Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0606-1302. The contractual start date was in August 2007. The final report began editorial review in May 2013 and was accepted for publication in July 2014. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none.
Disclaimer
this report contains transcripts of interviews conducted in the course of the research and contains language that may offend some readers.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Marshall et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Cognitive remediation workstream: IMproving PArticipation in Cognitive Therapy (IMPACT) – a randomised controlled trial
This chapter has been published in a shorter format as Drake R, Day J, Picucci R, Warburton J, Larkin W, Husain N, et al. A naturalistic, randomized, controlled trial of cognitive remediation combined with cognitive–behavioural therapy after first-episode non-affective psychosis. Psychol Med 2014;44:1889–99. Available on CJO2013. doi:10.1017/S0033291713002559.
Background: Schizophrenia can be an intractable illness and so it is important to understand how far combining therapies creates synergy. Cognitive remediation (CR), which improves neuropsychological deficits, might combine well with cognitive–behavioural therapy (CBT), which improves symptoms.
Hypothesis: Following a first episode of non-affective psychosis, CR will enhance the efficacy and efficiency of subsequent CBT.
Methods: Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition (DSM-IV) non-affective psychosis patients aged 18–35 years who were on waiting lists for routine CBT from NHS early-intervention services were randomised to receive either computerised CR over 12 weeks supported by a trained support worker or time-matched social contact (SC). All then received 6–30 weeks of CBT. The primary outcome was the Psychotic Symptom Rating Scales, blind-rated at baseline, after remediation (12 weeks) and after CBT (42 and 54 weeks). Secondary outcomes included duration of CBT, cognition, insight, other symptoms, relapse and self-esteem.
Results: There was no significant difference in psychotic symptoms between the remediation group and the comparison group [coefficient 0.3, 95% confidence interval (CI) –0.4 to 1.1; p = 0.39]. However, duration of CBT was shorter after remediation [median seven sessions, interquartile range (IQR) 2–12 sessions] than after SC (median 13 sessions, IQR 4–18 sessions; p = 0.011) and linked to better insight (p = 0.02). Global cognition did not improve significantly more after remediation (p = 0.20) but executive function did (Wisconsin Card Sort Task; p = 0.012). No other outcomes differed significantly.
Discussion: The duration of CBT after CR was substantially shorter than after an active control, perhaps mediated by improved neuropsychological function. Remediation was delivered by staff with minimal training and thus CR might considerably reduce the costs of CBT.
Introduction
Combination therapy has proven fruitful in many difficult-to-treat conditions. It has been widely touted as a means of targeting otherwise intractable symptomatic, social and cognitive outcomes of schizophrenia. 1–3 However, studies of combination therapy (especially of non-pharmacological interventions) are complex, expensive and difficult to organise. Typically, such trials have studied multiple interventions embedded within new service designs4–7 or complex programmes of care. 6–8 Although these studies have shown promising findings, the therapeutic offering is often so complex that it is difficult to extract precise information about the synergistic benefits, or otherwise, of combining specific interventions. 2 Studies of specific combinations of interventions are often open trials4–7,9–16 or have incompletely matched control conditions. 9–16 Hence there is a pressing need for further work in this area.
Cognitive remediation (CR) and cognitive–behavioural therapy for psychosis (CBTp) are good candidates for combination therapy in early schizophrenia for four reasons. First, both interventions have efficacy,1,16–18 even in the early stages of schizophrenia. 11,19 Systematic reviews show that CR improves cognitive measures across a range of domains,16 with effect sizes ranging from 0.15 to 0.65, whereas CBTp improves overall symptoms, with an effect size of 0.40 (95% CI 0.25 to 0.55). 17 Second, each intervention targets different but complementary aspects of the condition. Third, combining CR and CBTp makes sense in terms of service delivery, as the time spent waiting for CBTp from a specialist is a window of opportunity for computer-aided CR delivered by generic staff. Fourth, cognitive impairment is an obstacle to participation in CBTp, for example deficits in verbal memory (logical memory test) predict reduced CBTp efficacy. 20 It seems logical to offer CR to people with early psychosis before they have CBTp, as improved cognitive and social functioning arising from CR should lead to improved engagement with CBTp and ultimately to better outcomes.
In the NHS, early-intervention services look after all young adults with a first episode of psychosis and we therefore had a unique opportunity to examine the impact of CR combined with CBT in those who had suffered a recent first episode. This group is relatively unselected, as even those who will have a relatively good illness course are represented. It is also less socially and cognitively impaired and, being young and early in the illness career, perhaps neurophysiologically more plastic. Early-intervention services are expected to offer CBTp as part of their programme of care. 1,21 We planned a naturalistic trial, randomising service users to 12 weeks of CR or to a parallel time-matched SC control group, with both groups going on to receive CBTp from early-intervention services as part of usual care. CBTp offered in this way would usually last from 6 to 30 weeks, as determined by therapeutic need.
Our primary hypothesis was that CR would enhance the efficacy of CBTp, with symptoms reducing faster and further during CBTp preceded by CR. A secondary hypothesis was that CR would enhance the efficiency of CBTp, facilitating shorter courses or greater progress.
Methods
Participants
We identified potential participants among outpatients on waiting lists for CBTp from Lancashire Care NHS Foundation Trust’s and Pennine Care NHS Foundation Trust’s early-intervention services. Inclusion criteria were age 18–35 years and first episode of DSM-IV22 schizophreniform disorder, schizophrenia, schizoaffective disorder or delusional disorder, confirmed by semistructured interview. 23 Exclusion criteria were International Classification of Diseases, Tenth Edition (ICD-10)24 organic brain disease; DSM-IV substance abuse or dependence; primary diagnosis of DSM-IV substance-induced psychosis; and insufficient fluency in English to participate in neuropsychological assessment. All participants provided written informed consent and the study was approved by the Bolton NHS Local Research Ethics Committee (reference 08/H1009/76) and was consistent with the UK Research Governance Framework for Health and Social Care. 25
Outcome assessments
At baseline, demographic details (including self-ascribed ethnicity as a potential moderator of CR and CBTp outcome) and medication were recorded. The PSYRATS (Psychotic Symptom Rating Scales26) were the primary outcome measure, completed with other measures at baseline and after 12 and 42 weeks’ follow-up (Figure 1). The PSYRATS validly and reliably assess the severity of delusions and hallucinations in first-episode schizophrenia. 27 Intraclass correlation coefficients between assessors were > 0.99 for subtotals and the total score.
FIGURE 1.
Study design.
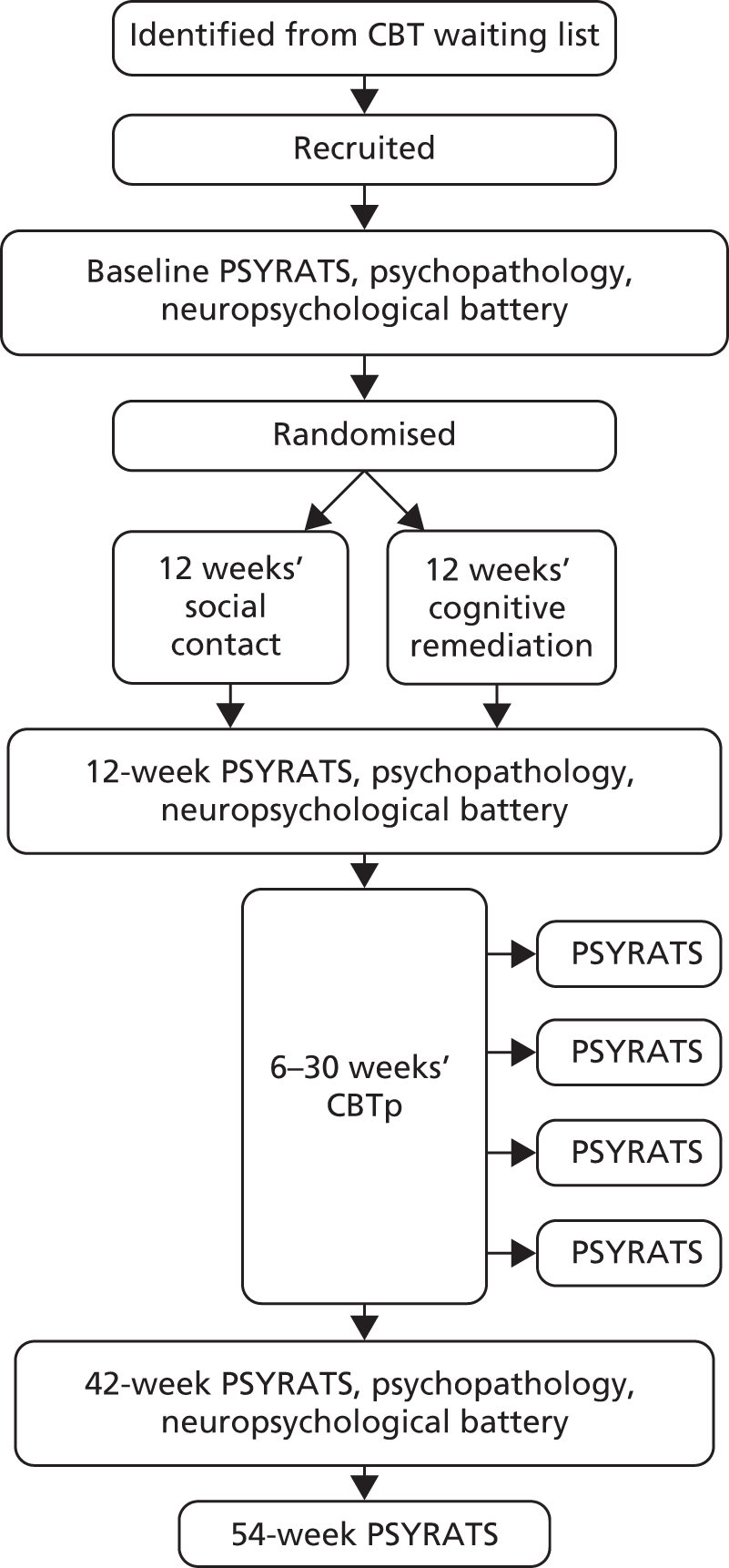
Assessments at baseline and 12 and 42 weeks included secondary measures of symptoms and function: the Positive and Negative Syndrome Scale (PANSS28), the Calgary Depression Scale for Schizophrenia (CDSS29), the Rosenberg Self-Esteem Scale30 and the Social and Occupational Functioning Assessment Scale (SOFAS31). A seven-item version of the Insight Scale (IS32), with the hospitalisation item dropped for this community sample, was scored from 0 to 14.
The PSYRATS were also rated at 6-week intervals during the CBTp envelope, at 12–42 weeks. Participants completed these interviews in person, or by telephone if they preferred. As CBTp was sometimes delayed or prolonged, and therefore incomplete by 42 weeks, the PSYRATS were also completed by telephone or in person at 54 weeks.
Cognitive–behavioural therapy for psychosis typically aims to progress from engagement, through examination of the nature of individual problems, to integration of problems into a formulation describing origins, schemata and maintaining mechanisms. At the end of CBTp, therapists assessed this progression using a five-point score. 33
An assessor blind to allocation extracted the number of sessions of CBTp from case records and identified readmission and relapse (defined, as elsewhere,34 as an exacerbation of psychotic symptoms lasting at least 2 weeks, leading to a change in management).
Neuropsychological assessments
Intelligence quotient (IQ) was assessed at baseline with the Wechsler Adult Intelligence Scale (WAIS) block design subtest and the Wechsler Test of Adult Reading (WTAR). 35 Secondary neuropsychological assessments of attention, executive function and memory were completed at baseline and at 12 and 42 weeks using the Wisconsin Card Sort Task (WCST),36 trail making,37 the 0-back version of the n-back task,38 paragraph recall39,40 and the Rey–Osterrieth Complex Figure Test41 (Table 1 contains details and rationales).
| Task | Neuropsychological rationale | Significance in previous CR trials |
|---|---|---|
| Computerised, meta-cognitive version of the Wisconsin Card Sort Task (MC-WCST) | Categories complete measures schema formation42 | Predicted a range of psychosocial outcomes43–45 |
| Free choice improvement measures meta-cognitive skill36 | ||
| Trail making A | Vigilance and motor speed | Predicted psychosocial outcome43,44 |
| Trail making B | Set alternation and working memory | Predicted psychosocial outcome43,44 |
| 0-back version of the n-back task | Basic CPT46 | CPT predicted psychosocial outcomes43,44,47 |
| Immediate and 30-minute paragraph recall | Memory skills related to structured narrative,48–51 expected to be important in CBTp | Predicted social skills improvement; predicted social function outcomes of linked rehabilitation programmes43,48,49,52 |
| Rey–Osterrieth Complex Figure Test | Accuracy of copying: visual–spatial function | Comparison with structured verbal immediate and delayed recall |
| 30-minute recall: visual memory |
Allocation
Within 3 days of initial assessment assessors faxed details of participants to identified administrators who were independent of the trial team and unaware of the hypothesis. They used randomly permuted variable blocks53 to allocate participants to the experimental group or the comparison group, masked from assessors, and then communicated allocation by telephone to the researchers providing the study interventions, before faxing them participants’ details.
Interventions
Cognitive remediation was provided in patients’ homes or service bases using the CIRCUITS software, (Reeder and Wykes, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, UK)54 run from a secure website via the internet or on a DVD. Based on a CR programme validated in schizophrenia patients,42,55 it used a colourful, engaging, virtual town as an environment, guiding participants through a sequence of tasks. This provided a social context for tasks, each requiring a specific mixture of skills (e.g. sustained attention, working memory, registration and recall, planning) and with specific criteria for progression. Early tasks prepared for later ones of increasing difficulty and complexity. Trainers supported participants and could enter their virtual environment with privileges that allowed them to modify the sequence.
Trainers were psychology graduates who had undergone 1 week of specific training (a regular open course provided by Clare Reeder and Til Wykes) to deliver CIRCUITS. CIRCUITS is based on well-established CR principles (Table 2).
| Principle | Procedure | Significance |
|---|---|---|
| Development of meta-cognitive (i.e. ‘thinking about thinking’) regulation and knowledge | Therapists’ and CIRCUITS prompts encourage participants to reflect on, learn about and develop strategies to systematically regulate their thinking and behaviour | Facilitates the transfer of new cognitive skills to a wide variety of situations within everyday life |
| Focus on transfer | Participants encouraged to define and work towards recovery-related goals and to consider how within-therapy cognitive skills can help improve everyday living skills | Aims to promote motivation and improved social functioning |
| Errorless learning | Therapists modulated difficulty to prevent repeated errors and participants progressed to harder tasks only once they were responding correctly | Prevents repeated errors that impair learning |
| Scaffolding | Trainers and the CIRCUITS tasks themselves initially provided strategies to support participants, but as difficulty increased this support was reduced | Forces participants to develop their capacity to strategise |
| Massed practice (frequent rehearsal) | Participants allowed to complete tasks between their sessions with trainers, with assignments reviewed at the start of sessions | Aiming for three to five 1-hour sessions per week and 40 hours’ intervention in 12 weeks |
The comparison condition was SC with support workers, with the duration of exposure matching that of exposure to the CR trainers. Both conditions provided interpersonal contact, warmth and unconditional positive regard within a professional relationship. Social activity (conversation, neurocognitively undemanding recreations) was the basis of sessions, although when necessary workers supported participants sufficiently to maintain their motivation to attend (i.e. non-directive listening concerning problems and symptoms).
Cognitive–behavioural therapy for psychosis was provided separately by therapists employed specifically for that purpose by the two NHS services who worked in seven different community clinics. All had specific CBTp training and were supervised by experienced senior CBTp therapists according to National Institute for Health and Care Excellence (NICE) standards. 1 CBTp quality was assessed using the Cognitive Therapy Scale for Psychosis (CTSpsy57), independently rated from randomly selected audio-taped sessions, provided that therapists thought it clinically appropriate and patients provided separate written consent. The mean CTSpsy score for seven CBTp interviews was 75.2% [standard deviation (SD) 14.5%].
Analysis
The effect of group on PSYRATS score was estimated using a mixed-effects model estimated using full information maximum likelihood with Stata version 11.2 (StataCorp LP, College Station, TX, USA). Allocation group and a group × time interaction term modelled the intervention’s effect. Time was entered as the square root of weeks from baseline; as PSYRATS scores tended to level off during follow-up this improved the model fit. 58 Demographic and other baseline potential confounders (lack of antipsychotic prescription, previous illicit substance use, diagnosis of schizophrenia) were removed by backward elimination, the criterion for retention being p < 0.20. PANSS, SOFAS and depression and self-esteem scales were analysed in the same way. Insight, in its mixed-effects model, was transformed towards normality by squaring the Insight Scale (IS) score. Insight changed relatively linearly over time and so weeks from baseline was entered as the time variable. A logistic regression against dropout with baseline variables as predictors assessed the pattern of missing data in relation to these variables.
As a categorical outcome, remission was defined as a PSYRATS score of zero. The relative risk of remission after CR was calculated and used to derive the number needed to treat/harm.
For neuropsychological measures, a global score was calculated. Individual baseline scores were transformed to normal distributions with a mean of 100 and SD of 15, with higher scores indicating better performance, before deriving a global score with the same characteristics. The same transformations were applied to 12- and 42-week follow-up scores, giving distributions with various means and SDs. The global score was then entered into a mixed-effects model.
In this relatively able population, WCST categories complete and complex figure copying (rather than recall; see Table 1) scores were too skewed by ceiling effects for this process. Categories complete and copying were modelled by ordinal logistic regression against follow-up scores, adjusting for baseline scores and potential confounders after backward elimination, clustering by clinic. To examine the sensitivity of ordinal logistic and linear regressions to the effect of dropout, all were repeated with cases probability weighted by a function of the risk of attrition, derived from the logistic regression against dropout. That is, the number of each case (n = 1) was multiplied by the reciprocal of the risk of completion of that type of case (calculated from a logistic regression using baseline characteristics to predict which cases would finish the study), so that cases of a type who more often dropped out appeared to be more frequent (e.g. apparent or weighted n = 1.43 if the probability of completion for a case with those demographic and clinical baseline characteristics was 0.7) to make up for the cases who did, in fact, drop out.
Time to relapse and readmission for allocation groups were compared by log-rank tests. Cox regressions adjusted hazard ratios (HRs) for potential demographic and clinical confounders, stratified by clinic.
Sample size
Assuming (after the trial by Eack and colleagues11) a PSYRATS effect size of 0.5, recruiting 64 participants provides > 80% power with a two-tailed alpha of 0.05, correlation of 0.5 between seven successive assessments and 25% dropout.
Results
Experimental and comparison group characteristics
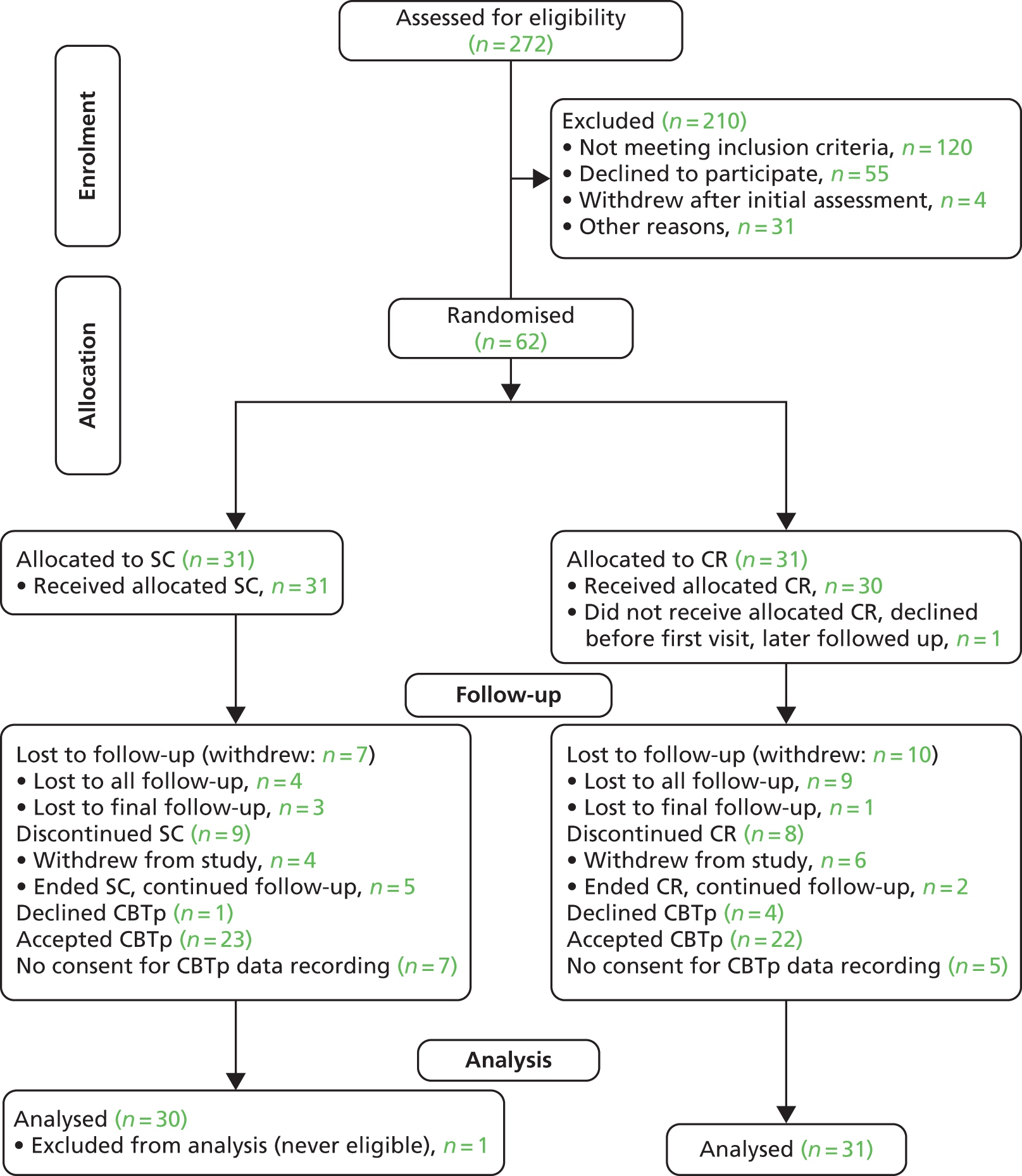
In total, 272 CBTp waiting list patients were screened for eligibility (Figure 2). Of these, 66 consented to participate, of whom four withdrew, leaving 62 to be randomised. One was subsequently excluded as a concealed history of previous psychosis rendered him ineligible and so 61 entered a modified intention-to-treat analysis (Table 3). Seven in the CR group and four in the SC group did not take maintenance antipsychotics (p = 0.51, Fisher’s exact test). No anticholinergics were prescribed and only one in each group took medication with high muscarinic receptor affinity (clozapine). Assessors accidentally discovered two participants’ allocation group during the trial, one from each group. CBTp therapists discovered eight participants’ allocation group (five CR group and three SC group) but their guesses at the allocation of the other participants were no better than chance (p = 0.43, Fisher’s exact test). Logistic regression against completion found no significant predictors of dropout.
FIGURE 2.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram showing participant flow through the trial.
| Variable | CR | SC |
|---|---|---|
| Gender, n (%) | ||
| Male | 21 (68) | 16 (53) |
| Ethnicity, n (%) | ||
| White | 23 (74) | 25 (83) |
| African and African Caribbean | 1 (3) | 2 (7) |
| South Asian | 5 (16) | 3 (10) |
| East Asian | 2 (7) | 0 (0) |
| Diagnosis, n (%) | ||
| Schizophrenia | 26 (84) | 26 (87) |
| Schizoaffective disorder | 5 (16) | 4 (13) |
| Age (years), mean (SD) | 24.7 (5.2) | 23.4 (4.4) |
| Full-time education (years), median (IQR) | 13 (11–14) | 13 (11–14) |
| PSYRATS, median (IQR) | 13 (8–22) | 16.5 (11–35) |
| PANSS, mean (SD) | 71.3 (13.9) | 69.5 (11.7) |
| IQ, mean (SD) | 105.4 (8.0) | 103.4 (10.0) |
| Global cognition, mean (SD) | 99.5 (13.7) | 100.5 (16.4) |
| WCST categories complete, median (IQR) | 5 (4–5) | 5 (4–5) |
| Complex figure copying, median (IQR) | 34 (32–36) | 34 (31–35) |
| Defined daily dose, median (IQR) | 0.75 (0–1.06) | 0.72 (0.31–1.00) |
There was no significant difference in exposure to CR trainers/exposure to support workers between the two groups (SC: median 390 minutes, IQR 145–610 minutes, CR: median 375 minutes, IQR 125–600 minutes, Mann–Whitney U-test, p = 0.84; SC: median 8.5 sessions, IQR 3–10 sessions, CR: median 9 sessions, IQR 4–13 sessions, Mann–Whitney U-test, p = 0.20).
Did cognitive remediation improve cognition?
Cognitive remediation did not predict differences in global cognition scores [intercept (baseline) –1.7, 95% CI –7.7 to 4.4, p = 0.59; gradient (group × time) –0.73, 95% CI –1.84 to 0.38, p = 0.20)]. However, after the intervention the CR group completed significantly more WCST categories: after CR, 74% completed five categories (range 4–5 categories); after SC, 63% completed five categories (range 2–5 categories) [adjusted odds ratio (OR) 2.9, 95% CI 1.3 to 6.9; p = 0.012]. By final follow-up these gains were lost (adjusted OR 0.7, 95% CI 0.2 to 2.5; p = 0.61). The median score for complex figure copying was 35 (IQR 33–36) after CR and 33 (IQR 29–35) after SC (p = 0.11, Mann–Whitney U-test). After removing an outlier with persistent, specific, severe visuospatial impairment (baseline copying score 12; range of other participants’ scores 24–36, mean 32.89, SD 3.89; Dixon’s Q59 0.50, p < 0.01), CR had a trend towards better scores at 12 weeks (adjusted OR 3.83, 95% CI 0.99 to 14.77; p = 0.052) but not at the final assessment (adjusted OR 1.6, 95% CI 0.3 to 7.7; p = 0.56).
Did cognitive remediation improve the efficacy of cognitive–behavioural therapy for psychosis?
Symptoms
Cognitive remediation was not associated with significantly lower PSYRATS scores over the period of study (Figure 3) (intercept –2.8, 95% CI –10.7 to 5.2, p = 0.50; gradient 0.3, 95% CI –0.4 to 1.1, p = 0.39). The effect of CR on the PANSS score was non-significant (intercept 3.8, 95% CI –2.7 to 10.3, p = 0.25; gradient –0.4, 95% CI –1.5 to 0.6, p = 0.44). Eight participants in each allocation group had full remission of symptoms on the PSYRATS (OR 0.96, 95% CI 0.26 to 3.51; p = 1.00, Fisher’s exact test), giving a number needed to harm with CR of 116 (95% CI ranging from a number needed to treat of 3 to a number needed to harm of 12).
FIGURE 3.
Mean AH and delusions subscale scores by visit for each allocation group. AH, Auditory Hallucinations; B/L, baseline.
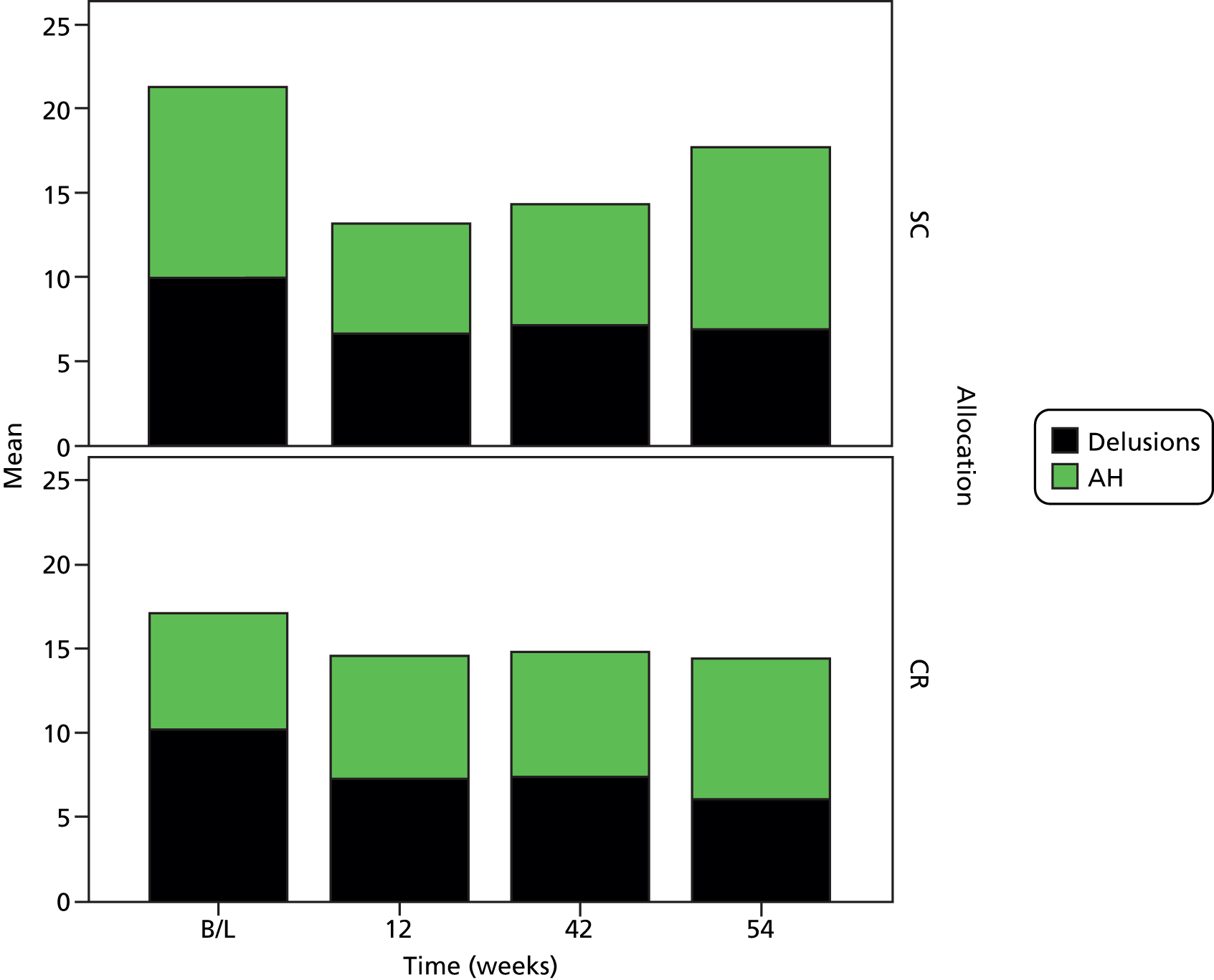
However, insight changed significantly more positively after CR than after SC (intercept –1.9, 95% CI –27.7 to 23.8, p = 0.88; gradient 0.55, 95% CI 0.08 to 1.01, p = 0.02). At 42 weeks the median insight score was 12 after both CR and SC, but the IQRs after CBTp (CR 9.5–13; SC 6–13) showed that fewer participants in the CR group had substantially impaired insight. There was no significant effect of CR on SOFAS score, depression or self-esteem.
Repeating multiple regressions for clinical and neuropsychological variables, weighting cases according to the probability of dropout, did not alter the significance of the results.
Relapse and readmission
Cognitive remediation had no significant effect on time to relapse (adjusted HR 1.4, 95% CI 0.5 to 3.5; p = 0.50) or readmission (adjusted HR 1.2, 95% CI 0.2 to 5.5; p = 0.84).
Did cognitive remediation improve the efficiency of cognitive–behavioural therapy for psychosis?
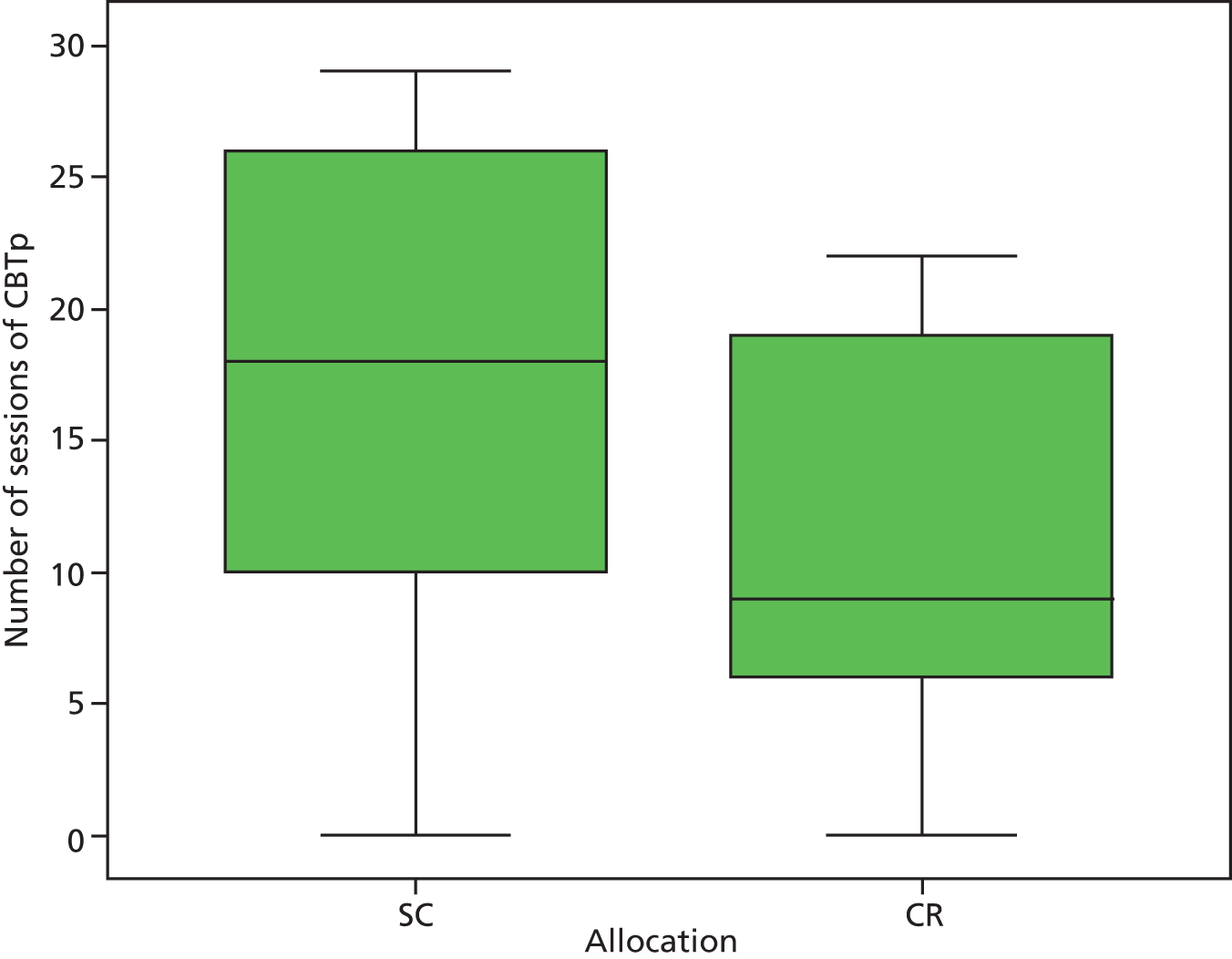
After CR, far fewer sessions of CBTp were required (Figure 4): a median of seven (IQR 2–12) sessions compared with a median of 13 (IQR 6–18) sessions for the SC group (p = 0.039, Mann–Whitney U-test). CR still predicted fewer sessions of CBTp after adjustment for potential confounders (coefficient –1.0, 95% CI –0.3 to –1.7; p = 0.011). Unmasking was not responsible: their allocation of 53 participants remained masked from therapists and the median number of sessions was six (IQR 1–12) after CR and 15 (IQR 7–19) after SC (p = 0.012, Mann–Whitney U-test). Allocation was unmasked for eight participants, for whom the median number of sessions was 14 (IQR 10–18.5) after CR and nine (IQR 3–18) after SC (p = 0.37, Mann–Whitney U-test).
FIGURE 4.
Number of sessions of CBTp by allocation group.
There was no evidence that level of formulation differed between the CR group and the SC group across all participants (adjusted OR 1.1, 95% CI 0.2 to 6.1; p = 0.88) or in masked patients alone (adjusted OR 0.7, 95% CI 0.2 to 2.3; p = 0.50).
Discussion
Although the hypothesis that CR would enhance the effect of CBTp on symptoms was rejected, the hypothesis that it would improve the efficiency of CBTp was supported. After CR, participants made the same amount of progress in half the number of CBTp sessions. If CBTp ended when a therapist judged that the client had progressed cognitively as far as possible, then better engagement because of improved insight and cognition after CR might have helped client and therapist reach this point more quickly. If symptom reduction determined when therapists ended CBTp, less efficient CBTp after SC could have led therapists to lengthen the duration of CBTp to compensate. Either would explain why efficiency rather than efficacy improved.
The CR group had fewer low scores on the IS at 42 weeks. The difference from the SC group became substantially greater after CBTp than before and so it appears most likely that CR enhanced CBTp and this led to relatively better insight. CR improved WCST performance, although this was not sustained. The WCST is one of the most common, robust and sensitive measures of CR outcome16 and frequently predicts progress in social skills and CR interventions,43 particularly categories complete (schema generation). 54 Silverstein and colleagues15 previously proposed that transient cognitive benefits could promote sustained changes in behaviour and performance during succeeding interventions, consistent with transient neuropsychological gains facilitating CBTp.
Global cognition did not improve but other neuropsychological measures might have been less accurate than the WCST. Short, less-demanding cognitive tasks were chosen for these process measures, mindful of the risk of alienating potential and actual participants and rendering the sample unrepresentative. Although these tasks had demonstrated validity in other trials (see Table 1), they lacked the rigor of lengthier cognitive batteries [e.g. the MATRICS Consensus Cognitive Battery (MCCB)],60 making them vulnerable to practice effects that could have concealed the benefits of CR. Nevertheless, other CR trials with more extensive batteries found that executive function effect sizes exceeded other types of cognition,55,61 suggesting that the difference was real.
One limitation of our study was that inexperienced therapists delivered CR. In addition, CBTp was delivered by NHS clinicians who, although qualified and supervised, were not research trained and supervised (although CBTp quality scores were reasonably good). Even so, we felt it important to accept these limitations to simulate routine clinical practice and make generalisation credible. One consequence of our naturalistic design was that the median number of CBT sessions was lower than that recommended by NICE guidelines;1 however, they still appeared sufficient to have an effect. A strength of the naturalistic trial design was that NHS early-intervention services take nearly all incident cases of schizophrenia in their catchment areas and so attendees at these services are reasonably representative of all those who might benefit from CBTp.
Another strength of our trial was that contact time was well matched between the CR group and the SC group, ensuring plausible matching of non-specific benefits of remediation. Even the most carefully designed randomised trials of combination therapy, for instance CR with social skills training9,14,15 or with vocational interventions,10,12,13 struggled to match exposure to the intervention and comparison conditions. 12 However, SC offered more opportunity for non-directive listening than task-oriented CR. This compensatory therapeutic effect could have led us to underestimate the specific benefits of CR.
Allocation was almost completely masked from assessors, whereas previous trials combining psychosocial interventions have often been open. 4–7,9–16 Although it is unclear how far measurement of neuropsychological performance or employment rates is affected by the open rating, previous meta-analysis of CBTp suggests that symptoms are sensitive, as limiting inclusion to studies with masking and other rigorous design features diminished the effect size of CBTp from 0.40 to 0.22. 17 Although it was difficult to mask allocation from CBTp therapists, they identified allocation in only 13% of participants. In fact, unmasking only attenuated the difference in CBTp length between the CR group and the SC group: the reduction in CBTp length after CR was greater in those participants with allocation still masked.
Overall, our findings suggest that CR delivered by relatively unskilled workers improved the efficiency of subsequent CBTp, enabling participants and therapists to achieve the same amount of progress in therapy in approximately half the number of sessions. The computer-aided CR approach that we selected was delivered by relatively inexpensive support workers within a typical clinical service. Labour costs for a course of CR were only £85. A substantial increase in the efficiency of CBTp implies that the same number of CBTp therapists could treat many more patients, whereas estimates from previous studies62,63 imply that the shortened duration of CBTp was equivalent to a saving of £335 per patient.
Key findings
-
Cognitive remediation after first episodes of non-affective psychosis improved executive function but no other neuropsychological test scores.
-
After CR, the duration of CBT was much shorter but there was no difference in efficacy.
-
At the end of CBT, those who had received CR had significantly better insight.
Chapter 2 Healthy living workstream: development of an intervention to encourage activity, improve diet and control weight gain (InterACT) and a randomised controlled trial
Reprinted from the International Journal of Nursing Studies, 49, Bradshaw T, Wearden A, Marshall M, Warburton J, Husain N, Pedley R, Escott D, Swarbrick C, Lovell K, Developing a healthy-living intervention for people with early psychosis using the Medical Research Council’s guidelines on complex interventions: Phase 1 of the HELPER – InterACT programme, 398–406. Copyright (2012), with permission from Elsevier.
Background: People with psychosis are at an increased risk of weight gain, contributing to poor physical health and early death.
Objectives: (1) Develop an effective and acceptable healthy-living intervention to prevent weight gain for people with psychosis, (2) evaluate the clinical effectiveness of the intervention and (3) evaluate the acceptability of the intervention.
Methods: The intervention was developed by synthesising the results of a number of inter-related studies including (1) a review of healthy-living intervention studies, (2) qualitative interviews with service users and health professionals, (3) identification of a theoretical model and (4) cultural adaptation of the intervention. It was tested with service users of two early-intervention services with a body mass index (BMI) ≥ 25 kg/m2, who were randomly allocated to the intervention group or usual care. The primary outcome was change in BMI from baseline to 12 months’ follow-up. Qualitative interviews were conducted with participants about their experience of the intervention.
Results: The intervention consisted of eight individual sessions with a trained support, time and recovery (STR) worker, supplemented by optional group activities. In total, 105 participants were recruited (54 intervention group, 51 usual-care group), with 89% retention at 12 months. There was a small but non-significant decrease in mean BMI at 12 months in the intervention group compared with no change in the usual-care group. The intervention was highly acceptable to participants.
Conclusion: The healthy-living intervention was associated with a small but non-significant reduction in BMI in the intervention group but not in the usual-care group.
Introduction
Individuals with psychosis have poorer physical health and die younger than other members of the general population. 64 Recent government policy has set targets for service providers to reduce this inequality. 65 One major contributory factor of poor physical health may be the rapid weight gain associated with the prescription of second-generation antipsychotic drugs. 66 A review by Foley and Morley67 showed average weight gain to be 5–6 kg after only 6–8 weeks of treatment with antipsychotic drugs, with unhealthy cardiometabolic changes already beginning to emerge. Commenting on the review, an editorial in the Lancet stated that in any other scenario the responsible physician would seek an alternative treatment. 68 However, for mental health professionals and their patients there are no realistic alternatives currently available. If, as the editor suggests, patients must continue to take antipsychotic medication, then there is an urgent need to develop interventions to help patients control their weight gain and protect themselves against the cardiometabolic effects and complications associated with weight gain.
Although conclusions from a Cochrane review showed that there is insufficient evidence to support the general use of pharmacological interventions for weight management in people with schizophrenia,69 there is sufficient evidence in support of non-pharmacological interventions. A meta-analysis of non-pharmacological weight management studies in people with psychosis70 showed that CBT and nutritional counselling were effective at reducing or slowing antipsychotic medication-induced weight gain. However, benefits rapidly attenuated once the intervention was discontinued. 71 An explanation for these modest effects and poor durability may lie in the development of the interventions. For the most part the interventions in the review were not obviously underpinned by theoretical models of behaviour change. For example, there was limited reporting of service users’ motivations for weight control, their beliefs about their ability to control their weight or the strategies that they would use to regulate their behaviour. Furthermore, it was not clear which (if any) of these factors were addressed in the interventions (and how). Additionally, the acceptability of interventions to service users and those delivering them was not assessed. Finally, only one study in the review had recruited patients in the early stages of treatment for psychosis.
In the last decade there has been growing interest in the development and evaluation of interventions, largely driven by the influential UK Medical Research Council (MRC) framework. 72 The MRC defines a ‘complex intervention’ as one in which there is ambiguity over the ‘active ingredients’ of the intervention and their optimal mix, and the phased development of such interventions has been advocated. 72 This involves the use of theoretical and empirical work to identify the active ingredients of the intervention (modelling), followed by an exploratory randomised controlled trial (RCT) to test the intervention, examine its delivery in routine settings and provide estimates of key trial parameters such as recruitment rates and effectiveness. In this chapter we will describe how, guided by the MRC framework,72 we have developed and tested the feasibility, acceptability and effectiveness of a novel lifestyle intervention that aims to help people with first-episode psychosis control their weight.
The overall aim of the workstream was to develop an evidence-based, acceptable, feasible and effective intervention to encourage activity, improve diet and control weight gain in people recovering from a first episode of psychosis and to evaluate its clinical effectiveness and cost-effectiveness in an exploratory randomised controlled study. We conducted two key phases.
Phase 1: development
Phase 1 had two key aims:
-
to identify the active ingredients of a healthy-living intervention to control weight in people after a first episode of psychosis
-
to develop an evidence-based, acceptable and feasible protocol and training programme for the delivery of a healthy-living intervention.
To develop the intervention we conducted a series of separate but inter-related studies, the findings of which were synthesised to produce a prototype healthy-living intervention (Figure 5). We (1) identified the evidence base by updating a systematic review, (2) identified the most appropriate theoretical model to underpin the intervention, (3) conducted qualitative interviews with service users to examine their beliefs about weight gain in psychosis, (4) conducted both focus groups and individual interviews with mental health professionals to examine their perspectives and (5) culturally adapted our intervention to be appropriate to the needs of the ethnically diverse population that we were working with. These data were synthesised by the trial team and the draft prototype healthy-living intervention was then subject to (6) a stakeholder consensus group to finalise the intervention.
FIGURE 5.
Development of the intervention. SRM, self-regulation model.

Systematic review
Aim
The study aimed to update a systematic review of the RCT literature and then conduct a meta-regression to identify:
-
the types and relationships between components of non-pharmacological interventions used to improve healthy-lifestyle behaviours (all interventions used to improve healthy-lifestyle behaviours including exercise, diet and weight maintenance) in people with psychosis
-
the overall clinical effectiveness of non-pharmacological interventions for people with psychosis
-
the factors associated with effective interventions such as setting (inpatient/community), delivery mode (group/individual) and personnel delivering the interventions.
Methods
We drew on a recently published high-quality systematic review of non-pharmacological management studies of antipsychotic medication-related weight gain. 70 We supplemented this by searching for any additional RCTs using the Cochrane Central Register of Controlled Trials (CENTRAL). The search of CENTRAL was then amplified by searches of MEDLINE, EMBASE, PsycINFO, the Health Technology Assessment database, the Allied and Complementary Medicine Database, Science Citation Index and Social Sciences Citation Index, the National Research Register and the System for Information on Grey Literature in Europe. The reference lists of all identified studies and review papers were searched for other relevant publications up to 2007 to identify recent additional literature. Searches (available from the authors) utilised a mixture of subject headings and free-text terms.
Inclusion criteria
-
Study design. RCTs in which the majority (> 50%) of the participants were diagnosed with a psychotic disorder.
-
Study context and population. Populations eligible for inclusion included any adult group with a psychotic mental health problem using any criteria (e.g. ICD-10 criteria,24 DSM-IV criteria22 or case note diagnosis) seeking treatment with a non-pharmacological intervention to improve healthy living or prevent weight gain. All settings including community, primary care, specialist outpatient and inpatient and non-clinical settings were eligible.
-
Interventions. Lifestyle/healthy-living/health-education interventions focusing on healthy eating and/or physical activity or a combination of both delivered on either a group or an individual basis.
-
Outcomes. Primary outcome measures included self-reported and externally assessed aerobic fitness, blood pressure, weight, BMI, flouval antioxidants, abdominal girth measurement, waist-to-hip ratio and/or other outcomes of measured compliance with recognised healthy-living guidelines. Secondary outcomes included psychiatric symptoms, quality of life, self-esteem, self-efficacy, medication compliance, medication dosage, attitude or belief change, numbers lost to follow-up and number of participants approached who declined to participate in the study.
Exclusion criteria
Studies that focused exclusively on smoking cessation or reducing the use of illicit substances, non-randomised studies and those not written in the English language were excluded.
Data extraction and quality assessment
Eligibility judgements and data extraction were carried out independently by two reviewers. No formal measure of the reliability of data extraction was calculated but disagreements were resolved by discussion with members of the trial team.
Analysis
We completed a meta-analysis of these studies using random-effects modelling to provide an overall pooled measure of effect on BMI rather than weight as in the review by Álvarez-Jiménez and colleagues. 70 In addition, we conducted a meta-regression on intervention moderators to examine the relationship between components of the intervention and outcomes, to determine potentially critical ingredients.
Results
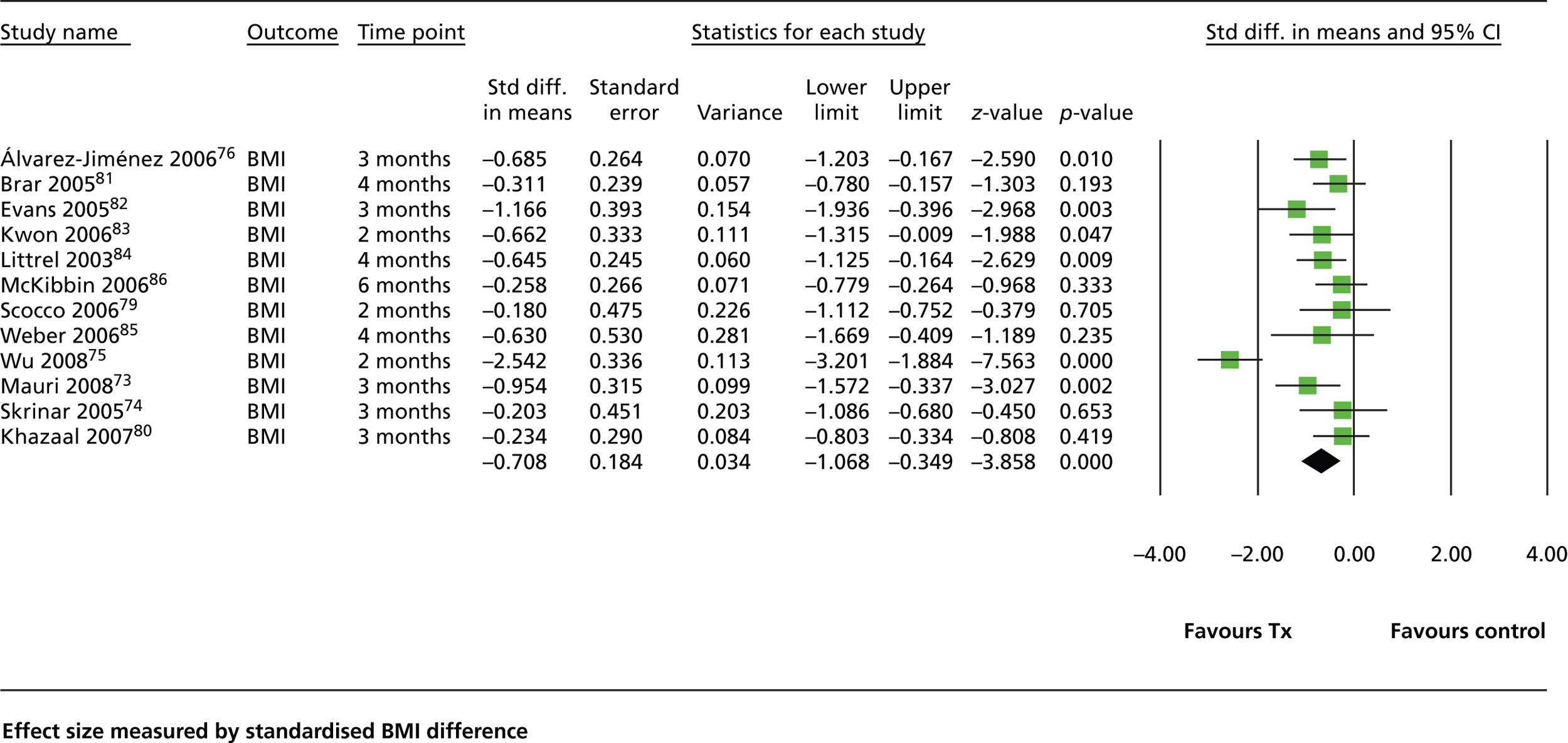
Three additional studies were identified. 73–75 We identified 12 studies in total73,75–85 Eleven compared the intervention with routine care and one80 used brief nutritional education as a control. The review by Álvarez-Jiménez and colleagues70 found that weight-management interventions resulted in a mean weight difference of 2.56 kg compared with treatment as usual (TAU). No difference in efficacy was shown between interventions delivered to groups and interventions delivered to individuals or between CBT interventions and nutritional counselling. Similar to the review of Álvarez-Jiménez and colleagues,70 our analysis showed that weight-management interventions are moderately effective, resulting in an average change in BMI of approximately 1 point (–0.708, 95% CI –1.07 to –0.349; Figure 6). We found no difference between studies that were CBT based and studies based on other therapeutic approaches. Unlike the study by Álvarez-Jiménez and colleagues,70 our analysis showed a trend towards greater effectiveness for individual interventions compared with group interventions [standardised mean difference (SMD) –1.04 (95% CI –1.68 to –0.41) vs. SMD –0.376 (95% CI –0.61 to –0.14) respectively]. We also found that studies in which supervised exercise actually formed part of the intervention showed a trend towards greater effectiveness than those that merely advised or encouraged exercise [SMD –1.153 (95% CI –2.57 to 0.27) vs. SMD –0.533 (95% CI –0.73 to –0.32) respectively]. In general, the effect of these interventions attenuated rapidly once the intervention had ceased. None of the reports indicated that the components of the intervention were underpinned by a specific psychological model of behaviour change and only one study had focused specifically on people with early psychosis. 76
FIGURE 6.
Forest plot showing the effectiveness of healthy-living interventions. Std diff., standard difference; Tx, treatment.
Key findings
-
Weight management interventions are moderately effective, with an average change in BMI of approximately 1 point or an average weight loss of 4% body weight. Effects seem to attenuate once the intervention stops, although most studies did not conduct follow-up assessments beyond 3 months.
-
Interventions that aimed to prevent weight gain were more effective than those that targeted weight loss.
-
Interventions that were delivered to individuals were more effective than those delivered solely to groups.
-
Interventions that have a supervised exercise component may be more effective than those that do not.
-
None of the interventions was underpinned by a specific psychological model of behaviour change.
Qualitative interviews with service users
Aim
To explore users’ views of illness and treatment beliefs, healthy living, preferences with regard to healthy-living interventions and barriers to and facilitators of healthy-living interventions.
Methods
All patients from an early-intervention service in the north-west of England (n = 260) were contacted to participate in interviews to ensure inclusion of a range of patient baseline characteristics (i.e. age, gender and ethnicity) to ensure a diversity of views. Semistructured interviews with consenting participants were conducted in patients’ homes by the researcher. An interview guide (see Appendix 1) was designed by the trial team to ensure exploration of key areas, including illness and treatment beliefs, beliefs about psychosis, obesity and weight control, antipsychotic medication and its side effects, current dietary, exercise and smoking habits and barriers to and facilitators of healthy-living interventions, and to establish preferences about key aspects of a potential healthy-living intervention including content, setting, format and delivery of the intervention. Interviews were digitally recorded and lasted between 30 and 45 minutes.
Analysis
Interviews were transcribed verbatim and data were analysed using framework analysis. 87 An initial coding framework was developed and transcripts were checked against the framework to ensure that there were no significant omissions. Codes in each interview were examined across individual transcripts as well as across the entire data set and allocated to the framework. Using the constant comparative method of analysis,88 broader categories used linked codes across interviews. Data were interpreted and analysed within the framework to distil, interpret and structure component statements about illness beliefs, healthy living and the intervention. Direct quotes are given an ID number and other characteristics have been removed to ensure anonymity.
Results
In total, 13 service users were interviewed. 10 were male and the mean age was 25.5 years (range 19–32 years). With regard to ethnicity, two were black African, eight were white British and three were South Asian. Ten participants were taking antipsychotic medication, one was taking a mood stabiliser and two were not taking any medication. Eight were unemployed, three were employed part-time, one was employed full-time and one was a student.
Three key themes in the data related to:
-
illness and treatment beliefs about weight gain and the role of medication
-
current lifestyles and perceptions of health risks
-
preferences regarding the type of healthy-living intervention.
These are briefly summarised in the following sections.
Illness and treatment beliefs
Service users’ descriptions about the onset of their psychosis and their initial reaction to symptoms reflected the individualistic and varied nature of their experiences. Two themes emerged from these explanations: (1) a response to stress resulting from life events and (2) the use of illicit substances including cannabis and ecstasy. Participants reported that lack of sleep, stress and stigma made their symptoms worse whereas a good sleep pattern and social support improved symptoms.
Participants taking antipsychotic medication expressed positive views regarding its helpfulness. The key negative views about medication were that it resulted in increased appetite, weight gain, tiredness and lethargy. All participants said that they had gained weight since starting antipsychotic treatment. The average self-reported weight gain was 10.5 kg. Participants had been given information about possible weight gain but had not understood why this was likely to happen or what they should do to prevent it.
The doctor mentioned it. He said that when you’re taking this medication you could put on a bit of weight . . . because the water content in your body increases and so when you feel hungry you sort of want to eat more, there’s an increase of water in your body and you put on weight but it’s easily counteracted if you remain fit and active and if you take regular exercise it’ll automatically go.
SU10
Some participants believed that they had not received sufficient information to manage the side effects, including increased appetite, which led to binge eating, and drowsiness, leading to reduced levels of physical activity, both of which were believed to have contributed to weight gain.
Current lifestyles and perceptions of health risks
Most participants felt that they had a fairly healthy lifestyle in relation to dietary intake and level of physical activity and thought that this was important to prevent further weight gain, although more than half smoked. Participants believed that early in their recovery from the episode of psychosis the amount of physical activity they had undertaken had reduced but that this had improved as recovery progressed. Weight gain was not seen as posing a current health risk but could in the future. Participants’ awareness of the degree and risk of weight gain was limited. Two participants viewed their weight gain as positive, as they had previously been underweight. Most were confident that they could manage weight gain through diet and exercise. Motivation to lose weight included the desire to stay fit and healthy and to regain their pre-illness body image.
I think I’m not too overweight but not where I want to be, really . . . about 12.5 stone, something like that. I’m not particularly bothered about the weight, it’s more like the beer belly kind of look, you know? I’d rather be . . . you know, I don’t want to be some big muscly . . . it’s the appearance. It’s the not the weight aspect, it’s the appearance, really.
SU7
Preferences regarding the type of healthy-living intervention
There was a mixed response to the question of whether a healthy-living intervention should be delivered on a group or an individual basis. Participants who placed greater importance on social interaction favoured a group intervention. No specific preference was expressed about who should facilitate the intervention and suggestions included a community mental health nurse, a support, time and recovery (STR) worker or an expert such as a physical fitness trainer or dietitian. Participants wanted the intervention to be cost neutral to them and, therefore, based locally. A strong preference was expressed for an intervention that could provide the opportunity to undertake physical activity, rather than being focused on education. Potential barriers to success expected by service users included financial barriers to participation, work hours, problems with memory and concentration and fluctuating motivation.
I think something cardiovascular. If people have got weight gain then the best way to maintain a healthy heart and to burn these calories off . . . because I think the medication makes people retain the weight much more worse than what they would do normally so you need to do things like running and rowing machines, things like that, access to the gym . . . Or maybe even learning how to do a few exercises at home for people who have got, I don’t know, agoraphobia and stuff like that where they’ve got a fear of going to places and meeting new people, maybe exercises at home that they can do.
SU13
Key findings
-
Most participants had gained weight and they attributed weight gain to an increased appetite and drowsiness from taking antipsychotic medication.
-
Most participants felt that managing the increase in their appetite and staying physically active to prevent further weight gain were important issues to them.
-
Participants perceived a low level of risk to their current health because of the weight that they had gained but many were sufficiently concerned about it to be taking some active steps to manage it and would be motivated to accept additional help were it to be made available.
-
Participants expressed specific preferences regarding the type of healthy-living intervention that would be acceptable, which needed to be active, promote physical activity, be geographically accessible, be cost neutral and be delivered flexibly on an individual or a group basis according to personal preference.
Qualitative interviews and focus groups with health professionals
Aim
To explore health professionals’ views of their respective roles and responsibilities in the area of physical health care and the feasibility and acceptability of a healthy-living intervention for people experiencing a first episode of psychosis.
Methods
All qualified health professionals from three early-intervention service teams in a north-west of England early-intervention service were asked to participate in the study. Consenting participants were given the option of a telephone interview or a focus group. An interview guide (see Appendix 2) was developed by the trial team to ensure exploration of key areas with regard to the healthy-living intervention, including participants’ views of their respective roles and responsibilities in the area of physical health care, the need for and capacity to deliver the suggested intervention and barriers to and facilitators of its uptake in various settings (e.g. community, secondary, primary). Interviews and focus group were digitally recorded.
Analysis
Interviews were transcribed verbatim. The interview data were analysed using the principles of framework analysis. 87
Results
In total, 14 health professionals consented (eight were interviewed individually and six attended a focus group). All health professionals recognised the importance of monitoring their clients’ physical health, which included their weight, and were in agreement that their clients’ lifestyle should be monitored. They felt that any healthy-living intervention should focus on food intake, exercise, alcohol use, smoking and substance misuse. Participants felt that they had received insufficient training on physical health and health promotion. The majority were aware of weight gain in service users as an important and distressing side effect.
I’ve got quite a few young women who’ve been quite resistant to some medications because of the likely increase in weight and things like that.
HP4
Case managers welcomed the idea of a specific healthy-living intervention and clearly highlighted the benefits to patients. Health professionals felt that the healthy-living intervention should identify service users’ current health behaviours and provide information about improving health and assistance with behaviour change. Health professionals felt that the key components of a healthy-living intervention should address (1) healthy eating, including buying healthy foods on a budget, cooking skills and recipes, (2) the risks of weight gain and how to monitor weight, (3) exercise: what is available, physically possible, affordable and accessible, (4) dental hygiene, (5) substance misuse and (6) physical health monitoring such as blood checks.
The ideal format of an intervention was seen as a mix of group and individual sessions, which should be informal, relaxed, local and delivered within a sufficient timescale to allow for behaviour change and the maintenance of new behaviours. Although health professionals recognised the benefits of a healthy-living intervention, they felt that they did not have the necessary time or expertise to deliver the intervention and felt that STR workers were most appropriately placed to do so.
Well my own personal, if it was up to, you know like you say in my power I’d be looking at kind of it being you know like a couple of the STR workers who are particularly interested in that and leave it up to and empowering them, letting them you know have, putting out the guidelines where they want to go, because again they tend to have, you know, a better relationship.
HP1
Key findings
-
Health professionals felt that the monitoring of clients’ physical health and lifestyle was part of their day-to-day work but they were less optimistic about their own role in providing a healthy-living intervention.
-
The ideal format of an intervention was seen as a mix of group and individual sessions, which should be informal, relaxed, local and on a sufficient time scale to allow for behaviour change and the maintenance of new behaviours.
Theoretical framework
The common-sense model (CSM) of self-regulation89 was chosen as the theoretical framework for the healthy-living intervention because it provides guidance on both motivation and the implementation of behaviour change. In the context of rapid weight gain in early psychosis, the CSM would suggest that people’s motivation to lose weight, and the behaviours selected to pursue that goal, depend on their personal beliefs about, or models of, the weight gain – what is causing it, what its consequences will be and how controllable it is. These personal models are influenced by information gained from abstract (knowledge-based) and concrete (experiential) sources. Furthermore, the CSM suggests that people are more likely to change (e.g. to eat more healthily and to exercise more) if they develop belief-congruent,90 ‘if–then’ action plans that they can use to regulate their behaviour. Feedback plays an important role in the CSM, as it suggests that behavioural attempts to regulate weight gain will be appraised and will feed back into both beliefs and subsequent behaviours. Thus, the model suggests combining motivational approaches (including education to bring about belief change) with action-planning interventions and the opportunity to review the effectiveness of changed behaviour. 90 Therefore, the CSM suggested that when planning our intervention we should begin by assessing participants’ own perceptions of their health and weight gain. By doing so we could then ensure that information about healthy living that was provided would be tailored to the needs of each individual. There would also need to be monitoring of the impact of behavioural feedback (e.g. increasing activity levels) on beliefs and of belief change on behaviour. A variety of techniques to modify perceptions would be incorporated to help service users benefit from abstract (knowledge-based) information derived from their own experiences of weight gain. Action planning would be incorporated, with goals set in accordance with motivational beliefs, plans would be developed to regulate behaviour (including using helpful cues and overcoming potential barriers) and explicit monitoring of the effectiveness of behaviour would be carried out.
Cultural adaptations
The area where the study was conducted had a large South Asian population, particularly among the younger members of the community who were likely to access the early-intervention service. In recognition of the importance of culture in influencing lifestyle factors such as diet and exercise, we used the relevant literature91 and consulted with one of the authors (NH) who has expertise in developing and delivering culturally sensitive interventions. Using both sources we identified the key issues that would need to be considered when delivering the intervention to young South Asians. Cultural adaptations to the intervention included recognition of the need to address the importance of understanding the wider family’s view of health education and to involve them when developing action plans. There was a need to translate written materials related to the delivery of the intervention into the predominant language of Urdu, which was still spoken by older family members. Some of the healthy living materials that were developed needed to have culturally specific advice such as information on healthy eating in specific communities. Personnel involved in the delivery of the intervention needed to have a cultural understanding and awareness of the ethnic groups with whom they were working.
Synthesis
The research team held a 2-day workshop meeting in August 2008 to synthesise the data collected and model the draft intervention. The synthesis meeting was conducted in two stages. In the first stage members of the study team presented the findings of each of the preliminary studies, which were then summarised (Table 4). The second stage involved interactive exercises and discussions, focusing on the possible content, duration and delivery of the intervention, drawing on the evidence presented in Table 4. The aim of this stage was to determine the optimum content, delivery and duration of the intervention as well as who should deliver it, what training they would require and any additional materials that might be needed (Table 5).
| Systematic review and meta-regression | Qualitative interviews with users experiencing first-episode psychosis | Qualitative interviews with health professionals working in early-intervention services | Core dimensions of the self-regulation model | Incorporation of cultural issues |
|---|---|---|---|---|
| An individual intervention is likely to be more effective than a group intervention | Interventions need to be free and geographically accessible | Case managers think that they do not have the time to deliver a healthy-living intervention | Partly individualised | Need to recognise the role of families in decision-making |
| Lifestyle interventions are effective in promoting weight loss but this only borders on clinical significance and falls below this at 6 months’ follow-up | Preference for an active intervention rather than a purely educational intervention | STR workers or assistant case managers are more likely to be delivering the more time-consuming and active interventions Active interventions are important |
Needs measures of representations | Workers delivering the intervention would need some training in cultural awareness Materials need to be translated into key languages, particularly for the relatives of South Asian participants |
| Length of follow-up is insufficient to see longer-term gains and there was rapid attenuation of effects over time In terms of key components, actual exercise seems to enhance outcome The difference in effect size between preventative trials and weight loss trials is minimal |
Mixed views on group vs. individual interventions Information provided about healthy living was from variable sources and of variable content Service users perceived a low level of risk from the weight gained |
Case managers thought that promoting healthy living in service users was important Case managers had clear ideas about what the content of a healthy-living intervention should be Early intervention around healthy eating is important Cost and accessibility Healthy-living interventions can be individualised in a group setting |
Development and monitoring of action plans Variety of methods for presenting information Focus on behaviour and emotion |
Need to explore families’ as well as individuals’ explanatory models of poor health As an engagement strategy for the intervention, emphasise the cultural sensitivity of the intervention |
| Component | Systematic review | User interviews | Professionals interviews/focus groups | Self-regulation model | Cultural adaptations | Incorporated into the intervention |
|---|---|---|---|---|---|---|
| Content |
|
|
|
|
|
|
|
|
|
|
|||
| Format: Individual/group/mixed model |
|
|
|
|
|
|
| Who should deliver the intervention |
|
|
|
|
|
|
| Setting of the intervention |
|
|
|
|
|
|
| Number/duration of sessions |
|
|
|
|
|
|
In summary, the draft healthy-living intervention that we developed was to be delivered in eight individual sessions over a 12-month period by a STR worker specially trained for this purpose. To promote consistency we produced a manual, outlining the content and key activities to be followed in each session. In addition to individual sessions, optional active group sessions were offered to encourage people to make healthy-lifestyle changes. The intervention was culturally adapted to meet the needs of young people from the South Asian community and strategies for behaviour change are underpinned by the principles of the common sense model. 89 Information about healthy living, including a range of healthy recipes, was provided for participants in a booklet.
Stakeholder consultation
The final stage of the development work involved presenting the draft intervention to a range of stakeholders including service users (n = 3), carers (n = 2) and professionals (n = 3) to elicit their views regarding its acceptability and to gather suggestions for the group activities and the duration of the intervention. There was agreement that the intervention was both acceptable and feasible. Two key suggestions emerged: (1) to develop a website that had similar intervention to the healthy-living book and (2) that we should involve service users in the running of the groups.
Following the stakeholder consultation we started to develop materials to support the delivery of our intervention. These included a training manual for the STR workers who would deliver the intervention, a healthy-living booklet for participants in the study and a website (see www.helper-interact.co.uk/: website active at time of writing). We also recruited four service users who were interested in running groups for the study and organised a workshop to train both the STR workers to deliver the individual intervention and the service users to support the STR workers when delivering group activities.
The healthy-living intervention
The healthy-living intervention included eight individual sessions over a 12-month period with an emphasis on facilitating participatory exercise and dietary change through the development and implementation of patient-led action plans. These sessions were delivered by a STR worker trained in the delivery of the intervention. To facilitate implementation of exercise and dietary change a range of optional active group sessions was offered by the STR worker for those who prefer a group-based activity. To optimise engagement, choice and self-management, a booklet was given to all participants providing educational advice, action plans, goals, details of the group sessions, healthy-eating recipes on a budget, etc. (see Appendix 3). A strong emphasis was placed on maximising carer/family engagement.
Individual sessions
The intervention comprised eight individual sessions, five in the first 3 months followed by two in months 4–6 and a final session in months 7–12 to ensure that gains were incorporated into the individual’s lifestyle. The decision to include eight individual sessions was based on recommendations given during the synthesis day event, as there were no data to suggest an appropriate number of sessions (see Table 5). In brief, sessions one and two focused on eliciting health beliefs and developing a collaborative individualised action plan and goals for change. Such goals took account of previously and currently enjoyed activities that were exercise related. Sessions three to five focused on the implementation of the action plan (and, when agreed, the inclusion of family/carer involvement). Collaborative problem-solving identified and facilitated solutions to barriers to implementing the action plan. STR workers accompanied clients to particular activities (gym, swimming, etc.) to maximise implementation. Sessions six and seven focused on progress and monitoring of the goals and action plan and adaptions made as necessary. Session eight focused on working with the user to try to ensure that implementation is embedded within his or her everyday routine.
Group sessions
In addition to the individual sessions a range of optional group sessions, some of which were already being delivered by the early-intervention service teams (e.g. football, cycling), was delivered by STR workers, who also set up a range of group sessions including walking and cycling groups.
The healthy-living intervention booklet
A healthy-living intervention booklet was given to all participants and provided accurate information about healthy living, including government guidelines on exercise and diet; strategies for implementing such changes into usual daily routines; culturally varied recipes; barriers to and facilitators of implementing healthy living that are specific to the participants’ mental health problems, including fatigue and motivation; a section for families and carers; and information on local activities. Following the suggestion from the stakeholder group during the development work, a website was commissioned and developed by a service user (see www.helper-interact.co.uk/).
Training
Training was provided by the trial team to three STR workers and four service users and consisted of a 3-day intensive training programme. The training was accompanied by a training handbook that included detailed session-by-session outlines (see Appendix 4). The training focused on all aspects of delivering the intervention from initial assessment to elicitation of health beliefs, developing collaborative action plans and goals, engaging family/carers, monitoring and collaborative problem-solving and overcoming barriers to implementation and running groups. A significant portion of the training was spent practising the above skills using fictitious but typical cases to enhance learning and skill. Because of staff turnover the training was repeated on two occasions.
Supervision
Clinical supervision for the STR workers was provided on a 2-weekly basis by a member of the trial team.
Phase 2: evaluation of a healthy-living intervention to control weight in people taking antipsychotic medication after a first episode of psychosis
In phase 2 we aimed to determine the uptake, adherence and clinical effectiveness of a healthy living intervention designed to reduce weight gain. We conducted an exploratory RCT, comparing the intervention with treatment as usual in two early intervention services for psychosis in England. Evidence from this phase has been published elsewhere. 92
A summary of the work is described below.
Aims and objectives
Our overall aim was to determine the feasibility, acceptability and effectiveness of our developed health living intervention utilising an exploratory RCT.
Objectives
-
To estimate the effect size of a healthy intervention by comparing outcomes between:
-
individuals receiving the intervention with treatment as usual
-
the subgroup of the intervention arm prescribed olanzapine or clozapine at randomisation compared with the remainder of the intervention group.
-
-
To examine recruitment rates, uptake and adherence to the intervention.
-
To determine the direct costs associated with the intervention.
Methods
Design
An exploratory single blind RCT using a parallel group design to compare a healthy living intervention plus TAU with TAU only.
Participants
Current users of early intervention services in the north-west of England experiencing a first episode of psychosis in the preceding 3 years with a BMI of ≥ 25 kg/m2, or ≥ 24 kg/m2 for individuals from the South Asian community. 92
Procedure
Case managers screened their personal caseloads to identify service users meeting study criteria. All potentially eligible individuals were provided with a study information sheet. Those service users consenting to be contacted were contacted by a researcher and offered an appointment to assess their eligibility. Randomisation of eligible individuals entering the trial was undertaken by an electronic software program (Open Source Clinical Data Management System: OpenCDMS, University of Manchester, Manchester, UK, www.opencdms.org) and stratified by whether or not the individual had commenced olanzapine or clozapine medication in the preceding 6 months. Randomisation outcomes were concealed from the researchers (who remained blind when undertaking assessments) but were communicated to the trial manager, who notified the STR worker if allocated to treatment. Researchers reported unblindings if these occurred during the follow-up assessments conducted at 6 and 12 months.
Interventions
Healthy-living intervention
Full details of the development of the healthy-living intervention are reported elsewhere. 93 The format of the intervention constituted eight individual sessions with a support time recovery worker (who had undertaken a 3-day intervention training course), across a 1-year period and supported by an accompanying intervention manual/website. The content of the individual sessions involved the elicitation of existing health beliefs, psychoeducation, the development of personalised goals/action plans and an ongoing review of goal achievement progress. These sessions were supplemented by optional STR worker-supported group activities such as cycling and cooking.
Treatment as usual
Individualised case management in the early intervention service and enhanced care planning.
Measures
The primary outcome was BMI. Secondary outcomes included physical activity levels, depression, diet, quality of life, medication usage, health status and health economics. Assessments were conducted at baseline, 6-month and 12-month follow-up. (For full details, see Lovell et al. 92)
Results
A total of 971 participants were caseload screened (for flow diagram see figure 1 in Lovell et al. 92). Potentially eligible participants providing consent to be contacted were approached by the researcher (n = 148), of whom 105 individuals providing consent and meeting eligibility criteria were randomised. Of the 54 individuals randomised to the intervention (51 to TAU), five withdrew. The follow-up completion rate at 12 months was 88.6%.
Results found no significant effects between the intervention and TAU group. However, the effect of the intervention was larger (effect size 0.54, not significant) in 15 (28%) intervention and 10 (20%) TAU participants who were taking olanzapine or clozapine at randomisation.
Discussion
We found that our healthy-living intervention had a small but non-significant reduction in BMI compared with TAU. We found that in those participants who were taking olanzapine or clozapine at randomisation, there was a larger but non-significant effect. We successfully recruited to target and achieved an 89% retention rate at 12-month follow-up rate. Of the intervention participants, 78% completed 6–8 sessions. Overall costs of health and social care services used were lower but not statistically significant in the intervention group, compared with the TAU group.
Qualitative acceptability interviews
Aim
The aim of the qualitative acceptability interviews was to explore the acceptability of the healthy-living intervention from the perspective of those receiving the intervention.
Methods
To explore acceptability from the patients’ perspective, all patients randomised into the intervention arm were asked to participate in an interview to ensure inclusion of a range of patient baseline characteristics (i.e. age, gender and ethnicity to ensure a diversity of views). Semistructured interviews with consenting participants were conducted by a researcher. An interview guide (see Appendix 5) was developed by the trial team to ensure exploration of key areas including timing, content and duration of the intervention, STR workers’ qualities and barriers to and facilitators of the intervention.
Analysis
For acceptability the tapes were transcribed verbatim. Data were analysed using a framework analysis,94 as described in the section on interviews with service users/health professionals.
Results
Of the 49 participants randomised to the intervention, 25 were included in the acceptability study. The age range of participants was 17–39 years (SD 5.72 years) and 15 were male. Recorded interviews were of 24–64 minutes’ duration (mean 41.4 minutes). Participants who participated in the acceptability interview undertook a significantly greater number of intervention sessions (mean 7.3 sessions, SD 1.2 sessions) than those who did not (mean 5.8 sessions, SD 2.6 sessions). However, there was no significant difference in BMI at 12 months’ follow-up between those who did and those who did not take part in the acceptability interview.
Two main themes emerged from the analysis: (1) views on the delivery method and (2) the STR worker.
Views on the delivery method
Participants felt that they had worked collaboratively with their STR worker to set individualised and achievable goals and associated action plans. Goal-setting appeared to be viewed positively as a tool to motivate behaviour change:
Once you reach the 3-month goal, it gives you this spur to go for the 6 months.
Pt.8, M
The healthy-living education provided was widely regarded as helpful by participants regardless of their level of pre-existing knowledge, serving either as a first introduction to how to live healthily or as a useful ‘refresher’. Participants largely appreciated the written materials provided (e.g. the booklet) because they could be quickly accessed. However, some struggled to make use of such materials because of a dislike of reading or because of literacy problems; these individuals felt that the intervention could be improved through an option for more practical forms of learning (e.g. cookery courses) or by offering the information on a DVD. The trial website was poorly utilised but it was unclear whether this was because of a lack of acceptability or because of participants not having been made aware of it.
Service users’ willingness to join in with group activities was mixed; whereas some found group activities helpful, others declined the offer, suggesting that group activities could be stigmatising or could compromise confidentiality. Those valuing group activities often emphasised the social benefits, such as making friends and improving social skills.
Undertaking joint sports activities with the STR worker provided a confidence boost and/or motivation to try physical activities that participants would have felt unable to attempt alone. For example, one individual who suffered from agoraphobia progressed from walking around the ‘block’ with his STR worker to joining a local walking group. Some individuals wanted further opportunities to undertake joint activities with their STR worker and a small number reported disappointment that they had not received the opportunity to do this.
Monitoring tools such as food diaries and goal progress ratings were regarded as motivational and educational. One individual was able to re-evaluate the cause of her weight gain after completing a food diary:
When I wrote down the diary – this is one of the ideas – of what I was eating I realised it was what I was eating that was making my weight go up and not just taking the pills.
Pt.3, F
Participants found the experience of being weighed as part of the study follow-up procedures helpful, often because they did not have access to good-quality scales at home.
Service users largely felt that the length and spacing of sessions was appropriate, with only a small number expressing a preference for a longer or more intense intervention. Home visits from the STR (as opposed to meetings at a clinic, etc.) helped participants to fit the sessions into their daily routine.
The support, time and recovery worker
Participants described an individualised intervention into which they could have significant input, for example goal-setting, the pace of lifestyle changes and choice of activities. Delivery of a tailored intervention appeared to be related to the STR workers’ ability to listen and respond to individual needs and preferences:
It was nice because it was just someone supporting you with what you wanted to do rather than supporting you with what someone else told you to.
Pt.2, M
The small number of individuals who were less positive about the intervention appeared to have received a less individualised intervention. For example, one individual felt that his STR worker had failed to get to know him, with the consequence that she had tried to motivate him to undertake sports activities that he disliked.
The quality of the relationship with the STR worker appeared to be a key factor in the engagement of participants in the intervention. It was clear that many participants liked and enjoyed meeting their worker:
I saw [him/her], like someone nice, like, a friend I would see now and again.
Pt.23, F
Participants appreciated the flexible, understanding and non-judgemental attitude of their STR worker and often felt that they shared common ground, helping to put them at ease. One individual spoke positively about being able to cancel intervention sessions without fear of a negative response:
[He/she] understood where I was coming from. And I never felt . . . it wasn’t like phoning me doctor or perhaps, you know when I used to work, like phoning my boss and saying, ‘Oh, I can’t come in’; I never felt that intimidation.
Pt.9, M
Conclusions
-
Goal-setting was a helpful tool, facilitating positive lifestyle changes.
-
Although extremely useful for some, written materials may not suit all learning styles. An educational DVD or a practical course may provide an accessible alternative.
-
Although some individuals find group physical activities motivational, others regard them negatively, for example finding that they lack confidentiality. One-to-one joint sports activities appear to be more widely acceptable.
-
Written monitoring tools can help individuals to re-evaluate their behaviours and motivate positive changes to their lifestyle. Future interventions could include weight monitoring within the intervention package.
-
Home visits were convenient for participants. Eight sessions were largely felt to be appropriate; however, a minority of individuals would have preferred a more intensive intervention.
-
The key to the intervention appeared to be related to the STR workers’ ability to build high-quality relationships with service users by being flexible and non-judgemental and by recognising and responding to the individual needs and preferences of participants.
Overall conclusions of the workstream
-
Evidence is accumulating that weight gain is a serious problem for people with psychosis, leading to cardiometabolic problems and early death, and it is increasingly recognised that this must be addressed.
-
We used information drawn from a variety of sources (a systematic review and interviews with service users, case managers and key stakeholders) to design a theory-based intervention specifically for people with recent-onset psychosis.
-
In an exploratory trial of the intervention, we were able to train STR workers to deliver the intervention, to recruit to target, to retain participants and to achieve a high rate of follow-up and the intervention was acceptable to participants.
-
The effect of the intervention on reducing weight or controlling weight gain was small and not statistically significant, although it could be argued that we set ourselves too difficult a task by focusing on people at particular risk of weight gain (those with recent-onset psychosis) who were already overweight or obese.
-
In the subgroup taking two particular antipsychotic medications, olanzapine and clozapine, the effect of our intervention on reducing weight was larger, suggesting that future work should focus attention on this subgroup of service users.
Chapter 3 Substance misuse workstream: the Asking about Substance use and Psychosis – Ideas, Reactions and Experiences (ASPIRE) study and the Rethinking Choices After Psychosis (ReCAP) randomised controlled trial
Reprinted from Social Science & Medicine, 70, Lobban F, Barrowclough C, Jeffery S, Bucci S, Taylor K, Mallinson S, Fitzsimmons M, Marshall M, Understanding factors influencing substance use in people with recent onset psychosis: a qualitative study, 1141–1147. Copyright (2010), with permission from Elsevier.
Objectives: To optimise and evaluate integrated motivational interviewing and cognitive–behavioural therapy (MiCBT) for substance misuse.
Methods: Qualitative interviews were conducted with 19 young people with recent-onset psychosis to identify factors influencing the use of substances. Themes were used to inform the development of a phase-specific MiCBT intervention focused on harm reduction for young people with psychosis. The intervention was evaluated in a pragmatic single-blind randomised controlled trial (RCT) in which 110 participants were randomly allocated to one of three conditions: a brief MiCBT intervention (up to 12 sessions over 4.5 months) with standard care from an early-intervention service; a long MiCBT intervention (up to 24 sessions over 9 months) with standard care; or standard care alone. The primary outcome was change in cannabis use as measured by the timeline follow-back (TLFB).
Results: Thematic analysis of the transcripts from the qualitative interviews identified four key themes: influence of perceived norms on behaviour; attributions for initial and ongoing drug-taking behaviour; changes in life goals affecting drug use; and beliefs about the links between mental health and drug use. The outcomes of the RCT are not yet available but data indicate good retention and engagement rates: the majority of participants (n = 60, 80%) engaged with the therapy and we retained 76 (69%) participants until the end of the trial.
Conclusion: This is a methodologically robust study that will produce results that are generalisable to mental health services.
Introduction
Large numbers of people with psychosis use substances. Cannabis is the most widely used drug, with particularly high prevalence rates in people experiencing their first episode of psychosis. 95 There is evidence that cannabis use is associated with worse individual outcomes and with greater societal costs. Negative consequences of use include more suicidal behaviour, violence, social instability, non-adherence with treatment, service disengagement and an increased likelihood of relapse and hospitalisation. 96–99 For young people who experience psychosis, continued cannabis use can exacerbate the symptoms of psychosis and may maintain and aggravate the disorder. 99,100 Furthermore, these risks are present even at levels of consumption that would be considered unremarkable in the general population, suggesting that psychosis brings an increased sensitivity to cannabis. 101,102
It is widely believed that the pattern of illness established during the ‘critical period’ after a first episode determines the long-term prognosis of the condition. 103 Thus, repeated psychotic episodes associated with cannabis use may increase the risk of treatment-resistant symptoms and precipitate an irreversible decline in social functioning, leading to lifelong reliance on health and welfare services. Hence there is a strong case for targeting people at an earlier stage of the illness when patterns of cannabis use are not so well established78 and any deterioration associated with prolonged use has not set in. Additionally, there are some indications that it may be easier to motivate patients to reduce cannabis use if intervention occurs at an early stage of the psychosis. For example, in Melbourne, Australia, it has been reported that 50% of young people using cannabis at admission to early-intervention services voluntarily cease use within the first 6–10 weeks. 104
Although the negative consequences of cannabis use are well documented, there are very few interventions of proven efficacy for people who use cannabis and have psychosis. A Cochrane review of psychosocial interventions for people with substance misuse and severe mental illness105 found ‘no compelling evidence to support any one psychosocial treatment over another to reduce substance use (or improve mental state) by people with serious mental illnesses’ (p. 3), although the review did note some support for integrated MiCBT based on pilot work.
To date, four RCTs have evaluated interventions aimed at reducing cannabis use in young people with recent-onset psychosis,106–109 all of them small (sample sizes ranging from 47 to 103) and with intervention periods ranging from 3 to 6 months. One intervention utilised motivational interviewing (MI) only107 and the rest combined MI and CBT, one as a group intervention. 109 None of the studies investigating integrated MiCBT found an advantage of MiCBT over the control conditions in terms of reducing cannabis use or improving clinical outcomes, although in one study the treatment group reported better quality of life post treatment. 109 In the MI-only study107 there was a modest post-treatment reduction in number of joints smoked per week but this was not maintained at 12 months’ follow-up.
To take forward this area of research we optimised MiCBT to make it phase specific to the ‘critical period’ of early psychosis, developed phase-appropriate accompanying psychoeducational materials and designed a RCT to evaluate the intervention. Our aims were to compare a long-term intervention (24 sessions delivered over 9 months) with a brief intervention (12 sessions delivered over 4.5 months), both aimed at reducing cannabis use in a sample of people with recent-onset psychosis, and to examine the impact of the interventions on a range of clinical outcomes. Each intervention had the same focus: increasing motivation to reduce or to be abstinent from problematic cannabis use and planning, implementing and consolidating change. Thus, there were no differences between the two approaches other than the number of sessions scheduled and the time available to achieve each stage of therapy.
We recognised that to optimise MiCBT a greater understanding of the psychological factors that influence the use of substances was necessary, in particular an understanding of the self-reported reasons for initiating, continuing and stopping substance use in young people with psychosis. Previous investigations have involved people with more established psychosis, have been largely questionnaire based and have focused more narrowly on self-reported reasons for substance use. 110–112 There is evidence that patterns of, and motivations for, substance misuse may differ during first-episode psychosis. 113 We therefore conducted an in-depth qualitative investigation of experiences of substance use in young people with psychosis.
Our hypotheses were as follows:
-
Some young people who have used substances before or during the first episode will discontinue spontaneously for reasons that can inform the development a phase-specific psychological intervention.
-
The MiCBT intervention will be efficacious in reducing substance use in people with early psychosis.
-
A brief version of the MiCBT intervention (12 sessions) will be as efficacious as a longer version (24 sessions).
Phase 1: Asking about Substance use and Psychosis: Ideas, Reactions and Experiences (ASPIRE) – a qualitative investigation of the reasons why young people continue/discontinue using substances following psychosis
Introduction
In this stage of the research we aimed to access the psychological factors associated with substance use in first-episode psychosis, specifically the factors that enable or present barriers to stopping using substances. We investigated reasons for substance use; why people stop or do not stop using substances; and why some people start to use substances again following a period of abstinence. The exploratory nature of this work made a qualitative design ideal. We chose the grounded theory approach as our aim was to generate or discover a theory114 of substance use in first-episode psychosis. Our ultimate aim was to use the theory generated from this qualitative enquiry to aid in the development of a phase-specific intervention for substance use in early psychosis.
Methods
Participants were recruited from an early-intervention service based in the north-west of England between January and September 2008. Purposive sampling was used to achieve variation in age, gender and ethnic background to ensure that the sample would reflect the whole service population. Inclusion criteria were:
-
acceptance into the early-intervention service for treatment of psychosis (i.e. not including patients from the ‘high-risk’ group)
-
a period of 3 months when the client used substances on at least 2 days of each week for half of the weeks in the 3-month period. This 3-month period will be either at present or in the past, as far back as 6 months before the first contact with the early-intervention service
-
working knowledge of the English language
-
aged > 16 years.
Participant eligibility was checked using a substance use checklist that detailed use of substances (amount and frequency of use) over the preceding 3 months and the substance use modules of the Structured Clinical Interview for DSM-IV Axis 1 Disorders (SCID). 23
Interviews took place once eligibility was confirmed and informed consent obtained. Interviews were conducted at a place chosen by the participant, typically their own home. The main interview was topic guided (see Appendix 6) and lasted between 60 and 90 minutes. The study followed an iterative approach with the topic guide developing as the study progressed to reflect the developing inductive theories. This practice is in line with a grounded theory approach. 115 All interviews were digitally recorded and transcribed verbatim.
Analysis
The qualitative data were analysed by a team comprising two academic researchers, three clinical academics with psychological perspectives, one specialist nurse therapist and a non-clinical academic with expertise in qualitative methodology. One member of this analysis team had previous experience of using mental health services and of substance misuse.
Interview transcripts were read and preliminary coding was conducted concurrently with data collection. To ensure transparency and reliability, all transcripts were read and analysed by the interviewer and coded by at least two members of the team. The analysis process was managed using NVivo software (version 9; QSR International, Warrington, UK). Team members discussed coding and their interpretation of the transcripts in detail to refine codes and identify key themes emerging from the data. The original transcripts were then reread, coded and indexed. The themes highlight that which is shared by interviewees, but we have also acknowledged data that are at odds with these themes. 116 The team approach to analysis allowed inconsistencies between the data and themes to be debated, refined and reflected in the final presentation of the main themes.
Results
Participants
Nineteen participants were interviewed and their interviews were transcribed and labelled A–S to preserve participant anonymity. All participants had experienced a psychotic episode and were within 3 years of first contact with the early-intervention service. Ten participants (53%) reported currently misusing substances at the time of the interview and the remaining nine (47%) reported recent use. All participants currently were or had previously been regular cannabis users and for 13 (68%) cannabis was the primary drug of use. Eleven participants (58%) were regular users of more than one substance. Other drugs commonly used were amphetamine, cocaine, ecstasy, heroin, methadone and diazepam. The median age of participants was 23 years (range 18–35 years) and the majority (n = 15, 79%) were male and described themselves as white British (n = 17, 89%). Twelve participants were unemployed (63%) and two (11%) were students. The gender and ethnic mix of the sample are representative of the larger population of young people in the early-intervention service from whom they were recruited, which is predominantly white British and has a ratio of males to females of > 2 : 1.
Key themes
Analysis identified four key themes in participants’ accounts of factors influencing their substance abuse:
-
the influence of perceived drug norms on behaviour
-
attributions for initial and ongoing drug-taking behaviour
-
changes in life goals affecting drug use
-
beliefs about the links between mental health and drug use.
Theme 1: the influence of perceived drug norms on behaviour
When participants were questioned about how they began using drugs they often talked about how ‘normal’ drug taking was in their neighbourhood or community. Drug taking was often described as an integral part of local landscapes. For example, one participant (M) reported that ‘everybody I know takes drugs’. Another (E) stated that:
Cannabis is probably used even more than people even think it is, it’s like there’s so much that I’ve witnessed that only in a small town like [university town] and like where I’m from and stuff, but it, it just makes me feel that it’s everywhere in this country.
Participants who contributed to this theme perceived little stigma attached to drug taking. They saw drug use as a commonplace and socially acceptable behaviour in their communities. For some participants, drug taking was about subverting perceived social norms rather than being part of ‘normal’ culture. They believed that drug taking offered them a way out of ‘normative worlds’ and into a more exciting life. As participant A stated:
I’d rather live this life than **** do nothing all my life, to be honest. At least I’ve done sommat.
Some drugs were perceived as being more acceptable than others. One amphetamine user (R) explained:
Heroin’s always seemed to me as a really dark evil drug that made people, what I call wrong people, you know thieve them, do anything basically to get more heroin, even if they had to kill and stuff.
Other drugs, particularly cannabis, were perceived as being likely to improve social behaviour. Participant A reported:
It [cannabis] keeps us out of trouble to be honest and that’s what it used to do when we were younger as well, I mean, it stops you from going out and doing, causing riots basically or you go into like a bus shelter and you’d have a smoke there or sommat.
It was apparent that there was a tension between the acceptability of personal drug use and the morality of promoting drug use to family or friends. Participants generally agreed that it was wrong to encourage other people to take drugs and that drug use was not an activity they would like to see their own family, particularly their children, engage in.
Theme 2: attributions for initial and ongoing drug-taking behaviour
Some, but not all, participants identified specific reasons for their drug use. Among those who were able to identify specific reasons there were two types of attribution: internal attributions (i.e. that drug and/or alcohol use was an active personal choice) and external attributions (i.e. that substance use was due to the influence of others). Those people who made internal attributions described seeking out information and weighing up the pros and cons to make their decisions, summed up by participant J as:
No one told me I should do this, I’ve never had drugs pushed on me . . . I’ve always knowingly gone into it, but erm, essentially it’s fun, it’s a **** great time sometimes.
In brief, this group found substance use to be ‘fun’. They also reported beneficial effects of substance use on interpersonal relationships, using drugs as a way of connecting with other people and fitting in, both by reducing anxiety and improving social performance. As participant I explained:
I never liked being normal from the age of 12, I’d rather be drunk, you know it were drink at first, and erm, I used to get drunk at first, to become a louder person, ‘cause I were always a quiet, and speed makes you not quiet and it gives you confidence and all that, all things that I needed really, or that I thought I needed, ‘cause I weren’t a confident person so I, yeah it were probably to give me the confidence to talk to other people and have fun.
For those who attributed substance use to external factors, some described being coerced into it by other people whereas others described a more passive course, with substance use initiated and maintained because of exposure to substances as part of a social network.
Theme 3: changes in life goals affecting drug use
In many accounts a key reason for reducing or stopping substance misuse was a change in personal life goals, especially an increase in the perceived value of health, disposable income and close family relationships. These reasons were largely identified by older participants who had reduced their use of substances and who were reflecting on the reasons for this. For example, one participant (J) said:
I don’t believe that I could possibly cope with my job and my relationships and so on and I don’t want to risk them, and so I don’t do it any more. I want to erm, make new circles of friends and I’d rather go to dinner parties these days than to a rave, you know, I’d like to have a nice steak dinner in a restaurant more than I’d like to take a pill.
Younger participants who were still using drugs identified their current goals and existing social networks as barriers to changing their drug use, recognising that drastic changes to these would be needed to allow them to make changes, as the following exchange shows:
All my friends do it, they all take drugs, they all smoke weed and not one of them has ever thought of quitting or even if they have they’ve not a chance they ever will, same for me.
So you don’t think you could actually do it?
I’d need to be taken away, like erm, you could put me in a hospital say for 2 weeks, I’d need to go away for a month, where I can’t get hold of any, you know, I can’t go out and get it or touch it, basically cold turkey, ‘cause if I’m around it and around people I can’t say no.
Some participants did experience significant life events that led to sudden and dramatic changes in behaviour. One participant described how family members intervened and took away his daughter:
Me mum and dad took me daughter off me, fetched her back here and they said I had a choice of staying with [ex-partner] and staying on the drugs, or coming home, getting myself ok clean and having me life with me daughter, so I decided to move out, get meself clean and sort meself out.
Theme 4: beliefs about the links between mental health and drug use
Many participants had theories about the connection between drugs and the onset of psychosis that were clearly influencing their behaviour. Some drew on sophisticated formulations in which drug use mediated between biological and/or life events and the onset of mental health problems, whereas others reported substance use to be a way of coping with existing mental health problems such as anxiety and depression. At the same time there was awareness that drug use could exacerbate symptoms and make problems worse. As participant S reported:
It just helps to bury your problems just for that day and then, but you don’t realise that your problems just stay there that are waiting for you to come back out again, they don’t just like disappear when you want them to, that’s all I would say with drugs, just buries it for you a couple of days, a couple of hours or what have you, and then it just comes back, it just keeps on building and building, adding more problems to it, you just keep on building it up to the day it just erupts.
The themes that emerged from this work develop our understanding of patterns of drug use in people with early psychosis and can be used to inform interventions at a number of levels. The changes in life goals that are associated with changes in substance-use behaviour lend support to the use of harm reduction approaches. Findings support the use of psychological approaches that help people to identify and acknowledge the positive reasons for their drug use, to explore the potential negative effects, including the impact on mental health problems and to find alternative strategies to achieve desired goals. In particular, the importance of peer group identity needs to be acknowledged and the role of drug use in facilitating inclusion into a desired social group needs to be examined. Finally, educational materials are unlikely to be helpful unless they resonate with personal experience and are offered in forms and via media that appeal to this age group.
Development of the intervention
The intervention that we sought to optimise and evaluate was originally developed for use in the Motivational Interventions for Drugs and Alcohol misuse in Schizophrenia (MIDAS) trial. 117,118 The MIDAS trial included people with substance use of any type (including use of alcohol above safe limits) who had established psychosis (i.e. who were not experiencing their first episode of psychosis) and the intervention was, therefore, adapted to be relevant for a first-episode population with cannabis use as its main focus. The intervention integrates the recognised therapeutic approaches of MI, CBT and relapse prevention. MI was included because it helps people to recognise the advantages and disadvantages of substance use, including the role of substances in psychotic experiences; it places responsibility for changes in behaviour on the individual, while encouraging the therapist to elicit and reinforce the person’s motivation for change until they reach an action stage of planned substance reduction. CBT helps the person plan and achieve change, including, when appropriate, helping him or her to understand his or her psychotic experiences, and it offers alternative strategies for managing symptoms that can take the place of substance misuse. Relapse prevention identifies the obstacles that arise in managing problematic substance use behaviours, reviews the factors that may trigger relapse of problematic substance use at different stages of recovery and presents procedures for teaching effective cognitive and behavioural coping strategies to deal with problematic substance use. The benefit of integrating MI, CBT and relapse prevention is in ensuring that all elements of the service user’s problems are given attention and that any negative interactions between mental health and substance-use problems can be formulated and addressed while taking into account the person’s motivation to address or reduce his or her substance use. We expected that in some participants motivation to reduce substance use would be low, and MiCBT, with its formulation-driven approach, is sufficiently flexible to work with other client-led problems if the initial attempts to increase the client’s motivation for change in substance use are unsuccessful.
The therapeutic model
The heuristic model underpinning the intervention (Figure 7) was also developed for use in the MIDAS trial. The model combines aspects of Marlatt and Gordon’s social cognitive theory of problematic substance use119 with elements of Blanchard and colleagues’ affect regulation model. 120 The model, shown here as applicable to people with co-occurring psychosis and cannabis use, proposes that certain situations and cues trigger cannabis use-related thoughts, which, in the absence of alternative strategies and in the context of low self-efficacy for resisting use and positive expectancies from use, make the person vulnerable and more likely to use cannabis. This interaction between situations and cognitive/emotional reactions becomes the basis of a repeated cycle that maintains the cannabis use.
FIGURE 7.
The therapeutic model.
Within this context, poor problem-solving abilities and limitations in obtaining pleasure in ways other than through cannabis use increase the likelihood of the learned expectations of the positive benefits of repeated cannabis use being reinforced. We have found in clinical practice that the contributing role of cannabis use to psychosis may not be as significant to the individual as to other concerned people, that is family or mental health services. Rather, cannabis use is likely to be associated with more positive than negative expectancies, leading to a situation in which cannabis use is maintained. Such perceived positive benefits may include, for example, mood enhancement, relief from boredom, anxiety reduction, opportunities for social interaction with other cannabis users, ‘time out’ from problems, the blocking out of unpleasant thoughts, general feelings of well-being, release from unpleasant self-consciousness and performance enhancement through physiological effects on arousal or other mechanisms.
Using the model as a template facilitates the therapist in completing a detailed functional analysis of the links between the person’s psychosis, associated difficulties and continued cannabis use. To achieve this, the therapist elicits information at distinct stages of the therapeutic intervention. The model assists the therapist to elicit self-motivational statements from the individual regarding the negative aspects of continued substance use and to then feed this back through a shared understanding.
The intervention
We recognised the need for therapy to explore and resolve ambivalence before engaging in change strategies. As such, the therapy has three specific phases: (1) building engagement; (2) exploring and resolving ambivalence in the direction of changing problematic cannabis use; and (3) the use of recognised psychological intervention strategies to facilitate changing problematic cannabis use in the context of psychosis (Table 6). Each phase of therapy comprises therapeutic strategies (stages) specific to that particular phase. The accompanying manual (available from Professor Christine Barrowclough on request) describes how to deliver therapy appropriate to individual need. For example, the service user who identifies early in therapy, that is in sessions 1–4, that he or she wants to commit to making a change in his or her cannabis use would not need to focus on phase 2 strategies designed to explore and resolve ambivalence. The service user who is engaged in therapy but who remains ambivalent about changing his or her cannabis use, however, will continue to be engaged in phase 2 strategies designed to explore his or her ambivalence.
| Phase 1 | Engagement | Goals |
|---|---|---|
| Stage 1 | Building engagement |
|
| Stage 2 | Eliciting an explanatory model of psychosis and cannabis use |
|
| Stage 3 | Identifying key values and development of list of goals |
|
| Stage 4 | Discussing information about psychosis and cannabis use |
|
| Phase 2 | Formulation | Goals |
| Stage 5 | Exploring the relationship between the symptoms of psychosis and cannabis use |
|
| Stage 6 | Identification of how problematic cannabis use fits into life concerns and satisfactions |
|
| Stage 7 | Developing a shared understanding/formulation |
|
| Stage 8 | Discussing and identifying possible change options |
|
| Stage 9 | Revisiting the formulation and developing a change plan |
|
| Phase 3 | Action | Goals |
| Stage 10 | Integrated CBT for cannabis use and psychosis |
|
| Stage 11 | Harm-minimisation strategies |
|
| Stage 12 | CBT for distressing mental health difficulties |
|
| Stage 13 | Developing therapy summary |
|
We optimised the intervention used in the MIDAS trial by making it phase specific for the ‘critical period’ of early psychosis, in which the patterns of, and motivations for, substance misuse appear to differ from those seen in the later stages. 113 In a first-episode population, the prevalence of street drug use is high and our qualitative work confirmed that issues such as drug use being perceived as a normalising social behaviour would have to be addressed. We used the findings from the qualitative study to develop appropriate and youth-friendly information resources suitable for this population, using materials from early-intervention services in other countries as a starting point.
In the MIDAS trial we found that people who made changes during therapy were more likely to maintain this change at follow-up. We therefore adapted the intervention to include greater use of behavioural experiments (experimenting with making changes) earlier in the therapy. For those not committed to making change, the behavioural experiments were an opportunity to test out their positive cannabis beliefs, for example ‘If I don’t use cannabis until 8.00 pm rather than when I wake up (11.00 am) I will not be able to manage my anxieties’. Belief ratings were elicited both before and after the experiments. Ways of managing the situation differently during the experiments were discussed. Conclusions from the experiments were discussed with the aim being to explore positive expectancies of cannabis use.
Our qualitative work showed that some young people with psychosis are ambivalent about any potential negative consequences to their mental health as a consequence of their cannabis use. They may associate a number of positive expectancies with cannabis use such as aiding sleep, reducing anxiety and fitting in with others socially. Furthermore, cannabis use might be a significant aspect of their social identity given its extensive use within the social network.
We recognised that service users with early psychosis are typically young people entering mental health services for the first time. They come into early-intervention services with a variety of expectations, previous experience and information. Many may not necessarily be actively seeking help. We therefore expected that in some cases service users may be reluctant to participate in a dialogue with the therapist about their concerns or even tolerate the presence of the therapist for periods of time.
The aims of the first phase of MiCBT are therefore to engage the service user in the therapeutic process, begin a discussion of their understanding of psychosis and cannabis use and identify their key values and goals. Several potential barriers to engagement exist in this group. Their relatively young age may impact on their readiness to engage. They may be unfamiliar with talking openly with somebody older or seemingly in a position of authority, resulting in caution or fear about discussing personal matters. There may be a perception that the therapist disapproves of their substance use and they may mistakenly believe that the point of therapy is to persuade them to give up smoking cannabis completely. The therapy, therefore, explores these perceptions in the early engagement stage to decrease resistance and prevent early termination of therapy.
The intervention seeks to gain an understanding of how the service user believes that he or she has arrived at his or her current situation with regard to involvement with early-intervention services. Thus, there is an increased focus on the participant’s explanatory model.
As the service users will have only recently had their first episode of psychosis, they may not as yet have had the opportunity to access any psychoeducation. Eliciting their current understanding helps the therapist to know what information will be useful to discuss with them in the later stages of therapy when using psychoeducation to build motivation for change.
Development of educational materials
Our preliminary qualitative work highlighted the need for any psychoeducational materials to be relevant to young people and to be grounded in personal experience when possible. We produced several information leaflets to be used during therapy. These provided easily digestible information on reasons that people have given for using cannabis, measuring amounts of cannabis use in comparison to use in the general population, potential negative consequences of cannabis use and managing cannabis withdrawal (see Appendix 7).
In addition to these leaflets we developed two short animations to be watched during stage 4 of the therapy – ‘Providing and discussing information on psychosis and cannabis use’. These animations, in DVD format, were based on transcripts from the Asking about Substance use and Psychosis – Ideas, Reactions and Experiences (ASPIRE) study previously outlined and were thus aimed specifically at service users within early-intervention services who have had an episode of psychosis and who also use cannabis. The animations were developed in a user-friendly format and consist of two segments of film, each 3 minutes in length, in which two animated characters talk about their experience of cannabis use and psychosis.
The style of the animation was inspired by the Lifeline.org.uk publications developed by Mark Holland and Mike Linnel, in particular the Out of your Head guides (see www.exchangesupplies.org/shopdisp_A37.php; accessed 19 November 2014).
To develop the DVD two team members reviewed the 19 interviews transcribed for the ASPIRE study. 121 Sentences fitting within the themes identified by ASPIRE were highlighted and extracted. From these extracts four stories were constructed and scripts developed. Our aim was for the scripts to be believable, for the characters within them to be ‘recognisable’ and for service users to be able to identify with them and with their stories.
Scripts were reviewed by a local consultant dual diagnosis nurse and other team members before being sent for animation by the graphic design team at the Media Centre at the University of Manchester. Two of the scripts were eventually animated (see Appendix 8 for a still image from the DVD) and voiceovers were recorded by actors with appropriate regional accents. Copies of the DVD are available on request from the authors.
Our plan was for the therapist and service user to watch the whole DVD or clips together to generate discussion. The DVD was also used as an aid to engagement by making a change from the usual format of talking and listening. For example, the therapist might ask, ‘In what ways do you think [the character] could improve his current situation?’ or ‘In what ways are [the character’s] experiences similar to your own?’
Following our preliminary work, which led to the optimisation of the MiCBT intervention to make it ‘phase specific’ and the development of phase-appropriate psychoeducational materials, we conducted a pragmatic trial of the intervention within early-intervention services. Our aim was to evaluate the intervention and to calculate the likely sample size required for a definitive multicentre trial.
Phase 2: a phase-specific psychological therapy for people with problematic cannabis use following a first episode of psychosis: the Rethinking Choices After Psychosis (ReCAP) trial
Methods
Design
We conducted a pragmatic RCT of brief MiCBT plus standard care compared with longer-term MICBT plus standard care and standard care alone. The trial was overseen by independent data monitoring and trial steering committees and is reported in accordance with the CONSORT guidelines for non-pharmacological trials. 93 Ethical approval was obtained from the Cumbria and Lancashire B Research Ethics Committee (reference 08/H1015/82). All participants gave written informed consent before taking part.
Participants
Participants were recruited from early-intervention services in five mental health trusts in the north-west of England between January 2009 and April 2011. Inclusion criteria were current involvement with early-intervention services; age 16–35 years; meeting DSM-IV criteria for non-affective psychotic disorder;40 a DSM-IV diagnosis of cannabis dependence or abuse;40 a history of cannabis use of at least 1 day per week in at least half of the weeks in the 3 months prior to assessment; having stable accommodation (i.e. not street homeless or roofless); possessing sufficient English to reliably complete the assessments; no significant history of organic factors implicated in the aetiology of psychotic symptoms; and an ability to give informed consent.
Estimating the sample size
For the primary outcome of frequency of cannabis use, if the intervention was successful we would expect to see a clinically significant reduction equivalent to 5 days of use relative to the control group. 108 Assuming a SD of 5 at baseline, a sample of 29 participants in each of the three groups was required to have a 90% chance of detecting this difference in three pairwise comparisons using a significance level of 0.05 (nQuery Advisor, 2012; Statistical Solutions, Saugus, MA, USA).
Procedure
Potential participants were identified by referral from care co-ordinators. The research team worked proactively with early-intervention services to identify services users who might be potentially eligible for the trial. This included attending team meetings to request referrals and meeting one-to-one with care co-ordinators to review their entire caseload to make sure no-one was missed. The first approach to the patient came from the care co-ordinator. After a complete description of the study had been provided to the client, giving plenty of opportunity for him or her to ask questions and go away to talk to other people about the study, written informed consent of those agreeing to participate was obtained by a trained research assistant. Diagnostic and substance use eligibility was confirmed by research assistants using the SCID. 22 A substance use checklist was used to determine the frequency and amount of substance use, including alcohol use. Participants were assessed on multiple measures before randomisation to one of the three arms of the trial.
Randomisation
Random allocation to brief therapy plus standard care, long therapy plus standard care or standard care alone was performed using openCDMS, an independent remote service. Participants were randomly allocated within each of the trusts to one of the treatment arms using computer-generated randomised permuted blocks, with randomly varying block sizes of 2, 4, 6 and 8, after stratifying by variables believed to be predictive of treatment participation or outcome (e.g. gender, living with family vs. not living with family). Notification of randomisation was immediately communicated to the trial manager and trial therapists.
Intervention
The psychological therapy was integrated MiCBT, optimised for use with a first-episode cannabis-using population. Participants in the brief intervention condition were offered up to 12 sessions of MiCBT over 4.5 months; participants in the long-term intervention condition were offered up to 24 sessions over 9 months. Both interventions were delivered at the participants’ preferred location, typically their own home. Sessions were audiotaped with the participants’ consent in order to be used for the evaluation of treatment fidelity and for therapist supervision. Therapy was delivered in accordance with the treatment manual, which is available on request from Professor Christine Barrowclough.
The therapy stages have been described earlier. Both interventions progressed through the same 13 steps outlined in Table 6. However, the long intervention allowed more time to develop the change plan and particularly the use of CBT within the plan. Progress during therapy was communicated to the care co-ordinator at two liaison meetings attended by both the therapist and the participant. These took place in the middle and towards the end of therapy.
Standard care from the early-intervention services involved in the study is compliant with the Policy Implementation Guide122 and includes intensive case management, crisis response, behavioural family therapy and cognitive therapy for persistent symptoms.
Training and monitoring of trial therapists
The trial therapists were two nurse therapists and one clinical psychologist, all of whom had experience in conducting CBT with people with first-episode psychosis.
The following training standards were met before the therapists commenced delivery of the intervention:
-
Met minimum training standards for the practice of CBT as set out by the British Association for Behavioural and Cognitive Psychotherapies (BABCP). Full details can be found on the BABCP website (see www.babcp.org.uk; accessed 19 November 2014).
-
Completed training in MI and supervised practice of MI with at least two clients with psychosis.
-
Achieved a ‘competence’ rating for at least one therapy tape rated on the Motivational Interviewing Treatment Integrity (MITI) scale. 123
Treatment was assessed in supervision using recorded therapy sessions, discussion of individual cases and feedback on the use of MI using MITI ratings. Treatment fidelity was assessed by an independent rater with previous experience of delivering and assessing MI and CBT. A sample of 20 digital recordings, each from a different participant, was randomly selected from a pool of 289 recorded sessions. Fidelity was rated on the MI-CBT fidelity scale. 124 This scale consists of 16 items rated as either ‘compliant’ or ‘not compliant’.
Assessments
All assessments were conducted by research assistants who were blind to treatment allocation, normally in the participant’s home, but occasionally at an early-intervention service venue, doctor’s surgery or community building. Before carrying out the assessments, research assistants received extensive training in conducting psychological and substance use assessments and in collecting hair samples. They were asked to record all instances of ‘unblinding’ (inadvertently becoming aware of treatment allocation) and were required to report them to the trial manager immediately. To maintain the blinding, research and therapy staff were housed in different locations, assessment and therapy data were stored separately and participants and care co-ordinators were reminded not to divulge information that might lead to the ‘unblinding’ of the research assistants. In total, 56 instances of ‘unblinding’ were recorded involving 38 different participants. For the majority of these a new ‘blind’ assessor was allocated. When this was not possible (on seven occasions) the assessment was conducted ‘unblinded’ and assessments were rated by a blind rater via digital recording.
Data on substance use, symptoms and illness history and demographic information were collected at baseline by self-report. Care co-ordinators provided additional information on substance use to corroborate these self-reports. Outcomes were assessed after completion of treatment (at 4.5 and 9 months for all groups to prevent ‘unblinding’) and 18 months after treatment allocation.
Substance use
The primary trial outcome was number of days abstinent from cannabis in the last 90 days as determined by the TLFB assessment. 125
The TLFB assessment, administered by the research assistants, uses an annotated calendar that is personalised for each participant. Significant events or regular patterns (e.g. ‘payday’) are recorded on the calendar and the calendar then serves as a memory cue for participants as they try to recall daily alcohol and drug use. For each of the 90 days the researcher records the number of drinks consumed, the quantity (in millilitres) and the strength of alcohol (which is later converted into alcohol units) and the type, amount (in grams) and cost of the drugs consumed. Participants were instructed to be as accurate as possible but when recall was difficult they were instructed to provide their best guess.
Additional substance use outcomes, also determined by the TLFB assessment, included total consumption of cannabis (in grams) over the preceding 30 days and average daily weight (in grams) of cannabis consumed per cannabis-using day, number of days abstinent from all substances and percentage change from baseline in amount of cannabis used. The TLFB assessment has good reliability and validity in dual-diagnosis populations. 118,126,127 The Readiness to Change Questionnaire (RTCQ)128 was used to assess motivation to change substance use. The Reasons for Substance Use in Schizophrenia scale110 was used to examine reasons for cannabis use and the Cannabis Experiences Questionnaire129 and Inventory of Drug Use Consequences130 assessed the self-reported consequences of use.
Care co-ordinators completed a brief version of the TLFB assessment (reporting participants’ use of substances per week for the previous 13 weeks) and the Drug Use Scale of the clinician rating scales131 to validate participants’ self-reports. These were conducted at baseline and at 4.5, 9 and 18 months. Additionally, 29% of the participants gave hair samples, which were subsequently examined by a specialist hair analysis company (Concateno TrichoTech Ltd, Cardiff, UK) for the presence of cannabis.
Symptoms and functioning
The PANSS28 was used to assess the positive, negative and general symptoms associated with psychosis. Distress in relation to symptoms was assessed using the PSYRATS. 26 Functioning was assessed using the Global Assessment of Functioning (GAF) scale. 22 Anxiety was assessed using the Beck Anxiety Inventory (BAI)132 and insight was assessed using the Birchwood IS. 32 Depression was examined using the CDSS,29 with participants also being asked to report previous instances of serious self-harm. Attitudes towards prescribed medication were assessed using the Drug Attitude Inventory (DAI),133 which can be used to categorise respondents as either ‘adherent’ or ‘non-adherent’ to medication.
All assessors rated 10 ‘gold standard’ video-recorded PANSS interviews before conducting trial assessments. Mean intraclass correlation coefficients were high, indicating excellent inter-rater reliability (positive subscale 0.87; negative subscale 0.86; general subscale 0.87; total 0.89; global assessment of functioning 0.94). Ratings were monitored throughout the trial as part of supervision and intraclass correlation coefficients remained high (positive subscale 0.90; negative subscale 0.85; general subscale 0.90; total 0.95).
Relapse and hospitalisation
Data on relapses and hospitalisations were obtained from participant psychiatric case notes. The frequency and duration of relapses and hospitalisations in the 9 months before the trial and during the 18-month intervention and follow-up period were recorded. ‘Relapse’ was defined as an exacerbation of psychotic symptoms that lasted for > 2 weeks and which resulted in a change in patient management (increased observation by the clinical team, increase in antipsychotic medication or both). The start and end dates of each relapse were recorded along with verbatim extractions from the notes detailing changes in symptoms and clinical management. The start and end dates of all hospital admissions for psychiatric purposes were also recorded. Admissions made for preplanned changes in medication were not included.
Information on antipsychotic medication and duration of untreated psychosis (DUP) was also obtained from case notes.
Before accessing participant case notes, research assistants were trained to protocol and extracted hospitalisation and relapse data from six test cases. Inter-rater reliability across assessors was excellent, with 100% agreement on admission (yes/no), number of admissions and number of weeks admitted. Intraclass correlation coefficients for relapse variables were also high, with 0.86 obtained for relapse (yes/no) and 0.97 for number of relapses.
Results
Participants
A total of 325 patients were identified as being potentially eligible. Of these, 138 declined to be screened for eligibility and 43 did not meet eligibility criteria; 144 consented to take part, of whom 34 withdrew consent before randomisation; 110 completed all baseline assessments and were randomised. Of these, 38 were allocated to brief therapy, 37 to long therapy and 35 to TAU. Data on the primary outcome were collected for 83 (75.5%) participants at 4.5 months, 79 (71.8%) participants at 9 months and 76 (69.1%) participants at 18 months. Full data on relapse and hospitalisation were available for 108 participants (98.2%). Figure 8 shows the participant flow through the trial.
FIGURE 8.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram showing participant flow through the trial. DNA, did not attend.
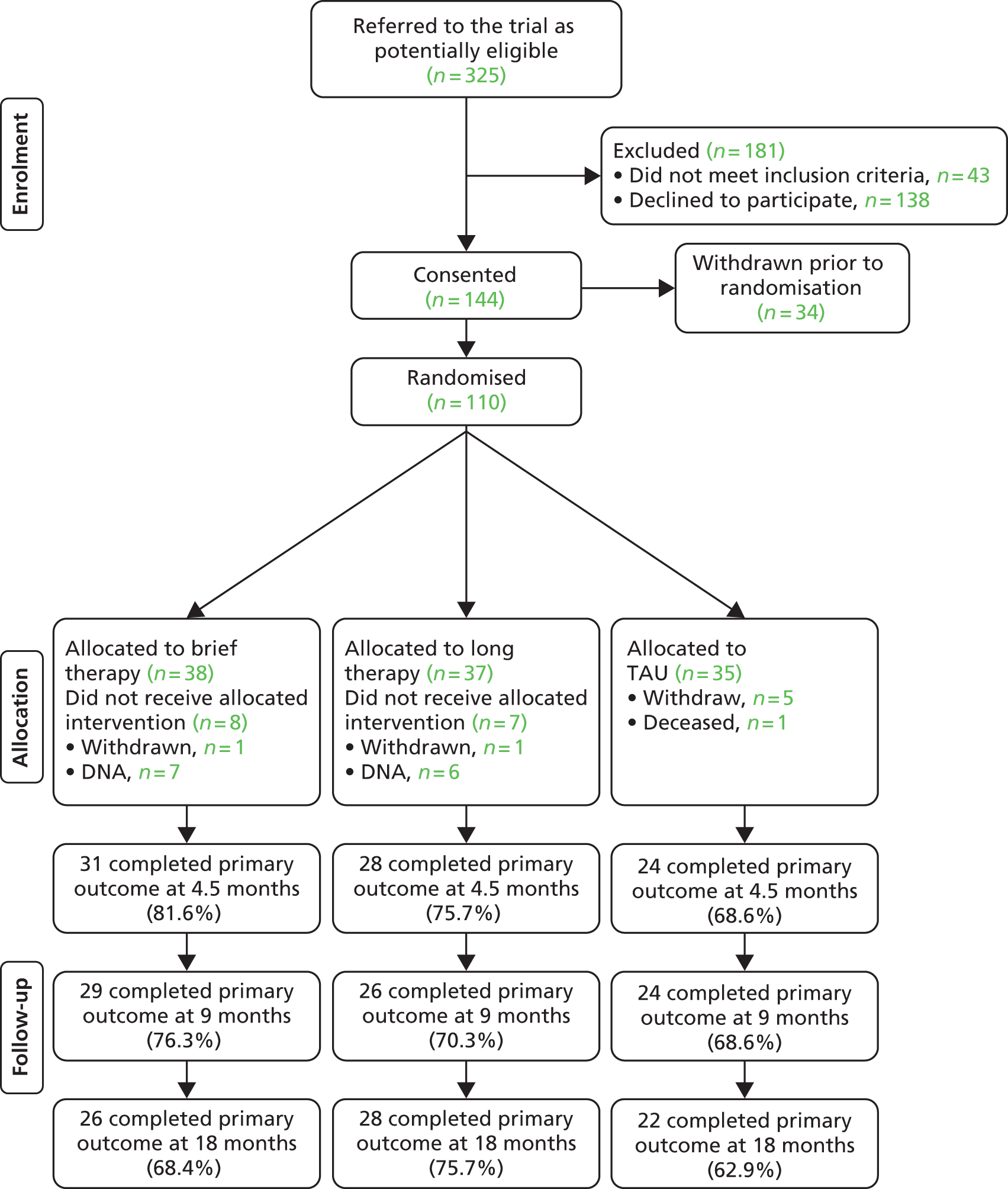
Tables 7 and 8 present baseline demographic, psychiatric and substance use information for participants in each trial arm. Participants were mostly male and aged in their mid-20s. The majority were unemployed. They had a history of psychosis of < 18 months and had been using cannabis for around 10 years, having begun using cannabis in their early teenage years. The majority (n = 100, 90.9%) met criteria for cannabis use dependence. Participants had been using cannabis on 59 out of the 90 previous days on average (SD 27.5 days), consuming an average of 107 g of cannabis in the same time period. The average amount of cannabis per cannabis-using day was 1.7 g (SD 1.5 g). The majority of participants were smoking mostly skunk (78.2%), with 20% smoking mostly resin and the remainder (1.8%) smoking equal amounts of each. More than four out of five participants were contemplating changing their cannabis use at entry to the study according to the RTCQ, with 53.2% identified as being at the ‘contemplation’ stage and 31.2% at the ‘action’ stage.
| Variable | TAU (n = 35) | Brief therapy (n = 38) | Long therapy (n = 37) |
|---|---|---|---|
| Age (years), mean (SD) | 23.4 (3.8) | 24.9 (5.6) | 24.1 (5.4) |
| Gender: male, n (%) | 30 (85.7) | 34 (89.5) | 34 (91.9) |
| Living arrangements, n (%) | |||
| Alone/house-share/hostel | 15 (42.9) | 14 (36.8) | 15 (40.5) |
| With partner or family | 20 (57.1) | 24 (63.2) | 22 (59.5) |
| Ethnicity, n (%) | |||
| White | 33 (94.3) | 35 (92.1) | 34 (91.9) |
| Black and minority ethnic | 2 (5.7) | 3 (7.9) | 3 (8.1) |
| Attended higher education, n (%) | 21 (60.0) | 16 (42.1) | 19 (51.4) |
| Employment, n (%) | |||
| Unemployed/retired | 27 (77.1) | 30 (78.9) | 32 (86.5) |
| Employed/self-employed | 4 (11.4) | 2 (5.3) | 2 (5.4) |
| Student | 4 (11.4) | 6 (15.8) | 3 (8.1) |
| History of psychosis (months), median (range) | 17.2 (2.3–57.0) | 13.4 (1.4–59.6) | 17.5 (1.8–62.8) |
| DUP, n (%) | |||
| < 4 months | 17 (54.8) | 13 (37.1) | 10 (30.3) |
| > 4 months | 14 (45.2) | 22 (62.9) | 23 (69.7) |
| Adherent to medication (DAI), n (%) | 25 (71.4) | 30 (78.9) | 30 (81.1) |
| Diagnosis (SCID), n (%) | |||
| Schizophrenia | 18 (51.4) | 20 (52.6) | 16 (43.2) |
| Schizophreniform disorder | 3 (8.6) | 3 (7.9) | 3 (8.1) |
| Schizoaffective disorder | 3 (8.6) | 5 (13.2) | 5 (13.5) |
| Delusional disorder | 3 (8.6) | 2 (5.3) | 4 (10.8) |
| Substance-induced psychosis | 3 (8.6) | 2 (5.3) | 1 (2.7) |
| Psychotic disorder NOS | 5 (14.3) | 6 (15.8) | 8 (21.6) |
| PANSS score, mean (SD) | |||
| Positive | 14.9 (3.1) | 15.4 (4.5) | 14.8 (4.7) |
| Negative | 14.1 (5.4) | 15.1 (3.8) | 13.0 (4.9) |
| General | 32.7 (6.8) | 35.6 (7.1) | 33.8 (7.2) |
| GAF score, mean (SD) | 37.9 (9.0) | 35.1 (7.2) | 39.0 (10.5) |
| CDSS score, mean (SD) | 5.7 (5.5) | 7.7 (4.6) | 7.3 (3.9) |
| Anxiety (BAI), mean (SD) | 14.8 (10.9) | 17.1 (11.7) | 20.3 (12.8) |
| Relapsed (9 months pre baseline), n (%) | 18 (51.4) | 16 (42.1) | 17 (45.9) |
| Admitted (9 months pre baseline), n (%) | 7 (20.0) | 6 (15.8) | 10 (27.0) |
| History of cannabis use (years), mean (SD) | 9.0 (4.3) | 10.3 (5.3) | 10.4 (5.5) |
| Substance use disorder (SCID), n (%) | |||
| Cannabis abuse | 2 (5.7) | 5 (13.2) | 3 (8.1) |
| Cannabis dependence | 33 (94.3) | 33 (86.8) | 34 (91.9) |
| Alcohol abuse | 3 (8.6) | 2 (5.3) | 2 (5.4) |
| Alcohol dependence | 1 (2.9) | 2 (5.3) | 0 (0) |
| Variable | TAU (n = 35) | Brief therapy (n = 38) | Long therapy (n = 37) | Comparison |
|---|---|---|---|---|
| Median (min.–max.) | Median (min.–max.) | Median (min.–max.) | χ2, p-value | |
| Proportion of days abstinent from cannabis (%) | 42.2 (0–88.9) | 21.1 (0–92.2) | 26.7 (0–6.7) | 1.4, 0.50 |
| Total cannabis use (g) | 87.8 (7.5–322.5) | 108.3 (1.2–640.0) | 42.8 (1.0–377.4) | 4.4, 0.11 |
| Average amount of cannabis used per cannabis-using day (g) | 1.3 (0.3–9.4) | 1.5 (0.1–7.1) | 1.2 (0.1–4.2) | 5.3, 0.069 |
| Proportion of days abstinent from all substances (%) | 31.1 (0–84.4) | 13.9 (0–82.2) | 22.2 (0–81.1) | 1.4, 0.49 |
Validity of substance use self-reports
Comparisons of participants’ self-reported cannabis use on the TLFB with hair samples and care co-ordinator reports (abbreviated TLFB and clinician drug use scales) indicated adequate concurrent validity. The agreement between cannabis use identified in hair samples and cannabis use reported by participants was κ = 0.61. The mean agreement between participant self-reports and care co-ordinator reports averaged across the four assessment time points was κ = 0.49. It should be acknowledged that the percentage of patients (71%) from whom we were unable to take hair samples is relatively high. This group consists of both those who refused to give hair and those with insufficient head hair to take an adequate sample. The remaining sample may therefore be subject to unknown bias. There were significant (p < 0.001) associations between participant self-reports and care co-ordinator reports with regard to total weight (in grams) of cannabis consumed at each time point, with an average intraclass correlation coefficient of 0.61. There were also significant associations between care co-ordinator reports on the Drug Use Scale of the clinician rating scales and weight of cannabis consumed as reported by participants (ρ = 0.44).
Treatment delivered and treatment fidelity
Brief therapy was better attended than long therapy, with 57.9% of those allocated to brief therapy attending at least 75% of the sessions offered compared with just 29.7% of those allocated to long therapy attending at least 75% of the sessions offered. The median number of therapy sessions delivered to participants was 9.75 for brief therapy and 11.0 for long therapy. Fifteen participants (20%) attended two or fewer therapy sessions, seven allocated to long therapy and eight allocated to brief therapy.
The number of items rated as compliant on the treatment fidelity scales ranged from 14 out of 16 (87.5%) to 16 out of 16 (100%) across the 20 digitally recorded sessions rated.
Discussion
We have conducted the largest trial to date of integrated MiCBT in a first-episode cannabis-using population. Our aim was to evaluate the effectiveness of MiCBT and compare the efficacy of a brief intervention (4.5 months) with that of a longer one (9 months), hypothesising that the MiCBT intervention would be efficacious in reducing substance use in people with early psychosis and that the brief version would be as efficacious as the longer version.
The outcomes of the trial are not yet available and we have therefore outlined the trial, described the sample at baseline and reported recruitment and retention rates. Indications are that despite the challenges associated with conducting trials with this client group the study has been successful in recruiting and retaining participants over a long follow-up period. We worked closely with the early-intervention teams to set up the study and identify potentially eligible participants and employed assertive engagement strategies once participants were recruited, seeing them in their own homes, arranging appointments flexibly but with persistence and conducting repeat visits for missed therapy and research appointments when necessary.
The refusal rate for participants identified as potentially eligible to take part was higher than those in recently conducted RCTs that had shorter intervention and follow-up periods109–111 but was in line with rates obtained from previously conducted studies. 107 We note that this was a non-treatment-seeking sample and all service users identified by their case managers as cannabis users were considered for inclusion. We do not have information on the service users who refused to participate or to be screened for eligibility and therefore cannot compare them with those included in the study. This may reduce the generalisability of our findings. It may be that those who declined the invitation to participate were more likely to be at a pre-contemplation stage of change. We found that > 80% of those randomised were contemplating changing their cannabis use at baseline. It is logical to assume that those who were contemplating change were more likely to agree to take part than those who were not.
Our assertive engagement strategies resulted in good retention for follow-up assessment. Almost 70% of the sample was retained at 18 months’ follow-up. Many of those who were lost to follow-up had moved away without leaving a forwarding address and were, therefore, not contactable. Take up of therapy was satisfactory, with 80% attending at least two sessions. Brief therapy was better attended than long therapy with twice the number of brief therapy participants attending 75% of the sessions offered. The median number of therapy sessions attended was similar in the two therapy arms, indicating that a brief form of the intervention may be more acceptable than a longer form. Qualitative interviews with therapy participants will seek to establish why brief therapy was better received and why some people refused therapy when offered it.
The sample is comparable with those in the four earlier RCTs of interventions with young cannabis-using recent-onset psychosis patients,106–109 three of which had not reported their results at the time that the ReCAP trial commenced,107–109 and to early-intervention service users generally: the majority were male, in their 20s and unemployed and had been using cannabis since their mid-teens. We recruited from services in various geographical locations encompassing both urban and rural populations and we are, therefore, confident that the sample is representative of our target population and that the results will be generalisable. We note that frequency of cannabis use, measured by percentage days abstinent, was higher in this trial than the previously reported trials with regard to percentage days abstinent. The amount of cannabis consumed is difficult to compare between the trials because of methodological differences, but ReCAP participants appeared to be smoking approximately 50% more cannabis than CapOpus participants. 108 Rates of abuse/dependence on other substances were much lower in the ReCAP trial than in the CapOpus trial (< 10% vs. approximately 50%), which may indicate that our sample was to some extent a ‘purer’ sample of cannabis users and more in line with the study by Bonsack and colleagues,107 which excluded those who were dependent on other substances and purposely recruited ‘heavier’ cannabis users. Rates of abuse and dependence on other substances were not reported in the other two trials and comparisons cannot be made.
The three previously conducted trials of integrated MiCBT in early psychosis106,108,109 found that the intervention did not confer an advantage over the control conditions in terms of reducing cannabis use or improving clinical outcomes. The results of the ReCAP trial will, therefore, be an important addition to the evidence base; with the longest intervention of all of the trials and the longest follow up, the results will be crucial in determining whether MiCBT can reduce cannabis use or a new therapeutic approach to cannabis use in psychosis will be required. It is perhaps noteworthy in this context that none of the recent trials, including the ReCAP trial, has involved families, possibly because of the constraints on recruitment of selecting only those who have regular contact with relatives. However, two studies that have found benefits from psychological therapy for people with psychosis and substance use have included a family-based component. 134,135
The strengths of this study include the high-quality randomisation process, the single-blind design, the range of substance use, symptom and functional outcomes and the 18-month follow-up period. The majority of participants engaged with the intervention and we were able to follow up a high proportion of participants. However, a significant minority (one in five) did not engage well with the intervention despite our assertive approach and future research should seek to understand influences on engagement in therapy as well as treatment preferences overall. Limitations include the potential bias resulting from early-intervention service users under-reporting substance use to their case managers and therefore not being considered eligible for referral; likewise, some potential participants may not have perceived their cannabis use to be problematic and declined to take part. Nonetheless, as the largest RCT conducted to date of an intervention to reduce cannabis use in first-episode psychosis and the only trial to compare brief therapy with longer therapy, we expect the results of this trial to be of considerable interest to academics and clinicians concerned with the development of an evidence base for the treatment of this client group.
Chapter 4 Discussion
We developed and evaluated three interventions aimed at reducing relapse/deterioration in physical health in young people with recent-onset psychosis. The first of these, CR, was hypothesised to enhance the efficacy of CBT, with symptoms predicted to reduce faster and further during CBT preceded by CR. A secondary hypothesis was that CR would enhance the efficiency of CBT, facilitating shorter courses or greater progress. Our hypotheses were partially supported. CR was not associated with significantly lower PSYRATS scores over the period of study. The effect of CR on PANSS score was also non-significant, although insight changed significantly more positively after CR than after SC. However, the hypothesis that CR would improve the efficiency of CBT was supported. After CR, participants made the same amount of progress in half the number of CBT sessions. There was no significant effect of CR on SOFAS score, depression, self-esteem, time to relapse or readmission. Our findings suggest that CR delivered by relatively unskilled workers can improve the efficiency of subsequent CBT, enabling participants and therapists to achieve the same amount of progress in therapy in approximately half the number of sessions. A substantial increase in the efficiency of CBT implies that the same number of CBTp therapists could treat many more patients. Our main recommendation for future research is that a definitive trial of CR-aided CBT should be conducted, with improvement in the efficiency of CBT as the primary outcome measure.
The healthy-living stream aimed to develop an evidence-based acceptable, feasible and effective intervention to encourage activity, improve diet and control weight gain in people recovering from a first episode of psychosis and to evaluate the clinical effectiveness and cost-effectiveness of the newly developed intervention in an exploratory RCT. We succeeded in these aims; early qualitative work confirmed that managing weight gain and staying physically active to prevent weight gain were important issues to service users and the intervention was carefully designed in collaboration with service users to be sensitive to their needs. Participants in the trial engaged well with the intervention and found it to be acceptable. This was reflected in excellent retention throughout the trial, with fewer than 10% of participants lost to follow-up. The healthy-living intervention was associated with a small reduction in BMI compared with the TAU group but the effect size was small (0.11) and the difference was not statistically significant. It could be that a more intensive intervention or more structured exercise programmes are required to achieve a larger effect. The small effect may also have resulted from our focus on weight loss in participants who were already obese rather than prevention of weight gain; this may have been a particularly difficult group to treat. There was evidence to suggest that the intervention may be more effective in participants taking olanzapine and clozapine. It could be that people may be more motivated to lose weight after having experienced medication-associated weight gain or it may be that the effects of dietary change and an increase in activity are larger in this group. Further research is needed to distinguish between the two possibilities. We did not consider measures of cardiovascular vulnerability such as high-density lipoprotein/low-density lipoprotein cholesterol ratio and levels of triglycerides as outcomes that our intervention may have had an impact on. Future research should consider these as outcomes in addition to BMI.
Health- and social-care costs were lower in the TAU group but the difference was not statistically significant and given the limited clinical effectiveness of the intervention it is not clear that the difference would be sufficient to offset the additional costs of providing the intervention. However, full cost data were not available for all participants who completed follow-up and further analysis is planned.
Our main recommendation for research is that further work should be carried out to improve the effectiveness of the healthy-living intervention, particularly focusing on those who are taking olanzapine and clozapine.
The substance use stream optimised an existing intervention aimed at reducing substance use, changing the focus to cannabis use and making it phase specific to the early-intervention period. The ReCAP trial was a well-designed, well-conducted trial that was successful in recruiting, engaging and retaining a representative sample of young cannabis users. In total, 80% engaged with therapy and almost 70% were retained until the 18-month follow-up. Outcome data are not yet available but will be of significant interest to academics and clinicians in the field.
Considering the trials overall, we anticipated that if a phase-specific intervention was effective we would see a trend in that trial towards reduced relapse rates. This was not the case. We also anticipated that there might be a trend towards lower relapse rates in the intervention groups across all three trials. This was also not the case. There was little evidence overall that any of the interventions had an impact on relapse, even at a trend level (Table 9).
| Intervention | Intervention group, n (%) | Control group, n (%) | Comparison |
|---|---|---|---|
| CR | 8 (26.7) | 11 (35.5) | χ2(1) = 0.55, p = 0.457 |
| Healthy living | 15 (30.6) | 12 (27.9) | χ2(1) = 0.08, p = 0.776 |
| MiCBT | 24 (32.4) | 13 (37.1) | χ2(1) = 0.24, p = 0.628 |
Future work
A common theme from all three trials was the interest in research shown by service users and staff in early-intervention services. This was manifest from the beginning in the eagerness of service users to participate in the qualitative research leading to the development of the interventions. It was also apparent in the openness of early-intervention services to our research teams and the willingness of early-intervention service staff and managers to publicise and support the research. We conclude that early-intervention services are particularly conducive to ongoing research programmes and would recommend that consideration be given to funding an ongoing programme of phase-specific research in early-intervention services.
Acknowledgements
This project was funded by the Programme Grants for Applied Research programme (RP-PG-0606–1302). This report presents independent research commissioned by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the NIHR Evaluation, Trials and Studies Coordinating Centre (NETSCC), the Programme Grants for Applied Research programme or the Department of Health. The views and opinions expressed by the interviewees in this publication are those of the interviewees and do not necessarily reflect those of the authors, the NHS, the NIHR, NETSCC, the Programme Grants for Applied Research programme or the Department of Health.
The ReCAP research assistants and therapists were Lee Fitzpatrick, Christine Kenny, Joanne Holding, Tom Weavers, Vera Azarova, Lauren Hampson and Lisa Lloyd.
Contributions of authors
Professor Max Marshall (Medical Director, Professor of Community Psychiatry) was the lead applicant and chief investigator with overall responsibility for the programme grant and contributed to all aspects of writing the report.
Professor Christine Barrowclough (Professor of Clinical Psychology) was a co-applicant, was responsible for the design and supervision of the substance-misuse intervention and trial and contributed to the writing of this report.
Dr Richard Drake (Senior Lecturer in Psychiatry) was a co-applicant, was responsible for the design and supervision and statistical analysis of the CR trial and contributed to the writing of this report.
Dr Nusrat Husain (Senior Lecturer in Psychiatry) was a co-applicant, was responsible for the cultural adaptation of interventions and contributed to the writing of this report.
Dr Fiona Lobban (Associate Director of the Spectrum Centre for Mental Health Research) was a co-applicant, was responsible for the development of the self-regulatory model of illness, supervised the substance misuse intervention and contributed to the writing of this report.
Professor Karina Lovell (Professor of Mental Health Nursing) was a co-applicant, was responsible for developing and supervising the healthy-living intervention and trial and contributed to the writing of this report.
Professor Alison Wearden (Professor of Health Psychology) was a co-applicant, was responsible for design of the healthy-living programme and supervision of the intervention and contributed to the writing of this report.
Dr Tim Bradshaw (Senior Lecturer) contributed to the development, delivery and supervision of the healthy-living intervention and contributed to the writing of this report.
Christine Day (Research Assistant) contributed to devising the assessments for the CR trial, recruited and assessed participants and prepared the data for analysis and contributed to the writing of this report.
Mike Fitzsimmons (Nurse Therapist) was responsible for the development and delivery of the substance-use intervention and contributed to the writing of this report.
Rebecca Pedley (Research Assistant) contributed to the day-to-day management of the InterACT trial, implemented the recruitment strategy and led on the acceptability qualitative interviews and contributed to the writing of this report.
Ruth Picucci (Research Assistant) delivered the CR intervention, produced and analysed the intervention CBTp quality and masking data and contributed to the writing of this report.
Dr Alicia Picken (Research Assistant) was involved in refining the experimental and control interventions in the CR trial as well as delivering the remediation intervention and contributed to recruitment and the writing of this report.
Dr Warren Larkin (Clinical Director, Children and Families Network, Lancashire Care NHS Foundation Trust) managed the NHS service component of the CR trial and supervised the CBTp therapists and contributed to the writing of this report.
Barbara Tomenson (Statistician) was responsible for analysing data from the substance use and healthy-living intervention trials and contributed to the writing of this report.
Mr Jeff Warburton (Assistant Network Director, Children and Families Network, Lancashire Care NHS Foundation Trust) was a co-applicant, was responsible for liaison with clinicians and service users and for facilitating recruitment within Lancashire Care early-intervention services and contributed to the writing of this report.
Dr Lynsey Gregg (Research Fellow) was the trial manager for the ReCAP trial, was responsible for supervising the research team, the day-to-day management of the trial, data management and statistical analysis and contributed to the writing of this report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health.
References
- Schizophrenia: Core Interventions in the Treatment and Management of Schizophrenia in Adults in Primary and Secondary Care. London: NICE; 2009.
- Bird V, Premkumar P, Kendall T, Whittington C, Mitchell J, Kuipers E. Early intervention services, cognitive–behavioural therapy and family intervention in early psychosis: systematic review. Br J Psychiatry 2010;197:350-6. http://dx.doi.org/10.1192/bjp.bp.109.074526.
- Lehman AF, Kreyenbuhl J, Buchanan RW, Dickerson FB, Dixon LB, Goldberg R, et al. The schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2003. Schizophr Bull 2004;30:193-217. http://dx.doi.org/10.1093/oxfordjournals.schbul.a007071.
- Bertelsen M, Jeppesen P, Petersen L, Thorup A, Ohlenschlaeger J, le Quach P, et al. Five-year follow-up of a randomized multicenter trial of intensive early intervention vs. standard treatment for patients with a first episode of psychotic illness. Arch Gen Psychiatry 2008;65:762-71. http://dx.doi.org/10.1001/archpsyc.65.7.762.
- Melle I, Larsen TK, Haahr U, Friis S, Johannesen JO, Opjordsmoen S, et al. Prevention of negative symptom psychopathologies in first-episode schizophrenia: two-year effects of reducing the duration of untreated psychosis. Arch Gen Psychiatry 2008;65:634-40. http://dx.doi.org/10.1001/archpsyc.65.6.634.
- Guo X, Zhai J, Liu Z, Fang M, Wang B, Wang C, et al. Effect of antipsychotic medication alone vs. combined with psychosocial intervention on outcomes of early-stage schizophrenia: a randomized, 1-year study. Arch Gen Psychiatry 2010;67:895-904. http://dx.doi.org/10.1001/archgenpsychiatry.2010.105.
- Hogarty GE, Flesher S, Ulrich R, Carter M, Greenwald D, Pogue-Geile M, et al. Cognitive enhancement therapy for schizophrenia – effects of a 2-year randomized trial on cognition and behavior. Arch Gen Psychiatry 2004;61:866-76. http://dx.doi.org/10.1001/archpsyc.61.9.866.
- Roder V, Mueller DR, Mueser KT, Brenner HD. Integrated Psychological Therapy (IPT) for schizophrenia: is it effective?. Schizophr Bull 2006;32:S81-93. http://dx.doi.org/10.1093/schbul/sbl021.
- Bowie CR, McGurk SR, Mausbach B, Patterson TL, Harvey PD. Combined cognitive remediation and functional skills training for schizophrenia: effects on cognition, functional competence, and real-world behavior. Am J Psychiatry 2012;169:710-18. http://dx.doi.org/10.1176/appi.ajp.2012.11091337.
- Greig TC, Zito W, Wexler BE, Fiszdon J, Bell MD. Improved cognitive function in schizophrenia after one year of cognitive training and vocational services. Schizophr Res 2007;96:156-61. http://dx.doi.org/10.1016/j.schres.2007.07.003.
- Eack SM, Greenwald DP, Hogarty SS, Cooley SJ, DiBarry AL, Montrose DM, et al. Cognitive enhancement therapy for early-course schizophrenia: effects of a two-year randomized controlled trial. Psychiatr Serv 2009;60:1468-76. http://dx.doi.org/10.1176/ps.2009.60.11.1468.
- Lindenmayer JP, McGurk SR, Mueser KT, Khan A, Wance D, Hoffman L, et al. A randomized controlled trial of cognitive remediation among inpatients with persistent mental illness. Psychiatr Serv 2008;59:241-7. http://dx.doi.org/10.1176/ps.2008.59.3.241.
- McGurk SR, Mueser KT, Feldman K, Wolfe R, Pascaris A. Cognitive training for supported employment: 2–3 year outcomes of a randomized controlled trial. Am J Psychiatry 2007;164:437-41. http://dx.doi.org/10.1176/ajp.2007.164.3.437.
- Silverstein SM, Spaulding WD, Menditto AA, Savitz A, Liberman RP, Berten S, et al. Attention shaping: a reward-based learning method to enhance skills training outcomes in schizophrenia. Schizophr Bull 2009;35:222-32. http://dx.doi.org/10.1093/schbul/sbm150.
- Silverstein SM, Hatashita-Wong M, Solak BA, Uhlhaas P, Landa Y, Wilkniss SM, et al. Effectiveness of a two-phase cognitive rehabilitation intervention for severely impaired schizophrenia patients. Psychol Med 2005;35:829-37. http://dx.doi.org/10.1017/S0033291704003356.
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry 2011;168:472-85. http://dx.doi.org/10.1176/appi.ajp.2010.10060855.
- Wykes T, Steel C, Everitt B, Tarrier N. Cognitive behavior therapy for schizophrenia: effect sizes, clinical models, and methodological rigor. Schizophr Bull 2008;34:523-37. http://dx.doi.org/10.1093/schbul/sbm114.
- Wykes T. Review: cognitive remediation improves cognitive functioning in schizophrenia. Evid Based Ment Health 2008;11. http://dx.doi.org/10.1136/ebmh.11.4.117.
- Tarrier N, Lewis S, Haddock G, Bentall R, Drake R, Kinderman P, et al. Cognitive–behavioural therapy in first-episode and early schizophrenia. 18-month follow-up of a randomised controlled trial. Br J Psychiatry 2004;184:231-9. http://dx.doi.org/10.1192/bjp.184.3.231.
- Penades R, Catalan R, Pujol N, Puig O, Guarch J, Masana G, et al. Is memory impairment a rate limiter in cognitive behavioural therapy for chronic schizophrenia?. Psychother Psychosom 2010;79:129-30. http://dx.doi.org/10.1159/000276378.
- The Mental Health Policy Implementation Guide. London: Department of Health; 2001.
- Diagnostic and Statistical Manual for Mental Disorders. Washington, DC: American Psychiatric Association; 1994.
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders – Clinician Version (SCID-CV). Arlington, VA: American Psychiatric Press; 1997.
- World Health Organization . International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) Version for 2010 n.d. http://apps.who.int/classifications/icd10/browse/2010/en#/VI (accessed 2 December 2012).
- Research Governance Framework for Health and Social Care. London: Department of Health; 2005.
- Haddock G, McCarron J, Tarrier N, Faragher EB. Scales to measure dimensions of hallucinations and delusions: the Psychotic Symptom Rating Scales (PSYRATS). Psychol Med 1999;29:879-89. http://dx.doi.org/10.1017/S0033291799008661.
- Drake R, Haddock G, Tarrier N, Bentall R, Lewis S. The Psychotic Symptom Rating Scales (PSYRATS): their usefulness and properties in first episode psychosis. Schizophr Res 2007;89:119-22. http://dx.doi.org/10.1016/j.schres.2006.04.024.
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987;13:261-76. http://dx.doi.org/10.1093/schbul/13.2.261.
- Addington D, Addington J, Maticka-Tyndale E. Assessing depression in schizophrenia: the Calgary Depression Scale. Br J Psychiatry Suppl 1993;22:39-44.
- Rosenberg M. Society and the Adolescent Self Image. Princeton, NJ: Princeton University Press; 1965.
- Goldman HH, Skodol AE, Lave TR. Revising axis V for DSM-IV: a review of measures of social functioning. Am J Psychiatry 1992;149:1148-56. http://dx.doi.org/10.1176/ajp.149.9.1148.
- Birchwood M, Smith J, Drury V, Healy J, Macmillian F, Slade M. A self-report Insight Scale for psychosis – reliability, validity and sensitivity to change. Acta Psychiatr Scand 1994;89:62-7. http://dx.doi.org/10.1111/j.1600-0447.1994.tb01487.x.
- Tarrier N, Haddock G, Hofmann SG, Thompson MC. Treating Chronic and Severe Mental Disorders: A Handbook of Empirically Supported Interventions. New York, NY: Guilford Press; 2001.
- Bebbington PE, Craig T, Garety P, Fowler D, Dunn G, Colbert S, et al. Remission and relapse in psychosis: operational definitions based on case-note data. Psychol Med 2006;36:1551-62. http://dx.doi.org/10.1017/S0033291706008579.
- Wechsler D. WMS-III Manual: Wechsler Memory Scale. San Antonio, TX: Psychological Corporation; 1997.
- Koren D, Seidman LJ, Poyurovsky M, Goldsmith M, Viksman P, Zichel S, et al. The neuropsychological basis of insight in first-episode schizophrenia: a pilot metacognitive study. Schizophr Res 2004;70:195-202. http://dx.doi.org/10.1016/j.schres.2004.02.004.
- Reitan RM. Manual for Administration of Neuropsycholigcal Test Batteries for Adults and Children. Tucson, AZ: Neuropsychology Laboratory; 1979.
- Cohen JD, Forman SD, Braver TS, Casey SJ, Servan-Schreiber D, Noll DC. Activation of the prefrontal cortex in a nonspatial working memory task with functional MRI. Hum Brain Mapp 1993;1:293-304. http://dx.doi.org/10.1002/hbm.460010407.
- Cunje A, Molloy DW, Standish TI, Lewis DL. Alternate forms of logical memory and verbal fluency tasks for repeated testing in early cognitive changes. Int Psychogeriatr 2007;19:65-7. http://dx.doi.org/10.1017/S1041610206003425.
- Lezak M. Neuropsychological Assessment. New York, NY: Oxford University Press; 1983.
- Silverstein SM, Osborn LM, Palumbo DR. Rey–Osterrieth Complex Figure Test performance in acute, chronic, and remitted schizophrenia patients. J Clin Psychol 1998;54:985-94. http://dx.doi.org/10.1002/(SICI)1097-4679(199811)54:7<985::AID-JCLP12>3.0.CO;2-G.
- Wykes T, Newton E, Landau S, Rice C, Thompson N, Frangou S. Cognitive remediation therapy (CRT) for young early onset patients with schizophrenia: an exploratory randomized controlled trial. Schizophr Res 2007;94:221-30. http://dx.doi.org/10.1016/j.schres.2007.03.030.
- Kurtz MM. Neurocognition as a predictor of response to evidence-based psychosocial interventions in schizophrenia: what is the state of the evidence?. Clin Psychol Rev 2011;31:663-72. http://dx.doi.org/10.1016/j.cpr.2011.02.008.
- Wykes T, Huddy V. Cognitive remediation for schizophrenia: it is even more complicated. Curr Opin Psychiatry 2009;22:161-7. http://dx.doi.org/10.1097/YCO.0b013e328322fbf4.
- Penades R, Catalan R, Puig O, Masana G, Pujol N, Navarro V, et al. Executive function needs to be targeted to improve social functioning with cognitive remediation therapy (CRT) in schizophrenia. Psychiatry Res 2010;177:41-5. http://dx.doi.org/10.1016/j.psychres.2009.01.032.
- Cornblatt BA, Keilp JG. Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophr Bull 1994;20:31-46. http://dx.doi.org/10.1093/schbul/20.1.31.
- Gold JM, Goldberg RW, McNary SW, Dixon LB, Lehman AF. Cognitive correlates of job tenure among patients with severe mental illness. Am J Psychiatry 2002;159:1395-402. http://dx.doi.org/10.1176/appi.ajp.159.8.1395.
- Bell M, Bryson G, Greig T, Corcoran C, Wexler BE. Neurocognitive enhancement therapy with work therapy – effects on neuropsychological test performance. Arch Gen Psychiatry 2001;58:763-8. http://dx.doi.org/10.1001/archpsyc.58.8.763.
- Penades R, Boget T, Catalan R, Bernardo M, Gasto C, Salamero M. Cognitive mechanisms, psychosocial functioning, and neurocognitive rehabilitation in schizophrenia. Schizophr Res 2003;63:219-27. http://dx.doi.org/10.1016/S0920-9964(02)00359-6.
- Penades R, Catalan R, Salamero M, Boget T, Puig O, Guarch J, et al. Cognitive remediation therapy for outpatients with chronic schizophrenia: a controlled and randomized study. Schizophr Res 2006;87:323-31. http://dx.doi.org/10.1016/j.schres.2006.04.019.
- Sartory G, Zorn C, Groetzinger G, Windgassen K. Computerized cognitive remediation improves verbal learning and processing speed in schizophrenia. Schizophr Res 2005;75:219-23. http://dx.doi.org/10.1016/j.schres.2004.10.004.
- Fiszdon JM, Choi J, Goulet J, Bell MD. Temporal relationship between change in cognition and change in functioning in schizophrenia. Schizophr Res 2008;105:105-13. http://dx.doi.org/10.1016/j.schres.2008.06.010.
- Dallal GE. First Random Generator 2007. www.randomization.com/ (accessed 20 January 2009).
- Reeder C, Smedley N, Butt K, Bogner D, Wykes T. Cognitive predictors of social functioning improvements following cognitive remediation for schizophrenia. Schizophr Bull 2006;32:S123-31. http://dx.doi.org/10.1093/schbul/sbl019.
- Wykes T, Reeder C, Landau S, Everitt B, Knapp M, Patel A, et al. Cognitive remediation therapy in schizophrenia: randomised controlled trial. Br J Psychiatry 2007;190:421-7. http://dx.doi.org/10.1192/bjp.bp.106.026575.
- Wykes T, Reeder C. Cognitive Remediation Therapy for Schizophrenia: Theory and Practice. London: Routledge; 2005.
- Haddock G, Devane S, Bradshaw T, McGovern J, Tarrier N, Kinderman P, et al. An investigation into the psychometric properties of the Cognitive Therapy Scale for Psychosis (Cts-Psy). Behav Cogn Psychother 2001;29:221-33. http://dx.doi.org/10.1017/S1352465801002089.
- Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modelling Using Stata. College Station, TX: Stata Press; 2005.
- Rorabacher DB. Statistical treatment for rejection of deviant values – critical values of Dixon’s ‘Q’ parameter and related subrange ratios at the 95% confidence level. Anal Chem 1991;63:139-46. http://dx.doi.org/10.1021/ac00002a010.
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry 2008;165:203-13. http://dx.doi.org/10.1176/appi.ajp.2007.07010042.
- Dickinson D, Tenhula W, Morris S, Brown C, Peer J, Spencer K, et al. A randomized, controlled trial of computer-assisted cognitive remediation for schizophrenia. Am J Psychiatry 2010;167:170-80. http://dx.doi.org/10.1176/appi.ajp.2009.09020264.
- Kuipers E, Fowler D, Garety P, Chisholm D, Freeman D, Dunn G, et al. London East Anglia randomised controlled trial of cognitive–behavioural therapy for psychosis – III: follow-up and economic evaluation at 18 months. Br J Psychiatry 1998;173:61-8. http://dx.doi.org/10.1192/bjp.173.1.61.
- Startup M, Jackson MC, Evans KE, Bendix S. North Wales randomized controlled trial of cognitive behaviour therapy for acute schizophrenia spectrum disorders: two-year follow-up and economic evaluation. Psychol Med 2005;35:1307-16. http://dx.doi.org/10.1017/S0033291705005003.
- Tiihonen J, Lönnqvist J, Wahlbeck K, Klaukka T, Niskanen L, Tanskanen A, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet 2009;374:620-7. http://dx.doi.org/10.1016/S0140-6736(09)60742-X.
- National Schedule of Reference Costs 2010–11 for NHS Trusts and PCTs Combined. London: Department of Health; 2011.
- McCloughen A, Foster K. Weight gain associated with taking psychotropic medication: an integrative review. Int J Ment Health Nurs 2011;20:202-22. http://dx.doi.org/10.1111/j.1447-0349.2010.00721.x.
- Foley DL, Morley KI. Systematic review of early cardiometabolic outcomes of the first treated episode of psychosis. Arch Gen Psychiatry 2011;68:609-16. http://dx.doi.org/10.1001/archgenpsychiatry.2011.2.
- Tiihonen J, Lönnqvist J, Wahlbeck K, Klaukka T, Niskanen L, Tanskanen A, et al. No mental health without physical health. Lancet 2011;377. http://dx.doi.org/10.1016/S0140-6736(11)60211-0.
- Faulkner G, Cohn T, Remington G. Interventions to reduce weight gain in schizophrenia. Cochrane Database Syst Rev 2007;1.
- Álvarez-Jiménez M, Hetrick SE, González-Blanch C, Gleeson JF, McGorry PD. Non-pharmacological management of antipsychotic-induced weight gain: systematic review and meta-analysis of randomised controlled trials. Br J Psychiatry 2008;193:101-7. http://dx.doi.org/10.1192/bjp.bp.107.042853.
- Álvarez-Jiménez M, Martínez-García O, Pérez-Iglesias R, Ramírez ML, Vázquez-Barquero JL, Crespo-Facorro B. Prevention of antipsychotic-induced weight gain with early behavioural intervention in first-episode psychosis: 2-year results of a randomized controlled trial. Schizophr Res 2010;116:16-9. http://dx.doi.org/10.1016/j.schres.2009.10.012.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337.
- Mauri M, Simoncini M, Castrogiovanni S, Iovieno N, Cecconi D, Dell’Agnello G, et al. A psychoeducational program for weight loss in patients who have experienced weight gain during antipsychotic treatment with olanzapine. Pharmacopsychiatry 2008;41:17-23. http://dx.doi.org/10.1055/s-2007-992148.
- Skrinar GS, Huxley NA, Hutchinson DS, Menninger E, Glew P. The role of a fitness intervention on people with serious psychiatric disabilities. Psychiatr Rehabil J 2005;29:122-7. http://dx.doi.org/10.2975/29.2005.122.127.
- Wu RR, Zhao JP, Jin H, Shao P, Fang MS, Guo XF, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA 2008;299:185-93. http://dx.doi.org/10.1001/jama.2007.56-b.
- Álvarez-Jiménez M, Gonzalez-Blanch C, Vázquez-Barquero JL, Pérez-Iglesias R, Martínez-García O, Pérez-Pardal T, et al. Attenuation of antipsychotic-induced weight gain with early behavioral intervention in drug-naive first-episode psychosis patients: a randomized controlled trial. J Clin Psychiatry 2006;67. http://dx.doi.org/10.4088/JCP.v67n0812.
- Jean-Baptiste M, Tek C, Liskov E, Chakunta UR, Nicholls S, Hassan AQ, et al. A pilot study of a weight management program with food provision in schizophrenia. Schizophr Res 2007;96:198-205. http://dx.doi.org/10.1016/j.schres.2007.05.022.
- Addington J. The promise of early intervention. Early Interv Psychiatry 2007;1:294-307. http://dx.doi.org/10.1111/j.1751-7893.2007.00043.x.
- Scocco P, Longo R, Caon F. Weight change in treatment with olanzapine and a psychoeducational approach. Eat Behav 2006;7:115-24. http://dx.doi.org/10.1016/j.eatbeh.2005.08.003.
- Khazaal Y, Fresard E, Rabia S, Chatton A, Rothen S, Pomini V, et al. Cognitive behavioural therapy for weight gain associated with antipsychotic drugs. Schizophr Res 2007;91:169-77. http://dx.doi.org/10.1016/j.schres.2006.12.025.
- Brar JS, Ganguli R, Pandina G, Turkoz I, Berry S, Mahmoud R. Effects of behavioral therapy on weight loss in overweight and obese patients with schizophrenia or schizoaffective disorder. J Clin Psychiatry 2005;66:205-12. http://dx.doi.org/10.4088/JCP.v66n0208.
- Evans S, Newton R, Higgins S. Nutritional intervention to prevent weight gain in patients commenced on olanzapine: a randomized controlled trial. Aust N Z J Psychiatry 2005;39:479-86.
- Kwon JS, Choi JS, Bahk WM, Yoon KC, Hyung KC, Chul SY, et al. Weight management program for treatment-emergent weight gain in olanzapine-treated patients with schizophrenia or schizoaffective disorder: a 12-week randomized controlled clinical trial. J Clin Psychiatry 2006;67. http://dx.doi.org/10.4088/JCP.v67n0405.
- Littrell KH, Hilligoss NM, Kirshner CD, Petty RG, Johnson CG. The effects of an educational intervention on antipsychotic-induced weight gain. J Nurs Scholarsh 2003;35:237-41. http://dx.doi.org/10.1111/j.1547-5069.2003.00237.x.
- Weber M, Wyne K. A cognitive/behavioral group intervention for weight loss in patients treated with atypical antipsychotics. Schizophr Res 2006;83:95-101. http://dx.doi.org/10.1016/j.schres.2006.01.008.
- McKibbin CL, Patterson TL, Norman G, Patrick K, Jin H, Roesch S, et al. A lifestyle intervention for older schizophrenia patients with diabetes mellitus: a randomized controlled trial. Schizophr Res 2006;86:36-44.
- Ritchie J, Spencer L, O’Connor W, Ritchie J, Lewis J. Qualitative Research in Practice: A Guide for Social Science Students and Researchers. London: Sage Publications; 2003.
- Corbin J, Strauss A. Grounded theory research: procedures, canons and evaluative criteria. Qual Sociol 1990;13:3-21. http://dx.doi.org/10.1007/BF00988593.
- Leventhal H, Meyer D, Nerenz D, Rachman S. Medical Psychology. New York, NY: Pergamon; 1980.
- McAndrew LM, Musumeci-Szabo TJ, Mora PA, Vileikyte L, Burns E, Halm EA, et al. Using the common sense model to design interventions for the prevention and management of chronic illness threats: from description to process. Br J Health Psychol 2008;13:195-204. http://dx.doi.org/10.1348/135910708X295604.
- Bhui K. Culture and complex interventions: lessons for evidence, policy and practice. Br J Psychiatry 2010;197:172-3. http://dx.doi.org/10.1192/bjp.bp.110.082719.
- Lovell K, Wearden A, Bradshaw T, Tomenson B, Pedley R, Davies L, et al. An exploratory randomized controlled study of a healthy living intervention in early intervention services for psychosis: the INTERvention to Encourage ACTivity, Improve Diet, and Reduce Weight Gain (INTERACT) study. J Clin Psychiatry 2014;75:498-505. http://dx.doi.org/10.4088/JCP.13m08503.
- Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med 2008;148:295-309. http://dx.doi.org/10.7326/0003-4819-148-4-200802190-00008.
- Ritchie J, Spencer L, Bryman A, Burgess RG. Analyzing Qualitative Data. Abingdon: Routledge; 1994.
- Koskinen J, Lohonen J, Koponen H, Isohanni M, Miettunen J. Rate of cannabis use disorders in clinical samples of patients with schizophrenia: a meta-analysis. Schizophr Bull 2010;36:1115-30. http://dx.doi.org/10.1093/schbul/sbp031.
- Conus P, Lambert M, Cotton S, Bonsack C, McGorry PD, Schimmelmann BG. Rate and predictors of service disengagement in an epidemiological first-episode psychosis cohort. Schizophr Res 2010;118:256-63. http://dx.doi.org/10.1016/j.schres.2010.01.032.
- Schimmelmann BG, Conus P, Cotton S, Kupferschmid S, McGorry PD, Lambert M. Prevalence and impact of cannabis use disorders in adolescents with early onset first episode psychosis. Eur Psychiatry 2012;27:463-9. http://dx.doi.org/10.1016/j.eurpsy.2011.03.001.
- Turner MA, Boden JM, Smith-Hamel C, Mulder RT. Outcomes for 236 patients from a 2-year early intervention in psychosis service. Acta Psychiatr Scand 2009;120:129-37. http://dx.doi.org/10.1111/j.1600-0447.2009.01386.x.
- Zammit S, Moore TH, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, et al. Effects of cannabis use on outcomes of psychotic disorders: systematic review. Br J Psychiatry 2008;193:357-63. http://dx.doi.org/10.1192/bjp.bp.107.046375.
- Gonzalez-Pinto A, Alberich S, Barbeito S, Gutierrez M, Vega P, Ibanez B, et al. Cannabis and first-episode psychosis: different long-term outcomes depending on continued or discontinued use. Schizophr Bull 2011;37:631-9. http://dx.doi.org/10.1093/schbul/sbp126.
- Curran HV, Brignell C, Fletcher S, Middleton P, Henry J. Cognitive and subjective dose–response effects of acute oral delta 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology (Berl) 2002;164:61-70. http://dx.doi.org/10.1007/s00213-002-1169-0.
- Kavanagh DJ, Waghorn G, Jenner L, Chant DC, Carr V, Evans M, et al. Demographic and clinical correlates of comorbid substance use disorders in psychosis: multivariate analyses from an epidemiological sample. Schizophr Res 2004;66:115-24. http://dx.doi.org/10.1016/S0920-9964(03)00130-0.
- Birchwood M, Todd P, Jackson C. Early intervention in psychosis. The critical period hypothesis. Br J Psychiatry Suppl 1998;172:53-9.
- Edwards J, Elkins K, Hinton M, Pitman K, Harrigan S, Athanosopoulos O, et al. Cannabis use following initial treatment of first-episode psychosis. International Congress on Schizophrenia Research, Whistler, BC, Canada, April 2001. Schizophr Res 2001;49.
- Cleary M, Hunt G, Matheson S, Siegfried N, Walter G. Psychosocial interventions for people with both severe mental illness and substance misuse. Cochrane Database Syst Rev 2008;1.
- Edwards J, Elkins K, Hinton M, Harrigan SM, Donovan K, Athanasopoulos O, et al. Randomized controlled trial of a cannabis-focused intervention for young people with first-episode psychosis. Acta Psychiatr Scand 2006;114:109-17. http://dx.doi.org/10.1111/j.1600-0447.2006.00783.x.
- Bonsack C, Gibellini Manetti S, Favrod J, Montagrin Y, Besson J, Bovet P, et al. Motivational intervention to reduce cannabis use in young people with psychosis: a randomized controlled trial. Psychother Psychosom 2011;80:287-97. http://dx.doi.org/10.1159/000323466.
- Hjorthoj CR, Fohlmann A, Larsen AM, Gluud C, Arendt M, Nordentoft M. Specialized psychosocial treatment plus treatment as usual (TAU) versus TAU for patients with cannabis use disorder and psychosis: the CapOpus randomized trial. Psychol Med 2013;43:1499-510. http://dx.doi.org/10.1017/S0033291712002255.
- Madigan K, Brennan D, Lawlor E, Turner N, Kinsella A, O’Connor JJ, et al. A multi-center, randomized controlled trial of a group psychological intervention for psychosis with comorbid cannabis dependence over the early course of illness. Schizophr Res 2013;143:138-42. http://dx.doi.org/10.1016/j.schres.2012.10.018.
- Gregg L, Barrowclough C, Haddock G. Development and validation of a scale for assessing reasons for substance use in schizophrenia: the ReSUS scale. Addict Behav 2009;34:830-7. http://dx.doi.org/10.1016/j.addbeh.2009.03.004.
- Schofield D, Tennant C, Nash L, Degenhardt L, Cornish A, Hobbs C, et al. Reasons for cannabis use in psychosis. Aust N Z J Psychiatry 2006;40:570-4. http://dx.doi.org/10.1080/j.1440-1614.2006.01840.x.
- Spencer C, Castle D, Michie PT. Motivations that maintain substance use among individuals with psychotic disorders. Schizophr Bull 2002;28:233-47. http://dx.doi.org/10.1093/oxfordjournals.schbul.a006934.
- Rolfe TJ, McGory P, Cocks J, Longley T, Plowright D. Cannabis use in first-episode psychosis: Incidence and short-term outcome. Schizophr Res 1999;36:313-14.
- Glaser BG. The Discovery of Grounded Theory: Strategies for Qualitative Research. New York, NY: Aldine de Gruyter; 1967.
- Charmaz K. Constructing Grounded Theory. Thousand Oaks, CA: Sage Publications; 2006.
- Silverman D. Doing Qualitative Research: A Practical Handbook. London: Sage Publications; 2005.
- Barrowclough C, Haddock G, Beardmore R, Conrod P, Craig T, Davies L, et al. Evaluating integrated MI and CBT for people with psychosis and substance misuse: recruitment, retention and sample characteristics of the MIDAS trial. Addict Behav 2009;34:859-66. http://dx.doi.org/10.1016/j.addbeh.2009.03.007.
- Barrowclough C, Haddock G, Wykes T, Beardmore R, Conrod P, Craig T, et al. Integrated motivational interviewing and cognitive behavioural therapy for people with psychosis and comorbid substance misuse: randomised controlled trial. BMJ 2010;341. http://dx.doi.org/10.1136/bmj.c6325.
- Marlatt GA, Gordon JR. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. New York, NY: Guilford Press; 1985.
- Blanchard JJ, Squires D, Henry T, Horan WP, Bogenschutz M, Lauriello J, et al. Examining an affect regulation model of substance abuse in schizophrenia. The role of traits and coping. J Nerv Ment Dis 1999;187:72-9. http://dx.doi.org/10.1097/00005053-199902000-00002.
- Lobbana F, Barrowclough C, Jeffery S, Bucci S, Taylor K, Mallinson S, et al. Understanding factors influencing substance use in people with recent onset psychosis: a qualitative study. Soc Sci Med 2010;70:1141-7. http://dx.doi.org/10.1016/j.socscimed.2009.12.026.
- The Mental Health Policy Implementation Guide. London: Department of Health; 2001.
- Moyers TB, Martin T, Christopher PJ, Houck JM, Tonigan JS, Amrhein PC. Client language as a mediator of motivational interviewing efficacy: where is the evidence?. Alcohol Clin Exp Res 2007;31:40-7. http://dx.doi.org/10.1111/j.1530-0277.2007.00492.x.
- Haddock G, Beardmore R, Earnshaw P, Fitzsimmons M, Nothard S, Butler R, et al. Assessing fidelity to integrated motivational interviewing and CBT therapy for psychosis and substance use: the MI-CBT fidelity scale (MI-CTS). J Ment Health 2012;21:38-4. http://dx.doi.org/10.3109/09638237.2011.621470.
- Sobell LC, Sobell MB, Litten RZ, Allen J. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Totawa, NJ: Humana Press; 1992.
- Hjorthoj CR, Hjorthoj AR, Nordentoft M. Validity of timeline follow-back for self-reported use of cannabis and other illicit substances – systematic review and meta-analysis. Addict Behav 2012;37:225-33. http://dx.doi.org/10.1016/j.addbeh.2011.11.025.
- Hjorthoj CR, Fohlmann A, Larsen A-M, Arendt M, Nordentoft M. Correlations and agreement between delta-9-tetrahydrocannabinol (THC) in blood plasma and timeline follow-back (TLFB)-assisted self-reported use of cannabis of patients with cannabis use disorder and psychotic illness attending the CapOpus randomized clinical trial. Addiction 2012;107:1123-31. http://dx.doi.org/10.1111/j.1360-0443.2011.03757.x.
- Rollnick S, Heather N, Gold R, Hall W. Development of a short ‘readiness to change’ questionnaire for use in brief, opportunistic interventions among excessive drinkers. Br J Addict 1992;87:743-54. http://dx.doi.org/10.1111/j.1360-0443.1992.tb02720.x.
- Barkus E, Lewis S. Schizotypy and psychosis-like experiences from recreational cannabis in a non-clinical sample. Psychol Med 2008;38:1267-76. http://dx.doi.org/10.1017/S0033291707002619.
- Blanchard KA, Morgenstern J, Morgan TJ, Lobouvie EW, Bux DA. Assessing consequences of substance use: psychometric properties of the inventory of drug use consequences. Psychol Addict Behav 2003;17:328-31. http://dx.doi.org/10.1037/0893-164X.17.4.328.
- Drake RE, Mueser KT, McHugo GJ, Sederer L, Dickey B. Outcomes Assessment in Clinical Practice. Baltimore, MD: Williams & Wilkins; 1996.
- Beck AT, Brown G, Epstein N, Steer RA. An inventory for measuring clinical anxiety – psychometric properties. J Consult Clin Psychol 1988;56:893-7. http://dx.doi.org/10.1037/0022-006X.56.6.893.
- Hogan TP, Awad AG, Eastwood R. A self-report scale predictive of drug compliance in schizophrenics: reliability and discriminative validity. Psychol Med 1983;13:177-83. http://dx.doi.org/10.1017/S0033291700050182.
- Barrowclough C, Haddock G, Tarrier N, Lewis SW, Moring J, O’Brien R, et al. Randomized controlled trial of motivational interviewing, cognitive behavior therapy, and family intervention for patients with comorbid schizophrenia and substance use disorders. Am J Psychiatry 2001;158:1706-13. http://dx.doi.org/10.1176/appi.ajp.158.10.1706.
- Naeem F, Kingdon D, Turkington D. Cognitive behavior therapy for schizophrenia in patients with mild to moderate substance misuse problems. Cogn Behav Ther 2005;34:207-15. http://dx.doi.org/10.1080/16506070510010684.
Appendix 1 The InterACT trial service user interview topic guide
The HELPER trial: the healthy living intervention
First qualitative interview schedule prompt sheet
-
Can you tell me when your mental health problems first started?
Probe
-
What do you remember about the onset of your problems?
-
What symptoms were you experiencing?
-
What did you think was happening to you at the time?
-
How did you react initially to experiencing symptoms?
-
What do you think caused the problems that you have experienced?
-
How is life different now to before your mental health problems started?
-
-
What sort of things have you noticed make your mental health problems either better or worse?
Probe
-
Do you think that there is anything that you or anyone else can do to help control your symptoms?
-
Can you identify any particular situations/triggers that make your symptoms worse?
-
How do you cope with these situations?
-
-
What if any medication are you taking at the moment?
Probe
-
Can you tell me the name and dosage of your medication?
-
How long have you been taking this medication?
-
Do you manage to take your medication exactly as it is prescribed?
-
How do you make sure that you remember to take it?
-
Do you feel that taking the medication helps you in any way?
-
Is there anything that you don’t like about taking medication?
-
-
If not taking medication
Probe
-
Why do you not take medication? Is it because you have never been asked to or is it a decision on your part?
-
How would you feel about taking medication to help your mental health problems if your doctor asked you to?
-
-
What information have you been given regarding medication?
Probe
-
What information have they been given?
-
Who gave them this information/how did they obtain it?
-
Do they know about any potential side effects?
-
Do they experience any unpleasant side effects, how much do these bother them and how do they cope with them?
-
-
What sort of things do you think are important for maintaining a healthy lifestyle?
Probe
-
What do you do at the moment to stay fit and healthy?
-
Have there been any changes to your lifestyle since you first experienced mental health problems?
-
-
Has your lifestyle changed in any way since you first developed mental health problems?
-
Tell me a bit about the types of things you like to eat
-
Do you eat regular meals at set times or are you more likely to graze on snacks between meals?
-
Can you tell me how many portions of fruit or vegetables you eat in a typical day?
-
How often do you eat fried food?
-
What type of bread do you prefer?
-
How much coke or how many other soft drinks do you have in an average day?
-
-
Do you smoke cigarettes?
-
If yes, how many in a typical day?
-
How long have you smoked for?
-
-
Do you drink alcohol?
-
If yes, how much do you drink on an average day?
-
-
Do you take any drugs that haven’t been prescribed by a doctor, such as cannabis?
-
If yes, what do you take and how much in a typical day/week?
-
-
-
Do you consider that you have any problems with your weight?
Probe
-
What do you consider these problems to be?
-
Have they ever tried to lose/gain weight?
-
How did they do this and how successful was it?
-
-
Would you like to make any changes to your current lifestyle in order to make it healthier?
Probe
-
What changes if any would you like to make?
-
-
What help or advice (if any) would you like to have in order for you to make healthy changes to your lifestyle?
Probe
-
Who do you think is the best person to give this advice, e.g. CPN, GP, practice nurse?
-
Where would they like to receive advice, e.g. hospital, GP surgery?
-
-
What type of healthy living intervention would you like to receive?
-
If free access to a gym or other fitness facility was made available do you think that you would use it?
-
Would you prefer to go with a group of people or alone?
-
Where do you think the best place to deliver the intervention would be (probe – mental health setting, community setting, other)?
-
What time of day would you prefer to attend a healthy living group (probe – afternoon, morning, early evening; fixed time, flexible times; how long, frequency of sessions; rationale for choice)?
-
What you think would be the best way to run the intervention (probe – explicitly here, i.e. games, live demonstrations, e.g. healthy eating, workbooks, small group work, discussion, bringing in some experts, e.g. dieticians, hands on, e.g. practical such as an actual cooking class; open or closed group)?
-
Who do you think are the best people to deliver the intervention (probe – MHW, users, experts in health promotion, etc.)?
-
What do you think are the things that will make the intervention successful in helping people to develop a healthier lifestyle (probe – free access to exercise facilities, etc.) and what things might get in the way of it being successful [probe – reasons and solutions, i.e. what do you think might help us overcome these (barriers) or how can we make sure we do this (facilitators)]?
-
-
Is there anything else that you would like to tell me about the service you are receiving? Or anything about the type of healthy living intervention that you would like to receive?
Thank you for giving up your time to see me today. It has been very helpful talking to you.
Appendix 2 The InterACT trial health professional interview topic guide
The HELPER trial: the healthy living intervention
Interview/focus group schedule for early intervention services/primary care staff
-
Introduction and presentation/information sheet on synthesis of our findings from phase 1 (i.e. content elements).
-
What are your views of your roles and responsibilities in the physical health care of people with psychosis (probe – reasons, i.e. policy, professional, etc.)?
-
What training have you received in providing health education advice to service users about healthy living (probe – adequacy of training; mandatory or voluntary)?
-
What training have you received in assessing and managing physical health problems (probe – adequacy of training; mandatory or voluntary)? Do you routinely assess for unwanted effects of medication (probe – how do they do this, what side effects do they assess for)?
-
Would you be prepared to run healthy living groups within the early intervention service (probe – exactly who, resource implications, training, etc.)?
-
If healthy living groups were to be delivered by the early intervention service, what would help them to run smoothly (probe – venue, group leader, time of day)?
-
Do you think there might be any obstacles to the healthy living groups running smoothly in the early intervention service (probe – pressure of work, service user apathy, not a priority, etc.)?
Appendix 3 The InterACT trial healthy living intervention booklet
Appendix 4 The InterACT trial support, time and recovery worker training guide
Appendix 5 The InterACT trial acceptability interview topic guide
Interview schedule, version 1 (14 February 2012)
Introduction
-
Explanation of ethics, consent and confidentiality of interview and analysis.
-
Explanation of objective of acceptability substudy.
-
Structure and duration of the interview.
-
Any questions?
Clarify – talking about intervention rather than the research.
Context
-
Tell me about the healthy living intervention?
-
Can you tell me about your experiences while taking part in the healthy living intervention? Probe – Individual and groups or individual only? If individual only why not groups?
Process
-
What were your initial expectations of the healthy living intervention?
-
What were the specific advantages and disadvantages of the healthy living intervention?
Probe
-
Time commitments, timing of appointments, travel, etc.
-
(What specific thing achieved change?)
-
-
What specifically did you like/dislike about the healthy living programme?
Probe
-
What did you think about the goal-setting and action plans?
-
How realistic were the goals you set?
-
How well did you feel you achieved your goals.
-
If you did the programme again, would you do anything differently?
-
(What specific element/thing achieved change?)
-
-
Were you provided with information about the website?
Probe
-
Did you use it? If not, why not?
-
How many times did you go on to it?
-
Did you find it useful?
-
How did you find getting access to the internet?
-
Did it help with the intervention?
-
-
Were you provided with information about a booklet to help with the intervention?
Probe
-
Did you use it?
-
How many times did refer to it?
-
Did you find it useful?
-
Did it help you achieve your goals?
-
-
Which would/did you prefer, the booklet or the website? Why?
-
STR worker – how did you get on with your STR worker? Good points? Things that could be improved?
Probe specific intervention factors (i.e. goal-setting, action plans, implementation of action plan, interventions and non-specific factors such as warmth, empathy, listening)
-
Some people have said that it was useful for them that their STR worker knew about their past – what you think about this? Did you find this useful? Why?
-
Some people have said that they found it useful that their STR worker knew about their mental health whereas others did not – how do you feel about this?
-
-
What was your experience of your family/carer being involved in your treatment?
Probe
-
Were family involved? If so, what were the advantages and disadvantages of your family being involved? How helpful was it?
-
-
Did the STR worker offer to see your family or other people involved in your care, for example close friends? If so, what was the outcome? How did you feel about them being involved?
Outcome
-
Do you feel that you have benefited from being on the programme?
Probe
-
Any specific benefits for healthy living or other benefits for functioning (social, occupational/school, private, leisure, family relationships), mood/depression, anxiety, stress, the way you feel about yourself, your appearance?
-
(What specific thing achieved change?)
-
-
Would you recommend this programme to other people with similar problems to your own?
Probe
-
Yes/no answers on reasons for their response.
-
(What specific thing would you recommend to someone about the programme or is it the programme as a whole?)
-
-
Is there anything that you would add to or change about the intervention?
Probe
-
For example, some people have said that a weekly weigh-in would be useful whereas others have said that they would not find this useful? What do you think?
-
-
Is there anything else that you would like to say about the healthy living intervention?
Ending
-
Thank participant for their time and information.
-
Next steps: would they like a copy of the transcript to be sent to them for validity checking?
-
A one-page copy of results will be sent to all participants.
Appendix 6 The Asking about Substance use and Psychosis: Ideas, Reactions and Experiences (ASPIRE) trial topic guide
I’d like to talk to you today about your drug use and what you think about drugs. I am interested in hearing all about your experiences and ideas.
I should remind you that everything you tell me will stay confidential unless I have serious concerns about your or other people’s welfare. What are your thoughts about this?
-
How do you feel about being here today? Any questions before we begin?
-
How are things going for you at the moment in general?
I thought it might be easier for me to draw a timeline of when you have used drugs in the past up to now. This is so I can get a better idea of what was going on for you at those times. I’ve got a few questions here that it would also be good to talk through.
[Fill in timeline for drug use]
-
I’d be interested to know about how your drug use has changed over the past months/years. Is that okay? In what ways has your drug use changed?
-
Reflect on pattern.
-
First occasion of use.
-
Current use.
-
Peaks/dips.
-
Related events/context.
-
Good things/bad things.
-
When, why, who/what, what drugs, feelings, motivations, pros/cons.
-
Thoughts, feelings, beliefs, social environment.
-
Triggers to think about giving up.
-
-
You’ve probably picked up lots of messages from your friends, family and doctors about drugs. What kind of messages have you heard?
-
Opinions about the messages.
-
Feelings about messages.
-
Spiritual/faith healers.
-
Opinions about other people’s use.
-
Different substances, situations, reasons for use, ways of voicing opinions.
-
-
What do you see for yourself in the future?
-
How would you like things to be different?
-
Drug use.
-
Reasons.
-
Timescale.
-
Possible influences.
-
-
(If not yet mentioned) People have lots of opinions about whether drugs and mental health are connected. Have you got any opinions about that?
-
I think we’ve covered most of what I planned. Is there anything else that you’d like to talk about?
-
How did you find it going through all this?
Reflections, affirmations, summaries, open questions
Appendix 7 The Rethinking Choices After Psychosis (ReCAP) trial psychoeducational materials
Facts about cannabis
How does your cannabis compare?
Managing withdrawal symptoms
Appendix 8 Example image from the Rethinking Choices After Psychosis (ReCAP) trial DVD

Appendix 9 The IMproving PArticipation in Cognitive Therapy (IMPACT) trial protocol
A randomised, controlled trial of cognitive remediation
Introduction
Cognitive Remediation (CR) is a method for improving neuropsychological function in schizophrenia. In essence the intervention is a form of ‘brain training’, consisting of regular practice on a number of mental puzzles. In clinical trials, CR has been shown to improve:
-
executive function (Kurtz et al. , 2001, 2004; Penades et al. , 2006; Reeder et al. , 2004; Twamley et al. , 2003; Wykes et al. , 1999, 2007);
-
attention (e.g. Kurtz et al. , 2001, 2004; Medalia et al. , 1998; Silverstein et al. , 2005, 2007; Twamley et al. , 2003);
-
long term memory (Kurtz et al. , 2001, 2004; Penades et al. , 2006; Twamley et al. , 2003; Wykes et al. , 1999);
-
insight into cognitive problems (Granholm et al. , 2005); and
-
positive symptoms (Reeder et al. , 2004).
Whilst previous meta-analyses have reached conflicting conclusions about the effect size of the intervention (Pilling et al. , 2002), more recent trials have produced increasingly positive results, as the intervention and its associated outcome measures have become more sophisticated and specific. Furthermore, there is evidence for combining CR with other interventions in trials of rehabilitation for the severe and long term mental illness (Bell et al. , 2007; Mueser & McGurk, 2007; Silverstein et al. , 2005, 2007).
We hypothesise that CR will enhance the efficacy of the cognitive behavioural therapy (CBT) by improving cognitive functioning, especially attention and executive functions, (such as set shifting). If so, we expect that those who receive CR will received greater benefit from CBT, by virtue of being able to acquire more complex skills and reaching a better understanding of their problems. We therefore predict that they will score lower on the Psychotic SYmptom RATing Scales (PSYRATS; Haddock et al., 1999), which measures the psychotic symptoms targeted by CBT. We also predict they will get better more quickly and score more highly on a scale that describes the quality of engagement in CBT, designed by Professor Gillian Haddock (personal communication). The scale is already being used in an MRC-funded trial of cognitive behavioural therapy and motivational interviewing for drug misuse conducted at the University of Manchester. Other secondary measures will include: other symptoms; neuropsychological function; cognitive insight; attitudes to illness; vocational achievement; and self-concept.
Methods
Sample
64 patients on the waiting list for CBT delivered via Lancashire Care’s Early Intervention Service (EIS).
-
Inclusion criteria: DSM 4 schizophreniform disorder, schizophrenia, schizoaffective disorder, delusional disorder or psychosis NOS; age 18-35; capacity to consent.
-
Exclusion criteria: DSM 4 substance dependence; organic brain disease.
Procedure
Recruitment
Participants will be recruited via contact with NHS staff, who will first approach patients about assessment. Participants will be recruited from the existing waiting list or via the allocation procedures for the CBT service at the EIS. After identification they will be approached, informed about the trial – including being given a patient information sheet. Their capacity to consent will be assessed and they will be given 24 hours to decide whether to take part. Should they consent they will be asked to sign a consent form before their baseline clinical assessment (see: Assessments, below).
Allocation
After the baseline assessment participants will be allocated randomly by telephone contact with an independent administrator to receive CR or the active control treatment (see: Intervention, below).
Interventions
The CR Intervention
This is based on the commercially available CIRCUITS package delivered via the internet or CD. This is in turn based on the intervention used in Wykes et al. , (1999), used as the basis for Drake et al.’s (2007) CR trial which is used to power this trial.
Participants complete 40 hours of neuropsychological tasks in the form of increasingly complex puzzles (e.g. find a route around a map to a designated objective) or socially framed tasks (e.g. write applying to attend a given event, using a framework provided by the programme), all displayed using attractive, interactive graphics. These tasks are spread over the 12 weeks of the intervention, delivered at the subject’s own pace. This typically takes the form of 3–4 one hour sessions per week.
Participants will attend community services if they have no access to home computers. A therapist will assist the progress of those engaged in the intervention by helping them attend the community services, monitoring and recording all participants’ progress and supervising if necessary.
The Control Intervention
This (Befriending) will consist of time-matched, non-directive social contact from a Support, Training and Rehabilitation worker provided through the EIS service.
Standard Care
As part of standard care in the EIS both groups will receive input from a case manager and a psychiatrist, who will provide a standard package of psychosocial care and medication management. Thus the care participants receive from the EIS will not be fundamentally altered.
CBT
After CR or befriending participants will proceed to CBT, delivered as part of the Lancashire Care EIS. There is a waiting list for this treatment which currently exceeds the period required to administer cognitive rehabilitation. Thus neither control or intervention patients will be disadvantaged in terms of access to CBT by their participation in the trial. The therapists are suitably qualified in delivering specialist CBT for psychosis and exclusively employed to provide it. This service’s CBT consists of 12–30 weekly sessions of approximately one hour aiming to engage participants; improve their use of coping strategies for their symptoms; progress to investigating the schemata underlying them; and then develop an overall formulation, using a normalising rationale but incorporating relapse-prevention strategies. CBT model fidelity will be rated on a randomly selected sub-sample using the CTS modified for psychosis (Devane et al. , 1998).
Therapists will be asked to record the number and date of sessions and score overall progress during therapy on a suitably modified Haddock scale of CBT-complexity. After CBT the assessor will rate the participants’ scores from notes, to maximise reliability of this outcome.
Assessments
Baseline
Symptoms will be recorded with the PSYRATS (Haddock et al. , 1999), Calgary Depression Scale for Schizophrenia (CDSS, Addington et al. , 1996) and PANSS (Kay et al. , 1987); this will take 35 minutes.
Cognitive insight will be rated using the Beck Cognitive Insight Scale (Beck et al. 2005), insight using the Birchwood Insight Scale (Birchwood et al. , 1994) and self esteem using the Rosenberg Self Esteem Scale (Rosenberg, 1962). Beliefs about illness will be rated using a modified form of the Illness Perception Questionnaire (IPQ, Lobban et al. , 2005). This will take about 15 minutes.
Overall illness severity and social dysfunction will be recorded with the Clinical Global Impression and Global Assessment of Functioning symptom and social functioning subscales (GAF-S & -F). This will take 1 minute.
At a second interview, neuropsychological function will be rated with measures of:
-
executive function and metacognition (Metacognitive Wisconsin Card Sort Test, Koren et al., 2004);
-
vigilance (Continuous Performance Test and Trailmaking A, Reitan & Reitan 1958);
-
set alternation (Trailmaking B, Reitan & Reitan 1958);
-
long term memory (the Logical Memory Test and Rey–Osterieth Complex Figure Test);
-
IQ (Wechsler Test of Adult Reading and Wechsler Adult Intelligence Scale (revised) block design subscale).
This will take about 40 minutes.
After First Intervention
After the intervention (after 12 weeks) the same measures, apart from the WTAR & WAIS, will be re-rated by the assessor, from whom allocation group (CR or control) will be masked.
During and After CBT
During CBT the assessor will re-rate the PSYRATS every six weeks, up to thirty weeks. At thirty weeks they will also rate the same measures as for the second interviews. Working Alliance Inventory score (Couture et al. , 2006) and Denver Recovery Markers Inventory (MHCD, 2006) will also be included.
Analysis
The main outcome will be the PSYRATS. Curves will be fitted to the longitudinal data using mixed effects models estimated by maximum likelihood methods. Allocation group (to CR or befriending) and potential confounders will be independent variables and the data will be clustered by cognitive–behavioural therapist.
The pattern of any missing data (e.g. due to drop-out) will be analysed using logistic regression to test whether the assumptions underlying this type of modelling (i.e. data ‘missing-at-random’) are met.
The principal secondary outcomes will be number of sessions and CBT complexity score. Others, adjusted for multiple testing, will include neuropsychological measures, global illness severity & dysfunction, attitudes to illness, cognitions and self esteem. In the event of positive results the effect of including process measures related to neuropsychological variables and attitudes will be examined.
Power
Based on a t-test of final interview scores, with an alpha value of 0.05 and power of 0.80, 64 participants allows detection of a difference between groups of effect size d of 0.50 (PS v 2.1.31, Dupont & Plummer 1997; using methodology from Dupont & Plummer 1990). This difference represents 8.1 points in PSYRATS totals (based on data from a first-episode sample in Lewis et al. , 2002).
The same data-set shows that there is substantial independence between measurements made at successive points in follow-up six weeks or more apart. Thus one may make the approximation that data from the n = 6 follow-up interviews (at six week intervals; see Figure 1) will each contribute independently to estimating outcome, which in turn would reduce the standard deviation for the control mean across the repeated measures by 1/√n. This approximation reduces the minimum detectable difference of approximately 3.4 points in PSYRATS total (about 25% of the controls’ overall mean score for the repeated measures).
Unpublished data from a recent trial in 67 schizophrenia-spectrum psychosis sufferers of cognitive remediation combined with social cognition training compared to control supportive therapy (Eack and colleagues, in press) found benefits of d 0.48 for a composite neuropsychological measure similar to the tasks proposed here and d 0.51 for symptoms.
References
Addington D, Addington J, Atkinson M. (1996) A psychometric comparison of the Calgary Depression Scale for Schizophrenia and the Hamilton Depression Rating Scale. Schizophr Res. 19, (2–3) 205–12.
Bell MD, Greig TC, Zito B, Wexler BW (2007) An RCT of neurocognitive enhancement therapy with supported employment: employment outcomes at 24 months. Schizophrenia Bulletin, 33, (2) 420.
Couture SM, Roberts DL, Penn DL, Cather C, Otto MW & Goff D. (2006). Do baseline client characteristics predict the therapeutic alliance in the treatment of schizophrenia? J Nerv Ment Dis. , 194, (1) 10–4.
Denver Recovery Markers Inventory, (2006) Mental Health Centre of Denver, 4141 E Dickenson Place, Denver, CO 80222.
Devane SM, Haddock G, Lancashire S, Baguley I, Butterworth T, Tarrier N, James A & Molyneux P. The clinical skills of community psychiatric nurses working with patients with severe and enduring mental health problems: an empirical analysis (1998) J Adv Nursing, 27, 253–60.
Drake RJ, Corcoran R, Kaiser SL, Smallman R, Aslam M, Lewis SW (2007). A pilot study of CR for poor insight in non-affective psychosis: a minimized, controlled trial. Schizophrenia Bulletin, 33, (2) 428.
Dupont WD & Plummer WD: (1990) Power and Sample Size Calculations: A Review and Computer Program. Controlled Clinical Trials; 11:116–28.
Eack SM, Hogarty GE, Cooley SS, DiBarry AL, Hogerty SS, Greenwald DP, Montrose DM & Keshavan MS. Cognitive Enhancement Therapy for Early Course Schizophrenia: Effects of a Two-Year Randomised Controlled Trial. Schizophrenia Bulletin (in press).
Granholm E, McQuaid JR, McClure FS, Auslander LA, Perivoliotis D, Pedrelli P, Patterson T & Jeste DV. (2005) A randomised, controlled trial of cognitive behavioral social skills training for middle-aged and older outpatients with chronic schizophrenia. Am J Psych, 162, 520–9.
Haddock G, McCarron J, Tarrier N & Faragher EB. (1999). Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS). Psychol Med. , 29, (4) 879–89.
Kay SR, Fiszbein A & Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. (1987). Schizophr Bull., 13, (2) 261–76.
Kurtz MM, Moberg PJ, Gur RC & Gur RE (2004) Approaches to cognitive remediation of neuropsychological deficits in schizophrenia: a review and meta-analysis. Neuropsychology Review, 11, 197–210.
Kurtz MM, Moberg PJ, Mozley LH, Swanson CL, Gur RC & Gur RE (2001) Effectiveness of an attention- and memory-training program on neuropsychological deficits in schizophrenia. Neurorehabilitation & Neurorepair, 15, 75–80.
Lewis S, Tarrier N, Haddock G, Bentall R, Kinderman P, Kingdon D, Siddle R, Drake R, Everitt J, Leadley K, Benn A, Grazebrook K, Haley C, Akhtar S, Davies L, Palmer S, Faragher B, Dunn G. (2002). Randomised controlled trial of cognitive-behavioural therapy in early schizophrenia: acute-phase outcomes. Br J Psychiatry Suppl., 43:s91–7.
Lobban F, Barrowclough C, Jones S. (2005). Assessing cognitive representations of mental health problems. I. The illness perception questionnaire for schizophrenia. Br J Clin Psychol. , 44, (Pt 2):147–62.
Medalia A, Aluma M, Tryon W & Merriam AE (1998) Effectiveness of attention training in schizophrenia. Schizophrenia Bulletin, 24, 147–52.
Mueser KT & McGurk S (2007) 2-3 year outcomes of a randomised controlled trial of cognitive training and supported employment for people with severe mental illness. Schizophrenia Bulletin, 33, (2) 448.
Penades R, Catalan R, Salamero M, Boget T, Puig O, Guarch J & Gasto C. (2006). Cognitive remediation therapy for outpatients with chronic schizophrenia: a controlled and randomised study. Schizophr Res. , 87, (1–3) 323–31.
Pilling S, Bebbington P, Kuipers E, Garety P, Geddes J, Martindale B, Orbach G and Morgan C (2002) Psychological treatments in schizophrenia: II. Meta-analysis of randomised controlled trials of social skills training and cognitive remediation. Psychological Medicine, 32, 783–91.
Reeder C, Newton E, Frangou S & Wykes T (2004). Which executive skills should we target to affect social functioning and symptom change? A study of a cognitive remediation therapy program. Schizophrenia Bulletin, 30, 87–100.
Silverstein SM, Berten S, Savitz A, Spaulding W & Menditto A (2007) Attention shaping procedures as a motivation-based intervention to improve attentiveness and skill acquisition in chronic schizophrenia patients. Schizophrenia Bulletin, 33, (2) 460.
Silverstein SM, Hatashita-Wong M, Solak BA, Uhlhaas P, Landa Y, Wilkniss SM, Goicochea C, Carpiniello K, Schenkel LS, Savitz A & Smith TE (2005). Effectiveness of a two-phase cognitive rehabilitation intervention for severely impaired schizophrenia patients. Psychol Med. , 35, (6) 829–37.
Twamley EW, Jeste DV & Bellack AS (2003) A review of cognitive training in schizophrenia. Schizophrenia Bulletin, 29, 359–82.
Wykes T, Reeder C, Corner J, Williams C & Everitt B. (1999). The effects of neurocognitive remediation on executive processing in patients with schizophrenia. Schizophr Bull. , 25, (2) 291–307.
Wykes T, Reeder C, Landau S, Everitt B, Knapp M, Patel A & Romeo R. (2007) Cognitive remediation therapy in schizophrenia: Randomised controlled trial. Br J Psychiatry. , 190, 421–7.
Appendix 10 The InterACT trial protocol
1. Title
1.1 Programme title
The HELPER programme (HEalthy Living and Prevention of Early Relapse).
1.2 Stream title
Stream 3: A Healthy Living Intervention to encourage activity, improve diet, and control weight gain in people with a first episode of psychosis within the past three years.
1.3 Trial acronym
INTERACT Trial (INTERvention to encourage ACTivity, improve diet, and control weight gain).
2. Introduction
2.1 Background
The life expectancy of adults with schizophrenia is reduced by about 15 years when compared with the general population (Hennekens et al. , 2005), and while some of this premature mortality is accounted for by suicide, approximately 62% of all deaths are attributable to natural causes (Harris & Barraclough, 1998). Adults with a diagnosis of psychosis are twice as likely as members of the general population to suffer from ischaemic heart disease, stroke, hypertension, epilepsy or diabetes by the age of 55 years (Hippisley-Cox & Pringle, 2005).
This poor physical health may be explained both by the side effects of antipsychotic medication (Marder et al. , 2004) and by the unhealthy lifestyles that many individuals with psychosis lead (Brown, Inskip & Barraclough, 2000). People with psychosis have been shown to take less exercise (McCreadie, 2003), eat poorer diets (McCreadie et al. , 1998) and to be significantly more likely to smoke (Brown et al. , 1999) than members of the general population. They are more likely to be obese (Homel et al. , 2002) and show a higher prevalence of metabolic syndrome (Sacks, 2004) and diabetes mellitus (Bushe & Holt, 2004). Patients taking second-generation antipsychotics, which are frequently prescribed to control symptoms of psychosis, are at particular risk of weight gain (Green et al. , 2000). The risk of antipsychotic induced weight gain may be even higher among young people experiencing a first episode of psychosis (Zipursky et al. , 2005). Recent guidance from the Department of Health (2006) has therefore highlighted the need to develop and evaluate health education programmes for people who have recently had a first episode of psychosis.
2.2 Development of the intervention
In deciding on the form of our intervention, which will be a pragmatic Phase II exploratory trial (Craig et al. , 2008) we carried out four pieces of work (collectively termed Phase 1 work), and then synthesised the findings. Firstly, we reviewed studies already in the literature, focussing on controlled interventions. Secondly, we carried out two qualitative studies, one with service users and one with case managers, to discover their views on the content, format, setting and mode of delivery of the proposed intervention. Thirdly, we reviewed the key features of Leventhal’s self-regulatory model in order to provide a theoretical underpinning for our intervention. Finally, we considered how to make our intervention culturally sensitive for delivery in a multi-cultural environment.
A systematic review and meta-analysis of ten non-pharmacological interventions to manage antipsychotic induced weight gain (Álvarez-Jiménez et al. , 2008) concluded that healthy living interventions for this population are effective, although the effect size was rather small, with an average reduction of about 4% of body weight. Only two of the interventions reviewed by Álvarez-Jiménez et al. were for first-episode psychosis, many of the studies had small samples, and some failed to describe their interventions adequately. Our own systematic review (HELPER team, 2008) of 12 trials, focussed on reduction in BMI, which we would argue is a better outcome measure than weight as there is less heterogeneity. We concluded that while some interventions have been effective, more work is needed to determine the ideal content of interventions. Analysis of group versus individual interventions suggested an advantage for individualised interventions, and evidence is emerging that interventions with a supervised exercise component may be superior to those in which exercise is merely prescribed or is absent. There is little evidence on long-term effects of the interventions, but what evidence there is suggests that the difference between treated and control participants tails off after about 2 months, suggesting a need for booster sessions and longer term follow up. A final observation is that none of the interventions in the literature to date was explicitly informed by any theoretical framework or model of behaviour change. We concluded from our literature review that there is a need for a phase-specific intervention to reduce BMI; that at least some components of the intervention should be individualised; that the intervention should include supervised exercise; that there should be post-intervention booster sessions; and that there should be long term follow up.
According to Blackburn (1995), health benefits can be seen with a body weight reduction of 5% or more, so this should be the minimum goal for any intervention. For a man aged 16–24 of UK average height (177 cm) (Health Survey for England 2006, Latest Trends) with a BMI in the overweight but not obese range of 27 (weight 84.6 kg), a loss of 5% of body weight (to 80.36 kg) would equate to a reduction in BMI of 1.35 points. We therefore decided that, in order for our intervention to have clinically meaningful effects, we should aim for a reduction in BMI of at least 1.5 points.
The most important features to emerge from our interviews with service users were that there is a general willingness to undertake a healthy living programme, that the intervention must be accessible in terms of both travel and cost, but that the actual place of the intervention was of less importance. Some service users would prefer a group intervention, with social activities, while others would prefer individual sessions. Mainly for reasons of time constraints, case managers were not enthusiastic about delivering the intervention themselves, and felt that whoever delivered the intervention would need additional training. There was agreement that the intervention needed to target both diet and exercise and that it needed to be enjoyable and client-centred. In order to further tailor the intervention to the views of service-users, we have decided to use people with lived experience of early psychosis as facilitators for some of the group activities.
Leventhal’s self-regulatory model (Leventhal, Nerenz & Steele, 1984) states that when people encounter a threat to their health (such as the threat posed by weight gain in first-episode psychosis) their behavioural response to that threat is generated by their personal perceptions or model of the threat. These personal perceptions are formed from both concrete perceptual experiences (such as previous experience of the health threat) and from abstract information (such as information from health care professionals and health education) and, if maladaptive, are more amenable to change if information comes from a both concrete and abstract sources. Threats to health may be actual (such as a current illness) or future risks (such as the threat posed by weight gain).The self-regulatory model suggests that it is important to take into account people’s personal models when prescribing (or developing collaboratively with patients) treatment programmes aimed at reducing health threats. Empirical work over the years since the model has developed has shown that health threats are conceptualised along a number of underlying dimensions, which can now be measured (Moss-Morris et al. , 2002), and used to inform individualised treatment programmes. Furthermore, recent work has started to adapt these established measures to enable the assessment of risk perceptions of potential threats to health (Cameron, 2008; Figueiras & Alves, 2007), and the intention to adopt preventive behaviours. The model prescribes the setting of goals, the use of well-specified action plans, and emphasises the roles of appraisal and feedback in modifying perceptions and behaviours. All of these elements are to be incorporated into our intervention.
Our intervention will take account of ethnic differences in diabetes and cardiovascular disease risk and of cultural factors with the potential to impact on both exercise behaviour and dietary change. There is evidence that BMI cut-offs for overweight, obesity and health risk are different in South Asian and other populations. Specifically, members of the South Asian population begin to be at risk of diabetes and cardiovascular disease at a lower BMI (≥ 24) than do members of the general population in the UK (Huxley et al. , 2008; Obesity in Asia Collaboration, 2007) and we will take account of this when determining our entry criteria and when evaluating progress during the intervention. Exercise prescriptions will be agreed with the participants, and participants will be invited to involve family members in their exercise programme should they so wish. Dietary recommendations will take account of cultural and ethnic differences in diet; dietary recommendations will be based on food groups and will be made in consultation with dieticians experienced in working with culturally diverse populations. We will endeavour to make our intervention materials relevant, comprehensible and acceptable to participants from different backgrounds. After the intervention, we will seek the views of participants on its acceptability.
2.3 Summary
To summarise, our Phase 1 work has led us to design a twelve-month healthy living intervention for patients who have experienced their first episode of psychosis in the past 3 years, who are attending an early intervention service, and who have a BMI of ≥ 25 (24 for people of South Asian origin). The intervention will focus on increasing activity and improving diet, and will:
-
aim to reduce BMI by 1.5 points at six months after the start of the intervention and to maintain this loss at the one year follow up;
-
consist of a mixed programme of individual and group sessions delivered over the first six months (the intervention proper);
-
provide a booster session in the second six months to reduce the risk of relapse;
-
contain an exercise component;
-
be delivered by a support time recovery worker (STR worker) who will utilise community resources and other professionals already working with this population, and will be assisted by service-users or ex service-users acting as group facilitators;
-
tailor treatment to patients own personal perceptions of weight gain and the risks associated with it;
-
be adapted to take account of cultural factors.
3. Objectives of the trial
The objectives of the INTERACT Trial are
-
To determine the clinical effectiveness of the intervention in reducing body mass index (BMI) by 1.5 points
-
To assess the long term effects of the intervention at 12 months post-baseline.
-
To assess the cost effectiveness of the intervention
-
To assess the acceptability of the intervention to participants
4. Design and brief outline of trial
This is a randomised controlled trial of a 12-month Healthy Living Intervention incorporating a booster session plus standard care versus standard care alone in an Early Intervention Service for patients with a first episode of psychosis in the past three years. The unit of randomisation will be the individual patient. The intervention will contain both individual and group components, the components of which will be delivered on a rolling programme by a specially trained Support Time Worker (STR), working with ex service-user facilitators. The STR worker may also enrol the assistance of others already working with this group (such as occupational therapists) where appropriate. The primary outcome will be change in BMI at 12 months post-baseline; secondary outcomes will include activity levels, a measure of quality of diet, and relapse of psychosis. The components of the trial are described in more detail below.
5. Participants
5.1 Inclusion criteria
The following people will be eligible for the trial:
-
aged 16 to 35 years
-
with a diagnosis of schizophrenia, schizophreniform disorder, schizoaffective disorder, delusional disorder, brief reactive psychosis, or psychosis not otherwise specified, determined using a checklist of criteria and review of case notes (OPCRIT http://sgdp.iop.kcl.ac.uk/opcrit/)
-
first episode of psychosis occurred within the three years preceding the trial
-
current user of an early intervention service
-
have stable accommodation (i.e. not street homeless or roofless)
-
able to give informed consent
-
have a BMI of ≥ 25, or a BMI of ≥ 24 for service users from the South Asian community (Huxley et al. , 2008; Obesity in Asia Collaboration, 2007)
5.2 Exclusion criteria
People will be excluded from the INTERACT trial if they:
-
have a diagnosis of substance dependence or abuse as determined from a review of case notes. Sub-diagnostic levels of substance use or abuse will not exclude people from the trial.
-
have a significant history of organic factors implicated in the aetiology of psychotic symptoms.
6. Recruitment procedures
The INTERACT trial is part of the HELPER programme of three clinical trials for people recovering from a first episode of psychosis, the other two streams being a cognitive remediation intervention and an intervention to reduce cannabis use. An integrated approach to recruitment across all three streams will be used. Research assistants attached to each trial will work as a team and will recruit eligible participants for all three trials. A trial manager will coordinate the work of the research assistants.
Patients will be recruited from the Lancashire and Mersey Care Early Intervention Services. Mental Health Research Network Clinical Studies Officers, together with members of the research assistant team will approach all Case Managers working for these services and ask to screen their entire caseloads for potentially eligible patients, using inclusion criteria 1–7 above. Having identified the list of possible patients, this will be shared with the case manager to check that criteria 1–7 are likely to be fulfilled. In particular, it may be necessary to obtain supplementary information on BMI. At this stage a preliminary decision will be made as to which patients might be suitable for each of the three HELPER programme trials so that patients can be approached about potential participation in a specific trial. Patients who are known to have a substance use problem will be approached about the cannabis use trial. Patients with a BMI of ≥ 25/24 and no known substance use problem will be approached about the INTERACT intervention. Patients who are awaiting cognitive therapy will be approached about the cognitive remediation intervention. Patients will be allocated a referral number for each trial at this point.
Participants identified as potentially suitable for the INTERACT trial will be given (by their case manager at a routine meeting) or sent (in the post) a copy of the INTERACT trial patient information sheet, together with a covering letter introducing the trial from their case manager. Ideally, we will encourage case managers to speak personally to potential patients about the trial and to obtain written consent from the patient for a member of our research team to contact him or her. To facilitate good working relations with case managers, our research assistants will be based with the case managers for at least part of the working week. In addition, the covering letter will have a tear off slip which potential participants can return to the research assistant to give permission to be contacted by telephone or letter, or in person at the Early Intervention Service offices, to discuss the trial further. Potential participants will have the opportunity to ask questions about the trial and will have at least one week to decide whether or not they wish to enter the trial.
If the patient agrees to be assessed, an appointment for an assessment interview will be made with the research assistant. Assessment will take place either in the patient’s home, at the Early Intervention Service, or in another location of the patient’s choice, by agreement. Potential participants under the age of 18 will have the opportunity to have a parent or guardian present during the assessment. Before the assessment begins, the trial will be explained again, another opportunity to ask questions will be given, and written informed consent to participate will be obtained. For participants under the age of 16, the written informed consent of a parent or guardian will also be required.
If the patient fulfils criteria for the INTERACT trial, he or she will be randomised into this intervention (see below). If the patient does not fulfil criteria for the INTERACT trial, he or she will be given information about the other two HELPER trials.
The INTERACT intervention will be offered as a ‘rolling programme’ of individual sessions with the option of a range of group sessions. Patients will be able to join the optional group sessions at different points in the programme. This will prevent the long delays which could occur for some patients while waiting for a group to be recruited.
7. Randomisation procedure and protection against bias
Group allocation will normally take place within one day after the baseline assessment has been carried out. Patients will be allocated on an individual basis by a person or organisation independent of the trial, by referring to one of two randomisation lists produced using PsyGrid before the start of the trial. The procedure will use randomised permuted blocks, with randomly varying block sizes of 2, 4, 6 and 8, after stratification for whether or not the patient has started to take olanzapine or clozapine in the past 6 months. There will therefore be two strata in total, and a separate randomisation list for each. A standard operating procedure for the randomisation of patients will be written. The research assistant who performed the assessment will supply the randomisation service with patient’s name and date of birth, and whether the patient has started to take olanzapine or clozapine in the past 6 months. The patient will be allocated to the next vacant position on the appropriate randomisation list, which will give the patient a unique study identifier and group allocation.
The patient’s study identifying number and group allocation will be informed to the STR worker and to the patient, but only the study identifying number will be supplied to the research assistant, who will remain blind to patient randomisation from this point on. To ensure that researchers remain blind to patient allocation, a blinding protocol will be adhered to by both researchers and STR workers. Level of blindness will be assessed by asking researchers to ‘guess’ the patient’s allocation after they have completed the final assessment.
A letter will be sent to the patient’s Case Manager, informing him or her of the arm to which the patient has been randomised.
8. Detailed description of the treatments, protocol for the intervention, therapist intervention manuals and patient materials
8.1 The active intervention
The INTERACT intervention will comprise of 8 individual sessions over a 12 month period with an emphasis on facilitating participatory exercise and dietary change through the development and implementation of patient led action plans. These sessions will be delivered by STR workers who will be trained in the delivery of the intervention. The STR workers may also be able to access other workers (e.g. occupational therapists) as a resource for the intervention. To facilitate implementation of exercise and dietary change a range of optional active group sessions (e.g. football, walking, netball groups, and a cooking group) will be offered by the STR worker for those who prefer a group based activity. The STR workers will be assisted in the running of the groups by service user or ex-service user facilitators. To optimise engagement, choice and self management a booklet will be given to all participants providing educational advice, action plans, goals, details of the group sessions, healthy eating recipes on a budget etc. A strong emphasis will be placed on maximising carer/family engagement.
8.1.a Individual sessions
The intervention will comprise a total of 8 individual sessions, 5 sessions in the first 3 months followed by 2 sessions in months 4–6 and a final session in months 10–12 to ensure gains are incorporated into the individual’s life style. In brief sessions 1 and 2 will focus on the elicitation of health beliefs and the collaborative development of individualised action plan and goals for change. A focussed discussion will elicit the goals for change, goals will be collaborative, measurable and feasible such as ‘To go to the gym 3 times per week’, ‘to increase the number of fruit and vegetables I eat by 50%’. Such goals will take account of previously and currently enjoyed activities which are exercise related, whether the participant has a preference for group or individual activity, carer/family involvement to assist with changes, and information on local activities which are geographically accessible and affordable. Sessions 3–5 will focus on the implementation of the action plan, (and where agreed the inclusion of family/carer involvement). Collaborative problem solving will identify and facilitate solutions to barriers of implementing the action plan. These sessions may also involve the STR worker accompanying clients to a particular activity for the first time (ie gym, swimming etc) to maximise implementation. Sessions 6 and 7 will focus on progress and monitoring of the goals stated in sessions 1 & 2 and adaption’s made as necessary. Session 8 will focus on working with the user to ensure that implementation is embedded within their everyday routine.
8.1.b Group Sessions
In addition to the individual sessions a range of optional group sessions will be delivered by the STR worker and co-facilitated by a service user on 3–5 occasions weekly for 2 hours and will incorporate some of the following ‘team exercise games’, lunch groups involving cooking and eating a healthy lunch, country walks, etc. Families and carers will be welcomed to attend the groups. Group activities will alternate between different geographical locations to maximise participation.
8.1.c The INTERACT Intervention Booklet
A healthy living intervention booklet will be given to all participants and will provide accurate information about healthy living, including governmental guidelines on exercise and diet, strategies for implementing such changes into usual daily routines, culturally varied recipes, barriers and facilitates to implementing healthy living which are specific to the participants mental health problems including fatigue and motivation, a section for families and carers and information of local activities. The booklet will have a Flesch readability ease score of between 60 and 70 and will be translated into Urdu.
8.1.d. Training of STR workers and facilitators, training materials and training handbook
Training for the STR workers will be provided by the trial team and a user and will consist of a 3 day intensive training programme. On the third day of this training, the STR workers will be joined by service user facilitators who are going to be working with the groups, and who will receive one day’s training. The training will be accompanied by a training handbook with detailed session by session outlines. The training will focus on all aspects of delivering the intervention from initial assessment, elicitation of health beliefs, developing collaborative action plans and goals, engaging family/carers, monitoring and collaborative problem solving to overcome barriers to implementation. A significant portion of the training will be spent practising the above skills using fictitious but typical cases to enhance learning and skill. In addition the STR workers will receive training on the running of groups.
8.1.e. Supervision of STR workers and facilitators.
Clinical supervision for the STR workers and service-user facilitators will be provided on a two weekly basis by members of the research team (TB, AW, KL).
8.2 Standard Care
The EIS works individually with each service user and his or her family to address problems/needs that are identified during his or her assessment/reassessments; all service users have enhanced care coordination and all should have a specific care plan personalized to their needs. Each service user would be seen on a regular basis; this may be weekly, fortnightly or more or less frequently by his or her case manager depending on the stage of the intervention and the need.
The case managers are supported by support time recovery workers who are commissioned and supervised by the case managers to do specific work, often for time limited periods. The service users are also offered cognitive behaviour based interventions by case managers. Case managers may consult with and obtain input from psychological therapists/psychologists who may themselves also be involved in delivering formal CBT.
9. Assessments and measures
9.1 Timing and administration
There will be three assessment points: Baseline assessment prior to randomisation into the trial; at 6 months; and final assessment at the end of one year. The primary outcome point will be at one year.
Assessments will be administered by the trial research assistant(s). While the majority of measures are self-report measures, in accordance with a blinding protocol every effort will be made to keep research assistants blind to randomisation codes so that post-intervention and long-term follow up assessments can be carried out blind to treatment allocation. Any unblindings will be recorded, and we will the degree of blindness maintained at the end of the trial (see Section 7 above).
9.2 Measures
The primary outcome will be change in BMI at one year post-baseline. This will be determined by measuring the participants’ height at baseline assessment and weighing the participant using the same set of scales at each assessment. The formula weight in kg/height in metres2 will then be applied to determine BMI. The intervention will be deemed successful if a patient has reduced his or her BMI by at least 1.5 points at one year follow up.
Secondary outcomes will be: weight loss at 6 months (ie end of the intervention proper); change in waist circumference, level of daily activity as measured by a standard activity level measure which has been validated for use in this population (short form IPAQ, Craig et al. , 2003; Faulkner et al. , 2006); adherence to the agreed exercise programme; relapse of psychosis, as determined from a review of case notes; health status and quality of life as measured by the EQ-5D (Rabin & de Charro, 2001) and the MOS SF-36 (Ware & Sherbourne, 1992); food frequency diary, with cultural adaptations (Crozier et al. , 2008).
Measures of predicted process variables and mediators of change will be; a measure of representations of weight gain and intentions to take preventive/remedial action (Cameron, 2008; Figuieras & Alves, 2006); a measure of perceptions of antipsychotic medication (Fialko et al. , 2008);. Depression will be measured using the Calgary depression scale (Addington et al. , 1996) as a potential predictor of response to the intervention; reduction in depression may also be an outcome of interest. We will also measure adherence to medication (Brief Adherence Rating Scale, BARS; Byerly et al. , 2008) and keyworker rating of adherence to medication on four point scale (Fialko et al. , 2008).
9.3 Schedule of assessments and measures
| Assessment measure | Baseline | 6 months | 12 months |
|---|---|---|---|
| Height, weight, BMI, waist-circumference | ✗ | ✗ | ✗ |
| Level of daily activity (short form IPAQ) | ✗ | ✗ | ✗ |
| Relapse of psychosis from notes | ✗ | ✗ | |
| Adherence to agreed exercise programme | ✗ | ✗ | |
| Quality of life (EQ-5D) | ✗ | ✗ | ✗ |
| Health status (SF-36) | ✗ | ✗ | ✗ |
| Food frequency diary | ✗ | ✗ | ✗ |
| Perceptions of weight gain | ✗ | ✗ | ✗ |
| Perception of antipsychotic medication | ✗ | ✗ | ✗ |
| The Illness Perceptions Questionnaire for Schizophrenia (IPQ-S) | ✗ | ✗ | ✗ |
| Depression (Calgary) | ✗ | ✗ | ✗ |
| Adherence to medication (BARS) | ✗ | ✗ | ✗ |
| Keyworker rating of adherence to medication | ✗ | ✗ | ✗ |
| Economic Analysis | ✗ | ✗ | ✗ |
9.4 Training and supervision of research assistants
Our research assistant(s) will be trained to administer the outcome measures in a standardized way. They will receive regular debriefing and supervision which will focus both on the integrity of the assessment procedures and on any health and safety or other issues arising from the assessments.
9.5 Risk assessments and health and safety
Risk assessments will be carried out prior to each assessment visit, in accordance with the procedures of the Early Intervention Services from which the patients are recruited. No RA will visit any patient in the absence of an up-to-date risk assessment. In order to maintain blindness, RAs will not have direct access to risk assessments.
10. Power analysis
Our trial is powered on the basis that a reduction in BMI of 1.5 points would be of clinical significance, and would be feasible to achieve. Our systematic review of weight loss interventions suggests a common standard deviation for change in BMI of 2 points, giving an anticipated effect size of 0.75. Using a simple t-test to compare intervention and control groups, we would require a total sample size of 78,or two groups of 39, for our finding to have 90% power to be significant at the p < .05 level (nQuery Advisor). Our systematic review shows that the anticipated drop out and loss to follow up rate will be low, at around 10%. Allowing for loss to follow up of 10% of the sample recruited, we would require 86 patients at baseline (43 allocated to each group).
Because the intervention arm of our trial includes optional group components, there is some potential variability in the actual intervention received by participants allocated to the intervention arm. To account for the possibility that some participants will attend groups and others will not, thus adding an additional factor to be taken into account in the analysis, we have increased the sample size suggested by our power calculation by 20% to 104 (52 in each group).
11. Economic assessment
The aims of the economic evaluation will be to explore whether the intervention has the potential to be cost effective and inform the design of a Phase III RCT. The costs will include the costs of the intervention (staff, facilities, equipment and materials), the costs of primary, community and secondary health service use, the costs of other social care service use and the costs of alternative and personal health expenditure. The data will be collected from patients using service use forms adapted from other studies. Unit costs will be collected from local and trial accounts (to estimate the costs of the intervention) and published databases (PSSRU, DH Reference costs). The unit costs will be supplemented by published literature where necessary. The main outcome for the analysis will be quality adjusted life years (QALYs). Two estimates of QALYs will be calculated from health status measured the EQ-5D and by the SF36 and associated population tariffs. Service use and health status data will be collected at each scheduled assessment from baseline to the end of follow up. The analysis plan for the economic assessment can be seen at 12.3b.
12. Analysis plan
12.1 Data entry and checking
Data spreadsheets will be set up by IT staff at the Mental Health Research Network. Data will be entered into data spreadsheets (one for each time point) by research assistants as they collect it. Data entry will be centralised using PsyGrid software so that RAs from each of the three HELPER trials can enter data online onto a central server. The primary outcome measure (weight and height before and after the intervention) and secondary outcome measures (weight at one-year follow up, activity levels, measure of quality of diet) will be double entered by two research assistants. Other data will be checked and cleaned by the research assistant before analysis.
12.2 Data protection and confidentiality
Paper copies of questionnaires will be stored in locked filing cabinets at the University of Manchester. Details of patient randomisation will be stored separately from all other data and password protected.
Monitoring arrangements
12.3 Analysis
12.3a Analysis of primary and secondary outcomes and process measures
Analysis of the primary and secondary outcome measures will be as randomised, i.e. on an intention to treat basis. Longitudinal data will be modelled using a mixed-effects model. Analyses to estimate treatment effects will be variations on analysis of covariance (depending on whether the outcome is binary or quantitative), with covariates including whether the patients is taking olanzapine/clozapine or not and baseline BMI. The effects of the optional group therapy will be explored using recently-developed causal inference methods to evaluate the role of mediators and other process variables in complex intervention studies – see TenHave et al. , 2007; Dunn & Bentall, 2007; Emsley, White & Dunn, 2009.
Distinguishing between attrition from treatment and attrition from the trial is rarely shown in academic papers. In keeping with the philosophy of ITT we will make every effort to follow people up regardless of whether they have been lost to treatment and distinguish between attrition (adherence) from treatment and attrition from the trial. As those who are not adherent with the treatment protocol tend to be less adherent with the assessments protocol, we will establish special procedures and make targeted efforts to assess the treatment dropouts at all scheduled time points.
Where data are missing, they will be modelled on the assumption that they are missing at random. Logistic regression will be used to test the validity of this assumption, and to calculate a probability of providing complete data, with baseline variables as predictors. These analyses would then be used to create inverse probability weights for subsequent analyses on outcome variables.
The effects of possible mediating variables will be tested by assessing their effect on outcome and on the significance of the treatment effect when they are added as additional covariates in the above analyses.
12.3b Analysis of economic data
Statistical analysis (regression, structural equation modelling) will be used to identify key covariates and potential mediators or moderators of costs. Cost effectiveness acceptability analysis will be used to explore the potential cost effectiveness of the intervention compared to standard care. All comparisons of costs and QALYs, and all cost effectiveness acceptability analyses will be adjusted for baseline covariates, mediators and moderators. The QALYs measured by the EQ5D and SF36 will be compared to assess whether they affect the results of the cost effectiveness acceptability analyses and identify whether one is more likely to be reliable, relevant, sensitive and valid than the other in this population, for this type of intervention.
13. Adverse events procedure and withdrawal of patients from the intervention
Patients will be withdrawn from the intervention under the following circumstances:
-
development or exacerbation of a medical condition which means that the patient is unable to continue, or that continued participation in the trial endangers the patient’s health
-
Withdrawal from intervention does not have to mean withdrawal from the study. Follow up assessments will still be made provided the patient is willing and able to undertake them, and every attempt will be made to maintain the blinding of the assessor.
14. Time schedule for trial
Ethical approval will be sought in January 2009. The STR worker will be trained from March to June 2009, and resources gathered (directory of local community resources, contacts made with other workers etc.) to design and implement the rolling programme of activities. Arrangements will be put in place to start recruitment from April 2009. Patients will be randomised into the trial over a period of eighteen months, starting in June 2009 and ending in December 2010. The intervention will last for 12 months, so that the last intervention groups will finish at the end of December 2011, with the last follow up assessments occurring within one month of that date.
15. Trial governance and management
15.1 Ethical approval
Ethical approval will be sought from the appropriate NRES committee.
15.2 Insurance and indemnity
A procedure to ensure the health and safety of trial workers will be appended to the protocol.
15.3 Trial management and oversight
Members of the HELPER INTERACT research team will meet monthly, and will report to the HELPER Programme Management group at their three-monthly meetings.
There will be two independent committees overseeing the work of the entire HELPER programme – a Trial Steering Committee (TSC) and an independent Data Monitoring and Ethics Committee (DMEC).
The DMEC will consist of members external to the study team including a statistician, a clinician and an expert in health services trials. A DMEC report template will be devised for reporting purposes and agreed by the DMEC committee prior to the commencement of the study.
16. Protocol amendments
Any amendments to the protocol which take place after the trial starts will be recorded in this protocol.
17. Ancillary studies
The agreement of the trial management team will be necessary for the adoption of ancillary studies. Such studies might include an investigation of the impact of the programme on significant others of participants.
18. References
Addington, D., Addington, J., & Atkinson, M. (1996). A psychometric comparison of the Calgary expression Scale for Schizophrenia and the Hamilton Depression Rating Scale. Schizophrenia Research, 19, 205–12.
Álvarez-Jiménez, M., Hetrick, S.E., González-Blanch, C., Gleeson, J.F., & McGorry, P.D. (2008). Non-pharmacological management of antipsychotic-induced weight gain: systematic review and meta-analysis of randomised controlled trials. British Journal of Psychiatry, 193, 101–107.
Blackburn, G. (1995). Effect of degree of weight loss on health benefits. Obesity Research, 3, (suppl.2):s211–216.
Booth, M.L. (2000). Assessment of Physical Activity: An International Perspective. Research Quarterly for Exercise and Sport, 71, 114–20.
Brown, S., Birtwistle, J., Roe, L., et al. (1999). The unhealthy lifestyle of people with schizophrenia. Psychological Medicine, 29, 697–701.
Brown, S., Inskip, H., & Barraclough, B. (2000). Causes of the excess mortality of schizophrenia. British Journal of Psychiatry, 177, 212–217.
Bush, C,. & Holt, R. (2004). Prevalence of diabetes and impaired glucose tolerance in patients with schizophrenia. British Journal of Psychiatry, 184, (suppl.47):s67–71.
Byerly, M.J., Nakonezny, P.A., & Rush, A.J. (2008). The Brief Adherence Rating Scale (BARS) validated against electronic monitoring in assessing the antipsychotic medication adherence of outpatients with schizophrenia and schizoaffective disorder. Schizophrenia Research, 100, 60–69.
Cameron, L.D. (2008). Illness risk representations and motivations to engage in protective behaviour: The case of skin cancer risk. Psychology and Health, 23, 91–112.
Craig, C.L., Marshall, A.L., Sjostrom, M., Bauman, A.E., Booth, M.L., Ainsworth, B.E., et al. (2003). International physical activity questionnaire: 12-country reliability and validity. Medicine and Science in Sports and Exercise, 35, 1381–1395.
Craig, P., Dieppe, P., Macintyre, S., Michie, S., Nazareth, I., & Petticrew, M. (2008). Developing and evaluating complex interventions: the new Medical Research Council guidance. British Medical Journal, 337:a1655, doi: 10.1136/bmj.a1655 (Published 29 September 2008)
Crozier, S. R., Inskip, H., Barker, M., Lawrence, W., Cooper, C., & Robinson, S. (2008). Development of a shortened FFQ to assess a ‘prudent’ dietary pattern amongst women in Southampton. Proceedings of the Nutrition Society (in press).
Department of Health. (2006). Choosing Health: Supporting the physical health needs of people with severe mental illness. Commissioning Framework. DH, London.
Dunn, G. & Bentall, R. (2007). Modelling treatment-effect heterogeneity in randomised controlled trials of complex interventions (psychological treatments). Statistics in Medicine 26, 4719–45.
Emsley, R., White, I. & Dunn, G. (2009). Mediation and moderation of treatment effects in randomised controlled trials of complex interventions. Statistical Methods in Medical Research, in press.
Faulkner, G., Cohn, T., & Remington, G. (2006). Validation of a physical activity assessment tool for individuals with schizophrenia. Schizophrenia Research, 82, 225–231.
Fialko, l., Garety, P., Kuipers, E., Dunn, G., Bebbington, P., Fowler, D., Freeman, D. (2008). A large-scale validation study of the Medication Adherence Rating Scale (MARS). Schizophrenia research, 100, 53–59.
Figueiras, M.J., & Alves, N.C. (2007). Lay perceptions of serious illnesses: an adapted version of the Revised Illness Perception Questionnaire (IPQ-R) for healthy people. Psychology and Health, 22, 143–158.
Green, A.I., Jayendra, K.P., Goisman, R.M., Allison, D.B., & Blackburn, G. (2000). Weight gain from novel Antipsychotic drugs: Need for action. General Hospital Psychiatry, 22, 224–235.
Health Survey for England (2006). Latest Trends. http://www.ic.nhs.uk/pubs/hse06trends
Harris, E.C., & Barraclough, B. (1998). Excess mortality of mental disorder. British Journal of Psychiatry, 173, 11–53.
Hennekens, C.H., Hennekens, A.R., Hollar, D., & Casey, D.E. (2005). Schizophrenia and increased risks of cardiovascular disease. American Heart Journal, 150, 1115–1121.
HELPER Team. (2008). A systematic review of weight management interventions for people with psychosis. Unpublished.
Hippisley-Cox, J., & Pringle, M. (2005). Health inequalities experienced by people with schizophrenia and manic depression: analysis of general practice data in England and Wales. Report to the Disability Rights Commission. University of Nottingham UK: Disability Rights Commission.
Homel, P., Casey, D., & Allison, D.B. (2002). Changes in body mass index for individuals with and without schizophrenia, 1987–1996. Schizophrenia Research, 55, 277–284.
Huxley, R., James, W.P.T., Barzi, F., Patel, J.V., Lear, S.A., et al. , on behalf of the Obesity in Asia Collaboration. (2008). Ethnic compaisons of the cross-sectional relationships between measures of body size with diabetes and hypertension. Obesity Reviews, 9, (Suppl 1): 53–61.
Leventhal, H., Nerenz, D., & Steele, D. (1984). Illness representations and coping with health threats In: Baum, A., Taylor, S., & Singer, J. (Eds.). Handbook of Psychology and Health. New Jersey: Hillsdale.
Marder, S.R., Essock, S.M., Miller, A.L., Buchanan, R.W., Casey, D.E., Davis, E.M., et al. (2004). Physical health monitoring of patients with schizophrenia. American Journal of Psychiatry, 161, 1334–1349.
McCreadie, R.G. (2003). Diet, smoking and cardiovascular risk in people with schizophrenia. British Journal of Psychiatry, 183, 534–539.
McCreadie, R.G., MacDonald, E., Blacklock, C., et al. (1998). Dietary intake of schizophrenic patients in Nithsdale, Scotland, case-control study. British Medical Journal, 317, 784–785.
Moss-Morris, R., Weinman, J., Petrie, K., Horne, R., Cameron, L., & Buick, D. (2002). The Revised Illness Perception Questionnaire (IPQ-R). Psychology and Health, 17, http://www.informaworld.com/smpp/title∼content=t713648133∼db=all∼tab=issueslist∼branches=17 - v171 – 16.
nQuery Advisor. URL: http://www.statsol.ie/nquery/nquery.htm
Obesity in Asia Collaboration. (2007). Waist circumference thresholds provide an accurate and widely applicable method for the discrimination of diabetes. Diabetes Care, 30, 3116–8.
OPCRIT http://sgdp.iop.kcl.ac.uk/opcrit/
Rabin, R., & de Charro, F. (2001). EQ-5D: a measure of health status from the Euro-Qol Group. Annals of Medicine, 33, 337–343.
Sacks, F.M. (2004). Metabolic syndrome: epidemiology and consequences. The Journal of Clinical Psychiatry, 65, (Suppl,18):3–12.
Ten Have, T.R., Joffe, M.M., Lynch, K.G., Brown, G.K., Maisto, S.A. & Beck, A.T. (2007). Causal Mediation Analyses with Rank Preserving Models. Biometrics 63, 926–34.
Ware, J.E., & Sherbourne, C.D. (1992). The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care, 30, 473–83.
Zipursky, R.B., Hongbin, G.U., Green, A.I., Perkins, D.O., Tohen, M.F., McEvoy, J.P., et al. (2005). Course and predictors of weight gain in people with first-episode psychosis treated with olanzapine or haloperidol. British Journal of Psychiatry, 187, 537–543.
Appendix 11 The Rethinking Choices After Psychosis (ReCAP) trial protocol
Title: A phase-specific psychological therapy for people with problematic cannabis use following a first episode of psychosis.
Aim of study: To evaluate a phase-specific psychological therapy (intergrated Motivational Interviewing and Cognitive Behaviour Therapy, MiCBT) designed to reduce cannabis use in people who have recently experienced a first-episode of psychosis. For young, early psychosis clients we hypothesise that a brief intervention (12 sessions) is as efficacious as longer term therapy (24 sessions) when compared with treatment as usual.
Background: People with psychosis have a higher rate of substance use disorders than comparable non-psychotic comparison groups. The rate is high amongst young people who have recently had their first episode of psychosis (FEP) (Cantwellet al. , 1999; Lambertet al. , 2005). In this population (FEP), cannabis is the most commonly used illicit drug, with typical rates of 35–45% for current cannabis use (Barneset al. , 2006; Lambertet.al., 2005; Wadeet al. , 2006). Young people with psychosis who use cannabis are at increased risk of: delayed remission, relapse, suicidal behaviour, violence, social instability and homelessness (Barnes et al. , 2005; Cleghornet al. , 1991; Linszen, 1994, Sorbaraet al. , 2003; Verdouxet al. , 2001). These risks are present even at levels of consumption that would be considered unremarkable in the general population, suggesting that psychosis brings an increased sensitivity to cannabis (Curranet al. , 2002; Bellacket al. , 2007).
It is particularly worrying when problematic cannabis becomes ingrained in the period immediately after a first episode of psychosis (Bellacket al. , 2007). It is widely believed that the pattern of illness established during this ‘critical period’ determines the long-term prognosis of the condition (Birchwoodet al. , 1998). Thus repeated psychotic episodes, induced by cannabis use, may increase the risk of treatment resistant symptoms, and precipitate an irreversible decline in social functioning, leading to life-long reliance on health and welfare services.
Unfortunately people with psychosis and substance misuse (‘dual diagnosis’) tend not to be well served by mental health services, even though treatment of dual diagnosis is a major NHS priority (Weaveret al. , 2003). Care often arrives too late in the day, after treatment resistance has set in, problematic patterns of substance misuse are well-established and motivation for change is low (Teesonet al. , 2000; Bakeret al. , 2002). Our own experience concords with the literature: in our earlier evaluations of therapy for people with dual diagnosis, we found that entrenched co-morbid substance use problems required intensive long term contact with a therapist (i.e. from 9–12 months duration) (e.g., Barrowcloughet al. , 2001; Haddocket al. , 2003). Thus, there is a strong case for targeting people at an earlier stage of the illness when cannabis use is not so well established, and the deterioration consequent on prolonged use has not set in.
As yet there are no interventions with clearly demonstrated acceptability, efficacy and effectiveness for people with substance misuse following a first episode. However, there are some indications that it may be easier to motivate patients to reduce cannabis if we intervene at an early stage of the psychosis. For example, in Melbourne, Australia it has been reported that 50% of young people using cannabis at admission to early intervention services voluntarily cease use within the first 6–10 weeks (Edwardset al. , 2006). This research group recently reported a clinical trial where 47 FEP patients using cannabis in the 4 weeks prior to assessment were randomised to 10 sessions of either psycho-education or a cognitive behavioural intervention. Over time there was significant reduction in cannabis use in both groups was found. Methodological issues limit the generalizability of this trial: the sample size was small and, there was no treatment as usual control group, meaning that we cannot measure the intervention improvement against any spontaneous reduction that occurred without treatment. Additionally, in a recent review of interventions for cannabis use in non-dual diagnosis samples, it was concluded that ‘response rates, particularly abstinence from cannabis, leave much room for improvement’ (Denis et al. , 2006). Thus it remains unclear whether brief psychological intervention is effective for this client group.
To take forward this area of research we propose to evaluate two hypotheses, concerning the treatment of cannabis use in people who have recently had a first episode of psychosis. The first hypothesis is that a long term intervention (24 sessions delivered over 9 months) will be more effective in reducing cannabis use than treatment as usual. The second hypothesis is that a brief intervention (12 sessions delivered over 4.5 months) will be as effective as the long-term intervention.
Summary of trial and proposed trial design
Summary of trial: People who have had a first episode of psychosis and who are under the care of an EIS service in one of the participating sites, who also have a co-morbid cannabis misuse or dependence problem will be randomly allocated to one of 3 conditions:
-
Long (9 months) treatment in addition to standard psychiatric care
-
Brief (4.5 months) treatment in addition to standard psychiatric care
-
Or standard care alone (SCA) – ‘Treatment as Usual’.
The therapy – integrated Motivational Interviewing (MI) and Cognitive Behaviour Therapy (MiCBT) – consists of MI to increase motivation for change, and cognitive behaviour therapy to assist the implementation of change and relapse prevention strategies. The MiCBT which patients receive will be matched to their stage of change during the treatment phase.
Proposed trial design: A rater blind randomised controlled trial using geographically defined samples of patients from different localities to ensure good generalizability of the study. This is a pragmatic rather than explanatory trial carried out to evaluate the benefits of two levels of psychological treatment over standard available care.
Planned trial interventions
The integrated MiCBT will match the focus of therapy to the patient’s stage of change regarding cannabis use.
For all clients initially substance use will be approached within the context of the client’s lifestyle and mental health problems, and the intervention will be described as an approach to identifying helpful lifestyle changes (too much of an emphasis on cannabis use in the initial stages of the intervention may be detrimental to the motivational processes in clients not ready for change). With the client’s permission, later sessions will focus on examining the role of cannabis and how it relates to problems and desired goals. Using the motivational interviewing style, the therapist will seek to understand the client’s frame of reference and build up a shared model of how cannabis relates to their mental health and other life concerns. Once client commitment to change is secured, the therapist will assist the client to make a plan for cannabis cessation or reduction. Cognitive behavioural techniques will be used to assist with the implementation of change. These will include identifying and problem solving for high risk situations; CBT for coping with symptoms associated with substance use; and relapse prevention. Whilst the intervention will use a range of strategies to develop and consolidate the client’s motivation to reduce cannabis, we do not expect all clients to move through stages at the same pace and some may be unwilling to change their substance use. As noted below, these clients will be supported in selecting a goal that is of high priority for them whilst the therapist will seek further opportunities to review substance use within this context. The care co-ordinator will be invited to 3 meetings (beginning, middle, end of therapy) to ensure good communication between the intervention and the EIS.
From our previous studies, we anticipate that there will be considerable variation in the number of sessions participants opt to attend; that some patients will require assertive outreach (unlimited repeat appointments; flexibility in timing and location of sessions; tracking of change in residence) in order to maintain engagement; and that engagement may wax and wane over the treatment period. The number of sessions attended will be controlled for statistically in the analyses.
Mindful of the client’s stage of change regarding cannabis, for both Long and Brief conditions the therapist will attempt to progress through the following steps:
Building engagement – developing a relationship:
-
Discussion with client about current concerns and how they have arrived at current situation particularly looking at mental health issues. Development of a wants list and problem list looking at obstacles to progress and identification of how their life values fit within these.
-
Exploration of their explanatory model of psychosis (onset, symptoms and consequences). Feedback information from assessments to further explore their understanding.
-
Exploration of how cannabis use fits into life generally and then elicitation of understanding of how cannabis use fits into their explanatory model of psychosis.
-
Provision of information about cannabis and mental health, feedback of information from substance use assessments and where appropriate further monitoring of links between cannabis and mental health.
-
Development of a shared understanding/formulation outlining links between psychosis, difficulties in life and cannabis use.
-
Based on formulation, discussion of possible change options, including change in cannabis use, to address difficulties identified by client.
-
Development of a change plan. Whilst abstinence will be the primary target of intervention, it will be the client’s choice whether they wish to plan for abstinence, reduction including harm reduction, or no change in substance use. If the latter option is selected then the therapist will help the client to focus on a goal that is of importance to the client and where possible the therapist will use this alternative goal as a context in which to continue to review the role of cannabis use and to facilitate motivation for change.
-
Development of a maintenance and relapse prevention plan.
Differentiating the Brief and Long Interventions: Clients in the Brief intervention condition will be offered up to 12 sessions of MiCBT over 4.5 months; clients in the Long term intervention condition will be offered up to 24 sessions over 9 months. Both interventions will attempt to progress through the 8 steps delineated above. However, the Long intervention will potentially allow:
-
More time to work through all the above steps. Those who have low motivation may be unlikely to proceed through all the steps in 12 sessions.
-
More time to develop the change plan and particularly the use of CBT within the plan.
-
Where clients are unwilling to change their substance use, more time to work on alternative goals and to develop motivation for changing substance use within these different contexts.
The MiCBT treatments will follow written protocols developed in our previous studies (Barrowcloughet al. , 2008), and further modified for this study by making it phase-specific for the ‘critical period’ of early psychosis. Therapists will undergo initial training and then supervision throughout the trial, and were participants give permission, sessions will be audio taped for the purpose of rating treatment fidelity.
Standard care from the Early Intervention Services involved in the study is compliant with the Policy Implementation Guide and includes intensive case management, crisis response, behavioural family therapy, and cognitive therapy for persistent symptoms.
Procedures for recruitment
Staff will be informed about the study prior to its commencement via presentations to teams and information sheets etc.
Identifying potentially eligible participants
All clients within the EIS of participating NHS trusts will be sent a letter from the head of the EIS service outlining the study and containing the Patient Information Sheet. The letter will inform clients that a) some clients may be approached by their care co-ordinator to ask if they would be interested in finding out more details about the study from one of the research workers, b) any client wishing to find out more should let their care co-ordinator know and the care co-ordinator will then pass on their name to the research worker. In this way, all clients will have the opportunity to discuss the research with a research worker and find out if they are potentially eligible.
For service users who agree to be contacted, the research worker will discuss the study with them and ensure that they have available a copy of the information sheet. Those consenting to be screened will be asked to complete an initial screening interview to evaluate inclusion/exclusion criteria. The interview will consist of the drug screen section of the SCID pertaining to cannabis.
Recruiting participants
Those service users who give informed consent and who fulfil the screen criteria will be recruited into the study. Once the service user consents to participation, letters will be sent to their GP, consultant and key worker.
Allocation of participants to trial groups
The trial will involve patients from the Early Intervention Services of two large NHS mental health trusts (Manchester Mental Health and Social Care NHS trust and Lancashire Care foundation NHS trust). In order to avoid treatment centre confounding, participants will be randomly allocated within each of the trusts to the three groups (MiCBT and Standard Care Long; MiCBT and Standard Care Brief; Standard Care alone) after stratifying by variables believed to be predictive of treatment participation or outcome: gender and living with family vs. not living with family. Allocation will be done using randomised permuted blocks with a randomly varying block size and will be the responsibility of the trial statistician.
Sample size
Our primary outcome measure will be number of days abstinent using the Time Line Follow Back. In a comparison of the Standard Care Alone vs Long and Brief treatments, with a sample size of 45 in each group and allowing for 20% attrition, the study would have 80% power to detect an effect size of 0.7 (a moderate to large effect size) with a significance level of .05. This effect size would be the equivalent of a 20% difference in days abstinent between no treatment and treatment groups which is in line with data from previous studies.
For the secondary outcome of the GAF, our previous research has shown that if the intervention is successful we would expect to see an 8 point improvement on this scale relative to the control group. Assuming a standard deviation of about 12 at baseline, a sample of 45 participants in each of the three groups is required to have an 80% chance of detecting this difference in two separate comparisons (short intervention vs. control; long intervention vs. control) using a Bonferoni-corrected significance level of 0.025. Hence for this exploratory trial we propose a total sample size of 45 per group (135 in total).
Proposed methods for protecting against bias
Blindness: Independent and blind assessment of the groups will be carried out. Blindness will be maximised by ensuring patient data are stored in separate offices, locating research and therapy staff in separate offices, and third party management of appointments to avoid appointment clashes.
Reliability of raters: Independent assessors will have a training period to ensure that they meet an identified standard of competence and level of reliability on the instruments used for the outcomes. These standards will be monitored by the project team. Independent checks on inter-rater reliability to check for rater ‘drift’ will be carried out during the trial.
Treatment Integrity: Treatment fidelity and skilfulness for the experimental condition (MiCBT) will be ensured by a) using therapists who have prior experience of using psychological treatments with psychosis clients, b) using therapists who meet the British Association of Behavioural and Cognitive Psychotherapies criteria for accreditation, c) by having a 3 month training period for therapists, d) weekly supervision using audiotaped therapy sessions provided by a therapist experienced in delivering MiCBT to psychosis clients. All therapy sessions during the trial will be audiotaped subject to the patients’ consent to permit independent fidelity ratings of taped sessions. Treatment dose in terms of number of therapy sessions attended will be closely monitored and documented, with good attendance facilitated by patients being offered treatment at the location of choice (hospital, home, clinic); by assertive methods of patient tracking; by offering an unlimited (within time constraints of the treatment period) number of repeat appointments when patients fail to attend.
Inclusion/exclusion criteria
-
Meeting DSM-IV criteria for schizophrenia, schizophreniform disorder, schizoaffective disorder, delusional disorder, or psychosis not otherwise specified using a checklist of criteria and review of case notes; and confirmation of this diagnosis using the Structured Clinical Interview for DSMIV Axis 1 disorders (see elaboration below)
-
DSM-IV diagnosis of cannabis dependence or abuse using the appropriate sections of the Structured Clinical Interview
-
A history of cannabis use of at least ×1 day per week in at least half the weeks in the 3 months prior to assessment (Those who are misusing other illicit substances or alcohol will not be excluded)
-
Age 16–35
-
Having stable accommodation (i.e. not street homeless or roofless)
-
Possessing sufficient English to reliably complete the assessments
-
Able to give informed consent
-
No significant history of organic factors implicated in the aetiology of psychotic symptoms.
Elaboration regarding criterion a
We acknowledge the complexity of diagnostic issues with recent onset psychosis. Clients with a diagnosis of Brief Reactive Psychosis will be included at the screening stage since often this develops into full Psychosis. Information from all such cases and any others where there is diagnostic uncertainty will be reviewed by the trial psychiatrists who will decide whether diagnostic criteria are met and those included will be further reviewed diagnostically at the end of the study.
Context of the research study
The proposed study is part of an NIHR funded programme of research ‘The HELPER programme: Healthy Living and Prevention of Relapse’. The programme focuses on developing interventions to target known risk factors for relapse and poor health, in young people with early psychosis. The interventions are linked by a common theoretical framework, the Self-regulatory Model of Illness Management and designed for optimum delivery by the national network of Early Psychosis Teams. The Self Regulation Model (SRM) (Leventhalet al. , 1984) proposes that people are problem solvers whose coping strategies are attempts to close the gap between their current health (as they see it) and the best health state they believe they can achieve. As part of the research program we intend to collect data to determine how far the SRM can be applied to people with psychosis. Hence the inclusion in this study of measures common to the programme.
Outcome measures
To be completed by blind and independent assessors at baseline, 4.5 months, 9 months and 18 months.
(i) Primary outcome – Cannabis use change: Measures of severity and frequency of cannabis and other illicit substances derived from Time Line Follow Back (TLFB) will be used. The TLFB is the most well researched method of collecting reliable and valid retrospective self reports of daily substance use in terms of drugs (Fals-Stewartet al. , 2000). We have found it to be acceptable and practical for use with this client group in a prior study (Barrowcloughet al. , 2001), with good concurrent validity in terms of independent ratings from patient case managers. The primary outcome will be frequency (calculated as change in percentage days abstinent (PDA)). A secondary outcome obtained from the TLFB assessments will be severity (percentage change in amount of use (PAU)) for cannabis relative to use at pre-treatment baseline. Information from collaterals including care co-ordinators will be used where possible using a similar TLFB method and the Clinician Rating Scales (Drakeet al. , 1996). Additionally the validity of the self reports will be assessed using hair analysis from a random sample of 25% of participants at pre, post, and follow up assessment points. Agreement to give a hair sample will not be an inclusion criteria.
(ii) Additional Secondary outcomes
Symptoms, functioning and service engagement:
Symptomatology: PANSS (Kay et al. , 1989) to assess severity of symptoms; PSYRATS (Haddocket al. , 1999) to assess distress regarding symptoms.
Relapse: Two methods of assessing the frequency and duration of relapse will be used: 1) number and duration of hospital admissions identified from hospital record systems, 2) number and duration of exacerbations of symptoms lasting longer than 2 weeks and requiring change in patient management (operationally defined, Barrowcloughet al. , 1999).
Global Assessment of Functioning (GAF-D: APA, 1994): Measure of global level of functional disability, 0–100 with anchor points. Widely used and good reliability.
Service Engagement Scale (Taitet al. , 2002): A 14 item measure of client engagement with services, with subscales of availability, collaboration, help seeking and treatment adherence. Disengagement with services is a widely acknowledged clinical problem with people with dual diagnosis. For 0, 12, 24 months.
Calgary Depression Scale (Addington and Addington, 1997): This is a 9-item scale designed to assess depressed mood in schizophrenia.
Deliberate self harm assessment: This is a structured assessment of whether an episode of near-fatal DSH has occurred in the previous time period (2 minutes), as used in the CUtLASS trial. (Both substance use and psychosis are risk factors for DSH).
Attitudes to Medication Questionnaire (Hayward, 1995).
Brief Illness Perception Questionnaire (Lobbanet al. , 2005).
Schedule for the Assessment of Insight (David, 1990).
Health status: EQ-5D and Sf6D 9 (Brooks, 1996; Jenkinson & Layte, 1997). This measure is included for two purposes: Firstly, to explore the potential impact of the interventions on the health staus and quality adjusted life years of patients. Secondly, to collect data to inform an economic analysis of the interventions included in the overall HELPER programme. For the full programme, we will examine how baseline characteristics of service users affect the cost effectiveness of the interventions and economic models (decision analytic models) will be developed to explore the potential impact of each of the interventions.
Use of health and social care services: Data will be collected from case notes and assessment about the range and frequency of health and social care service use. The data will be collected using the data collection forms developed for the MIDAS trial of combined motivational and cognitive behaviour therapy for people with schizophrenia and substance abuse. Again, this measure is included for two purposes: First to explore the potnetial impact of the intervention on the health status and quality adjusted life years of patients. Secondly, to collect data to inform an economic analysis of the interventions included in the overall HELPER programme.
Substance use outcomes:
Readiness to change: The Readiness to Change questionnaire (Rollnicket al. , 1992).
Consequences of use: The Inventory of Drug Use Consequences (Blanchardet al. , 2003) a 12 item self report questionnaire assessing consequences of use.
Cannabis Experiences Questionnaire (CEQ): A measuring the subjective experiences of cannabis (Barkuset al. , 2006).
RESUS-D: Reasons for drug use questionnaire (Gregget al. , 2009).
Planned analyses
At eighteen month follow up we will assess the impact of the intervention on the primary outcomes. For the secondary outcomes we will use a one-tailed 0.1 level of significance, rather than the conventional 0.05 level of significance. This is because in this phase (exploratory trial) we are principally concerned to ensure that we do not reject an active intervention at this early stage of the development process. The study will permit us to calculate the likely sample size required for a definitive multi-centre trial.
All statistical analyses will be carried out using Stata (currently version 10). The general analysis strategy will be based on the intention-to-treat principle and will involve the use of analyses of covariance (the exact form depending on the nature of the outcome variable under consideration). Treatment effects will be assessed after allowing for stratification and other baseline covariates thought a priori to be predictive of outcome. Careful consideration will be given to the influences of non-adherence and drop-out.
References
- Addington D., Addington J, Schissel B. A Depression Rating Scale for Schizophrenics. Schizophrenia Research 1990;3:247-51.
- Global Assessment of Functioning GAF-D, Diagnostic and Statistical Manual of Mental Disorders. Washington, DC; 1994.
- Baker A., Lewin T., Reichler H., Clancy R., Carr V., Garrett R., et al. Evaluation of a motivational interview for substance use within psychiatric in-patient services. Addiction 2002;97:1329-37.
- Barnes T.R., Mutsatsa S.H., Hutton S.B., Watt H.C., Joyce E.M. Co-morbid substance use and age at onset of schizophrenia. British Journal of Psychiatry 2006;188:237-42.
- Barkus E., Stirling J., Hopkins R.S., Lewis S. Cannabis-induced psychotic like experiences are associated with high schizotypy. Psychopathology 2006;39:175-8.
- Barrowclough C., Nothard S., Haddock G., Moring J., Allott R., Earnshaw P., et al. MiCBT: An Integrated Psychological Therapy for People with Psychosis and Substance Use 2008.
- Barrowclough C., Tarrier N., Lewis S., Sellwood W., Mainwaring J., Quinn J., et al. Randomised controlled effectiveness trial of a needs based psychosocial intervention service for carers of people with schizophrenia. British Journal of Psychiatry 1999;174:505-11.
- Barrowclough C., Haddock G., Tarrier N., Lewis S., Moring J., O’Brien R., et al. Randomised controlled trial of motivational interviewing, cognitive behavioural therapy and family intervention for patients with co-morbid schizophrenia and substance use disorders. American Journal of Psychiatry 2001;158:1706-13.
- Bellack A., Bennett M., Gearon J. Behavioural treatment for substance abuse in people with serious and persistent mental illness. New York: Routledge; 2007.
- Birchwood M, Todd P, . Early intervention in psychosis. The critical period hypothesis. British Journal of Psychiatry Supplement 1998;172:53-9.
- Blanchard K.A., Morgestern J., Morgan T.J., Labouvie E.W., Bux D.A. Assessing consequences of substance use: psychometric properties of the inventory of drug use consequences. Psychology of Addictive Behaviours 2003;17:328-31.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72.
- Cantwell R., Brewin J., Glazebrook C., Dalkin T., Fox R., Harrison G. Prevalence of substance misuse in first-episode psychosis. British Journal of Psychiatry 1999;174:150-3.
- Cleghorn J.M., Kaplan R.D., Szechtman B., Szechtman H., Brown G.M., Franco S. Substance abuse and schizophrenia: effect on symptoms but not on neurocognitive function. Journal of Clinical Psychiatry 1991;52:26-30.
- Curran H.V., Brignell C., Fletcher S., Middleton P., Henry J. Cognitive and subjective dose-response effects of acute oral Delta 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology 2002;164:61-70.
- David A. Insight and psychosis. British Journal of Psychiatry 1990;156:798-80.
- Drake R.E., Mueser K.T., McHugo G.J., Sederer L.I., Dickey B. Baltimore: Williams & Wilkins; 1996.
- Edwards J., Elkins K., Hinton M., Harrigan S.M., Donovan K., Athanasopoulosu O., et al. Randomised controlled trial of a cannabis-focused intervention for young people with first-episode psychosis. Acta Psychiatrica Scandinavica 2006;114:109-17.
- Fals-Stewart W., O’Farrell T., Freitas T., McFarlin S.K., Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. Journal of Consulting and Clinical Psychology 2000;68:134-4.
- Gregg L., Barrowclough C., Haddock G. Development and validation of a scale for assessing reasons for substance use in schizophrenia: the ReSUS scale 2009.
- Haddock G., McCarron J., Tarrier N., Faragher E.B. Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS). Psychological Medicine 1999;29:879-8.
- Haddock G., Barrowclough C., Tarrier N., Moring J., O’Brien R., Schofield N., et al. Randomised controlled trial of cognitive–behaviour therapy and motivational intervention for schizophrenia and substance use: 18 month carer and economic outcomes. British Journal of Psychiatry 2003;183:418-26.
- Hayward P., Chan N., Kemp R., Youle S., David A. Medication self-management: a preliminary report on an intervention to improve medication compliance. Journal of Mental Health 1995;4:511-7.
- Jenkinson C., Layte R., Jenkinson D., Lawrence K., Petersen S., Paice C., et al. Development and testing of the UK SF-12 (short term health survey). Journal of Health Services Research and Policy 1997;2:14-8.
- Kay S., Fiszbein A., Opler L. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin 1988;13:261-75.
- Denis C, Lavie E, Fatséas M, Auriacombe M. Psychotherapeutic interventions for cannabis abuse and/or dependence in outpatient settings. Cochrane Database of Systematic Reviews 2006.
- Lobban F., Barrowclough C., Jones S. Assessing cognitive representations of mental health problems. I. The illness perception questionnaire for schizophrenia. British Journal of Clinical Psychology 2005;44:147-62.
- Lambert M., Conus P., Lubman D.I., Wade D., Yuen H., Moritz S., et al. The impact of substance use disorders on clinical outcome in 643 patients with first-episode psychosis. Acta Psychiatr Scand 2005;112:141-8.
- Leventhal H., Nerenz D., . A Handbook of Psychological and Health 1984:219-52.
- Linszen D., Dingemans P., Lenior M. Cannabis abuse and the course of recent-onset schizophrenic disorders. Arch Gen Psychiatry 1994;51:273-4.
- Rollnick S., Heather N., Gold R., Hall W. Development of a short readiness to change questionnaire for use in brief opportunistic interventions. British Journal of Addiction 1992;87:743-54.
- Sorbara F., Liraud F., Assens F., Abalan F., Verdoux H. Substance use and the course of early psychosis: a two year follow-up of first-admitted subjects. Euro Psychiatr 2003;18:133-6.
- Tait L., Birchwood M., Trower P. A new scale (SES) to measure Engagement with community mental health services. Journal of Mental Health 2002;11:191-8.
- Teeson, , Teeson M., Hall W., Lynskey M., . Alcohol- and drug-use disorders in Australia: implications of the National Survey of Mental Health and Well-Being. Aust. N.Z. J. Psychiatry 2000;34:206-13.
- Verdoux H., Liraud F., Gonzales B. Predictors and outcome characteristics associated with suicidal behaviour in early psychosis: a two-year follow-up of first-episode subjects. Acta Psychiatr Scand 2001;103:347-54.
- Wade D., Harrigan S., Edwards J., Burgess P.M., Whelan G., McGorry P.D. A 15-month prospective follow-up study of substance misuse in first-episode psychosis. British Journal of Psychiatry 2006;189:229-34.
- Weaver T., Madden P., Charles V., Stimson G., Renton A., Tyrer P, et al. Co-morbidity of substance misuse and mental health illness in community mental health and substance misuse services. British Journal of Psychiatry 2003;183:304-13.
List of abbreviations
- ASPIRE
- Asking about Substance use and Psychosis – Ideas, Reactions and Experiences
- BABCP
- British Association for Behavioural and Cognitive Psychotherapies
- BAI
- Beck Anxiety Inventory
- BARS
- Brief Adherence Rating Scale
- BMI
- body mass index
- CBT
- cognitive–behavioural therapy
- CBTp
- cognitive–behavioural therapy for psychosis
- CDSS
- Calgary Depression Scale for Schizophrenia
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CR
- cognitive remediation
- CSM
- common-sense model
- CTSpsy
- Cognitive Therapy Scale for Psychosis
- DAI
- Drug Attitude Inventory
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition
- DUP
- duration of untreated psychosis
- EIS
- early-intervention service
- EQ-5D
- European Quality of Life-5 Dimensions
- GAF
- Global Assessment of Functioning
- HELPER
- HEalthy Living and Prevention of Early Relapse
- HR
- hazard ratio
- ICD-10
- International Classification of Diseases, 10th revision
- IMPACT
- IMproving PArticipation in Cognitive Therapy
- InterACT
- INTERvention to Encourage ACTivity, Improve Diet and Reduce Weight Gain
- IPAQ
- International Physical Activity Questionnaire
- IQ
- intelligence quotient
- IQR
- interquartile range
- IS
- Insight Scale
- MCS
- mental component summary
- MET
- metabolic equivalent
- MI
- motivational interviewing
- MiCBT
- integrated motivational interviewing and cognitive–behavioural therapy
- MIDAS
- Motivational Interventions for Drugs and Alcohol misuse in Schizophrenia
- MITI
- Motivational Interviewing Treatment Integrity
- MRC
- Medical Research Council
- NICE
- National Institute for Health and Care Excellence
- openCDMS
- Open Source Clinical Data Management System
- OR
- odds ratio
- PANSS
- Positive and Negative Syndrome Scale
- PCS
- physical component summary
- PSYRATS
- Psychotic Symptom Rating Scales
- RCT
- randomised controlled trial
- ReCAP
- Rethinking Choices After Psychosis
- RTCQ
- Readiness to Change Questionnaire
- SC
- social contact
- SCID
- Structured Clinical Interview for DSM-IV Axis 1 Disorders
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- SMD
- standardised mean difference
- SMR
- standardised mortality ratio
- SOFAS
- Social and Occupational Functioning Assessment Scale
- STR
- support, time and recovery worker
- TAU
- treatment as usual
- TLFB
- timeline follow-back
- WAIS
- Wechsler Adult Intelligence Scale
- WCST
- Wisconsin Card Sort Task
- WTAR
- Wechsler Test of Adult Reading