-
Children and young people from the MCRN Young Person’s Advisory Group at Alder Hey reviewed and commented upon the leaflets.
-
The revised children’s and young people’s leaflets were then reviewed by the ADRIC steering committee.
-
Following this review the leaflets were forwarded to the Paediatric Medicines Expert Advisory Group at the MHRA to mirror the review process that we had conducted for the parents’ leaflets.
We approached the Royal College of Paediatricians and Child Health to ask if it would consider hosting the parents’ leaflet on the publicly available Medicines for Children website (www.medicinesforchildren.org.uk). Including the leaflet on this website offers the advantage of making the leaflet available widely for parents and clinicians, as all hosted leaflets are freely available for download by anyone accessing the website. The leaflet was reviewed by the Patient Information Leaflet Committee, which requested that it be piloted to assess the suitability of the leaflet for use in clinical practice before its inclusion on the website.
Piloting the parent leaflet
The pilot study took place in Alder Hey. We conducted brief structured interviews with doctors and nurses after they had actually used the leaflet in the routine interactions that they had with families when they usually explained about any suspected ADRs.
Sample
The leaflet was used on 17 occasions. There were three further occasions when doctors declined to use the leaflet: either because they did not feel an ADR had occurred or they did not feel it was appropriate to introduce the concept of an ADR to a family at that particular time. Excerpts from interviews are presented to evidence our conclusions; in these excerpts omitted speech is indicated by [. . .] and explanatory text by [text], and excerpts are coded ‘N’ (nurse) or ‘D’ (doctor).
Pilot study results: development of a leaflet for parents
The pilot study indicated that from the perspective of clinicians, the leaflet was well designed and useful in supporting communication between parents and clinicians following a suspected ADR in a child. Clinicians commented that the leaflet was ‘very well laid out and [. . .] jargon free’ (P06:N) and ‘simple to read’ (P09:N). They also reported that the leaflet supported conversations with families by prompting clinicians to include information that they would not normally have covered when discussing ADRs with parents. In this way, clinicians indicated that the leaflet helped them to offer additional reassurance to parents. Some clinicians also felt the leaflet empowered parents to ask questions that they might not have otherwise voiced or felt able to ask: ‘Some of our other families [who had not seen the leaflet] [. . .] mightn’t have thought of those things or they are thinking “oh [. . .] it is all right to ask this and ask that” ’ (P06:N). All clinicians said they would use the leaflet again: ‘Oh yeah, I would be quite happy to do it again’ (P02:N); ‘Yeah I would use it again’ (P12:D).
Pilot study feedback and leaflet validation
The results of the pilot study were fed back to:
-
the Patient Information Committee at the Royal College of Paediatricians and Child Health
-
the Medicines Management Committee at Alder Hey
-
the ADRIC Steering Group (including members of the MHRA).
The leaflet for parents is now available at www.medicinesforchildren.org.uk/search-for-a-leaflet/side-effects-from-childrens-medicines/.
Methods: clinicians’ perspectives on communicating with families about suspected adverse drug reactions in children
The methods were similar to those used in Chapter 7 , with the key elements noted below.
We purposively sampled clinicians across three sites for maximum variation241 in terms of professional role (nurse, doctor or pharmacist), length of practice, seniority and specialty. Site 1 was a specialist regional paediatric hospital that had been actively involved in the ADRIC studies of the prevalence and characteristics of paediatric ADRs. 20,22 We were aware that clinicians at this hospital may have a heightened awareness of the subject because of the ADRIC studies. Therefore, to enhance the transferability of our findings we included two additional sites (sites 2 and 3), which were paediatric units in local district general hospitals.
Procedure
The researcher (JA) made initial contact with a senior research lead at each site, who helped the researcher to access a list of eligible clinicians. JA then contacted clinicians by e-mail with a brief outline of the study and an invitation to take part. Where a clinician did not reply they were sent one reminder e-mail. Clinicians who expressed an interest in the study were provided with a participant information sheet and their written consent was sought. One of two experienced qualitative researchers (JA and AR) conducted the interviews with each clinician at the hospitals where the clinicians worked.
A topic guide was developed to steer the interviews and this was adapted to reflect the different roles of each professional group in the sample. The topic guide included prompts to elicit clinicians’ definitions and experiences of ADRs; their perceptions of how families’ experienced ADRs; their accounts of communicating about potential ADRs with families, both at the time a medicine was prescribed and following a suspected ADR; and their accounts of recording and reporting ADRs. Interviews were audio-recorded and transcribed. The researchers also kept field notes of each interview detailing the interview context, including the setting and observations and reflections on the interview process.
Results: clinicians’ perspectives on communicating with families about suspected adverse drug reactions in children
We invited 90 clinicians to participate; 46 did not respond to an initial and reminder invitation and one doctor agreed to take part but we were unable to arrange an interview. Non-respondents included doctors (n = 28), nurses (n = 15) and pharmacists (n = 3). We audio-recorded interviews with 42 clinicians, which included doctors (n = 26), nurses (n = 12) and pharmacists (n = 4). The mean length since registration to practice of our sample was 18.5 years (range 3–36 years). Table 36 shows other sample demographics (interviews lasted approximately 70 minutes). We found no difference in participant characteristics or study findings between sites. In the following sections we present preliminary findings from our analysis.
| Professional role | Level/grade | Specialty | Length of time since registration |
|---|---|---|---|
| Doctor | Non-consultant | General paediatrics | 5–10 years |
| Doctor | Non-consultant | General paediatrics | < 5 years |
| Pharmacist | Consultant | General paediatrics | Missing |
| Nurse | Missing | General paediatrics | Missing |
| Nurse | Staff nurse | Paediatric A&E | Missing |
| Nurse | Specialist nurse practitioner | Paediatric oncology | > 10 years |
| Pharmacist | Senior | General paediatrics | > 10 years |
| Doctor | Consultant | Paediatric neurology | > 10 years |
| Doctor | Non-consultant | Community paediatrics | 5–10 years |
| Pharmacist | Senior | General paediatrics and research | > 10 years |
| Pharmacist | Senior | General paediatrics | 5–10 years |
| Nurse | Sister | Missing | > 10 years |
| Nurse | Specialist nurse practitioner | Pain and sedation | > 10 years |
| Nurse | Student | General medicine | N/A |
| Doctor | Missing | Missing | Missing |
| Doctor | Consultant | Paediatric allergies | > 10 years |
| Doctor | Consultant | Paediatric nephrology | > 10 years |
| Doctor | Consultant | Paediatric neurology | > 10 years |
| Doctor | Consultant | Paediatric general surgeon | > 10 years |
| Doctor | Consultant | General paediatrics | > 10 years |
| Doctor | Consultant | Paediatric nephrology | > 10 years |
| Doctor | Consultant | Paediatric respiratory/cystic fibrosis/allergy/asthma | > 10 years |
| Doctor | Consultant | Neonatal | > 10 years |
| Nurse | RSCN | Paediatric A&E | 5–10 years |
| Nurse | Sister | Paediatric | > 10 years |
| Doctor | Missing | Paediatric A&E | > 10 years |
| Nurse | Sister | Paediatric | > 10 years |
| Doctor | Non-consultant | Paediatric A&E | < 5 years |
| Doctor | Consultant | Paediatric cardiology | > 10 years |
| Nurse | Staff nurse | General paediatrics | < 5 years |
| Doctor | Consultant | Paediatric anaesthetist | > 10 years |
| Nurse | Sister | Paediatric diabetes | > 10 years |
| Doctor | Consultant | Paediatric respiratory | > 10 years |
| Nurse | Missing | General paediatrics | > 10 years |
| Doctor | Consultant | General paediatrics | > 10 years |
| Doctor | Consultant | Paediatric anaesthetist | > 10 years |
| Doctor | Consultant | Missing | Missing |
| Doctor | Consultant | Paediatric rheumatology | > 10 years |
| Doctor | Consultant | General paediatrics | > 10 years |
| Doctor | Consultant | Paediatric rheumatology | > 10 years |
| Doctor | Consultant | Oncology | Missing |
| Doctor | Consultant | Paediatric rheumatology | > 10 years |
Why communicate about adverse drug reactions?
Clinicians indicated that they viewed providing both prospective and retrospective information to families about ADRs as an important part of their clinical practice. They also talked of the need to optimise adherence to medicines and pointed to how prospective communication about ADRs helped to guard against the possibility that parents may refuse a medicine in the future because their child had experienced an ADR about which that they had not been warned. Only a few clinicians described how discussing ADRs prospectively was important in order to actively involve parents in their child’s clinical care.
In their accounts of retrospective communication following a suspected ADR, clinicians more commonly pointed to the importance of the active involvement of parents. For example, a few clinicians mentioned how discussing ADRs retrospectively was important in order to overcome the circumstances of the ADR. Clinicians also spoke of how important it was to ensure that parents were adequately briefed about the ADR and the medicine implicated in its causation so that parents could pass on this information in future interactions with health teams.
Definitions of adverse drug reactions
In some cases, clinicians indicated that although they recognised and responded clinically to possible side effects to a medicine, they did not necessarily consciously ‘label’ these symptoms as an ADR. The descriptions of ADRs used by clinicians reflect the clinicians’ thresholds for communication with parents. However, they did not mirror formal definitions of ADRs used in pharmacovigilance, such as in the MHRA Yellow Card system for reporting suspected reactions. We also noticed that some clinicians used the terms allergic and side effect interchangeably, and that this informed how and what clinicians communicated to families.
How much information to communicate about adverse drug reactions?
Clinicians’ accounts pointed to factors that led them to constrain or filter the information that they communicated to families about ADRs. These constraints reflected commonly accepted practice and ‘rules of thumb’ in communicating about medicines, as well as their judgements about the characteristics of the drug reaction, such as how severe, common or expected clinicians perceived an ADR to be. Clinicians also indicated that they constrained their prospective communication about ADRs in order to promote adherence to medicines: ‘If we read out the list of all potential reactions to parents, paracetamol for example, no-one would take paracetamol’ (23:D). A common theme reported by clinicians was the need to balance the information they gave to parents about ADRs with the need to avoid causing parents any unnecessary anxiety at the time a medicine was prescribed.
Who should discuss suspected adverse drug reactions with families
Clinicians’ views about who should discuss ADRs with parents were varied, although most emphasised that the member of staff who was ‘seeing the child’ (09:D) or responsible for the child’s care should also be the one who takes responsibility for communicating about ADRs. The clinical severity of a suspected ADR also influenced clinicians’ views about who should discuss a suspected ADR with a family retrospectively. Clinicians thought that nurses were well placed to discuss relatively minor, expected and transient ADRs with families, whereas ADRs that were more serious required a discussion between doctors and parents.
Discussion
This is the first study to report clinicians’ perceptions of discussing ADRs with parents. Similar to the findings from studies about other populations,263–267 the clinicians in this study reported that communication about ADRs was generally important in clinical practice. However, in specific cases they described filtering the information that they discussed with families. Clinicians’ views about what constituted an ADR also influenced their communication. When clinicians felt ADRs were mild, transient and expected they reported either not discussing these with families or constraining the information that they gave families. Evidence from our study of parents’ experiences of ADRs reported in Chapter 7 suggests that the level of parental anxiety and concern may relate more to suboptimal communication than to the perceived severity of an ADR. For example, parents of children with cancer were reassured and confident managing in suspected ADRs, even although the ADRs such children experienced could be severe. In contrast, parents of children who experienced relatively minor ADRs reported feeling confused, dismissed and abandoned. 29
Limitations of the study
This study explored clinicians’ accounts of discussing ADRs with families and may be subject to recall bias. In addition, during interviews, we found clinicians tended to drift into normative or idealised descriptions rather that giving specific accounts of what they did in practice when discussing ADRs with families. Because of this, it is difficult to establish to what extent clinicians’ accounts reflect day-to-day practice.
Strengths of the study
-
Wide range of participants, including doctors, pharmacists and nurses. 267
-
Multicentred (specialist and non-specialist).
-
Everyday interactions on busy wards in which the relationship with doctors was mainly episodic discrete rather than long term.
Conclusion
Taken together, the findings of this chapter and Chapter 7 point to a mismatch between clinicians’ and parents’ accounts of communication about ADRs. Although the clinicians spoke of the importance of communicating with parents about ADRs, parents pointed to deficiencies in clinicians’ approaches to communication. Our initial analyses suggest that this mismatch may relate, at least in part, to decisions made by clinicians about what and when to discuss ADRs with parents. Some of the difficulties experienced by parents may also have their roots in the different ways in which ADR terminology is used by clinicians. Our preliminary results suggest that clinicians use terms that describe ADRs in ways that reflect whether they need to tell parents about the incident, or whether they need to modify clinical management. The same terms are used by pharmacovigilance specialists with a broader drug safety agenda. This variation in the use of terminology may be one explanation for the low rates of reports to spontaneous pharmacovigilance systems. Further analysis is needed to clarify the results of these findings.
Conclusion and future research directions
Adverse Drug Reactions In Children was conceived and developed in 2006, which was the first year of operation of the NIHR MCRN and shortly before the European Regulation on Better Medicines for Children became law in January 2007. 268 The programme was an ambitious one and aimed, for the first time, to investigate the whole spectrum from when and where ADRs in children occurred to the development of solutions to reduce the burden of ADRs. As originally conceived, the key component parts were:
-
Identification To identify the nature of ADRs in children and the drugs causing these ADRs in hospitalised patients.
-
Quantification To determine the proportion of hospital admissions related to ADRs, and those occurring in hospital; to estimate the whole burden associated with local, specialist regional and national paediatric care.
-
Evaluation To evaluate the risk factors for ADRs, including OLUL use of medicines.
-
Intervention To develop tools and guidelines for improving the recognition and ultimately reducing the burden of ADRs through enhanced prevention.
At the time of submission, two important studies had been conducted by Pirmohamed et al. 198 in the adult population. First, a prospective study of 18,820 admissions to two adult hospitals showed that 6.5% of the admissions were due to ADRs, which led to death in 0.15% of adult patients (equivalent to 5700 deaths per year in the UK) at a cost to the NHS of £500M. 198 A further study, examining ADRs occurring in 3728 patients in hospital, showed that 15.8% of adult patients experienced an ADR after admission to hospital. 59 Importantly, > 70% of ADRs, in both the hospital admission study and the inpatient study, were potentially avoidable. Although the burden of disease and use of drugs in children is considerably lower than in adults, the majority of drugs are not evaluated in children, leading to a much higher proportion of drugs being used OLUL, with the consequent safety concerns. 3,269 Previous studies addressing ADRs in children had considerable limitations. The populations studied were poorly described, heterogeneous and the methodologies used to determine ADRs were unsatisfactory or inadequately reported, making incidence estimates highly unreliable. Assessment of causality, severity and avoidability was generally absent or inadequate and there was no assessment of risk factors for ADRs in children. There was a clear rationale for the first two studies proposed in ADRIC, which would be conducted at the largest children’s hospital in Europe with 12,600 children admitted per year and long term, each study conducted over 1 year.
Similarly, although systematic reviews of studies to identify ADRs in children had been conducted previously, there was a need for a systematic review, which was fully comprehensive and went beyond attempting to describe the aggregate incidence of ADRs, to examine the nature of ADRs in children and the drugs associated with them, and the quality of the methods used to detect, assess and avoid these ADRs.
We anticipated detecting a proportion of ADRs, which had not been previously described and which were unknown or unpredictable, from the known pharmacology of the drug and thus, originally planned to develop screening tools to improve recognition of such ADRs and of known ADRs. However, during the course of the first two studies, such unknown ADRs were not detected and we did not feel that development of such a screening tool was appropriate. However, a number of other problems were identified by the early studies, which required the development of tools for the benefit of patients in the NHS. These included the need to develop entirely new, validated tools to assess causality and avoidability, which became a focus of the later studies in ADRIC. In addition, the interviews with families whose children had experienced an ADR highlighted a disturbing degree of distress associated with this and some concern from them about the ways in which this diagnosis had been communicated by their health professionals. An additional focus of the later parts of the programme was to understand this better by conducting interviews with clinicians about how they communicate ADRs to families and to develop information leaflets for clinicians and families to help guide these discussions.
The prevalence of ADRs associated with admission to hospital was around 2.9%, lower than reported in a similar study in adults (6.5%). 198 When we assessed the origin of the prescriptions for the drugs responsible, the majority originated either directly from hospital or from protocols led by hospital practitioners. This also differed from experience in adults. Although many of the ADRs were predicted and the families were expecting them, for example in the children being treated with immunosuppressive therapy, other signals were more disturbing. These included the cases of respiratory depression associated with drugs used to treat status epilepticus and bleeding post tonsillectomy in children treated perioperatively with corticosteroids to prevent vomiting.
The most surprising findings were from the study of ADRs in hospital. First, we found that the incidence of ADRs in children is much greater than in adults. We included probable and definite ADRs, which gave an incidence of 17.7%; a comparable study, published by our group in adults,59 reported an incidence of 14.7% but this included ADRs classified as definite, probable or possible. If we included possible ADRs in our numerator, the comparable figure is that > 25% children in hospital experience an ADR. We also observed that the risk of experiencing an ADR was increased over six times in children who had a general anaesthetic during their admission and over half of the drugs implicated in all ADRs were used either perianaesthetic or post anaesthetic. These drug groups have been under-represented in previous studies and our findings have important implications for all clinicians concerned with the perioperative and postoperative management of children, in particular in view of the recent moves to ambulatory surgery in the UK and elsewhere.
The signals in studies 1 and 2 (see Chapters 2 and 3 ) of ADRs associated with drugs used during and after surgery are the most novel and concerning findings of this programme. In study 2, we were able to include only children who had been in hospital for > 48 hours. Most children who have surgery are in hospital for shorter periods than this and a very high proportion are discharged on the day of surgery. The anaesthetic care of children receiving their surgery as ‘day cases’ is adapted to ensure adequate control of pain and vomiting in the home environment. One example of this is use of a single dose of a corticosteroid to control vomiting over the ensuing 24 hours period, which in turn may contribute to postoperative bleeding following procedures such as tonsillectomy. Our study has highlighted a need to understand better the morbidities associated with anaesthesia and surgery in children.
The nested case–control study found that drugs used OLUL were more likely to be implicated in an ADR than authorised medicines. This again highlights the implications for how drugs are used in children. Furthermore, we observed that drugs licensed for use in children but given to a child below the minimum age or weight had the greatest risk of being implicated in an ADR, emphasising the importance of studies to provide pharmacokinetic data in children of different ages.
The LCAT is now available and is being used in research and clinical practice. Further work is being conducted to assess, by a randomised controlled trial, whether or not a short training package in the use of the tool – available online – enhances this assessment. We believe that the LCAT is the best tool currently available for assessment of ADR causality. Importantly, it has been developed, not only for children, but also for assessment of ADRs in adults. The Liverpool AAT is at an earlier stage of development, but we have learnt much from the development of the LCAT, in particular the visual algorithm is user friendly and allows a more rapid assessment. By providing a simple tool that can be used by all clinicians, the ADRIC programme has made an important contribution to the future assessment of ADRs. Like LCAT, the Liverpool AAT has been developed to provide this assessment of ADRs in both children and adults.
We have learnt much during the course of this programme, about the problems in communicating ADRs to children and families. The materials produced to aid these discussions have either undergone full assessment and user testing (the information leaflet to inform clinicians’ discussions) or will shortly do so, and we will make these leaflets available via the internet. More could be done to provide information to young people, for example via smartphone apps.
The ADRIC programme of research has highlighted a number of important implications for future research. The end of the ADRIC programme symposium included a workshop on the research implications of the burden of ADRs in paediatrics, and the key research questions from this are highlighted in Appendix 7 . Following discussion within the ADRIC Steering Group, the recommendations for future research are prioritised below:
-
Risk–benefit evaluation There is a need to explore the balance of drug safety compared with drug efficacy and, potentially, there has been too much focus on safety and monitoring at the expense of potential benefit. More research is necessary to assess the values that parents and children place on the use of different medicines and the risks that they will find acceptable within these contexts. Indeed, the conceptual framework within which the balance between safety and efficacy of medicines and individual drugs presents is an important area for further research, and poses the question whether the weighting between efficacious outcomes and safety of patients is appropriately calibrated. This subject is currently less understood in children than adults. There is a clear need for innovative means to interrogate and evaluate the risk and benefit associated with use of drugs in children. Methodological developments mean that this theme could be rigorously addressed, for example through the use of discrete choice experiments incorporating the views of parents, adolescents and younger children, regulators and industry. Important questions also include how do the decision-making differences between children and parents for particular drugs differ, and how the individual’s perspective may vary between the younger child and adolescent.
-
Evaluation of ADRIC outputs Two key ADRIC outputs are the LCAT and the Liverpool AAT. Although these tools are at different stages of development there is potential for further research with both. Following validation, the Liverpool AAT will be used to assess the avoidability of the ADRIC admissions study cases and the results will be compared with the Hallas54 assessments carried out in Chapter 2 . Similarly, for the inpatient study cases we will aim to assess a proportion of the cases using the new Liverpool AAT. This will help us to identify potentially avoidable ADRs. There is the potential to evaluate the use of both tools in a variety of settings including other paediatric and adult hospitals and clinical trial AE reporting. The applications of these tools in clinical practice and education could be explored. For example, a learning package which incorporates the LCAT has been developed in order to explore whether or not it can be used in this context to improve the causality assessment skills of clinicians.
-
Dose optimisation There is a clear need to reduce the burden of ADRs in children, and one component strategy is to optimise dosing in children and young people. A clear first step will be a comprehensive review of the literature to identify how extrapolations from adults to children are achieved, which methods have or have not been used, and an evaluation of the utility of those methods that have been used. The findings will help to inform a gold standard practice for extrapolation, which can be targeted towards drugs identified as high risk within ADRIC and other relevant studies, and in combination with alongside pharmacokinetic/pharmacodynamic (PK/PD) evaluation studies. Alongside studies of risk–benefit evaluation, enhanced methodologies for extrapolation would make a significant contribution to the potential of adaptive licensing within the regulatory framework for drugs in children.
-
Enhanced ADR monitoring associated with surgery and anaesthesia in children The signals in studies 1 and 2 (see Chapters 2 and 3 ) of ADRs associated with drugs used during and after surgery are the most novel and concerning findings of this programme, and have important implications for further research. Our study has highlighted a need to understand better the morbidities associated with anaesthesia and surgery in children. This will require a study that can follow up children in the community and in the home setting to assess the incidence of ADRs following surgery, and compare these according to the anaesthetic and postoperative drugs, surgical procedures and their comorbidities. Such an observational study could lead to an assessment of which children should be discharged on the day of surgery and, for those who are, randomised controlled trials will be able to assess the most appropriate treatment regimens to prevent pain, vomiting and other postoperative complications.
-
Evaluation of strategies for communication about ADRs We identified that families have unmet information needs following a suspected ADR in a child or young person and responded to this through the development of a series of leaflets. Further research should focus on the development of communication strategies, supported by the use of leaflets and other media. The reasons for the mismatch in the perceptions of families and clinicians about how ADRs are communicated should be investigated, inclusive of the uncertainties around terminology. The evaluation of any strategies developed should take into consideration how improvements in communication have a direct impact on the individuals involved (e.g. improved health outcomes for the patient) as well as their indirect impacts, for example, improve rates of ADR reporting by families and clinicians.
-
Quantification of ADRs in children in other settings The ADRIC programme generated rich data in a number of settings relevant to paediatric practice, but there remain significant gaps in the knowledge of the quantification of ADRs in children in a variety of settings. In addition to understanding the burden of ADRs in particular settings, such as theatres and critical care, and populations (most notably neonates), there is also a need to undertake comprehensive studies of the burden and impact of long-term side effects, which was not an objective within the ADRIC programme.
Finally, ADRIC has provided an important focus on this important and neglected area of paediatric medicine. It has provided the most comprehensive assessment to date of the size and nature of this problem in children presenting to, and cared for in, hospital, and the outputs that have resulted will improve the management and understanding of ADRs in children and adults within the NHS.
Acknowledgements
We would like to thank members of the ADRIC Steering Group: Professor Sir Alasdair Breckenridge (chairperson), Professor Deborah Ashby, Dr Julia Dunne, Dr June Raine and Professor Michael Rieder.
Contributions of authors
Rosalind L Smyth (Head, Institute of Child Health, University of Liverpool) Conceived and designed the studies, supervised all aspects of the conduct of the studies and analysis of the data, planned, led and contributed to all aspects of writing of the report.
Matthew Peak (Director of Research, Alder Hey Children’s NHS Foundation Trust) Conceived and designed the experiments and contributed to the writing of this report.
Mark A Turner (Head of Research and Development, Liverpool Women’s Hospital) Conceived and designed the experiments, performed the experiments, analysed the data and contributed to the writing of this report.
Anthony J Nunn (Honorary Professor, University of Liverpool) Conceived and designed the experiments, performed the experiments and contributed to the writing of this report.
Paula R Williamson (Head of Biostatistics, University of Liverpool) Conceived and designed the experiments, performed the experiments, analysed the data, contributed reagents/materials/analysis tools and contributed to the writing of this report.
Bridget Young (Professor and Director of Communication Skills, University of Liverpool) Conceived and designed the experiments, analysed the data and contributed to the writing of this report.
Janine Arnott (Research Associate, University of Liverpool) Performed the experiments, analysed the data and contributed to the writing of this report.
Jennifer R Bellis (Research Pharmacist, Alder Hey Children’s NHS Foundation Trust) Conceived and designed the experiments, performed the experiments, analysed the data and contributed to the writing of this report.
Kim A Bird (Research Nurse, Alder Hey Children’s NHS Foundation Trust) Conceived and designed the experiments, performed the experiments and contributed to the writing of this report.
Louise E Bracken (Research Pharmacist, Alder Hey Children’s NHS Foundation Trust) Performed the experiments, analysed the data and contributed to the writing of this report.
Elizabeth J Conroy (Research Statistician, University of Liverpool) Analysed the data and contributed to the writing of this report.
Lynne Cresswell (Research Statistician, University of Liverpool) Conceived and designed the experiments, performed the experiments, analysed the data, contributed reagents/materials/analysis tools and contributed to the writing of this report.
Jennifer C Duncan (Research Pharmacist, Alder Hey Children’s NHS Foundation Trust) Performed the experiments.
Ruairi M Gallagher (Clinical Research Fellow, University of Liverpool) Conceived and designed the experiments, performed the experiments, analysed the data, contributed reagents/materials/analysis tools and contributed to the writing of this report.
Elizabeth Gargon (Assistant Systematic Reviewer, University of Liverpool) Conceived and designed the experiments, performed the experiments, analysed the data, contributed reagents/materials/analysis tools and contributed to the writing of this report.
Hannah Hesselgreaves (Research Associate, University of Liverpool) Performed the experiments.
Jamie J Kirkham (Research Statistician, University of Liverpool) Conceived and designed the experiments, performed the experiments, analysed the data, contributed reagents/materials/analysis tools and contributed to the writing of this report.
Helena Mannix (Research Nurse, Alder Hey Children’s NHS Foundation Trust) Performed the experiments.
Rebecca MD Smyth (Systematic Reviewer, University of Liverpool) Conceived and designed the experiments, performed the experiments, analysed the data, contributed reagents/materials/analysis tools and contributed to the writing of this report.
Signe Thiesen (Clinical Research Fellow, University of Liverpool) Conceived and designed the experiments, performed the experiments, analysed the data, contributed reagents/materials/analysis tools and contributed to the writing of this report.
Munir Pirmohamed (Head of the Department of Molecular and Clinical Pharmacology, University of Liverpool) Conceived and designed the experiments, performed the experiments, analysed the data, contributed reagents/materials/analysis tools and contributed to the writing of this report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health.
Publications
Bellis JR. Adverse Drug Reactions in Children – The Contribution of Off-label and Unlicenced Prescribing. PhD thesis. Liverpool: University of Liverpool; 2013.
Bellis JR, Kirkham JJ, Nunn AJ, Pirmohamed M. Adverse drug reactions and off-label and unlicensed medicines in children: a prospective cohort study of unplanned admissions to a paediatric hospital. Br J Clin Pharmacol 2013;77:545–53.
Bellis J, Kirkham J, Thiesen S, Conroy E, Bracken L, Mannix H, et al. Adverse drug reactions and off-label and unlicensed medicines in children: a nested case-control study of inpatients in a pediatric hospital. BMC Medicine 2013;11:238.
Thiesen S, Conroy EJ, Bellis JR, Bracken LE, Mannix HL, Bird KA, et al. Incidence, characteristics and risk factors of Adverse Drug Reactions (ADRs) in hospitalised children: a prospective observational cohort study of 6601 admissions. BMC Med 2013;11:237.
Arnott J, Hesselgreaves H, Nunn AJ, Peak M, Pirmohamed M, Smyth RL, et al. Enhancing communication about paediatric medicines: lessons from a qualitative study of parents’ experiences of their child’s suspected adverse drug reaction. PLOS ONE 2012;7:e46022.
Arnott J, Hesselgreaves H, Nunn AJ, Peak M, Pirmohamed M, Smyth RL, et al. What can we learn from parents about enhancing participation in pharmacovigilance? Br J Clin Pharmacol 2012;75:1109–17.
Gallagher RM, Mason JR, Bird KA, Kirkham JJ, Peak M, Williamson PR, et al. Adverse drug reactions causing admission to a paediatric hospital. PLOS ONE 2012;7:e50127.
Mason J, Pirmohamed M, Nunn T. Off-label and unlicensed medicine use and adverse drug reactions in children: a narrative review of the literature. Eur J Clin Pharmacol 2012;68:21–8.
Smyth RMD, Gargon E, Kirkham J, Cresswell L, Golder S, Smyth R, et al. Adverse drug reactions in children: a systematic review. PLOS ONE 2012;7:e24061.
Gallagher RM, Bird KA, Mason JR, Peak M, Williamson PR, Nunn AJ, et al. Adverse drug reactions causing admission to a paediatric hospital: a pilot study. J Clin Pharm Therapeut 2011;36:194–9.
Gallagher RM, Kirkham JJ, Mason JR, Bird KA, Williamson PR, Nunn AJ, et al. Development and inter-rater reliability of the Liverpool Adverse Drug Reaction Causality Assessment Tool. PLOS ONE 2011;6:e28096.
References
- World Health Organization (WHO) . International drug monitoring: the role of national centres. WHO Technical Report Series 1972;498.
- European Medicines Agency with its Paediatric Committee . 5-Year Report to the European Commission. 2012. http://ec.europa.eu/health/files/paediatrics/2012–09_pediatric_report-annex1–2_en.pdf (accessed 14 May 2013).
- Turner S, Nunn AJ, Fielding K, Choonara I. Adverse drug reactions to unlicensed and off-label drugs on paediatric wards: a prospective study. Acta Paediatr 1999;88:965-8. http://dx.doi.org/10.1111/j.1651-2227.1999.tb00191.x.
- Barr J, Brenner-Zada G, Heiman E, Pareth G, Bulkowstein M, Greenberg R, et al. Unlicensed and off-label medication use in a neonatal intensive care unit: a prospective study. Am J Perinatol 2002;19:67-72. http://dx.doi.org/10.1055/s-2002-23557.
- Kearns GL, Abdel-Rahman S, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology-drug disposition, action, and therapy in infants and children. N Engl J Med 2003;349:1157-67. http://dx.doi.org/10.1056/NEJMra035092.
- Wysowski DK, Pollock ML. Reports of death with use of propofol (Diprivan) for nonprocedural (long-term) sedation and literature review. Anesthesiology 2006;105:1047-51. http://dx.doi.org/10.1097/00000542-200611000-00027.
- Todd GR, Acerini CL, Ross-Russell R, Zahra S, Warner JT, McCance D. Survey of adrenal crisis associated with inhaled corticosteroids in the United Kingdom. Arch Dis Child 2002;87:457-61. http://dx.doi.org/10.1136/adc.87.6.457.
- Mulhall A, de Louvois J, Hurley R. Chloramphenicol toxicity in neonates: its incidence and prevention. BMJ (Clin Res Ed) 1983;287:1424-7. http://dx.doi.org/10.1136/bmj.287.6403.1424.
- Levi N, Bastuji-Garin S, Mockenhaupt M, Roujeau JC, Flahault A, Kelly JP, et al. Medications as risk factors of Stevens–Johnson syndrome and toxic epidermal necrolysis in children: a pooled analysis. Pediatrics 2009;123:e297-304. http://dx.doi.org/10.1542/peds.2008-1923.
- Smyth RL, Ashby D, O’Hea U, Ashby D, O’Hea U, Burrows E, et al. Fibrosing colonopathy in cystic fibrosis: results of a case–control study. Lancet 1995;346:1247-51. http://dx.doi.org/10.1016/S0140-6736(95)91860-4.
- Turner S, Longworth A, Nunn AJ, Choonara I. Unlicensed and off label drug use in paediatric wards: prospective study. BMJ 1998;316:343-5. http://dx.doi.org/10.1136/bmj.316.7128.343.
- Avery AJ, Anderson C, Bond CM, Fortnum H, Gifford A, Hannaford BC, et al. Evaluation of patient reporting of adverse drug reactions to the UK ‘Yellow card scheme’: literature review, descriptive and qualitative analyses, and questionnaire surveys. Health Technol Assess 2011;15.
- The European Parliament and the Council of the European Union . Regulation (EC) No. 1901/2006 of the European Parliament and of the Council of 12 December 2006 on medicinal products for paediatric use and amending Regulation (EEC) No. 1768/92, Directive 2001/20/EC, Directive 2001/83/EC and Regulation (EC) No. 726/2004. OJEU 2006;49:1-19.
- The European Parliament and the Council of the European Union . Regulation (EU) No. 1235/2010 of the European Parliament and of the Council of 15 December 2010 amending, as regards pharmacovigilance of medicinal products for human use, Regulation (EC) No. 726/2004 laying down Community procedures for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency, and Regulation (EC) No 1394/2007 on advanced therapy medicinal products. OJEU 2010;53:1-16.
- Children & Young People’s Outcome Forum . Report of the Children &Amp; Young Peoples Outcome Forum n.d. www.dh.gov.uk/health/files/2012/07/CYP-report.pdf (accessed 14 May 2013).
- The European Parliament and the Council of the European Union . Commission Implementing Regulation (EU) No. 520/2012 of 19 June 2012 on the performance of pharmacovigilance activities provided for in Regulation (EC) No. 726/2004 of the European Parliament and of the Council and Directive 2001/83/EC of the European Parliament and of the Council. OJEU 2012;55:5-25.
- Medicines and Healthcare products Regulation Agency (MHRA) . Safety Information n.d. http://yellowcard.mhra.gov.uk/ (accessed August 2011).
- Anon . Patient yellow card scheme launched nationwide. Pharm J 2005;275.
- Smyth RMD, Gargon E, Kirkham J, Cresswell L, Golder S, Smyth R, et al. Adverse drug reactions in children: a systematic review. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0024061.
- Gallagher RM, Bird KA, Mason JR, Peak M, Williamson PR, Nunn AJ, et al. Adverse drug reactions causing admission to a paediatric hospital: a pilot study. J Clinical Pharm Ther 2011;36:194-9. http://dx.doi.org/10.1111/j.1365-2710.2010.01194.x.
- Gallagher RM, Mason JR, Bird KA, Kirkham J, Peak M, Williamson PR, et al. Adverse drug reactions causing admission to a paediatric hospital. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0050127.
- Thiesen S, Conroy EJ, Bellis JR, Bracken LE, Mannix HL, Bird KA, et al. Incidence, characteristics and risk factors of Adverse Drug Reactions (ADRs) in hospitalised children: a prospective observational cohort study of 6601 admissions. BMC Med 2013;11. http://dx.doi.org/10.1186/1741-7015-11-237.
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239-45. http://dx.doi.org/10.1038/clpt.1981.154.
- Avner M, Finkelstein Y, Hackam D, Koren G. Establishing causality in pediatric adverse drug reactions: use of the Naranjo probability scale. Paediatr Drugs 2007;9:267-70. http://dx.doi.org/10.2165/00148581-200709040-00007.
- Ferner RE, Aronson JK. Preventability of drug-related harms: part I – a systematic review. Drug Saf 2010;33:985-94. http://dx.doi.org/10.2165/11538270-000000000-00000.
- Hakkarainen KM, Andersson Sundell K, Petzold M, Hagg S. Methods for assessing the preventability of adverse drug events: a systematic. Drug Saf 2012;35:105-26. http://dx.doi.org/10.2165/11596570-000000000-00000.
- Fortnum H, Lee AJ, Rupnik B, Avery A, Collaboration YCS. Survey to assess public awareness of patient reporting of adverse drug reactions in Great Britain. J Clin Pharm Ther 2011;37:161-5. http://dx.doi.org/10.1111/j.1365-2710.2011.01273.x.
- Lorimer S, Cox A, Langford NJ. A patient’s perspective: the impact of adverse drug reactions on patients and their views on reporting. J Clin Pharm Ther 2012;37:148-52. http://dx.doi.org/10.1111/j.1365-2710.2011.01258.x.
- Arnott J, Hesselgreaves H, Nunn AJ, Peak M, Pirmohamed M, Smyth RL, et al. Enhancing communication about paediatric medicines: lessons from a qualitative study of parents’ experiences of their child’s suspected adverse drug reaction. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0046022.
- Bellis JR, Kirkham JJ, Nunn AJ, Pirmohamed M. Adverse drug reactions and off-label and unlicensed medicines in children: a prospective cohort study of unplanned admissions to apaediatric hospital. Br J Clin Pharmacol 2013;77:545-53. http://dx.doi.org/10.1111/bcp.12222.
- Bellis JR. Adverse Drug Reactions in Children – The Contribution of Off-label and Unlicenced Prescribing. Liverpool: University of Liverpool; 2013.
- McKenzie MW, Marchall GL, Netzloff ML, Cluff LE. Adverse drug reactions leading to hospitalization in children. J Pediatr 1976;89:487-90. http://dx.doi.org/10.1016/S0022-3476(76)80560-4.
- Mitchell AA, Lacouture PG, Sheehan JE, Kauffman RE, Shapiro S. Adverse drug reactions in children leading to hospital admission. Pediatrics 1988;82:24-9.
- Impicciatore P, Choonara I, Clarkson A, Provasi D, Pandolfini C, Bonati M. Incidence of adverse drug reactions in paediatric in/out-patients: a systematic review and meta-analysis of prospective studies. Br J Clin Pharmacol 2001;52:77-83. http://dx.doi.org/10.1046/j.0306-5251.2001.01407.x.
- Clavenna A, Bonati M. Adverse drug reactions in childhood: a review of prospective studies and safety alerts. Arch Dis Child 2009;94:724-8. http://dx.doi.org/10.1136/adc.2008.154377.
- Le J, Nguyen T, Law AV, Hodding J. Adverse drug reactions among children over a 10-year period. Pediatrics 2006;118:555-62. http://dx.doi.org/10.1542/peds.2005-2429.
- Jonville-Bera AP, Giraudeau B, Blanc P, Beau-Salinas F, Autret-Leca E. Frequency of adverse drug reactions in children: a prospective study. Br J Clin Pharmacol 2002;53:207-10. http://dx.doi.org/10.1046/j.0306-5251.2001.01535.x.
- Hawcutt DB, Mainie P, Riordan A, Smyth RL, Pirmohamed M. Reported paediatric adverse drug reactions in the UK 2000–2009. Br J Clin Pharmacol 2012;73:437-46. http://dx.doi.org/10.1111/j.1365-2125.2011.04113.x.
- Medicines and Healthcare products Regulatory Agency (MHRA) . Reporting Suspected Adverse Drug Reactions 2010. www.mhra.gov.uk/Safetyinformation/Reportingsafetyproblems/Reportingsuspectedadversedrugreactions/index.htm (accessed 28 June 2010).
- Hazell L, Shakir SAW. Under-reporting of adverse drug reactions: a systematic review. Drug Saf 2006;29:385-96. http://dx.doi.org/10.2165/00002018-200629050-00003.
- Easton KL, Parsons BJ, Starr M, Brien JE. The incidence of drug-related problems as a cause of hospital admissions in children. Med J Aust 1998;169:356-9.
- Martinez-Mir I, Garcia-Lopez M, Palop V, Ferrer JM, Estan L, Rubio E, et al. A prospective study of adverse drug reactions as a cause of admission to a paediatric hospital. Br J Clin Pharmacol 1996;42:319-24. http://dx.doi.org/10.1046/j.1365-2125.1996.04076.x.
- Yosselson-Superstine S, Weiss T. Drug-related hospitalization in paediatric patients. J Clin Hosp Pharm 1982;7:195-203.
- Schumock GT, Thornton JP. Focusing on the preventability of adverse drug reactions. Hosp Pharm 1992;27.
- Buajordet I, Wesenberg F, Brors O, Langslet A. Adverse drug events in children during hospitalization and after discharge in a Norwegian university hospital. Acta Paediatr 2002;91:88-94. http://dx.doi.org/10.1111/j.1651-2227.2002.tb01647.x.
- Oshikoya KA, Njokanma OF, Chukwura HA, Ojo IO. Adverse drug reactions in Nigerian children. Paediatr Perinat Drug Ther 2007;8:81-8. http://dx.doi.org/10.1185/146300907X199858.
- Lamabadusuriya SP, Sathiadas G. Adverse drug reactions in children requiring hospital admission. Ceylon Med J 2003;48:86-7.
- Impicciatore P, Mohn A, Chiarelli F, Pandolfini C, Bonati M. Adverse drug reactions to off-label drugs on a paediatric ward: an Italian prospective pilot study. Paediatr Perinat Drug Ther 2002;5:19-24. http://dx.doi.org/10.1185/146300902322125118.
- Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet 2000;356:1255-9. http://dx.doi.org/10.1016/S0140-6736(00)02799-9.
- DataPharm Communications . Electronic Medicines Compendium 2010. www.medicines.org.uk/emc/ (accessed 1 March 2010).
- British National Formulary for Children (2008/2009). London: BMJ Group, Pharmaceutical Press and RCPCH Publications; 2009.
- Turner S, Choonara I. Unlicensed drug use in children in the UK. Paediatr Perinat Drug Ther 1997;1:52-5.
- Gallagher RM, Kirkham JJ, Mason JR, Bird KA, Williamson PR, Nunn AJ, et al. Development and inter-rater reliability of the Liverpool Adverse Drug Reaction Causality Assessment Tool. PLOS ONE 2011;6. http://dx.doi.org/10.1371/journal.pone.0028096.
- Hallas J, Harvald B, Gram LF, Grodum E, Brøsen K, Haghfelt T, et al. Drug related hospital admissions: the role of definitions and intensity of data collection, and the possibility of prevention. J Intern Med 1990;228:83-90. http://dx.doi.org/10.1111/j.1365-2796.1990.tb00199.x.
- Rawlins MD, Thompson JW, Davies DM. Textbook of Adverse Drug Reactions. Oxford: Oxford University Press; 1977.
- Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm 1992;49:2229-32.
- The Health and Social Care Information Centre . Hospital Episode Statistics 2010. http://hesonline.nhs.uk (accessed 10 December 2010).
- Zopf Y, Rabe C, Neubert A, Hahn EG, Dormann H. Risk factors associated with adverse drug reactions following hospital admission. Drug Saf 2008;31:789-98. http://dx.doi.org/10.2165/00002018-200831090-00007.
- Davies EC, Green CF, Taylor S, Williamson PR, Mottram DR, Pirmohamed M. Adverse drug reactions in hospital in-patients: a prospective analysis of 3695 patient episodes. PLOS ONE 2009;4. http://dx.doi.org/10.1371/journal.pone.0004439.
- Le PM, Stewart K, Dooley M. The ten most common adverse drug reactions (ADRs) in oncology patients: do they matter to you?. Support Care Canc 2004;12:626-33.
- Sung L, Nathan PC, Lange B, Beyene J, Buchanan GR. Prophylactic granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor decrease febrile neutropenia after chemotherapy in children with cancer: a meta-analysis of randomized controlled trials. J Clin Oncol 2004;22:3350-6. http://dx.doi.org/10.1200/JCO.2004.09.106.
- Sasse Emma C, Sasse Andre D, Brandalise Sílvia R, Clark Otavio Augusto C, Richards S. Colony-stimulating Factors for Prevention of Myelosuppressive Therapy-induced Febrile Neutropenia in Children with Acute Lymphoblastic Leukaemia. Chichester: John Wiley & Sons; 2005.
- Kelly ME, Juern AM, Grossman WJ, Schauer DW, Drolet BA. Immunosuppressive effects in infants treated with corticosteroids for infantile hemangiomas. Arch Dermatol 2010;146:767-74. http://dx.doi.org/10.1001/archdermatol.2010.90.
- Shepherd RW, Turmelle Y, Nadler M, Lowell JA, Narkewicz MR, McDiarmid SV, et al. Risk factors for rejection and infection in pediatric liver transplantation. Am J Transplant 2008;8:396-403. http://dx.doi.org/10.1111/j.1600-6143.2007.02068.x.
- Toruner M, Loftus EV, Harmsen WS, Zinsmeister AR, Orenstein R, Sandborn WJ, et al. Risk factors for opportunistic infections in patients with inflammatory bowel. Gastroenterology 2008;134:929-36. http://dx.doi.org/10.1053/j.gastro.2008.01.012.
- Gluck T, Kiefmann B, Grohmann M, Falk W, Straub RH, Scholmerich J. Immune status and risk for infection in patients receiving chronic. J Rheumatol 2005;32:1473-80.
- Czarnetzki C, Elia N, Lysakowski C, Dumont L, Landis BN, Giger R, et al. Dexamethasone and risk of nausea and vomiting and postoperative bleeding after tonsillectomy in children: a randomized trial. JAMA 2008;300:2621-30. http://dx.doi.org/10.1001/jama.2008.794.
- Steward DL, Grisel J, Meinzen-Derr J. Steroids for improving recovery following tonsillectomy in children. Cochrane Database Syst Rev 2011;8.
- Lewis SR, Nicholson A, Cardwell ME, Siviter G, Smith AF. Nonsteroidal anti-inflammatory drugs and perioperative bleeding in paediatric tonsillectomy. Cochrane Database Syst Rev 2013;7.
- Goldman AC, Govindaraj S, Rosenfeld RM. A meta-analysis of dexamethasone use with tonsillectomy. Arch Otolaryngol Head Neck Surg 2000;123:682-6. http://dx.doi.org/10.1067/mhn.2000.111354.
- Stewart WA, Harrison R, Dooley JM. Respiratory depression in the acute management of seizures. Arch Dis Child 2002;87:225-6. http://dx.doi.org/10.1136/adc.87.3.225.
- McIntyre J, Robertson S, Norris E, Appleton R, Whitehouse WP, Phillips B, et al. Safety and efficacy of buccal midazolam versus rectal diazepam for emergency. Lancet 2005;366:205-10. http://dx.doi.org/10.1016/S0140-6736(05)66909-7.
- McMullan J, Sasson C, Pancioli A, Silbergleit R. Midazolam versus diazepam for the treatment of status epilepticus in children and young adults: a meta-analysis. Acad Emerg Med 2010;17:575-82. http://dx.doi.org/10.1111/j.1553-2712.2010.00751.x.
- Appleton R, Macleod S, Martland T. Drug management for acute tonic–clonic convulsions including convulsive status epilepticus in children. Cochrane Database Syst Rev 2008;16.
- Neubert A, Dormann H, Weiss J, Egger T, Criegee-Rieck M, Rascher W, et al. The impact of unlicensed and off-label drug use on adverse drug reactions in paediatric patients. Drug Saf 2004;27:1059-67. http://dx.doi.org/10.2165/00002018-200427130-00006.
- Santos D, Clavenna A, Bonati M, Coelho H. Off-label and unlicensed drug utilization in hospitalized children in Fortaleza, Brazil. Eur J Clin Pharmacol 2008;64:1111-18. http://dx.doi.org/10.1007/s00228-008-0543-1.
- Horen B, Montastruc JL, Lapeyre-Mestre M. Adverse drug reactions and off-label drug use in paediatric outpatients. Br J Clin Pharmacol 2002;54:665-70. http://dx.doi.org/10.1046/j.1365-2125.2002.t01-3-01689.x.
- Kramer MS, Hutchinson TA, Flegel KM, Naimark L, Contardi R, Leduc DG. Adverse drug reactions in general pediatric outpatients. J Pediatr 1985;106:305-10. http://dx.doi.org/10.1016/S0022-3476(85)80314-0.
- Bellis J, Kirkham J, Thiesen S, Conroy E, Bracken L, Mannix H, et al. Adverse drug reactions and off-label and unlicensed medicines in children: a nested case-control study of inpatients in a paediatric hospital. BMC Med 2013;11. http://dx.doi.org/10.1186/1741-7015-11-238.
- Powell-Jackson PR, Tredger JM, Williams R. Hepatotoxicity to sodium valproate: a review. Gut 1984;25:673-81. http://dx.doi.org/10.1136/gut.25.6.673.
- Rashed AN, Wong IC, Cranswick N, Tomlin S, Rascher W, Neubert A. Risk factors associated with adverse drug reactions in hospitalised children: international multicentre study. Eur J Clin Pharmacol 2012;68:801-10. http://dx.doi.org/10.1007/s00228-011-1183-4.
- Sharfstein JM, North M, Serwint JR. Over the counter but no longer under the radar: pediatric cough and cold medications. N Engl J Med 2007;357:2321-4. http://dx.doi.org/10.1056/NEJMp0707400.
- Srinivasan A, Budnitz D, Shehab N, Cohen A. Infant deaths associated with cough and cold medications. Two states, 2005. Morb Mortal Wkly Rep 2007;56:1-4.
- Demers P, Fraser D, Goldbloom RB, Haworth JC, LaRochelle J, MacLean R, et al. Effects of tetracyclines on skeletal growth and dentition: a report by the Nutrition Committee of the Canadian Paediatric Society. Can Med Assoc J 1968;99:849-54.
- Bateman DN, Rawlins MD, Simpson JM. Extrapyramidal reactions with metoclopramide. BMJ 1985;291:930-2. http://dx.doi.org/10.1136/bmj.291.6500.930.
- Mason J, Pirmohamed M, Nunn T. Off-label and unlicensed medicine use and adverse drug reactions in children: a narrative review of the literature. Eur J Clin Pharmacol 2012;68:21-8. http://dx.doi.org/10.1007/s00228-011-1097-1.
- Cuzzolin L, Atzei A, Fanos V. Off-label and unlicensed prescribing for newborns and children in different settings: a review of the literature and a consideration about drug safety. Expert Opin Drug Saf 2006;5:703-18. http://dx.doi.org/10.1517/14740338.5.5.703.
- Kimland E, Odlind V. Off-label drug use in pediatric patients. Clin Pharmacol Ther 2012;91:796-801. http://dx.doi.org/10.1038/clpt.2012.26.
- S.3187 112th Congress (2011–12): Food and Drug Administration Safety and Innovation Act 2012.
- British National Formulary for Children. London: BMJ Group, Pharmaceutical Press and RCPCH Publications; 2009.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344-9. http://dx.doi.org/10.1016/j.jclinepi.2007.11.008.
- Farrokhi S, Nahvi H, Pourpak Z, Moin M, Majdinasab P, Gholami K, et al. Adverse drug reactions in a Department of Pediatric Surgery. J Trop Pediatr 2006;52:72-3. http://dx.doi.org/10.1093/tropej/fmi058.
- van den Bemt PMLA, Egberts ACG, Lenderink AW, Verzijl JM, Simons KA, van der Pol WSCJM, et al. Risk factors for the development of adverse drug events in hospitalized patients. Pharm World Sci 2000;22:62-6. http://dx.doi.org/10.1023/A:1008721321016.
- Camargo A, Cardoso Ferreira M, Heineck I. Adverse drug reactions: a cohort study in internal medicine units at a university hospital. Eur J Clin Pharmacol 2006;62:143-9. http://dx.doi.org/10.1007/s00228-005-0086-7.
- Diez L. Assessing the willingness of parents to pay for reducing postoperative emesis in children. PharmacoEconomics 1998;13:589-95. http://dx.doi.org/10.2165/00019053-199813050-00011.
- Arnott J, Hesselgreaves H, Nunn AJ, Peak M, Pirmohamed M, Smyth RL, et al. What can we learn from parents about enhancing participation in pharmacovigilance?. Br J Clin Pharmacol 2012;75:1109-17. http://dx.doi.org/10.1111/j.1365-2125.2012.04441.x.
- Epstein RS, Huang SM. The many sides of off-label prescribing. Clin Pharmacol Ther 2012;91:755-8. http://dx.doi.org/10.1038/clpt.2012.37.
- Knibbe CAJ, Krekels EHJ, Danhof M. Advances in paediatric pharmacokinetics. Expert Opin Drug Met 2011;7:1-8. http://dx.doi.org/10.1517/17425255.2011.539201.
- Wester K, Jönsson AK, Spigset O, Druid H, Hägg S. Incidence of fatal adverse drug reactions: a population based study. Br J Clin Pharmacol 2008;65:573-9. http://dx.doi.org/10.1111/j.1365-2125.2007.03064.x.
- World Health Organization (WHO) . Safety of Medicines. A Guide to Detecting and Reporting Adverse Drug Reactions 2002. http://apps.who.int/medicinedocs/en/d/Jh2992e/#Jh2992e.
- Aagaard L, Christensen A, Hansen EH. Information about adverse drug reactions reported in children: a qualitative review of empirical studies. Br J Clin Pharmacol 2010;70:481-91. http://dx.doi.org/10.1111/j.1365-2125.2010.03682.x.
- Easton K, Chapman C, Brien JE. Frequency and characteristics of hospital admissions associated with drug-related problems in paediatrics. Br J Clin Pharmacol 2004;57:611-15. http://dx.doi.org/10.1111/j.1365-2125.2003.02052.x.
- Major S, Badr S, Bahlawan L, Hassan G, Khogaoghlanian T, Khalil R, et al. Drug-related hospitalization at a tertiary teaching center in Lebanon: incidence. Clin Pharmacol Ther 1998;64:450-61. http://dx.doi.org/10.1016/S0009-9236(98)90076-5.
- Santos R, Benjamin G, Paje-Villar E. Drug-related hospitalization among pediatric patients in a tertiary hospital Santo Tomas. J Med 2000;49:141-52.
- Duczmal E, Breborowicz A. Adverse drug reactions as a cause of hospital admission. Prz Pediat 2006;36:14-8.
- Ganeva M, Gancheva T, Lazarova R, Tzvetanova Y, Hristakieva E. A prospective study of adverse drug reactions in a dermatology department. Meth Find Exp Clin Pharmacol 2007;29:107-12. http://dx.doi.org/10.1358/mf.2007.29.2.1075348.
- Fattahi F, Pourpak Z, Moin M, Karemenejad A, Khotaei GT, Mamishi S, et al. Adverse drug reactions in hospitalized children in a department of infectious diseases. J Clin Pharmacol 2005;45:1313-18. http://dx.doi.org/10.1177/0091270005281205.
- Gill AM, Leach HJ, Hughes J, Barker C, Nunn AJ, Choonara I. Adverse drug reactions in a paediatric intensive care unit. Acta Paediatr (Oslo, Norway:1992) 1995;84:438-41. http://dx.doi.org/10.1111/j.1651-2227.1995.tb13667.x.
- Al-Tajir GK, Kelly WN. Epidemiology, comparative methods of detection, and preventability of adverse drug events. Ann Pharmacother 2005;39:1169-74. http://dx.doi.org/10.1345/aph.1E559.
- Baniasadi S, Fahimi F, Shalviri G. Developing an adverse drug reaction reporting system at a teaching hospital. Basic Clin Pharmacol Toxicol 2008;102:408-11. http://dx.doi.org/10.1111/j.1742-7843.2008.00217.x.
- Choonara IA, Harris F. Adverse drug reactions in medical inpatients. Arch Dis Child 1984;59:578-80. http://dx.doi.org/10.1136/adc.59.6.578.
- Dharnidharka VR, Kandoth PN, Anand RK. Adverse drug reactions in pediatrics with a study of in-hospital intensive surveillance. Indian Pediatr 1993;30:745-51.
- dos Santos DB, Coelho HL. Adverse drug reactions in hospitalized children in Fortaleza, Brazil. Pharmacoepidemiol Drug Saf 2006;15:635-40. http://dx.doi.org/10.1002/pds.1187.
- dos Santos L, Martinbiancho J, Silva M, da Silva R. Adverse drug reactions in general pediatrics units of a university hospital. Lat Am J Pharm 2009;28:695-9.
- Easton-Carter K, Chapman C, Brien J. Adverse drug reactions in paediatrics: are we getting the full picture?. J Pharm Pract Res 2003;33:106-10.
- Gonzalez-Martin G, Caroca CM, Paris E. Adverse drug reactions (ADRs) in hospitalized pediatric patients. A prospective study. Int J Clin Pharmacol Ther 1998;36:530-3.
- Jha N, Bajracharya O, Namgyal T. Prevalence of adverse drug reactions with commonly prescribed drugs in different hospitals of Kathmandu Valley. Kathmandu Univ Med J 2007;5:504-10.
- Leach H. Adverse Drug Reactions in Children 1998.
- Mitchell AA, Goldman P, Shapiro S, Slone D. Drug utilization and reported adverse reactions in hospitalized children. Am J Epidemiol 1979;110:196-204.
- Maistrello I, Di Pietro P, Renna S, Boscarini M, Nobili A. A surveillance-oriented medical record as a source of data for both drug and quality of care surveillance. Pharmacoepidemiol Drug Saf 1999;8:131-9. http://dx.doi.org/10.1002/(SICI)1099-1557(199903/04)8:2<131::AID-PDS397>3.0.CO;2-8.
- Martinez-Mir I, Garcia-Lopez M, Palop V, Ferrer JM, Estañ L, Rubio E, et al. A prospective study of adverse drug reactions in hospitalised children. Br J Clin Pharmacol 1999;15:681-8.
- Shockrollah F. Adverse drug and medical instrument reactions in a paediatric intensive care unit. Allergy 2009;64.
- Vazquez de la Villa A, Luna del Castillo JD, Galdo Munoz G, Puche Canas E. Adverse reactions caused by drugs in pediatrics. An Esp Pediatr 1989;31:49-53.
- Cirko-Begovic A, Vrhovac B, Bakran I. Intensive monitoring of adverse drug reactions in infants and preschool children. Eur J Clin Pharmacol 1989;36:63-5. http://dx.doi.org/10.1007/BF00561025.
- Easton-Carter KL, Chapman CB, Brien JE. Emergency department attendances associated with drug-related problems in paediatrics. J Paediatr Child Health 2003;39:124-9. http://dx.doi.org/10.1046/j.1440-1754.2003.00103.x.
- Juntti-Patinen L, Kuitunen T, Pere P, Neuvonen PJ. Drug-related visits to a district hospital emergency room. Basic Clin Pharmacol Toxicol 2006;98:212-17. http://dx.doi.org/10.1111/j.1742-7843.2006.pto_264.x.
- Kaushal R, Goldmann DA, Keohane CA, Christino M, Honour M, Hale AS, et al. Adverse drug events in pediatric outpatients. Ambul Pediatr 2007;7:383-9. http://dx.doi.org/10.1016/j.ambp.2007.05.005.
- Menniti-Ippolito G, Raschetti R, Da Cas R, Giaquinto C, Cantarutti L. Active monitoring of adverse drug reactions in children. Italian Paediatric Pharmacosurveillance Multicenter Group. Lancet 2000;355:1613-14. http://dx.doi.org/10.1016/S0140-6736(00)02219-4.
- Planchamp F, Nguyen KA, Vial T, Nasri S, Javouhey E, Gillet Y, et al. Active drug monitoring of adverse drug reactions in pediatric emergency department. Arch Pediatr 2009;16:106-11. http://dx.doi.org/10.1016/j.arcped.2008.11.013.
- Sanz E, Boada J. Adverse drug reactions in paediatric outpatients. Int J Clin Pharmacol Res 1987;7:169-72.
- Munoz MJ, Ayani I, Rodriguez-Sasiain JM, Gutierrez G, Aguirre C. Adverse drug reaction surveillance in pediatric and adult patients in an emergency room. Med Clin (Barc) 1998;111:92-8.
- Woods CG, Rylance ME, Cullen RE, Rylance GW. Adverse reactions to drugs in children. Br Med J (Clin Res Ed) 1987;294:869-70. http://dx.doi.org/10.1136/bmj.294.6576.869-a.
- Zahraoui M. Study for developing intensive pharmacovigilance system at pediatric emergency department. Fund Clin Pharmacol 2010;24.
- Haffner S, Von Laue N, Wirth S, Thürmann PA. Detecting adverse drug reactions on paediatric wards: intensified surveillance versus computerised screening of laboratory values. Drug Saf 2005;28:453-64. http://dx.doi.org/10.2165/00002018-200528050-00008.
- Speranza N, Lucas L, Telechea H, Santurio A, Giachetto G, Nanni L. Adverse drugs reactions in hospitalized children: a public health problem. Drug Saf 2008;31:885-960.
- Kushwaha KP, Verma RB, Singh YD, Rathi AK. Surveillance of drug induced diseases in children. Indian J Pediatr 1994;61:357-65. http://dx.doi.org/10.1007/BF02751889.
- McKenzie MW, Stewart RB, Weiss CF, Cluff LE. A pharmacist-based study of the epidemiology of adverse drug reactions in children. Am J Hosp Pharm 1973;30:898-903.
- Hewitt J. Drug-related unplanned readmissions to hospital. Aust J Hosp Pharm 1995;25:400-3.
- Ives TJ, Bentz EJ, Gwyther RE. Drug-related admissions to a family medicine inpatient service. Arch Intern Med 1987;147:1117-20. http://dx.doi.org/10.1001/archinte.147.6.1117.
- McDonnell PJ, Jacobs MR. Hospital admissions resulting from preventable adverse drug reactions. Ann Pharmacother 2002;36:1331-6. http://dx.doi.org/10.1345/aph.1A333.
- Van der Hooft CS, Sturkenboom MCJM, Van Grootheest K, Kingma HJ, Stricker BHC. Adverse drug reaction-related hospitalisations: a nationwide study in The Netherlands. Drug Saf 2006;29:161-8. http://dx.doi.org/10.2165/00002018-200629020-00006.
- Bordet R, Gautier S, Le Louet H, Dupuis B, Caron J. Analysis of the direct cost of adverse drug reactions in hospitalised patients. Eur J Clin Pharmacol 2001;56:935-41. http://dx.doi.org/10.1007/s002280000260.
- Kunac DL, Kennedy J, Austin N, Reith D. Incidence, preventability, and impact of Adverse Drug Events (ADEs) and potential ADEs in hospitalized children in New Zealand: a prospective observational cohort study. Paediatr Drugs 2009;11:153-60. http://dx.doi.org/10.2165/00148581-200911020-00005.
- Le J, Nguyen T, Law AV, Hodding J. Retrospective analysis of adverse drug reactions in pediatrics over a 10-year period. Pharmacotherapy 2005;25.
- Whyte J, Greenan E. Drug usage and adverse drug reactions in paediatric patients. Acta Paediatr 1977;66:767-75. http://dx.doi.org/10.1111/j.1651-2227.1977.tb07987.x.
- Pouyanne P, Haramburu F, Imbs JL, Bégaud B. Admissions to hospital caused by adverse drug reactions: cross sectional incidence study. BMJ 2000;320. http://dx.doi.org/10.1136/bmj.320.7241.1036.
- Al-Olah YH, Al Thiab KM. Admissions through the emergency department due to drug-related problems. Ann Saudi Med 2008;28:426-9. http://dx.doi.org/10.4103/0256-4947.51671.
- Schneeweiss S, Hasford J, Gottler M, Hoffmann A, Riethling AK, Avorn J. Admissions caused by adverse drug events to internal medicine and emergency. Eur J Clin Pharmacol 2002;58:285-91. http://dx.doi.org/10.1007/s00228-002-0467-0.
- Van der Hooft CS, Dieleman JP, Siemes C, Aarnoudse AJ, Verhamme KM, Stricker BH, et al. Adverse drug reaction-related hospitalisations: a population-based cohort study. Pharmacoepidemiol Drug Saf 2008;17:365-71. http://dx.doi.org/10.1002/pds.1565.
- Seidl L, Thornton G, Smith J, Cluff L. Studies on the epidemiology of adverse drug reactions III. Reactions in patients on a general medical service. Bull Johns Hopkins Hosp 1966;119:299-315.
- Classen DC, Pestotnik SL, Evans RS, Burke JP. Computerized surveillance of adverse drug events in hospital patients. JAMA 1991;266:2847-51. http://dx.doi.org/10.1001/jama.1991.03470200059035.
- Fincham JE. Pilot study of ADR reporting by physicians: Phase II. ASHP Annual Meeting 1989:1-6.
- Ramesh M, Pandit J, Parthasarathi G. Adverse drug reactions in a south Indian hospital: their severity and cost involved. Pharmacoepidemiol Drug Saf 2003;12:687-92. http://dx.doi.org/10.1002/pds.871.
- Smidt NA, McQueen EG. Adverse reactions to drugs: a comprehensive hospital inpatient survey. N Z Med J 1972;76:397-401.
- Jose J, Rao PG. Pattern of adverse drug reactions notified by spontaneous reporting in an Indian. Pharmacol Res 2006;54:226-33. http://dx.doi.org/10.1016/j.phrs.2006.05.003.
- Barstow L, Vorce-West T, Butcher B. Comparative Study of Three Voluntary ADR Reporting Systems 1988.
- Kaushal R, Bates DW, Landrigan C, McKenna KJ, Clapp MD, Federico F, et al. Medication errors and adverse drug events in pediatric inpatients. JAMA 2001;285:2114-20. http://dx.doi.org/10.1001/jama.285.16.2114.
- Takata GS, Mason W, Taketomo C, Logsdon T, Sharek PJ. Development, testing, and findings of a pediatric-focused trigger tool to identify medication-related harm in US children’s hospitals. Pediatrics 2008;121:e927-35. http://dx.doi.org/10.1542/peds.2007-1779.
- Takata GS, Taketomo CK, Waite S. Characteristics of medication errors and adverse drug events in hospitals. Am J Health Syst Pharm 2008;65:2036-44. http://dx.doi.org/10.2146/ajhp070557.
- Uppal R, Jhaj R, Malhotra S. Adverse drug reactions among inpatients in a north Indian referral hospital. Natl Med J India 2000;13:16-8.
- Wang JK, Herzog NS, Kaushal R, Park C, Mochizuki C, Weingarten SR. Prevention of pediatric medication errors by hospital pharmacists and the potential benefit of computerized physician order entry. Pediatrics 2007;119:e77-85. http://dx.doi.org/10.1542/peds.2006-0034.
- Buckley MS, Erstad BL, Kopp BJ, Theodorou AA, Priestley G. Direct observation approach for detecting medication errors and adverse drug. Pediatr Crit Care Med 2007;8:145-52. http://dx.doi.org/10.1097/01.PCC.0000257038.39434.04.
- Benkirane RR, Abouqal R, Haimeur CC, S Ech Charif El Kattani SS, Azzouzi AA, Mdaghri Alaoui AA, et al. Incidence of adverse drug events and medication errors in intensive care units: a prospective multicenter study. J Patient Saf 2009;5:16-22. http://dx.doi.org/10.1097/PTS.0b013e3181990d51.
- Agarwal S, Classen D, Larsen G, Tofil NM, Hayes LW, Sullivan JE, et al. Prevalence of adverse events in pediatric intensive care units in the United States. Pediatr Crit Care Med 2010;11:568-78. http://dx.doi.org/10.1097/PCC.0b013e3181d8e405.
- Neubert A, Dormann H, Weiss J, Criegee-Rieck M, Ackermann A, Levy M, et al. Are computerised monitoring systems of value to improve pharmacovigilance in paediatric patients?. Eur J Clin Pharmacol 2006;62:959-65. http://dx.doi.org/10.1007/s00228-006-0197-9.
- Weiss J, Krebs S, Hoffmann C, Werner U, Neubert A, Brune K, et al. Survey of adverse drug reactions on a pediatric ward: a strategy for early and detailed detection. Pediatrics 2002;110:254-7. http://dx.doi.org/10.1542/peds.110.2.254.
- Imbs JL, Pouyanne P, Haramburu F, Welsch M, Decker N, Blayac JP, et al. Iatrogenic medication: estimation of its prevalence in French public hospitals. Therapie 1999;54:21-7.
- Doomra R, Gupta S. Intensive adverse drug reaction monitoring in various speciality clinics of a tertiary care hospital in North India. J Med Toxicol Legal Med 2001;4:1-4.
- Phan H, Leder M, Fishley M, Moeller M, Nahata M. Off-label and unlicensed medication use and associated adverse drug events in a pediatric emergency department. Pediatr Emerg Care 2010;26:424-30. http://dx.doi.org/10.1097/PEC.0b013e3181e057e1.
- Calderon-Ospina C, Orozco-Diaz J. Adverse drug reactions as the reason for visiting an emergency department. Rev Salud Publica (Bogota) 2008;10:315-21. http://dx.doi.org/10.1590/S0124-00642008000200012.
- Dennehy CE, Kishi DT, Louie C. Drug-related illness in emergency department patients. Am J Health Syst Pharm 1996;53:1422-6.
- Doval DC, Nath C, Gulati A, Bhargava KP. A survey of adverse effects of drugs in an outpatient population. Indian J Public Health 1981;25:133-8.
- Prince BS, Goetz CM, Rihn TL, Olsky M. Drug-related emergency department visits and hospital admissions. Am J Hosp Pharm 1992;49:1696-700.
- Rebelo Gomes E, Fonseca J, Araujo L, Demoly P. Drug allergy claims in children: from self-reporting to confirmed diagnosis. Clin Exp Allergy 2008;38:191-8.
- Sharma H, Aqil M, Imam F, Alam MS, Kapur P, Pillai KK. A pharmacovigilance study in the department of medicine of a university teaching hospital. Pharm Pract 2007;5:46-9.
- Stoukides CA, D’Agostino PR, Kaufman MB. Adverse drug reaction surveillance in an emergency room. Am J Hosp Pharm 1993;50:712-14.
- Valladares J, Ferrer JM, Palop V, Rubio E, Morales Olivas FJ. Adverse reactions to medications in patients in ambulatory otorhinolaryngology. Acta Otorrinolaringol Esp 1992;43:213-17.
- Otero Lopez MJ, Bajo Bajo A, Maderuelo Fernandez JA, Dominguez-Gil Hurle A. Preventable adverse drug effects at an emergency department. Rev Clin Esp 1999;199:796-805.
- Smith KM, McAdams JW, Frenia ML, Todd MW. Drug-related problems in emergency department patients. Am J Health Syst Pharm 1997;54:295-8.
- Menniti-Ippolito G, Raschetti R, Da Cas R, Giaquinto C, Cantarutti L. Active monitoring of adverse drug reactions in children. Italian Paediatric Pharmacosurveillance Multicenter Group. Lancet 2000;355:1613-14.
- Martys CR. Adverse reactions to drugs in general practice. Br Med J 1979;2:1194-7. http://dx.doi.org/10.1136/bmj.2.6199.1194.
- Lemer C, Bates DW, Yoon C, Keohane C, Fitzmaurice G, Kaushal R. The role of advice in medication administration errors in the pediatric ambulatory setting. J Patient Saf 2009;5:168-75. http://dx.doi.org/10.1097/PTS.0b013e3181b3a9b0.
- Miller GC, Britth HC, Valenti L. Adverse drug events in general practice patients in Australia. Med J Aust 2006;184:321-4.
- Mulroy R. Iatrogenic disease in general practice: its incidence and effects. Br Med J 1973;2:407-10. http://dx.doi.org/10.1136/bmj.2.5863.407.
- Lewinski D, Wind S, Belgardt C, Plate V, Behles C, Schweim HG. Prevalence and safety-relevance of drug-related problems in German community. Pharmacoepidemiol Drug Saf 2010;19:141-9. http://dx.doi.org/10.1002/pds.1861.
- Knopf H, Du Y. Perceived adverse drug reactions among non-institutionalized children and adolescents in Germany. Br J Clin Pharmacol 2010;70:409-17. http://dx.doi.org/10.1111/j.1365-2125.2010.03713.x.
- Campbell WH, Johnson RE, Senft RA, Azevedo DJ. Adverse drug reactions in a disadvantaged population. J Community Health 1978;3:205-15. http://dx.doi.org/10.1007/BF01349383.
- Agbabiaka TB, Savović J, Ernst E. Methods for causality assessment of adverse drug reactions: a systematic review. Drug Saf 2008;31:21-37. http://dx.doi.org/10.2165/00002018-200831010-00003.
- Arimone Y, Bidault I, Colignon AE, Gerardin M, Guy C, Haramburu F, et al. Updating of the French causality assessment method. Fund Clin Pharmacol 2010;24.
- Laine L, Goldkind L, Curtis SP, Connors LG, Yanqiong Z, Cannon CP. How common is diclofenac-associated liver injury? Analysis of 17,289 arthritis patients in a long-term prospective clinical trial. Am J Gastroenterol 2009;104:356-62. http://dx.doi.org/10.1038/ajg.2008.149.
- Turner WM. The Food and Drug Administration algorithm. Special workshop: regulatory. Drug Inform J 1984;18:259-66.
- Kling A, Mjorndal T, Rantapaa-Dahlqvist S. Sepsis as a possible adverse drug reaction in patients with rheumatoid arthritis treated with TNF alpha antagonists. J Clin Rheumatol 2004;10:119-22. http://dx.doi.org/10.1097/01.rhu.0000128734.07926.8e.
- Macedo AF, Marques FB, Ribeiro CF, Teixeira F. Causality assessment of adverse drug reactions: comparison of the results obtained from published decisional algorithms and from the evaluations of an expert panel. Pharmacoepidemiol Drug Saf 2005;14:885-90. http://dx.doi.org/10.1002/pds.1138.
- Bradford Hill A. The environment and disease: association or causation?. Proc R Soc Med 1965;58:295-300.
- Irey NS. Tissue reactions to drugs. Am J Pathology 1976;82:613-47.
- Arimone Y, Begaud B, Miremont-Salame G, Fourrier-Reglat A, Moore N, Molimard M, et al. Agreement of expert judgment in causality assessment of adverse drug reactions. Eur J Clin Pharmacol 2005;61:169-73. http://dx.doi.org/10.1007/s00228-004-0869-2.
- Jones JK, Strom Bl. Pharmacoepidemiology. New York, NY: John Wiley & Sons; n.d.
- Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18,820 patients. BMJ 2004;329:15-9. http://dx.doi.org/10.1136/bmj.329.7456.15.
- Garcia-Cortes M, Lucena MI, Pachkoria K, Borraz Y, Hidalgo R, Andrade RJ. Evaluation of Naranjo Adverse Drug Reactions Probability Scale in causality assessment of drug-induced liver injury. Aliment Pharmacol Ther 2008;27:780-9. http://dx.doi.org/10.1111/j.1365-2036.2008.03655.x.
- Kane-Gill SL, Kirisci L, Pathak DS. Are the Naranjo criteria reliable and valid for determination of adverse drug reactions in the intensive care unit?. Ann Pharmacother 2005;39:1823-7. http://dx.doi.org/10.1345/aph.1G177.
- Fleiss JL. Measuring nominal scale agreement among many raters. Psychol Bull 1971;76:378-82. http://dx.doi.org/10.1037/h0031619.
- Altman DG. Practical Statistics for Medical Research. London: Chapman & Hall; 1991.
- Cicchetti DV, Feinstein AR. High agreement but low kappa: II. Resolving the paradoxes. J Clin Epidemiol 1990;43:551-8. http://dx.doi.org/10.1016/0895-4356(90)90159-M.
- Feinstein AR, Cicchetti DV. High agreement but low kappa: I. The problems of two paradoxes. J Clin Epidemiol 1990;43:543-9. http://dx.doi.org/10.1016/0895-4356(90)90158-L.
- Lantz CA, Nebenzahl E. Behavior and interpretation of the kappa statistic: resolution of the two paradoxes. J Clin Epidemiol 1996;49:431-4. http://dx.doi.org/10.1016/0895-4356(95)00571-4.
- Danan G, Benichou C. Causality assessment of adverse reactions to drugs. 1. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol 1993;46:1323-30. http://dx.doi.org/10.1016/0895-4356(93)90101-6.
- Koh Y, Li SC. A new algorithm to identify the causality of adverse drug reactions. Drug Saf 2005;28:1159-61. http://dx.doi.org/10.2165/00002018-200528120-00010.
- Pharmacovigilance: Ensuring the Safe Use of Medicines – WHO Policy Perspectives on Medicines. Geneva: WHO; 2004.
- European Medicines Agency . The ENCePP Code of Conduct for Scientific Independence and Transparency in the Conduct of Pharmacoepidemiological and Pharmacovigilance Studies 2010. www.encepp.eu/code_of_conduct/documents/CodeofConduct_Rev2.pdf (accessed 18 November 2013).
- Expert Group on Safe Medication Practices . Creation of a Better Medication Safety Culture in Europe: Building up Safe Medication Practices 2006. www.coe.int/t/e/social_cohesion/soc-sp/medication%20safety%20culture%20report%20e.pdf (accessed 18 November 2012).
- Hakkarainen KM, Hedna K, Petzold M, Hägg S. Percentage of patients with preventable adverse drug reactions and preventability of adverse drug reactions: a meta-analysis. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0033236.
- Aronson JK, Ferner RE. Preventability of drug-related harms: part II – proposed criteria, based on frameworks that classify adverse drug reactions. Drug Saf 2010;33:995-1002. http://dx.doi.org/10.2165/11538280-000000000-00000.
- Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ 2010;182:E839-42. http://dx.doi.org/10.1503/cmaj.090449.
- Dormann H, Criegee-Rieck M, Neubert A, Egger T, Geise A, Krebs S, et al. Lack of awareness of community-acquired adverse drug reactions upon hospital. Drug Saf 2003;26:353-62. http://dx.doi.org/10.2165/00002018-200326050-00004.
- Ducharme MM, Boothby LA. Analysis of adverse drug reactions for preventability. Int J Clin Pract 2007;61:157-61. http://dx.doi.org/10.1111/j.1742-1241.2006.01130.x.
- Olivier P, Caron J, Haramburu F, Imbs JL, Jonville-Béra AP, Lagier G, et al. Validation of a measurement scale: example of a French Adverse Drug Reactions Preventability Scale. Therapie 2005;60:39-45. http://dx.doi.org/10.2515/therapie:2005005.
- Pound P, Britten N, Morgan M, Yardley L, Pope C, Daker-White G, et al. Resisting medicines: a synthesis of qualitative studies of medicine taking. Soc Sci Med 2005;61:133-55. http://dx.doi.org/10.1016/j.socscimed.2004.11.063.
- ‘t Jong GW, Choonara I, Stricker BH, van den Anker JN. Lack of effect of the European guidance on clinical investigation of medicines in children. Clin Pharmacol Ther 2002;71.
- Mukattash TL, Millership JS, Collier PS, McElnay JC. Public awareness and views on unlicensed use of medicines in children. Br J Clin Pharmacol 2008;66:838-45. http://dx.doi.org/10.1111/j.1365-2125.2008.03290.x.
- Yoon EY, Clark SJ, Butchart A, Singer D, Davis MM. Parental preferences for FDA-approved medications prescribed for their children. Clin Pediatr 2011;50:208-14. http://dx.doi.org/10.1177/0009922810385105.
- Grinyer A. Young adults with cancer: parents’ interactions with health care professionals. Eur J Cancer Care 2004;13:88-95. http://dx.doi.org/10.1111/j.1365-2354.2004.00458.x.
- Berry DC. Interpreting information about medication side effects: differences in risk perception and intention to comply when medicines are prescribed for adults or young children. Psychol Health Med 2004;9:227-34. http://dx.doi.org/10.1080/13548500410001670753.
- Medicines and Healthcare products Regulatory Agency (MHRA) . Healthcare Professional Reporting: Adverse Drug Reactions n.d. http://yellowcard.mhra.gov.uk/ (accessed August 2010).
- Smith CC, Bennett PM, Pearce HM, Harrison PI, Reynolds DJ, Aronson GK, et al. Adverse drug reactions in a hospital general medical unit meriting notification to the Committee on Safety of Medicines. Br J Clin Pharmacol 1996;42:423-9. http://dx.doi.org/10.1046/j.1365-2125.1996.04376.x.
- Vallano A, Cereza G, Pedros C, Agustí A, Danés I, Aguilera C, et al. Obstacles and solutions for spontaneous reporting of adverse drug reactions in the hospital. Br J Clin Pharmacol 2005;60:653-8. http://dx.doi.org/10.1111/j.1365-2125.2005.02504.x.
- Anderson C, Krska J, Murphy E, Avery A. The importance of direct patient reporting of suspected adverse drug reactions: a patient perspective. Br J Clin Pharmacol 2011;72:806-22. http://dx.doi.org/10.1111/j.1365-2125.2011.03990.x.
- Aagaard L, Lars Hougaard N, Ebba Holme H. Consumer reporting of adverse drug reactions: a retrospective analysis of the Danish Adverse Drug Reaction Database from 2004 to 2006. Drug Saf 2009;32:1067-74. http://dx.doi.org/10.2165/11316680-000000000-00000.
- McLernon DJ, Bond CM, Hannaford PC, Watson MC, Lee AJ, Hazell L, et al. Adverse drug reaction reporting in the UK: a retrospective observational comparison of yellow card reports submitted by patients and healthcare professionals. Drug Saf 2010;33:775-88. http://dx.doi.org/10.2165/11536510-000000000-00000.
- Tobaiqy M, Stewart D, Helms PJ, Williams J, Crum J, Steer C, et al. Parental reporting of adverse drug reactions associated with attention-deficit hyperactivity disorder (ADHD) medications in children attending specialist paediatric clinics in the UK. Drug Saf 2011;34:211-19. http://dx.doi.org/10.2165/11586050-000000000-00000.
- Blenkinsopp A, Wilkie P, Wang M, Routledge PA. Patient reporting of suspected adverse drug reactions: a review of published literature and international experience. Br J Clin Pharmacol 2007;63:148-56. http://dx.doi.org/10.1111/j.1365-2125.2006.02746.x.
- van Grootheest K, de Jong-van den Berg L. Patients’ role in reporting adverse drug reactions. Expert Opin Drug Saf 2004;3:363-8. http://dx.doi.org/10.1517/14740338.3.4.363.
- Ekins-Daukes S, Irvine D, Wise L, Fiddes S. The yellow card scheme: evaluation of patient reporting of suspected adverse drug reactions. Pharmacoepidemiol Drug Saf 2006;15.
- van Hunsel F, van der Welle C, Passier A, van Puijenbroek E, van Grootheest K. Motives for reporting adverse drug reactions by patient-reporters in the Netherlands. Eur J Clin Pharmacol 2010;66:1143-50. http://dx.doi.org/10.1007/s00228-010-0865-7.
- ‘t Jong GW, Eland IA, Sturkenboom MCJM, van den Anker JN, Stricker BHC. Unlicensed and off label prescription of drugs to children: population based cohort study. BMJ 2002;324:1313-14. http://dx.doi.org/10.1136/bmj.324.7349.1313.
- Ball LK, Evans G, Bostrom A. Risky business: challenges in vaccine risk communication. Pediatrics 1998;101:453-8. http://dx.doi.org/10.1542/peds.101.3.453.
- Bellaby P. Communication and miscommunication of risk: understanding UK parents’ attitudes to combined MMR vaccination. BMJ 2003;327:725-8. http://dx.doi.org/10.1136/bmj.327.7417.725.
- Casiday RE, Cox AR. Restoring confidence in vaccines by explaining vaccine safety monitoring: is a targeted approach needed?. Drug Saf 2006;29:1105-9. http://dx.doi.org/10.2165/00002018-200629120-00002.
- Mills E, Jadad AR, Ross C, Wilson K. Systematic review of qualitative studies exploring parental beliefs and attitudes toward childhood vaccination identifies common barriers to vaccination. J Clin Epidemiol 2005;58:1081-8. http://dx.doi.org/10.1016/j.jclinepi.2005.09.002.
- Gust DA, Darling N, Kennedy A, Schwartz B. Parents with doubts about vaccines: which vaccines and reasons why. Pediatrics 2008;122:718-25. http://dx.doi.org/10.1542/peds.2007-0538.
- Hobson-West P. Understanding vaccination resistance: moving beyond risk. Health Risk Soc 2003;5:273-83. http://dx.doi.org/10.1080/13698570310001606978.
- Kai J. What worries parents when their preschool children are acutely ill, and why: a qualitative study. BMJ 1996;313:983-6. http://dx.doi.org/10.1136/bmj.313.7063.983.
- Patton MQ. Qualitative Research and Evaluation Methods. London: Sage Publications; 2002.
- Patton MQ. Enhancing the quality and credibility of qualitative analysis. Health Serv Res 1999;34:1189-208.
- Bowen GA. Naturalistic inquiry and the saturation concept: a research note. Qual Res 2008;8:137-52. http://dx.doi.org/10.1177/1468794107085301.
- Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P. Qualitative research methods in health technology assessment: a review of the literature. Health Technol Assess 1998;2.
- Boeije H. Analysis in Qualitative Research. London: Sage Publications Ltd.; 2010.
- Charmaz K. Grounded theory. Objectivist and Constructivist Methods. London: Sage Publications; 2003.
- Glaser B, Strauss A. Discovery of Grounded Theory. Chicago, IL: Aldine Publishing; 1967.
- Strauss A, Corbin C. Basics of Qualitative Research. Thousand Oaks, CA: Sage Publications; 1998.
- Bryman A, Burgess R. Analysis in Qualitative Research. London: Routledge; 2004.
- Pope C, Ziebland S, Mays N. Analysing Qualitative Data. BMJ 2000;320. http://dx.doi.org/10.1136/bmj.320.7227.114.
- Lincoln Y, Guba E. Naturalistic Inquiry. Newbury Park, CA: Sage Publications; 1985.
- Walker D, Myrick F. Grounded theory: an exploration of process and procedure. Qual Health Res 2006;16:547-59. http://dx.doi.org/10.1177/1049732305285972.
- Hammersely M. Social Research. London: Sage Publications; 1992.
- Denzin N. The Research Act in Sociology. Chicago, IL: Aldine; 1970.
- Janesick VJ, Denzin NK, Lincoln YS. Handbook of Qualitative Research. London: Sage Publications; 1994.
- Britten N. Patients’ ideas about medicines: a qualitative study in a general practice population. Br J Gen Pract 1994;44:465-8.
- Leickly FE, Wade SL, Crain E, Kruszon-Moran D, Wright EC, Evans R. Self-reported adherence, management behavior, and barriers to care after an emergency department visit by inner city children with asthma. Pediatrics 1998;101. http://dx.doi.org/10.1542/peds.101.5.e8.
- Duclos CW, Eichler M, Taylor L, Quintela J, Main DS, Pace W, et al. Patient perspectives of patient–provider communication after adverse events. Int J Qual Health Care 2005;17:479-86. http://dx.doi.org/10.1093/intqhc/mzi065.
- Gallagher TH, Studdert D, Levinson W. Disclosing harmful medical errors to patients. N Engl J Med 2007;356:2713-19. http://dx.doi.org/10.1056/NEJMra070568.
- McLernon DJ, Bond CM, Lee AJ, Watson MC, Hannaford PC, Fortnum H, et al. Patient views and experiences of making adverse drug reaction reports to the Yellow Card Scheme in the UK. Pharmacoepidemiol Drug Saf 2011;20:523-31. http://dx.doi.org/10.1002/pds.2117.
- Anderson C, Gifford A, Avery A, Fortnum H, Murphy E, Krska J, et al. Assessing the usability of methods of public reporting of adverse drug reactions to the UK Yellow Card Scheme. Health Expect 2012;15:433-40. http://dx.doi.org/10.1111/j.1369-7625.2011.00686.x.
- Tarn DM, Paterniti DA, Williams BR, Cipri CS, Wenger NS. Which providers should communicate which critical information about a new medication? Patient, pharmacist, and physician perspectives. J Am Geriatr Soc 2009;57:462-9. http://dx.doi.org/10.1111/j.1532-5415.2008.02133.x.
- Sleath B, Carpenter D, Sayner R, Ayala GX, Williams D, Davis S, et al. Child and caregiver involvement and shared decision-making during asthma pediatric visits. J Asthma 2011;48:1022-31. http://dx.doi.org/10.3109/02770903.2011.626482.
- Dy S, Purnell T. Key concepts relevant to quality of complex and shared decision-making in health care: a literature review. Soc Sci Med 2012;74:582-7. http://dx.doi.org/10.1016/j.socscimed.2011.11.015.
- Glass K, Wills C, Holloman C, Olson J, Hechmer C, Miller CK, et al. Shared decision making and other variables as correlates of satisfaction with health care decisions in a United States national survey. Patient Educ Counsel 2012;88:100-5. http://dx.doi.org/10.1016/j.pec.2012.02.010.
- Gravel K, Legare F, Graham ID. Barriers and facilitators to implementing shared decision-making in clinical practice: a systematic review of health professionals’ perceptions. Implement Sci 2006;1. http://dx.doi.org/10.1186/1748-5908-1-16.
- The European Parliament and the Council of the European Union . Regulation (EC) No. 1901/2006 of the European Parliament and of the Council of 12 December 2006 on medicinal products for paediatric use and amending Regulation (EEC) No. 1768/92, Directive 2001/20/EC, Directive 2001/83/EC and Regulation (EC) No 726/2004. OJEU 2006;49:1-19.
- Temple ME, Robinson RF, Miller JC, Hayes JR, Nahata MC. Frequency and preventability of adverse drug reactions in paediatric patients. Drug Saf 2004;27:819-29. http://dx.doi.org/10.2165/00002018-200427110-00005.
- Bégaud B, Evreux JC, Jouglard J, Lagier G. Imputation of the unexpected or toxic effects of drugs. Actualization of the method used in France. Therapie 1985;40:111-18.
- Bates DW, Boyle DL, Vander Vliet MB, Schneider J, Leape L. Relationship between medicationerrors and adverse drug events. J Gen Intern Med 1995;10:199-205. http://dx.doi.org/10.1007/BF02600255.
- Hutchinson TA, Leventhal JM, Kramer MS, Karch FE, Lipman AG, Feinstein AR. An algorithm for the operational assessment of adverse drug reactions. II. Demonstration of reproducibility and validity. JAMA 1979;247:633-8. http://dx.doi.org/10.1001/jama.242.7.633.
- Strand L, Morley P, Cipolle R, Ramsey R, Lamsam GD. Drug-related problems: their structure andfunction. Ann Pharmacother 1990;24:1093-7.
- Stephens MDB, Stephens MDB, Talbot JC, Routledge PA. The Detection of New Adverse Reactions. Chichester: John Wiley & Sons; 1998.
- Dartnell JGA, Anderson RP, Chohan V, Galbraith KJ, Lyon ME, Nestor PJ, et al. Hospitalisation for adverse events related to drug therapy: incidence, avoidability and costs. Med J Aust 1996;164:659-62.
- Kramer MS, Leventhal JM, Hutchinson TA, Feinstein AR. An algorithm for the operational assessment of adverse drug reactions. I. Background, description, and instructions for use. JAMA 1979;242:623-32. http://dx.doi.org/10.1001/jama.242.7.623.
- Naranjo CA, Busto U, Kalant H, Roschlau W. Principles of Medical Pharmacology. Toronto: BC Decker; 1989.
- Lau PM, Stewart K, Dooley MJ. Comment: hospital admissions resulting from preventable adverse drug reactions. Ann Pharmacother 2003;37:303-4. http://dx.doi.org/10.1345/aph.1A333a.
- Zandieh SO, Goldmann DA, Keohane CA, Yoon C, Bates DW, Kaushal R. Risk factors in preventable adverse drug events in pediatric outpatients. J Pediatr 2008;152:225-31. http://dx.doi.org/10.1016/j.jpeds.2007.09.054.
- Kunac DL, Reith DM. Preventable medication-related events in hospitalised children in New Zealand. N Z Med J 2008;121:17-32.
- Karch FE, Lasagna L. Toward the operational identification of adverse drug reactions. Clin Pharmacol Ther 1977;21:247-54.
- Meyboom RHB, Royer RJ. Causality classification at pharmacovigilance centers in the Europeancommunity. Pharmacoepidemiol Drug Safety 1992;1:87-9. http://dx.doi.org/10.1002/pds.2630010207.
- Thomas EJ, Brennan TA. Incidence and types of preventable adverse events in elderly patients:population based review of medical records. BMJ 2000;320:741-4. http://dx.doi.org/10.1136/bmj.320.7237.741.
- Lacouture PG, Mitchell AA, Sheehan J, Shapiro S. Adverse drug reactions leading to hospital admission. Pediatric Research 1986;20.
- Jones JK. Adverse drug reactions in the community health setting: approaches to recognizing, counseling, and reporting. Fam Community Health 1982;5:58-67. http://dx.doi.org/10.1097/00003727-198208000-00009.
- Michel DJ, Knodel LC. Program coordinated by a drug information service to improve adversedrug reaction reporting in a hospital. Am J Hosp Pharm 1986;43:2202-5.
- Venulet J, Ciucci AG, Berneker GC. Updating of a method for causality assessment of adversedrug reactions. Int J Clin Pharmacol Ther Toxicol 1986;24:559-68.
- Dangoumau J, Evreux JC, Jouglard J. Methode d’imputabilite’des effets indesirables desmedicaments. Therapie 1978;33:373-81.
- Blanc S, Levenberger P, Berger JP, Brooke EM, Schelling JL. Judgements of trained observers on adverse drug reactions. Clin Pharmacol Ther 1979;25:493-8.
- Smidt NA, McQueen EG. Adverse drug reactions in a general hospital. N Z Med J 1973;78.
- Evans RS, Pestotnik SL, Classen DC, Horn SD, Bass SB, Burke JP. Preventing adverse drug events in hospitalized patients. Ann Pharmacother 1994;28:523-7.
- Seidl LG, Thornton GF, Cluff LE. Epidemiological studies of adverse drug reactions. Am J Pub Health 1965;55:1170-5. http://dx.doi.org/10.2105/AJPH.55.8.1170.
Appendix 1 Databases searched
| Database | Period covered |
|---|---|
| MEDLINE via Ovid | 1950 to October 2010 |
| EMBASE via NHS Evidence Health Information Resource | 1980 to October 2010 |
| CINAHL via NHS Evidence Health Information Resources | 1981 to October 2010 |
| Science Citation Index | 1990 to October 2010 |
| Biological Abstracts | 1926 to October 2010 |
| International Pharmaceutical Abstracts | 1970 to October 2010 |
| Toxicology Literature Online – USA National Library of Medicine | Searched October 2010 |
| Iowa Drug Information Service | 1966 to October 2010 |
| Allied and Complementary Medicine Database | 1985 to October 2010 |
| General Practice Research Database | 1987 to October 2010 |
| Database of Systematic Reviews (The Cochrane Library) | Searched October 2010 |
| Database of Abstracts of Reviews of Effects | Searched October 2010 |
| Health Technology Assessment programme | Searched October 2010 |
| National Institutes of Health | Searched October 2010 |
| EMA | Searched October 2010 |
| US FDA | Searched October 2010 |
| ClinicalTrials.gov | Searched October 2010 |
| Agency for Healthcare Research and Quality | Searched October 2010 |
| Incidence and Prevalence | Searched November 2010 |
Appendix 2 Search strategy
-
exp Child/
-
exp Adolescent/
-
(young adj (person$ or people or adult$ or individual$ or women or woman or men or man)).ti,ab.
-
(child$ or adolescen$ or kid or kids or youth$ or youngster$ or minor or minors or teen$ or juvenile$ or student$ or pupil$ or boy$ or girl$).ti,ab.
-
exp Students/
-
Puberty/
-
Pediatrics/
-
(infan$ or newborn$ or new born$ or baby$ or babies or child$ or schoolchild$ or kid or kids or toddler$ or adoles$ or teen$ or boy$ or girl$ or minor$ or juvenil$ or youth$ or kindergar$ or nurser$ or puber$ or prepuber$ or pre puber$ or pubescen$ or prepubescen$ or pre pubescen$ or pediatric$ or paediatric$ or schoolage$).ti,ab.
-
9. side effect$.ti,ab.
-
10. (drug induced or drug related or drug safety).ti,ab.
-
11. tolerability.ti,ab.
-
12. toxicity.ti,ab.
-
13. Harm$.ti,ab.
-
14. adrs.ti,ab.
-
15. (adverse adj2 (effect or effects or reaction or reactions or event or events or outcome or outcomes)).ti,ab.
-
16. (toxic adj3 (effect$ or reaction$ or event$ or outcome$)).ti,ab.
-
17. exp product surveillance, postmarketing/ or exp adverse drug reaction reporting systems/ or exp drug toxicity/ or exp abnormalities, drug induced/ or exp drug hypersensitivity/
-
18. incidence/ or prevalence/
-
19. (incidence$ or prevalence$ or occurrence or admission$ or admitted or visit$ or hospitalisation or hospitalised or hospitalization or hospitalized).ti,ab.
-
20. (drug$ or pharmaceutical$ or medicin$).ti,ab.
-
21. Pharmaceutical Preparations/
-
22. (herbal$ or plant or plants or herb or herbs or aromatherap$ or aroma therap$).ti,ab.
-
23. Medicine, Chinese Traditional/ or Plant Preparations/ or Plants, Medicinal/ or Plant Extracts/ or Drugs, Chinese Herbal/
-
24. Aromatherapy/
-
25. Health Care Surveys/
-
26. Retrospective Studies/
-
27. Prospective Studies/
-
28. Cohort Studies/
-
29. Observational stud$.ti,ab.
-
30. (prospectiv$ adj3 review$).ti,ab.
-
31. (prospectiv$ adj3 stud$).ti,ab.
-
32. (retrospectiv$ adj3 stud$).ti,ab.
-
33. (retrospectiv$ adj3 review$).ti,ab.
-
34. population-based stud$.ti,ab.
-
35. cohort stud$.ti,ab.
-
36. incidence stud$.ti,ab.
-
37. Sn.fs.
-
38. Ep.fs.
-
39. monitor$.ti,ab.
-
40. surveillance.ti,ab.
Appendix 3 Study characteristics
| Study | Country | Study duration/design | Clinical setting | Population | Causality assessment | Avoidability assessment |
|---|---|---|---|---|---|---|
| ADRIC | UK | 12 months Prospective |
Causing admission, large tertiary – paediatric hospital | Children | Naranjo LCAT |
Hallas 199054 |
| Agarwal (2010)164 | USA | 4 months Retrospective |
In hospital, PICU | Children | Not reported | ADEs assessed, non-preventable = ADR. Determined by individual sites based on local interpretations; in general was based on the premise that the ADE may have been avoidable, given the appropriate implementation of evidence-based medicine and/or appropriate use of available services |
| Al-Olah (2008)147 | Saudi Arabia | 28 days Prospective |
Causing admission, emergency department | Children and adults | Naranjo | Definite preventable and definite non-preventable defined as three evaluators in agreement; possible preventable and possible non-preventable – two in agreement |
| Al-Tajir (2005)109 | United Arab Emirates | 12 months Prospective |
Causing admission, in hospital and in community, general paediatric ward | Children and adults | Naranjo score algorithm | Schumock 199244 |
| Baniasadi (2008)110 | Iran | 12 months Prospective |
Causing admission and in hospital, multidisciplinary hospital | Children and adults | Naranjo score algorithm | Schumock 199244 |
| Barstow (1988)156 | USA | 4 months Prospective |
Paediatric units | Children and adults | Not reported | Not reported |
| Benkirane (2009)163 | Morocco | 3 months Prospective |
In hospital, intensive care unit | Children and adults | Not reported | ADEs assessed, non-preventable = ADR |
| Bordet (2001)142 | France | 18 months Prospective |
Causing admission and in hospital General paediatric ward |
Children and adults | Begaud 1985270 | Not reported |
| Buajordet (2002)45 | Norway | 5 months Prospective |
Causing admission, in hospital and in community, general paediatric ward | Children | Naranjo score algorithm | Not reported |
| Buckley (2007)162 | USA | 12 days Prospective |
In hospital, PICU | Children | Not reported | ADEs assessed using Bates 1995,271 non-preventable ADE = ADR |
| Calderon-Ospina (2008)170 | Colombia | 12 days Prospective |
In community, A&E visits | Children and adults | WHO | Schumock 199244 |
| Campbell (1978)187 | USA | 48 months Prospective |
In community, medical care contacts | Children and adults | Not reported | Not reported |
| Choonara (1984)111 | UK | 6 months Prospective |
In hospital, general paediatric ward | Children | Seidl 1966150 | Six avoidable because three doses prescribed too high, one treatment not necessary, two applications of pharmacological principles would have prevented reactions |
| Cirko-Begovic (1989)124 | Croatia | 3 months Prospective |
In community, general paediatric outpatient unit | Children | Hutchinson 1979272 | Not reported |
| Classen (1991)151 | USA | 18 months Prospective |
Causing admission, acute care referral hospital | Children and adults | Naranjo score algorithm | Not reported |
| Dennehy (1996)171 | USA | 1 month Retrospective |
In community, emergency department | Children and adults | Strand 1990273 | Considered preventable if they could have been avoided through appropriate prescribing, outpatient monitoring or patient compliance |
| Dharnidharka (1993)112 | India | 18 months Prospective |
In hospital, paediatric unit | Children | Stephens 1998274 | Not reported |
| Doomra (2001)168 | India | 15 months Prospective |
In hospital and in community General paediatric outpatient unit |
Children and adults | Naranjo score algorithm | Not reported |
| dos Santos (2009)114 | Brazil | 2 years Prospective |
In hospital, general paediatric ward | Children | Naranjo | Not reported |
| dos Santos (2006)113 | Brazil | 5 months Prospective |
In hospital, general paediatric ward | Children | WHO | Not reported |
| Doval (1981)172 | India | Not reported Prospective |
In community, outpatient department | Children and adults | Not reported | Not reported |
| Duczmal (2006)105 | Poland | Not reported Retrospective |
Causing admission, paediatric department | Children | Naranjo | Not reported |
| Easton (1998)41 | Australia | 56 days Prospective |
Causing admission, medical ward | Children | Naranjo score algorithm | Schumock 199244 |
| Easton (2004)102 | Australia | 22 weeks Prospective |
Causing admission, specialist paediatric teaching hospital and general regional teaching hospital | Children | Dartnell 1996275 | Schumock 199244 |
| Easton-Carter (2003)115 | Australia | 18 weeks Prospective |
In community, emergency department | Children | Dartnell 1996275 | Schumock 199244 |
| Easton-Carter (2003)125 | Australia | 39 weeks Prospective and retrospective |
In hospital, general paediatric ward | Children | Naranjo score algorithm | Schumock 199244 |
| Farrokhi (2006)92 | Iran | 5 months Prospective |
In hospital, paediatric surgery | Children | Not reported | Not reported |
| Fattahi (2005)107 | Iran | 5 months Prospective |
Causing admission and in hospital Paediatric disease referral centre, paediatric infectious diseases department |
Children | WHO | Not reported |
| Fincham (1989)152 | USA | Not reported Not reported |
Causing admission and in hospital; hospital and private practice | Children and adults | Not reported | Not reported |
| Gallagher 201120 | UK | 2 weeks Prospective |
Causing admission, large tertiary – paediatric hospital | Children | Naranjo | Hallas 199054 |
| Ganeva 2007106 | Bulgaria | 5 years Prospective |
Causing admission, dermatology and venereology | Children and adults | Naranjo score algorithm | Not reported |
| Gill (1995)108 | UK | 28 months Prospective |
Causing admission and in hospital, PICU | Children | Kramer 1979276 | Not reported |
| Gonzalez-Martin 1998116 | Chile | 1 year Prospective |
In hospital, paediatric wards | Children | Naranjo score algorithm | Naranjo 1989277 |
| Haffner (2005)134 | Germany | 91 days; 80 days; overlap of 52 days Prospective |
Causing admission and in hospital ICU, general paediatric ward, department of paediatrics |
Children | WHO | Not reported |
| Hewitt (1995)138 | Australia | 4 months Retrospective |
Causing admission, general teaching hospital | Children and adults | Not reported | Not reported |
| Horen (2002)77 | France | Not reported Prospective |
In community, office-based practice | Children | Begaud 1985270 | Not reported |
| Imbs (1999)167 | France | 1 day Prospective |
In hospital, departments of medicine, surgery and geriatrics | Children and adults | Two members of the pharmacovigilance team validated each ADR | Not reported |
| Impicciatore (2002)48 | Italy | 9 months Prospective |
Causing admission and in hospital, paediatric unit | Children | WHO – confirmed by author | Not reported |
| Ives (1987)139 | USA | 1 year Retrospective |
Causing admission, family medicine inpatient service at hospital | Children and adults | Naranjo score algorithm | Not reported |
| Jha (2007)117 | Nepal | 5 months Prospective |
In hospital, general paediatric ward | Children and adults | Naranjo score algorithm | Not reported |
| Jonville-Bera (2002)37 | France | 1 week Prospective |
Causing admission, in hospital and in community, paediatric wards, A&E, private paediatricians | Children | Begaud 1985270 | Not reported |
| Jose and Rao (2006)155 | India | 12 months Prospective |
Causing admission, in hospital and in community, various departments (not stated) | Children and adults | Naranjo score algorithm | Lau 2003278 |
| Juntti-Patinen (2006)126 | Finland | 6 months Prospective |
In community, emergency department visits | Children and adults | WHO | Not reported |
| Kaushal (2001)157 | USA | 36 days Prospective |
In hospital, general paediatric ward | Children and adults | Naranjo score algorithm | Not reported |
| Kaushal (2007)127 – two citations (Zandieh 2008279) | USA | 2 month blocks Prospective |
In community, office-based practice | Children | Not reported | Not reported |
| Kramer (1985)78 | Canada | 1 year Prospective |
In community, private group practice | Children | Kramer 1979276 | Highly preventable – realistic non-drug alternative available Probably preventable – safer alternative drug available or lower dosage/possibly preventable – dose might have been modified/unpreventable – would not have changed the choice or dose of drug |
| Kunac – two citations (2008,280 2009143) | New Zealand | 12 weeks Prospective |
Causing admission, paediatric | Children | Naranjo score algorithm | Schumock 199244 |
| Kushwaha (1994)136 | India | 2 years Prospective |
In community, department of paediatrics | Children | Not reported | Not reported |
| Lamabadusuriya (2003)47 | Sri Lanka | 11 months Prospective |
Causing admission, medical ward | Children | Naranjo score algorithm | Not reported |
| Le – two citations (2005,144 200636) | USA | 10 years Retrospective |
Causing admission and in hospital, children’s hospital | Children | Definite; probable; possible; conditional | Not reported |
| Leach (1998)118 | UK | 14 months Prospective |
In hospital, regional ICU, a general medical ward, cardiac ICU and cardiac medical ward | Children | Naranjo 1981,23 Karch 1977,281 and Kramer 1979276 | Not reported |
| Lemer (2009)182 | USA | 10 months Prospective |
In community, attending GP | Children | ADEs assessed, non-preventable = ADR | Not reported |
| Lewinski (2010)185 | Germany | 3 months Prospective |
In community, community pharmacy | Children and adults | Strand 1990273 | Not reported |
| Maistrello (1999)120 | Italy | 6 months Prospective |
In hospital, emergency ward, infectivology ward, general paediatric ward, pneumology ward | Children | Not reported | Not reported |
| Major (1998)103 | Lebanon | 6 months Prospective |
Causing admission, medical, paediatric | Children and adults | Naranjo score algorithm | Not reported |
| Martinez-Mir – two citations (1996,42 1999121) | Spain | 105 days; and 99 days Prospective |
Causing admission and in hospital Paediatric hospital; paediatric isolation ward, lactants B ward |
Children | Spanish Drug Surveillance Scheme (Meyboom 1992)282 | Not reported |
| Martys (1979)181 | UK | 2 years Prospective |
In community, general practice | Children and adults | Not reported | Not reported |
| McDonnell (2002)140 | USA | 11 months Retrospective |
Causing admission, university affiliated teaching hospital | Children and adults | Naranjo score algorithm | Adapted from Schumock 199244 |
| McKenzie (1973)137 | USA | 8 months Prospective |
Causing admission and in hospital University affiliated teaching hospital, paediatric medicine services |
Children | Definite – directly attributable to drug Probable – a known direct relationship Possible – nebulous aspects that could be explained by the illness; no reference provided |
Not reported |
| McKenzie (1976)32 | USA | 3 years Prospective |
Causing admission and in hospital University affiliated teaching hospital |
Children | Definite – directly attributable to drug Probable – a known direct relationship Possible – temporally related to drug; no reference provided |
Not reported |
| Menniti-Ippolito (2000)128 | Italy | 1 year Prospective |
In community, family paediatricians | Children | Not reported | Not reported |
| Miller (2006)183 | Australia | 10 months Prospective |
In community, general practice | Children and adults | Not reported | Thomas 2000283 |
| Mitchell (1979)119 | USA | 4 years Prospective |
In hospital, general medical, oncology, NICU | Children | Not reported | Not reported |
| Mitchell (1988)33 – two citations (Lacouture 1986)284 | USA | 11 years Prospective |
Causing admission, teaching and community hospitals | Children | Definite – clear implicated drug caused the reaction Possible – other factors might have caused the reaction |
Not reported |
| Mulroy (1973)184 | UK | 1 year Prospective |
In community, general practice | Children and adults | Not reported | Not reported |
| Munoz (1998)131 | Spain | 25 months Prospective |
In community, emergency room | Children | Karch 1977281 | Not reported |
| Neubert (2004)75 | Germany | 8 months Prospective |
In hospital, paediatric isolation ward | Children | Naranjo score algorithm | Schumock 199244 |
| Neubert (2006)165 | Germany | 6 months Prospective |
In hospital, paediatric isolation ward | Children and adults | Naranjo score algorithm | Not reported |
| Oshikoya (2007)46 | Nigeria | 3 years Both |
Causing admission and in hospital General paediatric ward |
Children | Jones 1982285 | Done but no reference provided |
| Otero Lopez (1999)178 | Spain | 6 months Prospective |
In community, emergency department | Children and adults | Karch-Lasagna modified algorithm that uses the Spanish Pharmacovigilance System | Schumock 199244 |
| Phan (2010)169 | USA | 5 months Retrospective |
In community, emergency department | Children | Naranjo | Not reported |
| Planchamp (2009)129 | France | 6 months Prospective |
In community, emergency department | Children | Begaud 1985270 | Olivier 2005216 |
| Pouyanne (2000)146 | France | 14 days Prospective |
Causing admission, medical, public hospital | Children and adults | Not reported | Not reported |
| Prince (1992)173 | USA | 4 months Retrospective |
In community, emergency department | Children and adults | Michel 1986286 | Not reported |
| Ramesh (2003)153 | India | 7 months Prospective |
Causing admission and in hospital, memorial hospital | Children and adults | WHO | Not reported |
| Rebelo Gomes (2008)174 | Portugal | 4 months Prospective |
In community, general paediatric outpatient unit | Children | Not reported | Not reported |
| Santos (2000)104,108 – two citations | Philippines | 3 months Prospective |
Causing admission, paediatric unit | Children | Naranjo score algorithm | Not reported |
| Sanz (1987)130 | Spain | 6 months Prospective |
In community, general practice, outpatient paediatricians | Children | Karch 1977,281 Venulet 1986,287 Dangoumau 1978,288 Kramer 1979,276 Naranjo 198123 and Blanc 1979289 | Not reported |
| Schneeweiss (2002)148 | Germany | 2 years and 5 months Prospective |
Causing admission, internal medicine or emergency departments of all hospitals | Children and adults | Begaud 1985270 | Not reported |
| Seidl (1966)150 | USA | 3 months Prospective |
Causing admission and in hospital, general medical service | Children and adults | Documented confirmatory rechallenge test or a lab result indicating the unwanted effect. Probable – improvement or cessation of symptoms upon withdrawal of drug | Not reported |
| Sharma (2007)175 | India | 4 months Prospective |
In community, medicine outpatient department | Children and adults | WHO | Not reported |
| Shockrollah (2009)122 | Iran | 3 months Prospective |
In hospital, ICU | Children | Not reported | Not reported |
| Smidt and McQueen – two citations (1972,154 1973290) | New Zealand | 6 months Prospective |
Causing admission and in hospital, general hospital | Children and adults | Not reported | Not reported |
| Smith (1997)179 | USA | 1 month Retrospective |
In community, emergency department | Children and adults | Not reported | Not reported |
| Speranza (2008)135 | Uruguay | 1 week Prospective |
Causing admission and in hospital Paediatric hospital |
Children | Karch 1977281 | Not reported |
| Stoukides (1993)176 | USA | 6 months Retrospective |
In community, emergency department | Children and adults | Not reported | Not reported |
| Takata (2008)158 | USA | 3 months Retrospective |
In hospital, paediatric hospitals | Children | Not reported | Assessed but no detail provided, non-preventable ADE = ADR |
| Takata (2008)159 | USA | 6 months Prospective |
In hospital, paediatric teaching hospitals | Children | Naranjo score algorithm | Assessed but no detail provided, non-preventable ADE = ADR |
| Telechea (2010)a | Uruguay | 2 months Prospective |
In hospital, ICU | Children | Karch 1977281 | Not reported |
| Turner (1999)3 | UK | 13 weeks Prospective |
In hospital, surgical ward, medical ward, neonatal surgical ward, cardiac ICU, general paediatric intensive care units | Children | Choonara 1984134 | Not reported |
| Uppal (2000)160 | India | 3 years Prospective |
In hospital, general paediatric ward | Children and adults | Karch 1977281 | Not reported |
| Valladares (1992)177 | Spain | 4 years Prospective |
In community, ear, nose and throat outpatient unit | Children and adults | Karch 1977281 | Not reported |
| Van der Hooft (2006)141 | Netherlands | 1 year Retrospective |
Causing admission, academic and general hospitals | Children and adults | Not reported | Not reported |
| Van der Hooft (2008)149 | Netherlands | 1 year Retrospective |
Causing admission and in hospital, Integrated Primary Care Information Database | Children and adults | WHO | Hallas 199054 |
| Vazquez de la Villa (1989)123 | Spain | 12 months Prospective |
In hospital, paediatrics service | Children | Naranjo score algorithm | Not reported |
| Wang (2007)161 | USA | 3 months Prospective |
In hospital, ICU, general paediatric ward, NICU | Children | Not reported | ADEs assessed, non-preventable = ADR |
| Weiss (2002)166 | Germany | 8 months Prospective |
In hospital, paediatric isolation ward | Children | Adapted Naranjo 1981,23 Evans 1994291 | Avoidable or tolerated – toxicity, drug interactions, secondary effects Unavoidable – idiosyncratic or allergic reactions and intolerance No reference |
| Whyte (1977)145 | UK | 10 months Prospective |
In hospital, causing admission Paediatric unit |
Children | Not reported | Not reported |
| Woods (1987)132 | UK | 26 weeks Prospective |
In community, infant care and educational establishments | Children | Not reported | Not reported |
| Yosselson-Superstine (1982)43 | Israel | 7 months Prospective |
Causing admission, general paediatric ward | Children | Seidl 1965,292 Seidl 1966,127 McKenzie 1973,115 McKenzie 1976,32 Whyte 1977112 | Not reported |
| Zahroui (2010)133 | Morocco | 7 months Prospective |
In community, visits to A&E | Children | Not reported | Not reported |
Appendix 4 Liverpool Avoidability Assessment Tool glossary
Appropriate management plan(s) This could include any local, national or international guideline available, for example hospital guidelines, NICE, Scottish Intercollegiate Guidelines Network (SIGN), British Thoracic Society (BTS), the British Society for Paediatric and Adolescent Rheumatology (BSPAR), World Health Organization (WHO) or the National Guideline Clearinghouse (NGC). For example, in the case of postoperative nausea and vomiting, appropriate management plans could include Alder Hey Children’s NHS Trust guideline on postoperative nausea and vomiting, or the Association of Paediatric Anaesthetists of Great Britain and Ireland (APA) guideline on the prevention of postoperative vomiting in children.
Known preventative strategies Prophylactic or concomitant medicines or any necessary monitoring.
Information about the ADR and its avoidance: does the management plan mention any preventative measures to be taken to avoid the ADR, including medicines to be given prophylactically or concomitantly or any necessary monitoring, etc. (electrolytes, full blood count or blood pressure)? A management plan may or may not contain information regarding prevention of ADRs but more often than not they contain no information regarding the prevention of ADRs.
Other information sources Examples include the British National Formulary for Children (BNFC), SmPC, advice from colleagues, history from the parents/patients or information from a journal article, etc.
Unassessable The case could not be assessed owing to lack of information about the case and/or treatment or conflicting information.
Not avoidable The ADR was unavoidable based on the information available at the time of the reaction. There are four scenarios that lead to an ADR being categorised as ‘not avoidable’:
-
If the reaction was unpredictable and there was no known history of previous similar reaction or allergy to the drug.
-
If there was an appropriate management plan with information about the ADR and its avoidance and it was followed.
-
If there was no appropriate management plan, with information about the ADR and its avoidance available, there were no other information sources available to consult and there was no information in the history available for prevention of the ADR.
-
If there was no appropriate management plan, with information about the ADR and its avoidance available but there were other information sources available to consult or information in the history available for prevention of the ADR and appropriate action was taken to avoid the ADR.
Possibly avoidable There was no appropriate management plan available to follow but there were other information sources or information in the history available to prevent the ADR and these were not followed.
Definitely avoidable There were known preventative strategies or an appropriate management plan was available with information about the avoidance of the ADR but the strategies and or management plan were not followed.
Guide to questions in the avoidability tool
Is there sufficient information available about the case and the treatment to allow assessment?
If the answer is ‘yes’ then there is sufficient information available about the case and the treatment, and the assessor can proceed to the next question; if the answer is ‘no’, either due to lack of information or conflicting information, the case becomes ‘unassessable’.
Was the reaction predictable on the basis of the known pharmacology of the drug(s)?
This question relates to whether the ADR is predictable on the basis of known pharmacology, as there is lots of unknown pharmacology. If the answer is ‘no’ then you proceed to the question asking if there was a known history of a previous similar reaction. If the answer is ‘yes’ then you proceed down the left-hand side of the flow diagram, which asks questions regarding availability of appropriate management plans and if they were followed.
Was there a known history of allergy or previous similar reaction to the drug?
The purpose of this question is to establish if the patient has experienced a similar reaction in the past, and answering ‘no’ to the question takes you to ‘not avoidable’, as an unpredictable reaction where the patient had no previous history of occurrence means that the reaction could not have been prevented on this occasion. In theory this reaction could be avoided in the future.
Were other information sources, or information in the history available for prevention of the adverse drug reaction that could have been followed?
This is an important question to establish if there was something else that could have been done to avoid the ADR either by consulting a more senior clinician for advice or looking in other reference source examples including (but not limited to) BNFC, SmPC, consulting the parents or conducting a quick search for journal article, etc. If the answer is ‘no’ to this question then the reaction is categorised as ‘not avoidable’; if the answer is ‘yes’ then you proceed to the next question.
Was appropriate action taken to avoid the adverse drug reaction?
This question allows the reaction to be categorised as ‘not avoidable’ if appropriate action was taken to avoid the ADR but it occurred anyway, and in cases for which other information sources were available but the appropriate action was not taken, i.e. answering ‘no’ to the question, the ADR is categorised as ‘possibly avoidable’.
Were there known preventative strategies and/or appropriate management plan(s) available with information about the adverse drug reaction and its avoidance?
This question is designed to establish if there was an appropriate treatment guideline available. This could include any local, national or international guideline available: for example hospital guidelines, NICE, SIGN, BTS, WHO, NGC. If there was information available regarding the management of the condition but the guidance made no reference to the ADR or its prevention then by answering ‘no’ to the question you are directed to answer the question about whether other information sources were available. This allows for the application of other measures. If the answer is ‘yes’ to this question then you proceed to the next question below.
Were the strategies and/or management plan(s) followed?
If there was an appropriate management plan available and it contained information about the avoidance of the ADR but it was not followed then this would mean that answering ‘no’ to this question would categorise the ADR as ‘definitely avoidable’; if the answer is ‘yes’ that the drug(s) was used in accordance with the management plan then the ADR is categorised as ‘not avoidable’.
Appendix 5 Participant characteristics
| IDa | Child age (years)b | Child gender | Ranked IMD scoresc | Type of drug associated with suspected ADR | Body system affected by suspected ADR |
|---|---|---|---|---|---|
| AP1 | 3–5 | Female | 403 | Antibiotics | Skin and mucous membranes |
| AP2/AC2 | 12+ | Male | 10,787 | NSAID | Musculoskeletal |
| AP3 | 3–5 | Male | 306 | Corticosteroids, cytotoxics | Haematological |
| AP4/AC4 | 12+ | Female | 2482 | Cytotoxics | Gastrointestinal |
| AP5 | 0–2 | Female | 12,821 | Antibiotics | Skin and mucous membranes |
| AP6 | 0–2 | Male | 1574 | Cytotoxics | Haematological |
| AP7 | 0–2 | Male | 15,485 | Corticosteroids, cytotoxics | Haematological, immune system |
| AP8 | 3–5 | Female | 383 | Vaccines | Skin and mucous membrane |
| AP9/AC9 | 6–11 | Male | 6091 | Corticosteroids | Immune |
| AP10 | 0–2 | Female | 12,223 | Vaccines | Immune/infection |
| AP11 | 6–11 | Female | 16,778 | Antibiotics | Skin and mucous membranes |
| AP12 | 3–5 | Female | 271 | Antiepileptic | Hepatic |
| AP13 | 0–2 | Male | NA | Antibiotics | Skin and mucous membranes |
| AP14/AC14 | 6–11 | Male | 19,865 | Opioid analgesia | Nervous |
| AP15/AC15 | 12+ | Female | 24,299 | Opioid analgesia plus other postoperative analgesia | Nervous |
| AP16 | 0–2 | Female | 24,447 | Opioid analgesia | Skin and mucous membranes |
| AP17/AC17 | 12+ | Male | 108 | Opioid analgesia | Gastrointestinal |
| AP18 | 12+ | Male | NA | Antibiotics | Manifestation was flushing of skin but underlying cause was immune |
| AP19 | 0–2 | Male | 18,461 | Antibiotics | Manifestation was flushing of skin but underlying cause was immune |
| AP20 | 6–11 | Female | 14,971 | Drugs used in status epilepticus | Nervous |
| AP21/AC21 | 12+ | Male | 19,823 | Opioid analgesia | Gastrointestinal |
| AP22/AC22 | 6–11 | Male | 29,022 | Opioid analgesia | Respiratory |
| AP23 | 3–5 | Male | 5171 | Opioid analgesia | Gastrointestinal |
| AP24 | 12+ | Male | NA | Corticosteroid | Cardiovascular |
| AP25/AC25 | 6–11 | Male | 26,028 | Opioid analgesia | Nervous |
| AP26/AC26 | 12+ | Female | 11,667 | Drugs affecting the cardiovascular system | Nervous |
| AP27/AC27 | 6–11 | Female | 24,071 | Antibiotics; non-opioid analgesia | Skin and mucous membranes |
| YCP1 | 12+ | Male | 32,210 | Immunological products and vaccines | Endocrine |
| YCP2 | 12+ | Male | 17,251 | Drugs used for attention deficit disorder | Neurological |
| YCP3 | 12+ | Female | 20,387 | Immunological products and vaccines | Haematological |
| YCP4 | 12+ | Male | 31,691 | Non-opioid analgesia | Renal |
| YCP5 | 12+ | Female | 20,737 | Immunological products and vaccines | Neurological, musculoskeletal, gastrointestinal, skin and mucous membranes, mental health |
| YCP6 | 12+ | Female | 31,439 | Immunological products and vaccines | Neurological, immune, musculoskeletal |
| YCP7 | 6–11 | Female | NA | Respiratory | Mental health |
| YCP8 | 12+ | Female | 29,831 | Immunological products and vaccines | Musculoskeletal, neurological |
| YCP9 | 6–11 | Male | 22,922 | Immunological products and vaccines | Gastrointestinal |
| YCP10 | 12+ | Female | 30,656 | Immunological products and vaccines | Neurological, musculoskeletal, immune |
| YCP11 | 0–2 | Male | 31,508 | Immunological products and vaccines | Haematological |
| YCP12 | 12+ | Female | 30,775 | Immunological products and vaccines | Immune, neurological |
| YCP13 | 2–6 | Male | 9436 | Respiratory | Behavioural changes |
| YCP14 | 2–6 | Male | 31,612 | Respiratory | Behavioural changes |
| YCP15 | 6–11 | Male | 29,750 | Drugs used for attention deficit disorder | Neurological |
| YCP16 | 12+ | Male | 25,366 | Insulin | Behaviour changes, gastrointestinal |
| YCP17 | 6–11 | Female | 15,380 | Antibiotic | Skin and mucous membranes |
| EC12 | 12+ | Female | 19,959 | Opioid and other analgesia | Gastrointestinal/skin and mucous membranes |
| EC13 | 12+ | Female | 20,454 | Opioid analgesia | Neurological |
| EC14 | 6–11 | Male | 15,771 | Cytotoxics | Gastrointestinal |
| EC15 | 12+ | Male | NA | Opioid and other analgesia | Gastrointestinal/skin and mucous membranes |
| EC16 | 12+ | Male | 24,092 | Anaesthetics | Gastrointestinal |
| EC17 | 12+ | Male | 2350 | Anaesthetic | Neurological/gastrointestinal |
| EC18 | 12+ | Male | 26,978 | Opiate analgesia | Withdrawal |
| EC19 | 12+ | Female | NA | Intravenous paracetamol | Collapsed/fainted? Need to review notes |
Appendix 6 Study protocols
Appendix 7 Research Implications from Adverse Drug Reactions In Children
Outputs from a discussion group: what are the research implications of the burden of adverse drug reactions in paediatrics?
Symposium: Drug Safety in Children – Adverse Drug Reactions In Children
Atlantic Tower by Thistle Hotel, Liverpool
Friday 26 April 2013
14.00–15.00
Participants
Professor Munir Pirmohamed (Moderator), Professor Matthew Peak, Professor Sir Alasdair Breckenridge, Professor Deborah Ashby, Professor Michael W Beresford, Dr Andrew Rose, Dr Jamie Kirkham, Dr Sudeep Pushpakom, Dr Amitabh Shankar, Dr Petr Jirasek, Dr Virginia Ramos-Martin, Charlie Orton, Norkasihan Ibrahim, Mohammed Amali, Dave Delaney, Catherine Birch, Beth Conroy.
| Key implication | Key discussion points | Next steps summary |
|---|---|---|
| Dosing | Work with industry to develop adaptive licensing. In literature how do researchers extrapolate from adults to children? Explore what has been used, what has not been used and utility of methodologies. Use findings to develop a gold standard practice for extrapolation and apply to drugs identified as high risk within ADRIC Take forward drugs highlighted by ADRIC as a focus for further study, monitor the PK/PD of these drugs and aim to develop risk models – they may not differ across age |
|
| ADRIC outputs | LCAT and avoidability tools The discussion focused primarily on the LCAT as this tool is fully completed:
|
|
| Quantification | There are knowledge gaps in the quantification of ADRs in a variety of settings not included in the ADRIC study:
What lessons from the adult studies can be extrapolated to children? |
|
| Risk–benefit evaluation | There is a need to explore the balance of safety vs. efficacy. Is there too much focus on safety in monitoring at the expense of potential benefit? Is the weight between efficacious outcomes and safety of patients always sensible? This subject is currently less understood in children than adults. For further work, one paediatric area could be focused on, e.g. paediatric oncology What are parental views of risk–benefit evaluation? What are the children’s views? How do the decision making differences between children and parents for particular drugs differ? Does the benefit/risk comprehension and decision differ with age, e.g. from pre-school to adolescence? |
|
| Interventions | Development of interventions that could reduce harm to be given alongside high-risk drugs identified by ADRIC |
|
| Monitoring | ADRIC has highlighted a need to understand better the morbidities associated with anaesthesia and surgery in children. This requires a study that can follow-up and monitor children in the community and home setting to assess the incidence of ADRs following surgery, compare these according to the anaesthetic and postoperative drugs, surgical procedures and their comorbidities. An observational study could lead to an assessment of which children should be discharged on the day of surgery and for those who are, RCTs will be able to assess the most appropriate treatment regimens to prevent pain, vomiting and other postoperative complications Explore active reporting, passive reporting and the difference between them Communicate and collaborate with industry in the development and refinement of monitoring systems within paediatric pharmacovigilance |
|
Appendix 8 Leaflets
Reproduced with permission from Medicines for Children; Version 1, December 2013. © NPPG, RCPCH and WellChild 2011, all rights reserved.
List of abbreviations
- %EA
- exact agreement percentages
- %ED
- percentage of extreme disagreement
- A&E
- accident and emergency
- AAT
- avoidability assessment tool
- ADE
- adverse drug event
- ADR
- adverse drug reaction
- ADRIC
- Adverse Drug Reactions In Children
- AE
- adverse event
- Alder Hey
- Alder Hey Children’s NHS Foundation Trust
- BNF-C
- British National Formulary for Children
- BTS
- British Thoracic Society
- CAMHS
- Child and Adolescent Mental Health Services
- CAT
- causality assessment tool
- CI
- confidence interval
- EMA
- European Medicines Agency
- EMC
- Electronic Medicines Compendium
- EU
- European Union
- FDA
- US Food and Drug Administration
- GA
- general anaesthesia
- HDU
- high-dependency unit
- HR
- hazard ratio
- IMD
- Index of Multiple Deprivation
- IQR
- interquartile range
- IRR
- inter-rater reliability
- LCAT
- Liverpool Causality Assessment Tool
- LOS
- length of stay
- MA
- marketing authorisation
- MCRN
- Medicines for Children Research Network
- MDT
- multidisciplinary team
- MeSH
- medical subject heading
- MHRA
- Medicines and Healthcare products Regulatory Agency
- NGC
- National Guideline Clearinghouse
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NSAID
- non-steroidal anti-inflammatory drug
- OLUL
- off-label and/or unlicensed
- OR
- odds ratio
- PICU
- paediatric intensive care unit
- PK/PD
- pharmacokinetic/pharmacodynamic
- PONV
- postoperative nausea and vomiting
- RR
- relative risk
- SIGN
- Scottish Intercollegiate Guidelines Network
- SmPC
- summary of product characteristics
- STROBE
- Strengthening the Reporting of Observational Studies in Epidemiology
- TCU
- transitional care unit
- WHO
- World Health Organization