Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0407-10056. The contractual start date was in September 2008. The final report began editorial review in December 2013 and was accepted for publication in November 2014. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Jane Nixon has received post-doctoral fellowship grant funding from the Smith & Nephew Foundation; Jane Nixon, E Andrea Nelson, Claudia Rutherford, Susanne Coleman and Julia Brown have received grant funding from the Worldwide Universities Network Leeds Fund for International Research Collaborations; Delia Muir has received consultancy funding from Smith & Nephew PLC on behalf of the Pressure Ulcer Research Service User Network for the UK (PURSUN UK) for patient and public involvement input into educational materials; and Jane Nixon, Claudia Rutherford and Carol Dealey have received grant funding from Mölnlycke Health Care. Carol Dealey is a member of an expert advisory board which advises Mölnlycke on the use of dressings for pressure ulcer prevention. We confirm that the report content is acceptable to the other funding bodies (Smith & Nephew Foundation and the Worldwide Universities Network Leeds Fund for International Research Collaborations) and that there are no competing proprietorial interests in respect of the tools and methods set out in the monograph.
Dedication to Professor Donna Lamping
It was with great sadness that the team learned of Donna's illness in 2010 and her passing in 2011 at age 58. Donna was an international expert in the field of health psychology, health status and quality of life assessment. Educated and trained in centres of excellence in Canada and the USA, she moved to the London School of Hygiene and Tropical Medicine in 1992 where she established her position as an international leader in the field. Donna was an inspiration to the team, making a major contribution to the conception, design and gold standard evaluation of the Pressure Ulcer Quality of Life (PU-QOL) studies in her role as a grant co-applicant and through PhD supervision of Claudia Rutherford (née Gorecki). We feel privileged to have worked with her and dedicate this monograph to her memory.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Nixon et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Introduction
Pressure ulcers are defined as ‘localised injury to the skin and/or underlying tissue usually over a bony prominence, as a result of pressure, or pressure in combination with shear’ (p.16). 1 They are a widespread2–4 and costly health-care issue. 5–8 Pressure ulcers represent a major burden to patients and carers and have a detrimental effect on patients’ quality of life. 9,10
For the past two decades pressure ulcers have been identified in successive Department of Health policies as a key quality indicator,11,12 with associated guidelines for prevention13,14 and treatment. 15 There have been widespread changes in clinical practice during this period including the introduction of systematic risk assessment processes, investment in pressure-relieving mattresses, and quality improvement initiatives. However, reflecting the belief that the development of pressure ulcers remains an indicator of service quality that impacts on patients and health-care costs, more recently the Department of Health have set out the ambitious aim of eliminating all avoidable pressure ulcers in NHS-provided care,16 developed a Commissioning for Quality and Innovation Payment Framework to facilitate this,17 identified pressure ulcers as a high-impact action for nursing and midwifery18 and incorporated them into the national Operating Framework. 19 Despite the prominence and profile afforded the problem, the research basis to inform practice in this area is limited, partly because we do not understand the clinical and organisational risks sufficiently well and partly because of the dearth of high-quality randomised controlled trials of preventative and treatment interventions. 14,15,20 Our programme of work was established to provide the foundation for the development of an evidence base for practice through improved identification of patients at risk of pressure ulcer development and improved methods of evaluating outcomes that are important to patients.
In 2004, UK costs associated with pressure ulcer prevention and treatment were estimated to be £1.4–2.1B annually, equivalent to 4% of total NHS expenditure,5 because of increased length of hospital stay, hospital admission, community nursing, treatments (reconstruction surgery/mattresses/dressings/technical therapies) and complications (serious infection). Litigation is also a burden on NHS resources and is predicted to increase because of both general societal trends and changes in the law, which has led to investigation of severe pressure ulcers by government agencies to detect institutional and professional neglect of vulnerable adults. 21,22 The NHS focus on pressure ulcer prevention is mirrored elsewhere. In the USA, for example, health insurance companies have incentivised prevention through widespread changes to reimbursement policies. Hence, health-care providers are liable for the treatment costs arising from pressure ulcers that develop during care (organisation-acquired avoidable pressure ulcers). 17
Pressure ulcer classification
Numerous classification systems have been developed to categorise the severity of pressure ulcers. Before the start of the programme grant (in 2008) the two most commonly used systems classified pressure ulcers through four levels (1–4) of ‘stage’ or ‘grade’. 15–17 The descriptors ranged from non-blanching erythema of intact skin at level 1 (stage 1/grade 1) to full-thickness tissue loss at the most severe level (stage 4/grade 4). 23–25 In 2009 the American National Pressure Ulcer Advisory Panel (NPUAP) and European Pressure Ulcer Advisory Panel (EPUAP) developed joint guidelines and a revised classification system. 1 The two main differences were the use of the term ‘category’ (to distance the description from the ordinal properties assumed by the use of stage and grade) and the inclusion of two new descriptors: ‘unstageable’ and ‘deep tissue injury’. In the following chapters we have adopted the use of the NPUAP/EPUAP (2009) classification and use the term ‘category’ (with Arabic numerals rather than roman for ease of reading) to classify pressure ulcers in general reference to the literature and the terms ‘stage’ or ‘grade’ when reporting directly from individual studies, to accurately report the classification system used by the authors. In addition, the terms ‘superficial’ and ‘severe’ pressure ulcers are used to summarise pressure ulcer severity. A superficial ulcer is a category 2 ulcer and the term ‘severe’ is used to describe category 3, category 4 and unstageable pressure ulcers.
For clarification, the programme excluded consideration of ulcers caused by medical devices (e.g. nasogastric tubes, surgical drains, oxygen masks, urinary catheters, cannulas and prosthetic limbs).
Summary of the programme of research
The Pressure UlceR Programme Of reSEarch (PURPOSE) was developed by a clinical/academic research collaborative to address a number of research questions. The areas of work were organised into two themes with the following aims:
-
theme 1 – to reduce the impact of pressure ulcers on patients through:
-
early identification of patients at risk of developing pressure ulceration and
-
improved identification and investigation of patients at risk of progression to severe pressure ulceration
-
-
theme 2 – to reduce the impact of pressure ulcers on patients through the development of methods to capture patient-reported health-related quality of life (HRQoL) and health utilities for routine clinical use and in clinical trials.
Theme 1 focused on improving our understanding of risk factors and risk assessment and consisted of three work packages with the following objectives:
-
work package 1 – pain: to determine the extent of pressure area and pressure ulcer pain and explore the role of pain as a predictor of category 2 and above pressure ulcers in acute hospital and community populations
-
work package 2 – severe pressure ulcers: to identify individual and organisational factors that contribute to the development of severe pressure ulcers and develop a critical incident/adult neglect investigation methodology for their review
-
work package 3 – pressure ulcer risk assessment: to agree a pressure ulcer risk factor Minimum Data Set to underpin the development and validation of an evidence-based Risk Assessment Framework to guide decision-making about the risk of developing pressure ulceration and the risk of progression to more severe ulceration.
Theme 2 had a focus on the development of patient-reported outcome (PRO) measures and consisted of two work packages with the following objectives:
-
work package 4 – pressure ulcer quality of life: to determine outcomes important to patients who develop pressure ulcers and develop a psychometrically rigorous PRO measure that is reliable and valid and suitable for use in the NHS.
-
work package 5 – pressure ulcer quality of life utility instrument: to create a preference-based index that could be used to generate utility values suitable for use in cost–utility-based economic evaluations of pressure ulcer prevention and treatment interventions.
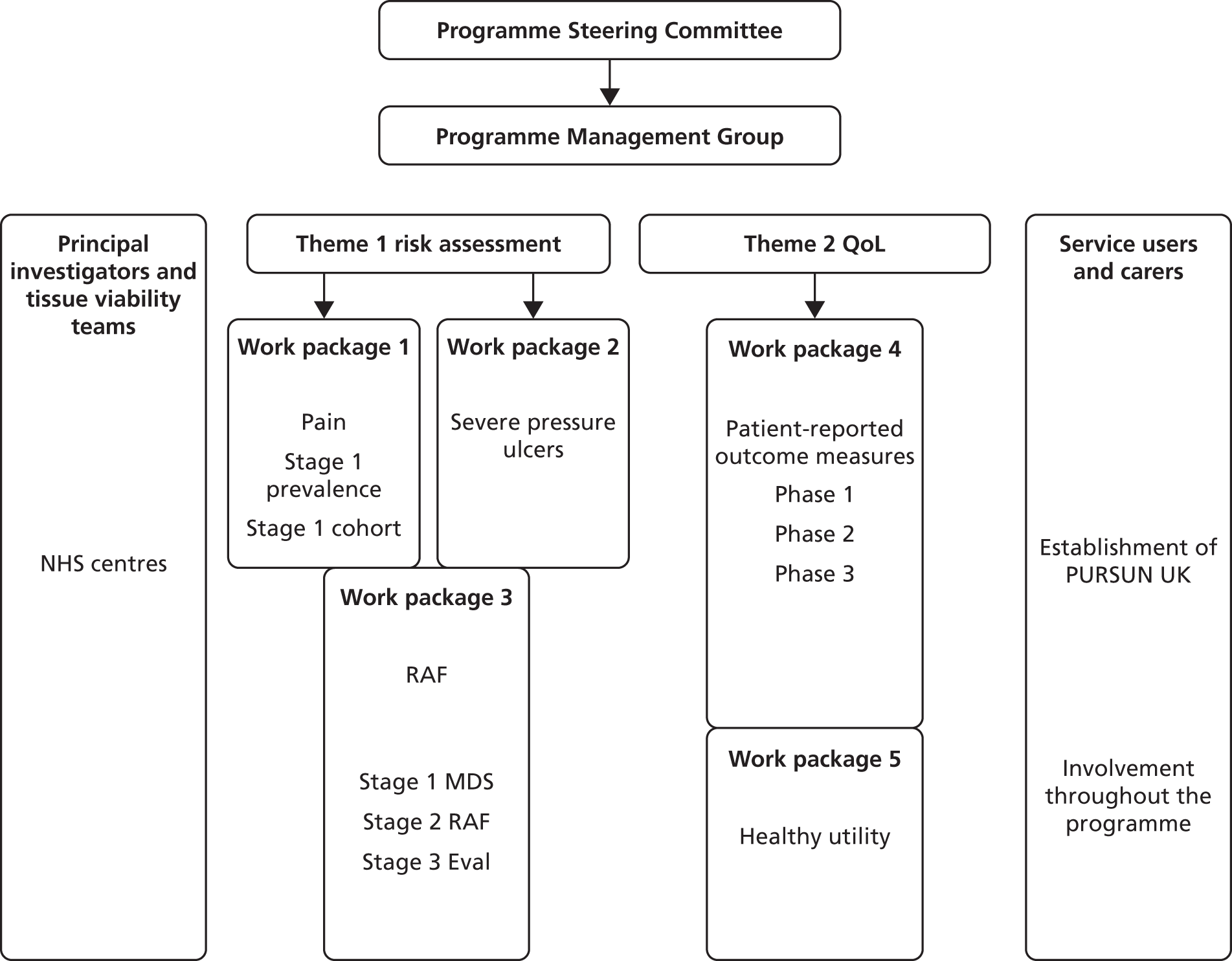
Both themes 1 and 2 were planned to progress in parallel. Within each theme, planning ensured that the early work contributed to later studies. Work packages 1 and 2 contributed to work package 3, and work package 4 contributed to work package 5 (Figure 1). In addition, within each work package we utilised a range of research methods in sequential phases including (for example) systematic reviews, prevalence studies, prospective cohort study, case study consensus methods, psychometric evaluation and time trade-off (TTO) task valuations of health states (Table 1).
FIGURE 1.
Outline of the programme. Eval, evaluation; MDS, minimum data set; PURSUN UK, Pressure Ulcer Research Service User Network UK; QoL, quality of life; RAF, Risk Assessment Framework.
| WP1 – pain (see Chapter 3) | WP2 – severe pressure ulcers (see Chapter 4) | WP3 – risk assessment (see Chapter 5) | WP4 – pressure ulcer quality of life (see Chapter 6) | WP5 – pressure ulcer quality of life utility instrument (see Chapter 7) |
|---|---|---|---|---|
|
Development of the research programme
During the course of the programme, delivery enhancements to the original proposal were made including NHS capacity development, patient and public involvement (PPI) and additional reviews and methodological research.
NHS research capacity development
Patient populations in pressure ulcer research are characterised by high levels of comorbidity, and are distributed across multiple care environments. This poses challenges in study design, recruitment and follow-up. The programme grant application was underpinned by a strong network of NHS collaborators across 13 acute and community NHS trusts (see Figure 1). During the programme of research (2008–13) this was further developed through support from the West Yorkshire Comprehensive Local Research Network, National Institute for Health Research (NIHR) portfolio adoption and incorporation onto the Dermatology and Primary Care portfolios, facilitating access to service support costs through the NIHR Comprehensive Local Research Networks and the participation of 30 acute and community NHS trusts (see Appendix 1 and Acknowledgements).
Patient and public involvement in pressure ulcer research
In addition, our original PPI plan was limited and this was significantly enhanced. We established a partnership with service users through the set-up of the UK Pressure Ulcer Research Service User Network (PURSUN UK) (see Figure 1 and Chapter 2). Service-user involvement underpinned our development and delivery of the programme of research as well as the dissemination and identification of ongoing research priorities. Indeed, PURSUN UK made a major contribution to the design, conduct and interpretation of the research, with the use of innovative involvement activities (see Table 1 and Chapter 2).
Additional reviews and methodological research
The original programme of work was enhanced through an additional two systematic reviews and five methodological substudies (see Table 1) addressing methodological issues with wider relevance to the applied health research field.
Structure of this report
Within each work package there are a number of components, including systematic reviews, primary research and methodological substudies. In total, 21 pieces of work have been undertaken, as illustrated in Table 1.
The monograph is structured as follows:
-
Chapter 2 provides an overview of the PPI activities, innovation and support
-
Chapters 3–7 present the rationale and research (including substudies), PPI and implementation components of each work package
-
Chapter 8 describes the wider benefits accrued through the PURPOSE programme award and 5-year investment period and summarises the key findings and their implications for practice, PPI, policy and research.
Chapter 2 Patient and public involvement
Chapter written by Delia Muir, Susanne Coleman, Lyn Wilson, Nikki Stubbs, Justin Keen, Elizabeth McGinnis and Jane Nixon.
Background
The importance of PPI within PURPOSE was recognised at the start of the programme. PPI in pressure ulcer research has not been strong to date and the project team identified an opportunity to address this through the programme. PPI can contribute at all stages of the research process, from commissioning and priority setting through to dissemination and implementation. 26–28 There is a danger, however, that PPI activities can become tokenistic, particularly when driven by ‘top-down’ policy initiatives rather than a genuine desire to learn from, and with, service users. 29
In recent years there has been a growth in PPI literature;26 however, inconsistencies in reporting and the variety of research methodologies covered by this literature make comparing studies and establishing quality challenging. 26,27 Recent reviews do highlight some general good practice principles. Shippee and colleagues30 describe four key components of involvement: (1) service user initiation (i.e. preparation, negotiating roles, establishing shared interests and goals), (2) building reciprocal relationships between service users and researchers (i.e. establish service users as valued members of the team rather than an optional addition), (3) co-learning (i.e. development of both service users and researchers) and (4) feedback (i.e. ongoing, iterative evaluation of PPI processes). This review also highlights the importance of involvement starting as early as possible in the research process. In addition, advocates of the co-production approach (used mainly in a service design and delivery context) highlight the need for peer support networks; to recognise and build on people’s strengths; and to build communities. 31
There is a paucity of literature related specifically to PPI in pressure ulcer research. The only other pressure ulcer PPI initiative that we are aware of is the James Lind Alliance Pressure Ulcer Partnership. This ran in parallel to this programme and focused on setting future research questions rather than on involvement in carrying out that research (see Supporting further research for additional information).
The PURPOSE project team set out to develop mechanisms for working in partnership with service users, to have a positive impact on the research methods and outputs for all strands of the programme. In line with the principles set out above, we also wanted to ensure that involvement would be a positive and rewarding experience for the individuals taking part.
Challenges
In the early stages of the programme PPI proved challenging. The project team tried to identify individuals with experience of pressure ulcers or people managing the risk of pressure ulceration (e.g. people with chronic conditions that limit mobility or people who have experienced periods of very acute illness). During the first year, two service users were identified (through clinical members of the project team) and agreed to be members of the PURPOSE steering committee. Finding more people proved difficult for a number of reasons. First, there was no infrastructure to support PPI in this field, unlike in some other areas that have established PPI networks that can support recruitment to research and offer guidance on working with particular populations. Some health and social care charities also support research and promote PPI activities. However, pressure ulcers are a cross-specialty problem; they are secondary to other serious illnesses/conditions and do not fit easily into existing national/charitable structures.
Despite efforts to recruit through generic PPI networks, our only success in identifying service users was through the project team’s clinical contacts. This approach raised some ethical considerations. There were concerns that service users might feel obliged to become involved to ‘repay’ good care or for fear of future care being affected. It was also felt that there needed to be a clear distinction between ‘caring for patients’ and ‘working with service users’. Clinical members of the team recruiting and supporting their own patients could potentially blur these boundaries and create unequal relationships within the team. This was a consideration when developing a PPI recruitment strategy for the programme.
Another challenge was provided by the complex health needs of many people with experience of pressure ulcers or pressure ulcer risk. As pressure ulcers affect people with serious long-term conditions or acute illness/injury, many service users with relevant experience may be unable to take part in traditional involvement activities.
To address these challenges, a PPI officer post was created. The aim of this post was to develop a pressure ulcer-focused service user network, which would both facilitate PPI throughout the programme and build PPI capacity within pressure ulcer research more generally.
The Pressure Ulcer Research Service User Network UK
The Pressure Ulcer Research Service User Network UK was established in 2010 and now has 18 members. The network is made up of service users and carers with personal experience of pressure ulceration and/or risk of pressure ulceration. Members have been identified through:
-
local, generic PPI groups/networks
-
snowball recruitment, whereby existing PURSUN UK members contact friends, colleagues or family members
-
advertising meetings and events in the local media and via e-mail networks
-
engaging with charities that focus on a topic related to pressure ulcer risk, such as conditions that limit mobility, for example the Multiple Sclerosis Society and Spina bifida, Hydrocephalus, Information, Networking, Equality (SHINE)
-
social media (@PURSUN_UK)
-
the distribution of PURSUN UK leaflets alongside recruitment of study participants
-
tissue viability nurse specialists.
Concerns about clinical members of the research team approaching their own patients, thereby blurring roles and boundaries, have been addressed by the introduction of the PPI officer as a neutral party. Nurses in practice hand out information about PURSUN UK and service users then have the option to contact the PPI officer for further discussions and induction to the network if desired.
The Pressure Ulcer Research Service User Network UK (www.pursun.org.uk) has a minimum of two management meetings a year at which a core group of the most active members consider the direction of the network, the terms of reference, recruitment, the website and other network materials. Research involvement opportunities are sent out via the mailing list as they arise, for example invitations to help interpret data, become co-authors or input into the study methods.
All members of PURSUN UK are prepared for involvement through a minimum of one induction meeting with the PPI officer (either face-to-face or by telephone). During this meeting service users are encouraged to discuss the skills and experience that they bring to the group, as well as any support that they may need. The remit of PURSUN UK is also discussed along with practical issues such as payment of fees and expenses. Ongoing support is provided based on the individual needs of each member. Many of the core group have also been through a more in-depth process of preparation based on the Patient Learning Journey model. 32 The Patient Learning Journey model was originally developed by the Leeds Institute for Medical Education as a way of preparing service users for involvement in the education of health professionals. The model was adapted for use in a research context, keeping the original Patient Learning Journey principles of sharing stories with other service users in a safe, facilitated environment and working together to identify themes within those experiences. Participants are also encouraged to think about which aspects of their stories they feel happy sharing with professionals and how best to communicate key messages. This novel approach to research preparation aims to help service users recognise the expertise that they have developed through their personal experiences. It also helps with group forming, encouraging empathy and peer support. Further development opportunities have been sought for members where possible, such as conference attendance and local research training. Offering a range of development opportunities has been useful: some members have travelled to large, national conferences whereas others have found shorter, local events or one-to-one meetings more manageable.
Pressure UlceR Programme Of reSEarch involvement activities
Between 2008 and 2010, PPI was limited by our ability to recruit service users. Following the establishment of PURSUN UK in late 2010, involvement activities increased across the programme. Furthermore, the methodology and focus of each work package have guided the nature of involvement. An overview of PPI activities at different stages of the programme of research is given in the following sections. Involvement in individual PURPOSE studies is also discussed in subsequent chapters.
Programme management
The PURPOSE steering committee includes two service user members. This led to the identification of the need for further PPI not only in the PURPOSE programme but also in the field of pressure ulcer research more generally. This recommendation was supported by the steering committee and led to the decision to appoint a PPI officer. A service user was involved in the recruitment process for the PPI officer post, including being a member of the interview panel.
Protocol and patient information leaflet development
Members of PURSUN UK have formally reviewed all of the PURPOSE study protocols via the PURPOSE steering committee. In addition, PURSUN UK members have made more detailed contributions to the design of the risk assessment (see Chapter 5) and Pressure Ulcer Quality of Life – Utility Index (PUQOL-UI) (see Chapter 7) studies. This has been through contributing to study protocols and advising on the development of patient information leaflets.
Data interpretation
The results of the pain studies (see Chapter 3) have been presented to PURSUN UK. Members have helped to put the pain results in context from a service user perspective and consider next steps for the research. They also worked with the project team to interpret qualitative data from the severe pressure ulcer study (see Chapter 4). This was achieved through a workshop utilising video and role play to make the interpretation process engaging and accessible for service users with little or no experience of data analysis and interpretation. This workshop is described in more detail in Chapter 4 (see Patient and public involvement).
Staff training
Case studies based on the real experiences of PURSUN UK members were developed. These were then used as part of the Pressure Ulcer Risk Primary or Secondary Evaluation Tool (PURPOSE-T; a pressure ulcer risk assessment tool) pretest training sessions with nurses (see Chapter 5, Phase 3: development of a new conceptual framework and theoretical causal pathway for pressure ulcer development). This allowed nurses to apply the tool to authentic case studies, in a safe learning environment.
Instrument development
Pressure Ulcer Research Service User Network UK has had considerable input into developing PURPOSE-T, with particular focus on making the tool acceptable for patients in practice. Its involvement was integrated within the consensus methodology and had a direct influence on the items included in the tool. This is discussed further in Chapter 5. Members also reviewed the Pressure Ulcer Quality of Life (PU-QOL) instrument (see Chapter 6), providing feedback about clarity, comprehension, design, layout and item wording. This process led to some modifications to the PU-QOL instrument (e.g. clarification of instructions, revisions to the wording of some items). Members with experience of living with a pressure ulcer have also been involved in developing the PUQOL-UI (see Chapter 7). This involved giving feedback on the questionnaire through ‘think out loud’ interviews and document review.
Dissemination and knowledge transfer
Three members of PURSUN UK are currently contributing to a paper in which their real-life narratives are used to illustrate findings from the pain studies and emphasise the relevance to clinical practice. They will be co-authors on the paper. Video podcasts are also being developed with service users. Their stories will be combined with input from clinicians and used to highlight key messages from the programme. The videos will be available online.
Implementation
Members of PURSUN UK reviewed the user manual for the PU-QOL instrument. One member is also involved in piloting a new method for investigating the development of severe pressure ulcers in practice (following on from the work described in Chapter 4).
Wider impact of the Pressure Ulcer Research Service User Network UK
In addition to PPI throughout the programme, PURSUN UK has begun to impact the wider tissue viability and PPI communities, as described in the following sections.
Professional development activities
Members of PURSUN UK have been invited to speak about their experiences at several events. Locally, this has included training for tissue viability link nurses, presenting to PURPOSE principal investigators, speaking at the launch of the NIHR Bradford Wound Prevention & Treatment Healthcare Technology Co-operative and working with medical students. Nationally, members have presented at the Tissue Viability Society conference, tissue viability education events and the INVOLVE (a national PPI advisory group) conference.
We have developed an effective model for presenting service users’ experiences in which the PPI officer interviews a member of PURSUN UK in front of a live audience. This provides an alternative to a traditional presentation for people who do not feel confident presenting personal experiences in that way. This model has received very positive feedback from both audiences and the service users involved. We have found that real-life stories are extremely powerful and can create a common focus for professionals from a variety of backgrounds.
Collaboration with industry
Medical devices play an important role in pressure ulcer prevention and treatment. With this in mind, PURSUN UK has collaborated with industry partners on projects such as education days and product development workshops. This collaboration has helped to diversify the involvement opportunities offered to PURSUN UK members and has been useful in terms of members’ personal development, as it has given people an insight into another aspect of tissue viability research. This work has also generated some funds for PURSUN UK, moving the network towards a sustainable model post PURPOSE.
Supporting further research
One member of PURSUN UK is a co-applicant on PRESSURE 2 [a NIHR Health Technology Assessment (HTA) programme-funded trial comparing two mattresses; http://medhealth.leeds.ac.uk/info/423/skin/1717/pressure_2 (accessed 31 August 2015)]. The wider network both helped to develop this trial and continues to be involved with it. PURSUN UK has also been a partner in the James Lind Alliance Pressure Ulcer Partnership, with members contributing to the prioritisation of pressure ulcer treatment and prevention uncertainties. These uncertainties are publicly available to inform future research [see www.jlapressureulcerpartnership.co.uk (accessed 20 February 2015)].
Developing materials
A website has been developed by PURSUN UK [see www.pursun.org.uk (accessed 20 February 2015)]. In addition, PURSUN UK has contributed to the international consensus document Optimising Wellbeing in People Living with a Wound, published by Wounds International [see www.woundsinternational.com/clinical-guidelines/international-consensus-optimising-wellbeing-in-people-living-with-a-wound (accessed 20 February 2015)].
Developing and sharing patient and public involvement methods
Developing a completely new service user network has given us the opportunity to be creative in our approach and develop innovative involvement models. These models have been shared with the UK PPI community. The PPI model used as part of the severe pressure ulcer study (see Chapter 4, Patient and public involvement) has been presented at three national conferences (INVOLVE, Involving People Wales and Tissue Viability Society) and forms part of an INVOLVE video resource on PPI in data interpretation and analysis [see www.invo.org.uk/resource-centre/conference/involve-conference-gallery/ (accessed 20 February 2015)]. A video about the Severe Pressure Ulcer PPI event was also made by PURSUN UK and has been widely disseminated online [see https://youtu.be/bgg6zkbILrg (accessed 21st July 2015)]. The novel approach of using the Patient Learning Journey as a model for service users contributing to research rather than health education has also been included as a case study in the INVOLVE training and development guidelines [see www.invo.org.uk/training-case-study-13-2/ (accessed 20 February 2015)].
Media
Working with service users has enabled us to more effectively engage with local and national media. Members of PURSUN UK have been interviewed for the Yorkshire Evening Post [see www.yorkshirepost.co.uk/news/at-a-glance/general-news/yorkshire-group-spearheads-bedsores-care-drive-1-3786988 (accessed 20 February 2015)] and the Daily Mail [see www.dailymail.co.uk/health/article-2093904/Bed-sores-How-does-local-hospital-compare.html (accessed 20 February 2015)] and we have found that journalists are more likely to run a health-related story if it has a real-life, human interest aspect to it.
Discussion
Our patient and public involvement methods
The growing role of PPI throughout the PURPOSE programme has been described. This likely reflects the situation in other research projects that need to recruit service users once the project is already under way, especially when projects span a number of years. This may be because of recruitment challenges or because it is determined that additional input from a particular group of people is needed or may be because of existing service user partners stepping down. Introducing service users to studies that are already under way can be challenging, particularly when relationships have already been formed within the project team.
Although members of PURSUN UK were not involved at the grant application stage and were therefore not part of setting the programme themes, they have found common ground with both each other and the project team. These shared goals have made collaboration possible. We recognise that early involvement is considered good practice30 and that there are areas throughout the PURPOSE programme (particularly in the early stages of programme delivery) where additional PPI might have been useful, for example involvement in the qualitative part of the PU-QOL study. However, the lack of a PPI infrastructure in the field made this difficult. The establishment of PURSUN UK and associated innovative approaches to PPI has addressed this need and is just one way in which PURPOSE intends to leave a legacy beyond the life of the programme.
Facilitating PPI effectively requires specialist skills. The creation of the PPI officer post brought specialist engagement expertise, dedicated time, innovative solutions, continuity and a single point of contact for service users. This enabled us to provide the individualised support that members of PURSUN UK require to be actively involved, for example briefing and debriefing meetings; information technology tuition and support; peer support opportunities; and practical support such as accessible venues and large-print documents.
The development of the PURSUN UK network has allowed us to move from ad hoc PPI activities at the start of the programme to a more strategic approach. Furthermore, we have found that members of PURSUN UK have helped to bridge the gap between research and practice, for example putting the PURPOSE findings in a NHS context and thinking about how findings can have the most impact. They have also highlighted continued gaps in the research and unanswered questions from the service user perspective, which will be taken forward in a future programme of work.
Our model involves a small number of service users in programme management and working with the wider network at key milestones throughout each study; this has worked well throughout the programme. We recognise that not all service users will feel comfortable in formal research environments such as the PURPOSE steering committee meetings. More informal meetings and workshops, which focus on the service user perspective, have proved invaluable. These meetings have highlighted key issues such as the importance of patient engagement in pressure ulcer prevention and treatment; the anxiety and stigma that can be felt as a result of a pressure ulcer; and the need to raise awareness of pressure ulcers with both patients and professionals.
To effectively engage with this group we have adopted a highly flexible and innovative methodology. We have used an asset-based approach. 31 This means building on the strengths of network members by adapting our research processes, rather than risk excluding people from traditional PPI activities. For example:
-
the use of role play and video to facilitate PPI in the interpretation of data from the severe pressure ulcer study (see Chapter 4, Patient and public involvement)
-
the adaptation of the Patient Learning Journey model32 for use in a research context
-
the use of a live interview model as an alternative to traditional presentations
-
the addition of a service user group to the consensus methodology used in the risk assessment study (see Chapter 5, Service user group participants)
-
individualised support for steering committee members, including one-to-one debriefs with the PPI officer
-
the integration of service user narratives into the dissemination of the quantitative pain studies.
The value of the Pressure Ulcer Research Service User Network UK to the service users involved
The reciprocal nature of engagement has been central to the success of PPI in this programme. In addition to PPI having a positive impact on research processes and outputs, service users have also reported that it has been a positive and rewarding experience for them. People have commented on increased confidence, self-worth, knowledge of research and awareness of their own health. They have valued the peer support that PURSUN UK provides and the opportunities to enter into an equal dialogue with researchers and clinicians.
I’ve loved putting my input into the work the group are doing. I had to give up everything [because of my severe pressure ulcer] and it has given me something to do. I feel like I’m back in the world again!
PURSUN UK member
PURSUN is a safe place to learn from sharing experiences with each other, and the comfort that comes from knowing that it is a safe environment cannot be underestimated. This should be acknowledged, even if it wasn’t originally on the radar of those wanting to set up a service user group
PURSUN UK member
The networks formed as part of their work with PURSUN UK have led to other opportunities for people, including paid positions. For example, people have become involved in teaching activities elsewhere in the University of Leeds and have joined research projects/service user groups in other topic areas.
Conclusion
This chapter outlines both the challenges and advantages of engaging with a previously seldom-heard group. A mixture of established good practice techniques and innovative PPI approaches has allowed us to move beyond the PPI plan outlined in our grant application and beyond what others have achieved in this field. Although we have worked exclusively within pressure ulcer research, the strategies outlined here could help service users and researchers work together in other contexts.
Chapter 3 Work package 1: pain
Chapter written by Jane Nixon, Isabelle L Smith, Michelle Collinson, Elizabeth McGinnis, Michelle Briggs, Sarah Brown, Susanne Coleman, Carol Dealey, Delia Muir, E Andrea Nelson, Rebecca Stevenson, Nikki Stubbs, Lyn Wilson and Julia M Brown.
Abstract
Introduction: Patients with pressure ulcers have reported that pain is their most distressing symptom and that pain at ‘pressure areas’ was experienced before the clinical manifestation of pressure ulcers but that the pain was ignored by nurses. The primary aim of the research was to determine the extent of pressure area and pressure ulcer pain and explore the role of pain as a predictor of category 2 and above pressure ulcers in acute hospital and community populations.
Methods: The pain work package comprised three research projects: (1) a nested multicentre pain prevalence study in three NHS acute hospital trusts, including all inpatients; (2) a nested pain prevalence survey in two community NHS trust localities incorporating a comparison of case-finding methods, including only patients with pressure ulcers; and (3) a multicentre prospective cohort study of pressure ulcer risk factors in acute hospital and community patients.
Results: In the hospital prevalence study a total of 3397 patients in nine acute hospitals were included in routine pressure ulcer prevalence audits and, of these, 2010 (59.2%) participated in the nested pain prevalence study. The community routine pressure ulcer prevalence audit identified 287 patients with pressure ulcers and, of these, 176 (61.3%) participated in the nested pain prevalence study. The overall prevalence of pressure ulcers was 0.58 per 1000 adult population, with differences observed between localities (locality 1 = 0.77 and locality 2 = 0.40). The unattributed pressure area-related pain prevalence was 16.3% (327/2010) in the hospital population, which included patients with and without pressure ulcers. In the hospital population with no observable pressure ulcers, 12.6% (223/1769) reported unattributed pressure area-related pain. The prevalence of unattributed pressure area-related pain in patients with pressure ulcers was 43.2% (104/241) in hospital patients and 75.6% (133/176) in the community patients. The detailed pain assessment of 160 hospital and 37 community patients identified pressure area-related pain on skin areas assessed as normal as well as all grades of pressure ulcer. The distribution of pain intensity measured using a 0–10 nominal rating scale was similar for all grades. The dominant type of pain in hospital patients was inflammatory pain (70.3% torso and 60.3% limb), whereas in the community patients neuropathic pain was dominant (54.5% torso and 61.1% limb). The cohort study of 632 acutely ill hospital and community patients identified significant evidence that the presence of pain at a skin site (assessed as normal, altered but intact or category 1) is an independent predictor for developing a category 2 or above pressure ulcer in four multivariable models: a priori logistic regression model, overdispersion logistic regression model and an accelerated failure time model for analyses conducted on a patient level and a multilevel logistic regression model for the analysis conducted on a skin-site level.
Conclusions: We have identified that a significant minority of hospital inpatients without pressure ulcers suffer pressure area-related pain, that approximately 40% of hospital patients and 75% of community patients with pressure ulcers report pain, that pain severity is not related to the severity of the ulcer and that both inflammatory and neuropathic pain are observed. Differences in pressure ulcer prevalence rates highlight the need for effective case ascertainment in the community setting. We have also established that the presence of pain (on skin areas assessed as normal, altered but intact or category 1 pressure ulcer) increases the risk of development of category 2 and above pressure ulcers and accelerates the time to their development. This is an area of practice that requires improved pain assessment; the incorporation of pain into risk assessment; preventative interventions in response to pain; and treatment strategies to alleviate pain.
Introduction
Our pre-programme grant qualitative work33,34 and systematic review of the pressure ulcer quality-of-life literature9 found that patients with pressure ulcers report that pain is their most distressing symptom. In addition, the work highlighted that pain at ‘pressure areas’ (see Definition of terms) was experienced by patients before the clinical manifestation of pressure ulcers but that the pain was ignored by nurses. Patients blamed nurses when a pressure ulcer developed subsequently, because of the lack of action. ‘Patients felt that they were responsible for communicating pain and that their care provider was responsible for attending to it, but patients’ views and concerns did not always prompt action and many healthcare professionals dismissed patients’ reports of pain at pressure areas’. 9,33,35
As part of the programme grant we carried out a mixed-methods systematic review, in which qualitative and quantitative studies of patients’ reports of pressure ulcer pain were identified and synthesised36 (see Chapter 6, Pressure ulcer-related pain: systematic review). Pain was reported as debilitating, reducing the individual’s ability to participate in physical and social activities, adopt comfortable positions, move, walk and undergo rehabilitation. 36 Patients with pressure ulcer pain described their experience as ‘endless pain’ characterised by a constant presence, needing to keep still and equipment and treatment pain. 9,34,37 This confirmed the importance of pain as a feature of living with a pressure ulcer.
Reviews of the epidemiological literature carried out by Girouard and colleagues38 and Pieper and colleagues39 identified eight studies reporting the prevalence of pain associated with pressure ulcers in study populations ranging from 20 to 186 participants, in diverse settings including hospitals and community and palliative care. In the four largest studies (> 100 participants), pressure ulcer pain prevalence estimates ranged from 37% to 66%. 40–43 The reviews highlight the limitations of the existing literature, including small sample sizes, the use of non-validated measures of pain, including nurse-assessed pain outcomes, and an absence of studies that report the dominant types of pain: nociceptive pain (inflammatory) and neuropathic pain (resulting from nerve damage or tissue ischaemia). 44 “Understanding the characteristics of pain is important as successful pain management depends upon using interventions that address the cause(s) of the pain. A further problem with research in the field is that pain reports are limited to Category 2 and above PUs [pressure ulcers]. 35,38,39,45 Pain associated with Category 1 PUs is not reported in most studies, nor is the presence of pain at ‘pressure areas.’” Despite patient reports that pain at ‘pressure areas’ preceded pressure ulcer development, our risk factor systematic review46 (see Chapter 5) did not identify any risk factor studies that included pain as a candidate risk factor in univariate or multivariable analysis.
‘In summary, qualitative evidence identifies pain preceding PU development and in PU management [as an important issue for patients]. Previous epidemiological research has focused on patients with existing PUs and a limitation of the literature is the lack of evidence relating to the extent of pain preceding PU development, the extent of pain associated with Category 1 PUs (the most prevalent PU Category), the type of pain (i.e. inflammatory or neuropathic)’45 and the relationship between pain at ‘pressure areas’ and subsequent category 2 pressure ulcer development. We therefore proposed to determine both the extent of the problem and explore the role of pain as a predictor of pressure ulcer development in acute hospital and community populations.
Research overview
Work package 1 comprised the following pain prevalence and cohort studies:
-
the prevalence of pressure area-related and pressure ulcer pain in hospitalised patients (see Pain prevalence in the hospital population)
-
the prevalence of pressure area-relataed and pressure ulcer pain in community patients (see Pain prevalence in the community population), including a substudy comparing community pressure ulcer case-finding methods (see see Routine pressure ulcer audit: community setting)
-
pain cohort study exploring the role of pain as a predictor of category 2 pressure ulcers in acute hospital and community populations (see Pain and pressure ulcer risk: cohort study)
Definition of terms
This is the first pain research undertaken in patient populations with and without pressure ulcers. To describe pain in the study populations, we developed and used the following four terms: (1) pressure area; (2) pressure area-related pain; (3) pressure ulcer pain and (4) unattributed pressure area-related pain as follows (see Glossary for description of terms):
-
Pressure area. A body site where pressure ulcers commonly develop; most commonly these include the sacrum, buttocks, ischial tuberosities, hips, heels, ankles and elbows.
-
Pressure area-related pain. Defined as pain, soreness or discomfort on any pressure area.
-
Pressure ulcer pain. Defined as pain, soreness or discomfort on a body site with an observable pressure ulcer of category 1 or above.
-
Unattributed pressure area-related pain. Defined as pain, soreness or discomfort reported by patients on a pressure area/pressure ulcer but in which the body site is not specified/recorded. 35
Pain prevalence in hospital and community populations
To assess the extent of pressure area-related and pressure ulcer pain we undertook two cross-sectional studies in three acute and two community NHS trusts to estimate prevalence. In the hospital setting we were able to nest the pain prevalence study into routine annual pressure ulcer audit methods and pain was assessed for all patients able to respond to pain screening questions, including those with and those without pressure ulcers. Our original plan (see the protocol in Appendix 3) assumed that community prevalence methodology was similar to long-standing and well-established acute hospital methods, with nurses undertaking a comprehensive skin assessment of each patient. 47 However, the two participating community trusts had developed different case-finding methods48 and this, together with the scale of the data collection task in the community setting, led to an adaptation of the original plan and limited the pain prevalence estimates to the patient population with pressure ulcers, which is reflected in the objectives.
Objectives
Pain prevalence in the hospital population
Objectives were to:
-
estimate the unattributed pressure area-related pain prevalence in a hospital population of patients with and without pressure ulcers
-
estimate the pressure area-related pain prevalence in patients with no observable pressure ulcers
-
estimate the pressure ulcer pain prevalence in patients with pressure ulcers
-
describe the intensity and type of pressure area-related and pressure ulcer pain in a hospital population of patients with and without pressure ulcers
-
explore the association between pain intensity, type of pain and pressure ulcer classification in a hospital population of patients with and without pressure ulcers.
Pain prevalence in the community population
Objectives were to:
-
estimate the prevalence of unattributed pressure area-related pain within a community population of patients with pressure ulcers
-
assess the intensity and type of pressure area-related and pressure ulcer pain within a community population of patients with pressure ulcers
-
describe the intensity and type of pressure area-related and pressure ulcer pain within a community population of patients with pressure ulcers
-
explore the association between pain intensity, type of pain and pressure ulcer classification within a community population of patients with pressure ulcers
-
compare and contrast community pressure ulcer prevalence case-finding methods.
Methods
Study design
We undertook nested, multicentre, cross-sectional studies in three acute hospital NHS trusts35 and two large community trusts in England45 embedding the pain prevalence study into routine pressure ulcer audits. To identify patients who had unattributed pressure area-related pain, questions about pain were added to the routine annual pressure ulcer prevalence audits undertaken in the participating NHS trusts. To estimate the prevalence of pressure area-related and pressure ulcer pain and to explore the association between pain and pressure ulcer classification, patients who reported pain were invited and consented to undergo a full pain assessment (see Appendix 5 for the consent form).
Nesting the pain prevalence study within routine pressure ulcer prevalence audits meant that we collected data for the total eligible patient population in each setting. The routine NHS audits collect unlinked anonymous data and patient consent is not required to ensure that accurate pressure ulcer prevalence data are obtained for the total eligible population. Nesting the study within routine pressure ulcer prevalence audits, however, limited the number of data items that could be collected. Furthermore, in the community the two trusts defined their denominator population differently and adopted different pressure ulcer case-finding methods. 48
Setting
Three acute NHS hospital trusts took part. One trust included three district general hospitals. The other two NHS trusts were large teaching hospitals and together included four main and two satellite hospitals. This meant that the patient population consisted of those in general secondary care and regional/supraregional specialist services from a total of nine hospitals.
The community NHS trusts consisted of locality 1, serving an urban population of 292,179, and locality 2, serving a rural population of 311,991. 49
Each NHS community trust provides general and tissue viability specialist nursing care to patients residing in their own homes and residential homes as well as community/rehabilitation/hospice inpatient facilities. In addition, each trust provides tissue viability specialist nursing care to patients residing in nursing home settings.
Routine pressure ulcer audit: hospital setting
Eligibility
The population included ‘all inpatients of 18 years of age or older who were in hospital on the date of the participating Trusts’ PU prevalence audit. Patients in paediatric, obstetric and psychiatric care settings were excluded from the study as the prevalence of PU in these settings is very low, and hence the data collection to information burden ratio is unacceptably high in these settings.’50
Patient identification method
Routine pressure ulcer prevalence audits in the participating acute trusts included training of a responsible nurse for each ward, completion of an audit form for each inpatient at 06.00 on the audit day, cross-referencing of the number of occupied beds on each ward and the number of audit forms submitted by an audit team and verification of data by an audit team comprising the tissue viability team and members of the mattress suppliers of the participating NHS trusts (as part of their mattress supply contract). The date of the prevalence audit for each hospital was determined locally.
Routine pressure ulcer audit: community setting
Eligibility
The target population was all patients aged ≥ 18 years who were identified as having a pressure ulcer. Patients in paediatric, obstetric and psychiatric community care settings were excluded.
Patient identification method
A number of challenges are faced when determining ‘community prevalence’: (1) defining the time period for data collection, (2) defining the term ‘community’ for case ascertainment to estimate the numerator and (3) defining the denominator population. ‘In the UK, within each locality there are six key healthcare providers in the community including community nursing services, residential homes, rehabilitation units, specialist palliative care units, nursing homes and General Practitioners. NHS community trusts provide general and specialist community nursing services to patients residing at home and also tissue viability specialist nursing to high risk patients and those with complex wounds residing in independent sector residential and nursing home facilities. Residential home facilities provide only social care and therefore a patient in this setting with a pressure ulcer would be referred to the community nursing service. Rehabilitation units, specialist palliative care units and nursing home facilities include ‘nursing care’ and only complex patients are referred to the community nursing service. General Practitioners usually refer patients with a pressure ulcer to community nursing services. To establish true community prevalence would require named patient data from each health-care provider. However, this is not achievable without considerable resource’48 and the data burden and use of named patient data in routine audits is not considered justified for the gain in precision of prevalence estimates.
Both localities completed data collection over a 6- to 8-week period. ‘The two localities applied different methods for case finding as per their local pressure ulcer audit practice.’48 Locality 1 requested that community nurses assess all of the patients on their community nursing caseload and that a nominated nurse in each residential home, specialist palliative care unit, rehabilitation unit and nursing home in the locality assess all inpatients/residents to identify patients with pressure ulcers. 47 An audit form was completed for each patient (i.e. those with and those without pressure ulcers). Locality 2 identified patients with known pressure ulcers from the community nursing caseload records and the community nurses completed an audit form only for those patients identified as having a pressure ulcer48 [note that patients treated by a general practitioner only (i.e. not also under the care of a general or specialist community nursing service) were not identified in the case-finding method by either locality 1 or locality 2]. In both trusts each patient identified through case finding as having a pressure ulcer had a tissue viability team member visit to verify the skin assessment recorded by the community nurse.
Pain prevalence eligibility criteria
Pain prevalence inclusion criteria
In addition to the standard pressure ulcer audit data, the ward/community nurses were asked to consider whether each patient was able to report the presence or absence of pain. Patients who were considered able to report pain were eligible for inclusion in the pain prevalence study and were asked two screening questions (see following section on assessments) relating to pressure area-related pain by a member of the tissue viability team.
Pain prevalence exclusion criteria
Patients were excluded from the pain prevalence study when it was considered ethically or clinically inappropriate by the ward nurse/clinical team, for example very sick patients or those for whom death was considered to be imminent. When patients were assessed as not able to report pain, this was recorded along with the reason for ineligibility.
Detailed pain assessment inclusion criteria and consent
Patients in the hospital and community settings who answered ‘yes’ to both pain screening questions were provided with a verbal explanation of the detailed pain assessment component of the study and a written information leaflet (see Appendix 4) by the tissue viability team member and were then invited to take part in a full pain assessment. Consenting patients underwent a detailed pain and skin assessment (see Detailed pain assessment).
Assessments
Unlinked anonymised individual patient audit data were recorded by a designated ward or community nurse trained in the use of the data collection form and skin assessment as part of the preparation for the audit. Data recorded included place of assessment (hospital/community and ward specialty, patient’s own home, nursing home, residential home, hospice, community bed), date of birth, gender, height, weight, ethnicity, mobility and risk assessment scale total score (using either the Waterlow score50 or the Braden scale51 as per local policy). Skin was assessed using the 1998 EPUAP24 classification and recorded for a minimum of 13 skin sites (sacrum, left and right buttocks, ischial tuberosities, hips, heels, elbows and ankles). The 1998 EPUAP classification (and not the revised EPUAP/NPUAP 2009 version)1 was used as this was the version in routine use at the participating centres/localities. In addition, the presence of an unstageable pressure ulcer, other type of wound or normal skin were confirmed or skin status was recorded as not applicable (e.g. amputation) or unable to assess.
When patients were assessed as not able to report pain this was recorded along with the reasons for ineligibility. When ward/community staff indicated that the patient was well and able to report pain, a member of the tissue viability team asked the patient two pain screening questions as follows:35,45
-
At any time, do you get pain, soreness or discomfort at a pressure area (prompt: back, bottom, heels, elbows or other as appropriate to the patient)? (Yes or no)
-
Do you think this is related to either your pressure ulcer OR lying in bed for a long time OR sitting for a long time? (Yes or no)
These questions were adapted from the case screening questions used in a large postal survey of pain prevalence in the UK. 35,52 Unlinked anonymous individual patient data were recorded for both questions. The site of the pain, soreness or discomfort was not recorded (i.e. the pressure area/pressure ulcer pain was unattributable to individual body sites).
Detailed pain assessment
Patients who answered ‘yes’ to both pain screening questions and who consented to further assessment underwent a detailed pain and skin assessment by a member of the tissue viability team that included pain intensity, type of pain and skin status/grade of ulcer (as above) on a minimum of 13 skin sites (as above). The patient risk profile was assessed using the Braden scale subscales to allow description of the patient population and comparison with the wider literature.
‘Pain was assessed by asking patients to report the pain intensity (for most severe pain over the past week) for all pressure area sites using a numerical rating scale of 0–10.’45,53,54 Patients were also asked to identify their ’most painful torso and limb skin sites and these were assessed using the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) Pain Scale. 55 The LANSS Scale is a clinically validated tool which allows assessment of neuropathic and inflammatory pain [and has been used in a wide variety of clinical settings55]. It consists of a brief assessment and is easy to score in the clinical setting. The questionnaire contains 5 symptom items and 2 clinical sensory testing items associated with neuropathic pain.’45 The responses to each of the seven items are scored and summed to provide a total score. If the LANSS total score is < 12, neuropathic mechanisms are unlikely and the pain is classified as inflammatory pain. If the LANSS total score is ≥ 12, neuropathic mechanisms are likely to be contributing to the pain and it is classified as neuropathic.
Staff training and preparation
Ward and community nurses were trained locally as per local trust standard pressure ulcer audit practice. Members of the tissue viability team were trained in study procedures, including pain assessment and skin assessments, by the programme manager (LW). No formal inter-rater reliability assessment was undertaken as previous research has demonstrated high agreement between specialist nurses and clinical research nurses in skin assessment and pressure ulcer classification. 56
Data processing
All data returned to the Clinical Trials Research Unit (CTRU) for data processing were anonymous. Data were entered into a bespoke MACRO (version 3; MACRO, Infermed, London, UK) database and range and consistency data checks were carried out to assess the accuracy of the data.
Sample size
For the priori sample size we planned to use a minimum of two acute NHS trusts with an estimated patient population of 2000 and two community NHS trusts with an estimated community nursing caseload of 6000 community patients; therefore, it was planned to include approximately 8000 patients in the prevalence audit.
The a priori sample size was based on the following assumptions:
-
the prevalence of pressure ulcers in hospital patients is 10% and in community patients is 5%2
-
30% of patients have pressure ulcers of grades 2–4/unstageable ulcers, of whom 25–50% would report pressure-area related pain40–43
-
70% of patients have pressure ulcers of grade 1, of whom 10–30% would report pressure area-related pain
-
90% of hospital and 95% of community patients have no pressure ulcers
-
2.5–5% of patients without pressure ulcers would report pressure area-related pain.
Based on these assumptions we estimated that between 259 and 555 patients would report pressure area-related pain (i.e. that 3–7% of patients would report pressure area-related pain; Table 2). A sample of 8000 patients would enable us to estimate a pressure area-related pain prevalence of 3% to within ±0.38% (n = 7742) and a pressure area-related pain prevalence of 7% to within ±0.56% (n = 7975).
| Setting | Pressure ulcer status | Pressure area-related pain | ||
|---|---|---|---|---|
| n | % | |||
| Hospital (n = 2000) | No PU (90%; n = 1800) | 45–90 | 2.5–5 | |
| PU (10%; n = 200) | Category 1 (70%; n = 140) | 14–42 | 10–30 | |
| Categories 2–4 (30%; n = 60) | 15–30 | 25–50 | ||
| Community (n = 6000) | No PU (95%; n = 5700) | 142–285 | 2.5–5 | |
| PU (5%; n = 300) | Category 1 (70%; n = 210) | 21–63 | 10–30 | |
| Categories 2–4 (30%; n = 90) | 22–45 | 25–50 | ||
Analysis
Analysis included data summaries and no inferential statistical testing was planned or undertaken. The denominator for the acute hospital pressure ulcer prevalence was the total inpatient population. The community pressure ulcer prevalence was calculated per 1000 of the estimated total population of adults aged ≥ 18 years for each site (240,038 locality 1 population aged ≥ 18 years; 251,891 locality 2 population aged ≥ 18 years). 49
‘Percentages were calculated using the total number of patients from the relevant population as the denominator (i.e. including all patients with missing data for that variable).’45 When another skin condition or chronic wound was indicated, the specific skin site was excluded from the analysis. ‘All analyses were carried out using SAS software [version 9.2; SAS Institute Inc., Cary, NC, USA]. All percentages were rounded to 1 decimal place. Means, medians, standard deviations [SDs] and ranges were summarised to one more decimal place than the data collected.’45
Type of pain was determined using the results of the seven-item LANSS scale,5 with the responses to each of the seven items scored and summed to provide a total score. Pain was classified as inflammatory pain when the LANSS total score was < 12 and neuropathic pain if the LANSS total score was ≥ 12.
Ethical approval
The studies were approved by the Leeds Central Research Ethics Committee prior to data collection (reference number 09/H1313/14).
Results
Pressure ulcer prevalence: hospital population
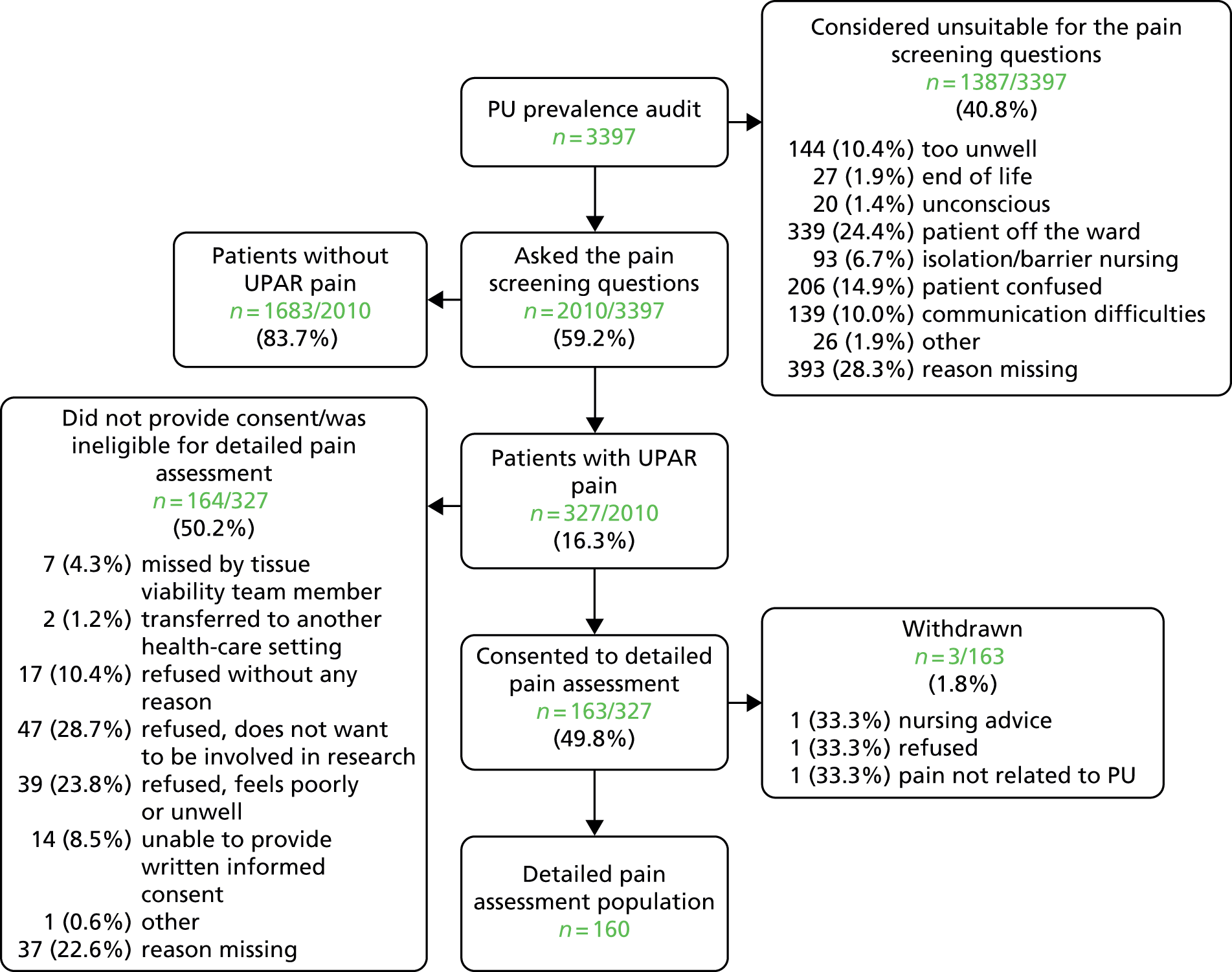
Data collection was undertaken between 15 September 2009 and 3 March 2010. From across nine acute hospitals, a total of 3397 patients (see Appendix 1) were included in the routine pressure ulcer prevalence surveys and this is our target hospital population. 50 Figure 2 details the flow of patients through each stage of the process. The number of patients audited by specialty is presented in Table 3.
FIGURE 2.
Participant flow: hospital population. PU, pressure ulcer; UPAR, unattributed pressure area-related.
| Specialty | Numbers of participants | % |
|---|---|---|
| Medicine | 1348 | 39.7 |
| Surgery | 868 | 25.6 |
| Elderly medicine | 380 | 11.2 |
| Orthopaedic and trauma | 305 | 9.0 |
| Oncology | 211 | 6.2 |
| Critical care | 179 | 5.3 |
| Rehabilitation | 79 | 2.3 |
| Burns | 15 | 0.4 |
| Clinical decision units | 8 | 0.2 |
| Missing | 4 | 0.1 |
| Total | 3397 | 100 |
The median age of patients was 70 years [mean 65.8 (SD 19.23), range 18–103 years]. The numbers of men and women were similar (48.7% male; 1655/3397) and 7.2% (243/3397) were non-Caucasian. 35 In total, 53.9% of patients (1830/3397) were assessed using the Waterlow scale and of these 1062 (58.0%) were classified as ‘at risk’ (score of ≥ 10); 46.1% of patients (1567/3397) were assessed using the Braden scale and of these 532 (34.0%) were classified as ‘at risk’ (score of ≤ 18) (Table 4).
| Characteristic | Total hospital prevalence population | Pain prevalence population | Detailed pain assessment population |
|---|---|---|---|
| Total population, n | 3397 | 2010 | 160 |
| Age (years) | |||
| Median | 70.0 | 68.0 | 69.0 |
| Mean (SD) | 65.8 (19.23) | 64.8 (18.57) | 66.2 (18.23) |
| Range | 18.0–103.0 | 18.0–100.0 | 18.0–99.0 |
| Male, n (%) | 1655 (48.7) | 980 (48.8) | 80 (50.0) |
| ‘At risk’, n/N (%) | |||
| Waterlow | 1062/1830 (58.0) | 504/997 (50.6) | |
| Braden | 532/1567 (34.0) | 263/1013 (26.0) | 114/160 (71.3) |
| Ethnicity, n (%) | |||
| White | 2963 (87.2) | 1774 (88.3) | 154 (96.3) |
| Other | 243 (7.2) | 118 (5.9) | 4 (2.5) |
| Missing | 191 (5.6) | 118 (5.9) | 2 (1.3) |
| Patients with PUs, n (%) | 502 (14.8) | 241 (12.0) | 75 (46.9) |
| Total number of PUs | 1066 | 491 | 139 |
| Number of PUs per patient | |||
| Median | 1.0 | 1.0 | 1.0 |
| Mean (SD) | 2.1 (1.63) | 2.0 (1.44) | 1.9 (1.23) |
| Range | 1.0–13.0 | 1.0–9.0 | 1.0–5.0 |
| Grade of PUs reported, n (%) | |||
| Grade 1 | 752 (70.5) | 357 (72.7) | 97 (69.8) |
| Grade 2 | 237 (22.2) | 100 (20.4) | 32 (23.0) |
| Grade 3 | 45 (4.2) | 18 (3.7) | 4 (2.9) |
| Grade 4 | 18 (1.7) | 10 (2.0) | 3 (2.2) |
| Unstageable | 14 (1.3) | 6 (1.2) | 3 (2.2) |
Of the 3397 patients included, 502 (14.8%) were reported to have 1066 pressure ulcers [median 1.0, mean 2.1 (SD 1.63), range 1–13 per patient]. The majority (70.5%; 752/1066) of reported pressure ulcers were grade 1, approximately one-fifth were grade 2 (22.2%; 237/1066) and a small percentage (7.2%; 77/1066) were grades 3–4/unstageable. 35
Pain prevalence: hospital population
Of the 3397 hospital patients in the pressure ulcer audit sample, 2010 (59.2%) were considered well enough to respond to the pain questions and hence were eligible for the pain prevalence study (see Figure 2).
The pain prevalence population demographics were similar to those of the total hospital prevalence population (see Table 4). The median age of patients was 68 years [mean 64.8 (SD 18.57), range 18–100 years], almost half (980/2010; 48.8%) were male and 122 (6.1%) were non-Caucasian. In total, 49.6% (997/2010) were assessed using the Waterlow scale and of these 504 (50.6%) were classified as ‘at risk’ (score of ≥ 10); 50.4% (1013/2010) were assessed using the Braden scale and of these 263 (26.0%) were classified as ‘at risk’ (score of ≤ 18).
A total of 241 patients (12.0%) were reported to have 491 pressure ulcers [median 1.0, mean 2.0 (SD 1.44), range 1–9 per patient]. As shown in Table 4, there were similar grades of pressure ulcers in both the total hospital prevalence population and the pain prevalence population. The majority (357/491; 72.7%) of reported pressure ulcers in the pain prevalence population were grade 1, 20.4% (100/491) were grade 2 and 6.9% (34/491) were grades 3–4/unstageable.
‘Of the 2010 people asked the pain questions, 327 said yes to both questions, indicating they had pain on one or more skin sites with or without a PU, providing an overall UPAR [unattributed pressure area-related] pain prevalence of 16.3%’ (see Figure 2). In total, 1769 patients did not have any pressure ulcers and 223 of these patients ‘reported pain, an UPAR pain prevalence of 12.6%. Of the 241 people with PUs, 104 patients reported pain at one or more PU site, an UPAR pain prevalence of 43.2%.’35
Detailed pain assessment: hospital population
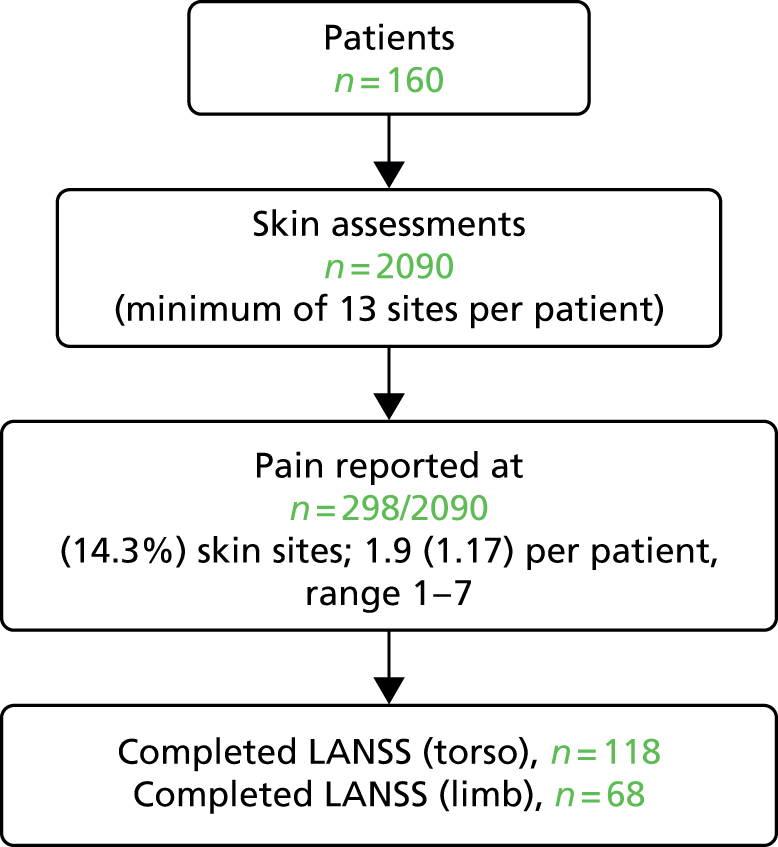
Of the 327 who answered ‘yes’ to both pain screening questions, 164 (50.2%) were not able, or declined, to participate in the full pain assessment and 163 (49.8%) consented. Three patients were subsequently withdrawn and therefore the analysis population of eligible patients with unattributed pressure area-related pain who participated in the detailed pain assessment was 160 (Figure 3).
FIGURE 3.
Participant flow: detailed pain and skin assessments: hospital population.
The median age of these 160 patients was 69.0 years [mean 66.2 (SD 18.23), range 18–99 years] and half (80/160; 50.0%) were men. Almost three-quarters (114/160; 71.3%) of patients with unattributed pressure area-related pain were assessed as ‘at risk’ on the Braden scale and four (2.5%) were non-Caucasian. A total of 75 (46.9%) patients were reported to have 139 pressure ulcers [median 1.0, mean 1.9 (SD 1.23), range 1–5 per patient] (see Table 4).
A total of 2090 skin sites were assessed (see Figure 3), with 1933 skin sites assessed as normal, 139 assessed as pressure ulcers and skin status was not able to be assessed for 18 sites. The majority (69.8%) of reported pressure ulcers were grade 1, 23.0% (32/139) were grade 2 and 7.2% (10/139) were grades 3–4/unstageable (see Table 4).
Pain was reported by 157 patients on 298 skin sites (mean 1.9, SD 1.17, range 1–7 per patient). This included pressure area-related pain reported on 9.8% (190/1933) of skin sites assessed as ‘normal’ and pressure ulcer pain for 68.0% (66/97) of grade 1 pressure ulcers, 84.4% (27/32) of grade 2 pressure ulcers and 90.0% (9/10) of grades 3–4/unstageable ulcers (Table 5). The worst pain intensity reported by each patient ranged from 1 to 10, with a mean of 5.4 (SD 2.30) and median of 5.0.
| Skin classification | Yes, n (%) | No, n (%) | Missing, n (%) | Total, n (%) |
|---|---|---|---|---|
| Normal skin | 190 (9.8) | 1730 (89.5) | 13 (0.7) | 1933 (100.0) |
| Grade 1 | 66 (68.0) | 30 (30.9) | 1 (1.0) | 97 (100.0) |
| Grade 2 | 27 (84.4) | 5 (15.6) | 0 (0.0) | 32 (100.0) |
| Grade 3 | 4 (100.0) | 0 (0.0) | 0 (0.0) | 4 (100.0) |
| Grade 4 | 3 (100.0) | 0 (0.0) | 0 (0.0) | 3 (100.0) |
| Unstageable | 2 (66.7) | 1 (33.3) | 0 (0.0) | 3 (100.0) |
| Unable to assess | 2 (22.2) | 1 (11.1) | 6 (66.7) | 9 (100.0) |
| Classification missing | 4 (44.4) | 5 (55.6) | 0 (0.0) | 9 (100.0) |
| Total | 298 (14.3) | 1772 (84.8) | 20 (1.0) | 2090 (100.0) |
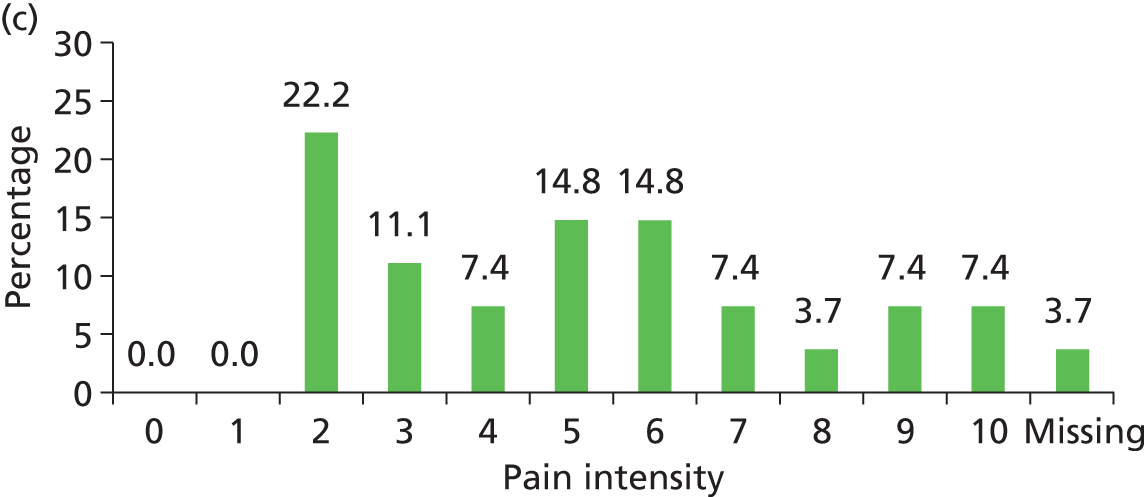
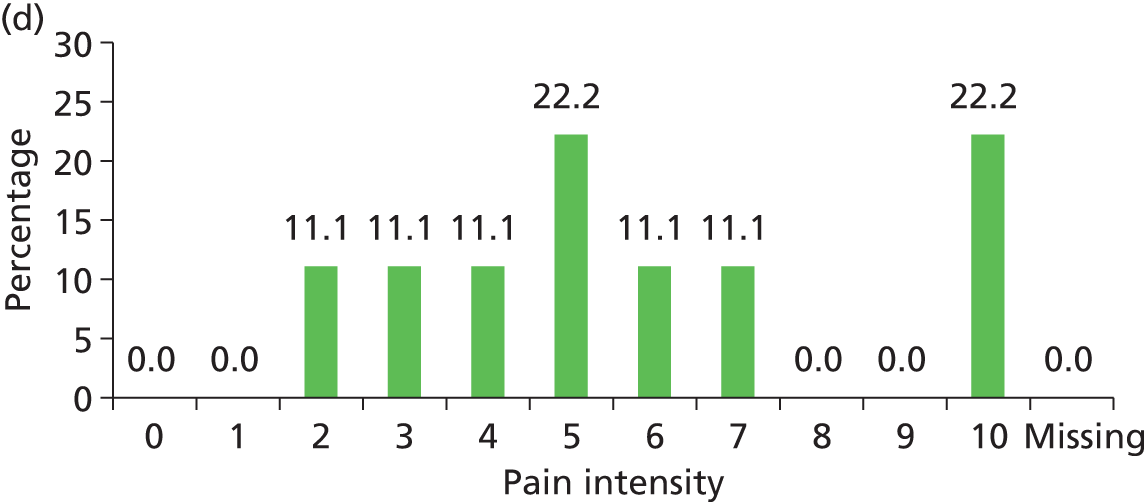
The distribution of pain intensity is similar for each grade of pressure ulcer (Figure 4). In total, 128 patients identified one skin site for LANSS assessment (89 torso and 39 limb skin sites) and 29 patients identified both a torso and a limb skin site for LANSS assessment, providing 118 torso and 68 limb LANSS assessments. Nociceptive pain was dominant in both torso and limb skin sites, with 70.3% (83/118) of painful torso skin sites and 60.3% (41/68) of painful limb skin sites scoring < 12 on the LANSS assessment (Table 6). Neuropathic pain was observed on skin assessed as normal as well as for all grades of pressure ulcer (see Table 6).
FIGURE 4.
Pain intensity by skin classification: hospital population. (a) Normal skin; (b) grade 1 pressure ulcers; (c) grade 2 pressure ulcers; and (d) grades 3–4/unstageable pressure ulcers.


| Location | Skin classification | Nociceptive, n (%) | Neuropathic, n (%) | Missing, n (%) | Total, n (%) |
|---|---|---|---|---|---|
| Torso | Normal skin | 56 (76.7) | 17 (23.3) | 0 (0.0) | 73 (100.0) |
| Grade 1 | 12 (54.5) | 8 (36.4) | 2 (9.1) | 22 (100.0) | |
| Grade 2 | 12 (70.6) | 5 (29.4) | 0 (0.0) | 17 (100.0) | |
| Grade 3 | 2 (66.7) | 1 (33.3) | 0 (0.0) | 3 (100.0) | |
| Grade 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Unstageable | 1 (100.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | |
| Missing | 0 (0.0) | 1 (50.0) | 1 (50.0) | 2 (100.0) | |
| Totala | 83 (70.3) | 32 (27.1) | 3 (2.5) | 118 (100.0) | |
| Limb | Normal skin | 27 (61.4) | 16 (36.4) | 1 (2.3) | 44 (100.0) |
| Grade 1 | 11 (73.3) | 3 (20.0) | 1 (6.7) | 15 (100.0) | |
| Grade 2 | 1 (20.0) | 4 (80.0) | 0 (0.0) | 5 (100.0) | |
| Grade 3 | 0 (0.0) | 1 (100.0) | 0 (0.0) | 1 (100.0) | |
| Grade 4 | 1 (100.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | |
| Unstageable | 1 (100.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | |
| Missing | 0 (0.0) | 0 (0.0) | 1 (100.0) | 1 (100.0) | |
| Totalb | 41 (60.3) | 24 (35.3) | 3 (4.4) | 68 (100.0) |
Pressure ulcer prevalence: community population
Locality 1 collected data between 8 February and 2 April 2010 and locality 2 collected data between 12 April and 7 May 2010. Figure 5 shows the patient flow through the stages of the process. 45
FIGURE 5.
Participant flow: community population. Grade 1-U, grade 1 or above. a, Locality 1 audited all patients on the caseload whereas locality 2 audited all patients with existing pressure damage. Adapted from McGinnis et al. 45 © 2014 McGinnis et al. ; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. 45
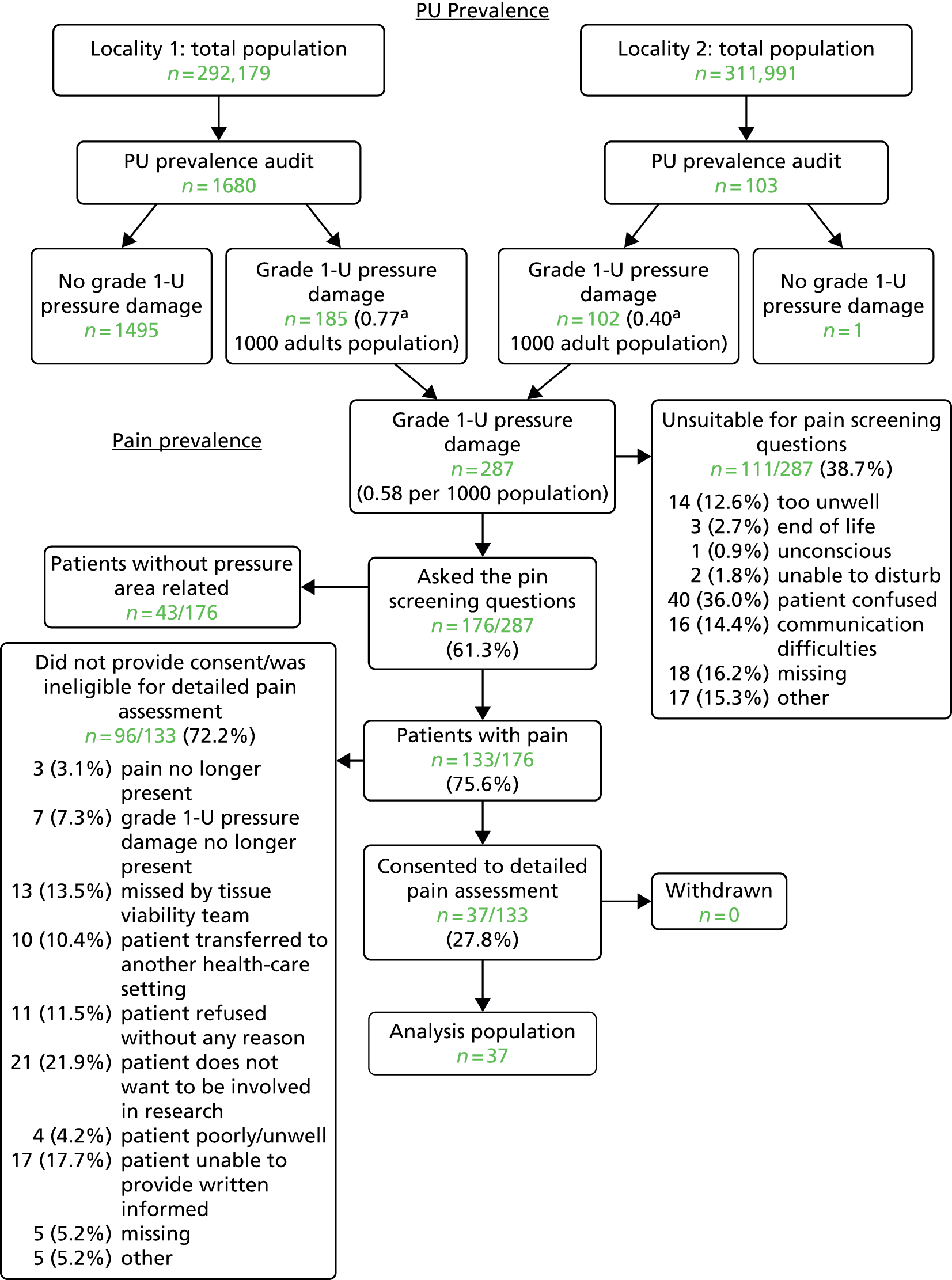
‘The two community NHS Trusts identified 287 patients with Grade 1–4/Unstageable pressure damage. The case finding methods resulted in differing prevalence rates. In locality 1, 1680 patients were assessed, and of these 185 patients were assessed as having a pressure ulcer ≥ Grade 1, a prevalence rate of 0.77 per 1000 [(185/240,038) × 1000 adults]. In locality 2, 102 patients were identified from the community nursing caseloads and assessed as having a Grade ≥ 1 pressure ulcer, a prevalence rate of 0.40 per 1000 [(102/251,891) × 1000 adults]’45. A notable difference between the two sites, and one that could also contribute to the difference in reported prevalence, was the patients’ place of residence. In locality 1, 93 out of 185 (50.3%) patients were resident in a nursing home, whereas in locality 2 only five out of 103 (4.9%) patients were resident in a nursing home.
The median age of patients with pressure ulcers was 81 years (mean 77.8, SD 13.44, range 23–106 years), just over one-third of patients were male (100/287; 34.8%),45 89.6% (251/280) were assessed as being ‘at risk’ using either the Waterlow scale or the Braden scale and only 1.4% (4/287) were non-Caucasian (Table 7).
| Characteristic | Total community prevalence population | Pain prevalence population | Detailed pain assessment population |
|---|---|---|---|
| Total population, n | 287 | 176 | 37 |
| Age (years) | |||
| Median | 81.0 | 79.0 | 75.0 |
| Mean (SD) | 77.8 (13.44) | 76.2 (13.27) | 72.6 (15.31) |
| Range | 23.0–106.0 | 23.0–99.0 | 23.0–98.0 |
| Male, n (%) | 100 (34.8) | 71 (40.3) | 9 (24.3) |
| ‘At risk’, n/N (%) | |||
| Waterlow | 38/38 (100) | 16/16 (100) | |
| Braden | 213/242 (88.0) | 132/156 (84.6) | 25/37 (67.6) |
| Ethnicity | |||
| White | 272 (94.8) | 171 (97.2) | 37 (100.0) |
| Other | 4 (1.4) | 2 (1.1) | 0 (0.0) |
| Missing | 11 (3.8) | 3 (1.7) | 0 (0.0) |
| Place of assessment, n (%) | |||
| Own home | 134 (46.7) | 108 (61.4) | 26 (70.3) |
| Nursing home | 98 (34.1) | 44 (25.0) | 6 (16.2) |
| Residential home | 36 (12.5) | 10 (5.7) | 3 (8.1) |
| Rehabilitation unit | 12 (4.2) | 9 (5.1) | 1 (2.7) |
| Specialist palliative care unit | 5 (1.7) | 4 (2.3) | 1 (2.7) |
| Missing | 2 (0.7) | 1 (0.6) | 0 (0.0) |
| Total number of PUs | 440 | 285 | 54 |
| Number of PUs per patient | |||
| Median | 1.0 | 1.0 | 1.0 |
| Mean (SD) | 1.5 (0.83) | 1.6 (0.88) | 1.5 (0.65) |
| Range | 1.0–5.0 | 1.0–5.0 | 1.0–3.0 |
| Grade of PUs reported, n (%) | |||
| Grade 1 | 155 (35.2) | 87 (30.5) | 20 (37.0) |
| Grade 2 | 177 (40.2) | 118 (41.4) | 17 (31.5) |
| Grade 3 | 63 (14.3) | 45 (15.8) | 8 (14.8) |
| Grade 4 | 32 (7.3) | 25 (8.8) | 5 (9.3) |
| Unstageable | 13 (3.0) | 10 (3.5) | 4 (7.4) |
The 287 patients with pressure ulcers were reported to have 440 ulcers [median 1, mean 1.5 (SD 0.83), range 1–5 per patient]. About one-third of pressure ulcers (155/440; 35.2%) were grade 1, 40.2% (177/440) were grade 2 and 24.5% (108/440) were grades 3–4/unstageable. 45
Pain prevalence: community population
Of the 287 patients with pressure ulcers, 176 (61.3%) were asked the pain screening questions. The median age of patients with pressure ulcers was 79.0 years (mean 76.2, SD 13.27, range 23–99 years), 40.3% were male (71/176), 86.0% (148/172) were assessed as being ‘at risk’ using either the Waterlow scale or the Braden scale and only 1.1% (2/176) were non-Caucasian (see Table 7).
The 176 patients with pressure ulcers were reported to have 285 pressure ulcers [median 1, mean 1.6 (SD 0.88), range 1–5 per patient]. Under one-third of pressure ulcers (87/285; 30.5%) were grade 1, 41.4% (118/285) were grade 2 and 28.1% (80/285) were grades 3–4/unstageable. The prevalence of unattributed pressure area-related pain in the community patient population who had existing pressure ulcers was 75.6% (133/176) (see Figure 5).
Detailed pain assessment: community population
‘Of the 133 patients with unattributed pressure area-related pain, 96 were not able or declined to participate in the full pain assessment [see Figure 5]. Therefore, the analysis population of eligible patients who consented to the detailed pain assessment was 27.8% (37/133) of the population reporting pain.’45
The median age of these 37 patients was 75.0 years (mean 72.6, SD 15.31, range 23–98 years). Most (70.3%) patients were assessed in their own home, with the remainder assessed in residential or nursing homes, rehabilitation units or palliative care units. Nine patients (24.3%) were male and all were white (see Table 7). 45
‘A total of 481 skin sites were assessed [Figure 6], including 427 skin sites assessed as normal and 54 PUs (mean 1.5 per patient, SD 0.65, range 1–3). Approximately a third of PUs were Grade 1 (37.0%; n = 20/54), Grade 2 (31.5%; n = 17/54) and Grade 3–4/U (31.5%; n = 17/54) [see Table 7], with 29 (53.7%) located on a torso skin site and 25 (46.3%) located on a limb skin site.’45
FIGURE 6.
Participant flow: detailed pain and skin assessments: community population. PU, pressure ulcer. Reproduced from McGinnis et al. 45 © 2014 McGinnis et al. ; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited.
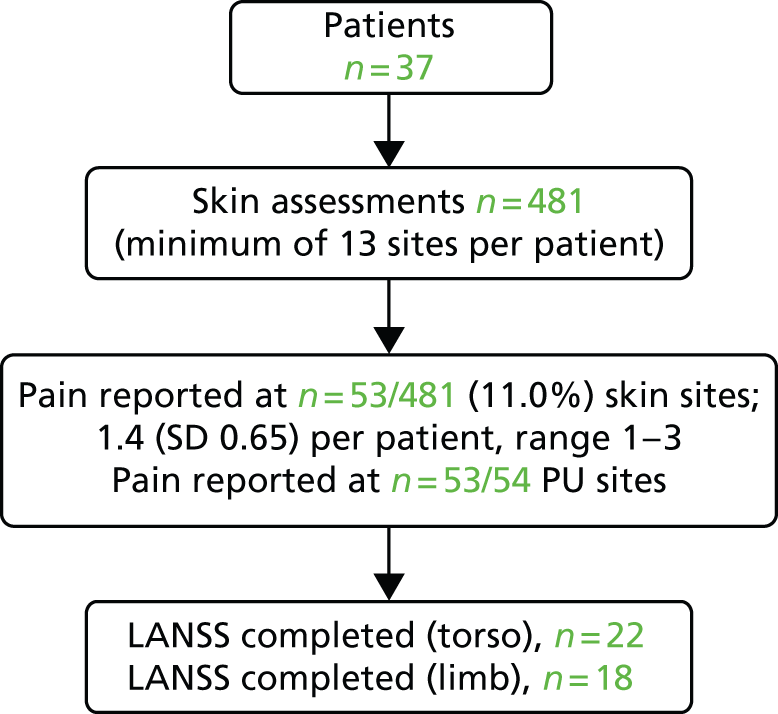
The 37 patients reported pain on 53 out of 481 (11.0%) skin sites [median 1.0, mean 1.4 (SD 0.65), range 1–3 per patient]. ‘No pressure area related pain was reported on normal skin whilst patients reported PU pain for 98.1% (n = 53/54) of all PUs [Table 8]. Pain intensity ranged from 1–10, with a mean of 6.4 (SD 2.53) and median of 7.0. There is a slightly skewed distribution of pain intensity with very similar pain levels for each grade of PU [Figure 7]. Thirty-one patients identified one skin site for LANSS assessment (n = 19 torso and n = 15 limb) and six patients identified both a torso and a limb skin site for LANSS assessment providing a total of 22 torso and 18 limb LANSS assessments. Neuropathic pain was slightly dominant in both torso and limb skin sites, with 54.5% (n = 12/22) of torso PUs and 61.1% (n = 11/18) of limb PUs scoring ≥ 12 on the LANSS assessment’ (Table 9).
| Yes, n (%) | No, n (%) | Total, n (%) | |
|---|---|---|---|
| Normal skin | 0 (0.0) | 427 (100.0) | 427(100.0) |
| Grade 1 | 19 (95.0) | 1 (5.0) | 20 (100.0) |
| Grade 2 | 17 (100.0) | 0 (0.0) | 17 (100.0) |
| Grade 3 | 8 (100.0) | 0 (0.0) | 8 (100.0) |
| Grade 4 | 5 (100.0) | 0 (0.0) | 5 (100.0) |
| Unstageable | 4 (100.0) | 0 (0.0) | 4 (100.0) |
| Total | 53 (11.0) | 428 (89.0) | 481 (100.0) |
FIGURE 7.
Pain intensity by skin classification: community population. (a) grade 1; (b) grade 2; and (c) grades 3–4/unstageable. Reproduced from McGinnis et al. 45 © 2014 McGinnis et al. ; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited.

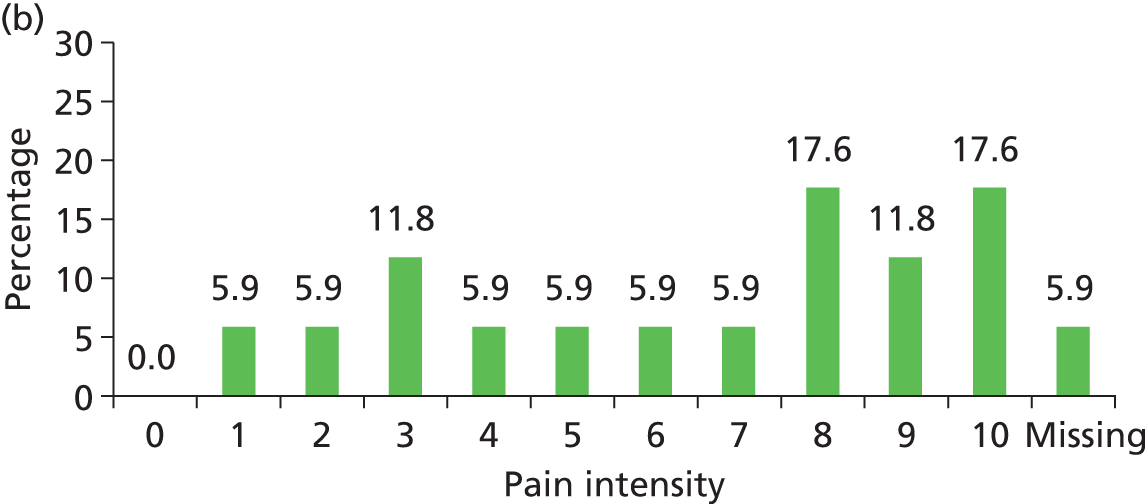
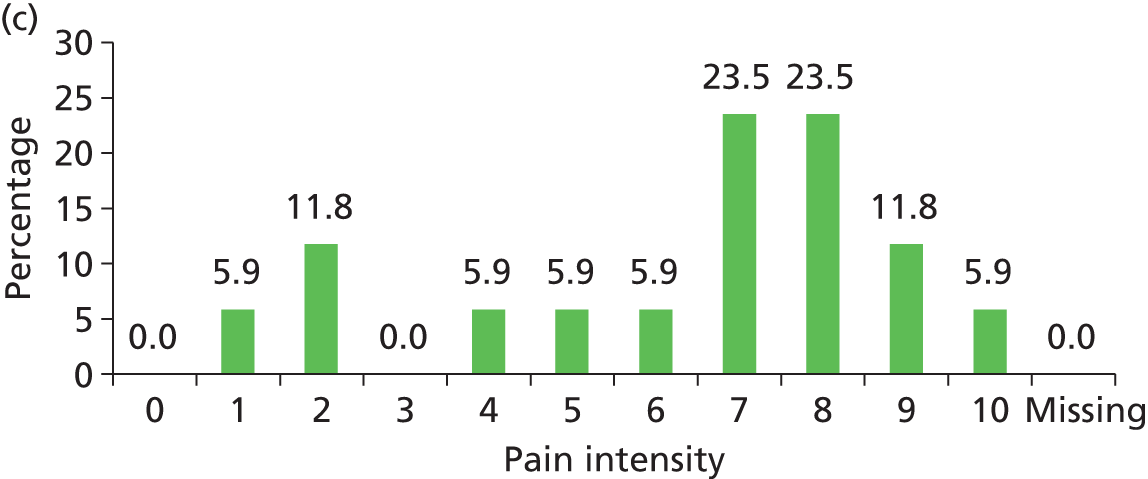
| Location | Skin classification | Nociceptive, N (%) | Neuropathic, N (%) | Missing, N (%) | Total, N (%) |
|---|---|---|---|---|---|
| Torso | Grade 1 | 3 (42.9%) | 4 (57.1%) | 0 (0.0%) | 7 (100.0%) |
| Grade 2 | 3 (33.3%) | 6 (66.7%) | 0 (0.0%) | 9 (100.0%) | |
| Grade 3 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Grade 4 | 4 (80.0%) | 1 (20.0%) | 0 (0.0%) | 5 (100.0%) | |
| Unstageable | 0 (0.0%) | 1 (100.0%) | 0 (0.0%) | 1 (100.0%) | |
| Totala | 10 (45.5%) | 12 (54.5%) | 0 (0.0%) | 22 (100.0%) | |
| Limb | Grade 1 | 2 (50.0%) | 2 (50.0%) | 0 (0.0%) | 4 (100.0%) |
| Grade 2 | 1 (20.0%) | 4 (80.0%) | 0 (0.0%) | 5 (100.0%) | |
| Grade 3 | 2 (33.3%) | 3 (50.0%) | 1 (16.7%) | 6 (100.0%) | |
| Grade 4 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Unstageable | 1 (33.3%) | 2 (66.7%) | 0 (0.0%) | 3 (100.0%) | |
| Totalb | 6 (33.3%) | 11 (61.1%) | 1 (5.6%) | 18 (100.0%) |
Pain and pressure ulcer risk: cohort study
To explore the role of pain as a predictor of category 2 pressure ulcer development we undertook a multicentre prospective cohort study in acute and community NHS trusts.
Aims and objectives
The main aim of this study was to explore the role of pain as an early predictor of category 2 pressure ulcer development.
Objectives were to:
-
assess whether the presence/absence of pressure area-related pain is a predictor of category 2 or above pressure ulcer development, after adjusting for other known variables
-
explore the relationship between skin classification category and reported pain
-
identify variables that are independently predictive of category 2 or above pressure ulcer development.
Methods
Study design
We undertook a multicentre prospective cohort study. We recorded the presence of key risk factors, skin status and pain at baseline with twice-weekly follow-up for up to 30 days from registration to identify the development of new category 2 or above pressure ulcers (see Appendix 6 for the study protocol).
Setting
Hospital patients were recruited from vascular, trauma, orthopaedic and medical/elderly wards and community NHS patients were recruited from their place of normal residence (own/residential/nursing home) and community inpatient facilities.
Eligibility criteria
Inclusion criteria
Patients were eligible for study inclusion if they met all of the following criteria:
-
there was evidence of acute illness through one or more of the following:
-
acute vascular, orthopaedic, medical or care of the elderly admission to secondary care hospital
-
recent hospital discharge to home/intermediate care/community care
-
existing community nursing patient with deterioration in overall condition or onset of acute illness
-
new referral to community nursing because of acute illness, deterioration in existing condition or care package breakdown
-
-
age ≥ 18 years
-
at high risk of pressure ulcer development because of one or more of the following:
-
bedfast/chairfast and completely immobile/very limited mobility51
-
localised skin pain on any pressure area skin site
-
category 1 pressure ulcer on any pressure area skin site
-
-
able to give their written informed consent to participate
-
expected to be able to comply with follow-up schedule.
Exclusion criteria
Patients were excluded from the study if one or more of the following criteria applied:
-
obstetric, paediatric, day-case surgery or psychiatric patients in both acute and community settings
-
unable to provide written informed consent
-
unable to comply with follow-up assessment schedule
-
deemed by the attending health-care professional to be too unwell to be approached and/or complete the study assessment schedule
-
unable to report the presence/absence of pain (e.g. unconscious)
-
with two or more category 2 or above pressure ulcers on any key pressure area skin sites (sacrum, buttocks, heels or hips).
Screening
Participating research sites were required to complete a log of all patients screened for eligibility. The following anonymised information was collected: age, gender, ethnicity and reason not eligible for study participation or the reason eligible but declined.
Recruitment and consent
When eligibility was indicated by the attending clinical team, patients were flagged to a member of the trust tissue viability team. A full verbal explanation of the study patient information leaflet (see Appendix 7) was provided for the patient to consider, including detailed information about the rationale, design and personal implications of the study. Assenting patients were then invited to provide written informed consent/witnessed consent (see Appendix 8) and eligibility was confirmed prior to registration using a central 24-hour telephone registration system.
Assessments
At registration, baseline demographics, skin status, pain status, analgesic use, mattress intervention, the Braden subscales51 and risk factors, including diabetic status, presence of other chronic wounds, history of weight loss and body mass index (BMI) were recorded.
Skin status
Thirteen key skin sites (sacrum, left and right buttocks, ischial tuberosities, hips, heels, elbows and ankles) were assessed for pressure ulcers and for the presence, duration and intensity of pain. Pressure ulcers were classified as categories 1–4 or unstageable. 1 In addition, as general skin condition is predictive of category 2 pressure ulcer development,37,57,58 observations of any alteration to intact skin (e.g. redness, scar, excoriation, dry, scaly) were recorded as ‘A’ and the presence of healthy skin was confirmed (see Appendix 6).
Pain status
To determine whether or not patients had localised skin pain on any pressure area skin site they were asked the two screening questions and, when pain was indicated, a detailed pain assessment was completed (see Pain prevalence in hospital and community populations, Methods).
Pressure ulcer interventions
All participating trusts had pressure ulcer prevention policies and guidelines, which included risk assessment, mattress and repositioning guidance. The cohort study participants received pressure ulcer prevention interventions as allocated by the attending clinical teams and as per local policies and guidelines. We recorded mattress provision, which was categorised as non-pressure relieving (e.g. domestic mattress or silicone fibre overlay), static pressure relieving (e.g. high-specification foam, viscoelastic foam, air filled, gel filled) and dynamic pressure relieving (e.g. alternating pressure, low air loss, air fluidised).
Frequency of assessments
A maximum of eight follow-up assessments were undertaken to establish the primary end point for patients who continued to be at high risk of pressure ulcer development during the 30-day period. Patients were followed up across health-care settings (i.e. hospital patients discharged home and community patients admitted to hospital) until 30 days from registration or they were assessed as being no longer at high risk, withdrawal or death. At follow-up, skin status, Braden subscales, pain status, analgesic use, mattress provision and serious adverse events were recorded.
Staff training and preparation
Consistent with methods used for the pain prevalence study, members of the tissue viability team were trained and no formal assessment of inter-rater reliability was undertaken (see Pain prevalence in hospital and community populations, Methods).
Data processing
Data processing methods were consistent with those described in Pain prevalence in hospital and community populations (see Methods). In addition, data queries and missing data were chased until the data were confirmed as correct or unavailable. Batch validation and consistency data checks were also carried out to check the accuracy of the data.
Sample size
For risk factor studies using logistic regression it is recommended that at least 10 patients with the event of interest are needed for reliable estimation of effects. 59 Our aim was to assess whether or not the presence of localised skin pain is predictive of new category 2 (or above) pressure ulcer development, after adjusting for the effects of other known risk factors.
We prespecified seven risk factors identified from our risk factor systematic review and emerging conceptual framework46 (see Chapter 5): age, diabetes status, nutritional status, Braden mobility subscale score, presence of skin alterations, presence of a category 1 ulcer on any site and patient setting (hospital or community). In addition, because a patient’s perception of pain is likely to be affected by the use of analgesics or other forms of pain relief, this was also included as a prespecified covariate, resulting in a model potentially including nine factors (i.e. pain, the seven prespecified risk factors and analgesic use).
A nine-factor model would therefore require a minimum of 90 patients to develop a new pressure ulcer of category 2 or above. In the absence of prospective data for community-based patient populations,46 the sample size estimate was based on previous research in acute hospital patients,37,58,60 suggesting that approximately 15% of patients would develop a new pressure ulcer of category 2 or above within 30 days of entering the study. Based on this assumption and allowing for potential loss to follow-up of 5%, we estimated that we would require 632 patients to be recruited to this study.
A further consideration in appraising the sample size estimate was the prevalence of pain at study entry. As no previous work in this field had been undertaken we considered a range of prevalence rates. Table 10 shows the largest difference in pressure ulcer incidence that could be detected with a minimum of 80% power if 10% or 20% of patients reported pain at study entry. We estimated pressure ulcer event rates in the patients without pain of 10% and 15% for each case and assumed that patients with pain at study entry are more likely to develop a new ulcer than those without pain at entry.
| Total n | Baseline pain | PU incidence | PU incidence | |||||
|---|---|---|---|---|---|---|---|---|
| With pain, n (%) | Without pain, n (%) | With pain, % | Without pain, % | Difference, % | With pain, % | Without pain, % | Difference, % | |
| 600 | 60 (10) | 540 (90) | 23.2 | 10.0 | 13.2 | 30.0 | 15.0 | 15.0 |
| OR 2.719 (95% CI 1.402 to 5.217) | OR 2.429 (CI 1.332 to 4.428) | |||||||
| 600 | 120 (20) | 480 (80) | 19.8 | 10.0 | 9.8 | 26.2 | 15.0 | 11.2 |
| OR 2.222 (95% CI 1.296 to 3.809) | OR 2.021 (CI 1.248 to 3.244) | |||||||
We estimated that, if we recruited 600 patients (after accounting for 5% loss to follow up) with 60 (10%) of them having pain on study entry, this would allow us to detect a statistically significant difference (p < 0.05) of 13.2% between those with and those without pain using a chi-square test (80% power, 5% significance) if 10% of patients without pain and 23.2% of those with pain developed a new pressure ulcer within the 30-day follow-up period, corresponding to an odds ratio (OR) of 2.72 [95% confidence interval (CI) 1.40 to 5.27].
As this was an exploratory study and there was uncertainty around the assumptions made to estimate the sample size, the proportion of patients with pain at baseline and the incidence of pressure ulcer development was monitored by the statistical team and chief investigator and reported to a subgroup of the programme steering committee.
End point definition
The primary end point was defined as the development of a new category 2 or above pressure ulcer on any skin site after registration and before the end of follow-up. End of follow-up was defined as no longer at high risk (see Inclusion criteria), patient transferred to a non-participating setting, death or the end of study follow-up (30 days), whichever was the earliest event.
The secondary end point was defined as the time in days after registration to development of the first new category 2 or above pressure ulcer or to the end of follow-up for patients who were not observed to develop a new category 2 or above pressure ulcer during follow-up. Patients who were not observed to develop a new category 2 or above pressure ulcer were censored at the end of follow-up.
Analysis population
The defined analysis population was all patients for whom a primary end point could be determined, that is, patients with at least one follow-up skin assessment completed.
Analysis methods
Primary end point analysis
Univariate logistic regression was conducted to assess the candidate variables for inclusion in a multivariable model. Candidate variables were considered statistically significant if the p-value of the associated likelihood ratio test was < 0.1.
A multivariable analysis was then conducted to build a logistic regression model for the odds of developing a new category 2 or above pressure ulcer, using forwards and backwards stepwise variable selection. Candidate variables were included in the model if their inclusion led to a reduction in deviance with a corresponding p-value of < 0.1 for the associated likelihood ratio test; similarly, candidate variables were retained in the model if their exclusion led to an increase in deviance with a corresponding p-value of > 0.1 for the associated likelihood ratio test. The candidate variables of interest, defined at baseline (study entry) and based on a conceptual framework46 (see Chapter 5), were age, diabetes status, history of weight loss, Braden mobility subscale score (category 3 or 4 vs. category 1 or 2), presence of skin alterations, presence of a category 1 pressure ulcer, setting (hospital, community), use of analgesics/pain relief and presence of pain on a skin site assessed as healthy, altered or a category 1 pressure ulcer.
The primary analysis focused on using unconditional logistic regression61 to determine whether or not the presence or absence of pain at study entry was predictive of the development of a new category 2 or above pressure ulcer, after allowing for the other a priori factors of interest. Parameter estimates (ORs), their 95% CIs and associated p-values are presented. Methods for assessing the appropriateness of the model and the influence of observations were applied (e.g. the Hosmer and Lemeshow goodness of fit test62).
Overdispersion analysis
An additional analysis was carried out to determine if there was overdispersion in the model and, if so, whether or not this could be explained by the inclusion of other variables for which data have been collected, irrespective of whether or not the presence of pain is an important predictor of pressure ulcer development. Further variables were assessed for inclusion in the final logistic regression model obtained in the primary analysis using forwards and backwards stepwise variable selection. Candidate variables were retained in/excluded from the model if the p-value was < 0.1 for the associated likelihood ratio test. An assessment of whether or not the variables included in the primary analysis were still significant was also undertaken as part of the variable selection process. The other baseline candidate variables assessed for inclusion were gender, BMI, Braden scale domains (activity, friction, moisture, nutrition and sensory perception), the presence of a category 2 or above pressure ulcer, the presence of a chronic wound and mattress category.
The relationship between skin classification and the presence or absence of pain was examined using descriptive statistics (mainly cross-tabulations). Baseline data for each patient were tabulated using frequencies and summary statistics. Missing or unobtainable data were noted. Characteristics were also summarised by whether the patient presented in an acute hospital or a community setting. Correlations between the explanatory variables were also examined.
Time-to-event analysis
The relationship between presence or absence of pain at study entry and time to onset of a new category 2 or above pressure ulcer was initially investigated using Cox proportional hazards regression. 63 The assumptions of the Cox proportional hazards model were assessed using log-log plots, Cox–Snell residuals and Schoenfeld residuals and by fitting a time-dependent covariate term in the model for the presence of pain. 64 However, the proportional hazards assumptions of the model obtained did not hold and an accelerated failure time model65 was fitted. Unlike the Cox proportional hazards model, the accelerated failure time model is a parametric model that required the distribution of the hazard function to be prespecified and, for this analysis, the gamma distribution was the most appropriate distribution. As for the logistic regression analysis, univariate analyses were conducted to determine which variables were associated with time to onset of a new category 2 or above pressure ulcer and were therefore candidates for the multivariable analyses. The final multivariable accelerated failure time model was obtained using forwards and backwards stepwise variable selection. Candidate variables were retained in/excluded from the model if the p-value was < 0.1 for the associated likelihood ratio test. The results are presented as parameter estimates (ratio of the time to onset of a category 2 or above pressure ulcer) with 95% CIs and associated p-values. The time to onset of a category 2 or above pressure ulcer was also assessed using cumulative incidence functions.
Skin site analysis
The relationship between presence or absence of pain at baseline on a healthy, altered or category 1 pressure ulcer skin site and the development of a new category 2 or above pressure ulcer at the same skin site was examined, taking account of the nesting of skin sites within patients using multilevel logistic regression (specifically, a two-level random-intercept logistic model was used). Univariate analyses and a multivariable analysis using forwards and backwards variable selection were conducted as for the primary logistic regression analysis. Candidate variables were included in the model if their inclusion led to a reduction in deviance with a corresponding p-value of < 0.1 for the associated likelihood ratio test; similarly, candidate variables were retained in the model if their exclusion led to an increase in deviance with a corresponding p-value of > 0.1 for the associated likelihood ratio test. The results are presented as parameter estimates (ORs), their 95% CIs and associated p-values.
Results
In total, 3819 patients were assessed for eligibility for the study and 634 patients were registered between 26 October 2009 and 17 November 2011. There were 26 recruiting centres across 18 NHS trusts in England, with the number of patients registered at each centre ranging from 1 to 86 (see Appendix 1). The centres consisted of eight teaching hospitals (four acute NHS trusts), 10 general hospitals (four acute and two community NHS trusts) and eight community care NHS trusts.
Of the 634 patients who were registered to the study, a primary end point could not be determined for 32 because they did not have any follow-up visits. Therefore, the analysis population included a total of 602 patients (Figure 8).
FIGURE 8.
Cohort study: participant flow. PU, pressure ulcer.
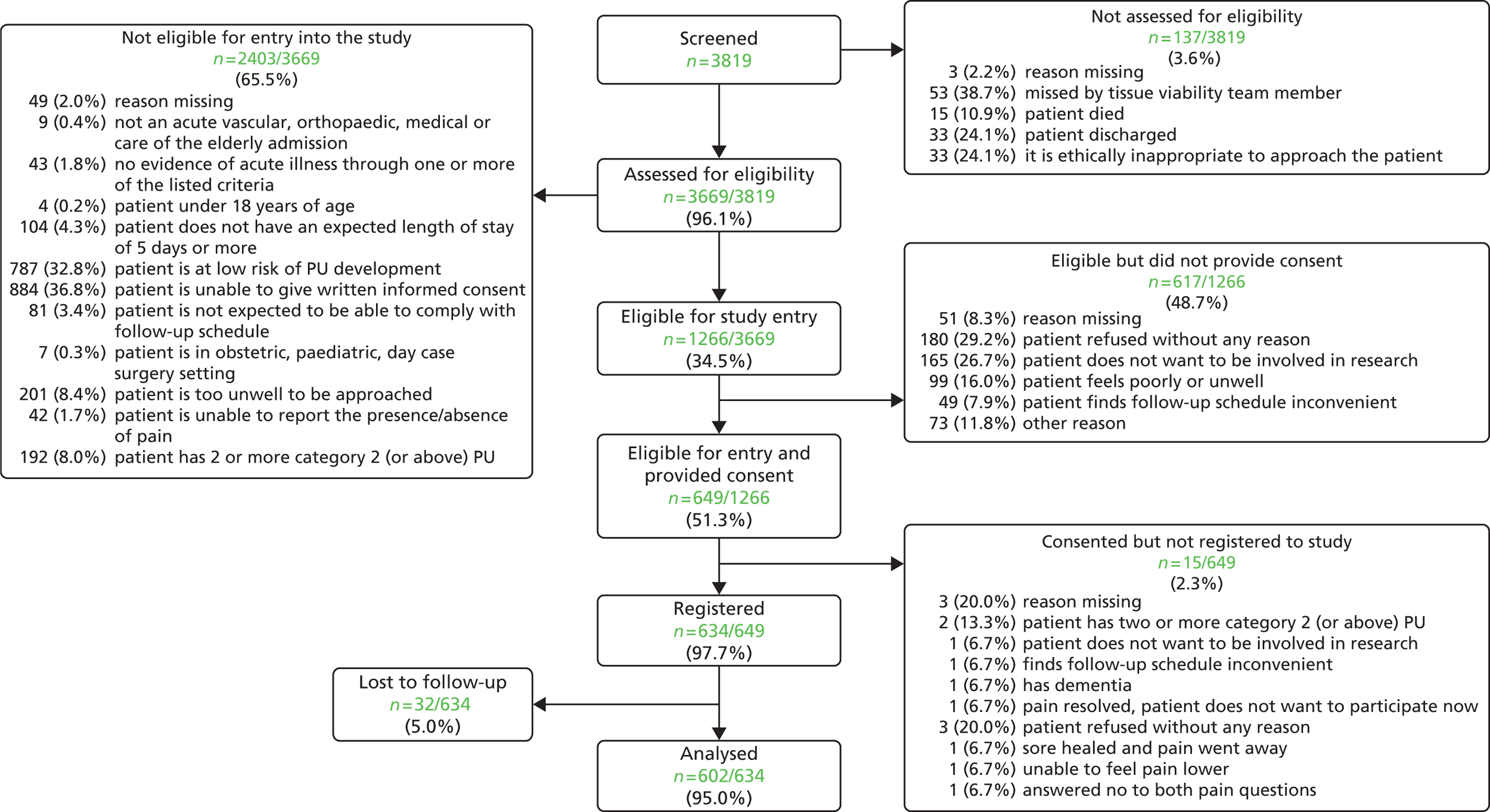
Baseline characteristics
In total, 397 (65.9%) patients were registered from the acute setting and 205 (34.1%) patients were registered from the community setting, half of whom were located in rehabilitation inpatient settings (104/205, 50.7%; Table 11).
| Specialty or place assessed | Acute (n = 397), n (%) | Community (n = 205), n (%) | Total (n = 602), n (%) |
|---|---|---|---|
| Vascular | 42 (10.6) | 0 (0.0) | 42 (7.0) |
| Orthopaedic | 155 (39.0) | 0 (0.0) | 155 (25.7) |
| Medical | 90 (22.7) | 0 (0.0) | 90 (15.0) |
| Elderly | 32 (8.1) | 0 (0.0) | 32 (5.3) |
| Medical/elderly | 78 (19.6) | 0 (0.0) | 78 (13.0) |
| Patient’s own home | 0 (0.0) | 49 (23.9) | 49 (8.1) |
| Nursing home | 0 (0.0) | 27 (13.2) | 27 (4.5) |
| Residential home | 0 (0.0) | 18 (8.8) | 18 (3.0) |
| Rehabilitation unit | 0 (0.0) | 104 (50.7) | 104 (17.3) |
| Other place assessed | 0 (0.0) | 7 (3.4) | 7 (1.2) |
Patient characteristics are detailed in Tables 12 and 13. In summary, across both groups there were fewer male patients (38.7%), all but one patient was Caucasian (99.8%) and one-quarter of patients were diabetic (25.4%). The median age of patients was 80 years (range 21–101 years). One-quarter of patients had a history of weight loss (24.4%), with 10.6% assessed as underweight by BMI and 22.3% assessed as obese. In total, 21.1% of patients had a chronic wound. The majority of patients recruited to the study had alterations to intact skin (62.0%), almost half had a category 1 pressure ulcer (48.2%) over one-quarter had a category 2 ulcer (27.2%) and 4.0% had a category 3, 4 or unstageable ulcer.
| Characteristic | Acute (n = 397) | Community (n = 205) | Total (n = 602) |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 75.6 (12.9) | 80.7 (11.7) | 77.3 (12.7) |
| Median (range) | 79 (21–101) | 83 (30–100) | 80 (21–101) |
| Sex, n (%) | |||
| Male | 156 (39.3) | 77 (37.6) | 233 (38.7) |
| Female | 241 (60.7) | 128 (62.4) | 369 (61.3) |
| Ethnicity, n (%) | |||
| Caucasian | 396 (99.7) | 205 (100.0) | 601 (99.8) |
| Non-Caucasian | 1 (0.3) | 0 (0.0) | 1 (0.2) |
| Is the patient diabetic, n (%) | |||
| Yes | 98 (24.7) | 55 (26.8) | 153 (25.4) |
| No | 299 (75.3) | 149 (72.7) | 448 (74.4) |
| Missing | 0 (0.0) | 1 (0.5) | 1 (0.2) |
| History of weight loss, n (%) | |||
| Yes | 95 (23.9) | 52 (25.4) | 147 (24.4) |
| No | 302 (76.1) | 152 (74.1) | 454 (75.4) |
| Missing | 0 (0.0) | 1 (0.5) | 1 (0.2) |
| BMI | |||
| Mean (SD) (kg/m2) | 27.1 (9.3) | 26.0 (9.9) | 26.7 (9.5) |
| Median (range) (kg/m2) | 25 (11–94) | 24 (11–111) | 25 (11–111) |
| Underweight (< 18.5 kg/m2), n (%) | 38 (9.6) | 26 (12.7) | 64 (10.6) |
| Normal weight (18.5 to < 25 kg/m2), n (%) | 143 (36.0) | 81 (39.5) | 224 (37.2) |
| Overweight (25 to < 30 kg/m2), n (%) | 106 (26.7) | 50 (24.4) | 156 (25.9) |
| Obese (≥ 30 kg/m2), n (%) | 98 (24.7) | 36 (17.6) | 134 (22.3) |
| Missing, n (%) | 12 (3.0) | 12 (5.9) | 24 (4.0) |
| Chronic wounds, n (%) | |||
| Yes | 75 (18.9) | 52 (25.4) | 127 (21.1) |
| No | 322 (81.1) | 152 (74.1) | 474 (78.7) |
| Missing | 0 (0.0) | 1 (0.5) | 1 (0.2) |
| Skin alterations at baseline, n (%) | |||
| Yes | 242 (61.0) | 131 (63.9) | 373 (62.0) |
| No | 155 (39.0) | 74 (36.1) | 229 (38.0) |
| Category 1 at baseline, n (%) | |||
| Yes | 198 (49.9) | 92 (44.9) | 290 (48.2) |
| No | 199 (50.1) | 113 (55.1) | 312 (51.8) |
| Existing category or above at baseline, n (%) | |||
| Yes | 116 (29.2) | 48 (23.4) | 164 (27.2) |
| No | 281 (70.8) | 157 (76.6) | 438 (72.8) |
| Pain at a healthy, altered or category 1 skin site, n (%) | |||
| Yes | 301 (75.8) | 163 (79.5) | 464 (77.1) |
| No | 96 (24.2) | 42 (20.5) | 138 (22.9) |
| Worst skin status at baseline, n (%) | |||
| Healthy intact skin | 45 (11.3) | 25 (12.2) | 70 (11.6) |
| Alterations to intact skin | 99 (24.9) | 55 (26.8) | 154 (25.6) |
| Category 1 | 137 (34.5) | 77 (37.6) | 214 (35.5) |
| Category 2 | 98 (24.7) | 42 (20.5) | 140 (23.3) |
| Category 3 | 10 (2.5) | 3 (1.5) | 13 (2.2) |
| Category 4 | 2 (0.5) | 2 (1.0) | 4 (0.7) |
| Unstageable | 6 (1.5) | 1 (0.5) | 7 (1.2) |
| Analgesic use, n (%) | |||
| Yes | 366 (92.2) | 182 (88.8) | 548 (91.0) |
| No | 31 (7.8) | 23 (11.2) | 54 (9.0) |
| Mattress category, n (%) | |||
| Non-pressure relieving | 5 (1.3) | 31 (15.1) | 36 (6.0) |
| Static pressure-relieving | 168 (42.3) | 105 (51.2) | 273 (45.3) |
| Dynamic pressure-relieving | 224 (56.4) | 68 (33.2) | 292 (48.5) |
| Missing | 0 (0.0) | 1 (0.5) | 1 (0.2) |
| Braden subscale | Acute (n = 397), n (%) | Community (n = 205), n (%) | Total (n = 602), n (%) |
|---|---|---|---|
| Sensory perception | |||
| Very limited | 5 (1.3) | 3 (1.5) | 8 (1.3) |
| Slightly limited | 68 (17.1) | 15 (7.3) | 83 (13.8) |
| No impairment | 324 (81.6) | 187 (91.2) | 511 (84.9) |
| Moisture | |||
| Constantly moist | 2 (0.5) | 3 (1.5) | 5 (0.8) |
| Very moist | 25 (6.3) | 13 (6.3) | 38 (6.3) |
| Occasionally moist | 105 (26.4) | 55 (26.8) | 160 (26.6) |
| Rarely moist | 265 (66.8) | 134 (65.4) | 399 (66.3) |
| Activity | |||
| Bedfast | 96 (24.2) | 8 (3.9) | 104 (17.3) |
| Chairfast | 221 (55.7) | 92 (44.9) | 313 (52.0) |
| Walks occasionally | 76 (19.1) | 81 (39.5) | 157 (26.1) |
| Walks frequently | 4 (1.0) | 24 (11.7) | 28 (4.7) |
| Mobility | |||
| Completely immobile | 9 (2.3) | 12 (5.9) | 21 (3.5) |
| Very limited | 204 (51.4) | 58 (28.3) | 262 (43.5) |
| Slightly limited | 142 (35.8) | 89 (43.4) | 231 (38.4) |
| No limitation | 42 (10.6) | 46 (22.4) | 88 (14.6) |
| Nutrition | |||
| Very poor | 13 (3.3) | 6 (2.9) | 19 (3.2) |
| Probably inadequate | 130 (32.7) | 28 (13.7) | 158 (26.2) |
| Adequate | 163 (41.1) | 95 (46.3) | 258 (42.9) |
| Excellent | 91 (22.9) | 76 (37.1) | 167 (27.7) |
| Friction and shear | |||
| Problem | 62 (15.6) | 39 (19.0) | 101 (16.8) |
| Potential problem | 295 (74.3) | 118 (57.6) | 413 (68.6) |
| No apparent problem | 40 (10.1) | 48 (23.4) | 88 (14.6) |
| Total score | |||
| At risk (≤ 18) | 324 (81.6) | 115 (56.1) | 439 (72.9) |
| Not at risk (> 18) | 73 (18.4) | 90 (43.9) | 163 (27.1) |
Pain was reported on healthy, altered and category 1 skin sites by 77.1% of patients (see Table 12). Only 3.5% of patients were completely immobile, with the majority of patients assessed as having either very limited (43.5%) or slightly limited (38.4%) mobility according to the Braden mobility subscale (see Table 13).
The majority of patients were receiving either a static pressure-relieving (45.3%) or a dynamic pressure-relieving (48.5%) mattress. In the community 15.1% of mattresses were described as non-pressure relieving whereas in the hospital setting this was the case for only 1.3% of patients (see Table 12).
Primary end point analysis
The primary end point was whether or not a patient developed a new category 2 or above pressure ulcer on any skin site after registration and before the end of follow-up. Skin sites with a category 2 or above pressure ulcer or recorded as not applicable/unable to assess (e.g. amputated limb or bandage/dressing in situ) at baseline were excluded from the primary end point analysis. The overall incidence of new category 2 or above pressure ulcers in the analysis population was 152 out of 602 (25.2%).
A total of 464 (77.1%) of the study population reported pressure area pain on skin assessed clinically as normal, altered or with a category 1 pressure ulcer and, of these, 130 (28.0%) developed a category 2 (or above) pressure ulcer compared with 22 (15.9%) patients with no pain at baseline (Table 14).
| Characteristic | Develops new PU (n = 152) | Does not develop new PU (n = 450) | Total (n = 602) |
|---|---|---|---|
| Age | |||
| Mean (SD) (years) | 78.1 (12.0) | 77.1 (13.0) | 77.3 (12.7) |
| Median (range) (years) | 81 (25–99) | 80 (21–101) | 80 (21–101) |
| < 65 years, n (%) | 21 (22.1) | 74 (77.9) | 95 (15.8) |
| 65–74 years, n (%) | 29 (25.4) | 85 (74.6) | 114 (18.9) |
| 75–84 years, n (%) | 51 (24.9) | 154 (75.1) | 205 (34.1) |
| ≥ 85 years, n (%) | 51 (27.1) | 137 (72.9) | 188 (31.2) |
| Sex, n (%) | |||
| Male | 68 (29.2) | 165 (70.8) | 233 (38.7) |
| Female | 84 (22.8) | 285 (77.2) | 369 (61.3) |
| Ethnicity, n (%) | |||
| Caucasian | 151 (25.1) | 450 (74.9) | 601 (99.8) |
| Non-Caucasian | 1 (100.0) | 0 (0.0) | 1 (0.2) |
| Is the patient diabetic, n (%) | |||
| Yes | 47 (30.7) | 106 (69.3) | 153 (25.4) |
| No | 105 (23.4) | 343 (76.6) | 448 (74.4) |
| Missing | 0 (0.0) | 1 (100.0) | 1 (0.2) |
| History of weight loss, n (%) | |||
| Yes | 38 (25.9) | 109 (74.1) | 147 (24.4) |
| No | 114 (25.1) | 340 (74.9) | 454 (75.4) |
| Missing | 0 (0.0) | 1 (100.0) | 1 (0.2) |
| BMI | |||
| Mean (SD) (kg/m2) | 27.0 (10.0) | 26.6 (9.3) | 26.7 (9.5) |
| Median (range) (kg/m2) | 25 (11–91) | 25 (11–111) | 25 (11–111) |
| Underweight (< 18.5 kg/m2), n (%) | 16 (25.0) | 48 (75.0) | 64 (10.6) |
| Normal weight (18.5 to < 25 kg/m2), n (%) | 61 (27.2) | 163 (72.8) | 224 (37.2) |
| Overweight (25 to < 30 kg/m2), n (%) | 30 (19.2) | 126 (80.8) | 156 (25.9) |
| Obese (≥ 30 kg/m2), n (%) | 38 (28.4) | 96 (71.6) | 134 (22.3) |
| Missing, n (%) | 7 (29.2) | 17 (70.8) | 24 (4.0) |
| Chronic wounds, n (%) | |||
| Yes | 45 (35.4) | 82 (64.6) | 127 (21.1) |
| No | 107 (22.6) | 367 (77.4) | 474 (78.7) |
| Missing | 0 (0.0) | 1 (100.0) | 1 (0.2) |
| Skin alterations at baseline, n (%) | |||
| Yes | 109 (29.2) | 264 (70.8) | 373 (62.0) |
| No | 43 (18.8) | 186 (81.2) | 229 (38.0) |
| Category 1 at baseline, n (%) | |||
| Yes | 105 (36.2) | 185 (63.8) | 290 (48.2) |
| No | 47 (15.1) | 265 (84.9) | 312 (51.8) |
| Existing category 2 or above at baseline, n (%) | |||
| Yes | 54 (32.9) | 110 (67.1) | 164 (27.2) |
| No | 98 (22.4) | 340 (77.6) | 438 (72.8) |
| Pain at a healthy, altered or category 1 skin site, n (%) | |||
| Yes | 130 (28.0) | 334 (72.0) | 464 (77.1) |
| No | 22 (15.9) | 116 (84.1) | 138 (22.9) |
| Analgesic use, n (%) | |||
| Yes | 137 (25.0) | 411 (75.0) | 548 (91.0) |
| No | 15 (27.8) | 39 (72.2) | 54 (9.0) |
| Braden mobility subscale, n (%) | |||
| Completely immobile | 5 (23.8) | 16 (76.2) | 21 (3.5) |
| Very limited | 61 (23.3) | 201 (76.7) | 262 (43.5) |
| Slightly limited | 63 (27.3) | 168 (72.7) | 231 (38.4) |
| No limitation | 23 (26.1) | 65 (73.9) | 88 (14.6) |
| Setting, n (%) | |||
| Acute | 98 (24.7) | 299 (75.3) | 397 (65.9) |
| Community | 54 (26.3) | 151 (73.7) | 205 (34.1) |
The results of the univariate analysis are presented in Table 15. Factors that had a statistically significant association with the odds of developing a new category 2 or above pressure ulcer included presence of skin alterations (OR 1.79, 95% CI 1.20 to 2.66; p = 0.0045), presence of at least one category 1 pressure ulcer (OR 3.20, 95% CI 2.63 to 4.74; p < 0.0001) and presence of pain on a healthy, altered or category 1 skin site at baseline (OR 2.05, 95% CI 1.25 to 3.38; p = 0.0047). There was marginal evidence that diabetic status was associated with the odds of developing a new category 2 or above pressure ulcer, with the odds higher for patients with diabetes than for patients without diabetes (OR 1.45, 95% CI 0.97 to 2.18; p = 0.0722). The following factors were not statistically significant: age, history of previous weight loss, Braden mobility subscale score, setting and analgesic use. Therefore, there was no evidence of an association between these factors and the odds of developing a new category 2 or above pressure ulcer.
| Covariate | OR | 95% CI | p-value |
|---|---|---|---|
| Age (continuous) | 1.01 | 0.99 to 1.022 | 0.3936 |
| Age (categorical) (reference = ‘< 65 years’) | |||
| ≥ 85 years | 1.31 | 0.73 to 2.35 | 0.4192 |
| 65–74 years | 1.20 | 0.63 to 2.29 | 0.3885 |
| 75–84 years | 1.17 | 0.65 to 2.08 | 0.2996 |
| Diabetic statusa (yes vs. no) | 1.45 | 0.97 to 2.18 | 0.0722 |
| History of weight lossb (yes vs. no) | 1.04 | 0.68 to 1.60 | 0.8462 |
| Braden mobility subscale score (1 or 2 vs. 3 or 4) | 1.21 | 0.84 to 1.76 | 0.3055 |
| Skin alterations (yes vs. no) | 1.79 | 1.20 to 2.66 | 0.0045 |
| Category 1 PU (yes vs. no) | 3.20 | 2.63 to 4.74 | < 0.0001 |
| Setting (acute vs. community) | 0.92 | 0.62 to 1.35 | 0.6576 |
| Analgesic use (yes vs. no) | 0.87 | 0.46 to 1.62 | 0.6542 |
| Pain on healthy, altered or category 1 skin site (yes vs. no) | 2.05 | 1.25 to 3.38 | 0.0047 |
| Covariates considered for overdispersion analysis | |||
| Gender (female vs. male) | 0.72 | 0.49 to 1.04 | 0.0780 |
| BMI (continuous) | 1.00 | 0.99 to 1.02 | 0.6702 |
| Braden sensory subscale (reference = no impairment) | |||
| Slightly limited | 0.92 | 0.54 to 1.58 | 0.6886 |
| Very limited | 0.41 | 0.05 to 3.40 | |
| Braden moisture subscale (reference = rarely moist) | |||
| Occasionally moist | 1.83 | 1.22 to 2.74 | 0.0277 |
| Very moist | 1.11 | 0.51 to 2.44 | |
| Constantly moist | 2.39 | 0.39 to 14.55 | |
| Braden activity subscale (reference = bedfast) | |||
| Chairfast | 1.83 | 1.05 to 3.19 | 0.0775 |
| Walks occasionally | 1.43 | 0.77 to 2.65 | |
| Walks frequently | 0.75 | 0.23 to 2.40 | |
| Braden nutrition subscale (reference = excellent) | |||
| Adequate | 0.93 | 0.59 to 1.47 | 0.6058 |
| Probably inadequate | 1.26 | 0.77 to 2.06 | |
| Very poor | 1.10 | 0.37 to 3.23 | |
| Braden friction and shear subscale (reference = no apparent problem) | |||
| Potential problem | 1.06 | 0.62 to 1.82 | 0.8036 |
| Problem | 1.22 | 0.64 to 2.36 | |
| Mattress category (reference = dynamic high-risk pressure relieving) | |||
| Static risk pressure relieving | 1.28 | 0.87 to 1.87 | 0.2430 |
| Non-pressure relieving | 0.95 | 0.41 to 2.17 | |
| Chronic wound | 1.89 | 1.24 to 2.88 | 0.0032 |
| Category 2 or above PU | 1.70 | 1.15 to 2.53 | 0.0083 |
The final multivariable model obtained from the variable selection carried out included three variables: the presence of a category 1 pressure ulcer (OR 3.25, 95% CI 2.17 to 4.86; p < 0.0001), the presence of skin alterations (OR 1.98, 95% CI 1.30 to 3.00; p = 0.0014) and the presence of pain on a healthy, altered or category 1 skin site at baseline (OR 1.56, 95% CI 0.93 to 2.63; p = 0.0931) (Table 16). Therefore, there was significant evidence that the presence of a category 1 pressure ulcer and presence of skin alterations are risk factors for developing a category 2 or above ulcer. After adjusting for these risk factors, there was marginal evidence that the presence of pain is a further risk factor for developing a category 2 or above pressure ulcer.
| Covariate | OR | 95% CI | p-value |
|---|---|---|---|
| Category 1 PU (yes vs. no) | 3.25 | 2.17 to 4.86 | < 0.0001 |
| Skin alterations (yes vs. no) | 1.98 | 1.30 to 3.00 | 0.0014 |
| Pain on healthy, altered or category 1 skin site (yes vs. no) | 1.56 | 0.93 to 2.63 | 0.0931 |
Overdispersion analysis
The model obtained in the primary end point analysis was further developed by considering the inclusion of other variables for which data were collected using further forwards and backwards stepwise variable selection. The results of the univariate analyses are shown in Table 15.
The final multivariable model obtained from the further variable selection carried out included six variables. These included (1) the presence of a category 1 pressure ulcer (OR 3.20, 95% CI 2.11 to 4.85; p < 0.0001), (2) the presence of skin alterations (OR 1.90, 95% CI 1.24 to 2.91; p = 0.0032), (3) the presence of pain on a healthy, altered or category 1 skin site at baseline (OR 1.85, 95% CI 1.07 to 3.20; p = 0.0271), (4) the presence of a category 2 ulcer (OR 2.09, 95% CI 1.35 to 3.23; p = 0.0009), (5) the presence of a chronic wound (OR 1.66, 95% CI 1.04 to 2.62, p = 0.0277; Table 17). In addition, (6) there was significant evidence that Braden activity was related to the odds of developing a new category 2 or above pressure ulcer (p = 0.0476). As shown in Table 17, the odds of developing a category 2 or above ulcer were greater for patients who were chairfast than for patients who were bedfast (OR 1.86, 95% CI 1.03 to 2.29) whereas there was no evidence that patients who walk occasionally or frequently were more likely to develop a category 2 or above pressure ulcer than patients who were bedfast (the 95% CIs for the ORs straddle 1). After adjusting for these risk factors, there was significant evidence that the presence of pain is a further risk factor for developing a category 2 or above pressure ulcer.
| Covariate | OR | 95% CI | p-value |
|---|---|---|---|
| Category 1 PU | 3.20 | 2.11 to 4.85 | < 0.0001 |
| Skin alterations | 1.90 | 1.24 to 2.91 | 0.0032 |
| Pain on a healthy, altered or category 1 skin site | 1.85 | 1.07 to 3.20 | 0.0271 |
| Category 2 PU | 2.09 | 1.35 to 3.23 | 0.0009 |
| Braden activity: chairfast vs. bedfast | 1.86 | 1.03 to 3.36 | 0.0476 |
| Braden activity: walks occasionally vs. bedfast | 1.19 | 0.62 to 2.29 | |
| Braden activity: walks frequently vs. bedfast | 0.71 | 0.21 to 2.46 | |
| Chronic wound | 1.66 | 1.06 to 2.62 | 0.0277 |
Correlations between explanatory variables were examined and each of the comparisons yielded low associations. Table 18 shows the associations between the explanatory variables. Cross-tabulations of explanatory variables were also produced for further information and are shown in Appendix 9.
| Explanatory variable 1 | Explanatory variable 2 | Correlation coefficient | Level of association |
|---|---|---|---|
| Category 1 PU | Skin alterations | –0.07 | Low |
| Category 1 PU | Pain on a category 0, 1 or A skin site | 0.19 | Low |
| Category 1 PU | Category 2 PU | –0.02 | Low |
| Category 1 PU | Braden activity | –0.01 | Low |
| Category 1 PU | Chronic wound | 0.09 | Low |
| Skin alterations | Pain on a category 0, 1 or A skin site | 0.11 | Low |
| Skin alterations | Category 2 PU | –0.02 | Low |
| Skin alterations | Braden activity | –0.10 | Low |
| Skin alterations | Chronic wound | 0.07 | Low |
| Pain on a category 0, 1 or A skin site | Category 2 PU | –0.21 | Low |
| Pain on a category 0, 1 or A skin site | Braden activity | –0.16 | Low |
| Pain on a category 0, 1 or A skin site | Chronic wound | 0.07 | Low |
| Category 2 PU | Braden activity | –0.02 | Low |
| Category 2 PU | Chronic wound | 0.04 | Low |
| Braden activity | Chronic wound | –0.15 | Low |
Time-to-event analysis
The variables that were statistically significantly (at the 10% level) associated with the time to onset of a new category 2 or above pressure ulcer in the univariate analyses were age [acceleration factor (AF) 1.01, 95% CI 1.00 to 1.03; p = 0.0354], Braden mobility subscale score (AF 1.37, 95% CI 1.00 to 1.87; p = 0.0498), the presence of a category 1 pressure ulcer (AF 2.63, 95% CI 1.93 to 3.58; p < 0.0001) and the presence of pain on a healthy, altered or category 1 skin site (AF 2.68, 95% CI 1.86 to 3.86; p < 0.0001). In addition, there was marginal evidence that the presence of skin alterations was associated with the time to onset of a new category 2 or above pressure ulcer (AF 1.40, 95% CI 0.99 to 1.97; p = 0.0593; Table 19).
| Factor | Ratioa of time to develop new category 2 or above PU | 95% CI | p-value |
|---|---|---|---|
| Age (continuous) | 1.01 | 1.00 to 1.03 | 0.0354 |
| Skin alterations (yes vs. no) | 1.40 | 0.99 to 1.97 | 0.0593 |
| Analgesic use (yes vs. no) | 1.05 | 0.61 to 1.83 | 0.8577 |
| Braden mobility (1 or 2 vs. 3 or 4) | 1.37 | 1.00 to 1.87 | 0.0498 |
| Category 1 PU (yes vs. no) | 2.63 | 1.93 to 3.58 | < 0.0001 |
| Diabetic (yes vs. no) | 1.24 | 0.85 to 1.80 | 0.2591 |
| History of previous weight loss (yes vs. no) | 0.90 | 0.62 to 1.30 | 0.5715 |
| Pain (yes vs. no) | 2.68 | 1.86 to 3.86 | < 0.0001 |
| Setting (acute vs. community) | 1.13 | 0.81 to 1.58 | 0.4652 |
The final accelerated failure time multivariable model obtained from forwards and backwards variable selection included the covariates presence of a category 1 pressure ulcer and presence of pain on a healthy, altered or category 1 skin site and so both are risk factors for reducing the time to develop a category 2 or above pressure ulcer (Table 20). Unlike the primary analysis, the presence of skin alterations has not been included in the final model but was shown to have a significant association with time to onset of a new category 2 or above pressure ulcer in the univariate analysis and therefore may explain why skin alterations were not included in the final model. The cumulative incidence function for the presence of skin alterations is very similar to the cumulative incidence function for the presence of pain on a healthy, altered or category 1 pressure ulcer skin site, although the cumulative incidence functions indicate that there is a larger difference between pain categories than between skin alteration categories. The final model shows that patients are likely to develop a new category 2 or above pressure ulcer 2.32 times faster if they have a category 1 pressure ulcer at baseline than if they do not have a category 1 pressure ulcer at baseline (AF 2.32, 95% CI 1.73 to 3.12; p < 0.0001). In addition, patients are likely to develop a new category 2 or above pressure ulcer 2.28 times faster if they have pressure area-related pain at a healthy, altered or category 1 skin site than if they do not have pressure area-related pain at a healthy, altered or category 1 skin site (AF 2.28, 95% CI 1.59 to 3.27; p < 0.0001).
| Factor | Ratioa of time to develop new category 2 or above PU | 95% CI | p-value |
|---|---|---|---|
| Category 1 PU (yes vs. no) | 2.32 | 1.73 to 3.12 | < 0.0001 |
| Pain (yes vs. no) | 2.28 | 1.59 to 3.27 | < 0.0001 |
Cumulative incidence functions for the presence of skin alterations, the presence of a category 1 pressure ulcer and the presence of pain on a healthy, altered or category 1 skin site at baseline are presented in Figure 9.
FIGURE 9.
Cumulative incidence plots. (a) Cumulative incidence plot of time to develop a new category 2 pressure ulcer by presence of skin alterations at baseline; (b) cumulative incidence plot of time to develop a new category 2 pressure ulcer by presence of a category 1 PU at baseline; and (c) cumulative incidence plot of time to develop a new category 2 pressure ulcer by presence of pain on a healthy, altered or category 1 pressure ulcer skin site at baseline.
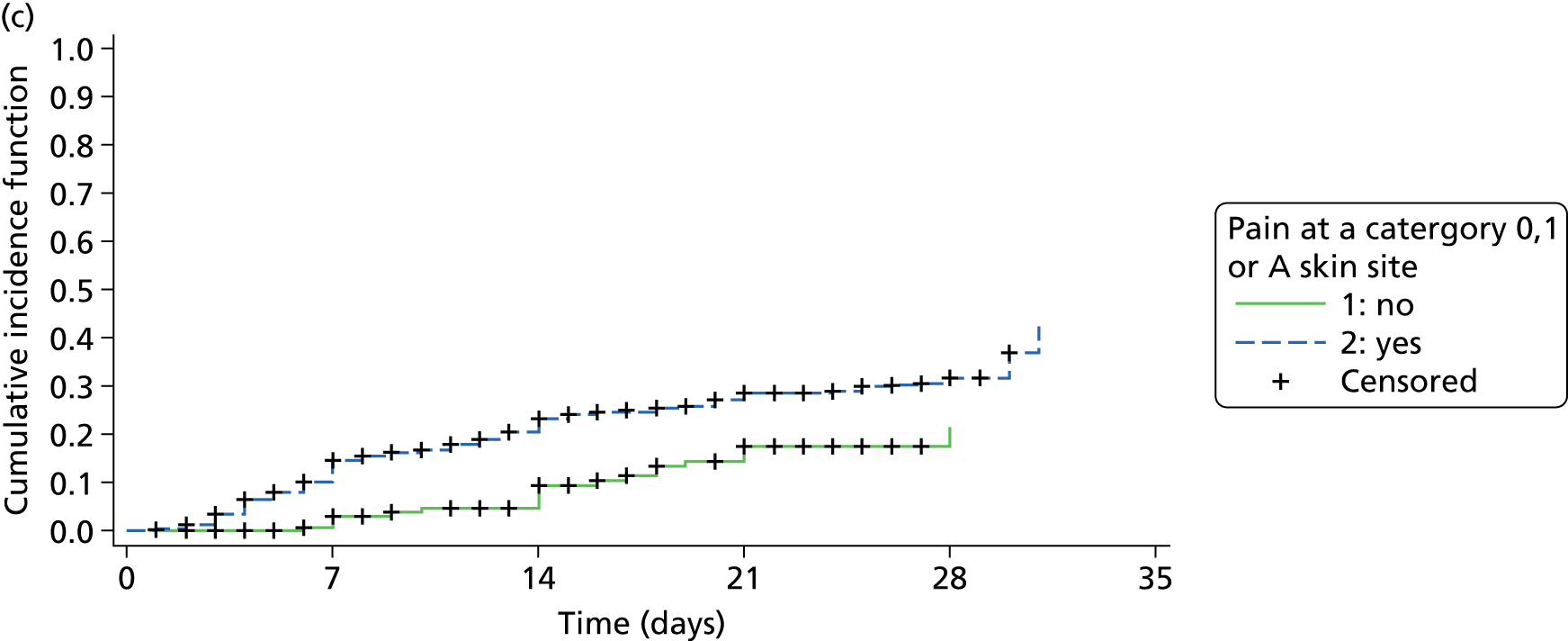
Skin site-level analysis
The analysis population of 602 patients had a combined total of 7863 potential skin sites assessed (Table 21), of which the majority (77.5%) were observed as being healthy skin sites. Pain was reported more frequently with more severe skin status, that is, 63.1% of category 1 skin sites were observed to have pain compared with 40.3% of skin sites with alterations and 6.4% of healthy skin sites (see Table 21).
| Skin classification | Pain yes, n (%) | Pain no, n (%) | Missing, n (%) | Total, n (%) |
|---|---|---|---|---|
| Normal skin | 390 (6.4) | 5700 (93.5) | 6 (0.1) | 6096 (77.5) |
| Skin alterations | 342 (40.3) | 504 (59.4) | 3 (0.4) | 849 (10.8) |
| Category 1 | 351 (63.1) | 205 (36.9) | 0 (0.0) | 556 (7.1) |
| Category 2 | 116 (78.4) | 32 (21.6) | 0 (0.0) | 148 (1.9) |
| Category 3 | 13 (100.0) | 0 (0.0) | 0 (0.0) | 13 (0.2) |
| Category 4 | 3 (75.0) | 1 (25.0) | 0 (0.0) | 4 (0.1) |
| Unstageable | 6 (85.7) | 1 (14.3) | 0 (0.0) | 7 (0.1) |
| Unable to assess | 6 (7.6) | 52 (65.8) | 21 (26.6) | 79 (1.0) |
| Not applicable | 2 (2.3) | 26 (29.9) | 59 (67.8) | 87 (1.1) |
| Other wound | 0 (0.0) | 6 (85.7) | 1 (14.3) | 7 (0.1) |
| Classification missing | 0 (0.0) | 0 (0.0) | 17 (100.0) | 17 (0.2) |
| Total | 1229 (15.6) | 6527 (83.0) | 107 (1.4) | 7863 (100.0) |
Of the total 7863 skin sites, 7483 (95.2%) were evaluable in the analysis, that is, all skin sites that were observed as being healthy, altered or category 1 at baseline and which had at least one follow-up assessment (i.e. the end point could be derived for that skin site). Overall, 223 (3.0%) of the evaluable skin sites developed a new category 2 or above pressure ulcer, and the incidence for skin sites with pain at baseline was higher at 10.3% than that for skin sites with no pain at baseline (1.7%) (Table 22). Similarly, the incidence of a new category 2 or above pressure ulcer was observed to increase with the severity of skin status at baseline (i.e. the incidence for skin sites with a category 1 pressure ulcer at baseline was 18.2% compared with 6.4% for sites with skin alterations and 1.1% for healthy skin sites at baseline).
| Variable | New PU (n = 223, 3.0%), n (%) | No new PU (n = 7260, 97%), n (%) | Total (n = 7483), n (%) |
|---|---|---|---|
| Pain | |||
| Yes | 111 (10.3) | 966 (89.7) | 1077 (14.4) |
| No | 112 (1.7) | 6294 (98.3) | 6406 (85.6) |
| Skin status | |||
| Healthy intact skin | 68 (1.1) | 6014 (98.9) | 6082 (81.3) |
| Alterations to intact skin | 54 (6.4) | 792 (93.6) | 846 (11.3) |
| Category 1 | 101 (18.2) | 454 (81.8) | 555 (7.4) |
The results of the univariate analysis are presented in Table 23. Factors that had a statistically significant association with the odds of developing a new category 2 or above pressure ulcer were skin status, which consisted of two levels – skin alterations (OR 6.29, 95% CI 4.21 to 9.40; p < 0.0001) and category 1 pressure ulcer (OR 27.34, 95% CI 18.5 to 40.4; p < 0.0001) – and the presence of pain at baseline on a healthy, altered or category 1 skin site (OR 8.68, 95% CI 6.30 to 11.97; p < 0.0001). The following factors were not statistically significant: age, diabetic status, history of previous weight loss, Braden mobility subscale score, setting and analgesic use. Therefore, there was no evidence of an association between these factors and the odds of developing a new category 2 or above pressure ulcer at the skin site level.
| Covariate | OR | 95% CI | p-value |
|---|---|---|---|
| Age (continuous) | 1.01 | 0.99 to 1.02 | 0.4808 |
| Diabetic statusa (no vs. yes) | 0.81 | 0.54 to 1.21 | 0.3070 |
| History of weight lossb (no vs. yes) | 1.03 | 0.68 to 1.57 | 0.8914 |
| Braden mobility subscale score (1 or 2 vs. 3 or 4) | 1.14 | 0.80 to 1.64 | 0.4714 |
| Skin status (reference = healthy skin) | |||
| Skin alterations | 6.29 | 4.21 to 9.40 | < 0.0001 |
| Category 1 PU | 27.34 | 18.5 to 40.4 | < 0.0001 |
| Setting (acute vs. community) | 0.91 | 0.63 to 1.32 | 0.6148 |
| Analgesic use (no vs. yes) | 1.33 | 1.74 to 2.39 | 0.3350 |
| Pain on healthy, altered or category 1 skin site (yes vs. no) | 8.68 | 6.30 to 11.97 | < 0.0001 |
The final multivariable model obtained from the variable selection carried out included two variables: skin status, which consisted of two levels – skin alterations (OR 4.65, 95% CI 3.01 to 7.18; p < 0.0001) and category 1 pressure ulcer (OR 17.30, 95% CI 11.09 to 27.00; p < 0.0001) – and the presence of pain on a healthy, altered or category 1 skin site (OR 2.25, 95% CI 1.53 to 3.29; p < 0.0001; Table 24). Therefore, there was significant evidence that skin status is a risk factor for developing a category 2 or above pressure ulcer after adjusting for between-patient variation. After adjusting for skin status and between-patient variation there was strong evidence that the presence of pain is a further risk factor for developing a category 2 or above pressure ulcer.
| Covariate | OR | 95% CI | p-value |
|---|---|---|---|
| Skin status (reference = healthy skin) | |||
| Skin alterations | 4.65 | 3.01 to 7.18 | < 0.0001 |
| Category 1 PU | 17.30 | 11.09 to 27.00 | < 0.0001 |
| Pain (yes vs. no) | 2.25 | 1.53 to 3.29 | < 0.0001 |
Discussion
The prevalence studies are the first to assess pressure area-related pain in large representative hospital and community populations. In the hospital population, of 2010 patients asked the pain screening questions, 327 indicated that they had pain on one or more pressure areas (skin site not recorded), providing an overall unattributed pressure area-related pain prevalence of 16.3%. The importance of the inclusion of patients without pressure ulcers is emphasised by the finding that 12.6% (223/1769) of hospital patients without pressure ulcers reported pressure area-related pain. 35 There are no other published reports of this type of pain. The prevalence of unattributed pressure area-related pain in patients with pressure ulcers was 43.2% (104/241) in hospital patients and 75.6% (133/176) in community patients. This is similar to the results of other smaller studies reporting pressure ulcer pain prevalence, with prevalence ranging from 37% to 100%,40–43,66,67 and is comparable to the prevalence of pain in other chronic wounds in European populations. 43,68–70
The detailed pain assessment of 160 hospital and 37 community patients identified pressure area-related pain on skin areas assessed as normal as well as on all grades of pressure ulcer. ‘The distribution of pain intensity measured using a 0–10 nominal rating scale was similar for all grades, which is consistent with pain intensity in other disease states, where the severity of illness is not necessarily related to patients’ reports of pain intensity. 68,71
It is noteworthy that in the community patient population, none of the patients reported pressure area related pain on a skin site assessed as normal’45 whereas, of the 75 hospital detailed pain assessment patients with a pressure ulcer, 30 (40%) did report pressure area-related pain on a skin site assessed as normal.
The dominant type of pain in hospital patients was inflammatory pain (70.3% torso and 60.3% limb skin sites) whereas in the community patients neuropathic pain was dominant (54.5% torso and 61.1% limb skin sites). This is consistent with the prevalence of neuropathic pain in leg ulcer patients. For example, in a prospective longitudinal cohort study of painful leg ulceration, 43.5% of respondents (n = 96) reported neuropathic pain. 68 It is noteworthy that both inflammatory and neuropathic pain was observed for normal skin and all grades of pressure ulcer for both hospital and community patients. We did not record the duration of the pain or pressure ulcer and this may be related to the type of pain and is an area of further study.
The ‘limitations of the overall pain prevalence estimate of unattributed pain are that: skin assessment data were recorded by clinical staff, which has inherent limitations37,56,72 and may have resulted in over or under-reporting of pressure ulcers or misclassification of Grade or extent of tissue damage, particularly at Grade 1, which is prone to misclassification;’45,56 the pain prevalence data were recorded at the patient level and not by skin site; we were not able to record pain treatment and therefore the quality of pain management may differ between wards/hospital/community settings and impact on pain reports; and the methodology used meant that a significant proportion of hospital (40.8%) and community (38.7%) patients were not able to participate in the pain prevalence study because of illness (too unwell, end of life, unconscious), difficulty in assessing pain (confused or communication difficulty) or unavailability (unable to disturb, off the ward, in isolation).
Prevalence studies provide a good measure of the extent of chronic long-lived disease and are less reliable in the measurement of short-lived disease. Both pressure ulcers and pain can be both long-lived and short-lived and as such there are general limitations associated with prevalence estimates for these conditions. In the hospital population, pressure ulcer prevalence rates are affected by staff training and awareness, admission/discharge rates and case mix, and in the interpretation of hospital prevalence rates these factors need to be taken into account. 2,73–75
‘Prevalence studies in the community are challenging and time-consuming to undertake. Few have been undertaken4,76,77 and the true extent of the pressure ulcer problem at a population level is not well quantified. In this study, in two localities in the North of England we report a prevalence . . . of 0.77 people with pressure ulcers per 1000 adult population [locality 1] and . . . 0.40 per 1000 adult population [locality 2]. The prevalence rates are similar to those reported by Vowden and Vowden,4 who reported a community prevalence rate of 0.66 per adult population.’47
‘Several previous studies have reviewed the methods for data collection for determining pressure ulcer prevalence and incidence. 2,73,74 This study provides further evidence that different methods used for case ascertainment result in potentially important variations in the prevalence reported. Locality 1 collected data for a much larger group of patients where pressure ulcer status not considered prior to assessment, whereas locality 2 collected data only for patients known to have a pressure ulcer.’47 A notable difference between the two localities resulting from the different method applied was the location of patients assessed. In locality 1, 50.3% of patients were in nursing homes, whereas in locality 2 only 4.9% of patients were in nursing homes. ‘As indicated in the methodology section, in the UK, nursing homes would normally treat patients with pressure ulcers themselves and only patients with complex wounds are referred to the community nursing service.’48 In locality 1 all nursing home residents were included in the prevalence study whereas in locality 2 only those patients known to community nursing services were included, ‘highlighting the importance of clear methodological descriptions and effective case ascertainment for study comparison and establishing the true prevalence of pressure ulcers’ in a community setting. 48
Our sample size estimates were based on limited pressure ulcer pain prevalence data,2,15 with no available data for grade 1 or normal skin. The prevalence rates observed were much higher than those estimated in patients with grade 1 and normal skin. In addition, the sample size calculation planned for the inclusion of 6000 patients from two community NHS trusts. However, this was an approximation as estimating the denominator population for these trusts was difficult as no data were available regarding the number of patients on the community nursing caseload. As a result of this and because of differences in the implementation of pressure ulcer prevalence audit methods in the two localities, the number of community patients included in the prevalence audit was significantly less than planned: locality 1 audited 1680 patients including those on the community nursing caseload and in nursing homes, residential homes and palliative care environments, whereas locality 2 audited only those 102 patients who were on the community nursing caseload and were known to have a pressure ulcer. In addition, both community trusts asked the pain questions only of those patients with pressure ulcers, whereas in the acute trusts the pain questions were asked of all patients, regardless of whether or not they had a pressure ulcer. As a result, 2010 patients were asked the pain questions in the acute trusts compared with 176 in the community trusts. Because of the differing methodologies employed by the acute and community trusts, the two populations cannot be combined and therefore the confidence boundaries for the overall unattributed pressure area-related pain prevalence provided in the sample size calculation have been updated post hoc for the two settings. The hospital patient population of 2010 patients allows us to estimate the unattributed pressure area-related pain prevalence of 16.3% to within ±1.7%. The community sample of 176 patients allows us to estimate the unattributed pressure area-related pain prevalence of 75.6% to within ±6.4%.
Cohort study
The pain cohort study is the first risk factor study to investigate the role of pain as a factor independently predictive of subsequent category 2 (or above) pressure ulcer development. It found that pain was an independent predictor of category 2 (or above) pressure ulcer development in high-risk hospital and community patients with acute illness.
Building on our previous research in the field37,60,72,78 and in our attempts to maximise the potential event rate and so minimise the required sample size, the pressure ulcer incidence rate of 25.2% was higher than predicted. This was achieved by the inclusion of patients with evidence of acute illness and at ‘high risk’, where ‘high risk’ defined as one or more of the following: bedfast/chairfast and completely immobile/very limited mobility;51 localised skin pain on any pressure area skin site; and category 1 pressure ulcer on any pressure area skin site. The incidence rate is comparable to that in other reports of the incidence of category 2 and above pressure ulcers. In our systematic review of risk factor studies46 (see Chapter 5), 19 studies reported incidence rates for grade/stage 2 and above pressure ulcers, ranging from 10.1% to 45.7% in heterogeneous patient populations. Of importance in terms of generalisability was our recruitment of community patients. The observed incidence rate in the community patient population was 26.3%, although it is noteworthy that the majority of ‘community’ patients were recruited from rehabilitation units, with only small numbers recruited in the home.
The age of our patient population was higher than expected from our previous prospective research,40–43 with a median of 81 years and 31.2% of those recruited aged > 85 years. The age profile is, however, consistent with the community pressure ulcer prevalence population (median 81 years; see Pain prevalence in hospital and community populations, Results) and suggests that the population is representative of high-risk patients.
An unexpected finding was the high proportion of patients (77.1%) who reported pain at baseline. Although a number of these patients also had category 1 pressure ulcers, the extent of the problem was underestimated in our sample size estimate (the sample size assumption was that 10% of patients would report pain at baseline). The sample size calculation assumed that at inception 15% of patients would have pain and that those would be an incidence rate of 10% in those patients with pain at baseline and 24.4% in those without pain. However, the incidence rate for those who reported pressure area pain on skin assessed clinically as normal, altered or category 1 was observed to be 28.0% and the incidence rate for those patients with no pain at baseline was observed to be 15.9%. Assuming an incidence of 28.0% for those with pain and 15.9% for those without pain, and that the proportion of patients with pain is equal to 77.1%, we would have required a reduced sample size of 535 patients to detect a difference between those with pain and those without pain at baseline with 80% power. Therefore, based on the data observed, we had an increase in power to 84% to detect a statistically significant difference in pressure ulcer incidence between those with pain and those without pain at baseline.
There was significant evidence that the presence of pain at a skin site is an independent predictor for developing a category 2 or above pressure ulcer, after adjusting for skin status (i.e. healthy, skin alterations, category 1 pressure ulcer) at baseline, across all four multivariable models.
At a patient level, the presence of pain on at least one skin site (healthy, altered or category 1 skin status) increased the odds of developing a category 2 or above pressure ulcer by 30 days of follow-up (primary end point) and also reduced the time to develop a category 2 or above ulcer compared with if pain is not present after adjusting for skin status.
At a skin-site level, the presence of pain is a predictor for developing a category 2 or above pressure ulcer on the same skin site by 30 days of follow-up, after adjusting for skin status and between-patient variation.
The other risk factors that emerged throughout the multivariable analyses included the presence of a category 1 pressure ulcer at baseline, consistent with all four previous studies,37,60,79,80 which have included this as a variable in multivariable modelling. The presence of alterations to intact skin has also emerged in multivariable modelling in 9 of 10 studies where it has been included as a variable46 (see Chapter 5, Research overview).
The study was designed to incorporate key quality criteria for the conduct and reporting of risk factor/prognostic factor studies81–89 to promote generalisability and minimise bias. The study design, including an a priori sample size estimate and data monitoring, ensured that there was a sufficient number of events to undertake robust statistical modelling incorporating key risk factors determined through systematic review and the development of a conceptual framework. The primary outcome, the development of a new category 2 or above pressure ulcer, provides the most reliable outcome measure. 56 Clear inclusion and exclusion criteria were applied, screening logs were maintained to assess the generalisability of the study population and trained clinical research nurses undertook all baseline and follow-up assessments providing high-quality data and minimising loss to follow-up. Patients were recruited from both acute and community settings, which were representative of UK ‘standard care’. All centres had pressure ulcer prevention and management policies and guidelines in place, including risk assessment, mattress provision, turning (and so on). The majority of patients received the recommended National Institute for Health and Care Excellence (NICE) standard mattress provision of either a high-specification foam mattress or an alternating pressure mattress15 and members of the research team did not alter standard care provision as determined by the local ward/community teams, who remained responsible for clinical care.
The limitations of the study included a lack of blinded outcome assessment. This could have been achieved if baseline and follow-up assessments had been undertaken by two different research nurses; however, there was not the funding or capacity within the tissue viability teams for this approach. It is feasible that the research nurses could have introduced bias to the outcome assessment. The feasibility of using photography for independent blind outcome assessment is currently being determined as part of the HTA programme-funded PRESSURE 2 trial [for details see http://medhealth.leeds.ac.uk/info/423/skin/1717/pressure_2 (accessed 13 July 2015)].
It is acknowledged that the patient population is not representative of the general NHS population because of exclusion of patients who had cognition problems, patients who were very sick or terminally ill and patients who either were unable to provide consent or it was considered unethical to approach. However, as pain is a symptom of underlying inflammation and/or nerve damage we suggest that the results are generalisable to the wider population and that efforts to assess pain, soreness and discomfort are made for all patients using pain assessment methods established for this group of patients. 90–92
It is also acknowledged that patients with darkly pigmented skin were under-represented in the study population. As indicated above, however, as pain is a symptom of underlying inflammation and/or nerve damage we suggest that the results are generalisable to the wider population.
Patient and public involvement in the pain workstream
As with all of the PURPOSE studies, high-level service user input has been present throughout via the steering committee. The majority of study-specific involvement has focused on interpreting and disseminating findings. The results of the pain studies were presented to PURSUN UK who reported that the work echoed many of its members’ own experiences. PURSUN UK members felt that pain is an important and often overlooked area and as such it is important that the results of this study are disseminated to front-line health professionals. They gave the project team feedback from the service user perspective on questions that remained unanswered, for example the lack of clear pain management strategies for pressure ulcers and the difficulties of assessing pressure area pain in complex, at-risk patients.
Three PURSUN UK members with experience of pressure ulcer/pressure area pain have worked with the PPI officer to develop written vignettes about their experiences. These narratives aim to illustrate the importance of the pain studies and put the findings in a real-life context. The vignettes will be included in a forthcoming publication, co-authored by three members of PURSUN UK and aimed at clinical nurses.
Conclusion
A major advantage of prevalence surveys is that they provide a general estimate of the extent of a problem. The results of this study provide a very strong indication that pressure area-related pain affects a significant minority of patients without pressure ulcers in hospital populations and that a substantial proportion of patients with pressure ulcers report pain. Pain severity is not related to severity of the ulcer and both inflammatory and neuropathic pain are observed. We nested the pain prevalence studies within routine pressure ulcer prevalence audits and in the community setting the case-finding method used was not standard. We observed different prevalence rates in the two localities, highlighting the importance of clear methodological descriptions and effective case ascertainment for study comparison and in establishing the true prevalence of pressure ulcers in a community setting.
We have also established that the presence of pain (on skin areas assessed as healthy, altered but intact or category 1) increases the probability of category 2 and above pressure ulcer development and accelerates the time to ulcer development. This is an area of practice that requires improved assessment, incorporation into risk assessment and treatment strategies to alleviate pain and reduce category 2 pressure ulcer development.
Chapter 4 Severe pressure ulcer study
Chapter written by Justin Keen, Susanne Coleman, Carol Dealey, Elizabeth McGinnis, Delia Muir, E Andrea Nelson, Malcolm Patterson, Lisa Pinkney, Nikki Stubbs, Lyn Wilson and Jane Nixon.
Abstract
Introduction: There is good evidence that pressure ulcer risks are associated with patients’ health status or their behaviour. There is also suggestive evidence that the organisation of treatment and care can influence patients’ risks. The principle research objective of this work package was to understand the ways in which the organisational context influences the development of severe pressure ulcers. A second, practical objective was to identify ways in which root cause analyses of reportable pressure ulcers could be improved on, to maximise the chances of learning from them.
Methods: The severe pressure ulcer work package comprised three pieces of work: (1) a retrospective case study based on eight patients who developed severe pressure ulcers; (2) a patient involvement workshop with PURSUN UK; and (3) development of a methodology for root cause analyses of critical incidents.
Results: For seven of the eight patients in the retrospective case study the best explanation of the evidence was that the general organisational context played a significant role in severe pressure ulcer development. In four accounts specific events contributed to development. One patient’s severe pressure ulcer was deemed to be unavoidable. Service users found the interactive workshop format, and the use of a ‘simulated patient’ account within it, valuable. A methodology for root cause analysis, rooted in current NHS practice but including novel components, can be used to improve the quality of the insights captured.
Conclusions: Severe pressure ulcers were more likely to develop in contexts characterised by one or more of clinicians failing to listen to patients or carers, clinicians failing to recognise and respond to clear signs that a patient had a pressure ulcer or was at risk of developing one, and services not being effectively co-ordinated. Presenting research data in live and interactive formats can make the interpretation process more engaging and accessible to service users and support meaningful dialogue between service users and professionals. Current best NHS practice in root cause analyses of reportable pressure ulcers should be augmented by interviews with patients and carers and by the construction of narratives based on key events. Our findings suggest that there is a need to move away from identification of root causes and towards broader explanations of events, based on identifying the ‘best fit’ between the available evidence and the explanations available in the patient safety literature.
Background
There are two distinct ways of thinking about patients’ risks of developing pressure ulcers. The first is based on the assumption that all pressure ulcer risks are associated with patients’ health status or their behaviour. Clinicians should therefore focus on identifying patients who are at risk, assess the nature and scale of their risks and design clinical interventions to reduce them. 46 This approach, which highlights the importance of risk assessment, informed our work on pain (see Chapter 3) and risk assessment (see Chapter 5). The second way of thinking starts from a different assumption, which is that the quality of treatment and care can also influence patients’ risks of developing pressure ulcers. Some environments are riskier than others so that patients who are at risk are more likely to develop pressure ulcers in settings where there is poor-quality treatment and care. The events at Mid Staffordshire NHS Foundation Trust, where at one point dozens of pressure ulcers were being reported every month, help to underline the significance of this point. 93
This study is rooted in the second way of thinking. It informs a number of Department of Health policies. For example, category 2 or above pressure ulcers, as rated on the EPUAP/NPUAP 1–4 scale,1 are classed as reportable incidents in official guidelines. 94 Category 3 and 4 pressure ulcers have to be reported as serious untoward incidents. Pressure ulcers are one of four patient safety indicators in the NHS Safety Thermometer and there are incentive payments for avoiding pressure ulcers in the Commissioning for Quality and Innovation (CQUIN) framework. 95 The NHS has a ‘no avoidable pressure ulcers’ goal and, as a result, pressure ulcer prevention is classed as a high-impact action for nursing and midwifery. 96 Yet there is limited evidence about the ways in which care processes influence the development of pressure ulcers and severe pressure ulcers. This study therefore focuses on the ways in which care processes influence the development of pressure ulcers by reconstructing events leading to the development of severe pressure ulcers in eight patients.
Aims and objectives
To implement national policies and reduce the incidence of pressure ulcers, clinicians need to understand how and why their actions increase or decrease the likelihood that patients will develop them. Our principal research objective was, accordingly, to explain the influence of organisational context on the development of pressure ulcers. Beyond this, we stated in the programme proposal that we would investigate the implications of our findings for the conduct of root cause analyses. A second, practical objective was therefore to identify ways in which root cause analyses of reportable pressure ulcers could be improved on, to maximise the chances of learning from them.
Research overview
The severe pressure ulcer work package comprised three pieces of work. The first was an empirical study designed to improve our understanding of the ways in which care processes influence the development of severe pressure ulcers, in which we constructed detailed retrospective accounts of the development of severe pressure ulcers in eight patients. The second presents the design and conduct of a patient involvement workshop, drawing on one of the accounts from the empirical study. The third sets out the development of a methodology for root cause analyses of reportable pressure ulcers.
Retrospective study of the development of severe pressure ulcers
Aim
We undertook an empirical study designed to improve our understanding of the development of severe pressure ulcers by constructing detailed retrospective accounts of the development of severe pressure ulcers in eight patients.
Methods
Design
The research design was substantially influenced by two arguments. The first stemmed from discussions at the start of the study. Although our principal objective concerned the effect of organisational context on the development of severe pressure ulcers, we realised that we could not simply assume that a relationship between the two existed and could be studied empirically. Indeed, the available empirical evidence is limited, but the literature offers three distinct explanations,97,98 namely:
-
pressure ulcers develop following a mistake made by an individual clinician99,100
-
they develop as a result of a sequence of otherwise unconnected mistakes101–103
-
there are systemic weaknesses in the organisation and delivery of care, such that the regime is one in which pressure ulcers are more likely to develop. 104–106
We decided that it would be necessary to discriminate between these candidate explanations to establish whether, and how, the organisational context helped to explain the development of severe pressure ulcers or alternatively played no role. We should also include two other logical possibilities, in order to identify their role in explaining development or eliminate them from consideration, namely (1) the behaviour of clinicians had no effect on the development of a pressure ulcer, which would have developed whatever they had done, and (2) there was an alternative explanation, which had not previously been reported or hypothesised.
The second argument flowed from the nature of the domain that we were studying. Severe pressure ulcers occur relatively rarely and can develop in a wide range of settings over periods of days or weeks. It is not currently possible to predict who will develop them and who will not; it is only possible to identify people who have already developed a severe pressure ulcer. A prospective study was therefore not feasible. We opted to identify patients who had developed severe pressure ulcers and to reconstruct what had happened to them. This led us to adopt a retrospective case study design. A process-tracing case study method was used to capture the experiences of eight individuals who had developed severe pressure ulcers. 107 Accounts of their experiences were developed, which were then compared and contrasted to identify common features and hence common explanations. The two arguments taken together led us to develop a novel research design.
Setting
Eight patients were recruited in six NHS trusts in Yorkshire, England. Four patients’ accounts occurred wholly or mainly in acute hospitals, three mainly in their own homes and one in a combination of a community hospital and an acute hospital rehabilitation ward. The decision to recruit eight patients was pragmatic: each account took approximately 4 months from initial interview to completion of analysis and we were therefore able to complete eight accounts with the resources available to this study.
Eligibility
Inclusion criteria
The study method was piloted with the first patient, who presented with few comorbidities, on the basis that the patient’s problems would be less likely to be confounded with organisational factors.
Subsequent patients were recruited from participating acute and community trusts if they:
-
had a current or previous category 3 or 4 pressure ulcer
-
were a hospital inpatient, hospital outpatient, intermediate care patient or community patient under the care of community nursing services.
Recruitment was designed to maximise the variation and presentation of severe (category 3 and 4) pressure ulcers, including anatomical site (e.g. heel, sacrum, buttocks).
Exclusion criteria
Patients were excluded if:
-
it was considered ethically inappropriate to approach them, for example those whose death was imminent
-
they were unable to tell the story (narrative) of their experience.
Recruitment and consent
Participants were sampled partly to maximise the diversity of individuals and the contexts in which they developed severe pressure ulcers and partly purposively (see Appendix 10). Eligible patients were identified by members of the local tissue viability nurse teams at one of the six study sites in Yorkshire, England, who informed them about the study and provided them with a study information leaflet and an ‘agree to be contacted by the researcher’ form (see Appendix 11). Consent to participate was obtained from individuals and, when appropriate, also from their main carers (see Appendix 12).
Data collection
Data were collected by a field researcher (LP) with a non-clinical background from five sources, namely interviews with individuals who had developed a severe pressure ulcer (and, when relevant, their main carers), interviews with clinical and other staff who had been involved in their care, clinical records, other documents relevant to the account such as critical incident reports, and relevant local policy documents (e.g. on the conduct of skin risk assessments) (Figure 10, stage 1). A parallel review of patient notes was undertaken by a tissue viability nurse at each study site.
FIGURE 10.
Analysis and review of individual accounts.
Patients were interviewed first and invited to give their account of the reasons why, in their view, their severe pressure ulcer had developed. Interviews were semistructured and lasted between 30 and 90 minutes. They were digitally recorded and transcribed.
The patient interview was used to direct the next phase of data collection, which involved accessing and reviewing nursing, medical and therapist notes, clinical incident reports and other documents (e.g. staff rotas for key periods of time in the patients’ accounts). An initial analysis of the documents was undertaken and, on the basis of the analysis and the patient account, an initial interview schedule was drawn up. The analysis was also used to identify members of staff who were likely to be able to provide useful information about the development of the severe pressure ulcer. After the initial interviews, the researcher discussed the emerging possible explanations with the site tissue viability nurse specialist and a list of further interviewees was agreed. It is worth noting that, for this and subsequent analyses, the focus was on understanding how severe pressure ulcers developed, but a range of contextual information was provided in interviewees’ responses and in the documentation provided that could be used to discriminate between the explanations identified earlier.
Interviewees for hospital-based accounts included matrons, ward nurses, health-care assistants, ward clerks, ward managers, physiotherapists and consultants. In community settings, interviewees included district nurses, home care assistants and therapists (Table 25). At least seven interviews were conducted for each account except for the eighth account, in which an individual developed a severe pressure ulcer in her own home after a fall and few health professionals had useful information about her circumstances. Few professionals were directly involved in identification of and response to her ulcer, and comprehensive notes were available about her assessment and treatment only once she came into contact with health services. In total, 70 interviews were conducted across the eight accounts. Judgements were made about the time period that each account needed to cover and the extent of the documentation that was needed. In some instances, both were extended when it became clear that the histories were longer or more complicated than at first appeared.
| Account | Individual | Carer | TVN | District nurse | Staff nurse | HCA | Consultant | Junior doctor | Physiotherapist | Occupational therapist | Ward clerk | Liaison nurse | Ward manager | Quality assurance manager | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 14 | |||
| 2 | 1 | 1 | 2 | 3 | 1 | 1 | 1 | 1 | 1 | 12 | |||||
| 3 | 1 | 1 | 2 | 1 | 1 | 1 | 7 | ||||||||
| 4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |||||||
| 5 | 1 | 1 | 2 | 2 | 3 | 1 | 1 | 1 | 12 | ||||||
| 6 | 1 | 1 | 2 | 1 | 1 | 1 | 7 | ||||||||
| 7 | 1 | 1 | 1 | 1 | 2 | 2 | 8 | ||||||||
| 8 | 1 | 1 | 1 | 3 |
Analysis
Transcripts of patients’ interviews were reviewed and key passages that set out their accounts of events were included verbatim at the start of each account. This was done partly because the patient (and in some cases also the carer) was the only person who had been present throughout and partly to guard against losing sight of the ‘patient’s voice’ in subsequent analysis. A Microsoft Access® 2010 database (Microsoft Corporation, Redmond, WA, USA) was then created for each account and used to organise key decisions and actions into a chronological sequence. Patient- and carer-derived data were recorded in one column, clinician interview data in a second and clinical record and other documentary sources in a third (see Figure 10, stage 2). The presentation of data in parallel columns made it possible to develop a chronological account of events, identify consistencies and inconsistencies between different data sources and assess the ‘strength’ of evidence available about key events, reflected in the number and quality of sources. These data were used as the basis of a single, provisional timeline of events.
The site principal investigator, who in each case was a nurse with a specialist interest in tissue viability, undertook a parallel review, based solely on available patient records and on other available documentation, including local guidelines and critical incident reports. The review followed the guidance for reviews of critical incidents in the NHS. 94 The investigator wrote a report, identifying key decisions and actions in chronological order, including departures from local guidelines. The field researcher and site principal investigator then met and compared their accounts, identifying consistencies and inconsistencies (e.g. actions that the nurse judged as important that were not included in the researcher’s account). The timeline in each initial account was revised in light of additional facts or insights generated (see Figure 10, stage 3).
Refinement of the accounts
The initial summaries of each account, supported by transcripts of all of the interviews conducted, were reviewed by a subgroup of nursing members of the research team, one independent hospital-based and one independent community-based tissue viability nurse specialist and one of the co-chief investigators (JN) (see Figure 10, stage 4). The subgroups met and reviewed the summaries and transcripts, identifying points where, in their professional opinion, there was a departure from ‘good usual care’ (e.g. category 2 pressure ulcer was recorded in the nursing notes but no additional action to treat the ulcer or prevent deterioration was reported). The meetings were recorded and transcribed.
The subgroups were asked to make summative judgements about the best explanation for the development of a severe pressure ulcer for each account. The method for this stage of the study drew on Yin’s108 strategy for eliminating hypotheses in case study research. The subgroups were invited to select one or more of the following five explanations for the development of a severe pressure ulcer:
-
it could not have been avoided
-
there was a single precipitating event
-
there was a sequence of precipitating events
-
the organisational context made development more likely
-
there was another explanation, not covered by the first four.
The second, third and fourth explanations were derived pragmatically from the literature on patient safety, with each representing a major class of explanation for adverse events. 109,110 The other two – the first and fifth explanations – were logical alternatives to the first three (i.e. the organisation of care played no role and there was an explanation that was not predicted by any of the three theories). We did not define key terms such as ‘precipitating event’ and ‘organisational context’ on the basis that subgroup members were expected to articulate the reasons why they opted for different explanations and in so doing would provide their own definitions in each account.
Viewed in the context of patient safety studies, this approach is novel, taking the study away from a narrow focus on root causes (i.e. only allowing causal explanations of events to be considered) and towards the broader classes of explanations in the safety literature. There is a technical point here, which is that the explanations are not based on causal relationships but on identifying the ‘best fit’ between the available data and one of the explanations.
The discussion leading up to the summative judgements, and the judgements themselves, were included in the revised accounts. The accounts at this stage therefore had three discrete sections, namely the patient’s account, the interpreted timeline and nurses’ summative judgement. The subgroups sometimes made queries about details of the accounts. After each meeting the queries were checked by going back to primary data sources and accounts were amended as appropriate.
The last two stages of the analysis were reviews of the individual accounts by a non-clinical co-chief investigator (JK) and then by an organisational psychologist (MP) who had not been involved in the earlier stages (see Figure 10, stage 5). The reviews focused on the coherence of each account (i.e. the extent to which the patient’s explanation and/or the nurses’ judgements made sense of the available evidence).
In the final step in the analysis, the accounts were compared with one another to identify themes that were common across the accounts, even though the details of the individuals, their pressure ulcers and the care settings varied widely. The themes were analysed inductively to develop a mid-range theory of the reasons why patients develop severe pressure ulcers. 109
Results
The study demonstrates that it is possible to develop detailed retrospective accounts of events and to use them to judge which of five possible explanations best fits the available evidence. The large volumes of data collected and included in the timeline appear to have minimised problems that might have arisen as a result of ‘missing data’. However, as we note in the discussion, the results may still be subject to a number of biases.
The eight accounts
The eight patients were selected, in part, to maximise diversity (Table 26). Unsurprisingly, then, there were marked differences in the details of their treatment and care and different explanations were offered by those interviewed for the development of severe pressure ulcers.
| Account | Individual | Setting |
|---|---|---|
| 1 | 38-year-old woman with paraplegia | Acute hospital, surgical ward |
| 2 | 65-year-old woman with a long-term chronic neurological condition and undiagnosed infection | Acute hospital, medical ward |
| 3 | 75-year-old man with multiple chronic health problems and acute infection | Community hospital, rehabilitation ward |
| 4 | 37-year-old woman with a long-term degenerative congenital neurological condition | At home |
| 5 | 90-year-old man with multiple chronic health problems and undiagnosed acute illness | Acute hospital, surgical ward |
| 6 | 39-year-old woman in hospital for acute undiagnosed postoperative surgical complications | Acute hospital, surgical ward |
| 7 | 65-year-old man with quadriplegia | At home, respite care and acute hospital |
| 8 | 89-year-old woman who fell at home | At home |
Seven of the eight – the exception being number 8 – exhibited widely recognised risk factors and had complex treatment and care needs. In a number of accounts some staff who were interviewed blamed the patients, on the basis that they had not complied with advice on managing their risks (e.g. shifting position regularly). But patients themselves, in the same accounts, generally pointed to specific actions or omissions, such as the failure to be turned regularly overnight, to be provided with a specialised mattress or to act on patients’ comments about their own risks.
Participants approached interviews in very different ways. Some patients and carers were very clear in their own minds about what had gone wrong whereas others were very reluctant to criticise any aspect of their care. Similarly, some accounts involved individuals moving between wards, or an account developed against a background of a major ward reorganisation. The significance attributed to these moves varied, including, for example, the failure to transfer notes with patients, which placed receiving staff at a disadvantage, and staff feeling harassed because they were working in an unfamiliar environment during a reorganisation.
Elimination of hypotheses
The diverse group of individuals all had the same outcome: a severe pressure ulcer. In one account (number 8), the review teams judged that the development of the pressure ulcer was unavoidable, because the individual concerned developed a severe pressure ulcer in her own home, before any health professional saw her. In another account (number 3) there was a single precipitating event and in three other accounts (numbers 2, 4 and 6) there was a sequence of events. But the clinical subgroup and subsequent reviewers all judged that the organisational context made development more likely in seven of the eight accounts (Table 27). It is possible that the organisational context made the ‘key events’ in accounts 2–4 and 6 more likely, although we cannot make causal inferences with any confidence on the basis of our evidence. The evidence suggests that the term ‘organisational context’ covers two distinct concepts. The first concerns the prevailing cultures in the settings where severe pressure ulcers developed (see Cross-patient themes). The second relates to what one might term the functional characteristics of those settings, particularly nursing staff shortages, staff who (justifiably or not) did not believe that they had enough time for proper treatment and care and wider organisational issues, including contemporaneous reconfigurations of services in four of the accounts.
| Account | Unavoidable | Single/isolated event | Sequence of events | Environment made development more likely | Other explanation |
|---|---|---|---|---|---|
| 1 | ✓ | ||||
| 2 | ✓ | ✓ | |||
| 3 | ✓ | ✓ | |||
| 4 | ✓ | ✓ | |||
| 5 | ✓ | ||||
| 6 | ✓ | ✓ | |||
| 7 | ✓ | ||||
| 8 | ✓ |
Cross-patient themes
The process of eliminating hypotheses, and the analysis of common themes across the eight individuals, led to the identification of three broad themes. First, the ‘voices’ of those who developed severe pressure ulcers, and of their carers when they were involved, were not heard by staff. As noted earlier, the individuals themselves behaved differently and had different relationships with clinical staff, but failures to heed information were evident in several accounts. There were examples of patients making repeated appeals for pain and discomfort to be addressed and expressing concerns about their own well-being that were not heeded over periods of hours or even days. In some instances, these appeals seem to have been dismissed by staff, that is, they were heard but were not taken seriously. Patients were also blamed for the development of their pressure ulcers on the basis that they did not comply with instructions that they were given, and were branded as ‘difficult’, even when they had a cognitive impairment.
Second, there were failures to recognise and act on warning signs. Risk assessments were not undertaken when they should have been and, in some cases, they were undertaken only several days after admission to an acute hospital ward. Evidence of pre-existing clinical risks in records was not acted on in six of the seven cases in which the environment was judged to have made development more likely. Action was not taken promptly when overt evidence, including the presence of a category 2 pressure ulcer, was identified. In interviews, there were a number of instances of staff blaming colleagues for these failures. Conversely, there was evidence of poor documentation, so that adherence with patients’ care plans was not recorded and, in some instances, direct evidence of skin redness or a pressure ulcer was not recorded. Some health-care assistants, who provided direct care, observed that they lacked the appropriate training to identify and record risks or were not allowed to record them.
Third, there were co-ordination failures, between patients, carers and staff, between staff in the same setting, between staff in different settings in the same organisation (e.g. two wards) and between staff in different organisations. Sometimes this was manifested as interprofessional communication failure and sometimes there was poor communication between the same professional groups in two locations. One example of the latter came in a postoperative setting where risks were not properly communicated between the anaesthetic recovery unit and the postoperative ward. In other accounts, records were not moved with an individual so that key information was not available in a new setting. Again, in staff interviews these problems were often recognised but in contexts in which interviewees were defensive, tending to blame others rather than taking responsibility for their individual or collective actions.
It would be possible to interpret these co-ordination failures as clear evidence of failure by individuals or teams. But there is a corollary to this point: nurses and health-care assistants, in particular, could find themselves working in conditions in which they had limited information about individuals and their risks (e.g. patients had an unknown diagnosis) or in which records had not travelled with a patient from another location. It is possible, therefore, that individual members of staff behaved reasonably in the contexts in which they found themselves; the problems lay more with the overall co-ordination of treatment and care.
The larger and broader finding – the mid-range theory arising from the findings – is that individuals developed severe pressure ulcers in environments where there were problems with the prevailing culture. This cultural explanation binds together the otherwise separate points made above. In most accounts there was a combination of problems – some staff blaming colleagues or describing patients as ‘difficult’, poor documentation and failures to act on repeated clear warning signs (i.e. to step up care provision when a superficial pressure ulcer was observed), so that a severe pressure ulcer was ‘allowed’ to develop. In these contexts responsibilities were not clear and staff interviews pointed to problems with team working, extending beyond the specific accounts that we were producing.
Discussion
This study sought to improve our understanding of the ways in which the organisational context contributes to the development of severe pressure ulcers and in doing so to discriminate between alternative explanations for their development in the research literature. The principal explanation is that severe pressure ulcers are more likely to develop in particular organisational contexts. The contexts were characterised by one or more of (1) clinicians failing to listen to patients’ or carers’ observations about their risks or the quality of their treatment and care, (2) clinicians failing to recognise and respond to clear signs that a patient had a pressure ulcer or was at risk of developing one and (3) services not being effectively co-ordinated. These can all be interpreted as failures in the governance of the services in the settings studied.
As noted in the methods section the study was designed in significant part to minimise biases in data collection and analysis. The study suggests that a novel method, based on tracing back the course of events retrospectively from a known outcome, can be used to reconstruct key events. The resulting accounts can be subjected to detailed review and used to discriminate between alternative explanations for those events, in the process preserving the ‘voices’ of the individuals affected. That said, it is important to stress that there are a number of sources of bias, starting with selection bias: although the sampling strategy maximised diversity, the eight accounts are of individuals who were willing and able to consent to participate. The initial presentation of the timelines and the backgrounds of the analysts and reviewers are also potential sources of bias. A study team with a different clinical or disciplinary background might have arrived at different judgements, for example a team with a background in human factors psychology might have placed greater weight on single events or sequences of events. Using a retrospective design, there is also a risk of hindsight bias, particularly in terms of reviewers assuming that staff must have known more than they actually did and should therefore have acted differently. 110 The sequential and iterative review process has, we hope, served to minimise these biases but we cannot say that they have been eliminated.
We can place our findings in the context of the patient safety literature. Reason111 points out that investigations of accidents, across many industries, have changed significantly over the last 50 years. An early focus on equipment failure gave way, in the 1970s and 1980s, to a focus on human error and then more recently to accounts that focused on systems and cultural issues. In spite of this, many patient safety studies today focus on explanations based on narrowly defined human factors and relatively few focus on the wider organisational context. 112 The findings reported here do not support the kinds of explanation that might have been advanced in the first two periods or by researchers focusing on human factors in clinical decision-making today. They are, though, consistent with explanations that emphasise systems and culture. This point is worth emphasising: our findings suggest that there is a need to move away from identification of root causes and towards broader explanations of events, based on identifying the ‘best fit’ between the available evidence and the explanations available in the patient safety literature.
As we noted earlier, explanations tend to emphasise either systems-based or cultural explanations. The results of this study suggest that, for people who developed severe pressure ulcers, both were important. In relation to systems-based explanations, the evidence about the poor co-ordination of services is broadly consistent with the Institute of Medicine’s arguments in To Err Is Human, namely that many safety failures are essentially system failures. 113 Drawing on the work of Perrow110 and others, the Institute argued that accidents are more likely in systems that are inherently complex – having many interconnected elements. The findings in this study support the observation that there were co-ordination failures between services that were loosely coupled with one another (i.e. that are managed independently but need to co-ordinate with one another). For example, there were communication failures between wards at times when there were major ward reorganisations, so that key information was not passed on. Similarly, one of the community-based accounts revealed that the individual was in receipt of a hospital service that community staff were unaware of and hence could not take into account in risk assessment or care planning.
The findings cannot be wholly explained as co-ordination failures. The failure to listen properly to patients – and even dismissing their concerns – and to act when there was a superficial pressure ulcer present emphasises the importance of prevailing cultural norms. The evidence suggests that the environments where severe pressure ulcers developed were ones where staff were under time pressure, where there were problematic relationships between staff groups and where staff were defensive and prepared to attribute failures to colleagues or to the ‘difficult’ behaviour of patients. Clinicians adopted risky work routines that were not appropriate for the vulnerable patients who were in their care. Severe pressure ulcers developed in contexts in which risky practices had become the norm – in which there was normalisation of deviance. 114 This resonates with wider concerns about the culture in parts of the NHS in England, where staff have been defensive and quick to blame others. 46 The implication is that the effective prevention of severe pressure ulcers requires staff to adopt appropriate behavioural norms, including effective communication with patients, a commitment to the thorough assessment of risks and prompt action when things go wrong.
Finally, we have noted that there were often discrepancies between patient accounts, staff interviews and records. No one source provided all of the key information needed to understand what had happened and, as noted, accounts could conflict with another. This provides a clue about a potentially important weakness of existing root cause analysis processes. At present, NHS guidance recommends reviews of clinical records and interviews with staff but not interviews with patients and carers, without whose testimony some of the accounts would have been incomplete and might well have been interpreted wrongly, leading to the wrong conclusions and hence the wrong remedial interventions.
Patient and public involvement
In Chapter 2 we described the creation of PURSUN UK. Members of PURSUN UK were invited to contribute to the interpretation of some of the findings from the retrospective study (see Retrospective study of the development of severe pressure ulcers). As we noted there, the evidence and interpretations provided by patients who had developed severe pressure ulcers proved to be important in the analysis. Patients and their carers were the only people who were present throughout; clinicians could be aware only of parts of each account. There was, though, a risk that the analysis, in ensuring that the accounts were accurate and coherent from a nursing perspective, might lose sight of patients’ and carers’ viewpoints. We therefore wanted to establish whether or not our accounts were ‘true and fair’ as perceived by patients with experience of having pressure ulcers.
Patients have only rarely been directly involved in the interpretation of research evidence, as opposed to being sources of evidence, in health services research. 115 A recent review of the literature on PPI found that involvement activities tended to focus on the early stages of research, such as identifying research priorities and aspects of study design. Only six studies were identified as having significant PPI in analysis or interpretation. 26
Aim and objectives
To design and conduct a patient involvement workshop, drawing on one of the accounts from the empirical study. The workshop had two distinct purposes:
-
to assess the face validity of the account from the point of view of a group of service users
-
to disseminate the findings of the project to those service users.
Methods
Design: Pressure Ulcer Research Service User Network UK workshop model
We organised a workshop that was developed and facilitated by the PURPOSE PPI officer, the severe pressure ulcer study field researcher and one member of PURSUN UK. The workshop was designed along the broad lines of a public enquiry – albeit a benign one – in which participants would be invited to act as ‘expert witnesses’ in a case that was presented to them.
Three types of material were prepared before the workshop. First, one patient’s account of her health problem and treatment was used to create a brief for a simulated patient. The PURSUN UK member with specialist expertise in health-related role play took on the role of the patient from the case. The researcher conducting the inquiry then interviewed the simulated patient about their experiences. This was presented live at the workshop. Various simulated patient models have been used in the UK since the late 1970s, typically in communication skills training or assessment for health professionals. 32 The approach was adapted here for use in a research context. Second, professionals’ accounts of events were filmed and edited into short videos. Here, actors were given a brief, prepared by the workshop facilitators, and asked to improvise a piece to camera (they did not read from a transcript, in part to avoid using verbatim quotes, which might have led to the patient being identified). Third, a visual timeline of events was presented using Prezi software (Prezi, Budapest).
The workshop was held on 17 May 2012 in Leeds. It was attended by nine members of PURSUN UK, six members of the research project team and two NHS PPI managers.
Workshop evaluation
The workshop was, as far as we were aware, innovative and we were unsure how successful it would be. We therefore decided to evaluate it. Five participants took part in videoed interviews and one further audio-recorded interview was carried out by the PURPOSE PPI officer. The interviews took place both during the workshop, to provide immediate reactions, and afterwards, giving participants time to reflect before commenting. Three participants also chose to provide written feedback. Themes from the interviews and written feedback were then collated by the PURPOSE PPI officer. Participants were also offered the opportunity to provide anonymous feedback through a neutral third party but in the event this option was not used. Clips from the videoed interviews are available at http://youtu.be/bgg6zkbILrg (accessed 24 February 2015).
Results
The workshop model
Participants found the metaphor of a public enquiry useful as it helped to convey the design of the field study and the rationale for the ‘expert witness’ roles in the workshop. Participants found the simulated patient interview engaging and valued the interactive nature of the session. One member of PURSUN UK commented that she would not have become involved in the project if it had required her to take part in a complex, paper-based exercise. The live interview also provided a snapshot into a part of the research process that few had previously experienced. As the simulated patient stayed ‘in role’ during discussions, workshop participants were able to briefly step into the shoes of the interviewer, asking follow-up questions and checking assumptions.
Impact on workshop participants
Service users reported an increased understanding of research processes in general as a result of the workshop. Some members also said that it had made them think more about their own and their family’s health, particularly in relation to preventing pressure ulcers. Some service users also reported an increased feeling of empathy for the health professionals dealing with such complex cases.
Members of the project team valued the dialogue with service users in a non-clinical context, despite some initial concerns about working in this way. Everyone felt that the collaborative nature of the workshop was important, particularly the fact that academics and nurses got to hear service users’ opinions first hand.
Face validity
The workshop also provided us with feedback – admittedly for just one of the eight accounts – about the validity of the account. Members of PURSUN UK arrived at a similar interpretation of events as the nurses and other experts involved in the formal analysis reported in Retrospective study of the development of severe pressure ulcers.
The research team had already identified a need to involve patients and carers in the critical review of severe pressure ulcers in the NHS (see Implementation project: the review of critical incidents). This was supported by PURSUN UK members and one member has worked with the study team during 2013. PURSUN UK also highlighted the importance of patient/carer engagement in pressure ulcer prevention and the role that professionals can play in facilitating that engagement. Conversely, we recognise that the severe pressure ulcer case study findings were filtered through the materials prepared for the workshop. This was necessary partly to make the findings accessible and partly to protect the anonymity of the individual whose account was used.
Conclusions
Thinking carefully about how PPI activities are designed and facilitated is important as the format affects people’s ability and willingness to contribute. Presenting research data in live and interactive formats can make the interpretation process more engaging and accessible and support meaningful dialogue between service users and professionals. The use of applied performance techniques, as described here, provides one model for doing this.
Implementation project: the review of critical incidents
The programme grant proposal included a commitment to investigate the implications of the study findings for the conduct of root cause analyses of occurrences of severe pressure ulcers.
Aim and objective
The aim was to develop a methodology that can be used to review reportable pressure ulcers in the NHS in England. The objective was to develop and test a method for reviewing severe pressure ulcers that incorporated two key features, namely consideration of organisational explanations for their development and eliciting information from patients. The first feature was derived from the main study and the second was an extension of the PPI study.
Overview of methods
The work was undertaken by two of the clinical experts who had been involved in the empirical study, the PURPOSE PPI officer, the programme chief investigator and the severe pressure ulcer study lead. A further member was co-opted who had a dual role, as a member of PURSUN UK, having recently experienced a severe pressure ulcer, and as a senior analytical researcher in a NHS organisation. An expert in adult safeguarding was also consulted in the course of the project.
The scope of the project was defined as follows: to devise an investigation process and template suitable for use in the NHS; to pilot this in both acute and community care settings; and to establish the added value of including the patient’s voice and any differences in findings compared with the traditional root cause analysis process and the value of the findings for meaningful action planning and process changes. The methodology should also encourage open contributions, to learn what could be done better in future, learn what good practice we could disseminate and gain feedback on prevention and management interventions.
Based on the findings of the empirical study, the team took the view that the review methodology should include:
-
a narrative of events associated with the development of a pressure ulcer, captured in conversations with patients and/or relatives/carers and with staff with knowledge of relevant events
-
a timeline of events based on information from patient records
-
identification of good practice – practices that patients might reasonably have expected
-
insights from the empirical study (e.g. about the systemic nature of organisational risks)
-
coverage of resource issues, to take into account current NHS resource constraints.
Initially, the team reviewed investigation methods currently used in the NHS, including the Herringbone and 5 Whys? methods,116 both of which were endorsed by the National Patient Safety Agency before it was closed down. Strengths and weaknesses of current NHS root cause analysis practices have been described by Nicolini and colleagues117 and their findings were used to inform our process.
We devised a method to direct data collection and the construction of patient narratives as follows: each scene was presented in terms of ‘actions’ taken by the actors, ‘constraints’ imposed on them, the ‘information’ they held and/or passed on and the ‘decisions’ they reached. This was initially tested informally using the patient member’s own experience. The process can be summarised as:
-
beginning, which covers setting up the study team, establishing the narrative or story of what happened from the patient notes and identifying the people who can contribute to the study
-
gathering, which involves the conversations with staff, patients and carers and reviewing the narrative in the light of their contributions
-
analysing, which is the process of sense making of the information gathered, looking at risks, good usual care and the constraints on the incident
-
reporting, which draws the analysis together into findings and actions.
The two clinical team members consulted with their host organisations and obtained agreement to pilot the method. In preparation, a workshop took place with the tissue viability link nurses during their training day. The nurses were given information from the records of a member of PURSUN UK relating to the development of a severe pressure ulcer. They were asked to use a draft template to produce a timeline of events. In parallel, a conversation then took place between an investigator and the member of PURSUN UK, during which he described his account of events. The nurses at the workshop then considered the differences between the two accounts and reflected on the experience of involving a patient in the process. Some practical issues were raised, including what to do if the patient was unable or unwilling to contribute (or there were no relatives available) and whether or not the process would increase the chances of litigation. Overall, though, the nurses felt that including the patient’s account added information that they would not otherwise have obtained and added value to the review process. Reviewing the event, it was noted that the systemic issues that were central to the empirical study were not identified by the nurses at the workshop. We judged that it would be necessary to provide prompts in any guidance for investigators, to encourage them to focus on systemic explanations for the development of pressure ulcers.
The template for recording events and the guidance for the investigator were revised in the light of the workshop. The two clinical team members then identified two patients and their carers within their own organisations who were willing to take part in a further pilot review process. Conversations took place and information was collected from patient records. The usual NHS root cause analysis process was carried out concurrently and separately. Data that might identify any individuals involved were removed prior to sharing with the project team. Details of time resources were considered and findings were compared with those of the usual root cause analysis process.
Results
Findings and reflections
The substantive findings of the reviews were consistent with those from the retrospective study (Table 28). Both reviews using the new method identified additional contributing decisions and actions, over and above those identified in the parallel root cause analyses. The patients and carers did not identify the key decisions and actions themselves; their descriptions allowed the investigators to do so. In both cases the patients and carers valued the opportunity to ‘tell their own story’.
| Incident | Root cause analysis findings | New investigation framework findings |
|---|---|---|
| Community pressure ulcer incident |
|
|
| Hospital pressure ulcer incident |
|
|
Feedback from ward staff
A meeting was held with the staff from the ward where the hospital pressure ulcer incident had taken place. This was to feed back the findings of the pilot investigation and to evaluate the process by eliciting staff views. Generally, staff thought that the process was more thorough than the current root cause analysis process and was more informative about the reasons why the pressure ulcer developed. They felt that the patient’s account not only told them a lot about the development of the pressure ulcer but also gave them important feedback about other aspects of care. There were observations in the patient’s account that they could not have obtained from any other source (e.g. an existing sore on the ankle). Some staff felt that it made ‘uncomfortable reading’ but that it was to be expected when a category 3 pressure ulcer had occurred. They agreed that it was no more uncomfortable than the current process. They acknowledged the value of more staff involvement in the process as people may take more note of the outcome of an investigation if they are part of the process. They also noted that nurses are currently carrying out root cause analysis without any training. Finally, the idea that an independent person should lead the investigation (i.e. not someone working on that ward) was supported.
Conclusions
Our conclusions, based on the pilot work, are that:
-
There is value in involving patients and carers in root cause analysis – they can provide important information that is not recorded in notes or reported by staff.
-
A narrative approach also has value. Underpinning this point, we note that nurses at the workshop and review events instinctively focused on root causes, that is, on cause–effect relationships, such as failure to turn a patient frequently enough. The findings of the empirical study and of this implementation study suggest that, rather than focus on root causes, teams and independent investigators should be encouraged to consider systemic explanations, that is, they should consider different ways of interpreting the available evidence, ranging from ‘one-off’ errors by staff to wider cultural issues that need to be addressed.
-
Root cause analyses should be co-ordinated by someone who is independent of the setting in which incidents occur and who has clinical knowledge that provides credibility and the capacity to help local teams to identify lessons that can be learned.
Summary
The severe pressure ulcer work package comprised three pieces of work. The first was a retrospective case study of eight patients who developed severe pressure ulcers. The second was a patient involvement workshop with PURSUN UK. The third focused on the development of a methodology for the root cause analysis of critical incidents.
The main field study set out to understand the ways in which the organisational context influences the development of severe pressure ulcers. For seven of the eight patients the best explanation of the evidence was that the general organisational context played a significant role in severe pressure ulcer development. In four accounts specific events contributed to development. One patient’s severe pressure ulcer was deemed to be unavoidable. We found that severe pressure ulcers were more likely to develop in contexts characterised by one or more of clinicians failing to listen to patients or carers, clinicians failing to recognise and respond to clear signs that a patient had a pressure ulcer or was at risk of developing one, and services not being effectively co-ordinated.
The patient involvement workshop was an additional study that was not described in the programme grant proposal. Service users found the interactive workshop format, and the use of a ‘simulated patient’ account within it, valuable. We found that presenting research data in live and interactive formats can make the interpretation process more engaging and accessible to service users and can support meaningful dialogue between service users and professionals.
We also found that a methodology for root cause analysis, rooted in current NHS practice but including novel components, can be used to improve the quality of the insights captured. On the basis of our three pieces of work, we conclude that current best NHS practice in root cause analysis of reportable pressure ulcers should be augmented by interviews with patients and carers and by the construction of narratives based on key events. Our findings suggest that there is a need to move away from identification of root causes and towards broader explanations of events, based on identifying the ‘best fit’ between the available evidence and the explanations available in the patient safety literature.
In conclusion, this study has not led us to a model or template that can be used for the analysis of the development of severe pressure ulcers or other incidents. Rather, it reinforces the view, articulated by Francis93 and others,97 that reviews are more likely to be effective if (1) they pay closer attention to details of the functional, or systems-based, components and also (2) they are used in ways that promote learning rather than blame. We have pointed in this chapter, and in the development of a risk assessment tool (see Chapter 5), to the importance of collecting the right information and collating and presenting it systematically. This is only worthwhile if clinical teams working in settings where severe pressure ulcers develop reflect on, and work on, their local cultures. No tool yet invented will substitute for this internal reflection and commitment to change practice.
Chapter 5 Risk assessment
Chapter written by Susanne Coleman, E Andrea Nelson, Isabelle L Smith, Sarah Brown, Julia M Brown, Lyn Wilson, Delia Muir, Justin Keen, Carol Dealey, Elizabeth McGinnis, Nikki Stubbs and Jane Nixon.
Abstract
Introduction: Increasing evidence makes it timely to reconsider which risk factors should be considered in pressure ulcer risk assessment and how to prompt an escalation of interventions for secondary prevention and treatment. The primary aim of the risk assessment work package of the programme was to agree a pressure ulcer risk factor Minimum Data Set to underpin the development and validation of a Risk Assessment Framework for use in clinical practice. The work package consisted of five phases incorporating a systematic review, a consensus study, conceptual framework development, design and pre-testing of the framework and clinical evaluation of the framework.
Methods: (1) A systematic review of primary research to identify pressure ulcer risk factors; (2) a consensus study using a modified nominal group technique based on the RAND/UCLA (Research and Development, University of California in Los Angeles) appropriateness method, incorporating an expert group, review of the pressure ulcer evidence and the views of PURSUN UK to agree a draft pressure ulcer risk factor Minimum Data Set and develop a Risk Assessment Framework; (3) development of a pressure ulcer conceptual framework and theoretical causal pathway, building on the phase 2 consensus study; (4) design and pre-testing of the draft Risk Assessment Framework using cognitive pre-testing methods, to assess and improve its acceptability and usability with clinical nurses; and (5) clinical evaluation of the reliability, validity, data completeness and clinical usability of the Risk Assessment Framework through field testing of 230 patients by expert and community/ward-based nurses.
Results: (1) The systematic review of primary research identified 15 risk factor domains and 46 related subdomains. The review indicated that there were three primary risk factor domains of mobility/activity, skin/pressure ulcer status and perfusion (including diabetes), but suggested that no single factor can explain pressure ulcer development. Other risk factor domains emerged less consistently. (2) The consensus study facilitated the agreement of risk factors and assessment items for the Minimum Data Set (including immobility, pressure ulcer and skin status, perfusion, diabetes, skin moisture, sensory perception and nutrition), allowing the development of a draft Risk Assessment Framework incorporating all Minimum Data Set items. (3) The new pressure ulcer conceptual framework incorporated five key components (mechanical boundary conditions, physiology and repair, mechanical properties of the tissue, geometry of the tissue/bone, and transport and thermal properties) and their impact on internal strains, stresses and damage thresholds. The theoretical causal pathway for pressure ulcer development identified direct causal factors, key indirect causal factors and other potential indirect causal factors for pressure ulcer development. (4) The design and pre-testing of the Risk Assessment Framework led to improved usability over the course of the three pre-test sessions, as demonstrated by increased data completeness and appropriate pathway allocation. (5) The field test demonstrated that inter-rater and test–retest agreement for the PURPOSE-T was ‘very good’ for the assessment decision overall as determined by kappa. The percentage agreement for the assessment of ‘problem/no problem’ for the eight risk factors (mobility, skin, previous pressure ulcer, sensory perception, perfusion, nutrition, moisture and diabetes) ranged from 79.1% to 94.2% for inter-rater reliability and from 87.0% to 93.9% for test–retest reliability. Convergent validity, assessed by comparison with the same or similar constructs on other risk assessment scales (Braden and Waterlow), demonstrated moderate to high associations. In addition, field notes recorded by the expert nurses highlighted positive and problem aspects of using the tool in the clinical environment. A follow-up consensus process allowed consideration of evidence from the pain work package and an extension of the pressure ulcer and skin status assessment items to include pressure area-related pain at the full assessment stage of the PURPOSE-T.
Conclusion: The work package led to the development of a new Risk Assessment Framework, the PURPOSE-T, incorporating the Minimum Data Set; a screening stage to target assessment towards those in need; a full assessment stage; use of colour (rather than a score) to describe risk in terms of a personal profile to help in the planning of appropriate interventions; and decision pathways that make a clear distinction between patients with an existing pressure ulcer(s) (or scarring from previous ulcers) who require secondary prevention and treatment and those at risk who require primary prevention. The final PURPOSE-T framework has content, face and construct validity, with good inter-rater reliability and very good test–retest reliability.
Background
It is not appropriate to prevent pressure ulcers by subjecting all patients to resource-intensive interventions (such as repositioning by nurses or expensive mattresses) that may impact on their quality of life (e.g. by disturbing sleep) and cause harm by diverting nursing time from other areas, hence we must target care to those patients for whom it is likely to do more good than harm. Targeting patients for whom pressure ulcer prevention interventions are needed is achieved by considering the patients’ characteristics, a process known as risk assesssment. It is noteworthy that this is an individualistic approach to determining if someone is likely to develop a pressure ulcer, in contrast to the interaction/context-based explanantions identified as also being important in our study of severe pressure ulcers (see Chapter 4). Regardless of context, risk assessment is widely accepted as being essential for pressure ulcer prevention1,14,23 as it allows ‘at-risk’ patients to be identified so that preventative interventions can be put in place to reduce the risk of ulcer development.
In clinical practice, risk assessment scales are commonly used to give some structure to the assessment process, in preference to clinical ‘assessment’ or ‘judgement’ of risk. Nixon and McGough118 noted that there were > 40 pressure ulcer risk assessment scales, with the majority being developed from a literature review, expert opinion and/or adaptation of an existing scale. They noted that there were only seven ‘original’ scales. Many were developed in the 1970s and 1980s when the epidemiological evidence was limited by the primary research, including there being few studies considering the relative contribution of individual risk factors. This led to the inconsistent inclusion of risk factors between different scales, with the variables most frequently incorporated being continence/moisture, nutrition/appetite and mobility. 118 This raises concern about the lack of agreement about what should be included in risk assessment scales to adequately identify risk and, as a result, the validity of those scales. 118,119 In addition, existing scales tend to use ordinal scoring systems in which a comparison is made between the patient and a standard reference value to allocate a level of risk (e.g. high risk, moderate risk, at risk), with equal weighting usually given to included risk factors despite the fact that some may be more predictive than others. It has been argued that pressure ulcer risk assessment scales need to be developed on the basis of multivariable analyses to identify factors that are independently associated with pressure ulcer development118,120,121 and to advance our understanding of the relative contribution that different risk factors make to pressure ulcer development. Gold standard methods for the development of risk stratification tools include multivariable modelling (either from single studies or from meta-analysis from a number of studies) to identify items for a risk tool, with subsequent model testing on a ‘new’ prospective target population. 122 This type of tool development has in the main been undertaken only in single-centre populations, with methodological limitations including inadequate sample sizes for both model derivation and testing. 123–125
From a practical perspective there are also issues with the use of existing risk assessment scales. Many were designed to identify risk status in patients without pressure ulcers, but in practice are often used for all patients, including those with and without pressure ulcers and do not distinguish between these groups. This is a limitation as nurses could discount an existing pressure ulcer in their clinical assessment and fail to instigate suitable secondary prevention and treatment interventions, which could lead to deterioration and the development of a more severe pressure ulcer. 126 This resonates with findings from the severe pressure ulcer study (see Chapter 4). This is important as the pain cohort study (see Chapter 3) and a recent systematic review of pressure ulcer risk factors,46 conducted as part of this programme grant, indicated that the presence of a category 1 pressure ulcer is a key predictor of the subsequent development of a category 2 or above pressure ulcer, increasing the odds by two- to threefold. 126
Increasing evidence makes it timely to reconsider which risk factors should be considered in pressure ulcer risk assessment, how these should be assessed and the overall assessment process in the development of a Risk Assessment Framework. Furthermore, the systematic review46 conducted as part of this programme highlighted the need to agree a pressure ulcer risk factor Minimum Data Set and further develop a pressure ulcer conceptual framework. These would encourage the use of consistent factors across studies, facilitating meta-analysis, provide a standardised data set for case-mix adjustment and provide the fundamental components for pressure ulcer risk assessment in clinical practice.
Aim
The overall aim of this work package was to agree a pressure ulcer risk factor Minimum Data Set to underpin the development and validation of a Risk Assessment Framework for use in clinical practice. (Note: as outlined in Chapter 1, Pressure ulcer development, the Risk Assessment Framework is not intended for the prevention or management of ulcers caused by medical devices as the primary risk factor for such ulcers is the presence of the device.)
Research overview
The methodological approach to the development of the Minimum Data Set and Risk Assessment Framework comprised five distinct phases: (1) developing the evidence base, (2) a consensus study, (3) conceptual framework development, (4) design and pre-testing and (5) clinical evaluation (Figure 11).
FIGURE 11.
Risk Assessment Framework: related studies. MDS, minimum data set; PU, pressure ulcer; RAF, Risk Assessment Framework.

Phase 1: systematic review of patient risk factors for pressure ulcer development
To provide the foundation for the risk assessment work package and to ensure consideration of potential risk factors for inclusion in the Minimum Data Set and Risk Assessment Framework, a systematic review of primary research was undertaken to identify risk factors that are independently predictive of pressure ulcer development in adult patient populations.
Methods
This Methods section has been largely reprinted with minor modifications from Int J Nurs Stud, vol. 50, Coleman S, Gorecki S, Nelson E, Closs S, Defloor T, Halfens R, et al. Patient risk factors for pressure ulcer development: systematic review, pp. 974–1003, 2013,46 with permission from Elsevier.
Design
The approach was based on the systematic review methods recommended for questions of effectiveness20,127 and adapted to identify risk factor studies, with consideration of the methodological limitations including bias and confounding associated with observational studies. 83,85
Study eligibility
Methodological quality criteria were integrated into the inclusion and exclusion criteria of the systematic review, developed from principles of good research conduct in observational studies and randomised controlled trials that minimise bias. 82,86,89,128
Inclusion criteria
Studies were included if they met the following criteria: primary research; adult study populations in any setting; outcome was the development of a new pressure ulcer(s); prospective cohort study, retrospective record review or a controlled trial; length of follow-up of at least 3 days, with the exception of operating room studies for which no minimal time period was set; outcome clearly defined as grade/stage 1 or above pressure ulcer23,129 or equivalent; multivariable analyses were undertaken to identify factors affecting pressure ulcer outcome; and the unit of analysis was the patient.
Exclusion criteria
Studies were excluded as follows: paediatric study populations; cross-sectional or case study designs; patient recall, patient self-report or an analysis of general practitioner records to assess outcome; or duplicate publications of a patient data set. Cohort studies (prospective and record reviews) were excluded from the review if > 20% of the study sample was excluded from analysis for reasons including withdrawal, death, loss to follow-up and missing records. 82,83,86,89 Controlled trials were excluded unless the following minimum criteria applied: randomised allocation to treatment and intention-to-treat analyses. 127,128 No language restriction was applied.
Data sources
Fourteen electronic databases were searched, from inception until March 2010: Allied and Complementary Medicine Database (AMED), British Nursing Index (BNI), MEDLINE, EMBASE, PsycINFO, Cumulative Index to Nursing and Allied Health Literature (CINAHL), The Cochrane Library, ProQuest, Networked Digital Library of Theses and Dissertations, International Theses in Progress, Theses Canada Portal, Australian Digital Theses Program, Russian Academy of Sciences Bibliographies and Index to Theses. The search strategy (see Appendix 14) sought to identify all published and unpublished research studies investigating risk factors for the development of pressure ulcers. The search strategy was designed with guidance from the collaborative team and included pressure ulcer search terms,130 Ovid maximum sensitivity filters for prognosis and aetiology or harm and an Ovid maximum sensitivity filter for randomised controlled trials. 127
In addition, we hand searched specialist journals and conference proceedings, contacted 13 experts, searched the UK national research websites and performed a citation search on all included studies and systematic reviews identified in the search (see Appendix 14).
Study selection
Abstracts were screened for relevance by one reviewer (CG) and checked by a second (JN). Articles assessed as potentially relevant were obtained in full and reviewed against the eligibility criteria by one reviewer (CG or SC) and checked by another (JN). When the statistical methods were unclear and eligibility could not be determined, statistical review was undertaken (JB). Disagreements were dealt with through consensus.
Data extraction
When studies fulfilled the eligibility criteria data were extracted by a single reviewer (CG or SC) and checked by a second reviewer (JN). When data were missing from the publication, attempts were made to contact the authors. When duplicate publications of patient data sets were identified, the most detailed report was used for data extraction. Experts in the field were asked to review/data extract abstracts and articles not published in English (see Acknowledgements).
Quality assessment
There are no guidelines for the quality assessment of risk factor studies and so we developed an assessment framework based on guidelines for assessing quality and risk of bias in prognostic studies and on methodological considerations in the analysis, meta-analysis and publication of observational studies. 81–89 Each study was appraised by two reviewers (JN, SC) and the following methodological limitations were noted when present: baseline characteristics not adequately described; inadequate measurement of risk factors (e.g. record review); inappropriate cut-points used for continuous data; and time-dependent covariates included in the analysis without appropriate adjustment.
In addition, specific consideration was given to the following criteria:
-
Is there a sufficient number of events (rule of thumb: ≥ 10 events per risk factor)?
-
Are there sufficient data to assess the adequacy of the methods and analysis?
-
Is the strategy for model building (i.e. inclusion of variables) appropriate and based on a conceptual framework?
-
Is the selected model adequate for the design?
Each of the four criteria was assessed to see whether or not they were met (yes/no/partial/unsure), which provided a structured approach for the classification of overall study quality. We classified studies as being of high, moderate, low and very low quality using the following criteria:
-
high-quality studies: ‘yes’ for all criteria
-
moderate-quality studies: ‘yes’ for criterion 1 and at least two other criteria
-
low-quality studies: ‘no’ for criterion 1 and ‘no’ or ‘partial’ for two other criteria
-
very low-quality studies: ‘no’ for criterion 1 and ‘no’ or ‘partial’ for all three remaining criteria.
Data synthesis
Meta-analysis of the data was not feasible for this review because of heterogeneity in the study designs, patient populations, risk factor descriptors, interventions used and outcomes reported. As the main aim was to identify risk factors rather than quantify the effect size of the relationship between these factors and pressure ulcer development, a narrative synthesis was carried out. 127
For each study, all factors entered into multivariable modelling and those that emerged as significant (p ≤ 0.05) were identified. For studies using stepwise regression, we included non-significant factors (p ≥ 0.05) if these were reported in the final model as being independently associated with pressure ulcer development.
Risk factors were categorised into domains and subdomains by collating related factors from the source articles into a grouping (domain). An example of a domain and subdomain would be the domain of skin/ulcer status and the subdomains of stage/grade 1, existing pressure ulcer, previous pressure ulcer and general skin status. Evidence tables (see Appendix 15 for an example), were generated for each risk factor subdomain, with a summary narrative synthesis by subdomain and domain. For each subdomain, the total number of studies entering the variable, the total number of studies in which the variable emerged in the multivariable analyses and the quality of the studies are summarised. In the evidence tables (see Appendix 15 for an example), grade and stage of pressure ulcer are recorded as reported in individual studies.
Results
The numbers of studies considered and meeting the eligibility criteria are shown in the study flowchart (Figure 12). The 5437,57,60,79,80,125,131–178 included studies included 34,449 patients from acute and community populations. 46
FIGURE 12.
Flowchart of studies. Reprinted from Int J Nurs Stud, vol. 50, Coleman S, Gorecki C, Nelson EA, Close SJ, Defloor T, Halfens R, Farrin A, Brown J, Schoonhoven L, Nixon J. Patient risk factors for pressure ulcer development: systematic review. 2013; 974–1003,46 with permission from Elsevier.
Study quality
The included studies comprised seven high-quality, 10 moderate-quality, 27 low-quality and 10 very low-quality studies (Table 29). The low- and very low-quality studies had inadequate numbers of pressure ulcers and other methodological limitations.
| Study and country | Study population | Other inclusion criteria | Design and analysis method | Number in final model (PU%), number developing PU and stage/grade | Results: n risk factors (n in model), model risk factor names | p-valuea | ORb | CIb | Overall study qualityc and limitation notes |
|---|---|---|---|---|---|---|---|---|---|
| Allman et al. 199579 USA |
286 patients Setting: acute care hospital Specialty: multiple |
Admitted to hospital within previous 3 days, aged ≥ 55 years, expected LOS in bed or chair ≥ 5 days, had a hip fracture, expected LOS (hospital) ≥ 5 days. Excluded patients with stage 2 or above PU, Friday admissions, active skin disease that would interfere with PU assessment and previous enrolment to study. Consent required | Cohort Backward stepwise Cox regression |
286 (12.9%), 37 stage ≥ 2 | 9 (5) | LQS Insufficient number of events |
|||
| Non-blanchable erythema if intact sacral skin | 0.05 | 7.5 | 1.0 to 59.1 | ||||||
| Immobility | 0.02 | 2.4 | 1.1 to 4.9 | ||||||
| Dry sacral skin | 0.04 | 2.3 | 1.0 to 5.2 | ||||||
| Decreased body weight | 0.03 | 2.2 | 1.1 to 4.5 | ||||||
| Lymphopenia | 0.003 | 4.9 | 1.7 to 13.9 | ||||||
| Baldwin and Ziegler 1998131 USA |
36 patients Setting: acute care hospital Specialty: trauma |
Adults aged 15–60 years, hospitalised because of severe trauma, previously healthy, did not require burn fluid resuscitation and expected LOS (hospital) ≥ 1 week | Cohort Forward logistic regression |
36 (30.6%), 11 stage ≥ 1 | 7 (2) | VLQS Baseline characteristics not reported; sample size too small; insufficient number of events |
|||
| Braden mobility subscore | 0.02 | 0.3 | 0.1 to 0.8 | ||||||
| Braden moisture subscore | 0.04 | 3.0 | 1.1 to 8.3 | ||||||
| Bates-Jensen et al. 2007132 USA |
35 non-surgical patients Setting: nursing home Specialty: elderly/geriatric |
Long-stay residents in two nursing homes eligible for a larger nutrition trial (not referenced) and provided informed written consent | Cohort Generalised logistic regression |
35 (45.7%), 16 stage ≥ 2 | 5 (2) | LQS Inadequate sample size resulting in wide CIs |
|||
| Subepidermal moisture (at 1 week) | ≤ 0.05 | 1.0 | 1.004 to 1.012 | ||||||
| Total Braden scale score | ≤ 0.05 | 6.8 | 0.6 to 72.3 | ||||||
| Baumgarten et al. 2004133 USA |
2285 non-surgical patients Setting: long-term nursing care/nursing home Specialty: NR |
Patients aged ≥ 65 years, newly admitted to nursing home, black or white skin colour, consent or relative assent. Excluded if previously resided in a nursing home or chronic care facility for ≥ 8 days in the year before the nursing home admission | Cohort Cox proportional hazards model |
1938 (23.2%), 450 stage ≥ 2 | 12 (3) | MQS All risk factors are categorical data rather than continuous data; 20% missing data in the final model |
|||
| Black race | 0.032 | 1.3 | 1.0 to 1.7 | ||||||
| Number of ADL dependencies | 0.001 | 1.4 | 1.3 to 1.5 | ||||||
| PU on admission | 0.001 | 1.8 | 1.4 to 2.3 | ||||||
| Bergquist and Frantz 1999134 USA |
1711 non-surgical patients Setting: community/home care Specialty: elderly/geriatric |
Home health-care agency, aged ≥ 60 years, no PU on admission, non-hospice, non-intravenous therapy. Consent not required | Record review Stepwise Cox proportional hazards model |
1567 (3.2%), 55 stage ≥ 2 | 45 (10) | LQS Record review; insufficient number of events; inadequate measurement of risk factors |
|||
| Limited to wheelchair | 0.0198 | 2.8 | 1.2 to 6.5 | ||||||
| ADL dressing | < 0.001 | 2.7 | 1.5 to 4.8 | ||||||
| Incontinence bowel and/or bladder | 0.0195 | 2.8 | 1.2 to 6.8 | ||||||
| Braden mobility subscore | < 0.001 | 5.2 | 2.4 to 11.1 | ||||||
| Anaemia | 0.0021 | 4.0 | 1.6 to 9.5 | ||||||
| Adult child primary caregiver | < 0.001 | 5.8 | 2.1 to 15.9 | ||||||
| Male | 0.0281 | 1.9 | 1.1 to 3.2 | ||||||
| Recent fracture | 0.0019 | 3.5 | 1.6 to 7.6 | ||||||
| Oxygen use | < 0.001 | 3.9 | 2.1 to 7.6 | ||||||
| Skin drainage | < 0.001 | 6.6 | 2.3 to 19.2 | ||||||
| Bergstrom and Braden 1992135 USA |
200 non-surgical patients Setting: long-term nursing care/nursing home Specialty: elderly/geriatric |
Consecutive patient admissions to teaching nursing home were screened and included if aged > 65 years, at risk of PU development (Braden score ≤ 17), free of existing PU, estimated LOS > 10 days. Consent required from patient or family | Cohort Logistic regression (backward elimination) |
200 (73.5%), 147 stage ≥ 1; (38.5%), 77 stage ≥ 2 Model 1: stage ≥ 1; model 2: stage ≥ 2; model 3: stage = 1 |
Model 1: 10 (5) | MQS No CIs reported |
|||
| Braden scale score | < 0.01 | NR | NR | ||||||
| Diastolic BP | < 0.01 | NR | NR | ||||||
| Temperature | NS | NR | NR | ||||||
| Age | NS | NR | NR | ||||||
| Protein (% RDA) | < 0.05 | NR | NR | ||||||
| Model 2: 10 (4) | |||||||||
| Braden scale score | < 0.001 | NR | NR | ||||||
| Age | < 0.05 | NR | NR | ||||||
| Systolic BP | < 0.01 | NR | NR | ||||||
| Protein (% RDA) | NS | NR | NR | ||||||
| Model 3: 10 (4) | |||||||||
| Braden scale score | < 0.01 | NR | NR | ||||||
| Diastolic BP | < 0.01 | NR | NR | ||||||
| Temperature | < 0.05 | NR | NR | ||||||
| Iron (% RDA) | < 0.01 | NR | NR | ||||||
| Bergstrom et al. 1996136 USA |
843 patients Setting: multiple Specialty: multiple |
Patients from two nursing homes, two university hospitals and two VAMCs, aged ≥ 19 years, no PU on admission, admitted for care within 72 hours | Cohort Logistic regression |
843 (12.8%), 108 stage ≥ 1 Model 1: age, gender, race, Braden scale score and preventative measures; model 2: mobility, activity and presence or not of 13 listed primary diagnoses; model 3: Braden scale total score and presence or not of 13 listed primary diagnoses |
Model 1: 6 (3) | HQS | |||
| Braden scale score | < 0.001 | 1.3 | 1.2 to 1.4 | ||||||
| Age | < 0.001 | 1.0 | 0.95 to 0.98 | ||||||
| Race | 0.012 | 2.7 | 1.3 to 6.0 | ||||||
| Model 2: 15 (3) | |||||||||
| Braden mobility subscore | < 0.001 | 1.7 | 1.3 to 2.3 | ||||||
| Braden activity subscore | 0.004 | 1.5 | 1.1 to 1.9 | ||||||
| Cardiovascular disease | 0.023 | 2.5 | 1.1 to 5.5 | ||||||
| Model 3: 14 (1) | |||||||||
| Braden total score | < 0.001 | 1.4 | 1.3 to 1.5 | ||||||
| Berlowitz and Wilking 1989137 USA |
185 non-surgical patients Setting: chronic care hospital Specialty: medicine |
All admissions to chronic care hospital (requiring medical, skilled nursing or rehabilitative services) with chronic medical conditions. Patients excluded if died or discharged within 1 week of admission or required transfer to an acute care hospital within 24 hours of admission (i.e. had PU at baseline). Consent not required – record review | Cohort Stepwise logistic regression |
185 (10.8%), 20 stage ≥ 2 | 11 (3) | LQS Insufficient number of events; data collection relied on clinical staff; only partial reporting of baseline characteristics |
|||
| Cerebrovascular accident | < 0.05 | 5.0 | 1.7 to 14.5 | ||||||
| Bed or chair bound | < 0.05 | 3.8 | 1.0 to 14.0 | ||||||
| Impaired nutritional intake | < 0.05 | 2.8 | 1.0 to 17.9 | ||||||
| Bostrom et al. 1996138 USA |
112 patients Setting: multiple Specialty: multiple |
Medical and surgical patients admitted to three hospitals (tertiary, general, community) aged ≥ 18 years, able to give consent and expected LOS (hospital) ≥ 48 hours | Cohort Logistic regression |
112 (8.04%), 9 stage ≥ 1 | 7 (1) | VLQS Insufficient number of events; analysis reporting inadequate; no CIs reported; time-dependent variables included in the analysis |
|||
| Number of layers between patient and mattress | 0.001 | NR | NR | ||||||
| Bourdel-Marchasson et al. 2000139 France |
672 patients Setting: acute care hospital Specialty: elderly/geriatric |
Patients recruited from university hospital wards and geriatric units (with > 40% of inpatients aged > 65 years), including neurology, gastroenterology, orthopaedic or vascular surgery, internal and geriatric medicine. Patients aged > 65 years, in acute phase of a critical illness, unable to move or eat independently, no PU on admission. Consent requirement not reported | RCT Cox proportional hazards model |
672 (44.5%), 299 stage ≥ 1 | NR (5) | MQS Full details of modelling not provided; adequate number of events is assumed as large number of events (n = 299) |
|||
| Hypoalbuminaemia | < 0.001 | 1.1 | 1.0 to 1.1 | ||||||
| Lower limb fracture | < 0.001 | 2.7 | 1.8 to 4.1 | ||||||
| Norton score 5–10 vs. > 14 | 0.04 | 1.3 | 1.0 to 1.6 | ||||||
| Kuntzman score | 0.003 | 1.2 | 0.3 to 4.6 | ||||||
| Control vs. nutritional intervention | 0.04 | 1.6 | 1.0 to 2.4 | ||||||
| Boyle and Green 2001140 UK |
534 patients Setting: ICU Specialty: intensive care |
ICU patients not consented. PUs developing after day 1 of admission included in the analysis; those with PU on admission excluded | Cohort Parametric survival regression (Weibull) |
534 (5.2%), 28 grade ≥ 1 | 7 (2) | LQS Baseline characteristics not reported; insufficient number of events |
|||
| Coma/unresponsiveness/paralysed and sedated | 0.001 | 4.2 | 30 to 77 | ||||||
| Cardiovascular instability | 0.035 | 2.7 | 4 to 70 | ||||||
| Brandeis et al. 1994141 USA |
4232 non-surgical patients Setting: long-term nursing care/nursing home Specialty: elderly/geriatric |
Residents aged > 60 years, admitted to nursing home during 1988 and 1989, no PU on admission and at 3 months’ FU (baseline assessment) Eligible residents remained in the home for ≥ 3 months after baseline assessment up to 21 months. Consent not required – record review | Cohort Pooled logistic regression |
4232 (12.9%), 546 stage ≥ 2 Model 1, high-incidence homes: 1322 (19.3%), 255 stage ≥ 2; model 2, low-incidence homes: 1365 (6.5%), 89 stage ≥ 2 |
Model 1: 15 (4) | HQS Record review |
|||
| Ambulation difficulty | < 0.001 | 3.3 | 2.0 to 5.3 | ||||||
| Faecal incontinence | < 0.001 | 2.5 | 1.6 to 4.0 | ||||||
| Diabetes | < 0.006 | 1.7 | 1.2 to 2.5 | ||||||
| < 0.001 | 2.2 | 1.5 to 3.3 | |||||||
| Model 2: 15 (3) | |||||||||
| Ambulation difficulty | < 0.001 | 3.6 | 1.7 to 7.4 | ||||||
| ADL feeding | < 0.001 | 3.5 | 2.0 to 6.3 | ||||||
| Male | < 0.007 | 1.9 | 1.2 to 3.6 | ||||||
| Chan et al. 2005142 Singapore |
666 patients Setting: acute care hospital Specialty: multiple |
All hospital inpatients on census date, aged > 18 years. Excluded infectious disease wards, aggressive psychiatric or airborne infectious patients, patients with existing ulcers | Cohort Logistic regression |
666 (8.1%), 54 stage ≥ 1 | 23 (1) | LQS Only partial reporting of baseline characteristics; inadequate reporting of analysis and modelling; inadequate number of events |
|||
| Braden score | 0.001 | ||||||||
| (Braden score 12–15) | 0.001 | 7.0 | 3.5 to 17.1 | ||||||
| (Braden score 6–11) | 0.001 | 12.5 | 4.5 to 34.6 | ||||||
| Cobb et al. 1997143 USA |
123 patients Setting: acute care hospital Specialty: intensive care |
Aged > 18 years, weight ≤ 290 lb, no pre-existing PU, expected LOS 1–2 weeks, determined to be at risk based on Braden scale score. Consent required. All hospital wards and ICU of a large military hospital | RCT Wilcoxon test |
123 (16.3%), 20 stage ≥ 1 | 4 (2) | VLQS Inadequate reporting of analysis methods; no CIs reported; insufficient number of events |
|||
| Hypertension | 0.03 | NR | NR | ||||||
| Weight | 0.05 | NR | NR | ||||||
| Compton et al. 2008144 German |
713 patients Setting: acute care hospital, non-surgical Specialty: intensive care |
Patients without a PU on admission to the medical ICU between April 2001 and December 2004. Patients in the ICU for < 72 hours were excluded from analysis | Record Review | 698 (17%), 121 grades 2–4 | 32 (6) | LQS Record review; large number of events but used 32 variables in the model; no CIs reported |
|||
| Male gender | 0.014 | 1.8 | NR | ||||||
| Moist skin | 0.001 | 2.4 | NR | ||||||
| Oedematous skin | 0.002 | 2.2 | NR | ||||||
| Centralised circulation | 0.001 | 2.4 | NR | ||||||
| Mottled skin | 0.016 | 2.0 | NR | ||||||
| Reddened skin | 0.001 | 2.3 | NR | ||||||
| Defloor and Grypdonck 200557 Belgium |
1772 non-surgical patients Setting: long-term nursing care/nursing home Specialty: elderly/geriatric |
All inpatients in 11 long-term care facilities during the 4-week study period | RCT Stepwise logistic regression |
1458 Model 1: 302/1458 (20.7%) grade ≥ 1; model 2: 171/1458 (11.7%) grade ≥ 2 |
Model 1: 19 (3) | HQS Limitation is the partial reporting of baseline characteristics |
|||
| Braden sensory perception subscore | 0.02 | 0.8 | 0.6 to 1.0 | ||||||
| Skin condition | < 0.001 | 1.5 | 1.2 to 1.9 | ||||||
| Existing PU | < 0.001 | 2.3 | 1.4 to 3.5 | ||||||
| Model 2: 19 (4) | |||||||||
| Braden activity subscore | 0.03 | 0.7 | 0.5 to 1.0 | ||||||
| Braden sensory perception subscore | 0.02 | 0.7 | 0.6 to 1.0 | ||||||
| Skin condition | < 0.001 | 1.6 | 1.3 to 2.1 | ||||||
| Existing PU | 0.01 | 1.9 | 1.1 to 3.0 | ||||||
| De Laat et al. 2007145 Netherlands |
399 patients Setting: acute care hospital Specialty: intensive care |
Patients admitted into ICU with expected LOS > 48 hours, without PU on admission and screened within 48 hours of admission. Consent not required | Cohort Cox proportional hazards model |
399 (35.1%), 140 grade ≥ 2 | 11 (3) | MQS Ward staff recorded data; no CIs reported; time-dependent covariates included in the analysis |
|||
| Preventative transfers | < 0.001 | 0.2 | NR | ||||||
| Shock/resuscitation | < 0.001 | 1.5 | NR | ||||||
| Friction/shear | 0.02 | 1.3 | NR | ||||||
| Donnelly 2006146 UK |
240 hip fracture patients Setting: acute care hospital Specialty: elderly/geriatric |
Aged ≥ 65 years on day of injury, new fractured hip (injury < 48 hours ‘old’), able to undergo tests and assessment procedures. Patient consent required | RCT Cox proportional hazards model |
239 (16.3%), 39 grade ≥ 1 | 20 (1) | LQS Insufficient number of events; no CI reported |
|||
| Control group (standard mattress) | 0.001 | 4.6 | NR | ||||||
| Ek 1987147 Sweden |
515 non-surgical patients Setting: chronic care hospital Specialty: medicine |
Consecutive patients admitted to a long-term medical ward who were hospitalised for > 3 days, with or without a PU at baseline. Consent requirement not reported | Cohort Logistic regression |
515 (7.6%), 39 ≥ stage 1-equivalent PU Model 1: baseline measures; model 2: variables on day of PU or if PU free in fourth week of care |
Model 1: 8 (1) | VLQS Partial reporting of baseline characteristics; inadequate reporting of methods; insufficient number of events; no CIs reported |
|||
| Norton mobility | < 0.05 | NR | NR | ||||||
| Model 2: 8 (2) | |||||||||
| General physical condition | < 0.01 | NR | NR | ||||||
| Norton activity | < 0.01 | NR | NR | ||||||
| Ek et al. 1991148 Sweden |
501 non-surgical patients Setting: acute care hospital Specialty: medicine |
Newly admitted long-term medical ward admissions who remained in hospital for > 3 weeks. Patient consent required | RCT Multiple regression |
495 (10.1%), 51 ≥ stage 1-equivalent PU | NR (4) | VLQS Partial reporting of baseline characteristics; inadequate reporting of methods and analysis; no CIs reported; adequacy of number of events cannot be assessed |
|||
| Albumin | < 0.001 | NR | NR | ||||||
| Norton mobility | < 0.001 | NR | NR | ||||||
| Norton activity | < 0.001 | NR | NR | ||||||
| Food intake | < 0.05 | NR | NR | ||||||
| Feuchtinger et al. 2006149 Germany |
175 surgical patients Setting: acute care hospital Specialty: cardiac surgery |
Aged ≥ 18 years, scheduled for cardiac surgery with electrocardiogram, not included in another study. Consent required | RCT Logistic regression |
175 (14.3%), 25 grade ≥ 1 | 13 (1) | LQS Inadequate reporting of analysis; insufficient number of events; no CIs reported |
|||
| Renal insufficiency | 0.05 | NR | NR | ||||||
| Fife et al. 2001150 USA |
186 patients Setting: ICU Specialty: intensive care |
Patients admitted to neuro-ICU (acute SCI/head injuries/gunshot wounds/CVAs). No consent required (apart for photographs). Excluded if more than two PUs on initial assessment, discharge from unit < 24 hours after admission, diagnosis of brain death or life support pending organ donation, no evaluation by nursing staff within 12 hours after admission | Cohort Stepwise logistic regression |
149 (15.4%), 23 stage ≥ 2 | 11 (2) | LQS Insufficient number of events; ORs and CIs not reported |
|||
| Braden score | 0.002 | NR | NR | ||||||
| Age | 0.043 | NR | NR | ||||||
| Goodridge et al. 1998151 Canada |
330 non-surgical patients Setting: acute care hospital Specialty: elderly/geriatric |
Medical/elderly of tertiary care and long-term care facilities, aged > 65 years, within 48–96 hours of admission. Excluded pre-existing dermal ulcers, terminal stage cancer, acute/chronic renal failure | Cohort Stepwise logistic regression |
330 (9.7%), 32 stage ≥ 1 | 5 (1) | VLQS Partial presentation of baseline data; nutritional factors collected but not analysed; analysis reporting inadequate; no CI reported; insufficient number of events; time-dependent variable included in the analysis |
|||
| Number of prevention strategies used prior to PU appearance | < 0.001 | 1.4 | NR | ||||||
| Gunningberg et al. 2001152 Sweden |
146 hip fracture patients Setting: acute care hospital Specialty: trauma |
Patients with hip fracture, aged ≥ 65 years, admitted without a PU, assessments carried out in A&E or orthopaedic department. Not sure about consent – assume not | Record review Logistic regression |
146 (36.9%), 54 stage ≥ 1 | 3 (1) | MQS Partial reporting of baseline characteristics and analysis reporting inadequate; no CI reported |
|||
| Advanced age | 0.03 | 1.1 | NR | ||||||
| Halfens et al. 2000153 Netherlands |
320 patients Setting: acute care hospital Specialty: multiple |
Three hospitals; patients from surgical, neurological, orthopaedic and internal medicine wards. No PU on admittance, Caucasian, probable LOS (hospital) ≥ 10 days. Consent required | Cohort Stepwise logistic regression |
320 (14.7%), 47 grade ≥ 1 | 16 (4) | LQS Partial reporting of baseline characteristics; insufficient number of events |
|||
| Braden sensory perception subscore | < 0.01 | 3.7 | 1.4 to 9.3 | ||||||
| Age | < 0.01 | 2.3 | 1.4 to 3.9 | ||||||
| Braden friction/shear subscore | < 0.01 | 2.3 | 1.4 to 4.0 | ||||||
| Braden moisture subscore | < 0.01 | 2.1 | 1.2 to 3.5 | ||||||
| Hatanaka et al. 2008154 Japan |
149 non-surgical patients Setting: acute care hospital Specialty: respiratory |
Bedridden patients hospitalised for a respiratory disorder, required constant attentive care or needed a considerable amount of assisted care | Cohort Cox proportional hazards model |
149 (25.5%), 38 grade ≥ 2 | NR (5) | LQS Clinical data collection method not reported and number of factors entered into the stepwise procedure not reported, therefore adequacy of number of events cannot be assessed |
|||
| Haemoglobin | 0.006 | 1.2 | 1.1 to 1.4 | ||||||
| C-reactive protein | 0.042 | 1.9 | 1.0 to 3.9 | ||||||
| Albumin | 0.021 | 0.4 | 0.2 to 0.9 | ||||||
| Age | 0.953 | 1.0 | 0.97 to 1.03 | ||||||
| Gender | 0.379 | 0.7 | 0.3 to 1.7 | ||||||
| Inman et al. 1999155 Canada |
149 patients Setting: ICU Specialty: intensive care |
Aged ≥ 17 years, APACHE II score ≥ 15, expected LOS (ICU) ≥ 3 days. Patients excluded if PUs at baseline, not expected to survive, admitted for compassionate care or ICU transfer. Consecutive admissions randomised – not concealed allocation, consent procedure not detailed | RCT Stepwise logistic regression |
144 (25.7%), 37 stage ≥ 1 | 9 (2) | VLQS Poor quality reporting; insufficient number of events; limited number of risk factors; inadequate statistical reporting and the independent variable is a composite score that includes the dependent variable; p-values, ORs and CIs not reported; data reporting by ward staff; time-dependent variables included in the analysis (LOS and increase in SURE score) |
|||
| LOS in ICU | NR | NR | NR | ||||||
| Increase in SURE score | NR | NR | NR | ||||||
| Kemp et al. 1993156 USA |
84 non-surgical patients Setting: multiple Specialty: elderly/medical |
Patients recruited from hospital inpatient (general medicine and geriatric medicine) and long-term care facilities. Included if aged ≥ 65 years, Braden scale score ≤ 16 and PU free. Consent requirements not detailed | RCT Cox regression |
84 (39.3%), 33 stage ≥ 1 | 11 (2) | LQS Inadequate number of events; CIs not reported |
|||
| Overlay type | 0.018 | NR | NR | ||||||
| Average Braden mobility subscore | < 0.001 | NR | NR | ||||||
| Lindgren et al. 2004157 Sweden |
548 mixed patients Setting: acute care hospital Specialty: multiple |
Elective and acute medical or surgical patients admitted to 21 wards in university hospital, aged > 17 years, expected LOS (hospital) ≥ 5 days, for patients undergoing surgery expected time on operating table ≥ 1 hour and PU free. Verbal consent required (patient or relative). Consecutive patients admitted in 3 defined days included up to maximum of nine per week | Cohort Multiple stepwise logistic regression |
530 (11.7%) 62 stage ≥ 1 Model 1: total sample; model 2: medical patients: 244 (8.6%) 21 stage ≥ 1; model 3: surgical patients: 286 (14.3%) 41 stage ≥ 1 |
Model 1: 13 (5) | LQS Insufficient number of events; time-dependent covariate was included in the analysis |
|||
| Mobility RAPS scale | 0.011 | 0.5 | 0.3 to 0.9 | ||||||
| Length of hospitalisation | 0.002 | 1.0 | 1.0 to 1.1 | ||||||
| Age | 0.014 | 1.0 | 1.0 to 1.1 | ||||||
| Weight | 0.006 | 1.0 | 0.9 to 1.0 | ||||||
| Surgical treatment | < 0.001 | 4.8 | 2.0 to 11.4 | ||||||
| Model 2: 13 (3) | |||||||||
| Mobility RAPS scale | 0.001 | 0.4 | 0.2 to 0.6 | ||||||
| Length of hospitalisation | 0.029 | 1.0 | 1.00 to 1.04 | ||||||
| Diastolic BP | 0.026 | 1.0 | 0.9 to 1.0 | ||||||
| Model 3: 13 (3) | |||||||||
| Serum albumin RAPS scale | 0.029 | 0.5 | 0.3 to 0.9 | ||||||
| Length of hospitalisation | 0.027 | 1.0 | 1.0 to 1.1 | ||||||
| Weight | 0.002 | 1.0 | 0.9 to 1.0 | ||||||
| Marchette et al. 1991158 USA |
161 surgical patients Setting: acute care hospital Specialty: intensive care |
Patients aged > 59 years in ICU after surgery. Consent not required | Record review Discriminant analysis |
161 (39.1%), 63 ≥ stage 2-equivalent PUs | NR (5) | VLQS Inadequate reporting of methods and analysis; no CIs reported; included time-dependent variables in the analysis; adequacy of number of events cannot be assessed |
|||
| Skin redness | < 0.001 | NR | NR | ||||||
| Days static air mattress for prevention | < 0.001 | NR | NR | ||||||
| Faecal incontinence | 0.0013 | NR | NR | ||||||
| Diarrhoea | 0.0019 | NR | NR | ||||||
| Preoperative albumin | 0.0028 | NR | NR | ||||||
| Nijs et al. 2009159 Belgium |
520 patients Setting: acute care hospital, surgical Specialty: intensive care |
Expected LOS in surgical ICU of an acute hospital > 24 hours. Excluded if aged < 16 years and admitted for burn injuries | Cohort Multivariate logistic regression |
463 (28.9%), 134 grades 2–4 | 19 (9) | MQS Full details of modelling not provided; adequate number of events is assumed as large number of events |
|||
| Dopamine < 5 µg/kg/minute | 0.003 | 6.1 | 1.9 to 19.5 | ||||||
| Medical history of vascular disease | < 0.001 | 4.5 | 2.0 to 10.2 | ||||||
| Intermittent haemodialysis or continuous veno-venous haemofiltration | 0.045 | 3.8 | 1.0 to 13.9 | ||||||
| Adequate prevention | 0.002 | 6.0 | 1.9 to 18.6 | ||||||
| Frequency of turning six or more times a day or alternating mattress | < 0.001 | 30.2 | 12.2 to 74.8 | ||||||
| Turning | < 0.001 | 6.7 | 2.7 to 16.4 | ||||||
| Use of sedatives | 0.006 | 0.3 | 0.1 to 0.7 | ||||||
| Body temperature ≥ 38.5 °C | 0.029 | 0.2 | 0.2 to 0.9 | ||||||
| Sitting in chair | < 0.001 | 0.1 | 0.0 to 0.3 | ||||||
| Nixon et al. 200637 UK |
1972 patients Setting: acute care hospital Specialty: multiple |
Aged ≥ 55 years, admitted to orthopaedic, vascular, medical or care of elderly wards, acute or elective, expected LOS ≥ 7 days, limited activity or mobility, existing grade 2 PU. Consent required | RCT, Logistic regression |
1971 (10.5%), 207 grade ≥ 2 | 13 (7) | HQS Minor limitation – number of patients in final model NR |
|||
| Hospital | 0.02 | ||||||||
| Acute admission | < 0.001 | 3.7 | 2.3 to 5.9 | ||||||
| Baseline wound | < 0.001 | 3.0 | 1.7 to 5.1 | ||||||
| Baseline skin trauma | 0.05 | 1.7 | 1.0 to 2.8 | ||||||
| Baseline grade 1 | 0.001 | 2.0 | 1.3 to 2.9 | ||||||
| Age | 0.03 | 1.0 | 1.002 to 1.04 | ||||||
| Diabetes | 0.047 | 1.6 | 1.0 to 2.6 | ||||||
| Nixon et al. 200760 UK |
109 surgical patients Setting: acute care hospital Specialty: multiple |
Aged > 55 years, expected LOS ≥ 5 days, scheduled for elective major general or vascular or acute orthopaedic surgery (average surgical time ≥ 90 minutes), with or without PU at baseline. Consent required | Cohort Forward stepwise logistic regression |
97 (15.5%), 15 grade ≥ 2 | 8 (4) | LQS Inadequate number of events; included time-dependent variables in the analysis |
|||
| Preoperative albumin | 0.009 | 0.8 | 0.7 to 1.0 | ||||||
| Grade 1 equivalent | 0.008 | 7.0 | 1.7 to 29.5 | ||||||
| Weight loss | 0.092 | 0.3 | 0.1 to 1.2 | ||||||
| Minimum diastolic BP | 0.205 | 1.0 | 0.9 to 1.0 | ||||||
| Okuwa et al. 2006160 Japan |
259 non-surgical patients Setting: long-term nursing care/nursing home Specialty: elderly/geriatric |
Patients admitted to long-term care facility, aged ≥ 65 years, bedfast, without lower-extremity PU, LOS (hospital) ≥ 14 days, at risk of developing PU. Consent required (patient or family) | Cohort Forward stepwise Cox regression |
259 (12.7%), 33 stage ≥ 2 | 9 (3) | LQS Inadequate number of events; time-dependent variables reported |
|||
| Ankle brachial index | < 0.001 | 0.1 | 0.0 to 0.2 | ||||||
| Length of bedfast period | 0.003 | 3 | 1.5 to 6.0 | ||||||
| Male gender | 0.001 | 1 | 1.004 to 1.015 | ||||||
| Olson et al. 1996161 USA |
149 patients Setting: acute care hospital Specialty: multiple |
Medical and surgical inpatients aged ≥ 18 years, no PU on admission, expected LOS (hospital) ≥ 5 days. Consent required | Cohort Stepwise logistic regression |
143 (13.9%), 20 stage ≥ 1 | 11 (3) | LQS Insufficient number of events |
|||
| Haemoglobin | 0.0731 | NR | NR | ||||||
| Hours in bed | 0.0551 | NR | NR | ||||||
| Pulse pressure | 0.3022 | NR | NR | ||||||
| Ooi et al. 1999162 USA |
5518 non-surgical patients Setting: long-term nursing care/nursing home Specialty: elderly/geriatric |
Nursing home residents free from PUs at baseline and 3-month FU assessment. Excluded residents in homes with < 50 beds. Consent not required – record review | Record review Logistic regression backward elimination |
5518 (11.4%), 629 stage ≥ 2 | 6 (6) | MQS Record review and limited range of risk factors considered (e.g. do not include mobility in the model) |
|||
| Age | 0.0081 | 1 | 1.00 to 1.03 | ||||||
| Diabetes | 0.0106 | 1.4 | 1.1 to 1.7 | ||||||
| Faecal/urine incontinence | < 0.001 | 1.6 | 1.2 to 2.0 | ||||||
| Transfers | < 0.001 | 1.5 | 1.2 to 1.8 | ||||||
| Medicaid payments | 0.0623 | 1.2 | 1.0 to 1.4 | ||||||
| Facility effects | |||||||||
| Facility effects intermediate risk | < 0.001 | 1.6 | 1.3 to 2.0 | ||||||
| Facility effects high risk | < 0.001 | 1.9 | 1.5 to 2.4 | ||||||
| Pancorbo Hidalgo and Garcia Fernandez 2001163 Spain |
187 patients Setting: acute care hospital Specialty: multiple |
Patients at risk of PUs (Gosnell score ≤ 12) and aged > 70 years, admitted to internal medicine, ICU, general surgery and orthopaedic wards | Cohort Logistic regression |
187 (16.6%), 31 stage ≥ 1 Model 1: stage ≥ 1; model 2: stage ≥ 2 |
Model 1: 16 (9) | LQS Article was translated so unable to undertake detailed quality assessment; limitations based on inadequate number of events; time-dependent variables included in the analysis |
|||
| LOS | < 0.05 | 1.1 | 1.1 to 1.2 | ||||||
| Gosnell score | < 0.05 | 1.2 | 1.1 to 1.2 | ||||||
| Incontinence | < 0.05 | 2.2 | 1.7 to 2.9 | ||||||
| Skin alterations diminished | < 0.05 | 1.4 | 1.0 to 1.9 | ||||||
| Highest systolic BP | < 0.05 | 1 | 0.9 to 1.0 | ||||||
| Lowest diastolic BP | < 0.05 | 1.1 | 1.06 to 1.13 | ||||||
| Low skinfold thickness | < 0.05 | 1.3 | 1.0 to 1.6 | ||||||
| Diminished lymphocytes | < 0.05 | 1.2 | 1.0 to 1.5 | ||||||
| Low haemoglobin | < 0.05 | 2.2 | 1.3 to 3.9 | ||||||
| Model 2: (10) | |||||||||
| LOS | < 0.05 | 1.2 | 1.1 to 1.2 | ||||||
| Gosnell score | < 0.05 | 1.1 | 1.1 to 1.2 | ||||||
| Incontinence | < 0.05 | 1.2 | 1.1 to 1.2 | ||||||
| Activity diminished | < 0.05 | 2 | 1.2 to 3.5 | ||||||
| Highest systolic BP | < 0.05 | 1 | 0.9 to 1.0 | ||||||
| Lowest diastolic BP | < 0.05 | 1.1 | 1.0 to 1.1 | ||||||
| Low skinfold thickness | < 0.05 | 1.4 | 1.0 to 1.9 | ||||||
| Diminished lymphocytes | < 0.05 | 1.5 | 1.1 to 2.0 | ||||||
| Low haemoglobin | < 0.05 | 3 | 1.5 to 6.1 | ||||||
| Use of alternating overlay (at-risk patients) | < 0.05 | 2.7 | 1.0 to 6.9 | ||||||
| Perneger et al. 2002164 Switzerland |
1190 patients Setting: acute care hospital Specialty: multiple |
All newly admitted patients to mixed specialties within a teaching hospital (with or without PU at baseline). Consent not required | Cohort Multivariate proportional hazards model |
1190 (10.8%), 129 stage ≥ 1 | 10 (3) | HQS Limitation is the partial reporting of baseline characteristics |
|||
| Braden/Norton mobility subscore | 0.006 | 1.4 | 1.1 to 1.8 | ||||||
| Braden friction/shear subscore | 0.034 | 1.5 | 1.0 to 1.8 | ||||||
| Age 16–59 years | |||||||||
| (Age 60–69 years) | 1.5 | 0.8 to 2.2 | |||||||
| (Age 70–79 years) | 2.5 | 1.5 to 4.4 | |||||||
| (Age 80–89 years) | 3.8 | 2.3 to 6.4 | |||||||
| (Age 90–96 years) | 5.2 | 2.6 to 10.6 | |||||||
| Rademakers et al. 2007165 Netherlands |
722 hip fracture patients Setting: acute care Specialty: trauma |
Hip fracture patients admitted to level 1 trauma centre. Excluded those aged < 60 years, (multiple) high-energy trauma (fall from higher than ground level; road traffic accident), initial conservative treatment, interhospital transfer, presence of PUs on admission, pathological fractures and recurrent fractures | Record review Multivariate logistic regression |
722 (29.6%), 214 stage ≥ 2 | 10 (5) | MQS Large sample size but limited number of risk factors considered and not based on a conceptual framework (no nutrition or skin moisture factors); inadequate measurement of risk factors (record review) |
|||
| Diabetes | 0.021 | 1.7 | 1.1 to 2.7 | ||||||
| Postoperative urinary tract infection | 0.004 | 1.9 | 1.2 to 2.9 | ||||||
| Postoperative hip dislocation | 0.009 | 2.7 | 1.3 to 5.6 | ||||||
| ASA class III/IV | 0.001 | 4.2 | 2.9 to 6.1 | ||||||
| Time to surgery > 12 hours | 0.008 | 1.7 | 1.2 to 2.6 | ||||||
| Reed et al. 200380 USA |
2771 non-surgical patients Setting: chronic care hospital Specialty: medicine |
Record review identifying mobility impaired, admitted to the chosen hospital wards between 1 July 1994 and 1 October 1997, LOS ≥ 1 week. Consent not required, grade 3 and 4 PUs reported | Record review Forward stepwise logistic regression |
2771 (14.7%), 406 stage ≥ 2 | 7 (6) | HQS Record review |
|||
| Low albumin levels | 0.014 | 1.4 | 1.1 to 1.8 | ||||||
| Confusion | 0.001 | 1.5 | 1.2 to 1.8 | ||||||
| Do not resuscitate order | < 0.001 | 1.5 | 1.2 to 1.9 | ||||||
| Urinary catheter on admission | < 0.001 | 1.6 | 1.4 to 1.8 | ||||||
| Malnutrition | < 0.001 | 1.7 | 1.3 to 2.2 | ||||||
| Stage 1 PU | < 0.001 | 3.1 | 2.4 to 4.1 | ||||||
| Rose et al. 2006166 Canada |
111 patients Setting: acute care hospital Specialty: intensive care |
Consecutive admissions to university hospital ICU. Consent not reported | Cohort Multiple regression |
111 (43.2%), 48 stage ≥ 1 | NR (3) | VLQS Abstract only; inadequate information on methodology and analysis; no p-values or CIs reported |
|||
| Skin quality | NR | NR | NR | ||||||
| Restricted movement | NR | NR | NR | ||||||
| Temperature | NR | NR | NR | ||||||
| Salzberg et al. 1999167 USA |
226 SCI patients Setting: acute care hospital Specialty: trauma |
SCI with a neurological deficit attributable to damage of the spinal cord, excluding the cortices and brainstem, defined by ICD-9-CM, acute SCI as a result of a trauma, survival ≥ 14 days following acute SCI, level of SCI between C4 and S1 | Record review Model 1 forward stepwise linear regression; model 2 Cox proportional hazards model |
226 (38.5%), 87 stage ≥ 1 | Model 1: 8 (3) | MQS Limited because record review and no CIs reported |
|||
| Extent of paralysis | < 0.001 | NR | NR | ||||||
| Moisture | < 0.001 | NR | NR | ||||||
| Serum creatinine | 0.007 | NR | NR | ||||||
| Model 2: 8 (8) | |||||||||
| Extent of paralysis | < 0.001 | NR | NR | ||||||
| Moisture | 0.003 | NR | NR | ||||||
| Serum creatinine | 0.006 | NR | NR | ||||||
| Incontinence | < 0.001 | NR | NR | ||||||
| Albumin | 0.028 | NR | NR | ||||||
| Mobility | 0.002 | NR | NR | ||||||
| Pulmonary disease | 0.014 | NR | NR | ||||||
| Level of activity | 0.036 | NR | NR | ||||||
| Sayar et al. 2009168 Turkey |
140 patients Setting: acute care hospital Specialty: intensive care |
Surgical and medical ICU patients. Within 1–2 hours of admission to the ICU, the Waterlow scale was administered; patients scoring ‘at risk’ and ‘very high risk’ were included | Cohort Multiple stepwise logistic regression |
140 (14.3%), 20 stage ≥ 1 | 6 (2) | LQS Insufficient number of events |
|||
| LOS | < 0.001 | 1.2 | 1.1 to 1.3 | ||||||
| Activity level | 0.005 | 0.3 | 0.2 to 0.7 | ||||||
| Schnelle et al. 1997169 USA |
105 non-surgical patients Setting: long-term nursing care/nursing home Specialty: elderly/geriatric |
Incontinent nursing home residents, consent required. Exclusion criteria were presence of stage 2 or above PU at baseline, use of a catheter, LOS < 60 days | Cohort Stepwise multiple regression |
91 (20.9%), 19 stage ≥ 1 Model 1: stage ≥ 1 severity index = NR; model 2: stage ≥ 1 only = NR |
Model 1: NR (2) | LQS Insufficient number of events and analysis reporting inadequate; no p-values or CIs reported |
|||
| Bed mobility | NR | NR | NR | ||||||
| Blanchable erythema severity | NR | NR | NR | ||||||
| Model 2: NR (1) | |||||||||
| Blanchable erythema severity | NR | NR | NR | ||||||
| Schoonhoven et al. 2002170 Netherlands |
223 surgical patients Setting: acute care hospital Specialty: multiple |
Patients scheduled for surgery expected to exceed 4 hours (post-recruitment exclusion if surgery lasted < 4 hours) | Cohort Multiple logistic regression |
208 (10.1), 21 grade ≥ 2 | 12 (1) | LQS Baseline characteristics not reported; insufficient number of events |
|||
| Length of surgery (minutes) | < 0.05 | 1.0 | 1.0035 to 1.0087 | ||||||
| Schultz et al. 1999171 USA |
413 surgical patients Setting: acute care hospital Specialty: mixed |
Patients scheduled for inpatient care, aged ≥ 18 years, with surgery scheduled to last > 2 hours in lithotomy or supine position. Excluded those with PUs at baseline, severe chronic skin problems or receiving only local anaesthesia | RCT Logistic regression |
413 (21.5%), 89 stage ≥ 1 | 7 (5) | HQS Risk factors recorded by operating room and ward staff although outcome data were assessed by research assistants |
|||
| Age | 0.005 | 1.1 | 1.0 to 1.1 | ||||||
| Presence of diabetes | 0.013 | 2.5 | 1.2 to 5.3 | ||||||
| Less body mass | 0.015 | 0.9 | 0.9 to 1.0 | ||||||
| Use of the study mattress | 0.044 | 1.9 | 1.0 to 3.7 | ||||||
| Admission Braden score | 0.013 | 0.8 | 0.7 to 1.0 | ||||||
| Serpa and Santos 2007172 Brazil |
170 patients Setting: private hospital Specialty: NR |
Age ≥ 18 years, no PU at admission, hospitalised for ≥ 24 hours, total Braden scale score, admitted to two private hospitals, agreed to participate. Exclusion criteria were chronic renal failure, dialysis treatment for > 1 month or the presence of hepatic insufficiency accompanied by ascites | Cohort Multivariate logistic regression |
170 (NR), NR | 16 (5) | LQS Unable to assess in detail as abstract and author communication available only; low-quality study based on assumed inadequate number of events; definition of stage of PU unknown |
|||
| Subjective Global Assessment (for Nutrition) | < 0.001 | ||||||||
| Albumin | < 0.001 | ||||||||
| Urea | < 0.001 | ||||||||
| Age | < 0.001 | ||||||||
| Institution | < 0.001 | ||||||||
| Stordeur et al. 1998173 Belgium |
174 surgical patients Setting: acute care hospital Specialty: cardiac/vascular |
Consecutive patients, aged ≥ 16 years who underwent cardiac or vascular surgery, minimum LOS (hospital) > 5 days. Excluded patients who died. Not sure about consent – assume not | Cohort Stepwise logistic regression |
163 (29.5%), 48 stage ≥ 2 | 16 (3) | LQS Insufficient number of events; CIs not reported |
|||
| Postoperative Braden score | < 0.001 | NR | NR | ||||||
| Haemoglobin concentration at admission | < 0.001 | NR | NR | ||||||
| Postoperative steroid therapy | 0.02 | NR | NR | ||||||
| Suriadi et al. 2007174 Indonesia |
105 patients Setting: ICU Specialty: intensive care |
Admitted to ICU for ≥ 24 hours and expected LOS (ICU) ≥ 3 days, bedfast or unable to walk, free from PUs, informed consent (by patient or family). Excluded patients who were physically incapable of participating (difficult to identify skin condition daily as patient could not be manipulated) or who did not wish to participate | Cohort Multivariate logistic regression |
105 (33.3%), 35 stage ≥ 1 | 6 (4) | LQS Insufficient number of events |
|||
| Interface pressure | < 0.001 | 17.6 | 4.1 to 74.3 | ||||||
| Skin moisture | 0.002 | 8.2 | 2.2 to 30.9 | ||||||
| Smoking > 10 cigarettes per day | 0.001 | 12.7 | 2.8 to 56.7 | ||||||
| Body temperature | 0.001 | 102 | 7.7 to 98.8 | ||||||
| Suriadi et al. 2008125 Japan |
253 patients Setting: acute care hospital Specialty: intensive care |
ICU patients aged > 18 years, admitted at least 24 hours before study enrolment, bedfast, no existing PU, able to give informed consent and of Indonesian origin | Cohort Logistic regression model |
253 (28.4%), 72 stage ≥ 1 | Unknown (3) | MQS Inadequate reporting of analysis and modelling; adequate number of events is assumed as large number of events |
|||
| Interface pressure | 2.2 | 1.6 to 2.9 | |||||||
| Body temperature | 2 | 1.7 to 2.5 | |||||||
| Cigarette smoking | 1.6 | 1.1 to 2.5 | |||||||
| Tourtual et al. 1997175 USA |
291 non-surgical patients Setting: acute care hospital Specialty: medicine, elderly/geriatric |
All patients admitted to four nursing units within an acute hospital, consent given. Baseline PU status not recorded | Cohort Forward stepwise logistic regression |
291 (21.6%), 63 stage ≥ 1 heel PU | 17 (2) | LQS Insufficient number of events; CIs not reported |
|||
| Braden friction and sheer subscore | 0.01 | NR | NR | ||||||
| Braden moisture subscore | 0.007 | NR | NR | ||||||
| Vanderwee et al. 2009176 Belgium |
235 patients Setting: nursing home Specialty: elderly non-surgical |
Nursing home patients with no PU (grades 2–4 according to EPUAP), able to be repositioned, expected LOS > 3 days in the nursing home and with non-blanchable erythema at pressure points on the skin | RCT Multivariate Cox regression analysis |
235 (18.7%), 44 grade ≥ 2 | 16 (6) | LQS Insufficient number of events |
|||
| Age > 80 to 90 years | 0.16 | 0.6 | 0.3 to 1.2 | ||||||
| Age > 90 years | 0.015 | 0.4 | 0.2 to 0.8 | ||||||
| CVA | 0.042 | 1.9 | 1.1 to 3.7 | ||||||
| Urinary incontinence | 0.004 | 0.2 | 0.1 to 0.6 | ||||||
| Dual incontinence | 0.086 | 0.5 | 0.2 to 1.1 | ||||||
| Contractures | 0.04 | 2 | 1.0 to 4.0 | ||||||
| Hypotension | 0.002 | 3.4 | 1.6 to 7.5 | ||||||
| Watts et al. 1998177 USA |
148 patients Setting: acute care Specialty: trauma |
Victims of blunt or penetrating injury, traumatic injury, aged ≥ 15 years, LOS ≥ 2 days and no pre-existing PU | Cohort Logistic regression |
148 (20. 3%), 30 stage ≥ 1 | 20 (1) | VLQS Baseline characteristics not reported; insufficient number of events and presentation of analysis; inadequate measurement of risk factors; no CI or p-value reported |
|||
| Braden mobility subscore | NR | 7.5 | NR | ||||||
| Yepes et al. 2009178 Colombia |
150 patients Setting: acute care hospital Specialty: intensive care |
Patients without PUs on admission, hospitalised for > 48 hours in the ICU and with any of the following risk factors for PUs: intubated and on mechanical ventilation, with vasopressor support | Cohort Multivariate logistic regression |
150 (26.7%), 40 stage ≥ 2 | 8 (3) | LQS Insufficient number of events; time-dependent variable included in the analysis |
|||
| Infection | 0.023 | 2.9 | 1.2 to 7.2 | ||||||
| ICU LOS | 0.005 | 1.1 | 1.1 to 1.2 | ||||||
| APACHE II | 0.044 | 1.1 | 1 to 1.1 |
Emerging risk factor domains/subdomains
The review identified 15 risk factor domains and 46 subdomains (Table 30). The number and quality of studies in which associated risk variables emerged in multivariable modelling and the number and quality of studies in which associated risk variables did not emerge are detailed in Table 30 [full evidence tables are available at: http://medhealth.leeds.ac.uk/downloads/download/657/systematic_review_evidence_tables (accessed August 2015)]. The review highlighted three primary risk factor domains:
-
Mobility/activity (immobility), with the subdomains of mobility subscales, mobility/activity, activities of daily living (ADL) and activity (bedfast/chairfast/immobile descriptors) emerging most consistently.
-
Skin/ulcer status, with the subdomain of stage/grade 1 pressure ulcer emerging most consistently. General skin status was also found to be important but the diverse variables (e.g. dry sacral skin, mottled skin, unhealthy skin, skin redness, baseline skin trauma) made interpretation difficult.
-
Perfusion, with the subdomain of diabetes emerging strongly in the high-/moderate-quality studies. Evidence from the large number of other perfusion-related variables suggests that factors that impair circulation increase the probability of pressure ulcer development but the evidence is limited by the large range of variable descriptors and study quality. Further research is needed in this area.
| Domain summary: variable significant/total number studies entered variable (%) | Number and quality of studies variable significant in multivariable modela | Number and quality of studies variable non-significant in multivariable modela |
|---|---|---|
| Mobility/activity subdomains | ||
| Risk Assessment Scale mobility subscale 8/14 studies (57.1%) |
1 HQS – Perneger et al.164 3 LQS – Bergquist and Frantz;134 Lindgren et al.;157 Kemp et al.156 4 VLQS – Baldwin and Ziegler;131 Watts et al.;177 Ek;147 Ek et al.148 |
1 MQS – Salzberg et al.167 4 LQS – Vanderwee et al.;176 Tourtual et al.;175 Pancorbo Hidalgo and Garcia Fernandez;163 Halfens et al.153 1 VLQS – Bostrom et al.138 |
| Risk Assessment Scale activity subscale 1/16 studies (6.2%) |
1 VLQS – Ek et al.148 | 3 HQS – Defloor and Grypdonck;57 Perneger et al.;164 Nixon et al.37 1 MQS – Salzberg et al.167 7 LQS – Bergquist and Frantz;134 Vanderwee et al.;176 Tourtual et al.;175 Pancorbo Hidalgo and Garcia Fernandez;163 Halfens et al.;153 Lindgren et al.;157 Kemp et al.156 4 VLQS – Baldwin and Ziegler;131 Watts et al.;177 Bostrom et al.;138 Ek147 |
| Activity (bedfast/chairfast/immobile) descriptors 6/11 (54.5%) |
1 MQS – Nijs et al.159 5 LQS – Schnelle et al.;169 Olson et al.;161 Allman et al.;79 Berlowitz and Wilking;137 Okuwa et al.160 |
2 MQS – De Laat et al.;145 Baumgarten et al.133 3 LQS – Fife et al.;150 Bergquist and Frantz;134 Donnelly146 |
| Mobility/activity ADL 4/7 (57.1%) |
1 HQS – Brandeis et al.141 1 MQS – Ooi et al.162 1 LQS – Sayar et al.168 1 VLQS – Rose et al.166 |
1 MQS – Rademakers et al.165 2 LQS – Bergquist and Frantz;134 Donnelly146 |
| General ADL 2/4 (50%) |
1 MQS – Baumgarten et al.133 1 LQS – Bergquist and Frantz134 |
1 HQS – Brandeis et al.141 1 LQS – Berlowitz and Wilking137 |
| RAS friction and shear 4/12 (33.3%) |
1 HQS – Perneger et al.164 1 MQS – De Laat et al.145 2 LQS – Tourtual et al.;175 Halfens et al.153 |
1 HQS – Defloor and Grypdonck57 4 LQS – Bergquist and Frantz;134 Vanderwee et al.;176 Lindgren et al.;157 Kemp et al.156 3 VLQS – Baldwin and Ziegler;131 Watts et al.;177 Bostrom et al.138 |
| Factors affecting mobility 6/13 (46.1%) |
3 MQS – Rademakers et al.;165 Salzberg et al.;167 Bourdel-Marchasson et al.139 3 LQS – Boyle and Green;140 Bergquist and Frantz;134 Vanderwee et al.176 |
1 HQS – Defloor and Grypdonck57 1 MQS – De Laat et al.145 5 LQS – Fife et al.;150 Sayar et al.;168 Tourtual et al.;175 Berlowitz and Wilking;137 Feuchtinger et al.149 |
| Interface pressures 2/2 (100%) |
1 MQS – Suriadi et al.125 1 LQS – Suriadi et al.174 |
|
| Skin/PU status subdomains | ||
| Stage/grade 1 4/4 (100%) |
2 HQS – Reed et al.;80 Nixon et al.37 2 LQS – Allman et al.;79 Nixon et al.60 |
|
| Existing PU 2/5 (40%) |
1 HQS – Defloor and Grypdonck57 1 MQS – Baumgarten et al.133 |
1 HQS – Nixon et al.37 2 LQS – Tourtual et al.;175 Stordeur et al.173 |
| Previous PUs 0/2 (0%) |
2 LQS – Allman et al.;79 Halfens et al.153 | |
| General skin status 9/10 (90%) |
2 HQS – Defloor and Grypdonck;57 Nixon et al.37 5 LQS – Compton et al.;144 Schnelle et al.;169 Allman et al.;79 Pancorbo Hidalgo and Garcia Fernandez;163 Bates-Jensen et al.132 2 VLQS – Rose et al.;166 Marchette et al.158 |
1 LQS – Boyle and Green140 |
| Perfusion subdomains | ||
| Diabetes 5/12 (41.6%) |
3 HQS – Schultz et al.;171 Brandeis et al.;141 Nixon et al.37 2 MQS – Rademakers et al.;165 Ooi et al.162 |
7 LQS – Compton et al.;144 Vanderwee et al.;176 Berlowitz and Wilking;137 Stordeur et al.;173 Halfens et al.;153 Feuchtinger et al.;149 Donnelly146 |
| Vascular disease 4/6 (66.6%) |
1 MQS – Nijs et al.159 3 LQS – Vanderwee et al.;176 Berlowitz and Wilking;137 Feuchtinger et al.149 |
2 LQS – Tourtual et al.;175 Donnelly146 |
| Circulation 3/6 (50%) |
3 LQS – Compton et al.;144 Olson et al.;161 Okuwa et al.160 | 1 HQS – Defloor and Grypdonck57 2 LQS – Tourtual et al.;175 Feuchtinger et al.149 |
| Blood pressure 6/11 (54.5%) |
1 MQS – Bergstrom and Braden135 4 LQS – Boyle and Green;140 Vanderwee et al.;176 Pancorbo Hidalgo and Garcia Fernandez;163 Nixon et al.60 1 VLQS – Cobb et al.143 |
5 LQS – Fife et al.;150 Suriadi et al.;174 Olson et al.;161 Lindgren et al.;157 Donnelly146 |
| Smoking 2/4 (50%) |
1 MQS – Suriadi et al.125 1 LQS – Suriadi et al.174 |
2 LQS – Feuchtinger et al.;149 Donnelly146 |
| Oedema 1/4 (25%) |
1 LQS – Compton et al.144 | 1 MQS – Nijs et al.159 2 LQS – Bergquist and Frantz;134 Donnelly146 |
| Haematological measures subdomains | ||
| Urea and electrolytes 2/4 (50%) |
1 MQS – Salzberg et al.167 1 LQS – Serpa and Santos172 |
2 LQS – Berlowitz and Wilking;137 Okuwa et al.160 |
| Protein 1/3 (33.3%) |
1 LQS – Hatanaka et al.154 | 1 LQS – Sayar et al.168 1 VLQS – Marchette et al.158 |
| Albumin 7/11 (63.6%) |
1 HQS – Reed et al.80 1 MQS – Bourdel-Marchasson et al.139 3 LQS – Serpa and Santos;172 Hatanaka et al.;154 Nixon et al.60 2 VLQS – Ek et al.;148 Marchette et al.158 |
2 MQS – Bergstrom and Braden;135 Salzberg et al.167 2 LQS – Lindgren et al.;157 Kemp et al.156 |
| Lymphopenia 2/2 (100%) |
2 LQS – Allman et al.;79 Pancorbo Hidalgo and Garcia Fernandez163 | |
| Haemoglobin 6/11 (54.5%) |
1 HQS – Nixon et al.37 5 LQS – Hatanaka et al.;154 Bergquist and Frantz;134 Olson et al.;161 Stordeur et al.;173 Pancorbo Hidalgo and Garcia Fernandez163 |
1 MQS – Gunningberg et al.152 4 LQS – Serpa and Santos;172 Feuchtinger et al.;149 Nixon et al.;60 Okuwa et al.160 |
| Moisture subdomains | ||
| Moisture subscales 4/12 (33.3%) |
1 MQS – Salzberg et al.167 2 LQS – Tourtual et al.;175 Halfens et al.153 1 VLQS – Baldwin and Ziegler131 |
2 HQS – Defloor and Grypdonck;57 Perneger et al.164 3 LQS – Bergquist and Frantz;134 Vanderwee et al.;176 Kemp et al.156 3 VLQS – Watts et al.;177 Bostrom et al.;138 Ek147 |
| Urinary incontinence 1/7 (14.3%) |
1 LQS – Vanderwee et al.176 | 1 HQS – Brandeis et al.141 2 MQS – Salzberg et al.;167 Baumgarten et al.133 3 LQS – Bergquist and Frantz;134 Halfens et al.;153 Donnelly146 |
| Faecal incontinence 2/11 (18.2%) |
1 HQS – Brandeis et al.141 1 VLQS – Marchette et al.158 |
1 HQS – Reed et al.80 1 MQS – Baumgarten et al.133 7 LQS – Boyle and Green;140 Fife et al.;150 Suriadi et al.;174 Olson et al.;161 Allman et al.;79 Halfens et al.;153 Donnelly146 |
| Dual incontinence 3/5 (60.0%) |
1 MQS – Ooi et al.162 2 LQS – Bergquist and Frantz;134 Vanderwee et al.176 |
1 MQS – Baumgarten et al.133 1 LQS – Tourtual et al.175 |
| Incontinence other 1/1 (100%) |
1 LQS – Pancorbo Hidalgo and Garcia Fernandez163 | |
| Urinary catheter 1/3 (33.3%) |
1 HQS – Reed et al.80 | 2 LQS – Compton et al.;144 Berlowitz and Wilking137 |
| Skin moisture 3/5 (60.0%) |
3 LQS – Suriadi et al.;174 Compton et al.;144 Bergquist and Frantz134 | 1 MQS – De Laat et al.145 1 LQS – Halfens et al.153 |
| Body temperature domain | ||
| Body temperature 5/8 (62.5%) |
3 MQS – Nijs et al.;159 Suriadi et al.;125 Bergstrom and Braden135 1 LQS – Suriadi et al.174 1 VLQS – Rose et al.166 |
2 LQS – Vanderwee et al.;176 Feuchtinger et al.149 1 VLQS – Ek147 |
| Nutrition subdomains | ||
| Nutritional scales 1/14 (7.1%) |
1 LQS – Serpa and Santos172 | 3 HQS – Defloor and Grypdonck;57 Perneger et al.;164 Nixon et al.37 6 LQS – Vanderwee et al.;176 Tourtual et al.;175 Pancorbo Hidalgo and Garcia Fernandez;163 Halfens et al.;153 Lindgren et al.;157 Kemp et al.156 4 VLQS – Baldwin and Ziegler;131 Watts et al.;177 Bostrom et al.;138 Ek147 |
| Food intake 4/7 (57.1%) |
1 HQS – Brandeis et al.141 1 MQS – Bergstrom and Braden135 1 LQS – Berlowitz and Wilking;137 1 VLQS – Ek et al.148 |
1 HQS – Defloor and Grypdonck57 1 MQS – De Laat et al.145 1 LQS – Bergquist and Frantz134 |
| Malnourishment 1/3 (33.3%) |
1 HQS – Reed et al.80 | 2 LQS – Schoonhoven et al.;170 Donnelly146 |
| Weight 4/12 (33.3%) |
3 LQS – Allman et al.;79 Lindgren et al.;157 Nixon et al.60 1 VLQS – Cobb et al.143 |
1 MQS – Bergstrom and Braden135 5 LQS – Yepes et al.;178 Boyle and Green;140 Compton et al.;144 Olson et al.;161 Kemp et al.156 2 VLQS – Inman et al.;155 Watts et al.177 |
| BMI 2/9 (22.2%) |
1 HQS – Schultz et al.171 1 LQS – Fife et al.150 |
2 HQS – Defloor and Grypdonck;57 Brandeis et al.141 5 LQS – Serpa and Santos;172 Compton et al.;144 Vanderwee et al.;176 Feuchtinger et al.;149 Lindgren et al.157 |
| Arm measurements 1/3 (33.3%) |
1 LQS – Pancorbo Hidalgo and Garcia Fernandez163 | 2 LQS – Serpa and Santos;172 Allman et al.79 |
| Other measures 0/4 (0%) |
2 LQS – Yepes et al.;178 Compton et al.144 2 VLQS – Inman et al.;155 Watts et al.177 |
|
| Age domain | ||
| Increasing age 12/32 (37.5%) |
4 HQS – Schultz et al.;171 Perneger et al.;164 Bergstrom et al.;136 Nixon et al.37 3 MQS – Ooi et al.;162 Bergstrom and Braden;135 Gunningberg et al.152 5 LQS – Serpa and Santos;172 Hatanaka et al.;154 Vanderwee et al.;176 Halfens et al.;153 Lindgren et al.157 |
2 HQS – Defloor and Grypdonck;57 Brandeis et al.141 2 MQS – De Laat et al.;145 Baumgarten et al.133 12 LQS – Chan et al.;142 Yepes et al.;178 Fife et al.;150 Compton et al.;144 Bergquist and Frantz;134 Tourtual et al.;175 Olson et al.;161 Allman et al.;79 Berlowitz and Wilking;137 Feuchtinger et al.;149 Kemp et al.;156 Nixon et al.60 4 VLQS – Inman et al.;155 Watts et al.;177 Goodridge et al.;151 Cobb et al.143 |
| Sensory perception domain | ||
| Braden sensory perception subscale 2/9 (22.2%) |
1 HQS – Defloor and Grypdonck57 1 LQS – Halfens et al.153 |
1 HQS – Perneger et al.164 3 LQS – Vanderwee et al.;176 Tourtual et al.;175 Kemp et al.156 3 VLQS – Baldwin and Ziegler;131 Watts et al.;177 Bostrom et al.138 |
| Mental status subdomains | ||
| Mental status subscales 1/5 (20%) |
1 HQS – Perneger et al.164 | 1 HQS – Defloor and Grypdonck57 2 LQS – Pancorbo Hidalgo and Garcia Fernandez;163 Donnelly146 1 VLQS – Ek147 |
| Mental status study-specific measures 1/8 (12.5%) |
1 HQS – Reed et al.80 | 1 HQS – Brandeis et al.141 1 MQS – Baumgarten et al.133 5 LQS – Bergquist and Frantz;134 Sayar et al.;168 Pancorbo Hidalgo and Garcia Fernandez;163 Halfens et al.;153 Donnelly146 |
| Race domain | ||
| Race 2/5 (40%) |
1 HQS – Bergstrom et al.136 1 MQS – Baumgarten et al.133 |
1 HQS – Brandeis et al.141 2 LQS – Bates-Jensen et al.;132 Chan et al.142 |
| Gender domain | ||
| Gender 4/15 (26.6%) |
4 LQS – Compton et al.;144 Bergquist and Frantz;134 Okuwa et al.;160 Hatanaka et al.154 | 2 HQS – Brandeis et al.;141 Bergstrom et al.136 1 MQS – Baumgarten et al.133 6 LQS – Chan et al.;142 Serpa and Santos;172 Boyle and Green;140 Fife et al.;150 Lindgren et al.;157 Donnelly146 2 VLQS – Inman et al.;155 Goodridge et al.151 |
| General health status subdomains | ||
| ASA 1/2 (50%) |
1 MQS – Rademakers et al.165 | 1 LQS – Donnelly146 |
| APACHE II 1/4 (25%) |
1 LQS – Yepes et al.178 | 1 MQS – Nijs et al.159 1 LQS – Compton et al.144 1 VLQS – Inman et al.155 |
| Norton score measures 0/3 (0%) |
2 HQS – Defloor and Grypdonck;57 Perneger et al.164 1 VLQS – Ek147 |
|
| Chronic wounds 1/2 (50%) |
1 HQS – Nixon et al.37 | 1 LQS – Nixon et al.60 |
| Other factors 8/26 (30.8%) |
3 HQS – Schultz et al.;171 Reed et al.;80 Nixon et al.37 2 MQS – Rademakers et al.;165 Nijs et al.159 2 LQS – Yepes et al.;178 Lindgren et al.157 1 VLQS – Marchette et al.158 |
2 HQS – Defloor and Grypdonck;57 Brandeis et al.141 2 MQS –Salzberg et al.;167 De Laat et al.145 12 LQS – Bates-Jensen et al.132 Chan et al.;142 Serpa and Santos;172 Schoonhoven et al.;170 Fife et al.;150 Compton et al.;144 Bergquist and Frantz;134 Halfens et al.;153 Feuchtinger et al.;149 Nixon et al.;60 Okuwa et al.;160 Donnelly146 2 VLQS – Inman et al.;155 Watts et al.177 |
| Medication domain | ||
| Medication 3/10 (30%) |
1 MQS – Nijs et al.159 2 LQS – Bergquist and Frantz;134 Stordeur et al.173 |
1 HQS – Brandeis et al.141 6 LQS – Yepes et al.;178 Schoonhoven et al.;170 Compton et al.;144 Vanderwee et al.;176 Olson et al.;161 Donnelly146 |
| Risk factor subdomains | ||
| Braden scale total score 7/16 (43.75%) |
2 HQS – Schultz et al.;171 Bergstrom et al.136 1 MQS – Bergstrom and Braden135 4 LQS – Bates-Jensen et al.132 Chan et al.;142 Fife et al.;150 Stordeur et al.173 |
6 LQS – Yepes et al.;178 Serpa and Santos;172; Bergquist and Frantz;134 Tourtual et al.;175 Kemp et al.;156 Donnelly146 3 VLQS – Baldwin and Ziegler;131 Watts et al.;177 Goodridge et al.151 |
| Other scales 3/7 (42.8%) |
1 MQS – Bourdel-Marchasson et al.139 1 LQS – Pancorbo Hidalgo and Garcia Fernandez163 1 VLQS – Inman et al.155 |
4 LQS – Compton et al.;144 Sayar et al.;168 Stordeur et al.;173 Lindgren et al.157 |
Other risk factor domains that were less consistently associated with pressure ulcer development included nutrition, moisture, age, haematological measures, general health status, sensory perception and mental status. Additionally, only a small number of studies included body temperature and immunity and these factors require further research. Finally, the evidence related to race and gender as risk factors was equivocal.
The review46 indicates that there are three primary risk factor domains of mobility/activity, skin/ulcer status and perfusion (including diabetes) but suggests that there is no single factor that can alone explain pressure ulcer development; rather, there is a complex interplay of factors that increases pressure ulcer probability. Although immobility is included in existing pressure ulcer risk assessment tools, the inclusion of skin/ulcer status and perfusion (including diabetes) is not universal. This highlights the need to reconsider the risk factors included in pressure ulcer risk assessment tools.
It is noteworthy that there were a large number of potential risk factors –15 domains and 46 subdomains including over 250 named variables. Furthermore, there was a lack of comparable data fields for measurement of the same constructs and key risk factors were not routinely recorded in all studies. 46 These limitations prevented meta-analysis to identify an item pool for a risk stratification tool and a key recommendation of the review was the development of a Minimum Data Set for pressure ulcer research and institutional cohorts to facilitate future large-scale multivariate analyses and meta-analysis.
Phase 2: consensus study
Although the systematic review46 provided a foundation for the ongoing work, there remained gaps in the evidence base and a lack of agreement over the key risk factors and data items to summarise patient risk. This highlighted the need to consult with experts in the pressure ulcer field about the relevance of the evidence to clinical practice and risk assessment and about other pertinent scientific (physiological and biomechanical) evidence that ought to be considered to agree the risk factors and items to be included in the Minimum Data Set and Risk Assessment Framework (see Appendix 16 for study protocol).
Aim and objectives
The Aims and objectives, Methods and Results sections are largely reproduced, with amendments, from Coleman S, Nelson E, Keen J, Wilson L, McGinnis E, Dealey C, et al. Developing a pressure ulcer risk factor minimum data set and risk assessment framework, J Adv Nurs, with permission from John Wiley & Sons. 126 © 2014 The Authors. Journal of Advanced Nursing. Published by John Wiley & Sons Ltd. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License.
The aim of this study was to develop a draft pressure ulcer risk factor Minimum Data Set and Risk Assessment Framework for pre-testing and clinical evaluation.
The objectives were to:
-
agree a list of patient characteristics to form a Minimum Data Set suitable for routine collection of key risk factors in adult patient populations
-
develop a Risk Assessment Framework incorporating the Minimum Data Set with:
-
a simple screening stage to quickly identify not-at-risk patients
-
a detailed full assessment stage for patients who are at potential/actual risk or who have an existing pressure ulcer
-
decision pathways [i.e. not currently at risk, primary prevention (at risk) or secondary prevention and treatment pathway (with pressure ulcer)].
-
Methods
Design
A consensus study using a modified nominal group technique based on the RAND/UCLA appropriateness method179 was used. This incorporated face-to face interaction and pre- and post-meeting questionnaire completion with an expert group, as well as face-to-face interaction with a service user group (PURSUN UK; see Chapter 2).
Expert group participants
The expert group comprised international clinical/academic leaders identified through their publication record in pressure ulcer or relevant research (see Acknowledgements). The involvement of international expert group members was facilitated by additional funding from a Leeds University World Universities Network grant. The group was purposively sampled to include the perspectives of nurses, doctors, bioengineers, epidemiologists and individuals with organisational development and decision science expertise. A multispecialty group was developed to take account of a wide range of opinions. 180 Seventeen members were recruited to allow for attrition, as 12 was considered the optimum number in terms of preventing co-ordination problems whilst maximising reliability. 181
Service user group participants
The service user group involved members of PURSUN UK (see Chapter 2). All members were invited to take part and seven people have been involved throughout the project. This includes people with experience of having a pressure ulcer or living with pressure ulcer risk as well as carers.
Data collection
The consensus process incorporated an initial expert group meeting and an initial PURSUN UK meeting followed by two consensus cycles. It was envisaged that the first consensus cycle would consider the Minimum Data Set and the second cycle would consider the Risk Assessment Framework; however, at the initial expert group meeting it was apparent that there were difficulties with considering the Minimum Data Set and Risk Assessment Framework separately as the two are interlinked. Discussion at the meeting highlighted the need to identify the key pressure ulcer risk factors and assessment items (i.e. the way in which the risk factors are measured) that would be included in the Minimum Data Set and incorporated in the Risk Assessment Framework. Therefore, the first consensus cycle focused on agreeing the risk factors to be included in the Minimum Data Set and Risk Assessment Framework and the second consensus cycle focused on the assessment items (Figure 13). The study was approved by the University of Leeds School of Healthcare Research Ethics Committee. Informed consent was gained from expert group members prior to participation (see Appendices 17–22 for participant information and consent forms).
FIGURE 13.
Overview of the consensus cycle. PU, pressure ulcer. Reprinted from Coleman S, Nelson EA, Keen J, Wilson L, McGinnis E, Dealey C, et al. Developing a pressure ulcer risk factor minimum data set and risk assessment framework. J Adv Nurs 2014;70:2339–52. 126 © 2014 The Authors. Journal of Advanced Nursing. Published by John Wiley & Sons Ltd. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non-commercial and no modifications or adaptations are made.
Reviewing the pressure ulcer risk factor evidence was an important element of the study and was integrated throughout all cycles of the consensus process. 46 The systematic review46 provided evidence regarding the current state of knowledge surrounding pressure ulcer risk factors but the group also considered wider scientific evidence that was drawn from the expertise of the group. The relevance of the evidence to clinical practice, as well as the practicalities of pressure ulcer risk assessment, were also considered by the group.
Questionnaires were completed by all expert group members privately before and after the cycle 1 and 2 meetings (see Figure 13). In each questionnaire, participants were asked to rate their level of support for statements (relating to the inclusion of risk factors/assessment items in the Minimum Data Set and Risk Assessment Framework) on a 9-point Likert scale, with 1 indicating strong disagreement and 9 indicating strong agreement. Each statement was preceded by a relevant summary of the pressure ulcer systematic review evidence, as well as a summary of expert group discussions, a summary of PURSUN UK group discussions (as applicable) and follow-up/explanatory notes (as applicable). Electronic links to the full systematic review evidence tables and the full summary of the preceding expert group discussions were also available within the questionnaires. This allowed the research team to identify areas of agreement, uncertainty and disagreement before each meeting and to schedule the discussion agenda accordingly. The completion of the questionnaire after the meeting allowed individuals to change their ratings in light of discussions and/or when necessary allowed questionnaire items to be clarified and amended.
Questionnaires were administered and completed using a commercial online survey platform. Participants were asked to complete the questionnaire within 2 weeks of initial posting. One or two reminders were sent (and on one occasion, because of a holiday period, a third reminder was sent) to participants who had not completed the questionnaire within the allotted 2-week period. The surveys were closed to response at 10 weeks following initial posting.
All expert group meetings were led by trained facilitators and were audiotaped. Unlike a traditional RAND/UCLA method in which the first face-to-face meeting occurs following questionnaire completion, an initial face-to-face meeting was undertaken to review the pressure ulcer evidence and consider the views of the group to inform the development of the cycle 1 risk factor questionnaire. 182 At cycle 1 and 2 expert group meetings (see Figure 13), the pre-meeting collective questionnaire responses were anonymously fed back to the group. Members were also provided with a reminder report of their individual questionnaire responses and a copy of the summary of the discussions of the previous expert group meeting. The questionnaire results highlighted areas of agreement and areas of uncertainty and disagreement, which provided a focus for the group discussions to ascertain whether there was genuine uncertainty or disagreement or if there was ambiguity in the wording of the questionnaire.
As pressure ulcer risk assessment practice is part of routine care there was a need to explore the acceptability of proposed risk assessment elements to patients and carers; this was undertaken through facilitated PURSUN UK meetings. PURSUN UK members’ views were fed back to the expert group at the subsequent meeting (cycles 1 and 2) and through the cycle 2 pre-meeting questionnaire.
Data analysis
The researcher (SC) listened to the audio tapes of the expert group meetings and read the associated transcripts in total to ensure completeness. The data were then coded, with categories based on the pressure ulcer risk factor systematic review, in keeping with a directed content analysis approach. 183 As new themes emerged from the expert group discussions further codes were added. A summary report of each meeting was generated by the researcher. The report was reviewed by the facilitators and members of the working group (subgroup of the expert group that comprised the local team and incorporated three academic nurses, three clinical nurses, one PPI officer and one member with organisational development expertise) to ensure that it reflected group discussions.
Careful notes were taken throughout the PURSUN UK meetings and a summary of the discussions was written by the researcher (SC). The summary was circulated to the facilitator and group participants to ensure that it reflected the discussions at the meeting.
Questionnaire statements were summarised using the median group response as a measure of central tendency. In keeping with the RAND/UCLA appropriateness methods and other studies179,184–186 Likert-scale group median responses for each statement were categorised into three tertiles. For this study, the categories were 1–3, disagree; 4–6, uncertain; and 7–9, agree. Within-group agreement was measured using the RAND disagreement index,179 which considers the dispersion of individual scores and identifies areas of disagreement (when panellists rate at both ends of the Likert scale). An index of > 1 indicates disagreement.
Using the group median response and the disagreement index for each statement (regarding risk factors/assessment items) the following principles were applied following post-meeting questionnaire completion:
-
group medians of 1–3 without disagreement would be excluded
-
group medians of 7–9 without disagreement would be included
-
when the disagreement index was > 1 or when the median was 4–6, group medians would be excluded but noted as potential areas for further research.
Results
The expert group comprised nine female and eight male participants. There was 100% (n = 68/68) completion of questionnaires, with 77.9% (n = 53/68) completed within the 2-week allotted time period [13.2% (9/68) were completed up to 1 week late; 2.9% (2/68) up to 4 weeks late; 1.5% (1/68) up to 6 weeks late; 1.5% (1/68) up to 7 weeks late; and 2.9% (2/68) up to 8 weeks late]. In total, there was 86.3% attendance at the face-to-face meetings (17/17 attended the first meeting, 13/17 attended the second meeting and 14/17 attended the third meeting). The results concerning the risk factors (cycle 1) and assessment items (cycle 2) of the Minimum Data Set and Risk Assessment Framework are detailed in the following sections.
Cycle 1: risk factors
The expert group agreed that three risk factors should be incorporated into the screening stage of the Minimum Data Set and Risk Assessment Framework for the assessment of all patients: immobility, existing pressure ulcer and previous pressure ulcer. Table 31 shows the changes in questionnaire responses between the pre-meeting questionnaire and the post-meeting questionnaire.
| Risk factor | Pre-meeting questionnaire responses | Post-meeting questionnaire responses | ||
|---|---|---|---|---|
| Group median | Disagreement index | Group median | Disagreement index | |
| Immobility status | 9.00a | 0.00 | 9.00a | 0.00 |
| Existing PU status | 9.00a | 0.13 | 9.00a | 0.00 |
| Previous PU status | 7.00a | 0.29 | 8.00a | 0.29 |
| General skin status | 5.00b | 1.87c | 3.00d | 0.74 |
| Sensory perception | 4.00b | 0.68 | 3.00d | 0.72 |
| Acute illness | 5.00b | 0.59 | 3.00d | 0.54 |
| Infection | 5.00b | 0.98 | 2.00d | 0.33 |
| Body temperature | 5.00b | 0.97 | 2.00d | 0.29 |
| Nutrition | 5.00b | 0.55 | 2.00d | 0.75 |
| Friction and shear | 2.00d | 0.16 | 2.00d | 0.29 |
| Chronic wounds | 3.00d | 0.65 | 2.00d | 0.29 |
| Diabetes | 4.00b | 0.55 | 2.00d | 0.37 |
| Summary measure GHS | 2.00d | 0.20 | 2.00d | 0.13 |
| Perfusion | – | – | 2.00d | 0.75 |
| Albumin | 3.00d | 0.48 | 2.00d | 0.29 |
| Skin moisture | 4.00b | 1.61c | 2.00d | 0.29 |
| Dual incontinence | 5.00b | 1.70c | 2.00d | 0.33 |
| Medication | 3.00d | 0.33 | 1.00d | 0.02 |
| Mental health status | 2.00d | 0.65 | 1.00d | 0.13 |
| Age | 4.00b | 0.67 | 1.00d | 0.16 |
| Race | 2.00d | 0.49 | 1.00d | 0.02 |
| Gender | 1.00d | 0.29 | 1.00d | 0.02 |
| Haemoglobin | 2.00d | 0.37 | 1.00d | 0.16 |
| Pitting oedema | 3.00d | 0.67 | 1.00d | 0.13 |
| Blood pressure | 3.00d | 0.67 | – | – |
| Smoking | 2.00d | 0.37 | – | – |
| Cardiovascular disease | 3.00d | 0.67 | – | – |
The expert group agreed that 11 risk factors, namely immobility, existing pressure ulcer, previous pressure ulcer, general skin status, perfusion, skin moisture, dual incontinence, diabetes, sensory perception, nutrition and albumin level, should be incorporated into the full assessment stage of the Minimum Data Set and Risk Assessment Framework for patients who were considered to be at potential/actual risk or who have an existing pressure ulcer identified at the screening stage. Table 32 shows the changes in questionnaire responses between the pre-meeting questionnaire and the post-meeting questionnaire. A summary of the key discussion points relating to uncertain (group median 4–6) risk factors is detailed in Table 33. After reviewing the evidence, the post-meeting questionnaire was revised and blood pressure, smoking and cardiovascular disease were combined into a general category of ‘perfusion’.
| Risk factor | Pre-meeting questionnaire responses | Post-meeting questionnaire responses | ||
|---|---|---|---|---|
| Group median | Disagreement index | Group median | Disagreement index | |
| Immobility status | 9.00a | 0.16 | 9.00a | 0.00 |
| Existing PU status | 9.00a | 0.13 | 9.00a | 0.16 |
| Previous PU status | 7.00a | 0.40 | 8.00a | 0.16 |
| General skin status | 8.00a | 0.23 | 8.00a | 0.29 |
| Skin moisture | 8.00a | 0.29 | 8.00a | 0.33 |
| Diabetes | 8.00a | 0.29 | 8.00a | 0.33 |
| Nutrition | 7.00a | 0.67 | 8.00a | 0.16 |
| Perfusion | – | – | 8.00a | 0.40 |
| Albumin | 7.00a | 0.20 | 7.00a | 0.45 |
| Sensory perception | 8.00a | 0.29 | 7.00a | 0.29 |
| Dual incontinence | 8.00a | 0.19 | 7.00a | 0.33 |
| Friction and shear | 5.00b | 1.10c | 6.00b | 0.52 |
| Chronic wounds | 6.00b | 0.42 | 6.00b | 0.37 |
| Medication | 5.00b | 0.41 | 5.00b | 0.08 |
| Acute illness | 7.00a | 0.07 | 5.00b | 0.59 |
| Infection | 5.00b | 1.10c | 5.00b | 0.41 |
| Body temperature | 7.00a | 0.52 | 5.00b | 0.88 |
| Pitting oedema | 6.00b | 0.30 | 5.00b | 1.04c |
| Age | 5.00b | 0.49 | 5.00b | 0.50 |
| Summary measure GHS | 4.00b | 0.62 | 4.00b | 0.65 |
| Haemoglobin | 5.00b | 0.32 | 3.00d | 0.72 |
| Mental health status | 5.00b | 0.72 | 2.00d | 0.75 |
| Race | 2.00d | 0.49 | 1.00d | 0.13 |
| Gender | 2.00d | 0.29 | 1.00d | 0.02 |
| Blood pressure | 5.00b | 0.52 | – | |
| Smoking | 5.00b | 0.59 | – | – |
| Cardiovascular disease | 6.00b | 0.42 | – | – |
| Uncertain risk factors | Key discussion points from the expert group meetings |
|---|---|
| Friction and shear |
|
| Acute illness, infection, body temperature (elements of general health status) |
|
| Chronic wound |
|
| Pitting oedema |
|
| Medication |
|
| Age |
|
Using the decision rules highlighted in the methods section, the Minimum Data Set and Risk Assessment Framework comprised only those risk factors for which there was agreement (group median 7–9 without disagreement). The progression of risk factors through the consensus study is detailed in Figure 14 (see also Tables 31 and 32). This shows that of the original 15 risk factor domains and 46 subdomains identified in the systematic review,46 26 risk factors were considered to potentially warrant inclusion in the Minimum Data Set and Risk Assessment Framework and progressed to consensus cycle 1.
FIGURE 14.
Risk factor progression. BP, blood pressure; MDS, minimum data set; PU, pressure ulcer; RAF, Risk Assessment Framework. Reprinted from Coleman S, Nelson EA, Keen J, Wilson L, McGinnis E, Dealey C, et al. Developing a pressure ulcer risk factor minimum data set and risk assessment framework. J Adv Nurs 2014;70:2339–52. 126 © 2014 The Authors. Journal of Advanced Nursing. Published by John Wiley & Sons Ltd. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non-commercial and no modifications or adaptations are made.
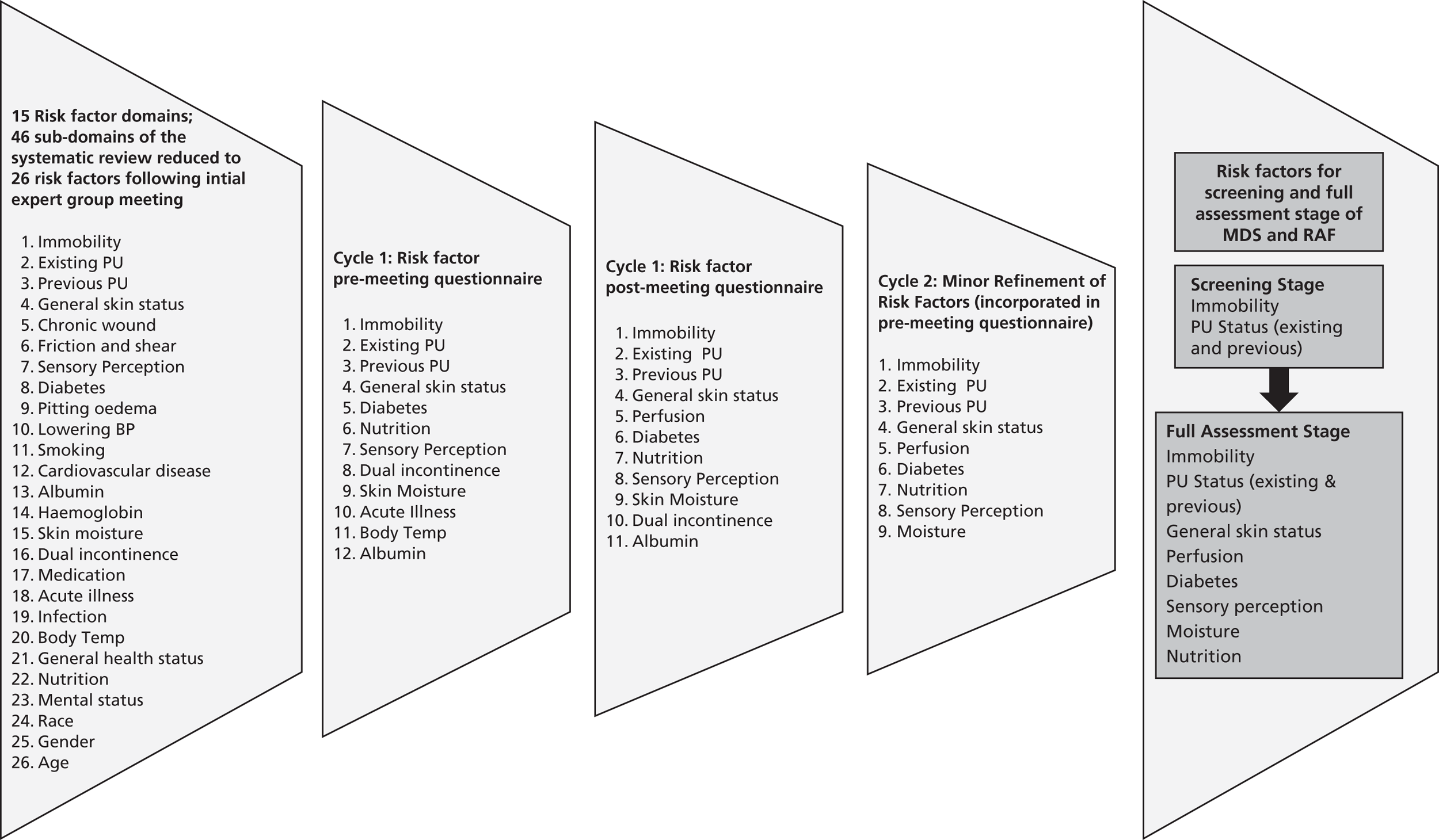
The risk factors for inclusion were mainly agreed in the cycle 1 post-meeting questionnaire but there were some refinements of the risk factors in the cycle 2 pre-meeting questionnaire. The expert group had agreed that albumin should be included at the second stage of the assessment (see Table 32). However, at a subsequent PURSUN UK meeting, concern was raised about the need to undertake an additional blood test for the assessment of albumin. In light of this, expert group members were asked whether there was a clinical indication for undertaking an additional blood test to measure albumin for patients to establish the level of pressure ulcer risk and it was concluded that this was unnecessary. The expert group also concluded that skin moisture and dual incontinence could be combined into one measure.
Cycle 2: assessment items for risk factors
There was support (group median 7–9 without disagreement) for all statements in the cycle 2 questionnaire concerning the assessment items of the Minimum Data Set and Risk Assessment Framework. However, following discussion at the cycle 2 meeting, the expert group felt that some changes should be made to specific items. As the group were content with the majority of the pressure ulcer risk factor Minimum Data Set items highlighted in the cycle 2 pre-meeting questionnaire, the post-meeting questionnaire focused on items that required adjustment. The agreed assessment items for the screening and full assessment stage are detailed in Table 34. In addition, the expert group agreed that the Risk Assessment Framework would facilitate the identification of a risk profile for each patient, rather than condense the risk from different aspects into a single score. This would support care planning, with interventions selected in response to specific risk factors.
| Risk factor | Mobility |
|---|---|
| Screening stage | |
| Mobility |
|
| PU status |
|
| Full assessment stage | |
| Immobility items to incorporate the frequency of independent movement, e.g. |
|
| Immobility items to incorporate the magnitude of independent movement, e.g. |
|
| Immobility items to incorporate general, clinically relevant descriptions of movement, e.g. |
|
| Sensory perception |
|
| PU (existing and previous PU) |
|
| General skin status |
|
| Perfusion |
|
| Diabetes |
|
| Moisture |
|
| Frequency | |
| Nutrition |
|
Draft Risk Assessment Framework
Using the results from cycle 1 and 2 of the study, an initial draft of the Risk Assessment Framework was produced (see Appendix 23) incorporating the screening and full assessment stage and decision pathways of the assessment process. This underwent further graphic design prior to pre-testing.
Phase 3: development of a new conceptual framework and theoretical causal pathway for pressure ulcer development
The Aims and objectives, Methods and Results sections are largely reproduced, with amendments, from Coleman S, Nixon J, Keen J, Wilson L, McGinnis E, Dealey C, et al. A new pressure ulcer conceptual framework, J Adv Nurs, with permission from John Wiley & Sons. 187 © 2014 The Authors. Journal of Advanced Nursing. Published by John Wiley & Sons Ltd. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License.
By bringing together the relevant fields of enquiry and clarifying key risk factors for pressure ulcer development, the consensus study provided an opportunity to undertake an additional piece of work to review and enhance the pressure ulcer conceptual framework. 1 This was to bridge the gap between the epidemiological, physiological and biomechanical evidence and enhance our understanding of the role of individual risk factors in pressure ulcer development. This was not part of the programme outline initially but emerged as an additional output of the work.
Aim and objectives
The aim was to develop a new pressure ulcer conceptual framework. Specific objectives were to:
-
review and update the biomechanical elements of the NPUAP/EPUAP 2009 conceptual framework
-
develop a theoretical causal pathway for pressure ulcer development
-
map risk factors identified in the consensus study to the updated conceptual framework.
Methods
The expert group reconvened in an additional facilitated meeting which was planned so that members had access to the outcomes of the consensus study, the evidence of the systematic review46 and risk factor/causal factor terminology before the face-to-face meeting. Familiarity with the risk factor/causal factor terminology allowed us to explore the role of the risk factors in the pressure ulcer causal pathway. This was facilitated by consideration of definitions from Brotman and colleagues:188
-
risk factor – a variable with a significant statistical association with a clinical outcome
-
independent risk factor – a risk factor that retains its statistical association with the outcome when other established risk factors for the outcome are included in a statistical model
-
non-independent risk factor – a risk factor that loses its statistical association with the outcome when other established risk factors for the outcome are included in a statistical model.
Brotman and colleagues188 suggest that a causal factor is a risk factor that has a causal relationship with a clinical outcome and is defined experimentally (known to affect outcome) rather than statistically. They make a distinction between direct and indirect causal factors:
-
Direct causal factor – directly impacts the outcome (or the likelihood of the outcome).
-
Indirect causal factor – impacts the outcome (or affects its likelihood of occurrence) by changing a direct causal factor. If the direct causal factor is prevented from changing then changes in the outcome will not be produced.
In our work we further categorised indirect causal factors into key indirect causal factors (for which the epidemiological/wider scientific evidence and/or clinical resonance was stronger) and other indirect causal factors. Meeting discussions were audio recorded and transcribed, allowing key themes to be identified.
Data analysis
In addition to considering the outcomes of the consensus study (see Phase 2: consensus study), the researcher (SC) listened to the audio tapes of the conceptual framework expert group meeting discussions and read the associated transcripts in total to ensure completeness. The analysis allowed the researcher (SC) to draft the new pressure ulcer conceptual framework and theoretical causal pathway, which was circulated to the expert group by e-mail to ensure content validity.
Results
The expert group discussions led to amendments to the existing NPUAP/EPUAP conceptual framework,1 as illustrated in Figure 15. Most notably, it was recognised that, although mechanical properties of the tissues and geometry (morphology) of the tissues and underlying bones impact on the internal strains and stresses (as an example, subjects who are either very emaciated or very obese will have enhanced strains and stresses within the soft tissues), their impact was considered to be more relevant to the susceptibility of the individual (i.e. impacting on the damage threshold). Furthermore, transport (perfusion and lymphatic drainage) also impacts on the damage threshold of the individual and this would also be affected by temperature in terms of vasodilation/vasoconstriction, thereby affecting tissue perfusion. The underlying physiology of an individual will also have an impact on his or her repair capacity and this was an important consideration that was captured in the amended conceptual framework (see Figure 15).
FIGURE 15.
Enhanced NPUAP/EPUAP (2009) factors that influence susceptibility for pressure ulcer development. Reproduced from Coleman, Nixon, Keen, Wilson, McGinnis, Dealey et al. A new pressure ulcer conceptual framework. J Adv Nurs 2014;70:2222–34. 187 © 2014 The Authors. Journal of Advanced Nursing Published by John Wiley & Sons Ltd. This is an open access article under the terms of the TODO: clickthrough URLCreative Commons Attribution-NonCommercial-NoDerivs License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non-commercial and no modifications or adaptations are made.
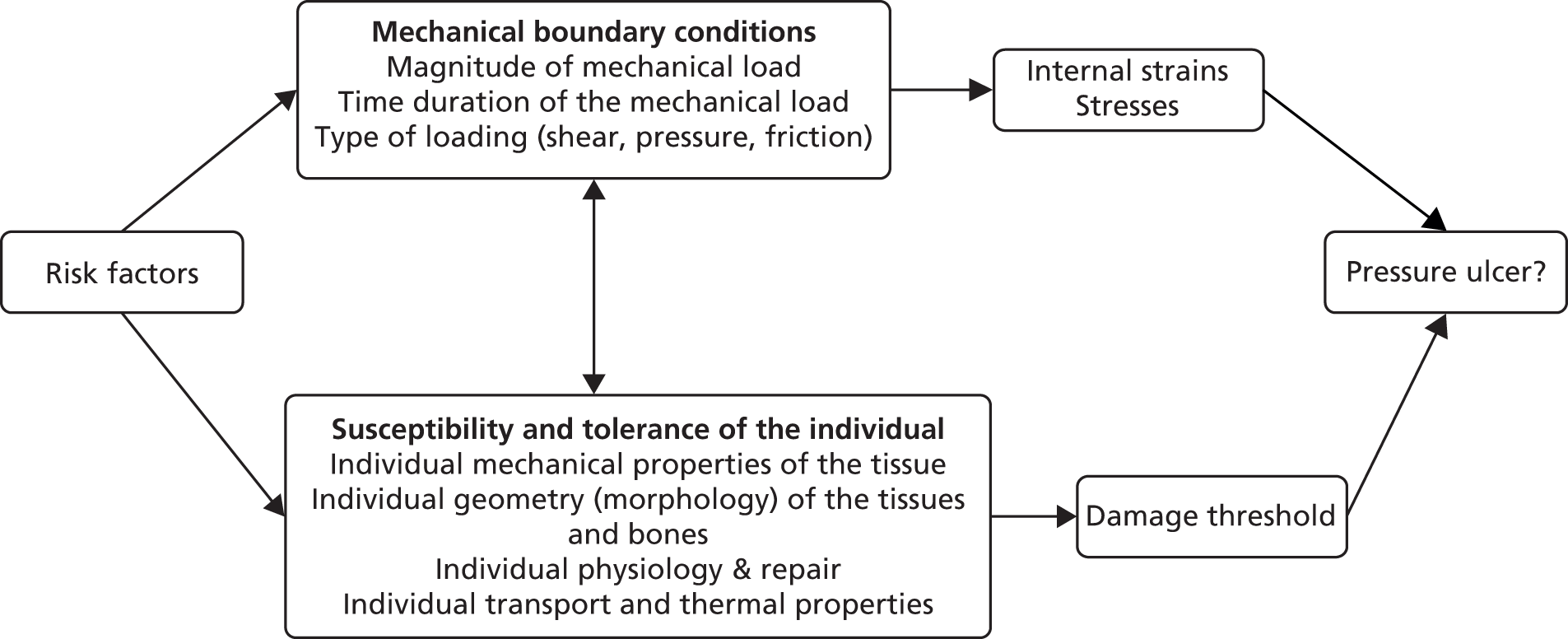
The theoretical schema of a proposed causal pathway for pressure ulcer development detailing the direct, key indirect and other potential indirect causal factors is illustrated in Figure 16. Table 35 shows the mapping of the direct causal factors and key indirect causal factors against the key components of the enhanced 2009 NPUAP/EPUAP conceptual framework. Although it was recognised that the presence and weighting of specific risk factors may vary in relation to the anatomical site of the pressure ulcer, it was not possible to delineate the evidence to skin site-level risk factors.
FIGURE 16.
Theoretical schema of proposed causal pathway for pressure ulcer development. PU, pressure ulcer. The solid arrows show the causal relationships between the key indirect causal factors, direct causal factors and the outcome. Broken arrows show the causal relationships between other potential indirect causal factors and key indirect causal factors and between direct causal factors. Broken arrows also demonstrate inter-relationships between direct causal factors and indirect causal factors. Reproduced from Coleman, Nixon, Keen, Wilson, McGinnis, Dealey et al. A new pressure ulcer conceptual framework. J Adv Nurs 2014;70:2222–34. 187 © 2014 The Authors. Journal of Advanced Nursing Published by John Wiley & Sons Ltd. This is an open access article under the terms of the TODO: clickthrough URLCreative Commons Attribution-NonCommercial-NoDerivs License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non-commercial and no modifications or adaptations are made.
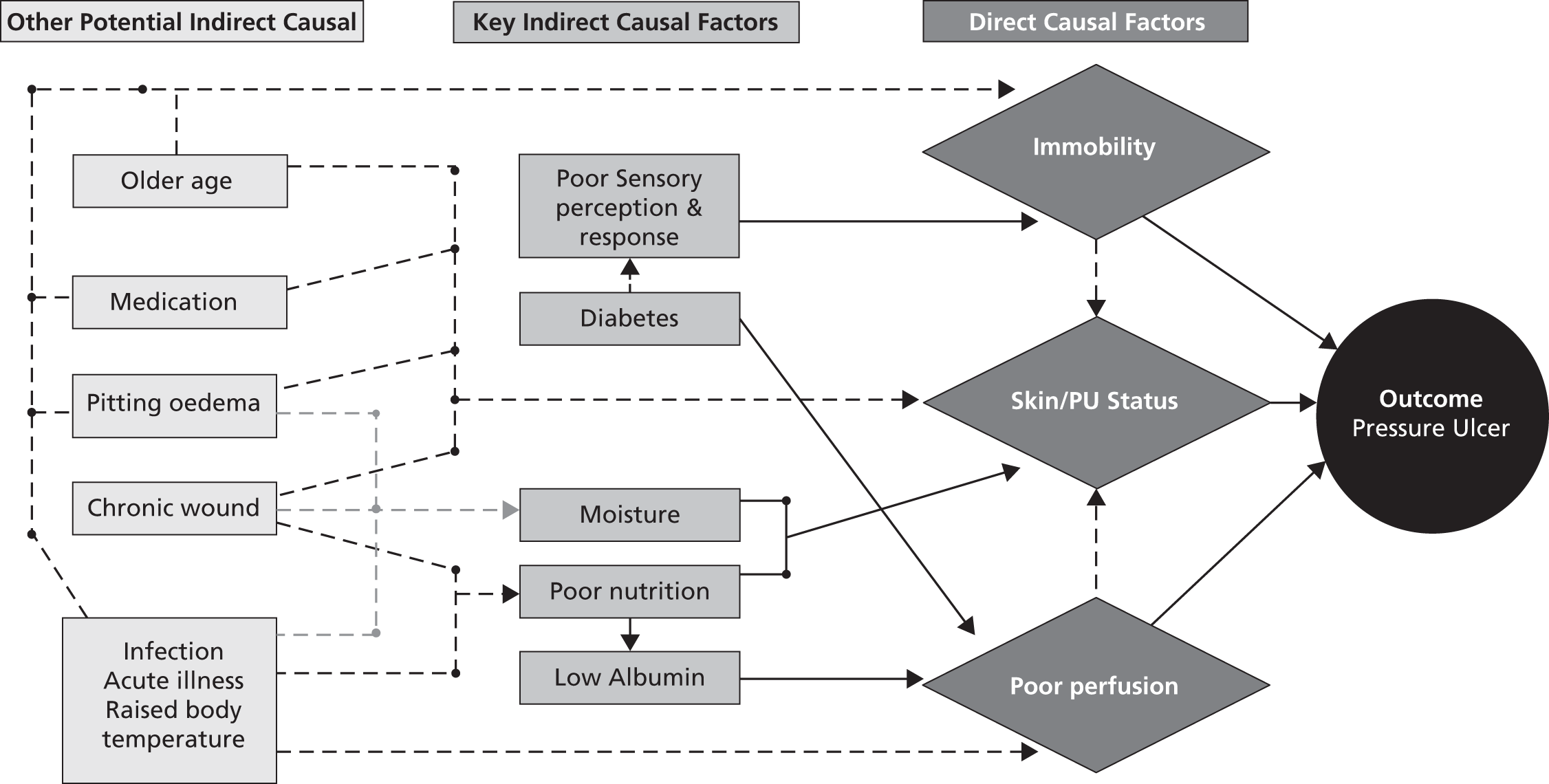
| Risk factor | Mechanical boundary conditions: type of loading (shear, pressure, friction) and magnitude and duration of mechanical load | Individual geometry (morphology) of the tissue and bones | Individual mechanical property of the tissues | Individual transport and thermal properties | Individual physiology and repair |
|---|---|---|---|---|---|
| Immobility | ✗ | ||||
| Skin/PU status | ✗ | ✗ | ✗ | ✗ | |
| Poor perfusion | ✗ | ✗ | |||
| Poor nutrition | (✗) in extreme cases | (✗) in extreme cases | ✗ | ✗ | |
| Moisture | ✗ | ✗ | |||
| Poor sensory perception and response | (✗) through immobility | ||||
| Diabetes | (✗) through sensory perception | (✗) through perfusion | |||
| Low albumin level | (✗) through perfusion |
Direct causal factors
Three characteristics were classified as direct causal factors: immobility, skin/ulcer status and perfusion. Immobility is a necessary condition for pressure ulcer development and is therefore considered a direct causal factor (see Figure 16); through its impact on mechanical boundary conditions (see Table 35) it directly impacts the outcome (or the likelihood of the outcome). Of note is that friction and shear is not specified as a patient characteristic but rather as a characteristic of the mechanical boundary condition (see Table 35).
Identifying whether skin/ulcer status (incorporating existing and previous pressure ulcers and general skin status) and poor perfusion represent a direct or indirect risk factor is less straightforward. It could be assumed that they are indirect factors as without some degree of immobility a pressure ulcer would not develop. However, this is not in keeping with the definitions of causal factors and oversimplifies the complex interplay of factors required to lead to tissue damage. There is strong epidemiological/wider scientific evidence that poor perfusion and skin/ulcer status reduce patients’ tolerance to pressure and increase the likelihood of pressure ulcer development, suggesting that they are direct causal factors, and this may explain why some immobile patients develop pressure ulcers whereas others do not.
Further insight was gained by mapping skin/ulcer status and poor perfusion to the conceptual framework; it was apparent that they were clearly implicated in the susceptibility and tolerance aspect of the framework (see Table 35). Skin/ulcer status mapped to the individual geometry (morphology) of the tissue and bones, the mechanical property of the tissues, the transport and thermal properties and the physiology and repair aspects of the framework. Perfusion mapped to the individual transport and thermal properties and the physiology and repair elements of the framework and is related to factors that impair circulation. Within the expert group it was recognised that oxygen-carrying capacity was important in maintaining healthy tissues, although it was also recognised that other factors such as the delivery of nutrients and waste removal were important, and at present it is difficult to determine the most important factors relating to perfusion. Further confirmatory research is needed to more clearly ascertain the aetiological mechanisms of importance.
Key indirect causal factors
Moisture, sensory perception, diabetes, low albumin level and poor nutrition were considered key indirect causal factors as they impact the outcome (or affect its likelihood of occurrence) by changing a direct causal factor (see Figure 16).
Other potential indirect causal factors
The theoretical conceptual schema (see Figure 16) was further developed to include other indirect causal factors to illustrate the potential relationships and the impact of diverse factors that may be involved in the causal pathway. However, it is recognised that the inter-relationships among potential and key indirect causal factors are complex and require further elucidation. Other potential indirect causal factors include those with weak or limited epidemiological/wider scientific evidence but which are thought to impact on key indirect and direct causal factors. These include age, medication and pitting oedema as well as other factors relating to general health status including infection, acute illness, raised body temperature and chronic wound.
New pressure ulcer conceptual framework
Having considered the causal pathway for pressure ulcer development (see Figure 16) and mapped the direct and key indirect causal factors for ulcer development against the components of the enhanced conceptual framework (see Table 35), a new conceptual framework is proposed that enables the epidemiological evidence to be linked to the conceptual framework (Figure 17). The new framework shows that there is a relationship between the mechanical boundary conditions and the susceptibility and tolerance of the individual. The risk factors that impact the mechanical boundary conditions and the susceptibility and tolerance of the individual are detailed in the framework and are based on the direct causal factors of immobility, skin/ulcer status and poor perfusion as well as on the key indirect causal factors of poor sensory perception and response, diabetes, poor nutrition, moisture and low albumin level.
FIGURE 17.
New pressure ulcer conceptual framework. PU, pressure ulcer. Reproduced from Coleman, Nixon, Keen, Wilson, McGinnis, Dealey et al. A new pressure ulcer conceptual framework. J Adv Nurs 2014;70:2222–34. 187 © 2014 The Authors. Journal of Advanced Nursing Published by John Wiley & Sons Ltd. This is an open access article under the terms of the TODO: clickthrough URLCreative Commons Attribution-NonCommercial-NoDerivs License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non-commercial and no modifications or adaptations are made.

For simplicity, the risk factors are represented under the elements that they predominantly affect (either mechanical boundary conditions or susceptibility and tolerance of the individual), but the broken line running under the risk factors indicates that some risk factors may have an effect on both sides of the framework, which is more clearly articulated in the theoretical schema (see Figure 16) and risk factor mapping (see Table 35). The absence of risk factors on either the individual susceptibility and tolerance or the mechanical boundary conditions side of the framework would affect the likelihood of pressure ulcer development [i.e. a patient with good perfusion may be able to tolerate higher levels of immobility (without developing a pressure ulcer) than a patient with poor perfusion].
Phase 4: design and pre-testing of the Risk Assessment Framework (incorporating the risk factor Minimum Data Set)
The consensus study identified the risk factors and assessment items and suggested a structure for the Risk Assessment Framework (incorporating the risk factor Minimum Data Set). With these elements agreed we engaged the support of a graphic designer (see Acknowledgements) to develop the new Risk Assessment Framework in a way that would facilitate ease of use. This incorporated the use of colour to aid clinical decision-making. To ensure that clinicians/nurses understood and were able to use the Risk Assessment Framework, the next study of the work package was undertaken – pre-testing of the Risk Assessment Framework (see Appendix 24 for study protocol).
Study aim
The aim was to assess and improve the acceptability, usability, format, design, clarity, comprehension, language and data completeness of the draft Risk Assessment Framework (incorporating the risk factor Minimum Data Set) with clinical nurses.
Methods
Cognitive pre-testing methods were used to evaluate how clinical nurses interpreted questions, response categories and instructions while using the draft Risk Assessment Framework. 189 This was conducted over three pre-test sessions and incorporated three focus groups and 12 ‘think out loud’ interviews, estimated as the number required for data saturation. It was anticipated that focus groups with nurses in similar roles (e.g. staff nurses, senior nurses) would facilitate greater understanding of the usability of the Risk Assessment Framework, allowing group members to ‘spark ideas off one another’, which might lead to greater disclosure. 190
In addition, one-to-one think out loud interviews191 were undertaken to allow the researcher (SC) to identify specific problems with the risk assessment tool that would be amenable to resolution by modification. The study was conducted to allow analysis of and adjustment to the Risk Assessment Framework to be undertaken between pre-test sessions so that three different versions of the tool could be pre-tested and improvements made in an interative process.
Participants
For the pre-test we recruited nurses from a large acute teaching hopsital trust, a district general hospital and two primary care trusts. Purposive sampling was undertaken to ensure that tissue viability nurses, staff nurses and sisters from hospital and community settings were recruited from each of the four participating sites. Participants included those who had an interest in tissue viability (e.g. a link nurse or member of a local pressure ulcer or wound care working group). The study was approved by the University of Leeds School of Healthcare Research Ethics Committee. Informed consent was obtained prior to participation in the study (see Appendices 25 and 26 for the patient information leaflet and consent form).
Data collection
The three facilitated pre-test sessions were undertaken away from the clinical environment and involved 8–12 nurses from the four participating sites, who were grouped by job role (staff nurse, sister/charge nurse and tissue viability nurse specialist/research nurse) to facilitate openness, as the use of heterogeneous groups can lead to inhibition in raising issues that do not seem to be shared by others. 190 This was thought to be particularly important for this group as a hierarchy might have stifled disclosure (e.g. a staff nurse might not want to disagree with the views of his/her ward sister). Having nurses from different centres minimised familiarity, which can lead to participants relying on ‘taken for granted’ assumptions. 190 At the pre-test session, the nurses were trained in how to use the Risk Assessment Framework and were then randomly allocated to either a focus group or a one-to-one think out loud interview.
Training involved a short presentation and demonstration on how to use the draft Risk Assessment Framework with a simulated patient. Each nurse then completed the draft Risk Assessment Framework using case studies and vignettes (see Appendix 27) that were accompanied by photographs of pressure areas and ulcers. The vignettes were appropriate to the nurses’ area of practice (i.e. community nurses used vignettes of community patients). The vignettes were co-developed by the project lead, the project team and members of PURSUN UK to ensure that they were realistic and clinically relevant. Nurses were encouraged to ask questions throughout the training session.
The sessions were planned to ensure that four to eight nurses192 per pre-test were assigned to the focus group. Each was asked to complete the Risk Assessment Framework again, using three case studies relevant to their area of practice. Nurse participants were encouraged to highlight any areas of the Risk Assessment Framework form that they found confusing. A co-facilitator assessed data completeness and listed areas where data items were not completed or were not completed as required, as well as areas noted by the nurses as confusing. The focus group meeting then convened to discuss the use of the Risk Assessment Framework. The meeting was moderated by two facilitators and audio recorded. The moderator promoted group interaction and guided discussions using a topic guide (see Appendix 28), which considered the usability of the Risk Assessment Framework and any areas of confusion regarding its use. This was informed by the data completeness assessment.
Up to four nurses from each session were assigned to the one-to-one think out loud interviews. A topic guide (see Appendix 29) was used and the researcher first guided the nurses though the think out loud technique. Once the nurse were content with the approach, they were asked to complete the Risk Assessment Framework again in the presence of the researcher using three vignette case studies appropriate to their area of practice. The researcher encouraged the nurses to vocalise their thoughts as they completed the Risk Assessment Framework. This allowed specific issues relating to difficulty in interpreting items or confusion about aspects of the Risk Assessment Framework to be identified. The interviews were audio recorded.
Analysis
Data completeness of the Risk Assessment Framework forms was undertaken by calculating the percentage of item-level missing data, the percentage of decision pathways allocated and the percentage of item-level missing data for those for whom a decision pathway was allocated. The appropriateness of the allocated decision pathway was also assessed based on the decision rules of the Risk Assessment Framework and the item responses for each assessment.
The focus group meetings and the think out loud interviews were audiotaped and transcribed. The researcher listened to the audio tapes and read the transcripts to ensure accuracy and to ensure that a good overview of the discussions had been achieved. The data were then coded, which was directed by the risk factor items of the Risk Assessment Framework, using a directed content analysis approach. 183 The emphasis was on identifying themes across the focus groups and think out loud interviews that impacted on the application of the Risk Assessment Framework in clinical practice. A summary report of each meeting was reviewed by the facilitators to ensure that it reflected the discussions that had taken place. The report was considered by a working group (consisting of clinical and academic leaders in the pressure ulcer field) and adjustments were made to the draft Risk Assessment Framework, which was pre-tested at the subsequent session in an iterative process. Following pre-testing, the Risk Assessment Framework was also reviewed by PURSUN UK and the consensus study expert group.
Results
The pre-test sessions were well attended by 34 nurses from acute (n = 16) and community (n = 18) settings. Over the three pre-test sessions, 101 Risk Assessment Framework assessments were undertaken using vignette case studies by 11 tissue viability/research nurses (n = 32 Risk Assessment Framework assessments), 12 staff nurses (n = 36 Risk Assessment Framework assessments) and 11 sisters (n = 33 Risk Assessment Framework assessments). At each pre-test session, four nurses undertook the think out loud interviews and seven or eight nurses attended the focus groups. Table 36 details the level of data completion for each pre-test session, which can be seen to improve as the Risk Assessment Framework was amended over the three pre-test sessions.
| RAF assessment concluding at step 1 screening | Pre-test 1: number of related items requiring completion PA | Pre-test 1 (TVNs/RNs): items completed, % (n/N) | Pre-test 2: number of related items requiring completion PA | Pre-test 2 (staff nurses): items completed, % (n/N) | Pre-test 3: number of related items requiring completion PA | Pre-test 3 (sisters): items completed, % (n/N) |
|---|---|---|---|---|---|---|
| Mobility | 4 | 100 (24/24) | At least 1 of 4 | 100.0 (10/10) | At least 1 of 4 | 100.0 (8/8) |
| Skin/PU status | 2 | 66.7 (8/12) | At least 1 of 4 | 90.0 (9/10) | At least 1 of 4 | 100.0 (8/8) |
| Decision pathway allocated | 1 | 0 (0/6) | 1 | 100.0 (10/10) | 1 | 87.5 (7/8) |
| Number of RAF assessments concluding at step 1 | 76.2 (32/42) | 96.7 (29/30) | 95.8 (23/24) | |||
| RAF assessment including step 1 (screening) and step 2 (full assessment) | Pre-test 1: number of related items requiring completion PA | Pre-test 1 (TVNs/RNs): items completed, % (n/N) | Pre-test 2: number of related items requiring completion PA | Pre-test 2 (staff nurses): items completed, % (n/N) | Pre-test 3: number of related items requiring completion PA | Pre-test 3 (sisters): items completed, % (n/N) |
| Mobility (first stage) | 4 | 93.3 (97/104) | At least 1 of 4 | 96.2 (25/26) | At least 1 of 4 | 100.0 (25/25) |
| Skin/PU status (first stage) | 2 | 98.1 (51/52) | AA | 100.0 (3/3) | AA | 100.0 (1/1) |
| Movement matrix | 1 | 100 (26/26) | 1 | 100.0 (26/26) | 1 | 96.0 (24/25) |
| Sensory perception | 1 | 96.2 (25/26) | 1 of 2 | 100.0 (26/26) | 1 of 2 | 100.0 (25/25) |
| Current DSA – listed sites | 15 | 71.5 (279/390) | 13 | 75.4 (255/338) | 13 | 97.2 (316/325) |
| Current DSA – other sites | AA | 0 (0/0) | AA | 50.0 (1/2) | AA | 0 (0/0) |
| Current PU | AA | 84.2 (16/19) | AA | 83.3 (20/24) | AA | 80.0 (20/25) |
| Previous PU history | AA | 75.0 (9/12) | AA | 77.8 (7/9) | 1 of 2 (if yes 3, AA) | 85.3 (29/34) |
| Scarring | 2 | 55.8 (29/52) | AA | 100.0 (1/1) | AA | 100.0 (1/1) |
| Perfusion | 2 | 92.3 (48/52) | At least 1 of 3 | 73.1 (19/26) | At least 1 of 3 | 100.0 (25/25) |
| Nutrition | 4 | 76.9 (80/104) | At least 1 of 5 | 100.0 (26/26) | At least 1 of 5 | 100.0 (25/25) |
| Moisture | 1 (if yes 2 as applicable) | 74.1 (40/54) | 1 of 3 | 84.6 (22/26) | 1 of 3 | 100.0 (25/25) |
| Diabetes | 1 | 100.0 (26/26) | As applicable | 100.0 (5/5) | 1 of 2 | 100.0 (25/25) |
| Decision pathway allocated | 1 of 3 | 53.8 (14/26) | 1 of 3 | 96.2 (25/26) | 1 of 3 | 100.0 (25/25) |
| Total RAF assessments including steps 1 and 2 | 78.5 (740/943) | 81.7 (461/564) | 96.6 (566/586) | |||
| Overall totala | 78.4 (772/985) | 82.5 (490/594) | 96.6 (589/610) | |||
| Overall totalb | 83.7 (417/498) | 84.4 (481/570) | 96.7 (587/607) |
An inappropriate decision pathway was allocated when an assessment detailed the presence of a ulcer and the case–study patient should have been allocated to the ‘pressure ulcer category 1 or above or scarring’ pathway but was allocated to the ‘at-risk’ pathway. Uncertainty about the appropriateness of the allocated pathway related to missing data, for example a patient was allocated to the ‘not currently at risk’ pathway but the skin assessment items were not fully completed and hence there was a possibility that a higher pathway was appropriate.
Figure 18 illustrates how the levels of missing data decreased over the three pre-test sessions overall and when a decision pathway was allocated. Figure 19 illustrates how the number of decision pathways allocated increased notably from the first to the second pre-test. Table 37 presents the appropriateness of the decision pathways allocated according to the decision rules of the Risk Assessment Framework and the item responses for each assessment.
FIGURE 18.
Percentage of missing data at each pre-test session.
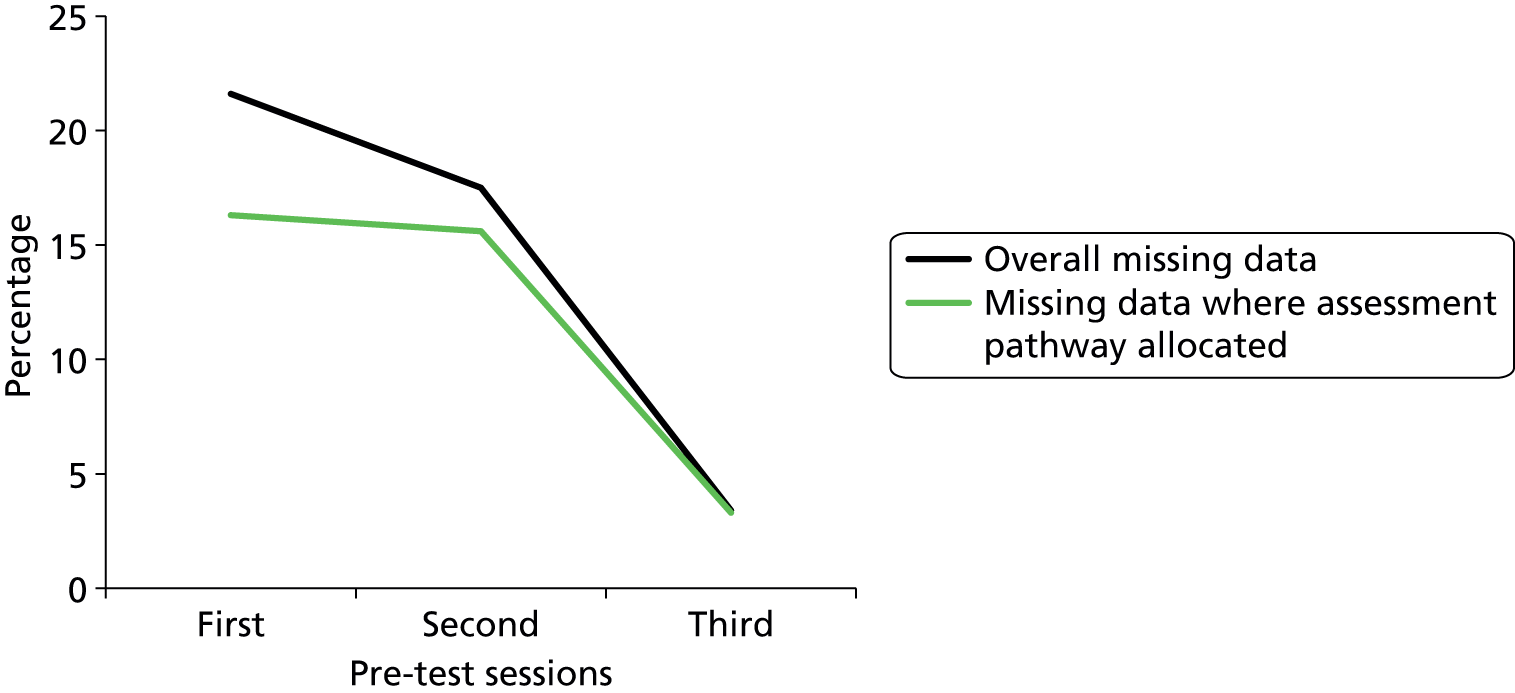
FIGURE 19.
Percentage of decision pathways allocated at each pre-test session.
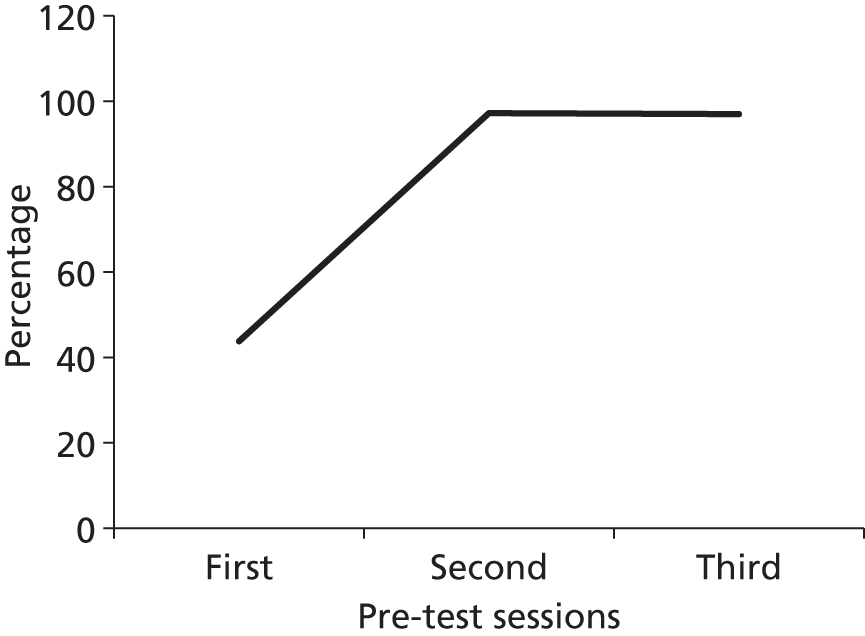
| Decision pathway allocation | Pre-test session 1 (TVNs/RNs), % (n/N) | Pre-test session 2 (staff nurse), % (n/N) | Pre-test session 3 (Sisters), % (n/N) |
|---|---|---|---|
| Appropriate pathway allocation | 78.6 (11/14) | 91.4 (32/35) | 90.6 (29/32) |
| Inappropriate pathway allocation | 7.1 (1/14) | ||
| Pathway allocated but some uncertainty in appropriateness because of missing data items | 14.3 (2/14) | 8.6 (3/35) | 9.4 (3/32) |
Changes made to the Risk Assessment Framework between pre-test sessions in response to the analysis of data completeness, think out loud interviews and focus groups are summarised in Figure 20 and related to three main areas: flow and format, decision support and wording of specific items. An example of the changes made to these main areas between pre-test sessions is shown in Figures 21–23 in relation to step 1 of the assessment. It should be acknowledged that, following these changes, some nurses still completed the step 1 skin/ulcer items despite not needing to. This could be related to the use of case studies in the pre-test sessions in which information on skin/ulcer status was readily available, whereas in clinical practice this information may be less obvious.
FIGURE 20.
Changes to the Risk Assessment Framework following each pre-test session. PU, pressure ulcer; PURAF, Pressure Ulcer Risk Assessment Framework; RAG, red, amber, green.
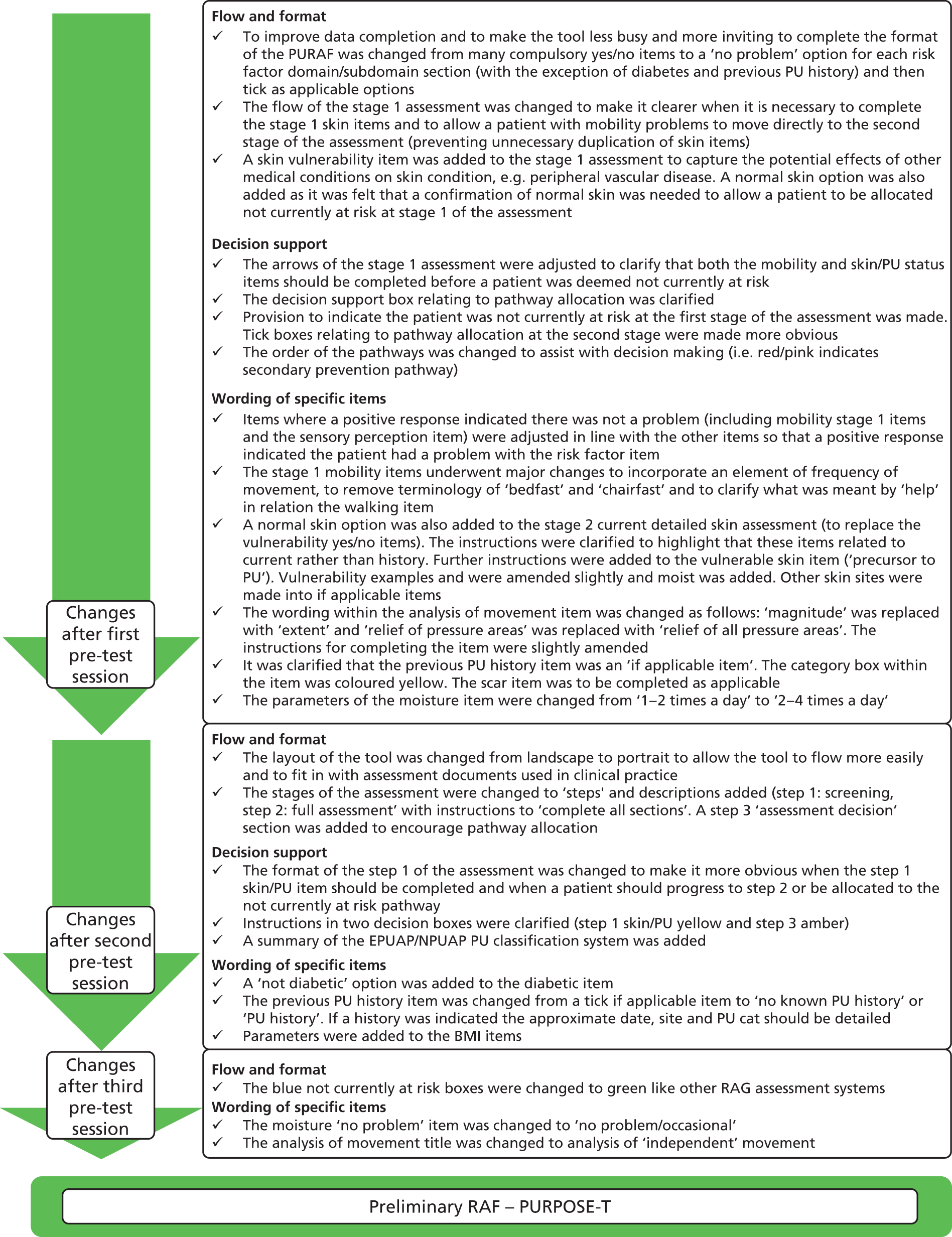
FIGURE 21.
Pre-test session 1 draft Risk Assessment Framework. Please note that the Risk Assessment Framework incorporates the use of colour. These examples have been adapted with the colour key provided.
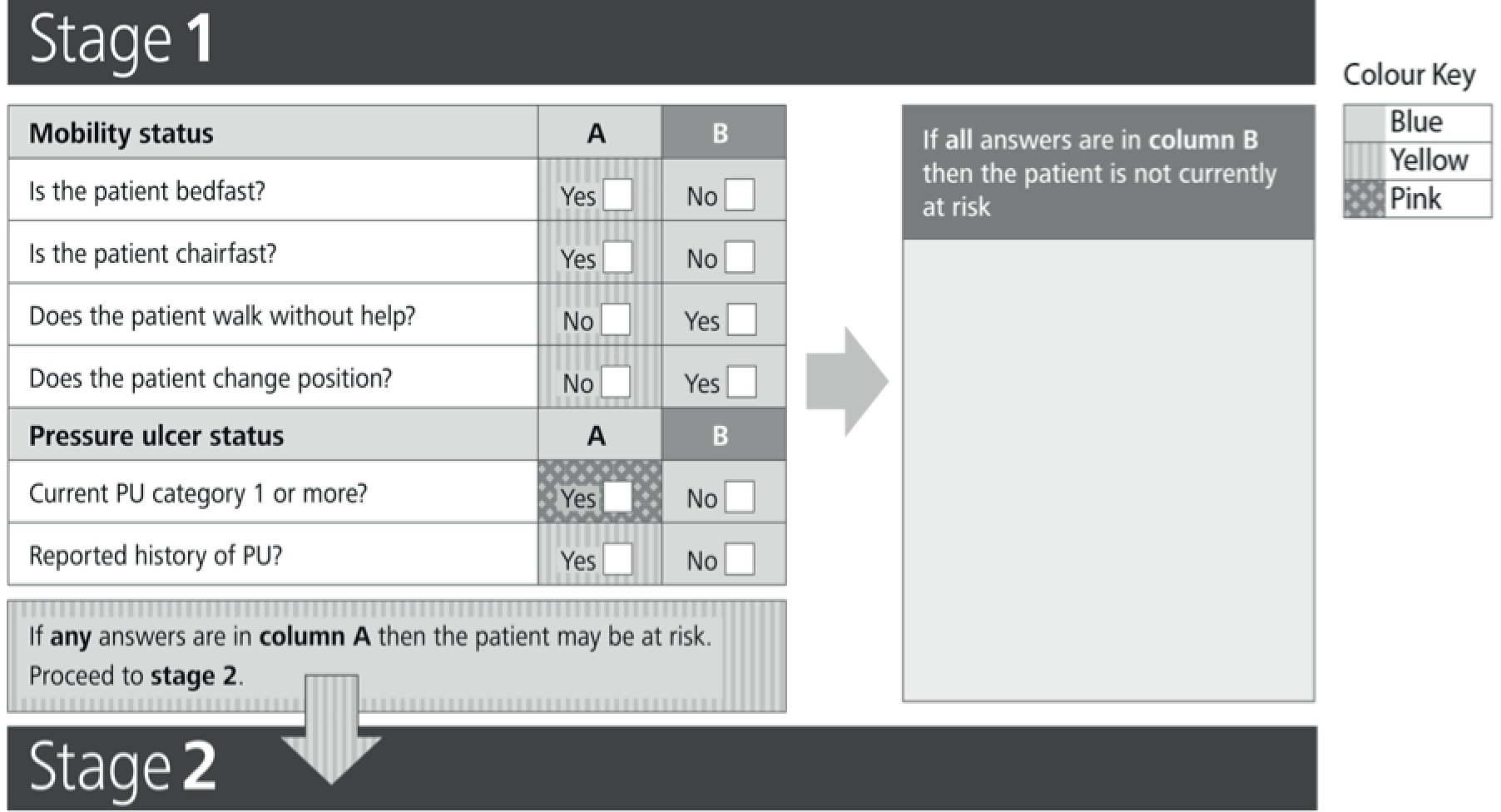
FIGURE 22.
Pre-test session 2 draft Risk Assessment Framework. Please note that the Risk Assessment Framework incorporates the use of colour. These examples have been adapted with the colour key provided.
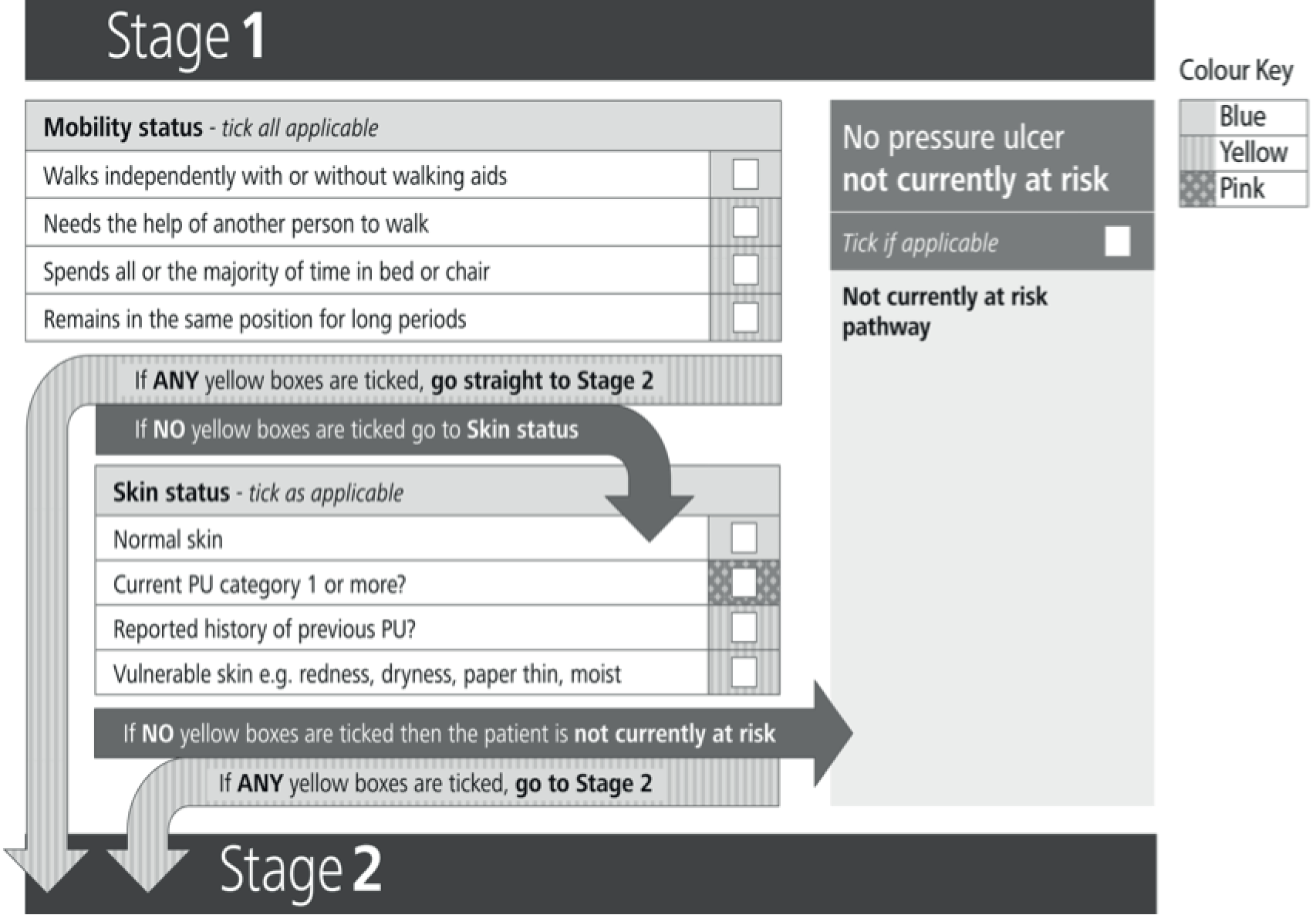
FIGURE 23.
Pre-test session 3 draft Risk Assessment Framework. Please note that the Risk Assessment Framework incorporates the use of colour. These examples have been adapted with the colour key provided.

Other notable changes made over the course of the pre-test session relate to the move from landscape to portrait orientation to improve the flow of the tool and the development of specific items (e.g. the terminology relating to ‘bedfast’ and ‘chairfast’ in the step 1 mobility items was found to be confusing and there was a need to incorporate an element of frequency to the items, which were subsequently amended and tested at the next session). The think out loud participants from the first pre-test also highlighted that items for which a positive response indicated that the patient did not have a problem were confusing. This related to step 1 mobility items and the step 2 sensory perception item and changes were made to the Risk Assessment Framework used at subsequent sessions.
The first pre-test focus group felt that there should be some provision within step 1 of the Risk Assessment Framework to enable nurses to use their clinical judgement in terms of other significant risk factors (which may be exceptions to the rule) that they should take into account when considering if a patient should progress to the more detailed step 2 assessment. This could relate to the severity of a risk factor (e.g. terminally ill patients, severe diabetes, perfusion problems and severe nutritional problems). Having ‘other items’ at step 1 was considered by the working group but there was concern that the screening stage could become too large. Taking into account the causal pathway for pressure ulcer development, it was decided that a ‘vulnerable skin’ item would be included instead to focus the assessment on the potential impact that other medical conditions might have on the skin, rather than on the presence or absence of many different conditions.
The data completeness assessment (see Table 36 and Figure 18) showed poor decision pathway allocation in the first pre-test. The corresponding focus group discussions highlighted confusion over where to indicate the pathway allocation; some nurses had attempted to indicate a pathway on the form although they were clearly unsure of where to do this. This alerted us to a significant omission and lack of clarity within the Risk Assessment Framework and the need to include a response box within the ‘not currently at risk’ pathway at the first stage of the assessment and to make the pathway allocation tick boxes at stage 2 of the assessment more obvious. In addition, the think out loud interviews in the first pre-test session highlighted an issue relating to the ordering of the decision pathway boxes in the first draft Risk Assessment Framework, that is, the first pathway (left) being the blue ‘not currently at risk pathway’, the second pathway (middle) being the orange primary prevention pathway and the third pathway (right) being the red secondary prevention/treatment pathway, and the resultant possibility of ticking the primary prevention pathway before getting to the secondary prevention/treatment pathway. It was suggested that, as ‘red trumps orange’, the boxes should be reordered so that the red one was first, and this was undertaken for the second pre-test (see Figures 21–23).
The review of the Risk Assessment Framework by PURSUN UK and the expert group (following pre-testing) led to a final change to the Risk Assessment Framework. Although members of PURSUN UK felt that the Risk Assessment Framework was clear and understandable, they raised concern about the wording of the sensory perception item; this related to the ‘ability to feel and respond’ aspect of the item. The group agreed that the patient might be able to fulfil only one of these requirements, which should be considered a problem, but the wording suggested that it would be a problem only if the patient could not do both. They felt that the terminology should be ‘feel and/or respond’. This led to the wording of the sensory perception item being reconsidered at the subsequent expert group meeting and amendments being made.
The pre-test facilitated the development of the preliminary Risk Assessment Framework (see Appendix 30), which was easily understood by clinical nurses. The Risk Assessment Framework was subsequently named the PURPOSE-T and will be further evaluated in clinical practice.
Phase 5: clinical evaluation of the PURPOSE-T (Risk Assessment Framework incorporating the risk factor Minimum Data Set)
To enable the provisional Risk Assessment Framework (PURPOSE-T) to be used with confidence in clinical practice, the fifth phase of the work package examined the fundamental properties of the instrument. This involved a field test to assess its reliability, convergent validity and clinical usabilty (see Appendix 31 for the study protocol).
Aims
The aims of field test 1 were to assess the:
-
data completeness and clinical usability of the PURPOSE-T
-
inter-rater and test–retest reliability of the PURPOSE-T
-
convergent validity and known groups validity of the PURPOSE-T.
Methods
Design
The PURPOSE-T was evaluated through field testing using observational descriptive methods. Hospital inpatients and community nursing patients were invited to participate. Demographic characteristics were collected and individual pressure ulcer risk was assessed for all patients. Paired assessments were undertaken simultaneously using the PURPOSE-T, one by a ward/community nurse and one by an expert nurse, with each nurse remaining blind to the corresponding assessment. A blinded retest was undertaken by the same expert nurse at a follow-up visit.
In addition, the expert nurses involved in data collection kept field notes of their experience of using the PURPOSE-T in clinical practice and comments from ward/community nurses during their use of the PURPOSE-T. The field notes were summarised and used to inform design amendments and issues of importance for implementation.
Description of the preliminary PURPOSE-T
The PURPOSE-T (field test version) incorporates a three-step assessment process:
-
step 1 – screening assessment of mobility and skin status
-
step 2 – full assessment of analysis of independent movement, sensory perception, detailed skin assessment, previous pressure ulcer history, perfusion, nutrition, moisture and diabetes
-
step 3 – assessment decision of ‘no pressure ulcer not currently at risk’, ‘no pressure ulcer but at risk’ and ‘pressure ulcer category 1 or above or scarring from previous pressure ulcer’.
The tool is colour coded as follows to facilitate decision-making:
-
blue – ‘no problem’ with risk factor
-
yellow – ‘problem’ that may impact on pressure ulcer risk
-
orange – ‘problem’ that puts the patient at risk and requires primary prevention
-
pink – patient has a pressure ulcer or scar from a previous pressure ulcer and requires secondary prevention/treatment.
The assessment decision is also colour coded using the RAG (red/amber/green) rating as follows:
-
red – ‘pressure ulcer category 1 or above or scarring from previous pressure ulcer’
-
amber – ‘no pressure ulcer but at risk’
-
green – ‘no pressure ulcer not currently at risk’.
At step 1 there are four mobility options with ‘tick all applicable’ instructions. If only the blue-coded criterion ‘walks independently with or without walking aids’ is ticked the instructions are to progress to step 1 skin status. If any other mobility criteria (which are all coded yellow) are ticked, the instructions are to progress to step 2 (see Appendix 30).
The step 1 skin status item also has four options with ‘tick all applicable’ instructions. If only the blue-coded ‘normal skin’ option is ticked the instructions are to allocate the patient to the green assessment decision – the ‘no pressure ulcer not currently at risk’ pathway. If any other skin status options are ticked (coded yellow and pink), the instructions are to progress to step 2 full assessment (see Appendix 30).
Step 2 includes assessment of the following:
-
analysis of independent movement: five options, including four coded orange (with varying limitations to frequency and extent of independent movement) and one coded yellow (making major position changes frequently)
-
detailed skin assessment of 13 skin sites (with the option for ‘other’ skin sites), with three options for each, including ‘normal skin’ coded blue, ‘vulnerable skin’ coded orange and ‘pressure ulcer category 1’ coded pink
-
previous pressure ulcer history: two options, including ‘no known pressure ulcer history’ coded blue and ‘pressure ulcer history’ coded yellow, with presence of scar (if applicable only) coded pink
-
sensory perception: two options, including ‘no problem’ coded blue and ‘patient is unable to feel and/or respond to discomfort from pressure’ coded orange
-
perfusion: three options, including ‘no problem’ coded blue and two options coded orange: ‘conditions affecting central circulation, for example shock, heart failure and hypotension’ and ‘conditions affecting peripheral circulation, for example peripheral vascular/arterial disease’
-
nutrition: five options, including ‘no problem’ coded blue and four options coded yellow: ‘unplanned weight loss’, ‘poor nutritional intake’, ‘low BMI’ and ‘high BMI’
-
moisture: three options, including ‘no problem/occasional’ coded blue and two options coded yellow: ‘frequent’ and ‘constant’
-
diabetes: two options, including ‘not diabetic’ coded blue and ‘diabetic’ coded yellow.
Step 3 involves allocation of an assessment decision as outlined in Table 38.
| Colour code | Assessment | Assessment decision |
|---|---|---|
| Any pink | Pressure ulcer of category 1 or above or scarring from previous pressure ulcer | Red: secondary prevention and treatment pathway |
| Any orange (but no pink) | No pressure ulcer but at risk | Amber: primary prevention pathway |
| Only yellow and blue | Nurse to consider risk factors present and decide | Amber: primary prevention pathway OR green: not currently at risk pathway |
Nurse eligibility and preparation
A nurse was defined as an expert if he or she was a member of the participating trusts’ tissue viability teams (tissue viability nurse consultant/specialist/clinical research nurse). Participating expert nurses attended an initiation training day at which the PURPOSE-T was presented, the instruction manual was provided and they used the PURPOSE-T through vignettes and role play until they were confident in how to use it. In practice, all expert nurses involved in recruitment and data collection were clinical research nurses with specialist tissue viability knowledge gained through their role in other PURPOSE programme research projects.
The expert nurses in the acute sector identified a range of wards, sought verbal permission from ward managers to undertake the research and arranged a mutually convenient date with a qualified member of the ward team to undertake training and patient assessment. In the community sector the expert nurses sought volunteers from the community nursing service and arranged a mutually convenient time to undertake training and patient assessment.
All participating ward/community nurses underwent training in the use of the PURPOSE-T from the expert nurses. This included a full explanation of the PURPOSE-T and the instruction manual followed by an invitation to undertake an assessment using the same vignettes that were used by the expert group nurses in their training, so that they were familiar with the instrument. Either the ward/community nurse or the ward/community team budget received a per-patient or a per-hour payment to cover the funding required to release the ward/community nurse from usual clinical duties.
Patient eligibility
Inclusion criteria
-
Age ≥ 18 years.
-
Inpatient in the acute setting or community nursing patient in the community setting.
-
Provide written informed consent/verbal witnessed consent/consultee agreement.
-
Expected to be available for the PURPOSE-T retest.
Exclusion criteria
-
Patients in obstetric, paediatric, day case surgery or psychiatric settings (acute or community).
-
Patients deemed by the attending health-care professional to be too unwell to be approached and/or complete the study assessment schedule.
Sampling strategy
Patients were purposively sampled ensuring a similar number of hospital and community patients and representation of patients across four broad levels of risk (as defined by their mobility and ulcer status) as follows:
-
no mobility restrictions
-
some mobility/activity limitations
-
bedfast/chairfast
-
pressure ulcer category 1 or above.
Each ward/community nurse was asked to identify four patients on his or her caseload, one from each of the four broad levels of risk when possible.
Recruitment and consent
Ward-/community-based nurses identified suitable patients from their area of practice. A full verbal explanation of the study and a patient information leaflet (see Appendix 32) were provided by the attending clinical staff or a member of the tissue viability team and assenting patients were then invited to provide informed, written consent (see Appendix 33). When patients were capable of giving consent but physically unable to complete the written aspects of the consent form, witnessed consent was obtained (see Appendix 34). In addition, to ensure that the study population was representative of the clinical population assessed in the course of usual care, when patients lacked capacity ethical approval was given for consultee agreement (see Appendices 35 and 36). Assessment of eligibility and informed consent was undertaken by a member of the tissue viability team. Patients who both were eligible for study participation and provided informed consent/consultee agreement were registered centrally using the CTRU automated 24-hour telephone registration system.
Data collection/assessments
Each patient recruited to the field test was assessed by only one pair of assessors.
At baseline, demographic and clinical data were recorded for each patient by the expert nurse. Baseline data included type of NHS facility (hospital/intermediate care/community nursing team), type of admission/referral (e.g. elective/acute), ward specialty (hospital patients only), date of birth, gender and ethnicity. Clinical assessment included the subscales of the Braden scale51 and the Waterlow scale. 50
At baseline the PURPOSE-T was completed and recorded by a member of the ward/community team and the expert nurse, blind to each other’s assessment. This incorporated the detailed skin assessment and, when applicable, pressure ulcer classification. 1 The blinding was maintained through the design of a sealable research form. Both nurses were instructed to complete their assessment and seal the form prior to collection.
Finally, the expert nurse undertook a second visit and completed the PURPOSE-T and recorded clinically relevant changes to the patient’s condition since the baseline assessment. The PURPOSE-T assessment was carried out blind to the baseline assessment, again maintained through the sealed research form.
The length of the test–retest interval was planned to be short enough to ensure that clinical change in the pressure ulcer was unlikely to occur but sufficiently long to ensure that the expert nurse did not recall his or her responses from the first assessment. Nurses were asked to plan their retest visit between 1 and 3 days after the baseline visit for hospital patients and between 1 and 7 days after the baseline visit for community patients, taking into account the anticipated recovery/deterioration/stability of each patient’s condition and, for hospital patients, length of stay.
The expert nurses involved in data collection also kept field notes of their experience of using the PURPOSE-T in clinical practice.
Analysis considerations
Study definitions of risk
The PURPOSE-T identifies three groups of patients: those patients who are not currently at risk of developing a pressure ulcer, those patients who have no pressure ulcer but who are ‘at risk’ and require primary prevention and those patients with an existing pressure ulcer/scar who require secondary prevention/treatment. For the purposes of describing the study population and to assess convergent validity with other risk assessment tools, ‘at risk’ is defined as all patients ‘who have no pressure ulcer but who are at risk’ and all patients who have a ‘pressure ulcer category 1 or above or scarring from previous pressure ulcers’. A patient is therefore defined as ‘not at risk’ if his or her outcome within the raw data was recorded as ‘no pressure ulcer not currently at risk’. The cut-point used to identify patients at risk was ≤ 18 for the Braden scale193 and ≥ 10 for the Waterlow scale. 50
Sample size
In the study population we aimed to recruit approximately 25% of patients ‘not at risk’ and 75% ‘at risk’. In a two-rater study, the numbers of subjects required to detect a statistically significant kappa (two-sided p-value ≤ 0.05) with 90% power and 75% assessed as being ‘at risk’, assuming a null hypothesis value for kappa, are given in Table 39.
| Kappa to detect | Null value | Number of required patients (90% power) |
|---|---|---|
| 0.7 | 0.4 | 114 |
| 0.7 | 0.5 | 231 |
| 0.7 | 0.6 | 793 |
| 0.8 | 0.4 | 64 |
| 0.8 | 0.5 | 103 |
| 0.8 | 0.6 | 199 |
| 0.8 | 0.7 | 536 |
| 0.9 | 0.4 | 41 |
| 0.9 | 0.5 | 58 |
| 0.9 | 0.6 | 89 |
| 0.9 | 0.7 | 159 |
To establish whether the tool gives a high degree of beyond-chance agreement, we tested against a null value of 0.6. With 90% power, 199 patients were required. To allow for withdrawal/non-compliance in paired assessments of 15%, we aimed to recruit 230 patients.
No examples of formal sample size estimation methods for the evaluation of screening instruments were identified in the literature. Therefore, literature relating to the psychometric evaluation of rating scales was considered. The ‘rule of thumb’ recommendation of 5–10 patients for every item in a questionnaire was used to estimate the sample size of 115–230 patients. 194,195 The proposed sample size of 230 to assess the inter-rater reliability of the instrument, with > 95% expert nurse data compliance (based on previous research experience), was expected to provide a sufficient number of patients to assess the validity of the risk assessment instrument.
Analysis methods
Data completeness was assessed for each element of the PURPOSE-T including the percentage of missing item-level data and risk categories allocated.
We produced kappa (with 95% CI), prevalence-adjusted bias-adjusted kappa (PABAK) and the maximum value of kappa (κmax. ) statistics to assess the inter-rater and test–retest reliability for agreement of risk status overall (i.e. at risk/not at risk); cross-tabulations of overall risk status by rater/retest were produced. We also examined the extent of agreement for individual PURPOSE-T items using cross-tabulations by type of rater/retest. In addition, we produced kappa (with 95% CI) and weighted kappa statistics to assess the inter-rater reliability for agreement of the PURPOSE-T outcome on the 3-point scale (no risk, at risk, current pressure ulcer or pressure ulcer scarring) and produced cross-tabulations of PURPOSE-T outcome by type of rater/retest. We used guidelines to interpret kappa analysis as detailed in Table 40. 196,197
| Value of kappa | Strength of agreement |
|---|---|
| < 0.20 | Poor |
| 0.21–0.40 | Fair |
| 0.41–0.60 | Moderate |
| 0.61–0.80 | Good |
| 0.81–1.00 | Very good |
Table 41 details the psychometric tests undertaken. Convergent validity assesses the degree to which constructs (or scores on a measure) expected to be related are, in fact, related. The degree to which assessment of ‘at risk’ and ‘not at risk’ is related to risk assessment status as assessed using the Braden and Waterlow risk assessment scales was determined, using cross-tabulations.
| Test property | Definition/test | Criteria |
|---|---|---|
| Data quality; acceptability/data completeness | The extent to which PURPOSE-T items are completed and used to allocate a risk category; quality of data is assessed by data completeness for each element of the PURPOSE-T and a risk category |
|
| Clinical usability | Compliance with the recommended completion guidelines |
|
| Inter-rater reliability | Inter-rater reliability assesses the extent to which the PURPOSE-T results obtained by two or more raters agree for the same population |
|
| Test–retest reliability | Test–retest reliability assesses the stability of the PURPOSE-T over a period of time in which the patient’s condition is not expected to change | |
| Content validity | The extent to which a scale measures what it intends to measure |
|
| Convergent validity (between-scale analysis – analyses against external criteria) | Evidence that PURPOSE-T constructs are correlated with other measures of the same or similar constructs, assessed on the basis of correlations between the measure and other similar measures (Braden scale and Waterlow scale) |
|
| Known group differences | The ability of PURPOSE-T risk categories to differentiate known groups, assessed by comparing PURPOSE-T risk categories for subgroups who are expected to differ on the construct being measured (significant differences between known groups or differences of expected magnitude) (e.g. elective/acute patients) |
In addition, cross-tabulations of corresponding items between the PURPOSE-T and the Braden scale and/or the Waterlow scale were produced and correlation coefficients were calculated. The Spearman rank correlation coefficient was used when each of the items being compared had more than two levels, for example the Braden activity and Braden mobility subscales each have four levels and were both compared with the PURPOSE-T analysis of independent movement (which had been reduced to a 3-point scale). The phi correlation coefficient was calculated when dichotomous variables were compared, for example risk status on the Braden scale compared with risk status on the PURPOSE-T. For exploratory purposes, the following hypotheses were used as guides to the magnitude of correlations, as opposed to pass/fail benchmarks: high correlation r > 0.7; moderate correlation r = 0.3–0.7; low correlation r < 0.3. 198,199 Moderate to high correlations (r ≥ 0.3) were predicted.
Known-group comparisons are used to evaluate the clinical utility of instruments or assessment tools. This method assesses the extent to which the overall assessment or items are able to discriminate between subgroups of patients known to differ in terms of clinical presentations. 200 A chi-square test for independence (used to compare the frequencies of cases found in the various categories of one variable across the different categories of another variable) was planned to determine whether or not type of hospital patient (e.g. elective vs. acute) was related to PURPOSE-T risk group as assessed by the expert nurse at baseline. However, because of the small number of elective patients this was not appropriate. No other known group was prespecified.
Field notes
The field notes were summarised and used to inform design amendments and issues of importance for implementation.
Results
In total, 394 patients were screened for eligibility for the study and 230 patients were registered to the study between 3 October 2012 and 25 January 2013 (Figure 24) from four secondary care acute hospital NHS trusts (comprising five recruiting hospitals) and four community NHS trusts, with numbers of patients registered at each centre ranging from 14 to 54 (see Appendix 1).
FIGURE 24.
Flow of participants.
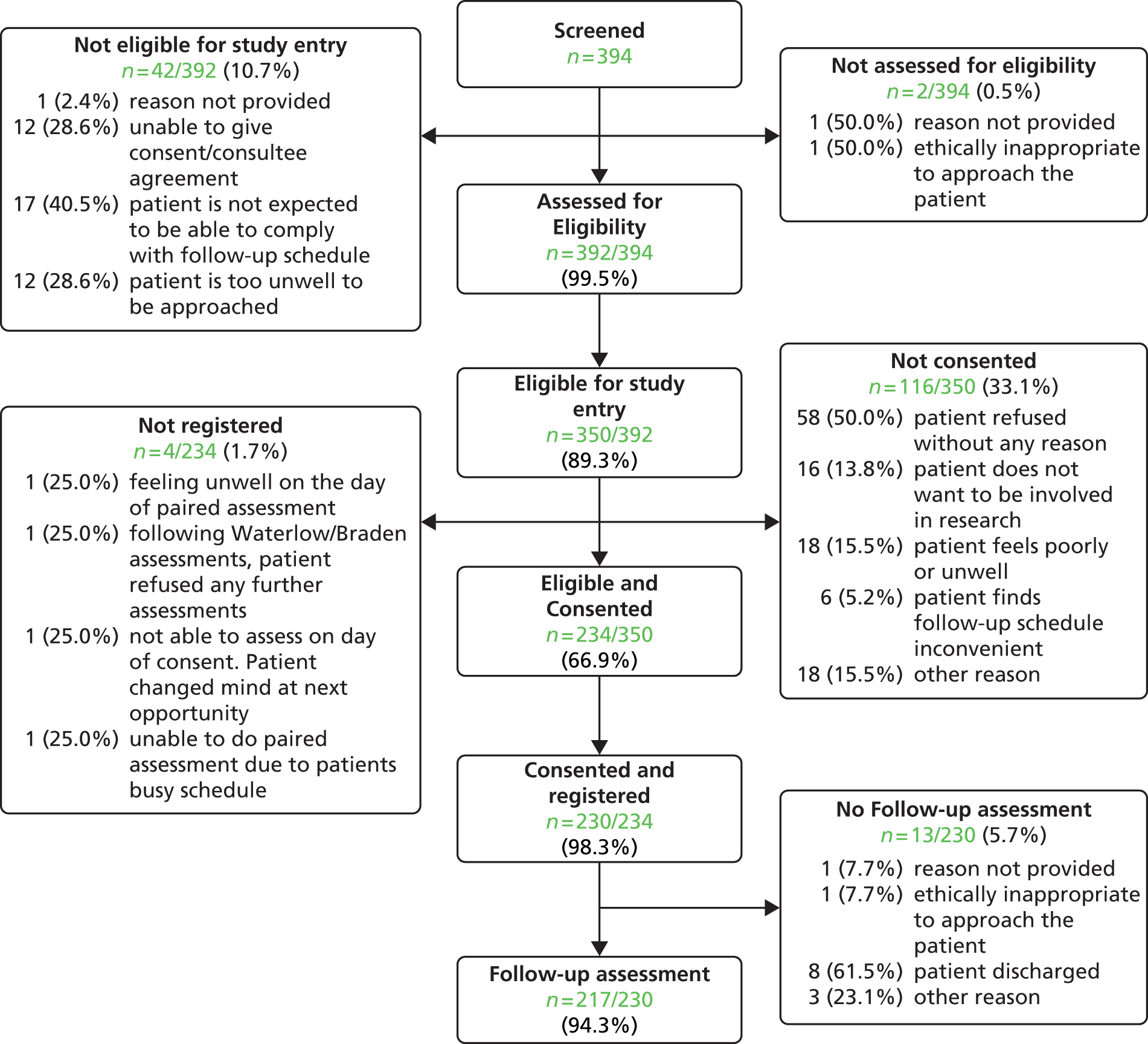
All of the 230 patients recruited were assessed in part or full using the PURPOSE-T, providing a total of 230 paired assessments undertaken by 11 expert nurses and 73 ward/community nurses. A median of three patients were assessed by each expert nurse and ward/community nurse pair. There was good representation from each of the four broad levels of risk with 53 (23.0%) patients having no mobility restrictions, 70 (30.4%) having some mobility/activity limitations, 49 (21.3%) who were bedfast/chairfast and 58 (25.2%) with an existing pressure ulcer of category 1 or above as reported at registration.
In total, 122 (53.0%) patients were recruited from community settings and 108 (47.0%) were recruited from secondary care hospital settings (Table 42). Of the 230 patients registered, 217 (94.3%) had retest assessments completed by the expert nurse (see Figure 24).
| Specialty or place assessed | Acute setting (n = 108), n (%) | Community setting (n = 122), n (%) | Total (n = 230), n (%) |
|---|---|---|---|
| Patient’s own home | – | 47 (38.5) | 47 (20.4) |
| Nursing home | – | 3 (2.5) | 3 (1.3) |
| Residential home | – | 8 (6.6) | 8 (3.5) |
| Rehabilitation unit | – | 57 (46.7) | 57 (24.8) |
| Other place assesseda | – | 7 (5.7) | 7 (3.0) |
| Medical | 26 (24.1) | – | 26 (11.3) |
| Care of the elderly | 3 (2.8) | – | 3 (1.3) |
| General/urological/gynaecological surgery | 19 (17.6) | – | 19 (8.3) |
| High-dependency unit | 1 (0.9) | – | 1 (0.4) |
| Oncology | 3 (2.8) | – | 3 (1.3) |
| Orthopaedic | 19 (17.6) | – | 19 (8.3) |
| Plastics | 2 (1.9) | – | 2 (0.9) |
| Renal | 12 (11.1) | – | 12 (5.2) |
| Spinal injury | 4 (3.7) | – | 4 (1.7) |
| Thoracic surgery | 4 (3.7) | – | 4 (1.7) |
| Vascular surgery | 15 (13.9) | – | 15 (6.5) |
The patient population comprised 99 (43.0%) men and 131 (57.0%) women. The median age was 77 years (range 19–102 years), the majority of patients were Caucasian (n = 224; 97.4%) and 79 (34.3%) had no activity limitation (i.e. were able to walk independently with or without a walking aid) (Table 43).
| Variable | PU at baselinea (n = 60) | No PU at baselinea (n = 169) | Missing PU statusa (n = 1b) | Totalc (n = 230) |
|---|---|---|---|---|
| Age (years) | ||||
| Mean (SD) | 73.8 (15.9) | 72.1 (18.3) | 72.6 (17.6) | |
| Median (range) | 76 (29–98) | 78 (19–102) | 78 (78–78) | 77 (19–102) |
| Sex, n (%) | ||||
| Male | 27 (27.3) | 72 (72.7) | 0 (0.0) | 99 (43.0) |
| Female | 33 (25.2) | 97 (74.0) | 1 (0.8) | 131 (57.0) |
| Ethnicity, n (%) | ||||
| Caucasian | 58 (25.9) | 165 (73.7) | 1 (0.4) | 224 (97.4) |
| Other | 2 (33.3) | 4 (66.7) | 0 (0.0) | 6 (2.6) |
| Setting, n (%) | ||||
| Community | 37 (30.3) | 84 (68.9) | 1 (0.1) | 122 (53.0) |
| Secondary care hospital | 23 (21.3) | 85 (78.7) | 0 (0.0) | 108 (47.0) |
| Mobility status PURPOSE-T step 1, n (%) | ||||
| Walks independently with or without walking aids | 10 (12.7) | 69 (87.3) | 0 (0.0) | 79 (34.3) |
| Needs help of another person to walk | 6 (22.2) | 21 (77.8) | 0 (0.0) | 27 (11.7) |
| Spends all/majority of time in bed/chair | 16 (28.1) | 40 (70.2) | 1 (1.8) | 57 (24.8) |
| Remains in same position for long periods | 28 (42.4) | 38 (57.6) | 0 (0.0) | 66 (28.7) |
| Not completed | 0 (0.0) | 1 (100.0) | 0 (0.0) | 1 (0.4) |
| Braden score, n (%) | ||||
| At risk (≤ 18) | 35 (41.2) | 50 (58.8) | 0 (0.0) | 85 (37.0) |
| Not at risk (> 18) | 25 (17.2) | 119 (82.1) | 1 (0.7) | 145 (63.0) |
| Waterlow total score, n (%) | ||||
| At risk (≥ 10) | 60 (31.1) | 132 (68.4) | 1 (0.5) | 193 (83.9) |
| Not at risk (< 10) | 0 (0.0) | 37 (100.0) | 0 (0.0) | 37 (16.1) |
| PURPOSE-T risk categorisation, n (%) | ||||
| Secondary prevention/treatment pathway | 60 (83.3) | 12 (16.7) | 0 (0.0) | 72 (31.3) |
| Primary prevention pathway | 0 (0.0) | 111 (100) | 0 (0.0) | 111 (48.3) |
| Not currently at risk pathway | 0 (0.0) | 46 (97.9) | 1 (2.1) | 47 (20.4) |
Based on the PURPOSE-T assessment carried out at baseline by the expert nurses, there were 60 (26.1%) patients who presented with a category 1 or above pressure ulcer. Within the community setting, 37 (30.3%) patients presented with a category 1 or above ulcer whereas, among secondary care hospital patients, 23 (21.3%) presented with a category 1 or above ulcer (see Table 43). There were a total of 96 pressure ulcers across the 60 patients including 21 (21.9%) category 1, 56 (58.3%) category 2, six (6.3%) category 3, six (6.3%) category 4 and seven (7.3%) unstageable ulcers (Table 44).
| Variable | Expert nurse baseline assessment | Ward/community nurse assessment | Expert nurse follow-up assessment |
|---|---|---|---|
| Total population | 230 | 230 | 217 |
| Number of patients with vulnerable skin | 152 | 156 | 144 |
| Number of patients with history of PU | 61 | 56 | 61 |
| Number of patients with PU | 60 | 62a | 56 |
| Total number of PUs | 96 | 98 | 87 |
| Total number of PUs per patient | |||
| Mean (SD) | 1.6 (1.1) | 1.7 (1.2)a | 1.6 (0.9) |
| Median (range) | 1 (1–7) | 1 (1–7) | 1 (1–5) |
| Categories of reported PUs, n (%) | |||
| Category 1 | 21 (21.9) | 22 (22.4) | 14 (16.1) |
| Category 2 | 56 (58.3) | 41 (41.8) | 50 (57.5) |
| Category 3 | 6 (6.3) | 5 (5.1) | 9 (10.3) |
| Category 4 | 6 (6.3) | 10 (10.2) | 5 (5.7) |
| Unstageable | 7 (7.3) | 7 (7.1) | 9 (10.3) |
| Missing | 0 (0.0) | 13 (13.3) | 0 (0.0) |
| Total | 96 | 98 | 87 |
In relation to the ‘at risk’ status of the patient population, the Waterlow scale score identified 193 (83.9%) patients as ‘at risk’, the PURPOSE-T identified 183 (79.6%) patients as ‘at risk’ (i.e. requiring primary or secondary prevention/treatment) and the Braden scale score identified 85 (37.0%) patients as ‘at risk’. The Braden scale score identified 145 patients as ‘not at risk’ of whom 25 (17.2%) had an existing pressure ulcer, whereas none of the patients with a pressure ulcer was assessed as ‘not at risk’ by either the Waterlow scale score or the PURPOSE-T (see Table 43).
Data completeness and usability
Data completeness and usability were assessed by quantifying compliance with the completion guidelines for steps 1, 2 and 3 (Tables 45–48) and data completeness (Table 49) and analysing the qualitative reports from the expert nurses (see Summary of expert nurse field notes).
| PURPOSE-T section | Expert nurse baseline assessment | Ward/community nurse assessment | Expert nurse retest |
|---|---|---|---|
| Step 1 mobility, n (%) | |||
| Completed | 229 (99.6) | 229 (99.6) | 217 (100.0) |
| Not completed | 1 (0.4) | 1 (0.4) | 0 (0.0) |
| Total | 230 (100.0) | 230 (100.0) | 217 (100.0) |
| Progression to step 1 skin status, n (%) | |||
| Mobility step 1 assessment – no mobility limitation | |||
| Appropriate completion of step 1 skin status | 78 (33.9) | 84 (36.5) | 63 (29.0) |
| Inappropriate non-completion of step 1 skin status | 1 (0.4) | 3 (1.3) | 0 (0.0) |
| Mobility step 1 assessment – mobility limitation | |||
| Appropriate completion of step 1 skin status | 114 (49.6) | 83 (36.1) | 117 (53.9) |
| Inappropriate non-completion of step 1 skin status | 36 (15.7) | 59 (25.7) | 37 (17.1) |
| Mobility step 1 assessment – not assessed | |||
| Completion of step 1 skin status | 0 (0.0) | 1 (0.4) | 0 (0.0) |
| Non-completion of step 1 skin status | 1 (0.4) | 0 (0.0) | 0 (0.0) |
| Total | 230 (100.0) | 230 (100.0) | 217 (100.0) |
| Progression to step 2 to full assessment, n (%) | |||
| Potential risk identified at step 1 | |||
| Appropriate completion of step 2 | 185 (80.4) | 185 (80.4) | 179 (82.5) |
| Inappropriate non-completion of step 2 | 0 (0.0) | 1 (0.4) | 0 (0.0) |
| Not at risk at step 1 | |||
| Appropriate non-completion of step 2 | 35 (15.2) | 32 (13.9) | 35 (16.1) |
| Inappropriate completion of step 2 | 9 (3.9) | 12 (5.2) | 3 (1.4) |
| Step 1 not completed | |||
| Step 2 completed | 1 (0.4) | 0 (0.0) | 0 (0.0) |
| Total | 230 (100.0) | 230 (100.0) | 217 (100.0) |
| Step 1/step 3 assessment decision allocated at step 1 or 3, n (%) | |||
| Appropriate pathway | 226 (98.3) | 219 (95.2) | 215 (99.1) |
| Inappropriate pathway | 4 (1.7) | 10 (4.3) | 2 (0.9) |
| No pathway selected | 0 (0.0) | 1 (0.4) | 0 (0.0) |
| Total | 230 (100.0) | 230 (100.0) | 217 (100.0) |
| PURPOSE-T decision pathway | Colour of boxes ticked, n (%) | Total, n (%) | ||
|---|---|---|---|---|
| At least one pink box ticked | No pink boxes and at least one orange box ticked | Only blue and yellow boxes ticked | ||
| PU category 1 or above or scarring | 72 (31.3) | 0 (0.0) | 0 (0.0) | 72 (31.3) |
| No PU, but at risk | 0 (0.0) | 109 (47.4) | 2 (0.9) | 111 (48.3) |
| No PU, not currently at risk | 0 (0.0) | 4 (1.7) | 43 (18.7) | 47 (20.4) |
| Total | 72 (31.3) | 113 (49.1) | 45 (19.6) | 230 (100.0) |
| PURPOSE-T decision pathway | Colour of boxes ticked, n (%) | Total, n (%) | ||
|---|---|---|---|---|
| At least one pink box ticked | No pink boxes and at least one orange box ticked | Only blue and yellow boxes ticked | ||
| PU category 1 or above or scarring | 63 (27.4) | 0 (0.0) | 0 (0.0) | 63 (27.4) |
| No PU, but at risk | 5 (2.2) | 107 (46.5) | 2 (0.9) | 114 (49.6) |
| No PU, not currently at risk | 0 (0.0) | 5 (2.2) | 47 (20.4) | 52 (22.6) |
| Missing | 0 (0.0) | 1 (0.4) | 0 (0.0) | 1 (0.4) |
| Total | 68 (29.6) | 113 (49.1) | 49 (21.3) | 230 (100.0) |
| PURPOSE-T decision pathway | Colour of boxes ticked, n (%) | Total, n (%) | ||
|---|---|---|---|---|
| At least one pink box ticked | No pink boxes and at least one orange box ticked | Only blue and yellow boxes ticked | ||
| PU category 1 or above or scarring | 68 (31.3) | 0 (0.0) | 0 (0.0) | 68 (31.3) |
| No PU, but at risk | 2 (0.9) | 104 (47.9) | 1 (0.5) | 107 (49.3) |
| No PU, not currently at risk | 0 (0.0) | 0 (0.0) | 42 (19.4) | 42 (19.4) |
| Total | 70 (32.3) | 104 (47.9) | 43 (19.8) | 217 (100.0) |
| Construct | Number of items requiring completion | Expert nurse baseline assessment, % (n/N) | Ward/community nurse assessment, % (n/N) | Expert nurse follow-up assessment, % (n/N) | Denominator (i.e. number of items expected to have been completed) |
|---|---|---|---|---|---|
| Step 1 screening | |||||
| Mobility | 1 of 4 | 99.6 (229/230) | 99.6 (229/230) | 100.0 (217/217) | All patients, as all were required to complete step 1 mobility |
| Skin status | 1 of 4 (if required) | 98.7 (78/79) | 96.6 (84/87) | 100.0 (63/63) | All patients for whom only the blue box was ticked in step 1 mobility |
| Assessment decision | 1 | 95.3 (41/43) | 85.7 (36/42) | 100.0 (38/38) | All patients for whom only the blue box was ticked in both step 1 mobility and step 1 skin status |
| Step 2 full assessment | |||||
| Number of step 2 assessments | 195 | 197 | 182 | ||
| Step 1 mobility | 1 of 4 | 99.5 (194/195) | 99.5 (196/197) | 100.0 (182/182) | All patients who progressed to step 2, as all were required to complete step 1 mobility |
| Step 1 skin status | 1 of 4 (if required) | 97.7 (43/44) | 96.3 (52/54) | 100.0 (28/28) | All patients for whom only the blue box was ticked in step 1 mobility who progressed to step 2 |
| Analysis of independent movement | 1 of 5 | 99.0 (193/195) | 99.0 (195/197) | 98.9 (180/182) | All patients who progressed to step 2 |
| Sensory perception and response | 1 of 2 | 96.9 (189/195) | 94.9 (187/197) | 98.4 (179/182) | |
| Current detailed skin assessment | 13 | 95.5 (2421/2535) | 95.3 (2440/2561) | 97.5 (2307/2366) | 13 (number of main skin sites) times the no. of patients who progressed to step 2 |
| Previous PU history | 1 of 2 | 99.0 (193/195) | 95.9 (189/197) | 98.4 (179/182) | All patients who progressed to step 2 |
| Previous PU details | At least 1 | 66.7 (40/60) | 54.7 (29/53) | 57.4 (35/61) | All patients reported to have a PU history in previous construct |
| Perfusion | At least 1 | 97.9 (191/195) | 97.5 (192/197) | 97.3 (177/182) | All patients who progressed to step 2 |
| Nutrition | At least 1 | 99.0 (193/195) | 99.5 (196/197) | 97.8 (178/182) | |
| Moisture | 1 of 3 | 99.5 (194/195) | 97.0 (191/197) | 96.7 (176/182) | |
| Diabetes | 1 of 2 | 99.0 (193/195) | 95.9 (189/197) | 96.7 (176/182) | |
| Decision pathway allocated | 1 of 3 | 100.0 (195/195) | 99.0 (195/197) | 100.0 (182/182) | |
| Step 1 and step 2 for those who completed step 2 | 96.5 (4239/4394) | 95.4 (4236/4441) | 97.2 (3979/4093) | Sum of all denominators for those who progressed to step 2 | |
Progression/non-progression to step 2 was completed in line with the recommended assessment flow for 220 (95.7%) patients, including 185 (80.4%) patients who were appropriately assessed at step 2 and 35 (15.2%) patients who were allocated a decision pathway after completion of step 1 and who did not require step 2 assessments. There was one (0.4%) patient for whom no step 1 assessment was completed and there were nine (3.9%) patients who progressed to step 2 assessments when it was not required (see Table 45). It is of note that three of these nine patients were allocated to the ‘no pressure ulcer but at risk’ pathway after their step 2 assessments; one of these patients had at least one skin site assessed as vulnerable skin in step 2 but had not had this skin status selected at step 1, whereas the other two patients were assessed as ‘at risk’ because the expert nurses had assessed them as having a condition affecting peripheral circulation in step 2.
The expert nurses allocated a step1/step 3 decision pathway to all 230 (100%) patients, with 226 (98.3%) allocated a decision pathway as per the decision rules and four (1.7%) allocated a pathway incorrectly as at least one orange box on the PURPOSE-T had been ticked but the patients had been allocated to the ‘not currently at risk’ pathway (see Table 46).
The follow-up expert nurse step 1 assessments were completed in line with the recommended assessment flow for 180 (82.9%) assessments (see Table 45). In 37 (17.1%) assessments the expert nurses completed the step 1 skin status when this was not required (i.e. patient already identified as having a mobility limitation).
Ward/community nurse assessment
The ward/community nurse form completion was similar to the expert nurse form completion. Step 1 assessments were completed in line with the recommended assessment flow for 167 (72.6%) patients. In 59 (25.7%) assessments the ward/community nurse completed the step 1 skin status assessment when this was not required (i.e. patient already identified as having a mobility limitation).
Progression/non-progression to step 2 was completed in line with the recommended assessment flow for 217 (94.3%) patients, including 185 (80.4%) patients who were appropriately assessed at step 2 and 32 (13.9%) patients who were allocated a decision pathway after completion of step 1 and who did not require step 2 assessments. There was one (0.4%) patient who should have progressed to step 2 and but did not and there were 12 (5.2%) patients who progressed to step 2 when it was not required, although it is of note that three of these patients were allocated to the ‘no pressure ulcer but at risk’ pathway after their step 2 assessments (see Table 45). The ward/community nurses allocated a step1/step 3 decision pathway to 229 (99.6%) patients, with 219 (95.2%) allocated a decision pathway as per the decision rules and 10 (4.3%) allocated a pathway incorrectly; there were five patients for whom at least one pink box had been ticked but the ‘no pressure ulcer but at risk’ decision pathway had been selected and there were five patients for whom at least one orange box had been ticked but the ‘no pressure ulcer not currently at risk’ decision pathway had been selected (see Table 47). It is noteworthy that the ward/community nurses also allocated the majority of patients to the ‘not at risk’ decision pathway when they completed only yellow and blue boxes (47/49, 95.9%; see Table 47).
At follow-up, progression/non-progression to step 2 was completed by the expert nurses in line with the recommended assessment flow for 214 (98.6%) patients (see Table 45). Only three (1.4%) patients progressed to step 2 when it was not required and they were subsequently assessed as ‘not currently at risk’.
At follow-up, the expert nurses allocated a step 1/step 3 decision pathway to all 217 (100%) patients, with 215 (99.1%) patients allocated as per the decision rules and two (0.9%) patients allocated a pathway inappropriately as the expert nurses had ticked a pink box on the PURPOSE-T form but selected the ‘no pressure ulcer but at risk’ decision pathway (see Table 48).
It is noteworthy that when only yellow and blue boxes were completed the expert nurses allocated the majority of patients to the ‘not at risk’ decision pathway at baseline (43/45; 95.6%) and follow-up (42/43; 97.7%) (see Tables 46 and 48).
Inter-rater reliability
There were 230 paired assessments available for comparison; at step 1 mobility there was a total of 228 paired assessments (Table 50) and at the step 2 assessments there were 191 paired assessments (Table 51). To assess the inter-rater reliability, the maximum number of paired assessments available was used. Non-compliance with the recommended assessment flow was not taken into account to maximise the use of all available data, that is, the population used for the assessment of step 2 inter-rater reliability consisted of 191 patients for whom the step 2 assessment was completed by both raters, irrespective of whether or not they progressed to step 2 in line with the recommended assessment flow.
| Expert nurse | Ward/community nurse, n (%) | ||||
|---|---|---|---|---|---|
| Walks independently with/without walking aids | Needs help of another person to walk | Spends all or majority of time in bed or chair | Remains in same position for long periods | Total | |
| Walks independently with or without walking aids | 72 (31.6) | 1 (0.4) | 5 (2.2) | 0 (0.0) | 78 (34.2) |
| Needs the help of another person to walk | 4 (1.8) | 14 (6.1) | 8 (3.5) | 1 (0.4) | 27 (11.8) |
| Spends all or the majority of time in bed or chair | 9 (3.9) | 5 (2.2) | 37 (16.2) | 6 (2.6) | 57 (25.0) |
| Remains in the same position for long periods | 2 (0.9) | 2 (0.9) | 29 (12.7) | 33 (14.5) | 66 (28.9) |
| Total | 87 (38.2) | 22 (9.6) | 79 (34.6) | 40 (17.5) | 228 (100.0) |
| Expert nurse baseline | Ward/community nurse, n (%) | |||
|---|---|---|---|---|
| Completed: appropriate (possible risk in step 1) | Completed: inappropriate (missing/not at risk in step 1) | Not completed | Total | |
| Completed: appropriate (possible risk in step 1) | 179 (77.8) | 3 (1.3) | 3 (1.3) | 185 (80.4) |
| Completed: inappropriate (missing/not at risk in step 1) | 3 (1.3) | 6 (2.6) | 1 (0.4) | 10 (4.3) |
| Not completed | 3 (1.3) | 3 (1.3) | 29 (12.6) | 35 (15.2) |
| Total | 185 (80.4) | 12 (5.2) | 33 (14.3) | 230 (100.0) |
To compare skin assessments the ‘worst’ skin status recorded on the PURPOSE-T (i.e. from step 1 or step 2) was used and there were a total of 230 paired assessments (Table 52).
| Expert nurse | Ward/community nurse, n (%) | ||||
|---|---|---|---|---|---|
| Normal skin | Vulnerable skin | PU category | Missing | Total | |
| Normal skin | 54 (23.5) | 11 (4.8) | 1 (0.4) | 2 (0.9) | 68 (29.6) |
| Vulnerable skin | 11 (4.8) | 82 (35.7) | 8 (3.5) | 0 (0.0) | 101 (43.9) |
| PU category | 0 (0.0) | 7 (3.0) | 53 (23.0) | 0 (0.0) | 60 (26.1) |
| Missing | 0 (0.0) | 1 (0.4) | 0 (0.0) | 0 (0.0) | 1 (0.4) |
| Total | 65 (28.3) | 101 (43.9) | 62 (27.0) | 2 (0.9) | 230 (100.0) |
Decision pathway
There was agreement in the decision pathway between the expert nurse and ward/community nurse for 187 (81.7%) paired assessments (Table 53). The corresponding simple kappa statistic of 0.71 (95% CI 0.63 to 79) and weighted kappa of 0.76 (95% CI 0.69 to 0.83) indicate good agreement between the raters (although the 95% CI for the weighted kappa straddles the ‘good’ and ‘very good’ cut-off values).
| Expert nurse | Ward/community nurse, n (%) | |||
|---|---|---|---|---|
| PU category 1 or above or scarring | No PU but at risk | No PU not currently at risk | Total | |
| PU category 1 or above or scarring | 54 (23.6) | 18 (7.9) | 0 (0.0) | 72 (31.4) |
| No PU but at risk | 9 (3.9) | 91 (39.7) | 10 (4.4) | 110 (48.0) |
| No PU not currently at risk | 0 (0.0) | 5 (2.2) | 42 (18.3) | 47 (20.5) |
| Total | 63 (27.5) | 114 (49.8) | 52 (22.7) | 229 (100.0) |
When classified dichotomously as ‘at risk’/’not at risk’ there was agreement between the expert nurse and ward/community nurse for 214 (93.4%) paired assessments (Table 54). The corresponding simple kappa statistic of 0.81 (95% CI 0.71 to 0.90), PABAK of 0.87 and κmax. of 0.94 indicate very good agreement between raters (although the 95% CI for the simple kappa statistic straddles the ‘good’ and ‘very good’ cut-off values).
| Expert nurse | Ward/community nurse, n (%) | ||
|---|---|---|---|
| At risk | Not at risk | Total | |
| At risk | 172 (75.1) | 10 (4.4) | 182 (79.5) |
| Not at risk | 5 (2.2) | 42 (18.3) | 47 (20.5) |
| Total | 177 (77.3) | 52 (22.7) | 229 (100.0) |
Mobility
There was overall agreement between the expert nurse and the ward/community nurse for 156 (68.4%) paired assessments of step 1 mobility, with agreement that there was ‘no problem’ (i.e. walks independently with or without walking aids) or that there was a ‘problem’ for 207 (90.8%) paired assessments (see Table 50). At step 2 there was absolute agreement across the five possible categories for the analysis of independent movement for 113 (59.2%) paired assessments, with agreement that there was ‘no problem’ (i.e. moves frequently and major position changes) and that there was a ‘problem’ for 165 (86.4%) paired assessments (Table 55).
| Expert nurse | Ward/community nurse, n (%) | ||||||
|---|---|---|---|---|---|---|---|
| Moves frequently and major position changes | Moves frequently and slight position changes | Moves occasionally and major position changes | Moves occasionally and slight position changes | Does not move | Not completed | Total | |
| Moves frequently and major position changes | 46 (24.1) | 3 (1.6) | 3 (1.6) | 2 (1.0) | 1 (0.5) | 1 (0.5) | 56 (29.3) |
| Moves frequently and slight position changes | 2 (1.0) | 5 (2.6) | 7 (3.7) | 9 (4.7) | 1 (0.5) | 0 (0.0) | 24 (12.6) |
| Moves occasionally and major position changes | 10 (5.2) | 4 (2.1) | 20 (10.5) | 10 (5.2) | 0 (0.0) | 1 (0.5) | 45 (23.6) |
| Moves occasionally and slight position changes | 1 (0.5) | 6 (3.1) | 7 (3.7) | 37 (19.4) | 2 (1.0) | 0 (0.0) | 53 (27.7) |
| Does not move | 0 (0.0) | 1 (0.5) | 1 (0.5) | 4 (2.1) | 5 (2.6) | 0 (0.0) | 11 (5.8) |
| Not completed | 2 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.0) |
| Total | 61 (31.9) | 19 (9.9) | 38 (19.9) | 62 (32.5) | 9 (4.7) | 2 (1.0) | 191 (100.0) |
Skin status
For the three possible ‘worst recorded’ skin categories, there was absolute agreement for 189 (82.2%) paired assessments, with agreement that there was ‘no problem’ (i.e. normal skin) or that there was a ‘problem’ for 204 (88.7%) paired assessments (see Table 52).
At step 2 there was agreement between raters for no known pressure ulcer history and pressure ulcer history for 160 (83.8%) paired assessments (Table 56).
| Expert nurse baseline | Ward/community nurse, n (%) | |||
|---|---|---|---|---|
| No known PU history | PU history | Not completed | Total | |
| PU history | ||||
| No known PU history | 116 (60.7) | 9 (4.7) | 4 (2.1) | 129 (67.5) |
| PU history | 15 (7.9) | 44 (23.0) | 1 (0.5) | 60 (31.4) |
| Not completed | 1 (0.5) | 0 (0.0) | 1 (0.5) | 2 (1.0) |
| Total | 132 (69.1) | 53 (27.7) | 6 (3.1) | 191 (100.0) |
| No problem | Patient unable to feel and/or respond appropriately to discomfort from pressure | Not completed | Total | |
| Sensory perception | ||||
| No problem | 123 (64.4) | 7 (3.7) | 8 (4.2) | 138 (72.3) |
| Patient unable to feel and/or respond appropriately to discomfort from pressure | 17 (8.9) | 28 (14.7) | 2 (1.0) | 47 (24.6) |
| Not completed | 6 (3.1) | 0 (0.0) | 0 (0.0) | 6 (3.1) |
| Total | 146 (76.4) | 35 (18.3) | 10 (5.2) | 191 (100.0) |
| No problem | Problem | Not completed | Total | |
| Nutrition | ||||
| No problem | 82 (42.9) | 9 (4.7) | 0 (0.0) | 91 (47.6) |
| Problem | 25 (13.1) | 74 (38.7) | 0 (0.0) | 99 (51.8) |
| Not completed | 0 (0.0) | 1 (0.5) | 0 (0.0) | 1 (0.5) |
| Total | 107 (56.0) | 84 (44.0) | 0 (0.0) | 191 (100.0) |
| No | Yes | Not completed | Total | |
| Unplanned weight loss | ||||
| No | 136 (71.2) | 8 (4.2) | 0 (0.0) | 144 (75.4) |
| Yes | 23 (12.0) | 23 (12.0) | 0 (0.0) | 46 (24.1) |
| Not completed | 1 (0.5) | 0 (0.0) | 0 (0.0) | 1 (0.5) |
| Total | 160 (83.8) | 31 (16.2) | 0 (0.0) | 191 (100.0) |
| No | Yes | Not completed | Total | |
| Poor nutritional intake | ||||
| No | 118 (61.8) | 10 (5.2) | 0 (0.0) | 128 (67.0) |
| Yes | 17 (8.9) | 45 (23.6) | 0 (0.0) | 62 (32.5) |
| Not completed | 1 (0.5) | 0 (0.0) | 0 (0.0) | 1 (0.5) |
| Total | 136 (71.2) | 55 (28.8) | 0 (0.0) | 191 (100.%) |
| No | Yes | Not completed | Total | |
| Low BMI | ||||
| No | 163 (85.3) | 6 (3.1) | 0 (0.0) | 169 (88.5) |
| Yes | 8 (4.2) | 13 (6.8) | 0 (0.0) | 21 (11.0) |
| Not completed | 0 (0.0) | 1 (0.5) | 0 (0.0) | 1 (0.5) |
| Total | 171 (89.5) | 20 (10.5) | 0 (0.0) | 191 (100.0) |
| No | Yes | Not completed | Total | |
| High BMI | ||||
| No | 160 (83.8) | 4 (2.1) | 0 (0.0) | 164 (85.9) |
| Yes | 16 (8.4) | 10 (5.2) | 0 (0.0) | 26 (13.6) |
| Not completed | 1 (0.5) | 0 (0.0) | 0 (0.0) | 1 (0.5) |
| Total | 177 (92.7) | 14 (7.3) | 0 (0.0) | 191 (100.0) |
| Not diabetic | Diabetic | Not completed | Total | |
| Diabetic status | ||||
| Not diabetic | 135 (70.7) | 2 (1.0) | 5 (2.6) | 142 (74.3) |
| Diabetic | 1 (0.5) | 45 (23.6) | 1 (0.5) | 47 (24.6) |
| Not completed | 2 (1.0) | 0 (0.0) | 0 (0.0) | 2 (1.0) |
| Total | 138 (72.3) | 47 (24.6) | 6 (3.1) | 191 (100.0) |
Step 2 other risk factors
There was agreement between raters that there was ‘no problem’ or that there was a ‘problem’ for 151 (79.1%) paired assessments, with disagreement for 24 (12.6%) paired assessments (see Table 56).
There was agreement between raters for ‘no problem’ and ‘problem’ for 156 (81.7%) paired assessments. In terms of the individual nutritional assessments, there was agreement between raters for 159 (83.2%) paired assessments for unplanned weight loss, 163 (85.3%) paired assessments for poor nutritional intake, 176 (92.1%) paired assessments for low BMI and 170 (89.0%) paired assessments for high BMI (see Table 56).
There was agreement between raters for diabetic status for 180 (94.2%) paired assessments, with disagreement for 3 (1.6%) paired assessments (see Table 56).
There was absolute agreement across the four possible categories for 125 (65.4%) paired assessments, with agreement that there was ‘no problem’ or a ‘problem’ for 139 (72.8%) paired assessments (Table 57).
| Expert nurse | Ward/community nurse, n (%) | |||||
|---|---|---|---|---|---|---|
| No problem | Conditions affecting central circulation | Conditions affecting peripheral circulation | Conditions affecting both central and peripheral circulation | Not completed | Total | |
| No problem | 88 (46.1) | 8 (4.2) | 12 (6.3) | 0 (0.0) | 1 (0.5) | 109 (57.1) |
| Conditions affecting central circulation | 12 (6.3) | 15 (7.9) | 2 (1.0) | 2 (1.0) | 1 (0.5) | 32 (16.8) |
| Conditions affecting peripheral circulation | 12 (6.3) | 2 (1.0) | 21 (11.0) | 1 (0.5) | 1 (0.5) | 37 (19.4) |
| Conditions affecting both central and peripheral circulation | 0 (0.0) | 3 (1.6) | 4 (2.1) | 1 (0.5) | 1 (0.5) | 9 (4.7) |
| Not completed | 4 (2.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (2.1) |
| Total | 116 (60.7) | 28 (14.7) | 39 (20.4) | 4 (2.1) | 4 (2.1) | 191 (100.0) |
There was absolute agreement across the three possible categories for 145 (75.9%) paired assessments, with agreement that there was ‘no problem’ or a ‘problem’ for 155 (81.2%) paired assessments (Table 58).
| Expert nurse | Ward/community nurse, n (%) | ||||
|---|---|---|---|---|---|
| No problem/occasional | Frequent (two to four times a day) | Constant | Not completed | Total | |
| No problem/occasional | 133 (69.6) | 14 (7.3) | 2 (1.0) | 3 (1.6) | 152 (79.6) |
| Frequent (two to four times a day) | 15 (7.9) | 11 (5.8) | 3 (1.6) | 1 (0.5) | 30 (15.7) |
| Constant | 0 (0.0) | 7 (3.7) | 1 (0.5) | 0 (0.0) | 8 (4.2) |
| Not completed | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 1 (0.5) |
| Total | 148 (77.5) | 32 (16.8) | 6 (3.1) | 5 (2.6) | 191 (100.0) |
Test–retest reliability
To assess the test–retest reliability between the baseline and the retest expert nurse assessments, the maximum number of paired assessments available was used. From a possible 217 paired assessments, four were excluded as a ‘change in condition’ form was received, providing an analysis population of 213 paired assessments. The median number of days between the baseline and the retest expert nurse assessment was three (range 1–7). There were 213 paired assessments available for comparison of the decision pathways (Table 59), 212 paired assessments for comparison of step 1 mobility (Table 60) and 177 paired assessments for comparison of the step 2 assessments (Table 61). To compare skin assessments the ‘worst’ skin status recorded on the PURPOSE-T (i.e. from step 1 and step 2) was used and there was a total of 213 paired assessments (Table 62).
| Expert nurse baseline | Expert nurse follow-up, n (%) | |||
|---|---|---|---|---|
| PU category 1 or above or scarring | No PU but at risk | No PU not currently at risk | Total | |
| PU category 1 or above or scarring | 64 (30.0) | 5 (2.3) | 0 (0.0) | 69 (32.4) |
| No PU but at risk | 3 (1.4) | 95 (44.6) | 5 (2.3) | 103 (48.4) |
| No PU not currently at risk | 0 (0.0) | 4 (1.9) | 37 (17.4) | 41 (19.2) |
| Total | 67 (31.5) | 104 (48.8) | 42 (19.7) | 213 (100.0) |
| Expert nurse baseline | Expert nurse follow-up, n (%) | ||||
|---|---|---|---|---|---|
| Walks independently with/without walking aids | Needs the help of another person to walk | Spends all or the majority of time in bed or chair | Remains in the same position for long periods | Total | |
| Walks independently with/without walking aids | 58 (27.4) | 2 (0.9) | 7 (3.3) | 2 (0.9) | 69 (32.5) |
| Needs help of another person to walk | 2 (0.9) | 15 (7.1) | 7 (3.3) | 1 (0.5) | 25 (11.8) |
| Spends all or the majority of time in bed or chair | 2 (0.9) | 3 (1.4) | 41 (19.3) | 9 (4.2) | 55 (25.9) |
| Remains in same position for long periods | 0 (0.0) | 1 (0.5) | 11 (5.2) | 51 (24.1) | 63 (29.7) |
| Total | 62 (29.2) | 21 (9.9) | 66 (31.1) | 63 (29.7) | 212a (100.0) |
| Expert nurse baseline | Expert nurse follow-up, n (%) | |||
|---|---|---|---|---|
| Completed: appropriate (at risk in step 1) | Completed: inappropriate (not at risk in step 1) | Not completed: appropriate (not at risk in step 1) | Total | |
| Completed: appropriate (at risk in step 1) | 171 (80.3) | 1 (0.5) | 3 (1.4) | 175 (82.2) |
| Completed: inappropriate (missing/not at risk in step 1) | 3 (1.4) | 2 (0.9) | 0 (0.0) | 5 (2.3) |
| Not completed: appropriate (not at risk in step 1) | 1 (0.5) | 0 (0.0) | 32 (15.0) | 33 (15.5) |
| Total | 175 (82.2) | 3 (1.4) | 35 (16.4) | 213a (100.0) |
| Expert nurse baseline | Expert nurse follow-up, n (%) | |||
|---|---|---|---|---|
| Normal skin | Vulnerable skin | PU category | Total | |
| Normal skin | 56 (26.3) | 3 (1.4) | 1 (0.5) | 60 (28.2) |
| Vulnerable skin | 8 (3.8) | 84 (39.4) | 3 (1.4) | 95 (44.6) |
| PU category | 0 (0.0) | 6 (2.8) | 51 (23.9) | 57 (26.8) |
| Missing | 0 (0.0) | 1 (0.5) | 0 (0.0) | 1 (0.5) |
| Total | 64 (30.0) | 94 (44.1) | 55 (25.8) | 213 (100.0) |
Decision pathway
There was agreement over the decision pathway between the expert nurse at baseline and the expert nurse at retest for 196 (92.0%) paired assessments (see Table 59). The corresponding simple kappa statistic of 0.87 (95% CI 0.81 to 0.93) and weighted kappa of 0.89 (95% CI 0.84 to 0.94) indicate very good agreement between the two assessments. When classified dichotomously as ‘at risk’/’not at risk’ there was agreement between the expert nurse at baseline and the expert nurse at retest for 204 (95.8%) paired assessments (Table 63). The corresponding simple kappa statistic of 0.87 (CI 0.78 to 0.95), PABAK of 0.92 and κmax. of 0.99 indicate very good agreement between raters (although the 95% CI for the simple kappa statistic straddles the ‘good’ and ‘very good’ cut-off values).
| Expert nurse baseline | Expert nurse follow-up, n (%) | ||
|---|---|---|---|
| At risk | Not at risk | Total | |
| At risk | 167 (78.4) | 5 (2.3) | 172 (80.8) |
| Not at risk | 4 (1.9) | 37 (17.4) | 41 (19.2) |
| Total | 171 (80.3) | 42 (19.7) | 213 (100.0) |
Mobility
There was overall agreement across the four ‘worst recorded’ categories for step 1 mobility for 165 (77.8%) paired assessments made by the expert nurse at baseline and at retest, with agreement that there was ‘no problem’ (i.e. walks independently with or without walking aids) or a ‘problem’ for 197 (92.9%) paired assessments (see Table 60). At step 2 there was absolute agreement across the five possible categories for the analysis of independent movement for 114 (64.4%) paired assessments, with agreement that there was ‘no problem’ (i.e. moves frequently and major position changes) or a ‘problem’ for 147 (83.1%) paired assessments (Table 64).
| Expert nurse baseline | Expert nurse follow-up, n (%) | ||||||
|---|---|---|---|---|---|---|---|
| Moves frequently and major position changes | Moves frequently and slight position changes | Moves occasionally and major position changes | Moves occasionally and slight position changes | Does not move | Not completed | Total | |
| Moves frequently and major position changes | 35 (19.8) | 5 (2.8) | 8 (4.5) | 0 (0.0) | 0 (0.0) | 1 (0.6) | 49 (27.7) |
| Moves frequently and slight position changes | 7 (4.0) | 6 (3.4) | 2 (1.1) | 7 (4.0) | 0 (0.0) | 0 (0.0) | 22 (12.4) |
| Moves occasionally and major position changes | 6 (3.4) | 6 (3.4) | 31 (17.5) | 2 (1.1) | 0 (0.0) | 0 (0.0) | 45 (25.4) |
| Moves occasionally and slight position changes | 2 (1.1) | 8 (4.5) | 4 (2.3) | 33 (18.6) | 2 (1.1) | 0 (0.0) | 49 (27.7) |
| Does not move | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.1) | 9 (5.1) | 0 (0.0) | 11 (6.2) |
| Not completed | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.6) | 1 (0.6) |
| Total | 50 (28.2) | 25 (14.1) | 45 (25.4) | 44 (24.9) | 11 (6.2) | 2 (1.1) | 177 (100.0) |
Skin status
For the three possible ‘worst recorded’ skin categories, there was absolute agreement for 191 (89.7%) paired assessments, with agreement that there was ‘no problem’ (i.e. normal skin) or a ‘problem’ for 200 (93.9%) paired assessments (see Table 62). At step 2 there was agreement between ratings of ‘no known pressure ulcer history’ and ‘pressure ulcer history’ for 165 (93.2%) paired assessments (Table 65).
| Expert nurse baseline | Expert nurse follow-up, n (%) | |||
|---|---|---|---|---|
| No known PU history | PU history | Not completed | Total | |
| PU history | ||||
| No known PU history | 110 (62.1) | 4 (2.3) | 3 (1.7) | 117 (66.1) |
| PU history | 3 (1.7) | 55 (31.1) | 0 (0.0) | 58 (32.8) |
| Not completed | 2 (1.1) | 0 (0.0) | 0 (0.0) | 2 (1.1) |
| Total | 115 (65.0) | 59 (33.3) | 3 (1.7) | 177 (100.0) |
| No problem | Patient unable to feel and/or respond appropriately to discomfort from pressure | Not completed | Total | |
| Sensory perception | ||||
| No problem | 118 (66.7) | 6 (3.4) | 2 (1.1) | 126 (71.2) |
| Patient unable to feel and/or respond appropriately to discomfort from pressure | 10 (5.6) | 36 (20.3) | 0 (0.0) | 46 (26.0) |
| Not completed | 3 (1.7) | 1 (0.6) | 1 (0.6) | 5 (2.8) |
| Total | 131 (74.0) | 43 (24.3) | 3 (1.7) | 177 (100.0) |
| No problem | Problem | Not completed | Total | |
| Nutrition | ||||
| No problem | 77 (43.5) | 7 (4.0) | 3 (1.7) | 87 (49.2) |
| Problem | 12 (6.8) | 77 (43.5) | 0 (0.0) | 89 (50.3) |
| Not completed | 0 (0.0) | 0 (0.0) | 1 (0.6) | 1 (0.6) |
| Total | 89 (50.3) | 84 (47.5) | 4 (2.3) | 177 (100.0) |
| No | Yes | Not completed | Total | |
| Unplanned weight loss | ||||
| No | 127 (71.8) | 4 (2.3) | 3 (1.7) | 134 (75.7) |
| Yes | 10 (5.6) | 32 (18.1) | 0 (0.0) | 42 (23.7) |
| Not completed | 0 (0.0) | 0 (0.0) | 1 (0.6) | 1 (0.6) |
| Total | 137 (77.4) | 36 (20.3) | 4 (2.3) | 177 (100.0) |
| No | Yes | Not completed | Total | |
| Poor nutritional intake | ||||
| No | 114 (64.4) | 6 (3.4) | 3 (1.7) | 123 (69.5) |
| Yes | 8 (4.5) | 45 (25.4) | 0 (0.0) | 53 (29.9) |
| Not completed | 0 (0.0) | 0 (0.0) | 1 (0.6) | 1 (0.6) |
| Total | 122 (68.9) | 51 (28.8) | 4 (2.3) | 177 (100.0) |
| No | Yes | Not completed | Total | |
| Low BMI | ||||
| No | 152 (85.9) | 0 (0.0) | 3 (1.7) | 155 (87.6) |
| Yes | 3 (1.7) | 18 (10.2) | 0 (0.0) | 21 (11.9) |
| Not completed | 0 (0.0) | 0 (0.0) | 1 (0.6) | 1 (0.6) |
| Total | 155 (87.6) | 18 (10.2) | 4 (2.3) | 177 (100.0) |
| No | Yes | Not completed | Total | |
| High BMI | ||||
| No | 143 (80.8) | 4 (2.3) | 3 (1.7) | 150 (84.7) |
| Yes | 4 (2.3) | 22 (12.4) | 0 (0.0) | 26 (14.7) |
| Not completed | 0 (0.0) | 0 (0.0) | 1 (0.6) | 1 (0.6) |
| Total | 147 (83.1) | 26 (14.7) | 4 (2.3) | 177 (100.0) |
| Not diabetic | Diabetic | Missing | Total | |
| Diabetic status | ||||
| Not diabetic | 123 (69.5) | 0 (0.0) | 6 (3.4) | 129 (72.9) |
| Diabetic | 3 (1.7) | 43 (24.3) | 0 (0.0) | 46 (26.0) |
| Not completed | 2 (1.1) | 0 (0.0) | 0 (0.0) | 2 (1.1) |
| Total | 128 (72.3) | 43 (24.3) | 6 (3.4) | 177 (100.0) |
Other step 2 risk factors
There was agreement between ratings that there was ‘no problem’ or a ‘problem’ for 154 (87.0%) paired assessments (see Table 65).
There was agreement between ratings that there was ‘no problem’ or a ‘problem’ for 154 (87.0%) paired assessments. In terms of the individual nutritional assessments, there was agreement between ratings for 159 (89.9%) paired assessments for unplanned weight loss, 159 (89.8%) paired assessments for poor nutritional intake, 170 (96.0%) paired assessments for low BMI and 165 (93.2%) paired assessments for high BMI (see Table 65).
There was agreement over diabetic status for 166 (93.8%) paired assessments (see Table 65).
There was absolute agreement across the four possible categories of perfusion for 138 (78.0%) paired assessments, with agreement that there was ‘no problem’ or ‘problem’ for 154 (87.0%) paired assessments (Table 66).
| Expert nurse baseline | Expert nurse follow-up, n (%) | |||||
|---|---|---|---|---|---|---|
| No problem | Conditions affecting central circulation | Conditions affecting peripheral circulation | Conditions affecting both central and peripheral circulation | Not completed | Total | |
| No problem | 87 (49.2) | 0 (0.0) | 8 (4.5) | 0 (0.0) | 4 (2.3) | 99 (55.9) |
| Conditions affecting central circulation | 6 (3.4) | 19 (10.7) | 3 (1.7) | 4 (2.3) | 0 (0.0) | 32 (18.1) |
| Conditions affecting peripheral circulation | 1 (0.6) | 3 (1.7) | 28 (15.8) | 2 (1.1) | 0 (0.0) | 34 (19.2) |
| Conditions affecting both central and peripheral circulation | 0 (0.0) | 1 (0.6) | 3 (1.7) | 4 (2.3) | 0 (0.0) | 8 (4.5) |
| Not completed | 3 (1.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.6) | 4 (2.3) |
| Total | 97 (54.8) | 23 (13.0) | 42 (23.7) | 10 (5.6) | 5 (2.8) | 177 (100.0) |
There was absolute agreement across the three possible categories of moisture for 155 (87.6%) paired assessments, with agreement that there was ‘no problem’ or a ‘problem’ for 159 (89.8%) paired assessments (Table 67).
| Expert nurse baseline | Expert nurse follow-up, n (%) | ||||
|---|---|---|---|---|---|
| No problem/occasional | Frequent (two to four times a day) | Constant | Not completed | Total | |
| No problem/occasional | 126 (71.2) | 7 (4.0) | 0 (0.0) | 5 (2.8) | 138 (78.0) |
| Frequent (two to four times a day) | 5 (2.8) | 24 (13.6) | 1 (0.6) | 0 (0.0) | 30 (16.9) |
| Constant | 0 (0.0) | 3 (1.7) | 5 (2.8) | 0 (0.0) | 8 (4.5) |
| Not completed | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.6) | 1 (0.6) |
| Total | 131 (74.0) | 34 (19.2) | 6 (3.4) | 6 (3.4) | 177 (100.0) |
Convergent validity
PURPOSE-T step 1
The mobility assessment at step 1 of the PURPOSE-T was compared with the Braden mobility and activity subscales using dichotomous scales (i.e. ‘problem’ or ‘no problem’). The step 1 mobility assessment on the PURPOSE-T was found to have a moderate association with both the Braden mobility subscale and the Braden activity subscale, with phi correlation coefficients of 0.60 and 0.66 respectively (Table 68).
| PURPOSE-T step 1 mobility | Braden mobility, n (%) | Correlation coefficient | ||
|---|---|---|---|---|
| No limitation | Slightly/very limited/completely immobile | Total | ||
| No problem | 69 (30.1) | 10 (4.4) | 79 (34.5) | Phi 0.60 – moderate |
| Problem | 37 (16.2) | 113 (49.3) | 150 (65.5) | |
| Total | 106 (46.3) | 123 (53.7) | 229 (100.0) | |
| PURPOSE-T step 1 mobility | Braden activity, n (%) | Correlation coefficient | ||
| Walks frequently | Walks occasionally, chairfast or bedfast | Total | ||
| No problem | 56 (24.5) | 23 (10.0) | 79 (34.5) | Phi 0.66 – moderate |
| Problem | 11 (4.8) | 139 (60.7) | 150 (65.5) | |
| Total | 67 (29.3) | 162 (70.7) | 229 (100.0) | |
| PURPOSE-T sensory response and perception | Braden sensory perception, n (%) | Correlation coefficient | ||
| No impairment | Slightly, very or completely limited | Total | ||
| No problem | 134 (70.9) | 7 (3.7) | 141 (74.6) | Phi 0.74 – high |
| Patient unable to feel and/or respond appropriately to discomfort from pressure | 11 (5.8) | 37 (19.6) | 48 (25.4) | |
| Total | 145 (76.7) | 44 (23.3) | 189 (100.0) | |
| PURPOSE-T nutrition | Braden nutrition, n (%) | Correlation coefficient | ||
| Excellent or adequate | Probably inadequate or very poor | Total | ||
| No problem | 93 (48.2) | 1 (0.5) | 94 (48.7) | Phi 0.58 – moderate |
| Problem | 47 (24.4) | 52 (26.9) | 99 (51.3) | |
| Total | 140 (72.5) | 53 (27.5) | 193 (100.0) | |
| PURPOSE-T nutrition | Waterlow malnutrition screening tool: patient eating poorly or lack of appetite, n (%) | Correlation coefficient | ||
| Yes | No | Total | ||
| Problem | 64 (33.7) | 35 (18.4) | 99 (52.1) | Phi 0.60 – moderate |
| No problem | 6 (3.2) | 85 (44.7) | 91 (47.9) | |
| Total | 70 (36.8) | 120 (63.2) | 190 (100.0) | |
| PURPOSE-T nutrition: poor nutritional intake | Braden nutrition, n (%) | Correlation coefficient | ||
| Probably inadequate or very poor | Excellent or adequate | Total | ||
| Yes | 50 (25.9) | 12 (6.2) | 62 (32.1) | Phi 0.82 – high |
| No | 3 (1.6) | 128 (66.3) | 131 (67.9) | |
| Total | 53 (27.5) | 140 (72.5) | 193 (100.0) | |
| PURPOSE-T nutrition: poor nutritional intake | Waterlow malnutrition screening tool: patient eating poorly or lack of appetite, n (%) | Correlation coefficient | ||
| Yes | No | Total | ||
| Yes | 57 (30.0) | 5 (2.6) | 62 (32.6) | Phi 0.79 – high |
| No | 13 (6.8) | 115 (60.5) | 128 (67.4) | |
| Total | 70 (36.8) | 120 (63.2) | 190 (100.0) | |
| PURPOSE-T nutrition: low BMI | Waterlow build or weight for height, n (%) | Correlation coefficient | ||
| BMI < 20 kg/m2 | BMI ≥ 20 kg/m2 | Total | ||
| Yes | 21 (11.0) | 0 (0.0) | 21 (11.0%) | Phi 0.72 – high |
| No | 16 (8.4) | 154 (80.6) | 170 (89.0%) | |
| Total | 37 (19.4) | 154 (80.6) | 191 (100.0%) | |
| PURPOSE-T nutrition: high BMI | Waterlow build or weight for height, n (%) | Correlation coefficient | ||
| BMI ≥ 30 kg/m2 | BMI < 30 kg/m2 | Total | ||
| Yes | 22 (11.5) | 4 (2.1) | 26 (13.6) | Phi 0.74 – high |
| No | 9 (4.7) | 156 (81.7) | 165 (86.4) | |
| Total | 31 (16.2) | 160 (83.8) | 191 (100.0) | |
The ‘worst’ overall PURPOSE-T skin status was compared with the ‘worst’ Waterlow skin status. There was a high association observed between the PURPOSE-T skin status and the Waterlow skin status, with a Spearman rank correlation coefficient of 0.83 (Table 69).
| PURPOSE-T step 2 analysis of independent movement | Braden mobility, n (%) | Correlation coefficient | |||
|---|---|---|---|---|---|
| Completely immobile | Very or slightly limited | No limitation | Total | ||
| Does not move | 7 (3.6) | 4 (2.1) | 0 (0.0) | 11 (5.7) | Spearman rank 0.62 – moderate |
| Moves occasionally and slight or major position changes or moves frequently with slight position changes | 1 (0.5) | 96 (49.7) | 26 (13.5) | 123 (63.7) | |
| Moves frequently and major position changes | 0 (0.0) | 12 (6.2) | 47 (24.4) | 59 (30.6) | |
| Total | 8 (4.1) | 112 (58.0) | 73 (37.8) | 193 (100.0) | |
| PURPOSE-T step 2 analysis of independent movement | Braden activity, n (%) | Correlation coefficient | |||
| Bedfast | Chairfast or walks occasionally | Walks frequently | Total | ||
| Does not move | 6 (3.1) | 5 (2.6) | 0 (0.0) | 11 (5.7) | Spearman rank 0.55 – moderate |
| Moves occasionally and slight or major position changes or moves frequently with slight position changes | 15 (7.8) | 102 (52.8) | 6 (3.1) | 123 (63.7) | |
| Moves frequently and major position changes | 0 (0.0) | 31 (16.1) | 28 (14.5) | 59 (30.6) | |
| Total | 21 (10.9) | 138 (71.5) | 34 (17.6) | 193 (100.0) | |
| PURPOSE-T skin status | Waterlow skin status, n (%) | Correlation coefficient | |||
| Healthy | Tissue paper, dry, oedematous, clammy | Discoloured grade 1 or broken spots grades 2–4 | Total | ||
| Normal skin | 47 (20.6) | 18 (7.9) | 0 (0.0) | 65 (28.5) | Spearman rank 0.83 – high |
| Vulnerable skin | 11 (4.8) | 79 (34.6) | 13 (5.7) | 103 (45.2) | |
| PU category | 1 (0.4) | 0 (0.0) | 59 (25.9) | 60 (26.3) | |
| Total | 59 (25.9) | 97 (42.5) | 72 (31.6) | 228 (100.0) | |
| PURPOSE-T moisture | Braden moisture, n (%) | Correlation coefficient | |||
| Rarely or occasionally moist | Very moist | Constantly moist | Total | ||
| No problem/occasional | 154 (79.4) | 2 (1.0) | 0 (0.0) | 156 (80.4) | Spearman rank 0.67 – moderate |
| Frequent (two to four times a day) | 18 (9.3) | 12 (6.2) | 0 (0.0) | 30 (15.5) | |
| Constant | 0 (0.0) | 4 (2.1) | 4 (2.1) | 8 (4.1) | |
| Total | 172 (88.7) | 18 (9.3) | 4 (2.1) | 194 (100.0) | |
PURPOSE-T step 2
The PURPOSE-T analysis of independent movement was compared with the Braden mobility and activity subscales. The analysis of independent movement was observed to be moderately associated with both the Braden mobility subscale and the Braden activity subscale, with corresponding Spearman rank correlation coefficients of 0.62 and 0.55 respectively (see Table 69).
The sensory perception and response assessment on the PURPOSE-T was compared with the Braden sensory perception score. A high association between the two sensory perception assessments was observed, with a corresponding phi correlation coefficient of 0.74 (see Table 68).
A moderate association was observed between ‘problem’/‘no problem’ on the PURPOSE-T nutrition construct and the Braden nutrition subscale (dichotomised to ‘excellent or adequate’/‘probably inadequate or very poor’), with a phi correlation coefficient of 0.58, and between ‘problem’/‘no problem’ on the PURPOSE-T nutrition construct and the Waterlow malnutrition screening tool (part c: eating poorly or has a lack of appetite), with a phi correlation coefficient of 0.60 (see Table 68).
A high association was observed between ‘poor nutritional intake’ (‘yes’/‘no’) on the PURPOSE-T and the Braden nutrition subscale (dichotomised to ‘excellent or adequate’/‘probably inadequate or very poor’), with a phi correlation coefficient of 0.82. A high association was also observed between ‘poor nutritional intake’ (‘yes’/‘no’) on the PURPOSE-T and the Waterlow malnutrition screening tool (part c: eating poorly or has a lack of appetite), with a phi correlation coefficient of 0.79; between ‘low BMI’ (‘yes’/‘no’) on the PURPOSE-T and the Waterlow build/weight for height construct, with a phi correlation coefficient of 0.72; and between ‘high BMI’ (‘yes’/‘no’) on the PURPOSE-T and the Waterlow build/weight for height construct, with a phi correlation coefficient of 0.74.
The PURPOSE-T moisture assessment was compared with the Braden moisture assessment. A moderate association was observed between the moisture assessment on the PURPOSE-T and the Braden moisture assessment, with a Spearman rank correlation coefficient of 0.67 (see Table 69).
Assessment decision
A moderate association was observed between the overall risk status on the PURPOSE-T and the Waterlow scale score for all patients, as assessed by the expert nurse at baseline, with a phi correlation coefficient of 0.63 (Table 70).
| PURPOSE-T risk status | Waterlow risk status, n (%) | Correlation coefficient | ||
|---|---|---|---|---|
| At risk (≥ 10) | Not at risk (< 10) | Total | ||
| At risk | 175 (76.1) | 8 (3.5) | 183 (79.6) | Phi 0.63 – moderate |
| Not at risk | 18 (7.8) | 29 (12.6) | 47 (20.4) | |
| Total | 193 (83.9) | 37 (16.1) | 230 (100.0) | |
| PURPOSE-T risk status | Braden risk status, n (%)a | Correlation coefficient | ||
| At risk (≥ 10) | Not at risk (< 10) | Total | ||
| At risk | 50 (29.6) | 73 (43.2) | 123 (72.8) | Phi 0.40 – moderate |
| Not at risk | 0 (0.0) | 46 (27.2) | 46 (27.2) | |
| Total | 50 (29.6) | 119 (70.4) | 169 (100.0) | |
There was a moderate association observed between the overall risk status on the PURPOSE-T and the Braden scale score for pressure ulcer-free patients, as assessed by the expert nurse at baseline, with a phi correlation coefficient of 0.40.
Summary of expert nurse field notes
The field notes (incorporating the views of expert nurses using the tool as well as the views of some ward/community nurses who provided feedback to the expert nurses, although exact numbers are unknown) described positive and problem aspects of using the PURPOSE-T in practice, as detailed in Table 71.
| Characteristic | Positive aspects of using the PURPOSE-T | Problem aspects of using the PURPOSE-T |
|---|---|---|
| Layout |
|
|
| Format |
|
|
| Content |
|
|
| Usability |
|
|
In addition, other problematic aspects of assessment that are common to all risk assessment instruments were also reported including:
-
lack of knowledge of pressure ulcer classification
-
difficulty assessing mobility when the patient is unable to communicate and when the patient has been seen for only a short period before assessment
-
difficulty assessing sensory perception
-
difficulty assessing medical history in the community setting
-
difficulty assessing poor nutritional intake
-
difficulty assessing BMI in the community setting.
Final amendments to the PURPOSE-T
Field test amendments
The field test results informed revisions and the production of the final PURPOSE-T and associated user manual. Revisions included:
-
increasing the font size and spacing of the skin assessment section by moving the ‘vulnerable skin’ descriptors to the classification box
-
further clarification of examples of skin vulnerability relating to skin redness
-
amendment of the flow of step 2 by moving the skin assessment section
-
simplification of the ‘previous pressure ulcer history’ item, encouraging nurses to record the number of previous pressure ulcers and to give a detailed account of the pressure ulcer that left a scar or the worst category pressure ulcer rather than all previous pressure ulcers.
Changes to the user manual were undertaken to reflect the changes made to the PURPOSE-T, detailed above. In addition, further guidance was included in the manual relating to:
-
parameters of weight loss and time periods
-
nutritional intake and support
-
assessment of BMI.
Consideration of new evidence from the pain cohort study
In the original programme grant timelines the pain cohort study should have concluded before the start of the risk assessment work package. In practice this did not happen because of the late start of the pain cohort study and the extended recruitment period required to deliver the study. Instead, there were some preliminary expert group discussions about pain in the expert group meetings and we adjusted the original consensus study to enable later consideration of the pain cohort study results.
As before, the consensus process involved a face-to-face meeting with PURSUN UK members. PURSUN UK members recognised pain as an important sign, noting that the results of the pain study reflected people’s personal experiences of pain (e.g. feeling discomfort before redness appears on the skin). They supported the inclusion of pain in the PURPOSE-T.
The expert group element was conducted by questionnaire alone. Expert group members privately completed an initial questionnaire that incorporated the results of the pain cohort study, the views of PURSUN UK and follow-up notes. They were asked to consider this evidence and rate their level of support for the inclusion of pain at the screening and full assessment stages of the PURPOSE-T (on a 9-point Likert scale) and to make comments regarding this. The results of the initial questionnaire, including the median group response, disagreement index and anonymised expert group comments (as well as the evidence included in the initial pain questionnaire), were incorporated into a follow-up questionnaire, allowing expert group members to consider the views of others before privately re-rating their level of support for the inclusion of pain in the PURPOSE-T. The results of this follow-up pain questionnaire determined whether or not pain was included in the PURPOSE-T, following the same criteria used in the original consensus study (see Phase 2: consensus study, Data analysis).
The results indicated that, although there was uncertainty regarding the inclusion of pain at the screening stage of the assessment, there was support for its inclusion at the full assessment stage and it was subsequently incorporated as an extension to the pressure ulcer and skin assessment section of the PURPOSE-T.
Discussion
A new Risk Assessment Framework – PURPOSE-T [incorporating a risk factor Minimum Data Set; see http://medhealth.leeds.ac.uk/accesspurposet (accessed July 2015)] – was developed to enhance pressure ulcer risk assessment practice. We were unable to use gold standard methods for the development of a risk stratification tool using multivariable modelling because of the lack of standard recording of key risk factors and appropriate data sets to identify items for a risk tool, with subsequent model testing on a ‘new’ prospective target population. 122 Rather, we undertook a systematic review incorporating a narrative synthesis of pressure ulcer risk factors to provide the foundation for a consensus study to agree a risk factor Minimum Data Set for inclusion in a new Risk Assessment Framework. This will facilitate the routine and standardised recording of risk factors in clinical practice and can be used for modelling and ongoing development. The PURPOSE-T underwent rigorous pre-testing and field testing with expert and ward/community nurses and has good face, content and construct validity and good and very good inter-rater and test–retest reliability respectively.
The systematic review allowed the risk factors that are independently associated with pressure ulcer development to be identified,46 providing a clearer notion of the critical pressure ulcer risk factors. However, there are remaining gaps in the literature for some potentially important risk factors, which require further research. In addition, pressure ulcer risk factors were inconsistently represented in the modelling of the primary studies of the systematic review and this limits both the interpretation and the overall conclusions. Other limitations of the literature include poor reporting, heterogeneity of patient populations, use of different outcomes, lack of differentiation between ulcer sites and the observation of mainly superficial pressure ulcers. Although the evidence of the systematic review provides a good insight into the risk factors associated with pressure ulcer development at a population level, it does not fully explain the underlying pathology of pressure ulcer development, and wider scientific evidence, and its relevance to clinical practice, must also be considered. Finally, it is acknowledged that, in the absence of a standard method for appraising the quality of risk factor research, we developed study-specific criteria and categorised studies as high, medium, low and very low quality, which has a number of inherent limitations.
The consensus study allowed the evidence of the systematic review to be carefully reviewed by an expert group, taking into account the wider scientific evidence, its relevance to clinical practice and the views of PURSUN UK. The consensus methods were particularly useful in allowing the expert group to agree the key risk factors to summarise patient risk (i.e. those that were considered to increase the probability of pressure ulcer development). Although the methods were also useful for identifying the key principles of the assessment items, they were inappropriate for considering the specific wording of items. Of note is the agreement that the risk factors and assessment items should be the same for the Minimum Data Set and the Risk Assessment Framework (i.e. no additional risk factor information was considered necessary for assessment in clinical practice). It was acknowledged that risk factors excluded from the Minimum Data Set and Risk Assessment Framework may still have a role in the pressure ulcer causal pathway through their relationship with the agreed risk factors and may be important at an individual patient level (e.g. the use of sedative medication may limit a patient’s mobility/activity and this would be addressed in the related items of the Risk Assessment Framework). Whereas some of the agreed risk factors emerged as primary risk factors in the systematic review [immobility, existing pressure ulcer, general skin status, perfusion (including diabetes)], others, although still important, emerged less consistently (moisture, nutrition, sensory perception) and two risk factors (previous pressure ulcer and pain) did not emerge in the systematic review. Previous pressure ulcer was included on the basis of service user opinion and theoretical bioengineering evidence (particularly relating to scarring) rather than on the basis of the epidemiological evidence. Pain was included following the availability of the results of the pain cohort study. Although all acknowledged that the epidemiological evidence was derived from a single study, its inclusion was influenced by the strength of the multivariable modelling, general pathophysiological principles and service user opinion. Conversely, albumin, which emerged more consistently in the systematic review and was initially agreed by the expert group for inclusion (in the full assessment stage of the Minimum Data Set and Risk Assessment Framework), was subsequently excluded because of concerns raised by PURSUN UK. In these examples, when the group diverged from the scientific evidence, the reasons were in keeping with some of those previously reported including clinical experience and patient preference. 201
The integration of the PURSUN UK perspective throughout the study proved invaluable and to our knowledge is the first study to use such an approach. Whereas others using consensus methods have incorporated patient/carer representation in their expert groups,202,203 we decided to use an alternative approach when developing the study methodology. This was because of a concern that the complexity of the epidemiological and wider scientific evidence, as well as the complex nature of facilitating a mixed group of patients and professionals, could have impeded the patients’ and carers’ input into the process. Difficulties in involving patients and carers in the development of technical and clinical guidelines have been raised previously204 and for this study there seemed to be more value in devoting whole meetings to patient/carer insights, with particular emphasis on the acceptability of elements of assessment. This allowed us to consider the views of a larger number of service users. We were conscious of the need to integrate PURSUN UK members’ perspectives into the consensus process and this was carried out by feedback at the expert group meetings or inclusion of their comments into questionnaires, so that the group could consider the patient/carer perspective alongside other evidence.
Although the consensus study involved an expert group with considerable experience, a limitation of consensus methodology relates to reliability and whether or not the results of this study are representative of the views of other experts in the field. Raine and colleagues182 proposed a new approach to developing clinical guidelines that includes checking the representativeness of the group’s ratings with a large similarly composed group. As it is our intention to continually update the Risk Assessment Framework, further work is currently being planned to validate the Minimum Data Set and Risk Assessment Framework through consultation with a larger group. This will also allow new evidence to be brought forward and integrated into the work. Another difficulty associated with consensus methods relates to validity and assessing whether or not the judgements made by the group are ‘good’. 181 Although we developed a consensus method (incorporating group expertise, relevant evidence and group facilitation) to facilitate ‘good judgements’ regarding the inclusion of risk factors and assessment items in the risk factor Minimum Data Set and Risk Assessment Framework, we were unable to assess this at the time of conducting the study. This should be assessed in future modelling work and the ongoing development of PURPOSE-T to establish whether the judgements of the consensus study are correct.
Building on the work of the consensus study we were able to develop a theoretical causal pathway for pressure ulcer development and a new conceptual framework, to bring together the epidemiological, physiological and biomechanical evidence, enhancing our understanding of the role of individual risk factors in pressure ulcer development. However, there is remaining uncertainty about how varying combinations of risk factors and their parameters (e.g. varying levels of mobility, nutrition, moisture) impact on pressure ulcer outcome as well as aetiological mechanisms of importance (e.g. uncertainty about the specific mechanisms of importance relating to perfusion). The importance of individual risk factors may also vary in relation to body site (e.g. a patient with peripheral vascular disease may have reduced tolerance to pressure to their heels but not to their trunk areas). The development of the conceptual framework through the combination of bioengineering and epidemiological expertise and evidence also highlights that currently the methods that we have to assess the direct and indirect causal factors involved in pressure ulcer development, including the mechanical boundary conditions and factors affecting tissue tolerance (geometry, mechanical properties of tissue, transport and thermal properties and physiology and repair), are very crude clinical assessments. The work provides a foundation for a programme of bioengineering and translational research to develop improved assessment techniques with greater precision for clinical use.
The pre-test allowed us to identify areas of confusion and improve the usability and acceptability of the Risk Assessment Framework for clinical nurses. It could be argued that undertaking a pre-test using vignette case studies is no substitute for assessing the Risk Assessment Framework in clinical practice. However, assessing and improving the acceptability of the Risk Assessment Framework with clinical nurses was considered a necessary and logical step to ensure face and content validity before evaluation in clinical practice with real patients. The vignettes were developed to be realistic, with input from the clinical members of the project team and members of PURSUN UK. The focus groups and think out loud interviews were held in a pleasant environment and were carefully planned to encourage disclosure among participants, which would not have been possible in a busy clinical area. In addition, topic guides were used by trained facilitators, group numbers were conducive to facilitation, nurses from different trusts were grouped according to job role and participants were fully briefed and had opportunities to ask questions before the actual interviews/focus groups.
The pre-test facilitated changes to the Risk Assessment Framework relating to three main areas, including the flow and format of the tool, decision support and the wording of specific items. This led to the development of a preliminary Risk Assessment Framework – PURPOSE-T – in readiness for clinical evaluation.
The field test of the PURPOSE-T involved 230 patients who were assessed by both expert and ward/community nurses. Apart from previous pressure ulcer history, the level of data completion for expert and ward/community nurse assessments for each construct on the PURPOSE-T was high at > 90%. The inter-rater and test–retest agreement was ‘very good’ for the assessment decision overall as determined by kappa. The percentage agreement for the assessment of ‘problem/no problem’ for the eight risk factors (mobility, skin, previous pressure ulcer, sensory perception, perfusion, nutrition, moisture and diabetes) ranged from 79.1% to 94.2% for inter-rater reliability and from 87.0% to 93.9% for test–retest reliability. Moderate to high associations were demonstrated for convergent validity, assessed by comparison with the same or similar constructs on other risk assessment scales (Braden and Waterlow). A known group comparison was not possible because of the small number of patients recruited from elective wards. In addition, field notes recorded by the expert nurses highlighted positive and problem aspects of using the tool in the clinical environment. Negative aspects included difficulties in assessing some of the PURPOSE-T items and concerns about reliability, but these were not evidenced in the formal evaluation of inter-rater and test–retest reliability.
It is of note that both expert and ward/community nurses allocated the majority of patients (> 95%) to the ‘not at risk’ category, with only ‘yellow’ and ‘blue’ boxes completed (see Tables 45–47). This means that these patients did not have skin, sensory perception, perfusion or major mobility problems but were characterised by minor mobility limitations with or without nutritional deficits, moisture problems or a history of previous pressure ulcers (with no scar). This is interesting because these factors do not emerge consistently in multivariable modelling (see Phase 1: Systematic review of patient risk factors for pressure ulcer development, Emerging risk factor domains/subdomains), were still judged to be important in the consensus development process, but colour coded as ‘yellow’ (i.e. requiring clinical judgement). It may be that in practice they are judged to be not important in the absence of the other key risk factors. The next stage of the development process will involve the dissemination of the PURPOSE-T into routine NHS care and this will facilitate large-scale multivariable modelling and predictive validity testing, allowing further refinement of the tool.
The main differences between the PURPOSE-T and other widely used risk assessment tools are as follows:
-
a risk factor Minimum Data Set is incorporated to facilitate multivariable modelling
-
involves a screening stage for all patients and a full assessment stage for those at potential/actual risk or with an existing pressure ulcer. This allows those who are obviously not at risk to be quickly identified, preventing the need for a more detailed full assessment, which will save time in clinical practice
-
a risk profile is identified for each patient (rather than a score condensed from different aspects of risk) to support care planning, with interventions selected in response to specific risk factors
-
there is incorporation of the symptom of pain as a risk factor
-
colour is used to aid decision-making
-
there is a clear distinction between primary and secondary prevention: patients with an existing pressure ulcer or scarring from a previous ulcer are allocated to a secondary prevention and treatment pathway. This has the potential to facilitate escalation of interventions to prevent deterioration in existing pressure ulcers and promote healing
-
development was based on a systematic review of the risk factor evidence and the pain cohort study
-
development involved international and interdisciplinary experts in the field
-
the tool was developed in partnership with service users.
Patient and public involvement in the risk assessment work package
Pressure Ulcer Research Service User Network UK members have been involved at various stages throughout this work package:
-
involvement in the consensus study (with particular emphasis on the acceptability of pressure ulcer risk assessment elements for patients)
-
contribution to the development of the case studies for the Risk Assessment Framework pre-test study
-
reviewing the Risk Assessment Framework following the pre-test
-
supporting the development of the Risk Assessment Framework clinical evaluation study, particularly relating to the development of patient information leaflets.
This project has provided some specific examples of the impact of PPI. The impact can be clearly seen in changes that were made to the Risk Assessment Framework as a direct result of PURSUN UK members’ input, such as the exclusion of albumin, the inclusion of pain and a previous severe pressure ulcer and changes to the wording of the sensory perception domain. PURSUN UK members also highlighted the need to adapt the Risk Assessment Framework so that it can be used by patients and carers at home. This is being incorporated into our next programme of work.
Conclusions
The risk assessment work package comprising a systematic review of pressure ulcer risk factors, consensus study, conceptual framework development, design and pre-test and clinical evaluation led to the development and validation of a new Risk Assessment Framework, the PURPOSE-T [see http://medhealth.leeds.ac.uk/purposet (accessed July 2015)], with an underpinning risk factor Minimum Data Set. The PURPOSE-T comprises two stages of assessment, the screening stage for all patients and the full assessment stage for patients at potential/actual risk or with an existing pressure ulcer. It facilitates the identification of a risk profile rather than a condensed score and allows patient to be allocated to a not currently at risk, primary prevention (at risk) or secondary prevention and treatment pathway (existing pressure ulcer or scarring from a previous pressure ulcer). The next stage of the development process will involve dissemination of the PURPOSE-T into routine NHS care, which will facilitate large-scale multivariable modelling and predictive validity testing, allowing refinement of the tool. The conceptual framework also provides a foundation for a programme of bioengineering and translational research to develop improved assessment techniques with greater precision for clinical use. The work package makes a key contribution to the pressure ulcer field and has the potential to directly impact risk assessment in clinical practice. The research methodologies utilised may also have a broader application to other relevant areas of health-care research.
Chapter 6 Development and evaluation of a patient-reported pressure ulcer health-related quality of life instrument
Chapter written by Claudia Rutherford, Julia M Brown, Michelle Briggs, Susanne Coleman, Carol Dealey, Elizabeth McGinnis, E Andrea Nelson, Nikki Stubbs, Lyn Wilson, Delia Muir and Jane Nixon.
Abstract
Introduction: Patient-reported outcome instruments are used to inform patient care and provide a strong evidence base for new treatments that incorporate patient perspectives. However, no PRO instruments for assessing the impact of pressure ulcers on HRQoL are available. Therefore, we aimed to (1) develop a conceptual framework of HRQoL specific to pressure ulceration, (2) develop a self-report HRQoL instrument for use with patients with pressure ulcers and (3) undertake a comprehensive evaluation of the fundamental psychometric measurement properties of the new instrument.
Methods: We used gold standard methods to develop and evaluate a new PRO instrument for people with pressure ulcers (PU-QOL) instrument. In phase 1 we developed a conceptual framework describing the impact of pressure ulcers on HRQoL using three sources: a systematic review of the pressure ulcer HRQoL literature, clinical expertise and qualitative data from 30 patient interviews. In phase 2 we developed a provisional instrument. First, we used the conceptual framework to form the basis of the PU-QOL scales. Next, we generated a pool of questions representing all outcomes from the conceptual framework. These questions were generated from the phase 1 patient interviews, from two further systematic reviews of pressure ulcer pain and existing chronic wound measures and by asking experts. The questions were then brought together to produce a draft instrument. Finally, we pre-tested the provisional instrument using mixed methods (cognitive interviews with 35 patients and Rasch measurement theory). In phase 3 we undertook psychometric evaluation in two field tests. In field test 1 we undertook item reduction and testing of scale formation; assessment of differential item functioning to determine the optimal mode of administration (Rasch measurement theory); and assessment of acceptability, scaling assumptions, reliability and validity (classical test theory) using PU-QOL data from 285 patients. In field test 2 we undertook psychometric evaluation of the item-reduced version of the instrument on PU-QOL data from an additional 229 patients, using both Rasch measurement theory and classical test theory, to test scale targeting, item response categories, item fit, response bias, acceptability, scaling assumptions, reliability and validity.
Results: Our conceptual model includes four HRQoL domains (symptoms, physical functioning, psychological well-being, social participation) divided into 13 subdomains. The final PU-QOL consists of 10 scales to measure pain, exudate, odour, sleep, vitality, mobility/movement, daily activities, emotional well-being, self-consciousness and appearance, and participation. We established that a self-administration mode is not suitable for hospital inpatients with pressure ulcers and it is therefore intended for administration following a user manual, with respondents rating the amount of ‘bother’ attributed on a 3-point scale. The final PU-QOL evaluation provides preliminary evidence in support of measurement reliability and validity; Cronbach’s alpha values for the PU-QOL scales ranged from 0.89 to 0.97 and hypothesised correlations between PU-QOL and Short Form questionnaire-12 items (SF-12) scores (r > 0.30) were consistent with predictions.
Conclusions: We have identified HRQoL outcomes that are important to people with pressure ulcers and developed a conceptual framework of HRQoL and the PU-QOL instrument, reflecting the conceptual domains. The PU-QOL instrument provides a standardised method for assessing the impact of pressure ulcers and for quantifying the benefits of associated interventions from the patient perspective, thus far lacking in this area. It can be used in research with adults with any type of pressure ulcer and is suitable for all UK health-care settings. Further work is needed to provide evidence in support of score interpretation and to explore the utility of the PU-QOL in routine practice.
Introduction
Health-related quality of life
A patient’s health status can be measured through various concepts, including symptomatic outcomes (i.e. pain), effect on ability to carry out daily tasks and more complex concepts such as HRQoL. HRQoL is a multidimensional construct that encompasses four primary domains: psychological, physical, social and role functioning and issues relating to well-being. 205,206 Assessment of HRQoL is no longer just a relevant end point of clinical trials but is often carried out in routine clinical practice and is considered essential to understanding the quality of health care. 207 HRQoL data provide information about the impact of a specific disease and subsequently increase awareness of, and the ability to address, the needs and concerns most important to patients. As such, assessment of HRQoL is particularly relevant in disease areas in which there is a significant impact on HRQoL and a significant treatment burden, such as chronic wounds.
Impact of pressure ulcers on health-related quality of life
The impact of pressure ulcers on HRQoL is substantial, although few studies contain empirical data to substantiate this assumption. 9 Our pre-programme systematic review9 identified that the majority of work to date has been mainly qualitative and that pressure ulcers severely compromise patient functioning: they can affect sleep, rehabilitation, mobility and psychological, physical and social aspects of patients’ lives. 34,208,209 They also cause patients substantial pain. However, the pain is often underestimated by health-care professionals:210 patients described how their ulcer-related pain was largely unrecognised by health-care professionals, how their reports of pain were ignored and how their ulcer-related pain was rarely formally assessed,9 findings consistent with those from the severe pressure ulcer study (see Chapter 4).
Health-related quality of life not only is related to the presence of a pressure ulcer but also is affected by the treatments that patients undergo to either prevent or treat a pressure ulcer. Because of the complexity of pressure ulcers, health-care professionals face the challenge of providing effective preventative and treatment interventions. The choice of intervention depends on the purpose, for example pressure damage prevention using pressure-reducing/-offloading devices and repositioning, skin protection from moisture or wound treatments to promote healing. NHS practice guidance is focused on identifying patients at risk through risk assessment of all patients on admission to acute hospital and community nursing services (see Chapter 5), implementing preventative care (e.g. specialist mattresses, turning, skin care) and using interventions to halt damage and promote healing (e.g. mattresses, dressings, nutritional supplements). 1,11,14,15,211 However, these interventions can affect patient functioning and cause significant treatment burden. 33 For example, the frequency and regularity of dressing changes may affect a patient’s daily routine, increase fatigue, restrict mobility and cause additional pain or discomfort.
Our pre-programme work has identified factors within the wider health-care context that may contribute to reduced or improved HRQoL, such as satisfaction with health care received, inconsistencies in the health care provided (i.e. different methods between nurses, wards and/or hospitals) and the relationship between patient and health-care provider. 9,10 These contributory factors relating to service organisation were also evident from the severe pressure ulcer study (see Chapter 4). Assessment of HRQoL and other contributory outcomes can facilitate patient–health-care provider communication and provide information required for effective health-care planning and ulcer management. In addition to improving patient health care, assessment of HRQoL can be important for indicating how satisfied patients are with the health care received, indicators that are important for treatment and health-care effectiveness.
Measuring health-related quality of life in people with pressure ulcers
Assessment of HRQoL is considered subjective in nature and therefore best measured by directly asking the person involved through the use of PRO instruments or rating scales. The best PRO instruments are designed to probe people in a structured, formal way to give reproducible, meaningful, quantitative assessments from a personal perspective of how they feel and function. 212 PRO instruments may be generic, designed to measure concepts that are relevant across different diseases, outcomes, treatments and populations, or disease/condition specific, designed to assess the impact of a specific disease or condition on HRQoL, with the goal of detecting clinically important changes. 213
The use of PRO instruments has become increasingly important in many disease areas214,215 and there has been a growth in instruments to evaluate HRQoL in some chronic skin conditions. However, established PRO instruments are currently not available for use with patients with pressure ulcers. 216 PRO instruments are used to inform and monitor the performance of patient care and health-care delivery and are important for providing a strong evidence base for new treatments that incorporate patient perspectives and cost-effectiveness. Cochrane reviews highlight the lack of reliable evidence for the clinical effectiveness of a majority of pressure ulcer treatments. 20 Further, few studies in this field include PROs as study outcomes9 and national and international prevention and treatment guidance is not mandated. 1,15 When HRQoL outcomes have been assessed, generic or chronic wound-specific measures have been used. 216 However, despite common conceptual domains between pressure ulcer and chronic wound HRQoL models, existing PRO instruments do not adequately represent pressure ulcer-specific HRQoL outcomes (e.g. content differs at the subdomain and item level; important components such as issues stemming from treatments and symptoms, mobility, sleep, embarrassment and physical appearance are not well represented),216 questioning their appropriateness for use in pressure ulcer research. Moreover, assessment of outcomes in clinical trials of pressure ulcer intervention effectiveness either has been limited to conventional clinical outcomes (i.e. prevention or healing) or has used limited, inappropriate (i.e. not fit for purpose) or inadequately validated instruments. 9,72 Importantly, clinical decision-making is not informed by high-quality studies based on patients’ perspectives and cost-effectiveness.
We need a systematic way of considering (1) patients’ priorities for interventions and (2) health economic evaluation (see Chapter 7 for work to derive a preference-based measure for use in cost–utility analysis). A PRO instrument specific to pressure ulcers could facilitate clinician–patient communication, shared decision-making and training of new staff; identify and prioritise patient problems and preferences; monitor changes or outcomes of treatment; measure the performance of health-care providers; and facilitate clinical audit. 217–220
Aim and objectives
The principal aim of this work package was to develop a PRO measure of HRQoL specific for people with pressure ulcers, the PU-QOL instrument. This would provide a standardised method for evaluating patients’ needs and self-reports of the impact of pressure ulcers and their treatment on HRQoL.
Specific objectives were to:
-
identify HRQoL outcomes relevant to patients with any category of pressure ulcer and the relative ulcer burden
-
develop a PRO measure of HRQoL specific to pressure ulcers that is acceptable, reliable and valid and suitable for use in the UK.
Research overview
Several sequential pieces of reseach were undertaken to develop and evaluate the PU-QOL instrument (Figure 25):
-
phase 2: instrument construction and pre-testing comprising:
-
-
– review of patient interview transcripts from phase 1b (see Item generation from patients)
-
– systematic review of existing chronic wound instruments literature216 (see Existing chronic wound instruments: systematic review)
-
– systematic review of pressure ulcer-related pain literature36 (see Pressure ulcer-related pain: systematic review)
-
– item generation from experts (see Item generation from experts)
-
-
preliminary PU-QOL construction (see Preliminary PU-QOL construction)
-
pre-testing through semistructured cognitive interviews with patients222 (see Pre-testing)
-
-
phase 3: psychometric evaluation in two parts:
-
field test 1: item reduction and scale formation; mode of administration substudy (see Field test 1 and mode of administration substudy); acceptability, scaling assumptions, reliability and validity [classical test theory (CTT)]
-
field test 2: a comprehensive psychometric evaluation of the final version, including scale targeting, item response categories, item fit, response bias, acceptability, scaling assumptions, reliability and validity, using both Rasch and traditional psychometric methods223 (see Field test 2: final psychometric evaluation).
-
FIGURE 25.
Flow diagram of the research undertaken to develop and evaluate the PU-QOL instrument.

International PRO instrument guidelines and criteria were consulted to ensure high quality and standardisation for PU-QOL development. 192,212,224,225 Collaboration was sought from members of EPUAP and from 29 acute and primary care NHS trusts around the UK.
Phase 1: conceptual framework development
Various parts are reprinted from Int J Nurs Stud, vol. 47, Gorecki C, Lamping DL, Brown JM, Madill A, Firth J, Nixon J. Development of a conceptual framework of health-related quality of life in pressure ulcers: a patient-focused approach, pp. 1525–34, 2010,221 with permission from Elsevier.
When developing a new PRO instrument, the construct intended for measurement (in this case quality of life associated with pressure ulceration) needs to be clearly defined and the content (items) needs to reflect the construct. The first phase of the project involved developing a conceptual framework, by tapping into three sources. First, a systematic review and narrative analysis of the HRQoL outcomes literature relevant to pressure ulcers was undertaken (work predating the programme). 9 The review generated HRQoL issues, which were grouped into HRQoL domains, formulating a working conceptual framework. Second, in-depth qualitative interviews were undertaken with a sample of patients with pressure ulcers. From the information obtained from the patient interviews and the third source, expert opinion, a final conceptual framework was produced.
Pre-programme systematic review overview
We undertook a systematic review (pre programme) of both the qualitative and quantitative pressure ulcer and HRQoL literature to identify the impact of pressure ulcers and associated interventions on HRQoL. 9 We included studies describing the impact on HRQoL from direct patient reports. From 31 studies, including 2463 participants with pressure ulcers aged between 17 and 96 years, we extracted 293 findings, which were divided into 46 categories and 11 themes. Our working conceptual framework consisted of HRQoL themes (physical, social and psychological impact, symptoms, general health) and other impacts of pressure ulcers (health-care professional–client relationships, need for vs. effect of interventions, impact on others, financial impact, perceived aetiology, need for knowledge). 9 Importantly, the systematic review highlighted that pressure ulcers severely impact on patients’ HRQoL and that there was no PRO instrument available to assess pressure ulcer-specific HRQoL outcomes.
Qualitative study
An important consideration when developing PRO instruments is conceptualisation and content of the instrument. Developers of disease-/condition-specific PRO instruments often utilise both top-down (e.g. literature review) and bottom-up (e.g. qualitative data) approaches to develop the conceptual framework to ensure that those aspects of HRQoL that are most important to patients with the underlying condition are reflected. Having used the top-down approach (see Pre-programme systematic review overview) we then undertook a qualitative study (see Appendix 38 for the study protocol) utilising the bottom-up approach.
Aim
The aim of this study was to undertake in-depth qualitative interviews with a sample of patients with pressure ulcers. The information obtained would be used to develop a conceptual framework of HRQoL specific to pressure ulcers. Specific objectives were to:
-
identify HRQoL outcomes relevant and important to patients with grade 1, superficial and severe pressure ulcers
-
identify whether HRQoL outcomes for patients with grade 1, superficial and severe pressure ulcers are the same in relation to the impact of interventions
-
gain insight into the relative ulcer burden and what it is like to live with a pressure ulcer.
Methods
Design
Top-down (literature review) and bottom-up (qualitative data) approaches were combined to develop the conceptual framework. The top-down approach involved developing a working conceptual framework of HRQoL in pressure ulcers, based on a systematic review of the quantitative and qualitative pressure ulcers and HRQoL literature. 9 The bottom-up approach involved further qualitative work to elicit information pertaining to the impact of pressure ulcers on HRQoL and specific domain components. A multidisciplinary expert group, including seven tissue viability nurse specialists, a chronic pain specialist and five outcome methodologists (see Acknowledgements), reviewed the qualitative results and final conceptual domains.
Participants
Patients from both acute and primary care were included because of the high pressure ulcer prevalence in both settings and the need to obtain perspectives from people in both settings as interventions can differ between settings. Adult patients with a pressure ulcer of any severity,1 duration or location or a pressure ulcer that had recently healed (within 3 months) were included if they were aged ≥ 18 years, from a hospital, rehabilitation or community setting and under the care of a tissue viability nurse specialist and were able to reflect on and share their experience and provide informed consent to participate. Patients were excluded if they did not currently have a pressure ulcer or one that had healed within the previous 3 months or were unconscious, confused, cognitively impaired or unable to speak English.
A purposive sampling method was devised, with sampling of patients targeted to key factors to reflect the range and diversity of the target population, including age (< 70 years and ≥ 70 years), ulcer severity (superficial and severe129) and location (torso and limb sites) and health-care setting (hospital and community). A minimum of five patients per key factor were consecutively sought and found. 221
Recruitment and data collection
Eligible patients were identified and approached to participate by members of the tissue viability teams at participating hospitals and community services, who provided information (see Appendix 39) about the rationale, design and personal implications of the study and the ‘agree to be contacted by the researcher’ form (see Appendix 40). Following information provision, patients had as much time as they needed to consider participation. After receiving a signed agreement to be contacted, the researcher (CG) carried out an interview at the patient’s home or hospital ward, which was audio recorded and transcribed verbatim.
We conducted individual face-to-face semistructured interviews, guided by an interview schedule (e.g. questions to confirm or refute the importance of the working framework domains), open-ended questions to elicit relevant new information (e.g. ‘Is there anything else that you want to add about how your pressure ulcers may have impacted you?’) and clarifying questions (e.g. ‘Do you think [that] is only because of your pressure ulcer or possibly resulting from a combination of things?) to ensure that issues reported were in fact the result of pressure ulceration (see Appendix 41). The provisional HRQoL domains were revised as new information emerged from the data, refining the working framework deductively, and were incorporated into discussion in subsequent interviews to confirm the importance of new HRQoL issues.
Data analysis
First, the researcher read the transcripts while listening to the recording to confirm the accuracy of transcription and to obtain an overview of the data collected. Any first impressions and interpretations were noted, including thoughts about the main HRQoL domain components, general feelings about the interview and audio cues from the patient that would be lost in transcription. Preliminary analysis was carried out after the first three patients had been interviewed to assess whether the interview schedule’s HRQoL domains were consistent with the emerging themes and to identify any gaps in information. Then, two researchers (CG, JF) conducted thematic content analysis manually of textual data from the first four interviews, identifying HRQoL issues within the transcripts and coding to a provisional coding schema developed using a combined inductive (codes arising from transcripts) and deductive (codes developed from the interview schedule) approach. The provisional coding schema was refined during subsequent stages of the analysis; data collection and coding were conducted iteratively in multiple rounds of interviews so that subsequent data collection was informed by earlier coding and confirmed in later interviews.
Expert opinion
Health-related quality of life components that emerged from the patient interviews were reviewed by a multidisciplinary expert group (see Acknowledgements). Any issues mentioned infrequently were discussed and those that were agreed not to be clinically relevant were either excluded or consolidated with related components (e.g. various negative emotions such as irritated and distressed were consolidated with ‘negative mood changes’) rather than being retained as separate components in the conceptual framework. 221 Following data analysis, the group reviewed the final conceptual framework with the view towards making a distinction between components that addressed the impact of pressure ulcers on HRQoL and other contributory factors that may affect HRQoL. 10
Ethical approval
The study was approved by the North West Research Ethics Committee prior to data collection (reference number 07/H1010/60).
Results
Thirty-two patients with pressure ulcers from seven acute and primary care settings in England and Northern Ireland during December 2007 to October 2008 consented to participate. However, two patients were recruited twice and were not interviewed and so the final sample included 30 interviews (a record of those approached to participate and refusals was not made). Participants ranged in age from 22 to 94 years (mean age 62.2 years), 18 (60%) were male and 19 had other chronic conditions (e.g. eight had a spinal cord injury and three had multiple sclerosis). Patients represented different settings (n = 17 hospital or rehabilitation; n = 13 community), ulcer severity (n = 12 superficial; n = 15 severe; n = 3 mixed severity), numbers of pressure ulcers (n = 13 had more than one ulcer), ulcer duration (few days up to 4 years) and sites (n = 15 sacrum; n = 14 heel; others on the lower back, buttocks, ankles, hips, back of head and elbow). 221
We identified both HRQoL outcomes and contributory factors that affect pressure ulcer-related HRQoL. Contributory factors included six experience-of-care and 10 individual patient factors. Adults with pressure ulcers have concerns about treatment and wound management, treatment burden, communication difficulties, their ability to cope with functional limitations, poor support networks and other health problems and comorbidities. 10 However, as the intention was to develop a HRQoL instrument, a distinction between HRQoL outcomes and contributory factors (such as motivation and satisfaction with health care received) that may affect HRQoL was made (see Expert opinion), resulting in a defined, conceptualised and operationalised pressure ulcer-specific HRQoL conceptual framework.
The pressure ulcer-specific conceptual framework consists of four domains and 13 subdomains: symptoms (pain and discomfort, exudate, odour), physical functioning (mobility, daily activities, general malaise, sleep), psychological well-being (mood, anxiety and worry, self-efficacy and dependence, appearance and self-consciousness) and social functioning (isolation, participation)221 (Figure 26). This study provides qualitative evidence on HRQoL components that are important from the perspective of patients with pressure ulcers, an essential step when developing new PRO measures. The conceptual framework provides the basis for the development of the new pressure ulcer-specific measure of HRQoL.
FIGURE 26.
Pressure ulcer-specific HRQL conceptual framework. PU, pressure ulcer. Reprinted from Int J Nurs Stud, vol. 47, pp. 1525–34, 2010, Gorecki C, Lamping DL, Brown JM, Madill A, Firth J, Nixon J. Development of a conceptual framework of health-related quality of life in pressure ulcers: a patient-focused approach,221 with permission from Elsevier.
Phase 2: item generation, instrument construction and pre-testing
Various parts are largely reprinted from J Pain Symptom Manage, vol. 42, Gorecki C, Closs J, Nixon J, Briggs M. Patient-reported pressure ulcer pain: a mixed methods systematic review, pp. 443–59, 2011,36 with permission from Elsevier; and from Int J Nurs Stud, vol. 51, Gorecki C, Lamping DL, Alvari Y, Brown JM, Nixon J, Patient-reported outcome measures for chronic wounds with particular reference to pressure ulcer research: a systematic review, pp. 157–65, 2014,216 with permission from Elsevier.
The second phase of this project was the development of the PU-QOL instrument. Three sources were utilised to generate the list of candidate items for the instrument: patient interview transcripts (see Item generation from patients); systematic reviews of the pain literature and existing chronic wound PRO instruments (see Item generation from existing instruments and pressure ulcer pain literature); and experts in the field (see Item generation from experts). The item list was used to construct a preliminary version of the PU-QOL (see Preliminary PU-QOL construction), which was pre-tested with a sample of patients with pressure ulcers using mixed methods [cognitive interviews and Rasch measurement theory (RMT) (see Pre-testing)]. Based on information obtained from patients and expert opinion, the pre-test version was revised accordingly.
Item generation from patients
Item generation involved developing an exhaustive list of potential items (item pool) for each domain within our conceptual framework. Content (patient words verbatim) from the phase 1 patient interviews (see Qualitative study) was used to generate items. All content was grouped into HRQoL domains, with each domain comprising a number of items describing slightly different components. We took the inclusive approach retaining all content if reported more than once. Interview data were an excellent source for generating items as items using patients’ words and representing variable components across the broad spectrum of pressure ulcer-specific HRQoL outcomes were identified.
Item generation from existing instruments and pressure ulcer pain literature
We undertook two systematic reviews, first, to review generic, pressure ulcer-specific and chronic skin wound-specific PRO instruments used to assess HRQoL in patients with pressure ulcers or other similar chronic skin wounds216 and, second, to review patient reports of pressure ulcer-associated pain, descriptions of the pain experience and the impact on patients’ lives. 36
Existing chronic wound instruments: systematic review
Despite the impact on HRQoL, no research has been undertaken to determine the availability of PRO instruments, either generic or condition specific, and their suitability for use in pressure ulcer research. We developed a pressure ulcer-specific HRQoL conceptual framework (see Qualitative study). The conceptual framework provides a structured and formal framework against which the content of available PRO instruments can be assessed.
Aim
The aim of the systematic review was to identify generic, pressure ulcer-specific and chronic skin wound-specific PRO instruments used to assess HRQoL in patients with pressure ulcers or other chronic skin wounds and determine how useful or appropriate they are, based on their content, for use with patients with pressure ulcers in assessing HRQoL outcomes.
Methods
Studies of any design were included if PRO measures were used to assess HRQoL or related concepts in adult patient populations presenting with any grade of pressure ulcer or other chronic wounds, from hospital, rehabilitation or community health settings within Europe, North America or Australia. Studies were excluded if (1) HRQoL was assessed by the health-care provider or proxy (i.e. not patient reported); (2) they used an instrument intended primarily for other medical conditions, in which pressure ulcers are a secondary outcome (e.g. Life Situation Questionnaire); (3) they used an instrument assessing mediating or contributory outcomes only (e.g. Inventory of Socially Supportive Behaviours, personality scales, locus of control, Coping Response Inventory, Mini Mental State Examination for screening cognition); (4) HRQoL was assessed using a single-item rating scale [i.e. visual analogue scale (VAS)]; or (5) they were limited to paediatric populations or wounds caused by trauma (e.g. burns). Abstracts from conference proceedings were excluded unless additional information was provided by the authors216 (see Appendix 42 for data sources and search strategies).
One researcher screened abstracts for relevance. Studies assessed as potentially relevant or studies whose relevance was ambiguous were obtained in full for further scrutiny. Two researchers independently assessed potentially relevant studies against the inclusion criteria. Studies that did not meet the inclusion criteria were excluded from further analysis. 216
Measures without evidence of any development or validation process (i.e. ad hoc instruments with no formal reliability or validity testing) were excluded. Additional quality components were not used as a threshold for the inclusion of instruments as the intention was to provide a descriptive summary of the content domains of existing instruments used to assess HRQoL in patients with pressure ulcers and other chronic wounds. However, empirical evidence for reliability and validity was a minimum requirement for inclusion of PRO instruments. 216
Data were independently extracted by two reviewers. We cross-checked data extraction for errors, omissions and consistency between extractions. Disagreements or discrepancies were discussed between the two researchers and confirmed with a psychometrics expert (DL). We had intended to extract data on the development and evaluation of PRO instruments to allow the appraisal of the measurement properties216 for PRO instruments that had at least 75% of pressure ulcer-specific content (at both domain and item level) and no more than 25% of non-relevant content. As none of the identified measures met these criteria, the measurement properties were not extracted and assessed.
Our analysis systematically determined the extent to which PRO instruments covered the pressure ulcer-specific conceptual framework (see Figure 26). 216 Items from identified instruments were mapped to the conceptual framework to determine content of relevance to pressure ulcers. Those considered relevant could be included in the item pool.
Results
Three generic and 14 chronic wound instruments were identified but no pressure ulcer-specific instruments (Figure 27). 216 None of the available instruments cover all HRQoL domains that are important in pressure ulcers. One condition-specific instrument, the Venous Leg Ulcer instrument, matched most closely conceptually, but failed to represent three important domains and contained items not specific to pressure ulcers. 216 Although a potentially valuable source for generating items, few items were assessed as pressure ulcer specific (i.e. they were worded to assess the impact of other conditions and not specifically the impact of pressure ulcers) or were items already generated from patient reports.
FIGURE 27.
Flow of studies: existing instrument systematic review. a, Duplication of instruments across studies. PROM, patient-reported outcome measure. Reprinted from the International Journal of Nursing Studies, vol. 51, pp. 157–65, 2014, Gorecki C, Lamping DL, Alvari Y, Brown JM, Nixon J, Patient-reported outcome measures for chronic wounds with particular reference to pressure ulcer research: a systematic review,216 with permission from Elsevier.
Pressure ulcer-related pain: systematic review
Pressure ulcers can cause patients considerable pain and discomfort; however, little is known about how pressure ulcer pain affects patients’ everyday lives.
Aim
The aim of this systematic review was to identify and synthesise all research that included verbal patient reports of pressure ulcer-associated pain, including descriptions of the pain experience, intensity and quality and impact, to interpret the complexities of the pain experienced from pressure ulcers. Specific objectives were to describe specific characteristics of pressure ulcer pain and determine how it affects patients’ lives. 36
Methods
Studies were included if the study sample consisted of adult patients with any category of pressure ulcer from any setting with any existing comorbidity and the study used qualitative methods to obtain patient reports of their experience of pressure ulcer pain. Studies using mixed-method designs were included only if pressure ulcer-specific findings were reported separately from mixed wound findings or quantitative methods to assess pain used existing validated outcome measures in which pain descriptors were available. Studies that used patient-reported HRQoL instruments were considered if a pain scale was included and the results were reported. Studies were excluded if the study sample consisted of those with mixed wounds, pressure ulcer-associated pain was not patient reported (i.e. proxy assessment) or data were collected using ratings or a VAS to obtain pain severity scores. No upper age, gender or language restrictions were applied (see Appendix 42 for data sources and search strategies). 36
Study selection methods were consistent with those described in Existing chronic wound instruments: systematic review.
Individual quality components of study methodology were not used as a threshold for the selection of primary studies. We included all available data but assessed the appropriateness of each study by making a judgement about whether a study used appropriate methods for addressing our review questions and for ensuring that findings about the pressure ulcer pain experience were indeed from the patient perspective (e.g. whether data collection methods were appropriate for helping patients express their views and how pressure ulcer pain affects them). 36
Two reviewers independently extracted findings in the form of direct patient quotes and allocated findings to defined categories. A category was determined by grouping common findings (i.e. findings that reflected similar phenomena or variables). Categories that were sufficiently similar in meaning were generated into synthesised themes. Synthesis of findings and categories was reviewed by three reviewers until consensus. Any descriptions relating to pressure ulcer-associated pain, including descriptions of the pain experience, intensity, quality and impact, could be included as items.’36
Results
‘Ten studies were included: six qualitative and four quantitative studies (Figure 28). These included 108 adults with pressure ulcers. We produced a biopsychosocial model of the pressure ulcer pain experience, including five domains: communicating the pain, feeling the pain, impact of pain, self-management and professional management. The findings of the review suggest that, to achieve the best possible outcomes that are important to patients, improved communication of pain experienced between the individual and health-care professional and across disciplines, interventions to help control or reduce pressure ulcer pain, patient-centred concerns and systemic barriers need to be considered when managing pressure ulcers to ensure more effective pressure ulcer pain management in the future.’36 These findings are consistent with those from the severe pressure ulcer study (see Chapter 4).
FIGURE 28.
Flow of studies: pain systematic review.
With regard to potential items, similar to the existing instrument systematic review, pain-related issues had already been identified from patient reports and no new descriptors were added to the item pool.
Item generation from experts
As patients with pressure ulcers receive specialist care from health-care professionals who have a vast range of experience in treating patients with pressure ulcers and therefore probable insight into patients’ experiences, a clinical expert group reviewed the item pool generated from patient interviews. The group consisted of three community and acute care tissue viability nurse specialists (LW, NS, EM), three nurses with extensive experience undertaking pressure ulcer research (JN, EAN, CD), one chronic wound pain specialist (MB) and one nurse with experience of health-care policy development (SC) (see Acknowledgements). Items were grouped by domains and the focus of the review was on item grouping and relevance, content and wording and clinical importance. Items with similar content were highlighted and the accuracy of domain categorisation (item grouping) was discussed. Additional items were added if necessary. Items were retained if they were considered clinically relevant, not too similar (redundant items were combined or removed) or to pertain to HRQoL (not measuring other constructs such as personality or satisfaction). Final item elimination decisions were based on consideration of item problems in combination.
At this stage an important conceptual decision was made to include pressure ulcer symptoms of pain, exudate and odour into the pressure ulcer-specific model. These symptoms are important consequences of having a pressure ulcer but initially were not considered HRQoL outcomes and were therefore excluded from the original item pool. Counting the frequency or assessing the intensity of symptoms may not be an adequate measure of HRQoL,226 but the impact of symptoms and the meaning that they have for individuals is an important aspect of HRQoL assessment and therefore is considered important for inclusion in a pressure ulcer-specific PRO instrument. Patient comments pertaining to these outcomes were identified in the qualitative work and items were added to represent these symptoms. The amended item pool and content mapping were reviewed by a group of seven health outcome methodologists (see Acknowledgements), who focused on the constructs measured (e.g. whether or not items for each domain were representative of the construct being measured) and item wording (i.e. whether any items were confusing, ambiguous, double-barrelled). The process of item generation resulted in an initial pool of 118 items (see Appendix 43).
Construction of the preliminary Pressure Ulcer Quality of Life tool
The development of the preliminary PU-QOL instrument involved careful stepwise construction with consideration of the design, layout and instructions, framing of questions, response format and recall period to ensure that the way that the PU-QOL was presented was tailored to the characteristics of patients with pressure ulcers (i.e. understood by and relevant to the intended population).
Instructions, design and layout
Consistent with recommendations,227 general information pertaining to what the PU-QOL instrument is about and instructions about how questions should be answered were placed at the beginning of the instrument. Instructions specific to individual questions were placed close to the relevant question. Instructions were brief and clear and bold font was used to highlight important components (e.g. ‘during the past week’ and ‘tick all that apply’). A statement ensuring confidentiality was included to encourage accurate reporting.
The PU-QOL instrument was designed as a double-sided A4-size booklet on white paper. Font size 12 was chosen as respondents are largely elderly people with some visual impairment. Questions were grouped into item sets (scales; see Framing of questions) and numbered, not crowded or split between two pages, with horizontal response formats (see Response options) attached, and ended with a thank you. 227
Framing of questions
The US Department of Health and Human Services Food and Drug Administration (FDA) guidance for developing PRO instruments212 recommends that items should adequately cover important conceptual domains, relate to the instrument’s objectives, use words that are familiar to patients and not be confrontational, upsetting or ambiguous. These recommendations were considered when constructing the PU-QOL instrument to ensure clearly formulated and precise items.
Operationalisation
The item pool (n = 118) was transformed into scales through a process known as operationalization, in which logically related items are grouped or blocked into scales based on their conceptual meaning to represent coherent clinically meaningful constructs. 225 Each scale represented one of the 13 subdomains within the pressure ulcer-specific HRQoL conceptual framework (see Figure 26) and produced a scale score rather than an overall total score. The intention was to enable the monitoring of changes within patients on these 13 outcomes rather than a global HRQoL change.
Item stem
As the PU-QOL is a condition-specific instrument, the item stem had to be worded in a way that focused patients’ thinking towards their pressure ulcer so that any bother attributed related to pressure ulcer impact rather than impact from other health problems that the patient might have (i.e. the item stem needed to be salient to people with pressure ulcers rather than relating to overall health status). This was considered important as people who develop pressure ulcers usually have a multitude of health problems or comorbidities that may affect the same outcomes that pressure ulcers affect, such as pain. The item stem for each question was, ‘During the past week, because of your pressure ulcer, how much were you bothered by . . .’, which was followed by the item content (e.g. feeling uncomfortable).
Response options
The Likert-scale response method is commonly used in PRO measurement and was the chosen method for the PU-QOL instrument. When constructing Likert scales the number of response categories to use and how they should be labelled needs to be considered. 212 The response option descriptors chosen for the PU-QOL instrument relate to the amount of bother attributed (e.g. ‘How much have you been bothered by . . .?’) rather than the frequency of the outcome (e.g. a symptom might be frequent but might not necessarily be bothersome). ‘Bother’ was a term that was frequently used by patients during the qualitative interviews (see Qualitative study). Both response formats (frequency of the problem and amount of bother attributed) were presented to participants during pre-testing. Each item uses four discrete response options scored with successive integer scores (e.g. 0 = no bother at all to 3 = a lot of bother). These imply a continuum of increasing impact (bother), from less (no bother) to more (a lot of bother). This assumption was tested by examination of threshold ordering in subsequent testing (see Phase 3: field testing).
Time frame
Important disease changes/progression and memory error (recall bias) need to be considered when choosing a time frame. A recall period of the past week was chosen on clinical grounds, as changes in pressure ulcer severity and symptomology often occur over days and thus a longer recall period would risk not capturing relevant changes in HRQoL. Events that occurred over a month ago may no longer be relevant or may have been resolved or treated.
Mode of administration
With regard to mode of administration there is essentially a choice between interviewer administered (i.e. face-to-face, telephone) and patient self-completed (e.g. postal survey, during clinic visit). Each method has its advantages and disadvantages and the current evidence is inconsistent in differentiating the superiority of one method over the other in terms of quantity and quality of response. 228 We considered that patients with pressure ulcers (i.e. acutely ill, elderly) may have difficulty with self-completion, but as the PU-QOL instrument is intended for pressure ulcer intervention effectiveness research, and there is cost–benefit associated with self-completion methods in clinical trials that require large samples,229 the decision was made to develop a self-completed version in the first instance. The suitability of this method was determined during field test 1 (see Field test 1 and mode of administration substudy).
Pre-testing
Pre-testing is key in PRO instrument development. We undertook a pre-test study to evaluate patients’ understanding of the items, instructions, response options and recall period, determining whether readability was appropriate for the target population and confirming the completeness of concepts covered by items212 (see Appendix 44 for the study protocol).
Aim
The aim of this study was to pre-test the preliminary PU-QOL version using cognitive interviewing and RMT methods to identify and resolve problems with layout, time frame, response options, framing of items and administration mode. The study was also designed to determine whether or not readability was appropriate for patients with pressure ulcers and confirm content (i.e. the need for additional items or elimination/rewording of other items) prior to formal psychometric evaluation,222 hence it being described as ‘pre-testing’.
Methods
Design
Mixed methods were used to pre-test the preliminary PU-QOL tool. The intention was to identify and resolve any problems with the way in which the PU-QOL tool was constructed, by comparing RMT230 findings with the findings from cognitive interviews for consistency. 222 The intention was to reduce respondent burden and decrease data errors and non-response because of poor design and layout and unclear, misunderstood or irrelevant items to ensure that the PU-QOL was relevant to and understood by people with pressure ulcers.
Participants
Adult patients from acute and primary care settings were included if they had a pressure ulcer of any severity,1 duration or location; were aged ≥ 18 years; were from a hospital, rehabilitation or community setting; and were able to read and write in English. Patients without a pressure ulcer or who were unconscious, confused, cognitively impaired, unable to speak English or deemed ethically inappropriate to approach (e.g. death was imminent) were not eligible.
The sampling method for the pre-test was consistent with that detailed in phase 1 (see Qualitative study, Sampling).
Recruitment and data collection
The recruitment method for the pre-test was consistent with that detailed above in phase 1 (see Qualitative study, Recruitment and data collection; see also Appendix 45 for the patient information leaflet and consent form).
Structured face-to-face cognitive interviews were undertaken to gain an understanding of how patients interpret and understand individual questions (i.e. whether questions are understood as intended) and produce their answers. 195 Emphasis was on comprehension (i.e. clarity, language), retrieval from memory and response judgements (i.e. frequency judgements, logic decisions). Interviews were conducted in patients’ homes, clinics or wards, as determined by the each patient’s circumstances at the time of interview.
Two interviewing techniques were employed; however, the first three participants who were asked to think aloud (spontaneous conversation) while completing the PU-QOL reported that the method made completion difficult. Therefore, the remaining participants completed the preliminary PU-QOL instrument without researcher assistance. They were instructed to flag/mark items that they found confusing, difficult to understand, upsetting/intrusive or annoying while completing the PU-QOL and to consider the format, design and response options. The preliminary version contained 118 items and took around 22 minutes to complete. Many items were similar, with slight variations in wording; however, these were retained and presented to patients for consideration.
Following completion, the researcher (CG), guided by a standard set of questions and probes (see Appendix 46), sought to elicit the cognitive processes employed by patients while completing the PU-QOL instrument. Patients were asked to give feedback on their understanding of each question and associated response categories and instructions and to verbalise how they had gone about producing their answers, with particular emphasis on retrieval from memory and subsequent judgements and decisions. During debriefing interviews the researcher took notes, fed back to patients to ensure comprehension of responses and reviewed recorded interviews, making notes on structured data extraction forms. 222
Data analysis
An analysis schema was developed based on the Question Appraisal System (QAS-99). 231 The QAS-99 is a coding tool that focuses on the cognitive demands required for answering a question, and potentially problematic item characteristics that may lead to response error, such as content, layout/length, time frame and response options, were identified across interviews. Problems from the patient interviews included misunderstanding of the item stem, response options or instructions; unclear wording (i.e. patients used expressions such as ‘should’, ‘needs to’, ‘must’); and negative comments about an item (e.g. ‘that item upset me’, ‘that item is annoying, it’s like the previous one’). The focus of the analysis was on identifying dominant trends across interviews (i.e. problems that occurred repeatedly) and key findings (i.e. problems identified in a single interview, but indicating a potentially problematic issue). 222
Rasch measurement theory provides a formal method for evaluating scale functioning against a sophisticated mathematical measurement model. 230 The Rasch model defines how a set of items should perform to generate reliable and valid measurements232 and evaluates the legitimacy of summing items to generate measurements. 230,233 In a Rasch analysis, the extent to which observed data (patients’ actual responses to scale items) are concurrent with (‘fit’) predictions of those responses from the Rasch model is examined, with the difference between expected and observed scores indicating the degree to which rigorous measurement is achieved. 234 The expected response structure is a probabilistic Guttman pattern, which assumes that, for people with the same ability, the probability of endorsing an easy item is higher than the probability of endorsing a more difficult item and vice versa. 235 When a rating scale is used to discriminate between those with different abilities, someone with higher ability is expected to affirm all items endorsed by a person with lower ability in addition to items representative of higher ability. RMT was used to examine the PU-QOL instrument’s response options, the appropriateness of the item series and biases arising because of question ordering.
We compared the cognitive interview and Rasch analysis findings in an interactive and iterative process to identify potential strengths and weaknesses of PU-QOL items and to guide decision-making about further revisions to items and the questionnaire design/layout.
As there is no standard method for using cognitive interview data to modify PRO instruments,236 the outcome methodologists (see Acknowledgements) discussed and resolved aggregated findings (both within and across interviews) after each patient interview round in an iterative process. This was carried out on a consensus, item-by-item basis to decide whether to retain, revise, eliminate or add items or make changes to the design and layout, with particular weight given to the same comments by several patient respondents. Occasionally, a single negative remark led to an item revision (e.g. a remark signalling a serious misunderstanding of an item). A group of clinical experts (see Acknowledgements) reviewed the revisions made to ensure clinical relevance. Expert appraisal assisted in avoiding bias that would be introduced when relying solely on the judgement of one researcher in determining the implications of the cognitive interview findings. 222
Pressure Ulcer Research Service User Network UK members with experience of living with a pressure ulcer were invited to review the final pre-test version of the PU-QOL instrument and feed back on clarity and comprehension, design and layout and item wording. Responses from PURSUN UK members was fed back to the clinical expert group and incorporated into the final pre-test version.
Ethical approval
The pre-test study was approved by the North West Research Ethics Committee prior to data collection (reference number 08/H1010/112).
Results
We screened 134 patients from 11 acute and community NHS sites across England from April 2009 to September 2009 in three rounds of cognitive interviews. Of those screened, 66 were considered eligible and 35 were recruited to the three rounds (10, 10 and 15 respectively). Patients ranged in age from 36 to 85 years (mean age 65 years) with half (49%) aged ≥ 70 years. In total, 16 (46%) were men and 18 (51%) had an additional chronic condition (e.g. spinal cord injury). Patients represented different settings (n = 19 hospital; n = 4 rehabilitation; n = 12 community) and ulcer severity (n = 13 superficial; n = 18 severe; n = 4 mixed severity), duration (2 weeks up to 5 years) and skin site (n = 33 sacrum/buttocks; n = 13 heel; others: lower back, groin, hips, back of thighs and ankles). 222
Cognitive interviews identified five key problem areas: content, instruction/layout/length, recall period, response options and administration mode. For example, patients reported that there were too many items about odour – ‘How many different words do you need for smell, you could remove a lot of these’ – or items used words that were too sensitive (e.g. ‘dirty smell’) or not commonly understood (e.g. ‘foisty’). Revisions focused on using words that patients use (e.g. ‘pressure sore’ instead of ‘pressure ulcer’). 222 Participants preferred responding in terms of how bothered they were about a particular problem rather than simply reporting on the frequency. We also found that almost half of the sample (n = 15; 43%) needed some assistance with completing the instrument, which led us to change the mode of administration from self-complete to interview administered to ensure suitability across the wide spectrum of pressure ulcer patients. The optimal mode of administration was tested empirically during the first field test (see Field test 1 and mode of administration substudy).
The Rasch analysis highlighted problems with the response options (i.e. the 4-point item response options were not supported; disordered thresholds were found in 74 of 90 items, indicating that the proposed scoring function was not working as intended). However, as the Rasch analysis was preliminary, it was considered premature at this stage to make changes to the response options until further empirical evidence could be obtained. 222 Consistent with the qualitative findings, the Rasch analysis identified item redundancy; examination of item locations indicated that some items clustered at similar locations (e.g. the item ‘lacking self-esteem’ was considered similar to the items ‘feeling self-conscious’ and ‘lacking confidence’ by both methods and was subsequently removed).
The results guided changes to layout, administration mode and content (e.g. item selection and deletion to reduce respondent burden, data errors and non-response). Feedback from PURSUN UK members led to some additional clarification of the instructions and revisions to the item wording for two items (removed item ‘upset’; merged items ‘concerned’ and ‘worried’ into one item).
Preliminary Pressure Ulcer Quality of Life instrument
The final preliminary PU-QOL instrument consisted of 13 scales (87 items): pain (11 items), exudate (eight items), odour (six items), sleep (six items), malaise (three items), mobility (11 items), daily activities (nine items), mood (seven items), anxiety (three items), self-consciousness and appearance (seven items), autonomy (three items), isolation (four items) and participation (nine items). It was intended for interview administration and responses are given in terms of the amount of bother attributed (‘During the past week, how much have you been bothered by . . .?’) during the past week on a 4-point response scale (0 = not at all to 3 = a lot).
Phase 3: field testing
Various parts are largely reproduced from Gorecki et al. 223 © 2013 Gorecki et al.; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
To enable the PU-QOL instrument to be used with confidence in clinical practice and future research it must be shown to meet psychometric standards for reliable and valid measurement. The third phase of this project involved a full psychometric evaluation of some of the fundamental measurement properties of the final pre-test version of the PU-QOL instrument. 223 It included two field tests and a mode of administration substudy. The first field test was carried out to identify any items with poor psychometric performance for possible elimination (item reduction) and establish the optimal mode of administration for the instrument (i.e. patient self-completion or researcher administered). The second field test involved a full psychometric evaluation (e.g. tests for scale targeting, item response categories, scaling assumptions, acceptability, reliability and validity) of the item-reduced version of the instrument (see Appendix 44 for the study protocol).
Field test 1 and mode of administration substudy
The first field test was required to establish the feasibility and acceptability of the PU-QOL instrument, produce a shorter version if appropriate (i.e. reduce the instrument length without losing measurement precision) and identify subscales and test scaling assumptions.
Aims
This study aimed to:
-
confirm the feasibility and acceptability of the instrument
-
produce a scientifically robust shorter version by selecting items that perform best against established psychometric criteria
-
examine the legitimacy of summing items into scales and test scaling assumptions
-
carry out a preliminary evaluation of the reliability and validity of the shorter item-reduced version
-
empirically investigate the optimal mode of administration (i.e. establish whether the PU-QOL instrument can be developed for use with both self-completion and interview-administered modes or whether two mode-specific instruments are required).
Methods
Design
A field test was carried out to evaluate the PU-QOL instrument’s response format, scales and items in an independent sample of patients with pressure ulcers. Part of the field test included a mode of administration substudy to provide empirical support for the chosen mode of administration (i.e. interview administered) for the PU-QOL instrument. Initially, the intention was for the PU-QOL instrument to be self-completed; however, pre-testing identified problems with completion rates (see Pre-testing, Results), questioning the appropriateness of a self-completed instrument for patients with pressure ulcers.
Participants
Patients from selected acute and community NHS trusts in England and Scotland were included in the field test and substudy if they were aged ≥ 18 years and were hospital, intermediate care including rehabilitation, nursing home or community patients with an existing pressure ulcer of any category, location or duration and were able to provide informed consent to participate. 223 Patients were excluded if they had only moisture lesions or were unconscious, confused or cognitively impaired, it was deemed ethically inappropriate to approach them (e.g. death was imminent), they did not speak or understand English or they were unable to provide informed consent. To ensure equivalent samples in both administration mode groups, the eligibility criteria were adapted for the substudy to include only patients able to read and write in English (i.e. patients able to self-complete).
Between 200 and 250 patients with pressure ulcers were purposively sampled ensuring balanced representation of patients across ulcer categories (superficial and severe) and location (torso and limb skin sites), settings (acute and community), age (< 70 years and ≥ 70 years) and gender. 223 No formal sample size estimation methods for the evaluation of PRO instruments were found. The ‘rule of thumb’ sample size recommendation for psychometric analyses of new summated scales is five to 10 subjects per item, to reduce the effect of chance. 194,195 Following this recommendation, if we take the longest potential summated scale, assessing pain, which contains 11 items, a 110-patient sample would be required. For the Rasch analysis, a sample of 200–250 patients was sought. This estimate was based on a need for sample selection across the full range of measurement. Sample membership to five class interval groups [i.e. different levels (class intervals) of a person factor, e.g. ulcer severity] of around 50 patients in each group is suggested. 233,237
A subsample (60–100 patients) was recruited to the mode of administration substudy. It was anticipated that up to 100 patients would be required to meet the data requirement for the differential item functioning (DIF) analysis and to account for the likelihood of missing data from the self-complete group. RMT methods are able to provide useful exploratory data in small samples (n > 30). 238
Recruitment and data collection
Consecutive patients were identified and approached to participate in the study by attending clinical teams at participating trusts. Screened patients who were eligible were provided with a study information leaflet and consent form (see Appendix 47). Patients who provided informed consent but who were unable to self-complete the PU-QOL instrument were registered into the field test study. Those who were eligible and able to self-complete the PU-QOL instrument were registered and enrolled into the substudy.
The PU-QOL instrument was administered to all patients by tissue viability team members or the researcher, following the PU-QOL user manual. The user manual was developed to provide information about how to administer the PU-QOL questionnaire and encourage standardisation across administration. It outlines the administration procedure, includes some helpful question and answers and provides scoring information. Patients enrolled to the substudy and randomised to the self-completion group were provided with the PU-QOL instrument and instructed to complete it on their own. Completion took approximately 16 minutes.
Substudy randomisation
Substudy registrants were randomised by telephone on a 2 : 1 basis to either the self-completion or the interview-administered mode of administration. The 2 : 1 ratio was used to account for the likelihood of there be being more missing data from self-completed questionnaires. Randomisation was stratified by age (< 70 and ≥ 70 years) and ulcer severity (superficial vs. severe).
Analytical methods
Conventionally, PRO instruments or rating scales have been developed and evaluated according to traditional psychometric standards derived from CTT. 195,239 CTT comprises a set of principles and related statistical techniques for developing and testing measures (e.g. PRO instruments) to determine how successful they are at estimating unobservable (e.g. HRQoL) variables of interest. 240 However, some concerns have been raised about existing PRO instruments developed according to CTT: they may be cumbersome for respondents, be burdensome for clinical use, not be applicable over the continuum of care or across research settings, suffer from floor and ceiling effects and/or lack a standardised scoring metric to allow comparisons across health conditions. 241–244
More recent advances in psychometrics have seen the development and application of modern psychometric methods such as RMT to supplement traditional approaches to rating scale development. 244 Both RMT and traditional psychometric methods were used to psychometrically evaluate the PU-QOL instrument. Using both methods would allow the selection of scale items that are free of bias, confirm the legitimacy of summing scale items to generate measurements (Rasch) and determine whether or not the measurements produced are valid and reliable in line with proposed US FDA criteria for reliability and validity. 212 Table 72 presents full details of the tests and criteria used in the psychometric evaluation.
| Property | Definition of psychometric property | Criteria Rasch methods | Criteria traditional methods |
|---|---|---|---|
| Data quality – acceptability/data completeness | The extent to which scale items are scored and total scores can be computed. Acceptability determined by data quality; assessed by completeness of item- and scale-level data (percentage of missing data for each item; percentage of people for whom a scale score is computed245) and score distributions (floor/ceiling effects and skew of scale scores) |
|
|
| Scaling assumptions | The extent to which it is legitimate to sum a set of items, without weighting or standardisation, to produce a total score. Summing item scores is considered legitimate when items:
|
|
|
| Item response categories | The extent to which item response categories work in a logical hierarchy reflecting the measurement continuum within the frame of reference of the scale. Respondents with high levels of the trait measured by the scale are expected to endorse high-scoring responses, whereas individuals with low levels would consistently endorse low-scoring responses. Disordered thresholds occur if respondents fail to use the response options in a manner consistent with the level of the trait being measured249 |
|
|
| Targeting | The extent to which the range of the variable measured by the scale matches the range of that variable in the study sample. This involves examination of score distributions at both the item and the scale level within the whole sample and also by disease severity subgroups. Evidence of matched scale-to-sample targeting focused around the scale’s best point of measurement. A well-targeted sample is one in which the person distribution closely matches the item distribution (person locations should be covered by items and item locations should be covered by persons) when they are both calibrated on the same metric scale250 |
|
|
| Reliability | The ability of a measure to yield the same score at each administration, assuming that all things are equal (i.e. true change has not occurred) and the extent to which scale scores are free from random error | ||
| Internal consistency | The extent to which items comprising a scale measure the same construct (e.g. homogeneity of the scale) | ||
| Test–retest reliabilitya | The stability of a measuring instrument; assessed by administering the instrument to respondents on two different occasions and examining the correlation between the test and the retest scores. This indicates the strength of the relationship between scores at baseline and time 2 (retest 2–7 days post baseline administration) |
|
|
| Validity | The extent to which a scale measures what it intends to measure; a scale may be reliable but consistently measure the wrong thing255 (e.g. demonstrating that a set of items intended to measure pain has good reliability merely indicates that the items are getting the same true score but not necessarily tapping into the true pain score240) |
|
|
| Content validity | The extent to which the content (items) of a scale is representative of the conceptual construct that it is intended to measure. Consideration of item sufficiency and the target population is essential, including systematic comparison with existing standards, well-accepted theoretical definitions, expert opinions and interviews with individuals at whom the measure is targeted212 |
|
|
| Construct validity | Indicates the degree to which a measure represents what it is intended to represent | ||
| Within-scale analysis | Evidence that a single entity (distinct construct) is being measured and that items can be combined to form a scale score. Item fit statistics imply that an item is not working as intended in a scale and may be regarded as not measuring the scale’s intended construct. Three-item fit statistics indicate the extent to which observed data (actual responses to scale items) accord with (fit) responses expected for groups of responders across the trait (class intervals): fit residuals, chi-square statistics and item characteristic curves.256 For meaningful interpretation, findings are considered together and in the context of their clinical usefulness as an item set |
|
|
| RMT purports that the aim of scale items is to mark out the construct as a continuum on which people can be measured. Measurement continuum implies that individual scale items are located across a continuum in the same way that the location of individual people is spread across the continuum241 |
|
||
| A problem with the local dependence of items can be found by response dependency. The assumption of local independence implies that once the Rasch factors have been extracted (final scales) no leftover patterns in the residuals should be present. Response dependency is when items are linked in some way such that the response to one item will determine the response to another |
|
||
| Between-scale analysis | |||
| Criterion validity | A special type of construct validity in which stronger hypotheses are made possible by the availability of a criterion or ‘gold standard’ measure |
|
|
| Convergent validitya | Evidence that the scale is correlated with other measures of the same or similar constructs; assessed on the basis of correlations. Correlations are expected to vary according to the degree of similarity between the constructs measured by each instrument. Specific hypotheses are formulated based on the proximity of constructs and predictions tested on the basis of correlations |
|
|
| Discriminant validitya | Evidence that the scale is not correlated with measures of different constructs; assessed on the basis of correlations with measures of different constructs (e.g. age, gender) to determine whether responses are biased by these variables |
|
|
| Known-groups differencesa | The ability of a scale to differentiate known groups; assessed by comparing scores for subgroups known to differ on the construct being measured (significant differences between known groups or difference of expected magnitude). Note: The PU HRQL literature is not well established and therefore was limited for identifying clinical parameters to formulate known groups |
|
|
| DIF (item bias) | A technique for investigating conditional relationships between item response and group membership.260 It is based on the assumption that respondents with a similar knowledge or ability (determined by total scale scores) should perform or respond in similar ways to individual items regardless of characteristics such as gender, age or ethnicity, Groups to be studied are selected based on theoretical considerations about whether or not the construct studied is hypothesised to have the same conceptual meaning across groups. DIF occurs when people from different groups (e.g. gender) with the same latent trait (e.g. pain) have a different probability of giving a certain response to an item |
|
|
| Responsiveness | The ability of a scale to detect clinically significant change following treatment of known efficacy; assessed by within-person change scores from before to after treatment and by calculating an effect size statistic (mean change score divided by the SD of the pre-treatment score) |
|
|
First, RMT methods230 were used to investigate the PU-QOL instrument items within the context of the instrument, response options, appropriateness of item series (i.e. item content, response bias, dimensionality, precision) and question ordering (item fit) to evaluate how well items, scales and response options work to measure what they are intended to measure.
A Rasch analysis, using the Andrich rating scale model,264 was performed using RUMM2030 software (RUMM Laboratory, Perth, WA, Australia), comprising targeting of the sample to items, ordering of response options (i.e. ordering of item thresholds)249 and item-fit statistical indicators (i.e. fit residual and chi square256 and spread of item locations;256,258 see Table 72). PU-QOL data were tested against model expectations and any deviations from model expectations were examined to determine whether or not scale attributes could be improved. Final decisions on item inclusion/exclusion were made according to appraisals of the analyses of the observed data against measurement criteria described in Table 72, and clinical relevance (the extent to which items within proposed scales are clinically cohesive), as opposed to examinations carried out singularly or sequentially.
Response rate, data quality and DIF analyses265 were undertaken to establish measurement equivalence across the two mode of administration groups (self-completion and interview administered). DIF provides a method of exploring conditional relationships between item response and group membership by examining the significance of differences observed between different levels (class intervals) of a person factor (e.g. administration mode group). 260 Groups to be studied are selected based on theoretical considerations about whether or not the construct studied is hypothesised to have the same conceptual meaning across groups.
To determine whether or not the PU-QOL instrument fulfilled fundamental prerequisites for rigorous measurement as defined by traditional psychometric criteria and the US FDA guidance,212 the Rasch model-developed PU-QOL scales underwent a preliminary psychometric evaluation using standard psychometric tests. 212,225,250,254,263,266 The scales were examined for acceptability and data quality, scaling assumptions, targeting, reliability and construct validity against prespecified criteria (see Table 72). As RMT provides a formal method of testing the degree to which rigorous measurement is achieved by PRO scales, use of factor analysis to determine scale structure was not deemed necessary. The psychometric tests were performed using SPSS 15.0 software (SPSS Inc., Chicago, IL, USA).
Ethical approval
The two field tests were approved by the North West Research Ethics Committee prior to data collection (reference number 08/H1010/112).
Results
Sample
A total of 989 patients were screened for participation in the first field test from 21 hospitals, 10 community services and one hospice. Of those screened, eligibility was assessed for 787 (79.6%); 416 were considered eligible (52.9%) and, of those eligible, 285 (68.5%) consented to participate (Figure 29). Cognitive impairment was the main reason for ineligibility (38.8%). Those able to self-complete were included in the substudy (n = 75), with 54 randomised to the self-completion group and 21 randomised to the researcher-administered group; the remaining 210 participants were registered to the main study. The main study analysis population included those able to self-complete and randomised to the researcher-administered group in the substudy (n = 21) plus those registered to the main researcher-administered study (n = 206). The final analysis populations after exclusions included 70 participants in the substudy and 227 in the main study (see Figure 29). Table 73 presents the characteristics of the final analysis samples.
FIGURE 29.
Assessment flow chart for the substudy and field test 1.

| Characteristic | Substudy (n = 70) | Field test 1 (n = 227) | Field test 2 (n = 229) |
|---|---|---|---|
| Age (years), range (mean, SD) | 21–93 (64, 15) | 24–98 (72, 13.5) | 20–103 (71.3, 16.5) |
| Gender, n (%) | |||
| Male | 47 (67.1) | 90 (39.6) | 119 (52.0) |
| Female | 23 (32.9) | 137 (60.4) | 110 (48.0) |
| Ethnicity, n (%) | |||
| White | NA | 223 (98.2) | 227 (99.1) |
| Asian | NA | 1 (0.4) | 2 (0.9) |
| Black/African | NA | 2 (0.9) | 0 |
| Chinese | NA | 0 | 0 |
| Not stated | NA | 1 (0.4) | 0 |
| Setting, n (%) | |||
| Hospital (surgery) | 38 (54.3)a | 99 (43.6) | 62 (27.1) |
| Hospital (medicine) | 21 (9.3) | 74 (32.3) | |
| Community | 32 (45.7) | 107 (47.1) | 88 (38.4) |
| Missing | 0 | 0 | 5 (2.2) |
| Pressure ulcer severity, n (%) | |||
| Category 1 | 40 (57.1)b | 38 (11.3) | 76 (18.1) |
| Category 2 | 144 (42.9) | 170 (40.5) | |
| Category 3/4 | 30 (42.9) | 153 (42.7) | 170 (40.5) |
| Missing | 0 | 1 (0.3) | 4 (0.9) |
| Pressure ulcer risk classification, n (%) | |||
| Short term | NA | 39 (17.2) | 36 (15.7) |
| New medium to long term | NA | 71 (31.3) | 87 (38.0) |
| Ongoing long term | NA | 116 (51.1) | 103 (45.0) |
| Missing | NA | 1 (0.4) | 3 (1.3) |
| Marital status, n (%) | |||
| Single (includes divorced, separated, widowed) | NA | 59 (26.0) | 71 (31.0) |
| Married | NA | 85 (37.4) | 77 (33.6) |
| Cohabiting | NA | 81 (35.7) | 75 (32.8) |
| Missing | NA | 2 (0.9) | 6 (2.6) |
| Living arrangements, n (%) | |||
| Live alone | NA | 84 (37.0) | 86 (37.6) |
| Cohabit with carer | NA | 63 (27.8) | 51 (22.3) |
| Cohabit with other | NA | 61 (26.9) | 48 (21.0) |
| Missing | NA | 19 (8.4) | 44 (19.2) |
| Education, n (%) | |||
| No formal education | NA | 129 (56.8) | 125 (54.6) |
| GCSE or equivalent | NA | 39 (17.2) | 40 (17.5) |
| A-Level or equivalent | NA | 25 (11.0) | 16 (7.0) |
| Degree or higher | NA | 15 (6.6) | 21 (9.2) |
| Missing | NA | 19 (8.4) | 27 (11.8) |
Mode of administration substudy
The substudy provided qualitative and empirical evidence for the selection of the most appropriate administration mode for people with pressure ulcers. Qualitative findings highlighted difficulties with patient self-completion. PU-QOL forms returned with missed items were examined to investigate patterns in missing responses and the following observations were noted. Of the 19 self-completed PU-QOLs with missing data, four respondents wrote ‘n/a’ next to items missed; six completed only one item per scale; five missed items at random; two missed a page; one missed items from only the daily activities scale; and one mostly missed items at the beginning. Of the three researcher-administered PU-QOLs with missing data, one had one item missed and one had two items missed; in the other case the patient asked to stop completing the PU-QOL because of feeling ill, resulting in a large amount of items (n = 26) missed towards the end. No obvious patterns in responses emerged. We concluded that, if length was an issue for missing data, we would expect to see more missing data towards the end of the questionnaire (i.e. when patients became tired or fed up with completing a long questionnaire). However, this pattern was not observed. Rather, patients were not responding to items as instructed, resulting in missing data.
Investigation of response rate and data quality indicated a difference in response rate between administration methods: 90.7% of the self-completed PU-QOL forms compared with 100% of the administered PU-QOL forms were returned, an overall high response rate. A difference was also observed in data quality: a large proportion of PU-QOL forms were returned with missing data in the self-completed group (Table 74), supporting the pre-test findings (see Pre-testing, Results). Inspecting returned PU-QOL forms by setting, missing data were observed mostly on forms that were self-completed by patients in hospital (see Table 74), although preliminary DIF analysis indicated that administration mode did not impact on the way that community patients responded to PU-QOL items, supporting the equivalence of self-completed and interview-administered versions in community populations. The DIF analysis was an important methodological step for highlighting areas warranting further investigation if pursuing a self-completed version in the future. Based on the substudy findings we continued evaluating only a researcher-administered version.
| Characteristic | Self-completed (n = 49) | Administered (n = 21) | Totala (n = 70) |
|---|---|---|---|
| PU-QOL forms with missing data, n (%) | 19 (38.8) | 3 (14.3) | 22 (31.4) |
| Total number of PU-QOL items missed (range 1–87 items per PU-QOL), n (%) | 619 (14.5) | 29 (1.6) | 648 (10.6) |
| Age | |||
| < 70 years | |||
| Number with missing data | 12/25 | 2/14 | 14/39 |
| Number (%) of items missed | 336 (15.5) | 3 (0.3) | 345 (10.2) |
| ≥ 70 years | |||
| Number with missing data | 7/24 | 1/7 | 8/31 |
| Number (%) of items missed | 283 (13.6) | 26 (4.3) | 309 (11.5) |
| Health-care setting | |||
| Acute | |||
| Number with missing data | 16/26 | 2/12 | 18/38 |
| Number (%) of items missed | 604 (26.7) | 28 (2.7) | 632 (19.1) |
| Community | |||
| Number with missing data | 3/23 | 1/9 | 4/32 |
| Number (%) of items missed | 15 (0.8) | 1 (0.1) | 16 (0.6) |
Item reduction and scale formation: Rasch analysis
The Rasch analysis detected important limitations of the PU-QOL scales, resulting in minor modifications. 223 It detected that the four-category item scoring function did not work as intended for one or more items within one or more scales, as demonstrated by disordered thresholds. For the other items, for which the response categories were working as intended, thresholds were close to being disordered, suggesting that people had difficulty distinguishing between the categories ‘a little bother’ and ‘quite a bit of bother’. This provided good evidence that items would benefit from having fewer response categories. Consequently, all scale items were subjected to a post hoc rescoring by collapsing adjacent categories (so that all items had three response categories). Reanalysis of the data demonstrated that all thresholds were now correctly ordered, producing scales with new categories (0 = no bother, 1 = little bother, 2 = a lot of bother).
Another important finding was suboptimal scale-to-sample targeting. 223 There were significant floor effects; the largest frequency of patients was often at the floor of the scale ranges (‘least bother’), suggesting that the scales might provide limited information about people at the extremes of the sample distribution (those with the least disability or impairment). Ideally, there should be a good match between the scale range and the sample range, with people falling within the range of the items. However, the ordering of scale items along each variable was clinically sensible, providing evidence of the construct validity of each scale variable.
Three items had notable criterion failures as defined by a fit residual level outside ±2.5, high chi-square values with significant p-values and adherence to the item characteristic curve (significantly underdiscriminating or overdiscriminating). Few items exceeded residual correlations of +0.3, implying that the responses to items are independent of each other and locally independent, or –0.3, suggesting no redundant items. Departures from item fit expectation were relatively small but when considered in combination resulted in six items being removed (see Appendix 43). Person separation index values indicated good to reasonable reliability for scales distinguishing between responders on each scale variable.
At this stage, items that were considered clinically important but which did not fit into existing scales were retained as single items (e.g. itchiness). Scales that did not meet requirements for reliable and valid measurement were either conceptually combined (e.g. items representing mood, anxiety, autonomy and isolation were combined into an emotional well-being scale) or had items added [e.g. three items, determined from patients transcripts (see Item generation from patients), were added to the vitality and malaise scale to produce a six-item scale], reducing the instrument from 13 to 10 scales. The final scales and items are presented in Appendix 43.
Preliminary psychometric evaluation: traditional analysis
The results of the psychometric evaluation using traditional psychometric tests supported the PU-QOL scales as being reliable and valid measures of pressure ulcer-symptoms, physical and social functioning and psychological well-being. 223 The criteria were satisfied for most psychometric properties evaluated. Briefly, data quality was high (scale scores were computable for 93–99.6% of respondents) and scaling assumptions were satisfied [mostly similar mean item scores; corrected item–total correlation (ITC) ranges 0.525–0.920]. Scale-to-sample targeting was good [scale scores spanned the scale range but were notably skewed for three scales (value outside ±1.0), mean scores were near the scale mid-point for six of nine scales and ceiling effects were negligible; however, floor effects exceeded the 15% criterion for two of nine scales]. Internal consistency reliability was high, as demonstrated by Cronbach’s alpha values (range 0.893–0.962). The ITCs, alpha coefficient and homogeneity coefficient (inter-item correlation mean and range) provide evidence of the internal construct validity of the PU-QOL scales. A full psychometric evaluation was planned for field test 2.
Field test 2: final psychometric evaluation
The second field test was used to perform a full psychometric evaluation of the item-reduced version of the PU-QOL in a large independent sample of patients with pressure ulcers223 (see Appendix 44 for the study protocol).
Aim
The study aimed to provide researchers and clinicians with a comprehensive evaluation of some of the fundamental psychometric measurement properties of the final (10-scale/83-item) PU-QOL instrument, including scale targeting, item response categories, item fit, response bias, acceptability, scaling assumptions, reliability and validity.
Methods
Design
The second quantitative field test was undertaken to carry out a comprehensive psychometric evaluation of the final PU-QOL instrument, in a large independent sample of patients with pressure ulcers. Consistent with methods used in the first field test (see Field test 1 and mode of administration substudy, Design), a Rasch analysis was performed first on all PU-QOL scales, followed by traditional psychometric tests (see Table 72), in line with current US FDA guidance. 212
Participants
The eligibility criteria for the second field test were consistent with those used in the first field test (see Field test 1 and mode of administration substudy, Eligibility).
A total of 200–250 patients with pressure ulcers was purposively sampled, consistent with methods described in Field test 1 and mode of administration substudy (see Sampling). This provided sufficient subjects for test–retest analysis; correlations at levels expected in test–retest situations (r ≥ 0.80) can be estimated with reasonable precision (95% CIs of ±0.1) with relatively few subjects. 267,268
Recruitment and data collection
The recruitment method for the second field test was consistent with that detailed in Field test 1 and mode of administration substudy (see Recruitment and data collection; see also Appendix 48 for the patient information leaflet and consent forms).
A questionnaire pack containing the PU-QOL and the SF-12 was administered to all participants. The SF-12v2 Acute, English (UK) version was used269 to minimise respondent burden. This is a generic measure that asks respondents to rate their health and functioning during the past week on eight domains: physical functioning, role physical, bodily pain, general health, energy/fatigue, social functioning, role emotional and mental health.
A subsample of 50–60 patients completed a second PU-QOL 2–7 days after the first to evaluate test–retest reliability. The test–retest interval had to be short enough to ensure that clinical change in the pressure ulcer was unlikely to occur but sufficiently long so that respondents did not recall their responses from the first administration; a short test–retest interval is necessary to evaluate stability per se, rather than clinical change in the pressure ulcer. The tissue viability team member returned to administer the second PU-QOL to all patients who agreed to the second administration, either on the ward or at their home, within the specified time frame.
Analytical methods
Consistent with methods used in the first field test (see Field test 1 and mode of administration substudy, Analytical methods), both RMT and traditional psychometric methods were used to psychometrically evaluate the final PU-QOL instrument. Table 72 presents full details of the tests and criteria used in the psychometric evaluation.
The Rasch analysis was consistent with methods used during the first field test (see Field test 1 and mode of administration substudy, Analytical methods, and Table 72). Additional tests for person fit and uniform and non-uniform DIF in relation to four clinical subgroupings [age (< 70 years and ≥ 70 years), gender (male and female), ulcer location (torso, limb, both) and health-care setting (hospital and community)] were considered during the final psychometric evaluation (see Table 72).
The final Rasch scales underwent a psychometric evaluation using the same traditional psychometric tests examined during field test 1 (see Field test 1 and mode of administration substudy, Analytical methods) plus additional tests for reliability (test–retest) and validity (convergent and discriminant validity and known-groups differences) (see Table 72).
Missing data were not imputed. The frequency of missing data was determined and items with a response rate of < 90% were investigated.
Results
Sample
In total, 879 patients were screened for study participation, of whom eligibility was assessed for 717; of these, 391 were considered to be eligible of whom 231 consented to participate. The final analysis population was 229 after exclusions (Figure 30). Table 73 presents the characteristics of the analysis sample.
FIGURE 30.
Assessment flow chart for field test 2.

Rasch analysis
The measurement properties of the PU-QOL scales were largely supported as demonstrated by items that mapped out continua of increasing intensity and are located along those continua in a clinically sensible order. Scale items work well together to define single variables, albeit some item misfit, local dependence and items exhibiting DIF were detected. For example, DIF was demonstrated in three items (Table 75); however, the deviations from model expectations were marginal, suggesting that item performance across the four clinical subgroups is stable and that these groups can be measured on a common ruler.
| Scale items | Threshold | Location | FR | CS | p-value | Person–item correlations | DIF age | DIF gender | DIF setting | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Uniform | Non-uniform | Uniform | Non-uniform | Uniform | Non-uniform | |||||||
| Pain scale (n = 180; 4 Class interval; PSI = 0.814) | ||||||||||||
| Uncomfortable | Ordered | –1.104 | –0.771 | 6.527 | 0.089 | < 0.3 | + | + | + | + | + | + |
| Tenderness | Ordered | –1.069 | –0.192 | 3.708 | 0.295 | < 0.3 | + | + | + | + | + | + |
| Annoying | Ordered | –0.670 | –1.817 | 7.508 | 0.057 | < 0.3 | + | + | + | + | + | + |
| Red raw | Ordered | 0.219 | –0.145 | 2.065 | 0.559 | < 0.3 | + | + | + | + | + | + |
| Stinging | Ordered | 0.388 | –0.496 | 2.817 | 0.421 | < 0.3 | + | + | + | + | + | + |
| Burning | Ordered | 0.482 | 0.328 | 0.314 | 0.957 | < 0.3 | + | + | + | + | + | + |
| Throbbing | Ordered | 0.729 | 0.692 | 4.205 | 0.240 | < 0.3 | + | + | + | + | + | + |
| Stabbing | Ordered | 1.024 | 0.519 | 6.468 | 0.091 | < 0.3 | + | + | + | + | + | + |
| Exudate scale (n = 59; 2 Class interval – people with only superficial PUs removed, if retained n = 95; PSI = 0.598) | ||||||||||||
| Dressing off | Disordered | –0.751 | 0.804 | 0.020 | 0.887 | < 0.3 | + | + | + | + | + | + |
| Staining | Ordered | –0.398 | –0.758 | 0.367 | 0.544 | < 0.3 | + | + | + | + | + | + |
| Weeping | Ordered | –0.356 | –0.342 | 0.670 | 0.413 | < 0.3 | + | + | + | + | + | + |
| Sticky | Ordered | –0.266 | –0.129 | 0492 | 0.483 | < 0.3 | + | + | + | + | + | + |
| Messy | Ordered | –0.006 | –1.264 | 4.607 | 0.032 | < 0.3 | + | + | + | + | + | + |
| Running | Ordered | 0.252 | –0.269 | 0.255 | 0.613 | < 0.3 | + | + | + | + | + | + |
| Bleeding | Ordered | 0.683 | 0.552 | 1.493 | 0.222 | < 0.3 | + | + | + | + | + | + |
| Pus | Ordered | 0.843 | 1.566 | 2.599 | 0.107 | < 0.3 | + | + | + | + | + | + |
| Odour scale (n = 21; 2 Class interval – people with only superficial PUs removed, if retained n = 27; PSI = 0.486) | ||||||||||||
| Unpleasant | Ordered | –1.303 | –0.039 | 1.340 | 0.247 | < 0.3 | + | + | + | + | + | + |
| Lingering | Ordered | –0.207 | –0.915 | 2.186 | 0.139 | < 0.3 | + | + | + | + | + | + |
| Pungent | Ordered | –0.187 | 0.330 | 1.195 | 0.274 | < 0.3 | + | + | + | + | + | + |
| Stench | Ordered | 0.047 | –0.566 | 0.273 | 0.602 | < 0.3 | + | + | + | + | + | + |
| Putrid | Ordered | 0.745 | –0.465 | 0.404 | 0.525 | < 0.3 | + | + | + | + | + | + |
| Sickening | Ordered | 0.906 | 0.640 | 2.591 | 0.108 | < 0.3 | + | + | + | + | + | + |
| Sleep scale (n = 133; 3 Class interval; PSI = 0.719) | ||||||||||||
| Comfortable position | Ordered | –0.907 | 0.777 | 0.468 | 0.792 | < 0.3 | + | + | + | + | + | + |
| Sleep in one position | Ordered | –0.058 | 2.639a | 3.239 | 0.198 | < 0.3 | + | + | + | + | + | + |
| Interrupted sleep | Ordered | 0.027 | –1.144 | 9.530 | 0.009 | < 0.3 | + | + | + | + | + | + |
| Not getting amount of sleep needed | Ordered | 0.065 | –1.789 | 13.303 | 0.001a | < 0.3 | + | + | + | + | + | + |
| Kept awake | Ordered | 0.422 | –1.485 | 6.333 | 0.042 | < 0.3 | + | + | + | + | + | + |
| Trouble falling asleep | Ordered | 0.451 | 1.427 | 6.838 | 0.033 | < 0.3 | + | + | + | + | + | + |
| Mobility and movement scale (n = 130; 3 Class interval; PSI = 0.505) | ||||||||||||
| Pushing up to sitting | Ordered | –0.457 | –0.123 | 3.303 | 0.192 | < 0.3 | + | + | + | + | + | + |
| Adjusting in bed | Ordered | –0.349 | –0.832 | 6.928 | 0.031 | 0.498 | + | + | + | + | + | + |
| Difficulty sitting | Ordered | –0.155 | 2.310 | 0.990 | 0.610 | < 0.3 | + | + | + | + | + | + |
| Difficulty turning/moving in bed | Ordered | –0.138 | –0.454 | 3.079 | 0.214 | 0.498 | + | + | + | + | + | + |
| Walking slowed | Ordered | –0.006 | –0.501 | 6.426 | 0.040 | 0.701 | + | + | + | + | 0.00 | + |
| Difficulty standing for long periods | Disordered | 0.165 | –0.060 | 1.008 | 0.604 | < 0.3 | + | + | + | + | + | + |
| Limited in ability to walk | Ordered | 0.168 | 0.198 | 4.790 | 0.091 | 0.701 | + | + | + | + | 0.00 | + |
| Difficulty transferring | Ordered | 0.201 | 0.747 | 0.378 | 0.828 | < 0.3 | + | + | + | + | + | + |
| Limited ability to go up/down stairs | Disordered | 0.572 | 0.475 | 0.954 | 0.621 | < 0.3 | + | + | + | + | + | + |
| Activity scale (n = 95; 2 Class interval; PSI = 0.102) | ||||||||||||
| Regular activities | Disordered | –0.299 | 0.956 | 0.652 | 0.419 | < 0.3 | + | + | + | + | + | + |
| Washing | Ordered | –0.298 | 1.564 | 0.097 | 0.756 | < 0.3 | + | + | + | + | + | + |
| Shopping | Disordered | –0.230 | –1.446 | 1.825 | 0.177 | < 0.3 | + | + | + | + | + | + |
| Toileting | Ordered | –0.125 | 0.962 | 0.055 | 0.815 | < 0.3 | + | + | + | + | + | + |
| Dressing | Ordered | –0.003 | 0.281 | 5.084 | 0.024 | < 0.3 | + | + | + | + | + | + |
| Jobs around house | Disordered | 0.059 | –0.814 | 2.247 | 0.134 | < 0.3 | + | + | + | + | + | + |
| Doing things you enjoy | Ordered | 0.334 | 0.872 | 2.002 | 0.157 | < 0.3 | + | + | + | + | + | + |
| Being emotionally close | Disordered | 0.561 | –0.263 | 1.642 | 0.200 | < 0.3 | + | + | + | + | + | + |
| Vitality scale (n = 98; 2 Class interval; PSI = 0.557) | ||||||||||||
| Tired | Ordered | –0.500 | –0.327 | 0.992 | 0.319 | 0.49 | + | + | + | + | + | + |
| Fatigued | Ordered | –0.493 | –2.177 | 7.987 | 0.005 | 0.49 | + | + | + | + | + | + |
| Energy reduced | Ordered | –0.148 | 0.104 | 0.824 | 0.364 | < 0.3 | + | + | + | + | + | + |
| Unwell/poorly | Ordered | 0.338 | 1.624 | 0.415 | 0.521 | < 0.3 | 0.00 | + | + | + | + | + |
| Appetite reduced | Ordered | 0.804 | 1.133 | 1.164 | 0.281 | < 0.3 | + | + | + | + | + | + |
| Emotional well-being scale (n = 181; 4 Class interval; PSI = 0.846) | ||||||||||||
| Fed up | Ordered | –1.478 | 1.109 | 2.428 | 0.489 | < 0.3 | + | + | + | + | + | + |
| Frustrated | Ordered | –1.055 | –1.298 | 9.373 | 0.025 | < 0.3 | + | + | + | + | + | + |
| Annoyed/irritated | Ordered | –0.673 | 1.542 | 4.816 | 0.186 | < 0.3 | + | + | + | + | + | + |
| Physically dependent | Ordered | –0.598 | 0.558 | 2.208 | 0.530 | < 0.3 | + | + | + | + | + | + |
| Miserable | Ordered | –0.441 | –1.073 | 7.850 | 0.049 | < 0.3 | + | + | + | + | + | + |
| Anxious | Ordered | –0.298 | 1.223 | 7.749 | 0.052 | 0.560 | + | + | + | + | + | + |
| No control | Ordered | –0.120 | –2.261 | 7.078 | 0.069 | < 0.3 | + | + | + | + | + | + |
| Burden/nuisance | Ordered | –0.113 | –0.096 | 3.332 | 0.343 | < 0.3 | + | + | + | + | + | + |
| Concerned/worried | Ordered | –0.104 | 0.795 | 0.719 | 0.867 | 0.560 | + | + | + | + | + | + |
| Angry | Disordered | 0.164 | –0.735 | 4.103 | 0.250 | < 0.3 | + | + | + | + | + | + |
| Missing out | Ordered | 0.223 | –1.209 | 5.481 | 0.140 | < 0.3 | + | + | + | + | + | + |
| Depressed | Ordered | 0.235 | –2.361 | 9.588 | 0.022 | < 0.3 | + | + | + | + | + | + |
| Lonely | Ordered | 0.891 | –0.832 | 1.770 | 0.621 | 0.519 | + | + | + | + | + | + |
| Cut off/isolated | Ordered | 0.926 | –1.345 | 3.520 | 0.318 | 0.519 | + | + | + | + | + | + |
| Others avoided | Disordered | 2.442 | 0.117 | 5.294 | 0.152 | < 0.3 | + | + | + | + | + | + |
| Self-consciousness and appearance scale (n = 100; 2 Class interval; PSI = 0.529) | ||||||||||||
| Helpless | Ordered | –1.268 | –0.517 | 0.784 | 0.376 | < 0.3 | + | + | + | + | + | + |
| Lacking confidence | Ordered | –0.654 | –0.025 | 0.143 | 0.705 | < 0.3 | + | + | + | + | + | + |
| Self-conscious | Ordered | –0.465 | 0.114 | 1.388 | 0.239 | 0.415 | + | + | + | + | + | + |
| Embarrassed | Ordered | –0.290 | 0.077 | 0.731 | 0.393 | 0.415 | + | + | + | + | + | + |
| Feeling physically unattractive | Ordered | 0.727 | –1.131 | 3.061 | 0.080 | < 0.3 | + | + | + | + | + | + |
| Lack understanding from others | Ordered | 0.928 | 1.137 | 4.227 | 0.040 | < 0.3 | + | + | + | + | + | + |
| Uneasy being close to others | Ordered | 1.022 | –0.283 | 1.007 | 0.315 | < 0.3 | + | + | + | + | + | + |
| Participation scale (n = 82; 2 Class interval; PSI = 0.435) | ||||||||||||
| Restricted where you go out | Disordered | –0.912 | –0.962 | 2.095 | 0.148 | < 0.3 | + | + | + | + | + | + |
| Difficulty going out | Ordered | –0.877 | –0.801 | 0.991 | 0.319 | < 0.3 | + | + | + | + | + | + |
| Restricted how you long stay out | Disordered | –0.664 | –0.227 | 0.424 | 0.515 | < 0.3 | + | + | + | + | + | + |
| Holiday/weekend | Disordered | –0.016 | 0.403 | 0.690 | 0.406 | < 0.3 | + | + | + | + | + | + |
| Give up hobbies/leisure | Disordered | 0.188 | –0.387 | 1.193 | 0.275 | < 0.3 | + | + | + | + | + | + |
| Participate family gatherings | Disordered | 0.356 | 0.342 | 0.689 | 0.407 | 0.694 | + | + | + | + | + | + |
| Meeting family/friends | Disordered | 0.428 | 0.116 | 0.156 | 0.693 | 0.694 | + | + | + | + | + | + |
| Plan going out around PU care | Disordered | 0.501 | 0.163 | 0.151 | 0.698 | < 0.3 | + | + | + | + | + | + |
| Time involved caring for PU | Ordered | 0.995 | 0.387 | 0.608 | 0.436 | < 0.3 | + | + | + | + | + | + |
The Rasch analysis detected important limitations of some PU-QOL scales. It detected that the three-category item scoring function did not work as intended for 16 out of 82 scale items (see Table 75). Some item locations indicated areas on the continuum within the scale range measured where the measurement could be improved (i.e. at extreme ends of the scale range). As the sample sizes for these scales were quite small, major modifications to items and the scoring function were deemed premature without additional empirical evidence.
Another limitation pertains to the sample distribution. For most scales the sample was not normally distributed (normal distribution is neither expected nor wanted as sample distribution is an empirical finding rather than a requirement, but it does suggest that assumptions about the distribution of people and the variables measured in populations should not be made). 250 The largest frequency of patients was often at the floor of the scale ranges (‘least bother’), suggesting suboptimal targeting of the PU-QOL scales to the study sample. Ideally, there should be a good match between the scale range and the sample range, with people falling within the range of the items (see Table 75). For the symptom scales, the targeting can be justified as not all patients with pressure ulcers are expected to have problems with symptoms and so it is clinically reasonable that these people would fall outside the scale range. Importantly, when people have symptom bother, there need to be items within the scales that will discriminate symptom bother and, in this instance, the symptom scales perform this function.
Traditional psychometric analysis
The traditional psychometric analysis supported the final PU-QOL scales as being reliable and valid measures of pressure ulcer symptoms, physical and social functioning and psychological well-being. The criteria were satisfied for most psychometric properties evaluated. Briefly, data quality was high (scale scores were computable for 95.6–99.6% of respondents; Table 76) and scaling assumptions were satisfied (mean scale scores and SDs were mostly similar to scale mid-points; Table 77). All item–own scale correlations were high (corrected ITC ranges 0.511–0.940; see Table 77), satisfying the recommended criterion (> 0.3), thus providing support that items within scales measured a common underlying construct (a corrected ITC > 0.3 indicates that items within each scale contain a similar proportion of information).
| Scale | Data completeness | Targeting | ||||||
|---|---|---|---|---|---|---|---|---|
| Computable scale score (%) | Possible score (range)a | Range mid-point | Observed score (range) | Mean score | SD | F/C effect (%)b | Skewness | |
| Pain | 95.6 | 0–16 | 8 | 0–16 | 6.14 | 4.586 | 15.2/3.9 | 0.396 |
| Exudate | 98.3 | 0–15 | 7.5 | 0–15 | 2.09 | 3.494 | 57.0/0.9 | 1.898 |
| Odour | 99.6 | 0–12 | 6 | 0–12 | 0.97 | 2.850 | 83.0/4.3 | 3.144 |
| Sleep | 99.6 | 0–12 | 6 | 0–12 | 4.66 | 4.302 | 10.7/4.1 | 0.434 |
| Vitality | 98.3 | 0–10 | 5 | 0–10 | 2.72 | 3.217 | 27.0/2.2 | 0.896 |
| Mobility | 97.8 | 0–18 | 9 | 0–17 | 7.08 | 5.377 | 1.5/0.4 | 0.362 |
| Daily activities | 95.6 | 0–16 | 8 | 0–14 | 3.67 | 4.389 | 3.9/0.4 | 1.058 |
| Emotional Well-being | 95.2 | 0–30 | 15 | 0–28 | 10.15 | 9.190 | 8.3/1.3 | 0.673 |
| Appearance & self-consciousness | 96.5 | 0–14 | 7 | 0–14 | 2.53 | 3.632 | 38.7/2.2 | 1.566 |
| Participation | 95.6 | 0–18 | 9 | 0–18 | 5.66 | 6.264 | 6.2/0.4 | 0.587 |
| Scale | Internal consistency (Cronbach’s alpha) | SEM | 95% CI | Mean IIC | IIC (range) | Scaling assumptions (corrected ITC) (range) | Test–retest reproducibility | ||
|---|---|---|---|---|---|---|---|---|---|
| ICC consistency | ICC absolute | Correlation | |||||||
| Pain | 0.893 | 0.453 | 6.50 to 8.29 | 0.482 | 0.235–0.663 | 0.525–0.703 | 0.803 | 0.805 | 0.804 |
| Exudate | 0.907 | 0.233 | 1.63 to 2.55 | 0.544 | 0.316–0.715 | 0.511–0.752 | 0.622 | 0.625 | 0.622 |
| Odour | 0.969 | 0.187 | 0.60 to 1.35 | 0.841 | 0.716–0.934 | 0.794–0.940 | 0.681 | 0.680 | 0.700 |
| Sleep | 0.920 | 0.327 | 4.01 to 5.30 | 0.657 | 0.491–0.805 | 0.681–0.846 | 0.822 | 0.816 | 0.824 |
| Vitality | 0.900 | 0.275 | 2.18 to 3.27 | 0.638 | 0.488–0.902 | 0.628–0.898 | 0.735 | 0.738 | 0.736 |
| Mobility | 0.927 | 1.069 | 6.20 to 10.59 | 0.586 | 0.226–0.912 | 0.666–0.799 | 0.873 | 0.864 | 0.879 |
| Daily activities | 0.952 | 1.374 | 4.83 to 10.57 | 0.710 | 0.407–0.904 | 0.583–0.899 | 0.866 | 0.872 | 0.870 |
| Emotional Well-being | 0.934 | 0.931 | 9.71 to 13.42 | 0.486 | 0.242–0.780 | 0.537–0.761 | 0.829 | 0.820 | 0.832 |
| Appearance & self-consciousness | 0.894 | 0.271 | 1.99 to 3.07 | 0.557 | 0.371–0.789 | 0.617–0.755 | 0.812 | 0.814 | 0.814 |
| Participation | 0.932 | 0.719 | 4.23 to 7.09 | 0.601 | 0.359–0.877 | 0.599–0.861 | 0.627 | 0.639 | 0.634 |
Scale-to-sample targeting was good, apart from where skew was clinically reasonable [scale scores spanned the scale range but were notably skewed for four scales (value outside ±1.0), mean scores were near the scale mid-point for 6 out of 10 scales and ceiling effects were negligible; however, floor effects exceeded the 15% criterion for 4 out of 10 scales; see Table 76]. Internal consistency reliability was high, as demonstrated by Cronbach’s alpha values for all PU-QOL scales exceeding the standard criterion of 0.7 (range 0.893–0.969; see Table 77). ITCs ranged from 0.511 to 0.940, fulfilling the recommended criterion of > 0.3. Finally, test–retest correlations for eight out of 10 scales exceeded 0.7 (see Table 77); two scales had correlations below the recommended criterion, but marginally, thus mostly fulfilling the recommended minimum criteria and indicating good scale stability.
Evidence of the internal construct validity of the PU-QOL scales is provided by moderate to high ITCs, high Cronbach’s alpha values and moderate to high inter-item correlations (means > 0.48 and ranges between 0.226 and 0.934 indicate that PU-QOL scale items were mostly correlated with scale scores; see Table 77), indicating that each scale measures a single construct. Correlations between PU-QOL scales and hypothesised related scales of the SF-12 were consistent with most predictions (Table 78), providing support for PU-QOL scales measuring what they intend to measure; moderate to high correlations (r > 0.30) were predicted. Correlations between PU-QOL scales and sociodemographic variables (age, gender) were consistent with predictions (r < 0.30; see Table 78), thus suggesting that responses to PU-QOL scales are not biased by age or gender.
| Scale | Convergent validity | Discriminant validity | Known groupsc – PU severityd | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SF-12 PF scale ra | SF-12 SF scale ra | SF-12 RF scale ra | SF-12 MH scale ra | SF-12 pain item ra | SF-12 fatigue item ra | PU-QOL pain item ra | PU-QOL QOL item ra (n) | Gender rb (n) | Age rb (n) | Mean score (n) | p-value (95% CI) | |
| Pain | –0.03 | –0.08 | –0.13 | –0.04 | 0.48e,f | 0.06 | 0.79f | 0.38f (206) | 0.13f (214) | 0.11f (214) | 0.895 | |
| Category 1 | 5.36 (14) | (2.85 to 7.86) | ||||||||||
| Category 2 | 5.81 (77) | (4.78 to 6.83) | ||||||||||
| Category 3/4 | 5.51 (68) | (4.49 to 6.54) | ||||||||||
| Exudate | – | – | – | – | – | – | – | 0.25g (216) | 0.08f (225) | –0.14f (224) | 0.000h | |
| Category 1 | 0.64 (14) | (–0.43 to 1.72) | ||||||||||
| Category 2 | 1.07 (81) | (0.55 to 1.60) | ||||||||||
| Category 3/4 | 3.26 (72) | (2.31 to 4.21) | ||||||||||
| Odour | – | – | – | – | – | – | – | 0.20g (217) | 0.05f (228) | –0.14f (227) | 0.004h | |
| Category 1 | 0.07 (14) | (–0.08 to 0.23) | ||||||||||
| Category 2 | 0.28 (82) | (–0.05 to 0.61) | ||||||||||
| Category 3/4 | 1.60 (72) | (0.77 to 2.43) | ||||||||||
| Sleep | – | – | – | – | – | – | – | 0.32f (171) | 0.21f (178) | 0.10f (178) | 0.774 | |
| Category 1 | 4.89 (9) | (1.36 to 8.42) | ||||||||||
| Category 2 | 4.49 (65) | (3.41 to 5.57) | ||||||||||
| Category 3/4 | 4.02 (54) | (2.84 to 5.20) | ||||||||||
| Vitality | –0.24e | –0.12 | –0.37e | –0.01 | 0.37e | 0.36f | – | 0.52f (135) | 0.03f (137) | –0.16f (137) | 0.036h | |
| Category 1 | 1.22 (9) | (–0.40 to 2.84) | ||||||||||
| Category 2 | 1.82 (50) | (1.02 to 2.62) | ||||||||||
| Category 3/4 | 3.25 (48) | (2.26 to 4.24) | ||||||||||
| Mobility | –0.49e | –0.30 | –0.45e | –0.10 | 0.40h | 0.49e | – | 0.39f (37) | 0.04f (39) | 0.22f (39) | 0.137 | |
| Category 1 | 5.00 (4) | (–1.62 to 11.62) | ||||||||||
| Category 2 | 4.36 (11) | (1.94 to 6.79) | ||||||||||
| Category 3/4 | 8.31 (13) | (4.82 to 11.80) | ||||||||||
| ADL | –0.39h | –0.36h | –0.389e | –0.17 | 0.53e | 0.14 | – | 0.35f (48) | –0.05f (49) | –0.19f (49) | 0.094 | |
| Category 1 | 1.60 (5) | (–0.66 to 3.86) | ||||||||||
| Category 2 | 1.73 (11) | (–1.06 to 4.51) | ||||||||||
| Category 3/4 | 4.63 (24) | (2.81 to 6.44) | ||||||||||
| EWB | –0.21h | –0.25e | –0.37e | –0.44h | 0.29e | 0.38e | – | 0.58f (133) | 0.16f (135) | –0.15f (135) | 0.001h | |
| Category 1 | 4.13 (8) | (1.39 to 6.86) | ||||||||||
| Category 2 | 7.41 (46) | (4.98 to 9.84) | ||||||||||
| Category 3/4 | 13.28 (47) | (10.39 to 16.16) | ||||||||||
| ASC | –0.22e | –0.30e | –0.29e | –0.40h | 0.32e | 0.23e | – | 0.50f (176) | 0.23f (179) | –0.03f (178) | 0.014h | |
| Category 1 | 0.92 (12) | (–0.42 to 2.26) | ||||||||||
| Category 2 | 1.85 (62) | (1.02 to 2.68) | ||||||||||
| Category 3/4 | 2.52 (58) | (2.43 to 4.71) | ||||||||||
| Social participation | –0.34e | –0.46e | –0.38e | –0.07 | 0.03 | 0.26h | – | 0.51f (75) | 0.01f (76) | –0.29f (76) | 0.018h | |
| Category 1 | 3.67 (6) | (–1.55 to 8.88) | ||||||||||
| Category 2 | 2.55 (22) | (0.43 to 4.66) | ||||||||||
| Category 3/4 | 7.35 (31) | (4.84 to 9.87) | ||||||||||
The scales for exudate and odour were able to differentiate known groups as predicted; a significant step increase in mean score by pressure ulcer severity groups was observed (see Table 78). All other tests of known group differences were considered exploratory; significant step increases were observed in scores for the scales measuring vitality, daily activities, emotional well-being and self-consciousness when patients were grouped by pressure ulcer severity. In contrast, no step increase in scores was observed for the scales measuring pain, sleep, mobility and movement and participation. For all scales apart from the sleep scale, the mean score on HRQoL outcomes for category 1 ulcers was lower than that for category 3/4 ulcers, suggesting that HRQoL outcomes are worse for people with severe ulcers than for those with superficial category 1 ulcers. It is important to note that the category 1 ulcer sample sizes were small (range 4–14 patients) and therefore the known groups validity results are preliminary and further empirical evidence is required to have confidence that the PU-QOL scales can detect small differences in the constructs being measured.
Final Pressure Ulcer Quality of Life instrument
The final version of the PU-QOL consists of 10 scales (82 items) measuring symptoms, physical functioning, psychological well-being and social participation specific to pressure ulcers [see Appendix 43; the final PU-QOL instrument can be accessed at http://medhealth.leeds.ac.uk/puqol-ques (accessed July 2015)]. An additional item is included to assess pain severity. There are three symptom scales measuring pain (eight items plus one descriptive pain severity item), exudate (eight items) and odour (six items) and one descriptive itchiness item; four physical functioning scales measuring sleep (six items), movement and mobility (nine items), daily activities (eight items) and vitality (five items); two psychological well-being scales measuring emotional well-being (15 items) and self-consciousness and appearance (seven items); and one social participation scale (nine items). Patients rate the amount of ‘bother’ attributed (e.g. ‘During the past week, how much have you been bothered by . . . because of your pressure sore?’) on a 3-point response scale (0 = no bother; 1 = little bother; 2 = a lot of bother). In addition, respondents are given an alternative response option enabling them to state that they are affected by the issue described in the item but that it is not related to their ulcer (‘I have this problem but not because of my pressure ulcer’); this is treated as descriptive information and is not part of the scale score. Scales can be selected for use depending on the nature of the research and scale items can be summed to produce scores, without weighting or standardisation. Scores for each domain are calculated as the sum of each individual item score within that scale, which is then converted to a metric of 0–100; a lower score indicates better outcome. Imputation of missing data, based on methods undertaken for scoring the Short Form questionnaire-36 items (SF-36),270 can be undertaken, provided that at least 50% of items are complete for an individual.
The PU-QOL instrument is appropriate for use in adults across the range of levels of pressure ulcer severity and type (location and duration) and UK health-care settings and is suitable for group comparison. It is intended for interview administration, supported by a user manual providing practical information necessary for administration and scoring. The PU-QOL instrument and user manual are freely available through the University of Leeds CTRU website [see http://medhealth.leeds.ac.uk/puqol-ques (accessed July 2015)].
Discussion
This research established the impact of pressure ulcers on HRQoL, determined the need for a pressure ulcer-specific PRO instrument and developed and psychometrically evaluated such an instrument. In our work predating the PURPOSE programme we systematically reviewed the HRQoL literature in the pressure ulcer field and found that it is mainly qualitative with an emphasis on pain and physical functioning impairment rather than a comprehensive exploration of issues that are important to patients. 9 Potential sources of bias arise because of the use of small sample sizes (n ≤ 10) and under-representation of people with superficial ulcers, the elderly (aged > 70 years) and those acutely ill or with various comorbidities. Some HRQoL outcomes that are unique to pressure ulceration were highlighted but these outcomes are currently not systematically included as outcomes in clinical trials. Therefore, the pressure ulcer literature is unconvincing in terms of robust evaluation of the impact of pressure ulcers and treatments on HRQoL (i.e. quantitative studies designed to explore HRQoL in pressure ulceration had used measures not developed or validated for use with patients with pressure ulcers). 216 Further, no pressure ulcer-specific PRO instruments exist,216 highlighting the need for outcome measures that can accurately depict the impact of pressure ulcers on HRQoL.
The PU-QOL instrument was developed to provide a formal method for capturing issues that are most important to patients with pressure ulcers from their perspectives. New instrument construction needs to be underpinned with a strong conceptual base to ensure valid measurement, one that adequately defines the variables and relationships conceptually and gives operational meaning that guides the development (or selection) of PRO instruments. 212 Building on our earlier work9 we found that pressure ulcers impact all aspects of HRQoL, severely compromise patient functioning and cause significant burden, pain and increased discomfort as a result of treatment. 36,221 We developed a pressure ulcer-specific conceptual framework of HRQoL that includes conceptual domains for symptoms; difficulty with range of movement and mobility; limitations in daily activities; psychological functioning; and ability to participate socially. 221 These constructs are similar to those in generic HRQoL models;206,271,272 however, our framework incorporates additional components specific to pressure ulceration (e.g. symptoms; appearance and self-consciousness). The development of our conceptual framework was hampered by the poor quality and quantity of the existing literature9 and required further qualitative interviews with patients and consultation with experts. Elucidation of conceptual domains that are important to patients with pressure ulcers provides a useful framework for designing future research and consequently improving the quality of research in the pressure ulcer field by inclusion of a pressure ulcer-specific PRO instrument.
Mixed methods, including feedback from patients through cognitive interviews and a Rasch analysis of PU-QOL data, were effective for identifying problems with PU-QOL items early in the development process. Overall, patient input was the most important element of the development process. Despite undertaking two systematic reviews of the literature (pressure ulcer pain and existing chronic wound PRO instruments) intended to generate items, only a few descriptive words for pressure ulcer-related pain were added to the item pool; the majority of the item content was generated from patient interviews. The empirically derived pressure ulcer-specific conceptual framework informed the development of the PU-QOL instrument.
Scale development and item reduction were primarily guided by RMT, which provided a vehicle for the detection of items deviating from model expectations with the intention of improving scale attributes. Final decisions on item inclusion were made according to appraisals of the analyses of the observed data against measurement criteria and clinical relevance, as opposed to examinations carried out singularly or sequentially. A preliminary evaluation of the Rasch-produced scales using traditional psychometric methods supported the PU-QOL instrument as being reliable and valid. In addition, an empirical investigation of the optimal mode of administration of the PU-QOL instrument revealed that self-completion was not suitable for patients with pressure ulcers. Consequently, the mode of administration was changed to interview administered to ensure that the PU-QOL instrument would be applicable to a wider range of people with pressure ulcers and potentially yield higher-quality data.
The final psychometric evaluation supported the PU-QOL scales as being valid and reliable for use in clinical trials according to US FDA criteria;212 however, some important limitations were identified. The Rasch analysis highlighted that further work is required before the PU-QOL scales can be used as the main PRO measure in future clinical trials or other research. Measurement precision could be improved by developing items that span a wider measurement range and, in the process, maximising the potential of the PU-QOL instrument to detect change. The appropriateness of the PU-QOL instrument for use in individual decision-making requires further investigation. The measurement precision may need strengthening to enable assessment of individual patients in clinical practice (e.g. revisit the qualitative work to add items to extend the measurement at the floor/ceiling scale range; further work to ensure that the PU-QOL scales differentiate between different levels of ulcer severity). Longitudinal studies should be undertaken to assess the responsiveness of the PU-QOL instrument over time and following treatment, as clinical studies evaluating the effectiveness of various ulcer treatments and interventions require accurate detection of true change. Further research is also needed to investigate self-completion and electronic (e.g. ePRO) mode of administration methods, the feasibility of use in specific subgroups, support for score interpretation and the utility of the PU-QOL instrument in routine practice; to develop proxy measures and language translations given the prevalence of cognitively impaired patients with pressure ulcers (e.g. almost 40% of screened patients in field test 1 and 30% in field test 2 were ineligible because of cognitive impairment, consistent with findings from the pain prevalence study;48 see Chapter 3); and to carry out an economic evaluation (see Chapter 7).
The final PU-QOL instrument consists of independent scales for assessing symptoms and physical, psychological and social functioning specific to pressure ulcers. The PU-QOL instrument can be included as one outcome measure among others in future pressure ulcer research on the proviso that studies have built in a parallel psychometric analysis to indicate the performance (psychometric evaluation) of the scales in future samples. Currently, the PU-QOL scales are most appropriate for patients with severe pressure ulcers, as demonstrated by a lack of items to represent people with little or no bother as a result of pressure ulcers. The exudate and odour scales are not intended for people with superficial ulcers (category 1).
The PU-QOL instrument provides a means to comprehensively assess the impact of pressure ulcers and a way of quantifying the benefits of ulcer interventions. It may also provide a key to discussions between health-care providers and patients about impact that are currently not being held. Patients in our interviews repeatedly reflected on their relationship with their tissue viability nurse, stating that the issues discussed during our interviews were not issues that they had previously discussed with their nurse. The lack of inclusion of HRQoL outcomes in previous pressure ulcer research is supported by the literature. Thus, PU-QOL data could facilitate patient–health-care provider communication and increase understanding of the impact of pressure ulcers on individuals, which ultimately could lead to adjustments in care delivery to meet patient needs. They may also highlight important patient-orientated differences between interventions to justify resource allocation. This is particularly important for changing practice through mandated NICE guidance. Specifically, the perceived value of pressure ulcer interventions and evaluating PROs associated with treatment and relative burden must have a robust evidence base212,224,273 to help inform decisions about the most appropriate ulcer management, policies and health-care delivery in the pressure ulcer field.
Methodological issues and study limitations
This is the first outcome measure specific to pressure ulcers, reflecting the domains in a pressure ulcer-specific conceptual framework of HRQoL outcomes, content that differs from that for other chronic wounds. However, this study had some limitations. In the qualitative phase of PU-QOL development, in-depth interviews were used to develop and refine the content of the scales. Additional qualitative methods such as focus groups and interviews with the carers of people with pressure ulcers may have provided a further opportunity to combine findings. Further, the questioning was intended to elicit the worst aspect of having a pressure ulcer. This line of questioning may have resulted in valuable information about patient experiences when pressure ulcer symptoms and other aspects are managed well being missed. Better use of qualitative questioning would have resulted in the inclusion of patients with healed or close to healed pressure ulcers, asking them asked about the entirety of their experience, with more thought given to covering the full spectrum of the pressure ulcer experience (e.g. experience of when treatment was effective and when pressure ulcer impact was milder/not at its worst and words to describe the benefit/ulcer improvement). This may have helped to improve the measurement range by including items that represented milder ulcer impact/bother.
The validity testing of the PU-QOL scales was limited, in part by a lack of appropriate validating measures and the inability to formulate hypotheses to enable known group difference testing. The literature is limited about the roles that pressure ulcer severity, duration and location play in affecting HRQoL outcomes. Such gaps in knowledge limit the ability to develop strong hypotheses to evaluate known group validity. Further, the SF-12v2 is a generic measure that has not been developed or validated for use with people with pressure ulcers. Given the uncertainty about the appropriateness of the SF-12 for use with people with pressure ulcers, this was included in the validation process on an exploratory basis.
Pressure Ulcer Research Service User Network UK
Pressure Ulcer Research Service User Network UK members were involved in reviewing the different versions of the PU-QOL instrument and associated materials throughout the development process. One of the considerations when developing the PU-QOL user manual was ensuring that the instrument itself is used in a way that is acceptable to patients. With that in mind, PURSUN UK members were invited to review the manual. To help facilitate this review process, the researcher set a series of questions designed to help guide people through a fairly complex document.
Members were also asked to give general comments about the PU-QOL instrument itself. Comments made highlighted:
-
the importance of reassuring patients that there is no right or wrong answer and that their perception is what is important
-
areas that could be clearer (e.g. instructions)
-
the importance of anonymity for this particular population and the need to be clear about how the results of the instrument will be used.
Throughout the review process, the perspective of PURSUN UK has been balanced with the need to adhere to international guidelines on the development of outcome measures. This required an open dialogue between members and the researcher. PURSUN UK members said that they valued the fact that the researcher was honest about aspects that could not be changed and gave clear reasons for this rather than simply disregarding their input.
Conclusion
This study makes important contributions to the pressure ulcer and wider health measurement fields. The PU-QOL instrument provides a means to comprehensively assess pressure ulcer impact and quantify the benefits of pressure ulcer interventions from the patient perspective for research use, thus far lacking in this area. Scientifically rigorous PRO measurement needs to become more commonplace in the pressure ulcer field so that the goal of pressure ulcer management can be to enhance and maintain the HRQoL of people with pressure ulcers. Subject to further development, the PU-QOL instrument is a tool that can be used to evaluate whether or not pressure ulcer treatments and the health care given achieve this, outcomes that are ultimately best judged by patients themselves. Future use of the PU-QOL instrument will provide the data necessary for its further development.
Chapter 7 Deriving a preference-based measure for use in cost–utility analyses of pressure ulcer interventions
Chapter written by David M Meads, Carolyn Czoski Murray, Claudia Rutherford, Carol Dealey, Elizabeth McGinnis, Nikki Stubbs, Lyn Wilson, Jane Nixon, Claire T Hulme and Christopher McCabe.
Abstract
Introduction: Cost–utility analysis has become the gold standard for economic evaluation. In some therapeutic areas where the use of the European Quality of Life-5 Dimensions (EQ-5D) is found to be inappropriate, condition-specific utility measures are developed with the aim of providing a more accurate assessment of the impact of conditions and to provide a more sensitive measure of the benefit of interventions. The aim of this study was to create a preference-based index from the PU-QOL instrument that could be used to generate utility values suitable for use in cost–utility-based economic evaluations.
Methods: The methods employed to achieve this followed those used to value the three-level EQ-5D. Specifically, we conducted time trade-off task valuations of health states derived from selected PU-QOL items with a sample (n = 200) of the general population. A secondary study was conducted to validate the item selection and assess the psychometrics of the new index.
Results: Seven items were selected from the PU-QOL instrument for inclusion in the index on the basis of best practice psychometric and Rasch methods. Of the large number of potential health states constructed from the items and response option variants, 52 were valued by the general population with the remaining health state values being predicted using ordinary least squares and random-effects regression models. Although both models exhibited satisfactory predictive power and acceptably low levels of error, the random-effects model is recommended for use. The secondary study analysis indicated that item selection for the PUQOL-UI was appropriate and that the index was acceptable to patients and had adequate levels of validity.
Conclusions: The PUQOL-UI is a seven-item instrument that will complement the PU-QOL instrument and will deliver pressure ulcer-specific utility values for use in cost–utility analysis.
Introduction
In this chapter we report the design, implementation and analysis of a valuation study for the PUQOL-UI, undertaken to facilitate the use of the PU-QOL instrument (see Chapter 6) as an outcome measure in clinical trials of pressure ulcer prevention and treatment interventions, by supporting its use in cost-effectiveness analyses. The chapter starts by providing some background on cost-effectiveness analysis for health-care resource allocation and the use of generic compared with condition-specific utility measures (CSUMs) in this context. It then goes on to describe the PU-QOL instrument, the process of identifying a short-form version and additional changes to the response format used in the PU-QOL, to develop a measure that it would be feasible to use in a health state valuation study. This is followed by a description of the design and implementation of the health state valuation study and analysis of the data obtained to produce a health state utility algorithm for the PU-QOL instrument. The fourth chapter component describes a study that was conducted to test the PUQOL-UI item selection and provide an assessment of the psychometric properties of the measure. The strengths and weaknesses of the research and priorities for further work are outlined in the discussion.
Cost-effectiveness analysis for health-care resource allocation
Cost–utility analysis has become the gold standard for economic evaluation in many countries as it allows a combined evaluation of the cost of health technologies along with a measure of the quality and survival benefits that they confer. Another important advantage of cost–utility analysis is that it allows the comparison of technology cost-effectiveness across therapeutic areas. The most common way to capture health state utility values for use in cost–utility analysis is to employ multi-attribute utility questionnaires. Such multi-attribute utility instruments as the EQ-5D,274 Short Form questionnaire-6 Dimensions (SF-6D)275 and Health Utilities Index (HUI)276 are commonly included in clinical studies for this purpose. Utility (or ‘preference-based’) measures typically produce a range of values from 1, representing full or perfect health, through 0, representing death, to minus infinity, with negative values denoting health states considered worse than death. These utility value weights are combined with survival information to calculate quality-adjusted life-years (QALYs), which are the basis of cost–utility analysis. One year spent in a health state of full or perfect health (utility = 1) is equal to 1 QALY.
In its updated health technology appraisal guidance, NICE273 states that health effects for cost-effectiveness analyses should be expressed in terms of QALYs and that the EQ-5D is the preferred utility measure on which QALYs should be based. As different utility measures may yield substantively divergent utility values for the same individual, the use of a common metric and tool with which to measure it is necessary to ensure that comparative analyses and resource allocation decisions across therapeutic areas (and time) are possible.
European Quality of Life-5 Dimensions three-level version
The EQ-5D-3L (EQ-5D three-level version) includes five domains (questions): mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each question has three response options (‘no problems’, ‘some problems’ and ‘extreme problems’) and utility values range from 1 (full health) to –0.59, based on a tariff scoring system generated in the UK. 277 Although the EQ-5D is quick and relatively easy to complete, its lack of breadth may mean that it is not a suitable instrument for capturing health effects in some populations. NICE acknowledges this and states that alternatives can be used if there is evidence that the EQ-5D lacks content or construct validity or responsiveness in the target population. These shortcomings may be present in patients with pressure ulcers. A qualitative examination of the impact of pressure ulceration on patient quality of life (see Chapter 6, Qualitative study)221 suggests that there are a number of domains of relevance to patients that are omitted from the EQ-5D. These include ulcer smell and exudate, general malaise, fatigue and sleep impairment and a significant emotional and social impact encompassing self-consciousness and social isolation. Furthermore, at least one item is inappropriate for a proportion of people with pressure ulcers, specifically those using wheelchairs. Typically, people with pressure ulcers have an antecedent condition that impairs mobility, which can then lead to the development of ulcers. A potentially high proportion of this group will use wheelchairs and this subgroup of patients may find it difficult to answer the EQ-5D mobility question (with response options ‘I have no problems walking about’, ‘I have some problems walking about’, ‘I am confined to bed’). Although wheelchair users have significant problems walking, they are not confined to bed; thus, the item is invalidated for this group. There are a number of qualitative references highlighting this issue278–280 although the EuroQol group (understandably) does not permit revisions to the measure to make it more acceptable in this group. 279
The EQ-5D has rarely been employed in published studies including patients with pressure ulcers281,282 and an adequate test of the validity of the measure in this group has not yet been conducted. Although there are few data available on the performance of the EQ-5D in pressure ulcer studies, there is evidence that the EQ-5D is unresponsive to health status change in general wound care. For example, Jull and colleagues283 found that the measure could not distinguish between a group of venous leg ulcer patients whose wounds had and had not healed. Although a new five-level version of the EQ-5D has now been developed, it includes additional response categories and not domains and as such is unlikely to confer better measurement scope in this group than the three-level version.
Condition-specific utility
As a result of limitations of the EQ-5D in some therapeutic areas, a number of CSUMs have been developed from existing condition-specific quality of life measures. These include measures in cancer,284 incontinence,285 asthma,286 mental health,287 dementia288 and pulmonary hypertension. 289 The main aim of these CSUMs is to provide a more accurate assessment of the impact of conditions and a more sensitive measure of the benefit of interventions. Although there is a dearth of evidence to corroborate the promise of CSUMs in this regard, the growth in their numbers continues.
Reducing the incidence of pressure ulcers and improving outcomes for those who experience them are key targets for the NHS. 17 To facilitate this there is an identified need to conduct clinical studies of interventions and to establish the value of these interventions. 14,15 However, in view of the potential limits of the EQ-5D as a tool for capturing the health effects of pressure ulcers, there is a clear argument for the development of a CSUM for use in this population. No CSUM currently exists for use in pressure ulcer prevention and care. Alternative sources of utility values are limited to other generic preference-based measures or a predictive algorithm developed using a generic preference-based measure and clinical information,290 which would not meet the standard of NICE reference case data. 273,291 This chapter describes the generation of a CSUM from the newly developed PU-QOL instrument (see Chapter 6). The PU-QOL instrument allows a comprehensive assessment of the impact of pressure ulcers on patients’ HRQoL, providing information to help improve patient health care and patient HRQoL and a tool for use in intervention effectiveness research. It is already in use in clinical studies. It is likely that a CSUM, based on the items within the PU-QOL, would provide a more appropriate, valid and sensitive assessment of patients’ preferences associated with pressure ulcers than a generic utility measure and would therefore be a more useful tool in cost-effectiveness analysis of associated interventions.
Aim and objective
The aim of this PURPOSE programme work package was to derive a preference-based utility index (PUQOL-UI) from the PU-QOL, enabling the collection of utility values from the PU-QOL and therefore the calculation of QALYs for the purpose of economic evaluation. We also sought to conduct a preliminary validation of the PUQOL-UI.
Research overview
Two studies were conducted in this work package: study A (valuation study) involved the identification of items for inclusion in the PUQOL-UI and the completion of a general population valuation study followed by modelling to identify the PUQOL-UI scoring tariff; study B involved the collection of data from patients using a revised (attribution-free) form of the PU-QOL that would allow verification of item selection and assessment of the psychometric properties of the PUQOL-UI.
Valuation study: general population survey
The generation of a CSUM requires that health states comprising subsets of items from the target measure are ‘valued’ using preference elicitation techniques (such as the TTO method). Although debate continues about who is the most appropriate source of such values, NICE273 currently prefers these to come from the general population rather than from patients with the condition in question. This section describes the valuation survey and regression modelling used to generate the PUQOL-UI (see Appendix 49 for the study protocol).
Aim and objectives
The aim of the valuation study was to derive a preference-based utility index (PUQOL-UI) from the PU-QOL, enabling the collection of pressure ulcer-specific utility values. Specific objectives were to:
-
test the acceptability of a revised form of the PU-QOL with a small group of patients
-
select PU-QOL items for inclusion in the PUQOL-UI
-
design and conduct a valuation survey with the general population
-
conduct analysis to derive the PUQOL-UI scoring tariff.
Methods
The Pressure Ulcer Quality of Life instrument
The PU-QOL instrument is a multidimensional measure of the impact of pressure ulcers on HRQoL (see Chapter 6 for a full description of the PU-QOL instrument and details on the development work). It includes 83 items and consists of 10 domains covering pain, exudate, odour, sleep, vitality, mobility/movement, ADL, emotional well-being, self-consciousness and appearance and participation outcomes and a single descriptive item on itchiness. Each item has a recall period of ‘the past week’ and three response options: ‘no bother at all’, ‘a little bother’ and ‘a lot of bother’. In addition, respondents are given an alternative response enabling them to state that they are affected by the issue described in the item but that it is not related to their ulcer (‘I have this problem but not because of my pressure ulcer’). The items and domains were identified on the basis of extensive interviews with patients followed by appropriate field testing. The results of these stages indicate that the PU-QOL instrument has face, content and construct validity and provides a reliable and comprehensive assessment of HRQoL in this group.
The incorporation of the ‘attribution’ question format was driven by a desire to create a measure that could be used within the individual patient consultation as well as for measuring change at the patient population level. It was also motivated by the fact that pressure ulcers tend to be a corollary of an underlying health condition (which impairs patient mobility) and a desire for pressure ulcer impact not to be subsumed by patients’ responses to their general health status (see Chapter 6, Preliminary PU-QOL construction, for a full justification of this approach). The fact that the PU-QOL instrument asks respondents to focus on the impact associated with their pressure ulcer, requesting that they disregard their general health level, represented a methodological challenge to the generation of the utility index.
The standard approach to health state valuation locates health states described in the descriptive system (the PU-QOL instrument in the current case) on the health utility scale using methods such as TTO and standard gamble. It assumes that all relevant health attributes (i.e. those that will impact on the value attached to health) are captured in the HRQoL descriptive system. Attributable condition-specific measures, such as the PU-QOL instrument, explicitly set aside attributes of health that the respondent does not consider to be impacted by the health condition of interest and thus are not a disaggregation of global health but a disaggregation of one component of global health – those aspects of health that they currently consider to be affected by the condition of interest [the pressure ulcer(s) in the current case].
To relate the information provided by the attributable condition-specific measure to global health in which the QALY scale is anchored, it is necessary to specify the relationship between the condition-specific measure descriptors and the other domains of health that impact on the value attached to a health state. When the domains of health in the attributable condition-specific measure are completely independent of the other domains of global health, then obtaining values for attributable health states would produce estimates of the decrement from full health as a result of the ‘attributable condition-specific health status’ – in this case the pressure ulcer(s). Thus, a valuation of the PU-QOL in its original format would deliver values representing utility decrements associated with pressure ulcer(s) but not the starting point of the individual on the utility scale. To deal with this issue we created a separate, revised version of the PU-QOL instrument question stem, removing the ‘because of your pressure ulcer’ attribution, and removed the response option ‘I have this problem but not because of my pressure ulcer’.
Study design
Figure 31 (A: valuation study) outlines the stages required for the generation of the utility index. It was first necessary to verify the acceptability of the revised PU-QOL instrument, then select a subset of items for the utility index, generate the health states and conduct the valuation study. The valuation study methods followed those employed in the UK EQ-5D measurement and valuation of health study;277 specifically, we used the TTO task in a general population sample. Study B (described in Validation study) generated data enabling both the psychometric testing of the new utility index and verification of item selection.
FIGURE 31.
Schematic of study design.
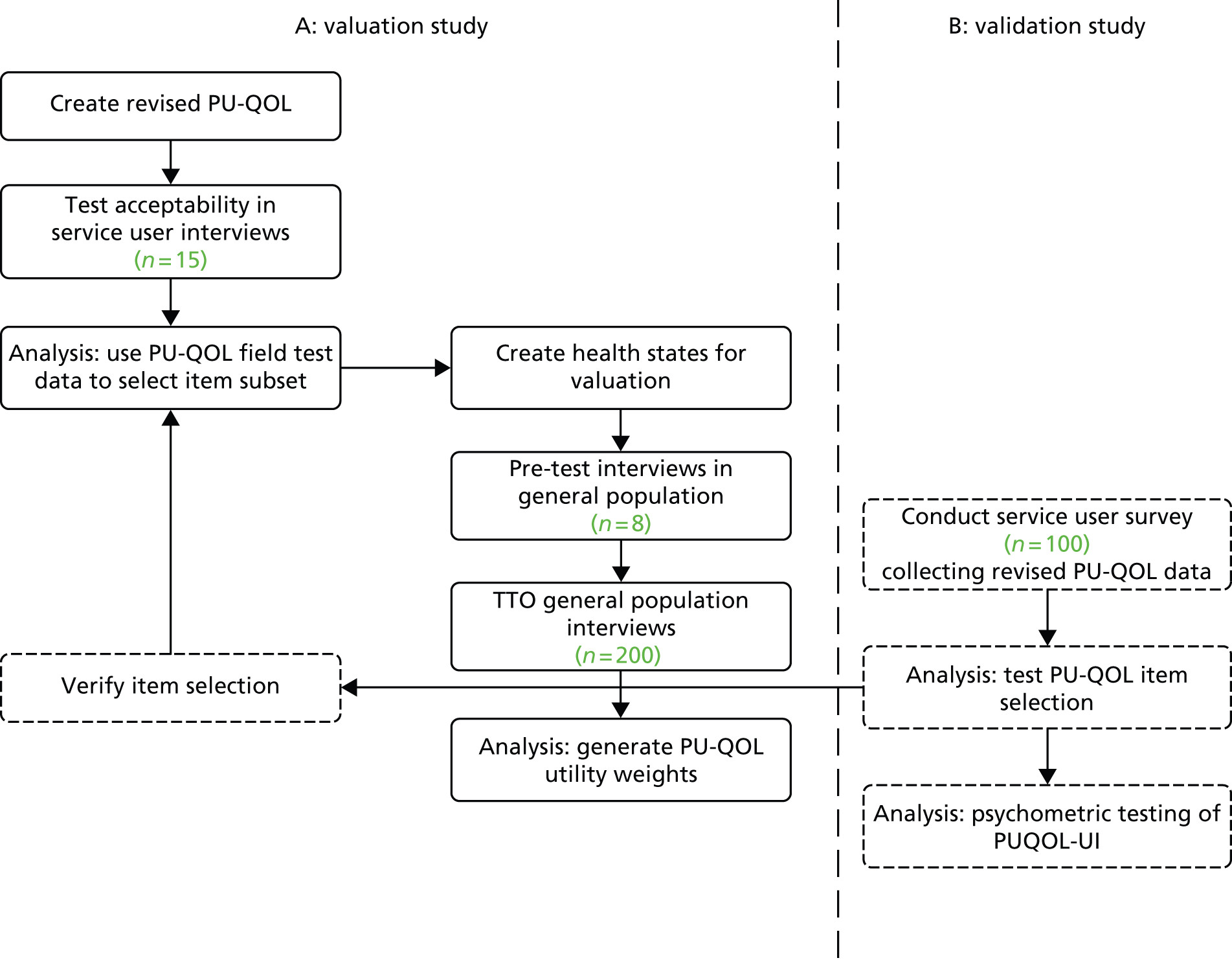
Acceptability of the revised Pressure Ulcer Quality of Life instrument
Before the health states were generated for the valuation interviews, it was necessary to check the acceptability of the revised PU-QOL instrument with a small group of people who have experience of pressure ulcers. This involved conducting a small number (n = 16) of semistructured, face-to-face interviews with people who currently had or had experienced an ulcer. Participants were recruited through local support groups and PURSUN UK members and were interviewed by an experienced qualitative researcher (see Appendix 50 for the study information leaflet). Participants completed the revised PU-QOL instrument and were then asked whether or not they found the instrument easy to understand and complete and whether or not there were aspects or questions that they found confusing. Participants were asked specifically whether or not the new question stem (after the removal of the attribution) made sense in each domain. The interview responses were analysed by two qualitative researchers who came to a consensus about the acceptability of the reviewed measure and any necessary changes.
Item selection
To generate the health states for valuation it was necessary to identify a reduced version of the instrument as the original PU-QOL instrument had too many potential health states (i.e. n = 384) for direct valuation to be feasible. Table 79 compares the domains included in the PU-QOL instrument and EQ-5D, highlighting those that could be considered to overlap and those that are unique. The aim of the item reduction process was to select a number of items that captured both the important generic health impact of pressure ulcers and the antecedent health condition and also the more nuanced, condition-specific health impacts associated with pressure ulcers. However, this had to be balanced with how much information is presented in the health states as overburdening valuation survey respondents would lead to poor data quality. Creating a CSUM also requires achieving balance between the inclusion of nuanced items, capturing elements not captured by generic measures, and equally including only items that are important to patients (considering the impact of concurrent health conditions) and that members of the general population would be willing to trade time to ameliorate.
| PU-QOL domain | Number of items | EQ-5D overlap |
|---|---|---|
| Pain | 9 | ✗ |
| Exudate (leakage) | 8 | |
| Odour | 6 | |
| Sleep | 6 | |
| Mobility | 9 | ✗ |
| ADL | 8 | ✗ |
| Vitality | 5 | |
| Emotional well-being | 15 | ✗ |
| Self-consciousness and appearance | 7 | |
| Social functioning/participation | 9 | ✗ |
| Total | 82a |
The reduced form was constructed using the methods reported by Brazier and Rowan292 for the development of a number of CSUMs – including the Asthma Quality of Life – 5 Dimensions instrument293,294 – and followed best practice recommendations. This involved secondary analysis of the PU-QOL field test 2 data set (see Chapter 6, Phase 3: field testing) and the use of traditional psychometric and Rasch analyses to identify a reduced PU-QOL instrument (of between seven and 10 items) that incorporated important condition-specific dimensions of the full version. It was necessary to ensure that the items selected represented a range of severity (i.e. not all mild or all severe items), fitted the Rasch model, were valid and had good discriminatory power. Table 73 describes the data set used for the analysis, generated during field test 2 of the PU-QOL instrument. In field test 2, patients also completed global items on health and the SF-12v2 health status measure, which was converted to SF-6D utility values. 275 Along with these data, information was collected on ulcer category and care setting and demographics. As the PU-QOL instrument is a new instrument there were no available data on its ability to detect change over time (i.e. responsiveness). However, cross-sectional analysis of pressure ulcer category provided a proxy for this desirable attribute.
Analysis
Rasch measurement theory295 is a valuable tool for the development and refinement of instruments and has several advantages over CTT, such as factor analysis250,296–298 (see Chapter 6, Pre-testing, Data analysis for additional discussion). A Rasch analysis is often employed to identify reduced forms of instruments that will be used in preference valuation studies. 288,299,300 The Rasch model is a special case of the latent-trait item response theory model and places response data for each individual and each item on the same spectrum of severity (logit scale). According to the model, the probability that an individual will respond in a certain way to a particular item is a logistic function of the relative distance between the item location (parameter) and the person location (parameter), and only a function of these two factors. Persons and items are plotted on the same logit scale on the basis of the difference in their location on the underlying spectrum. This difference governs the probability of the expected response for a person, of a given severity, on a question of a given severity. If the observed data do not deviate significantly from the expected responses, then the items fit the Rasch model. The Rasch analysis and traditional psychometric analysis criteria used for item selection are listed in the following sections. A similar method of item selection was employed in the original development and refinement of the PU-QOL instrument, although here we also used factor analysis.
Rasch analysis
-
Degree of fit to the Rasch model – chi-square probability and fit residual (items with non-significant chi-square values and residuals < ±2.5 are candidates for selection).
-
Differential item functioning based on age and gender such that bias by these factors is minimised (items with no DIF are candidates for selection).
-
Item logit position on each construct’s measurement continuum such that items with a range of severity (spanning the entire measurement range) can be identified (items that collectively represent a wide spread of the latent trait are candidates for selection).
-
Ordered response category thresholds (items with correctly functioning response categories are candidates for selection).
Traditional psychometric analysis
-
Distribution of scores and presence of floor/ceiling effects (items with no floor/ceiling effect are candidates for selection).
-
Item–total correlation (items with an ITC of > 0.4 are candidates for selection).
-
Principal components factor analyses (items having a moderate to high factor loading within a subscale are candidates for selection).
-
Ability to discriminate between pressure ulcer severity groups – t-tests for superficial compared with severe ulcer patient scores (highly discriminatory items are candidates).
-
Pearson correlations with SF-6D and a global PU-QOL item [‘How would you rate your overall quality of life because of your pressure sore(s)’] (items with moderate to high correlations are candidates).
An initial item selection process sought to identify a candidate item from each of the 10 existing PU-QOL instrument domains. A second selection iteration sought to ensure a balance between scope (number of items) and feasibility of valuing the resultant health states, removing items that were not considered of sufficient importance. Results from these analyses were considered together with qualitative information about the relative importance of each item and domain. Members of the PURPOSE team considered the candidate items before reaching a consensus on the items to be included in the PUQOL-UI.
Analyses were conducted in SPSS and RUMM2030.
Valuation
Selection of health states for valuation
The design of health state valuation studies is not currently informed by definitive experimental design theory, but rather by good practice conventions. In line with these conventions, we selected a set of core health states (n = 37) for use in the valuation study by constructing an orthogonal array for the PUQOL-UI health state space. This array included the full health state of the PUQOL-UI, which automatically takes the value of 1.0 in the TTO method used for the study, so that one fewer states (n = 36) are valued. This orthogonal array was then supplemented with additional states to ensure broad coverage of the health state space described by the PUQOL-UI. To this end, we also selected the following candidate states: the corner states (n = 7) of the descriptive system; the inverse corner states (n = 7); and the PITS state (n = 1), in which each domain of the PUQOL-UI is at level 3 (‘a lot of bother’). The corner states are those in which one domain is at the most severe level and all others are at the least severe. In contrast, the inverse corner states place one domain at the lowest level of severity and set all others at the highest level of severity. All states were reviewed for face validity/feasibility and a conventional ‘backing-off’ procedure was used to identify a feasible health state (i.e. the fewest dimension changes possible were implemented). In the backing-off procedures, five additional health states were created. These were 2222222, 2222122, 2122222, 3332332 and 3232333, where level 1 refers to ‘no bother’, level 2 to ‘a little bother’ and level 3 to ‘a lot of bother’.
For the purposes of validating the estimated utility models an additional small sample of states was identified using a second orthogonal array. Eight states observed in the second orthogonal array but not in the original array were chosen at random for inclusion in the valuation survey, but not in the estimation sample. In addition to the 51 states already included, this produced a total of 59 states to be valued in the valuation survey.
There is no definitive guidance on the number of observations per state required to estimate utility models, with substantial variation observed in published studies. 274,275,301 Informed by work by one of the authors of the current study, we chose a target of 25 observations per health state on the grounds that the central limit theorem indicates that 25 observations is sufficient to provide a robust estimate of the mean value. This figure is considerably less than that in the UK measurement and valuation of health study277 but more than that in the UK SF-6D valuation study.
On the basis of experience with previous valuation studies and piloting of the interview schedule (see Appendix 51) it was decided to elicit no more than nine valuations from each respondent, to ensure that respondent burden did not impair the quality of the data collected. Surveys were undertaken in 15 distinct geographical regions in the counties of East, West and South Yorkshire and Lincolnshire in the UK. Health state descriptions were randomly allocated across eight batches – four batches consisted of eight health state cards, with the remaining batches using nine health state cards.
Preference elicitation
Preference elicitation was conducted in interviews with a sample (n = 200) of the general population. Individuals were recruited door-to-door and offered a £5 voucher as a ‘thank you’ for participating. Consent was gained from the individuals before they completed the interviews. Interviews were delivered in the home, face-to-face, with responses input directly into a laptop. The screen guided respondents through the tasks, adapting to their responses. However, the interviewer was present to make sure that the respondents understood the task and to answer any queries.
The interview consisted of four sections. In the first section, basic socioeconomic information about the respondent was collected and the respondent was asked to complete the EQ-5D questionnaire. The second section required the respondent to rank the health state cards in the allocated batch, plus their own health and ‘dead’, from ‘worst’ to ‘best’. Subsequently, they were asked to locate each of these states on a VAS between 0 (‘dead’) and 100 (‘full health’), such that the score and the difference between scores represented how good or bad they felt a health state was and how much better or worse it was than the health states below and above it on the scale.
The third section of the interview entailed a TTO valuation for each of the states (eight or nine plus one ‘test’ state) in the allocated batch using a laptop-based TTO sliding scale as a visual prop. The TTO was based on that used in the measurement and valuation of health study,277 with a 10-year life expectancy. 274 The smallest time increment allowed by the script and prop was 1 month.
The TTO task is a standard economic technique to elicit individuals’ strength of preference for various health states. 302 In the TTO task, individuals choose between two certain options: full length of life (10 years, after which they die) in the health state to be valued or a shorter period in ‘full health’ (after which they die). The amount of time (months, years) to be spent in full health is varied until the respondent can no longer easily decide which option they prefer (the point of indifference), signalling the end of the exercise. The final utility value assigned to the health state being valued is given by the time spent in full health divided by the time spent in the health state (in this case 10 years). Thus, if the respondent was indifferent between living for 5 years in full health and living for 10 years in the health state being presented, the utility of that health state would be 5/10 = 0.50. The ‘ping-pong’ technique was employed to vary the amount of time in full health offered. The upper anchor employed in this study was ‘full health’ rather than ‘perfect health’ (as used in the measurement and valuation of health study278). The former is a more realistic and imaginable proposition. We presented the best pressure ulcer health state as the upper anchor, which states ‘no bother’ on all dimensions, as a representation of ‘full health’. Figure 32 shows a screenshot from the TTO survey.
FIGURE 32.
Screenshot of the TTO interview question. Reproduced with permission from Accent London, London, UK.
The formulation of the TTO for health states worse than death was that proposed by Torrance and colleagues:302 if a respondent felt that they would rather die immediately than live for any amount of time in a given health state, the survey adapted to offer them a choice between immediate death and an amount of time in the health state being valued followed by the remaining time in full health. The longer the amount of time in full health required to compensate the poor health state, the worse the health state being valued is perceived. The utility value in this case is calculated as follows:
In line with best practice, respondents were given information regarding pressure ulceration so that they based their interview responses on informed preferences. We chose not to include pictorial information on ulcers as it was difficult to identify pictures of pressure ulcers that corresponded with the full range of health states being valued.
The fourth section of the interview asked the interviewer to record how well he or she considered the respondent had understood the interview exercises, particularly the TTO questions. All interviews were carried out by experienced interviewers who had previous experience of using the computer-based TTO prop. In addition, the interviewers received additional training from one of the authors with substantial experience of undertaking health state valuation interviews. The responses to the ranking, scaling and TTO questions were automatically captured by the computer-based prop and communicated directly to the independent research company that carried out the interviews on behalf of the study investigators. The survey company was responsible for ensuring that recruitment was stratified such that the sample was representative (with consideration of gender, age, ethnicity and income) of the general population. The interviews lasted around 50 minutes. University ethical committee approval was gained before the survey began.
Analysis: modelling the health state valuations
The aim of the study was to construct a model that would predict the health state values for all 2187 states in the PUQOL-UI descriptive system from the measured health state data on the 52 health states in the estimation sample.
The standard model structure for a statistical inference health state valuation model is:
where i = 1,2,. . .,n represents the individual health states of the descriptive system and j = 1,2,. . .,m represents the individual respondents to the survey. Uij is the TTO valuation for health state i provided by respondent j. These valuations are modelled as a function of the sum of two items: xij, the vector of dummy variables for each response level on each domain of the descriptive system, and rij, a vector of interaction terms (which also depend on the levels of different domains). Finally, εij is the error term. Aside from the function g, the only remaining parameters requiring estimation relate to the coefficients on the dummy variables (β) and on the interaction terms (θ).
Using the PUQOL-UI, level 1 (‘no bother’) as the baseline for each domain so that the vector xi contains 14 dummy variables that indicate when each of the seven domains take one of the other two levels (level 2 ‘a little bother’ and level 3 ‘a lot of bother’). The dummy variables take on a value of 1 when the health state being valued includes that domain at the specified level and a value of 0 otherwise. For a simple linear model, the intercept represents the value of full health and the value of any impaired health state is calculated by summing the coefficients of the ‘on’ dummy variables and subtracting this from 1.0.
Regression analysis of health state preference data has tended to estimate either ordinary least squares regression (assumes independent error terms have a zero mean and constant variance) or random-effects regression. The assumption for ordinary least squares regression means that the 1500 observations from 200 respondents are treated as though they were provided by 1500 distinct respondents. By contrast, the random-effects model acknowledges that the error term may not be independent of the respondent and thus separates out a within-respondent and between-respondent error term, that is:
where uj is a respondent-specific variation and is random across individuals and eij is the residual error term for valuation i by individual j.
We report person-level main-effects models for the ordinary least squares and random-effects specification. The models are assessed according to the standard goodness of fit tests; the sign and logical ordering of parameters; and the predictive performance measured using the mean absolute error statistic, both within the estimation sample and for the validation sample.
Patient and public involvement
The research plan was presented to members of PURSUN UK at a meeting in January 2012. At that meeting the proposed valuation interviews were discussed in detail. PURSUN UK members expressed some concerns about the ability of the general public to imagine that they had a pressure ulcer (as required by the NICE273 reference case TTO methods). This resulted in a dialogue about the need to balance the views of people with specific health-related experiences with those of the general public. Two members of the network volunteered to contribute to the patient information sheets, interview schedules and study protocols in more detail. Members of PURSUN UK who currently had or who had previously experienced a pressure ulcer were also involved in testing the revised PU-QOL instrument interviews.
As with the PU-QOL study, there was a need to balance the input of service users with accepted methods and guidelines in this field. Again, we found that the best way to manage this was to have an open and honest conversation with the group. Once it became apparent that more development work was needed (i.e. identifying a short-form version of the PU-QOL and additional changes to the question format), the input of PURSUN UK members proved invaluable. Members not only were able to try out and provide feedback on the new instrument, they also helped to recruit more participants to the study through their networks.
Results
Acceptability of the revised Pressure Ulcer Quality of Life instrument
Sixteen people were included in the interview testing of the revised PU-QOL instrument. All interviewees had previous experience of at least one pressure ulcer, lasting from 1 week up to 8 months, and three had a pressure ulcer at the time of the interview. The age of the sample ranged from 27 to 66 years (mean age 57 years) and 10 (62.5%) of the interviewees were male.
In general, the revised instrument was well received and the removal of the attribution aspect of the instrument caused no issues for comprehension or completion. However, some specific issues were raised about the instrument. For example, some respondents had difficulty deciding whether they had ‘a lot of bother’ or ‘no bother at all’ with carrying out some of the described activities either because they never did them or because someone else always did them for them. A few of the interviewees felt that the ordering of the different questionnaires was important. In cases in which both (attribution-free) generic and condition-specific measures with attribution were used, generic measures should be completed before condition-specific measures to avoid confusing respondents.
We were interested in whether or not the use of ‘bothered’ as a description was helpful and whether or not other terms such as ‘affected’ would be more suitable. The respondents either were indifferent or expressed positive approval of the current terminology.
Item selection
For the purposes of the item reduction analyses, the PU-QOL items were scored as follows: ‘no bother’ = 0, ‘a little bother’ = 1 and ‘a lot of bother’ = 2. For the Rasch analyses, when respondents reported that they ‘have this problem but not because of their pressure sore’, scores were recoded to ‘0’. This was to ensure that these respondents were not excluded from the analysis. However, as the Rasch analysis excludes extreme scores, a reduced sample was encountered regardless. For the traditional psychometric analyses (except where indicated), respondents’ scores on these items were coded as ‘missing’; this was to ensure that items were selected that reflected the greatest impact of pressure ulceration (without dilution of the general health considerations). As the current version of the PU-QOL instrument asked patients to think only about the impact of their pressure ulcer on aspects of their quality of life, there were significant floor effects in some domains (especially the non-pressure ulcer-specific domains), making item selection challenging.
Table 80 provides the correlations between the PU-QOL subscales and the global item, ‘How would you rate your overall quality of life because of your pressure ulcer?’ The lowest correlations were observed with the most obvious condition-specific scales: odour and exudate (leakage). The highest correlations were observed with the emotional well-being, participation and malaise/vitality scales. This finding suggests that the two most obvious pressure ulcer-specific dimensions have a weak impact on overall HRQoL whereas the emotional impact appears to be more significant. The implications for item selection were that we considered combining items from the odour and exudate domains to augment the response data and impact and that the inclusion of two items from the emotional well-being scale might be warranted. Given less weight in the selection process, but of interest nevertheless, were the correlations between the PU-QOL subscales and the SF-6D (Table 81), which exhibited a similar pattern as the correlations seen with global HRQoL.
| PU-QOL subscales | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pain | Exudate | Odour | Sleep | Mobility | Daily activities | Malaise/vitality | Emotion | Self-consciousness | Participation | |
| Correlation coefficient | 0.386a | 0.251a | 0.177a | 0.354a | 0.414b | 0.344b | 0.543a | 0.576a | 0.493a | 0.527a |
| p-value | 0.000 | 0.000 | 0.009 | 0.000 | 0.011 | 0.017 | 0.000 | 0.000 | 0.000 | 0.000 |
| n | 206 | 216 | 217 | 166 | 37 | 48 | 135 | 133 | 176 | 75 |
| PU-QOL subscales | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pain | Exudate | Odour | Sleep | Mobility | Daily activities | Malaise/vitality | Emotion | Self-consciousness | Participation | |
| Correlation coefficient | 0.386a | 0.251a | 0.177a | 0.354a | 0.414b | 0.344b | 0.543a | 0.576a | 0.493a | 0.527a |
| p-value | 0.000 | 0.000 | 0.009 | 0.000 | 0.011 | 0.017 | 0.000 | 0.000 | 0.000 | 0.000 |
| n | 206 | 216 | 217 | 166 | 37 | 48 | 135 | 133 | 176 | 75 |
Table 82 provides the item-by-item performance on the Rasch and traditional psychometric selection parameters. In general, few items suffered low factor loadings in factor analysis and none had ITCs below 0.4. Substantial floor effects were observed on items in several scales (i.e. exudate, odour, daily activities and self-consciousness). In the pressure ulcer-specific dimensions, it is possible that this was a result of the item issue not causing a lot of bother (hence scored ‘0’).
| PU-QOL scale and items | Low PCF loading | Floor effect ≥ 50%a | Discriminate between PUsb | R – global itemc | R – SF-6Dc | Rasch misfit (p < 0.01) | Rasch residual ±2.5 | Disordered threshold | DIF sex (p < 0.01) | DIF age (p < 0.01) |
|---|---|---|---|---|---|---|---|---|---|---|
| Pain | None | None | None | None | None | |||||
| Feeling uncomfortable | N | 0.318d | –0.295d | 0.008 | ||||||
| Tenderness | N | 0.277d | –0.238d | |||||||
| Annoying pain or discomfort | N | 0.373d | –0.269d | |||||||
| Red raw | 58 | N | 0.239d | –0.193e | ||||||
| Stinging | N | 0.294d | –0.173e | |||||||
| Burning | 61 | N | 0.188d | –0.178e | ||||||
| Throbbing | 55 | N | 0.290d | –0.255d | ||||||
| Stabbing pains | 68 | N | 0.211d | –0.302d | ||||||
| Tingling | 60 | N | 0.235d | –0.156e | 0.000 | |||||
| Exudate (leakage) | None | None | None | None | None | |||||
| Weeping | 71 | N | 0.191d | –0.151e | ||||||
| Staining | 73 | Y | 0.274d | –0.148 | ||||||
| Messy | 78 | Y | 0.191d | –0.166e | ||||||
| Causing dressing to come off | 79 | Y | 0.183d | –0.185e | Y | |||||
| Running | 84 | Y | 0.160e | –0.175e | ||||||
| Sticky | 78 | Y | 0.161e | –0.058 | ||||||
| Bleeding | 83 | Y | 0.142e | –0.015 | ||||||
| Pus | 89 | N | 0.089 | –0.071 | Y | |||||
| Odour | None | None | None | |||||||
| An unpleasant smell | 84 | Y | 0.171e | –0.116 | ||||||
| A stench or stink | 90 | Y | 0.179d | –0.157e | ||||||
| A pungent smell | 89 | Y | 0.185d | –0.144 | ||||||
| A lingering smell | 90 | Y | 0.179d | –0.149 | ||||||
| A sickening smell | 91 | N | 0.171e | –0.087 | ||||||
| A putrid smell | 91 | N | 0.186d | –0.176e | ||||||
| Sleep | None | None | None | |||||||
| Trouble finding a comfortable position | N | 0.234d | –0.227d | |||||||
| Having to sleep in one position (e.g. your back or side) | N | 0.268d | –0.193e | 2.64 | ||||||
| Not getting the amount of sleep that you needed | 55 | N | 0.285d | –0.204e | 0.001 | |||||
| Interrupted sleep (e.g. restless sleep or being woken up during your sleep) | 52 | N | 0.296d | –0.256d | 0.009 | |||||
| Being kept awake | 61 | N | 0.248d | –0.239d | ||||||
| Trouble falling asleep | 61 | N | 0.243d | –0.254d | ||||||
| Mobility | None | None | ||||||||
| Feeling that your walking was slowed down | Y | 0.249e | –0.474d | |||||||
| Feeling limited in your ability to walk | N | 0.242e | –0.526d | |||||||
| Difficulty adjusting yourself in bed | N | 0.381d | –0.369d | |||||||
| Difficulty turning or moving around in bed | N | 0.410d | –0.318d | |||||||
| Difficulty pushing up to a sitting position | N | 0.370d | –0.441d | |||||||
| Feeling limited in your ability to go up and down stairs | 73 | N | 0.241 | –0.223 | Y | |||||
| Difficulty standing for long periods | 57 | Y | 0.211 | –0.538e | Y | |||||
| Difficulty sitting (e.g. sitting up in bed or a chair) | N | 0.327d | –0.219e | |||||||
| Difficulty transferring (e.g. from a bed to a chair or to a car) | N | 0.324d | –0.311d | |||||||
| Daily activities | None | None | None | |||||||
| Being able to wash yourself in your usual way (e.g. hand wash, bath, shower) | 69 | Y | 0.287d | –0.334d | ||||||
| Doing shopping | 73 | Y | 0.259e | –0.566d | Y | Y | ||||
| Doing your regular daily activities (e.g. work, volunteering, religious service, clubs, university) | 71 | Y | 0.325d | –0.332d | Y | Y | ||||
| Being able to go to the toilet | 66 | N | 0.381d | –0.351d | ||||||
| Doing jobs around the house (e.g. cooking, housework, DIY) | 71 | N | 0.228 | –0.384d | Y | |||||
| Getting dressed or undressed | 65 | N | 0.327d | –0.283d | ||||||
| Doing things that you enjoy (e.g. reading a book, watching a movie, using a computer) | 81 | N | 0.367d | –0.366d | ||||||
| Being emotionally close or affectionate with loved ones (e.g. able to cuddle, being intimate) | 89 | N | 0.394d | –0.380d | Y | |||||
| Vitality/malaise | None | None | None | |||||||
| Feeling that your appetite has reduced | 76 | N | 0.340d | –0.437d | Y | |||||
| Feeling that your energy levels have been reduced | 55 | Y | 0.418d | –0.372d | ||||||
| Feeling tired | 51 | Y | 0.453d | –0.350d | ||||||
| Feeling fatigued | 52 | N | 0.467d | –0.335d | ||||||
| Feeling unwell or poorly | 69 | N | 0.533d | –0.351d | Y | |||||
| Emotional well-being | None | |||||||||
| Feeling fed up | Y | 0.406d | –0.323d | Y | ||||||
| Feeling frustrated | Y | 0.380d | –0.366d | |||||||
| Feeling annoyed or irritated | Y | 0.312d | –0.276d | Y | ||||||
| Feeling miserable | Y | 0.462d | –0.413d | |||||||
| Feeling physically dependent on others | Y | 0.479d | –0.396d | |||||||
| Feeling concerned or worried | 53 | Y | 0.419d | –0.257d | Y | |||||
| Feeling anxious | Y | 0.461d | –0.417d | Y | ||||||
| Feeling depressed | 62 | Y | 0.428d | –0.358d | ||||||
| Feeling like you have no control over your life because of your sore | 60 | Y | 0.500d | –0.348d | ||||||
| Feeling like a burden or nuisance to others | 56 | Y | 0.458d | –0.460d | Y | |||||
| Feeling angry | 70 | Y | 0.439d | –0.318d | Y | |||||
| Feeling like you were missing out | 63 | Y | 0.426d | –0.422d | ||||||
| Feeling lonely | 73 | Y | 0.387d | –0.401d | Y | |||||
| Feeling cut off or isolated from others | 75 | Y | 0.389d | –0.403d | Y | |||||
| Feeling that people avoided you or treated you differently now | 91 | Y | 0.234d | –0.282d | Y | |||||
| Self-consciousness and appearance | None | None | None | None | None | |||||
| Feeling helpless | 60 | Y | 0.508d | –0.462d | ||||||
| Lacking in confidence | 69 | Y | 0.414d | –0.427d | ||||||
| Feeling self-conscious | 72 | N | 0.422d | –0.393d | ||||||
| Feeling embarrassed | 75 | N | 0.319d | –0.336d | ||||||
| Feeling physically unattractive | 83 | N | 0.312d | –0.366d | ||||||
| Feeling uneasy being close to or around other people | 85 | Y | 0.288d | –0.312d | ||||||
| Feeling a lack of understanding from those close to you | 84 | Y | 0.243d | –0.232d | Y | |||||
| Social functioning/participation | None | None | None | |||||||
| Being restricted to where you could go out | Y | 0.476d | –0.446d | Y | ||||||
| Being unable to get away for a holiday or make a trip at the weekend | 62 | Y | 0.388d | –0.418d | Y | |||||
| Having to give up on hobbies or leisure activities | 65 | Y | 0.403d | –0.434d | Y | Y | ||||
| Difficulty going out | Y | 0.430d | –0.583d | |||||||
| Being restricted in how long you could stay out | 58 | Y | 0.356d | –0.411d | Y | |||||
| Having to plan going out around pressure sore care | 79 | Y | 0.305d | –0.303d | Y | |||||
| The amount of time involved in caring for your sore | 79 | Y | 0.211d | –0.302d | Y | |||||
| Being unable to participate in family gatherings or activities | 75 | Y | 0.440d | –0.385d | ||||||
| Difficulty meeting up or seeing family and/or friends | 73 | Y | 0.404d | –0.406d | Y |
In the more generic dimensions this was probably because the issue was not attributed to a pressure ulcer and was therefore scored ‘0’/coded missing – useable scores for the daily activities and mobility scales were very low for this reason. Notably, a number of items did not discriminate between ulcer categories (superficial vs. severe). All of the pain and sleep scale items, most mobility items, around half of the daily activities items and half of the vitality items failed to discriminate between groups. It is not clear why this would be the case although it is possible that some clinical features of superficial pressure ulcers may render the experience of them indistinguishable from the experience of severe pressure ulcers. Chapter 3 discusses why pain intensity may not be associated with ulcer category. For the mobility and daily activities scales, this finding may be an artefact of the low average scores because of the floor effects.
In general, most scales and items fit the Rasch model well, with few items exhibiting significant chi-square values, high residuals or DIF. However, there were exceptions, with the pain scale having three misfitting items, the emotional well-being scale incurring some DIF and the participation scale exhibiting a relatively high number of disordered thresholds.
The items selected based on qualitative and quantitative assessments are included in Table 83 along with the justification for selection. Two items were included from the emotional dimension as the analysis indicated that this was one of the most significant impacts of pressure ulcers. Given that these 11 candidate items would result in a possible 177,147 health states (311) for valuation, we explored ways to reduce or combine items further. We combined the exudate and odour item into one joint item; however, further item reduction was indicated (Table 84).
| PUQOL-UI item number | PU-QOL scale/dimension | Original PU-QOL item number and description | Justification for item inclusion |
|---|---|---|---|
| 1 | Pain | 3. Annoying pain or discomfort |
|
| 2 | Exudate | 10. Weeping from your pressure ulcer |
|
| 3 | Odour | 18. An unpleasant smell from your pressure ulcer |
|
| 4 | Sleep | 27. Interrupted sleep (e.g. restless sleep or being woken up during your sleep) |
|
| 5 | Mobility | 31. Difficulty adjusting yourself in bed |
|
| 6 | Daily activities/self-care | 39. Difficulty being able to wash yourself in your usual way (e.g. hand wash, bath, shower) |
|
| 7 | Malaise/vitality | 49. Feeling that your energy levels have been reduced |
|
| 8 | Emotional well-being | 60. Feeling depressed |
|
| 9 | Emotional well-being | 62. Feeling like a burden or nuisance on others |
|
| 10 | Self-consciousness | 69. Lacking in confidence |
|
| 11 | Participation | 78. Difficulty going out |
|
| Dimension | Item | Justification for removal |
|---|---|---|
| Exudate and odour | Weeping or an unpleasant smell from your pressure sore (combined item) | Although this appears to be the most ‘condition specific’ of the dimensions, the results indicate that the issue may have a relatively limited impact on quality of life. Weeping and odour items had high floor effects (71% and 84% respectively) and low correlations with the overall PU-QOL (0.191 and 0.171 respectively). These correlations were the lowest observed with global pressure ulcer quality of life, even when combined into one variable |
| Sleep | Interrupted sleep (e.g. restless sleep or being woken up during your sleep) | The item did not discriminate between ulcer categories and had a low correlation with global pressure ulcer quality of life. It is likely that a large proportion of the impact captured in the item will also be captured by the item relating to vitality |
| Self-consciousness | Lacking in confidence | It is likely that the impact of this item is captured by others. The items on depression and being a nuisance were combined into one variable and correlated with this item to determine the extent to which it captures unique impact. As the correlation was relatively high (r = 0.59) it was concluded that this item provides little additional information and should be removed |
Thus, after the second iteration of item reduction, a final PUQOL-UI measure consisting of seven items was settled on [available from the University of Leeds CTRU website: http://medhealth.leeds.ac.uk/puqol-ques (accessed July 2015). Despite the level of item reduction, the Rasch item map suggests that the seven remaining items span a relatively wide range on the latent trait continuum (Figure 33). A non-trivial level of floor effect remains but this is likely to be related to the response data being attributable only. Despite the loss of the odour and exudate items, the PUQOL-UI retains domains that are highly pertinent for this group, capturing moving in bed, energy levels and the sense of being a burden on others. This is in addition to important generic health impacts such as pain and self-care.
FIGURE 33.
Item map for the final PUQOL-UI.

Health state valuation survey
In total, 200 valuation interviews were successfully completed in June 2013. Of the sample, 54% of respondents were female, 51% were married or in a civil partnership and 67% were in some form of employment or full-time education. Over half of the sample (56%) had completed secondary education and 38.5% had completed post-secondary education. In terms of self-rated health, 82.5% reported their health as ‘good’ or ‘very good’ and only 1.5% reported their health as ‘very poor’. Table 85 reports the mean, minimum and maximum values and the number of observations for each of the health states in the estimation sample. Figure 34 presents a histogram of the utility values.
| State | Mean | Minimum | Maximum | n |
|---|---|---|---|---|
| 1111113 | 0.829 | 0.28 | 1 | 25 |
| 1111131 | 0.866 | 0.48 | 1 | 26 |
| 1111222 | 0.871 | 0.1 | 1 | 25 |
| 1111311 | 0.871 | 0.48 | 1 | 25 |
| 1112131 | 0.718 | –0.6 | 1 | 25 |
| 1112313 | 0.745 | 0.28 | 1 | 25 |
| 1113111 | 0.829 | –0.01 | 1 | 25 |
| 1121133 | 0.646 | 0 | 1 | 24 |
| 1123321 | 0.870 | 0.3 | 1 | 25 |
| 1131111 | 0.775 | 0 | 1 | 49 |
| 1131211 | 0.793 | 0 | 1 | 25 |
| 1133112 | 0.691 | 0.28 | 1 | 25 |
| 1211311 | 0.798 | 0 | 1 | 25 |
| 1212113 | 0.742 | –0.38 | 1 | 24 |
| 1221111 | 0.860 | 0 | 1 | 25 |
| 1223212 | 0.761 | 0 | 1 | 25 |
| 1311111 | 0.876 | 0 | 1 | 25 |
| 1311132 | 0.781 | 0.28 | 1 | 25 |
| 1312221 | 0.830 | 0.33 | 1 | 25 |
| 1331323 | 0.735 | 0 | 1 | 25 |
| 1333111 | 0.727 | 0 | 1 | 24 |
| 1333333 | 0.402 | –0.74 | 1 | 25 |
| 2121331 | 0.686 | 0 | 1 | 25 |
| 2122122 | 0.836 | 0.38 | 1 | 25 |
| 2122222 | 0.808 | 0.28 | 1 | 26 |
| 2131113 | 0.784 | –0.38 | 1 | 25 |
| 2132211 | 0.747 | –0.48 | 1 | 25 |
| 2211231 | 0.806 | 0.38 | 1 | 25 |
| 2213123 | 0.728 | 0.28 | 1 | 25 |
| 2222122 | 0.727 | 0 | 1 | 26 |
| 2222222 | 0.877 | 0.43 | 1 | 25 |
| 2311112 | 0.730 | –0.9 | 1 | 25 |
| 2313311 | 0.638 | –0.21 | 1 | 25 |
| 3111111 | 0.766 | 0.28 | 1 | 25 |
| 3111121 | 0.700 | 0 | 1 | 25 |
| 3111312 | 0.707 | 0.18 | 1 | 25 |
| 3113111 | 0.724 | 0 | 1 | 25 |
| 3113233 | 0.510 | –0.21 | 1 | 24 |
| 3133333 | 0.358 | –0.9 | 1 | 25 |
| 3231121 | 0.672 | 0 | 1 | 26 |
| 3232332 | 0.508 | –0.38 | 1 | 26 |
| 3232333 | 0.444 | –0.9 | 0.93 | 24 |
| 3313333 | 0.367 | –0.9 | 1 | 24 |
| 3321213 | 0.613 | 0.28 | 1 | 25 |
| 3322111 | 0.667 | 0 | 1 | 25 |
| 3331333 | 0.353 | –0.9 | 1 | 25 |
| 3332332 | 0.506 | –0.6 | 1 | 26 |
| 3333133 | 0.469 | –0.9 | 1 | 25 |
| 3333313 | 0.436 | –0.9 | 1 | 51 |
| 3333331 | 0.317 | –0.9 | 0.9 | 25 |
| 3333333 | 0.375 | –0.9 | 1 | 200 |
FIGURE 34.
Histogram of utilities in the model estimation data set. Mean 0.65, SD 0.376, n = 1500.
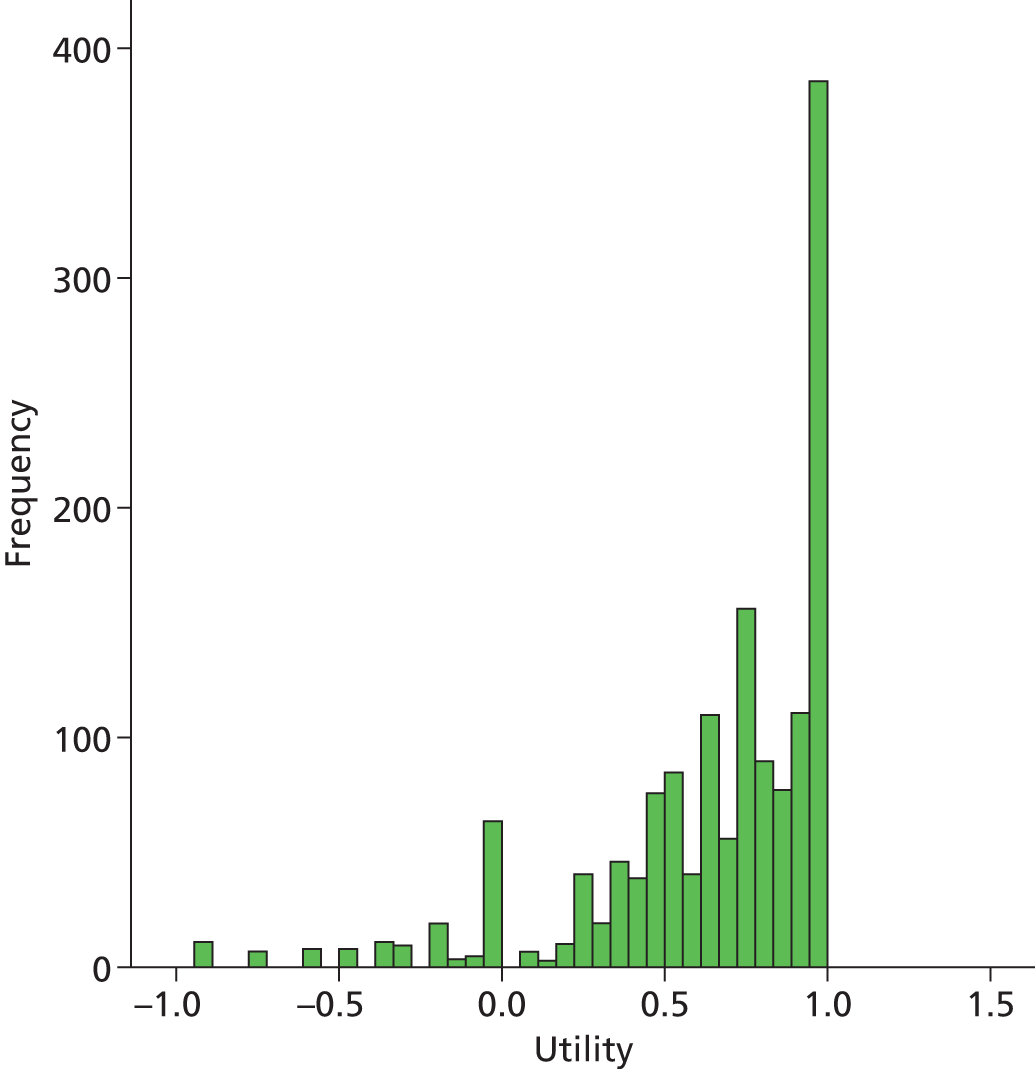
Table 86 reports the parameter coefficients for the main-effects ordinary least squares and random-effects models, as well as the significance values for each parameter. It also reports the adjusted R2, the mean absolute error for each model and the t-test for the expected value of the errors being significantly different from zero. Note that the parameters for the models are identical at three decimal places, with some changes in the significance of some of the coefficients. It is also worth noting that we fitted terms for interactions between level 3 on each domain. Although the resulting models produced significant coefficients on some of the interaction dummies – notably the burden and depression interaction – the coefficients on a number of main effects became non-significant and lost their logical ordering. Therefore, we have not reported these models in detail.
| Dimension and level | OLS regression model | RE regression model | ||
|---|---|---|---|---|
| β (SE) | Significance | β (SE) | Significance | |
| Constant | 1.00 | 1.00 | ||
| Pain 2 | –0.056 (0.027) | 0.036 | –0.056 (0.024) | 0.018 |
| Pain 3 | –0.138 (0.023) | 0.000 | –0.138 (0.018) | 0.000 |
| Mobility/adjusting self in bed 2 | –0.045 (0.025) | 0.075 | –0.045 (0.022) | 0.038 |
| Mobility/adjusting self in bed 3 | –0.074 (0.022) | 0.001 | –0.074 (0.022) | 0.001 |
| Self-care 2 | –0.047 (0.027) | 0.075 | –0.047 (0.020) | 0.017 |
| Self-care 3 | –0.106 (0.022) | 0.000 | –0.106 (0.021) | 0.000 |
| Energy 2 | –0.049 (0.025) | 0.055 | –0.049 (0.021) | 0.020 |
| Energy 3 | –0.100 (0.022) | 0.000 | –0.100 (0.019) | 0.000 |
| Depression 2 | –0.030 (0.026) | 0.243 | –0.030 (0.021) | 0.152 |
| Depression 3 | –0.072 (0.023) | 0.002 | –0.072 (0.020) | 0.000 |
| Burden 2 | –0.006 (0.026) | 0.821 | –0.006 (0.021) | 0.779 |
| Burden 3 | –0.095 (0.023) | 0.000 | –0.095 (0.020) | 0.000 |
| Social participation 2 | –0.038 (0.026) | 0.139 | –0.038 (0.020) | 0.050 |
| Social participation 3 | –0.093 (0.023) | 0.000 | –0.093 (0.022) | 0.000 |
| n | 1500 | 1500 | ||
| Mean absolute error | ||||
| Estimation | 0.064 | 0.064 | ||
| Validation | 0.081 | 0.081 | ||
| Ljung–Box test | 23.49 | 0.432 | 23.49 | 0.432 |
The mean absolute error records how far away the mean predicted value of a state (using the regression results) is from the mean observed value of a state. The lower the mean absolute error, the better the fit for that state. In general, the more states are within a specified distance from the observed values, the better the fit of the model. Figure 35 plots the proportion of all estimated states (n = 51) that have mean absolute errors below a given tolerance, as we vary that tolerance. As the parameters for both the ordinary least squares and the random-effects models are nearly identical, the mean absolute error curves are indistinguishable and so we have not plotted these separately. Figure 36 plots the observed and predicted health state values and prediction errors for the states in the estimation sample. Figure 37 does the same for eight states in the validation data set. It also reports the mean absolute error for the predictions of the validation state mean utilities. Table 87 reports the summary statistics for the health states in the validation sample.
FIGURE 35.
Mean absolute error curve. Proportion of predictions with target mean absolute error range.
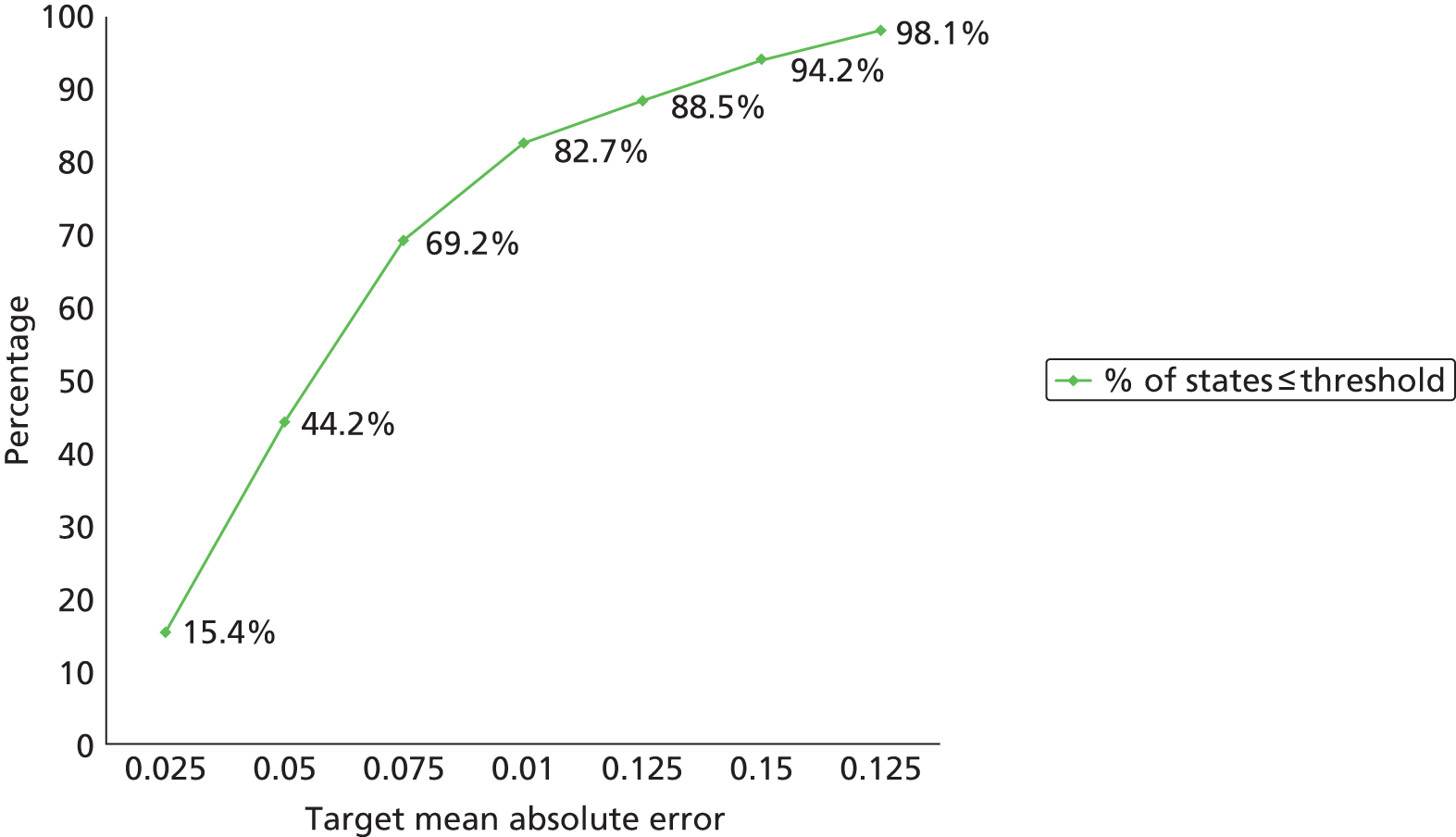
FIGURE 36.
Observed and predicted health state utilities with prediction errors. MAE, mean absolute error.
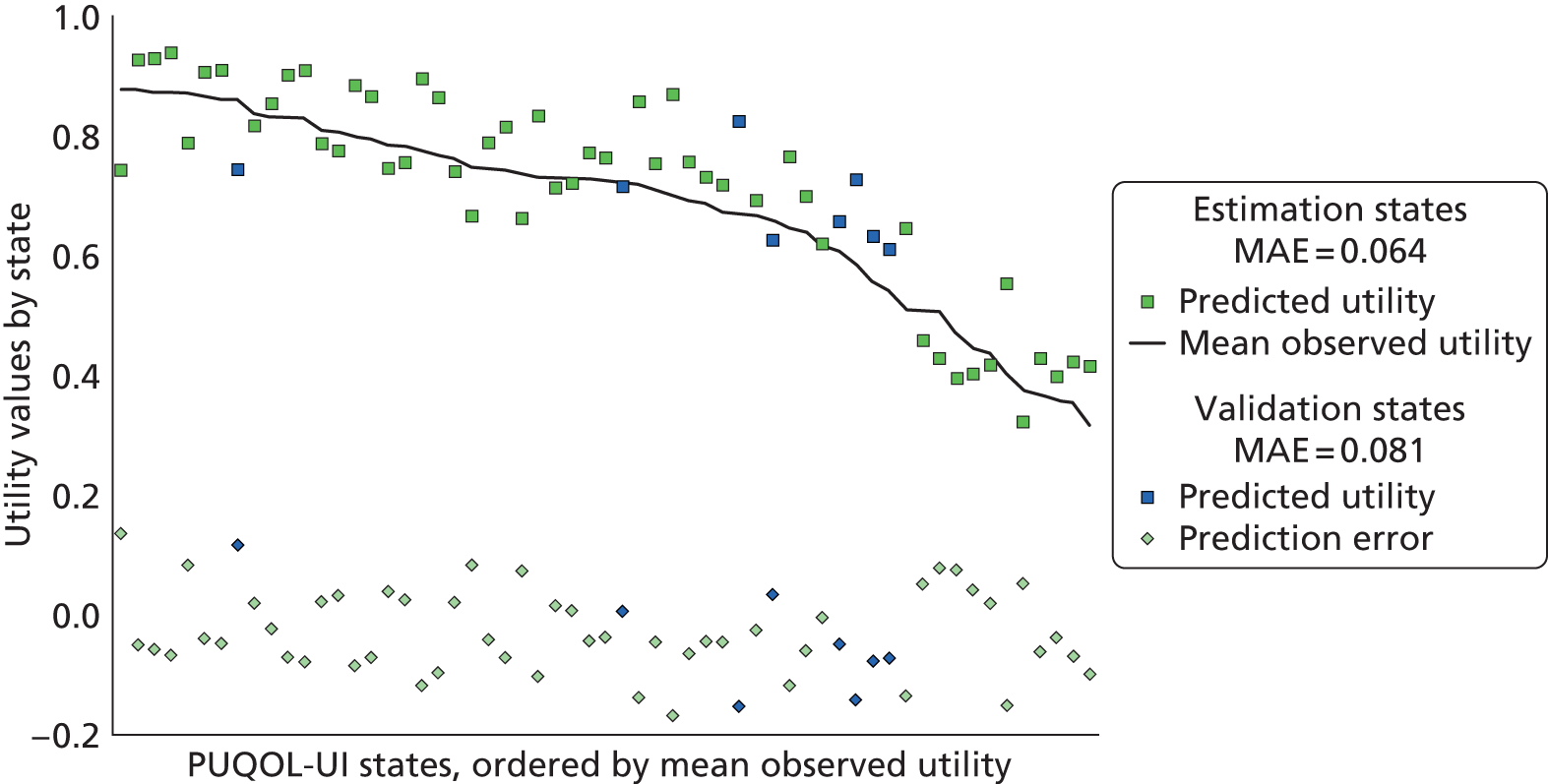
FIGURE 37.
Mean and predicted health state utilities and errors: validation data set. MAE, mean absolute error.
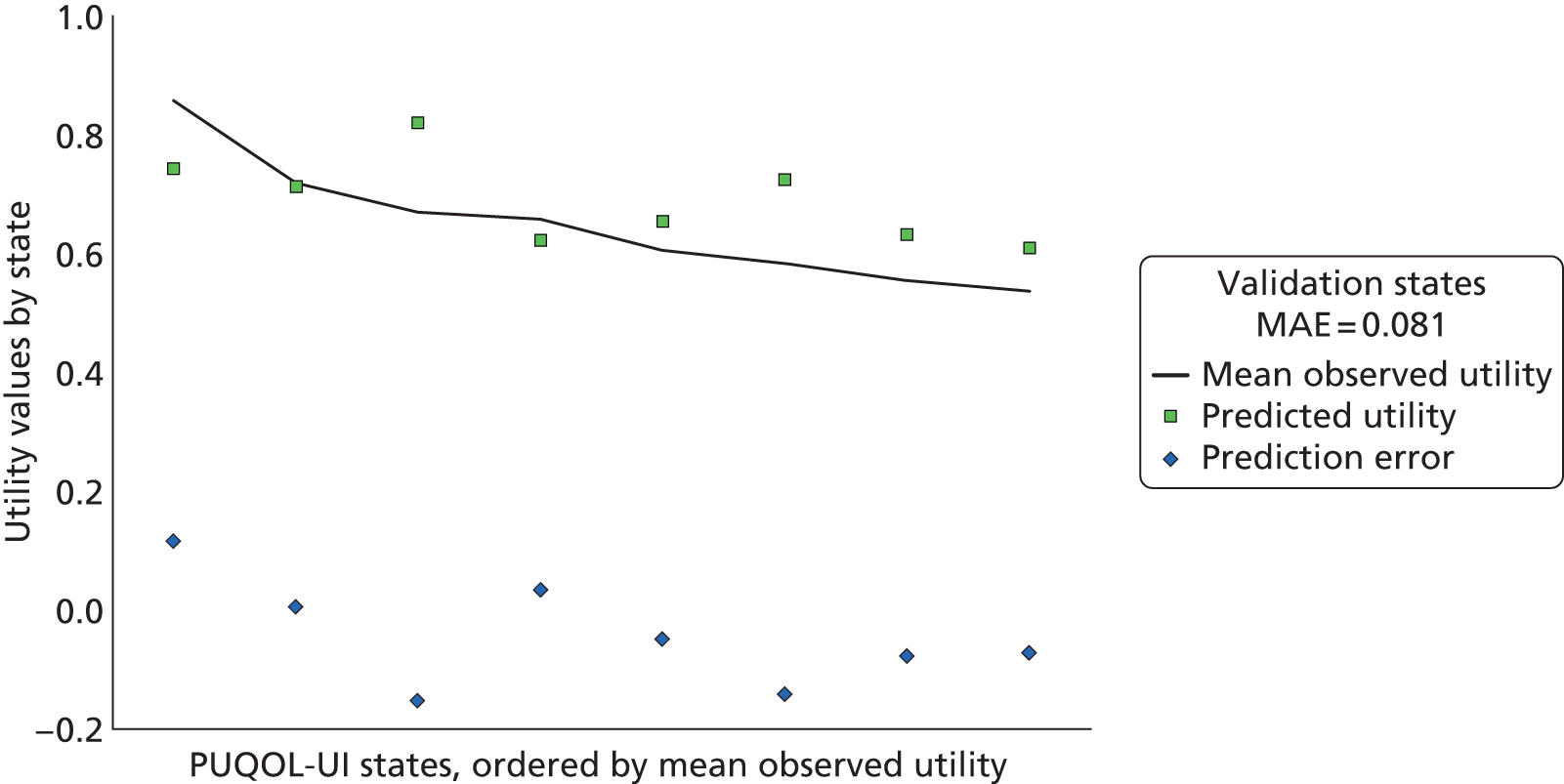
| State | Mean | Minimum | Maximum | n |
|---|---|---|---|---|
| 1212232 | 0.86 | 0.82 | 1.00 | 25 |
| 1333312 | 0.54 | 0.47 | 0.82 | 25 |
| 2112323 | 0.67 | 0.50 | 0.93 | 25 |
| 2232211 | 0.72 | 0.63 | 0.95 | 24 |
| 3122312 | 0.61 | 0.42 | 0.88 | 26 |
| 3133221 | 0.56 | 0.47 | 0.72 | 25 |
| 3213113 | 0.66 | 0.42 | 0.88 | 25 |
| 3311222 | 0.58 | 0.47 | 0.72 | 25 |
Validation study
The analysis for identifying the PU-QOL item subset to construct the valuation health states was based on data generated using the original ‘pressure ulcer-attributable’ wording of the measure, which does not reflect the ‘attribution-free’ format of the PUQOL-UI. Although there are no empirical studies in this space to confirm this, it is possible that, when the attribution is removed, the performance of the PUQOL-UI items may change. The validation study (study B) aimed to generate a data set using the revised (attribution-free) PU-QOL instrument to enable item analysis, to identify whether the performance of the selected items is adversely affected by the removal of attribution. The data set would also allow preliminary validation of the PUQOL-UI (see Appendix 52 for the study protocol).
Aim and objectives
The aim of the validation study was to validate the PUQOL-UI (in terms of item selection and psychometric performance). Specific objectives were to:
-
collect data on the revised attribution-free PU-QOL
-
verify the item selection for the PUQOL-UI using attribution-free PU-QOL data
-
conduct a preliminary validation of the PUQOL-UI.
Methods
Study design
A sample of NHS patients with pressure ulcers completed the researcher-administered revised PU-QOL instrument in hospital. The sample size was dependent on that required to obtain robust estimates from the Rasch analysis. Linacre238 proposed that for most purposes a sample size of 100 (range 64–144) will provide 95% confidence of item calibration within ±0.5 logits. In this study we aimed for 95% confidence and therefore a sample of 100 patients was recruited across multiple sites between July and November 2013.
Data collection
Patients who consented to participate (see Appendix 53 for the patient information sheet and consent form) completed the revised (attribution-free) 82-item PU-QOL and the EQ-5D-3L (see Appendix 54 for the questionnaire booklet). These were interviewer administered by research nurses in the inpatient setting. A set of sociodemographic and pressure ulcer-related questions was also completed. The latter included clinical grading of the pressure ulcer by the nurse. In cases in which the patient had more than one pressure ulcer the nurse returned the highest category of any of the pressure ulcers present. Ethical committee approval was gained for the study (National Research Ethics Service Committee North East – York; reference number 13/NE/0152).
Item selection analyses
The item analysis described in Valuation study: general population survey, Analysis was conducted again although on this occasion emphasis was on evaluating the items that had been selected for the PUQOL-UI rather than item selection. In addition, correlations were conducted with the EQ-5D rather than with the SF-6D. The internal consistency of the PUQOL-UI was evaluated as was criterion validity (through correlations with the EQ-5D).
Pressure Ulcer Quality of Life – Utility Index validation
The PUQOL-UI was scored employing the random-effects algorithm and the following analyses were conducted:
-
examination of utility score descriptives and distribution
-
assessment of criterion validity through correlations with the EQ-5D
-
assessment of the ability of the PUQOL-UI to discriminate between pressure ulcer severity and general health groups.
Analyses were conducted in RUMM2030 and Stata version 12 (StataCorp LP, College Station, TX, USA).
Results
Sample
In total, 100 patients with a current pressure ulcer participated in the study, including 52 patients from acute hospital NHS settings and 48 from community hospital, residential and home care settings (see Appendix 1 for recruitment by participating centre). The sample was well balanced in terms of gender, age, educational status and pressure ulcer category. The sample was also similar for these attributes to that which provided the data for the item selection analysis, thus ensuring that comparison is possible. The sample details are provided in Table 88.
| Characteristic | n |
|---|---|
| Gender | |
| Male | 51 |
| Female | 49 |
| Age (years) | |
| Mean (SD) | 77.16 (15.33) |
| Range | 22.67–101.67 |
| Ethnicity | |
| White | 98 |
| Black or black British | 2 |
| Highest level of education | |
| University or college or equivalent | 13 |
| Intermediate between secondary level and university | 23 |
| Secondary school | 55 |
| Primary school (or less) | 9 |
| Wheelchair user | |
| Yes | 50 |
| No | 50 |
| Pressure ulcer duration (months) | |
| Mean (SD) | 9.91 (17.3) |
| Range | 0.23–96.0 |
| More than one pressure ulcer | |
| Yes | 14 |
| No | 86 |
| Pressure ulcer category | |
| 1 | 14 |
| 2 | 37 |
| 3 | 21 |
| 4 | 24 |
| Missing | 4 |
| Self-rated pressure ulcer severity | |
| Very severe | 11 |
| Severe | 19 |
| Moderate | 36 |
| Mild | 18 |
| Very mild | 13 |
| Missing | 3 |
Item analyses
A summary of the item analyses is included in Table 89. The analysis did not identify any significant problems with the seven items selected for the PUQOL-UI that would warrant their exclusion from the measure. None of the PUQOL-UI items misfit the Rasch model, had low factor loadings or low ITCs. The items showed minimal floor effects and all but one of the items (‘Difficulty going out’) correlated significantly with the EQ-5D. Correlations with the global health item were generally lower although this likely relates to the number of data points available. Only one of the selected items discriminated between pressure ulcer category groups. However, this was the case for all of the items in the pain, sleep, mobility and malaise/vitality dimensions when all items were analysed. A number of items in the exudate and odour dimensions discriminated between pressure ulcer groups. However, as in the initial item selection, high floor effects were observed in these dimensions (range 68–94% in the exudate and 87–97% in the odour dimensions) as well as low correlations with global health and as such their exclusion from the PUQOL-UI is vindicated. Only one item in the daily activities and emotional well-being dimensions and two items in the self-esteem scales discriminated between pressure ulcer category groups. These may have been candidates for inclusion in the utility index; however, it is possible, given the number of statistical tests conducted, that the significant differences are spurious.
| PU-QOL scale and items | Low PCF loadinga | Floor effect ≥ 50%b | ITC | Discriminate between PUsc | R – global item | R – EQ-5D |
|---|---|---|---|---|---|---|
| Pain | ||||||
| Annoying pain or discomfort | 0.710 | N | 0.289d | 0.313d | ||
| Mobility | ||||||
| Difficulty adjusting yourself in bed | 0.764 | N | 0.224e | 0.339d | ||
| Daily activities | ||||||
| Being able to wash yourself in your usual way (e.g. hand wash, bath, shower) | 0.741 | N | 0.170 | 0.357d | ||
| Vitality/malaise | ||||||
| Feeling that your energy levels have been reduced | 0.795 | N | 0.339d | 0.250e | ||
| Emotional well-being | ||||||
| Feeling depressed | 55.10 | 0.778 | N | 0.328d | 0.411d | |
| Feeling like a burden or nuisance to others | 0.743 | N | 0.166 | 0.368d | ||
| Social functioning/participation | ||||||
| Difficulty going out | 0.827 | Y | 0.128 | 0.138 | ||
Overall, the item analysis based on the attribution-free PU-QOL did not identify any performance issues with the PUQOL-UI items nor an alternative configuration of the measure that was clearly preferable to the one that was chosen. Therefore, the PUQOL-UI format and item make-up is confirmed as an acceptable and valid basis of pressure ulcer health states for valuation. Although of secondary importance, the scale properties were also found to be adequate with a reasonable level of item inter-relatedness (Cronbach’s alpha = 0.74), with good fit to the Rasch model with no individual item misfit.
Pressure Ulcer Quality of Life – Utility Index validation
The PUQOL-UI exhibited a greater number of missing values than the EQ-5D. This might in part be explained by the greater number of items in the full PU-QOL and associated respondent fatigue, which may be not be an issue when the seven-item PUQOL-UI is completed. The range of observed PUQOL-UI scores (Table 90) were close to the possible range of the measure (0.322–1) in this sample but with no evidence of a floor or ceiling effect that might jeopardise the responsiveness of the measure. The PUQOL-UI scores were significantly higher than the EQ-5D scores. Correlations with the EQ-5D were moderate (r = 0.54; p < 0.001; n = 82) indicating a moderate level of shared variance.
| PUQOL-UI | EQ-5D | |
|---|---|---|
| n | 84 | 94 |
| Mean (SD) | 0.70 (0.18) | 0.19 (0.37) |
| Range | 0.32 to 0.99 | –0.59 to 0.85 |
| Pressure ulcer category, mean (SD) | ||
| Superficial (categories 1–2) | 0.72 (0.17) | 0.24 (0.36) |
| Severe (categories 3–4) | 0.67 (0.19) | 0.15 (0.38) |
| p-valuea | 0.17 | 0.28 |
| Self-rated pressure ulcer severity, mean (SD) | ||
| Mild | 0.78 (0.16) | 0.29 (0.36) |
| Moderate | 0.72 (0.17) | 0.25 (0.34) |
| Severe | 0.58 (0.17) | 0.04 (0.40) |
| p-valueb | < 0.001 | 0.023 |
| Self-rated general health, mean (SD) | ||
| Good | 0.75 (0.18) | 0.23 (0.40) |
| Moderate | 0.72 (0.16) | 0.26 (0.35) |
| Poor | 0.58 (0.18) | 0.03 (0.40) |
| p-value | 0.006 | 0.073 |
| Number of current pressure ulcers, mean (SD) | ||
| 1 | 0.72 (0.18) | 0.21 (0.38) |
| > 1 | 0.58 (0.18) | 0.07 (0.30) |
| p-value | 0.012 | 0.206 |
Neither the PUQOL-UI nor the EQ-5D were able to distinguish (to a statistically significant degree) between patients grouped according to pressure ulcer category (categories 1 and 2 vs. categories 3 and 4). However, the PUQOL-UI was able to discriminate between patients grouped according to number of pressure ulcers, self-reported severity of ulcer and self-reported health. The discriminatory power of the PUQOL-UI appeared to be greater than that of the EQ-5D, which could not discriminate between self-rated general health groups or by number of current pressure ulcers. This suggests that the new utility index is a valid measure of health state preferences in people with pressure ulcers.
Discussion
The PU-QOL instrument is a comprehensive measure of HRQoL that captures a wide range of clinical, personal, social and broader role effects of pressure ulcers. Having been developed with a view to utilisation in both the clinical and the population research setting, it asks respondents to focus on the component of their health that is attributable to their pressure ulcer. Both the scope of the instrument and the attribution form of the questions created challenges to the development of a short-form preference-based version of the instrument that could be used in the economic evaluation of pressure ulcer prevention and treatment technologies. The challenge from the instrument attribution would not have arisen had a non-attribution format been employed. However, the team involved in the development of the PU-QOL had specified a priori a desire to create a measure that captured only pressure ulcer-specific impact and that because of the prevalence of significant comorbidities in this group it was necessary for the instrument to enquire only about pressure ulcer impact. 221
The challenge represented by the scope of the PU-QOL instrument is one common to many short-form development projects and, in line with other researchers, we used psychometric assessment and Rasch analysis to identify a reduced set of domains and levels that were strongly related to measured global health. The short form consists of seven domains and is thus within the accepted scope for health state valuation – based on the psychological literature which reports that people can process between five and nine pieces of information in arriving at a decision. Although a number of the more clinical domains of the PU-QOL were dropped from the short version, this was based on analysis of the data rather than on any prior judgement on the researchers’ part.
The concern with the attribution form of the PU-QOL related to the feasibility of respondents parsing the value of pain, depression, burden, etc. attributable to their pressure ulcer if they had comorbidities that also impacted on these domains of HRQoL. Therefore, we undertook a small interview study of people with pressure ulcers and asked them to complete the PU-QOL questions without the attribution format. Respondents had no difficulty completing the revised question format and did not report problems with its meaning or relevance in the absence of the ‘attribution’ question format. On this basis we moved forward with the development of the short-form instrument – the PUQOL-UI.
Having decided to proceed with the valuation of the PUQOL-UI we had to consider whether to obtain patient or general population values for the health states it described. Although it is increasingly accepted that the description of the impact of a condition on an individual’s health should come from relevant service users, it is still recommended that, when valuations are intended to inform population health-care resource allocation decisions, these should come from the general population rather than from the patients. Therefore, we chose the general population as the population to sample for the health state valuation survey. It would be interesting to know the location and the magnitude of any differences between patient and general population values for health states described by the PUQOL-UI. However, the additional valuation surveys necessary to address this question were beyond the resources available to this study.
In line with recommendations from NICE,273 we chose to use the TTO method for obtaining health state values, using props and timelines based on the UK measurement and valuation of health study,277 which produced the algorithm for the EQ-5D. Like the measurement and valuation of health study,277 we undertook an interviewer-administered survey to enable us to have more confidence in the quality of the data obtained, both through interviewer training and reporting back on interviewee comprehension.
The recommended utility algorithm is, perhaps surprisingly, a simple linear additive model. However, this was the model that best fit the data and produced acceptable predictive performance in both the estimation and the validation data set. Additional work to assess whether or not more sophisticated model specifications, such as generalised linear models and two-part models, produce better-performing algorithms could be undertaken. However, the random-effects main-effects regression model performs acceptably and is comparable in many ways with widely used utility algorithms such as the SF-6D.
Additional efforts were expended in checking that the item selection process had been valid. This was perhaps not critical as the psychometric properties of a utility measure can be established only ex post when the utility weights are available and as item selection based on unweighted item responses provides only an idea of which items are most suitable. However, the item analysis conducted in the validation study did verify that the items selected for inclusion in the PUQOL-UI were a good representation of the long form of the measure and confirmed the validity of the ‘attribution-free’ format. Psychometric analyses also suggested that the PUQOL-UI has adequate levels of discriminatory power and may have greater power than the EQ-5D. Further work is required to compare the performance of the EQ-5D and that of the PUQOL-UI to determine whether or not the condition-specific measure indeed confers measurement benefits and also to determine the responsiveness of the PUQOL-UI over time. PUQOL-UI values were much higher than those observed on the EQ-5D. It is likely that this is an artefact of the much greater range on the EQ-5D and the lower values drawing the mean lower. The EQ-5D has a much lower bound than the PUQOL-UI and this may be because it describes more severe health states (e.g. ‘I am unable to wash or dress myself’ in the EQ-5D vs. ‘Having a lot of bother washing yourself in the usual way’ in the PUQOL-UI).
Conclusions
The development of a condition-specific, preference-based measure for use in economic evaluations of pressure ulcer preventative and treatment interventions has been a success. A brief measure – the PUQOL-UI – has been identified and valued using general population TTO tasks. The subsequent modelling identified a robust algorithm that delivers the utility values necessary for cost–utility analysis. The PUQOL-UI complements the PU-QOL instrument as the latter delivers a comprehensive assessment of patient quality of life; investigators should consider employing both instruments in future pressure ulcer studies. Further work is necessary to establish the responsiveness of the PUQOL-UI, especially compared with generic preference-based measures. Future methodological work should explore the impact of condition attribution in health measurement and valuation, especially when comorbidities exist in the population of interest.
The final PUQOL-UI instrument and the associated utility scoring algorithm syntax (in SPSS and Stata) are available from http://medhealth.leeds.ac.uk/puqol-ques (accessed July 2015).
Chapter 8 Conclusions, wider impacts and recommendations
Summary
The PURPOSE clinical and academic partnership has been successful in developing and delivering a world-leading applied health research programme in the field of pressure ulcer prevention. The PURPOSE programme has addressed fundamental issues identified by patients and provides the foundation for the development of evidence-based, patient-centred practice in the field and a future clinical trials portfolio.
Our pain prevalence study has indicated that hospital and community patients experience both pressure area-related and pressure ulcer pain and results from our cohort study indicate that pain is independently predictive of category 2 (and above) pressure ulcer development. This is the first cohort study to have explored pain as a risk factor and supports patient reports from previous qualitative work9 that pain had preceded the clinical presentation of their pressure ulcer.
Similarly, our work on severe pressure ulcers is the first study to have investigated different explanations for their development, linking the literature on pressure ulcer development to the broader literature on patient safety, the management of clinical risks and the investigation of reportable incidents. There is an unhelpful divide between those such as Francis,93 who stress the importance of organisational and cultural explanations for adverse events, and the bulk of the academic literature, which continues to focus on errors made by individual clinicians. 112 Our findings show that it is possible to reconcile these perspectives: severe pressure ulcers are more likely to occur where individuals fail to respond to clear signs, when there are wider problems with the contexts in which they are working. This has practical implications for the conduct of root cause analyses in the NHS. Analyses currently focus on identifying specific decisions or actions and encourage teams to attach blame to individuals. Broader organisational and cultural considerations should be included in root cause analyses.
The pressure ulcer risk factor systematic review highlighted the need for high-quality risk factor research in the field, common standards for the definition of key risk factors and improved data sets underpinned by a conceptual model for the development and testing of prediction models. We therefore developed a risk factor Minimum Data Set and used this to form the basis of a Risk Assessment Framework, the PURPOSE-T, using consensus methods underpinned by the best available evidence. Decisions incorporated the views of service users. The risk assessment work incorporated key findings from the pain and severe pressure ulcer work packages, namely the inclusion of pain in the Risk Assessment Framework and designing the framework to distinguish between primary prevention and secondary prevention/treatment decision pathways.
The development of PROs for the assessment of quality of life and health utilities provide tools for use in future effectiveness research and patient-directed treatment plans that are both evidence based and focused on important outcomes for patients.
Related themes: practice implementation
A number of related themes have emerged from the results of the work:
-
Patient’s reports of pain preceding pressure ulcer development are dismissed by nurses (quality of life work package).
-
Patients reported that pain is their most distressing symptom, but little priority was given to pain, which is not systematically assessed or treated effectively (quality of life work package).
-
Pain is a common problem and is reported by patients with pressure ulcers but also by a small number of patients without pressure ulcers on ‘at-risk’ skin sites (pain work package).
-
The presence of pain is a predictor of subsequent category 2 pressure ulcer development (pain work package).
-
Severe pressure ulcers were more likely to develop when:
-
– nurses failed to listen to patients/carers
-
– nurses failed to recognise deterioration in condition or acknowledge the presence of an existing pressure ulcer (severe work package).
-
-
Current risk assessment does not make a distinction between those patients who have no pressure ulcers but who are at risk and require primary prevention and those patients who have an existing pressure ulcer or scarring from a previous pressure ulcer who require secondary prevention and treatment (risk assessment work package).
-
Severe pressure ulcers developed when there was no response or escalation of care despite clear signs that a pressure ulcer was developing (blanching redness to category 1) or deteriorating (e.g. from category 1 to category 2) (severe work package).
-
Severe pressure ulcers developed in situations in which effective pressure relief was difficult to achieve, for example because of a patient’s capacity to understand and respond to advice or because of physical limitations such as contractures. The problems were not, though, attributable solely to the complexity of the problems. Severe pressure ulcers developed in cultural contexts in which some staff were prepared to blame patients for what had happened.
-
Skin status is a key risk factor for pressure ulcer development, including alterations to intact skin, and critically the presence of a category 1 pressure ulcer is predictive of category 2 and above pressure ulcer development (pain and risk work packages).
-
Service provision, particularly in the community setting, is fragmented and not patient focused. This was evident in the prevention of pressure ulcers in community patients (severe pressure ulcer work package) and in the treatment of pressure ulcers (quality of life work package), with service provision identified as a factor that had contributed to severe pressure ulcer development and also impacted on quality of life because of a lack of consideration of patients’ needs in the service delivery and organisation of treatment (e.g. unspecified dressing visit times). The severe pressure ulcer findings showed that patients’ voices were not sought, or were not heard, when problems arose.
Together, the issues identified from the individual PURPOSE work packages highlight limitations of the standard ‘assess, plan, implement and evaluate’ model of care. This has led to the development of an active monitoring model of care – Pressure Ulcer Prevention Pathways (PUPPs). PUPPs incorporates risk assessment using the PURPOSE-T (including skin status and pain), the allocation of patients to primary and secondary prevention pathways and active monitoring of individual patients’ skin response to preventative interventions. It details required actions and escalation in response to deterioration and pressure ulcer development [see http://medhealth.leeds.ac.uk/accesspurposet (accessed July 2015)].
Wider impacts
In a field characterised by little high-quality research and investment, this programme grant has led to a number of positive impacts that extend beyond the scope of the original award.
Patient and public involvement
The PPI activity and the setting up of PURSUN UK has led to many benefits, as detailed in Chapter 2, with examples of innovative engagement. An outcome of the programme grant is the continuation of a sustainable network of service users who are committed to the ongoing development of public and professional awareness of the impact of pressure ulcers and a strong user-driven and supported research agenda.
Isolating the impact of PPI within a research study is notoriously challenging. 303,304 Those challenges are magnified here given the complex nature of a programme grant, in which individual studies feed into and influence each other. The severe pressure ulcer study was the only study in which PPI was formally evaluated. This proved to be very valuable and is something that we would do for the entire programme in future. Despite the challenges we have been able to identify a number of ways in which the involvement of PURSUN UK was helpful (described throughout the monograph and summarised in Table 91). We identified these points through the formal severe pressure ulcer PPI evaluation; through ongoing, informal feedback from service users and the project team; by looking at documentation such as e-mails and meeting minutes; and through feedback during the drafting of the monograph.
| Area of impact | Examples |
|---|---|
| Impact on members of PURSUN UK |
|
| Impact on the project team |
|
| Management/oversight of the research |
|
| Conduct of the research |
|
| Dissemination/implementation |
|
| Development of new research |
|
National Health Service research capacity and capability
The design and delivery of large multicentre projects has extended our original network of NHS research-active centres that have the capacity and research workforce with the clinical knowledge and experience of recruiting the ‘hard-to-reach’ patient population.
We have had considerable support from a number of NIHR Clinical Local Research Networks and the NIHR Dermatology Specialty Group in terms of set-up and project delivery and this has been maintained with subsequent funding for a NIHR HTA programme-funded trial (PRESSURE 2), with seamless transition of the NIHR infrastructure in key centres.
The programme of studies has facilitated the development of a cohort of tissue viability nurse specialists as new principal investigators as well as partnership with dermatologists who have supported the programme as principal investigators. In addition, the clinical research nurse roles established during the 5-year period have resulted in a number of benefits for the NHS, including the retention of senior clinical nurses, development opportunities for clinical research nurses through local and national dissemination activities and the development of clinical expertise leading to promotion.
The programme grant has also supported the development of three of the programme grant co-applicants (E McGinnis, N Stubbs and L Wilson), who have made a significant contribution to the design and delivery of our nationally and internationally relevant programme of work whilst maintaining substantive NHS nurse specialist/consultant posts. Again, the programme funding supporting their involvement created development opportunities through local part-time secondment opportunities into nurse specialist roles and has enabled the development of capacity, clinical expertise and a team approach to tissue viability service delivery.
Academic research capacity and capability
The programme grant award has led to the development of a cohesive clinical academic research group in Leeds with an ongoing portfolio of research and grant development.
The programme grant award has made a contribution to the successful promotion of four of the co-applicants to professor (J Nixon, EA Nelson, C Dealey and M Briggs), including two promotional chairs and an Honorary Professorship at the Russell Group Universities. This is a major achievement from a professional nursing perspective, which as a profession has low numbers of professorial appointments and very few promotional chairs.
The PURPOSE programme has also supported the development of the programme manager, four research fellows, through their registration as part-time PhD students [C Rutherford (née Gorecki), S Coleman, L Pinkney and C Szoski-Murray], and a trial co-ordinator, who commenced medical school and graduated as a medical doctor (R Stevenson). To date, we have had two successful PhD completions (Rutherford and Coleman).
International collaborations
We have established strong international collaborations through systematic reviews and consensus methods and established the foundation for future collaborative programmes of translational research at the basic science and implementation stages of the translational pathways.
Emerging National Institute for Health Research portfolio
The infrastructure and tools developed during the PURPOSE programme are underpinning an ongoing portfolio of NIHR-funded research. In 2012 the team were successful in a trial grant application to the NIHR HTA programme (PRESSURE 2), securing the clinical academic partnerships and NHS infrastructure for a further 4-year period. The PRESSURE 2 trial is utilising the PURPOSE-T as a method of recording the pressure ulcer risk factor Minimum Data Set and the PU-QOL and PUQOL-UI as secondary outcome measures. Substudies will assess the predictive validity of the PURPOSE-T and the responsiveness of the PU-QOL instrument.
Dissemination and knowledge transfer
We developed a dissemination and knowledge transfer plan for the PURPOSE programme including electronic communications, investigator meetings, publications (see Acknowledgements, Publications), local, national and international conference presentations (see Acknowledgements, List of presentations and posters), web-based resources and curriculum development.
Dissemination activity is ongoing. For example, in relation to web-based resources we are currently developing freely available dissemination materials including podcasts, slide packs, case studies and resources for root cause analysis training and posters for use at local events for general dissemination, training and education by NHS providers. In addition, we have set up web access for the three tools that we have developed during this programme of research: the PURPOSE-T, PU-QOL and PUQOL-UI. Web-based access includes copyright agreements and permissions for the use of data for ongoing validation research and guidelines for international translation and validation.
Recommendations
Implications for patient and public involvement in research
-
Patient and public involvement requires explicit commitment to involving service users and their perspectives throughout every aspect of the research process.
-
Presenting research data in live and interactive formats can make the interpretation process more engaging and accessible to service users and support meaningful dialogue between service users and professionals.
Implications for clinical practice development
-
Front-line health-care professionals should respond to patient symptoms including pain (soreness and discomfort), alterations to intact skin and category 1 pressure ulcers and instigate/escalate care provision.
-
Patients with pressure ulcers should have pressure ulcer pain assessment, including type of pain, to inform treatment.
-
In circumstances in which clinicians do not have the skills necessary to address needs, patients should be referred to appropriate colleagues.
-
Some clinicians blamed patients for the development of severe pressure ulcers. In circumstances in which provision of effective pressure ulcer prevention interventions is impacted by a patient’s mental capacity or physical disability, advice (consultation) should be sought from colleagues with appropriate multidisciplinary specialist expertise and a problem-solving approach adopted.
-
The development of an electronic version of the PURPOSE-T in health-care settings would facilitate large-scale multivariable modelling and refinement of the PURPOSE-T.
-
Implementation of key research findings may be facilitated through the use of the PUPPS active monitoring model of care, which incorporates risk assessment using the PURPOSE-T (including skin status and pain), allocation of patients to primary and secondary prevention pathways and active monitoring of individual patients’ skin response with required actions and escalation in response to deterioration and pressure ulcer development [see http://medhealth.leeds.ac.uk/accesspurposet (accessed July 2015)].
Implications for quality, safety and health service management
-
To maximise learning, root cause analysis could be extended in two ways:
-
interview patients and carers to capture their accounts of events
-
increase awareness of the possibility that staff are working in contexts in which risky practices are tolerated and be able to assess whether or not this is the case.
-
-
It is important to co-ordinate services effectively so that pressure ulcer risks are communicated to everyone involved (patients, carers, all members of the multidisciplinary team).
-
Service reconfiguration/ward reorganisation planning needs to ensure continuity of clinical leadership and oversight/delivery of clinical care to high-risk patients.
-
Development of a standardised case ascertainment method in the community setting is required.
Implications for future research
Pain
-
Replication of the pain cohort study is required.
-
The impact of including pain as an indicator for the escalation of preventative interventions requires investigation.
Severe pressure ulcers
-
The severe pressure ulcer study is the first of its kind and findings should be confirmed by further empirical research.
-
There may be merit in studying ‘best practice’ settings to better understand how patients’ and organisational risks are identified and effectively acted on.
Risk assessment
-
Development of objective measurement methods for mechanical boundary conditions, individual susceptibility and tissue tolerance and early indicators of damage.
-
Further evaluation of the PURPOSE-T is required including sensitivity and specificity in different patient populations; impact on decision-making/processes of care; and effectiveness in reducing pressure ulcer incidence in practice.
-
The pressure ulcer risk factor Minimum Data Set should be incorporated into future research.
-
Development of appraisal methods for risk factor research.
-
Development of a lay person version of the PURPOSE-T that can be used by patients and carers to facilitate self-assessment.
-
The impact of including skin status as an indicator for the escalation of preventative interventions requires investigation.
Pressure ulcer quality of life
-
The PU-QOL instrument requires further evaluation through an assessment of responsiveness.
-
The PU-QOL can be used as an outcome measure in future pressure ulcer research (e.g. clinical trials and observational studies) on the proviso that studies have built in a parallel psychometric analysis to indicate the performance (psychometric evaluation) of the scales in future samples.
-
The PU-QOL instrument requires translation and validation for international utilisation.
Pressure ulcer cost–utility analysis
-
The PUQOL-UI can be used in pressure ulcer prevention and treatment trials to enable cost–utility analyses.
-
Further research is required to determine the responsiveness of the PUQOL-UI.
-
Further research is required to establish the benefits of the PUQOL-UI (and other CSUMs) over generic utility measures; this must take into consideration the impact that CSUMs may have on decision-making and efforts to achieve allocative efficiency.
-
Further research is required to determine the extent to which patients completing HRQoL measures consider (and are able to consider) ‘disease-attributable’ impact only.
Acknowledgements
Participants
We wish to express our gratitude to all of the patients who gave up their time and participated in this research programme.
Programme steering committee members
-
Chairperson 2009–10: Professor Dame Jill Macleod-Clark, Deputy Dean, Faculty of Medicine, University of Southampton, UK.
-
Chairperson 2011–13: Professor Elaine McColl, Director, Newcastle Clinical Trials Unit, University of Newcastle, UK.
External/independent members
-
Ms Juliette Cosgrove, Lead Nurse Patient Safety, Leeds Teaching Hospitals NHS Trust, UK.
-
Professor Amit Gefen, Professor in Biomedical Engineering, Faculty of Engineering, Tel Aviv University, Israel.
-
Professor Zena Moore, Associate Professor, Director of Nursing Research, Royal College of Surgeons in Ireland, Dublin.
-
Mr Alan Pickersgill, service user.
-
Mr Brian Rawson, service user.
-
Dr Lisette Schoonhoven, Assistant Professor in Nursing Science, Scientific Institute for Quality of Healthcare (IQ healthcare), Radboud University Medical Center, the Netherlands, and Reader at the Faculty of Health Sciences, University of Southampton, UK.
-
Professor Justin Waring, Professor of Health Systems and Policy, Nottingham University Business School, UK.
Pressure Ulcer Research Service User Network UK
We would like to sincerely thank all members of PURSUN UK for their continued hard work and dedication. Particular thanks go to the following people for their valuable input into the PURPOSE programme studies: Dr Philip Bell, Ms Carole Bennett, Dr Claire Ginn, Mr Bryan McArdle, Mrs Andrea McGoverin, Mr Alan Pickersgill, Mr Brian Rawson, Mrs Yvonne Rawson, Mrs Claire Roberts, Mrs Patricia Slater, Ms Kay Walker and Mr Ken Watson.
Chapter 3
We would like to sincerely thank Dr Carly Rivers who was the Senior Trial Co-ordinator during the set-up and delivery of the pain prevalence research projects and Caroline Fallon of ArjoHuntleigh (ArjoHuntleigh House, Bedfordshire, UK) who collaborated in the co-ordination and delivery of the acute trust prevalence surveys.
Chapter 4
We would like to sincerely thank Mrs Jackie Hansford, formerly of Bradford Teaching Hospitals NHS Foundation Trust, who was a co-applicant and made a decisive contribution to study conception and design and Dr Claire Ginn, who is a member of PURSUN UK and who made a critical contribution to the development of a methodology for root cause analyses of critical incidents.
Chapter 5
Co-authors on related papers
-
Professor Jose Closs, Professor of Nursing Research, School of Healthcare, University of Leeds, UK.
-
Professor Tom Defloor, Department of Nursing, University of Ghent, Belgium.
-
Professor Amanda Farrin, Professor of Clinical Trials and Evaluation of Complex Interventions, CTRU, University of Leeds, UK.
-
Dr Ruud Halfens, Department of Health Services Research, CAPHRI School for Public Health and Primary Care, Maastricht University, the Netherlands.
Members expert group and co-authors on papers
-
Professor Dan Bader, Faculty of Health Sciences, University of Southampton, UK.
-
Professor Dan Berlowitz, Bedford VA Hospital and Boston University School of Public Health, MA, USA.
-
Associate Professor Janet Cuddigan, College of Nursing Omaha Division, University of Nebraska Medical Center, NE, USA.
-
Professor Dawn Dowding, Columbia University School of Nursing, Columbia University, NY, USA.
-
Professor Amit Gefen, Department of Biomedical Engineering, Faculty of engineering, Tel Aviv University, Israel.
-
Dr Edward Jude, MD, MRCP, Tameside Hospital NHS Foundation Trust, University of Manchester, UK.
-
Associate Professor Cees Oomens, Biomedical Engineering Faculty, Eindhoven University of Technology, the Netherlands.
-
Dr Lisette Schoonhoven, Assistant Professor of Nursing Science, Scientific Institute for Quality of Healthcare (IQ healthcare), Radboud University Medical Center, the Netherlands, and Reader at the Faculty of Health Sciences, University of Southampton, UK.
-
Professor Jos Schols, Department of Family Medicine and Department of Health Services Research, CAPHRI School for Public Health and Primary Care, Maastricht University, the Netherlands.
-
Professor Peter Vowden, MD, FRCS, Clinical Director, NIHR Wound Prevention and Treatment Healthcare Technology Co-operative, Bradford Teaching Hospitals NHS Foundation Trust, UK.
Chapter 6
Multidisciplinary expert group
Tissue viability specialists
-
Professor Jane Nixon, Professor Andrea E Nelson, Professor Carol Dealey, Dr Elizabeth McGinnis, Mrs Susanne Coleman, Mrs Lyn Wilson and Mrs Nikki Stubbs.
Chronic pain specialist
-
Professor Michelle Briggs.
Outcome methodologists
-
Dr Yasmene Alavi, Research Fellow, Health Services Research Unit, London School of Hygiene & Tropical Medicine, UK.
-
Associate Professor Stefan Cano, Senior Lecturer in Psychometrics, Clinical Neurology Research Group, Peninsula College of Medicine and Dentistry, UK.
-
Professor Katerina Hilari, Reader, Joint Research Director and Senior Tutor for Research, Division of Language and Communication Science, City University London, UK.
-
Professor Donna Lamping, Professor of Psychology, Health Services Research Unit, London School of Hygiene & Tropical Medicine, UK.
-
Dr Sarah Smith, Senior Research Fellow, Health Services Research Unit, London School of Hygiene & Tropical Medicine, UK.
Co-authors on related papers
-
Dr Anna Madill, Reader in Qualitative Inquiry, Institute of Psychological Sciences, University of Leeds, UK.
-
Dr Jill Firth, Consultant Nurse in Rheumatology/Clinical Governance Lead, Visiting Senior Research Fellow, School of Healthcare, University of Leeds, UK.
-
Professor Jose Closs, Professor of Nursing Research, School of Healthcare, University of Leeds, UK.
-
Dr Yasmene Alavi, Research Fellow, Health Services Research Unit, London School of Hygiene & Tropical Medicine, UK.
Chapter 7
-
Teresa McGarry and Chris Heywood from Accent Research Agency, London, UK, for their help in running the valuation interviews.
Co-authors on related papers
-
Dr Richard Edlin, Senior Lecturer in Health Systems, School of Population Health, University of Auckland, New Zealand.
Research team members at participating sites
We are grateful to all of the clinical research team members and co-authors from the 30 NHS trusts who participated in this research and made it a part of their busy daily schedule. Without the commitment and support of the principal investigators (PIs), tissue viability nurse specialists (TVNS) and clinical research nurses (CRNs) in gaining local permissions, recruiting patients and collecting data this research programme would not have been possible.
-
NHS Ayrshire and Arran: Margo Henry (CRN).
-
Basildon and Thurrock University Hospitals NHS Foundation Trust: Gupta Indi (PI).
-
University Hospitals Birmingham NHS Foundation Trust: Prof Carol Dealey (PI), Lynne Ellocks (CRN) and Jeannette Marshall (CRN).
-
Bradford Teaching Hospitals NHS Foundation Trust: Kathryn Vowden (PI) and Janet McGowan (CRN).
-
Burton Hospitals NHS Foundation Trust: Linda Rafter (PI).
-
Calderdale and Huddersfield NHS Foundation Trust: Helen Fearnley (PI) and Catherine Smith (CRN).
-
County Durham Primary Care Trust and Darlington Primary Care Trust: Richard Buckland (PI).
-
Harrogate and District NHS Foundation Trust: Dr Alison Layton (PI) and Margaret Broome (CRN).
-
James Paget University Hospitals NHS Foundation Trust: Ingrid Salvery (PI) and Tina Dyble (CRN).
-
NHS Kirklees: Tracy Conroy (PI), Alison Murphy (TVNS), Geraldine Thompson (TVNS) and Jackie Ward (CRN).
-
NHS Leeds: Nikki Stubbs (PI), Lindsey Worstenholme (CRN), Karen Lamb (CRN), Rachael Lee (CRN) and Julie Thackrey (CRN).
-
Leeds Teaching Hospitals NHS Trust: Sally Blundell (CRN), Jimmy Choo (CRN), Andrea Dyer (TVNS), Elizabeth McGinnis (PI) and Kira Meethan (CRN).
-
University Hospitals of Leicester NHS Trust: Aiden Dunphy (CRN), Emma Alderman (CRN), Sandra Kazembe (CRN), Bianca Ngwenya (CRN) and Karen Wheafer (PI).
-
NHS Leicester County and Rutland Community Health Services: Lynne Spencer (PI).
-
Marie Curie Hospice: Jean Gordon (PI).
-
Mid Yorkshire Hospitals NHS Trust: Beverly Hemingway (CRN) and Caron Storey (CRN).
-
Norfolk and Norwich University Hospital: Helen May (PI) and Susanne McDonald (CRN).
-
Northern Devon Healthcare NHS Trust: Melanie Hucker (PI), Geraldine Belcher (CRN), Jane Hunt (CRN) and Amanda Skinner (CRN).
-
Northern Lincolnshire and Goole Hospitals NHS Foundation Trust: Helen Blagg (PI).
-
North Tees and Hartlepool Hospitals NHS Foundation Trust: Christine Russel (PI).
-
Northumbria Healthcare NHS Foundation Trust: Val Henderson (PI), Louise Jones (CRN) and Sean Phillips (CRN).
-
North Yorkshire and York NHS Trust: Joyce Simms (PI).
-
Scarborough and North East Yorkshire Healthcare NHS Trust: Samantha Haigh (PI).
-
Southern Health NHS Foundation Trust: Chantelle Mitchell (CRN).
-
South Tyneside NHS Foundation Trust: Jeannette Milne (PI).
-
Sussex Community NHS Trust: Claire Cox (PI), Kate Porter (CRN) and Nina Walters (CRN).
-
Wakefield Primary Care Trust: Joanne Newbald (PI) and Claire Brown (TVNS).
Complementary funding
-
TRANslating Science into Clinically Relevant Information for Pressure-ulcer Therapies and Risk-assessment (TranSCRIPTaR) Network. J Nixon, P Brooks, A Dehghani, JM Brown, E Andrea Nelson, C Gorecki, S Coleman, M Fader, D Bader, A Gefen, C Oomens, L Bilston, T Defloor, L Schoonhoven, D Berlowitz, D Margolis. Worldwide Universities Network, University of Leeds Fund for International Research Collaboration, £14,070 over 18 months. This funding enabled participation of international experts in the development of the pressure ulcer minimum dataset and Risk Assessment Framework (see Chapter 5).
-
Systematic reviews of pressure ulcer risk factors and patient quality of life. J Nixon. Postdoctoral Nursing Research Fellowship from the Smith & Nephew Foundation, £109,280.
-
The original work for the risk factor systematic review (see Chapter 5) was undertaken as part of the Smith & Nephew Foundation but was unpublished. The search was updated and the review methodology, evidence tables and narrative synthesis were developed and published as part of the programme grant.
Contributions of authors
Professor Jane Nixon (Professor of Tissue Viability and Clinical Trials Research) was the programme chief investigator and lead for the pain studies. She led the conception of the PURPOSE programme; contributed to the design, protocol development, protocol implementation and interpretation of data for the pain, severe pressure ulcer, risk assessment, PU-QOL and PUQOL-UI studies; and was involved in drafting Chapters 1, 3 and 8 and revising Chapters 2 and 4–7 critically for intellectual content. She also gave approval to the final version of the monograph and was the guarantor for Chapters 2, 3, 5 and 6.
Professor E Andrea Nelson (Professor of Wound Healing) was a programme co-applicant. She was involved in the conception of the PURPOSE programme; contributed to the design, protocol development, protocol implementation and interpretation of data for the pain, severe pressure ulcer, risk assessment and PU-QOL studies; and was involved in revising Chapters 1–8 critically for intellectual content. She also gave approval to the final version of the monograph.
Dr Claudia Rutherford (née Gorecki) (Research Fellow, Psychometrics) was a programme co-applicant. She was involved in the conception of and was the study lead for the PU-QOL studies including the design, protocol development, co-ordination of all approvals and acquisition of data, data analysis, data interpretation and drafting of Chapter 6; contributed to the design, protocol development, data acquisition and drafting of the risk factor systematic review (part of Chapter 5); and contributed to protocol development and interpretation of data for the PUQOL-UI study and revision of Chapter 7 critically for intellectual content. She was also involved in the co-ordination of the monograph and gave approval to the final version.
Dr Susanne Coleman (Programme Manager) was the study lead for the risk assessment studies including design, protocol development, co-ordination of approvals, acquisition of data and data analysis and interpretation and drafting of Chapter 5; contributed to the design, protocol development, protocol implementation and interpretation of data for the pain, severe pressure ulcer and PU-QOL studies; and contributed to the revision of Chapters 3, 4 and 6 critically for intellectual content. She also gave approval to the final version of the monograph.
Ms Delia Muir (Patient and Public Involvement Officer) was the PPI lead for all projects, set up and co-ordinated the Pressure Ulcer Service User Network UK (PURSUN UK), integrated service user involvement and drafted Chapter 2 and the PPI activity for all work packages (see Chapters 1, 3–8). She also gave approval to the final version of the monograph.
Professor Justin Keen (Professor of Health Politics) was a programme co-applicant. He was involved in the conception of and was the study lead for the severe pressure ulcer studies including design, protocol development, data analysis and data interpretation and drafting of Chapter 4. In addition, he contributed to the design, protocol development, protocol implementation and interpretation of data for the risk assessment study and the revision of Chapter 5 critically for intellectual content. He was the guarantor for Chapter 4 and gave approval to the final version of the monograph.
Professor Christopher McCabe (Professor of Health Economics) was a programme co-applicant. He was involved in the conception of and was the study lead for the PUQOL-UI studies including design, protocol development, protocol implementation, data analysis and data interpretation and the revision of Chapter 7 critically for intellectual content. He was the guarantor for Chapter 7 and gave approval to the final version of the monograph.
Professor Carol Dealey (Honorary Professor of Tissue Viability and Senior Nurse Researcher) was a programme co-applicant. She was involved in the conception of the PURPOSE programme; contributed to the design, protocol development, protocol implementation, local co-ordination of approvals and data acquisition for University Hospitals Birmingham NHS Foundation Trust for all work packages; was involved in the interpretation of data for Chapters 3–6; and was involved in revising Chapters 1–8 critically for intellectual content. She also gave approval to the final version of the monograph.
Professor Michelle Briggs (Professor of Nursing) was a programme co-applicant. She was involved in the conception of the PURPOSE programme; contributed to the design, protocol development, protocol implementation and interpretation of data for the pain study; contributed to drafting and revising of Chapter 3 critically for intellectual content; and contributed to the design, protocol development, protocol implementation, data analysis (systematic reviews) and interpretation of data for the PU-QOL study and the revision of Chapter 6 critically for intellectual content. She also gave approval to the final version of the monograph.
Ms Sarah Brown (Principal Medical Statistician) was involved in the design and supervision of the statistical analysis and interpretation of data for the pain cohort study and the Pressure Ulcer Risk Assessment Framework field test and contributed to drafting and revising Chapters 3 and 5 critically for intellectual content. She also gave approval to the final version of the monograph.
Mrs Michelle Collinson (Senior Medical Statistician) was involved in the design, statistical design and protocol development for all of the pain studies and the statistical analysis and interpretation of data for the two pain prevalence studies and contributed to drafting and revising Chapter 3 critically for intellectual content.
Professor Claire T Hulme (Professor of Health Economics) was involved in the design, protocol development, co-ordination of approvals, data acquisition, data analysis and data interpretation for the PUQOL-UI study and contributed to drafting and revising Chapter 7 critically for intellectual content. She also gave approval to the final version of the monograph.
Mr David M Meads (Lecturer in Health Economics) was involved in the design, protocol development, co-ordination of approvals, data acquisition, data analysis and data interpretation for the PUQOL-UI study and contributed to drafting and revising Chapter 7 critically for intellectual content. He also gave approval to the final version of the monograph.
Dr Elizabeth McGinnis (Tissue Viability Nurse Consultant) was a programme co-applicant. She was involved in the conception of the PURPOSE programme; contributed to the design, protocol development, protocol implementation and local co-ordination of approvals and data acquisition for Leeds Teaching Hospitals NHS Trust for all work packages; was involved in the interpretation of data for and contributed to the drafting of Chapters 2–7 critically for intellectual content. She also gave approval to the final version of the monograph.
Dr Malcolm Patterson (Senior Research Fellow) was involved in the design, data analysis and data interpretation from an organisational psychology perspective for the severe pressure ulcer study and contributed to drafting and revising Chapter 4 critically for intellectual content. He also gave approval to the final version of the monograph.
Ms Carolyn Czoski-Murray (Research Fellow, Health Economics) was involved in the design, protocol development, co-ordination of approvals, data acquisition, data analysis and data interpretation for the PUQOL-UI study and contributed to drafting and revising Chapter 7 critically for intellectual content. She also gave approval to the final version of the monograph.
Ms Lisa Pinkney (Research Officer) was involved in the design, protocol development, co-ordination of approvals, data acquisition and all field work, data analysis and data interpretation for the severe pressure ulcer study and contributed to drafting and revising Chapter 4 critically for intellectual content. She also gave approval to the final version of the monograph.
Ms Isabelle L Smith (Medical Statistician) was involved in the statistical design and analysis and interpretation of data for the pain cohort study and the Pressure Ulcer Risk Assessment Framework field test and contributed to drafting and revising of Chapters 3 and 5 critically for intellectual content. She also gave approval of the final version to the monograph.
Dr Rebecca Stevenson (Trial Co-ordinator and Medical Student) was involved in protocol development, co-ordination of approvals, case report forms and database design, co-ordination of data acquisition and interpretation of analysis for the pain study, was the lead for the community prevalence substudy analysis and contributed to drafting and revising Chapter 3 critically for intellectual content. She also gave approval to the final version of the monograph.
Mrs Nikki Stubbs (Tissue Viability Nurse Specialist) was a programme co-applicant. She was involved in the conception of the PURPOSE programme; contributed to the design, protocol development, protocol implementation, local co-ordination of approvals and data acquisition for Leeds Community Healthcare NHS Trust for all work packages; and was involved in the interpretation of data for and the revision of Chapters 2–7 critically for intellectual content. She also gave approval to the final version of the monograph.
Mrs Lyn Wilson (Programme Manager and Tissue Viability Nurse Specialist) contributed to the design, protocol development, protocol implementation and central co-ordination of approvals and centre set-up for all studies; was involved in the local co-ordination of approvals and data acquisition for Mid Yorkshire Hospitals NHS Trust; and was involved in the interpretation of data for and the revision of Chapters 2–7 critically for intellectual content. She also gave approval of the final version to the monograph.
Professor Julia M Brown (Professor of Clinical Trials Research) was a programme co-applicant. She was involved in the conception of the PURPOSE programme; contributed to the design, protocol development, protocol implementation and interpretation of data for the pain, risk assessment and PU-QOL studies; and contributed to the revision of Chapters 3, 5 and 6 critically for intellectual content. She also gave approval to the final version of the monograph.
Publications
Chapter 2
Muir D. Patient and public involvement in pressure ulcer research. J Tissue Viability 2011;20:132–3.
Muir D. The Pressure Ulcer Research Service User Network UK (PURSUN UK). Eur Wounds Manag Assoc J 2011;11:26.
Coleman S, Muir D, Rawson B and Rawson Y. Patient involvement in risk tool development. Nursing Times 2015;111:17–19.
Chapter 3
Briggs M, Collinson M, Wilson L, Rivers C, McGinnis E, Dealey C, et al. The prevalence of pain at pressure areas and pressure ulcers in hospitalised patients. BMC Nurs 2013;12:19.
Stevenson R, Collinson M, Henderson V, Wilson L, Dealey C, McGinnis E, et al. The prevalence of pressure ulcers in community settings: an observational study. Int J Nurs Stud 2013;50:1550–7.
McGinnis E, Briggs M, Collinson M, Wilson L, Dealey C, Brown J, et al. Pressure ulcer related pain in community populations: a prevalence survey. BMC Nurs 2014;13:16.
Chapter 4
Keen J. Normal accidents: learning how to learn about safety. In Exworthy M, Powell M, Peckham S, Hann A, editors. Shaping Health Policy: Case Study Methods and Analysis. Bristol: Policy Press; 2011. pp. 107–18.
Pinkney L, Nixon J, Wilson L, McGinnis E, Stubbs N, Dealey C, et al. Why do patients develop severe pressure ulcers? A retrospective case study. BMJ Open 2014;4:e004303.
Chapter 5
Coleman S, Gorecki C, Nelson EA, Closs J, Defloor T, Halfens R, et al. Patient risk factors for pressure ulcer development: a systematic review. Int J Nurs 2013;50:974–1003.
Coleman S, Nixon J, Keen J, Wilson L, McGinnis E, Dealey C, et al. A new pressure ulcer conceptual framework. J Adv Nurs 2014;70:2222–34.
Coleman S, Nelson EA, Keen J, Wilson L, McGinnis E, Dealey C, et al. Developing a pressure ulcer risk factor minimum data set and risk assessment framework. J Adv Nurs 2014;70:2339–52.
Chapter 6
Gorecki CA, Brown JM, Briggs M, Nixon J. The evaluation of 5 search strategies in retrieving qualitative patient-reported electronic data of the impact of pressure ulcers on quality of life. J Adv Nurs 2010;66:645–52.
Gorecki CA, Lamping DL, Brown JM, Madill A, Firth J, Nixon J. Development of a conceptual framework of health-related quality of life in pressure ulcers: a patient focused approach. Int J Nurs Stud 2010;47:1525–34.
Gorecki C, Closs J, Nixon J, Briggs M. Patient-reported pressure ulcer pain: a mixed methods systematic review. J Pain Symptom Manage 2011;42:443–59.
Gorecki C, Lamping D, Nixon J, Brown J, Cano S. Applying mixed methods to pre-test the Pressure Ulcer Quality of Life (PU-QOL) instrument. Qual Life Res J 2012;21:441–51.
Gorecki C, Nixon J, Madill A, Firth J, Brown J. What influences the impact of pressure ulcers on health-related quality of life? A qualitative patient-focused exploration of contributory factors. J Tissue Viability 2012;21:3–12.
Gorecki C, Brown J, Cano S, Lamping D, Briggs M, Coleman S, et al. Development and validation of a new patient-reported outcome measure for patients with pressure ulcers: the PU-QOL instrument. Health Qual Life Outcomes 2013;11:95.
Gorecki C, Nixon J, Lamping D, Alvari Y, Brown J. Patient-reported outcome measures for chronic wounds with particular reference to pressure ulcer research: a systematic review. Int J Nurs Stud 2014;51:157–65.
Rutherford C, Nixon J, Brown JM, Lamping DL, Cano SJ. Using mixed methods to select optimal mode of administration for a patient-reported outcome instrument for people with pressure ulcers. BMC Med Res Methodol 2014;14:22.
Overall programme
Nixon J, Wilson LM, Coleman S, Gorecki C, Muir D, Pinkney L, et al. Pressure ulcer programme of research – PURPOSE. Eur Wounds Manag Assoc J 2012;12:21–4.
Research nurses
Choo J, Blundell S, McGinnis E. Ethical issues and challenges in pressure ulcer research – the research nurses’ perspective. J Tissue Viability 2012;21:105–8.
Hemingway B, Storey C. The experience of two registered nurses adapting to the role of a clinical research nurse. Nurs Stand 2013;27:62–8.
Theses
Gorecki CA. The Development and Validation of a Patient-Reported Outcome Measure of Health-Related Quality of Life for Patients with Pressure Ulcers: PUQOL Project. PhD thesis. Leeds: University of Leeds; 2011.
Coleman SB. The Development of a Pressure Ulcer Risk Assessment Framework and Minimum Data Set. PhD thesis. Leeds: University of Leeds; 2014.
Fell JS. A Secondary Analysis of Pain Presentation and Analgesic Use in the PURPOSE Pressure Ulcer Cohort Study. MSc thesis. Leeds: University of Leeds; 2014.
List of presentations and posters
Chapter 2
Muir D, Nixon J. A different type of expertise; patient and public involvement in pressure ulcer research. 14th Annual EPUAP Conference, Oporto, Portugal, September 2011 (oral presentation).
Muir D, McGoverin A, Rawson B. The Pressure Ulcer Research Service User Network. PURPOSE Principal Investigators Training Day, Leeds, UK, December 2011 (oral presentation).
Muir D, Bennett C. Pressure ulcer patient stories. Tissue Viability Society Conference, Kettering, UK, April 2012 (oral presentation, invited speaker).
Muir D, Bell P, Bennett C, Pickersgill A, Rawson Y, Rawson B, et al. Patient and public involvement in pressure ulcer research: lessons learnt and next steps. Tissue Viability Society Conference, Kettering, UK, April 2012 (oral presentation).
Muir D, Pinkney L, McGoverin A, Parker S. Performing an enquiry: an innovative model for involving people in the interpretation of research data. INVOLVE Conference, Nottingham, UK, November 2012 (workshop).
Muir D. Bringing research to life: involving service users in the severe pressure ulcer project. Tissue Viability Society Conference, Kettering, UK, April 2013 (poster presentation).
Muir D. Bringing research to life: involving service users in the severe pressure ulcer project. Involving People Annual Meeting, Cardiff, UK, March 2013 (poster presentation).
Muir D, Rawson B, Rawson Y. Living with a pressure ulcer – a patient and carer perspective. Stop the Pressure Student Conference, Lincoln, UK, October 2013 (oral presentation, invited speaker).
Chapter 3
Nixon J, Wilson L, on behalf of the PURPOSE Project Team. Pressure ulcer pain suffering: issues raised in a multi-centre prevalence. 13th EPUAP Open Meeting, Birmingham, UK, September 2010 (oral presentation, invited speaker).
Nixon J, on behalf of the PURPOSE Project Team. Understanding prevalence of localized pressure ulcer related pain. Tissue Viability Society Conference, Kettering, UK, April 2011 (oral presentation, invited speaker).
Nixon J, on behalf of the PURPOSE Pain Team. Pressure ulcer pain prevalence in community populations: prevalence survey. 14th Annual EPUAP Conference, Oporto, Portugal, September 2011 (oral presentation).
Briggs M, on behalf of the PURPOSE Project Team. The prevalence of pain and pressure ulcers in hospitalized patients; results of a national survey. British Pain Society Annual Scientific Meeting, Liverpool, UK, April 2012 (poster; awarded best poster prize).
Stevenson R, Collinson M, Henderson V, Cozens J, Nixon J. Pressure ulcers in the community: a multicentre prevalence study. 15th Annual EPUAP Conference, Cardiff, UK, September 2012 (oral presentation; winner of the student oral competition).
Nixon J, on behalf of the PURPOSE Pain Team. The prevalence of pressure area and pressure ulcer pain in hospitalised patients. 13th NPUAP Biennial Conference, Houston, TX, USA, February 2013 (poster presentation).
McGinnis E, Nixon J, Briggs M, Collinson M, Wilson L, Rivers C, et al. Prevalence of pressure ulcer pain in community patients. 13th NPUAP Biennial Conference, Houston, TX, USA, February 2013 (poster presentation).
Nixon J. Is pain a predictor of category 2 pressure ulcers? Results of the PURPOSE Pain Cohort Study. Tissue Viability Society Conference, Kettering, UK, April 2013 (oral presentation, invited speaker).
Nixon J, Smith I, Brown S, Nelson EA, McGinnis E, Stubbs N, et al. , on behalf of the PURPOSE Pain Cohort Group. Is pain a predictor of category 2 pressure ulcers? Results of the PURPOSE Pain Cohort Study. 16th Annual EPUAP Conference, Vienna, Austria, September 2013 (oral presentation).
Muir D, Briggs M, McGinnis E and Nixon N. Pain and pressure ulcer development: the service user perspective. TVS Conference, York, 2014 (oral presentation).
Nixon J, Smith I. Is pain a predictor of Category 2 pressure ulcers? Analysis of skin site level data from the PURPOSE Pain Cohort Study, TVS Annual Conference, York, April 2014 (oral presentation).
Smith I, Brown S, McGinnis E, Stubbs N, Nixon J on behalf of the PURPOSE Pain Cohort Group. Is pain a predictor of Category 2 pressure ulcers? Analysis of skin site level data from the PURPOSE Pain Cohort Study, 17th EPUAP Meeting, Stockholm, August 2014 (oral presentation).
Smith I, Brown S, McGinnis E, Stubbs N, Nixon J on behalf of the PURPOSE Pain Cohort Group. What is the extent of pain suffering, and is pain predictive of pressure ulcer development? 25th Conference of the European Wound Management Association, London, May 2015 (oral presentation, invited speaker).
Chapter 4
Pinkney L, Keen J, Nixon J. Why do patients develop severe pressure ulcers? Leeds Institute of Health Sciences Research Postgraduate Symposium, Leeds, UK, June 2010 (oral presentation).
Pinkney L, Keen J, Nixon J. Why do patients develop severe pressure ulcers? BMJ International Forum on Quality and Safety in Healthcare, Amsterdam, Netherlands, April 2011 (poster presentation).
Pinkney L, on behalf of the Severe Pressure Ulcer Project Team. Do organizations cause pressure ulcers? An exploratory review. 14th Annual EPUAP Conference, Oporto, Portugal, September 2011 (oral presentation).
Keen J, on behalf of the Severe Pressure Ulcer Project Team. Severe pressure ulcers: how organisational contexts influence their development. Tissue Viability Society Conference, Kettering, UK, April 2013 (oral presentation).
Dealey C, Keen K, Nixon J, on behalf of the Severe Pressure Ulcer Project Team. Why do patients develop severe pressure ulcers? 16th Annual EPUAP Conference, Vienna, Austria, September 2013 (oral presentation).
Keen J. Why do patients develop severe pressure ulcers? EWMA, London, May 2015 (oral presentation).
Chapter 5
Coleman S, Nixon J, Gorecki C, Nelson EA, on behalf of the PURE Collaborative Group. A systematic review of pressure ulcer risk factors. 14th Annual EPUAP Conference, Oporto, Portugal, September 2011 (oral presentation).
Coleman S, Wilson L, on behalf of the PURAF Project Team. Pressure ulcer risk assessment. Building Blocks of Wound Care Conference North East Tissue Viability Nurses Regional Group, Leeds, UK, September 2011 (oral presentation, invited speaker).
Coleman S. The development of pressure ulcer minimum data set (PU-MDS) using consensus methods. Postgraduate Research Conference, Leeds, UK, December 2012 (oral presentation)
Colman S, on behalf of the PURAF Project Team. Systematic review of pressure ulcer risk factors. 13th NPUAP Biennial Conference, Houston, TX, USA, February 2013 (poster presentation).
Coleman S. From systematic review to clinical practice – risk factor domains to be considered in pressure ulcer risk assessment. Tissue Viability Society Conference, Kettering, UK, April 2013 (oral presentation, invited speaker).
Coleman S, Nixon J, Nelson EA, Farrin A, on behalf of the PURAF Study Group. From systematic review to clinical practice: using consensus methods to develop a Pressure Ulcer Risk Assessment Framework (PURAF). 16th Annual EPUAP Conference, Vienna, Austria, September 2013 (oral presentation).
Coleman S, Nixon J, Nelson EA, Farrin A, on behalf of the PURAF Study Group. The design and pre-testing of a Pressure Ulcer Risk Assessment Framework (PURAF). 16th Annual EPUAP Conference, Vienna, Austria, September 2013 (oral presentation).
Coleman S, Stubbs N, McGinnis E and Nixon J on behalf of the PUPPs and PURPOSE T Implementation Team. Pressure Ulcer Prevention Pathways (PUPPs) and Pressure Ulcer Risk Primary Or Secondary Evaluation Tool (PURPOSE T). TVS, York, April 2014 (workshop).
Coleman S and Nixon J. Translational gap: measuring risk factors in clinical practice, 2nd EPUAP Focus Meeting on Skin Health and Microclimate. Southampton, April 2014, (oral presentation, invited speaker).
Nixon J and Coleman S. Pressure ulcer recognition and prevention: the value of risk assessment. Stop the pressure student conference, Leeds, June 2014 (oral presentation, invited speaker).
Coleman S and Nixon J on behalf of the PUPPs and PURPOSE T Implementation Team. Active monitoring model of care incorporating PURPOSE T Workshop, 17th EPUAP Meeting, Stockholm, Aug 2014 (oral presentation, invited speaker).
Coleman S on behalf of the PURPOSE RAF Project Team. Risk factors in context: from conceptual framework to risk assessment in practice, 17th EPUAP Meeting, Stockholm, August 2014 (oral presentation, invited speaker).
Coleman S. The development of PURPOSE T. Pressure ulcer research: dissemination and implementation conference, Leeds, February 2015 (oral presentation, invited speaker).
Coleman S behalf of the PUPPs and PURPOSE T Implementation Team. PURPOSE T Master Class. Academic Health Science Network, Patient Safety Collaborative: Pressure Damage learning collaborative, Sussex, May 2015 (workshop, invited speaker).
Coleman S and McGinnis E on behalf of the PUPPs and PURPOSE T Implementation Team. Evidenced-based pressure ulcer risk assessment and implementation in clinical practice. EWMA 2015, London, May 2015 (workshop).
Coleman S, Muir D, Rawson B and Rawson Y. Involving patients in pressure ulcer prevention. Patient Safety Congress, Birmingham, July 2015 (oral presentation).
Nixon J and Coleman S, Translation of pressure ulcer risk factor research into practice. Posture and Mobility Group Conference, Leeds, July 2015 (oral presentation, invited speaker).
Chapter 6
Gorecki C, Brown J, Nelson EA, Briggs M, Dealey C, Schoonhoven L, et al. A systematic review of pressure ulcers and quality of life. EPUAP Open Meeting, Oxford, UK, August 2007 (oral presentation).
Gorecki C, Brown J, Nelson EA, Briggs M, Dealey C, Schoonhoven L, et al. A systematic review of pressure ulcers and quality of life. 14th Annual Conference of the International Society of Quality of Life, Toronto, Canada, October 2007 (poster presentation).
Gorecki C, Brown J, Briggs M, Nixon J. Evaluation of 5 search strategies to locate subjective patient-reported HRQoL data. 14th Annual Conference of the International Society of Quality of Life, Toronto, Canada, October 2007 (poster presentation).
Gorecki C, Brown J, Lamping D, Nelson EA, Briggs M, Dealey C, et al. Pressure ulcers and quality of life: systematic review and preliminary results from a qualitative study. Tissue Viability Society Annual Conference, Peterborough, UK, April 2008 (oral presentation, invited speaker).
Gorecki C, on behalf of the PUQOL Project Team. Existing outcome measures used in pressure ulcers. 12th Annual EPUAP Open Meeting, Amsterdam, the Netherlands, September 2009 (oral presentation).
Gorecki C, Brown J, Lamping D, Madill A, Firth J, Nixon J, on behalf of the PUQOL Project Team. Health-related quality of life in pressure ulceration: development of a conceptual framework. 16th Annual Conference of the International Society for Quality of Life Research, New Orleans, LA, USA, October 2009 (oral presentation).
Gorecki C, Brown J, Lamping D, Nixon J. Using cognitive interviewing to improve a newly developed health-related quality of life patient-reported outcome for people with pressure ulcers. 16th Annual Conference of the International Society for Quality of Life Research, New Orleans, LA, USA, October 2009 (poster presentation).
Gorecki C, Closs J, Nixon J, Briggs M. Patient-reported pressure ulcer-associated pain: a mixed-methods systematic review. British Pain Society Annual Scientific Meeting, Manchester, UK, April 2010 (poster presentation).
Briggs M, Gorecki C, Nixon J, Closs SJ. Words used to describe pressure ulcer pain: the results of a systematic review and qualitative synthesis. 13th EPUAP Open Meeting, Birmingham, UK, September 2010 (oral presentation).
Gorecki C. EPUAP Novice Award Lecture 2010. What constitutes health-related quality of life in pressure ulcers and how do we measure it? 13th EPUAP Open Meeting, Birmingham, UK, September 2010 (oral presentation, invited speaker).
Gorecki C, Lamping DL, Nixon J, Brown J, Cano S. The benefits of mixed methods in scale development I: the added value of Rasch analysis in pre-testing. 17th Annual Conference of the International Society for Quality of Life Research, London, UK, October 2010 (poster presentation).
Gorecki C, Nixon J, Lamping DL, Brown J, Cano S. The benefits of mixed methods in scale development II: selecting optimal mode of administration. 17th Annual Conference of the International Society for Quality of Life Research, London, UK, October 2010 (oral presentation, invited speaker).
Firth J, Briggs M, Nelson EA, Gorecki C. Health-related quality of life in patients with rheumatoid arthritis and foot ulceration: care pathways and experiences of care provision. British Health Professionals in Rheumatology Conference, Brighton, UK, April 2011 (poster presentation).
Gorecki C. Challenges in measuring HRQoL in pressure ulcers: development of a PRO measure. Tissue Viability Society Conference, Kettering, UK, April 2011 (oral presentation).
Claudia Gorecki. Development of a patient-reported outcome measure: impact of pressure ulcers on HRQoL. Royal College of Nursing International Conference, Harrogate, UK, May 2011 (oral presentation).
Briggs M, Firth J, Nelson EA, Gorecki, C. Pain and foot ulceration in rheumatoid arthritis; how do patients describe the experience? British Pain Society Annual Conference, Edinburgh, UK, June 2011 (oral presentation).
Gorecki C. Application of mixed methods in early rating scale development. International Rasch Expert Group Meeting, Dubrovnik, Croatia, June 2011 (oral presentation, invited speaker).
Gorecki C, Nixon J, on behalf of the PUQOL Project Team. PU-QOL: a patient-reported outcome measure of health-related quality of life for patients with pressure ulcers. 14th Annual EPUAP Conference, Oporto, Portugal, September 2011 (oral presentation).
Nelson EA, Nixon J, Coleman S, Gorecki C. Pressure ulcer epidemiology, pain and quality of life. 4th Congress of the World Union of Wound Healing Societies, Yokohama, Japan, September 2012 (oral presentation).
Nixon J, Gorecki C, on behalf of the PUQOL Project Team. Final version of a patient-reported outcome measure of health-related quality of life for patients with pressure ulcers (PUQOL). 13th NPUAP National Biennial Conference, Houston, TX, USA, February 2013 (poster presentation).
Rutherford C on behalf of the PUQOL project team. A patient-reported outcome measure of health-related quality of life for patients with pressure ulcers: the PU-QOL instrument. The 4th Australasian Wound and Tissue Repair Society Conference, Queensland, Australia, May 2014 (oral presentation).
Chapter 7
Czoski-Murray C, Meads D, Edlin R, Hulme C, Gorecki C, Nixon J, et al. Constructing a utility algorithm for the Pressure Ulcer Quality of Life – Utility Instrument (PuQol-UI). TVS conference, York, April 2014 (oral presentation).
Overall programme
Nixon J, on behalf of the PURPOSE Collaborative Group. Pressure UlceR Programme Of ReSEarch. Tissue Viability Society Annual Conference, Llandudno, UK, April 2009 (oral presentation).
Nixon J, Keen J, McCabe C, Nelson A, Dealey C, Briggs M, et al. PURPOSE – Pressure UlceR Programme Of reSEarch. Nursing and Midwifery Conference, Leeds, UK, May 2009 (poster presentation).
Nixon J, on behalf of the PURPOSE Collaborative Group. Pressure UlceR Programme Of ReSEarch. Yorkshire and the Humber Directors of Nursing Network Meeting, York, UK, June 2009 (oral presentation).
Nixon J, on behalf of the PURPOSE Collaborative Group. Pressure UlceR Programme Of ReSEarch. A Practical Guide to Reducing Healthcare Associated Pressure Ulcers, Manchester, UK, July 2009 (oral presentation, invited speaker).
Wilson L, Coleman S, on behalf of the PURPOSE Collaborative Group. Pressure UlceR Programme Of reSEarch (PURPOSE). Wounds UK 2009 Wound Care Conference, Harrogate, UK, November 2009 (oral presentation).
Nixon J Wilson L, Coleman S Gorecki C Nelson A, on behalf of the PURPOSE team. Pressure UlceR Programme Of reSEarch (PURPOSE). 13th EPUAP Open Meeting, Birmingham, UK, September 2010 (poster presentation).
Muir D, Nixon J, on behalf of the PURPOSE team. A different type of expertise; patient and public involvement in pressure ulcer research. Royal College of Nursing International Nursing Research Conference, Harrogate, UK, May 2011 (Symposium: programmatic research in pressure ulcer prevention; update on progress and how we are addressing challenges).
Nelson EA, Coleman S, Nixon J, on behalf of the PURPOSE team. Challenges in identifying risk factors for pressure ulceration: systematic review of risk factors and problems addressed. Royal College of Nursing International Nursing Research Conference, Harrogate, UK, May 2011 (Symposium: programmatic research in pressure ulcer prevention; update on progress and how we are addressing challenges).
Nixon J, on behalf of the PURPOSE Pain Team. Pressure ulcer pain suffering: issues raised in a multi-centre pain prevalence study. Royal College of Nursing International Nursing Research Conference, Harrogate, UK, May 2011 (Symposium: programmatic research in pressure ulcer prevention; update on progress and how we are addressing challenges)
Nixon J, Wilson L, Coleman S, Gorecki C, Nelson EA, on behalf of the PURPOSE team. Pressure UlceR Programme Of reSEarch – PURPOSE. Royal College of Nursing International Nursing Research Conference, Harrogate, UK, May 2011 (Symposium: programmatic research in pressure ulcer prevention; update on progress and how we are addressing challenges)
Nixon J, Choo J, McGinnis E, Nelson EA, on behalf of the PURPOSE team. Pressure UlceR Programme Of reSEarch (PURPOSE). 2nd International Nursing Research Conference, University of Malaya, Malaysia, February 2012 (poster presentation).
Nelson EA, Nixon J, Coleman S, Gorecki C. Pressure ulcer epidemiology, pain and quality of life. 4th Congress of the World Union of Wound Healing Societies, Yokohama, Japan, September 2012 (oral presentation).
Wilson L. High impact actions – PURPOSE. E4E Conference, Mid Yorkshire Hospitals NHS Trust, UK, September 2012 (oral presentation).
McGinnis E, Stubbs N, Coleman S, Muir D, Ginn C, Hinchcliffe S, Nixon J. Pressure ulcer research: dissemination and implementation conference, LGI, Leeds, 5 February 2015 (oral presentation).
Research nurses
Storey C, Hemingway B. Reflections on the clinical research nurse role. Tissue Viability Society Conference, Kettering, UK, April 2011 (poster presentation; awarded best poster prize).
Choo J, Blundell S, McGinnis E. Ethical issues and challenges in pressure ulcer research – the research nurses’ perspective. Tissue Viability Society Conference, Kettering, UK, April 2012 (poster presentation).
Awards
-
EPUAP Senior Investigator Award 2012 – Professor Carol Dealey.
-
EPUAP Novice Investigator Award 2010 – Dr Claudia Rutherford (née Gorecki).
-
Poster prize – Storey C, Hemingway B. Reflections on the clinical research nurse role. Tissue Viability Society Conference, Kettering, April 2011.
-
Poster prize – Briggs M, on behalf of the Project Team. The prevalence of pain and pressure ulcers in hospitalized patients; results of a national survey. British Pain Society Annual Scientific Meeting, Liverpool, April 2012.
-
Student oral competition – Stevenson R, Collinson M, Henderson V, Cozens J, Nixon J. Pressure ulcers in the community: a multicentre prevalence study. 15th Annual EPUAP Conference, Cardiff, September 2012.
-
Poster prize – McGinnis E, Nixon J, Briggs M, Collinson M, Wilson L, Rivers C, et al. Prevalence of pressure ulcer pain in community patients. 13th NPUAP National Biennial Conference, Houston, TX, USA, February 2013.
Data sharing statement
Requests for data should be made to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health.
References
- Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. Washington, DC: NPUAP; 2009.
- Kaltenthaler E, Whitfield MD, Walters SJ, Akehurst RL, Paisley S. UK, USA and Canada: how do their pressure ulcer prevalence and incidence data compare?. J Wound Care 2001;10:530-5. http://dx.doi.org/10.12968/jowc.2001.10.1.26039.
- Pieper B. Pressure Ulcers: Prevalence, Incidence, and Implications for the Future. Washington, DC: NPUAP; 2012.
- Vowden K, Vowden P. The prevalence, management, equipment provision and outcome for patients with pressure ulceration identified in a wound care survey within one English health care community. J Tissue Viability 2009;18:20-6. http://dx.doi.org/10.1016/j.jtv.2008.11.001.
- Bennett G, Dealey C, Posnett J. The cost of pressure ulcers in the UK. Age Ageing 2004;33:230-5. http://dx.doi.org/10.1093/ageing/afh086.
- Dealey C, Posnett J, Walker A. The cost of pressure ulcers in the United Kingdom. J Wound Care 2012;21:261-6. http://dx.doi.org/10.12968/jowc.2012.21.6.261.
- Schuurman J, Schoonhoven L, Defloor T, van Engelshoven I, van Ramshorst B, Buskens E. Economic evaluation of pressure ulcer care: a cost minimization analysis of preventive strategies. Nurs Econ 2009;27:390-40.
- Severens J, Habraken J, Duivenvoorden S, Frederiks C. The cost of illness of pressure ulcers in the Netherlands. Adv Skin Wound Care 2002;15:72-7. http://dx.doi.org/10.1097/00129334-200203000-00008.
- Gorecki C, Brown J, Nelson E, Briggs M, Schoonhoven L, Dealey C, et al. Impact of pressure ulcers on quality of life in older patients: a systematic review. J Am Geriatr Soc 2009;57:1175-83. http://dx.doi.org/10.1111/j.1532-5415.2009.02307.x.
- Gorecki C, Nixon J, Madill A, Firth J, Brown J. What influences the impact of pressure ulcers on health-related quality of life? A qualitative patient-focused exploration of contributory factors. J Tissue Viability 2012;21:3-12. http://dx.doi.org/10.1016/j.jtv.2011.11.001.
- Pressure Sores – a Key Quality Indicator: A Guide for NHS Purchasers and Providers. London: Department of Health; 1993.
- Essence of Care: Patient-Focused Benchmarking for Health Care Practitioners. London: Department of Health; 2001.
- Pressure Ulcer Risk Assessment and Prevention. London: NICE; 2001.
- Pressure Relieving Devices (CG7). London: NICE; 2003.
- Pressure Ulcer Management: Cost Impact Report (CG29). London: NICE; 2005.
- NHS 2010–2015, Good to Great, Preventative People Centred, Productive. London: Department of Health; 2009.
- Using the Commissioning for Quality and Innovation (CQUIN) Payment Framework for the NHS England. London: Department of Health; 2008.
- National Institute for Innovation and Improvement . High Impact Actions for Nursing and Midwifery 2009. www.institute.nhs.uk/option,com_joomcart/Itemid,194/main_page,document_product_info/products_id,731.html (accessed 20 October 2013).
- The Operating Framework for the NHS in England 2012/13. London: Department of Health; 2011.
- The Cochrane Collaboration . The Cochrane Library 2009. www.cochranelibrary.com/about/about-the-cochrane-library.html (accessed 6 July 2015).
- Safeguading Vulnerable Groups Bill (HL). London: The Stationery Office; 2006.
- Clinical Governance and Adult Safeguarding. London: Department of Health; 2010.
- Pressure Ulcers in adults: Prediction and Prevention. Quick Reference Guide for Clinicians. Rockville, MD: Agency for Healthcare Research and Quality; 1992.
- Pressure Ulcer Prevention Guidelines. Oxford: EPUAP; 1998.
- National Pressure Ulcer Advisory Panel . Position Statement: Stage 1 Assessment in Darkly Pigmented Skin 1998. www.npuap.org/positn4.htm (accessed 9 March 2015).
- Boote J, Wong R, Booth A. ‘Talking the talk or walking the walk?’ A bibliometric review of the literature on public involvement in health research published between 1995 and 2009 [published online ahead of print 4 October 2012]. Health Expect 2012.
- Brett J, Staniszewska S, Mockford C, Seers K, Herron-Marx S, Bayliss H. The Piricom Study: A Systematic Review of the Conceptualisation, Measurement, Impact and Outcomes of Patients and Public Involvement in Health and Social Care Research. London: University of Warwick; 2010.
- Staley K. Exploring Impact: Public Involvement in NHS, Public Health and Social Care Research. Eastleigh: INVOLVE; 2009.
- Ward P, Thompson J, Barber R, Armitage C, Boote J, Cooper C, et al. Critical perspectives on ‘consumer involvement’ in health research: epistemological dissonance and the know-do gap. J Sociol 2009;46:63-82. http://dx.doi.org/10.1177/1440783309351771.
- Shippee N, Domecq Garces J, Prutsky Lopez G, Wang Z, Elraiyah T, Nabhan M, et al. Patient and service user engagement in research: a systematic review and synthesized framework published online ahead of print 3 June 2013]. Health Expect 2013. http://dx.doi.org/10.1111/hex.12090.
- Stephens L, Ryan-Collins J, Boy D. Co-Production: A Manifesto for Growing the Core Economy. New Economics Foundation 2008. http://s.bsd.net/nefoundation/default/page/-/files/Co-production.pdf (accessed 20 September 2013).
- Morris P, Dalton E, McGoverin A, Symons J, Bradbury H, Frost N, et al. Beyond Reflective Practice. Oxford: Routledge; 2010.
- Spilsbury K, Nelson A, Cullum N, Iglesias C, Nixon J, Mason S. Pressure ulcers and their treatment and effects on quality of life: hospital inpatient perspectives. J Adv Nurs 2007;57:494-50. http://dx.doi.org/10.1111/j.1365-2648.2006.04140.x.
- Hopkins A, Dealey C, Bale S. Patient stories of living with a pressure ulcer. J Adv Nurs 2006;56:345-53. http://dx.doi.org/10.1111/j.1365-2648.2006.04007.x.
- Briggs M, Collinson M, Wilson L, Rivers C, McGinnis E, Dealey C, et al. The prevalence of pain at pressure areas and pressure ulcers in hospitalised patients. BMC Nurs 2013;12. http://dx.doi.org/10.1186/1472-6955-12-19.
- Gorecki C, Closs J, Nixon J, Briggs M. Patient-reported pressure ulcer pain: a mixed methods systematic review. J Pain Symptom Manage 2011;42:443-59. http://dx.doi.org/10.1016/j.jpainsymman.2010.11.016.
- Nixon J, Cranny G, Iglesias C, Nelson EA, Hawkins K, Phillips A, et al. Randomised, controlled trial of alternating pressure mattresses compared with alternating pressure overlays for the prevention of pressure ulcers: PRESSURE (pressure relieving support surfaces) trial. BMJ 2006;332:1413-16. http://dx.doi.org/10.1136/bmj.38849.478299.7C.
- Girouard K, Harrison M, VanDenKerkof E. The symptom of pain with pressure ulcers: a review of the literature. Ostomy Wound Manage 2008;54:30-42.
- Pieper B, Langemo D, Cuddigan J. Pressure ulcer pain: a systematic literature review and National Pressure Ulcer Advisory Panel White Paper. Ostomy Wound Manage 2009;55:16-31.
- Dallam L, Smyth C, Jackson B, Krinsky R, O’Dell C, Rooney J, et al. Pressure ulcer pain: assessment and quantification. J Wound Ostomy Continence Nurs 1995;22:211-15. http://dx.doi.org/10.1097/00152192-199509000-00007.
- Eriksson E, Hietanen H, Asko-Seljavaara S. Prevalence and characteristics of pressure ulcer: a one-day patient population in a Finnish city. Clin Nurs Special 2000;14:199-225. http://dx.doi.org/10.1097/00002800-200005000-00006.
- Hatcliffe S, Dawe R. Clinical audit. Monitoring pressure sores in a palliative care setting. Int J Palliative Nurs 1996;2:182-6.
- Lindholm C, Bergsten A, Berglund E. Chronic wounds and nursing care. J Wound Care 1999;8:5-10. http://dx.doi.org/10.12968/jowc.1999.8.1.25828.
- Woolf C. Pain: moving from symptom control toward mechanism specific pharmacologic management. Ann Intern Med 2004;140:441-51.
- McGinnis E, Briggs M, Collinson M, Wilson L, Dealey C, Brown J, et al. Pressure ulcer related pain in community populations: a prevalence study. BMC Nurs 2014;13. http://dx.doi.org/10.1186/1472-6955-13-16.
- Coleman S, Gorecki C, Nelson E, Closs J, Defloor T, Halfens R, et al. Patient risk factors for pressure ulcer development: systematic review. Int J Nurs Stud 2013;50:974-1003. http://dx.doi.org/10.1016/j.ijnurstu.2012.11.019.
- Vanderwee K, Clark M, Dealey C, Gunningberg L, Defloor T. Pressure ulcer prevalence in Europe: a pilot study. J Eval Clin Pract 2007;13:227-35. http://dx.doi.org/10.1111/j.1365-2753.2006.00684.x.
- Stevenson R, Collinson M, Henderson V, Wilson L, Dealey C, McGinnis E, et al. The prevalence of pressure ulcers in community settings: an observational study. Int J Nurs Stud 2013;50:1550-7. http://dx.doi.org/10.1016/j.ijnurstu.2013.04.001.
- Population Estimates for UK, England and Wales, Scotland and Northern Ireland – Mid 2010. London: ONS; 2011.
- Waterlow J. The Waterlow card for the prevention and management of pressure sores: towards a pocket policy. Care Sci Pract 1988;6:8-12.
- Bergstrom N, Braden BJ, Laguzza A, Holman V. The Braden scale for predicting pressure sore risk. Nurs Res 1987;36:205-10. http://dx.doi.org/10.1097/00006199-198707000-00002.
- Elliott AM, Smith BH, Penny KI, Smith WC, Chambers WA. The epidemiology of chronic pain in the community. Lancet 1999;354:1248-52. http://dx.doi.org/10.1016/S0140-6736(99)03057-3.
- Dworkin R, Turk D, Wyrwich K, Beaton D, Cleeland C, Farrar J, et al. Interpreting the Clinical Importance of Treatment Outcomes in Chronic Pain Clinical Trials: IMMPACT recommendations. J Pain 2008;9:105-21. http://dx.doi.org/10.1016/j.jpain.2007.09.005.
- The Assessment of Pain in Older People: National Guidelines. Consice Guidance to Good Practice Series, No. 8. London: Royal College of Physicians; 2007.
- Bennett M. The LANSS pain scale: the Leeds assessment of neuropathic symptoms and signs. Pain 2001;92:147-57. http://dx.doi.org/10.1016/S0304-3959(00)00482-6.
- Nixon J, Thorpe H, Barrow H, Phillips A, Nelson EA, Mason SA, et al. The reliability of pressure ulcer classification and diagnosis. J Adv Nurs 2005;50:613-23. http://dx.doi.org/10.1111/j.1365-2648.2005.03439.x.
- Defloor T, Grypdonck MF. Pressure ulcers: validation of two risk assessment scales. J Clin Nurs 2005;14:373-82. http://dx.doi.org/10.1111/j.1365-2702.2004.01058.x.
- Schoonhoven L, Haalboom J, Bousema M, Algra A, Grobbee D, Grypdonck M, et al. The prevention and pressure ulcer risk score evaluation study. Prospective cohort study of routine use of risk assessment scales for prediction of pressure ulcers. BMJ 2003;325:797-801. http://dx.doi.org/10.1136/bmj.325.7368.797.
- Machin D, Campbell S. The Design of Studies for Medical Research. Chichester: John Wiley & Sons; 2005.
- Nixon J, Cranny G, Bond S. Skin alterations of intact skin and risk factors associated with pressure ulcer development in surgical patients: a cohort study. Int J Nurs Stud 2007;44:655-63. http://dx.doi.org/10.1016/j.ijnurstu.2006.02.010.
- Stoltzfus J. Logistic regression: a brief primer. Acad Emerg Med 2011;18:1099-104. http://dx.doi.org/10.1111/j.1553-2712.2011.01185.x.
- Hosmer D, Lesmeshow S. Applied Logistic Regression. Hokoben, NJ: John Wiley; 2000.
- Cox D. Regression models and life-tables. J R Stat Soc Ser B 1972;34:187-220.
- Kay R. Goodness of fit methods for the proportional hazards regression model: a review. Rev Epidemiol Sante Publique 1984;32:185-98.
- Wei L. The accelerated failure time model: a useful alternative to the Cox regression model in survival analysis. Stat Med 1992;11:1871-9. http://dx.doi.org/10.1002/sim.4780111409.
- Quirino J, Santos VLC, Quednau TJP, Martins APF, Lima P, Almeida MRM. Pain in pressure ulcers. Wounds 2003;15:381-9.
- Szor JK, Bourguignon C. Description of pressure ulcer pain at rest and at dressing change. J Wound Ostomy Continence Nurs 1999;26:115-20.
- Briggs M, Bennett MI, Closs SJ, Cocks K. Painful leg ulceration: a prospective, longitudinal cohort study. Wound Repair Regen 2007;15:186-91. http://dx.doi.org/10.1111/j.1524-475X.2007.00203.x.
- Hofman D, Ryan TJ, Arnold F, Cherry GW, Lindholm C, Bjellerup M, et al. Pain in venous leg ulcers. J Wound Care 1997;6:222-4.
- Nemeth KA, Harrison MB, Graham ID, Burke S. Pain in pure and mixed aetiology venous leg ulcers: a three-phase point prevalence study. J Wound Care 2003;12:336-40. http://dx.doi.org/10.12968/jowc.2003.12.9.26532.
- VanDenKerkhof E, Hopman W, Carley M, Kuhnke J, Harrison M. Leg ulcer nursing care in the community: a prospective cohort study of the symptom of pain. BMC Nurs 2013;12. http://dx.doi.org/10.1186/1472-6955-12-3.
- Nixon J, Nelson EA, Cranny G, Iglesias CP, Hawkins K, Cullum NA, et al. Pressure relieving support surfaces: a randomised evaluation. Health Technol Assess 2006;10. http://dx.doi.org/10.3310/hta10220.
- Baharestani M, Black J, Carville K, Clark T, Cuddigan J, Dealey C, et al. Dilemmas in measuring and using pressure ulcer prevalence and incidence: an international consensus. Int Wound J 2009;6:97-104. http://dx.doi.org/10.1111/j.1742-481X.2009.00593.x.
- Bethell E. Incidence and prevalence data: can we ensure greater accuracy. J Wound Care 2002;11:285-8. http://dx.doi.org/10.12968/jowc.2002.11.8.26429.
- Dealey C. The size of the pressure sore problem in a teaching hospital. J Adv Nurs 1991;16:663-70. http://dx.doi.org/10.1111/j.1365-2648.1991.tb01724.x.
- Barbenel J, Jordan M, Nicol S, Clark M. Incidence of pressure-sores in the Greater Glasgow health board area. Lancet 1977;310:548-50. http://dx.doi.org/10.1016/S0140-6736(77)90676-6.
- Srinivasaiah N, Dugdall H, Barrett S, Drew P. A point prevalence survey of wounds in north-east England. J Wound Care 2007;16:413-16. http://dx.doi.org/10.12968/jowc.2007.16.10.27910.
- Nixon J, McElvenny D, Mason S, Brown J, Bond S. A sequential randomised controlled trial comparing a dry visco-elastic polymer pad and standard operating table mattress in the prevention of post-operative pressure sores. Int J Nurs Stud 1998;35:193-20. http://dx.doi.org/10.1016/S0020-7489(98)00023-6.
- Allman RM, Goode PS, Patrick MM, Burst N, Bartolucci AA. Pressure ulcer risk factors among hospitalized patients with activity limitation. JAMA 1995;273:865-70. http://dx.doi.org/10.1001/jama.1995.03520350047027.
- Reed RL, Hepburn K, Adelson R, Center B, McKnight P. Low serum albumin levels, confusion, and fecal incontinence: are these risk factors for pressure ulcers in mobility-impaired hospitalized adults?. Gerontology 2003;49:255-9. http://dx.doi.org/10.1159/000070407.
- Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using ‘optimal’ cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst 1994;86:829-35. http://dx.doi.org/10.1093/jnci/86.11.829.
- Altman DG. Systematic reviews in health care: systematic reviews of evaluations of prognostic variables. BMJ 2001;323:224-8. http://dx.doi.org/10.1136/bmj.323.7306.224.
- Egger M, Smith GD, Schneider M, Egger M, Smith GD, Altman DG. Systematic Reviews in Health Care: Meta-Analysis in Context. London: BMJ Publishing Group; 2001.
- Harrell F, Lee K, Matchar D, Reichert T. Regression models for prognostic prediction: advantages, problems and suggested solutions. Cancer Treat Rep 1985;69:1071-7.
- Hayden J, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006;144:427-37. http://dx.doi.org/10.7326/0003-4819-144-6-200603210-00010.
- Maltoni M, Caraceni A, Brunelli C, Broeckaert B, Chrsitakis N, Eychmueller S, et al. Prognostic factors in advanced cancer patients: evidence-based clinical recommendations – a study by the Steering Committee of the European Association for Palliative Care. J Clin Oncol 2005;23:6240-8. http://dx.doi.org/10.1200/JCO.2005.06.866.
- Peduzzi P, Concato J, Feinstein A, Holford T. The importance of events per independent variable in proportional hazards regression analysis II. Accuracy and precision of regression estimates. J Clin Epidemiol 1995;48:1503-10. http://dx.doi.org/10.1016/0895-4356(95)00048-8.
- Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med 2006;25:127-41. http://dx.doi.org/10.1002/sim.2331.
- STROBE . STROBE Statement: Strengthening the Reporting of Observational Studies in Epidemiology n.d. www.strobe-statement.org/PDF/STROBE-Checklist-Version3.pdf (accessed 9 March 2015).
- Corbett A, Husebo B, Malcangio M, Staniland A, Cohen-Mansfield J, Aarsland D, et al. Assessment and treatment of pain in people with dementia. Nat Rev Neurol 2012;8:264-74. http://dx.doi.org/10.1038/nrneurol.2012.53.
- Hadjistavropolous T, Herr K, Turk D, Fine P, Dworkin R, Helme R, et al. An interdisciplinary expert consensus statement on assessment of pain in older persons. Clin J Pain 2007;23:S1-43. http://dx.doi.org/10.1097/AJP.0b013e31802be869.
- Zwakhalen S, Hamers J, Abu-Saad H, Berger M. Pain in elderly people with severe dementia: a systematic review of behavioural pain assessment tools. BMC Geriatr 2006;6. http://dx.doi.org/10.1186/1471-2318-6-3.
- Francis RC. Independent Inquiry into Care Provided by Mid-Staffordshire NHS Foundation Trust, January 2005–March 2009. London: The Stationary Office; 2013.
- National Framework for Reporting and Learning from Serious Incidents Requring Investigation. London: NPSA; 2010.
- Delivering the NHS Safety Thermometer CQUIN 2012/13. London: Department of Health; 2012.
- National Institute for Innovation and Improvement . High Impact Actions. Your Skin Matters n.d. www.institute.nhs.uk/building.capability/hia.supporting.info/your.skin.matters.html (accessed 12 August 2013).
- Leape L, Berwick D, Clancy C, Conway J, Gluck P, Guest J, et al. Transforming healthcare: a safety imperative. Qual Saf Health Care 2009;18:424-8. http://dx.doi.org/10.1136/qshc.2009.036954.
- Reason J. Managing the Risks of Organisational Accidents. Farnham: Ashgate; 1997.
- van Gaal B, Schoonhoven L, Hulscher M, Mintjes J, Borm GF, Koopmans R, et al. The design of the SAFE or SORRY? study: a cluster randomised trial on the development and testing of an evidence based inpatient safety program for the prevention of adverse events. BMC Health Serv Res 2009;9. http://dx.doi.org/10.1186/1472-6963-9-58.
- van Gaal B, Schoonhoven L, Vloet L, Mintjes J, Borm GF, Koopmans R, et al. The effect of the SAFE or SORRY? programme on patient safety knowledge of nurses in hospitals and nursing homes: a cluster randomised trial. Int J Nurs Stud 2010;47:1117-25. http://dx.doi.org/10.1016/j.ijnurstu.2010.02.001.
- Chaves L, Grypdonck M, Defloor T. Pressure ulcer prevention in homecare: do Dutch homecare agencies have an evidence-based pressure ulcer protocol?. J Wound Ostomy Continence Nurs 2006;33:273-80. http://dx.doi.org/10.1097/00152192-200605000-00008.
- Dopierala L, Szewczyk M, Cierzniakowska K, Cwajda J, Popow A, Wyrzykowska M. Level of preparation for preventive procedures and pressure ulcer treatment in health care units from the Kujawsko-Pomorski region. Adv Med Sci 2007;52:81-4.
- Schubert M, Clarke S, Glass T, Schaffert-Witvliet B, De Geest S. Identifying thresholds for relationships between impacts of rationing of nursing care and nurse- and patient-reported outcomes in Swiss hospitals: a correlational study. Int J Nurs Stud 2009;46:884-93. http://dx.doi.org/10.1016/j.ijnurstu.2008.10.008.
- Castle N. Administrator turnover and quality of care in nursing homes. Gerontologist 2001;41:757-67. http://dx.doi.org/10.1093/geront/41.6.757.
- Kennedy M. Improving pressure ulcer prevention in a nursing home: action research. Br J Community Nurs 2005;10:S6-1. http://dx.doi.org/10.12968/bjcn.2005.10.Sup4.20144.
- Yang K. Relationships between nurse staffing and patient outcomes. J Nurs Res 2003;11:149-58. http://dx.doi.org/10.1097/01.JNR.0000347631.87723.de.
- George A, Bennett A. Case Studies and Theory Development in the Social Sciences. Cambridge, MA: MIT Press; 2005.
- Yin R. Case Study Research: Design and Methods. London: Sage; 2008.
- Pawson R. Middle range realism. Eur J Sociol 2000;41:283-325. http://dx.doi.org/10.1017/S0003975600007050.
- Perrow C. Normal Accidents. Princeton, NJ: Princeton University Press; 1999.
- Reason J. The Human Condition. Farnham: Ashgate; 2008.
- Waring J, Rowley E, Dingwall R, Palmer C, Murcott T. A narrative review of the UK’s patient safety research portfolio. J Health Serv Res Policy 2010;15:26-32. http://dx.doi.org/10.1258/jhsrp.2009.009042.
- To Err Is Human. Washington, DC: IoM; 1999.
- Vaughan D. The Challenger Launch Decision. Chicago, IL: University of Chicago Press; 1996.
- Entwistle V. Involving service users in qualitative analysis: approaches and assessment. Health Expect 2010;13:111-12. http://dx.doi.org/10.1111/j.1369-7625.2010.00611.x.
- Nicolini D, Waring J, Mengis J. Policy and practice in the use of root cause analysis to investigate clinical adverse events: mind the gap. Soc Sci Med 2011;73:217-25. http://dx.doi.org/10.1016/j.socscimed.2011.05.010.
- Nicolini D, Waring J, Mengis J. The challenges of undertaking root cause analysis in health care: a qualitative study. J Health Serv Res Policy 2011;16:34-41. http://dx.doi.org/10.1258/jhsrp.2010.010092.
- Nixon J, McGough A, Morison M. The Prevention and Treatment of Pressure Ulcers. Edinburgh: Mosby; 2001.
- Gould D, Goldstone L, Gammon J, Kelly D, Maidwell A. Establishing the validity of pressure ulcer risk assessment scales: a novel approach using illustrated patient scenarios. Int J Nurs Stud 2002;39:215-28. http://dx.doi.org/10.1016/S0020-7489(01)00012-8.
- Bridel J. Risk assessment. J Tissue Viability 1994;4:84-5. http://dx.doi.org/10.1016/S0965-206X(14)80224-6.
- Cullum N, Deeks J, Fletcher A, Long A, Mouneimne H, Sheldon T, et al. The prevention and treatment of pressure sores: how effective are pressure-relieving interventions and risk assessment for the prevention and treatment of pressure sores?. Effective Health Care Bull 1995;2:1-16.
- Steyerberg E. Clinical Prediction Models: a Practical Approach to Development, Validation, and Updating. New York, NY: Springer; 2010.
- Page K, Barker A, Kamar J. Development and validation of a pressure ulcer risk assessment tool for acute hospital patients. Wound Repair Regen 2011;19:31-7. http://dx.doi.org/10.1111/j.1524-475X.2010.00647.x.
- Slowikowski G, Funk M. Factors associated with pressure ulcers in patients in a surgical intensive care unit. J Wound Ostomy Continence Nurs 2010;37:619-26. http://dx.doi.org/10.1097/WON.0b013e3181f90a34.
- Suriadi, Sanada H, Sugama J, Thigpen B, Subuh M. Development of a new risk assessment scale for predicting pressure ulcers in an intensive care unit. Nurs Crit Care 2008;13:34-43. http://dx.doi.org/10.1111/j.1478-5153.2007.00250.x.
- Coleman S, Nelson EA, Keen J, Wilson L, McGinnis E, Dealey C, et al. Developing a pressure ulcer risk factor minimum data set and risk assessment framework. J Adv Nurs 2014;70:2339-52. http://dx.doi.org/10.1111/jan.12444.
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Healthcare. York: Centre for Reviews and Dissemination, University of York; 2009.
- Schulz K, Altman DG, Moher D. CONSORT Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c332.
- Pressure Ulcer Treatment Guidelines. Oxford: EPUAP; 1999.
- Cullum N, Nelson EA, Flemming K, Sheldon T. Systematic reviews of wound care management: beds, compression, laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy. Health Technol Assess 2001;5. http://dx.doi.org/10.3310/hta5090.
- Baldwin KM, Ziegler SM. Pressure ulcer risk following critical traumatic injury. Adv Wound Care 1998;11:168-73. http://dx.doi.org/10.1016/s1361-3111(98)80058-7.
- Bates-Jensen BM, McCreath HE, Kono A, Apeles NC, Alessi C. Subepidermal moisture predicts erythema and stage 1 pressure ulcers in nursing home residents: a pilot study. J Am Geriatr Soc 2007;55:1199-205. http://dx.doi.org/10.1111/j.1532-5415.2007.01261.x.
- Baumgarten M, Margolis D, van Doorn C, Gruber-Baldini AL, Hebel JR, Zimmerman S, et al. Black/white differences in pressure ulcer incidence in nursing home residents. J Am Geriatr Soc 2004;52:1293-8. http://dx.doi.org/10.1111/j.1532-5415.2004.52358.x.
- Bergquist S, Frantz R. Pressure ulcers in community-based older adults receiving home health care. Prevalence, incidence, and associated risk factors. Adv Wound Care 1999;12:339-51.
- Bergstrom N, Braden B. A prospective study of pressure sore risk among institutionalized elderly. J Am Geriatr Soc 1992;40:747-58. http://dx.doi.org/10.1111/j.1532-5415.1992.tb01845.x.
- Bergstrom N, Braden B, Kemp M, Champagne M, Ruby E. Multi-site study of incidence of pressure ulcers and the relationship between risk level, demographic characteristics, diagnoses, and prescription of preventive interventions. J Am Geriatr Soc 1996;41:22-30. http://dx.doi.org/10.1111/j.1532-5415.1996.tb05633.x.
- Berlowitz DR, Wilking SV. Risk factors for pressure sores. A comparison of cross-sectional and cohort-derived data. J Am Geriatr Soc 1989;37:1043-50. http://dx.doi.org/10.1111/j.1532-5415.1989.tb06918.x.
- Bostrom J, Mechanic J, Lazar N, Michelson S, Grant L, Nomura L. Preventing skin breakdown: nursing practices, costs, and outcomes. Appl Nurs Res 1996;9:184-8. http://dx.doi.org/10.1016/S0897-1897(96)80057-7.
- Bourdel-Marchasson I, Barateau M, Rondeau V, Dequae-Merchadou L, Salles-Montaudon N, Emeriau J, et al. A multi-center trial of the effects of oral nutritional supplementation in critically ill older inpatients. GAGE Group. Groupe Aquitain Geriatrique d’Evaluation. Nutrition 2000;16:1-5. http://dx.doi.org/10.1016/S0899-9007(99)00227-0.
- Boyle M, Green M. Pressure sores in intensive care: defining their incidence and associated factors and assessing the utility of two pressure sore risk assessment tools. Aust Crit Care 2001;14:24-30. http://dx.doi.org/10.1016/S1036-7314(01)80019-9.
- Brandeis GH, Ooi WL, Hossain M, Morris JN, Lipsitz LA. A longitudinal study of risk factors associated with the formation of pressure ulcers in nursing homes. J Am Geriatr Soc 1994;42:388-93. http://dx.doi.org/10.1111/j.1532-5415.1994.tb07486.x.
- Chan EY, Tan SL, Lee CK, Lee JY. Prevalence, incidence and predictors of pressure ulcers in a tertiary hospital in Singapore. J Wound Care 2005;14:383-8. http://dx.doi.org/10.12968/jowc.2005.14.8.26820.
- Cobb GA, Yoder LH, Warren JB. Pressure Ulcers: Patient Outcomes on a KinAir Bed or EHOB Waffle Mattress. Fort Sam, Houston, TX: Brooke Army Medical Centre; 1997.
- Compton F, Hoffmann F, Hortig T, Strauss M, Frey J, Zidek W, et al. Pressure ulcer predictors in ICU patients: nursing skin assessment versus objective parameters. J Wound Care 2008;17:417-20. http://dx.doi.org/10.12968/jowc.2008.17.10.31304.
- De Laat E, Pickkers P, Schoonhoven L, Verbeek A, Feuth T, Van Achterberg T. Guideline Implementation Results in a Decrease of Pressure Ulcer Incidence in Critically Ill Patients n.d. http://dx.doi.org/10.1097/01.ccm.0000257072.10313.56.
- Donnelly J. A Randomised Controlled Trial Comparing the Heelift Suspension Boot® With Standard Care in the Prevention of Pressure Ulcers on the Heels of Older People With Fractured Hips 2006.
- Ek A-C. Prediction of pressure sore development. Scand J Caring Sci 1987;1:77-84. http://dx.doi.org/10.1111/j.1471-6712.1987.tb00603.x.
- Ek A-C, Unosson M, Larsson J, Von Schenck H, Bjurulf P. The development and healing of pressure sores related to the nutritional state. Clin Nutr 1991;10:245-50. http://dx.doi.org/10.1016/0261-5614(91)90002-T.
- Feuchtinger J, de Bie R, Dassen T, Halfens R. A 4-cm thermoactive viscoelastic foam pad on the operating room table to prevent pressure ulcer during cardiac surgery. J Clin Nurs 2006;15:162-7. http://dx.doi.org/10.1111/j.1365-2702.2006.01293.x.
- Fife C, Otto G, Capsuto EG, Brandt K, Lyssy K, Murphy K, et al. Incidence of pressure ulcers in a neurologic intensive care unit. Crit Care Med 2001;29:283-90. http://dx.doi.org/10.1097/00003246-200102000-00011.
- Goodridge DM, Sloan JA, LeDoyen YM, McKenzie JA, Knight WE, Gayari M. Risk-assessment scores, prevention strategies, and the incidence of pressure ulcers among the elderly in four Canadian health-care facilities. Can J Nurs Res 1998;30:23-44.
- Gunningberg L, Lindholm C, Carlsson M, Sjoden P-O. Reduced incidence of pressure ulcers in patients with hip fractures: a 2-year follow-up of quality indicators. Int J Qual Health Care 2001;13:399-407. http://dx.doi.org/10.1093/intqhc/13.5.399.
- Halfens RJG, Van Achterberg T, Bal RM. Validity and reliability of the Braden scale and the influence of other risk factors: a multi-centre prospective study. Int J Nurs Stud 2000;37:313-19. http://dx.doi.org/10.1016/S0020-7489(00)00010-9.
- Hatanaka N, Yamamoto Y, Ichihara K, Mastuo S, Nakamura Y, Watanabe M, et al. A new predictive indicator for development of pressure ulcers in bedridden patients based on common laboratory tests results. J Clin Pathol 2008;61:514-18. http://dx.doi.org/10.1136/jcp.2007.050195.
- Inman KJ, Dymock K, Fysh N, Robbins B, Rutledge FS, Sibbald WJ. Pressure ulcer prevention: a randomized controlled trial of 2 risk-directed strategies for patient surface assignment. Adv Wound Care 1999;12:72-80.
- Kemp M, Kopanke D, Tordecilla L, Fogg L, Shott S, Matthiesen V, et al. The role of support surfaces and patient attributes in preventing pressure ulcers in elderly patients. Res Nurs Health 1993;16:89-96. http://dx.doi.org/10.1002/nur.4770160203.
- Lindgren M, Unosson M, Fredrikson M, Ek A-C. Immobility – a major risk factor for development of pressure ulcers among adult hospitalized patients: a prospective study. Scand J Caring Sci 2004;18:57-64. http://dx.doi.org/10.1046/j.0283-9318.2003.00250.x.
- Marchette L, Arnell I, Redick E. Skin ulcers of elderly surgical patients in critical care units. Dimens Crit Care Nurs 1991;10:321-9. http://dx.doi.org/10.1097/00003465-199111000-00006.
- Nijs N, Toppets A, DeFloor T, Bernaerts K, Milisen K, Van Den Berghe G. Incidence and risk factors for pressure ulcers in the intensive care unit. J Clin Nurs 2009;18:1258-66. http://dx.doi.org/10.1111/j.1365-2702.2008.02554.x.
- Okuwa MM, Sanada HPRW, Sugama JP, Inagaki MP, Konya CPRW, Kitagawa AP, et al. A prospective cohort study of lower-extremity pressure ulcer risk among bedfast older adults. Adv Skin Wound Care 2006;19:391-7. http://dx.doi.org/10.1097/00129334-200609000-00017.
- Olson B, Langemo D, Burd C, Hanson D, Hunter S, Cathcart-Silberberg T. Pressure ulcer incidence in an acute care setting. J Wound Ostomy Continence Nurs 1996;23:15-20.
- Ooi WL, Morris JN, Brandeis GH, Hossain M, Lipsitz LA. Nursing home characteristics and the development of pressure sores and disruptive behaviour. Age Ageing 1999;28:45-52. http://dx.doi.org/10.1093/ageing/28.1.45.
- Pancorbo Hidalgo PL, Garcia Fernandez FP. Risk factors for the development of pressure ulcers among hospitalized elderly patients. Gerokomos 2001;12:175-84.
- Perneger TV, Rae A-C, Gaspoz J-M, Borst F, Vitek O, Heliot C. Screening for pressure ulcer risk in an acute care hospital: development of a brief bedside scale. J Clin Epidemiol 2002;55:498-504. http://dx.doi.org/10.1016/S0895-4356(01)00514-5.
- Rademakers LMF, Vainas T, van Zutphen SWA, Brink PRG, van Helden SH. Pressure ulcers and prolonged hospital stay in hip fracture patients affected by time-to-surgery. Eur J Trauma Emerg Surg 2007;33:238-44. http://dx.doi.org/10.1007/s00068-007-6212-8.
- Rose P, Cohen R, Amsel R. Development of a scale to measure the risk of skin breakdown in critically ill patients. Am J Crit Care 2006;15:337-41.
- Salzberg CA, Byrne DW, Kabir R, van Niewerburgh P, Cayten CG. Predicting pressure ulcers during initial hospitalization for acute spinal cord injury. Wounds 1999;11:45-57.
- Sayar S, Turgut S, Dogan H, Ekici A, Yurtsever S, Demirkan F, et al. Incidence of pressure ulcers in intensive care unit patients at risk according to the Waterlow scale and factors influencing the development of pressure ulcers. J Clin Nurs 2009;18:765-74.
- Schnelle JF, Adamson GM, Cruise PA, Al-Samarrai N, Sarbaugh FC, Uman G, et al. Skin disorders and moisture in incontinent nursing home residents: intervention implications. J Am Geriatr Soc 1997;45:1182-8. http://dx.doi.org/10.1111/j.1532-5415.1997.tb03767.x.
- Schoonhoven L, DeFloor T, van der Tweel I, Buskens E, Grypdonck M. Risk indicators for pressure ulcers during surgery. Appl Nurs Res 2002;16:163-73. http://dx.doi.org/10.1053/apnr.2002.34145.
- Schultz A, Bien M, Dumond K, Brown K, Myers A. Etiology and incidence of pressure ulcers in surgical patients. AORN J 1999;70:437-40. http://dx.doi.org/10.1016/S0001-2092(06)62325-9.
- Serpa LF, Santos VLC. Assessment of the nutritional risk for pressure ulcer development through Braden scale. The 39th Annual Wound, Ostomy and Continence Nurses Annual Conference. J Wound Ostomy Continence Nurs 2007;34:S4-6. http://dx.doi.org/10.1097/01.WON.0000270854.08299.7c.
- Stordeur S, Laurent S, D’Hoore W. The importance of repeated risk assessment for pressure sores in cardiovascular surgery. J Cardiovasc Surg 1998;39:343-9.
- Suriadi, Sanada H, Sugama J, Kitagawa A, Thigpen B, Kinosita S, et al. Risk factors in the development of pressure ulcers in an intensive care unit in Pontianak, Indonesia. Int Wound J 2007;4:208-15. http://dx.doi.org/10.1111/j.1742-481X.2007.00315.x.
- Tourtual DM, Riesenberg LA, Korutz CJ, Semo AH, Asef A, Talati K, et al. Predictors of hospital acquired heel pressure ulcers. Ostomy Wound Manag 1997;43:24-8.
- Vanderwee K, Grypdonck M, Bacquer DD, DeFloor T. The identification of older nursing home residents vulnerable for deterioration of grade 1 pressure ulcers. J Clin Nurs 2009;18:3050-8. http://dx.doi.org/10.1111/j.1365-2702.2009.02860.x.
- Watts D, Abrahams E, MacMillan C, Sanat J, Silver R, VanGorder S, et al. Insult after injury: pressure ulcers in trauma patients. Orthop Nurs 1998;17:84-91. http://dx.doi.org/10.1097/00006416-199807000-00012.
- Yepes D, Molina F, Leon W, Perez E. Incidence and risk factors associated with the presence of pressure ulcers in critically ill patients. Med Intensiva 2009;33:276-81. http://dx.doi.org/10.1016/S0210-5691(09)72195-3.
- Fitch K, Bernstein S, Aguilar M, Burnand B, LaCalle J, Lazaro P, et al. The RAND/UCLA Appropriateness Method User’s Manual. Santa Monica, CA: RAND Corporation; 2001.
- Hutchings A, Raine R. A systematic review of factors affecting the judgments produced by formal consensus development methods in health care. J Health Serv Res Policy 2006;11:172-9. http://dx.doi.org/10.1258/135581906777641659.
- Murphy M, Black N, Lamping D, McKee C, Sanderson C, Askham J, et al. Consensus development methods and their use in clinical guideline development. Health Technol Assess 1998;2.
- Raine R, Sanderson C, Black N. Developing clinical guidelines: a challenge to current methods. BMJ 2005;331:631-3. http://dx.doi.org/10.1136/bmj.331.7517.631.
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005;15:1277-88. http://dx.doi.org/10.1177/1049732305276687.
- Kroger E, Tourigny A, Morin D, Cote L, Kergoat M, Lebel P, et al. Selecting process quality indicators for the integrated care of vulnerable older adults affected by cognitive impairment or dementia. BMC Health Serv Res 2007;7. http://dx.doi.org/10.1186/1472-6963-7-195.
- Scott E, Black N. Appropriateness of cholecystectomy in the United Kingdom – a consensus panel approach. Gut 1991;32:1066-70. http://dx.doi.org/10.1136/gut.32.9.1066.
- Shiffman R, Shekelle P, Overhage J, Slutsky J, Grimshaw J, Deshpande A. Standardised reporting of clinical practice guidelines: a proposal from the conference on guidance standardization. Ann Intern Med 2003;139:493-8. http://dx.doi.org/10.7326/0003-4819-139-6-200309160-00013.
- Coleman S, Nixon J, Keen J, Wilson L, McGinnis E, Dealey C, et al. A new pressure ulcer conceptual framework. J Adv Nurs 2014;70:2222-34. http://dx.doi.org/10.1111/jan.12405.
- Brotman D, Walker E, Lauer M, O’Brien R. In search of fewer independent risk factors. Arch Intern Med 2005;165:139-45. http://dx.doi.org/10.1001/archinte.165.2.138.
- Collins D. Pretesting survey instruments: an overview of cognitive methods. Qual Life Res 2003;12:229-38. http://dx.doi.org/10.1023/A:1023254226592.
- McColl E, Fayers P, Hays R. Assessing Quality of Life in Clinical Trials. New York, NY: Oxford University Press; 2005.
- Willis G. Cognitive Interviewing: A Tool for Improving Questionnaire Design. Thousand Oaks, CA: Sage; 2005.
- Kitzinger J. Qualitative research: introducing focus groups. BMJ 1995;311:299-302. http://dx.doi.org/10.1136/bmj.311.7000.299.
- Bergstrom N, Braden BJ, Kemp M, Champagne M, Ruby E. Predicting pressure ulcer risk: a multi-site study of the predictive validity of the Braden scale. Nurs Res 1998;47:261-9. http://dx.doi.org/10.1097/00006199-199809000-00005.
- Blazeby J, Sprangers M, Cull A, Groenvold M, Bottomley A. EORTC Quality of Life Group: Guidelines for Developing Questionnaire Modules, 3rd Edition Revised 2002. http://groups.eortc.be/qol/sites/default/files/archives/guidelines_for_developing_questionnaire-_final.pdf (accessed 9 March 2015).
- Nunnally J, Bernstein I. Psychometric Theory. New York, NY: McGraw-Hill; 1994.
- Bland J. Measuring Health and Disease: Cohen’s Kappa. York: Department of Health Sciences, University of York; 2008.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. http://dx.doi.org/10.2307/2529310.
- Burnand B, Kernan W, Feinstein A. Indexes and boundaries for ‘quantitative significance’ in statistical decisions. J Clin Epidemiol 1990;143:1278-84. http://dx.doi.org/10.1016/0895-4356(90)90093-5.
- Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas 1960;20:37-46. http://dx.doi.org/10.1177/001316446002000104.
- Kerlinger F. Foundations of Behavioural Research. New York, NY: Holt, Rinehart, and Winston; 1973.
- Raine R, Sanderson C, Hutchings A, Carter S, Larkin K, Black N. An experimental study of determinants of group judgments in clinical guideline development. Lancet 2004;364:429-37. http://dx.doi.org/10.1016/S0140-6736(04)16766-4.
- Jackson A, Hettinga D, Mead J, Mercer C. Using consensus methods in developing clinical guidelines for exercise in managing persistent low back pain. Physiotherapy 2009;95:302-11. http://dx.doi.org/10.1016/j.physio.2009.08.001.
- Rycroft-Malone J. Formal consensus: the development of a national clinical guideline. Qual Health Care 2001;10:238-44. http://dx.doi.org/10.1136/qhc.0100238.
- Rolls K, Elliott D. Using consensus methods to develop clinical practice guidelines for intensive care: the intensive care collaborative project. Aust Crit Care 2008;21:200-15. http://dx.doi.org/10.1016/j.aucc.2008.08.003.
- Fallowfield L. The Quality of Life: Missing Measurement in Health Care. London: Souvenir Press; 1990.
- Ware JE. The status of health assessment 1994. Annu Rev Public Health 1995;16:327-54. http://dx.doi.org/10.1146/annurev.pu.16.050195.001551.
- Greenhalgh J, Meadows K. The effectiveness of the use of patient-based measures of health in routine practice in improving the process and outcomes of patient care: a literature review. J Eval Clin Pract 1999;5:401-16. http://dx.doi.org/10.1046/j.1365-2753.1999.00209.x.
- Fox C. Living with a pressure ulcer: a descriptive study of patients’ experiences. Br J Community Nurs 2002;7. http://dx.doi.org/10.12968/bjcn.2002.7.Sup1.12954.
- Langemo DK, Melland H, Hanson D, Olson B, Hunter S. The lived experience of having a pressure ulcer: a qualitative analysis. Adv Skin Wound Care 2000;13:225-35.
- Rastinehad D. Pressure ulcer pain. Journal Wound Ostomy Continence Nurs 2006;33:252-7. http://dx.doi.org/10.1097/00152192-200605000-00005.
- McInnes E, Jammali-Blasi A, Bell-Syer S, Dumville J, Cullum N. Support surfaces for pressure ulcer prevention. Cochrane Database Syst Rev 2011;13.
- US Department of Health and Human Services Food and Drug Administration . Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labelling Claims 2009. www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm (accessed 9 March 2015).
- Patrick DL, Deyo RA. Generic and disease-specific measures in assessing health status and quality of life. Med Care 1989;27:217-32. http://dx.doi.org/10.1097/00005650-198903001-00018.
- Fitzpatrick R, Davey C, Buxton MJ, Jones DR. Evaluating patient-based outcome measures for use in clinical trials. Health Technol Assess 1998;2.
- Garratt A, Schmidt L, Mackintosh A, Fitzpatrick R. Quality of life measurement: bibliographic study of patient assessed health outcome measures. BMJ 2002;324:1417-22. http://dx.doi.org/10.1136/bmj.324.7351.1417.
- Gorecki C, Nixon J, Lamping DL, Alavi Y, Brown JM. Patient-reported outcome measures for chronic wounds with particular reference to pressure ulcer research: a systematic review. Int J Nurs Stud 2014;51:157-65. http://dx.doi.org/10.1016/j.ijnurstu.2013.03.004.
- Browne J, Jamieson L, Lawsey J, van der Meulen J, Black N, Cairns J, et al. Patient Reported Outcome Measures (Proms) in Elective Surgery 2007. www.lshtm.ac.uk/hsru/research/PROMs-Report-12-Dec-07.pdf (accessed 14 September 2011).
- Fayers P. Evaluating the effectiveness of using PROs in clinical practice: a role for cluster-randomised trials. Qual Life Res 2008;17:1315-21. http://dx.doi.org/10.1007/s11136-008-9391-9.
- Greenhalgh J. The applications of PROs in clinical practice: what are they, do they work, and why?. Qual Life Res 2009;18:115-23. http://dx.doi.org/10.1007/s11136-008-9430-6.
- Velikova G, Booth L, Smith A, Brown P, Lynch P, Brown J. Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomised controlled trial. J Clin Oncol 2004;22:714-24. http://dx.doi.org/10.1200/JCO.2004.06.078.
- Gorecki C, Lamping DL, Brown JM, Madill A, Firth J, Nixon J. Development of a conceptual framework of health-related quality of life in pressure ulcers: a patient-focused approach. Int J Nurs Stud 2010;47:1525-34. http://dx.doi.org/10.1016/j.ijnurstu.2010.05.014.
- Gorecki C, Lamping D, Nixon J, Brown J, Cano S. Applying mixed methods to pretest the Pressure Ulcer Quality of Life (PU-QOL) instrument. Qual Life Res 2012;21:441-51. http://dx.doi.org/10.1007/s11136-011-9980-x.
- Gorecki C, Brown J, Cano S, Lamping D, Briggs M, Coleman S, et al. Development and validation of a new patient-reported outcome measure for patients with pressure ulcers: the PU-QOL instrument. Health Qual Life Outcomes 2013;11. http://dx.doi.org/10.1186/1477-7525-11-95.
- Rothman M, Burke L, Erickson P, Kline Leidy N, Patrick D, Petrie C. Use of existing patient-reported outcome (PRO) instruments and their modification: the ISPOR good research practices for evaluating and documenting content validity for the use of existing instruments and their modification PRO tasck force report. Value Health 2009;12:1075-83. http://dx.doi.org/10.1111/j.1524-4733.2009.00603.x.
- Streiner D, Norman G. Health Measurement Scales: A Practical Guide to their Development and Use. Oxford: Oxford University Press; 2003.
- Skevington SM, Sartorius N, Amir M, Sartorius N, Orley J, Kuyken W, et al. Developing methods for assessing quality of life in different cultural settings – the history of the WHOQOL instruments. Soc Psychiatry Psychiatr Epidemiol 2004;39:1-8. http://dx.doi.org/10.1007/s00127-004-0700-5.
- Sudman S, Bradburn N. Asking Questions: A Practical Guide to Questionnaire Design. San Francisco, CA: Jossey-Bass; 1982.
- McColl E, Jacoby A, Thomas L, Soutter J, Bamford C, Steen N, et al. Design and use of questionnaires: a review of best practice applicable to surveys of health service staff and patients. Health Technol Assess 2001;5. http://dx.doi.org/10.3310/hta5310.
- Fayers P, Hays R. Assessing Quality of Life in Clinical Trials: Methods and Practice. Oxford: Oxford University Press; 2005.
- Rasch G. Probabilistic Models for Some Intelligence and Attainment Tests. Chicago, IL: University of Chicago; 1960.
- Willis G, Lessler J. Question Appraisal System: QAS-99. Rockville, MD: Research Triangle Institute; 1999.
- Andrich D. Distinctions between Assumptions and Requirements in Measurements in the Social Sciences. North Holland: Elsevier Science Publications BV; 1989.
- Andrich D. Rasch Models for Measurement. Beverly Hills, CA: Sage; 1988.
- Cano S, Barrett L, Zajicek J, Hobart J. Beyond the reach of traditional analyses: using Rasch to evaluate the DASH in people with multiple sclerosis. Mult Scler J 2011;17:214-22. http://dx.doi.org/10.1177/1352458510385269.
- Andrich D, Brandon-Tuma N. Sociological Methodology. San Fransisco, CA: Jossey-Bass; 1985.
- Christodoulou C, Junghaenel DU, DeWalt DA, Rothrock N, Stone AA. Cognitive interviewing in the evaluation of fatigue items: results from the patient-reported outcomes measurement information system (PROMIS). Qual Life Res 2008;17:1239-46. http://dx.doi.org/10.1007/s11136-008-9402-x.
- Andrich D, Luo G, Sheridan B. Interpreting RUMM2020. Perth, WA: RUMM Laboratory; 2004.
- Linacre J. Sample size and item calibration stability. Rasch Meas Trans 1994;7.
- Allen M, Yen W. Introduction to Measurement Theory. Monterey, CA: Brooks/Cole; 1979.
- DeVellis R. Classical test theory. Med Care 2006;44:S50-9. http://dx.doi.org/10.1097/01.mlr.0000245426.10853.30.
- Hobart J, Cano S, Zajicek J, Thompson A. Rating scales as outcome measures for clinical trials in neurology: problems, solutions, and recommendations. Lancet Neurol 2007;6:1094-5. http://dx.doi.org/10.1016/S1474-4422(07)70290-9.
- Lipscomb J, Donaldson M, Hiatt R. Cancer outcomes research and the arenas of application. JNCI Monogr 2004;33:1-7. http://dx.doi.org/10.1093/jncimonographs/lgh038.
- Reeve B, Hays R, Chang C, Perfetto E. Applying item response theory to enhance health outcomes assessment. Qual Life Res 2007;16:195-9. http://dx.doi.org/10.1007/s11136-007-9220-6.
- Tennant A, McKenna S, Hagell P. Application of Rasch analysis in the development and application of quality of life instruments. Value Health 2004;7:S22-6. http://dx.doi.org/10.1111/j.1524-4733.2004.7s106.x.
- McHorney CA, Ware J, Lu J, Sherbourne C. The MOS 36-item short-form health survey (SF-36): III. Tests of data quality, scaling assumptions and reliability across diverse patient groups. Med Care 1994;32:40-66. http://dx.doi.org/10.1097/00005650-199401000-00004.
- WHOQOL Group . The World Health Organization Quality of Life Assessment (WHOQOL): development and general psychometric properties. Soc Sci Med 1998;46:1569-85. http://dx.doi.org/10.1016/S0277-9536(98)00009-4.
- Ware J, Snow K, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Boston, MA: New England Medical Centre; 1997.
- Likert R. A technique for the measurement of attitudes. Arch Psychol 1932;22:5-55.
- Hagquist C, Andrich D. Is the Sense of Coherence instrument applicable on adolescents? A latent trait analysis using Rasch modelling. Pers Individ Dif 2004;36:955-68. http://dx.doi.org/10.1016/S0191-8869(03)00164-8.
- Hobart J, Cano S. Improving the evaluation of therapeutic interventions in multiple sclerosis: the role of new psychometric methods. Health Technol Assess 2009;13. http://dx.doi.org/10.3310/hta13120.
- McHorney CA, Tarlov A. Individual-patient monitoring in clinical practice: are available health status surveys adequate?. Qual Life Res 1995;4:293-307. http://dx.doi.org/10.1007/BF01593882.
- Hays R, Anderson R, Revicki D. Psychometric consideration in evaluating health-related quality of life measures. Qual Life Res 1993;2:441-9. http://dx.doi.org/10.1007/BF00422218.
- Lohr KN, Aaronson NK, Alonso J, Burnam MA, Patrick DL, Perrin EB, et al. Evaluating quality-of-life and health status instruments: development of scientific review criteria. Clin Ther 1996;18:979-92. http://dx.doi.org/10.1016/S0149-2918(96)80054-3.
- Scientific Advisory Committee of the Medical Outcomes Trust . Assessing health status and quality-of-life instruments: attributes and review criteria. Qual Life Res 2002;11:193-205. http://dx.doi.org/10.1023/A:1015291021312.
- Kaplan R, Bush J, Berry C. Health status: types of validity and the Index of Well-being. Health Serv Res 1976;11:478-507.
- Wright B, Masters G. Rating Scale Analysis: Rasch Measurement. Chicago, IL: MESA; 1982.
- Tennant A, Conaghan P. The measurement model in rheumatology: what is it and why use it? When should it be applied, and what should one look for in a Rasch paper?. Arthritis Rheum 2007;57:1358-62. http://dx.doi.org/10.1002/art.23108.
- Hobart J, Riazi A, Thompson A, Styles I, Ingram W, Vickery P, et al. Getting the measure of spasticity in multiple sclerosis: the Multiple Sclerosis Spasticity Scale (MSSS-88). Brain 2006;129:224-34. http://dx.doi.org/10.1093/brain/awh675.
- Smith E. Detecting and evaluating the impact of multidimensionality using item fit statistics and principal components analysis of residuals. J Appl Meas 2002;3:205-31.
- Teresi J, Ramirez M, Lai J, Silver S. Occurences and sources of differential item fuctioning (DIF) in patient-reported outcome measures: description of DIF methods, and review of measures of depression, quality of life and general health. Psychol Sci Q 2008;50.
- Kazis L, Anderson J, Meenan R. Effect sizes for interpreting changes in health status. Med Care 1989;27:S178-89. http://dx.doi.org/10.1097/00005650-198903001-00015.
- Cano SJ, Browne JP, Lamping DL, Roberts AH, McGrouther DA, Black NA. The Patient Outcomes of Surgery – Head/Neck (POS-head/neck): a new patient-based outcome measure. J Plast Reconstr Aesthet Surg 2006;59:65-73. http://dx.doi.org/10.1016/j.bjps.2005.04.060.
- Lamping DL, Schroter S, Marquis P, Marrel A, Duprat-Lomon I, Sagnier PP. The community-acquired pneumonia symptom questionnaire: a new, patient-based outcome measure to evaluate symptoms in patients with community-acquired pneumonia. Chest 2002;122:920-9. http://dx.doi.org/10.1378/chest.122.3.920.
- Andrich D. Rating formulation for ordered response categories. Psychometrika 1978;43:561-73. http://dx.doi.org/10.1007/BF02293814.
- Zumbo BD. A Handbook on the Theory and Methods of Differential Item Functioning (DIF): Logistic Regression Modeling as a Unitary Framework for Binary and Likert-Type (Ordinal) Item Scores. Ottawa, ON: Directorate of Human Resources Research and Evaluation, Department of National Defense; 1999.
- Lamping DL, Schroter S, Kurz X, Kahn SR, Abenhaim L. Evaluation of outcomes in chronic venous disorders of the leg: development of a scientifically rigorous, patient-reported measure of symptoms and quality of life. J Vasc Surg 2003;37:410-19. http://dx.doi.org/10.1067/mva.2003.152.
- Fleiss J. Reliability of Measurements. The Design and Analysis of Clinical Experiments. New York: John Wiley; 1986.
- Guyatt G, Walter S, Norman G. Measuring change over time: assessing the usefulness of evaluative instruments. J Chronic Disabil 1987;4:171-8. http://dx.doi.org/10.1016/0021-9681(87)90069-5.
- Ware J, Kosinski M, Turner-Bowker D. How to Score Version 2 of the SF-12 Health Survey. Lincolm, RI: Quality Metric Incorporated; 2002.
- Ware J, Kosinski M, Keller SD. Physical and Mental Component Summary Measures: A Users’ Manual. Boston, MA: Health Institute, New England Medical Centre; 1994.
- Bowling A. Measuring Health: A Review of Quality of Life Measurement Scales. Berkshire: Open University Press; 2005.
- Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life: a conceptual model of patient outcomes. JAMA 1995;273:59-65. http://dx.doi.org/10.1001/jama.1995.03520250075037.
- Guide to the Methods of Technology Appraisal. London: NICE; 2008.
- EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- Brazier J, Usherwood T, Harper R, Thomas K. Deriving a preference-based single index from the UK SF-36 health survey. J Clin Epidemiol 1998;51:1115-28. http://dx.doi.org/10.1016/S0895-4356(98)00103-6.
- Torrance GW, Furlong W, Feeny D, Boyle M. Multi-attribute preference functions. Health Utilities Index. Pharmacoeconomics 1995;7:503-20. http://dx.doi.org/10.2165/00019053-199507060-00005.
- Dolan P. Modeling valuations for EuroQoL health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- Hulme C, Long A, Kneafsey R, Reid G. Using the EQ-5D to assess health-related quality of life in older people. Age Ageing 2004;33:504-7. http://dx.doi.org/10.1093/ageing/afh178.
- Malley J, Caiels J, Fox D, McCarthy M, Smith N, Beadle-Brown J, et al. A Report on the Developmental Studies for the National Adult Social Care User Experience Survey. Canterbury: PSSRU, University of Kent; 2010.
- Report on NICE Citizens Council Meeting. Quality Adjusted Life Years (QALYs) and the Severity of Illness. London: NICE; 2008.
- Ashby R, Dumville J, Soares M, McGinnis E, Stubbs N, Torgerson D, et al. A pilot randomised controlled trial of negative pressure wound therapy to treat grade III/IV pressure ulcers [ISRCTN69032034]. Trials 2012;28. http://dx.doi.org/10.1186/1745-6215-13-119.
- Essex HN, Clark M, Sims J, Warriner A, Cullum N. Health-related quality of life in hospital inpatients with pressure ulceration: assessment using generic health-related quality of life measures. Wound Repair Regen 2009;17:797-805. http://dx.doi.org/10.1111/j.1524-475X.2009.00544.x.
- Jull A, Parag V, Walker N, Rodgers A. Responsiveness of generic and disease-specific health-related quality of life instruments to venous ulcer healing. Wound Repair Regen 2010;18:26-30. http://dx.doi.org/10.1111/j.1524-475X.2009.00556.x.
- Versteegh M, Leunis A, Uyl-de Groot C, Stolk E. Condition-specific preference-based measures: benefit or burden?. Value Health 2012;15:504-13. http://dx.doi.org/10.1016/j.jval.2011.12.003.
- Brazier J, Czoski-Murray C, Roberts J, Brown M, Symonds T, Kelleher C. Estimation of a preference-based index from a condition-specific measure: the King’s health questionnaire. Med Decis Making 2008;28:215-25.
- Yang Y, Brazier J, Tsuchiya A, Young T. Estimating a preference-based index for a 5-dimensional health state classification for asthma derived from the asthma quality of life questionnaire. Med Decis Making 2011;31:281-91. http://dx.doi.org/10.1177/0272989X10379646.
- Mavranezouli I, Brazier J, Rowan D, Barkham M. Estimating a preference-based index from the Clinical Outcomes in Routine Evaluation – Outcome Measure (CORE-OM): valuation of CORE-6D. Med Decis Making 2013;33:381-95. http://dx.doi.org/10.1177/0272989X12464431.
- Mulhem B, Rowan D, Brazier J, Smith S, Romeo R, Tait R, et al. Development of DEMQOL-U and DEMQOL-PROXY-U: generation of preference-based indices from DEMQOL and DEMQOL-PROXY for use in economic evaluation. Health Technol Assess 2013;17.
- McKenna S, Ratcliffe J, Meads D, Brazier J. Development and validation of a preference based measure derived from the Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR) for use in cost utility analyses. Health Qual Life Outcomes 2008;21. http://dx.doi.org/10.1186/1477-7525-6-65.
- Thein H, Gomes T, Krahn M, Wodchis W. Health status utilities and the impact of pressure ulcers in long-term care residents in Ontario. Qual Life Res 2009;19:81-9. http://dx.doi.org/10.1007/s11136-009-9563-2.
- McCabe C, Eldin R, Meads D, Brown C, Kharroubi S. Constructing indirect utility models: some observations on the principles and practice of mapping to obtain health state utilities. Pharmacoeconomics 2013;31:635-41. http://dx.doi.org/10.1007/s40273-013-0071-4.
- Brazier J, Rowan D. NICE DSU Technical Support Document 11: Alternatives to EQ-5D for Generating Health State Utility Values 2011. www.nicedsu.org.uk/TSD11%20Alternatives%20to%20EQ-5D_final.pdf (accessed 9 March 2015).
- Young T, Rowan D, Brazier J, Norquist J, Ambegaonkar B, Sazonov V. Developing preference-based health measures: using Rasch analysis to generate health state values. Qual Life Res 2010;19:907-17. http://dx.doi.org/10.1007/s11136-010-9646-0.
- Young T, Yang Y, Brazier J, Tsuchiya A. The use of Rasch analysis in reducing a large condition-specific instrument for preference valuation: the case of moving from AQLQ to AQL-5D. Med Decis Making 2011;31:195-210. http://dx.doi.org/10.1177/0272989X10364846.
- Rasch G. On General Laws and the Meaning of Measurement in Psychology. Berkeley, CA: University of California Press; 1961.
- Luquet C, Chau N, Guillemin F, Nadif M, Moreau T, Gavillot C, et al. A method for shortening instruments using the Rasch model. Validation on a hand functional measure. Rev Epidemiol Sante Publique 2001;49:273-86.
- Prieto L, Alonso J, Lamarca R. Classical test theory versus Rasch analysis for quality of life questionnaire reduction. Health Qual Life Outcomes 2003;1. http://dx.doi.org/10.1186/1477-7525-1-27.
- Waugh R, Chapman E. An analysis of dimensionality using factor analysis (true-score theory) and Rasch measurement: what is the difference? Which method is better?. J Appl Meas 2005;6:80-99.
- Brazier J, Rowan D, Mayranezouli I, Tsuchiya A, Young T, Yang Y, et al. Developing and testing methods for deriving preference-based measures of health from condition-specific measures (and other patient-based measures of outcome). Health Technol Assess 2012;16. http://dx.doi.org/10.3310/hta16320.
- Kowalski J, Rentz A, Walt J, Lloyd A, Lee J, Young T, et al. Rasch analysis in the development of a simplified version of the National Eye Institute Visual-Function Questionnaire-25 for utility estimation. Qual Life Res 2012;21:323-34. http://dx.doi.org/10.1007/s11136-011-9938-z.
- McCabe C, Stevens K, Roberts J, Brazier J. Health state values for the HUI 2 descriptive system: results from a UK survey. Health Econ 2005;14:231-44. http://dx.doi.org/10.1002/hec.925.
- Torrance G, Thomas W, Sackett D. A utility maximisation model for the evaluation of health care programs. Health Serv Res 1972;7:118-33.
- Barber R, Boote J, Parry G, Cooper C, Yeeles P, Cook S. Can the impact of public involvement on research be evaulated? A mixed methods study. Health Expect 2012;15:229-41. http://dx.doi.org/10.1111/j.1369-7625.2010.00660.x.
- Wyatt K, Carter M, Mahtani A, Barnard A, Hawton A, Britten N. The impact of consumer involvement in research: an evaluation of consumer involvement in the London primary care studies programme. Fam Pract 2008;25:154-61. http://dx.doi.org/10.1093/fampra/cmn019.
Appendix 1 Recruitment by participating centre
| Name of trust | Name of centre | Pain prevalence | Pain cohort | Severe project | PURAF field test | PU-QOL qualitative study | PUQ-OL pre-test | PU-QOL field test 1 | PU-QOL field test 2 | PUQOL-UI method substudy | Trust total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Leeds Teaching Hospitals NHS Trust | Leeds General Infirmary | 730 | 57 | 1 | 16 | 8 | 9 | 11 | 9 | 6 | 1810 |
| St James’s University Hospital | 760 | 52 | 1 | 42 | 3 | 10 | 4 | ||||
| Chapel Allerton Hospital | 52 | 9 | |||||||||
| Wharfedale Hospital | 25 | 4 | 1 | ||||||||
| 2. Newcastle and North Tyneside PCT | 1680 | 1 | 2 | 4 | 19 | 1706 | |||||
| 3. University Hospitals Birmingham NHS Foundation Trust | Queen Elizabeth Hospital | 447 | 59 | 22 | 4 | 45 | 25 | 15 | 1047 | ||
| Selly Oak Hospital | 417 | 12 | 1 | ||||||||
| 4. Mid Yorkshire Hospitals NHS Trust | Pinderfields General Hospital | 443 | 86 | 1 | 14 | 6 | 7 | 27 | 18 | 8 | 1167 |
| Pontefract Hospital | 225 | 8 | 1 | 3 | 2 | ||||||
| Dewsbury and District Hospital | 298 | 7 | 4 | 1 | |||||||
| Rehabilitation Hospital Queen Elizabeth House | 8 | ||||||||||
| 5. Northumberland Care Trust (Northumbria Healthcare Trust as of 1 April 2011) | 103 | 41 | 14 | 30 | 30 | 23 | 241 | ||||
| 6. Leeds Community Healthcare NHS Trust | 27 | 2 | 54 | 3 | 24 | 15 | 13 | 138 | |||
| 7. Sussex Community NHS Trust | Sussex Community NHS Trust | 34 | 12 | 97 | |||||||
| Brighton General Hospital | 51 | ||||||||||
| 8. Calderdale and Huddersfield NHS Foundation Trust | Calderdale Royal Hospital | 16 | 5 | 38 | |||||||
| Huddersfield Royal Infirmary | 13 | 1 | 3 | ||||||||
| 9. Calderdale and Huddersfield PCT | 2 | 3 | 3 | 8 | |||||||
| 10. Kirklees PCT | 43 | 17 | 17 | 77 | |||||||
| 11. Bradford Teaching Hospitals NHS Foundation Trust | Bradford Royal Infirmary | 26 | 1 | 30 | 20 | 77 | |||||
| 12. County Durham and Darlington PCT | 28 | 3 | 4 | 18 | 15 | 68 | |||||
| 13. Harrogate and District NHS Foundation Trust | Harrogate District Hospital | 37 | 14 | 3 | 3 | 57 | |||||
| 14. Wakefield District PCT | 5 | 20 | 13 | 7 | 45 | ||||||
| 15. University Hospitals of Leicester NHS Trust | Leicester Royal Infirmary | 16 | 2 | 10 | 28 | ||||||
| 16. Norfolk and Norwich University Hospitals NHS Foundation Trust | James Paget Hospital | 11 | 26 | ||||||||
| Norfolk and Norwich University Hospital | 10 | 5 | |||||||||
| 17. North Lincolnshire and Goole NHS Foundation Trust | Scunthorpe General Hospital | 4 | 18 | ||||||||
| Diana Princess of Wales Hospital | 10 | 4 | |||||||||
| 18. North Devon District Hospital | North Devon District Hospital | 17 | 17 | ||||||||
| 19. Leicester and Rutland PCT | 2 | 6 | 3 | 11 | |||||||
| 20. South Tyneside NHS Foundation Trust | South Tyneside NHS Foundation Trust | 10 | 10 | ||||||||
| 21. Walsall PCT | 5 | 5 | 10 | ||||||||
| 22. Scarborough and North East Yorkshire NHS Trust | Scarbourgh General Hospital | 1 | 7 | 9 | |||||||
| Bridlington and District Hospital | 1 | ||||||||||
| 23. Burton Hospitals NHS Foundation Trust | Queen’s Hospital | 7 | 7 | ||||||||
| 24. Southern Health NHS Foundation Trust | Southern Health NHS Foundation Trust | 6 | 6 | ||||||||
| 25. Southport and Ormskirk Hospital NHS Trust | Southport and Formby District General Hospital | 2 | 4 | 6 | |||||||
| 26. Belfast Health and Social Care Trust | Musgrave Park Hospital SCI Unit | 4 | 5 | ||||||||
| Royal Victoria Hospital, Belfast | 1 | ||||||||||
| 27. Ayrshire and Arran Health Board | Crosshouse Hospital | 2 | 2 | ||||||||
| 28. North Tees and Hartlepool Hospitals NHS Foundation Trust | University Hospital of Hartlepool | 0 | 1 | 2 | |||||||
| University Hospital of North Tees | 1 | 0 | |||||||||
| 29. North Yorkshire and York PCT | 1 | 1 | |||||||||
| 30. Marie Curie | Marie Curie Hospice Bradford | 1 | 1 | ||||||||
| Total | 5180 | 634 | 8 | 230 | 32 | 35 | 285 | 231 | 100 | 6735 |
Appendix 2 Pressure Ulcer Research Service User Network UK information leaflet
Appendix 3 Pain prevalence study reduced format protocol
Appendix 4 Pain prevalence study patient information leaflet
Appendix 5 Pain prevalence study witnessed consent form
Appendix 6 Pain cohort study reduced format protocol
Appendix 7 Pain cohort study patient information leaflet
Appendix 8 Pain cohort study consent forms
Appendix 9 Cross-tabulations of explanatory variables
| Category 1 pressure ulcer | Skin alterations, n (%) | Total, n (%) | |
|---|---|---|---|
| Yes | No | ||
| Yes | 170 (28.2) | 120 (19.9) | 290 (48.2) |
| No | 203 (33.7) | 109 (18.1) | 312 (51.8) |
| Total | 373 (62.0) | 229 (38.0) | 602 (100.0) |
| Category 1 pressure ulcer | Pain on a category 0, 1 or A skin site, n (%) | Total, n (%) | |
|---|---|---|---|
| Yes | No | ||
| Yes | 247 (41.0) | 43 (7.1) | 290 (48.2) |
| No | 217 (36.0) | 95 (15.8) | 312 (51.8) |
| Total | 464 (77.1) | 138 (22.9) | 602 (100.0) |
| Category 1 pressure ulcer | Category 2 pressure ulcer, n (%) | Total, n (%) | |
|---|---|---|---|
| Yes | No | ||
| Yes | 76 (12.6) | 214 (35.5) | 290 (48.2) |
| No | 88 (14.6) | 224 (37.2) | 312 (51.8) |
| Total | 164 (27.2) | 438 (72.8) | 602 (100.0) |
| Category 1 pressure ulcer | Braden activity score, n (%) | Total, n (%) | |||
|---|---|---|---|---|---|
| Bedfast | Chairfast | Walks occasionally | Walks frequently | ||
| Yes | 51 (8.5) | 145 (24.1) | 88 (14.6) | 6 (1.0) | 290 (48.2) |
| No | 53 (8.8) | 168 (27.9) | 69 (11.5) | 22 (3.7) | 312 (51.8) |
| Total | 104 (17.3) | 313 (52.0) | 157 (26.1) | 28 (4.7) | 602 (100.0) |
| Category 1 pressure ulcer | Chronic wound, n (%) | Total, n (%) | |
|---|---|---|---|
| Yes | No | ||
| Yes | 72 (12.0) | 218 (36.2) | 290 (48.2) |
| No | 55 (9.1) | 257 (42.7) | 312 (51.8) |
| Total | 127 (21.1) | 475 (78.9) | 602 (100.0) |
| Skin alterations | Pain on a category 0, 1 or A skin site, n (%) | Total, n (%) | |
|---|---|---|---|
| Yes | No | ||
| Yes | 301 (50.0) | 72 (12.0) | 373 (62.0) |
| No | 163 (27.1) | 66 (11.0) | 229 (38.0) |
| Total | 464 (77.1) | 138 (22.9) | 602 (100.0) |
| Skin alterations | Category 2 pressure ulcer, n (%) | Total, n (%) | |
|---|---|---|---|
| Yes | No | ||
| Yes | 99 (16.4) | 274 (45.5) | 373 (62.0) |
| No | 65 (10.8) | 164 (27.2) | 229 (38.0) |
| Total | 164 (27.2) | 438 (72.8) | 602 (100.0) |
| Skin alterations | Braden activity score, n (%) | Total, n (%) | |||
|---|---|---|---|---|---|
| Bedfast | Chairfast | Walks occasionally | Walks frequently | ||
| Yes | 55 (9.1) | 196 (32.6) | 99 (16.4) | 23 (3.8) | 373 (62.0) |
| No | 49 (8.1) | 117 (19.4) | 58 (9.6) | 5 (0.8) | 229 (38.0) |
| Total | 104 (17.3) | 313 (52.0) | 157 (26.1) | 28 (4.7) | 602 (100.0) |
| Skin alterations | Chronic wound, n (%) | Total, n (%) | |
|---|---|---|---|
| Yes | No | ||
| Yes | 87 (14.5) | 286 (47.5) | 373 (62.0) |
| No | 40 (6.6) | 189 (31.4) | 229 (38.0) |
| Total | 127 (21.1) | 475 (78.9) | 602 (100.0) |
| Pain on a category 0, 1 or A skin site | Category 2 pressure ulcer, n (%) | Total, n (%) | |
|---|---|---|---|
| Yes | No | ||
| Yes | 103 (17.1) | 361 (60.0) | 464 (77.1) |
| No | 61 (10.1) | 77 (12.8) | 138 (22.9) |
| Total | 164 (27.2) | 438 (72.8) | 602 (100.0) |
| Pain on a category 0, 1 or A skin site | Braden activity score, n (%) | Total, n (%) | |||
|---|---|---|---|---|---|
| Bedfast | Chairfast | Walks occasionally | Walks frequently | ||
| Yes | 71 (11.8) | 239 (39.7) | 128 (21.3) | 26 (4.3) | 464 (77.1) |
| No | 33 (5.5) | 74 (12.3) | 29 (4.8) | 2 (0.3) | 138 (22.9) |
| Total | 104 (17.3) | 313 (52.0) | 157 (26.1) | 28 (4.7) | 602 (100.0) |
| Pain on a category 0, 1 or A skin site | Chronic wound, n (%) | Total, n (%) | |
|---|---|---|---|
| Yes | No | ||
| Yes | 105 (17.4) | 359 (59.6) | 464 (77.1) |
| No | 22 (3.7) | 116 (19.3) | 138 (22.9) |
| Total | 127 (21.1) | 475 (78.9) | 602 (100.0%) |
| Category 2 pressure ulcer | Braden activity score, n (%) | Total | |||
|---|---|---|---|---|---|
| Bedfast | Chairfast | Walks occasionally | Walks frequently | ||
| Yes | 30 (5.0) | 78 (13.0) | 50 (8.3) | 6 (1.0) | 164 (27.2) |
| No | 74 (12.3) | 235 (39.0) | 107 (17.8) | 22 (3.7) | 438 (72.8) |
| Total | 104 (17.3) | 313 (52.0) | 157 (26.1) | 28 (4.7) | 602 (100.0) |
| Category 2 pressure ulcer | Chronic wound, n (%) | Total, n (%) | |
|---|---|---|---|
| Yes | No | ||
| Yes | 39 (6.5) | 125 (20.8) | 164 (27.2) |
| No | 88 (14.6) | 350 (58.1) | 438 (72.8) |
| Total | 127 (21.1) | 475 (78.9) | 602 (100.0) |
| Braden activity | Chronic wound, n (%) | Total, n (%) | |
|---|---|---|---|
| Yes | No | ||
| Bedfast | 15 (2.5) | 89 (14.8) | 104 (17.3) |
| Chairfast | 63 (10.5) | 250 (41.5) | 313 (52.0) |
| Walks occasionally | 36 (6.0) | 121 (20.1) | 157 (26.1) |
| Walks frequently | 13 (2.2) | 15 (2.5) | 28 (4.7) |
| Total | 127 (21.1) | 475 (78.9) | 602 (100.0) |
Appendix 10 Severe pressure ulcer study reduced format protocol
Appendix 11 Severe pressure ulcer study participant information leaflet and agree to research contact form
Appendix 12 Severe pressure ulcer study consent form
Appendix 13 Severe pressure ulcer study topic guide
‘Why do patients develop severe pressure ulcers?’ study
Researchers: Lisa Pinkney, Professor Justin Keen, Dr Jane Nixon
Address: Centre for Health and Social Care, University of Leeds, Leeds, LS2 9LJ
Tel.: 0113 343 0828
Interview topic guide: patients
(Verbal introduction . . .) Have you any questions about this study? Are you happy to start the interview?
This interview will be unstructured and informal and guided by you, not by a set of questions. However, as an opening question . . .
Introductory question: Why do you think you developed a severe pressure ulcer?
Some topics that will be covered but which are only tentative topics and which will be developed as the research progresses:
-
background/history of events
-
severe pressure ulcer description
-
timeline of events – micro, mezzo and macro levels
-
interpersonal level
-
people involved
-
support systems – people, services
-
clinical risks
-
unexpected events
-
communication
-
service involvement.
Appendix 14 Search strategies and data sources
Search strategy for the systematic review of patient risk factors for pressure ulcer development
Four electronic databases, AMED, MEDLINE, EMBASE and CINAHL, were searched from inception until March 2010 through the Ovid web gateway from their inception using the search template detailed below. The search plan included pressure ulcer search terms and Ovid maximum sensitivity filters for prognosis and aetiology or harm.
-
decubitus.sh.
-
skin ulcer.sh,tw.
-
exp decubitus ulcer/
-
decubitus ulcer$.tw.
-
PU$.tw.
-
pressure damage$.tw.
-
pressure sore$.tw.
-
bed sore$.tw.
-
or/1-8
-
exp cohort-studies/
-
exp risk/
-
(odds and ratio$).mp. [mp=title, original title, abstract, name of substance word, subject heading word]
-
(relative and risk$).mp. [mp=title, original title, abstract, name of substance word, subject heading word]
-
(case and control$).mp. [mp=title, original title, abstract, name of substance word, subject heading word]
-
or/10-14
-
incidence.tw.
-
exp mortality/
-
Follow-Up Studies/
-
prognos$.tw.
-
predict$.tw.
-
course.tw.
-
Survival Analysis/
-
or/16-22
-
9 and 15
-
9 and 23
-
24 or 25
-
case report.sh.
-
historical article.pt.
-
review of reported cases.pt.
-
review, multicase.pt.
-
letter.pt.
-
comment.pt.
-
editorial.pt.
-
or/27-33
-
26 not 34
-
limit 35 to humans
The first 200 retrieved abstracts were screened and key words from non-relevant papers were identified and used to further refine the search (i.e. increase specificity):
-
leg ulcer.mp. [mp=ab, hw, ti, it, sh, tn, ot, dm, mf, nm]
-
varicose ulcer.mp. [mp=ab, hw, ti, it, sh, tn, ot, dm, mf, nm]
-
pilonidal.tw.
-
surgical flaps.mp. [mp=ab, hw, ti, it, sh, tn, ot, dm, mf, nm]
-
skin transplantation$.mp. [mp=ab, hw, ti, it, sh, tn, ot, dm, mf, nm]
-
burn$.mp. [mp=ab, hw, ti, it, sh, tn, ot, dm, mf, nm]
-
gunshot.mp. [mp=ab, hw, ti, it, sh, tn, ot, dm, mf, nm]
-
corneal ulcer.mp. [mp=ab, hw, ti, it, sh, tn, ot, dm, mf, nm]
-
exp dentistry/
-
peptic ulcer.mp. [mp=ab, hw, ti, it, sh, tn, ot, dm, mf, nm]
-
duodenal ulcer.mp. [mp=ab, hw, ti, it, sh, tn, ot, dm, mf, nm]
-
stomach ulcer.mp. [mp=ab, hw, ti, it, sh, tn, ot, dm, mf, nm]
-
fistula$.mp. [mp=ab, hw, ti, it, sh, tn, ot, dm, mf, nm]
-
bite.tw.
-
or/37-50
-
36 not 51
Hand searching
The following specialist journals and conference proceedings were hand searched.
Journals
-
Journal of Tissue Viability, 1990 to present.
-
Journal of Wound Care, 1991 to present.
-
European Pressure Ulcer Advisory Panel reviews, volume 1, issue 2, 1999 to volume 7, issue 2, 2006.
Conference proceedings
-
Proceedings of the 1st European Conference on Advances in Wound Management, September 1991, Cardiff, UK.
-
Proceedings of the 2nd European Conference on Advances in Wound Management, October 1992, Harrogate, UK.
-
Proceedings of the 3rd European Conference on Advances in Wound Management, October 1993, Harrogate, UK.
-
Proceedings of the 4th European Conference on Advances in Wound Management, September 1994, Copenhagen, Denmark.
-
Proceedings of the 5th European Conference on Advances in Wound Management, November 1995, Harrogate, UK.
-
Proceedings of the 6th European Conference on Advances in Wound Management, October 1996, Amsterdam, the Netherlands.
-
Proceedings of the 7th European Conference on Advances in Wound Management, November 1997, Harrogate, UK.
-
Proceedings of the 8th European Conference on Advances in Wound Management, April 1998, Madrid, Spain.
-
Proceedings of the 9th European Conference on Advances in Wound Management, November 1999, Harrogate, UK.
-
Proceedings of the 10th European Conference on Advances in Wound Management, May 2000, Stockholm, Sweden.
-
Proceedings of the 11th Conference of the European Wound Management Association, May 2001, Dublin, Ireland.
-
Proceedings of the 12th Conference of the European Wound Management Association, May 2002, Granada, Spain.
-
Proceedings of the 13th Conference of the European Wound Management Association, May 2003, Pisa, Italy.
-
Proceedings of the 15th Conference of the European Wound Management Association, September 2005, Stuttgart, Germany.
-
Proceedings of the 16th Conference of the European Wound Management Association, May 2006, Prague, Czech Republic.
-
Proceedings of the European Wound Management Association and Journal of Wound Care Conference, April 1997, Milan, Italy.
-
Proceedings of the European Wound Management Association and Journal of Wound Care Autumn Conference, November 1998, Harrogate, UK.
-
2nd World Union of Wound Healing Societies Meeting, July 2004, Paris, France.
-
Journal of Wound Healing 2nd Conference, September 2005, Stuttgart, Germany.
-
Wounds UK Conference, November 2004, Harrogate, UK.
-
1st European Pressure Ulcer Advisory Panel Open Meeting, September 1997, Oxford, UK.
-
2nd European Pressure Ulcer Advisory Panel Open Meeting, September 1998, Oxford, UK.
-
3rd European Pressure Ulcer Advisory Panel Open Meeting, September 1999, Amsterdam, the Netherlands.
-
4th European Pressure Ulcer Advisory Panel Open Meeting, September 2000, Pisa, Italy.
-
5th European Pressure Ulcer Advisory Panel Open Meeting, September 2001, Le Mans, France.
-
6th European Pressure Ulcer Advisory Panel Open Meeting, September 2002, Budapest, Hungary.
-
7th European Pressure Ulcer Advisory Panel Open Meeting, September 2003, Tampere, Finland.
-
8th European Pressure Ulcer Advisory Panel Open Meeting, May 2005, Aberdeen, Scotland.
-
European Tissue Repair Society, Focus Meeting, November 2000, St Anne’s College, Oxford, UK.
-
European Tissue Repair Society, Annual Conference, September 2001, Cardiff, UK.
-
European Tissue Repair Society, Focus Meeting, September 2002, Nice, France.
-
13th Annual European Tissue Repair Society Meeting, September 2003, Amsterdam, the Netherlands.
-
European Tissue Repair Society, Focus Meeting, March 2005, Southampton, UK.
Appendix 15 Example evidence table for subdomain oedema
| Author and year | Study quality | Study limitation notes | Study design | PU events/sample | Specific variable | OR | CI | Study population |
|---|---|---|---|---|---|---|---|---|
| aCompton et al. 2008144 | Low-quality study | Record review. Large number of events but used 32 variables in the model. No CIs reported | Record Review | 121/698 | Skin condition oedematous skin | 2.245 | NR | Acute care hospital, ICU, non-surgical |
| Nijs et al. 2009159 | Moderate-quality study | Full details of modelling not provided. Adequate number of events is assumed as large number of events | Cohort | 134/463 | Pitting oedema | NR | NR | Acute care hospital, ICU, surgical |
| Bergquist and Frantz 1999134 | Low-quality study | Record review and insufficient number of events. Inadequate measurement of risk factors | Record Review | 55/1567 | Oedema | NR | NR | Community/home care, elderly/geriatric, non-surgical |
| Donnelly 2006146 | Low-quality study | Insufficient number of events and no CIs reported | RCT | 39/239 | Oedema | HR 1.023 | NR | Acute care hospital, elderly/geriatric, hip fracture |
Appendix 16 Consensus study reduced format protocol
Appendix 17 Consensus study pressure ulcer Minimum Data Set nominal group participant information sheet
Appendix 18 Consensus study pressure ulcer Minimum Data Set nominal group consent form
Appendix 19 Consensus study Pressure Ulcer Risk Assessment Framework nominal group participant information sheet
Appendix 20 Consensus study Pressure Ulcer Risk Assessment Framework nominal group consent form
Appendix 21 Consensus study pressure ulcer Minimum Data Set wider consultation participant information sheet
Appendix 22 Consensus study Pressure Ulcer Risk Assessment Framework wider consultation participant information sheet
Appendix 23 Initial draft Risk Assessment Framework with underpinning Minimum Data Set
N/A, not applicable; PU, pressure ulcer. Reprinted from Coleman S, Nelson EA, Keen J, Wilson L, McGinnis E, Dealey C, et al. Developing a pressure ulcer risk factor minimum data set and risk assessment framework. J Adv Nurs 2014;70:2339–52. 126 © 2014 The Authors. Journal of Advanced Nursing. Published by John Wiley & Sons Ltd. This is an open access article under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non-commercial and no modifications or adaptations are made.
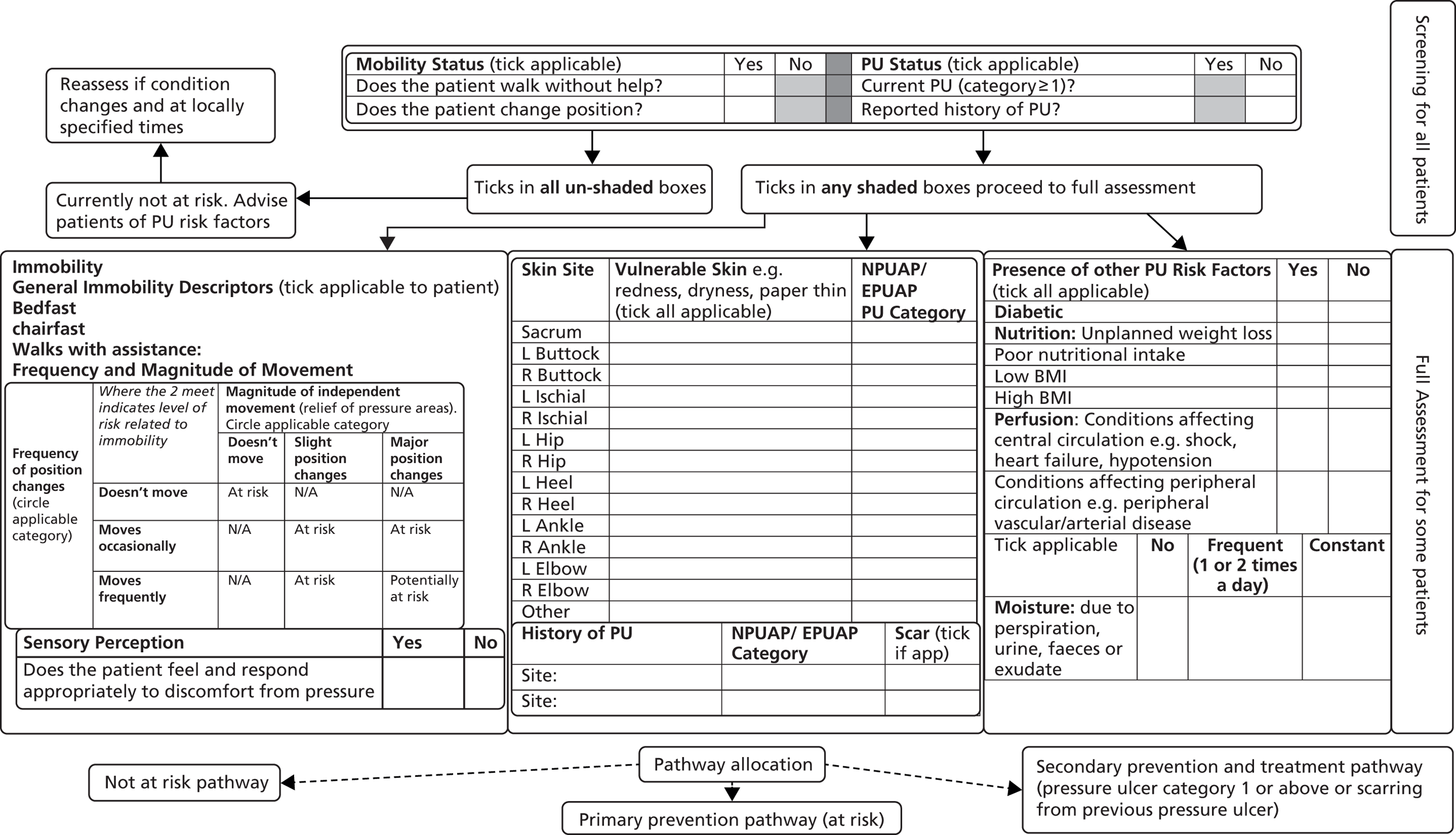
Appendix 24 Pressure Ulcer Risk Assessment Framework pre-test study reduced format protocol
Appendix 25 Pressure Ulcer Risk Assessment Framework pre-test study participant information sheet
Appendix 26 Pressure Ulcer Risk Assessment Framework pre-test study consent form
Appendix 27 Pressure Ulcer Risk Assessment Framework pre-test study case studies
Appendix 28 Pressure Ulcer Risk Assessment Framework focus group topic guide
-
Introduction of moderators and group members by name.
-
The overall aims of the study and how the focus group contributes to this will be explained by the moderator.
-
Aims of the session – to consider the acceptability of using the Pressure Ulcer Risk Assessment Framework (PURAF) incorporating:
-
what was liked about the PURAF
-
what was disliked about the PURAF
-
usability of the PURAF and how nurses found using the PURAF overall (were there any confusing areas)
-
whether nurses anticipate any problems in using the PURAF in clinical practice.
-
-
Ground rules – Everyone will have chance to speak and be heard. There are not right or wrong answers. The moderator will remind the group that the meeting will be audio-taped, answer any questions and confirm that everyone is happy to proceed with the meeting.
-
Ice breaker – discussion in pairs of what was liked about the PURAF and list on flip chart and group feedback.
-
Group discussion of what was disliked about the PURAF and list on flip chart.
-
Group discussion of the usability of the PURAF and how the nurses found using the framework overall (were there any confusing areas). The moderator will use the data completeness forms taken from the training element to inform discussions. Note on flip chart.
-
Group discussion of any anticipated problems with using the PURAF in clinical practice. Note on flip chart.
Appendix 29 Pressure Ulcer Risk Assessment Framework pre-test study think out loud topic guide
-
Introduction of researcher to nurse.
-
Aims of the interview are to identify any specific items that cause confusion when using the PURAF.
-
Ground rules – There are not right or wrong answers. The researcher will remind the nurse to ‘think out loud’ as he or she completes the PURAF. The researcher will remind the nurse that the interview will be audio-taped, answer any questions and confirm that he or she is happy to proceed with the interview.
-
The nurse completes the PURAF again using a case study with photographs and the researcher encourages the nurse to ‘think out loud’ as he or she does this.
Appendix 30 Preliminary Risk Assessment Framework: PURPOSE-T
Please note that the PURPOSE-T incorporates the use of colour to aid decision-making. This version of the tool has been adapted with the colour key included. It is not intended for implementation and is not the final version of the tool.
Appendix 31 Pressure Ulcer Risk Assessment Framework field test 1 reduced format protocol
Appendix 32 Pressure Ulcer Risk Assessment Framework field test 1 participant information sheet
Appendix 33 Pressure Ulcer Risk Assessment Framework field test 1 participant consent form
Appendix 34 Pressure Ulcer Risk Assessment Framework field test 1 witnessed consent form
Appendix 35 Pressure Ulcer Risk Assessment Framework field test 1 consultee information sheet
Appendix 36 Pressure Ulcer Risk Assessment Framework field test 1 consultee declaration
Appendix 37 PURPOSE-T user manual
Appendix 38 Pressure Ulcer Quality of Life qualitative study reduced format protocol
Appendix 39 Pressure Ulcer Quality of Life qualitative study patient information leaflet and consent form
Appendix 40 Pressure Ulcer Quality of Life qualitative study agree to researcher contact form
Appendix 41 Pressure Ulcer Quality of Life qualitative study interview schedule
Introduction to project and present study
-
Thank you for volunteering and ask questions at any time.
-
Background and explanation of project.
-
Confirmation of agreeing to tape-record the interview.
-
Obtain informed written consent.
Introduction to the Pressure Ulcer Quality of Life development project
The PU-QOL is a larger project, undertaken by a national group of researchers including myself, that is intended to develop and evaluate a questionnaire to measure quality of life in people who have a pressure ulcer. In order to develop such a questionnaire, it is important to obtain information directly from the people who have experience of the problem. To find out what impact a pressure ulcer and pressure ulcer treatments have on quality of life, I am interviewing around 24 people who have experienced a pressure ulcer to find out what quality of life issues are most important to them. From the quality of life issues already identified, I have grouped them into seven main themes. I will ask you to comment on these themes towards the end of our discussion.
Tape recording and anonymity
[Check comfortable, do they need toilet, glass of water; clarify what name to use]
[Spoken by interviewer] I would like to make a tape recording of this interview as that will help make sure that I catch everything you say. We think it is better than my taking notes. Before we start, can I just confirm that you are happy with that? Now what will happen to this tape is that I will take it back and our conversation will be typed out in full. When we do that we make sure that there is nothing in the document that could identify you, so, for example, your name or the name of the hospital or ward would be blanked out. Similarly, names of any other people that you mention will also be blanked out or changed so that both you and they can remain anonymous.
Clinical data and impact of pressure ulcers and interventions
[Opening discussion prompts]
-
You have a sore area, can you tell me about it? What is it like to live with a pressure ulcer?
-
The ward staff tell me that you have a sore area. What can you tell me about it?
-
Can you tell me a little bit about yourself? Why are you in hospital?
-
Do you know why you got the pressure sore?
-
How did you know that you had a pressure sore?
-
Some people experience discomfort around their sore such as an ache or discomfort when it is being dressed. Do you feel anything?
-
What kind of symptoms have you experienced?
Current situation
-
Where the patient is living – is this your permanent living arrangement or just short term? If short term, what is the long-term plan for living arrangements?
-
Do you get any help at home with daily care from health-care professionals or family/friends? Is this related to your pressure sore?
General health
-
How would you describe your general health?
-
Try to think back to the time just before you developed your pressure sore. What was your life like then?
-
What do you know about pressure ulcers (establish pressure ulcer history and current knowledge)?
Their pressure ulcer
-
When did you notice any skin problems? Was a pressure ulcer present on admission or did you develop a pressure ulcer in hospital?
-
When did it start/how long did it last (i.e. ongoing or healed)?
-
Location(s); what stage did it progress to? How many did you develop?
-
What were you told about the pressure ulcer? How did you feel?
-
Have you seen it? If yes, what did you think about it? How did it make you feel? If no, has anyone else seen it? How have they described it to you?
-
What has been the reaction of others when you told them about your pressure ulcer?
-
Do you have any worries about it?
Treatments
-
Have you received any treatment on that area? What about anything else to help that area? (Dressings, creams, others)? If so, which ones?
-
Frequency of wound care (i.e. how often do you have dressing changes)?
-
Who performs the wound care (i.e. dressing changes)?
-
Can you tell me about your experience of the wound care treatments that you received (i.e. symptoms, acceptability, satisfaction)?
-
Have any aspects of your life been affected by the wound care you received?
-
What do you perceive have been the benefits of your treatments?
-
How has your pressure sore been attended to; what sorts of things have been done? And who did this? What was it like?
-
In terms of being in hospital for your pressure sore treatment, what is that like for you?
Pressure ulcer experience and impact
-
What is the biggest problem that your pressure ulcer has caused you?
-
How has it affected your everyday life/your relationships?
-
How has your life changed since your ulcer developed? Anything else?
-
What areas of your life have been most affected since you developed your pressure ulcer? In what way?
-
What kinds of things are more difficult for you to do? Any other tasks?
-
How does the pressure ulcer affect your ability to move?
-
How does it affect you at work?
-
Does the pressure ulcer have an impact on your psychological well-being?
Prompts for further information
-
Is there anything else that I haven’t asked you about your sore that you think that I should know?
-
You have mentioned . . ., can you tell me more about what it has been like for you?
-
Is there anything else that you would like to tell me about your pressure ulcer or treatments?
-
Is there anything else about the sore that you think researchers and people who provide pressure sore care need to know?
-
What would you like us to know about how it has affected you from your perspective?
Discussion of existing quality of life main themes
[Spoken by interviewer] Conversations with other people with pressure ulcers have identified various issues and problems associated with having a pressure ulcer. Some of these issues include:
-
perceived pressure ulcer aetiology (reasons pressure ulcer developed, risks)
-
impact of pressure ulcer on daily living – physical
-
symptoms/consequences of the pressure ulcer
-
psychological well-being
-
social impact of pressure ulcer
-
impact of treatments/wound care
-
the nurse–patient relationship
-
impact of pressure ulcer on health
-
impact on others
-
need for knowledge about the pressure ulcer
-
need for treatment vs. effect of treatment on patient.
-
From our conversation and from these themes, can you see anything that we have missed?
-
Have you experienced anything else that we have not covered today?
-
Is there anything else that you want to add about your experience?
-
From all the things that we have talked about today, what is the single most important thing?
End of interview
[Collect clinical data]
-
Nurse contact: name and telephone.
-
Trust name; hospital, acute or community.
-
Age or date of birth.
-
Gender.
-
Marital status.
-
Ethnicity.
Close the discussion; thank the patient for their time and involvement; explain how their information will be used.
Appendix 42 Pressure Ulcer Quality of Life study search strategies and data sources
Search strategies for review of existing patient-reported outcome instruments used in pressure ulcers and chronic wounds
Searches of the following electronic databases were performed from inception until May 2012 using the search strategies below: MEDLINE, EMBASE, PsycINFO, CINAHL, BNI and AMED.
Pressure ulcer terms
-
decubitus.sh
-
skin ulcer.sh
-
exp decubitus ulcer
-
decubitus ulcer$.tw
-
pressure ulcer$.tw.
-
pressure damage$.tw
-
pressure sore$.tw
-
bed sore$.tw
-
skin ulcer$.tw
-
or/1-9
Chronic wound terms
-
chronic wound$.tw
-
leg ulcer$.tw
-
foot ulcer$.tw
-
venous ulcer$.tw
-
necrotic wound$.tw
-
ischaemic ulcer$.tw
-
arterial ulcer$.tw
-
fungating wound$.tw
-
diabetic ulcer$.tw
-
varicose vein$.tw
-
dehisced wound$.tw
-
pilonidal.tw
-
or/11-22
-
10 or 23
Quality of life terms
-
(wellbeing or well being).ti,ab,tw,sh,kw
-
(hrql or hrqol or qol or hql or hqol).ti,ab,tw,sh,kw
-
exp quality of life
-
quality of living.tw
-
(health status or health state$).ti,ab,tw,sh,kw
-
(satisfaction or life satisfaction or satisfaction with life).tw
-
(attitude$ or emotion$ or feeling$ or mood$).tw
-
((psycho$ or social) adj (adjust$ or adap$ or function$)).tw
-
(cope$ or coping).tw
-
exp emotion
-
exp psychological
-
exp adaptation, psychological
-
exp acceptance, psychological
-
symptom$.tw,ab,sh,kw
-
exp pain
-
pain.tw
-
comfort$.tw
-
acceptab$.tw
-
discomfort.tw
-
exp quality of sleep
-
sleep.tw
-
exp smell
-
smell$.tw
-
odo?r$.tw
-
exudat$.tw
-
or/25-49
-
(instrument$ or questionnaire$ or survey$ or measure$).kw,ab,ti
-
(patient outcome$ or patient reported outcome$ or PRO$).ti,ab,tw,sh,kw
-
health measurement$.ti,ab,tw,sh,kw
-
(categor$ scal$ or linear scal$ or linear analog$ scal$ or visual scal$ or magnitude estimat$).ti,ab.
-
or/51-55
-
22 and 50
-
24 and 55
-
56 or 57
Refinement terms
-
historical article.pt.
-
review.pt.
-
(systematic adj review$).ti,ab,pt
-
(meta adj analysis).ti,ab
-
audit.ti,ab,pt
-
case report.tw,sh,mp,pt
-
(case adj stud$).ti,ab,pt
-
exp guidelines
-
letter.pt.
-
comment.pt.
-
editorial.pt.
-
burn$.tw
-
digital ulcer$.tw
-
buruli ulcer$.tw
-
spider bite$.tw
-
or/59-73
-
58 not 74
-
limit 75 to humans, adult
The Cochrane Library and Web of Knowledge (WOK) databases were searched using ‘PU’ or ‘pressure sore’ and ‘quality of life’ topic words. To find relevant PRO measures not detected in the electronic bibliographic search, we hand searched specialist journals and relevant conference proceedings, contacted experts, searched the Patient-Reported Outcome and Quality of Life Database [PROQOLID; see www.qolid.org/ (accessed 9 March 2015)] and performed a citation search on all included studies and systematic reviews identified.
Pressure ulcer-related pain search strategy
Searches of the following electronic databases were performed from inception until April 2010 using the search strategies below: MEDLINE, EMBASE, PsycINFO, CINAHL, BNI and AMED.
Pressure ulcer terms
-
decubitus.sh
-
skin ulcer.sh
-
exp decubitus ulcer
-
decubitus ulcer$.tw
-
pressure ulcer$.tw.
-
pressure damage$.tw
-
pressure sore$.tw
-
bed sore$.tw
-
skin ulcer$.tw
-
or/1-9
Pain terms
-
exp pain
-
pain.tw,ti,ab,sh,kw
-
discomfort.tw
-
uncomfortable.tw
-
hurt$.tw
-
unpleasant.tw
-
throb$.tw
-
ach$.tw
-
or/11-18
Existing measures terms
-
((pain) adj2 (questionnaire or measure$ or assess$ or survey or outcome or instrument$)).tw,ab,sh
-
(mcgill pain questionnaire or MPQ).tw
-
(brief pain inventory or BPI).tw
-
or/20-22
Qualitative methodology terms
-
qualitative.tw,ab,ti,pt,sh
-
finding$.tw
-
interview$.tw,ab
-
experience$.ti,ab,tw
-
descri$.tw,ab
-
or/24-28
-
10 and 23
-
10 and 19 and 29
-
30 or 31
Refinement terms
-
(intensity rating scale$).tw
-
(numerical rating scale or NRS).tw
-
(verbal rating scale$ or VRS).tw
-
(visual analogue scale$ or VAS).tw
-
(facial recognition scale or FRS).tw
-
(present pain intensity or PPI).tw
-
historical article.pt.
-
review.pt.
-
(systematic adj review$).ti,ab,pt
-
(meta adj analysis).ti,ab
-
audit.ti,ab,pt
-
exp guidelines
-
letter.pt.
-
comment.pt.
-
editorial.pt.
-
leg ulcer.mp
-
varicose vein$.mp
-
pilonidal.tw
-
digital ulcer.mp
-
skin transplant$.mp
-
burn$.mp
-
buruli ulcer.mp
-
diabetic ulcer.mp
-
stomach ulcer.mp
-
bite.tw
-
or/33-57
-
32 not 58
The Cochrane Library and WOK databases were searched using ‘PU’ or ‘pressure sore’ and ‘pain’ topic words. A citation search was performed on all included studies and relevant systematic reviews.
Hand searching
The following specialist journals were hand searched:
-
Journal of Tissue Viability, 1990–2010
-
Journal of Wound Care, 1991–2010
-
Wounds Repair and Regeneration, 2000–10
-
EPUAP review, 1999–2010
-
International Wound Journal, 2004–10
-
European Wound Management Association Journal, 2001–May 2010
-
Journal of Health and Quality of Life Outcomes, 1999–2010
-
Journal of the American Medical Association archive collection of ‘Quality of Life’, 1998–2010.
The following conference proceedings were hand searched:
-
European Conference on Advances in Wound Management, 1991–2000
-
Conference of the European Wound Management Association, 2001–6
-
Proceedings of the European Wound Management Association and Journal of Wound Care, 1997–8
-
2nd World Union of Wound Healing Societies Meeting, 2004
-
Journal of Wound Healing 2nd Conference, 2005
-
Wounds UK Conference, 2004
-
EPUAP Open Meeting, 1997–2007
-
European Tissue Repair Society, Focus Meeting, 2000–5
-
Conference of the International Society of Quality of Life Research, 1997–2007
The following dissertation databases were searched from inception to April 2010:
-
ProQuest Dissertations & Theses
-
Networked Digital Library of Theses and Dissertations
-
International Theses in Progress
-
Theses Canada Portal
-
Australian Digital Theses Program
-
Index to Theses
-
Russian Academy of Sciences Bibliographies.
Appendix 43 Pressure Ulcer Quality of Life study items through the development process
| Domain and scales | Reduced item list field test 1 (n = 87) – post pre-test | Original item list pre-tested (n = 118) |
|---|---|---|
| Symptoms | ||
| Pain and discomfort | Feeling uncomfortable | Feeling uncomfortable |
| Annoying pain or discomfort | Annoying pain or discomfort | |
| Itchiness | Itchiness | |
| Tenderness | Tenderness | |
| Niggling | ||
| Soreness | ||
| A dull achea | Aching | |
| Pins and needles | ||
| Tinglingb | Tingling | |
| Throbbing | Throbbing | |
| Nagging | ||
| Shooting | ||
| Stinging | Stinging | |
| Stabbing pains | Stabbing | |
| Electric shocks | ||
| Red raw | Red raw | |
| Burning | Burning | |
| Exudate | Weeping | Weeping |
| Oozing | ||
| Running | Running | |
| Sticky | Sticky | |
| Slimy | ||
| Wet | ||
| Messy | Messy | |
| Staining | Staining | |
| Causing dressing to come off | Causing dressing to come off | |
| Gungy | ||
| Pus | Pus | |
| Bleeding | Bleeding | |
| Odour | Unpleasant smell | Unpleasant smell |
| Lingering smell | Lingering smell | |
| Dirty smell | ||
| Foisty smell | ||
| Stench or stink | Stench | |
| Stink | ||
| Stale smell | ||
| Pungent smell | Pungent smell | |
| Sickening smell | Sickening smell | |
| Putrid smell | Putrid smell | |
| Physical functioning | ||
| Mobility and movement | Difficulty sitting up in bed | Difficulty sitting up in bed |
| Difficulty adjusting yourself in bed | Difficulty adjusting yourself in bed | |
| Difficulty turning or moving in bed | Difficulty turning in bed | |
| Difficulty pushing up to a sitting position | Difficulty pushing up to a sitting position | |
| Difficulty sitting in one position for long periodsa | Difficulty sitting in one position for long periods | |
| Difficulty standing for long periods | Difficulty standing for long periods | |
| Difficulty transferring (e.g. from bed to a chair or to a car) | Difficulty transferring from bed to a chair | |
| Feeling limited in your ability to walk | Feeling limited in your ability to walk | |
| Feeling limited in your ability to go up and down stairs | Feeling limited in your ability to go up and down stairs | |
| Feeling limited in how far you were able to walka | Feeling limited in how far you were able to walk | |
| Feeling that your walking was slowed down | Feeling that your walking was slowed down | |
| ADL | Being able to wash yourself in your usual way (e.g. hand wash, bath, shower) | Washing yourself in the bath or shower |
| Getting dressed or undressed | Getting dressed or undressed | |
| Doing jobs around the house (e.g. cooking, housework, DIY) | Doing housework | |
| Doing gardeninga | Doing gardening | |
| Doing shopping | Doing shopping | |
| Being able to go to the toilet | Going to the toilet | |
| Being able to travel or drive a car | ||
| Doing things that you enjoy (e.g. reading a book, watching a movie, using a computer) | Doing things that you enjoy | |
| Getting up and about to do things that you enjoy | ||
| Being emotionally close or affectionate with loved ones | Being intimate with loved ones | |
| Doing your regular daily activities (e.g. work, volunteering, religious service, clubs, university) | Doing usual work | |
| General vitality | Feeling that your appetite has reduced | Feeling that your appetite has reduced |
| Feeling unwell or poorly | Feeling unwell or poorly | |
| Feeling that your energy levels have been reducedc | Feeling that your energy levels have been reduced (e.g. feeling tired, fatigued) | |
| Feeling tiredc | ||
| Feeling fatiguedc | ||
| Sleep | Trouble falling asleep | Trouble falling asleep |
| Interrupted sleep (e.g. restless sleep or being woken up during your sleep) | Restless sleep | |
| Being kept awake | Being kept awake | |
| Being woken up during the night | ||
| Not getting the amount of sleep that you needed | Not getting the amount of sleep that you needed | |
| Having to sleep in one position (e.g. your back or side) | Having to sleep in one position | |
| Trouble finding a comfortable position | Trouble finding a comfortable position | |
| Psychological well-being | ||
| Mood | Feeling frustrated | Feeling frustrated |
| Feeling fed up | Feeling fed up | |
| Feeling annoyed or irritated | Feeling annoyed | |
| Feeling irritated | ||
| Feeling bad tempered | ||
| Feeling angry | Feeling angry | |
| Feeling miserable | Feeling miserable | |
| Feeling downa | Feeling down | |
| Feeling depressed | Feeling depressed | |
| Anxiety and worry | Feeling fearful | |
| Feeling afraid | ||
| Feeling upseta | Feeling upset | |
| Feeling concerned or worried | Feeling concerned | |
| Feeling worried | ||
| Feeling anxious | Feeling anxious | |
| Feeling surprised | ||
| Feeling shocked | ||
| Self-efficacy and dependence | Feeling like a burden or nuisance on others | Feeling like a burden or nuisance on others |
| Feeling like you have no control over your life because of your sore | Feeling like you have no control over your life | |
| Feeling physically dependent on others | Feeling physically dependent on others | |
| Appearance/self-consciousness | Feeling helpless | Feeling helpless |
| Feeling a lack of self-esteem | ||
| Feeling self-conscious | Feeling self-conscious | |
| Lacking in confidence | Feeling a lack of self-confidence | |
| Feeling embarrassed | Feeling embarrassed | |
| Feeling physically unattractive | Feeling physically unattractive | |
| Feeling disinterested in socialising | ||
| Feeling uneasy being close to or around other people | Feeling uneasy being close to people | |
| Feeling worried about how others will react to your ulcer | ||
| Feeling a lack of understanding from those close to you | Feeling a lack of understanding from those close to you | |
| Social functioning | ||
| Isolation | Feeling left out | |
| Feeling isolated | ||
| Feeling cut off or isolated from others | Feeling cut off | |
| Feeling lonely | Feeling lonely | |
| Feeling like you were missing out | Feeling like you were missing out | |
| Feeling like people avoided you or treated you differently now | Feeling like people avoided you or treated you differently now | |
| Participation | ||
| Difficulty going out | Difficulty going out | |
| Being unable to meet up with others | ||
| Difficulty meeting up or seeing family and/or friends | Difficulty seeing family and/or friends | |
| Being unable to participate in family gatherings or activities | Being unable to participate in family gatherings or activities | |
| Having to plan going out around ulcer care | Having to plan going out around ulcer care | |
| Being unable to do things spontaneously | ||
| Having to give up on hobbies or leisure activities | Giving up on hobbies or leisure activities | |
| Being restricted to where you could go out | Being restricted to where you could go out | |
| Being restricted to how long you could stay out | Being restricted to how long you could stay out | |
| Being unable to get away for a holiday or take a trip at the weekend | Being unable to get away for a holiday or take a trip at the weekend | |
| The amount of time involved in caring for your ulcer | The amount of time involved in caring for your ulcer | |
Appendix 44 Pressure Ulcer Quality of Life pre-test and field test study reduced format protocol
Appendix 45 Pressure Ulcer Quality of Life pre-test study patient information leaflet and consent form
Appendix 46 Pressure Ulcer Quality of Life pre-test study interview schedule
General introduction
Thank you for participating in this interview. Your feedback about the questionnaire will help us to develop a questionnaire that is easy to understand and simple to complete. The purpose of this interview is to get feedback from you on a quality of life questionnaire, specifically the questionnaire’s layout and instructions, and help us to determine whether any of the specific questions are confusing or ambiguous and might need rewording. I would like to tape-record this interview so that I remember everything that you tell me. Do I have your permission to record the interview? As well as recording, I will be taking notes.
Introduction: think aloud method
The interview format will be what we call a ‘think aloud’ process. What this involves is asking you to think out loud while you read and complete the questionnaire. Now, thinking out loud may be new and unfamiliar to you, but please know that there are no right or wrong answers so feel free to say anything that you’re thinking. I am only interested in knowing what is going through your mind when you read the questions and try to find the most appropriate answer to represent your experience. To explain this process to you in more detail, as you read the questionnaire and answer the questions, I would like you to tell me out loud any thoughts that go through your mind. For example, as you read the questionnaire, you might be thinking that a particular sentence is a little confusing or difficult to understand so I would want you to tell me this. You might also find that a particular question is not clear and you’re not entirely sure what the question is asking you; this I would also like you to tell me. So basically any thoughts about what you are reading and thinking while completing the questionnaire. Do you have any questions? We will now start the actual interview.
-
I noticed that you hesitated – can you tell me what you were thinking?
-
Talk me through what you are thinking while answering the question.
Introduction: debrief probing method
As part of this interview, I’m going to ask you to complete a questionnaire about the impact of pressure ulcers on quality of life. I will leave you for a little while (approx. 10–15 minutes) so that you can complete the questionnaire on your own. Take as much time as you need to read and complete the questionnaire. While you complete the questionnaire I would like you to circle, mark or underline any words that you do not understand or any individual questions that you find to be confusing, intrusive or ambiguous; basically, mark any problems that you find with the questionnaire. Feel free to write any comments in the margins and note any questions or problems that arise while you are completing the questions. There are no right or wrong answers. I am only interested in knowing which questions may be problematic in terms of wording, understanding and so on. After you complete the questionnaire I will come back and you can tell me about how it was for you completing the questionnaire. I will also ask you some questions about what you were thinking while you completed the questions and we can discuss any questions that you circled or marked and discuss any comments that you made. Do you have any questions before we get started?
Probing questions
Content and instruction probes
-
What do you think this questionnaire is about?
-
What to you is ‘(quality of life)’? Determine if anything is omitted.
-
Any areas of your life that your pressure ulcer affects that the questionnaire didn’t ask you about?
-
Did you understand the instructions? Was any part confusing or difficult to understand?
Layout probes
-
What do you think about the layout of the questionnaire? (i.e. general format, stem, items)
-
What do you think about the length of the questionnaire?
Response option probes
-
How easy or hard was it to tell the difference between each response choice?
-
You chose ‘(a little bother)’ as your answer, what does ‘(a little bother)’ mean to you?
-
Would you change any questions to make them easier to understand? What would you do?
Time frame probes
-
When answering the questions, did you compare now to how you were a week ago?
-
When you read ‘in the last week’, which days did you think of? (Which day.)
-
Would you have responded differently to this question(s) if I had asked you about your experience over the last 14 days or 30 days instead of only the last week?
-
Did you think mostly about your experience on specific days or times of day, or what was typical for you over the last week? (If specific day/times of day.) Can you tell me more about what made you think about those specific days/times?
Item stem probes: item by item and overall (comprehension/interpretation/recall)
-
What do the words ‘(difficulty with general movement)’ mean to you?
-
Describe your general movement or describe a typical day and the activities that you might do. Did you consider all these when you answered question 1?
-
Can you tell me in your own words what you think question ‘(1a)’ is asking?
-
How would you say this question in your own words?
-
How easy/hard was this question to answer? How would you reword it to make it easier?
-
Do you find any of the questions sensitive?
-
Are the questions worded in the language that you would use?
-
How did you arrive at that answer?
-
For question ‘(1a)’ you chose ‘(response option)’ How did you get that answer?
-
How well do you remember this? (Test recall of the relevant information.)
-
How do you remember this? (Study recall strategy.)
-
How hard was it answer? (Determine level of difficulty/likelihood of estimation/guessing.)
-
Was this hard/easy to answer? (Determine comprehension and overall ability to recall.)
Patients with multiple pressure ulcers
-
For patients with more than one pressure ulcer, go through each question and ask them which pressure ulcer they thought about when they answered this question.
-
While completing the questions, did you think about the pressure ulcer that was most bothersome? Or did you answer thinking about overall combined affect?
Finally, what could we do, if anything, to improve this questionnaire or any specific questions when we use them in the future with other people like you?
Closing
Thank you for taking the time to complete this questionnaire and for talking with me about what it was like for you. Now that we have completed the interview, do you have any questions?
Appendix 47 Pressure Ulcer Quality of Life field test 1 patient information leaflet and consent form
Appendix 48 Pressure Ulcer Quality of Life field test 2 patient information leaflet and consent forms
Appendix 49 Pressure Ulcer Quality of Life: Utility Index study reduced format protocol
Appendix 50 Pressure Ulcer Quality of Life: Utility Index general population valuation study information leaflet
Appendix 51 Pressure Ulcer Quality of Life: Utility Index valuation study interview schedule
Appendix 52 Pressure Ulcer Quality of Life: Utility Index methodology study reduced format protocol
Appendix 53 Pressure Ulcer Quality of Life: Utility Index methodology study patient information sheet and consent form
Appendix 54 Pressure Ulcer Quality of Life: Utility Index methodology study patient questionnaire
Appendix 55 Original application
Patient and public involvement section
Summary of proposal funded
We planned to ensure PPI via service user membership of the Programme Management Group and Steering Committee. We proposed to approach people via patient forums and clinical links, such as local tissue viability nurses and spinal injury units. We aimed to identify two service user members for each committee and prepare them for their roles via informal, supportive meetings with experienced team members. Meetings would be focused around the needs of the service users; both in terms of practical issues (e.g. timings, venue etc.) and the format of the meetings (e.g. language, chairing, agenda setting etc.).
Discrepancies between PPI activities undertaken and proposal funded
We did not establish service user membership of the Programme Management Group.
PPI activities undertaken that were not proposed
We have worked with service users in many other ways throughout the programme. The establishment of a PURPOSE PPI officer post enabled us to engage with service users and undertake more in depth PPI activities than set out in the funding application. For example:
-
We have set up a service user research network which will continue to support research in this field beyond the life of PURPOSE.
-
We have undertaken a series of meetings, workshops and consultations which focused on the service user perspective and have allowed input into all of the PURPOSE studies.
-
We undertook an evaluation of PPI in the Severe Pressure Ulcer Study.
-
We have involved service users in interpretation, dissemination and implementation of research findings.
-
We have shared our learning and PPI methods with the tissue viability and PPI research communities.
-
On-going support and mentorship has been offered to service users and development opportunities, such as conferences and training, have been provided where possible.
Pain studies
Summary of proposal funded
The pain work package proposed to determine the prevalence, type and severity of localised pressure ulcer pain in ‘pressure areas’ in patients with clinically assessed normal skin, erythema, superficial and severe pressure ulcers and explore the role of pain as an early predictor of Category 2 (or above) pressure ulcers. We planned: i) multicentre prevalence to determine the extent of pressure area related pain, nested within routine annual pressure ulcer prevalence audits in acute and community NHS Trusts and ii) a multi-centre prospective cohort study.
We estimated that we would require 4,000 hospital and 2,700 community patients for the prevalence survey, requiring us to piggy-back this work onto routine prevalence surveys in 4–5 acute and 2–3 community Trusts, and 340 patients for the prospective cohort study (based upon a model including 8 factors and an incidence rate of 25%).
Discrepancies between research undertaken and proposal funded
Prevalence: we revised our sample size estimates in response to Board comments and planned to undertake nested prevalence in a minimum of 2 acute and 2 community NHS Trusts with an expected sample size of 2,000 hospital and 6,000 community patients. However, the community nursing caseloads estimated by the community trusts at the grant application stage were inflated and our original plan assumed that the community prevalence methodology was similar to long-standing and well established acute hospital methods where nurses undertake a comprehensive skin assessment of each patient. This was not the case for one of the Trusts, with the two participating community Trusts using different case finding methods. This together with the scale of the data collection task in the community setting led to an adaptation of the original plan as follows: a) the community and acute prevalence studies were conducted and analysed separately, b) the community pain prevalence estimates included only those patients with pressure ulcers and c) the total survey population was 5180.
Prospective cohort study: in the grant application we had planned to recruit patients into the cohort study from the prevalence surveys. In practice this was not practical due to the organisational demands of Trust-wide pressure ulcer prevalence audits. We amended the sample size estimate to 632 in response to Board comments, with the addition of analgesic use as a risk factor in the planned model and a reduced incidence rate (model including nine factors and an incidence rate of 15%). We planned to undertake additional exploratory analyses to: i) assess the relationship between pain as measured by the two assessment methods (numerical rating scale and LANSS) and pressure ulcer development and ii) assess the relationship between changes in pain over time and the time to pressure ulcer development by treating pain as a time-dependent co-variate in a Cox proportional hazards model. This secondary analysis has not yet been undertaken.
Research undertaken that was not proposed
Prevalence: we undertook a sub-study comparing community pressure ulcer case finding methods.
Cohort analysis: In the grant application we proposed one multi-variable model using logistic regression. We undertook four analyses including the a priori logistic regression along with an over-dispersion logistic regression model and an Accelerated Failure Time model conducted at a patient level, and a multi-level regression model for analysis conducted at a skin site level.
Severe pressure ulcer study
Summary of proposal funded
The work package had two objectives, namely, (1) identify individual and organisational factors which contribute to the development of severe pressure ulcers, and, (2) develop a critical incident/adult neglect methodology for their review. A retrospective case study design was proposed, based on the retrospective review of the events leading to patients developing severe pressure ulcers. The study would draw on the methods used by Perrow (1999) and Vaughan (1996), both of whom pieced together accounts of major accidents from a range of sources, including documents and interviews with people who had been present when the accidents occurred. Patients would be identified via critical incident and adult protection referrals, and then purposively sampled, in order to maximise the range of patient and service characteristics. Competing explanations for the development of severe pressure ulcers would be evaluated using Yin’s ‘elimination of hypotheses’ method (2008).
The results would be fed back to local NHS teams responsible for critical incidents and for adult protection issues. It was envisaged that organisational risk factors, identified in this study, would be integrated with patient risk factors (in Study 3), and integrated into a single Minimum Data Set. A critical incident/adult neglect review protocol would be developed. Implementation would involve pilot work at two local sites, and then roll out to other participating centres. Study recommendations would be more widely disseminated.
Discrepancies between research undertaken and proposal funded
The only significant discrepancy concerns the identification of organisational risk factors, and the integration of those risk factors with the Minimum Data Set in Study 3. The study did not identify organisational risk factors that could be integrated in the manner originally envisaged. However, the findings did inform the design of the Risk Assessment Framework and which incorporated decision pathways which make a clear distinction between patients ‘at risk’ and those with an existing pressure ulcer who require secondary prevention and treatment, with escalation of interventions to prevent deterioration in existing pressure ulcers.
Research undertaken that was not proposed
A PPI led workshop was undertaken, where members of PURSUN UK were invited to contribute to the interpretation of some of the study findings. The workshop included video and role play to make the interpretation process engaging and accessible for service users with little or no experience of data analysis and interpretation.
References
Perrow C. Normal Accidents. New edition ed. Princeton: Princeton University Press; 1999.
Vaughan D. The Challenger Launch Decision. Chicago: University of Chicago Press; 1996.
Yin R. Case Study Research: Design and Methods. London: Sage; 2008.
Risk assessment
Summary of proposal funded
The risk assessment work package proposed to agree a pressure ulcer risk factor Minimum Data Set and use to underpin the development, implementation and evaluation of an evidence-based Risk Assessment Framework including safety flagging to guide decision making about risk of superficial pressure ulcers and risk of progression to severe pressure ulcers. The Risk Assessment Framework would adopt a stepwise approach with basic screening questions to quickly distinguish patients who are clearly not at risk and those who require more detailed risk assessment and would enable meaningful assessment and documentation of risk (incorporating anticipated patient need).
The proposal outlined work to update the pressure ulcer risk factor systematic review and using this along with the results from the severe pressure ulcer study and the pain studies, undertake a consensus study to agree a patient level risk factor Minimum Data Set. Using the Minimum Data Set we would go on to develop the Risk Assessment Framework and pilot, evaluate and implement the tool.
Discrepancies between research undertaken and proposal funded
In the original proposal the implementation and evaluation element of the Risk Assessment Framework development involved preliminary pilot work in one acute and one community trust with roll-out to other participating centres: the intended strategies included multi-disciplinary working groups, local guideline development, engagement of opinion leaders and incorporation into routine assessment/record keeping processes and the delivery of a package to support training and competency assessment in practice. Feedback from the local implementation teams would be used to refine the Risk Assessment Framework with ongoing consultation by the expert group. The intended evaluation included assessment of reliability (inter-rater and test re-test), face and content validity (assessed by local multi-disciplinary groups), compliance (via planned prevalence audits) and acceptability (through qualitative interviews with clinically based nursing staff).
The research actually undertaken provided a more structured approach, focussing on the evaluation and improvement of the Risk Assessment Framework to facilitate long-term implementation. Following the systematic review and the consensus study a pre-test with clinical nurses was undertaken to assess and improve the acceptability usability, format, design, clarity, comprehension, language and data completeness of the Risk Assessment Framework. This was considered an important and logical step in the tools development as it allowed improvements to be made prior to clinical evaluation. The clinical evaluation was undertaken in four acute and four community NHS Trusts and incorporated tissue viability nurses/research nurses and ward/community nurses using the Risk Assessment Framework with patients in a field test study. This allowed evaluation of the Risk Assessment Framework in relation to its inter-rater and test re-test reliability, convergent and known groups validity, data completeness and clinical usability. The more structured emphasis on the evaluation and improvement of the Risk Assessment Framework in the delivered studies of the programme will facilitate long-term implementation of the Risk Assessment Framework into routine NHS care.
Research undertaken that was not proposed
-
We gained additional funding from the World Universities Network to enable international membership to the multi-disciplinary expert group (two US, three Netherland, one Israel, and 11 UK) of the consensus study.
-
We integrated the views of service users (via PURSUN UK) into the consensus study methodology to ensure the acceptability of proposed assessment elements for patients was considered.
-
Following on from the consensus study we undertook an additional piece of work to develop a new conceptual framework and theoretical causal pathway for pressure ulcer development. This allowed us to bridge the gap between the epidemiological, physiological and biomechanical evidence.
Pressure ulcer quality of life
Summary of proposal funded
The work package proposed to develop and validate a psychometrically rigorous, patient-reported outcome (PRO) measure of HRQoL in patients with pressure ulcers (the PU-QOL instrument) that is reliable and valid, and suitable for use in the NHS. We planned to: i) develop a conceptual framework based on evidence from a systematic review of the PRO and HRQoL literatures, qualitative interviews with patients, and expert opinion; ii) produce a draft questionnaire and pre-test it using cognitive interviewing and a computerised appraisal tool; the Questionnaire Understanding Aid (QUAID); and iii) evaluate the psychometric properties of the PU-QOL in two-stage field testing using both modern and traditional psychometrics, including: a preliminary field test (item reduction) to identify items with poor psychometric properties for possible elimination and identify subscales, and a final (psychometric evaluation) field test to evaluate the reliability and validity of the item-reduced version of the PU-QOL instrument.
Discrepancies between research undertaken and proposal funded
We developed a researcher administered instrument and not a self-complete questionniare following an optimal mode-of-administration sub-study.
For the pre-test anaysis, we proposed to use the QUAID to analyse the cognitive interview data. However, instead we used a coding tool, the Quality Appraisal System (QAS-99), that focuses on the cognitive demands required for answering a question and potential item characteristics that may lead to response error. We also used Rasch analysis to examine the PU-QOL instruments’ response options, appropriateness of the item series, and biases due to question ordering and compared the cognitive interview and Rasch analysis findings to guide decision-making about further revisions to items and questionnaire design/layout.
For the psychometric evaluation phase, we proposed to use factor analysis, however instead we used Rasch analysis to investigate hypothesised scales. Rasch measurement theory provides a formal method of testing the degree to which rigorous measurement is achieved by rating scales, therefore use of factor analysis to determine scale structure was not deemed necessary.
Research undertaken that was not proposed
-
We undertook a sub-study to investigate optimal mode-of-administration for the PU-QOL instrument (between patient self-complete and interviewer-administration).
-
We completed two additional systematic reviews: 1) to explore content from existing chronic wound HRQoL outcome measures and 2) to explore patient reports of pressure ulcer-related pain.
-
We undertook an additional preliminary Rasch analysis on pre-test data to investigate PU-QOLs’ response options, appropriateness of the item series, and biases due to question ordering prior to large scale field testing.
Pressure ulcer cost utility
Summary of proposal funded
This work package proposed to assess the value patients place on the prevention and cure of pressure ulcers and to develop a pressure ulcer-specific health utility measure. We planned to: identify items for inclusion in the utility index from the PU-QOL instrument using Rasch measurement theory; undertake a valuation survey involving patients using a choice-based approach (the standard gamble); use modelling techniques to derive and test the utility algorithm.
Discrepancies between research undertaken and proposal funded
There were two main discrepancies between the research proposed and that undertaken: we used Time Trade Off and not Standard Gamble to elicit preferences; and the valuation exercise was conducted with the general population rather than patients. These changes were in part driven by updated guidance on technology appraisal by the National Institute for Health and Clinical Excellence which reaffirmed two key recommendations for valuation studies; that valuations should come from the general population and not patients with the condition; and that the valuation method should match that employed in the valuation of the EQ-5D (i.e. Time Trade Off). It also became apparent that a valuation survey would be particularly challenging in this patient group given that many were quite poorly, frail and elderly. The latter fact presented both practical and methodological issues.
Research undertaken that was not proposed
The validation study (study B) was additional research that was not originally proposed. This was felt necessary because the PU-QOL instrument incorporated an ‘attribution’ question format and this represented a methodological challenge to the generation of the utility index. We, therefore, undertook a validation sub-study which involved a patient survey (n = 100) with completion of a revised (attribution free) PU-QOL along with the EQ-5D allowing us to verify the item selection process and also allowing a psychometric assessment of the PUQOL-UI. These analyses enhance the overall body of work and the survey data will present future methodological research opportunities.
Glossary
- Algorithm
- The equation that enables conversion of responses on the health state classification system (i.e. the Pressure Ulcer Quality of Life Utility Index) to health state utility values.
- Backing-off procedure
- The revision of the valuation design (in particular the corner states) to avoid implausible health states.
- Construct/variable
- The main topic or outcome under investigation, for example quality of life.
- Corner state/s
- When one domain only is at the most severe level and all others are at the least severe level.
- Direct causal factor
- A factor that directly impacts the outcome (or the likelihood of the outcome).
- Domain
- The higher-level grouping of related topics/issues that are closely associated to the outcome of interest (or variable). For example, a domain of the construct quality of life could be ‘physical functioning’.
- Domain level
- The ‘response’ level of the measure being considered, that is, level 1 refers to ‘no bother’, level 2 refers to ‘a little bother’ and level 3 refers to ‘a lot of bother’.
- Health-related quality of life
- A multidimensional construct that represents an individual’s perception of how a given disease or medical condition and its treatment affect, at a minimum, his or her psychological, physical and social functioning.
- Independent risk factor
- A risk factor that retains its statistical association with the outcome when other established risk factors for the outcome are included in a statistical model.
- Indirect causal factor
- A factor that impacts the outcome (or affects its likelihood of occurrence) by changing a direct causal factor. If the direct causal factor is prevented from changing, then changes in the outcome will not be produced.
- Instrument
- Encompasses any method used to measure the variable or construct of interest, including rating scales, questionnaires, clinical assessments and electronic devices.
- Inverse corner state/s
- When one attribute only is at the least severe level. These states provide information on the weight of the domain.
- Item
- Refers to a single question intended to assess or measure a particular variable. A variable is usually measured by an instrument or a scale consisting of multiple items representing aspects relevant to the particular variable.
- Minimum Data Set
- A list of key data that should be recorded in all settings to allow comparison of patient groups.
- Non-independent risk factor
- A risk factor that loses its statistical association with the outcome when other established risk factors for the outcome are included in a statistical model.
- Patient-reported outcome
- A measurement of any aspect of a patient’s health status that comes directly from the patient without any interpretation of the patient’s response by physicians or others, about how he or she functions or feels in relation to a health condition or its therapy.
- Ping-pong procedure
- Interview technique when eliciting preferences in which the number of years to be traded switches alternatively from high to low offers until the point of indifference is reached.
- PITS
- A term used to denote the worst health state in which all of the domains are at the most severe level.
- Pressure area
- A body site where pressure ulcers commonly develop; most commonly these include the sacrum, buttocks, ischial tuberosities, hips, heels, ankles and elbows.
- Pressure area-related pain
- Pain, soreness or discomfort in any pressure area.
- Pressure ulcer pain
- Pain, soreness or discomfort at a body site with an observable pressure ulcer.
- Quality of life
- A multidimensional construct referring to all aspects of a person’s well-being influenced by the person’s perceived level of satisfaction in a variety of circumstances.
- Response options
- The choices available to select when answering a particular question, used to quantify items. For example, ‘not at all’/’a little’/’moderately’/’quite a bit’/’extremely’ are response categories for the Short Form questionnaire-12 items health status instrument.
- Risk factor
- A variable with a significant statistical association with a clinical outcome.
- Subdomain
- A further breakdown of the domain into its constituent parts, for example ‘walking’ could be a subdomain of the higher-level domain ‘physical functioning’.
- Time trade-off
- A preference elicitation technique in which respondents consider how much time in a given ill-health state they are willing to trade for less time in full or perfect health.
- Unattributed pressure area-related pain
- Pain, soreness or discomfort reported by patients on a pressure area/pressure ulcer but in which the body site is not specified/recorded.
- Utility
- Health state utility values typically range from 1 (perfect health) through 0 (dead) to minus infinity. They are used to weight life-years to produce quality-adjusted life-year estimates for use in cost–utility analyses.
List of abbreviations
- ADL
- activities of daily living
- AF
- acceleration factor
- AMED
- Allied and Complementary Medicine Database
- BMI
- body mass index
- BNI
- British Nursing Index
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CRN
- clinical research nurse
- CSUM
- condition-specific utility measure
- CTRU
- Clinical Trials Research Unit
- CTT
- classical test theory
- DIF
- differential item functioning
- EPUAP
- European Pressure Ulcer Advisory Panel
- EQ-5D
- European Quality of Life-5 Dimensions
- EQ-5D-3L
- European Quality of Life-5 Dimensions three-level version
- FDA
- Food and Drug Administration
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ITC
- item–total correlation
- LANSS
- Leeds Assessment of Neuropathic Symptoms and Signs
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NPUAP
- National Pressure Ulcer Advisory Panel
- OR
- odds ratio
- PABAK
- prevalence-adjusted bias-adjusted kappa
- PI
- principal investigator
- PPI
- patient and public involvement
- PRO
- patient-reported outcome
- PUPPs
- Pressure Ulcer Prevention Pathways
- PU-QOL
- Pressure Ulcer Quality of Life
- PUQOL-UI
- Pressure Ulcer Quality of Life – Utility Index
- PURAF
- Pressure Ulcer Risk Assessment Framework
- PURPOSE
- Pressure UlceR Programme Of reSEarch
- PURPOSE-T
- Pressure Ulcer Risk Primary Or Secondary Evaluation Tool
- PURSUN UK
- Pressure Ulcer Research Service User Network UK
- QALY
- quality-adjusted life-year
- QAS-99
- Question Appraisal System
- RAND/UCLA
- Research and Development, University of California in Los Angeles
- RMT
- Rasch measurement theory
- SD
- standard deviation
- SF-6D
- Short Form questionnaire-6 Dimensions
- SF-12
- Short Form questionnaire-12 items
- TTO
- time trade-off
- TVNS
- tissue viability nurse specialist
- VAS
- visual analogue scale
- WOK
- Web of Knowledge