Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0407-10177. The contractual start date was in September 2008. The final report began editorial review in April 2014 and was accepted for publication in May 2015. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Sébastien Barbarot reports grants from Le Collège des Enseignants en Dermatologie de France (CEDEF) during the conduct of the study and grants from Astellas, personal fees from GlaxoSmithKline, personal fees from Sinclair Pharma, personal fees from Astellas, grants from the Foundation for Atopic Dermatitis, Pierre Fabre Laboratory, and grants from Pierre Fabre Laboratory as an investigator outside the submitted work. Hywel Williams is Deputy Director of the National Institute for Health Research Programme, chairperson of the HTA Commissioning Board, and Programme Director for the Health Technology Assessment programme.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Nankervis et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background and aims
What is eczema?
Eczema, commonly referred to as ‘atopic eczema’ or ‘atopic dermatitis’, is a chronic inflammatory skin condition characterised by an itchy red rash. Eczema can affect any part of the body but it typically settles in the skin creases such as the folds of elbows or behind the knees; the face is commonly involved in infants and in adults. Eczema lesions vary in appearance from collections of fluid in the skin (vesicles) to gross thickening of the skin (lichenification) on a background of poorly demarcated redness. Other features such as crusting, scaling, cracking and swelling of the skin can occur. 1 Dry skin that results in impaired barrier function is also a key feature of eczema. Eczema is associated with other atopic diseases such as seasonal allergic rhinitis (hay fever) and asthma. Around 30% of people with eczema develop asthma and 35% develop allergic rhinitis. 2 Atopic eczema typically starts in early life, with about 80% of cases starting before the age of 5 years. 3
When is eczema ‘atopic’?
Although the word ‘atopic’ is often used when describing eczema, up to two-thirds of people with eczema do not have measurable levels of circulating allergen-specific immunoglobulin E (IgE) antibodies, which are a necessary criterion to denote a person as ‘atopic’. 4 The relationship between atopy and eczema is unclear. Atopy is more common in more severe disease in hospital populations than in community populations and atopy is also more common in affluent than in non-affluent countries. It has been suggested that the relationship between eczema and atopy might be apparent only because of shared causes for both conditions. 5
The nomenclature for allergy6 has been revised and is now based on the mechanisms that initiate and mediate allergic reactions. The term ‘atopic eczema’ should be used only when IgE sensitisation has been confirmed by allergen-specific IgE antibodies in the blood or by a positive skin-prick test to common allergens such as house dust mite. 7 As this review includes cases of eczema based purely on a clinical diagnosis, the term ‘eczema’ is used throughout to refer to what is more often described as ‘atopic dermatitis’ or ‘atopic eczema’.
How is eczema defined in clinical studies?
Quite often no definition of eczema is given in clinical studies, which leaves the reader guessing as to what sort of people were studied. Eczema is a difficult disease to define as the clinical features are highly variable. This variability can relate to the skin rash morphology (e.g. it can be dry and thickened or weeping and eroded), location (e.g. it commonly affects the cheeks in infants and skin creases in older children) and time (it can be bright red one day and apparently gone a couple of days later) (Figure 1). There is no specific diagnostic test for all people with typical eczema to serve as a reference standard; diagnosis is therefore a clinical one.
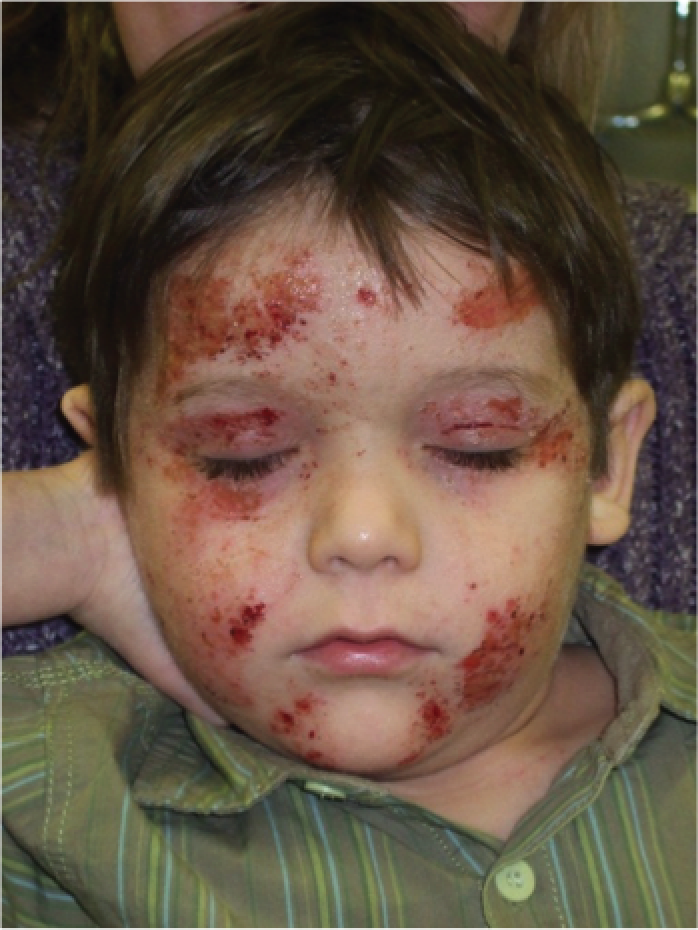
FIGURE 1.
Facial eczema. Severe eczema with signs of weeping, crusting and scratching. Image reproduced with permission.
Until the late 1970s at least 12 synonyms for eczema-like conditions were in common usage in the dermatological literature and it is not certain whether physicians were all referring to the same disease when using these terms. A major milestone in describing the main clinical features of eczema was the Hanifin and Rajka diagnostic criteria of 1980. 8 These consensus criteria are frequently cited in clinical trial articles, thereby providing a degree of confidence that researchers are referring to a similar disease. Scientifically developed refinements of the Hanifin and Rajka diagnostic criteria have been developed by a UK Working Party9 and these criteria have been validated widely10 and used throughout the world and in National Institute for Health and Care Excellence (NICE) guidance. To qualify as a case of eczema, the person must have an itchy skin condition plus three or more of the following:
-
past involvement of the skin creases, such as the bends of elbows or behind the knees
-
personal or immediate family history of asthma or hay fever
-
tendency towards a generally dry skin
-
onset under the age of 2 years (except when aged ≤ 4 years)
-
visible flexural dermatitis as defined by a photographic protocol.
Binary or continuous disease?
It is unclear whether eczema is an ‘entity’ in itself or whether it is part of a continuum when considered at a population level. 11 Although it may be appropriate to ask the question, ‘How much atopic eczema does he/she have?’ as opposed to ‘Does he/she have atopic eczema – yes or no?’, most population and clinical studies require a categorical cut-off.
Is it all one disease?
It is quite possible that there are distinct subsets of eczema, for example those with filaggrin mutations,12,13 which may lead to more persistent and severe disease. When assessing which treatments are going to be effective for eczema it is still sensible to consider the clinical disease as one condition. As more evidence about the different phenotypes of eczema is collected (e.g. those who are definitely atopic with raised circulating IgE levels to allergens or those with severe disease and associated asthma),11 sensitivity analyses can be carried out to evaluate whether such subdivisions are useful for predicting treatment response.
The prevalence of atopic eczema
Eczema is a very common problem. The International Study of Asthma and Allergies in Childhood (ISAAC) has been collecting data using standardised questionnaires combined with physical examinations since 1992 [see http://isaac.auckland.ac.nz/ (accessed 12 December 2015)]. Data from just under half a million children and adolescents who participated in both phase I (1992–4) and phase III (1999–2004) have shown that eczema is a truly global problem. The most recent phase revealed that prevalence among 6- to 7-year-olds ranged from 0.9% in Jodhpur, India, to 22.5% in Quito, Ecuador. Among 13- to 14-year-olds the prevalence ranged from 0.2% in Tibet, China, to 24.6% in Barranquilla, Colombia. Prevalence in the UK for 6- to 7-year-olds was 16% and for 13- to 14-year-olds was 10.6%. ISSAC has shown that eczema prevalence has increased globally by just under 2%. 14 Rising rates of prevalence have been found in many developing countries, whereas a ‘levelling off’ or decrease in prevalence has been reported for developed countries. The increased prevalence in developing countries could be for a number of reasons. One review postulated that the increase in use of soaps, shower gels and other harsh cleaning products, often seen in countries undergoing rapid development, could contribute towards this rise in prevalence. 15 In the UK the incidence of eczema has been assessed once, through a large primary care database in 2005; it was found to affect around one in nine people at some point in the year. 16
Age
Eczema is more common in childhood, particularly in the first 5 years of life. 17 The prognosis of eczema shows a mixed picture, with one study18 showing a 90% clearance rate for children within 10 years, and other studies finding the rate to be around 60% by age 16 years. These figures may still not reflect the true level of eczema clearance, as many people relapse at some point in their life. Eczema prognosis may differ between the community setting (where the majority of cases are mild) and the hospital setting (where cases tend to be more severe). One study found that 10% of hospital eczema patients still suffer as adults. Adults constitute around one-third of all those with eczema in a general practice community. 17 Adults also tend to represent a more persistent and severe subset of cases.
Severity distribution
Most cases of childhood eczema are mild. A study from 1998 by Emerson and colleagues19 found that 84% of 1760 children aged 1–5 years from four urban and semi-urban general practices in Nottingham were mild cases (as defined globally by the examining physician), with 14% of cases in the moderate category and 2% in the severe category. However, there has been little research regarding the severity distribution of eczema, even though this would provide useful information for allocating health resources (as the costs of managing severe eczema are disproportionally large in comparison to the costs of managing mild eczema).
How does eczema affect people?
Direct morbidity has been estimated in several studies using generic dermatology quality-of-life scales. 20,21 Impairment of quality of life has been found to be directly proportional to the severity of eczema. 20 It has been found that atopic eczema usually accounts for the worst scores compared with other dermatological disease. Specific aspects of a child’s life that are affected by atopic eczema are:
-
itch and associated sleep disturbance
-
ostracism by other children and parents
-
the need for special clothing and bedding
-
avoidance of activities such as swimming, which other children can enjoy
-
the need for frequent applications of greasy ointments and visits to the doctor.
Family disturbance is also considerable, with sleep loss and the need to take time off work for visits to health-care professionals. 21,22 Eczema in infancy incorporating sleep disruption is associated with an increased risk of mental health issues at age 10 years. 23
Economic costs
In financial terms, the cost of atopic eczema is potentially very large. The costs associated with the management of eczema are largely indirect, such as workdays lost by parents and travel costs for heath-care appointments, with much of this expense being met by the family of the person with eczema. There have been a number of studies in the UK, the Netherlands and the USA that have shown the costs to vary between country and according to severity. 16,24,25
What causes eczema?
Genetics
There is strong evidence to suggest that genetic factors are important in the predisposition to eczema. In addition to family studies, twin studies have shown a much higher concordance for monozygotic (identical) (85%) than for dizygotic (non-identical) (21%) twins. 26 Mutations that occur in the filaggrin (filament-aggregating-protein) gene give rise to a faulty filaggrin protein that results in dry skin and an increased risk of developing eczema, as well as more severe and persistent disease and associated asthma. 13
Environment
Although genetic factors are probably a very important factor for disease predisposition, there are numerous general and specific clues that point strongly to the crucial role of the environment in disease expression. 27 The recent, large increases in the prevalence of atopic eczema are difficult to explain purely in genetic terms. 15 It has been shown that atopic eczema is more common in wealthier families. 28,29 It is unclear whether this positive social class gradient is a reflection of indoor allergen exposures or whether it reflects a whole constellation of other factors associated with ‘development’. The observation that many cases of atopic eczema improve spontaneously around puberty is also difficult to explain in genetic terms alone. Recent large birth cohort studies14 have not found any protective effect of elder siblings, conflicting with earlier studies. 30 The original observation that increasing family size was associated with decreased eczema prevalence led to the ‘hygiene hypothesis’, which proposed that children in larger families were protected from expressing atopy because of frequent exposure to infections. 31 The role of exposure to microbes and allergens in the environment is still being debated.
Migrant studies also point strongly to the role of environmental factors in eczema development. It has been shown that 14.9% of black Caribbean children living in London develop atopic eczema (according to the UK diagnostic criteria) compared with only 5.6% of similar children living in Kingston, Jamaica. 25 Other migrant studies reviewed elsewhere have consistently recorded large differences in ethnic groups migrating from warmer climates to more prosperous cooler countries. 32
Further work has suggested that the tendency to develop eczema could be programmed at birth and could be related to factors such as maternal tobacco exposure. 33 Specific risk factors for eczema expression in the environment include furry pets; however, there is evidence that these can also have protective effects. 34 Allergic factors such as exposure to the house dust mite could be important, but non-allergic factors such as exposure to nylon clothing, dust or shampoo may also be important. 35
Pathophysiology
A number of molecular mechanisms and cell types are thought to be important in atopic eczema and these are reviewed in detail elsewhere. 36 Microscopically, the characteristic appearance of eczema is fluid between the cells in the epidermis (spongiosis). When severe, this fluid eventually disrupts the adjacent cells in the epidermis to form small collections of fluid, which are visible to the naked eye as vesicles. In the chronic phase, atopic eczema is characterised by gross thickening of the epidermis (acanthosis) and a lymphocytic infiltrate in the dermis. The pathophysiology of atopic eczema may be related to abnormal gene expression of immune cells as they infiltrate and remain in the mucosal surfaces and skin. There appears to be a failure to switch off the natural predominance of type 2 helper (Th2) lymphocytes that normally occurs in infancy, leading to an abnormal response of cytokines (chemical messengers) to a variety of stimuli. This failure to achieve the normal balance of type 1 helper (Th1) and Th2 cells may be a result of mutations in the interleukin-18 gene37 or other genes,38 for example those that produce receptors for the innate immune system. Defects in the composition of the skin barrier leading to dry skin and enhanced penetration of irritants and allergens are also thought to be critical. 39,40
Does eczema clear with time?
Although the tendency towards a dry and irritable skin is probably lifelong, the majority of children with atopic eczema appear to ‘grow out’ of their disease, at least to a point at which the condition no longer requires active medical care. A detailed review of studies that have determined the prognosis of atopic eczema has been reported elsewhere. 3 About 60% of childhood cases are clear or symptom free in early adolescence, although many such apparently clear cases are likely to recur in adulthood, often as hand eczema. The strongest and most consistent factors that appear to predict more persistent atopic eczema are early onset, severe widespread disease in infancy, concomitant asthma, wheezing or hay fever, and a family history of atopic eczema. 33
How is eczema treated in the UK?
In 2007 NICE41 published guidance for the management of atopic eczema in children aged < 12 years. The Scottish Intercollegiate Guidelines Network (SIGN)42 published guidance for eczema management in 2011 that covers adults and children.
National Institute for Health and Care Excellence guidance
The NICE guidance41 covers diagnosis, assessment and management of eczema and information on eczema. The evidence was systematically reviewed and opinions from clinicians, researchers and consumers were used to develop the clinical guidelines.
The guidelines recommend a holistic approach at each consultation, taking into account the severity of the atopic eczema and the child’s quality of life, including everyday activities, sleep, and psychosocial well-being.
The guidelines give a clear list of the first-line (emollients and topical corticosteroids), second-line (topical calcineurin inhibitors) and third-line (systematic treatments and phototherapy) treatments based on need and severity.
The mainstay of treatment for all severities of eczema is emollients. The guidelines emphasise the importance of using emollients even when the skin is clear. It also recommends allowing the patient a choice of emollients for washing, bathing and moisturising.
Topical corticosteroids should be tailored to the severity and area of eczema and should be used once or twice daily for an appropriate length of time. Topical steroids are an important tool for eczema treatment, although care regarding the duration of treatment, site and age of the person treated was emphasised. Potent steroids are not recommended for use in children aged < 12 months without specialist dermatologist supervision.
Oral antihistamines should not be used routinely in the management of atopic eczema in children. However, if sleep disturbance (for the child or their parents/carers) becomes significant during an exacerbation of eczema (flare), health-care professionals should offer a 7- to 14-day trial of an age-appropriate sedating antihistamine (for children aged ≥ 6 months). This treatment can be repeated during subsequent flares if successful.
Occlusive medicated dressings and dry bandages should not be used to treat infected atopic eczema in children.
Phototherapy or systemic treatments should be initiated in children with atopic eczema only after assessment and documentation of the severity of atopic eczema and quality of life.
Health-care professionals should spend time educating children with atopic eczema and their parents or carers about atopic eczema and its treatment.
Scottish Intercollegiate Guidelines Network guidance
The SIGN guidance42 covers diagnosis, referral, management and patient education. The evidence for the SIGN guidelines came from a systematic review of the literature from 2004 to 2009, involving five bibliographic databases. The analysis of the evidence was conducted by the SIGN committee using standard forms, with any additional evidence submitted by members of the committee, including key reviews outside the search period. A review of studies on the issues facing patients was conducted and presented to the committee.
The guidelines give the following key recommendations:
-
Emollients should be given on a continuous basis, even when topical corticosteroids are being used.
-
Topical corticosteroids should be applied once a day, and twice-weekly treatment should be considered when those with moderate to severe eczema have frequent relapses.
-
Topical calcineurin inhibitors are recommended for use in children aged ≥ 2 years if topical corticosteroids are not controlling the eczema or the level of use of topical corticosteroid could lead to adverse effects.
-
Topical antibiotics are not recommended for the treatment of non-infected eczema.
Other recommendations included:
-
As a precaution topical calcineurin inhibitors should not be applied to skin that appears actively infected.
-
Patients with non-infected moderate to severe eczema should be advised to cover affected areas with dry wrap dressings to provide a physical barrier to scratching and improve retention of emollient.
-
Swabs of potential Staphylococcus aureus carriage sites (of both the patient and family members) should be considered in patients with recurrent infection.
-
In patients with atypical features, or when there is concern about possible streptococcal infection, skin swabs of affected areas should be considered.
-
Short-term use of sedating antihistamines at night-time should be considered in patients with atopic eczema when there is debilitating sleep disturbance.
-
When an irritant effect is suspected, patients should be advised to avoid biological washing powders, fabric conditioners and fragranced products such as soaps and shower gels.
-
Dietary exclusion is not recommended for management of atopic eczema in patients without confirmed food allergy.
-
When there is suspicion of food allergy in infants or children with atopic eczema, general practitioners (GPs) should refer to an allergist or paediatrician with a special interest in allergy.
-
Exercise caution when using herbal medicines and be wary of any herbal product that is not labelled in English or does not come with information about safe usage.
The guidelines also detail what information can reasonably be expected by a patient during diagnosis and treatment.
How is care organised in the UK?
Most children with atopic eczema in the UK are managed by a primary care team, with around 4–10% of children with atopic eczema referred to a dermatologist for further advice. 19
The quality of service provided by secondary care has been audited by the British Association of Dermatologists. Although most departments provided a high-quality service, some aspects of care, such as the administration of simple standardised record forms, could be improved. 43 The audit found that the outcomes may not be as good as some doctors believe, with the improvements in quality of life and numbers of adults returning to work not meeting the working standards. 43
Adherence (or, more correctly, concordance) seems to be a major cause of apparent treatment failure. A survey conducted in Nottingham found that most parents worry that topical steroids cause adverse effects, although many were not able to distinguish between weak and strong ones. 44
The National Eczema Society [see www.eczema.org (accessed 12 October 2015)] is the UK’s largest self-help organisation for people with eczema. It has a well-organised information service and national network of activities geared to help those with eczema and their families. Sources of alternative care abound in the community, especially with the increased access to the internet, ranging from the highly professional to elaborate, expensive diagnostic and therapeutic measures of dubious value.
How are the effects of atopic eczema captured in clinical trials?
Outcome measures used in trials have recently been reviewed. 45 Most outcome measures have incorporated some measure of itch as assessed by a doctor at periodic reviews or patient self-completed diaries. Other more sophisticated methods of objectively recording itch have been tried. 46 Composite outcome measures are most often used. These usually incorporate measures of extent of atopic eczema and several physical signs such as redness, scratch marks, thickening of the skin, scaling and dryness. Such signs are typically mixed with symptoms of sleep loss and itching and variable weighting systems are used. It has been shown that measuring surface area involvement in atopic eczema is fraught with difficulties,27 which is not surprising considering that eczema is, by definition, ‘poorly defined erythema’. A systematic review of named outcome measure scales for atopic eczema45 found that, of the 20 named scales in current use, only three have been validated adequately enough to be recommended for use in clinical trials: Severity Scoring of Atopic Dermatitis (SCORAD),47 Eczema Area and Severity Index (EASI)48 and the Patient-Oriented Eczema Measure (POEM). 49 Quality-of-life measures specific to dermatology include the Dermatology Quality of Life Index50–52 and Skindex53,54
Why is a scoping systematic review still needed?
Keeping up to date with the rapidly increasing evidence base for eczema treatment is challenging.
The predecessor to this review by Hoare and colleagues55 gave an overview of the evidence, with an assessment of quality and the implications for both practice and research. The review was well received and provided major contributions to eczema guidelines around the world. This updated version of the review has the same aim of giving a succinct, clinically relevant overview and identifying the major research gaps.
The treatment landscape has also radically changed in the past decade, with many new treatments now in routine clinical use and new treatment regimens regularly being advocated. Even though the pace of eczema research has steadily increased, with more relevant Cochrane and non-Cochrane systematic reviews being published, there is still considerable uncertainty about the effectiveness of the prevention and treatment of atopic eczema. This is unsurprising as there are still sizeable ‘holes’ in the web of up-to-date systematic reviews and identifying randomised controlled trials (RCTs) that fill in these holes has previously been difficult and time-consuming. Although there have been a good number of systematic reviews of specific eczema treatments over the past 14 years, many treatments have never been reviewed, and the scoping nature of this updated systematic review helps to redress this imbalance.
The high disease burden and concerns regarding adverse effects coupled with the profusion of treatments delivered in different care settings are all reasons why an up-to-date scoping systematic review of atopic eczema treatments is needed.
This updated review will also contribute to identifying, prioritising and generating further primary, secondary and methodological research.
Summary of the problem of atopic eczema
-
The terms ‘atopic eczema’ and ‘atopic dermatitis’ are synonymous and when ‘atopy’ has not been tested for, the term ‘eczema’ should be used.
-
The definition of eczema is a clinical one based on itching, redness and involvement of the skin creases.
-
About 20% of people seen in hospitals with clinically typical eczema are not ‘atopic’.
-
Eczema affects about 15–20% of UK children.
-
About 80% of cases in the community are mild.
-
Adults make up about one-third of all cases in a given community.
-
Eczema accounted for the largest burden of disability life-adjusted years for skin diseases in the 2010 World Health Organization global burden of diseases project. 56
-
The constant itch and resultant skin damage in eczema can lead to a poor quality of life for people with eczema and their families.
-
The economic costs of eczema are high.
-
Genetic and environmental factors are both critical for disease expression.
-
Non-allergic factors may be just as important as allergic factors in determining disease expression and persistence.
-
The immune system and skin barrier abnormalities are both important in explaining the pathological processes of atopic eczema.
-
About 60% of children with atopic eczema are apparently clear or free of symptoms by adolescence.
-
Early onset, severe disease in childhood and associated asthma/hay fever are predictors of a worse prognosis.
-
Current first-line treatment in the UK includes emollients and topical corticosteroids.
-
Second-line treatments include topical calcineurin inhibitors and ultraviolet light.
-
Third-line treatments include systemic immunomodulatory treatments such as ciclosporin and azathioprine.
-
Most people with eczema are managed by their primary care team.
-
Some people with eczema seek alternative treatments, such as complementary therapies.
-
An up-to-date systematic review is needed to map out in which areas high-quality research has been conducted to date, with the aim of resolving some areas of uncertainty and identifying knowledge gaps to be addressed by further primary and secondary research.
Research questions asked in this review
The remit of this project was to provide a summary of RCTs of eczema with the main aim of providing useful clinical information for health-care professionals, commissioners and people with eczema and their families, and also to identify research gaps for further primary, secondary or methodological research. It is also hoped that the review will be of some use to health-care providers, physicians involved in the care of people with eczema and also people with eczema and their families by placing current treatments in context within the current evidence base. The main research questions asked in this review are therefore:
-
What treatment recommendations can be made by summarising the available RCT evidence using narrative and quantitative methods? The main outputs for this question are detailed summaries of available RCT evidence for different interventions for atopic eczema along with the review authors’ interpretations of the data based on the quality of that evidence, the magnitude of the treatment effect and the clinical relevance of the evidence.
-
What therapeutic interventions have the RCTs of atopic eczema covered so far? The main output for this question is a summary of research gaps for further research, with research commissioners, charities and researchers as the main target audience.
A question- or data-driven review?
The very broad-ranging scoping nature of this review implies that it cannot be hypothesis driven. Trying to answer similar questions for each of the 92 or so interventions used for the treatment of atopic eczema would be impossible in one short report.
This updated review is still unashamedly data driven. It is a review that aims to map out what has been done in terms of RCTs in atopic eczema to date and to reflect and comment on the coverage of already-researched areas in relation to questions that are commonly asked by physicians and their patients.
The authors are aware that there is a danger that a data-driven review can serve to amplify and perpetuate current trends in evaluating minor differences between a profusion of similar pharmacological products. The authors have mitigated against this inevitable hazard by drawing attention to gaps that have not been addressed when summarising the reported studies and also by including a section on unanswered questions in the discussion and conclusions section of this report, based on a recently completed James Lind Alliance Priority Setting Partnership. 57
Chapter 2 Methods
General methods structure
This review uses methods developed by the Cochrane Collaboration when possible. 58 The review follows the general structure of and guidance from the National Institute for Health Research (NIHR) reports of systematic reviews and closely follows the methods used in the NIHR Health Technology Assessment (HTA) programme report published in 200055 for continuity. This previous HTA report serves as the published protocol for this review and therefore an additional separate protocol was not published.
Types of studies included in the review
Only RCTs of treatments for eczema were included in the data summaries as other forms of evidence are associated with higher risks of bias. For a RCT to be included, it needed to be prospective and randomise participants diagnosed with eczema to two or more groups. In addition, the RCT had to be concerned with therapeutic issues in relation to the treatment of atopic eczema.
Provocation studies that evaluated cellular or biochemical responses to substances such as histamine were not included. Studies of possible increased incidence of drug adverse effects in atopic people compared with non-atopic people were also excluded. Studies also had to include at least one clinical outcome. Therefore, studies that reported only changes in blood tests or cellular mechanisms were excluded.
Study participants
Studies were included if participants (of any age) had eczema meeting diagnostic criteria (e.g. Hanifin and Rajka criteria,8 UK Working Party criteria9 or similar) or had been diagnosed by a physician. Terms used to identify trial participants with definite and possible eczema and those definitely not having eczema are shown in Table 1. Those studies using terms in the ‘definitely not eczema’ category, such as ‘allergic contact eczema’, were excluded. Those studies using terms in the ‘possible eczema’ category, such as ‘childhood eczema’, were scrutinised by one of the authors and included only if the description of the participants clearly indicated eczema (i.e. itching and flexural involvement).
| Definite eczemaa | Possible eczemab | Not eczemac |
|---|---|---|
|
|
|
Main outcome measures
Changes in patient-rated symptoms of eczema such as itching (pruritus) or sleep loss were used when possible. Global severity, as rated by patients or their physician, was also sought. If these outcomes were not available, then global changes in composite rating scales using a published named scale (Table 2 provides information on the most commonly used scales) or, when not possible, the authors’ modification of existing scales or new scales developed within the study were summarised. Quality of life, using any named scale or, when not possible, the authors’ modification of existing scales, and adverse events were also included if reported.
| Name | Scale | Usually assessed by | Assessment of symptoms | Assessment of signs | Extent | Comments |
|---|---|---|---|---|---|---|
| SCORAD47 | 0–103 | Physician | Pruritus, sleep disturbance | Erythema, oedema/induration/papulation, oozing/crusting/weeping/exudation, excoriation, lichenification, dryness | % body surface area | Used mostly in Europe. Recommended for use in clinical trials in one systematic review of outcome measures45 |
| EASI48 | 0–72 | Physician | Not assessed | Erythema, oedema/induration/papulation, excoriation, lichenification | % body surface area | Mostly used in America. Recommended for use in clinical trials in one systematic review of outcome measures45 |
| Six Area, Six Sign Atopic Dermatitis (SASSAD)59 | 0–108 | Physician | Not assessed | Erythema, oozing/crusting/weeping/exudation, excoriation, lichenification, dryness, cracking/fissuring | Assessment at defined body sites | Measures signs only and therefore it can be used when blinded outcome assessment by a trained observer is needed |
| POEM49 | 0–28 | Patient or carer | Pruritus, sleep disturbance | Oozing/crusting/weeping/exudation, dryness, cracking/fissuring, flaking, bleeding | Not assessed | Recommended for use in clinical trials in one systematic review of outcome measures45 |
| Atopic Dermatitis Area and Severity Index (ADASI)60 | 0–15 | Physician | Pruritus | Erythema, oedema/induration/papulation, oozing/crusting/weeping/exudation, lichenification, scaling | % body surface area affected | Test–retest reliability and sensitivity to change have not been tested45 |
| Leicester Sign Score (LSS)61 | 0–150 | Physician | None | Erythema, excoriation, lichenification, dryness, cracking/fissuring | Assessment at defined body sites | Interobserver reliability and test–retest reliability have not been tested45 |
| Investigator’s Global Assessment Of Disease Activity (IGADA)62 | Clear to very severe | Physician | None | Erythema, oedema/induration/papulation, oozing/crusting/weeping/exudation, excoriation, lichenification, scaling | Assessment at defined body sites | Interobserver reliability and test–retest reliability have not been tested. Adequate sensitivity to change has been shown45 |
| Three-Item Severity (TIS) score63 | 0–9 | Physician | None | Erythema, oedema/induration/papulation, excoriation | Not assessed | Adequate construct validity has been shown45 |
Secondary outcome measures
Secondary outcomes measures were changes in individual signs of atopic eczema as assessed by a physician, for example:
-
erythema (redness)
-
purulence (pus formation)
-
excoriation (scratch marks)
-
xerosis (skin dryness)
-
scaling
-
lichenification (thickening of the skin)
-
fissuring (cracks)
-
exudation (weeping serum from the skin surface)
-
pustules (pus spots)
-
papules (spots that protrude from the skin surface)
-
vesicles (clear fluid or ‘water blisters’ in the skin)
-
crusts (dried serum on the skin surface)
-
infiltration/oedema (swelling of the skin)
-
induration (a thickened feel to the skin).
For the original review, the selection of outcome measures was explored in more detail with a focus group of consumers when developing the review methods. The secondary outcomes were not altered for the updated review to allow synthesis of the evidence. This work also forms part of a larger body of research on developing core outcome sets for eczema clinical trials known as the Harmonising Outcome Measures for Eczema (HOME) initiative (see the following section),57 in which there has been considerable patient and public involvement.
Dealing with different outcome measures
Eczema outcomes have historically been measured in many different ways. In 2007, a systematic review of eczema outcomes45 identified 20 different named scales for measuring the severity of eczema and concluded that only three of these (SCORAD, EASI and POEM) could be recommended for use in clinical trials on the basis of the available validation studies. The eczema severity outcome measures cover different signs and symptoms of eczema as well as working out the extent of the eczema in different ways. These differences often make direct comparison of trial results difficult or impossible. To improve the situation, the HOME initiative was formed. 64,65 This initiative is ongoing and involves and welcomes contributions from researchers, clinicians, patients and the pharmaceutical industry. The aim of this initiative is to decide on a set of core outcome measures that should be included in all RCTs. These core outcomes will cover the agreed domains of signs, symptoms, quality of life and long-term control. The HOME initiative has so far recommended that the signs and symptoms for eczema be recorded using EASI. 66 Trials are not restricted to the core outcome measures but should always include them to facilitate direct comparisons of the results of RCTs in the future.
Search strategy
Electronic searching
To retrieve all RCTs on atopic eczema treatments in accordance with the inclusion criteria, a systematic and mainly electronic search was carried out. The Cochrane Handbook for Systematic Reviews of Interventions58 was used as a template.
The following electronic databases were searched from the end of 2000 up to and including August 2013:
-
MEDLINE
-
EMBASE with its higher yield of non-English reports
-
Cochrane Central Register of Controlled Trials
-
The Cochrane Skin Group Specialised Trials Register
-
Latin American and Caribbean Health Sciences (LILACS) database
-
Allied and Complementary Medicine Database (AMED)
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL).
Disease terms for atopic eczema [as a text word and medical subject heading (MeSH) term] are shown in Appendix 1. Possible trials were identified from each of the six databases as follows:
-
MEDLINE (OvidSP) – the Cochrane Collaboration ‘highly sensitive electronic search string’ for RCTs was used (see Appendix 1). Publications from 2000 to 31 August 2013 were searched and yielded > 4000 references using the disease search terms in Appendix 1.
-
EMBASE (OvidSP) – because of the different format of this database an alternative search strategy was employed (see Appendix 1). Publications from 2000 to 31 August 2013 were searched and yielded > 2000 references using the same eczema terms as for MEDLINE.
-
Cochrane Central Register of Controlled Trials – The Cochrane Library 2013 was searched for controlled trials within the Cochrane Central Register of Controlled Trials section by exploding the disease-specific search terms separated by the Boolean ‘AND’ with the advanced search option. These included clinical controlled trials (quasi randomisation) and RCTs (randomisation).
-
Cochrane Skin Group Specialised Register – this was searched with the disease-specific terms and the kind help of the Cochrane Skin Group Trials Search Coordinator.
-
www.controlled-trials.com was searched for completed and ongoing RCTs using the terms ‘atopic dermatitis’, ‘atopic eczema’ and ‘eczema’. This meta-register of trials contains the entries from the following databases:
-
ISRCTN register (international) – copy of the ISRCTN register
-
Action Medical Research (UK) – subset of the ISRCTN register
-
Medical Research Council (UK) – subset of the ISRCTN register
-
US National Institutes of Health ClinicalTrials.gov register (international) – subset of randomised trial records.
-
Handsearching
As there are > 200 specialist dermatology journals and none specific to atopic eczema, separate handsearching was not carried out for this report. Some trials published in journals not listed in the bibliographic databases searched, or published within the body of a letter to the editor, might therefore have been missed. However, specialist dermatology journals are handsearched by the Cochrane Skin Group and these are included in the Cochrane Skin Group Specialised Register of trials, which was searched. This includes the results of handsearching the following dermatology journals over varying dates: Cutis, Acta Dermato-Venereologica Supplementum, Archives of Dermatology, British Journal of Dermatology, Clinical and Experimental Dermatology, International Journal of Dermatology, Journal of Investigative Dermatology and Journal of the American Academy of Dermatology.
In addition, conference proceedings of previous symposia such as the Atopic Dermatitis Symposia and all meeting abstracts for the annual meetings of the Society for Investigative Dermatology, European Academy of Dermatology and British Association of Dermatologists were handsearched by one of the authors and the results made available to the Cochrane Skin Group Specialised Register. Furthermore, one of the authors has been prospectively handsearching five dermatology journals (Clinical Experimental Dermatology, British Journal of Dermatology, Journal of the American Academy of Dermatology, Journal of Investigative Dermatology and Paediatric Dermatology) since January 1998 for atopic eczema treatment trials.
Other trial sources
In addition to checking citations in retrieved RCTs, additional trials were sought by personal contact with atopic eczema researchers and by writing to 15 pharmaceutical companies with a product (commercially available or in development) related to atopic eczema treatment.
Filtering
The initial search yielded 7168 references. A manual filtering process was conducted to assess whether each reference fitted the preliminary labels of ‘trial’ and ‘eczema’.
Not all references had abstracts and therefore studies were included if their title related to an eczema trial, to avoid premature exclusion. In cases of uncertainty, the full paper was requested and scrutinised by one of the authors and discussed with a second senior author as necessary. Papers excluded were categorised by reason for exclusion by one reviewer; this was checked by a second reviewer in cases of uncertainty. When a trial fitted the inclusion criteria but was found to be published only as an abstract, the trial was excluded from the review. All papers were catalogued on a specialised referencing database (EndNote X7; Thomson Reuters, CA, USA).
Non-English-language studies
Non-English-language studies were screened by international colleagues (see Acknowledgements) and full data extraction performed for those meeting the inclusion criteria.
Identifying treatments with no randomised controlled trials
A data-driven review can identify only treatments for which some evidence exists and therefore there may be other treatments that are currently used throughout the world for atopic eczema but which are not necessarily supported by RCTs.
Data assessment
Papers meeting the inclusion criteria were subject to data extraction with a view to pooling data or producing a narrative summary. Data extraction forms were developed by one of the authors. Data extraction was carried out independently by two authors for all included trials. One author checked the two sets of data extraction for discrepancies and then consulted the original papers to resolve these with the second data extractor or a third arbitrator when unclear.
Study quality
The methodological quality of each study was assessed using the Cochrane Collaboration risk-of-bias assessment tool,58 with potential sources of bias evaluated:
-
method of generating the randomisation sequence
-
concealment of the allocation sequence
-
blinding of participants, study personnel and outcome assessments
-
other issues including incomplete outcome data and selective outcome reporting and the extent to which the primary analysis included all participants initially randomised (i.e. an intention-to-treat analysis).
The method of generation of the randomisation sequence, allocation concealment and blinding have been consistently shown to predict bias in effect estimates67 and so these have been tabulated throughout the review to assist readers in making comparisons between studies. When assessing the risk of bias for blinding, a trial was assessed as low risk if the primary outcome assessor was reported as blinded even if other parties, such as the participants, were not. When the trials are summarised, standard statements about the collective risk of bias have been used (Table 3). This allows readers to see which aspects of the study reporting were deficient for each treatment. Because of the sheer size of this scoping review, the report authors were not blinded to the identity of the RCT authors when assessing quality or carrying out data extraction.
| Collective risk-of-bias descriptions for summary statements | Basis for description |
|---|---|
| Overall low risk of bias | Method of generating the randomisation sequence, concealment of the allocation sequence and blinding were assessed as low risk for all of the trials summarised |
| Overall unclear risk of bias | Method of generating the randomisation sequence, concealment of the allocation sequence and blinding were assessed as unclear risk for all of the trials summarised |
| Overall high risk of bias | Method of generating the randomisation sequence, concealment of the allocation sequence and blinding were assessed as high risk for all of the trials summarised |
| Mostly low risk of bias | A clear majority of the method of generating the randomisation sequence, concealment of the allocation sequence and blinding were assessed as low risk of bias |
| Mostly unclear risk of bias | A clear majority of the method of generating the randomisation sequence, concealment of the allocation sequence and blinding were assessed as unclear risk of bias |
| Mostly high risk of bias | A clear majority of the method of generating the randomisation sequence, concealment of the allocation sequence and blinding were assessed as high risk of bias |
| A mixed risk of bias | The assessments were a fairly even distribution of risk of bias for method of generating the randomisation sequence and concealment of the allocation sequence |
Quantitative data synthesis
Pooling of the data did not make sense clinically for any of the interventions and so no pooling of quantitative data was undertaken.
Methods of presenting results
Summarising the evidence for treatments and harms from 287 RCTs covering at least 92 different interventions in a way that would be helpful to health-care commissioners, providers and physicians is challenging. There is always a conflict in such a situation of providing too much information, resulting in loss of the general picture, or of omitting important details in some specific areas.
Readers are encouraged to read the original studies when doubt occurs over the reported data or the report authors’ conclusions. One of the report authors has led the development of a database of eczema RCTs [the Global Resource for EczemA Trials (GREAT) database68] in which information about and links to the included and excluded studies in this review can be found.
In many of the studies it would have been impractical to document every outcome and therefore the report authors have highlighted:
-
patient-rated global improvement or itch or sleep loss
-
global severity score based on several skin signs, or individual skin sign scores.
In some studies evaluating multiple outcomes, statistically significant post hoc tests were highlighted in the paper’s conclusions or abstract. The report authors have mitigated against this bias by stating whether the results were from a post hoc test.
If pre-existing systematic reviews were identified for any of the interventions, these were highlighted at the beginning of the relevant sections.
Separating trial data from authors’ opinions
The report authors have been careful to make a clear distinction between the facts abstracted from individual studies and the respective authors’ interpretations of what those results or lack of results mean. Thus, actual data on efficacy and possible harms have been clearly separated from the authors’ ‘overall implications for research and practice’ sections. In the comments sections of the risk-of-bias tables, the report authors have also commented on possible sources of bias.
Chapter 3 Results
Included studies
There were 287 RCTs of eczema treatments included in this update review (Table 4) (see Chapters 4–13). The results from the previous review of RCTs of eczema treatment55 were also included in this review for every treatment for which new RCTs were reported. When no new RCTs were included from the updated search, the treatment was not discussed and the reader is directed to the previous review of eczema treatments. 55
| Treatment | Page number |
|---|---|
| Topical corticosteroids and topical immunomodulators | 25 |
| Topical corticosteroids compared with placebo | 26 |
| Topical corticosteroids compared with active treatments | 28 |
| Tacrolimus compared with placebo | 30 |
| Tacrolimus compared with active treatments | 32 |
| Pimecrolimus compared with placebo | 37 |
| Pimecrolimus compared with active treatments | 39 |
| Tacrolimus compared with pimecrolimus | 42 |
| Topical calcineurin inhibitors used concurrently with topical corticosteroids | 44 |
| Topical corticosteroids with occlusive therapy | 45 |
| Emollients and other topical treatments | 51 |
| Emollients | 51 |
| Bath additives | 58 |
| Furfuryl palmitate | 59 |
| Pill mask | 60 |
| Black seed oil | 61 |
| Rosmarinic acid | 62 |
| Hippophae rhamnoides | 62 |
| Shale oil | 63 |
| Vitreoscilla filiformis | 64 |
| Miltefosine | 65 |
| Opiate receptor antagonist | 66 |
| Topical vitamin B12 | 67 |
| WBI-1001 cream (Welichem Biotech Inc.) | 68 |
| Carbohydrate-derived fulvic acid | 70 |
| Protease inhibitor SRD441 (Serentis Ltd) | 71 |
| Raffinose | 72 |
| Atopiclair™ (Graceway Pharmaceuticals) | 73 |
| Farnesol and xylitol | 75 |
| Levomenol and heparin | 76 |
| Bacterial antigens | 77 |
| Camomile extract | 78 |
| Camellia oil | 79 |
| Cipamphylline cream | 80 |
| Lipoxin A4 | 81 |
| N-acetyl-L-hydroxyproline | 82 |
| Nalmefene hydrochloride monohydrate | 83 |
| Licochalcone A | 84 |
| AR-GG27 | 85 |
| Antimicrobials including antibiotics, antiseptics and antifungals | 89 |
| Antibiotics | 90 |
| Fusidic acid | 90 |
| Mupirocin | 92 |
| Tetracycline | 94 |
| Clarithromycin | 95 |
| Antiseptics | 95 |
| Triclosan | 95 |
| Bleach baths | 96 |
| Antifungals | 98 |
| Ketoconazole | 98 |
| Miconazole | 99 |
| Itraconazole | 100 |
| Antihistamines and mast cell stabilisers | 105 |
| Cetirizine | 106 |
| Loratidine | 107 |
| Fexofenadine | 107 |
| Ketotifen and epinastine | 108 |
| Chlorpheniramine | 110 |
| Topical doxepin | 111 |
| Topical sodium chromoglycate | 112 |
| Dietary interventions | 115 |
| Probiotics, prebiotics and synbiotics | 116, 122, 123 |
| Borage oil | 125 |
| Evening primrose oil | 126 |
| Fish oil/soybean oil | 126 |
| Docosahexaenoic acid | 127 |
| Hempseed oil | 128 |
| Vitamin D | 129 |
| Hypoallergenic formula | 132 |
| Non-pharmacological interventions | 137 |
| Specialised clothing | 137 |
| Education | 142 |
| Support groups | 149 |
| Ion-exchange water softener | 152 |
| Staying in a different climate | 153 |
| House dust mite desensitisation | 154 |
| Extra visits to the doctor | 155 |
| Vaccines | 156 |
| Phototherapy | 161 |
| Ultraviolet B | 161 |
| Ultraviolet A | 162 |
| Ultraviolet A vs. ultraviolet B | 164 |
| Phototherapy combined with other treatments | 166 |
| Full-spectrum light therapy | 168 |
| Excimer laser | 168 |
| Systemic immunomodulatory agents | 171 |
| Azathioprine | 171 |
| Ciclosporin | 173 |
| Methotrexate | 176 |
| Oral prednisolone | 177 |
| Mycophenolate mofetil | 178 |
| Montelukast | 179 |
| Systemic immunotherapy | 182 |
| Mepolizumab | 184 |
| Omalizumab | 185 |
| Intravenous immunoglobulin | 186 |
| Oral pimecrolimus | 188 |
| Complementary therapies | 193 |
| St John’s wort | 193 |
| Acupuncture | 195 |
| Hypnotherapy | 197 |
| Chinese herbal medicine | 199 |
| Homeopathy | 201 |
| Other herbal medicines | 202 |
| Japanese traditional medicine | 203 |
| Hwangryunhaedoktang | 204 |
| Balneotherapy | 204 |
| Progressive muscle relaxation | 205 |
| Other interventions not covered elsewhere | 209 |
| Autologous blood therapy | 209 |
| Tandospirone citrate | 210 |
| Oral naltrexone | 211 |
Excluded studies
Eczema prevention trials have not been included in this review as the body of evidence is substantial. Prevention of eczema has been covered in 40 systematic reviews69 and one overview of the reviews70 (see Chapter 1), which is itself a component of the eczema prevention work programme of this grant.
The details of the excluded studies are shown in Figure 2. Further details of eczema trials that were excluded in the final stages as they were reported only as abstracts are provided in Appendix 2. A more comprehensive archive of the excluded papers can be found in the GREAT database. 71
FIGURE 2.
Flow chart of the filtering process. AD, atopic dermatitis; AE, adverse event. a, Duplicate references that were removed before being filtered are included in this total.

Chapter 4 Topical corticosteroids and topical immunomodulators
Background
Topical corticosteroids have been one of the cornerstones of the treatment of atopic eczema for > 50 years.
Hydrocortisone was first used as a skin treatment in 1952, when it was found to improve various dermatoses when applied topically. 72 Since then, another 30 or so compounds have been developed, each in different formulations (e.g. creams, oily creams or ointments) and in combination with other ingredients such as antibiotics. Topical corticosteroids vary in strength (as measured by their ability to constrict blood vessels rather than their clinical anti-inflammatory or skin thinning effect) from very mild [e.g. hydrocortisone acetate (HC45, Reckitt Benckiser Healthcare)] to very strong fluorinated products [e.g. clobetasol propionate (Dermovate®, GlaxoSmithKline)]. Systemic adverse effects are rare and include suppression of the hypothalamic–pituitary–adrenal axis, Cushing syndrome and osteoporosis. Local adverse effects include spread of untreated fungal infection, irreversible striae (stretch marks), prominent fine blood vessels, contact dermatitis, perioral dermatitis, worsening of acne and mild loss of pigmentation. The adverse effect that undoubtedly causes the most concern is that of skin thinning. 73
More recently, topical calcineurin inhibitors have been developed and are now being used to treat some dermatoses, including eczema, as an alternative to or in combination with topical corticosteroid treatment. Topical tacrolimus (Protopic®, Astellas Pharma) was first available in 2001 in the USA to treat moderate to severe eczema. Pimecrolimus (Elidel®, Meda) was licensed for the treatment of mild to moderate eczema in the USA in 2002. Both tacrolimus and pimecrolimus selectively inhibit the activation of T cells. 74,75 T cells play a key role in the characteristic inflammation of the skin in eczema. Pimecrolimus has also been trialled as an oral treatment for eczema, which is discussed later in this review (see Chapter 11). Evidence to date on the potential harms of topical calcineurin inhibitors has not shown serious adverse effects of skin thinning or stretch marks as seen with prolonged or inappropriate use of topical corticosteroids. Evidence that tacrolimus and pimecrolimus may increase the risk of some cancers prompted the US Food and Drug Administration to issue a ‘black box’ warning. 76 Long-term trials to assess the potential harms of these treatments have now been completed77 and another trial is still ongoing. 78
Scope of this chapter
This chapter covers the following treatments:
-
topical corticosteroids:
-
compared with placebo
-
compared with active treatments (except topical immunomodulators)
-
once-daily compared with twice-daily applications
-
-
topical immunomodulators:
-
tacrolimus
-
compared with placebo
-
compared with other active treatments
-
-
pimecrolimus
-
compared with placebo
-
compared with other active treatments
-
-
tacrolimus compared with pimecrolimus
-
-
topical calcineurin inhibitors combined with topical corticosteroid
-
topical corticosteroids with occlusive therapy.
Topical corticosteroids
Topical corticosteroids compared with placebo
Studies
Thirteen trials were published before 200055 (see Appendix 3).
Fourteen new trials97–110 have been reported since 2000. Fluticasone was compared against placebo in four of the trials98,106–108 and hydrocortisone butyrate was compared against placebo in two of the trials. 101,103 Placebo-controlled trials were reported for fluocinonide,104,110 fluocinolone acetonide,97 triamcinolone acetonide,100 desonide,99 methylprednisolone aceponate,105 clobetasol propionate109 and betamethasone 17-valerate in combination with fusidic acid. 102
Assessment of risk of bias for the new studies
There was enough detail about the method of randomisation to allow assessment of the risk of bias in only six97,102,103,107,108,110 out of 14 trials and all were assessed as being at low risk. There was enough detail about the method used to generate the allocation sequence to allow assessment of the risk of bias in only one97 out of the 14 trials and this was assessed as low risk. There was enough detail about blinding to allow assessment of the risk of bias in five97,103,105,106,109 of the 14 trials and all five were assessed as being at low risk.
Benefits
Reactive treatment regimens
Fairly high proportions of participants using topical corticosteroids compared with placebo successfully responded to treatment as assessed by the Investigator’s Global Assessment (IGA), ranging from 39% to 75% for topical corticosteroid treatment compared with 11–36.4% for placebo treatment. 97–99,101–104,109,110 Definitions of ‘success’ varied, but the most common was an IGA score of 0 or 1 at the end of treatment, with two trials also adding that there had to be a ≥ 2 point improvement from baseline as well. 99,101,103 The participant global assessment and the pruritus assessments were similar to the IGA for the reactive treatment regimens.
Proactive treatment regimens
There were fairly large differences in the proportion of participants who did not have a flare of their eczema during 16 weeks of maintenance treatment (‘keep control’ treatment for 2 consecutive days a week), which ranged from 27.5% to 87.1% for topical corticosteroid treatment and from 19.4% to 65.8% for placebo. 105–107
Harms
Reactive treatment regimens
There was one serious adverse event reported, which was not thought to be related to study treatment,99 and four withdrawals from treatment due to adverse events,98,99 some of which were reported to be probably or possibly related to treatment. 99 One mention of skin changes was reported by a participant on placebo. 100 Mild hypopigmentation was reported for two participants being treated with moderate potency (class IV, US system) fluocinolone acetonide. 97
Proactive treatment regimens
Adverse events were reported in 14–45% of the treatment groups. Two trials105,106 reported that skin atrophy was not seen; however, one trial108 reported that two participants showed skin changes often seen before atrophy (telangiectasia). Overall, a small number of adverse events assessed as being related to the trial medication were reported. One trial107 reported four serious adverse events of erysipelas (skin infection) and two cases of exacerbation of eczema. One trial106 reported two cases of possible adrenal suppression.
Overall implications for research and practice
The evidence base for topical corticosteroids used to treat eczema is mature and there is plenty of good evidence that topical corticosteroids are beneficial and safe if used correctly. Therefore, there is now no reasonable clinical or ethical justification for comparing a new topical corticosteroid against placebo, as this will not give any new clinically relevant information. Although the number of trials of topical corticosteroids compared with placebo has decreased considerably in the last decade, the trials that have been reported are mostly still plagued by the same lack of methodological detail, with non-existent or inappropriately short follow-up assessment periods. Most of the money spent on this type of trial in the last decade amounts to research wastage. 111 Trials that compare a new topical corticosteroid against other active treatment options and seek to answer the question, ‘Which is the best topical corticosteroid and treatment regimen for my patient?’ are still desperately needed.
Reactive treatment regimens
There is strong evidence that continuous use of eczema treatments will result in a large beneficial effect compared with placebo. However, without follow-up periods after treatment cessation, trials fail to provide information regarding the potential ability of an intervention to provide, or continue to provide, beneficial effects. This is an important question to address in the future.
Proactive treatment regimens
There is strong evidence that, in the short term, ‘getting control’ using 2–3 weeks of continuous treatment and then ‘keeping control’ with treatment on 2 consecutive or evenly spaced days a week has a large beneficial effect.
Topical corticosteroids compared with active treatments (except topical immunomodulators)
Studies
There were 40 trials of topical corticosteroids compared with other topical corticosteroids and four trials of topical corticosteroids compared with other active agents reported before 200055 (see Appendix 3).
A total of 12 trials112–122 were reported from 2000 onwards, with two trials reported together in one report. 113 Ten of these trials compared novel topical corticosteroids with standard topical corticosteroids and one trial118 compared a topical corticosteroid with and without the addition of a penetration-enhancing drug. One trial122 compared continuous treatment with treatment 4 days a week with clobetasone butyrate ointment [0.05% weight by weight (w/w)]. This trial report was very short and there were very few details provided about the trial. Nine of these trials112–117,122 compared treatments exclusively in children and three trials118–120 did not report the age of the participants. Six of the trials113,116–118,121 had > 100 participants and six112,114,115,119,120,122 had ≤ 50 participants. The treatment regimens varied, with one trial providing 7 days of treatment as required over an 18-week period116 one trial providing either twice daily treatment for 8 weeks or twice daily treatment for four consecutive days per week for 8 weeks,122 and the other trials providing continuous treatment for periods from 2 weeks to 42 days. 112–115,117–120
An additional four trials123–126 were reported that compared a new active treatment against a topical corticosteroid as the ‘standard treatment’ comparator. These are discussed in the chapters in which the active treatments are discussed. This section does not include trials that compared topical corticosteroids with topical immunomodulators. These trials are discussed separately in the topical immunomodulators section later in this chapter.
An additional trial by Sillevis Schmitt and colleagues122 was reported that compared two different treatment regimens of clobetasone butyrate ointment (0.05%): twice a day every day for 8 weeks and twice a day for 4 days a week for 8 weeks. Forty children with eczema were randomised. The trial report was very short and very few details were provided about the trial.
Assessment of risk of bias for the new studies
Table 5 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Cato 2001118 | Low risk | Unclear risk | Unclear risk | |
| Kim 2013121 | Unclear risk | Unclear risk | Unclear risk | |
| Kirkup 2003113 | Unclear risk | Unclear risk | Unclear risk | |
| Lebrun-Vignes 2000114 | Unclear risk | Unclear risk | Unclear risk | |
| Prado de Oliveira 2002112 | Unclear risk | Unclear risk | Unclear risk | |
| Ruzicka 2012119 | Unclear risk | Unclear risk | Unclear risk | |
| Schlessinger 2006117 | Unclear risk | Unclear risk | High risk | |
| Sillevis Smitt 2000122 | Unclear risk | Unclear risk | Unclear risk | |
| Thomas 2002116 | Low risk | Unclear risk | Low risk | |
| Trookman 2011120 | Unclear risk | Low risk | Unclear risk | Intention-to-treat method not stated |
| Wong 2008115 | Unclear risk | Unclear risk | Unclear risk |
Benefits
There was no evidence of any significant differences between the topical corticosteroids being compared except in two identically designed trials113 assessing fluticasone propionate (0.05%) compared with hydrocortisone (1%) or hydrocortisone butyrate (0.1%). The participants were experiencing an eczema flare on entry to the trial. The study treatment was used daily for 2–4 weeks (to bring the flare under control) and then intermittently to maintain control (for up to 12 weeks). Fluticasone propionate showed a statistically significant benefit over hydrocortisone and hydrocortisone butyrate. Compared with hydrocortisone butyrate, fluticasone propionate resulted in a greater percentage of participants being ‘much improved’ after the acute treatment phase; however, for both treatments this was high (84% vs. 98%, respectively).
The trial by Thomas and colleagues116 assessed the differences between short bursts of potent topical corticosteroids and longer bursts of milder topical corticosteroids. The study evaluated the number of relapses, number of scratch-free days, severity, number of undisturbed nights and quality of life but no statistically significant differences were found.
The trial by Cato and colleagues,118 involving the manufacturers of laurocapram, found a greater reduction in induration, pruritus and erythema as well as global disease improvement when using triamcinolone acetonide combined with laurocapram than when using triamcinolone acetonide alone or vehicle.
A within-person trial by Wong and colleagues115 compared hydrocortisone (1%) combined with the antifungal agent miconazole with hydrocortisone (1%) alone in children with active eczema affecting the knees and elbows. Two dermatologists rated the relief of symptoms from photographs in terms of which treatment gave a better response or whether there was no difference. The inter-rater variability between the two outcome assessors’ ratings was high and the scores did not show a significant difference between the treatments. The participants rated their response to the treatments, with 10 stating that hydrocortisone in combination with miconazole gave a better response, 15 reporting a better response with hydrocortisone only and four noticing no difference. No significant difference was found in the amount of topical corticosteroids used.
The trial by Sillevis Smitt and colleagues122 found a significant improvement in eczema severity for participants using clobetasone butyrate ointment (0.05%) for just 4 days a week in relation to participants using the treatment continuously. The difference between the improvement in SCORAD scores in each group was 6.7 [standard deviation (SD) 3.03; p = 0.03] in favour of the 4 days a week treatment regimen.
The trial by Ruzicka and colleagues119 compared a new mometasone furoate oil-in-water cream containing 33% of water with the mometasone furoate commercially available preparation containing 5% of water in a 2-week intra-individual study including 20 adults with eczema. They found no significant difference in disease severity between the groups, assessed using a non-validated score. The new formulation seemed to be preferred by participants.
The trial by Kim and colleagues121 compared mometasone furoate in a new multi-lamellar emulsion containing pseudoceramide (aimed at enhancing skin penetration) against methylprednisolone aceponate in a 2-week intra-individual study including 175 children with moderate to severe eczema. The disease severity improvement ratio assessed by Physician Global Assessment (PGA) was 82.62 SD ± 21.62% in the mometasone furoate in multi-lamellar emulsion group and 68.32 SD ± 24.05% in the methylprednisolone aceponate group (p ≤ 0.0001). Pruritus showed a more significant improvement in the mometasone furoate group than in the methylprednisolone group (p ≤ 0.0001). Sixteen participants were excluded from the analysis.
An industry-funded trial by Trookman and Rizer120 compared desonide hydrogel with desonide ointment in a 4-week single-blind study including 46 participants. The trial authors did not compare the changes in severity between the two groups making it impossible to assess the results of the trial. Patient rankings of absorbability and (lack of) greasiness were significantly higher among patients receiving desonide hydrogel than among those receiving desonide ointment.
Harms
No serious adverse events linked to the study treatments were reported. The levels and types of adverse events possibly or definitely related to treatment varied considerably in these trials. The harms that are commonly associated with topical corticosteroid use, such as skin thinning, were not reported, although none of the trials looked at consistent long-term use.
Five trials114,115,119,120,122 reported that there were no adverse events during the trial.
Once-daily compared with twice-daily application
There were three trials published before 200055 (see Appendix 3). No new studies have been published since 2000.
Overall implications for research and practice
The publication of new trials comparing topical corticosteroids has dramatically slowed in the last 10 years, possibly because of emerging competitors such as the topical calcineurin inhibitors. The few trials reported, mostly with manufacturer involvement, still do not seem to directly address the knowledge gaps about the best choices of topical corticosteroids. There is a small amount of evidence that combining a topical corticosteroid with a penetration-enhancing chemical shows an increased benefit,118 but the trial was not clear enough about the methodology used to assess the risk of bias and so this must be treated with caution until stronger, long-term treatment evidence becomes available. There is some very weak evidence that pulsed treatment is more effective than continuous treatment. 122
As all of the trials comparing an active treatment that is not another topical corticosteroid or topical immunomodulator use the topical corticosteroid as the ‘standard treatment comparator’, they have been discussed in the chapters of this report that cover the treatments they have been compared against. Three of the trials123,124,126 are discussed in Chapter 5; the other is discussed in Chapter 10. 125
Topical immunomodulators
There were four trials of topical immunomodulatory treatment published before 200055 (see Appendix 3).
Tacrolimus compared with placebo
Studies
Twelve new trials,127–137 two of which were published together in one report,128 of tacrolimus compared with placebo have been reported since 2000. The treatment regimens can be divided into reactive regimes (treatment started when signs and symptoms appear or worsen) and proactive regimes (treatment used intermittently to prevent the eczema recurring).
Reactive treatment regimens
Eight trials127–132,136 of tacrolimus compared with placebo were reported. Participants in all eight trials were instructed to apply the treatment twice daily, with treatment durations ranging from 2 to 12 weeks. Treatment was not continued if the eczema went into ‘remission’ in four127–129 of the eight trials. Five127–129,136 of the trials were industry sponsored and three130–132 did not report funding sources. The number of participants in the eight trials ranged from 14 to > 600, with five127–129,131 of the trials having > 200 participants.
Proactive treatment regimens
Tacrolimus was compared against vehicle in four trials. 133–135,137 We did not find any comparisons of proactive treatment with tacrolimus against proactive use of topical corticosteroids. Three of the included trials were multicentre with sample sizes of around 200–250 participants. Of these three trials, one133 was conducted in the USA and two134,135 were conducted in Europe. One133 of these trials included children and adults and gave the treatments three times a week for 40 weeks whereas two trials, one in adults135 and one in children,134 provided the treatments twice a week for 1 year. All participants in the three trials were allowed to use non-medicated emollients. A smaller open-label trial137 included 70 children and adults and compared the use of either tacrolimus or emollient treatment for 1 month (treatment regimen not reported). After induction of remission using tacrolimus, the participants used their usual emollient twice a day and their usual topical corticosteroid (up to 10 g per week) for the maintenance phase.
Assessment of the risk of bias for the new studies
Reactive treatment regimens
Sufficient detail about the generation of the allocation sequence was available in two129,130 out of eight trials to allow an assessment of the risk of bias. Both of these trials were assessed as having a low risk of bias. Sufficient detail about allocation concealment was not given in any of the trials. Sufficient detail about blinding was available in two132,136 out of eight trials to allow an assessment of the risk of bias. One of these trials136 was assessed as being at low risk of bias and the other trial132 was assessed as being at high risk of bias.
Benefits
Reactive treatment regimens
For continuous treatment of eczema with tacrolimus (until clearance or for a set period of time) there is evidence of a large reduction of pruritus after 2–4 days when using the treatment twice a day. The reduced levels of pruritus persisted for the length of treatment. There was also evidence from three of the trials127–129 of a large reduction in severity compared with placebo, with the proportion of participants with an IGA of 0 or 1 at the end of treatment ranging from 36.8% to 49.7% for tacrolimus compared with 6.9–29% for placebo. The improvements in participant-assessed severity were in line with the improvement in pruritus severity.
Proactive treatment regimens
The data on participants’ dermatology-related quality of life were conflicting in the two trials134,135 that reported this outcome. One trial134 reported that the treatment groups were comparable for Infant Dermatology Life Quality Index (IDLQI) and Children’s Dermatology Life Quality Index (CDLQI) scores at the end of the trial and the other trial135 reported a reduction from 9.3 to 3.6 for the tacrolimus group and from 9.3 to 6.8 in the placebo group, with lower scores equating to a better quality of life. The difference in these scores was not compared statistically. Two of the trials reported that 50% of the participants on tacrolimus compared with 30% on placebo134 and 56.9% of the participants on tacrolimus compared with 29.6% on placebo135 experienced no flares during the year of treatment. Another trial133 reported that there were significantly more flare-free treatment days using tacrolimus (177 vs. 134; p = 0.003) and a significantly longer time to first flare [169 days, 95% confidence interval (CI) 113 to 225 days for tacrolimus treatment vs. 43 days, 95% CI 31 to 113 days for vehicle). One trial137 specifically assessed recurrence of pruritus by visual analogue scale (VAS). In this trial, the cumulative itch recurrence (defined as an increase in VAS itch score of > 20 points) was 23.8% (95% CI 10.7% to 52.9%) in the tacrolimus group and 100% in the emollient group after 1 month.
Harms
Reactive treatment regimens
The most common adverse events were application site stinging or burning and skin irritation. In some trials this was more common in the placebo group and for others it was more common in the tacrolimus group. Serious adverse events were not mentioned. Two130,136 of the trials did not report any information on adverse events.
Proactive treatment regimens
Pruritus and impetigo were more common in the treatment group than in the placebo group in one134 of the trials. There was a low level of serious adverse events; of these, two were reported as being related to tacrolimus (application site infection and eczema herpeticum). In another trial,135 application site pruritus was fairly common and evenly spread between the tacrolimus treatment group and the placebo treatment group.
Overall implications for research and practice
As a collection of trials that are likely to have been conducted in a methodologically robust way, it is a shame that the trial reports did not provide enough detail to make a true assessment of the risk of bias. A lack of longer-term follow-up assessment after the treatment was stopped also restricts the usefulness of this evidence.
Reactive treatment regimens
There is strong evidence for short-term treatment with tacrolimus compared with placebo: continuous treatment with tacrolimus twice a day provides a large beneficial effect by reducing the pruritus and severity of eczema relatively quickly. What is not clear is how long this beneficial effect continues after stopping treatment, as the trials did not follow up the participants for more than a few weeks once treatment had stopped. None of the trials used treatment once daily, which has already been shown to be as effective as twice-daily treatment for corticosteroids. This is an important question for both people with eczema and health-care commissioners.
Proactive treatment regimens
There is strong evidence for a short-term beneficial effect from ‘getting control’ and ‘keeping control’ with tacrolimus compared with placebo.
Tacrolimus compared with active treatments
Studies
Tacrolimus compared with hydrocortisone
Six new trials were reported,138–142 one of which included a single-centre extended trial that was published separately. 143 Four138–140 of the six trials were particularly large, multicentre, multinational, manufacturer-sponsored trials that compared tacrolimus, often at both the lower 0.03% and stronger 0.1% concentrations, against various different preparations of hydrocortisone, particularly 1% hydrocortisone acetate (weak potency) or 0.1% hydrocortisone butyrate (moderate potency). Two138,139 of the six trials involved only children, with treatments being applied every day for 3 weeks; participants were not followed up for > 2 weeks after the end of treatment. One140 of the four trials in adults was conducted at a single centre and gave treatment for 7 days to clear the eczema and then participants used the treatments as needed to treat flares for 6 months, with follow-up for 1 year. Another trial in adults was very small and had mainly mechanistic goals. 142 The other two trials, by Reitamo and colleagues138 and Caproni and colleagues,141 administered treatment twice a day for 3 weeks; Reitamo and colleagues138 also followed up the participants 2 weeks after the end of treatment.
Tacrolimus compared with other topical corticosteroids
The first ‘acute’ phase of a trial by Breneman and colleagues133 compared twice-daily application of the topical corticosteroid alclometasone dipropionate ointment (0.05%) with twice-daily tacrolimus ointment (0.03%) for 4 days.
Two small crossover trials144,145 compared a moderate-potency topical corticosteroid against tacrolimus (0.1%). The trial by Gradman and Wolthers145 comparing tacrolimus (0.1%) against mometasone furoate (0.1%) in 20 children aged 5–12 years was primarily concerned with measuring the effect of the treatments on short-term growth. The trial by Nivenius and colleagues144 compared tacrolimus (0.1%) against clobetasol butyrate for eyelid eczema. Twenty-five adult participants (age range 18–70 years) with moderate eczema (specifically those with eyelid eczema and keratoconjunctivitis) applied the treatments twice daily for 3 weeks.
Four trials104,146–148 compared potent topical corticosteroids against tacrolimus (0.1%) to treat moderate to severe eczema. The trial by Bieber and colleagues148 compared a relatively new topical preparation of methylprednisolone aceponate against tacrolimus (0.1%) twice a day for a minimum of 2 weeks and a maximum of 3 weeks, with cleared areas treated for an additional 7 days. The 265 children randomised had moderate to severe eczema and were experiencing an acute flare on entry to the trial. The other two trials by Doss and colleagues,146,147 both industry funded, compared tacrolimus (0.1%) against fluticasone propionate (0.005%). The first trial146 involved 568 adults with moderate to severe facial eczema involving at least 10% of the skin surface area. Treatments were used twice daily for 3 weeks or until clearance. After this period, participants could stop treatment if the eczema had cleared, use the same treatment once a day or switch to the other treatment group (with twice-daily applications) and still be blinded. The second, non-inferiority trial147 compared second-line use of tacrolimus (0.1%) or fluticasone propionate in 479 children with moderate to severe eczema who had not had an adequate response to previous topical corticosteroid treatment. A within-person trial by Del Rosso and Conte104 compared once-daily fluocinonide cream (0.1%) against twice-daily tacrolimus ointment (0.1%) applied to the target sites for 4 weeks; in this trial it was reported that the investigator was blinded and only seven adult participants out of 30 with various dermatoses had moderate eczema.
One manufacturer-supported trial by Neumann and colleagues149 randomly assigned adults with moderately severe eczema to tacrolimus (0.1%) or standard treatment with topical corticosteroids and emollients. There was no standard treatment regimen and participants were observed using their own treatment patterns. The participants were followed up for 6–20 months.
A trial examining tacrolimus and fusidic acid compared with fluticasone propionate and fusidic acid by Hung and colleagues150 is discussed in Chapter 6.
Tacrolimus compared with other active comparators
The study by Pacor and colleagues151 compared 3 mg/kg of oral ciclosporin once a day with topical tacrolimus (0.1%) twice a day over 42 days using a ‘double dummy’ technique to achieve blinding. The 30 participants aged 13–45 years had moderate to severe eczema that had only partially resolved using topical corticosteroids. No other treatment for eczema was allowed during the study except 10 mg of cetirizine once or twice a day to help with itching.
Assessment of risk of bias for the new studies
Table 6 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Tacrolimus vs. hydrocortisone | ||||
| Antiga 2011142 | Low risk | Unclear risk | Unclear risk | |
| Caproni 2007141 | Unclear risk | Unclear risk | Unclear risk | No intention-to-treat analyses |
| Reitamo 2002138 (hydrocortisone butyrate) | Unclear risk | Unclear risk | Unclear risk | Known potential side effects may have unintentionally unblinded some stakeholders |
| Reitamo 2002138 (hydrocortisone acetate) | Unclear risk | Unclear risk | Unclear risk | Known potential side effects may have unintentionally unblinded some stakeholders |
| Reitamo 2004139 | Unclear risk | Unclear risk | Unclear risk | Known potential side effects may have unintentionally unblinded some stakeholders |
| Reitamo 2005140 (Mandelin 2010143) | Unclear risk | Unclear risk | Low risk | |
| Tacrolimus vs. other topical corticosteroids | ||||
| Bieber 2007148 | Unclear risk | Unclear risk | Unclear risk | |
| Breneman 2008133 (acute phase only) | Unclear risk | Unclear risk | Unclear risk | |
| Del Rosso 2007104 | Unclear risk | Unclear risk | Unclear risk | |
| Doss 2009146 | Unclear risk | Unclear risk | Low risk | |
| Doss 2010147 | Unclear risk | Unclear risk | Unclear risk | Unclear what numbers of participants were included in each analysis |
| Gradman 2007145 | Low risk | Unclear risk | Low risk | Results very difficult to interpret |
| Neumann 2008149 | Unclear risk | Unclear risk | Unclear risk | |
| Nivenius 2007144 | Unclear risk | Unclear risk | Unclear risk | Unclear whether five out of 25 participants were included in the analyses |
| Tacrolimus vs. other active treatments | ||||
| Pacor 2004151 | Unclear risk | Unclear risk | Low risk | |
Benefits
Tacrolimus compared with hydrocortisone
Six trials compared tacrolimus against hydrocortisone, two in children139,152 and four in adults. 138,140–142
Reitamo and colleagues138,139 compared mild-potency hydrocortisone acetate (1%) against tacrolimus in two trials in children with moderate to severe eczema. The difference in reduction of severity over 3 weeks of treatment, assessed using EASI, ranged from 55.2% to 76.7% for tacrolimus and from 36.0% to 47.6% for hydrocortisone acetate (1%).
The potent topical corticosteroid hydrocortisone butyrate (0.1%) was compared with tacrolimus in three138,140,142 out of four of the trials in adults. The participants in the two larger trials138,140 had moderate to severe eczema. These two trials reported conflicting results. One trial by Reitamo and colleagues138 reported that hydrocortisone butyrate gave a greater reduction in severity than tacrolimus (0.03%) and tacrolimus (0.1%) over 3 weeks of twice-daily continuous use, regardless of clearance. The other trial by Reitamo and colleagues140 reported a significant benefit in terms of reduction in severity for tacrolimus after 3 months of treatment (primary outcome) and also after 6 months of treatment ‘as required’ for flares, after initial continuous treatment for 7 days. An additional publication of the trial data from one centre in Finland,143 including follow-up of the participants for 1 year, also showed this superior benefit of tacrolimus at 6 months but not at 1 year. Two trials141,142 by the same team, which were mostly concerned with biochemical and safety outcomes, compared the mild-potency hydrocortisone (1%) preparation against tacrolimus in participants with a SCORAD score of > 15. These trials, which each analysed < 25 participants, had conflicting results. In one of the trials141 tacrolimus was found to be superior to hydrocortisone for post-treatment reduction in severity measured using SCORAD scores (p = 0.027). However, in the other trial,142 although the tacrolimus-treated group had a greater reduction in SCORAD scores, there were no significant differences reported between the groups. The time frame for ‘post treatment’ was not reported.
Tacrolimus compared with other topical corticosteroids
In the trial by Breneman and colleagues,133 4 days of treatment with the low-potency topical corticosteroid alclometasone dipropionate (0.05%) gave a statistically significant reduction in EASI scores from baseline compared with treatment with tacrolimus (0.03%) (p = 0.03), a significant reduction in itch score (alclometasone dipropionate group from 5.9 to 3.2, tacrolimus group from 6.2 to 4.2; p = 0.0009) and a significant reduction in percentage body surface area affected (p = 0.02). There was no significant difference in the proportion of participants achieving an IGA of ‘clear’ or ‘almost clear’. Few participants in the whole population achieved this after the 4 days of treatment. None of these were prespecified outcome end points for the trial.
For moderately potent topical corticosteroids, Gradman and Wolthers145 found that both treatments cleared the eczema completely; however, the trial population’s low baseline eczema severity should be taken into account when considering the impact of this treatment. No between-treatment comparisons seem to have been made. This trial focused on the growth of the children, with growth impairment being a previously noted adverse side effect of treatment with topical corticosteroids, and did not find a significant difference in growth per week between twice-daily tacrolimus (0.1%) treatment and mometasone furoate (0.1%) treatment in mild to moderate eczema. The trial by Nivenius and colleagues144 reported no significant difference between twice-daily clobetasone butyrate (0.05%) and tacrolimus (0.1%) treatment for the reduction in eczema score or blepharitis scores over a 3-week period. The combined eczema and blepharitis score just reached statistical significance, with a difference between the treatments of –2.39 (95% CI –4.79 to 0.00; p = 0.05).
The study by Bieber and colleagues148 reports that the primary outcome of a static IGA taken at the end of treatment showed no statistically significant difference between the potent topical corticosteroid methylprednisolone aceponate and tacrolimus. The secondary outcome of reduction in severity using EASI scores at 7 and 14 days was significantly better for methylprednisolone aceponate; however, there was no significant difference between treatments in reduction of severity after 3 weeks. The reduction in itch was significantly better using methylprednisolone aceponate throughout the 3 weeks; this was also a secondary outcome.
In the study comparing the use of fluticasone propionate (0.005%) with tacrolimus to treat facial eczema146 there was a significant difference in the primary outcome of response rate (proportion of participants with a ≥ 60% reduction in modified EASI score from baseline to day 21) in favour of tacrolimus, with 93.3% compared with 87.8% of participants responding, respectively (p = 0.026). The participant- and also investigator-assessed facial global response to treatment were both statistically significantly in favour of tacrolimus treatment [64% vs. 55% of participants (p = 0.014) and 88% vs. 79% of participants (p = 0.043), respectively], whereas the participant-assessed pruritus scores were not significantly different.
Tacrolimus was reported to be non-inferior to fluticasone propionate (0.005%) for treating all areas of the body excluding the eyelids in children. 147 For the primary outcome of response rate (proportion of participants with a ≥ 60% reduction in modified EASI score from baseline to day 21) there was a difference in the full analysis set, in which withdrawals were counted as non-responders with a lower 95% confidence limit of –11.3%, which was within the predefined limit of 15%.
No significant difference was reported in the reduction of severity or body surface area affected in the trial by Neumann and colleagues149 comparing tacrolimus against standard topical corticosteroid treatment, which was prescribed according to individual participant severity. It was reported that treatment usage was slightly higher in the topical corticosteroids group during the trial.
Tacrolimus compared with other active comparators
In the trial by Pacor and colleagues151 both groups showed large reductions in the severity of eczema at the end of the trial (day 42) but there were no significant difference between the two treatments, with tacrolimus resulting in a reduction in mean SCORAD score from 69.0 points at baseline to 7.3 at day 42 and ciclosporin resulting in a reduction in mean SCORAD score from 73.7 to 8.6 at day 42. The severity of eczema was significantly reduced using tacrolimus compared with ciclosporin at days 14, 21, 28 and 35 but was not significantly reduced after run-in or 7 days. The trial did specify a particular time point for assessment of the severity of eczema outcome.
For participant-assessed itching, assessed using a 4-point ordinal scale, there was a statistically significant difference in favour of tacrolimus on days 7, 14 and 21. This difference never went above –0.65 (95% CI –0.91 to –0.40).
For participant-assessed sleep loss, also assessed using a 4-point ordinal scale, there was a statistically significant difference in favour of tacrolimus only on days 7 and 21, with the mean difference on day 7 being the largest at –0.4 (95% CI –0.64 to –0.15). For participant-assessed erythema, also assessed using a 4-point ordinal scale, there was a statistically significant difference in favour of tacrolimus on days 7, 14 and 21.
All of these outcomes showed a statistically significant difference in favour of tacrolimus when the ‘area under the curve’ was calculated over the whole 42 days.
Harms
Tacrolimus compared with topical corticosteroids
All of the trials reported higher rates of application site burning and often pruritus in the tacrolimus groups compared with the hydrocortisone groups. In the trials that reported the proportion of participants experiencing adverse events,146–148 levels were high for both treatments, with around 20% more in the tacrolimus group experiencing adverse events. This difference was always reported to be because of the higher level of application site burning and pruritus for tacrolimus treatment.
Tacrolimus compared with other active comparators
In the trial by Pacor and colleagues151 four out of 15 participants from each group reported adverse events. Those in the tacrolimus group all reported skin burning and those in the ciclosporin group reported gastric irritation (n = 1) and headache (n = 3). Serum creatinine levels were higher in the tacrolimus group (70.72–141.44 µmol/l) but not outside normal levels.
Overall implications for research and practice
Tacrolimus compared with topical corticosteroids
The evidence for tacrolimus treatment compared with topical corticosteroids is mixed. The treatment regimens and treatment comparisons vary in this collection of trials. Some of the trials do not make clinically relevant treatment comparisons, such as tacrolimus compared with the low-potency topical corticosteroid hydrocortisone acetate in children with moderate to severe eczema, which is likely to undertreat those randomised to the topical corticosteroid group.
Tacrolimus compared with other active treatments
The evidence from one small trial151 comparing tacrolimus with ciclosporin in participants with moderate to severe eczema is not sufficient to determine whether tacrolimus, a topical immunomodulator, is a viable alternative to ciclosporin, a systemic immunomodulator, which is usually used only for the most severe cases of eczema.
This trial showed some potential benefit of tacrolimus over ciclosporin over the first month of treatment, which then disappeared after 1.5 months, the end of treatment. This is not surprising given the long time to initial response to treatment for ciclosporin and it is a great shame that this trial did not include a longer follow-up period, ideally ≥ 6 months, to provide a fair comparison. The absolute decreases in the severity of eczema were likely to have been clinically significant for both treatments, which helps to confirm the benefit of both treatments for moderate to severe eczema. In clinical practice, a topical corticosteroid regimen is nearly always in place when systemic immunomodulators are started to ‘buffer’ this slow response rate.
More long-term research will be needed before a comparison between the potential harms of these two treatments can be made. Trials or other study designs such as cohort observational studies looking at combining tacrolimus and systemic immunomodulators for the most severe cases of eczema should ideally be carried out as well as longer-term RCTs.
Pimecrolimus compared with placebo
Studies
Reactive treatment regimens
Pimecrolimus was compared against vehicle using a regimen of twice-daily continual treatment until clearance at the first signs and symptoms of a flare or recurrence of eczema in five trials published since 2000. Four out of the five trials, three in children153–155 and one in adults,156 were large multicentre, industry-sponsored studies that provided treatment for 6 months or up to a year. The fifth trial was a much smaller single-centre trial157 conducted in the Czech Republic that lasted a year and did not report funding or sponsorship details. Use of emollients and rescue topical corticosteroids instead of study treatment in the event of a flare was allowed in all five trials.
A regimen of continual treatment until eczema clearance or for a set period of time was used in 11 trials. 158–168 Participants in all 11 trials were instructed to apply the treatment twice daily for a period anywhere from 7 days to 26 weeks, with 6 weeks being most common. Treatment was not continued if the eczema went into ‘remission’ in four158–160,162 of the 11 trials. Nine of the eleven trials158–166 were industry sponsored or funded and two trials167,168 did not report funding sources. The number of participants in the 11 trials ranged from 19 to > 500, with eight158–160,162–166 trials having > 150 participants.
Proactive treatment regimens
A proactive treatment regimen that aimed to prevent the recurrence of eczema at sites previously successfully cleared using a burst of continuous treatment with either topical corticosteroid or topical immunomodulatory agents was used in two manufacturer-supported trials153,169 reported since 2000. One of these trials169 randomised 74 participants to twice-daily treatment for 3 weeks on the cleared eczema sites and worsening eczema sites. The other trial153 used treatment for at least 7 days when eczema signs and symptoms of eczema were present for 24 weeks.
Assessment of risk of bias for the new studies
Reactive treatment regimens
Sufficient detail about the generation of the allocation sequence was available in nine153–156,158,159,161,164,165 out of 16 reactive treatment trials to allow an assessment of the risk of bias. All of these trials were assessed as having a low risk of bias. Sufficient detail about blinding was available in five153,156,158,161,168 out of the 16 trials to allow an assessment of the risk of bias. All of these trials were assessed as having a low risk of bias. Sufficient detail about concealment of the allocation system was available in one of the trials158 and this trial was assessed as having a low risk of bias.
Proactive treatment regimens
Sufficient detail about blinding was available in one out169 of two proactive treatment trials to allow an assessment of the risk of bias. This trial was assessed as having a low risk of bias. Sufficient detail about the method of generating the allocation sequence and the concealment of the allocation sequence was available in both153,169 proactive treatment trials to allow an assessment of the risk of bias. Both trials were assessed as having a low risk of bias.
Benefits
Reactive treatment regimens
For twice-daily continual treatment until clearance at the first signs and symptoms of a flare or recurrence of eczema, there was a statistically significant reduction (p < 0.003 from day 3 onwards) in pruritus on a scale of 0–3 (units not reported) for pimecrolimus compared with placebo (reduction of 0.9 for pimecrolimus compared with an increase of 0.3 for placebo) in one trial. 156 A statistically significant difference in the proportion of participants assessing their eczema as ‘completely or well controlled’ at the end of the treatment period (62/96 for pimecrolimus vs. 34/96 for placebo) was reported in the same trial. Participant assessments of pruritus and overall eczema severity were not assessed as outcomes in the other three trials. 153,154,157 All four trials reported a significant difference in the number of participants who had no flares over 6 months of treatment, ranging from 18.8% to 40.7% of participants on placebo compared with 44.8–71% of participants on pimecrolimus. Other outcomes assessing flares such as number of flares or time to first flare showed statistically significant benefits for pimecrolimus. The proportion of participants who did not use topical corticosteroids over 6 months of treatment was reported in three154,156,157 out of four of the trials and ranged from 57.4% to 66.7% for the pimecrolimus group and from 15.4% to 31.6% for the placebo group. The trial by Wahn and colleagues155 reported that the proportion of participants who did not use topical corticosteroid treatment after 1 year was 63.7% and 34.8% for infants in the pimecrolimus and placebo groups, respectively, and 57.4% and 31.6% for children in the pimecrolimus and placebo groups, respectively. The mean number of days on topical treatment was also assessed in all four trials and showed statistically significant differences in favour of pimecrolimus.
For continuous twice-daily treatment of eczema with pimecrolimus until clearance or for a set period of time, there was evidence of a large treatment effect for pimecrolimus compared with placebo for reduction of pruritus, which then persisted for the length of treatment. 159,162,165 There was also evidence of large treatment effects compared with placebo for the IGA, with the proportion of participants with an IGA score of 0 or 1 at the end of treatment ranging from 11% to 74.5% for pimecrolimus compared with 0–51.9% for placebo. 159–163,166 Participant-assessed improvement in severity also showed a large treatment effect in line with the improvement in pruritus severity. 163
Proactive treatment regimens
The participant-assessed overall self-assessment score was reported for the severe eczema (IGA ≥ 4) subgroup in the trial by Zuberbier and colleagues. 153 A statistically significant result for itching, loss of sleep and disease in favour of pimecrolimus was reported but it is not clear how many participants were included in these assessments. The number of days spent on topical corticosteroid ‘rescue’ treatment was not significantly different but was statistically significant, with a mean of 10% of the study time on topical corticosteroids in the pimecrolimus group compared with 19% of the study time on vehicle (9% difference, 95% CI 14.1% to 3.7%; p = 0.0009). A significant improvement in parents’ eczema-related quality of life was reported for pimecrolimus treatment in the same trial. Participants’ dermatology-related quality of life was reported to be not significantly different. In both proactive treatment regimen trials153,169 the severity of eczema measured using the EASI score showed a statistically significant difference in favour of pimecrolimus.
Harms
For both proactive and reactive treatment regimens, most events were mild or moderate and there were very few serious adverse events or withdrawals because of adverse events. Most of the events consisted of common symptoms such as nasopharyngitis or headache and were not thought to be related to the trial treatment. There were a handful of cases of eczema herpeticum or herpes simplex, with more cases in the treatment groups. Application site burning was reasonably common, although it was not consistently more common in the treatment or placebo group.
Overall implications for research and practice
As with topical corticosteroids compared with placebo, thousands of participants have taken part in topical calcineurin inhibitor compared with placebo trials and the evidence base has matured. There is strong evidence that using a calcineurin inhibitor confers clinically relevant benefits compared with a placebo. More trials of tacrolimus or pimecrolimus compared with placebo are not needed.
Although these trials were generally better reported than many in this review, there is still a lack of clear reporting of the method of allocation concealment. Raised awareness of the importance of a full and clear description of this aspect of methodology is essential for all trial reports to increase confidence in the results of trials.
Although the treatment effects appear fairly substantial, it is important to note that the placebo groups showed reasonable benefits from being in the trials as well. The relative merits of adding in another active treatment compared with encouraging increased adherence to a patient’s current topical treatments merits further research.
Randomised controlled trials are not appropriate for detecting rare or slow to develop adverse events and other research is being conducted to evaluate long-term safety as these treatments have not been in routine clinical use for very long. It is clear that local application site reactions such as burning or stinging are common when using pimecrolimus, but the evidence from these trials points to a lack of clarity about whether these adverse events are being caused by the active treatment, the vehicle or both.
Reactive treatment regimens
For short-term and long-term continuous treatment for a set period of time or until remission there is strong evidence of a moderate to large beneficial effect of using pimecrolimus compared with placebo. A significant reduction in pruritus is a major benefit, although it is not clear if this results from the improvement in eczema severity or is the result of a particular mechanism of action by pimecrolimus. For long-term treatment of eczema by using treatment twice daily at the first signs and symptoms of eczema until clearance, large reductions in the number of flares and frequency of use of topical corticosteroids as a rescue medication are evident. As use of pimecrolimus has been compared with placebo and essentially involves three treatments for complete control in some people with eczema, it is unclear whether this is of distinct clinical benefit compared with a similar regimen using topical corticosteroids, which is essentially very close to current clinical reality.
Proactive treatment regimens
Only two relatively small trials have tested a proactive treatment regimen for pimecrolimus compared with placebo, although both were of low risk of bias. 153,169 For long-term treatment (≥ 6 months) there was some evidence of benefit from a proactive regimen compared with placebo in one trial for a subgroup of people whose head and neck eczema was problematic. 153 For short-term continuous treatment after induction of remission, the evidence of benefit is weak but does show a modest treatment effect for mild or moderate eczema. 169
Pimecrolimus compared with active treatments
Studies
Pimecrolimus compared with topical corticosteroids
Two new trials170,171 compared pimecrolimus against betamethasone and one new trial compared pimecrolimus against triamcinolone acetonide. 172 The trial by Luger and colleagues170 compared five different doses of pimecrolimus against betamethasone 17-valerate and placebo in a within-person dose-ranging study. In total, 260 adults with at least moderate eczema and 5–30% body surface area coverage applied treatment twice daily to all affected areas (except on the head and neck) until clearance for up to 3 weeks. The trial by Jensen and colleagues171 compared twice-daily application of pimecrolimus (1%) against betamethasone cream (0.1%). This within-person trial, involving 15 adults with mild to moderate eczema, compared the treatments on the upper limbs for 3 weeks. A multicentre, multinational trial by Luger and colleagues172 involving 658 adults with moderate to severe eczema compared pimecrolimus (1%) against triamcinolone (0.1%) with hydrocortisone acetate (1%) for the face, neck and intertriginous regions. Treatment was applied twice daily until complete clearing and itching cessation, after which the treatment was restarted if inflammation occurred, for a period of 12 months.
Pimecrolimus compared with other active comparators
An industry-funded within-person study by Frankel and colleagues173 in the USA compared three times daily ceramide–hyaluronic acid emollient foam application against twice-daily application of pimecrolimus cream (1%) over a 4-week period. The trial included 30 participants who had been affected by mild to moderate eczema for at least 1 year. The treatments were applied to an investigator-selected target area of eczema. A very small single-blind industry-funded trial by Emer and colleagues174 compared a new lipid-rich topical medical device cream (applied three times daily) with pimecrolimus cream (applied twice daily). The trial lasted for 4 weeks and involved 20 participants.
Assessment of risk of bias for the new studies
Table 7 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Pimecrolimus vs. topical corticosteroids | ||||
| Jensen 2009171 | Unclear risk | Unclear risk | Unclear risk | Only 15 participants and so not likely to be powered to detect any differences in efficacy. Mostly interested in skin barrier effects |
| Luger 2001170 | Unclear risk | Unclear risk | Unclear risk | |
| Luger 2004172 | Unclear risk | Unclear risk | Low risk | |
| Pimecrolimus vs. other active comparators | ||||
| Emer 2011174 | Unclear risk | Low risk | Low risk | Single-blind, very small study. Objective outcome assessors were blinded; however, participants were not |
| Frankel 2011173 | Unclear risk | Unclear risk | Low risk | Patients and study co-ordinator who dispensed medication were unblinded and treatments involved different treatment regimens. Objective outcome assessors were blinded |
Benefits
Pimecrolimus compared with topical corticosteroids
The trial of pimecrolimus compared with triamcinolone acetonide by Luger and colleagues172 was primarily concerned with long-term safety and tolerability, particularly the rate of infections. Triamcinolone acetonide and hydrocortisone acetate (for the face, neck and intertriginous areas) treatment was reported to be significantly more effective at reducing severity, assessed using EASI scores, at all time points assessed during 1 year. The proportion of participants rated as being moderately clear or better according to the investigator’s assessment was significantly higher in the topical corticosteroid group at all time points apart from at 13 months. Topical corticosteroids were not used at all during the trial by 135 out of 328 participants using pimecrolimus. There were no significant differences between the groups in time to first remission and time to first recurrence.
Another trial by Luger and colleagues170 was a dose-ranging study of pimecrolimus (0.05%, 0.2%, 0.6% and 1%) compared with the standard topical corticosteroid comparator betamethasone 17-valerate (0.1%) and also placebo. All of the doses of pimecrolimus were significantly less effective than betamethasone 17-valerate for reduction in EASI score, reduction in pruritus and proportion of participants who assessed their eczema to be moderately clear or better (> 50% improvement). The most potent concentration of pimecrolimus, 1%, was still markedly less effective than betamethasone, although this was not formally statistically compared in the trial report.
A trial by Jensen and colleagues171 mostly concerned with assessing the change in skin structure also compared pimecrolimus (1%) against betamethasone (0.1%). This trial also did not compare the treatment groups against each other for severity or pruritus. The graphically presented results show a marked difference in favour of betamethasone, which peaked at 4 weeks, 1 week after treatment was stopped.
Pimecrolimus compared with other active comparators
In the trial by Frankel and colleagues173 there was not very much difference between the treatment groups in the severity of eczema using the IGA, with 12 out of 28 participants ‘clear’ and 11 out of 28 ‘almost clear’ by week 4 in the emollient foam group compared with 10 out of 28 ‘clear’ and 10 out of 28 ‘almost clear’ in the pimecrolimus group. There was also no difference in the level of improvement from baseline to week 4 (67.9% emollient foam group vs. 63.1% pimecrolimus group). Investigator-assessed severity was mirrored by participant-assessed severity. It was also reported that there were no significant differences between treatments over time in symptom scores for erythema, infiltration, excoriation, lichenification and scaling for the target lesions in both groups, but no absolute values were reported. In the trial by Emer and colleagues174 there were no significant differences between the two groups in disease severity assessed by IGA.
Harms
Pimecrolimus compared with topical corticosteroids
Of the two trials170,171 comparing pimecrolimus with betamethasone, one171 did not report adverse events and the other170 reported that a few systemic adverse events occurred that were not related to treatment. Application site reactions were the most common adverse event. There were no adverse events that were specifically attributed to the trial treatments.
Pimecrolimus compared with other active comparators
Adverse events were monitored during the trial by Frankel and colleagues;173 however, no adverse events were recorded. It is also reported that were no cutaneous side effects such as irritation or atrophy. In the trial by Emer and colleagues174 there were also no adverse events reported.
Overall implications for research and practice
Pimecrolimus compared with topical corticosteroids
An accurate assessment of the risk of bias for these trials was almost impossible as the trial reports failed to describe the method of randomisation, allocation concealment and blinding in enough detail. Sweeping statements that assume prior knowledge of the trial protocol are common. This must be kept in mind when assessing the evidence.
All of the trials that compared pimecrolimus against topical corticosteroids had safety and tolerability as the primary outcomes and so the data on efficacy in these trials, which suggest that topical corticosteroids such as betamethasone are more effective than pimecrolimus, need to be treated with some caution. To compound this problem, some of the pimecrolimus trials did not formally compare the two treatment groups. The evidence base for the efficacy of pimecrolimus compared with topical corticosteroids is still weak. The evidence from the trials on the potential harms of treatment, except for confirming that application site burning is common for pimecrolimus, is far from clear.
Trials of long-term treatment using the minimum amount of treatment needed, and paying more attention to the outcomes that are most important to those with eczema, are still needed to compare these treatments. There is still not enough evidence to suggest which topical corticosteroid or topical immunomodulator is most appropriate in common clinical eczema scenarios. The move to looking at proactive treatment regimens is welcomed and needs to become more pragmatic still when comparing these treatments.
Pimecrolimus compared with other active comparators
Two very small trials173,174 failed to show any hint of a benefit of a ceramide–hyaluronic acid emollient foam or a lipid-rich topical medical device cream over pimecrolimus in the 4 weeks of treatment, even for the participant-subjective outcomes in these single-blind trials. The trials did not report any adverse events but the small numbers of participants and short treatment duration make it unlikely that adverse events would be captured effectively. It is not clear whether the participants were allowed to use any other treatments during the trials, which makes interpretation of the trial results difficult.
Tacrolimus compared with pimecrolimus
Studies
Five new multicentre, manufacturer-sponsored trials, three in children175,176 and two175,177 in adults, compared tacrolimus with pimecrolimus twice daily. Four175,176 of the trials gave treatment for up to 6 weeks and one177 trial gave treatment for 13 days. One175 of the two trials in adults compared tacrolimus ointment (0.1%) with pimecrolimus cream (1%) twice a day until 1 week after complete clearance of the affected area or for 6 weeks in 413 participants with mild to very severe eczema. The other trial177 involved 37 adults and was primarily concerned with pharmacokinetic outcomes. Three trials175,176 involving a total of 793 children compared tacrolimus (0.03%) against pimecrolimus (1%) for up to 6 weeks. One trial175 recruited only participants with mild eczema, another176 recruited only those with moderate eczema and the third175 recruited participants with moderate to very severe eczema.
Different regimens of tacrolimus or pimecrolimus
The industry-funded trial by Ruer-Mulard and colleagues178 compared once-daily pimecrolimus (1%) with twice-daily pimecrolimus (1%) for up to 16 weeks in 268 children aged ≥ 2 years, after inducing remission in all participants.
The trial by Reitamo and colleagues179 compared twice-daily tacrolimus (0.03%) with once-daily tacrolimus (0.03%), using a placebo for the once-a-day group for the second application of treatment. The 53 children aged 3–24 months had eczema that required mild-potency topical corticosteroids on entry to the trial. The trial treatment was applied for 14 days; on the first and last days, only one application of study treatment was given, applied by the investigator. This trial focused on pharmacokinetics.
A multicentre trial conducted in the USA by Ling and colleagues180 compared pimecrolimus (1%) twice a day with pimecrolimus (1%) four times a day for 1 week, after which participants could choose to continue on either treatment for a further 2 weeks. Forty-nine participants were randomised aged ≥ 11 years with eczema that affected ≥ 30% of the body, a pruritus score of at least 2 out of 3 and an IGA of ≥ 2.
Assessment of risk of bias for the new studies
Table 8 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Tacrolimus vs. pimecrolimus | ||||
| Draelos 2005177 | Unclear risk | Unclear risk | Low risk | |
| Kempers 2004176 | Low risk | Low risk | Low risk | Participants who violated the study protocol were not included in the final analyses |
| Paller 2005175 (three trials) | Low risk | Low risk | Low risk | Unclear whether all those who withdrew were included in the analyses or not |
| Different regimens of pimecrolimus or tacrolimus | ||||
| Ling 2005180 | Unclear risk | Unclear risk | Low risk | Intention-to-treat population of all participants randomised used for all analyses |
| Reitamo 2009179 | Unclear risk | Unclear risk | Unclear risk | Not powered for statistical comparisons of the treatment group as the trial was primarily concerned with pharmacokinetic profiles of the treatments |
| Ruer-Mulard 2009178 | Low risk | Unclear risk | Low risk | The sample size was not based on statistical power calculations |
Benefits
In the three trials by Paller and colleagues175 the combined analysis of the reduction in severity measured by EASI score from baseline to the end of treatment was significantly greater in the tacrolimus group than in the pimecrolimus group (54.1% vs. 34.9%; p < 0.0001). Tacrolimus also achieved a significantly greater treatment success rate (IGA of disease activity of 0 or 1) of 40% compared with 22% in the pimecrolimus group at the end of the trial (p = 0.001). A significant difference in participant assessment of itch in favour of tacrolimus was also reported, although this was not as marked as the improvements in eczema severity.
The trial by Kempers and colleagues,176 sponsored by the manufacturer of tacrolimus, reported a statistically significant reduction in pruritus in favour of pimecrolimus whereas the three trials by Paller and colleagues,175 sponsored by the manufacturer of pimecrolimus, did not report any significant difference between the groups.
The small pharmacokinetic trial by Draelos and colleagues177 sponsored by the manufacturer of pimecrolimus did not statistically compare the efficacy results from the trial. The intensity of pruritus in both of the treatment groups reduced from around half of the participants having mild or moderate pruritus to three-quarters of the participants having absent or mild pruritus. One out of 18 participants (5.6%) in the pimecrolimus group and two out of 19 (10.5%) in the tacrolimus group achieved whole-body treatment success (defined as an IGA of 0 or 1 at the end of treatment). Five out of 18 (27.8%) in the pimecrolimus group and five out of 19 (26.3%) in the tacrolimus group achieved head and neck area treatment success (defined as an IGA of 0 or 1 at the end of treatment).
Different regimens of tacrolimus and pimecrolimus
The trial by Reitamo and colleagues179 did not compare or analyse data from the different treatment groups for the clinician’s global assessment. The decreases from baseline for the three surface area stratification groups were reported as –66.7, –51.8 and –60.9 for tacrolimus treatment once a day and –66.7, –75.9 and –59.5 for tacrolimus treatment twice a day.
The multinational trial by Ruer-Mulard and colleagues178 reported that the relapse rate was lower in the twice-daily pimecrolimus group (9.9%) than in the once-daily pimecrolimus group (14.7%). The time to relapse was not significantly different. The severity of eczema reduced from 83.2% for participants having an IGA of 0 or 1 at the start of randomised treatment to 62.1% in the twice-daily pimecrolimus group and 59.5% in the once-daily pimecrolimus group at the end of the trial.
The trial by Ling and colleagues180 found no significant differences in pruritus relief or reduction in severity between pimecrolimus twice daily and pimecrolimus four times daily at the end of the trial; however, as the participants had a choice as to the number of times they could use the study treatment after the end of the first week it is difficult to interpret the results.
Harms
The trials by Paller and colleagues175 and the trial by Kempers and colleagues176 all reported that there were adverse events such as application site burning and pruritus but that there were no differences in the rate of adverse events between the two treatments.
Different regimens of tacrolimus and pimecrolimus
Both trials comparing once-daily with twice-daily pimecrolimus178,179 reported frequent adverse events such as nasopharyngitis, cough, pyrexia and minor infections. There were a few treatment-related adverse events reported in both trials relating to application site burning, pruritus and dermatitis. The trial by Ruer-Mulard and colleagues178 reported that nasopharyngitis occurred slightly more frequently in the twice-daily group than in the once-daily group (14.9% vs. 8.2%, respectively) but tests for statistical significance were not reported.
The trial by Ling and colleagues,180 which compared pimecrolimus twice daily with pimecrolimus four times daily, reported that there were no serious adverse events; there was one withdrawal as a result of study treatment (application site burning to 90% of the total body surface). The number of participants experiencing at least one adverse event was five in the four times a day group and 10 in the twice-daily group. The number of participants experiencing at least one treatment-related adverse event was three in the four times a day group and four in the twice-daily group.
Overall implications for research and practice
Although pimecrolimus and tacrolimus have now been compared ‘head to head’ in several trials, it is important to remember that they are licensed for different ranges of severity of eczema. Pimecrolimus is not licensed for severe eczema as evidence has shown that it is not as potent as tacrolimus (0.1%).
None of the trials comparing treatment regimens for pimecrolimus and tacrolimus have so far attempted to address clinically important questions such as the optimal treatment regimen for patients with moderate eczema.
Trials looking at optimal treatment regimens need to be much longer and use regimens that mirror pragmatic clinical use with an active ‘standard practice’ comparator treatment.
Topical corticosteroids in combination with topical calcineurin inhibitors
Studies
One new trial by Meurer and colleagues181 compared a combination of fluticasone propionate (with hydrocortisone acetate for the face neck and hands) and pimecrolimus with fluticasone propionate with hydrocortisone acetate and vehicle cream. This multinational trial randomised 376 children aged between 2 and 17 years to treatments applied twice a day for 4 weeks. Those children who were clear or almost clear after 4 weeks of treatment were observed for a further 12 weeks to assess time to relapse.
Another new within-person multicentre trial by Hebert and colleagues182 compared treatment with tacrolimus (0.1%) ointment used concurrently with desoximetasone (0.25%) ointment with tacrolimus ointment used concurrently with the vehicle of desoximetasone, twice daily until clear or for 21 days. Eighty-two adults with eczema in the target lesions of ≥ 8/15 for total symptom score had their left and right sides randomised to treatment. There was no follow-up of participants beyond 21 days.
Assessment of risk of bias for the new studies
Table 9 provides the risk-of-bias assessment for the new studies.
Benefits
The trial by Meurer and colleagues,181 which was primarily concerned with safety, combined pimecrolimus and fluticasone and compared this treatment with fluticasone only. The trial found no significant differences between the treatments except for an increased time to relapse in what appeared to be a post hoc subgroup analysis of only those participants who were ‘clear’ of eczema as assessed by the IGA at the end of treatment.
In the trial by Hebert and colleagues182 the reduction in participant-assessed pruritus over 21 days was significantly better in the combined treatment group than with tacrolimus alone (p = 0.006); however, the scores were not reported and only 69 out of 82 participants were included in the pruritus assessment because of missed visits. The percentage of participants with a score of 0 (no pruritus) at baseline was 58% for combination treatment and 61% for tacrolimus treatment; this compared with 84% for combination treatment and 71% for tacrolimus treatment after 3 days. This was reported as statistically significant in favour of combination treatment (p = 0.04). A difference of 0.3 (95% Cl 0.1 to 0.5) between the groups in the physician’s global assessment score at day 21 was reported, which was significantly significant in favour of the combination treatment. For the total symptom score for the target lesions, the difference between the reduction in scores after 21 days was 0.8 (95% Cl 0.4 to 1.2) in favour of combination treatment (p = 0.0002).
Harms
The trial by Meurer and colleagues181 found a relatively high rate of infections and infestations (25–30% of participants) in both treatment groups. Bronchitis was far more common in the pimecrolimus and topical corticosteroid group (2.7% vs. 0%). The proportion of participants experiencing adverse events suspected to be related to the study drug was slightly higher on combination treatment (6.3%) than with the topical corticosteroid-only treatment (4.4%). For adrenal suppression, 54 participants (28.4%) on combination treatment and 44 participants (24.0%) on the topical corticosteroid-only treatment were suspected cases, but the majority of patients were confirmed not to be suppressed.
Hebert and colleagues182 reported that five participants withdrew from the trial because of ‘non-compliance’, ‘protocol violation’ or an ‘adverse event’, but the number of participants effected by adverse events and the nature of the events was not reported.
Overall implications for research and practice
There is not enough convincing evidence that combining topical corticosteroids and topical calcineurin inhibitors confers any short-term beneficial effects compared with topical corticosteroids alone. One large trial in children181 and a smaller trial in adults182 based their positive conclusions on the results of post hoc subgroup analysis or complete case analysis that excluded many participants.
Topical corticosteroids with occlusive therapy
Studies
No trials involving wet wrap bandages for the treatment of eczema were reported before 2000. Five new trials involving the use of wet wrap bandages were reported after 2000. 183–187
A left–right within-person comparison trial by Foelster-Holst and colleagues183 compared prednicarbate ointment and tubular bandages soaked in warm water and then covered with dry dressings with prednicarbate ointment alone for 48–72 hours. All of the 24 adults and children had experienced an acute episode of atopic eczema and also used an emollient.
A single-centre UK trial by Hindley and colleagues184 compared conventional treatment [hydrocortisone (1%) or stronger steroids] under wet wraps with hydrocortisone (1%) and emollients. Fifty children aged 4–27 months (eligibility was from 3 months to 5 years) with eczema that scored ≥ 15 using SCORAD scores were randomised to treatment. The children were assessed over a 4-week period but it was not clear whether they were using the study treatment for the whole 4 weeks.
A four-arm trial conducted in Hong Kong, China, by Pei and colleagues185 compared a one-tenth dilution of mometasone furoate ointment (0.1%) only for 4 weeks (first group) and the same treatment with wet wraps under dry wraps for the second 2 out of 4 weeks (second group) with a one-tenth dilution of fluticasone propionate (0.005%) only for 4 weeks (third group) and the same treatment with wet wraps under dry wet wraps for the second 2 out of 4 weeks (fourth group). The 40 children randomised were aged between 1 and 15 years with active eczema with a severity of at least 40–144 using a composite scale despite being treated with UK class II or stronger topical corticosteroids plus soap substitutes and emollients.
The single-centre trial by Schnopp and colleagues186 compared mometasone furoate (0.1%) with wet wraps against the vehicle for mometasone furoate with wet wraps. The wet wraps were applied only to a test area of eczema twice daily for 5 days, but not at night, and the mometasone furoate was applied morning and evening. Basic adjuvant treatment was allowed, although there were no details of what was deemed acceptable. Twenty children aged 2–17 years with exacerbated atopic eczema were randomised into the trial.
A small pilot trial conducted in the UK by Beattie and Lewis-Jones187 compared treatment with hydrocortisone and wet wraps with treatment with hydrocortisone without wet wraps. The treatment was applied twice a day for the first week and once a day for the second week. Participants could use emollients freely as long as a 20-minute gap between application of the hydrocortisone and application of the emollient was observed. All participants used emollients only for a third week. The 19 participants were aged ≤ 5 years and had atopic eczema with > 30% body surface area affected and no clinical evidence of infection.
Assessment of risk of bias for the new studies
Table 10 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Beattie 2004187 | Low risk | Unclear risk | Unclear risk | |
| Foelster-Holst 2006183 | Unclear risk | Unclear risk | High risk | Number of withdrawals and numbers included in the analyses not reported |
| Hindley 2006184 | Unclear risk | Unclear risk | Low risk | 5/28 in the wet wrap group withdrew for ‘non-compliance’ and were not included in the analyses |
| Pei 2001185 | Unclear risk | Unclear risk | High risk | 13/40 participants withdrew, 10 because of a > 50% improvement in eczema. Numbers included in the analyses were not reported |
| Schnopp 2002186 | Unclear risk | Unclear risk | Unclear risk | Number of withdrawals and numbers included in the analyses not reported |
Benefits
In the trial of prednicarbate ointment and wet wraps by Foelster-Holst and colleagues183 only the local severity of eczema using SCORAD scores was reported. The prednicarbate and wet wrap group improved by 4.4 SCORAD points whereas the prednicarbate-only group improved by 3.0 SCORAD points after 48–72 hours. This was reported to be a statistically significant difference (p = 0.011) in favour of prednicarbate with wet wraps. The baseline local SCORAD score was an average of 12.0 (SD 1.04). The participants had not been allowed any topical corticosteroid treatment in the 2 days before the treatment.
The trial by Hindley and colleagues184 found no significant difference between conventional treatment with wet wraps and conventional treatment only after 4 weeks for the primary outcome of severity of eczema measured by SCORAD. ‘Tolerability’, ‘ease of application’ and ‘efficacy’, each on a 5-point Likert scale, were stated to be outcomes, but no results were reported.
The trial by Pei and colleagues185 reported the severity of eczema using a composite scale; however, the groups were not compared against each other. The number of participants analysed for each group was not reported after the second randomisation. The trial reports that the two groups that used wet wraps continued to improve compared with baseline whereas the two groups that did not use wet wraps plateaued.
The trial by Schnopp and colleagues186 measured the severity of eczema using local SCORAD scores. Over 5 days the severity of eczema decreased from 10.6 to 2.5 in the mometasone group and from 11.1 to 4.0 in the vehicle group. This was a significantly better improvement in severity in the mometasone furoate and wet wraps group compared with the vehicle and wet wraps group (p < 0.01). The nurses and carers rated the wet wraps more difficult to use in a questionnaire.
The pilot trial by Beattie and Lewis-Jones187 found that there was a greater mean reduction in Six Area, Six Sign Atopic Dermatitis (SASSAD) score without wet wraps (8 more SASSAD points than with wet wraps) after the 2-week treatment period (95% CI for difference –18 to + 2; p = 0.11). For quality of life measured using the IDLQI, the group without wet wraps had a greater median decrease (5 more IDLQI points) than the group with wet wraps (95% CI for difference –10 to + 3; p = 0.24). The Dermatitis Family Impact score showed no significant difference between the treatments.
Harms
Two trials185,186 did not report adverse events, one trial187 reported that two participants withdrew because of folliculitis and one trial183 reported that there were no withdrawals and no adverse effects. The trial by Hindley and colleagues184 reported that recruitment was stopped early because of clinically significant adverse event differences between the treatment groups, although it was not clear what these were. It was also reported that 5 out of 28 of the participants using the wet wraps needed antibiotic treatment for infected eczema compared with none in the group not using wet wraps.
Overall implications for research and practice
These five small, short-term and poorly reported trials do not currently provide evidence of a beneficial effect of combining wet wrap treatment with topical corticosteroid treatment, but this may be because of their small size and design flaws. The between-group results were not properly compared in one trial,185 making interpretation of the evidence difficult.
Summary for topical corticosteroids and topical immunomodulators
Topical corticosteroids compared with placebo
-
There were 13 trials reported before 2000 and the trials that did report the magnitude of benefit suggested a large treatment effect of topical corticosteroids compared with placebo.
-
Nine trials reported from 2000 onwards that gave a continuous short- to medium-term course of treatment provide further evidence of the large beneficial effect of topical corticosteroid treatment compared with placebo.
-
There is evidence from four trials that used an initial 4-week continuous treatment regimen to ‘get control’ followed by a 16-week period of twice-weekly treatment on consecutive or evenly spaced days to ‘keep control’ of a large beneficial effect of topical corticosteroids compared with placebo.
Topical corticosteroids compared with active treatments (except topical immunomodulators)
-
There were 40 trials reported before 2000 comparing topical corticosteroids against other topical corticosteroids. The trial evidence was mixed and it was difficult to provide a summary as none of the trials compared all of the main topical corticosteroids together. Fluticasone propionate and mometasone furoate were found to be reasonably equivalent to older topical corticosteroids when used once daily.
-
Eleven new trials reported from 2000 onwards compared a new topical corticosteroid against another commonly used topical corticosteroid. These trials still do not compare more than two treatments and only add to the previous mixed results seen before 2000. Of these:
-
one trial provided some very weak evidence that pulsed treatment is more effective than continuous treatment with the same topical corticosteroid, which needs further research
-
one trial provided some evidence that adding a penetration-enhancing chemical to topical corticosteroid treatment was more beneficial than topical corticosteroid alone
-
one trial provided some evidence that mometasone furoate in multilamellar emulsion was more beneficial than methylprednisolone aceponate.
-
-
Four trials reported after 2000 compared a topical corticosteroid against a different active comparator. All of these trials used the topical corticosteroid as the standard comparator and these trials are discussed in the relevant sections of this review.
Topical immunomodulators
Tacrolimus compared with placebo
-
There were two well-reported trials of tacrolimus compared with vehicle reported before 2000, one in children and one in adults. These trials provided evidence of a short-term large beneficial effect for tacrolimus compared with vehicle, regardless of the concentration used.
-
Eight trials of tacrolimus compared with placebo given twice daily for 2–12 weeks were reported from 2000 onwards. These trials provided evidence of a benefit for tacrolimus compared with placebo.
-
Three large multicentre trials and one small open-label trial of tacrolimus compared with placebo given after initial treatment to gain control of the eczema provided evidence of a benefit for tacrolimus compared with placebo for the prevention of flares.
Tacrolimus compared with other active treatments
-
There were no trials of tacrolimus compared with other active treatments reported before 2000.
-
There were six new trials of tacrolimus compared with hydrocortisone reported from 2000 onwards. The two trials of hydrocortisone acetate compared with tacrolimus provide evidence that tacrolimus is more beneficial for moderate to severe eczema. However, this is not a clinically appropriate comparison of treatments. The three trials comparing the more potent hydrocortisone butyrate against tacrolimus gave conflicting results, with the hydrocortisone treatment being more beneficial for continuous treatment for 3 weeks and tacrolimus being more beneficial when using treatment ‘as required’ for flares over 6 months after an initial 7-day continuous treatment.
-
There were eight trials of tacrolimus compared with other topical corticosteroids comparing different treatments, regimens and areas of the body. There was some evidence from one trial that tacrolimus (0.1%) was more beneficial than fluticasone propionate (0.005%) for facial eczema in adults and evidence from another trial of the non-inferiority of tacrolimus (0.1%) compared with fluticasone propionate (0.005%) on all areas of the body.
-
One trial compared tacrolimus against ciclosporin, which is discussed in Chapter 11.
Pimecrolimus compared with placebo
-
There were no trials of pimecrolimus compared with placebo reported before 2000.
-
There were four trials of continuous treatment with pimecrolimus compared with placebo at the first signs and symptoms of a flare or recurrence of eczema until clearance. These trials provide evidence of a large beneficial effect of pimecrolimus.
-
There were 11 trials, mostly with industry involvement, of reactive continuous treatment until eczema clearance or for a set period of time, which was usually 6 weeks. These fairly well-reported trials provide evidence of a large beneficial effect of pimecrolimus, particularly for the reduction of pruritus.
-
There were two small but well-reported trials of the use of either a proactive 3-week continuous treatment or ‘as-needed’ bursts of continuous treatment over 6 months after induction of remission. The short-term (3-week) continuous treatment provided weak evidence of benefit for pimecrolimus compared with placebo for mild to moderate eczema over 6 months. The long-term (as-needed) treatment provided evidence of a beneficial effect over 6 months only for a subgroup of those with problematic head and neck eczema.
Pimecrolimus compared with other active treatments
-
There were no trials of pimecrolimus compared with topical corticosteroids reported before 2000.
-
The three new trials that compared pimecrolimus against topical corticosteroids had safety and tolerability as primary outcomes. The mostly methodologically unclear trials provide evidence that the topical corticosteroids betamethasone 17-valerate and triamcinolone acetonide are more beneficial than pimecrolimus in adults with moderate to severe eczema.
-
One very small trial compared pimecrolimus against an emollient foam over 4 weeks and found no evidence of a significant difference between the treatments.
-
One very small trial reported in 2011, with a high risk of bias for blinding, compared pimecrolimus against a lipid-rich topical cream and found no evidence of a significant difference between the treatments.
Tacrolimus compared with pimecrolimus
-
There were no trials of pimecrolimus compared with tacrolimus reported before 2000.
-
Five trials compared tacrolimus against pimecrolimus. Overall, these trials had a mostly low risk of bias and provide some evidence of benefit for pimecrolimus treatment compared with tacrolimus treatment.
Topical calcineurin inhibitors combined with topical corticosteroid
-
There were no trials of topical calcineurin inhibitors combined with topical corticosteroids reported before 2000.
-
One large, methodologically unclear trial in children that was primarily concerned with safety did not provide any evidence of a short-term significant difference in benefit when pimecrolimus and a topical corticosteroid were combined over 4 weeks of treatment. There was a significant difference in relapse rates over 12 weeks in favour of the combined treatment from a, probably post hoc, subgroup of all those clear at the end of treatment.
-
One small, methodologically unclear trial in adults provides some weak evidence of benefit for the combination of tacrolimus (0.1%) and a topical corticosteroid.
Topical corticosteroids with occlusive therapy
-
There were no trials involving occlusive therapy in combination with topical corticosteroids reported before 2000.
-
There were five new RCTs reported after 2000 but they were all small, very short term and methodologically unclear. The results do not provide clear evidence of a significant benefit from the addition of occlusive therapies to topical corticosteroids.
Chapter 5 Emollients and other topical treatments
Background
Many topical treatments have been tried in people with eczema. This chapter summarises new RCT evidence on topical eczema treatments that do not fit into other categories in this review.
Existing systematic reviews
There is one systematic review focusing on emollients by Tarr and Iheanacho. 188 The SIGN,42 NICE41 and American Academy of Dermatology (AAD)94 guidelines have all covered emollients. A review of reducing pruritus for eczema93 covers emollients, topical doxepin and sodium chromoglycate and a Cochrane review189 covers evening primrose oil and borage oil for eczema treatment.
Emollients
Studies
Five trials were reported before 2000 that tested emollients for eczema55 (see Appendix 3).
Most of the 15 new trials123,126,190–202 looking at emollients reported after 2000 compared twice-daily treatment with an emollient (often applied concurrently with topical corticosteroid treatment) with treatment with topical corticosteroids alone, other emollients or, in one case, no treatment. Most trials had a treatment length of around 4–6 weeks and none of the trials gave treatment for > 2 months. Nearly all of the trials included participants with mild to moderate eczema, with only three trials123,126,191 including participants with severe eczema.
Albolene®
One ‘equivalence’ trial of 60 patients with mild eczema sponsored by the manufacturer of the over-the-counter emollient Albolene® (DSE Healthcare Solutions) compared Albolene against a prescription device emollient (MimyX™, Stiefel Laboratories), with concurrent use of topical triamcinolone (0.1%) cream in both groups, twice daily for 4 weeks. 190
Assessment of risk of bias
Table 11 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Draelos 2009190 | Unclear risk | Unclear risk | Unclear risk | No details of a sample size calculation or margin for the claim of ‘parity’ between treatments |
Benefits
Both the investigators and the participants assessed the severity of eczema using the same 6-point ordinal scale from ‘none’ to ‘severe’. The author reported that there were no statistically significant differences between the treatments for eczema severity assessed by either the investigators or the participants.
Harms
It was reported that no adverse events occurred during the trial.
Exomega milk
A 6-week unblinded trial funded by the manufacturers of Exomega milk® (Pierre Fabre Limited) randomised 173 infants with moderate to severe eczema to Exomega milk applied twice daily on non-inflammatory areas of skin or no equivalent treatment. 191 All of the participants were allowed to use topical corticosteroids during the trial to treat inflammatory lesions. The primary outcome was steroid sparing (decrease in topical corticosteroid use).
A smaller trial,192 also involving the Pierre Fabre Laboratories, included 76 infants and children aged from 6 months to 2 years with mild to moderate eczema. Participants were randomised to use either Exomega milk or a cleaning bar (A-Derma®) all over the body, twice a day, for 2 months. All participants were allowed to use topical corticosteroids during the trial.
Assessment of risk of bias
Table 12 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Giordano-Labadie 2006192 | Unclear risk | Unclear risk | Unclear risk | |
| Grimalt 2007191 | Unclear risk | Unclear risk | Low risk | The intention-to-treat population did not include seven participants from the emollient group and four from the control group because of protocol or inclusion criteria violations and no follow-up data |
Benefits
The study by Grimalt and colleagues191 showed a significant difference between the steroid-sparing effect of the two treatments after 21 days and 42 days of treatment. After 42 days, the Exomega milk group had used 8.56 g (SD ± 1.74 g) of a potent topical corticosteroid whereas the control group had used 14.7 g (SD ± 2.08 g), a –41.8% difference (p < 0.05). The consumption of moderate-potency corticosteroids was not significantly different between the groups. The severity of eczema measured using SCORAD scores fell in both groups by just over half of the baseline value, from 35.63 to 15.96 in the emollient group and from 35.96 to 16.45 in the control group, showing no difference between the groups. Although not prespecified as an outcome, the proportions of participants with dryness (p = 0.015, percentage of participants with dryness in each group not reported) and moderate to severe dryness (33% vs. 61.5%; p = 0.007) were reported as significantly lower in the emollient group after 21 days. Dryness was not significant at 42 days (20.25% vs. 36.36%).
The smaller trial by Giordano-Labadie and colleagues192 found no significant difference between the groups in the severity of eczema measured using SCORAD scores after 28 or 56 days of treatment. The authors report a significant reduction in the emollient group and the control group for both xerosis (–35.9% vs. –68.6%; p = 0.01) and pruritus (–41.6% vs. –65.7%; p = 0.01) after 2 months of treatment, although these were not prespecified outcomes. There was a significant reduction in quality of life, a prespecified outcome measured using the CDLQI, after 2 months in the emollient group (from 2.24 to 1.18) compared with the control group (from 1.59 to 1.4; p = 0.01).
Harms
Grimalt and colleagues191 reported a number of adverse events possibly related to the treatment; three were mild, three were moderate and two were serious and led to treatment discontinuation. The nature of these adverse events was not reported. The trial report states that all adverse events resolved without sequelae.
Giordano-Labadie and colleagues192 did not report any information about adverse events.
Urea and glycerine emollients
Two virtually identical trials by Loden and colleagues,193,194 each lasting for 30 days, compared a glycerine cream (20%) with its vehicle (with glycerine substituted with water) and a cream containing urea (4%) and sodium chloride (4%). For the earlier trial193 the treatment was applied only twice a day to a patch of dry skin identified by the dermatologist. For the second trial194 participants were allowed to use the treatments as much as necessary and at least once a day. The first trial was primarily concerned with physical markers of efficacy but also measured skin dryness. The second trial was more concerned with efficacy and measured both participant- and investigator-assessed skin dryness, as well as participant-assessed degree of stinging, smarting, itching and dryness/irritation.
A trial by Bissonnette and colleagues195 compared a urea moisturiser (5%) against a urea lotion (10%) but did not include a control arm. The trial included 100 adults aged > 18 years with mild eczema (SCORAD score of < 30) and treatments were applied twice daily for 42 days.
A trial by Amichai and Grunwald196 compared the liquid soap Axera™ (Perrigo-Pharma), containing 12% ammonium lactate and 20% urea, with a commercially available liquid soap for showering over a 3-week period. No other emollients or soaps were permitted during the trial but participants could continue to use their current eczema treatments. The study included 36 adults and children aged 3–40 years with mild to moderate eczema, diagnosed according to the UK Working Party’s criteria. 9
Assessment of risk of bias
Table 13 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Amichai 2009196 | Unclear risk | Unclear risk | Unclear risk | |
| Bissonnette 2010195 | Unclear risk | Unclear risk | Unclear risk | |
| Loden 2001193 | Unclear risk | Unclear risk | Unclear risk | |
| Loden 2002194 | Unclear risk | Unclear risk | Unclear risk |
Benefits
The trial report by Loden and colleagues193 gives very little detail and the two graphs that present the data on dryness score appear to show very different baseline scores for the three treatment groups, with the urea group having a noticeably higher baseline score than the glycerine and placebo groups. No details are given about the method of randomisation or whether allocation concealment took place and the difference in baseline values raises doubts about these procedures. Although no detailed data are presented, the trial report states that after 30 days’ treatment the urea treatment group had a significantly lower dryness score than the glycerine treatment group (p = 0.021). It is unclear whether this refers to the difference between the final dryness scores or the difference between the change in dryness scores.
The second trial by Loden and colleagues194 used a dermatologist-assessed dryness scale, with no statistically significant differences reported between urea cream and glycerine cream and between glycerine cream and placebo cream. For participant assessment of dryness at the end of treatment there was no significant difference between the urea and glycerine groups (89% vs. 85% of participants rating the dryness as ‘improved’; p = 0.77). The proportion of participants rating the dryness as ‘improved’ was significantly higher in the glycerine cream group than in the placebo group (89% vs. 69%; p = 0.019). Again, no detailed data are presented, including any baseline scores or demographics.
The trial by Bissonnette and colleagues195 did not find a statistically significant difference in eczema severity between the urea cream (5%) and the urea lotion (10%) after 42 days of treatment (19.76% vs. 19.23% reduction in mean SCORAD scores). The trial report states that the urea cream (5%) had better cosmetic acceptability than the urea lotion (10%).
Amichai and Grunwald196 reported significant reductions for the urea and ammonium liquid soap (Axera) compared with the commercially available liquid soap in scaling (urea and ammonium soap: from 1.63 to 0.68, ‘placebo’ soap: from 1.75 to 1.42; p < 0.0001), skin dryness scaling (urea and ammonium soap: from 1.88 to 0.77, ‘placebo’ soap: from 1.83 to 1.25; p < 0.0001), redness (urea and ammonium soap: from 0.58 to 0.14, ‘placebo’ soap: from 0.62 to 0.53; p = 0.03) and participant-assessed itching (urea and ammonium soap: from 1.38 to 0.32, ‘placebo’ soap: from 1.83 to 0.92; p < 0.001). The participants rated the urea and ammonium soap significantly better for its non-sticky texture and for the improvement of skin smoothness; however, no data were provided for this outcome.
Harms
Information about adverse events was not recorded in the first trial by Loden and colleagues. 193
Adverse events that could possibly be related to study treatment were recorded and graded in the second trial by Loden and colleagues. 194 The report states that adverse skin reactions were significantly lower in the glycerine group than in the urea group, with 10% in the glycerine group experiencing moderate to severe stinging compared with 24% in the urea group (p < 0.0006).
In the trial by Bissonnette and colleagues,195 22 out of the 100 participants experienced at least one adverse event. Five adverse events were reported as being possibly related to study treatment and no participant experienced more than two adverse events. Three participants withdrew from the study because of adverse events, two in the urea lotion group because of irritant contact dermatitis and pruritus and one in the urea moisturiser group because of erythema.
In the trial by Amichai and Grunwald196 one participant in the Axera group had a mild transient skin irritation related to using the soap.
Lipid emollient
One trial by Wiren and colleagues197 conducted in Sweden compared an emollient with 20% lipid content (Canoderm® cream, ACO Hud) with no treatment until relapse or 6 months. All 55 adults with eczema who were recruited into the trial initially used the topical corticosteroid betamethasone (0.01%) for 3 weeks to induce remission. Only those participants who had ‘cleared eczema’ according to an assessment by a dermatologist (n = 44) were then randomised to the maintenance period of emollient or no treatment. The aim of the trial was to assess whether emollient use prolonged the time spent in remission from eczema.
Assessment of risk of bias
Table 14 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Wiren 2009197 | Unclear risk | Unclear risk | Unclear risk | Primary author employed by ACO Hud, the makers of the study treatments |
Benefits
The median time to first relapse was > 6 months for the emollient group compared with 30 days when using no treatment. This difference in time to relapse was statistically significant, with a relative risk reduction of 53% and number needed to treat of 2.8.
Harms
No information about adverse events was reported.
Sunflower oleodistillate emollient
Two industry-sponsored trials123,198 from the same group compared a sunflower oleodistillate (2%)-containing emollient (Stelatopia®; Mustela DermoPediatrie, Laboratoires Expanscience) with a topical corticosteroid for 3 weeks.
In the first trial, by Msika and colleagues,123 86 infants and young children aged from 4 months to 48 months with mild to moderate eczema were randomised to five groups. Each group received a treatment based on a moderate-potency topical corticosteroid (Tridesonit®; CS Dermatologie) (0.05%) once or twice daily or every other day combined or not with application of the sunflower emollient twice daily for 21 days. Overall, two groups received topical corticosteroids alone and three groups received topical corticosteroids plus emollient. The second trial, by De Belilovsky and colleagues,198 compared twice-daily application of Stelatopia with the mild-potency topical corticosteroid hydrocortisone butyro-propionate cream (1 mg/g) in infants and young children aged from 4 months to 4 years with mild to moderate eczema.
Assessment of risk of bias
Table 15 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| De Belilovsky 2011198 | Unclear risk | Unclear risk | Unclear risk | The trial was single blinded |
| Msika 2008123 | High risk | Unclear risk | Unclear risk | The trial report does not state what statistical cut-off point they have used for claims of ‘significance’. The trial was single blinded |
Benefits
In the trial by Msika and colleagues,123 the overall comparison of the five groups found no significant differences in disease severity changes assessed by SCORAD scores or in quality of life (SCORAD scores were reduced by 58–75% from baseline to 3 weeks in the five groups). There was no difference between using topical corticosteroids twice daily without sunflower emollient and using topical corticosteroids every other day plus sunflower emollient twice daily. Because of the similar decrease in eczema severity in these two groups, the trial authors suggested that using a sunflower oleodistillate-containing emollient produced a topical steroid-sparing effect of 75%. However, there was no statistical comparison between the topical corticosteroids once-daily group and the topical corticosteroids plus sunflower oleodistillate-containing emollient group in the study, which is the most appropriate and real-life situation. Furthermore, there was also no difference between using topical corticosteroids twice daily alone and using topical corticosteroids twice daily plus sunflower emollient. Thus, the conclusion could also have been that using sunflower emollient produced no topical corticosteroid-sparing effect at all in this case.
In the trial by De Belilovsky and colleagues,198 there were also no significant difference between groups in disease severity changes assessed by SCORAD scores at 3 weeks (change from 37.2 to 11 in the topical corticosteroid group and from 36.9 to 9.4 in the emollient group) or in quality of life, suggesting that sunflower emollient could have an anti-inflammatory effect.
Emollients containing ceramide
An industry-sponsored multicentre trial by Sugarman and Parish126 compared twice-daily application of a ceramide-dominant barrier repair formulation (EpiCeram) against the topical corticosteroid fluticasone propionate (0.05%) (Cutivate™; GlaxoSmithKline) on affected areas in body folds. The trial included 121 infants and children aged from 6 months to 18 years. All of the participants used the emollient lotion Cetaphil™ (Galderma Laboratories) on unaffected areas of skin.
A trial by Berardesca and colleagues200 compared a lipid mixture containing ceramide-3, cholesterol, palmitic acid and oleic acid in water-in-oil with nanoparticles with the same lipid mixture in combination with topical corticosteroids. Out of a trial population of 508 participants, 91 participants had eczema. All participants applied the treatment once or twice a day until healing had occurred or for a maximum of 8 weeks.
A trial by Draelos201 compared a ceramide-based emollient against a hyaluronic acid-based foam, the details of which are discussed later in this chapter.
A within-person trial by Simpson and Dutronc202 compared a body wash and moisturiser containing ceramide (Restoraderm®; Galderma (UK) Ltd) in addition to standard eczema treatment with standard eczema treatment alone. The trial included 127 adults and children aged > 3 years with mild to moderate eczema according to IGA, randomised to emollient treatment twice daily on one side of the body and no emollient treatment on the other side, for an unreported length of time.
Assessment of risk of bias
Table 16 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Berardesca 2001200 | Unclear risk | Unclear risk | Unclear risk | |
| Draelos 2011201 | Unclear risk | Unclear risk | Unclear risk | |
| Simpson 2011202 | Unclear risk | Unclear risk | Unclear risk | |
| Sugarman 2009126 | Unclear risk | Unclear risk | High risk | Many participants had recently used fluticasone propionate before the trial, leading to it being impossible to blind the trial |
Benefits
The industry-funded trial by Sugarman and colleagues126 did not make it clear whether this was a superiority or an equivalence trial, although the stated aim of the trial seems to have been to prove equivalence. Similar improvements in eczema severity (measured by SCORAD score), pruritus and sleep loss were observed in both groups. The relative reductions in eczema severity, measured using SCORAD score, were fairly large, with the emollient group decreasing from 37.2 to 18.5 and the fluticasone propionate group decreasing from 34 to 12 (estimated from a graph). The reductions in pruritus were also fairly large, with the emollient group decreasing from 6.1 to 2.8 and the fluticasone propionate group decreasing from 5.6 to 2.1 (estimated from a graph). The sleep loss assessments showed a decrease from 4.1 to 1.4 in the emollient group and from 4.1 to 0.7 in the fluticasone propionate group.
The trial by Berardesca and colleagues200 reported a statistically significant difference in favour of combined treatment with emollient and topical corticosteroids compared with emollient alone for pruritus after 8 weeks (p = 0.018), overall disease severity after 4 weeks (p = 0.007), dryness and scaling, but no detailed data are provided.
The trial by Simpson and Dutronc202 found a significant reduction in eczema severity for the ceramide-containing emollient compared with no emollient after 7 (p = 0.0003) and 15 (p = 0.0043) days while using standard eczema treatment. This difference was not significant at days 21 and 28. There were no absolute values reported. The mean change was not explicitly stated but appears to have been no more than –1.0 in the no treatment group and –1.4 in the ceramide-containing emollient group, as determined from a graph.
Harms
Berardesca and colleagues200 did not report any information on adverse events. In the trial by Sugarman and colleagues126 there were no serious adverse events and four participants in the emollient group had a worsening of eczema that required rescue medication. Simpson and Dutronc202 did not report information about adverse events.
Hyaluronic acid-based emollient
A within-person trial by Draelos201 compared a hyaluronic acid-based foam emollient (Hyaltopic™; Onset Therapeutics) against a ceramide-based emollient (EpiCeram) used twice daily for 4 weeks. The participants used the treatment on either their arms or legs, one limb per treatment, and used randomised barrier treatment on the rest of each side of their body. The 20 adults randomised had mild to moderate eczema as assessed by IGA and symmetrically distributed lesions
Assessment of risk of bias
Table 17 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Draelos 2011201 | Unclear risk | Unclear risk | Unclear risk |
Benefits
There was a significant preference for the hyaluronic acid-based emollient for the aesthetics (‘worked better, ‘less odour’, ‘rubs into skin easier’, ‘more soothing’) of the treatment. There was no significant difference for ‘would prefer to keep using’. The treatments were not statistically compared against each other for severity of eczema or individual signs and symptoms of eczema.
Harms
It was reported that there were no problems with safety for both treatments.
Overall implications for research and practice
Although it is pleasing to note an increase in the number of emollient trials since the 2000 report,55 the lack of reporting of methodological detail is disappointing. Some studies have tried to demonstrate sparing of use of topical corticosteroids, presumably on the rationale that the latter may exhibit side effects such as skin atrophy if used inappropriately. Although skin atrophy and systemic effects have been associated with prolonged use of potent topical corticosteroids in the past, these adverse effects are probably rare nowadays with appropriate use in the context of eczema management. Furthermore, reducing cutaneous inflammation by using topical corticosteroids is probably a way to improve cutaneous barrier functionality. None of the studies involving topical corticosteroids to date have shown an increase in skin atrophy caused by topical corticosteroids. It is important that studies aiming to show topical steroid sparing demonstrate that such sparing is not achieved at the expense of lack of eczema control. One new study has suggested that regular emollients used once disease has been controlled by topical corticosteroids may reduce the frequency of flares and time to next flare, an important finding that needs to be replicated in larger studies that include those with mild, moderate and severe eczema. Despite a lot of interest in emollients that have been designed to contain specific ‘barrier repair’ ingredients, there is no clear evidence to date that any of these more expensive preparations are superior to simple cheaper emollients. There is some evidence, however, that some emollients such as aqueous cream, which contains sodium lauryl sulfate, may harm the skin barrier203 and more refined mechanistic studies to identify which emollients are helpful and which are not are needed before large-scale comparative trials are carried out. Research into the effectiveness of increasing compliance with regard to the use of emollients, such as allowing patients to choose their own emollient from a range of consistencies, and how long should be left after applying an emollient before applying a topical corticosteroid is needed. Emollient application from birth is now being considered as an intervention to prevent or at least delay the onset of eczema. 204
Bath additives
One trial involving an antibacterial bath additive was reported before 200055 (see Appendix 3).
Two new trials on antibacterial bath additives were reported after 2000. 205,206
The trial by Huang and colleagues,205 which compared 0.005% bleach baths and mupirocin ointment intranasally for all family members against bathing without bleach and petroleum (placebo) intranasally for all family members, is discussed in Chapter 6.
A trial carried out in Japan by Shibagaki and colleagues206 compared a bath additive containing eucalyptus extract, oat extract and oily moisturising ingredients such as a synthetic pseudoceramide with and without a derivative of diamide (a chemical oxidant that affects cell signalling). The trial randomised 21 participants with eczema to use 30 ml of the treatment dissolved in 180–200 l of hot water for 5 minutes at least four times a week for 3–6 weeks. It was not clear what ages the participants were.
Assessment of risk of bias
Table 18 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Shibagaki 2005206 | Unclear risk | Unclear risk | Unclear risk | Analysis of change in severity of eczema was carried out on an intention-to-treat basis; however, change in pruritus was not carried out on an intention-to-treat basis. Six participants from the diamide group and one from the group that did not contain diamide withdrew |
Benefits
There was no significant difference between the treatment groups for the investigator assessment of improvement. A significant difference was reported in the number of participants assessed as having an improvement in their itching scores (six out seven in the diamide derivative group compared with two out of seven in the control group). This was a per-protocol analysis that included only 7 out of 13 participants in the diamide derivative group and seven out of eight in the control group. All of the withdrawn participants were withdrawn because they did not use the treatment four or more times a week.
Harms
There was no information on adverse events included in this report.
Overall implications for research and practice
It is unclear from this small trial206 whether adding a diamide derivative to bath emollients improves eczema. The study was not powered appropriately to pick up anything but very large changes. The significant difference in rates of improvement in pruritus reported for the per-protocol population of 14 participants is highly susceptible to attrition bias given the differential dropout rate. Much larger, pragmatic and long-term trials are needed to evaluate the possible additional benefit of bath emollients in eczema. It is possible that the emollient effects from adding moisturisers to the bath are minimal compared with leave-on emollients applied after bathing. 188
Furfuryl palmitate
Furfuryl palmitate is an antioxidant compound, which can neutralise (block the activity of) free radicals. Higher levels of free radicals are associated with cellular damage. There is interest in antioxidant supplementation for eczema as these compounds may stop the skin cell damage that occurs in eczematous skin.
Studies
No RCTs on furfuryl palmitate for eczema were reported before 2000.
One new trial was reported after 2000. 207 This new trial by Tripodi and colleagues207 compared an emollient with the antioxidants furfuryl palmitate, superoxide dismutase, 18-beta-glycyrrhetinic acid, vitamin E and alpha-bisabolol against the same treatment without furfuryl palmitate. The trial randomised 117 children aged 3 months to 14 years with eczema according to the UK Working Party’s criteria9 to treatment twice a day for 2 weeks, using one fingertip unit for every patch of skin the area of two adult hands. In total, 109 participants completed the trial, 21 of whom took medications not permitted by the trial protocol. Participants did not change their lifestyle and diet during the trial. Participants were not allowed to have used any topical or systemic treatments for eczema for 1 week before randomisation and during the trial.
Assessment of risk of bias
Table 19 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Tripodi 2009207 | Low risk | Low risk | Low risk | Intention-to-treat analyses were used (102/117) but it is not clear from the report why seven participants who violated the study protocol were not included in this number as the trial report stated that they should have been |
Benefits
This trial surprisingly reported that the emollient base without furfuryl palmitate showed better efficacy than the furfuryl palmitate emollient, with 70% (38/54) of the base emollient group compared with 29% (14/48) of the furfuryl palmitate group having a ≥ 20% reduction in baseline SCORAD scores at the end of the 2-week study. The investigator- and participant-assessed efficacy results supported this finding, with the emollient base cream being significantly more effective than the furfuryl palmitate cream.
Harms
No formal data were provided in the trial report about adverse events. The authors report in the discussion that some of the participants using the furfuryl palmitate emollient cream reported itching and burning sensations after application. There was no particular difference in the number of withdrawals from the trial; however, 14 out of 53 of the participants in the furfuryl palmitate group used other topical or systemic treatments compared with seven out of 56 in the control group.
Overall implications for research and practice
This methodologically robust trial207 based on a formal sample size calculation clearly shows that the addition of furfuryl palmitate in an emollient base seems to confer a negative effect for eczema. It is a credit to the trial authors and journal editors for publishing this negative study, which might save money from being spent on the further pursuit of this treatment.
Pill mask
Studies
No trials involving pill masks for eczema were reported before 2000.
One new trial was reported after 2000. 208 This manufacturer-funded, open, three-arm trial by Palombo and colleagues208 compared a pill mask containing a chitosan-derived anti-inflammatory compound ATOBIOL, tocotrienols and hyaluronic acid against a lamellar active emulsion containing ATOBIOL or petroleum ointment only. Thirty-five children applied the study treatments after using bath oils or used petroleum ointment only, twice a day for the first 8 days. After this treatment period, all participants used triamcinolone (0.1%) either once a day or twice a week (the abstract and main text do not agree) and the lamellar gel only. It is not entirely clear from the trial report exactly what the ‘pill mask’ is in this trial, except that it is a topical method of delivery for medication.
Assessment of risk of bias
Table 20 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Palombo 2004208 | Unclear risk | Unclear risk | High risk |
Benefits
Erythema and pruritus, scaling and crusting were assessed on a VAS and both of these scores were also combined to make the ‘clinical score’. The improvement in clinical score after 4 weeks compared with baseline was reported as 58% in the pill mask group compared with the petroleum ointment, 82% in the pill mask group compared with the lamellar gel group and 64% in the petroleum group compared with the lamellar gel group. The erythema and pruritus, scaling and crusting scores were reported in graphs, but the scores were not compared between groups.
Harms
No information about adverse events was reported.
Overall implications for research and practice
The lack of methodological and treatment detail, the low number of participants and the unclear analysis of the results between groups mean that the potential benefits or harms of this topical pill mask cannot be adequately assessed from this one trial. 208
Black seed oil
The seeds of Nigella sativa, a medicinal herb, are used to make an oil that has been used in traditional medicine practices in parts of Asia and the Middle East. Pharmacological research has found that components of the oil have antimicrobial and anti-inflammatory properties.
Studies
There were no RCTs for black seed oil topical treatment before 2000.
One new study was reported after 2000. 209 This within-person study involving 20 people with eczema was conducted by Stern and Bayerl209 in Germany. An ointment containing 15% black seed oil was applied on one arm and the base treatment was applied on the other arm twice a day for 28 days. The application of the two treatments was randomised to the participants’ left or right side. No other creams or ointments were allowed on the arms during the study.
Assessment of risk of bias
Table 21 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Stern 2002209 | Unclear risk | Unclear risk | High risk | Black seed oil had a smell that was disliked by some participants and so it is likely that blinding was broken at least for some of the participants |
Benefits
A modified SCORAD score was used to record the overall severity of eczema; there was only a slight decrease in severity in both treatment groups, with no significant difference between the groups. The intensity of pruritus was measured on a VAS; there was hardly any change in either group compared with baseline and no significant difference between the groups at the end of treatment.
Harms
It was reported that there were no adverse events during the trial.
Overall implications for research and practice
It is likely that many of the participants, and possibly the outcome assessors, in this small trial209 will have known which treatment they were using. Neither the black seed oil treatment nor the base ointment changed the severity of eczema or pruritus levels. The smell of the black seed oil was reported as unacceptable. It seems that the addition of black seed oil to topical eczema treatments is not a good potential candidate for eczema treatment.
Rosmarinic acid
Rosmarinic acid is a plant-derived compound. Laboratory research has shown rosmarinic acid to have antiviral, antibacterial, anti-inflammatory and antioxidant properties. It is found in many culinary herbs such as peppermint and rosemary.
Studies
No trials on rosmarinic acid for eczema were reported before 2000.
One new trial was reported after 2000. 210 This study from Korea by Lee and colleagues210 compared a cream containing rosmarinic acid (0.3%) against the vehicle cream applied twice daily for 8 weeks in 21 participants. It is not clear whether this was a within-person trial or a parallel-group trial as the trial report describes the area being treated as ‘half an elbow flexure’.
Assessment of risk of bias
Table 22 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Lee 2008210 | Unclear risk | Unclear risk | Unclear risk |
Benefits
After treatment for 8 weeks the severity of eczema decreased from a mean SCORAD score of 7.37 to 3.27 in the rosmarinic acid group and from a mean SCORAD score of 6.49 to 5.63 in the placebo group. The individual symptom scores for erythema, oedema/papulation, oozing/crusting, lichenification and local pruritus were all reported to have significantly decreased after 8 weeks of treatment compared with baseline in the rosmarinic acid group. As no between-group comparisons were reported, it is not known whether rosmarinic acid showed a beneficial effect in comparison to the vehicle cream; however, nearly all severity and symptom scores for both groups were almost the same at 8 weeks.
Harms
The trial report states that there were no reactions to rosmarinic acid at 4 or 8 weeks and that no adverse reactions such as erythema, burning and pruritus were observed.
Overall implications for research and practice
The lack of methodological clarity and appropriate analyses of the results in this trial210 mean that the trial does not provide any evidence of a beneficial effect of rosmarinic acid.
Hippophae rhamnoides
Hippophae rhamnoides (common sea buckthorn) is a flowering plant native to Europe and Asia. Compounds derived from H. rhamnoides berries have been shown to have many different properties, including immunomodulatory and antimicrobial.
Studies
One trial involving H. rhamnoides for eczema was reported before 2000.
One new trial was reported after 2000. 211 This trial by Thumm and colleagues,211 conducted in Germany, included 58 Caucasian participants who were randomised to one of three creams for 28 days: H. rhamnoides (20%), H. rhamnoides (10%) or Miglyol® (CREMER OLEO) (placebo). The vehicle cream for all three treatments was based on beeswax, glycerine and paraffin.
Assessment of risk of bias
Table 23 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Thumm 2000211 | Unclear risk | Unclear risk | Unclear risk |
Benefits
The severity of eczema significantly improved in all three groups, but the degree of improvement appeared comparable (improvement of 10.98 points for H. rhamnoides (20%), 9.52 points for H. rhamnoides (10%) and 13.76 points for Miglyol cream), although this was not statistically analysed. These results were closely mirrored by the quality-of-life scores as well as participant-assessed signs and symptoms of eczema (redness, itching, dryness and general skin condition).
Harms
No information was given in the trial report about adverse events.
Overall implications for research and practice
This short-term trial211 did not show any significant benefit of adding H. rhamnoides to an emollient base.
Shale oil
Shale oil is produced when oil shale rocks are heated to a high temperature. The oil can undergo further processing to form sulfonated shale oil, which comes in dark and pale forms. It has been used topically for other skin diseases such as acne and psoriasis and some studies have shown it to have anti-inflammatory and antimicrobial properties.
Studies
No RCTs on shale oil for eczema were reported before 2000.
One new trial was reported after 2000. 212 This manufacturer-sponsored trial conducted in Germany by Korting and colleagues212 compared a cream containing sodium bituminosulfonate (4%) (Ichthosin® cream; Ichthyol-Gesellschaft) (pale sulfonated shale oil) with the vehicle cream in 99 children aged 0–12 years with mild to moderate eczema. All participants applied the treatments three times a day for 4 weeks and no concomitant medications were allowed during the trial. Skincare products were allowed on unaffected skin.
Assessment of risk of bias
Table 24 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Korting 2010212 | Low risk | Unclear risk | High risk |
Benefits
The severity of eczema was assessed using the authors’ own severity assessment of erythema, crusts, excoriations, scales, lichenification and itch, and the percentage area affected in each body site, combined into one score, with a maximum possible score of 45, as for other measures such as EASI or SCORAD. A statistically significant reduction in severity of 8.9 (SD ± 7.4) was found after 4 weeks of using the pale sulfonated shale oil cream compared with a reduction of 1.3 (SD ± 8.3) using the vehicle cream, which was also present after 1 week. Participant-assessed tolerability also showed a significant difference in favour of pale sulfonated shale oil, with 73 participants in the pale sulfonated shale oil group compared with 42 participants in the vehicle group rating the treatment as ‘very good’. All individual signs and symptoms assessed, including itch, were reported as significantly better in the pale sulfonated shale oil group from 2 weeks onwards.
Harms
There were no serious adverse events reported during this trial. The other adverse events reported resulted in the participants withdrawing from the study: two in the pale sulfonated shale oil group, because of pruritus, erythema and spreading of eczema, and four in the vehicle group, one because of bacterial superinfection and three who experienced itch plus worsening/spreading of eczema or erythema.
Overall implications for research and practice
Even though the two treatments were matched for colour, the slight odour of the shale oil cream means that there is a risk that participants and possibly the investigators who were outcome assessors were unblinded. Nevertheless, this trial212 demonstrated a reasonable beneficial effect of adding pale sulfonated shale oil to a topical cream for the treatment of mild to moderate eczema in Caucasian children, with no evidence of any particular adverse events. It is important that this trial is followed up with further long-term, large-scale research that incorporates a range of skin tones and pays attention to blinding the outcome assessors of the objective outcomes by making sure that the cream is not applied close to an assessment.
Vitreoscilla filiformis
Vitreoscilla filiformis is an aerobic bacterium found in sulfurous thermal springs. Laboratory research has shown that extracts from V. filiformis have anti-irritant properties.
Studies
No RCTs on V. filiformis for eczema were reported before 2000.
Two new trials were reported after 2000. 213,214 These two manufacturer-funded trials by Guéniche and colleagues213,214 compared a cream with V. filiformis lysate (5%) against vehicle cream. The first trial213 involved 13 participants with mild to moderate eczema, who used the treatments on either side of the body twice daily for 4 weeks. The second trial214 involved 75 participants (aged 6–70 years) with mild eczema and a history of atopy.
Assessment of risk of bias
Table 25 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Guéniche 2006213 | Unclear risk | Unclear risk | Low risk | |
| Guéniche 2008214 | Unclear risk | Unclear risk | Low risk | Areas to be treated were predefined; however, it is not clear whether this differed from one participant to another or whether the person predefining the treatment areas was aware of treatment allocation |
Benefits
In the first study213 a statistically significant but relatively small difference in the severity of eczema of around half a point using the EASI score (p = 0.012) and the modified EASI score (p = 0.008), which included itch scores, was reported after 28 days of treatment.
In the second, larger, study,214 the V. filiformis lysate cream showed a significant beneficial effect on eczema severity and pruritus after 30 days. Mean SCORAD scores at the end of treatment were 24.9 in the placebo group and 15.1 in the V. filiformis group (p = 0.004). Baseline scores were 29 (SD ±9.7) in the placebo group and 31 (SD ±11.9) in the V. filiformis group. The severity scores were analysed over time and also gave a significant result in favour of the bacterial lysate cream.
Harms
The first study213 reported the most common adverse event as pricking and burning sensations (at the same rate for both treatments). The authors suggest that this may have been caused by the composition of the vehicle cream, which was not designed specifically for eczema. The trial also reported that few participants reported dryness for both sides of the body.
Although it was reported in the second study214 that adverse events were monitored, no information was given on whether any occurred or not.
Overall implications for research and practice
These trials213,214 suggest a possible beneficial effect of V. filiformis on eczema severity. It is not clear if participants were allowed any other eczema treatments during the trials and so we are left guessing whether the beneficial treatment effect seen is the result of use of the V. filiformis lysate cream or an increase in the use of standard eczema treatments. Frustratingly, information on adverse event monitoring is not provided at all for the second, larger, trial, making it impossible to weigh up benefits compared with any potential harms. With so little practical information in the context of potential use in a clinical setting, more independent research with a longer treatment period, full information on any adverse events and a clear report of any concomitant eczema treatments used are needed before the true potential of this treatment can be assessed.
Miltefosine
Background
Miltefosine is a phospholipid analogue that was originally developed as a chemotherapy treatment. It has also been found to have antiprotozoal activity and it is effective against both visceral and cutaneous leishmaniasis.
Studies
No RCTs on miltefosine for eczema were reported before 2000.
One new trial was reported after 2000. 215 This part industry-sponsored within-person trial by Dolle and colleagues215 compared a solution containing miltefosine against a hydrocortisone solution for 3 weeks. The dose for both treatments was gradually increased from two drops per lesion once a day for the first week to two drops per lesion twice a day for the second and third weeks. Sixteen adults with moderate to severe eczema according to the criteria of the Hanifin and Rajka8 and having at least two comparable lesions were recruited.
Assessment of risk of bias
Table 26 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Dolle 2010215 | Unclear risk | Unclear risk | Unclear risk |
Benefits
The severity of the eczema lesions treated with hydrocortisone improved slightly more than the severity of the miltefosine-treated lesions after 3 weeks of treatment. The primary outcome of ‘improvement’ in eczema severity, defined as a > 1.5-point drop in Three-Item Severity (TIS)63 score, occurred in 10 out of 16 participants for the miltefosine-treated lesion. The results of the primary outcome for hydrocortisone treatment were reported only in a graphical form, although this appears to show a greater reduction in severity for the hydrocortisone group. Four weeks after treatment was stopped, the hydrocortisone-treated lesions increased in severity by a median of 0.5 TIS points to 2 (minimum 1.5, maximum 3), whereas the miltefosine-treated lesions decreased by a median of 2 TIS points to exactly the same TIS score as the hydrocortisone-treated lesions.
Harms
There were a relatively high number of local topical adverse events related to the treatments, with miltefosine treatment producing more of the adverse events (10/16 participants affected) than hydrocortisone treatment (7/16 participants affected). These adverse events included pruritus, burning, tingling and dry skin. Dry skin was seen only with miltefosine treatment. There were no withdrawals because of adverse events and no systemic adverse events.
Overall implications for research and practice
Although the trial authors focused on the deterioration of the hydrocortisone-treated lesions after treatment was stopped, the rate of deterioration was slow and the lesions only reached the same severity as those lesions treated with miltefosine. It is interesting that miltefosine seemed to show a perpetuating beneficial effect on the severity of eczema, although it was slower than the hydrocortisone treatment to take effect. With the lack of methodological clarity and wide CIs, the evidence for treatment of eczema with miltefosine is poor. This early trial has not provided a promising signal to justify further trials.
Opiate receptor antagonists
Opiate receptor antagonists have been used mainly as a treatment for alcohol and opioid dependence. As the opiate receptor antagonist naltrexone can suppress pruritus, it has been examined for its potential to treat pruritus associated with eczema.
Studies
No RCTs assessing opiate receptor antagonists for eczema were reported before 2000.
One new trial was reported after 2000. 216 This multicentre crossover trial by Bigliardi and colleagues216 in Germany compared naltrexone cream against vehicle cream to be applied for up to 28 days when the participants were experiencing intense symptoms of itching. Forty-five adults with eczema and experiencing bouts of itching of > 50 mm on a 100-mm VAS were randomised. All participants were allowed to use topical eczema treatments as rescue medication, except in an itching intensity monitoring period.
Assessment of risk of bias
Table 27 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Bigliardi 2007216 | Unclear risk | Unclear risk | Unclear risk |
Benefits
A 52.6-mm (26.7%) difference between the decrease in pruritus intensity score for the two treatments in favour of naltrexone was reported for the 40 out of 45 participants who made up the full analysis set. This was reported as a statistically significant treatment effect between the treatment groups of p = 0.047. No confidence limits were given for any of the results in the trial. The results of the per-protocol analysis of 39 participants were similar. This trial did not record the severity of eczema.
Harms
Information on adverse events was not reported.
Overall implications for research and practice
Although a statistically significant effect on pruritus appears to be a positive result, this poorly reported trial216 does not provide enough data to support the adoption of naltrexone. The severity of the eczema in the two treatment groups and the amount of topical corticosteroid used is not reported and, although pruritus levels tend to decrease as the severity of eczema decreases, this trial on its own is not robust evidence of a beneficial effect of naltrexone. Trials that measure the severity of eczema as well as pruritus and document any concurrent eczema treatment are needed.
Topical vitamin B12
Studies
No RCTs looking at topical vitamin B12 were reported before 2000.
Two new trials have been reported since 2000. 217,218 These two within-person trials,217,218 both industry funded, compared cyanocobalamin (0.07%) cream with a base cream. In the trial by Stücker and colleagues,217 48 adults aged 18–70 years applied the treatments twice a day for 8 weeks. In the trial by Januchowski,218 22 children aged from 6 months to 18 years (no severity inclusion criteria stated) were randomised to treatment for 4 weeks, but it was not clear how often they were instructed to use the treatment.
Assessment of risk of bias
Table 28 provides the risk-of-bias assessment for the new studies.
Benefits
The trial by Stücker and colleagues217 found that there was a significant decrease in eczema severity after 8 weeks of topical vitamin B12 treatment compared with the base cream, as measured using a modified SASSAD score (maximum of 240 points). The mean decrease for topical vitamin B12 was 55.34 (standard error of the mean 5.74) whereas the mean decrease for the base cream was 28.87 (standard error of the mean 4.86; p < 0.0002). This significant difference was seen from week 4 onwards. Participant- and investigator-assessed efficacy both showed a significant difference in favour of topical vitamin B12 treatment after 8 weeks when the responses were grouped as ‘effective’ or ‘non-effective’ (p < 0.005).
The trial by Januchowski218 also reported a significant decrease in eczema severity, measured using modified SCORAD scores (maximum of 27 points), for topical vitamin B12 cream compared with the base cream after 4 weeks of treatment. Taking data from a graph, the decrease in total SCORAD scores for topical B12 treatment after 4 weeks was 4.5 whereas that for the placebo treatment was 1.7 (p = 0.011). The objective SCORAD score mirrored this result and the subjective SCORAD score also showed a significant difference, although not as pronounced, by week 4.
Harms
In the trial by Stücker and colleagues,217 33 cutaneous events were reported, which were all mild except for one, which involved a moderate reaction after applying the placebo cream. Of these cutaneous events, four cases were considered ‘probably related’ and two cases were ‘possibly related’ to the application of vitamin B12.
In the trial by Januchowski,218 only one adverse event was reported, which resulted in withdrawal from the trial. The participant had a reaction to both the active and placebo treatments but the nature of the reaction was not reported.
Overall implications for research and practice
The two trials217,218 appear to show a significant beneficial effect of topical vitamin B12 cream on the severity of eczema. The lack of CIs for the results presented in both trials means that there is no way to judge the statistical robustness of the results. There were a number of adverse events relating to skin irritation, probably caused by topical vitamin B12. Whether or not to try this topical treatment will probably remain a decision based on individual circumstance and preference.
WBI-1001
Studies
No RCTs looking at WBI-1001 were reported before 2000.
Two new trials were reported after 2000. 219,220 The trial by Bissonnette and colleagues in 2010,219 reported as a research letter, compared the synthetic compound 2-isopropyl-5-[(E)–2-phenylethenyl] benzene-1,3-diol (WBI-1001; Welichem Biotech Inc.) at both 0.5% and 1.0% concentrations against vehicle cream. The 37 participants had eczema scores of ≤ 12 on the EASI scale and scored 2 (mild) or 3 (moderate) for IGA; they were randomised to one of the three treatment groups and applied the treatment twice daily for 4 weeks.
Bissonnette and colleagues220 published a second trial in 2012, again comparing the same topical treatment WBI-1001 at 0.5% and 1% concentrations with vehicle as the placebo. This was a larger and slightly longer trial. In total, 148 adults aged 18–65 years with ‘chronic’ eczema for ≥ 6 months, diagnosed according to the criteria of Hanifin and Rajka,8 a body surface area of 3–20% and an eczema severity of mild to severe (IGA 2–4) were included.
Assessment of risk of bias
Table 29 provides the risk-of-bias assessment for the new studies.
Benefits
The earlier trial by Bissonnette and colleagues219 stated that all efficacy analyses were not planned before database lock and so should be considered post hoc. Change in the severity of eczema from baseline measured by IGA, SCORAD and EASI showed significant differences in favour of WBI-1001. For the IGA after 4 weeks’ treatment, the percentage decreases from baseline were 5.6% for placebo, 38.9% for WBI-1001 0.5% and 45.8% for WBI-1001 1%. Active treatment (WBI-1001 0.5% and 1.0%) was significantly beneficial compared with placebo (p = 0.003). The severity of eczema measured by EASI and SCORAD closely mirrored these results. Body surface area also showed almost the same significant percentage decreases by week 4. Pruritus scores showed the same pattern but no between-group analyses were reported.
The larger trial by Bissonnette and colleagues in 2012220 reported a significant reduction in pruritus at day 42 from baseline for the WBI-1001 0.5% group (29.8%) and the WBI-1001 1.0% group (66.9%) compared with placebo (9.5%) (p < 0.001 for both WBI-1001 treatments). There was also a significant reduction in eczema severity at day 42 from baseline, measured using IGA, for both of the WBI-1001 treatments (0.5% and 1.0%) compared with placebo (WBI-1001 0.5%: –1.3 SD ± 0.97, 95% CI –1.2 to –0.5; WBI-1001 1.0%: –1.8 SD ± 1.02, 95% CI –1.6 to –0.9; placebo: –0.5 SD ± 0.89, 95% CI was not reported) (p < 0.001 for both WBI-1001 treatments). Significant reductions in eczema severity as measured by EASI and SCORAD were also reported for the WBI-1001 creams compared with placebo.
Harms
In the earlier trial by Bissonnette and colleagues219 there were no serious adverse events and no withdrawals as a result of adverse events. One participant in the WBI-1001 group had a T-wave anomaly, although it is not reported if this was related to treatment. Two participants in the placebo group and one in the WBI-1001 group had mild papules and two participants in the placebo group had pruritus.
Two serious adverse events were reported in the trial by Bissonnette and colleagues220 published in 2012: one case of cellulitis in the WBI-1001 0.5% group and one case of acute cholecystitis in the WBI-1001 1.0% group. Neither of these events was reported as being related to study treatment. Nine participants stopped treatment because of adverse events. In the placebo group four events were eczema and one was worsening eczema. Two events, one of eczema and one of contact dermatitis, were reported in the WBI-1001 0.5% group and two events of contact dermatitis were reported in the WBI-1001 1.0% group.
Overall implications for research and practice
The small Phase 2 trial,219 which did not prespecify the efficacy outcomes reported, offered a hint that it may be worth carrying out a larger, long-term Phase 3 trial of WBI-1001. The trial was not designed to determine clinical efficacy and included a small number of participants. The same group conducted a larger, slightly longer-term trial aimed at assessing the clinical benefits and harms of WBI-1001 and found clear evidence of benefit compared with placebo for clinician-assessed severity of eczema. The trial did not use any patient-reported outcome measures such as itching as separate outcomes. Longer trials that use a pragmatic comparator such as topical corticosteroids or topical immunomodulators and measure patient-reported outcomes are now needed to be clearer about whether this treatment could be useful in routine clinical care. Adverse events such as contact dermatitis should also be assessed in more detail.
Carbohydrate-derived fulvic acid
Fulvic acids are formed during organic matter biodegradation. A few studies have investigated the antifungal and antibacterial properties of fulvic acids, but this has mainly been in laboratory settings.
Studies
No RCTs of carbohydrate-derived fulvic acid treatments for eczema were reported before 2000.
Gandy and colleagues221 compared carbohydrate-derived fulvic acid in an emollient base with the emollient base only in 36 participants aged > 2 years. The participants applied the emollient twice daily for 4 weeks on the affected area. All participants were allowed to use Epizone (VanDyk Pharmaceutical Products) (an emollient buffered with acetic acid) as needed.
Assessment of risk of bias
Table 30 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Gandy 2011221 | Unclear risk | Unclear risk | Unclear risk | Two participants who completed the trial were excluded from the analysis for use of concomitant medication |
Benefits
Although the report states that there were statistically significant decreases in the carbohydrate-derived fulvic acid group for investigator global response to treatment, investigator-assessed severity of disease and participant-assessed severity of disease, there were also significant decreases in the emollient placebo group. The trial does not report between-group differences but it seems unlikely from the data that there were any statistically significant differences between the treatments.
Harms
The only adverse event reported was a short-lived burning sensation after treatment, although the report does not state which treatment was involved.
Overall implications for research and practice
This short trial without a clear methodology seems to show no additional benefit of adding carbohydrate-derived fulvic acid to emollient treatment. The trial did not include two participants in the final analyses as they used concomitant treatments. The extent to which participants and investigators, both of whom were the outcome assessors, were blinded is not known. Perhaps reassuringly, this trial adds to evidence that regular emollient use improves the severity of eczema.
Protease inhibitor SRD441
Studies
No RCTs looking at the topical matrix metalloproteinase and aureolysin inhibitor SRD441 for eczema were reported before 2000.
One new trial was reported after 2000. This industry-funded study by Foelster-Holst and colleagues222 compared SRD441 against vehicle in 93 adults with mild to moderate atopic eczema confirmed by a dermatologist. The participants used SRD441 cream (1 mg/g) or vehicle cream on all affected and commonly affected areas twice a day for 28 days. The trial was run in 13 centres in Germany, Bulgaria and Finland.
Assessment of risk of bias
Table 31 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Foelster-Holst 2010222 | Low risk | Low risk | Unclear risk | The extent to which any of the stakeholders were blinded is unclear |
Benefits
There was no significant difference between the treatments for the primary outcome of rate of ‘success’ (defined as an IGA score of 0 or 1) at day 21. There were also no significant differences in any of the secondary outcomes, which included time to resolution of the primary exacerbation (IGA score of 0 or 1), IGA score (all visits), participant-assessed total pruritus over the previous 24 hours, number of participants requiring rescue medication and quality of life measured using the Dermatology Life Quality Index (DLQI).
Harms
In total, 60.0% (n = 27) of the SRD441 group and 70.8% (n = 34) of the vehicle group experienced adverse events. Of these, 40.0% (n = 18) in the SRD441 group and 58.3% (n = 28) in the vehicle group were possibly or probably related to study treatment. The adverse events related to treatment were mostly application site reactions and occurred at roughly equal rates in both groups. Seven participants in the SRD441 group and 11 participants in the vehicle group withdrew because of adverse events. The main reason for withdrawal from treatment was application site reactions.
Overall implications for research and practice
This is a clearly reported and methodologically sound trial. 222 Both the vehicle control and the study treatment were poorly tolerated, precipitating a higher than expected withdrawal rate because of adverse events. Although problems with the vehicle control may have masked any potential beneficial effects elicited by SRD441, this trial shows no evidence of benefit for SRD441 in the treatment of eczema.
Raffinose
Raffinose is an indigestible oligosaccharide composed of galactose, fructose and glucose that is abundant in plants, with high concentrations found in legumes and whole grains.
Studies
No RCTs looking at the oligosaccharide raffinose for eczema were reported before 2000.
One new trial was reported after 2000. 223 This trial, conducted in France by Misery and colleagues,223 compared a lipiderm cream with raffinose (1%) added (Tefirax®; Laboratoire G-pharm) against the lipiderm cream alone. Participants were instructed to apply as much as necessary for 3 days and as needed for persistent symptoms of pruritus. The 11 adults in the trial all had eczema and current pruritus.
Assessment of risk of bias
Table 32 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Misery 2005223 | Unclear risk | Unclear risk | Unclear risk |
Benefits
The short trial report223 does not give any detailed data apart from a graph of pruritus intensity on the first application and a graph of the mean pruritus intensity for all applications of treatment (67 applications between 11 participants). After treatment, six out of 11 participants reported a benefit from the cream containing raffinose, four participants reported a benefit from both treatments and one participant reported no benefit from either treatment. The report states that the study is too small to analyse whether the treatment showed significant benefit and no between-group analyses were reported. Although the primary outcome results (intensity of pruritus) were presented, the results of other specified outcomes were not reported.
Harms
The report states that the cream containing raffinose produced application site burning.
Overall implications for research and practice
It is debatable whether a crossover trial223 of 11 participants, which the trial authors admit was not appropriately sized to assess the treatment’s potential benefits, just serves to confuse more than aid those looking for evidence of treatment benefits. If the relative merits or otherwise of topically applied raffinose are to be considered at all, an appropriately designed trial will need to be conducted.
Atopiclair
Atopiclair is medical device emollient cream containing 2% glycyrrhetinic acid and shea butter as well as other ingredients in a hydrolipid base.
Studies
There were no trials on Atopiclair reported before 2000.
Five new trials were reported after 2000. 199,224–227
Children
One trial conducted in Italy by Patrizi and colleagues226 compared Atopiclair™ (Graceway Pharmaceuticals), Atopiclair light (oil-in-water formulation containing a lower concentration of key ingredients) and vehicle for the treatment of childhood eczema. The 60 participants aged 2–17 years with mild to moderate eczema applied the treatments three times a day for 43 days.
A larger trial by Boguniewicz and colleagues227 also compared Atopiclair against vehicle using the same treatment regimen (three times a day for 43 days). In this larger trial, 142 children aged from 6 months to 12 years with mild to moderate eczema applied the treatments to affected areas and those areas likely to be affected during the trial.
A 3-week trial by Miller and colleagues,199 conducted in the USA, compared Atopiclair with EpiCeram™ (Ceragenix Pharmaceuticals) and Aquaphor healing ointment® (Beiersdorf). Thirty-nine children aged 2–17 years with mild to moderate eczema applied the treatments three times a day using the smallest amount needed.
Adults
Two trials, one with 30 participants224 and one with 218 articipants,225 both with very similar methodologies, compared Atopiclair against a vehicle cream (placebo) in adults, who were instructed to apply the cream three times a day. In the small study by Belloni and colleagues,224 participants applied the treatments for 5 weeks. Only those with light/fair skin without a recent suntan and a Rajka and Langeland228 diagnostic criteria score of 3.0–7.5 were enrolled.
Assessment of risk of bias
Table 33 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Children | ||||
| Boguniewicz 2008227 | Low risk | Unclear risk | Unclear risk | |
| Miller 2011199 | Unclear risk | Unclear risk | Low risk | |
| Patrizi 2008226 | Low risk | Unclear risk | Low risk | |
| Adults | ||||
| Abramovits 2006225 | Low risk | Unclear risk | Unclear risk | |
| Belloni 2005224 | Low risk | Low risk | Low risk | Power calculations to determine study size were not performed |
Benefits
Children
The smaller multicentre trial by Patrizi and colleagues226 showed a significant difference in the primary outcome of ‘success’ (defined as an IGA score of 0 or 1) after 22 days of treatment. The Atopiclair group had an 80% (16/20) ‘success’ rate compared with 16.6% (3/20) for the Atopiclair light group and 26.3% (5/20) for the vehicle group. Atopiclair had significantly more successes than the vehicle treatment (p < 0.0001) and Atopiclair light (p = 0.001). The change in participant-assessed pruritus from baseline was significant only for Atopiclair light compared with vehicle (p < 0.05 using Tukey’s test). The data on pruritus were reported only in a graph, which showed roughly a mean decrease from baseline of 24 points for Atopiclair compared with 1.5 for Atopiclair light and no discernible decrease or increase in the vehicle group.
In the trial by Boguniewicz and colleagues,227 for all of the secondary end points, apart from the need for rescue medication, there was a statistically significant difference between the two treatment groups at all time points. The mean change in severity of eczema measured using EASI at day 22 was – 5.15 SD ± 7.24 in the Atopiclair group and 0.84 SD ± 3.52 in the placebo group.
The severity of eczema measured using IGA at day 22 (primary end point) was significantly reduced in the Atopiclair group compared with the vehicle group (p < 0.001). For the 26 out of 139 participants (18.7%) in the whole population who needed rescue medication to treat a flare (six participants in the Atopiclair group and 20 participants in the vehicle group), the mean duration of rescue treatment was 4.17 days (range 3–8 days) in the Atopiclair group and 5.65 days (range 2–12 days) in the vehicle group.
In the trial by Miller and colleagues199 there was a very clear lack of statistical significance between the three emollients after 21 days of treatment. The trial measured eczema severity using the IGA, EASI and participant-assessed global assessment of improvement. Itching also did not show any statistically significant differences between Atopiclair and EpiCeram or the Aquaphor healing ointment. The trial also looked at costs and reported that the Aquaphor healing ointment was 47 times more cost-effective than Atopiclair and EpiCeram. All treatments had a 15–40% ‘success’ rate (0 or 1, clear or almost clear IGA, ≥ 75% improvement in EASI score from baseline) after 21 days of treatment.
Adults
In the trial by Belloni and colleagues,224 the affected area and itch score were improved after 21 days of Atopiclair treatment compared with baseline values. No between-group comparison was performed.
A much larger, multicentre trial was conducted by Abramovits and colleagues,225 enrolling participants with mild to moderate eczema on the Rajka and Langeland228 scale and scoring at least 40 mm out of 100 mm on a VAS for itch. After 50 days, the end of the trial, there was a significant difference in the level of itch in a target lesion in favour of Atopiclair. The Atopiclair group reduced by 58 mm and the vehicle group reduced by 20 mm on the VAS (p < 0.0001). The percentage body surface area affected improved significantly more in the Atopiclair group throughout the trial. The severity of eczema measured using the EASI score (mean ± SD) improved significantly using Atopiclair treatment (3.82 ± 3.44) compared with the vehicle treatment (0.15 ± 4.78; p < 0.0001) at day 22. The mean difference was –3.67 (95% CI –4.789 to –2.543; p < 0.0001) at day 22, but this was also significant from day 8 throughout the trial.
Harms
Children
The trial by Patrizi and colleagues226 reported that 10% of the participants in each of the two Atopiclair treatment groups and 20% of the vehicle group experienced at least one adverse event. One adverse event in the Atopiclair group was judged to be probably related to treatment and two events in the vehicle group were judged to be possibly related. Five out of the nine adverse events reported resulted in the treatment being stopped. There were no serious adverse events reported for this trial. In the trial by Boguniewicz and colleagues227 there was an average of 0.83 events per participant in the Atopiclair group compared with 0.80 events per participant in the vehicle group. Adverse events judged to be probably related to treatment were reported in 16.6% of the vehicle group compared with 12.5% of the Atopiclair group. There were no serious adverse events thought to be related to treatment. In the trial by Miller and colleagues199 no adverse events were observed.
Overall implications for research and practice
Three of the five trials show a significant improvement in IGA for participants treated with Atopiclair compared with those treated with vehicle, with two also reporting an improvement in EASI score. 225–227 No conclusions can be drawn from the trial by Belloni and colleagues224 as no between-treatment comparison was performed. One trial showed no difference between Atopiclair and two other emollients for IGA and EASI. 199
Overall, there is reasonable evidence of benefit for Atopiclair compared with vehicle. Further trials comparing Atopiclair against other active treatments are required and these should ideally be independent from the manufacturers of any interventions involved.
Farnesol and xylitol
Farnesol is an acyclic sesquiterpene alcohol that occurs naturally in plants and is present in many essential oils, such as citronella and neroli; research has also shown it to have antibacterial properties. Xylitol is a sugar alcohol, commonly used as a sweetener, which has been found to have antibacterial properties. Laboratory research has shown that a combination of farnesol and xylitol can inhibit the growth of some bacteria.
Studies
No RCTs looking at farnesol or xylitol for eczema were reported before 2000.
One new trial was reported after 2000. 229 This within-person trial by Katsuyama and colleagues229 compared an oil-in-water 17% moisturiser cream containing 0.02% farnesol and 5% xylitol against the oil-in-water 17% moisturiser cream only. The treatments were applied for 7 days by 17 participants to their left and right forearms.
Assessment of risk of bias
Table 34 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Katsuyama 2005229 | Unclear risk | Unclear risk | Unclear risk |
Benefits
Although this trial was focused on physiological indicators of efficacy and numbers of S. aureus, changes in dryness, redness, excoriation, scaling and papules were assessed by a dermatologist. The trial reported no significant differences between the treatments; however, no detailed data were presented in the report.
Harms
It was reported that no adverse events occurred during the trial.
Overall implications for research and practice
The changes in dermatologist-assessed skin condition were not significantly different between the two treatments.
Levomenol and heparin
Levomenol (bisabolol) is a monocyclic sesquiterpene alcohol present in many essential oils. It is one of the primary components of chamomile (Matricaria chamomilla) essential oil. Levomenol can cause contact dermatitis in some people but laboratory studies have shown it may have anti-inflammatory, anti-irritant and antimicrobial properties. Heparin is most commonly used as anticoagulant (a blood thinner) to prevent the formation of blood clots, but has been investigated for its potential to treat many allergic diseases. It has been suggested that it has a role in defence against invading microbes and other foreign substances.
Studies
No RCTs that assessed a combination of levomenol and heparin for eczema were reported before 2000.
One new trial was reported after 2000. This trial by Arenberger and colleagues, published separately in both German230 and English,231 compared a cream containing a combination of levomenol and heparin with a cream containing levomenol alone, a cream containing heparin alone and a cream without any active ingredient.
Assessment of risk of bias
Table 35 provides the risk-of-bias assessment for the new study.
Benefits
The primary outcome of intensity of itching (on a VAS) was significantly reduced in the combined levomenol and heparin group compared with the levomenol, heparin and vehicle groups. The most significant difference (mean ± SD) was between the vehicle group and the combined levomenol and heparin group at week 8 (24.3 mm ± 2.1 mm, 95% CI 20.2 mm to 28.5 mm), although the two separate levomenol and heparin groups were not compared against vehicle. The severity of eczema measured by SCORAD mirrored the pruritus results, with the most significant difference (mean ± SD) being between the combined levomenol and heparin group and the vehicle group after 8 weeks of treatment (14.6 ± 1.3, 95% CI 12.8 to 17.1). The participants were asked to rate the efficacy of the treatment on a 4-point scale and the combined treatment had the highest percentage of assessments rated as ‘good’ or ‘very good’ (97%). This assessment was not analysed across treatment groups, except in a subgroup of children aged 0–12 years, with 100% in the combined group and 42.9% in the vehicle group assessing the treatment as ‘good’ or very good’ (p = 0.002). It is not clear whether this subgroup was prespecified or not from the trial report.
Harms
One participant in the heparin group reported a transient increase in itching, which the investigators assessed as most likely being caused by the participant’s eczema. No other adverse events were reported.
Overall implications for research and practice
There is reasonable evidence from this one trial230,231 of benefit from combined heparin and levomenol treatment compared with vehicle and some evidence that the combination is significantly more effective than each of the treatments given separately. The subgroup analysis of children aged ≤ 12 years must be treated with caution as the numbers of participants were low and it is not clear whether this was a post hoc analysis or not. It may be worth comparing this treatment against a more pragmatic comparator of ‘standard care’ with emollients and topical corticosteroids to obtain a clearer picture of its potential benefit.
Bacterial antigens
Lantigen B (Bruschettini Srl) is a mixture of the lysate of six (inactivated) bacterial strains that commonly cause respiratory tract infections.
Studies
No RCTs that assessed a topical bacterial antigen suspension for eczema were reported before 2000.
One new trial was reported after 2000. 232 This trial by Mora and colleagues232 compared a topical suspension containing Lantigen B against a placebo solution used twice a day for 3 months on the eczema lesions, using one drop per year of age. Eighty children aged between 2 and 6 years with external auditory eczema lesions were randomised.
Assessment of risk of bias
Table 36 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Mora 2004232 | Unclear risk | Unclear risk | Unclear risk |
Benefits
The trial report states that the clinical efficacy scores were lower for the antigen suspension than for the placebo in the second study period, which is possibly referring to the period of 1 year after finishing treatment, but no values were reported. For the 3-month treatment period, the clinical efficacy score decreased from 7.1 to 3.4 in the antigen group and from 7.3 to 6.4 in the placebo group; however, the difference between the groups was not statistically analysed.
Harms
The authors reported that no participants experienced side effects.
Overall implications for research and practice
The trial report232 does not give enough detail about the trial methodology and results to be able to gather any good evidence of benefits or harms of this topical bacterial antigen treatment.
Chamomile extract
Chamomile contains a number of different chemicals thought to have biological activity, including bisabolol, which was discussed earlier in this chapter.
Studies
No trials of chamomile extract for eczema were published before 2000.
One new trial involving camomile extract was reported after 2000. 233 This within-person trial by Patzelt-Wenczler and Ponce-Poschl233 randomised 72 participants with eczema whose severity was described as ‘at least moderate’. Participants applied Kamillosan® cream (Dales Pharmaceuticals Ltd) (containing 2% ethanolic extract of chamomile flowers), vehicle or hydrocortisone twice daily to a specified arm. The severity of eczema was recorded as the sum of the pruritus, erythema and desquamation scores. The IGA scores for each arm separately were also recorded. Five individual signs of oedema, papules/pustules, lichenification, excoriation and fissures were each assessed on a 4-point scale and combined into one score. Adverse events were also measured. It is not clear how long the participants used the interventions for; however, the participants were followed up for 2 weeks from baseline.
Assessment of risk of bias
Table 37 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Patzelt-Wenczler 2000233 | Unclear risk | Unclear risk | High risk | It is likely that the participants knew which intervention was which because of the difference in colour and aroma between Kamillosan and the placebo or hydrocortisone |
Benefits
The trial report233 does not compare the interventions against each other for any of the outcomes measured. The assessment of Kamillosan, hydrocortisone or the vehicle cream, which has emollient properties in its own right, did not show any big differences in effect apart from hydrocortisone not performing quite as well as the other two comparators. This is not surprising as the potency of the hydrocortisone used (0.5%) falls well below the potency required to effectively treat nearly all cases of eczema.
Harms
The only information given on adverse events in this trial was that three participants in the combined Kamillosan/placebo group withdrew early because of intolerability.
Overall implications for research and practice
This trial fails to compare the interventions and hence does not provide any evidence regarding the use of Kamillosan for eczema. A methodologically robust trial of Kamillosan compared with other topical treatments that pays greater attention to recording adverse events is needed to better inform the many people with eczema who buy this relatively expensive treatment over the counter and the clinicians who are asked to give advice on this treatment.
Camellia oil
Camellia (japonica) oil is derived from the seeds of Camellia japonica, a flowering shrub found in parts of East Asia. It is frequently used in cosmetics, including those designed for use on the skin.
Studies
No trials involving camellia oil were published before 2000.
One new trial involving camellia oil was published after 2000. 234 This small crossover study by Hamada and colleagues234 investigated the use of a spray containing camellia oil against a spray containing purified water. Forty-two participants with eczema described as ‘less than moderate’ used the spray in addition to their usual care, as desired, for 2 weeks. After 2 weeks, participants switched to the other spray.
Assessment of risk of bias
Table 38 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Hamada 2008234 | Unclear risk | Unclear risk | High risk | The trial was reported as double blind; however, camellia oil is pale yellow and has an aroma and so it is unlikely that blinding was totally successful |
Benefits
The camellia oil spray showed significant benefits for itching (p < 0.01) and moisturising (p < 0.01) compared with the purified water spray. The amount of ointment being used was significantly decreased when using camellia oil.
Harms
There were no adverse events reported in this trial. 234
Overall implications for research and practice
This one small trial234 hints at the potential benefit of this treatment and has not reported any adverse events. A large trial that addresses the issue of blinding an intervention that has a distinctive aroma is needed to assess this treatment further.
Cipamfylline cream
Cipamfylline is a theophylline analogue that acts as an inhibitor of phosphodiesterase-4, which is found in high levels in the leucocytes of people with eczema.
Studies
There were no trials of topical cipamfylline cream reported before 2000.
One new trial of cipamfylline cream has been reported since 2000. 124 This trial by Griffiths and colleagues124 compared cipamfylline cream (1.5 mg of cipamfylline per gram of cream) used up to a maximum of 2 g of cream per day against hydrocortisone 17-butyrate cream (0.1%) or vehicle of cipamfylline. The 103 adults with eczema according to the Hanifin and Rajka8 criteria, who had stable symmetrical lesions on the arms, applied up to 2 g of study treatment per day for 14 days.
Assessment of risk of bias
Table 39 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Griffiths 2002124 | Low risk | Unclear risk | Low risk |
Benefits
For the primary outcome of eczema severity measured using a total severity score, cipamfylline cream was significantly more effective than vehicle after 14 days (mean difference 1.67, 95% CI 1.06 to 2.28; p < 0.001) and hydrocortisone 17-butyrate was significantly more effective than cipamfylline cream (mean difference –2.10, 95% CI –2.93 to –1.27; p < 0.001). For both the investigator- and the participant-assessed overall response, the cipamfylline cream was significantly more effective than the vehicle and hydrocortisone 17-butyrate cream was significantly more effective than the cipamfylline cream. For participant-assessed pruritus after 14 days, the cipamfylline cream was significantly more effective than the vehicle and hydrocortisone 17-butyrate cream was significantly more effective than the cipamfylline cream. The participants found the hydrocortisone 17-butyrate cream to be significantly more cosmetically acceptable than the cipamfylline cream and the cipamfylline cream significantly more acceptable than the vehicle. For those who only needed emollient on treated areas after 14 days of study treatment, there was no significant difference in the relapse rate after 7 days for cipamfylline cream compared with vehicle. The relapse rate was significantly lower after hydrocortisone 17-butyrate cream compared with cipamfylline cream (p = 0.022).
Harms
There was no difference in cutaneous adverse events assessed as possibly or probably related to trial treatment on the treatment sites in either group (p = 0.13 for both treatment comparison groups). In the cipamfylline/vehicle comparison group, 29 (55.8%) participants reported 63 adverse events in total. In the hydrocortisone/cipamfylline group, 20 (40.8%) participants reported 41 adverse events in total. The adverse events were mostly application site reactions, including itching, stinging or burning, and drug reactions.
Overall implications for research and practice
This trial has clearly placed cipamfylline cream as less effective than hydrocortisone cream but more effective than vehicle for both participant-assessed and objective outcomes. The methodology of the trial was fairly clear and robust enough to exclude further testing of topical cipamfylline cream. Topical cipamfylline may have some limited short-term benefits for those with difficult to manage eczema because of steroid phobia, steroid resistance or contraindications for steroids, but it should be used with caution as there are no data on the safety of long-term treatment with this cream.
Lipoxin A4
Lipoxin A4 antagonises many cell responses evoked by pathogens and pro-inflammatory mediators, acting to counter-regulate inflammation. Lipoxin A4 also inhibits the production of leukotriene and interleukins. Lipoxin A4 stable analogues have been designed to mimic this function; one such drug is 15(R/S)-methyl-lipoxin A4.
Studies
There were no trials of lipoxin A4 reported before 2000.
One new trial conducted in China by Wu and colleagues235 compared 15(R/S)-methyl-lipoxin A4 0.1% cream against mometasone furoate 0.1% cream and also a placebo of distilled water in 1% dimethyl sulfoxide mixed with the identical cream base as used for the 15(R/S)-methyl-lipoxin A4. All treatments were applied to the face twice a day for 10 days using cotton sticks. Sixty participants with infantile acute or subacute facial eczema were randomised, 20 to each group.
Assessment of risk of bias
Table 40 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Wu 2013235 | Low risk | Unclear risk | Unclear risk | Two out of 60 participants were excluded from the analyses as they used concomitant medication |
Benefits
The efficacy of 15(R/S)-methyl-lipoxin A4 cream was not directly compared with that of mometasone furoate cream or placebo in the trial report, making it impossible to assess the results of the trial. It was reported that of the six components of the Severity Scale Score, the 15(R/S)-methyl-lipoxin A4 cream significantly reduced erythema and pruritus/scratching at day 3, papulation, vesiculation and scaling at day 5 and lichenification at day 10 compared with baseline. Mometasone furoate cream significantly reduced erythema, papulation, vesiculation, scaling and pruritus/scratching at day 3. Placebo significantly reduced scaling at day 5.
Harms
No clinical adverse events were reported and none of the safety tests, including full blood count and kidney and liver function tests as well as an electrocardiogram, showed any significant differences compared with baseline for all three treatment groups.
Overall implications for research and practice
There is no attempt to compare the results of one treatment against another in this trial report. An appropriate analysis of between-group differences is needed before being able to assess this potential treatment.
N-acetyl-L-hydroxyproline
N-acetyl-L-hydroxyproline has previously been used to treat rheumatoid arthritis and osteoarthritis, both orally and topically. The effects when used topically for eczema have not been ascertained.
Studies
There were no trials of N-acetyl-L-hydroxyproline reported before 2000.
One new trial of N-acetyl-L-hydroxyproline has been reported since 2000. 236 This within-person trial conducted in Japan randomised 15 adults with slight eczema as assessed by a dermatologist according to the guidelines of the Japanese Dermatological Association to N-acetyl-L-hydroxyproline 1% cream and the vehicle cream for 4 weeks, with the treatments being applied twice daily to each forearm.
Assessment of risk of bias
Table 41 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Hashizume 2013236 | Unclear risk | Unclear risk | Unclear risk |
Benefits
The only clinically relevant efficacy outcome assessed was the reduction in pruritus, assessed using a 100-mm VAS; however, the change in pruritus was not compared between the two treatments. The control treatment resulted in a reduction in pruritus from 27.1 SD ± 5.9 mm at baseline to 19.6 SD ± 6.0 mm at 4 weeks whereas the N-acetyl-L-hydroxyproline treatment resulted in a reduction in pruritus from 27.8 SD ± 5.9 mm at baseline to 16.4 SD ± 5.0 mm at week 4. The only scores to be statistically compared between groups were the pruritus scores at 4 weeks (p = 0.07).
Harms
It was reported that there were no adverse events, defined as no inflammation, no irritation and no allergic reactions, during the trial.
Overall implications for research and practice
This trial report does not appropriately compare the results of the two treatment groups and so does not provide any evidence about the comparative effectiveness of this potential treatment.
Nalmefene hydrochloride monohydrate (SRD174)
Nalmefene hydrochloride monohydrate (SRD174) is a µ-opioid receptor antagonist. It is similar to naltrexone but has a greater affinity for opioid receptors.
Studies
There were no trials of nalmefene hydrochloride monohydrate (SRD174) reported before 2000.
A new crossover trial compared SRD174 cream against vehicle cream. 237 The participants had to have active and pruritic eczema covering a body surface area of ≤ 20% and at least three episodes of moderate to severe pruritus defined as ≥ 40 on a 101-point VAS in the 7 days prior to randomisation. The study treatments were applied when a participant experienced an itch of ≥ 40 on a 101-point VAS during two treatment periods of 7 days each. The 62 participants randomised had to identify a target area of highest intensity and treat both the target area and other areas of bothersome itch. Participants could treat more than one episode of itch in a day provided that the episodes were > 8 hours apart and the total amount of study drug used in a day was less than one 10-g tube.
Assessment of risk of bias
Table 42 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Herzog 2011237 | Low risk | Unclear risk | Unclear risk |
Benefits
The primary outcome of the sum of pruritus intensity difference between 0 and 4 hours was 210.7 (SD ± 20.4) in the SRD174 group and 212 (SD ± 20.2) in the vehicle group, a difference of –1.3 (95% CI –25.9 to 23.3; p = 0.91).
None of the secondary efficacy end points tested (sum of pruritus intensity difference between 0 and 8 hours, pruritus intensity difference at each assessed time point) demonstrated a statistically significant or clinically important difference between the test product and the vehicle. Change in EASI score during each time period, IGA during each treatment period, quality of sleep recorded during each treatment period, pruritus relief, time to achieve > 30%, > 50% and 80% reduction in itch sensation, time to achieve a reduction in itch sensation to below a VAS score of 40 and use of rescue medication for pruritus were also not significantly different between the groups.
Harms
There was a higher incidence of adverse events in the SRD174 group than in the vehicle group: 22 (36.7%) participants reported a treatment-emergent adverse event in the SRD174 group and 14 (23.3%) participants reported a treatment-emergent adverse event in the vehicle group.
Overall implications for research and practice
This trial, although lacking some methodological clarity, does not provide any supportive evidence of a potential benefit for this treatment for any of the large number of outcomes. The trial authors are also clear in the report that there is no evidence of benefit for this treatment. It is probably not worth pursuing this treatment further.
Licochalcone A
Studies
There were no trials of licochalcone A reported before 2000.
A new within-person trial by Udompataikul and Srisatwaja238 compared a cream containing licochalcone A (0.025%) in a ceramide and linoleic acid lipid base (12% omega-6-fatty acids, 0.05% ceramide and 10% glycerine) for 6 weeks against hydrocortisone 1% lotion for 4 weeks followed by 2 weeks of ‘cream base’, which was not described further. Thirty children aged 2–15 years with mild to moderate eczema (SCORAD 1–40) that was present in the flexures on both sides of the body applied each treatment to one side of the body twice daily. Before starting treatment, those taking oral treatments had a washout period of 4 weeks and those using topical treatments had a washout period of 2 weeks.
Assessment of risk of bias
Table 43 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Udompataikul 2011238 | Unclear risk | Low risk | Unclear risk |
Benefits
The trial report stated that there were no significant differences between treatments in the proportion of participants who rated their satisfaction as ‘good’ or ‘excellent’ (licochalcone A cream: 10/26 excellent, 12/26 good, total 84.6%; hydrocortisone/base cream: 12/26 excellent, 10/26 good, total 84.6%). It is clear that there were some differences if those rating their satisfaction as ‘good’ or ‘excellent’ were assessed separately. There was no significant difference between groups in the reduction of the severity of eczema, measured using SCORAD (p = 0.199), although it was unclear which data this statistical comparison referred to. The last 2 weeks of the trial are described as a follow-up phase to evaluate relapse, but the participants were still using the active licochalcone A treatment on one side of their body. There was no significant difference between the treatments in relapse rate in the follow-up period using a Kaplan–Meier survival analysis (p = 0.240).
Harms
The trial report stated that there were no side effects of either treatment.
Overall implications for research and practice
Although the trial authors appear to suggest that the trial provides evidence that treatment with licochalcone A cream is beneficial compared with hydrocortisone, there was no evidence of superiority from this trial.
AR-GG27
Studies
There were no trials of AR-GG27® (Giuliana SpA) reported before 2000.
A new single-centre trial by Patrizi and colleagues239 compared AR-GG27 cream, which contains many different ingredients, with placebo cream, which contained 10 ingredients that are in the AR-GG27 cream and citric acid. Sixty children aged from 2 months to 15 years with pityriasis alba on the face and/or limbs and/or trunk, and eczema diagnosed using the Hanifin and Rajka8 criteria, with xerosis and pruritus present, were randomised to either AR-GG27 cream or placebo cream applied twice daily about 12 hours apart on affected and perilesional areas for 30 days. No other topical or systemic treatments were allowed during the trial, including sun exposure.
Assessment of risk of bias
Table 44 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Patrizi 2012239 | Unclear risk | Unclear risk | Unclear risk | 8/60 participants not included in the analysis |
Benefits
The intensity of itching (mean ± SD) in the intention-to-treat population was significantly reduced in the AR-GG27 group (–2.048 mm ± 2.330 mm) compared with the placebo group (–0.388 mm ± 2.22 mm) after 15 days of treatment (p = 0.011). The significant reduction in itch after 15 days was also reported in the population of participants who began the trial with itching (AR-GG27 group n = 18, placebo group n = 11). The severity of eczema (mean ± SD) using the IGA was significantly reduced in the AR-GG27 group (–6.30 ± 3.27) compared with the placebo group (–2.80 ± 3.19) after 15 days of treatment (p = 0.0007). This was also significant after 30 days of treatment.
Harms
There were no serious adverse events reported. There were seven adverse events in six participants in the placebo group, with one case of urticaria and one case of worsening eczema and pityriasis alba. These two events, which were reported as being possibly correlated with treatment, caused discontinuation. It is not reported whether these two events occurred in the same participant or not. Five of the events were not related to study treatment and were mild.
Overall implications for research and practice
This trial provides some evidence of short-term clinical benefit from using AR-GG27 cream compared with placebo. The trial methodology is unclear and it is not known how this treatment compares with any of the current standard treatments for eczema. Also, as the participants had both pityriasis alba and eczema, it is unclear how much beneficial effect would be seen when applied to those with only eczema. If this treatment is trialled again for eczema, particular attention needs to be paid to the adverse effects of the treatment.
Summary of emollients
Emollients
-
Five trials of emollients were reported before 2000, which found no significant difference between using one emollient and using another, some evidence of benefit for using an emollient in addition to a topical corticosteroid compared with a topical corticosteroid alone and evidence of benefit for using an emollient containing urea (10%) compared with the vehicle base, but no evidence of any difference in beneficial effect for different concentrations of urea.
-
There were 12 new trials of emollients reported from 2000 onwards:
-
Four trials, three large and one small, with an overall unclear risk of bias, did not provide any evidence of benefit from using one emollient compared with another emollient. Participants were allowed to use topical corticosteroid treatment as well as the emollients in two of the trials.
-
Four trials, three small and one medium sized, with a mostly unclear risk of bias, did not provide evidence of benefit or equivalence for emollient treatment compared with topical corticosteroid treatment.
-
One small trial, with a mostly unclear risk of bias, did not provide any evidence of benefit for a combination of emollient and topical corticosteroid compared with emollient alone.
-
One medium-sized trial, with a mostly unclear risk of bias, provided evidence of benefit for an emollient compared with no treatment. All participants were allowed to use topical corticosteroids.
-
One moderately sized trial, with an overall unclear risk of bias, provided evidence of benefit for an emollient compared with a cleansing wash.
-
One small proactive therapy trial, with an overall unclear risk of bias, provided evidence of benefit for an emollient compared with no emollient after induction of remission with topical corticosteroids.
-
Bath additives
-
One trial of bath additives published before 2000 compared Oilatum® with Oilatum® Plus (Stiefel Laboratories) (which contained an added antiseptic) and found some evidence of a beneficial effect of the emollient/antiseptic combination compared with Oilatum alone.
-
There were two trials of bath additives reported since 2000:
-
One trial, with a mostly unclear risk of bias, compared a dilute bleach bath and a once-a-month mupirocin treatment of the nares against placebo in children with infected eczema and found a significant beneficial effect for the bleach and mupirocin treatment.
-
Another trial, with a mostly unclear risk of bias, did not find any significant benefit from using a bath additive containing a diamide derivative compared with the same bath additive without the diamide derivative.
-
-
We did not find any trials comparing a bath additive in which there is no antimicrobial component against no bath additive, nor did we find any trials comparing bath emollients against direct application of emollients to the skin after bathing.
Summary of other topical treatments
-
There were no trials for the other topical treatments summarised in this chapter up to 2000.
Cipamfylline cream
-
One trial, with a mostly low risk of bias, provided evidence of a modest benefit from using cipamfylline cream compared with vehicle. However, it also provided evidence that hydrocortisone 17-butyrate was more beneficial than cipamfylline cream.
Camellia oil (Camellia japonica extract)
-
One small trial, with a mostly unclear risk of bias, provided evidence of benefit for camellia oil spray for 2 weeks compared with placebo spray.
Furfuryl palmitate (antioxidant)
-
One small trial, with an overall low risk of bias, provided evidence of benefit for an emollient when furfuryl palmitate was removed (vehicle) compared with the emollient when furfuryl palmitate was added.
Atopiclair
-
There were five trials overall, four funded by the makers of Atopiclair, with a mixed risk of bias. Three of the five trials showed improvements for the Atopiclair group compared with vehicle, one showed no difference between Atopiclair and other available emollients and one trial failed to compare groups.
SRD441 (protease inhibitor)
-
One small industry-funded trial, with a mostly low risk of bias, did not provide evidence of benefit for the protease inhibitor SRD441 compared with vehicle.
Vitamin B12
-
Two small trials, one with a mostly low risk of bias and one with a mostly unclear risk of bias, provided evidence of benefit for topical vitamin B12 cream compared with vehicle.
WBI-1001 (an inhibitor of T-cell inflammatory cytokine secretion)
-
Two small trials, with a mostly unclear risk of bias, provided evidence of benefit for WBI-1001 (0.5%) and (1.0%) compared with vehicle, although the smaller trial did not prespecify outcomes before data lock.
Other topical treatments [Hippophae rhamnoides, black seed oil, pill mask, rosmarinic acid, Vitreoscilla filiformis, shale oil, miltefosine, opiate receptor antagonist, carbohydrate-derived fulvic acid, raffinose, farnesol and xylitol, bacterial antigens, chamomile extract, heparin and levomenol, 15(R/S)-methyl-lipoxin A4, N-acetyl-L-hydroxyproline, nalmefene hydrochloride monohydrate (SRD174)]
-
Each of these treatments were tested in one trial reported from 2000 onwards. None of the trials found any evidence of benefit for the treatment tested compared with placebo or, in the case of licochalcone A, compared with hydrocortisone.
Chapter 6 Antimicrobials including antibiotics, antiseptics and antifungal agents
Background
Antimicrobials refer to a group of agents that share the common aim of reducing the possibility of infection and sepsis. Antibiotics are often derived from moulds or are made synthetically and are absorbed into the body with the aim of killing bacteria (bactericidal) or preventing their multiplication (bacteriostatic). Antibiotics can be given parenterally (intramuscularly, intravenously), orally, or applied topically to the skin in the form of a cream or ointment. Antiseptics on the other hand are substances that are applied to the skin but not absorbed significantly and which are able to reduce the possibility of infection. Disinfectants can destroy micro-organisms including bacteria on non-living objects such as toilets. Antifungal agents are drugs that share the common property of killing or inhibiting the growth of fungi, including yeasts. Antifungals can be given intravenously, orally or topically.
Rationale
The relationship between secondary infection or skin colonisation with the bacterium S. aureus and atopic eczema disease activity has been debated for many years. People with atopic eczema carry S. aureus in about 90% of clinically involved areas and about 75% of clinically uninvolved areas. S. aureus represents about 90% of the total aerobic bacterial flora of such individuals compared with 30% in normal skin. The density of S. aureus tends to increase with the clinical severity of the atopic eczema lesions. It has been suggested that the dry skin of atopic eczema is deficient in certain inhibitory fatty acids, which may encourage growth of the organism. S. aureus may also show enhanced adherence properties to skin cells, which has been shown when comparing atopic eczema sufferers with normal control subjects. 240,241 Other studies reviewed elsewhere242,243 have suggested that the balance of pathogenic and synbiotic bacterial species on the skin is altered in atopic eczema, resulting in an agitated skin microbiome.
Few clinicians would dispute that grossly infected atopic eczema with oozing and sore pus spots requires treatment with some form of antibiotic or antiseptic, and that the bacteria are contributing at least in part to that particular flare-up. However, the role of S. aureus in non-clinically infected atopic eczema skin or for borderline infection (e.g. with just redness and oozing) is far from clear and the definition of what constitutes ‘clinically infected atopic eczema’ among physicians is also not clear. Skin swabs taken for bacteriological culture are of little use because of the almost universal colonisation of atopic eczema skin with S. aureus, although such swabs may reveal additional bacteria such as streptococci species.
If S. aureus does play a pathogenic role in atopic eczema, then this could be mediated in a number of ways including direct chemical irritation, a non-specific reaction of the protein A component of the bacterium to immune cells and by the production of specific exotoxins called superantigens. Superantigens are capable of activating large populations of T lymphocytes distant from the site of colonisation, giving rise to widespread eczematous inflammation.
Although in many cases of non-clinically infected atopic eczema, the presence of S. aureus could be considered as an ‘innocent bystander’, which has simply colonised a dry and broken skin surface, there is at least some rationale for considering the role of S. aureus in more acute forms of atopic eczema. This has led to the use of many antimicrobial compounds, such as oral antibiotics that are active against S. aureus given in short or prolonged courses, topically applied antibiotics and antiseptic agents applied directly or by mixing with emollients applied directly to the skin or within bath additives.
Existing systematic reviews
The efficacy of antimicrobials and antiseptics for eczema has been reviewed in a Cochrane review that was published in 2008 by Birnie and colleagues. 244 This has since been updated in 2010, although not as a Cochrane review, with a new search ending in March 2009. 245 A systematic review in 2007, with a search end date in September 2005, assessed the safety of topical therapies for atopic dermatitis and this included topical antibiotics and antiseptic treatments. 80 All three of the current eczema guidelines from the AAD,94 SIGN42 and NICE41 cover antimicrobials and antiseptics.
Scope of this chapter
This chapter is divided into different sections describing the antibiotic, antiseptic and antifungal treatments for which RCTs have been published:
-
antibacterials:
-
topical: fusidic acid, mupirocin, tetracycline
-
oral: clarithromycin, tetracycline
-
-
antiseptics: triclosan, bleach
-
antifungals:
-
oral: itraconazole, ketoconazole
-
topical: ciclopirox olamine, ketoconazole shampoo, miconazole.
-
Antibacterials (topical)
Fusidic acid
Fusidic acid (Fucidin®; Leo Laboratories Ltd) is a bacteriostatic agent that inhibits bacterial protein synthesis. Its biological action is attributed to its effect on Gram-positive bacteria such as staphylococcus and streptococcus species.
Studies
There were no studies looking at fusidic acid for eczema before the year 2000.
Three new studies have emerged since 2000. 102,150,246 One of the trials, by Ravenscroft and colleagues,246 is discussed in more detail later in this chapter (see Mupirocin).
A four-arm, open-label, parallel-group trial conducted in a single centre in Taiwan by Hung and colleagues150 compared four treatments or treatment combinations: 0.05% fluticasone propionate with 2% fusidic acid cream; 0.05% fluticasone propionate; 0.03% tacrolimus ointment with 2% fusidic acid; and 0.03% tacrolimus ointment. All 60 participants had eczema diagnosed according to the Hanifin and Rajka8 criteria, without overt infection.
Larsen and colleagues102 conducted a European parallel-group multicentre trial consisting of three arms. The trial had industry sponsorship. The three arms consisted of Fucicort® Lipid cream (20 mg/g fusidic acid plus 1 mg/g betamethasone 17-valerate) (LEO Pharma), Fucicort® in a new lipid cream formulation and the lipid cream vehicle alone. The constituents of the new lipid cream were not reported. The 629 participants with a clinical diagnosis of infected eczema aged ≥ 6 years applied the treatments to all areas of eczema apart from the face, twice a day for 2 weeks. If required, participants could use hydrocortisone in a lipocream (Mildison®; Yamanouchi Pharmaceutical Co.) for facial lesions. The participants were also allowed to use an emollient cream (Locobase®; Yamanouchi Pharmaceutical Co.) on the areas not being treated with the trial medication.
Assessment of risk of bias
Table 45 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Hung 2007150 | Unclear risk | Unclear risk | High risk | Design and power calculation of trial not reported but non-inferiority is claimed |
| Larsen 2007102 | Low risk | Unclear risk | Unclear risk | Non-inferiority trial but used to claim superiority over vehicle as well |
| Ravenscroft 2003246 | Low risk | High risk | Low risk | Powered to evaluate the change in carriage rates of fusidic acid resistant S. aureus |
Benefits
As reported later in the section on mupirocin, the trial by Ravenscroft and colleagues246 showed a difference in reduction in eczema severity as assessed by the participant-assessed global severity score between those treated with mupirocin and betamethasone and those treated with fusidic acid and betamethasone.
The trial by Hung and colleagues150 found no statistically significant differences in severity (p = 0.81) after 2 weeks of treatment between the group using tacrolimus only and the group using tacrolimus and fusidic acid. There was also no statistically significant difference in severity after 2 weeks between the group treated with fluticasone propionate only and the group using fluticasone propionate and fusidic acid (p = 0.82). There was also no significant difference between these groups after 8 weeks.
The non-inferiority trial by Larsen and colleagues102 reported that the lipid formation of Fucicort was not inferior to the Fucicort cream after 2 weeks of treatment. The difference in the total severity score between the treatments was 0.23% (95% CI –3.83% to 4.30%). However, the Fucicort lipid preparation was superior to the vehicle preparation alone, with an estimated treatment difference for the total severity score of 48.3% (95% CI 41.0% to 55.7%; p < 0.001).
Harms
The only adverse events reported in the trial by Ravenscroft and colleagues246 were minor skin irritation in two participants treated with mupirocin and one participant treated with fusidic acid.
In the trial by Hung and colleagues150 information about adverse events was not reported. Two participants who used a treatment with fusidic acid added were found to have fusidic acid-resistant strains of S. aureus on their skin at the end of the study period.
In the trial by Larsen and colleagues102 the proportion of adverse events in each group was similar; however, only the nature and distribution of the adverse events relating to the skin reactions were reported.
Overall implications for research and practice
The largest trial on fusidic acid serves only to deliver a different vehicle preparation of a topical corticosteroid and fusidic acid preparation to market and is not clinically relevant as there is no comparison with another active treatment. 102 The two remaining trials,150,246 which compare different active treatment regimens, were not designed with a primary research question to investigate the effect of fusidic acid on the severity of eczema and so may not be appropriately powered to answer this question. These trials also have a high risk of bias because of inadequate blinding. For clinically infected eczema there is currently no evidence of additional benefit from adding fusidic acid to other topical treatments over adding mupirocin. 246 For non-infected eczema, there is no evidence of benefit from adding fusidic acid to short-term topical corticosteroid treatment. Trials that seek to pragmatically answer the question, ‘Which is the most effective treatment regimen for reducing severity and clearing infection in infected eczema?’, are needed to give clinicians the most relevant information for clinical practice. The ChildRen with Eczema, Antibiotic Management (CREAM) trial (HTA 09/118/03) is currently addressing this question [see www.nets.nihr.ac.uk/projects/hta/0911803 (accessed 14 January 2016)].
Mupirocin
Mupirocin (Bactroban®; GlaxoSmithKline) is an antibiotic that is bacteriostatic at low concentrations and bactericidal at high concentrations. It is effective against Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA).
Studies
One trial was reported before 200055 (see Appendix 3).
Four new studies have emerged since the 2000 HTA report. 55 One of the trials, by Huang and colleagues,205 which used topical mupirocin applied to nasal carriage sites as part of an anti-infective intervention that included bleach baths, is discussed in more detail in the antiseptics section of this chapter.
A trial by Gong and colleagues247 conducted in China compared a dual treatment regimen of the topical corticosteroid hydrocortisone butyrate (Pandel®; Tianjing Yaoye Ji-tuan Co., Ltd) followed by topical mupirocin 1–2 hours later against the dual treatment regimen of the topical corticosteroid hydrocortisone butyrate and the base ointment only 1–2 hours later. The treatments were applied every morning for 28 days. The 119 participants were aged between 2 and 65 years and had eczema as defined by the Hanifin and Rajka8 criteria.
A UK trial by Ravenscroft and colleagues246 compared 2% fusidic acid plus 0.1% betamethasone cream with mupirocin ointment plus 0.1% betamethasone cream. The treatments were applied to all affected areas twice daily for 2 weeks. The trial included 46 participants from the community who had eczema that warranted the use of potent topical corticosteroids for 2 weeks according to an assessing clinician.
A three-arm trial by Canpolat and colleagues248 compared hydrocortisone used concurrently with mupirocin or hydrocortisone with emollient only as a control. All treatments were applied twice daily to affected areas for up to 7 days. The potency of the hydrocortisone and the mupirocin ointment were not reported. Eighty-three infants aged from 6 months to 2 years with mild to moderate eczema based on the Hanifin and Rajka8 criteria and with 2–30% body surface area involvement were randomised.
Assessment of risk of bias
Table 46 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Canpolat 2012248 | Unclear risk | Unclear risk | Low risk | Although the term ‘randomised’ is used, the method of randomisation is not reported and it is stated that those in the control group were infants whose parents did not want to use pharmacological treatments on their child |
| Gong 2006247 | Unclear risk | Unclear risk | Low risk | Although the trial report states that an intention-to-treat population was analysed, it is unclear which analyses this was used for |
| Ravenscroft 2003246 | Low risk | High risk | Low risk | Powered to evaluate the change in carriage rates of fusidic acid-resistant S. aureus |
Benefits
The trial by Gong and colleagues247 did not find any difference in eczema severity, measured using the EASI scoring system, between treatment with mupirocin plus hydrocortisone butyrate and treatment with hydrocortisone butyrate alone in the whole trial population. The mupirocin group decreased from a score of 13.9 (SD 8.4) to 1.4 (SD 2.3) and the control group decreased from 13.6 (SD 8.5) to 2.5 (SD 5.2). In what appears to be a post hoc subgroup analysis, a significant beneficial effect was reported from the addition of mupirocin when the EASI severity score was ≥ 7 on the seventh day of treatment; however, this effect was not apparent during the rest of the treatment period. The rates of colonisation by S. aureus showed no significant difference and were reported as matching the improvements in eczema severity.
In the trial by Ravenscroft and colleagues246 the primary objective was not to investigate clinical improvements in eczema; however, the severity of eczema was assessed using both objective and subjective outcomes. A modification of Costa and colleagues’ simple scoring system,249 with a maximum score of 98, showed no significant difference in the change in severity between the two combinations of treatment after 2 weeks. Participant-assessed global severity also showed no significant difference in the change in severity after 2 weeks. Both treatments showed clinically relevant reductions in severity, from 23 to 6 for the fusidic acid group and from 28.5 to 8 for the mupirocin group for the objective severity measure. There was a strong correlation between improvement in eczema severity and reduction in carriage of S. aureus (p = 0.866) analysed over three time points for the entire trial population, but this was not significant when assessed for each individual participant.
The trial by Canpolat and colleagues248 did not include any patient-reported outcomes. There was a significant difference in eczema severity, measured by the EASI scoring system, for the mupirocin and hydrocortisone group compared with the hydrocortisone-only group at the end of treatment (day 8) [4.2 (range 2–6) vs. 5.1 (range 2–7)]. The emollient-only group had an EASI score of 5.5 (range 2–8) at the end of treatment. For eczema measured by the SCORAD system, at day 8 the difference between the emollient-only group [30 (range 23–34)] and the hydrocortisone-only group [27 (range 20–33); p = 0.014] and between the hydrocortisone-only group [range 27 (20–33)] and the hydrocortisone and mupirocin group [26 (range 21–32); p = 0.006], was significant. There was a significant difference in treatment success, defined as a ≥ 50% reduction in lesion severity scores (measured using EASI/SCORAD), between the emollient-only group (36%) and the hydrocortisone-only group (65%) and the hydrocortisone and mupirocin group (74%) (p = 0.014 and p = 0.006, respectively) after 60 days.
Harms
No information about adverse events was reported for the trial by Gong and colleagues. 247 In the trial by Ravenscroft and colleagues246 minor skin irritation was reported for 1 out of 28 participants treated with fusidic acid and 2 out of 18 participants treated with mupirocin. No participants stopped study treatment early. The trial by Canpolat and colleagues248 did not report any information about adverse events, despite stating that they would record these in the trial.
Overall implications for research and practice
In the trial by Gong and colleagues247 many methodological aspects are unclear, such as prespecified outcomes and the amount of study treatments and co-treatments applied. The trial does show a beneficial effect from adding mupirocin, but this must be treated with caution as so little methodological information is reported. The trial by Ravenscroft and colleagues246 provided no evidence of benefit for the addition of antibiotics to steroid treatment for non-infected eczema over a 2-week period. It is important to remember that this trial was powered to answer the question, ‘Does treatment with a topical corticosteroid/fusidic acid combination lead to an increased rate of carriage of fusidic acid-resistant S. aureus’, and may not be as appropriate for assessing any potential clinical benefit. The trial by Canpolat and colleagues248 also provides some evidence of benefit by statistical comparison, but the magnitude of effect appears quite small for the treatment period and it is possible that some participants may have been added to the control group because of their preference for not using pharmacological treatment. The long-term follow-up results seem to provide more clinically relevant reductions for steroid or combined treatment than for emollient, but the hydrocortisone-only and hydrocortisone and mupirocin groups are not compared and participants could use other eczema treatments, the levels of use of which are not reported.
The current trials do not provide any convincing evidence of benefit for the addition of mupirocin to topical corticosteroid treatment compared with topical corticosteroid treatment only for non-infected eczema. Indeed, one trial highlights that it is important to carefully consider the use of antimicrobial treatment as it carries risks associated with antimicrobial resistance. Researching antimicrobials for clinically non-infected eczema is unlikely to be taken forward given the lack of beneficial signals to date and concerns about promoting antimicrobial resistance, and research on antimicrobial therapy should concentrate on clinically overt, secondarily infected eczema.
Tetracycline
Tetracycline is a broad-spectrum antibiotic produced by Streptomyces species of Actinobacteria.
Studies
No trials investigating tetracycline for eczema were found before 2000.
One new trial has been reported since 2000. 250 This trial, conducted in the Netherlands by Schuttelaar and Coenraads,250 compared a combination of the moderate-potency topical corticosteroid triamcinolone acetonide (0.1%) plus topical tetracycline (3%) against triamcinolone acetonide alone. The 44 participants with moderate to severe clinically non-infected eczema (SCORAD score of ≥ 25) diagnosed according to the Hanifin and Rajka8 criteria applied the treatment twice daily all over the body for 2 weeks. After the 2 weeks of randomised treatment, all participants were then treated with 0.1% triamcinolone acetonide for a further 6 weeks and followed up to assess maintenance treatment.
Assessment of risk of bias
Table 47 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Schuttelaar 2008250 | Low risk | Low risk | Low risk |
Benefits
The trial reported that there were no significant differences in the severity of eczema measured by both objective SCORAD and SASSAD scores after 2 weeks, although the differences in the scores were not reported. No significant differences in severity between the two treatment groups were found in the 6-week maintenance period. No participant-assessed outcomes were recorded for this trial. The tetracycline and triamcinolone acetonide combination was reported as having a significantly better rate of antibacterial efficacy, with 14 out of 22 participants having their colonisation with S. aureus eradicated, compared with 5 out of 22 participants in the placebo group.
Harms
The authors reported a low to moderate level of folliculitis in both groups but there is no information whether this occurred in the RCT phase, the maintenance phase of therapy or the maintenance open-label period.
Overall implications for research and practice
This fairly well-reported trial did not find any benefit of adding tetracycline to topical corticosteroid treatment in people with eczema without overt signs of clinical infection. Although bacterial counts were reduced in the intervention group, these were not matched by clinical benefit, raising doubts about whether the bacteria (S. aureus) are playing a pathogenic role. Additional research evidence on combining tetracycline with topical corticosteroids in uninfected eczema is probably not needed.
Antibacterial (oral)
Clarithromycin
One trial by Capella and colleagues251 compared oral montelukast against a ‘standard’ treatment combination of clarithromycin, cetirizine and mometasone furoate. No data were presented for clarithromycin treatment alone and so it is impossible to draw any conclusions about the use of clarithromycin for eczema on its own from this one trial.
Antiseptics
Triclosan
This antiseptic and disinfectant is widely used in everyday items such as toothpaste, chopping boards and rubbish bags.
Studies
Two trials were reported that tested triclosan before 200055 (see Appendix 3).
Two new trials have been reported since 2000. A small manufacturer-sponsored trial by Tan and colleagues252 of a 1% triclosan-containing emollient compared with vehicle emollient was conducted in 60 participants aged between 12 and 40 years. All participants were required to use 0.025% betamethasone valerate cream once a day for 27 days as well as the study treatment; after this, participants could choose to discontinue betamethasone valerate use. It was reported that most participants did use the topical corticosteroid during the trial period.
A small manufacturer-sponsored trial by Breneman and colleagues253 compared a soap bar containing triclosan (1.5%) against a ‘placebo’ soap bar that did not contain any antibacterials. Fifty participants (age not reported) with moderate eczema defined using the Hanifin and Rajka8 criteria were randomised to wash their whole body at least once a day for 63 days using the treatment. The participants had their other eczema treatments standardised so that all participants used a non-medicated cleansing bar, non-medicated moisturising cream and only 0.025% triamcinolone acetonide as the topical corticosteroid treatment. For the last 21 days of treatment, no topical corticosteroid was allowed.
Assessment of risk of bias
Table 48 provides the risk-of-bias assessment for the new studies.
Benefits
The trial by Tan and colleagues252 measured the use of topical corticosteroids and noted that the triclosan group used significantly less than the vehicle group. However, no data were reported to support this statement. The primary outcome, which was the number of participants achieving a ≥ 20-point improvement on the SCORAD index from baseline, was statistically significantly different between the groups (triclosan group, n = 9; vehicle group, n = 4; p < 0.05). The secondary outcome, change in eczema severity from baseline using the SCORAD score, was significantly different only at day 14 (triclosan group –8.86, vehicle group –4.75, 95% CI –8.58 to 0.32; p > 0.05).
The trial report by Breneman and colleagues253 contained mostly summary results with no additional data, including no baseline data. The trial report states that, for itching, the participants using the soap bar containing triclosan experienced less itching than those using the placebo soap bar and that this effect was carried through the final 21 days of treatment without use of topical corticosteroids. For the dermatologist global assessment, no baseline scores are provided on the graph and the scores do not appear to differ between the groups, but no statistical analysis between groups is provided. The report states that there was a significant difference in favour of the triclosan-containing soap bar for disease extent and severity components of SASSAD but the total SASSAD scores are not reported.
Harms
A quarter of the study population in the trial by Tan and colleagues252 reported adverse events, but only four adverse events were considered to be related to treatment. Three participants in the triclosan group experienced application site stinging and one participant in the vehicle group experienced application site pruritus.
In the trial by Breneman and colleagues253 it was reported that there was only one study-related event. One participant withdrew because of worsening of eczema but it was not stated which treatment group they were in. No other information about adverse events was reported.
Overall implications for research and practice
Although the sample size in the trial by Tan and colleagues252 was powered as per an appropriate calculation, it is not clear what previous work the assumption of a SCORAD response rate of 90% for the triclosan group and 50% for the vehicle group was based on and this perhaps seems overly optimistic. Also, the amount of the study emollient used in each group, an essential factor in assessing the comparative effectiveness of these two active treatments, was not recorded. The trial report by Breneman and colleagues253 provides so few data that it is impossible to interpret the slight beneficial effect reported for the triclosan soap bar. The two pre-2000 previous trials added triclosan and benzalkonium chloride together, making it impossible to assess the impact of triclosan alone, and these trials were also difficult to interpret. Until trials that assess triclosan with clear, appropriate methodology are published it is impossible to assess the potential benefits and harms of this antimicrobial agent.
Bleach baths
Common household bleach should not be applied to the skin as it can cause burns on contact and is toxic if ingested. However, research has investigated the use of extremely small amounts of a certain form of bleach. The amount of bleach added to the bath makes the concentration very dilute.
Studies
There were no studies looking at bleach baths for eczema before 2000.
One new trial was reported after 2000. 205 This trial, by Huang and colleagues,205 compared a regimen of half a cup of bleach in a bath (0.005%) twice weekly for 5–10 minutes and topical mupirocin applied to the nares of the nose for 5 consecutive days per month with a regimen of half a cup of water in the bath twice weekly for 5–10 minutes and petroleum ointment applied to the nares of the nose for 5 consecutive days per month. The treatment regimen was followed for 3 months and 31 children aged from 6 months to 17 years with moderate to severe infected eczema were randomised. Children who were currently or recurrently using topical antimicrobials were excluded from the trial. In addition to the participants, all other members in each participant’s household had to apply mupirocin (treatment arm) or petroleum (placebo arm) intranasally twice a day for 5 consecutive days of the month.
The participants could bathe without the treatment as often as they wished. All of the participants had to use a stable treatment regimen of emollients and topical corticosteroids throughout the trial. Additionally, patients received Cefalexin (Keflex®; Flynn Pharma) at 50 mg/kg per day (maximum daily dose 2 g) divided into three daily doses for 2 weeks.
Assessment of risk of bias
Table 49 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Huang 2009205 | Unclear risk | Unclear risk | Low risk | Severity and percentage body surface area affected were higher in the treatment group at baseline |
Benefits
This trial by Huang and colleagues205 reported a statistically significant difference in the change in severity compared with baseline between the treatments, as assessed using the EASI score, favouring the bleach bath and mupirocin for the area of the body in contact with the bath water. This was based on a post hoc subgroup analysis at 1 month and 3 months [bleach bath and mupirocin group (mean ± standard error) –15.3 ± 3.8, placebo group (mean ± standard error) –3.2 ± 1.6; p = 0.004]. The baseline EASI scores were quite different between the groups [bleach bath and mupirocin group (mean ± SD) 22.1 ± 13.3, placebo group (mean ± SD) 16.6 ± 9.8] as was the body surface area affected [bleach bath and mupirocin group (mean ± SD) 37.8 ± 21.6, placebo group (mean ± SD) 28.1 ± 18.2] and it was unclear if these baseline imbalances were adjusted for in the final analyses. For the IGA, the treatment groups were significantly different at month 1 (p = 0.024) but not at month 3. There was a decrease of 67% in eczema severity score measured using the IGA in the bleach bath and mupirocin group and a 15% decrease in the placebo group at month 3.
Harms
One participant in the treatment group developed irritation and itching; he then failed to comply with the treatment regimen and subsequently developed a community-acquired MRSA infection, was hospitalised and received intravenous antibiotics; he resumed the study once he had recovered.
Overall implications for research and practice
The use of dilute bleach baths and 5 out of 28 days of intranasal mupirocin application (by participants and members of their household) resulted in a significant improvement in eczema severity over 3 months. Because of this, the low cost of treatment and ease of administration this intervention shows promise. The evidence from this trial should be treated with caution, however, as baseline severity and body surface area were very different at baseline, being higher in the treatment group. The analysis of the area of the body submerged in the bath against the head and neck was a post hoc analysis and was unlikely to have been powered correctly. It is interesting that the IGA does not show the same significant difference between groups. As the mupirocin and bleach baths were trialled only as a combined treatment, it is impossible to know how effective each treatment would be on its own. It is disappointing that no participant-assessed outcomes were reported. Only one adverse event was reported; a patient who failed to follow the treatment regime after developing skin irritation and pruritus subsequently developed an MRSA infection for which hospitalisation and intravenous antibiotics were needed. Although this could easily be just ‘bad luck’, the potential side effects of this intervention need greater scrutiny. Although there is not enough strong evidence from one trial, this intervention is worth pursuing in larger, long-term treatment trials.
Antifungal agents
Rationale for antifungal agents
The role of fungi and yeasts in eczema is not clear. Although fungal infections such as athlete’s foot (tinea pedis) can result in secondary eczematisation, secondary fungal infection co-existing or superimposed on atopic eczema lesions is apparently uncommon. However, it has been suggested that allergic sensitisation to Malassezia yeast species, which is common on the scalp and head, may contribute to some patterns of atopic eczema affecting the head and neck in adults. Although the role of fungi and yeasts in atopic eczema is tenuous, some have used antifungal agents combined with topical corticosteroids and some have used antifungals in shampoo or tablet form in an attempt to improve atopic eczema, and these will be discussed at the end of this chapter.
Ketoconazole (oral and topical)
Ketoconazole is an antifungal agent available in oral and topical forms. The topical form (Nizoral® shampoo; Janssen-Cilag) is used in shampoos for dandruff and scalp psoriasis but is not currently licensed for the treatment of eczema.
In July 2013, the European Medicines Agency’s Committee for Medicinal Products for Human Use issued a statement that oral medicines containing ketoconazole should no longer be used for the treatment of fungal infections because of safety concerns regarding the risk of developing hepatotoxicity and adrenal insufficiency, and the potential for fatal drug interactions. 254 As such, the use of ketoconazole in developed countries has been largely superseded by newer azoles, such as itraconazole (Sporanox®; Janssen-Cilag), because of a lower risk of liver toxicity and drug interactions.
Studies
One trial involving ketoconazole shampoo was reported before 200055 (see Appendix 3).
Two trials of oral ketoconazole were reported after 2000. A trial in Finland by Lintu and colleagues255 compared 200 mg of oral ketoconazole per day with placebo in 80 adults with eczema who were also shown to be sensitive to the fungi Pityrosporum orbiculare, Candida albicans or Saccharomyces cerevisiae. The treatment was given for 30 days and the participants were followed up 3 months after treatment. Topical treatment with 1% hydrocortisone was allowed during the trial as long as the same brand was used throughout.
A trial in Sweden by Back and Bartosik256 compared 200 mg of oral ketoconazole per day against placebo for 3 months in 32 adults with eczema and specific serum antibodies to Malassezia furfur or P. orbiculare above 3.5 kU/l and an elevated serum IgE level (> 400 kU/l).
Assessment of risk of bias
Table 50 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Back 2001256 | Unclear risk | Unclear risk | Unclear risk | |
| Lintu 2001255 | Unclear risk | Unclear risk | Unclear risk | The participants were allowed to use stronger topical treatment in the follow-up period if needed. Many of the withdrawals were a result of exacerbation of eczema and these were not included in the analyses |
Benefits
The trial by Lintu and colleagues255 looked at the severity of eczema using the SCORAD index, total serum IgE level, sensitivity and allergy, and presence of P. orbiculare, C. albicans or S. cerevisiae. This trial gave results of the improvement within each treatment group after the treatment period but did not compare the two treatment groups against each other. The mean SCORAD score in the ketoconazole group reduced by 7.9 (SD 13.1) points, whereas that in the placebo group reduced by 2.9 (15.3) points. The trial report states that the data for the follow-up period, when study treatment had stopped, were not reliable for therapeutic effect because of the use of extra topical treatment. No participant-assessed outcomes were reported for this trial.
The trial by Back and Bartosik256 did not find any significant difference in the severity of eczema measured using the SCORAD index after 3 months of treatment (p = 0.533), but the actual SCORAD values were regrettably not reported. The report states that the use of betamethasone was correlated with the improvement in the placebo group in the second and third months (r = 0.66, p = 0.013) but not in the ketoconazole group (r = 0.15, p = 0.61), but no values were reported. The participants’ evaluation of eczema improvement was reported as not significantly different between the groups, but no data were reported.
Harms
The trial by Lintu and colleagues255 did not report any information about adverse events. The trial by Back and Bartosik256 reported that there were only rare adverse events in the ketoconazole group, with two participants complaining of intense dreams, nausea and abdominal pain.
Overall implications for research and practice
The evidence for or against oral ketoconazole is difficult to interpret as neither trial reported the appropriate data for evaluating whether one treatment arm was better than the other. Both trials also allowed all participants to use topical corticosteroids as required and have not provided enough data on the levels used to be certain whether any beneficial or harmful effects seen are likely to be from use of ketoconazole or use of rescue treatment with topical corticosteroids. Difficulties with recruitment to these trials were mentioned because of pre-treatment ‘washout’ of topical ketoconazole being required. Potential participants were satisfied with their current treatment of topical ketoconazole, especially ketoconazole shampoo, and topical corticosteroids as required and so were not motivated to join the trial. This would indicate little need to pursue treatment alternatives such as oral ketoconazole. There is no good evidence of benefit for oral ketoconazole from these trials and it is probably not an area where further trials are needed.
Miconazole (topical)
Miconazole is an imidazole agent that is used topically to treat fungal infections. When taken orally it has also been shown to be effective against some forms of leishmaniasis and it also has some antibacterial properties.
Studies
One trial involving miconazole was reported before 200055 (see Appendix 3).
One trial has been reported since 2000. A within-person trial by Wong and colleagues115 in Hong Kong compared a combined treatment regimen of miconazole and 1% hydrocortisone against 1% hydrocortisone only, applied twice daily for 2 weeks. The trial included 30 children aged between 5 and 14 years with eczema, defined according to the UK Working Party’s criteria,9 symmetrically distributed (at knees or elbows), of whom 80% were classified as severe using the Nottingham Eczema Severity Score. 257
Assessment of risk of bias
Table 51 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Wong 2008115 | Unclear risk | Unclear risk | Unclear risk |
Benefits
The trial mainly relied on participants reporting which treatment gave better relief of eczema symptoms after 2 weeks of treatment and after another 6 weeks with no treatment. Two independent dermatologists also assessed outcomes using photographs. The two dermatologists gave vastly different interpretations of the eczema photographs, with one recording nearly all as ‘no difference’ and the other being equally split between ‘better with miconazole’, ‘better with hydrocortisone only’ and ‘no difference’, so this method proved to be unreliable and therefore difficult to interpret. The participants also reported no differences between the treatments using the same scoring system. There was also no difference in topical corticosteroid-free days between the two treatment groups in the follow-up period.
Harms
The trial report stated that there were no reported side effects.
Overall implications for research and practice
This small and poorly reported trial did not show any evidence of a beneficial effect of a topical combination of hydrocortisone and miconazole over hydrocortisone alone. The trial did not use any reliable objective or subjective measures of eczema severity.
Itraconazole (oral)
Itraconazole belongs to the triazole group of antifungal medications. It has a broad spectrum of action against fungi including yeasts and dermatophytes. It is similar to fluconazole but also treats Aspergillus infections.
Studies
There were no trials before 2000 looking at the use of itraconazole for eczema.
One new, small, three-arm trial258 concentrating on the head and neck area compared 200 mg or 400 mg of itraconazole against placebo in adults whose head and neck eczema was more severe than the eczema elsewhere on their body. The treatment was given for only 7 days but the follow-up period lasted for 105 days. The results reported do not include the follow-up at 105 days and concentrate on day 7 and day 14 measurements, thus giving only a short-term picture of any treatment effect. If a participant required adjuvant treatment during the trial then the trial protocol was violated and the participant was withdrawn.
Assessment of risk of bias
Table 52 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Svejgaard 2004258 | Unclear risk | Unclear risk | Unclear risk |
Benefits
The severity of eczema in the head and neck region was significantly reduced compared with baseline for both 200 mg and 400 mg of itraconazole. Comparison of improvement between all three groups showed a statistically significant difference only for 200 mg of itraconazole (mean difference of 4.5, extrapolated from a graph) compared with placebo (mean difference of 16, extrapolated from a graph) at day 14 (p = 0.0318). For the primary success criterion, measured using the SCORAD index, 8 out of 35 participants using itraconazole reported a reduction in severity of > 50% compared with baseline compared with 2 out of 18 participants using placebo. Both the participant and investigator global assessment were reported as showing no significant differences in overall improvement in eczema severity, but the complete data for these outcomes were not reported.
Harms
This trial reported no adverse events in the itraconazole groups and there were no withdrawals because of adverse events. Over half of each treatment group withdrew, mainly because of exacerbations of eczema requiring additional treatment. Most of the withdrawals occurred after study treatment had stopped.
Overall implications for research and practice
Giving itraconazole as the first-line treatment without the use of adjuvant treatments resulted in over half of the trial population withdrawing from the study, mostly between days 14 and 56 because of the need for additional treatments. A post hoc decision to report the results for day 14 and draw conclusions about this 7-day treatment course accordingly diverts attention away from the potential unsuitability of a 7-day course of itraconazole as a long-term treatment for eczema. The number of participants was small and no formal power calculation was reported. Although there is some evidence of a beneficial effect of 200 mg of itraconazole after 14 days, the 400-mg dose appears to be no more effective than placebo, a result that is counterintuitive and raises the suspicion that the positive finding at 14 days for the lower-dose group was just a chance finding. Given that another similar oral antifungal, ketoconazole, has failed to provide any clear benefit for people with mainly head and neck eczema, further trials of oral antifungals are probably not a priority.
Ciclopirox olamine (topical)
Ciclopirox olamine is a hydroxypyridine antifungal agent. It has been shown to be highly effective against Malassezia species, which have been implicated in difficult-to-treat atopic eczema in the head and neck area.
Studies
There were no studies on ciclopirox olamine for eczema before the year 2000.
One study was reported after 2000. This trial, by Mayser and colleagues,259 compared 1% topical ciclopirox olamine against the base cream, which was applied twice daily to the head and neck region for 28 days. The 50 randomised participants had had moderate to severe eczema for at least 6 months. The eczema was defined according to the Hanifin and Rajka8 criteria and an IGA of score of ≥ 3, and all presented with ≥ 10% coverage in the head and neck region. All of the participants had to have at least a class I reaction for specific IgE to M. sympodialis and M. furfur and enterotoxin A and B. The trial followed the participants until 2 weeks after treatment had stopped.
Assessment of risk of bias
Table 53 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Mayser 2006259 | Unclear risk | Unclear risk | Low risk | Concomitant medication given to the groups appears to have differed. Only 29 out of 50 participants included in the analysis |
Benefits
Only 29 out of the 50 participants who completed the study were included in the analysis. The assessments of eczema severity on the head and neck using the IGA tool and EASI showed significant between group differences at 28 days compared with baseline.
Harms
The trial report did not provide any information about adverse events or other potential harms.
Overall implications for research and practice
The results of this trial should be treated with caution because of the high dropout rate and failure to perform an intention-to-treat analysis. In a group with moderate to severe eczema, failure to permit rescue therapy was perhaps unethical given the lack of a priori evidence of benefit of ciclopirox olamine; instead, the use of rescue therapy could have been recorded as a study outcome. The results of this one small, short-term trial in those with moderate to severe eczema on the head and neck and sensitive to M. sympodialis, M. furfur and enterotoxin A and B do not provide any clear evidence of benefit from ciclopirox olamine, but do provide some weak evidence of a worsening in severity when the treatment is stopped. Given the lack of a beneficial signal in this study, further research with ciclopirox olamine is probably not a priority.
Summary of antimicrobials including antiseptics and antifungals
Antibiotics
Topical fusidic acid
-
There were no trials on fusidic acid reported before 2000.
-
For non-infected eczema, one trial reported in 2003, with a mostly high risk of bias, did not provide any evidence of benefit for a combination of fusidic acid (2%) and topical corticosteroid compared with a combination of mupirocin and topical corticosteroid.
-
For non-infected eczema, one four-arm trial reported in 2007, with a mostly unclear risk of bias and a high risk of blinding bias, did not provide any evidence of benefit for a combination of fusidic acid (2%) and topical corticosteroid treatment compared with topical corticosteroid alone or for a combination of fusidic acid (2%) and topical tacrolimus compared with topical tacrolimus alone.
-
The largest trial, reported in 2007, with a mixed risk of bias, provided evidence of benefit for a combination of fusidic acid and betamethasone 17-valerate in a lipid base compared with the lipid base alone for people with infected eczema. This trial also compared a combination of fusidic acid and betamethasone 17-valerate in a cream base against the same treatments in the lipid base and provided evidence of non-inferiority.
Topical mupirocin
-
One small pre 2000 trial, with a mostly unclear risk of bias, provided evidence of a large beneficial effect for mupirocin ointment compared with placebo in people whose eczema was not overtly infected. The participants could use emollients and topical corticosteroids.
-
Three trials, two small and one moderately sized, reported in 2003, 2012 and 2006, respectively, and with a mixed risk of bias, did not provide any evidence of benefit from using mupirocin in combination with topical corticosteroids compared with topical corticosteroids alone in people with non-infected eczema. One of these trials primarily evaluated the carriage of fusidic acid-resistant S. aureus.
-
A fourth small trial, reported in 2009, with a mostly unclear risk of bias, provided evidence for combined mupirocin treatment of the nostrils and bleach baths against compared with placebo for children with infected eczema.
Topical tetracycline
-
There were no trials using tetracycline for eczema reported before 2000.
-
One small trial reported in 2008, with an overall low risk of bias, did not provide any evidence of benefit for a combination of tetracycline and topical corticosteroid compared with topical corticosteroid alone in people with clinically non-infected eczema.
Clarithromycin
-
One trial compared oral montelukast against a ‘standard’ treatment combination of clarithromycin, cetirizine and mometasone furoate. No data were presented for clarithromycin treatment alone and so it is impossible to draw any conclusions about the use of clarithromycin for eczema on its own from this one trial.
Antiseptics
Triclosan
-
Two very small trials reported pre 2000 provided evidence of benefit for a bath additive containing 2% triclosan and benzalkonium chloride (6% w/w) compared with the same bath additive without antiseptics.
-
Two small trials funded by the manufacturer reported in 2000 and 2010, with a mostly unclear risk of bias, provided evidence of benefit for a triclosan 1.5%- or 1.0%-containing soap bar or emollient, respectively, compared with vehicle in people with eczema that was not infected. One of the trials provided hardly any data in the trial report.
-
The four trials using triclosan for eczema have been designed and reported in a way that makes it difficult to interpret the results and therefore there is no clear evidence on the benefits or harms of triclosan for eczema.
Bleach baths
-
There were no trials of bleach baths reported before 2000.
-
One small trial reported in 2009, with a mostly unclear risk of bias, provided evidence of a benefit at 1 month in a post hoc subgroup analysis, but not at 3 months, for bleach baths once a week and a 5-day treatment of mupirocin to the nostrils once a month compared with placebo.
Antifungals
Oral ketoconazole
-
There were no trials of oral ketoconazole for eczema reported before 2000.
-
Two small trials reported in 2001, with an overall unclear risk of bias, did not provide any evidence of benefit for oral ketoconazole (200 mg) compared with placebo in people with eczema who were sensitive to fungi.
Topical miconazole
-
One small trial reported pre 2000, with a mostly unclear risk of bias, did not provide any evidence of benefit for a combination of miconazole, topical corticosteroid and ketoconazole shampoo compared with topical corticosteroid and shampoo.
-
One very small trial reported in 2008, with an overall unclear risk of bias, did not provide any evidence of benefit for a combination of miconazole and topical hydrocortisone compared with hydrocortisone alone.
Oral itraconazole
-
One small trial reported in 2004, with an unclear risk of bias, did not find any evidence of benefit for itraconazole compared with placebo.
Topical ciclopirox olamine
-
One trial reported in 2006, with a mostly unclear risk of bias, did not find any evidence of benefit for topical ciclopirox olamine compared with the base cream.
Chapter 7 Antihistamines and mast cell stabilisers
Background
Antihistamines have long been prescribed for atopic eczema in the belief that they reduce itching by blocking the action of histamine on its receptors in the skin. The role of histamine in mediating pruritus in atopic eczema is unclear and it may play only a small part. There are four types of histamine receptor; however, current antihistamines have mainly been developed to target the H1 and H2 receptors, which are both found in the skin. Most antihistamines that have been trialled as treatments for atopic eczema are H1 receptor antagonists. H1 antihistamines can be further subdivided into those with a sedating action (e.g. chlorpheniramine) and those with a less sedating action (e.g. cetirizine). Although lack of sedation may be desirable in the daytime, it is often stated that antihistamines are effective in atopic eczema only if they are sedative. It is suggested that sedating antihistamines are effective because of their central sedating effect rather than because of any action on peripheral histamine blockade. Regardless of how antihistamines might work in atopic eczema, it is useful to consider the evidence of whether they help at all.
Mast cell stabilisers block a calcium channel essential for mast cell degranulation, preventing the release of histamine and related mediators.
Existing systematic reviews
The NICE,41 SIGN42 and AAD94 guidelines and associated evidence reviews cover antihistamines. A systematic review covering the safety of eczema treatments80 also covers topical doxepin. A systematic review of interventions to reduce itching for eczema93 also covers many of the antihistamines in this chapter.
Scope of this chapter
This chapter covers the following treatments
-
H1 antihistamines (less sedating)
-
loratidine (oral)
-
ketotifen (oral)
-
epinastine (oral)
-
cetirizine (oral)
-
fexofenadine (oral)
-
-
Antihistamine (non-sedating)
-
Olopatadine hydrochloride (oral)
-
-
H1 antihistamines (sedating)
-
chlorpheniramine (oral)
-
doxepin (topical)
-
-
mast cell stabiliser
-
sodium chromoglycate (topical)
-
Antihistamines
Cetirizine (oral) (less sedating)
Cetirizine (Zirtek™; UCB Pharma) is a potent antihistamine and is used in adults and children.
Studies
Five trials involving cetirizine were reported before 200055 (see Appendix 3).
One new trial by Diepgen and colleagues260 compared cetirizine oral solution (10 mg/ml, 0.25 mg/kg) against placebo twice daily for 18 months. The trial included 817 children aged 1–2 years who had had active eczema for at least a month before recruitment and who had at least one parent who had a history of asthma, atopic eczema or allergic rhinitis. The trial was primarily looking at the rate of development of asthma in the infants but also assessed the effect of the intervention on the eczema.
One new trial by Capella and colleagues251 compared oral montelukast against a ‘standard’ treatment combination of clarithromycin, cetirizine and mometasone furoate. No data were presented for cetirizine treatment alone and so it is impossible to draw any conclusions about the use of cetirizine for eczema on its own from this trial.
Assessment of risk of bias
Table 54 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Diepgen 2002260 | Unclear risk | Unclear risk | Unclear risk | Powered to detect the primary outcome, which was not relevant to eczema |
Benefits
Diepgen and colleagues260 did not provide many methodological details in the trial report, instead referring the reader to a previous publication containing this information. 261 Cetirizine was described as having a similar appearance and taste to the placebo. The severity of eczema and use of mild and moderate to potent corticosteroids (secondary outcomes) did not significantly differ between the cetirizine group and the placebo group during the trial. The only significant differences between the groups were a lower use of other oral H1 antihistamines in the cetirizine group and a lower rate of development of urticaria in the cetirizine group, with the latter not being listed as an outcome of the trial in the report.
Harms
Very few details were provided about the adverse events recorded in the trial by Diepgen and colleagues260 apart from the levels of urticaria, for which there was a beneficial effect of treatment with cetirizine.
Overall implications for research and practice
The large, long-term trial by Diepgen and colleagues260 failed to find any significant difference between the groups in the severity of eczema or any topical corticosteroid-sparing effect from the use of 0.25 mg/kg of cetirizine twice daily. The lower rate of use of other H1 antihistamines in the cetirizine group makes it difficult to draw conclusions as antihistamines can be used to treat a variety of other, mostly allergic diseases, the level of which may have differed between the two groups, although the levels of sensitisation to milk, egg, grass pollen and house dust mite were similar between the groups.
Loratidine (oral) (less sedating)
Loratidine is a non-sedating second-generation H1 antihistamine used to treat allergies. In the UK it is sold over the counter (Clarityn®; Merck Sharp & Dohme Ltd) as well as being available on prescription.
Studies
Three trials involving loratidine were reported before 200055 (see Appendix 3).
Only one new trial262 used loratidine for the treatment of eczema and this was as the comparator to test the addition of modified Jiawei Danggui Decection (a type of Chinese medicine). As loratidine was used in both groups, this trial cannot be used to assess the effectiveness of loratidine.
Fexofenadine (oral) (less sedating)
Fexofenadine (Telfast®; Sanofi) is used for hay fever and other allergic conditions with similar symptoms. It is not as sedating as some other H1 antihistamines.
Studies
No studies of fexofenadine were reported before 2000.
Two trials involving fexofenadine were reported after 2000. One new trial, by Kawashima and colleagues,263 compared fexofenadine hydrochloride (60 mg) given twice daily (morning and evening) for 1 week against placebo. The study population of 411 adults had a diagnosis of eczema according to the Japanese Dermatological Association criteria26 and a pruritus score between 4 and 8 after 3 days of placebo treatment prior to enrolment. All participants received placebo for 1 week prior to the trial and used hydrocortisone butyrate (0.1%) twice a day for the placebo period before randomisation and during the treatment period.
A multicentre trial by Nakagawa and Kawashima264 compared fexofenadine hydrochloride (30 mg or 60 mg twice a day, depending on the age of the participant) against another antihistamine, ketotifen (1 mg twice a day), for 4 weeks. In total, 190 children aged 7–15 years with an average score of ≥ 2 for itching in the 3 days before allocation and requiring 0.1% hydrocortisone butyrate on ≥ 70% of their body were randomised.
Assessment of risk of bias
Table 55 provides the risk-of-bias assessment for the new studies.
Benefits
The trial by Kawashima and colleagues263 found a significant benefit of fexofenadine for itching as judged by the participants and also as measured by the ratio of area of pruritus to body surface area, assessed by an investigator.
In the trial by Nakagawa and colleagues264 there were no significant differences between the treatment groups in the mean change in itching score, daily change in itchiness, improvement of rash, participant assessment of the eczema and rate of adverse events.
Harms
In the trial by Kawashima and colleagues263 the number of participants who experienced adverse events was approximately the same in each group (48 in the fexofenadine group and 45 in the placebo group), with no serious adverse events and one withdrawal because of adverse events in each group. The most common adverse events reported were drowsiness, increases in serum bilirubin and glutamic-pyruvic transaminase, and positive urinary protein. The incidence of these events was similar between the two groups.
There were no serious adverse events reported in the trial by Nakagawa and Kawashima. 264
Overall implications for research and practice
Without an objective measure of severity in the trial by Kawashima and colleagues263 and because of the likelihood that judging the surface area of pruritus could be extremely difficult for an investigator, it is difficult to assess the significance of the efficacy results from this trial. Longer-term trials with at least one validated objective measure of eczema severity are needed. The trial by Nakagawa and Kawashima264 does not provide any evidence of benefit for fexofenadine compared with ketotifen.
There is not yet any positive RCT evidence for this treatment in children, in whom drowsiness is sometimes less of a problem or even a potential benefit for reducing sleep loss as a result of eczema.
Ketotifen fumarate and epinastine hydrochloride (oral) (less sedating)
Ketotifen fumarate (Zaditen®; Swedish Orphan Biovitrum) functions as a mast cell stabiliser and has been used as a treatment for chronic idiopathic urticaria because of its antipruritic properties. Similarly, epinastine hydrochloride is both an antihistamine and a mast cell stabiliser.
Studies
Two trials on ketotifen fumarate were reported before 200055 (see Appendix 3). There were no studies on epinastine hydrochloride reported before 2000.
Two trials involving ketotifen fumarate, one of which used epinastine hydrochloride as the comparator, have been published since 2000. The trial by Nakagawa and Kawashima264 compared fexofenadine against ketotifen fumarate and is discussed in the previous section.
The trial by the Epinastine Hydrochloride Dry Syrup Clinical Study Group265 compared epinastine hydrochloride (10 mg in 1 g of dry syrup) 1.0 g/day (body weight 14–24 kg) or 2.0 g/day (body weight > 24 kg) against ketotifen fumarate (1.38 mg in 1 g of dry syrup) 1.2 g/day (body weight 14–24 kg) or 2.0 g/day (body weight > 24 kg). The treatments were given as dry syrup for 4 weeks. The trial included 163 children aged up to 15 years. The trial was blinded using a double dummy design so that each group received one dose of treatment and one dose of placebo per day. Children who had been using mild or moderate topical corticosteroid for at least 1 week and who had an itching score of ≥ 2 were included.
Assessment of risk of bias
Table 56 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Epinastine Hydrochloride Dry Syrup Clinical Study Group 2004265 | Unclear risk | Unclear risk | Unclear risk |
Benefits
This trial carried out in Japan measured itching using three different outcomes and severity using two separate outcomes. The objective of the trial was to prove the non-inferiority of epinastine hydrochloride to ketotifen fumarate. The trial found no significant differences between the treatments at 2 or 4 weeks in terms of itching or severity of rash in a per-protocol population of 148 children.
Harms
There was a high rate of adverse events in the ketotifen fumarate group, with 22 out of 78 participants undergoing events that were considered to be related to the study treatment, compared with 9 out of 84 in the epinastine hydrochloride group. In particular, drowsiness (seven in the epinastine hydrochloride group and 18 in the ketotifen fumarate group) and nasopharyngitis (14 in the epinastine hydrochloride group and 11 in the ketotifen fumarate group) were common problems.
Overall implications for research and practice
The trial by the Epinastine Hydrochloride Dry Syrup Clinical Study Group265 was the only RCT found that investigated the use of epinastine hydrochloride for the treatment of eczema. This trial was short term and did not compare the treatments against a placebo or another non-antihistamine comparator. Both of the treatments considered resulted in fairly high levels of adverse events, most commonly drowsiness and nasopharyngitis. The evidence for a reduction in itching in adults using ketotifen fumarate has previously (published before 2000) been contradictory, with one trial providing evidence of a reduction from baseline after 3 months in adults and another not providing any evidence of a difference in itching or erythema compared with placebo after 4 months in children. 55 Longer-term studies comparing ketotifen fumarate and epinastine hydrochloride with other active eczema treatments in common use are needed to clarify whether their use for treating eczema is beneficial, especially when weighed against the level of adverse events.
Olopatadine hydrochloride (oral) (non-sedating)
Studies
One ‘double dummy’ multicentre trial conducted in Japan266 randomised 305 children aged 7–16 years with eczema according to the Japanese Dermatological Association criteria26 to olopatadine hydrochloride (10 mg/day) as a tablet or ketotifen fumarate dry syrup (2 mg per day) for 2 weeks. The children had to have an eczema lesion on the head, neck or face that was expected to be cleared by hydrocortisone butyrate ointment and at least a mild pruritus score (score of 2), with the score being different between night-time and daytime. All participants used hydrocortisone ointment twice a day in an observation period before starting the trial.
Assessment of risk of bias
Table 57 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Kawashima 2011266 | Unclear risk | Unclear risk | Unclear risk |
Benefits
The primary outcome of change in pruritus score and the secondary outcomes of change in global score assessed by a clinician and response score for itching, measured by participants on a 5-point narrative scale, were not significantly different between treatments.
Harms
In the olopatadine hydrochloride group, 29 out of 152 (19.1%) participants reported adverse events, including 18 adverse drug reactions. In the ketotifen fumarate group, 37 out of 153 (24.2%) participants reported adverse events, including 10 adverse drug reactions. None of the events were serious or severe.
Overall implications for research and practice
This trial of olopatadine compared with ketotifen fumarate, which was reported to be a non-inferiority trial, did not provide any evidence of a difference between the two treatments in the reduction of pruritus or eczema severity in children. It is impossible to assess whether or not olopatadine has any clinically relevant benefit for eczema compared with other treatments such as topical corticosteroids and emollients. Both treatment groups changed by an average of < 1 point on a 5-point scale for all outcomes measured. This raises doubts about the clinical relevance of both treatments. The lack of change could be because the severity of the eczema was potentially quite low at baseline because of the 1-week treatment period with topical corticosteroids before starting the study treatment.
Chlorpheniramine (oral) (sedating)
Chlorpheniramine (Piriton®; GlaxoSmithKline) is a relatively weak sedative antihistamine that is used for the prevention of rhinitis and urticaria.
Studies
One trial involving chlorpheniramine was reported before 200055 (see Appendix 3).
One new trial by Munday and colleagues267 compared chlorpheniramine elixir 2.5 ml (those aged 1–5 years) or 5 ml (those aged 6–12 years) before bedtime every evening with placebo for a period of 1 month. Participants were allowed a second dose after 3 hours. After 2 weeks, if sleeplessness was still present the dose could be doubled to 5 ml (those aged 1–5 years) or 10 ml (those aged 6–12 years) before bedtime every evening. The trial included 155 children aged 1–12 years with atopic eczema. All participants were given Unguentum M emollient (100 g) (Almirall Hermal GmbH), Efcortelan® (30 g) (GlaxoSmithKline) and hydrocortisone (1%) cream to use as necessary.
Assessment of risk of bias
Table 58 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Munday 2002267 | Unclear risk | Unclear risk | Unclear risk | Twelve participants left the study because of withdrawal of parental consent |
Benefits
This trial assessed an intention-to-treat population of 151 participants and did not find any significant differences in the investigator- and participant-assessed severity of itching score, assessed on a 5-point scale; participant-assessed sleeplessness because of itching and scratching; participant-assessed daytime drowsiness; investigator-assessed severity of eczema for excoriation; dryness; lichenification; exudation and crusting assessed on a VAS; and adherence with treatment by weighing the medication. The only significant finding was a reduction in erythema in the chlorpheniramine group.
Harms
Twenty participants out of 151 reported a total of 29 adverse events, of which none was serious. The rate of events was the same in the chlorpheniramine and placebo groups. The trial report did not provide any details about the nature of the adverse events. Three participants were reported to have withdrawn because of adverse events.
Overall implications for research and practice
The weight of evidence from one small study published before 2000 and one large study published in 2002 suggests that there is no beneficial effect of chlorpheniramine for eczema, although the studies were both short term. The fact that the large trial by Munday and colleagues267 attempted to detect a beneficial effect using several different outcomes, with none of these showing a beneficial effect, is all the more convincing. As yet, there is no evidence that using chlorpheniramine has a beneficial effect and some fairly strong evidence to suggest that it has no beneficial effect for eczema.
Doxepin (topical) (sedating)
Doxepin (a tricyclic antidepressant) has powerful antihistamine properties by antagonising the H1 and H4 receptors. It is available in oral and topical formulations (Xepin®; Cambridge Healthcare Supplies).
Studies
Four trials involving topical doxepin for eczema were reported before 200055 (see Appendix 3).
One new trial, by Lee and colleagues,268 compared topical doxepin (5%) cream applied four times a day for 7 days against placebo. The trial included 44 adults with eczema who had moderate to severe daily pruritus for at least 1 week before entering the trial.
Assessment of risk of bias
Table 59 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Lee 2006268 | Unclear risk | Unclear risk | Unclear risk | Intention-to-treat analysis not used |
Benefits
This small trial by Lee and colleagues,268 carried out in a Korean population, showed a significant effect of doxepin for the relief of itching. The study concentrated on itching outcomes, using two different measures, and also used EASI scores to assess the severity of eczema. As in the previous trials on this treatment,55 a significant improvement in pruritus was found but no significant effect on the severity of eczema. A 15.5% improvement in pruritus on day 1 in the doxepin group was found, rising to 42.6% by day 7. This was reported to be statistically significant in favour of doxepin but the data for the placebo group were not reported.
Harms
The most common adverse event was erythema and xerosis at the site of application, which affected five participants in the doxepin group and three participants in the placebo group. Drowsiness was also a problem for two participants in the doxepin group, with one of these participants withdrawing from the trial.
Overall implications for practice and research
The results of this trial in a Korean population are in agreement with those of the previously reported trials by Drake and colleagues,269,270 which had a very similar protocol. This trial also looked at 7 days of treatment and noted problems with drowsiness and application site erythema and xerosis. These additional data expand the evidence base for this treatment by confirming the results of the trials by Drake and colleagues. 269,270 Future trials could focus on the long-term relief of itching and levels of adverse events in comparison with other active treatments for the relief of itching, although it seems unlikely that these will be pursued. A clearer picture in specific groups such as infants and young children would be valuable to inform the best use of this treatment.
Mast cell stabilisers
Sodium chromoglycate (topical)
Sodium chromoglycate has been used as an inhaled powder for the treatment of asthma for over 30 years and has a very strong safety profile. The mechanism of action is partly the result of the drug inhibiting the release of inflammatory mediators from mast cells. It is now being investigated as a treatment for diseases such as eczema, for which it is added to topical preparations.
Studies
Ten trials of sodium chromoglycate for eczema were reported before 200055 (see Appendix 3).
One new trial, by Stainer and colleagues,271 compared topical sodium chromoglycate (4%) (Altoderm™; Thornton & Ross Ltd) against topical lotion vehicle, applied twice daily for 12 weeks. The trial included 114 children aged 2–12 years who were diagnosed with atopic eczema according to the UK Working Party’s criteria9 and who also had a SCORAD score between 25 and 60. The same diagnostic criteria were required at both of two assessments carried out 14 days apart. Also, overall skin condition and itching were required to be assessed as a score of at least 2 on a scale of 0–3 on at least four separate days within the 14-day baseline period.
Assessment of risk of bias
Table 60 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Stainer 2005271 | Low risk | Low risk | Unclear risk |
Benefits
The trial by Stainer and colleagues271 showed a significant difference in the severity of eczema in favour of sodium chromoglycate as measured by SCORAD scores and the participant-assessed overall skin condition scores. Interestingly, the participant-assessed itching and sleep loss scores were not significantly different between the topical sodium chromoglycate group and the vehicle group. The lack of reduction in itching differs from the results found in previous studies using different formulations of sodium chromoglycate.
Harms
The rate of participant-reported adverse events was quite high, at 66 out of 114, but there was no difference between the groups (34 placebo group, 32 sodium chromoglycate group). Eleven participants had an adverse event that was considered to be treatment related, four in the placebo group and seven in the sodium chromoglycate group. No severe adverse events were reported; however, there were more withdrawals assessed as being possibly, probably or highly related to the study treatment in the sodium chromoglycate group, with five in the sodium chromoglycate group and one in the placebo group.
Overall implications for research and practice
The body of evidence for topical sodium chromoglycate treatment is still mixed; however, a medium-sized methodologically robust trial in children did not find any beneficial effect on itching or sleep loss. This could be because of the formulation used or the population included in this study, with participants having to have a moderately high level of itching on at least 4 days out of 14 to be included in the study, as in some of the previous trials. The higher rate of withdrawal in the topical sodium chromoglycate group because of adverse events that were assessed as being possibly, probably or highly related to the study treatment needs further research, although there appears to be a low number of events possibly, probably or highly related to the study treatment overall.
Summary of antihistamines
Cetirizine (oral) (less sedating)
-
Four trials, two small and two medium sized, involving cetirizine for eczema were reported before 2000. The largest of these trials provided evidence of benefit for cetirizine but only at four times the normal dose. The other three trials did not provide any evidence of benefit.
-
One very large trial published in 2005, with an overall unclear risk of bias, provided evidence of no benefit of long-term twice-daily cetirizine (0.25 mg/kg) treatment.
Loratadine (oral) (less sedating)
-
Three trials involving loratadine were reported before 2000. Two trials, one small and one very small, with an overall unclear risk of bias, provided evidence of benefit for loratadine compared with placebo. The largest trial did not provide any evidence of benefit for loratadine compared with cetirizine.
-
Only one new trial, published in 2008, used loratadine for the treatment of eczema, comparing loratadine with and without the addition of modified Jiawei Danggui Decection (a type of Chinese medicine). As loratadine was used in both groups, this trial cannot be used to assess the effectiveness of loratadine.
Fexofenadine (oral) (less sedating)
-
No trials of fexofenadine were reported before 2000.
-
One large trial reported in 2006, with an overall unclear risk of bias, provided evidence of benefit for fexofenadine (60 mg twice daily) compared with placebo.
-
A further medium-sized trial reported in 2006, with an overall unclear risk of bias, did not provide any evidence of benefit for fexofenadine (30 or 60 mg twice daily) compared with ketotifen (1 mg once daily).
Ketotifen and epinastine (oral) (less sedating)
-
Two small trials on ketotifen compared with placebo reported pre 2000 gave conflicting results.
-
There were no trials involving epinastine for eczema reported before 2000.
-
One medium-sized trial reported in 2003, with an overall unclear risk of bias, provided evidence of the non-inferiority of ketotifen to epinastine.
Olopatadine hydrochloride (oral) (non-sedating)
-
There were no trials involving olopatadine hydrochloride for eczema treatment reported before 2000.
-
One large trial reported in 2011, with an overall unclear risk of bias, did not show a difference for children treated with olopatadine hydrochloride compared with ketotifen fumarate in terms of itch or eczema severity.
Chlorpheniramine (oral) (sedating)
-
One very small trial involving chlorpheniramine, with missing baseline data, was reported pre 2000; however, this trial did not compare the chlorpheniramine-only group with the placebo group and so did not provide any information on the possible benefit of chlorpheniramine compared with placebo.
-
One medium-sized trial reported in 2002, with an overall unclear risk of bias, did not provide any evidence of benefit for chlorpheniramine compared with placebo.
Doxepin (topical) (sedating)
-
Four fairly well-reported manufacturer-sponsored trials involving topical doxepin, two large and two small, were reported before 2000. Two of the trials provided evidence of benefit for topical doxepin compared with placebo and two did not.
-
One small trial reported in 2006, with an overall unclear risk of bias, provided evidence of benefit for doxepin compared with placebo.
Sodium chromoglycate (topical)
-
Ten trials of sodium chromoglycate were reported before 2000.
-
One new medium-sized trial, with a mostly low risk of bias, provided evidence of benefit for sodium chromoglycate (4%) compared with vehicle.
Chapter 8 Dietary interventions
Background
Polyunsaturated fatty acids are essential components of all cell membranes. There are two families of such essential fatty acids (EFAs): omega-6 fatty acids (n-6) (e.g. linoleic and arachidonic acid) and omega-3 fatty acids (n-3) [e.g. eicosapentaenoic acid (EPA)]. Eicosanoids are signalling molecules derived from fatty acids that play an important part in the inflammatory and immunological processes of atopic eczema. Alterations in linoleic acid metabolism have been demonstrated in some patients with atopic eczema, suggesting that a defect in the enzymatic conversion of this EFA by δ-6-desaturase might be responsible for defects in the lipid barrier of the skin and a decreased production of anti-inflammatory metabolites in the skin. These observations provide the rationale for dietary supplementation with EFAs in atopic eczema.
Supplementation with bacteria that may confer benefit (probiotics) or oligosaccharides that encourage the growth of beneficial bacteria (prebiotics) has become a focus of eczema research in the last decade. Evidence of changes in the balance of the gut microflora in those with eczema compared with those without eczema raised interest in interventions that could redress the balance, in the hope of a decrease in eczema severity.
Existing systematic reviews
There have been 20 systematic reviews of dietary interventions for established eczema. Five reviews79,272–275 specifically examine probiotic supplementation, a Cochrane review276 and subsequent non-Cochrane updated review277 specifically evaluate dietary exclusions, another Cochrane review278 specifically explores dietary supplementation and other reviews35,41,42,93,94,279–283 cover more than one dietary intervention. A Cochrane review of evening primrose oil and borage oil supplementation has also recently been published. 189
Scope of this chapter
The trials included in this chapter cover the following treatments:
-
probiotics
-
prebiotics
-
synbiotics
-
EFA supplementation
-
borage oil
-
evening primrose oil
-
fish oil (omega-3)/soybean oil (omega-6)
-
docosahexaenoic acid (DHA)
-
hempseed oil
-
-
oral vitamins D and E
-
goat’s/ass’s milk
-
hypoallergenic formula
Probiotics
Probiotics are live micro-organisms that can confer a health benefit on the host. The mechanism of action may involve reducing inflammation and permeability in the gut and modifying the microbiota or modulating immune responses in the host.
Mixed-strain probiotics
Studies
No RCTs were reported up to the year 2000 for mixed-strain probiotic treatment for eczema.
Five trials have been published since 2000. The trial by Rosenfeldt and colleagues284 compared a powdered mixture of lyophilised Lactobacillus rhamnosus 19070–2 and Lactobacillus reuteri DSM 122460 against powdered skimmed milk powder as a placebo. Fifty-eight children aged 1–13 years with eczema diagnosed using the UK Working Party’s criteria9 were given the active and the placebo treatment twice daily for 6 weeks in a randomised order, with a 6-week washout phase in between.
Sistek and colleagues285 compared a powdered mixture of L. rhamnosus and Bifidobacterium lactis against a placebo of microcrystalline cellulose. Sixty-two children aged 1–10 years were randomised to once-daily treatment for 12 weeks. Some older children took the treatments in the opaque capsules, but most children took the treatment as the powder mixed with water. The participants were followed for 16 weeks.
The trial by Cukrowska and colleagues286 specifically recruited children aged < 24 months who had eczema and symptoms of cow’s milk allergy when taking cow’s milk protein and who were currently not having milk in their diet or being breastfed. The trial compared a mixture of lyophilised Lactobacillus casein LOCK0900, Lactobacillus casein LOCK0908 and Lactobacillus species LOCK0919 on hydrolysed casein against a placebo of hydrolysed casein. Both treatments were taken for 3 months.
Yesilova and colleagues287 conducted an 8-week study comparing a mixture of four probiotic strains (Bifidobacterium bifidum, Lactobacillus acidophilus, Lactobacillus casei and Lactobacillus salivarius) with a ‘placebo’ of skimmed milk powder. Forty children aged 1–13 years with moderate to severe eczema according to the Hanifin and Rajkas8 criteria were randomised. Participants did not use any eczema medication during the 2 weeks before enrolment to the study. It is not clear whether the participants were allowed to use other treatments during the study.
The trial by Iemoli and colleagues288 compared a combination of two probiotic strains, L. salivarius LS01 and Bifidobacterium breve BR03, given twice daily for 12 weeks against placebo in 48 adults with eczema.
Assessment of risk of bias
Table 61 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Cukrowska 2008286 | Unclear risk | Unclear risk | Unclear risk | |
| Iemoli 2012288 | Unclear risk | Unclear risk | Unclear risk | |
| Rosenfeldt 2003284 | Unclear risk | Unclear risk | Unclear risk | |
| Sistek 2006285 | Low risk | Unclear risk | Low risk | |
| Yesilova 2012287 | Unclear risk | Unclear risk | Unclear risk | Disease severity was different between the groups at baseline |
Benefits
The trial by Rosenfeldt and colleagues284 showed no significant difference in the change in severity and itching during probiotic treatment compared with placebo treatment. The trial analysed the subgroup of participants who had one or more positive skin-prick tests and raised IgE and found a significant difference between the groups in the change in eczema severity from baseline. It is not clear whether this was a preplanned subgroup analysis.
The trial by Sistek and colleagues285 did not find any significant difference in change in severity from baseline between probiotic treatment and placebo treatment in the whole trial population when the results were adjusted because of significantly higher eczema severity in the treatment group at baseline. A significant difference in change in severity from baseline was found in a post hoc subgroup analysis of 43 children who were found to be sensitised to food (geometric mean ratio 0.73, 95% CI 0.54 to 1.00; p = 0.047). These results were also mirrored for the number of food-sensitised children who had an improvement in SCORAD score at the end of the 12 weeks of treatment (probiotic treatment 18/19, placebo group 15/24; p = 0.01). A post hoc analysis of those children who were not sensitised to food did not show any significant differences between groups.
The trial by Cukrowska and colleagues286 did not find any significant difference between the treatment groups in the number of participants with improved severity compared with the number of participants with no improvement or deterioration, either at the end of treatment (3 months) or after 8 months, for the whole treatment population, who all displayed symptoms of allergy to cow’s milk. A significant difference between the groups at the end of treatment was found in an identical post hoc analysis of participants with ‘IgE-dependant eczema’, with the number of participants in this subgroup not reported (odds ratio 11, 95% CI 1.108 to 112.08; p = 0.0329). No significant difference between the groups was found in an identical post hoc analysis of participants with ‘IgE-independent eczema’; again, the number of participants in this subgroup was not reported.
The trial by Yesilova287 reported a significant difference in SCORAD eczema severity from baseline to 8 weeks between the probiotic group (from 35.4 SD ± 13.4 to 12.4 SD ± 7.2) and the placebo group (from 28.1 SD ± 6.1 to 15.3 SD ± 5.1; p = 0.0015).
In the trial by Iemoli and colleagues,288 the changes in severity were not compared between the two groups, making it impossible to assess the results of the trial. The authors simply stated that there was a significant reduction in SCORAD score in the mixed probiotic group from baseline to the end of the study (from 46.2 to 29.4; p < 0.001) and not in the placebo group (from 45 to 40.2; not significant) and that this effect continued for 2 months after suspension of treatment. They also stated that there were significant changes in quality of life as measured by the DLQI in the probiotic group but not in the placebo group.
Single-strain probiotics
Studies
No trials were reported before 2000 on single-strain probiotic treatment for eczema.
Sixteen289–304 new trials were reported after 2000. Nearly all of the 16 trials reported compared a probiotic against an inactive placebo or occasionally against no treatment. Treatment was given for periods ranging from 4 weeks to 1 year (mostly 8 or 12 weeks) and some trials followed up the participants for a few more weeks. Three trials290,291,297 administered the probiotic in a hydrolysed cow’s milk formula and compared this against hydrolysed milk formula only in infants. Only one trial compared two strains of probiotics in hydrolysed milk formula against each other and a placebo of maltodextrin in a three-arm comparison trial. 302 In 13289–293,295–298,300,302 of the 16 trials the participants were children, particularly infants. Only three trials,294,299,301 conducted in Japan and Italy, included adult participants.
Assessment of risk of bias
Table 62 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| L. rhamnosis GG | ||||
| Brouwer 2006291 | Unclear risk | Unclear risk | Unclear risk | |
| Fölster-Holst 2006292 | Unclear risk | Unclear risk | Unclear risk | |
| Grüber 2007289 | Low risk | Unclear risk | Low risk | |
| Nermes 2011290 | Unclear risk | Unclear risk | Unclear risk | |
| Viljanen 2005293 | Low risk | Unclear risk | Unclear risk | |
| Other lactobacilli | ||||
| Drago 2012301 | Low risk | Low risk | Unclear risk | |
| Gøbel 2010300 | Unclear risk | Unclear risk | Unclear risk | |
| Gore 2012302 | Unclear risk | Unclear risk | Unclear risk | It is unclear whether intention-to-treat rules have been used in this study |
| Han 2012304 | Low risk | Low risk | Unclear risk | |
| Moroi 2011294 | Unclear risk | Unclear risk | Unclear risk | |
| Torii 2011296 | Low risk | Unclear risk | Unclear risk | |
| Weston 2005303 | Unclear risk | Unclear risk | Unclear risk | |
| Woo 2010295 | Unclear risk | Unclear risk | Unclear risk | |
| Bifidobacterium | ||||
| Isolauri 2000297 | Unclear risk | Unclear risk | Unclear risk | |
| Taniuchi 2005298 | Unclear risk | Unclear risk | Unclear risk | |
| Yoshida 2010299 | Unclear risk | Unclear risk | Unclear risk | |
Lactobacillus rhamnosis GG
Benefits
The trial by Nermes and colleagues290 found no significant difference in eczema severity between L. rhamnosis GG in extensively hydrolysed casein formula and the same formula only at either 1 month or 3 months (end of treatment). The trial by Grüber and colleagues289 found no significant differences in either severity or itching at any time point between placebo and L. rhamnosis GG groups.
In the trial by Brouwer and colleagues291 the change in severity of eczema was not significantly different between the L. rhamnosis in extensively hydrolysed whey formula group, the L. rhamnosis GG in extensively hydrolysed whey formula group and the extensively hydrolysed whey formula-only group and the change from baseline was significant in all three groups. In the trial by Fölster-Holst and colleagues292 there was no significant difference between the L. rhamnosis GG group and the placebo group in change in eczema severity, itching and sleep loss, quality of life and use of corticosteroids at any time point. The trial by Viljanen and colleagues293 found no significant difference in change in eczema severity in the whole population between the L. rhamnosis GG group, the mix of five probiotics group and the placebo group and also in subgroups of participants who were cow’s milk allergy positive, cow’s milk allergy negative and IgE association negative. There was a significant difference in change in eczema severity in the subgroup of participants who were IgE association positive; however, this was not reported as being a prespecified subgroup analysis.
Harms
Three of the trials290,291,293 did not report any data on adverse events. The two trials289,292 that reported adverse events did not find any serious adverse events. Other adverse events included lower respiratory tract infections, ear, nose and throat infections, gastrointestinal complaints, nausea and vomiting and other infections, but all of these were not significantly different between the treatment groups.
Other lactobacilli
Lactobacillus sakei KCTC 10755BP
The trial by Woo and colleagues295 compared Lactobacillus sakei KCTC 10755BP in microcrystalline cellulose twice daily for 12 weeks with placebo in children aged 2–10 years with stable, moderate to severe eczema (SCORAD score of > 25). All participants were told to bathe for 10 minutes a day and then apply emollients; they could also use prednicarbate (0.1%) as required during the trial. Although the severity of eczema, reported to be adjusted for SCORAD score and cytokines, was significantly different between the groups after 12 weeks of treatment, the trial report gives statements that seem to conflict with this result and make the results of this trial impossible to interpret.
Lactobacillus fermentum VRI-003 PCC
The trial by Weston and colleagues303 in Perth, Australia, compared treatment with Lactobacillus fermentum VRI-003 PCC twice a day for 8 weeks against placebo in 56 children aged between 6 and 18 months with moderate to severe eczema (modified SCORAD score of ≥ 25). The change in severity of eczema was recorded and decreased in both groups: the probiotics group decreased by a median of 17 (25th to 75th percentile: 9.8 to 24.6) and the placebo group decreased by a median of 12 (25th to 75th percentile: –5 to 20); however, a between-group analysis of these decreases was not reported. The percentage of participants with an improved SCORAD score at week 16 was reported as 92% in the probiotics group and 63% in the placebo group. There were no particular differences between the groups in parents’ perception of the eczema, both during treatment and after stopping treatment. Dermatitis Family Impact scores also hardly differed between the interventions. No specific adverse events were reported; however, one child’s vomiting did cause concern for the parents.
Lactobacillus acidophilus NCFM (ATCC700396)
A government-funded trial by Gøbel and colleagues300 in Denmark compared L. acidophilus NCFM (ATCC 700396) treatment once a day for 8 weeks against treatment with Bifidobacterium animalis subspecies Lactis Bi-07 (ATCC SD5220) or placebo. The trial included 50 children aged 7–24 months with eczema involving continuous itching. All but one participant had a first-degree relative with allergy. No significant differences were found between the groups in terms of change in severity of eczema, with or without adjustment for confounding factors: gender, predisposition to allergy, age and whether IgE values had increased. The analysis of severity included all 50 participants who were randomised to treatment. Objective SCORAD scores were also reported to show no differences. It is not clear whether this was a post hoc analysis. Data on adverse events were not reported.
Lactobacillus paracasei K71 (heat killed)
A government-funded trial carried out in Japan by Moroi and colleagues294 compared a once-daily dose of heat-killed Lactobacillus paracasei K71 for at least 12 weeks against placebo in 34 adults with mild to moderate eczema. There was no significant difference between the groups in change in eczema severity or change in itch scores or quality of life. There was greater use of topical corticosteroids in the placebo group but this was not significant. One participant in the treatment group suffered from headache and nausea and four participants in the placebo group had six adverse events (headache, toothache, diarrhoea, stomach ache, nausea and vomiting).
Lactobacillus paracasei CNCM I-2116
A trial by Gore and colleagues302 compared a once-daily dose of L. paracasei CNCM I-2116 with a once-daily dose of Bifidobacterium lactis CNCM I-3446 or placebo for 3 months in 137 babies aged 3–6 months with physician-diagnosed eczema. All participants were fed an extensively hydrolysed whey formula during the study. There was an open observational group including excluded babies (those who were exclusively breastfed and those whose parents did not want to substitute the study formula). In the highly selected trial population there was no significant difference in severity of eczema measured by the SCORAD index between randomised groups at any time point up to age 3 years. Results were similar when analysis was controlled for allergen sensitisation or when only sensitised infants were analysed. No steroid-sparing effect, measured using frequency of use and potency of topical steroids, was observed between groups. At the 4-week visit, 42 out of 137 (30.7%) parents reported some difficulties (e.g. green loose stools, increased vomiting, feed refusal or colic) related to the change in formula and 24 out of 137 (17.5%) had stopped using the study formula.
Lactobacillus acidophilus L-92
A trial by Torii and colleagues296 conducted in Japan compared heat-treated L. acidophilus L-92 given for 8 weeks against placebo in 60 children with eczema who were tolerant to cow’s milk. The severity of eczema, measured using an adaptation of the Atopic Dermatitis Area and Severity Index (ADASI), significantly decreased over time in the treatment group compared with the placebo group (p = 0.0474). No other measures of clinical benefit were performed. No information about adverse events was reported.
Lactobacillus salivarius LS01
A trial by Drago and colleagues conducted in Italy301 compared Lactobacillus salivarius LS01 given twice daily for 16 weeks against a placebo of maltodextrin in 38 adults with moderate to severe eczema. The authors did not compare the changes in severity between the two groups, making it impossible to assess the comparative effectiveness of the treatments being tested. They stated only that there was a significant reduction in SCORAD score in the probiotic group from baseline to the end of the study (from 27.6 to 13.1; p < 0.001) but not in the placebo group (from 24.3 to 20.1; not significant). Likewise, the authors reported significant changes in quality of life, measured using the DLQI, in the probiotic group [from 8.28 SD ± 1.79 at baseline to 4.57 SD ± 1.11 after 8 weeks (p = 0.02) and 4.42 SD ± 0.27 after 16 weeks (p = 0.04)] but not in the placebo group. No significant adverse events were recorded.
Lactobacillus plantarum CJLP133
A 16-week trial by Han and colleagues304 conducted in South Korea compared twice-daily Lactobacillus plantarum CJLP133 for 12 weeks against placebo in 118 children aged 1–13 years with eczema according to the Hanifin and Rajkas8 criteria. Disease severity measured by the SCORAD index was significantly lower in the probiotic group than in the placebo group after 14 weeks (20.4 vs. 25.6; p = 0.044). Changes in disease severity measured by the SCORAD index were significantly higher in the probiotic group than in the placebo group at 14 weeks (9.1 vs. 1.8; p = 0.004). The changes were also significantly different at 16 weeks (7.6 vs. 2.6; p = 0.041). Overall, the changes were significantly different in the intention-to-treat population and in patients who did not use topical steroids but not in patients who did use topical steroids as a rescue treatment during the study. However, the total amounts of topical corticosteroids used through the trial in the probiotic and placebo groups were the same. Adverse events were not reported in this study.
Bifidobacterium
Bifidobacterium breve YY
A small pilot study carried out in Japan with an 8-week duration299 compared twice-daily treatment with capsules of live lyophilised B. breve YY against placebo in 24 adults with eczema diagnosed according to the Japanese Dermatological Association26 criteria. Severity measured by objective SCORAD score and quality of life were both reported as significantly different between the groups, with a decrease from a mean objective SCORAD score of 33.7 to 28.9 in the treatment group of 16 adults compared with a decrease from a mean objective SCORAD score of 21.8 to 21.1 in the placebo group of eight adults. The baseline values for both total and objective SCORAD scores and quality of life, as well as other indicators of severity such as thymus and activation-regulated chemokine and serum IgE levels, were significantly higher in the treatment group at baseline, which calls into question the randomisation process and makes the changes in severity and quality of life in this trial impossible to interpret. No data on adverse events were reported for this trial.
Bifidobacterium lactis Bb-12
A trial by Isolauri and colleagues297 randomised 27 exclusively breastfed infants to three treatment groups: B. lactis Bb-12-supplemented extensively hydrolysed formula, L. rhamnosis GG-supplemented extensively hydrolysed formula or unsupplemented extensively hydrolysed formula. It is not clear how long the participants were given treatment for but all participants were weaned on to the formula at the start of the study. The probiotic-supplemented formulas both showed significant decreases in eczema severity after 2 months compared with the unsupplemented formula, which showed an increase in eczema severity [B. lactis Bb-12: from median of 12 to 0 (range 0–3.8); L. rhamnosis GG: from median of 14.5 to 1 (range 0.1–8.7); unsupplemented formula: from median of 10 to 13.4 (range 4.5–18.2)]. All baseline values were extrapolated from a graph in the trial report. Interestingly, all three treatment groups had a median SCORAD score of 0 (range 0–6.6) at 6 months.
Bifidobacterium breve M-16V
A trial by Taniuchi and colleagues298 compared B. breve M-16V with placebo. The trial included participants with cow’s milk allergy proven by clinical symptoms, a positive radioallergosorbent test (RAST), a positive skin-prick test and < 30% Bifidobacterium in their intestinal microflora after being fed casein-hydrolysed formula milk for at least 1 week. The planned outcome of eczema severity was not reported and so it is impossible to assess the impact of this probiotic on eczema.
Overall implications for research and practice for mixed-strain and single-strain probiotics
There is reasonably strong evidence from three trials284–286 that mixtures of probiotic strains do not show any benefit over placebo for children with eczema, whether or not they have symptoms of cow’s milk allergy or are sensitised to one or more allergens. All three trials undertook subgroup analyses that were not prespecified and reported a significant benefit for those children with raised IgE levels. Only one small trial comparing a mixture of four different probiotic strains compared with skimmed milk powder in children reported a significant difference in change in disease severity between the probiotic group and the placebo group. 287 However, although not compared statistically, the severity of eczema in the active treatment group was noticeably higher than in the placebo group at baseline and it was unclear whether this imbalance had been adjusted for in the statistical comparisons. In the absence of any trials specifically designed and powered to test mixtures of probiotic strains in people with eczema who are proven as ‘atopic’ with raised circulating IgE antibodies, it is impossible to assess whether probiotics are of true benefit over placebo. In reality, routine tests for atopy are not conducted, especially in primary care, and with up to two-thirds of those with eczema not ‘atopic’ there is only limited potential for mixtures of probiotic strains for the treatment of established eczema.
Probiotic strains of lactobacilli mostly do not show any good evidence of significant benefit over placebo in addition to standard treatments for eczema over a period of 8–12 weeks. Most of the trials do not give enough information about the methodology used, especially for allocation concealment, blinding and which outcome and statistical analyses were prespecified. The trials have mostly been small and often raise questions about the generalisability of the results, as selective trial populations have been studied. All five trials on L. rhamnosis have not shown any evidence of clinical benefit for eczema compared with placebo and probably now equate to evidence of no benefit, given the overall weight of evidence. One moderately sized trial involving L. paracasei in unselected infants provided no evidence of benefit of this treatment compared with placebo. Another trial involving L. plantarum provided some weak evidence of benefit compared with placebo. The only potential evidence of benefit may be for people with truly ‘atopic’ eczema, as suggested by a few reported subgroup analyses; however, this requires further specific research as none of these subgroups appears to have been prespecified. Even though there is a large amount of ‘noise’ in these trials because of a lack of clarity of reporting, it seems likely that, with the majority of trials on lactobacilli probiotics not showing any significant benefit for the treatment of eczema, there is no beneficial effect to be found.
Prebiotics
Prebiotics are short-chain carbohydrates (oligosaccharides) that stimulate the growth of beneficial gut bacteria such as lactobacilli and bifidobacteria species.
Studies
No RCTs involving prebiotics were reported before 2000.
Two new trials have been published since 2000. A trial conducted in Japan by Shibata and colleagues305 included children under 3 years of age with atopic eczema defined according to the criteria of the Japanese Dermatological Association. 26 The trial compared oral kestose, an oligosaccharide that encourages the growth of bifidobacteria, every day for 12 weeks against no treatment. The participants were able to use topical corticosteroids during the trial.
A trial by Ghanei and colleagues306 carried out in Iran compared fructo-oligosaccharides and inulin in powder added to milk against a placebo of dextrin powder. The trial included 90 children aged between 7 and 24 months who were full term and a normal birth weight. The children had been delivered by caesarean section and diagnosed with eczema by a physician and had started solid food by the age of 6 months. The treatment was given every day for 90 days (5 g for those aged 7–12 months, 7.5 g for those aged 13–18 months and 10 g for those aged 19–24 months).
Assessment of risk of bias
Table 63 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Ghanei 2011306 | Unclear risk | Unclear risk | Unclear risk | Baseline severity of eczema in the active group was twice as high as that in the control group. No intention-to-treat analysis reported and final number analysed was only 70 out of 90 randomised |
| Shibata 2009305 | Low risk | Unclear risk | Low risk |
Benefits
The trial by Shibata and colleagues305 showed a significant reduction in eczema severity for the participants using the kestose probiotic compared with those using the placebo after 12 weeks, with a median SCORAD score in the kestose group of 19.5 (range 25.6–22.0) and in the placebo group of 37.5 (range 25.5–43.5; p < 0.001). The baseline SCORAD values were 41.3 (range 36.2–46.4) in the kestose group and 38.3 (range 26.3–41.8) in the placebo group and, although the change in severity of each group was not compared, it does seem to show a greater decrease in the kestose group; however, no analysis for this was performed. This apparent benefit was reflected in the measurement of eczema severity using the Intensity of Atopic Dermatitis (maximum score 12 points), although, again, no analysis of the difference between the groups in change in severity was carried out.
The trial by Ghanei and colleagues306 reported a significant difference between the groups in eczema severity at the end of treatment; however, again, no comparison was made of the change in eczema severity between the groups over the treatment period. This change was reported as significant for both groups and so it is unlikely that there is a significant benefit from the fructo-oligosaccharide and inulin powder compared with placebo for the treatment of eczema.
Harms
The trial by Shibata and colleagues305 did not report information on adverse events. In the trial by Ghanei and colleagues306 there was one report of diarrhoea lasting for > 1 week in the placebo group; no other information was reported.
Overall implications for research and practice
The lack of information on the relative use of other eczema treatments by participants and the appropriate comparisons of eczema severity, coupled with a large difference in baseline eczema severity in one trial, make interpretation of the clinical significance of these trials very difficult. The relatively large number of withdrawals of those not taking the treatment for > 75 out of 90 days in the trial by Ghanei and colleagues306 may be masking potential problems with the treatment, such as unpalatability or even adverse events. Trials that pay detailed attention to randomisation, allocation concealment and blinding of all stakeholders, especially the outcome assessors and the participants, are needed. The age range of the participants in these trials also poses a problem as there is a large change in the prevalence of eczema over the age range from 6 months to 3 years.
Synbiotics
Synbiotics are optimal combinations of prebiotics and probiotics.
Studies
No trials involving synbiotics were reported up to the year 2000.
Eight trials307–314 involving synbiotics were reported from 2000. The eight trials on synbiotics all tested different combinations of probiotics and prebiotics. All but two trials309,313 compared the synbiotics against an inactive placebo or a placebo of the hydrolysed formula given to both groups. These other two trials compared the synbiotic against the probiotic or the prebiotic only.
Assessment of risk of bias
Table 64 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Farid 2011312 | Unclear risk | Unclear risk | Unclear risk | Relatively large number of withdrawals (12/40), who were not included in the final analyses |
| Gerasimov 2010307 | Low risk | Unclear risk | Low risk | |
| Hattori 2003310 | Unclear risk | Unclear risk | Unclear risk | |
| Murosaki 2006311 | Unclear risk | Unclear risk | High risk | |
| Passeron 2006309 | Unclear risk | Unclear risk | Unclear risk | |
| Shafiei 2011314 | Low risk | Unclear risk | Low risk | Not clear whether the five participants who withdrew were included in the analyses |
| van der Aa 2010308 | Low risk | Low risk | Low risk | Industry sponsored (Danone) |
| Wu 2012313 | Low risk | Unclear risk | Low risk |
Benefits
Farid and colleagues312 found a significant difference in change in eczema severity according to SCORAD scores from baseline to 4 weeks [treatment group mean –29.51 (SD 19.09) vs. control group mean –11.06 (SD 10.96); p = 0.001] and 8 weeks [treatment group mean –39.2 (SD 24.22) vs. control group mean –20.10 (SD 8.63); p = 0.005], but the difference was not significant between week 4 and week 8.
Gerasimov and colleagues307 found a significant difference in change in eczema severity from baseline after 8 weeks of treatment [L. acidophilus DDS-1 and B. lactis UABLA-12 combined with fructo-oligosaccharide group mean –14.2 (SD 9.9) vs. placebo group mean –7.8 (SD 7.7); p = 0.001]. It is not clear whether the analysis was carried out on an intention-to-treat basis including the five participants in the treatment group and one in the placebo group who withdrew during the trial.
Van der Aa and colleagues308 compared a mixture of B. breve M-16V and galacto-/fructo-oligosaccharide against placebo and found no significant difference in change in eczema severity after 12 weeks in the whole trial population. There was, however, a significant difference in change in severity within the IgE-associated eczema subgroup of 45 participants (synbiotic group –18.1 SD ± 1.6 vs. control group –13.5 SD ± 1.6; p = 0.04), although it was not clear whether this was a preplanned analysis.
Hattori and colleagues310 also found a significant difference in change in eczema severity from baseline, measured using cutaneous signs, between B. breve M-16V with raffinose and placebo (p = 0.0344). This analysis involved only 15 participants and the median change in score from baseline was –3.5 (range 0 to –6) for the synbiotics group compared with –1 (range 0 to –4) for the placebo group.
Murosaki and colleagues311 and Passeron and colleagues309 reported no significant difference in change in severity of eczema, itching or quality of life for synbiotic treatment compared with placebo311 or probiotic. 309 Shafiei and colleagues314 reported no significant difference in change in eczema severity between synbiotic and placebo treatment for 2 months.
Wu and colleagues313 compared a mixture of L. salivarius plus prebiotic (fructo-oligosaccharide) for 8 weeks against prebiotic alone in 60 children with moderate to severe eczema. They found a significant difference in eczema severity measured by the SCORAD index between the experimental group and the control group at 8 weeks (27.4 vs. 36.3; p = 0.022). No significant differences were found between groups for quality of life assessed by an unvalidated scale or for medication use.
Harms
These trials reported a range of levels of adverse events. Only the trial by Hattori and colleagues310 did not report any information about adverse events. Serious adverse events in the probiotics group consisted of one burn and one croup and those in the placebo group consisted of one head injury and two food poisoning cases. 307 Two serious adverse events that resulted in hospitalisation were a case of respiratory syncytial virus and a severe allergic reaction to cow’s milk. 308 Farid and colleagues312 reported no significant adverse events but did not give any further data on adverse events. Passeron and colleagues309 reported no serious adverse events and three cases of abdominal pain, two in the synbiotics group and one in the probiotics group. Murosaki and colleagues311 reported one case of nausea in the placebo group. Van der Aa and colleagues308 reported that 91.1% of participants in the synbiotic group and 84.1% of participants in the placebo group experienced adverse events but that none of these was considered to be related to treatment; one participant used antibiotics in the synbiotics group and five participants used antibiotics in the placebo group, but this was not significantly different between the groups. Gerasimov and colleagues307 reported that 60.5% (n = 26) in the synbiotics group and 51.5% (n = 24) in the placebo group experienced adverse events. The trial by Shafiei and colleagues314 did not specifically report adverse events; however, two children were withdrawn because of diarrhoea in a twin. Wu and colleagues313 reported mild diarrhoea in two patients in the synbiotics group.
Overall implications for research and practice
A feature of these trials appears to be the level to which ‘data mining’ is taking place, that is, trying to find a significant result using outcomes and subgroups that were not originally planned. The evidence is conflicting over whether synbiotics are beneficial for eczema. This may be because of the differences in the combinations of probiotics and prebiotics tested; however, the lack of methodological detail and small trial populations also mean that the evidence is not convincing.
Essential fatty acid supplementation
Borage oil
Borage oil is extracted from the seeds of the borage plant (Borago officionalis) and has high levels of omega-6 fatty acids, higher than evening primrose oil and blackcurrant seed oil.
Studies
Five trials involving borage oil were reported before 200055 (see Appendix 3).
One new trial involving borage oil was reported after 2000. This trial, by Takwale and colleagues,315 compared borage oil capsules (23% gamma-linoleic acid) with liquid paraffin (adults) or olive oil (children), given twice daily for 12 weeks. In total, 151 participants were randomised.
Assessment of risk of bias
Table 65 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Takwale 2003315 | Low risk | Low risk | Unclear risk | Although it is reported that the participants were blinded and that the capsules for the treatments ‘matched’, it is not reported whether the outcome assessor for severity of eczema was blinded |
Benefits
This trial315 focused on participant-assessed outcomes of itching, sleep loss and irritability as well as response to treatment, tolerability and need for topical corticosteroids. The trial also objectively measured the severity of eczema using SASSAD scores. The treatments were given for a reasonably long period of time to potentially see an effect; however, this trial failed to show any significant differences between the treatments for any of these outcomes. There was also no significant difference between the groups in the need for topical corticosteroids.
Harms
Many different adverse events were reported, with the most common being upper respiratory tract infection, which affected 26% of the borage oil group and 38% of the placebo group. The next most common events were diarrhoea (7% of the borage oil group and 11% of the placebo group) and skin sepsis (7% of the borage oil group and 13% of the placebo group). The report does not comment on whether any of the adverse events reported are related to the study treatment.
Evening primrose oil (oral)
Studies
Six trials involving evening primrose oil were reported up to 200055 (see Appendix 3).
One new trial316 by Senapati and colleagues was reported in 2008. Oral evening primrose oil [8–10% gamma-linolenic acid (GLA) per 500-mg capsule] was compared with oral sunflower oil (300-mg capsules) for 5 months. The 65 participants took different numbers of capsules per day, split into two doses, ranging from one to four capsules for those aged < 1 year and 12 capsules for those aged ≥ 16 years. Participants were always given the maximum dose applicable.
Assessment of risk of bias
Table 66 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Senapati 2008316 | Low risk | Unclear risk | Unclear risk | Serious concern of attrition bias as analysis included only the first 25 participants from each group |
Benefits
Both groups showed a significant difference in total severity score (extent, intensity, dryness and itching) between baseline and 5 months. The number of participants who ‘improved’ after 5 months (≤ 75% of their baseline score) compared with baseline was 96% (24/25 participants) for treatment with evening primrose oil and 32% (8/25 participants) for placebo treatment (difference between groups p = 0.00001). None of the other outcome measures was compared between groups and so it is not clear whether there were any other significant differences between treatments.
Harms
It was reported that no treatment-related adverse events occurred during the trial.
Fish oil (omega-3)/soybean oil (omega-6)
Fish oils contain the omega-3 fatty acids EPA and DHA. EPA and DHA are precursors to a number of substances that have been shown to reduce inflammation. Soybean oil is one of the most widely consumed cooking oils and contains high levels of omega-6 fatty acids.
Studies
Three trials involving fish oil were reported before 200055 (see Appendix 3).
One new trial involving fish oil was reported after 2000. This trial, by Mayser and colleagues,317 compared two 10% lipid emulsions, one derived from fish oil (n-3) and one derived from soybean oil (n-6). These were delivered by intravenous infusion twice a day for 10 days. The trial involved 22 participants with moderate to severe eczema according to the Hanifin and Rajka8 criteria, with > 10% body surface area involvement.
Assessment of risk of bias
Table 67 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Mayser 2002317 | Unclear risk | Unclear risk | Unclear risk | Withdrawals were replaced with more randomised participants but it is not clear whether the withdrawals were included in the analysis |
Benefits
This small industry-funded trial in hospitalised participants found a short-term benefit of fish oil over soybean oil, which started at day 6 of the infusion treatment and disappeared when the infusions were stopped, resulting in some cases of a relapse of eczema. In the initial few days of treatment, the fish oil infusion showed some hint of benefit and from day 6 to day 10 there was a significant difference in favour of fish oil infusion.
Harms
One participant given the fish oil infusion experienced vertigo, whistling in the ears and pallor after the infusion. One participant given the soybean oil infusion experienced hypertriglyceridaemia. Three participants in the fish oil group and one in the soybean oil group had an oily taste in their mouth during the infusion.
Docosahexaenoic acid
This acid belongs to the group of n-3 polyunsaturated fatty acids and inhibits T-cell activation and proliferation and reduces the numbers of granulocytes circulating in the blood.
Studies
Docosahexaenoic acid had not previously been tested as a treatment in its own right before 2000.
One new trial involving DHA was reported after 2000. This trial, by Koch and colleagues,318 recruited 54 adults aged between 18 and 40 years who had eczema according to the Hanifin and Rajka8 criteria. The participants received either 5.35 g of DHA and 0.37 g of EPA as ethyl esters per day or 4.17 g of caprylic acid and 2.84 g of capric acid per day for 8 weeks as seven capsules per day.
Assessment of risk of bias
Table 68 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Koch 2008318 | Unclear risk | Low risk | Unclear risk | Intention-to-treat principle was not used for the analyses |
Benefits
This fairly small trial318 found no significant difference between the groups in the severity of eczema measured using the SCORAD index. There was a decrease in the median SCORAD score of 20 for the DHA treatment group and 12.4 for the placebo treatment group in the participants who completed the trial at week 20.
Harms
The authors reported that three participants experienced mild abdominal discomfort in the DHA group. It does not appear from the trial report that there were any withdrawals because of adverse events. Of the 53 participants, 44 completed the entire study course. Six patients failed to complete the study, two because of ‘hospitals stays’ and four because of ‘non-compliance’. Three patients were excluded from the analysis, one because of ‘excessive sunbathing’ and two because of a lack of interruptions to the trial protocol regarding the use of standard medication.
Hempseed oil
Up to 35% of the weight of hempseed is an edible oil with a high EFA content (approximately 80%), including omega-6 (55%) and omega-3 (22%) fatty acids. This oil is extracted and often used as a dietary supplement.
Studies
Hempseed oil had not previously been tested as a treatment for eczema before 2000.
One new trial involving hempseed oil was reported after 2000. This crossover trial by Callaway and colleagues319 compared cold pressed hempseed oil against cold pressed extra virgin olive oil. Twenty adults aged between 25 and 60 years with eczema according to the Hanifin and Rajka8 criteria and a body mass index of < 30 kg/m2 were included. Participants supplemented their diet with one type of oil for 8 weeks and, following a 4-week wash-out period, then supplemented their diets with the other type of oil for 8 weeks.
Assessment of risk of bias
Table 69 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Callaway 2005319 | Low risk | Unclear risk | High risk | Lead author had a financial interest in the production and sale of hempseed oil. Four out of the 20 participants withdrew and were not included in the analysis |
Benefits
This trial did not show any significant benefit from taking cold pressed hempseed oil for eczema.
Harms
Only 16 patients (one male, 15 female) completed the study. Of the withdrawals, three occurred in the first week for personal reasons and one patient withdrew in week 14 because of the taste of the hempseed oil. However, the trial reported no adverse events from the treatments in the trial.
Overall implications for research and practice
The large fairly well-reported trial on borage oil315 showing no evidence of benefit for objective and participant-assessed outcomes, similar to the results found in a previous large trial,320 adds to the weight of evidence showing no beneficial effect of borage oil for eczema. The questions around whether there is a subgroup of the population with eczema who may respond to borage oil and whether increased adherence may show beneficial effects have not been addressed.
The additional small study on oral evening primrose oil316 showed a significant benefit of taking evening primrose oil compared with a placebo of sunflower oil. The potential bias introduced by analysing only the first 25 participants from each treatment group, and the lack of clarity about whether the outcome assessors were blinded to treatment allocation, means that this result must be treated with caution. This trial does not change the overall body of evidence on evening primrose oil for eczema, with the largest and best-reported trials not providing any evidence of benefit and other trials providing conflicting results (ranging from no hint of benefit to a 10–20% improvement).
A small pilot trial317 showed a short-term but marked improvement in eczema in a small number of participants treated with fish oil and a slower to develop, but longer-lasting improvement in participants treated with soybean oil. It is not easy to interpret whether these results are clinically significant because of the lack of a control group of standard care and a placebo. A concern with this trial is the addition of new randomised participants when participants were withdrawn as it is not clear which participants were included in the final analysis. Further trials on these treatments should evaluate whether they can be taken orally as infusions may not be as readily acceptable to patients and take more resources to administer.
The trial on DHA318 showed a hint of a modest beneficial effect of DHA compared with EPA on eczema severity after the treatment had been stopped. Larger, longer-term trials are needed that concentrate on participant-assessed outcomes such as quality of life and acceptability of treatment, as well as on objective and subjective measures of severity.
The very small pilot trial on hempseed oil,319 in which the participants would have very easily been able to determine which treatment they were taking, did not show any significant benefit from hempseed oil for skin dryness and itchiness as assessed by the participants.
Vitamins D and E (oral)
As phototherapy is beneficial for eczema, is has been hypothesised that vitamin D supplementation may be beneficial for those living with eczema at higher latitudes, as vitamin D deficiency is more common. The possible association between vitamin D levels, eczema prevalence and severity is unclear. 321–325
Studies
No trials reported before 2000 involved oral vitamin D as a treatment for eczema.
Three trials were reported after 2000, with one trial examining oral ergocalciferol (vitamin D2),326 one examining cholecalciferol (vitamin D3) and one examining vitamin D3 and vitamin E supplementation.
The small pilot trial by Sidbury and colleagues,326 reported in a letter, compared 1000 IU per day of ergocalciferol with placebo for 1 month in children aged 2–13 years. The eczema must have had a winter onset or exacerbation and the trial was conducted in the winter. All but one child had mild eczema (EASI score between 10 and 18.6). The 11 children randomised into the trial were allowed to continue all current treatments but were not allowed to start any new treatments and were not allowed to travel to temperate climates during the trial.
The trial by Amestejani and colleagues327 compared 1600 IU per day of cholecalciferol for 2 months with placebo in adults and adolescents aged ≥ 14 years. In total, 60 participants diagnosed with eczema using the Hanifin and Rajka8 criteria and with a mean SCORAD score of 25 were randomised; however; only the 54 participants who completed the study were included in the analysis. Usual treatments (emollients, topical corticosteroids, oral antihistamines) were permitted during the study period in both treatment groups.
The trial by Javanbakht and colleagues328 randomised 52 adult participants with eczema according to the Hanifin and Rajka8 criteria into four treatment groups: 1600 IU per day of vitamin D3 taken concurrently with placebo; 600 IU per day of vitamin E taken concurrently with placebo; 1600 IU per day of vitamin D3 taken concurrently with 600 IU per day of vitamin E; and placebo. The participants took the treatments once daily, separately, with a meal (vitamin D or placebo as one capsule, vitamin E or placebo as two soft gels). All participants could use standard prescription eczema treatments during the trial.
Assessment of risk of bias
Table 70 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Amestejani 2012327 | Low risk | Unclear risk | Unclear risk | Intention-to-treat principle was not used for the analyses |
| Javanbakht 2011328 | Low risk | Low risk | Low risk | It is not clear whether the intention-to-treat principle was used for the analysis |
| Sidbury 2008326 | Unclear risk | Low risk | Unclear risk |
Benefits
In the study by Sidbury and colleagues,326 four out of five children who took vitamin D2 and one out of six children in the placebo group improved by one category on the IGA scale compared with baseline. One out of five children who took vitamin D2 and four out of six children in the placebo group did not change score on the IGA scale compared with baseline. One out of six children in the placebo group worsened by one category on the IGA scale.
In the trial by Amestejani and colleagues,327 the efficacy of vitamin D3 was not statistically compared with that of placebo in the trial report, making it impossible to assess the results of the trial. Disease severity in the vitamin D3 group measured by the SCORAD index showed a significant improvement between baseline and the end of the study (from 24.8 to 15.3; p = 0.01). Likewise, disease severity in the vitamin D3 group measured by the TIS score also showed a significant improvement. No significant reduction in eczema severity was observed in the placebo group.
In the study by Javanbakht and colleagues,328 four small groups were included (n = 13) yet the trial failed to compare the changes in disease severity between the groups. The rates of SCORAD score improvement from baseline were 34.8% for the vitamin D3 plus placebo group, 35.7% for the vitamin E plus placebo group, 64.3% for the vitamin D3 plus vitamin E group (p = 0.004) and 28.9% for the placebo group. The changes in the pruritus VAS in the four groups were from 5.7 to 5, from 6.2 to 3.4, from 5.5 to 2 and from 7 to 4, respectively. The changes in the sleeplessness VAS were from 2.3 to 1.4, from 4.3 to 1.3, from 3.2 to 1 and from 1.1 to 1, respectively. The percentage decrease in topical corticosteroid use was 66.8%, 70.2%, 88.7% and 37.5%, respectively (p = 0.05).
Harms
Information about adverse events was not reported for these trials.
Overall implications for research and practice
These trials are too small and have not been analysed appropriately to provide any good evidence about whether oral vitamin D or vitamin E supplementation is beneficial or not for eczema. However, there is enough of a signal that a full trial of oral vitamin D or vitamin E supplementation may be worth pursuing. Without additional research evidence, the role of vitamin D or vitamin E supplementation remains unclear.
Goat’s and ass’s milk
Cow’s milk allergy can exacerbate eczema symptoms and, especially in infants, it is important that the nutrients available in cow’s milk, such as calcium, are replaced.
Studies
Goat’s milk and ass’s milk had not previously been tested as treatments for eczema before 2000.
One new trial involving goat’s and ass’s milk was reported after 2000. This crossover trial by Vita and colleagues329 randomised 28 children aged from 6 months to 3 years with active (SCORAD score of > 20) atopic eczema and a clinical history of cow’s milk allergy. The children were randomised to goat’s milk or ass’s milk for 6 months and then switched to the other milk for a further 3 months.
Assessment of risk of bias
Table 71 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Vita 2007329 | Unclear risk | Unclear risk | Low risk |
Benefits
This small trial329 found a large magnitude of effect for ass’s milk compared with goat’s milk, with a median SCORAD score reduction of 34 points for ass’s milk and 11 points for goat’s milk. Despite the participants being aware of the milk that they were drinking, their assessment of skin symptoms also showed the same result. Treatment with ass’s milk resulted in a 4.5-point decrease on a VAS whereas treatment with goat’s milk resulted in a 0.5-point decrease. Treatment with goat’s milk also resulted in 23 out of 26 participants having a positive reaction to a double-blind placebo-controlled food challenge whereas only one participant out of 26 had a positive reaction to ass’s milk.
Harms
No specific details about adverse events were reported; however, it was stated that one participant had to withdraw because of a systemic reaction to goat’s milk involving shortness of breath, sneezing and severe generalised urticaria.
Overall implications for research and practice
This one small crossover trial329 in a very specific eczema population provides some interesting but fairly weak evidence against the use of goat’s milk for children with eczema and a cow’s milk allergy because of a lack of a beneficial effect and high levels of development of sensitivity to the goat’s milk. Ass’s milk appears to show a large beneficial effect in this population but this needs further confirmation in trials comparing ass’s milk with the commonly used cow’s milk substitute of soy milk.
Hypoallergenic formula
Studies
Hypoallergenic milk formula had not previously been tested as a treatment for eczema before 2000.
Two new studies were reported after 2000. 330,331 A crossover trial by Leung and colleagues330 involving hypoallergenic formula was reported in 2004. This trial randomised 15 children aged < 3 years and taking at least 500 ml of soy or cow’s milk-based formula to a hypoallergenic formula that was lactose and sucrose free (Neocate®, SHS International) or the child’s pre-existing milk formula. Each formula milk was given for 6 weeks in a randomised order.
Jin and colleagues331 conducted a 12-week study comparing a partially hydrolysed whey and casein formula milk with conventional cow’s milk formula in 113 babies with mild to moderate eczema according to the Hanifin and Rajka8 criteria. Twenty-seven infants dropped out of the study (16 infants in the experimental group because of the taste of the formula being unacceptable to them, the introduction of solid food, or suffering from other severe diseases that required additional therapy and 11 infants in the control group because of their eczema not improving).
Assessment of risk of bias
Table 72 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Jin 2011331 | Low risk | Unclear risk | Unclear risk | It is unclear whether intention-to-treat rules have been used in this study |
| Leung 2004330 | Unclear risk | Unclear risk | High risk | Dermatologist who assessed eczema severity was not blinded |
Benefits
In the Leung study,330 eczema severity, measured using the SCORAD index, showed a significant treatment by period interaction of 7.23 points (p = 0.012); however, it did not did not show a statistically significant treatment difference (3.97 points; p = 0.274). For the individual components of the SCORAD index, intensity and pruritus showed a statistically significant treatment by period interaction (p = 0.036 for both). The caregiver global health assessment did not show a statistically significant difference for either the treatment by period interaction or the treatment difference.
In the Jin study,331 there was a significant difference between the groups in eczema severity measured by the SCORAD index and the Japanese Dermatological Association Scoring System after 12 weeks. Eczema severity, measured using the SCORAD index, was 13 in the experimental group and 20 in the placebo group, resulting in a significant difference (p = 0.001). The number of flare-ups was significantly decreased in the experimental group but not in the control group (but results were not compared between the groups). The trial report did not state clearly the disease severity at baseline in the two groups.
Harms
These trials did not report whether or not there were any adverse events. In the trial by Jin and colleagues331 there were no differences in nutritional status between groups after 12 weeks.
Overall implications for research and practice
These two trials have given some evidence of the benefit of hypoallergenic formulas for the reduction of eczema severity, especially in very young children. More independent studies are needed to confirm these results.
Summary of dietary interventions
Probiotics
-
There were no trials involving probiotics for eczema treatment reported up to 2000.
-
Twenty-one trials were conducted after 2000 and showed varied results depending on the strains of probiotics examined:
-
Five small trials, with a mostly unclear risk of bias, provided evidence of benefit for mixed-strain probiotics compared with placebo, mostly in post hoc subgroups of participants with raised IgE levels.
-
Five trials, three small, one medium and one large, with a mostly unclear risk of bias, did not provide any evidence of benefit for L. rhamnosis GG compared with placebo or vehicle.
-
One small trial, with an overall unclear risk of bias, provided evidence of benefit for L. sakei KCTC 10755BP compared with placebo.
-
One small trial, with an overall unclear risk of bias, did not provide any evidence of benefit for L. fermentum VRI-033 PCC compared with placebo.
-
One small trial, with an overall unclear risk of bias, did not provide any evidence of benefit for L. acidophilus NCFM (ATCC 700396) compared with placebo.
-
One small trial, with an overall unclear risk of bias, did not provide any evidence of benefit for heat-killed L. paracasei K71 compared with placebo.
-
One large trial, with an overall unclear risk of bias, did not provide evidence of benefit for L. paracasei CNCM I-2116 compared with placebo in infants.
-
One small trial, with a mostly unclear risk of bias (low risk of bias for the method of randomisation), provided evidence of benefit for L. acidophilus L-92 compared with placebo.
-
One medium trial, with a mostly low risk of bias, provided evidence of benefit for L. plantarum CJLP133 compared with placebo.
-
One small trial, with an overall unclear risk of bias, did not provide any evidence of benefit for L. salivarius LS01 compared with placebo.
-
One small trial with an overall unclear risk of bias, did not provide any clear evidence for live lysophilised B. breve YY versus placebo in adults.
-
One small trial, with an overall unclear risk of bias, provided evidence of benefit for B. lactis Bb-12 supplemented formula or L. rhamnosis GG supplemented formula compared with unsupplemented formula in previously exclusively breastfed infants.
-
One small trial, with an overall unclear risk of bias, did not provide any evidence for B. breve M-16 supplemented formula compared with unsupplemented formula in participants with a proven allergy to cow’s milk.
-
Prebiotics
-
There were no trials involving prebiotics (substances that promote the growth of beneficial micro-organisms in the gut) for treating established eczema reported up to 2000.
-
Two small trials reported after 2000, one with a mostly low risk of bias and the other with an overall unclear risk of bias, provided evidence of benefit for prebiotic treatment compared with placebo.
Synbiotics
-
There were no trials involving synbiotics (a combination of probiotics and prebiotics) for eczema reported up to 2000.
-
Eight trials were reported after 2000 and provided mixed evidence of benefit for synbiotics:
-
Six small trials, with a mostly unclear or low risk of bias, provide mixed evidence for synbiotics compared with placebo. Three trials provided evidence of benefit and three trials did not. The two largest trials, with a mostly low risk of bias, gave conflicting results.
-
One small trial, with an overall unclear risk of bias, did not provide any evidence of benefit for synbiotic treatment compared with probiotic treatment.
-
One small trial, with a low risk of bias, provided evidence of benefit for synbiotic treatment compared with prebiotic treatment alone in children.
-
Essential fatty acid supplementation
Borage oil
-
There were five trials involving borage oil for eczema compared with placebo reported before 2000. The largest trial did not provide any evidence of benefit except in one post hoc subgroup of ‘good compliers’. The four small trials were split, with two providing evidence of benefit and two not providing evidence of benefit.
-
One large trial, reported in 2003, with a mostly low risk of bias, did not provide evidence of any benefit for borage oil compared with placebo (olive oil for children and liquid paraffin for adults). This trial supports the evidence suggesting a lack of benefit of borage oil in the general eczema population.
Evening primrose oil
-
Six trials, with a mostly unclear risk of bias, investigating evening primrose oil compared with placebo were reported up to 2000 and provided conflicting results. The two largest and best-reported trials did not provide any evidence of benefit. The results of the four smaller trials ranged from not providing any evidence of benefit to evidence of a modest 10–20% benefit of oral evening primrose oil.
-
One small trial reported in 2008, with a mostly unclear risk of bias, provided evidence of benefit for oral evening primrose oil compared with placebo (sunflower oil). This trial does not clarify the evidence base for oral evening primrose oil, which on balance is thought to be ineffective for the treatment of eczema.
Fish oil (omega-3)/soybean oil (omega-6)
-
Three trials involving fish oil were reported up to 2000. The largest and best-reported independent trial did not provide any evidence of benefit for fish oil compared with placebo. The two smaller trials provided evidence of benefit for fish oils compared with placebo, with a particularly large magnitude of benefit in one.
-
One very small trial reported in 2002, with an overall unclear risk of bias, provided evidence of benefit for fish oil compared with soybean oil given as twice-daily intravenous infusions. This trial does not clarify the existing evidence base for fish oil.
Docosahexaenoic acid
-
There were no trials of DHA (an omega-3 fatty acid) as a treatment in its own right for eczema reported up to 2000.
-
One small trial reported in 2008, with a mostly unclear risk of bias, did not provide any evidence of benefit for DHA compared with a mixture of caprylic acid and capric acid.
Hempseed oil
-
There were no trials involving hempseed oil for eczema reported up to 2000.
-
One small trial reported in 2005, with a mixed risk of bias, did not provide any evidence of benefit for hempseed oil compared with cold pressed extra-virgin olive oil.
Oral vitamins D and E
-
There were no trials involving oral vitamin D (ergocalciferol or cholecalciferol) for eczema reported up to 2000.
-
Three small trials were reported after 2000 and did not provide evidence of benefit of oral vitamin D or E:
-
One very small pilot trial, with a mostly unclear risk of bias, did not provide any evidence of benefit for ergocalciferol compared with placebo.
-
Two small trials, with a low or unclear risk of bias, did not provide sufficient evidence of benefit for cholecalciferol compared with placebo or vitamin E or cholecalciferol plus vitamin E in adolescents and adults.
-
Goat’s/ass’s milk
-
There were no trials involving goat’s or ass’s milk for eczema reported up to 2000.
-
One small trial reported in 2007, with a high risk of bias for blinding, provided evidence of benefit for ass’s milk compared with goat’s milk. The participants had eczema and a clinically relevant cow’s milk allergy.
Hypoallergenic formula
-
There were no trials involving hypoallergenic formula for established eczema reported up to 2000.
-
One very small trial reported in 2004, with a high risk of bias for blinding, provided evidence of benefit for hypoallergenic formula compared with infants’ previous soy- or cow’s milk-based formula.
-
One moderately sized trial reported in 2011, with an overall unclear risk of bias, provided evidence of benefit for hypoallergenic formula compared with conventional cow’s milk formula in infants.
Chapter 9 Non-pharmacological interventions
Background
Existing systematic reviews
There have been nine systematic reviews35,244,245,332–337 and three guidelines (NICE,41 SIGN42 and AAD94) that include non-pharmacological interventions. Eight of these41,42,94,332–336 assessed psychological and/or educational interventions for eczema; five41,42,94,245,337 assessed textiles and/or specialised clothing for eczema; and four35,41,42,94 assessed the reduction of allergens, mostly aeroallergens.
Scope of this chapter
The trials included in this chapter cover the following treatments:
-
specialised clothing
-
education
-
for adults
-
for children with eczema and their parents
-
nurse-led clinics
-
support groups
-
e-health portal
-
-
stress management
-
ion-exchange water softeners
-
living in a different climate
-
house dust mite reduction
-
additional visits to the doctor
-
vaccines.
Specialised clothing
Wearing fabrics next to the skin has been identified as a physical irritant that can be a trigger for eczema. Wool may cause irritation because of the ‘spiky’ nature of its fibres and even cotton has been shown to cause irritation when moist. Cotton, silk or smooth man-made fibres have been used to create specialised clothing for people with eczema. Some specialised clothing also has antimicrobials, such as silver, added to the material.
Studies
Three trials involving specialised clothing were reported before 200055 (see Appendix 3).
Silk
Three new trials assessing the efficacy of DermaSilk® fabric (Espère Healthcare Ltd) were identified. 338–340
In 2007, Koller and colleagues338 published a trial comparing DermaSilk arm tubes against ordinary silk arm tubes. Twenty-two children with mild to moderate eczema were included in the trial. For the initial 2 weeks, arms were covered in either silk fabric or DermaSilk fabric; for the remaining 10 weeks, one arm was covered with a cotton arm tube and the other with a DermaSilk tube. The fabric tubes were randomised to be worn on the left or right arm so that the interventions were compared within the same person. The fabric tubes were worn all day and washed daily. The severity of the eczema in the area covered by the fabric tubes was measured using local SCORAD scores by an assessor who was not aware of the allocation of the interventions. Participants in the study were not allowed to use any antimicrobial or anti-inflammatory treatments during the trial.
A double-blind, within-person randomised trial by Stinco and colleagues339 published in 2008 compared DermaSilk sleeves against the same fabric without the impregnated antimicrobial for 28 days in 30 children and adults with eczema. The ages of the participants ranged from 3 to 31 years. The fabric arm tubes were identical apart from the seam colour. No one with an acute infection was included in this trial. Local SCORAD scores for the arm and participant-assessed pruritus were measured up to 28 days.
A trial by Fontanini and colleagues340 compared a DermaSilk long-sleeve top and trousers against a cotton long-sleeve top and trousers. Twenty-two infants who were aged < 18 months and who had eczema were randomised to wear the garments every day for 2 years, except in the summer and on other very hot days. All of the families taking part were given mite-impermeable mattress and pillow encasings for both the parents’ and the children’s beds. All of the children were treated with mometasone furoate as needed during the trial.
Assessment of risk of bias
Table 73 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Fontanini 2013340 | Unclear risk | Unclear risk | High risk | Although the trial report states that both the parents and the investigators did not know which garments the children were wearing, DermaSilk can easily be differentiated |
| Koller 2007338 | Unclear risk | Unclear risk | Low risk | |
| Stinco 2008339 | Low risk | Unclear risk | Low risk | Labelling the interventions using red and green may have biased the responses and assessment of the intervention despite blinding |
Benefits
In the study by Koller and colleagues,338 a statistically significant difference in both objective and subjective severity scores between DermaSilk and the ordinary silk and cotton was reported after 4 weeks and was still present at 12 weeks. This represented a difference in the median scores of 2 points out of 18 for the local SCORAD score and 3 points out of 10 for the subjective severity score.
In the study by Stinco and colleagues,339 both the severity of eczema under the area covered by the fabrics and participant-assessed itching were significantly better with DermaSilk fabric than with the same fabric without the impregnated antimicrobial. The difference in the pruritus score was 1.88 SD ± 1.7 (p < 0.0001) on a 10-point VAS and the difference in severity measured by the SCORAD index was a reduction of 10.98 SD ± 11.9 (p < 0.0001) more in the DermaSilk group than in the DermaSilk without antimicrobial group.
In the trial by Fontanini and colleagues,340 both the total number of tubes of topical corticosteroid used over 2 years [3.0, interquartile range (IQR) 1.0 to 6.0 in the cotton group and 1.2, IQR 0.7 to 1.5 in the DermaSilk group] and the number of tubes of topical corticosteroid used per month (0.17, IQR 0.09 to 0.33 in the cotton group and 0.07, IQR 0.05 to 0.09 in the DermaSilk group) were significantly lower in the DermaSilk group than in the cotton group using non-parametric Somer’s D coefficient (p = 0.023 and p = 0.000, respectively), but only the number of tubes per month was significant using the Mann–Whitney test (p = 0.006). There was also a statistically significant difference between the groups in the parent-reported satisfaction rating of ‘satisfied’ or ‘not satisfied’ with the reduction in itching, with nine out of nine parents of those using DermaSilk satisfied compared with five out of 11 parents of those using cotton clothing satisfied (Fisher’s exact test p = 0.014). The severity of eczema was not assessed in this trial.
Ethylene vinyl alcohol fibre
Two new small studies341,342 from Japan reported since 2000 looked at ethylene vinyl alcohol fibre fabric as underwear and compared it with cotton underwear.
Thirty participants who were being well maintained on standard eczema treatment participated in the 8-week crossover study by Ozawa and colleagues. 341 Each participant wore each fabric (ethylene vinyl alcohol fibre and cotton) for 4 weeks without a washout period in between the different fabrics. It is not known how much of the day and night the participants were instructed to wear the underwear and how often they were asked to wash it.
A small parallel-group trial342 of 24 children aged 3–9 years also compared ethylene vinyl alcohol fibre fabric with cotton underwear. The children were not allowed to use topical corticosteroids throughout the study.
Assessment of risk of bias
Table 74 provides the risk-of-bias assessment for the new studies.
Benefits
The study by Ozawa and colleagues341 reported no significant difference between the fabrics in the severity of eczema at the end of the study. This was the only clinical efficacy outcome measured.
In the study by Yokoyama and colleagues342 there was also no significant difference in the severity of eczema, including sleep loss and itching, between ethylene vinyl alcohol fibre fabric and cotton underwear.
Harms
Adverse events were not reported in the study by Ozawa and colleagues. 341 The study by Yokoyama and colleagues342 reported one case of increased itching of the areas of the legs not covered by the fabric and one case of increased itching before bed, but decreased sleep loss. It was reported that most adverse events were not related to study treatment.
Textiles with added silver
Padycare®
Gauger and colleagues343 in Germany compared Padycare® (TEXAMED® GmbH), a polyamide and LYCRA® (INVISTA, Wichita, KS, USA) material with silver filaments woven in to produce a silver content of 20% in total, with a cotton textile. Both were worn day and night for 2 weeks as either an all-in-one suit for infants or a long-armed and long-legged suit. All 68 participants had eczema of moderate severity (SCORAD score of at least 20). The severity of eczema, daytime pruritus, sleep loss, quality of life, functionality and wearing comfort were all assessed. The study is reported as being double blind but it is stated only that the investigators were blinded and this is further confirmed as the participants did not wear the garments to the consultations. It is very unlikely that the participants, who were key outcome assessors, could have been blinded.
Assessment of risk of bias
Table 75 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Gauger 2006343 | Unclear risk | Unclear risk | High risk |
Benefits
This trial reported no significant differences in eczema severity, day- and night-time pruritus and sleep loss, skin condition and quality of life at the end of the 2-week study. The participant-assessed improvement in pruritus was significantly greater in the silver textile group than in the cotton group (p = 0.02).
Harms
The trial reported that there were no adverse events related to the textiles.
X-STATIC®
Juenger and colleagues344 compared undergarments containing silver-coated nylon fibres (X-STATIC®; Noble Biomaterials) against either undergarments without the silver-coated fibres or a medium-potency topical corticosteroid (prednicarbate ointment) in a three-phase trial. Thirty participants were randomised to three groups; each group (n = 10) used a different comparator (silver textile, silver-free textile or prednicarbate ointment) in the first 14 days; all participants then used the silver undergarments in the second 14 days and all groups could use only prednicarbate as needed in the third 14-day phase. All participants could use prednicarbate as often as required throughout the trial. The amount of prednicarbate used was recorded along with the severity of eczema using the SCORAD index, participant-assessed pruritus and participant-assessed disease control on a 4-point scale.
Assessment of risk of bias
Table 76 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Juenger 2006344 | Low risk | Low risk | Unclear risk |
Benefits
This three-phase trial344 with only 10 participants per group was difficult to interpret. The severity of eczema measured using the SCORAD index over the first 2 weeks of the trial showed a significant benefit for silver textile (p = 0.003) and regular prednicarbate ointment (p = 0.014) use over the use of non-silver textiles. In these same 2 weeks, the silver textile group used almost as much prednicarbate ointment as the prednicarbate group (135 g vs. 145 g) whereas the non-silver textile group used hardly any prednicarbate (13 g).
In the second 2 weeks of the trial, when all of the participants wore the silver textile, the group who originally wore the non-silver textile improved significantly compared with the group who originally used prednicarbate (p = 0.037). In this phase, the group who continued to wear the silver textile also improved. In this phase, all three groups used very little prednicarbate (silver group 10 g, non-silver group 0 g, prednicarbate ointment 30 g).
In the final 2 weeks of the trial, all groups used only prednicarbate when necessary. In this period the group who had worn the silver textile for 4 weeks worsened in severity significantly more than the group who had worn the non-silver textile for 2 weeks and then the silver textile for 2 weeks. In this final phase the silver textile group used 45 g of prednicarbate, the non-silver textile group used 0 g of prednicarbate and the prednicarbate group used 90 g of prednicarbate.
There was a significant improvement in participant-rated itching in the silver textile group and the non-silver textile group over the weeks that they were wearing the silver textile. The prednicarbate group had the lowest absolute value of the three groups for the time that they were wearing the sliver textile, but this was not a significant change from the rest of the trial period for this group. In terms of participant-rated disease control, a high number of participants rated their disease control as ‘complete or well controlled’ for the 4 weeks.
Harms
One participant had 1 g/l of silver in their urine at day 28, which disappeared at day 56. No silver deposits were found on the skin or the mucous membranes.
Garments with added tourmaline
A trial by Kim and colleagues345 compared undergarments made of anion textile, pure polyester fibres containing nanoparticles of crushed tourmaline, with undergarments made of pure cotton. Fifty-two adults and children aged between 2 and 30 years with mild to severe eczema were randomised to wear the undergarments all of the time for 4 weeks. The use of topical or systemic treatments for eczema during the trial was not permitted.
Assessment of risk of bias
Table 77 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Kim 2012345 | Unclear risk | Unclear risk | Unclear risk | 8/52 participants dropped out and it is not clear whether these participants were included in the analyses |
Benefits
The improvement in eczema severity from baseline to week 4 was significantly greater in the anion group than in the cotton group (from 47.2 SD ± 14.0 to 36.1 SD ± 16.5 in the anion group and from 41.8 SD ± 16.3 to 37.7 SD ± 17.2 in the cotton group; p = 0.0308). The sleep loss and itching components of the SCORAD score were also significantly different for the same comparison (p = 0.0064) whereas the objective components of the SCORAD score were not significantly different. The participants were asked to rate the wearing comfort of the textiles: 76% (n = 19) in the anion group and 74% (n = 14) in the cotton group gave a rating of ‘comfortable’.
Harms
The trial report stated that no adverse events were reported from wearing the undergarments. No further details about adverse events were reported.
Overall implications for research and practice
The study of specialised clothing is challenging because of the inability to blind participants. Interpreting the clinical significance of participant-rated outcomes in the presence of a lack of blinding raises concerns about information/detection bias as patients often have high expectations of benefit from these garments. The studies on specialised clothing collated for this review provide some evidence of potential benefits without any harms. Unfortunately, when potential bias in the subjective results, because of most participants being aware of their treatment allocation, is taken into account, the results have to be interpreted with caution. The objective measures of severity assessed by blinded outcome assessors can be given more weight. DermaSilk showed statistically significant benefits for the severity of eczema in comparison to ordinary silk and cotton and DermaSilk fabric without antimicrobial, but the time period of the trials was very short for such a chronic, long-term condition. The numbers of participants included in the trials were low and the absolute point differences were so small that they may not be clinically meaningful. The X-STATIC silver textile trial is difficult to interpret because of confounding from topical corticosteroid use. One participant in this trial had silver in their urine, which had disappeared by the end of the trial. The ethylene vinyl alcohol fibre did not show even a hint of efficacy for eczema, although this has been assessed only in a Japanese population to date. The silver textile Padycare and the anion textile with tourmaline both resulted in a significant difference in participant-rated improvement in pruritus, a measure that is prone to detection bias for an unblinded intervention. The anion textile with tourmaline also provided evidence of significant benefit for the severity of eczema compared with cotton, but this was not significant when only the objective severity measures were assessed.
Specialised clothing is a relatively expensive treatment both in direct monetary terms and in terms of hidden indirect costs (for increased washing of clothing, having to have multiple changes of garments and rapidly growing children needing bigger sizes). For clinicians, guideline writers and people with eczema and their families to be confident that this clothing has a favourable cost–benefit ratio, more rigorous long-term trials that compare these textiles against standard care in a pragmatic setting are needed. The CLOTHing for the relief of Eczema Symptoms (CLOTHES) trial (ref. HTA 11/65/01)346 is currently running in the UK, comparing the addition of silk clothing to normal eczema treatment against normal eczema treatment alone.
Education
Efforts to improve the quality of life of eczema sufferers and their families by teaching them more about the nature and treatment of eczema have been formalised into education programmes in some countries (such as Germany). The education can take the form of a ‘one-off’ session or regular group or individual sessions.
Interventions covering education are heterogeneous and it would not be appropriate to attempt to summarise all of the trials together as one. We have therefore divided the trials into what might be considered useful groupings for the UK health-care system. Education delivered in sessions outside the clinic is considered separately to education and treatment delivered in the clinic by the same health-care professional. Support groups that deliver education indirectly with or without clinical input are also considered separately. The final group considered is online participant-led education and remote, real-time treatment advice.
Adults
Studies
No trials involving education for adults with eczema were reported before 2000.
Two new trials347,348 have been published since 2000.
One trial347 in the Netherlands including 54 adults aged 18–35 years compared a 2-week intensive day school programme made up of two 3-hour sessions per day over 10 days in groups of five participants with communal breaks allowed against normal outpatient appointments. The education covered coping with stress, social and psychological aspects, alternative medicine, allergies and aggravating factors, dealing with itching and habit reversal. Dermatological treatment was allowed three times a week during the programme. The participants were followed for 40 weeks, with outcomes measured being quality of life [measured using the Marburger Neurodermitis-Fragebogen (Marburg Atopic Dermatitis Questionnaire)349], amount of sick leave and the number of medical consultations and time taken for the consultations.
The trial by Armstrong and colleagues348 randomised 80 adults aged ≥ 18 years with eczema to either a paper pamphlet or an online video with information about eczema including clinical manifestations, environmental factors, bathing and hand washing, moisturiser vehicles and common treatment modalities. The pamphlet or online video could be used as often as the participant wished for 12 weeks. Participants’ knowledge was assessed before the intervention and after the 12 weeks of the trial. The severity of eczema was assessed using POEM49 and participants’ satisfaction with the material was also assessed.
Assessment of risk of bias
Table 78 provides the risk-of-bias assessment for the new studies.
Benefits
In the trial by Span and colleagues347 the intensive day school programme group showed a significant improvement in quality of life assessed using the Marburg Atopic Dermatitis Questionnaire349 compared with the control group after 40 weeks. The education group reported a median score decrease of –16 (25th to 75th percentile: –28.2 to –4.7) whereas the control group reported a median score decrease of –3 (25th to 75th percentile: –17 to 5.5), resulting in a median difference of –13 points (p = 0.03). The trial also reported a difference between the treatment groups in sick leave at 10 weeks, with the education group showing an average reduction of –6.7 compared with an average increase of 7 in the control group, which did not reach statistical significance (p = 0.09) and was not apparent at the end of the trial. The number of outpatient visits was not significantly different between the two groups, but the time taken in the consultations was reduced for those who had participated in the education programme and after 40 weeks showed some benefit (difference in the mean reduction –7.2; p = 0.06).
The participants in the trial by Armstrong and colleagues348 found significantly greater satisfaction from the online video than from the paper pamphlet (p = 0.0086). The level of knowledge in the online video group was also significantly better than that in the pamphlet group (video group 3.05, pamphlet group 1.85; p = 0.011). The severity of eczema was also significantly reduced after 12 weeks in the online video group compared with the paper pamphlet group (video group 3.30 SD ± 3.15, pamphlet group 1.03 SD ± 3.75; p = 0.0043).
As it is very unlikely that there was any blinding of any of the outcome assessors for either of these trials, the significance of these measures, apart from possibly the level of knowledge assessment, should be treated with caution. Whether or not increased knowledge translates into better eczema relief is still unclear.
Harms
The level of adverse events from educational interventions is not clear from the existing studies as these two trials did not measure or describe potential harms or costs in terms of time.
Children with eczema and their parents and carers
Studies
One trial involving education for parents of infants and children with eczema was reported before 200055 (see Appendix 3).
Six new studies reported since 2000350–355 have looked at eczema education sessions for children, parents and carers, with all using slightly different approaches. Overall, the interventions in this section are quite diverse with regard to the components and intensity of the interventions, with time spent on the education ranging from 15 minutes to 2 weeks.
One study in North Carolina by Shaw and colleagues350 of 151 children with eczema used an individual, tailored session with a trained eczema educator lasting for 15 minutes at the initial visit; the level of follow-up was dependant on the severity of eczema. This intervention was compared against standard care by a resident and attending paediatric dermatologist. The educator was available 24 hours a day to respond to queries. The children’s quality of life and severity of eczema were measured but it is not clear when these measurements were taken. Both the educator and the parent/caregiver were not blinded to the intervention and it is unclear whether or not there were any other investigators who assessed the objective outcome measurements or whether they were blinded or not.
A study by Staab and colleagues352 in 2002 looked at a parental training programme. Ninety-three participants received 2-hour training sessions (once a week for 6 weeks) and 111 participants did not receive any training. The intervention was delivered by a multidisciplinary team including a paediatrician, a psychologist and a dietitian. The content of the course included education on relaxation, triggers, dealing with itching and sleep disturbances, child nutrition and food allergies, treatment of symptoms, coping and self-management, and daily skin care. Those in the control group were offered the opportunity to attend the education sessions after the trial period. The severity of eczema, quality of life, coping, treatment costs and treatment behaviour were assessed after 1 year.
A larger trial conducted in Germany in 2006 by Staab and colleagues351 investigated the use of a standardised group education programme in parents of 518 infants and children aged from 3 months to 7 years and 185 children aged 8–12 years and in 120 adolescents aged 13–18 years. The training programme involved a paediatrician or dermatologist, psychologist, nurse and dietitian providing education on eczema including relaxation, triggers, dealing with itching and sleep disturbances, stage-related treatment of symptoms, self-management, daily skin care and coping strategies. The intervention consisted of 2-hour sessions once a week for 6 weeks. The participants were followed up 1 year after the start of the study.
The trial reported by Grillo and colleagues353 involved 61 children with eczema and their parents. The trial compared a 2-hour group education session in addition to normal care with normal care alone. The education consisted of understanding atopic eczema, trigger factors, investigations, basic skin care, practical sessions on wet wrapping and cream application, information on topical corticosteroids and complementary therapies. During the sessions there was time for questions, sharing experiences and ideas. The severity of eczema and quality of life were assessed at 4 weeks and 12 weeks.
The trial by Futamura and colleagues354 involved 59 children with eczema aged from 6 months to 6 years and their parents. This assessor-blinded trial compared a 2-day education programme for children and their parents with standard treatment against standard treatment alone. The programme consisted of lectures, group discussions and one practical session. Participants in the active group stayed in hospital for the treatment.
The trial by Kardorff and colleagues355 involved 30 children aged 3–6 years. One group was educated about eczema using a 10-minute active demonstration with a physical skin model and the other group received only verbal instruction as in normal routine care.
Assessment of risk of bias
Table 79 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Futamura 2013354 | Low risk | Low risk | Unclear risk | |
| Grillo 2006353 | Low risk | Unclear risk | High risk | |
| Kardorff 2003355 | Unclear risk | Unclear risk | Low risk | |
| Shaw 2008350 | Low risk | Low risk | Unclear risk | Participants lost to follow-up were not included in the analyses |
| Staab 2002352 | Unclear risk | Unclear risk | High risk | |
| Staab 2006351 | Low risk | Low risk | High risk | Participants lost to follow-up were not included in the analyses. A large number of participants dropped out, differentially more in the no education group |
Benefits
Patient-reported outcomes
For sleeplessness, measured using a 0–10 scale, the trial by Futamura and colleagues354 found a greater reduction in the education group than in the control group, with a difference of 3.4 SD ± 2.4 in the education group compared with 1.5 SD ± –3.4 over 6 months (p = 0.048). Pruritus, also measured using a 0–10 scale, was significantly different at 3 months (education group 4.0 SD ± 2.3 vs. control group 1.6 SD ± 2.5; p = 0.001), but the difference was not quite significant at 6 months (p = 0.056).
Clinically assessed severity
Four351,352,354,355 of the six trials reported significant beneficial effects of the educational interventions on the severity of eczema. The larger study by Staab and colleagues351 reported significant between-group differences for reduction in eczema severity, measured using the SCORAD index, for all three age groups. The adolescents (aged 13–18 years) receiving the educational intervention had a mean reduction in eczema severity from 43.1 to 23.4 (mean difference −19.7, 95% CI −23.7 to −15.7) over 12 months whereas the no treatment group had a mean reduction in eczema severity from 40.4 to 35.2 (mean difference −5.2, 95% CI −10.5 to 0.1), showing a mean difference between treatment groups of –14.5 (95% Cl –21.2 to –7.9; p < 0.0001). The older children (aged 8–12 years) in the educational intervention group had a mean reduction in eczema severity from 41.8 (SD 16.6) to 25.8 (SD 17.7) whereas those given no treatment had a mean reduction in eczema severity from 40.4 (SD 15.1) to 32.6 (SD 16.5), showing a mean difference between treatment groups of –8.2 (95% Cl –13.6 to –2.8; p = 0.003). The infants and young children (aged 3 months to 7 years) in the educational intervention group had a mean reduction in eczema severity from 41.4 (SD 16.6) to 23.7 (SD 16.7) whereas those having no treatment had a mean reduction in eczema severity from 40.6 (SD 15.2) to 28.4 (SD 16.5), showing a mean difference between treatment groups of –5.2 (95% Cl –8.2 to –2.2; p = 0.0002). The other trial by Staab and colleagues352 found no significant difference in severity of eczema, measured using the SCORAD index, at 1 year (p = 0.43).
In the trial by Futamura and colleagues,354 the primary outcome of the difference in severity of eczema after 6 months, measured using the SCORAD index, showed a statistically significant reduction in severity for the education group compared with the control group (mean difference 10, 95% CI 2.3 to 17.7; p = 0.01). There was no significant difference between the groups in the amount of topical corticosteroid used. The trial by Kardorff and colleagues355 also reported a significant reduction in the severity of eczema in the education group compared with the control group after 42 days (skin model group from 38.6 SD ± 6.1 to 14.1 SD ± 4.3 vs. control group from 38.8 SD ± 5.8 to 19.8 SD ± 5.9; p < 0.006).
Quality of life
There was a reduction in quality-of-life scores (better quality of life) in the education programme groups compared with the control groups in three351–353 of the six trials. In the trial by Grillo and colleagues353 the CDLQI at 12 weeks reduced by 7.35 points in the intervention group (education session in addition to normal care) and 2.61 points in the control group (normal care only) (p = 0.004). Both studies by Staab and colleagues,351,352 which used very similar interventions, found some significant reductions in quality-of-life scores using a German scale with five separate subscales, which had been previously validated. In the smaller 2002 trial352 the confidence in medical treatment subscale was the only one to show a notable benefit of the education programme (p = 0.016, alpha level set at 0.01 after correction for the number of scales). In the much larger trial in 2006,351 the youngest age group (3 months to 7 years) showed a significant benefit of education for all five subscales but the difference in point reduction was small (between 1 and 3 for all scales). The 8–12 years age group showed a benefit of education for the confidence in medical treatment, emotional coping and acceptance of disease subscales but again the point reductions were between 1 and 3 for these scales.
In one trial,351 an assessment of how itching behaviour was affected by education in those aged 8–18 years found that catastrophisation (thoughts of not being able to cope) significantly improved but that coping did not improve. Statistical logistic regression showed that education was found to be the biggest predictor of change in treatment behaviour.
Harms
The level of adverse effects from educational interventions is not clear from the existing studies as none of the trials reported any information about potential harms, except that by Futamura and colleagues,354 which found that there were no adverse events related to topical corticosteroids.
Overall implications for research and practice
Although not being able to blind participants and sometimes assessors to the treatment allocation may lead to risk of bias, the methodology of these trials was of good quality.
For adults, the intensive education course proposed by Span and colleagues347 would probably have meant taking 2 weeks off work, which could lead to an improvement in many chronic conditions. As one of the most important confounding factors, it is important that any future trial uses a carefully chosen comparator. Further, large pragmatic studies on intensive education programmes are needed to help to confirm these encouraging results. Studies in other age groups of this format of education should now be explored. The format of education should be taken into account for adults as it seems to have a significant impact on the benefits seen. Offering a choice with regard to how the education is delivered, for example online or face-to-face, may be a good way to maximise the benefits for individuals. Additional research evidence comparing two or three different formats of education with normal practice is needed.
Taken as a whole, education programmes for parents, young children and adolescents appear to confer some benefits, with the greatest body of evidence being the reduction of eczema severity or improvements in disease-related quality of life or both. Patient-reported symptoms of eczema such as sleeplessness and itching have not been considered sufficiently and deserve more attention, despite the methodological difficulties because of participants being unblinded. The effects of education appear to be complex and may be related to age, presence or absence of parents or children, format, providers and setting of the education. Further research that investigates the most effective elements and format of delivery of education programmes for different eczema groups is needed to give clarity to the most effective use of educational programmes for eczema. It is worth noting that in these trials it was the adolescents, who were educated mostly without their parents, who saw the greatest effect of education programmes on eczema severity, although a large number of dropouts were not included in the analyses. This age group is also more likely than younger children to spontaneously improve and so research targeting this often difficult-to-treat age group must now be pursued. Whether an educational intervention developed in one country can be adopted in another country with different cultural norms is another important aspect that requires exploration, as is the cost-effectiveness of such interventions.
Dermatology nurse consultations
The role of nurses in eczema, as well as for other dermatological conditions, has evolved in the last 10 years, which has resulted in specialist nurses taking on a much more autonomous role within clinics. For some specialist nurses, such as nurse consultants, this has meant having their own list of patients to manage through consultations.
Studies
No trials were published before 2000.
Three new trials356–358 have been published since 2000.
A trial carried out in the UK by Chinn and colleagues357 compared an intervention in which participants had a 30-minute consultation with a newly qualified dermatology nurse with no consultation. The trial included 225 children with eczema recruited from general practice. The consultation involved an individual treatment plan, establishing the parents’ and child’s knowledge about eczema, demonstrations of treatment application, advice about treatments, triggers, bathing and self-management, advice about the UK’s National Eczema Society and an offer of continued support by telephone and/or a further appointment. Participants were given written information about the topics in the consultation. Those in the control group were offered the same consultation intervention at the end of the trial period. Participant- or caregiver-rated eczema severity and both child and family quality of life were assessed at 4 weeks and 12 weeks after the intervention.
A trial in the Netherlands by Schuttelaar and colleagues358 compared consultations with a dermatologist with consultations with a nurse practitioner in 160 children with eczema who had been given a new referral from general practice. No details about the dermatologist consultations were given in the report apart from the duration of the consultations, which was 20 minutes initially and then 10 minutes for each subsequent consultation required based on eczema severity and a 5-minute telephone call for allergy test results. The nurse consultations lasted for 30 minutes initially, with follow-up visits lasting for 20 minutes. A routine follow-up appointment took place after 2 weeks, either as a second visit or as a 10-minute telephone consultation. Subsequent consultations depended on eczema severity. The nurse provided education as part of the intervention, either in the consultations or as a 2-hour group session with up to eight participants.
An Australian trial by Moore and colleagues356 compared one 90-minute ‘workshop’ with a dermatology nurse consultant with an average 40-minute consultation with a dermatologist in 165 children with a new referral for eczema. The nurse-led workshop involved a history being taken and SCORAD scores being obtained, an examination, a management plan, a demonstration of techniques for applying treatments and prescriptions and equipment cards being obtained. The dermatologist consultation involved a history being taken and SCORAD scores being obtained, an examination, a management plan, a demonstration of techniques for applying treatments and prescription and equipment cards being issued. Eczema severity and types of treatments used were assessed at 4 weeks.
Assessment of risk of bias
Table 80 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Chinn 2002357 | Low risk | Unclear risk | Unclear risk | |
| Moore 2009356 | Low risk | Low risk | High risk | There was a greater number of severe participants in the group allocated to a consultation with a dermatologist. Did not use an intention-to-treat principle for the analysis |
| Schuttelaar 2010358 | Low risk | Low risk | High risk |
Benefits
In the trial by Chinn and colleagues357 a 30-minute dermatology nurse consultation in a primary care setting did not appear to have any effect on the children’s quality of life. The impact of eczema on the family improved after 4 weeks compared with having no consultation (mean difference in Family Dermatitis Index score –0.79, 95% CI 1.62 to 0.04; p = 0.06) but this effect was not present at 12 weeks and such a small overall difference may not carry any clinical significance. It is not clear whether there was any effect on the severity of eczema as no data other than baseline were provided in the report.
In the trial by Schuttelaar and colleagues358 both the course of consultations with a nurse practitioner with 3 years’ experience and the course of consultations with a dermatologist resulted in significant improvements compared with baseline for both the infants’ and children’s dermatology quality-of-life scores and the family impact scores (all had reductions of around 5 points to half of the baseline values); however, none of these measures showed any significant difference between the interventions. The severity of eczema also showed significant improvement from baseline for both types of consultation (a reduction of 13 points in the objective SCORAD scores in both groups) but there was no significant difference between the interventions.
The nurse-led workshops in the study by Moore and colleagues356 were found to confer a statistically significant decrease in eczema severity compared with the dermatologist consultations, with a mean difference in SCORAD score between the treatment groups of –9.93 (95% Cl –14.57 to –5.29; p < 0.001). It is not reported whether the SCORAD assessors were blinded but it is unlikely that this was the case. It is also worth noting that baseline eczema severities were skewed, with more severe cases in the dermatologist consultation group, and that the nurse clinic was already well established.
Overall implications for research and practice
One trial357 based in two GP practices with a newly qualified dermatology nurse did not find any significant benefit of the intervention on quality of life in 225 patients. A possible confounder in this trial was that participants were already receiving treatment before baseline, which could have positively affected the baseline scores for quality of life, leaving little room to detect any further improvements conferred by the intervention. It would have been useful to see the effect of the intervention on the severity of eczema but these data were not reported. There is also no indication from the trial by Schuttelaar and colleagues358 that an experienced dermatology nurse practitioner compared ‘head to head’ with a dermatologist had a more beneficial effect, although this is not evidence of equivalence as this was a superiority trial. It is reassuring to note that both health-care professionals had a significant positive impact on the people with eczema that they treated. The positive results from a nurse-led clinic in Australia356 should be interpreted with caution as there were many methodological issues, such as differences in the ways that the separate nurse and dermatologist clinics were run, that could lead to bias. Good-quality evidence of the particular benefits of nurse-led clinics and dermatologist-led clinics is needed to inform dermatology services as effectively and economically as possible in the future.
Support groups
Support groups of people who have or who care for someone with a common condition are often formed both through the NHS and outside it. These groups offer a good diversity of methods of support and some of the larger groups offer helplines and written or online educational information. In the UK, the National Eczema Society is the largest national support group for those with eczema.
Studies
No studies on support groups were reported before 2000.
One new trial was reported after 2000. This small, poorly reported trial359 compared participants attending fortnightly 90-minute support group sessions (over a 6-month period) with a control group. The trial randomised 36 families with a child aged between 2 and 16 years with moderate to severe eczema. It was not clear whether the control group had any intervention or not during the study. For the support group intervention, the children were taken into a separate session that involved free time to play followed by a small amount of education about eczema and its treatment, a short discussion around a theme and then activities around this theme. They then joined the parent group towards the end of the session and showed them what they had been doing. The adult session involved a theme being explained alongside written text about the theme; the parents could then discuss their own experiences around that theme but they were also free to discuss things other than the chosen theme. Session co-ordinators acted as arbiters. The children’s sessions were run by a child psychiatrist and assisted by volunteer medical students; the adult sessions were run by a senior dermatologist assisted by two other dermatologists.
Assessment of risk of bias
Table 81 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Blessmann Weber 2008359 | Unclear risk | Unclear risk | High risk | Participants who did not complete the study were not included in the analysis |
Benefits
The frequency of pruritus decreased in the support group compared with baseline (p = 0.023) but it is unclear whether this was statistically significant compared with the control group. The influence of itching on participant mood was statistically significantly reduced in the support group compared with the control group after the intervention, but the data show a relatively small difference [values after the intervention: support group 3.63 (SD ±3.30), control group 6.19 (SD ±3.54); p = 0.03].
The overall CDLQI was reported as being statistically significantly different between the groups after the intervention (p = 0.01). The domains of leisure (p = 0.04) and personal relationships (p = 0.02) were reported as being statistically significantly improved in their own right for the support group compared with the control group. No data were given for these reported statistical differences.
Harms
Adverse events were not reported for this trial.
Overall implications for research and practice
The most interesting result from the only trial359 to have looked at the role of support groups in eczema is that quality of life and effect of itching on mood of the children with eczema significantly improved whereas impact of eczema on the whole family did not. The children participated in their own separate group session with only their peers and the support group co-ordinators. This trial hints at the potential benefit of this kind of child-focused, semistructured support group for children with eczema, although which particular aspect or aspects of the group support may be conferring this benefit needs much more detailed investigation. This trial did not give enough methodological detail to be confident of the results. Support groups exist in many different forms and provide many different kinds of support and other services. Detailed, pragmatic but thorough trial research around eczema supports groups is needed to make the most effective use of these resources.
E-health portal
As access to the internet increases, more health services are exploring ways of offering all or part of their service online. Direct access to care via the internet, termed ‘e-health’ or ‘telemedicine’, has been shown to have small to moderate benefits on health outcomes for the management of chronic illnesses.
Studies
One trial360 conducted in the Netherlands compared access to a personal eczema portal against standard face-to-face care by a dermatologist. The personal eczema portal provided internet-guided monitoring, self-management training, general information about eczema and personal information about each patient’s treatment regimen. Participants could monitor their eczema via digital photographs and self-reported data such as a VAS for sleeping and itching and a diary of topical treatment. The participants using the portal could have e-consultations with a dermatology nurse, who could consult a dermatologist if needed. In total, 109 adults with moderate eczema and 90 parents of children aged 0–6 years with moderate eczema were randomised.
Assessment of risk of bias
Table 82 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| van Os-Medendorp 2012360 | Unclear risk | Low risk | High risk | It is not clear how many participants were included in the intention-to-treat population |
Benefits
There were no significant differences between the treatment groups in quality of life, measured using the IDLQI (p = 0.45 interaction over time), and the intensity of itching (p = 1.00 interaction over time). For the severity of eczema, there was a significant difference between treatment groups (p = 0.04 interaction over time) but it is not clear which treatment the difference was in favour of. The results for the severity of eczema were not significantly different at any individual time point during the trial.
Harms
No information was reported about adverse events in this trial.
Overall implications for research and practice
The authors claim from the results of this trial that e-health is as effective as standard care for clinical outcomes, even though the trial was designed to assess whether there was a large enough economic saving (€150) to outweigh the cost of the e-health service compared with standard care alone. The trial found a significant economic cost saving for e-health compared with standard care; however, the confidence limits for this figure were too wide for this trial to provide any firm evidence.
Stress management
Studies
A trial by Schut and colleagues361 conducted in Germany compared a standardised cognitive behavioural therapy stress management programme with a waiting list control group. Twenty-eight participants with eczema diagnosed according to the Hanifin and Rajka8 criteria took part in the trial for 10–14 weeks. The age of the participants was not reported. The stress management programme consisted of four 3-hour sessions over 2 weeks, in groups of six to eight participants, which covered cognitive restructuring and enhancing problem-solving skills. A booster session was then given 3 weeks after the last session. The trial was primarily concerned with endocrine stress levels but measured clinical outcomes as well.
Assessment of risk of bias
Table 83 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Schut 2013361 | Low risk | Unclear risk | Low risk |
Benefits
The trial reported that there was no significant difference between the treatment groups in the severity of eczema, measured using the SCORAD index (p = 0.179). Although the data for baseline were not reported, the values for the severity of eczema appear to have been significantly more severe in the stress management programme group at baseline according to the graph of the severity of eczema during the trial.
Harms
The authors did not report any information about adverse events.
Overall implications for research and practice
This very small, short-term trial does not provide any evidence of benefit in terms of improving the severity of eczema using cognitive behavioural therapy. Future trials in this area need to be larger with longer-term follow-up and assess patient-reported outcomes such as quality of life and sleep loss to gain a true picture of the potential of this psychological technique.
Ion-exchange water softeners
Anecdotal evidence that eczema in soft water areas is less severe362,363 and that people who emigrate find their eczema suddenly getting much better or worse have led to suspicions that the hardness of the water could have an impact on the severity of eczema.
Studies
No trials involving ion-exchange water softeners were published before 2000.
One new trial involving ion-exchange water softeners was reported after 2000. This study, conducted in the UK by Thomas and colleagues,364 included 336 children with moderate to severe eczema who were living in hard water areas. The trial compared usual eczema care with usual eczema care plus an ion-exchange water softener to soften the water for bathing and washing. The trial used a parallel-group design and the primary outcome was analysed at 12 weeks. At the end of the study, participants were ‘crossed over’ for 4 weeks to allow the participants in the control group to experience the intervention and to monitor the potential decline in beneficial effects in the intervention group. The nurse who recorded these observations was blinded; however, the participants were not.
Assessment of risk of bias
Table 84 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Thomas 2011364 | Low risk | Low risk | Low risk | Objective outcome assessors were blinded; however, participants were not |
Benefits
The severity of eczema after 12 weeks was not significantly different in the water softener group compared with the control group. The CIs for this blinded objective outcome were very tight, making a robust argument for the lack of additional benefit of softening the water using an ion-exchange water softener for eczema severity (mean difference in SASSAD scores at 12 weeks –0.66, 95% Cl –1.37 to 2.69). In contrast to the blinded primary outcome, unblinded participant- or carer-assessed secondary outcomes did show small statistically significant beneficial effects of the water softeners. It is likely that these positive effects were a result of the bias introduced by the participants’ awareness of treatment allocation as expectation in the effectiveness of the water softeners was high.
Harms
Adverse events were not formally recorded; however, the parents of three participants reported an exacerbation of eczema, which they thought may have been due to the softened water. Two water softener units were removed early for this reason.
Overall implications for research and practice
This large, methodologically robust trial364 of ion-exchange water softeners for eczema clearly shows no benefit of the softeners for the severity of eczema. The significant differences in favour of the water softeners in three of the participant- or carer-assessed outcomes were small and are unlikely to be clinically relevant given the detection bias resulting from participants and carers being aware of the intervention that they were allocated to. This trial gives a robust message that ion-exchange water softeners cannot be recommended as an effective treatment for eczema.
Living in a different climate
Sending people to a warmer climate to treat conditions such as atopic eczema is currently practised in countries such as Norway.
Studies
No trials involving staying in another climate were published before 2000.
One new trial has been published since 2000. This open trial365 involving 61 participants in Norway investigated the effect of sending school children to Gran Canaria compared with remaining in Norway for a duration of 1 month. The children who went to Gran Canaria had to go to school, which included 1 hour of gymnastics a day, and also had to bathe in seawater for 1–2 hours a day. The children in the control group continued attending school as usual, with only 2–4 hours of gymnastics a week. No requirement to bathe in seawater was reported for this group. The control group were offered a trip to Gran Canaria at the end of the study. The severity of eczema and quality of life were measured in all of the children when the Gran Canaria group returned to Norway after 1 month and again 3 months later.
Assessment of risk of bias
Table 85 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Byremo 2006365 | High risk | Unclear risk | High risk | Groups exposed to different complex interventions, making analysis of results difficult |
Benefits
A significant improvement in the severity of eczema after 1 month, measured using the SCORAD index, was reported in the Gran Canaria group, with the score decreasing from a mean of 37.15 (95% CI 29.40 to 44.90) to 21.18 (95% CI 17.24 to 25.13) in the Gran Canaria group and from a mean of 36.84 (95% CI 30.00 to 43.69) to 30.62 (95% CI 24.13 to 37.11) in the control group (p = 0.045). The improvement was sustained for 3 months after the children’s return. Although quality of life improved significantly in the Gran Canaria group, it is not reported whether this was significant in comparison with the control group.
Harms
It was reported that some of the participants in the Gran Canaria group had mild sunburn. No adverse event data for the control group were provided.
Overall implications for research and practice
It is very difficult to decide how much clinical significance this treatment approach has because of the many confounding factors that could have affected the severity of eczema that were different between the two groups, such as bathing in seawater and the amount of exercise undertaken. The application of sun cream in the Gran Canaria group could potentially have had an effect on eczema because of its emollient effect. Both the inclusion criteria and the method of randomisation were not clear and so the generalisability of the treatment effects to the wider eczema population is hard to gauge from this trial. Even if genuinely effective, these benefits could be attributed to the psychological effects of going to a different country, the effect of ultraviolet light on skin inflammation and vitamin D synthesis, saltwater bathing, a changed diet or altered allergen exposure. In the light of the economic and social implications of removing a person with eczema and possibly other family members from their normal life, choosing this treatment over other options must be very carefully considered.
House dust mite reduction
A possible link between house dust mite sensitisation, which is relatively common in people with eczema, and the severity of eczema symptoms has been suggested. 366 Measures to reduce house dust exposure, such as intense vacuuming and mattress encasings, are usually targeted at the bedroom as this environment has the highest potential for long periods of close contact with higher levels of house dust mite allergen.
Studies
Four trials involving house dust mite reduction were reported before 200055 (see Appendix 3).
Three new trials367–369 have been reported since 2000. Ricci and colleagues369 randomised a group of 41 children aged from 2 to 10 years and sensitised to food or inhalant allergens to either ‘recommended’ house dust mite reduction measures (mattress and pillow encasings, hot wash of bedding at least once a week, vacuuming the living room and bedroom at least twice a week, carpets vacuumed at least once a week or removed and no pets) or no recommendations (normal cleaning patterns) for 2 months. After this all participants followed the house dust mite reduction recommendations for a further 10 months. The severity of eczema was assessed using SCORAD scores and total dust mite load and the specific load of Dermatophagoides pteronyssinus and D. farinae was measured.
A larger Dutch mite avoidance study by Oosting and colleagues368 was reported in which 86 participants with eczema and sensitised to house dust mites were randomised to either mite-impermeable mattress, duvet and pillow encasings made of GORE-TEX® material or cotton ‘placebo’ encasings for 1 year on all beds in the participants’ bedroom. The clinical severity and extent of eczema were assessed using the Leicester Sign Score. 61 Sensitivity to house dust mite was measured by intradermal and patch testing alongside total and specific IgE. It is not clear whether the participants were adults, children or both.
The third trial by Gutgesell and colleagues367 randomised only 20 participants with eczema and sensitised to house dust mite to either allergen-impermeable polyurethane encasings and acaricide (mite-killing) spray made up of tannic acid and benzyl benzoate or cotton ‘placebo’ encasings and ‘placebo’ acaricide spray (water with traces of ethanol) for a year. The severity of eczema was assessed using the SCORAD index; daytime pruritus and pruritus-induced sleeplessness were assessed using a VAS, and participant-assessed skin status and the amount of topical corticosteroids used were both recorded. It was unclear whether the participants were children, adults or both.
Assessment of risk of bias
Table 86 provides the risk-of-bias assessment for the new studies.
Benefits
All three trials367–369 failed to show any benefit of house dust mite reduction interventions for eczema compared with placebo or normal cleaning practices over a period of up to 12 months. The trial by Ricci and colleagues369 showed a mean decrease in SCORAD scores from 33 to 24 in the first month for the mite avoidance group compared with a mean decrease from 27 to 22 in the placebo group. After the additional 10 months of the mite avoidance intervention for all participants, the groups had SCORAD values of 16 and 17, respectively. In the trial by Gutgesell and colleagues,367 the SCORAD values in both groups fluctuated throughout the year, with no overall trend, and were almost identical, with a difference of only 3 points between the groups (p = 0.901). Pruritus-induced sleeplessness (p = 0.399), daytime pruritus (p = 0.799) and the participant-assessed skin status (p = 0.583) also showed no marked differences between the groups. The Dutch mite avoidance study by Oosting and colleagues368 reported a significant reduction in the house dust mite load in the GORE-TEX encasing group whereas the placebo group load did not change significantly. This effect does not seem to translate into clinical benefit, however, as sleeplessness, itching, disease activity and extent all decreased by only a few points in both groups, with no significant difference between the groups.
The three trials367–369 gave only scant details of the trial methodology, with none reporting the method of randomisation and allocation concealment. It is unlikely that 2 months of intervention in the trial by Ricci and colleagues369 is long enough to be of clinical relevance when testing house dust mite reduction for a long-term condition such as eczema. On the other hand, two other trials367,368 with a duration of 1 year saw no significant effects in favour of house dust mite reduction interventions.
Harms
None of these trials specifically reported adverse events, although one participant withdrew from the trial because of sweating-induced exacerbation of eczema, attributed to the allergen-impermeable encasings.
Overall implications for research and practice
Applying encasings to a bed and having to wash or vacuum more frequently with a high-quality vacuum cleaner may be fairly achievable in many cases and, if proven to be of significant benefit, could have great potential for the treatment of eczema. However, a complex intervention such as in the trial by Ricci and colleagues,369 which could potentially add a large physical and mental burden to members of the family (because of extra housework, strict regimens and even loss of treasured soft toys), raises questions about the balance between the effectiveness of the intervention and quality of life for the participant and his or her family.
No long-lasting, significant clinical benefits of any of these interventions have yet been shown, but the trials have so far lacked methodological clarity and the important question of the impact on quality of life must be addressed in any future trials on reduction of house dust mites. There is a need for simple, pragmatic, long-term clinical trials of individual house dust mite interventions with blinded outcome assessments. Given that increased exposure to allergens can sometimes induce tolerance, it is also important to explore whether reducing allergen levels from different baseline levels actually induces more harm than good by periodically increasing sensitisation.
Additional visits to a doctor
It has been found that patient adherence to treatment has a tendency to increase around the time of follow-up visits; this has been termed ‘white coat compliance’. 370,371 Increased adherence to a treatment can result in increased benefit of the treatment. 372
Studies
There were no trials involving additional clinic visits reported before 2000.
One new trial was reported after 2000. This trial, by Sagransky and colleagues,373 reported as a short communication, involved 30 children treated with topical tacrolimus 0.03% daily for 4 weeks. The participants were randomised in an open manner to have either one extra visit to the clinician 1 week after starting treatment or no extra visit. All participants visited the clinician after 4 weeks of treatment.
Assessment of risk of bias
Table 87 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Sagransky 2010373 | Unclear risk | Unclear risk | High risk | Only 26/30 participants completed the trial and were included in the analysis and so no intention-to-treat analysis was carried out |
Benefits
This pilot trial373 found a significant improvement in eczema severity assessed by EASI scores, IGA score and participant-assessed itch in both groups, but there were no significant differences between those who had an extra visit to the clinician at 4 weeks and those who did not. The mean difference in percentage improvement in severity measured using EASI scores (extra visit group 76% vs. control group 45%; p = 0.06) showed some difference; however, only 26 out of the 30 participants randomised were included in the analysis.
Harms
The extra visit group reported three adverse events and the control group reported two adverse events, none of which was assessed as being related to treatment.
Overall implications for research and practice
This small, unblinded pilot trial,373 powered to detect large differences in treatment effect, did not find any significant beneficial effect of one extra visit to the doctor. The trial also found no correlation between adherence and reduction in severity and no correlation between baseline severity and adherence, but the study was underpowered to exclude even moderate differences. Given that visits to the doctor are expensive, future trials of adherence enhanced by extra clinic visits should include an economic evaluation. Although the unblinded nature of the intervention may raise concerns about performance bias, in the context of this intervention blinding is not desirable as the intervention is clearly intended to increase adherence/performance. Measuring treatment adherence is challenging, especially in the case of emollients, where the amount applied will depend on severity and the current level of control. Developing new methods for accurately measuring treatment adherence will be a difficult but necessary move to further investigate the influence of the number of doctor visits on eczema severity.
Vaccines
Observational studies have suggested that children exposed to unpasteurised milk (which contains harmful bacteria) may be less likely to develop eczema than control subjects. 374 This has led researchers to investigate whether vaccinating people with some forms of mycobacteria can improve eczema severity by altering the immune response.
Studies
There were no studies involving vaccines for eczema reported before 2000.
Four new trials involving vaccines for the treatment of established eczema were reported after 2000. The trial by Arkwright and David,375 reported in 2001, followed 41 participants aged 5–18 years with moderate to severe eczema for 3 months following a single vaccination or placebo injection. The severity of eczema and potency of topical corticosteroids used were recorded at 1 and 3 months after vaccination.
Another similar trial376 by the same group compared a vaccination with heat-inactivated Mycobacterium vaccae against a placebo vaccination. The 56 participants were aged between 2 and 6 years and had moderate to severe eczema. The severity of eczema scale included the extent of involvement and severity (dermatitis score), with a maximum score of 300 points. The potency of topical corticosteroids used was recorded at 1, 3 and 6 months after the vaccination.
A multicentre, parallel-group trial in the UK and Croatia by Berth-Jones and colleagues377 was reported in 2006. This trial involved 166 children aged between 5 and 16 years with moderate to severe eczema, diagnosed according to the UK Working Party’s criteria. 9 Children were randomised to receive a single injection containing either heat-inactivated M. vaccae or placebo. The participants were followed up for 24 weeks with assessments at 4, 8, 12 and 24 weeks. The participants could not make any changes to their eczema medication in the 4 weeks prior to randomisation and could not use any topical or systemic immunomodulatory treatments, Chinese traditional medicine or phototherapy during the trial.
A trial by Brothers and colleagues378 conducted in New Zealand also compared heat-inactivated M. vaccae immunisation (three injections at 2-week intervals) with placebo in 129 children aged 5–16 years with moderate to severe eczema but otherwise good general health. The participants could keep using their other treatments for eczema or discontinue them, as long as they were not tacrolimus, ciclosporin, methotrexate, pimecrolimus, ultraviolet A (UVA) or ultraviolet B (UVB) phototherapy, systemic corticosteroids, high-dose inhaled steroids or traditional Chinese medicines. The participants were followed up for 6 months after immunisation.
Assessment of risk of bias
Table 88 provides the risk-of-bias assessment for the new studies.
Benefits
The first trial in children and adolescents aged 5–18 years375 showed a greater reduction in eczema severity and surface area affected in the M. vaccae vaccination group than in the placebo group, with the dermatitis score significantly improving from month 1 and at month 3 (total mean change from baseline 41 points vs. 10 points; p < 0.01) and the surface area affected also significantly improved by month 3 (total mean change from baseline 17% vs. 0%; p < 0.01).
The trial in children aged 2–6 years376 failed to show any significant difference between M. vaccae and placebo, although it must be noted that the M. vaccae group had significantly more severe disease than the placebo group at baseline (p = 0.05; difference in mean dermatitis score of 9 points). Neither of these trials found a significant decrease or increase in the potency of topical corticosteroids used in the trial.
The largest trial, carried out with children and adolescents aged 5–16 years in the UK and Croatia,377 reported a mean decrease in eczema severity after 12 weeks, measured by SASSAD, of 9.4 in the 1-mg M. vaccae group, 7.0 in the 0.1-mg M. vaccae group and 8.8 in the placebo group. The decrease in the 1.0-mg group was statistically significant compared with the placebo group (95% CI –4.3 to 5.4; p > 0.05). There were significant differences in favour of the 0.1-mg M. vaccae group compared with placebo and also both M. vaccae groups combined compared with placebo for sleep disturbance after 8 weeks. The trial report states that there were no significant differences in all of the other outcomes of change in severity of eczema at 24 weeks, change in body surface area affected, participant-assessed global assessment of response, pruritus severity and frequency of use of topical corticosteroids.
The trial by Brothers and colleagues378 reported that there was no significant change in eczema severity, measured using SASSAD, between the placebo group and the immunisation group at 3 months (p = 0.77) and 6 months (p = 0.70) after immunisation. There was also no significant difference in the extent of eczema (p = 1.0) at 3 months. There was also no significant difference in the change in quality of life, sleep disturbance and frequency and potency of topical corticosteroid use, but the data for these outcomes were not reported.
Harms
Both trials by Arkwright and David375,376 showed a very similar pattern of adverse events. There were no systemic adverse events reported in either trial; however, approximately half of the participants (13/21 and 13/29, respectively) who were vaccinated with M. vaccae developed a temporary localised red lump at the injection site.
In the trial by Berth-Jones and colleagues,377 103 participants reported 260 adverse events. The most common adverse event was reported as eczema (53 participants) followed by infected eczema (24 participants). Forty-one participants (25%) reported one or more adverse events assessed as at least being possibly related to treatment. There were five serious adverse events assessed as not being related to study treatment.
The trial by Brothers and colleagues378 reported that 47% of the participants had a local injection site reaction and that 75% of these had received the M. vaccae vaccine.
Overall implications for research and practice
The evidence from these four trials, comprising almost 400 participants, with a low risk of bias, indicates that there is insufficient evidence of benefit for vaccination with M. vaccae for atopic eczema treatment. Additional research evidence on M. vaccae vaccination for treating eczema is not needed.
Summary of non-pharmacological interventions
Specialised clothing
-
There were three small trials involving specialised clothing for eczema reported up to 2000. One of these trials found evidence of benefit for clothing made from cotton compared with clothing made from two other fibres and another trial found evidence of benefit for warp knits compared with jersey knits. The third trial found evidence of benefit for gel-filled absorbent core nappies compared with cellulose absorbent core nappies for nappy rash but not for eczema.
-
Eight trials reported after 2000 covered four different types of specialised clothing:
-
Three trials, with a mostly low and unclear risk of bias, found evidence of benefit for silk clothing (DermaSilk).
-
One trial, with a high risk of bias for blinding, provided evidence of benefit for clothing containing silver (Padycare) and one trial, with a mostly low risk of bias, was difficult to interpret and therefore did not provide evidence of benefit for clothing containing silver (X-STATIC).
-
Two trials, with a mostly high risk of bias, did not provide any evidence of benefit for clothing made from ethylene vinyl alcohol fibre.
-
One trial, with an overall unclear risk of bias, provided evidence of benefit for anion textile with added tourmaline.
-
Education
-
One small unblinded trial was published before 2000 and provided evidence of benefit for an educational intervention given by a nurse compared with no education.
-
Twelve trials reported after 2000, with an overall mixed risk of bias, covered different educational approaches and provided some evidence of benefit for educational approaches.
E-Health portal
-
One small trial, with a mixed risk of bias, did not provide any clear evidence of benefit for an online health-care portal compared with standard care.
Stress management
-
There were no trials of stress management for eczema reported up to 2000.
-
One very small trial reported in 2013, with a mostly low risk of bias, did not provide any evidence for the effectiveness of a stress management treatment for eczema based on cognitive behavioural therapy compared with no treatment.
Ion-exchange water softeners
-
There were no trials of ion-exchange water softeners for eczema reported up to 2000.
-
One large trial reported in 2011, with an overall low risk of bias, provided evidence of no benefit from the use of an ion-exchange water softener in the home compared with no water softener.
Living in a different climate
-
There were no trials of living in a different climate for eczema reported up to 2000.
-
One small trial, with a mostly high risk of bias, provided evidence of possible benefit of living in a warmer climate compared with staying at home.
House dust mite reduction
-
There were four small trials, with a mostly unclear risk of bias, reported up to 2000. The results were conflicting, with only one of the trials providing evidence of benefit for a house dust mite reduction intervention (mite-impermeable encasings, intensive/high-filtration vacuuming, acaricide spray) compared with placebo (mite-permeable encasings, normal cleaning patterns, vacuuming with reduced suction).
-
Three small trials, with an overall unclear risk of bias, did not provide any evidence of benefit for interventions to reduce house dust mites (more frequent vacuuming, hot washing of bedding and soft toys, removing pets, mite-impermeable mattress and bedding encasings, acaricide spray) compared with placebo (normal patterns of cleaning and washing, allowing pets, mite-permeable encasings, placebo spray).
Additional visits to a doctor
-
There were no trials of additional visits to a doctor for eczema reported up to 2000.
-
One very small trial published in 2010, with a high risk of bias for blinding, provided no evidence of benefit of one extra visit to the doctor compared with no extra visit.
Vaccines
-
There were no trials of vaccination for treating eczema reported up to 2000.
-
Three small trials published after 2000, with an overall low risk of bias, did not provide any evidence of benefit for vaccination with M. vaccae for treating established eczema.
Chapter 10 Phototherapy treatment
Background
Phototherapy treatment with UVA or UVB light is used in moderate to severe cases of eczema. Phototherapy is often used as an alternative to other long-term treatment regimens such as topical corticosteroids. Patients are usually treated in hospital two or three times a week for several weeks and sometimes months.
Scope of this chapter
This chapter covers the following phototherapy treatments:
-
UVB treatments
-
UVA treatments
-
UVA treatments compared with UVB treatments
-
phototherapy in combination with other treatments
-
full-spectrum light therapy
-
excimer laser (form of UV laser).
Studies
Six trials involving phototherapy were reported before 200055 (see Appendix 3).
Ultraviolet B treatments
Studies
A left/right within-person trial by Selvaag and colleagues,381 reported in 2005, compared standard UVB fixed-dose increments against UVB using skin reflectance guided dosing. The trial included 20 adults aged 16–38 years with mild to moderate eczema. Treatment was given for up to 6 weeks and was stopped early if a participant’s SCORAD score fell to < 10 on either side of the body. The whole of the face was given standard UVB treatment. Emollients and topical corticosteroids were allowed during the trial as long as they were used symmetrically. It was not reported how many treatments were given per week.
Assessment of risk of bias
Table 89 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Selvaag 2005381 | Unclear risk | Unclear risk | High risk |
Benefits
The severity of eczema was recorded using the SCORAD index. It was reported that no significant difference was found in the reduction of eczema severity between the two treatment regimens, but no detailed data were reported. The times taken for the SCORAD scores to reduce to < 10 were reported. The standard treatment regimen had a median time (5th and 95th percentiles) of 3.5 weeks (1.5, 6.0) and the skin reflectance regimen had a median time (5th and 95th percentiles) of 3.0 weeks (2.0, 5.5). The cumulative UVB dose was significantly lower for the skin reflectance regimen (median 39 × 10 mJ/cm2; p < 0.01) than for the standard regimen (median 124 × 10 mJ/cm2). The initial UVB dose was reported as higher for the skin reflectance regimen, with a median of 3.4 standard erythemal dose (SED) (×10 mJ/cm2 at 298 nm using the CIE erythema action spectrum) compared with 2.6 SED (×10 mJ/cm2 at 298 nm using the CIE erythema action spectrum) for the standard regimen, but this was not statistically significant.
Harms
Data on adverse events were not reported for this trial.
Overall implications for research and practice
The potential to administer low-dose UV radiation and achieve the same clinically beneficial effects as reported with high-dose UV radiation is encouraging but requires confirmation in larger pragmatic studies. This one small trial381 indicates that this could be achievable; however, the results must be treated with caution. Problems with the study include the lack of blinding, the claim of equivalence of non-inferiority being based on a very small sample size and no methodological details about the study design being reported. Such an important question should be examined in detail in an appropriately designed and powered trial to give patients and clinicians clear guidelines on the best treatment regimen for UV phototherapy.
Ultraviolet A treatments
Studies
Broadband UVA treatment is used alone or in combination with broadband UVB treatment. More recently, UVA treatment has been combined with other agents such as the photosensitiser psoralen plus ultraviolet A (PUVA)], which is a naturally occurring chemical found in the common fig and celerys as well as other plants and seeds. UVA is also being combined with photo(chemo)therapeutic agents in UVA1 phototherapy treatment, narrowband UVA therapy and extracorporeal photopheresis. Narrowband UVA and UVA1 phototherapy (high-intensity, long-wavelength UVA 340–400 nm) are currently used for eczema as they have a high output and narrow emission spectrum and so are expected to be the most efficacious and safe versions of UVA for eczema treatment.
A trial by Dittmar and colleagues382 in 2001 compared three different doses of UVA1 phototherapy against each other. The low-dose group was given a maximum single dose of 20 J/cm2 and a maximum cumulative dose of 300 J/cm2; the medium-dose group received a maximum single dose of 65 J/cm2 and a maximum cumulative dose of 975 J/cm2; and the high-dose group received a maximum single dose of 130 J/cm2 and a maximum cumulative dose of 1840 J/cm2. The treatment was given five times a week for 3 weeks. The trial randomised 34 adults with eczema and a SCORAD score of > 30. No other treatments except for emollient were permitted during the trial. The trial appeared to compare the treatments using a parallel-group design, but this is not specifically stated.
A crossover trial by Tzaneva and colleagues383 compared UVA1 phototherapy with PUVA provided on an outpatient basis. The 40 participants were given UVA1 phototherapy at doses of 20 J/cm2 unless the minimal erythema dose was below this, in which case this value was used initially with an incremental increase of 10 J/cm2 for each subsequent treatment, up to a maximum of 70 J/cm2 as long as there was no erythema. The PUVA treatment (1.2 mg/kg) was given 2 hours before UVA exposures, which had a starting dose of 70% of the minimal phototoxic dose. The UVA dose was increased by 20% of the minimal phototoxic dose if there was no erythema and 10% if there was barely perceptible erythema. Other than the study treatment, participants were allowed to use only emollients.
Assessment of risk of bias
Table 90 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Dittmar 2001382 | Unclear risk | Unclear risk | Unclear risk | 9/34 participants withdrew and it was not clear whether these were included in the analyses. Large differences in baseline immediate pigmentation dose and severity |
| Tzaneva 2010383 | Low risk | Unclear risk | Unclear risk | 17/40 participants withdrew from the trial and were not included in any subsequent analyses |
Benefits
In the trial by Dittmar and colleagues382 comparing three different doses of UVA phototherapy, the low-dose group did not significantly improve in severity from baseline, with a reduction in SCORAD score from 54 to 46. The medium- and high-dose groups significantly improved compared with baseline, reducing from 56.29 to 40.16 and from 70.81 to 33.94, respectively. No between-group severity analyses were reported. No other efficacy outcomes were reported.
The trial by Tzaneva and colleagues383 primarily measured length of remission after each phototherapy treatment. The PUVA treatment group had a median time to relapse of 12 weeks (IQR 4–24 weeks) whereas the UVA1 treatment group had a median time to relapse of 4 weeks (IQR 4–12 weeks), which was reported as statistically significant (p = 0.012). Severity was reported as a secondary outcome. The SCORAD scores in the PUVA group decreased from a mean of 62.5 at baseline to 36 after 10 exposures and to 28.8 after 15 exposures. The SCORAD scores in the UVA1 group decreased from a mean of 63.7 at baseline to 46.9 after 10 exposures and to 40.1 after 15 exposures. The mean ± SD percentage reductions in SCORAD scores from baseline were 54.3% ± 25.7% for PUVA and 37.7% ± 22.8% for UVA1. The difference between the groups was statistically significant.
Harms
The trial report by Dittmar and colleagues382 stated that no adverse events were observed. In the trial by Tzaneva and colleagues383 only minor adverse events were reported. Two participants treated with UVA1 and nine treated with PUVA reported mild palmoplantar erythema. Seven participants treated with UVA1 reported heat and burning after treatment. Folliculitis was reported by one participant using UVA1 and by two participants using PUVA. Two participants using PUVA reported photo-onycholysis (nail degradation).
Overall implications for research and practice
The optimal dosing regimen for treatment with UVA1 is still unclear. The trial by Dittmar and colleagues382 has large disparities in both baseline eczema severity and immediate pigmentation dose, and provides no between-group analysis. The trial does provide a hint of a positive dose–response relationship, which should be treated with caution. Further methodologically robust research should attempt to clarify the optimal treatment regimen for phototherapy with a clinically realistic duration of treatment.
The trial of PUVA treatment383 showed a modest superior effect of PUVA over UVA1 treatment after 15 treatments; however, this is a relatively short treatment period. The particularly striking result was the significantly longer length of remission that PUVA induces compared with UVA1. Inducing long periods of remission in a chronic, long-term condition such as eczema is vitally important. PUVA therapy is time-consuming, even more so than phototherapy on its own, and so this must be taken into account.
Short-term adverse events appear to be mild and occur at low levels for both UVA1 and PUVA treatment, with some indication that UVA1 has a slightly better safety profile than PUVA.
Ultraviolet A compared with ultraviolet B treatments
Studies
A trial by Reynolds and colleagues384 randomised 73 adults with eczema that was not considered to be mild to either narrowband UVB, broadband UVA or visible light phototherapy. All participants had treatment twice a week for a total of 24 treatments. All participants were allowed to use emollients (emulsifying ointment or aqueous cream were advised as some emollients absorb UV radiation) and topical corticosteroids (except very potent ones) 2 weeks before and during the trial.
A left/right within-person trial by Majoie and colleagues385 compared narrowband UVB (311 nm) against medium-dose UVA1 (350–400 nm) given three times per week for 8 weeks. Thirteen adults with a symmetrical eczema distribution were included. For narrowband UVB, the first dose was 70% of the minimal erythema dose; the dose was increased by 20% if there was no erythema or by 10% if the previous dose produced slight erythema. For UVA1 phototherapy, the first dose was 30 J/cm2, and this was increased to 45 J/cm2 in two treatments. The dose was decreased if the reaction was too strong. No other topical treatments except for emollients were allowed during the trial. The face was excluded from the analyses.
A crossover trial by Gambichler and colleagues386 compared UVA1 phototherapy with narrowband UVB phototherapy each given three times a week for 6 weeks. The trial randomised 47 participants with eczema diagnosed according to the Hanifin and Rajka8 criteria, with a SCORAD score of ≥ 20. For UVA1 treatment the dose was 50 J/cm2. For narrowband UVB treatment, the first dose was 70% of the minimal erythema dose and this was increased by 10–20% per session up to a maximum of 1.2 J/cm2 for skin type II or by 1.5 J/cm2 for skin type III or IV. Any prospective participants with an abnormal photosensitivity to UVA1 were not included in the trial. Participants were allowed to use emollients and moisturisers during the trial.
Assessment of risk of bias
Table 91 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Gambichler 2009386 | Low risk | Unclear risk | Low risk | Only 28/47 participants were included in the intention-to-treat analyses |
| Majoie 2009385 | Unclear risk | Unclear risk | Unclear risk | |
| Reynolds 2001384 | Low risk | Unclear risk | Low risk |
Benefits
The trial by Reynolds and colleagues384 reported that 19 out of 21 participants in the UVB group had a reduction in itch over the treatment period compared with 12 out of 19 in the UVA group. The number of participants who improved in the visible light group was not reported, but it was stated that the UVA and UVB groups had higher numbers of improvers than the visible light group. In total, 15 out of 21 in the UVB group and 10 out of 19 in the UVA group had an improvement in sleep at the end of treatment. Again, the number of improvers in the visible light group was not provided but the UVA and UVB groups were reported to have higher proportions of improvers than this group. The total disease activity score improved in the UVA group by a mean of 4.4 points (95% Cl –1.0 to 9.8) more than the visible light group and in the UVB group by a mean of 9.4 points (95% Cl 3.6 to 15.2) more than the visible light group. All three groups started with a similar mean baseline severity score (UVA group 32.3, UVB group 29.8, visible light group 30.8). The maximum severity score obtainable was 90. These data were extrapolated from graphs.
In the trial by Majoie and colleagues385 the participant-assessed reduction in itch over 12 weeks was similar for the two treatments. The medium-dose UVA1 group fell from 5.8 to 2.7 and the narrowband UVB group fell from 5.9 to 2.3. In the between-group analysis it was reported that no significant difference was found, but no data were provided. Eczema severity was recorded using the Leicester Sign Score. 61 The medium-dose UVA1 group fell from 19 to 10 and the narrowband UVB group fell from 18 to 9. In the between-group analysis it was reported that no significant difference was found, but no data were provided.
The trial by Gambichler and colleagues386 found no significant differences in the reduction in pruritus, eczema severity measured using SASSAD and quality of life measured using Skindex-29. For pruritus, the UVA1 group had a 16% SD ± 61.8% reduction at the end of treatment whereas the narrowband UVB group had a 25.2% SD ± 30.5% reduction for treatment at the end of treatment (p = 0.5). Eczema severity was reported as the mean relative reduction in SASSAD score after 6 weeks. The UVA1 group had a 43.7% SD ± 31.4% reduction at the end of treatment whereas the narrowband UVB group had a 39.4% SD ± 24.1% reduction at the end of treatment (p = 0.5). Quality of life was also reported as the mean relative reduction after 6 weeks. The UVA1 group had a 12.7% SD ± 18.8% reduction at the end of treatment whereas the narrowband UVB group had a 16.5% SD ± 17.6% reduction at the end of treatment (p = 0.1).
Harms
In the trial by Reynolds and colleagues384 two participants withdrew because of ‘burning’ (one in the narrowband UVB group, one in the visible fluorescent light group) and four withdrew because of ‘exacerbation of eczema’ (one in the narrowband UVB group, two in the broadband UVA group and one in the visible fluorescent light group). A further three participants withdrew because of ‘dislike of treatment’ (two in the broadband UVA group and one in the visible fluorescent light group). The trial by Majoie and colleagues385 did not report any data about adverse events. In the trial by Gambichler and colleagues386 one participant in the UVA1 group and four in the narrowband UVB group developed mild erythema.
Overall implications for research and practice
Both UVA and UVB phototherapy appear to reduce pruritus and the severity of eczema after a course of treatment, although broadband UVA therapy did not appear to fare as well as narrowband UVB or UVA1 therapy. The length of time for which these benefits are sustained after the cessation of phototherapy has not yet been addressed; as there does not seem to be much of a difference in the efficacy of UVA compared with UVB, this is now a key research gap. Phototherapy is labour intensive for all involved and some of the adverse effects such as heat loading can be difficult to cope with, especially for children. There is a large degree of variability observed in treatment response, which is evident from the wide deviations in severity score reductions in the trial by Gambichler and colleagues. 386 Trials involving two different phototherapy treatments are easier to blind than trials of phototherapy compared with other treatments.
Ultraviolet A/B treatments compared with or in combination with other active treatments
Studies
A trial by Valkova and Velkova387 compared phototherapy with UVA/UVB against phototherapy with UVA/UVB combined with the topical corticosteroids fluticasone and hydrocortisone butyrate. Thirty-one adults and children aged between 8 and 45 years with moderate to severe eczema were randomised and underwent UV treatment five times a week, with one group also applying topical corticosteroids twice a day, five times a week. The length of time that the study treatment was given was not reported.
A left/right within-person trial by Tzung and colleagues388 compared narrowband UVB alone against narrowband UVB in combination with 1% pimecrolimus, twice daily for 6 weeks. The trial randomised 26 children aged 5–17 years to either half-body UVB and whole-body pimecrolimus or whole-body UVB and half-body pimecrolimus. The first dose of UVB treatment was 70% of the minimal erythema dose and then percentage-based increments up to a maximum of 1.5 J/cm2 were carried out.
A multicentre, two-arm trial by Heinlin and colleagues389 compared synchronous balneotherapy, in which the participants were immersed in a bath containing dead sea salts at a concentration of 10% and given UVB (311 nm) phototherapy, with UVB (311 nm) phototherapy only. In total, 180 adults with dermatologist-diagnosed eczema were given treatment according to a dose escalation schedule for their skin type. The bathing time for the synchronous balneotherapy increased in line with the schedule for the phototherapy. Participants started with three to five sessions a week and underwent 35 sessions in total.
The trial by Granlund and colleagues390 is discussed in Chapter 11.
Assessment of risk of bias
Table 92 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Granlund 2001390 | Unclear risk | Unclear risk | High risk | |
| Heinlin 2011389 | Unclear risk | Low risk | High risk | |
| Tzung 2006388 | Unclear risk | Unclear risk | Low risk | Objective outcome assessors were blinded; however, participants were not |
| Valkova 2004387 | Unclear risk | Unclear risk | High risk |
Benefits
In the trial by Valkova and Velkova387 no between-treatment comparisons were reported. There was a large reduction in the severity of itch in the phototherapy and topical corticosteroid combination group, from a mean ± SD of 235.7 ± 16.9 to 78.6 ± 18.7 after treatment. In the phototherapy-only group the very low score of 3 ± 13.6 increased to 5 ± 12.3 after treatment. Sleep loss decreased in the phototherapy and topical corticosteroid combination group, from a mean of 50 ± 20.2 to 21.4 ± 11.4 after treatment. In the phototherapy-only group the score decreased from 76 ± 23.5 to 11 ± 8 after treatment. Overall eczema clinical severity decreased in the phototherapy and topical corticosteroid combination group, from 395.4 ± 35 to 36.9 ± 7.3 after treatment. In the phototherapy-only group the score was 360.4 ± 37.6 at baseline and 37.9 ± 6.7 after treatment.
In the trial by Tzung and colleagues388 the primary outcome of change in eczema severity was measured using EASI scores. For combination treatment compared with pimecrolimus alone, there was no significant difference in the reduction of severity from baseline (p = 0.084). This was also the case for combination treatment compared with narrowband UVB treatment alone (p = 0.059). The combination treatment and each of the treatments alone all reduced the baseline severity of eczema by around 50%. No absolute values were given for severity in the trial report. All three treatments also reduced the severity of pruritus by around 3 points. Again, no absolute values for pruritus were given.
The trial by Heinlin and colleagues389 reported quality of life, measured using the Sickness Impact Profile, which was evaluated by the patient. There was no significant difference in mean quality of life between the treatment groups at the end of treatment [synchronous balneotherapy 4.6 (SD 6.8) vs. phototherapy only 4.0 (SD 5.5); p = 0.98]. Disease-specific quality of life was measured using the Freiburg Quality of Life Index and it was reported that there was no significant difference between the groups at the end of treatment. The participants assessed their global impression of treatment on a 6-point scale from ‘very good to ‘very bad’ and the proportion of participants with a score of ‘good or ‘very good’ at the end of treatment was statistically significantly different between the groups (synchronous balneotherapy 73.6% vs. phototherapy only 55.4%; p = 0.002) and was also significantly different at 1 and 6 months after the end of treatment. There was a statistically significant difference in the reduction from baseline in the severity of eczema (primary outcome), measured using the SCORAD index, between the synchronous balneotherapy group [61.8 (SD 14.1) to 25.6 (SD 22.0)] and the phototherapy-only group [61.5 (SD 12.4) to 34.6 (22.3)] at the end of treatment (after 35 treatments) (p = 0.004).
Harms
The adverse events reported were erythema with skin tenderness, burning, skin xerosis, uncomfortable heat load and intense sweating. 387 All of these events except for skin xerosis were reported with a frequency of ‘five or less’ but it was unclear whether this was ‘events’ or ‘participants affected’. Skin xerosis was reported with a frequency of 10 for the phototherapy-only treatment group and five for the combination treatment group.
In the trial by Tzung and colleagues,388 two participants in the whole-body narrowband UVB and half-body pimecrolimus group had intractable generalised pruritus and tender erythema after the UVB treatment.
The trial by Heinlin and colleagues389 reported that 30 participants in the synchronous balneotherapy group experienced 46 adverse events compared with 24 participants in the phototherapy group who experienced 31 adverse events. Eleven out of 46 events in the synchronous balneotherapy group were ‘definitely’ or ‘probably’ related to the trial treatment whereas 10 out of 31 events in the phototherapy group were ‘definitely’ or ‘probably’ related to the trial treatment. The most common events related to trial treatment were erythema and light dermatoses. Eight of the adverse events were serious (synchronous balneotherapy, n = 2; phototherapy only, n = 6), but none of these was classed as related to the trial treatment. Eight participants withdrew before the end of the trial because of adverse events (synchronous balneotherapy, n = 2; phototherapy only, n = 6).
Overall implications for research and practice
Although no formal comparative analyses were reported, it is obvious from the reduction in scores that there was no difference between a combination of UVA/UVB and topical corticosteroids and UVA/UVB alone. A huge disparity in baseline itch scores leads to questions about the method of randomisation and allocation concealment and makes it impossible to interpret the impact of the treatments on itch compared with each other. One small trial of balneotherapy combined with UVB phototherapy compared with UVB phototherapy only gives some evidence of benefit from the addition of balneotherapy; however, as there is no mention of a blinded severity of eczema outcome assessor for this trial, this evidence must be treated with caution until appropriately blinded trials are carried out to confirm this beneficial effect.
Full-spectrum light treatments
Studies
One trial by Byun and colleagues391 compared full-spectrum light (320–5000 nm) for eight irradiations (twice a week for 4 weeks) plus emollient against emollient only twice a week for 4 consecutive weeks. The 38 children randomised into the trial were all Korean with a SCORAD score of > 25 and skin type III or IV. The children were not allowed to use any other treatments during the trial.
Assessment of risk of bias
Table 93 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Byun 2011391 | Unclear risk | Unclear risk | High risk |
Benefits
The participants assessed their own clinical improvement in the trial, with 75% of the full-spectrum light group recording a ‘good’ or ‘excellent’ response on a 4-point scale compared with 50% of the emollient-only control group. The severity of eczema was measured using the SCORAD index. The score in the full-spectrum light group reduced from a mean of 47.87 at baseline to 30.76 at week 8. The score in the control group reduced from a mean of 39.79 at baseline to 33.8 at week 8. Although a significant reduction from baseline was reported in the full-spectrum light group, no between-group analyses were reported.
Harms
No serious adverse events were reported. In the full-spectrum light group, 6 out of 20 participants reported erythema, 6 out of 20 reported dryness, 4 out of 20 reported pruritus and 2 out of 20 reported burning. Six out of 20 participants also reported a transient exacerbation of eczema in the first 2 weeks.
Overall implications for research and practice
This trial391 was reported as open. It is not clear whether the SCORAD assessor was blinded and the baseline SCORAD scores were noticeably higher at baseline in the full-spectrum light group than in the control group. This means that the results should be treated with caution. The trial appears to show a reasonable improvement in eczema severity matched by the participants’ assessment of their own response to treatment. With only a small number of participants and a narrow range of skin types and a common heritage, much larger studies on mixed populations are needed before any recommendations about the use of full-light phototherapy can be made.
Excimer laser (form of ultraviolet laser)
Studies
A within-person trial from the Netherlands by Brenninkmeijer and colleagues125 compared once-daily clobetasol propionate (0.05%) against twice-weekly 200 mW/cm excimer laser for 10 weeks. The trial involved 13 participants with atopic eczema (diagnosed according to the millennium criteria392) and more than four symmetrical prurigo nodules.
Assessment of risk of bias
Table 94 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Brenninkmeijer 2010125 | Low risk | Unclear risk | High risk | Only 10/13 participants included in the analysis |
Benefits
A blinded physician assessment of individual signs of eczema found a statistically significant difference in favour of excimer laser treatment at 14, 22 and 34 weeks after starting treatment, which in all cases was after the end of the 10-week treatment period. There was a mean absolute decrease in the score over 34 weeks of 6 points on a 15-point scale in the excimer laser group and of 4.1 points in the clobetasol propionate group. Pruritus, assessed on a VAS, showed a 63% improvement in the excimer laser group and a 49% improvement in the clobetasol propionate group over the entire 34 weeks of the trial. The difference between the pruritus scores was reported as non-significant for weeks 14, 22 or 34, with the absolute values being only 1 point apart on a 10-point scale over this time period.
Harms
Excimer laser treatment resulted in a fairly high level of adverse events, although there were no reported serious adverse events. Adverse events included four reports of a burning sensation, five reports of erythema, two reports of vesicles and one report of blistering. All 10 participants analysed for the trial experienced hyperpigmentation at the treatment sites. Two participants withdrew because of an exacerbation of eczema that required systemic treatment. It is unclear which treatment group the patients withdrew from, although the exacerbations were described as being unlikely to be related to the study treatment.
Overall implications for research and practice
This very small trial125 hints at the potential for using clobetasol propionate laser treatment for a relatively short-term period to confer long-lasting beneficial effects compared with a moderate-potency topical corticosteroid. However, the level of adverse events is a serious cause for concern.
Summary of phototherapy
-
There were six trials of phototherapy treatment reported up to 2000 comparing different UVA or UVB treatments and regimens. The trials were small and poorly reported but did provide some evidence of a large treatment benefit.
-
Twelve trials were published after 2000; all were small but they showed some weak evidence of a large and rapid treatment benefit of phototherapy.
Ultraviolet B
-
One very small trial, with a high risk of bias for blinding, did not provide any evidence of benefit for UVB treatment with fixed doses guided by skin reflectance of red (660 nm) and green (555 nm) wavelengths to calculate the highest dose not eliciting erythema compared with standard UVB fixed-dose increments.
Ultraviolet A
-
One small trial, with an overall unclear risk of bias, compared high-dose UVA1 with medium-dose or low-dose UVA1, but failed to compare the treatment group results against each other.
-
One small trial, with a mostly unclear risk of bias, provided evidence of benefit for PUVA compared with UVA1 for length of remission and reduction in eczema severity.
Ultraviolet A compared with ultraviolet B
-
One very small trial, with an overall unclear risk of bias, did not provide any evidence of benefit for medium-dose UVA1 (350–400 nm) compared with narrowband UVB (311 nm).
-
One small trial, with a mostly low risk of bias, compared narrowband UVB with broadband UVA or visible light and failed to compare the treatment group results against each other.
-
One small trial, with a mostly low risk of bias, did not provide any evidence of benefit for UVA1 compared with narrowband UVB.
Phototherapy in combination with other active treatments
-
One small trial, with a high risk of bias for blinding, did not provide any evidence of benefit for UVA/UVB combined with the topical corticosteroids fluticasone and hydrocortisone butyrate compared with UVA/UVB treatment alone.
-
One very small trial, with a high risk of bias for blinding, did not provide any evidence of benefit for narrowband UVB alone compared with narrowband UVB in combination with 1% pimecrolimus.
-
One moderately sized trial, with a mixed risk of bias, did not provide any evidence of benefit for synchronous balneotherapy with UVB phototherapy compared with UVB phototherapy alone.
Phototherapy compared with other active treatments
-
One small trial, with a high risk of bias for blinding, provided evidence of benefit for oral ciclosporin (initial dose 4 mg/kg/day) compared with combined UVA/UVB treatment.
Full-spectrum light therapy
-
One small trial, with a high risk of bias for blinding, did not provide any evidence of benefit for full-spectrum light therapy applied with emollients compared with emollient treatment alone.
Excimer laser (form of ultraviolet laser)
-
One very small trial, with a mixed risk of bias, provided evidence of benefit for twice-weekly excimer laser treatment for 10 weeks compared with once-daily clobetasol propionate (0.05%) treatment.
Chapter 11 Systemic immunomodulatory agents
Background
Systemic immunomodulatory agents are third-line treatments considered when other interventions are not adequately controlling the eczema.
Existing systematic reviews
The NICE guidelines for the management of atopic eczema41 include most of the treatments in this chapter, apart from mepolizumab, omalizumab, immunotherapy and montelukast.
A systematic review with a search ending in August 2005, which covers all of the systemic immunomodulatory agents included in the NICE guidelines,41 was published by Schmitt and colleagues393 in 2007. This review concluded that, because of the weight of evidence of effectiveness, ciclosporin should be considered as the preferred option for third-line treatment of severe eczema. A systematic review by Schram and colleagues,394 with a search ending in 2009, reviewed the off-label use of azathioprine, including use for severe eczema. Two reviews93,395 examined trials of biological therapies and reviewed their use for eczema and two reviews396,397 examined desensitisation treatments (systemic immunotherapy) for eczema.
Scope of this chapter
This chapter covers the following systemic immunomodulatory agents:
-
azathioprine (oral)
-
ciclosporin (oral)
-
methotrexate
-
systemic corticosteroids:
-
prednisolone
-
-
mycophenolate mofetil
-
montelukast
-
systemic immunotherapy (desensitisation)
-
biological therapies:
-
mepolizumab
-
omalizumab
-
-
intravenous immunoglobulin
-
pimecrolimus (oral).
Azathioprine
The systemic immunosuppressant azathioprine has been used to prevent rejection following organ transplantation and to treat steroid-responsive diseases such as inflammatory bowel disease and vasculitis. It is sometimes used as a topical steroid-sparing agent. Azathioprine is converted to a purine synthesis inhibitor, which inhibits the production of lymphocytes, especially B and T cells. Thiopurine methyltransferase levels are checked before treatment to enable adjustment of the individual starting dose. Azathioprine is now commonly used for cases of severe eczema for medium- to long-term control.
Studies
There were no trials involving azathioprine reported before 2000.
Three new trials have been published since 2000. A double-blind placebo-controlled crossover trial by Berth-Jones and colleagues398 compared azathioprine against placebo in 37 adult participants with eczema that seriously affected their quality of life, despite the daily use of topical corticosteroids. The treatments were taken consecutively, with each treatment taken continuously for 3 months; the order of treatment was randomly allocated. The use of topical corticosteroids was permitted during the trial, except for very potent corticosteroids.
Another double-blind trial by Meggitt and colleagues399 allocated participants to groups using minimisation. This trial compared azathioprine suspension given once a day for 12 weeks with a placebo suspension using the same regimen. Sixty-three adults with moderate to severe eczema that had been stable in the recent past were enrolled. The ratio of participants given azathioprine to participants given placebo was 2 : 1 and therefore 42 participants were given azathioprine.
One small single-blind trial, reported by Schram and colleagues394 in 2011, compared azathioprine (1.5–2.5 mg) against methotrexate (10–22.5 mg) for 12 weeks in 43 adult patients with severe eczema who were unresponsive to or intolerant of ciclosporin. This trial is discussed in more detail later in this chapter (see Ciclosporin).
Assessment of risk of bias
Table 95 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Berth-Jones 2002398 | Low risk | Low risk | Unclear risk | Intention-to-treat population did not include two participants who attended only the baseline assessment |
| Meggitt 2006399 | Low risk | Low risk | Low risk | Intention-to-treat population did not include participants who attended only the baseline assessment |
| Schram 2011394 | Low risk | Low risk | Low risk | The study was underpowered to provide evidence of equivalent efficacy between groups |
Benefits
The trial by Berth-Jones and colleagues398 showed statistically significant improvements in eczema when using azathioprine compared with placebo, measured using the SASSAD scale. 59 For the participant-assessed outcomes of itching, sleep disturbance and disruption of work/daytime activity, the difference in mean improvement between azathioprine and placebo was statistically significant only for disruption of work/daytime activity. There was no evidence of carry-over effects of taking azathioprine (analysis of covariance = 0.8), despite the lack of a washout period between treatments.
In the trial by Meggitt and colleagues,399 the results for the primary outcome, change in severity of eczema over 12 weeks, showed a 5.4-point (17%) difference [95% CI 1.4 to 9.3 (4.3% to 29%)] in favour of azathioprine. This difference was less than the difference of 30% that the trial was powered to detect, based on pilot studies. For participant-rated itch and sleep loss over 12 weeks there were only small differences in scores, although the significance of these reductions is not clear. For quality of life there was a 3.5-point difference (95% CI 0.3 to 6.7) in favour of azathioprine, but it is very difficult to interpret the significance of this reduction. The investigator- and participant-assessed severity scores showed significant differences in favour of azathioprine, with the investigator assessment having the greatest significance.
The study by Schram and colleagues394 provided evidence of benefit of 12 weeks of treatment with both methotrexate and azathioprine, but there was no significant difference in efficacy between the two treatments (SCORAD 50, p = 0.76; at least mild IGA score, p = 0.74; mean IGA score, p = 0.2; mean PGA score, p = 0.95; mean EASI score, p = 0.95; sleeplessness, p = 0.24; itch, p = 0.78).
Harms
In the trial by Berth-Jones and colleagues398 the most frequent adverse events were gastrointestinal (nausea, vomiting, diarrhoea, abdominal pain, bloating and anorexia). Treatment with azathioprine resulted in more adverse events, some serious enough to result in four withdrawals from treatment. In comparison, there were no withdrawals in the placebo group. The study reported that treatment with azathioprine resulted in transient mild neutropenia, lymphopenia and transient elevation of liver enzymes in eight participants. Placebo treatment resulted in transient elevation of liver enzymes in two participants. It was reported that one of these elevations of liver enzymes, near the end of the study, would have been severe enough to require withdrawal; however, it was not clear which group this participant was in.
The trial by Meggitt and colleagues399 reported that the most frequent adverse event was nausea, which mainly occurred in participants taking azathioprine (51% of this group) and resulted in seven participants having their doses reduced and four withdrawals in the azathioprine group. Another two participants withdrew from the azathioprine group because of hypersensitivity to the treatment. One participant withdrew from the placebo group because of headaches and malaise.
Overall implications for research and practice
Two fairly high-quality trials398,399 comparing azathioprine with placebo in adults, with sufficient power to detect a 25% improvement in SASSAD scores, have shown significant benefit in terms of the severity of eczema and disruption of work/daytime activity. There is not yet any clear evidence of benefit for itching and sleep disturbance, which may be of much more importance to some people with severe eczema when weighed against the potential harms of this treatment. The treatment was only given for 3 months and may not yet have reached its full therapeutic potential,399 which can be established only by conducting trials over a longer period of time. There is insufficient evidence to deduce whether the benefit provided by azathioprine is equivalent to that provided by methotrexate in adults, as there have not been any non-inferiority or equivalence trials.
As yet, there appear to be no trials looking at the use of azathioprine in children with eczema. Given that azathioprine treatment is increasingly used for children with severe eczema, it is important that high-quality RCTs involving children are conducted. These future trials need to pay attention to the azathioprine regimens currently being used in clinical practice to maximise the applicability of the results.
Ciclosporin
Ciclosporin (Neoral®; Novartis) is a systemic immunomodulator that is used to treat severe eczema. There has been previous evidence from controlled trials that it is beneficial compared with placebo, in particular for the relief of itching. Relapse can be very rapid after discontinuation of treatment.
Studies
Fifteen trials were reported before 200055 (see Appendix 3).
Eight new trials have been published since 2000. 151,390,400–405
A trial by Czech and colleagues400 included 106 adults with severe eczema and compared high (300 mg/day) and low (150 mg/day) doses of oral ciclosporin, regardless of body weight.
Bemanian and colleagues401 compared 3 months of daily ciclosporin at doses of 4 mg/kg against one intravenous immunoglobulin infusion of 2 g/kg in 16 participants with severe eczema.
A trial by Granlund and colleagues,390 which included 72 adults with severe eczema, compared UVAB light therapy against ciclosporin using cycles of treatment, with 2 weeks of topical treatment between each cycle, for 1 year.
A small double-blind trial by Pacor and colleagues151 of 30 adolescents and adults compared a dose of 3 mg/kg of oral ciclosporin daily with topical tacrolimus (0.1%) ointment applied twice a day for 42 days using a double-dummy technique to ensure blinding.
One small, well-reported trial by Schmitt and colleagues402 included 38 adults with severe eczema and compared a 2-week tapering dose of oral prednisolone with a constant daily dose of ciclosporin for 6 weeks. This mimics the treatment regimens frequently used in clinical practice. No other topical or systemic treatments apart from emollient, prednicarbate (0.25%) and antihistamines (at the dose taken before the study) were allowed.
A feasibility crossover trial by Kwon and colleagues,405 reported in 2013, included 10 patients aged > 12 years and compared ciclosporin treatment with and without glucosamine supplementation. Treatments were given once a day for 2 weeks followed by crossover to the other treatment for 2 weeks. This 4-week cycle was continued for 6 months and there were no washout periods because of concern about rebound exacerbation of eczema. The severity of eczema was assessed at the end of each 2-week period of treatment.
A trial by El-Khalawany and colleagues404 compared methotrexate against ciclosporin for 12 weeks in 40 children aged > 8 years with severe eczema that had been unresponsive or poorly responsive to topical therapy or phototherapy. Blinding procedures were not reported in this study. No dropouts were reported.
The trial by Haeck and colleagues403 compared mycophenolate mofetil with ciclosporin and is discussed later in this chapter (see Mycophenolate mofetil). This trial found mycophenolate mofetil to be non-inferior to ciclosporin.
Assessment of risk of bias
Table 96 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Bemanian 2005401 | Unclear risk | Unclear risk | Unclear risk | Very small sample size and exclusion criteria not specified. Participants were hospitalised for treatment. Blinding not reported and not likely |
| Czech 2000400 | Low risk | High risk | Low risk | |
| El-Khalawany 2013404 | Low risk | Unclear risk | High risk | Intention-to-treat population not used |
| Granlund 2001390 | Unclear risk | Unclear risk | High risk | |
| Haeck 2011403 | Low risk | Unclear risk | Low risk | All participants initially received a course of ciclosporin at a higher dose than the trial treatment |
| Kwon 2013405 | Unclear risk | Unclear risk | Unclear risk | Very small sample size. Intention-to-treat analysis not described |
| Pacor 2004151 | Unclear risk | Unclear risk | Unclear risk | Trial not long enough to see the full potential effect of ciclosporin |
| Schmitt 2010402 | Low risk | Low risk | Low risk | Study terminated early and so the number analysed was underpowered (only 38 out of the 66 needed) |
Benefits
The trial by Czech and colleagues400 did not compare the different doses of ciclosporin investigated against standard body weight-dependent dosing, was of short duration and involved only a short follow-up period The authors concluded that there appears to be no difference between these treatment regimens; however, without another comparator, such as standard topical treatment, the clinical relevance of this trial is lost.
Four trials151,390,401,402 compared body weight-dependent doses of ciclosporin against other active treatments. The trial by Bemanian and colleagues401 showed a significant improvement in the severity of eczema according to SCORAD scores for ciclosporin compared with intravenous immunoglobulin by day 30, which was sustained until the end of the study on day 90. Use of topical corticosteroids was allowed to control flare-ups but no data were provided on the amount of corticosteroids used in each group. The immunoglobulin was given only once compared with continuous treatment with ciclosporin and so it is difficult to make a direct comparison; however, immunoglobulin is much more expensive than ciclosporin and requires hospitalisation to administer.
In the trial by Granlund and colleagues,390 the amount of time spent in remission (reduction in disease activity assessed by SCORAD to ≤ 50% of the baseline) was significantly higher for the ciclosporin group and the speed of reduction in eczema severity was faster. Overall, there was no difference in quality of life and after the first complete treatment cycle (10 weeks) there was no significant difference in severity between the treatments, pointing to a similar relapse rate. The trial set out to measure how much emollient and topical corticosteroid was used, but these data were not reported.
In the trial by Pacor and colleagues,151 the assessments of itching, sleep loss and erythema by participants showed a significant improvement with topical tacrolimus compared with ciclosporin between day 7 and day 21; tacrolimus ointment was also significantly more effective in terms of eczema severity as assessed by SCORAD scores between day 14 and day 35. These differences were not present at any other points in the trial. The trial reported that there were no exacerbations of eczema during the 3-month follow-up.
The trial by Schmitt and colleagues402 assessed the number of participants in stable remission at the end of treatment. Because of rebound/exacerbation of eczema, half (11/21) of the participants taking prednisolone and six out of 17 of the participants taking ciclosporin dropped out before the study was stopped prematurely. Significantly more of the participants achieved stable remission on ciclosporin. Both treatments improved the severity of eczema but were not significantly different from each other at the end the trial period or after a further 12 weeks. For those who responded to the treatments initially, 89% on prednisolone relapsed and 45% on ciclosporin relapsed during the 12-week follow-up.
By combining the severity of eczema SCORAD scores for all 2-week periods on each treatment, the trial by Kwon and colleagues405 reported that ciclosporin combined with glucosamine was significantly more beneficial than ciclosporin alone from 1.5 months of treatment onwards.
In the trial by El-Khalawany and colleagues404 there was no statistically significant difference between groups in the reduction in SCORAD score at 12 weeks (SCORAD mean absolute reduction was 26.2 in the methotrexate group and 25.0 in the ciclosporin group; p = 0.93).
Harms
The trials published since 2000 have compared ciclosporin against different active agents. In each of these trials, the number of people taking ciclosporin who reported adverse events was fairly high; however, the rates were about the same for tacrolimus, UVAB phototherapy and intravenous immunoglobulin. It is worth noting that there were more withdrawals because of treatment failure/exacerbation of eczema for UVAB phototherapy and prednisolone. The treatment duration in these eight trials varied from 2 weeks to 1 year. For these time periods the levels of liver- and kidney-related adverse events reported appear to be very low.
Overall implications for research and practice
The previous review55 provided good evidence that oral ciclosporin is of significant benefit compared with placebo. It is reassuring that there have now been trials comparing ciclosporin with other active treatments for eczema. These studies suggest that a short course of ciclosporin might be more effective than intravenous immunoglobulin or oral prednisolone. 401,402 For oral prednisolone treatment, there is evidence that this lack of benefit results from serious eczema exacerbations when oral prednisolone has to be stopped, rather than because it is less effective when being taken. There was some evidence that UVAB phototherapy,390 or methotrexate,404 is not significantly more beneficial than a short course of ciclosporin. Topical tacrolimus showed more favourable results than ciclosporin in one small, short-term study,151 but this needs clarification from much larger methodologically robust trials. It is not possible to assess whether or not ciclosporin combined with glucosamine is actually more beneficial than ciclosporin alone as the trial demonstrating a beneficial effect was very small and of an unusual design. 405 This raised doubts about which treatment effect was being measured, the treatment just given or the treatment given in the 2 weeks before, because of a lag in the reduction of the SCORAD score.
Methotrexate
Methotrexate is a folic acid antagonist that targets several T-cell activities. It is a common drug that has been used for decades in inflammatory rheumatic diseases and severe psoriasis.
Studies
No trials of methotrexate were reported before 2000.
Two new studies have been published since 2000. One small single-blind trial, reported by Schram and colleagues394 in 2011, compared methotrexate with azathioprine for 12 weeks in 43 adult patients with severe eczema that was unresponsive or intolerant to ciclosporin. The trial by El-Khalawany and colleagues404 is discussed in the section on ciclosporin, earlier in this chapter.
Assessment of risk of bias
Table 97 provides the risk-of-bias assessment for the new studies.
Benefits
In the trial by Schram and colleagues,394 both treatments improved eczema severity but were not significantly different from each other at 12 weeks. The mean reduction in SCORAD score for methotrexate was 22.7 points and for azathioprine was 22.2 points. The proportion of patients with a SCORAD score reduction of ≥ 50% was 40.0% in the methotrexate group and 45.4% in the azathioprine group. There were also no differences between the two groups at 12 weeks in patient-reported outcomes – itch assessment using a VAS, POEM scale, quality-of-life scale – and in the use of concomitant steroids during the study.
Harms
In the trial by Schram and colleagues,394 no serious adverse events were reported in either group. One patient in the methotrexate group dropped out after 4 weeks because of nausea and fatigue and three patients were withdrawn in the azathioprine group. Abnormalities in blood count were significantly more frequent in the azathioprine group [n = 17 (77%) vs. n = 6 (30%); p = 0.002]. In the trial by El-Khalawany and colleagues,404 common adverse effects in the methotrexate group included anaemia (30%), fatigue (30%), abnormal liver function (25%), nausea and vomiting (20%) and glossitis with oral ulceration (20%). In the ciclosporin group, common complications included fatigue (45%), leucopoenia (35%), headache (25%), anaemia (20%) and flu-like symptoms (20%). None of the adverse events reported necessitated discontinuing or decreasing the dose of the drug and all had resolved when followed up.
Overall implications for research and practice
These two trials are underpowered. Additionally, the study by El-Khalawany and colleagues404 had some methodological weaknesses and used a dose that could well have been subtherapeutic. Consequently, larger, clearly reported, clinically relevant studies are needed to properly compare the benefits of these drugs in adults and children with severe eczema. Based on the findings in these two small trials, there is no evidence that methotrexate is significantly more beneficial than azathioprine or ciclosporin. Adverse events for methotrexate do not appear, from the current trial evidence, to be any less significant than those for either azathioprine or ciclosporin, but long-term evidence for the safety of these treatments is lacking. There is definitely a need for further trials to assess the efficacy and safety of methotrexate for the treatment of eczema.
Prednisolone
Prednisolone is a systemic corticosteroid widely used to treat a variety of health conditions such as asthma, pyoderma gangrenosum and inflammatory bowel disease. This treatment needs to be slowly tapered when stopping.
Studies
One trial involving oral prednisolone was published before 200055 (see Appendix 3).
One small, well-reported trial by Schmitt and colleagues,402 published in 2010, included 38 adults with severe eczema and compared a 2-week tapering dose of oral prednisolone with a constant daily dose of ciclosporin for 6 weeks. This mimics the treatment regimens frequently used in clinical practice. The only treatments permitted were emollients, prednicarbate (0.25%) and antihistamines at the dose taken before the study. The trial looked at the number of participants in stable remission at the end of treatment.
Assessment of risk of bias
Table 98 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Schmitt 2010402 | Low risk | Low risk | Low risk | Study terminated early and so the number analysed was underpowered (only 38 out of the 66 required) |
Benefits
Significantly more of the participants on ciclosporin than on prednisolone achieved stable remission. Both treatments improved eczema severity but were not significantly different from each other at the end of the treatment period or after a further 12 weeks. For those who responded to the treatments initially, 89% on prednisolone relapsed and 45% on ciclosporin relapsed during the 12-week follow-up.
Harms
This trial was stopped early because of the unexpectedly large number of participants who withdrew because of exacerbation of their eczema (15/38 participants, two of whom needed to be hospitalised). Most other adverse events noted in the study were mild; however, both groups included a few participants who had reversible hypertension. No increases in creatinine levels were reported. Although standardisation of the concomitant treatments makes analysis of the trial results easier, this is very unlikely to compare to a normal clinical situation.
Overall implications for research and practice
Prednisolone cannot be used for long periods of time without a significant risk of side effects. The trial by Schmitt and colleague402 seems to suggest that short courses also lead to high rates of eczema relapse, some of which can be very severe (10/15 participants who relapsed were on prednisolone). As this treatment was also not as effective as ciclosporin, and the trial was underpowered, these results should be treated with caution. Whether or not prednisolone can be useful as an emergency rescue treatment on top of other third-line treatments has yet to be investigated in a RCT, but based on the results of these two trials the use of prednisolone for the treatment of eczema should be very carefully considered.
Mycophenolate mofetil
Mycophenolate mofetil is an immunosuppressant that works by preventing T cells and B cells dividing.
Studies
No trials of mycophenolate mofetil were reported before 2000.
One new trial was reported after 2000. This small industry-funded single-centre non-inferiority trial from the Netherlands403 compared 1440 mg/day of enteric-coated mycophenolate sodium against 3 mg/kg/day of ciclosporin for 30 weeks. All participants were treated for a 6-week run-in period with 5 mg/kg/day of ciclosporin. The participants were also followed up for 12 weeks after the treatment stopped. Only the assessing physician was blinded. Fifty adults with eczema, assessed according to the Hanifin and Rajka8 criteria, who were not responding adequately to potent topical corticosteroids were randomised.
Assessment of risk of bias
Table 99 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Haeck 2011403 | Low risk | Unclear risk | High risk | All participants were treated for a 6-week run-in period with a higher dose of ciclosporin than that used in the trial |
Benefits
The participants did not report any significant differences in itch and sleep loss during the trial, but the data were not reported for this outcome. The severity of eczema, measured using the SCORAD index after 10 weeks, was comparable in both study arms (difference 0.8, 95% CI –4.4 to 6.0) until the end of the maintenance phase.
There were fewer participants with a high quality-of-life score in the mycophenolate mofetil group after 6 weeks of treatment, but there were no significant differences for the rest of the trial.
Harms
There were no serious adverse events reported for this trial. Hypertrichosis was reported for 62% of the ciclosporin group, whereas fatigue (46%) and flu-like symptoms (34%) were reported for the mycophenolate mofetil group. Anomalies in laboratory tests were recorded when they occurred more than twice during the trial; of those reported, two had a notable difference in numbers affected in each treatment group: magnesium decreased by 27% (n = 7) in the ciclosporin-only group and by 4% (n = 1) in the mycophenolate mofetil group and blood pressure increased by 15% (n = 4) in the ciclosporin-only group and 0% in the mycophenolate mofetil group. All laboratory abnormalities were reported to be transient in both treatment groups.
Overall implications for research and practice
This trial provides some evidence of reasonable quality that, in an objective measurement of the severity of eczema, mycophenolate sodium is not inferior to ciclosporin. As only the assessing physician was blinded to treatment, the participants’ assessments of quality of life, itching and sleep loss must be treated with some caution; however, they did not appear to show a significant difference between the two treatments. It is important to bear in mind that all participants used ciclosporin first and the trial then compared the two treatments as maintenance treatment. Consequently, this trial does not provide evidence about the use of mycophenolate sodium for initial symptom reduction or long-term efficacy, The trial report states that there is evidence that mycophenolate mofetil sustains improvements in eczema after the treatment is stopped, but insufficient data for this were provided for independent verification of this claim.
Montelukast
Montelukast (Singulair®; Merck Sharp & Dohme) is a specific antagonist of cysteinyl-leukotriene receptor 1. Chemicals produced by mast cells and eosinophils bind to this receptor to mediate responses associated with inflammation. Montelukast is currently used to treat asthma and seasonal allergic rhinitis.
Studies
There were no trials involving montelukast published before 2000.
Six new trials have been published since 2000. 251,406–410
Montelukast compared with placebo
Four trials comparing montelukast with placebo have been reported.
The trial by Pei and colleagues406 in Hong Kong, China, compared a 5-mg once-a-day dose of montelukast against a placebo (chewable ascorbic acid) taken daily for 4 weeks. Fifteen participants aged 6–16 years were given the treatments in a crossover design: all participants were given one treatment in a randomised order and then, after a 2-week break, were given the other treatment. All participants used 70% light liquid paraffin as a soap substitute, aqueous cream as an emollient and clobetasone butyrate (0.05%) cream twice daily during the study.
Three trials compared 10 mg of montelukast daily against placebo in adults. Two of these studies407,408 looked at moderate to severe disease and both were supported by Merck Sharpe & Dohme, which markets montelukast. Both trials compared 10 mg per day of montelukast against placebo after a washout period of 2 weeks in which all participants took only placebo. The trial by Veien and colleagues,408 which included 59 participants aged 16–70 years with moderate to severe eczema, did not allow any other topical or systemic treatments during the study, which ran for 4 weeks of treatment. The trial by Friedmann and colleagues407 gave treatment for 8 weeks.
The third trial409 was carried out in adults with mild to moderate eczema and used a crossover design to compare 10 mg daily of montelukast with placebo for 4 weeks. The eight participants were allowed class V (potent) or weaker topical corticosteroids, emollients and antihistamines.
Montelukast compared with active treatments
Two trials compared montelukast with other active treatments.
An open, randomised parallel-group trial from Bangladesh by Rahman and colleagues410 compared hydrocortisone (1%) and antihistamine with a 5-mg (participants aged ≤ 14 years) or a 10-mg once-a-day dose of montelukast for 4 weeks in 31 participants aged ≥ 6 years.
Another randomised, single-blind trial by Capella and colleagues251 compared 10 mg per day of montelukast and placebo topical and tablet treatment against cetirizine, clarithromycin and topical mometasone furoate (0.1%) or topical methylprednisolone aceponate (0.1%) in 32 adults with moderate to severe eczema.
Assessment of risk of bias
Table 100 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Capella 2001251 | Unclear risk | Unclear risk | Unclear risk | |
| Friedmann 2007407 | Low risk | Low risk | Low risk | |
| Pei 2001406 | High risk | High risk | Unclear risk | Baseline severity not comparable between groups; more severe in the montelukast first group |
| Rahman 2006410 | Unclear risk | Unclear risk | High risk | |
| Veien 2005408 | Unclear risk | Unclear risk | Unclear risk | |
| Yanase 2001409 | High risk | High risk | Low risk | Only a 2-day washout between treatments. Participants do not appear to have been blinded |
Benefits
Montelukast compared with placebo
In the trial by Pei and colleagues,406 for each group the greatest decrease in eczema severity using a score of six eczema signs across eight areas of the body (maximum score of 144) was during the first treatment phase [group A decreased by 22.9 points (IQR –35.6 to –18.7 points) on placebo and group B decreased by 20.0 points (IQR –55.5 to –13.5 points) on montelukast], with a much smaller decrease in the second treatment phase (p = 0.043). There were no significant differences in quality of life or extent of disease. This trial included children who were already using a class II (moderate-potency) topical corticosteroid, emollients and soap substitutes; however, their eczema was still not being adequately controlled. All participants in the trial were instructed to use clobetasone butyrate twice daily during the trial and this seems most likely to have been responsible for the changes in severity seen, potentially masking any benefit from montelukast.
In the trial by Veien and colleagues,408 the severity of eczema was measured using a modified EASI score (which included pruritus scores). No significant differences between the groups were found. The trial by Friedmann and colleagues407 administered treatment for 8 weeks and measured the severity of eczema using SASSAD scores in addition to participant- and clinician-assessed response to treatment, severity of itching and severity of sleep loss, No significant differences were observed. Although the quantity of topical corticosteroids used was reported, and does not appear to differ greatly between groups, the report is difficult to interpret.
The results of the very small trial by Yanase and colleagues409 are difficult to interpret but appear to show a significant difference in severity (ADASI) scores between treatments (p = 0.014). It is not clear for most of the results whether between-group or within-group differences are being reported.
Montelukast compared with other active treatments
In the trial by Rahman and colleagues410 the severity of eczema was measured using SCORAD scores and showed a significant difference between groups in favour of montelukast (p = 0.01). Not much detail about the study design was reported, including whether any other treatments such as emollients were permitted or whether the groups were comparable at baseline. This positive result should be treated with caution as the trial was not blinded.
There were no significant differences between the treatments in the trial by Capella and colleagues251 in terms of reducing the severity of eczema. Although reported as a single-blind trial, it was not clear who was blinded. This trial is discussed in more detail in Chapter 7.
Harms
According to the available evidence, montelukast does not appear to result in any significant harms after short-term use. One of the trial reports did not give details about whether there were any adverse events. 409 One trial stated that there were no withdrawals because of adverse events but did not give any other safety data. 406 Three trials reported either no adverse events or no adverse events related to the study treatments. 251,408,410 The trial by Friedmann and colleagues407 reported one serious adverse event of septicaemia, at the end of the trial, in a participant in the montelukast group (who rapidly recovered). There was also one withdrawal because of dizzy spells in a participant in the montelukast group. The authors also stated that there had been other adverse events that were mild (respiratory tract infections, headaches, flares of eczema, mild gastrointestinal disturbances) but that these occurred in both treatment groups at comparable rates.
Overall implications for research and practice
There have now been a number of small RCTs comparing montelukast with placebo. Only two of these trials can be given serious credit for their results, as the others all use concomitant medications in their trials, required for ethical reasons, but often to a level that interferes with interpretation of any potential beneficial effects of montelukast. These trials, both of good methodological quality, fail to demonstrate a beneficial effect of montelukast over placebo. Although much larger, longer-term studies are needed to fully explore the question of whether montelukast is effective, it is unlikely that these will now be funded.
The two small trials comparing montelukast with active treatments were not performed in a methodologically rigorous manner. The favourable result in the unblinded study by Rahman and colleagues410 is not enough evidence to suggest that this treatment be considered in routine clinical practice.
Systemic immunotherapy (desensitisation)
Immunotherapy aims to desensitise the immune system to one or more specific allergens or to produce a more general desensitisation to raised levels of IgE. Levels of sensitivity to house dust mite, as measured by circulating IgE antibodies in the blood, skin-prick tests or atopy patch tests, are relatively high amongst people with eczema, and there have been links made between sensitivity to house dust mite and eczema severity, implying that such sensitivity may be playing a role in the disease. 366 One treatment approach is to reduce exposure to allergens such as house dust mite and another is to desensitise by deliberately exposing people to small amounts of allergen until tolerance develops. Desensitisation using the allergen to which they display sensitivity has been tried in selected patients to reduce the severity of eczema.
Studies
Three trials involving systemic immunotherapy were published before 200055 (see Appendix 3).
Four new studies have been published since 2000. 411–414
A small double-blind trial by Silny and Czarnecka-Operacz411 compared specific immunotherapy using house dust mite allergens (two common species) or grass pollen by injection with placebo (histamine injections). Twenty participants who had sensitisation to grass or house dust mite as well as eczema, 10 in each group, were treated for 1 year.
One small unblinded trial by Sanchez-Caraballo and Cardona-Villa,412 reported in 2012, compared specific immunotherapy using subcutaneous house dust mite allergens once a month for 1 year plus standard treatment (emollients, topical steroids, tacrolimus and oral steroids if needed) with standard treatment alone in 65 children and adults with eczema and sensitisation to house dust mite allergens (two common species). There were four dropouts in the control group and one in the experimental group.
A trial by Novak and colleagues,413 reported in 2012, compared specific immunotherapy using subcutaneous house dust mite allergens every 6 weeks plus standard treatment (emollients, topical steroids, pimecrolimus and oral steroids if needed) for 18 months with standard treatment alone in 168 adults with moderate to severe eczema and sensitisation to house dust mite allergens (two common species). Participants were randomised 2 : 1 to immunotherapy plus standard treatment or standard treatment alone. There were 37 dropouts in the experimental group (out of 112 participants) and 18 dropouts in the control group (out of 56 participants).
A blinded trial by Pajno and colleagues414 included 56 children aged from 5 to 16 years with chronic eczema that had not spontaneously improved before 5 years of age and with a proven house dust mite IgE-mediated sensitivity. The trial compared sublingual immunotherapy using a solution containing house dust mite (allergens Der p1 and Der f1) against a placebo solution. The immunotherapy dose was gradually titrated from 100 to 1000 and finally to 10,000 RAST units per ml and was given at the highest concentration (five drops, three times a week) for 18 months. The severity of eczema, parent-assessed overall symptoms of eczema, the amount of rescue medication used and any local or systemic adverse events were recorded.
Assessment of risk of bias
Table 101 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Novak 2012413 | Low risk | Unclear risk | Unclear risk | |
| Pajno 2007414 | Low risk | Unclear risk | Low risk | Efficacy analyses using intention-to-treat principles not carried out. Eight participants withdrew during the trial |
| Sanchez-Caraballo 2012412 | Unclear risk | Unclear risk | High risk | The intention-to-treat population was not used for analysis |
| Silny 2006411 | Unclear risk | Unclear risk | Unclear risk | The trial was very small and severity was measured by the point index, which is not as sensitive as other measures |
Benefits
The trial by Silny and Czarnecka-Operacz411 reported a significant difference in eczema severity and extent of skin inflammation between treatments in favour of specific immunotherapy. It is not clear whether any other treatments for eczema were permitted during the trial.
The trial by Sanchez-Caraballo and Cardona-Villa412 reported a significant improvement in disease severity, measured using the SCORAD index, in the experimental group compared with the control group after 6 months (p = 0.03), but the magnitude of benefit cannot be found in the reported data. An unvalidated patient-reported subjective score was also used to assess disease severity in this study and there was a significant difference in severity between treatments in favour of specific immunotherapy (p = 0.01). Moreover, after 1 year of follow-up, a reduction in the use of topical steroids and tacrolimus was presented in the experimental group compared with the control group (p = 0.02).
The trial by Novak and colleagues413 reported no significant differences between the treatment groups in disease severity measured by SCORAD scores, quality of life measured by the DLQI or use of basic medication during the trial. Post hoc analysis performed in the severe subgroup of patients (SCORAD score of > 50 at baseline) found a statistically significant reduction in the median total SCORAD score over time, with the immunotherapy plus standard care group having a 21% improvement compared with standard treatment only (p = 0.02).
The trial by Panjo and colleagues414 showed no significant difference between the two groups for participant-/carer-assessed severity of eczema over 18 months. The sublingual immunotherapy group performed significantly better than the placebo group when the severity of eczema was assessed by SCORAD scores, but only from 9 months onwards until the end of the trial at 18 months. At 9 months the immunotherapy group had improved by an average of 12 SD ± 3.8 points and the placebo group by an average of 4 SD ± 3.5 points (p = 0.0025); this difference only reduced slightly by 18 months. The sublingual immunotherapy group used significantly less rescue medication than the placebo group over the trial period. Patient with mild to moderate eczema (SCORAD score of < 40) gained the most benefit, with a significant benefit of sublingual immunotherapy for eczema severity compared with placebo; participants with severe eczema did not show a significant benefit of sublingual immunotherapy for any of the outcomes.
Harms
The trial by Silny and Czarnecka-Operacz411 reported that there were no clinically significant systemic adverse events. Eight participants in the immunotherapy group and six participants in the placebo group experienced worsening of skin inflammation that required mild topical corticosteroids.
In the trial by Sanchez-Caraballo and Cardona-Villa,412 16 local immediate reactions were observed in 11 patients in the first 3 months of treatment, whereas no systemic reactions were recorded. It was unclear in which groups these adverse events were observed.
In the trial by Novak and colleagues,413 local reactions of mild intensity were reported in 39% of participants in the immunotherapy group and 35% in the placebo group.
In the trial by Pajno and colleagues,414 two participants experienced generalised itching and flares about 1 hour after being given sublingual immunotherapy, which needed treatment with intramuscular chlorpheniramine and betamethasone. They were rechallenged three times and then excluded. Tiredness was reported by six participants in the immunotherapy group and one participant in the placebo group. One participant in the immunotherapy group reported headaches. There were local, delayed reactions in the build-up of dosing for one participant, who experienced swelling of the mouth, lips and face, and a further three participants experienced oral itching.
Overall implications for research and practice
Four trials reported after 2000 provide contradictory evidence. The largest did not find any benefit for the addition of house dust mite-specific immunotherapy in sensitised adult patients, except in a post hoc subgroup of only those with severe eczema. 413 Two smaller trials in children contradict each other. In one trial of sublingual immunotherapy treatment414 the results from 9 to 18 months do provide evidence of benefit, but there is a lack of evidence of benefit over the entire 18 months. This trial also found no treatment effect for those with severe eczema. The other trial in children did not find any evidence of benefit. 412 The smallest trial provided evidence of benefit for immunotherapy, but with only 20 participants and a questionable placebo treatment (histamine) this does not add much weight to the evidence. 411
The evidence base for this treatment is far from clear. A better picture of which severities of eczema may benefit and whether there is a difference in effectiveness between adults and children is still needed. There is some evidence that there is a steroid-sparing effect of immunotherapy but exactly why this occurs is unclear. As immunotherapy appears to take a long time to show effectiveness, future trials should ideally include longer durations of treatment and longer follow-up periods to investigate the full potential of immunotherapy. A pragmatic trial should consider approaches to standardise concomitant medication for eczema during the initial treatment phase, to avoid losing unacceptable numbers of participants because of an initial lack of efficacy.
The adverse events reported here are similar to those observed in previous trials on desensitisation and there are some individuals who cannot use immunotherapy because of serious reactions such as acute exacerbation of eczema or allergic reactions. This is an important consideration and requires further specific research using methodologies more appropriate to assessing harms.
Mepolizumab
Mepolizumab is a humanised monoclonal antibody to interleukin 5, which is a key cytokine in eosinophil production in the bone marrow.
Studies
There were no trials involving mepolizumab published before 2000.
One new trial by Oldhoff and colleagues415 compared mepolizumab with placebo in 43 adults with eczema who were experiencing a flare. The treatment was given in two single doses, 7 days apart. The only other treatments allowed were non-medicated emollients, bath oils and hydrocortisone acetate (1%) for the face. Those who had not responded by day 16 were allowed fluticasone propionate (0.05%) once daily.
Assessment of risk of bias
Table 102 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Oldhoff 2005415 | Unclear risk | Unclear risk | Unclear risk | The sample size was powered to detect only large treatment effects. The treatment and follow-up durations were short for the nature of eczema |
Benefits
Of the 40 participants evaluated after 2 weeks, there were no significant differences between the treatments according to the physician’s global assessment (p = 0.115), severity measured by SCORAD scores (p = 0.293) and itching.
Harms
Adverse events were only very briefly discussed in the trial report. The report states that there were some mild and transient adverse events in the mepolizumab group and that they did not differ from those seen in the placebo group.
Overall implications for research and practice
This trial has failed to show any clinically relevant effects of mepolizumab. It is too early to conclude with certainty that mepolizumab does not have any treatment effect, as this one trial was powered to detect only large treatment effects over a short period. That said, mepolizumab is a very expensive treatment and so it may be difficult to justify its use, even if future trials show a moderate short- or long-term benefit.
Omalizumab
The recombinant humanised monoclonal antibody (IgG1κ) omalizumab has been shown to lower blood serum levels of free IgE. Omalizumab has shown efficacy and a good safety profile when tested in allergic asthma, rhinitis and food allergy. It is also used in immunotherapy to help prevent type I hypersensitivity reactions.
Studies
There were no trials involving omalizumab published before 2000.
One new trial has been published since 2000. This small mechanistic trial416 compared omalizumab [0.016 mg/kg/IgE (IU/ml) every 4 weeks] with placebo for 16 weeks in 20 participants aged 12–60 years. Emollients, hydrocortisone acetate (1%) and diflucortolone valerate (0.1%) were all permitted.
Assessment of risk of bias
Table 103 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Heil 2010416 | Unclear risk | Unclear risk | Low risk | Only 20 participants and designed as a mechanistic study; not clear whether it was powered to detect a clinically relevant effect |
Benefits
No significant differences were found in the secondary outcomes of eczema severity measured using the EASI scale, IGA score and investigator’s assessment of itch, but detailed data were not reported.
Harms
The trial reported a high level of adverse events; however, there were no serious adverse events or deaths and no withdrawals because of adverse events. Fourteen participants reported 19 adverse events, with 10 of the participants being in the omalizumab group. The adverse events reported were diverse and included vertigo, injection site reaction and migraine thought to be related to taking omalizumab.
Overall implications for research and practice
This very small trial416 that did not show any benefit of taking omalizumab and which reported a high level of adverse events should be treated with caution. Much larger, longer-term, studies are needed before this treatment can be considered for routine clinical practice.
Intravenous immunoglobulin
Treatment with intravenous immunoglobulin has immunomodulatory and anti-inflammatory properties and has been shown to be effective for several immune-mediated conditions.
Studies
One trial involving intravenous immunoglobulin was published before 200055 (see Appendix 3).
Three RCTs401,417,418 of 2 g/kg intravenous infusions of immunoglobulin have been reported since 2000. One fairly well-reported trial by Paul and colleagues417 in 10 adults compared immediate intravenous immunoglobulin treatment for 2 days against standard care (emollients and topical corticosteroids).
The trial by Bemanian and colleagues401 compared oral ciclosporin against intravenous immunoglobulin (2 g/kg over 4–8 hours) in 16 participants with severe eczema who were hospitalised for treatment. Blinding was not reported, but as the ciclosporin was taken daily for 3 months it seems unlikely that blinding could have taken place.
The industry-funded trial by Jee and colleagues418 compared 3 months of intravenous immunoglobulin treatment given in monthly injections (2 g/kg per month) to hospitalised patients against a ‘placebo’ of general topical moisturising lotion, 1% hydrocortisone cream and oral antihistamines for itching. The intravenous immunoglobulin group could also use the same treatments as the placebo group and all participants could also use an emollient ointment or steroid-free hydrophilic cream as an adjuvant to treatment.
Assessment of risk of bias
Table 104 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Bemanian 2005401 | Unclear risk | Unclear risk | Unclear risk | Very small sample size and exclusion criteria not specified. Participants were hospitalised for treatment. Blinding not reported and not likely |
| Jee 2011418 | Unclear risk | Unclear risk | Unclear risk | Baseline values for SCORAD and total IgE are much higher in the intravenous immunoglobulin group than in the placebo group |
| Paul 2002417 | Low risk | Low risk | Low risk | Very small sample size may not allow detection of small differences. Previous use of systemic treatments may have confounded the results |
Benefits
In the trial by Paul and colleagues,417 the severity of eczema was measured using SCORAD scores and a participant-assessed global disease measure at day 30, after which the standard care group received the intravenous immunoglobulin treatment and the participants were assessed again at day 60. The study found no significant difference in eczema severity between the two groups after 30 days (p = 0.4) and the participant-assessed global disease measure was also not significantly different between groups, with little change for the delayed treatment group and a slight improvement for the intravenous immunoglobulin group.
The trial by Bemanian and colleagues401 reported a significant difference in eczema severity in favour of ciclosporin (p = 0.005), which was evident by day 30. The severity of eczema did decrease in both groups over the 90 days of the trial but the clinical significance of the trial is difficult to gauge as the improvement may have resulted from the close monitoring and attention given in the trial.
The trial by Jee and colleagues418 reported no significant differences in participant assessments of itching and sleep loss. A significant difference in favour of intravenous immunoglobulin treatment for change in SCORAD score after 3 months of treatment and 3 months after treatment was stopped was reported.
Harms
The trials by Paul and colleagues417 and Bemanian and colleagues401 did not report any information about serious adverse events for participants on intravenous immunoglobulin treatment. Adverse events of hirsutism and herpetic keratoconjunctivitis occurred in one participant each taking ciclosporin. 401 The trial by Jee and colleagues418 reported that 5 out of 30 participants in the intravenous immunoglobulin group withdrew because of adverse events including headache and nausea. Two out of 10 participants in the placebo group withdrew for personal reasons.
Overall implications for research and practice
Intravenous immunoglobulin therapy is an expensive and resource-intensive treatment (because of the need for secondary care resources) that to date does not have any good-quality evidence of benefit in comparison to other systemic immunomodulatory agents. The trial by Jee and colleagues418 reported that intravenous immunoglobulin is beneficial compared with a standard treatment regimen; however, baseline eczema severity and atopy (assessed by total IgE level) were considerably higher in the intravenous immunoglobulin group at baseline and the very small number of participants in the standard care group raises serious concerns about the validity of these results. The participants in the trial did not report any significant differences in sleep loss and itching. The use of this treatment for eczema in clinical practice should be carefully considered until good-quality evidence of the benefits and harms becomes available.
Oral pimecrolimus
Studies
There were no trials of oral pimecrolimus reported before 2000.
One trial has been reported since 2000. This industry-funded multicentre trial by Wolff and colleagues419 compared three different doses of oral pimecrolimus (20 mg, 40 mg and 60 mg) against a placebo given twice daily as divided doses for 12 weeks. In total, 103 adults with moderate to very severe eczema were randomised to one of four treatment groups. All participants were allowed to use emollients and 1% hydrocortisone throughout the trial and for 7 days before the trial. There was a 12-week follow-up phase after treatment during which the participants could use only standard eczema treatment.
Assessment of risk of bias
Table 105 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Wolff 2005419 | Low risk | Low risk | Low risk |
Benefits
A statistical comparison between treatment groups of the percentage of participants with a pruritus score of ≤ 1 and the IGA score was not reported. The percentage of participants achieving ‘complete control’ (0) or ‘good control’ (1) for the participant assessment of the response to treatment was significantly different between the placebo group (27%) and the 60-mg pimecrolimus group (62%) after 6 weeks of treatment. The severity of eczema, measured using EASI, was significantly different after 6 and 12 weeks of treatment for the three pimecrolimus treatment groups combined compared with placebo (overall superiority using analysis of covariance test: p = 0.042 at week 7, p = 0.263 at week 12). The 40-mg and 60-mg pimecrolimus groups were also reported as being superior using the same test at week 7.
Harms
This trial reported that there were no differences in the overall incidence of adverse events between treatment groups. Adverse events of nausea and feeling hot occurred and presented a dose–response relationship with pimecrolimus. Two participants reported serious adverse events. One participant taking 60 mg of pimecrolimus a day, who had a family history of type 1 and type 2 diabetes and a body mass index of 33.3 kg/m2, had an elevated fasting blood glucose level. Another participant in the placebo group had chest and abdominal pain, an abnormal electrocardiogram and sinus bradycardia.
Overall implications for research and practice
This trial of different doses of oral pimecrolimus compared with placebo did not provide much convincing evidence that any dose < 60 mg per day gave a meaningful clinical benefit on top of standard treatment alone, taking into account the known risks of this treatment. The trial did follow up participants for 3 months after the treatment was stopped but did not present these results, which leaves the long-term benefits and harms of oral pimecrolimus still unknown.
Summary of systemic immunomodulatory agents
Azathioprine
-
There were no trials of azathioprine for eczema reported before 2000.
-
Three trials were reported after 2000:
-
Two small trials, with a mostly low risk of bias, found evidence of a large beneficial effect of 3 months of treatment with azathioprine compared with placebo in adults.
-
One small trial, with an overall low risk of bias, did not provide any evidence of benefit of azathioprine compared with methotrexate.
-
-
Azathioprine treatment has a high burden of adverse events such as nausea, vomiting and diarrhoea, which may limit its use. Serious adverse events such as elevated liver enzymes, neutropenia and lymphopenia present a risk of harm from long-term use.
-
There have not yet been any trials of azathioprine treatment in children with eczema.
Ciclosporin
-
There were 10 trials of oral ciclosporin for eczema reported before 2000. These provided evidence of a strong short-term beneficial effect of ciclosporin compared with placebo.
-
Serious adverse effects, especially renal damage and hypertension, indicate that long-term use of ciclosporin presents a significant risk of harm. Even for short-term treatment there is no evidence that having strategic treatment interruptions are beneficial in decreasing the risk of adverse events.
-
Three small and very small trials reported after 2000, with a mixed risk of bias, provided evidence of significant benefit for oral ciclosporin (doses ranged from 2.7 to 4 mg/kg per day) compared with topical tacrolimus, intravenous immunoglobulin or phototherapy treatment.
-
One trial reported in 2000, with a high risk of bias for allocation concealment, did not provide any evidence of benefit for body weight-dependent dosing of ciclosporin compared with standard ciclosporin treatment. This trial did not compare ciclosporin against another treatment comparator.
-
One small trial reported in 2010, with an overall low risk of bias, provided evidence of benefit for remission for ciclosporin compared with a 2-week tapering dose of oral prednisolone. This trial was forced to close early because of the unexpectedly high number of relapses requiring hospitalisation or withdrawal.
-
One small trial reported in 2011, with a mostly low risk of bias, provided evidence of non-inferiority for ciclosporin compared with mycophenolate mofetil.
-
One very small trial reported in 2013, with an overall unclear risk of bias, did not provide evidence of benefit for ciclosporin plus glucosamine compared with ciclosporin alone.
-
One small trial reported in 2012, with a mixed risk of bias, did not provide any evidence of benefit for ciclosporin compared with methotrexate.
Methotrexate
-
There were no studies involving methotrexate before 2000.
-
Two small trials were reported after 2000:
-
One trial, with an overall low risk of bias, did not provide any evidence of benefit for azathioprine compared with methotrexate.
-
One trial, with a mixed risk of bias, did not provide any evidence of benefit for 12 weeks of treatment with low-dose methotrexate compared with treatment with low-dose ciclosporin in children with severe eczema.
-
Prednisolone
-
There were no trials involving oral prednisolone reported before 2000.
-
One small trial reported in 2010, with an overall low risk of bias, involving a 2-week tapering course of oral prednisolone treatment provided no evidence of benefit of prednisolone compared with ciclosporin for 6 weeks. A higher proportion of participants using prednisolone relapsed during the 6-week treatment phase and in the 12 weeks after this. The trial was forced to close early because of the unexpectedly high number of relapses requiring hospitalisation or withdrawal.
Montelukast
-
There were no trials involving montelukast for eczema reported before 2000.
-
Four trials, two small and two very small, reported after 2000, with a mixed risk of bias, gave conflicting evidence of benefit for montelukast compared with placebo.
-
Two small trials reported in 2001 and 2007, with a mostly unclear risk of bias, gave conflicting results for montelukast compared with a standard treatment regimen of topical corticosteroids, antihistamines and antibiotics (for only one of the trials).
Systemic immunotherapy (desensitisation)
-
One medium-sized trial in 2012 involving house dust mite desensitisation in sensitised adult patients with eczema, with a mixed risk of bias, provided no evidence of benefit for specific systemic immunotherapy.
-
Two small trials in 2006 and 2012 involving house dust mite desensitisation in sensitised patients with eczema, with a mixed risk of bias, provided evidence of benefit for specific systemic immunotherapy.
Mepolizumab
-
There were no trials involving mepolizumab for eczema reported before 2000.
-
One small trial in 2005, with an overall unclear risk of bias, did not find any evidence of benefit for mepolizumab compared with placebo for clinically relevant outcomes.
Omalizumab
-
There were no trials involving omalizumab for eczema reported before 2000.
-
One small trial in 2010, with a mostly unclear risk of bias, did not provide any evidence of benefit for omalizumab compared with placebo, considering the clinically relevant outcomes only.
Intravenous immunoglobulin
-
One small trial reported before 2000, with an overall unclear risk of bias, provided evidence of benefit for intravenous immunoglobulin compared with intravenous albumin.
-
Two trials, reported in 2002 and 2011, provided conflicting evidence of benefit for intravenous immunoglobulin compared with standard treatment (topical corticosteroid and emollients and oral antihistamines in one of the trials). The largest and much longer-term trial, with an overall unclear risk of bias, did not provide evidence of benefit for 3 months of treatment (2 mg/kg/month).
-
One very small trial reported in 2005, with an overall unclear risk of bias, provided evidence of benefit for ciclosporin compared with intravenous immunoglobulin treatment.
Pimecrolimus (oral)
-
There were no trials involving oral pimecrolimus reported before 2000.
-
One trial in 2005 involving three different doses of pimecrolimus (20 mg, 40 mg or 60 mg per day in two divided doses) provided evidence of benefit for the highest dose of pimecrolimus (60 mg) compared with placebo after 7 weeks of treatment. However, development of this treatment was halted because of concerns over carcinogenicity. 420
Chapter 12 Complementary therapies
Background
We define complementary therapies as a group of therapeutic and diagnostic disciplines that exist largely outside the institutions where conventional health care is taught and provided. This chapter includes all trials of interventions that met this definition at the time of writing the review.
Existing systematic reviews
A technology report94 accompanying the AAD guidelines covered hypnotherapy, Chinese herbal medicine, homeopathy and evening primrose oil. The NICE guidelines41 covered homeopathy, massage, hypnotherapy and aromatherapy. The SIGN guidelines42 covered homeopathy and acupuncture. A review of systemic treatment for severe atopic eczema in 2007,393 a review of treatments for eczema to relieve pruritus in 201293 and a Cochrane review of Chinese herbal medicine for atopic eczema in 2013421 reviewed the trials of Chinese herbal medicine. A review of complementary/alternative treatment in dermatology in 2002282 also assessed hypnotherapy. Three systematic reviews282,422,423 covered homeopathy.
Scope of this chapter
This chapter covers the following treatments:
-
St John’s wort
-
acupuncture
-
acupressure
-
hypnotherapy
-
aromatherapy/massage
-
Chinese herbal medicine
-
homeopathy
-
other herbal medicine
-
Japanese traditional medicine
-
Hwangryunhaedoktang
-
balneotherapy
-
progressive muscle relaxation.
St John’s wort (Hypericum) (topical)
St John’s wort is the common name given to a genus of herbs found in Europe. The plants are used to create a herbal preparation now used in mainstream medicine in many European countries for treating mild to moderate depression, but this use is not currently recommended by NICE. 424 The exact mechanism of action of St John’s wort is unclear. For topical treatment, the flavonoids and tannins may be possible active components.
Studies
No studies using topical St John’s wort for eczema were reported before 2000.
One study involving St John’s wort was reported after 2000. This small within-person study by Schempp and colleagues425 compared St John’s wort (1.5%) (Hypericum perforatum L.) cream with a placebo cream in 21 participants aged 12–59 years with ‘subacute’ eczema, defined as a SCORAD score of < 80. The creams were applied twice daily for 4 weeks.
Assessment of risk of bias
Table 106 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Schempp 2003425 | Unclear risk | Unclear risk | Unclear risk | The intention-to-treat analysis excluded three participants who had used the treatment for < 10 days or who did not have efficacy data. Although the creams were colour matched, It was unclear if other differences were controlled for |
Benefits
At the end of the trial period of 28 days the mean ± SD change in eczema severity, as assessed using objective SCORAD scores, was –5.4 ± 4.9 for the St John’s wort group compared with –2.3 ± 3.3 for the placebo group. 425 This was a statistically significant reduction for St John’s wort compared with placebo (p = 0.022). This significant reduction in severity was also reported between the treatments at weeks 1 and 4. Participant-assessed skin tolerability and cosmetic acceptability were described as ‘good or excellent’ for both treatments, but no further information was provided.
Harms
There were four acute episodes of eczema, which led to withdrawal from treatment for three participants reported in this study. 425 It was not reported whether these exacerbations of eczema were thought to be related to both or one of the treatments. One of the three participants also developed contact eczema on the area treated by the placebo, which was thought to be probably related to the vehicle cream. There were no serious adverse events reported.
Overall implications for research and practice
The results of this one small pilot study425 are potentially encouraging; however, the lack of information about allocation concealment and blinding and the lack of a formal sample size calculation means that the results must be treated with caution. It is important that any future studies of St John’s wort compare it against emollients and topical corticosteroids to enable a clearer judgement to be made on its potential clinical usefulness.
Acupuncture
Studies
No trials involving acupuncture were reported before 2000.
One new trial involving acupuncture was reported after 2000. This observer-blinded single-centre industry-funded trial by Pfab and colleagues426 compared acupuncture twice weekly for 33 days against a control of a study examination visit only, with no acupuncture. The 10 participants who were randomised had to have a history of eczema for > 10 years, a SCORAD score of > 20 and allergic rhinitis with sensitisation to Phleum pratense and D. pteronyssinus.
Assessment of risk of bias
Table 107 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Pfab 2011426 | Unclear risk | Unclear risk | High risk |
Benefits
The baseline itch intensity, measured on a VAS from 0 to 100, was markedly different between the groups, with mean ± SD itch intensity being 11 ± 7 in the non-acupuncture group and 55 ± 22 in the acupuncture group. The change in itch intensity from baseline to day 15 and day 30 was not statistically compared. There were no significant differences between the treatment groups in change in eczema severity from baseline to 15 days or 30 days (change from baseline to 30 days: acupuncture group –5.6 ± 17.6, non-acupuncture group 3.5 ± 3.4).
Harms
No adverse events were reported for this trial.
Overall implications for practice and research
This very small trial with very little methodological detail and a huge disparity in baseline pruritus scores does not provide any evidence of benefit for acupuncture. Until a larger, methodologically rigorous trial is conducted, the use of acupuncture for eczema remains unclear.
Acupressure
Studies
No trials involving acupressure were reported before 2000.
One new trial involving acupressure was reported after 2000. This single-centre trial compared acupressure in addition to standard treatment against standard treatment only. The 15 adult participants, who had not used acupressure or acupuncture in the previous year, were treated for 4 weeks. Those randomised to acupressure were taught the technique and applied pressure to the large intestine point 11 using a 1.2-mm titanium ‘acupellet’ for 3 minutes, three times a week.
Assessment of risk of bias
Table 108 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Lee 2012427 | Unclear risk | Unclear risk | High risk | 3/15 participants were lost to follow-up and were not included in the analyses |
Benefits
Compared with baseline, the reduction in pruritus (p = 0.04) and severity of eczema measured using the IGA score (p = 0.03) and the lichenification score from the EASI measure (p = 0.03) was greater in the acupressure group than in the standard treatment-only group. For itching, three out of seven participants in the acupressure group did not change (0–25% change) and four out of seven improved (≥ 25% improvement); in the control group five out of five participants in the control group did not change (0–25% change). For eczema severity measured using IGA, two out of seven in the acupressure group did not change and five out of seven improved (≥ –1 point); in the control group two out of five worsened (≥ +1 point), one out of five did not change and two out of five improved (≥ –1 point).
Harms
It was reported that there was no adverse events during the trial.
Overall implications for research and practice
As the authors of the trial point out, although this trial reported a statistically significant benefit for acupressure compared with no acupressure, as there were so few patients and no ‘placebo’ such as sham acupressure, a larger trial needs to be carried out to see whether this beneficial effect can be confirmed. Until then, there is insufficient evidence of benefit for acupressure.
Hypnotherapy
Hypnotherapy and biofeedback used to develop relaxation techniques with or without mental imagery may be beneficial in the management of atopic eczema to distract from the symptoms associated with the itch–scratch cycle.
Studies
One trial involving hypnotherapy was reported before 200055 (see Appendix 3).
One new trial involving hypnotherapy was reported after 2000. This trial in Germany,428 which compared 12 sessions of hypnotherapy (1 hour in duration) with no hypnotherapy, included 33 adults diagnosed with eczema by a dermatologist. The participants were assessed once a week, with the last assessment 1 week after the last treatment session.
Assessment of risk of bias
Table 109 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Senser 2004428 | Unclear risk | Unclear risk | High risk |
Benefits
The severity of eczema, measured by SCORAD scores, increased by 32% in the control group and decreased by 40% in the hypnotherapy group from baseline to 1 week after the last hypnotherapy session was given. The difference between the two treatments appears to have been analysed but the significance was unclear as it appears to have been reported as p = 0.000. Eczema-related quality of life, measured using the Marburg Atopic Dermatitis Questionnaire,349 showed a 26% improvement for the hypnotherapy group, also reported as p = 0.000. The percentage change in quality of life for the control group was not given; however, it was reported to be insignificant. Pruritus, scratch intensity and subjective skin condition, all recorded by participants on a VAS, showed an improvement in the hypnotherapy group and a worsening in the control group compared with baseline. The difference between the groups was highly significant for all of these outcomes (p = 0.000).
Harms
No adverse events were reported for this trial.
Overall implications for practice and research
This trial of hypnotherapy shows a striking level of benefit for eczema; however, the results of this one trial must be treated with caution, especially given the high risk of detection/information bias and other concerns regarding possible selection and performance bias because of the poorly reported nature of the study. It is unclear whether any attempt at blinding of the outcome assessors was made, but it would seem almost impossible to blind the participants, who were recording the subjective outcomes. A group of people with eczema who are not on any treatment at all is very unlikely to match reality, where nearly all people with eczema are at a minimum at least using an emollient. It appears that the control group in this trial did not use any treatment and not surprisingly deteriorated during the trial, making the difference between the control group and those having hypnotherapy seem more significant than it is likely to be in a normal clinical setting. Although it is unlikely that participants on a hypnotherapy trial could ever be blinded to their treatment allocation, blinding of outcome assessors and the inclusion of a purely objective outcome measure where possible will be essential in any future hypnotherapy trial. With two small trials on hypnotherapy showing some positive indications of benefit, a large, pragmatic and methodologically robust trial of hypnotherapy should now be conducted.
Aromatherapy/massage
It is possible that massage therapy may be beneficial in childhood eczema as a stress-reducing and enjoyable interaction between parent and child. Massage may also increase peripheral circulation (which may be defective in eczema) or may be a way of increasing compliance with topical treatments.
Studies
There were no RCTs of aromatherapy for eczema reported before the year 2000, although the abstract of the one new trial reported below was mentioned in the previous review. 55
Anderson and colleagues429 compared massage administered by the mother using essential oils in massage oil against massage administered by the mother using massage oil without essential oils. All participants bathed daily and essential oils were added to the bath only for the essential oils group. A therapist visited the home of each participant once every week and performed 30 minutes of massage on the participant and counselled the participant and the mother together. The trial consisted of 16 white children aged 3–7 years from middle-class backgrounds with professional working mothers, living as a family with their natural father. The participants’ GP, therapist and mother all separately assessed the children’s general improvement using an ordinal scale from 0 (no improvement) to 10 (considerable improvement) and the mother assessed night-time and daytime disturbance using the same scale.
Assessment of risk of bias
Table 110 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Anderson 2000429 | Unclear risk | Unclear risk | High risk | Although the participants were not told which group they were in, it is likely that the essential oils’ aroma would unblind many participants and possibly the GP performing the blinded outcome assessments |
Benefits
Parent-assessed improvement was not significantly different between the treatment groups. Mother-assessed night-time and day-time disturbance also showed no significant difference in improvement between the groups after 8 weeks of treatment. All of the above assessments showed a statistically significant improvement within the treatment groups; however, there did not appear to be any statistically or clinically significant differences between the treatment groups, although no other between-group analyses were reported.
Harms
Adverse events were not reported for this trial. The possibility that patients may develop allergic contact dermatitis to the aromatherapy constituents was mentioned.
Overall implications for research and practice
It is impossible to draw conclusions from this trial about the use of either aromatherapy oils or massage for children with eczema. The addition of counselling and massage could have masked any potential beneficial effects of the aromatherapy. Small numbers of a very select population of people with eczema were all treated with massage therapy, with one group using aromatherapy in addition. It was also highly likely that the assessors of all but one of the outcomes (parent and therapist) were aware of the group to which the child was allocated. If there is serious interest in exploring further the possible benefits of massage in eczema, a much larger and pragmatic trial of a more representative population is needed, which adds a simple combined massage with aromatherapy oils regimen to standard care.
Chinese herbal medicine
Chinese herbal medicine forms part of a system that includes oral and/or topical Chinese herbs, acupuncture, diet and exercise for both treatment and prophylaxis of disease. Medicinal plants of various kinds can be taken orally, usually in combination with others as a decoction by boiling them in water and drinking the ‘tea’ produced, or can be applied directly to the skin.
Prescriptions are individually determined based on an overall assessment of the patient including pulse, appearance of the tongue and disease features, hence standardised formulae are not generally prepared.
Studies
Four studies were reported before 2000, which compared different Chinese herbal preparations against placebo55 (see Appendix 3).
Six new trials have been published since 2000. 262,430–434
Hon and colleagues430 compared a five-herb concoction (containing Flos lonicerae, Herba menthae, Cortex moutan, Rhizoma attactylodis and Cortex phellodendri) against placebo given in capsules for 12 weeks. The placebo capsules were assessed by an expert panel to ensure that they were indistinguishable from the herbal capsules. Eighty-five children and young adults aged between 5 and 21 years with moderate to severe eczema (SCORAD score of > 15) took three capsules twice a day.
Another industry-sponsored study, by Shapira and colleagues,431 compared a herbal preparation of Siberian ginseng, Achillea millefolium and Lamium album against placebo, both taken orally three times a day for 2 weeks. The trial was conducted at community-based clinics and included 49 participants aged > 12 months with moderate eczema, diagnosed using criteria closely resembling the UK Working Party criteria. 9 Participants were allowed to use emollients and topical corticosteroids as long as they were using them before entering the trial and they were not altered during the trial.
Shi and colleagues262 conducted an open trial of 47 participants from China, comparing modified Jiawei Danggui Decection against no treatment. Both groups took 10 mg of loratadine per day and used hydrocortisone butyrate (0.1%) once a day for 4 weeks. The active treatment group were also treated with 250 ml of Jiawei Danggui Decection, but the treatment regimen was not reported.
A trial carried out in Taiwan by Cheng and colleagues432 compared Xiao-Feng-San (a mixture of 13 different plant materials in granular form dispersed in warm water) against a placebo of caramel, lactose and starch, which had a similar taste to the active treatment. One packet (the dose was dependent on age) was taken three times a day for 8 weeks. The participants had eczema on > 20% of their body surface area, were described as ‘refractory’ and had no active infection or exudation.
A pilot trial by Choi and colleagues433 in Korea compared 2.5 g of TJ-15, a traditional compound of Chinese herbs containing Scutellaria baicalensis root, Gardenia jasminoides fruit, Coptis chinensis rhizome and Phellodendron amurense, against a mixture of 1.25 g of TJ-15 and 1.25 g of TJ-17, containing Alisma orientalis root tuber, Poria cocos mycelium, Atractylodes lancea rhizome, Cinnamomum cassia branch and Polyporus umbellatus mycelium. Twenty-four participants (age not reported) with eczema diagnosed according to the Hanifin and Rajka8 criteria and a ‘Dampness-Heat’ pattern, diagnosed by a Korean traditional medicine specialist, were randomised. The dose was taken three times a day, 90 minutes after a meal, for 4 weeks. All participants were given guidance on the application of non-steroidal emollients. All participants were also given dietary and environmental recommendations.
In 2000 an open study by Henderson and colleagues,434 sponsored by the manufacturers, compared a Chinese herbal preparation PSE101, which was made up as tea, against the same herbal preparation (PSE222) taken as lacquered granules, to avoid the palatability problems associated with the herbal preparation as a tea. As no other treatment was tested, such as a placebo or different active treatment, this trial was not suitable for assessing the clinical efficacy of the herbal preparation. Interestingly, no participant assessment of palatability appears to have been reported, although the trial authors conclude that the granules are better tolerated. This trial is not discussed further.
Assessment of risk of bias
Table 111 provides the risk-of-bias assessment for the new studies.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Cheng 2011432 | Low risk | Low risk | Low risk | |
| Choi 2012433 | Low risk | Unclear risk | Unclear risk | |
| Henderson 2000434 | High risk | High risk | High risk | |
| Hon 2007430 | Low risk | Low risk | Unclear risk | |
| Shapira 2005431 | Unclear risk | Unclear risk | Unclear risk | |
| Shi 2008262 | Low risk | High risk | High risk |
Benefits
In the trial by Hon and colleagues,430 the severity of eczema decreased in each group by around 10 points after 12 weeks, with no significant difference between the herbal treatment and the placebo. Quality of life, measured using the CDLQI, improved by > 30% in the herbal medicine group compared with hardly any improvement in the placebo group over 12 weeks of treatment, which was a statistically significant difference (p = 0.008). The decrease in the number of days using topical corticosteroid did not differ significantly between groups; however, the amount of rescue medication (mometasone furoate) used by the herbal medicine group was significantly less than that used by the placebo group after 12 weeks in the 80 participants primarily using mometasone furoate.
In the trial by Shapira and colleagues,431 patients in both treatment groups experienced a fall of around 15–20 points in eczema severity after 2 weeks of treatment, with no significant difference between the groups. Subjective daytime pruritus and sleep loss, measured as part of SCORAD, also showed no significant difference between the groups when the results were separated from the objective outcome measures.
In the trial by Shi and colleagues,262 56.0% (14/25) of participants in the treatment group had a ≥ 70% improvement in SASSAD score compared with 22.7% (5/22) in the control group (p < 0.05). The trial report mentions measuring ‘scratch’ using a VAS, but does not report the results.
Cheng and colleagues432 found significantly greater improvements from baseline in the mean clinical lesion score in the group using Xiao-Feng-San compared with the group using placebo after 4, 8 and 12 weeks of treatment [79.1 points (SD 5.7 points) vs. 13.5 points (SD 7.56 points) at 8 weeks]. Improvement from baseline in pruritus, sleep and skin surface damage scores was significantly greater in the Xiao-Feng-San group compared to the placebo group at 4, 8 and 12 weeks. Improvement from baseline in erythema score was also significantly greater in the Xiao-Feng-San group than in the placebo group at 4 and 8 weeks.
The trial by Choi and colleagues433 did not compare Chinese herbs against other eczema treatments or placebo and so the trial cannot provide any clinically relevant data on the benefits or harms of Chinese herbal medicine treatment. There was no statistically significant difference between the groups in reduction in eczema severity, measured using EASI and SCORAD. The magnitude of the reduction in eczema severity using the SCORAD index was large (TJ-15 –24.9 SD ± 13.7; TJ-15 and TJ-17 –27.2 SD ± 8.9).
Harms
None of the five trials262,430–433 documented any change in blood chemistry or renal function. One participant had a transient elevation of aspartate aminotransferase, which returned to normal levels within 8 weeks after the treatment was stopped. Other adverse events that occurred significantly more in the active treatment groups were diarrhoea and visits to the GP. It was not clearly reported whether the adverse events were thought to be related to the study treatment.
Overall implications for research and practice
There is evidence of striking levels of benefit from modified Jiawei Danggui Decection, although as the amount of hydrocortisone and loratadine used in each treatment group was not reported this result should be treated with caution, particularly as the steps to reduce bias were so poorly described. 262 No difference in the effect on severity of eczema was found between the five-herb concoction (containing F. lonicerae, H. menthae, C. moutan, R. attactylodis and C. phellodendri) and placebo. 430 The trial reported an improvement in quality of life only in the participants treated with the herbal concoction and there also appeared to be a specific steroid-sparing effect for those using mometasone furoate. The effect on quality of life deserves further scrutiny in trials of Chinese herbal medicine; however, the steroid-sparing effect does not appear to have been a prespecified outcome. The Xiao-Feng-San treatment also had a beneficial effect on the severity of eczema after 8 weeks of treatment, which was still apparent 4 weeks later. 432 Siberian ginseng, A. millefolium and L. album did not show any beneficial effect over placebo. 431 The trial comparing TJ-15 against a TJ-15 and TJ-17 mixture did not provide any evidence that one was superior to the other, but as both treatments seemed to provide a good level of eczema severity reduction it would be useful to see these treatments tested against standard mainstream eczema treatments in larger trials. 433 With a total of four positive trials of Chinese herbal medicines, further clearly reported multinational large trials with blinded outcome assessments at the core, and which pay attention to subjective outcomes such as quality of life, are advised. Quality control is a key issue in developing such interventions.
Homeopathy
Homeopathy involves diluting a substance repeatedly with alcohol or distilled water. These solutions are used to ‘treat’ many conditions, including many chronic conditions such as eczema. NICE currently does not recommend homeopathy for the treatment of any health condition [see www.nhs.uk/conditions/homeopathy/pages/introduction.aspx (accessed 15 January 2016)].
Studies
One study involving homeopathy was reported before 200055 (see Appendix 3).
One new trial involving homeopathy was reported after 2000. This blinded trial from Germany,435 which included 24 adults aged between 18 and 35 years with eczema covering at least 20% of their body, compared individualised homeopathic treatment against placebo for 32 weeks. The participants could not use other treatments (including corticosteroids and antihistamines) during the trial.
Assessment of risk of bias
Table 112 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Siebenwirth 2009435 | Unclear risk | Unclear risk | Unclear risk | A large number of participants withdrew from the trial (10/24) and it is not clear whether they were included in the final analyses |
Benefits
No difference or only small differences between the groups in the change from baseline scores for eczema severity were shown. This outcome was measured using Costa and colleagues’249 multiparameter atopic dermatitis score and the ADASI and SCORAD scores. The Marburg Atopic Dermatitis Questionnaire,349 list of complaints and the global assessment of treatment success by both the physician and the participants all showed virtually no difference or only small differences between groups in the change from baseline scores.
Harms
The 14 adverse events recorded in nine participants were distributed fairly evenly between the groups and included herpes simplex, cough and influenza, which all occurred in both groups.
Overall implications for research and practice
This trial of homeopathy for eczema is not large enough or methodologically rigorous enough to provide any evidence about the benefits or harms of homeopathy for eczema.
Other herbal treatments
Studies
No studies involving other herbal treatments were reported before 2000.
One trial involving a herbal treatment was reported after 2000. Klövekorn and colleagues436 conducted a placebo-controlled within-person trial in south Germany. A herbal topical cream available in Germany as Ekzevowen derma®, in the UK as Linderma® and in the USA as Dermavex® (Weber & Weber GmbH & Co.) was used as the treatment. The cream contained 5 g per 100 g of each of the alcohol-based extracts of Mahonia aquifolium, Viola tricolour and Centella asiatica. All 88 adults used herbal cream on one side of the body and placebo cream on the other, in a randomised order, twice daily on the areas affected by eczema for 4 weeks. The trial was reported as double blind but it was not reported who was blinded. The interventions were in identical tubes and were described as being similar in appearance.
Assessment of risk of bias
Table 113 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Klövekorn 2007436 | Low risk | Low risk | Unclear risk |
Benefits
No significant difference in reduction of eczema severity was found when the entire population was analysed. A post hoc subgroup of 64 participants treated when the mean outside temperature was ≤ 10°C did show a significant reduction in severity for the herbal cream group compared with the placebo cream group (p = 0.019). There were no significant differences in participant-assessed pruritus and efficacy and the improvements recorded were all quite modest.
Harms
There were 33 adverse events reported during this trial, none of which was serious. Of these, 31 were reported as being not related to the study treatment and did not occur at the test sites. Two adverse events, one in the treatment group and one in the vehicle cream group, occurred at the test sites and required cessation of study treatment.
Overall implications for research and practice
This herbal cream does not show any indication of having any benefit over the vehicle cream alone for both the objective and subjective outcomes. One post hoc subgroup analysis did show a significant benefit when the cream was used when the outside temperature was ≤ 10°C; however, pragmatically, even if this significant result was replicated in a trial designed to confirm this, a treatment that is effective only over a certain temperature range is of very limited use in normal clinical practice.
Japanese traditional medicine
Studies
No studies involving Japanese traditional medicine were reported before 2000.
One new study involving Japanese traditional medicine was reported after 2000. This manufacturer-sponsored trial by Kobayashi and colleagues437 compared a herbal tea, Hochu-ekki-to (containing 10 species of medicinal plants), against placebo for 24 weeks. Ninety-one participants with eczema according to the Japanese Dermatological Association criteria26 and a delicate (Kikyo) constitution took the interventions twice a week for 24 weeks.
Assessment of risk of bias
Table 114 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Kobayashi 2010437 | Unclear risk | Unclear risk | Unclear risk | Not clear if the active herbal ‘tea’ could be distinguished from the ‘placebo’ tea in any way |
Benefits
A significant reduction in the amount of topical treatments used (steroids or tacrolimus), adjusted for potency, was reported for the Hochu-ekki-to group compared with the placebo group. No significant difference was found in the number of participants achieving a score of 0 on the skin severity scale. However, there were significantly fewer participants taking Hochu-ekki-to (1/37, 3%) whose use of topical corticosteroids increased by ≥ 50% by the end of treatment than those taking placebo (7/39, 18%).
Harms
The number of adverse events was noticeably higher in the Hochu-ekki-to group (13/40 participants and 33 events vs. 12/44 participants and 20 events). The adverse events were described as ‘moderate’ events such as nausea and diarrhoea; however, the number of events per participant is not known but is likely to have been more than one as the majority of participants suffered adverse events, particularly in the Hochu-ekki-to group. Slight increases in laboratory tests were also reported, the most common of which was for eosinophilia (three participants in the Hochu-ekki-to group and four participants in the placebo group).
Overall implications for research and practice
Despite the fact that treatment with Hochu-ekki-to was given for 24 weeks, the trial authors suspected that a longer time period would be required for this treatment to become effective. No significant beneficial effect was seen in comparison to placebo within this 24-week treatment period. However, Hochu-ekki-to appears to show a significant steroid-sparing effect. This effect would need to be confirmed in a future trial in which either other concomitant treatments were not allowed or, more likely, the amounts of other concomitant treatments used were closely recorded. It is also unclear whether blinding was assured, as no information about whether the placebo was distinguishable from the active treatment was given.
Hwangryunhaedoktang
A protocol for a RCT by Kim and colleagues438 comparing Hwangryunhaedoktang (a traditional Korean medicine that is licensed for use by the Korean Food and Drug administration) against placebo was published in 2011 as an open access paper. The protocol is very detailed and the authors are to be congratulated on putting such valuable information clearly in the public domain. We await the full trial report with interest.
Balneotherapy
Studies
No studies involving balneotherapy for eczema were reported before 2000.
One new study involving balneotherapy was reported after 2000. This unblinded trial439 compared Comano spa water (Trentino, Italy) balneotherapy against topical corticosteroid treatment for 2 weeks. It was not clear whether, or how often, the corticosteroid treatment group bathed. Comano spa water, which contains various microelements with calcium and magnesium, was heated to 36–37 °C and participants bathed in this water for 20 minutes twice a day. The participants in the topical corticosteroid group used either methylprednisolone aceponate (those aged < 2 years) or mometasone furoate (those aged > 2 years), once daily for 2 weeks. The only other treatment allowed for all participants was emollient.
Assessment of risk of bias
Table 115 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Farina 2011439 | Low risk | Unclear risk | High risk |
Benefits
A statistically significant difference between treatments was observed for severity of eczema, measured using the SCORAD index, with a bigger reduction for those using topical corticosteroids than for those in the balneotherapy group after 2 weeks of treatment (mean ± SD 46% ± 7.71% vs. 26% ± 9.4%; p < 0.03). There were no significant differences between groups after 2 weeks of treatment for severity of eczema measured using IGA and also for pruritus, participants’ global assessment and quality of life. There was a statistically significant reduction in pruritus and the participants’ global assessment in favour of balneotherapy 4 months after the end of treatment.
Harms
There were no major side effects reported for this study. The most common adverse event reported was a mild erythema and burning sensation, which affected 23 out of 25 participants in the balneotherapy group. None of these participants stopped or altered their treatment.
Overall implications for research and practice
It is unclear what component of Comano spa water is thought to have an effect on eczema. It is clear from the trial that 2 weeks of balneotherapy is not as beneficial as 2 weeks of topical corticosteroid use in reducing the severity of eczema. The severity of eczema data were not available 4 months after the end of treatment, when itching and participants’ global assessment had significantly improved for those receiving balneotherapy compared with those receiving topical corticosteroids. A further confounding factor is the lack of clarity about the circumstances of the spa treatment, such as whether the participants were resident at the spa during the treatment or not.
Progressive muscle relaxation
Studies
No studies involving progressive muscle relaxation for eczema were reported before 2000.
One new study involving progressive muscle relaxation was reported after 2000. This trial by Bae and colleagues440 compared the addition of a progressive muscle relaxation technique to standard treatment with standard treatment alone. The 25 participants were aged between 12 and 40 years and had a diagnosis of eczema according to the Hanifin and Rajka8 criteria and an EASI score of ≥ 10. One group of participants used audio and visual progressive muscle relaxation programmes at home twice a day for 4 weeks under controlled heating and lighting conditions and without eating or drinking anything except water and also used their standard treatments. The other group used only their standard eczema treatments. It was recommended to the participants that they did not drink alcohol or caffeine-containing beverages.
Assessment of risk of bias
Table 116 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Bae 2012440 | Unclear risk | Unclear risk | Unclear risk |
Benefits
The treatment groups were not statistically compared for any of the outcomes and so there was no evidence about the relative benefits of progressive muscle relaxation. The improvement in the progressive muscle relaxation group was significant compared with baseline for sleep loss and pruritus. It was stated that this was not the case for the standard treatment only group, but the data are not shown. The reduction in eczema severity, measured using EASI scores, was significant in both groups compared with baseline, but from the graph provided it appears that there was no significant difference between the treatments after 1 month of treatment.
Harms
No information about adverse events was reported.
Overall implications for research and practice
There are so few methodological details provided and little appropriate analysis of the results in this trial that it is impossible to obtain any useful information about the potential benefits and harms of progressive muscle relaxation.
Summary of complementary therapies
St John’s wort
-
There were no trials involving topical St John’s wort for eczema reported up to 2000.
-
One very small trial reported in 2003, with an overall unclear risk of bias, provided evidence of benefit for treatment with St John’s wort compared with placebo.
Acupuncture
-
There were no trials involving acupuncture for eczema reported up to 2000.
-
One very small trial reported in 2011, with a high risk of bias for blinding, did not provide any evidence of benefit for acupuncture compared with a study visit only. Participants all had both eczema and allergic rhinitis.
Acupressure
-
One small trial reported in 2012 provided no convincing evidence of benefit for acupressure and standard treatment compared with standard treatment alone.
Hypnotherapy
-
One small trial reported pre 2000, with a high risk of bias for blinding, provided evidence of benefit for hypnotherapy compared with a placebo discussion about eczema without mentioning symptom control.
-
One small trial reported in 2004, with a high risk of bias for blinding, provided evidence of benefit for hypnotherapy compared with no hypnotherapy.
Aromatherapy/massage
-
Only an abstract of the trial summarised below was reported before 2000.
-
One very small trial reported in 2000, with a high risk of bias for blinding, did not provide any evidence of benefit for massage treatment with aromatherapy oils compared with massage treatment without aromatherapy oils.
Chinese herbal medicine
-
Four trials of Chinese herbal medicine compared with placebo were reported up to 2000. All four trials were small, with an overall unclear risk of bias. Only one of the four trials provided evidence of benefit for a Chinese herbal medicine compared with placebo, yet all four tested very similar combinations of herbs.
-
Four small trials reported after 2000, with a mixed risk of bias, provided some evidence of benefit for oral Chinese herbal medicine compared with placebo or no treatment.
-
One small trial reported in 2000, with an overall high risk of bias, compared the same Chinese herbal treatment taken as either lacquered granules or as a tea. This trial did not include a control group and did not provide any clinically relevant evidence of benefit for this Chinese herbal medicine.
-
One small trial reported in 2013, with a mostly unclear risk of bias, compared one Chinese herbal medicine against the same medicine combined with another Chinese herbal medicine. This trial did not include a control group and did not provide any clinically relevant evidence of benefit for these Chinese herbal medicines.
Homeopathy
-
One trial protocol was reported pre 2000 for a study comparing homeopathy with placebo. A published full trial report has not been found for this trial protocol.
-
One very small trial reported in 2009, with an overall unclear risk of bias, did not provide any evidence of benefit for homeopathy compared with placebo.
Other herbal treatments
-
There were no trials involving other herbal treatments reported before 2000.
-
One small trial reported in 2007, with a mostly low risk of bias, did not provide any evidence of benefit for a herbal cream containing extracts of Mahonia aquifolium, Viola tricolour and Centella asiatica compared with placebo, except when the outside temperature was ≤ 10°C, in a subgroup analysis that was probably post hoc.
Japanese traditional medicine
-
There were no trials involving Japanese traditional medicine reported before 2000.
-
One small trial reported in 2010, with an overall unclear risk of bias, provided evidence of benefit for Hochu-ekki-to (a combination of 10 different medicinal plants) compared with placebo.
Balneotherapy
-
There were no trials involving balneotherapy before 2000.
-
One small trial reported in 2013, with a mixed risk of bias, provided evidence of greater benefit for the comparator (topical corticosteroid treatment) than for balneotherapy.
Progressive muscle relaxation
-
There were no trials involving progressive muscle relaxation for eczema before 2000.
-
One very small trial reported in 2012, with an overall unclear risk of bias, did not provide any evidence of benefit for progressive muscle relaxation in addition to standard eczema treatment compared with standard eczema treatment alone.
Chapter 13 Other interventions not covered elsewhere
Background
Novel interventions that have been used successfully for other chronic conditions are often tested for efficacy in eczema in the hope of finding additional effective treatments. This chapter covers all trials using interventions that do not fit neatly into the other treatment categories covered in this review.
Existing systematic reviews
There have been no systematic reviews covering any of the interventions discussed in this section.
Scope of this chapter
The treatments covered in this chapter are:
-
autologous blood therapy
-
tandospirone citrate
-
oral naltrexone
-
Polypodium leucotomos.
Autologous blood therapy
Autologous blood therapy involves taking a small amount of a patient’s own blood and reinjecting it. Variations of the technique include adding other substances to the blood or concentrating a particular component, such as platelets, before reinjection. This technique has been used for some joint conditions such as tendinopathy. The aim of the procedure is to provide cellular and humoral mediators to induce healing.
Studies
There were no trials involving autologous blood therapy reported before 2000.
Two new trials have been published since 2000. A trial by Kief441 was unable to provide evidence on the efficacy of autologous blood therapy for eczema because it compared two ‘active’ interventions (a modified method of autologous blood therapy against standard autologous blood therapy).
A placebo-controlled study by Pittler and colleagues442 included 31 adults with non-exudative eczema defined using the Hanifin and Rajka8 criteria who were not using potent topical corticosteroids. Treatment involved taking a participant’s blood and reinjecting it intramuscularly, with the procedure being performed once a week for 4 weeks. The participants were allowed to continue using their standard treatments during the trial. The outcome assessor was blinded by not being allowed in the room while the treatment was being given. Participants were blinded by means of a bedsheet drape while the treatment was being administered. Participants were assessed at 5 and 9 weeks after the start of the trial.
Assessment of risk of bias
Table 117 provides the risk-of-bias assessment for the new studies.
Benefits
The trial by Kief441 reported no significant differences between autologous blood therapy and modified autologous blood therapy. Both groups in the trial displayed significantly decreased eczema severity, assessed by SCORAD scores, and an improvement in DLQI score.
Pittler and colleagues442 found that there was a significant decrease in eczema severity in the autologous blood therapy group compared with the placebo group (saline injections) after 9 weeks, with a mean difference in change in SASSAD scores from baseline between the groups of 13.5 points (95% Cl 6.6 to 20.4 points; p < 0.001). This significant difference was also present at 5 weeks, shortly after treatment had ended. There were no significant differences between the groups in the participant-assessed outcomes of DLQI, pruritus, skin appearance and sleep loss. The trial report stated that there were no clinically significant results with regard to the use of other topical treatments, although no data were provided.
Harms
The trial by Kief441 reported that no adverse events occurred.
In the trial by Pittler and colleagues,442 6 out of 15 participants treated with autologous blood therapy reported adverse events and 7 out of 15 participants treated with saline placebo reported adverse events. One participant had bruising in the antecubital region and another had an itching sensation on the face. All adverse events were reported as minor and transient.
Overall implications for research and practice
One small trial442 found evidence of a significant reduction in the severity of eczema after 9 weeks when using autologous blood therapy for 4 weeks in addition to standard treatment compared with standard treatment alone. This preliminary study suggests that a larger and longer-term trial that includes children might be worthwhile. Particular attention should be paid to participant and assessor blinding and measurement of concomitant treatments in such a study. Future research should also explore the apparent lack of beneficial effect on quality of life, sleep loss, pruritus and other participant-assessed outcomes. It will be important to focus on whether this intervention is practical and whether it is realistic only for patients with severe disease. Another small trial441 compared two slightly different methods of autologous blood therapy against each other but did not include a control group. Without a control group as a reference, the results of this trial do not provide any clinically relevant evidence for autologous blood therapy.
Tandospirone citrate
Tandospirone citrate is a potent selective partial 5-HT1A (serotonin) receptor agonist that binds to the 5-HT1A receptor, thereby activating it. It is marketed mainly in Japan and China, where it is used as an antianxiety treatment and an antidepressant.
Studies
There were no trials reported before 2000.
One new trial was reported after 2000. This small pilot trial in Japan443 compared tandospirone citrate (10 mg, three times a day) in addition to standard corticosteroid or antihistamine treatment against standard corticosteroid or antihistamine treatment alone in 37 participants for 4 weeks.
Assessment of risk of bias
Table 118 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Kawana 2010443 | Unclear risk | Unclear risk | Unclear risk |
Benefits
No significant difference was seen in severity of eczema, loss of sleep and mental stress between those treated with tandospirone citrate and those not treated with tandospirone citrate. A significant improvement in participant-assessed mood state, recorded considering the previous 3 days, was seen for the tandospirone group compared with the no treatment group. Data from the 37 participants were compared against ‘normal’ data from imaginary healthy control subjects for this mood state outcome.
Harms
No information on adverse events was provided in the trial report.
Overall implications for research and practice
Although there is a possibility that this serotonin receptor agonist could be beneficial in the treatment of eczema, this pilot trial has not provided much additional evidence.
Oral naltrexone
Naltrexone is an opiate receptor antagonist that is used to treat alcohol and drug dependence. As opiate membrane receptors have been implicated in the brain’s processing of pruritus, this treatment has been suggested as being potentially suitable for eczema.
Studies
There were no trials involving oral naltrexone reported before 2000.
One new trial has been reported since 2000. This trial by Malekzad and colleagues444 compared twice-daily treatment with 25 mg of oral naltrexone with placebo for 2 weeks. Thirty-eight adults aged 24–85 years, who had to have pruritus associated with eczema, were randomised. All participants continued with their other eczema medication during the trial.
Assessment of risk of bias
Table 119 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Malekzad 2009444 | Unclear risk | Unclear risk | Unclear risk | Those who withdrew were not included in the analysis – 3/18 in the naltrexone group and 2/20 in the placebo group |
Benefits
The participant assessment of pruritus, measured on a VAS, decreased from a mean ± SD of 8.11 ± 1.4 to 3.3 ± 1.6 in the naltrexone group and from 7.8 ± 1.6 to 5.6 ± 2.1 in the placebo group after 1 week of treatment. This was a statistically significant difference (p < 0.005) using a Mann–Whitney test. The pruritus decreased to a mean ± SD of 1.3 ± 1.4 in the naltrexone group and 4.5 ± 2.8 in the placebo group after 2 weeks of treatment, which was also statistically significant.
Harms
Two participants in the naltrexone group experienced treatment-related adverse events, including sedation and nausea; in the discussion of the report, dizziness, vomiting, headache and cramps are also reported. One participant in the placebo group reported nausea. It was reported that there was no significant difference in adverse events between the treatment groups; however, no formal statistical calculations were reported. It was not reported whether any of the participants who experienced adverse events withdrew from the trial.
Overall implications for research and practice
This trial gave some evidence of a significant, short-term reduction in pruritus. However, the trial included a small number of participants and was methodologically unclear. Larger, methodologically clear and robust trials will be needed before this benefit can be confirmed. The levels of adverse events in this trial do not appear to be a cause for concern but, again, this trial was not large enough to detect serious adverse events.
Polypodium leucotomos
Polypodium leucotomos is a fern found in tropical and subtropical parts of Central and South America, where it has been used in traditional medicine. Laboratory studies have demonstrated that P. leucotomos extracts have antioxidant and photoprotective properties. 445
Studies
There were no trials involving P. leucotomos for eczema reported before 2000.
One new multicentre trial conducted in Spain compared capsules containing P. leucotomos extract (Anapsos®, Especialidades Farmacéuticas Centrum S.A.) against placebo capsules in 105 children aged 2–17 years. 446 The treatment dose was dependent on age (children aged < 6 years 240 mg/day, 6–12 years 360 mg/day, > 12 years 480 mg/day) and the treatment was given daily for 6 months. All participants could use a thin layer of methylprednisolone aceponate 0.1% in emulsion to treat flares, and dry skin was treated with moisturising cream after a bath or shower. Participants who needed systemic therapy were given deflazacort (0.25–1.5 mg/kg/day). Pruritus was treated with desloratadine.
Assessment of risk of bias
Table 120 provides the risk-of-bias assessment for the new study.
| Trial | Sequence generation | Allocation concealment | Blinding | Other potential sources of bias |
|---|---|---|---|---|
| Ramírez-Bosca 2012446 | Unclear risk | Unclear risk | Unclear risk |
Benefits
Topical corticosteroid use (percentage of days) was not significantly different between the groups, although the statistical results were not reported. There were no significant differences in eczema severity, measured using SCORAD and IGA, or time to first flare, with a median of 0.5 months in both groups. There was a significant reduction in oral antihistamine use (median percentage of days) (P. leucotomos group 4.5%, placebo group 13.6%; p = 0.038), but this was not a prespecified outcome for the trial.
Harms
There were 218 adverse events in the P. leucotomos group and 223 in the placebo group, most of which were reportedly mild. There were 14 participants in the placebo group and seven participants in the P. leucotomos group with moderate adverse events. One serious adverse event occurred in the placebo group (diarrhoea with dehydration). One participant in the placebo group withdrew because of an adverse event, but the severity was not documented nor whether it was related to trial treatment.
Overall implications for research and practice
This trial failed to demonstrate any benefit of taking a P. leucotomos extract compared with placebo for severity of eczema or reduction in the use of topical corticosteroids. A significant reduction in the use of antihistamines for those taking the P. leucotomos extract is slightly encouraging; however, this was not a prespecified outcome and reduction in the use of antihistamines could have more than one cause. A trial specifically assessing this treatment’s ability to reduce pruritus compared with other routinely used treatments would be needed to assess its potential benefit.
Summary of interventions not covered elsewhere
Autologous blood therapy
-
There were no trials involving autologous blood therapy reported up to 2000.
-
Two small trials involving autologous blood therapy, with a mixed risk of bias, were reported after 2000. One of these trials provided evidence of benefit for 4 weeks of treatment compared with placebo. The other trial compared two types of blood therapy (no control group) and did not provide any evidence of benefit for one treatment compared with the other.
Tandospirone citrate
-
There were no trials involving tandospirone citrate reported before 2000.
-
One small trial reported in 2010, with an overall unclear risk of bias, found no significant benefits of the addition of tandospirone citrate to standard treatment with topical corticosteroids and antihistamines compared with standard treatment with topical corticosteroids and antihistamines alone.
Oral naltrexone
-
There were no trials involving oral naltrexone reported before 2000.
-
One small trial, with an overall unclear risk of bias, was reported in 2009 and provided evidence of benefit for oral naltrexone treatment compared with placebo.
Polypodium leucotomos
-
One moderately sized trial, reported in 2012, did not provide any evidence of benefit for P. leucotomos compared with placebo.
Chapter 14 Discussion
This systematic review of eczema treatments sits within a programme of work on eczema and other dermatoses. The review both complements and has helped to inform other research on eczema treatment: prioritising unanswered research questions; the HOME initiative; and the GREAT database (see the companion report447). The rapidly expanding and important area of primary eczema prevention has been separately reviewed as part of the wider programme of work (see the companion report447).
Main findings
The body of evidence evaluating specific eczema treatments has grown considerably since the last review published in 2000. 55 Regrettably, many widely used treatments such as emollients or bandages still lack a strong evidence base. Theoretically, one could argue that they are established treatments and so do not require evaluation. Yet history suggests, through various examples (such as the universal use of cryotherapy for plantar warts, which is no better than topical salicylic acid448), that simple widespread use of a mode of therapy may be a weak defence for an evaluation bypass. This is not to suggest that universal treatments such as emollients should be stopped or restricted on the basis of a lack of RCT evidence, as this would limit patient choice and paradoxically favour the use of powerful treatments such as ciclosporin that have been evaluated thoroughly by RCTs. Instead, the juxtaposition of widespread use and limited evidence should prompt a renewed and critical interest in evaluating such treatments.
Box 1 sets out the authors’ opinion on the value of the evidence base for the interventions considered. When interpreting Box 1, it is important to point out that our classification of treatment options into four categories, such as ‘evidence of benefit to support’, is not the same as a positive recommendation for widespread use or otherwise, as that is the remit of guideline developers. Box 1 is also not intended as a substitute for scrutinising the original studies in the context of local guideline and policy development.
(Based on at least one good-quality RCT or a large body of evidence and a clinically useful finding. We defined a ‘good-quality’ trial as well designed and well reported and with a magnitude of benefit deemed by the authors to be clinically relevant and a ‘large body of evidence’ as enough trials with consistent evidence of a clinically relevant benefit, despite some limitations in reporting.)
Topical treatments to treat eczema-
Topical corticosteroids more beneficial than vehicle.
-
Topical tacrolimus more beneficial than mild-potency topical corticosteroids, mainly in children with moderate to severe eczema.
-
Tacrolimus more beneficial than moderate topical corticosteroids for moderate to severe facial eczema in adults.
-
Pimecrolimus more beneficial than vehicle, mainly in children with mild to moderate eczema.
-
Tacrolimus more beneficial than pimecrolimus, in children and adults with eczema of all severities.
-
Atopiclair emollient more beneficial than vehicle, in children and adults with mild to moderate eczema
-
Topical corticosteroids 2 days a week more beneficial than vehicle, mainly in adults and children with moderate to severe eczema (all over).
-
Tacrolimus 2 or 3 consecutive days a week more beneficial than vehicle, in children and adults with mild to severe eczema.
-
Topical pimecrolimus 2 or 3 consecutive days a week more beneficial than vehicle, mainly in children with mild to severe eczema.
-
Many forms of UV light therapy have been tested against each other and other active treatments and in various combinations in mainly small trials. Trying to summarise the many comparisons that have been carried out is difficult but, overall, light therapy appears to be effective, with the best evidence favouring narrowband UVB.
-
Ciclosporin more beneficial than placebo, mainly in adults with severe eczema.
-
Azathioprine more beneficial than placebo, in adults with moderate to severe eczema.
-
Educational interventions more beneficial then no education, mainly in children with moderate to severe eczema.
(At least one good-quality RCT or several less well-reported RCTs that consistently failed to show a convincing benefit for overall disease activity. We defined a ‘good-quality’ trial as well designed and well reported and large enough to exclude a clinically useful benefit and ‘several less well-reported RCTs’ as several trials with no evidence of benefit to give confidence in there being no clinically relevant benefit, despite less clear reporting.)
Topical treatments to treat eczema-
Twice-daily vs. once-daily topical corticosteroids.
-
Topical corticosteroids in combination with antibiotics for eczema that is not clinically infected.
-
Other topical treatments: protease inhibitor SRD441 in adults with mild to moderate eczema; emollient with furfuryl palmitate in children; cipamfylline cream in adults with eczema on the arms.
-
M. vaccae vaccine, mainly in children with moderate to severe eczema
-
Probiotics for treating eczema, mainly in children with unspecified disease severity
-
Avoidance of enzyme washing powders, mainly in adults with mild to moderate eczema
-
Ion-exchange water softening devices, in children with moderate to severe eczema
-
Dietary supplements rich in linoleic acid (such as evening primrose oil, borage oil), mainly in adults and children with eczema of unknown severity
(Insufficient or contradictory RCT evidence that does not fall into the other two categories. We acknowledge that some RCTs may have been missed and some RCTs may not have been included as they did not fulfil our inclusion criteria)
Topical treatments to treat eczema-
Emollients to reduce the severity of eczema, prevent flares and reduce the need for other eczema treatments
-
Topical corticosteroids in combination with antibiotics for eczema that is infected
-
Wet wraps on top of topical corticosteroids
-
Antiseptic bath additives
-
Topical antifungals
-
Other topical treatments such as WBI-1001 cream, topical coal tar, topical vitamin B12, V. filiformis lysate cream
-
Oral prednisolone
-
Methotrexate
-
Mycophenolate mofetil
-
Immunotherapy (desensitisation)
-
Biological therapies: omalizumab, mepolizumab
-
Oral sedating and non-sedating antihistamines
-
Other interventions: oral pimecrolimus, oral naltrexone, autologous blood therapy, tandospirone citrate, full-spectrum light therapy, excimer laser
-
Intravenous immunoglobulin
-
Montelukast
-
Specialised clothing (silk or synthetic fibres with or without antibiotics)
-
Environmental interventions: house dust mite reduction and desensitisation, staying in a different climate
-
Different approaches to the organisation of care such as additional visits to the doctor or nurse-led clinics
-
Support groups, e-health management
-
Dietary interventions such as prebiotics, dietary restrictions and synbiotics
-
Complementary therapies such as Chinese herbal treatment, hypnotherapy, massage therapy, aromatherapy, acupuncture, acupressure, other herbal treatments
-
Psychological therapies such as stress reduction techniques and biofeedback
-
Balneotherapy (salt baths)
-
Dilution of topical corticosteroids
-
Impregnated bandages (zinc paste bandages)
-
Routine patch testing
-
Other bathing practices such as avoidance of soaps and frequency of bathing
Treatments covered only in the previous systematic review55 are in italics.
How has the evidence base changed since the review in 2000?
Interventions with reasonable established efficacy
Topical calcineurin inhibitors, educational interventions and azathioprine have entered the category of reasonable evidence of benefit since the previous review in 2000.
Only a few trials were reported on topical calcineurin inhibitors up to 2000 as these drugs were newly developed at the time. The number of trials on pimecrolimus and tacrolimus has grown to a combined total of > 50 by the end of August 2013. As a significant number of these trials are large and of reasonable duration and carry a fairly low risk of bias, it is clear that these treatments are of benefit compared with placebo treatments (plain grease or cream base).
Educational interventions were tested in only one trial up to 2000. Since 2000, seven trials covering various educational interventions have been performed. Although these have provided evidence of benefit, the active component of these interventions is unclear.
Although azathioprine was in use for severe eczema before 2000, no RCTs regarding its use had been published. The two trials published since the previous review have bolstered confidence that azathioprine provides a significant benefit for adults with severe eczema compared with placebo, although there is still no trial evidence for children or including comparisons with other active treatments such as maximum topical therapy or other systemic treatments.
Additional trials of oral ciclosporin and topical corticosteroids have further strengthened the already existing evidence of benefit for these treatments but, again, with comparisons mainly against placebo treatment, which limits their clinical relevance.
Interventions with insufficient evidence to make recommendations
Since 2000, many novel dietary, non-pharmacological, complementary and other topical or systemic interventions have been the subject of one or two very small or small trials. Mainly because of the lack of detailed reporting, the risk of bias for these trials has been mostly unclear. This coupled with few participants has generally resulted in insufficient evidence of benefit for these treatments. It is a pity that so much effort has been wasted on producing one inconclusive small and poorly reported trial after another, as some of the interventions tested could have been useful and have been discarded on the false belief that clinically useful benefits have been ruled out. 449
Most of the treatments highlighted in the previous review as having insufficient evidence, because of small sample sizes and poor reporting, have regrettably not been the subject of further trials. Those treatments for which additional trials have been published have not clarified whether or not the treatments are of genuine clinical benefit. This is the case for antihistamines and dietary restrictions for established eczema, which are still in routine use.
Interventions for which randomised controlled trial evidence does not support a clinically useful benefit
Despite the general improvement in methodology and reporting of RCTs of eczema treatments since the previous review, there have been very few trials published that provide convincing evidence of no benefit. Proving that something is not beneficial is a difficult task when traditional frequentist approaches are used to challenge a null hypothesis of no treatment effect, but it is possible to exclude a minimum level of clinical benefit in studies that find no difference between treatments by the narrowness of the CI around that treatment difference. Since 2000, topical antibiotic/steroid combinations compared with topical corticosteroids alone for non-infected eczema, probiotics, evening primrose oil and borage oil have been the subject of additional trials, which in the views of the authors have resulted in an evidence base that is large and robust enough to amount to reasonable evidence of no benefit.
Interventions that previously did not have randomised controlled trial evidence
There are now far fewer treatments in use in the UK with no RCT evidence available. It is pleasing to find RCTs of widely used treatments for severe eczema, such as oral azathioprine. Ion-exchange water softening devices have been ruled out for treatment of eczema in one definitive RCT. 364 Other interventions such as organisation of care, specialised clothing and wet wrap bandages have now also been tested to some extent in RCTs, although there is not yet enough evidence from these trials to be clear whether these interventions are of benefit or not.
Interventions with no randomised controlled trial evidence
One of the most pressing remaining evidence gaps is whether the routine use of allergy testing (including patch testing, RAST, skin-prick tests and aeroallergen tests) followed by avoidance of allergens yielding positive reactions results in improved eczema control. The universal advice of soap avoidance and arguments regarding bathing frequency also require further critical scrutiny. It is salutary that these issues are all found on the list of prioritised eczema treatment uncertainties from the recent Eczema Priority Setting Partnership57 (see Table 121).
Coverage and clinical relevance
Since the previous review,55 there has been a slight increase in trials of non-pharmacological interventions such as specialised clothing and education, non-steroidal topical preparations and complementary therapies. Disappointingly, there are still too many placebo studies, which are largely uninformative for clinical practice.
It is also worthy to note the mismatch research conducted by investigators and pharmaceutical companies against the most pressing questions summarised in the Eczema Priority Setting Partnership57 between clinicians and patients, highlighted in Table 121.
| Group | Uncertainty |
|---|---|
| Shared priorities | What is the best and safest way of using topical steroids for eczema: frequency of application, potency, length of time, alternating with other topical treatments and age limits for treatment? |
| What is the long-term safety of applying steroids to the skin for eczema? | |
| What role might food allergy tests play in treating eczema? | |
| Which emollient is the most effective and safe in treating eczema? | |
| Patient and carer priorities | What is the best psychological treatment for itching/scratching in eczema? |
| What is the best way for people with eczema to wash: frequency of washing, water temperature, bath vs. shower? | |
| What are the best and safest natural products to apply to the skin for eczema? | |
| How much does avoidance of irritants and allergens help people with eczema? | |
| What is the role of diet in treating eczema: exclusion diets and nutritional supplements? | |
| Health-care professional priorities | Which is most effective in the management of eczema: education programmes, GP care, nurse-led care, dermatologist-led care or multidisciplinary care? |
| Which is safer and more effective for treating eczema: steroids or calcineurin inhibitors? | |
| How effective are interventions to reduce skin infections in the management of eczema? | |
| Which should be applied first when treating eczema, emollients or topical steroids? | |
| What is the best and safest way of using drugs that suppress the immune system when treating eczema? |
Difficult body sites
Sensitive areas of the body can be problematic to treat, as the skin is thin and the potential for treatments such as topical corticosteroids to cause local side effects, such as skin thinning, is greater.
Despite the need for good evidence on treating sensitive sites, hardly any of the trials in this review evaluated treatment of these areas specifically. Most trials of topical corticosteroids have used low-potency hydrocortisone acetate for such areas instead of the treatment being tested. Typically, outcomes are measured over the whole body, thereby giving no specific indication of the success or otherwise of treatment on sensitive sites. One area to have been given specific attention is the head and neck, for which trials of antifungal agents have not provided any convincing evidence of additional benefit for non-infected eczema. Another study of eyelid dermatitis failed to show any clear benefit of topical tacrolimus over topical corticosteroids. Trials of pimecrolimus have provided some evidence of benefit for the head and neck region compared with placebo and topical corticosteroids.
Combinations of treatments
It is encouraging that there has been progress in testing treatments in addition to ‘standard care’ since 2000, which has made the analysis of the potential benefits and harms of a treatment much easier in the context of routine clinical practice.
Validity and robustness of results
Missed studies
Although there is a possibility that a mainly electronic search of reference databases will miss certain RCTs because of misclassification, the authors have attempted to mitigate this risk by searching both the main bibliographic databases (MEDLINE and EMBASE) and several smaller, specialist databases (CINAHL, AMED and LILACS).
Author bias
The blinding of the review authors to the authors of the trials was not practically possible and it is possible that some bias has occurred in the narrative summary of results. The authors have used a standardised approach using a tool for assessing risk of bias in addition to their own comments and a consistent approach for separating the actual results from their interpretation.
External validity
The population of those with eczema in the trials published does not reflect the majority of the eczema population, as most trials were conducted in secondary care. Most people with eczema never reach secondary care and so would not have had the opportunity to be recruited into these trials. This raises serious concerns about the external validity of the results of most eczema treatment trials for people seen in primary care.
Quality of reporting
Study design issues
The quality of reporting was assessed using the Cochrane Collaboration risk-of-bias tool,58 with an assessment of the risk of bias for the generation of the randomisation sequence, allocation concealment and blinding undertaken. Whether an intention-to-treat analysis was used was also assessed. The original review55 did not use the Cochrane Collaboration tool, but did make a very similar assessment of the quality of reporting for the same criteria that have been shown to lead to biased estimates of treatment effects. 450
The trials in the original review55 were generally of very poor quality. The trials since 2000 are mixed, with notable differences in the quality of reporting between the treatment categories. In particular, concealment of the allocation sequence was often poorly reported and some trials confused blinding of outcome assessment with allocation concealment, making it impossible to assess whether either had taken place to a satisfactory level.
The use of validated scales for physician measurement of severity or composite measurement has risen considerably since 2000. Named scales that have been used are also far more likely to be ones that have been tested to some degree. There is still widespread heterogeneity in the choice of outcome measures, rendering any form of meta-analysis very difficult and underscoring the need for core outcome sets for eczema, as are being developed by the HOME initiative. 64,65
There has been a modest improvement in the number of trials reporting participant-assessed outcomes such as itching and sleep loss, but these were almost always secondary outcomes and the results were very often presented with insufficient detail.
Studies still need to be longer
Although eczema typically lasts for several years or a lifetime, many eczema trials investigate short-term treatments lasting from 1 to 12 weeks. There is an argument for short-term trials and that is to test the speed of induction of remission – studies could arguably be of 1–2 weeks’ duration as most patients or parents would like to see some evidence of benefit within 48–72 hours. But the short-term studies that keep appearing are neither one thing or the other in that they attempt to claim efficacy based on changes reported over a period of say 6 weeks – too long for assessing the induction of remission and too short for assessing the duration of disease remission. Some improvement in trial duration has occurred in those trials of the maintenance of remission (using topical corticosteroids and calcineurin inhibitors) lasting from 6 months to 1 year. Running a clinical trial is generally a costly and lengthy business, with 1–2 years of set-up before the first participant is recruited, so it is understandable that the length of follow-up becomes a critical cost driver when conducting trials in this area.
Inappropriate comparators
Several areas of eczema treatment research have now matured to a point where there is a very strong body of evidence which shows that a treatment or a class of treatments is of more benefit for those with eczema than a placebo or not giving the treatment. Unfortunately, it has been common over the past 14 years for more trials comparing a treatment or a class of treatments against placebo or no treatment to be conducted, despite overwhelming evidence of efficacy from previous placebo- or vehicle-controlled studies. The total number of vehicle-controlled studies for topical pimecrolimus, for example, now exceeds 15 and yet not a single comparative trial has been published at the time of writing this report that compares this newer treatment to the most obvious and least expensive existing comparator, that is, mild topical corticosteroids. One can appreciate the need for two or three pivotal efficacy studies testing new treatments against placebo in different populations such as children and adults or different ethnic groups who may respond differently, but > 15 trials is excessive and possibly unethical. It is possible that some placebo trials are used as marketing tools so that sceptical clinicians become engaged with a new product. It is therefore important that trial participants, funders and ethics committees scrutinise the need for additional placebo- or vehicle-controlled studies in the context of existing evidence.
Separate publications from the same study
A number of authors presented the results of a single trial in multiple publications, each highlighting a specific population (e.g. children) or outcomes. Although these publications can serve to make researchers with specific interests more aware of trial evidence, they are potentially serious sources of bias, as many do not mention that they make up part of a larger trial or they lack clarity and detail if they do. The review authors had to scrutinise trial publications to be certain not to include the results of any trial participants twice.
Chapter 15 Summary and conclusions
Strengths and weaknesses of this review
This updated review has used a clear and robust method for identifying RCTs for inclusion, combined with a wide set of inclusion criteria, minimising the potential for selection bias by the review authors. Trying to answer similar questions for the 92 or so interventions used for the treatment of eczema would be impossible in one short report. Therefore, we have taken an approach that is a hybrid between a scoping review451 and a full systematic review, in the hope that this will spin off more detailed Cochrane reviews. We cannot be sure that we have found all relevant RCTs despite our rigorous searches. Although we are not aware of any unpublished RCTs, there is good evidence that a large proportion of research never gets published, particularly negative studies. 452 This means that our review is likely to depict a more positive picture of the true body of evidence than truly exists. The references were filtered by only one author, in consultation with another author in cases of uncertainty. This does not reach the gold standard of two or more authors independently screening all references and could have led to some studies being missed. We did not contact authors for missing data as this was not practical for so many trials within the time and resources available. Such data, if forthcoming, would probably not have altered any of our narrative conclusions considering that so many of our studies were RCTs of single interventions. No meta-analyses were performed as very few of the included studies shared common outcomes or treatment comparisons.
Implications for health care
The strength of evidence supporting the various interventions has already been reported in the separate chapters dealing with the diverse intervention groups. The strength of evidence in relation to those interventions that are commonly used in the UK is summarised narratively in Box 1. This box refers to evidence on the short-term control of eczema, except where mentioned. It is now up to guideline developers such as NICE and SIGN to use the evidence summarised in this report to update their treatment guidelines. 41,42
Future research priorities
Primary research
Although not unique to eczema, perhaps the biggest priority for future research is to better understand why researchers across the world continue to conduct small, poorly planned, unregistered and poorly reported trials on people who have volunteered to participate in such studies. This is despite the presence of overwhelming evidence on the value of ensuring that all trials are registered in the public domain before recruitment starts and that all of the essential features of the completed studies are reported, so that readers can make judgements about their quality and utility. International organisations such as the International Society of Atopic Dermatitis, HOME, the Expert Resource Group of the American Academy of Dermatology and the European Task Force on Atopic Dermatitis are all in a good position to encourage better-quality trials.
In addition to understanding why researchers continue to undertake trials without consideration of the existing evidence base and what mistakes to avoid, perhaps the largest gap for primary research exists in conducting clinical trials in primary care, where most patients are seen in the UK.
Priority areas for future primary research have already been highlighted by a priority-setting partnership between health-care professionals and patients in the accompanying Programme Grant for Applied Research report447 and are summarised in Table 121. Specific examples of new RCTs with accompanying cost-effectiveness analyses that could be undertaken in line with the priorities from the priority-setting partnership are outlined below:
-
evaluation of allergy tests followed by avoidance of allergens guided by such tests in high-risk and unselected participants
-
evaluation relating to emollients, especially to gain a better understanding of which are beneficial and which are potentially harmful203
-
evaluation of expensive ceramide-containing preparations compared with cheaper petrolatum-based products
-
evaluation of whether daily or less frequent washing and avoidance of soap is really necessary for people with eczema
-
evaluation of the optimum use of topical corticosteroids over longer periods, especially with a view to documenting whether skin thinning and problems associated with systemic absorption, such as clinically relevant adrenal gland suppression, really do exist with modern recommended usage patterns
-
evaluation of topical tacrolimus 0.1% compared with potent topical corticosteroids for flare prevention in those with moderate to severe disease, especially if accompanied by a cost-effectiveness analysis420
-
comparison of topical pimecrolimus with low-potency corticosteroids for the prevention of flares in people with mild eczema in the community
-
evaluation of educational interventions for health-care providers as well as patients
-
head-to-head comparisons of systemic treatment for severe eczema in children.
Most of these new RCT ideas should ideally be carried out in a primary care population as well as secondary care.
Although this report deals strictly with the treatment of established disease, disease prevention should not be overlooked for eczema as it remains a distinct possibility as elaborated on in our eczema prevention work programme of our companion monograph447 (see chapter 1, eczema prevention work programme).
Secondary research
This scoping review attempts to provide a panoramic overview of primary and secondary research on eczema treatment. It appears that expansion of secondary research for eczema is outstripping the availability of RCT evidence in many areas of eczema treatment. Several Cochrane reviews of eczema either have been completed or are in progress (Box 2), which will provide a more detailed and in-depth analysis of specific interventions and which will be updated as new evidence becomes available. Some other non-Cochrane reviews have been well reported and are mapped for further reference on the Centre of Evidence Based Dermatology resource section [see www.nottingham.ac.uk/dermatology (accessed 6 November 2015)]. Overviews of existing single intervention systematic reviews, such as the one conducted for eczema prevention,70 are also needed, as is the application of mixed-treatment comparisons for understanding more about the crucial missing treatment comparisons that are needed to inform clinical practice.
-
Chinese herbal medicine for atopic eczema. 421
-
Dietary exclusions for established atopic eczema. 276
-
Interventions to reduce Staphylococcus aureus in the management of atopic eczema. 244
-
Psychological and educational interventions for atopic eczema in children. 453
-
Oral evening primrose oil and borage oil for eczema. 189
-
Topical pimecrolimus for eczema. 89
-
Dietary supplements for established atopic eczema. 278
-
Probiotics for treating eczema. 273
-
Oral H1 antihistamines as monotherapy for eczema. 454
-
Topical tacrolimus for atopic dermatitis. 455
-
Specific allergen immunotherapy for the treatment of atopic eczema. 456
-
House dust mite reduction and avoidance measures for treating eczema. 457
-
Different strategies for using topical corticosteroids for established eczema. 458
-
Leukotriene receptor antagonists for atopic dermatitis (no published protocol to date).
-
Complementary and alternative medicine treatments for atopic eczema. 459
-
Systemic immunosuppressive therapies in atopic eczema (no published protocol to date).
-
Oral H1 antihistamines as ‘add-on’ therapy to topical treatment for eczema (no published protocol to date).
Methodological research
Is eczema more than one disease? Although the clinical definition of eczema is relatively well established, it is possible that eczema represents a constellation of different diseases. These diseases may be differentiated into an exophenotype (based on observable characteristics such as discoid pattern or associated asthma) or their endotype (based on measurable underlying pathological and genetic mechanisms). In the future, these endotypes may be identifiable using genetic biomarkers. Different exo- and endophenotypes may respond differently to treatments and researchers are encouraged to explore such subtypes in planned subgroup analyses where possible.
Trial design
Progress in trial design is moving forward and has provided some novel methodologies that may merit use for specific research questions in eczema treatment. Adaptive designs could well inform the early assessment of new treatments and dose-finding studies. RCTs combined with observational studies could lend themselves well to a condition that waxes and wanes so frequently over time. Cohort studies from which multiple RCTs are conducted may provide a more holistic picture of how cumulative treatment changes the course of a disease such as eczema. The use of routine databases and biobanks also provide possibilities to conduct large pragmatic trials with the potential to stratify disease.
The greatest challenge is in the field of outcome measures, where significant progress has been made through international consensus in identifying the core domains of symptoms, signs, quality of life and long-term control that should be measured in all future RCTs of eczema. 449 Instruments of sufficient truth, discrimination and feasibility probably exist for all four domains with the exception of long-term control, which requires a lot of further fundamental thought on the underlying concepts and how they are best measured.
Methods to prioritise future research
The development of priority-setting partnerships is a methodology that is itself evolving, as is the use of economic modelling to identify the expected health benefits of additional evidence in prioritising research. 56
Key points
-
Randomised controlled trials of interventions for eczema have often not answered the questions of most importance to patients and their carers.
-
The lack of studies in primary care is still a major problem as the majority of people with eczema are managed in primary care.
-
Several treatments for eczema now have a sound evidence base.
-
Perhaps the greatest benefit identified in this review is the use of twice-weekly anti-inflammatory treatment to maintain disease remission.
-
There are still major gaps in current knowledge about commonly used treatments such as emollients and impregnated bandages.
-
This updated review has identified many of the same methodological issues noted by the original review in 2000,55 including the lack of trial registration, poor reporting according to Consolidated Standards of Reporting Trials guidelines, inadequate sample size, trials of too short duration, inappropriate analysis of within-group changes only and lack of patient-reported outcomes.
-
All of these deficiencies can be easily overcome if investigators, sponsors, ethics committees, journals, patients and funders are made aware of the existing evidence base described in this review and commit to full trial registration and complete trial reporting.
-
The creation of the GREAT database,68 an international database of all RCTs and systematic reviews of eczema treatments freely in the public domain, should facilitate future trial prioritisation and systematic reviews for the benefit of patients.
Acknowledgements
The authors wish to thank the following people for their helpful contribution to this report.
Searching
-
Mrs Elizabeth Doney, Cochrane Skin Group, University of Nottingham.
-
Miss Jordan Samuels, University of Nottingham.
-
Mrs Sherie Smith, University of Nottingham.
-
Staff of the Greenfield Medical Library, University of Nottingham.
Translators
-
Chinese articles – Dr Sherman Gu, Dr Kyle Tang and Dr Sam Feng.
-
Dutch articles – Dr Gudula Kirtschig and Dr Alexandra Erven.
-
French articles – Dr Louise Lansbury.
-
German articles – Dr Katja Boehm.
-
Italian articles – Dr Alida DePase.
-
Japanese articles – Dr Yukihiro Ohya and Dr Masaki Futamura.
-
Korean articles – Dr Euiseok Kim.
-
Norwegian articles – Dr Liv Reinar.
-
Farsi (Persian) articles – Dr Alireza Firooz.
-
Polish articles – Dr Margorzata Bala.
-
Russian articles – Dr Viktoria Eleftheriadou.
-
Slovakian and Czech articles – Dr Ivan Chromej.
-
Spanish articles – Dr Martin-Clavijo.
-
Spanish and Portuguese articles – Mrs Maxine Whitton, MBE.
Copy-editor
-
Mrs June Cody.
Proofreaders and peer reviewers
-
Dr Mary Glover, Dr Sherman Gu, Dr Fiona Meredith, Dr Matt Ridd, Mrs Amanda Roberts.
This report presents independent research funded by the NIHR under its Programme Grants for Applied Research funding scheme (RP-PG-0407-10177). The views expressed in this report are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Contributions of authors
Helen Nankervis (Research Associate) performed the searches, checked for eligible studies, obtained hard copies of the studies, abstracted data from the included studies, updated the background and methods sections, wrote the results sections and was involved in writing the discussion and conclusions sections of the review.
Kim S Thomas (Professor of Applied Dermatology Research) provided expert methodological advice on the structure and content of the review and was involved in writing the abstract, scientific summary and some sections of the review.
Finola M Delamere (Managing Editor of the Cochrane Skin Group) updated and designed the search strategies and gave expert input and assistance in performing the searches, commented on all sections of the review and the review structure and layout, was involved in supervising Helen Nankervis and gave expert methodological input.
Sébastien Barbarot (Dermatologist specialising in Paediatric Dermatology) abstracted data from some of the included studies and updated sections of the review with studies from 2011 and 2013. He also gave expert methodological and clinical advice.
Natasha K Rogers (Research Associate) abstracted data from studies, contributed to maintenance of GREAT Database, and contributed to writing and editing the review.
Hywel C Williams (Professor of Dermato-Epidemiology) wrote the study proposal for the original review, supervised Helen Nankervis, commented on all sections of the review as well as the structure and layout, was involved in writing some sections of the review, particularly the summaries and the discussion, and gave expert clinical input.
Data sharing statement
The data presented in this report are also included in the appendices of the review and in the GREAT database at www.greatdatabase.org.uk (accessed 9 March 2016).
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health.
References
- Archer CB, Williams HC. Atopic Dermatitis. Cambridge: Cambridge University Press; 2000.
- Luoma R, Koivikko A, Viander M. Development of asthma, allergic rhinitis and atopic dermatitis by the age of five years. A prospective study of 543 newborns. Allergy 1983;38:339-46. http://dx.doi.org/10.1111/j.1398-9995.1983.tb04128.x.
- Williams HC, Wuthrich B, Williams HC. Atopic Dermatitis. Cambridge: Cambridge University Press; 2000.
- Flohr C, Johansson SG, Wahlgren CF, Williams H. How atopic is atopic dermatitis?. J Allergy Clin Immunol 2004;114:150-8. http://dx.doi.org/10.1016/j.jaci.2004.04.027.
- Flohr C, Weiland SK, Weinmayr G, Bjorksten B, Braback L, Brunekreef B, et al. The role of atopic sensitization in flexural eczema: findings from the International Study of Asthma and Allergies in Childhood Phase Two. J Allergy Clin Immunol 2008;121:141-7. http://dx.doi.org/10.1016/j.jaci.2007.08.066.
- Johansson SG, Hourihane JO, Bousquet J, Bruijnzeel-Koomen C, Dreborg S, Haahtela T, et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy 2001;56:813-24. http://dx.doi.org/10.1034/j.1398-9995.2001.t01-1-00001.x.
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol 2004;113:832-6. http://dx.doi.org/10.1016/j.jaci.2003.12.591.
- Hanifin JM, Rajka G. Diagnostic features of atopic-dermatitis. Acta Derm Venereol 1980;92:44-7.
- Williams HC, Burney PGJ, Hay RJ, Archer CB, Shipley MJ, Hunter JJA, et al. The UK Working Party’s diagnostic criteria for atopic dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. Br J Dermatol 1994;131:383-96. http://dx.doi.org/10.1111/j.1365-2133.1994.tb08530.x.
- Brenninkmeijer EE, Schram ME, Leeflang MM, Bos JD, Spuls PI. Diagnostic criteria for atopic dermatitis: a systematic review. Br J Dermatol 2008;158:754-65. http://dx.doi.org/10.1111/j.1365-2133.2007.08412.x.
- Williams HC, Williams HC. Atopic Dermatitis. Cambridge: Cambridge University Press; 2000.
- Brown SJ, Irvine AD. Atopic eczema and the filaggrin story. Semin Cutan Med Surg 2008;27:128-37. http://dx.doi.org/10.1016/j.sder.2008.04.001.
- van den Oord RA, Sheikh A. Filaggrin gene defects and risk of developing allergic sensitisation and allergic disorders: systematic review and meta-analysis. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b2433.
- Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI. ISAAC Phase Three Study Group . Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol 2009;124:1251-8. http://dx.doi.org/10.1016/j.jaci.2009.10.009.
- Williams HC, Stewart A, von Mutius E, Cookson W, Anderson HR. International Study of Asthma and Allergies in Childhood (ISAAC) Phase One and Three Study Groups . Is eczema really on the increase worldwide?. J Allergy Clin Immunol 2008;121:947-54. http://dx.doi.org/10.1016/j.jaci.2007.11.004.
- Simpson CR, Newton J, Hippisley-Cox J, Sheikh A. Trends in the epidemiology and prescribing of medication for eczema in England. J R Soc Med 2009;102:108-17. http://dx.doi.org/10.1258/jrsm.2009.080211.
- Herd RM, Tidman MJ, Prescott RJ, Hunter JA. Prevalence of atopic eczema in the community: the Lothian Atopic Dermatitis study. Br J Dermatol 1996;135:18-9. http://dx.doi.org/10.1111/j.1365-2133.1996.tb03600.x.
- Vickers CFH. The natural history of atopic eczema. Acta Derm Venereol (Stockh) 1980:113-15.
- Emerson RM, Williams HC, Allen BR. Severity distribution of atopic dermatitis in the community and its relationship to secondary referral. Br J Dermatol 1998;139:73-6. http://dx.doi.org/10.1046/j.1365-2133.1998.02316.x.
- Ben-Gashir MA, Seed PT, Hay RJ. Quality of life and disease severity are correlated in children with atopic dermatitis. Br J Dermatol 2004;150:284-90. http://dx.doi.org/10.1111/j.1365-2133.2004.05776.x.
- Herd RM, Williams HC. Atopic Dermatitis. Cambridge: Cambridge University Press; 2000.
- Meltzer LJ, Moore M. Sleep disruptions in parents of children and adolescents with chronic illnesses: prevalence, causes, and consequences. J Pediatr Psychol 2008;33:279-91. http://dx.doi.org/10.1093/jpepsy/jsm118.
- Schmitt J, Apfelbacher C, Chen CM, Romanos M, Sausenthaler S, Koletzko S, et al. Infant-onset eczema in relation to mental health problems at age 10 years: results from a prospective birth cohort study (German Infant Nutrition Intervention plus). J Allergy Clin Immunol 2010;125:404-10. http://dx.doi.org/10.1016/j.jaci.2009.10.055.
- Mancini AJ, Kaulback K, Chamlin SL. The socioeconomic impact of atopic dermatitis in the United States: a systematic review. Pediatr Dermatol 2008;25:1-6. http://dx.doi.org/10.1111/j.1525-1470.2007.00572.x.
- Schuttelaar ML, Vermeulen KM, Coenraads PJ. Costs and cost-effectiveness analysis of treatment in children with eczema by nurse practitioner vs. dermatologist: results of a randomized, controlled trial and a review of international costs. Br J Dermatol 2011;165:600-11. http://dx.doi.org/10.1111/j.1365-2133.2011.10470.x.
- Saeki H, Furue M, Furukawa F, Hide M, Ohtsuki M, Katayama I, et al. Guidelines for management of atopic dermatitis. J Dermatol 2009;36:563-77. http://dx.doi.org/10.1111/j.1346-8138.2009.00706.x.
- Williams HC. Atopic eczema. BMJ 1995;311:1241-2. http://dx.doi.org/10.1136/bmj.311.7015.1241.
- Williams HC, Strachan DP, Hay RJ. Childhood eczema: disease of the advantaged?. BMJ 1994;308:1132-5. http://dx.doi.org/10.1136/bmj.308.6937.1132.
- Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol 2011;131:67-73. http://dx.doi.org/10.1038/jid.2010.251.
- McNally NJ, Williams HC, Phillips DR, Strachan DP. Is there a geographical variation in eczema prevalence in the UK? Evidence from the 1958 British Birth Cohort Study. Br J Dermatol 2000;142:712-20. http://dx.doi.org/10.1046/j.1365-2133.2000.03416.x.
- Strachan DP. Hay fever, hygiene, and household size. BMJ 1989;299:1259-60. http://dx.doi.org/10.1136/bmj.299.6710.1259.
- Burrell-Morris C, Williams HC, Williams HC. Atopic Dermatitis. Cambridge: Cambridge University Press; 2000.
- Peters JL, Boynton-Jarrett R, Sandel M. Prenatal environmental factors influencing IgE levels, atopy and early asthma. Curr Opin Allergy Clin Immunol 2013;13:187-92. http://dx.doi.org/10.1097/ACI.0b013e32835e82d3.
- Langan SM, Flohr C, Williams HC. The role of furry pets in eczema: a systematic review. Arch Dermatol 2007;143:1570-7. http://dx.doi.org/10.1001/archderm.143.12.1570.
- Langan SM, Williams HC. What causes worsening of eczema? A systematic review. Br J Dermatol 2006;155:504-14. http://dx.doi.org/10.1111/j.1365-2133.2006.07381.x.
- Bieber T. Atopic dermatitis. N Engl J Med 2008;358:1483-94. http://dx.doi.org/10.1056/NEJMra074081.
- Novak N, Kruse S, Potreck J, Maintz L, Jenneck C, Weidinger S, et al. Single nucleotide polymorphisms of the IL18 gene are associated with atopic eczema. J Allergy Clin Immunol 2005;115:828-33. http://dx.doi.org/10.1016/j.jaci.2005.01.030.
- Lange J, Heinzmann A, Zehle C, Kopp M. CT genotype of promotor polymorphism C159T in the CD14 gene is associated with lower prevalence of atopic dermatitis and lower IL-13 production. Pediatr Allergy Immunol 2005;16:456-7. http://dx.doi.org/10.1111/j.1399-3038.2005.00277.x.
- Williams HC, Chalmers JR, Simpson EL. Prevention of atopic dermatitis. F1000 Med Rep 2012;4. http://dx.doi.org/10.3410/M4-24.
- Hanifin JM, Rogge JL. Staphylococcal infections in patients with atopic dermatitis. Arch Dermatol 1977;113:1383-6. http://dx.doi.org/10.1001/archderm.1977.01640100061009.
- Atopic Eczema in Children: Management of Atopic Eczema in Children from Birth up to the Age of 12 Years. London: NICE; 2007.
- Management of Atopic Eczema in Primary Care. Edinburgh: SIGN; 2011.
- Schofield J, Grindlay D, Williams H. Skin Conditions in the UK: a Health Care Needs Assessment. Nottingham: Centre of Evidence Based Dermatology, University of Nottingham; 2009.
- Charman CR, Morris AD, Williams HC. Topical corticosteroid phobia in patients with atopic eczema. Br J Dermatol 2000;142:931-6. http://dx.doi.org/10.1046/j.1365-2133.2000.03473.x.
- Schmitt J, Langan S, Williams HC. European Dermato-Epidemiology Network . What are the best outcome measurements for atopic eczema? A systematic review. J Allergy Clin Immunol 2007;120:1389-98. http://dx.doi.org/10.1016/j.jaci.2007.08.011.
- Wootton CI, Koller K, Lawton S, O’Leary C, Thomas KS. Are accelerometers a useful tool for measuring disease activity in children with eczema? Validity, responsiveness to change, and acceptability of use in a clinical trial setting. Br J Dermatol 2012;167:1131-7. http://dx.doi.org/10.1111/j.1365-2133.2012.11184.x.
- Severity scoring of atopic dermatitis: the SCORAD index . Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 1993;186:23-31. http://dx.doi.org/10.1159/000247298.
- Hanifin J, Thurston M, Omoto M, Cherill R, Tofte S, Graeber M. The Eczema Area and Severity Index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol 2001;10:11-8. http://dx.doi.org/10.1034/j.1600-0625.2001.100102.x.
- Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol 2004;140:1513-19. http://dx.doi.org/10.1001/archderm.140.12.1513.
- Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol 1995;132:942-9. http://dx.doi.org/10.1111/j.1365-2133.1995.tb16953.x.
- Lewis-Jones MS, Finlay AY, Dykes PJ. The Infants’ Dermatitis Quality of Life Index. Br J Dermatol 2001;144:104-10. http://dx.doi.org/10.1046/j.1365-2133.2001.03960.x.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol 1994;19:210-16. http://dx.doi.org/10.1111/j.1365-2230.1994.tb01167.x.
- McHenry PM, Williams HC, Bingham EA. Management of atopic eczema. Joint Workshop of the British Association of Dermatologists and the Research Unit of the Royal College of Physicians of London. BMJ 1995;310:843-7. http://dx.doi.org/10.1136/bmj.310.6983.843.
- Williams HC. On the definition and epidemiology of atopic dermatitis. Dermatol Clin 1995;13:649-57.
- Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technol Assess 2000;4.
- Hay RJ, Johns NE, Williams HC, Bolliger IW, Dellavalle RP, Margolis DJ, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol 2014;134:1527-34. http://dx.doi.org/10.1038/jid.2013.446.
- Batchelor JM, Ridd MJ, Clarke T, Ahmed A, Cox M, Crowe S, et al. The Eczema Priority Setting Partnership: a collaboration between patients, carers, clinicians and researchers to identify and prioritize important research questions for the treatment of eczema. Br J Dermatol 2013;168:577-82. http://dx.doi.org/10.1111/bjd.12040.
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions 2011. www.cochrane-handbook.org (accessed 27 October 2015).
- Berth-Jones J. Six Area, Six Sign Atopic Dermatitis (SASSAD) severity score: a simple system for monitoring disease activity in atopic dermatitis. Br J Dermatol 1996;135:25-30. http://dx.doi.org/10.1111/j.1365-2133.1996.tb00706.x.
- Bahmer FA. ADASI score: Atopic Dermatitis Area and Severity Index. Acta Derm Venereol Suppl (Stockh) 1992;176:32-3.
- Neame RL, Berth-Jones J, Kurinczuk JJ, Graham-Brown RA. Prevalence of atopic dermatitis in Leicester: a study of methodology and examination of possible ethnic variation. Br J Dermatol 1995;132:772-7. http://dx.doi.org/10.1111/j.1365-2133.1995.tb00725.x.
- Sugarman JL, Fluhr JW, Fowler AJ, Bruckner T, Diepgen TL, Williams ML. The objective severity assessment of atopic dermatitis score: an objective measure using permeability barrier function and stratum corneum hydration with computer-assisted estimates for extent of disease. Arch Dermatol 2003;139:1417-22. http://dx.doi.org/10.1001/archderm.139.11.1417.
- Wolkerstorfer A, De Waard van der Spek FB, Glazenburg EJ, Mulder PG, Oranje AP. Scoring the severity of atopic dermatitis: three item severity score as a rough system for daily practice and as a pre-screening tool for studies. Acta Derm Venereol 1999;79:356-9. http://dx.doi.org/10.1080/000155599750010256.
- Schmitt J, Williams H. HOME Development Group. Harmonising Outcome Measures for Eczema (HOME). Report from the First International Consensus Meeting (HOME 1), 24 July 2010, Munich, Germany. Br J Dermatol 2010;163:1166-8. http://dx.doi.org/10.1111/j.1365-2133.2010.10054.x.
- Schmitt J, Spuls P, Boers M, Thomas K, Chalmers J, Roekevisch E, et al. Towards global consensus on outcome measures for atopic eczema research: results of the HOME II meeting. Allergy 2012;67:1111-17. http://dx.doi.org/10.1111/j.1398-9995.2012.02874.x.
- Williams HC, Schmitt J. Harmonising Outcome Measures for Eczema (HOME) 2013. www.homeforeczema.org (accessed 27 October 2015).
- Savovic J, Jones H, Altman D, Harris R, Juni P. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta-epidemiological studies. Health Technology Assess 2012;16. http://dx.doi.org/10.3310/hta16350.
- University of Nottingham . Global Resource for EczemA Trials 2013. www.greatdatabase.org.uk (accessed 27 October 2015).
- Futamura M, Thomas KS, Grindlay DJC, Doney E, Torley D, Williams H. Mapping systematic reviews on atopic eczema – an essential resource for dermatology professionals and researchers. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0058484.
- Foisy M, Boyle RJ, Chalmers JR, Simpson EL, Williams HC. Overview of reviews. The prevention of eczema in infants and children: an overview of Cochrane and non-Cochrane reviews. Evid Based Child Health 2011;6:1322-39. http://dx.doi.org/10.1002/ebch.827.
- Nankervis H, Maplethorpe A, Williams HC. Mapping randomized controlled trials of treatments for eczema – the GREAT database (the Global Resource of EczemA Trials: a collection of key data on randomized controlled trials of treatments for eczema from 2000 to 2010). BMC Dermatol 2011;11. http://dx.doi.org/10.1186/1471-5945-11-10.
- Sulzberger MB, Witten VH. The effect of topically applied compound F in selected dermatoses. J Invest Dermatol 1952;19:101-2. http://dx.doi.org/10.1038/jid.1952.72.
- Charman C. Clinical evidence: atopic eczema. BMJ 1999;318:1600-4. http://dx.doi.org/10.1136/bmj.318.7198.1600.
- Vafadari R, Kraaijeveld R, Weimar W, Baan CC. Tacrolimus inhibits NF-kappaB activation in peripheral human T cells. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0060784.
- Zuberbier T, Chong SU, Grunow K, Guhl S, Welker P, Grassberger M, et al. The ascomycin macrolactam pimecrolimus (Elidel, SDZ ASM 981) is a potent inhibitor of mediator release from human dermal mast cells and peripheral blood basophils. J Allergy Clin Immunol 2001;108:275-80. http://dx.doi.org/10.1067/mai.2001.116865.
- Temeck J. Protopic and Elidel Presentation for Regulatory Briefing 2005 n.d. www.fda.gov/ohrms/dockets/ac/05/briefing/2005-4089b2_01_02_DPDD%20Consult.pdf (accessed 7 March 2016).
- Cury Martins J, Martins C, Aoki V, Gois AF, Ishii HA, da Silva EM. Topical tacrolimus for atopic dermatitis. Cochrane Database Syst Rev 2015;7. http://dx.doi.org/10.1002/14651858.cd009864.pub2.
- A Pediatric Longitudinal Evaluation to Assess the Long-Term Safety of Protopic for the Treatment of Atopic Dermatitis (APPLES) n.d. https://clinicaltrials.gov/ct2/show/NCT00475605 (accessed 12 December 2015).
- Schmitt J, von Kobyletzki L, Svensson A, Apfelbacher C. Efficacy and tolerability of proactive treatment with topical corticosteroids and calcineurin inhibitors for atopic eczema: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol 2011;164:415-28. http://dx.doi.org/10.1111/j.1365-2133.2010.10030.x.
- Callen J, Chamlin S, Eichenfield LF, Ellis C, Girardi M, Goldfarb M, et al. A systematic review of the safety of topical therapies for atopic dermatitis. Br J Dermatol 2007;156:203-21. http://dx.doi.org/10.1111/j.1365-2133.2006.07538.x.
- Green C, Colquitt JL, Kirby J, Davidson P, Payne E. Clinical and cost-effectiveness of once-daily versus more frequent use of same potency topical corticosteroids for atopic eczema: a systematic review and economic evaluation. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8470.
- Green C, Colquitt JL, Kirby J, Davidson P. Topical corticosteroids for atopic eczema: clinical and cost effectiveness of once-daily vs. more frequent use. Br J Dermatol 2005;152:130-41. http://dx.doi.org/10.1111/j.1365-2133.2005.06410.x.
- Schiffner R, Schiffner-Rohe J, Landthaler M, Stolz W. Treatment of atopic dermatitis and impact on quality of life: a review with emphasis on topical non-corticosteroids. Pharmacoeconomics 2003;21:159-79. http://dx.doi.org/10.2165/00019053-200321030-00002.
- Chen SL, Yan J, Wang FS. Two topical calcineurin inhibitors for the treatment of atopic dermatitis in pediatric patients: a meta-analysis of randomized clinical trials. J Dermatol Treat 2010;21:144-56. http://dx.doi.org/10.3109/09546630903401470.
- Fleischer AB, Boguniewicz M. An approach to pruritus in atopic dermatitis: a critical systematic review of the tacrolimus ointment literature. J Drugs Dermatol 2010;9:488-98.
- El-Batawy MM, Bosseila MA, Mashaly HM, Hafez VS. Topical calcineurin inhibitors in atopic dermatitis: a systematic review and meta-analysis. J Dermatol Sci 2009;54:76-87. http://dx.doi.org/10.1016/j.jdermsci.2009.02.002.
- Yan J, Chen SL, Wang XL, Zhou W, Wang FS. Meta-analysis of tacrolimus ointment for atopic dermatitis in pediatric patients. Pediatr Dermatol 2008;25:117-20. http://dx.doi.org/10.1111/j.1525-1470.2007.00600.x.
- Ashcroft DM, Dimmock P, Garside R, Stein K, Williams HC. Efficacy and tolerability of topical pimecrolimus and tacrolimus in the treatment of atopic dermatitis: meta-analysis of randomised controlled trials. BMJ 2005;330. http://dx.doi.org/10.1136/bmj.38376.439653.D3.
- Ashcroft DM, Chen LC, Garside R, Stein K, Williams HC. Topical pimecrolimus for eczema. Cochrane Database Syst Rev 2007;4. http://dx.doi.org/10.1002/14651858.cd005500.pub2.
- Hebert AA. Review of pimecrolimus cream 1% for the treatment of mild to moderate atopic dermatitis. Clin Ther 2006;28:1972-82. http://dx.doi.org/10.1016/j.clinthera.2006.12.014.
- Garside R, Stein K, Castelnuovo E, Pitt M, Ashcroft D, Dimmock P, et al. The effectiveness and cost-effectiveness of pimecrolimus and tacrolimus for atopic eczema: a systematic review and economic evaluation. Health Technol Assess 2005;9. http://dx.doi.org/10.3310/hta9290.
- Iskedjian M, Piwko C, Shear NH, Langley RG, Einarson TR. Topical calcineurin inhibitors in the treatment of atopic dermatitis: a meta-analysis of current evidence. Am J Clin Dermatol 2004;5:267-79. http://dx.doi.org/10.2165/00128071-200405040-00006.
- Sher LG, Chang J, Patel IB, Balkrishnan R, Fleischer AB. Relieving the pruritus of atopic dermatitis: a meta-analysis. Acta Derm Venereol 2012;92:455-61. http://dx.doi.org/10.2340/00015555-1360.
- Hanifin JM, Cooper KD, Ho VC, Kang S, Krafchik BR, Margolis DJ, et al. Guidelines of care for atopic dermatitis, developed in accordance with the American Academy of Dermatology (AAD)/American Academy of Dermatology Association ‘Administrative Regulations for Evidence-Based Clinical Practice Guidelines’. J Am Acad Dermatol 2004;50:391-404. http://dx.doi.org/10.1016/j.jaad.2003.08.003.
- Braham SJ, Pugashetti R, Koo J, Maibach HI. Occlusive therapy in atopic dermatitis: overview. J Dermatol Treat 2010;21:62-7. http://dx.doi.org/10.3109/09546630902911854.
- Devillers AC, Oranje AP. Efficacy and safety of ‘wet-wrap’ dressings as an intervention treatment in children with severe and/or refractory atopic dermatitis: a critical review of the literature. Br J Dermatol 2006;154:579-85. http://dx.doi.org/10.1111/j.1365-2133.2006.07157.x.
- Paller AS, Nimmagadda S, Schachner L, Mallory SB, Kahn T, Willis I, et al. Fluocinolone acetonide 0.01% in peanut oil: therapy for childhood atopic dermatitis, even in patients who are peanut sensitive. J Am Acad Dermatol 2003;48:569-77. http://dx.doi.org/10.1067/mjd.2003.174.
- Eichenfield LF, Miller BH. Cutivate Lotion Study Group . Two randomized, double-blind, placebo-controlled studies of fluticasone propionate lotion 0.05% for the treatment of atopic dermatitis in subjects from 3 months of age. J Am Acad Dermatol 2006;54:715-17. http://dx.doi.org/10.1016/j.jaad.2005.10.063.
- Hebert AA, Cook-Bolden FE, Basu S, Calvarese B, Trancik RJ. Desonide Hydrogel Study Group . Safety and efficacy of desonide hydrogel 0.05% in pediatric subjects with atopic dermatitis. J Drugs Dermatol 2007;6:175-81.
- Pellanda C, Weber M, Bircher A, Surber C. Low-dose triamcinolone acetonide in the phytocosmetic lichtena reduces inflammation in mild to moderate atopic dermatitis. Dermatology 2005;211:338-40. http://dx.doi.org/10.1159/000088504.
- Abramovits W, Oquendo M. Hydrocortisone butyrate 0.1% lipocream in pediatric patients with atopic dermatitis. Skinmed 2010;8:72-9.
- Larsen FS, Simonsen L, Melgaard A, Wendicke K, Henriksen AS. An efficient new formulation of fusidic acid and betamethasone 17-valerate (fucicort lipid cream) for treatment of clinically infected atopic dermatitis. Acta Derm Venereol 2007;87:62-8. http://dx.doi.org/10.2340/00015555-0174.
- Matheson R, Kempers S, Breneman D, Draelos Z, Johnson CE, Loss R, et al. Hydrocortisone butyrate 0.1% lotion in the treatment of atopic dermatitis in pediatric subjects. J Drugs Dermatol 2008;7:266-71.
- Del Rosso JQ, Conte ET. An investigator-blinded evaluation of fluocinonide 0.1% cream in the treatment of atopic dermatitis and psoriasis vulgaris. Cosmetic Dermatol 2007;20:545-52.
- Peserico A, Stadtler G, Sebastian M, Fernandez RS, Vick K, Bieber T. Reduction of relapses of atopic dermatitis with methylprednisolone aceponate cream twice weekly in addition to maintenance treatment with emollient: a multicentre, randomized, double-blind, controlled study. Br J Dermatol 2008;158:801-7. http://dx.doi.org/10.1111/j.1365-2133.2008.08436.x.
- Hanifin J, Gupta AK, Rajagopalan R. Intermittent dosing of fluticasone propionate cream for reducing the risk of relapse in atopic dermatitis patients. Br J Dermatol 2002;147:528-37. http://dx.doi.org/10.1046/j.1365-2133.2002.05006.x.
- Berth-Jones J, Damstra RJ, Golsch S, Livden JK, Van Hooteghem O, Allegra F, et al. Twice weekly fluticasone propionate added to emollient maintenance treatment to reduce risk of relapse in atopic dermatitis: randomised, double blind, parallel group study. BMJ 2003;326. http://dx.doi.org/10.1136/bmj.326.7403.1367.
- Glazenburg EJ, Wolkerstorfer A, Gerretsen AL, Mulder PG, Oranje AP. Efficacy and safety of fluticasone propionate 0.005% ointment in the long-term maintenance treatment of children with atopic dermatitis: differences between boys and girls?. Pediatr Allergy Immunol 2009;20:59-66. http://dx.doi.org/10.1111/j.1399-3038.2008.00735.x.
- Breneman D, Fleischer AB, Kaplan D, Lebwohl M, Miller B, Pariser D, et al. Clobetasol propionate 0.05% lotion in the treatment of moderate to severe atopic dermatitis: a randomized evaluation versus clobetasol propionate emollient cream. J Drugs Dermatol 2005;4:330-6.
- Woods MT, Brown PA, Baig-Lewis SF, Simpson EL. Effects of a novel formulation of fluocinonide 0.1% cream on skin barrier function in atopic dermatitis. J Drugs Dermatol 2011;10:171-6.
- Chalmers I, Glaziou P. Avoidable waste in the production and reporting of clinical research. Lancet 2009;374:86-9. http://dx.doi.org/10.1016/S0140-6736(09)60329-9.
- Prado de Oliveira ZN, Cuce LC, Arnone M. Comparative evaluation of efficacy, tolerability and safety of 0.1% topical momethasone furoate and 0.05% desonide in the treatment of childhood atopic dermatitis. An Bras Dermatol 2002;77:25-33.
- Kirkup ME, Birchall NM, Weinberg EG, Helm K, Kennedy CT. Acute and maintenance treatment of atopic dermatitis in children – two comparative studies with fluticasone propionate (0.05%) cream. J Dermatol Treat 2003;14:141-8. http://dx.doi.org/10.1080/09546630310013388.
- Lebrun-Vignes B, Legrain V, Amoric J, Taieb A. Comparison of efficacy of 0.1 p. 100 micronized desonide cream and 0.05 p. 100 methasone dipropionate cream in the treatment of atopic dermatitis of childhood and effect on plasma cortisol levels. Ann Dermatol Venereol 2000;127:590-5.
- Wong AWK, Hon EKL, Zee B. Is topical antimycotic treatment useful as adjuvant therapy for flexural atopic dermatitis: randomized, double-blind, controlled trial using one side of the elbow or knee as a control. Int J Dermatol 2008;47:187-91. http://dx.doi.org/10.1111/j.1365-4632.2008.03414.x.
- Thomas KS, Armstrong S, Avery A, Po ALW, O’Neill C, Young S, et al. Randomised controlled trial of short bursts of a potent topical corticosteroid versus prolonged use of a mild preparation for children with mild or moderate atopic eczema. BMJ 2002;324:768-71. http://dx.doi.org/10.1136/bmj.324.7340.768.
- Schlessinger J, Miller B, Gilbert RD, Plott RT, Vanos Study Group. An open-label adrenal suppression study of 0.1% fluocinonide cream in pediatric patients with atopic dermatitis. Arch Dermatol 2006;142:1568-72. http://dx.doi.org/10.1001/archderm.142.12.1568.
- Cato A, Swinehart JM, Griffin EI, Sutton L, Kaplan AS. Azone enhances clinical effectiveness of an optimized formulation of triamcinolone acetonide in atopic dermatitis. Int J Dermatol 2001;40:232-6. http://dx.doi.org/10.1046/j.1365-4362.2001.01161.x.
- Ruzicka T, Willers C, Wigger-Alberti W. Efficacy and patient-reported outcomes of a new mometasone cream treating atopic eczema. Skin Pharmacol Physiol 2012;25:305-12. http://dx.doi.org/10.1159/000341809.
- Trookman NS, Rizer RL. Randomized controlled trial of desonlde hydrogel 0.05% versus desonide ointment 0.05% in the treatment of mild-to-moderate atopic dermatitis. J Clin Aesthet Dermatol 2011;4:34-8.
- Kim DH, Lee HJ, Park CW, Kim KH, Lee KH, Ro BI, et al. The clinical efficacy of mometasone furoate in multi-lamellar emulsion for eczema: a double-blinded crossover study. Ann Dermatol 2013;25:17-22. http://dx.doi.org/10.5021/ad.2013.25.1.17.
- Sillevis Smitt JH, Spuls PI, van Leent EJ, de Vries HJ, Mulder PG, Glazenburg EJ, et al. Should children with constitutional eczema be treated with corticosteroids continuously or in pulse? A prospective, randomised, double blind study with clobetason butyrate. Nederland Tijdschr Dermatol Venereol 2000;10:204-5.
- Msika P, De Belilovsky C, Piccardi N, Chebassier N, Baudouin C, Chadoutaud B. New emollient with topical corticosteroid-sparing effect in treatment of childhood atopic dermatitis: SCORAD and quality of life improvement. Pediatr Dermatol 2008;25:606-12. http://dx.doi.org/10.1111/j.1525-1470.2008.00783.x.
- Griffiths CE, Van Leent EJ, Gilbert M, Traulsen J. Cipamyflline Study Group . Randomized comparison of the type 4 phosphodiesterase inhibitor cipamfylline cream, cream vehicle and hydrocortisone 17-butyrate cream for the treatment of atopic dermatitis. Br J Dermatol 2002;147:299-307. http://dx.doi.org/10.1046/j.1365-2133.2002.04894.x.
- Brenninkmeijer EE, Spuls PI, Lindeboom R, van der Wal AC, Bos JD, Wolkerstorfer A. Excimer laser vs. clobetasol propionate 0.05% ointment in prurigo form of atopic dermatitis: a randomized controlled trial, a pilot. Br J Dermatol 2010;163:823-31. http://dx.doi.org/10.1111/j.1365-2133.2010.09858.x.
- Sugarman JL, Parish LC. Efficacy of a lipid-based barrier repair formulation in moderate-to-severe pediatric atopic dermatitis. J Drugs Dermatol 2009;8:1106-11.
- Paller A, Eichenfield LF, Leung DY, Stewart D, Appell M. A 12-week study of tacrolimus ointment for the treatment of atopic dermatitis in pediatric patients. J Am Acad Dermatol 2001;44:47-5. http://dx.doi.org/10.1067/mjd.2001.109813.
- Hanifin JM, Ling MR, Langley R, Breneman D, Rafal E. Tacrolimus ointment for the treatment of atopic dermatitis in adult patients: part I, efficacy. J Am Acad Dermatol 2001;44:28-3. http://dx.doi.org/10.1067/mjd.2001.109810.
- Chapman MS, Schachner LA, Breneman D, Boguniewicz M, Gold MH, Shull T, et al. Tacrolimus ointment 0.03% shows efficacy and safety in pediatric and adult patients with mild to moderate atopic dermatitis. J Am Acad Dermatol 2005;53:177-85. http://dx.doi.org/10.1016/j.jaad.2005.04.061.
- Dou X, Liu L, Xie Z, Chen L, Li L, Feng S, et al. The impact of tacrolimus ointment on health-related quality of life of Chinese adult and pediatric patients with atopic dermatitis. J Clin Dermatol 2006;35:50-2.
- Otsuki M, Kawashima M, Shibata Y, Nakagawa H, Harada S. Efficacy and safety of FK506 (tacrolimus) ointment in children with atopic dermatitis: Phase III double-blinded comparison with vehicle ointment. J Clin Therapeut Med 2003;19:569-95.
- Rahman MF, Rashid MM, Sikder AU, Akhtar N, Banu LA, Wahab MA, et al. Efficacy of topical tacrolimus in atopic dermatitis. J Pakistan Assoc Dermatol 2008;18:84-92.
- Breneman D, Fleischer AB, Abramovits W, Zeichner J, Gold MH, Kirsner RS, et al. Intermittent therapy for flare prevention and long-term disease control in stabilized atopic dermatitis: a randomized comparison of 3-times-weekly applications of tacrolimus ointment versus vehicle. J Am Acad Dermatol 2008;58:990-9. http://dx.doi.org/10.1016/j.jaad.2008.02.008.
- Thaci D, Reitamo S, Gonzalez Ensenat MA, Moss C, Boccaletti V, Cainelli T, et al. Proactive disease management with 0.03% tacrolimus ointment for children with atopic dermatitis: results of a randomized, multicentre, comparative study. Br J Dermatol 2008;159:1348-56. http://dx.doi.org/10.1111/j.1365-2133.2008.08813.x.
- Wollenberg A, Reitamo S, Girolomoni G, Lahfa M, Ruzicka T, Healy E, et al. Proactive treatment of atopic dermatitis in adults with 0.1% tacrolimus ointment. Allergy 2008;63:742-50. http://dx.doi.org/10.1111/j.1398-9995.2008.01683.x.
- Granlund H, Remitz A, Kyllonen H, Lauerma AI, Reitamo S. Treatment of lichenified atopic eczema with tacrolimus ointment. Acta Derm Venereol 2001;81:314-15. http://dx.doi.org/10.1080/00015550152573083.
- Takeuchi S, Saeki H, Tokunaga S, Sugaya M, Ohmatsu H, Tsunemi Y, et al. A randomized, open-label, multicenter trial of topical tacrolimus for the treatment of pruritis in patients with atopic dermatitis. Ann Dermatol 2012;24:144-50. http://dx.doi.org/10.5021/ad.2012.24.2.144.
- Reitamo S, Rustin M, Ruzicka T, Cambazard F, Kalimo K, Friedmann PS, et al. Efficacy and safety of tacrolimus ointment compared with that of hydrocortisone butyrate ointment in adult patients with atopic dermatitis. J Allergy Clin Immunol 2002;109:547-55. http://dx.doi.org/10.1067/mai.2002.121832.
- Reitamo S, Harper J, Bos JD, Cambazard F, Bruijnzeel-Koomen C, Valk P, et al. 0.03% Tacrolimus ointment applied once or twice daily is more efficacious than 1% hydrocortisone acetate in children with moderate to severe atopic dermatitis: results of a randomized double-blind controlled trial. Br J Dermatol 2004;150:554-62. http://dx.doi.org/10.1046/j.1365-2133.2004.05782.x.
- Reitamo S, Ortonne JP, Sand C, Cambazard F, Bieber T, Fölster-Holst R, et al. A multicentre, randomized, double-blind, controlled study of long-term treatment with 0.1% tacrolimus ointment in adults with moderate to severe atopic dermatitis. Br J Dermatol 2005;152:1282-9. http://dx.doi.org/10.1111/j.1365-2133.2005.06592.x.
- Caproni M, Torchia D, Antiga E, Terranova M, Volpi W, del Bianco E, et al. The comparative effects of tacrolimus and hydrocortisone in adult atopic dermatitis: an immunohistochemical study. Br J Dermatol 2007;156:312-19. http://dx.doi.org/10.1111/j.1365-2133.2006.07609.x.
- Antiga E, Volpi W, Torchia D, Fabbri P, Caproni M. Effects of tacrolimus ointment on Toll-like receptors in atopic dermatitis. Clin Exp Dermatol 2011;36:235-41. http://dx.doi.org/10.1111/j.1365-2230.2010.03948.x.
- Mandelin J, Remitz A, Virtanen H, Reitamo S. One-year treatment with 0.1% tacrolimus ointment versus a corticosteroid regimen in adults with moderate to severe atopic dermatitis: a randomized, double-blind, comparative trial. Acta Derm Venereol 2010;90:170-4. http://dx.doi.org/10.2340/00015555-0803.
- Nivenius E, van der Ploeg I, Jung K, Chryssanthou E, van Hage M, Montan PG. Tacrolimus ointment vs. steroid ointment for eyelid dermatitis in patients with atopic keratoconjunctivitis. Eye (Lond) 2007;21:968-75. http://dx.doi.org/10.1038/sj.eye.6702367.
- Gradman J, Wolthers OD. Short-term growth in children with eczema during treatment with topical mometasone furoate and tacrolimus. Acta Paediatr 2007;96:1233-7. http://dx.doi.org/10.1111/j.1651-2227.2007.00363.x.
- Doss N, Reitamo S, Dubertret L, Fekete GL, Kamoun MR, Lahfa M, et al. Superiority of tacrolimus 0.1% ointment compared with fluticasone 0.005% in adults with moderate to severe atopic dermatitis of the face: results from a randomized, double-blind trial. Br J Dermatol 2009;161:427-34. http://dx.doi.org/10.1111/j.1365-2133.2009.09143.x.
- Doss N, Kamoun MR, Dubertret L, Cambazard F, Remitz A, Lahfa M, et al. Efficacy of tacrolimus 0.03% ointment as second-line treatment for children with moderate-to-severe atopic dermatitis: evidence from a randomized, double-blind non-inferiority trial vs. fluticasone 0.005% ointment. Pediatr Allergy Immunol 2010;21:321-9. http://dx.doi.org/10.1111/j.1399-3038.2009.00895.x.
- Bieber T, Vick K, Fölster-Holst R, Belloni-Fortina A, Städtler G, Worm M, et al. Efficacy and safety of methylprednisolone aceponate ointment 0.1% compared to tacrolimus 0.03% in children and adolescents with an acute flare of severe atopic dermatitis. Allergy 2007;62:184-9. http://dx.doi.org/10.1111/j.1398-9995.2006.01269.x.
- Neumann E, Amtage D, Bruckner-Tuderman L, Mockenhaupt M. A single-center open-label long-term comparison of tacrolimus ointment and topical corticosteroids for treatment of atopic dermatitis. J Dtsch Dermatol Ges 2008;6:548-53. http://dx.doi.org/10.1111/j.1610-0387.2008.06641.x.
- Hung SH, Lin YT, Chu CY, Lee CC, Liang TC, Yang YH, et al. Staphylococcus colonization in atopic dermatitis treated with fluticasone or tacrolimus with or without antibiotics. Ann Allergy Asthma Immunol 2007;98:51-6. http://dx.doi.org/10.1016/S1081-1206(10)60859-9.
- Pacor ML, Di Lorenzo G, Martinelli N, Mansueto P, Rini GB, Corrocher R. Comparing tacrolimus ointment and oral cyclosporine in adult patients affected by atopic dermatitis: a randomized study. Clin Exp Allergy 2004;34:639-45. http://dx.doi.org/10.1111/j.1365-2222.2004.1907.x.
- Reitamo S, Van Leent EJ, Ho V, Harper J, Ruzicka T, Kalimo K, et al. J Allergy Clin Immunol 2002;109:539-46. http://dx.doi.org/10.1067/mai.2002.121831.
- Zuberbier T, Heinzerling L, Bieber T, Schauer U, Klebs S, Brautigam M. Steroid-sparing effect of pimecrolimus cream 1% in children with severe atopic dermatitis. Dermatology 2007;215:325-30. http://dx.doi.org/10.1159/000107627.
- Sigurgeirsson B, Ho V, Ferrandiz C, Andriano K, Grinienko A, Jimenez P, et al. Effectiveness and safety of a prevention-of-flare-progression strategy with pimecrolimus cream 1% in the management of paediatric atopic dermatitis. J Eur Acad Dermatol Venereol 2008;22:1290-301. http://dx.doi.org/10.1111/j.1468-3083.2008.02785.x.
- Wahn U, Bos JD, Goodfield M, Caputo R, Papp K, Manjra A, et al. Efficacy and safety of pimecrolimus cream in the long-term management of atopic dermatitis in children. Pediatrics 2002;110. http://dx.doi.org/10.1542/peds.110.1.e2.
- Meurer M, Fölster-Holst R, Wozel G, Weidinger G, Jünger M, Bräutigam M, et al. Pimecrolimus cream in the long-term management of atopic dermatitis in adults: a six-month study. Dermatology 2002;205:271-7. http://dx.doi.org/10.1159/000065863.
- Salavec M, Buckova H. First experiences with 1% pimecrolimus cream therapy in prevention of atopic eczema flares in children. Čes-Slov Derm 2004;79:3-7.
- Gollnick H, Kaufmann R, Stough D, Heikkila H, Andriano K, Grinienko A, et al. Pimecrolimus cream 1% in the long-term management of adult atopic dermatitis: prevention of flare progression. A randomized controlled trial. Br J Dermatol 2008;158:1083-93. http://dx.doi.org/10.1111/j.1365-2133.2008.08484.x.
- Hoeger PH, Lee KH, Jautova J, Wohlrab J, Guettner A, Mizutani G, et al. The treatment of facial atopic dermatitis in children who are intolerant of, or dependent on, topical corticosteroids: a randomized, controlled clinical trial. Br J Dermatol 2009;160:415-22. http://dx.doi.org/10.1111/j.1365-2133.2008.08928.x.
- Murrell DF, Calvieri S, Ortonne JP, Ho VC, Weise-Riccardi S, Barbier N, et al. A randomized controlled trial of pimecrolimus cream 1% in adolescents and adults with head and neck atopic dermatitis and intolerant of, or dependent on, topical corticosteroids. Br J Dermatol 2007;157:954-9. http://dx.doi.org/10.1111/j.1365-2133.2007.08192.x.
- Leung DY, Hanifin JM, Pariser DM, Barber KA, Langley RG, Schlievert PM, et al. Effects of pimecrolimus cream 1% in the treatment of patients with atopic dermatitis who demonstrate a clinical insensitivity to topical corticosteroids: a randomized, multicentre vehicle-controlled trial. Br J Dermatol 2009;161:435-43. http://dx.doi.org/10.1111/j.1365-2133.2009.09145.x.
- Ho VC, Gupta A, Kaufmann R, Todd G, Vanaclocha F, Takaoka R, et al. Safety and efficacy of nonsteroid pimecrolimus cream 1% in the treatment of atopic dermatitis in infants. J Pediatr 2003;142:155-62. http://dx.doi.org/10.1067/mpd.2003.65.
- Eichenfield LF, Lucky AW, Boguniewicz M, Langley RG, Cherill R, Marshall K, et al. Safety and efficacy of pimecrolimus (ASM 981) cream 1% in the treatment of mild and moderate atopic dermatitis in children and adolescents. J Am Acad Dermatol 2002;46:495-504. http://dx.doi.org/10.1067/mjd.2002.122187.
- Kaufmann R, Fölster-Holst R, Höger P, Thaçi D, Löffler H, Staab D, et al. Onset of action of pimecrolimus cream 1% in the treatment of atopic eczema in infants. J Allergy Clin Immunol 2004;114:1183-8. http://dx.doi.org/10.1016/j.jaci.2004.08.015.
- Kaufmann R, Bieber T, Helgesen AL, Andersen BL, Luger T, Poulin Y, et al. Onset of pruritus relief with pimecrolimus cream 1% in adult patients with atopic dermatitis: a randomized trial. Allergy 2006;61:375-81. http://dx.doi.org/10.1111/j.1398-9995.2005.00977.x.
- Fowler J, Johnson A, Chen M, Abrams K. Improvement in pruritus in children with atopic dermatitis using pimecrolimus cream 1%. Cutis 2007;79:65-72.
- Leo HL, Bender BG, Leung SB, Tran ZV, Leung DY. Effect of pimecrolimus cream 1% on skin condition and sleep disturbance in children with atopic dermatitis. J Allergy Clin Immunol 2004;114:691-3. http://dx.doi.org/10.1016/j.jaci.2004.05.037.
- Aschoff R, Schwanebeck U, Brautigam M, Meurer M. Skin physiological parameters confirm the therapeutic efficacy of pimecrolimus cream 1% in patients with mild-to-moderate atopic dermatitis. Exp Dermatol 2009;18:24-9. http://dx.doi.org/10.1111/j.1600-0625.2008.00756.x.
- Bangert C, Strober BE, Cork M, Ortonne JP, Luger T, Bieber T, et al. Clinical and cytological effects of pimecrolimus cream 1% after resolution of active atopic dermatitis lesions by topical corticosteroids: a randomized controlled trial. Dermatology 2011;222:36-48. http://dx.doi.org/10.1159/000321711.
- Luger T, Van Leent EJ, Graeber M, Hedgecock S, Thurston M, Kandra A, et al. SDZ ASM 981: an emerging safe and effective treatment for atopic dermatitis. Br J Dermatol 2001;144:788-94. http://dx.doi.org/10.1046/j.1365-2133.2001.04134.x.
- Jensen JM, Pfeiffer S, Witt M, Brautigam M, Neumann C, Weichenthal M, et al. Different effects of pimecrolimus and betamethasone on the skin barrier in patients with atopic dermatitis. J Allergy Clin Immunol 2009;123:1124-33. http://dx.doi.org/10.1016/j.jaci.2009.03.032.
- Luger TA, Lahfa M, Fölster-Holst R, Gulliver WP, Allen R, Molloy S, et al. Long-term safety and tolerability of pimecrolimus cream 1% and topical corticosteroids in adults with moderate to severe atopic dermatitis. J Dermatolog Treat 2004;15:169-78. http://dx.doi.org/10.1080/09546630410033781.
- Frankel A, Sohn A, Patel RV, Lebwohl M. Bilateral comparison study of pimecrolimus cream 1% and a ceramide-hyaluronic acid emollient foam in the treatment of patients with atopic dermatitis. J Drugs Dermatol 2011;10:666-72.
- Emer JJ, Frankel A, Sohn A, Lebwohl M. A bilateral comparison study of pimecrolimus cream 1% and a topical medical device cream in the treatment of patients with atopic dermatitis. J Drugs Dermatol 2011;10:735-43.
- Paller AS, Lebwohl M, Fleischer AB, Antaya R, Langley RG, Kirsner RS, et al. Tacrolimus ointment is more effective than pimecrolimus cream with a similar safety profile in the treatment of atopic dermatitis: results from 3 randomized, comparative studies. J Am Acad Dermatol 2005;52:810-22. http://dx.doi.org/10.1016/j.jaad.2004.12.038.
- Kempers S, Boguniewicz M, Carter E, Jarratt M, Pariser D, Stewart D, et al. A randomized investigator-blinded study comparing pimecrolimus cream 1% with tacrolimus ointment 0.03% in the treatment of pediatric patients with moderate atopic dermatitis. J Am Acad Dermatol 2004;51:515-25. http://dx.doi.org/10.1016/j.jaad.2004.01.051.
- Draelos Z, Nayak A, Pariser D, Shupack JL, Chon K, Abrams B, et al. Pharmacokinetics of topical calcineurin inhibitors in adult atopic dermatitis: a randomized, investigator-blind comparison. J Am Acad Dermatol 2005;53:602-9. http://dx.doi.org/10.1016/j.jaad.2005.06.013.
- Ruer-Mulard M, Aberer W, Gunstone A, Kekki OM, Lopez Estebaranz JL, Vertruyen A, et al. Twice-daily versus once-daily applications of pimecrolimus cream 1% for the prevention of disease relapse in pediatric patients with atopic dermatitis. Pediatr Dermatol 2009;26:551-8. http://dx.doi.org/10.1111/j.1525-1470.2009.00981.x.
- Reitamo S, Mandelin J, Rubins A, Remitz A, Makela M, Cirule K, et al. The pharmacokinetics of tacrolimus after first and repeated dosing with 0.03% ointment in infants with atopic dermatitis. Int J Dermatol 2009;48:348-55. http://dx.doi.org/10.1111/j.1365-4632.2009.03853.x.
- Ling M, Gottlieb A, Pariser D, Caro I, Stewart D, Scott G, et al. A randomized study of the safety, absorption and efficacy of pimecrolimus cream 1% applied twice or four times daily in patients with atopic dermatitis. J Dermatolog Treat 2005;16:142-8. http://dx.doi.org/10.1080/09546630510033159.
- Meurer M, Eichenfield LF, Ho V, Potter PC, Werfel T, Hultsch T. Addition of pimecrolimus cream 1% to a topical corticosteroid treatment regimen in paediatric patients with severe atopic dermatitis: a randomized, double-blind trial. J Dermatolog Treat 2010;21:157-66. http://dx.doi.org/10.3109/09546630903410158.
- Hebert AA, Koo J, Fowler J, Berman B, Rosenberg C, Levitt J. Desoximetasone 0.25% and tacrolimus 0.1% ointments versus tacrolimus alone in the treatment of atopic dermatitis. Cutis 2006;78:357-63.
- Foelster-Holst R, Nagel F, Zoellner P, Spaeth D. Efficacy of crisis intervention treatment with topical corticosteroid prednicarbat with and without partial wet-wrap dressing in atopic dermatitis. Dermatology 2006;212:66-9. http://dx.doi.org/10.1159/000089025.
- Hindley D, Galloway G, Murray J, Gardener L. A randomised study of ‘wet wraps’ versus conventional treatment for atopic eczema. Arch Dis Child 2006;91:164-8. http://dx.doi.org/10.1136/adc.2004.050831.
- Pei AY, Chan HH, Ho KM. The effectiveness of wet wrap dressings using 0.1% mometasone furoate and 0.005% fluticasone proprionate ointments in the treatment of moderate to severe atopic dermatitis in children. Pediatr Dermatol 2001;18:343-8. http://dx.doi.org/10.1046/j.1525-1470.2001.01952.x.
- Schnopp C, Holtmann C, Stock S, Remling R, Fölster-Holst R, Ring J, et al. Topical steroids under wet-wrap dressings in atopic dermatitis – a vehicle-controlled trial. Dermatology 2002;204:56-9. http://dx.doi.org/10.1159/000051811.
- Beattie PE, Lewis-Jones MS. A pilot study on the use of wet wraps in infants with moderate atopic eczema. Clin Exp Dermatol 2004;29:348-53. http://dx.doi.org/10.1111/j.1365-2230.2004.01583.x.
- Tarr A, Iheanacho I. Should we use bath emollients for atopic eczema?. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b4273.
- Bamford JT, Ray S, Musekiwa A, van Gool C, Humphreys R, Ernst E. Oral evening primrose oil and borage oil for eczema. Cochrane Database Syst Rev 2013;4. http://dx.doi.org/10.1002/14651858.cd004416.pub2.
- Draelos ZD. An evaluation of prescription device moisturizers. J Cosmet Dermatol 2009;8:40-3. http://dx.doi.org/10.1111/j.1473-2165.2009.00422.x.
- Grimalt R, Mengeaud V, Cambazard F. Study Investigators’ Group . The steroid-sparing effect of an emollient therapy in infants with atopic dermatitis: a randomized controlled study. Dermatology 2007;214:61-7. http://dx.doi.org/10.1159/000096915.
- Giordano-Labadie F, Cambazard F, Guillet G, Combemale P, Mengeaud V. Evaluation of a new moisturizer (Exomega milk) in children with atopic dermatitis. J Dermatolog Treat 2006;17:78-81. http://dx.doi.org/10.1080/09546630600552216.
- Loden M, Andersson AC, Andersson C, Frodin T, Oman H, Lindberg M. Instrumental and dermatologist evaluation of the effect of glycerine and urea on dry skin in atopic dermatitis. Skin Res Technol 2001;7:209-13. http://dx.doi.org/10.1034/j.1600-0846.2001.070401.x.
- Loden M, Andersson AC, Anderson C, Bergbrant IM, Frodin T, Ohman H, et al. A double-blind study comparing the effect of glycerin and urea on dry, eczematous skin in atopic patients. Acta Derm Venereol 2002;82:45-7. http://dx.doi.org/10.1080/000155502753600885.
- Bissonnette R, Maari C, Provost N, Bolduc C, Nigen S, Rougier A, et al. A double-blind study of tolerance and efficacy of a new urea-containing moisturizer in patients with atopic dermatitis. J Cosmet Dermatol 2010;9:16-21. http://dx.doi.org/10.1111/j.1473-2165.2010.00476.x.
- Amichai B, Grunwald MH. A randomized, double-blind, placebo-controlled study to evaluate the efficacy in AD of liquid soap containing 12% ammonium lactate + 20% urea. Clin Exp Dermatol 2009;34:e602-4. http://dx.doi.org/10.1111/j.1365-2230.2009.03273.x.
- Wiren K, Nohlgard C, Nyberg F, Holm L, Svensson M, Johannesson A, et al. Treatment with a barrier-strengthening moisturizing cream delays relapse of atopic dermatitis: a prospective and randomized controlled clinical trial. J Eur Acad Dermatol Venereol 2009;23:1267-72. http://dx.doi.org/10.1111/j.1468-3083.2009.03303.x.
- De Belilovsky C, Roo-Rodriguez E, Baudouin C, Menu F, Chadoutaud B, Msika P. Natural peroxisome proliferator-activated receptor-alpha agonist cream demonstrates similar therapeutic response to topical steroids in atopic dermatitis. J Dermatolog Treat 2011;22:359-65. http://dx.doi.org/10.3109/09546634.2010.499932.
- Miller DW, Koch SB, Yentzer BA, Clark AR, O’Neill JR, Fountain J, et al. An over-the-counter moisturizer is as clinically effective as, and more cost-effective than, prescription barrier creams in the treatment of children with mild-to-moderate atopic dermatitis: a randomized, controlled trial. J Drugs Dermatol 2011;10:531-7.
- Berardesca E, Barbareschi M, Veraldi S, Pimpinelli N. Evaluation of efficacy of a skin lipid mixture in patients with irritant contact dermatitis, allergic contact dermatitis or atopic dermatitis: a multicenter study. Contact Dermatitis 2001;45:280-5. http://dx.doi.org/10.1034/j.1600-0536.2001.450505.x.
- Draelos ZD. A clinical evaluation of the comparable efficacy of hyaluronic acid-based foam and ceramide-containing emulsion cream in the treatment of mild-to-moderate atopic dermatitis. J Cosmet Dermatol 2011;10:185-8. http://dx.doi.org/10.1111/j.1473-2165.2011.00568.x.
- Simpson E, Dutronc Y. A new body moisturizer increases skin hydration and improves atopic dermatitis symptoms among children and adults. J Drugs Dermatol 2011;10:744-9.
- Danby SG, Al-Enezi T, Sultan A, Chittock J, Kennedy K, Cork MJ. The effect of aqueous cream BP on the skin barrier in volunteers with a previous history of atopic dermatitis. Br J Dermatol 2011;165:329-34. http://dx.doi.org/10.1111/j.1365-2133.2011.10395.x.
- Centre of Evidence Based Dermatology, University of Nottingham . Barrier Enhancement Eczema Prevention (The BEEP Study) n.d. www.beepstudy.org/ (accessed 6 November 2015).
- Huang JT, Abrams M, Tlougan B, Rademaker A, Paller AS. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics 2009;123:e808-14. http://dx.doi.org/10.1542/peds.2008-2217.
- Shibagaki N, Inozume T, Ando N, Kitamura R, Mizutani M, Nagasaka A, et al. Clinical efficacy of bath additive containing a diamide derivative in patients with atopic dermatitis. Nishi Nihon Hifuka 2005;67:152-9. http://dx.doi.org/10.2336/nishinihonhifu.67.152.
- Tripodi S, Di Rienzo Businco A, Panetta V, Pingitore G, Volterrani A, Frediani T, et al. Lack of efficacy of topical furfuryl palmitate in pediatric atopic dermatitis: a randomized double-blind study. J Investig Allergol Clin Immunol 2009;19:204-9.
- Palombo P, Morganti P, Fabrizi G, Guarneri F, Valenzano F, Feng XZ. A special pill-mask to re-hydrate the skin affected by atopic dermatitis. J Appl Cosmet 2004;22:87-9.
- Stern T, Bayerl C. Black seed oil ointment – a new approach for the treatment of atopic dermatitis?. Aktuelle Dermatol 2002;28:74-9. http://dx.doi.org/10.1055/s-2002-25234.
- Lee J, Jung E, Koh J, Kim YS, Park D. Effect of rosmarinic acid on atopic dermatitis. J Dermatol 2008;35:768-71. http://dx.doi.org/10.1111/j.1346-8138.2008.00565.x.
- Thumm E, Stoss M, Bayerl C, Schurholz T. Randomized trial to study efficacy of a 20% and 10% Hippophae rhamnoides containing creme used by patients with mild to intermediate atopic dermatitis. Aktuelle Dermatol 2000;26:285-90.
- Korting HC, Schollmann C, Cholcha W, Wolff L. Collaborative Study Group . Efficacy and tolerability of pale sulfonated shale oil cream 4% in the treatment of mild to moderate atopic eczema in children: a multicentre, randomized vehicle-controlled trial. J Eur Acad Dermatol Venereol 2010;24:1176-82. http://dx.doi.org/10.1111/j.1468-3083.2010.03616.x.
- Guéniche A, Hennino A, Goujon C, Dahel K, Bastien P, Martin R, et al. Improvement of atopic dermatitis skin symptoms by Vitreoscilla filiformis bacterial extract. Eur J Dermatol 2006;16:380-4.
- Guéniche A, Knaudt B, Schuck E, Volz T, Bastien P, Martin R, et al. Effects of nonpathogenic gram-negative bacterium Vitreoscilla filiformis lysate on atopic dermatitis: a prospective, randomized, double-blind, placebo-controlled clinical study. Br J Dermatol 2008;159:1357-63. http://dx.doi.org/10.1111/j.1365-2133.2008.08836.x.
- Dolle S, Hoser D, Rasche C, Loddenkemper C, Maurer M, Zuberbier T, et al. Long-term reduction in local inflammation by a lipid raft molecule in atopic dermatitis. Allergy 2010;65:1158-65. http://dx.doi.org/10.1111/j.1398-9995.2010.02341.x.
- Bigliardi PL, Stammer H, Jost G, Rufli T, Buchner S, Bigliardi-Qi M. Treatment of pruritus with topically applied opiate receptor antagonist. J Am Acad Dermatol 2007;56:979-88. http://dx.doi.org/10.1016/j.jaad.2007.01.007.
- Stücker M, Pieck C, Stoerb C, Niedner R, Hartung J, Altmeyer P. Topical vitamin B12 – a new therapeutic approach in atopic dermatitis – evaluation of efficacy and tolerability in a randomized placebo-controlled multicentre clinical trial. Br J Dermatol 2004;150:977-83. http://dx.doi.org/10.1111/j.1365-2133.2004.05866.x.
- Januchowski R. Evaluation of topical vitamin B12 for the treatment of childhood eczema. J Altern Complement Med 2009;15:387-9. http://dx.doi.org/10.1089/acm.2008.0497.
- Bissonnette R, Chen G, Bolduc C, Maari C, Lyle M, Tang L, et al. Efficacy and safety of topical WBI-1001 in the treatment of atopic dermatitis: results from a phase 2A, randomized, placebo-controlled clinical trial. Arch Dermatol 2010;146:446-9. http://dx.doi.org/10.1001/archdermatol.2010.34.
- Bissonnette R, Poulin Y, Zhou Y, Tan J, Hong HC, Webster J, et al. Efficacy and safety of topical WBI-1001 in patients with mild to severe atopic dermatitis: results from a 12-week, multicentre, randomized, placebo-controlled double-blind trial. Br J Dermatol 2012;166:853-60. http://dx.doi.org/10.1111/j.1365-2133.2011.10775.x.
- Gandy JJ, Snyman JR, van Rensburg CE. Randomized, parallel-group, double-blind, controlled study to evaluate the efficacy and safety of carbohydrate-derived fulvic acid in topical treatment of eczema. Clin Cosmet Investig Dermatol 2011;4:145-8. http://dx.doi.org/10.2147/CCID.S23110.
- Foelster-Holst R, Reitamo S, Yankova R, Worm M, Kadurina M, Thaci D, et al. The novel protease inhibitor SRD441 ointment is not effective in the treatment of adult subjects with atopic dermatitis: results of a randomized, vehicle-controlled study. Allergy 2010;65:1594-9. http://dx.doi.org/10.1111/j.1398-9995.2010.02417.x.
- Misery L, Liege P, Cambazard F. Evaluation of efficacy and tolerance of a cream containing raffinose during atopic dermatitis. Nouvelles Dermatol 2005;24:339-41.
- Belloni G, Pinelli S, Veraldi S. A randomised, double-blind, vehicle-controlled study to evaluate the efficacy and safety of MAS063D (Atopiclair) in the treatment of mild to moderate atopic dermatitis. Eur J Dermatol 2005;15:31-6.
- Abramovits W, Boguniewicz M. Adult Atopiclair Study Group . A multicenter, randomized, vehicle-controlled clinical study to examine the efficacy and safety of MAS063DP (Atopiclair) in the management of mild to moderate atopic dermatitis in adults. J Drugs Dermatol 2006;5:236-44.
- Patrizi A, Capitanio B, Neri I, Giacomini F, Sinagra JL, Raone B, et al. A double-blind, randomized, vehicle-controlled clinical study to evaluate the efficacy and safety of MAS063DP (Atopiclair) in the management of atopic dermatitis in paediatric patients. Pediatr Allergy Immunol 2008;19:619-25. http://dx.doi.org/10.1111/j.1399-3038.2008.00724.x.
- Boguniewicz M, Zeichner JA, Eichenfield LF, Hebert AA, Jarratt M, Lucky AW, et al. MAS063DP is effective monotherapy for mild to moderate atopic dermatitis in infants and children: a multicenter, randomized, vehicle-controlled study. J Pediatr 2008;152:854-9. http://dx.doi.org/10.1016/j.jpeds.2007.11.031.
- Rajka G, Langeland T. Grading of the severity of atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1989;144:13-4.
- Katsuyama M, Kobayashi Y, Ichikawa H, Mizuno A, Miyachi Y, Matsunaga K, et al. A novel method to control the balance of skin microflora Part 2. A study to assess the effect of a cream containing farnesol and xylitol on atopic dry skin. J Dermatol Sci 2005;38:207-13.
- Arenberger P, Drozenová H, Hladícova M, Holcova S. Additive effect of heparin and levomenol against atopic dermatitis. Aktuelle Dermatol 2010;36:217-21. http://dx.doi.org/10.1055/s-0029-1244081.
- Arenberger P, Arenbergerova M, Drozenová H, Hladíkova M, Holcova S. Effect of topical heparin and levomenol on atopic dermatitis: a randomized four-arm, placebo-controlled, double-blind clinical study. J Eur Acad Dermatol Venereol 2011;25:688-94. http://dx.doi.org/10.1111/j.1468-3083.2010.03950.x.
- Mora R, Bellussi L, Passali FM, Crippa B, Mora F, Cordone MP, et al. Efficacy of a topical suspension of bacterial antigens for the management of recurrent eczema in children. Med Sci Monit 2004;10.
- Patzelt-Wenczler R, Ponce-Poschl E. Proof of efficacy of Kamillosan(R) cream in atopic eczema. Eur J Med Res 2000;5:171-5.
- Hamada M, Gyoutoku T, Sato S, Matsuda T, Kinukawa N, Furue M. The usefulness of camellia oil spray for treatment of atopic dermatitis. Nishi Nihon Hifuka 2008;70:213-18. http://dx.doi.org/10.2336/nishinihonhifu.70.213.
- Wu S-H, Chen X-Q, Liu B, Wu H-J, Dong L. Efficacy and safety of 15(R/S)-methyl-lipoxin A4 in topical treatment of infantile eczema. Br J Dermatol 2013;168:172-8. http://dx.doi.org/10.1111/j.1365-2133.2012.11177.x.
- Hashizume E, Nakano T, Kamimura A, Morishita K. Topical effects of N-acetyl-L-hydroxyproline on ceramide synthesis and alleviation of pruritus. Clin Cosmet Investig Dermatol 2013;6:43-9. http://dx.doi.org/10.2147/CCID.S39370.
- Herzog JL, Solomon JA, Draelos Z, Fleischer A, Stough D, Wolf DI, et al. A randomized, double-blind, vehicle-controlled crossover study to determine the anti-pruritic efficacy, safety and local dermal tolerability of a topical formulation (SRD174 cream) of the long-acting opiod antagonist nalmefene in subjects with atopic dermatitis. J Drugs Dermatol 2011;10:853-60.
- Udompataikul M, Srisatwaja W. Comparative trial of moisturizer containing licochalcone A vs. hydrocortisone lotion in the treatment of childhood atopic dermatitis: a pilot study. J Eur Acad Dermatol Venereol 2011;25:660-5. http://dx.doi.org/10.1111/j.1468-3083.2010.03845.x.
- Patrizi A, Raone B, Raboni R, Neri I. Efficacy and tolerability of a cream containing AR-GG27(R) (sorbityl furfural palmitate) in the treatment of mild/moderate childhood atopic dermatitis associated with pityriasis alba. A double-blind, placebo-controlled clinical trial. G Ital Dermatol Venereol 2012;147:1-8.
- Cho SH, Strickland I, Boguniewicz M, Leung DY. Fibronectin and fibrinogen contribute to the enhanced binding of Staphylococcus aureus to atopic skin. J Allergy Clin Immunol 2001;108:269-74. http://dx.doi.org/10.1067/mai.2001.117455.
- Cho SH, Strickland I, Tomkinson A, Fehringer A, Gelfand E, Leung D. Preferential binding of Staphylococcus aureus to skin sites of Th2-mediated inflammation in a murine model. J Investig Dermatol 2001;116:658-63. http://dx.doi.org/10.1046/j.0022-202x.2001.01331.x.
- Lin YT, Wang CT, Chiang BL. Role of bacterial pathogens in atopic dermatitis. Clin Rev Allergy Immunol 2007;33:167-77. http://dx.doi.org/10.1007/s12016-007-0044-5.
- Schommer NN, Gallo RL. Structure and function of the human skin microbiome. Trends Microbiol 2013;21:660-8. http://dx.doi.org/10.1016/j.tim.2013.10.001.
- Birnie AJ, Bath-Hextall FJ, Ravenscroft JC, Williams HC. Interventions to reduce Staphylococcus aureus in the management of atopic eczema. Cochrane Database Syst Rev 2008;3. http://dx.doi.org/10.1002/14651858.cd003871.pub2.
- Bath-Hextall FJ, Birnie AJ, Ravenscroft JC, Williams HC. Interventions to reduce Staphylococcus aureus in the management of atopic eczema: an updated Cochrane review. Br J Dermatol 2010;163:12-26. http://dx.doi.org/10.1111/j.1365-2133.2010.09743.x.
- Ravenscroft JC, Layton AM, Eady EA, Murtagh MS, Coates P, Walker M, et al. Short-term effects of topical fusidic acid or mupirocin on the prevalence of fusidic acid resistant (FusR) Staphylococcus aureus in atopic eczema. Br J Dermatol 2003;148:1010-17. http://dx.doi.org/10.1046/j.1365-2133.2003.05292.x.
- Gong JQ, Lin L, Lin T, Hao F, Zeng FQ, Bi ZG, et al. Skin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: a double-blind multicentre randomized controlled trial. Br J Dermatol 2006;155:680-7. http://dx.doi.org/10.1111/j.1365-2133.2006.07410.x.
- Canpolat F, Erkocoglu M, Tezer H, Kocabas CN, Kandi B. Hydrocortisone acetate alone or combined with mupirocin for atopic dermatitis in infants under two years of age – a randomized double blind pilot trial. Eur Rev Med Pharmacol Sci 2012;16:1989-93.
- Costa C, Rilliet A, Nicolet M, Saurat JH. Scoring atopic dermatitis: the simpler the better?. Acta Derm Venereol 1989;69:41-5.
- Schuttelaar ML, Coenraads PJ. A randomized, double-blind study to assess the efficacy of addition of tetracycline to triamcinolone acetonide in the treatment of moderate to severe atopic dermatitis. J Eur Acad Dermatol Venereol 2008;22:1076-82. http://dx.doi.org/10.1111/j.1468-3083.2008.02716.x.
- Capella GL, Grigerio E, Altomare G. A randomized trial of leukotriene receptor antagonist montelukast in moderate-to-severe atopic dermatitis of adults. Eur J Dermatol 2001;11:209-13.
- Tan WP, Suresh S, Tey HL, Chiam LY, Goon AT. A randomized double-blind controlled trial to compare a triclosan-containing emollient with vehicle for the treatment of atopic dermatitis. Clin Exp Dermatol 2010;35:e109-12. http://dx.doi.org/10.1111/j.1365-2230.2009.03719.x.
- Breneman DL, Hanifin JM, Berge CA, Keswick BH, Neumann PB. The effect of antibacterial soap with 1.5% triclocarban on Staphylococcus aureus in patients with atopic dermatitis. Cutis 2000;66:296-300.
- European Medicinal Agency . European Medicines Agency Recommends Suspension of Marketing Authorisations for Oral Ketoconazole. Benefit of Oral Ketoconazole Does Not Outweigh Risk of Liver Injury in Fungal Infections 2013. www.ema.europa.eu/docs/en_GB/document_library/Press_release/2013/07/WC500146613.pdf.
- Lintu P, Savolainen J, Kortekangas-Savolainen O, Kalimo K. Systemic ketoconazole is an effective treatment of atopic dermatitis with IgE-mediated hypersensitivity to yeasts. Allergy 2001;56:512-17. http://dx.doi.org/10.1034/j.1398-9995.2001.056006512.x.
- Back O, Bartosik J. Systemic ketoconazole for yeast allergic patients with atopic dermatitis. J Eur Acad Dermatol Venereol 2001;15:34-8. http://dx.doi.org/10.1046/j.1468-3083.2001.00207.x.
- Emerson RM, Charman CR, Williams HC. The Nottingham Eczema Severity Score: preliminary refinement of the Rajka and Langeland grading. Br J Dermatol 2000;142:288-97. http://dx.doi.org/10.1046/j.1365-2133.2000.03300.x.
- Svejgaard E, Larsen PØ, Deleuran M, Ternowitz T, Roed-Petersen J, Nilsson J. Treatment of head and neck dermatitis comparing itraconazole 200 mg and 400 mg daily for 1 week with placebo. J Eur Acad Dermatol Venereol 2004;18:445-9. http://dx.doi.org/10.1111/j.1468-3083.2004.00963.x.
- Mayser P, Kupfer J, Nemetz D, Schafer U, Nilles M, Hort W, et al. Treatment of head and neck dermatitis with ciclopiroxolamine cream – results of a double-blind, placebo-controlled study. Skin Pharmacol Physiol 2006;19:153-8. http://dx.doi.org/10.1159/000092596.
- Diepgen TL. Early Treatment of the Atopic Child Study Group . Long-term treatment with cetirizine of infants with atopic dermatitis: a multi-country, double-blind, randomized, placebo-controlled trial (the ETAC trial) over 18 months. Pediatr Allergy Immunol 2002;13:278-86. http://dx.doi.org/10.1034/j.1399-3038.2002.01047.x.
- Wahn U. on behalf of the ETAC® study group. Allergic factors associated with the development of asthma and the influence of cetirizine in a double-blind, randomised, placebo-controlled trial: first results of ETAC®. Pediatr Allergy Immunol 1998;9:116-24. http://dx.doi.org/10.1111/j.1399-3038.1998.tb00356.x.
- Shi YJ, Zhang CM, Ma DM. Clinical study on treatment of atopic dermatitis by integrated traditional Chinese and Western medicine. Zhongguo Zhong Xi Yi Jie He Za Zhi 2008;28:686-8.
- Kawashima M, Tango T, Noguchi T, Inagi M, Nakagawa H, Harada S. Addition of fexofenadine to a topical corticosteroid reduces the pruritus associated with atopic dermatitis in a 1-week randomized, multicentre, double-blind, placebo-controlled, parallel-group study. Br J Dermatol 2003;148:1212-21. http://dx.doi.org/10.1046/j.1365-2133.2003.05293.x.
- Nakagawa H, Kawashima M. Efficacy and safety of fexofenadine hydrochloride in pediatric patients with atopic dermatitis in a phase III, randomized, double-blind, multi-center comparative study. Nishi Nihon Hifuka 2006;68:553-65. http://dx.doi.org/10.2336/nishinihonhifu.68.553.
- Epinastine Hydrochloride Dry Syrup Clinical Study Group . [Phase III study on the efficacy and safety of epinastine hydrochloride dry syrup in pediatric patients with atopic dermatitis – double-blind comparative study of epinastine hyderochloride dry syrup with ketotifen fumarate dry syrup.]. Nishinihon J Dermatol 2004;66:60-79. http://dx.doi.org/10.2336/nishinihonhifu.66.60.
- Kawashima M, Nakagawa H. Olopatadine hydrochloride in children: evidenced efficacy and safety for atopic dermatitis treatment in a randomized, multicenter, double-blind, parallel group comparative study. Nishi Nihon Hifuka 2011;73:278-89. http://dx.doi.org/10.2336/nishinihonhifu.73.278.
- Munday J, Bloomfield R, Goldman M, Robey H, Kitowska GJ, Gwiezdziski Z, et al. Chlorpheniramine is no more effective than placebo in relieving the symptoms of childhood atopic dermatitis with a nocturnal itching and scratching component. Dermatology 2002;205:40-5. http://dx.doi.org/10.1159/000063138.
- Lee HJ, Park CO, Lee JH, Lee KH. The antipruritic effect of topical doxepin cream in patients with atopic dermatitis. Korean J Dermatol 2006;44:309-14.
- Drake LA, Cohen L, Gillies R, Flood JG, Riordan AT, Phillips SB, et al. Pharmacokinetics of doxepin in subjects with pruritic atopic dermatitis. J Am Acad Dermatol 1999;41:209-14. http://dx.doi.org/10.1016/S0190-9622(99)70051-4.
- Drake LA, Fallon JD, Sober A. Relief of pruritus in patients with atopic dermatitis after treatment with topical doxepin cream. The Doxepin Study Group. J Am Acad Dermatol 1994;31:613-16. http://dx.doi.org/10.1016/S0190-9622(94)70225-X.
- Stainer R, Matthews S, Arshad SH, McDonald S, Robinson J, Schapira C, et al. Efficacy and acceptability of a new topical skin lotion of sodium cromoglicate (Altoderm) in atopic dermatitis in children aged 2–12 years: a double-blind, randomized, placebo-controlled trial. Br J Dermatol 2005;152:334-41. http://dx.doi.org/10.1111/j.1365-2133.2004.06303.x.
- Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, Murrell DF, Tang ML. Probiotics for the treatment of eczema: a systematic review. Clin Exp Allergy 2009;39:1117-27. http://dx.doi.org/10.1111/j.1365-2222.2009.03305.x.
- Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, Murrell DF, Tang ML. Probiotics for treating eczema. Cochrane Database Syst Rev 2008;4. http://dx.doi.org/10.1002/14651858.cd006135.pub2.
- Michail SK, Stolfi A, Johnson T, Onady GM. Efficacy of probiotics in the treatment of pediatric atopic dermatitis: a meta-analysis of randomized controlled trials. Ann Allergy Asthma Immunol 2008;101:508-16. http://dx.doi.org/10.1016/S1081-1206(10)60290-6.
- Lee J, Seto D, Bielory L. Meta-analysis of clinical trials of probiotics for prevention and treatment of pediatric atopic dermatitis. J Allergy Clin Immunol 2008;121:116-21. http://dx.doi.org/10.1016/j.jaci.2007.10.043.
- Bath-Hextall FJ, Delamere FM, Williams HC. Dietary exclusions for established atopic eczema. Cochrane Database Syst Rev 2008;1. http://dx.doi.org/10.1002/14651858.cd005203.pub2.
- Bath-Hextall F, Delamere FM, Williams HC. Dietary exclusions for improving established atopic eczema in adults and children: systematic review. Allergy 2009;64:258-64. http://dx.doi.org/10.1111/j.1398-9995.2008.01917.x.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, Williams HC. Dietary supplements for established atopic eczema. Cochrane Database Syst Rev 2012;2. http://dx.doi.org/10.1002/14651858.cd005205.pub3.
- Flohr C, Pascoe D, Williams HC. Atopic dermatitis and the ‘hygiene hypothesis’: too clean to be true?. Br J Dermatol 2005;152:202-16. http://dx.doi.org/10.1111/j.1365-2133.2004.06436.x.
- Fiocchi A, Bouygue GR, Martelli A, Terracciano L, Sarratud T. Dietary treatment of childhood atopic eczema/dermatitis syndrome (AEDS). Allergy 2004;59:78-85. http://dx.doi.org/10.1111/j.1398-9995.2004.00653.x.
- van Gool CJ, Zeegers MP, Thijs C. Oral essential fatty acid supplementation in atopic dermatitis – a meta-analysis of placebo-controlled trials. Br J Dermatol 2004;150:728-40. http://dx.doi.org/10.1111/j.0007-0963.2004.05851.x.
- Ernst E, Pittler MH, Stevinson C. Complementary/alternative medicine in dermatology: evidence-assessed efficacy of two diseases and two treatments. Am J Clin Dermatol 2002;3:341-8. http://dx.doi.org/10.2165/00128071-200203050-00006.
- Chafen JJ, Newberry SJ, Riedl MA, Bravata DM, Maglione M, Suttorp MJ, et al. Diagnosing and managing common food allergies: a systematic review. JAMA 2010;303:1848-56. http://dx.doi.org/10.1001/jama.2010.582.
- Rosenfeldt V, Benfeldt E, Nielsen SD, Michaelsen KF, Jeppesen DL, Valerius NH, et al. Effect of probiotic Lactobacillus strains in children with atopic dermatitis. J Allergy Clin Immunol 2003;111:389-95. http://dx.doi.org/10.1067/mai.2003.389.
- Sistek D, Kelly R, Wickens K, Stanley T, Fitzharris P, Crane J. Is the effect of probiotics on atopic dermatitis confined to food sensitized children?. Clin Exp Allergy 2006;36:629-33. http://dx.doi.org/10.1111/j.1365-2222.2006.02485.x.
- Cukrowska B, Ceregra A, Rosiak I, Klewicka E, Slizewska K, Motyl I, et al. The influence of probiotic Lactobacillus casei and paracasei strains on clinical status of atopic eczema in children with food allergy on cow’s milk proteins. Pediatria Wspolczesna 2008;10:67-70.
- Yesilova Y, Calka O, Akdeniz N, Berktas M. Effect of probiotics on the treatment of children with atopic dermatitis. Ann Dermatol 2012;24:189-93. http://dx.doi.org/10.5021/ad.2012.24.2.189.
- Iemoli E, Trabattoni D, Parisotto S, Borgonovo L, Toscano M, Rizzardini G, et al. Probiotics reduce gut microbial translocation and improve adult atopic dermatitis. J Clin Gastroenterol 2012;46:S33-40. http://dx.doi.org/10.1097/MCG.0b013e31826a8468.
- Grüber C, Wendt M, Sulser C, Lau S, Kulig M, Wahn U, et al. Randomized, placebo-controlled trial of Lactobacillus rhamnosus GG as treatment of atopic dermatitis in infancy. Allergy 2007;62:1270-6. http://dx.doi.org/10.1111/j.1398-9995.2007.01543.x.
- Nermes M, Kantele JM, Atosuo TJ, Salminen S, Isolauri E. Interaction of orally administered Lactobacillus rhamnosus GG with skin and gut microbiota and humoral immunity in infants with atopic dermatitis. Clin Exp Allergy 2011;41:370-7. http://dx.doi.org/10.1111/j.1365-2222.2010.03657.x.
- Brouwer ML, Wolt-Plompen SAA, Dubois AEJ, van der Heide S, Jansen DF, Hoijer MA, et al. No effects of probiotics on atopic dermatitis in infancy: a randomized placebo-controlled trial. Clin Exp Allergy 2006;36:899-906. http://dx.doi.org/10.1111/j.1365-2222.2006.02513.x.
- Fölster-Holst R, Muüller F, Schnopp N, Abeck D, Kreiselmaier I, Lenz T, et al. Prospective, randomized controlled trial on Lactobacillus rhamnosus in infants with moderate to severe atopic dermatitis. Br J Dermatol 2006;155:1256-61. http://dx.doi.org/10.1111/j.1365-2133.2006.07558.x.
- Viljanen M, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, et al. Probiotics in the treatment of atopic eczema/dermatitis syndrome in infants: a double-blind placebo-controlled trial. Allergy 2005;60:494-500. http://dx.doi.org/10.1111/j.1398-9995.2004.00514.x.
- Moroi M, Uchi S, Nakamura K, Sato S, Shimizu N, Fujii M, et al. Beneficial effect of a diet containing heat-killed Lactobacillus paracasei K71 on adult type atopic dermatitis. J Dermatol 2011;38:131-9. http://dx.doi.org/10.1111/j.1346-8138.2010.00939.x.
- Woo SI, Kim JY, Lee YJ, Kim NS, Hahn YS. Effect of Lactobacillus sakei supplementation in children with atopic eczema-dermatitis syndrome. Ann Allergy Asthma Immunol 2010;104:343-8. http://dx.doi.org/10.1016/j.anai.2010.01.020.
- Torii S, Torii A, Itoh K, Urisu A, Terada A, Fujisawa T, et al. Effects of oral administration of Lactobacillus acidophilus L-92 on the symptoms and serum markers of atopic dermatitis in children. Int Arch Allergy Immunol 2011;154:236-45. http://dx.doi.org/10.1159/000321110.
- Isolauri E, Arvola T, Sutas Y, Moilanen E, Salminen S. Probiotics in the management of atopic eczema. Clin Exp Allergy 2000;30:1604-10. http://dx.doi.org/10.1046/j.1365-2222.2000.00943.x.
- Taniuchi S, Hattori K, Yamamoto A, Sasai M, Hatano Y, Kojima T, et al. Administration of Bifidobacterium to infants with atopic dermatitis: changes in fecal microflora and clinical symptoms. J Appl Res 2005;5:387-96.
- Yoshida Y, Seki T, Matsunaka H, Watanabe T, Shindo M, Yamada N, et al. Clinical effects of probiotic Bifidobacterium breve supplementation in adult patients with atopic dermatitis. Yonago Acta Med 2010;53:37-45.
- Gøbel R, Larsen N, Mølgaard C, Jakobsen M, Michaelsen KF. Probiotics to young children with atopic dermatitis: a randomized placebo-controlled trial. Int J Probiotics Prebiotics 2010;5:53-9.
- Drago L, Toscano M, De Vecchi E, Piconi S, Iemoli E. Changing of fecal flora and clinical effect of L. salivarius LS01 in adults with atopic dermatitis. J Clin Gastroenterol 2012;46:56-63. http://dx.doi.org/10.1097/MCG.0b013e318265ef38.
- Gore C, Custovic A, Tannock GW, Munro K, Kerry G, Johnson K, et al. Treatment and secondary prevention effects of the probiotics Lactobacillus paracasei or Bifidobacterium lactis on early infant eczema: randomized controlled trial with follow-up until age 3 years. Clin Exp Allergy 2012;42:112-22. http://dx.doi.org/10.1111/j.1365-2222.2011.03885.x.
- Weston S, Halbert A, Richmond P, Prescott SL. Effects of probiotics on atopic dermatitis: a randomised controlled trial. Arch Dis Child 2005;90:892-7. http://dx.doi.org/10.1136/adc.2004.060673.
- Han Y, Kim B, Ban J, Lee J, Kim BJ, Choi BS, et al. A randomized trial of Lactobacillus plantarum CJLP133 for the treatment of atopic dermatitis. Pediatr Allergy Immunol 2012;23:667-73. http://dx.doi.org/10.1111/pai.12010.
- Shibata R, Kimura M, Takahashi H, Mikami K, Aiba Y, Takeda H, et al. Clinical effects of kestose, a prebiotic oligosaccharide, on the treatment of atopic dermatitis in infants. Clin Exp Allergy 2009;39:1397-403. http://dx.doi.org/10.1111/j.1365-2222.2009.03295.x.
- Ghanei N, Siassi F, Zandieh F, Rahimi A. Effectiveness of prebiotic in atopic dermatitis reduction in 7–24 months old children living in Isfahan. J Isfahan Med Sch 2011;28:679-87.
- Gerasimov SV, Vasjuta VV, Myhovych OO, Bondarchuk LI. Probiotic supplement reduces atopic dermatitis in preschool children: a randomized, double-blind, placebo-controlled, clinical trial. Am J Clin Dermatol 2010;11:351-61. http://dx.doi.org/10.2165/11531420-000000000-00000.
- van der Aa LB, Heymans HS, van Aalderen WM, Sillevis Smitt JH, Knol J, Ben Amor K, et al. Effect of a new synbiotic mixture on atopic dermatitis in infants: a randomized-controlled trial. Clin Exp Allergy 2010;40:795-804. http://dx.doi.org/10.1111/j.1365-2222.2010.03465.x.
- Passeron T, Lacour JP, Fontas E, Ortonne JP. Prebiotics and synbiotics: two promising approaches for the treatment of atopic dermatitis in children above 2 years. Allergy 2006;61:431-7. http://dx.doi.org/10.1111/j.1398-9995.2005.00956.x.
- Hattori K, Yamamoto A, Sasai M, Taniuchi S, Kojima T, Kobayashi Y, et al. Effects of administration of bifidobacteria on fecal microflora and clinical symptoms in infants with atopic dermatitis. Arerugi 2003;52:20-3.
- Murosaki S, Yamamoto Y, Yotsumoto H, Kubo H, Nomoto S, Usuki K, et al. Effects of intake of syrup supplemented with nigerooligosaccharides and heat-killed Lactobacillus plantarum L-137 on skin symptom and immune function in patients with atopic dermatitis. Jpn J Clin Pharmacol Therapeut 2006;34:1087-96.
- Farid R, Ahanchian H, Jabbari F, Moghiman T. Effect of a new synbiotic mixture on atopic dermatitis in children: a randomized-controlled trial. Iran J Pediatr 2011;21:225-30.
- Wu KG, Li TH, Peng HJ. Lactobacillus salivarius plus fructo-oligosaccharide is superior to fructo-oligosaccharide alone for treating children with moderate to severe atopic dermatitis: a double-blind, randomized, clinical trial of efficacy and safety. Br J Dermatol 2012;166:129-36. http://dx.doi.org/10.1111/j.1365-2133.2011.10596.x.
- Shafiei A, Moin M, Pourpak Z, Gharagozlou M, Aghamohamadi A, Sajedi V, et al. Synbiotics could not reduce the Scoring of Childhood Atopic Dermatitis (SCORAD): a randomized double blind placebo-controlled trial. Iran J Allergy Asthma Immunol 2011;10:21-8.
- Takwale A, Tan E, Agarwal S, Barclay G, Ahmed I, Hotchkiss K, et al. Efficacy and tolerability of borage oil in adults and children with atopic eczema: randomised, double blind, placebo controlled, parallel group trial. BMJ 2003;327. http://dx.doi.org/10.1136/bmj.327.7428.1385.
- Senapati S, Banerjee S, Gangopadhyay DN. Evening primrose oil is effective in atopic dermatitis: a randomized placebo-controlled trial. Indian J Dermatol Venereol Leprol 2008;74:447-52. http://dx.doi.org/10.4103/0378-6323.42645.
- Mayser P, Mayer K, Mahloudjian M, Benzing S, Kramer HJ, Schill WB, et al. A double-blind, randomized, placebo-controlled trial of n-3 versus n-6 fatty acid-based lipid infusion in atopic dermatitis. JPEN J Parenter Enteral Nutr 2002;26:151-8. http://dx.doi.org/10.1177/0148607102026003151.
- Koch C, Dolle S, Metzger M, Rasche C, Jungclas H, Ruhl R, et al. Docosahexaenoic acid (DHA) supplementation in atopic eczema: a randomized, double-blind, controlled trial. Br J Dermatol 2008;158:786-92. http://dx.doi.org/10.1111/j.1365-2133.2007.08430.x.
- Callaway J, Schwab U, Harvima I, Halonen P, Mykkanen O, Hyvonen P, et al. Efficacy of dietary hempseed oil in patients with atopic dermatitis. J Dermatolog Treat 2005;16:87-94. http://dx.doi.org/10.1080/09546630510035832.
- Henz BM, Jablonska S, van de Kerkhof PC, Stingl G, Blaszczyk M, Vandervalk PG, et al. Double-blind, multicentre analysis of the efficacy of borage oil in patients with atopic eczema. Br J Dermatol 1999;140:685-8. http://dx.doi.org/10.1046/j.1365-2133.1999.02771.x.
- Heimbeck I, Wjst M, Apfelbacher CJ. Low vitamin D serum level is inversely associated with eczema in children and adolescents in Germany. Allergy 2013;68:906-10. http://dx.doi.org/10.1111/all.12167.
- Chiu YE, Havens PL, Siegel DH, Ali O, Wang T, Holland KE, et al. Serum 25-hydroxyvitamin D concentration does not correlate with atopic dermatitis severity. J Am Acad Dermatol 2013;69:40-6. http://dx.doi.org/10.1016/j.jaad.2013.01.010.
- Wang SS, Hon KL, Kong AP, Pong HN, Wong GW, Leung TF. Vitamin D deficiency is associated with diagnosis and severity of childhood atopic dermatitis. Pediatr Allergy Immunol 2014;25:30-5. http://dx.doi.org/10.1111/pai.12167.
- Mutgi K, Koo J. Update on the role of systemic vitamin D in atopic dermatitis. Pediatr Dermatol 2013;30:303-7. http://dx.doi.org/10.1111/j.1525-1470.2012.01850.x.
- Samochocki Z, Bogaczewicz J, Jeziorkowska R, Sysa-Jędrzejowska A, Glińska O, Karczmarewicz E, et al. Vitamin D effects in atopic dermatitis. J Am Acad Dermatol 2013;69:238-44. http://dx.doi.org/10.1016/j.jaad.2013.03.014.
- Sidbury R, Sullivan AF, Thadhani RI, Camargo CA. Randomized controlled trial of vitamin D supplementation for winter-related atopic dermatitis in Boston: a pilot study. Br J Dermatol 2008;159:245-7. http://dx.doi.org/10.1111/j.1365-2133.2008.08601.x.
- Amestejani M, Salehi BS, Vasigh M, Sobhkhiz A, Karami M, Alinia H, et al. Vitamin D supplementation in the treatment of atopic dermatitis: a clinical trial study. J Drugs Dermatol 2012;11:327-30.
- Javanbakht MH, Keshavarz SA, Djalali M, Siassi F, Eshraghian MR, Firooz A, et al. Randomized controlled trial using vitamins E and D supplementation in atopic dermatitis. J Dermatolog Treat 2011;22:144-50. http://dx.doi.org/10.3109/09546630903578566.
- Vita D, Passalacqua G, Di Pasquale G, Caminiti L, Crisafulli G, Rulli I, et al. Ass’s milk in children with atopic dermatitis and cow’s milk allergy: crossover comparison with goat’s milk. Pediatr Allergy Immunol 2007;18:594-8. http://dx.doi.org/10.1111/j.1399-3038.2007.00567.x.
- Leung TF, Ma KC, Cheung LT, Lam CW, Wong E, Wan H, et al. A randomized, single-blind and crossover study of an amino acid-based milk formula in treating young children with atopic dermatitis. Pediatr Allergy Immunol 2004;15:558-61. http://dx.doi.org/10.1111/j.1399-3038.2004.00197.x.
- Jin YY, Cao RM, Chen J, Kaku Y, Wu J, Cheng Y, et al. Partially hydrolyzed cow’s milk formula has a therapeutic effect on the infants with mild to moderate atopic dermatitis: a randomized, double-blind study. Pediatr Allergy Immunol 2011;22:688-94. http://dx.doi.org/10.1111/j.1399-3038.2011.01172.x.
- de Bes J, Legierse CM, Prinsen CA, de Korte J. Patient education in chronic skin diseases: a systematic review. Acta Derm Venereol 2011;91:12-7. http://dx.doi.org/10.2340/00015555-1022.
- Ahnert J, Müller J, Löffler S, Vogel H. Patient and parental education programs for atopic neurodermatitis. Evidence-based literature review on their effectiveness in the rehabilitation of children and adolescents with atopic dermatitis. Monatsschr Kinderheilkd 2010;158:586-91. http://dx.doi.org/10.1007/s00112-010-2169-5.
- Chida Y, Steptoe A, Hirakawa N, Sudo N, Kubo C. The effects of psychological intervention on atopic dermatitis. A systematic review and meta-analysis. Int Arch Allergy Immunol 2007;144:1-9. http://dx.doi.org/10.1159/000101940.
- Ersser SJ, Latter S, Sibley A, Satherley PA, Welbourne S. Psychological and educational interventions for atopic eczema in children. Cochrane Database Syst Rev 2007;3. http://dx.doi.org/10.1002/14651858.cd004054.pub2.
- Moore E, Williams A, Manias E, Varigos G. Nurse-led clinics reduce severity of childhood atopic eczema: a review of the literature. Br J Dermatol 2006;155:1242-8. http://dx.doi.org/10.1111/j.1365-2133.2006.07534.x.
- Vlachou C, Thomas KS, Williams HC. A case report and critical appraisal of the literature on the use of DermaSilk in children with atopic dermatitis. Clin Exp Dermatol 2009;34:e901-3. http://dx.doi.org/10.1111/j.1365-2230.2009.03672.x.
- Koller DY, Halmerbauer G, Böck A, Engstler G. Action of a silk fabric treated with AEGIS in children with atopic dermatitis: a 3-month trial. Pediatr Allergy Immunol 2007;18:335-8. http://dx.doi.org/10.1111/j.1399-3038.2006.00511.x.
- Stinco G, Piccirillo F, Valent F. A randomized double-blind study to investigate the clinical efficacy of adding a non-migrating antimicrobial to a special silk fabric in the treatment of atopic dermatitis. Dermatology 2008;217:191-5. http://dx.doi.org/10.1159/000141648.
- Fontanini C, Berti I, Monasta L, Longo G. DermaSilk in long-term control of infantile atopic dermatitis: a double blind randomized controlled trial. G Ital Dermatol Venereol 2013;148:293-7.
- Ozawa M, Numata I, Watabe A, Koizumi H, Memezawa A, Kikuchi K, et al. Effect of underwear made from MEDIELE on skin barrier function of atopic dermatitis patients in winter season. Skin Res 2008;7:475-81.
- Yokoyama Y, Kimata H, Mitarai S, Hirano S, Shirakawa T. Ethylene vinyl alcohol (EVOH) fiber compared to cotton underwear in the treatment of childhood atopic dermatitis: a double-blind randomized study. Indian Pediatr 2009;46:611-14.
- Gauger A, Fischer S, Mempel M, Schaefer T, Foelster-Holst R, Abeck D, et al. Efficacy and functionality of silver-coated textiles in patients with atopic eczema. J Eur Acad Dermatol Venereol 2006;20:534-41. http://dx.doi.org/10.1111/j.1468-3083.2006.01526.x.
- Juenger M, Ladwig A, Staecker S, Arnold A, Kramer A, Daeschlein G, et al. Efficacy and safety of silver textile in the treatment of atopic dermatitis (AD). Curr Med Res Opin 2006;22:739-50. http://dx.doi.org/10.1185/030079906X99990.
- Kim SH, Hwang SH, Hong SK, Seo JK, Sung HS, Park SW, et al. The clinical efficacy, safety and functionality of anion textile in the treatment of atopic dermatitis. Ann Dermatol 2012;24:438-43. http://dx.doi.org/10.5021/ad.2012.24.4.438.
- Harrison EF, Haines RH, Cowdell F, Sach TH, Dean T, Pollock I, et al. A multi-centre, parallel group superiority trial of silk therapeutic clothing compared to standard care for the management of eczema in children (CLOTHES Trial): study protocol for a randomised controlled trial. Trials 2015;16. http://dx.doi.org/10.1186/s13063-015-0921-9.
- Span L, Molier L, Coenraads PJ, Jaspers JP, Fidler V, Tupker R. Intensive daycare for young adults with atopic eczema. Nederland Tijdschr Dermatol Venereol 2001;11:279-83.
- Armstrong AW, Kim RH, Idriss NZ, Larsen LN, Lio PA. Online video improves clinical outcomes in adults with atopic dermatitis: a randomized controlled trial. J Am Acad Dermatol 2011;64:502-7. http://dx.doi.org/10.1016/j.jaad.2010.01.051.
- Stangier U, Gieler U, Ehlers A, Gieler U, Stangier U, Brähler E. A Psychological Perspective of Skin Disorders. Göttingen, Germany: Hogrefe Press; 1993.
- Shaw M, Morrell DS, Goldsmith LA. A study of targeted enhanced patient care for pediatric atopic dermatitis (STEP PAD). Pediatr Dermatol 2008;25:19-24. http://dx.doi.org/10.1111/j.1525-1470.2007.00575.x.
- Staab D, Diepgen TL, Fartasch M, Kupfer J, Lob-Corzilius T, Ring J, et al. Age related, structured educational programmes for the management of atopic dermatitis in children and adolescents: multicentre, randomised controlled trial. BMJ 2006;332:933-8. http://dx.doi.org/10.1136/bmj.332.7547.933.
- Staab D, von Rueden U, Kehrt R, Erhart M, Wenninger K, Kamtsiuris P, et al. Evaluation of a parental training program for the management of childhood atopic dermatitis. Pediatr Allergy Immunol 2002;13:84-90. http://dx.doi.org/10.1034/j.1399-3038.2002.01005.x.
- Grillo M, Gassner L, Marshman G, Dunn S, Hudson P. Pediatr Dermatol 2006;23:428-36. http://dx.doi.org/10.1111/j.1525-1470.2006.00277.x.
- Futamura M, Masuko I, Hayashi K, Ohya Y, Ito K. Effects of a short-term parental education program on childhood atopic dermatitis: a randomized controlled trial. Pediatr Dermatol 2013;30:438-43. http://dx.doi.org/10.1111/pde.12105.
- Kardorff B, Schnelle-Parker G, Kardorff M, Wahlen M, D’Orville IH, Dorittke P. Successful reduction of the SCORAD score by a short-time teaching method using a simplified skin model in children with atopic eczema in a 6-week comparison. J Dtsch Dermatol Ges 2003;1:451-6. http://dx.doi.org/10.1046/j.1439-0353.2003.02021.x.
- Moore EJ, Williams A, Manias E, Varigos G, Donath S. Eczema workshops reduce severity of childhood atopic eczema. Australas J Dermatol 2009;50:100-6. http://dx.doi.org/10.1111/j.1440-0960.2009.00515.x.
- Chinn DJ, Poyner T, Sibley G. Randomized controlled trial of a single dermatology nurse consultation in primary care on the quality of life of children with atopic eczema. Br J Dermatol 2002;146:432-9. http://dx.doi.org/10.1046/j.1365-2133.2002.04603.x.
- Schuttelaar ML, Vermeulen KM, Drukker N, Coenraads PJ. A randomized controlled trial in children with eczema: nurse practitioner vs. dermatologist. Br J Dermatol 2010;162:162-70. http://dx.doi.org/10.1111/j.1365-2133.2009.09502.x.
- Blessmann Weber M, De Tarso Da Luz Fontes Neto P, Prati C, Soirefman M, Mazzotti NG, Barzenski B, et al. Improvement of pruritus and quality of life of children with atopic dermatitis and their families after joining support groups. J Eur Acad Dermatol Venereol 2008;22:992-7. http://dx.doi.org/10.1111/j.1468-3083.2008.02697.x.
- van Os-Medendorp H, Koffijberg H, Eland-de Kok PC, van der Zalm A, de Bruin-Weller MS, Pasmans SG, et al. E-health in caring for patients with atopic dermatitis: a randomized controlled cost-effectiveness study of internet-guided monitoring and online self-management training. Br J Dermatol 2012;166:1060-8. http://dx.doi.org/10.1111/j.1365-2133.2012.10829.x.
- Schut C, Weik U, Tews N, Gieler U, Deinzer R, Kupfer J. Psychophysiological effects of stress management in patients with atopic dermatitis: a randomized controlled trial. Acta Derm Venereol 2013;93:57-61. http://dx.doi.org/10.2340/00015555-1415.
- McNally NJ, Williams HC, Phillips DR, Smallman-Raynor M, Lewis S, Venn A, et al. Atopic eczema and domestic water hardness. Lancet 1998;352:527-31. http://dx.doi.org/10.1016/S0140-6736(98)01402-0.
- Miyake Y, Yokoyama T, Yura A, Iki M, Shimizu T. Ecological association of water hardness with prevalence of childhood atopic dermatitis in a Japanese urban area. Environ Res 2004;94:33-7. http://dx.doi.org/10.1016/S0013-9351(03)00068-9.
- Thomas KS, Dean T, O’Leary C, Sach TH, Koller K, Frost A, et al. A randomised controlled trial of ion-exchange water softeners for the treatment of eczema in children. PLOS Med 2011;8. http://dx.doi.org/10.1371/journal.pmed.1000395.
- Byremo G, Rod G, Carlsen KH. Effect of climatic change in children with atopic eczema. Allergy 2006;61:1403-10. http://dx.doi.org/10.1111/j.1398-9995.2006.01209.x.
- Hallai N, Gawkrodger DJ. Patch testing to aeroallergens, especially house dust mite, is often positive in atopics with eczema of the hands and face. J Eur Acad Dermatol Venereol 2009;23:728-30. http://dx.doi.org/10.1111/j.1468-3083.2009.03189.x.
- Gutgesell C, Heise S, Seubert S, Seubert A, Domhof S, Brunner E, et al. Double-blind placebo-controlled house dust mite control measures in adult patients with atopic dermatitis. Br J Dermatol 2001;145:70-4. http://dx.doi.org/10.1046/j.1365-2133.2001.04283.x.
- Oosting AJ, de Bruin-Weller MS, Terreehorst I, Tempels-Pavlica Z, Aalberse RC, de Monchy JG, et al. Effect of mattress encasings on atopic dermatitis outcome measures in a double-blind, placebo-controlled study: the Dutch mite avoidance study. J Allergy Clin Immunol 2002;110:500-6. http://dx.doi.org/10.1067/mai.2002.126791.
- Ricci G, Patrizi A, Specchia F, Menna L, Bottau P, D’Angelo V, et al. Effect of house dust mite avoidance measures in children with atopic dermatitis. Br J Dermatol 2000;143:379-84. http://dx.doi.org/10.1046/j.1365-2133.2000.03666.x.
- Cramer JA, Scheyer RD, Mattson RH. Compliance declines between clinic visits. Arch Intern Med 1990;150:1509-10. http://dx.doi.org/10.1001/archinte.1990.00390190143023.
- Feldman SR, Camacho FT, Krejci-Manwaring J, Carroll CL, Balkrishnan R. Adherence to topical therapy increases around the time of office visits. J Am Acad Dermatol 2007;57:81-3. http://dx.doi.org/10.1016/j.jaad.2007.04.005.
- Kinney MA, Feldman SR. What’s new in the management of psoriasis?. G Ital Dermatol Venereol 2009;144:103-17.
- Sagransky MJ, Yentzer BA, Williams LL, Clark AR, Taylor SL, Feldman SR. A randomized controlled pilot study of the effects of an extra office visit on adherence and outcomes in atopic dermatitis. Arch Dermatol 2010;146:1428-30. http://dx.doi.org/10.1001/archdermatol.2010.368.
- Perkin M, Strachan DP. Which aspects of the farming lifestyle explain the inverse association with childhood allergy?. J Allergy Clin Immunol 2006;117:1374-81. http://dx.doi.org/10.1016/j.jaci.2006.03.008.
- Arkwright PD, David TJ. Intradermal administration of a killed Mycobacterium vaccae suspension (SRL 172) is associated with improvement in atopic dermatitis in children with moderate-to-severe disease. J Allergy Clin Immunol 2001;107:531-4. http://dx.doi.org/10.1067/mai.2001.113081.
- Arkwright PD, David TJ. Effect of Mycobacterium vaccae on atopic dermatitis in children of different ages. Br J Dermatol 2003;149:1029-34. http://dx.doi.org/10.1111/j.1365-2133.2003.05557.x.
- Berth-Jones J, Arkwright PD, Marasovic D, Savani N, Aldridge CR, Leech SN, et al. Killed Mycobacterium vaccae suspension in children with moderate-to-severe atopic dermatitis: a randomized, double-blind, placebo-controlled trial. Clin Exp Allergy 2006;36:1115-21. http://dx.doi.org/10.1111/j.1365-2222.2006.02558.x.
- Brothers S, Asher MI, Jaksic M, Stewart AW. Effect of a Mycobacterium vaccae derivative on paediatric atopic dermatitis: a randomized, controlled trial. Clin Exp Dermatol 2009;34:770-5. http://dx.doi.org/10.1111/j.1365-2230.2008.03153.x.
- Meduri NB, Vandergriff T, Rasmussen H, Jacobe H. Phototherapy in the management of atopic dermatitis: a systematic review. Photodermatol Photoimmunol Photomed 2007;23:106-12. http://dx.doi.org/10.1111/j.1600-0781.2007.00291.x.
- Gambichler T, Breuckmann F, Boms S, Altmeyer P, Kreuter A. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol 2005;52:660-70. http://dx.doi.org/10.1016/j.jaad.2004.08.047.
- Selvaag E, Caspersen L, Bech-Thomsen N, Wulf HC. Optimized UVB treatment of atopic dermatitis using skin reflectance measurements. A controlled, left–right comparison trial. Acta Derm Venereol 2005;85:144-6. http://dx.doi.org/10.1080/00015550410024085.
- Dittmar HC, Pflieger D, Schopf E, Simon JC. [UVA1 phototherapy. Pilot study of dose finding in acute exacerbated atopic dermatitis]. Hautarzt 2001;52:423-7. http://dx.doi.org/10.1007/s001050051336.
- Tzaneva S, Kittler H, Holzer G, Reljic D, Weber M, Honigsmann H, et al. 5-Methoxypsoralen plus ultraviolet (UV) A is superior to medium-dose UVA1 in the treatment of severe atopic dermatitis: a randomized crossover trial. Br J Dermatol 2010;162:655-60. http://dx.doi.org/10.1111/j.1365-2133.2009.09514.x.
- Reynolds NJ, Franklin V, Gray JC, Diffey BL, Farr PM. Narrow-band ultraviolet B and broad-band ultraviolet A phototherapy in adult atopic eczema: a randomised controlled trial. Lancet 2001;357:2012-16. http://dx.doi.org/10.1016/S0140-6736(00)05114-X.
- Majoie IM, Oldhoff JM, van Weelden H, Laaper-Ertmann M, Bousema MT, Sigurdsson V, et al. Narrowband ultraviolet B and medium-dose ultraviolet A1 are equally effective in the treatment of moderate to severe atopic dermatitis. J Am Acad Dermatol 2009;60:77-84. http://dx.doi.org/10.1016/j.jaad.2008.08.048.
- Gambichler T, Othlinghaus N, Tomi NS, Holland-Letz T, Boms S, Skrygan M, et al. Medium-dose ultraviolet (UV) A1 vs. narrowband UVB phototherapy in atopic eczema: a randomized crossover study. Br J Dermatol 2009;160:652-8. http://dx.doi.org/10.1111/j.1365-2133.2008.08984.x.
- Valkova S, Velkova A. UVA/UVB phototherapy for atopic dermatitis revisited. J Dermatolog Treat 2004;15:239-44. http://dx.doi.org/10.1080/09546630410035338.
- Tzung TY, Lin CB, Chen YH, Yang CY. Pimecrolimus and narrowband UVB as monotherapy or combination therapy in children and adolescents with atopic dermatitis. Acta Derm Venereol 2006;86:34-8.
- Heinlin J, Schiffner-Rohe J, Schiffner R, Einsele-Krämer B, Landthaler M, Klein A, et al. A first prospective randomized controlled trial on the efficacy and safety of synchronous balneophototherapy vs. narrow-band UVB monotherapy for atopic dermatitis. J Eur Acad Dermatol Venereol 2011;25:765-73. http://dx.doi.org/10.1111/j.1468-3083.2010.03857.x.
- Granlund H, Erkko P, Remitz A, Langeland T, Helsing P, Nuutinen M, et al. Comparison of cyclosporin and UVAB phototherapy for intermittent one-year treatment of atopic dermatitis. Acta Derm Venereol 2001;81:22-7. http://dx.doi.org/10.1080/000155501750208137.
- Byun HJ, Lee HI, Kim B, Kim MN, Hong H, Choi Y, et al. Full-spectrum light phototherapy for atopic dermatitis. Int J Dermatol 2011;50:94-101. http://dx.doi.org/10.1111/j.1365-4632.2010.04663.x.
- Bos JD, Van Leent EJ, Sillevis Smitt JH. The millennium criteria for the diagnosis of atopic dermatitis. Exp Dermatol 1998;7:132-8. http://dx.doi.org/10.1111/j.1600-0625.1998.tb00313.x.
- Schmitt J, Schakel K, Schmitt N, Meurer M. Systemic treatment of severe atopic eczema: a systematic review. Acta Derm Venereol 2007;87:100-11. http://dx.doi.org/10.2340/00015555-0207.
- Schram ME, Roekevisch E, Leeflang MM, Bos JD, Schmitt J, Spuls PI. A randomized trial of methotrexate versus azathioprine for severe atopic eczema. J Allergy Clin Immunol 2011;128:353-9. http://dx.doi.org/10.1016/j.jaci.2011.03.024.
- Sanchez J, Ramirez R, Diez S, Sus S, Echenique A, Olivares M, et al. Omalizumab beyond asthma. Allergol Immunopathol (Madr) 2012;40:306-15. http://dx.doi.org/10.1016/j.aller.2011.09.011.
- Pajno GB, Finegold I. SIT beyond respiratory diseases. Ann Allergy Asthma Immunol 2011;107:395-400. http://dx.doi.org/10.1016/j.anai.2011.04.011.
- Compalati E, Rogkakou A, Passalacqua G, Canonica GW. Evidences of efficacy of allergen immunotherapy in atopic dermatitis: an updated review. Curr Opin Allergy Clin Immunol 2012;12:427-33. http://dx.doi.org/10.1097/ACI.0b013e328354e540.
- Berth-Jones J, Takwale A, Tan E, Barclay G, Agarwal S, Ahmed I, et al. Azathioprine in severe adult atopic dermatitis: a double-blind, placebo-controlled, crossover trial. Br J Dermatol 2002;147:324-30. http://dx.doi.org/10.1046/j.1365-2133.2002.04989.x.
- Meggitt SJ, Gray JC, Reynolds NJ. Azathioprine dosed by thiopurine methyltransferase activity for moderate-to-severe atopic eczema: a double-blind, randomised controlled trial. Lancet 2006;367:839-46. http://dx.doi.org/10.1016/S0140-6736(06)68340-2.
- Czech W, Bräutigam M, Weidinger G, Schöpf E. A body-weight-independent dosing regimen of cyclosporine microemulsion is effective in severe atopic dermatitis and improves the quality of life. J Am Acad Dermatol 2000;42:653-9. http://dx.doi.org/10.1067/mjd.2000.103815.
- Bemanian MH, Movahedi M, Farhoudi A, Gharagozlou M, Seraj MH, Pourpak Z, et al. High doses intravenous immunoglobulin versus oral cyclosporine in the treatment of severe atopic dermatitis. Iran J Allergy Asthma Immunol 2005;4:139-43.
- Schmitt J, Schäkel K, Fölster-Holst R, Bauer A, Oertel R, Augustin M, et al. Prednisolone vs. ciclosporin for severe adult eczema. An investigator-initiated double-blind placebo-controlled multicentre trial. Br J Dermatol 2010;162:661-8. http://dx.doi.org/10.1111/j.1365-2133.2009.09561.x.
- Haeck IM, Knol MJ, Ten Berge O, van Velsen SG, de Bruin-Weller MS, Bruijnzeel-Koomen CA. Enteric-coated mycophenolate sodium versus cyclosporin A as long-term treatment in adult patients with severe atopic dermatitis: a randomized controlled trial. J Am Acad Dermatol 2011;64:1074-84. http://dx.doi.org/10.1016/j.jaad.2010.04.027.
- El-Khalawany MA, Hassan H, Shaaban D, Ghonaim N, Eassa B. Methotrexate versus cyclosporine in the treatment of severe atopic dermatitis in children: a multicenter experience from Egypt. Eur J Pediatr 2013;172:351-6. http://dx.doi.org/10.1007/s00431-012-1893-3.
- Kwon HB, Ahn BJ, Choi Y, Jin SY, Cheong KA, Lee J, et al. Combination of glucosamine improved therapeutic effect of low-dose cyclosporin A in patients with atopic dermatitis: a pilot study. J Dermatol 2013;40:207-10. http://dx.doi.org/10.1111/1346-8138.12003.
- Pei AY, Chan HH, Leung TF. Montelukast in the treatment of children with moderate-to-severe atopic dermatitis: a pilot study. Pediatr Allergy Immunol 2001;12:154-8. http://dx.doi.org/10.1034/j.1399-3038.2001.012003154.x.
- Friedmann PS, Palmer R, Tan E, Ogboli M, Barclay G, Hotchkiss K, et al. A double-blind, placebo-controlled trial of montelukast in adult atopic eczema. Clin Exp Allergy 2007;37:1536-40. http://dx.doi.org/10.1111/j.1365-2222.2007.02811.x.
- Veien NK, Busch-Sørensen M, Stausbøl-Grøn B. Montelukast treatment of moderate to severe atopic dermatitis in adults: a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol 2005;53:147-9. http://dx.doi.org/10.1016/j.jaad.2004.12.011.
- Yanase DJ, David-Bajar K. The leukotriene antagonist montelukast as a therapeutic agent for atopic dermatitis. J Am Acad Dermatol 2001;44:89-93. http://dx.doi.org/10.1067/mjd.2001.111352.
- Rahman ML, Choudhury AM, Islam MM. Effectiveness of montelukast in the treatment of atopic dermatitis. Mymensingh Med J 2006;15:85-8.
- Silny W, Czarnecka-Operacz M. Specific immunotherapy in the treatment of patients with atopic dermatitis – results of double blind placebo controlled study. Pol Merkur Lekarski 2006;21:558-65.
- Sanchez-Caraballo JM, Cardona-Villa R. Clinical and immunological changes of immunotherapy in patients with atopic dermatitis: randomized controlled trial. ISRN Allergy 2012. http://dx.doi.org/10.5402/2012/183983.
- Novak N, Bieber T, Hoffmann M, Fölster-Holst R, Homey B, Werfel T, et al. Efficacy and safety of subcutaneous allergen-specific immunotherapy with depigmented polymerized mite extract in atopic dermatitis. J Allergy Clin Immunol 2012;130:925-31. http://dx.doi.org/10.1016/j.jaci.2012.08.004.
- Pajno GB, Caminiti L, Vita D, Barberio G, Salzano G, Lombardo F, et al. Sublingual immunotherapy in mite-sensitized children with atopic dermatitis: a randomized, double-blind, placebo-controlled study. J Allergy Clin Immunol 2007;120:164-70. http://dx.doi.org/10.1016/j.jaci.2007.04.008.
- Oldhoff JM, Darsow U, Werfel T, Katzer K, Wulf A, Laifaoui J, et al. Anti-IL-5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitis. Allergy 2005;60:693-6. http://dx.doi.org/10.1111/j.1398-9995.2005.00791.x.
- Heil PM, Maurer D, Klein B, Hultsch T, Stingl G. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course – a randomized, placebo-controlled and double blind pilot study. J Dtsch Dermatol Ges 2010;8:990-8. http://dx.doi.org/10.1111/j.1610-0387.2010.07497.x.
- Paul C, Lahfa M, Bachelez H, Chevret S, Dubertret L. A randomized controlled evaluator-blinded trial of intravenous immunoglobulin in adults with severe atopic dermatitis. Br J Dermatol 2002;147:518-22. http://dx.doi.org/10.1046/j.1365-2133.2002.04833.x.
- Jee SJ, Kim JH, Baek HS, Lee HB, Oh JW. Long-term efficacy of intravenous immunoglobulin therapy for moderate to severe childhood atopic dermatitis. Allergy Asthma Immunol Res 2011;3:89-95. http://dx.doi.org/10.4168/aair.2011.3.2.89.
- Wolff K, Fleming C, Hanifin J, Papp K, Reitamo S, Rustin M, et al. Efficacy and tolerability of three different doses of oral pimecrolimus in the treatment of moderate to severe atopic dermatitis: a randomized controlled trial. Br J Dermatol 2005;152:1296-303. http://dx.doi.org/10.1111/j.1365-2133.2005.06674.x.
- Williams HC. Preventing eczema flares with topical corticosteroids or tacrolimus: which is best?. Br J Dermatol 2011;164:231-3. http://dx.doi.org/10.1111/j.1365-2133.2011.10205.x.
- Gu S, Yang AW, Xue CC, Li CG, Pang C, Zhang W, et al. Chinese herbal medicine for atopic eczema. Cochrane Database Syst Rev 2013;9. http://dx.doi.org/10.1002/14651858.cd008642.pub2.
- Simonart T, Kabagabo C, De Maertelaer V. Homoeopathic remedies in dermatology: a systematic review of controlled clinical trials. Br J Dermatol 2011;165:897-905. http://dx.doi.org/10.1111/j.1365-2133.2011.10457.x.
- Ernst E. Homeopathy for eczema: a systematic review of controlled clinical trials. Br J Dermatol 2012;166:1170-2. http://dx.doi.org/10.1111/j.1365-2133.2012.10994.x.
- Depression: The Treatment and Management of Depression in Adults. London: NICE; 2009.
- Schempp CM, Hezel S, Simon JC. Topical treatment of atopic dermatitis with Hypericum cream. A randomised, placebo-controlled, double-blind half-side comparison study. Hautarzt 2003;54:248-53.
- Pfab F, Athanasiadis GI, Huss-Marp J, Fuqin J, Heuser B, Cifuentes L, et al. Effect of acupuncture on allergen-induced basophil activation in patients with atopic eczema:a pilot trial. J Altern Complement Med 2011;17:309-14. http://dx.doi.org/10.1089/acm.2009.0684.
- Lee KC, Keyes A, Hensley JR, Gordon JR, Kwasny MJ, West DP, et al. Effectiveness of acupressure on pruritus and lichenification associated with atopic dermatitis: a pilot trial. Acupunct Med 2012;30:8-11. http://dx.doi.org/10.1136/acupmed-2011-010088.
- Senser C, Habermüller M, Revenstorf D. Hypnotherapy in atopic dermatitis. Aktuelle Dermatol 2004;30:103-8. http://dx.doi.org/10.1055/s-2004-814483.
- Anderson C, Lis-Balchin M, Kirk-Smith M. Evaluation of massage with essential oils on childhood atopic eczema. Phytother Res 2000;14:452-6. http://dx.doi.org/10.1002/1099-1573(200009)14:6<452::AID-PTR952>3.0.CO;2-4.
- Hon KL, Leung TF, Ng PC, Lam MC, Kam WY, Wong KY, et al. Efficacy and tolerability of a Chinese herbal medicine concoction for treatment of atopic dermatitis: a randomized, double-blind, placebo-controlled study. Br J Dermatol 2007;157:357-63. http://dx.doi.org/10.1111/j.1365-2133.2007.07941.x.
- Shapira MY, Raphaelovich Y, Gilad L, Or R, Dumb AJ, Ingber A. Treatment of atopic dermatitis with herbal combination of Eleutherococcus, Achillea millefolium, and Lamium album has no advantage over placebo: a double blind, placebo-controlled, randomized trial. J Am Acad Dermatol 2005;52:691-3. http://dx.doi.org/10.1016/j.jaad.2003.05.008.
- Cheng HM, Chiang LC, Jan YM, Chen GW, Li TC. The efficacy and safety of a Chinese herbal product (Xiao-Feng-San) for the treatment of refractory atopic dermatitis: a randomized, double-blind, placebo-controlled trial. Int Arch Allergy Immunol 2011;155:141-8. http://dx.doi.org/10.1159/000318861.
- Choi IH, Kim S, Kim Y, Yun Y. The effect of TJ-15 plus TJ-17 on atopic dermatitis: a pilot study based on the principle of pattern identification. J Altern Complement Med 2012;18:576-82. http://dx.doi.org/10.1089/acm.2011.0208.
- Henderson CA, Morris A, Wilson A, Ilchyshyn A. An open study comparing the efficacy of two different Chinese herbal therapy formulations in atopic eczema and their effects on circulating activated T-lymphocytes. J Dermatolog Treat 2000;11:91-6. http://dx.doi.org/10.1080/09546630050517478.
- Siebenwirth J, Lüdtke R, Remy W, Rakoski J, Borelli S, Ring J. Effectiveness of a classical homeopathic treatment in atopic eczema. A randomised placebo-controlled double-blind clinical trial. Forsch Komplementmed 2009;16:315-23. http://dx.doi.org/10.1159/000242434.
- Klövekorn W, Tepe A, Danesch U. A randomized, double-blind, vehicle-controlled, half-side comparison with a herbal ointment containing Mahonia aquifolium, Viola tricolor and Centella asiatica for the treatment of mild-to-moderate atopic dermatitis. Int J Clin Pharmacol Ther 2007;45:583-91. http://dx.doi.org/10.5414/CPP45583.
- Kobayashi H, Ishii M, Takeuchi S, Tanaka Y, Shintani T, Yamatodani A, et al. Efficacy and safety of a traditional herbal medicine, Hochu-ekki-to in the long-term management of Kikyo (delicate constitution) patients with atopic dermatitis: a 6-month, multicenter, double-blind, randomized, placebo-controlled study. Evid Based Complement Alternat Med 2010;7:367-73. http://dx.doi.org/10.1093/ecam/nen003.
- Kim NK, Lee DH, Seo HS, Sun SH, Oh YL, Kim JE, et al. Hwangryunhaedoktang in adult patients with atopic dermatitis: a randomised, double-blind, placebo-controlled, two-centre trial – study protocol. BMC Complement Altern Med 2011;11. http://dx.doi.org/10.1186/1472-6882-11-68.
- Farina S, Gisondi P, Zanoni M, Pace M, Rizzoli L, Baldo E, et al. Balneotherapy for atopic dermatitis in children at Comano spa in Trentino, Italy. J Dermatolog Treat 2011;22:366-71. http://dx.doi.org/10.3109/09546634.2010.512950.
- Bae BG, Oh SH, Park CO, Noh S, Noh JY, Kim KR, et al. Progressive muscle relaxation therapy for atopic dermatitis: objective assessment of efficacy. Acta Derm Venereol 2012;92:57-61. http://dx.doi.org/10.2340/00015555-1189.
- Kief H. A prospective, controlled study on the efficacy of the treatment with AHIT and treatment with patient’s blood in atopic dermatitis. Aktuelle Dermatol 2007;33:216-27. http://dx.doi.org/10.1055/s-2007-966541.
- Pittler MH, Armstrong NC, Cox A, Collier PM, Hart A, Ernst E. Randomized, double-blind, placebo-controlled trial of autologous blood therapy for atopic dermatitis. Br J Dermatol 2003;148:307-13. http://dx.doi.org/10.1046/j.1365-2133.2003.04921.x.
- Kawana S, Kato Y, Omi T. Efficacy of a 5-HT1a receptor agonist in atopic dermatitis. Clin Exp Dermatol 2010;35:835-40. http://dx.doi.org/10.1111/j.1365-2230.2009.03771.x.
- Malekzad F, Arbabi M, Mohtasham N, Toosi P, Jaberian M, Mohajer M, et al. Efficacy of oral naltrexone on pruritus in atopic eczema: a double-blind, placebo-controlled study. J Eur Acad Dermatol Venereol 2009;23:948-50. http://dx.doi.org/10.1111/j.1468-3083.2009.03129.x.
- Zattra E, Coleman C, Arad S, Helms E, Levine D, Bord E, et al. Polypodium leucotomos extract decreases UV-induced Cox-2 expression and inflammation, enhances DNA repair, and decreases mutagenesis in hairless mice. Am J Pathol 2009;175:1952-61. http://dx.doi.org/10.2353/ajpath.2009.090351.
- Ramírez-Bosca A, Zapater P, Betlloch I, Albero F, Martiínez A, Díaz-Alperi J, et al. Polypodium leucotomos extract in atopic dermatitis: a randomized, double-blind, placebo-controlled, multicenter trial. Actas Dermosifiliogr 2012;103:599-607. http://dx.doi.org/10.1016/j.ad.2012.01.008.
- Thomas KS, Batchelor JM, Bath-Hextall F, Chalmers JR, Clarke T, Crowe S, et al. A programme of research to set priorities and reduce uncertainties for the prevention and treatment of skin disease. Programme Grants Appl Res 2016.
- Cockayne S, Curran M, Denby G, Hashmi F, Hewitt C, Hicks K, et al. EVerT: cryotherapy versus salicylic acid for the treatment of verrucae – a randomised controlled trial. Health Technol Assess 2011;15. http://dx.doi.org/10.3310/hta15320.
- Williams H, Seed P. Inadequate size of ‘negative’ clinical trials in dermatology. Br J Dermatol 1993;128:317-26. http://dx.doi.org/10.1111/j.1365-2133.1993.tb00178.x.
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408-12. http://dx.doi.org/10.1001/jama.1995.03520290060030.
- Armstrong R, Hall BJ, Doyle J, Waters E. Cochrane Update. ‘Scoping the scope’ of a Cochrane review. J Public Health (Oxf) 2011;33:147-50. http://dx.doi.org/10.1093/pubmed/fdr015.
- Sridharan L, Greenland P. Editorial policies and publication bias: the importance of negative studies. Arch Intern Med 2009;169:1022-3. http://dx.doi.org/10.1001/archinternmed.2009.100.
- Ersser SJ, Cowdell F, Latter S, Gardiner E, Flohr C, Thompson AR, et al. Psychological and educational interventions for atopic eczema in children. Cochrane Database Syst Rev 2014;1. http://dx.doi.org/10.1002/14651858.cd004054.pub3.
- Apfelbacher CJ, van Zuuren EJ, Fedorowicz Z, Jupiter A, Matterne U, Weisshaar E. Oral H1 antihistamines as monotherapy for eczema. Cochrane Database Syst Rev 2013;2. http://dx.doi.org/10.1002/14651858.CD007770.pub2.
- Cury Martins J, Martins C, Aoki V, Leonardi-Bee J, Gois AFT, Ishii H, et al. Topical tacrolimus for atopic dermatitis (protocol). Cochrane Database Syst Rev 2012;5. http://dx.doi.org/10.1002/14651858.CD009864.
- Calderon M, Boyle RJ, Nankervis H, García Nunez I, Williams H, Durham S. Specific allergen immunotherapy for the treatment of atopic eczema. Cochrane Database Syst Rev 2010;10. http://dx.doi.org/10.1002/14651858.CD008774.
- Nankervis H, Smith E, Boyle RJ, Rushton L, Williams HC, Hewson D, et al. House dust mite reduction and avoidance measures for treating eczema (protocol). Cochrane Database Syst Rev 2010;3. http://dx.doi.org/10.1002/14651858.CD008426.
- Moed H, Yang Q, Oranje AP, Panda S, van der Wouden JC. Different strategies for using topical corticosteroids for established eczema (protocol). Cochrane Database Syst Rev 2012;10. http://dx.doi.org/10.1002/14651858.CD010080.
- Jadotte Y, Santer M, Vakirlis E, Schwartz RA, Bauer A, Gundersen D, et al. Complementary and alternative medicine treatments for atopic eczema (protocol). Cochrane Database Syst Rev 2014;1. http://dx.doi.org/10.1002/14651858.CD010938.
Appendix 1 Search strategies
MEDLINE (Ovid) Cochrane Collaboration highly sensitive search string
-
random$.mp.
-
factorial$.mp.
-
(crossover$ or cross-over$).mp.
-
placebo$.mp. or PLACEBO/
-
(doubl$ adj blind$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
(singl$ adj blind$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
(assign$ or allocat$).mp.
-
volunteer$.mp. or VOLUNTEER/
-
Crossover Procedure/
-
Double Blind Procedure/
-
Randomized Controlled Trial/
-
Single Blind Procedure/
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12
-
exp Dermatitis, Atopic/
-
atopic dermatitis.mp.
-
atopic eczema.mp.
-
exp NEURODERMATITIS/
-
neurodermatitis.mp.
-
infantile eczema.mp.
-
childhood eczema.mp.
-
(besnier$ and prurigo).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
eczema.mp. or exp Eczema/
-
21 or 17 or 20 or 15 or 14 or 22 or 18 or 16 or 19
-
23 and 13
EMBASE (Ovid) search string
-
random$.mp.
-
factorial$.mp.
-
crossover$.mp.
-
placebo$.mp. or PLACEBO/
-
(doubl$ adj blind$).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name]
-
(singl$ adj blind$).mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name]
-
assign$.mp.
-
volunteer$.mp. or VOLUNTEER/
-
Crossover Procedure/
-
Double Blind Procedure/
-
Randomized Controlled Trial/
-
Single Blind Procedure/
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12
Appendix 2 Randomised controlled trials of eczema treatments in abstract form only
| Study | Interventions |
|---|---|
| Gromert N, Axelsson I. Lactobacillus reuteri effect in atopic eczema in childhood. European Society for Paediatric Gastroenterology, Hepatology, and Nutrition Annual Meeting, Budapest, Hungary. J Pediatr Gastroenterol Nutr 2009;48:E148–9 | L. reuteri dietary supplementation, placebo |
| Rossi AM, Ortonne JP, Guillet G, Dubertret L, Lahfa M, Griffiths C, et al. Efficacy and safety comparison of desonide 0.05% lotion versus fluocortolone 0.5% ointment in atopic dermatitis. Ann Dermatol Venereol 2002;129:1S420 | Desonide lotion 0.05%, fluocortolone ointment |
| Barba JF. Pimecrolimus cream 1% is effective, well tolerated and safe in infants/children with atopic eczema of the face. J Eur Acad Dermatol Venereol 2003;17:182 | Pimecrolimus 1%, vehicle |
| Sugai J, Kakurai M, Otsuki M, Nakagawa H. Combination therapy with 0.1% tacrolimus ointment and cetirizine for facial atopic dermatitis. J Eur Acad Dermatol Venereol 2003;17:17 | Tacrolimus and cetirizine combined |
| Smith CH, Ormerod AD. Pimecrolimus cream 1% once-daily maintenance therapy for the prevention of relapse in mild to moderate atopic dermatitis in adults. Br J Dermatol 2005;153:37 | Pimecrolimus 1%, vehicle |
| Thaci D, Hengge UR, Goertz HP, Rossi AB. Treatment effects of 1% pimecrolimus ointment formulations in adult patients with atopic dermatitis. J Eur Acad Dermatol Venereol 2005;19:218 | Pimecrolimus ointment (three different formulations – X, Y and Z), vehicle |
| Del Rosso J, Bikowski J, Hawkes S, Sanglay L. A double-blind, randomized comparative assessment of efficacy and skin tolerability in patients using either a branded wash versus a soap-based cleanser. J Am Acad Dermatol 2006;54(Suppl. 3):AB64 | Branded skin cleanser, soap-based cleanser |
| Freeman S, Day R, Williams K, Liauw W. A new treatment for atopic dermatitis: a randomized double-blind placebo-controlled study. J Am Acad Dermatol 2006;54(Suppl. 3):AB3 | Auranofin (gold compound) and mometasone ointment, mometasone ointment, vehicle |
| Friedlander SF, Loss R, Schlessinger J, Potts A. A phase 3 double-blind, randomized, vehicle-controlled study of desonide foam in pediatric and adolescent patients with mild to moderate atopic dermatitis. J Am Acad Dermatol 2006;54(Suppl. 3):AB84 | Desonide foam, vehicle foam |
| Hanifin J. A novel topical nuclear factor kappa-B decoy: results from a phase I/II trial in atopic dermatitis. J Am Acad Dermatol 2006;54(Suppl. 3):AB4 | Nuclear factor kappa-B decoy, placebo |
| Hoey S, Catney D, Maguire S, Mckenna K. Fixed low dose versus increasing dose of ultraviolet B-TL01 in the treatment of atopic eczema. Br J Dermatol 2006;155:121–2 | UVB TL01 (standard increasing-dose regimen), UVB TL01 (fixed-dose regimen) |
| Laumann A, Lai S, Lucky A, Schlessinger J, Jarratt M, Jones T, et al. The efficacy and safety of MimyX Cream in reducing the risk of relapse in atopic dermatitis. J Invest Dermatol 2006;126:45 | MimyX cream (contains palmitic acid monoethanolamide and other lipids) and emollient, emollient only (control) |
| Mraz S, Miller B, Bucko A, Tschen E. A multicenter, double-blind, placebo-controlled study of the effectiveness of kiwi fruit extract in adults with atopic dermatitis of moderate severity. J Am Acad Dermatol 2006;54(Suppl. 3):AB3 | Oral kiwi extract, placebo |
| Ramon G, Cambazard F. Evaluation of the corticosteroid-sparing effect of a new emollient in 162 atopic infants. 4th European Association of Dermatology and Venereology (EADV) Spring Symposium, Saariselka, Lapland, Finland, 2006. Abstract P-009 | Emollient milk containing Rhealba® extracts (Pierre Fabre), no emollient (control) |
| Beutner K, Jones T, Bucko A, Loss R. A randomized, double-blind, vehicle-controlled, multi-center study to evaluate the safety and efficacy of topically applied AN0128 cream, 1% for the treatment of mild to moderate atopic dermatitis. J Am Acad Dermatol 2007;56(2 Suppl. 2):AB72 | AN0128 cream (Anacor), vehicle cream |
| Eichenfield LF. A randomized, double-blind, placebo-controlled, parallel group study of nanocrystalline silver (NPI 32101) cream in pediatric atopic dermatitis (AD). J Am Acad Dermatol 2007;56(2 Suppl. 2):AB75 | Nanocrystalline silver (NPI 32101, Westaim Holdings Limited) cream 1%, nanocrystalline silver (NPI 32101) cream 2%, vehicle cream |
| Hanifin J, Hultsch T, Paller A, Eichenfield L. The demographic profile of a large population of infants with atopic dermatitis: a longitudinal study on development of asthma and allergies. J Am Acad Dermatol 2007;56(2 Suppl. 2):AB68 | Pimecrolimus 1%, vehicle |
| Bautista LC, Mendoza A, Sumpaico M, Recto M, Castor M, De Leon J. The effects of fish oil supplementation on serum levels of interleukin 10 and total immunoglobulin E among pediatric patients with atopic dermatitis: a randomized controlled single blind clinical trial. Ann Allergy Asthma Immunol 2010;105:A126 | Fish oil, no treatment |
| Kaur M, Feldman S, Clark A, Inabinet R. Adherence to topical hydrocortisone 17-butyrate 0.1% using different vehicles in adults with atopic dermatitis. J Am Acad Dermatol 2007;56(2 Suppl. 2):AB74 | Topical hydrocortisone 17-butyrate cream, topical hydrocortisone 17-butyrate ointment, topical hydrocortisone 17-butyrate lipocream |
| Kuznecovs I, Jegina K, Kuznecovs S. Atorvastatin and polyprenol effect on atopic dermatitis: pathogenesis links in adult patients. Allergy 2010;65(Suppl. s92):75 | Atorvastatin with polyprenol, placebo |
| Melamed IR, Robinson L, Heffron M. The benefit of montelukast in atopic dermatitis induced by food allergies. J Allergy Clin Immunol 2010;125(2 Suppl. 1):AB93 | Montelukast, placebo |
| Rikken G, Gertner J. SUn13834 in the treatment of subjects with atopic dermatitis. J Invest Dermatol 2010;130:S69 | SUN13834 (chymase inhibitor) (Asubio Pharma), placebo |
| Bishop M, Vukovic-Wysocki I, Qaqundah P, Poulin Y. A 5-year randomized study to investigate the safety of pimecrolimus cream 1% in the treatment of mild-to-moderate atopic dermatitis in infants: Clinical safety. J Am Acad Dermatol 2011;64(2 Suppl. 1):AB56 | Pimecrolimus cream, topical corticosteroid-based regimen |
| Bostoen J, Geusens B, Bracke S, Dekeyser S, Lambert J. Follow-up on the effect of a patient educational programme: early results of a prospective randomized controlled trial in psoriasis and atopic dermatitis. Br J Dermatol 2011;165:e34–5 | Education, no education |
| Rubio-Gomis E, Martinez-Mir I, Morales-Olivas F, Martorell-Aragones A, Palop-Larrea V, Bernalte-Sese A, et al. Randomized controlled, double blind trial of topical twice weekly fluticasone propionate maintenance treatment to reduced risk of relapse in mild or moderate atopic dermatitis (AD) in children. Basic Clin Pharmacol Toxicol 2012;111:30–1 | Fluticasone propionate, vehicle |
| Yuen NS, Ibrahim K, Begum S. Does order of application of emollient and topical corticosteroids make a difference to severity in children with atopic eczema? Eur J Pediatr Dermatol 2012;22:14–15 | Topical corticosteroids followed by emollients, emollients followed by topical corticosteroids |
| Zane LT, Gogoleva T, Heerinckx FA, Jermano JA. Safety and efficacy of AN2728 and AN2898 ointments in a phase 2a bilateral study of mild-to-moderate atopic dermatitis. J Invest Dermatol 2012;132:S90 | AN2728, AN2829 (Anacor Pharmaceuticals) |
| Alex P, Payne A, Desai A, Centola M, Thomas S, Yesudas T. HAT-01, a novel herbal preparation, is superior to corticosteroids and pimecrolimus for the treatment of moderate to severe atopic dermatitis. J Am Acad Dermatol 2013;68(4 Suppl. 1):AB76 | HAT-01 herbal preparation (Haus Bioceuticals Inc.), placebo |
| Fukuie T, Matsumoto K, Narita M, Nomura I, Tokura Y, Ohya Y. Does proactive management of atopic dermatitis affect sensitization or tolerance? A randomized controlled study. Allergy 2013;68:37 | Proactive treatment with topical corticosteroids, reactive treatment with topical corticosteroids |
| Lee KC, West D, Holbrook J, Kwasny M, Lio P. Novel use of a cooling pillow for treatment of severe head and neck atopic dermatitis. J Am Acad Dermatol 2013;68(4 Suppl. 1):AB77 | Chillow (a water-filled, naturally cooling pillow) (Soothsoft Ltd), participant’s normal pillow |
| Nunez C, Hogan D, Humphrey M, Zhang P, Lisante T, Doshi U. A colloidal oatmeal OTC cream is as clinically effective as a prescription barrier repair cream for the management of mild to moderate atopic dermatitis in African American children. J Am Acad Dermatol 2013;68(4 Suppl. 1):AB73 | Colloidal oatmeal cream (over the counter), prescription barrier repair cream |
| Beck LA, Thaci DH, Graham JD, Bieber NM, Rocklin T, Ming R, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med 2014;371:130–9 | Monoclonal antibody, placebo |
Appendix 3 Studies published before 200055
Click on section headings to access the previous 2000 NIHR HTA report. 55
Topical corticosteroids compared with placebo
Topical corticosteroids were compared with placebo in 13 trials published before 2000, which were included in the original review. Most trial reports had a poor quality of reporting, with very little methodological detail provided, and nearly all of the trials were of < 1 month’s duration. Most notably, there were no RCTs looking at betamethasone 17-valerate compared with placebo, but this topical corticosteroid is used as the standard comparator for new topical corticosteroids.
Topical corticosteroids compared with active treatments (except topical immunomodulators)
Forty trials were reported before 2000; however, an overall summary of the evidence from these trials was not provided at that time as the trials did not compare all of the possible contenders for the most effective and safest topical corticosteroid. Most of the trials were described as being of poor quality and they also tended to mix eczema participants with participants who had other conditions. Fluticasone propionate and mometasone propionate given once daily were compared against ‘older’ agents given twice daily and demonstrated reasonable equivalence. Many trials that introduced a ‘me-too’ product claimed equivalence by erroneously assuming that no evidence of statistical difference is the same as evidence of equivalence.
Many trials compared topical corticosteroids, usually as the standard comparator, with other active treatments. Most of the trials did not include a placebo comparator, making it difficult to gauge the level of clinical effect. Several trials compared a topical corticosteroid with a combination of a topical corticosteroid with another active treatment, usually a topical antimicrobial. None of these trials involving combined treatment demonstrated any additional benefit over the topical corticosteroid alone.
Topical immunomodulatory agents
There were four trials of topical immunomodulatory treatment published before 2000.
Other topical treatments
One trial by Harper and colleagues comparing Oilatum against Oilatum Plus containing triclosan and benzalkonium chloride antimicrobials was published in 1995. There was a difference in the change from baseline scores of 9.0 in the Oilatum Plus group and 2.7 in the Oilatum group after 4 weeks of treatment. The participant-rated scores did not show any significant differences.
Five other RCTs were reported before 2000. Two studies comparing the use of two different emollients – Moisturel™ (Warner Chilcott) which is not available in the UK, and Eucerin™ (Beiersdorf AG), which is used in addition to topical corticosteroids – found no significant difference between them. These two trials, which were very short and poorly reported, did not show any significant difference between one emollient and the other when used in the presence of a moderately potent topical corticosteroid. One manufacturer-sponsored study used Cetaphil with corticosteroid compared with corticosteroid alone. This study reported that regularly using emollient in addition to topical corticosteroid may result in small increases in treatment response. Two poorly reported trials on emollients containing urea showed some benefit over vehicle and one study did not show any difference in benefit for different concentrations of urea.
Antimicrobials
One crossover trial by Lever and colleagues conducted in Scotland in 1988 compared topical mupirocin ointment with placebo for 2 weeks. The participants were also able to use topical corticosteroids and emollients. There was a significant decrease in eczema severity for mupirocin treatment compared with placebo treatment for the first 2-week treatment period. There appears to have been a strong carry-over effect from the treatment phase of this trial.
One crossover trial by Harper and colleagues and one parallel-group trial by Holland and colleagues, both published in 1995, compared the bath emollient Oilatum against Oilatum Plus, which has 2% triclosan added as well as another antiseptic (6% w/w benzalkonium chloride). Both trials had a trial period of 4 weeks. The change in severity scores for the two treatments were not compared and baseline scores were not reported in the trial by Harper and colleagues. There was no statistically significant difference in severity between the groups in the trial by Holland and colleagues. Both trials had low numbers of participants and relatively high numbers of dropouts, who were not included in the analyses.
One parallel-group trial from Sweden by Broberg and Faergemann compared a treatment regimen of a miconazole and hydrocortisone cream applied twice daily to the neck and use of ketoconazole shampoo twice a week with a treatment regimen of a hydrocortisone cream and the shampoo base.
Antihistamines
Three trials of loratidine, all given as 10 mg a day, were reported. Two trials compared loratidine against placebo and one three-arm trial compared loratidine against hydroxyzine or placebo.
Two trials of ketotifen were published before 2000. One study in adults and one in children compared ketotifen against placebo. The study in children administered treatment for 4 months and reported no significant differences between the groups in redness of the skin, day itch, night itch or asthma symptoms, as reported by parents. Fifteen out of the 42 children in the trial had eczema. In the adult trial, treatment was administered for 3 months and a significant reduction in itch in both groups compared with baseline on a scale of 1–3 was reported. This trial also measured itch, sleep loss, erythema, lichenification and overall efficacy of treatment.
Five studies of cetirizine were reported before 2000, three of which compared cetirizine with placebo. The largest of the placebo trials, which was relatively well reported, showed possible benefit from cetirizine only at 40 mg, four times the normal dose, and at the expense of some sedation. The other trials were missing important pieces of methodological information making them difficult to interpret.
One multiple crossover trial compared chlorpheniramine and cemitidine against chlorpheniramine or placebo in adolescents and adults administered each treatment for 3 weeks. This underpowered small trial did not show any significant benefit of taking chlorpheniramine alone or in combination with cemitidine.
Four trials of doxepin were reported: two compared topical doxepin against placebo; one compared 2.5% hydrocortisone plus 5% doxepin, 0.1% triamcinolone plus 5% doxepin, 2.5% hydrocortisone only and 0.1% triamcinolone only; and one compared 2.5% doxepin hydrochloride only and 2.5% doxepin hydrochloride plus 0.025% triamcinolone acetonide. All four trials were sponsored by the manufacturer and conducted by the same group of researchers. There was some evidence of a reduction of itch in the first 24–48 hours for doxepin compared with vehicle but this became non-significant after 1 week. None of the trials demonstrated a clinically useful benefit at time points > 1 week. The trials reported drowsiness and stinging as adverse events, which led to differential dropouts.
Ten small trials of topical chromoglycate, all compared with placebo and lasting between 4 and 12 weeks, were conflicting. Most of the positive trials were from the same group of triallists and used a solution rather than a semi-solid formulation.
Dietary interventions
Five trials on borage oil were published before 2000, of which four were small and one was large and well reported. Two of the small trials suggested that there was an improvement in eczema with borage oil and two did not. The large trial did not find any evidence of benefit, not even a suggestion. A post hoc analysis of a subgroup of this large trial, with an unknown number of the most compliant participants and those with blood test changes, showed some beneficial effect. As this analysis was post hoc and the reasons for differences in compliance were not reported, this would need to be explored in a RCT with participants who showed an increase in GLA metabolites.
A large independently funded trial that compared fish oil against placebo did not find any hint of a difference between the groups. Two smaller studies showed a possible benefit of fish oils, with one of these trials showing a very large magnitude of benefit.
Nine trials involving evening primrose oil were published before 2000. Two large well-reported studies showed no evidence of benefit whereas the four moderately sized trials reported conflicting results, with one trial providing evidence of no benefit and another providing evidence of a 10–20% benefit for some outcome measures compared with placebo. Three small studies reported a benefit of taking evening primrose oil orally for eczema.
Non-pharmacological interventions
Only one trial of an educational intervention was reported before 2000. This trial compared an additional nurse education session on top of usual dermatologist care with usual care in 50 infants and young children. A modest benefit was seen for the participants in the intervention group after 3 months. The trial was not blinded, did not use intention-to-treat analyses and did not adjust for different baseline values; therefore, the results should be treated with some caution.
Three small studies compared desensitisation using house dust mite extracts, one in oral suspension and two as injections, with placebo. Two of the studies recruited participants with eczema who a had positive skin-prick test to house dust mite allergen. Neither of these studies found any significant differences in outcomes between the desensitisation group and the placebo group. However, this lack of association could have been a result of the small sample sizes, a strong placebo effect from regular injections or the use of house dust mite reduction measures in the control group.
Four trials on house dust mite reduction were reported, which gave varying results. None of the trials reported the method of randomisation and whether allocation concealment took place. Two of the studies found a significant beneficial effect of intensive high-filtration vacuuming, mattress encasings made of GORE-TEX and acaricide spray on the severity of eczema in children. Another study of Japanese infants not allergic to house dust mite using mattress and quilt encasings found that, in the control group, just over twice as many developed serological evidence of sensitivity to house dust mites, but the study did not look at the impact on severity of eczema. These trials were not pragmatic and were often very intensive. None of the trials reported adverse events.
Three trials involving specialised clothing were reported. The trial by Diepgen and colleagues assessed four different materials of varying fibre roughness. Cotton was found to be significantly more comfortable than the two heaviest, roughest fibres. A second trial by Diepgen and colleagues compared seven different fabrics of varying fibre roughness, yarn roughness and fabric weave. Irritation was higher for warp knits than for jersey knits but there was no difference between cotton and polyester of fine fibre construction. Sweating was found to reduce comfort for all fabrics. The final study compared cellulose with gel nappies for infants with atopic eczema. Napkin dermatitis was reduced in the group using nappies with absorbent gel, but overall there was no difference in the severity of eczema.
Phototherapy
Before 2000, only six RCTs of phototherapy were reported. The only two trials of PUVA treatment could not be included as it was unclear whether the participants had eczema in one of the trials and the subgroup of eczema participants was inseparable in the results of the other trial. The trials were generally small and left and right side of body comparisons were carried out without blinding. The beneficial effects seen were large with a rapid onset and the lack of effect on the placebo side of the body seemed to point to a lack of systemic effect. The trials found that mild skin redness and burning was a common side effect. Information on the long-term risk of cancer was not available from the trials.
Systematic immunomodulatory agents
Twelve trials involving ciclosporin were reported before 2000, two trials of oral ciclosporin and 10 trials of topical ciclosporin. There was good evidence that topical ciclosporin was of benefit compared with placebo; however, the adverse events reported in these trials make it clear that it would not be justifiable to use ciclosporin for long-term treatment.
One trial involving intravenous immunoglobulin was published before 2000 and showed some evidence of benefit but this result would need to be confirmed with further research evidence.
Four trials involving systemic immunotherapy were published before 2000 [three of these trials are in the non-pharmacological treatments chapter (p. 82)55 under house dust mite hyposensitisation].
One trial involving oral prednisolone was published before 2000.
Complementary therapies
Four RCTs were reported before 2000 comparing different Chinese herbal preparations against placebo, all with similar methodologies. Only one of these studies showed a significant beneficial effect of the herbal preparation.
One trial protocol reported before 2000 compared classical homeopathic treatment with placebo for 8 months, in which the homeopathic doctor was free to change remedies, dosages or potencies if required. It appears that this protocol relates to the full trial report described in this updated review.
One trial comparing hypnotherapy with biofeedback or discussion only in children was reported before 2000. There was some reduction in surface damage and lichenification for hypnotherapy compared with discussion only but no difference for hypnotherapy compared with biofeedback. There was no difference in erythema reduction between all of the interventions.
Appendix 4 Topical corticosteroids compared with active treatments
| Trial | Intervention A | Intervention B | Intervention C | Comments on interventions | Country | Number of participants randomised | Inclusion criteria | Outcomes | Comments on outcomes | Main reported results | Quality of reporting |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cato 2001118 | TNX combination; 0.05% triamcinolone acetonide, not stated for laurocapram. Applied twice daily for 2 weeks | TN 0.05% applied twice daily for 2 weeks | AN applied twice daily for 2 weeks | A dose of 0.01% laurocapram is mentioned in the report but not specifically stated as being the dose used in the study | 150 (n = 50 TNX group, n = 50 TN group, n = 50 AN group) | Chronic atopic dermatitis with a minimum of three stable or worsening atopic dermatitis lesions present for at least 1 year | Global evaluation of overall change in disease status relative to baseline (6-point scale, with 1 = cleared/100% clearing of signs/symptoms, 2 = marked improvement/75% to < 100%, 3 = moderate improvement/50–75%, 4 = slight improvement/< 50%, 5 = no change, 6 = exacerbation) Severity of signs and symptoms of disease (erythema, induration and pruritus on a 7-point scale, with 0 = absent to 6 = markedly severe) |
Significantly higher degree of improvement in the TNX group for signs and symptoms of atopic eczema (erythema, induration and pruritus). Greater overall improvement in disease status compared with treatment with TN or AN | The method of randomisation was described and was adequate. Allocation concealment was not reported. Intention-to-treat analysis not reported | ||
| Kim 2013121 | MLE topically applied to skin lesions on one side of the body once a day for 2 weeks | Methylprednisolone aceponate topically applied to skin lesions on the other side of the body once a day for 2 weeks | Patients taking a systemic corticosteroid had a wash-out period of 2 weeks. Patients who applied a topical corticosteroid had a wash-out period of 1 week | Korea | 175 patients were enrolled, number randomised not stated, 159 patients were included in the analysis | Patients with eczema and moderate to severe manifestations. All patients showed eczematous skin lesions, presenting symmetrically | PGA (calculated from scales of erythema, vesiculation, pruritus and burning/pain; each parameter was judged on a 0–3 scale, with 0 = no symptoms, 1 = mild symptoms, 2 = moderate symptoms and 3 = severe symptoms) Clinical efficacy assessed by the PGA improvement ratio (%) = [(PGAday1 – PGAdayn)/PGAday1] × 100 TEWL improvement ratio (%) = [(TEWLday1 – TEWLdayn)/TEWLday1] × 100 Pruritus (assessed subjectively using 10 VASs that the patients scored) VAS improvement ratio Adverse events |
Comparison of PGA score, TEWL ratio and VAS score at baseline with those at days 4, 8 and 15 showed a significant improvement in both groups. MLE group significantly better than methylprednisolone group (74.8% vs. 47.8%; p < 0.05). VAS improvement was better in the MLE group | Randomisation and allocation concealment not described. Double-blind study but no description of blinding or parties blinded. No intention-to-treat analysis described | ||
| Kirkup 2003113 | Study A: FP and HC. Applied twice daily to affected areas for 2–4 weeks until stabilised, then applied intermittently for up to 12 weeks | Study B: FP and HCB. Applied twice daily to affected areas for 2–4 weeks until stabilised, then applied intermittently for up to 12 weeks | Use of regular inhaled or intranasal corticosteroids was permitted | Up to seven countries | Study A n = 137 (n = 70 FP, n = 67 HC); study B n = 128 (n = 66 FP, n = 62 HCB) | Age 2–14 years; experiencing a flare of moderate to severe atopic eczema; total atopic dermatitis score of ≥ 6 | (Primary) Total atopic dermatitis score: extent of body surface area affected and severity, calculated as the sum of three signs (erythema, excoriation and lichenification), each graded 0–3 at a particularly troublesome site (target area). The same target area was used throughout the study. The formula for the total atopic dermatitis score was as follows (max. = 21): number of body areas affected (out of possible 12 body areas) + sum of scores for target area (max. 9) Participant-assessed intensity of rash Adverse events Participant-assessed daytime itch Participant-assessed sleep disturbance |
Total atopic dermatitis score at the end of the acute and maintenance phases was significantly lower following treatment with FP than following treatment with either HC or HCB. Acute phase difference vs. HC: –2.39, 95% CI –3.47 to –1.31 (p < 0.001); vs. HCB: –1.25, 95% CI –2.46 to –0.05 (p = 0.042). Maintenance phase difference vs. HC: –1.88, 95% CI –3.20 to –0.56 (p = 0.006); vs. HCB: –1.39, 95% CI –2.72 to –0.05 (p = 0.042) | Description of randomisation and allocation concealment not provided | ||
| Lebrun-Vignes 2000114 | Micronized desonide cream 0.1%, days 1–5 twice daily (in hospital), days 6 and 7 once daily, then alternate days until day 15 | Betamethasone dipropionate cream 0.05%, days 1–5 twice daily (in hospital), days 6 and 7 once daily, then alternate days until day 15 | All participants had their hygiene standardised: mild soap, emollient, antiseptic foam solution (0.1% hexamidine and 0.3% chlorocresol) | France | 29 participants (n = 15 desonide group, n = 14 betamethasone group) | Age ≤ 8 years, severe eczema; no infection warranting hospital admission; available for follow-up for 30 days | Percentage body surface area involved (Wallace’s rule of nines) Lesion score (0–3 scale, with 0 = no improvement, 1 = slight improvement, 2 = moderate improvement, 3 = considerable improvement for each of the following: erythema, pruritus, discharge, excoriation, lichenification; max. score 15) Relapse after stopping treatment (yes/no) Plasma cortisol levels (comparative blinding assays) |
Both treatments effective for lesion score and affected body surface area. Plasma cortisol levels: days 0–5 – significantly reduced in both groups: desonide group –4.74 mg/ml (p = 0.01), betamethasone group –13.06 mg/ml (p < 0.0001); days 0–20: desonide group –7.38 mg/ml (p = 0.002); days 0–30: desonide group –3.18 mg/ml (p = 0.06). Difference between groups at day 5: p = 0.01 (greater reduction in desonide group); difference between groups at day 20: p = 0.06 (greater reduction in desonide group) | Method of randomisation and allocation concealment not reported. Intention-to-treat analysis not reported | ||
| Prado de Oliveira 2002112 | Mometasone furoate cream 0.1% once a day after a bath for up to 42 days | Desonide cream 0.05% once a day after a bath for up to 42 days | Participants were not allowed antibiotics, antihistamines, topical emollients, other corticosteroids or drugs shown in a laboratory to be associated with hepatotoxicity or which could induce an increase in hepatic enzymes | Brazil | 25 participants (n = 13 mometasone, n = 12 desonide) | Age 2–12 years; atopic eczema with at least 6% involved body surface area; total disease score not < 8; erythema score not < 2; no other clinically significant disease | Signs and symptoms (erythema, lichenification, desquamation, excoriation, pruritus) each graded as 0 = absent (no sign/symptom), 1 = slight (sign/symptom present), 2 = moderate (sign/symptom present, well defined and uncomfortable, although still tolerable), 3 = intense, (sign/symptom difficult to tolerate, interfering with daily activities and/or sleep) Global severity (1 = disappearance of lesions 100%, 2 = notable improvement 75–100%, 3 = moderate improvement 50–75%, 4 = slight improvement < 50%, 5 = no alteration, 6 = exacerbation) Involved body surface area Laboratory examinations (total blood count, glycaemia, TGO and TGP) Cutaneous atrophy (each of: thinning of the skin, striae, shiny skin, telangiectasia, loss of elasticity, loss of normal lines on the cutaneous surface on a 0–3 scale, with 0 = absent, 1 = slight, 2 = moderate, 3 = intense) Vital signs (heart frequency, blood pressure, respiratory frequency) |
Efficacy and tolerablity: both treatments were similar Signs of mild atrophy: mometasone furoate treatment = 4, desonide treatment = 2. No significant differences between values at 42 days (using Freidmann and Mann–Whitney tests) |
Method of randomisation and allocation concealment not reported. Intention-to-treat analysis not reported | ||
| Ruzicka 2012119 | Mometasone furoate 0.1% (a newly developed oil-in-water cream with a water content of 33% – Monovo® Cream (Almirall Hermal GmbH). Each area was treated with 2 mg/cm2 mometasone furoate corresponding to an amount of 200–600 µl. The cream was applied once daily by the patients themselves at home for a period of 2 weeks | Mometasone furoate 0.1% (a commercially available preparation with a marginal water content of < 5% – Ecural® Fettcreme [Schering-Plough (Merck Sharp & Dohme Ltd)]. Each area was treated with 2 mg/cm2 mometasone furoate corresponding to an amount of 200–600 µl. The cream was applied once daily by the patients themselves at home for a period of 2 weeks | Adequate application of cream was verified by weighing the returned amount of product and by the records in a treatment diary. If a significant deviation from the expected need was suspected on day 8, the patient was advised to adjust the dose. Patients were instructed to avoid activities causing excessive sweating (e.g. sunbathing, visiting public swimming pools during the study period). Patients were not allowed to use additives such as bath or shower oils or any cosmetic preparations in the test areas. The treatment area was defined as the entire ventral forearm or the entire ventral lower leg. At least one lesional region had to cover ≥ 20 cm2 | Not stated | 20 patients (10 males, 10 females) | Mild to moderate atopic eczema (defined as a SCORAD score of ≥ 5 with an erythema score of ≥ 2 and a lichenification, dryness and itching score of ≥ 1 each and an Erlangen atopy score sum not < 10 points – the difference in local score values in the treatment fields was not allowed to exceed the level of 3); at least two comparable lesional areas on opposite extremities (forearm or lower leg) | Total clinical assessment score (sum of the individual severity of clinical symptoms of SCORAD: erythema, oedema/papulation, oozing/crusts, excoriations, lichenification, dryness and itching) on a 4-point scale, with where 0 = no symptoms and 3 = severe symptoms Stratum corneum hydration via corneometry Rate of efficient skin penetration (estimated from responses to a patient question: ‘Has the test product been absorbed enough so that you would put on clothes again’) Patient satisfaction (assessed by a questionnaire on cosmetic properties and patient satisfaction) on a 5-point scale: completely applicable, extensively applicable, somehow applicable, less applicable and not applicable Health-related quality of life using the DLQI Safety – evaluated on the basis of medical history and physical evaluation |
Both preparations were equally effective and well tolerated. The new formulation was preferred by the patients | Method of randomisation not described. Allocation concealment not stated. The study was double blind but no other details are given. Intention-to-treat analysis was performed | ||
| Schlessinger 2006117 | Flucinonide cream 0.1% once daily for 2 weeks | Flucinonide cream 0.1% twice daily for 2 weeks | Not stated | 126 participants (once daily n = 63, twice daily n = 63) | Clinically diagnosed with atopic eczema; ≥ 20% body surface area affected; age 3 months to < 18 years; normally functioning HPA axis | Rate of incidence of adrenal suppression (serum cortisol level ≤ 18 µg/dl 30 minutes after intravenous cosyntropin stimulation) Specific skin safety evaluations (presence or absence of skin atrophy, telangiectasia, transparency, loss of elasticity, loss of normal skin markings, thinning, striae, pigmentation changes, bruising) Severity of eczema [clear/almost clear, improved (but less than clear/almost clear), no improvement, worsened] |
Suppression of HPA axis: once daily = none in the two youngest cohorts, twice daily = three in the two oldest cohorts (1/15 cohort 1, 2/16 cohort 2). Severity of eczema: > 90% of participants (all four cohorts) for both groups showed an improvement in disease severity | Method of randomisation and allocation concealment not reported. Intention-to-treat analysis not clear | |||
| Silevis Smitt 2000122 | Clobetasol butyrate ointment 0.05% applied twice a day for 4 consecutive days per week (on the other 3 days used an emollient) for 8 weeks | Clobetasol butyrate ointment 0.05% applied every day, twice a day, for 8 weeks | Not stated | 40 participants | Children with atopic eczema | Severity of eczema (SCORAD score) | Severity of eczema (SCORAD score): baseline, mean (min. to max.) – continuous group 34.8 (16.4 to 53.5), pulse group 33.8 (15.2 to 54.8). Improvement – SCORAD score was 6.7 (SE 3.03) points lower in the pulse group than in the continuous group (p = 0.03). This improvement in the pulse group began as early as the second week of treatment | Method of randomisation not described. Allocation concealment was not reported. Intention-to-treat analysis not reported. It should be noted that information was taken from a short article only | |||
| Thomas 2002116 | Low-potency topical corticosteroid (hydrocortisone 1%) applied twice daily for 7 days; 7-day course given as required for 18 weeks | Potent topical corticosteroid (betamethasone valerate 0.01%) applied twice daily for 3 days (then white soft paraffin twice daily for 4 days); 7-day course given as required for 18 weeks | UK | 207 participants. Mild group n = 104 (hospital n = 17, community n = 87); potent group n = 103 (hospital n = 16, community n = 87) | Age 1–15 years, atopic eczema as defined by the Hanifin and Rajka modified criteria (UK Working Party criteria9); mild or moderate eczema in the month before inclusion in the study | (Primary) Number of scratch-free days (scratch score: ‘How much has your eczema made you scratch today?’, with 1 = not at all to 5 = all the time). Scratch free = scratch scores of < 2 (Primary) Number of relapses during the study period (scratch score: ‘How much has your eczema made you scratch today?’, with 1 = not at all to 5 = all the time). Relapse = scratch score of ≥ 2 for at least 3 consecutive days) (Secondary) Short-term control (median duration of first relapse and median duration of first remission (Secondary) Number of undisturbed nights (Secondary) Disease severity (SASSAD score) (Secondary) Quality of life (Children’s Life Quality Index and Dermatitis Family Impact scores) (Secondary) Number of treatment failures (treatment failure = use of concurrent medication or lost to follow-up) |
Other outcomes (secondary): skin thickness at elbow and knee creases, lateral aspect of the forearm and the back of the calf (20 MHz B-mode ultrasound scanner) | No differences between the mild group and the potent group for all outcomes. Median number of scratch-free days: mild group = 118.0, potent group = 117.5, difference = 0.5 (95% Cl –2.0 to 4.0) (p = 0.53). Median number of relapses: mild group = 1.0, potent group = 1.0. Both groups showed clinically important improvements for disease severity and quality of life | Method of randomisation described. Allocation concealment unclear. Intention-to-treat population defined and used for the analyses | ||
| Trookman 2011120 | Desonide hydrogel 0.05%. A thin layer of medication was rubbed into the affected areas twice daily, morning and evening | Generic desonide ointment 0.05%. A thin layer of medication was rubbed into the affected areas twice daily, morning and evening | Affected area had to be cleansed and patted dry with a soft towel before the medication was applied | Not stated | 84 patients were screened and 46 patients were enrolled, of whom 44 completed the study (desonide hydrogel group n = 22, desonide ointment group n = 22). Two patients voluntarily withdrew before randomisation | Clinically verified atopic dermatitis, at least one mild to moderate atopic lesion at the start of the 4-week trial | EASI score (erythema, oedema, induration and papulation, excoriations and lichenification were assessed at each site and scored on a scale of 0–3, with 0 = absent and 3 = severe; the composite score ranged from 0, no disease, to 72, severest disease on all body areas) Body surface area involvement Target lesion assessment scored on a 5-point scale, with 0 = clear and 4 = severe atopic dermatitis Subjective irritation symptoms (burning/stinging, dryness and itching on a 4-point scale, with 0 = absent and 3 = severe) Corneometry Transepidermal water loss |
At weeks 2 and 4 participants also completed a vehicle preference questionnaire | Desonide hydrogel 0.05% was shown to be as effective as generic desonide ointment 0.05% in reducing the signs and symptoms of mild to moderate atopic dermatitis in patients aged 12–65 years over a 4-week study period | Randomised to treatment group according to a stratified randomisation scheme. No other details of randomisation given. Allocation was concealed from the investigator. Single-blind (investigator) study. Intention-to-treat analysis not reported | |
| Wong 2008115 | Hydrocortisone 1% cream plus miconazole cream twice daily to the affected areas, approximately 12 hours apart, for 2 weeks | Hydrocortisone 1% cream twice daily to the affected areas, approximately 12 hours apart, for 2 weeks | No concomitant medication allowed. Only usual moisturisers and cleansers allowed | Hong Kong, China | 30 participants | Diagnosis of atopic eczema according to the UK Working Party criteria;9 age between 5 and 14 years (criterion not stated); active eczema equally affecting the knees or elbows; asked whether the participant had used an antimycotic in the 3 months before the trial | Participant-assessed relief of symptoms at 2 weeks Dermatologist-assessed change in clinical signs (from photographs) after 2 weeks Number of topical corticosteroid-free days (6-week follow-up period) |
The addition of an antimycotic did not provide any enhanced benefit compared with standard treatment as shown by all three study outcomes | Method of randomisation, allocation concealment and intention-to-treat analysis not reported |
Appendix 5 Topical corticosteroids combined with topical immunomodulators
| Trial | Intervention A | Intervention B | Comments on interventions | Country | Number of participants randomised | Inclusion criteria | Outcomes | Comments on outcomes | Main reported results | Quality of reporting |
|---|---|---|---|---|---|---|---|---|---|---|
| Hebert 2006182 | Tacrolimus 0.1% ointment on top of desoximetasone 0.25% ointment applied twice daily until clear or for 21 days | Tacrolimus 0.1% ointment on top of vehicle of desoximetasone ointment applied twice daily until clear or for 21 days | Treatments applied to affected areas on the left side or right side. Half of the participant population applied double active treatment to one side of the body and single active treatment to the other side. The remaining participants applied the double active treatment to the other side | USA | 82 participants | Age ≥ 18 years; any race; clinical diagnosis of eczema of at least 2 months; total symptom score of the target lesions ≥ 8; subjects needed two bilateral symmetrical target lesions for evaluation at each visit | Total symptom score for the target lesions, mean change from baseline to day 21 (max. score 5; erythema, lichenification, pruritus, scaling/dryness and oozing/crusting each on a scale of 0–3 with half values) Skin atrophy and telangiectasia of the target lesions (scale of 0–3 with half values) Global evaluation at day 21 (visit to visit) (scale from 1 = clear to 6 = severe) Participant-assessed disease control (from 0 = complete control to 3 = uncontrolled) Participant-assessed pruritus from baseline to day 7 (on 0–3 scale with half values) (carried out at the visit – treatment applied under supervision and graded after 20–30 minutes) Participant-assessed burning (on 0–3 scale with half values) (carried out at the visit – treatment applied under supervision and graded after 20–30 minutes) |
Desoximetasone and tacrolimus in combination was superior to tacrolimus alone (p = 0.0002) using the summary score for erythema, lichenification, pruritus, scaling/dryness, and oozing/crusting. Pruritus: less pruritus in the desoximetasone and tacrolimus group than in the tacrolimus-only group (p = 0.04) | Method of randomisation and allocation concealment described. Intention to treat used for the analyses but a definition was not given | |
| Meurer 2010181 | Fluticasone propionate 0.05% (and hydrocortisone acetate 1% for face, neck and intertriginous areas) and pimecrolimus 1% cream applied to the eczema lesions twice daily for 4 weeks. Participants with an IGA score of 0 or 1 entered a 12-week observation period | Fluticasone propionate 0.05% (and hydrocortisone acetate 1% for face, neck and intertriginous areas) and vehicle cream applied to the eczema lesions twice daily for 4 weeks. Participants with an IGA score of 0 or 1 entered a 12-week observation period. | Italy, USA, South Africa, Canada, Germany | Treatment period 376 participants, but only 373 patients received at least one application of study medication (safety population) (n = 190 pimecrolimus and topical corticosteroid group, n = 183 topical corticosteroid group). Observational period 273 participants (n = 138 pimecrolimus and topical corticosteroid group, n = 135 topical corticosteroid group) | Age 2–17 years; severe atopic eczema (IGA of ≥ 4); eczema affecting ≥ 5% of the total body surface area excluding the face | (Primary) Incidence rates of predefined adverse events of interest associated with the use of corticosteroids in participants with severe atopic eczema during the treatment period (Kaplan–Meier – time to first occurrence) (Secondary) Incidence rates of all other adverse events (including local tolerability of the study treatment, bacterial, viral or fungal infections) (Secondary) Time to relapse (relapse = IGA of ≥ 2) (Secondary) Time to treatment success (success defined as IGA of 0 ‘clear’ or 1 ‘almost clear’) (Secondary) Treatment success and improvement (Secondary) Improvement in extent and severity of eczema (EASI, max. score 72) (Secondary) Pruritus relief (success = absent ‘0’ or mild ‘1’) |
(Secondary) Improvement in health-related quality of life | Erythematous rash more frequent in the pimecrolimus and topical corticosteroid group. No other adverse events of interest showed a difference between groups. Efficacy: all outcomes showed no difference between groups apart from time to relapse in participants who were clear at the end of treatment, which was longer in the pimecrolimus and topical corticosteroid group, with a marked improvement in facial eczema | Method of randomisation not described. Allocation concealment not reported. Intention-to-treat analysis used for the efficacy analyses and described, but exact numbers analysed not given |
Appendix 6 Topical corticosteroids combined with occlusive therapy
| Trial | Intervention A | Intervention B | Comments on interventions | Country | Number of participants randomised | Inclusion criteria | Outcomes | Comments on outcomes | Main reported results | Quality of reporting |
|---|---|---|---|---|---|---|---|---|---|---|
| Beattie 2004187 | Hydrocortisone with wet wrap bandages. Hydrocortisone applied once daily in the morning and wet wraps applied twice daily for the first week and then only at night for the second week, for 2 weeks | Hydrocortisone without wet wrap bandages. Hydrocortisone applied twice daily for 2 weeks | All participants instructed to apply emollients as often as they liked throughout the study with a 20-minute delay from the application of hydrocortisone. In the third week, all participants used emollients only | Not stated | 19 participants (n = 10 wet wraps group, n = 9 no wet wraps group) | Age < 5 years; atopic eczema on > 30% of body surface area; no clinical evidence of infection | (Primary) SASSAD score (Secondary) IDLQI (Secondary) Dermatitis Family Impact score Amount of emollients and topical corticosteroid used (weight of tubes) |
SASSAD (mean decrease, 95% Cl): at 2 weeks – difference between groups 8 (–18 to 2) (p = 0.11); IDLQI (median change): wet wrap group 2, no wet wrap group 7 (95% CI for difference –10 to 3) (p = 0.24); Dermatitis Family Impact: wet wrap group 2, no wet wrap group 5 (95% CI for difference –14 to 2) (p = 0.42) | Method of randomisation and allocation concealment described but the descriptions are not clear | |
| Foelster-Holst 2006183 | Coverflex® (Hartmann) tubular bandage and prednicarbate ointment. One arm or leg treated with the ointment and covered with Coverflex moistened by soaking in warm water and covered with dry dressings; 48–72 hours of treatment | Prednicarbate ointment. One arm or leg treated with the ointment; 48–72 hours of treatment | All arms or legs treated were also rubbed with the emollient Alfason Basis Cresa® (Astellas Pharma Europe BV) | Not stated | 24 participants randomised (n = 13 Coverflex and prednicarbate group, n = 11 prednicarbate group) | Adults and children (aged 6–16 and 18–63 years) with an acute episode of atopic eczema; no systemic treatment in the 7 days before recruitment and no topical corticosteroids in the 2 days before recruitment | Physician-assessed local SCORAD score (erythema, papulation, lichenification, exudation, excoriation and dryness on a 4-point scale, with 0 = absent, 1 = mild, 2 = moderate, 3 = severe) | In comparison to prednicarbate alone, the decrease in local SCORAD score in the Coverflex plus prednicarbate group was significantly better. The reduction in severity of eczema was significantly better in the Coverflex plus prednicarbate group (p < 0.011) | No detail given on method of randomisation and no information provided about whether blinding took place. Intention-to-treat analysis not reported. The exact timings of assessments and follow-up visits are not clearly reported | |
| Hindley 2006184 | Wet wrap treatment and conventional treatment: 1% hydrocortisone (or stronger steroid) under wet wraps; 1% hydrocortisone and emollients as required when not using wet wraps. Treatment applied daily, 24 hours a day for 1 week, then 12 or 24 hours per day depending on progress assessed by the ‘education nurse’ | Conventional treatment: emollients, 1% hydrocortisone, more potent steroid if necessary. Emollients applied at least three times daily and when skin dry; 1% hydrocortisone as required applied twice daily; more potent steroid if necessary | Both groups given information/advice about allergen control, emollient application, application of wet wraps (if required), other treatments given by ‘education nurse’ | UK | 50 (n = 28 wet wraps, n = 22 conventional treatment) | Participants aged 3 months to 5 years; eczema diagnosed according to Hanifin and Rajka8 criteria; SCORAD score of > 15 | Efficacy (5-point scale from ‘none’ to ‘very good’) Tolerability (5-point scale from ‘very poor’ to ‘very good’) Ease of application (5-point scale from ‘very difficult’ to ‘very easy’) (Primary) SCORAD score at treatment end |
Mean change in SCORAD score: no significant difference between groups at 4 weeks or in timescale of improvement. Amount of topical corticosteroid used similar in both groups. Wet wrap group had significantly more skin infections requiring antibiotics. Carers rated wet wraps as less easy to apply than conventional treatment | The method of randomisation is described as ‘unmarked envelopes’; however, there no further details given about the envelopes. Allocation concealment not mentioned. Some of the baseline characteristics were quite different between the two groups, pointing to possible bias in the randomisation process. It is stated that intention-to-treat analyses could not be performed as some participants withdrew | |
| Pei 2001185 | Mometasone furoate ointment (one-tenth dilution of 0.1%) with wet wraps (wet Tubifast layer with a dry Tubifast layer over; Seton Healthcare Group) applied once a day to the affected areas after a bath for 4 weeks (group II) or with wet wraps (wet tubifast layer with a dry tubifast layer over) for the second 2 weeks (groups IV) | Fluticasone propionate (one-tenth dilution of 0.005%) applied once a day to the affected areas after a bath for 4 weeks (group I) or with wet wraps for the second 2 weeks (group III) | Participants could enter the second 2 weeks of the trial only if their eczema had failed to improve by > 50% from baseline. All participants had their other treatments standardised from 2 weeks before the start of the study (included emulsifying ointment as soap, petroleum as emollient and 0.005% fluocinolone acetonide applied twice daily to the affected areas). Petroleum was applied to unaffected areas throughout the study | Hong Kong, China | 40 participants (n = 21 fluticasone propionate ointment, n = 19 mometasone furoate). Number of participants in each group (I–IV) not stated | Atopic eczema diagnosed using the UK Working Party criteria;9 age 1–15 years; active disease despite being treated with UK class II or stronger topical corticosteroids as well as soap substitutes and emollients; severity score of ≥ 40 (max. 144) | Disease severity score (six signs – erythema, oedema/papulation, oozing/crusting, excoriation, lichenification, dryness each scored at eight body areas on a 0–3 scale, with 0 = none, 1 = mild, 2 = moderate, 3 = severe, max. 144, max. for each area 18) Disease extent score (rule of nines) Subjective assessment of impact of atopic eczema on daily life (effects on school, work, play, social life, choice of clothing, sleep, itching, pain on a scale of 0–3, higher score = more severe) |
Disease severity (comparison to baseline): significant improvement after 2 weeks of open application (p = 0.043). Wet wraps further improved severity of eczema (p < 0.05). Wet wraps were well tolerated. Mometasone furoate 0.1% and fluticasone propionate 0.005% were both effective at treating eczema. Wet wraps useful as they resulted in further improvement of refractory eczema | Method of randomisation partially described but unclear. Allocation concealment described. Intention-to-treat analysis not reported | |
| Schnopp 2002186 | Wet wraps (Tubifast™ bandages) and mometasone furoate 0.1% applied only to test area. Wet wraps applied twice daily for 5 days (none at night), mometasone furoate 0.1% applied morning and evening | Wet wraps (Tubifast bandages; Mölnlycke Health Care Ltd) and vehicle applied only to test area. Wet wraps applied twice daily for 5 days (none at night), vehicle applied morning and evening | Basic adjuvant therapy allowed on all other body areas | Not stated | 20 participants; not stated how many randomised to each treatment group | Age 2–17 years; exacerbated atopic eczema according to Hanifin and Rajka8 criteria | Severity of eczema using local SCORAD score (erythema, oedema/papulation, oozing/crusts, excoriations, lichenification and subjective local pruritus each graded on a 4-point scale, with 0 = absent, 1 = mild, 2 = moderate, 3 = severe, max. 18) Barrier function using transepidermal water loss S. aureus colonisation |
Improvement in atopic eczema: mometasone group significantly better than vehicle group (p < 0.01). Transepidermal water loss improved: no significant difference between groups. S. aureus colonisation decreased during first 3 days of treatment, then decreased further at day 5 on steroid-treated lesions | Method of randomisation not described. Allocation concealment not stated. Intention-to-treat analysis not reported. The aim of the trial was to study the effect of wet wrap dressings; however, both groups were given wet wrap dressings, which makes interpreting the study difficult |
Appendix 7 Pimecrolimus compared with active treatments
| Trial | Intervention A | Intervention B | Intervention C | Comments on interventions | Country | Number of participants randomised | Inclusion criteria | Outcomes | Comments on outcomes | Main reported results | Quality of reporting |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Emer 2011174 | Thin layer of pimecrolimus cream 1% was applied twice daily for 4 weeks to the study physician-chosen target eczematous area located on one side of the body | Topical medical device cream [Eletone (Mission Pharmacal Company) containing a high lipid content that utilises a specialised hydrolipid technology; reverse phase formulation of 70% lipids dispersed in 30% outer phase of water] was applied three times daily for four weeks on the study physician-chosen symmetrical target eczematous area on the opposite side of the body | Patients were instructed not to bath or shower for at least 8 hours after application of the study treatment | 20 participants | Male or female; aged ≥ 2 years; clear diagnosis of mild to moderate atopic dermatitis for at least 1 year; symmetrical target eczematous areas on opposite sides of the body; PGA of at least ‘mild’ severity for each selected target area. Females were included if they had a negative urine pregnancy test at baseline and agreed to use adequate birth control during the study | (Primary) percentage of subjects achieving a PGA score of clear (0) or almost clear (1) (Primary) PGA of target lesions (0 = clear, 1–5 = increasing severity) (Secondary) PGA score changes at each study visit (Secondary) Change in TLSS at each visit to assess the severity of erythema, papulation/infiltration, excoriation and lichenification (0 = clear, 1–3 = increasing severity) (Secondary) Subject's self-assessment using an IRB-approved 4-point (0–3) scale (half points not accepted) (Secondary) Standardised digital photography (Secondary) Safety evaluations through patient history and physical examination (Secondary) Adverse events |
75% of subjects were rated ‘clear’ or ‘almost clear’ by PGA for both medications after 4 weeks: pimecrolimus 15/20 (75%), topical medical device cream 15/20 (75%). Percent improvement of PGA from randomisation: pimecrolimus 72.50%, topical medical device cream 71.67% (p = 0.9283). PGA scores decreased significantly from baseline for both treatments (p = 0.004). There were no significant differences between the groups for PGA scores throughout the study (p = 0.8236). No cutaneous side effects were noted | Method of randomisation described. Allocation concealed from study investigators. Single-blind study as only the study investigators were blinded. Intention-to-treat analysis not described but data from all 20 participants were analysed | |||
| Frankel 2011173 | Ceramide–hyaluronic acid-based emollient foam applied three times daily on a study physician-chosen symmetrical target eczematous area on the opposite side of the body to intervention B for 4 weeks | Pimecrolimus cream 1%. A thin layer was applied twice daily to a study physician-chosen target eczematous area located on the opposite side of the body to Intervention A for 4 weeks | Patients were advised not to bathe or shower for at least 8 hours after application of study treatment. Patients applied ceramide–hyaluronic acid-based emollient on one side of the body and pimecrolimus cream on the opposite side for the same 4 weeks | USA | 30 participants; 28 participants completed the study | Male or female; age > 2 years; clear diagnosis of mild to moderate atopic dermatitis for at least 1 year with symptoms on the extremities; symmetrical target eczematous areas on opposite sides of the body; IGA of at least mild severity for each selected target area; negative urine pregnancy test and agreeing to use adequate birth control throughout the study (females) | PGA (0 = no inflammatory signs of atopic dermatitis to 5 = severe erythema and papulation/infiltration with oozing/weeping TLSS; erythema, papulation/infiltration, excoriation, lichenification and scaling using a grading system that ranged from 0 = none to 3 = severe Participant self-assessment (a 4-point scale ranging from 0 = complete disease control to 3 = uncontrolled disease) Itching severity scale (VAS) Product preference at the end of the study Safety evaluations Adverse events |
Photography was used to record the location and size of chosen target areas throughout the study | Both pimecrolimus and ceramide-hyaluronic acid foam showed efficacy in mild to moderate atopic dermatitis | Method of randomisation and allocation concealment not clearly reported. Intention-to-treat population not reported. Single-blind study (investigator) | |
| Jensen 2009171 | Pimecrolimus cream 1% applied to the same upper limb twice daily for 3 weeks | Betamethasone cream 0.1% applied to the same upper limb twice daily for 3 weeks | No systemic treatment for atopic dermatitis was allowed. Emollients and other non-pharmaceutical interventions were allowed only on non-target lesions and were monitored by the investigator | Not stated | 15 participants | Adult participants with mild to moderate atopic eczema; target lesion score of 3–8 (0–12 scale) for both right and left sides; symmetrical lesions that do not differ by > 1 point between sides, upper limbs at least 10% affected | pEASI (only upper limbs evaluated) Participant assessment of pruritus (VAS 1–10) |
Immunohistochemistry revealed that the enhanced epidermal proliferation in atopic dermatitis, possibly as a consequence of inflammation, was reduced by 49% by pimecrolimus. The 74% reduction of epidermal proliferation by betamethasone may be considered a suppression exceeding the normal level, possibly leading to thinning of the epidermis. Both treatments induced the differentiation markers involucrin, loricrin and water-binding filaggrin | Data were extracted from the study abstract and therefore trial information was limited | ||
| Luger 2001170 | SDZ ASM 981 cream 0.05%, 0.2%, 0.6% and 1.0% applied twice daily on all affected areas except for the head and neck for up to 3 weeks or until complete clearance if this was sooner | Vehicle cream applied twice daily on all affected areas except for the head and neck for up to 3 weeks or until complete clearance if this was sooner | Betamethasone 17-valerate cream 0.1% applied twice daily on all affected areas except for the head and neck for up to 3 weeks or until complete clearance if this was sooner | Multinational (Belgium, Denmark, Finland, Germany, the Netherlands, Norway, UK) | 260 participants (vehicle n = 43, ASM 0.05% n = 42, ASM 0.2% n = 46, ASM 0.6% n = 42, ASM 1.0% n = 45, betamethasone 17-valerate n = 42) | Men and women aged ≥ 18 years who had given their written informed consent; suffering from atopic dermatitis according to the diagnostic criteria of Hanifin and Rajka8 of at least moderate severity according to the Rajka and Langeland228 grading system and affecting between 5% and 30% of the total body surface area | Adapted EASI score (excluding head and neck region): erythema, infiltration or papules, excoriation and lichenification were assessed on a 4-point scale, with 0 = none, 1 = mild, 2 = moderate, 3 = severe Pruritus assessed on a 4-point scale, with 0 = none, 1 = mild, 2 = moderate, 3 = severe; assessed as the intensity of the itch in the previous 24 hours Participant-assessed atopic eczema at the end of the study assessed on a 7-point scale, with 0 = normal or 100% clear, 1 = almost/90–99% clear, 2 = marked improvement or 75–89% clear, 3 = moderate improvement or 50–74% clear, 4 = slight improvement or 25–49% clear, 5 = unchanged or < 25% clear, 6 = worsened Adverse events |
Betamethasone 17-valerate group had the highest rate of moderately clear or better participants. SDZ ASM 981 1.0%, 0.6% and 0.2% creams were significantly more effective than the vehicle cream. The 0.05% SDZ ASM 981 cream failed to show a significant effect. The 1.0% SDZ ASM 981 cream showed the greatest improvement compared with all concentrations of SDZ ASM 981 Betamethasone 17-valerate was more effective than any SDZ ASM 981 cream concentration in reducing pruritus. SDZ ASM 981 1.0%, 0.6% and 0.2% creams were associated with a greater increase from baseline to end point in the number of participants with pruritus rated as ‘absent’ or ‘mild’ compared with vehicle Participant assessment of eczema at the end of the study showed a higher proportion of participants to be moderately clear or better (> 50% improvement) in the SDZ ASM 981 1.0%, 0.6% and 0.2% cream groups compared with vehicle All treatments were less effective in the more severe participants |
Method of randomisation, allocation concealment and blinding not adequately described | ||
| Luger 2004172 | Pimecrolimus cream 1% applied to all affected areas twice daily until complete clearance and itching had stopped, then treatment restarted if inflammation recurred. Persistent lesions could be treated while stopping treatment of cleared lesions, for 12 months | Triamcinolone acetonide 0.1% (trunk and limbs) and hydrocortisone acetate 1% (face, neck and intertriginous areas) applied to all affected areas twice daily until complete clearance and itching had stopped, then treatment restarted if inflammation recurred. Persistent lesions could be treated while stopping treatment of cleared lesions, for 12 months | Use of emollients during the study was encouraged. Only antihistamines allowed as concomitant treatment | Multinational (Belgium, Canada, Denmark, Finland, France, Germany, the Netherlands, Norway, UK) | 658 participants (pimecrolimus n = 328, topical corticosteroids n = 330) | Age 18–79 years; atopic eczema diagnosed according to the UK Working Party criteria;9 moderate to severe eczema (Rajka and Langeland228 scale); ≥ 5% of body surface area affected | The primary objective of the study was to investigate long-term safety and tolerability | Majority of participants used study treatment continuously over the 1-year treatment period. In patients with an affected body surface area > 30%, rate of all skin infections was significantly lower in the pimecrolimus group (95% Cl of the treatment difference –25.3% to –3.4%). The most common application site reaction was burning (transient, mild or moderate) (pimecrolimus group 25.9%, topical corticosteroids 10.9%. Three participants on topical corticosteroids reported skin striae. There were no clinically significant systemic or treatment-related adverse events. Efficacy: better with continuous topical corticosteroid treatment. Participants who completed the trial were similarly well controlled in both groups. There was no topical corticosteroid use in the 1-year treatment period in 42% of the pimecrolimus group | Method of randomisation and allocation concealment not reported. Intention to treat not used for the analyses |
Appendix 8 Tacrolimus compared with active treatments
| Trial | Intervention A | Intervention B | Intervention C | Comments on interventions | Country | Number of participants randomised | Inclusion criteria | Outcomes | Comments on outcomes | Main reported results | Quality of reporting |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antiga 2010142 | Tacrolimus ointment 0.1% applied twice daily for 21 days | Hydrocortisone-17-butyrate ointment 0.1% applied twice daily for 21 days | 24 patients (tacrolimus group n = 12, hydrocortis one group n = 12) | Male or female; any ethnic group; adults; atopic dermatitis that requires treatment according to the Hanifin and Rajka8 criteria; SCORAD score of ≤ 15; informed consent to contraception during the study until 4 weeks after its conclusion; 2-week pharmacological washout | SCORAD score Immunohistochemical parameters |
Both treatments improved signs and symptoms of atopic dermatitis in all patients analysed. No significant difference between treatment groups | Method of randomisation described. Allocation concealment not stated. Blinding not reported. Unclear whether intention-to-treat analysis was used but analysis was carried out only on patients who completed the study | ||||
| Bieber 2007148 | Tacrolimus 0.03% ointment applied twice daily, morning and evening, to all affected body surface areas for a minimum of 2 weeks and a maximum of 3 weeks and cleared areas treated for an additional 7 days post clearance | Methylprednisolone aceponate 0.1% applied once daily in the evening to all affected body surface areas for a minimum of 2 weeks and a maximum of 3 weeks and cleared areas treated for an additional 7 days post clearance | The methylprednisolone aceponate group also applied a vehicle ointment in the morning to maintain blinding | Germany, Italy, Spain | 265 participants randomised (n = 129 methylprednisolone aceponate 0.1%, n = 136 tacrolimus 0.03%) | Acute flare of atopic dermatitis according to the IGA (≥ 4: ‘severe’ or ‘very severe’); history of moderate to severe atopic dermatitis for at least 1 year; age 2–15 years at baseline; affected body surface area minimum of 5%; avoidance of excessive exposure to natural or artificial sunlight | Static IGA dichotomised into ‘treatment success’ (score clear or almost clear at the end of treatment) and ‘no success’ (score worse than almost clear or missing) EASI Affected body surface area Participant assessment of quality of sleep (100-mm VAS, with 0 mm = ‘no itch/slept well’ and 100 mm = ‘worst itch imaginable/slept badly’) Cost-effectiveness Participant-/parent-assessed intensity of itch during previous 24 hours mEASI, which integrates the participant assessment of itch, the CDLQI and a scaled participant assessment of the change of disease from baseline |
Adverse events were also recorded | For the IGA at the end of treatment (primary), the difference between the two groups was not statistically significant (p = 0.9314). The difference between the groups in change from baseline for EASI was significant after 7 days (p = 0.0352) and 14 days (p = 0.0214) of treatment but not at day 21 (p = 0.0667). For participant assessment of itch, the mean intensity of itch declined substantially from baseline to the end of treatment and was particularly pronounced in the methylprednisolone aceponate group. The change in assessment of itch was already statistically significant in favour of methylprednisolone aceponate by day 4 (p = 0.026) (day 7 p = 0.0006, day 14 p = 0.0007, day 21 p = 0.0004). The improvement in quality of sleep in the methylprednisolone aceponate group was significantly better than that that with tacrolimus at day 14 (p = 0.0409) and at the end of treatment (p = 0.0094) | Method of randomisation was not described. Allocation concealment was not reported. Intention-to-treat analysis not clearly stated. Full analysis set was used for the analyses | |
| Breneman 2005109 | Clobetasol propionate 0.05% lotion applied twice daily for 2 weeks (thin coating on the target lesion and other affected areas) | Clobetasol propionate 0.05% emollient cream applied twice daily for 2 weeks (thin coating on the target lesion and other affected areas) | Placebo (vehicle lotion) applied twice daily for 2 weeks (thin coating on the target lesion and other affected areas) | No topical treatments (except suncream on the face) allowed during the study | Not stated | 229 participants (but stated as 224 ‘enrolled’ earlier in the paper) (n = 96 clobetasol propionate lotion, n = 100 clobetasol propionate emollient cream, n = 33 placebo) | Stable moderate to severe atopic eczema; total dermatological sum score = at least 6 in the target area and at least three of the following: pruritus, flexural lichenification, linearity in adults with a variety of skin lesions, chronic or chronically relapsing dermatitis, personal or family history of atopy; concomitant medical/dermatological disorders that interfere with an accurate evaluation of eczema | (Primary) Success rate (Global Severity Scale: 0–1 = ‘success’, 2–4 = ‘failure’) (Secondary) Global Severity Scale (full scale) (Secondary) Dermatological sum score (Secondary) Individual disease scores for erythema, induration/papulation, lichenification, oozing/crusting, dryness/scaling (when two sign or symptoms were described, the score from the most severe was chosen when the scores did not agree) Adverse events |
Clobetasol propionate lotion was significantly more effective than placebo (vehicle lotion). Clobetasol propionate lotion was comparable to clobetasol propionate emollient cream. Clinical success 2 weeks after treatment cessation was higher for clobetasol propionate lotion than for clobetasol propionate emollient cream | Method of randomisation described. Allocation concealment unclear. Intention-to-treat population described but its use in the analyses is unclear | |
| Caproni 2007141 | Tacrolimus 0.1% ointment applied twice daily for 21 days | Hydrocortisone 1% ointment applied twice daily for 21 days | Not stated | 20 | Adults; male or female; any ethnic group; no legal impediment; atopic dermatitis requiring treatment according to the Hanifin and Rajka8 criteria; SCORAD score of ≥ 15; ability to give written informed consent; ability to follow the study instructions; informed consent to contraception during the study and until 4 weeks afterwards | Change in SCORAD values | This trial was mainly focused on the immunohistochemistry of the treatments; the outcomes for this are not documented | The tacrolimus group showed a statistically significant reduction in post-treatment SCORAD score compared with the hydrocortisone group (p = 0.027) | The method of randomisation was not stated and no mention of allocation concealment or blinding | ||
| Doss 2009146 | Fluticasone propionate 0.005% ointment applied twice daily on facial eczema lesions for 3 weeks or until clearance | Tacrolimus 0.1% ointment applied twice daily on facial eczema lesions for 3 weeks or until clearance | All other eczema lesions treated with open-label fluticasone 0.005% ointment. For 21 days after the initial 3 weeks, the participants could stop treatments if the facial lesions had cleared; stay on the same treatment once a day; or swap treatment using it twice daily (still blinded) | Belgium, Finland, France, Morocco, Romania, Tunisia | 568 participants (n = 288 tacrolimus, n = 280 fluticasone propionate) | Moderate to severe facial atopic eczema; moderate to severe atopic eczema (Rajka and Langeland228 score 4.5–9); age ≥ 16 years; atopic eczema covering at least 10% of the face (head, neck, cleavage and nape); no more than two flares on the face in the past 12 months | (Primary) Response rate (≥ 60% reduction in mLEASI score; mLEASI includes facial erythema, max. 90) from baseline to day 21 (participants who withdrew were all counted as ‘non-responders’) (Secondary) Presence of facial erythema (Secondary) Participant-assessed facial pruritus (100-mm VAS) (Secondary) Participant-assessed global clinical response for facial lesions (7-point scale) (Secondary) Physician-assessed global clinical response for facial lesions (7-point scale) (Secondary) Number of participants who required a switch of treatment (Secondary) Adverse events |
Response rate: tacrolimus 93%, fluticasone 88%; difference p = 0.0026. mLEASI: better improvements in components of mLEASI for tacrolimus than for fluticasone. Facial erythema and pruritus improved in both groups. Global clinical response: ‘marked improvement’ or better: tacrolimus 88%, fluticasone 79%. Number switching to the other treatment after 21 days: fluticasone to tacrolimus 9%, tacrolimus to fluticasone 4.5%. Adverse events: more in the tacrolimus group (mostly application site burning). Safety: in line with the product’s expected characteristics | The method of randomisation was described but slightly unclear. Allocation concealment not described. Intention-to-treat principle used for the analyses | ||
| Doss 2010147 | Tacrolimus 0.03% ointment applied to all affected areas except eyelids until clearance, up to 3 weeks. All participants who responded to treatment could apply treatment once a day to the remaining lesions for another 3 weeks | Fluticasone 0.005% ointment applied to all affected areas except eyelids until clearance, up to 3 weeks. All participants who responded to treatment could apply treatment once a day to the remaining lesions for another 3 weeks | Any participant who had a flare-up in the second 3-week period could resume twice-daily treatment | Belgium, Finland, France, Morocco and Tunisia | 479 participants (n = 240 tacrolimus, n = 239 fluticasone) | Age 2–15 years; moderate to severe atopic eczema (Rajka and Langeland228 score of ≥ 4.5); inadequate response to topical corticosteroids; women of childbearing age must use adequate contraception during the study and for 4 weeks after | (Primary) Response rate (percentage of participants with a ≥ 60% improvement in the mEASI at the end of week 3 compared with day 1). Withdrawals at day 21 were counted as non-responders (Secondary) mEASI score at each visit (Secondary) Severity of pruritus (change from day 1, 100-mm VAS) (Secondary) Sleep quality at each visit (Secondary) Investigator’s global assessment of clinical response at each visit after day 1 (Secondary) Participant/parent global assessment of clinical response at each visit after day 1 (Exploratory analysis) Incidence of new flares during days 21–42 |
Adverse events were also monitored. A post hoc analysis was carried out of partial mEASI for four body regions (head and neck, upper limbs, trunk, lower limbs). Calculated by taking the sum of the four symptoms scores for each region multiplied by the area factor | Response rate: tacrolimus 86.3%, fluticasone 91.5%. Lower limit of the 95% CI 11.8% (exceeds the –15% non-inferiority limit). Investigator’s global assessment of (moderate or better improvement): tacrolimus 93.6%, fluticasone 92.4%. Median pruritus scores: tacrolimus 84.0%, fluticasone 91.5%. Sleep quality: both groups 92%. New flare-ups after day 21 (percentage of participants): tacrolimus 5.5%, fluticasone 11.3%. Mean time to new flare-ups after day 21: tacrolimus 6.5 SD ± 5.0 days, futicasone 8.6 SD ± 5.2 days | Method of randomisation not clearly reported. Allocation concealment not reported. Full analysis set described but it is unclear which analyses this was used for | |
| Gradman 2007145 | Mometasone furoate 0.1% ointment (Elocon®; Schering-Plough A/S) applied once daily at approximately 1900 for 2 weeks | Tacrolimus 0.1% ointment applied twice daily at approximately 0700 and 1900 for 2 weeks | Emollient was applied twice daily during the 2-week run-in, washout and run-out periods. No other treatments allowed during the study | Not stated | 20 participants | Prepubertal (Tanner stage 1 of pubertal development); diagnosed with eczema according to the Hanifin and Rajka8 criteria | EASI score (max. 72) Lower leg length (knemometer) mEASI score (max. score 90), which includes a participant assessment of pruritus |
Mean lower leg length growth rate reduction: mometasone furoate 0.09 mm/week, tacrolimus 0.06 mm/week (F = 1.12, p = 0.35). No statistically significant effects of eosinophil protein X and crossed-linked N-telopeptides found | Method of randomisation and allocation not clear | ||
| Mandelin 2010143 | Tacrolimus 0.1% ointment applied to all affected body areas for a flare until 7 days after clearance, as many times as required in 1 year | Hydrocortisone butyrate ointment 0.1% (trunk and extremities) and hydrocortisone acetate ointment 0.1% (head and neck) applied to affected body areas, as prescribed, for a flare until 7 days after clearance, as many times as required in 1 year | Topical and systemic corticosteroids (other than the study treatments) were not permitted during the study for the treatment of atopic eczema. Also not permitted were topical and systemic antimicrobials that could influence the efficacy results, systemic antihistamines, coal tar, ultraviolet radiation treatments, hypnotics and sedatives and immunosuppressive agents. Participants were advised to minimise the exposure of treated areas to sunlight | 80 participants (n = 40 tacrolimus group, n = 40 hydrocortisone group) | Age ≥ 18 years; moderate to severe atopic eczema (Rajka and Langeland criteria228) | Response rate after 3 months of treatment (proportion of participants with ≥ 60% improvement in mEASI) Clinical efficacy (PGA and participant’s global assessment, eczema score for the head and neck) |
Affected body surface area, EASI score and transepidermal water loss decreased in both groups at months 6 and 12. Efficacy scores: tacrolimus was superior for all efficacy scores at month 6 and for the head and neck at month 12 | Method of randomisation not described. Allocation concealment not reported. Results presented for the intention-to-treat population, but not explicitly stated | |||
| Neumann 2008149 | Protopic ointment/tacrolimus 0.1% ointment (P group). The topical therapy was applied thinly on affected skin once to twice daily (depending on condition of the skin) | Standard therapy/topical corticosteroid group (S group). The topical therapy was applied thinly on affected skin once to twice daily (depending on condition of the skin) | Germany | Total n = 50, of whom 40 were included in the analysis (n = 20 protopic group, n = 20 standard group) | Male and female participants with moderately severe atopic dermatitis according to the criteria of Hanifin and Rajka;8 age ≥ 16 years | Participant severity in terms of EASI. Four different skin regions (head/neck, arm, leg, trunk) were evaluated with respect to the extent of atopic dermatitis and the morphological criteria (erythema, infiltration/papules, excoriation and lichenification; 0 = none, 1 = mild, 2 = moderate, 3 = severe) Percentage body surface area affected |
The improvement in skin condition did not show a statistically significant difference between treatments. Ointment usage was slightly higher in the standard group. Emollient usage: no regular pattern could be demonstrated | Intention-to-treat population and blinding criteria not described | |||
| Nivenius 2007144 | Tacrolimus 0.1% ointment applied twice a day for 3 weeks (2-week washout periods before and after treatment) | Clobetasone butyrate 0.05% applied twice a day for 3 weeks (2-week washout periods before and after treatment) | No topical, periorbital or systemic anti-inflammatory treatments other than the study treatment were allowed during both the treatment and the washout periods | Sweden | 25 participants | Clinical diagnosis of keratoconjunctivitis; eyelid eczema; need for topical and/or periorbital steroid in the previous 6 months; ability to have 2 weeks of washout (no anti-inflammatory and/or anti-infective treatments) | Eyelid eczema severity Blepharitis severity Conjunctivitis and keratitis Participant-assessed eye region discomfort Intraocular pressure Bacterial presence on eyelids Cytokine levels |
Both treatments were effective at reducing signs and symptoms of eczema. Tacrolimus had a near superior benefit in terms of eczema total skin score (p = 0.05) | Method of randomisation and allocation concealment not stated. Intention-to-treat analysis not reported | ||
| Pacor 2004151 | Oral ciclosporin 3 mg/kg and placebo of tacrolimus ointment. Ciclosporin given once a day, placebo of tacrolimus given twice a day, for 42 days | Tacrolimus 0.1% ointment and placebo of ciclosporin. Tacrolimus given twice a day, placebo of ciclosporin given once a day, for 42 days | Rescue medication for itching: 10 mg of cetirizine (one to two tablets a day). No other treatments allowed during the trial | Not stated | 30 participants (n = 15 ciclosporin, n = 15 tacrolimus) | Confirmed diagnosis of moderate or severe atopic eczema (based on the Rajka and Langeland228 criteria); treated with topical corticosteroids and had partial improvement; age 13–45 years (criterion not stated) | Severity of eczema (SCORAD, max. 103) Participant-assessed itch (0 = no itch, 1 = mild itch, 2 = moderate itch, 3 = severe itch) Participant-assessed sleep loss connected with eczema (0 = none, 1 = mild, 2 = moderate, 3 = severe) Participant-assessed erythema (0 = no erythema, 1 = mild erythema, 2 = moderate erythema, 3 = severe erythema) Serum and total IgE Blood eosinophil count Blood markers for haematological, biochemical, electrolyte, renal and hepatic function |
SCORAD score: day 14 – scores in both groups decreased but the tacrolimus group had significantly lower scores than the ciclosporin group; day 42 – overall SCORAD score significantly lower in the tacrolimus group (area under the curve assessment p < 0.001). Itching at day 42 (area under the curve): p = 0.003 in favour of tacrolimus. Sleep loss at day 42 (area under the curve): p = 0.01 in favour of tacrolimus. Erythema at day 42 (area under the curve): p = 0.005 in favour of tacrolimus. Number of days without use of rescue medication: tacrolimus 82.5 days, ciclosporin 76.5 days (p = 0.03) | Method of randomisation not reported. Allocation concealment was unclear. Intention-to-treat analysis not reported | ||
| Reitamo 2002138 | Tacrolimus 0.1% ointment applied in a thin layer twice daily to areas of actively diseased skin | Tacrolimus 0.03% ointment applied in a thin layer twice daily to areas of actively diseased skin | Hydrocortisone acetate 1% applied in a thin layer twice daily to areas of actively diseased skin | Six European countries and Canada | 560 participants were randomised and received at least one application of ointment | Male and female children aged 2–15 years with a diagnosis of atopic dermatitis on the basis of the criteria of Hanifin and Rajka8 were eligible for study participation. Participants were also required to have an atopic dermatitis severity grading of moderate to severe according to the criteria of Rajka and Langeland228 and disease involvement of at least 5% but no more than 60% of the total body surface area | mEASI (max. score 72). Investigators rated erythema, oedema–induration–papulation, excoriations and lichenification on a scale from 0 to 3 and estimated the percentage of the total body surface area affected by atopic dermatitis (0–100%) for four body regions (head and neck, trunk, upper limbs and lower limbs) Participant-assessed intensity of itching using a 10-cm VAS (during the previous 24 hours), with 0 cm indicating ‘no itch’ and 10 cm indicating ‘worst itch imaginable’ Investigator-assessed overall clinical improvement in the physician’s global evaluation, with ‘cleared’ = 100% improvement, ‘excellent’ = 90–99% improvement, ‘marked’ = 75–89% improvement, ‘moderate’ = 50–74% improvement, ‘slight’ = 0–29% improvement, ‘worse’ = improvement of < 0% Adverse events were monitored on an ongoing basis |
The mEASI mean area under the curve as a percentage of baseline showed 0.03% and 0.1% tacrolimus to be significantly more effective than 1% hydrocortisone acetate (p < 0.001) Both tacrolimus concentrations were significantly more effective than 1% hydrocortisone acetate; of the two concentrations tested, 0.1% was more effective than 0.03% Transient skin burning was the only adverse event to show a higher incidence in the tacrolimus treatment group |
Exact method of generating the randomisation sequence was not stated. Allocation concealment was not explicitly stated but was implied. Intention-to-treat principle used for the analyses and defined | ||
| Reitamo 2002138 | Tacrolimus 0.03% ointment applied in a thin layer twice daily to all areas of actively diseased skin. Participants were instructed to continue treatment for the entire 3-week treatment period, regardless of whether clearance occurred | Tacrolimus 0.1% ointment applied in a thin layer twice daily to all areas of actively diseased skin. Participants were instructed to continue treatment for the entire 3-week treatment period, regardless of whether clearance occurred | Hydrocortisone 17-butyrate 0.1% ointment applied in a thin layer twice daily to all areas of actively diseased skin. Participants were instructed to continue treatment for the entire 3-week treatment period, regardless of whether clearance occurred | Eight European countries | Total n = 571 | Male and female participants aged 16–70 years with a diagnosis of atopic dermatitis on the basis of the criteria of Hanifin and Rajka;8 atopic dermatitis severity grading of moderate to severe according to the criteria of Rajka and Langeland228 and disease involvement of at least 5% of the total body surface area. The main exclusion criterion was a serious skin disorder other than atopic dermatitis that required treatment. All participants gave written informed consent | Investigators rated erythema, oedema, induration, papulation, excoriations and lichenification on a scale of 0–3 and estimated the percentage of the total body surface area affected by atopic dermatitis (0–100%) for four body regions (head and neck, trunk, upper limbs and lower limbs) Participants assessed the intensity of itching experienced during the previous 24 hours using a 10-cm VAS, with 0 cm indicating ‘no itch’ and 10 cm indicating worst itch imaginable, to calculate the mEASI. The mEASI is identical to the EASI except that in the latter an assessment of itching is not included Investigators also assessed overall clinical improvement in the physician’s global evaluation of clinical response. ‘Cleared’ indicated an improvement of 100%, ‘excellent’ an improvement of 90–99%, ‘marked’ an improvement of 75–89%, ‘moderate’ an improvement of 50–74%, ‘slight’ an improvement of 30–49%, ‘no appreciable improvement’ an improvement of 0–29% and ‘worse’ an improvement of < 0% Adverse events |
The median mEASI mean area under the curve as a percentage of baseline was 47.0%, 36.5%, and 36.1% for participants who received 0.03% tacrolimus, 0.1% tacrolimus and 0.1% hydrocortisone 17-butyrate, respectively. There was no statistically significant difference between 0.1% tacrolimus and 0.1% hydrocortisone 17-butyrate; however, the lower improvement in mEASI for 0.03% tacrolimus was statistically significant when compared with 0.1% tacrolimus (p < 0.001) or hydrocortisone 17-butyrate (p = 0.002). Skin burning and pruritus at the application site showed a higher incidence in the tacrolimus treatment groups than in the hydrocortisone butyrate group (p < 0.05). Laboratory parameters showed no treatment differences and no marked changes over time | Method of randomisation and allocation concealment not clearly described but adequate measures are implied in the description. Intention-to-treat analysis and blinding both clearly described | ||
| Reitamo 2004139 | Tacrolimus 0.03% ointment applied in a thin layer to all affected body surfaces once daily over a 3-week period | Tacrolimus 0.03% ointment applied in a thin layer to all affected body surfaces twice daily over a 3-week period | HA 1% ointment applied in a thin layer to all affected body surfaces twice daily over a 3-week period | 11 European countries | Total n = 624 (tacrolimus ointment once daily n = 207, twice daily n = 210 or 1% HA twice daily n = 207) | The participants were required to have a grading of moderate to severe atopic dermatitis as defined by the scoring system of Rajka and Langeland.228 Participants were also required to have disease involvement of 5–100% of the total body surface area | (Primary) Percentage change between baseline and the end of treatment in the mEASI. The mEASI is identical to the EASI but includes an additional assessment of itch Participant- or parent-assessed intensity of itching during the previous 24 hours using a 10-cm VAS, with 0 cm indicating ‘no itch’ and 10 cm representing the ‘worst itch imaginable’ (this was converted to an ordinal scale and combined with other scores to complete the mEASI score) Physician’s global evaluation of clinical response. Investigators were instructed to use ‘cleared’ to indicate improvement of 100%, ‘excellent’ for improvement of 90–99%, ‘marked’ for 75–89% improvement, ‘moderate’ for 50–74% improvement, ‘slight’ for 30–49% improvement, ‘no appreciable improvement’ for 0–29% improvement and ‘worse’ for worsening of the condition The participant or parent evaluated the change from baseline of the atopic dermatitis (how it looked, felt, appeared to others) as ‘much better’, ‘better’, ‘slightly better’, ‘same’, ‘slightly worse’, ‘worse’ or ‘much worse’ The quality of sleep during the previous night was rated using a 10-cm VAS, with 0 cm indicating ‘slept badly’ and 10 cm indicating ‘slept well’ Additional end point included the EASI score Adverse events |
Tacrolimus 0.03% ointment both once or twice daily was significantly better than 1% HA (p < 0.001) for mEASI. Tacrolimus 0.03% ointment twice a day was significantly better than 0.03% tacrolimus once a day (p = 0.007) for mEASI For severe atopic dermatitis, twice-daily 0.03% tacrolimus ointment was significantly better than once-daily ointment (p = 0.001) Transient mild to moderate skin burning occurred significantly more often in the 0.03% tacrolimus groups (p = 0.028) but resolved in most cases within 3–4 days Laboratory parameters: no clinically relevant changes |
Method of randomisation not reported. Allocation concealment not stated. Intention-to-treat principle used for the analyses | ||
| Reitamo 2005140 | Tacrolimus 0.1% ointment applied in a thin layer to all affected areas twice daily until 7 days after clearance of eczema each time a flare of eczema occurred for 6 months | Hydrocortisone butyrate 0.1% (trunk and extremities) and hydrocortisone acetate 1% (head and neck) applied in a thin layer to all affected areas twice daily until 7 days after clearance of eczema each time a flare of eczema occurred for 6 months | Bath oils and non-medicated emollients applied 2 hours after applying study medication. Participants could not use topical corticosteroids for eczema, systemic corticosteroids, systemic antimicrobials, sedating antihistamines, coal tar, UV radiation, hypnotics and sedatives or systemic immunosuppressive agents. Washout phases ranging from 5 days to 6 weeks before the start of treatment were employed for these treatments | 12 European counties | Only intention-to-treat population given (n = 487 tacrolimus, n = 485 corticosteroids) | Age ≥ 18 years; diagnosis of atopic eczema according to the criteria of Hanifin and Rajka;8 moderate to severe atopic eczema (at least 4.5 on the Rajka and Langeland228 scale) | (Primary) Response rate at month 3 [responder = 60% improvement in mEASI score (max. score 90); 60% improvement chosen as considered clinically relevant] (Secondary) Response rate at other time points (Secondary) mEASI score (max. 90) (Secondary) EASI score (max. 72) (Secondary) Physician’s global evaluation of clinical response (Secondary) Participant’s evaluation of global response (Secondary) Physician’s assessment of individual signs of atopic eczema |
Other secondary outcomes: total affected body surface area, participant assessment of itch and quality of sleep, number of days of treatment as a percentage of days in the study | Response to treatment at month 3: tacrolimus 72.6%, corticosteroids 52.3% (p < 0.001). Tacrolimus showed greater improvement in mEASI, EASI, affected body surface area and physician and participant assessments of global response. Number of participants experiencing skin burning: tacrolimus 52.4%, corticosteroids 13.8% (p < 0.001). Most cases were mild to moderate and decreased quickly after the first week. There was no increase in malignancies or infections over time in either group | Method of randomisation not explicitly described. Allocation concealment not reported. Intention-to-treat principle used for the analysis but not defined |
Appendix 9 Tacrolimus compared with pimecrolimus
| Trial | Intervention A | Intervention B | Intervention C | Comments on interventions | Country | Number of participants randomised | Inclusion criteria | Outcomes | Comments on outcomes | Main reported results | Quality of reporting |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Draelos 2005177 | Pimecrolimus 1% applied twice daily for 13 days | Tacrolimus 0.1% applied twice daily for 13 days | USA | 37 (n = 18 pimecrolimus, n = 19 tacrolimus) | Adult participants; moderate to severe atopic eczema – IGA 3–5; minimum total body surface area affected of 40% (first 12 participants) or 30% (all other participants enrolled) | Blood concentrations of study treatments Overall IGA (6-point scale, with 0 = clear and 5 = very severe disease) Head and neck only IGA (6-point scale, with 0 = clear and 5 = very severe disease) Participant-assessed overall pruritus (scale from 0 to 3) for 24 hours before visit Adverse events including local application site reactions using a cosmetic acceptability questionnaire and intensity on a 10-cm VAS |
Overall treatment success at day 13: pimecrolimus group 1/18 (5.6%), tacrolimus group 2/19 (10.5%). Head and neck treatment success at day 13: pimecrolimus group 5/18 (27.8%), tacrolimus group 5/19 (26.3%). Results are for severe eczema and may not be applicable to mild or moderate eczema | No details of the method of randomisation or allocation concealment were given. It was stated that the study was not designed for statistical hypothesis testing and therefore it is not surprising that intention to treat is not mentioned. This does, however, make interpretation of the study difficult | |||
| Kempers 2004176 | Pimecrolimus 1% cream applied twice daily until complete clearance of disease or until the week 6 visit | Tacrolimus 0.03% ointment applied twice daily until complete clearance of disease or until the week 6 visit | 141 participants (n = 71 pimecrolimus, n = 70 tacrolimus) | Age 2–17 years; moderate atopic eczema (IGA = 3) | (Primary) Local tolerability (application site reactions) and formulation attributes (questionnaire – questions required responses such as ‘yes/no’ or ‘excellent/very good/good/fair/poor’; if ‘yes’, participants were asked to provide further details including type of reaction – categorised as ‘erythema/irritation’, ‘itching’, warmth/stinging/burning’ and ‘other’) (Secondary) IGA (0 = clear to 5 = very severe disease) Participant-assessed pruritus for the 24-hour period before the clinic visit (0 = no itching/scratching to 3 = bothersome itching/scratching that disturbs sleep) Signs and symptoms of eczema (oozing/crusting, hyperpigmentation, dry skin/xerosis) Percentage of total affected body surface area Adverse events |
Incidence of erythema/irritation was significantly greater using tacrolimus (p = 0.039). Erythema/irritation lasting > 30 minutes was significantly less common in the pimecrolimus group (p = 0.073). Incidence of warmth, stinging and burning was similar in both groups; however, reactions lasting > 30 minutes were fewer in the pimecrolimus group than in the tacrolimus group (p < 0.001). More participants receiving pimecrolimus rated ease of application as ‘excellent’ or ‘very good’ than those receiving tacrolimus (p < 0.020). Pimecrolimus cream had better formulation attributes and local tolerability than tacrolimus ointment while providing similar efficacy and overall safety in paediatric participants with moderate atopic eczema | Method of randomisation was described and allocation concealment was unclear. Intention-to-treat population was described and used for the analyses | ||||
| Paller 2005175 | Tacrolimus 0.1% ointment. A thin layer of study medication was applied twice daily to the affected area(s) for up to 6 weeks or until 1 week after the affected area(s) was completely cleared | Pimecrolimus 1% cream. A thin layer of study medication was applied twice daily to the affected area(s) for up to 6 weeks or until 1 week after the affected area(s) was completely cleared | Not stated | Total n = 226 (n = 112 tacrolimus 0.1%, n = 114 pimecrolimus 1%). Participants evaluable for safety n = 225 | Paediatric participants aged 2–15 years of age; atopic dermatitis disease severity score of moderate to very severe; met the clinical criteria of Hanifin and Rajka8 for the diagnosis of atopic dermatitis; disease over at least 5% of total body surface area | (Primary) EASI score IGADA (a six-category scale including the following categories: clear, almost clear, mild, moderate, severe and very severe) Participant assessment of itch using a VAS graded on a scale of 0 cm (no itch) to 10 cm (worst itch imaginable). Completed by parent or guardian Percentage of total body surface area affected; four body regions included (head/neck, upper limbs, trunk and lower limbs). Adverse events |
Tacrolimus ointment was more effective than pimecrolimus cream at the end of the study in children with moderate/severe disease assessed using EASI (p = 0.04). Tacrolimus was more effective than pimecrolimus for IGADA, improvement in percentage of total body surface area affected and improvement in itch scores (p ≤ 0.05), with a faster onset of action. There was no significant difference in the incidence of adverse events | Randomisation conducted by telephone through a centralised randomisation centre. Investigator was blinded. Participants and the study co-ordinator were aware of the treatment assignment as commercially available products were used. Intention-to-treat data were specified; however, this was as a combined figure with two other cohorts of the same study | |||
| Paller 2005175 | Tacrolimus 0.03% ointment. A thin layer of study medication was applied twice daily to the affected area(s) for up to 6 weeks or until 1 week after the affected area(s) was completely cleared | Pimecrolimus 1% cream. A thin layer of study medication was applied twice daily to the affected area(s) for up to 6 weeks or until 1 week after the affected area(s) was completely cleared | Not stated | Total n = 426 (n = 209 tacrolimus 0.03%, n = 217 pimecrolimus 1%) | Paediatric participants aged 2–15 years with atopic dermatitis that was mild in severity; met the clinical criteria of Hanifin and Rajka8 for the diagnosis of atopic dermatitis; disease over at least 5% of total body surface area | (Primary) EASI score IGADA (a six-category scale including the following categories: clear, almost clear, mild, moderate, severe and very severe) Participant assessment of itch using a VAS graded on a scale of 0 cm (no itch) to 10 cm (worst itch imaginable). Completed by parent or guardian Percentage of total body surface area affected; four body regions included (head/neck, upper limbs, trunk and lower limbs) Adverse events |
Tacrolimus ointment was more effective than pimecrolimus cream at week 1 in children with mild disease measured using EASI (p = 0.04). Tacrolimus was more effective than pimecrolimus for IGADA, improvement in percentage of total body surface area affected and improvement in itch scores (p ≤ 0.05), with a faster onset of action. There was no significant difference in the incidence of adverse events | Randomisation conducted by telephone through a centralised randomisation centre. Investigator was blinded. Participants and the study co-ordinator were aware of the treatment assignment as commercially available products were used. Intention-to-treat data were specified as combined data with that from two other cohorts of the same study | |||
| Paller 2005175 | Tacrolimus 0.1% ointment. A thin layer of study medication was applied twice daily to the affected area(s) for up to 6 weeks or until 1 week after the affected area(s) was completely cleared | Pimecrolimus 1% cream. A thin layer of study medication was applied twice daily to the affected area(s) for up to 6 weeks or until 1 week after the affected area(s) was completely cleared | USA | Total n = 413 (n = 210 tacrolimus 0.03%, n = 203 pimecrolimus 1%) | At least 16 years of age; mild to very severe atopic dermatitis; met the clinical criteria of Hanifin and Rajka8 for the diagnosis of atopic dermatitis; disease over at least 5% of total BSA | (Primary) EASI score IGADA (a six-category scale including the following categories: clear, almost clear, mild, moderate, severe and very severe) Participant assessment of itch using a VAS graded on a scale of 0 cm (no itch) to 10 cm (worst itch imaginable). Completed by parent or guardian Percentage of total body surface area affected; four body regions included (head/neck, upper limbs, trunk and lower limbs) Adverse events |
Tacrolimus ointment was more effective than pimecrolimus cream at the end of the study in adults, measured using EASI (p < 0.0001). Tacrolimus was more effective than pimecrolimus for IGADA, improvement in percentage of total body surface area affected and improvement in itch scores (p ≤ 0.05), with a faster onset of action. There was no significant difference in the incidence of adverse events | Randomisation conducted by telephone through a centralised randomisation centre. Investigator was blinded. Participants and the study co-ordinator were aware of the treatment assignment as commercially available products were used. Intention-to-treat data were specified as a combined figure with two other cohorts of the same study | |||
| Different regimens of the same topical calcineurin inhibitor | |||||||||||
| Ling 2005180 | Pimecrolimus 1% cream applied four times a day for 1 week, then a choice of two to four times a day for 2 weeks | Pimecrolimus 1% cream applied twice a day (placebo twice a day) for 1 week then a choice of two to four times a day for 2 weeks | No topical or systemic treatments (other than the study drug) permitted. Bland emollients could be used on lesional skin from day 8 onwards but not within 1 hour of study treatment application | USA | 49 participants (n = 24 pimecrolimus four times daily, n = 25 pimecrolimus twice daily) | Age ≥ 11 years; diagnosed with atopic eczema according to the Hanifin and Rajka8 criteria; ≥ 30% affected body surface area; IGA of ≥ 2; pruritus score of at least 2 (0–3) | (Primary) Safety and tolerability (adverse events, physical examination including vital signs, haematology and chemistry laboratory measurements) IGA (treatment success = IGA score of 0 or 1) EASI score Participant-assessed pruritus severity score (daily, 0 = no itching/scratching, 3 = uncontrolled disease) Participant-assessed atopic eczema control (0 = complete control, 3 = uncontrolled) |
A substudy assessing pharmacokinetic outcomes was run within this study | Adverse events: number (%) of participants reporting adverse events – pimecrolimus four times daily 3 (12%), pimecrolimus twice daily 4 (17%). No significant difference between groups for frequency, type or severity of events. Median number of applications for days 8–21 (choice of two to four times a day application) remained at four times a day. Both treatment regimens improved the signs and symptoms of atopic eczema to a similar degree according to EASI scores, IGA scores, percentage of affected BSA and participant-assessed control of eczema (biochemical substudy results not extracted) | Method of randomisation and allocation concealment not stated. Intention-to-treat population used and defined. Method of blinding not described | |
| Reitamo 2009179 | Tacrolimus 0.03% ointment. Participants received a single application of study medication on days 1 and 14. On the remaining study days (days 2–13), according to randomisation, treatment consisted of morning treatment and evening treatment with tacrolimus ointment | Tacrolimus 0.03% ointment and placebo. Participants received a single application of study medication on days 1 and 14. On the remaining study days (days 2–13), according to randomisation, treatment consisted of morning treatment with tacrolimus ointment and evening treatment with placebo | Pharmacokinetics trial carried out in Canada, Finland, Ireland, Latvia and UK | 53 participants (n = 28 once-daily tacrolimus ointment, n = 25 twice-daily tacrolimus ointment) | Infants aged 3–24 months with atopic dermatitis requiring treatment with mid-potency topical corticosteroids; at least 5% of body surface area affected by atopic dermatitis at baseline | Physician’s global evaluation of clinical response Physician’s assessment of individual signs and symptoms EASI score Adverse events were monitored and reported by the parent or observed by the physician |
Minimal systemic tacrolimus exposure: 97% of blood samples assayed contained tacrolimus concentrations < 1 ng/ml and 20% were below the lower limit of quantification (0.025 ng/ml). Mean apparent half-life of tacrolimus was 80 SD ± 35 hours (range 25–175 hours). There were no clinically significant changes in laboratory values and the most frequently reported adverse events were minor infections and local skin irritations | Method of randomisation and allocation concealment not described. Intention-to-treat analysis not described | |||
| Ruer-Mulard 2009178 | Pimecrolimus 1% cream. Participants received treatment in the morning and in the evening for up to 16 weeks | Pimecrolimus 1% cream and vehicle. Participants received pimecrolimus cream in the morning and vehicle in the evening for up to 16 weeks | A bland emollient was applied daily throughout the study as a standard care for dry skin | Multi-national | 268 | Open-label phase: male or female paediatric outpatients aged ≥ 2 and ≤ 17 years; mild to severe atopic dermatitis affecting ≥ 5% of the total body surface area; severity of atopic dermatitis confirmed at the screening visit by an IGA score of ≥ 2. Double-blind phase: disease remission (IGA = 0) by the end of the 6-week open-label period or earlier or decrease in IGA score of at least 1 point by the end of the open-label period, without experiencing a disease relapse | Primary end point of the double-blind phase was disease relapse Efficacy was evaluated using the IGA at each scheduled and unscheduled visit (IGA score: 0 = clear, 1 = almost clear, 2 = mild disease, 3 = moderate disease, 4 = severe erythema, 5 = very severe disease) Intensity of pruritus over the 24 hours preceding an unscheduled visit (pruritus score: 0 = none, 1 = mild, 2 = moderate, 3 = severe) EASI score, assessed at the screening visit and at visit 4 Adverse events |
Relapse rate was lower in the twice-daily dose group than in the once-daily dose group, but analysis of the time to disease relapse did not show a statistically significant difference between treatment arms (hazard ratio 0.64, 95% CI 0.31 to 1.30). Treatment of active atopic dermatitis lesions with pimecrolimus 1% cream twice daily, followed by the once-daily dosing regimen was sufficient to prevent subsequent atopic dermatitis relapses over 16 weeks in paediatric participants | Method of randomisation was adequately described. Allocation concealment was not clearly reported. Blinding was adequately described | ||
Appendix 10 Other topical treatments
| Trial | Intervention A | Intervention B | Intervention C | Comments on interventions | Country | Number of participants randomised | Inclusion criteria | Outcomes | Comments on outcomes | Main reported results | Quality of reporting |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abramovits, 2006225 | MAS063DP emollient cream self-administered three times per day (morning, afternoon and evening) to affected areas and those areas prone to be affected for up to 50 days | Vehicle emollient cream self administered three times per day (morning, afternoon and evening) to affected areas and those areas prone to be affected for up to 50 days | Not stated | 218 participants (n = 145 MAS063DP, n = 73 vehicle) | Age ≥ 18 years; diagnosed with mild to moderate atopic dermatitis according to the Hanifin and Rajka8 criteria and Rajka and Langeland228 scale; score of at least 40 mm on a VAS scale for itch (100-mm scale, with 0 mm = no itch, 100 mm = worst possible itch); women of child-bearing potential had a negative test for pregnancy and had to agree to use adequate birth control throughout the study | (Primary) EASI score at day 22 EASI score at other time points Itch (target lesion) on a VAS Percentage of affected body surface area IGA of clinical response from baseline Need for rescue medication in event of a flare Adverse events Participant global assessment of clinical improvement from baseline |
Other outcomes: participant assessment of itch over total body (improvement from baseline on a 4-point scale, with 1 = worse to 3 = total resolution); participant opinion of acceptability of the study cream | MAS063DP was statistically (p < 0.0001) more effective than vehicle in all outcomes at all time points. Incidence of rash: 2.1% in the MAS063DP group vs. 5.5% in the vehicle group. Two participants discontinued MAS063DP because of an adverse event. Participant global assessment: day 22 – MAS063DP group 77% vs. vehicle group 21% had a participant global assessment of ‘good improvement’ or better (chi-square test p < 0.0001). More participants had an improvement in itch over their total body in the MAS063DP group than in the vehicle group (p < 0.0001) | Method of randomisation was described and is good. Allocation concealment not reported. Intention-to-treat population used in the analyses and compared with the per-protocol population | ||
| Amichai 2009196 | Liquid soap (Axera) containing 12% ammonium lactate and 20% urea was used once daily for 3 weeks whilst showering | Placebo commercial liquid soap used for 3 weeks | The placebo soap had a similar odour and cleaning properties to the intervention soap. All patients continued all other systemic or topical medication but avoided any other soap or emollients | 36 patients randomised [n = 24 group A (active), n = 12 group B (placebo)] | Male or female; mild to moderate stable atopic dermatitis diagnosed according to criteria proposed by the UK Working Party;9 age range of participants was 3–40 years but the paper does not state if this was a specific inclusion criterion | Scaling, skin dryness and redness (score of 0–4, with 0 = none, 1 = mild, 2 = moderate, 3 = severe and 4 = worsened) Patient-assessed subjective itch [score of 0–3, with 0 = none, 1 = mild only (occasional), 2 = moderate (many times during the day) and 3 = severe (troublesome itching both day and night] Patient assessment of liquid soap (based on the parameters of skin smoothness, odour quality and stickiness using a score of 1–3, with 1 = very good, 2 = good, 3 = bad) |
After 3 weeks there were significant improvements in investigator-rated scaling, skin dryness and redness in the study group compared with the placebo group (scaling p < 0.0001, skin dryness p < 0.0001, redness p = 0.03). Subjective patient itch improved in the study group compared with the control group (p < 0.001) | No description of randomisation method, allocation concealment or blinding. Not stated whether intention-to-treat principle was used | |||
| Arenberger 2010, 2011,230,231 | Combination of levomenol (0.3 g/100 g cream) and heparin (20,000 IU/100 g cream) cream (group A) applied to the affected eczematous areas twice daily for 8 weeks | Levomenol cream (0.3 g/100 g cream) (group B) applied to the affected eczematous areas twice daily for 8 weeks | Heparin cream (20,000 IE/100 g cream) (group C) applied to the affected eczematous areas twice daily for 8 weeks | Group D = base cream vehicle, applied to the affected eczematous areas twice daily for 8 weeks. Base vehicle was a cortisone-free cream base manufactured as an oil-in-water emulsion | Not stated | 280 patients were screened and 278 participants were randomised (n= 79 group A, n = 80 group B, n = 78 group C, n = 41 group D) | Adults and children with atopic eczema (no specific inclusion criteria based on severity of the atopic dermatitis); age up to 60 years (inclusion of children was explicitly approved of) | (Primary) Itching (100-mm VAS) Severity of eczema (SCORAD index) Physician-rated global assessment (VAS) Participant-rated global assessment (verbal rating scale – ‘no activity’, ‘low’, ‘moderate’, ‘good’ and ‘very good)’ Participant-rated global tolerability (‘very bad’, ‘bad’, ‘acceptable’, ‘good’, ‘very good’) Adverse effects Local tolerance (included the assessment of eczemas, blisters/urticaria, ulcers, excoriation and folliculitis) |
Severity (SCORAD index) and pruritus: combination treatment (group A) was superior to the treatments alone and the control group (analysis of covariance p < 0.00000007). The improvement in pruritus in the combination treatment group approximately equated to the cumulative effect of the two individual treatments. Mean improvements in itching: group A –41.3 mm, group B –13.3 mm, group C –21.3 mm, group D +0.6 mm (95% CIs for comparisons: group A vs. B 7.1 to 13.5, group A vs. C 2.9 to 9.2, group A vs. D 10.4 to 18.3) | Method of randomisation described (ratio 2 : 2 : 2 : 1). Allocation concealment achieved. Physicians, patients, statistician and the study sponsor were fully blinded until statistical assessment was complete. Intention-to-treat population used for the analyses | |
| Belloni 2005224 | MAS063D (Atopiclair) cream applied the first time following visit 1 and then three times a day for 5 weeks | Vehicle-only cream applied the first time following visit 1 and then three times a day for 5 weeks | The vehicle-only cream was described as the emollient cream base that is used as the vehicle for MAS063D | Not stated | 30 participants | Fair/light skin without recent suntan; age > 16 years; mild to moderate eczema (Hanifin and Rajka8 criteria referenced but not specifically stated as being used); grading according to the Rajka and Langeland228 criteria of 3.0–7.5; > 20% cutaneous body surface area involvement; written informed consent given; female sexually active participants required to test negative in a pregnancy test and to agree to use birth control during the study and for 2 weeks afterwards | Severity of atopic dermatitis (graded according to the Rajka and Langeland228 criteria) Percentage of body surface area affected Area and severity using the EASI score Participant-assessed itch score using a VAS (10 cm, without anchor points) Hours of sleep Adverse events observed by clinicians or reported by participants |
Questions to the participants were given in a consistent way according to the structure of the case report form | In the vehicle-only group there was a significant change in itch score (p < 0.05) at visit 4 (end of treatment) compared with baseline. There were no significant changes for any of the other outcomes assessed. In the MAS063D group, statistically significant improvements between visits 1 and 4 were found for the itch score, affected area score and EASI score. In the MAS063D group, 33% of participants stated that they ‘would’ use the cream again and 60% stated that they ‘may’ use it again. In the vehicle group, 60% of participants stated that they ‘may’ use the cream again and 33% stated that they ‘would not’ use it again | Method of randomisation and allocation concealment both described and adequate. Intention-to-treat population used for the analyses | |
| Berardesca 2001200 | Skin lipid mixture containing ceramide-3, cholesterol, palmitic acid and oleic acid in a water-in-oil vehicle with nanoparticles (Reposital®/Alfason® Repair/Lactobase Repair®/Nouriva™ Repair; Yamanouchi) applied one to two times a day until healing occurred or for a maximum of 8 weeks | Skin lipid mixture (see intervention A) and topical corticosteroids applied one to two times a day until healing occurred or for a maximum of 8 weeks | Not stated | 91 participants with atopic eczema out of 508 in total for the trial (n = 206 allergic contact dermatitis, n = 283 irritant contact dermatitis) | Atopic eczema, irritant contact dermatitis or allergic contact dermatitis | Overall disease severity (visual 4-point scale, with 0 = none, 1 = mild, 2 = moderate, 3 = severe) Dryness (visual 4-point scale, with 0 = none, 1 = mild, 2 = moderate, 3 = severe) Scaling (visual 4-point scale, with 0 = none, 1 = mild, 2 = moderate, 3 = severe) Erythema (visual 4-point scale, with 0 = none, 1 = mild, 2 = moderate, 3 = severe) Pruritus (visual 4-point scale, with 0 = none, 1 = mild, 2 = moderate, 3 = severe) Fissuring (visual 4-point scale, with 0 = none, 1 = mild, 2 = moderate, 3 = severe) |
(Only for the participants with atopic eczema) Compared with baseline, both groups improved in all measures taken at week 4 and week 8. Between the two groups: statistically significant improvement in favour of combined therapy for dryness and scaling | Whether a true method of randomisation was used is unclear as the term ‘randomised’ is not used. Allocation concealment was not mentioned and seems unlikely. Intention-to-treat analysis not stated | |||
| Bigliardi 2007216 | 1% Naltrexone cream applied when experiencing severe pruritus for up to 28 days | Placebo cream (vehicle for naltrexone – Excipial cream; Galderma) applied when experiencing severe pruritus for up to 28 days | Participants were allowed to use topical corticosteroids as rescue medication except in the itching intensity measuring periods | Switzerland | 45 participants | Age ≥ 18; atopic eczema; bouts of itching recorded as > 50 mm on a 100-mm VAS (strong intensity) | Location of pruritus attacks (participant recorded) Itch sensation during attacks Use of rescue medication Side effects SPID = SUMi = 1 to n[VAS(i) – VAS(Baseline)] where i = 1 is the first pruritus measurement and n is the last measurement. The mean of three itching attacks was calculated for each time point |
No residual (carry-over) effect found in the analysis | Naltrexone cream had a 29.4% better effect than the placebo cream for sum of the pruritus intensity difference (per-protocol set). Naltrexone cream = median 46 minutes for a reduction of 50% in itching; placebo cream = median 74 minutes for a reduction of 50% in itching | Method of randomisation and allocation concealment not reported. Full analysis set used for the analysis but not defined | |
| Bissonnette 2010195 | Urea 5% moisturiser applied twice a day, morning and evening, on the trunk and limbs for 42 days | Urea 10% lotion | The base emollients of the two treatments have different compositions so this study compares two completely different treatments | Not stated | 100 participants (n = 50, 5% urea; n = 50, 10% urea) | Age ≥ 18 years; diagnosed with atopic eczema (SCORAD score of ≤ 30) | Efficacy (SCORAD index) Safety (5-point tolerance scale, with 1 = very good, 2 = good, 3 = average, 4 = poor, 5 = very poor) Safety (evaluation of adverse events) |
Mean SCORAD score: 5% urea – day 0 to day 42 reduction 19.76%, 10% urea – day 0 to day 42 reduction 19.23% (p < 0.001). No difference between the treatments in reduction of SCORAD scores. Both treatments were well tolerated (three withdrawals because of adverse events). For cosmetic acceptability, significantly more participants preferred the 5% urea moisturiser than the 10% urea lotion | Method of randomisation and allocation concealment were not stated. Intention to treat not used but data to be included in the analyses were clearly described | ||
| Bissonette 2012220 | Synthetic compound 2-isopropyl-5-[(E)–2-phenylethenyl] benzene-1,3-diol (WBI-1001) 0.5% cream applied twice daily for 4 weeks | Synthetic compound 2-isopropyl-5-[(E)–2-phenylethenyl] benzene-1,3-diol (WBI-1001) 1% cream applied twice daily for 4 weeks | Placebo – vehicle cream applied twice daily for 4 weeks | WBI-1001 shows non-steroidal anti-inflammatory properties | Not stated | 37 participants (numbers allocated to each group not stated) | Between 1% and 10% of the body surface area affected (excluding the face, groin, scalp and genital regions); EASI score of < 12; IGA score of 2 (mild) or 3 (moderate) | Severity of eczema (EASI score) Severity of eczema (SCORAD index) IGA Affected body surface area Pruritus (10-cm VAS) Adverse events |
The efficacy analyses should be considered as post hoc as they were carried out before database lock | No serious adverse events were reported. The pharmacokinetic analyses showed that WBI-1001 was only minimally absorbed. The severity of eczema was significantly reduced in the WBI-1001 groups compared with the placebo group as measured by SCORAD and EASI, IGA and pruritus | Method of randomisation not described. Allocation concealment not reported, Intention-to-treat analysis not reported. Not stated which parties were blinded |
| Boguniewicz 2008227 | MAS063DP (Atopiclair) cream applied to skin affected by atopic eczema, skin that had been affected by atopic eczema in the past or skin that could reasonably be affected by atopic eczema during the course of the study. Cream applied three times daily (morning, afternoon and evening) for 43 days | Vehicle cream applied to skin affected by atopic eczema, skin that had been affected by atopic eczema in the past or skin that could reasonably be affected by atopic eczema during the course of the study. Cream applied three times daily (morning, afternoon and evening) for 43 days | It is stated that the emollient base of the vehicle cream was ‘similar’ to the MAS063DP base | USA | 142 participants (n = 72 MAS063DP, n = 70 vehicle) | Girls and boys aged from 6 months to 12 years; diagnosed with atopic eczema according to the Hanifin and Rajka8 criteria; IGA of 2 (mild) or 3 (moderate); at least 5% body surface area affected by atopic eczema at study entry; a sore of at least 40 mm on a VAS from 0 to 100 mm for itch; participant/caregiver agreement to refrain from using other topical and systemic medications (including phototherapy) | (Primary) IGA at day 22 (Secondary) IGA at other time points (Secondary) Participant/caregiver assessment of pruritus on a VAS (Secondary) EASI score at all time points (Secondary) Participant/caregiver assessment of global response from baseline (Secondary) Onset of itch relief and duration of action (Secondary) Need for rescue medication in the event of a flare |
MAS063DP is safe and effective monotherapy in infants and children with mild to moderate atopic eczema. MAS063DP applied three times daily resulted in rapid improvement with resolution of pruritus and was well tolerated | Method of generation of the randomisation procedure was described and was adequate. Allocation concealment was not reported. Intention-to-treat analysis was carried out | ||
| De Belilovsky 2011198 | 2% Sunflower oleodistillate emollient cream (Stelatopia) applied to the entire body twice a day for 3 weeks | Hydrocortisone butyropropionate cream (CENEO®; Pensa) (1 mg/g) applied to the affected lesions twice a day for 3 weeks | Spain | 80 participants (n = 40emollient group, n = 40 hydrocortisone group) | Age 4 months to 4 years; mild to moderate atopic eczema (clinical definition = acute lesions in the folds of the elbows and/or knees and/or surfaces of the limbs and/or cheeks); SCORAD score between 15 and 60 | (Primary) SCORAD score measured at days 0, 7 and 21 (Secondary) IGA of eczema flare-ups at day 21 (Secondary) Quality of Life (IDLQI and Dermatitis Family Impact questionnaires) (Secondary) Individual components of the SCORAD assessment (extent of lesions, erythema, oedema/papulation, oozing/crusting, excoriation, lichenification, dry skin in healthy areas, pruritus, sleep loss) |
SCORAD, hydrocortisone vs. emollient: day 0: 37.2 vs. 36.9, day 7: 18.9 (–49%) vs. 19.2 (–48%), day 21: 11 (–70%) vs. 9.4 (–75%) (p < 0.01 vs. day 0). Quality of life (IDLQI/Dermatitis Family Impact): improvement at day 21: hydrocortisone group 65%/67%, emollient group 72%/75% (p < 0.01 vs. day 1) | Method of randomisation only partially described. Allocation concealment not reported. Intention-to-treat analysis not reported | |||
| Dolle 2010215 | 6% Miltefosine solution (Miltex®; Baxter Oncology GmbH) applied topically (two drops of solution per affected lesion) once daily for the first week, then twice daily for the second and third weeks | 1% Hydrocortisone solution (Hydrogelan®; GALENpharma GmbH) applied topically (two drops of solution per affected lesion) once daily for the first week, then twice daily for the second and third weeks | All of the participants had moderate to severe atopic eczema (as per the SCORAD index) | Not stated | 16 participants (only 12 participants consented to skin biopsies) | Atopic eczema according to the diagnostic criteria of Hanifin and Rajka;8 age ≥ 18; two comparable skin lesions | (Primary) Local clinical response: decline by > 1.5 in TIS score (score of 0–3 for each of erythema, oedema/papulations, excoriations, with 0 = none, 1 = mild, 2 = moderate, 3 = severe) Transepidermal water loss Maximum temperature of the treated lesions Epidermal atrophy (measured by transepidermal thickness) CD4+ T-cell infiltration of the lesions FoxP3+ protein expression |
All assessed lesions had a TIS score of 5–7 before treatment | Both treatments caused a significant decrease in TIS score; however, only miltefosine showed a carry-over effect after stopping treatment. Both treatments reduced the infiltration of CD4+ T cells | Method of randomisation and allocation concealment not reported. Intention-to-treat analysis not reported | |
| Draelos 2009190 | Abolene (over-the-counter moisturiser) with triamcinolone 0.1% cream for moderate eczema applied twice daily for 4 weeks | MimyX (prescription device moisturiser) with triamcinolone 0.1% cream for moderate eczema applied twice daily for 4 weeks | Moisturiser put on top of topical corticosteroid. Participants allowed to use their preferred cleanser throughout the trial. No other moisturisers allowed on the limbs for the duration of the trial | Not stated | 60 participants, 30 with dermatologist-assessed mild eczema, 30 with dermatologist-assessed moderate eczema | Symmetrical mild to moderate eczema (as assessed by a dermatologist) of the arms or legs | (Primary) Abolene and MimyX parity in treating mild eczema (Secondary) Abolene and MimyX parity in treating moderate eczema in combination with triamcinolone cream |
Parity between the over-the-counter moisturiser Abolene and the prescription device moisturiser MimyX was proven. This was based on investigator and participant assessments | Method of randomisation and allocation concealment not reported. Intention-to-treat principle not used for the analyses | ||
| Draelos 2011201 | Hyaluronic acid-based emollient foam (Hylatopic) applied twice daily to the target site for 4 weeks | Ceramide-containing emulsion cream (EpiCeram) applied twice daily to the target site for four weeks | Split-body study. Patients were randomised to apply intervention A to one limb and intervention B to the opposite limb. Patients were instructed to treat all of the non-target areas with the randomised barrier therapy for that side of the body to yield a split-body design | 20 subjects randomised | Age ≥ 18 years; investigator-assessed mild to moderate atopic dermatitis defined by IGA with symmetrically distributed target lesions on the arms or legs – each target lesion required a minimum score of 3 on a 6-point target lesion severity scale (0 = none, 1 = minimal, 2 = mild, 3 = moderate, 4 = moderately severe, 5 = severe) at baseline; minimum total body surface area involvement, including the investigator-evaluated target lesions, of 10%; participant agreed to avoid all other topical medications and moisturisers during the study period and for 2 weeks prior to study enrolment | Investigator-assessed overall eczema severity Investigator-assessed erythema, scaling, lichenification, excoriation, itching, stinging and burning Subject-evaluated skin appearance for redness, peeling, dryness, stinging/burning and overall skin irritation Survey to report which features of the two products the subjects favoured (including spreadability, moisturisation, soothing ability, skin absorption and lack of odour) Subject-assessed belief of which product was most effective, which they would be willing to pay for and which they preferred |
A 6-point ordinal rating scale was used for both investigator and subject assessments (0 = none, 1 = minimal, 2 = mild, 3 = moderate, 4 = moderately severe, 5 = severe) | Both formulations showed a statistically significant improvement in clinical signs and symptoms of atopic dermatitis by week 4 (p < 0.001). The hyaluronic acid-based formulation showed a statistically significant improvement in overall severity by week 2 (p = 0.016) whereas the ceramide-containing emulsion cream did not (p = 0.155). Subjects significantly favoured the hyaluronic acid foam in terms of ability to spread, ability to moisturise, ease of use and lack of odour. The foam was also favoured for effectiveness and the ability to soothe | Method of randomisation and allocation concealment not described. The authors reported that the study was double blind. The evaluating investigator was blinded but it is not clear which other parties were blinded | ||
| Foelster-Holst 2010222 | SRD441 (a topical matrix metalloproteinase inhibitor) (1 mg/g) applied to all affected body areas and commonly affected sites twice daily for up to 28 days | Vehicle applied to all affected body areas and commonly affected sites twice daily for up to 28 days | Germany, Bulgaria, Finland | 93 participants (n = 45 SRD441 group, n = 48 vehicle group) | Age ≥ 18 years; dermatologist-confirmed diagnosis of atopic eczema; mild to moderate atopic eczema at randomisation (IGA of 2 or 3); ≤ 20% of total body surface area affected; current exacerbation of atopic eczema (requiring a ‘step up’ in medication) | (Primary) Success rate at day 21 (success defined as IGA of 0 or 1) (0 = clear, 1 = almost clear, 2 = mild, 3 = moderate, 4 = severe, 5 = very severe) (Secondary) Time to resolution of primary exacerbation (IGA of 0 or 1) (Secondary) IGA score at each visit (Secondary) Participant-assessed total pruritus over previous 24 hours (‘none’, ‘mild’, ‘moderate’, ‘severe’) (Secondary) Number of subjects requiring rescue medication (Secondary) Quality of life (DLQI – 10-question questionnaire with scoring for each question as follows: 3 = very much, 2 = a lot, 1 = a little) Safety (adverse events, physical examinations, clinical laboratory measures, change from baseline in investigator’s visual assessment) |
Success rate (IGA): no significant difference between groups at day 21 – SRD441 11.1%, vehicle 12.5% (p = 1.000). Secondary outcomes did not show any clinical or other significant differences. In total, 18 participants withdrew because of an adverse event (n = 7 SRD441, n = 11 vehicle). Number (%) of participants reporting an adverse event: SRD441 27 (60.0), vehicle 34 (70.8) | Method of randomisation and allocation concealment reported. Intention-to-treat population used for the analyses but not defined | |||
| Gandy 2011221 | CHD-FA 3.5% in an emollient buffered to pH 4.8 applied twice daily for 4 weeks to affected areas | Placebo emollient buffered to pH 4.8 applied twice daily for 4 weeks to affected areas | Epizone A® emollient buffered with acetic acid used as needed | Not stated | 36 (n = 18 CHD-FA, n = 18, placebo) | Healthy males or females with known eczema; age > 2 years; females using a reliable contraception method if of childbearing age; written informed consent | Investigator-assessed global response to treatment [7-point scale, with 0 = completely clear, 1 = almost clear (about 90%), 2 = marked improvement (75%), 3 = moderate improvement (50%), 4 = slight improvement (25%), 5 = no change (moderate to severe disease) 6 = worse] Investigator assessment of severity of disease (5-point scale, with 0 = absent, 1 = mild, 2 = moderate, 3 = moderately severe, 4 = severe) Severity of eczema assessed by the participant (VAS from 0 to 100 mm) Blood tests (liver function tests, full blood count, kidney function tests) Clinical examination and electrocardiography Adverse events Laboratory investigations |
All safety parameters stayed within normal limits. There were significant differences for severity and erythema compared with baseline in the placebo and CHD-FA groups. There was a significant difference for scaling compared with baseline in the placebo group | No description of randomisation process or allocation concealment. The title states that this was a double-blind trial but there is no description of who was blinded. It is unclear if the intention-to-treat population was used or not. It is stated that two patients were excluded from the analysis for using concomitant medications; however, the number in the data tables appears to be the number randomised | ||
| Giordano-Labadie 2006192 | Moisturising milk (Exomega) applied all over the body twice a day for 2 months | Standard cleansing bar (A-Derma) applied all over the body twice a day for 2 months | At least 1 week washout of no application of topical therapy prior to randomisation for all participants. Only topical corticosteroids were allowed during the study | Not stated | 76 participants (n = 37 = Exomega milk, n = 39 standard cleansing bar) | Age 6 months to 2 years; mild to moderate atopic eczema (SCORAD score < 35) | Severity of eczema (SCORAD) Tolerance (0 = excellent/no side effects, 1 = satisfactory/minor unwanted side effects, 2 = mediocre/occurrence of unwanted side effects requiring reduction of frequency of application, 3 = unsatisfactory/occurrence of unwanted side effects requiring stoppage of treatment) Topical steroid use Quality of life (CDLQI; max. score 30) |
SCORAD: after 2 months decrease in SCORAD index in Exomega milk group not statistically significantly (p = 0.051). There was a significant difference between groups at 2 months for xerosis (p = 0.01) and pruritus (p = 0.01) in favour of the treatment group. There was a significant improvement in quality of life after 2 months in the Exomega milk group (p = 0.0011). Good to excellent tolerance: 97% participants | Method of randomisation, allocation concealment and intention-to-treat analysis not described | ||
| Griffiths 2002124 | Cipamfylline cream (1.5 mg/g), maximum application of 2 g (four fingertip units) per day for up to 14 days | Hydrocortisone 17-butyrate 0.1% cream, maximum application of 2 g (four fingertip units) per day for up to 14 days | Vehicle of cipamfylline cream, maximum application of 2 g (four fingertip units) per day for up to 14 days | Canada, Denmark, the Netherlands and the UK | 103 participants (n = 54 cipamfylline/vehicle group, n = 49 cipamfylline/hydrocortisone 17-butyrate group) | Men and women aged ≥ 18 years, diagnosis of atopic eczema according to the Hanifin and Rajka8 criteria; stable, symmetrical lesions of atopic eczema on both arms with a minimum total severity score of 6 for a selected larger lesion; women of childbearing potential had a negative pregnancy test and used adequate contraception throughout the study | (Primary) Investigator-assessed absolute change in TSS from baseline to end of treatment (TSS = severity of erythema, oedema/papulation, oozing/crusting, excoriations, lichenification on a 4-point scale, with 0 = absent, 1 = mild, 2 = moderate, 3 = severe) (Secondary) Investigator-assessed overall response to treatment since commencement of treatment as recorded on days 3, 7, 14 (‘worse’, ‘no change’, ‘minimal improvement’, ‘moderate improvement’, ‘marked improvement’ or ‘completely cleared’) (Secondary) Participant-assessed overall response to treatment (Secondary) Participant-assessed cosmetic acceptability (‘unacceptable’, ‘acceptable’, ‘good’ or ‘very good’) (Secondary) Participant-assessed severity of pruritus of the target lesions on a 10-cm VAS (ranging from ‘no itching’ to ‘worst possible itching’) (Secondary) Proportion of participants relapsing defined as the requirement for treatment of atopic dermatitis on the target lesions |
There was a statistically significant reduction in the TSS from baseline to the end of treatment for all three treatments (p < 0.002). In the cipamfylline/vehicle group the difference between treatments was statistically significant in favour of cipamfylline (1.67, 95% CI 1.06 to 2.28; p < 0.001). In the cipamfylline/hydrocortisone 17-butyrate group the difference between treatments was statistically significant in favour of hydrocortisone 17-butyrate (–2.10, 95% CI –1.27 to –2.93; p < 0.001) | Randomisation procedure described. Allocation concealment appears to have happened, although this is not as clear. Intention-to-treat population was used and clearly defined | ||
| Grimalt 2007191 | Emollient (Exomega lotion) applied twice a day (after morning cleansing and before bed in the evening) for 6 weeks in sufficient amount on the dry, non-inflammatory areas of the skin over the whole body | Control (no emollient) | Both groups applied topical corticosteroids of high (micronised desonide 0.1% cream) or moderate (desonide 0.1% cream) potency on the inflammatory lesions according to the investigators’ regular practice | Not stated | 173 participants randomised (n = 91 emollient group, n = 82 control group) | Male and female infants aged < 12 months with moderate to severe atopic eczema with a SCORAD score of 20–70 | (Primary) Steroid-sparing effect (measured as amount of topical corticosteroid used) (Secondary) Severity of eczema (SCORAD) (Secondary) Participant and parent quality of life (French version of the IDLQI and Dermatitis Family Impact) Physician-assessed global tolerance (only for emollient group) on a 4-point scale from 1 = no sign of tolerance to 4 = intolerance signs leading to treatment discontinuation Adverse events |
The amount of topical corticosteroid used in 6 weeks decreased by 7.5% (not significant) and 42% (p < 0.05) in the control and the emollient group, respectively. The SCORAD index and infant and parent quality of life significantly improved (p < 0.0001) in both groups | Some information given about the method of randomisation and the reason for not blinding the study is also stated. Intention-to-treat population used for the analysis, although it was not fully described | ||
| Guéniche 2006213 | V. filiformis bacterial extract in a base cream containing glyceryl mono/distearate and polyethylene glycol stearate, isoparaffin cyclopentadimethylsiloxane (5% V. filiformis biomass) was applied in a thin layer to an investigator-defined area twice daily for 4 weeks | Base cream containing glyceryl mono/distearate and polyethylene glycol stearate, isoparaffin cyclopentadimethylsiloxane was applied in a thin layer to an investigator-defined area twice daily for 4 weeks | Base cream not designed specifically for atopic skin. Areas for treatment were symmetrical: left side/right side | Not stated | 13 participants | Atopic dermatitis; mild to moderate according to Rajka and Langeland228 criteria; 5–60% total body surface area involvement | Investigator-assessed erythema, oedema–induration–papulation, excoriations and lichenification (4-point scale from 0 to 3) and estimated total body surface area affected (0–100%) in four body areas: head and neck, trunk, upper limbs and lower limbs Participant-assessed itch intensity (in previous 24 hours on a 10-cm VAS, with 0 cm = no itch and 10 cm = worst itch imaginable) mEASI (includes an assessment of itch) (max. score 90) Investigator-assessed global evaluation of clinical response (cleared = 100% improvement, excellent = 90–99% improvement, marked = 75–89% improvement, moderate = 50–74% improvement, slight = 30–49% improvement, no appreciable improvement = 0–29% improvement, worse = worsening of the condition) Adverse events (any undesirable experience that occurred during the trial) |
A beneficial effect was seen after 2 weeks of treatment, which increased after this point | Method of randomisation and allocation concealment not reported. Intention-to-treat population defined but it is not clear which analyses were carried out using this population | ||
| Guéniche 2008214 | 5% V. filiformis lysate cream applied twice daily in a thin layer on predefined areas for 30 days | Vehicle cream applied twice daily in a thin layer on predefined areas for 30 days | Not stated | 75 (n = 37 5% V. filiformis lysate cream, n = 38 vehicle cream) | Age 6–70 years; diagnosis of mild atopic eczema according to the Hanifin and Rajka8 criteria; history of atopy (e.g. allergic rhinitis and/or allergic asthma, atopic eczema, type I sensitivity to aeroallergens); presenting with dry and/or scaling skin | Severity of eczema (SCORAD index) Participant-assessed pruritus (VAS) Participant-assessed sleep loss (VAS) Transepidermal water loss Skin microflora (counts per 1 cm2 of skin surface) Adverse events |
Severity of eczema: 5% V. filiformis lysate cream compared with vehicle – p = 0.0044 in favour of 5% V. filiformis lysate cream. Pruritus: 5% V. filiformis lysate cream compared with vehicle – p = 0.0171 in favour of 5% V. filiformis lysate cream. Sleep loss: 5% V. filiformis lysate cream significantly decreased sleep loss from day 0 to day 29 (p = 0.0074). Skin microflora: 5% V. filiformis lysate cream reduced S. aureus on the skin. Skin barrier: transepidermal water loss decreased significantly in both groups from baseline with no difference between the groups | Both randomisation and allocation concealment described. Intention-to-treat principle used for the analysis | |||
| Hamada 2008234 | Camellia oil spray (Atopico® Skin Health Care Oil) applied as often as desired for 2 weeks (then used purified water spray for 2 weeks) | Purified water spray applied as often as desired for 2 weeks (then used camellia oil spray for 2 weeks) | No modification to participants’ standard care | Japan | 42 participants | Patients with atopic dermatitis who fulfilled the atopic dermatitis criteria of the Japanese Dermatological Society;26 severity less than moderate, which was based on the atopic dermatitis guideline of the Ministry of Health, Labour and Welfare in Japan | Change in eczema severity evaluated by the physician Change of applied ointment dose assessed by the patient Moisturising affect assessed by the patient Frequency of use of the spray assessed by the patient Effectiveness of the treatment assessed by the patient Itch score assessed by the patient |
Changes in the severity of clinical manifestations: no difference between groups. Participants’ impressions of the treatments: significant difference between groups in favour of the camellia oil spray (p < 0.01) | Method of randomisation not described. Allocation concealment not reported. Intention-to-treat population does not appear to have been used for the analyses | ||
| Hashizume 2013236 | 1% AHYP in a cream with aqua, squalane, glycerin, glyceryl stearate, stearic acid, steareth-20, cetyl alcohol, arginine, dimethicone, sorbitan stearate, 1,2-hexanediol, caprylyl glycol and phenoxyethanol applied to one forearm twice daily for 4 weeks | Control cream containing aqua, squalane, glycerin, glyceryl stearate, stearic acid, steareth-20, cetyl alcohol, arginine, dimethicone, sorbitan stearate, 1,2-hexanediol, caprylyl glycol and phenoxyethanol applied to the other forearm twice daily for 4 weeks | The study was carried out in winter. Subjects were asked not to use any other new medicines to treat their skin apart from their usual treatment and not to change or add any emollient or medicine during the study | Japan | Slight atopic dermatitis diagnosed by a dermatologist in accordance with the atopic dermatitis treatment guidelines of the Japanese Dermatological Association26 | Transepidermal water loss Pruritus change (pruritus intensity evaluated using a 100-mm VAS, with 0 mm = no itch and 100 mm = maximum itch) Dermatological observations Safety |
No adverse events were observed. Transepidermal water loss was increased in the control cream-treated region of the forearm (p < 0.05) after 4 weeks whereas there was no change in the AHYP-treated region of the forearm. Pruritus intensity was decreased in the AYHP-treated forearm between 0 and 4 weeks (p < 0.05) but there was no change in the control-treated forearm | Randomisation method and allocation concealment not described. The study was blinded but method of blinding and parties blinded were not stated. Intention-to-treat population not described | |||
| Herzog 2011237 | SRD174 cream (nalmefene hydrochloride monohydrate, a μ-opiate receptor antagonist; strength 0.95% w/w anhydrous nalmefene base with isopropyl myristate, glycerol, hydroxyethylcellulose and phenoxyethanol supplied in a 10-g tube). When the subject experienced an itch of ≥ 40 on a 101-point VAS during the treatment periods he or she had to identify a target area of highest intensity and treat both the target area and other areas of bothersome itch. The severity of the target area itch was then assessed using the 101-point VAS at 15, 30, 45 and 60 minutes and 2, 3, 4, 6 and 8 hours after administration of the trial medication Once a recording of itch intensity was complete (8 hours) another area could be treated. Subjects could treat more than one episode of itch in a day provided (1) the episodes were > 8 hours apart and (2) the total amount of study drug used in a day was less than one tube (10 g) | Vehicle cream, identical to the SRD174 cream but without the nalmefene. When the subject experienced an itch of ≥ 40 on a 101-point VAS during the treatment periods he or she had to identify a target area of highest intensity and treat both the target area and other areas of bothersome itch. The severity of the target itch was then assessed using the 101-point VAS at 15, 30, 45 and 60 minutes and 2, 3, 4, 6 and 8 hours after administration of the trial medication Once a recording of itch intensity was complete (8 hours) another area could be treated. Subjects could treat more than one episode of itch in a day provided (1) the episodes were > 8 hours apart and (2) the total amount of study drug used in a day was less than one tube (10 g) | SRD174 cream is white to off-white and supplied in a collapsible aluminium tube. The vehicle cream had the same ingredients with the exception that it contained no nalmefene. All labelling and packaging for the two study drugs was identical. Subjects applied either vehicle or SRD174 in the first treatment period of 7 days and the opposite treatment for the second treatment period of 7 days following a washout period (length of washout not stated). During the assessment period (0–8 hours) following treatment, subjects were asked not to reapply the study medication to the target itch or other areas. Subjects were also asked not to apply any emollients or rescue medication during this time and particularly for at least the first 4 hours. If itch intensity was < 40 on the VAS they were asked not to apply trial medication. They were asked to wait for the itch to alleviate spontaneously or for it to worsen such that the VAS score was ≥ 40. They would then be eligible to apply the study medication. When waiting was not acceptable, the subjects could use their standard emollient or moisturiser. This would be recorded as a concomitant medication | Not stated | 136 subjects were screened and 62 were randomised (n = 31 SRD174 in the first treatment phase, n = 31 vehicle in the first treatment phase) | Male and female; age ≥ 18 years; recurrent, persistent episodes of moderate to severe atopic dermatitis pruritus defined as ≥ 40 on a 101-point pruritus VAS; active and pruritic atopic dermatitis covering a body surface area ≤ 20%; subjects had adequately recorded at least three episodes of moderate to severe pruritus defined as VAS score of ≥ 40 in the 7 days prior to randomisation | (Primary) Period mean of the SPID from 0 to 4 hours based on participant assessment of itch intensity at 15, 30, 45 and 60 minutes and 2, 3 and 4 hours post study drug administration compared with baseline (Secondary) SPID from 0 to 8 hours Pruritus intensity difference at each assessed time point Change in EASI score during each time period IGA during each treatment period Quality of sleep recorded during each treatment period Average daily pruritus score during each treatment period Eppendorf Itch Questionnaire |
Other secondary end points included pruritus relief; time to achieve a > 30%, > 50% and 80% reduction of itch sensation; time to reduction of itch sensation to VAS score of < 40; and use of rescue medication for pruritus | Least square means (SDs) for SPID: SRD174 210.7 (20.4), vehicle 212.1 (20.2), difference –1.3 (95% CI –25.9 to 23.3). None of the secondary efficacy end points demonstrated a statistically significant or clinically important difference between the test product and the vehicle. The SRD174 cream was well tolerated but there was a higher incidence of adverse events in the SRD174 group: SRD174 22 (36.7% of subjects), vehicle 14 (23.3% of subjects) | Randomisation method described. Allocation concealment not described. Study was double blind but it was not clear who was blinded. Intention-to-treat analysis was used | |
| Januchowski 2009218 | Vitamin B12 cream (0.07% by weight of cyanocobalamin in a moisturising base) applied for 4 weeks | Placebo cream (moisturising base only) applied for 4 weeks | Not stated | 22 | Age 6 months to 18 years; eczema; ability to understand the consent process | Severity of eczema (modified SCORAD index, max. score 27) | Treatment with topical vitamin B12 significantly improved the skin in comparison to the placebo cream at 2 weeks (p = 0.02) and 4 weeks (p = 0.01) | Method of randomisation described but slightly unclear. Allocation concealment was unclear. Intention-to-treat analysis not reported | |||
| Katsuyama 2005229 | ‘Oil-in-water’ cream containing 17% moisturiser, 0.2% farnesol (3,7,11-tri-methyl-2,6,10-dodecatrien-1-ol, molecular weight 222.37) and 5% xylitol (xylo-pentane-1,2,3,4,5-pentol, molecular weight 152.15) was applied to the left or right forearm for 7 days | (Placebo) ‘Oil-in-water’ cream containing 17% moisturiser was applied to the left or right forearm for 7 days | Not stated | 17 participants | Atopic eczema of mild to moderate severity on the arms | Dermatologist-assessed changes in skin condition (dryness, scaling excoriation, redness and papules) Skin conductance Transepidermal water loss Skin microflora |
Ratio of S. aureus in the total bacteria at the test site after 1 week: farnesol and xylitol cream significantly decreased compared with before application (p = 0.007) and with placebo cream (p = 0.045). Mean skin conductance of farnesol and xylitol cream test patches was significantly increased after application compared with before application (p = 0.0001) and with placebo cream (p = 0.002) | No description of method of randomisation or allocation concealment. Intention-to-treat population not reported | |||
| Korting 2010212 | Oil-in-water cream with 4% sodium bituminosulfonate (Ichthosin cream) (pale sulfonated shale oil) applied three times a day for 4 weeks | Vehicle cream (propylene glycol, glycerol monostearate, cetyl alcohol, medium-chain triglycerides, macrogol-1000-glycerol monostearate, white vaselin (‘white soft paraffin’, Ichthyol-Gesellschaft, Hamburg, Germany), purified water and, for adjustment for colour, caramel and quinoline yellow) applied three times a day for 4 weeks | Skin-care products were allowed on unaffected skin. Concomitant medications of any kind were not allowed | Germany | 99 participants (n = 51 pale shale oil, n = 48 vehicle) (safety population) | Caucasian; mild to moderate atopic eczema (total score of ≤ 21); age 0–12 years | (Primary) Total score – erythema, crusts, excoriations, scales, lichenification and itch (each scored from 0 to 5, with 0 = non-existent, 1 = very mild, 2 = mild, 3 = moderate, 4 = severe, 5 = very severe) Topographical distribution (0 = not affected, 1 = 1–33%, 2 = 34–66%, 3 = 67–100%) for the areas of face and neck, head, torso front, torso back, arms, hands and wrists, legs, feet. Topographical score halved as in EASI. Total score max. = 42 (Secondary) Reduction of total score after 1 and 2 weeks of treatment (Secondary) Reduction in signs and symptoms after 1, 2 and 4 weeks of treatment (Secondary) Reduction in topographical distribution after 1, 2 and 4 weeks Participant- and investigator-assessed tolerability of the study treatment (‘very good’, ‘good’, ‘medium’, ‘moderate’ or ‘bad’) Adverse events |
Total score: baseline – vehicle cream 13.0 SD ± 3.1, shale oil cream 13.4 SD ± 3.7; 1 week – vehicle cream reduction of 1.3 SD ± 5.9, shale oil cream reduction of 5.6 SD ± 4.3; 4 weeks – vehicle cream 11.7 SD ± 8.6, shale oil cream 4.5 SD ± 7.4 (p < 0.0001). Tolerability (at end of treatment): better in the shale oil group than in the vehicle cream group according to investigators (p < 0.0001) and participants/parents (p = 0.0051) | Method of randomisation described. Allocation concealment not reported. Intention-to-treat population used for the efficacy analyses and defined | ||
| Lee 2008210 | Rosmarinic acid-containing cream (0.3% rosmarinic acid) applied to the elbow flexures twice daily, in the morning and evening, for 8 weeks | Vehicle cream applied to the elbow flexures twice daily, in the morning and evening, for 8 weeks | Use of topical or systemic corticosteroids strictly forbidden during the trial | Korea | 21 | Affected by eczematous lesions on elbows (this was taken as moderate atopic eczema according to the SCORAD index); diagnosis of atopic eczema according to the criteria of Hanifin and Rajka8 | Severity of eczema (SCORAD index) Local pruritus Transepidermal water loss Safety |
SCORAD index: erythema on antecubital fossa significantly reduced at weeks 4 and 8 (p < 0.05). Transepidermal water loss on the antecubital fossa significantly reduced at 8 weeks compared with before treatment (p < 0.05). Participant questionnaire: improvements in dryness, pruritus and general eczema symptoms | The method of randomisation was not described. Allocation concealment was not reported. Intention-to-treat analysis not reported | ||
| Loden 2001193 | Glycerine 20% cream applied twice daily for 30 days over one part of the body identified as dry by the dermatologist | Urea cream (4% urea and 4% sodium chloride) applied twice daily for 30 days over one part of the body identified as dry by the dermatologist | Placebo cream (same as the glycerine cream with water replacing the glycerine) applied twice daily for 30 days over one part of the body identified as dry by the dermatologist | The urea cream did not have the same constituents as the glycerine and placebo creams. The participants were asked to replace their normal moisturisers with the treatment cream | Not stated | 110 | Atopic dermatitis according to the criteria of Hanifin and Rajka8 and with no other significant concurrent illness and no known allergy to ingredients in the test creams | Dryness of the skin [scaling, roughness, redness and cracks (fissures) in the identified area were scored from 0 to 4 and the sum of the severity score was calculated; max. score 16] Transepidermal water loss Skin capacitance |
No difference in transepidermal water loss was found between the glycerine cream and the placebo cream, whereas a lower value was found in the urea cream group. No difference in skin capacitance was found. The clinical assessment of dryness showed urea to be superior to glycine in treating eczema | Details of randomisation and blinding are not given and there is no mention of intention-to-treat analysis | |
| Loden 2002194 | Glycerine 20% cream. Participants used as much cream as desired and at least once daily for 30 days | Urea 4% cream. Participants used as much cream as desired and at least once daily for 30 days | Placebo cream. Participants used as much cream as desired and at least once daily for 30 days | Participants were allowed to continue to use topical corticosteroids but noted their use in their diary | Not stated | 197 participants randomised (n = 68 glycerine group, n = 63 urea group, n = 66 placebo group) | Atopic dermatitis | Participant-assessed degree of smarting, stinging, itching and dryness/irritation on a 5-point scale (0–4) after 2 weeks of treatment Participant-assessed skin dryness at the end of treatment (14-cm VAS) Dermatologist-assessed dry skin [DASI: scaling, roughness, redness and cracks (fissures) in the worst affected area in each of four body regions were scored from 0 to 4 and the size of the area involved was estimated; DASI = sum of the severity score × percentage area affected (max. score of 1600)] |
Adverse skin reactions such as smarting were felt significantly less among participants using glycerine cream then among participants using urea cream (10% vs. 24% severe or moderate smarting; p < 0.0006). No differences were found regarding skin reactions such as stinging, itching and dryness/irritation. There were equal effects on skin dryness as judged by the participants and the dermatologist | No details were given about the method of randomisation. Allocation concealment and intention-to-treat analysis were not reported | |
| Miller 2011199 | Glycyrrhetinic acid-containing barrier repair cream composed of glycyrrhetinic acid 2%, hyaluronic acid, Vitis vinifera (grapevine) extract, telmesteine and Butyrospermum parkii (Shea butter). The smallest amount needed to cover the area was used so that it would rub in easily. The cream was applied three times daily (morning, afternoon and evening) to all affected areas for 3 weeks | Ceramide-dominant barrier repair cream consisting of a 3 : 1 : 1 molar mixture of a synthetic ceramide, free fatty acids and cholesterol. The smallest amount needed to cover the area was used so that it would rub in easily. The cream was applied three times daily (morning, afternoon and evening) to all affected areas for 3 weeks | Petrolatum-based skin protectant moisturiser (over the counter). The smallest amount needed to cover the area was used so that it would rub in easily. The moisturiser was applied three times daily (morning, afternoon and evening) to all affected areas for 3 weeks | Subjects were given a study treatment diary and were instructed to note the times that they administered the treatment doses each day. Each moisturiser was distributed in a plain white jar so that subjects and investigators were blinded | USA | 39 | Age 2–17 years; mild to moderate atopic eczema; a rating of 2–3 on a 5-point IGA severity scale as well as ≥ 1% of overall body surface area involvement including facial and intertriginous skin | IGA (scale 0–4) Body surface area involvement (scale 0–100) Investigator’s global assessment of improvement (scale 0–6) Itch intensity (VAS with no itch on the left and most intense itch on the right) EASI (scale 0–72) Subject global assessment of improvement (0 = completely clear and 6 = worsening of disease) |
There were no statistically significant differences between the groups at any time point. The petroleum-based moisturiser was 47 times more cost-effective than the other two treatments | Method of randomisation and allocation concealment not reported. Investigators and subjects were blinded but this was limited as the physical properties of the treatments were different. Intention-to-treat principle was applied | |
| Misery 2005223 | Lipiderm cream containing 1% raffinose (Tefirax) applied as frequently as necessary for 3 days and when needed for persistent symptoms of pruritus | Lipiderm cream without raffinose applied as frequently as necessary for 3 days and when needed for persistent symptoms of pruritus | Tefirax had blue packaging and the Lipiderm cream had red packaging | France | 11 participants | Diagnosed with atopic dermatitis; age between 6 and 60 years; presence of pruritus; atopic eczema on the body without infection | (Primary) Intensity of pruritus (VAS from 0 to 10) Assessment of improvement of study treatment area between 5 minutes before treatment and 5 minutes after application of treatment Amount of study treatment used Investigator assessment of erythema, xerosis, lichenification, scaling, exudation, presence of pustules (rated individually on a 6-point scale, with 0 = absent, 0.5 = presence suspected, 1 = very mild, 2 = mild, 3 = moderate, 4 = severe) Adverse events Amount of repeated application because of the recurrence of pruritus |
Six participants reported good efficacy results with Tefirax; four participants reported symptom improvements for both Terifax and Lipiderm; and one participant reported no improvement for either cream. No significant difference found between the study treatments. This could be because of the small size of the study | Intention-to-treat population not described | ||
| Mora 2004232 | Lantigen B (a topical suspension of bacterial antigens), 1 drop per year of age, given twice a day for 3 consecutive months in the external acoustic duct | Placebo (physiological solution), 1 drop per year of age, given twice a day for 3 consecutive months in the external acoustic duct | Not stated | 80 children were followed up for a period of 1 year. Number randomised is not explicitly stated | Recurrent external auditory atopic dermatitis; age 2–6 years at the time of the first examination | Severity [Rajka and Langeland228 scale, which considers the intensity (itch level – mild, moderate, severe), extent (affected percentage of the total external ear surface – < 20%, 20–60%, > 60%) and course (length of remission – > 3 months of remission in 1 year, < 3 months in 1 year and persistent) of the lesions, each on a scale of 1–3; severe atopic eczema 8–9, moderate 5–7, mild 3–4] Frequency of allergies (skin-prick test) Total serum IgE |
In the Lantigen B group, using the Wilcoxon test, there was an improvement in the clinical items measured. Participants were allowed to receive concomitant medications to treat acute episodes, which may have partly contributed to the positive results obtained | Method of randomisation not stated and concealment of allocation not reported. Intention-to-treat analysis not reported | |||
| Msika 2008123 | Emollient containing 2% sunflower oleodistillate applied twice daily for 21 days (groups B, D and E) | Topical 0.05% corticosteroid (desonide) applied for 21 days: groups A and B = twice daily, groups C and D = once daily (morning), group E = once every other day (morning) | Not stated | 86 participants (group A n = 18, group B n = 17, group C n = 15, group D n = 17, group E n = 19 | Age 4 months to 48 months; free from application of topical corticosteroids for the 8 days prior to study entry; inflammatory phase moderate to severe atopic eczema | Severity of eczema (SCORAD index) Investigator global evaluation (‘completely agree’, ‘quite agree’, ‘not very agree’, ‘not agree’, ‘no opinion’) Quality of life (DLQI and Dermatitis Family Impact) Tolerance |
All of the groups improved (SCORAD index and quality of life) at the end of treatment. Therefore, the emollient cream twice a day combined with topical corticosteroid once every other day was as effective as topical corticosteroid once or twice a day on its own (topical corticosteroid-sparing effect). The emollient cream had a significant impact on quality of life and lichenification and excoriation | Method of randomisation unclear and allocation concealment not reported. Intention-to-treat analysis not reported | |||
| Palombo 2004208 | Imbibed pill mask containing the chitosan-derived anti-inflammatory compound ATOBIOL (5 mg/g) solubilised in a lamellar active emulsion of tocotrienols and hyaluronic acid applied 3 minutes after washing using a bath oil (hydrogenated polydecene, silica dimethyl silylate, oleth-3) twice a day for 8 weeks | Lamellar active emulsion of the chitosan-derived compound ATOBIOL (5 mg/g) applied 3 minutes after washing using a bath oil (hydrogenated polydecene, silica dimethyl silylate, oleth-3) twice a day for 8 weeks | Petroleum ointment applied 3 minutes after washing using a bath oil (hydrogenated polydecene, silica dimethyl silylate, oleth-3) twice a day for 8 weeks | After 8 days of treatment, all participants were given triamcinolone 0.1% ointment twice a week and lamellar gel only twice a day | Not stated | 36 participants (n = 15 pill mask group, n = 12 vehicle group, n = 9 petroleum ointment group) | Children | Clinical score (erythema, scaling, crusting and pruritus on a VAS) | After 4 weeks: improvement from starting point – 58% in pill mask group vs. the petroleum group, 82% in pill mask group vs. lamellar gel group, 64% in petroleum group vs. lamellar group | Method of randomisation and allocation concealment not reported. Intention-to-treat analysis not reported. Lack of blinding reported | |
| Patrizi 2008226 | MAS063DP (Atopiclair) applied to all areas of eczema three times a day for 43 days | MAS060 (Atopiclair light), which is in an oil-in-water formulation with no preservatives and at a lower concentration (specifically for the paediatric population), applied to all areas of eczema three times a day for 43 days | Vehicle applied to all areas of eczema three times a day for 43 days | Participants could not use topical or systemic treatments or phototherapy in the washout or study period | Italy | 60 participants (n = 20 MAS063DP, n = 20 MAS060, n = 20 vehicle) | Age 2–17 years; diagnosed with atopic eczema according to the Hanifin and Rajka8 criteria; IGA of 2 (mild) or 3 (moderate) at study entry; ≥ 5% affected body surface area | (Primary) IGA at day 22 (on a scale from 0 to 5, with 0 = clear, 5 = severe disease) (Secondary) Participant-/caregiver-assessed pruritus (0–100-mm VAS) (Secondary) IGA at days 8, 15, 29 and 43 (Secondary) EASI score (max. score 72) (Secondary) Use of rescue medication (Secondary) Participant-/caregiver-assessed acceptability of study treatment |
IGA at day 22: difference between groups – MAS063DP compared with MAS060 p < 0.0001, MAS063DP compared with vehicle p = 0.001, MAS060 compared with vehicle not significant. Statistically significant results maintained until the end of the study | Method of randomisation described and adequate. Allocation concealment not reported. Description of blinding adequate | |
| Patrizi 2012239 | 1 mg/cm2 AR-GG27 cream (Dermolichtena; Giuliana SpA) containing aqua, PEG-8 Beeswax, caprylic/capric triglyceride, dicaprylyl ether, isostearyl isostearate, diisopropyl sebacate, glycerin, Butyrospermum parkii butter, Zea mays, Calendula officinalis extract, tapioca starch, glycyrrhetinic acid, rhizobian gum, sodium hyaluronate, sorbityl furfural palmitate, citric acid, sodium hydroxymethyl glycinate, betain, inositol, trehalose, bisabolol, phenoxyethanol, allantoin, diethylhexyl syringylidenemalonate, carbomer, beta-sitosterol, pentaerythrityl tetra-di-t-butyl hydroxycinnamate, dimethylmethoxy cromanol, Olea europea leaf extract, Rosmarinus officinalis extract and tocotrienols. Cream was applied twice daily (approx. 12 hours apart) on the affected and perilesional areas for a period of 30 days | 1 mg/cm2 placebo cream containing aqua, PEG-8 Beeswax, caprylic/capric triglyceride, isostearyl isostearate, glycerin, tapioca starch, carbomer, citric acid, sodium hydroxymethylglycinate, phenoxyethanol. Cream applied twice daily (approx. 12 hours apart) on the affected and perilesional areas for a period of 30 days | Treatment with topical steroids or topical calcineurin inhibitors had to be stopped for at least 15 days. Any general therapy and phototherapy or sun exposure was stopped at least 30 days before. Emollients were stopped at least 7 days before. No other local or systemic treatments were allowed as well as sun exposure, during the trial | Not stated | 60 patients were enrolled and randomised (n = 30 AR-GG27, n = 30 placebo) | Patients aged between 2 months and 15 years; normal social and dietary habits; affected by pityriasis alba located on the face and/or limbs and/or the trunk; atopic dermatitis previously diagnosed on the basis of the Hanifin and Rajka8 criteria; xerosis was present as well as pruritus | IGA – average change between the 15th day of treatment and baseline in the two groups Overall disease severity based on IGA (erythema, excoriation, desquamation) Effectiveness of AR-GG27 for pityriasis alba (based on the severity of three clinical signs: erythema, excoriation and desquamation using a subjective 5-point scale, with 0 = absent, 1 = almost absent, 2 = mild, 3 = moderate and 4 = severe) Changes in pruritus severity using a 10-cm VAS, with 0 cm = no itching and 10 cm = worst imaginable itching Safety |
Statistically significant difference in the group treated with AR-GG27 compared with placebo after 15 days (p = 0.0007) and 30 days (p = 0.005). Itching was reduced after 15 days in the AR-GG27 group compared with placebo (p = 0.01) in the whole trial population and also in those who had itching at baseline | Randomisation method not described. Allocation concealment not described. Patients, caregivers, investigators and clinical staff were blinded to treatment. Intention-to-treat analysis was used | ||
| Patzelt-Wenczler 2000233 | Kamillosan cream (containing 2% ethanolic extract of camomile flowers) applied to the affected areas of the arm twice daily | Hydrocortisone 0.5% cream applied to the affected areas of the arm twice daily | Vehicle cream applied to the affected areas of the arm twice daily | Participants randomised as follows: group 1: left arm = Kamillosan, right arm = hydrocortisone; group 2: left arm = Kamillosan, right arm = placebo; group 3: left arm = hydrocortisone, right arm = Kamillosan; group 4: left arm = placebo, right arm = Kamillosan. Additional treatment of eczema was not allowed | Not stated | 72 participants | Atopic eczema on both arms (must have pruritus, lichenification in the joint flexures and chronic course); moderate eczema (sum score of pruritus, erythema and desquamation of ≥ 3 on a scale from 0 to 9) | Efficacy (sum scores of pruritus, erythema and desquamation Individual symptoms (oedema, papules/pustules, lichenification, excoriation, fissures (4-point scale, with 0 = missing, 1 = low, 2 = marked, 3 = severe) Investigator-assessed global assessment (each arm separately: ‘very good/good’, ‘satisfactory’, ‘insufficient’, ‘unassessable’) Tolerability (unspecified interrogated adverse events) |
After 2 weeks of treatment, Kamilosan was slightly superior to 0.5% hydrocortisone and there was a marginal difference compared with placebo | Method of randomisation described. Allocation concealment not stated. Intention-to-treat analysis not reported | |
| Shibagaki 2005206 | A bath additive containing a diamide derivative, eucalyptus extract, oat extract and oily moisturising ingredients such as a synthetic pseudoceramide; 30 ml in 180–200 l of hot water for 5 minutes for four or more times a week for 3–6 weeks | A bath additive containing eucalyptus extract, oat extract and oily moisturising ingredients such as a synthetic pseudoceramide (intervention A without the diamide derivative); 30 ml in 180–200 l of hot water for 5 minutes four or more times a week for 3–6 weeks | Japan | 21 | Participants with atopic dermatitis; lesion with dryness; no complications affecting the evaluation of the study | Skin scores (from 0 = none to 4 = severe) of dryness, desquamation, itching and scratch marks, erythema and papules pre and post intervention Transepidermal water loss on the flexural area of the forearm pre and post intervention Stratum corneum hydration on the flexural area of the forearm pre and post intervention Investigator evaluation Participant self-evaluation Comprehensive evaluation Itching scores (from 0 = none to 4 = severe) before, during and after bathing pre and post intervention from itching diary in participants who used additive four or more times a week |
A comprehensive evaluation was conducted using skin scores, investigator evaluation and participant self-evaluation; however, further details of the manner in which these assessments were combined were not provided | Method of randomisation not described. Allocation concealment not reported. Intention-to-treat principle not used | |||
| Simpson 2011202 | CRM containing ceramides, humectants, emollients and occlusives to be applied twice daily on the designated side of the body with no moisturiser on the other side | No moisturiser applied to the side of the body that was not designated to CRM | Subjects were instructed to continue their routine course of care with topical steroids. They were then instructed to apply CRM to the designated side of the body and no moisturiser to the other side of the body | USA | 127 patients were enrolled and randomised. A subset of 42 patients was enrolled for corneometry/transepidermal water loss measurements | Male or female; age ≥ 3 years; diagnosed with mild to moderate atopic dermatitis as rated by IGA | mEASI (adapted to a split-body design in which the constant weighted values were reduced by 50%) Skin hydration (transepidermal water loss) Patient satisfaction |
A more rapid decrease in overall disease severity was observed on days 7, 14 and 21 (p < 0.05) compared with steroid treatment alone | Method of randomisation and allocation concealment not described. Single-blinded (evaluator) study. Intention-to-treat analysis not reported | ||
| Stern 2002209 | Black seed oil 15% ointment applied twice daily for 28 days | No further description of ‘base treatment’, applied twice daily for 28 days | Total length of trial 4 weeks; no additional creams or ointments were allowed on either arm | Germany | 20 | ‘Volunteers’ | Severity/intensity and pruritus Transepidermal water deficit Skin moisture/hydration of stratum corneum pH value Subjective rating of easiness of application and properties for care |
No significant differences between black seed oil ointment and ‘base treatment’. No differences in subjective rating of easiness of application and properties for care between black seed oil and ‘base treatment’ but smell of black seed oil was remarked on (in negative sense) | No adequate description of the method of randomisation, allocation concealment and blinding | ||
| Stücker 2004217 | Topical vitamin B12 cream (0.07% cyanocobalaminin DAB) applied twice daily (morning and evening) for 8 weeks | Placebo cream applied twice daily (morning and evening) for 8 weeks | Germany | 48 | Age 18–70 years; atopic eczema for at least 2 years; three/four major criteria of the UK Working Party criteria9 fulfilled | (Primary) Placebo-corrected efficacy (modified SASSAD score, max. score per body half 240, ‘exudation’ changed to ‘infiltration’) Investigator assessment of efficacy Participant assessment of efficacy Assessment of tolerability of the treatment |
One investigator took all of the efficacy measurements to minimise variability | Modified SASSAD score: topical vitamin B12-treated side 55.34 SD ± 5.74 SEM, placebo-treated side 28.87 SD ± 4.86 SEM (p < 0.001). End of study: investigator- and participant-assessed ratings of the treatments – topical vitamin B12 ‘good’ = 58%, ‘very good’ = 59%; placebo – ‘moderate’ = 89%, ‘poor’ = 87% | Method of randomisation described and adequate. Allocation concealment not reported. Intention-to-treat analysis was unclear; missing values were replaced by last value carried forward | ||
| Sugarman 2009126 | Ceramide-dominant barrier repair formulation applied to the eczema lesions twice daily for 28 days | Fluticasone propionate 0.05% (hydrocortisone 2.5% for the face and body folds) applied to the eczema lesions twice daily for 28 days | All participants used Cetaphil lotion twice daily on unaffected skin | Not stated | 121 participants (n = 59 ceramide formulation, n = 62 fluticasone propionate) | Dermatologist-diagnosed moderate to severe atopic eczema (severity measured by SCORAD index); age 6 months to 18 years | (Primary) Severity (SCORAD index) at 28 days (0 = none, 72 = severe) (Primary) Pruritus score at 28 days (scale 0–10, with 0 = none, 10 = severe) (Primary) Sleep habit at 28 days (scale 0–10, with 0 = none, 10 = severe) (Secondary) Participant-/family-assessed improvement (no change, improved, worsening) IGA (scale 0–4, with 0 = clear, 1 = almost clear, 2 = mild, 3 = moderate, 4 = severe) (Secondary) Excellent improvement as per the IGA and SCORAD index |
Ceramide formulation improved clinical disease severity, decreased pruritus and improved sleep habits at 14 and 28 days of treatment. Fluticasone propionate showed a significantly greater improvement at 14 days but at 28 days there was no significant difference | Method of randomisation and allocation concealment not stated. Intention-to-treat principle used for the analyses | ||
| Thumm 2000211 | 20% Sea buckthorn kernel oil cream (H. rhamnoides) applied for 28 days | 10% Sea buckthorn kernel oil cream (H. rhamnoides) applied for 28 days | Placebo: 20% Miglyol cream (caprylic/capric triglyceride) applied for 28 days | Cream for all three groups was based on bees wax, glycerine and paraffin | Germany | 58 | Clinical diagnosis according to Hanifin and Rajka8 criteria; atopic dermatitis with light to severe symptoms; SCORAD score of < 60 | Dermatologist assessment of percentage of affected skin areas A clinician-rated score that assessed redness, oedema/papules, exudates/scabbing, excoriation, lichenification and dryness Participant-reported extent of pruritus and sleep loss on a VAS Transepidermal water deficit Moisture in the stratum corneum DLQI – measuring quality of life Participant diaries: daily reporting on VAS scale of redness, pruritus, dryness and general well-being |
Skin condition improved by all preparations. SCORAD score increased in all three groups (p > 0.05). Transepidermal water loss decreased in all three groups (p < 0.05). Skin moisture increased in all three groups (p < 0.05). Quality of life improved in all three groups (p < 0.05). Redness, itching, dryness and general condition improved in all three groups but the worst results were seen in the placebo group | No reporting on randomisation procedure nor on allocation concealment | |
| Tripodi 2009207 | Emollient cream with furfuryl palmitate and the antioxidants superoxide dismutase, 18-beta-glycyrrhetinic acid, vitamin E and alpha-bisabolol. Applied twice a day for 2 weeks only on eczematous skin, one fingertip unit per area the size of two adult hands with fingers together | Emollient cream with the antioxidants superoxide dismutase, 18-beta-glycyrrhetinic acid, vitamin E and alpha-bisabolol. Applied twice a day for 2 weeks only on eczematous skin, one fingertip unit per area the size of two adult hands with fingers together | Not stated | 117 participants (n = 57 furfuryl palmitate group, n = 60 emollient group) | Age 3 months to 14 years; diagnosed with atopic eczema according to the UK Working Party criteria;9 no topical or systemic treatments at least 1 week prior to starting the study; no changes to participants’ usual lifestyle and dietary habits 1 week before and thought the study period | Severity of eczema (SCORAD index: mild eczema < 25, moderate eczema 25–50, severe eczema > 50) Efficacy (by questionnaire: ‘worsening’, ‘inexistent’, ‘poor’, ‘good’, ‘very good’) Tolerability (by questionnaire: ‘poor’, ‘good’ or ‘very good’) Atopic status (skin-prick tests for standard panel of commercial extracts including D. pteronyssinus, Parietaria judaica, olive, cat dander, Alternaria, hen’s egg, wheat and cod fish; prick by prick method used for cow’s milk) |
Both groups: significant reduction (p < 0.001) in SCORAD score after 14 days. Per-protocol analysis: emollient cream = bigger reduction. Intention-to-treat analysis of treatment success (≥ 20% reduction in SCORAD score): furfuryl palminate group 29% (number needed to treat = 2.4, 95% Cl 1.6 to 4.6), emollient group 70%. Emollient cream without furfuryl palminate found to be more effective by parents and paediatricians. Tolerability = no differences between groups | Method of randomisation described and adequate. Allocation concealment described. Intention-to-treat population reported and described | |||
| Udompataikul 2011238 | LA in a ceramide and linoleic acid lipid base formulation (Eucerin) consisting of 0.025% LA, 12.00% omega-6-fatty acids, 0.05% ceramide 3 and 10.00% glycerine was applied twice daily on one side of the body for 6 weeks | Hydrocortisone 1% cream was applied on the opposite side of the body for 4 weeks followed by the cream base for 2 weeks. The number of times a day that the treatments were applied was not stated | The LA cream also contained paraffinum liquidum, octyldodecanol, hydrogenated castor oil, dimethicone, Glycyrrhiza inflata, sorbitan isostearate, methoxy PEG-22 and 45/dodecyl glycol copolymer and BHT. The cream base lotion consisted of 10% propylene glycol, 5% mineral oil, 2% cetostearyl, 0.05% BHT and 0.1% ethylenediaminetetra-acetic acid disodium. A washout period of up to 4 weeks was required for patients taking oral medications (e.g. corticosteroids and antihistamines). A washout period of up to 2 weeks was required for patients receiving topical medications (corticosteroids, calcineurin inhibitors and moisturisers) | Not stated | 30 patients randomised, 26 completed the protocol | Children aged 2–15 years; mild to moderate atopic dermatitis diagnosed by the Hanifin and Rajka8 criteria (mild to moderate was defined as scores of 1–40 on the SCORAD index); skin lesions on both flexural areas of the body; included patients had to have been through a washout of up to 4 weeks if they were taking oral medications (e.g. corticosteroids, antihistamines) and up to 2 weeks if they were receiving topical medications (corticosteroids, calcineurin inhibitors and moisturisers) | SCORAD index (modified as each treatment was on a different side of the body – scores multiplied by 2) Global self-evaluation (graded as ‘excellent’, ‘good’, ‘fair’ and ‘minimal or no change’) Adverse events Relapse rate – measured using a survival analysis programme |
Baseline SCORAD index – about 28 on both sides. Response rate to both agents: 73.33%. No statistically significant difference between groups in reduction of SCORAD index. More rapid resolution of oedema and erythema in the hydrocortisone-treated side but this was not significant. There was no significant difference for the LA-treated side. Relapse rate was higher in the hydrocortisone group than in the LA group but this was not significant. No side effects were observed in either group | Method of randomisation not described. Allocation was concealed as assignment of treatment group was carried out by a third party. The study was single blind (investigator). Not stated whether intention-to-treat population was used | ||
| Wiren 2009197 | Canoderm 5% cream (lipid content around 20%). Moisturiser was to be applied to the identified area (previously treated with topical corticosteroid in the acute phase) twice daily until relapse of eczema or for 6 months | No treatment – patients had to abstain from using any topical formulation until relapse of eczema or for 6 months | There was an acute phase to the study that involved patients being randomised to betamethasone cream [either Betnoderm® 0.01% (ACO HUD AB) or Betnovat® 0.01% (GlaxoSmithKline)] on an identified area for 3 weeks. Those achieving ‘cleared eczema’ according to the assessment of a dermatologist (except lichenification) could then be randomised again to either emollient (intervention A) or no treatment (intervention B) in a maintenance phase lasting 6 months. Other body areas could be treated with topical treatment throughout the study | Sweden | Acute phase n = 55 (n = 27 Betnoderm, n = 28 Betnovat). Maintenance phase n = 44 (n = 2 emollient, n = 22 no treatment) | Age 18–65 years; atopic eczema diagnosed according to the Hanifin and Rajka8 criteria or by the clinic; one lesion that was typical on an easily inspected site (e.g. arms, legs, chest); degree of eczema at identified site with a score of ≥ 6 (Atopic Dermatitis Severity Index) | Time to relapse of atopic eczema, calculated as the time from entry into the maintenance phase until a relapse occurred (Secondary) Transepidermal water loss after 3 weeks of the maintenance phase |
Maintenance phase: median time to relapse – emollient > 180 days (length of study), no treatment 30 days. Eczema free during the whole maintenance phase: emollient 68%, no treatment 32% | Method of randomisation and allocation concealment not reported. Blinding not stated. Intention-to-treat population used for the analyses and defined |
Appendix 11 Antimicrobial and antifungal agents
| Trial | Intervention A | Intervention B | Intervention C | Comments on interventions | Country | Number of participants randomised | Inclusion criteria | Outcomes | Comments on outcomes | Main reported results | Quality of reporting |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Back 2001256 | Ketoconazole (oral) (200 mg/day) taken as one dose per day for 3 months | Placebo taken as one dose per day for 3 months | There are no details on the composition of the placebo; only stated that it was taken in ‘capsule’ form | Sweden | 32 | Adults with atopic eczema diagnosed according to the Hanifin and Rajka8 criteria and with specific serum IgE antibodies to M. furfur/P. orbiculare (> 3.5 kU/l) and elevated serum IgE (> 400 kU/l) | Clinical improvement measured by SCORAD index at baseline, 1 month and 3 months Change in total IgE at baseline, 1 month and 3 months Change in specific IgE at baseline, 1 month and 3 months Quantity of betamethasone used Participant-assessed evaluation of improvement |
The outcomes did not appear to be prestated | The improvement in clinical score (SCORAD) for the ketoconazole group did not differ significantly from that for the placebo group; however, the improvements in the second and third months correlated to the amount of betamethasone used in the placebo group (r = 0.66, p = 0.013), but not in the ketoconazole group (r = 0.15, p = 0.61). There was a decrease in total serum IgE levels and specific IgE levels to M. furfur/P. orbiculare and C. albicans. These decreases were not significant at the end of month 1 but were highly significant at the end of the study (month 3) for specific IgE to M. furfur/P. orbiculare. The decreases in total IgE and specific IgE to C. albicans were close to significance levels | The exact process of generating the randomisation sequence was not stated. Allocation concealment was described as the ‘code’ being kept secret by the pharmacy until all participants had completed the final clinical examination and the case report forms were handed in. The data were not analysed according to the intention-to-treat principle | |
| Breneman 2010253 | Antibacterial soap containing 1.5% triclosan (Safeguard®; Procter & Gamble). The whole body was washed at least once a day for 63 days (42 days = treatment period, 21 days = regression period) | Placebo soap bar (not antibacterial). The whole body was washed at least once a day for 63 days (42 days = treatment period, 21 days = regression period) | For the standardisation period (14 days) and for the entire length of the trial all participants used a non-medicated cleansing bar and a non-medicated moisturising cream and could not use systemic/topical antibiotics or antimicrobial/antibacterial soap, lotion, cream and shampoo. Participants were also given 0.025% triamcinolone acetonide in place of all other topical corticosteroids. In the treatment period the participants were allowed to use 0.025% triamcinolone acetonide as needed. Regression period – no topical corticosteroid allowed, but study treatment continued | Not stated | 50 participants; group allocations not stated | Atopic eczema according to Hanifin and Rajka8 criteria; moderate severity according to Rajka and Langeland228 scale; Fitzpatrick skin types I–IV; active lesions (presence of combinations of erythema, scales, lichenification, crusting and excoriation) | Severity of eczema (SASSAD score) including itching assessment and body surface area assessment (7-point scale, with 0 = 0% affected, 6 = 90–100% affected) Total aerobic micro-organisms Reductions in S. aureus Dermatologist-assessed change in global eczema severity (–5 = severe worsening, 0 = no change, 5 = total clearing) Amount of topical corticosteroid used (weight of tubes) |
Antimicrobial soap resulted in a significantly better improvement in the extent and severity of eczema skin lesions compared with placebo soap. There were reductions in S. aureus for those with S. aureus at baseline and total aerobic micro-organisms correlated with the improvement in eczema | Method of randomisation and allocation concealment not stated. Intention-to-treat analysis not reported | ||
| Canpolat 2012248 | Hydrocortisone acetate applied by parents as a thin coating to affected areas twice daily at least 2 hours before bathing for up to 7 days | Hydrocortisone plus mupirocin ointment applied by parents as a thin coating to affected areas twice daily at least 2 hours before bathing for up to 7 days. Hydrocortisone was applied morning and evening whereas mupirocin was applied at noon and at night | Emollient – no further details given | Before all medication, swab cultures were obtained from lesions for S. aureus colonisation. Non-steroidal immunosuppressants (pimecrolimus), other investigational drugs, systemic corticosteroids and UV light therapy as well as concomitant topical medications (including other topical corticosteroids, topical H1 and H2 antihistamines and other topical antimicrobials) were not allowed during the treatment period. Use of sunscreen was allowed and application of non-medicated emollients was permitted on non-treatment areas. Use of cosmetics on treatment sites was not allowed. It appears from the trial report that the use of oral antihistamines was not allowed during the trial; however, the information in the report is unclear | Not stated | 83 (n = 30 control emollient only, n = 26 steroid, n = 27 steroid plus mupirocin) | Infants aged between 6 months and 2 years; diagnosis of mild to moderate (Rajka and Langeland228 severity scale) atopic dermatitis based on the Hanifin and Rajka8 criteria involving 2–30% of the body surface area | SCORAD index [including an assessment by a physician of objective signs (extent and intensity) and subjective symptoms (pruritus and sleep disturbance) compiled on an analogue scale by parents] EASI score including assessment of disease extent and percentage involved body surface area Treatment success (defined as > 50% recovery of the lesions or > 50% decrease in EASI or SCORAD scores) |
65% (17/26) of patients were treated successfully with hydrocortisone based on SCORAD and EASI scores. There was a significant improvement in patients using hydrocortisone plus mupirocin ointment [74% (20/27)]. The improvement from baseline in EASI score was significantly greater in the hydrocortisone group and the combined hydrocortisone/mupirocin group than in the emollient treated patients (36%) (p = 0.0187 and p = 0.012 respectively) | Randomisation not clearly described. No description of allocation concealment. Authors state that the study was double blind but parents were unblinded. No description of intention-to-treat population although number analysed appears to be the number randomised | |
| Gong 2006247 | Mupirocin ointment (Bactroban) and hydrocortisone butyrate ointment. Mupirocin applied once every morning at 0800–0900 and then hydrocortisone butyrate applied between 1000 and 1100 | Base ointment and hydrocortisone butyrate ointment. Base ointment applied once every morning at 0800–0900 and then hydrocortisone butyrate applied between 1000 and 1100 | The order of the ointments could not be changed and there had to be 2–3 hours between applications of the two ointments, but the timings were flexible | China | 119 participants (n = 5 mupirocin group, n = 61 control group) | Participants diagnosed using the Hanifin and Rajka8 criteria; age 2–65 | Severity (EASI score) Global therapeutic effect (4-point scale) Colonisation rates Colonisation density |
At the end of treatment there was no significant difference (p > 0.05) in global therapeutic effect between the two groups. In the participants with eczema with a clinical score of > 7, the therapeutic effect in the mupirocin group was significantly greater than that in the control group (p < 0.05) on the seventh day of treatment | Method of generation of the randomisation sequence was not described. Allocation concealment was unclear. Blinding is well described and the numbers of participants are given for all the outcomes. Clear reporting of means and SDs allows the analyses to be accurately interpreted. There are a few possibly post hoc subgroup analyses | ||
| Hung 2007150 | Fluticasone propionate 0.05% cream either with fusidic acid 2% cream (group I) or without (group II) applied to all effective areas, twice daily (morning and evening), for 8 weeks | Tacrolimus 0.03% ointment either with fusidic acid 2% cream (group III) or without (group IV) applied to all affected areas, twice daily (morning and evening), for 8 weeks | The use of medicated soaps and detergents was not permitted during the study. The only topical treatments that were allowed were the participants’ own moisturisers, which they were told to apply immediately after bathing. Oral antihistamines were given to all participants | Taiwan | 60 participants (n = 15 fluticasone, n = 15 tacrolimus, n = 15 fluticasone and fusidic acid, n = 15 tacrolimus and fusidic acid) | Atopic eczema diagnosed according to the criteria of Hanifin and Rajka;8 use of topical or systemic corticosteroids in the 4 weeks before entry to the study; use of topical or systemic antibiotics in the 4 weeks before entry to the study; overt secondary infection that required oral antibiotic therapy; moderate to severe eczema according to the Rajka and Langeland228 severity scale at entry | Overall clinical severity (SCORAD index; max. score 103) Local severity (modified local SCORAD index – score of 0–3, with 0 = absent, 3 = severe, for each of erythema/darkening, oedema/papulation, oozing/crusts, excoriation, lichenification/prurigo, local dryness; max. score 18) Colonisation rate of S. aureus Colonisation density of S. aureus Levels of staphylococcal enterotoxin A-specific IgE Levels of staphylococcal enterotoxin B-specific IgE |
It is not clear whether any of these outcomes were prespecified from the structure of the trial report | Topical tacrolimus was comparable to fluticasone for reduction of SCORAD scores; however, tacrolimus was slower to eradicate S. aureus. Complementary treatment with fusidic acid did not result in any additional benefit over tacrolimus and fluticasone alone. Two participants developed fusidic acid-resistant S. aureus during the study after 8 weeks of fusidic acid treatment | Method of randomisation not described. Allocation concealment not reported. Intention-to-treat population used for the analyses | |
| Huang 2009205 | Half a cup of 0.005% bleach in a bath twice weekly for 5–10 minutes and mupirocin ointment applied twice daily for 5 consecutive days per month | Half a cup of water in the bath twice weekly for 5–10 minutes and Vaseline applied twice daily for 5 consecutive days per month (placebo) | All household members had to use the mupirocin/Vaseline treatment. The participants were allowed to bathe in addition to the treatment baths as much as desired throughout the trial. All participants used a stable treatment regimen using topical corticosteroids and emollients for the duration of the study | Not stated | 31 participants (n = 15 treatment, n = 16 placebo) | Age 6 months to 17 years; moderate to severe eczema (IGA determined); bacterial skin infection as characterised by weeping, oozing and/or pustules | (Primary) EASI score [validated composite score, with 0 = clear, 72 (max. score) = very severe]. Calculated from the percentage body surface area affected and physician assessment of individual signs score IGA (0 = clear, 1 = almost clear, 2 = mild, 3 = moderate, 4 = severe, 5 = very severe) Antibiotic sensitivity of bacteria from the nares and worst infected skin lesion (antibiotics tested = amoxicillin, amoxicillin–clavulanate, oxacillin, cephalexin, trimethoprim–sulfamethoxazole, erythromycin, clindamycin and mupirocin) Adverse events |
Participants were removed from the study if they developed an allergic reaction | Prevalence of community-acquired MRSA: skin 7.4%, nares 4% (lower than hospital centre population at 75–85%). Bleach bath and mupirocin group had a significantly greater mean reduction in EASI score from baseline than the placebo group at 1 month and 3 months. Head and neck area (not treated by the baths): no difference between groups. Body and extremities (treated by baths): decreased at 1 and 3 months in the bleach bath and mupirocin group compared with the placebo group | The method of randomisation was described. Allocation concealment was unclear. Intention-to-treat principle was used for the analyses and a definition was given | |
| Larsen 2007102 | Fucicort [fusidic acid (20 mg/g) and betamethasone 17-valerate (1 mg/g)] lipid cream applied to all eczematous areas except the face, twice a day for 2 weeks | Fucicort [fusidic acid (20 mg/g) and betamethasone 17-valerate (1 mg/g)] applied to all eczematous areas except the face, twice a day for 2 weeks | Lipid cream vehicle applied to all eczematous areas except the face, twice a day for 2 weeks | For treatment of the face, group I topical corticosteroid were used if needed. For non-treatment areas, emollient cream was used (Locobase®) | Six European counties | 629 participants (n = 275 Fucicort lipid group, n = 264 Fucicort group, n = 90 vehicle) | Age ≥ 6 years; clinical diagnosis of infected eczema; diagnosis of atopic eczema according to the Hanifin and Rajka8 criteria; target lesion at least 4 × 4 cm; target lesion must have a minimum score of 1 for each of erythema, oedema/papulation, oozing/crusting, excoriation; negative pregnancy test and agreement to use an adequate method of contraception during the study (women of childbearing age) | TSS (4-point scale from ‘absent’ to ‘severe involvement’ for each of erythema, oedema/papulation, oozing/crusting, excoriation; max. score 12) Participant-assessed overall treatment efficacy for the whole treatment area (excluding face) relative to baseline (6-point scale) Investigator-assessed overall treatment efficacy for the whole treatment area (excluding face) relative to baseline (6-point scale) Participant-assessed cosmetic acceptability (4-point scale) Compliance (weight of returned study treatment) Bacterial susceptibility to antibiotics Successful bacterial response |
TSS at the end of treatment: Fucicort lipid cream group 82.9%, Fucicort cream group 82.7%, vehicle group 33.0%. Successful bacteriological response: Fucicort lipid cream group 89.7%, Fucicort cream group 89.6%, vehicle group 25.0%. Fucicort lipid cream of similar efficacy to Fucicort cream and superior to vehicle | Method of randomisation described. Allocation concealment not described. Intention-to-treat population used for some of the analyses but not defined | |
| Lintu 2001255 | Ketoconazole tablets (Nizoral®) (200 mg) taken once daily for 30 days | Placebo taken once daily for 30 days | Topical treatment with emollients or 1% hydrocortisone was allowed provided that the same brand was used throughout the 30-day treatment period | Finland | 80 participants (40 in each group) | Age ≥ 18 years with previous skin-prick test or positive RAST to Pityrosporum ovale, C. albicans or S. cerevisiae and current atopic eczema diagnosed using the Hanifin and Rajka8 criteria | Severity (SCORAD index): extent estimated as the percentage of the body surface area affected with adult measures (A); erythema, papulation, excoriation, dryness, crusts and lichenification assessed on a scale of 0–3 (none, mild, moderate and severe) (B); and pruritus and sleep disturbances assessed on a scale from 0 (none) to 10 (severe) (C). Calculated as A/5 + 7B/2 + C (max. score 103) Total serum IgE Specific IgE to P. orbiculare, C. albicans and S. cerevisiae Presence of P. orbiculare, C. albicans and S. cerevisiae (culture) Allergy to P. orbiculare, C. albicans and S. cerevisiae (skin-prick test – positive reaction was regarded as at least 50% of the diameter of the histamine wheal, which had to be at least 3 mm) |
A significant improvement in the SCORAD values was seen in the ketoconazole group at the second visit (treatment end) compared with the first visit (before treatment) (p < 0.0005, n = 36), but not in the placebo group. Of the individual determinants of the SCORAD index, itching (p < 0.05), the extent of dermatitis (area percentage), excoriation, lichenification (p < 0.01), erythema, papulation and dryness (p < 0.05) improved significantly in the ketoconazole group. In the placebo group only the extent of dermatitis (area percentage) decreased significantly (p < 0.05). In the ketoconazole group the number of positive P. ovale cultures decreased from 60% to 31% (n = 35) whereas the corresponding figures in the placebo group, were a decrease from 64% to 56% (n = 39). The clinical response was most significant in female participants with positive yeast cultures | No information was given on the method of randomisation, blinding or whether the intention-to-treat principle was used for the analyses | ||
| Ravenscroft 2003246 | Fusidic acid 2%/betamethasone 0.1% cream applied to all affected areas twice daily for 2 weeks | Topical mupirocin ointment and betamethasone 0.1% cream applied to all affected areas twice daily for 2 weeks | All participants also given a standardised emollient (Diprobase® cream; Schering-Plough) | UK | 46 participants (n = 28 fusidic acid group, n = 18 mupirocin group) | Atopic eczema that on examination by a dermatologist appeared to warrant the use of a potent topical steroid for 2 weeks | Dermatitis severity (modified version of Costa and colleagues’ simple scoring method249): sum of scores of 0–6 for the worst affected area of each of the following: erythema, oedema, vesicles, exudation, crusts, excoriation, scale and lichenification); pruritus and sleep loss each assessed on a 0–10 scale; and distribution assessed on a 0–3 scale for each of 10 areas (max. score 98 – 70% for worst area and symptoms and 30% for distribution) Participant-assessed global severity (scale 0–10, with 0 = no eczema, 10 = worst eczema imaginable) Prevalence of carriage of fusidic acid-resistant S. aureus at eczema sites Prevalence of carriage of fusidic acid-sensitive and -resistant S. aureus at eczema sites Prevalence of carriage in the nares of fusidic acid-resistant S. aureus Prevalence of carriage in the nares of fusidic acid-sensitive and -resistant S. aureus |
Both groups showed similar clinical improvement after 1 and 2 weeks. Overall median clinical improvement was paralleled by reduction in prevalence and population density of fusidic acid-sensitive and -resistant S. aureus | The method of randomisation is described; however, the method is open to potential bias. Allocation concealment not described. Intention to treat is not mentioned; however, no participants withdrew | ||
| Schuttelaar 2008250 | TT (0.1%, 3%) ointment applied twice daily all over the body for 2 weeks | T 0.1% ointment applied twice daily all over the body for 2 weeks | All participants used 0.1% T for 6 weeks after the 2-week RCT period to assess maintenance treatment | The Netherlands | 44 participants (n = 22 TT ointment, n = 22 T ointment) | Diagnosis of atopic eczema according to Hanifin and Rajka8 criteria; moderate to severe eczema (≥ 25 objective SCORAD score) | (Primary) Objective SCORAD score at week 2 (Primary) SASSAD score at week 2 (Secondary) Objective SCORAD score at weeks 4 and 8 (Secondary) Bacterial load (‘successful’ efficacy defined as pretreatment pathogen eradicated) |
Eczema severity: no significant difference between the two treatments. Clinically relevant improvements in both groups compared with baseline at week 2. Bacterial colonisation: TT group improvement 14/22 (63.6%), T group improvement 5/22 (22.7%) | Method of randomisation and allocation concealment both described and adequate. Intention-to-treat analysis not stated but dropouts in the maintenance phase were not included in the analyses | ||
| Svejgaard 2004258 | Itraconazole 200 mg daily Two capsules (itraconazole, placebo) were taken in the morning and two (itraconazole, placebo) in the evening with a meal, every day for 7 days | Itraconazole 400 mg daily (100-mg capsules). Two capsules (itraconazole , itraconazole) were taken in the morning and two (itraconazole, itraconazole) in the evening with a meal, every day for 7 days | Placebo (100-mg capsules). Two capsules (placebo, placebo) were taken in the morning and two (placebo, placebo) in the evening with a meal, every day for 7 days | Not stated | 53 participants (n = 18 itraconazole 200 mg, n = 17 itraconazole 400 mg, n = 18 placebo) | Age between 18 and 50 years; atopic eczema involving the head and neck; diagnosis of atopic eczema according to Hanifin and Rajka8 criteria; at least four clinical signs out of erythema, oedema/papulation, oozing/crusting, excoriation, lichenification and/or dryness; head and neck area total intensity score greater than total intensity score of remaining body surface area; generally good health; negative urine pregnancy test for women | (Primary) SCORAD index (head, neck and upper thorax to a line through the nipples) (Primary) SCORAD index (body except head and neck region) Participant-assessed global evaluation (whole body) (1 = cured, 2 = markedly improved, 3 = moderately improved, 4 = no change, 5 = deterioration) Investigator-assessed global evaluation (whole body) (1 = cured, 2 = markedly improved, 3 = moderately improved, 4 = no change, 5 = deterioration) Hypersensitivity to Malassezia Cross-reactivity between fungi (Malassezia and C. albicans) Participant-assessed sleep loss and itching (0–100-mm VAS) |
SCORAD index – number of participants with > 50% reduction in scores | At 7 and 14 days: significant improvement in head and neck region SCORAD index for 400 mg of itraconazole (p = 0.0385 and p = 0.0134, respectively) and 200 mg of itraconazole (p = 0.0140 and p = 0.0006, respectively). Placebo group improved slightly (p = 0.0785). At day 14 there was a significant difference in favour of 200 mg of itraconazole compared with placebo (p = 0.0318) | Method of randomisation and allocation concealment not stated. Intention-to-treat analysis carried out on all participants receiving treatment; however, exact numbers in the intention-to-treat population were unclear | |
| Tan 2010252 | 1% Triclosan-containing leave-on emollient cream applied to the whole body twice daily for 41 days | Vehicle cream applied to the whole body twice daily for 41 days | Both groups applied 0.025% betamethasone valerate cream in a thin layer over the eczematous areas once a day for the first 27 days, before application of study treatment | Singapore | 60 participants (n = 30 triclosan-containing emollient, n = 30 vehicle cream) | Age between 12 and 40 years; atopic eczema according to the Hanifin and Rajka8 criteria; mild to moderate eczema according to the SCORAD index | (Primary) SCORAD response on day 27 (response defined as a reduction of ≥ 20 points from baseline) (Secondary) Change in SCORAD index from baseline (Secondary) Use of topical corticosteroids |
Day 14: significant decrease in SCORAD index from baseline for the triclosan-containing emollient compared with vehicle (p < 0.05). Day 27: improved mean reduction, but no longer significant (p > 0.05). Mean amount of topical corticosteroid applied was significantly lower for the emollient group than for the control group (p = 0.40). Day 27: overall benefit of triclosan-containing emollient not significant | Method of randomisation described and adequate. Allocation concealment not reported. Intention-to-treat principle used for data analysis, but not defined | ||
| Wong 2008115 | Hydrocortisone 1% cream plus miconazole cream twice daily to the affected areas, approximately 12 hours apart, for 2 weeks | Hydrocortisone 1% cream twice daily to the affected areas, approximately 12 hours apart, for 2 weeks | No concomitant medication allowed. Only usual moisturisers and cleansers allowed | Hong Kong, China | 30 participants | Diagnosis of atopic eczema according to the UK Working Party criteria;9 age between 5 and 14 years (criterion not stated); active eczema equally affecting the knees or elbows; asked whether the participant had used an antimycotic in the 3 months before the trial | Participant-assessed relief of symptoms at 2 weeks Dermatologist-assessed change in clinical signs (from photographs) after 2 weeks Number of topical corticosteroid-free days (6-week follow-up period) |
The addition of an antimycotic did not provide any enhanced benefit compared with standard treatment as shown by all three study outcomes | Method of randomisation and allocation concealment not reported. Intention-to-treat analysis not reported |
Appendix 12 Antihistamine and mast cell stabilisers
| Trial | Intervention A | Intervention B | Comments on interventions | Country | Number of participants randomised | Inclusion criteria | outcomes | Main reported results | Quality of reporting |
|---|---|---|---|---|---|---|---|---|---|
| Diepgen 2002260 | Cetirizine oral solution (10 mg/ml) given at a dose of 0.25 mg/kg twice daily for 18 months | Placebo solution given twice daily for 18 months | Placebo described as matching (similar in appearance and taste) | 12 European countries and Canada | 817 participants (795 treated and had follow-up information collected; n = 398 cetirizine group, n = 397 placebo group) | Infants aged 1–2 years with active symptoms of atopic eczema for at least 1 month before inclusion and at least one parent/sibling with a history of atopic eczema, allergic rhinitis or asthma | Severity of eczema using SCORAD index Consumption of concomitant topical and systemic medications (Primary) Onset of asthma in the study period In this trial the evaluation of pruritus was incorporated into the SCORAD scale |
The severity of eczema as measured by the SCORAD index decreased significantly (p < 0.001) in both groups. Mild topical corticosteroids were used in 41.6% of participants (placebo group 41.6%, cetirizine group 41.7%) and moderate to potent topical corticosteroids in 55.0% (56.4% and 53.5% respectively). In a subgroup of infants with a SCORAD score of ≥ 25 this corticosteroid-sparing effect was statistically significant (placebo group 35.1% of days vs. cetirizine group 25.8% of days; Mann–Whitney test p = 0.014) | There are no details of the method of randomisation or which parties were blinded given. Analysis population appears to be an intention-to-treat population |
| Epinastine Hydrochloride Dry Syrup Clinical Study Group 2004265 | Epinastine hydrochloride dry syrup + placebo. 1 g containing epinastine hydrochloride 10 mg. Dose 1.0 g/day (body weight 14–24 kg) or 2.0 g/day (body weight > 24 kg), epinastine hydrochloride given twice a day and placebo once a day for 4 weeks | Ketotifen fumarate dry syrup + placebo. 1 g containing ketotifen fumarate 1.38 mg. Dose 1.2 g/day (body weight 14–24 kg) or 2.0 g/day (body weight > 24 kg), ketotifen fumarate given twice a day and placebo once a day for 4 weeks | Not stated | 162 participants (n = 84 epinastine hydrochloride group, n = 78 ketotifen fumarate group) | Children with atopic dermatitis; age ≤ 15 years; whose body weight ≥ 14 kg; applied moderate or mild rank corticosteroid ointment for ≥ 1 week; itching score before allocation ≥ 2 | (Primary) Degree of pruritus at week 4 (Secondary) Degree of pruritus at week 2 (Secondary) Degree of rash at week 2 (Secondary) Degree of rash at week 4 (Secondary) Pruritus scores on the itching questionnaire at week 2 (Secondary) Pruritus scores on the itching questionnaire at week 4 |
Degree of pruritus at week 4: the results proved the non-inferiority of epinastine hydrochloride compared with ketotifen fumarate. Secondary outcomes: no difference between groups | Unclear as paper reported in Japanese | |
| Kawashima 2003263 | Fexofenadine hydrochloride 60 mg given twice daily (morning and evening) for 1 week | Placebo given twice daily (morning and evening) for 1 week | All participants received placebo for 1 week prior to the randomised trial. All participants used 0.1% hydrocortisone butyrate twice a day during the placebo period before randomisation and during the randomised treatment period | Japan | 411 participants (n = 207 fexofenadine group, n = 204 placebo group) | Age ≥ 16 years; diagnosis of atopic eczema according to the Japanese Dermatological Association criteria;26 self-assessed pruritus score from 4 to < 8 for the 3 days of placebo treatment prior to randomisation; participant capable of entering accurate pruritus diary entries | (Primary) Participant-assessed mean change in pruritus score from baseline (difference between 3-day participant selection period and 6.5 days of the randomised treatment period (Secondary) Time course changes in diurnal and nocturnal pruritus scores (separately assessed) (Secondary) Investigator-assessed changes in the ratio of pruritus area to body surface area (Secondary) Subgroup analysis of mean change in pruritus score from baseline (based on pruritus severity during 3-day subject selection period) Adverse events Laboratory assessments and physical examinations also performed including echocardiographic QTc interval |
Severity of pruritus significantly decreased in the fexofenadine group compared with placebo (p = 0.0005). Fexofenadine significantly improved diurnal (p = 0.0001) and nocturnal (p = 0.013) pruritus compared with placebo | Method of randomisation and method of allocation concealment described. Intention-to-treat population used for the efficacy analysis |
| Kawashima 2011266 | Olopatadine hydrochloride (5 mg oral dose) given twice daily for 2 weeks | Ketotifen fumarate (dry syrup 1 g) given twice daily for 2 weeks | Not stated | 305 participants | Patients with atopic dermatitis; age 7–16 years, weight > 20 kg | Change in the itching score Efficacy Adverse events |
Demonstrated non-inferiority in the olopatadine group compared with the ketotifen fumarate group. Adverse events incidence: olopatadine group 19.1% (29/152 patients), ketotifen group 24.2% (37/153 patients). Adverse drug reactions: olopatadine group 11.8% (18/152 patients), ketotifen group 6.5% (10/153 patients) | Method of randomisation, allocation concealment and blinding were not adequately described | |
| Lee 2006268 | 5% doxepin cream applied four times a day for 7 days | Vehicle cream applied four times a day for 7 days | Not stated | 44 participants | Atopic eczema; moderate to severe daily pruritus for at least 1 week | Physician global assessment of relief of pruritus Pruritus severity (VAS) Pruritus relief (VAS) EASI score |
Significant improvement in the doxepin group compared with the placebo group for pruritus | No description of the blinding or randomisation methods | |
| Munday 2002267 | Chlorpheniramine maleate BP elixir (2 mg/5 ml). Dose of 2.5 ml (1–5 years) or 5 ml (6–12 years) given before bedtime every evening, with a second dose being able to be given after 3 hours. After 2 weeks, if sleeplessness was still present, the dose could be doubled to 5 ml (1–5 years) or 10 ml (6–12 years) before bedtime every evening | Placebo elixir. Dose of 2.5 ml (1–5 years) or 5 ml (6–12 years) given before bedtime every evening, with a second dose being able to be given after 3 hours. After 2 weeks, if sleeplessness was still present, the dose could be doubled to 5 ml (1–5 years) or 10 ml (6–12 years) before bedtime every evening | All participants were given 100 g of Urgentium M emollient (Almirall Hermal GmbH) and 30 g of Efcortelan 1% hydrocortisone cream (GlaxoSmithKline) to use as necessary | UK and Poland | 155 participants were recruited | Established atopic eczema with nocturnal itching and scratching; age range 1–12 years | Investigator (day 1)/participant (throughout the study) assessed severity of itching (5-point scale from ‘none’ to ‘severe’) Participant-assessed severity of daytime drowsiness (5-point scale) Participant-assessed episodes of sleeplessness caused by itching and scratching Investigator-assessed severity of atopic eczema: erythema, excoriation, dryness, lichenification, exudation and crusting (digital VAS) Compliance (weights of study medication, hydrocortisone and emollient) Adverse events The participants recorded all of the details in a diary |
Chlorpheniramine was no more effective than placebo for relief of symptoms | The method of randomisation was not described and allocation concealment was not stated. The intention-to-treat population was used in the analyses but it unclear why the intention-to-treat population included four participants less than were recruited. The data collected were described in great detail including 95% CIs |
| Nakagawa 2006264 | Fexofenadine 30 mg (7–11 years) or 60 mg (12–15 years) given twice daily for 4 weeks | Ketotifen fumarate dry syrup 1 mg given twice daily for 4 weeks | Not stated | 190 participants | Not stated | Change in itching score Severity of rashes Participant impression |
Mean change in itching: fenofexadine (n = 77) –0.50 (95% Cl –0.61 to –0.38,) ketotifen fumarate (n = 85) –0.58 (95% Cl –0.70 to –0.45). Ketotifen fumarate is not inferior to fenofexadine | Method of randomisation and allocation concealment not adequately described | |
| Stainer 2005271 | Altoderm (4% sodium chromoglycate w/w) was generously rubbed into affected areas gently until it turned white and eventually clear, twice daily for 12 weeks | Vehicle cream (control) was generously rubbed into affected areas gently until it turned white and eventually clear, twice daily for 12 weeks | All participants were allowed to continue existing treatments for atopic eczema and other allergic diseases | Not stated | 114 participants (n = 58 Altoderm group, n = 56 placebo group) | Age 2–12 years; diagnosed with atopic eczema according to the UK Working Party criteria;9 SCORAD score of 25–60 inclusive at both of two clinics 14 days apart; overall skin condition and itching of at least 2 on a 0–3 scale on at least 4 days in the 14-day baseline period | (Primary) Change in SCORAD score (primary time point 12 weeks) (Secondary) Participant-assessed overall skin condition (Secondary) Participant-assessed itching (Secondary) Participant-assessed sleep disturbance (Secondary) Mean symptom score (mean of overall skin condition, itching and sleep disturbance) For all outcomes, baseline value was the mean of the 14-day baseline period |
Altoderm n = 58, placebo n = 56 in the intention-to-treat analysis. SCORAD score (mean ± SD): baseline – Altoderm 41.0 ± 9.0, placebo 40.4 ± 8.73. After 12 weeks – Altoderm reduced by 13.2 (35%), placebo reduced by 7.6 (20%). Difference 5.6 (95% CI 1.0 to 10.3) (p = 0.018, statistically significant) | Method of randomisation and allocation concealment described. Intention- to-treat population used for the analyses and described |
Appendix 13 Dietary interventions
| Trial | Intervention A | Intervention B | Intervention C | Comments on interventions | Country | Number of participants randomised | Inclusion criteria | Outcomes | Comments on outcomes | Main reported results | Quality of reporting |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amestejani 2012327 | Cholecalciferol (vitamin D), 1600 IU. Patients took one capsule and two soft gels for 60 days | Placebo (capsule was filled with starch). Patients took one capsule and two soft gels for 60 days | The placebo vitamin D capsule was identical to the vitamin D capsule in size and colour. Patients could use prescribed routine treatments for atopic dermatitis including emollients, topical corticosteroids and oral antihistamines depending on the severity of disease and physician prescription | Not stated | 60 patients randomised (n = 30 vitamin D group, n = 30 placebo group). A total of 53 patients remained in the study | Age ≥ 14 years; no systemic diseases; no concomitant systemic pyretic or inflammatory processes (other than diabetes mellitus and chronic viral hepatitis | Severity of atopic dermatitis based on SCORAD index (mild < 15, moderate 15–40, severe > 40) Severity of atopic dermatitis based on TIS score (mild form 0–2, moderate 3–5, severe 6–9) Serum 25-hydroxyvitamin D determined by radioimmunoassay |
Vitamin D group showed significant improvement for patients with mild, moderate and severe atopic dermatitis according to SCORAD and TIS scores (p < 0.05) | Method of randomisation reported. Allocation concealment not described. The clinicians carrying out the clinical evaluations were blinded but not stated whether other parties were blinded. The study was a double-blind study. No intention-to-treat analysis was carried out | ||
| Brouwer 2006291 | Nutrilon Pepti (extensively hydrolysed whey formula) with L. rhamnosis, 3 × 108 colony-forming units/g of powder (5 × 109 colony-forming units/100 ml of formula), given for 3 months | Nutrilon Pepti (extensively hydrolysed whey formula) with Lactobacillus GG, 3 × 108 colony-forming units/g of powder (5 × 109 colony-forming units/100 ml of formula), given for 3 months | Nutrilon Pepti (control), no probiotic bacteria in the control formula, given for 3 months | During the baseline period prior to randomisation all participants used Nutrilon Pepti for a period of 3–5 weeks | Not stated | 53 participants | Age < 5 months; exclusively formula fed at enrolment; fulfilled the Hanifin and Rajka8 criteria for atopic eczema; suspected of having cow’s milk allergy | Symptom scores (SCORAD index) | The change in SCORAD score was not affected by the use of probiotics. The differences in SCORAD scores at randomisation and the subsequent decrease in SCORAD scores during treatment between groups were not significant | Method of randomisation not described. Allocation concealment not reported. Intention-to-treat analysis not reported | |
| Callaway 2005319 | Hempseed oil (cold pressed, bottled without any additives), 30 ml (2 tbsp) per day. Taken once every day for 8 weeks | Olive oil (cold-pressed extra virgin, bottled without any additives), 30 ml (2 tbsp) per day. Taken once every day for 8 weeks | The two oils differed in colour, smell and taste | Not stated. Conducted at latitude 63 degrees north | 20 participants (number in each group not provided) | Body mass index < 30 kg/m2; age between 25 and 60 years; diagnosis of atopic dermatitis according to Hanifin and Rajka8 criteria | Participant perception of changes in skin dryness or itching (0 = no dryness or itching, 5 = severe dryness or itching, sleep disturbance) Participant-reported use of dermal medication (0 = no medication, 5 = regular use) Transepidermal water loss Change in fatty acid concentrations (molar percentages of the fatty acid methyl ester profile) |
Both skin dryness and itchiness improved (p = 0.027) and dermal medication usage decreased (p = 0.024) after hempseed oil intervention | Details about randomisation and blinding are given but are hard to interpret and there is no mention of intention-to-treat analysis | ||
| Cukrowska 2008286 | Mixture of lyophilised Lactobacillus casein LOCK0900, Lactobacillus casein LOCK 0908, Lactobacillus species LOCK0919 on hydrolysed casein, 109 cells/day, given for 3 months | Hydrolysed casein, not extracted, given for 3 months | Poland | 60 participants | Children with symptoms of allergy on cow’s milk protein who were on a no milk diet or who were fed naturally; age > 24 months; no antibiotics or probiotics for at least 6 months before study commencement | Severity of atopic eczema according to the SCORAD index at 0, 3 and 8 months | Method of randomisation not described. Allocation concealment not reported. Intention-to-treat principle not used | ||||
| Drago 2012301 | Probiotic L. salivarius LS01, 1 × 109 colony-forming units/g in maltodextrin taken twice a day for 16 weeks | Placebo – maltodextrin taken twice a day for 16 weeks | Supplements were stored as freeze-dried powder in sachets, which the patient dissolved in water or any cold liquid. During the study, patients were allowed to use only oral antihistamines or different emollient creams. None of the patients changed their diet during the study and they were asked to avoid any fermented food product containing live micro-organisms | Not stated | 38 patients (n = 19 probiotic group, n = 19 placebo group) | Age 18–46 years; moderate/severe atopic dermatitis | SCORAD index DLQI IgE in serum Measurement of cytokines Quantification of cultivable bacteria in faecal samples Molecular identification of L. salivarius LS01 Compliance – measured using dose counts whereby returned sachets were counted by the clinical investigators |
NS | The probiotic group showed a significant improvement in SCORAD and DLQI at the end of treatment compared with the placebo group (SCORAD p < 0.0001, DLQI p = 0.021) | Method of randomisation described. Allocation concealment described. Double blind study – parties not stated, although the clinical investigator was blind to treatment group and the powders were matched for size, shape and volume of contents. Intention to treat not described | |
| Farid 2011312 | Probiotics (combination of seven strains) taken for 8 weeks | Placebo taken for 8 weeks | Not stated | 40 participants; number allocated to each group not stated | Mild to severe atopic eczema; age 3 months to 7 years | Severity of eczema (SCORAD index) IL-4 and IFN-γ (assessed on the supernatant after polyclonal stimulation of mononuclear cells culture) |
SCORAD index: the probiotic group showed a significantly greater reduction than the placebo group (p = 0.001). There was no significant effect of the probiotics on cytokine levels | Method of randomisation not described. Allocation concealment not reported. Intention-to-treat analysis not reported. Study was double blind but not stated which parties were blinded | |||
| Fölster-Holst 2006292 | L. rhamnosus GG, 5 × 109 colony-forming units, given twice daily for 8 weeks | Placebo (microcrystalline cellulose), no probiotic bacteria in the placebo, given twice daily for 8 weeks | The two interventions were stated as being identical in appearance | Germany | 54 | Fulfilled the Hanifin and Rajka8 criteria for eczema | Severity of disease (SCORAD index) Evaluation of pruritus and sleep loss Parent’s global evaluation of skin condition and pruritus Use of corticosteroids Use of antihistamines Parent-assessed health-related quality of life (five dimensions) |
The difference in severity score between both groups was not significant at any time point. No statistically significant difference between groups for corticosteroid potency at inclusion or during the study. For corticosteroid use at baseline compared with week 8, the L. rhamnosus GG group reduced by 2.7 applications per week and the placebo group reduced by 0.8 applications per week. No significant differences were found in any of the health-related quality-of-life dimensions after week 4 and week 8 in both groups | The methods for generating randomisation and allocation concealment were not stated. Although the trial was stated to be double blind, the parties who were blinded were not stated. It appears that an intention-to-treat analysis was not performed | ||
| Gerasimov 2010307 | Probiotic formulation of L. acidophilus DDS-1 and B. lactis UABLA-12 with fructo-oligosaccharide in a rice maltodextrin powder. Single dose (one-quarter of a teaspoon/approx. 1 g) = 5 million colony-forming units of L. acidophilus DDS-1 and B. lactis UABLA-12 and 50 mg of fructo-oligosaccharie. Each dose was reconstituted in tepid water, juice or baby food and taken immediately twice a day for 8 weeks | Placebo powder of pure rice maltodextrin. Single dose (one-quarter of a teaspoon/approx.. 1 g) = pure rice maltodextrin. Each dose was reconstituted in tepid water, juice or baby food and taken immediately twice a day for 8 weeks | Not stated | 96 participants (n = 48 probiotic group, n = 48 placebo group) | Age 12–36 months; atopic eczema diagnosed according to the Hanifin and Rajka8 criteria; moderate to severe disease; parental or guardian to understand the requirements and consent to the trial; direct telephone access | (Primary) Percentage change in SCORAD score at week 8 (Secondary) Changes in Infant’s Dermatitis Quality of Life scores at weeks 2, 4 and 8 (Secondary) Changes in Dermatitis Family Impact scores at weeks 2, 4 and 8 (Secondary) Frequency of use of topical corticosteroids (Secondary) Amount of topical corticosteroids used (Secondary) Absolute number and percentage of peripheral blood lymphocyte subsets at week 8 |
Percentage change in SCORAD score at week 8: probiotic group 33.7%, placebo group 19.4% (p = 0.001). Mean (SD) change in SCORAD score at week 8: probiotic group –14.2 (9.9), placebo group –7.8 (7.7) (p = 0.001) | Method of randomisation described. Allocation concealment unclear. Intention-to-treat analysis not reported | |||
| Ghanei 2011306 | Prebiotics (fructo-oligosaccharide and inulin powder), 0.8 g per 100 ml of milk, total daily dose 5 g for age 7–12 months, 7.5 g for age 13–18 months, 10 g for age 19–24 months. Taken for a total of 90 days | Placebo (dextrin) powder, dosing same as intervention A. Taken for a total of 90 days | Iran | 90 participants | Atopic dermatitis based on diagnosis made by a physician; age 7–24 months; normal weight at birth; gestational age 37–42 weeks; caesarean delivery; beginning solid foods after 6 months; willingness of mother to co-operate with the trial | Severity of eczema (SCORAD index) Side effects |
The difference between mean total SCORAD scores of the intervention group (4.3 SD ± 9.6) and control group (19.4 SD ± 13.0) after treatment was statistically significant (p < 0.001) | Method of randomisation described. Allocation concealment not stated. No intention-to-treat analysis. Some description of blinding | |||
| Gøbel 2010300 | L. acidophilus NCFM (ATCC 700396), 1010 colony-forming units/day. Taken once a day for 8 weeks | B. animalis ssp. lactis Bi-07 (ATCC SD5220), 1010 colony-forming units/day. Taken once a day for 8 weeks | Placebo (filler only – cellulose, silicon dioxide, rice-maltodextrin). Taken for 8 weeks | Denmark | 50 participants (n = 17 L. acidophilus NCFM group, n = 17 B. animalis ssp. lactis Bi-07 group, n = 16 placebo group). A significantly higher number of boys were randomised into the NCFM group | Atopic eczema diagnosed by a GP or dermatologist; experiencing continuous itching/pruritus at time of inclusion. Children recruited were between 7 and 24 months – specific criteria for inclusion not given. 49/50 children had a mother, father or sibling with an allergy (one child’s data not available as adopted) – specific criteria not given | (Primary) Severity of eczema (SCORAD index) (Secondary) Total IgE (Secondary) Specific IgE (Secondary) Faecal calprotectin (Secondary) Cytokines IL-10, IFN-γ, IL-31 |
Severity of eczema (SCORAD index): no beneficial effects of either probiotic found. Post hoc analysis found a beneficial effect of Bi-07 in conjunction with a decrease in the levels of IFN-γ and IL-10, making this probiotic of possible interest. No effect on inflammatory markers or faecal calprotectin was found in this study. Objective SCORAD index (post hoc?) did not show any significant differences between groups | Method of randomisation and allocation concealment not reported. Intention-to-treat analysis not clearly reported | ||
| Gore 2012302 | Dairy elimination diet (standard formula changed to extensively hydrolysed whey formula) plus a daily supplementation of lyphophilised powder L. paracasei (CNCM I-2116), 1010 colony-forming units per day. One sachet of L. paracasei per day plus the extensively hydrolysed formula for 3 months | Dairy elimination diet (standard formula changed to extensively hydrolysed whey formula) plus a daily supplementation of lyphophilised powder B. lactis (CNCM I-3446), 1010 colony-forming units per day. One sachet of B. lactis per day plus the extensively hydrolysed formula for 3 months | Placebo (maltodextrin). One sachet of placebo per day along with the extensively hydrolysed formula for 3 months | At the baseline visit there was a run-in period of 2 weeks when standardised skin treatment was commenced (1% hydrocortisone ointment twice a day, emollients/moisturisers two to four times a day and bath emollient). Other fermented or probiotic-containing products were avoided during the treatment period | Not stated | 328 infants screened, 272 met the inclusion criteria, 208 enrolled. Of these, 137 were randomised (n = 45 L. paracasei group, n = 45 B. lactis group, n = 47 placebo group). A total of 71 were put into an observational group (n = 22 continued exclusive breastfeeding, n = 49 continued established formula) | Physician-diagnosed eczema; SCORAD score of ≥ 10; age 3–6 months; good general health; normal growth; consuming ≥ 200 ml of standard formula a day | SCORAD index before and after the 12-week intervention SCORAD index at all other visits Eczema severity using the SCORAD index Infant’s Dermatitis Quality of Life Index (10-item questionnaire, Likert responses) Respiratory and allergic symptoms (using an interviewer-administered validated respiratory questionnaire) Allergic sensitisation (specific serum IgE to cow’s milk, egg, peanut, house dust mite, cat and dog) Adverse events |
Also measured urinary eosinophilic protein and carried out gastrointestinal permeability testing and detection of probiotic study strains in faeces | SCORAD index pre/post intervention: no significant difference observed between groups (p = 0.7). No differences were found for secondary outcomes. No difference was observed in SCORAD score between randomised and observational groups | Method of randomisation described. Allocation concealment not described. Parties blinded not stated. ‘Double blind’ listed as a key word, although this information could not be deduced from the main body of the paper. The paper states that intention-to-treat analysis was carried out but it is difficult to confirm this from the results presented |
| Grüber 2007289 | Lactobacillus GG > 5 × 109 colony-forming units per capsule, given twice a day for 12 weeks | Placebo capsules given twice a day for 12 weeks | Participants were instructed to reconstitute the contents of the capsule in milk or water but not tea or food heated to > 37°C | Germany | 106 (n = 54 Lactobacillus group, n = 48 placebo group) | Infants aged 3–12 months; visible atopic eczema according to the standard criteria; symptoms of eczema for ≥ 4 weeks; current severity of mild to moderate eczema (severity scoring/SCORAD score of 15–40) | (Primary) Symptom load score, mean reduction from baseline to week 12 [sum of SCORAD (mild ≤ 25, moderate 26–50 and severe 51–104) and MEDSCORE, an assessment of use of rescue medication – 2 points for each use of rescue medication in the week prior to assessment] Cumulative use of rescue medication (weighing tubes of medication) Compliance |
SCORAD score (mean, SD): Lactobacillus group 24.6 SD ± 8.8, placebo group 23.6 SD ± 7.8. Symptom load at 12 weeks: no statistically significant group differences. Also no statistically significant group differences for improvement when data stratified for age, severity or use of rescue medication. No significant between-group differences found for the use of rescue medication. Newly developed allergic sensitisation against hen’s egg or cow’s milk: Lactobacillus group 18.8% vs. placebo group 10% | Method of randomisation reported and adequate. Allocation concealment not reported. Intention-to-treat population used for the analysis | ||
| Han 2012304 | L. plantarum CJLP133, 0.5 × 1010 colony-forming units, given twice a day for 12 weeks after an initial washout period with placebo for 2 weeks. Afterwards, all participants had a 2-week follow-up period without any study medication | Placebo preparation, given twice a day for 12 weeks after an initial washout period with placebo for 2 weeks. Afterwards, all participants had a 2-week follow-up period without any study medication | During the 16-week study period patients were instructed not to consume any fermented food products containing live micro-organisms. Parents were instructed to bathe the children once daily in warm water and to treat their atopic dermatitis lesions frequently with emollients. Topical corticosteroids were not used unless the VAS for itch and insomnia was ≥ 7. 0.25% prednicarbate was offered to patients requiring rescue medication. The tube was weighed at each visit for quantitative measurements. Doses of medication were stored in airtight alu-bags at 4°C until administration | Not stated | 208 participants assessed for eligibility and 118 randomised (n = 58 probiotic group, n = 60 placebo group) | Patients aged 1–13 years with atopic dermatitis based on the criteria defined by Hanifin and Rajka;8 SCORAD scores ranged from 20 to 50 (not clear if this was an inclusion criterion or the distribution of scores of the enrolled patients) | SCORAD index assessed by the same paediatric allergist Pruritus on a scale of 1–10 (subjective score) Insomnia on a scale of 1–10 (subjective score) Eosinophil count Serum total IgE level and specific IgE levels to D. pteronyssinus and D. farinae, egg white, cow’s milk, soy, wheat and peanut. Measured at week 2 and at week 14 Cytokine measurements Microbial composition of L. plantarum in faecal samples |
SCORAD score at week 14 was lower in the probiotic group than in the placebo group (p = 0.044). No statistical differences were found in the use of topical corticosteroids between the groups (p = 0.815). The total eosinophil count was significantly lower at the end of treatment in the probiotic group than at baseline (p = 0.023). Logarithmic IFN-γ and IL-4 were significantly decreased after the intervention compared with baseline in the probiotic group (p < 0.001 and p = 0.049, respectively) | Randomisation method and allocation concealment described. Double-blind study. Intention-to-treat and per-protocol analyses carried out | ||
| Hattori 2003310 | Oral administration of B. breve M-16V strain in hydrolysed casein milk with raffinose, 5.0 × 109 colony-forming units per day or 1.5 × 1010 colony-forming units per day. Taken for 1 month | Hydrolysed casein milk taken for 1 month | Japan | 15 participants | Infants with atopic dermatitis after feeding with hydrolysed casein milk for > 2 weeks whose gut microflora ratio of Bifidobacterium was < 30% of total microflora | The proportion of Bifidobacterium in the faecal microflora 1 month after bifidobacteria administration The proportion of aerobic bacteria in the faecal microflora 1 month after bifidobacteria administration The level of faecal trehalase after bifidobacteria administration Allergic symptom score consisting of eczema, diarrhoea and respiratory symptoms 1 month after bifidobacteria administration |
No significant change in the level of stool trehalase in each group. Significant difference in the change in allergic symptom score between the experimental group and the control group (p = 0.0344/0.0189) | Method of randomisation not described. Allocation concealment not reported. Intention-to-treat analysis unclear. There were no excluded participants | |||
| Iemoli 2012288 | L. salivarius LS01 DSM 2275 and B. breve BR03 DSM 16604, 1 × 109 colony-forming units/g each in maltodextrin, given twice daily for 12 weeks | Placebo – maltodextrin, matched for shape, size and volume of contents with the intervention, given twice daily for 12 weeks | Supplements were stored as stable freeze-dried powder in sachet packets (provided by Probiotical Spa, Novara, Italy). Compliance was measured through a dose count (returned sachet packets). During the study patients were allowed to use oral antihistamines or emollient cream and were asked to limit exposure to the sun as much as possible, at least in the first 3 months. Patients were not asked to change their diet but were asked to avoid any fermented food products containing live micro-organisms and antibiotics administration | Italy | 60 patients recruited, 48 patients randomised (n = 32 active group, n = 16 placebo group) | Adult patients suffering from atopic dermatitis; no other inclusion criteria stated | SCORAD score (mild = 0–15, moderate = 16–40, severe > 40) DLQI measured by the patient Peripheral blood mononuclear cell immunophenotypic analysis of Th1, Th2, Th17 and regulatory T-cell lymphocyte subpopulations Plasma lipopolysaccharide concentration Cultivable bacteria in faecal samples |
SCORAD index was measured by a single, blinded investigator. DLQI was measured by the patient | Patients receiving probiotics showed a significant improvement in the SCORAD index (p < 0.0001) and DLQI (p = 0.021) from baseline. These changes were not observed in the placebo group | Method of randomisation not described. Allocation concealment not described. Double blind but parties blinded not stated. Intention-to-treat analysis used | |
| Isolauri 2000297 | Extensively hydrolysed whey formula supplemented with B. lactis Bb-12, 1 × 109 colony-forming units per gram (participants were weaned on to the formula) | Extensively hydrolysed whey formula supplemented with Lactobacillus GG, 3 × 108 colony-forming units per gram (participants were weaned on to the formula) | Extensively hydrolysed whey formula (participants were weaned on to the formula) | The strains of bacteria were selected in view of their documented safety and efficacy in controlling acute infantile diarrhoea | Not stated | 27 (n = 9 in each group) | Fulfilling the Hanifin and Rajka8 criteria for atopic eczema in children; eczema had begun during exclusive breastfeeding and the infant had had no exposure to any infant formula or substitute formula before enrolment; infants also had to tolerate the assigned formulas; enrolled at weaning when still fully breastfed | (Primary) Extent (estimates using the rule of nines), intensity (sum of the scores for erythema, oedema and/or papules, excoriation, lichenification and dryness) and subjective symptoms (pruritus and sleep loss) of atopic eczema, as SCORAD index (extent/5 + 3.5 × intensity + subjective score) (Secondary) Serum concentrations of soluble cell surface molecules and cytokines/chemokines (Secondary) Urinary concentrations of methyl-histamine and eosinophilic protein X |
SCORAD index, reflecting the extent and severity of atopic eczema, was a median of 16 (IQR 7–25) during breastfeeding. After 2 months, a significant improvement in skin condition occurred in participants given probiotic-supplemented formulas compared with the unsupplemented group (χ2 = 12.27, p = 0.002) | Many details of the study design are not reported. There are not details for the method of randomisation or blind. Intention-to-treat is not mentioned | |
| Javanbakht 2011328 | One 1600 IU calciferol (vitamin D3) capsule and two vitamin E placebo softgels (identical to vitamin E capsules but filled with mineral oil) daily. Group D: one capsule (vitamin D3) and two softgels (vitamin E supplement) to be taken with meals once a day for 60 days | Synthetic all-rac-alpha-tocopherol softgels (vitamin E) and vitamin D placebo (identical to the vitamin D capsules but filled with starch). Group E: one capsule (vitamin D placebo) and two softgels (vitamin E) (400 IU and 200 IU) to be taken with meals once a day for 60 days | Cholecalciferol (vitamin D3) capsule and synthetic all-rac-alpha-tocopherol softgels (vitamin E). Group DE: one capsule (vitamin D3) (1600 IU) and two softgels (vitamin E) (400 IU and 200 IU) to be taken once daily with meals for 60 days | The fourth group (group P) received one capsule (vitamin D placebo) and two softgels (vitamin E placebo) once daily with meals for 60 days. Patients were told to take the capsules with meals and to take vitamins D and E separately. Patients could use the prescribed routine treatments of atopic dermatitis including emollients, topical corticosteroids and oral antihistamines | Not stated | 52 participants randomised (n = 13 group D, n = 13 group E, n = 13 group DE, n = 13 group P) | Participants with atopic dermatitis diagnosed based on Hanifin and Rajka’s criteria;8 SCORAD score 10–70; normal hepatic and renal functions | SCORAD score Individual components of SCORAD (erythema, oedema, oozing, excoriation, lichenification, dryness) Subjective symptoms (pruritus, sleeplessness) Serum 25-hydroxy vitamin D3 level Plasma alpha-tocopherol level Topical corticosteroid usage |
Percent reduction in SCORAD after 60 days: group D (vitamin D plus vitamin E placebo) 34.8%, group E (vitamin E plus vitamin D placebo) 35.7%, group DE (vitamin D and vitamin E) 64.3% (p = 0.004). Objective SCORAD score showed significant improvement. There was a positive correlation between SCORAD score and intensity, objective, subjective and extent (p < 0.001). There was a significant negative association between plasma alpha-tocopherol and SCORAD, intensity, objective and extent (p = 0.02) | Randomisation method described. Allocation concealed. Double-blind study. Intention-to-treat analysis unclear | |
| Jin 2011331 | phCMF. The proteins whey and casein were partially hydrolysed to about 3500 Da by molecular weight. The ratio of whey to casein was 60% : 40%. Infants were given this formula for 12 weeks | Conventional CMF. Infants were given this formula for 12 weeks | Parents of the study subjects were advised to hold the introduction of other solid food throughout the study. The use of drugs including topical corticosteroids (mometasone furoate) and oral sedating antihistamines remained unchanged | Not stated | 113 subjects randomised (n = 72 phCMF, n = 41 CMF) | Healthy infants aged < 6 months; mild to moderate atopic dermatitis (defined by the criteria of Hanifin and Rajka8 where mild to moderate atopic dermatitis is defined as 10–50% of the whole body surface being involved); infants who tolerated exclusive CMF feeding or breastfeeding < 10% of the time. All of the infants included had no symptoms of cow’s milk allergy | JDASS (based on EASI) SCORAD allergy profile (total blood eosinophils, total/specific IgE, Th1/Th2 cytokine profiles and percentage of regulatory T cells) Growth status (length, weight, head circumference and chest circumference) Distribution of skin lesions (upper limb, lower limb, trunk and pate) |
Atopic dermatitis severity scores were significantly reduced in the phCMF group compared with the CMF group after 12 weeks (p < 0.05). Severity scores in the phCMF group were significantly reduced at weeks 4, 8 and 12 compared with enrolment (p < 0.05). No significant improvement was observed at any time point in the CMF group (p > 0.05). The number of atopic dermatitis flare-ups was significantly decreased in the phCMF group (p = 0.002) | Method of randomisation described. Allocation concealment not stated. Study was double blind, although parties blinded were not stated. Intention-to-treat analysis not stated, although the analysis was carried out on the number completing the study and not the number randomised | ||
| Koch 2008318 | DHA (5.4 g per day). Seven capsules taken a day for 8 weeks | Isoenergetic control [caprylic acid (4.17 g per day) and capric acid (2.84 g per day)]. Seven capsules taken a day for 8 weeks | Participants could use their standard therapy (emollients, topical corticosteroids, oral antihistamines) | Not stated | 53 participants (n = 28 DHA group, n = 25 control group) | Atopic eczema according to Hanifin and Rajka8 criteria; age 18–40 years | Severity of eczema (SCORAD index) Clinical safety (liver enzymes, complete blood count, serum lipids) IgE production from peripheral blood mononuclear cells Activation of monocytes and B cells Plasma fatty acid composition |
DHA group: significant clinical improvement of eczema (especially SCORAD score) – baseline 37.0 (17.9–48.0), week 8 28.5 (17.6–56.2). Placebo: no significant clinical improvement of eczema – baseline 25.4 (17.2–63.0), week 8 33.4 (10.7–56.2). Anti-CD40 IL4-mediated IgE synthesis of peripheral blood mononuclear cells: significant reduction in DHA group only. Modified activation status of peripheral blood mononuclear cells: both groups. DHA group: increase in plasma n-3 polyunsaturated fatty acids, decrease in n-6/n-3 polyunsaturated fatty acid ratio | Method of randomisation unclear; however, allocation concealment described. Intention-to-treat analysis not reported. Numbers of participants used for the analysis did not include withdrawals | ||
| Leung 2004330 | Neocate (a hypoallergenic, milk-, lactose- and sucrose-free, complete elemental formula with free amino acids; SHS International) given for 6 weeks | Participants’ pre-existing formula (control) given for 6 weeks | Advised not to give any dairy- or soy-based products to participants throughout the study. All other treatments remained unchanged throughout (mometasone furoate for all participants if needed) | Not explicitly stated (Chinese children) | 15 | Age < 3 years; recruited from paediatric allergy and dermatology clinics in a hospital; atopic eczema diagnosed according to Hanifin and Rajka8 criteria; drinking at least 500 ml of cow’s milk- or soy-based formula | Severity of eczema (SCORAD index) Caregiver-assessed global health score (VAS from 1 = worst to 9 = best) Eosinophil protein X concentration (expressed as a quotient to creatinine (sensitivity 3 µg/l) |
Results given for participants completing the study (n = 11). All changes (median) in scores and eosinophil protein X concentration not statistically significant. No carry-over effects found for SCORAD index or its components and the global health score, but some for eosinophil X protein (p = 0.05) | Method of randomisation not described and allocation concealment unclear. Intention-to-treat principle not used for the analyses | ||
| Mayser 2002317 | Fish oil-derived (n-3) 10% lipid emulsion by peripheral intravenous infusion – 100 ml twice daily (120 minutes per infusion) for 10 days | Soybean oil-derived (n-6) 10% lipid emulsion by peripheral intravenous infusion – 100 ml twice daily (120 minutes per infusion) for 10 days | Both groups were given a low-fat diet (1800 kcal per day) during the study. Both groups allowed to use only bland emollients during treatment with the study drug. In the 4-week follow-up period after receiving the study drug, both groups were allowed only 2–5% urea emulsifying ointments and specific treatments if necessary to control a reoccurrence of eczema | Not stated | 22 participants (n = 11 fish oil group, n = 11 soybean oil group) | Moderate to severe atopic eczema diagnosed according to the criteria of Hanifin and Rajka;8 ≥ 10% total body surface area involvement using the rule of nines | Dermatologist- assessed severity of eczema (score 0–4 for erythema, infiltration, desquamation on each of head, breast, back, abdomen, anogenital region, upper arms, forearms, hands, upper and lower thighs, feet; max. score 44) Participant-assessed clinical status (9-point scale, with 1 = worst to 10 = best for lesion appearance, impairment of daily life, pruritus, burning and pain; max. score 50) Levels of lipid mediators T-cell function Clinical safety (alanine aspartate aminotransferase, alkaline phosphatase, serum creatinine, blood glucose, total cholesterol, triglycerides, complete blood count) |
Both groups: marked improvement compared with baseline. Days 6, 7, 8 and 10: eczema severity score was better in the fish oil group than in the soybean oil group (p < 0.05). Free arachidonic acid: no substantial change in both groups. Plasma-free EPA, total bound EPA, membrane EPA/AA ratio: increased markedly in the fish oil group. Alongside this, EPA-derived lipid mediators appeared and lymphocyte functions were unaffected. Post-treatment period (4 weeks): relapse in some participants after n-3 PUVA infusion. Marked long-term improvement in the soybean oil group | Method of randomisation not adequately described and the statement ‘substitutions were made according to the random list’ when discussing dropouts makes the study difficult to interpret. Allocation concealment not stated. Intention-to-treat analysis not described | ||
| Moroi 2011294 | Heat-killed L. paracasei K71. Daily dose approx. 2 × 1011 colony-forming units of L. paracasei and 400 mg of dextrin NSD300 totalling 500 mg. Taken once a day dissolved in about 100 ml of water, tea or coffee for at least 12 weeks (length of time on treatment not explicitly stated) | Placebo. Daily dose 500 mg of dextrin NSD300 and 0.45 mg of carotene (to aid blinding). Taken once a day dissolved in about 100 ml of water, tea or coffee for at least 12 weeks (length of time on treatment not explicitly stated) | The participants were told not to change the kinds of topical treatments used if possible during the study | Japan | 34 participants (n = 17 L. paracasei group, n = 17 placebo group) | Japanese adults aged 20–65 years; diagnosis of mild or moderate atopic eczema according to Japanese Dermatological Association criteria26 | Skin severity (Japanese Dermatological Association criteria: sum of eruption intensity on a scale of 0–4, with 0 = no symptoms, 1 = mild, 2 = moderate, 3 = severe, 4 = extremely severe, for each of five body areas – head and neck, anterior trunk, posterior trunk, upper limbs, lower limbs) Itch intensity – (100-mm VAS, with 0 = no itch, 100 = worst itch imaginable) Quality of life (Skindex-16 – Japanese edition) Tolerability and safety (incidence and frequency of study treatment-related adverse events) |
Skin severity: L. paracasei group – change from baseline to week 8 p < 0.05, change from baseline to week 12 p < 0.01; placebo group – no significant changes. No evidence of effect of the L. paracasei diet on itch and quality of life. Use of topical corticosteroids: placebo group 1.9 times greater than the L. paracasei group but not a significant difference | Method of randomisation not clearly described. Allocation concealment not reported. Intention-to-treat analysis not explicitly stated and does not appear to have been used | ||
| Murosaki 2006311 | Nigero-oligosaccharides and heat-killed L. plantarum L-137. 10 g of syrup contained 3 g of nigero-oligosaccharides and 10 mg of heat-killed L. plantarum L-137. 10 g taken once a day for 12 weeks | Matching maltose syrup without nigero-oligosaccharides or heat-killed L. plantarum L-137 as placebo. 10 g taken once a day for 12 weeks | The patients continued with the same therapy used before the trial | Not stated | 45 participants (n = 22 placebo, n = 23 intervention) | Participants with moderate or severe atopic dermatitis | Health-related quality-of-life score pre and post intervention Investigator evaluation: clinical usefulness score (1 = useful to 5 = unfavourable) Cell surface expression of CD64 on monocytes pre and post intervention Total serum IgE pre and post intervention Peripheral blood eosinophil levels pre and post intervention Patient self-evaluation: itching score (1 = severe to 5 = none) pre intervention and after 4, 8 and 12 weeks |
Itching score: no difference between groups. Severe eczema participants: difference between groups (p = 0.088). Clinical findings: no difference between groups | Method of randomisation not described. Allocation concealment not reported | ||
| Nermes 2011290 | Extensively hydrolysed casein formula with L. rhamnosis GG (ATCC 53103), 5.0 × 107 colony-forming units/g to achieve 3.4 × 109 colony-forming units/day | Extensively hydrolysed casein formula without supplementation | Finland | 39 participants (n = 19 probiotics and formula group, n = 20 formula group); 37 participants completed the trial | Atopic dermatitis according to the criteria of Hanifin and Rajka8 | Severity of eczema (SCORAD index) IgE-mediated rates of sensitisation to common food allergens (cow’s milk, raw hen’s egg, gliadin) Total number of circulating immunoglobulin-secreting cells (IgA, IgG, IgM) Proportion of cells expressing CD19 and CD27 (flow cytometry) Microbiota characterisation (quantitative polymerase chain reaction) Qualitative polymerase chain reaction Number of infections during the study |
Proportion of IgA- and IgM-secreting cells (baseline-adjusted ratios) at 1 month: IgA-secreting cells – probiotics and formula group vs. formula only 0.59 (95% Cl 0.36 to 0.99) (p = 0.044), IgM-secreting cells – probiotics and formula group vs. formula only 0.53 (95% Cl 0.29 to 0.96) (p = 0.036). Proportions of CD19-positive and CD27-positive B cells increased in the probiotics and formula infant group but not in the formula-only group. No significant differences in bifidobacterial species between groups. Skin bacterial counts of Bifidobacterium genus vs. Clostridium coccoides were similar in both groups | Description of randomisation and allocation concealment not provided. Intention-to-treat analysis not reported. The study was double blind but it did not state which parties were blinded | |||
| Passeron 2006309 | L. rhamnosis Lcr35 and prebiotic-specific preparation and metabolites secreted by L. rhamnosis Lcr35 (contains skimmed milk powder, potato starch and lactose). Per 1.5-g dose, 1.2 × 109 colony-forming units of L. rhamnosis. The prebiotic dose contained, 0.344 g of skimmed milk powder (33% bovine protein 52% lactose), 0.759 g of potato starch and 0.397 g of lactose. Taken three times a day for 3 months | L. rhamnosis Lcr35. Per 1.5-g dose, 1.2 × 109 colony-forming units of L. rhamnosis. Taken three times a day for 3 months | France | 48 participants (n = 24 synbiotic group, n = 24 prebiotic group) | Age 2–12 years; atopic eczema according to UK Working Party criteria;9 total SCORAD score of > 14; not presenting with a flare of eczema | Total SCORAD score (max. score 103; moderate = 16–39, severe = ≥ 40) Objective SCORAD score (without the subjective pruritus/sleep loss component; max. score 83) IGA (6-point scale, with 0 = clear, 1 = almost clear, 2 = mild, 3 = moderate, 4 = severe, 5 = very severe) Number of flares Possible side effects Participant-/parent-assessed efficacy (eczema worse/unchanged/improved) |
Mean total SCORAD score: prebiotic group 39.3 before treatment, 20.7 after 3 months’ treatment (p < 0.0001). Difference between the two groups after 3 months’ treatment not statistically significant (p = 0.535). Use of ointment: no statistically significant difference between groups (p = 0.966). Tolerance was excellent in both groups. Analysis of whole population at month 3 showed comparable results for objective and total SCORAD scores | Method of randomisation and allocation concealment not reported. Intention to treat not used for the primary analyses, but whole population assessed for comparison of month 3 data | |||
| Rosenfeldt 2003284 | Lactobacillus strains (lyophilised L. rhamnosus 19070–2 and L. reuteri DSM 122460), 1010 colony-forming units per 1-g dose, given twice daily for 6 weeks dissolved in 2.5–5 ml of any liquid acceptable to the participant | Placebo (skimmed milk powder), 0.28 g of bovine protein and 0.72 g of dextrose anhydrate per 1-g dose, given twice daily for 6 weeks dissolved in 2.5–5 ml of any liquid acceptable to the participant | 6-week washout period between the two active treatment phases | Denmark | 58 participants | Age 1–13 years; diagnosis of atopic eczema based on the UK Working Party diagnostic criteria9 | Severity of eczema (SCORAD index; max. score 80, mild = 0–15, moderate = 16–40, severe = > 40) Participant- or caregiver-assessed eczema evaluation (‘generally better’, ‘better’, ‘unchanged’ or ‘worse’) Serum IgE Serum eosinophil cationic protein Level of cytokines from peripheral blood mononuclear cells |
Participant- or caregiver-assessed severity of eczema: 56% improvement in eczema after treatment with Lactobacillus, 15% improvement in eczema after treatment with placebo (p = 0.001). Total SCORAD score: no significant change. Extent of eczema: Lactobacillus group decreased from 18.2% to 13.7% (p = 0.02). For allergic participants (at least one positive skin-prick test and elevated IgE levels), treatment response more pronounced and in this group the SCORAD score decreased (p = 0.02 compared with non-allergic participants) | Method of randomisation only partially described. Allocation concealment and intention-to-treat analysis not reported | ||
| Senapati 2008316 | Evening primrose oil, 500-mg capsules with 8–10% of gamma-linoleic acid and 10 IU of vitamin E. Taken for 5 months: one to four capsules per day for those aged up to 1 year, five to six capsules per day for those aged 2–5 years, seven to eight capsules per day for those aged 6–10 years, nine to 10 capsules per day for those aged 11–16 years, 12 capsules per day for those aged > 16 years | Placebo (sunflower oil), 300-mg capsules with 10 IU of vitamin E. Taken for 5 months: one to four capsules per day for those aged up to 1 year, five to six capsules per day for those aged 2–5 years, seven to eight capsules per day for those aged 6–10 years, nine to 10 capsules per day for those aged 11–16 years, 12 capsules per day for those aged > 16 years | Participants were always given the maximum acceptable dose to avoid the effect of varying dose on response | India | 65 participants (n = 29 evening primrose oil group, n = 36 placebo group); n = 26 and n = 27, respectively, completed the study | Diagnosed with atopic eczema according to Hanifin and Rajka8 criteria | Extent of disease (one area/< 20% body surface area = 1, two or three areas/up to 40% body surface area = 2, more than two or three areas/> 40% body surface area = 3) Intensity of disease (Intensity Item Score Aggregate: erythema, oedema/papulation, vesiculation/oozing/crusting, excoriation, scaling and lichenification each on a scale from 0 to 3, with 0 = absence, 1 = mild, 2 = moderate, 3 = severe. IISA 1–6 = 1, IISA 7–12 = 2, IISA 13–18 = 3) Dryness on uninvolved areas (0 = absence, 1 = mild, 2 = moderate, 3 = severe) Itching (0 = absence, 1 = mild, 2 = moderate, 3 = severe) Total score (sum of four major parameters – extent, intensity, dryness, itching; score 1–4 = mild, 5–8 = moderate, 9–12 = severe) Change in total score from baseline (up to and including 25% of baseline = marked improvement, > 25% up to 50% = moderate improvement, > 50% up to 75% = mild improvement, > 75% up to 99% = marginal improvement, 100% = static, more than baseline = deterioration, ‘no improvement’ = marginal improvement, static and deterioration) |
At the end of month 5, 96% (24 participants) in the evening primrose group and 32% (eight participants) in the placebo group showed an improvement. Difference between groups p = 0.00001. No significant adverse events reported | Method of randomisation described and adequate. Allocation concealment not reported. Intention-to-treat analysis not reported. Only the first 25 participants from each group were analysed for simplicity | ||
| Shafei 2011314 | Synbiotic preparation (seven strains of probiotic plus prebiotic). One sachet contained a 10-mg mixture of probiotics = 1 × 109 colony-forming units of seven strains of probiotics, and 990 mg of fructo-oligosaccharides. Taken once a day, mixed with breast milk, water, formula or solid food for up to 2 months | Placebo: 1000 mg of sucrose. Taken once a day mixed with breast milk, water, formula or solid food for up to 2 months | The synbiotic and placebo supplements were image matched and identical in appearance, taste and smell. Usual treatment of atopic eczema was allowed including usual bathing habits, moisturising cream (Eucerin) and topical corticosteroid (1% hydrocortisone) | Iran | 41 patients were initially included (n = 20 synbiotic group, n = 21 placebo group). Five patients did not complete the study (three in the placebo group and two in the synbiotic group). In total, 36 patients completed the trial | Age 1–36 months; atopic eczema according to the Hanifin and Rajka8 criteria; moderate to severe eczema (SCORAD score of > 25) | Total SCORAD score Objective SCORAD score Total IgE level in peripheral blood Sensitivity to common food allergens and aeroallergens (skin-prick tests – wheal diameter of 3 mm greater than negative control considered a positive reaction) Subjective parent-assessed pruritus and sleep loss Complete blood count |
No significant difference between placebo group and synbiotic group for mean decrease in total SCORAD score: synbiotic group 24.2, placebo group 22.3. No difference between groups in the mean decrease in total SCORAD according to different demographic, clinical and paraclinical subgroups | Method of randomisation described and allocation concealment reported. Intention-to-treat analysis not reported. All study investigators blinded to treatment | ||
| Shibata 2009305 | Oral kestose (oligosaccharide) – 1 g of 97% 1-kestose and < 3% nystose (glucose-[fructose]3) per day (< 1 year) or 2 g per day (> 1 year), taken every day for 12 weeks | Placebo – 1 g of maltose (glucose-glucose) per day (< 1 year) or 2 g per day (> 1 year), taken every day for 12 weeks | All participants were permitted to use topical steroids | Japan | n = 15 kestose group, n = 15 placebo group | Age < 3 years; diagnosis of atopic eczema according to the Japanese Dermatological Association26 (all three of pruritus, typical morphology and distribution, chronic relapsing course) | ISAD (sum of intensity of each of erythema, papules, typical morphology, distribution, chronically relapsing course on a 4-point scale, with 0 = absence, 1 = mild, 2 = moderate, 3 = severe) SCORAD index (objective only; max. score 83) Total IgE (using human IgE enzyme-linked immunosorbent assay) Allergen-specific IgE (ImmunoCAP) |
SCORAD score (medians): kestose group significantly lower than placebo group at week 6 (25.3 vs. 36.4; p = 0.004) and week 12 (19.5 vs. 37.5; p < 0.001). Improvement in SCORAD score and bifidobacteria count: no significant correlation | Method of randomisation described. Allocation concealment not reported. Intention-to-treat analysis not reported and the one participant who withdrew was not included in the analysis | ||
| Sidbury 2008326 | Ergocalciferol (vitamin D2), 1000 IU. Taken once daily for 1 month | Placebo taken once daily for 1 month | Participants could continue with all previous treatment including emollients but could not start any new treatments during the study. No travel to temperate climates during the study | USA | 11 participants(n = 5 vitamin D group, n = 6 placebo group) | Children (no specific criteria) (age range of included participants 2–13 years); no specific severity criteria given (10/11 participants had mild atopic eczema according to an EASI score of 10–18.6) | IGA (6-point scale, with 1 = clear 6 = very severe) change from baseline | Study suggests potential short-term benefits for vitamin D2 supplementation. Longer studies are needed to confirm this result | Method of randomisation described but unclear. Allocation possibly described but the report is unclear. Intention-to-treat analysis not reported | ||
| Sistek 2006285 | Probiotic (L. rhamnosis and B. lactis), 2 × 1010 colony-forming units per gram. Powder given once daily mixed with water for 12 weeks (some older children took the powder in its opaque capsule) | Placebo (microcrystalline cellulose). Powder given once daily mixed with water for 12 weeks (some older children took the powder in its opaque capsule) | New Zealand | 62 participants eligible (n = 29 probiotic, n = 30 placebo) | Age 1–10 years; atopic eczema present of at least 6 months’ duration; atopy – at least one positive skin-prick test (wheal size ≥ 3 mm) or one positive RAST test (≥ 7 kU/l) to any common food or environmental allergens; SCORAD score of ≥ 10; stable atopic eczema (change in SCORAD score of not more than 11 points between the visit 2 weeks before treatment and baseline) – if not stable the participant was seen for an extra visit 2 weeks later | SCORAD score Family and personal history of allergic disease (participant reported – diary) Medication use (participant reported – diary) Changes in lifestyle/housing (participant reported – diary) |
For assessment of the SCORAD score the assessors independently scored children until there was a high level of agreement on the parameters of the SCORAD index | SCORAD geometric mean at baseline: probiotic group 26.0 (95% CI 21.9 to 30.8), placebo group 35.1 (95% CI 28.9 to 42.8), difference significant. After adjustment for baseline differences, no significant improvement in atopic dermatitis at 12 weeks: SCORAD geometric mean ratio 0.80 (95% Cl 0.62 to 1.04) (p = 0.10). Food-sensitised children: improvement in the probiotics group: SCORAD geometric mean ratio 0.73 (95% Cl 0.54 to 1.00) (p = 0.047) | Method of randomisation described but slightly unclear. Allocation concealment not reported. Intention-to-treat population used for the analyses but a definition not reported | ||
| Takwale 2003315 | Borage oil (23% gamma-linoleic acid). Adults took four capsules (920 g of gamma-linoleic acid) and children took two capsules twice daily for 12 weeks | Liquid paraffin (adults) and olive oil (children), to avoid possible laxative effects of liquid paraffin, taken twice daily for 12 weeks | England | 151 participants(n = 91 borage oil group, n = 60 placebo group) | Children aged > 2 years and adults; diagnosis of atopic eczema according to the Hanifin and Rajka8 criteria; fertile women had to use effective contraception | (Primary) Mean change in Total Sign Score at 12 weeks using the SASSAD scoring system (erythema, exudation, excoriation, dryness, cracking and lichenification each graded 0–3 at each of six sites: hands, feet, arms, legs, head, neck and trunk; max. score 108) Participant-assessed itching on a 10-cm VAS Participant-assessed sleep loss on a 10-cm VAS Participant-assessed irritability on a 10-cm VAS Participant-assessed treatment response relative to baseline (5-point scale: ‘worse’, ‘same’, ‘improved’, ‘much improved’, ‘cleared’) Need for topical steroids (5-point scale, with 1 = none, 2 = occasionally, 3 = alternate days, 4 = once daily, 5 = twice daily) Participant-assessed overall tolerability of the treatment (4-point scale) |
Blood samples for biochemistry and haematology also taken at screening, week 2 and end of treatment | Mean SASSAD score in the intention-to-treat population (n = 140): borage oil group – from 30 to 27 at the end of treatment, placebo group – from 28 to 23 at the end of treatment. Mean difference 1.4 (95% Cl –2.2. to 5.0). Mean SASSAD score in the per-protocol population (n = 124): borage oil group – from 30 to 27 at the end of treatment, placebo group – from 28 to 24 at the end of treatment. Mean difference 0.6 (95% Cl –3.1 to 4.3) | Method of randomisation and allocation concealment explained and adequate. Intention-to-treat method used and clearly defined. SDs presented and narrow | ||
| Taniuchi 2005298 | Lyophilised B. breve M-16V strain, 5 × 109 or 15 x 109 colony-forming units per day. Taken orally for 3 months | No treatment | The B. breve preparation did not contain any milk proteins | Not stated | 17 participants (n = 10 B. breve group, n = 7 no treatment group) | Atopic eczema based on Hanifin and Rajka8 criteria; cow’s milk allergy (positive RAST or skin-prick test and positive allergic symptoms when given cow’s milk/improvement of allergic symptoms on an elimination diet); faecal microflora containing < 30% Bifidobacterium after being fed a casein hydrolysed formula (New-MA-1) for at least 2 weeks | Participant-/parent-reported allergic symptom score (total obtained from cutaneous symptom score and scores for gastrointestinal symptoms, respiratory symptoms and utilisation of ointments on a scale from 0 to 3, with 3 = most severe) Atopic eczema score (modified Kimata’s score – erythema, lichenification and cracking on a scale from 0 to 2, with 2 = most severe, assessed on four body regions – face, trunk, arms and legs; subjective symptoms of itching and sleep loss on a scale from 0 to 3) |
Proportion of B. breve in the faecal microflora: B. breve group – proportion increased after 3 months of treatment whereas proportion of aerobic bacteria decreased – associated with a significant improvement in allergic symptoms compared with baseline; no treatment group – no significant changes in faecal microflora. Total allergic score: no significant changes for the whole study period | Method of randomisation, allocation concealment and blinding not described | ||
| Torii 2011296 | Milk components supplemented with 100 mg of heat-treated L. acidophilus (L-92) (at a concentration equivalent to a bacterial count of approximately 1.5 ×1011) and 900 mg of dextrin for an 8-week period | Milk components supplemented with 1000 mg of dextrin (placebo) | Each patient and his or her parents not to change the patient’s lifestyle or skincare regimen during the study period | Not stated | 60 children were enrolled (n = 30 placebo, n = 30 L-92); 29 patients in each group completed the study and 50 patients were included in the analysis (n = 24 placebo, n = 26 L-92) | Tolerance to cow’s milk; no evidence of skin infection, including infectious impetigo or dermatomycosis at enrolment; no recent history of antibiotic use; clear steroid dependency for maintaining skin condition; no complication with seasonal allergic rhinitis; no habit of consuming materials that may affect the intestinal microbiota, including medicine for intestinal disorders and fermented foods such as fermented milk | Atopic eczema severity evaluated using ADASI score (range 1–18) Symptom medication score calculated as the sum of the ADASI score and the medication score [sum of the product of the intensity factor and the amount (expressed in grams) of each steroid ointment used in a 4-week period] White blood cell count, number of eosinophils and serum C-reactive protein concentration Serum total IgE concentration Serum thymus and activation-regulated cytokine concentration |
Ns | Orally administered L-92 significantly improved the symptoms of atopic dermatitis in Japanese children. L-92 also affected the serum concentrations of thymus and activation-regulated chemokine in a time-dependent manner | Method of randomisation described. No description of allocation concealment. The study was double blind but the parties blinded were not stated. Intention-to-treat analysis not described | |
| van der Aa 2010308 | Synbiotic mixture of B. breve M-16V and 90% short-chain galacto-oligosaccharide + 10% long-chain fructo-oligosaccharide suspended in partially hydrolysed whey-based formula (Nutrilon Pepti®, Nutricia). B. breve M-16V = 1.3 × 109 colony-forming units/100 ml; 90% short-chain galacto-oligosaccharide + 10% long-chain fructo-oligosaccharide = 0.8 g/100 ml. Taken for 12 weeks | Partially hydrolysed whey-based formula (Nutrilion Pepti®). Taken for 12 weeks | Only emollients, topical corticosteroids or antibiotics allowed during the study | The Netherlands | 90 participants (n = 46 synbiotic group, n = 44 placebo group) | Full-term infants; age 0–7 months; fulfilling Hanifin and Rajka8 criteria for atopic eczema; exclusively bottle fed at the time of enrolment; SCORAD score of > 15 | (Primary) Change in the severity (SCORAD index) of eczema after 12 weeks compared with baseline (Secondary) Change in topical corticosteroid usage (change in class of topical corticosteroid) (Secondary) Frequency of topical corticosteroid use (days per week) (Secondary) Total serum IgE (Secondary) Specific IgE against food and inhalant allergens (Secondary) Serum eosinophilic granulocytes (Secondary) Change in microbiota composition, faecal short-chain fatty acids, lactate and pH |
(Secondary) Stool frequency and consistency | SCORAD index improvement: no difference between groups. Microbiota composition (%) (synbiotic group vs. placebo group): bifidobacteria 54.7% vs. 30.1% (p < 0.001), Clostridium lituseburense/histolyticum 0.5% vs. 1.8% (p = 0.02), Eubacterium rectale/Clostridium coccoides 7.5% vs. 38.1% (p < 0.001). Infants with IgE-associated atopic eczema subset (n = 48): improvement in SCORAD index was significantly greater in the synbiotic group at week 12: –18.1 vs. –13.5 (p = 0.04) | Method of randomisation described. Allocation concealment not explicitly stated but implied | |
| Viljanen 2005293 | Lactobacillus GG, 5 × 109 colony-forming units. One capsule content mixed with food, twice daily | Mixture of Lactobacillus GG, L. rhamnosus, B. breve, Propionibacterium freudenreichii ssp. shermanii JS, 5 × 109 colony-forming units for both Lactobacillus GG and L. rhamnosus, 2 × 108 colony-forming units for B. breve and 2 × 109 colony-forming units for P. freudenreichii ssp. shermanii JS. One capsule content mixed with food, twice daily | Placebo (microcrystalline cellulose only). One capsule content mixed with food, twice daily | Participants were instructed to apply emollients daily and use 1% topical hydrocortisone as needed up to a maximum of 2 weeks at a time | Finland | 252 participants (n = 85 Lactobacillus GG, n = 85 mixture, n = 82 placebo) | Age < 12 months; symptoms suggesting cow’s milk allergy; must have eczema | Severity (SCORAD index – extent scored from 0 to 100, intensity estimated with the help of reference photographs, score of 0–3 for each of erythema, oedema/papules, oozing/crusts, excoriation, lichenification, skin dryness and score of 0–10 for each of participant-assessed pruritus and sleep loss. Extent/5 + subjective symptoms. Max. score 103) (Exploratory) Interactions between treatment and different baseline characteristics (IgE association and SCORAD index) |
Whole group: SCORAD index decreased by 65%. No difference in SCORAD index between treatment groups straight after treatment or 4 weeks after treatment. No difference in SCORAD index for participants with cow’s milk allergy. SCORAD index in IgE-sensitised group participants: Lactobacillus GG group –26.1 vs. placebo group –19.8 (p = 0.036) from baseline to 4 weeks post treatment. Exclusion of participants who took antibiotics during the study reinforced these findings | Some details of a good method of randomisation. Allocation concealment not stated. Intention-to-treat analysis not described; however, the analyses were carried out only on the participants who completed the study. It is stated that randomisation did not work well as the SCORAD index at baseline differed between groups | |
| Vita 2007329 | Goat’s milk. First group = 6 months, second group = 3 months | Ass’s milk. First group = 6 months, second group = 3 months | Timed for the trial to stop before the summer to avoid the confounding factor of improvement of eczema during the summer months | Italy | 28 participants (n = 14 ass’s milk, n = 14 goat’s milk) | Age 6 months to 3 years; clinical history of cow’s milk allergy; positive prick-by-prick test to cow’s milk extract; positive double-blind placebo-controlled food challenge to cow’s milk; active atopic eczema (SCORAD score of > 20) | (Primary) SCORAD score (mild ≤ 25, moderate > 25 and < 50, severe ≥ 50) Allergic status to ass’s and goat’s milk at the end of the study (double-blind placebo-controlled food challenge) Participant-assessed skin symptoms during the last 4 weeks (VAS from 0 = no symptoms to 10 = very severe symptoms) Milk proteins (sodium dodecyl sulphate polyacrylamide gel electrophoresis) |
Ass’s milk: improvement in SCORAD score and participant-assessed skin symptoms (p < 0.03 vs. baseline and intergroup). Goat’s milk: no measurable clinical effect. End of study: positive food challenge – ass’s milk 1/26, goat’s milk 23/26 | Method of randomisation described and adequate but allocation concealment not reported. Intention-to-treat analysis not reported | ||
| Weston 2005303 | Probiotic (L. fermentum VRI-003 PCC). Powder containing 1 billion colony-forming units. Two sachets of powder per day for 8 weeks | Maltodextrin (control group). Two sachets of powder per day for 8 weeks | Australia | 56 participants randomised and received treatment (n = 28 probiotic group, n = 28 placebo group) | Age 6–18 months; moderate to severe atopic eczema meeting Hanifin and Rajka8 criteria; modified SCORAD score of ≥ 25 | Change in severity of eczema (SCORAD index, modified SCORAD index) Change in family quality of life (Dermatitis Family Impact questionnaire) Change in reported topical corticosteroid usage Compliance (parent-reported ‘sachet chart’) Parental impression of the treatment (at the end of treatment – ‘better’, ‘worse’, ‘unchanged’) |
Single investigator performed all of the SCORAD assessments | Reduction in SCORAD index over time: probiotics group – significant (p = 0.03), placebo group – not significant compared with baseline. Probiotics group – 92% of participants (n = 24) had a better SCORAD value at week 16. Placebo group – 63% (n = 17) had a better SCORAD value at week 16. Difference – p = 0.01. Number of participants with mild eczema at the end of the study: probiotic group 54% (n = 14), placebo group 30% (n = 8) | Method of randomisation described and allocation concealment implied but not explicitly stated. Intention-to-treat analysis does not appear to have been used | ||
| Woo 2010295 | L. sakei KCTC0755BP in microcrystalline cellulose, 5 × 109 colony-forming units per dose. Two doses per day for 12 weeks | Microcrystalline cellulose placebo (identical in appearance). Two doses per day for 12 weeks | None of the participants changed their diet during the trial. Participants were allowed to use a topical corticosteroid (0.1% prednicarbate) during the study. All participants were asked to bathe for to 10 minutes every day and then apply emollient after bathing during the study | Not stated | 88 participants (n = 45 probiotic, n = 43 placebo) | Age 2–10 years; atopic eczema-dermatitis syndrome for at least 6 months; SCORAD index > 25; change in SCORAD index not > 10% within 2 weeks | Severity of eczema (SCORAD index, objective score max. 83, objective and subjective score max. 103) (mild = < 25, moderate = 25–50, severe = > 50) Participant/parent assessment of tolerance Participant/parent assessment of efficacy Serum levels of chemokine CCL17 and CCL27 (enzyme-linked immunosorbent assay) Total and specific IgE levels |
Total SCORAD index (adjusted for baseline values): probiotic group scores were lower than placebo group scores (p = 0.01). Change in scores from baseline: probiotic group 13.1 (31%), placebo group 5.2 (13%) (p = 0.08). Significant differences in favour of the probiotic group were observed for the proportion of participants with improvement of at least 30% and 50% | Method of randomisation not described. Allocation concealment not reported. Full analysis set used for the analyses | ||
| Wu 2012313 | Synbiotic group (probiotic and prebiotic): L. salivarius and fructo-oligosaccharide. Each capsule contained 475 mg of fructo-oligosaccharide and 25 mg of live probiotic (2 × 109 colony-forming units). One capsule was taken orally twice daily for 8 consecutive weeks | Control group (prebiotic only): fructo-oligosaccharide and maize starch. Each capsule contained 475 mg of fructo-oligosaccharide plus 25 mg of maize starch. One capsule was taken orally twice daily for 8 consecutive weeks | Routine therapy for atopic dermatitis continued throughout the trial including use of topical corticosteroids and topical calcineurin inhibitors. Nerisone® fatty ointment was used (1 g contained 1 mg of diflucortolone valerate; Intendis Manufacturing SpA). A topical calcineurin inhibitor (pimecrolimus 1% cream) was used for skin lesions on the face and inguinal areas. Patients who received systemic steroid therapy or had a SCORAD score of ≥ 25 were discontinued from the trial | Taiwan | 60 children (n = 30 synbiotic group, n = 30 prebiotic group) | Age 2–14 years (not stated as an inclusion criterion but this is the age range in the included population); moderate to severe atopic dermatitis according to the Hanifin and Rajka8 diagnostic criteria (SCORAD score > 25) and persistently showed atopic dermatitis symptoms for at least 4 days before diagnosis | Clinical efficacy: SCORAD scores at weeks 0, 4, 8 and 10 Extent (using individual component of the SCORAD index) Intensity (using individual component of the SCORAD index) Pruritus on a VAS from 0 to 10 points Sleep loss on a VAS from 0 to 10 points Quality of life – 13-point quality-of-life assessment on a 5-point scale, with 0 = none, 1 = seldom (less than three times per month), 2 = sometimes, 3 = usually, 4 = every day, recorded by parents Frequency of medication use – measured as part of the 13-point quality-of-life assessment on a 5-point scale |
SCORAD score at 8 weeks: synbiotic group 27.4 SD ± 12.7, control group 36.3 SD ± 14.9 (p = 0.022). Atopic dermatitis intensity was significantly lower in the synbiotic group at 8 weeks (p = 0.013). Severity of atopic dermatitis: number (%) of children with mild atopic dermatitis: synbiotic group 14/27 (52%), control group 8/27 (30%) (p = 0.024). Medication use frequency was significantly reduced in the treatment group at 8 weeks compared with 4 weeks | Method of randomisation and allocation concealment described. Double-blind design. Intention-to-treat principle applied | ||
| Yesilova 2012287 | Four types of probiotic bacteria. Two bags containing 2 × 109 of B. bifidum, L. acidophilus, L. casei and L. salivarius (ProBiotik pur; Ella Farma) taken daily for 8 weeks | Placebo: skimmed milk powder (Dairy Inc., Enka Milk Joint Stock Company) and dextrose (Havana Chemistry, Pharmaceutical Medical Limited) taken daily for 8 weeks | Not stated | 40 paediatric patients (n = 20 probiotic group, n = 20 placebo group) | Moderate to severe SCORAD index score; age 1–13 years; absence of any other disease; used no medication including antihistamines and corticosteroids for 14 days prior to the study; absence of gastrointestinal malabsorption | SCORAD index Total IgE levels Eosinophil cationic protein Cytokine levels |
Probiotic intervention effectively reduced the SCORAD index in paediatric patients with atopic dermatitis | Method of randomisation given but no description of the method. Allocation concealment stated but not described. Study was double blind but no details given. No description of intention-to-treat population, although it looks as though this was used because the number analysed was the same as the number randomised despite one patient dropping out | |||
| Yoshida 2010299 | Lyophilised powder of live B. breve strain YY, > 20 billion colony-forming units per day. One capsule taken after breakfast and one capsule taken after dinner | Placebo powder. One capsule taken after breakfast and one taken capsule after dinner | Pretreatment medication remained unchanged throughout the study for nearly all of the participants | Not stated | 24 participants (n = 16 B. breve group, n = 8 placebo group) | Adults; diagnosed according to the Japanese Dermatological Association criteria26 | Severity of eczema (SCORAD index) Bacterial content of gut flora (faecal sampling) Serum IgE Eosinophils Plasma thymus and activation-regulated chemokine Health-related quality of life (Skindex-29-J Japanese version) |
Objective SCORAD index: significantly improved in the B. breve group compared with the placebo group. Quality of life: total score significantly improved in the B. breve group. Faecal sampling: the proportion of B. breve found in the B. breve group was increased. Serum IgE, eosinophils and thymus and activation-regulated chemokine/chemokine CCL17: no significant changes | Method of randomisation described but open to query as it is not clear if a truly random allocation was used. Allocation concealment was not explicitly stated but was implied from the report. Intention-to-treat analysis not reported |
Appendix 14 Non-pharmacological treatments
| Trial | Intervention A | Intervention B | Intervention C | Comments on interventions | Country | Number of participants randomised | Inclusion criteria | Outcomes | Comments on outcomes | Main reported results | Quality of reporting |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arkwright 2001375 | M. vaccae, heat-inactivated (SRL 172) in buffer solution, 0.3-ml suspension containing 1010 M. vaccae/ml. Given once via an intradermal injection into an eczema-free zone on the left deltoid | Buffer solution, 0.3-ml suspension. Given once via an intradermal injection into an eczema-free zone on the left deltoid | England | 41 participants (n = 21 SRL 172 group, n = 20 placebo group) | Moderate to severe eczema | Severity of eczema (SASSAD score) Potency of topical steroid used |
No significant improvement in the surface area affected by eczema in the placebo group. There was a significant reduction in surface area affected by eczema in the SRL 172 group compared with the placebo group at month 1 (p = 0.01) and 3 months after vaccination (p = 0.01). There was a significant reduction in the severity of eczema in the SRL 172 group compared with the placebo group at month 1 (p = 0.001), which persisted until the end of the trial | Method of randomisation and allocation concealment described and good. Blinding described and good. It is not clear from the information provided whether the analyses were carried out on an intention-to-treat basis. The power of the study was stated but was calculated. retrospectively | |||
| Arkwright 2003376 | Heat-inactivated M. vaccae in phosphate buffer (SRP299), 0.3-ml suspension containing 10 mg/ml of M. vaccae. One 0.1-ml (1 mg) intradermal injection on the left deltoid at the beginning of the trial into normal skin | Phosphate buffer only as placebo. One 0.1-ml (1 mg) intradermal injection on the left deltoid at the beginning of the trial into normal skin | UK | 56 participants (n = 27 placebo group, n = 29 M. vaccae group) | Moderate to severe atopic eczema; aged 2–6 years | Investigator-assessed severity of atopic eczema (extent of involvement, severity – erythema, excoriation, exudation and lichenification on a 3-point scale, with 1 = mild, 2 = moderate, 3 = severe for each body region. Max. score 300) Potency of topical corticosteroid |
One investigator carried out all of the assessments | M. vaccae group: 38–54% reduction in surface area involved (p = 0.005) at all time points. Placebo group not significantly different | Method of randomisation and allocation concealment well described. Intention-to-treat analysis not described; however, there were no dropouts or withdrawals | ||
| Armstrong 2011348 | Online education eczema video about clinical manifestations of atopic eczema, environmental factors, bathing and handwashing, moisturiser vehicles, common treatment modalities. To be viewed as many times as desired in 12 weeks but at least once | Paper pamphlet containing the same information as the online video. To be viewed as many times as desired in 12 weeks but at least once | Participants in the video group were given instructions on how to access the video and had to prove they could do so | Not stated | 80 participants (n = 40 video group, n = 40 pamphlet group) | Age ≥ 18 years (adults); atopic eczema according to the criteria of Hanifin and Rajka;8 English speaking; able to view online videos | Severity of eczema (POEM) Improvement in participant knowledge Overall satisfaction with the educational material |
Improvements in knowledge about atopic eczema (changes from baseline): video group 3.05, pamphlet group 1.85 (p = 0.011). Improvement in clinical score (POEM): video group 3.30, pamphlet group 1.03 (p = 0.0043). Usefulness of the interventions: no significant difference between groups (p = 0.77). The online video was found to be significantly more appealing than the paper pamphlet (p = 0.0086) | Method of randomisation and allocation concealment described. Intention to treat used for the analyses. Unclear whether there was any blinding | ||
| Berth-Jones 2006377 | Heat-inactivated M. vaccae in phosphate buffer (SRP299), 1 mg or 0.1 mg of M. vaccae in a 0.1-ml intradermal injection. Given once at the randomisation visit | Placebo (phosphate-buffered saline) injection, 0.1-ml intradermal injection. Given once at the randomisation visit | The M. vaccae injection was slightly cloudy whereas the placebo injection was colourless and clear | Not stated | 166 participants | Males and females aged 5–16 year; atopic eczema diagnosed using the UK Working Party criteria;9 moderate to severe disease severity as defined by the Rajka and Langeland228 criteria (the protocol was changed to state that the participants had to have a SASSAD score of at least 20), females of child-bearing potential were required to provide a negative pregnancy test and use effective birth control | (Primary) Change in severity of atopic eczema as assessed by the SASSAD score at week 12 (Secondary) Change in severity of atopic eczema as assessed by the SASSAD score at week 24 (Secondary) Change in affected body surface area assessed using the rule of nines (Secondary) Participant-rated global assessment of response on a 5-point scale (Secondary) Pruritus severity on a 5-point scale (Secondary) Sleep disturbance on a 4-point scale (Secondary) Frequency of use of topical corticosteroids on a 5-point scale |
At week 12, the change in severity between the high-dose group and the placebo group was 0.6 points in favour of the high-dose group (95% CI –4.3 to 5.4) (p > 0.05). There were no statistically significant changes in any of the other outcomes except for changes in sleep disturbance at week 8 in favour of the low-dose group compared with the placebo group and both M. vaccae groups combined (p < 0.05) | The randomisation procedure was partially described but did not include the method of sequence generation. Allocation concealment was not described and it is not clear whether this was in place or not. The trial was described as double blind but the second party blinded is not clear. This paper followed CONSORT guidelines | ||
| Blessman Weber 2008359 | Support group, 90-minute fortnightly meetings for 6 months (children aged < 16 years were in a separate group to their relatives until near the end of the session, when the two groups joined together) | Control, not stated | There is no information in the paper to explain what treatment, if any, the control group received | Not stated; however, appears to be Brazil | 36 participants | Girls and boys aged 2–16 years, atopic eczema defined by the Hanifin and Rajka8 criteria as moderate or severe (scores between 4.5 and 7.5 for moderate and between 8 and 9 for severe); not responded appropriately to conventional treatments | Quality of life Pruritus Gender Age |
Significant change in the pattern of pruritus in the treatment group, with a larger number of participants reporting only weekly itch after follow-up as opposed to daily itch at baseline (p = 0.023). Improvement in quality of life in the leisure (p = 0.04) and personal relationship (p = 0.02) domains when the intervention group was compared with the control group | Method of generating the randomisation sequence and concealment of allocation not stated. Intention-to-treat analysis not carried out | ||
| Brothers 2009378 | Heat-inactivated M. vaccae injection, 250 µg/ml in phosphate-buffered saline. One injection of 12.5 µg (0.05 ml) at 2-week intervals, with a total of three injections | Placebo (phosphate-buffered saline). One injection of 0.05 ml at 2-week intervals, with a total of three injections | Participants could use (or discontinue) their standard atopic dermatitis medications during the study as long as they were not listed in the exclusion criteria | New Zealand | 1826 expressions of interest, 198 assessed for eligibility, 129 randomised (n = 64 M. vaccae group, n = 65 placebo group) | Children aged 5–16 years; moderate to severe atopic dermatitis (defined using the UK Working Party criteria,9 with severity defined by the Rajka and Langeland228 criteria); good general health | (Primary) Change in SASSAD score from baseline to 3 months (Primary) Change in SASSAD score in the AVAC group relative to the placebo group (Secondary) Relative change in severity of atopic dermatitis between baseline and 6 months (Secondary) Relative change in the extent of atopic dermatitis between baseline, 3 months and 6 months using the rule of nines (Secondary) Change in topical corticosteroid use between baseline, 3 months and 6 months. Frequency of use in the previous week using a 5-point scale and potency graded as mild, moderately potent, potent and very potent (Secondary) Change in quality of life between baseline, 3 months and 6 months using the CDLQI (Secondary) Change in sleep and pruritus between baseline, 3 months and 6 months using 4- and 5-point symptom scales respectively |
Secondary outcomes also included change in total serum IgE level between baseline and 2 and 3 months, local injection site reactions and safety assessments [including full blood count, liver and renal function tests, urinalysis, physical examination and urinary pregnancy test (if there was childbearing potential)] | No significant difference between the two groups for change in severity of atopic dermatitis at 3 and 6 months (3 months p = 0.77, 6 months p = 0.70). No significant change in extent of disease at 3 months (p = 1.0). Local injection site reactions occurred in 47% of patients; 75% of these had received M. vaccae | Method of randomisation and allocation concealment described. Double-blind study. Intention-to-treat analysis not described and analysis was carried out on the number of patients completing the study not the number randomised | |
| Byremo 2006365 | A stay in a subtropical climate – Gran Canaria (climate therapy); 30 minutes in the sun at the start, increasing to 3–4 hours at the end of the stay. Sunscreen (factor 15) was used during the whole stay. Bathed in seawater for 1–2 hours per day. Duration of stay 4 weeks, with follow-up of 3 months | (Control) Stay at home in a subarctic climate – Norway. Follow-up of 3 months | Half of each group were investigated in the spring and the other half of each group were investigated in the autumn to account for seasonal variations | Norway and Gran Canaria | 61 participants (n = 32 Gran Canaria group, n = 29 Norway group). Allocations by season, not including withdrawals: spring – n = 13 Norway, n = 14 Gran Canaria;autumn – n = 13 Norway, n = 16 Gran Canaria | Based on clinical information from referring doctors. Children (age range for inclusion not stated) | Severity of atopic eczema (SCORAD index) Total IgE level Skin-prick tests (positive = 3-mm larger than negative control after 15 minutes) with histamine chloride (10 mg/ml) as the positive control. Tested for birch, timothy and mug worth pollen, cat, dog and horse dander, house dust mite (Dermatophagoides herbarum) and Cladosporium herbarum Serum eosinophilic cationic protein level Skin bacterial colonisation Quality of life (CDLQI) Use of pharmacological treatment |
SCORAD score: decreased in Gran Canaria group (p < 0.0005) much more than control group (data not given). CDLQI: improved in Gran Canaria group (p < 0.0005) but not in control group. Bacterial skin colonisation (S. aureus): Gran Canaria group – decreased from 23/30 (77%) to 12/30 (40%) (p = 0.001) after 1 month and 12/30 (40%) after 3 months (p = 0.005); did not decrease in control group. Use of topical corticosteroids: decreased in Gran Canaria group but not in control group | Method of randomisation and allocation concealment described but unclear. Intention-to-treat analysis not reported | ||
| Chinn 2002357 | Dermatology nurse consultation, one 30-minute session | Control group (no consultation) | Sessions delivered by a recently trained dermatology nurse holding the ENB 393 certificate in dermatology. Consultation involved the following: establish knowledge of eczema (parent and child); demonstration of techniques for applying medication; individual treatment plan; knowledge of how, when and where to apply topical treatments; avoidance of irritants [soap, wool, long fingernails, sudden changes in temperature, dust mite precautions (especially if the participant has asthma)]; advice on bathing and use of emollients; discussion about wet wraps (if needed); practical advice on self-management at school; offer of continued support (telephone/further appointments); advice about the National Eczema Society; written information on discussion topics provided. Control group offered nurse consultation after 12-week follow-up questionnaire returned | Not stated | 235 (n = 119 nurse consultation, n = 116 control) | Age 6 months to 16th birthday; diagnosis of eczema using British Association of Dermatologists guidelines (contains UK diagnostic criteria); new cases/requesting repeat prescription for atopic eczema | Quality of Life (using CDLQI or IDLQI: 10 questions with four possible responses scored from 0 to 3; max. score 30, high score = poor quality of life) Impact on the family (Family Dermatitis Index) Participant-/carer-assed severity of present eczema (5-point scale: ‘none’, ‘fairly good’, ‘average’, ‘severe’, ‘extremely severe’) |
All quality of life and family impact measures were skewed at baseline. Mean differences in IDLQI and CDLQI scores between the nurse consultation group and the control group were small at 4 and 12 weeks (p > 0.05). Improvement in family impact at 4 weeks: nurse consultation group better than control group (p < 0.06) | Method of randomisation described. Allocation concealment not reported | ||
| Fontanini 2013340 | DermaSilk long-sleeve top and trousers, which covered the trunk, arms and legs entirely, worn every day for 24 months, except during the summer and on very hot days in other seasons | Cotton long-sleeve top and trousers worn every day for 24 months, except during the summer and on very hot days in other seasons | All participants were given mite-impermeable mattress and pillow encasings to use during the trial for both the child’s and the parents’ bed. All participants used mometasone furorate as needed | Italy | 22 participants; baseline data reports nine participants in the DermaSilk group and 11 participants in the cotton group – it is not clear which group the other two participants were in | Infants aged < 18 months with eczema | Use of topical corticosteroids (patient-reported quantity used and area treated) Length of time (months) that the intervention was worn Parent-reported satisfaction with treatment, expressed as ‘satisfied’ or ‘dissatisfied’ with reduction in itching |
Severity of eczema was recorded only at baseline and was not an outcome of the trial | Use of topical corticosteroids was significantly lower in the DermaSilk group than in the cotton group (p = 0.006). The parent evaluation of satisfaction with the itching reduction was also significantly better in the DermaSilk group | The methods for generating the randomisation sequence and allocation concealment were not reported. Although it is stated that both the parents and the investigators were not aware of which garments the children were wearing, no further details are reported and it seems very unlikely that at least the parents, who would have been washing and helping to dress the children, would not have been fairly certain which garment their child had been allocated | |
| Futamura 2013354 | A 2-day residential (in hospital) education programme consisting of 2 hours of lectures on epidemiology, diagnosis, pathophysiology, treatment, genuine side effects of topical corticosteroids, skin care including method of application, and allergen avoidance; 2 hours of group discussion on skin management at home; and three 20-minute practical sessions on caring for the child’s skin in the bathroom. The participants also received an information booklet | Normal treatment regimen (control). Participants received the same information booklet as the education group | Standard eczema treatment continued in both groups. This consisted of emollients and topical corticosteroids of the appropriate potency, both used according to guidelines. Mixtures of emollients and topical calcineurin inhibitors were not allowed. Switching topical corticosteroid and the use of antibiotics or antihistamines was allowed only according to allergist judgement. Topical corticosteroids were used until complete resolution of the eczema and then the application was decreased by 1 day per month. If eczema relapsed, daily application of topical corticosteroids was reinstated | Japan | 59 participants (n = 29 education group, n = 30 control group) | Children aged 6 months to 6 years; moderate to severe eczema; required topical corticosteroid application daily | (Primary) Change in severity of eczema at 6 months measured by SCORAD score (Primary) Eczema severity measured using objective SCORAD score (post hoc analysis) (Secondary) Change in symptom scores for pruritus (scale 0–10) (Secondary) Change in symptom scores for sleeplessness (scale 0–10) (Secondary) Parental quality-of-life score, measured using the Dermatitis Family Impact score. (Secondary) Corticosteroid anxiety, measured using the question ‘How much are you worrying about applying corticosteroids to your child’s skin?’, with 1 = no anxiety, 5 = very anxious (Secondary) Corticosteroid usage at 3 and 6 months |
Objective SCORAD measurements were carried out by a single assessor from digital photographs of the whole body and a single representative lesion | Education group had a significantly lower severity of eczema at 6 months compared with the control group, measured using the SCORAD score (mean difference 10.0, 95% CI 2.3 to 17.7; p = 0.01) and the objective SCORAD score (mean difference 7.1, 95% CI 0.8 to 13.5; p = 0.03). Sleeplessness (mean difference 1.6, 95% CI 0.0 to 3.1; p = 0.048) and corticosteroid anxiety (p = 0.02) were both significantly better in the education group than in the control group at 6 months. There was no difference between groups for the amount of topical corticosteroid used and quality of life | The method of randomisation was unclear as two methods were described. Allocation concealment was acceptable. The primary outcome was blinded | |
| Gauger 2006343 | Silver-coated textile as verum (Padycare) consisting of micromesh material (82% polyamide, 18% lycra) with woven silver filaments with a silver content of 20% in total. Worn day and night next to the skin (except for consultations) for 2 weeks | Placebo: pure cotton textile of equal size. Worn day and night next to the skin (except for consultations) for 2 weeks | For infants, all-in-one suits were used. For children and adults, long-armed, long-legged suits were used | Germany | 68 participants (n = 37 silver-coated textile group, n = 31 placebo group) | Clinical diagnosis of eczema with moderate severity as measured by a SCORAD score of at least 20 | Severity (SCORAD score) Extent of eczema lesions (rule of nine) Daytime pruritus (VAS from 0 to 10) Sleep loss (VAS from 0 to 10) Quality of Life (German Instrument for the assessment of Quality of Life in Skin Diseases) Functionality and wearing comfort |
SCORAD score: no significant difference between the groups. Subjective assessment found a significant improvement in pruritus and skin condition in the silver-coated textile group compared with the placebo group (p < 0.001 and p < 0.003, respectively) after 2 weeks. Quality of life: no significant differences between the groups | The methods of generating the randomisation sequence and allocation sequence are not described. Study described as double blind; however, only one of the parties blinded is described and the other is unclear. Intention-to-treat analysis was carried out but was not described | ||
| Grillo 2006353 | Education programme and normal treatment regimen, 2-hour workshop (group), one session | Normal treatment regimen (control), one session | Education programme: understanding atopic eczema, allergic and non-allergic trigger factors, investigations, basic skin care, topical corticosteroids, complementary therapy, wet wrapping practical session and cream application practical session. Time was provided for questions and sharing experiences and ideas | Not stated | 61 children with eczema and their parents (n = 29 control group, n = 32 education group) | Physician-diagnosed atopic dermatitis | (Primary) Severity of eczema (SCORAD score) Quality of Life (IDLQI, CDLQI) Impact on the family |
Severity of eczema: education programme group significantly improved compared with control group at weeks 4 and 12. Quality of life: children aged 5–16 (small numbers) showed a significant improvement. No other significant differences in quality of life | Method of randomisation described and adequate. Allocation concealment not stated. Intention-to-treat analysis not described but some information on how missing data were dealt with was provided | ||
| Gutgesell 2001367 | Allergen-impermeable polyurethane encasings and acarcide spray (tannic acid and benzylbenzoate) used for 1 year | (Placebo) cotton allergen-permeable encasings and water spray with traces of ethanol used for 1 year | Not stated | 20 participants (not stated how many in each group) | Moderate to severe atopic eczema; sensitisation to house dust mite D. pteronyssinus (specific IgE antibodies with a RAST class > 3; CAP-FEIA method, Pharmacia) | Eczema severity (SCORAD index) Daytime pruritus (VAS) Pruritus-induced sleeplessness (VAS) Judgement of skin status (participant assessed) Amount of topical steroids used (weighing the tubes) |
Der p1 exposure: polyurethane encasing group showed a statistically significant reduction compared with placebo group. SCORAD score: no statistically significant difference between groups. Eosinophilic cationic protein: no statistically significant difference between groups. Less pruritus-induced sleeplessness in some participants in the polyurethane encasing group but no statistically significant difference between the groups | Method of randomisation and allocation concealment not reported. Intention-to-treat analysis not described | |||
| Juenger 2006344 | Undergarments made of silver textile material. Material consisted of 67% polyamide, 15% spandex and 18% silver thread (X-STATIC). Worn next to the skin as long-sleeved and long-legged undergarments. Group 1 wore the silver textile in phase 1 (days 1–14) and phase 2 (days 14–28). Phase 3 (days 28–56) was a post-treatment phase and the garments were not worn | Undergarments made of silver-free textile material. Material consisted of 67% polyamide, 15% spandex and 20% polyamide thread. Worn next to the skin from day 1 to day 14 and then from day 14 to day 28 the silver textile was worn. Phase 3 (days 28–56) was a post-treatment phase and the garments were not worn | Prednicarbate ointment (1 g contains 2.5 mg of prednicarbate). From day 1 to day 14 the ointment was applied at least once a day to eczematous areas. During phase 2 (days 14–28), the silver textile was worn. Phase 3 (days 28–56) was a post-treatment phase | For phase 1 (days 1–14) the three interventions were applied. For phase 2 (days 14–28) all groups wore undergarments made with silver textile. In phase 3 (days 28–56) all treatments were withdrawn except for prednicarbate ointment. Patients could apply prednicarbate as necessary throughout the study period. Its use was measured by weighing the tube before and after use | Germany | 30 participants (n = 10 silver group, n = 10 non-silver group, n = 10 prednicarbate group) | Clinically established diagnosis of acute atopic dermatitis; age > 24 months; consent of patient or patient’s parent/guardian | (Primary) Severity of eczema (change in SCORAD score days 0–14) (Primary) Frequency/severity of undesirable events (Secondary) Severity of eczema (SCORAD score) on days 3, 7, 28 and 56 (Secondary) Use of prednicarbate ointment from day 0 to day 28 (Secondary) Use of prednicarbate ointment from day 29 to day 56 (Secondary) Overall eczema disease control assessed by the participant or the participant’s parent/guardian (on a 0–3 scale rating the 7 days before assessment, with 0 = complete disease control, 4 = severe complaints) (Secondary) Participant or participant’s parent/guardian assessment of wearing comfort (19 multiple-choice questions on a scale of 1– 4, with 1 = no discomfort, 4 = severe complaints) |
Quality-of-life questionnaire, days 28 and 56 | SCORAD score: after 14 days, the silver group and the prednicarbate group improved significantly [from 74.6 to 29.9 (p = 0.005) and from 57.8 to 24.0 (p = 0.009), respectively]. Between 15 and 28 days, the silver group and the non-silver group improved [from 29.9 to 18.1 (p = 0.037) and from 48.2 to 24.1 (p = 0.015), respectively] and the prednicarbate group stayed at an improved level (from 24 to 23.5). Prednicarbate usage: 0–14 days – silver group 135 g, non-silver group 13 g, prednicarbate group 145 g; 15–28 days – silver group 10 g, non-silver group 0 g, prednicarbate group 30 g; follow-up period – silver group 45 g, non-silver group 0 g, prednicarbate group 90 g. Severity of pruritus was reduced by the silver textiles (p = 0.031); silver-free textiles and prednicarbate showed no significant improvements | Randomisation process described. Allocation concealment unclear. Not clear if anyone was blinded, although the study materials (silver textile and non-silver textile) were identical. Not stated whether the investigators were blinded to treatment group. It was stated that the intention-to-treat principle was used as the analysis was carried out on all randomised participants |
| Kardorff 2003355 | Active educational demonstration with Kardorff–Scnell–Parker skin model regarding skin care, 10 minutes, days 0 and 14 | Verbal instructions similar to those given during routine dermatological practice, 10 minutes, days 0 and 14 | Both groups received 14 days of steroid therapy. Initial treatment in the acute stage: moderately effective fourth-generation topical corticosteroid (prednicarbate 0.25%) applied twice daily for 14 days, followed by 8 days of tapering treatment: twice-daily skin care and topical steroidal cream every other day in the evening, alternating with conventional skin care | Not stated | 30 children (n = 15 group A, n = 15 group B) | SCORAD score Remission |
SCORAD score at days 0, 14 and 42 | SCORAD score of patients taught using the skin model was significantly reduced compared with the control group at day 42 (p < 0.006) | Method of randomisation not reported. Reporting of allocation concealment lacking. Single-blind study only | ||
| Kim 2012345 | Undergarments made from an anion textile (constructed from pure polyester filaments containing nano-sized, fine-crusted tourmaline powder). Subjects were asked to wear the undergarments at all times during the 4-week study period | Undergarments made of pure cotton. Subjects were asked to wear the undergarments at all times during the 4-week study period | Tourmaline is a crystal boron silicate mineral compounded with elements such as aluminium, iron, magnesium, sodium or potassium. Systemic and topical agents, including glucocorticoid, immunosuppressive and immunomodulatory agents, were not permitted. Only low-sedating antihistamines and topical emollients were permitted to treat pruritus | Not stated | 52 participants (n = 30 anion group, n = 22 cotton group) | Age between 2 and 30 years; mild to severe atopic dermatitis based on the diagnostic criteria of Hanifin and Rajka8 | SCORAD score Transepidermal water loss Stratum corneum hydration Skin erythema Wearing comfort (a questionnaire by Gauger et al.343 was used to assess wearing comfort) |
A significant decrease in SCORAD score was observed at the end of the study among patients in the anion group (mean SCORAD score decreased from 47.2 to 36.1). Improvements in mean transepidermal water loss, skin erythema and stratum corneum hydration were significantly greater in the anion group | Methods of randomisation and allocation concealment not described. Blinding not reported. Intention-to-treat analysis not reported | ||
| Koller 2007338 | DermaSilk arm tubes worn all day on one arm for 3 months | Silk arm tubes/cotton arm tubes. Silk tube worn all day on one arm for 2 weeks, then cotton tube worn all day on one arm for the remainder of the 3 months | None of the participants used topical or systemic antibiotic or anti-inflammatory agents throughout the study. All participants were educated about the Dermasilk characteristics and the importance of keeping the arm tubes on all day for the whole study and were advised to wash the arm tubes daily | Not stated | 22 participants | Age range 5–12 years (criterion not given); mild to moderate atopic eczema according to the Hanifin and Rajka8 criteria | Local severity (modified SCORAD score; highest intensity score for erythema, papulation, exudation, abrasions, lichenification, xerosis = 18 and highest subjective score = 10) | Local SCORAD score: difference between groups (all in favour of DermaSilk),mean (quartile 1 to quartile 3) – at 4 weeks: 6.5 (5 to 8) vs. 8 (7 to 9) (p < 0.002); at 8 weeks: 6 (5.25 to 7.75) vs. 8 (7 to 9) (p < 0.0001); at 12 weeks: 6 (5 to 6) vs. 8 (7.25 to 10) (p < 0.0001) | Method of randomisation not stated. Allocation concealment not stated. Intention-to-treat analysis not described | ||
| Moore, 2009356 | Dermatology nurse consultant-led workshop (history taken, examination and SCORAD score, management plan, demonstration of applying treatments, prescriptions and equipment cards obtained, information booklet given, video, nurse presentation), one 90-minute session | Dermatologist-led clinic (history taken, examination and SCORAD score, dermatologist reviewed the participant, management plan, demonstration of applying treatments, prescriptions and equipment cards obtained), one 40-minute (average) session | Australia | 165 participants (n = 80 nurse-led workshop, n = 85 dermatologist-led clinic) | Age ≤ 16 years; new referrals for management of atopic eczema | (Primary) Severity of eczema at 4 weeks (SCORAD index) (Secondary) Comparison of eczema treatments used |
SCORAD score: after 4 weeks, the mean reduction in SCORAD score was significantly higher in the nurse-led workshop group than in the dermatologist-led clinic (–9.93, 95% Cl –14.57 to –5.29; p < 0.001). Improvement from moderate to mild eczema was significantly greater in the nurse-led workshop group. Adherence was also greater in the nurse-led workshop group | Method of randomisation and allocation concealment clearly described. Intention-to-treat analysis not carried out | |||
| Oosting 2002368 | Gortex bedding system (mattress, pillow and duvet encasings) on all beds in the bedrooms of participants for 12 months | Placebo cotton mattress, pillow and duvet encasings on all beds in the participants’ bedroom for 12 months | Unloaded pore size of placebo encasings 0.08 × 0.1 nm (with 200-N force pore size of 0.1 × 0.1 nm). Allergen barrier of placebo encasings 15% compared with 98% for Gortex bedding system | The Netherlands | 86 participants (n = 45 Gortex bedding system group, n = 41 placebo group) | Diagnosis of atopic eczema; either or both of ≥ 0.7 house dust mite RAST, ≥ 0.7 skin test index; Der p1 or Der f1 concentration in mattress of ≥ 200 ng/g of dust; no encasings/willing to remove encasings for the study | (Primary) Clinical score (Leicester Sign Score: ‘disease activity’ erythema, purulence, excoriation or crusting, dryness or scaling, cracking or fissuring and lichenification scored from 0 to 3 at six defined body sites) (Primary) Clinical score (Leicester Sign Score – ‘extent of disease’ rule of nines, max. score 100%) (Secondary) Leicester Sign Score – ‘sleep and itching’ for the preceding 2 weeks on a VAS from 0 to 100 mm) (Secondary) Early allergic responses to Der p1 (intradermal test and atopy patch test) (Secondary) Total serum IgE level (Secondary) Specific serum IgE against Der p1 (Secondary) Total blood eosinophil count |
Outcomes for levels of dust and Der p1 do not appear to be prespecified | Der p1 allergen mattress concentration: Gortex bedding system group – after 12 months reduced by factor of 2.1 (p = 0.007). Der p1 and Der f1 combined allergen mattress concentration: Gortex bedding system group – after 12 months, reduced by factor of 2.5 (p = 0.005); placebo group – no significant change in allergen concentration. Treatment-induced changes – no significant difference between groups | Method of randomisation unclear. Allocation concealment not reported. Intention-to-treat analysis not described | |
| Ozawa 2008341 | Ethylene vinyl alcohol copolymer fibre underwear (MEDIELE®) worn for 4 weeks | Cotton underwear worn for 4 weeks | Frequency and length of wearing the difference fabrics per day were not stated. A washout period was not stated. The study was performed in the winter season from October to March | Japan | 30 participants | Patients with atopic eczema maintained in a relatively good condition by standard treatment | Skin findings (local SCORAD score) on the scapular region at 0, 2, 4, 6 and 8 weeks High-frequency conductance (for an index of the horny layer fluid volume) in the scapular region at 0, 2, 4, 6 and 8 weeks Quantity of transepidermal water loss in the scapular region at 0, 2, 4, 6 and 8 weeks |
The local SCORAD score consists of six items (erythema, oedema/papulation, excoriations, lichenification, oozing/crusts and dryness), each scored from 0 to 3. High-frequency conductance and the quantity of transepidermal water loss were measured as follows. Measurements were performed at room temperature (23 SD ± 1°C) and 50 SD ± 5% relative humidity. Each site was exposed to air for 15 minutes before measurement and measurements were carried out in a prone position. On measurement day it was prohibited for any ointment to be applied to the testing site. High-frequency conductance was performed with a 3.5-Hz high-frequency conductance measuring assembly (SKICON®–200EX, IBS). Measurement of transepidermal water loss was carried out with DermaLab® (Cortex Technology) | Local SCORAD score: no difference between groups. Skin conductance: no difference between groups. Transepidermal water loss: increased in the cotton underwear group; no change in the MEDIELE group | Insufficient details provided about blinding and randomisation. For test results, CIs and p-values, concrete numerical values are not described | |
| Ricci 2000369 | Dust mite avoidance measures recommended for 2 months. Recommendation of mattress and pillow encasings, hot wash of bedding once a weeks, living room and bedroom vacuumed at least twice a week, soft toys taken out of the bedroom or washed once a week, carpets vacuumed at least once a week/removed, no pets) (total trial duration 12 months) | No dust mite avoidance measures recommended for 2 months (continued normal cleaning patterns) (total trial duration 12 months) | First 2 months were randomised and controlled; for the following 10 months both groups were recommended the dust mite avoidance measures. Topical steroids and oral antihistamines only were given for an acute eczema relapse. Both groups did not restrict their diet or take systemic steroids | Not stated | 41 participants (n = 21 mite avoidance group, n = 20 control group) | Age 2–10 years; diagnosis of atopic eczema according to the Hanifin and Rajka8 criteria; all participants had raised total IgE antibodies to food or inhalant allergens (unclear whether this was a criterion) | Severity of eczema (SCORAD index) Dust load (mg/m2) Concentration of D. pteronyssinus (Der p1) and D. farinae (Der f1) (ng/m2 or µg/m2) |
One investigator performed the SCORAD assessments who was not involved in giving the dust mite avoidance advice. Two investigators collected the dust samples, one for each group | Mite avoidance group: Der p1 and Der f1 load in children’s beds: major decrease (from 393 to 94 ng/m2); Der p1 and Der f1 concentration in children’s beds: major decrease (from 1.84 to 0.73 µg/g of dust). After 12 months (for 10 of which both groups were recommended house dust mite avoidance measures) Der p1 and Der f1 load, concentration and clinical score had improved (similar in both groups) | Method of randomisation unclear and allocation concealment not reported. Intention-to-treat analysis not reported | |
| Sagransky 2010373 | One extra clinician visit at week 1. Subjects were scheduled follow-up visits at week 1 and week 4 | No intervention (one visit), that is, no extra visit at week 1. Subjects were scheduled for only one follow-up visit at week 4 | All participants had their eczema treated with application to the affected areas of topical tacrolimus 0.03% applied twice daily for 4 weeks | Not stated | 30 participants (n = 13 extra visit group, n = 17 control group). In total, 26 participants completed the study and were included in the analysis | Age 2–15 years; atopic eczema affecting > 5% body surface area; moderate to severe eczema according to IGA (scale 0–4) | Adherence (MEMS monitoring device; Aardex Corp.) EASI score IGA (0–4 scale) Itch intensity (100-m VAS) |
The MEMS cap had a microprocessor that recorded the date and time of every tube opening. 100% adherence required, two daily applications | No abstract. Both groups showed significant improvements in all assessments by week 4. The difference in percentage adherence between the groups did not reach statistical significance (Kruskal–Wallis test p > 0.05). No correlation was found between adherence and clinical outcomes or between baseline disease severity and adherence | Method of randomisation not reported. Allocation concealment not described. Not stated whether intention-to-treat principle was used but the number completing the study was analysed, not the number randomised | |
| Schut 2013361 | Standardised CBS programme. The programme focused on cognitive restructuring and enhancing problem-solving skills. Four 3-hour sessions in a 2-week period plus a booster session 3 weeks after the final lesson. Sessions included groups of six to eight people and were run by a certified female psychologist | No intervention | The control group received the treatment at the end of the study period. The study was mainly looking at endocrine stress levels. The whole study lasted for 10–14 weeks divided into four study periods: baseline (week 1), CBS (weeks 2–8), measurement of basal effects (week 9), induction of acute stress (weeks 10–14). The control group was offered CBS after the end of the trial | Germany | 28 participants were randomised [n = 14 CBS group (4 male, 10 female), n = 14 control group (4 male, 10 female)] | Diagnosis of atopic dermatitis according to the Hanifin and Rajka8 criteria | Severity of eczema (SCORAD score measured at week 1 and week 9 by a trained doctoral student and calibrated by a dermatologist before the study) Hypothalamic–pituitary–adrenal axis reactivity via the cortisol awakening response Psychological stress response via the Multidimensional Mood Questionnaire, which measures ‘mood’, ‘alertness’ and ‘calmness’ before and after acute stress |
The experimental group had a tentatively reduced cortisol awakening response after the stress management programme. The stress management programme group also remained calmer and showed lower salivary cortisol levels under acute stress. No effect was observed for the SCORAD score | Method of randomisation and allocation concealment described. Outcome assessors were blinded to treatment. Intention-to-treat analysis not stated | ||
| Schuttelaar 2010358 | Consultations with a dermatologist, 20 minutes on average for the initial consultation and then 10 minutes for each of the following consultations and 5-minute telephone consultations for allergy test results. The number of consultations depended on the severity of the eczema for each participant. No education given by the nurse | Consultations with a nurse practitioner, 30 minutes on average for the initial consultation and then 20 minutes for each of the following consultations. Education given by the nurse at each consultation or in a 2-hour group session with up to eight parents. A routine follow-up visit took place 2 weeks after the first consultation, which could have been a 10-minute telephone consultation. After this, the number of follow-up visits depended on the severity of the eczema of each of the participants | The participants receiving nurse practitioner consultations could contact the nurse practitioner for support, feedback or tips by mail or telephone. The nurse practitioner was supervised by an independent dermatologist if necessary | The Netherlands | 160 participants (n = 79 dermatologist group, n = 81 nurse practitioner group) | Age ≥ 16 years; new referrals for GPs; clinical diagnosis of atopic eczema according to the UK Working Party criteria9 | (Primary) Differences in quality of life of the child at month 12 compared with baseline (IDLQI/CDLQI; max. score 30, higher score = poorer quality of life) (Secondary) Differences in quality of life of the child at month 4 and month 8 (IDLQI/CDLQI) (Secondary) Differences in quality of life of the family (Dermatitis Family Impact questionnaire; max. score 30) at baseline, month 4 and month 12 (Secondary) Severity of eczema (objective SCORAD score; max. score 83) at baseline and months 4, 8 and 12 (Secondary) Participant satisfaction (Client Satisfaction Questionnaire-8; max. score 32, higher score = higher satisfaction). Completed by the participants at home and sent to the independent data entry office |
None of the between-group differences for the IDLQI or CDLQI at baseline or months 4, 8 or 12 were significant. Objective SCORAD and Dermatitis Family Impact scores showed significant improvements at 12 months in both groups. Participant satisfaction was significantly higher in the nurse practitioner group at all time points | Method of randomisation described. Allocation concealment reported. Intention-to-treat population described and used for the analyses | ||
| Shaw 2008350 | Individual session with an atopic eczema educator and standard care from the resident and attending paediatric dermatologist (educator session included more formal iteration of the treatment plan verbally and in writing). Initial educator visit lasted for 15 minutes. Length of follow-ups were dependent on severity. The educator was available for questions 24 hours a day by telephone or e-mail | Standard care from the resident and attending paediatric dermatologist (individual treatment plan explained verbally and in writing if necessary, focusing on the proper usage of medication and behavioural changes including bathing habits). Length of follow-ups were dependent on severity | Eczema educator also used standardised forms for proper use of medication and behavioural changes including bathing habits | USA | 151 participants (n = 77 eczema educator group, n = 74 standard care-only group) | People with atopic eczema; newborn to age 18 years; new and returning participants to general paediatric and atopic eczema clinic | Eczema severity measured by SCORAD index (area of skin involved, intensity of physical signs and subjective symptoms including itchiness and sleep loss) Quality of life measured using age-dependent standardised questionnaires: IDLQI for children aged < 4 years, CDLQI for children aged 4–18 years |
No significant difference in eczema severity or quality of life between the two groups | Method of randomisation described and good. Allocation concealment partially described but sealed envelopes not described as ‘opaque’, which could lead to potential bias | ||
| Span 2001347 | IDB. Three times a week the day started with individual dermatological treatment. Other programme sessions took place in a group setting, with one session lasting approximately 2.5 hours. There were communal coffee/tea breaks and lunch breaks, etc. Information was provided and discussion took place on pathophysiology, allergies and aggravating factors (two sessions) and asthma, diet and environment cleansing (two sessions); instruction was provided on ointment use and trial of ointments and creams (four sessions); information was provided and discussion took place on social and psychological aspects of eczema (two sessions); discussion took place about alternative medicine (one session), the role of stress and training in relaxation techniques (two sessions) and dealing with itch and training in habit reversal (two sessions); and discussion of atopic dermatitis took place with family members/partners (one session). The education programme was delivered by a multidisciplinary team (nurse specialist, social worker, medical psychologist and dermatologist) for 2 weeks (2 × 5 working days) to groups of five to six people | Regular outpatient treatment | The Netherlands | 53 participants (n = 31 IDB group, n = 22 control group) | Moderate to severe atopic dermatitis (SCORAD score> 20); age between 18 and 35 years | Total score of the MNF, sick leave and the number of outpatient visits Subdomains of the MNF Subjective and objective eczema scores (among others SCORAD) Scores in relation to time lost because of visits to outpatient clinics Self-care capacity (Appraisal of Self-Care Agency Scale) Quality of life (SF-36) Ointment use |
MNF total score showed a significant positive effect in the IDB group in the short and long term. Sick leave was significantly reduced in the IDB group in the short term. The time spent on medical consultation visits was significantly reduced in the IDB group compared with the control group, if a confidence threshold of < 10% was accepted, but the number of visits was not significant | Method of randomisation and allocation concealment not reported. Intention-to-treat principle appears not to have been used | |||
| Staab 2002352 | Parental training programme, six sessions of 2 hours each, once a week in the evening over 6 weeks | Control group: no parental training programme (this was offered after the study period) | Parental training programme: session 1 (paediatrician and psychologist) – introduction, basic medical information about atopic eczema, introduction of a relaxation technique; session 2 (paediatrician) – recognition and avoidance of trigger factors, daily skin care; session 3 (psychologist) – stress management, dealing with itching and scratching and sleep disturbances; session 4 (paediatrician) – stage-related treatment of symptoms, unconventional therapies; session 5 (dietitian) – general child nutrition, food allergies in atopic eczema, different forms of diets; session 6 (paediatrician and psychologist) – coping issues, self-management, problems with transfer to daily routine | Not stated | 204 participants (n = 93 parental training programme group, n = 111 control group) | Diagnosis of atopic eczema by a physician; eczema of at least 4 months’ duration; moderate to severe eczema (SCORAD score of > 20) conformed by clinical examination; diagnosis confirmed using Hanifin and Rajka8 criteria | Severity of eczema (SCORAD score) Quality of life (newly developed and pretested disease-specific questionnaire with 26 items on psychometric well-being, effects of disease on social life, emotional coping and acceptance of disease and ‘daily life’ generic questionnaire) Coping (Trier Scales of Coping: 37 items on rumination, seeking information about the disease, seeking social support, minimising disease-related threat, seeking support in religion) Treatment costs (previous 6 months of direct costs covered by health insurance) Treatment behaviour |
Treatment behaviour: significant effects of the training programme for regular use of emollients, using antiseptics, using topical steroids and antiseptics for exacerbations and reducing the use of unconventional therapies. There was also improved satisfaction with treatment and reduced rumination (ineffective, passive coping strategy). Treatment costs were significantly reduced in the training programme group | Method of randomisation not described. Allocation concealment not reported. Intention-to-treat analysis not reported | ||
| Staab 2006351 | Standardised atopic eczema group intervention programme (three groups: age 3 months to 7 years, parents and their children with atopic eczema, atopic eczema adolescents aged 13–18 years), 2-hour session once a week for 6 weeks | No intervention | Germany | 3 months to 7 years: n = 274 intervention group, n = 244 control group; parents and their children with atopic eczema: n = 102 intervention group, n = 83 control group; adolescents aged 13–18 years: n = 70 intervention group, n = 50 control group) | Atopic eczema of at least 3 months’ duration; diagnosis according to the Hanifin and Rajka8 criteria; SCORAD score of at least 20 | (Primary) Severity of eczema at 12 months (SCORAD score) Parent-assessed severity of eczema (‘skin detective’ – parents compared their child’s skin lesions with pictures evaluated by experts) (Primary) Parental quality of life (participants aged < 13 years) at 12 months using the German-validated questionnaire ‘Quality of life in parents of children with atopic dermatitis’, which has five separately interpretable subscales of psychomatic well-being, effects on social life, confidence in medical treatment, emotional coping and acceptance of disease Itching behaviour (two standardised questionnaires: JUCKKI for those aged 8–12 years; JUCKJU for those aged 13–18 years) |
Severity of eczema and subjective severity of eczema: significant improvements in the intervention group compared with the control group for all age groups. Quality of life: for parents of children aged < 7 years with atopic eczema, the results from five out of five subscales were significantly improved; for parents of children aged 8–12 years with atopic eczema, three out of five subscales were significantly improved | Method of randomisation and allocation concealment not stated. Intention-to-treat analysis not reported | |||
| Stinco 2008339 | DermaSilk (knitted fibron fabric with bonded antimicrobial AEM 5772/5; AEGIS) sleeves worn all night and all day for 28 days with one change per day | DermaSilk fabric sleeves without antimicrobial AEGIS AEM 5772/5 worn all night and all day for 28 days with one change per day | Participants were instructed to wash the sleeves in mild detergent and always to put the same colour sleeve on the same arm throughout the study (green seam or red seam). Both groups could use only an assigned emollient containing 5% lactic acid and 20% propylene glycol both on the study area and on the rest of the body. All participants were supplied with a non-irritating skin cleanser (no antimicrobials or antiseptics) | Not stated | 30 participants | Age range 3–31 years (no specific criterion given); atopic eczema diagnosed by the Hanifin and Rajka8 criteria; active atopic eczema on the arms with no sign of infection; 1-week washout prior to inclusion: topical corticosteroids or antibiotics on treatment areas (arms), topical antimycotics; 2-week washout before inclusion: systemic corticosteroids or antibiotics; 4-week washout before inclusion: topical calcineurin inhibitors; 8-week washout before inclusion: systemic immunosuppressant therapies except corticosteroids, investigational agents, UV light therapy or systemic antimycotics | Local SCORAD score (adapted for the arm) Parent/participant assessment of pruritus (10-cm VAS) |
Local SCORAD score, mean: both groups decreased significantly from baseline to day 28 (end of study) – DermaSilk: constant decrease from week to week; DermaSilk without antimicrobial: significant decrease for first 2 weeks only. Pruritus: decrease between day 0 and day 28 greater in the DermaSilk group | Randomisation described; however, allocation concealment was unclear. Intention-to-treat analysis not mentioned | ||
| Thomas 2011364 | Ion-exchange water softener unit. All water in the home was softened except one drinking water tap (optional), 12 weeks of continuous use | Usual care for 12 weeks | After 12 weeks, the intervention was removed from group A and provided to group B for a 4-week observational phase only | UK | 336 participants (n = 170 water softener group, n = 166 usual care group) | Age 6 months to 16 year; diagnosis of moderate to severe eczema according to UK Working Party criteria;9 minimum eczema severity score (SASSAD) of 10 points; must live in hard water area (200 mg/ml of calcium carbonate minimum); home suitable for straightforward water softener installation | (Primary) Difference in mean change in severity (SASSAD score) at 12 weeks Difference in proportion of time spent moving at night (surrogate for sleep loss and itchiness, recorded using an accelerometer) Proportion of participants with reasonable (≤ 20%), good (> 20% to 50%) or excellent (> 50%) improvement in SASSAD score Difference in amount of topical corticosteroid/calcineurin inhibitors used during the 12 weeks of the study (weight of medication) Difference in POEM score Difference in the number of totally controlled weeks and well controlled weeks (using number of days with eczema symptoms and number of days that topical corticosteroid was applied). Difference in mean change in health-related quality of life at 12 weeks |
Also measured difference in mean change in Dermatitis Family Impact score at 12 weeks (questionnaire) | Change in SASSAD score at 12 weeks: water softener group –5.0 (20% improvement); usual care group –5.7 (22% improvement); mean difference 0.66 (95% Cl –1.37 to 2.69) (p = 0.53). No between-group differences for use of topical corticosteroids and topical calcineurin inhibitors | Method of randomisation and allocation concealment clearly described. Intention-to-treat population used for the analysis and described | |
| van Os-Medendorp 2012360 | E-health by a personal eczema portal – patients had access to the password-protected portal including e-consultations with the dermatology nurse. Face-to-face visits to the dermatology nurse were possible in some cases when e-health was inadequate. Initial appointment with the dermatologist and dermatology nurse (for diagnosis and initiation of therapy, to start self-management training and to explain e-health). Follow-up visit with dermatologist 6 weeks later, then patients had access to the password-protected eczema portal. It was not clear how long participants had access to the intervention for | Control condition: usual care After randomisation there was an initial appointment with the dermatologist and dermatology nurse. The control group then received usual care consisting of about five follow-up visits for diagnosis, monitoring and treatment during the first year. There was also at least one additional visit for self-management training (promoting compliance, preventing exacerbations, coping with itching and managing psychosocial problems). It was not clear how long participants had access to the intervention for | The eczema portal also consisted of internet-guided monitoring and self-management training. General information was also available about atopic dermatitis and personal information about prescribed treatment and daily skin care. There was the facility for patients to monitor disease using digital photographs and self-reported data on skin status, VAS scores of sleeping and itching and a diary of topical treatment. These data were used by the dermatology nurses to support the patients/parents via e-consultations (the nurses could consult a dermatologist if necessary), giving advice and information to individual patients. Psychosocial aspects and quality of life could also be assessed | The Netherlands | 199 participants (control group: total n = 98, adults n = 53, parents of children n = 45; intervention group: total n = 101, adults n = 56, parents of children n = 45) | Parents of children with moderate atopic dermatitis aged 0–4 years (this was changed during the first year of inclusion to 0–6 years); adults with moderate atopic dermatitis aged ≥ 18 years; internet access | Disease-specific quality of life (DLQI/IDLQI) Self-reported data on skin status and itching using two parts of the shortened Impact of Chronic Skin Disease on Daily Life Extent questionnaire online Severity of atopic dermatitis measured for nine parts of the body (total score for the affected area ranged from 9 to 36) Intensity of itch using a VAS from 0 to 100 Direct costs of care, calculated by multiplying actual resource utilisation with unit costs, estimated according to the guidelines for pharmacoeconomic research Indirect costs of care, estimated using two modules of the online Health and Labour Questionnaire and applying the friction cost approach to account for reduced productivity during paid work or unpaid labour |
No significant differences in disease-specific quality of life, severity of atopic dermatitis and intensity of itching between groups at any time point | Randomisation was carried out using an online randomisation programme. No other details given. States that allocation sequence was concealed but no methodology provided. No blinding was possible. Intention-to-treat analyses were used | ||
| Yokoyama 2009342 | EVOH fibre underwear (short-sleeve garments, EVOH material only on the inner surfaces, cotton on the outer surfaces) | Cotton underwear (control) | Japan | 24 participants (n = 11 control group, n = 13 EVOH fibre group) | Age 3–9 years; diagnosed as having atopic eczema according to the Hanifin and Rajka8 criteria | Severity of eczema (SCORAD score, extent and intensity, pruritus and sleep loss) Objective SCORAD score (extent and intensity) for the area covered by the study garments Urinary growth hormone levels Urinary cortisol levels |
Objective SCORAD score in both groups: significantly decreased. SCORAD score and urinary cortisol decreased only in the EVOH fibre group (p < 0.01 and p < 0.05, respectively | Method of randomisation and allocation concealment clearly reported. Intention-to-treat analysis not reported; however, participants not included in the analyses clearly stated along with reasons for this |
Appendix 15 Phototherapy
| Trial | Intervention A | Intervention B | Intervention C | Comments on interventions | Country | Number of participants randomised | Inclusion criteria | Outcomes | Comments on outcomes | Main reported results | Quality of reporting |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Brenninkmeijer 2010125 | Xenon chloride gas 308-nm excimer laser (Talos®, Wavelight Laser Technology AG, Erlangen, Germany – 10- or 20-mm spot size, 200 mW/cm, 60 ns, 200 Hz). Laser spot size of 20 mm was used. Initial dose = minimal erythema dose, then increased stepwise every other treatment. In the case of adverse events, treatment was deferred until resolution and the dose was not increased. For blistering, the dose was decreased by one minimal erythema dose and treatment was deferred. Treatment was given twice weekly for 10 weeks | Clobetasol propionate 0.05% ointment, applied once daily to the specified lesions only for 10 weeks | Participants could use topical treatments on all other affected areas of the body except the selected extremities during the study | The Netherlands | 13 participants | Diagnosis of atopic eczema based on the millennium criteria;392 more than four symmetrical prurigo lesions on the upper or lower extremities that had persisted for > 6 months | (Primary) PAIS [number of nodules, excoriation, erythema, induration, pruritus (VAS) each scored from 0 to 3, with 0 = absent, 1 = mild, 2 = moderate, 3 = severe; max. score 15] (Secondary) PGA (0 = clear, 1 = almost clear, 2 = mild, 3 = moderate, 4 = severe) (Secondary) Patient Global Assessment (overall change from baseline: 0 = cleared or almost clear, 1 = marked improvement, 2 = moderate improvement, 3 = mild improvement, 4 = no change, 5 = worsening) Duration of remission (relapse = PAIS returned to ≥ 75% of the baseline value) Adverse events Compliance with treatment regimen Participant treatment preference at the end of the study |
Use of concomitant medication. Photodocumentation took place under standardised conditions. It is not clear from the report which outcome parameter these data were collected for | All outcomes significantly improved for both treatments at the end of active treatment (10 weeks). Follow-up: excimer laser showed more improvement than clobetasol propionate. For the excimer laser-treated sites there was a marked decrease in epidermal thickness and an increase in inflammatory infiltrate | Method of randomisation and allocation described. Intention-to-treat analysis not explicitly stated and seems unlikely as withdrawn participants were not included in the analysis | |
| Byun 2011391 | FSL phototherapy device (320–5000 nm) and emollient (Physiogel®; Stiefel-Korea Ltd). Phototherapy was administered for 20 minutes on the anterior side of the body and 20 minutes on the posterior side of the body. Each irradiation 530 J/cm2, which included 121 J/cm2 of UVA and 409 J/cm2 of visible and infrared light. Treatment given twice a week for 4 consecutive weeks (total eight irradiations) | Control (emollient – Physiogel), applied twice a day for 4 weeks | No other topical or systemic treatments were allowed during the study. The FSL phototherapy device generates full-spectrum light on a continuous wavelength spectrum of 320–5000 nm | Not stated | 38 participants (n = 20 FSL phototherapy group, n = 18 control group) | Moderate to severe eczema (SCORAD score > 25); all participants were Korean with skin phototype III or IV (specific criterion not stated) | Eczema severity (SCORAD score) Participant assessment of clinical improvement (4-point scale: 0–25% improvement = poor improvement, 26–50% improvement = fair response, 51–75% improvement = good response, 76–100% improvement = excellent response) Serum eosinophilic cationic protein level IgE level Levels of 22 different human cytokines (see paper for full list) |
Participant assessment of clinical improvement measured at week 8 | Mean SCORAD score: FSL group – significantly reduced after 4 weeks and then stayed reduced for another 4 weeks, control group – no significant change during the study. Participants rated ‘good to excellent’ improvement: FSL group 75% of participants, control group 50% of participants. No serious adverse events reported | Method of randomisation not described. Allocation concealment not reported. Intention-to-treat analysis not reported | |
| Dittmar 2001382 | High-dose UVA phototherapy. Maximum single dose 130 J/cm2, maximum cumulative dose 1840 J/cm2. Total of 15 treatments (five per week) | Medium-dose UVA phototherapy. Maximum single dose 65 J/cm2, maximum cumulative dose 975 J/cm2. Total of 15 treatments (five per week) | Low-dose UVA phototherapy. Maximum single dose 20 J/cm2, maximum cumulative dose 300 J/cm2. Total of 15 treatments (five per week) | Not stated | 34 participants (n = 11 low-dose group, n = 12 medium-dose group, n = 11 high-dose group) | Diagnosis of atopic eczema according to the Hanifin and Rajka criteria;8 aged > 18 years; SCORAD score > 30 | Severity of eczema (SCORAD score) | At the end of treatment (after 15 treatments): high-dose and medium-dose UVA had significantly reduced the SCORAD score. No significantly reduced SCORAD score for the low-dose group. All groups showed no statistically significant changes in IgE and eosinophil cationic protein and number of eosinophils in peripheral blood. No side effects reported | Method of randomisation, allocation concealment and intention-to-treat analysis not described | ||
| Gambichler 2009386 | UVA1 phototherapy, 50 J/cm2 per session. Treatment given three times per week for 6 weeks | Narrowband UVB. First dose was 70% of the minimal erythema dose. Increased by 10–20% per session (maximum dose 1.2 J/cm2 skin type II, 1.5 J/cm2 skin types III and IV). Treatment given three times per week for 6 weeks | A test to exclude abnormal photosensitivity to UVA1 was carried out before treatment | Germany | 47 participants (n = 22 UVA1, n = 25 narrowband UVB) | Diagnosed with extrinsic atopic eczema according to the Hanifin and Rajka8 criteria; SCORAD score of ≥ 20 | (Primary) Relative reduction in SASSAD score (Secondary) Reduction in VAS scores for pruritus (Secondary) Reduction of Skindex-29 (score range 30–150) (Secondary) Reduction in serological parameters |
No difference between the two treatments in terms of relative reduction in clinical scores: SASSAD score p = 0.5, pruritus score p = 0.5. No significant difference in Skindex-29 scores between treatments (p = 0.1). No significant differences in total IgE and eosinophil cationic protein following treatment with UVA1 (p = 0.3) or narrowband UVB (p = 0.9) | Method of randomisation reported but not detailed. Allocation concealment unclear. Intention-to-treat population used and definition given | ||
| Granlund 2001390 | Ciclosporin: initial dose 4 mg/kg/day, which could then be increased or decreased during the first two treatment cycles by 1 mg/kg/day at each scheduled visit to a maximum of 4 mg/kg/day and a minimum of 1 mg/kg/day. The lowest effective dose in the second cycle was chosen as a constant maintenance dose in subsequent cycles. Treatment was given for 8 weeks (treatment phase) followed by a period of only topical treatment until relapse or for at least 2 weeks (remission phase). One treatment phase followed by one remission phase was one treatment cycle. The total study time was 12 months and contained as many treatment cycles as needed to keep a participant in remission | UVAB phototherapy: initial dose depended on the participant’s skin type and on previous experience with UVAB phototherapy. Successive dose increments were performed at every other treatment visit according to a standard treatment schedule up to maximal doses of 15 J/cm2 of UVA and 0.26 J/cm2 of UVB. If remission occurred before the maximal dose was achieved, no further dose increments were performed. If erythema appeared the dose was reduced to the preceding dose. Treatment was administered two to three times a week. It was intended that participants should have at least 16 visits per cycle and no more than one cycle was allowed to be incomplete. Treatment was given for 8 weeks (treatment phase) followed by a period of only topical treatment until relapse or for at least 2 weeks (remission phase). One treatment phase followed by one remission phase was one treatment cycle. The total study time was 12 months and contained as many treatment cycles as needed to keep a participant in remission | Not stated | 72 participants were randomised (n = 36 in each group) | Age between 18 and 70 years with atopic eczema diagnosed according to the criteria outlined by Hanifin and Rajka;8 disease severity of 7–9 according to Rajka and Langeland228 | Number of days in remission (using SCORAD index, with remission being defined as ≤ 50% of the participant’s baseline value) Use of emollients and topical corticosteroids Participant-assessed overall assessment of efficacy at the end of each treatment phase (5-point scale, with 1 = very good, 2 = good, 3 = moderate, 4 = slight, 5 = none) Quality of Life using the Eczema Disability Index Adverse events |
Ciclosporin produced significantly more days in remission than UVAB phototherapy during the 1-year study period. At the end of the study no difference between the two groups was noted in terms of quality of life. A significant increase in serum creatinine occurred in two participants and seven participants developed mild or moderate hypertension during ciclosporin treatment | The method of randomisation was not described but a reasoning for the study not being blinded was given. Intention-to-treat analysis was clearly described | |||
| Heinlin 2011389 | sBPT: an anatomically shaped bathtub with a computer-controlled purification system for solution balneotherapy and a continuously adjustable and individually dispensable light console. Light consisted of TL-01 UVB lamps including a gauged dosimeter (311 nm). 10% Tomesa® solution (Dead Sea salt, the most important components being kalium 129,400 mg/kg, magnesium 82,000 mg/kg, natrium 52, 500 mg/kg, bromide 3980 mg/kg, calcium 3940 mg/kg and sulphate 510 mg/kg) as used as an analogue to the ion formation of the dead sea. The purification system required a reduction of the solution concentration to 10%. Starting doses were determined according to individual skin type. Each skin type had a dose escalation schedule. During treatment the dose per treatment unit was increased by simultaneously increasing the bathing time. Patients started with three to five sessions per week up to 35 sessions in total with increasing treatment duration. Sessions lasted from 15 minutes up to 30 minutes. This included a bathing time of at least 4 minutes before UV light started. Patients started treatment sessions with a short bath without phototherapy followed by sBPT. They turned over every 4 minutes to guarantee a constant all-over covering of the irradiated skin with the solution. Patients had to moisten their faces regularly with the salt solution | Narrowband UVB PT: TL-01 UVB lamps including a gauged dosimeter (311 nm). Patients started with three to five sessions per week up to 35 sessions in total with increasing treatment duration. Not clear how long the light therapy sessions lasted | The same treatment device was used for both interventions (an anatomically shaped therapy bath tub with a computer-controlled purification system for balneotherapy and a light console located above the tub. For the PT group, patients had to lie on a couch in the bathtub. They were treated by the same light console but without receiving synchronous bathing. Incremental steps to reach the final dose depended on the skin type of each patient and a patient’s individual acceptance (erythema threshold). During the treatment period patients were allowed to use emollients. UV therapy and specific systemic therapy for atopic dermatitis had to be stopped 4 weeks before the study and topical treatment 1 week before the study and they were disallowed during the treatment period. After completion of the treatment period, no limitation was put on type or duration of additional active treatments until the end of follow-up (6 months) | 180 randomised (n = 90 sBPT group, n = 90 PT group). In total, 177 were included in the safety population (n = 88 sBPT group, n = 89 PT group); 169 could be analysed in the intention-to-treat population | Atopic dermatitis diagnosed by a dermatologist; age ≥ 18 years; Caucasian ethnic background; SCORAD score at baseline > 35; written informed consent | SCORAD score – relative improvement from baseline to the end of therapy SIP evaluated by the patient’s global impression of therapy on a 6-step-Likert scale (improvement from very good to very bad, with 1 = very good to 6 = very bad) FLQA-d Willingness to pay Safety data/adverse events |
SCORAD score: at the end of therapy a clinically relevant and significant difference of 26.2% could be shown (p < 0.001) in favour of the sBPT group. After 6 months, sBPT was found to show statistically significant superiority. More patients withdrew early because of adverse events in the PT group: sBPT n = 2, PT n = 6 | Method of randomisation unclear. Allocation concealment fairly adequately described. No description of any blinding. Intention-to-treat analysis was used | |||
| Majoie 2009385 | Narrowband UVB (311 nm). First dose was 70% of the minimal erythema dose, then increases of 20% were given if the previous dose produced no erythema or 10% if the previous dose produced slight erythema. Treatment given three times per week for 8 weeks | Medium-dose UVA1 (350–400 nm). First dose 30 J/cm2, then increased to 45 J/ cm2 in two treatments. Dose was decreased if the reaction was too strong. Treatment given three times per week for 8 weeks | 4 weeks of follow-up after the treatment was ended | Not stated | 13 participants | Age range 20–56 years (criterion not stated); atopic eczema according to the Hanifin and Rajka8 criteria; symmetrical eczema distribution | Eczema severity (Leicester Sign Score: erythema, purulence, excoriation or crusting, dryness or scaling, cracking or fissuring and lichenification graded on a scale from 0 (none) to 3 (severe) at six body sites; score range 0–108) Participant-assessed itch (VAS from 0 = no itch to 10 = most intense itch imaginable) Epidermal cellular infiltrate: numbers of T cells, dendritic cells, eosinophils and neutrophils in the epidermis Dermal infiltrate: numbers of CD3+ (T cells), FoxP3+ (regulatory T cells), EG2 (eosinophils), CD1a (dendritic cells), AA1 (mast cells) and elastase (neutrophils) |
Both treatments significantly decreased atopic eczema severity (p < 0.01). Also decreased dermal cellular infiltrate. Percentage of FoxP3+ CD3+ T cells did not change after both treatments | Method of randomisation not clearly described. Allocation concealment not reported. Intention-to-treat analysis not described | ||
| Reynolds 2001384 | Narrowband UVB phototherapy. Whole body, 0.4 J/cm2 then percentage-based increments every week to a maximum of 1.5 J/cm2. Treatment given twice a week for 24 exposures | Broadband UVA phototherapy. Whole body 5 J/cm2 then percentage-based increments every week to a maximum of 15 J/cm2. Treatment given twice a week for 24 exposures | Visible light phototherapy, 5-minutes exposure rising to 15-minutes exposure (participants turned by 180° half-way through each exposure). Treatment given twice a week for 24 exposures | UK | 73 participants (n = 26 narrow band UVB, n = 24 broadband UVA, n = 23 visible light) | Age 16–65 years; diagnosis of atopic eczema as per UK Working Party criteria9 | (Primary) Change in total disease activity at treatment end (24 weeks) (erythema, papules, vesicles, excoriation, scaling or dryness and lichenification each graded from 0 to 3 at six body sites; max. score 90) (Primary) Change in extent of disease at treatment end (24 weeks) (Secondary) PGA (‘exacerbation of disease’, ‘no change’, ‘slight improvement’, ‘moderate improvement’, ‘marked improvement’ or ‘complete resolution’) (Secondary) Participant-assessed itching and sleep loss (10-cm VAS) (Secondary) Quantities of topical steroids used (weight of medication tubes) Adverse events |
Mean reduction in disease severity over 24 treatments: narrowband UVB 9.4 points (95% Cl 3.6 to 15.2), broadband UVA 4.4 points (95% Cl –1.0 to 9.8) more than visible light phototherapy. Mean percentage reduction in extent of disease over 24 treatments: narrowband UVB 6.7% (95% Cl 1.5% to 11.9%), broadband UVA –1.0% (95% Cl –5.3% to 3.3%) compared with visible light phototherapy. Small proportion of participants developed erythema after phototherapy or had a flare of eczema sufficient to withdraw from treatment | Description of randomisation and allocation concealment given. Intention-to-treat population defined and used for the analysis; however, different numbers of participants are stated for each data point | ||
| Selvaag 2005381 | Standard UVB light therapy. Fixed-dose increments: start dose 1.6 standard erythema doses (mJ/cm2). Dose increased by 25% each subsequent treatment. If erythema was induced, dose was not increased at the next session. Up to 6 weeks of treatment (treatment stopped if SCORAD score on either side of the body was < 10) | Skin reflectance-guided UVB light therapy. Measurements guided by skin reflectance taken on non-lesional skin between the shoulder blades and chest. Highest dose not eliciting erythema for each individual. Up to 6 weeks of treatment (treatment stopped if SCORAD score on either side of the body was < 10) | The whole of the face of all participants was given standard UVB treatment. Participants allowed to use topical steroids and emollients but only if used symmetrically. Use of topical corticosteroids stopped during UVB treatment if possible | Not stated | 20 participants | Mild to moderate atopic eczema; age range of participants 16–38 years (criterion for inclusion not given) | Severity of atopic eczema using SCORAD index Initial UVB dose Final UVB dose Cumulative UVB dose Adverse events |
Initial UVB dose was higher in the skin reflectance-guided treatment group than in the standard treatment group. Reduction in SCORAD score: no difference between the two treatments. Cumulative UVB dose was significantly lower in the skin reflectance-guided treatment group than in the standard treatment group | Method of randomisation not described. Allocation concealment not reported. Intention-to-treat analysis not reported | ||
| Tzaneva 2010383 | UVA1 phototherapy. Single exposure doses of 70 J/cm2, minimal erythema dose used if under this amount and increased by 10 J/cm2 on subsequent treatment days as long as there was no erythema to a maximum dose of 70 J/cm2. Total of 15 exposures (five times per week for 3 weeks) | Oral psoralen plus UVA photochemotherapy. Oral 5-MOP (1.2 mg/kg) given 2 hours before exposures. Starting dose for phototherapy was 70% of the minimal phototoxic dose. Increments of 20% of the minimal phototoxic dose were given at each treatment session if no erythema, 10% increments if barely perceptible erythema. Total of 15 exposures (five times per week for 3 weeks) | All participants allowed only unrestricted use of emollients. All exposures were performed on an outpatient basis and no maintenance treatments were given | Not stated | 40 participants | Atopic eczema fulfilling the Hanifin and Rajka8 criteria; severe generalised eczema (SCORAD score of ≥ 45); age ≥ 18 years | (Primary) Length of remission after each treatment (Secondary) Reduction in SCORAD score (Secondary) Tolerability of the two regimens |
Reduction in SCORAD score compared with baseline (mean ± SD): PUVA 54.3% ± 25.7%, UVA1 37.7% ± 22.8% (p = 0.041). Median length of remission: PUVA 12 weeks (IQR 4 to 26,) UVA1 4 weeks (IQR 4 to 12) (p = 0.012) | Method of randomisation described. Allocation concealment not reported. Intention-to-treat principle not used for the analyses | ||
| Tzung 2006388 | 1% pimecrolimus cream (whole body) and narrowband UVB (half body). Pimecrolimus – thin film applied to all skin lesions. nUVB – 70% of minimal erythema dose then percentage-based increments up to a maximum dose of 1.5 J/cm2. Treatment given for 6 weeks: pimecrolimus cream – twice daily, narrowband UVB – twice weekly | 1% pimecrolimus cream (half body) and narrowband UVB (whole body). Pimecrolimus – thin film applied to all skin lesions. nUVB – 70% of minimal erythema dose then percentage-based increments up to a maximum dose of 1.5 J/cm2. Treatment given for 6 weeks: pimecrolimus cream – twice daily, narrowband UVB – twice weekly | 26 participants [n = 12 pimecrolimus cream (whole body) and narrowband UVB (half body), n = 14 pimecrolimus cream (half body) and narrowband UVB (whole body)] | Age 5–17 years; moderate to severe atopic eczema with symmetrical distribution | (Primary) Change in EASI score Participant-/carer-assessed severity of pruritus (score for 24 hours before each visit on a 10-cm VAS) Adverse events Level of eosinophilic cationic protein Change in total IgE level |
EASI score and pruritus score reduced in both groups (p = 0.002 and p < = 0.004, respectively). No significant difference in efficacy between treatments at 6 weeks | Method of randomisation not stated. Allocation concealment not described. Intention-to-treat analysis not described. No mention of dropouts or withdrawals. Numbers of participants included in the analysis not stated | ||||
| Valkova 2004387 | UVA/UVB monotherapy. Starting UVA dose 2–2.5 J/cm2. Starting UVB dose 0.06 J/cm2 for phototype II and 0.08 J/cm2 for phototypes III and IV. Doses increased twice weekly by 1–2 J/cm2 for UVA and 0.02 J/cm2 for UVB if no preceding erythema to a maximum dose of 8 J/cm2 for UVA and 0.23 J/cm2 for UVB. Treatment given five times per week until remission achieved (minimum duration of treatment 2 months, maximum duration of treatment 8 months) | UVA/UVB monotherapy and topical corticosteroids (fluticasone propionate, hydrocortisone butyrate). Starting UVA dose 2–2.5 J/cm2. Starting UVB dose 0.06 J/cm2 for phototype II and 0.08 J/cm2 for phototypes III and IV. Doses increased twice weekly by 1–2 J/cm2 for UVA and 0.02 J/cm2 for UVB if no preceding erythema to a maximum dose of 8 J/cm2 for UVA and 0.23 J/cm2 for UVB. Topical corticosteroids applied twice a day. Phototherapy treatment given five times a week until remission achieved (minimum duration of treatment 2 months, maximum duration of treatment 8 months) | Both groups were allowed unlimited use of emollients | Not stated | 31 participants (n = 17 phototherapy, n = 14 phototherapy and topical corticosteroid) | Moderate to severe atopic eczema; adults and children (age range 8–45 years); fulfils diagnostic criteria of Hanifin and Rajka8 | Severity of eczema (Costa and colleagues’249 simple scoring method): erythema, oedema, infiltration, vesicles, pustules, crusts, excoriation, dryness, desquamation, lichenification, itch, loss of sleep | All participants were assessed by the same assessor | No difference between the two groups for efficacy (p = 0.904). Number of treatments – t = 2.5, p = 0.02 in favour of phototherapy and corticosteroid. Total UVB dose – t = 2.3, p = 0.03 in favour of phototherapy and topical corticosteroid. Duration of remissions (t = 0.9, p = 0.39) and frequency of side effects (data not given), no significant difference | Method of randomisation and allocation concealment not reported. Intention-to-treat analysis not described |
Appendix 16 Systemic immunomodulators
| Trial | Intervention A | Intervention B | Comments on interventions | Country | Number of participants randomised | Inclusion criteria | Outcomes | Comments on outcomes | Main reported results | Quality of reporting |
|---|---|---|---|---|---|---|---|---|---|---|
| Bemanian 2005401 | Oral ciclosporin, 4 mg/kg, given daily for 3 months | Intravenous immunoglobulin, 2 g/kg, given as a single dose as a slow intravenous infusion over 4–8 hours (one drop/kg/minute) after hospital admission | Both groups used emollients daily after a bath. For flare-ups, oral hydroxyzine (0.5 mg/kg at bedtime) and topical corticosteroids (short courses) were given. For clinically proved bacterial infection, systemic cephalexin or topical mupirocin was given | Not stated | 14 participants (n = 6 intravenous immunoglobulin group, n = 8 ciclosporin group) | Severe atopic eczema (SCORAD score of > 70); diagnosis by modified Hanifin and Rajka8 criteria; failure to respond to first- and second-line therapy | Severity of eczema (SCORAD score) | Both groups of participants were given skin-prick testing for common allergens including whole egg, trees, mites and cow’s milk | Positive skin-prick test: milk 62.5%, egg 75% (8/14 participants). RAST: cow’s milk 66.6%, egg 100%. Day 90: significant reduction in SCORAD score in the ciclosporin group compared with the intravenous immunoglobulin group (p = 0.005). There were no significant adverse reactions in either group | Method of randomisation and allocation concealment not described. Intention-to-treat analysis not reported |
| Berth-Jones 2002398 | Azathioprine 2.5 mg/kg/day. A single dose was given per day, usually in the morning, for 12 weeks | Matched placebo 2.5 mg/kg/day. A single dose was given per day, usually in the morning, for 12 weeks | Both treatments were supplied in sealed gelatin capsules identical in appearance, smell and taste | Not stated | 37 | Men or women aged ≥ 16 years; diagnosis of atopic eczema using the Hanifin and Rajka8 criteria; severity of disease such that quality of life was seriously impaired despite the daily use of a potent topical steroid; fertile women using an adequate contraception | (Primary) Objective assessment of disease activity (SASSAD score) Participant-assessed severity of pruritus (itching) using a VAS Participant-assessed degree of sleep disturbance using a VAS Participant-assessed disruption of work and daytime activity using a VAS Adverse events |
Haematological and biochemical monitoring was also carried out | Mean SASSAD score improved from 39.7 to 29.6 for the azathioprine group, an improvement of 10.2 (26%), whereas the score improved from 33.6 to 32.6 for the placebo group, an improvement of 1.0 (3%). The azathioprine group demonstrated larger improvements than the placebo group for all of the participant-assessed outcomes of sleep disturbance, disruption to work and daytime activities and itch. Only the disruption to work and daytime activities scores changed significantly (p = 0.02, using a paired t-test). There were no significant changes in any of the participant-assessed outcomes in the placebo groups | Method of randomisation and allocation concealment described and adequate. The intention-to-treat population did not include everyone who was randomised (participants who attended the baseline visit but not subsequent visits were not included) |
| Capella 2001251 | Oral montelukast (10 mg) and placebo tablets and pharmacologically inert, non-moisturising, non-greasy gel. Montelukast taken once a day, placebo tablet taken three times a day, placebo cream applied twice a day on atopic eczema lesions and xerotic skin for 6 weeks | Cetirizine (10 mg) and clarithromycin (250 mg) and topical mometasone furoate 0.1% or topical methylprednisolone aceponate 0.1% cream for atopic eczema lesions and topical emulsions containing urea or ammonium lactate for xerotic skin. Cetirizine taken twice a day for 6 weeks, clarithromycin taken twice a day for 10 days and topical preparations applied for 6 weeks | Not stated | 32 participants (16 in each group) | Adults (aged ≥ 18 years); moderate to severe atopic eczema (SCORAD score of ≥ 30); eczema to fulfil the Hanifin and Rajka8 criteria; consulted because of a flare-up and/or intolerance to and/or ineffectiveness of previous treatments | Severity of eczema (SCORAD score) Level of eosinophilic cationic protein Level of eosinophilic protein X Eosinophil and basophil counts Standard liver and kidney function tests |
Similar improvement in SCORAD values seen in both groups. Eosinophilic cationic protein and eosinophilic protein X levels were significantly reduced within each group | Method of randomisation, allocation concealment and intention-to-treat analysis all not described | ||
| Czech 2000400 | Ciclosporin (Neoral, microemulsion), 150 mg (low dose). First 2 weeks: 150 mg once a day; 3–8 weeks (if clinical response – total body surface area affected reduced by ≥ 50%) dose reduced by 50% (75 mg). After 8 weeks responders entered a 4-week follow-up phase and were randomised to stop treatment or to receive the last effective dose every second day | Ciclosporin (Neoral, microemulsion), 300 mg (high dose). First 2 weeks: 300 mg once a day; 3–8 weeks (if clinical response – total body surface area affected reduced by ≥ 50%) dose reduced by 50% (150 mg). After 8 weeks responders entered a 4-week follow-up phase and were randomised to stop treatment or to receive the last effective dose every second day | One large capsule (100 mg), one medium capsule (50 mg) and one small capsule (25 mg) taken each morning and each evening by all participants. In the low-dose group, large capsule = placebo. In the high-dose group, small capsule = placebo. Adjuvant therapy with corticosteroids, cytotoxic agents or photo/chemotherapy was not allowed during the study | Germany | 106 participants (n = 53 low-dose ciclosporin group, n = 53 high-dose ciclosporin group) | Men and women; age ≥ 18 years; diagnosis according to the Hanifin and Rajka8 criteria (at least three criteria fulfilled); severe atopic dermatitis not controlled by conventional therapy; minimum score of 10 on the Erlangen criteria of atopic diathesis; minimum value of 30 for the total body surface area score; minimum body weight 55 kg; women of childbearing age asked to use reliable contraception until 4 weeks after the study | (Primary) Difference in total body surface area affected after week 2 (calculated using a grading of 0 = absent, 1 = mild, 2 = moderate, 3 = severe for erythema, infiltration, vesiculation/papulation, dryness/scaling, excoriation/crusting at six body sites: arms, hands, legs, feet, head/neck and trunk; max. score 108) (Secondary) Differences in affected body surface area (Secondary) Itching (linear analogue scale from 0 to 100) (Secondary) Sleep loss (linear analogue scale from 0 to 100) (Secondary) Quality of life (slightly modified version of the DLQI) (Secondary) Differences in blood pressure (Secondary) Differences in serum creatinine levels |
(Secondary) Adverse events | Decrease in total symptom score after 2 weeks: low-dose group – from 59.0 to 39.3, high-dose group – from 60.7 to 33.2 (p < 0.05). Decrease in total symptom score to week 8: low-dose group 30.8, high-dose group 25.5 (p < 0.05). Positive effects observed for affected body surface area, itching, sleep loss and quality of life. Serum creatinine at week 2: low-dose group – 0.6% increase, high-dose group – 5.8% increase (p < 0.01). Serum creatinine at week 8: low-dose group – 1.1% increase, high-dose group – 6.0% increase (p < 0.01) | Method of randomisation described and adequate. Allocation concealment not stated and the nature of the blinding and parties blinded not described. Intention-to-treat population used for the analyses and well described |
| El-Khalawany 2013404 | Methotrexate, initial dose 5 mg (test dose), maintenance dose 7.5 mg. Administered orally (2.5 mg/tablet) in three divided doses with a 12 hour interval as a single weekly dose for 12 weeks | Ciclosporin, 2.5 mg/kg/day. Oral solution (100 mg/ml) diluted in juice and administered in two divided doses for 12 weeks | Methotrexate patients were supplemented with 400 g of folic acid once a week following the day of the methotrexate dose. All patients were asked to stop any topical or systemic treatment except emollients for at least 2 weeks prior to commencement of the study | Egypt | 40 children (n = 20 methotrexate, n = 20 ciclosporin) | Children aged 8–14 years; severe diagnosed atopic dermatitis; failed to be treated with topical therapy; unfit, unco-operative or poorly responsive to phototherapy | SCORAD score Clinical assessment Laboratory investigations |
Methotrexate group: mean ± SD SCORAD score at the beginning of the study 57.90 ± 3.21. This reduced to 29.35 ± 6.32 at the end of the study. The mean absolute reduction was 26.25 ± 7.03. Ciclosporin group: mean ± SCORAD score 56.54 ± 4.82 at the start of treatment and 31.35 ± 8.89 at the end of treatment. Mean absolute reduction was 25.02 ± 8.21. There was no statistically significant difference in the reduction in SCORAD score between the groups (p = 0.93) | Randomisation described; however, allocation concealment and intention-to-treat analysis were not described. No blinding described | |
| Friedmann 2007407 | Montelukast 10 mg daily for 8 weeks | Placebo daily for 8 weeks | Placebo is stated as ‘matching’. There was a 2-week single-blind period before randomisation when all participants took the placebo | Not stated | 60 participants (n = 30 montelukast group, n = 30 placebo group) | Eczema defined according to the Hanifin and Rajka8 criteria; moderate disease severity defined as a SASSAD score between 12 and 50 at visits 1 and 2 (the 2-week single-blind placebo phase); age 16–60 years | Investigator-assessed response to treatment (7-point scale) Participant-assessed response to treatment (7-point scale) Severity (SASSAD score) Proportion of skin involved in the disease Topical corticosteroid usage (5-point scale) Severity of pruritus (10-cm VAS) Severity of sleep loss (10-cm VAS) |
Adverse events were also recorded | There were no significant differences between the treatment groups in any of the parameters used to assess treatment response | The method for generating the randomisation was described and was good. Concealment of allocation was unclear. The blinding was described and was good |
| Granlund 2001390 | Ciclosporin: initial dose 4 mg/kg/day, which could then be increased or decreased during the first two treatment cycles by 1 mg/kg/day at each scheduled visit to a maximum of 4 mg/kg/day and a minimum of 1 mg/kg/day. The lowest effective dose in the second cycle was chosen as a constant maintenance dose in subsequent cycles. Treatment was given for 8 weeks (treatment phase) followed by a period of only topical treatment until relapse or for at least 2 weeks (remission phase). One treatment phase followed by one remission phase was one treatment cycle. The total study time was 12 months and contained as many treatment cycles as needed to keep a participant in remission | UVAB phototherapy: initial dose depended on the participant’s skin type and on previous experience with UVAB phototherapy. Successive dose increments were performed at every other treatment visit according to a standard treatment schedule up to maximal doses of 15 J/cm2 of UVA and 0.26 J/cm2 of UVB. If remission occurred before the maximal dose was achieved, no further dose increments were performed. If erythema appeared the dose was reduced to the preceding dose. Treatment was administered two to three times a week. It was intended that participants should have at least 16 visits per cycle and no more than one cycle was allowed to be incomplete. Treatment was given for 8 weeks (treatment phase) followed by a period of only topical treatment until relapse or for at least 2 weeks (remission phase). One treatment phase followed by one remission phase was one treatment cycle. The total study time was 12 months and contained as many treatment cycles as needed to keep a participant in remission | Finland and Norway | 72 participants were randomised (36 in each group) | Age between 18 and 70 years with atopic eczema diagnosed according to the criteria outlined by Hanifin and Rajka;8 a disease severity of 7–9 according to Rajka and Langeland228 | Number of days in remission (using the SCORAD score, with remission being defined as ≤ 50% of the participant’s baseline value) Use of emollients and topical corticosteroids Participant-assessed overall assessment of efficacy at the end of each treatment phase (5-point scale, with 1 = very good, 2 = good, 3 = moderate, 4 = slight, 5 = none) Physician-assessed overall assessment of efficacy Quality of life using the Eczema Disability Index Adverse events |
Ciclosporin produced significantly more days in remission than UVAB phototherapy during the 1-year study period. At the end of the study no difference between the two groups was noted in terms of quality of life. A significant increase in serum creatinine occurred in two participants and seven participants developed mild or moderate hypertension during ciclosporin treatment | Method of randomisation not described but a reasoning for the study not being blinded was given. Intention-to-treat analysis clearly described | ||
| Haeck 2011403 | Oral EC-MPS, 720 mg twice a day (1440 mg daily). After a 6-week run-in period of 5 mg/kg/day of oral CsA (intervention B), 720 mg twice a day (1440 mg/day) of EC-MPS was given for 30 weeks. Intervention A was then withdrawn and patients were followed up for a further 12 weeks | Oral CsA, 5 mg/kg/day split into two doses per day for the 6-week run-in period followed by 3 mg/kg/day for the 30-week treatment period. Intervention B was then stopped and there was a 12-week follow-up period | Participants were allowed class III (US classification) topical corticosteroids. In the case of disease exacerbation then classes III and I topical corticosteroids could be used. If this failed then short-term oral corticosteroids could be used (prednisolone 0.5 mg/kg for 1 week) for a maximum of two courses | The Netherlands | 55 patients recruited; five were excluded before randomisation; 50 patients randomised (n = 24 EC-MPS group, n = 26 CsA group) | Age ≥ 18 years; diagnosis of atopic dermatitis according to the criteria of Hanifin and Rajka;8 insufficient response to treatment with potent topical corticosteroids at the time of inclusion | Clinical disease activity measured using SCORAD score Serum thymus and activation-regulated chemokine levels Serum IgE and IgG antibody levels DLQI VAS for itch and sleeplessness Side effects |
This was a non-inferiority trial with the sample size calculated on the objective SCORAD score (margin of 10 points) | Objective SCORAD and serum thymus and activation-regulated chemokine levels were higher in the EC-MPS group in the first 10 weeks. Seven out of 24 participants in the EC-MPS group needed oral corticosteroids during the trial. Maintenance phase – disease activity was the same in both groups. Side effects in both groups were mild and transient. In the follow-up phase (no treatment) the disease activity in the ciclosporin group rose considerably compared with the EC-MPS group | Method of randomisation not described. Allocation concealment not stated. Only the observer was blinded. Intention-to-treat principle applied |
| Heil 2010416 | Omalizumab, 0.016 mg/kg/IgE (IU/ml) per 4 weeks for 16 weeks | Placebo for 16 weeks | Participants allowed to use emollients, hydrocortisone acetate 1% and diflucortolon valerate 0.1% cream | Not stated | 20 participants (n = 13 omalizumab group, n = 7 placebo group) | Age 12–60 years; clinical diagnosis of atopic eczema according to the Hanifin and Rajka8 criteria; serum IgE between 30 and 1300 IU/ml; at least one significantly positive ImmunoCAP; positive skin-prick test at the same positivity as the ImmunoCAP; IGA of ≥ 2 at randomisation; stable atopic eczema; active atopic eczema defined as IGA of ≥ 2 for ≥ 9 months per year | (Primary) Changes in immunohistochemistry, flow cytometry, immunohistology (Primary) Serum IgE levels (Secondary) Changes in skin tests (skin-prick tests, titrated skin-prick test, atopy patch test) (Secondary) Clinical course (IGA) (Secondary) Clinical course (EASI) (Secondary) Clinical course (Investigator’s Pruritus Severity Assessment) |
In vivo results: omalizumab did not significantly change the clinical course of eczema in this study. Omalizumab did improve atopy patch tests in some participants. Omalizumab raised the threshold allergen concentration required to produce a type 1 hypersensitivity reaction (titrated skin-prick tests) There were also in vitro results detailed in the report | Method of randomisation and allocation concealment not reported. Intention-to-treat population used for the analysis but not defined | |
| Jee 2011418 | IVIg therapy, 2 g/kg of body weight/month. Treatment given once a month for 3 months (12 weeks) by injection whilst hospitalised | Placebo – general topical moisturising lotion, 1% hydrocortisone cream and oral antihistamines for itching | All participants were allowed only steroid-free hydrophilic or emollient ointment on the skin as adjunctive treatment. All participants were allowed to take oral acetaminophen for headache or nausea and to use general moisturising cream or 1% hydrocortisone cream and could take oral antihistamines if they complained of skin itching. Assessments were conducted after each injection (V2, V3, V4) and 3 (V5) and 6 (V6) months after treatment | Not stated | 48 patients were enrolled; 40 participants were randomised (n = 30 IVIg group, n = 10 placebo group) | Moderate to severe atopic eczema as defined by the Hanifin and Rajka8 criteria; atopic eczema that had not responded to conventional therapy; > 30% body surface area affected; age > 2 years | SCORAD score Individual components of SCORAD index: total body surface area affected and subjective measurement of symptoms such as pruritus and loss of sleep Eosinophil cationic protein levels Serum IgE concentration IL-5, IFN-γ and ICAM-1 levels |
Disease severity index was significantly decreased at V5 compared with baseline (p < 0.05). There were no significant changes in total IgE level or total eosinophil count in peripheral blood at the last injection (V4) compared with baseline. The IL-5/IFN-γ ratio in Th1 and Th2 cells significantly decreased between baseline and V5, after which it increased such that the ratio at V6 was not significantly different from that at baseline. Compared with the level at baseline, the ICAM-1 level at V4 did not differ significantly but the level at V5 was lower | Randomisation not described. Allocation concealment not described. No description of any blinding. Intention-to-treat analysis not described | |
| Kwon 2013405 | Ciclosporin in combination with glucosamine (C regimen), < 3 mg/kg/day of ciclosporin and 100 mg/day of glucosamine C once a day for 2 weeks followed by a crossover to ciclosporin (S regimen) alone for 2 weeks. This cycle continued for 6 months | Ciclosporin alone (S regimen), < 3 mg/kg/day once a day for 2 weeks followed by a crossover to a combination of ciclosporin/glucosamine (C regimen) for 2 weeks. This cycle continued for 6 months | There was no washout between the periods because relapse or rebound exacerbation of atopic dermatitis with abrupt discontinuation of the administration drugs was of concern. Patients were allowed to apply the prescribed low-potency corticosteroid ointment | Not stated | 12 patients were included in the study, 10 were randomised and completed the study | Male and female patients; age > 12 years with atopic dermatitis recalcitrant to topical therapy | SCORAD score Levels of the cytokines IL-4, IL-5 and IFN-γ in peripheral blood mononuclear cells were included as secondary efficacy variables Adverse drug reactions |
Reduction in SCORAD score with the C regime was greater than that with the S regime. This difference increased over time. The glucosamine combination was predicted to cause an additive reduction in the mean per cent change in SCORAD index (approx. 6%) with decreasing IL-4 and IL-5 cytokine levels but without increasing treatment-related adverse events | Method of randomisation and allocation concealment not described. Intention-to-treat analysis not reported. Outcome assessors were blinded to treatment. Unclear whether other parties were blinded | |
| Meggitt 2006399 | Azathioprine suspension 30 mg/5ml (graduated dose regimen based on azathioprine pharmacogenetics), one dose a day for 12 weeks | Placebo suspension, one dose a day for 12 weeks | All participants were given the same range of topical corticosteroids to use throughout the trial (1% hydrocortisone, 0.025% and 0.1% betamethasone valerate). Emollients and topical corticosteroids could be used as normal except for highly potent topical corticosteroids | Not stated | 63 participants (n = 42 azathioprine group, n = 21 placebo group) | Age 16–65 years; moderate to severe atopic eczema according to the UK Working Party criteria9 | (Primary) Change in disease activity (SASSAD score; max. score 108) from baseline to 12 weeks (Secondary) Affected body surface area (rule of nines) (Secondary) Participant-assessed sleep loss (10-cm VAS) (Secondary) Participant-assessed itch (10-cm VAS) (Secondary) Participant- and investigator-assessed global change from baseline (complete resolution, striking improvement, moderate improvement, slight improvement, no change or worse) (Secondary) DLQI [max. score 30 (worst)] Soluble CD30 levels |
Adverse events and blood count, urea and serum biochemistry were also recorded | Mean disease activity (SASSAD score): week 12 – azathioprine 37% (12-unit) improvement, placebo 20% (6.6-unit) improvement, difference 17% (5.4-unit) improvement (95% Cl 4.3% to 29%). Significant improvements in participant-assessed itch, area of involvement, global assessment and quality of life were also observed for the azathioprine group compared with the placebo group | Method of randomisation and allocation concealment described. Intention-to-treat population used for the analyses and defined |
| Oldhoff 2005415 | Mepolizumab 750 mg in two single doses 7 days apart | Placebo in two single doses 7 days apart | All participants classed as ‘non responders’ (responders = PGA of 0–2 at day 14) at day 16 were given rescue medication of fluticasone propionate 0.05% once daily. Participants did not use any other medications except for non-medicated emollients, bath oil as needed and topical hydrocortisone acetate 1% on the face | Europe | 43 participants (n = 20 mepolizumab group, n = 23 placebo group) | Age range 18–57 years (inclusion criterion unknown); diagnosed with atopic eczema according to the Hanifin and Rajka8 criteria; experiencing a flare (SCORAD score 20–40) | (Primary) PGA at 14 days [6-point scale, with 0 = 100% clear, 1 = almost clear (90–99%), 2 = marked improvement (50–89%), 3 = modest improvement (< 50%), 4 = no change, 5 = worse] (responders = PGA of 0–2 at day 14) (Secondary) Intensity of pruritus (4-point scale, with 0 = no itching, 1 = occasional itch, 2 = fairly persistent itch, 3 = intolerable constant itch) (Secondary) Pruritus (VAS from 0 to 10, with 0 = no itch, 10 = most intense itch imaginable) (Secondary) SCORAD score Serum thymus and activation-regulated chemokine Peripheral blood eosinophil level |
Full blood count, blood chemistry and urinalysis were also conducted | Blood eosinophil count: mepolizumab group significantly reduced compared with the placebo group (p < 0.05). PGA was not statistically significant different between the two groups (p = 0.115). SCORAD score was not statistically significant different between the two groups (p = 0.293). Also no clinical success for pruritus and serum thymus and activation-regulated chemokine. However, ‘modest improvement’ according to the Physician’s Global Assessment (< 50% improvement) was significantly higher in the mepolizumab group than in the placebo group (p < 0.05) | Method of randomisation and allocation concealment not stated. Intention-to-treat analysis not described but does not appear to have been used as participants who withdrew were not included in the analysis |
| Pacor 2004151 | Oral ciclosporin 3 mg/kg and placebo of tacrolimus ointment. Ciclosporin given once a day, placebo of tacrolimus given twice a day, for 42 days | Tacrolimus 0.1% ointment and placebo of ciclosporin. Tacrolimus given twice a day, placebo of ciclosporin given once a day, for 42 days | Rescue medication for itching: 10 mg of cetirizine (one to two tablets a day). No other treatments allowed during the trial | Not stated | 30 participants (n = 15 ciclosporin group, n = 15 tacrolimus group) | Confirmed diagnosis of atopic eczema; moderate to severe eczema (based on the Rajka and Langeland228 criteria); treated with topical corticosteroids and had partial improvement; Age range 13–45 years (criterion not stated) | Severity of eczema (SCORAD score, max. score 103) Participant-assessed itch (0 = no itch, 1 = mild itch, 2 = moderate itch, 3 = severe itch) Participant-assessed sleep loss connected with eczema (0 = none, 1 = mild, 2 = moderate, 3 = severe) Participant-assessed erythema (0 = no erythema, 1 = mild erythema, 2 = moderate erythema, 3 = severe erythema) Serum and total IgE levels Blood eosinophil count Blood markers for haematological, biochemical, electrolyte, renal and hepatic function |
SCORAD score: day 14 – scores in both groups decreased but the tacrolimus group had a significantly lower score than the ciclosporin group; day 42: overall SCORAD score was significantly lower in the tacrolimus group (area under the curve assessment p < 0.001). Itching at day 42 (area under the curve): p = 0.003 in favour of tacrolimus. Sleep loss at day 42 (area under the curve): p = 0.01 in favour of tacrolimus. Erythema at day 42 (area under the curve): p = 0.005 in favour of tacrolimus. Number of days without use of rescue medication: tacrolimus group 82.5 days, ciclosporin group 76.5 days (p = 0.03) | Method of randomisation not reported. Allocation concealment unclear. Intention-to-treat analysis not reported | |
| Pajno 2007414 | Sublingual immunotherapy (glycerinated solution) containing Der p1 and Der f1. Three progressively increasing concentrations were administered: 100, 1000 and 10,000 RAST units/ml (10,000 RAST units concentration = 4.3 µg/ml of Der p1 and 3.5 µg/ml of Der f1). One drop (50 µl) was taken from the lowest concentration vial per day, increasing by one drop every day to five drops; this procedure was then repeated with the medium concentration vial and finally the highest concentration vial. The maintenance dose was five drops three times per week from the highest concentration vial for 18 months | Placebo solution, one drop (50 µl) per day from the lowest concentration vial, increasing by one drop every day to five drops; this procedure was then repeated with the medium concentration vial and finally the highest concentration vial. The maintenance dose was five drops three times per week from the highest concentration vial for 18 months | Allowed medication while on the study: 3 days of topical fluticasone propionate and/or oral hydroxyzine on demand for worsening pruritus, itching, oozing or oedema. For cutaneous superinfection: 6 days of clarithromycin (15 mg/kg/day). No other treatment, including moisturisers were allowed | Not stated | 56 participants (n = 28 immunotherapy group, n = 28 placebo group) | Children aged 5 to 16 years. Clinical history of chronic atopic eczema without evidence of spontaneous improvement at age 5 years. Sensitisation to house dust mite (IgE mediated) – assessed by positive skin prick test (wheal > 3 mm) and positive CAP-RAST assay (Class III or greater). Pollen sensitisation allowed (as long as no exacerbation of atopic eczema in the pollen season). If history of positive history or history suggestive of food allergy with positive skin test, then foods have to fully tolerated at the start of the study, confirmed by double blind placebo controlled food challenge. SCORAD = 8 or greater | Severity of eczema (SCORAD) Parent/carer assessed overall symptoms of eczema using the question ‘How was the eczema in this last month?’ (VAS, 0 = no symptoms at all, 10 = very severe symptoms) Total amount of rescue medication used (1 point = one dose of oral hydoxyzine or topical steroid, 2 points = one dose of oral clarithromycin in the 6 day course) Local or systemic reactions after administration of study treatment (rhinitis, conjunctivitis, itching/swelling in the mouth, lips, throat, or face, generalised itching, urticaria, cough and wheezing on a 0 to 3 scale, 0 = no symptoms, 3 = severe symptoms) |
Difference in SCORAD score from baseline was significant between the two treatment groups starting from 9 months of treatment (p = 0.025 ). Significant reduction in use of medications only in the sublingual immunotherapy group. Significant difference in the reported outcomes found only in mild to moderate eczema participants. Severe participants showed marginal benefits | Method of randomisation given and adequate but allocation concealment not reported. Intention-to-treat principle was not used for the efficacy analyses; however, an explanation for this was given: the effect of sublingual immunotherapy became detectable over months | |
| Paul 2002417 | Intravenous immunoglobulin, 2 g/kg; patients received an 8-hour infusion of 1 g/kg daily for 2 consecutive days | Delayed (for 30 days) intravenous immunoglobulin, 2 g/kg; patients received 30 days of routine care and observation followed by an 8-hour infusion of 1 g/kg daily for 2 consecutive days | For first 60 days up to 60 g/month of betamethasone dipropionate only allowed. Also, participants were told not to modify the amount of topical corticosteroid used compared with before the study | Not stated | 10 participants | Age 18–50 years; severe atopic eczema according to the Hanifin and Rajka8 criteria; eczema uncontrolled by conventional treatment (emollients, topical corticosteroids, phototherapy); minimum SCORAD severity = 50; stopped systemic therapy at least 1 month prior to study (ciclosporin, azathioprine, oral corticosteroids, phototherapy) | Severity of eczema lesions (SCORAD score) Participant-assessed global disease assessment (10-cm VAS, with 0 cm = total clearance, 10 cm = worst possible disease) |
SCORAD score did not differ between groups at day 30. Participant-assessed global severity showed no clinically significant difference at day 30. The mean percentage decrease in SCORAD score compared with baseline was 13% (95% CI 6% to 24%) at day 30 and 22% (95% CI 5% to 39%) at day 60 | Method of randomisation described and good. Allocation concealment not described; however, description of a centralised telephone allocation procedure suggests that the allocation was concealed adequately. Intention-to-treat analysis carried out but no definition given | |
| Pei 2001406 | Montelukast, 5 mg once a day for 4 weeks | Placebo (chewable ascorbic acid) once a day for 4 weeks | All participants had to use 70% light liquid paraffin as a soap substitute, aqueous cream as an emollient and 0.05% clobetisone butyrate cream twice daily. After 4 weeks there was a washout period of 2 weeks before participants crossed over to the second phase of the study | Hong Kong, China | 15 participants [not including dropouts: n = 6 group A (placebo then treatment), n = 5 group B (treatment then placebo)] | Age 6–16 years | Severity of eczema (sum of 0–3 score for each of erythema, oedema/papulation, oozing/crusting, excoriation, lichenification, and dryness on eight body areas: head and neck, front of trunk, back, genitalia, four limbs; max. score per area 18, max. score total 144) Extent of disease (estimate of percentage body surface area involved, with 9% each for head and neck, right upper limb and left upper limb, 18% each for right lower limb, left lower limb, back of trunk and front of trunk and 1% for genitalia) Subjective quality-of-life questionnaire similar to the CDLQI (translated into Chinese) with additional questions on relationships with family members and social life (max. score 52, 13 questions with a max. score of 4 for each) |
Severity of eczema (group labels in Table 2 of the paper appear to have been mixed up; the results have been extracted to match the narrative from the report): statistically significant improvement (p < 0.05) in the montelukast group compared with the placebo group | Method of randomisation described but it is unclear whether the method of allocation concealment was adequate enough (sealed envelopes). Intention-to-treat analysis not described | |
| Rahman 2006410 | Oral montelukast, 10 mg/day for those aged ≥ 14 years, 5 mg/day for those aged 6–14 years (one tablet per day) for 4 weeks | Conventional treatment (antihistamine and topical 1% hydrocortisone) for 4 weeks | Bangladesh | 31 participants (n = 16 montelukast group, n = 15 control group) | Atopic eczema diagnosed according to the Hanifin and Rajka criteria;8 age ≥ 6 years; at least 1 year of intermittent or persistent symptoms; SCORAD score of ≥ 30 | Severity of eczema (SCORAD score) Adverse events |
SCORAD score: montelukast group – statistically significant improvement (p = 0.003), placebo group – improvement, but not significant (p = 0.088). Pruritus, then sleep loss and also inflammatory signs were most influenced by montelukast treatment. Xerosis was not affected by montelukast treatment. No adverse events reported in the montelukast group | Method of randomisation not stated. Allocation concealment not reported | ||
| Schmitt 2010402 | Prednisolone, initial dose 0.5–0.8 mg/kg, tapering to nil within 2 weeks. Treatment given for 2 weeks (then placebo for 4 weeks) | Ciclosporin, 2.7–4.0 mg/kg constant daily dose for 6 weeks | All participants were told to apply emollients provided by the investigators twice a day and were not allowed to use any other topical or systemic treatments for eczema during the study. Participants were allowed to continue antihistamines at the same dose and concomitant use of topical prednicarbate 0.25% was allowed during the study | Germany | 38 participants (n = 21 prednisolone group, n = 17 ciclosporin group) | Age 18–55 years; diagnosed with eczema according to the UK Working Party criteria;9 severe eczema as per objective SCORAD score of ≥ 40, significantly decreased quality of life defined as DLQI score of ≥ 10; did not have adequate control with topical corticosteroids and topical calcineurin inhibitors | (Primary) Proportion of patients in stable remission at week 2 for prednisolone and week 6 for ciclosporin (at least 50% improvement in objective SCORAD score relative to baseline, SCORAD50) and no flare (≥ 75% of baseline objective SCORAD after previous response) within the 12-week follow-up (Secondary) Proportion of participants reaching SCORAD50 (Secondary) Proportion of participants relapsing during follow-up (Secondary) Mean change in SCORAD score under active treatment relative to baseline (Secondary) IGA (6-point Likert scale) (Secondary) Participant global assessment (6-point Likert scale) (Secondary) Proportion of participants with a ≥ 5-point improvement in the DLQI |
(Secondary) Overall participant satisfaction with treatment/medical care of eczema (100-unit VAS, with 0 = total dissatisfaction, 100 = maximal satisfaction) | Study was terminated early because of a higher than expected level of withdrawals (15/38). Thirty-eight participants were analysed: stable remission – prednisolone 1/21, ciclosporin 6/17 (p = 0.031) | Method of randomisation described. Allocation concealment not explicitly described but the details given suggest that it was performed |
| Schram 2011394 | Methotrexate 10–22.5 mg/week as a single oral dose. One dose per week with dose escalation at scheduled follow-up visits for 12 weeks. Dose escalation with 2.5–5 mg/scheduled visit was allowed until a dose of 22.5 mg/week was reached | Azathioprine 1.5–2.5 mg/kg/day in a single dose. One dose per day with dose escalation at scheduled follow-up visits for 12 weeks of 0.5 mg/kg/day until a maximum dose of 2.5 mg/kg/day was reached | Each patient receiving methotrexate also received 5 mg of folate 1 day after the methotrexate intake. Dosage was escalated if patients did not achieve at least a 25% reduction in disease activity at a study visit. The dosage could be decreased in case of abnormal findings on physical examination, abnormal laboratory markers and/or adverse events. After 12 weeks, dosages in responders were reduced to find the optimum dosage. Patients were allowed to continue or start with concomitant topical triamcinolone acetonide ointment for the body and hydrocortisone ointment for the face and oral antihistamines. Patients were allowed to receive a maximum of two courses of rescue medication [30 mg/day of oral prednisolone for 1 week and a 1-week reduction schedule (20–20–15–15–10–10–5 mg)] | 45 patients screened for eligibility, 43 patients randomised, 42 patients included in the intention-to-treat population | Patients with atopic eczema (with and without the presence of allergen-specific IgE) defined according to the millennium criteria and the UK Working Party criteria;9 age ≥ 18 years; graded by the Rajka and Langeland228 criteria as severe; unresponsive, contraindicated or intolerant to ciclosporin treatment; not previously treated with azathioprine or methotrexate | Primary efficacy outcome: mean, relative and absolute change in severity of atopic eczema at week 12 as assessed using the SCORAD index Number of patients with a SCORAD score reduction of ≥ 50% (SCORAD50) Number of patients achieving mild disease (defined as mild, minimal or no disease activity on IGA) IGA (6-point Likert scale, with 0 = clear, 1 = almost clear, 2 = mild disease, 3 = moderate disease, 4 = severe disease, 5 = very severe disease) Patient global assessment (6-point Likert scale, with 0 = clear, 1 = almost clear, 2 = mild disease, 3 = moderate disease, 4 = severe disease, 5 = very severe disease) Mean change in EASI score (range 0–72) Patient-orientated eczema measurement (range 0–28) |
Also measured itch and sleeplessness on a VAS, Skindex-17 (Dutch version of Skindex-17 used to measure change in quality of life – range 0–85 points, with higher scores indicating more significantly impaired quality of life), levels of TARC measured at baseline and week 12, amount of concomitant corticosteroids and number of courses of rescue medication used, safety and adverse events | At week 12, mean relative reduction in SCORAD score: methotrexate 42% (SD 18%), azathioprine 39% (SD 25%) (p = 0.52). Proportions of patients achieving at least mild disease and reductions in impact of quality of life, symptoms and levels of TARC were similar in both groups at weeks 12 and 24. No significant differences were found in the number and severity of adverse events. No serious adverse events occurred | Method of randomisation and allocation concealment described. The study was single blind. Intention-to-treat analysis was used | |
| Silny 2006411 | SIT performed using aluminium hydroxide-adsorbed allergen preparations administered by subcutaneous injection (Nexter Allergopharma) with either a mixture containing both D. pteronyssinus (50%) and D. farinae (50%) or grass pollens (100%) for 12 months | Placebo, 0.0125 or 0.125 mg/ml of histamine for 12 months | Poland | 20 participants | Atopic dermatitis and monovalent sensitisation to airborne allergens (house dust mites or grass pollens), confirmed by clinical symptoms, skin point tests and specific serum IgE level | Clinical score (point index of severity and extensiveness of skin inflammation) Serum concentration of total and allergen-specific IgE Immunological parameters such as eosinophil cationic protein, sIL-2R, IFN-γ, IL-4, IL-5 and IL-10 |
The mean clinical score in the SIT group before treatment was 87.6 SD ± 15.8 points and this decreased to 38.8 SD ± 34.4 points after 12 months of therapy (–55.8%; p < 0.01). In the placebo group the mean clinical score before treatment was 86.3 SD ± 15.7 points and after 12 months of therapy this increased to 111.9 SD ± 41.7 points (+29.7%; not significant). Comparative statistical analysis indicated a significant difference between the two groups in favour of participants treated with the active allergy vaccines (difference 65.3%; p < 0.01). Serum levels of specific IgE in the SIT group showed a tendency to decrease whereas those in the placebo group tended to increase. Serum concentrations of selected immunological parameters including eosinophil cationic protein, sIL-2R, IFN-γ, IL-4, IL-5 and IL-10 were monitored before and after treatment but did not show significant differences | Methods of randomisation and allocation concealment do not appear to have been described. Intention-to-treat population unclear. All participants were analysed | ||
| Veien 2005408 | Oral montelukast, 10 mg per day (one tablet a day) for 4 weeks | Placebo, one tablet a day for 4 weeks | No active topical or systemic treatment allowed during the study. All participants were given 2 weeks of placebo treatment as a washout period before being randomised to treatment | Not stated | 59 participants (n = 29 montelukast group, n = 30 placebo group) | Age between 16 and 70 years; diagnosis according to Hanifin and Rajka8 criteria; moderate to severe disease; 5–35% of body surface area affected; minimum score of 4.5 according to the Rajka and Langeland228 grading system | EASI score (Primary) modified EASI score (sum of EASI and pruritus scores) Pruritus score (10-cm VAS marked 0–3 at 2.5-cm intervals) |
No difference in efficacy between montelukast and placebo | Method of randomisation not reported. Allocation concealment described. Intention-to-treat population used and definition given | |
| Wolff 2005419 | Pimecrolimus, 20 mg, 40 mg or 60 mg twice daily for 12 weeks | Vehicle twice daily for 12 weeks | 12-week open-label follow-up phase (standard eczema treatments). Participants were allowed to use emollients and 1% hydrocortisone as needed for 7 days prior to randomisation and during the study | Austria, Belgium, Canada, Finland, Poland, the UK and the USA | 103 participants (n = 27 placebo group, n = 26 20-mg pimecrolimus group, n = 24 40-mg pimecrolimus group, n = 26 60-mg pimecrolimus group) | Adults; atopic eczema according to the Hanifin and Rajka8 criteria; moderate to very severe atopic eczema according to the IGA | (Primary) Change in EASI score after 6 weeks compared with baseline (Secondary) IGA (0 = clear, no inflammatory signs to 5 = very severe disease, severe erythema, papulation and infiltration) (Secondary) Participant assessment of disease control in the 7 days prior to assessment (0 = complete disease control to 3 = uncontrolled disease) (Secondary) Pruritus severity assessment (previous 24 hours) (0 = none to 3 = severe; no half-points permitted) |
Pimecrolimus: clear dose-dependent effect shown. Onset of efficacy: statistically significant at week 2. Greatest reduction in EASI score compared with baseline: 66.6% at week 7 in the 60-mg pimecrolimus group. Oral pimecrolimus was well tolerated. There were no signs of nephrotoxicity and induction of hypertension | Method of randomisation and allocation concealment described but slightly unclear. Intention-to-treat population described and used for the efficacy analyses | |
| Yanase 2001409 | Montelukast, 10 mg once a day for 4 weeks | Placebo for 4 weeks | 1-week washout period before starting treatment for all participants and a 2-day washout period before participants crossed over to the second phase of the study. Emollients, antihistamines and class V or weaker topical corticosteroids were allowed throughout the trial | Not stated | 8 participants | Diagnosis of mild to moderate atopic eczema according to the Hanifin and Rajka8 criteria; at least 1 year of intermittent/persistent symptoms of eczema; age ≥ 18 years; ADASI score 6–15; healthy or with non-life-threatening, clinically stable, concomitant diseases | Severity (ADASI score) (sum of scores of 0–3 including half marks for each of erythema, induration/papulation, excoriation, lichenification, scaling/dryness, erosion/oozing) | Significant difference between placebo and active agent (p = 0.014). No significant interaction between order and treatment. Atopic eczema scores (mean ± SD): placebo 8.7 ± 2.0, montelukast 6.8 ± 2.1. Scores tended to be higher with placebo | Method of randomisation and allocation concealment not reported. Intention-to-treat analysis not described |
Appendix 17 Complementary therapies
| Trial | Intervention A | Intervention B | Comments on interventions | Country | Number of participants randomised | Inclusion criteria | Outcomes | Comments on outcomes | Main reported results | Quality of reporting |
|---|---|---|---|---|---|---|---|---|---|---|
| Anderson 2000429 | Almond oil with three essential oils in a 1 : 1 : 1 ratio (2% dilution). Massaged into the arms, legs, front and back for 10 minutes by the mother of the participant once every day for 8 weeks | Almond oil massaged into the skin for 10 minutes by the mother of the participant once every day for 8 weeks | A therapist visited the home of each participant once every week and performed 30 minutes of massage on the participant and counselled the participant and the mother together. All participants bathed every day, with the addition of the essential oils (six drops) only for the essential oil group | Unclear | 16 participants | Middle-class socioeconomic background; professional working mother; living in a family unit with the natural father; age 3–7 years; eczema non-responsive to usual therapy and with no known causes (e.g. no birth in the family or home change); a period of separation from the mother before 3 years of age; all children attended school or preschool for at least half a day a week | Daytime irritation and night-time disturbance as assessed by the mother (0 = no disturbances, 10 = very many disturbances) General improvement score (10-point scale, with 0 = no improvement, 10 = considerable improvement) |
The clinical condition was rated separately by the GP, mother and therapist | There was a significant reduction in both the daytime irritation and night-time disturbance scores in both groups compared with pretreatment scores | Method of generating the randomisation sequence not stated and allocation concealment not described. Blinding and the problems with this were clearly stated |
| Bae 2012440 | PMR together with conventional treatments. PMR involves tensing and relaxing muscle groups in the arms, legs, face, abdomen and chest. Target muscle group is tensed for 10 seconds and then relaxed for 20 seconds. Patients performed PMR twice a day at home using video and audio programmes for 4 weeks under controlled room temperature and light conditions without eating or drinking (except water) | Control group: conventional treatments only | Conventional treatments included topical glucocorticoids, topical calcineurin inhibitors, topical emollients and antihistamines. Abstinence from drinking alcohol and caffeine-containing beverages was recommended during the study period. It was not stated whether this was just for the PMR group or for both groups. Compliance was assessed using checklists regarding performance of PMR. Systemic immunosuppressants and immunomodulatory drugs were prohibited in both groups | 25 patients were randomised, one was excluded (PMR group n = 14, control group n = 10) | Not stated | Confirmed diagnosis of atopic dermatitis according to the criteria of Hanifin and Rajka;8 at least moderate severity of eczema (EASI score of > 10); not explicitly stated as inclusion criteria but patients had no other concomitant dermatological, medical or psychological disorders except atopic manifestations – these included allergic asthma, allergic rhinitis and allergic keratoconjunctivitis | EASI score (range 0–72) Pruritus VAS (0 = no pruritus, 10 = the most severe pruritus) Loss of sleep VAS (0 = no loss of sleep, 10 = most severe loss of sleep Serum levels of nerve growth factor, neuropeptide Y, interleukin-4 (IL-4), IL-5 and IL-13 Beck Depression Inventory (a 21-item test – each item is scored from 0 to 3; overall score ranges from 0 to 63) State–Trait Anxiety Inventory (40 questions, 20 relating to state anxiety and 20 relating to trait anxiety; each question is scored from 1 to 4; scores for trait anxiety and state anxiety range from 20 to 80) Interaction Anxiousness Scale (15 items that span a range of anxiety-evoking situations; each item is scored from 0 to 4; total score ranges from 0 to 60) |
Study also used the Private Body Consciousness subscale, which assesses the attention to internal sensations such as dry mouth, hunger and body temperature. Rated on a 6-point scale, with 0 = an extremely uncharacteristic quality and 5 = an extremely characteristic quality | After therapy the degree of sleep loss and pruritus was significantly reduced in the PMR group (p < 0.001). The control group showed no significant difference. There were significant reductions in EASI score but there was no difference between the groups. State anxiety scores showed a significant improvement in the PMR group (p = 0.005) | No description of randomisation method or allocation concealment. No description of any blinding or intention-to-treat analysis |
| Cheng 2011432 | XFS a common Chinese herbal preparation. Powder packets (3 g) to be mixed in 120 ml of warm drinking water. Dose dependent on age: 3–7 years one pack, 8–12 years two packs, 13+ years three packs three times a day for 8 weeks | Placebo (caramel, lactose and starch). Powder packets of caramel, lactose and starch in a ratio of 2 : 1 : 1 mixed with 120 ml of warm drinking water. Age 3–7 years took one pack, age 8–12 years took two packs and age 13+ years took three packs every day for 8 weeks | Taiwan | 71 patients randomised (n = 47 intervention group, n = 24 placebo group). Two patients dropped out of the study at baseline; therefore, intention-to-treat population included69 participants (n = 46 intervention group, n = 23 placebo group) | Refractory atopic eczema diagnosed by recognised clinical criteria and extensive (not limited to the skin folds and covering > 20% of the body surface area) lichenified or erythematous papules or plaques of atopic dermatitis; no active exudation or infection; poor response to conventional treatment (topical steroids and oral antihistamines) | Physician-assessed clinical lesion score. The body surface was divided into 20 roughly equal areas and within each area a score of 0 (none) to 3 (severe) was given for erythema and surface damage. This was multiplied by the percentage affected area within each zone (1 = < 33% affected, 2 = between 34% and 66% affected, 3 = > 67% affected). The sum of the severity scores multiplied by the area scores provided a total body score for each feature (max. score 180) Physician-assessed erythema score (0 = none to 3 = severe for each body area) Physician-assessed surface damage score (0 = none to 3 = severe for each body area) Patient-rated severity of itching (0 = no itching, 1 = slight itching, 2 = moderate itching, 3 = severe itching, 4 = very severe itching) Patient-rated sleep disturbance (0 = no sleep interruptions, 1 = sleep interrupted once or twice, 2 = sleep interrupted three or four times, 3 = sleep interrupted more than five times, 4 = unable to sleep) Clinical investigations: full blood count, serum bilirubin, aspartate aminotransferase, alkaline phosphatase, albumin, urea and electrolytes, creatinine, calcium, phosphate, glucose, creatine phosphokinase, immunological markers (IgE, eosinophil count, eosinophil cationic protein, IL-5, IL-13), blood pressure and weight |
Patients were also asked to report any side effects. Patients showing persistent abnormalities in blood chemistry would be removed | Decrease in total lesion score in the treatment group at 8 weeks was significantly greater than that in the placebo group (p < 0.001). There was a significant difference between groups with regard to erythema, surface damage, pruritus and sleep scores in favour of the treatment group. This difference was still significant at the 12-week follow-up (4 weeks after treatment ended) for all outcome measures except erythema. No side effects were reported | Clear description of randomisation. Allocation concealment not completely clear. Both physicians and patients were blinded to treatment. Intention-to-treat population was clearly stated and analysis was carried out on this population | |
| Choi 2012433 | TJ-15 (2.5 g), a traditional Chinese herbal compound (S. baicalensis root 3.0 g, G. jasminoides fruit 2.0 g, C. chinensis rhizome 2.0 g and P. amurense bark 1.5 g) (Tsumura Co. Ltd). Doses were adjusted to the patient’s weight. Ingested 90 minutes after a meal, three times a day (total of 7.5 g a day) for 4 weeks | TJ-15 (1.25 g) plus TJ-17 (1.25 g) (another traditional herbal compound composed of A. orientalis root tuber 4.0 g, P. cocos mycelium 3.0 g, A. lancea rhizome 3.0 g, C. cassia branch 1.5 g and P. umbellatus mycelium 1.5 g) (Tsumura Co. Ltd). TJ-15 and TJ-17 were mixed and ingested 90 minutes after a meal, three times a day (total of 7.5 g a day) for 4 weeks | Certain dietary and environmental recommendations were made and moisturising methods were suggested for all participants. Participants were allowed to use only emollients, lotions and ointments that did not contain topical corticosteroids during the trial | Korea | 60 participants screened, 24 participants randomised | Atopic dermatitis diagnosed according to the Hanifin and Rajka8 criteria; diagnosed with the ‘Dampness-Heat’ pattern type of atopic dermatitis according to pattern identification by a traditional Korean medicine dermatology specialist; four of the following nine criteria had to be fulfilled: rapid change in signs and condition, excessive itching, vesicles, oozing, discomfort during defecation, gastric bloating and distension, red-brownish urine and discomfort when urinating, thickly coated and slippery red tongue, rapid pulse | (Primary) SCORAD score (Secondary) EASI score (Secondary) Symptoms measured by pattern identification/symptom differentiation (Secondary) Clinical manifestations of symptoms including erythema, oedema/papule, oozing/crust, excoriation, lichenification and dryness were recorded as secondary end points. Severity was grouped into severe = 3, moderate = 2, mild = 1 and absent = 0 Safety assessed by laboratory tests including AST, ALT, blood urea nitrogen and creatinine |
SCORAD and EASI scores showed greater improvement in the TJ-15 plus TJ-17 group than in the TJ-15 alone group (not statistically significant). Symptoms related to the Dampness-Heat pattern were reduced in both groups. No abnormalities were observed in AST, ALT, blood urea nitrogen or creatinine | Randomisation described. Allocation was stated to be concealed but the method was not clearly described. The study was double blind. No intention-to-treat analysis described | |
| Farina 2011439 | One or two daily baths of 20 minutes each with total immersion in an individual bathtub with Comano water heated to 36–37°C | Topical corticosteroid treatment (the use of methylprednisolone aceponate for those aged < 2 years or mometasone furoate for those aged > 2 years) applied once daily for 2 weeks | During the treatment period, any other treatment was prohibited apart from emollients | 104 participants (n = 54 balneotherapy group, n = 50 topical corticosteroid group) | Age 1–14 years; diagnosis of mild to moderate atopic dermatitis (IGA < 3); SCORAD score of < 40 | Atopic dermatitis severity using IGA on a 6-point scale (0 = no disease, 1 = almost absent, 2 = mild, 3 = moderate, 4 = severe and 5 = very severe) SCORAD score PGSA Intensity of pruritus (VAS 0–100) CDLQI Family Dermatitis Impact questionnaire Adverse events |
Treatment adhesion and pleasantness 4 months after the end of treatment, patients were asked again about course and severity, (PGSA and VAS pruritus, CDLQI and FDIQ). They were also asked about the use of topical corticosteroids in the last 4 months |
Week 2: balneotherapy and topical corticosteroid use resulted in a significant reduction of all parameters. SCORAD score (mean ± SD): balneotherapy group 26% ± 9.4%, topical corticosteroid group 46% ± 7.71% (p < 0.03). IGA, PGSA, CDLQI and FDIQ showed similar improvements. Month 4: the number and duration of relapses were less in the balneotherapy group than in the topical corticosteroid group | Method of randomisation described. No description of allocation concealment. Open-label study so no parties were blinded. Intention-to-treat analysis not described, although all participants completed the trial | |
| Henderson 2000434 | Chinese herbal therapy (preparation PSE101), four teabags per day | Chinese herbal therapy (preparation PSE222), four granule sachets per day | After reconstitution, both interventions had the same concentration of herbal extracts | Not stated | 32 participants (n = 16 PSE101 group, n = 16 PSE222 group) | Persistent moderate to severe atopic eczema; age 17–60 years (average 29.8 years); no change to routine treatment permitted; all systemic steroids, immunosuppressive drugs and phototherapy stopped ≥ 4 weeks before the study; using contraceptives if of childbearing age | Eczema severity (0–3 for redness and surface damage – vesiculation, crusting, excoriations, lichenification and involvement; 20 body areas scored for each with a total max. score of 180) Changes in activated lymphocyte levels |
Erythema and surface damage significantly reduced for both treatment groups at treatment end (8 weeks) | Method of randomisation not described in detail. Allocation concealment not stated. Intention-to-treat analysis not described | |
| Hon 2007430 | Traditional Chinese herbal medication [2 g of F. lonicerae, 1 g of H. menthae (Bohe), 2 g of C. moutan (Danpi), 2 g of R. attactylodis (Cangzhu), 2 g of C. phellodendri (Huangbai)], three capsules twice daily for 12 weeks | Placebo (cornstarch and caramel), three capsules twice daily for 12 weeks | Placebo was assessed by a panel using visual and tactile observations. The results of the panel suggested that the placebo met a defined quality and reached the sensory specification consistent with the herbal capsule. The placebo was not considered to exert any beneficial or adverse effects on atopic eczema | 85 participants (n = 42 traditional Chinese herbal medicine group, n = 43 placebo group) | Diagnosis of atopic eczema based on the Hanifin and Rajka criteria;8 age 5–21 years; SCORAD score of > 15 (moderate to severe disease) | (Primary) Changes in total SCORAD score (Primary) CDLQI at the end of treatment Adverse events Compliance with trial medication (counting and weighing of trial medication) Coexisting allergic rhinitis (allergic rhinitis score – symptoms of sneezing, watery rhinorrhoea, nasal congestion, itching nose, itching eyes and eye watering) |
Mean SCORAD score over 12 weeks: no significant difference between groups. Quality of Life (CDLQI): herbal medication group significantly improved compared with the placebo group at the end of treatment (p = 0.008) and 4 weeks after stopping treatment (p = 0.059). Amount of topical corticosteroid used: herbal medication group significantly reduced by a third (p = 0.024) | Method of randomisation described and good. Allocation concealment partly described. Intention-to-treat population used for the analyses but no definition given | ||
| Klövekorn 2007436 | Herbal cream containing alcohol-based extracts of M. aquifolium (Berberis aquifolium), V. tricolor and C. asiatica (5 g of each extract/100 g of ointment). A thin layer was applied twice daily to all affected areas for 4 weeks | Base (vehicle) cream. A thin layer was applied twice daily to all affected areas for 4 weeks | Southern Germany | 88 participants | Caucasian men and women; age between 18 and 65 years; mild to moderate atopic dermatitis diagnosed according to the Hanifin and Rajka8 criteria; severity measured according to the Rajka and Langeland228 criteria (score 3–7); severity of test sites (both elbow flexures or, if not, knee flexures) must differ by > 2 points on the severity summary score for the primary end point | (Primary) Summary score for erythema, oedema/papulation, oozing/crust, excoriation, lichenification of the target area (each symptom scored 0 = absent, 1 = mild, 2 = moderate, 3 = severe) (Secondary) Participant-assessed global assessment of effectiveness (6-point verbal scale, with 1 = symptoms completely resolved, 2 = symptoms markedly improved, 3 = symptoms moderately improved, 4 = symptoms slightly improved, 5 = symptoms unchanged, 6 = symptoms worse) (Secondary) Physician-assessed global assessment of effectiveness (6-point verbal scale, with 1 = symptoms completely resolved, 2 = symptoms markedly improved, 3 = symptoms moderately improved, 4 = symptoms slightly improved, 5 = symptoms unchanged, 6 = symptoms worse) (Secondary) Pruritus severity of the target area (10-cm VAS from 0 cm = no itch, 10 cm = worst itch imaginable) (Secondary) Individual Erythema, oedema/papulation, oozing/crust, excoriation, lichenification scores Adverse events Participant- and physician-assessed overall tolerability (4-point verbal scale, with 0 = excellent, 1 = good, 2 = moderate, 3 = poor) |
Primary and secondary end points not statistically significantly difference for herbal cream compared with vehicle cream. Post hoc subanalysis of participants treated when the outside mean temperature was ≤ 10°C (n = 64) showed a highly significant difference between the groups in the primary end point in favour of the herbal cream group | Method of randomisation described and adequate. Allocation concealment unclear. Intention-to-treat population used in the analysis; however, no exact definition given | ||
| Kobayashi 2010437 | Hochu-ekki-to (contains 10 species of medicinal plants; see comments), 7.5 g (6.4 g of hot water extracts). Treatment given twice a week for 24 weeks | Inactive placebo given twice a week for 24 weeks | Medicinal plants in Hochu-ekki-to: Ginseng radix (4.0 g), Atractylodis rhizoma (4.0 g), Astragali radix (4.0 g), Angelicae radix (3.0 g), Zizyphi fructus (2.0 g), Bupleuri radix (2.0 g), Glycyrrhizae radix (1.5 g), Zingiberus rhizoma (0.5 g), Cimicifugae rhizoma (1.0 g), Aurantii nobilisPericarpium (2.0 g) | Not stated | 91 participants (n = 43 Hochu-ekki-to group, n = 48 placebo group); 84 participants received medication and were analysed (n = 40 Hochu-ekki-to group, n = 44 placebo group); 77 participants completed the treatment course (n = 37 Hochu-ekki-to group, n = 40 placebo group) | Fulfilled the diagnostic criteria of the Japanese Dermatological Association for atopic eczema; age 20–40 years; Kikyo (delicate) constitution [≥ 18 points on the Kikyo questionnaire, including one major sign (easy fatigability or lack of perseverance = 10) and, 10 minor signs (2 points each) (susceptible to cold, delayed recovery from cold, vulnerable to other infectious diseases, susceptible to suppuration, recent very little eating, appetite loss, easily full stomach, refusal of food, diarrhoea, easy drowsiness after meals)] | Skin severity score (Atopic Dermatitis Severity Evaluation Committee of the Japanese Dermatological Association – erythema/acute papules, oozing/crusts and excoriations, lichenification/chronic papules and nodules each measured on a scale of 0–3 at each of five body sites; max. score 60 points) Amount of topical agents used Adverse events Laboratory values – IgE, lactate dehydrogenase and eosinophil counts Prominent efficacy (defined as skin severity score = 0) Aggravated rate (defined as a > 50% increase in use of topical agents since the beginning of the study) |
Total equivalent amount of topical agents (steroids or tacrolimus): significantly (p < 0.05) less in the Hochu-ekki-to group compared with the placebo group. Overall skin severity score: not statistically different between groups. Prominent efficacy rate (skin severity score = 0): Hochu-ekki-to group 7/37 (19%), placebo group 2/40 (5%). Aggravated rate (a > 50% increase in use of topical agents compared with baseline): Hochu-ekki-to group 1/37 (3%), placebo group 7/39 (18%). No statistical difference between groups for adverse events (only mild adverse events occurred) | Method of randomisation unclear. Allocation concealment reported but the methods not explained. Full analysis set used for the analysis and defined | |
| Lee 2012427 | Acupressure with standard care – subjects were taught to perform acupressure techniques using a 1.2-mm titanium acupellet (Lhasa OMS brand) at the large intestine 11 (LI11 located on the left arm lateral to the antecubital fossa) pressure point. Pressure was to be applied for 3 minutes, three times a week for 4 weeks | Control with standard care. No further details provided | All participants were encouraged to continue using prescription/over-the-counter medications or lotions | Not stated | 15 participants were enrolled (n = 8 acupressure group, n = 7 control group) | Adults; pruritic atopic dermatitis | Severity of itch using a 10-cm VAS IGA survey measured on a scale from 0 (clear) to 5 (very severe disease) EASI survey (score calculated from area of involvement, erythema, thickness, excoriations and lichenification) |
VAS – change between baseline and follow-up: acupressure group: significant decrease (p = 0.05), control group: no significant change. EASI lichenification – change between baseline and follow-up: acupressure group: significant decrease (p = 0.03). No significant change in overall EASI score Comparison between groups: greater decrease in VAS score in the acupressure group than in the control group (p = 0.04), greater decrease in IGA in the acupressure group than in the control group (p = 0.03), greater decrease in EASI lichenification in the acupressure group than in the control group (p = 0.03) |
||
| Pfab 2011426 | Acupuncture. Individual acupuncture points were chosen always including Quchi (LI 11), Hegu (LI 4), ZuSanLi (St 36) and Xuehai (Sp 10). Stainless steel needles (0.25 × 40 mm) were inserted by 2–3 cm for a period of 20 minutes. Acupuncture treatments were carried out twice a week for 33 days (10 sessions of acupuncture) | No acupuncture, study examination visits only at baseline, day 15 and day 33 | Patients were advised to continue their topical therapy as before the study. Potentially systemically active agents were not allowed. Topical class I and II corticosteroids were allowed | Not stated | 10 participants | History of atopic eczema; age > 10 years; SCORAD score of > 20; allergic rhinitis with sensitisation to P. pratense and D. pteronyssinus | Severity of eczema measured by SCORAD score Itch rated on a VAS between 0 and 100 Blood samples to measure basophil activation |
Mean itch intensity (VAS) was rated significantly lower in the acupuncture group than in the control group | No description of randomisation process or allocation concealment. Only the observer was blinded. Not reported whether intention-to-treat analysis was carried out | |
| Schempp 2003425 | St John’s wort (H. perforatum L.) cream 1.5% applied to the skin on the appropriate side of the body twice daily for 4 weeks | Placebo (vehicle) cream applied to the skin on the appropriate side of the body twice daily for 4 weeks | Placebo and St John’s wort creams were colour matched for blinding of treatment | Not stated | 21 participants | Age 12–59 years; diagnosis of subacute atopic dermatitis of limited extent (SCORAD score of < 80); willing to apply study medications according to the instructions; willing to complete self-assessment scales; willing to comply with the study conditions | (Primary) Clinical intensity of skin lesions (modified SCORAD score with subjective pruritus and sleep loss scores excluded – extent and intensity of erythema, papulation, crust, excoriation, lichenification and scaling, each scored on a 4-point scale, with 0 = none, 1 = mild, 2 = moderate, 3 = severe) (Secondary) Bacterial colonisation of skin lesions (4-point scale: ‘excellent’, ‘good’, ‘moderate’, ‘poor’) (at day 0 and day 28) (Secondary) Participant-assessed skin tolerance (4-point scale: ‘excellent’, ‘good’, ‘moderate’, ‘poor’) (at day 7, day 14 and day 28) (Secondary) Participant-assessed cosmetic acceptability of study medication (at day 7, day 14 and day 28) |
St John’s wort cream group significantly improved compared with the vehicle group at all visits (days 7, 14 and 28) (p < 0.05) | No descriptions of the method of randomisation or allocation concealment are given. Intention-to-treat population is well defined and used for the analyses | |
| Senser 2004428 | Hypnotherapy, 1 hour each session for 12 sessions | No treatment | Germany | n = 15 treatment group, n = 18 control group [following newspaper advertisement (volunteers)] | Participants diagnosed with atopic dermatitis by their dermatologist | SCORAD score DLQI Marburger Neurodermitis-Fragebogen (quality of life) Pruritus Scratch intensity Subjective skin condition (diaries, VAS) |
Participants in the hypnotherapy group showed a highly significant improvement in all variables (p < 0.01). SCORAD score: pre/post comparison showed 32% worsening of SCORAD values in the control group and 40% improvement of in the treatment group (highly significant). DLQI: pre/post comparison shows significant improvement in the treatment group and significant worsening in the control group. Quality of life: pre/post comparison showed around a 26% improvement in quality-of-life symptoms in the treatment group and not much change in the control group – no difference pre therapy but highly significant change post therapy. Pre/post comparison showed a worsening of pruritus, scratch intensity and subjective skin condition in the control group and an improvement in those in the treatment group (highly significant) | No description of randomisation procedure. No description of investigator blinding | ||
| Shapira 2005431 | Tri-herbal formulation containing Siberian ginseng (Eleutherococcus), A. millefolium and L. album taken orally three times per day for 2 weeks | Placebo taken orally three times per day for 2 weeks | Participant told not to change topical treatment. Moisturisers were allowed, as were topical corticosteroids if a participant was already using them before the study. Topical immunomodulators were not permitted. Participants were withdrawn if an eczema exacerbation called for oral steroids or they had a severe side effect | Not stated | 49 participants (after withdrawals: n = 22 tri-herbal cream group, n = 22 placebo group) | Moderate atopic eczema, defined as itchy skin plus three out of four of (i) flexor surface involvement, (ii) asthma, hay fever or atopia in a first-degree relative, (iii) dry skin and (iv) signs of eczema before the age of 2 years; age > 1 year | Severity of eczema (SCORAD score) | Response to treatment: tri-herbal medication – significant response for the objective and subjective scores. Participants were maintained in partial remission until the end of the follow-up. The placebo group also had a similar response. There was no significant difference between the two groups | Method of randomisation and allocation concealment not stated. Intention-to-treat analysis not reported | |
| Shi 2008262 | Loratidine (10 mg) and modified Jiawei Danggui Decection. Loratidine: one dose per day for 4 weeks; modified Jiawei Danggui Decection: dose not stated | Loratidine (10 mg), one dose per day for 4 weeks | Not stated | Not stated | 47 participants [n = 25 combined treatment group, n = 22 control (loratidine-only) group] | Atopic dermatitis according to the UK Working Party criteria;9 meeting a specific type of constituent (脾虛氣躁) according to the theory of traditional Chinese medicine | Interleukin 4, 10 and 12 levels SASSAD score |
Total effective rate: 14/25 (56%) in the combined group and 5/22 (22.7%) in the control group had a ≥ 70% improvement in SASSAD score. Difference between groups was significant (χ2 = 5.38, p < 0.05) | Method of randomisation described and adequate. Allocation concealment and blinding not reported | |
| Siebenwirth 2009435 | Individualised homeopathic remedies – alcoholic dilutions (potency LM6, LM12, LM18, LM24 and LM30) and saccharose globule (potency D6, D12, C30 and C200). Treatment given for 32 weeks (untreated baseline period of 4 weeks) | Placebo alcoholic dilutions or globule, individualised. Treatment given for 32 weeks (untreated baseline period of 4 weeks) | Interventions are individualised, therefore not reproducible results | Not stated | Not stated | Diagnosis of atopic eczema according to the Hanifin and Rajka8 criteria; age between 18 and 35 years; at end of baseline phase > 20% of skin surface affected; had the condition for at least 1 year; no treatment with corticosteroids (topical or systemic) or antihistamines or other therapies during the baseline phase | Costa and colleagues’249 multiparameter atopic dermatitis score (1–100) ADASI score SCORAD score Global assessments of treatment success Freiburger list of complaints (changes in subjective state – 78 questions regarding physical complaints) Marburger Neurodermitis-Fragebogen (113 questions on a 5-point scale, with 1 = not affected to 5 = very strongly affected) Global subjective assessment provided by patients as well as physician |
Also measured contacts with therapist | Individualised homeopathic remedies were not superior to placebo | Method of randomisation reported. Allocation concealment not clear. Double-blind study. Unclear whether intention-to-treat analysis was used |
Appendix 18 Other interventions not covered elsewhere
| Trial | Intervention A | Intervention B | Comments on interventions | Country | Number of participants randomised | Inclusion criteria | Outcomes | Comments on outcomes | Main reported results | Quality of reporting |
|---|---|---|---|---|---|---|---|---|---|---|
| Kawana 2010443 | Tandospirone citrate 10 mg three times per day for 4 weeks | No treatment | Participants were allowed to continue using oral antihistamines or topical corticosteroids if they were already using them before the study | Japan | 37 participants (n = 20 tandospirone citrate group, n = 17 no treatment group) | Inpatients or outpatients at the dermatology department, Nippon Medical School, Tokyo, Japan | Participant-assessed mood states (previous 3 days) using POMS (65 questions that measure six scales: tension–anxiety, depression–dejection, anger–hostility, fatigue, confusion, vigour) Severity of eczema (SCORAD score) Lost of sleep (VAS) (1 = slept very well, 10 = could not sleep at all) Mental stress (VAS) (1 = no sense of stress, 10 = intolerable stress) |
POMS: took all 37 participants with atopic eczema and compared their scores with those of 37 imaginary healthy controls with standard mood profiles estimated from a large number of healthy volunteers using mood profile conversion charts table with separate gender and age rankings | POMS score (tension and anxiety) and SCORAD score: significantly decreased for participants in the tandospirone citrate group but did not change significantly for untreated participants. Changes in tension–anxiety scores showed that the two groups responded significantly differently. Significant correlation between changes in the tension–anxiety scores and SCORAD scores. | Method of randomisation reported but not clear. Allocation concealment not reported. Intention-to-treat analysis not reported |
| Kief 2007441 | Modified autologous blood treatment (AHIT® preparation 02/4 – a cell suspension of autologous blood culture that was produced according to a patent) subcutaneously and orally, twice a week. Increase in dose and concentration for the treatment time of 1 year, followed by 1-year observational period | Standard autologous blood treatment with participant’s own blood plus sodium chloride solution with 1% benzyl alcohol for subcutaneous application, together with base solution containing water and lactic acid racemate (latter for oral use), twice a week for 2 years. Increase in dose and concentration for the treatment time of 1 year, followed by 1-year observational period | Germany | 86 participants (n = 44 AHIT group, n = 42 own blood group) | SCORAD ≥ 25; age 12–65 years; Hanifin and Rajka8 criteria; leading dermatologists made diagnosis | SCORAD score DLQI score Amount of eosinophils and total IgE Rhinomanometry and corneometry |
No side effects. Eosinophils and total IgE decreased in both groups. Rhinomanometry and corneometry: no difference comparing pre/post results. Statistically significant decrease in SCORAD score and increase in DLQI score in both groups (intergroup difference but not between groups). Good tolerability for both groups | Very bad methodological quality | ||
| Malekzad 2009444 | Naltrexone 25 mg twice daily for 2 weeks | Placebo, twice daily for 2 weeks | All participants were told to continue with their previous medication throughout the study | Not stated | 38 participants. (n = 20 placebo group, n = 18 naltrexone group) | Age 24–85 years; pruritus associated with atopic eczema; no opiate dependency or use in previous 2 weeks; no evidence of any acute or chronic liver or renal disease | Severity of pruritus (VAS on scale of 0–10) Adverse events |
Severity of pruritus: naltrexone was significantly more effective than placebo in decreasing VAS score after 1 week (p < 0.005) and 2 weeks (p < 0.001). Naltrexone might be considered as an adjunct treatment of pruritus | Description of randomisation and allocation concealment not provided. Intention-to-treat analysis not described | |
| Pittler 2003442 | Analogous blood therapy. Baseline = 1 ml of the participant’s own venous blood, week 1 = 2 ml, week 2 = 3 ml, week 3 = 2 ml, week 4 = 1 ml | Placebo therapy. Baseline = 1 ml of saline, week 1 = 2 ml, week 2 = 3 ml, week 3 = 2 ml, week 4 = 1 ml | All participants encouraged to continue using their normal treatment during the trial | UK | 31 participants. (n = 15 analogous blood therapy group, n = 15 placebo group; one participant dropped out – reason not given) | Age 16–65 years; non-excudative eczema according to the criteria of Hanifin and Rajka8 | (Primary) Severity of eczema (SASSAD) (max. score 108) (Secondary) DLQI score (Secondary) Participant-assessed pruritus (100-mm VAS) (Secondary) Participant-assessed quality of sleep (100-mm VAS) (Secondary) Skin appearance (100-mm VAS) Use of topical treatment Overall effectiveness and tolerability (5-point scale – ‘very good’ to ‘very poor’) |
Success of participant blinding also assessed | SASSAD score: mean reduction above placebo from baseline to week 9 (end of follow-up) 13.5 (95% Cl 6.6 to 20.4) (p < 0.001). Secondary outcomes: no significant differences between the groups for all secondary outcomes. Minor, transient adverse events: analogous blood therapy group n =6 participants, placebo group n = 7 participants | Method of randomisation and allocation concealment described. Intention-to-treat principle used for the analyses |
| Ramírez-Bosca 2012446 | PL extract capsules (Anapsos, 120-mg capsules). Patients were grouped by age: children aged < 6 years received two capsules in a single dose at night (240 mg/day); children aged 6–12 years received three capsules in two doses, one in the morning and two at night (360 mg/day); children aged > 12 years received four capsules in two doses, two in the morning and two at night (480 mg/day). The regime was taken for 6 months | Placebo capsules made by the same manufacturing company that made the PL capsules. Children aged < 6 years received two capsules in a single dose at night; children aged 6–12 years received three capsules in two doses, one in the morning and two at night; children aged > 12 years received four capsules in two doses, two in the morning and two at night. The regime was taken for 6 months | Methylprednisolone aceponate 0.1% in emulsion was applied in a thin layer on affected areas to treat flares. Dry skin was treated with moisturising cream after showering or bathing. Patients who required systemic corticosteroids were given deflazacort (0.25–1.5 mg/kg/day). Pruritus was treated with desloratadine (low sedating so less likely to affect the sleep loss component of the SCORAD index) | Spain | 105 patients randomised (n = 52 PL group, n = 53 placebo group) | Age between 2 and 17 years (inclusive); diagnosis of atopic dermatitis according to the Hanifin and Rajka8 criteria; SCORAD score of 20–40 (inclusive); requires or already using topical corticosteroids to treat flares | (Primary) Percentage of days on which topical corticosteroids were used in the 6 months of the trial (Secondary) Change in SCORAD index 1, 2, 3, 4, 5 and 6 months after the start of treatment (Secondary) Change in IGA 1, 2, 3, 4, 5 and 6 months after the start of treatment (Secondary) Time to first flare (time from randomisation to appearance of a new flare (Secondary) Changes in the incidence of flares (based on mean number of flares reported for the 2 months before the baseline visit) |
A flare was defined as an exacerbation that could be quantified, following evaluation of the patient’s diary, physical examination and an interview with the patient or tutor, with an IGA score of ≥ 4 or that required the use of topical corticosteroids for at least 3 days | PL extract use did not significantly reduce the mean (SD) percentage of days on which topical corticosteroids were used: PL group 11% (SD 12%), placebo group 12% (SD 11%). There was a significant reduction for oral histamine use (median percentage of days): PL group 4.5%, placebo group 13.6% (p = 0.038). The percentage of patients who used oral antihistamines was also lower in the PL group | Randomisation was computer generated. Allocation concealment not described. Study was double blind but blinding was not described. Intention-to-treat analysis was used as well as per-protocol analysis |
Appendix 19 Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2009 checklist
| Section/topic | Number | Checklist item | Reported on page |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis or both | Title page |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including, as applicable, background; objectives; data sources; study eligibility criteria, participants and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number | v, vi |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | xxxix, 7 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes and study design (PICOS) | 8 + 9 |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists and if and where it can be accessed (e.g. web address) and, if available, provide registration information including registration number | Previous HTA review, 11 |
| Eligibility criteria | 6 | Specify study characteristics (e.g. PICOS, length of follow-up) and report characteristics (e.g. years considered, language, publication status) used as criteria for eligibility, giving rationale | 11 + 12 |
| Information sources | 7 | Describe all information sources (e.g. databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | 14 + 15 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | Appendix 1 |
| Study selection | 9 | State the process for selecting studies (i.e. screening, eligibility, included in systematic review and, if applicable, included in the meta-analysis) | 15 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g. piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | 16 |
| Data items | 11 | List and define all variables for which data were sought (e.g. PICOS, funding sources) and any assumptions and simplifications made | 12–14 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was carried out at the study or outcome level) and how this information is to be used in any data synthesis | 16 |
| Summary measures | 13 | State the principal summary measures (e.g. risk ratio, difference in means) | Not applicable |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if carried out, including measures of consistency (e.g. I2) for each meta-analysis | Not applicable |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g. publication bias, selective reporting within studies) | Not applicable |
| Additional analyses | 16 | Describe methods of additional analyses (e.g. sensitivity or subgroup analyses, meta-regression), if carried out, indicating which were prespecified | Not applicable |
| Results | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | 22 + 23 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g. study size, PICOS, follow-up period) and provide the citations | Main body of report, 25–214 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome-level assessment (see item 12) | |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group, (b) effect estimates and confidence intervals, ideally with a forest plot | Not applicable |
| Synthesis of results | 21 | Present results of each meta-analysis carried out, including confidence intervals and measures of consistency | Not applicable |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15) | Not applicable |
| Additional analysis | 23 | Give results of additional analyses, if carried out [e.g. sensitivity or subgroup analyses, meta-regression (see Item 16)] | Not applicable |
| Discussion | |||
| Summary of evidence | 24 | Summarise the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g. health-care providers, users and policy makers) | 215–218 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g. risk of bias) and at review level (e.g. incomplete retrieval of identified research, reporting bias) | 220–223 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence and implications for future research | 219–222 |
| Funding | 223–225 | ||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g. supply of data) and the role of funders for the systematic review | iii |
Glossary
- Abstracted
- Pulling out essential information from published trial reports.
- Allocation
- Assignment to a treatment group.
- Atopy
- Predisposition to mount an excessive immune response.
- Balneotherapy
- Salt bathing.
- Bias
- Factors that may alter the outcome of a study.
- Blind
- When treatment allocation is unknown by the people taking part and the researchers conducting the trial. A trial can be double blind (whereby neither participants nor investigators know the treatment allocation) or single blind (whereby only one of these knows the treatment allocation).
- Calcineurin inhibitors
- Non-steroidal treatments that block a chemical that activates inflammation.
- Dermatoses
- Skin conditions.
- Emollient
- Non-cosmetic moisturisers that are designed to prevent and treat dry skin.
- Erythema
- Redness of the skin.
- Excoriations
- Destruction or removal of skin from scratching.
- Folliculitis
- Infection of the hair follicles.
- Genotype
- Genetic make-up of a person.
- Impetigo
- Contagious skin infection.
- Intention-to-treat analysis
- An assessment of participants according to their initial treatment assigned regardless of other factors (such as whether they dropped out or switched treatments).
- Intertriginous regions
- When areas of skin come into contact with each other, for example between the toes.
- Lysate
- Contents of cells.
- Meta-analysis
- The statistical combination of results from two or more separate studies.
- Nares
- Nostrils.
- Nasopharyngitis
- Common cold.
- Pityriasis alba
- Dry white patches.
- Pruritus
- Itching.
- Pulsed treatment
- Burst of continuous treatment with periods of no treatment in between.
- Pyrexia
- Fever.
- Random sequence generation
- Ensuring that there is an equal probability of being assigned to a control or a treatment group according to a predefined list.
- Randomised controlled trial
- A way to compare treatments – participants are randomly assigned to receive either the treatment being assessed or an alternative treatment, which may be a placebo or no treatment.
List of abbreviations
- AAD
- American Academy of Dermatology
- ADASI
- Atopic Dermatitis Area and Severity Index
- AMED
- Allied and Complementary Medicine Database
- CDLQI
- Children’s Dermatology Life Quality Index
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- DHA
- docosahexaenoic acid
- DLQI
- Dermatology Life Quality Index
- EASI
- Eczema Area and Severity Index
- EFA
- essential fatty acid
- EPA
- eicosapentaenoic acid
- GLA
- gamma-linolenic acid
- GP
- general practitioner
- GREAT
- Global Resource of EczemA Trials
- HOME
- Harmonising Outcome Measures for Eczema
- HTA
- Health Technology Assessment
- IDLQI
- Infant Dermatology Life Quality Index
- IGA
- Investigator’s Global Assessment
- IgE
- immunoglobulin E
- IQR
- interquartile range
- LILACS
- Latin American and Caribbean Health Sciences
- MRSA
- methicillin-resistant Staphylococcus aureus
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PGA
- Physician Global Assessment
- POEM
- Patient-Oriented Eczema Measure
- PUVA
- psoralen plus ultraviolet A
- RAST
- radioallergosorbent test
- RCT
- randomised controlled trial
- SASSAD
- Six Area, Six Sign Atopic Dermatitis
- SCORAD
- Severity Scoring of Atopic Dermatitis
- SD
- standard deviation
- SED
- standard erythemal dose
- SIGN
- Scottish Intercollegiate Guidelines Network
- TIS
- Three-Item Severity
- VAS
- visual analogue scale
- w/w
- weight by weight