Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0606-1048. The contractual start date was in August 2007. The final report began editorial review in July 2014 and was accepted for publication in September 2015. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Tylee et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Overview of the programme
We have previously published the rationale and protocol for the UPBEAT-UK programme,1 how we subsequently developed the intervention for the pilot randomised controlled trial (RCT) from the findings of the earlier work packages2 and the final protocol for the pilot RCT. 3 This chapter opens with a statement on patient and public involvement (see Patient and public involvement). We have then summarised the introductory sections of the published introductory papers1–3 (see Background), which has been updated with references to more recent literature. In Aims and objectives of the programme, the key objectives of the four work packages are outlined. Finally, all currently published papers from the UPBEAT-UK study are listed in Acknowledgments.
Patient and public involvement
We were able to recruit patient and public involvement representatives from mental health organisations (Service User Research Enterprise and Charlie Waller Memorial Trust), but we were unfortunately unable to recruit any patient representatives with coronary heart disease (CHD) to our programme over its duration, despite frequent attempts at contacting relevant organisations, such as the British Heart Foundation, through our research team members, including our cardiologist member. However, it would have been quite a commitment for a patient representative to give up 5–7 years and this may have been the limiting factor. This has been a disappointment, particularly as there could be an increasing role for providing such support from relevant third-sector organisations. The patient and public involvement initiative was in its infancy when these studies were set up; 10 years on we may have had a more positive response from patient-based voluntary organisations that would have become more familiar with the required task.
Background
Coronary heart disease and depressive disorders are two of the leading causes of burden of disease and disability, as measured by disability-adjusted life-years. It has been estimated, using data from the World Health Organization, that by 2030, unipolar depressive disorders and CHD will be the second and third leading cause of burden of disease, respectively, trailing only human immunodeficiency virus/acquired immunodeficiency syndrome. 4 Surprisingly, however, while there are several guidelines worldwide for the management of both conditions separately, there are no clear guidelines on how best to manage patients who have both comorbid conditions. A key recommendation of a specially convened US Preventive Task Force on the management of depression and CHD was to conduct RCTs of stepped care to generate much-needed evidence. 5 So far, RCTs have shown that treating depression in CHD slightly improves depressive symptoms and quality of life, but has no effect on mortality. 6 Furthermore, cardiac rehabilitation (CR) together with mental health treatments may reduce depression, CHD events and mortality risk. 7 Despite this, limitations in the current literature show that further research is needed to improve psychological and cardiac outcomes in these patients.
Coronary heart disease
Coronary heart disease, especially when chest pain is present, often causes functional limitation, distressing symptoms, is often life-threatening and requires long-term management, mostly in primary care. Many patients with CHD have a documented history of myocardial infarction (MI) or coronary artery disease shown at angiography. CHD also causes 70% of heart failure with fatigue and shortness of breath. CHD mainly comprises three groups of patients with: chronic stable angina (with exertional chest pain), post MI, and heart failure with a hierarchy of physical and emotional effects. The researchers at Imperial College London, on behalf of the Public Health Observatories of England, have developed a prevalence model. 8 They estimate the prevalence of CHD in primary care trusts and at local authority level to be 5.80%, although this number rises to 16.08% in people aged 65–74 years, and 21.91% in those ≥ 75 years. In the south London primary care trusts of Lambeth, Southwark and Lewisham, the prevalence is 3.30%, 3.50%, and 3.85%, respectively (≥ 15% in those aged 65–74 years and ≥ 22% in those ≥ 75 years). 8
Depression
Depression is a major public health problem responsible for around 100 million lost working days in England and Wales each year and costing £9B per annum. 9 In the UK, depression is a common reason for consulting a general practitioner (GP). Up to one-third of people who visit the GP have mental health problems, and 90% of those are treated only in primary care. 10
Depression incurs a 50% increase in the cost of long-term medical care after controlling for the severity of the physical illness,11 and this may relate to the link between depression and adverse health risk behaviours such as smoking, diet, lack of exercise and poor self-care. Depression may exacerbate the perceived severity of symptoms and this, in turn, can bring about an increase in health service utilisation. Treating depression and improving outcomes for depression has been shown to reduce health costs in people with physical illness. 11 Furthermore, collaborative care and individualised management of patients with depression and chronic conditions, such as diabetes and CHD, has shown to improve both medical outcomes and depression. 12
Depression and coronary heart disease comorbidity
Depression occurs in up to 20% of patients with CHD and depression increases the incidence and recurrence of CHD, acute coronary syndromes and mortality. 5 A systematic review by Nicholson et al. 13 places the pooled relative risk (RR) of future CHD associated with depression at 1.81; however, the authors stop short of calling depression an independent risk factor for developing CHD, owing to the heterogeneity of studies.
The clear association that exists between these two conditions has led to a long discussion about the precise nature of the relationship. Ageing (which increases the odds of both conditions), lifestyle factors, inflammation pathways, heart rate variability, impaired arterial repair and several genes, are just some of the mechanisms that could play a role. 14 Nevertheless, the link is well established. A review by the American Heart Association15 recently recommended that depression be considered an independent risk factor for adverse outcomes in patients with acute coronary syndrome, given the strength of the evidence in the current literature.
The presence of concurrent physical illness, such as CHD, is known to reduce the likelihood of major depression being recognised by GPs. 16 Diagnosing depression in elderly primary care patients is hampered by conditions such as heart disease and the drugs to treat these conditions, which can have mood-destabilising effects. 17 The natural history, morbidity and mortality of depression in primary care CHD populations are unknown.
As GP practices keep separate registers for their patients with heart failure, this programme of research is solely concerned with CHD and patients on CHD registers rather than patients on heart failure registers. This also means that the focus of research in terms of physical symptoms is purely on chest pain rather than on fatigue and dyspnoea.
Managing depression and coronary heart disease
Although there are several treatment options for managing depression in primary care that are endorsed by the National Institute for Health and Care Excellence (NICE)18 [e.g. antidepressant medication, supervised exercise, guided self-help, problem-solving, computerised cognitive–behavioural therapy (CBT), group or individual CBT or interpersonal therapy], the treatment preferences of CHD patients are unknown, as are primary care professional treatment preferences. A recent US working party on the management of depression in CHD concluded that RCTs comparing stepped depression care with treatment as usual (TAU) for patients with CHD and depression are needed. 5
It remains unclear how GPs and practice nurses (PNs) should best manage patients with depression and CHD. Previous research, which has been largely from the USA, has focused on treating depression with antidepressant medication, psychological treatment, case management and collaborative care. Because of the absence of available evidence in this area, we decided to conduct a systematic review and metasynthesis of available evidence in work package 1.
Medication and psychological treatment
Two large US-based trials19,20 have also provided an indication in post-hoc subgroup analyses that there may have been cardiovascular benefit from the management of the depression using antidepressant medication and it has been suggested that this may be owing to an effect on platelet activation. 21,22 Mortality seems to have been reduced in those whose depression improved or in those who took sertraline (Zoloft®, Pfizer) in one study. 23,24 This evidence was influential in the introduction of financial incentive payments to English GPs for screening consecutive CHD patients for comorbid depressive disorder under the General Medical Services Quality and Outcomes Framework (QOF), although this was abandoned in 2013.
Case management
Case management has been shown to improve outcomes for depression in primary health-care settings,25 but there has been no research to determine the cost-effectiveness in patients with CHD. Case management is ‘taking responsibility for following-up patients; determining whether patients were continuing the prescribed treatment as intended; assessing whether depressive symptoms were improving; taking action when patients were not adhering to guideline-based treatment or were not showing expected improvement’. 26 It consists of five essential components:25
-
identification of patients in need of services
-
assessing individual patient’s needs
-
developing personalised treatment plans
-
co-ordination of care
-
monitoring outcomes and altering care when favourable outcomes are not achieved.
Collaborative care
An early, large US-based multicentre study of stepped collaborative depression care showed positive results regarding depression in older people as did another US trial of collaborative depression care in diabetics in motivated patients. 27,28 However, the latter trial did not improve diabetic outcome. 28 Subsequently, during the life of the UPBEAT-UK programme, Katon et al. ,12 using collaborative care, were able to demonstrate in a groundbreaking study the improvement of depression, systolic hypertension, glycosylated haemoglobin and blood lipids. As they applied rigorous nurse care to the depression, hypertension, diabetes and lipid abnormalities, it is not clear to what extent the management of depression contributed to the improvement of the physical outcome measures. Since then, and again in the lifetime of the UPBEAT-UK programme, it has been demonstrated that collaborative care as a model works in an English setting for depressive disorder, albeit with a modest effect size. 29 Collaborative care usually requires the collaboration of psychiatrists or other mental health professionals working together with their primary care colleagues to supervise case management by dedicated case workers. These care workers are usually mental health professionals brought in for the purpose of overseeing the case management and liaising with the patient’s primary and secondary care workers.
The overall design of the UPBEAT-UK programme led to a pilot RCT in order to inform a future definitive RCT of the cost-effectiveness of PN-led personalised case management in primary care for patients with CHD and depression. A pilot RCT was necessary to determine whether or not case management by PNs for this population would be a feasible and acceptable intervention compared with TAU in terms of both depression and cardiac outcomes.
The first step was to judge the acceptability, feasibility and likely effect of case management to inform whether or not to conduct a future definitive RCT. As any future potential definitive RCT would be a complex intervention, it was necessary to follow Medical Research Council (MRC) guidelines30 for the development of a complex intervention using a programme of related work packages to develop and test a new nurse-led personalised case management practice for depression and CHD in primary care. The overall scientific framework for the UPBEAT-UK programme was the MRC framework for the development and testing of complex interventions. 30 This framework has four stages:
Stage 1: development concerns the identification of existing evidence in order to develop the intervention to a point where it can be expected to have a beneficial effect. Conducting a systematic literature review or metasynthesis is recommended if such a review does not already exist. This is followed by the identification and development of theory with a rationale for the proposed intervention and likely change process. The development of theory may build on existing evidence and be supplemented with new (often qualitative) research. This should then lead to a testable model with a specific intervention, process and likely outcomes.
Stage 2: feasibility and piloting involves assessing the feasibility and acceptability of the new complex intervention and of the evaluation methods, including acceptability, compliance, delivery, randomisation, recruitment, retention, and observed variability around changes in the primary outcome to inform the power calculation for a subsequent definitive RCT. The pilot RCT examines key criteria for a definitive RCT.
Stage 3: evaluation involves the evaluation of the intervention using appropriate design usually by a definitive RCT.
Stage 4: implementation involves the routine implementation of the new intervention, surveillance, monitoring and long-term follow-up.
Stages 1–4 should be seen as cyclical rather than linear, with results from any stage informing not just subsequent stages but previous stages in continuous improvement and increasingly higher-level evaluation. 30 The UPBEAT-UK programme mainly involves stages 1 and 2 outlined above.
The four inter-related UPBEAT-UK work packages are:
-
a review of previous work in the area and qualitative study of GP and PN treatment preferences for this patient group
-
a qualitative study of patients with CHD and comorbid depression treatment preferences
-
a pilot RCT of nurse-led case management for depressed primary care patients with CHD
-
a 4-year cohort study of patients with CHD.
In this introductory chapter, the detailed methods will be described in each work package chapter.
Aims and objectives of the programme
Work package 1
A review and metasynthesis of the existing literature and a qualitative study of health professionals’ perceptions of distress and depression in patients with CHD.
Objectives
-
To review and conduct a synthesis of existing literature on primary care management of CHD and depression.
-
To explore primary care professionals’ views on distress and depression in patients with CHD.
-
To explore their current management strategies and attitudes to a range of treatments in relation to this patient group.
-
To provide guidance on the design and implementation of a PN-led case management depression intervention.
Work package 2
Study of patients’ perceptions of distress and depression in patients with CHD.
Aims
The aim was to elicit patients’ perceptions of their psychological state as linked to their CHD and explore their views on appropriate treatments for distress or depression in the context of their CHD.
Sample
Up to 50 people were to be purposively sampled from the cohort study based on age, sex, practice, CHD status, and depression severity.
Work package 3
Pilot RCT of primary care case management for depressed patients with symptomatic CHD (sCHD).
Objectives
The objectives of this pilot were:
-
Clinical efficacy of case management.
To explore whether or not case management for primary care patients with sCHD and depression, when delivered by nursing professionals, may be more effective than TAU.
-
Sample size calculation.
To calculate estimates of the location of the mean and variability around the mean [standard deviation (SD)] for the primary outcome measure (depression).
-
To enable selection of the most appropriate primary and secondary outcome measures.
-
Integrity of the study protocol.
To test all procedures for a definitive effectiveness RCT, for example:
-
inclusion/exclusion criteria
-
training of staff in the administration and assessment of the intervention
-
to test data collection forms and questionnaires
-
to ensure the acceptability of the questionnaires to participants, along with comprehensibility, appropriateness, clarity and consistency
-
patient information documents and consent forms were also tested, as was inter-rater reliability between researchers.
-
-
Randomisation procedure.
To test the randomisation process and acceptability of randomisation to primary care professionals and participants.
-
Recruitment and consent.
To test the recruitment method and the consent rate for participants, and explore barriers to recruitment of both practices and participants.
-
To determine the acceptability of the intervention and the trial to practices and participants.
To determine the possible sources of contamination, and to develop a standardised manual for case management for use in the definitive RCT. To make an informal assessment of the degree to which the intervention can be standardised and whether or not therapist effects are likely to be a major factor.
Work package 4
Cohort study
Objectives
The objectives were:
-
to determine prevalence, incidence rate and risk factors of depression in primary care patients with CHD
-
to explore and describe the course, relationship, prognosis and current management of physical and depressive symptoms in primary care patients with CHD and comorbid depression over a 3- to 4-year period
-
to determine the effect of comorbid depression on mortality, symptom severity, quality of life, disability, pain, service use (at all levels) and service costs, and lost employment costs in primary care patients with CHD.
Chapter 2 General practitioners’ and practice nurses’ perceptions of distress and depression in patients with and without symptomatic coronary heart disease: literature review and qualitative interview study (work package 1)
Plan
In the previous, introductory, chapter, we described the existing evidence demonstrating putative mechanisms linking CHD and depression. We have described how depression has previously been associated with worse cardiac prognosis and an increased cardiac mortality. We described a need to better understand the relationship between the two disorders and a pressing global public health need to improve integrated primary care for people with both disorders.
In this chapter we describe how we gathered evidence from primary care professionals – GPs and PNs – concerning their current practice, views and experience of managing depression in people with CHD, which would chiefly be people who were included on their general practice-based QOF CHD registers. We also asked a sample of south London GPs and PNs how they currently managed such patients and if there was a need for an enhanced, probably PN-led, intervention in their own general practice for their registered CHD patients complaining of chest pain and depression and if so, what they would want from such a new stepped care intervention.
Prior to commencing this qualitative study, we searched for and synthesised existing qualitative and quantitative research in general practice on the management of people with depression and CHD and used this to inform the design of our own qualitative study, especially the topics for discussion. The evidence we gathered from the metasynthesis and both qualitative studies with professionals and patients would subsequently be used to inform the development of our UPBEAT-UK pilot/feasibility intervention according to MRC guidelines30 for the development of complex interventions. How the findings from these earlier studies informed the development of our feasibility/pilot study is described in Chapter 4.
Methods
Metasynthesis of published qualitative and quantitative research
Review aim
We considered that issues important to the effective primary care-based management of depression in general are likely be important when managing depression in people who also have CHD, but that there may also be additional factors to consider when both conditions are comorbid in an individual. By systematically investigating similarities and differences in previous findings across published studies of primary care professionals’ experience of managing depression, our metasynthesis could provide high-quality evidence to inform our proposed qualitative study focused on primary care professionals’ depression management for people with CHD. This review, therefore, aimed to identify barriers to and facilitators of the effective management of depression in people with or without comorbid physical health problems in primary care settings.
Review methods
Terms relating to depression, primary care and primary care professionals’ attitudes to depression were combined to form a search strategy (see Appendix 1), which was adapted for four databases (MEDLINE, EMBASE, PsycINFO and British Nursing Index and Archives). We also searched the reference lists of identified papers. This review was conducted early in the programme (search date 30 June 2008). We planned to use the findings to inform the GP and PN interviews reported in Qualitative interview study of general practitioners’ and practice nurses’ views and experience of managing depression in coronary heart disease. Hence we have not updated this review, but note that the paper in which the results were published31 has been highly cited. To obtain the most relevant and up-to-date evidence, we included only publications concerning studies that had been conducted in the UK and published after 2000. This was a pragmatic method of including a manageable number of studies and ensured we obtained data on current and relevant attitudes (2000 is after the publication of the National Service Framework for Mental Health32). We only included studies set in the UK as we felt that these would be most likely to inform an intervention that could be implemented in UK primary care; that is, studies conducted in other settings were excluded as we felt it would be difficult to understand the impact of different health-care delivery systems on their findings, which would impair their translation to UK primary care. Both qualitative and quantitative studies were included. We assessed the identified abstracts for relevance and then we quality rated them according to standard recognised criteria. 33,34 We did not use quality judgements to exclude papers but tested the strength of findings by examining whether or not they were supported by studies in the upper tertile of scores. 35 We extracted key themes and synthesised them using principles drawn from meta-ethnography24 and guidelines for producing narrative syntheses. 36
Review results
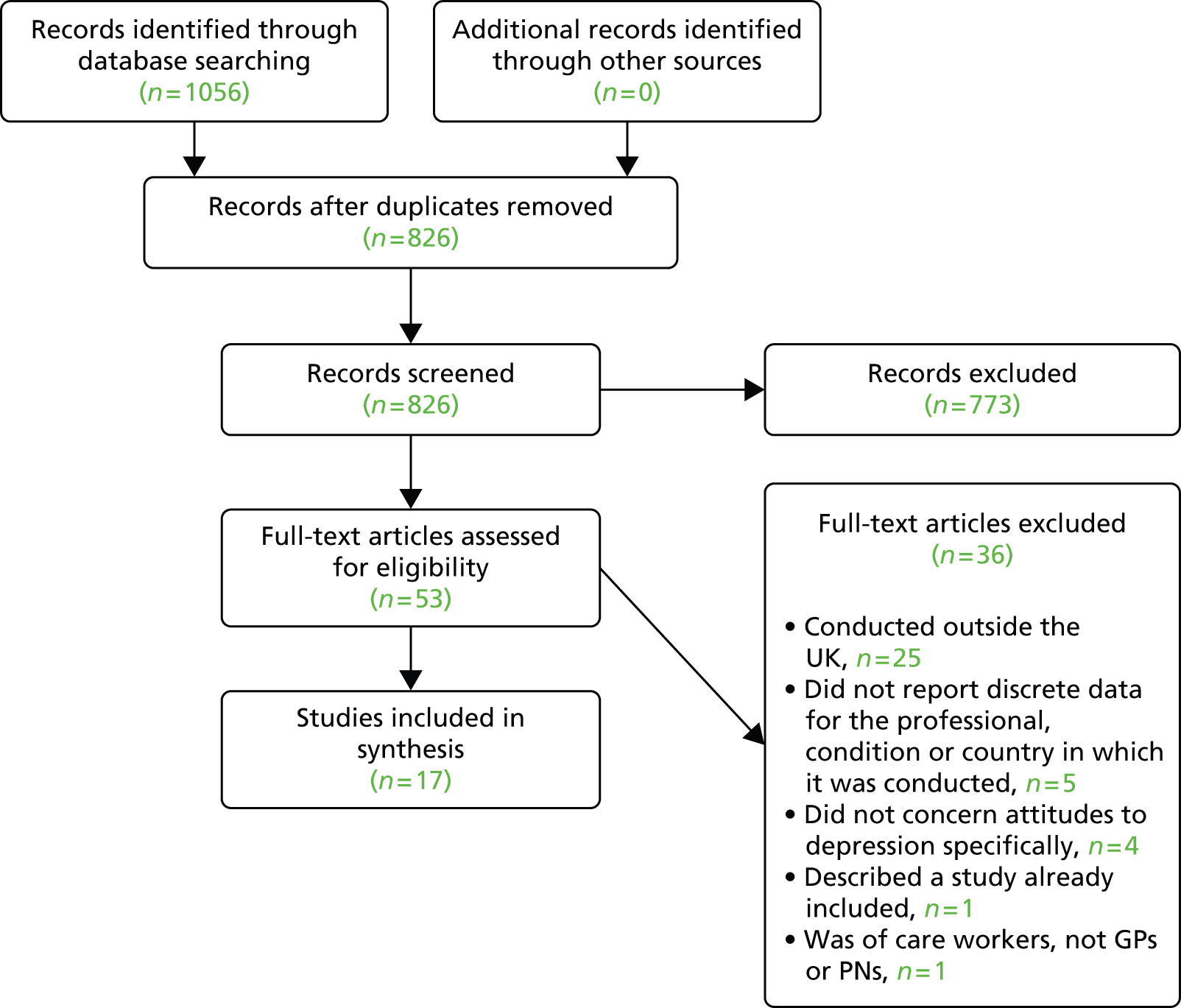
Our first finding was that no relevant paper concerning GP or PN views about depression and comorbid physical illness of any kind was identified, despite our use of a comprehensive search strategy. Seven qualitative37–43 and 10 quantitative studies of the management of primary depression were included. 44–53 Figure 1 shows the flow of studies through the review process.
FIGURE 1.
Flow of studies through review process. Reproduced from Barley et al. 31 © 2011 Barley et al; licensee BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Summary of review findings
Details of our metasynthesis findings have been published in an open access journal by Barley et al. 31 in 2011. Table 1 shows the included studies. The key themes and how they translate across the included studies are detailed in Table 2. Both tables were also published Barley et al. 2 In summary, we identified seven key themes, which were all supported by at least one good-quality study and by both qualitative and quantitative data. The first theme concerned professionals’ understanding of depression; depression was either seen as a normal response to life events, or biomedical explanations were given. The second theme concerned how clinicians recognise depression and highlighted how they struggle to distinguish between ‘normal’ distress and depression requiring treatment. Management options for depression made up the third theme, with clinicians expressing a preference for talking therapy over antidepressants, but also for being able to take a personalised approach. Shame and stigma around depression arose as a fourth theme; it was felt that this prevented some patients seeking help, but the authors of one study considered that this may be constructed to hide a reluctance to explore depression, with patients arising from a desire to avoid feelings of powerlessness when management options seem limited. A lack of interprofessional working within primary care was the main finding within our fifth theme. Our sixth theme showed that clinicians may have ambivalent attitudes to managing depression; this was reflected in our final theme which showed that although primary care clinicians felt that they needed more training in mental health, when this was offered, they did not take it up. Key findings in relation to these themes were that GPs and PNs consider depression and its diagnosis to be complex. There was ambivalence about the use of case finding tools in primary care settings, such as those that were recommended at the time for use in people with CHD and diabetes in the QOF. 56 However, most studies identified in our search did not discuss these as they had been conducted and published prior to the introduction of financial incentives under the QOF of the UK GP contract56 in 2006 when their use became routine.
| Reference | Participants (response rate) | Aim | Setting and sample selection | Methods of collecting clinician data and research perspective (quality assessment) |
|---|---|---|---|---|
| Qualitative studies | ||||
| Johnston et al., 200737 | 32 GPs (24%) | Identify issues of importance to GPs in depression management | 28 GP practices in and around Southampton (plus two GPs in Leicester) | Semistructured interviews; grounded theory (10/10) |
| Murray et al., 200638 | 18 GPs, 7 PNs | Identify perceptions of depression older people | 18 south London primary care teams in five Boroughs, purposive sampling based on setting (socioeconomic/ethnic groups served) and practice type | In-depth, semistructured interviews; grounded theory (9/10) |
| Burroughs et al., 200639 | 9 GPs, 3 PNs | Explore how primary care professionals and patients view late-life depression | One PCT in NW England, purposive sampling but criteria not stated | Semistructured interviews; constant comparison (7/10) |
| Maxwell et al., 200540 | 20 GPs | Explore GPs’ experience of recognising and managing depression | 11 practices from a ‘random’ sample of 55 in Scotland – four NHS board areas | Semistructured interviews; critical realist perspective (5/10) |
| Pollock and Grime, 200341 | 19 GPs | Investigate GPs’ views on consultation time and depression management | Eight West Midland practices – purposeful selection based on socioeconomic/geographical setting and patient list size | Semistructured interviews (8/10) |
| Chew-Graham et al., 200242 | 35 GPs in teaching practices (66%) | Explore GPs’ attitudes to the management of depression in deprived vs. affluent populations | 22 inner city GPs vs. 13 suburban and semirural GPs in NW England, purposive sampling based on practice size | Semistructured interviews; constant comparative qualitative analysis (9/10) |
| Rogers et al., 200143 | 10 GPs | Explore GPs’ views on depression management | Eight practices in Greater Manchester (inner city/suburban) | In-depth, semistructured interviews (5/10) |
| Quantitative studies | ||||
| Kendrick et al., 200544 | 17 GPs | Explore associations between GP treatment, depression severity and patient characteristics | Six GP practices in Southampton (nine practices approached) | Questionnaire (devised for this study) ratings of patient characteristics and GP treatment decisions completed following consultation (3/7) |
| Shiels et al., 200445 | Four GPs – three principals, one assistant | Compare GPs’ and male patients’ assessments of depression | One practice in a prosperous rural area of Cheshire | Questionnaire (devised for this study) completed following consultation (3/7) |
| Naji et al., 200446 | 442 PNs (56.2%) | Assess PNs’ knowledge, attitudes, training and management of depressed patients | One in two sample of Scottish general practices (428 practices) | Questionnaire – DAQ54 plus some questions developed for this study, postal survey (5/7) |
| Manning and Marr, 200347 | 202 GPs (50%) | Compare expectations of GPs and patients in the management of relapse of depression | GP practices ‘across the UK’ | Questionnaire – devised for this study by a market research company, postal request sent with link to online questionnaire (2/7) |
| Byng et al., 200348 | 274 GPs | Describe GPs’ beliefs about their management of depression | All GPs in Lambeth, Southwark and Lewisham Health Authority (inner city) | Likert scale questionnaire – (devised for this study, but piloted on 15 GPs) (4/7) |
| Oladinni, 200249 | 61 GPs (60%) | Assess the attitudes of GPs towards depression | Inner city GP surgeries in Lambeth (group and single handed), ‘randomly’ selected | Questionnaire (DAQ) – postal survey (2/7) |
| Telford et al., 200250 | 1703 GPs (48%) | Survey GPs’ perception of the availability/quality of primary care and community-based services for depressed people. Identify barriers to provision of services | 11 health authorities – one from each English region and one each from Northern Ireland, Wales and Scotland. Urban, rural, deprived and privileged | Questionnaire – devised for study, piloted on 131 GPs; postal survey (4/7) |
| Rothera et al., 200251 | 263 GPs (72%) | Examine the attitudes and practice of GPs in managing late-life depression | All 116 practices in Nottingham Health Authority | Postal survey (adapted for study from previous research) – responses to attitude statements and clinical vignettes (4/7) |
| Dowrick et al., 200052 | 40 GPs | Test hypotheses that measures of GPs’ confidence in identifying depression predict ability to identify depression and that GPs who prefer antidepressants prescribe more than those who prefer psychotherapy | Practices in Liverpool and Manchester | Questionnaire (DAQ), prescribing information, Likert scale depression ratings (3/7) |
| Livingston et al., 200053 | 31 GPs, 24 PNs (12% of practices approached) | Assess acceptability and feasibility of an educational package concerning management of depression in old age | 14 practices in West Essex, East Hertfordshire, Redbridge | Vignettes and questionnaire (adapted DAQ for older people) (baseline data only used in synthesis) (4/7) |
| Summary second order constructs | Extracted key themes (authors’ ‘own words’ or paraphrase)55 | Summary translation across studies |
|---|---|---|
| Professionals’ understanding of depression | Constructing and resisting boundaries between depression, the self and ‘normal’ sadness37Depression as a normal response to life events42Inconsistency between beliefs and practice43Concerns surrounding the medicalisation of social problems40Depression as ‘understandable reaction to distressing circumstances’38Aetiology of depression49Nature of depression49,52,53 | Depression may be seen as a normal reaction to distressing events or as pathology. Understandings of depression may conflict with management strategies |
| Recognising depression | Potential for secondary gain42GPs’ accounts of diagnosing and caring for patients with depression40GPs’ experiences of the diagnosis and management of depression43consultation length/time and disclosure41Presenting complaints38Distinguishing between depression and physical illness42Avoidance of psychosocial problems38Making the diagnosis39Accuracy of diagnosis44–46,52 | Recognising depression is a complex process involving non-explicit subjective processes. Some see patients as reluctant to talk about their mood. Somatisation is common. Somatisation and/or comorbidity may complicate diagnosis. Receiving or giving a diagnosis of depression may benefit patients and GPs |
| Management strategies and tools | GP goals and management approach37GPs’ accounts of diagnosing and caring for patients with depression40GPs experience of the diagnosis and management of depression43Importance of listening37Time as a barrier to listening42 Time and consultation length, disclosure, antidepressants, time management41Management of late-life depression in primary care39Antidepressant use44,47–49,52 Role of specialist services50 Managing depression46 |
Clinicians used antidepressants, talking therapies, listening and specialist services. Listening to depressed patients takes time; this may be a barrier to effective treatment, but one in-depth study contested this |
| Stigma and shame | Stigma and shame38depression still carries a stigma in this age group39 | Depression is perceived as stigmatising for some elderly people, especially those from ethnic minority groups |
| Relationships between professionals | Primary care relationships39PNs’ position in the practice38 Confusion over the role of and lack of access to specialist services43,46,50scarcity of counselling resources41 |
There is confusion between primary care staff concerning their roles and responsibilities in the diagnosis and management of depression, and about the role of specialist services, which seems focused around lack of access |
| Attitudes to managing depression | GP responses to chronic depression37Interactional difficulties with depressed people42Pessimistic about outcome43 Positive about outcome40,41 Lack of confidence in managing depression39,46,48,53 Ambivalence49,51 |
GPs and PNs experience frustration in managing depression. Some are confident about outcomes, but commonly there is ambivalence |
| Training needs | without understanding the framework which underpins GPs views on ‘depression’ . . . educational interventions directed at GPs will not improve patient outcome42Lack of training and knowledge39,46,50,51,53education efforts should focus on increasing GPs’ sense of therapeutic optimism and providing them with sufficient skill in and knowledge of a range of psychological procedures52GPs would benefit from educational programmes that promote awareness of current treatment guidelines47 | Many PNs and some GPs say they need more training in managing depression, but this is not a priority for them. Training should be grounded in professionals’ understandings of depression and should seek to improve attitudes to working with depressed people |
Not all information was available for each study. Quality assessment score: higher score represents higher quality, qualitative studies scores out of 10 on critical appraisal skills programme checklist;33 quantitative observational studies scores out of seven on a scale devised for this study (see Appendix 2; also published in our metasynthesis paper31).
All translated second-order constructs were supported by at least one good-quality study (bold references are studies scoring in top tertile of quality scores: qualitative studies ≥ 8/10, quantitative studies ≥ 4/7) and by both qualitative and quantitative studies.
The management of depression in a general practice setting is perceived as particularly complex when patients also present with concurrent social problems. GPs and PNs are very aware of the relationship between social problems and mood, but they are unsure of its exact nature and of their role in managing it. This uncertainty may be exacerbated by a lack of attention in guidelines concerning the influence of social problems on response to treatment. 54
There are ambivalent attitudes among GPs and PNs towards working with depressed people. This was reflected in a lack of confidence among some clinicians in their ability to manage mental health problems and the use of a limited number of management options. Most of these data are from GPs, perhaps because PNs may be less likely to manage depression than GPs; however, where depression is comorbid with physical illness PNs’ views may be more important since they are taking an increasing lead in long-term condition management and particularly since practices began to be reimbursed for screening their diabetic patients and patients with CHD for depression under QOF, although this payment has since ceased.
There was also evidence that GPs avoid giving a diagnosis of depression based on a belief that some patients, especially older people and those of Caribbean or South Asian ethnicity, will feel stigmatised by it. One high-quality study39 suggested, however, that concern about stigmatisation might be constructed to hide a reluctance to explore depression in order to avoid feelings of powerlessness when management options seem limited. At the time of the review, there were no data available to determine whether or not the same issues are important when managing patients with depression and comorbid physical illness. We, therefore, used the findings of this review to inform our planned study of GPs’ and PNs’ views and experience of managing depression in CHD.
Our review had demonstrated variation both between and within studies in views expressed concerning the management of depression in general. When considering the management of depression comorbid with CHD, we therefore decided to use a semistructured interview design. This would allow us to focus the interview on the topics that we considered important for informing our future intervention, while allowing participants to highlight topics important to them. Similarly, we realised that it would be important to use an iterative process for the data collection and analysis so that new themes arising in early interviews could be tested with later participants. The review findings also informed our topic guide (see Appendix 3). With the plan to develop a new intervention in mind, we ensured that each interview would cover three broad areas: defining depression in CHD, current management of depression in CHD and future management of depression in CHD. The key findings from our review related to each broad area were then used as prompts during the interview. In this way, we were able to test whether or not the findings of our review were relevant when considering depression in people who also had comorbid CHD and which issues would need to be addressed by the future CHD-specific intervention. For instance, when asking clinicians about how they defined depression in CHD, if they did not mention social problems (which we had found in our review to be important), we planned to prompt them to consider this issue. Hence, our metasynthesis allowed us to conduct a more relevant and focused qualitative study, which is described in the following section.
Qualitative interview study of general practitioners’ and practice nurses’ views and experience of managing depression in coronary heart disease
Study aim
This qualitative interview study was designed to help understand GPs’ and PNs’ views and experience of managing depression specifically when it is comorbid with CHD and to determine their preferences for our planned UPBEAT-UK nurse-led pilot intervention for people with CHD reporting chest pain, which could be angina or non-anginal chest pain, and depression.
Study methods
The initial sampling frame was the 16 GP practices participating in our cohort study, the design of which is described in Chapter 5. In recruiting the participants from these practices, we used a purposive, maximum variation approach to recruitment based on ethnicity, age, practice setting (inner city vs. suburban) and size (single handed vs. group). After several interviews, we noticed that participants often mentioned their involvement in our cohort study and we became concerned that this was an indication of heightened awareness of depression in CHD. From then on, only those clinicians from UPBEAT-UK practices who were not personally involved in the cohort study were interviewed. We also used a snowballing technique to identify GP and PN participants independent of the UPBEAT-UK research programme, that is, practices that we had not recruited to the cohort study.
We conducted individual semistructured interviews using a topic guide (see Appendix 3) based on the findings of our metasynthesis. We revised the topic guide iteratively: for instance, early participants introduced the problems of ‘erectile dysfunction’ and ‘housebound patients’ so we explored these topics with later participants. In order to ground opinions in practice, we asked participants to recall specific patients. We audio-recorded the interviews and transcribed them verbatim following written informed consent.
We conducted the interviews and analysis concurrently, and stopped recruitment at data saturation; that is, when no new themes or information emerged. We applied a staged procedure of thematic analysis to the data57 adding rigour to the process with the techniques of constant comparison. 58 Three researchers (EB, JM and PW) independently coded the first interview and met to discuss preliminary descriptive codes. Following this, two of the researchers (EB and JM) independently applied these and, where appropriate, new codes to the following four transcripts when consistency in coding was achieved. Descriptive codes were collated into themes and a preliminary explanatory framework devised. This was then used as the basis for coding and for informing future interviews. Data for each theme were gathered and coded using computer software (NVivo version 8; QSR International, Warrington, UK). The robustness of themes was tested by examining differences and similarities between coded data.
Study results
In total, EB interviewed 10 GPs and 12 PNs. Male and female GPs were recruited but no male PNs were identified. GPs and PNs appeared to have similar views. We have published the findings of this study, including illustrative quotes and participant details, in open access journal papers by Barley et al. 2,59 in 2012. The identified themes and topics discussed are listed in Box 1 and the main findings are summarised below.
Distress vs. depression.
Distress or depression in CHD.
-
vs. ‘general depression’.
-
vs. depression in other chronic diseases.
-
CHD severity.
-
Why some CHD patients become depressed.
-
Disease impact.
-
Individual difference.
-
Social factors.
-
Illness perceptions.
Impact of understandings on decisions to treat.
Theme: recognising and screening for depressionQOF questions.
-
Benefits of QOF questions.
Reservations about QOF.
Clinical judgement.
Theme: assessing the severity of depression Theme: current management of depression in CHDManagement goals.
Current management options.
Choosing management options.
-
Antidepressants.
-
Talking therapy.
-
Informal counselling.
-
Exercise.
-
Specialist NHS and community services.
-
Other issues influencing management choices.
Erectile dysfunction.
Housebound patients.
Theme: future intervention for depression in CHDType of intervention.
Timing of intervention.
Who would deliver the intervention.
Summary of study results
Current attitudes and practices
General practitioners and PNs expressed diverse views, which indicates uncertainty about this topic. However, for most of the themes, a majority view could be identified, even if some individuals also offered alternative opinions. For instance, most of the participants appeared to consider CHD depression similar to other types of depression. Distress and depression were viewed, by most, as lying on a continuum of severity and/or chronicity. Individuals may ‘naturally’ become distressed following a diagnosis of CHD or a cardiac event, but only when the distress becomes severe and enduring is it seen as depression requiring treatment.
Most participants used the QOF questions to screen for depression, followed by a more detailed questionnaire, such as the Patient Health Questionnaire (PHQ-9) or the Hospital Anxiety and Depression Scale (HADS); however, use of additional questionnaires was not routine, even when responses to the QOF questions were positive (indicating possible risk of depression or a need for further exploration). In several practices, PNs did not have access to the detailed questionnaires, suggesting that there may be ambivalence towards their use. That most participants stressed the importance of their clinical judgement both in assessing depression and making management decision supports this. These findings are also supported by a recent study which also showed that although GPs used the questionnaires, they preferred to rely on their ‘practical wisdom and clinical judgement’ to guide their assessments. 60
The clinicians identified a range of management options for CHD depression. Antidepressants and talking therapies were most often cited. This is not surprising since each of these treatments or a combination is known to be effective for depression in other populations. There is also some evidence that selective serotonin reuptake inhibitors may have a direct beneficial effect on platelets,61 although only one GP in our study, who is also an academic, mentioned this. However, in CHD depression, many considered antidepressants as a last resort. This appeared to be owing to a perceived reluctance in CHD patients to accept them, either because of fear of stigma associated with mental health problems or to a general dislike of medications, which is exacerbated when patients are already taking multiple medications for their heart condition. Data from patients are required to assess the validity of this perception.
Talking therapies were favoured; however, only a few GPs differentiated between types of therapy. This suggests a lack of clarity about the aims and process of different therapies, which may reduce their ability to make appropriate referrals. Some patients had been observed to reject talking therapy, again out of fear of being stigmatised. However, the main barrier to the use of talking therapy was a lack of availability. This has been reported previously. 42 It appears that the government’s Improving Access to Psychological Therapies (IAPT) programme has not yet reached these south London practices. 62
Access to other management options that clinicians felt would improve aspects of CHD depression, such as exercise on referral schemes, clubs to reduce loneliness and agencies to help with financial or housing problems, varied widely between the practices. Furthermore, there was variation between clinicians in their knowledge of the availability of such options. This may reflect variation in clinicians’ attitudes to managing problems that they believe are social in origin. Previous research39 has identified ‘therapeutic nihilism’ in which clinicians feel helpless in the face of the complex social problems which impact on their patients’ health. This attitude was seen in several participants in this study, although others actively sought to address social difficulties; one GP even expressed enjoyment in this aspect of her work. This latter attitude is encouraged in the government White Paper Our Health, Our Care, Our Say: A New Direction for Community Health Services. 63 This calls for greater integration of health and social care for patients with long-term conditions. One method may be ‘social prescribing’, which signposts patients to non-medical facilities and services available in the wider community, such as financial advice agencies and social clubs, which they can access to address the factors that influence their well-being. One practice involved in this study was providing this service, but our findings suggest that there is considerable scope to develop this for CHD depression in south London.
Informal counselling activities, such as reassurance and education, were performed by all the participants to some extent. With a few exceptions, most GPs were unwilling or unable to give much time to this. Some PNs reported that they did have the time, but even among these, there was doubt about how useful this was and many were left wondering how to progress and so would welcome guidance. Other nurses were open that they did not enjoy dealing with mental health problems or that they did not consider it to be part of their role either owing to lack of training or interest. These nurses would avoid raising the possibility of depression for fear of ‘opening a can of worms’ with which they felt unable to cope.
Future coronary heart disease depression intervention
This study has identified issues that should be addressed when designing a new CHD depression intervention. Findings from this study, along with those from previous work, may inform the type of intervention, the timing of the intervention and who should deliver the intervention.
Type of intervention
The participants were very clear that any intervention should be easily accessible. This was both in terms of being carried out locally, as they had observed a reluctance or inability in many patients to travel, and in terms of having clear and simple referral criteria so that clinicians would not be burdened by having to remember complex rules. Some participants reported difficulties remembering what treatment options and facilities were available for the multiple conditions that they managed; this could be a barrier to effective treatment.
The need for multiple treatment options was also stressed. This was so that options could be matched with the varying needs identified in individual CHD patients; because the participants recognised the importance of patient choice in improving adherence to treatment; and because clinicians value the opportunity to use their clinical judgement. Interventions that improve adherence to treatment were considered especially important as most of the clinicians considered that this was poor in CHD patients. However, although clinical judgement was valued, several nurses said they would favour the development of a protocol to guide their management decisions. This appeared to arise from their reported uncertainty as to what management options are available and appropriate for CHD depression.
Any new intervention should be evidence and theory based. Having goals and social support are known to be important to well-being. 64 Although no participant specifically mentioned goals, several noted that the causes of depression are specific to individuals and that to be effective, management plans should be patient driven. Evidence-based interventions that involve patients deciding which areas of their lives to address, such as problem-solving therapy, motivational interviewing or the technique of ‘SMART goals’, where individuals identify an area to change that is Specific, Measurable, Appropriate, Realistic and Timely, may therefore be useful in CHD depression and would most likely be accepted by GPs and PNs.
All participants mentioned the importance of social support. Some raised the role of the family in directing patients to care and helping with self-management. Most participants stressed the importance of combating isolation, which they felt is a determinant of depression in many CHD patients. This issue was considered to be especially relevant to housebound CHD patients, for whom, in many practices, no specific system of care had been devised. Identification by the patient of an individual who can support them in their self-management is a commonly used and effective strategy in several health-care interventions, for example weight loss and health training. 64 This type of intervention may be appropriate for many CHD patients and would be supported by GPs and PNs.
Cognitive habits are known to influence depression and, as such, CBT is now an established treatment for depression. 65 Similarly, in chronic disease, the ways in which individuals think about their illness and approach its management are known to influence outcome. 66,67 Given the strength of this evidence, it is surprising that cognitive factors were not discussed in detail or by all participants. It appears that some of the clinicians considered physical and mental health separately and were more focused on the former.
Some participants, however, did mention the importance of cognitions such as illness perceptions and motivation or commitment to change in improving mood or health in general. Some participants said they or a colleague within the practice had had training in brief cognitive interventions such as ‘10-minute CBT’68 or problem-solving therapy. 69 It appears that GPs and PNs vary in their understanding of and willingness to address cognitions when managing CHD depression. A new CHD depression intervention is likely to be most effective if it involves some level of cognitive change in the patient. Increasing self-efficacy, motivation, readiness and commitment to change, and changing illness perceptions have all been shown to improve outcomes in a range of chronic illness, including heart disease. 67 Some GPs and PNs may need education to accept such interventions.
Timing of the intervention
The participants were divided into whether an intervention for CHD depression would be most effective if delivered immediately following a cardiac event or several months later. The rationale for early intervention was to prevent the distress that they believed is commonly experienced following a CHD diagnosis or cardiac event from developing into a lasting depression. An early intervention should, therefore, focus on helping the patient come to terms with the feelings, observed by the clinicians, of shock and vulnerability and help them to adjust to changes in role or functioning. However, several clinicians felt that the majority of people recover without intervention. Previous research has shown that the medical condition and depression status of individuals within 6 months of MI fluctuate. 5 It has been suggested by an expert panel on depression and CHD5 that a population whose depression is likely to remit spontaneously may not be the best group in whom to test the hypothesis that reducing depression will reduce the risk of CHD-related mortality and morbidity.
However, the panel also point out that early treatment with antidepressants, as opposed to depression symptom interventions, may be appropriate, to the extent that the former may have direct cardiovascular benefit. A further argument for early intervention concerns the role of illness perceptions in disease progression, which is well documented. 67 This issue was raised by two participants who felt that patients could be disabled more by their perceptions than by their actual CHD severity. There is some trial evidence to suggest that early modification of illness perceptions may lead to improved outcomes. In a small RCT,70 inpatients who received a brief intervention designed to alter their perceptions about their MI had improved functional outcomes and reduced angina symptoms compared with controls at 3 months post discharge.
The participants’ rationale for a later intervention, that is, some months after an event or initial diagnosis, was that if spontaneous recovery had not occurred, CHD-associated distress would have become chronic and therefore could be described as depression that needed treatment. Patients had, therefore, not adapted to having CHD or to its effects. Effects, such as loss of function or role due to CHD, and symptoms, such as erectile dysfunction (which may occur any time in CHD and up to 5 years prior to diagnosis,71 were identified as particular predictors of depression. These may need to be addressed at this time. It was also suggested that after a few months, when their medical condition has stabilised, the support available to CHD patients, such as CR and outpatient appointments is reduced. This may increase patients’ risk of depression.
A complicating factor when deciding on the timing of a new intervention is that many participants were aware that their CHD patients were depressed prior to their diagnosis or cardiac event. This was explained in terms of the difficult social lives that many patients in south London lead; CHD was just another problem that added to their depression. An intervention that addressed multiple problems would therefore be necessary for such patients. This could be delivered at any time following diagnosis.
Findings from this study and from previous research therefore indicate that it is likely that different types of intervention may be more or less effective at different times following diagnosis or a cardiac event.
Who should deliver the intervention?
The participants of this study varied in their opinions as to who should deliver or manage a new CHD depression intervention, although no one suggested that a GP should lead. There was a majority opinion, however, that there is currently no suitable person within primary care, at least, not without training. It was felt important that the person delivering the intervention should be knowledgeable about and have experience of managing both CHD and depression.
The PNs were all experienced in managing the physical aspects of CHD, but their confidence and interest in dealing with psychological problems varied widely. This could be addressed through training. Nurse-led psychosocial interventions have been shown to be effective, including in heart disease. 72,73 However, many nurses complained of a lack of time. A previous study41 has shown that it is possible to have a flexible attitude to time management in primary care in order to manage depression effectively. This was supported by data from GPs and a PN in one practice where the policy was to be flexible with time in order to address their patients’ psychosocial needs. The observation from this study that PNs with 30-minute, as opposed to the more usual 15-minute, appointments often tended to be more willing to provide informal counselling to depressed CHD patients also supports this. If the new CHD depression intervention is to be delivered by PNs, it could be made attractive to them if it could be shown that it would save them time. This may be achieved by pointing out reports by PNs, gathered during this study, that they currently spend a lot of time delivering informal counselling despite being unsure that it is effective.
Finally, this study has identified that some primary care clinicians may be reluctant to raise the subject of depression with their CHD patients. In addition, several of the clinicians perceived that their patients were also unwilling to do this or to accept treatment. This may explain findings that depressed patients with comorbidity, including CHD, were less likely to be treated for depression. 74 In this study, one reason cited for this reluctance to discuss mood was fear of stigma, both in patients, who did not want to be stigmatised, and doctors, who did not want to create stigma. Stigma around mental health is well documented. 75 Some participants suggested that the use of screening questionnaires helped them to raise mental health issues in a non-threatening manner. This is supported by recent findings that patients liked their GPs using questionnaires as they saw them as an efficient and structured supplement to medical judgement and as evidence that the doctor was taking their problem seriously. 60
Other reasons for not addressing mood were lack of confidence or interest in treating mental health problems, prioritisation of physical health problems and a belief that ‘nothing could be done’ about the depression, which occurred mainly when depression was perceived to stem from social difficulties. These views suggest that, although they are very aware of the social difficulties experienced by their patients, some clinicians are still working with a biomedical model of health, where mental health and physical health problems are viewed as separate entities. This is despite the widespread acceptance of the biopsychosocial model76 as a more useful explanatory model of health. 77
Adoption of the biopsychosocial model may empower clinicians to manage mental health problems that they see as social in origin. Care systems built around an understanding that physical and mental health are interlinked and occur in a social context may lead clinicians to explore management options other than those that are traditional within primary care, for instance local clubs to combat isolation and advice agencies. Some of our participants were aware that such facilities may be useful, but there did not appear to be any system for identifying local facilities or for matching them to their patients’ needs. For this to work, such facilities must be available and accessible. This study shows that provision does vary within the limited geographical area of south London, but also that many clinicians are not fully aware of all the local facilities that are available to them. Any new intervention should aim to optimise use of existing facilities.
Therefore, as well as aiming to improve patient health, any new intervention for CHD depression should aim to increase clinicians’ awareness of the inter-relationship between physical and psychological health, and the social context in which it occurs. It should also be aimed at increasing primary care clinicians’ feelings of self-efficacy in managing complex psychological needs that may appear to be social in original.
Conclusions
The study suggests that for many GPs and PNs, only depression that is severe and chronic is considered to need treatment in CHD. QOF screening questions are valued in detecting depression, but use of these is not routinely followed up with a more detailed questionnaire, such as the PHQ-9, which may be more accurate. Routine use of more detailed questionnaires, especially by PNs, many of who currently do not have access to these, may increase identification of CHD depression.
The GPs and PNs in this study felt that managing depression in CHD was worthwhile. To be accepted by clinicians and patients, a new intervention should have clear referral criteria and be local to the practice. The participants valued their clinical judgement and recognised the importance of patient choice in successful management; a new intervention should offer a range of treatment options that clinicians and patients can select together. These should include options for problems identified as exacerbating or causing depression in CHD, such as erectile dysfunction and being housebound. This study suggests that housebound patients currently may not receive adequate psychological care. Many clinicians felt that exercise is useful in managing CHD depression; there may be potential to develop this as a management option.
Depression in CHD may be exacerbated by, or may exacerbate, distress or depression associated with social problems. Potential exists, in CHD depression management, for greater use of existing local resources to combat problems common in CHD, such as social clubs for loneliness or agencies for financial or housing advice. However, clinicians vary in their perceived responsibility and ability to manage such problems. Some may need to be supported and empowered to manage problems that they consider social in origin.
Similarly, despite good evidence that cognitions are important predictors of response to chronic disease and that changing cognitions may improve health outcomes, GPs and PNs appear to vary in their understanding of this. A new CHD depression intervention should include education for clinicians concerning the role of cognitions such as illness perceptions, self-efficacy and motivation in chronic disease management.
Factors associated with depression in CHD may vary according to the time elapsed following receipt of a diagnosis or a cardiac event. Distress or depression immediately following diagnosis or an event may resolve spontaneously in many patients. An early intervention to manage unhelpful illness perceptions may reduce the number of patients whose distress or depression does not resolve. In patients whose depression is persistent, a more complex intervention may be needed to address adjustment problems or social issues which are exacerbating the mood problems.
Finally, this study suggests that there is currently no suitable professional within primary care to deliver a CHD depression intervention, at least not without training. Some nurses say that they would be willing to be trained to do this, but others would need persuasion owing to a lack of interest in or perceived responsibility to manage mental health problems. Given their current heavy workload, all PNs would need to be convinced that any new CHD depression intervention was effective and would have timesaving benefits. Time may be saved by reducing the time spent in informal counselling, which the PNs report to be time-consuming and of uncertain benefit.
Summary: what we learned from reviewing existing literature and conducting our own qualitative study
The findings of our metasynthesis of studies of primary care management of depression in general and our qualitative study of primary care management of depression comorbid with CHD were complementary. On balance, GPs and PNs expressed uncertainty in the management of depression, citing lack of time, training and available management options that are acceptable to patients. That these findings were similar for our metasynthesis and this qualitative study indicates that GPs and PNs have similar struggles when managing depression whether or not it is comorbid with CHD. Social problems, in both studies, were seen as important contributing factors, which should be addressed; our qualitative study indicated that these might be especially important in CHD patients who often come from low socioeconomic groups. However, GPs and PNs are uncertain in their role in and responsibility for addressing social problems and existing resources are underused; a finding from both our review and this qualitative study which highlights the need for a new intervention to help address this. Nurses’ attitudes towards and confidence in managing depression varied enormously. This was a finding of both our review and qualitative study, and, as primary care patients with CHD commonly receive most of their care through nurse-led clinics, suggests that the development of interventions for depression in these patients should include sensitive consideration of nurses’ views.
By asking clinicians, in our qualitative study, to consider depression comorbid with CHD, we not only confirmed that the findings of our review were relevant when managing people with CHD, but also identified issues that may be particularly important to people living with CHD. For instance, erectile dysfunction is a common problem that is not addressed routinely despite clinician awareness that this can contribute to depression and that if people with CHD become housebound, they are unlikely to receive any help for depression.
Strengths and limitations
Through the inclusion of only recent studies conducted in the UK, we ensured that our metasynthesis produced findings relevant to current practice within the NHS; findings may be less relevant to those wishing to develop interventions in other health-care systems. The aim of our review was to identify broad themes concerning depression management in order to inform our qualitative study focusing on managing depression when it is comorbid with CHD and hence we excluded studies focusing on specific aspects of the management of major depression such as antidepressant use. These more specific aspects of management were, however, addressed in our qualitative study focusing on their relevance to people with comorbid CHD. As is the case with all metasyntheses, we had to make decisions concerning which studies to include; we have detailed our methods clearly but others conducting the same review may have selected different studies. The findings of our review and qualitative study were, however, concordant, indicating that our choices were appropriate and that we produced robust findings to inform our intervention.
The findings of our metasynthesis were extremely useful in ensuring that our qualitative interviews were focused on known barriers and facilitators to managing depression in the UK; this information was essential in ensuring that our intervention would be feasible to deliver in primary care. However, we also used an iterative process of data collection and analysis so that new topics could be explored in subsequent interviews. For instance, the idea that depression in some people with CHD may be compounded by erectile dysfunction or by being housebound had not been identified in our review. Such findings emphasised the need for a future intervention to be personalised in order to address the varying needs of individuals. A potential weakness is that our participants were all practising within south-east London; however, we were careful to recruit from areas of contrasting deprivation and affluence in order to elicit a range of experiences.
It should also be noted that, as in the case of all qualitative research, it is possible that our positionality influenced our final findings. For instance, the UPBEAT-UK programme was funded to consider the ‘problem’ of depression comorbid with CHD and this informed our methodology. Had we conducted a study about the impact of illness on well-being and health as a resource for daily living, our findings may have been different.
Finally, since we conducted our study, guidelines for the management of depression with a chronic physical health problem have been issued;18 these may impact on attitudes and practice in the future.
Conclusion
Our systematic review of existing literature describing GPs’ and PNs’ experience of managing depression in UK primary care identified a range of barriers and facilitators to delivering care that need to be considered when designing future interventions for depression. We were then able to determine whether or not similar barriers and facilitators were experienced by GPs and PNs when managing depression in patients who also have CHD by conducting our own qualitative study. This work addressed a gap in the literature highlighted by our review and helped us to determine GPs’ and PNs’ views concerning what an intervention for people with sCHD and comorbid depression should consist of. By seeking clinicians’ views directly, we were able to identify elements (such as the need for a flexible, personalised intervention which allowed clinicians to use both their clinical judgement and existing tools and resources) of a future intervention, which would make it not only effective (in terms of addressing care needs identified as unaddressed in these patients) but, importantly, feasible to deliver in current practice.
The following chapter describes how we also sought the views of patients with CHD and comorbid depression to inform our intervention. How this work was synthesised to develop an intervention to test is then detailed in Chapter 4.
Chapter 3 Perceptions of distress and depression in patients with symptomatic coronary heart disease: a qualitative study (work package 1)
Introduction
In the introductory chapter, we described how CHD and depression are both common health problems worldwide and are predicted to be the two top causes of global health burden and disability by 2020. We have described how depression has previously been associated with worse cardiac prognosis and an increased cardiac mortality. We described a need to better understand the relationship between the two disorders and a pressing global public health need to improve integrated primary care for people with both disorders.
Although the prevalence of depression in patients with CHD is high, little is known about how people with CHD and comorbid depression perceive these conditions and the relationship between them. Treatment trials for patients with CHD and depression have included antidepressant medication with some success in reducing mortality. 78,79 However, it is currently unclear whether or not patients with CHD would opt for other interventions recommended by NICE for depression, such as supervised exercise, guided self-help, problem-solving, group or individual CBT or interpersonal therapy. It is also unclear whether or not NICE guidelines for depression need modification for patients with concurrent CHD and depression.
Before designing a new primary care-based intervention by nurses for patients with CHD and concurrent depression we undertook qualitative research to explore patients’ perceptions of their depression in the context of CHD, their health-care preferences and own self-help strategies for coping with depression.
In this chapter we report on qualitative findings from a pilot study of five unstructured interviews with UPBEAT-UK cohort study patients with sCHD (chiefly chest pain) and symptoms of depression, as well as qualitative findings from a semistructured interview study with 30 UPBEAT-UK cohort study patients presenting with sCHD and symptoms of depression.
Aims
The study aimed to explore (1) primary care patients’ perceptions of links between their physical condition and mental health; (2) their experiences of living with depression and CHD; and (3) their own self-help strategies and attitudes to current personalised care (PC) interventions for depression.
Methods
The sampling frame was the UPBEAT-UK cohort study database of primary care patients with CHD. At the time of recruitment (November 2008–January 2009) this numbered 376. On recruitment to the cohort, study participants were given the option of being interviewed in depth about their experiences of CHD and how this affected them emotionally. From participants who agreed to be contacted, we purposively sampled for positive scores on the PHQ-9,80 which indicates symptoms of depression. We also sampled for maximum variation on sociodemographic factors: age, sex, ethnicity and occupational status.
At the time of sampling, 42 patients screened positive for symptoms of depression and of these, five were included in the pilot interview study. Of the remaining 37, one patient declined and three could not be contacted or had died. Of the remaining 33 patients, 30 were interviewed, at which point interviewing was stopped as data saturation had been reached.
We conducted five individual unstructured pilot interviews and 30 semistructured interviews. Topic guides were informed by the aims of the research, review of the literature, discussion with coauthors and findings from the pilot interview study. We audio-recorded all interviews and transcribed them verbatim following written informed consent. Transcribed interviews were entered into NVivo 8 qualitative software for analysis and data management.
All transcripts were analysed using a thematic approach81 involving a process of constant comparison between cases. 82 Analysis began alongside data collection, with ideas from early analysis informing later data collection in an iterative process. Analysis of individual transcripts commenced with open coding grounded in the data. This generated an initial coding framework, which was added to and refined, with material regrouped and recoded as new data were gathered. Codes were gradually built into broader categories through comparison across transcripts and higher-level recurring themes were developed. Data were scrutinised for disconfirming and confirming views across the range of participants. Discrepancies were resolved by discussion at regular UPBEAT-UK research meetings to ensure consistency.
Pilot interview study
Study results
Participants
Participant characteristics are recorded in Table 3.
| Age | Sex | Ethnicity | Occupational status |
|---|---|---|---|
| 57–59 years | Two male | Three white British | One full-time employment |
| One Afro-Caribbean | One disabled | ||
| Two female | One South Asian | Three retired |
Participants were given pseudonyms to protect their anonymity.
Seven key themes were identified:
-
taking control and individual coping strategies
-
the significance of having heart problems
-
ambivalent attitudes to accessing PC mental health services
-
services participants would like for heart disease and depression
-
future orientation (having achievable hopes and dreams for the future)
-
the positives
-
contributory or causative factors for distress/depression.
Taking control and individual coping strategies
Analysis of pilot interviews revealed the ways in which patients tried to gain control of their health and emotional well-being and the individual coping strategies they used. These included:
-
devising own fitness regime
-
going to the gym
-
studying health issues and alternative or complementary medicine
-
meditating
-
praying and being an active member of a religious community
-
learning specific relaxation techniques based on yoga and Buddhist philosophy
-
fishing and being in the natural environment (described as a yoga-like experience)
-
being able to drive
-
setting goals for exercise and pacing performance
-
getting an allotment and growing vegetables
-
volunteering at a hospital.
The following are illustrations of strategies used by participants: devising own fitness regime, going fishing and going to the gym. These examples not only illustrate the most cited strategies used but also individual differences in participant preferences for individual or group approaches to self-help.
Geoff described his fitness regime; he has a mini-gym in his sitting room and also goes fishing. He explained:
I’ve got equipment where I train, I’ve got the balls, I’ve got the weights, I’ve got a track that I run on, and I got legs . . . because of this operation, because I have got arthritis I got to strengthen my legs . . . and I got a thing to do my upper body. Yeah I train at least once per week.
When exercising and out walking, Geoff also wears a heart rate watch and he finds this source of ‘biofeedback’ helps him to pace and monitor his exercise, particularly when climbing hills. Geoff finds that being by the river, fishing and enjoying the natural environment a therapeutic experience in itself, he said:
Yeah, yeah, it’s like a yoga, you can go into your own trance can’t you, you can sit by the river and just imagine you’re doing anything, it’s like yoga.
Geoff had worked as a porter in the various Covent Garden markets for most of his life but since retiring he had built up a sizeable collection of books on alternative and complementary approaches to health. Geoff may have symptoms of depression, as detected by his PHQ score, but he is actively taking control of his health.
Another participant, June, talked about the ‘psychosocial’ benefits she gained from her regular trips to a gym where there was a special programme for heart disease patients:
. . . there is a group there [at the gym] and nine times out of ten we spend most of our time laughing!
This participant clearly enjoyed the fun she had with other people who had similar health problems and were trying to cope with the gym equipment and exercises through laughter.
The significance of having heart problems
For all five participants, having heart problems was not the health condition that concerned them the most. Three out of the five participants suffered from arthritis and this condition appeared to be causing more problems than heart disease owing to chronic pain and impaired mobility. One participant, June, was unable to work because of arthritis in her shoulder, arms, legs and ankles. She had already undergone five operations for arthritis.
Out of the five participants, Gurch seemed the most depressed or unhappy. Gentle probing about his heart problems (bypass surgery and a number of heart attacks) and how this had affected his feelings almost immediately led onto the topic that dominated the interview: his unhappy marriage and home life and his abuse of alcohol. The following exchange illustrates the problems Gurch had experienced:
But how did it [heart bypass surgery] affect you emotionally, what did you think and what did you feel?
At that time there are family problems.
Did you have family problems?
Yes family problems you know then I just take so much whisky you know drank you know.
All right so I understand that you were saying that you had family problems and that was why you were drinking a lot.
It was one of the causes you know, the family problem you know I don’t know (. . .) my wife is but I do not know at that time you know, what are right what are wrong, anyhow it were due to my family quarrelling.
A little later in the interview he reflected that at the time of his heart bypass surgery, nearly 23 years ago, he was ‘totally unhappy, just spending my time you know just passing my time’.
Like Gurch, four out of the five participants had experienced problems in their marital relationships that seemed to have affected them over a long period of time and caused them unhappiness, anxiety and depression. In comparison with their relationship issues, participants seemed almost blasé about their heart problems.
Ambivalence about accessing mental health services
The next theme explored participants’ ambivalence about accessing mental health services through their GP and these included:
-
cultural perceptions of depression can act as barrier to accessing help, for example in South Asian and Sikh communities
-
not knowing the meaning of ‘depression’
-
suffering seen as Karmic – in the hands of God
-
cannot talk to anyone in community (stigma?)
-
fearing negative perceptions of GPs as always ‘moaning and groaning’ (this idea is reinforced when doctor praises client for not moaning)
-
discomfort in talking about emotional issues
-
low self-esteem and a sense that their problems are not worthy of attention
-
the perception that their health is relatively good and that there are people in far worse situations
-
some people do not want to go down that path because it frightens them.
Excerpts from interviews with June and Ivy illustrated how health stoicism and fear of being viewed negatively by their GP acted as an inhibitor to accessing primary care health services. Although June suffered from asthma, arthritis, heart disease and possible depression she seemed reluctant to visit her GP other than for her regular 2-monthly check-ups. June explained ‘. . . there are some times when you are thinking to yourself that there are people a lot worse off than you!’.
Ivy had also adopted this approach – even though she was 79 years old and had heart disease, arthritis, balance problems and symptoms of depression. She explained:
To be honest, I hardly go near them [GP surgeries] . . . I mean they have got enough to do when they have got a whole lot of patients who are worse off than I am.
OK so do you think of yourself as being in quite good health?
Yeah.
You think ‘I’m OK I’m much better off than some people’?
Yeah, yeah.
And does that make you feel better?
Sometimes it does.
Although the strategies of stoicism and ‘counting your blessings’ that Ivy used can have some positive benefits (putting problems into perspective to combat feelings of anxiety about health) it can also act as an inhibiter to accessing health care.
June was concerned that her GP might perceive her negatively by making more frequent visits to the surgery. June explained:
I don’t want to come in too often!
Too often?
Even though they say they don’t get fed up, you don’t want them to get fed up, you know?
In relation to depression, a study by Priest et al. 83 found that stigma was still associated with depression, and patients felt ambivalent about consulting a family doctor. Some feared the GP would make negative evaluations of them or would be irritated or annoyed. With this in mind, June might be similarly reluctant to disclose her feelings of depression to her GP.
Services participants would like for heart disease and depression
In relation to the services participants would like for heart disease and depression, participants seemed more interested and comfortable in talking about services they would like for heart disease. The following suggestions were made:
-
more frequent (physical) check-ups at the GP surgery with specialist nurse and improved feedback
-
being able to request a check-up when needed
-
access to a gym with specialist cardiac trainer for attention, guidance and reassurance
-
the promotion of wrist heart rate monitors
-
some people were interested in having counselling
-
one participant had counselling and found it helpful.
Three out of the five participants had used a gym as part of their aftercare but only one participant, June, seemed to have attended sessions that were designed for people with heart problems. The other participants attended general fitness centres where they were assessed by a trainer and then given a set of exercises and left to get on with these on their own. These three participants all felt that a dedicated session would be preferable, particularly in the period immediately after their coronary incident when they needed the most reassurance about symptoms and safety while exercising. June’s experience of going to a dedicated gym session was an enjoyable one because there were a group of people ‘in the same boat’ and they had a good laugh. She also felt safe and reassured by the trainer when she had some cardiac symptoms. Even though this kind of CR might not be considered a psychological intervention, in the strictest sense, it may have some positive outcomes for patients suffering from mild depression and may also help to prevent a ‘cardiac invalid’ mind set.
Only two out of the five participants had received help for depression from their GP. None of the participants had received antidepressant medication, June had received information and Ivy had attended counselling sessions. Geoff had been offered group therapy but did not want to attend. Generally participants seemed cautiously positive about the idea of receiving one-to-one counselling while less positive about group therapy.
Future orientation (having achievable hopes and dreams for the future)
When asked if there was anything the participants enjoyed or were looking forward to doing with their lives, the following topics were discussed:
-
planning for a holiday with a friend
-
gaining skills needed for a new way of life
-
planning to retire and live abroad
-
wanting to learn how to use a laptop
-
volunteering at a hospital.
In a critical literature review of the relevance of hope in the trajectory of heart failure illness, Davidson et al. 84 explained:
. . . hope . . . defend[s] against despair by focusing on the future. Expectations can be directed toward relief from a difficult situation. To achieve this relief, people must have a sense of control over their environment and feel that they are capable of making decisions that alter their life circumstances.
In relation to hope, four out of the five participants gave examples of achievable personal goals. When talking about their personal dreams, participants looked relaxed and animated, and their body language changed. Even the unhappiest participant, Gurch, seemed a different person when talking about his activities as a volunteer with Parkinson disease patients at a London hospital. Besides a lot of other benefits, being a volunteer had given Gurch some future orientation. Gurch explains how this has affected him as follows:
It makes me feel good, when I help a person and I can see that they are suffering like that you know and I help ladies and gentlemen who are suffering from this disease how to do step by step.
You are looking a lot happier while you are talking to me about doing the volunteering you look quite happy.
Yes I am happy.
Another participant, James, talked about his hopes and dreams for the future as follows:
. . . we’ve got friends in Portugal that are living in a very beautiful kind of place and it is very nice to go and visit them and I think ‘this will do me fine’ nothing grand, just something with a bit of land. Errrh, yeah and this is why I am doing the allotment because I need to know that I can do it, if I want to.
James was close to retirement age and was seriously considering starting a new life abroad. He was taking some practical steps in preparation for his goal of having some land of his own to cultivate.
Similarly, June talked about her wish to go travelling as follows:
What I want to do at the moment is to go travelling and I can’t at the moment, I want to go away for a holiday but I will have to wait. That is what I want; I really, really want to go away.
June and a friend were hoping to go to Jamaica in a year’s time, health permitting.
Maintaining hope and encouraging a future orientation may be important factors to consider in devising interventions for patients with CHD and depression. Patients with CHD may need some help in either finding a future goal or in taking practical steps to achieve something they want to do.
The positives
Although all five participants had positive scores for the PHQ-280 and had suffered from periods of ‘unhappiness’ over the course of their lives, participants spoke about the positive aspects of their lives, their friendships, interests, hopes and dreams or about how they had successfully tackled personal problems, such as alcohol abuse. Of the five participants:
-
four had a close friend, group of friends and contact with family members
-
four mentioned things that they enjoyed doing
-
one participant had used his experience of heart disease as an opportunity for personal growth and improved care of self
-
the unhappiest participant was trying to improve his situation by volunteering and by trying to understand his unhappy relationship
-
two participants with alcohol problems had managed to either abstain or moderate their drinking behaviour.
One participant, James, thought that his heart disease had given him the opportunity for change and personal growth. James explained as follows:
I started the yoga and the Buddhism and a couple of the meditation techniques. It did change me quite a lot and I was quite happy about that change . . . I used to be somebody I didn’t like very much and now I am somebody who is OK, as far as I’m concerned. I used to treat people very badly and I used to argue about anything and would drink a lot and I have stopped doing a lot of those things really.
In the excerpt above James explained how having a heart attack had been a catalyst for change in his life. He had also changed his diet and lost 5 stone in weight.
Participants’ perceptions of what caused or contributed to feelings of distress and depression
Participants talked about the issues that they felt had contributed to or caused their depression and these included fear of dying, boredom, unhappy relationships, chronic pain and disability, low self-confidence, alcohol abuse, being bullied and being bereaved.
In the interview with Geoff, dying and fear of dying was a topic touched on in different ways. Geoff was understandably scared when he realised he was about to undergo open-heart surgery. Geoff also referred to his sense of frustration with other heart disease sufferers who were becoming ‘cardiac invalids’ because of their fear of dying and he gave examples of friends who had suddenly died from a heart attack, reminding him of his own mortality.
The following extract describes Geoff’s reaction when he first realised he was going to have a heart operation.
So I went up the hospital earlier about me stomach he looked at me diagrams and me sheets and all that and he said ‘do you want us to do this one first’ and I thought ‘what one is that?’ He said sort out yer valves in your artery and so I said Oh . . . !
So that was news to you was it?
Scary and all. So I said ‘OK then’ he said ‘do you want to know what the chances are of dying?’ and he said ‘six to one′ so I said ‘all right then.’ So he said it will be a little while before we get a spare bed so I come home here and then I got a phone call to say could you come in. So that’s when the scary part went in!
Geoff’s interview provided examples of how the reality of dying had affected and distressed him. Indeed, since his operation some years ago he had become very interested in health matters and had devised his own fitness regime that involved monitoring his heart rate, using a wrist monitor. One could speculate that Geoff’s anxieties had been channelled into monitoring his heart rate, as the following extracts might suggest:
You go fishing yeah and what does going fishing do for you? Why do you prefer going fishing to group therapy?
Because I can train. I got a hill like that (tilts hand) and I go up the hill but I don’t do that straight away but that’s a little bit of training that is getting me heart pumping I do it and when I reach the 120 heart murmurs I stop because I have to watch . . . that’s a good idea if you can get them free, a heart watch so if people are training and have just had open heart surgery and the doctor said ‘if you want your heart rate to be 120 you should look at your watch and if it says 120 and then I stop, stop going up the hill work it to about 112 and then I will carry on back up the hill, don’t forget I’ve got all me fishing gear with me and a barra [barrow].
And later in the interview he continued . . .
So when I go fishing there is a hill and it is a hill, but I want to work me heart so going up this hill is errh when I get to 120 . . . I done 130 actually and then I did stop. I wasn’t out of breath but I just thought ‘well that’s too much for me’ so I went and I looked at me clock and it was 130 and I thought ‘that’s quite good’, it went down to 112 and when it goes down to 112 the heart is (makes pumping sounds) just nicely beating . . .
Although Geoff was doing something positive about his health, his need for reassurance through biofeedback could be a manifestation of the anxiety he was experiencing. Geoff was not interested in attending group therapy sessions because he felt that listening to everyone’s symptoms of heart disease would make him worried for them (or perhaps remind him of his own health issues).
Discussion
The themes explored in this pilot study have revealed more similarities than differences among five participants who were purposively sampled for contrasting demographic backgrounds.
The participants did not want to be perceived as ‘moaning and groaning’ in relation to accessing health care and were mostly uncomfortable in talking about their feelings and ambivalent about accessing mental health services.
Although all of the participants had experienced unhappiness in their lives (mainly owing to relationship problems) they were all still trying to do the best they could and seemed to prefer taking their own courses of action to improve their lives, thereby preserving a sense of agency, control and future orientation.
For all of the participants, having heart disease and the symptoms of heart disease did not seem to be their main health concern. In contrast, chronic pain and impaired mobility caused by conditions such as arthritis seemed to affect participants the most.
Most participants would like to be able to attend dedicated gym sessions for people with heart problems so that they feel safe and supported. As dedicated sessions provide an opportunity for social interaction with other people with heart problems there is also an opportunity to make these fun and enjoyable and thereby emotionally therapeutic.
The main difference between the responses of the participants was evident with one participant, James, who seemed more comfortable about introspecting and talking about his feelings. James occupied the highest occupational status of the five participants and this difference in socioeconomic status/social class might indicate that he has access to and is familiar with psychological discourses, whereas the other four participants appeared to refer to ideas of stoicism in preference to introspection. There may well be issues of social class influencing the importance patients place on their feelings and whether or not these are appropriate and safe topics to discuss, either with a researcher or a health-care professional.
How the pilot study informed the main interview study
For the pilot study, an unfocused interview approach was adopted to allow participants to raise issues that were salient to them around the topic of CHD and depression. Findings from the pilot interviews and UPBEAT-UK literature reviews were used to inform the topic guide for the main interview study. This approach allowed us to balance topics identified in academic literature with patient-centred perspectives.
The researcher commenced the interviews by asking the following question: ‘So, if you wouldn’t mind just starting by telling me about when you first knew that you had some problems with your heart.’ Participants responded to this request by recounting personal accounts of their lives in the context of CHD and depression. This narrative approach worked well in the pilot study; it provided an opportunity to assess how participants perceived the connections between their physical, social and emotional worlds, in the context of illness. For this reason a narrative-based topic guide was developed for use in the main interview study.
Main interview study
Study results
RS interviewed 30 patients with CHD and symptoms of depression. The main findings from this study have been published in an open access journal paper by Simmonds et al. 85
The interviews were conducted using a narrative approach guided by topics informed by findings from the pilot interview study.
While recounting their stories of CHD and depression the following topics were explored:
-
participants’ perceptions of the relationship between their physical and mental health
-
participants’ experiences of living with depression
-
participants’ own self-help strategies for coping with depression
-
participants’ views and experiences of primary care interventions for depression
-
participants’ ideas for primary care-based interventions for depression that they would like to be made available.
Summary of results
Loss was a theme that underpinned all of the interviews in this study, as seen in Figure 2. Losses included interpersonal and health factors. 85
FIGURE 2.
Interpretative diagram of themes relating to participants’ perceptions of sCHD and distress/depression.
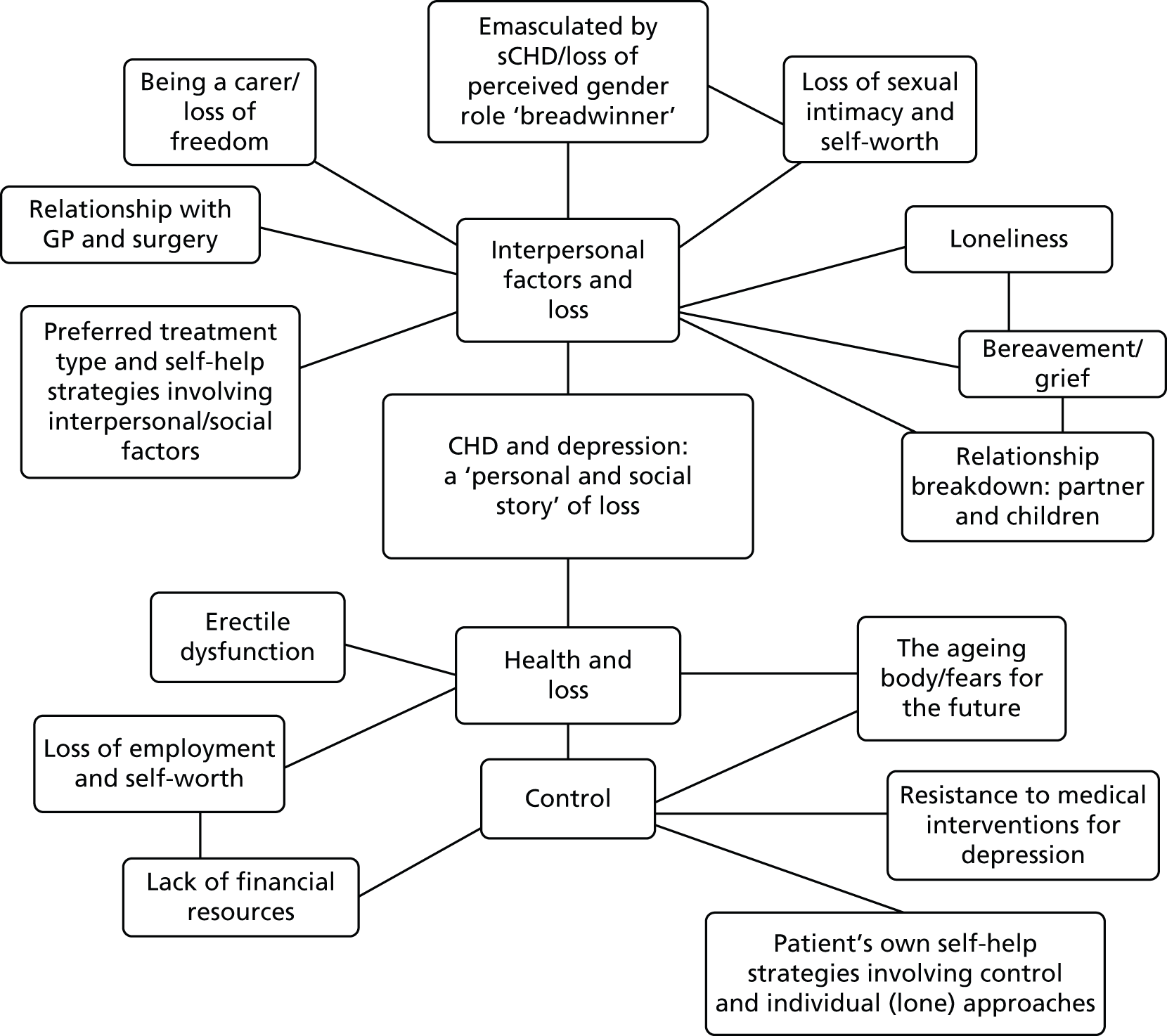
In relation to health, participants experienced a sense of loss that also impacted on feelings of agency and being in control of their lives. Loss of good health through CHD had impacted on employment status and financial security for some participants. Participants also communicated a loss of control in relation to their bodies, sense of gender identity and the ageing processes in general. Some participants tried to reclaim and exercise control by resisting the ‘medicalisation’ of their lives, particularly in relation to treatment interventions for depression, such as antidepressants. Participants also exercised control by developing their own self-help strategies in relation to depression. The kind of self-help activities participants preferred fell roughly into two types: (1) social and interpersonal, typically involving group activities and (2) lone activities, such as fishing or meditation.
Participants varied in the extent to which they attributed feelings of depression to their physical condition. Three levels of association between mental states and CHD were identified: (1) direct links made between CHD and depression; (2) indirect links between CHD and depression; and (3) weak links between CHD and depression. The three levels of association are summarised as follows.
Direct links made between coronary heart disease and depression
Of the 30 participants interviewed, 63% (19 participants) were male. Nearly half of these male participants (30% of the sample) reported feeling depressed, very depressed or suicidal after having a heart attack and explicitly stated a link between feeling depressed and having CHD.
The way men described their feelings was closely connected to the loss of perceived manliness (emasculation) and gender roles, which included loss of ability to work, erectile dysfunction and generally feeling ‘useless’.
Indirect links made between coronary heart disease and depression
Fewer direct links were made between CHD and depression by participants who reported a history of negative life events.
Factors contributing to or causing depression in this category were underpinned by feelings of interpersonal loss. The main issues that participants reported as ‘getting them down the most’ were:
-
marriage and partnership breakdown/unhappiness, problems with access to children after marital breakdown, loss of home/homelessness
-
bereavement issues through the death of a child, partner, parents and friends
-
being a full-time carer for wife, not having any support or respite and not being able to visit children in America.
Although partnership breakdown was reported as being a major, long-term, stressful experience by male and female participants alike, more male participants talked about the added stress of either losing touch or being denied access to their children. Destructive family politics were mentioned by a number of participants, both male and female, and these corrosive situations had caused a great deal of stress.
For participants who had talked about marital or relationship breakdown the breakdown had preceded CHD and participants partly attributed their feelings of distress or depression to this experience and partly to their physical condition. Experiencing stressful life events was seen more as a factor that caused participants’ heart disease rather than heart disease causing them to feel depressed. 86
Participants experienced bereavement as a severe loss and the source of ongoing distress. Some participants believed that there was a causal link between experiencing grief and heart disease. Parkes et al. 87 argued that the association between heart disease and bereavement is one that cuts across social class difference. Patients with CHD may need particular help in the case of bereavement.
One participant was a full-time carer for his wife, who was incapacitated through rheumatoid arthritis. Although this participant was in his eighties and had suffered a heart attack, he did all the housework, washing, cooking, shopping and personal care for this wife.
This participant reported feeling depressed about being trapped in the daily grind of being a carer and not being able to visit his children in America. His wife refused any respite care and her family were unsupportive. The loss that this participant was experiencing centred round a loss of freedom, which included the freedom to visit his children in America.
Weak links made between coronary heart disease and depression
A number of participants were coping with CHD and comorbid conditions including: asthma, psoriasis, chronic obstructive pulmonary disease (COPD), arthritis, diabetes, thrombosis, sarcoidosis, stroke, hearing loss, poor eyesight, diabetic blindness, memory loss, prostate problems, kidney problems, paroxysmal positional vertigo, morbid obesity, underactive thyroid and restless leg syndrome. Chronic painful conditions limited mobility and interfered with the daily activities of participants.
Conditions that caused pain, such as arthritis, seemed to distress participants the most. As there are no ‘cures’ for some health conditions participants were faced with a seemingly never-ending illness trajectory. These factors, coupled with the realities of ageing, made the future seem bleak for some participants. Links made between depression and CHD were weaker in participants coping with other painful health conditions. Participants with comorbidities seemed more depressed and attributed low mood to physical conditions that were painful and disabling, such as arthritis.
NHS interventions and services participants found helpful or unhelpful
Talking therapies
Participants seemed to prefer NHS services for CHD and depression that included either or both of the following: interpersonal talking approaches and the opportunity for social interaction and support. Services that had been offered to patients and were considered helpful included: CR, information and self-help, counselling, referral to a psychiatrist or therapist and supervised exercise.
Although a few participants had received a counselling therapy for depression, there appeared to be some lack of provision for counselling services in primary care. Other problematic issues raised in connection with counselling were: continuity of care/therapeutic alliance, service availability, effective signposting, crisis prevention and waiting lists.
Psychiatric services
One male participant who had been severely depressed post cardiac surgery was very appreciative of the help that he received from two psychiatrists. However, it was the talking part of the psychiatric support that he found most helpful, particularly in expressing his feelings and in communicating these to his wife. This participant felt that his marriage had become stronger because of this therapeutic approach.
A number of participants had attended a 6-week CR programme post MI. Everyone who attended CR found it a helpful and positive experience. Participants found CR programmes to be useful and enjoyable for the following reasons:
-
Mastery: patients taught to monitor their pulse rates, improve diet and lifestyles, and take back control of their bodies.
-
Confidence: supervised exercise programme built confidence – patients felt safe and reassured about any physical sensations they were experiencing.
-
Social support: patients enjoyed a group approach and supported each other.
As found in the pilot interview study, most participants in this study reported wanting to attend a similar supervised programme of exercise for patients with CHD rather than attending a normal fitness centre, where there may be a lack of supervision or group support.
Participants’ own coping strategies
Most participants had developed coping and self-management strategies to help themselves when they were ‘having a down day’ and these included:
-
Self-talk and thinking strategies: counsels self, counting one’s blessings, setting mini-goals and pacing progress, visualisation techniques (taught by therapist).
-
Meditation and yoga: a number of participants used meditation and yoga techniques for relaxation, to calm the mind and alleviate physical symptoms.
-
Religious practices/spiritual beliefs: provides social support, calming effect of praying and being in place of worship.
-
Exercise and hobbies: swimming, walking, fishing, lawn bowling, going to gym, Tai Chi.
-
Creative expression: writing poetry, listening to music.
-
Displacement activities: keeping busy, finding something to do.
Some of these strategies, such as goal-setting, self-talk/thinking strategies, and meditation and yoga could be developed in a primary care approach to treating concurrent CHD and depression.
Exercise
Exercise was a strategy used by participants to improve physical fitness, elevate mood and provide opportunities for social interaction. The kinds of physical activities mentioned were walking, swimming, Tai Chi, grass bowling, fishing and going to a gym. For some participants exercising also offered the opportunity to socialise with a friend and share experiences with family members.
Engaging in physical activities, hobbies and sports could be encouraged in primary care by identifying and addressing any barriers to access and through helping the patient to set goals, pace themselves and by monitoring and encouraging their progress.
NHS services for depression that participants did not find helpful
Antidepressants
Overall, participants were not enthusiastic about antidepressant medication for the following reasons: did not want antidepressants on medical record (stigmatising), incompatibility issues with other medications and unpleasant side effects.
Discussion
Most participants in this study had suffered heart attack(s) and other health/personal traumas that had impacted on every area of their lives. The losses experienced by participants were complex and multiple. For this reason the association participants made between CHD and depression varied substantially.
Male participants in the study made the strongest links between their physical state and mental health. Charmaz88 (p. 268) observed ‘illness can reduce a man’s status in masculine hierarchies, shift his power relations with women and raise his self-doubt about masculinity’. These observations were reflected in our study of men who felt emasculated by CHD. Men who suffer serious cardiac disease may need specific therapeutic support to help them find new ‘scripts’ through which they can perform positive masculine identities.
Participants who had suffered losses in their personal relationships through divorce and bereavement made weaker causal links between CHD and depression. These participants attributed depression mainly to the unhappiness and upheaval they had experienced in their lives. Together with patients who had multiple health conditions, these participants felt that they needed someone to talk to, who would get to know them, rather than take antidepressant medications. Patients who have lost a partner through death or divorce might find the experience of ageing and ill health a lonely process. These participants do not want to ‘burden’ their children with their concerns and they are reluctant to talk to their GP because they generally do not want to be perceived as ‘moaning and groaning’. Older CHD patients, living alone, may be more susceptible to depression if they lack an intimate confidante and someone they can share age-related anxieties with.
The theme of resistance underpinning reasons participants gave for not wanting to take antidepressants may be reflecting one way in which patients can reclaim some control. These patients may have been demonstrating resistance to the medicalisation of their lives and perhaps the resulting feelings of disempowerment this engendered. As a number of participants felt that their lives were already dominated by ‘taking pills’ perhaps they felt they could say ‘no’ to antidepressants, in contrast to the rest of their medication, which was probably essential to the functioning of their hearts. A diagnosis for depression as a ‘mental illness’ is a culturally powerful label that can have serious implications for how a person is perceived. Resisting antidepressants could be a way of resisting the meaning given to ‘depression’ via medical records and biomedical discourse. 89–92
Barriers to accessing care provided by GPs, such as an overly bureaucratic GP appointment system, a lack of patient privacy when upset and a lack of continuity of care can affect the quality of care for patients with mild to moderate depression. 93 Continuity of care, that allows the patient to develop a relationship over time with one GP, might also have the benefit of improving facilitation of conversations about emotions and feelings for patients who are distressed or depressed. Privacy and continuity of care may be important elements to consider when designing a primary care intervention for CHD and depression.
From care currently provided by the NHS for patients with CHD, CR courses and appropriately supervised exercise were rated highly by participants. However, the fact that participants enjoyed CR may not equate with a significant improvement in their depressive symptoms. A longitudinal study on the effect of CR on depressive symptomatology94 concluded that exercise itself is not effective in alleviating depression and that CR programmes should provide specific screening and treatment for depression. Doing something enjoyable or useful is important to most people and perhaps this should be seen as just one important ingredient in a holistic approach to improving mental health rather than a treatment for depression per se.
Although a theme of ‘loss’ underpins participants’ accounts of CHD and depression, this does not mean that participants’ lives were totally devoid of happiness and pleasure. Participants talked about enjoying rewarding relationships with grandchildren, going on holidays and day trips, doing voluntary work and in self-development. Despite having symptoms of distress/depression, most participants had hopes and dreams for the future, some of which could be achieved through support in goal-setting and realistic pacing. A primary care intervention that includes a mentoring approach, as part of a stepped care model for treating depression, could help patients identify and achieve some of their goals while enhancing a sense of self-determination and control over their lives. Table 4 summarises participants’ ideas and preferences for interventions that could be delivered in primary care.
| Type of activity | Translation to a primary care intervention | Similar approaches that can be adapted |
|---|---|---|
| Group-based exercise | Ongoing supervised exercise for people with sCHD: to achieve a sense of mastery, increased confidence and an opportunity for social support | Similar to CR programmes but ongoing |
| Individual approaches to exercise and other lone activities/interests of patient’s choice | One to one with a PN to identify realistic goals Support in pacing and achieving goals Empowering patients Improving sense of control and self-confidence |
Mentoring approach |
| Having someone to talk to who knows them – when they need it (within reason) – over an extended period of time | PN with CBT training Clinician continuity important Dealing with issues of loneliness by helping patient to make supportive social links in the community |
Similar to CBT or solution-based approaches to counselling, but less formal/medicalised and with more flexible time frames. Privacy is also important |
How findings from the interview studies informed the pilot intervention
A clear finding from the patient interview studies was that (1) participants were experiencing a range of social, physical and gender identity (erectile dysfunction) losses; (2) some participants preferred to do things on their own, whereas others preferred group activities; (3) most participants had hopes and dreams for the future or enjoyed some aspect of their lives; (4) participants felt disempowered by ill health and the medicalisation of their lives; (5) participants preferred therapeutic treatment approaches for depression; (6) participants were not enthusiastic about antidepressant medication; and (7) loneliness was a problem for a number of participants.
Given the diverse needs of the sample (older people with chronic health conditions who feel lonely, men who felt emasculated and/or want to work again and participants who had experienced a number of adverse life events) an intervention for patients with CHD and depression may need to adopt an individual approach that can accommodate the specific sociocultural and personal needs of patients. A holistic, case management/mentoring approach to depression in CHD could help patients to feel more empowered and in control of their lives, especially if they are supported to achieve personal and health goals.
While conducting the patient interview study, interim findings, participant suggestions and final conclusions were discussed iteratively with the UPBEAT-UK team. The final design of a primary care, nurse-led complex intervention was informed by both the GP and PN (see Chapter 2), and patient qualitative studies together with the UPBEAT-UK literature reviews.
Plans for the content and structure of a nurse-led complex intervention were discussed in-depth with patients (suffering from sCHD and depression) during a series of two focus groups. Adjustments to the design and content of the intervention were made according to recommendations from the focus groups.
Ethical approval
Bexley and Greenwich Ethics Committee gave ethical permission for the study (reference 07/H0809/38), and approval was obtained from NHS trust research governance offices in south-east and south-west London. The researcher (RS) consented the participants.
Chapter 4 Development and testing of an intervention for primary care patients with symptomatic coronary heart disease and depressive symptoms (work package 2)
Plan
The previous chapters detail how we gathered the views of patients, GPs and PNs as well as findings from published studies concerning requirements and preferences for a future intervention for people with sCHD and comorbid depressive symptoms. In this work package of the UPBEAT-UK programme, our aim was to develop and evaluate an intervention that would be feasible to deliver in UK primary care.
We conducted an iterative evidence review and synthesised data from previous work packages to develop the intervention, which we then modified informed by findings of a focus group study with people with sCHD and depressive symptoms and further literature review.
We then conducted an exploratory randomised controlled study to examine the acceptability, feasibility and potential costs of the new intervention, and to test the methods for a definitive trial.
Developing the intervention
Barley et al. 2 published a full account of the process that we used to develop the intervention in an open access journal in 2012. The key steps of the process, which followed MRC guidelines for developing complex interventions,30 are summarised here. This process is also depicted in Figure 3.
FIGURE 3.
The UPBEAT-UK study intervention development stages. Reproduced from Barley et al. 2 © 2012 Barley et al. ; licensee BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
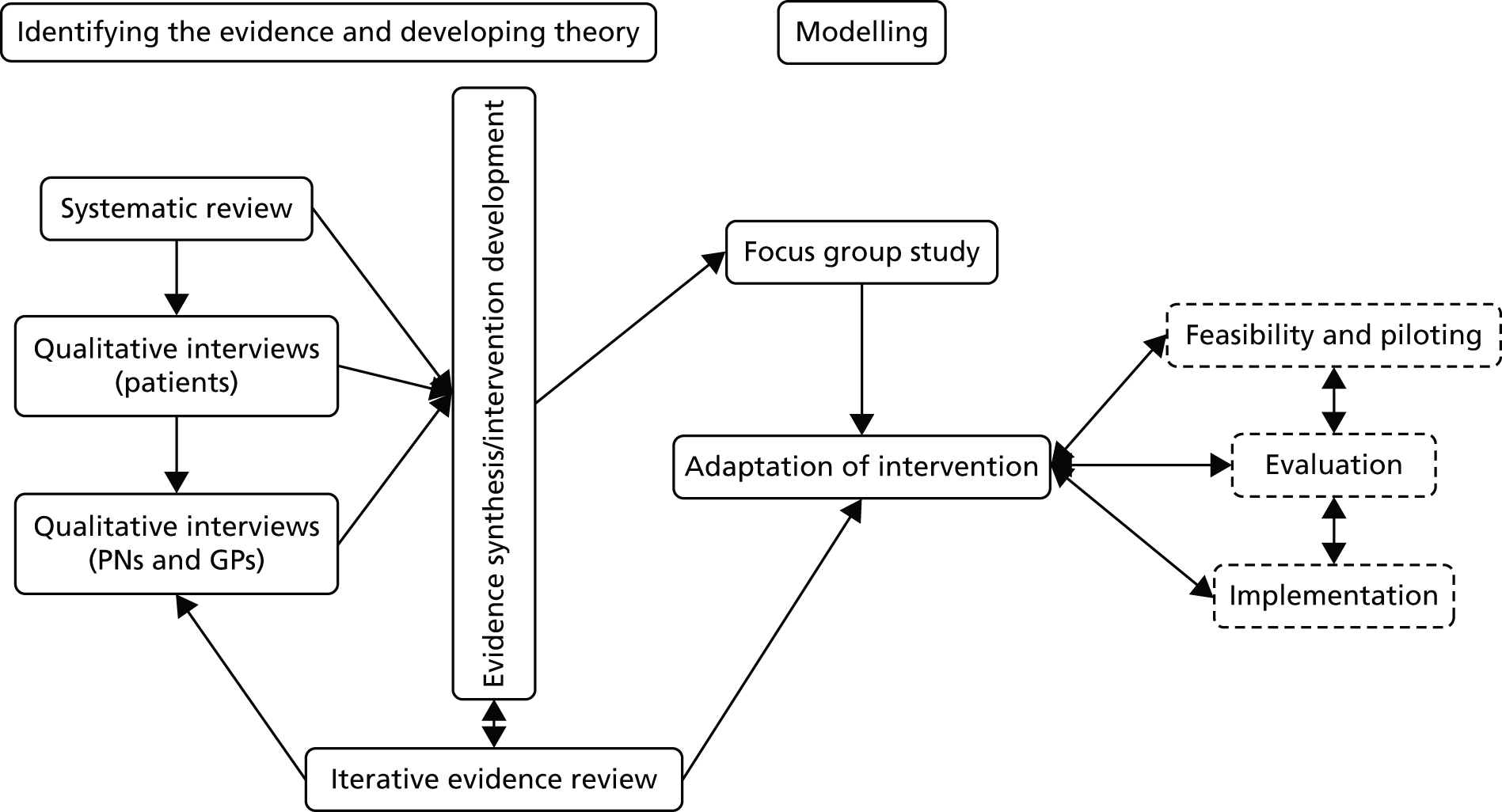
Phase 1: gathering evidence
This phase included the qualitative studies of patients and primary care clinicians, and the metasynthesis of published literature detailed in Chapters 2 and 3. Essentially, all three studies led to the conclusion that the needs of patients with CHD and depression are diverse and include psychosocial problems involving interpersonal and health/control losses that primary care staff are uncertain how to manage.
Phase 2: synthesis of findings from previous work packages and iterative literature review
Multidisciplinary team discussions involving GPs, psychiatrists, psychologists, nurses and a cardiologist, of our earlier studies led to agreement that the UPBEAT-UK intervention should:
-
improve depression, quality of life and cardiac outcomes in patients with CHD
-
help patients and clinicians to manage an individualised range of problems, including social problems
-
be nurse-led and feasible for delivery in primary care.
These conclusions were used to guide literature searches focused on identifying high-quality systematic reviews and evidence-based guidelines: we searched the Cochrane Database of Systematic Reviews, the Database of Abstracts of Reviews of Effectiveness and the NICE website. We also looked for evidence published subsequently to the reviews and guidelines using MEDLINE, EMBASE and PsycINFO. Findings from the evidence reviews were discussed and used to support choices for the intervention content. This is detailed in Table 5 (first published in our RCT development paper). 2
| Intervention should | Findings | Conclusions in relation to new intervention |
|---|---|---|
| Improve depression, quality of life and cardiac outcomes in patients with CHD | NICE guidelines18 for depression with a chronic physical health problem recommend a stepped care model involving psychological therapy and/or pharmacotherapy Two Cochrane reviews95,96 identify a range of psychological and pharmacological interventions; there is insufficient evidence to determine which elements are beneficial. No effect found on cardiac outcomes A well-conducted trial in the USA12 found that collaborative care improved depression and disease control in patients with CHD and/or diabetes |
Collaborative care is intensive; our empirical work suggested only a minimal intervention would be feasible A key ingredient of collaborative care is ‘case management’ (CM):97 a health worker follows up patients, assesses adherence, monitors progress, takes action when treatment is unsuccessful and delivers psychological support as part of a stepped care approach.25 This is a central aspect of the UK long-term conditions strategy98 so it is familiar to PNs CM allows PC but the processes by which change is expected to occur should be specified |
| Help patients and clinicians to manage an individualised range of problems, including social problems | The CHD and depression association is likely to be explained largely by behaviour.99,100 Behaviour change interventions for risk behaviours for CHD and depression have been delivered in primary care and shown benefit for smoking101 and alcohol intake.102 Not known if they are helpful for patients with CHD and depression Current UK policy103 promotes liaison between professionals and utilisation of existing resources to tackle depression and adverse health behaviours |
Training in specific behaviour change techniques and identification of existing local resources, such as social clubs, advice agencies and therapy services, would provide PNs with a ‘toolkit’ of resources that they could tailor according to patient need and preference Specification of interventions used will inform implementation and evaluation of the intervention |
| Be nurse-led and feasible for primary care | Case managers are often nurses, but studies lack details concerning implementation and process104 Evidence-based behaviour change interventions, such as goal-setting and action-planning, have been identified for use in primary care64 |
Pilot work should be undertaken to understand which aspects of case management are effective in CHD patients with depression and which outcomes should be targeted |
We discussed the meaning of these findings in the context of UK primary care, where an established component of chronic disease management is the provision of self-management support; this means enabling patients to take better care of themselves, for instance by providing information and helping them to change unhealthy behaviours.
Two important factors for behaviour change are known to be belief in the importance of an outcome and belief in capacity to succeed (self-efficacy). 105 This suggested to us that instead of focusing on generic CHD or depression risk factors, the new intervention should enable patients to specify their own goals, for instance stopping smoking or increasing social contact, so that work is directed towards outcomes important to patients.
Informed by our evidence review a ‘toolkit’ of behaviour change skills and existing local resources was developed to facilitate nurses, acting as case managers, to help the patient to increase their self-efficacy and achieve their desired outcomes.
The intervention we developed therefore was a PN-led PC intervention. It comprises the following:
Personalised care planning: the nurse acting as case manager conducts a standardised biopsychosocial assessment (including physical and mental health, difficulties with current treatment regimens, problems with daily activities and social problems) face to face with the patient either at the patient’s GP surgery or at their home, according to patient preference. Patients are helped to identify up to three problems that they consider contribute to their depression and which they most want to address. The nurse case managers, as applicable, provide information, signpost patients to existing resources (e.g. leisure centres, social clubs, IAPT services) and use evidence-based behaviour change techniques to help patients set and achieve goals. The underlying intention of the intervention is to increase the patient’s self-efficacy to achieve their desired goals (as opposed to goals determined by others such as symptom management or reduction of cardiac risk factors, a primary aim of many previous collaborative care projects). Details of the assessment and action plan are recorded in a ‘personalised health plan’, which the patient holds.
Follow-up care: follow-up interviews to determine progress and/or set new goals are conducted via telephone to conserve nurse time. This is initially weekly and then the frequency of contact is agreed between the patient and nurse case manager. Calls are planned to last 15 minutes.
The intervention process is detailed in Figures 4 and 5 (first published in our RCT development paper). 2
FIGURE 4.
Personalised care planning. The UPBEAT-UK intervention assessment stage and initial care planning. Reproduced from Barley et al. 2 © 2012 Barley et al. ; licensee BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
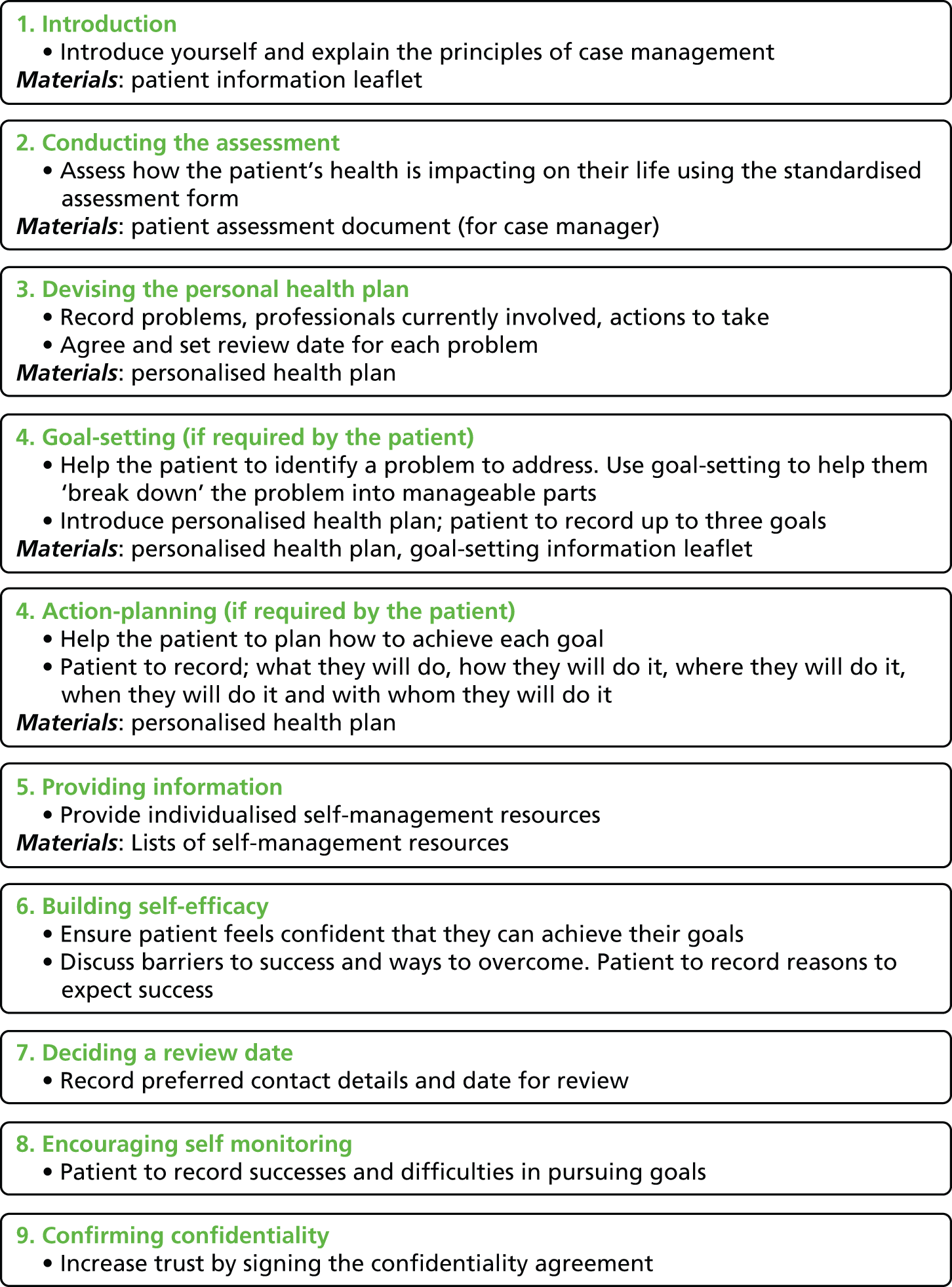
FIGURE 5.
Follow-up care. The UPBEAT-UK intervention follow-up care stage. Reproduced from Barley et al. 2 © 2012 Barley et al. ; licensee BioMed Central Ltd. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
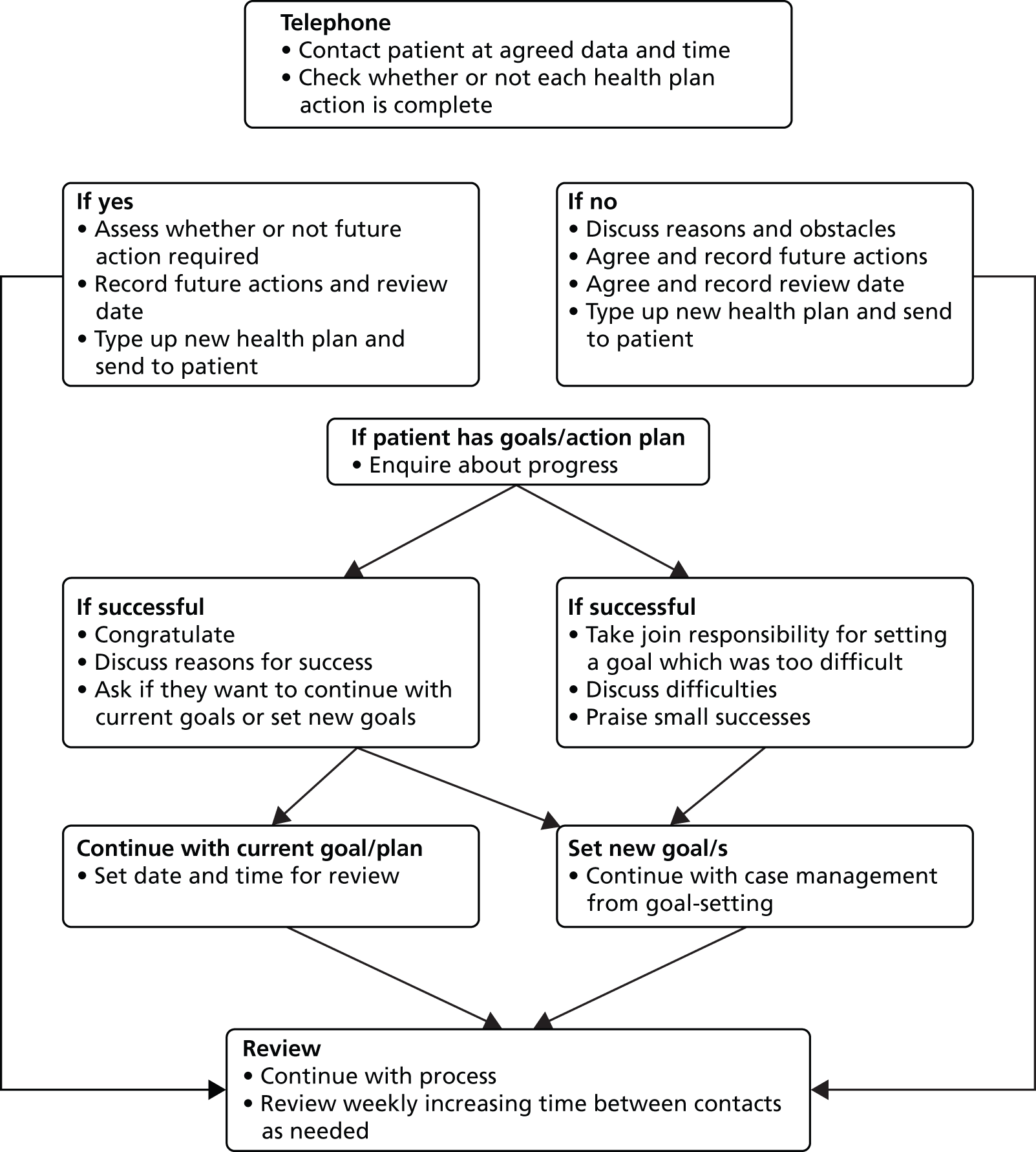
Phase 3: modelling
Having developed the intervention, and following MRC guidance,30 we now wanted to explore its acceptability among people with sCHD and depressive symptoms. Using maximum variation sampling to select patients varying by sex, age, ethnicity and borough of residence, we invited participants of the UPBEAT-UK cohort study to attend two focus groups.
In a short presentation, two researchers (RS and Zoe Fortune) explained the proposed intervention and the intervention materials (patient information leaflet, assessment form, personalised health plan) were provided. All participants gave informed consent and ethical approval was granted, along with that for the other qualitative studies, by Bexley and Greenwich Research Ethics Committee (07/H0809/38).
Sixteen patients agreed to take part. Thirteen actually participated; five out of six came for the first group and eight out of 10 came for the second group. Reasons for not attending were not elicited. Those who participated (53% male) were aged between 48 and 86 years (mean 71 years). All but two, who were African Caribbean, described themselves as white British.
Discussions were tape-recorded and transcribed by RS. Transcripts were entered into NVivo 8 qualitative software. Coding was informed by the aims of the study. The coding frame and themes were discussed within the research team; we used notes of key discussion points (verified by participants) to guide our analysis.
Key findings of the discussion and modifications to the intervention
The participants were very focused on their need for someone to confide in and felt that a case manager would help with this.
The concept of self-management, in which the case manager would help them to solve their own problems, did not seem to be well understood. We planned to examine this in our exploratory study.
Participants agreed that PNs were the correct people to act as case managers because of their understanding of heart disease and its associated problems. Nurses should also be able to provide the continuity of care that participants emphasised as important.
Some participants disliked the planned use of goal-setting. Rather, participants were focused on wanting case managers to be a source of social contact. Based on our work with PNs, who complained of lack of time to manage these patients, this did not seem feasible. Instead, we felt that goal-setting and action-planning could be used to help patients obtain social contact. Furthermore, our literature searches identified good evidence for the feasibility of goal-setting in primary care. 106 This highlighted to us the importance, when designing interventions, of utilising multiple sources of evidence. We planned to explore the acceptability of goal-setting further in our exploratory study.
Finally, a minor point was that some of the wording used on the assessment form was not clear. We had developed our care plan format from the framework proposed by the Department of Health as the standard assessment for adults;107,108 this grouped health-related domains such as ‘activities of daily living and mobility’ under headings such as ‘improved personal dignity and autonomy’. These higher-level headings appeared to confuse patients, so they were removed from intervention documents.
Discussion
Informed by the MRC framework for the design of complex interventions,30 we conducted empirical studies and iterative literature searching to identify evidence and theory to develop and model a new PN-led PC intervention to improve mood and cardiac outcomes in sCHD patients with depressive symptoms.
Our approach is only one possible approach to developing a complex intervention using the MRC framework. 30 For instance, others109 have drafted an intervention around a specific psychological theory and have tested theory-related hypotheses. However, we feel that a strength of our approach is that the intervention development was driven by the patients who will receive it and by the clinicians who will deliver it, with theory (i.e. self-efficacy theory and behaviour change theory) and evidence used to support choices concerning its content. The fact that all the studies reported here were funded by a single programme grant facilitated access to patients and allowed subsequent work to build on earlier findings in a timely fashion.
Furthermore, our approach led us to change our initial plans, which, as we have explained in Chapter 1, were to develop a nurse-led stepped care intervention. Our intervention development work clearly demonstrated a need for an intervention that could be tailored to the differing needs of individual patients; this could be included within a stepped care approach. It was also clear from this work that some PNs will need support to deliver an intervention for depression and that, since their workload is already very high, they would need convincing that any new intervention is feasible for them to deliver as well as effective for patients. Hence, with so much uncertainty around the feasibility of PN-led interventions for depression, we changed our plan to conduct a definitive RCT and decided to conduct an exploratory study which would provide this evidence and inform the best methods of a future definitive RCT.
The exploratory randomised controlled trial
In our exploratory study, two nurse researchers, independent of the participating practices, acted as case managers; one was a general and mental health nurse with experience of working in primary care, and the other was a general nurse who had subsequently qualified as a health psychologist. We also knew, from our cohort study, that CHD patients have high levels of comorbidity and that for some any cardiac event would have been several years ago; we therefore recruited only those patients with sCHD (i.e. reporting current chest pain) in order to ensure that they would understand the intervention in terms of their CHD. The study explored the acceptability, feasibility and potential costs of PN-led PC for primary care CHD patients who have depressive symptoms and current chest pain with the overarching aim to test the methods for a definitive trial. The full details of this study have been published in a peer reviewed paper by Barley et al. 110 Here we summarise our methods and findings and report additional exploratory analyses.
Aims and objectives
-
To examine the rate of participant recruitment and reasons for non-participation.
-
To examine research procedures such as consent, randomisation/blinding and data collection.
-
To explore possible differences between primary outcome measurements and explore the most appropriate secondary outcome measures in relation to patients’ reported problems.
-
To identify any trends between the groups in changes in self-efficacy and the impact of this on depression outcomes.
-
To determine the acceptability and feasibility of the intervention to practices and participants.
-
To explore whether or not the intervention can be standardised and whether or not therapist effects are likely to be important.
-
To explore potential costs of the intervention.
Methods
Design
The design was a patient-randomised pilot study with a control condition. We compared PN-led PC plus TAU for 6 months with TAU alone. The protocol for the study has been published;111 deviations from the protocol are explained in the published report of this study. 110 The study was reviewed and approved by the south-east London Research Ethics Committee (reference 10/H0808/5) and is registered with Current Controlled Trials International Standard Randomised Controlled Trial Number 21615909. The UPBEAT-UK Programme Grant Steering Committee, who decided that a data monitoring committee would not be necessary, oversaw the study.
Study setting
Practices in south London were recruited via the Greater London Primary Care Research Network (PCRN-GL). To be included, the practice had to keep a register of patients with CHD for the QOF112 and be willing to liaise over patients in the PC arm when necessary.
Participant eligibility and recruitment
Inclusion criteria were sCHD (registered on GP CHD QOF register and reporting chest pain), reporting depression symptoms. All patients on practice case registers for CHD were asked by their GP for consent to contact from a researcher. Those consenting were contacted by a researcher and assessed for depressive symptoms using the PHQ-280 and for symptoms of current chest pain using the modified Rose angina questionnaire. 113 Patients scoring ≥ 3 on the PHQ-2, and who reported currently experiencing chest pain (using the modified Rose angina questionnaire) were assessed further using the HADS. 114 If they scored ≥ 8 on the depression scale of HADS (HADS-D) they were eligible to participate. Those consenting to participate were then randomised to either the intervention (PN-led PC and general practice TAU) or control (general practice TAU). Patients who were temporary registrants or currently hospitalised, or who a GP from the practice deemed actively suicidal, suffering from psychotic depression or non-English speaking were excluded.
As estimation of an effect size was not the focus of this pilot study, we used only a preliminary sample size calculation. An end-of-study mean difference between intervention and control score of ≥ 3 on the HADS-D, assuming a pooled SD around mean scores of 3.5, would require 30 participants per group for 90% power at the 5% significance level. To allow for loss to follow-up, estimated at 25%, our plan was to recruit 80 participants (40 per arm) into the study. The target figure of 3 was based on consensus discussion among clinicians at planning meetings and the assumed SD of 3.5 was obtained from the baseline cohort study. The estimate of attrition of 25% was considered reasonably conservative; it was felt that higher levels would have indicated lack of feasibility for implementing and testing the intervention. We estimated from the results of the UPBEAT-UK cohort study1 that 10–15 practices each with around 10,000 patients would be needed to recruit 40 persons per arm.
Randomisation and blinding
Randomisation at patient level was conducted independently by the Mental Health and Neurosciences Clinical Trials Unit at King’s College London. A random permuted block design was used to balance the numbers between groups. PC group participants were randomly allocated to one of two nurse researchers acting as case managers.
To ensure that those responsible for outcome data collection were blind to participants’ allocation status, participants were asked at the beginning of each follow-up interview not to mention if they had been in contact with other study staff. The statistician was also kept uninformed of allocation status.
Outcome data collection
Research assistants who were blind to allocation collected data at baseline and at 1, 6 and 12 months post randomisation. Data were collected face to face at baseline and at follow-up via telephone.
The intervention: personalised care
This was delivered over 6 months, as detailed above.
Usual care
All patients received primary care TAU from their GP and/or PN; this may or may not include specific depression intervention such as antidepressant prescription or referral to talking therapy. The nature of TAU may vary between practices; we assumed that important differences in care delivery between the participating practices would be randomised between the groups.
Outcomes
Baseline demographic variables
All participants provided baseline demographic data including sex, age, ethnicity, socioeconomic status,115 employment and relationship status, living arrangement and lifestyle factors (e.g. smoking status, alcohol consumption, body mass index).
Aim 1: to examine participant recruitment
We made detailed records of recruitment rates. The number of participants at each stage of the study was documented and reasons for attrition were recorded.
Aim 2: to examine the study procedures
We recorded the number of randomisation errors [e.g. numbers of participants randomised despite being ineligible or who were randomised to the intervention (PC) but who did not receive it], and recorded rates and reasons for attrition and missing data for outcome measure at each time point.
Aim 3: to explore outcome measures
The preliminary primary outcome was the HADS-D. We observed depression status (response defined as ≥ 50% decrease in score from baseline at follow-up and remission defined as a score of < 8 at follow-up) and severity (continuous score). We also explored the PHQ-9116 as an alternative measure of depression severity and extracted the number of GP/PN consultations for depression, antidepressant prescriptions and referrals to talking therapy during the 12-month study period from participants’ medical records for both groups.
Our cohort study found that self-reported chest pain (measured using the modified Rose angina questionnaire) is associated with mood and social problems, so was also explored as a potential primary outcome for a future trial. The number of GP/PN consultations for heart-related problems during the 12-month study period was also extracted from participants’ medical records as a proxy measure of participants’ cardiac status in both groups.
Potential secondary outcomes explored were: anxiety [HADS – anxiety subscale (HADS-A)],114 well-being (Warwick–Edinburgh Mental Well-being Scale),117 quality of life [Short Form questionnaire-12 items (SF-12)],118 functional status (specific activity schedule),119 number of reported social problems120 and adherence to antidepressant medication (if relevant – adapted version of Morisky Adherence Index121). To try to capture between-patient variety in reported problems and needs, we used a validated patient-generated measure of problems, function and well-being – the Psychological Outcome Profiles Questionnaire. 122
To reflect on how these outcome measures relate to the problems that patients consider important or feasible to change, we explored the types of needs and problems identified by intervention patients in collaboration with their case manager. This information was extracted from the notes made by nurse case managers during consultations. The Brief Illness Perceptions Questionnaire (BIPQ)123 asks participants to ‘Please list in rank-order the three most important factors that you believe caused your illness’ – we also explored these responses.
Aim 4: to explore changes in self-efficacy
We used the General Self Efficacy Scale;124 the scale has 12 items designed to assess perceived self-efficacy in order to predict coping with daily hassles and adaption after life events, with a high score indicating greater self-efficacy (range 10–40). We also used the BIPQ123 to assess changes in perceptions about illness along the following dimensions: consequences, timeline (anticipated duration of illness), personal control, treatment control, identity (symptoms associated with the illness), illness concern, illness coherence (understanding of CHD) and emotional representations (emotional impact of CHD). Each of these eight items is scored 1–10. We examined General Self Efficacy Scale and BIPQ total scores as mediating factors for depression symptom reduction, remission and response.
Aim 5: to determine the acceptability and feasibility of the intervention
We recorded the time taken for assessment and the number and duration of follow-up telephone calls per patient, and explored participant satisfaction using an 11-item questionnaire devised for the study that was posted to the intervention patients after their 12-month follow-up.
Aim 6: to explore whether or not the intervention can be standardised and whether or not therapist effects are likely to be important
We developed a manual for the intervention, examined differences in patient outcomes between the two nurse researchers delivering the intervention and recorded nurse actions during the intervention.
Aim 7: to explore potential costs of the intervention
We calculated quality-adjusted life-year (QALY) gain using the European Quality of Life-5 Dimensions (EQ-5D). 125 The EQ-5D consists of five domains (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) and each is rated 1 (no problem), 2 (moderate problems) or 3 (major problems). UK values were applied to the distinct health states derived from the EQ-5D to estimate the utility value for each patient at each time point and area-under-the-curve methods were used to calculate the QALYs. 126
Economic costs were calculated from a societal perspective. PC costs included the time spent by PNs with patients in face-to-face assessments and subsequent telephone reviews. A unit cost of £36 per hour was attached to the average intervention duration for each patient. Other service use was recorded using the Client Service Receipt Inventory127 for the 6-month period preceding baseline, 6- and 12-month follow-ups. Health services included hospital inpatient and outpatient visits, GPs, psychiatrists, psychologists, physiotherapists, counsellors, nurses and other therapists. Unit costs were applied to service-use data using the NHS reference costs in 2009–10 prices128 and the 2010 Unit Costs of Health and Social Care. 129 In addition, data were collected on the weekly number of hours of help (i.e. personal or child care, help in/around and outside the house) received from friends and relatives of the patient. The unit cost of a home care worker was used as a proxy for costing informal care.
Medication use was recorded and costs were calculated based on the 2010 prices from the British National Formulary130 and the Prescription Cost Analysis. 131 The basic types of medication included psychological, cardiovascular (most common type), gastrointestinal, respiratory, eye, ear, nose and oropharynx drugs. Indirect costs of productivity loss because of CHD and comorbid depression were calculated using the human capital approach. For employed patients, productivity loss was the product of the days missed from work caused by sickness and the national mean daily wage in the UK,132 adjusted for full- or part-time working. However, only resource-use costs were considered in the cost-effectiveness analysis, as data on medication and sickness absence were self-reported and available for the baseline only (regarding the past 6 months).
Analysis
We conducted exploratory analyses using Stata version 11.2 (StataCorp LP, College Station, TX, USA). The intention-to-treat principle was used for all analyses. Owing to the exploratory nature of the analysis, p-values are reported for the preliminary primary outcome (HADS-D) only. We were fortunate to have a National Institute for Health Research-funded statistical fellow attached to our programme and were therefore able to conduct detailed exploratory analyses; these are explained fully in the published report of this study. 110 Essentially, we developed a single statistical model to estimate the difference in mean scores between participants randomised to PC and TAU across the three follow-up points (1, 6 and 12 months). Other exploratory analyses compared the median number of responders (≥ 50% decrease in score from baseline at follow-up) and remitters (score of < 8 at follow-up) according to the HADS-D score between groups and explored changes in self-efficacy, the effect of nurse contact time and therapist effects using t-tests and chi-squared tests.
A health economics analysis used multiple regression, incremental cost-effectiveness ratios and the net benefit approach to estimate mean differences in costs and QALYs. Non-parametric bootstrap analyses were conducted to account for the highly skewed distribution of the cost data; results were plotted on a cost-effectiveness plane and used to estimate cost-effectiveness acceptability curves.
Results
Aim 1: examining recruitment
Seventeen practices were approached by the PCRN-GL and agreed to participate. Practices were recruited between October 2010 and June 2011; practice recruitment was therefore completed in considerably less than the 12 months planned in the study proposal, indicating that recruitment of practices for a definitive trial would be feasible.
We have published patient recruitment details in a paper by Tylee et al. 111 We summarise it briefly here. Of the 3325 people on the 17 GP CHD registers, 1001 consented to be contacted by returning a letter to their GP. A brief screen by telephone found that 126 were eligible for assessment (PHQ-9 score of ≥ 3 and reporting current chest pain on the modified Rose angina questionnaire). Of the 126 who were eligible, 40 had a HADS score of < 8, two had experienced hallucinations, two had no current chest pain and one did not have sufficient English, therefore, 81 were found to be eligible. These were consented and randomised (41 to intervention, 40 to control). The screening process involved minimal effort (return of a letter and a brief telephone screen). Target recruitment was achieved within 8 months, which was considerably faster than expected (we had planned a 12-month recruitment period based on experience of other studies); recruitment of patients for a definitive RCT, therefore, seems promising.
Baseline demographic and lifestyle data are reported elsewhere. 110 There were 27 (66%) males in the PC group and 25 (63%) in the TAU group; mean age was 64.2 years (SD 13.0 years) in the PC group and 64.9 years (SD 8.5 years) in the TAU group. Any differences between the groups on the recorded demographic, lifestyle and outcome variables appeared small and the randomisation process appears to have produced balanced groups.
Past depression
Forty-eight participants reported having ever been diagnosed with depression (21/41 = 51% PC; 27/40 = 67% TAU). Of these, 12 had had one episode, seven had had two episodes, four had had three episodes and 23 reported having had four or more episodes (data on number of episodes were missing for two participants). Forty-six participants had previously received treatment for depression; of these, 41 had taken antidepressants and 29 had had talking therapy. Eighteen reported having received other treatment such as ‘anger management’, seeing a psychiatrist, electroconvulsive therapy, inpatient psychiatric care and relaxation and assertiveness courses. Our participants therefore represent a chronic and severe group.
Current depression
Twenty-four participants reported that they were currently receiving treatment for depression (9 in PC, 22%; 15 in TAU, 38%). According to the medical notes data, 13 in PC (32%) and 17 in TAU (43%) were taking some form of antidepressant medication at baseline. Despite being prescribed antidepressants, these participants were still reporting depressive symptoms. Nineteen participants reported their current episode had lasted > 12 months, two said it had lasted between 6 and 12 months, and three said it had lasted < 6 months.
Mean HADS-D scores [PC 11.6 (SD 3.3); TAU 11.4 (SD 3.0)] indicated moderate depression and mean PHQ-9 scores [PC 16.0 (SD 5.3); TAU 15.4 (SD 5.5)] indicated moderately severe depression in both groups. At baseline, according to the HADS-D, 21 (51.2%) participants in the PC group could be considered mild, 14 (34.2%) moderate and six (14.6%) severe. In the TAU group there were 19 (47.5%) mild, 15 (37.5%) moderate and six (15.0%) severe. For the PHQ-9, three (7.3%) were mild, 10 (24.4%) were moderate, 14 (34.2%) were moderately severe and 12 (29.3%) were severe in the PC group. In the TAU group, there were eight (20.0%) mild, eight (20.0%) moderate, 14 (35.0%) moderately severe and nine (22.5%) severe. The correlation between baseline HADS-D and PHQ-9 was r = 0.48 (p < 0.0001).
Coronary heart disease status
Patients were recruited if they reported current chest pain. Overall, 19 were current smokers and 53 were overweight or obese (see Table 8). Participants were also asked if they had high blood pressure and cholesterol, and diabetes; 56 out of 76 who responded (74%) said yes to high blood pressure (29/37 in PC; 27/39 in TAU), 42 out of 72 who responded (58%) said yes to high cholesterol (21/34 in PC; 21/38 in TAU) and 22 out of 80 who responded (28%%) said yes to diabetes (12/40 in PC; 10/40 in TAU).
Aim 2: examining study procedures
Randomisation
Three patients who were ineligible owing to no current chest pain were randomised in error (two in the intervention arm); reasons for this are unclear. Based on the intention-to-treat principle these were included in all analyses, however, we conducted a sensitivity analysis and found that there were no differences in our conclusions when these patients were omitted from the analyses.
Blinding
Over the course of the study, there were many staff changes, especially among the research assistants responsible for outcome data collection. It was therefore not possible to test formally whether or not those collecting data remained blinded to the patients’ allocation status. However, it was noted that some participants had reported contact with the case manager. Following the conduct of the main analyses, the statistician reported becoming unblinded; this was as a result of hearing that an additional participant had been randomised to PC.
Attrition
The Consolidated Standards of Reporting Trials diagram for the study is shown in Figure 6 and has been published. 110 By 12 months, six people in the intervention group had dropped out (two because they found participation upsetting, two because they felt too physically unwell to continue and two gave no reason) and one from the control group had dropped out (because they found participation upsetting). Two intervention group participants received baseline assessment but no intervention, as the nurses were unable to contact them. Overall, attrition was low (7/81 = 9%), with data collected at one or more follow-up points for 79 people (98%).
FIGURE 6.
The UPBEAT-UK pilot study Consolidated Standards of Reporting Trials diagram. Uncontactable means lost to follow-up. Reproduced from Barley et al. 111 © 2014 Barley et al. This is an open access article distributed under the terms of the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
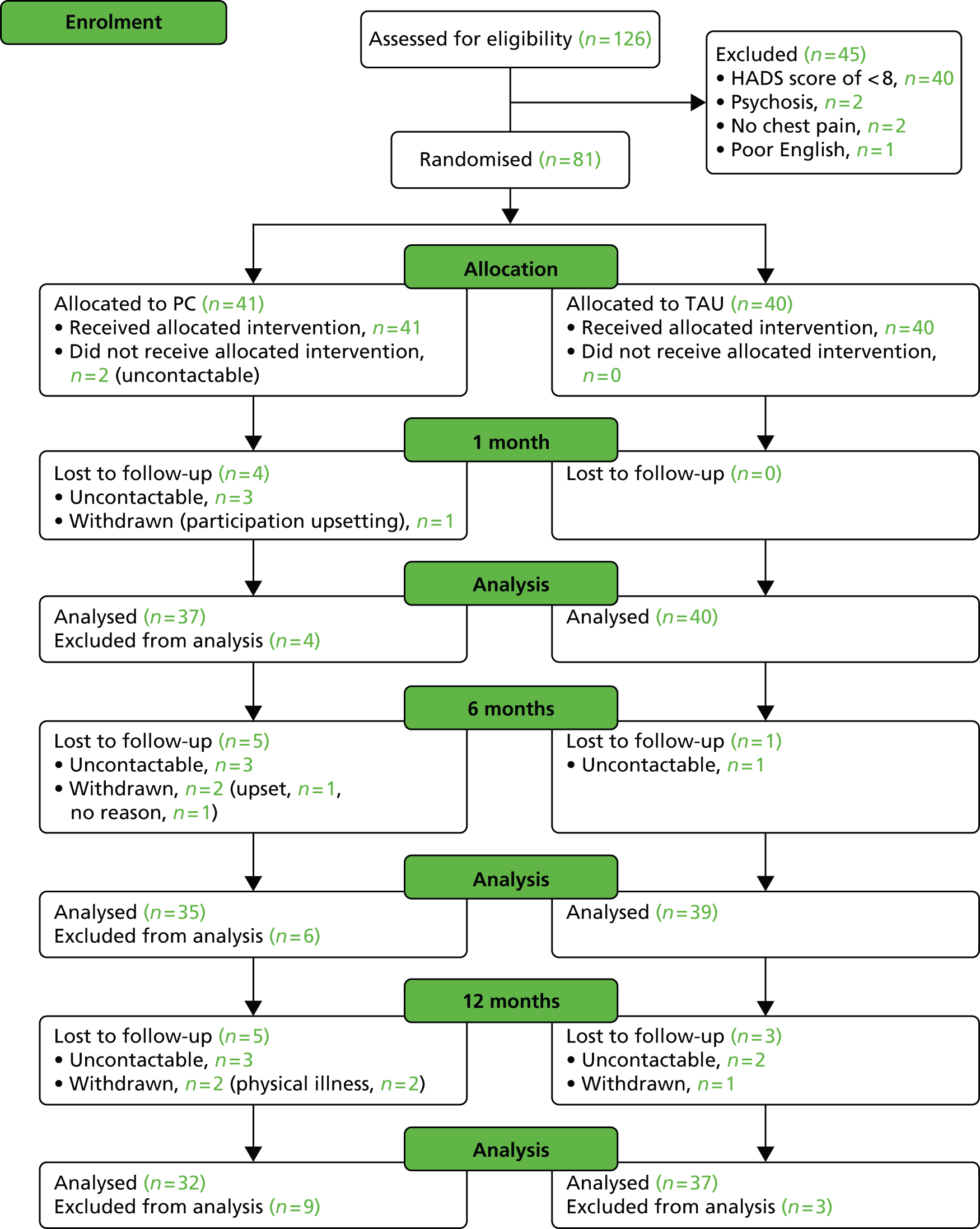
Across the study period, completion was better for the TAU group than the PC group (Table 6). Completion was better among non-drinkers and those that drank the most (> 11 units per week), as well as those who were retired compared with being in paid employment across time points. Characteristics of completers are described in Table 7, which lists the demographics of those who completed follow-up and those who did not. HADS-D scores (Table 8) were marginally lower at 1 and 6 months among non-completers; however, they evened out by 12 months. We compared the models from our analyses with an additional model that controlled for any variables that were associated with missing follow-ups; these analyses did not give us any reason to alter any of our conclusions.
| Measure | 1-month follow-up completed, n (%) | 6-month follow-up completed, n (%) | 12-month follow-up completed, n (%) |
|---|---|---|---|
| Randomisation | |||
| PC | 37 (90) | 35 (85) | 32 (78) |
| TAU | 40 (100) | 39 (98) | 37 (93) |
| Sex | |||
| Male | 48 (92) | 46 (88) | 44 (85) |
| Female | 29 (100) | 28 (97) | 25 (86) |
| Ethnicity | |||
| White | 64 (96) | 61 (91) | 57 (85) |
| Black | 3 (100) | 3 (100) | 3 (100) |
| Asian | 5 (100) | 4 (80) | 4 (80) |
| Other | 5 (83) | 6 (100) | 5 (83) |
| BMI | |||
| Underweight | 3 (100) | 3 (100) | 2 (67) |
| Normal | 19 (100) | 17 (90) | 15 (79) |
| Overweight | 22 (96) | 21 (91) | 21 (91) |
| Obese | 27 (90) | 28 (93) | 25 (83) |
| Smoke | |||
| Never | 20 (91) | 20 (91) | 18 (82) |
| Ex-smoker | 39 (98) | 37 (93) | 35 (88) |
| Current | 18 (95) | 17 (89) | 16 (84) |
| Units of alcohol | |||
| Does not drink | 38 (97) | 37 (95) | 35 (90) |
| 1–10 | 26 (90) | 24 (83) | 21 (72) |
| ≥ 11 | 13 (100) | 13 (100) | 13 (100) |
| Employment status | |||
| Paid employment | 10 (83) | 10 (83) | 10 (83) |
| Retired | 55 (100) | 52 (95) | 49 (89) |
| Housewife/husband | 4 (100) | 3 (75) | 3 (75) |
| Unemployed/student | 6 (86) | 7 (100) | 5 (71) |
| Relationship status | |||
| Married | 38 (95) | 37 (93) | 37 (93) |
| Cohabiting | 6 (75) | 7 (88) | 5 (63) |
| Widowed | 12 (100) | 12 (100) | 10 (83) |
| Separated | 4 (100) | 4 (100) | 4 (100) |
| Divorced | 11 (100) | 8 (73) | 8 (73) |
| Single/non-cohabiting partner | 6 (100) | 6 (100) | 5 (83) |
| Live with | |||
| Spouse | 26 (93) | 27 (96) | 25 (89) |
| Spouse and children | 14 (88) | 13 (81) | 13 (81) |
| Children | 7 (100) | 7 (100) | 6 (86) |
| Alone | 28 (100) | 25 (89) | 23 (82) |
| Other | 2 (100) | 2 (100) | 2 (100) |
| Place of residence | |||
| Owner occupied house/flat | 32 (97) | 32 (97) | 29 (88) |
| Privately rented house/flat | 8 (100) | 8 (100) | 7 (88) |
| House/flat rented from local authority | 32 (91) | 30 (86) | 29 (83) |
| Sheltered housing/warden control | 4 (100) | 3 (75) | 3 (75) |
| Other | 1 (100) | 1 (100) | 1 (100) |
| Measure | Completed | Not completed | ||||
|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |
| 1-month follow-up | ||||||
| Age (years) | 77 | 65.1 | 10.8 | 4 | 53.8 | 7.1 |
| IMD score | 77 | 26 | 13.9 | 4 | 26.6 | 10.2 |
| BMI (kg/m2) | 71 | 29.5 | 7.1 | 4 | 32.3 | 5.3 |
| Years in education | 74 | 12.7 | 8.4 | 4 | 14.3 | 5.3 |
| 6-month follow-up | ||||||
| Age (years) | 74 | 65.4 | 10.5 | 7 | 55.1 | 11.6 |
| IMD score | 74 | 26.1 | 14 | 7 | 25.6 | 10.8 |
| BMI (kg/m2) | 69 | 29.8 | 7.2 | 6 | 28 | 3.8 |
| Years in education | 72 | 12.0 | 4 | 6 | 11.8 | 3.1 |
| 12-month follow-up | ||||||
| Age (years) | 69 | 64.9 | 10.4 | 12 | 62.4 | 13.9 |
| IMD score | 69 | 26.3 | 14.4 | 12 | 24.2 | 8.6 |
| BMI (kg/m2) | 63 | 30 | 7 | 12 | 27.7 | 6.6 |
| Years in education | 67 | 12 | 4 | 11 | 11.7 | 3.2 |
| Measure | Completed | Not completed | ||||
|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |
| 1-month follow-up | ||||||
| HADS-D score | 77 | 11.6 | 3.2 | 4 | 10.0 | 2.3 |
| HADS-A score | 77 | 12.4 | 5 | 4 | 15.5 | 1.3 |
| PHQ-9 score | 76 | 15.7 | 5.4 | 4 | 16.3 | 5.2 |
| 6-month follow-up | ||||||
| HADS-D score | 74 | 11.7 | 3.2 | 7 | 10.3 | 2.4 |
| HADS-A score | 74 | 12.5 | 4.9 | 7 | 13.3 | 4.5 |
| PHQ-9 score | 73 | 15.9 | 5.4 | 7 | 13.6 | 5.1 |
| 12-month follow-up | ||||||
| HADS-D score | 69 | 11.5 | 3.1 | 12 | 11.6 | 3.4 |
| HADS-A score | 69 | 12.3 | 4.8 | 12 | 14.4 | 5.4 |
| PHQ-9 score | 68 | 15.8 | 5.2 | 12 | 15.4 | 6.6 |
Data collection
The maximum number of observations available was 81 at baseline, 77 at 1 month, 74 at 6 months and 69 at 12 months. Table 9 shows the number of (and percentage of available) missing scores for each questionnaire at each assessment point. Note: missing data for the Social Problems Questionnaire (SPQ) are not recorded because of confusion concerning whether items were missing or left out because they were not applicable (for analysis purposes no response was considered to mean ‘not applicable’ and therefore no problem in this area). Two of our outcome measures had no missing scores at any point: the modified Rose angina questionnaire and the specific activity schedule. The second question of the BIPQ (BIPQ2) had the most missing scores, with 14% missing at 6 months. For the other measures, between 5% and 10% of scores were missing at one or more assessment points for the General Self Efficacy Scale, the BIPQ (questions 3 and 4) and the Warwick–Edinburgh Mental Well-Being Scale; and < 5% of scores were missing for HADS-D, PHQ-9, BIPQ (questions 1, 5, 6, 7, 8), HADS-A and the SF-12 (mental and physical components). Therefore, our selected outcome measures appeared to be acceptable to the participants and would be feasible to use in definitive trial within a similar population.
| Measure | Baseline missing, n (%)a | 1 month missing, n (%)a | 6 months missing, n (%)a | 12 months missing, n (%)a |
|---|---|---|---|---|
| HADS-D | 0 | 0 | 1 (1.4) | 0 |
| PHQ-9 | 1 (1.2) | 2 (2.6) | 1 (1.4) | 0 |
| General Self Efficacy Scale | 8 (9.9) | 5 (6.5) | 6 (8.1) | 2 (2.9) |
| BIPQ1 | 0 | 2 (2.6) | 3 (4.1) | 0 |
| BIPQ2 | 4 (4.9) | 5 (6.5) | 10 (13.5) | 5 (7.2) |
| BIPQ3 | 2 (2.5) | 3 (3.9) | 5 (6.8) | 1 (1.5) |
| BIPQ4 | 5 (6.2) | 6 (7.8) | 5 (6.8) | 0 |
| BIPQ5 | 0 | 3 (3.9) | 3 (4.1) | 0 |
| BIPQ6 | 0 | 3 (3.9) | 3 (4.1) | 0 |
| BIPQ7 | 0 | 2 (2.6) | 3 (4.1) | 0 |
| BIPQ8 | 0 | 2 (2.6) | 3 (4.1) | 0 |
| HADS-A | 0 | 0 | 1 (1.4) | 0 |
| WEMWBS | 2 (2.5) | 5 (6.5) | 4 (5.4) | 3 (4.4) |
| SF-12 physical | 2 (2.5) | 1 (1.3) | 1 (1.4) | 1 (1.5) |
| SF-12 mental | 2 (2.5) | 1 (1.3) | 1 (1.4) | 1 (1.5) |
| Modified Rose angina questionnaire | 0 | 0 | 0 | 0 |
| Specific activity schedule | 0 | 0 | 0 | 0 |
Depression
Depression outcomes are shown in Table 10. Both groups showed some improvement in depression symptoms (HADS-D score) at all time points, with the mean score in both groups moving from indicating moderate depression severity at baseline to mild severity at 12 months. We saw a similar pattern using the PHQ-9, with mean scores in both groups indicating moderately severe depression at baseline reducing to moderate depression at 12 months.
| Outcomes | PC | TAU | Mixed-effects model |
|---|---|---|---|
| HADS-D severity, mean (SD) | |||
| Baseline | 11.6 (3.3) | 11.4 (3) | Mean difference –0.73 (95% CI –2.08 to 0.62; p = 0.29)a |
| 1 month | 11.0 (3.4) | 10.0 (4.5) | |
| 6 months | 10.3 (3.8) | 9.2 (4.6) | |
| 12 months | 9.5 (4.6) | 8.8 (4.8) | |
| HADS-D remission,b % (number of patients/total number) | |||
| Baseline | N/A | N/A | OR 2.67 (95% CI 0.71 to 10.4; p = 0.15)c |
| 1 month | 14 (5/37) | 30 (12/40) | |
| 6 months | 24 (8/34) | 36 (14/39) | |
| 12 months | 34 (11/32) | 41 (15/37) | |
| HADS-D response,d % (number of patients/total number) | |||
| Baseline | N/A | N/A | OR 1.33 (95% CI 0.38 to 4.61; p = 0.65)d |
| 1 month | 3 (1/37) | 10 (4/40) | |
| 6 months | 15 (5/34) | 21 (8/37) | |
| 12 months | 28 (9/323) | 24 (9/37) | |
| PHQ-9 severity (PHQ-9 scores) | |||
| Baseline | 16.0 (5.3) | 15.4 (5.5) | Mean difference –0.63 (95% CI –2.60 to 1.35; p = 0.54)b |
| 1 month | 14.8 (6.4) | 13.0 (6.8) | |
| 6 months | 13.4 (7.0) | 11.7 (6.5) | |
| 12 months | 12.6 (7.1) | 12.0 (6.9) | |
According to the HADS-D, there was a greater percentage of remitters in the TAU group compared with the PC group at 6 and 12 months; there was also a greater percentage of responders at 6 months in the TAU compared with the PC group, but by 12 months more PC group participants had responded. However, the mixed-effects models showed no significant differences between groups over time for any measure of depression and CIs were wide so an effect in favour of either group cannot be ruled out.
From the medical notes, across the 12-month study period, in the PC group, 31 participants (76%) saw their GP or PN regarding their mental health (total of 101 consultations recorded); in the TAU group, 29 participants (73%) made a mental health consultation (total of 102 mental health consultations recorded). Of those participants who were not treated for depression at baseline (i.e. no record of antidepressant prescription or talking therapy referral), three PC participants had received a prescription for an antidepressant [Citalopram (Cipramil®, Lundbeck), n = 2; Mirtazepine (Mirtazepine®, Merck Sharp & Dohme, Corp.), n = 1; one of these participants was also referred for ‘counselling’] and one additional PC group participant had been referred to a ‘psychiatric clinic’ by 12 months; no participants in the TAU group had a new referral for depression treatment or a new prescription for an antidepressant at the end of the study.
Chest pain
The most notable difference between the PC and TAU groups was in self-reported chest pain (modified Rose angina questionnaire). At 6 months the proportion of patients who no longer reported chest pain was 37% in the PC group versus 18% in the TAU group and at 12 months it was 31% in the PC group versus 19% in the TAU group. From the medical notes across the 12-month study period, in the PC group, 34 participants (83%) saw their GP or PN regarding their CHD (total of 158 consultations recorded); in the TAU group, 29 participants (73%) made a CHD consultation (total of 170 consultations recorded). It is not clear from the notes whether these were routine or emergency visits, so we examined recorded accident and emergency (A&E) visits.
In the PC group, 10 participants (six for heart problems, two for other state reasons, two no reason recorded) visited A&E (total 13 visits: nine heart problems, two other stated reasons, two no reason recorded). In the TAU group, 15 participants (four for heart problems, five for other state reasons, six no reason recorded) visited A&E (total 26 visits: seven heart problems, six other stated reasons, 13 no reason recorded). PC participants therefore made fewer A&E visits (24% in PC vs. 38% in TAU), although missing data concerning the reason for these visits makes this information difficult to interpret.
Preliminary secondary outcomes
At 6 and 12 months both groups improved on all outcomes (Table 11); these data have been published. 111 There was no evidence for an interaction between time point and study arm for any outcome, so a differential effect over time appears unlikely.
| Measure | 1-month follow-up | 6-months follow-up | 12-months follow-up | Mixed-effects model (where calculated) | |||
|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | ||
| Self-efficacy (GSES)a | 27.7 (7.0) | 27.1 (6.3) | 26.7 (8.1) | 28.2 (6.9) | 28.6 (6.7) | 27.9 (8.1) | –0.58 (–3.05 to 1.89)b |
| Illness perceptions (BIPQ total score) | 42.8 (13.3) | 44.4 (13.3) | 43.0 (13.3) | 40.9 (11.5) | 40.0 (14.8) | 43.0 (13.1) | –0.42 (–4.57 to 3.72)b |
| Anxiety (HADS-A) | 12.2 (4.3) | 11.3 (5.0) | 11.0 (5.0) | 10.4 (5.0) | 9.9 (4.9) | 9.5 (5.4) | –0.80 (–2.08 to 0.48)b |
| Chest pain (yes) (modified Rose angina questionnaire) | 27 (73%) | 29 (75%) | 22 (63%) | 32 (82%) | 22 (69%) | 30 (81%) | 2.21 (0.69 to 7.03)c |
| QoL (SF-12 physical)a | 31.6 (8.4) | 32.8 (9.3) | 31.3 (10.6) | 31.7 (9.1) | 32.4 (10.7) | 33.3 (9.2) | –0.04 (–2.85 to 2.77)b |
| QoL (SF-12 mental)a | 31.4 (10.8) | 30.8 (11.0) | 32.4 (11.2) | 35.4 (11.8) | 34.5 (11.6) | 33.6 (12.5) | 1.27 (–2.01 to 4.55)b |
| PSYCHLOPS | 14.6 (4.4) | 14.8 (4.9) | 13.1 (5.4) | 14.0 (5.9) | 13.6 (5.1) | 13.4 (5.4) | –0.39 (–2.20 to 1.42)b |
| Well-being (WEMWBS)a | 37.5 (11.1) | 36.1 (10.4) | 38.4 (12.2) | 39.6 (11.6) | 40.6 (11.2) | 39.6 (12.3) | 1.59 (–1.98 to 5.15)b |
| Morisky adherence index | |||||||
| High | 8 (29%) | 17 (52%) | 12 (57%) | 13 (46%) | 8 (38.1%) | 15 (60%) | |
| Intermediate | 19 (68%) | 13 (39%) | 9 (43%) | 12 (43%) | 12 (57%) | 10 (40%) | |
| Low | 1 (4%) | 3 (9%) | 0 (0%) | 3 (11%) | 1 (5%) | 0 (0%) | |
| Functioning (specific activity schedule) | |||||||
| 1 | 10 (27%) | 16 (40%) | 9 (26%) | 10 (26%) | 6 (19%) | 8 (22%) | |
| 2 | 8 (22%) | 2 (5%) | 7 (20%) | 7 (18%) | 7 (22%) | 7 (19%) | |
| 3 | 15 (41%) | 16 (40%) | 15 (43%) | 18 (46%) | 12 (38%) | 17 (46%) | |
| 4 | 4 (11%) | 6 (15%) | 4 (11%) | 4 (10%) | 7 (22%) | 5 (14%) | |
| SPQ, total n problems | |||||||
| 0 | 11 (26.8) | 3 (7.5) | 15 (36.6) | 8 (20.0) | 16 (39.0) | 9 (22.5) | |
| 1 | 7 (17.1) | 9 (22.5) | 10 (24.4) | 13 (32.5) | 8 (19.5) | 13 (32.5) | |
| 2 | 13 (31.7) | 8 (20.0) | 5 (4535) | 4 (10.0) | 5 (12.2) | 8 (20.0) | |
| 3 | 3 (7.3) | 8 (20.0) | 5 (12.2) | 8 (20.0) | 5 (12.2) | 5 (12.5) | |
| 4–8 | 7 (17.1) | 12 (30.0) | 6 (14.6) | 7 (17.5) | 7 (17.1) | 5 (12.5) | |
Aim 3: validity of outcome measures in comparison with participant-reported problems
Using the BIPQ, participants were asked to list the three most important problems that caused their illness (CHD). Sixty-one participants gave at least one reason (these data are published online as an appendix110). The most common reason given was ‘genetics or heredity’, followed by lifestyle factors such as smoking, poor diet and lack of exercise. Mood problems, especially stress and work-related stress were also mentioned, and comorbid or past health problems were also blamed. Four patients mentioned relationship problems and one mentioned financial problems.
Participants in the PC group (n = 41) identified 21 types of problem as contributing to their depression and which were addressed during the intervention (up to three problems per patient); most common were pain (chest and other pain, e.g. arthritis) (n = 18), lack of exercise (n = 17), difficulty sleeping (n = 13), anxiety (n = 11) and being overweight (n = 11). Reported problems and whether or not they were addressed during the intervention are published online as an appendix. 110
Participants therefore explained both their CHD and their depression in terms of wide-ranging problems that appeared similar for the two conditions; lifestyle problems in particular were associated with both.
Within our study, mood outcomes were assessed using the HADS (depression and anxiety) and the PHQ-9 (depression); however, we had no measure of change in lifestyle-related outcomes. The PC intervention was aimed at tackling the problems that each participant felt were important rather than addressing specific cardiac risk factors; however, in view of our current finding that patients do consider lifestyle factors known to be associated with CHD as contributing to their depression, inclusion of a measure of change in cardiac risk factors should be considered for a definitive trial of PC. It will be important to select a measure that captures the variation between participants in terms of which risk factors they want to address; a validated measure of goal attainment may therefore be appropriate.
Aim 4: exploring changes in self-efficacy
Self-efficacy improved over the course of the study (see Table 11). At 12 months, the PC group had a mean increase of 2.5 points versus 0.9 points in the TAU group, suggesting a greater increase in self-efficacy in the PC group; however, the mixed-effects model indicated no difference between groups over time (adjusting for baseline self-efficacy): mean difference –0.58 (95% CI –3.05 to 1.89).
At 6 and 12 months the overall illness perceptions score and most measured dimensions showed improvement in both groups, though differences between groups were small (Table 12). The mean improvement in overall score from baseline to 12 months was greater in the PC group than in the TAU group: 7.8 points versus 2.5 points. As expected, the biggest difference in mean improvement between the PC and TAU groups in dimension scores was in personal control (mean change in BIPQ from baseline = 1.5 for PC group vs. 1.1 for TAU at 6 months; and 1.1 for PC vs. 0.1 for TAU at 12 months); however, the mixed-effects model suggested no difference between groups over time (adjusting for baseline total BIPQ score): mean difference –0.42 (95% CI –4.57 to 3.72).
| BIPQ sections | PC | TAU | Mean difference | |||
|---|---|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | PC – TAU | 95% CIa | |
| Consequences (BIPQ1) | ||||||
| Baseline | 41 | 5.4 (3.1) | 40 | 6.1 (3.2) | –0.6 | –2.0 to 0.8 |
| 1 month | 35 | 5.9 (3.0) | 40 | 5.8 (3.0) | 0.1 | –1.3 to 1.5 |
| 6 months | 32 | 5.8 (2.9) | 39 | 5.3 (3.2) | 0.5 | –1.0 to 1.9 |
| 12 months | 32 | 5.6 (3.6) | 37 | 5.0 (3.2) | 0.6 | –1.0 to 2.2 |
| Timeline (BIPQ2) | ||||||
| Baseline | 39 | 9.3 (2.1) | 38 | 9.2 (2.5) | 0.1 | –0.9 to 1.2 |
| 1 month | 34 | 9.1 (2.0) | 38 | 9.2 (2.1) | 0.0 | –1.0 to 0.9 |
| 6 months | 29 | 9.2 (2.0) | 35 | 9.3 (2.0) | –0.1 | –1.1 to 0.9 |
| 12 months | 30 | 9.5 (1.5) | 34 | 9.2 (2.2) | 0.3 | –0.7 to 1.2 |
| Personal controlb (BIPQ3) | ||||||
| Baseline | 39 | 3.5 (3.3) | 40 | 3.5 (3.3) | 0.0 | –1.4 to 1.5 |
| 1 month | 35 | 4.6 (3.1) | 39 | 3.5 (3.5) | 1.1 | –0.4 to 2.6 |
| 6 months | 32 | 5.0 (3.5) | 37 | 4.6 (3.5) | 0.4 | –1.3 to 2.1 |
| 12 months | 32 | 4.6 (3.7) | 36 | 3.6 (3.6) | 0.9 | –0.9 to 2.7 |
| Treatment controlb (BIPQ4) | ||||||
| Baseline | 40 | 7.0 (2.9) | 36 | 6.9 (2.9) | 0.0 | –0.3 to 1.3 |
| 1 month | 35 | 7.4 (2.6) | 36 | 6.4 (3.8) | 1.0 | –0.6 to 2.5 |
| 6 months | 31 | 7.7 (2.3) | 38 | 6.6 (3.5) | 1.1 | –0.4 to 2.5 |
| 12 months | 32 | 7.5 (3.1) | 37 | 7.0 (3.6) | 0.4 | –1.2 to 2.1 |
| Identity (BIPQ5) | ||||||
| Baseline | 41 | 5.1 (8.1) | 40 | 5.5 (3.2) | –0.4 | –1.7 to 1.0 |
| 1 month | 34 | 4.7 (2.9) | 40 | 5.4 (3.3) | –0.7 | –2.1 to 0.8 |
| 6 months | 32 | 4.9 (3.2) | 39 | 4.7 (2.9) | 0.2 | –1.3 to 1.6 |
| 12 months | 32 | 5.1 (9.7) | 37 | 4.9 (3.2) | 0.1 | –1.4 to 1.6 |
| Illness concern (BIPQ6) | ||||||
| Baseline | 41 | 6.4 (3.7) | 40 | 6.1 (3.9) | 0.3 | –1.4 to 2.0 |
| 1 month | 34 | 6.3 (3.8) | 40 | 5.4 (3.9) | 0.9 | –0.9 to 2.7 |
| 6 months | 32 | 6.4 (3.7) | 39 | 4.5 (3.6) | 2.0 | 0.2 to 3.7 |
| 12 months | 32 | 4.7 (3.8) | 37 | 4.8 (4.0) | 0.0 | –1.9 to 1.8 |
| Illness coherenceb (BIPQ7) | ||||||
| Baseline | 41 | 6.0 (4.1) | 40 | 5.7 (3.6) | 0.4 | –1.3 to 2.1 |
| 1 month | 35 | 6.0 (3.7) | 40 | 6.3 (3.4) | –0.3 | –1.9 to 1.3 |
| 6 months | 32 | 6.4 (3.7) | 39 | 6.0 (3.9) | 0.4 | –1.4 to 2.2 |
| 12 months | 32 | 7.7 (3.2) | 37 | 6.8 (3.7) | 0.9 | –0.8 to 2.6 |
| Emotional representations (BIPQ8) | ||||||
| Baseline | 41 | 6.7 (3.4) | 40 | 6.1 (3.5) | 0.6 | –0.9 to 2.2 |
| 1 month | 35 | 5.8 (3.4) | 40 | 6.2 (3.1) | –0.4 | –1.9 to 1.1 |
| 6 months | 32 | 5.4 (3.5) | 39 | 4.6 (3.4) | 0.7 | –0.9 to 2.4 |
| Total scorec | ||||||
| Baseline | 36 | 47.8 (13.5) | 35 | 45.5 (14.1) | 2.3 | –4.3 to 8.8 |
| 1 month | 30 | 42.8 (13.3) | 35 | 44.4 (13.3) | –1.5 | –8.2 to 5.1 |
| 6 months | 28 | 43.0 (13.3) | 34 | 40.9 (11.5) | 2.1 | –4.2 to 8.4 |
| 12 months | 30 | 40.0 (14.8) | 33 | 43.0 (13.1) | –3.0 | –10.0 to 4.0 |
Controlling for changes in self-efficacy or overall illness perceptions had little effect on change in depression over time, whether or not considering depression severity, remission or response (Table 13). Since CIs were wide, change in favour of either PC or TAU cannot be ruled out.
| Depression | Mixed-effects modelsa (95% CI; p-value) | ||
|---|---|---|---|
| Time point | Self-efficacyb | Illness beliefsc | |
| Severity (mean difference) | –0.73 (–2.08 to 0.62; 0.29) | –0.88 (–2.11 to 0.36; 0.16) | –0.57 (–1.90 to 0.77; 0.40) |
| Remission (odds ratio) | 2.67 (0.71 to 10.05; 0.15) | 3.15 (0.64 to 15.50; 0.16) | 3.26 (0.94 to 11.24; 0.07) |
| Response (odds ratio) | 1.33 (0.38 to 4.61; 0.65) | 1.02 (0.26 to 3.95; 0.98) | 1.27 (0.34 to 4.66; 0.72) |
Post-hoc analyses
As anxiety symptoms were high at baseline, we also explored HADS-A score as mediator for improvement in depression. Controlling for anxiety slightly reduced the difference in depression symptoms between the groups over time: mean difference –0.43 (95% CI –1.48 to 0.63; p = 0.43). Controlling for anxiety considerably reduced the odds of remission in the TAU group versus PC group in favour of the PC group: odds ratio (OR) remission in TAU versus PC group 0.42, 95% CI 0.10 to 1.68; p = 0.22, which suggests that changes in anxiety symptoms may be a mediator for depression remission. The odds of depression response in the TAU group compared with the PC group were also slightly reduced when anxiety scores were controlled, although the odds were still in favour of TAU (OR 1.12, 95% CI 0.32 to 3.94; p = 0.86). None of these analyses showed a statistically significant effect; all changes in effect were small and CIs were wide so we cannot rule out benefit for PC or TAU.
Aim 5: exploring acceptability and feasibility of personalised care
Nurse time used for intervention
Intervention patients (n = 41) received a mean of 203 minutes (SD 100 minutes) of nurse time [78 minutes (SD 19 minutes) for assessment, 125 minutes (SD 91 minutes) in telephone follow-up calls over 6 months]. The mean number of follow-up calls was nine (SD five); the mean duration of calls was 14 minutes (SD 4 minutes). The nurses arranged a time to call the patient, but sometimes patients did not respond; there was considerable variation between patients in the number of failed follow-up contact attempts by nurses over the 6-month intervention period (range 0–32), but on average nurses made 2.8 calls for every successful contact.
Effect of intervention intensity
The amount of time spent talking to the nurse varied considerably between patients (range 74–406 minutes), so we used the median duration (167 minutes) to divide the participants into high (n = 20) and low (n = 19) ‘dose’ groups. There were no significant differences (p > 0.05) between the groups at baseline in depression [HADS-D mean: low-dose group 11.0 (SD 3); high-dose group 12 (SD 3.7)]. The magnitude of improvement in depression over time was greater for the high- compared with the low-dose group and fewer high-dose patients had chest pain at 6 and 12 months, although the mixed-effects models indicated little difference between the groups: depression mean difference –0.72, 95% CI –3.03 to 1.60; chest pain OR 0.34, 95% CI 0.01 to 7.53.
Patient satisfaction with personalised care
Of the 41 PC participants, 21 completed and returned the satisfaction questionnaire. The questionnaire and responses are shown in Table 14. On the whole, patients reported finding the different elements of the intervention (assessment, care plan and follow-up calls) helpful. They also found that the intervention helped them communicate with other health professionals such as their GP. Most respondents agreed or strongly agreed that the nurse was able to answer their questions about their mood, heart or other health problems and that they could understand the information given, and that the nurse provided support and encouragement and had a courteous manner. Of the 15 patients who responded to this question, all said that they would like their GP to offer a similar service.
| Question | Number of responses | |||||
|---|---|---|---|---|---|---|
| Yes | No | |||||
| I found the assessment meeting with nursea helpful | 18 | 1 | ||||
| My care plan has been helpful | 16 | 1 | ||||
| My telephone conversations with nursea were helpful | 15 | 2 | ||||
| My contact with nursea helped in consultations with my GP, PN or other health professional | 12 | 4 | ||||
| About right | Too little | Too much | ||||
| My level of contact with nursea was . . . | 11 | 5 | 2 | |||
| Strongly Agree | Agree | Neutral | Disagree | Strongly Disagree | N/A | |
| Nursea answered any questions I may have had about my heart problem | 4 | 9 | 2 | 0 | 0 | 1 |
| Nursea answered any questions I may have had about my low mood | 3 | 10 | 1 | 1 | 0 | 2 |
| Nursea answered any questions I may have had about other health issues | 4 | 9 | 1 | 1 | 0 | 2 |
| Nursea provided and explained information in an understandable way | 7 | 6 | 2 | 1 | 1 | 0 |
| Nursea provided support and encouragement | 8 | 6 | 1 | 1 | 1 | 0 |
| Nursea had a courteous personal manner | 12 | 3 | 1 | 0 | 1 | 0 |
| Yes | No | |||||
| Would you like your GP practice to provide a service such as this? | 15 | 0 | ||||
The patients were also asked which aspect of working with their nurse they had liked best and least. Seventeen patients responded regarding what they liked best: two patients liked ‘everything’ or ‘all’; several comments referred to the patient’s pleasure in having someone pleasant to talk to:
it’s just good to talk to somebody.
sympathetic helpful and friendly approach.
[Nurse] is a nice person and very pleasant.
Other comments were more specific and referred to the nurse’s ability to treat them as an individual when offering advice and understanding:
Their personal interest in me as a patient and not just a number, like you feel in hospital sometimes. Their interest in my problems and how they could help me with my problems and difficulties and trying to help, in getting me to understand my problems and difficulties and that there was a light at the end of the tunnel and they has been so good to me in that area and I’m hoping I can do their help worthy.
They always had a nice manner (sic). Listens very well and after what I’ve been through they had a good answer and good advice. Please thank [nurse] for their time.
their advice about routine – listening to radio and when you believe somebody care about you – here I am alone, no family (except my children), no friend. We need such as this service.
Regarding what they liked least: 10 patients responded ‘none’ or ‘N/A’ [not applicable]; one patient responded ‘forms’; another, who had been positive about the intervention, appeared to indicate that they had found participation difficult:
Knowing the value of time it was so difficult to convince myself that I was not wasting both (the Nurse’s) and my time operating this plan. I fought hard against this feeling.
This patient’s comment in the ‘liked best’ section seemed to suggest that they nevertheless valued the intervention, so their earlier comment may be reflective of their depressed state:
Honestly I didn’t really like any of it. I feared that I was too set in my regimes to open my soul and shortcomings. However I understood that it was important for me to proceed and tried to give it my full attention. But it wasn’t likeable.
Aim 6: exploring standardisation and therapist effects
Intervention fidelity
As planned, the nurse case managers used a range of nursing and behaviour change techniques to help patients address their problems. Classifiable behaviour change techniques reported by nurses were: general encouragement, information linking health and behaviour, goal-setting and action-planning, barrier identification and focus on past success. Other nurse-reported actions included lifestyle advice, signposting (e.g. to relevant local resources such as leisure or day centres), promoting adherence to therapy and supportive counselling.
There was also some evidence of collaborative care. The nurse case manager contacted the patient’s GP (10 patients), the patient’s PN (four patients), social services (one patient) or another professional, for example IAPT worker or other therapist, occupational therapist, community mental health nurse, physiotherapist, housing or benefits officer (17 patients). With the patient’s permission, the nurse case managers consulted a family member of four patients.
Therapist effects
The patients of Nurse 1 had a higher mean baseline HADS-D score (12.4 vs. 10.9, Wilcoxon rank-sum test; p = 0.07). However, the random-effects model (combining data from 1, 6 and 12 months) indicated little difference in the average therapist effect on the HADS-D score across the time points (adjusting for baseline HADS depression score): mean difference –0.86 (95% CI –2.81 to 1.10).
Regarding self-reported chest pain, of Nurse 1’s (registered general nurse and health psychologist) patients, 44% continued to report chest pain at 6 months, compared with 79% of Nurse 2’s (registered general and mental health nurse) patients (p = 0.03). In the random-effects model, the odds of reporting chest pain across the study period were higher for Nurse 2 than for Nurse 1 (OR 7.80, 95% CI 0.88 to 69.40).
Aim 7: examine the potential cost of personalised care
The average EQ-5D utility scores at baseline were slightly higher for the PC group (see Figure 7, which is also published elsewhere110), although the difference between groups was not statistically significant (95% CI –0.98 to 0.25; p = 0.40). By the 1-month follow-up, the TAU group had a higher utility score, and this difference was maintained up to the 12-month follow-up [95% CI –0.26 to 0.11; p = 0.422 (at 6-month follow-up: 95% CI –0.27 to 0.11, p = 0.408)]. In terms of QALYs, the TAU group showed an incremental QALY gain of 0.038 compared with PC over the 12-month treatment period. In Figure 7 the area between the two curves represents the QALY gain for the control group.
FIGURE 7.
European Quality of Life-5 Dimensions score and QALY gain. CM, nurse-led case management. Reproduced from Barley et al. 110 © 2014 Barley et al. This is an open access article distributed under the terms of the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
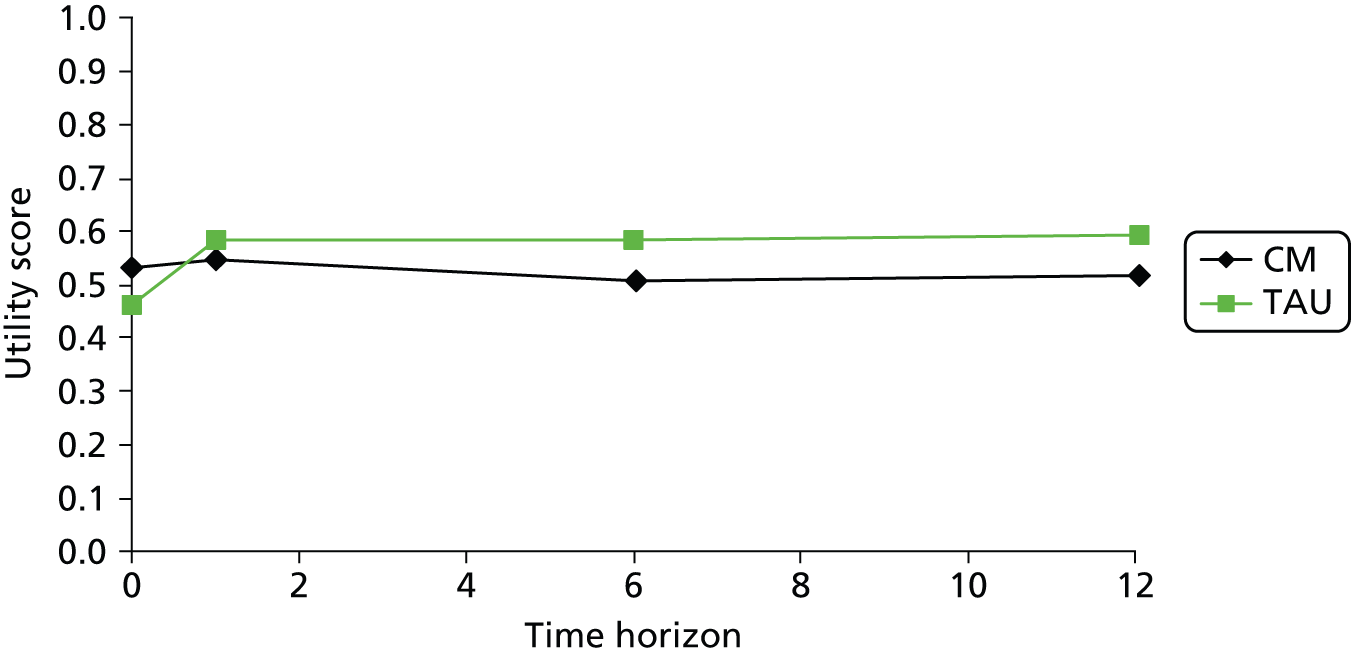
Service use and costs
Service use was fairly similar between the intervention and the control groups during the study period (Table 15, which is also published online as an appendix to our paper110). Hospital services were used more intensively by the TAU group than the PC group at all time points, with inpatient and outpatient care being the most frequently used services. The TAU group incurred higher inpatient costs than the PC group at each time point (particularly at baseline and at the 12-month follow-up). Few patients used day hospital services, but the costs incurred were high for both groups. The majority of patients received care from GPs and the costs of this were similar between the groups. Informal care was used slightly more among patients in the PC group than in the TAU group. Average total costs at each time point were lower for the PC group than for the TAU group. However, the differences were not statistically significant. For the PC group, the intervention itself accounted for only 6.7% of total costs.
| Type of service | PC | TAU | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (N = 41) | 6-month follow-up (N = 35) | 12-month follow-up (N = 32) | Baseline (N = 40) | 6-month follow-up (N = 39) | 12-month follow-up (N = 37) | |||||||
| na (%) | Meanb (SD) | na (%) | Meanb (SD) | na (%) | Meanb (SD) | na (%) | Meanb (SD) | na (%) | Meanb (SD) | na (%) | Meanb (SD) | |
| A&E | 7 (17) | 63 (0) | 4 (11) | 94 (63) | 2 (6) | 125 (89) | 13 (33) | 77 (38) | 8 (21) | 110 (73) | 6 (16) | 73 (26) |
| Day hospital | 3 (7) | 347 (309) | 1 (3) | 703c | 2 (6) | 440 (371) | 4 (10) | 233 (149) | 3 (8) | 238 (149) | 2 (5) | 396 (345) |
| Inpatient care | 11 (27) | 1768 (1609) | 1 (3) | 805c | 6 (19) | 1118 (704) | 14 (35) | 6105 (11,201) | 7 (18) | 1418 (1321) | 8 (22) | 3690 (4013) |
| Outpatient care | 30 (73) | 947 (2064) | 21 (60) | 253 (139) | 19 (59) | 664 (888) | 30 (75) | 1008 (1765) | 30 (77) | 694 (735) | 25 (68) | 565 (389) |
| GP | 37 (90) | 250 (518) | 27 (77) | 171 (165) | 27 (84) | 210 (383) | 36 (90) | 237 (288) | 36 (92) | 188 (233) | 32 (86) | 158 (218) |
| Psychiatrist | 0 (0) | – | 0 (0) | – | 0 (0) | – | 0 (0) | – | 1 (3) | 656c | 0 (0) | – |
| Other c/b doctor | 0 (0) | – | 0 (0) | – | 0 (0) | – | 1 (3) | 100c | 0 (0) | – | 0 (0) | – |
| District nurse | 0 (0) | – | 2 (6) | 238 (285) | 1 (3) | 146c | 1 (3) | 641c | 1 (3) | 366c | 3 (8) | 250 (339) |
| PN | 25 (61) | 15 (19) | 13 (37) | 14 (8) | 16 (50) | 14 (12) | 23 (58) | 25 (51) | 17 (44) | 21 (42) | 18 (49) | 11 (10) |
| Mental health nurse | 1 (2) | 112c | 0 (0) | – | 0 (0) | – | 0 (0) | – | 0 (0) | – | 0 (0) | – |
| Health visitor | 0 (0) | – | 0 (0) | – | 1 (3) | 100c | 0 (0) | – | 0 (0) | – | 0 (0) | – |
| Other nurse | 0 (0) | – | 1 (3) | 58c | 1 (3) | 0 (0) | – | 1 (3) | 19c | 1 (3) | 173 (.) | |
| Psychologist | 1 (2) | 972c | 2 (6) | 324 (0) | 1 (3) | 972c | 0 (0) | – | 1 (3) | 162c | 2 (5) | 162 (115) |
| Counsellor | 2 (5) | 569 (434) | 2 (6) | 88 (31) | 0 (0) | – | 5 (13) | 210 (189) | 4 (10) | 334 (197) | 2 (5) | 212 (258) |
| Occupational therapist | 0 (0) | – | 1 (3) | 42c | 1 (3) | 84c | 0 (0) | – | 0 (0) | – | 0 (0) | – |
| Physiotherapist | 3 (7) | 504 (436) | 0 (0) | – | 4 (13) | 194 (214) | 1(3) | 42c | 2 (5) | 53 (15) | 4 (11) | 562 (970) |
| Other therapist | 0 (0) | – | 0 (0) | – | 2 (6) | 28 (8) | 2 (5) | 42 (38) | 2 (5) | 57 (48) | 1 (3) | 100 (.) |
| Social worker | 0 (0) | – | 0 (0) | – | 0 (0) | – | 0 (0) | – | 1 (3) | 71c | 1 (3) | 107 (.) |
| Housing worker | 0 (0) | – | 1 (3) | 26c | 0 (0) | – | 0 (0) | – | 0 (0) | – | 0 (0) | – |
| Home care worker | 2 (5) | 227 (107) | 2 (6) | 1210 (0) | 1 (3) | 1814c | 2 (5) | 1361 (1069) | 1 (3) | 454c | 2 (5) | 6237 (8286) |
| Care attendant | 0 (0) | – | 3 (9) | 2407 (3487) | 1 (3) | 605c | 0 (0) | – | 0 (0) | – | 1 (3) | 1210 (.) |
| Support worker | 0 (0) | – | 0 (0) | – | 0 (0) | – | 1 (3) | 103c | 0 (0) | – | 1 (3) | 182 (.) |
| Voluntary worker | 0 (0) | – | 0 (0) | – | 1 (3) | 288c | 0 (0) | – | 1 (3) | 10c | 0 (0) | – |
| Day centre | 0 (0) | – | 2 (6) | 138 (172) | 0 (0) | – | 1 (3) | 518c | 3 (8) | 238 (219) | 1 (3) | 1042 (.) |
| Other c/b service | 4 (10) | 563 (222) | 0 (0) | – | 1 (3) | – | 4 (10) | 1929 (2342) | 0 (0) | – | 0 (0) | – |
| Personal care | 2 (5) | 150 (35) | 0 (0) | – | 0 (0) | – | 0 (0) | – | 0 (0) | – | 0 (0) | – |
| Help in/around home | 13 (32) | 337 (306) | 7 (20) | 332 (252) | 8 (25) | 428 (366) | 8 (20) | 259 (339) | 4 (10) | 388 (269) | 7 (19) | 150 (114) |
| Help outside home | 8 (20) | 233 (187) | 2 (6) | 63 (18) | 4 (13) | 50 (29) | 5 (13) | 75 (61) | 3 (8) | 75 (66) | 3 (8) | 100 (43) |
| Other help | 1 (2) | 750c | 0 (0) | – | 0 (0) | – | 3 (8) | 650 (677) | 0 (0) | – | 0 (0) | – |
| Total cost | – | 1773 (2498) | – | 832 (1383) | – | 1088 (1320) | – | 3604 (7852) | – | 1191 (1168) | – | 2014 (3246) |
Cost–utility analysis
Of the total 81 participants, cost and QALY data at each time point were available for 68 patients (84%). Cost–utility results yielded an incremental cost-effectiveness ratio of £29,921 per additional QALY. Cost-effectiveness plane and cost-effectiveness acceptability curves were produced from bootstrapped resamples. The distribution of the cost-effectiveness point estimates on the cost-effectiveness plane (Figure 8, which can also be found in Barley et al. 110) indicated a strong likelihood of cost savings for the PC group compared with the TAU group. The point estimate of the incremental cost-effectiveness ratio falls in the south-western quadrant, representing the situation where the PC group has reduced costs and worse outcomes. The second most likely result is that PC results in lower costs and better outcomes (south-east quadrant).
FIGURE 8.
Distribution of the cost-effectiveness point estimates on the cost-effectiveness plane. NE, north-east; NW, north-west; SE, south-east; SW, south-west. Source: reproduced from Barley et al. 110 © 2014 Barley et al. This is an open access article distributed under the terms of the Creative Commons Attribution License 4.0, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
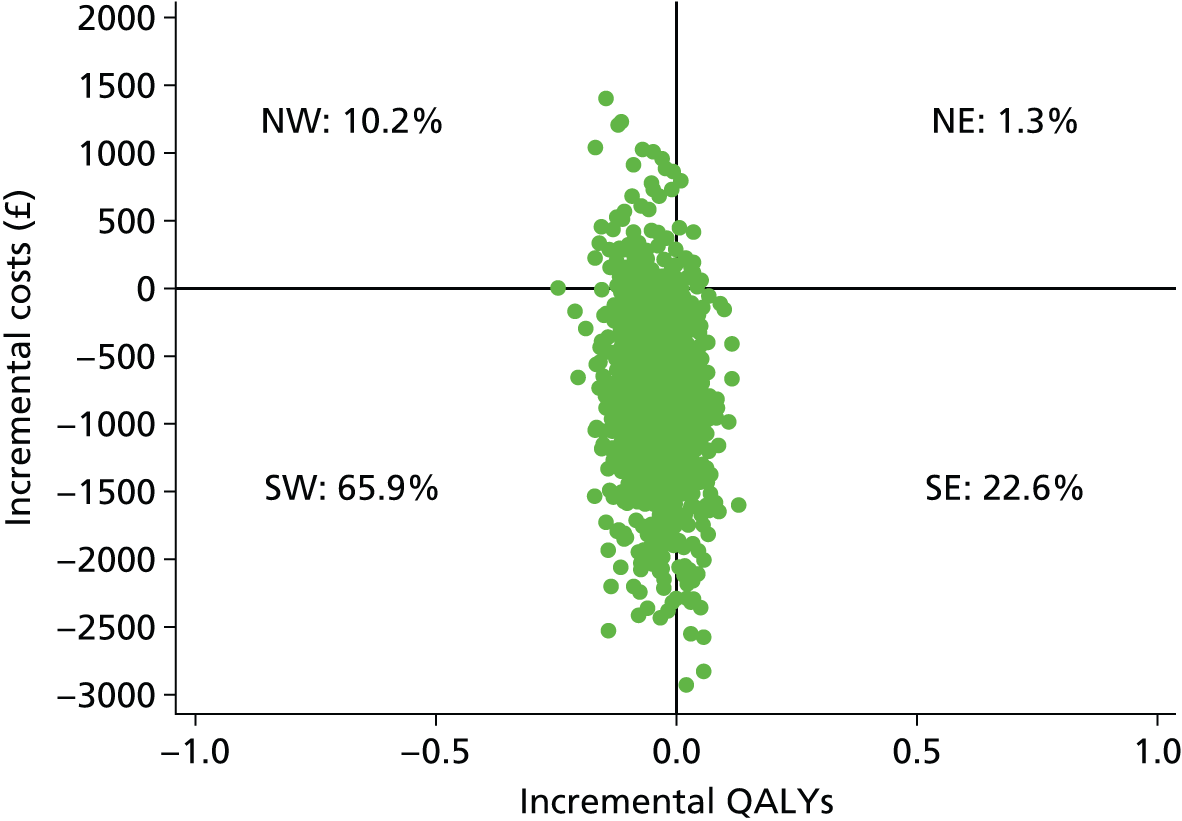
The cost-effectiveness acceptability curves (Figure 9) for the PC group compared with the TAU group was downward sloping. There is a greater likelihood of PC being the most cost-effective option up to a QALY threshold of £3035.
FIGURE 9.
Cost-effectiveness acceptability curves. c-e, cost-effective; p, probability. Source: reproduced from Barley et al. 110 © 2014 barley et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
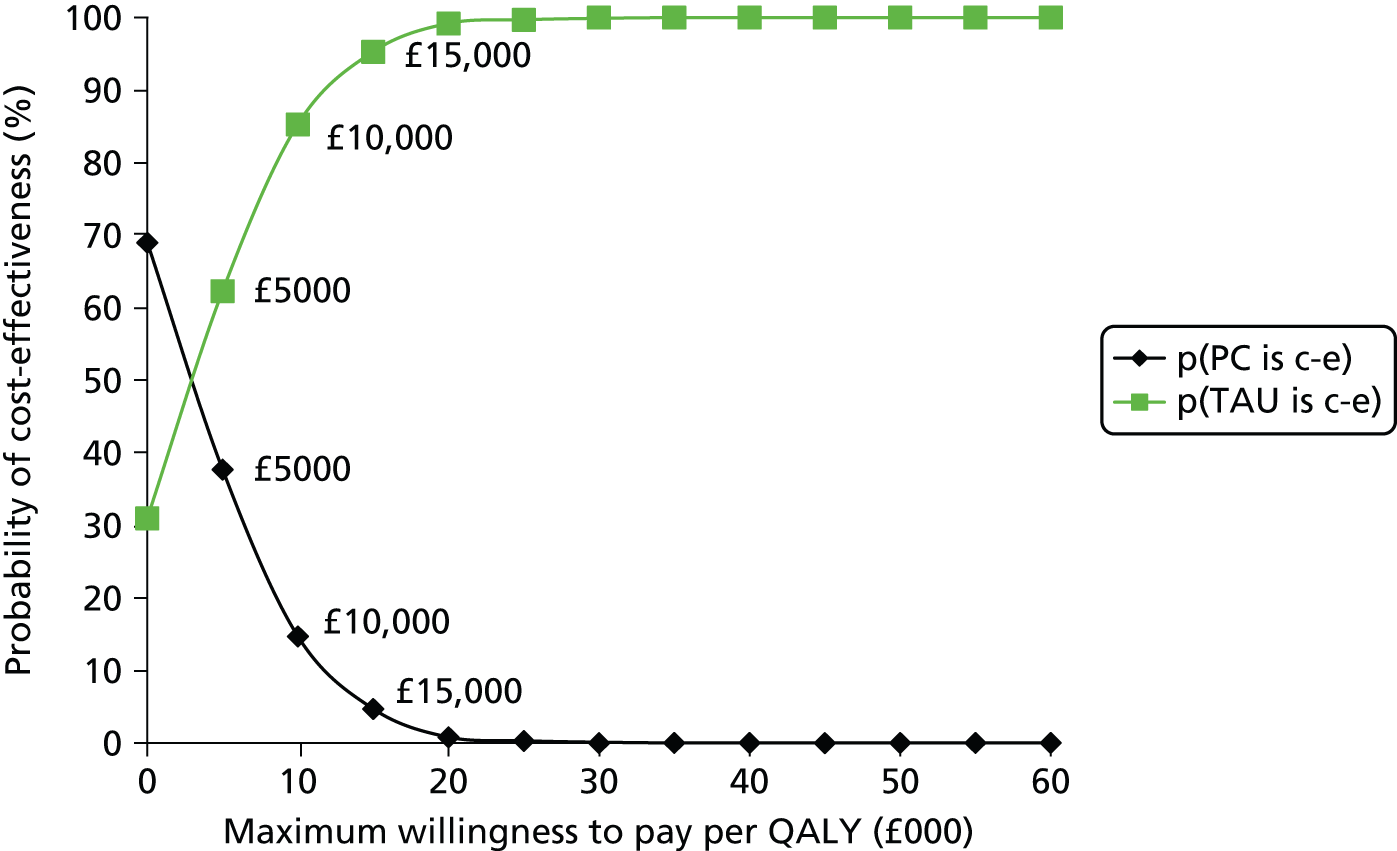
Discussion
We developed a PN-led PC intervention, which was designed to be easily implemented within practice in order to improve current primary care. In this patient-randomised pilot trial we explored the acceptability, feasibility and potential costs of the intervention for primary care CHD patients who have probable concurrent depression and current chest pain. We also explored the feasibility of the trial protocol to inform the methods of a definitive trial.
Feasibility and acceptability of personalised care
The PC intervention appeared to be feasible and acceptable for use in current primary care: a short amount of nurse time was needed, engagement with the nurse case managers was high and most of the PC group participants who returned our questionnaire reported satisfaction with all aspects. To further understand participants’ experience of the intervention, we have interviewed a subsample of 12 PC group participants. We used purposive sampling to select participants by age [>/≤ 60 years], sex, nurse case manager, depression (HADS-D >/≤ 10) and nurse case manager impression of depression response. Owing to research assistants leaving the programme for positions with longer-term prospects, these data are yet to be analysed.
As expected, this exploratory study confirmed the findings of our earlier qualitative work that patients with CHD and depression symptoms report a wide range of problems that they consider contribute to their low mood. The PC intervention enabled patients to identify and address these problems with the nurse case managers. This is an improvement on current care for these patients since management of depression and/or psychosocial problems is not routinely addressed in this population,31,59 despite recent routine depression screening under the QOF. 112 Compared with the widespread organisational change that would be needed for collaborative care interventions as trialled in the USA,12 our PC intervention appears to offer an enhanced form of TAU which could be implemented easily in current primary care practice. Evidence from the UK-based proactive care by PNs for people with depression and anxiety (ProCEED) trial133,134 that care reviews delivered by PNs acting as case managers were acceptable to patients with long-term depression supports this. 134
We explored a wide range of outcomes focusing on depression, as measured by the HADS-D as a potential primary outcome. Findings tended to show slight benefit for TAU compared with PC for depression symptoms, remission and response, except that at 12 months more PC participants had responded. However, differences between groups were very small and wide CIs mean that improvement with either PC or TAU cannot be ruled out. Our mixed-effects model indicated that PC would be unlikely to do much harm compared with TAU (mean difference –0.73), but could improve symptoms up to 2.1 points on the HADS-D (95% CI –2.08 to 0.62). We see little reason to change to a different outcome.
Accepted health psychology models agree on two factors important for behaviour change: belief in the importance of an outcome and belief in capacity to succeed (self-efficacy). Our intervention facilitated participants to work on outcomes important to them, and we used techniques such as action-planning to increase self-efficacy. Our data indicated that self-efficacy and illness perceptions, especially personal control, which is closely related to self-efficacy, were increased in those receiving PC but the difference from the increase in TAU group participants was not great.
Although both groups improved on all our measured outcomes, there were no large differences between groups, except in self-reported chest pain, which was also an inclusion criterion for the study. Our cohort study indicated that chest pain has a range of negative impacts and is therefore an important outcome to study.
Potential costs of personalised care
There were no great differences in service use and costs between PC and TAU, with the exception of inpatient care, which also accounted for a substantial proportion of total costs. Overall, it appears that PC reduced costs compared with TAU, but produced slightly lower benefits in terms of QALYs. This may appear counterintuitive given the other findings, but the utility scores underlying the QALY calculations were fairly similar and did not change much over time. Costs may have been underestimated owing to reliance on patient self-report in service use, the lack of medication and sick-leave data at all time points and the approach used to quantify informal care. Informal care constitutes a major cost driver in chronically ill populations. In this analysis, the ‘proxy good method’135 and the unit cost of home care worker was used to calculate informal care. However, in a future trial, an alternative cost, such as the national minimum wage, could be used to quantify informal care in the context of a sensitivity analysis. A future trial should also test whether or not a longer time horizon is needed for this particular patient group to benefit from an intervention of this kind. It may be considered unusual to include a full economic analysis in a feasibility study and, therefore, these results should be seen as exploratory.
Implications of clinical findings for a future trial of personalised care
An implication of the lack of difference between PC and TAU and the small degree of change in depression symptoms detected is that, if depression symptoms are to be used as a primary outcome in a definitive trial, a large sample size would be required to replicate difference of the order found here [e.g. using the HADS-D mean and SD (PC: mean 10.3, SD 4.6; TAU: mean 9.2, SD 4.6) at 6 months an achieved sample size of 368 per group would be required for 90% power at a 5% significance level (two-sided)]. This would be increased considerably if a cluster design were employed, which would be necessary to reduce contamination if PC were tested using PNs based in practice, and would also need to be increased to take account of attrition. On the other hand, a clinically significant effect of 3 on HADS-D, as originally proposed, would require fewer. The main implications for planning are the somewhat higher SD than that originally assumed (4.6 compared with 3.5), and the lower attrition rate (13% compared with 25%). The recruitment of practices to achieve these figures is another factor to be considered in planning. The fact that only a small improvement in depression symptoms was found over the entire sample is consistent with systematic review evidence indicating that even intensive evidence-based psychological treatments, such as CBT, problem-solving and pharmacological intervention with selective serotonin reuptake inhibitors, have only a small effect on depression in people with CHD. 96,136
Furthermore, our sample appears to represent a hard-to-treat group: the level of depression symptoms was high, more than half reported recurrent depression and more than one-quarter reported that they were receiving depression treatment at baseline and yet still reported depression symptoms. In addition, a large longitudinal cohort study (n = 1209)137 has found that pain, mediated by baseline severity of mood symptoms, was predictive of a worse course of depressive and anxiety disorders; all of our participants reported current chest pain, which was an inclusion criterion. A 3-point change in HADS-D score (which a trial of this size could have detected) after 6 months of relatively low-intensity intervention may therefore be an unrealistic expectation and a more intensive intervention for depression with engagement over a longer period may be needed for patients such as these. Our data, which suggest that receipt of more nurse time is associated with greater improvement in depression and self-reported chest pain, support this.
As well as chest pain, anxiety comorbid with depression is predictive of a worse depression outcome;138 our participants reported high anxiety levels and we found some evidence of anxiety as a mediator for depression improvement. In a future trial, more active treatment of anxiety should be tested, particularly when associated with chest pain.
The potential to detect differences between PC and TAU in our trial may have been reduced because TAU is itself an active intervention: at baseline 43% of the TAU group versus 32% of the PC group were prescribed antidepressants (according to their medical notes), and we were unable to control for this difference in our analyses. It is also possible that TAU may have been intensified during the trial: during our qualitative work GPs and PNs reported greater awareness of the problem of comorbid depression and CHD as a result of participation in our cohort study, the same may apply to participation in our RCT. This may have led to more than usual intervention in the TAU group, although from the medical notes it appeared that the number of mental health consultations were similar for both groups. In a future trial, changes in TAU during the trial should be recorded systematically.
We hypothesised that the intervention would increase self-efficacy to achieve desired outcomes which would lead to improved depression outcomes. However, the small recorded changes in self-efficacy and related illness perceptions had little effect on depression outcomes. This may have been expected: a difference between our intervention and that of others that have not found improved self-efficacy139 following self-management intervention is that our participants chose the outcomes on which to work on, that is, they identified the factors that they felt contributed to their low mood, rather than being required to work directly on their depression. A better examination of the theory behind our PC intervention would be to explore the effect of changes in self-efficacy on a measure of goal attainment, then test the effects of goal attainment on depression over the long term, although this would require a complex trial. Fewer PC compared with TAU participants visited A&E (24% vs. 38%), which may indicate increased self-efficacy in self-management in the PC group, though in a future trial a more robust measure than self-report of A&E attendance should be used to examine this, for instance Hospital Episode Statistics. 140
The possibility that our PC intervention may impact on reported chest pain requires further investigation. We are unable to determine whether or not self-reported chest pain in our trial participants was of cardiac origin. It is estimated that in half of all patients presenting with chest pain, the pain is of non-cardiac origin. 141 A high-quality review of psychological interventions for chest pain in patients with normal coronary anatomy (15 studies, 803 participants),142 suggests a modest to moderate benefit, especially for CBT and possibly hypnotherapy. Our trial indicates that non-pharmacological intervention may also be effective for chest pain in patients with CHD. Given the impact of chest pain on patients’ quality of life, mood and on the health service, self-reported chest pain may be an important primary outcome for a future trial. However, since determination of the causes of self-reported chest pain is complex, this should be supported by a more objective measure of cardiac status, such as heart rate variability, which is a predictor of a range of cardiac outcomes and associated with a number of psychological risk factors for CHD. 143
Lessons learnt concerning delivery of personalised care
The PC intervention was designed, informed by the findings from our qualitative work, to facilitate both patient choice and clinical judgement, so variation in intervention delivery was expected. However, we produced a manual for the intervention and the nurse case managers reported using its key elements of behaviour change interventions, signposting and liaison with other professionals. In weekly study group meetings, the multidisciplinary clinical team was satisfied that the PC was delivered as planned. Exploratory investigation of therapist effects indicated no difference in depression outcomes between the patients of the two nurse case managers, but that the patients of the nurse case manager with more health psychology experience may have had a greater reduction in self-reported chest pain. There was a very small sample size for this analysis, so this finding should be interpreted with caution, but it suggests that training in the behaviour change aspects of the intervention is important for case managers. However, other studies have shown that even nurses and GPs trained in behaviour change techniques may have difficulty applying them. 144,145 Further research into how best to train, or whether or not non-psychologists can be trained, to work more psychologically to increase the effectiveness of care is needed. A future trial of PC could test it as delivered by IAPT psychological well-being practitioners (PWPs).
An alternative approach would be to make greater effort to ensure that depressed patients managed using PC receive guideline-informed treatment. Multidisciplinary collaboration to ensure receipt of available, effective depression treatment has been a key element in a number of successful trials of complex interventions for depression in primary care patients. 12,146,147 In this trial, four participants in the PC group, compared with none in the TAU group, received new depression treatment (antidepressant treatment or psychological treatment) by the end of the trial. The nurse case managers often contacted other professionals involved in the care of the majority of PC group participants. However, the nurses reported difficulty in accessing some of the participants’ GPs and PNs (e.g. several telephone attempts needed, lack of response to e-mails); this limitation may be overcome by using case managers based within the GP surgery. However, even when contact was made, patients may not have received guideline-informed depression treatment (e.g. one GP was reluctant to prescribe antidepressants to a severely depressed patient despite advice from the nurse case manager because of expressed anxiety concerning multipharmacy and because IAPT services were unavailable in some areas). This suggests that in a future definitive trial, nurse case managers should be embedded within study practices to increase multidisciplinary collaboration (e.g. by planned times for discussion of cases with GPs and PNs, as is often the case in collaborative care) and means of ensuring that patients can access guideline-informed depression treatment should be predetermined.
Lessons learnt concerning the study protocol
Overall, the findings suggest that the trial protocol was feasible, with high levels of compliance and acceptability. For instance, attrition was < 10% and rates of missing data for most outcomes were low, despite the large number of measures. The PCRN-GL was responsible for practice recruitment, which was achieved well within our predicted time frame. This was helped by their prior knowledge of practices willing and able to conduct such research. Patient recruitment was also as expected and was in line with other studies of depression interventions in primary care. 146,148,149 Only around one-third of patients invited to participate by his or her GP provided consent to contact; this was also the case in our cohort study. Use of this ‘opt-in’ system appears to result in substantial loss of potential participants; however, this is the usual method of recruitment for studies conducted in UK primary care. All of the patients meeting our inclusion criteria at baseline agreed to be randomised. Low attrition and high acceptability suggest that people with CHD, depressive symptoms and chest pain are receptive to additional support.
Conclusions
We have developed an intervention that is acceptable to primary care CHD patients who have probable concurrent depression and current chest pain, and which is feasible for use in current practice. The PC intervention could, we think, be delivered by PNs, although training in behaviour change techniques will be necessary. IAPT PWPs may also be potential case managers, but they would need training in long-term condition management.
Depression symptoms in CHD patients who report chest pain appear difficult to treat, as evidenced by this trial and by previous work. 96 Our data suggest that more nurse time was associated with improved outcomes, so more intensive follow-up should be included in future use of the PC intervention.
In this trial, collaborative working to ensure that patients received guideline-informed depression intervention was not optimal; this may be improved by using case managers based within trial practices and having agreed methods for providing access to guideline-informed care. Other trials12,146 suggest that this will improve depression outcomes.
It is uncertain which outcomes, other than depression, should be used in a future trial of PC. Our data suggest that self-reported chest pain may be important, but given the complexities in the relationship between self-reported chest pain and cardiac outcome, use of this measure should be supplemented with a more objective measure of cardiac health, for instance heart rate variability.
Our data also suggest that the underlying theory of our PC intervention, that increased self-efficacy to achieve desired outcomes would lead to improvements in depression, needs further testing. Since participants chose a wide range of problems on which to work on, it was difficult to determine how many achieved their goals; a measure of goal attainment should be included in a future trial. However, changes in self-efficacy were small and more intensive psychological intervention may be needed to achieve greater improvement. Longer follow-up is likely to be needed to see impact on depression outcomes.
The trial protocol appeared, on the whole, to be successful with high levels of compliance and acceptability. However, we found, in common with other studies, that large numbers of patients have to be approached and screened for a sufficient number of CHD patients with both chest pain and depressive symptoms to be randomised. This makes such trials costly.
In so much as patients were able to address the wide variety of problems that trouble them, the PC intervention represents enhanced care, which may also be cheaper than TAU. If a fair test of the differences between this low-intensity, quality improvement intervention and TAU is to be made, in future trials it will also be important to monitor more closely any changes in TAU over the course of the trial which may occur owing to increased awareness in participating clinicians of the need to manage depression in people with CHD.
Chapter 5 Cohort study (work package 3)
Objectives and methods
In this chapter we describe the relationship between depression and chest pain in primary care patients recruited into the study from the GP QOF CHD registers. The aims, objectives and methods have been presented in detail elsewhere1 and will be summarised here.
Objectives
The objectives of the cohort study were to:
-
determine the prevalence, incidence rate and risk factors of depressive disorders in primary care patients with CHD
-
explore and describe the course and prognosis of physical and depressive symptoms among primary care patients with CHD over a 3-year period
-
determine the effect of depression on mortality, symptom severity and pain in primary care patients with CHD.
Service use, service costs and lost employment costs will be described separately.
Methods
The cohort study methods, instruments and analysis plan have been described elsewhere. 1 This was a naturalistic cohort study of primary care patients recruited from QOF CHD registers held by GP practices in south London. All participants on these registers were eligible for inclusion in the study as long as they were > 18 years of age and not temporarily registered with their GP. Eligible consenting patients were assessed at baseline and then followed up at 6-monthly intervals for a period of up to 4 years. We report on the 3-year follow-up data here. All patients were eligible for 36 months follow-up at the end of the study. A smaller population was eligible for a 4-year follow-up (and will be analysed in the future). The measures used at baseline and follow-up are shown in Table 16, previously published as table 1 in Tylee et al. 1 Not all measures have been used in the analyses presented here. For more details on the instruments used, see Appendix 4.
| Measure | Baseline | Follow-up |
|---|---|---|
| Modified Rose angina questionnaire (chest pain)113 | ✗ | ✗ |
| Guy’s Hospital Chest Pain Questionnaire150 | ✗ | ✗ |
| Specific activity scale119 | ✗ | ✗ |
| General Health Questionnaire-12 (psychological distress)151 | ✗ | |
| HADS114 | ✗ | ✗ |
| Clinical Interview Schedule – Revised (psychiatric symptoms)152 | ✗ | |
| PHQ-9 (depression)116 | ✗ | ✗ |
| EQ-5D (quality of life)153 | ✗ | ✗ |
| SF-12 (quality of life)118 | ✗ | ✗ |
| List of Threatening Experiences Questionnaire (life events)154 | ✗ | ✗ |
| SPQ120 | ✗ | ✗ |
| Client Service Receipt Inventory (health costs)127 | ✗ | ✗ |
| BIPQ123 | ✗ | ✗ |
| PSYCHLOPS (patient defined problems)155 | ✗ | ✗ |
| Rapid Estimate of Adult Literacy in Medicine (health literacy)156 | ✗ |
The reporting of chest pain by cohort members was recorded at each wave of follow-up. Chest pain is the cardinal symptom of CHD and the key to a clinical diagnosis of angina pectoris. We report here the prevalence rate of this physical symptom at baseline and the outcome in terms of cardiovascular events (e.g. MI) or continued exertional chest pain (i.e. patients reporting exertional pain on most occasions). We have analysed the relative roles of depression, anxiety and quality-of-life scores for these outcomes.
Chest pain assessment
We used the Rose questionnaire113 to assess the severity of the symptomatology (i.e. chest pain) of CHD. Devised in the 1960s for the purpose of detecting angina pectoris in field studies, it has been widely used since. The original validation criterion was agreement with CHD diagnosis in GP medical notes but since then it has been used as a useful predictor of subsequent cardiovascular events in the longer term. 157–160 On the other hand, the agreement between a positive response on the Rose and concurrent clinical testing for evidence of CHD can be poor. 161,162 It is used in both a long form in field studies and, as the short form of the Rose questionnaire has equivalent predictive power, the short form is often preferred. We describe how both the short form and the long form are rated below. Pain on exertion seems to be the key symptom to predict subsequent problems. 163
The short form is outlined in Table 17 below and the additional questions for the long form are shown in Table 18.
| Rose angina questionnaire item | Response required for scoring |
|---|---|
| 1. Do you ever have any pain or discomfort in your chest? (Rose question 1) | Yes |
| 2. When you walk at an ordinary pace on the level does this produce the pain? | No (grade 1)/yes (grade 2) |
| 3. When you walk uphill or hurry does this produce the pain? | Yes |
| Rose angina questionnaire item | Response required for scoring |
|---|---|
| 4. When you get any pain or discomfort in your chest on walking, what do you do? | Stop or slow down |
| 5. Does the pain or discomfort in your chest go away if you stand still? | Yes |
| 6. How long does it take to go away? | ≤ 10 minutes |
| 7. Diagram | |
| 7.1 Diagram cross section | Upper centre chest pain |
| 7.2 Diagram cross section | Middle centre chest pain |
| 7.3 Diagram cross section | Upper left chest pain and middle left chest pain |
To assess cardiac outcomes, all cardiology notes, investigations and interventions were collected from the GP notes by a medical doctor on the team (JP) and entered into an encrypted Microsoft Access® 2007 database (Microsoft Corporation, Redmond, WA, USA). The dates of each visit to a cardiologist, each investigation for a cardiac-related problem and each visit to a rapid access chest pain clinic, A&E or hospital admission for a cardiac investigation or intervention (e.g. bypass graft, angioplasty) were recorded. We also recorded any GP visit for cardiac-related problems or regular follow-ups. The data to be inputted and the specific definitions of cardiac investigations were decided before the hard copies were examined, with exception to the ‘rapid access chest pain clinic’ category. The GP notes were re-examined to gather this extra information and all data were entered into the Access database before any analysis of cardiac investigation data occurred. Numerous records per individual were condensed into variables that denoted: (a) the first cardiac investigation to occur within two time points would be recorded per person; and (b) the most severe cardiac investigation to occur within two time points would be recorded per person.
Thus, a cardiac outcome variable with seven hierarchical categories was created using a combination of the short form of the Rose angina questionnaire, the GP medical notes and publicly available mortality records:
-
no chest pain: score of 0 on the short Rose angina questionnaire
-
chest pain: positive response to the Rose angina questionnaire item
-
exertional chest pain: positive classification of grade 1 or grade 2 angina as defined by the short form Rose angina questionnaire
-
rapid access: any visit to the rapid access chest pain clinic, or any visit to A&E or emergency hospital admittance where cardiac-related chest pain was the diagnosis
-
bypass graft or angioplasty: intervention reported on GP medical notes or cardiologist/cardiology service notes
-
MI: occurring at any point during the follow-up period
-
death: any cardiovascular-related cause of death included (i.e. cardiovascular and cerebrovascular).
For the purpose of the longitudinal analysis, the most severe outcome was assigned to the time point at the end of the period where the outcome occurred. MIs and deaths had to be combined for some analyses, when there were insufficient numbers to fit full models. When linking cardiac investigation and mortality data with interview data at specific time points, deaths and cardiac investigations were coded into time points at the end of the 6-month window between interviews. If a participant had a recorded cardiac investigation within the 6 months before baseline, this was recorded in the baseline time point. All cardiac investigation data as described, with dates of the investigations, were merged into the cohort data set. The aim of this time assignment was to produce data where mental health status could be clearly identified in its temporal relation to cardiac outcomes (the actual date of death was, however, used for a more detailed and specific study of mortality).
Mortality data was gathered from a number of sources, including case notes and publicly available records. The deaths of the cohort participants were tracked in the first instance via the practice manager at the different GP surgeries, where we asked for date and cause of death. In those cases in which the participant had moved away from the surgery, we contacted the primary care support services within the specific boroughs. Further to this, we purchased death certificates through the health authorities relating to the specific boroughs. Where consent had been withdrawn we recorded the fact of death if known but not the reason. The censoring date was the date of death, the final interview date or the date when the interview would have taken place for those who were lost to follow-up. For the latter group, deaths were monitored using public sources up to their censoring date. If the reason for loss to follow-up was withdrawal of consent, the reasons for death were not sought unless available as part of the public record. An intermediate censoring date was also created, based on the 36-month interview date. Only deaths that occurred before that date were used when analysing the 36-month data.
A sample size of at least 800 participants was calculated to be adequate to allow modelling of associations between physical and mental illness. 1
Analyses
Descriptive statistics were used to summarise the cohort at baseline and logistic regression was used to estimate associations between outcome and predictor variables. 164
The incidence of depression was described from the raw data and separately by depression at baseline. The incidence rates are minimum rates, as missing time points (non-response) were coded as non-depression incident. Mechanisms of missingness were explored and the analyses were adjusted for variables associated with missing time points (age, ethnicity and cancer). The analysis was conducted under the assumption that data were missing at random.
Data were set up for a discrete-time survival analysis as per Singer and Willett. 165 Discrete-time survival analysis was chosen as the appropriate model to estimate the odds of an event and the time to an event occurring. Discrete-time (as opposed to the more common continuous time) survival analysis was chosen to reflect the sampling design of 6-monthly time intervals. For more information on discrete-time survival analyses please refer to Singer and Willett. 165
The baseline hazard was first modelled as unstructured, thereby allowing the odds of depression incidence to vary freely among time points; however, we chose to adopt a growth curve modelling framework for the odds of becoming depressed over time. This was estimated using Mplus version 7 (Muthén & Muthén, Los Angeles, CA, USA) and in a latent variable framework using robust maximum likelihood. For more information please see Muthén and Masyn. 166 This approach allows for both time-invariant and time-variant predictors to affect both the intercept and the change in odds with time (linear and quadratic terms). The assumption of proportional odds was tested by including time-varying effects of predictors and comparing nested models using the Satorra-Bentler scaled chi-squared difference test. 167 Model development started with an unconstrained ‘full’ model, with a number of variables included on a theoretical basis and inspired by our baseline paper (see List of variables tested). Parameters were tested using chi-squared difference testing and assessment of model fit statistics (Akaike information criterion and Bayesian information criterion), and the model presented is our best fitting constrained model.
List of variables tested
Depression at baseline, history of depression, sex, age, employment status, relationship status, alcohol intake, smoking status, EQ-5D items (problems with mobility/pain, self-care, usual activities, pain/discomfort), SPQ items covering a range of social problems, diabetes, Rose angina questionnaire, cardiac events during the cohort (including MI) and cardiac operations (bypass graft or angioplasty).
Standardised mortality ratios for those aged > 45 years were calculated in relation to the 2011 Office for National Statistics figures for heart disease (International Classification of Diseases, 10th edition), by age and sex. 168 Time at risk was assigned to 10-year age bands with a ‘lexis’ expansion using the stsplit command in Stata. Incident rates of mortality were analysed separately using Poisson regression modelling which took into account the time at risk. Covariates were selected to be the same as those considered in the primary analysis of cardiac outcome. An analysis was also performed using the outcome of ‘non-cardiovascular deaths’ with the same covariates to explore trends for other causes of death.
Results
Recruitment and retention of the study population
Recruitment
Recruitment into the cohort study is shown in Figure 10. Sixteen general practices from south London participated in the research programme, and from these practices 803 participants were recruited into the cohort study from the practice-held CHD registers. The study population represented 27% of those eligible to be included in the cohort study. 164
FIGURE 10.
Recruitment profile of the UPBEAT-UK cohort study.
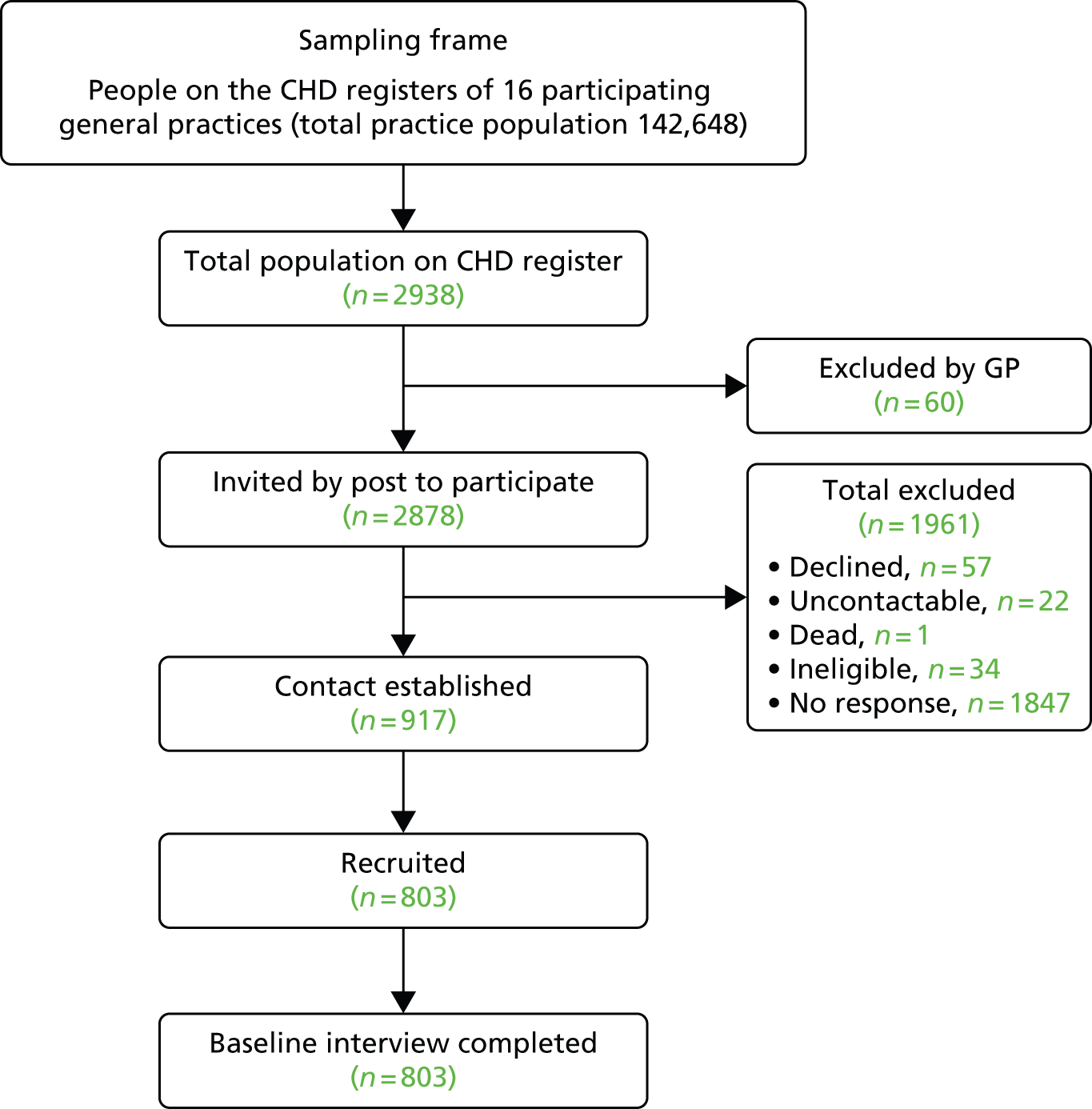
Retention of study population
The average follow-up time for 803 participants (561 men and 242 women) from 16 practices was 2.64 years and the median was 2.94 years; 141 (17.9%) were lost to (complete) follow-up or declined to be interviewed at some point during the 36-month follow-up period. A further 22 (2.8%) died of cardiac causes, 22 (2.8%) from vascular disease and a further 28 (3.64%) died from other causes. Table 19 shows the reasons for leaving the study prematurely.
| Reasons | n (%)a |
|---|---|
| Declined | |
| Poor health | 22 (2.79) |
| No longer interested | 46 (5.84) |
| Other | 27 (3.43) |
| Died | |
| Cardiac | 22 (2.79) |
| Stroke/vascular disease | 22 (2.79) |
| Cancer | 16 (2.03) |
| Other | 8 (1.02) |
| Unobtainable | 4 (0.51) |
| Lost to follow-up | 46 (5.84) |
| Total | 213 (27.04) |
Baseline characteristics of the study population
The baseline characteristics of the cohort population have been published in detail. 164 Tables 20–22 show the demographic, social and clinical characteristics of participants. Essentially, this was an older population (average age 71 years), predominantly male (70%), with 87.3% classifying themselves as ‘white’. Black and ethnic minorities made up 13% of participants. As would be expected, the majority were retired (77%). The average length of time since a diagnosis of CHD was made in the GP records was 10 years, and 44% reported ongoing chest pain. Social problems were common, with relationship problems being the most common reported social problem (38%). Levels of disability were also high in this population, with 53% reporting problems with pain or discomfort and 49% with mobility problems.
| Physical health status | n (%) |
|---|---|
| Patient reports suffering chest pains | 356 (44.3) |
| Primary diagnosis according to GP recordsa | |
| Documented MI | 336 (41.8) |
| Ischaemic heart disease | 374 (46.6) |
| Angina | 57 (7.1) |
| Heart failure | 3 (0.4) |
| Chest pain not otherwise specified | 10 (1.2) |
| Arrhythmias | 6 (0.8) |
| Other vascular causes or no diagnosis given | 14 (1.7) |
| Length of time since first recorded diagnosis of CHD and entry into study (years) | 10.36 (8.0)b |
| Comorbidc | |
| Diabetes mellitus | 200 (24.9) |
| Osteoarthritis | 134 (16.7) |
| COPD | 91 (11.3) |
| Chronic renal disease | 152 (18.9) |
| Asthma | 65 (8.1) |
| Hypertension | 445 (55.4) |
| Cancer | 96 (12.0) |
| Total number of comorbid medical illnessesd | |
| None | 296 (36.9) |
| One | 301 (37.5) |
| Two | 145 (18.1) |
| More than two | 61 (7.6) |
| BMI classificatione | |
| Normal | 180 (22.4) |
| Overweight | 343 (42.7) |
| Obese | 251 (31.3) |
| Missing data | 28 (3.5) |
| Characteristics | n (%) |
|---|---|
| Age (years) | 71.1 (10.9)a |
| Sex | |
| Male | 561 (69.9) |
| Ethnicity | |
| White | 701 (87.3) |
| Black | 33 (4.1) |
| Asian | 47 (5.9) |
| Other | 22 (2.7) |
| Employment status | |
| Paid employment | 148 (18.4) |
| Retired | 617 (76.8) |
| Housewife/husband | 2 (0.3) |
| Unemployed | 30 (3.7) |
| Relationship status | |
| Married/cohabiting | 508 (63.2) |
| Spouse/partner deceased | 150 (18.7) |
| Separated/divorced | 65 (8.1) |
| Single | 77 (9.59) |
| Usually live with | |
| Spouse/partner | 488 (60.8) |
| Children | 33 (4.1) |
| Alone | 236 (29.4) |
| Other | 43 (5.4) |
| Usual residence | |
| Owner occupier | 526 (65.5) |
| Private rental | 53 (6.0) |
| Housing association rental | 174 (21.7) |
| Sheltered housing | 18 (2.2) |
| Other | 23 (2.9) |
| Index of Multiple Deprivation score | 20.3 (14.0)a |
| n (%)a | |
|---|---|
| Social problemsb | |
| Housing problems | 43 (5.4) |
| Work problems | 73 (9.0) |
| Financial problems | 70 (8.7) |
| Social contact problems | 106 (13.2) |
| Problems with relatives | 89 (11.1) |
| Relationship problems | 302 (37.6) |
| Living alone | 29 (3.6) |
| Disabilityc | |
| Mobility problems | 391 (48.7) |
| Self-care problems | 101 (12.6) |
| Problems with usual activities | 237 (29.6) |
| Problems with pain/discomfort | 425 (53.0) |
| Problems with depression or anxiety | 196 (24.7) |
| Number of disability areasd | |
| No disability areas | 264 (33.2) |
| One to two disability areas | 285 (35.9) |
| More than two disability areas | 246 (30.9) |
Table 23 shows that our cohort study population contains approximately equal proportions of those with a completed MI and a current GP diagnosis of ischaemic heart disease with no history of MI. Similarly, equal proportions have and have not received some kind of surgical intervention.
| Cardiac status | n (%) |
|---|---|
| Report of chest pain (Rose angina questionnaire question 1) | |
| No | 447 (55.7) |
| Yes | 356 (44.3) |
| Primary GP coded CHD diagnosis | |
| MI | 339 (42.2) |
| IHD/angina | 431 (53.7) |
| Other | 33 (4.1) |
| Treatment (stent, angioplasty, CABG, pacemaker, ablation) | |
| No | 385 (47.9) |
| Yes | 418 (52.1) |
| Number of years from first coded coronary event, median (IQR), range | 8.5 (4.3–14.7), range: 0.1–46.2 |
Cardiac status at baseline is shown in Table 24.
| Baseline classification | n (%) |
|---|---|
| Short Rose angina questionnaire classification | |
| No chest pain | 447 (55.7) |
| Chest pain only | 132 (16.4) |
| Exertional pain (grade 1 or 2) | 214 (26.6) |
| Unclassifiable | 10 (1.3) |
| Full (long) Rose angina questionnaire classification | |
| No chest pain | 447 (55.7) |
| Chest pain only | 171 (21.3) |
| Grade 1 angina | 64 (8.0) |
| Grade 2 angina (requires pain on the level) | 47 (5.9) |
| Unclassifiable | 74 (9.2) |
Using the short Rose angina questionnaire, just over one-quarter of the cohort report exertional pain, which could indicate angina. Using the more stringent full Rose angina questionnaire criteria, 13.9% are likely to have a clinical diagnosis of angina (grade 1 or 2).
Discussion
A total of 44% of the sample stated that they currently experience chest pain. If this pain reflected just current angina pectoris, this figure would be very high, given that angina typically accounts for 4% of GP consultations and, in community samples of older people in Scotland, rates of angina pectoris using the Rose angina questionnaire are reported as 2.5% for men and 2.7% for women. 169 A meta-analysis of prevalence rates of angina in community samples in 31 countries showed a weighted mean of 5.7% for men and 6.7% for women – the highest rates from any of the countries were 14.4% for men and 15.7% for women. 170 Rates of angina following infarction are reported at 19%171 and there should not be any in the aftermath of successful revascularisation.
In fact, chest pain is commonly reported as a symptom in representative epidemiological studies, along with other types of pain. In one study, 12% of participants reported chest pain, compared with 41% reporting backache and 26% reporting headache. 172 The most common explanatory physical diagnosis for chest pain is oesophageal reflux. 173,174 There has been one epidemiological study in Australia of those with non-cardiac chest pain. 175 The authors noted that (1) the most common accompanying symptom was heartburn that suggests oesophageal disorder; (2) many did not seek help for it; and (3) quality of life was reduced for those reporting it with. 175,176 Non-specific chest pain is associated with an increased mortality rate. 177
It has long been recognised that many patients referred for investigation of chest pain have concurrent anxiety and depression syndromes. 173 Chest pain is indeed a recognised symptom for the diagnosis of a panic attack. 178 Case-level anxiety or depression has been stated to be a contraindication to expensive cardiology investigation, as few patients are found to have coronary disease. 179
Most of the work on the association of chest pain with psychiatric disorders has been carried out in cardiology patients. Our study population is different in that we have recruited our patients from a primary care-based register of CHD patients, some of whom are under the care of cardiologists. To our knowledge, there are no previously published studies on this topic based on GP register patients.
We are mainly reporting on the group identified as ‘exertional pain’ from the short Rose angina questionnaire, as there is adequate indication from published studies that this symptom is the most prognostically important for future events as described above. 163 The distribution of the data when classified by the short Rose angina questionnaire data also allows greater power in our analyses, and there are fewer missing responses than when using the long version. Multivariate analyses have been conducted to investigate baseline associations of short Rose classification.
Exertional pain versus non-exertional pain (risk factors)
The next analysis (Table 25) simultaneously compares those with chest pain with those with non-specific pain, and with those with exertional chest pain. Each paired analysis takes into account the third group.
| Sociodemographic and clinical predictors | Group 2: chest pain only | Group 3: exertional pain |
|---|---|---|
| Variable (adjusted risk ratio) | ||
| IMD score | 1.04* | |
| Depression | ||
| No | Reference | Reference |
| Yes | 3.29* | 2.32* |
| Anxiety | ||
| No | Reference | Reference |
| Yes | 2.96* | 2.26* |
| How much bodily pain? | 1.18 | 1.27* |
| Comorbidity: COPD | ||
| No | Reference | |
| Yes | 1.86 | |
| Primary GP coded CHD diagnosis | ||
| MI | Reference | |
| IHD/angina | 2.34* | |
| Other | 0.74 | |
| Total SPQ social problems | 1.36* | |
The variables entered were those baseline variables describing cohort members set out in the paper on baseline analyses. 164 They concern sociodemographic status, cardiac variables, self-reported cardiac risk factors, physical disease comorbidity and social problems.
Variables were included in the model if they provided information to an alpha threshold of p < 0.2. Variables in the model were age, sex, ethnicity, Index of Multiple Deprivation (IMD) score, smoking status, comorbidity of COPD, depression (positive on the HADS-D scale), anxiety (positive on the HADS-A scale), total number of SPQ social problems and ‘How much bodily pain’ (i.e. non-specific pain) in the past 4 weeks? (Question 21 on the Short Form Questionnaire 36-items quality-of-life scale.) Only those variables contributing with a significance threshold of p < 0.05 are reported above.
For continuous variables (age, IMD score and total SPQ social problems): with every one unit increase in IMD deprivation, the RR of being in the chest pain group compared with the no chest pain group increases by 3% (RR 1.03; p > 1).
For the categorical variables (sex, ethnicity, depression, COPD and primary GP coded CHD diagnosis): for those classified by the HADS as having depression compared with those without HADS depression, the RR of being in the chest pain group was 2.87, and being in the exertional pain group was 2.92, both compared with the no chest pain group.
The analysis in Table 25 was repeated using the full (long) Rose angina questionnaire classification variables: no pain (n = 447), pain in chest (n = 171), grade 1 and grade 2 angina (n = 111). The results, which are not reported here, were very similar.
Discussion
Notable in the results of this analysis is the absence of any of the cardiac variables or cardiac risk factors that we recorded as associations of chest pain (whether or not the chest pain was exertional) – in particular whether or not there is a history of a revascularisation procedure. The association with GP diagnosis of ischemic heart disease and exertional pain is compatible with the validation criteria of the Rose angina questionnaire. The association with COPD has clinical face validity – sufferers may experience discomfort when they exert themselves.
Depression, anxiety and social factors are the evident associations. The most potent association is the number of social problems rather than any individual social problem. For every additional social problem reported, the risk of exertional pain increases by 36%. Depression and anxiety are both associated with complaints of non-specific and exertional pain – the RR being higher for the exertional pain. Experience of panic and bodily pain remain independently associated with chest pain. Increasing report of bodily pain is associated with increased risk of being in both of the two chest pain categories.
The data from this cohort strongly suggest a psychosocial context for the complaint of chest pain whether or not it is anginal in type. As this is a cross-sectional analysis, direction of causality cannot be assumed. It is possible that having chest pain limits life to the extent that multiple social problems result, for example less social contact.
Chest pain and quality of life
Baseline analysis
The literature suggests that quality of life is impaired many years after MI for those with persisting symptoms,180 the effect being more marked in younger than older people. The same is reported for those with non-cardiac chest pain. 175 The EQ-5D is used in these analyses as a measure of health-related quality of life, a score can be obtained by summing the response to the five questions asked or from the accompanying visual analogue, in which the respondent makes a judgement of their quality of life now. We have chosen the latter, as one of the five questions asks about anxiety and depression and we wish to examine quality of life separately from mental state so far as is possible.
Examination of associations of baseline European Quality of Life-5 Dimensions visual analogue scale
Quality of life was thus recorded using the EQ-5D questionnaire visual analogue scale (EQ-5D-VAS). To examine the factors associated with quality of life, we used a multiple linear regression model and selected variables from a large pool of sociodemographic and clinical factors. Variables were selected using a stepwise selection procedure that retained all predictors if evidence for their association with the visual analogue scale was lower than p = 0.2.
The outcome, as well as all predictors used in this analysis, was collected by self-report questionnaire and at the same time point (baseline), and so this analysis can be seen as an examination into the factors associated with quality of life and, on an exploratory basis, an examination into the potential predictors of quality of life in our cohort. Owing to the positively skewed distribution of the EQ-5D-VAS, non-parametric bootstrapping using 200 replications was used in the analyses.
The results of this analysis are shown in Table 26–28.
| Statistic | Score (0–100) |
|---|---|
| Observations | 797 |
| Mean (SD) | 69.26 (18.76) |
| Median (IQR) | 70 (55–80) |
| Range | 4–100 |
| EQ-5D (summary score) | EQ-5D-VAS | HADS-D | HADS-A | |
|---|---|---|---|---|
| EQ-5D (summary score) | 1 | – | – | – |
| EQ-5D-VAS | 0.4801 | 1 | – | – |
| HADS-D | –0.5021 | –0.4694 | 1 | – |
| HADS-A | –0.4472 | –0.3715 | 0.6504 | 1 |
| Sociodemographic and clinical variables | Coefficient | SE | 95% CI (bias corrected) | p-value | Beta |
|---|---|---|---|---|---|
| Ethnicity (other vs. white) | –4.74* | 2.10 | –8.97 to –0.85 | 0.024 | –0.09 |
| BMI class | |||||
| Underweight/normal | – | – | – | – | – |
| Overweight | 1.19 | 1.61 | –2.04 to 4.33 | 0.462 | 0.03 |
| Obese | –3.04 | 1.81 | –6.70 to 0.23 | 0.093 | –0.08 |
| Smoking status | |||||
| Never | – | – | – | – | – |
| Ex-smoker | –2.47 | 1.45 | –5.25 to 0.67 | 0.088 | –0.06 |
| Current smoker | –5.19* | 2.27 | –9.36 to –0.96 | 0.022 | –0.09 |
| Rose angina questionnaire score | |||||
| No chest pain | – | – | – | – | – |
| Chest pain only | 0.031 | 1.72 | –3.09 to 3.39 | 0.986 | 0.001 |
| Angina (grade 1 and 2) | –3.90* | 1.67 | –7.30 to –0.65 | 0.020 | –0.09 |
| How much bodily pain?a | –3.85** | 0.47 | –4.83 to –3.02 | 0.000 | –0.29 |
| Received any past treatment for heart (including stent, angioplasty, CABG, pacemaker or ablation)? | –2.75* | 1.19 | –5.23 to –0.58 | 0.021 | –0.07 |
| Physical comorbidities | |||||
| Chronic kidney disease | –2.18 | 1.61 | –5.53 to 0.91 | 0.176 | –0.05 |
| Cancer | –5.63* | 2.26 | –9.87 to –0.74 | 0.013 | –0.10 |
| Psychological | |||||
| HADS-D (positive) | –9.71** | 2.36 | –13.55 to –4.72 | 0.000 | –0.17 |
| HADS-A (positive) | –4.80** | 1.63 | –7.76 to –1.30 | 0.003 | –0.11 |
The data in Table 27 show high correlation between anxiety and depression scores, and between EQ-5D scores and both. In the latter, high depression anxiety scores (bad) correlate inversely with high EQ-5D sores (good). Table 28 shows analyses that explore, at baseline, what factors influence the EQ-5D-VAS score in this cohort.
Summary of results
The following variables associated with baseline quality of life:
-
Ethnicity: white ethnic group had a 4.74 unit higher score (p = 0.024). This is a small standardised effect size (0.1).
-
Current smokers: current smokers scored, on average, lower by 5.19 units (p = 0.022).
-
Exertional chest pain: those with exertional chest pain had a lower quality-of-life score by 3.90 units (p = 0.020), a small effect size (0.09).
-
Bodily pain: those reporting bodily pain (an item taken from the SF-12) had, on average, a 3.85-point lower score on the EQ-5D-VAS (p < 0.001). This was a moderate effect size (0.28).
-
Treatment for heart problem: people who had received a treatment for their heart problem in the past had a lower quality-of-life score by –2.75 units (p = 0.021). This was a small effect size (0.07).
-
Cancer: cancer was associated with lower quality of life by 5.63 points (p = 0.013). This was a small effect size (0.1).
-
Depression: HADS-D (scoring ≥ 8 on the HADS-D scale) was associated with a 9.71-unit lower quality of life (p < 0.001). This was a small effect (0.17).
-
Anxiety: HADS-A (score of ≥ 8) was also associated with a lower score on the EQ-5D-VAS scale by 4.8 units (p = 0.003). This was a small effect (0.11).
Depression, anxiety and chest pain all impair quality of life at baseline.
Prevalence, incidence and course of depression
Prevalence of depression
The prevalence of depressive and anxiety disorders and the risk factors associated with a depressive disorder at baseline have been presented in detail elsewhere31 and will be summarised here. The prevalence of depressive and anxiety disorders in this population at baseline is shown in Table 29.
| Mental health status | n (%) |
|---|---|
| GP diagnosis of depression | 56 (7.0) |
| GP diagnosis of anxiety | 95 (11.8) |
| Depression and anxiety by CIS-R | |
| No disorder | 654 (81.4) |
| Severe depressive episode | 17 (2.1) |
| Moderate depressive episode | 14 (1.7) |
| Mild depressive episode | 23 (2.9) |
| Panic disorder | 4 (0.5) |
| Generalised anxiety disorder | 25 (3.1) |
| Mixed anxiety and depression | 66 (8.2) |
| HADS scores | |
| Mean depression score (SD) | 3.34 (3.5) |
| Depression score of ≥ 8 | 103 (12.8) |
| Mean anxiety score (SD) | 4.97 (4.5) |
| Anxiety score of ≥ 8 | 199 (24.8) |
The proportion of the population who had depression recorded as an active problem in GP records at recruitment into the cohort was 7%, whereas 4.6% of the population had an International Classification of Diseases, 10th edition, diagnosis of a depressive disorder (mild, moderate or severe) as defined by the Clinical Interview Schedule – Revised (CIS-R). According to the HADS-D scale, 12.8% of the population suffered from a depressive disorder at baseline (i.e. scored > 7 on the depression subscale).
The statistically significant associations between predictor variables and CIS-R-defined depression at baseline fell into three domains: living alone, disability and pain, and age (problems with living alone: adjusted OR for a CIS-R diagnosis of mild, moderate or severe depressive episode 5.49, 95% CI 2.11 to 13.40; p < 0.001; experiencing chest pain: adjusted OR for a CIS-R diagnosis of mild, moderate or severe depressive episode 3.27, 95% CI 1.58 to 6.76; p = 0.001; being disabled by pain or discomfort: adjusted OR for a CIS-R diagnosis of mild, moderate or severe depressive episode 3.39, 95% CI 1.42 to 8.10; p < 0.006; having problems carrying out usual activities: adjusted OR for a CIS-R diagnosis of mild, moderate or severe depressive episode 3.71, 95% CI 1.93 to 7.14; p < 0.001; and age: adjusted OR per year for a CIS-R diagnosis of mild, moderate or severe depressive episode 0.95, 95% CI 0.92 to 0.98; p < 0.001) (see Walters et al. 164). Chest pain was significantly associated with depression independently of other pains and discomfort.
Participants had an increased odds of having a GP-recorded diagnosis of depression if they reported problems with close relationships (adjusted OR for a GP diagnosis of depression 2.51, 95% CI 1.40 to 4.52; p = 0.002), reported having diabetes mellitus (adjusted OR for a GP diagnosis of depression 2.01, 95% CI 1.11 to 3.63; p = 0.02), were disabled by pain and discomfort (adjusted OR for a GP diagnosis of depression 1.95, 95% CI 1.04 to 3.68, p = 0.037), or were female (adjusted OR for a GP diagnosis of depression 1.88, 95% CI 1.04 to 3.37; p = 0.035) (see Walters et al. 164).
Incidence of depression and risk factors for incident depression
The incidence risk of developing a depressive disorder (as defined by scoring ≥ 8 on the HADS-D subscale) is shown in Table 30. The proportion of new cases of depression was similar at each time point for those who were not depressed at baseline. However, the proportion of incident cases of depression decreased across time points for those who were depressed at baseline. The cohort followed participants for a total 1942.3 years at risk. During this period there were 240 incident cases of depression giving an incident rate ratio of 123.6 per 1000 person-years at risk.
| Time point (months) | Not depresseda up to time point, n (%) | New cases of depression during time point, n (%) |
|---|---|---|
| Not depressed at baseline | ||
| 0 | 696 (100) | – |
| 6 | 620 (94) | 42 (6) |
| 12 | 569 (95) | 33 (5) |
| 18 | 510 (95) | 29 (5) |
| 24 | 477 (97) | 17 (3) |
| 30 | 434 (96) | 18 (4) |
| 36 | 383 (94) | 25 (6) |
| Total | 164 | |
| Depressed at baseline | ||
| 0 | 103 (100) | – |
| 6 | 45 (47) | 50 (53) |
| 12 | 31 (70) | 13 (30) |
| 18 | 25 (86) | 4 (14) |
| 24 | 17 (81) | 4 (19) |
| 30 | 10 (71) | 4 (29) |
| 36 | 9 (90) | 1 (10) |
| Total | 76 | |
The risk factors associated with the incidence of depression are shown in Table 31. The strongest predictor of incident depression was having had a documented MI at some point before the incident depression. Other predictors of incident depression were: suffering with depression at baseline or having a past history of depression, self-reported exertional chest pain, problems with disability and living alone.
| Predictors | OR | Logit-hazard function | SE | Estimate/SE | p-value |
|---|---|---|---|---|---|
| Time-invariant predictors with a time invariant effect a | |||||
| Depression at baseline vs. no depression at baseline | 5.39 | 1.69 | 0.20 | 8.57 | 0.000 |
| History of depression vs. no history of depression | 1.91 | 0.65 | 0.16 | 4.06 | 0.000 |
| Exertional pain vs. no chest pain | 3.38 | 1.21 | 0.29 | 4.20 | 0.000 |
| Problems with mobility/pain vs. no problems with mobility | 1.73 | 0.55 | 0.19 | 2.87 | 0.004 |
| Problems with usual activities vs. no problems with usual activities | 2.14 | 0.759 | 0.185 | 4.09 | 0.000 |
| Work problems vs. no work problems | 3.10 | 1.13 | 0.387 | 2.92 | 0.004 |
| Problems with living alone vs. no problems living alone | 1.79 | 0.58 | 0.28 | 2.05 | 0.040 |
| Age (centred at 70 years) per year | 0.99 | –0.001 | 0.007 | –0.16 | 0.872 |
| Ethnicity (non-white vs. white) | 1.27 | 0.24 | 0.22 | 1.11 | 0.266 |
| Comorbid cancer vs. no comorbid cancer | 0.80 | –0.22 | 0.25 | –0.86 | 0.390 |
| Time-invariant predictors with a time varying effect b | |||||
| Exertional pain vs. no chest pain (effect with time) | 0.69 | –0.37 | 0.16 | –2.29 | 0.022 |
| Work problems vs. no work problems (effect with time) | 0.75 | –0.28 | 0.10 | –2.97 | 0.003 |
| Time-varying predictors with a time invariant effect c | |||||
| History of MI preceding depression | 11.24 | 2.42 | 0.88 | 2.76 | 0.006 |
[Exertional pain was initially considered as a possible type 3 variable. However, in the final model it was better treated as a time-invariant (baseline only) predictor because of uncertainties in the longitudinal data recording. It was not always possible to establish the time sequence of the incidence of pain and depression and there were a large number of missing values, which greatly reduced the sample size.]
The course of depression in cohort participants is described in Table 32.
| Patterns of depression | No missing values, n (%) | Missing values during follow-up, n (%) | Missing at follow-up, n (%) | Total, n (%) |
|---|---|---|---|---|
| Depression at baseline | 54 (10.8) | 9 (13.0) | 40 (17.4) | 103 (12.9) |
| Always depressed | 18 (33.3) | 5 (55.6) | 21 (52.5) | 44 (42.7) |
| No depression at final follow-up | 19 (35.2) | 3 (33.3) | 14 (35.0) | 36 (35) |
| Improved then depressed at final follow-up | 17 (31.5) | 1 (11.1) | 5 (12.5) | 23 (22.3) |
| No depression at baseline | 446 (80.2) | 60 (87.0) | 190 (82.6) | 696 (87.1) |
| No depression at any point | 338 (75.8) | 45 (75.0) | 149 (78.4) | 532 (76.4) |
| Developed depression at final follow-up | 51 (11.4) | 9 (15.0) | 24 (12.6) | 84 (12.1) |
| Developed depression, not at final follow-up | 57 (12.8) | 6 (10.0) | 17 (8.9) | 80 (11.5) |
| Total | 500 (62.6) | 69 (8.6) | 230 (28.8) | 799 |
Of the 12.9% of participants depressed at baseline, 42.7% remained depressed throughout the 36-month follow-up period. Only 35% were not depressed at final follow-up. Of those not depressed at baseline, 23.6% developed depression at some point over the follow-up period.
The incidence and effect of depression and anxiety on cardiac outcomes and mortality
The incidence of cardiac outcomes is reported in two ways – first, the incidence of cardiac events over the follow-up period (and the effect of depression and anxiety) and, second, the continuing reporting of chest pain over that same period.
Table 33 shows the incidence of cardiac events over the 36-month follow-up. There were 44 total deaths that had a cardiovascular cause. Sixteen patients in the cohort had a MI during the follow-up period.
| Time point (months) | Bypass graft or angioplasty, n (%) | MI, n (%) | Cardiovascular death, n (%) | Any |
|---|---|---|---|---|
| Baseline | 12 (63.2) | 7 (36.8) | 0 (0.0) | 19 |
| 6 | 11 (73.3) | 0 (0.0) | 4 (26.7) | 15 |
| 12 | 11 (52.4) | 4 (19.0) | 6 (28.6) | 21 |
| 18 | 8 (36.4) | 3 (13.6) | 11 (50.0) | 22 |
| 24 | 15 (53.6) | 1 (3.6) | 12 (42.9) | 28 |
| 30 | 10 (76.9) | 0 (0.0) | 3 (23.1) | 13 |
| 36 | 10 (52.6) | 1 (5.3) | 8 (42.1) | 19 |
| Total | 77 (56.2) | 16 (11.7) | 44 (32.1) | 137 |
Table 34 shows the percentage of cardiac outcomes that were related to depression and anxiety. Although our initial analysis was done with depression, we found that for every cardiac outcome anxiety was more strongly related, especially in MI and cardiovascular death.
| Cardiac outcome | Depression (%) | Anxiety (%) |
|---|---|---|
| No chest pain | 7.2 | 15.5 |
| Chest pain | 17.8 | 34.4 |
| Exertional pain | 23.1 | 41.2 |
| Rapid access | 18.3 | 26.3 |
| Bypass graft/angioplasty | 18.4 | 24.7 |
| MI | 6.3 | 25.0 |
| CV death | 6.8 | 29.5 |
Table 35 shows the predictors of cardiac outcome with ‘no chest pain’ as comparator.
| Outcome | Relative risk ratio | 95% CI | p-value |
|---|---|---|---|
| Chest pain (Rose angina questionnaire category 1 at follow-up) | |||
| Time point (year) | 0.952 | 0.896 to 1.012 | 0.115 |
| Age in years | 0.988 | 0.970 to 1.006 | 0.193 |
| Sex (female vs. male) | 1.763 | 1.252 to 2.484 | 0.001 |
| Ethnic group (non-white vs. white) | 1.053 | 0.653 to 1.700 | 0.831 |
| Rose category 1 vs. no chest pain | 2.131 | 1.407 to 3.228 | < 0.001 |
| Rose category 2 vs. no chest pain | 3.059 | 1.802 to 5.192 | < 0.001 |
| Baseline depression (score of ≥ 8 on HADS-D) | 1.353 | 0.762 to 2.400 | 0.302 |
| Baseline anxiety (score of ≥ 8 on HADS-A) | 1.938 | 1.285 to 2.922 | 0.002 |
| Exertional chest pain (Rose category 2 at follow-up) | |||
| Time point (year) | 0.993 | 0.945 to 1.043 | 0.785 |
| Age in years | 0.986 | 0.972 to 0.999 | 0.044 |
| Sex (female vs. male) | 1.418 | 1.025 to 1.961 | 0.035 |
| Ethnic group (non-white vs. white) | 1.116 | 0.729 to 1.709 | 0.614 |
| Rose category 1 vs. no chest pain | 8.797 | 6.153 to 12.579 | < 0.001 |
| Rose category 2 vs. no chest pain | 12.610 | 7.987 to 19.871 | < 0.001 |
| Baseline depression (score of ≥ 8 on HADS-D) | 1.627 | 0.992 to 2.668 | 0.614 |
| Baseline anxiety depression (score of ≥ 8 on HADS-A) | 1.807 | 1.214 to 2.690 | 0.004 |
| Attendance at a rapid access clinic at follow-up with a cardiac cause | |||
| Age in years | 0.988 | 0.965 to 1.012 | 0.332 |
| Sex (female vs. male) | 0.887 | 0.524 to 1.501 | 0.655 |
| Ethnic group (non-white vs. white) | 1.455 | 0.965 to 1.012 | 0.332 |
| Rose category 1 vs. no chest pain | 4.439 | 2.538 to 7.762 | < 0.001 |
| Rose category 2 vs. no chest pain | 4.003 | 1.838 to 8.720 | < 0.001 |
| Baseline depression (score of ≥ 8 on HADS-D) | 1.749 | 0.837 to 3.656 | 0.137 |
| Baseline anxiety (score of ≥ 8 on HADS-A) | 1.155 | 0.634 to 2.101 | 0.638 |
| Cardiac intervention at follow-up (angioplasty, bypass grafting) | |||
| Age in years | 0.968 | 0.949 to 0.988 | 0.002 |
| Sex (female vs. male) | 0.794 | 0.431 to 1.461 | 0.458 |
| Ethnic group (non white vs. white) | 1.743 | 0.935 to 3.248 | 0.080 |
| Rose category 1 vs. no chest pain | 6.076 | 3.369 to 10.956 | < 0.001 |
| Rose category 2 vs. no chest pain | 7.512 | 3.736 to 15.104 | < 0.001 |
| Baseline depression (score of ≥ 8 on HADS-D) | 1.910 | 0.858 to 4.249 | 0.113 |
| Baseline anxiety depression (score of ≥ 8 on HADS-A) | 0.708 | 0.348 to 1.439 | 0.340 |
| MI/death at follow-up | |||
| Age in years | 1.089 | 1.037 to 1.143 | 0.001 |
| Sex (female vs. male) | 0.653 | 0.348 to 1.224 | 0.184 |
| Ethnic group (non-white vs. white) | 0.808 | 0.248 to 2.636 | 0.724 |
| Rose category 1 vs. no chest pain | 2.150 | 0.916 to 5.049 | 0.079 |
| Rose category 2 vs. no chest pain | 3.724 | 1.539 to 9.010 | 0.004 |
| Baseline depression (score of ≥ 8 on HADS-D) | 0.587 | 0.198 to 1.739 | 0.336 |
| Baseline anxiety depression (score of ≥ 8 on HADS-A) | 3.930 | 1.954 to 7.904 | < 0.001 |
Discussion
In terms of predictors of cardiac outcome status, chest pain and exertional chest pain were not associated with baseline depression status but were statistically significantly associated with baseline anxiety. There were no associations between attendance at a rapid access cardiac clinic for a cardiac reason and either baseline depression or anxiety.
There was no evidence of an association between baseline depression and having a MI or dying from a cardiac cause, but there was a statistically significant association with baseline anxiety and having a MI or dying from a cardiac cause. Rose angina questionnaire categories for chest pain at baseline were significantly and strongly associated with subsequent chest pain and exertional chest pain, especially the latter, and in the direction that would be expected, that is, both were associated with all cardiac outcome status compared with no chest pain with exertional chest pain having a stronger association with cardiac status (apart from attendance at a rapid access cardiac clinic) than chest pain. Women have an increased risk of chest pain and exertional pain (but no evidence for higher risk of other cardiac outcomes). Age was a significant predictor only of MI/death. The effect of adding covariates to an unadjusted mode, leading to the full model reported above, is shown in Table 36.
| Model | Chest pain | Exertional chest pain | Rapid access | Intervention | MI/death |
|---|---|---|---|---|---|
| (a) Unadjusted | 2.557** | 3.729** | 2.932** | 2.907** | 1.030 |
| (b) Adjusted for age, sex, ethnic group | 2.357** | 3.363** | 2.688** | 2.430** | 1.378 |
| (c) As (b) + Rose category at baseline | 1.951* | 2.231** | 1.892* | 1.592 | 1.177 |
| (d) As (c) + anxiety at baseline | 1.353 | 1.627* | 1.449 | 1.910 | 0.587 |
Sensitivity analyses
General practitioner practice was also included in the model as a sensitivity analysis as the practices were situated in areas with different sociodemographic characteristics and also had varying levels of loss to follow-up. The results did not differ significantly from the original model, apart from the exertional chest pain outcome, in which depression and anxiety had similar relative risk ratios (RRRs), both of which were significant (depression: RRR 1.702, 95% CI 1.025 to 2.827; p = 0.040; anxiety: RRR 1.573, 95% CI 1.027 to 2.411; p = 0.037).
The baseline number of comorbid medical illnesses and social problems as measured by the SPQ was added to the model. Depression was now no longer significant for chest pain (RRR 1.177, 95% CI 0.650 to 2.130; p = 0.591), although anxiety remained significant (RRR 1.730, 95% CI 1.132 to 2.643; p = 0.011). Total number of social problems was also related to chest pain (p = 0.001), but not the number of comorbidities. In terms of exertional chest pain, in this model, depression was no longer statistically significant (RRR 1.424, 95% CI 0.560 to 2.373; p = 0.174) although, again, anxiety remained statistically significant (RRR 1.600, 95% CI 1.059 to 2.413; p = 0.026). There was no change in associations for rapid access chest clinic or for cardiac interventions (neither significant), but social problems were significant for chest pain and exertional chest pain (p = 0.001 for both) and for cardiac interventions (p = 0.005), but not for other outcomes.
Two alternative definitions of depression were analysed:
-
Depression in the 6 months before the index cardiac event. The results of this analysis were similar to the main model, except that both depression and anxiety were statistically significantly associated with exertional chest pain (depression: RRR 1.85, 95% CI 1.291 to 2.663; p = 0.001; anxiety: RRR 1.867, 95% CI 1.349 to 2.585; p < 0.001). For other outcomes, the model could not be fitted owing to small sample sizes.
-
Cumulative burden of depression using the sum of follow-up periods, occurring before the index cardiac outcome, in which the patient had been depressed. Chest pain and exertional chest pain were both associated with depression and anxiety (depression: RRR 1.217, 95% CI 1.000 to 1.482; p = 0.05; anxiety: RRR 1.239, 95% CI 1.041 to 1.474; p = 0.016). However, other outcomes were similar to the main model except that cumulative anxiety was associated with attendance at a rapid access chest clinic (RRR 1.350, 95% CI 1.059 to 1.722; p = 0.015).
Redefining the cardiac outcome with rapid access chest clinic combined with any other interventions did not show any association with baseline depression or anxiety.
Mortality
There were 72 deaths altogether, of which 22 were from cardiac disease, 22 from vascular disease, 16 from cancer and eight were attributable to other causes; in four cases, the cause of death was not ascertainable. Of the 22 cardiac deaths, 14 were of men (2.5% of 561) and eight of women (3.3% of 242). Incidence rates of cardiac death were 12.7 per 1000 person-years for men and 9.3 per 1000 person-years for women. Standardised mortality ratios compared with the general population were 1.13 (95% CI 0.62 to 1.90) and 1.87 (95% CI 0.81 to 3.70), for men and women, respectively.
Table 37 shows a Poisson regression model for the rates of cardiovascular deaths. Only risk ratios for increasing age and a higher baseline anxiety were significant at p = 0.05. A sensitivity analysis using the CIS-R definition of depression and anxiety was similar, except that the risk ratio for depression was increased to 1.260 (95% CI 0.274 to 5.796; p = 0.766) and that for anxiety decreased (risk ratio 2.001, 95% CI 0.694 to 5.774, p = 0.199) and was no longer significant.
| Adjusted RR | 95% CI | p-value | |
|---|---|---|---|
| Female | 0.726 | 0.357 to 1.473 | 0.374 |
| Age (years) | 1.119 | 1.076 to 1.164 | 0.000 |
| Rose category | |||
| 1: chest pain only | 0.768 | 0.292 to 2.017 | 0.592 |
| 2: angina | 1.089 | 0.402 to 2.957 | 0.866 |
| BMI class | |||
| 2: overweight | 1.119 | 0.544 to 2.303 | 0.760 |
| 3: obese | 0.959 | 0.375 to 2.457 | 0.931 |
| Smoking status | |||
| 1: ex-smoker | 1.123 | 0.556 to 2.271 | 0.746 |
| 2: current smoker | 1.299 | 0.379 to 4.459 | 0.677 |
| Units alcohol | |||
| 1: 0 units | 0.881 | 0.430 to 1.80 | 0.730 |
| 2: 1–10 units | 1.012 | 0.379 to 2.701 | 0.982 |
| 3: 11 + units | 0.856 | 0.236 to 3.109 | 0.813 |
| SPQ total | 1.149 | 0.847 to 1.559 | 0.371 |
| Total comorbidities | 1.027 | 0.776 to 1.360 | 0.852 |
| Baseline depression | 0.313 | 0.069 to 1.431 | 0.134 |
| Baseline anxiety | 2.482 | 1.125 to 5.48 | 0.024 |
Table 38 shows a Poisson regression model for the rates of non-cardiovascular deaths. Only RRs for increasing age, male sex and higher baseline total comorbidities were significant at p = 0.05.
| Adjusted RR | 95% CI | p-value | |
|---|---|---|---|
| Female | 0.227 | 0.065 to 0.796 | 0.021 |
| Age (years) | 1.059 | 1.009 to 1.110 | 0.020 |
| Rose category | |||
| 1: chest pain only | 1.916 | 0.750 to 4.893 | 0.174 |
| 2: angina | –a | ||
| BMI class | |||
| 1: normal | 0.632 | 0.242 to 1.648 | 0.805 |
| 2: overweight | 0.549 | 0.169 to 1.789 | 0.320 |
| Smoking status | |||
| 1: ex-smoker | 0.894 | 0.367 to 2.176 | 0.805 |
| 2: current smoker | 0.392 | 0.042 to 3.055 | 0.348 |
| Units alcohol | |||
| 1: 0 units | 0.518 | 0.398 to 1.250 | 0.143 |
| 2: 1–10 units | 0.191 | 0.024 to 1.533 | 0.119 |
| 3: 11 + units | 1.099 | 0.322 to 3.749 | 0.879 |
| SPQ total | 0.952 | 0.598 to 1.514 | 0.834 |
| Total comorbidities | 1.391 | 1.015 to 1.907 | 0.040 |
| Baseline depression | 1.122 | 0.212 to 5.928 | 0.892 |
| Baseline anxiety | 0.415 | 0.076 to 2.255 | 0.309 |
The types and course of chest pain, and predictors of chest pain as an outcome
The effect of depression on continuing chest pain
This next series of analyses use data from the five-wave follow-up. We report on the frequency of cardiac events, the persistence of chest pain reported by respondents and the predictive power of depression and other psychosocial factors.
Chest pain type and frequency of report
As shown in Table 39, the amount of chest pain is reported, ranging from 0 to 100%, of the occasions when patients were interviewed using the Rose angina questionnaire. These proportions became the outcome variable to contrast with baseline predictors between those with no chest pain and those with non-exertional pain only.
| Full sample (N = 803), n (%) | |
|---|---|
| No chest pain | 345 (45) |
| Non-exertional pain only | 103 (14) |
| 1–25% | 90 (12) |
| 26–50% | 98 (13) |
| 51–75% | 43 (6) |
| 76–100% | 82 (11) |
| Total | 761 (91) |
In contrast to Table 39, which takes into account the whole cohort, subsequent analyses report on those who remained under follow-up who did not experience any of the events mentioned above. In addition, only those who had, at most, two missing chest pain responses out of the five possible over the follow-up period were included. Those with more than two missing responses were excluded. (Sensitivity analysis was carried out restricting it to one or fewer missing responses, and no differences were found, thus we selected this analysis, which includes a larger portion of the sample.) These two exclusions reduced the sample for analysis to n = 568. Baseline depression and anxiety scores were examined separately, and then quality of life, as measured by the EQ-5D-VAS, was entered into the analysis.
Predictors of non-exertional pain
Females and those with asthma comorbidity were most at risk of reporting non-exertional chest pain (Tables 40 and 41). Shorter amount of time on the GP CHD register is also predictive. Anxiety and depression, counterintuitively, do not have an effect. The addition of the quality-of-life measure does produce a significant finding, with a lower score being predictive of more pain report.
| Non-exertional chest pain (SD) | Non-exertional chest pain with inclusion of EQ-5D-VAS (SD) | |
|---|---|---|
| Chest pain only | 7.19** (2.62) | 7.34** (2.71) |
| Sex (female) | 2.74** (7.26) | 2.80** (7.14) |
| Asthma | 3.34* (1.87) | 3.34* (1.88) |
| Number of years since first coded coronary event | 0.95* (0.02) | 0.95* (0.02) |
| HADS-A | 1.81 (0.79) | 1.33 (0.62) |
| Age | 0.98 (0.02) | 0.98 (0.02) |
| Ethnicity (other vs. white) | 1.56 (0.78) | 1.49 (0.75) |
| Cancer | 1.79 (0.82) | 1.87 (0.88) |
| EQ-5D-VAS | 0.98* (0.01) |
| Non-exertional chest pain (SD) | Non-exertional chest pain with inclusion of EQ-5D-VAS (SD) | |
|---|---|---|
| Chest pain only | 7.08** (2.57) | 7.45** (2.75) |
| Sex (female) | 2.83** (0.96) | 2.67** (0.93) |
| Asthma | 3.41* (1.88) | 3.37* (1.88) |
| Number of years since first coded coronary event | 0.96* (0.02) | 0.95* (0.02) |
| HADS-D | 1.31 (0.93) | 0.77 (0.59) |
| Age | 0.98 (0.02) | 0.98 (0.02) |
| Ethnicity (other vs. white) | 1.54 (0.77) | 1.47 (0.74) |
| Cancer | 1.84 (0.83) | 1.92 (0.89) |
| EQ-5D-VAS | 0.97** (0.01) |
Exertional pain
The situation with exertional pain is quite different (Tables 42 and 43). Exertional pain reported at baseline had a massive effect in predicting subsequent reports of chest pain, as did, to a lesser extent, non-exertional pain. Depression and anxiety each had an additional effect on baseline pain. The length of time on the register was predictive, but in the opposite direction to non-exertional pain, meaning the longer time spent on the register, the more likely they were to report exertional pain. Older age was a weak risk factor. Addition of the quality-of-life measure eliminated the effect of depression, and weakened that of anxiety.
| Anxiety alone (SD) | Anxiety with inclusion of EQ-5D-VAS (SD) | |
|---|---|---|
| Chest pain only | 4.82** (1.15) | 4.64** (1.12) |
| Exertional chest pain | 28.93** (7.26) | 28.07** (7.14) |
| Number of years since first coded coronary event | 1.02 (0.01) | 1.02 (0.01) |
| HADS-A | 2.01** (0.44) | 1.65* (0.38) |
| Age | 0.99 (0.01) | 0.99 (0.01) |
| Ethnicity (other vs. white) | 0.96 (0.29) | 0.87 (0.27) |
| Cancer | 0.72 (0.24) | 0.75 (0.25) |
| EQ-5D-VAS | 0.98** (0.01) |
| Depression alone (SD) | Depression with inclusion of EQ-5D-VAS (SD) | |
|---|---|---|
| Chest pain only | 5.14** (1.22) | 5.00** (1.20) |
| Exertional chest pain | 29.82** (7.48) | 29.18** (7.41) |
| Number of years since first coded coronary event | 1.02* (0.01) | 1.02 (0.01) |
| HADS-D | 1.83* (0.50) | 1.30 (0.37) |
| Age | 0.99 (0.01) | 0.99 (0.01) |
| Ethnicity (other vs. white) | 0.89 (0.27) | 0.82 (0.25) |
| Cancer | 0.73 (0.24) | 0.75 (0.25) |
| EQ-5D-VAS | 0.98** (0.01) |
Economic analysis of the UPBEAT-UK programme
Objectives
To:
-
assess the long-term costs of care for a sample of patients with CHD and comorbid (a) baseline, and (b) subsequent depression compared with those without comorbid depression
-
identify predictors of these costs.
Methods
Health-care utilisation and costs
The primary measures for the economic analysis were health-care utilisation and total health-care and societal costs. Medical and informal (i.e. family care) resource utilisation was assessed using a questionnaire, the Client Service Receipt Inventory, developed by members of the Centre for the Economics of Mental and Physical Health. 127 The questionnaire is frequently used in mental and physical care evaluations. Medical utilisation included the number of contacts with hospital and community services and (where appropriate) the length of these contacts and the duration of stay for inpatient care. Hospital service utilisation consisted of outpatient, inpatient, A&E and day hospital visits. Community services mainly included contacts with GPs, practice and district nurses, other medical professionals (psychiatrist, other community-based doctor, community mental health nurse, health visitor, other nurse, psychologist, counsellor, occupational therapist, physiotherapist, ‘alternative’ medicine or therapy, other therapist) and care professionals (social worker, housing worker, home help/home care worker, care attendant, community support worker, voluntary worker, day centre/drop-in/social club, any other community-based service). Informal care involved assistance in daily activities by family members, relatives or friends (i.e. personal or child care, help in/around and outside the house) and was measured as the average weekly hours spent with the patient specifically because of their health problems.
Data were collected every 6 months for a period of 3 years between September 2007 and September 2012.
Total costs were calculated from both a health-care payer’s and a wider societal perspective, by multiplying the estimated resource use by the corresponding unit cost. Unit costs were applied to service-use data using the NHS Reference Costs for 2011–12181 and the 2012 Unit Costs of Health and Social Care. 129 The unit cost of a home care worker was used as a proxy for costing informal care. Wherever necessary, costs were inflated to 2012 prices using the inflation indices for hospital and community health services. 129 All costs were expressed in pounds sterling (GBP).
Indirect costs of productivity loss due to CHD and comorbid depression were not included in the analysis because of incomplete data on sickness absence from work, occupation and status or mode of employment (i.e. full time or part time). However, the average age was 71 years and most participants were retired. Therefore, productivity loss is not likely to be a major consideration.
Medication use was self-reported and was recorded for baseline only (for the past 6 months).
Missing values
Across the study period (six follow-ups), some data (overall approximately 15% of data items) on health-care utilisation were missing, mainly representing the missing number and/or duration of contacts. The approach adopted to handle the missing data was imputation by the mean values of the respective variables at each time point. Owing to the large sample size and the fact that total outcomes are presented at a mean level, it was decided to use the mean instead of median imputation. In three observations where the number and/or duration of contacts were reported but the type of service was missing, the imputed value was assumed to be the most frequently used service across the study period.
Sample characteristics
Differences in sociodemographic variables between CHD patients with/without comorbid baseline depression were analysed using a two-sided t-test and chi-squared test. Depression status was measured by the HADS-D at baseline and at each follow-up point. Patients were classified into depressed and non-depressed, using a cut-off score of ≥ 8 for possible depression. 114 At baseline, 13% of patients had depression.
Sociodemographic characteristics of the sample are shown in Table 44. Approximately two-thirds were male in both groups and the average age was 66 years and 71 years for the depressed and the non-depressed group, respectively. The majority of patients were married and retired, with only a small proportion (< 5%) cohabiting. In the non-depressed group, 53% received > 11 years of education while a similar proportion in the depressed group received < 10 years of education. Approximately half of either group were ex-smokers and consumed, on average, between 0 and 10 units of alcohol per week. The mean IMD score was 20 and 23, for the non-depressed and depressed group, respectively. The vast majority of the depressed patients reported chest pain, while most of the non-depressed patients did not have chest pain. Both groups had an average number of two additional comorbidities, with the most frequent ones being hypertension and diabetes. Approximately 65% of patients had depression when assessed by the CIS-R instrument. It is noticeable that approximately 80% of the depressed patients also had anxiety when measured by the HADS-A. Most of the differences were statistically significant at a 10% level (see Table 44).
| Baseline characteristics | p-value | Non-depressed (N = 696) | Depresseda (N = 103) | ||
|---|---|---|---|---|---|
| n (%) | Mean (SD) | n (%) | Mean (SD) | ||
| Age (years) | < 0.001 | 696 (87) | 71 (11) | 103 (13) | 66 (12) |
| Sex | 0.382 | ||||
| Male | 489 (70) | 68 (66) | |||
| Female | 207 (30) | 35 (34) | |||
| Ethnicity | 0.016 | ||||
| White | 617 (89) | 80 (78) | |||
| Black | 26 (4%) | 7 (7%) | |||
| Asian | 37 (5) | 10 (10) | |||
| Other | 16 (2%) | 6 (6) | |||
| Relationship status | < 0.001 | ||||
| Married/civil partner | 426 (61) | 51 (50) | |||
| Cohabiting | 27 (4) | 3 (3) | |||
| Spouse/partner deceased | 134 (19) | 16 (16) | |||
| Separated | 9 (1) | 17 (17) | |||
| Divorced | 36 (5) | 11 (11) | |||
| Single/non-cohabiting partner | 64 (9) | 13 (13) | |||
| Years in education | |||||
| Average number | 0.666 | 693 (100) | 12 (8) | 101 (98) | 12 (9) |
| ≤ 10 | 0.504 | 320 (46) | 54 (52) | ||
| > 11 | 368 (53) | 46 (45) | |||
| Employment status | < 0.001 | ||||
| Paid employment | 134 (19) | 14 (14) | |||
| Retired/housewife/househusband | 543 (78) | 74 (72) | |||
| Unemployed/student | 16 (2) | 13 (13) | |||
| Smoking status | < 0.001 | ||||
| Never | 215 (31) | 25 (24) | |||
| Ex | 405 (58) | 52 (50) | |||
| Current | 76 (11) | 26 (25) | |||
| Alcohol consumption (average/week) | 0.001 | ||||
| Does not drink | 181 (26) | 42 (41) | |||
| 0–10 units | 343 (49) | 40 (39) | |||
| 11–20 units | 99 (14) | 6 (6) | |||
| > 20 units | 71 (10) | 15 (15) | |||
| BMI (kg/m2) | 0.098 | 680 (98) | 28 (6) | 97 (94) | 29 (7) |
| IMD score | 0.028 | 696 (100) | 20 (14) | 103 (100) | 23 (13) |
| Number of CHD factors – 0/6 | 0.004 | 696 (100) | 3 (1) | 103 (100) | 3 (1) |
| Have chest pain | < 0.001 | ||||
| No | 419 (60) | 27 (26) | |||
| Yes | 277 (40) | 76 (74) | |||
| Total number of comorbidities | 0.097 | 696 (100) | 2 (1) | 103 (100) | 2 (1) |
| Currently active comorbidities | |||||
| Diabetes | 0.045 | 166 (24) | 34 (33) | ||
| Arthritis | 0.837 | 116 (17) | 18 (17) | ||
| COPD | 0.142 | 74 (11) | 16 (16) | ||
| Cancer | 0.655 | 85 (12) | 11 (11) | ||
| Osteoporosis | 0.295 | 22 (3) | 5 (5) | ||
| Stroke | 0.247 | 10 (1) | 6 (6) | ||
| CKD | 0.668 | 134 (19) | 18 (17) | ||
| Asthma | 0.531 | 55 (8) | 10 (10) | ||
| Hypertension | 0.688 | 384 (55) | 59 (57) | ||
| Parkinson’s disease | 7 (1) | ||||
| Multiple sclerosis | 2 (0) | ||||
| Depression | 0.681 | 38 (5) | 17 (17) | ||
| Anxiety | 0.351 | 9 (1) | 4 (4) | ||
| Both depression and anxiety | 0.104 | 8 (1) | 5 (5) | ||
| Anxiety (HADS) | < 0.001 | 118 (17) | 11 (2) | 81 (79) | 13 (3) |
| Depression (PHQ-9) | < 0.001 | 62 (9) | 72 (70) | ||
| Depression (CIS-R score of > 12) | < 0.001 | 73 (10) | 66 (64) | ||
Statistical analyses
Statistical analyses were carried out using Stata version 11. Regression analysis was used to estimate mean differences in service use and costs between groups. Baseline and follow-up costs were used as the dependent variables and the group identifier as the independent variable. To estimate whether or not the difference in costs between depressed and non-depressed patients was present at each time point, the HADS-D status was also assessed at consecutive follow-up times (Figure 11).
FIGURE 11.
Mean depression score (HADS-D) across time.
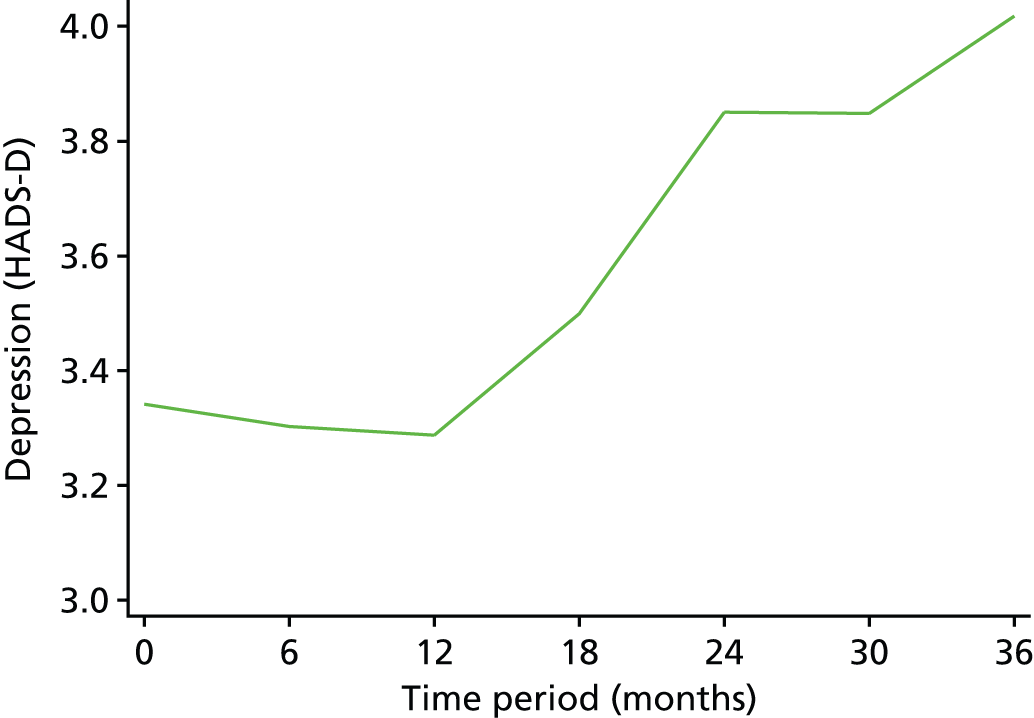
Sociodemographic and clinical baseline variables were tested for significance using simple ordinary least squares regression models. Each variable was regressed on the cumulative 3-year hospital care costs, community care costs, informal care costs, total health-care costs and total societal costs. Those variables with statistical significance at the 10% level (p < 0.10) in at least one of the above mentioned dependent variables were included in the final multivariate model to identify predictors of costs (Table 45). Certain other variables that were of interest, although not statistically significant in the first stage, were also included in the final models. Of the statistically significant variables, those with high numbers of missing values were excluded from the final models. The remaining variables were checked for correlation and those that were highly correlated (r ≥ 0.7) with each other were removed, one at a time, from the model. Two models were constructed using the cumulative health-care and societal costs as the dependent variables. The models included the following baseline covariates: sex, ethnicity, relationship status, age at referral, health literacy status, years in education, family history of depression or cardiac problems, smoking status, average units of alcohol per week, the body mass index score, number of CHD risk factors, depressive disorder assessed by the CIS-R, currently being treated for depression, anxiety status measured by the HADS, the IMD score, housing, working or financial problems, social contact and relationship problems, currently active comorbidities such as diabetes, arthritis, cancer, asthma, hypertension, COPD and chronic kidney disease. The models were also adjusted for informal care costs and total health-care costs at baseline. Approximately 100 observations had missing data on the health literacy variable. Thus, two additional models including all the above covariates except the health literacy variable were also run. To produce a more parsimonious model that aids interpretation, the backward elimination stepwise method was used. This method involved starting with all independent variables and then sequentially removing variables with the highest p-values until only those with p-values < 0.1 were included.
| Label | Missing values | Hospital costs | Community costs | Informal costs | Total health-care costs | Total societal costs | |
|---|---|---|---|---|---|---|---|
| 1 | Sex | 0 | * | * | |||
| 2 | Ethnicity | 0 | |||||
| 3 | Had chest pain | 0 | * | ||||
| 4 | Smoke | 0 | * | * | |||
| 5 | Diabetic | 4 | * | * | |||
| 6 | Family history of heart problems | 28 | |||||
| 7 | Family history of depression | 44 | |||||
| 8 | Received any treatment for depression | 602 | |||||
| 9 | Taken antidepressants | 620 | |||||
| 10 | Currently being treated for depression | 2 | |||||
| 11 | Drink alcohol | 1 | |||||
| 12 | Had a major financial crisis | 2 | |||||
| 13 | Marital status | 3 | * | * | * | ||
| 14 | Years in education | 6 | |||||
| 15 | Highest examination level achieved | 4 | |||||
| 16 | Employment status | 3 | |||||
| 17 | Retired owing to ill health | 187 | |||||
| 18 | Relationship status | 2 | |||||
| 19 | HADS-D score | 4 | * | * | * | * | |
| 20 | HADS-A score | 1 | |||||
| 21 | Depression status (PHQ-9) | 2 | * | * | * | * | * |
| 22 | EQ-5D summary score | 8 | * | * | * | * | |
| 23 | Housing problems | 0 | * | * | * | ||
| 24 | Work problems | 0 | * | * | * | ||
| 25 | Financial contacts | 0 | * | * | * | * | |
| 26 | Social contact problems | 0 | * | * | * | * | |
| 27 | Relative problems | 0 | |||||
| 28 | Relationship problems | 0 | * | * | * | * | |
| 29 | Health literacy | 116 | * | * | |||
| 30 | Age at referral (years) | 0 | * | * | |||
| 31 | Smoking status | 0 | * | ||||
| 32 | BMI | 22 | * | ||||
| 33 | IMD score | 0 | * | * | * | * | * |
| 34 | Number of CHD factors – 0/6 | 0 | * | * | * | * | |
| 35 | Number of episodes of depression | 602 | |||||
| 36 | Average units drink per week | 2 | |||||
| 37 | Currently active diabetes | 0 | * | * | * | ||
| 38 | Currently active arthritis | 0 | * | * | * | ||
| 39 | Currently active COPD | 0 | * | * | * | ||
| 40 | Currently active cancer | 0 | |||||
| 41 | Currently active osteoporosis | 771 | |||||
| 42 | Currently active stroke | 763 | * | * | * | * | |
| 43 | Currently active CKD | 0 | * | * | * | * | |
| 44 | Currently active asthma | 0 | |||||
| 45 | Currently active hypertension | 0 | |||||
| 46 | Currently active Parkinson’s disease | 796 | |||||
| 47 | Currently active MS | 801 | |||||
| 48 | Currently active depression | 678 | |||||
| 49 | Currently active anxiety with depression | 774 | |||||
| 50 | Currently active anxiety | 757 | |||||
| 51 | Total number of comorbidities | 0 | * | * | * | * | * |
| 52 | Number of comorbidities (0–1, 2–6) | 0 | * | * | * | * | * |
| 53 | Total health-care costs at baseline | 0 | * | * | * | * | * |
| 54 | Total societal costs at baseline | 0 | * | * | * | * | |
| 55 | Depression (CIS-R) at baseline | 0 | * | * | * | * |
It is commonly known that health-care data often violate the assumptions underlying ordinary least squares. Therefore, the models were run to test for the assumptions of normality and homoscedasticity of residuals. Both assumptions were violated and generalised linear models were used. The use of log transformation is common practice for dealing with skewed data, such as costs. However, log-scale results are of little interest in decision-making, while actual values (i.e. GBPs) are meaningful. Generalised linear models overcome the shortcomings of (log) ordinary least squares using the original scale of costs. 182 To use generalised linear models, the distribution of cost data and a function that specifies the relationship between the mean and the linear specification of the covariates (the so-called link function) must be specified. 182 The appropriate distribution of costs was identified by implementing the modified Park test. 183 The gamma distribution, with a log-link function, was applied to the final model. The log-link function means that the value of the exponentiated coefficients is interpreted as proportional changes of the 3-year total health-care and societal costs (Tables 46–49).
| Independent variables | Coefficient | Exponentiated coefficient | Standard error | 95% CI |
|---|---|---|---|---|
| Ethnicity (reference: white) | ||||
| Other | –0.5991 | 0.5492 | 0.1073 | 0.3745 to 0.8056 |
| Smoking status (reference: never) | ||||
| Ex-smoker | –0.2032 | 0.816 | 0.1004 | 0.641 to 1.0388 |
| Health literacy (reference: adequate) | ||||
| Inadequate | –0.4840 | 0.6163 | 0.1144 | 0.4282 to 0.8869 |
| Housing problems (SPQ) | 0.7307 | 2.0764 | 0.628 | 1.1478 to 3.7565 |
| Relationship problems (SPQ) | 0.2608 | 1.2979 | 0.1656 | 1.0106 to 1.6669 |
| Currently active comorbidities | ||||
| Currently active cancer | 0.4017 | 1.4943 | 0.2805 | 1.0343 to 2.1588 |
| Depression status (CIS-R) | 0.4856 | 1.6251 | 0.2896 | 1.1459 to 2.3046 |
| Total health-care costs (baseline) | 0.0001 | 1.0001 | 0.0000 | 1.0000 to 1.0001 |
| Independent variables | Coefficient | Exponentiated coefficient | Standard error | 95% CI |
|---|---|---|---|---|
| Ethnicity (reference: white) | ||||
| Other | –0.6530 | 0.5204 | 0.1026 | 0.3536 to 0.7661 |
| Housing problems (SPQ) | 0.6914 | 1.9965 | 0.6266 | 1.0792 to 3.6935 |
| Relationship problems (SPQ) | 0.2239 | 1.2508 | 0.1651 | 0.9656 to 1.6204 |
| Currently active comorbidities | ||||
| Currently active cancer | 0.4259 | 1.5309 | 0.2961 | 1.0477 to 2.2368 |
| Depression status (CIS-R) | 0.4135 | 1.5121 | 0.2705 | 1.0649 to 2.1472 |
| Total health-care costs (baseline) | 0.0001 | 1.0001 | 0.0000 | 1.0000 to 1.0001 |
| Independent variables | Coefficient | Exponentiated coefficient | Standard error | 95% CI |
|---|---|---|---|---|
| Ethnicity (reference: white) | ||||
| Other | –0.6391 | 0.5277 | 0.0983 | 0.3662 to 0.7604 |
| Housing problems (SPQ) | 0.6279 | 1.8736 | 0.5443 | 1.0602 to 3.311 |
| Relationship problems (SPQ) | 0.2345 | 1.2642 | 0.1604 | 0.9859 to 1.6212 |
| Had chest pain (Rose angina questionnaire) | 0.2068 | 1.2297 | 0.1500 | 0.9682 to 1.5618 |
| Currently active comorbidities | ||||
| Currently active CKD | 0.3008 | 1.3509 | 0.2083 | 0.9984 to 1.8277 |
| Currently active cancer | 0.4785 | 1.6136 | 0.2936 | 1.1296 to 2.3052 |
| Depression status (CIS-R) | 0.3959 | 1.4857 | 0.2510 | 1.0668 to 2.069 |
| Total health-care costs (baseline) | 0.0001 | 1.0001 | 0.0000 | 1.0000 to 1.0001 |
| Independent variables | Coefficient | Exponentiated coefficient | Standard error | 95% CI |
|---|---|---|---|---|
| Ethnicity (reference: white) | ||||
| Other | –0.6356 | 0.5296 | 0.0971 | 0.3696 to 0.7588 |
| Housing problems (SPQ) | 0.6051 | 1.8314 | 0.5235 | 1.0457 to 3.2073 |
| Relationship problems (SPQ) | 0.2327 | 1.2620 | 0.1578 | 0.9876 to 1.6126 |
| Had chest pain (Rose angina questionnaire) | 0.2054 | 1.2280 | 0.1475 | 0.9703 to 1.5541 |
| Currently active comorbidities | ||||
| Currently active CKD | 0.2948 | 1.3428 | 0.2040 | 0.997 to 1.8087 |
| Currently active cancer | 0.4644 | 1.5910 | 0.2850 | 1.1198 to 2.2604 |
| Depression status (CIS-R) | 0.4173 | 1.5178 | 0.2525 | 1.0955 to 2.103 |
| Total health-care costs (baseline) | 0.0001 | 1.0001 | 0.0000 | 1.0000 to 1.0001 |
Results
Health-care utilisation
Tables 50 and 51 show the cumulative resource use by depression status. Depressed patients utilised more health-care resources than non-depressed patients during the 3-year study period (87.6 vs. 59 total health-care contacts; 95% CI 5.4 to 51.8). In particular, 41% of the depressed patients used the A&E department, compared with 33% of the non-depressed patients, but the average number of attendances was 0.3 lower in the depressed group than in the non-depressed group. However, the mean number of attendances was higher for the depressed patients in all periods except the last year. The differences were not statistically significant.
| 1–3 years: service users | No depression (N = 696) | Depression (N = 103) | Difference, mean (95% CI) | Difference, mean duration (minutes) (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| n (%) | Mean (SD) | Mean duration (minutes) (SD) | n (%) | Mean (SD) | Mean duration (minutes) (SD) | |||
| A&E attendances | 228 (33) | 2.2 (3.3) | – | 42 (41) | 1.9 (1.4) | – | –0.3 (–1.3 to 0.7) | – |
| Day hospital attendances | 184 (26) | 2.8 (7.9) | – | 21 (20) | 7.4 (20.9) | – | 4.6 (0.1 to 9.1) | – |
| Inpatient stays | 255 (37) | 13.3 (20.9) | – | 46 (45) | 21.0 (40.1) | – | 7.8 (0.0 to 15.6) | – |
| Outpatient visits | 588 (84) | 12 (15.5) | – | 86 (83) | 17.6 (46.1) | – | 5.6 (0.7 to 10.6) | – |
| Informal care (hours per week) | 208 (30) | 17.7 (32.3) | – | 56 (54) | 19.6 (19.6) | – | 1.9 (–7.1 to 10.9) | – |
| GP visits | 645 (93) | 11.8 (11.3) | 44.8 (22.9) | 93 (90) | 13.2 (10.5) | 46.2 (29.7) | 1.4 (–1.1 to 3.8) | 1.4 (–3.8 to 6.6) |
| PN visits | 581 (83) | 7.7 (16.1) | 30.1 (21.4) | 73 (71) | 7.7 (10.7) | 31.4 (19.3) | 0.0 (–3.8 to 3.8) | 1.4 (–3.8 to 6.5) |
| District nurse visits | 76 (11) | 20.8 (39.9) | 25.4 (17.6) | 12 (12) | 22.8 (38.3) | 30.0 (26.8) | 2.0 (–22.5 to 26.5) | 4.6 (–7.2 to 16.3) |
| Other medical professional | 238 (34) | 7.9 (10.3) | 59.8 (60.0) | 37 (36) | 15.7 (24.5) | 95.5 (105.8) | 7.9 (3.3 to 12.4) | 79.8 (12.2 to 59.4) |
| Other care professional | 175 (25) | 67.7 (158.2) | 165.3 (194.1) | 32 (3) | 89.8 (149.0) | 200.7 (219.2) | 22.1 (–37.4 to 81.5) | 35.4 (–39.7 to 110.5) |
| 1–3 years: service users | No depression (N = 696) | Depression (N = 103) | Difference, mean (95% CI) | ||
|---|---|---|---|---|---|
| n (%) | Mean (SD) | n (%) | Mean (SD) | ||
| Hospital care contacts | 162 (23) | 7.5 (14.1) | 30 (29) | 10.0 (21.0) | 2.5 (–3.5 to 8.5) |
| Community care contacts | 210 (30) | 15.6 (52.3) | 32 (31) | 44.1 (138.2) | 28.5 (2.5 to 54.6) |
| Primary care contacts (includes contacts to GPs, practice and district nurses) | 655 (94) | 20.9 (26.3) | 93 (90) | 22.2 (21.3) | 1.3 (–4.3 to 6.9) |
| Secondary care contacts (includes hospital care and contacts to other medical or care professionals/specialists). | 630 (91) | 40.0 (95.7) | 91 (88) | 67.9 (128.5) | 27.9 (5.8 to 50.0) |
| Total health-care contacts | 658 (95) | 59.0 (102.7) | 94 (91) | 87.6 (134.5) | 28.6 (5.4 to 51.8) |
Patients with depression used inpatient care more and stayed in hospital significantly longer (7.8 days, 95% CI 0 to 15.6 days) than patients without depression. Approximately 5% of the patients were hospitalised for CHD reasons (1% of those had depressive symptoms). Nearly 80% of patients in both groups used outpatient care, with the depressed group using it more intensively (17.6 vs. 12 visits, 95% 0.7 to 10.9 visits). Patients in both groups made similar use of primary care, which included contacts with GPs, practice and district nurses, with the depressed group having a slightly higher frequency of contact. The mean duration of contacts with GPs and PNs was slightly, but not significantly, higher (1.4 minutes per patient) for the depressed patients than for the non-depressed patients. Patients with depression tended to spend approximately 5 minutes more with their GPs than non-depressed patients. Overall, secondary care, which included hospital care and care provided by other medical and care professionals (e.g. psychiatrists, psychologists, other community-based doctors, mental health nurses, social workers, housing workers and care attendants), was used more by the depressed group, with a significant difference of 27.9 contacts (95% CI 5.8 to 50 contracts) compared with the non-depressed group.
Resource use (by time point)
It was found that informal care was utilised more (54% vs. 30%) and more intensively (19.6 vs. 17.7 average hours per week per patient) by the depressed patients than by the non-depressed ones. Information on resource use by type of service and by group at each time point, separately, is given in Tables 52–58.
| Baseline: service users | No depression (N = 696) | Depression (N = 103) | Difference, mean (95% CI) | Difference, mean duration (minutes) (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| n (%) | Mean (SD) | Mean duration (minutes) (SD) | n (%) | Mean (SD) | Mean duration (minutes) (SD) | |||
| A&E attendances | 58 (8) | 2.2 (2.6) | – | 15 (15) | 3.9 (6.9) | – | 1.7 (–0.5 to 4.0) | – |
| Day hospital attendances | 21 (3) | 2.4 (2.4) | – | 3 (3) | 1.3 (0.6) | – | –1.1 (–4.0 to 1.8) | – |
| Inpatient stays | 73 (10) | 6.7 (8.8) | – | 15 (15) | 2.9 (2.8) | – | –3.9 (–8.4 to 0.7) | – |
| Outpatient visits | 452 (65) | 3.5 (6.1) | – | 70 (68) | 5.3 (9.1) | – | 1.8 (0.1 to 3.4) | – |
| Informal care (hours per week) | 83 (12) | 8.0 (13.7) | – | 19 (18) | 8.3 (15.8) | – | 0.3 (–6.9 to 7.4) | – |
| GP visits | 554 (80) | 2.7 (2.4) | 11.6 (6.6) | 81 (79) | 3.4 (2.4) | 11 (4.6) | 0.7 (0.2 to 1.3) | –0.6 (–2.1 to 0.9) |
| PN visits | 334 (48) | 2.1 (2.7) | 11.0 (6.7) | 52 (50) | 2.5 (2.6) | 12.5 (7.2) | 0.4 (–0.4 to 1.2) | 1.5 (–0.5 to 3.4) |
| District nurse visits | 21 (3) | 4.9 (10.1) | 19.8 (18.5) | 6 (6) | 5 (9.3) | 23.3 (19.4) | 0.1 (–9.4 to 9.6) | 3.6 (–14.3 to 21.4) |
| Other medical professional | 81 (12) | 6.2 (6.8) | 47.9 (33) | 18 (17) | 7.6 (14.1) | 45.6 (33.3) | 1.4 (–3.0 to 5.8) | –2.4 (–19.5 to 14.8) |
| Other care professional | 77 (11) | 23.0 (40.2) | 72.3 (59.9) | 13 (13) | 33.5 (53.3) | 75.1 (81.2) | 10.5 (–14.7 to 35.6) | 2.8 (–35.1 to 40.7) |
| 6 months: service users | No depression (N = 638) | Depression (N = 93) | Difference, mean (95% CI) | Difference, mean duration (minutes) (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| n (%) | Mean (SD) | Mean duration (minutes) (SD) | n (%) | Mean (SD) | Mean duration (minutes) (SD) | |||
| A&E attendances | 54 (8) | 2.0 (3.8) | – | 15 (16) | 2.6 (3.5) | – | 0.6 (–1.6 to 2.8) | – |
| Day hospital attendances | 29 (5) | 1.2 (0.5) | – | 4 (4) | 18.8 (35.5) | – | 17.6 (5.6 to 29.6) | – |
| Inpatient stays | 52 (8) | 5.3 (5.4) | – | 16 (17) | 12.5 (16.1) | – | 7.2 (2.1 to 12.4) | – |
| Outpatient visits | 350 (55) | 3.2 (3.4) | – | 61 (66) | 3.5 (4.3) | – | 0.3 (–0.7 to 1.3) | – |
| Informal care (hours per week) | 61 (10) | 8.7 (14.5) | – | 27 (29) | 8 (10.1) | – | –0.6 (–6.7 to 5.5) | – |
| GP visits | 506 (79) | 2.6 (2.1) | 11.2 (5.1) | 76 (82) | 4.0 (3.5) | 12.1 (6.8) | 1.4 (0.9 to 2.0) | 0.9 (–0.3 to 2.2) |
| PN visits | 326 (51) | 2.2 (3.4) | 9.5 (6.6) | 46 (49) | 3.2 (4.3) | 10.7 (7.1) | 1 (–0.1 to 2.1) | 1.2 (–0.9 to 3.3) |
| District nurse visits | 10 (2) | 15.3 (25.4) | 20.5 (6.4) | 5 (5) | 13.2 (8.7) | 16 (9.6) | –2.1 (–27.7 to 23.5) | –4.5 (–13.4 to 4.4) |
| Other medical professional | 57 (9) | 5.1 (6.1) | 45.3 (63.4) | 16 (17) | 10.1 (8) | 44.4 (26) | 5 (1.3 to 8.6) | –0.9 (–33.4 to 31.5) |
| Other care professional | 71 (11) | 17.6 (25.3) | 79.0 (67.2) | 16 (17) | 35.2 (46.2) | 108.8 (94.2) | 17.6 (1.0 to 34.1) | 29.8 (–10.2 to 69.8) |
| 12 months: service users | No depression (N = 622) | Depression (N = 91) | Difference, mean (95% CI) | Difference, mean duration (minutes) (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| n (%) | Mean (SD) | Mean duration (SD) | n (%) | Mean (SD) | Mean duration (minutes) (SD) | |||
| A&E attendances | 51 (8) | 1.7 (2.4) | – | 12 (13) | 2.3 (2.9) | – | 0.6 (–1.0 to 2.2) | – |
| Day hospital attendances | 34 (5) | 1.3 (0.7) | – | 0 (0) | – | – | – | – |
| Inpatient stays | 64 (10) | 10.2 (15.9) | – | 13 (14) | 17.0 (41.6) | – | 6.8 (–6.6 to 20.2) | – |
| Outpatient visits | 377 (61) | 3.4 (4.5) | – | 57 (63) | 6.3 (14.4) | – | 2.9 (1.0 to 4.8) | – |
| Informal care (hours per week) | 73 (12) | 9.4 (15.6) | – | 19 (21) | 9.6 (10.9) | – | 0.2 (–1.3 to 7.8) | – |
| GP visits | 470 (76) | 2.9 (2.6) | 11.2 (5.1) | 71 (78) | 4.1 (3.7) | 11.9 (6.2) | 1.2 (0.5 to 1.9) | 0.6 (–0.7 to 1.9) |
| PN visits | 283 (45) | 2.7 (5.5) | 11.2 (7.4) | 37 (41) | 1.9 (1.5) | 13.6 (12.5) | –0.8 (–2.5 to 1.0) | 2.4 (–0.4 to 5.2) |
| District nurse visits | 18 (3) | 12.7 (16) | 15.3 (8.1) | 5 (5) | 15.0 (10.7) | 13 (2.7) | 2.3 (–13.6 to 18.3) | –2.3 (–10.1 to 5.5) |
| Other medical professional | 65 (10) | 5.8 (8.8) | 39.2 (27.5) | 18 (20) | 7.9 (9.2) | 34.7 (22.5) | 2.1 (–2.6 to 6.8) | –4.4 (–18.5 to 9.6) |
| Other care professional | 68 (11) | 38.8 (67.5) | 102.2 (100.8) | 10 (11) | 31.0 (25.2) | 121.1 (118.8) | –7.8 (–51.0 to 35.3) | 18.9 (–50.6 to 88.5) |
| 18 months: service users | No depression (N = 574) | Depression (N = 95) | Difference, mean (95% CI) | Difference, mean duration (minutes) (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| n (%) | Mean (SD) | Mean duration (minutes) (SD) | n (%) | Mean (SD) | Mean duration (minutes) (SD) | |||
| A&E attendances | 41 (7) | 1.3 (1) | – | 13 (14) | 1.7 (1.4) | – | 0.4 (–0.4 to 1.1) | – |
| Day hospital attendances | 53 (9) | 2.3 (2.4) | – | 12 (13) | 3.8 (7.1) | – | 1.5 (–0.9 to 3.8) | – |
| Inpatient stays | 71 (12) | 9.7 (10.9) | – | 18 (19) | 16.1 (26.1) | – | 6.4 (–1.5 to 14.3) | – |
| Outpatient visits | 316 (55) | 3.8 (6.4) | – | 51 (54) | 4.0 (4.1) | – | 0.2 (–1.6 to 2) | – |
| Informal care (hours per week) | 76 (13) | 10.3 (20.8) | – | 27 (28) | 9.0 (9.2) | – | –1.3 (–9.5 to 7) | – |
| GP visits | 428 (75) | 2.9 (3.1) | 11.3 (6.9) | 71 (75) | 4.8 (5.3) | 13.6 (6.8) | 1.9 (1.0 to 2.7) | 2.3 (0.6 to 4.0) |
| PN visits | 272 (47) | 3.1 (7.3) | 10.5 (6.4) | 40 (42) | 2.7 (3.8) | 10.8 (6.4) | –0.4 (–2.8 to 1.9) | 0.3 (–1.8 to 2.5) |
| District nurse visits | 16 (3) | 10.0 (14.6) | 24.8 (17.6) | 8 (8) | 10.4 (10.8) | 15.6 (6.8) | 0.4 (–11.8 to 12.5) | –9.1 (–22.7 to 4.4) |
| Other medical professional | 60 (10) | 5.7 (5.7) | 35.2 (19.3) | 16 (17) | 2.9 (2.4) | 58.1 (48.5) | –2.8 (–5.7 to 0.1) | 23 (7.4 to 38.5) |
| Other care professional | 46 (8) | 55.8 (104.7) | 67.8 (44.2) | 11 (12) | 55.7 (67.1) | 88.2 (65.4) | 0.0 (–66.6 to 66.5) | 20.3 (–12.5 to 53.1) |
| 24 months: service users | No depression (N = 538) | Depression (N = 95) | Difference, mean (95% CI) | Difference, mean duration (minutes) (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| n (%) | Mean (SD) | Mean duration (minutes) (SD) | n (%) | Mean (SD) | Mean duration (minutes) (SD) | |||
| A&E attendances | 49 (9) | 1.2 (0.5) | – | 16 (17) | 1.8 (1.6) | – | 0.6 (0.1 to 1.2) | – |
| Day hospital attendances | 38 (7) | 1.7 (3.1) | – | 3 (3) | 1.3 (0.6) | – | –0.4 (–4.0 to 3.3) | – |
| Inpatient stays | 78 (14) | 5.9 (6.3) | – | 17 (18) | 14.1 (18.6) | – | 8.2 (3.1 to 13.3) | – |
| Outpatient visits | 307 (57) | 3.4 (4.1) | – | 58 (61) | 6.2 (13.2) | – | 2.8 (0.9 to 4.6) | – |
| Informal care (hours per week) | 71 (13) | 6.3 (7.2) | – | 27 (28) | 9.9 (10.5) | – | 3.6 (–0.1 to 7.3) | – |
| GP visits | 392 (73) | 3.0 (4.4) | 11.0 (4.5) | 72 (76) | 5.0 (4.8) | 13.5 (6.3) | 2.0 (0.8 to 3.1) | 2.5 (1.3 to 3.7) |
| PN visits | 243 (45) | 3.0 (7.7) | 10.9 (5.4) | 51 (54) | 3.5 (4.5) | 11.1 (5.0) | 0.5 (–1.7 to 2.6) | 0.2 (–1.4 to 1.8) |
| District nurse visits | 16 (3) | 18.8 (28.1) | 16.8 (8.9) | 4 (4) | 10.0 (10.5) | 23.8 (24.3) | –8.8 (–39.3 to 21.8) | 7.0 (–8.1 to 22) |
| Other medical professional | 63 (12) | 5.0 (7.9) | 37.7 (29.4) | 11 (12) | 6.5 (4.8) | 53.2 (30.5) | 1.6 (–3.3 to 6.5) | 15.5 (–3.7 to 34.8) |
| Other care professional | 34 (6) | 39.5 (89.2) | 96 (84.1) | 14 (15) | 63.9 (97) | 109.2 (107) | 24.3 (–34.2 to 82.8) | 13.2 (–45 to 71.5) |
| 30 months: service users | No depression (N = 498) | Depression (N = 100) | Difference, mean (95% CI) | Difference, mean duration (minutes) (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| n (%) | Mean (SD) | Mean duration (minutes) (SD) | n (%) | Mean (SD) | Mean duration (minutes) (SD) | |||
| A&E attendances | 48 (10) | 1.3 (1.1) | – | 15 (15) | 1.1 (0.4) | – | –0.2 (–0.7 to 0.4) | – |
| Day hospital attendances | 42 (8) | 4 (15.2) | – | 11 (11) | 3.2 (2.9) | – | –0.9 (–10.2 to 8.5) | – |
| Inpatient stays | 58 (12) | 8.4 (12.3) | – | 18 (18) | 15.1 (24.9) | – | 6.7 (–2 to 15.3) | – |
| Outpatient visits | 285 (57) | 3.4 (4.8) | – | 49 (49) | 6.4 (13.4) | – | 3 (1 to 5.1) | – |
| Informal care (hours per week) | 66 (13) | 6.9 (13.5) | – | 29 (29) | 9.3 (10.2) | – | 2.4 (–3.2 to 7.9) | – |
| GP visits | 348 (70) | 2.8 (3.3) | 11 (12.9) | 70 (70) | 3.4 (3.8) | 12.9 (7) | 0.6 (0 to 1.8) | 2 (0.7 to 3.2) |
| PN visits | 246 (49) | 2.3 (4.1) | 10.3 (5.3) | 46 (46) | 2.2 (2) | 11.5 (4.1) | –0.1 (–1.3 to 1.1) | 1.2 (–0.4 to 2.8) |
| District nurse visits | 15 (3) | 17.4 (20.1) | 15.3 (8.1) | 8 (8) | 5.5 (7.9) | 18.1 (8.4) | –11.9 (–27.4 to 3.6) | 2.8 (–4.7 to 10.3) |
| Other medical professional | 65 (13) | 5.5 (10.4) | 32.6 (29.2) | 12 (12) | 7.3 (6.6) | 45.4 (22.5) | 1.8 (–4.5 to 8) | 12.9 (–4.9 to 30.6) |
| Other care professional | 34 (7) | 43.3 (49.4) | 98.6 (104.9) | 17 (17) | 69.6 (95) | 107.1 (126) | 26.4 (–14.1 to 66.8) | 8.4 (–58.5 to 75.4) |
| 36 months: service users | No depression (N = 470) | Depression (N = 103) | Difference, mean (95% CI) | Difference, mean duration (minutes) (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| n (%) | Mean (SD) | Mean duration (minutes) (SD) | n (%) | Mean (SD) | Mean duration (minutes) (SD) | |||
| A&E attendances | 57 (12) | 1.5 (2.6) | – | 15 (15) | 1.2 (0.6) | – | –0.3 (–1.6 to 1.1) | – |
| Day hospital attendances | 32 (7) | 1.6 (1.2) | – | 6 (6) | 3.2 (4.4) | – | 1.5 (–0.2 to 3.3) | – |
| Inpatient stays | 54 (11) | 6.9 (8.8) | – | 17 (17) | 10.2 (22.2) | – | 3.4 (–3.9 to 10.7) | – |
| Outpatient visits | 280 (60) | 3.5 (5.6) | – | 61 (59) | 8.8 (22.1) | – | 5.3 (2.4 to 8.3) | – |
| Informal care (hours per week) | 52 (11) | 7.9 (11.6) | – | 26 (25) | 9.7 (14.5) | – | 1.9 (–4.1 to 7.9) | – |
| GP visits | 339 (72) | 2.6 (2.1) | 11.1 (6.2) | 73 (71) | 4.4 (11.8) | 13.9 (16.8) | 1.8 (0.5 to 3.2) | 2.8 (0.5 to 5.1) |
| PN visits | 232 (49) | 2.9 (7.1) | 10.9 (6.5) | 42 (41) | 3.2 (4.6) | 11 (5.3) | 0.3 (–2.0 to 2.5) | 0.1 (–2.0 to 2.2) |
| District nurse visits | 14 (3) | 7.9 (9.1) | 20.4 (14.5) | 5 (5) | 30.6 (31.9) | 19 (7.4) | 22.7 (3.5 to 41.8) | –1.4 (–15.8 to 13.1) |
| Other medical professional | 52 (11) | 4.3 (4.9) | 38.3 (40.1) | 14 (14) | 3.9 (5.8) | 49.6 (41.5) | –0.4 (–3.4 to 2.7) | 11.3 (–13 to 35.6) |
| Other care professional | 26 (6) | 38.7 (42) | 116.5 (99.2) | 15 (15) | 56.9 (68.7) | 165.1 (181.8) | 18.2 (–16.7 to 53.1) | 48.6 (–39.8 to 137.1) |
Costs
The cumulative costs for the 3-year study period are shown in Tables 59 and 60. Costs by type of service and by patient group at each time point for service users only (i.e. excluding those with zero costs) and for the whole sample are given in Tables 61–67 and Tables 68–74, respectively. In almost all types of service, patients with depression incurred higher costs than patients without depression. The difference was statistically significant for costs of outpatient services and contacts with other medical professionals as well as for total costs. The aggregated 3-year costs for service users were significantly higher for the depressed group than for the non-depressed group, by approximately £4000 (see Table 59). The highest costs were for inpatient care across the study period (Figure 12). At baseline the non-depressed group incurred higher inpatient care costs per patient than the depressed group (–£1739, 95% CI –£3790 to £312). However, baseline costs were not included in the calculation of the aggregate costs because the estimates referred to the 6-month period prior to the study initiation. In all other follow-ups, there was a positive difference of approximately £3000 in inpatient care costs between patients with and without depression, except in the 36-month follow-up, in which case the difference remained positive but at a lower level (£1534, 95% CI –£1772 to £4840) (see Tables 61–67).
| 1–3 years: service users | No depression (N = 696) | Depression (N = 103) | Difference, mean, £ (95% CI) | ||
|---|---|---|---|---|---|
| n (%) | Mean, £ (SD) | n (%) | Mean, £ (SD) | ||
| A&E attendances | 242 (35) | 183 (267) | 43 (42) | 159 (120) | –24 (–105 to 58) |
| Day hospital attendances | 182 (26) | 575 (1202) | 20 (19) | 1312 (3328) | 737 (23 to 1450) |
| Inpatient stays | 255 (37) | 6072 (9439) | 46 (45) | 9584 (18,167) | 3511 (–18 to 7041) |
| Outpatient visits | 588 (84) | 1669 (2161) | 86 (83) | 2451 (6411) | 782 (91 to 1472) |
| GP visits | 645 (93) | 484 (654) | 93 (90) | 560 (514) | 76 (–63 to 215) |
| PN visits | 580 (83) | 80 (264) | 73 (71) | 75 (96) | –5 (–66 to 56) |
| District nurse visits | 76 (11) | 443 (852) | 12 (12) | 411 (733) | –33 (–550 to 485) |
| Other medical professional | 225 (32) | 224 (390) | 36 (35) | 721 (1298) | 497 (285 to 709) |
| Other care professional | 164 (24) | 2178 (5111) | 31 (30) | 2978 (4592) | 800 (–1144 to 2745) |
| Total health-care costs | 658 (95) | 5282 (8730) | 94 (91) | 9204 (16,109) | 3923 (1770 to 6076) |
| Informal care (hours per week) | 208 (30) | 406 (742) | 56 (54) | 450 (509) | 43 (–164 to 251) |
| Total societal costs | 659 (95) | 5402 (8804) | 94 (91) | 9472 (16,219) | 4070 (1901 to 6240) |
| 1–3 years: service users | No depression (N = 696) | Depression (N = 103) | Difference, mean, £ (95% CI) | ||
|---|---|---|---|---|---|
| n (%) | Mean, £ (SD) | n (%) | Mean, £ (SD) | ||
| Hospital care costs | 439 (63) | 2037 (5202) | 49 (48) | 2990 (5217) | 953 (–587 to 2493) |
| Community care costs | 496 (71) | 457 (1423) | 60 (58) | 931 (2176) | 475 (66 to 883) |
| Primary care costs | 493 (71) | 239 (654) | 60 (58) | 257 (315) | 18 (–150 to 187) |
| Secondary care costs | 451 (65) | 2224 (5456) | 53 (51) | 3528 (5388) | 1303 (–251 to 2858) |
| Baseline: service users | No depression (N = 696) | Depression (N = 103) | Difference, mean, £ (95% CI) | ||
|---|---|---|---|---|---|
| n (%) | Mean, £ (SD) | n (%) | Mean, £ (SD) | ||
| A&E attendances | 58 (8) | 182 (216) | 15 (15) | 324 (571) | 142 (–42 to 326) |
| Day hospital attendances | 20 (3) | 404 (349) | 3 (3) | 610 (767) | 206 (–319 to 731) |
| Inpatient stays | 73 (10) | 3108 (3940) | 15 (15) | 1369 (1239) | –1739 (–3790 to 312) |
| Outpatient visits | 452 (65) | 493 (860) | 70 (68) | 737 (1271) | 244 (14 to 475) |
| GP visits | 553 (79) | 116 (186) | 80 (78) | 128 (103) | 12 (–301224 to 53) |
| PN visits | 332 (48) | 21 (47) | 52 (50) | 29 (37) | 8 (–5 to 21) |
| District nurse visits | 21 (3) | 119 (245) | 6 (6) | 98 (160) | –21 (–241 to 198) |
| Other medical professional | 72 (10) | 242 (329) | 18 (17) | 613 (1469) | 371 (–1 to 743) |
| Other care professional | 74 (11) | 1059 (4711) | 13 (13) | 833 (1159) | –226 (–2850 to 2397) |
| Total health-care costs | 649 (93) | 922 (1909) | 93 (90) | 1215 (1728) | 293 (–118 to 704) |
| Informal care (hours per week) | 83 (12) | 184 (316) | 19 (18) | 190 (364) | 6 (–158 to 170) |
| Total societal costs | 650 (93) | 944 (1933) | 94 (91) | 1240 (1726) | 296 (–117 to 710) |
| 6 months: service users | No depression (N = 638) | Depression (N = 93) | Difference, mean, £ (95% CI) | ||
|---|---|---|---|---|---|
| n (%) | Mean, £ (SD) | n (%) | Mean, £ (SD) | ||
| A&E attendances | 54 (8) | 163 (313) | 15 (16) | 214 (290) | 51 (–129 to 231) |
| Day hospital attendances | 28 (4) | 361 (266) | 4 (4) | 3069 (5682) | 2709 (728 to 4689) |
| Inpatient stays | 52 (8) | 2430 (2401) | 16 (17) | 5686 (7262) | 3257 (942 to 5571) |
| Outpatient visits | 348 (55) | 446 (469) | 60 (65) | 487 (596) | 41 (–93 to 174) |
| GP visits | 505 (79) | 103 (119) | 76 (82) | 172 (204) | 68 (36 to 101) |
| PN visits | 326 (51) | 19 (29) | 46 (49) | 31 (46) | 12 (2 to 21) |
| District nurse visits | 10 (2) | 327 (561) | 5 (5) | 236 (198) | –91 (–658 to 477) |
| Other medical professional | 54 (8) | 163 (363) | 16 (17) | 502 (656) | 339 (87 to 592) |
| Other care professional | 68 (11) | 551 (678) | 16 (17) | 955 (1583) | 404 (–101 to 909) |
| Total health-care costs | 583 (91) | 702 (1206) | 88 (95) | 1990 (4168) | 1288 (867 to 1710) |
| Informal care (hours per week) | 61 (10) | 200 (333) | 27 (29) | 185 (232) | –15 (–155 to 126) |
| Total societal costs | 587 (92) | 718 (1222) | 88 (95) | 2047 (4201) | 1329 (904 to 1754) |
| 12 months: service users | No depression (N = 622) | Depression (N = 91) | Difference, mean, £ (95% CI) | ||
|---|---|---|---|---|---|
| n (%) | Mean, £ (SD) | n (%) | Mean, £ (SD) | ||
| A&E attendances | 51 (8) | 141 (198) | 12 (13) | 192 (236) | 51 (–81 to 182) |
| Day hospital attendances | 34 (5) | 383 (582) | 0 (0) | 0 (0) | – |
| Inpatient stays | 64 (10) | 4644 (7175) | 13 (14) | 7720 (18,844) | 3076 (–2986 to 9138) |
| Outpatient visits | 377 (61) | 472 (629) | 57 (63) | 873 (2006) | 401 (141 to 661) |
| GP visits | 468 (75) | 115 (134) | 71 (78) | 189 (307) | 75 (33 to 117) |
| PN visits | 282 (45) | 26 (52) | 37 (41) | 21 (23) | –5 (–22 to 13) |
| District nurse visits | 18 (3) | 280 (420) | 5 (5) | 233 (171) | –48 (–474 to 358) |
| Other medical professional | 62 (10) | 194 (337) | 15 (16) | 416 (709) | 222 (–25 to 470) |
| Other care professional | 66 (11) | 1109 (2158) | 9 (10) | 866 (1152) | –243 (–1710 to 1224) |
| Total health-care costs | 587 (94) | 1102 (3161) | 82 (90) | 2218 (8332) | 1116 (156 to 2076) |
| Informal care (hours per week) | 73 (12) | 216 (359) | 19 (21) | 222 (252) | 5 (–169 to 180) |
| Total societal costs | 589 (95) | 1125 (3182) | 83 (91) | 2242 (8301) | 1117 (159 to 2075) |
| 18 months: service users | No depression (N = 574) | Depression (N = 95) | Difference, mean, £ (95% CI) | ||
|---|---|---|---|---|---|
| n (%) | Mean, £ (SD) | n (%) | Mean, £ (SD) | ||
| A&E attendances | 41 (7) | 110 (84) | 13 (14%) | 139 (118) | 29 (–30 to 88) |
| Day hospital attendances | 52 (9) | 343 (375) | 12 (13%) | 628 (1047) | 285 (–72 to 642) |
| Inpatient stays | 71 (12) | 4427 (4906) | 18 (19%) | 7319 (11,793) | 2892 (–686 to 6471) |
| Outpatient visits | 316 (55) | 529 (885) | 51 (54%) | 554 (576) | 25 (–227 to 277) |
| GP visits | 427 (74) | 116 (152) | 71 (75) | 222 (261) | 107 (64 to 150) |
| PN visits | 271 (47) | 34 (123) | 40 (42) | 27 (50) | –7 (–45 to 32) |
| District nurse visits | 16 (3) | 293 (552) | 8 (8) | 178 (198) | –115 (–536 to 307) |
| Other medical professional | 52 (9) | 159 (210) | 12 (13) | 168 (184) | 9 (–123 to 141) |
| Other care professional | 42 (7) | 2474 (5997) | 11 (12) | 1981 (1982) | –493 (–4198 to 3211) |
| Total health-care costs | 534 (93) | 1272 (3175) | 85 (89) | 2488 (6653) | 1216 (336 to 2096) |
| Informal care (hours per week) | 76 (13) | 236 (480) | 27 (28) | 207 (212) | –29 (–219 to 160) |
| Total societal costs | 537 (94) | 1298 (3178) | 85 (89) | 2554 (6664) | 1255 (375 to 2136) |
| 24 months: service users | No depression (N = 538) | Depression (N = 95) | Difference, mean, £ (95% CI) | ||
|---|---|---|---|---|---|
| n (%) | Mean, £ (SD) | n (%) | Mean, £ (SD) | ||
| A&E attendances | 49 (9) | 96 (42) | 16 (17) | 149 (135) | 53 (10 to 97) |
| Day hospital attendances | 38 (7) | 441 (435) | 3 (3) | 388 (319) | –53 (–575 to 469) |
| Inpatient stays | 78 (14) | 2731 (2831) | 17 (18) | 6418 (8484) | 3686 (1368 to 6004) |
| Outpatient visits | 307 (57) | 471 (570) | 58 (61) | 856 (1840) | 385 (132 to 637) |
| GP visits | 392 (73) | 116 (175) | 72 (76) | 225 (217) | 109 (63 to 154) |
| PN visits | 243 (45) | 33 (114) | 51 (54) | 34 (44) | 0 (–31 to 32) |
| District nurse visits | 16 (3) | 352 (536) | 4 (4) | 181 (169) | –170 (–750 to 410) |
| Other medical professional | 54 (10) | 111 (194) | 11 (12) | 386 (396) | 274 (117 to 431) |
| Other care professional | 26 (5) | 1578 (2883) | 13 (14) | 1523 (1658) | –55 (–1811 to 1700) |
| Total health-care costs | 490 (91) | 990 (1908) | 88 (93) | 2329 (4705) | 1338 (762 to 1916) |
| Informal care (hours per week) | 71 (13) | 145 (166) | 27 (28) | 227 (242) | 82 (–3 to 167) |
| Total societal costs | 494 (92) | 1003 (1918) | 89 (94) | 2371 (4704) | 1368 (793 to 1944) |
| 30 months: service users | No depression (N = 498) | Depression (N = 100) | Difference, mean, £ (95% CI) | ||
|---|---|---|---|---|---|
| n (%) | Mean, £ (SD) | n (%) | Mean, £ (SD) | ||
| A&E attendances | 48 (10) | 106 (90) | 15 (15) | 93 (29) | –13 (–60 to 34) |
| Day hospital attendances | 42 (8) | 672 (2116) | 11 (11) | 415 (296) | –256 (–1549 to 1037) |
| Inpatient stays | 58 (12) | 3854 (5537) | 18 (18) | 6874 (11,270) | 3020 (–887 to 6926) |
| Outpatient visits | 285 (57) | 468 (665) | 49 (49) | 886 (1867) | 418 (133 to 704) |
| GP visits | 348 (70) | 103 (127) | 70 (70) | 167 (198) | 64 (27 to 100) |
| PN visits | 245 (49) | 24 (90) | 46 (46) | 22 (19) | –2 (–28 to 24) |
| District nurse visits | 15 (3) | 395 (705) | 8 (8) | 104 (139) | –291 (–820 to 239) |
| Other medical professional | 50 (10) | 172 (337) | 11 (11) | 264 (298) | 92 (–128 to 313) |
| Other care professional | 29 (6) | 1007 (1804) | 16 (16) | 2207 (2831) | 1200 (–192 to 2593) |
| Total health-care costs | 461 (93) | 1032 (2656) | 87 (87) | 2585 (6036) | 1553 (768 to 2338) |
| Informal care (hours per week) | 66 (13) | 159 (310) | 29 (29) | 214 (234) | 55 (–73 to 183) |
| Total societal costs | 469 (94) | 1037 (2645) | 89 (89) | 2596 (6006) | 1560 (786 to 2333) |
| 36 months: service users | No depression (N = 470) | Depression (N = 103) | Difference, mean, £ (95% CI) | ||
|---|---|---|---|---|---|
| n (%) | Mean, £ (SD) | n (%) | Mean, £ (SD) | ||
| A&E attendances | 57 (12) | 120 (210) | 15 (15) | 99 (46) | –21 (–130 to 88) |
| Day hospital attendances | 31 (7) | 429 (335) | 6 (6) | 692 (555) | 263 (–76 to 601) |
| Inpatient stays | 54 (11) | 3140 (3965) | 17 (17) | 4675 (10,055) | 1534 (–1772 to 4840) |
| Outpatient visits | 280 (60) | 480 (781) | 61 (59) | 1217 (3075) | 737 (327 to 1147) |
| GP visits | 339 (72) | 107 (187) | 73 (71) | 291 (1202) | 184 (49 to 319) |
| PN visits | 232 (49) | 33 (142) | 42 (41) | 34 (63) | 1 (–43 to 45) |
| District nurse visits | 14 (3) | 185 (246) | 5 (5) | 599 (734) | 414 (–43 to 871) |
| Other medical professional | 51 (11) | 115 (170) | 13 (13) | 276 (584) | 161 (–25 to 346) |
| Other care professional | 25 (5) | 780 (849) | 15 (15) | 2950 (4433) | 2170 (336 to 4004) |
| Total health-care costs | 442 (94) | 896 (1931) | 91 (88) | 2559 (6324) | 1663 (952 to 2374) |
| Informal care (hours per week) | 52 (11) | 181 (267) | 26 (25) | 224 (333) | 44 (–95 to 183) |
| Total societal costs | 442 (94) | 917 (1941) | 94 (91) | 2539 (6253) | 1622 (919 to 2325) |
| Baseline: everyone | No depression (n = 696) | Depression (n = 103) | Difference, mean, £ (95% CI) |
|---|---|---|---|
| Mean, £ (SD) | Mean, £ (SD) | ||
| A&E attendances | 15 (80) | 47 (241) | 32 (8 to 56) |
| Day hospital attendances | 12 (89) | 18 (149) | 6 (–14 to 27) |
| Inpatient stays | 326 (1586) | 199 (668) | –127 (–438 to 184) |
| Outpatient visits | 320 (721) | 501 (1101) | 181 (19 to 342) |
| GP visits | 92 (172) | 99 (105) | 7 (–27 to 41) |
| PN visits | 10 (34) | 14 (30) | 5 (–2 to 12) |
| District nurse visits | 4 (46) | 6 (42) | 2 (–7 to 12) |
| Other medical professional | 25 (128) | 107 (644) | 82 (28 to 136) |
| Other care professional | 56 (301) | 105 (485) | 49 (–19 to 118) |
| Total health-care costs | 859 (1857) | 1097 (1681) | 237 (–143 to 618) |
| Informal care (hours per week) | 22 (124) | 35 (170) | 13 (–14 to 40) |
| Total societal costs | 881 (1883) | 1132 (1685) | 250 (–135 to 636) |
| 6 months: everyone | No depression (n = 638), mean, £ (SD) | Depression (n = 93), mean, £ (SD) | Difference, mean, £ (95% CI) |
|---|---|---|---|
| A&E attendances | 14 (101) | 35 (138) | 21 (–2 to 44) |
| Day hospital attendances | 16 (92) | 132 (1202) | 116 (21 to 211) |
| Inpatient stays | 198 (951) | 978 (3641) | 780 (438 to 1122) |
| Outpatient visits | 245 (412) | 320 (535) | 75 (–19 to 168) |
| GP visits | 82 (114) | 140 (196) | 59 (31 to 86) |
| PN visits | 10 (22) | 15 (36) | 6 (0 to 11) |
| District nurse visits | 5 (78) | 13 (68) | 8 (–9 to 24) |
| Other medical professional | 14 (114) | 86 (326) | 73 (38 to 107) |
| Other care professional | 59 (278) | 164 (735) | 106 (25 to 186) |
| Total health-care costs | 642 (1170) | 1883 (4078) | 1242 (846 to 1637) |
| Informal care (hours per week) | 19 (118) | 54 (149) | 35 (8 to 61) |
| Total societal costs | 661 (1188) | 1937 (4112) | 1276 (876 to 1676) |
| 12 months: everyone | No depression (n = 622), mean, £ (SD) | Depression (n = 91), mean, £ (SD) | Difference, mean, £ (95% CI) |
|---|---|---|---|
| A&E attendances | 12 (68) | 25 (105) | 14 (–3 to 30) |
| Day hospital attendances | 21 (160) | 0 (0) | –21 (–54 to 12) |
| Inpatient stays | 478 (2686) | 1103 (7398) | 625 (–176 to 1427) |
| Outpatient visits | 286 (541) | 547 (1638) | 261 (91 to 431) |
| GP visits | 86 (126) | 148 (282) | 62 (27 to 96) |
| PN visits | 12 (38) | 9 (18) | –3 (–11 to 5) |
| District nurse visits | 8 (84) | 13 (64) | 5 (–13 to 23) |
| Other medical professional | 19 (120) | 69 (320) | 49 (14 to 85) |
| Other care professional | 118 (777) | 86 (431) | –32 (–196 to 131) |
| Total health-care costs | 1040 (3081) | 1999 (7933) | 959 (70 to 1847) |
| Informal care (hours per week) | 25 (141) | 46 (144) | 21 (–10 to 52) |
| Total societal costs | 1065 (3106) | 2045 (7949) | 980 (87 to 1873) |
| 18 months: everyone | No depression (n = 574), mean, £ (SD) | Depression (n = 95), mean, £ (SD) | Difference, mean, £ (95% CI) |
|---|---|---|---|
| A&E attendances | 8 (36) | 19 (64) | 11 (2 to 20) |
| Day hospital attendances | 31 (149) | 79 (415) | 48 (3 to 94) |
| Inpatient stays | 548 (2251) | 1387 (5785) | 839 (184 to 1494) |
| Outpatient visits | 291 (707) | 298 (503) | 6 (–142 to 155) |
| GP visits | 86 (141) | 166 (245) | 80 (46 to 115) |
| PN visits | 16 (86) | 11 (35) | –4 (–22 to 13) |
| District nurse visits | 8 (101) | 15 (74) | 7 (–14 to 28) |
| Other medical professional | 14 (77) | 21 (84) | 7 (–10 to 24) |
| Other care professional | 181 (1729) | 229 (908) | 48 (–308 to 405) |
| Total healthcare costs | 1183 (3080) | 2226 (6336) | 1043 (235 to 1851) |
| Informal care (hours per week) | 31 (191) | 59 (146) | 28 (–13 to 68) |
| Total societal costs | 1214 (3090) | 2285 (6348) | 1070 (260 to 1881) |
| 24 months: everyone | No depression (n = 538), mean, £ (SD) | Depression (n = 95), mean, £ (SD) | Difference, mean, £ (95% CI) |
|---|---|---|---|
| A&E attendances | 9 (30) | 25 (78) | 16 (7 to 25) |
| Day hospital attendances | 31 (161) | 12 (83) | –19 (–52 to 14) |
| Inpatient stays | 396 (1441) | 1148 (4286) | 752 (289 to 1216) |
| Outpatient visits | 269 (490) | 522 (1493) | 254 (94 to 414) |
| GP visits | 84 (158) | 170 (212) | 86 (49 to 122) |
| PN visits | 15 (78) | 18 (36) | 3 (–13 to 19) |
| District nurse visits | 10 (108) | 8 (47) | –3 (–25 to 19) |
| Other medical professional | 11 (69) | 45 (179) | 33 (13 to 54) |
| Other care professional | 76 (708) | 208 (792) | 132 (–26 to 290) |
| Total health-care costs | 902 (1842) | 2157 (4568) | 1255 (720 to 1790) |
| Informal care (hours per week) | 19 (78) | 64 (164) | 45 (25 to 66) |
| Total societal costs | 921 (1858) | 2222 (4589) | 1301 (762 to 1839) |
| 30 months: everyone | No depression (n = 498), mean, £ (SD) | Depression (n = 100), mean, £ (SD) | Difference, mean, £ (95% CI) |
|---|---|---|---|
| A&E attendances | 10 (42) | 14 (35) | 4 (–5 to 13) |
| Day hospital attendances | 57 (636) | 46 (161) | –11 (–137 to 115) |
| Inpatient stays | 449 (2247) | 1237 (5372) | 788 (143 to 1434) |
| Outpatient visits | 268 (553) | 434 (1374) | 167 (4 to 329) |
| GP visits | 72 (116) | 117 (182) | 45 (17 to 73) |
| PN visits | 12 (64) | 10 (17) | –2 (–14 to 11) |
| District nurse visits | 12 (136) | 8 (47) | –4 (–31 to 24) |
| Other medical professional | 17 (118) | 29 (126) | 12 (–14 to 37) |
| Other care professional | 59 (489) | 353 (1370) | 295 (141 to 448) |
| Total health-care costs | 955 (2570) | 2249 (5693) | 1293 (583 to 2004) |
| Informal care (hours per week) | 21 (124) | 62 (158) | 41 (13 to 69) |
| Total societal costs | 976 (2578) | 2311 (5721) | 1334 (621 to 2047) |
| 36 months: everyone | No depression (n = 470), mean, £ (SD) | Depression (n = 103), mean, £ (SD) | Difference, mean, £ (95% CI) |
|---|---|---|---|
| A&E attendances | 15 (82) | 14 (39) | 0 (–17 to 16) |
| Day hospital attendances | 28 (136) | 40 (204) | 12 (–20 to 44) |
| Inpatient stays | 361 (1668) | 772 (4347) | 411 (–98 to 919) |
| Outpatient visits | 286 (647) | 721 (2434) | 435 (182 to 688) |
| GP visits | 77 (166) | 206 (1019) | 129 (32 to 227) |
| PN visits | 16 (101) | 14 (43) | –2 (–22 to 18) |
| District nurse visits | 6 (52) | 29 (195) | 24 (3 to 44) |
| Other medical professional | 12 (66) | 35 (220) | 22 (–1 to 46) |
| Other care professional | 41 (260) | 430 (1947) | 388 (205 to 571) |
| Total health-care costs | 842 (1884) | 2261 (5997) | 1418 (765 to 2071) |
| Informal care (hours per week) | 20 (105) | 57 (192) | 37 (10 to 63) |
| Total societal costs | 862 (1895) | 2317 (6014) | 1455 (799 to 2110) |
FIGURE 12.
Hospital services costs per patient (£, in 2011–12 prices) by cost category and by group for service users over time.

The vast majority of patients in both groups received outpatient care, and care from GPs and PNs. Outpatient care costs per patient were higher for patients with depression during the study period and significantly higher at 12, 24, 30 and 36 months, at around double the costs of the non-depressed group. However, the number of people who utilised the services was similar for both groups at each time period. Only a few patients used day hospital services at each time point; however, the costs incurred were high for both groups. This can be attributed to the high cost of some services, such as cataract operation. Average community care costs per patient were higher by £475 for patients with depression than for those without depression, and the difference was statistically significant (95% CI £66 to £883). Community costs and total health-care costs are shown in Figures 13–16. None of the differences in informal care costs during the study period was statistically significant. Societal costs are shown in Figures 17–21. Indirect costs included informal care, which was similar between groups, with slightly higher costs incurred by the depressed group (difference £43, 95% CI –£164 to £251), especially during the last three follow-up periods (see Figures 16 and 18).
FIGURE 13.
Community services costs per patient (£, in 2011–12 prices) by cost category and by group for service users over time.
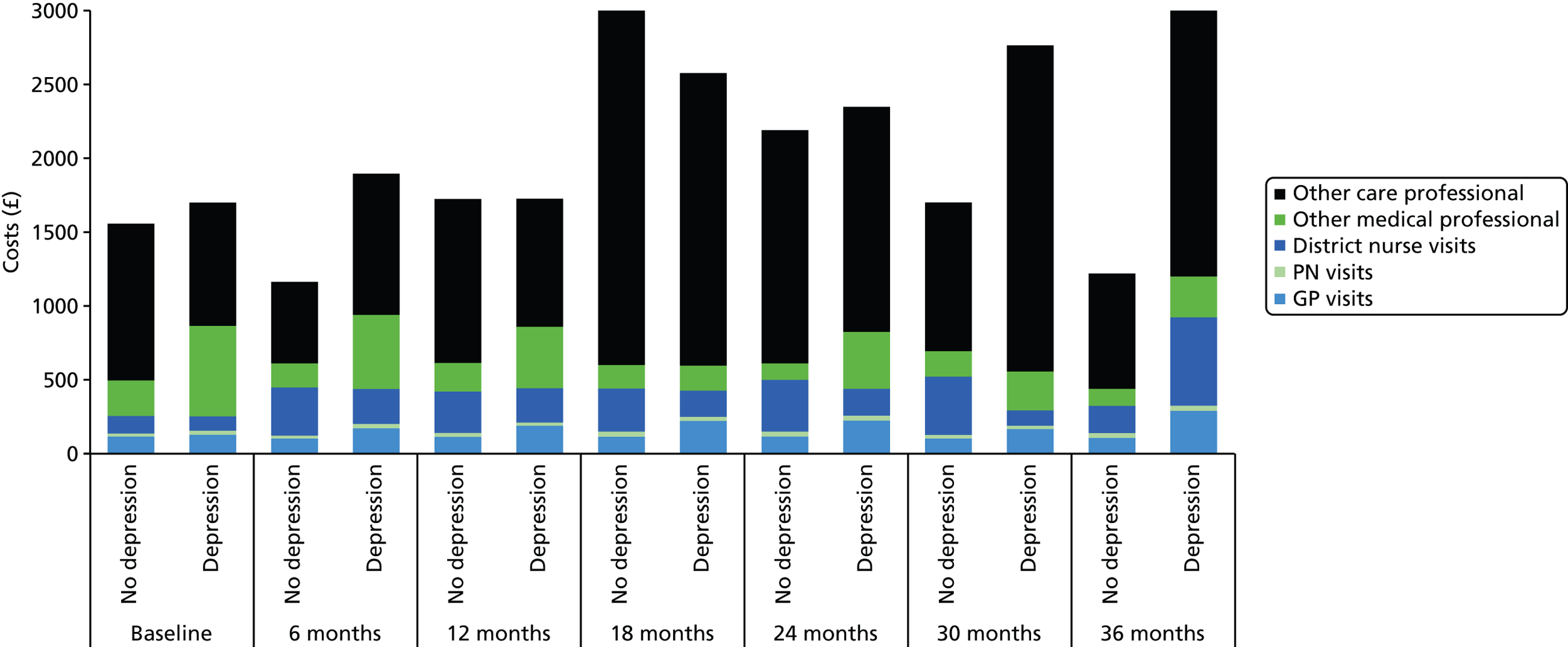
FIGURE 14.
Total health-care costs per patient (£, in 2011–12 prices) by group for service users over time.
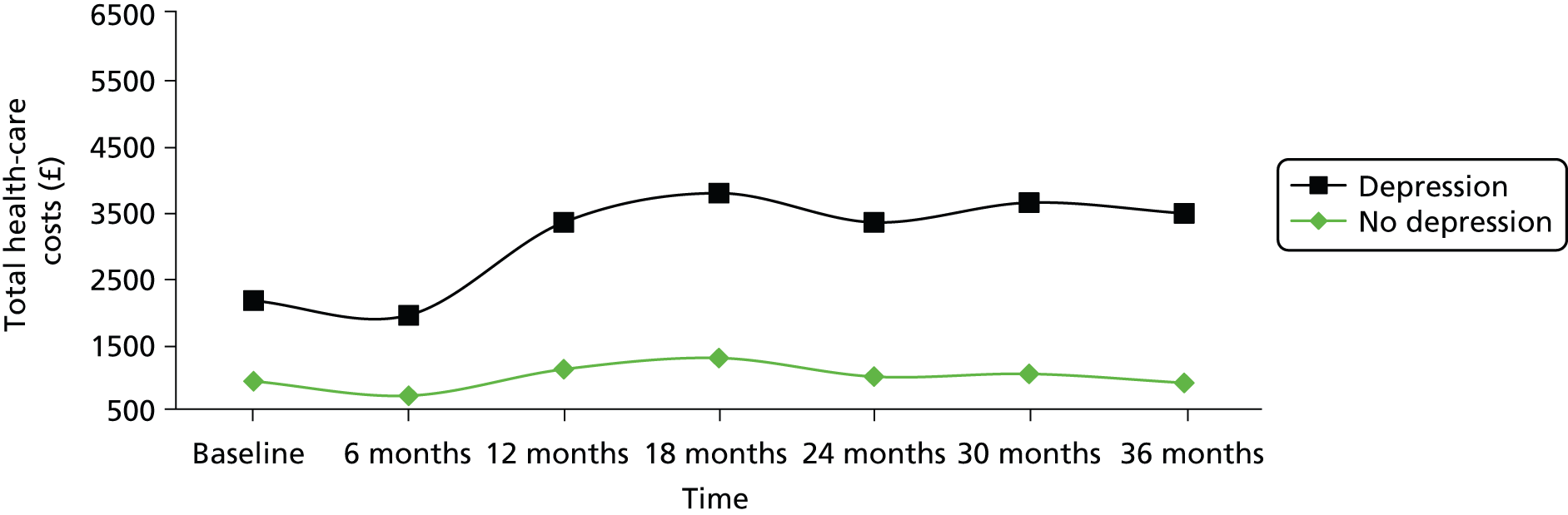
FIGURE 15.
Cumulative health-care costs per patient (£, in 2011–12 prices) by group for service users at 36 months.
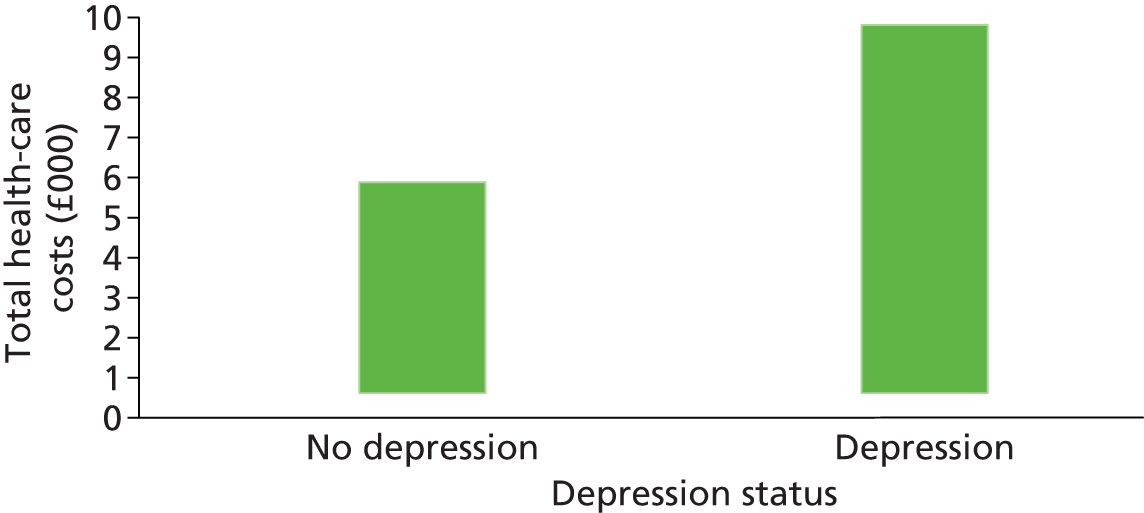
FIGURE 16.
Informal care costs per patient (£, in 2011–12 prices) by group for service users over time.
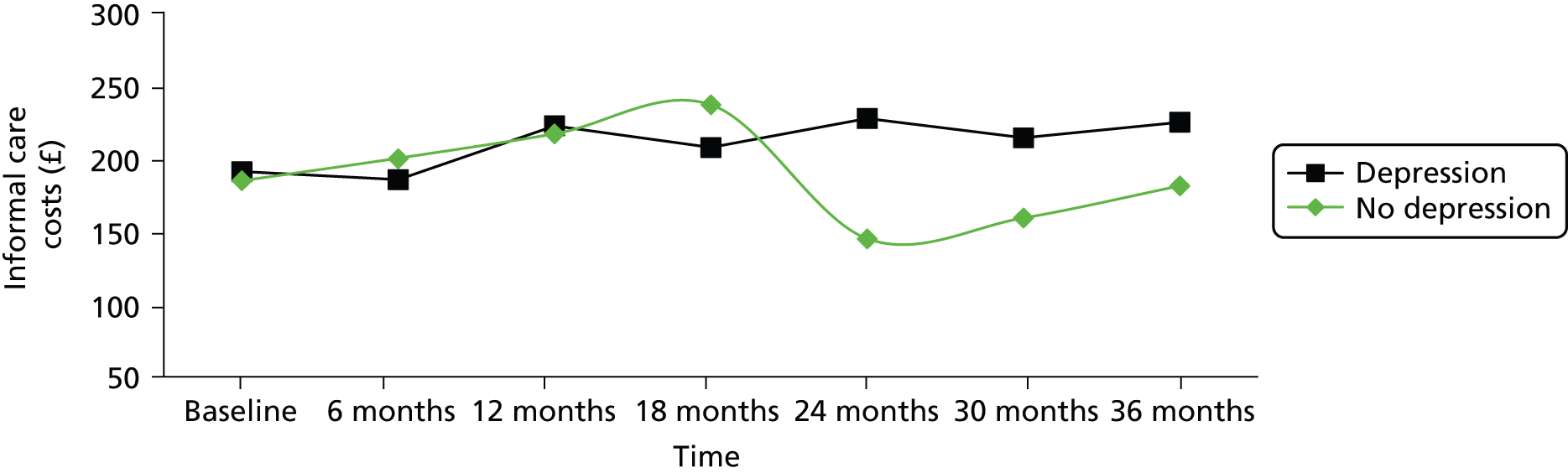
FIGURE 17.
Cumulative informal care costs per patient (£, in 2011–12 prices) by group for service users at 36 months.

FIGURE 18.
Total societal costs per patient (£, in 2011–12 prices) by group for service users over time.

FIGURE 19.
Cumulative societal costs per patient (£, in 2011–12 prices) by group for service users at 3 years.

FIGURE 20.
Grouped cumulative costs (£, in 2011–12 prices) by group and by cost category for service users at 36 months.
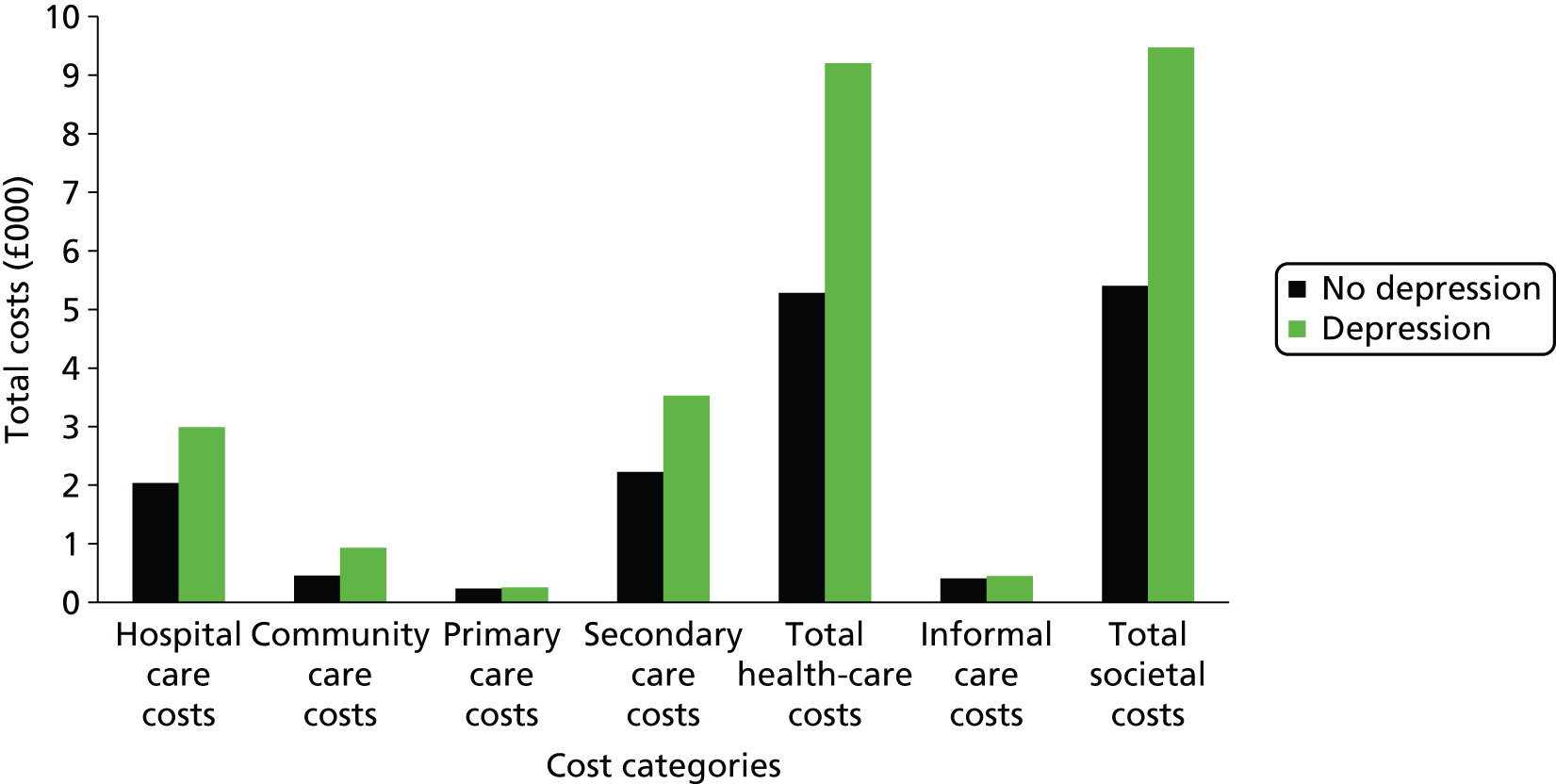
FIGURE 21.
Mean total societal costs per patient (£, in 2011–12 prices) across time.

Predictors of costs
Tables 46–49 show the output of the four generalised linear models, having accounted for the impact of depression status on costs by using the depressive disorder assessed by the CIS-R as an input variable. The results reveal that health literacy had a significant negative impact on total costs from the health-care perspective. It would decrease costs by approximately 40%. From both the health-care and societal perspective, ethnicity was significantly associated with total costs. More specifically, being Asian, black or an ethnicity other than white is predicted to reduce costs by approximately 45%. Similarly, higher costs were associated with the prevalence of depressive disorder (measure by the CIS-R), with housing and relationship problems, and with currently suffering from cancer.
When the health literacy variable was excluded from the first two models, health-care and societal costs were shown to be significantly and positively associated with having an additional comorbidity other than cancer, and this included chronic kidney disease. Another new factor that was present in these models was reported chest pain, based on responses to the Rose angina questionnaire for angina. The association this had with costs was positive and the magnitude was similar (+23%) from both perspectives. It is notable that smoking status is not now a significant predictor of total costs. Total health-care costs at baseline are predicted not to have any impact on the cumulative health-care or societal costs in any of the four models.
Discussion
The aim of the economic analysis was to estimate the cumulative health-care utilisation and costs of patients with cardiovascular heart disease with and without baseline and subsequent depressive symptoms. The cumulative cost per patient incurred by depressed patients was approximately double the cost incurred by non-depressed patients during the 3-year study period. Overall, inpatient care services dominated costs at baseline and follow-up, while the majority of patients in both groups received outpatient care and care from GPs and PNs.
Across the study period, total costs were increasing, except in the second half of the second year, when they declined dramatically (see Figure 21). Differences in costs between depressed and non-depressed patients were statistically significant at almost all time points during the study period. Statistically significant predictors of higher societal costs were depressive disorder (CIS-R), white ethnicity, housing problems, relationship problems, having reported cancer as an additional comorbidity and baseline health-care costs. The prevalence of depressive disorder measured by the CIS-R instrument predicted increases in total costs by approximately 50% in all four multivariate models. Inadequate health literacy was associated with reduced health-care cost and this may reflect poor access to care for this group.
There are several reasons why costs might have been under- or overestimated in this economic analysis. With regard to direct costs, medication costs were not taken into account because such data (self-reported) were available at baseline only. However, some patients were being treated for conspicuous depression and other comorbidities during the study. Although this is a limitation, antidepressant treatment is generally inexpensive, given that the most frequently used drugs are ‘off patent’. Another limitation is that indirect costs included only informal care. The high level of missing data in variables related to employment status and sickness absence made it impossible to calculate productivity losses, which is a very important component of indirect costs. Finally, the subgroup analysis was based on depression status as assessed by the HADS score.
Overall, the results could be considered as robust, as data on the important variables for this economic analysis (e.g. service use) were mostly complete, with relatively few having extensive missing data. Further analyses could consider the patterns of depression, examining the varying cost among the different patterns of depression and anxiety. Other future work could look at the economic impact of comorbid depression as a disorder rather than as a symptom-based condition. Similarly, the examination of the relationship between anxiety and costs would be of interest, considering that the majority of patients in the depressed group of this analysis also presented symptomatic anxiety (HADS-A). These findings could drive further research to investigating the excess cost of depression (and/or anxiety) in this patient group by conducting modelling studies on diagnostic and therapeutic approaches that might help to improve health-care outcomes and reduce total costs.
Chapter 6 Summary of the UPBEAT-UK programme
Introduction
This chapter discusses the overall findings of the entire UPBEAT-UK programme and discusses to what extent and how the combined findings from the inter-related work packages have worked synergistically and added to current knowledge about the associations between depression and CHD, and best approaches to manage people with CHD, chest pain and depressed mood.
This is the first time, to our knowledge, that a programme of inter-related work has been conducted with a broad range of people with varying levels of CHD, chest pain and depression/anxiety over 3 years in 33 south London general practices. At the same time, this location is a limitation when it comes to generalising to the country as a whole, as indicated by the lower prevalence of CHD (see Chapter 1, Patient and public involvement). It is also the first time, to our knowledge, that the perceptions of users and primary care professionals have been combined to create a new nurse-led form of PC comprising elements of case management and self-management theory for CHD register patients with chest pain and depression.
Most previous research on CHD and depression has been conducted with people who are immediately post cardiac event (usually MI) and who are being seen mainly in secondary care settings. The unique feature of the UPBEAT-UK programme was that we have been able to look at all new incident cardiac events and new incident depression over 3 years in a GP-registered CHD population and examine the factors predicting both cardiac and depressive outcomes. For all four work packages there is, of course, a limitation in generalisation because these were essentially volunteer practices.
In this chapter, we will discuss the combined achievements of the UPBEAT-UK programme and the implications for everyday general practice. It is striking that there is little in the way of available current national guidance for the management of CHD patients with chest pain and depression/anxiety. Existing guidelines tend to either be very non-specific or concern only one condition. However, many older patients have multiple conditions and require complex care pathways.
First, we will summarise the most important findings, which have been described and discussed in each previous chapter.
Overall, we consider the project to have been successful. The main findings were (1) that a much larger trial than we had anticipated would be needed to estimate to what extent the preference-led intervention we developed will be successful in treating depression in CHD patients in primary care; (2) an intervention that would deal with anxiety as well will perhaps improve outcomes; (3) the current primary care practice setting, with the many competing agendas and the generally limited skill levels, may not yet be geared towards delivering the type of integrated care that many of these patients need; and (4) perhaps psychological services such as IAPT are better placed to deliver such care. An IAPT trial seems to be the way forward.
Work package 1
The literature review and metasynthesis of existing literature was conducted at the very beginning of the programme in 2007/8, as this was necessary before we could conduct the qualitative study of professionals. The findings of the review have been published31 and have since been highly cited by other authors. It is likely that, since we conducted this review, further relevant research has been published and a future review should include this.
At the beginning of the programme we were unable to identify previous relevant work. We found several papers on depression in primary care but none on CHD, chest pain and depression in primary care.
The qualitative study with GPs and PNs also highlighted the paucity of research or guidelines on how best to manage people with CHD complaining of non-acute chest pain and depression. Consequently, our GP and nurse respondents had no guidance to cite and felt relatively untrained and ill-equipped in this area. In addition, while being very well aware of the frequent concurrence of social problems, they were unsure of what assistance may have been available. With some notable exceptions, GPs and PNs were unsure of their role in managing social problems.
Work package 2
The most striking finding of the qualitative study with patients was that there was such a breadth of ‘understandable’ psychosocial losses that were described by participants. This then highlighted an immediate mismatch between described user experience and the lack of relevant confidence and skills in the main primary care work force.
Work package 1 and 2 indicated the complexities involved with helping patients to try and improve coping with chronic physical illness and depression in a generalist environment. UK primary care, as busy as it is, is not easily geared towards providing this type of care.
This type of mismatch is likely to militate against full and frank discussion of such issues in every day practice. This, combined with a climate of reactive rather than proactive primary care, provided by increasing numbers of GP-salaried assistants as part of the move away from ‘named’ professional care, would make it more likely that a ‘collusion of anonymity’ could occur. 184
Work package 3
Our pilot RCT findings have coincided with a new national policy from the Department of Health that all older patients with long-term conditions should now have a ‘named GP’ responsible for their personalised case management. 185 We have shown an indication from our small pilot study that a named nurse providing PC combining elements of case management and self-management theory in which patient preference is prioritised appears to enhance both patients’ sense of self-efficacy and reduce the self-reporting of chest pain.
In testing the feasibility, acceptability and potential impact of named nurse PC, we have demonstrated that it is acceptable to patients – even if largely by short yet frequent telephone contact, which can be done in a reasonable amount of time – and it is potentially cost-effective, not least if it reduces reported chest pain and potentially attendance at a rapid access chest pain clinic or a 999 call-out and transfer by an ambulance to A&E for further tests.
We used a pilot RCT design186 to assess the likely benefits and harms of named nurse PC to allow inferences about the possible causal effects of our new intervention. The MRC framework30 recommends RCTs early in the development and testing of complex interventions. For pilot studies such as ours it has been suggested that the analysis should mainly be descriptive or focus on the CI estimates. 187 Pilot studies are described by Arain et al. 188 as a smaller version of a main study, to ensure components of the main study work well. They are ‘focused on the processes of the main study’ such as ‘recruitment, randomisation, treatment, and follow-up’. Pilot studies can be run as the first phase of the main study and results can be included in the overall analysis.
Overall the UPBEAT-UK pilot RCT aimed to:
-
identify whether or not UPBEAT-UK nurse personalised case management is feasible and acceptable, and determine any necessary modifications
-
test procedures for a potential future definitive RCT, especially in relation to eligibility criteria, randomisation procedures, allocation processes, and recruitment and retention rates
-
test approaches to assessing fidelity, process evaluation and outcome evaluation, to inform the design of a future potential definitive RCT including choice of primary and secondary outcome measures, and sample size calculation.
The UPBEAT-UK pilot RCT also needed to establish feasibility of the new nurse-led PC, that is, rigorously examine the potential usefulness and acceptability of the intervention and estimate relevant parameters for the evaluation strategy to inform a future definitive RCT. 188 Often there is no clear distinction in the literature between pilot and feasibility studies. 189 A literature review found that definitions of feasibility and pilot studies vary, but studies labelled ‘feasibility’ tend to use a flexible method compared with ‘pilot’ studies in practice. Arain et al. 188 suggest that feasibility studies are undertaken before a main study to ‘estimate important parameters’ for the design of a main study, such as the SD of the outcome measure for sample size calculation, characteristics and feasibility of the outcome measure, people’s readiness to be recruited and randomised, number of eligible participants, adherence and follow-up rates. Feasibility studies should ‘not evaluate the outcome of interest’ but instead be powered to estimate parameters such as recruitment or dropout rates. Feasibility or external pilot studies assess one or more of the following:186,189
-
the feasibility of key processes, such as eligibility criteria, and their ascertainment, randomisation procedures and their acceptability, retention and refusal rates, failure/success rates, adherence, appropriateness of the primary outcome measure or the acceptability of questionnaires
-
potential time and resource problems, such as administrative issues, staff training, the willingness and capacity of involved clinicians or centres, process times, for example for postage, preparing equipment, dealing with contingencies such as material breakdown, clinician absence
-
potential human and data management problems, including space for personnel and data collection forms, data entry and basic properties of the data such as missingness or variability
-
acceptability and safety of the intervention, dose, response, estimated treatment effect and estimated variance of the treatment effect for sample size calculation
-
the integrity of the study protocol.
We used a pragmatic approach to trial design with a specific emphasis on processes, to ensure external or ecological validity. 190,191 The intervention was conducted in a routine GP setting (general practice or patients’ homes), using little or no participant selection beyond being on a CHD register in the practice and the clinical indication of chest pain (exertional non-exertional) and depression, and specifically not excluding comorbidities that were common in general practice, such as diabetes, and may attenuate the treatment effect. This approach maximised real-world applicability of the findings, by allowing a direct translation of the outcomes to routine settings while also explaining processes occurring during the UPBEAT-UK intervention. The UPBEAT-UK intervention was manualised to allow fidelity evaluation, but was delivered with some degree of flexibility, for example with respect to session timing, to reflect real-world practice and the interests of our two nurses [one (Mark Haddad) is a community psychiatric nurse who has worked clinically as a community psychiatric nurse in a local south London practice for 20 years and the other nurse (EB) is a registered general nurse and has a background as a respiratory nurse in a London teaching hospital with subsequent training as a chartered practitioner health psychologist and who currently works mainly as a nurse researcher]. Although our pragmatic trial design focused on effectiveness in the ‘real world’, a more explanatory RCT may have resulted in a clearer idea about what the active ingredients and causal processes may have been. However, it has been argued that pragmatism and understanding mechanisms should not be viewed as mutually exclusive190 and our UPBEAT-UK findings should be able to answer both pragmatic and explanatory questions to some degree. The UPBEAT-UK pilot RCT was a pragmatic trial with explicit attention to processes, with several advantages and some disadvantages. We tested the intervention in everyday GP settings and one of the trial nurses (EB) chose to make home visits to build a better picture of the patient’s environment.
One disadvantage and potential barrier to change we encountered was that the GPs and PNs in the south London practices proved sometimes to be harder to engage or contact than had been expected, and this had not been discussed in advance or built in to the study. For example, one of our GPs decided not to prescribe an antidepressant despite advice that this may be beneficial from one of the trial nurses after supervision with an academic GP (AT) and two psychiatrists (AM and PW).
Our pilot study was not designed to detect any improvement over and beyond TAU for depressive symptoms and well-being, as we were not testing treatment effect and were focused on testing feasibility and acceptability. Our patients in the pilot study prioritised managing pain, lack of exercise and sleep problems. Strategies for pain relief seem to have resulted in a reduction of reported chest pain although this would need to be tested in a definitive trial.
Minimisation of selection and allocation bias was addressed by random allocation by the independent King’s Clinical Trials Unit. Randomisation appeared to achieve equal balancing of individual variables between groups. We reduced bias by collecting the baseline data before allocation. We addressed bias in the intervention by ensuring fidelity to the UPBEAT-UK pilot RCT manual was monitored to ensure that comparability of the intervention by our two nurses who jointly received weekly peer/group supervision (by AT, AM and PW). We minimised any bias in the follow-up data by ensuring that all participating patients were followed up and included in the analysis using an intention-to-treat approach to reduce the impact of selective attrition. We minimised bias in outcome data collection by the use of standardised objective assessments, rater training and supervision, and self-rating scales where possible and by using research assistants blind to treatment allocation.
A limitation common in similar trials was that blinding was achieved at only some levels: ideally the research participant, the care provider, the assessor and the statistician should all be blind. Although blinding participants and care providers prevents performance bias, that is, the preferential receipt of additional treatment in one group, it was not possible to achieve, as is so often the case. 192 Blinding research assistants ensures independent judgement about outcome193 but is difficult in practice, for example because participants disclose their allocation. We blinded our research assistants and asked them to record which group they thought participants were in and to notify us of any unblinding, which did happen on occasion. Our statistician became unblinded early on when the number of participants in the intervention group was mentioned in discussion – we had different numbers in each group (40 and 41) so it became obvious which group was which.
Although these methodological considerations are less relevant for the interpretation of a pilot study, such as UPBEAT-UK, non-blinded GPs and PNs, may cause post-randomisation confounding and biased outcomes through differential intensification of routine care in a definitive trial. 192 Differential intensification may have occurred when participants in the experimental treatment were sensitised to certain issues and talked about them to their GP or nurse. Equally, GPs and nurses who knew about a person’s assignment to the control condition and were convinced of the benefits of the experimental treatment may have encouraged access to additional non-study care, leading to intensification of routine treatment in the control group and thus decreasing the effect size of the intervention. The choice of a control condition is important for the trial’s internal validity, which can be threatened when the TAU group receives additional health-promoting behaviour, especially in trials of psychosocial interventions; although it is perfectly possible that the patients could have easily told their GPs or PNs if they saw them, there did not seem to be much interaction between them. 194 We used a TAU control and obtained printouts of GP consultations, referrals and treatment of both groups to assess what was received by the control group participants. As the UPBEAT-UK intervention was conducted independently of the usual GP practice staff, it is unlikely that there was any contamination. This would need careful consideration in any future definitive trial. TAU implies that anyone on GP CHD registers with chest pain and depression would routinely receive treatment, which may not be the case in reality. A more accurate term may have been ‘usual care’, which may or may not have happened. Comparing the UPBEAT-UK named nurse PC directly with existing general practice care for people on CHD registers with chest pain and depression may be problematic and may not even be possible if GP or nurse routine care involves individualised multiaspect multiprofessional case management for people with CHD and depression. It seems likely that we superimposed the experimental UPBEAT-UK treatment onto existing practice (whatever that is) to see if UPBEAT-UK nurse personalised case management has any potential added value that could be further tested in a definitive trial using a larger, more powered sample.
Recipients of UPBEAT-UK nurse PC would have received more attention (i.e. from the trial nurse) than the comparator group participants. They may have wanted to please their trial nurse after 6 months by inflating their self-rated improvement on the final outcome measure, which could be a possible limitation. Further non-specific factors may have been present, such as therapeutic alliance. We examined this by looking for any differences between our two nurses. A limitation of the study was that we did not record or rate consultations to assess alliance or attention. This makes it hard to assess the active ingredients.
Although our pilot was designed to assess feasibility and acceptability, rather than treatment effect on depression, a range of variables and events outside our PC may influence depressed mood. Confounding factors that have been empirically shown to impact on depression in people with CHD include medication changes, concurrent social problems and life events. Theoretically, these influences should be evenly distributed across randomised participants. However, their impact may still differ between groups depending on the chosen interventions and problems to be addressed. Hence, while it may be neither feasible nor necessary to control for all possible influences on depressed mood, a definitive trial might still aim to systematically assess the most prevalent influences on depressed mood. This would be a challenge, however, in a patient preference-led intervention such as UPBEAT-UK PC. Our GPs and nurses indicated that they knew that such problems abounded in their patients on CHD registers but were often at a loss to know what could be done about adverse social conditions.
All of these challenges will need to be addressed in a definitive RCT. Our pilot work does not address implementation issues involved in transferring the intervention to clinical practice. Use of a model such as May’s Normalisation Process Theory195 could be used to critically appraise the intervention. This would inform optimal implementation following demonstration of efficacy in a definitive RCT.
Work package 4
The aim of the cohort was to study the relationship over time between depression and symptoms of cardiac disease (chest pain) and outcomes (interventions, MI). We found the prevalence and incidence of depression to be lower than might be expected in such a cohort, but that of anxiety unexpectedly high. Nevertheless, the comorbidity of depression and anxiety in patients with CHD is worth taking into account, since patients suffering from conditions have a higher incidence of adverse outcomes and management strategies in these patients have so far failed to reduce this incidence.
It has long been noted that many patients referred for investigation of chest pain have concurrent anxiety and depression syndromes. 196 Chest pain is indeed a recognised symptom for the diagnosis of a panic attack. 173 Case-level anxiety or depression has been stated to be a contraindication to expensive cardiology investigation, as few are found to have coronary disease. 178 Most of the work on the association of chest pain with psychiatric disorders has been carried out in secondary care cardiology patients. To our knowledge there are few studies of the different types of chest pain experienced in people with known CHD in primary care. Our study population is different and there are no published studies on this topic in CHD register patients. In the UPBEAT-UK study, 44% of patients on GP CHD registers reported current chest pain. This was higher than previously reported. 162 This is an important finding, although it could reflect a selection bias in the opt-in recruitment process. Two-thirds of the reported pain was exertional, suggesting cardiac origin, and exertional pain was a strong predictor of MI and death. These patients with continuing exertional pain are under primary care follow-up, but not all were referred to secondary care. There is potentially a case to be made for more emphasis on referral to secondary care, with patients continuing to report exertional chest pain during their primary care follow-up appointments.
Our study of associations at baseline shows that both exertional and non-exertional chest pain were associated with psychosocial factors such as depression and anxiety. Causality between chest pain and psychosocial factors cannot be determined during cross-sectional analysis. However, the great strength of this cohort data set is that it is longitudinal and multiwave. This allows us to be clearer about the direction of the association, and to see whether risk factors are equally powerful if they are distal (baseline) or proximal (last measured data point). To our knowledge, there is no previous study in which the Rose questionnaire has been used serially every 6 months for 3 years.
There is a different risk factor profile for those with exertional pain over this time, compared with the smaller proportion of people with non-exertional pain. For the former, baseline depression, anxiety and impaired quality of life play a part, with anxiety as the more dominant risk factor as it persists even after using multivariate models, which include quality of life. For non-exertional pain, the profile of females with asthma and shorter time on the register at baseline is the one more strongly associated.
Although anxiety may seem understandable in patients with CHD and chest pain, our study suggests that this may be a malign additional symptom that needs to be considered in addition to depression. Therefore, GPs and PNs monitoring these patients should be aware of the role of anxiety in addition to chest pain in predicting continuing suffering and decreased quality of life.
Limitations of the cohort methodology include the self-selection of the general practices participating and the selection by response rate of 27%. We cannot gauge the bias resulting from the response rate, as we were not allowed access to data on any patients who refused to participate. The patients were interviewed every 6 months, reporting on how they were currently as well as over the previous 6 months, introducing a possible recall bias. The 6-month intervals could be underestimating the number of experiences of anxiety and depression, as patients could have a brief episode of either condition, which we would not have detected by this method. The physical health information of these patients has come from patient recall, use of the Rose angina questionnaire and GP records. Each of these could inaccurately represent the health status of the patient. We did not have the resources to have a physical examination of each patient, hence the need to rely on these proxies.
The role and utility of general practitioner coronary heart disease registers for research
This was the first complex programme of research on patients registered on GP CHD registers. We have been unable to find comparable data from other studies, as most previous research has been conducted in secondary care settings. The advantage of using GP CHD registers is that patients are readily accessible through their GP practices, which maintain these registers, and this has been a key benefit to research from the advent of the QOF. This made it much simpler for us to recruit patients with CHD in a primary care setting. However, we tested the accuracy of the CHD registers with our cardiologist team members, an exercise which had not been done before, and we can report for the first time that the accuracy of CHD registers was approximately 85%. The registered population, we have discovered, contains a heterogeneous group in terms of the degree of CHD and cardiac intervention they have received, and recency or history of onset. Registers could also be used to select specific groups of patients (e.g. MI). This makes them a unique and epidemiologically enriched population to study the effect of any factors of cardiac outcome. Our sample was older, contained more males, had high levels of comorbidity (e.g. 25% diabetic), and multiple social problems and was less likely to be from black and minority ethnic communities. The sample, therefore, reflects a typical general practice population and thus differs from previous research in this area.
The disadvantage of using GP CHD registers for epidemiological research, however, is that the members are survivors. Consequently, there is a survivor cohort bias. The fact that our sample was 27% of the potential CHD register population was not because of the CHD registers but because of the required opt-in procedure. Unfortunately, we are unable to examine this bias because we cannot access information about or interview the patients who chose not to opt in to the research. This is a common problem in primary care research. It is possible that those patients who did opt in to UPBEAT-UK were more symptomatic in terms of chest pain and anxiety, and perhaps patients who did not opt in may have been more depressed than those who did opt in. If those who did opt in were less depressed, it is possible they felt that the trial was less relevant to them. This is cross-sectional so we cannot infer causality, but further modelling could help elucidate the complex inter-relationships and pathways between chest pain, anxiety and depression. Chest pain was found to reduce health-related quality of life, and further study is needed to fully understand the impact. It is noteworthy that our PC intervention was found to reduce the likelihood of reporting chest pain at both 6 and 12 months. Further research would require sophisticated modelling techniques that model hypothesised causal relationships.
The relative importance of anxiety and depression in this patient group
When we originally applied for funding there was a lot of interest in depression and CHD. While we found that there was a modestly high prevalence of depression, the high incidence rate and episodic nature was more noteworthy. Surprisingly, we did not find from our data any evidence that depression is a strong predictor of adverse cardiac outcome. This is not consonant with previous research from secondary care. This may reflect the characteristics and possible selection bias in our sample. On the other hand, the high prevalence rates for anxiety in our sample were unexpected. It is possible that more anxious people want to be recruited into such trials. Anxiety rates stayed high throughout the 36 months. Baseline anxiety disorder was a stronger predictor of adverse cardiac outcome and cardiac death than baseline depression. This finding needs further assessment and it is potentially very important for further research to examine the possible links between anxiety and cardiac pathology, as much previous research has focused on mechanisms linking depression and cardiac disease. These findings also have implications for clinical practice and raise questions about the need for anxiety being given equal emphasis with depression in terms of case finding. Our cohort data set could be used to develop a valid anxiety scale for use by practitioners to follow up people with anxiety and CHD.
In our pilot RCT, patients were helped by the nurses to prioritise perceived problems relating to their CHD and depression: one of the top five problems prioritised by the patients was anxiety (another of the top five problems was insomnia, which may have been anxiety related) and we also found some evidence of anxiety as a mediator for depression improvement. In a future trial, more active treatment, such as a more formal type of CBT or problem-solving treatment for anxiety and depression, should be tested, particularly when associated with chest pain.
The programme as a whole and how the findings from each of the work packages informed each other
This programme has been successful because of a multidisciplinary team with research and clinical backgrounds working together for over 6 years, combining qualitative and quantitative approaches in an active dialogue with regular meetings.
Some common features emerged from the varied approaches: the importance of social problems, chest pain as an unpleasant symptom with adverse effects, and depression and anxiety. While professionals often see the last two as independent conditions to pick out and treat, depression and anxiety are often not seen as such by patients. For patients, these conditions are merged with their physical ill health and social difficulties and they want an integrated approach to treatment. This inevitably locates their treatment best in primary care. We postulate that the method in our pilot RCT addresses the need for such an integrative approach. Motivation for patients is important, and the high patient adherence to our PC demonstrates the need for continuity of care from a case manager. Feedback from our trial participants showed how much continuity was appreciated. A combination of goal attainment and a focus on symptom resolution for chest pain, anxiety and depression seems important.
We were unable to employ PNs on the pilot RCT as originally intended because many of them informed us that they had no spare capacity, as well as considering themselves not to have the sufficient mental health or behavioural health training for such an intervention. It became apparent that a new intervention would need new integrated systems of care involving the whole practice team rather than depending entirely on one group of professionals or one treatment approach. Our nurses often found it difficult to contact members of the practice team and regular meetings with practice staff would have improved communication. PNs have a long tradition of case management for patients on registers (e.g. asthma, diabetes) and many would need enhanced training in behavioural management and psychosocial care.
There is current national interest in enhancing the role of IAPT to provide integrated care for patients with long-term conditions and 13 national pathfinder pilots are under way, one of which is in the area of CHD. Much of this role is likely to fall to PWPs, who are often graduate psychologists with very little, if any, clinical training in relation to physical health problems (long-term conditions). PWPs, although well trained in providing low-intensity treatments and guided self-help approaches for anxiety and depression, would need much additional training and supervision in CHD prevention and management.
Our findings showed that overall costs were higher for those participants who were depressed at baseline, by approximately £5000 (i.e. almost double the cost of non-depressed participants). Differences between the two groups were significant across some services and non-significant across others, but the data highlight that people with depressive symptoms can use health services at a high rate and there are subsequent societal costs associated with this.
Conclusions
Nearly half of all patients on GP CHD registers were found to have current chest pain, and this was strongly associated with concurrent social problems. Patients who reported chest pain at the outset of the study were more likely to have further chest pain over the following 3 years and to have more adverse cardiac outcomes such as needing stent insertion, bypass graft surgery, having a MI or dying of a cardiac cause. While depression was common, episodic and associated with increased costs, anxiety disorder was more common and found to be a stronger predictor of worse cardiac outcome and mortality than depression.
Recommendations for further research
We propose several avenues for further research to build on our findings.
-
There is a pressing need to better understand the links. More sophisticated models linking the patterns of depression, anxiety and chest pain are needed to understand the associations between anxiety, chest pain and adverse cardiac outcome. We have a unique cohort data set to allow us to achieve this.
-
GPs and nurses seem currently uncertain how best to manage patients’ symptoms in the context of the many psychosocial problems in their CHD patients. PC promoting self-management proved to be acceptable and feasible, improved patient self-efficacy, reduced chest pain and was associated with fewer overall costs than usual care (e.g. fewer A&E attendances). PC combining case management and self-management with an extra emphasis on anxiety needs to be further piloted and definitively tested with PN, working closely with colleagues in practices. Many nurses told us they had little extra current capacity so this would need to be built into practice plans.
-
PC combining self-management with an extra emphasis on anxiety could also be piloted and definitively tested with PWPs in IAPT although they would need training and supervision in long-term conditions and behavioural change techniques.
-
Further research should identify the training needs of staff to support the roll-out and deliverability of the intervention.
-
The cohort data set is available for further analyses for researchers both in and out of King’s College London. Already we are collaborating with the Department of Psychological Medicine at King’s College London (Professor M Hotopf) and with the Department of General Practice at the University of Amsterdam (Professor H van Marwyck). The longitudinal nature of the data makes them a rare and valuable resource for hypothesis testing. The analyses for this report, reflecting our funded aims, concerned anxiety and depression, and their relationship to cardiovascular outcomes. Other variables (e.g. physical comorbidity, social stresses and risk behaviours such as smoking and alcohol) were included as covariables in that relationship but not studied over time in their own right. To emphasise, CHD register patients are insufficiently studied, given the frequency of reported chest pain.
-
Self-reporting through the installation of an application on a mobile phone or tablet could allow patients to record each incident of chest pain with concurrent mood and social circumstances. This information would certainly inform GP management better than patient recall every few months and could lead to a patient‘s greater self-efficacy.
-
The result of the pilot RCT does not as yet justify a move to a larger trial but its acceptability and feasibility without additional cost suggest that these comorbid patients would appreciate this approach. We would propose, as the next step, another small feasibility study in which an IAPT worker was compared with a PN in the therapeutic role. An up-to-date metasynthesis should be carried out to inform this and the content of the intervention should be as effective for anxiety as for depression.
Implications for practice
The implications arising from these research results are for the primary care teams who follow up patients on the CHD register.
Chest pain in general and angina specifically remains a common experience in these patients despite the time that has elapsed since the event that placed them on the register. We have shown that chest pain produces high risk for another cardiac event. It is thus important that those reporting pain are not lost to follow-up in the practice.
Anxiety is more common than depression in these patients and, although usually comorbid with depression, is the more potent predictor of the two of subsequent cardiac events. Consideration could be given to the inclusion of the assessment of anxiety in these patients by the primary care team who could provide a strategy of management if necessary.
These older patients appreciated an approach to dealing with their low or anxious mood that focused on their own needs using psychological support. All the indications were that additional medication to that being taken for their physical conditions were unwanted.
The qualitative research suggested that a ‘narrative of loss’ underlay low mood in many patients. Their cardiovascular disease had led to loss of occupational role, social engagement or ability to be independent and in men loss of self-worth accompanying diminished sexual performance. This insight might enable a therapeutic point of contact to be established with some patients.
Acknowledgements
The coapplicants of the programme grant were: A Tylee, Department of Primary Care and Public Health Sciences, King’s College London; M Ashworth, Department of Primary Care and Public Health Sciences, King’s College London; J Chambers, Guys and St Thomas Foundation Trust, King’s College London; M Leese, A Mann, P McCrone, J Murray, D Rose and P Walters, Health Service and Population Research Development, King’s College London.
The wider UPBEAT-UK team members were: M Ashworth; J Brown, Institute of Psychiatry, Psychology and Neuroscience, King’s College London; L Carvalho, University College London Research Department of Epidemiology; J Chambers; A Farmer, Institute of Psychiatry, Psychology and Neuroscience, King’s College London; Z Fortune, Institute of Psychiatry, Psychology and Neuroscience, King’s College London; M Haddad, City University London; S Hampshire, Institute of Psychiatry, Psychology and Neuroscience, King’s College London; R Lawton, Institute of Psychiatry, Psychology and Neuroscience, King’s College London; A Mehay, Prostate Cancer UK; J Mirza, Institute of Psychiatry, Psychology and Neuroscience, King’s College London; N Nikkheslat, Institute of Psychiatry, Psychology and Neuroscience, King’s College London; C Pariante, Institute of Psychiatry, Psychology and Neuroscience, King’s College London; R Phillips, Singapore Clinical Research Institute; L Rolland, Institute of Psychiatry, Psychology and Neuroscience, King’s College London; G Rowlands, Department of Primary Care and Public Health Sciences, King’s College London; H Sims, Institute of Psychiatry, Psychology and Neuroscience, King’s College London; and J Smith, Institute of Psychiatry, Psychology and Neuroscience, King’s College London.
André Tylee was partly funded by the National Institute for Health Research Mental Health Biomedical Research Centre at the South London and Maudsley Foundation Mental Health Trust and the Institute of Psychiatry, Psychology and Neuroscience at King’s College London.
We are grateful for the work of the UPBEAT-UK research assistant team: Aysha Begum, Sally Hampshire, Rebecca Lawton, Hannah Sims, Alison Smith, Joe Mirza, Zoe Fortune and Anita Mehay for collecting data from both patients and practices.
We would like to thank Professors Andrew Steptoe, Roger Jones, Peter Bower, Helen Lester and Christopher Dickens who provided independent Programme Steering Committee advice over the course of the programme.
The authors are grateful for the support and assistance of the PCRN-GL (Professors Gillian Rowlands and Brendan Delaney) and Dr Jo Burns in recruiting practices to the UPBEAT-UK study and to the 33 practices that kindly provided assistance with recruitment and ongoing support for the cohort and pilot RCT study. We would also like to thank the GPs and PNs from south London who agreed to be interviewed for the staff qualitative study and the 30 patients and their practices in south-east and south-west London for their help with the patient qualitative study. Above all, we would like to thank the 803 patients who agreed to be interviewed over 4 years, the 81 patients who took part in the pilot study and those who agreed to be interviewed for the qualitative studies.
We are particularly grateful to the UPBEAT-UK administrative staff: Julie Smith for overseeing the administration of UPBEAT-UK throughout the 6 years and Louise Rolland, who has joined in the last year and provided invaluable support for committees, oversight of financial statements and production of the final report.
Contributions of authors
André Tylee (Professor, academic general practice) was the chief investigator and was responsible for the design, conduct, supervision of entire programme team, interpretation of analysis and dissemination, drafting relevant chapters and co-ordination of the report including final draft after comments.
Elizabeth A Barley (Senior Lecturer, nursing) was responsible for the design, conduct, analysis and dissemination of the metasynthesis and qualitative study with professionals. Design, conduct as nurse care manager, interpretation of analysis and dissemination of pilot RCT. She was the UPBEAT-UK programme co-ordinator (last 2 years), overall interpretation of analysis and dissemination, drafting relevant chapters and commenting on full drafts.
Paul Walters (Consultant Psychiatrist, epidemiology) was responsible for the design, conduct, supervision of research assistants and trial nurses, analysis of cohort study and overall interpretation of other analyses and dissemination, drafting relevant chapters and commenting on full drafts. He was the UPBEAT-UK programme co-ordinator (first 4 years).
Evanthia Achilla (Research Fellow, health economics) was responsible for the design, conduct, analysis dissemination and drafting of the cost-effectiveness analysis.
Rohan Borschmann (Research Fellow, clinical psychology) was responsible for the interpretation of analysis and dissemination, editing of the report and commenting on relevant chapters.
Morven Leese (Emeritus Reader, biostatistics) was responsible for the design, conduct of statistical analysis and dissemination of results, drafting relevant chapters and commenting on full drafts.
Paul McCrone (Professor, health economics) was responsible for the design, conduct, analysis, dissemination and drafting of the cost-effectiveness analysis. He also commented on full drafts.
Jorge Palacios (Clinical Research Fellow, epidemiology) was responsible for the design, conduct, interpretation of analysis and dissemination of results. He also drafted and commented on relevant chapters.
Alison Smith (Research Fellow, social science) was responsible for the conduct of interviews, data collection, dissemination and commenting on relevant chapters.
Rosemary Simmonds (Research Associate) was responsible for the design, conduct and analysis of the qualitative study with patients. She also commented on relevant chapters.
Diana Rose (Professor, user-led research) was responsible for the design, conduct, analysis and oversight of the qualitative study with patients. She also commented on relevant chapters.
Joanna Murray (Senior Lecturer, health services and population research) was responsible for the design, conduct, analysis and oversight of the qualitative study with professionals. She also commented on relevant chapters.
Harm van Marwijk (Associate Professor, academic general practice) was responsible for the interpretation of the analysis, dissemination and commenting on full drafts.
Paul Williams (Research Fellow, biostatistics) was responsible for the design, conduct of the statistical analysis and dissemination of results. He also drafted and commented on relevant chapters.
Anthony Mann (Professor, epidemiological psychiatry) was responsible for the design, conduct, supervision of research assistants and trial nurses, interpretation of the analysis and dissemination of results, drafting relevant chapters, commenting on full drafts and co-ordination of report revisions.
Publications
Tylee A, Ashworth M, Barley E, Brown J, Chambers J, Farmer A, et al. UP-BEAT UK: a programme of research into the relationship between coronary heart disease and depression in primary care patients. BMC Fam Pract 2011;12:38.
Barley EA, Murray J, Walters P, Tylee A. Managing depression in primary care: a meta-synthesis of qualitative and quantitative research from the UK to identify barriers and facilitators. BMC Fam Pract 2011;12:47.
Barley EA, Walters P, Tylee A, Murray J. General practitioners’ and practice nurses’ views and experience of managing depression in coronary heart disease: a qualitative interview study. BMC Fam Pract 2012;13:1.
Barley EA, Haddad M, Simmonds R, Fortune Z, Walters P, Murray J, et al. The UPBEAT depression and coronary heart disease programme: using the UK Medical Research Council framework to design a nurse-led complex intervention for use in primary care. BMC Fam Pract 2012;13:119.
Tylee A, Haddad M, Barley E, Ashworth M, Brown J, Chambers J, et al. A pilot randomised controlled trial of personalised care for depressed patients with symptomatic coronary heart disease in South London general practices: the UPBEAT-UK RCT protocol and recruitment. BMC Psychiatry 2012;12:58.
Haddad M, Walters P, Phillips R, Tsakok J, Williams P, Mann A, et al. Detecting depression in patients with coronary heart disease: a diagnostic evaluation of the PHQ-9 and HADS-D in primary care, findings from the UPBEAT-UK study. PLOS ONE 2013;8:e78493.
Simmonds RL, Tylee A, Walters P, Rose D. Patients’ perceptions of depression and coronary heart disease: a qualitative UPBEAT-UK study. BMC Fam Pract 2013;14:38.
Rowlands GP, Mehay A, Hampshire S, Phillips R, Williams P, Mann A, et al. Characteristics of people with low health literacy on coronary heart disease GP registers in South London: a cross-sectional study. BMJ Open 2013;3:e001503.
Smith A, Fortune Z, Phillips R, Walters P, Lee GA, Mann A, et al. UPBEAT study patients’ perceptions of the effect of coronary heart disease on their lives: a cross-sectional sub-study. Int J Nurs Stud 2011;51:1500–6.
Lawton R, Seed P, Kordowicz M, Schofield P, Tylee A, Ashworth M. Using a patient-generated mental-health measure ‘PSYCHLOPS’ to explore problems in patients with coronary heart disease. Br J Gen Pract 2014;64:e354–63.
Barley EA, Walters P, Haddad M, Phillips R, Achilla E, McCrone P, et al. The UPBEAT nurse-delivered personalised care intervention for people with coronary heart disease who report current chest pain and depression: a randomised controlled pilot study. PLOS ONE 2014;9:e98704.
Walters P, Barley EA, Mann A, Phillips R, Tylee A. Depression in primary care patients with coronary heart disease: baseline findings from the UPBEAT UK Study. PLOS ONE 2014;9:e98342.
Data sharing statement
All available data can be obtained by contacting the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health.
References
- Tylee A, Ashworth M, Barley E, Brown J, Chambers J, Farmer A, et al. Up-BEAT UK: a programme of research into the relationship between coronary heart disease and depression in primary care patients. BMC Fam Pract 2011;12. http://dx.doi.org/10.1186/1471-2296-12-38.
- Barley EA, Haddad M, Simmonds R, Fortune Z, Walters P, Murray J, et al. The UPBEAT depression and coronary heart disease programme: using the UK Medical Research Council framework to design a nurse-led complex intervention for use in primary care. BMC Fam Pract 2012;13. http://dx.doi.org/10.1186/1471-2296-13-119.
- Haddad M, Walters P, Phillips R, Tsakok J, Williams P, Mann A, et al. Detecting depression in patients with coronary heart disease: a diagnostic evaluation of the PHQ-9 and HADS-D in primary care, findings from the UPBEAT-UK study. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0078493.
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLOS Med 2006;3. http://dx.doi.org/10.1371/journal.pmed.0030442.
- Davidson KW, Kupfer DJ, Bigger JT, Califf RM, Carney RM, Coyne JC, et al. Assessment and treatment of depression in patients with cardiovascular disease: National Heart, Lung, and Blood Institute Working Group Report. Psychosom Med 2006;68:645-50. http://dx.doi.org/10.1097/01.psy.0000233233.48738.22.
- Wang J, Hoffman B, Blumenthal J. Management of depression in patients with coronary heart disease: association, mechanisms, and treatment implications. Expert Opin Pharmacother 2011;12:85-98. http://dx.doi.org/10.1517/14656566.2010.513701.
- Rutledge T, Redwine L, Linke S, Mills P. A meta-analysis of mental health treatments and cardiac rehabilitation for improving clinical outcomes and depression among patients with coronary heart disease. Psychosom Med 2013;75:335-49. http://dx.doi.org/10.1097/PSY.0b013e318291d798.
- Walford H, Ramsay L, Soljak M, Majeed A. CVD Prevalence Modelling Briefing Document. London: Eastern Region Public Health Observatory and Imperial College London; 2011.
- Layard G. The Depression Report. A New Deal for Depression and Anxiety Disorders. London: London School of Economics and Political Science, Centre for Economic Performance, Mental Health Policy Group; 2006.
- RCGP Curriculum 2010, Statement 3.10 Care of People with Mental Health Problems. London: Royal College of General Practitioners; 2010.
- Katon W, Von Korff M, Lin E, Simon G, Ludman E, Bush T, et al. Improving primary care treatment of depression among patients with diabetes mellitus: the design of the pathways study. Gen Hosp Psychiatry 2003;25:158-68. http://dx.doi.org/10.1016/S0163-8343(03)00013-6.
- Katon W, Lin E, Von Korff M, Ciechanowski P, Ludman E, Young B, et al. Collaborative care for patients with depression and chronic illnesses. N Eng J Med 2010;363:2611-20. http://dx.doi.org/10.1056/NEJMoa1003955.
- Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146538 participants in 54 observational studies. Eur Heart J 2006;27:2763-74. http://dx.doi.org/10.1093/eurheartj/ehl338.
- Nemeroff C, Goldschmidt-Clermont P. Heartache and heartbreak – the link between depression and cardiovascular disease. Nat Rev Cardiol 2012;9:526-39. http://dx.doi.org/10.1038/nrcardio.2012.91.
- Lichtman J, Froelicher E, Blumenthal J, Carney R, Doering L, Frasure-Smith N, et al. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American Heart Association. Circulation 2014;129:1350-69. http://dx.doi.org/10.1161/CIR.0000000000000019.
- Tylee A, Freeling P, Kerry S, Burns T. How does the content of consultations affect the recognition by general practitioners of major depression in women?. Br J Gen Pract 1995;45:575-8.
- Volkers A, Nuyen J, Verhaak P, Schellevis F. The problem of diagnosing major depression in elderly primary care patients. J Affect Disord 2004;82:259-63. http://dx.doi.org/10.1016/j.jad.2003.11.003.
- Depression in Adults with a Chronic Physical Health Problem: Treatment and Management. London: National Institute for Health and Care Excellence; 2009.
- Nejtek VA, Brown ES, Khan DA, Moore JJ, Wagner JV, . Prevalence of mood disorders and relationship to asthma severity in patients at an inner-city asthma clinic. Ann Allergy Asthma Immunol 2001;87:129-33. http://dx.doi.org/10.1016/S1081-1206(10)62206-5.
- Bruce TO. Comorbid depression in rheumatoid arthritis: pathophysiology and clinical implications. Curr Psychiatry Rep 2008;10:258-64. http://dx.doi.org/10.1007/s11920-008-0042-1.
- NHS Centre for Reviews and Dissemination . Improving the recognition and management of depression in primary care. Effective Health Care Bull 2002;7:1-11.
- Hyde J, Calnan M, Prior L, Lewis G, Kessler D, Sharp D. A qualitative study exploring how GPs decide to prescribe anti-depressants. Br J Gen Pract 2005;55:755-62.
- Macdonald S, Morrision J, Maxwell M, Munoz-Arroyo R, Power A, Smith M, et al. ‘A coal-face option’: GPs’ persectives on the rise in antidepressant prescribing. Br J Gen Pract 2009;59:e299-307. http://dx.doi.org/10.3399/bjgp09X454106.
- Noblit GW, Hare RD. Meta-ethnography: Synthesizing Qualitative Studies. London: Sage Publications; 1988.
- Von Korff M, Goldberg D. Improving outcomes in depression: the whole process of care needs to be enhanced. BMJ 2001;7319:948-9. http://dx.doi.org/10.1136/bmj.323.7319.948.
- Mental and Neurological Disorder – Factsheet 265. Geneva: World Health Organisation; 2001.
- Afari N, Schmaling KB, Barnhart S, Buchwald D. Psychiatric comorbidity and functional status in adult patients with asthma. J Clin Psychol Med Settings 2001;8:245-52. http://dx.doi.org/10.1023/A:1011912712262.
- Brown ES, Vigil L, Khan DA, Liggin JDM, Carmody TJ, Rush AJ. A randomized trial of citalopram versus placebo in outpatients with asthma and major depressive disorder: a proof of concept study. Biol Psychiatry 2005;58:865-70. http://dx.doi.org/10.1016/j.biopsych.2005.04.030.
- Richards D, Hill J, Gask L, Lovell K, Chew-Graham C, Bower P, et al. Clinical effectiveness of collaborative care for depression in UK primary care (CADET): cluster randomised controlled trial. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f4913.
- Craig P, Dieppe P, Macintyre S, Mitchie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:979-83. http://dx.doi.org/10.1136/bmj.a1655.
- Barley E, Murray J, Walters P, Tylee A. Managing depression in primary care: a meta-synthesis of qualitative and quantitative research from the UK to identify barriers and facilitators. BMC Fam Pract 2011;12. http://dx.doi.org/10.1186/1471-2296-12-47.
- National Service Framework: Mental Health. London: Department of Health; 1999.
- 10 Questions to Help You Make Sense Qualitative of Research. Oxford: Public Health Resource Unit; 2006.
- von Elm E, Altman D, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. PLOS Med 2007;4. http://dx.doi.org/10.1371/journal.pmed.0040296.
- Feder GS, Hutson M, Ramsay J, Taket A. Women exposed to intimate partner violence: a meta-analysis of qualitative studies. Arch Intern Med 2006;166:22-37. http://dx.doi.org/10.1001/archinte.166.1.22.
- Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, et al. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. A Product from the ESRC Methods Programme 2006. www.lancaster.ac.uk/shm/research/nssr/research/dissemination/publications.php (accessed 2 May 2015).
- Johnston O, Kumar S, Kendall K, Peveler R, Gabbay J, Kendrick T. Qualitative study of depression management in primary care: GP and patient goals, and the value of listening. Br J Gen Pract 2007;57:872-9. http://dx.doi.org/10.3399/096016407782318026.
- Murray J, Banerjee S, Byng R, Tylee A, Bhugra D, Macdonald A, et al. Primary care professionals’ perceptions of depression in older people: a qualitative study. Soc Sci Med 2006;63:1363-73. http://dx.doi.org/10.1016/j.socscimed.2006.03.037.
- Burroughs H, Lovell K, Morley M, Baldwin R, Burns A, Chew-Graham C. ‘Justifiable depression’: how primary care professionals and patients view late-life depression? A qualitative study. Fam Pract 2006;23:369-77. http://dx.doi.org/10.1093/fampra/cmi115.
- Maxwell M. Women’s and doctors’ accounts of their experiences of depression in primary care: the influence of social and moral reasoning on patients’ and doctors’ decisions. Chronic Illn 2005;1:61-7. http://dx.doi.org/10.1177/17423953050010010401.
- Pollock K, Grime J. GPs’ perspectives on managing time in consultations with patients suffering from depression: a qualitative study. Fam Pract 2003;20:262-9. http://dx.doi.org/10.1093/fampra/cmg306.
- Chew-Graham CA, Mullin S, May CR, Hedley S, Cole H. Managing depression in primary care: another example of the inverse care law?. Fam Pract 2002;19:632-7. http://dx.doi.org/10.1093/fampra/19.6.632.
- Rogers A, May C, Oliver D. Experiencing depression, experiencing the depressed: the separate worlds of patients and doctors. J Ment Health 2001;10:317-33. http://dx.doi.org/10.1080/09638230020023840.
- Kendrick T, King F, Albertella L, Smith PWF. GP treatment decisions for patients with depression. Br J Gen Pract 2005;55:280-6.
- Shiels C, Gabbay M, Dowrick C, Hulbert C. Depression in men attending a rural general practice: factors associated with prevalence of depressive symptoms and diagnosis. Br J Psychiatry 2004;185:239-44. http://dx.doi.org/10.1192/bjp.185.3.239.
- Naji SA, Gibb J, Hamilton RJ, Lawton K, Palin AN, Eagles JM. How ready are practice nurses to participiate in the identification and management of depressed patients in primary care?. Primary Care Mental Health 2004;2:47-54.
- Manning C, Marr J. ‘Real-life burden of depression’ surveys – GP and patient perspectives on treatment and management of recurrent depression. Curr Med Res Opin 2003;19:526-31. http://dx.doi.org/10.1185/030079903125002117.
- Byng R, Weaver L, Bury C. GPs’ beliefs about their management of depression and needs for supporting change in practice. Primary Care Psychiatry 2003;8:121-5. http://dx.doi.org/10.1185/135525703125002153.
- Oladinni O. A survey of inner London general practitioners’ attitudes towards depression. Primary Care Psychiatry 2002;8:95-8. http://dx.doi.org/10.1185/135525702125001227.
- Telford R, Hutchinson A, Jones R, Rix S, Howe A. Obstacles to effective treatment of depression: a general practice perspective. Fam Pract 2002;19:45-52. http://dx.doi.org/10.1093/fampra/19.1.45.
- Rothera I, Jones R, Gordon C. An examination of the attitudes and practice of general practitioners in the diagnosis and treatment of depression in older people. Int J Geriatr Psychiatry 2002;17:354-8. http://dx.doi.org/10.1002/gps.603.
- Dowrick C, Gask L, Perry R, Dixon C, Usherwood T. Do general practitioners’ attitudes towards depression predict their clinical behaviour?. Psychol Med 2000;30:413-19. http://dx.doi.org/10.1017/S0033291799001531.
- Livingston G, Yard P, Beard A, Katona C. A nurse-coordinated educational initiative addressing primary care professionals’ attitudes to problem-solving in depression in older people – a pilot study. Int J Geriatr Psychiatry 2000;15:401-5. http://dx.doi.org/10.1002/(SICI)1099-1166(200005)15:5<401::AID-GPS121>3.0.CO;2-X.
- Hegarty K, Gunn J, Blashki G, Griffiths F, Dowell T, Kendrick T, et al. How could depression guidelines be made more relevant and applicable to primary care? A quantitative and qualitative review of national guidelines. Br J Gen Pract 2009;59:e149-56. http://dx.doi.org/10.3399/bjgp09X420581.
- Britten N, Campbell R, Pope C, Donovan J, Morgan M, Pill R. Using meta-ethnography to synthesise qualitative research: a worked example. J Health Serv Res Policy 2002;7:209-15. http://dx.doi.org/10.1258/135581902320432732.
- Revisions to the GMS Contract, 2006/7: Delivering Investment in General Practice. London: British Medical Association; 2006.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. http://dx.doi.org/10.1191/1478088706qp063oa.
- Glaser BG. Theoretical sensitivity: advances in the methodology of grounded theory. Mill Valley, CA: Sociology Press; 1978.
- Barley EA, Walters P, Tylee A, Murray JM. General practitioners’ and practice nurses’ views and experience of managing depression in coronary heart disease: a qualitative interview study. BMC Fam Pract 2012;13. http://dx.doi.org/10.1186/1471-2296-13-1.
- Dowrick C, Leydon GM, McBride A, Howe A, Burgess H, Clarke P, et al. Patients’ and doctors’ views on depression severity questionnaires incentivised in UK quality and outcomes framework: qualitative study. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b663.
- Serebruany VL, Suckow RF, Cooper TB, O’Connor CM, Malinin AI, Krishnan KR, et al. Sertraline antidepressant heart attack randomized trial. Relationship between release of platelet/endothelial biomarkers and plasma levels of sertraline and N-desmethylsertraline in acute coronary syndrome patients receiving SSRI treatment for depression. Am J Psychiatry 2005;162:1165-70. http://dx.doi.org/10.1176/appi.ajp.162.6.1165.
- Clarke DM. Implementing NICE guidelines for the psychological treatment of depression and anxiety disorders: the IAPT experience. Int Rev Psychiatry 2011;23:318-27. http://dx.doi.org/10.3109/09540261.2011.606803.
- Our Health, Our Care, Our Say: A New Direction for Community Services. London: Deparment of Health; 2006.
- Michie S, Rumsey N, Fussell A, Hardeman W, Johnston M, Newman S, et al. Improving Health: Changing Behaviour. NHS Trainer Handbook. London: Department of Health; 2008.
- National Collaborating Centre for Mental Health . Management of Depression in Primary and Secondary Care National Clinical Practice Guideline Number 23 2004. www.nice.org.uk/nicemedia/pdf/CG023fullguideline.pdf (accessed 15 July 2013).
- Petrie KJ, Weinman J. Why illness perceptions matter. Clin Med 2006;6:536-9. http://dx.doi.org/10.7861/clinmedicine.6-6-536.
- Rollnick S, Mason P, Butler C. Health Behaviour Change: A Guide for Practitioners. London: Churchill Livingstone; 1999.
- David L. Using CBT in General Practice: The 10 Minute Consultation. Oxfordshire: Scion Publishing; 2006.
- Mynors-Wallis L. Problem-Solving Treatment for Anxiety and Depression: A Practical Guide. Oxford and New York: Oxford University Press; 2005.
- Petrie KJ, Cameron LD, Ellis CJ, Buick D, Weinman J. Changing illness perceptions after myocardial infarction: an early intervention randomized controlled trial. Psychosom Med 2002;64:580-6. http://dx.doi.org/10.1097/00006842-200207000-00007.
- Jackson S. Sexual response in cardiovascular disease. J Sex Res 2009;46:233-6. http://dx.doi.org/10.1080/00224490902747693.
- Campbell NC, Ritchie LD, Thain J, Deans HG, Rawles JM, Squair JL. Secondary prevention in coronary heart disease: a randomised trial of nurse led clinics in primary care. Heart 1998;80:447-52. http://dx.doi.org/10.1136/hrt.80.5.447.
- Fahey T, Schroeder K, Ebrahim S, Glynn L. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev 2006;4. http://dx.doi.org/10.1002/14651858.cd005182.pub2.
- Kendrick T, Dowrick C, McBride A, Howe A, Clarke P, Maisey S, et al. Management of depression in UK general practice in relation to scores on depression severity questionnaires: analysis of medical record data. BMJ n.d.;338. http://dx.doi.org/10.1136/bmj.b750.
- Thornicroft G. Shunned: Discrimination Against People with Mental Illness. Oxford: Oxford University Press; 2006.
- Engel G. The need for a new medical model: a challenge for biomedicine. Science 1977;196:129-36. http://dx.doi.org/10.1126/science.847460.
- Borrell-Carrio F, Suchman AL, Epstein RM. The biopsychosocial model 25 years later: principles, practice, and scientific inquiry. Ann Fam Med 2004;2:576-82. http://dx.doi.org/10.1370/afm.245.
- Carney RM, Blumenthal JA, Freedland KE, Youngblood M, Veith RC, Burg MM, et al. Depression and late mortality after myocardial infarction in the Enhancing Recovery in Coronary Heart Disease (ENRICHD) study. Psychosom Med 2004;66:466-74. http://dx.doi.org/10.1097/01.psy.0000133362.75075.a6.
- Taylor CB, Youngblood ME, Catellier D, Veith RC, Carney RM, Burg MM, et al. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry 2005;62:792-8. http://dx.doi.org/10.1001/archpsyc.62.7.792.
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care 2003;41:1284-92. http://dx.doi.org/10.1097/01.MLR.0000093487.78664.3C.
- Miles M, Huberman A. Qualitative Data Analysis: An Expanded Sourcebook. Thousand Oaks, CA: Sage; 1994.
- Glaser BG, Strauss AL. The Discovery of Grounded Theory: Strategies for Qualitative Research. Chicago, IL: Aldine Transaction; 1967.
- Priest R, Vize C, Roberts A, Roberts M, Tylee A. Lay people’s attitudes to treatment of depression: results of opinion poll for Defeat Depression Campaign just before its launch. BMJ 1996;313:858-9. http://dx.doi.org/10.1136/bmj.313.7061.858.
- Davidson P, Dracup K, Phillips J, Daly J, Padilla G. Preparing for the worst while hoping for the best: the relevance of hope in the heart failure illness trajectory. J Cardiovasc Nurs 2007;22:159-65. http://dx.doi.org/10.1097/01.JCN.0000267821.74084.72.
- Simmonds RL, Tylee A, Walters P, Rose D. Patients’ perceptions of depression and coronary heart disease: a qualitative UPBEAT-UK study. BMC Fam Pract 2013;14. http://dx.doi.org/10.1186/1471-2296-14-38.
- Alderson SL, Foy R, Glidewell L, House AO. Patients understanding of depression associated with chronic physical illness: a qualitative study. BMC Fam Pract 2014;15:1-19. http://dx.doi.org/10.1186/1471-2296-15-37.
- Parkes C, Benjamin B, Fitzgerald R. Broken heart: a statistical study of increased mortality among widowers. Br Med J 1969;1:740-3. http://dx.doi.org/10.1136/bmj.1.5646.740.
- Charmaz K, Sabo D, Gordon D. Men’s Health and Illness: Gender, Power and the Body. Thousand Oaks, CA: Sage Publications; 1995.
- Arney W, Bergen B. Medicine and the Management of Living: Taming the Last Great Beast. Chicago, IL: University of Chicago Press; 1984.
- Barker P, Foucault M. Subversions of the Subject. Hertfordshire: Harvester Wheatsheaf; 1993.
- Silverman D. Discourses of Counselling. HIV Counselling as Social Interaction. London: Sage; 1997.
- Kendall G, Wickham G. Using Foucault’s Methods. London: Sage; 1999.
- Cronin E, Campbell S, Ashworth M, Hann M, Blashki G, Murray J, et al. A tale of two systems: perceptions of primary care for depression in London and Melbourne. Fam Pract 2009;26:210-20. http://dx.doi.org/10.1093/fampra/cmp017.
- Grace S, Abbey S, Ruxandra P, Shnek Z, Irvine J, Stewart D. Longitudinal course of depressive symptomatology after a cardiac event: effects of gender and cardiac rehabilitation. Psychosom Med 2005;67:52-8. http://dx.doi.org/10.1097/01.psy.0000151486.28349.70.
- Rees K, Bennett P, West R, Davey SG, Ebrahim S. Psychological interventions for coronary heart disease. Cochrane Database of Syst Rev 2004;2. http://dx.doi.org/10.1002/14651858.cd002902.pub2.
- Baumeister H, Hutter N, Bengel J. Psychological and pharmacological interventions for depression in patients with coronary artery disease. Cochrane Database Syst Rev 2011;9. http://dx.doi.org/10.1002/14651858.CD008012.pub3.
- Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med 2006;166:2314-21. http://dx.doi.org/10.1001/archinte.166.21.2314.
- Improving Chronic Disease Management. London: Department of Health; 2004.
- Hamer M, Molloy G, Stamatakis E. Psychological distress as a risk factor for cardiovascular events: pathophysiological and behavioral mechanisms. J Am Coll Cardiol 2008;52:2156-62. http://dx.doi.org/10.1016/j.jacc.2008.08.057.
- Whooley MA, de Jonge P, Vittinghoff E, Otte C, Moos R, Carney RM, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA 2008;300:2379-88. http://dx.doi.org/10.1001/jama.2008.711.
- Stead L, Bergson G, Lancaster T. Physician advice for smoking cessation. Cochrane Database Syst Rev 2008;2. http://dx.doi.org/10.1002/14651858.cd000165.pub3.
- Kaner E, Beyer F, Dickinson H, Pienaar E, Campbell F, Schlesinger C, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev 2007;2. http://dx.doi.org/10.1002/14651858.cd004148.pub3.
- Healthy Lives, Healthy People. London: Department of Health; 2011.
- Reilly S, Hughes J, Challis D. Case management for long-term conditions: implementation and processes. Ageing Soc 2010;30:125-55. http://dx.doi.org/10.1017/S0144686X09990183.
- Spanou C, Simpson S, Hood K, Edwards A, Cohen D, Rollnick S, et al. Preventing disease through opportunistic, rapid engagement by primary care teams using behaviour change counselling (PRE-EMPT): protocol for a general practice-based cluster randomised trial. BMC Fam Pract 2010;11. http://dx.doi.org/10.1186/1471-2296-11-69.
- Bodenheimer T, Handley MA. Goal-setting for behavior change in primary care: an exploration and status report. Patient Educ Couns 2009;76:174-80. http://dx.doi.org/10.1016/j.pec.2009.06.001.
- Independence, Well-being and Choice: Our Vision for the Future of Social Care for Adults in England. London: Department of Health; 2009.
- Common Assessment Framework for Adults: A Consultation on Proposals to Improve Information Sharing Around Multi-Disciplinary Assessment and Care Planning. London: Department of Health; 2009.
- Smith S, Murchie P, Devereux G, Jahnston M, Lee A, Macleod U, et al. Developing a complex intervention to reduce time to presentation with symptoms of lung cancer. Br J Gen Pract 2012;62:605-15. http://dx.doi.org/10.3399/bjgp12X654579.
- Barley EA, Walters P, Haddad M, Phillips R, Achilla E, McCrone P, et al. The UPBEAT nurse-delivered personalized care intervention for people with coronary heart disease who report current chest pain and depression: a randomised controlled pilot study. PLOS ONE 2014;9. http://dx.doi.org/10.1371/journal.pone.0098704.
- Tylee A, Haddad M, Barley E, Ashworth M, Brown J, Chambers J, et al. A pilot randomised controlled trial of personalised care for depressed patients with symptomatic coronary heart disease in south London general practices: the UPBEAT-UK RCT protocol and recruitment. BMC Psychiatry 2012;12. http://dx.doi.org/10.1186/1471-244X-12-58.
- Quality and Outcomes Framework Achievement Data 2008/09. West Yorkshire: Health and Social Care Information Centre; 2009.
- Rose G. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ 1962;27:645-58.
- Zigmund AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. http://dx.doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Neighbourhoods Statistical Release: The English Indices of Deprivation 2010. London: Department for Communities and Local Government; 2011.
- Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. http://dx.doi.org/10.1046/j.1525-1497.2001.016009606.x.
- Tennant R, Hiller L, Fishwick R, Platt S, Joseph S, Weich S, et al. The Warwick-Edinburgh Mental Well-being Scale (WEMWBS): development and UK validation. Health Qual Life Outcomes 2007;5. http://dx.doi.org/10.1186/1477-7525-5-63.
- Ware J, Kosinski M, Keller SD. A 12-Item Short-Form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. http://dx.doi.org/10.1097/00005650-199603000-00003.
- Goldman L, Hashimoto B, Cook EF, Loscalzo A. Comparative reproducibility and validity of systems for assessing cardiovascular functional class: advantages of a new specific activity scale. Circulation 1981;64:1227-34. http://dx.doi.org/10.1161/01.CIR.64.6.1227.
- Corney RH, Clare AW. The construction, development and testing of a self-report questionnaire to identify social problems. Psychol Med 1985;15:637-49. http://dx.doi.org/10.1017/S0033291700031494.
- Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986;24:67-74. http://dx.doi.org/10.1097/00005650-198601000-00007.
- Ashworth M, Shepherd M, Christey J, Matthews V, Wright K, Parmentier H, et al. A patient-centred psychometric instrument: the development of ‘PSYCHLOPS’ (‘Psychological Outcome Profiles’). Counsell Psychother Res 2004;4:27-31. http://dx.doi.org/10.1080/14733140412331383913.
- Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res 2006;60:631-7. http://dx.doi.org/10.1016/j.jpsychores.2005.10.020.
- Schwarzer R, Jerusalem M, Weinman J, Wright S, Johnston M. Measures in Health Psychology: A User’s Portfolio Causal and Control Beliefs. Windsor: NFER-Nelson; 1995.
- Hurst NP, Kind P, Ruta D, Hunter M, Stubbings A. Measuring health-related quality of life in rheumatoid arthritis: validity, responsiveness and reliability of EuroQOL (EQ-5D). Br J Rheumatol 1997;36:551-9. http://dx.doi.org/10.1093/rheumatology/36.5.551.
- Fenwick E, Byford S. A guide to cost-effectiveness acceptability curves. Br J Psychiatry 2005;187:106-8. http://dx.doi.org/10.1192/bjp.187.2.106.
- Beecham J, Knapp M, Thornicroft G. Measuring Mental Health Needs. London: Gaskell; 2001.
- NHS Reference Costs 2009–2010. London: Department of Health; 2011.
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: Personal Social Services Research Unit, University of Kent; 2010.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2010.
- Prescription Cost Analysis: England 2010. The Health and Social Care Information Centre; 2011.
- Statistical Bulletin: 2010 Annual Survey of Hours and Earnings. UK: Office for National Statistics; 2010.
- Buszewicz M, Griffin M, McMahon E, Beecham J, King M. Evaluation of a system of structured, pro-active care for chronic depression in primary care: a randomised controlled trial. BMC Psychiatry 2010;10. http://dx.doi.org/10.1186/1471-244X-10-61.
- Bennett M, Walters K, Drennan V, Buszewicz M. Structured pro-active care for chronic depression by practice nurses in primary care: a qualitative evaluation. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0075810.
- Koopmanschap M, van Exel J, van den Berg B, Brouwer W. An overview of methods and applications to value informal care in economic evaluations of healthcare. Pharmacoeconomics 2008;26:269-80. http://dx.doi.org/10.2165/00019053-200826040-00001.
- Dickens C, Cherrington A, Adeyemi I, Roughley K, Bower P, Garrett C, et al. Characteristics of psychological interventions that improve depression in people with coronary heart disease: a systematic review and meta-regression. Psychosom Med 2013;75:211-21. http://dx.doi.org/10.1097/PSY.0b013e31827ac009.
- Gerrits M, Vogelzangs N, van Oppen P, van Marwijk H, van Horst H, Penninx B. Impact of pain on the course of depressive and anxiety disorders. Pain 2012;153:429-36. http://dx.doi.org/10.1016/j.pain.2011.11.001.
- Penninx B, Nolen WJH, Lamers WA, Zitman F, Smit FG, Spinhoven JH, et al. Two-year course of depressive and anxiety disorders: results from the Netherlands Study of Depression and Anxiety (NESDA). J Affect Disord 2011;133:76-85. http://dx.doi.org/10.1016/j.jad.2011.03.027.
- Kennedy A, Bower P, Reeves D, Blakeman T, Bowen R, Chew-Graham C, et al. Implementation of self management support for long term conditions in routine primary care settings: cluster randomised controlled trial. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f2882.
- Health and Social Care Information Centre . Hospital Episode Statistics 2013 n.d. www.hscic.gov.uk/hes (accessed 4 March 2016).
- Lenfant C. Chest pain of cardiac and noncardiac origin. Metabolism 2010;59:S41-6. http://dx.doi.org/10.1016/j.metabol.2010.07.014.
- Kisely S, Campbell L, Yelland M, Paydar A. Psychological interventions for symptomatic management of non-specific chest pain in patients with normal coronary anatomy. Cochrane Database Syst Rev 2012;6. http://dx.doi.org/10.1002/14651858.cd004101.pub4.
- Bhattacharyya MR, Whitehead DL, Rakhit R, Steptoe A. Depressed mood, positive affect, and heart rate variability in patients with suspected coronary artery disease. Psychosom Med 2008;70:1020-7. http://dx.doi.org/10.1097/PSY.0b013e318189afcc.
- Butler C, Simpson S, Hood K, Cohen D, Pickles T, Spanou C, et al. Training practitioners to deliver opportunistic multiple behaviour change counselling in primary care: a cluster randomised trial. BMJ 2013;19. http://dx.doi.org/10.1136/bmj.f1191.
- Noordman J, Koopmansa B, Korevaara JC, van der Weijdenb T, van Dulmen S. Exploring lifestyle counselling in routine primary care consultations: the professionals’ role. Fam Pract 2013;30:332-40. http://dx.doi.org/10.1093/fampra/cms077.
- Morgan M, Dunbar J, Reddy P, Coates M, Leahy R. The TrueBlue study: is practice nurse-led collaborative care effective in the management of depression for patients with heart disease or diabetes. BMC Fam Pract 2009;10. http://dx.doi.org/10.1186/1471-2296-10-46.
- Aragonès E, Lluís Piñol J, Caballero A, López-Cortacans G, Casaus P, Maria Hernández J, et al. Effectiveness of a multi-component programme for managing depression in primary care: a cluster randomized trial. The INDI project. J Affect Disord 2012;142:297-305. http://dx.doi.org/10.1016/j.jad.2012.05.020.
- Richards DA, Lovell K, Gilbody S, Gask L, Torgerson D, Barkham M, et al. Collaborative care for depression in UK primary care: a randomized controlled trial. Psychol Med 2008;38:279-87. http://dx.doi.org/10.1017/S0033291707001365.
- Sharp D, Chew-Graham C, Tylee A, Lewis G, Howard L, Anderson I, et al. A pragmatic randomised controlled trial to compare antidepressants with a community-based psychosocial intervention for the treatment of women with postnatal depression: the RESPOND trial. Health Technol Assess 2010;14. http://dx.doi.org/10.3310/hta14430.
- Cooke RA, Smeeton N, Chambers JB. Comparative study of chest pain characteristics in patients with normal and abnormal coronary angiograms. Heart 1997;78:142-6. http://dx.doi.org/10.1136/hrt.78.2.142.
- Goldberg D, Williams P. A User’s Guide to the General Health Questionnaire. Windsor: NFER-Nelson; 1988.
- Lewis G. Assessing psychiatric disorder with a human interviewer or a computer. J Epidemiol Community Health 1994;48:207-10. http://dx.doi.org/10.1136/jech.48.2.207.
- Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med 2001;33:337-43. http://dx.doi.org/10.3109/07853890109002087.
- Brugha TS, Cragg D. The list of threatening experiences: the reliability and validity of a brief life events questionnaire. Acta Psychiatr Scand 1990;82:77-81. http://dx.doi.org/10.1111/j.1600-0447.1990.tb01360.x.
- Ashworth M, Robinson SI, Godfrey E, Shepherd M, Evans C, Seed P, et al. Measuring mental health outcomes in primary care: the psychometric properties of a new patient-generated outcome measure, ’PSYCHLOPS’(‘psychological outcome profiles’). Prim Care Mental Health 2005;3:261-70.
- Davis TC, Long SW, Jackson RH, Mayeaux EJ, George RB, Murphy PW, et al. Rapid estimate of adult literacy in medicine: a shortened screening instrument. Fam Med 1993;25:391-5.
- Graff-Iversen S, Selmer R, Løchen M-L. Rose angina predicts 23-year coronary heart disease mortality in women and men aged 40–49 years. Heart 2008;94:482-6. http://dx.doi.org/10.1136/hrt.2007.115931.
- Bodegard J, Erikssen G, Bjornholt J, Thelle D, Erikssen J. Possible angina detected by the WHO angina questionnaire in apparently healthy men with a normal exercise ECG: coronary heart disease or not? A 26 year follow up study. Heart 2004;90:627-32. http://dx.doi.org/10.1136/hrt.2003.012542.
- Cook DG, Shaper A, MacFarlane P. Using the WHO (Rose) angina questionnaire in cardiovascular epidemiology. Int J Epidemiol 1989;18:607-13. http://dx.doi.org/10.1093/ije/18.3.607.
- Owen-Smith V, Hannaford PC, Elliott AM. Increased mortality among women with Rose angina who have not presented with ischaemic heart disease. Br J Gen Pract 2003;53:784-9.
- Garber CE, Carleton RA, Heller GV. Comparison of ‘Rose Questionnaire Angina’ to exercise thallium scintigraphy: different findings in males and females. J Clin Epidemiol 1992;45:715-20. http://dx.doi.org/10.1016/0895-4356(92)90048-R.
- Lawlor D, Adamson J, Ebrahim S. Performance of the WHO Rose angina questionnaire in post-menopausal women: are all of the questions necessary?. J Epidemiol Community Health 2003;57:538-41. http://dx.doi.org/10.1136/jech.57.7.538.
- Lampe F, Whincup P, Wannamethee S, Ebrahim S, Walker M, Shaper A. Chest pain on questionnaire and prediction of major ischaemic heart disease events in men. Eur Heart J 1998;19:63-7. http://dx.doi.org/10.1053/euhj.1997.0729.
- Walters P, Barley EA, Mann A, Phillips R, Tylee A. Depression in primary care patients with coronary heart disease: baseline findings from the UPBEAT UK Study. PLOS ONE 2014;9. http://dx.doi.org/10.1371/journal.pone.0098342.
- Singer JD, Willett JB. It’s about time: using discrete-time survival analysis to study duration and the timing of events. J Educ Stat 1993;18:155-95. http://dx.doi.org/10.2307/1165085.
- Muthén BO, Masyn K. Discrete-time survival mixture analysis. J Educ Behav Stat 2005;30:27-58. http://dx.doi.org/10.3102/10769986030001027.
- Satorra A, Heijmans RDH, Pollock DSG, Satorra A. Innovations in Multivariate Statistical Analysis: A Festschrift for Heinz Neudecker. London: Kluwer Academic Publishers; 2000.
- Statistical Bulletin: Deaths registered in England and Wales (Series DR), 2011. London: Office for National Statistics; 2012.
- Murphy N, Simpson C, MacIntyre K, McAlister F, Chalmers J, McMurray J. Prevalence, incidence, primary care burden and medical treatment of angina in Scotland: age, sex and socioeconomic disparities: a population-based study. Heart 2006;92:1047-54. http://dx.doi.org/10.1136/hrt.2005.069419.
- Hemingway H, Langenberg C, Damant J, Frost C, Pyörälä K, Barrett-Connor E. Prevalence of angina in women versus men: a systematic review and meta-analysis of international variations across 31 countries. Circulation 2008;117:1526-36. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.720953.
- Maddox T, Reid K, Spertus J, Mittleman M, Krumholz H, Parashar S, et al. Angina at 1 year after myocardial infarction: prevalence and associated findings. Arch Intern Med 2008;168:1310-16. http://dx.doi.org/10.1001/archinte.168.12.1310.
- Von Korff M, Dworkin S, Le Resche L, Kruger A. An epidemiologic comparison of pain complaints. Pain 1988;32:173-83. http://dx.doi.org/10.1016/0304-3959(88)90066-8.
- Colgan S, Schofield P, Whorwell P, Bennett D, Brooks N, Jones P. Angina-like chest pain: a joint medical and psychiatric investigation. Postgrad Med J 1988;64:743-6. http://dx.doi.org/10.1136/pgmj.64.756.743.
- Tibbling L. Oesophageal dysfunction and angina pectoris in a Swedish population selected at random. Acta Med Scand Suppl 1981;644:71-4. http://dx.doi.org/10.1111/j.0954-6820.1981.tb03126.x.
- Eslick G, Jones M, Talley N. Non-cardiac chest pain: prevalence, risk factors, impact and consulting –a population-based study. Aliment Pharmacol Ther 2003;17:1115-24. http://dx.doi.org/10.1046/j.1365-2036.2003.01557.x.
- Eslick G, Coulshed D, Talley N. Review article: the burden of illness of non-cardiac chest pain. Aliment Pharmacol Ther 2002;16:1217-23. http://dx.doi.org/10.1046/j.1365-2036.2002.01296.x.
- Wilhelmsen L, Rosengren A, Hagman M, Lappas G. ‘Nonspecific’ chest pain associated with high long-term mortality: results from the primary prevention study in Göteborg, Sweden. Clin Cardiol 1998;21:477-82. http://dx.doi.org/10.1002/clc.4960210706.
- Diagnostic and Statistical Manual of Mental Disorders. Arlington, VA: American Psychiatric Association; 2000.
- Channer K, Papouchado M, James M, Rees J. Anxiety and depression in patients with chest pain referred for exercise testing. Lancet 1985;2:820-3. http://dx.doi.org/10.1016/S0140-6736(85)90805-0.
- Melville M, Lari M, Brown N, Young T, Gray D. Quality of life assessment using the short form 12 questionnaire is as reliable and sensitive as the short form 36 in distinguishing symptom severity in myocardial infarction survivors. Heart 2003;89:1445-6. http://dx.doi.org/10.1136/heart.89.12.1445.
- NHS Trusts and NHS Foundation Trusts Reference Cost Schedules 2011–12 (NSRC01). London: Department of Health; 2012.
- Barber J, Thompson S, Barber J, Thompson S. Multiple regression of cost data: use of generalised linear models. J Health Serv Res Policy 2004;9:197-204. http://dx.doi.org/10.1258/1355819042250249.
- Glick H, Doshi J, Sonnad S, Polsky D. Analyzing Cost. Economic Evaluation in Clinical Trials. New York, NY: Oxford University Press; 2007.
- Balint M. The Doctor, the Patient and the Illness. London: Pitman Medical; 1965.
- NHS Employers . GMS Contract Changes n.d. www.nhsemployers.org/your-workforce/primary-care-contacts/general-medical-services/gms-contract-changes (accessed 9 March 2015).
- Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol 2010;10. http://dx.doi.org/10.1186/1471-2288-10-1.
- Grimes DA, Schulz KF. Descriptive studies: what they can and cannot do. Lancet 2002;359:145-9. http://dx.doi.org/10.1016/S0140-6736(02)07373-7.
- Arain M, Campbell M, Cooper C, Lancaster G. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med Res Methodol 2010;10. http://dx.doi.org/10.1186/1471-2288-10-67.
- Lancaster G, Dodd S, Williamson P. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004;10:307-12. http://dx.doi.org/10.1111/j. 2002.384.doc.x.
- Dunn G. Pragmatic trials of complex psychosocial interventions: methodological challenges. Epidemiol Psychiatric Sci 2013;22:105-9. http://dx.doi.org/10.1017/S2045796013000048.
- Zwarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S, Haynes B, et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 2008;337. http://dx.doi.org/10.1136/bmj.a2390.
- Freedland KE, Mohr DC, Davidson KW, Schwartz JE. Usual and unusual care: existing practice control groups in randomized controlled trials of behavioral interventions. Psychosom Med 2011;73:323-35. http://dx.doi.org/10.1097/PSY.0b013e318218e1fb.
- Juni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ 2001;323:42-6. http://dx.doi.org/10.1136/bmj.323.7303.42.
- Mohr DC, Spring B, Freedland KE, Beckner V, Arean P, Hollon SD, et al. The selection and design of control conditions for randomized controlled trials of psychological interventions. Psychother Psychosom 2009;78:275-84. http://dx.doi.org/10.1159/000228248.
- May C, Murray E, Finch T, Mair F, Treweek S, Ballini L, et al. Normalization Process Theory On-Line Users’ Manual and Toolkit 2010. www.normalizationprocess.org (accessed 2 May 2013).
- Moser DK, McKinley S, Riegel B, Doering LV, Meischke H, Pelter M, et al. Relationship of persistent symptoms of anxiety to morbidity and mortality outcomes in patients with coronary heart disease. Psychosom Med 2011;73:803-9. http://dx.doi.org/10.1097/PSY.0b013e3182364992.
- Lawton R, Seed P, Kordowicz M, Schofield P, Tylee A, Ashworth M. Using a patient-generated mental-health measure ‘PSYCHLOPS’ to explore problems in patients with coronary heart disease. Br J Gen Pract 2014;64:e354-63. http://dx.doi.org/10.3399/bjgp14X680137.
- Rowlands GP, Mehay A, Hampshire S, Phillips R, Williams P, Mann A, et al. Characteristics of people with low health literacy on coronary heart disease GP registers in south London: a cross-sectional study. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2012-001503.
- McHorney CA, Ware JE, Raczek AE. The MOS 36-item short-form health status survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993;31. http://dx.doi.org/10.1097/00005650-199303000-00006.
Appendix 1 Search strategy for the metasynthesis of published qualitative and quantitative literature
-
exp Depression/ or depression.mp
-
depress$.mp.
-
1 AND 2
-
primary care.mp. OR Primary Health Care/
-
general practice.mp. OR Family Practice/
-
Health Personnel/
-
Medical staff/
-
Nurses/
-
general practitioners.mp. OR Physicians Family/
-
4 OR 5 OR 6 OR 7 OR 8 OR 9
-
“Attitude of Health Personnel"/ OR Attitude/ or attitude.mp.
-
belief.mp.
-
perception.mp. OR Perception/
-
11 OR 12 OR 13
-
3 AND 10 AND 14
-
limit 15 to English language and yr “2000 - 2008”
Appendix 2 Checklist devised to assess the quality of observational studies
(Answer items 1–5 ‘yes’ or ‘no’.)
Screening: was there a clear aim?
-
Was the selection of participants appropriate? (Consider source population, inclusion or exclusion criteria, methods of selection.)
-
Was the measurement of variables appropriate? (Consider validity and reliability of instruments/measures used.)
-
Was there appropriate control of bias? (Consider sources of bias, were appropriate methods outlined to deal with any issues such as recall bias, interviewer bias, non-responders, note response rate.)
-
Was the use of statistics appropriate? (Consider primary outcome stated a priori, note sample size.)
-
Was the study free of conflict of interest? (Consider declarations of conflict of interest or identification of funding sources.)
-
List any other limitations of the study.
Appendix 3 Topic guide for general practitioners and practice nurses
Context
Can you tell me a little about the practice and the population you serve?
Probes: SES [socioeconomic status], ethnicity, challenges, special interests, additional services, special interest in mental health, practice population size.
Coronary heart disease
How much of a problem is CHD in this practice?
Probes: high-risk groups; age; ethnicity; gender.
How do you manage your CHD patients?
Probes: recording, role of other professionals, frequency of visits, are there particulate difficulties in managing these patients?
Coronary heart disease and depression
Defining depression in coronary heart disease
It appears from research that many people with CHD may also be depressed. What is your experience of this?
Probes: direction of causality; which factors are important: symptoms, family, SES, gender, exercise; factors specific to CHD compared with other illnesses; do you routinely consider depression in these patients? Is it a special problem?
What are your views on the inclusion in the Quality and Outcomes Framework (QOF) of depression screening in patients with CHD?
Probes: is it a good/bad idea, workable in practice/difficult to do, relevant/irrelevant.
How would you make a distinction between distress and depression in someone with CHD?
Probes: for instance, distress that is perhaps a normal reaction to an adverse life event, and depressive illness that may require management? Criteria used; causes; patient factors; severity; course; outcome.
Do the patients share your views?
Current management of depression in coronary heart disease
Are you currently treating any of your patients for CHD and depression?
Probes: severity of CHD; severity of depression; risk factors; did the patient seek help for their depression? What was it about this patient that made you decide to treat them?
How do you feel about treating patients with CHD and depression?
Probe: is it any different from managing depression in other patients?
How do you/would you manage depression in someone with CHD?
Probes: available resources – medication, talking therapy, IAPT, lifestyle changes, complementary therapies, voluntary agencies, cardiac rehabilitation services; role of other professionals; successes; barriers; are there any issues specific to people with CHD compared with other illnesses? Is CHD treatment or depression treatment altered by comorbidity?
What would you hope to happen as a result of treating someone with CHD for depression?
Probes: improvement in mood; improvement in physical health; adherence to treatment; priorities.
What about discussing depression and treatment for depression with your CHD patients? Are there particular issues?
Probes: barriers; patient concerns; patient factors – ethnicity, gender, SES, age.
Future treatment of depression in coronary heart disease
We are aiming to design a programme of care for people with both CHD and depression. What options would you like to see included?
Probes: which would be most important? Who would deliver; role of other professionals/agencies; who would you refer; are any of these options especially suited to particular groups of patients? CHD specific versus generic.
Can you foresee any difficulties with any of the potential options?
How many of your current patients would benefit?
How do you think your CHD patients would feel about these options?
Any other issues
Finally, are there any other issues that we haven’t addressed and that you would like to mention?
Appendix 4 Instruments used in the UPBEAT-UK programme
Two measures were included at the behest of local GPs as part of an agreement for co-operation. They (Psychological Outcome Profiles Questionnaire and Rapid Estimate of Adult Literacy in Medicine) were analysed separately and led to publications, which are listed in the acknowledgments. 197,198 The third (Guy’s Hospital Questionnaire) was suggested by the cardiologist on the team but was abandoned because it was too difficult to administer over the telephone.
| Instrument | Author(s) | Description of instrument | Section of UPBEAT-UK used |
|---|---|---|---|
| BIPQ | Broadbent et al., 2006123 | 9-item scale designed to rapidly assess the cognitive and emotional representations of illness | Cohort study, RCT |
| Modified Rose angina questionnaire | Rose, 1962113 | Questionnaire investigating on chest pain, which is widely used to determine the presence of angina in clinical populations | Cohort study, RCT |
| REALM | Davis et al., 1993156 | A screening instrument designed to be used in public health and primary care settings to identify patients with low reading levels. It provides reading grade estimates for patients who read below a ninth grade level. The REALM can be administered in 1–2 minutes by personnel with minimal training | Cohort study |
| PSYCHLOPS | Ashworth et al., 2004122 | PSYCHLOPS is a brief one-page mental health outcome measure and can be used during the course of any psychotherapeutic intervention. It is patient generated and can be self-completed. It has questions on problems, function and well-being | Cohort study |
| CSRI forms 1 and 2 | Beecham and Knapp, 2001127 | The CSRI is a questionnaire for collecting retrospective information about study participants’ use of health and social care services, accommodation and living situation, income, employment and benefits. The service receipt section is the largest part of the questionnaire. The data collected through the CSRI can be used to calculate service costs and total costs of care | RCT |
| SPQ | Corney and Clare, 1985120 | A brief self-report questionnaire identifying social problems, difficulties and dissatisfactions. The SPQ covers housing, occupation, finance, social and leisure activities, child/parent and marital relationships, relationships with relatives, friends, neighbours and workmates, and legal problems | Cohort study, RCT |
| Life events questionnaire | Brugha and Cragg, 1990154 | Cohort study | |
| Short Form Questionnaire-36 items | McHorney et al., 1993199 | The SF-36 is a multipurpose, short-form health survey with only 36 questions. It yields an 8-point scale profile of functional health and well-being scores as well as psychometrically based physical and mental health summary measures and a preference-based health utility index | Cohort study |
| SF-12 | Ware et al., 1996118 | Abbreviated version of the SF-36 comprising only 12 items | RCT |
| EQ-5D | Hurst et al., 1997125 | The EQ-5D assesses respondents’ subjective quality of life – in reference to the assessment date only – across five life domains: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Overall health state is measured by a sixth item, which asks respondents to indicate their own health state on a scale from 0–100 (where 0 = ‘the worst imaginable health state’ and 100 = ‘the best imaginable health state’). Respondents are asked to take into account both their physical health and mental health when indicating the overall health state | Cohort study, RCT |
| PHQ-9 | Kroenke et al., 2001116 | Brief self-administered diagnostic instrument to detect depression | Cohort study, RCT |
| PHQ-2 | Kroenke et al., 200380 | The PHQ-2 is a shorter version of the PHQ-9 with two screening questions to assess the presence of a depressed mood and a loss of interest or pleasure in routine activities; a positive response to either question indicates further testing is required | RCT |
| HADS | Zigmund and Snaith, 1983114 | The HADS is a 14-item self-report scale for detecting states of depression and anxiety in outpatients. Respondents receive separate scores for depression and anxiety by summing scores from the appropriate items, with higher scores indicating higher levels of depression/anxiety. The HADS is useful for detecting change in a respondent’s emotional state, as well as for assessing presence or absence of clinically significant degrees of anxiety and depression | Cohort study, RCT |
| General Health Questionnaire-12 item | Goldberg and Williams, 1988151 | A self-administered screening questionnaire designed to detect those with a diagnosable psychiatric disorder | Cohort study |
| Specific Activity Scale | Goldman et al., 1981119 | Cohort study, RCT | |
| Morisky Adherence Questionnaire – adapted version | Morisky et al., 1986121 | A structured four-item self-report adherence measure addressing the barriers to medication taking | RCT |
| List of Threatening Experiences Questionnaire | Brugha and Cragg, 1990154 | Brief questionnaire investigating the presence and impact of a range of common stressful life events on respondents | RCT |
| CIS-R | Lewis, 1994152 | Interviewer completed psychiatric assessment | Cohort baseline |
| Warwick–Edinburgh Mental Well-being Scale | Tenant et al., 2007117 | Self-report well-being scale | RCT |
Appendix 5 The UPBEAT-UK study cohort audit trail
| 18 July 2013 | |
| Summary: | Contains every action undertaken on the UPBEAT COHORT data set since receiving the data set |
| Original data sets: | J:\Programme Grant\Rachel\UPBEAT cohort\Data |
| Final data sets: | J:\Programme Grant\Rachel\UPBEAT cohort\Data\Final data sets |
| Syntax: | J:\Programme Grant\Rachel\UPBEAT cohort\Data\Syntax |
| Other information: | J:\Programme Grant |
| Data version | Action | Syntax file |
|---|---|---|
| 1 | Data set set up (separately for each time point) | |
| Original data sets downloaded from MACRO by the Data Manager (Christopher Rowson) and emailed as a .csv file | ||
| Rachel Phillips was responsible for receiving all extractions up to and including the 24-month data | ||
| RP also wrote the instructions for cleaning the data sets and data checking processes | ||
| Paul Williams took over for the 30-month until 48-month (final) data sets, using these instructions | ||
| Original data sets as .csv files can all be found in the ‘Data’ folder under the relevant subfolders | ||
| Paul Williams was also responsible for collating the data sets and merging in any additional data | ||
| 1.1 | Data cleaning and scale scoring | |
| The same operations were undertaken for each data extraction (each time point) | ||
| All data cleaning were carried out in STATA version 11.2 | ||
| The instruction document (Document to outline the ordering of do files for UPBEAT data.doc) describes the cleaning process that transforms the .csv file into a cleaned STATA data set. e.g. for 30 month: | ||
| 30month_UPBEAT_Live20120821.csv | Extraction to STATA format | 0_insheet_30months.do |
| thirty_month.dta | Full cleaning process (including the start of data cleaning) | 1_30month.do |
| 1.2 | Data checking | |
| The instruction document (Document to outline the ordering of do files for UPBEAT data.doc) also describes the data checking process that checks for: data entry errors, correct number of participants, correct dates for the time between baseline and time point) | ||
| Any data errors that were flagged when conditions were not met were noted in the document (upbeat data discrepancies v2.2.xlsx) and amendments were written into the cleaning.do file) | ||
| e.g. for 30 months: | ||
| 30month.dta | Data errors flagged, recorded and amendments made to.do file | 1_30month.do |
| A list of ID numbers was then obtained and this was checked by the researchers to see whether or not this list matches the list of participants recorded on the ACCESS database | ||
| Any mis-matches flagged were recorded in the document (Access and Macro Follow ups at 30months FIXED.docx) and also (Date discrepancies CORRECTIONS.doc) | ||
| Any resulting amendments were translated into STATA code and the STATA data set was changed accordingly | ||
| 30month.dta | Changes to be made to the participants in the data set of the time point | 30m_amendments.do |
| If in the case the interview had been stored in the incorrect time point, the same.do file (30m_ammendments.do) extracts the data set, places it in the correct folder, and merges the record into the correct time point. In which case, the record would be stored as a separate data set in that folder, e.g.: | ||
| 30month_P14007.dta | ||
| Correct dates of the interviews were checked by calculating differences between their interview date and the interview date of their baseline visit. Any participants with substantial differences (> 1 month early, > 4 months late) were investigated | ||
| All reporting of date errors was recorded in the document (Date discrepancies CORRECTIONS.doc) | ||
| 30month.dta | Merge 30 month and baseline time point variables and create merged data set called base_30month.dta | Merging 30 months.do |
| base_30month.dta | Check time difference between time points, check outliers with researchers | initial_30months.do |
| 30month.dta | Make amendments to data set, resulting in a cleaned final data set with the same name | 30month_correctdates.do |
| A data cleaning checklist was compiled to keep track of progress for weekly meetings (Data cleaning check.xlsx) | ||
| 2 | data set set up (complete cohort data set) | |
| The process for combining the data sets was as follows: (1) make all the data sets (of each time point) compatible; (2) create a variable to distinguish the data sets; then (3) append these data sets | ||
| By appending data sets of the same observations, and mostly the same variables but different time points, we essentially create a data set in long format with many rows per participant. Matching occurs on the variables, not the unique identifiers as is normally done with merging. Appending has the advantage in this instance of creating a data set with fewer variables than what would have been produced in the wide format | ||
| 1. Making the data sets compatible | ||
| For each data set, variable names were shorted to remove the suffix containing the time point information | ||
| Any variable names that were not the same in all the time points were renamed accordingly for synchronisation | ||
| PYSCHLOPS variables that contained information about previous time points were dropped | ||
| Data sets used were from the folder: (J:\Programme Grant\Rachel\UPBEAT cohort\Data\Final data sets) | ||
| base_medic_complete.dta | ||
| 6month.dta | ||
| 12month1.dta | ||
| 18month.dta | ||
| 24month.dta | ||
| 30month.dta | ||
| 36month.dta | ||
| 42month.dta | ||
| 48month.dta | ||
| 2. Create a variable to distinguish time points | ||
| The variable <time point> was created, containing sequential values per data set (per time point) | ||
| data sets were saved locally | ||
| base.dta | ||
| 6.dta | ||
| 12.dta | ||
| 18.dta | ||
| 24.dta | ||
| 30.dta | ||
| 36.dta | ||
| 42.dta | ||
| 48.dta | ||
| 3. Append these data sets | ||
| All data sets were appended to the master data set (baseline) simultaneously. | ||
| base | append all other time points into this data set, and save as upbeat_cohort.dta | upbeat_cohort_merge.do |
| 2.1 | Data cleaning and scale scoring | |
| The process of data cleaning on the cohort data set involved (1) dropping all empty fields, (2) renaming and ordering variables appropriately, (3) ordering the data by patid and time point, and (4) inserting information into missing fields (e.g. gender was only recorded at baseline and so this information was carried across to other time points) | ||
| upbeat_cohort.dta | Data cleaning (1–4) | upbeat_cohort_clean.do |
| HADS anxiety missing values (> 55) to be classed as missing on variables (hads_anx anx_cat anx) | upbeat_cohort_clean.do | |
| Create ROSE classifications variable (called rose_short) | upbeat_cohort_clean.do | |
| Labels for GP practices | upbeat_cohort_clean.do | |
| Relabeling age variables to make explicit these were age at baseline (demographics questions collected only at baseline). The creation of a new age variable to record participant age throughout the data collection | upbeat_cohort_clean.do | |
| Relabeling comorbidity variables to make explicit these were age at baseline (demographics questions collected only at baseline) | upbeat_cohort_clean.do | |
| upbeat_cohort.dta | Check to see whether or not the all dates and time point make sense | upbeat_cohort_inspec.do |
| 2.2 | Merging additional data: (1) loss information (2) cardiac investigations information (3) depression pattern information | |
| Data from sources other than MACRO were entered into the cohort data set as follows | ||
| 2.2.1 | (1) Loss information | |
| Information regarding [(1) deaths, (2) withdrawals, and (3) loss to follow ups] on the whole sample was collected by Alison Smith and Rebecca Lawson | ||
| Specific categories were decided upon during weekly UPBEAT meetings with the whole team | ||
| Data was entered onto an excel spreadsheet (Cohort Main Spreadsheet – Deaths,LTFU,Withdrawal.xlsx) | ||
| The codebook for reasons of drop out is reported in the document (CODES FOR COHORT.doc) | ||
Data was imported into the upbeat cohort, with the following specification:
|
||
| upbeat_cohort.dta | Merge in the Loss data from (Cohort Main Spreadsheet – Deaths,LTFU,Withdrawal.xlsx) | upbeat_cohort_loss.do |
| Fill in demographic data into these new records | upbeat_cohort_loss.do | |
| Save data set as upbeat_cohort_v2.dta | upbeat_cohort_loss.do | |
| 2.2.2 | (2) Cardiac investigation information | |
| Information regarding cardiac investigations were collected from the GP notes for every participant in the cohort study and were inputted into an encrypted Microsoft Access database by Dr Jorge Palacios (Upbeat_Cohort_Med_Notes_2.mdb) | ||
| The specific data to be inputted and the specific definitions of cardiac investigations were decided before the hard copies were examined, with exception to the ‘Rapid Access Clinic’ category*. It was decided that data on rapid access would provide important information as to the severity of chest pain problems that a participant was experiencing (by the reasoning that if they used this service, then they must have experienced a level of severity higher than ‘ROSE Exertional pain’ but lower than the severity that required an intervention or even the severity defined by a cardiac event. This was decided upon by the UPBEAT team during weekly meetings. The GP notes were re-examined to gather this information and all data were inputted into the Access database before any analysis of cardiac investigation data occurred | ||
| * It was later suggested by the UPBEAT team that this ‘Rapid Access’ classification may not provide information regarding cardiac severity in the manner it was first thought to, and suggestions were made to remove it due to the following reasons: (1) it presented a very heterogeneous group of participants who had various reasons for accessing rapid access, of which the reasons were not recorded; (2) the results from the Rapid access service were not recorded so this could not be validated. Based on these two reasons, it cannot be used to determine with adequate accuracy the severity of heart problems as the other categories; and (3) participants access to the service was disparate among locations (as defined by GP practice) leading to potentially biased results dependent on where the participant lived | ||
| Access data was exported to a STATA data set file (data_cardiac_investigations.dta) | ||
| A codebook for the cardiac investigations was saved as a STATA data set file (tbl_cardiac intervention type.dta) | ||
| Data was imported into the upbeat cohort before any analysis, with the following specification: | ||
|
||
| 0 no chest pain | ||
| 1 chest pain | ||
| 2 exertional chest pain | ||
| 3 rapid access | ||
| 4 Bypass graft or angioplasty | ||
| 5 MI | ||
| 6 Cardiovascular death | ||
| The classification of a participant within a time point depended on their most severe data for that time point. Cardiovascular deaths (cardiac/stroke/vascular) were chosen over cardiac only deaths due to low numbers in order to increase statistical power. This ordinal variable was duplicated for the two definitions of cardiac investigation (1) first (2) severe. *After discussions regarding the appropriateness of the ‘rapid access’ classification, the two versions (first and severe) classifications were split further to include/exclude this rapid access | ||
| data_cardiac_investigations.dta | Clean data and save data set as cardiac_all_wide.dta | upbeat_cohort_cardiac outcomes |
| upbeat_cohort_v2.dta | Keep only variables: (id, time point and visit date). Reshape wide. Merge in the cardiac investigations data (cardiac_all_wide.dta) | upbeat_cohort_cardiac outcomes |
| Visit dates for time points 0 (baseline) – 8 (48 months) were available in wide format. An additional variable was created to mark the date exactly 6 months prior to their baseline visit. All cardiac investigations were assigned to their appropriate time point based on the window (Tn-1 to Tn) | upbeat_cohort_cardiac outcomes | |
| These cardiac investigations were then used to create the appropriate variables (1) first investigation per person per time point was coded into a variable, and (2) the most severe investigation per person per time point was coded into another variable. Dates of such investigations were retained alongside each of these. All other information was dropped. This data set was saved as cardiac_long.dta | upbeat_cohort_cardiac outcomes | |
| Merge in the cardiac investigations data (cardiac_long.dta) | upbeat_cohort_cardiac outcomes | |
| Outcome variables created for the ordinal cardiac problems (from no chest pain – chest pain – cardiac investigations – cardiovascular death). The two versions utilise the two definitions of cardiac investigation (1) first in time point (2) most severe in time point | upbeat_cohort_cardiac outcomes | |
| Cleaning of variables as new records per person were added | upbeat_cohort_cardiac outcomes | |
| Save data set as upbeat_cohort_v3.dta | ||
| 2.2.3 | (3) Depression pattern information | |
| Depression episodes as defined by the HADS depression scale (cut off 8 or more = positive) were extracted from the upbeat cohort into an Excel spreadsheet for the purpose of descriptively reporting the patterns of depression throughout the cohort up to 36 months | ||
| It was decided by the team that the combination of different patterns (start, end, fluctuating) and missingness should be collapsed as succinctly as possible | ||
| These coding for this was performed in Microsoft Excel in the file (depression table with analysis 2.0.xls) | ||
| A version of this that contained only the information on patterns and missingness was saved as a comma delimited file to be imported into STATA (dep_pat.csv) | ||
| The 3 variables chosen to describe a participant’s pattern of depression episodes were | ||
|
||
| upbeat_cohort_v3.dta | Merge in the patterns data from (dep_pat.csv) | upbeat_cohort_dep_patterns |
| Data cleaning | upbeat_cohort_dep_patterns | |
| Save data set as upbeat_cohort_v4.dta | upbeat_cohort_dep_patterns | |
| 3 | Creating a wide version of the data set | |
| To create a wide data set, first the long data set needed to be reduced as it contained 940 variables. A wide version of which would contain (8 × 940 variables) and it was decided appropriate to reduce this for simplicity | ||
| The method for creating a wide version of the data set consisted of (1) retaining only key variables, (2) add suffix ‘_’ to the end of variable names, and (3) reshape wide | ||
| A list of key variables of immediate interest was selected to be retained in the wide version of the data set (to save space). These variables along with others as requested were retained | ||
| upbeat_cohort_v4.dta |
|
upbeat_cohort_wide.do |
|
upbeat_cohort_wide.do | |
|
upbeat_cohort_wide.do | |
| Save data set as upbeat_cohort_v4_wide.dta | upbeat_cohort_wide.do | |
List of abbreviations
- A&E
- accident and emergency
- BIPQ
- Brief Illness Perception Questionnaire
- CBT
- cognitive–behavioural therapy
- CHD
- coronary heart disease
- CI
- confidence interval
- CIS-R
- Clinical Interview Schedule – Revised
- COPD
- chronic obstructive pulmonary disease
- CR
- cardiac rehabilitation
- EQ-5D
- European Quality of Life-5 Dimensions
- EQ-5D-VAS
- European Quality of Life-5 Dimensions visual analogue scale
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HADS-A
- Hospital Anxiety and Depression Scale – anxiety subscale
- HADS-D
- Hospital Anxiety and Depression Scale – depression subscale
- IAPT
- Improving Access to Psychological Therapies
- IMD
- Index of Multiple Deprivation
- MI
- myocardial infarction
- MRC
- Medical Research Council
- NICE
- National Institute for Health and Care Excellence
- OR
- odds ratio
- PC
- personalised care
- PCRN-GL
- Greater London Primary Care Research Network
- PHQ-9
- Patient Health Questionnaire
- PN
- practice nurse
- PPI
- patient and public involvement
- PWP
- psychological well-being practitioner
- QALY
- quality-adjusted life-year
- QOF
- Quality and Outcomes Framework
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RR
- relative risk
- RRR
- relative risk ratio
- sCHD
- symptomatic coronary heart disease
- SD
- standard deviation
- SF-12
- Short Form questionnaire-12 items
- SPQ
- Social Problems Questionnaire
- TAU
- treatment as usual