Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0608-10038. The contractual start date was in January 2010. The final report began editorial review in May 2015 and was accepted for publication in January 2016. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Peter Brocklehurst reports personal fees from Oxford Analytica, grants from the National Institute for Health and Care Excellence, grants and personal fees from the Medical Research Council (MRC), grants from the MRC, National Institute for Health Research (NIHR) Health Services and Delivery Research programme, NIHR Health Technology Assessment (HTA) programme, and Wellcome Trust outside the submitted work; and that he is chairperson of the NIHR HTA Maternal, Neonatal and Child Health Panel.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Knight et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
A comprehensive programme of study of maternal deaths has been undertaken in the UK for > 50 years,1,2 including confidential external review. This has contributed to major improvements in the quality of maternity care and a dramatic reduction in the maternal mortality rate,2,3 such that maternal deaths are now very rare. The latest figures from the confidential enquiry into maternal deaths show that only three women died from causes directly related to pregnancy for every 100,000 women giving birth,2 equating to fewer than 30 deaths per year. This rate has not changed significantly for more than 20 years, but this does not mean that pregnancy can be regarded as ‘safe’. Waterstone et al. 4 demonstrated in 1997–8 that 1200 cases of near-miss severe maternal morbidity occurred per 100,000 births in the South East Thames region, with a ratio of near-miss morbidity to death of more than 100 : 1. 4 It is now increasingly being recognised that in countries such as the UK, where maternal deaths are rare, the study of near-miss severe maternal morbidity (defined as a ‘severe life-threatening obstetric complication necessitating urgent medical intervention in order to prevent likely death of the mother’5) provides additional important information to aid disease prevention, treatment and service provision. 6,7
The advantages of an additional study of near-miss morbidity are several. Near-miss morbidity occurs more frequently, allowing for more rapid study completion and reporting of results owing to the larger number of cases identified. 6 The higher case numbers give studies of near-miss morbidity greater power to identify factors associated with disease incidence and hence generate recommendations to impact on disease prevention. 8 In addition, morbidity studies allow for the investigation of factors associated with poor disease outcomes. When information about fatal and non-fatal cases is compared, factors associated with progression from severe disease to death can be identified and management guidelines produced to help improve outcomes. 9 A further advantage of a national programme of study of near-miss morbidity is the ability to examine the quality of care of specific rare diseases10 and hence impact on patient safety. 11 The national collaboration of clinicians contributing to the UK Obstetric Surveillance System (UKOSS)10 provided a unique opportunity to undertake such a programme of study of near-miss severe maternal morbidity.
The only population-based study of near-miss maternal morbidity previously undertaken in England4 suggested that 1% of births are complicated by near-miss maternal morbidity. This illustrates the high burden of disease underlying maternal deaths in the UK, with an estimated 8000 women in the UK experiencing near-miss maternal morbidity each year compared with only approximately 80 who die from direct or indirect causes during pregnancy or in the first 6 weeks after the end of pregnancy. 2 Changes to the characteristics of women giving birth in the UK, including older age at childbirth,12 rising levels of obesity,13 more births to ethnic minority women14 and greater numbers of women with multiple pregnancies,15 each a reported risk factor for near-miss maternal morbidity, suggest that this rate is likely to rise.
At the time of commencement of this programme, the aim of health reform in England was ‘to develop a patient-led NHS that uses available resources as effectively and fairly as possible to promote health, reduce health inequalities and deliver the best and safest healthcare’. 16 Guidance for commissioners of maternity services stated that ‘this means providing high quality, safe and accessible services’. 17 Reducing the gap in infant mortality between socioeconomic groups was one of the key measures of the national Public Service Agreement health inequalities target18 and, as part of interventions to address this, the Department of Health also called for specific action to improve the quality of antenatal care. 19 Maternity care, safety and quality of maternity services remains a priority area in the NHS as recognised by the recent report on maternity services in a group of hospitals in the north of England20 and a recently announced review of maternity services by NHS England. 21
The aim of this programme of research into near-miss maternal morbidity was to address these priorities by providing evidence to underpin the development of preventative and management strategies for near-miss maternal morbidity, hence leading to improved quality of maternity care. Basic information, such as method and timing of diagnosis, use of specific interventions at the right time and in the right order, appropriate transfer to secondary care, seniority and discipline of carer and the impact of these factors on the outcomes for women and their babies, is largely lacking in this field. Exploring the role of these modifiable events yields important information to improve the quality and safety of maternity care.
Rationale
Although individually rare, when considered together, near-miss maternal morbidities represent a considerable burden to women, their families and health-care systems. Studies of rare disorders, such as specific near-miss maternal morbidities, require large collaborations to identify even a small number of cases. High-quality research is thus rarely undertaken, with studies frequently limited to retrospective hospital-based case series. Hence, clinical practice is rarely based on robust evidence and evidence-based guidelines for management are frequently lacking. We established the UKOSS in 2005 to enable the study of rare disorders of pregnancy, including near-miss maternal morbidity,10 and have shown that national data collection is feasible with the participation of all UK consultant-led maternity units in the UKOSS programme and clear identification of messages to improve patient outcomes. 11 However, in order to conduct a comprehensive programme of study to improve the care of women with near-miss maternal morbidity, this work needed to be expanded to address additional research questions through different methodologies.
Studies in other developed countries have investigated near-miss maternal morbidity through routine sources of data,22–25 but these studies are limited in their scope to identify risk factors by incomplete information on potential confounders. 26 In addition, the use of routine data means that these studies are unable to investigate diagnosis and management and hence provide evidence to improve clinical care. Only one population-based study has been conducted in England,4 although the small number of cases meant that the authors were unable to address research questions specific to individual morbidities and the impact of maternal characteristics, diagnosis and management on outcomes. This study only conducted follow-up to a maximum of 1 year following the event27 and did not explore women’s experiences. Surveillance studies of specific near-miss morbidities in the UK have been conducted through UKOSS,8,28–32 but further expanded work was required to conduct a comprehensive programme covering all the main causes of direct maternal death.
Studies of near-miss maternal morbidity using other methodologies, beyond hospital-based case series and surveillance using routine hospital data, are even more limited. The impact of basic factors on outcomes, such as pregnancy-related factors (e.g. multiple pregnancy following assisted reproduction), maternal characteristics (such as obesity), method of diagnosis (e.g. antenatal ultrasound or magnetic resonance imaging diagnosis of placenta accreta), timing of diagnosis [e.g. of acute fatty liver of pregnancy (AFLP) or haemolysis, elevated liver enzymes and low platelets (HELLP) syndrome], interventions (such as induction or augmentation of labour in women with uterine rupture) or other management techniques (e.g. brace sutures or arterial embolisation in severe peripartum haemorrhage), have not been systematically assessed for any of these conditions in national studies. All these factors are potentially modifiable and, hence, this programme sought to provide important information, currently lacking, to guide clinical practice and service provision and improve outcomes for women and their babies.
Aims and objectives
Aims
-
To implement a national programme of study of near-miss maternal morbidity to complement confidential enquiries into maternal deaths.
-
To use mixed methodologies to improve the evidence base for disease prevention and treatment and to inform commissioning of maternity services.
-
To use the data to develop recommendations for best practice to prevent and manage near-miss maternal morbidities.
Objectives
-
To determine the incidence of the specific morbidities most commonly leading to maternal death in the UK.
-
To assess the contribution of existing risk factors to disease incidence and identify steps that may be taken in clinical practice to address these factors to reduce incidence.
-
To describe how the conditions are managed and describe any variations in management, exploring the impact that different management strategies or interventions have on outcomes and costs, in order to make recommendations for best practice to improve outcomes for all women.
-
To describe the outcomes of the conditions for mother and infant and identify any groups in which outcomes differ.
-
To investigate which factors influence the risk of death and how these might be addressed to prevent death.
-
To explore whether an external confidential enquiry or a local review approach can be used to investigate and improve the quality of care for affected women.
-
To assess the longer-term impacts of near-miss maternal morbidity for women, their babies and families.
Workstreams
The aims and objectives were addressed in six workstreams.
Workstream 1: incidence, risk factors, management and outcomes of near-miss maternal morbidity, described in Chapters 3 and 4.
Workstream 2: factors contributing to case fatality, described in Chapter 6.
Workstream 3: addressing inequalities – focusing services for near-miss maternal morbidity, described in Chapter 7.
Workstream 4: working with hospitals to maximise the benefits of studies of near-miss maternal morbidity, described in Chapter 8.
Workstream 5: exploring the use of UKOSS data to conduct economic evaluation of treatments for near-miss morbidities, described in Chapter 5.
Workstream 6: long-term follow-up of women and their infants affected by near-miss morbidity, described in Chapters 2, 9 and 10.
Patient and public involvement
We set up an Advisory Group, consisting of women who had experienced severe morbidities in pregnancy, their partners and representatives of voluntary groups working in the maternity area. We held an annual face-to-face meeting, with e-mail discussion in the intervening periods. The first meeting took place in September 2010. The group, chaired by a public member, advised on developing the different component studies in a manner appropriate for women and so that they would provide most benefit in terms of improving maternity care. Members also helped recruit participants for the studies and publicised them through their networks when appropriate. Study progress and interim results of all studies were discussed with the group, who advised on additional analyses, interpretation and presentation prior to publication. The Advisory Group also suggested future research priorities based on their experiences and priorities and the findings of the programme. The activities of this group thus contributed throughout the component workstreams and their contribution was invaluable to the success of the programme.
Chapter 2 Unheard voices: women’s and their partners’ experiences of severe pregnancy complications
Background
Facts and figures are essential, but insufficient, to translate the data and promote the acceptance of evidence-based practices and policies . . . narratives, when compared with reporting statistical evidence alone, can have uniquely persuasive effects in overcoming preconceived beliefs and cognitive biases.
Meisel and Karlawish33
Up to 8000 women and their families each year have to cope with a life-threatening pregnancy complication and its aftermath. The causes of these ‘near-miss’ maternal morbidities are varied but include pre-eclampsia, haemorrhage, thrombosis and sepsis, and may in some cases require an emergency hysterectomy and/or result in pre-term delivery. 4 Mother and newborn are often separated, as women may have to spend time in intensive care or a high-dependency unit (HDU). Their babies may be born prematurely and require the neonatal intensive care unit (NICU).
Recent studies draw attention to the potential for long-term psychological and emotional impact on women of maternal morbidities. 34–38 In addition to their physical recovery, women can experience anxiety, isolation and flashbacks in the aftermath. Birth trauma can have lasting consequences that impact negatively on maternal, infant and family well-being. 39 Medically complicated pregnancies can also impact negatively on breastfeeding rates. 35,40
Women may be discharged from hospital having had major surgery and emergency treatment or time in intensive care, and follow-up is variable. Some may have lost their baby as a result of their illness. Babies delivered pre-term may need to spend long periods in NICU. These experiences are a long way away from normal birth.
Qualitative research allows a detailed exploration of the range of different reactions, emotions and experiences of care women encounter and the significance of the ‘near-miss’ in their life and their wider family. It may suggest avenues for service improvement that are not apparent from quantitative survey findings. The aim of this workstream was to explore the impacts of experiencing a near-miss obstetric emergency, in order to inform development of subsequent workstreams, provide a resource for women who have had a severe maternal morbidity and develop teaching and learning materials for NHS staff in order to help improve future care.
Research questions
-
What are the experiences of women who have near-miss maternal morbidity in the UK?
-
How does this experience impact on their social and family relationships and future childbearing plans?
-
Are there any aspects of the care experience that were particularly good or bad or could be improved?
Methods
The study was conducted using the standard methodology developed by the Health Experiences Research Group at the University of Oxford. 41 Interviews were conducted by a professional qualitative researcher.
Women who experienced a life-threatening complication in pregnancy (defined as ‘severe maternal illnesses which, without urgent medical attention, would lead to a mother’s death’5) were invited to take part in an interview study. We also invited the women’s partners to participate.
The sample
We aimed for a maximum variation sample of women living in the UK42,43 covering a wide range of conditions, based on the principal causes of direct maternal deaths identified in recent maternal death enquiry reports. 2 We sought to include women with a range of ethnic and socioeconomic backgrounds and at varying times after their ‘near-miss’ event. Interviews continued until thematic saturation was reached. Our overall sample included 36 women, 10 male partners and one lesbian partner (Table 1). The majority of partners were interviewed together.
| Characteristic | Number of participants |
|---|---|
| Age at the time of interview (years) | |
| 21–30 | 3 |
| 31–40 | 31 |
| 41+ | 13 |
| Age at time of near miss event (years) | |
| 21–30 | 11 |
| 31–40 | 31 |
| 41+ | 5 |
| Sex | |
| Women/mothers | 36 |
| Fathers/partners | 11 (10 men, one lesbian partner) |
| Occupation | |
| Professional | 21 |
| Other non-manual | 13 |
| Skilled manual | 4 |
| Unskilled manual | 2 |
| Other (such as housewife or student) | 7 |
| Ethnic group | |
| White British | 42 |
| British Pakistani | 1 |
| White other | 3 |
| British Somali | 1 |
| Time since near miss | |
| < 1 year | 9 |
| > 1 to < 2 years | 16 |
| 2–4 years | 16 |
| 5–9 years | 4 |
| 10+ years | 2 |
Recruitment packs were distributed through a number of routes to ensure a wide, varied sample. Routes included support groups, the National Childbirth Trust, social network forums [Mumsnet (www.mumsnet.com) and Netmums (www.netmums.com)], newspaper advertisement, intensive care clinicians contacted through the Intensive Care National Audit & Research Centre (ICNARC), an advertisement in the UKOSS newsletter and word of mouth. To try and reach a wider ethnic minority population, we had the recruitment packs translated into Bengali and distributed through a consultant in an east London hospital. Note, however, that the final sample had limited ethnic diversity (see Table 1). We sought to include as broad a range of conditions and time distance from the event as possible as we were keen to understand the longer-term effects of a near-miss maternal morbidity. All women had experienced a severe life-threatening complication in pregnancy [thrombosis or thromboembolism, hypertensive disorders of pregnancy, haemorrhage, amniotic fluid embolism (AFE) or sepsis]. Our sample included broad socioeconomic diversity. Interviews took place between 2010 and 2014.
After obtaining informed consent, one of the authors (LH) interviewed participants in the setting of their choice (usually their home). Participants were asked about their or their partner’s experiences of pregnancy and life-threatening illness. The interview started with an open ended narrative section during which respondents described what had happened, followed by a semistructured section with prompts to explore any relevant issues that had not already emerged, including their recovery and family life since their near-miss. The interviews were all audio- or video-taped and transcribed verbatim.
The analysis reported here focuses on the implications that emerged from the data for quality improvement, commissioning and clinical practice across the care pathway for women and their families. Verbatim quotations are used to illustrate themes that emerged from the data.
Analysis
The transcripts were read and reread, a coding frame was constructed and the data coded. Anticipated and emergent themes were then examined across the whole data set as well as in the context of each person’s interview. A qualitative interpretive approach was taken, combining thematic analysis with constant comparison. 44,45 NVivo 9 (QSR International, Warrington, UK) was used to facilitate the analysis.
Results
Implications for quality improvement, commissioning and clinical practice
This section pulls together all the implications for quality improvement, commissioning and clinical practice that have been identified from analysis of the data. Implications were identified across the care pathway:
-
the emergency
-
experiences of intensive care
-
transfer from intensive care
-
contact with the baby
-
communication
-
the aftermath
-
follow-up
-
support in the community
-
emotional recovery
-
counselling
-
impact on the family – partners
-
impact on the family – family and family life
-
information and support
-
good practice.
The emergency
-
Symptoms that lead to an unexpected or traumatic event in childbirth are varied; some have early warning signs but others are an emergency (to the mother or baby) that develops rapidly, out of the blue. However, both groups of women may find it difficult to adjust emotionally to their unexpected childbirth experience.
-
Some women may be hospitalised during their pregnancy for monitoring, which can be challenging but provides an opportunity for good communication and understanding of their condition.
-
If the baby suddenly arrives early, mothers may be especially emotionally unprepared for the arrival of their newborn:Kirsty, first baby
-
You know, I wasn’t ready. I wasn’t mentally prepared. I certainly wasn’t emotionally prepared. [Um] Luckily I’d had some time to sort out a Moses basket and [um] some clothes and nappies, but that was what the last 4 weeks were for. For reading the books. For doing some research. For decorating the nursery, you know, getting it all set up. That was going to be the joyous moment of preparing. And that was all taken away from me, I suppose. Well from us.
-
-
Women are reassured by calm professionalism from staff during their emergency.
Experiences of intensive care
-
Some mothers (or babies) may need to be in intensive care for a while. For a mother, waking up in intensive care can be very shocking. As new mothers they are often distressed at being separated from their baby. It would be helpful to develop protocols for women who require high dependency or intensive care to facilitate the baby being taken to visit their mother or vice versa. Rachel, third baby
-
You’re not as I would thought, in a place where may be there’s other people who have been through pregnancy related or birth related issues. I was in this, some old man ranting, there was a man ranting and pulling out his drips on one side. There was a man who’d had an accident opposite me. They brought in somebody who they discovered had E. coli [Escherichia coli] next to me and then she died next to me, and they were busy cleaning up.
-
-
Separation from their newborn baby can be very hard as mothers feel they are missing vital first steps. If it is not possible for women to see or be with their baby, they appreciate being kept in touch with the baby’s progress through photos, updates or contact with the paediatrician.
-
Many find it helpful to piece together what has happened to them, filling in the blanks, with the help of discussion with both staff and family and friends.
-
Being in an intensive therapy unit (ITU) can be very distressing; women often experience fear, humiliation and difficulties communicating.
Experiences of transfer from intensive care
-
Women who have suffered an unexpected event feel different from other new mothers. They feel more supported when information about what has happened has been handed over between staff and the team in the new setting shows that they are fully aware of what they have experienced.
-
Transfer from intensive care, although a positive step, can be difficult. Although there appears no ideal solution to step-down care:
-
Women who go to a high-dependency area alongside a labour ward find this helpful.
-
If women are in high-dependency care for an extended period, policies that allow visits from the wider family (e.g. grandparents) are appreciated.
-
-
Similarly, women can find transfer to the postnatal ward challenging:
-
If possible, a side room is the most comfortable place for women who have experienced a near-miss to be cared for.
-
If side rooms are not available or not appropriate in view of a woman’s clinical condition and potential safety concerns, a clear explanation of the reason for this is helpful.
-
Some women will experience significant problems with caring for themselves and their new baby in a side-room and will need extra help. Clara, first baby
-
And then through the night, I actually had what I kind of look back on, I felt like I was in a survival mode through that first night because I was left on my own with the baby. Now bearing in mind the baby had been looked after by the nurses at night. This is my first night out of intensive care. And my first night with the baby, all on my own, with no support whatsoever.
-
-
Personal support and empathy from individual staff members makes a real difference to how women cope with transfer and recovery.
-
Contact with the baby
-
Even when very ill, women want to be a mother to their new baby.
-
Sometimes babies will be in intensive care for days or weeks.
-
Many women face challenges in seeing their new baby and it would be helpful to develop protocols ahead of time for women who require high dependency or intensive care to facilitate the baby being taken to visit their mother or vice versa. Amy, first babyKirsty, first baby
-
You have this idealistic picture in your head, what it’s like when you’ve got a baby, that you’ll spend all the time cuddling them, and I didn’t feel I could do that, because just holding him to start with was just exhausting. So that was a really difficult sort of emotional battle really.
-
And obviously I didn’t see my son for 4 days. Such an odd feeling. I mean [not] expecting to have the baby so early and then I wasn’t a mother. I was just some useless person lying there.
-
-
When it is not possible for women to see or be with their baby, they appreciate being kept in touch with the baby’s progress through:
-
photos
-
regular verbal or written updates such as a diary of the baby’s day
-
direct contact with the paediatrician when the baby is ill.
-
-
Missing a baby’s ‘firsts’ is something women particularly notice; if at all possible they want to be there for important milestones such as the first feed.
-
Breastfeeding is very important for some women, particularly in the context where they may feel they have failed at normal childbirth.
-
Having a baby in a neonatal unit, after a severe obstetric emergency, is often challenging for women as they recover from their own illness. Sometimes they need to focus on getting well themselves, as well as being with their baby.
Communication
-
Women and their partners appreciate staff giving clear explanations in non-medical language at all stages before, during and after the emergency (Box 1):
-
When conditions are diagnosed in the non-emergency situation antenatally, being aware of what might happen helps women and their partners prepare and cope better afterwards.
-
During the emergency, repeated reassurance is appreciated.
-
Being listened to and being able to ask questions after the emergency is important for women to come to terms with what has happened. Naomi, first baby
I do vaguely recall someone sitting next to me holding my hand, and just saying, ‘It’s all right, you know, it’s going to fine. Just they need to get some blood into you’. And I don’t actually remember all of what she said. It’s more a memory present of there being somebody next to me, kind of trying to reassure me. So there was after that initial flurry of activity, I’m fairly certain that there was someone with me, trying to keep me calm. Which I greatly appreciated.
-
-
Partners/fathers can feel forgotten during and after the emergency:
-
Frequent updates from any member of staff can help them to feel less anxious and isolated.
-
Having another family member with them for support can help.
-
-
Knowing that staff have learned from a woman’s near-miss is reassuring for her.
Kerry and Sarah were both diagnosed with placenta praevia and didn’t feel doctors explained to them the severity of their condition. In contrast, Alex, who had the same condition, described excellent communication with her doctors who explained to her clearly why she needed to stay in hospital until her baby was born.
Kerry felt that doctors did not fully explain to her the risks of her placenta praevia. She wished they had sat her down and explained to her what a haemorrhage was and what to expect.
I would rather they had sat down and said are you aware of what haemorrhage is? What it means. Expect, this is how much … I just thought I haemorrhage was when you was bleeding and it just trickled slowly out of you, but didn’t stop. That to me was what a haemorrhage was. I didn’t expect it to be the biggest gush. I felt like, to look at the blood, I can see it now, I felt like every pint of blood in my body was on the floor it was that bad.
Kerry, second baby
Alex was told she was a walking time bomb. Doctors explained very well how serious her situation was in a way that allowed her to process little things at a time.
They did it very well. They explained the gravity of the situation but not in a way that would have complete…. I mean every time, it was almost like a drip feeding process. And I mean, it might not work for everyone, but it worked well for me, because it enabled me to process little things at a time.
Alex, second baby
The aftermath
-
What physical shape women are in when they get home will vary greatly; some will be recovering from major abdominal surgery, others from severe blood loss. Women find general practitioner (GP) support once they are discharged, and recognition of their particular childbirth experience, valuable to help them return to normal life.
-
Many women experience longer postnatal recovery periods than normal, although, on the whole, women did make a good recovery.
-
Women felt frustration at not being able to look after their new baby properly on their own.
-
Scars left over from the surgery caused distress for some women.
-
Women may face difficulties settling back into their previous social relationships with family, friends and their local community. Emma, second baby
-
But it is hard you know, when I left hospital people, people just stopped bothering and . . . I felt I really struggled with friends. I really struggled with people that made false promises, and I’ve still got a lot of upset towards them people that were all round and all there, when I was in hospital. But as soon as I came out they didn’t care. That’s when I really needed my friends. That’s when I really needed people to be there for me.
-
-
Women often feel isolated (physically and emotionally) when they first come out of hospital, unable to relate to others who have not experienced their trauma.
-
They feel that it is a taboo subject to raise at postnatal support groups, an experience which can be particularly isolating for first-time mothers.
-
Given the rarity of the conditions that lead to a near miss, or unexpected event, women can struggle to find anyone to talk to about it. Online support groups can be particularly valuable in providing access to other patient experiences.
Follow-up
-
Women who had a follow-up review at the hospital found this a positive experience to help their understanding of their emergency and their recovery.
-
When follow-up was not offered, women felt abandoned and were left with questions.
-
Women noted a number of things that were particularly helpful elements of the follow-up review:
-
Seeing and talking through their notes.
-
Answering questions about future pregnancies.
-
Sensitivity about the place where the review was conducted – returning to the antenatal clinic or labour ward or even hospital could be upsetting.
-
Flexible timing of the follow-up review – some women were not ready for this until several months or even years after the event.
-
An offer of counselling was helpful to some.
-
-
The follow-up programme with intensive care unit (ICU) staff that some women were involved with was considered a good model. Catherine, second baby
-
Huge, hugely helpful, because it made you feel, because after about 6 weeks I felt that everybody had kind of not forgotten about it, but moved on. You know, you’re alive you made it through it, you know, it’s time to put it behind you and everybody has moved on with their life and you’re just left with this yuk you know. And so to be able to be actually go into hospital and talk to people who knew, because even if you talk to some people who weren’t related to ICU, they still couldn’t really understand the trauma of what your body had gone through, having lost so much blood and what an impact that had on you.
-
Support in the community
-
Support from their GP and health visitors was highly valued by women who received it. It helped their physical and mental recovery. Kathryn, second baby
-
He’s seen me every 4 weeks. He put me in touch with support groups and things like that. And he was really patient, because I did keep going back. I thought I had illness after illness.
-
-
Those who did not receive support felt they could have done with:
-
practical help coping with their physical recovery and new babies or young children
-
emotional support as they came to terms with their emergency experience and its implications
-
help overcoming isolation from peers
-
early discussions around fears about future pregnancies/fertility
-
an awareness of how their emergency experience could impact on other family members.
-
Emotional recovery
-
Finding out what happened to them, and coming to terms with the seriousness of their illness and what a narrow escape they had had, was often very emotionally difficult for women.
-
Realising the severity of what they have been through can take time.
-
Understanding what had happened was very important; it helped mothers fill in the gaps in their memories and start to come to terms with their illness. Sally, third baby
-
I know from hearing through the grape vine that there was some kind of debrief that happened. That my case was kind of obviously looked into and sort of, they kind of had a look at what had went wrong. And I would have liked to have been involved in that. I would have liked to have had, not even a say I would have just liked to have known what had happened, because I still don’t know to this, to this day, quite what happened. I just kind of know just kind of bits and pieces.
-
-
Talking through events with clinicians at follow-up meetings, or going through their notes, can be valuable in helping women understand what has happened to them.
-
Coming to terms with their emergency experience can be difficult and take time. Anniversaries could stir up strong emotions. Emma, second baby
-
Before the [year] anniversary, that’s when I really panicked, because I just thought, well what am I going to do? I don’t know what I’m . . . It’s weird, it’s like I was in, in prison in my head . . . It was like I put on a front every day, and I don’t think many people saw past it, but my boyfriend did. And I know he worried for me, and he would just say to me, ‘You know, we need to get over this’. But not because he was like, oh get over it. It was because he could see how much underneath everything it was sort of eating me up and that was really hard.
-
Counselling
-
Women felt the need for counselling at different times after their emergency and there was great variation in when women felt ready to talk. Penny, first baby
-
The aftermath was so kind of overwhelming really, that at the time you just work through it, and its only afterwards you kind of think, you know, you stamp your foot a bit and say that was really unfair, that happened to me. You know, and so I was able to do all of that with, with the counsellor then, just at the time I needed it.
-
-
Many found counselling very helpful, in particular cognitive–behavioural therapy.
-
Others who had not received counselling said they wish they had received it as it would have helped them make sense of what they had been through.
-
Help may not be sought for long-term mental health issues; therefore, actively offering help may be beneficial.
Impact on the family
Partners
-
All of the partners/fathers we spoke to have been deeply affected by their partner’s life-threatening experiences; for some it has had a profound impact on their long-term mental health.
-
In situations for which an emergency delivery might be anticipated, such as when a woman has placenta praevia, explanation of what might happen helps partners prepare and cope subsequently.
-
Frequent updates during the emergency help partners/fathers feel less isolated and anxious. Joe, first baby
-
So there was this massive rush, alarms going everywhere, rush, shooting off and everything and they said, ‘Well you stay there’. And I’m thinking, what’s going on, you know, no one was telling me. And this is the bit I don’t like telling [my partner], is I was left there with blood everywhere, all over me, all over the floor, and they left me there for about 3 to 4 hours.
-
-
Personal touches of support from individual staff make a real difference to how partners cope.
-
Partners remember more about events than the woman who is ill, but still appreciate repeated explanations.
-
Partners/fathers can find seeing their partner in high dependency or intensive care very traumatic and may need support from staff and family members to:
-
enable them to visit their partner
-
understand that the situation is not hopeless – their partner may recover
-
come to terms with what has happened.
-
-
Long-term mental health problems in partners/fathers after a near-miss experience may have a big impact financially, practically and emotionally, and families may need additional support in this event.
-
Partners/fathers who experience mental health symptoms do not necessarily seek help, although they do feel that counselling, if offered, could be beneficial.
Family and family life
-
Women’s relationships with their partners are often put under severe strain and additional support may be required in this context.
-
Parenting advice can be important because:
-
Existing children can be severely affected by nearly losing a parent.
-
Building a relationship with the new baby following a near-miss event can be challenging even when the baby does not require neonatal unit care.
-
-
Issues around future fertility and family size can be complex:
-
Some women require support to come to terms with a loss of future fertility.
-
For others, the worry about the possibility of a near-miss event in a future pregnancy leads to a decision not to have further children and robust contraceptive advice can be important in these circumstances.
-
-
Mental health impacts for both mothers and fathers may require long-term management. Justin, third baby
-
And so, yes, it’s not nice. I get flashbacks every now and again. They used to be really bad, really, really bad, because visions are, meant to be a happy time picking your baby up. I get . . . it is visions of her being whizzed passed me, my wife. Doctors and nurses running and all of a sudden me baby is whizzed straight past and she’s going to special care baby unit, you know, in an incubator. It’s not nice. And that’s what visions come back all the time and haunt me.
-
-
Mental health and other impacts can lead to significant changes in career or life paths, which places an additional burden on parents.
-
Many women reported that they would have welcomed more support in the community, in particular access to mother and toddler or other parent’s groups where other women had similarly ‘abnormal’ birth experiences.
Information and support
-
Women and their families often needed a great deal of practical support as they recovered from their emergency.
-
Because of the rarity of these conditions, women often felt isolated when they came home and found it hard to access information.
-
Women wanted further information for various reasons and at various stages:
-
if possible, before the baby was born – to understand more about the condition they had been diagnosed with, and the risks to them and their baby
-
while in hospital – clear, jargon-free explanations of what has happened to them
-
after the emergency – practical information about what to expect during recovery.
-
-
Information tailored to new mothers, particularly in the case of hysterectomy, was highly valued.
-
Specialist online support was described as invaluable. Catherine, second baby
-
An oasis of somewhere that I could talk to women who’d been through exactly what I’d been through and to be able to pour out your grief feelings without being judged. It was like having a huge family of sisters . . . It is just a place to go that you can absolutely unload.
-
Good practice
-
Personal touches from individual staff can make a real difference to how women and their partners cope with the emergency and recovery.
-
Transfers within the hospital can be difficult and are made easier for women by:
-
considering both their critical care needs and their needs as a new mother
-
use of a single room if possible.
-
-
Reviewing their notes and/or a discussion with their consultant after an event can help women make sense of what happened.
-
Women find GP support once they are discharged valuable to help them return to normal life.
-
Explanations, often repeated, of what is happening are helpful to women and their partners at all stages of the emergency and recovery.
Teaching and learning points
The themes identified in the interviews were summarised as a series of teaching and learning resources. They are intended as a resource for clinicians across the care pathways of these women and their families, including midwives, obstetricians, nurses, anaesthetists, intensive care specialists, health visitors and GPs. Below are the key learning points from each of the summaries. A fuller description and clips are available on www.healthtalk.org. 46 Note that themes relevant to both analyses are repeated.
Good practice that makes a big difference
-
Personal touches from individual staff can make a real difference to how women and their partners cope with the emergency and recovery.
-
Transfers within the hospital can be difficult and are made easier for women by:
-
considering both their critical care needs and their needs as a new parent
-
use of a single room if possible.
-
-
Reviewing their notes and/or a discussion with their consultant after an event can help women make sense of what happened.
-
Women find GP support once they are discharged valuable to help them return to normal life.
-
Explanations, often repeated, of what is happening are helpful to women and their partners at all stages of the emergency and recovery.
Access to the baby after the emergency
-
Even when very ill, women want to be a mother to their new baby.
-
Many women face challenges in seeing their new baby and it would be helpful to develop protocols ahead of time for women who require high dependency or intensive care to facilitate the baby being taken to visit their mother or vice versa.
-
If it is not possible for women to see or be with their baby, they appreciate being kept in touch with the baby’s progress through:
-
photos
-
regular verbal or written updates such as a diary of the baby’s day
-
direct contact with the paediatrician when the baby is ill.
-
-
Missing a baby’s ‘firsts’ is something women particularly notice; if at all possible they want to be there for important milestones such as the first feed.
Transfer from critical care
-
Women who have suffered a near-miss feel different from other new mothers. They feel more supported when information about their condition has been handed over between staff and the team in the new setting show that they are fully aware of what they have experienced.
-
Transfer from intensive care, while a positive step, can be difficult. Although there appears no ideal solution to step-down care:
-
Women who go to a high-dependency area alongside a labour ward find this helpful.
-
If women are in high-dependency care for an extended period, policies that allow visits from the wider family (e.g. grandparents) are appreciated.
-
-
Similarly, women can find transfer to the postnatal ward challenging:
-
If possible, a side room is the most comfortable place for women who have experienced a near-miss to be cared for.
-
If side rooms are not available, a clear explanation of the reason for this is helpful.
-
Some women will experience significant problems with caring for themselves and their new baby in a side room and will need extra help.
-
-
Personal support and empathy from individual staff members makes a real difference to how women cope with transfer and recovery.
Information and understanding
-
Women and their partners appreciate staff giving clear explanations in non-medical language at all stages before, during and after the emergency:
-
When conditions are diagnosed in the non-emergency situation antenatally, being aware of what might happen helps women and their partners prepare and cope better afterwards.
-
During the emergency, repeated reassurance is appreciated.
-
Being listened to and being able to ask questions after the emergency is important for women to come to terms with what has happened.
-
-
Partners/fathers can feel forgotten during and after the emergency:
-
Frequent updates from any member of staff can help them to feel less anxious and isolated.
-
Having another family member with them for support can help.
-
-
Knowing that staff have learned from a woman’s near-miss is reassuring for her.
Support for partners/fathers
-
All the partners/fathers we spoke to have been deeply affected by their partner’s life-threatening experiences, for some it has had a profound impact on their long-term mental health.
-
In situations for which an emergency delivery might be anticipated, such as when a women has placenta praevia, explanation of what might happen helps partners prepare and cope subsequently.
-
Frequent updates during the emergency help partners/fathers feel less isolated and anxious.
-
Personal touches of support from individual staff make a real difference to how partners cope.
-
Partners remember more about events than the woman who is ill, but still appreciate repeated explanations.
-
Partners/fathers can find seeing their partner in high dependency or intensive care very traumatic and may need support from staff and family members to:
-
enable them to visit their partner
-
understand that the situation is not hopeless – their partner may recover
-
come to terms with what has happened.
-
-
Long-term mental health problems in partners/fathers after a near-miss experience may have a big impact financially, practically and emotionally, and families may need additional support in this event.
-
Partners/fathers who experience mental health symptoms do not necessarily seek help, although they do feel that counselling, if offered, could be beneficial.
Follow-up and counselling
-
Women who had a follow-up review at the hospital found this a positive experience to help their understanding and recovery.
-
When follow-up was not offered, women felt abandoned and were left with questions.
-
Women noted a number of things that were particularly helpful elements of the follow-up review:
-
Seeing and talking through their notes.
-
Answering questions about future pregnancies.
-
Sensitivity about the place where the review was conducted – returning to the antenatal clinic or labour ward or even hospital could be upsetting.
-
Flexible timing of the follow-up review – some women were not ready for this until several months or even years after the event.
-
An offer of counselling was helpful to some.
-
-
The follow-up programme with ICU staff that some women were involved with was considered a good model.
Long-term effects
-
Women’s relationships with their partners are often put under severe strain and additional support may be required in this context.
-
Parenting advice can be important because:
-
Existing children can be severely affected by nearly losing a parent.
-
Building a relationship with the new baby following a near-miss event can be challenging even when the baby does not require neonatal unit care.
-
-
Issues around future fertility and family size can be complex:
-
Some women require support to come to terms with a loss of future fertility.
-
For others, the worry about the possibility of a near-miss event in a future pregnancy leads to a decision not to have further children and robust contraceptive advice can be important in these circumstances.
-
-
Mental health impacts for both mothers and fathers may require long-term management.
-
Help may not be sought for long-term mental health issues; therefore, actively offering counselling may be beneficial.
-
Mental health and other impacts can lead to significant changes in career or life paths, which place an additional burden on parents.
-
Many women reported that they would have welcomed more support in the community. In particular:
-
access to mother and toddler or other parent’s groups where other women had similarly ‘abnormal’ birth experiences
-
GP and health visitor support for first-time mothers to help with feelings of isolation.
-
Conclusions
There has been little research about the long-term impact of traumatic birth and how best to help women. There is inconclusive evidence on the impact of debriefing programmes. However, we know that those who are most likely to be well after childbirth are women who had no complications, no worries about their labour and birth and information about their choices for care. 47 Women who experience a near-miss maternal morbidity have had none of these. For them, there is often no follow-up from hospital obstetric or midwifery staff. Primary care teams should routinely be made aware if a woman has had a near-miss event so they can offer the support these women may need and be aware these new mothers may be isolated from their peers. Therefore, potential support networks, GPs and health visitors need to be alert for mental health problems developing and mindful of the impact that the near-miss experience can have on the whole family (including partner and other children) and be prepared to offer advice about future pregnancies.
Although critical illness may be uncommon, it is a potentially devastating complication in pregnancy. 48 The obstetric population is changing, increasingly presenting clinicians with older mothers with pre-existing disorders and advanced chronic medical conditions. The 2011 report by the Maternal Critical Care Working Group49 acknowledged the many challenges of caring for critically ill obstetric patients. Multidisciplinary approaches are essential for these women and require urgent attention. 50 However, there is minimal guidance for nursing management of critically ill obstetric patients in the ITU. Critical care nurses report concerns about their competence and confidence in managing obstetric patients in the ICU, finding it hard to meet their needs. Special issues arise in terms of lactation support, emotional impact and communication with family members. 51,52 Midwives express anxiety about caring for critically ill pregnant women53 and further research is still needed into how to provide optimum care for critically ill pregnant and postpartum women.
All the partners/fathers we spoke to had been deeply affected by their partner’s life-threatening experiences. For some, it had a profound impact on their long-term mental health. In situations for which an emergency delivery might be anticipated, such as when a woman has placenta praevia, an explanation of what might happen helped partners prepare and cope subsequently. Staff might consider that frequent updates during the emergency help partners/fathers feel less isolated and anxious. Our study demonstrates that (often small) personal touches of support from individual staff can make a real difference to how partners cope. Although partners may remember more about events than the woman who is ill, they still appreciate repeated explanations. Partners/fathers can find seeing their partner in high dependency or intensive care very traumatic and may need support from staff and family members to enable them to visit their partner, understand that the situation is not hopeless and that their partner may recover, and to come to terms with what has happened.
Long-term mental health problems in partners/fathers after a near-miss experience may have a big impact financially, practically and emotionally, and families may need additional support in this event. They often felt that counselling could have been beneficial, if it had been offered. However, clinicians should take into account that partners/fathers who experience mental health symptoms do not necessarily seek help.
Chapter 3 Incidence, risk factors, management and outcomes of severe maternal morbidities
This chapter includes excerpts from Fitzpatrick KE, Hinshaw K, Kurunczuk JJ, Knight M, Risk factors, management, and outcomes of hemolysis, elevated liver enzymes, and low platelets syndrome and elevated liver ezymes, low platelets syndrome, Obstetrics and Gynecology, 123, 3, 618–27, reproduced with permission. 54
Background
Rare obstetric events are, by virtue of their rarity, difficult to study. Each clinician will see less than one affected woman per year, and perhaps only one in a clinical lifetime. As noted in Chapter 1, any study beyond a small case-series requires a large collaboration and thus management is rarely evidence based, and information for women and their families about the likely outcome and long-term prognosis of their condition is lacking. Hospital-based studies of these conditions require retrospective review of many years of data and are not necessarily representative of the condition in the current population as a whole because of changes over time and social and demographic differences in the populations served by individual hospital units as well as local differences in clinical practice. Fundamental questions, therefore, remain unanswered and the potential remains for significant improvements in the outcomes of these conditions for women and their babies by the collection of detailed national information. This workstream included a rolling programme of studies, thus enabling the study of risk factors, diagnosis and management, and relating these to outcomes of a range of near-miss maternal morbidities on a national basis. This co-ordinated programme of parallel studies had the additional advantage of preventing clinicians being burdened with multiple requests for information from different researchers. National studies of the following near-miss morbidities were conducted: AFE, placenta accreta/increta/percreta, HELLP/elevated liver enzymes and low platelets (ELLP) syndrome, and uterine rupture.
Amniotic fluid embolism
Amniotic fluid embolism although rare, remains one of the leading causes of direct maternal mortality in high-income countries,2,55,56 characterised by sudden unexplained cardiovascular collapse, respiratory distress and disseminated intravascular coagulation. The rarity of AFE together with the fact that clinical diagnosis of the condition is one of exclusion makes it difficult to obtain reliable information concerning incidence, risk factors, management and outcomes. Previous reviews56,57 suggest that the reported total incidence of AFE varies from 1.9 per 100,000 to 7.7 per 100,000 maternities; the reported incidence of fatal AFE cases varies from 0.4 per 100,000 to 1.7 per 100,000 while the case fatality rate owing to AFE ranges from 11% to 43%. There is little consistency in the factors reported to be associated with the occurrence of AFE and very limited data available regarding factors associated with serious maternal outcomes.
Placenta accreta/increta/percreta
Three variants of abnormally invasive placentation are recognised: placenta accreta, in which placental villi invade the surface of the myometrium; placenta increta, in which placental villi extend into the myometrium; and placenta percreta, in which the villi penetrate through the myometrium to the uterine serosa and may invade adjacent organs, such as the bladder. Placenta accreta, increta or percreta are associated with major pregnancy complications58 and are thought to be becoming more common59 owing to a number of factors including rising maternal age at delivery and an increasing proportion of deliveries by caesarean. 60,61 This finding is of particular concern in the context of increasing rates of caesarean delivery and older maternal age at childbirth. 62,63 However, the risk associated with these factors has not been quantified on a population basis in studies using robust clinical and pathological definitions. There is also limited information to guide the optimal management of this condition.
HELLP/ELLP syndrome
Haemolysis, elevated liver enzymes and low platelets syndrome is a serious complication of pregnancy that usually occurs in women who have signs of pre-eclampsia. 64 In the absence of haemolysis, the condition has been called ELLP syndrome. 65,66 Incidence estimates vary widely and there has been no comprehensive study of the risk factors for this complication. There is also debate over the optimal management of the syndrome, particularly with regard to women who develop the condition remote from term. 66,67
Uterine rupture
Uterine rupture is a complication of pregnancy associated with severe maternal and fetal morbidity and mortality. In high-income countries it most commonly occurs in women who have previously delivered by caesarean section. 68 This observation has led to debate about the optimal management of labour and delivery in women who have delivered by caesarean section in previous pregnancies. Women with a previous caesarean delivery have generally been encouraged to attempt a trial of labour in subsequent pregnancies,69 but recent reports of an increased risk of morbidity, particularly owing to uterine rupture, are thought to have contributed to a marked decrease in some countries in the number of women attempting vaginal birth after caesarean section. 70
Research questions
The following questions were addressed in all studies:
-
What is the incidence of the condition?
-
Are any factors (e.g. age, parity, obesity, ethnicity, socioeconomic status, multiple pregnancy, late or poor attendance for antenatal care) associated with an increased risk of developing the condition?
-
How is the condition managed in the UK?
-
What are the outcomes of the condition for women and their babies?
In addition, the following condition-specific questions were addressed.
Amniotic fluid embolism
-
What are the presenting features of the condition?
-
Are there any temporal trends in the incidence of the condition?
-
Are there specific factors, including timing of involvement of medical personnel and management strategies used, that are associated with adverse maternal outcomes?
Placenta accreta/increta/percreta
-
What are the risks associated with previous caesarean delivery and older maternal age?
-
What proportion of cases are diagnosed antenatally by ultrasound or MRI? Is antenatal diagnosis related to outcomes?
-
What proportion of cases have no attempt to remove their placenta, in an attempt to conserve their uterus or prior to hysterectomy? Do cases managed in this way have different outcomes?
HELLP/ELLP syndrome
-
What are the presenting features of the condition?
-
What proportion of antenatally diagnosed cases have a planned management of immediate delivery, delivery within 48 hours or expectant/conservative management? Are there any differences in outcomes according to planned management?
-
How are corticosteroids used to manage the syndrome in the UK?
-
Are there any differences in the characteristics, diagnostic features, presenting symptoms/signs, management and outcomes of women diagnosed with HELLP syndrome compared with women diagnosed with ELLP syndrome?
Uterine rupture
-
What are the common symptoms and signs noted prior to diagnosis of uterine rupture?
-
What proportion of ruptures occur in women who have previously delivered by caesarean section?
-
What are the risks associated with planned mode of delivery and labour induction and/or augmentation among women with a prior delivery by caesarean section?
-
What are the characteristics of the women who experience uterine rupture in the absence of a previous caesarean delivery?
UK Obstetric Surveillance System methodology
Case and control definitions
The case and control definitions used for each of the studies were as follows:
Amniotic fluid embolism
Cases were all women in the UK identified as having AFE defined as follows:
In the absence of any other clear cause:
EITHER
acute maternal collapse with one or more of the following features: acute fetal compromise, cardiac arrest, cardiac rhythm problems, coagulopathy, hypotension, maternal haemorrhage, premonitory symptoms (e.g. restlessness, numbness, agitation, tingling), seizure and shortness of breath, excluding women with maternal haemorrhage as the first presenting feature in whom there was no evidence of early coagulopathy or cardiorespiratory compromise
OR
women in whom the diagnosis was made at postmortem examination with the finding of fetal squames or hair in the lungs.
Controls were defined as the two women who did not have AFE and delivered immediately before other UKOSS study cases. 8,28,29,71–74
Placenta accreta/increta/percreta
Cases were all women in the UK identified as having placenta accreta/increta/percreta defined as either placenta accreta/increta/percreta diagnosed histologically following hysterectomy or postmortem, or an abnormally adherent placenta, requiring active management, including conservative approaches where the placenta is left in situ.
Controls were defined as the two women who did not have placenta accreta/increta/percreta and delivered immediately before each case in the same hospital.
HELLP/ELLP syndrome
Cases were any pregnant women in the UK identified as having new onset of the following:
elevated liver enzymes (serum aspartate aminotransferase ≥ 70 IU/l or gamma-glutamyl transferase ≥ 70 IU/l or alanine aminotransferase ≥ 70 IU/l)
AND
low platelets (platelet count < 100 × 109/l)
AND EITHER
haemolysis [abnormal (fragmented or contracted red cells) peripheral blood smear or serum lactate dehydrogenase levels ≥ 600 IU/l or total bilirubin ≥ 20.5 µmol/l)
OR
hypertension (systolic blood pressure ≥ 140 mmHg or a diastolic blood pressure ≥ 90 mmHg) OR proteinuria [1 + (0.3 g/l) or more on dipstick testing, a protein : creatinine ratio of ≥ 30 mg/mmol on a random sample, or a urine protein excretion of ≥ 300 mg per 24 hours].
Cases with haemolysis were classified as HELLP syndrome and those without haemolysis were classified as ELLP syndrome.
Controls were defined as the two women who did not have HELLP or ELLP syndrome and delivered immediately before each case in the same hospital.
Uterine rupture
Cases were all women in the UK identified as having a uterine rupture defined as a complete separation of the wall of the pregnant uterus, with or without expulsion of the fetus, involving rupture of membranes at the site of the uterine rupture or extension of the complete separation of the wall of the uterus into uterine muscle separate from any previous scar and endangering the life of the mother or fetus. Any asymptomatic palpable or visualised defect, noted incidentally at caesarean delivery for example, was excluded.
Controls were defined as any woman delivering a fetus or infant who had not had a uterine rupture and who had delivered by caesarean section in any previous pregnancy regardless of the mode of delivery of the current pregnancy.
Data collection
Cases of the specific near-miss morbidities were notified through the monthly card mailing of UKOSS from all hospitals with obstetrician-led maternity units in the UK. On reporting a case, clinicians were sent a data collection form requesting further details to confirm the case definition and ascertain information regarding potential risk factors, diagnosis, management and outcomes. AFE cases were identified between 1 February 2005 and 31 January 2014; placenta accreta/increta/percreta cases were identified between 1 May 2010 and 30 April 2011; HELLP/ELLP syndrome cases were identified between 1 June 2011 and 31 May 2012; and uterine rupture cases were identified between 1 April 2009 and 30 April 2010.
For the uterine rupture study, controls were obtained from a random sample of obstetrician-led maternity units in the UK in month 4 and month 12 of the study, weighted by the total number of births. The time and day on which reporting clinicians were asked to select controls were randomly identified using data on birth date and time from one county of England (Leicestershire) to try and provide a representative sample of women delivering during each 24-hour period and on different days of the week. Clinicians were then asked to complete a data collection form for these controls. For the placenta accreta/increta/percreta and HELLP/ELLP syndrome studies, clinicians who reported a case were asked to select and complete a data collection form for two controls, identified as the two women meeting the control definition.
All data requested were anonymous and obtained from the woman’s medical records. If complete forms were not returned, up to five reminders were sent. The data were double entered into a customised database. Duplicate reports were identified using information on the woman’s year of birth and expected date of delivery. Cases were checked to ensure that they met the case definition and controls were checked to confirm they had been selected correctly. When data were missing or a data validity check highlighted a problem, the reporting clinicians were contacted to provide or check the information.
Study power
Amniotic fluid embolism: the analysis had 80% power at the 5% level of statistical significance to detect odds ratios (ORs) of ≥ 1.7 and ≥ 2.6, assuming putative risk factors have a prevalence of 40% and 5%, respectively.
Placenta accreta/increta/percreta: the actual number of cases and controls identified during the study gave an estimated power of 80% at the 5% level of significance to detect ORs between 1.9 and 3.3, assuming a prevalence range for potential risk factors of between 5% and 40% in the control women.
HELLP/ELLP syndrome: the actual number of HELLP cases and controls identified gave an estimated power of 80% at the 5% level of significance to detect ORs between 1.8 and 2.9, assuming a prevalence range for potential risk factors of between 5% and 40% in the control women.
Uterine rupture: over the 13-month study period, we anticipated identifying 200 cases (based on an estimated incidence of 1 in 4000 maternities4) and 600 controls. A ratio of three controls per case was planned in the study proposal to maximise the power of the study given that uterine rupture is a rare condition and the number of cases would be limited by disease incidence. Assuming that 10% of women with a previous caesarean section delivering in the UK are induced with prostaglandin and/or receive oxytocin in their labour, and with a 3 : 1 ratio of controls to cases, 106 cases and 316 controls would give an estimated power of 80% at the 5% level of statistical significance to detect a 2.5-fold increase in the odds of uterine rupture in women with a previous caesarean section who have prostaglandin labour induction and/or oxytocin used in labour.
Analysis
The overall incidence with 95% confidence intervals (CIs) of each condition was calculated using the most recently available national birth data that corresponded most closely with the particular study period, as a proxy denominator for the number of maternities during the study period. Denominator data to calculate the incidence and 95% CIs of placenta accreta/increta/percreta in women with and without a previous caesarean delivery and in women with and without placenta praevia diagnosed prior to delivery were estimated using the proportions of women in these various categories observed in the control women together with the most recently available birth data. To calculate the incidence and 95% CIs of placenta accreta/increta/percreta in women with a previous caesarean with and without placenta praevia diagnosed prior to delivery, we used an estimate of the incidence of placenta praevia in women with a previous caesarean delivery (1.2%), derived from a 2010 systematic review. 75 To calculate the incidence with 95% CIs of uterine rupture in women with and without a previous caesarean section, the most recently available birth data were used together with an estimate of the proportion of women in the UK who had previously delivered by caesarean section (15%), derived from the rate in a group of population-based controls comprising women giving birth in the UK in 2005–6. 8 Information on the proportion of women with a previous caesarean delivery planning a vaginal or caesarean section delivery in their current pregnancy, estimated from that observed in the control women, was used to estimate the denominator for calculation of the incidence and 95% CI of uterine rupture according to planned mode of delivery in women with a previous caesarean section. Denominator data to allow calculation of the incidence and 95% CI of uterine rupture in women with a prior caesarean delivery planning a vaginal delivery according to whether labour was induced with or without prostaglandin and/or oxytocin were also estimated using the proportions observed in the control women.
Potential risk factors for each condition were investigated using unconditional logistic regression to estimate ORs and 95% CIs. A full regression model was developed by including both explanatory and potential confounding factors in a core model if there was a pre-existing hypothesis or evidence to suggest they were related to the condition in question; the findings are reported as adjusted odds ratios (aORs) and 95% CIs. Continuous variables were tested for departure from linearity by the addition of first-order fractional polynomials to the model and subsequent likelihood ratio testing. When there was evidence for non-linearity, continuous variables were presented and treated as categorical in the analysis. When there was no evidence of departure from linearity, continuous variables were presented as categorical for ease of interpretation, but have been treated as continuous linear terms when adjusting for them in the analysis. Plausible interactions were tested in the full regression model by the addition of interaction terms and subsequent likelihood ratio testing on removal, with a p-value of 0.01 considered evidence of significant interaction to account for multiple testing. The chi-squared test, Fisher’s exact test or Wilcoxon rank-sum test, as appropriate, were used to compare groups. Logistic regression using robust standard errors (SEs) to allow for the non-independence of infants from multiple births was used when comparing infant outcomes. All analyses were carried out using Stata statistical software version 11 (StataCorp LP, College Station, TX, USA).
Results
Amniotic fluid embolism
Incidence
There was a total of 120 confirmed cases of AFE, 23 of which were fatal (case fatality rate 19%, 95% CI 12% to 29%), in an estimated 7,001,438 maternities. 76–89 This represents a total incidence of 1.7 per 100,000 maternities (95% CI 1.4 to 2.1) and a fatal incidence of 0.3 per 100,000 maternities (95% CI 0.2 to 0.5).
Presentation
Amniotic fluid embolism presented at or before delivery in 53% (62/117) of women, at a median gestation of 39 weeks (range 28–42 weeks); the remaining women (n = 55) presented with AFE a median of 19 minutes after delivery (range 1 minute to 6 hours and 27 minutes) having delivered at a median gestation of 39 weeks (range 28–42 weeks). Diagnosis of AFE, including both antenatal and postnatal cases, was first considered a median of 33 minutes (range 0 minutes to 2 days) after presentation. Women with AFE who died were also significantly more likely than those who survived to present with cardiac arrest (87% vs. 36%; p < 0.001) and exhibit this feature as the first recognised symptom or sign of AFE (26% vs. 5%; p = 0.006); no other significant differences in presentation were found between these groups. The majority of women (91%, 108/119) had ruptured membranes at or before AFE presentation.
Risk factors
The characteristics of the women with AFE compared with the control women are shown in Table 2. Women aged ≥ 35 years had significantly raised odds of having AFE. The odds of having AFE were also significantly increased in women who had a multiple pregnancy, placenta praevia and induction of labour using any method.
| Risk factor | Number (%) of cases (N = 120) | Number (%) of controls (N = 3834) | uOR (95% CI) | p-value | aOR (95% CI)a | p-value |
|---|---|---|---|---|---|---|
| Sociodemographic factors | ||||||
| Age (years) | ||||||
| <35 | 74 (62) | 3062 (80) | 1 | 1 | ||
| ≥ 35 | 46 (38) | 766 (20) | 2.48 (1.71 to 3.62) | < 0.0001 | 2.15 (1.43 to 3.23) | 0.0002 |
| Missing | 0 (0) | 6 (0) | ||||
| Ethnic group | ||||||
| White | 92 (77) | 3064 (80) | 1 | 1 | ||
| Black or other minority ethnic group | 25 (21) | 699 (18) | 1.19 (0.76 to 1.87) | 0.4458 | 1.17 (0.72 to 1.91) | 0.5183 |
| Missing | 3 (3) | 71 (2) | ||||
| Socioeconomic group | ||||||
| Managerial and professional occupations | 42 (35) | 977 (25) | 1 | 1 | ||
| Other | 60 (50) | 2075 (54) | 0.67 (0.45 to 1.01) | 0.0529 | 0.81 (0.52 to 1.24) | 0.3306 |
| Missing | 18 (15) | 782 (20) | ||||
| BMI at booking (kg/m2) | ||||||
| < 30 | 90 (75) | 2797 (73) | 1 | 1 | ||
| ≥ 30 | 22 (18) | 674 (18) | 1.01 (0.63 to 1.63) | 0.9528 | 0.99 (0.60 to 1.62) | 0.9559 |
| Missing | 8 (7) | 363 (9) | ||||
| Smoking status | ||||||
| Never/ex-smoker | 99 (83) | 3045 (79) | 1 | 1 | ||
| Smoked during pregnancy | 20 (17) | 732 (19) | 0.84 (0.52 to 1.37) | 0.4842 | 1.12 (0.66 to 1.89) | 0.6829 |
| Missing | 1 (1) | 57 (1) | ||||
| Previous obstetric factors | ||||||
| Parity | ||||||
| 0 | 48 (40) | 1669 (44) | 1 | 1 | ||
| ≥ 1 | 72 (60) | 2160 (56) | 1.16 (0.80 to 1.68) | 0.4353 | 1.07 (0.72 to 1.60) | 0.7336 |
| Missing | 0 (0) | 5 (0) | ||||
| Current pregnancy factors | ||||||
| Multiple pregnancy | ||||||
| No | 106 (88) | 3786 (99) | 1 | 1 | ||
| Yes | 14 (12) | 48 (1) | 10.42 (5.57 to 19.48) | < 0.0001 | 7.75 (3.60 to 16.69) | < 0.0001 |
| Induction of labour using any method | ||||||
| No | 71 (59) | 2921 (76) | 1 | 1 | ||
| Yes | 49 (41) | 908 (24) | 2.22 (1.53 to 3.22) | < 0.0001 | 2.53 (1.70 to 3.75) | < 0.0001 |
| Missing | 0 (0) | 5 (0) | ||||
| Gestation age at delivery (weeks)b | ||||||
| Pre-term (< 37) | 99 (83) | 3414 (89) | 2.12 (1.25 to 3.60) | 0.0053 | 1.34 (0.71 to 2.55) | 0.3653 |
| Term (37–41) | 17 (14) | 276 (7) | 1 | 1 | ||
| Post term (≥ 42) | 3 (3) | 125 (3) | 3.45 (0.44 to 27.20) | 0.2401 | 0.51 (0.15 to 1.71) | 0.2742 |
| Missing | 1 (1) | 10 (0) | ||||
| Macrosomia (birthweight ≥ 4500 g)b | ||||||
| No | 109 (91) | 3742 (98) | 1 | 1 | ||
| Yes | 5 (4) | 77 (2) | 2.23 (0.88 to 5.62) | 0.0892 | 2.28 (0.88 to 5.92) | 0.0892 |
| Missing | 6 (5) | 6 (0) | ||||
| Placenta praevia | ||||||
| No | 116 (97) | 3797 (99) | 1 | 1 | ||
| Yes | 3 (3) | 22 (1) | 4.46 (1.32 to 15.12) | 0.0163 | 5.75 (1.64 to 20.19) | 0.0064 |
| Missing | 1 (1) | 15 (0) | ||||
| Placental abruption | ||||||
| No | 119 (99) | 3806 (99) | 1 | |||
| Yes | 0 (0) | 6 (0) | 3.93 (0 to 27.44) | |||
| Missing | 1 (1) | 22 (1) | ||||
Of the 55 women who presented with AFE postnatally, 11 (20%) had an instrumental vaginal delivery (ventouse or forceps) and 39 (71%) delivered by caesarean. Both instrumental vaginal delivery and caesarean delivery were associated with significantly raised odds of having AFE postnatally (aOR 9.51, 95% CI 3.17 to 28.51, and aOR 16.15, 95% CI 6.20 to 42.05, respectively).
Management and outcomes
Table 3 shows the factors used to manage coagulation following AFE presentation. Forty-four women (37%) did not receive any coagulopathy management. The variety of other management strategies used following AFE presentation is illustrated in Table 4. Thirty-one women (26%) had one and 21 women (18%) had more than one of these other management strategies.
| Number (%) of cases that had severe outcomea (N = 30) | Number (%) of cases that did not have severe outcomea (N = 90) | uOR (95% CI) | p-value | |
|---|---|---|---|---|
| Cryoprecipitate | ||||
| No | 24 (80) | 49 (54) | 1 | |
| Yes | 6 (20) | 41 (46) | 0.3 (0.11 to 0.80) | 0.0163 |
| Fresh-frozen plasma | ||||
| No | 13 (43) | 38 (42) | 1 | |
| Yes | 17 (57) | 52 (58) | 0.96 (0.41 to 2.20) | 0.9151 |
| Platelets | ||||
| No | 22 (73) | 54 (60) | 1 | |
| Yes | 8 (27) | 36 (40) | 0.55 (0.22 to 1.36) | 0.1929 |
| Factor VIIa | ||||
| No | 24 (80) | 70 (78) | 1 | |
| Yes | 6 (20) | 20 (22) | 0.88 (0.31 to 2.43) | 0.7981 |
| Fibrinogen | ||||
| No | 29 (97) | 89 (99) | 1 | |
| Yes | 1 (3) | 1 (1) | 3.07 (0.19 to 50.64) | 0.433 |
| Number (%) of cases that had severe outcomea (N = 30) | Number (%) of cases that did not have severe outcomea (N = 90) | uOR (95% CI) | p-value | |
|---|---|---|---|---|
| Hysterectomy | ||||
| No | 18 (60) | 71 (79) | 1 | |
| Yes | 12 (40) | 19 (21) | 2.49 (1.02 to 6.06) | 0.0441 |
| Other surgery for haemorrhageb | ||||
| No | 25 (83) | 81 (90) | 1 | |
| Yes | 5 (17) | 9 (10) | 1.8 (0.55 to 5.87) | 0.3296 |
| Exchange transfusion | ||||
| No | 30 (100) | 87 (97) | 1 | |
| Yes | 0 (0) | 3 (3) | 0.77 (0 to 7.33) | 0.8366 |
| Plasma exchange | ||||
| No | 30 (100) | 86 (96) | 1 | |
| Yes | 0 (0) | 4 (4) | 0.55 (0 to 4.58) | 0.6221 |
| Misoprostol | ||||
| No | 28 (93) | 86 (96) | 1 | |
| Yes | 2 (7) | 4 (4) | 1.54 (0.27 to 8.84) | 0.6309 |
| Hemabate | ||||
| No | 28 (93) | 84 (93) | 1 | |
| Yes | 2 (7) | 6 (7) | 1 (0.19 to 5.24) | 1 |
| Intrauterine balloons | ||||
| No | 29 (97) | 84 (93) | 1 | |
| Yes | 1 (3) | 6 (7) | 0.48 (0.06 to 4.18) | 0.5085 |
| Embolisation | ||||
| No | 30 (100) | 88 (98) | 1 | |
| Yes | 0 (0) | 2 (2) | 1.24 (0 to 16.10) | 1 |
| Tranexamic acid | ||||
| No | 29 (97) | 85 (94) | 1 | |
| Yes | 1 (3) | 5 (6) | 0.59 (0.07 to 5.23) | 0.6324 |
| Obstetrician present at time of event or arrived within 5 minutes | ||||
| No | 10 (33) | 28 (31) | 1 | |
| Yes | 17 (57) | 55 (61) | 0.87 (0.35 to 2.14) | 0.7541 |
| Missing | 3 (10) | 7 (8) | ||
| Anaesthetist present at time of event or arrived within 5 minutes | ||||
| No | 9 (30) | 26 (29) | 1 | |
| Yes | 18 (60) | 57 (63) | 0.91 (0.36 to 2.30) | 0.8457 |
| Missing | 3 (10) | 7 (8) | ||
| Obstetrician and/or anaesthetist present at time of event | ||||
| No | 11 (37) | 35 (39) | 1 | |
| Yes | 16 (53) | 47 (52) | 1.08 (0.45 to 2.62) | 0.8593 |
| Missing | 3 (10) | 8 (9) | ||
Twenty-three women (19%) died and seven (6%) of the women who survived had permanent neurological injury. Women with AFE who died or had permanent neurological injury were less likely than those who survived and did not have permanent neurological injury to have cryoprecipitate given, were more likely to have had a hysterectomy, had a shorter time interval between the AFE event and when the hysterectomy was performed (median interval 77 minutes, range 0–360 minutes vs. median interval 248 minutes, range 23 minutes to 12 days; p = 0.0315) and were more likely to be from black or other minority ethnic groups (aOR 2.85, 95% CI 1.02 to 8.00).
Women with AFE gave birth to a total of 134 infants (106 singletons and 14 twin pregnancies). There were four stillbirths (one antepartum and three intrapartum) and five early neonatal deaths among these infants, equating to a perinatal mortality rate of 67 per 1000 (95% CI 31 to 124), which is significantly higher than the national rate of 7.5 per 1000 [risk ratio (RR) 8.91, 95% CI 4.74 to 16.75]. Both mother and infant did not survive in four cases. Major complications were reported in 17 of the surviving infants, including 10 diagnosed with neonatal encephalopathy and three requiring phototherapy for severe jaundice.
HELLP syndrome
Incidence
There were 210 women who met the case definition in an estimated 799,003 maternities. 80,90,91 Of these, 129 had haemolysis and were considered to have HELLP syndrome, giving an incidence of 1.6 per 10,000 maternities (95% CI 1.3 to 1.9); a further 81 of the women met the case definition, but either they did not have haemolysis (n = 79) or it is unknown whether or not they had haemolysis (n = 2). These women were considered to have ELLP syndrome, giving an incidence of 1 per 10,000 maternities (95% CI 0.8 to 1.3).
Risk factors
The odds of having both HELLP and ELLP syndrome were significantly raised in nulliparous women and women who had a gestational hypertensive disorder in a previous pregnancy (HELLP aOR 3.47, 95% CI 1.49 to 8.09; ELLP aOR 4.66, 95% CI 1.37 to 15.9). Older women and women who had a multiple pregnancy also had significantly raised odds of having HELLP (aOR in women aged ≥ 35 years was 1.85, 95% CI 1.12 to 3.06; aOR in women with multiple pregnancy was 4.51, 95% CI 1.45 to 14.1) but not ELLP syndrome, noting the limited power of the analysis. There was also evidence that women of black or other minority ethnic groups and women who smoked during pregnancy had significantly reduced odds of ELLP (aOR in women of black or other minority ethnic groups was 0.42, 95% CI 0.19 to 0.95; aOR in women who smoked was 0.15, 95% CI 0.03 to 0.63), although no such association was apparent for HELLP, again noting the limited power of the analysis. The only significant interaction found was between essential hypertension and parity for HELLP syndrome; women who had essential hypertension had a raised odds of having HELLP syndrome if they were nulliparous but not if they were multiparous (aOR 6.46, 95% CI 1.62 to 25.83 in nulliparous women; aOR 0.23, 95% CI 0.01 to 1.95 in multiparous women).
Management and outcomes
Women with HELLP syndrome were significantly more likely than those with ELLP syndrome to have received magnesium sulphate [76% (98/129) vs. 62% (50/81); p = 0.028]. No other significant differences in management were seen between the women with either HELLP or ELLP syndrome (data not shown) and, therefore, the management of these women has been presented together. Of the women diagnosed antenatally with HELLP or ELLP syndrome, 51% (71/138) had a planned management of immediate delivery and 43% (60/138) had a planned management of delivery within 48 hours; only 7/138 (5%) had a planned attempt at expectant (conservative) management. Women who had a planned management of delivery within 48 hours were more likely than those who had planned immediate delivery to be nulliparous [83% (50/60) vs. 68% (48/71); p = 0.039], but no other differences in characteristics were found between these women. A total of 80% (166/208) were given antihypertensive medication.
Although there were no maternal deaths among the women diagnosed with ELLP syndrome, one woman with HELLP syndrome died (case fatality 0.8%, 95% CI 0.02% to 4.2%). No significant differences were found between the women who had a planned management of delivery within 48 hours of diagnosis and those who had a planned management of immediate delivery. There were no significant differences in the outcomes of HELLP according to whether women were managed with blood products or admitted to ITU.
There were two stillbirths among the 135 infants born to women with HELLP syndrome, equating to a perinatal mortality rate of 15 per 1000 total births (95% CI 2 to 53). Of the 87 infants born to women with ELLP syndrome, one died in the early neonatal period, equating to a perinatal mortality rate of 12 per 1000 total births (95% CI 0.3 to 62). There were no significant differences between the infants born to women who had a planned management of delivery within 48 hours of diagnosis and those born to women who had a planned management of immediate delivery. Of the seven infants born to the women who had a planned attempt at expectant management, none died but two had major complications relating to pre-term birth and three were small for gestational age.
Placenta accreta/increta/percreta
Incidence
There was a total of 134 confirmed cases of placenta accreta/increta/percreta in an estimated 798,634 maternities,81,92,93 representing an estimated incidence of 1.7 per 10,000 maternities (95% CI 1.4 to 2.0). Table 5 shows the estimated incidence of accreta/increta/percreta for various categories of women; incidence estimates range from 1 in 33,000 for women without a previous caesarean delivery to 1 in 20 for women with at least one previous caesarean delivery and placenta praevia diagnosed prior to delivery.
| Categorya | Number of women with placenta accreta/increta/percreta | Estimated number of maternities | Estimated incidence of placenta accreta/increta/percreta (95% CI) per 10,000 maternities |
|---|---|---|---|
| Women without a previous caesarean delivery | 21 | 678,839 | 0.3 (0.2 to 0.5) |
| Women with at least one previous caesarean delivery | 113 | 119,795 | 9 (8 to 11) |
| Women without placenta praevia diagnosed prior to delivery | 47 | 790,648 | 0.6 (0.4 to 0.8) |
| Women with placenta praevia diagnosed prior to delivery | 86 | 7986 | 108 (86 to 133) |
| Women with at least one previous caesarean delivery but without placenta praevia diagnosed prior to delivery | 30 | 118,357 | 3 (2 to 4) |
| Women with at least one previous caesarean delivery and placenta praevia diagnosed prior to delivery | 83 | 1438 | 577 (462 to 711) |
Risk factors
The odds of having placenta accreta/increta/percreta rose with increasing maternal age (aOR 1.15, 95% CI 1.06 to 1.24) for every 1-year increase in age. The odds of having placenta accreta/increta/percreta were also raised in women who had a previous caesarean delivery (aOR 14.41, 95% CI 5.63 to 36.85). There was evidence of an interaction between age and previous caesarean delivery. The raised odds associated with older maternal age were apparent only in women without a previous caesarean delivery (aOR 1.30, 95% CI 1.13 to 1.50 for every 1-year increase in age in women without a previous caesarean delivery compared with aOR 1.03, 95% CI 0.92 to 1.15 for every 1-year increase in age in women with a previous caesarean). Women who had other previous uterine surgery, such as myomectomy, also had an increased odds of having placenta accreta/increta/percreta (aOR 3.40, 95% CI 1.30 to 8.91), as did women who had an in vitro fertilisation (IVF) pregnancy (aOR 32.13, 95% CI 2.03 to 509.23). The odds of placenta accreta/increta/percreta were also raised in women who had placenta praevia diagnosed antenatally (aOR 65.02, 95% CI 16.58 to 254.96).
Diagnosis
Placenta accreta/increta/percreta was suspected prior to delivery in half of the women (66/133, 50%). The majority of the women who did not have placenta accreta/increta/percreta suspected antenatally presented with difficult or unsuccessful delivery of the placenta either at vaginal or caesarean delivery (52/65, 80%). Women who had placenta accreta/increta/percreta suspected antenatally were more likely than those who did not to be multiparous [98% (65/66) vs. 84% (56/67); p = 0.003], were more likely to have had a previous caesarean delivery [98% (65/66) vs. 72% (48/67); p < 0.001] and were more likely to have had placenta praevia diagnosed prior to delivery [97% (64/66) vs. 33% (22/67); p < 0.001]. Sixty-three (95%) suspected cases had both placenta praevia diagnosed antenatally and a previous caesarean delivery compared with 20 (30%) of the unsuspected cases (p < 0.001). There was a suggestion that the women who had placenta accreta/increta/percreta suspected antenatally had a greater severity of placental invasion as they were more likely to have a final diagnosis after delivery of placenta increta or percreta rather than accreta [43% (28/65) of suspected cases vs. 27% (18/67) of unsuspected cases; p = 0.051].
In total, 65% (87/133) of the women had a final diagnosis after delivery of placenta accreta, 5% (7/133) had a final diagnosis of placenta increta and 29% (39/133) had a final diagnosis of placenta percreta.
Management and outcomes
Women who had placenta accreta/increta/percreta suspected antenatally were more likely than those who did not to deliver by planned caesarean, have no attempt to remove any of their placenta around the time of delivery, have other therapy or therapies to prevent haemorrhage and be admitted to ITU/HDU (Table 6). Although they were less likely to have other therapy or therapies to treat haemorrhage, there was no significant difference in their median estimated total blood loss, the proportion who received a blood transfusion or who subsequently had a hysterectomy. However, subgroup analysis suggests that while antenatal diagnosis is not associated with a lower median estimated total blood loss or need for blood transfusion in women with a final diagnosis of placenta accreta [median estimated blood loss 3000 ml, range 300–14,435 ml in suspected cases vs. 3100 ml, range 200–15,000 ml in unsuspected cases (p = 0.9131); 84%, 31/37 of suspected cases had blood transfusion vs. 81%, 39/48 of unsuspected cases (p = 0.761)], there is such an association in those who had a final diagnosis of placenta increta or percreta [median estimated blood loss 2750 ml, range 250–10,514 ml in suspected cases vs. 6100 ml, range 1500–24,000 ml in unsuspected cases (p = 0.008); 59%, 16/27 of suspected cases had blood transfusion vs. 94%, 17/18 of unsuspected cases (p = 0.014)].
| Peripartum management/maternal outcome | n (%)a unless otherwise stated of cases suspected antenatally (N = 66) | n (%)a unless otherwise stated of cases not suspected antenatally (N = 67) | p-value |
|---|---|---|---|
| Planned mode of delivery | |||
| Vaginal | 2 (3) | 20 (30) | |
| Caesarean | 64 (97) | 46 (70) | < 0.001 |
| Attempt made to remove any of placenta around time of delivery | |||
| No | 27 (41) | 5 (7) | |
| Yes | 39 (59) | 62 (93) | < 0.001 |
| Hysterectomy performed | |||
| No | 23 (35) | 31 (46) | |
| Yes | 43 (65) | 36 (54) | 0.18 |
| Hysterectomy type | |||
| Total | 25 (58) | 18 (50) | |
| Subtotal | 18 (42) | 18 (50) | 0.469 |
| Other therapy(ies) to prevent haemorrhage | |||
| No | 17 (26) | 32 (48) | |
| Yes | 49 (74) | 34 (52) | 0.007 |
| Other therapy(ies) to treat haemorrhage | |||
| No | 33 (50) | 17 (25) | |
| Yes | 33 (50) | 50 (75) | 0.003 |
| Median estimated total blood loss in ml (range) | 3000 (250–14,435) | 3500 (200–24,000) | 0.126 |
| Estimated total blood loss (ml) | |||
| < 2500 | 30 (45) | 20 (30) | |
| ≥ 2500 | 36 (55) | 47 (70) | 0.063 |
| Blood products given | |||
| No | 17 (27) | 10 (15) | |
| Yes | 47 (73) | 56 (85) | 0.109 |
| Median units of whole or packed red cells transfused (range)b | 7 (0–24) | 7 (2–29) | 0.783 |
| Median units of fresh-frozen plasma transfused (range)b | 3.5 (0–13) | 4 (0–12) | 0.685 |
| Median units of platelets transfused (range)b | 0 (0–6) | 0 (0–4) | 0.813 |
| Median units of cryoprecipitate transfused (range)b | 0 (0–10) | 0 (0–10) | 0.848 |
| Median millilitres of cell salvaged blood transfused (range)b | 75 (0–8000) | 0 (0–1700) | < 0.001 |
| Admission to ITU/HDU | |||
| No | 13 (20) | 29 (43) | |
| Yes | 53 (80) | 38 (57) | 0.003 |
| Median duration of stay in ITU/HDU in days (range) | 2 (1–26) | 1.5 (1–19) | 0.617 |
A total of 102 (76%) women had an attempt made to remove their placenta around the time of delivery. The hysterectomy rate did not vary according to whether or not an attempt was made to remove any of the placenta (Table 7). As well as being more likely to have been diagnosed antenatally, in terms of characteristics, women who had no attempt to remove any of their placenta were more likely than those who did to have had a previous caesarean delivery [97% (31/32) vs. 80% (82/102); p = 0.025], were more likely to have had placenta praevia diagnosed prior to delivery [88% (28/32) vs. 57% (58/101); p = 0.002] and were more likely to have a final diagnosis of placenta increta or percreta rather than accreta [71% (22/31) vs. 24% (24/102); p < 0.001]; no other significant differences were found in other current pregnancy, previous obstetric or sociodemographic characteristics (data not shown). Despite being more likely to have a greater severity of placental invasion, the women who had no attempt to remove any of their placenta were less likely to have other therapy or therapies to treat haemorrhage, had a lower estimated total blood loss and were less likely to have a blood transfusion (see Table 7).
| Peripartum management/maternal outcome | n (%)a unless otherwise stated of cases who had no attempt to remove placenta around time of delivery (N = 32) | n (%)a unless otherwise stated of cases who did have an attempt to remove placenta around time of delivery (N = 102) | p-value |
|---|---|---|---|
| Caesarean delivery | |||
| No | 2 (6) | 14 (14) | |
| Yes | 30 (94) | 88 (86) | 0.356 |
| Hysterectomy performed | |||
| No | 11 (34) | 44 (43) | |
| Yes | 21 (66) | 58 (57) | 0.379 |
| Hysterectomy type | |||
| Total | 12 (57) | 31 (53) | |
| Subtotal | 9 (43) | 27 (47) | 0.771 |
| Other therapy(ies) to prevent haemorrhage | |||
| No | 7 (23) | 42 (42) | |
| Yes | 24 (77) | 59 (58) | 0.055 |
| Other therapy(ies) to treat haemorrhage | |||
| No | 24 (75) | 26 (26) | |
| Yes | 8 (25) | 75 (74) | < 0.001 |
| Median estimated total blood loss in ml (range) | 1750 (200–15,000) | 3700 (500–24,000) | 0.001 |
| Estimated total blood loss (ml) | |||
| < 2500 | 18 (56) | 32 (31) | |
| ≥ 2500 | 14 (44) | 70 (69) | 0.011 |
| Blood products given | |||
| No | 13 (43) | 14 (14) | |
| Yes | 17 (57) | 87 (86) | < 0.001 |
| Median units of whole or packed red cells transfused (range)b | 7 (3–24) | 7 (0–29) | 0.597 |
| Median units of fresh-frozen plasma transfused (range)b | 4 (0–13) | 4 (0–12) | 0.763 |
| Median units of platelets transfused (range)b | 0 (0–4) | 0 (0–6) | 0.583 |
| Median units of cryoprecipitate transfused (range)b | 0 (0–4) | 0 (0–10) | 0.402 |
| Median ml of cell salvaged blood transfused (range)b | 0 (0–8000) | 0 (0–5500) | 0.067 |
| Admission to ITU/HDU | |||
| No | 10 (31) | 32 (31) | |
| Yes | 22 (69) | 70 (69) | 0.99 |
| Median duration of stay in ITU/HDU in days (range) | 1.5 (1–26) | 2 (1–19) | 0.894 |
None of the women with placenta accreta/increta/percreta died. Four of the women lost or had their pregnancy terminated before 24 weeks’ gestation. The remaining 130 women gave birth to a total of 134 infants (126 singletons and four pairs of twins). There were no stillbirths and two early neonatal deaths among the 134 infants, equating to a perinatal mortality rate of 14.9 per 1000 (95% CI 1.8 to 52.8).
Uterine rupture
Incidence
There were 159 confirmed cases of uterine rupture in an estimated 852,206 maternities,82,92,94 representing an estimated incidence of 1.9 per 10,000 maternities (95% CI 1.6 to 2.2). Table 8 shows the estimated incidence of uterine rupture in different categories of women. Data collection forms were received for 448 controls (75% of those requested).
| Category | Number of women with a uterine rupture | Estimated number of maternities | Estimated incidence of uterine rupture (95% CI) per 10,000 maternities |
|---|---|---|---|
| Women without a previous caesarean delivery | 20 | 724,375 | 0.3 (0.2 to 0.4) |
| Women with a previous caesarean delivery | 139 | 127,831 | 11 (9 to 13) |
| Women with a previous caesarean delivery planning | |||
| Elective caesarean delivery in current pregnancy | 20 | 71,585 | 3 (2 to 4) |
| Vaginal delivery in current pregnancy | 116 | 56,246 | 21 (17 to 25) |
| Women with a previous caesarean delivery planning a vaginal delivery in current pregnancy and | |||
| laboured without prostaglandin inductiona or oxytocin used in labour | 52 | 41,622 | 13 (9 to 16) |
| labour induced with prostaglandins and/or oxytocin used in labour | 44 | 14,624 | 30 (22 to 40) |
| labour induced with prostaglandin and oxytocin not used in labour | 10 | 2812 | 36 (17 to 65) |
| laboured without prostaglandin inductiona but oxytocin used in labour | 28 | 10,124 | 28 (18 to 40) |
| labour induced with prostaglandin and oxytocin used in labour | 6 | 1687 | 36 (13 to 77) |
Risk factors for uterine rupture after prior delivery by caesarean section
A total of 139 (87%) of the uterine ruptures occurred in women who had previously delivered by caesarean section. Table 9 shows the characteristics of these women compared with control women. Women who had two or more previous caesarean deliveries had a higher odds of having a uterine rupture than women with only one previous caesarean delivery (aOR 3.02, 95% CI 1.16 to 7.85), as did women who had an interval of < 12 months compared with ≥ 24 months between their last caesarean section and their last menstrual period in their current pregnancy (aOR 3.12, 95% CI 1.62 to 6.02). There was no evidence to suggest a departure from linearity in the relationship between odds of rupture and number of caesarean deliveries, with the odds of rupture increasing by 3.02 (95% CI 1.62 to 5.63) for every one additional caesarean delivery. However, there was evidence of a non-linear relationship in the association between uterine rupture and caesarean section pregnancy interval, with the odds of rupture appearing to plateau for intervals beyond 12 months (Figure 1).
| Risk factor | n (%)a of cases with a previous caesarean (N = 139) | n (%)a of controls (N = 448) | Unadjusted OR (95% CI) | Adjustedb OR (95% CI) |
|---|---|---|---|---|
| Sociodemographic factors | ||||
| Age (years) | ||||
| < 35 | 94 (68) | 313 (70) | 1 | 1 |
| ≥ 35 | 45 (32) | 134 (30) | 1.12 (0.74 to 1.68) | 1.47 (0.89 to 2.45) |
| Ethnic group | ||||
| White | 94 (69) | 325 (75) | 1 | 1 |
| Non-white | 42 (31) | 111 (25) | 1.31 (0.86 to 2.00) | 1.12 (0.68 to 1.84) |
| Socioeconomic group | ||||
| Managerial and professional occupations | 33 (30) | 108 (32) | 1 | |
| Other | 77 (70) | 226 (68) | 1.12 (0.70 to 1.78) | |
| BMI at booking (kg/m2) | ||||
| < 25 | 56 (42) | 173 (40) | 1 | 1 |
| 25–29.9 | 43 (33) | 132 (31) | 1.01 (0.64 to 1.59) | 1.12 (0.65 to 1.91) |
| ≥ 30 | 33 (25) | 127 (29) | 0.8 (0.49 to 1.31) | 0.73 (0.41 to 1.30) |
| Previous obstetric and medical history | ||||
| Parity | ||||
| 1–2 | 116 (83) | 385 (86) | 1 | 1 |
| ≥ 3 | 23 (17) | 62 (14) | 1.23 (0.73 to 2.07) | 1.1 (0.57 to 2.14) |
| Number of previous caesarean deliveries | ||||
| 1 | 121 (87) | 368 (82) | 1 | 1 |
| ≥ 2 | 18 (13) | 79 (18) | 0.69 (0.40 to 1.20) | 3.02 (1.16 to 7.85) |
| Previous caesarean uterine incision type(s) | ||||
| All low transverse incisions | 120 (99) | 390 (98) | 1 | |
| Any non-low transverse incisions | 1 (1) | 8 (2) | 0.41 (0.05 to 3.28) | |
| Previous caesarean uterine closure type(s) | ||||
| All double | 75 (90) | 241 (91) | 1 | |
| All single | 5 (6) | 17 (6) | 0.95 (0.34 to 2.65) | |
| Mixture of double and single or other closure type | 3 (4) | 7 (3) | 1.38 (0.35 to 5.46) | |
| Previous uterine surgery | ||||
| No | 124 (90) | 394 (88) | 1 | 1 |
| Yes | 14 (10) | 52 (12) | 0.86 (0.46 to 1.60) | 0.92 (0.43 to 1.96) |
| Previous uterine performation | ||||
| No | 137 (100) | 446 (100) | ||
| Yes | 0 (0) | 1 (0) | ||
| Current pregnancy | ||||
| Twin pregnancy | ||||
| No | 139 (100) | 444 (99) | ||
| Yes | 0 (0) | 4 (1) | ||
| Interval between last caesarean section and last menstrual period (months) | ||||
| ≥ 24 | 71 (52) | 294 (67) | 1 | 1 |
| 12–23 | 35 (26) | 99 (22) | 1.46 (0.92 to 2.33) | 1.38 (0.80 to 2.38) |
| < 12 | 31 (23) | 48 (11) | 2.67 (1.59 to 4.50) | 3.12 (1.62 to 6.02) |
| Placenta praevia | ||||
| No | 136 (98) | 445 (99) | 1 | 1 |
| Yes | 3 (2) | 3 (1) | 3.27 (0.65 to 16.40) | 28.19 (4.03 to 197.39) |
| Macrosomia (birthweight ≥ 4000 g) | ||||
| No | 117 (89) | 382 (86) | 1 | 1 |
| Yes | 14 (11) | 62 (14) | 0.74 (0.40 to 1.36) | 0.85 (0.42 to 1.73) |
| Planned mode of delivery | ||||
| Elective caesarean section | 20 (15) | 250 (56) | 1 | 1 |
| Vaginal | 116 (85) | 198 (44) | 7.32 (4.40 to 12.19) | 19.37 (8.53 to 43.98) |
FIGURE 1.
Risk of uterine rupture according to the interval between the last caesarean section and start of current pregnancy. a, Adjusted for women’s age as a continuous variable, ethnicity, body mass index as a continuous linear term, parity as a continuous linear term, number of previous caesarean deliveries as a continuous linear term, previous uterine surgery, placenta praevia, macrosomia and planned mode of delivery.

The presence of placenta praevia increased the odds of rupture (aOR 28.19, 95% CI 4.03 to 197.39), although note that this finding is based on a very small number of women and should be interpreted with caution. The odds of rupture was also raised in women who planned to have a vaginal delivery in their current pregnancy compared with women who planned to deliver by elective caesarean section (aOR 19.37, 95% CI 8.53 to 43.98). This finding was irrespective of whether or not the women who planned to have a vaginal delivery had their labour induced and/or received oxytocin in labour (Table 10). However, the women who had prostaglandin labour induction and/or oxytocin used in labour appeared to have raised odds of rupture compared with the women who laboured without prostaglandin induction or oxytocin in labour (see Table 10). No significant interactions were found.
| Risk factor | n (%)a of cases with a previous caesarean (N = 139) | n (%)a of controls (N = 448) | Unadjusted OR (95% CI) | Adjustedb OR (95% CI) |
|---|---|---|---|---|
| Planned elective caesarean section delivery | 20 (17) | 250 (58) | 1 | 1 |
| Planned vaginal delivery and | ||||
| laboured without prostaglandin inductionc or oxytocin in labour | 52 (45) | 132 (31) | 4.92 (2.82 to 8.60) | 12.74 (5.44 to 29.87) |
| labour induced with prostaglandin and oxytocin not used in labour | 10 (9) | 9 (2) | 13.89 (5.06 to 38.10) | 35.91 (10.38 to 124.28) |
| laboured without prostaglandin inductionc but oxytocin in labour | 28 (24) | 33 (8) | 10.61 (5.38 to 20.91) | 35.36 (13.38 to 93.41) |
| labour induced with prostaglandin and oxytocin used in labour | 6 (5) | 5 (1) | 15.00 (4.21 to 53.48) | 52.05 (11.30 to 239.84) |
While dinoprostone (prostin, propess, prostaglandin E) was the agent used for all the control women induced with prostaglandin, dinoprostone was used in 82% and misoprostol in 18% of the women induced with prostaglandin who had a uterine rupture. Intrauterine death was the indication for induction for all of the women who received misoprostol.
A further exploratory analysis found that among women with a previous caesarean delivery, uterine rupture was no more likely to occur in women who had not had a previous vaginal delivery compared with those who had (79%, 110/139 of women with uterine rupture had not had a previous vaginal delivery compared with 74%, 331/446 of control women, aOR 1.43, 95% CI 0.65 to 3.13).
Outcomes
Two of the 159 women with a uterine rupture died, a case fatality of 1.3% (95% CI 0.2% to 4.5%). Fifteen (9%) women had a hysterectomy following uterine rupture, 10 (6%) women had one or more other organs damaged at rupture or removed during surgery and 69 (43%) women had other or additional morbidity following their uterine rupture.
Outcomes were known for 152 of the infants born to women with a uterine rupture. There were 15 stillbirths (12 antepartum, seven of which occurred prior to uterine rupture in women who were induced following intrauterine death, and three intrapartum) and 10 early neonatal deaths. Excluding the stillbirths that occurred prior to uterine rupture, the perinatal mortality rate was 124 per 1000 (95% CI 75 to 189 per 1000), significantly higher than the national rate of 7.5 per 1000 (RR 16.46, 95% CI 10.68 to 25.39 per 1000). 95 Major complications were reported in an additional 19 infants, including nine infants diagnosed with neonatal encephalopathy and six diagnosed with respiratory distress syndrome.
Conclusions and implications for practice
These studies identified several important messages for care and future research.
AFE: for every one woman with AFE who dies, four survive. Of the women with AFE who survive, 7% have permanent neurological injury. Outcome appears largely related to the severity of the clinical presentation. Women who die or have permanent neurological disability are more likely to have cardiac arrest at presentation and death most commonly occurs on the same day as the event. Women who die or have permanent neurological disability are more likely to have a hysterectomy, suggesting more intractable haemorrhage. Further investigation is needed to establish whether or not better and more rapid correction of coagulopathy, through the use of cryoprecipitate, fresh-frozen plasma, platelets and fibrinogen is associated with improved outcomes. Consideration needs to be given to whether or not earlier treatments, including correction of coagulopathy, can reverse the cascade of deterioration that seems to be present with AFE, and so improve survival in the most serious cases.
HELLP/ELLP syndrome: where there are sound reasons that a delay in delivery of women with HELLP or ELLP syndrome may be beneficial, for example to allow administration of corticosteroids for fetal lung maturation, this study suggests that a short delay, of up to 48 hours, may be considered. Current UK guidelines recommend corticosteroids when there is a risk of delivery prior to 35 completed weeks96 or planned caesarean delivery at < 39 completed weeks. Although we cannot comment on whether ELLP cases proceed to HELLP or whether they are different entities, it is clear that women with ELLP as well as HELLP have severe additional complications and, thus, ELLP should not be managed as a less severe form of HELLP. In particular, there is a high rate of eclampsia among both women with ELLP and HELLP, thus obstetricians should consider magnesium sulphate prophylaxis alongside delivery planning.
Placenta accreta/increta/percreta: women with both a prior caesarean delivery and placenta praevia have a high incidence of placenta accreta/increta/percreta (1 in every 20 women). In the absence of a completely sensitive and specific antenatal diagnostic technique,97 this highlights the importance of having a high index of suspicion of abnormal placental invasion and making preparations for delivery accordingly in this group of women. It also highlights the importance of preventing the first caesarean section in order to reduce the risk of subsequent placenta accreta/increta/percreta. Women with placenta accreta/increta/percreta who have no attempt to remove any of their placenta with the aim of conserving their uterus or prior to hysterectomy, have reduced levels of haemorrhage and reduced need for blood transfusion, supporting recommendation of this practice.
Uterine rupture: although uterine rupture is associated with significant maternal and perinatal mortality and morbidity, even among women with a previous caesarean section planning a vaginal delivery in their current pregnancy, it is rare, occurring in only one of every 500 women. For women with a previous caesarean section, the risk of uterine rupture increases not only with trial of labour but also with the number of previous caesarean deliveries, a short interval since the last caesarean section and labour induction and/or augmentation. These factors should be considered when counselling and managing the labour of women with a previous caesarean section.
Chapter 4 Severe maternal sepsis: identifying actions to address morbidity and mortality
This chapter includes excerpts from Acosta CD, Bhattacharya S, Tuffnell D, Kurinczuk JJ, Knight M. Maternal sepsis: a Scottish population-based case–control study. BJOG 2012;119:474–83. 98 © The Authors. BJOG An International Journal of Obstetrics and Gynaecology © 2012 RCOG, reproduced with permission.
Background
Maternal death from sepsis appears to be increasing in countries with advanced health-care systems. 2,99–101 Sepsis is estimated to cause 9.7% of maternal deaths in Africa, 11.6% in Asia and 7.7% in Latin America and the Caribbean combined. 102 In 2006–8, the UK maternal mortality rate from genital tract sepsis was 1.13/100,000 maternities, a rate not seen since the early 1970s,100,103 and in 2009–12, one-quarter of all maternal deaths were due to infectious causes. 2 Although the absolute risk of maternal death from sepsis is low, an increase in maternal mortality implies a greater number of women with severe, life-threatening illness. Recent work has suggested an approximate doubling of the incidence of maternal sepsis in the USA since 2003. 99
Key information gaps in the understanding of this pressing problem are the number of women affected, causative organisms, sources of infection and risk factors for severe sepsis and poor outcomes such as septic shock. Sepsis progresses along a spectrum of severity, so clarity about these factors has urgent implications for clinical management and infection control strategies to avoid preventable maternal deaths.
The objectives of this workstream were to estimate the incidence, investigate any temporal trends, describe the causative organisms and sources of infection, and identify the risk factors for severe maternal sepsis with the aim of informing strategies to improve outcomes for mothers and their babies through further development of guidelines for prevention and management of sepsis in pregnancy in the UK. The objectives were addressed using three complementary data sources: data from the Aberdeen Maternity and Neonatal Databank (AMND), ICNARC Case Mix Programme (CMP) database, and primary data collected using the UKOSS.
Methods
Data sources
Aberdeen Maternity and Neonatal Databank
All pregnancy-related events from the University of Aberdeen, Aberdeen Maternity Hospital, a tertiary-care maternity hospital for the NHS North of Scotland region, have been recorded in the AMND since 1950. The hospital is the only tertiary referral hospital in the region; it serves a large and well-defined geographical region and has approximately 5000 births per year. Data entry, coding protocols and consistency rates for internal validation and valid ranges of measurable variables are described in previous studies. 104–106
Intensive Care National Audit & Research Centre Case Mix Programme database
The ICNARC CMP is the national clinical audit for adult critical care units (including intensive care and combined intensive care and HDUs) in England, Wales and Northern Ireland. The CMP database contains pooled case mix data, collected from the first 24 hours following admission to the critical care unit and outcome data on consecutive admissions to units participating in the CMP. 107 The CMP database has been independently assessed to be of high quality. 108 All data used in this study were validated and from units that had been reporting to the CMP for at least 6 months. Support for the collection and use of patient-identifiable data without consent was obtained under Section 251 of the NHS Act 2006109 (approval number PIAG 2–10(f)/2005). Data were extracted on women who were reported to be pregnant or recently pregnant.
National birth statistics were used for comparison with CMP data in this study. Aggregate published data on maternal age, Index of Multiple Deprivation, multiple births and stillbirths were obtained from the Office for National Statistics (ONS) for England and Wales,110 and the Northern Ireland Statistics and Research Agency. 111 Data on ethnic groups were obtained from ONS and extrapolated for Northern Ireland based on the reported ethnic population distribution in the region. 112 Data on mode of delivery were obtained from Hospital Episode Statistics,113 StatWales114 and extrapolated for Northern Ireland based on published rates of mode of delivery in the region. 115
UK Obstetric Surveillance System
Primary data on severe maternal sepsis were collected using UKOSS. Data were collected as described in Chapter 3.
Analyses
Stata 11 statistical software was used for all analyses. Frequencies of demographic and clinical variables were tabulated for all studies and compared across groups using chi-squared tests for categorical variables and a Student’s t-test or Wilcoxon rank-sum test as appropriate for continuous variables.
Aberdeen Maternity and Neonatal Databank
An anonymised case–control study was conducted including all cases of maternal sepsis recorded in the AMND between 1986 and 2009. All cases were identified as those with an International Classification of Diseases, Ninth Edition (ICD-9) sepsis code: 038.0–038.9 (septicaemia), 634.0–639.0 (sepsis following abortion), 670.2 (puerperal sepsis) and 785.5 (septic shock). Severe (‘near-miss’) sepsis cases were identified as those with an ICD-9 code of septic shock or according to previously validated criteria defined by Martin et al. ,116 which were those with an additional ICD-9 code for acute organ dysfunction associated with sepsis. All other cases of sepsis are referred to as ‘uncomplicated’. All cases with an ICD-9 code for sepsis had a clinical diagnosis for systemic inflammatory response syndrome (SIRS) in addition to a culture-confirmed diagnosis of infection. Four controls per case selected from the AMND who did not have an ICD-9 code for sepsis were frequency matched to the cases on year of delivery.
The sample size of this study was limited by the population incidence of maternal sepsis and prevalence of exposures. As an illustration, with 103 cases (all cases occurring in the population over the study period) and four controls per case, the study had 90% power at p < 0.05 (two-sided) to detect a statistically significant OR of ≥ 2.3 associated with obesity, with a prevalence of exposure of 19%.
Univariable logistic regression analyses were carried out to initially identify demographic and clinical risk factors for uncomplicated maternal sepsis and severe sepsis; all p-values were unadjusted and two-sided, and a p-value of < 0.05 was considered statistically significant. Significant variables from univariable regression, or those factors that were plausible confounders, were included in a multivariable logistic regression model using a stepwise method. Potential confounding factors that were not significant at a p-value of < 0.05 in the initial multivariable regression (blood loss of ≤ 500 ml, pre-eclampsia and previous miscarriage) were removed from the final model. Interactions between demographic and clinical variables were assessed using likelihood ratio tests (LR-tests) with a significance level of p < 0.01; no interactions were identified in the final adjusted model. Only two cases occurred antenatally and removal of these cases did not significantly change the regression results, so the antenatal cases were retained in the final regression models. Multivariable regression results were adjusted for calendar time and all other factors in the model. Results are reported as unadjusted OR (uOR) and aOR with 95% CIs.
Research ethics committee approval for use of anonymised data was not required. Approval of the research protocol was obtained from the Steering Committee of the AMND before data extraction.
Intensive Care National Audit & Research Centre
A cohort study of all pregnant and ‘recently pregnant’ women who were reported in the CMP from 2008 to 2010, and who were either admitted with or developed severe sepsis within the first 24 hours of admission, was conducted. Recently pregnant women were defined as having been pregnant within 42 days of admission to the critical care unit. Severe sepsis was defined according to a modified version of the Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) clinical trial definition. 117 Septic shock was defined as severe sepsis with cardiovascular organ system dysfunction. 118 Readmissions of women to critical care during the same hospital stay were excluded.
The total number of maternal critical care admissions with severe sepsis was estimated based on the number of severe maternal sepsis admissions observed in the CMP database each year, the CMP reporting coverage rate for each year and the total number of adult general critical care units in England, Wales and Northern Ireland. Using all maternities in England, Wales and Northern Ireland as the comparison group, the RR with 95% CI for maternal critical care admission with severe sepsis was calculated for each variable.
The characteristics of cohort survivors were compared with those of non-survivors in a univariable logistic regression model. Based on previous literature and plausible confounding, variables were then modelled using multivariable logistic regression. Mortality was measured by death at ultimate discharge from acute hospital. Multivariable regression results were adjusted for calendar time and all other factors in the model. The continuous variables age and body mass index (BMI) were treated as categorical variables in the multivariable regression model as there was a large amount of missing information for BMI and there was evidence of departure from linearity for age. Missing data for BMI were accounted for by retaining a category for ‘unknown BMI’ within the BMI variable in all regression models. After accounting for missing BMI data, complete-case analyses were used as levels of missing data were very low for all other variables. Assuming a prevalence of exposure of at least 15% among survivors, the model had 80% power at p < 0.05 (two-sided) to detect a statistically significant OR of ≥ 3.5. The results are reported as uORs and aORs with 95% CIs.
UK Obstetric Surveillance System
A national prospective case–control study of all peripartum women diagnosed with severe sepsis (including septic shock), irrespective of the source of infection, was carried out in all obstetrician-led maternity units in the UK from 1 June 2011 to 31 May 2012.
As there is currently no standardised definition for severe sepsis in pregnant and peripartum women, the study definition for surveillance purposes was developed based on previous literature and by consensus of the UKOSS steering committee. 10 In the non-obstetric population, consensus definitions of sepsis severity (SIRS, sepsis, severe sepsis and septic shock) were developed in 1992. 119 However, these definitions and subsequent improvements are often not applicable to pregnant and peripartum women because clinical signs and symptoms of severe infection differ in this population. Specifically, SIRS can be a sign of ruptured membranes and changing biochemistry associated with labour and delivery, as well as a clinical marker of severe infection. Therefore, the clinical parameters of SIRS in the presence of an infection are often altered in the obstetric population. We adopted the ‘obstetric SIRS’ criteria from a 2001 study of severe obstetric morbidity4 and took into account clinical management (level 2 or level 3 critical care)120 and whether or not the woman died. The full case definition for this study is listed in Box 2. Controls were women who did not have severe sepsis and who delivered immediately before each case in the same hospital. For women transferred to higher-level hospitals, controls were drawn from the delivery hospital. The source population was, thus, all women giving birth in the UK.
Applied to women at any point in pregnancy and up to 6 weeks postpartum:
-
death related to infection or suspected infection
-
any woman requiring level 2 or level 3 critical care (or obstetric HDU-type care) with severe sepsis or suspected severe sepsis
-
a clinical diagnosis of severe sepsis:
-
temperature > 38 °C or < 36 °C, measured on two occasions at least 4 hours apart
-
heart rate > 100 b.p.m., measured on two occasions at least 4 hours apart
-
respiratory rate > 20 breaths per minute, measured on two occasions at least 4 hours apart
-
white cell count > 17 × 109/l or < 4 × 109/l or with > 10% immature band forms, measured on two occasions.
-
Level 2 care is defined as patients requiring more detailed observation or intervention, single failing organ system or postoperative care, and higher levels of care. Level 3 care is defined as patients requiring advanced respiratory support alone or basic respiratory support together with support of at least two organ systems. This level includes all complex patients requiring support for multiorgan failure.
The study included a descriptive analysis of the incidence, causative organisms, sources of infection and outcomes of severe sepsis, and a case–control analysis of factors associated with severe sepsis and septic shock. In order to assess risk factors for developing severe sepsis, all cases were compared with non-septic controls. To assess the risk of progression to septic shock, cases with a diagnosis of septic shock were compared with all other cases with severe sepsis that did not develop into septic shock.
The incidences of severe maternal sepsis and septic shock with 95% CIs were calculated using the number of maternities reported in the most recent national birth data78,90,91 as the denominator. Women with signs and symptoms of sepsis prior to delivery were classified as antepartum cases. Sources of infection, causative organisms, and sepsis severity characteristics were tabulated for all cases and stratified according to partum status, as pathogenesis is known to differ between pregnant and postpartum women. 121
For risk factor analyses, sociodemographic, medical history and delivery characteristics with a priori evidence of an association with sepsis were compared between cases and controls, and between cases with and without septic shock. Sources of infection and causative organisms were also assessed as risk factors in the latter comparison. The proportion of missing data in this study was very low; the only variables with substantial missing data (> 1%) were source and organism of infection and socioeconomic group. In order to account for the missing data for sources of infection, causative organisms and socioeconomic group, the subcategories of ‘unknown’ and ‘no laboratory-confirmed infection’ were included for these variables in all analyses.
The odds of severe sepsis and septic shock associated with each risk factor were estimated using univariable unconditional logistic regression and were then adjusted using multivariable unconditional logistic regression. For both the severe sepsis and septic shock outcome groups, factors were adjusted in two stages. First, all a priori sociodemographic and medical history factors, with the exception of previous caesarean delivery and previous pregnancy problem (as these were dependent on parity) and partum status (as the control population was only women who had delivered), were included in a primary model. Second, delivery factors were then adjusted for a priori risk factors using a more parsimonious approach in order to avoid overadjustment or substantial colinearity given the large number of variables. Results were adjusted only for a priori factors from the primary model that were known risk factors, were significant in the primary model at a p-value of < 0.05, or were plausible confounders as identified in previous literature. Delivery characteristics were evaluated for postpartum cases only, as this set of risk factors pertained specifically to delivery.
Results of both stages of adjustment are reported as uORs and aORs and their 95% CIs for severe sepsis. For ease of presentation of risk factors for progression to septic shock, results are reported only for factors included in the final adjusted models. LR-tests with a significance level of p < 0.01 were used to check for interactions between variables; no significant interactions were identified in the final adjusted models.
Within a 1-year study period, we anticipated approximately 316 cases of severe sepsis based on an estimated incidence of 4 per 10,000 maternities. 4 For the severe sepsis risk factor analysis, with two controls per case, and for a risk factor prevalence of at least 5% in control women, the study was estimated to have had 80% power at p < 0.05 (two-sided) to detect a statistically significant OR of ≥ 2.3. The actual number of cases and controls identified during the study period of 12 months generated an estimated power of 80% at the 5% level of significance to detect an OR of ≥ 2.1, for the same risk factor prevalence level. For the septic shock risk factor analysis, for a risk factor prevalence of at least 15% in women without septic shock, the analysis had 80% power at the 5% level of significance to detect an OR of ≥ 2.6.
Results
Aberdeen Maternity and Neonatal Databank
The total study population comprised 89 cases of uncomplicated maternal sepsis, 14 cases of severe maternal sepsis and 412 control women. The majority of all cases occurred postpartum and before hospital discharge [98.9% (n = 88) of women with uncomplicated sepsis and 92.9% (n = 13) of women with severe sepsis]. One woman had uncomplicated sepsis antepartum and one women with severe sepsis was diagnosed with an infection during delivery, which later progressed to septic shock postpartum. Sepsis case rates for the 23-year study period are shown in Figure 2. Although rates of severe sepsis remained relatively constant over the study period, rates of sepsis overall have increased significantly since 2003 (p = 0.002) compared with the previous two decades. Given the increase in awareness of sepsis, we cannot exclude that this represents an increase in diagnosis/reporting rather than an increase in disease.
FIGURE 2.
Incidence of all sepsis and severe sepsis cases per 1000 maternities from 1986 to 2009 in Aberdeen (3-year rolling averages). Reproduced with permission from Acosta et al. , © The Authors. BJOG An International Journal of Obstetrics and Gynaecology © 2012 RCOG.
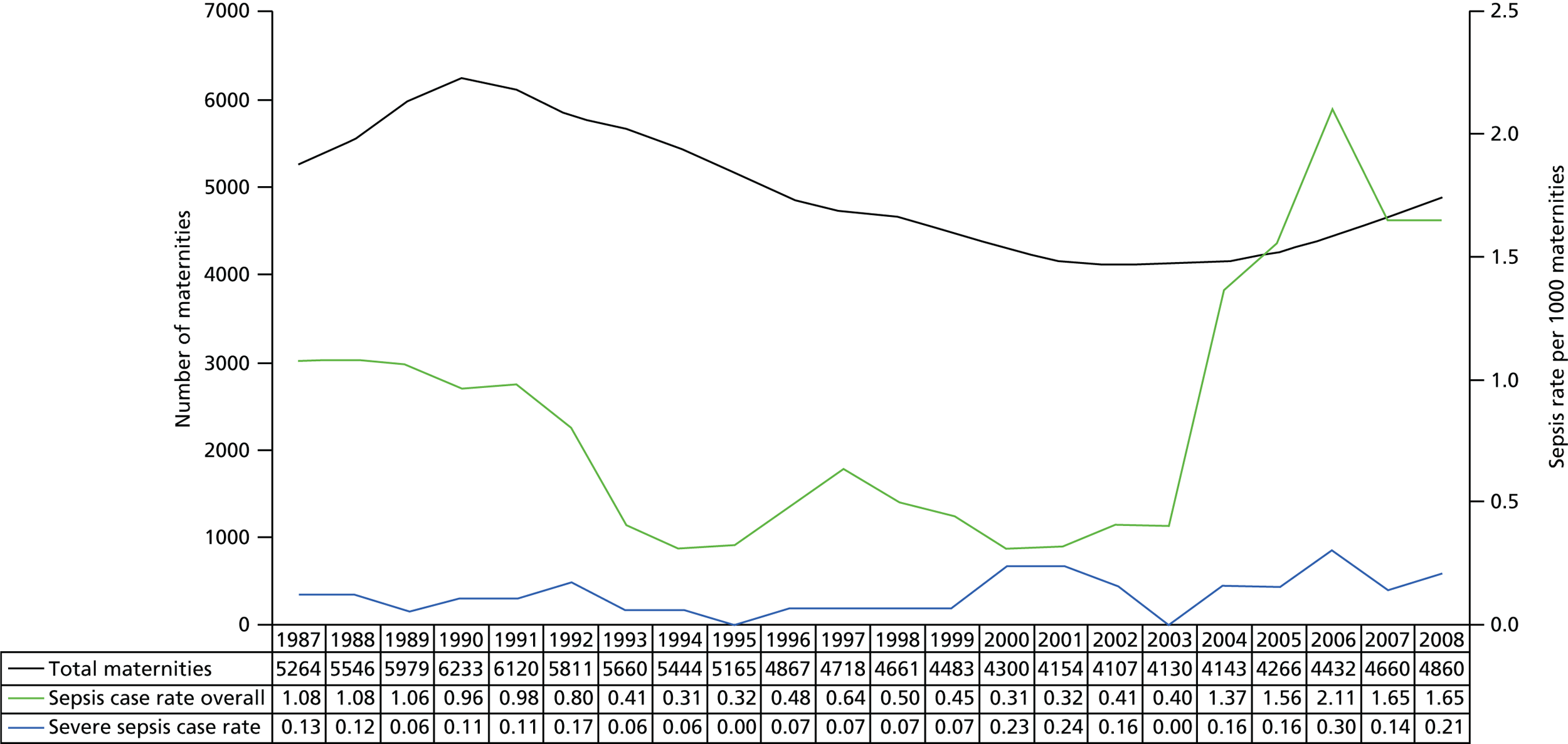
Over the 23-year study period, the proportion of spontaneous vaginal deliveries decreased while the proportion of operative vaginal deliveries and caesarean sections increased significantly (p < 0.001, p = 0.009 and p < 0.001, respectively). Despite these trends, sepsis case rates remained predominantly the highest among women who had a caesarean section throughout the study period, although rates increased in all modes from 2003 onwards. Antibiotic usage (before discharge from the labour ward) in the overall study population increased steadily from 1986 to 1996 (p < 0.001) and plateaued from 1997 to 2009. Although there was no significant difference between cases and controls in the total proportion of antibiotic usage across all modes of delivery (Table 11) among the women with sepsis who had a caesarean section, 38.5% received antibiotics during pregnancy or delivery, which was significantly lower than the 70.2% of control women who had a caesarean section and received antibiotics (p < 0.001). In addition, antibiotic usage among control women who had a caesarean section increased significantly over time (p = 0.003), whereas antibiotic usage among cases who had a caesarean section did not increase over time. There was no significant difference in antibiotic usage among women in the case and control groups who had a manual placenta removal or an operative vaginal delivery.
| Factor | Uncomplicated sepsis | Severe sepsis | ||||||
|---|---|---|---|---|---|---|---|---|
| uOR | 95% CI | aOR | 95% CI | uOR | 95% CI | aOR | 95% CI | |
| Demographic characteristics | ||||||||
| Age groups | ||||||||
| < 25 years | 4.94 | 2.64 to 9.22 | 5.15 | 2.43 to 10.90 | 5.10 | 1.32 to 19.78 | 10.17 | 1.86 to 55.52 |
| 25–34 years | 2.88 | 1.68 to 4.93 | 2.56 | 1.37 to 4.78 | 2.63 | 0.74 to 9.27 | 3.26 | 0.61 to 17.41 |
| > 34 years | 1a | 1a | 1a | 1a | ||||
| Parity | ||||||||
| 0 | 1a | 1a | 1a | 1a | ||||
| 1 | 7.13 | 3.90 to 13.01 | 7.44 | 3.64 to 15.23 | 4.47 | 1.10 to 18.15 | 6.31 | 0.94 to 42.55 |
| ≥ 2 | 3.54 | 1.91 to 6.56 | 6.29 | 2.88 to 13.77 | 4.15 | 1.17 to 14.72 | 12.04 | 2.07 to 69.90 |
| Late booking (second or third trimester) | 0.96 | 0.49 to 1.88 | – | – | ||||
| Marital status | ||||||||
| Married | 1a | 1a | ||||||
| Single supported | 1.17 | 0.65 to 2.13 | 0.74 | 0.16 to 3.41 | ||||
| Single unsupported | 1.52 | 0.71 to 3.26 | 0.82 | 0.10 to 6.54 | ||||
| Divorced or separated | 5.03 | 0.99 to 25.55 | – | – | ||||
| Medical characteristics | ||||||||
| Obese | 2.4 | 1.46 to 3.96 | 2.12 | 1.14 to 3.89 | 0.71 | 0.16 to 3.25 | ||
| Smoked during pregnancy | 1.46 | 0.86 to 2.48 | 0.30 | 0.04 to 2.36 | ||||
| Anaemia | 3.43 | 2.11 to 5.58 | 3.44 | 1.93 to 6.13 | 21.55 | 2.79 to 166.4 | 18.5 | 1.97 to 173.1 |
| Diabetes | 3.13 | 0.52 to 19.04 | – | – | ||||
| Any previous miscarriage | 2.41 | 0.88 to 6.60 | 5.56 | 1.12 to 27.61 | ||||
| Clinical delivery characteristics | ||||||||
| Premature rupture of membranes | 0.72 | 0.27 to 1.90 | 2.92 | 0.57 to 14.99 | ||||
| Type of membrane rupture | ||||||||
| Artificial | 1a | 1a | ||||||
| Spontaneous | 1.25 | 0.76 to 2.08 | 2.61 | 0.68 to 9.99 | ||||
| Induced labour | 1.05 | 0.65 to 1.70 | 3.54 | 1.16 to 10.75 | 3.92 | 1.02 to 15.35 | ||
| Prolonged labour | 1.30 | 0.77 to 2.20 | 1.55 | 0.46 to 5.17 | ||||
| Type of delivery | ||||||||
| Spontaneous vaginal | 1a | 1a | 1a | 1a | ||||
| Operative vaginalb | 1.13 | 0.59 to 2.15 | 2.20 | 1.02 to 4.87 | 2.89 | 0.47 to 17.54 | 6.39 | 0.72 to 56.46 |
| Caesarean section | 2.88 | 1.70 to 4.88 | 3.23 | 1.65 to 6.34 | 8.74 | 1.85 to 41.19 | 13.35 | 2.08 to 85.68 |
| Manual placenta removal | 1.22 | 0.44 to 3.35 | 2.16 | 0.26 to 17.68 | ||||
| ≥ 500 ml blood loss at delivery | 1.97 | 1.21 to 3.19 | 7.96 | 2.44 to 25.94 | ||||
| Perineal wound | 0.32 | 0.20 to 0.52 | 0.10 | 0.02 to 0.47 | ||||
| Antibiotics during pregnancy or deliveryc | 0.95 | 0.60 to 1.52 | 0.37 | 0.10 to 1.33 | ||||
| Severe pre-eclampsia | 2.69 | 1.40 to 5.18 | 3.35 | 0.89 to 12.65 | ||||
| Haemorrhage, placental abruption, or placenta praevia | 1.34 | 0.67 to 2.65 | 1.43 | 0.31 to 6.60 | ||||
| Pre-term birth | 3.00 | 1.58 to 5.74 | 2.46 | 1.11 to 5.47 | 3.47 | 0.92 to 13.13 | ||
After adjusting for changes over time and the other factors in each model, factors significantly associated with both uncomplicated sepsis and severe sepsis were younger age, multiparity, anaemia, operative vaginal delivery and caesarean section (compared with spontaneous vaginal delivery) (see Table 11). Obesity was significantly associated with uncomplicated sepsis; however, this association was not present with severe sepsis. Additionally, operative vaginal delivery and pre-term birth were associated with uncomplicated sepsis, whereas induced labour was associated with severe sepsis.
Intensive Care National Audit & Research Centre
In the CMP database between 2008 and 2010, there were 646 pregnant or recently pregnant women who met the case definition for severe sepsis, which represented 14.4% of maternal critical care unit admissions and 10.6% (n = 474) had septic shock. The absolute risk of maternal critical care admission with severe sepsis was 4.1 per 10,000 maternities (95% CI 2.9 to 5.6).
Characteristics of the cohort are shown in Table 12. Women had a significantly higher risk of admission to critical care with severe sepsis if they were aged < 20 years or ≥ 40 years than if they were aged 25–29 years. Increased risk of severe sepsis was also significantly and progressively associated with increasing deprivation based on area of residence.
| Critical care admissions with sepsis | All maternities | Relative risk (95% CI) | |
|---|---|---|---|
| Total, n (%) | 646 (100.0) | 2,186,818 | |
| Recently pregnant | 413 (63.9) | ||
| Maternal age (years), mean (SD) | 28.3 (6.9) | 29.0 (–) | |
| < 20 | 80 (12.4) | 129,167 (5.9) | 2.5 (1.9 to 3.3) |
| 20–24 | 133 (20.6) | 419,944 (19.2) | 1.3 (1.0 to 1.6) |
| 25–29 | 148 (23.0) | 602,716 (27.6) | 1a |
| 30–34 | 153 (23.7) | 600,694 (27.5) | 1.0 (0.8 to 1.3) |
| 35–39 | 95 (14.7) | 352,364 (16.1) | 1.1 (0.9 to 1.4) |
| ≥ 40 | 36 (5.6) | 81,933 (3.7) | 1.8 (1.2 to 2.6) |
| Black and other minority ethnic groups, n (%) | 154 (23.8) | 609,250 (27.9) | 0.9 (0.8 to 1.0) |
| IMD (quintiles),b n (%) | |||
| 1 (least deprived) | 63 (10.1) | 589,784 (27.6) | 1a |
| 2 | 75 (12.0) | 475,573 (22.2) | 1.5 (1.1 to 2.1) |
| 3 | 109 (17.5) | 395,137 (18.5) | 2.6 (1.9 to 3.5) |
| 4 | 148 (23.8) | 346,002 (16.2) | 4.0 (3.0 to 5.4) |
| 5 (most deprived) | 228 (36.6) | 330,916 (15.5) | 6.5 (4.9 to 8.5) |
| BMI (kg/m2), median (IQR)b (n = 312) | 26 (22–30) | ||
| History of immunosuppression, n (%) | 15 (2.3) | ||
| Weeks’ gestation | |||
| Antenatal, median (IQR) | 26 (20–31) | ||
| Postnatal, median (IQR) | 38 (31–41) | ||
| Recently pregnant women only | |||
| Parity,b n (%) | |||
| 0 | 193 (48.4) | ||
| 1 | 96 (24.1) | ||
| ≥ 2 | 110 (27.6) | ||
| Assisted conception,b n (%) | 24 (8.1) | ||
| Mode of delivery,c n (%) | |||
| Spontaneous vaginal | 98 (25.9) | 1,334,242 (61.0) | 1a |
| Assisted vaginal | 28 (7.4) | 273,340 (12.5) | 1.4 (0.9 to 2.1) |
| Caesarean section | 242 (64.0) | 535,999 (24.5) | 6.2 (4.9 to 7.8) |
| Unknown | 10 (2.7) | ||
| All multiple births (live births and stillbirths), n (%) | 28 (7.6) | 34,663 (1.6) | 4.4 (3.1 to 6.3) |
| Pregnancy outcomes, n (%) | |||
| Live births | 321 (77.7) | ||
| Stillbirths | 47 (11.4) | 11,697 (0.5%) | 21.3 (16.3 to 27.9) |
| First/second trimester loss | 25 (6.1) | ||
| Ectopic pregnancy | 10 (2.4) | ||
| Otherd | 2 (0.5) | ||
| Unknown | 10 (2.4) | ||
| Hysterectomy, n (%)b | 20 (5.4) | ||
| Days since delivery, median (IQR) | 3 (0–8) | ||
The source of infection could be identified from the primary reason for admission to the critical care unit for 598 women (92.6%). Frequencies of the reported source of infection among women admitted with severe sepsis are shown in Figure 3. The most common source of infection was pneumonia/respiratory infection (n = 257; 40%). Of these, only 27 were identified as laboratory confirmed cases of AH1N1 influenza (2009 and 2010 were AH1N1 influenza pandemic years).
FIGURE 3.
Source of infection among pregnant or recently pregnant women admitted to critical care units in England, Wales and Northern Ireland 2008–10.
Of all severe maternal sepsis admissions, 4.6% (n = 29) died. The absolute risk of acute hospital mortality of women admitted was 1.8 per 100,000 maternities (95% CI 1.1 to 2.8). Pneumonia/respiratory infection was the most common source of sepsis among women who died (n = 12; 41.4%). Factors associated with mortality are shown in Table 13. After adjustment for a priori factors and changes over time, deprivation, being overweight or obese, respiratory dysfunction and haematological dysfunction were significant independent risk factors for mortality.
| Characteristic | Severe sepsis survivors (n = 610), n (%) | Severe sepsis deaths (n = 29), n (%) | uOR (95% CI) | aOR (95% CI) |
|---|---|---|---|---|
| Recently pregnant | 387 (63.4) | 22 (75.9) | 1.8 (0.8 to 4.3) | 1.1 (0.2 to 3.0) |
| Maternal age (years) | ||||
| < 25 | 234 (38.4) | 5 (17.2) | 1a | 1a |
| 25–34 | 254 (41.7) | 15 (51.7) | 2.8 (1.0 to 7.7) | 2.2 (0.71 to 7.0) |
| ≥ 35 | 121 (19.9) | 9 (31.0) | 3.5 (1.1 to 10.6) | 3.3 (0.4 to 11.2) |
| Black and other minority ethnic groups | 147 (24.1) | 7 (24.1) | 1.0 (0.4 to 2.5) | 0.6 (0.21 to 1.6) |
| IMD quintiles 4 and 5 | 354 (58.1) | 17 (58.6) | 1.02 (0.5 to 2.2) | 2.6 (1.3 to 6.7) |
| BMI (kg/m2) | ||||
| Unknown | 317 (52 1) | 13 (44.8) | 0.8 (0.2 to 3.7) | 1.2 (0.2 to 9.1) |
| < 25 | 126 (20.7) | 3 (10.3) | 1a | 1a |
| ≥ 25 to < 30 | 90 (14.8) | 7 (241) | 3.3 (0.8 to 13.3) | 5.2 (1.4 to 18.9) |
| ≥ 30 | 76 (12.5) | 6 (20.7) | 3.5 (0.9 to 14.6) | 6.3 (1.5 to 27.0) |
| History of immunosuppression | 13 (2.1) | 2 (6.9) | 3.3 (0.7 to 15.7) | |
| Weeks’ gestation | ||||
| Antenatal | ||||
| ≥ 37 | 16 (7.0) | 0 (0.0) | – | |
| 25–36 | 104 (47.5) | 3 (42.9) | 1a | |
| < 25 | 99 (45.2) | 4 (57.1) | 1.4 (0.3 to 6.4) | |
| Postnatal | ||||
| ≥ 37 | 202 (54.9) | 14 (66.7) | 1a | |
| 25–36 | 114 (31.0) | 3 (14.3) | 0.4 (0.1 to 1.3) | |
| < 25 | 52 (14.1) | 4 (19.1) | 1.1 (0.4 to 3.5) | |
| Recently pregnant women only | ||||
| Parity | ||||
| 0 | 183 (48.9) | 9 (42.9) | 1a | |
| 1 | 88 (23.5) | 7 (33.3) | 1.6 (0.6 to 4.5) | |
| ≥ 2 | 103 (27.5) | 5 (23.8) | 1.0 (0.3 to 3.0) | |
| Assisted conception | 24 (8.7) | 0 (0.0) | ||
| Mode of delivery | ||||
| Spontaneous vaginal | 89 (23.6) | 8 (36.4) | 1a | |
| Assisted vaginal | 28 (7.4) | 0 (0.0) | – | |
| Caesarean section | 227 (60.2) | 12 (54.6) | 0.6 (0.2 to 1.5) | |
| Termination | 23 (6.1) | 2 (9.1) | 1.0 (0.2 to 4.9) | |
| Ectopic | 10 (2.7) | 0 (0.0) | – | |
| All multiple births | 24 (7.0) | 3 (15.0) | 2.3 (0.6 to 8.6) | |
| Stillbirth(s) | 42 (21.9) | 4 (33.3) | 1.8 (0.5 to 6.2) | |
| Hysterectomy | 16 (4.6) | 3 (15.0) | 3.6 (1.0 to 13.7) | |
| < 24 hours since delivery | 104 (27.6) | 8 (36.4) | 1.5 (0.6 to 3.7) | |
| Source of infectionb | ||||
| Pneumonia (chest infection) | 216 (35.4) | 12 (41.4) | 1.3 (0.6 to 2.7) | |
| Intrauterine infection | 69 (11.3) | 2 (6.9) | 0.6 (0.1 to 2.5) | |
| Pelvic infection | 47 (7.7) | 0 (0.0) | – | |
| UTI/pyelonephritis | 43 (7.1) | 0 (0.0) | – | |
| Number of organ system dysfunctions | ||||
| 1 | 221 (36.2) | 2 (6.9) | 1a | |
| 2 | 224 (36.7) | 8 (27.6) | 3.9 (0.8 to 18.7) | |
| ≥ 3 | 165 (27.1) | 19 (65.5) | 12.7 (2.9 to 55.1) | |
| Organ system dysfunctionc | ||||
| Cardiovascular | 444 (72.8) | 24 (82.8) | 1.8 (0.7 to 4.8) | |
| Respiratory | 349 (57.2) | 26 (89.7) | 6.5 (1.9 to 21.6) | 8.1 (1.8 to 36.0) |
| Metabolic acidosis | 322 (52.8) | 18 (62.1) | 1.5 (0.7 to 3.1) | |
| Renal | 39 (6.4) | 8 (27.6) | 5.6 (2.3 to 13.4) | 2.9 (0.9 to 9.3) |
| Haematological | 52 (8.5) | 11 (37.9) | 6.5 (2.9 to 14.6) | 5.7 (2.0 to 16.0) |
UK Obstetric Surveillance System
During the study period, there were a total of 365 confirmed cases of severe sepsis out of 780,537 maternities in the UK,11–13 representing an incidence of 4.7 per 10,000 maternities (95% CI 4.2 to 5.2). Seventy-one women (20%) developed septic shock, which represents an incidence of 0.91 per 10,000 maternities (95% CI 0.71 to 1.15). Data were collected on 757 controls.
Laboratory-confirmed infection was reported for 233 (63.8%) severe sepsis cases and a source of infection was identified for 270 cases (74.0%); 60 cases (16.4%) had neither a source of infection nor causative organism identified. The distribution of sources of infection, causative organisms and severity characteristics are shown in Table 14 and Figure 4. Overall, the largest proportion of cases was due to genital tract infection (31.0%) and the most common organism causing infection was Escherichia coli (21.1%). However, the distributions of both the infection source and the causative organism differed significantly between women with antepartum and postpartum sepsis (p < 0.0001 for both), as did the risk of septic shock. Readmission (for reasons other than delivery) also differed significantly between the two groups: 108 (48%) women with postpartum sepsis were readmitted, compared with six (5%) women with antepartum sepsis (p < 0.0001). Of all cases, 286 (78%) received level 2 or intensive care and five women died (see Table 14). Of the women who died, two had infection with E. coli and three women had an unknown causative organism. Twenty-nine women (8%) with severe sepsis had either a miscarriage or a termination of pregnancy. For women diagnosed with severe sepsis antenatally, 5 of 137 infants were stillborn (3.6%) and 7 died in the neonatal period (5.1%). Fifty-eight infants (42.3%) were admitted to the NICU.
| Characteristic | Antepartum, n (%) (N = 134) | Postpartum,a n (%) (N = 231) | p-value | Total, n (%) (N = 365) |
|---|---|---|---|---|
| Source of infection | < 0.0001 | |||
| Genital tract | 27 (20.2) | 86 (37.2) | 113 (31.0) | |
| Urinary tract | 45 (33.6) | 27 (11.7) | 72 (19.7) | |
| Wound | 0 (0.0) | 33 (14.3) | 33 (9.0) | |
| Respiratory | 12 (9.0) | 8 (3.5) | 20 (5.5) | |
| Other | 10 (7.5) | 22 (9.5) | 32 (8.8) | |
| Unknown | 40 (29.9) | 55 (23.8) | 95 (26.0) | |
| Organism | < 0.0001 | |||
| Escherichia coli | 33 (24.6) | 44 (19.1) | 77 (21.1) | |
| Group A Streptococcus | 2 (1.5) | 30 (13.0) | 32 (8.8) | |
| Group B Streptococcus | 13 (9.7) | 17 (7.4) | 30 (8.2) | |
| Other Streptococcus | 6 (4.5) | 15 (6.5) | 21 (5.7) | |
| Staphylococcus | 2 (1.5) | 21 (9.1) | 23 (6.3) | |
| Mixed organisms | 5 (3.7) | 14 (6.1) | 19 (5.2) | |
| Other | 12 (9.0) | 13 (5.6) | 25 (6.9) | |
| Unknown | 5 (3.7) | 1 (0.4) | 6 (1.6) | |
| No laboratory-confirmed infection | 56 (41.8) | 76 (32.9) | 132 (36.2) | |
| Severity | ||||
| Level 2 or ITU admission | 103 (76.9) | 183 (79.2) | 0.598 | 286 (78.4) |
| Level 2 admission | 64 (47.8) | 107 (46.3) | 0.79 | 171 (46.9) |
| ITU admissionb | 39 (29.1) | 75 (32.5) | 0.504 | 114 (31.2) |
| Septic shock | 16 (11.9) | 55 (23.8) | 0.006 | 71 (19.5) |
| Death | 2 (1.5) | 3 (1.3) | 0.915 | 5 (1.4) |
FIGURE 4.
Distribution of causative organisms according to (a) source of infection; and (b) mode of delivery. Stacked bars represent the number of cases with specific causative organisms according to infection source and mode of delivery categories. Data are mutually exclusive.

There were 296 cases with recorded dates and times for the first sign of SIRS and the severe sepsis diagnosis. For 245 (83%) severe sepsis cases and for 49 (85%) septic shock cases, there were < 24 hours between the first sign of SIRS and the diagnosis of severe sepsis, and for 264 (89%) severe sepsis cases and for 55 (95%) septic shock cases there were < 48 hours between the first sign of SIRS and the diagnosis of severe sepsis. Additionally, for 16 (50%) women with a group A streptococcal infection there were < 2 hours and for 24 (75%) women there were < 9 hours, between the first sign of SIRS and the diagnosis of severe sepsis.
A priori sociodemographic and medical history characteristics of women with severe sepsis compared with control women are listed in Table 15. After adjustment and compared with controls, women who were of black or other minority ethnic origin, were primiparous, had a pre-existing medical problem, or had a febrile illness or were taking antibiotics in the 2 weeks prior to presentation were at significantly increased odds of severe sepsis. There was no statistically significant association between premature rupture of membranes and severe sepsis in either antenatal cases (n = 20, aOR 1.72, 95% CI 0.98 to 3.02) or postnatal cases (Table 16). In addition to significant a priori factors, the following factors significantly increased the odds of severe sepsis in women with postpartum sepsis: having an operative vaginal delivery (aOR 2.49, 95% CI 1.32 to 4.70), having a pre-labour caesarean section (aOR 3.83, 95% CI 2.24 to 6.56), having a caesarean section after the onset of labour (aOR 8.06, 95% CI 4.65 to 13.97), or having a complication of delivery (aOR 1.69, 95% CI 1.09 to 2.63) (see Table 16). Of note, of all women who had a caesarean section, 96.6% of cases and 94.8% of controls received prophylactic antibiotics at delivery.
| Risk factor | Cases, n (%)a (N = 365) | Controls, n (%)a (N = 757) | χ2 p-value | uOR | 95% CI | aORb | 95% CI |
|---|---|---|---|---|---|---|---|
| Sociodemographic factors | |||||||
| Age (years) | < 0.001 | ||||||
| < 25 | 117 (32.0) | 158 (20.9) | 1.73 | 1.29 to 2.32 | 1.38 | 0.96 to 2.00 | |
| 25–34 | 186 (51.0) | 438 (57.9) | 1 | 1 | |||
| ≥ 35 | 62 (17.0) | 160 (21.1) | 0.91 | 0.65 to 1.28 | 1.00 | 0.67 to 1.51 | |
| Ethnic group | 0.003 | ||||||
| White | 221 (60.7) | 525 (69.5) | 1 | 1 | |||
| Black and other minority | 143 (39.3) | 230 (30.5) | 1.48 | 1.14 to 1.92 | 1.82 | 1.32 to 2.51 | |
| Socioeconomic group | 0.001 | ||||||
| Managerial and professional occupations | 68 (19.7) | 189 (25.6) | 1 | 1 | |||
| Intermediate | 63 (18.2) | 147 (20.0) | 1.19 | 0.79 to 1.79 | 1.17 | 0.73 to 1.88 | |
| Manual | 96 (27.8) | 224 (30.4) | 1.19 | 0.83 to 1.72 | 1.26 | 0.81 to 1.94 | |
| Unemployed | 29 (8.0) | 46 (6.1) | 1.75 | 1.02 to 3.01 | 1.56 | 0.82 to 2.97 | |
| Unknown | 109 (29.9) | 151 (19.9) | 2.00 | 1.38 to 2.91 | 1.63 | 1.02 to 2.61 | |
| Marital status | 0.006 | ||||||
| Single | 85 (23.3) | 124 (16.4) | 1.54 | 1.13 to 2.10 | 1.13 | 0.75 to 1.69 | |
| Married or cohabitating | 280 (76.7) | 630 (83.6) | 1 | 1 | |||
| Obstetric and medical factors | |||||||
| Late booking | 0.18 | ||||||
| Yes | 85 (23.3) | 150 (19.8) | 1.23 | 0.91 to 1.66 | 1.08 | 0.77 to 1.50 | |
| No | 280 (76.7) | 607 (80.2) | 1 | 1 | |||
| Parity | 0.001 | ||||||
| 0 | 197 (54.1) | 330 (43.6) | 1.53 | 1.19 to 1.96 | 1.6 | 1.17 to 2.20 | |
| ≥ 1 | 167 (45.9) | 427 (56.4) | 1 | 1 | |||
| Previous caesarean deliveries | 0.002 | ||||||
| Yes | 47 (12.9) | 96 (12.7) | 1.33 | 0.89 to 2.0 | |||
| No | 121 (33.2) | 330 (43.7) | 1 | ||||
| Previous pregnancy problems | 0.001 | ||||||
| Yes | 65 (18.0) | 141 (18.7) | 1.31 | 0.90 to 1.90 | |||
| No | 100 (27.6) | 284 (37.6) | 1 | ||||
| Multiple pregnancy | 0.036 | ||||||
| Yes | 10 (2.8) | 8 (1.1) | 2.63 | 1.03 to 6.73 | 2.8 | 0.81 to 9.72 | |
| No | 354 (97.3) | 746 (98.9) | 1 | 1 | |||
| Smoked during pregnancy | 0.103 | ||||||
| Yes | 99 (27.4) | 173 (22.9) | 1.27 | 0.95 to 1.69 | 1.13 | 0.81 to 1.57 | |
| No | 262 (72.6) | 581 (77.1) | 1 | 1 | |||
| BMI at booking (kg/m2) | 0.982 | ||||||
| < 18.5 | 15 (4.1) | 29 (3.8) | 1.1 | 0.58 to 2.11 | 0.71 | 0.32 to 1.60 | |
| 18.5 to < 25 | 159 (43.6) | 339 (44.8) | 1 | 1 | |||
| 25 to < 30 | 96 (26.3) | 196 (25.9) | 1.04 | 0.77 to 1.42 | 1.1 | 0.77 to 1.57 | |
| ≥ 30 | 95 (26.0) | 193 (25.5) | 1.05 | 0.77 to 1.43 | 1.2 | 0.83 to 1.74 | |
| Diabetes | 0.35 | ||||||
| Yes | 10 (2.7) | 29 (3.8) | 0.71 | 0.34 to 1.47 | 0.8 | 0.35 to 1.83 | |
| No | 355 (97.3) | 728 (96.2) | 1 | 1 | |||
| History of pyelonephritis/UTI | < 0.001 | ||||||
| Yes | 36 (9.9) | 33 (4.4) | 2.4 | 1.47 to 3.92 | 1.31 | 0.71 to 2.42 | |
| No | 329 (90.1) | 724 (95.6) | 1 | 1 | |||
| History of sexually transmitted infection | 0.029 | ||||||
| Yes | 26 (7.2) | 31 (4.1) | 1.8 | 1.05 to 3.09 | 1.63 | 0.91 to 2.90 | |
| No | 336 (92.8) | 723 (95.9) | 1 | 1 | |||
| Pre-existing medical problemsc | < 0.001 | ||||||
| Yes | 120 (32.9) | 171 (22.7) | 1.67 | 1.27 to 2.20 | 1.4 | 1.01 to 1.94 | |
| No | 245 (67.1) | 583 (77.3) | 1 | 1 | |||
| Invasive antenatal proceduresd | 0.57 | ||||||
| Yes | 5 (1.38) | 11 (1.46) | 0.94 | 0.32 to 2.73 | 0.66 | 0.18 to 2.42 | |
| No | 358 (98.6) | 742 (98.5) | 1 | 1 | |||
| Febrile illness or antibiotics in 2 weeks before presentation | < 0.001 | ||||||
| Yes | 153 (41.9) | 42 (5.6) | 12.29 | 8.45 to 17.86 | 12.1 | 8.11 to 18.0 | |
| No | 212 (58.1) | 715 (94.5) | 1 | 1 | |||
| Risk factor | Postpartum cases, n (%)a (N = 231) | Controls, n (%)a (N = 757) | p-value | uOR | 95% CI | aORb | 95% CI |
|---|---|---|---|---|---|---|---|
| Delivery factors | |||||||
| Premature rupture of membranes | 0.476 | ||||||
| Yes | 21 (13.7) | 74 (11.6) | 1.21 | 0.72 to 2.03 | 0.98 | 0.53 to 1.81 | |
| No | 132 (86.3) | 562 (88.4) | 1 | 1 | |||
| > 5 vaginal examinations | 0.04 | ||||||
| Yes | 53 (23.1) | 127 (17.1) | 1.46 | 1.02 to 2.09 | 1.09 | 0.66 to 1.79 | |
| No | 176 (76.9) | 615 (82.9) | 1 | 1 | |||
| Fetal blood sampling | 0.003 | ||||||
| Yes | 19 (8.2) | 27 (3.6) | 2.42 | 1.32 to 4.44 | 1.03 | 0.48 to 2.19 | |
| No | 212 (91.8) | 730 (96.4) | 1 | 1 | |||
| Fetal scalp electrode | 0.027 | ||||||
| Yes | 32 (13.9) | 67 (8.9) | 1.66 | 1.06 to 2.60 | 1.14 | 0.64 to 2.06 | |
| No | 199 (86.2) | 690 (91.2) | 1 | 1 | |||
| Labour induction | 0.563 | ||||||
| Yes | 62 (26.8) | 218 (28.8) | 0.91 | 0.65 to 1.26 | 1.13 | 0.75 to 1.70 | |
| No | 169 (73.2) | 539 (71.2) | 1 | 1 | |||
| Mode of delivery | < 0.001 | ||||||
| Spontaneous vaginal | 51 (25.5) | 443 (58.8) | 1 | 1 | |||
| Operative vaginal | 29 (14.5) | 100 (13.3) | 2.52 | 1.52 to 4.17 | 2.49 | 1.32 to 4.70 | |
| Pre-labour caesarean | 44 (22.0) | 119 (15.8) | 3.21 | 2.05 to 5.04 | 3.83 | 2.24 to 6.56 | |
| Caesarean after labour onset | 76 (38.0) | 92 (12.2) | 7.18 | 4.72 to 10.92 | 8.06 | 4.65 to 13.97 | |
| Complications of deliveryc | 0.46 | ||||||
| Yes | 79 (34.2) | 279 (36.9) | 0.89 | 0.65 to 1.21 | 1.69 | 1.09 to 2.63 | |
| No | 152 (65.8) | 478 (63.1) | 1 | 1 | |||
Multiple pregnancy (aOR 5.75, 95% CI 1.54 to 21.5) and group A Streptococcus as the causative organism (aOR 4.84, 95% CI 2.17 to 10.8) were significantly associated with an increase in the odds of progression from severe sepsis to septic shock. Before adjustment for group A streptococcal infection, spontaneous vaginal delivery (aOR 3.85, 95% CI 1.35 to 10.96) and operative vaginal delivery (aOR 3.12, 95% CI 1.03 to 9.57) were significantly associated with an over threefold increase in the odds of progression to septic shock.
Conclusions and implications for practice
In the AMND study we identified that obesity, younger maternal age, operative vaginal delivery and other known factors such as multiparity, anaemia, labour induction, caesarean section and pre-term birth were associated with maternal sepsis. Women with sepsis who had a caesarean section were less likely than controls to receive antibiotics, highlighting the need for prophylaxis. The association we observed between operative vaginal delivery and maternal sepsis emphasises the importance of strict aseptic technique and infection control measures in clinical practice. The association between obesity and maternal sepsis is also of clinical significance given the concurrent increase in maternal obesity in the UK. 13 However, this AMND analysis, despite using a large population database, had limited power to investigate factors associated with severe maternal sepsis. Therefore, we undertook further research, including an analysis of intensive care admission data and a national prospective cohort study, to comprehensively evaluate the magnitude of severe maternal sepsis morbidity.
We found that severe sepsis and septic shock morbidity are common among pregnant and recently pregnant women admitted to intensive care (one in seven and one in nine obstetric ICU admissions, respectively). We further identified several findings with clinical and health-care policy implications: pneumonia/respiratory infection is a leading source of sepsis irrespective of epidemic influenza periods; there are major significant disparities in socioeconomic status and the risk of severe sepsis; and deprivation, increased BMI, respiratory and haematological organ system dysfunctions are significant independent risk factors for mortality due to sepsis.
Forty per cent of women with severe sepsis had pneumonia/respiratory infection as the source of sepsis. Despite the significant influenza epidemic, which occurred from 2009 to 2010, only 10.5% (27/257) of pneumonia/respiratory infection cases were due to the epidemic AH1N1 strain.
These results indicate that in addition to genital tract infection, respiratory infection is a major source of severe maternal sepsis irrespective of an influenza epidemic. Immediate implications are that the significance of respiratory tract infection in pregnant and recently pregnant women should be recognised, and there is clearly a precedent to improve timely recognition of severe respiratory tract infection as well as genital tract sepsis in pregnant and recently pregnant women. Most maternal deaths and critical illnesses from severe sepsis occur because of a delay in recognition and diagnosis. 2 Obstetric and front-line clinicians should therefore maintain a high index of suspicion, as well as alert pregnant and recently pregnant women to the possible severity of any infection, in particular the clinical symptoms of respiratory and genital tract infection and the dangers of delay in seeking medical care. From an economic perspective, an improvement in recognition before onset of severe infection could have a substantial effect on intensive care resource utilisation.
Obesity is clearly a risk factor for sepsis mortality100 and ICU admission,122 but it was evident from the missing data in the ICNARC study that BMI was often not routinely recorded, which has subsequently been addressed in obstetric guidelines. 123
The UKOSS national cohort study found that for each maternal sepsis death in the UK, approximately 50 women have life-threatening morbidity from sepsis and the onset of severe sepsis from SIRS occurs very rapidly. Genital tract and urinary tract infections (UTIs) are the predominant sources of infection in women admitted to obstetric units with sepsis; all modes of operative delivery carry significant risks for severe sepsis and although the largest proportion of cases of severe sepsis is caused by E. coli, outcomes are significantly worse for women with group A streptococcal infection. Importantly, women who are treated with antibiotics in the perinatal period are at significant risk of severe sepsis, suggesting that a significant proportion of infections progress even following antibiotic treatment. These findings highlight a number of key messages for clinical practice in both primary and secondary care, with the high levels of life-threatening morbidity identified indicating that pregnant or recently pregnant women with suspected infection need closer attention than women who are not pregnant.
Severe sepsis in pregnancy presents in primary care and the previously undescribed association between antibiotic prescription in the perinatal period and risk of severe sepsis suggests that primary care practitioners should have a low threshold for referral of women in pregnancy with signs of infection. Over 40% of women with severe sepsis had a febrile illness or were taking antibiotics prior to presentation, which suggests that at least a proportion were not adequately diagnosed, treated or followed up. It cannot be assumed that antibiotics will prevent progression to severe sepsis and safety net checks, for example follow-up appointments or instructions to return if symptoms do not resolve, should therefore be in place to make sure a pregnant woman who has been treated for infection has recovered. Simply prescribing antibiotics alone may not be appropriate. This message applies equally to secondary care and there is a need to ensure that follow-up happens to ensure that treatment is effective.
As sepsis progresses along a spectrum of severity, the occurrence of life-threatening sepsis represents the severest end, with the exception of maternal death and, therefore, only the ‘tip of the iceberg’ of serious maternal morbidity. The rapid progression to severe sepsis highlights the importance of following the international Surviving Sepsis Campaign’s guidelines124 in pregnancy and the recommendation for administration of high-dose intravenous (i.v.) antibiotics within 1 hour of admission for anyone with suspected sepsis. 124
A challenge in all previous studies of maternal sepsis has been to assess the temporality of mode of delivery in relation to infection and sepsis. These studies show that after controlling for illness before delivery, as well as clinical risk factors such as premature rupture of membranes, all modes of operative delivery (operative vaginal, pre-labour caesarean and caesarean after the onset of labour) were independent risk factors for severe sepsis. Even though antibiotic prophylaxis at caesarean section is routine practice in the UK, these results suggest that women are still at heightened risk of severe sepsis, despite the administration of antibiotics, and emphasise the importance of attention to prophylaxis particularly in emergency deliveries. The risk associated with operative vaginal delivery suggests that there is a need for further investigation of the role of prophylactic antibiotics in a randomised controlled trial (RCT), as well as stringent attention to infection control measures for these deliveries. Further to this programme of work and as a direct consequence of these findings, a trial of a single dose of prophylactic antibiotic following operative vaginal delivery has been funded and is now in progress. 125
The different patterns of infection we observed in antenatal and postnatal women suggest that overall greater consideration needs to be given to the source of infection and, therefore, the most appropriate antibiotic to prescribe. This study highlights that UTIs remain an important cause of severe sepsis, particularly antenatally, so prompt treatment and follow-up in primary care to ensure that the infection is eradicated is important.
Our results indicate that although severe sepsis is more common following caesarean delivery, women delivering vaginally are at a heightened risk of group A streptococcal infection and those that are infected with group A Streptococcus are at significantly increased risk of progression to septic shock compared with women infected with another organism. Fifty per cent of proven group A streptococcal infections in our study population led to septic shock, with very rapid progression from the first sign of SIRS. This has a direct implication for decisions about the availability of rapid antigen diagnostic tests for group A Streptococcus in obstetrics. Although culture remains the gold standard for confirmation of group A Streptococcus, it takes 1–2 days to obtain results, which is significantly longer than the time course from the first signs of SIRS to septic shock for most women. In the absence of rapid diagnostics, a positive culture for group A Streptococcus should be reported urgently by telephone as soon as it is discovered in the laboratory and, prior to this, a clinical suspicion of group A Streptococcus should be regarded as a red flag for urgent action, including administration of antibiotics and very close monitoring. In addition, training about group A streptococcal infection should be routinely included in all obstetric emergency training courses.
In conclusion, this workstream emphasises that both primary and secondary care practitioners should remain aware that pregnant or recently pregnant women with suspected infection need closer attention than women who are not pregnant. Antibiotic prescription does not necessarily prevent progression to severe sepsis and women should be followed up to ensure recovery. The rapid progression to severe sepsis highlights the importance of following the international Surviving Sepsis Campaign guideline of administration of high-dose i.v. antibiotics within 1 hour of admission to hospital for anyone with suspected sepsis. Signs of severe sepsis, particularly with confirmed or suspected group A streptococcal infection, should be regarded as an obstetric emergency and should be routinely included in obstetric emergency training courses. Vigilant infection control at vaginal delivery should be maintained, with a potential role for prophylactic antibiotics at operative vaginal delivery. Future research should assess the efficacy of rapid antigen diagnostic tests for group A Streptococcus in obstetrics.
Chapter 5 Extending the uses of observational data on severe maternal morbidity: economic evaluation of second-line managements for postpartum haemorrhage
Background
Randomised controlled trials of treatments for conditions that are both rare and occur in emergency situations are particularly difficult owing to the time limitations in the emergency situation as well as the large collaboration needed to conduct studies of sufficient size. 126 Studies of effectiveness and particularly cost-effectiveness are thus rarely undertaken in this setting. National observational data, such as those obtained through UKOSS, have fewer of the biases classically attributed to many observational studies. 127 This information may therefore be used to explore effectiveness of different current treatments for near-miss maternal morbidities. In the absence of evidence from RCTs it is necessary to rely on these observational data, but given the known variation in the use of specific interventions, national recommendations will rely on these data supplemented by economic data. For example, providing uterine artery embolisation for postpartum haemorrhage (PPH) involves substantial capital outlay associated with the provision of a dedicated out-of-hours interventional radiology service. Therefore, investigating the utility of these observational data for economic evaluation is important.
The basic management of PPH consists of initial medical care and the use of uterotonic drugs and/or an intrauterine balloon. When these initial therapies fail, an escalated series of second-line therapies are currently implemented before hysterectomy is considered to avoid maternal death. These second-line therapies include uterine compression sutures, factor VIIa, pelvic vessel ligation and interventional radiological techniques, which have been widely introduced into clinical practice and recommended by national guidelines. 128 Little was known until recently about the effectiveness of these therapies in practice. 129 Kayem et al. 129 reported that uterine compression sutures and interventional radiology techniques experienced higher success rates than recombinant factor VIIa (rFVIIa) and pelvic vessel ligation using a cohort of women with PPH identified through UKOSS. The authors concluded that before recommending uterine compression sutures or interventional radiology as part of clinical practice, a full assessment of the costs and effects of the interventions should be conducted.
Conducting a cost-effectiveness analysis alongside observational studies as the sole vehicle for the economic evaluation is subject to problems such as selection bias and confounding, particularly confounding by indication. 130 However, the use of appropriate statistical methods to deal with this type of bias is recommended to estimate particular cost-effectiveness information using observational data (e.g. transition probabilities, costs associated to a health state, utility decrements) that can be synthesised alongside other sources of data in a decision economic model. 130–132 The aim of this workstream was to conduct a cost–utility analysis of second-line interventions for PPH using a decision-analytic model that synthesised effectiveness data from the national cohort UKOSS study, cost data obtained through interviews and the literature, and health-related quality-of-life information extracted from the literature.
Research questions
-
What is the relative cost-effectiveness of treatment for peripartum haemorrhage with uterine compression sutures, factor VIIa and major vessel ligation or embolisation?
-
What additional data should be collected in a primary study of therapies for near-miss maternal morbidity to inform an economic evaluation?
Methods
A cost–utility analysis was conducted from the UK NHS perspective to determine which second-line therapies for PPH represent value for money of scarce NHS resources. The main outcome measure used in this economic evaluation was quality-adjusted life-years (QALYs).
Structure of cost-effectiveness model
A decision-tree model of the acute clinical pathway of women (mean age 32 years; interquartile range 29–36 years) with PPH was developed using TreeAge Pro Suite 2015 software (TreeAge Software, Inc., Williamstown, MA, USA). This type of model is recommended for acute conditions that need immediate effective interventions. 133 A time horizon of 12 months was used in the economic model. Treatment success was defined as no requirement for either a further therapy to treat PPH or hysterectomy. The model commenced when first-line treatment for PPH failed and one of the four second-line treatments was used as potential therapies to stop the bleeding. If the second-line therapy was successful, the model stopped and costs and effects were calculated for that therapy. If second-line therapy failed, the woman either underwent hysterectomy or was treated with one of the remaining second-line therapies as a third-line therapy. After receiving third-line therapy, the final possible outcomes were successful treatment or hysterectomy. We assumed that if third-line therapy failed, women underwent hysterectomy and not a fourth-line treatment, as this is most likely in clinical practice. 2,128
Estimation of baseline probabilities
Baseline probabilities were extracted from the UKOSS study. 10,129 Table 17 presents the probabilities used in the model for each intervention that were computed as the number of occurrences divided by the total number of women receiving a particular therapy. Probabilities are presented either as intervention specific or common to all regimes. For the intervention-specific probabilities, the probability of a successful outcome after second-line treatment and the probability of hysterectomy after a failure of second-line treatment were extracted from the epidemiological study. The probability of a failure outcome after second-line therapy was estimated as residuals (i.e. 1 – probability of a successful outcome). The probability of having a particular third-line therapy was also estimated as the residual. The probabilities of having a particular third-line treatment and the outcomes after third-line treatment were common to all regimes. No clear evidence about the probability of use of a particular intervention as third line compared with the remaining ones was observed in the study by Kayem et al. 129 Therefore, we assumed that women were equally likely to receive one of the remaining therapies as third-line treatment. We pooled the number of hysterectomies performed across all interventions used as third line, to estimate the probability of having hysterectomy after third-line treatment.
| Second-line regime | Baseline probability | α | β | n | PSA distribution |
|---|---|---|---|---|---|
| Uterine compression sutures | |||||
| Successful outcome after second-line treatment | 0.704 | 140 | 59 | 199 | Beta |
| Failure outcome after second-line treatmenta | 0.296 | ||||
| Hysterectomy after a failure outcome post second-line treatment | 0.166 | 33 | 166 | 199 | Beta |
| Third-line treatment after a failure outcome post second-line treatmenta | 0.834 | ||||
| Pelvic vessel ligation | |||||
| Successful outcome after second-line treatmentb | 0.286 | ||||
| Failure outcome after second-line treatmenta | 0.714 | ||||
| Hysterectomy after a failure outcome post second-line treatment | 0.100 | 2 | 18 | 20 | Beta |
| Third-line treatment after a failure outcome post second-line treatmenta | 0.900 | ||||
| rFVIIa | |||||
| Successful outcome after second-line treatmentb | 0.291 | ||||
| Failure outcome after second-line treatmenta | 0.709 | ||||
| Hysterectomy after a failure outcome after second-line treatment | 0.452 | 14 | 17 | 31 | Beta |
| Third-line treatment after a failure outcome after second-line treatmenta | 0.548 | ||||
| Interventional radiology | |||||
| Successful outcome after second-line treatmentb | 0.857 | ||||
| Failure outcome after second-line treatmenta | 0.143 | ||||
| Hysterectomy after a failure outcome post second-line treatment | 0.091 | 2 | 20 | 22 | Beta |
| Third-line treatment after a failure outcome post second-line treatmenta | 0.909 | ||||
| Probability of having a particular intervention as third-line treatment (common to all regimes) | 0.333 | ||||
| Outcomes after third-line treatment (common to all regimes) | |||||
| Successful outcome after third-line treatmenta | 0.474 | ||||
| Hysterectomy after third-line treatment | 0.526 | 20 | 18 | 38 | Beta |
Incorporating treatment effect in the economic model
The treatment effect in the model was introduced by modifying the baseline probability of successful outcome after uterine compression sutures by a treatment modifier reflecting the treatment effect of uterine compression sutures against one of the remaining second-line therapies. The baseline probability of uterine compression was used because it was the most commonly used intervention among the 272 women identified in the UKOSS study. As treatment modifier, we used the OR of successful outcome after second-line treatment between pelvic vessel ligation, rFVIIa or interventional radiology compared with uterine compression sutures. We used the UKOSS data to obtain ORs from a logistic regression using a binary dependent variable indicating whether or not second-line therapy was successful and treatment indicators as well as a set of women’s characteristics and mode of labour as explanatories. The ORs (95% CIs) estimating treatment success for rFVIIa, pelvic vessel ligation and interventional radiology compared with uterine compression sutures were estimated to be 0.173 (0.068 to 0.469), 0.169 (0.053 to 0.537) and 2.533 (0.531 to 12.087), respectively. Baseline probabilities of successful outcome after second-line rFVIIa, pelvic vessel ligation and interventional radiology were converted first into odds before being multiplied by the OR and were converted back into probabilities when informing the parameters in the model.
Costs
The direct health-care costs associated with the second-line interventions were the only costs included in the model and were calculated using a detailed costing analysis. Any other health-care costs (e.g. length of stay, hospital readmissions, primary care visits) were assumed to be similar across interventions and, therefore, cancelling out in the calculation of the incremental costs and hence were excluded from the model. This is based on the clinical assumption that the outcomes of successful treatment of PPH do not differ in a treatment-dependent manner. The main resource use and cost drivers associated with the interventions were elucidated through interviews with a haematologist, two obstetricians and an anaesthetist all based in UK hospitals. The results from the interviews suggested that staff, equipment, key drugs and a set of general measures common to all regimes were the main cost drivers. General measures included common resources to all therapies such as intra-arterial monitoring, control venous pressure monitoring, active warming and fresh-frozen plasma. Unit costs associated with the resource use were primarily obtained from national sources when available134 and from the literature. If no published cost data were readily available, commercial companies were contacted to obtain such information. All unit costs were expressed in 2012–13 Great British pounds and discounting was not implemented given that only intervention costs were included in the model. A summary of the mean interventions costs used in the model is presented in Table 18.
| Mean estimate (£) | Range used in one-way sensitivity analysis (£) | |||
|---|---|---|---|---|
| ± 20% of mean estimate | ± 40% of mean estimate | ± 60% of mean estimate | ||
| Uterine compression sutures | ||||
| Intervention-specific drugs | 81 | |||
| Staff | 312 | |||
| Equipment | 34 | |||
| General measuresa | 2564 | |||
| Total | 2991 | 2393–3589 | 1795–4187 | 1196–4786 |
| Pelvic vessel ligation | ||||
| Staff | 934 | |||
| Equipment | 28 | |||
| General measuresa | 2564 | |||
| Total | 3525 | 2820–4230 | 2115–4935 | 1410–5640 |
| rFVIIa | ||||
| rFVIIa unit cost | 3935 | |||
| Staff | 295 | |||
| General measures | 2564 | |||
| Total | 6794 | 5435–8153 | 4076–9512 | 2718–10,870 |
| Interventional radiology | ||||
| Intervention-specific drugs | 366 | |||
| Staff | 2425 | |||
| Equipment | 1111 | |||
| Ambulance transfer | 261 | |||
| General measuresa | 2564 | |||
| Total | 6727 | 5382–8072 | 4036–9418 | 2691–10,763 |
| Hysterectomy | ||||
| Staff | 583 | |||
| Equipment | 46 | |||
| General measuresa | 2564 | |||
| Total | 3193 | |||
Base-case utility values
Evidence suggests that women who have a PPH but have a successful outcome after second- or third-line treatment experience a complete recuperation at 1-year follow-up with quality-of-life levels similar to women of the same age from the general population. Utility values associated with a successful outcome health state in the model were therefore extracted from responses to the European Quality of Life-5 Dimensions-3 Level (EQ-5D-3L) instrument135 in the Health Survey for England in 2011 from women aged < 40 years in the general population. The utility value of a successful outcome was based on 359 young women’s responses and we applied the UK value set to estimate utilities obtaining a mean (SE) utility value of 0.923 (0.008). We sought published sources to identify quality-of-life weights associated with women of childbearing age undergoing hysterectomy in an emergency situation by conducting a literature search from inception to May 2014 in MEDLINE, Cumulative Index to Nursing and Allied Health Literature and EMBASE using a combination of the terms quality of life, fertility, infertility and hysterectomy and found no evidence of published utilities. We searched the national health surveys in the UK and USA to explore whether or not such data could potentially be available. We found that in the 2003 US Medical Expenditure Panel Survey (MEPS),136 women who completed the EQ-5D-3L were asked whether or not they had undergone a hysterectomy (which may or may not have been related to PPH) in the previous year. Full details are available from the MEPS survey web site. 137 We identified 148 women under the age of 40 years who underwent a hysterectomy (for any reason) in the 2003 MEPS dataset and estimated a mean (SE) utility weight of 0.744 (0.024). We used the UK value set to calculate utilities from the EQ-5D-3L responses obtained in MEPS. 138 We had originally anticipated using data from the study reported in Chapter 9 to produce utility estimates, but the questionnaire response rate was too low to be considered representative.
Analytical methods
All analyses are carried out from the perspective of the UK NHS and are reported in terms of incremental cost-effectiveness ratios (ICERs) and net-benefit statistics. The primary health outcome measure in the economic analysis was QALYs but the results of the analysis are also presented using hysterectomy avoided. Therefore, ICER results are presented using cost per QALY gained and cost per hysterectomy avoided. QALYs for each treatment strategy were estimated attaching a utility value to the successful outcome or hysterectomy health states and assuming women spent 12 months in that state. Dominated strategies (one strategy is found to be both cheaper and produces more QALYs) were eliminated before conducting any incremental analysis. Uncertainty in the model parameters (probabilities, treatment effect and utility parameters) was evaluated through a probabilistic sensitivity analysis (PSA). 139 In PSA, each input parameter in the model is assigned a specific statistical distribution for which values are randomly drawn generating a large number of pairs of mean costs and effects for each strategy. These estimates are then used to create an empirical distribution of the differences in costs and effects between interventions.
We generated 10,000 random draws and used beta distributions for both probabilities and utilities. 140 As we had access to patient-level data to inform the uncertainty around treatment effects of the interventions, we used the empirical distribution of the ORs reported in Chapter 5, Sensitivity analysis, from 10,000 bootstrap samples for the PSA. We reported the PSA summary results for the total costs and effects per patient associated to each second-line regime to treat PPH for our base case in line with current recommendations. 140 Uncertainty around cost-effectiveness results is presented using cost-effectiveness acceptability curves (CEACs) that indicate the probability that a particular intervention is cost-effective for different thresholds of willingness to pay (WTP) for a QALY or hysterectomy avoided. Current thresholds of WTP for a QALY as suggested by National Institute for Health and Care Excellence (NICE) were used in this study to determine value for money. 141
To estimate the impact of individual parameters on the results of the analysis, we performed an analysis of covariance. Using this approach, the proportion of the sum of squares in the output parameters (i.e. incremental costs and incremental QALYs) explained by uncertainty in each input parameter was identified. 140
Costs were treated deterministically in the economic model, as there were no reliable stochastic estimates to inform SEs. We implemented a one-way sensitivity analysis to determine how the main base cost-effectiveness results varied when the total costs associated with uterine compression sutures, pelvic vessel ligation, rFVIIa and interventional radiology were increased/reduced by 20%, 40% and 60%, as informed by the ranges in Table 18.
Although our literature search found no published evidence on quality-of-life weights associated with women of childbearing age undergoing hysterectomy in an emergency situation, it identified proxy utilities associated with the health state of convalescence after hysterectomy in women with heavy menstrual bleeding. 142 In addition, the 2011 US MEPS143 had also available hysterectomy treatment information and data on the generic quality of life Short Form questionnaire-12 items144 that we translated into EQ-5D-3L utility values using a validated a mapping algorithm. 145 We evaluated the robustness of our original base-case utility estimates running the economic model using these two additional sources of utility information.
Results
Base-case summary results
Table 19 presents a summary of the base-case probabilistic analysis ranking all interventions in ascending order of costs. Both pelvic vessel ligation and rFVIIa were dominated strategies (more expensive and produce fewer QALYs/avoid fewer hysterectomies) by interventional radiology and uterine compression sutures. Therefore, pelvic vessel ligation and rFVIIa were removed and not evaluated further in the base case with the incremental analysis comparing interventional radiology versus uterine compression sutures. The interventional radiology strategy was more expensive but also produced more QALYs than uterine compression sutures, with an estimated incremental cost of £2827 (95% non-parametric CI £1909 to £3948) and incremental QALYs of 0.013 (95% CI –0.006 to 0.032). The ICER representing the value of the additional benefit of interventional radiology compared with uterine compression sutures was estimated to be £210,585 per QALY gained. This indicates that for current NICE thresholds of WTP for QALY gained, interventional radiology does not represent a good use of NHS resources. This was also corroborated by the results of the net monetary benefit analysis that indicated uterine compression sutures had the largest monetary benefit of £12,843, assuming a WTP of £20,000 per QALY gained. Similar results were obtained using hysterectomies avoided as the main outcome measure but in this case, interventional radiology had an estimated ICER of £37,533 per hysterectomy avoided.
| Second-line regime | Total cost strategy per patient (£, 2012–13 prices) | Outcome: QALYs | Outcome: hysterectomy avoided | ||||
|---|---|---|---|---|---|---|---|
| Total QALYs per strategy per patient | Cost per QALY gained (vs. compression sutures), £ | Net monetary benefit (WTP = £20,000), £ | Total hysterectomies avoided per strategy per patient | Cost per hysterectomy avoided (vs. compression sutures), £ | Net monetary benefit (WTP = £20,000), £ | ||
| Compression sutures | 4972 | 0.891 | - | 12,843 | 0.821 | - | 11,439 |
| Interventional radiology | 7798 | 0.904 | 210,585 | 10,284 | 0.896 | 37,533 | 10,118 |
| Pelvic vessel ligation | 8383 | 0.849 | Dominated | 8607 | 0.589 | Dominated | 3406 |
| rFVIIa | 10,216 | 0.828 | Dominated | 6349 | 0.470 | Dominated | –814 |
Analysis of uncertainty
The CEACs associated with uterine compression sutures and interventional radiology, using QALYs or hysterectomy avoided as measures of outcome in the economic evaluation, are presented in Figure 5. Using QALYs, the use of uterine compression sutures as a second-line treatment strategy always had a higher probability of being cost-effective than interventional radiology for any value of WTP for QALY gained. When using hysterectomy avoided as the outcome measure, interventional radiology had a higher probability of being cost-effective than uterine compression sutures at values of WTP > £30,000 per hysterectomy avoided.
FIGURE 5.
Cost-effectiveness acceptability curves indicating the probability that uterine compression sutures or interventional radiology is cost-effective for different values of WTP for health gained. (a) QALYs as the measure of outcome; and (b) hysterectomy avoided as the measure of outcome.
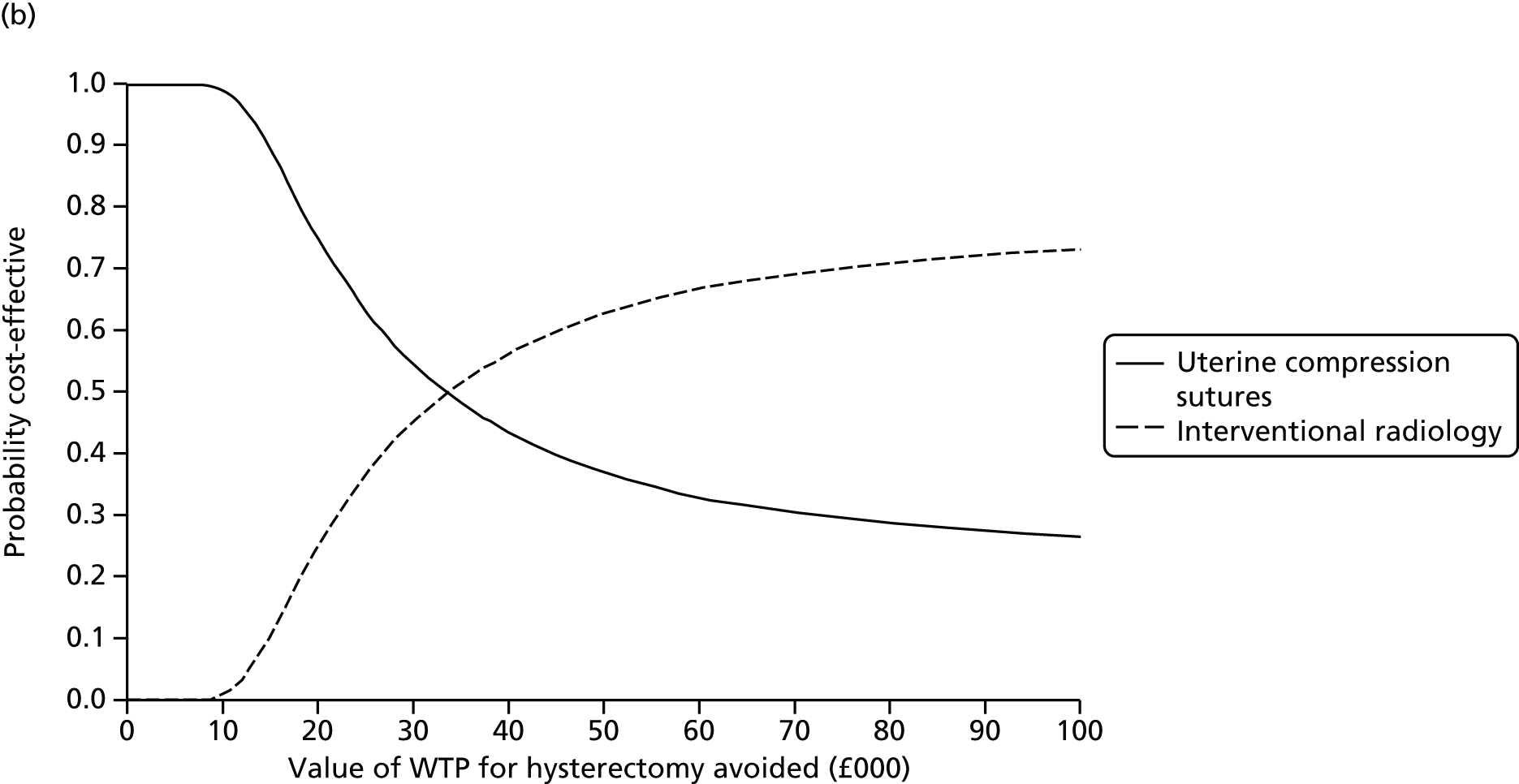
The results of the analysis of covariance suggested that the probability of success after uterine compression sutures had the largest contribution to the uncertainty around incremental costs explaining 71.7% of the variability of incremental costs and 19.8% of the variability in incremental QALYs. The utility estimate associated with the hysterectomy state contributed to 44.7% of the variability of the incremental QALYs. The remaining parameters did not contribute greatly to the uncertainty around cost-effectiveness results.
Sensitivity analysis
Reducing or increasing uterine compression suture costs by 20%, 40% or 60% did not affect the baseline cost-effectiveness results and uterine compression sutures were still the most cost-effective option for any threshold of WTP. A similar output was obtained when the costs of pelvic vessel ligation and rFVIIa were reduced/increased. Increasing the costs of interventional radiology improved the cost-effectiveness of uterine compression sutures to an almost asymptotic CEAC towards a probability of 1. However, if the costs of interventional radiology were reduced by 40%, it became the most cost-effective intervention for thresholds of WTP > £19,000 per QALY gained but asymptotically to a maximum probability of 70%. Interventional radiology became the most cost-effective intervention for any threshold of WTP per QALY gained if the costs of interventional radiology were reduced by 60%.
The CEACs of uterine compression sutures compared with interventional radiology using alternative utility estimates to inform the quality-of-life weights associated with women undergoing hysterectomy in an emergency situation suggest little impact on cost-effectiveness results compared with the base-case analysis.
Conclusions and implications for practice and service provision
In this study we have synthesised evidence on treatment effect, intervention costs and health-related quality of life using data from UKOSS and the literature of the main four second-line strategies for the care of PPH using a decision-analytic model (see Results).
This study suggested that, given the available evidence, use of uterine compression sutures was the most cost-effective strategy when compared with interventional radiology. Interventional radiology was more effective than uterine compression sutures but significantly more expensive, and both pelvic vessel ligation and rFVIIa were dominated strategies, indicating that they were less effective and more expensive than both uterine compression sutures and interventional radiology. Note, however, that this analysis was limited by the following factors: expert report and published sources (see Methods, Costs) were used to derive health-care costs, as we were not able to collect information from women directly about resource use, and utility estimates were based on data from general population studies of women post hysterectomy, rather than women specifically undergoing postpartum hysterectomy.
The sensitivity analysis suggested that the base-case results were robust to changes in the costs of uterine compression sutures, pelvic vessel ligation and rFVIIa. However, if the costs of interventional radiology were significantly reduced (e.g. as might be the case in hospitals already providing a 24-hour radiology service), it would become the most cost-effective intervention for the NHS. The evidence on health-related quality of life associated with health states in women of childbearing age undergoing hysterectomy in an emergency situation, as informed by our literature search, was limited. We used different proxy utility weights to inform this parameter in the model and the sensitivity analysis suggested that the base-case results were robust using different sources of utility information.
The treatment effect used to inform the effectiveness parameters in the model was estimated using logistic regression. Although we controlled for women’s characteristics and mode of delivery in the regression, it is likely that we have not removed confounding completely in this analysis. Residual confounding is a widely known, recognised problem when using observational data to estimate treatment effects. 146 It is possible that women treated with compression sutures were different in unmeasured ways from those treated with interventional radiology, pelvic vessel ligation and rFVIIa, and this is an important limitation in the study. Nevertheless, in the absence of RCTs, UKOSS was one of the best sources available to determine treatment effect of the treatments. This analysis would be strengthened by a systematic review of the literature to identify any other sources of data with which to estimate treatment effects. To recognise the current uncertainty associated with the estimates of treatment effect, we used the empirical distribution of the ORs obtained from the data using bootstrapping in the PSA. We also implemented a log-normal distribution (data not reported) to inform the uncertainty around this parameter but the main base-case cost-effectiveness results did not vary.
Only direct health-care costs associated with the second-line interventions were included in the model. We excluded any other health-care costs based on the premise that they were similar across interventions but also because of lack of follow-up resource-use and cost data available either from our work or the literature. In addition, possible costs to the extended family in terms of indirect costs were also omitted in our study. Future work should assess how our results would change if any of these resources were incorporated in the model if new evidence becomes available. These data items represent the key ones that should be considered as additional items in any future UKOSS observational studies comparing different treatment modalities for severe maternal morbidities.
The analysis of covariance results identified the probability of successful uterine compression sutures and the quality-of-life weight associated with the hysterectomy health state as the main parameters with the largest contribution to the uncertainty in incremental costs and QALYs of the main comparison between uterine compression sutures and interventional radiology. Prospective work should evaluate what additional research and funding would be necessary to reduce the uncertainty around these parameters using value-of-information methods.
The analytical techniques used here provide a model for the future of evidence synthesis using a decision-analytic model to conduct an economic evaluation in a situation in which RCTs are challenging; however, further work is required to strengthen the fundamental inputs into the analytical model.
Chapter 6 Factors associated with disease progression
Background
The UK led the world in the development of confidential enquiries into maternal deaths. Since the introduction of these confidential enquiries, maternal mortality has decreased 10-fold. 1 Data from UKOSS studies have been used to provide contextual information about underlying maternal morbidity to complement the detailed examination of mortality,3 but formal comparison between the information concerning women who suffered near-miss maternal morbidity and those who died from the same conditions had not been undertaken. The aim of this comparison was to quantify the risks associated with identified factors in order to inform policy and practice to improve survival. This workstream undertook this comparison through two studies.
The initial study compared data on women who died from specific maternal conditions obtained from the UK Confidential Enquiries into Maternal Deaths undertaken through the Centre for Maternal and Child Enquiries (CMACE) from 2003 to 20083,100 with UKOSS data and identified four maternal characteristics (age ≥ 35 years, obesity, belonging to unemployed or manual socioeconomic groups, and black Caribbean and African ethnic backgrounds) to be associated with progression from severe morbidity to death. However, the data available for women who died limited detailed investigation of factors potentially underlying these associations, in particular medical comorbidities, substance misuse, inadequate antenatal care and problems during current and previous pregnancies. Therefore, a subsequent unmatched case–control study further investigated the potential role of these factors in the progression from severe morbidity to direct maternal death among pregnant women in the UK using new and more detailed data on maternal deaths.
Research questions
-
What are the characteristics of women with fatal and non-fatal specific severe maternal morbidities?
-
What factors are associated with maternal death, including core demographic and pregnancy characteristics (such as maternal age, ethnicity, socioeconomic and smoking status, BMI, parity, multiple pregnancy, inadequate use of antenatal care, pre-existing medical conditions and current and previous pregnancy problems)?
Methods
Data sources
Centre for Maternal and Child Enquiries/UK Obstetric Surveillance System study
Information about women who died from specific causes (AFLP, antenatal pulmonary embolism, AFE, antenatal thromboembolic and haemorrhagic stroke, eclampsia) identified through the maternal deaths enquiry conducted by CMACE (2003–8)3,100 was compared with information on women with near-miss morbidity from the same conditions identified through UKOSS studies of near-miss morbidity. 28–30,74
The methodology of the confidential enquiries into maternal deaths conducted through CMACE has been described in detail previously. 3,100 In summary, cases of maternal death were reported to CMACE through several different sources and, in addition, ascertainment of cases was undertaken through linkage of routine birth and death vital statistics records. Cases of maternal death from eclampsia, antenatal pulmonary embolism, AFE, AFLP and antenatal stroke occurring between 2003 and 2008 were identified by interrogating the CMACE database and contacting the clinicians on the confidential enquiry panel responsible for assessing the cause of death where the exact cause of death was unclear in the database.
The UKOSS methodology is described in Chapter 3.
Mothers and Babies: Reducing Risk through Audits and Confidential Enquiries across the UK/UK Obstetric Surveillance System study
The second study used data from the more recent confidential enquiries into maternal death (2009–12) conducted through Mothers and Babies: Reducing Risk through Audits and Confidential Enquiries across the UK (MBRRACE-UK)2 and data on severe life-threatening complications from the UKOSS (2005–13), to investigate the association of medical comorbidities, current and previous pregnancy problems, substance misuse and inadequate use of antenatal care with direct maternal deaths in the UK. Five major causes of direct maternal deaths in the UK – eclampsia, pulmonary embolism, severe sepsis, AFE and peripartum haemorrhage – for which data were available from both databases, were included in this analysis.
Confidential enquiries into maternal deaths in the UK occurring since 2009 have been undertaken through the MBRRACE-UK collaboration. 2,147 Deaths of women during or after pregnancy are identified through a variety of sources, the majority being notified to the MBRRACE-UK office directly from the unit in which the maternal death occurred. Other sources include coroners/procurators fiscal or pathologists, local supervising authority midwifery officers, members of the public and inquest reports from the media. In addition, case ascertainment is cross-checked with routine birth and death vital statistics records from the ONS and the National Records of Scotland. For every death reported, basic demographic and clinical details are collected.
Analyses
Centre for Maternal and Child Enquiries/UK Obstetric Surveillance System study
Four hundred and seventy-six women who suffered from eclampsia, antenatal pulmonary embolism, AFE, AFLP and antenatal stroke, but survived, were compared with 100 women who died from the same conditions.
In order to investigate trends in continuous variables, unadjusted relative risks with 95% CIs were calculated across groups and Spearman’s rank-order correlation was used to examine the associations between age and BMI and the risk of death. We further investigated the potential factors underlying mortality differences in severe maternal morbidities by using a logistic regression analysis. Factors were included if there was a pre-existing hypothesis or evidence to suggest that they may be associated with maternal mortality. In order to adjust for any effect relating to the individual morbidities, we included an adjustment factor for each condition in the analysis. We developed a full regression model by including both potential explanatory and confounding factors. We tested continuous variables for departure from linearity by the addition of quadratic terms to the model and subsequent likelihood ratio testing. There was no evidence of departure from linearity. We calculated aORs with 95% CIs.
The factors included in the model were maternal age, parity, BMI, smoking during pregnancy, ethnicity and socioeconomic classification based on occupation. Data about the coexisting medical conditions of women who died were not included in the CMACE database for the entire time period and, thus, we could not robustly examine any putative association with maternal medical comorbidities. Occupation was classified according to the ONS socioeconomic classification,148 on the basis of the woman’s occupation, unless she was not in paid employment in which case the occupation of her partner was used. Ethnicity was categorised according to the UK census classification. 149
Data were missing for ethnicity and BMI for between 12% and 23% of women who died. We investigated two different methods of analysis to account for this. In a first analysis we included all participants, with creation of a categorical indicator variable for missing responses (missing indicator). The second analysis included all participants with missing responses by using multiple imputation. Missing data for BMI, type of employment, age, parity, smoking and ethnicity were imputed using chained equations. 150,151 The multiple imputation prediction model included all variables in the conceptual framework. In addition, indicator variables for the following characteristics were included in the prediction model – cause of death or morbidity and route of recruitment into the analysis (CMACE or UKOSS). Twenty imputed data sets were created and analysed together. Standard logistic regression models were fitted using Stata 10. The imputed data sets were analysed in Stata 10 using the ice suite of commands. 150
We assessed the additive effect of the presence of multiple risk factors on the risk of death in a model including all factors found to be significantly associated. Because of the limited statistical power of the study, binary variables were used for all maternal characteristics, using standard cut-off points ensuring that groups were not too small to provide unstable estimates. The final model included maternal age ≥ 30 years, BMI ≥ 30 kg/m,2 black Caribbean or African ethnicity, and unemployed, routine or manual occupation.
We used Stata 10 software for all analyses.
Mothers and Babies: Reducing Risk through Audits and Confidential Enquiries across the UK/UK Obstetric Surveillance System study
One hundred and thirty-five women who died from five conditions (eclampsia, pulmonary embolism, severe sepsis, AFE and peripartum haemorrhage) and 1661 women who suffered one of these five conditions but survived were compared using a multivariable logistic regression model. Fourteen known risk factors for severe maternal morbidity and mortality were included as independent variables: gestational diabetes, hypertensive disorders of pregnancy during the current pregnancy, anaemia, multiple pregnancy, inadequate use of antenatal care services, smoking, substance misuse, previous pregnancy problems, pre-existing medical conditions, parity, BMI, employment status, maternal age and ethnicity. 32,152–155 Women were categorised as having gestational diabetes, hypertensive disorders of pregnancy and anaemia during the current pregnancy, based on clinician-recorded conditions in the medical records. The variable ‘ethnicity’ was categorised according to the UK national census classification. 149 Maternal age, BMI and parity were each categorised into three groups. 156–159 In order to compare the findings with that of the previous study,160 we also categorised parity and BMI into binary variables and recategorised age and tested these in a separate regression model. Medical comorbidities were grouped initially as a single variable and subsequently into 18 categories (Box 3). This study had 80% power to detect an OR of ≥ 1.6 of maternal death at a significance level of p < 0.05 (two-sided) associated with the presence of medical comorbidities, which had a prevalence of 29% in the survivors.
Asthma.
Autoimmune diseases (e.g. systemic lupus erythematosus).
Known malignancies.
Cardiac problems (congenital and acquired).
Diabetes mellitus (type 1 or 2).
Diseases caused by blood-borne viruses (e.g. HIV and hepatitis B and C).
Endocrine disorders, excluding diabetes mellitus (e.g. hypothyroidism and hyperthyroidism).
Renal problems (e.g. pyelonephritis, nephrectomy and recurrent UTI).
Neurological disorders (e.g. migraine).
Mental health problems.
Haematological disorders (e.g. thalassemia, sickle cell anaemia, iron deficiency anaemia and procoagulant states).
Epilepsy.
Inflammatory disorders and allergic/atopic conditions (e.g. eczema and ulcerative colitis).
Essential hypertension.
Thrombotic events.
Musculoskeletal disorders (e.g. osteoarthritis and hip replacement).
Other infections, excluding blood-borne viruses (e.g. sexually transmitted infections, tuberculosis and group B streptococcal infection).
Treated for infertility.
HIV, human immunodeficiency virus.
We further investigated the role of medical comorbidities by examining different subgroups of comorbidity. We conducted univariable analyses to assess the association of the 18 specific pre-existing medical comorbidities with the outcome. Thirteen variables found to be associated with the outcome at p < 0.05 (two-tailed) and those identified as important factors associated with maternal morbidity and mortality in previous literature were included in a second multivariable logistic regression model (model 2). This was followed by an additional exploratory regression analysis to examine the factors that were associated with medical comorbidities in the study population.
A ‘risk factors’ score was generated to understand the additive odds associated with the presence of one or more factors that were found to be statistically significantly associated with maternal death. Using the same method as in the CMACE/UKOSS study, we assigned a score of one to each factor (see Analysis, Centre for Maternal and Child Enquiries/UK Obstetric Surveillance System study). We also calculated the population attributable fraction (PAF) for the ‘risk factors’ score and the individual factors using standard methods for calculating PAF in case–control studies. 161 All analyses were performed using Stata 13.
Results
Maternal characteristics associated with death during pregnancy and childbirth
Increasing maternal age and BMI were significantly associated with the risk of death in the CMACE/UKOSS analysis (Figure 6). Results from the multivariable models are presented in Table 20. The final model using a missing indicator analysis produced very similar effect estimates to those from the multiple imputation analysis. Women who were ≥ 30 years, black Caribbean or African, unemployed or with routine or manual occupation had higher odds of progressing to death. Women who had a BMI ≥ 30 kg/m2 were more likely to die from these severe maternal morbidities (aOR 1.71, 95% CI 0.91 to 3.19), although the increased odds were not statistically significant.
FIGURE 6.
Risk of death according to (a) age; and (b) BMI. Bars show risks with 95% CIs.
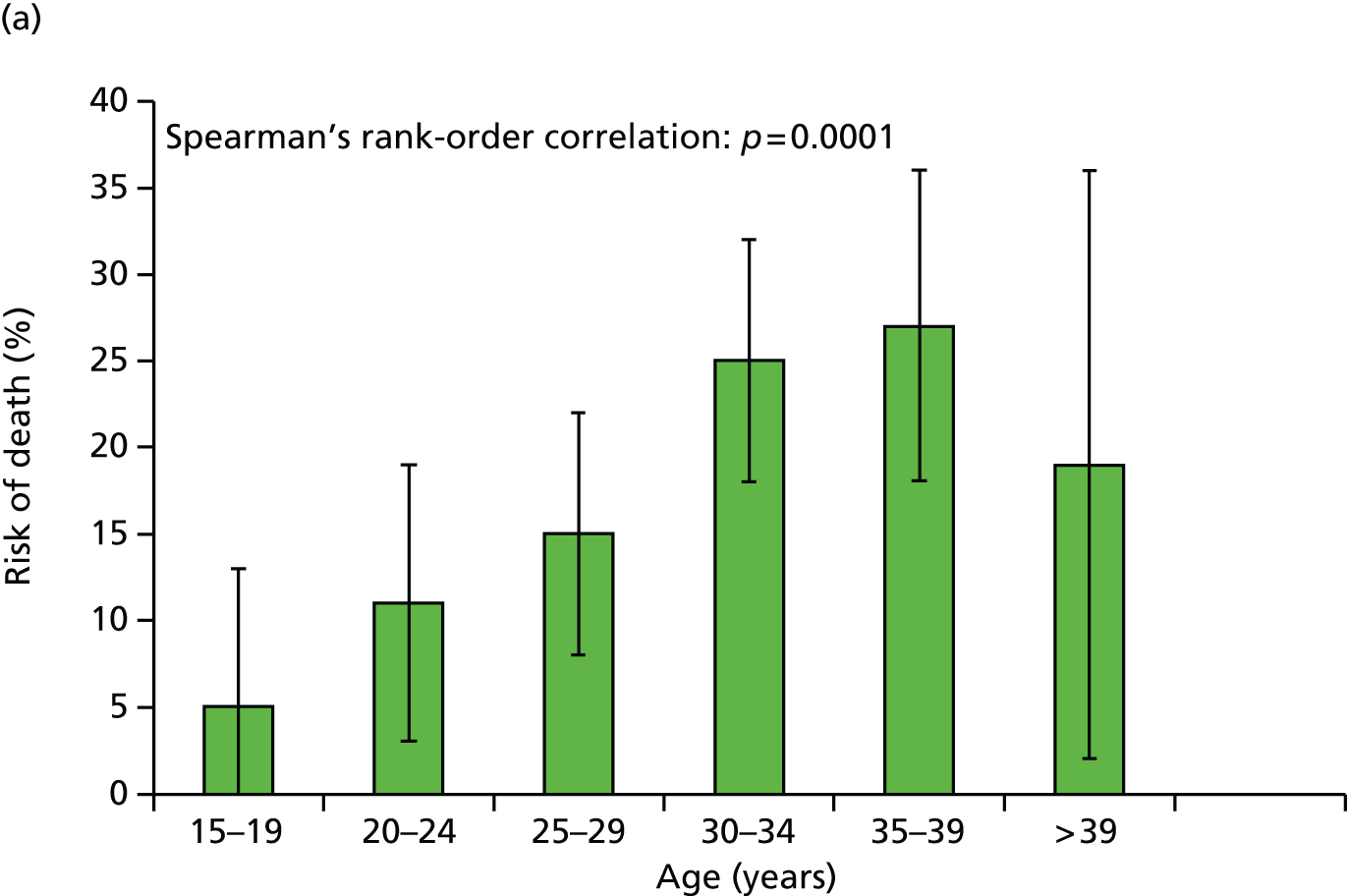
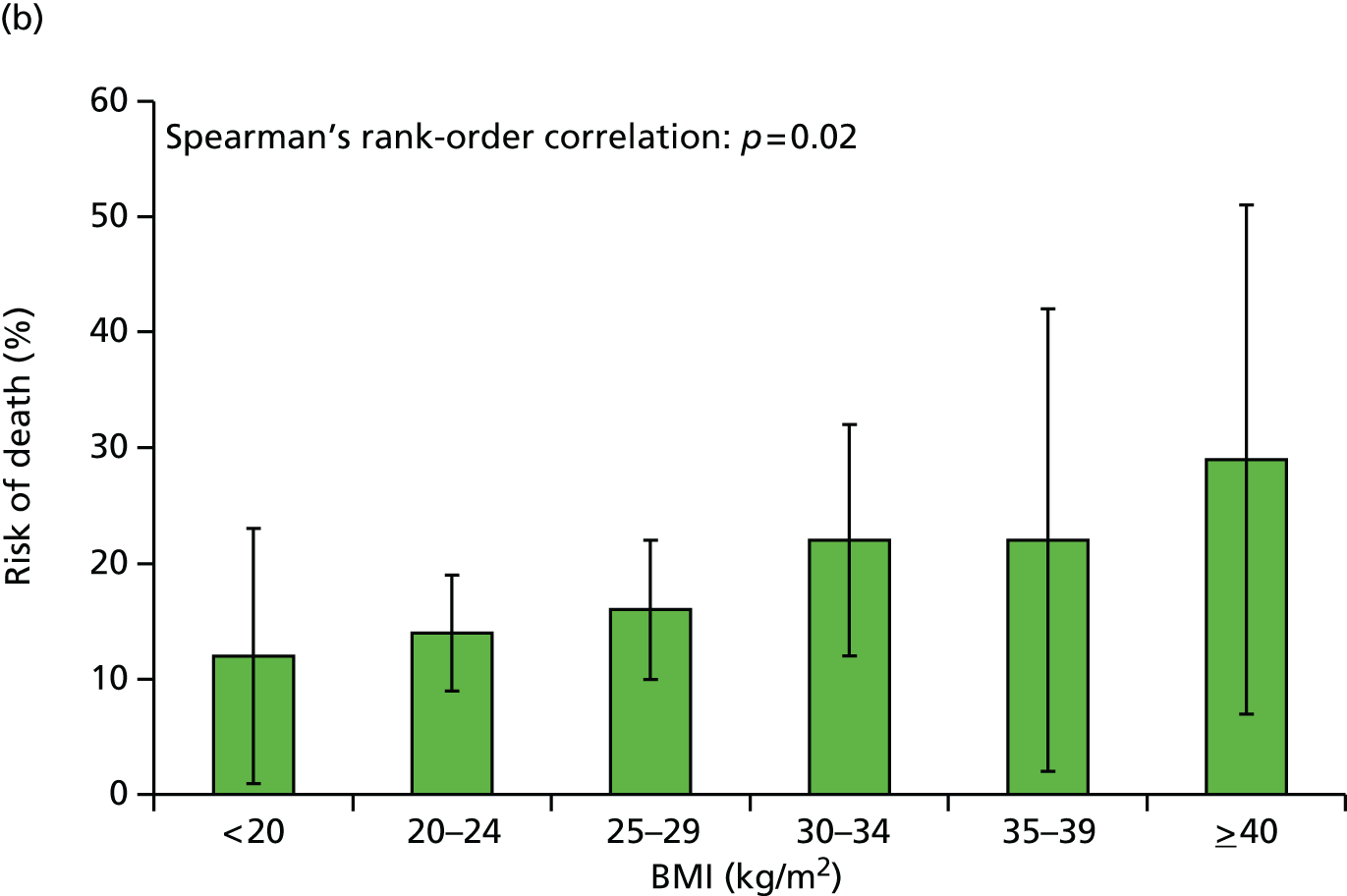
| Factor | Maternal deaths, n (%) (N = 100) | Survivors, n (%) (N = 476) | Crude ORa (95% CI) | aOR,a missing indicator model (95% CI) | aOR,a multiple imputation model (95% CI) |
|---|---|---|---|---|---|
| Age (years) | |||||
| < 30 | 32 (32) | 269 (57) | 1 | 1 | 1 |
| 30–34 | 40 (40) | 120 (25) | 2.80 (1.68 to 4.68) | 2.89 (1.56 to 5.36) | 2.58 (1.36 to 4.90) |
| ≥ 35 | 27 (27) | 85 (18) | 2.67 (1.51 to 4.71) | 2.04 (1.01 to 4.11) | 2.36 (1.22 to 4.56) |
| Missing | 1 (1) | 1 (0) | |||
| Parity | |||||
| Nulliparous | 46 (46) | 286 (60) | 1 | 1 | 1 |
| Multiparous | 53 (53) | 189 (40) | 1.74 (1.13 to 2.70) | 0.75 (0.44 to 1.28) | 0.76 (0.45 to 1.29) |
| Missing | 1 (1) | 1 (0) | |||
| Ethnicity | |||||
| White | 65 (64) | 352 (74) | 1 | 1 | 1 |
| Black Caribbean and African | 19 (20) | 45 (9) | 2.44 (1.34 to 4.44) | 2.40 (1.14 to 5.06) | 2.38 (1.15 to 4.92) |
| Other minority ethnic groups | 15 (15) | 72 (15) | 1.20 (0.64 to 2.23) | 1.16 (0.54 to 2.51) | 1.31 (0.65 to 2.76) |
| Missing | 1 (1) | 7 (1) | |||
| BMI (kg/m2) | |||||
| < 30 | 53 (53) | 327 (67) | 1 | 1 | 1 |
| ≥ 30 | 24 (24) | 84 (18) | 1.83 (1.06 to 3.14) | 1.57 (0.83 to 2.97) | 1.71 (0.91 to 3.19) |
| Missing | 23 (23) | 65 (14) | |||
| Occupational classification | |||||
| Managerial | 20 (25) | 113 (24) | 1 | 1 | 1 |
| Intermediate occupation | 19 (23) | 101 (21) | 1.06 (0.54 to 2.10) | 1.41 (0.65 to 3.07) | 1.33 (0.59 to 2.95) |
| Manual or unemployed | 42 (52) | 207 (43) | 1.14 (0.64 to 2.05) | 2.33 (1.13 to 4.80) | 2.19 (1.03 to 4.68) |
| Missing | 19 (19) | 55 (12) | |||
| Smoking during pregnancy | |||||
| Yes | 13 (14) | 89 (19) | 0.67 (0.35 to 1.26) | 0.62 (0.29 to 1.32) | 0.58 (0.27 to 1.26) |
| No | 82 (86) | 376 (79) | 1 | 1 | 1 |
| Missing | 5 (5) | 9 (2) | |||
Analysis of the combined effects of the risk factors present showed that the odds of death associated with these severe maternal morbidities increased progressively in the presence of more than one of the risk factors identified (Table 21), although of note was the high degree of uncertainty around the estimated odds associated with the presence of all four risk factors.
| Number of risk factors present | OR (95% CI) |
|---|---|
| 0 | 1 |
| 1 | 1.35 (0.67 to 2.75) |
| 2 | 2.77 (1.33 to 5.76) |
| 3 | 4.40 (1.76 to 11.0) |
| 4 | 8.45 (0.49 to 149) |
Role of medical comorbidities and other factors associated with direct maternal deaths
Medical comorbidities were found to be significantly associated with direct maternal deaths from eclampsia, pulmonary embolism, severe sepsis, AFE and peripartum haemorrhage, after controlling for a number of known risk factors for maternal mortality (Table 22). The adjusted odds of having pre-existing medical conditions were almost fivefold higher among women who died than women who survived (aOR 4.82, 95% CI 3.14 to 7.40). In addition, we also found substance misuse (aOR 10.16, 95% CI 1.81 to 57.04), inadequate use of antenatal care (aOR 15.87, 95% CI 6.73 to 37.41), previous pregnancy problems (aOR 2.21, 95% CI 1.34 to 3.62), hypertensive disorders of pregnancy during current pregnancy (aOR 2.44, 95% CI 1.31 to 4.52) and Indian ethnic background (aOR 2.70, 95% CI 1.14 to 6.43) to be independently associated with maternal deaths (see Table 22). At a population level, 70% (95% CI 66% to 73%) of the increased risk could be attributed to these six factors, the most important being medical comorbidities (PAF 48.9%, 95% CI 40.5% to 56.2%) followed by previous pregnancy problems, hypertensive disorders of pregnancy, inadequate use of antenatal care services, Indian ethnicity and substance misuse (see Table 24).
| Risk factors | Cases, n (%) (N = 135) | Controls, n (%) (N = 1661) | uOR (95% CI) | aORa (95% CI) |
|---|---|---|---|---|
| Age (years) | ||||
| < 20 | 7 (5.2) | 105 (6.3) | 0.83 (0.37 to 1.84) | 0.76 (0.29 to 2.03) |
| 20–34 | 84 (62.2) | 1044 (62.9) | 1 | 1 |
| ≥ 35 | 44 (32.6) | 512 (30.8) | 1.06 (0.73 to 1.56) | 0.94 (0.60 to 1.48) |
| Parity | ||||
| Nulliparous | 49 (36.3) | 692 (41.7) | 0.81 (0.55 to 1.19) | 1.05 (0.62 to 1.78) |
| 1–3 | 61 (45.2) | 697 (41.9) | 1 | 1 |
| > 3 | 23 (17.0) | 269 (16.2) | 0.98 (0.59 to 1.61) | 0.79 (0.43 to 1.43) |
| Missing | 2 (1.5) | 3 (0.2) | 7.62 (1.25 to 46.46) | 1.22 (0.02 to 67.56) |
| BMI (kg/m2) | ||||
| < 18.5 | 6 (4.4) | 45 (2.7) | 1.83 (0.76 to 4.41) | 1.56 (0.57 to 4.28) |
| 18.5–30 | 84 (62.2) | 1152 (69.4) | 1 | 1 |
| ≥ 30 | 35 (25.9) | 325 (19.6) | 1.48 (0.98 to 2.23) | 1.05 (0.66 to 1.70) |
| Missing | 10 (7.4) | 139 (8.3) | 0.99 (0.50 to 1.95) | 0.35 (0.13 to 0.94) |
| Multiple pregnancy | ||||
| No | 129 (95.5) | 1588 (95.6) | 1 | 1 |
| Yes | 4 (3.0) | 73 (4.4) | 0.67 (0.24 to 1.87) | 0.67 (0.22 to 2.01) |
| Missing | 2 (1.5) | 0 (0.0) | Omitted | |
| Gestational diabetes | ||||
| No | 118 (87.4) | 1605 (96.6) | 1 | 1 |
| Yes | 8 (5.9) | 50 (3.0) | 2.18 (1.01 to 4.70) | 1.43 (0.59 to 3.44) |
| Missing | 9 (6.7) | 6 (0.4) | 20.40 (7.14 to 58.29) | |
| Hypertensive disorders of pregnancy | ||||
| No | 106 (78.5) | 1555 (93.6) | 1 | 1 |
| Yes | 20 (14.8) | 100 (6.0) | 2.93 (1.75 to 4.93) | 2.44 (1.31 to 4.52) |
| Missing | 9 (6.7) | 6 (0.4) | 22.0 (7.69 to 62.98) | |
| Anaemia | ||||
| No | 123 (91.1) | 1632 (98.2) | 1 | 1 |
| Yes | 3 (2.2) | 23 (1.4) | 1.73 (0.51 to 5.84) | 2.39 (0.60 to 9.47) |
| Missing | 9 (6.7) | 6 (0.4) | 19.90 (6.97 to 56.82) | |
| Inadequate utilisation of antenatal careb | ||||
| No | 106 (78.5) | 1637 (98.6) | 1 | 1 |
| Yes | 21 (15.6) | 18 (1.1) | 18.02 (9.32 to 34.84) | 15.87 (6.73 to 37.41) |
| Missing | 8 (5.9) | 6 (0.3) | 20.59 (7.02 to 61.43) | |
| Smoking status | ||||
| Non-smoker | 95 (70.4) | 1285 (77.4) | 1 | 1 |
| Smoker | 27 (20.0) | 346 (20.8) | 1.05 (0.68 to 1.64) | 0.79 (0.45 to 1.39) |
| Missing | 13 (9.6) | 30 (1.8) | 5.86 (2.96 to 11.61) | 2.92 (0.93 to 9.14) |
| Substance misuse | ||||
| No | 123 (91.1) | 1651 (99.4) | 1 | 1 |
| Yes | 6 (4.4) | 4 (0.2) | 20.13 (5.61 to 72.29) | 10.16 (1.81 to 57.04) |
| Missing | 6 (4.4) | 6 (0.4) | 13.42 (4.27 to 42.24) | 2.94 (0.19 to 47.67) |
| Previous pregnancy problemsc | ||||
| No | 76 (56.3) | 1247 (75.1) | 1 | 1 |
| Yes | 56 (41.5) | 407 (24.5) | 2.26 (1.57 to 3.25) | 2.21 (1.34 to 3.62) |
| Missing | 3 (2.2) | 7 (0.4) | 7.03 (1.78 to 27.73) | 0.25 (0.01 to 5.32) |
| Pre-existing medical problemsd | ||||
| No | 37 (27.4) | 1175 (70.7) | 1 | 1 |
| Yes | 93 (68.9) | 481 (29.0) | 6.14 (4.13 to 9.12) | 4.82 (3.14 to 7.40) |
| Missing | 5 (3.7) | 5 (0.3) | 31.76 (8.81 to 114.45) | 18.93 (1.63 to 220.32) |
| Employment status | ||||
| Employed | 91 (67.4) | 1211 (72.9) | 1 | 1 |
| Unemployed | 17 (12.6) | 188 (11.3) | 1.20 (0.70 to 2.07) | 1.27 (0.65 to 2.47) |
| Missing | 27 (20.0) | 262 (15.8) | 1.37 (0.87 to 2.15) | 0.81 (0.44 to 1.51) |
| Ethnicity | ||||
| White European | 88 (65.2) | 1226 (73.8) | 1 | 1 |
| Indian | 8 (5.9) | 47 (2.8) | 2.37 (1.09 to 5.17) | 2.70 (1.14 to 6.43) |
| Pakistani | 7 (5.2) | 73 (4.4) | 1.34 (0.60 to 2.99) | 2.10 (0.86 to 5.11) |
| Bangladeshi | 2 (1.5) | 39 (2.4) | 0.71 (0.17 to 3.01) | 1.21 (0.27 to 5.45) |
| Other Asian | 4 (3.0) | 66 (4.0) | 0.84 (0.30 to 2.37) | 1.08 (0.36 to 3.27) |
| Black Caribbean | 3 (2.2) | 40 (2.4) | 1.04 (0.32 to 3.45) | 0.93 (0.24 to 3.64) |
| Black African | 13 (9.6) | 105 (6.3) | 1.72 (0.93 to 3.19) | 1.09 (0.51 to 2.31) |
| Other/mixed | 10 (7.4) | 65 (3.9) | 2.14 (1.06 to 4.32) | 1.46 (0.59 to 3.61) |
Eight out of the 18 medical comorbidities were significantly associated with maternal death (Table 23). The odds of pre-existing musculoskeletal disorders was 12 times higher and the odds of inflammatory/atopic disorders (excluding asthma) was 10-fold higher among women who died than women who survived. Women who died had five times higher odds of suffering from autoimmune diseases and infections, such as sexually transmitted infections, tuberculosis and group B Streptococcus infection, fourfold higher odds of having a pre-existing haematological disorder and more than three times higher odds of essential hypertension than the women who survived. The odds of suffering from mental health problems and asthma were more than two times higher among women who died than women who survived.
| Risk factors | Cases, n (%) (N = 135) | Controls, n (%) (N = 1661) | uOR (95% CI) | aORa (95% CI) |
|---|---|---|---|---|
| Asthma | ||||
| No | 111 (82.2) | 1562 (94.0) | 1 | 1 |
| Yes | 19 (14.1) | 94 (5.7) | 2.84 (1.68 to 4.83) | 2.36 (1.19 to 4.65) |
| Inflammatory disorders and allergic/atopic conditions (excluding asthma) | ||||
| No | 116 (85.9) | 1645 (99.0) | 1 | 1 |
| Yes | 14 (10.4) | 11 (0.7) | 18.05 (8.01 to 41.65) | 9.79 (3.50 to 27.36) |
| Haematological disorders | ||||
| No | 117 (86.7) | 1617 (97.4) | 1 | 1 |
| Yes | 13 (9.6) | 39 (2.3) | 4.61 (2.39 to 8.87) | 4.29 (1.88 to 9.76) |
| Infertility | ||||
| No | 122 (90.4) | 1640 (98.7) | 1 | 1 |
| Yes | 8 (5.9) | 16 (1.0) | 6.72 (2.82 to 16.02) | 3.08 (0.87 to 10.84) |
| Neurological disorders | ||||
| No | 125 (92.6) | 1650 (99.3) | 1 | 1 |
| Yes | 5 (3.7) | 6 (0.4) | 11.00 (3.31 to 36.55) | 3.83 (0.71 to 20.74) |
| Musculoskeletal disorders | ||||
| No | 120 (88.9) | 1648 (99.2) | 1 | 1 |
| Yes | 10 (7.4) | 8 (0.5) | 17.17 (6.65 to 44.30) | 12.65 (3.56 to 44.98) |
| Mental health problems | ||||
| No | 110 (81.5) | 1585 (95.4) | 1 | 1 |
| Yes | 20 (14.8) | 71 (4.3) | 4.06 (2.38 to 6.91) | 2.63 (1.28 to 5.44) |
| Infection (other than blood-borne viruses) | ||||
| No | 122 (90.4) | 1646 (99.1) | 1 | 1 |
| Yes | 8 (5.9) | 10 (0.6) | 10.79 (4.18 to 27.84) | 5.31 (1.63 to 17.23) |
| Essential hypertension | ||||
| No | 122 (90.4) | 1626 (97.9) | 1 | 1 |
| Yes | 8 (5.9) | 30 (1.8) | 3.55 (1.59 to 7.92) | 3.35 (1.25 to 9.00) |
| Thrombotic event | ||||
| No | 125 (92.6) | 1640 (98.7) | 1 | 1 |
| Yes | 5 (3.7) | 16 (1.0) | 4.10 (1.48 to 11.38) | 2.74 (0.68 to 11.08) |
| Autoimmune diseases | ||||
| No | 125 (92.6) | 1637 (98.6) | 1 | 1 |
| Yes | 5 (3.7) | 19 (1.1) | 3.45 (1.27 to 9.38) | 5.16 (1.63 to 16.39) |
| Cardiac disease (congenital or acquired) | ||||
| No | 126 (93.3) | 1632 (98.3) | 1 | 1 |
| Yes | 4 (3.0) | 24 (1.4) | 2.16 (0.74 to 6.32) | 1.32 (0.34 to 5.15) |
| Diabetes mellitus | ||||
| No | 127 (94.1) | 1641 (98.8) | 1 | 1 |
| Yes | 3 (2.2) | 15 (0.9) | 2.58 (0.74 to 9.04) | 2.09 (0.50 to 8.79) |
| Blood-borne viruses | ||||
| No | 127 (94.1) | 1644 (99.0) | 1 | |
| Yes | 3 (2.2) | 12 (0.7) | 3.24 (0.90 to 11.62) | Excluded |
| Renal problems | ||||
| No | 126 (93.3) | 1629 (98.1) | 1 | |
| Yes | 4 (3.0) | 27 (1.6) | 1.92 (0.66 to 5.56) | Excluded |
| Endocrine disorders | ||||
| No | 127 (94.1) | 1625 (97.8) | 1 | |
| Yes | 3 (2.2) | 31 (1.9) | 1.24 (0.37 to 4.11) | Excluded |
| Known malignancies | ||||
| No | 129 (95.6) | 1650 (99.3) | 1 | |
| Yes | 1 (0.7) | 6 (0.4) | 2.13 (0.25 to 17.84) | Excluded |
| Epilepsy | ||||
| No | 130 (96.3) | 1642 (98.9) | 1 | |
| Yes | 0 (0.0) | 14 (0.8) | 1 (omitted) | Excluded |
The odds associated with maternal death increased by more than three and a half times per unit increase in the ‘risk factor’ score (aOR 3.59, 95% CI 2.83 to 4.56; p < 0.001), after controlling for other variables that are not included in the score. On a population basis, 70% (95% CI 66% to 73%) of the increased risk associated with maternal death could be attributed to the six identified factors, the most important being medical comorbidities followed by previous pregnancy problems, hypertensive disorders of pregnancy, inadequate use of antenatal care services and Indian ethnicity (Table 24).
| Risk factors | PAF (%) | 95% CI |
|---|---|---|
| ‘Risk factors’ score | 69.8 | 66.1 to 73.0 |
| Specific factors | ||
| Medical comorbidities | 48.9 | 40.5 to 56.2 |
| Previous pregnancy problems | 21.1 | 11.7 to 29.5 |
| Hypertensive disorders of pregnancy | 12.0 | 7.7 to 16.1 |
| Inadequate use of antenatal care | 10.5 | 9.7 to 11.4 |
| Indian ethnicity | 2.9 | 0.3 to 5.5 |
| Substance misuse | 1 | 0.03 to 1.4 |
Conclusions and implications for policy and practice
The most recent UK Confidential Enquiries into maternal deaths (2009–12) showed that while direct maternal deaths related to obstetric causes have significantly decreased over the last 10 years, deaths due to indirect medical causes outnumber direct deaths by two to one and have remained unchanged since 2000–2. 2 We found six factors to be associated with maternal death from direct pregnancy complications after adjustment: inadequate use of antenatal care, substance misuse, medical comorbidities, hypertensive disorders of pregnancy, previous pregnancy problems and Indian ethnicity. Together, these account for 70% of the increased population risk. Belonging to other ethnic minority backgrounds was also associated with increased odds of death but the ORs were not statistically significant, possibly because of a smaller number of cases in each of these groups resulting in low statistical power. Specific medical comorbidities, including asthma, autoimmune diseases, inflammatory/atopic disorders, mental health problems, essential hypertension, haematological disorders, musculoskeletal disorders and infections were found to be associated with a higher risk of dying from the conditions included in this study. Medical comorbidities accounted for half of the increased risk of fatality in the study population.
Several studies have demonstrated individual medical comorbidities, such as pre-existing asthma, hypertension, malignancy, chronic ischaemic and congenital heart disease, chronic renal disease, systemic lupus erythematosus, hypercoagulability states, human immunodeficiency virus and diabetes mellitus, to be associated with both severe maternal morbidity and mortality,25,153,162–168 but the extent of the population risk attributable to medical comorbidities as a whole in the UK has not previously been quantified. Uptake of antenatal care was found to be poorer among women with medical comorbidities in our study population, which could increase the adverse effects associated with these conditions. Our findings suggest that the association of older maternal age and obesity with increased odds of dying observed in the CMACE/UKOSS study160 could be mediated to some extent through medical comorbidities. Unlike other studies,25,153 we did not find pre-existing diabetes mellitus and cardiac conditions to be associated with maternal death. Whether or not this reflects improved obstetric care of women with diabetes and cardiac disease is unclear and requires further research.
Hypertension during pregnancy has been shown in other analyses to be associated with an increased risk of intracerebral haemorrhage, eclampsia or end-organ dysfunction culminating in death. 169–172 Studies from different parts of the world, including the UK, show a higher risk of mortality among women who do not receive adequate antenatal care. 156,169,171,173 Although there is a debate about the role of antenatal care in preventing maternal deaths caused by acute conditions that emerge close to the time of delivery, its role in identifying pregnant women at high risk (such as women with hypertensive disorders, medical comorbidities, anaemia and infections) and lowering their risk of mortality is widely accepted. 174
Confidential enquiries into maternal deaths in the UK have identified that a number of women who die during or after pregnancy are substance misusers, although the association with maternal death has not been formally quantified before now. 100 Studies have shown that women who suffered problems during a previous pregnancy were more likely to develop severe morbidity in subsequent pregnancies. 4,154,175 There is mixed evidence about ethnic inequalities in maternal death. Although our first study160 found an association with black African and Caribbean ethnicity in the UK, Geller et al. 9 did not find any association with minority ethnicity in the USA. Among the ethnic minority groups examined in this study, the association with maternal death was higher among the Indian group, but only for sepsis and haemorrhage. This is likely to explain the difference between our findings and the previous UK study, which did not include these conditions. 160 Although factors such as pre-existing medical conditions, previous pregnancy problems and being of Indian origin cannot be altered, their adverse consequences can be potentially minimised through extra vigilance and proactive management.
The messages from this study can be used to inform actions to reduce maternal mortality throughout the developed world. Ongoing high-quality national surveillance programmes still have an important role to play in addressing new challenges in maternal health and care. Women from vulnerable populations in high-resource countries remain at increased risk of maternal death in the presence of severe maternal morbidities. This national study has identified six risk factors associated with maternal death from direct pregnancy complications: inadequate use of antenatal care services; substance misuse; medical comorbidities; hypertensive disorders of pregnancy; previous pregnancy problems; and Indian ethnicity. As noted earlier, maternal deaths arising from indirect (medical) causes now outnumber direct deaths arising from obstetric causes in most high-resource countries, with mortality rates either remaining static or increasing. 2,101,156,176,177 This study shows that medical comorbidities are also important factors associated with deaths arising from obstetric causes (direct deaths) in the UK; almost 50% of the population-attributable risk is associated with the presence of medical comorbidities. This highlights the importance of optimal care for women with pre-existing medical problems in pregnancy. Further studies are required to understand whether or not specific aspects of care could be improved to reduce maternal deaths among women with medical comorbidities.
Chapter 7 Inequalities in severe maternal morbidities: investigation of the roles of maternal age, ethnic group and socioeconomic status
Background
The characteristics of women giving birth are changing. Women are, on average, older, more likely to have been born outside the UK and more likely to suffer from pre-existing medical conditions than in the past. In addition, the impact of technologies such as IVF with ovum donation may contribute to an increasing incidence of pregnancies in women outside the normal reproductive age.
Addressing inequalities in health is an important aspect of public health policy in the UK, including a call for specific action to improve the quality of antenatal care. 19 Identifying and describing such inequalities is an important first step in developing strategies to address them. 19 An analysis of rates of selected near-miss morbidities identified through UKOSS has shown important inequalities in the occurrence of these disorders in ethnic minority women. 158 However, this study, because of its relatively small size, was not able to investigate in-depth potential causes for the observed differences between individual minority groups, only between ethnic minority women as a whole and white women. Recent work has also suggested poorer outcomes of pregnancy in older women178 and an over-representation of socially disadvantaged women among mothers who died. 3 Infant mortality is known to be much higher among routine and manual socioeconomic groups179,180 but social inequalities in near-miss maternal morbidity have not been investigated on a national basis in the UK. The aim of this workstream was to identify and investigate inequalities in maternal morbidity among specific population groups, with the aim of identifying actions to address any inequality identified.
Research questions
-
Are there differences in the incidence of near-miss maternal morbidity between women from different age, socioeconomic and ethnic groups?
-
What are the roles of differences in demographic, pregnancy-related and other factors, such as poor attendance for antenatal care, in any differences observed?
-
What near-miss maternal morbidities occur in women of ≥ 48 years giving birth in the UK? What proportion of pregnancies in these women follow assisted reproductive technologies? How are these women managed?
-
What are the outcomes of pregnancy for women from different age, socioeconomic and ethnic groups?
Methods
This workstream investigated inequalities in maternal morbidity in three specific groups: older mothers, women from routine and manual socioeconomic groups, and ethnic minority women. Information about the entire cohort of women with near-miss morbidity identified through UKOSS was analysed to determine rates of near-miss morbidity and the outcomes within subgroups. In addition, a cohort study was conducted, collecting information about all women ≥ 48 years of age delivering in the UK to describe comprehensively the morbidities, their management and pregnancy outcomes within this group.
Secondary data analysis
Two unmatched case–control analyses were conducted using existing UKOSS data collected between February 2005 and January 2013 to examine the associations of ethnic and socioeconomic groups with severe maternal morbidity in the UK. Two sets of cases and controls were used. Cases included in the analysis of socioeconomic inequalities were women who suffered from one of the following six conditions of severe maternal morbidity directly attributable to pregnancy causes: AFE,32 AFLP,30 eclampsia,28 peripartum hysterectomy,31 peripartum haemorrhage129 and uterine rupture. 181 For the ethnic inequality study, in addition to these six conditions, cases were included from a further five conditions: antenatal pulmonary embolism,29 stroke in pregnancy,74 placenta accreta,72 HELLP syndrome54 and severe sepsis. 99 These data sets represented the entirety of data available on direct maternal morbidities and control women at the time the analyses were conducted. The controls were women who delivered immediately before the cases in the same hospital. 158 In total, 1144 cases and 2256 controls were included in the socioeconomic inequality study182 and 1753 cases and 3310 controls were included in the ethnic differences study. 154 For the included sample sizes, assuming a prevalence of exposure of 1.1% (the prevalence of the smallest ethnic minority group, the Bangladeshi group), the analysis had 80% power to detect an OR of ≥ 1.8 associated with severe maternal morbidity at p < 0.05.
Maternal occupation (or husband’s/partner’s occupation if information about women’s occupational status was not available) was used to categorise women into four socioeconomic groups: managerial/professional, intermediate, routine/manual and unemployed (additional category), using the National Statistics Socio-economic Classification. 183 The ethnic groups were classified based on self-reported ethnicity noted in medical records according to the UK national census classification. 149 Potential confounders were those that were adjusted for in previous studies – pregnancy-related factors such as parity, problems during current and previous pregnancies, multiple pregnancy, medical comorbidities, utilisation of antenatal care, maternal age, BMI and smoking status. 152,155,158
Univariable analyses were conducted to assess the association of the exposure variables socioeconomic status and ethnicity, and other independent variables with the outcome (severe maternal morbidity). Multivariable unconditional logistic regression analyses were performed to control for confounders. For the socioeconomic analysis, the potential confounders were added to a model that included the outcome and socioeconomic status variable one at a time with subsequent likelihood ratio testing. All variables found to be associated with the outcome at p < 0.05 (two-tailed) and those identified as important confounders from the literature were included in the final model.
The multivariable models for ethnicity analysis included all variables that were found to be associated with the outcome at p < 0.1 (two-tailed) in the univariable analyses and those identified as important potential confounders in previous literature. Four models were built in a hierarchical fashion. Model 1 adjusted for anaemia in current pregnancy, diabetes in current pregnancy, previous pregnancy problems, pre-existing medical problems and parity. In model 2, smoking status and inadequate utilisation of antenatal care services were added to the other variables. Model 3 included socioeconomic status in addition to those variables included in model 2, and model 4 adjusted for all variables including women’s age and BMI. In addition to controlling for the known risk factors, this approach enabled understanding of their effects on the association between ethnicity and severe maternal morbidity.
Plausible interactions were tested by adding interaction terms and using LR-tests (p < 0.05) and no significant interactions were identified. Information on ethnicity was not available for 1.8% of the sample. Based on the method used in two previous studies, women with unknown ethnicity were included in the ‘white European’ group because the redistributed proportions matched more accurately with the estimated ethnic profiles in the UK population census. 158,184 Although for a majority of the independent variables the number of participants with missing data was < 1% and their distribution did not differ significantly between the cases and controls and among the ethnic groups, for four variables, ‘socioeconomic status’, ‘BMI’, ‘smoking’ and ‘previous pregnancy problems’, the proportion of participants with missing information was > 1%. The data were assumed to be not missing at random and thus coded ‘missing’ as a separate group for these variables. In addition, a series of sensitivity analyses were performed by assuming extreme scenarios and accordingly redistributing the missing observations into the extreme groups; this had no material effect on the findings.
The continuous variables were tested for deviations from linearity,185 which showed non-linear associations between the variables and the outcome. Thus, in the final model the variables were incorporated as categorical variables for the ease of interpretation of the ORs and the advantage of including and testing the missing data as a separate category. All analyses were carried out using Stata 11.
Primary data collection
A national, population-based cohort study was conducted using UKOSS as described in Chapter 3. The cohort included any pregnant woman in the UK of ≥ 20 weeks’ gestation who was of very advanced maternal age. Although very advanced maternal age has generally been used to refer to women aged ≥ 45 years, for pragmatic reasons so as to not overburden reporting clinicians, very advanced maternal age was defined for our purposes as women aged ≥ 48 years at their date of delivery. The cohort was identified through UKOSS between 1 July 2013 and 30 June 2014. The UKOSS reporting clinicians were also asked to identify comparison women, defined as the two pregnant women of ≥ 20 weeks’ gestation who were < 48 years of age at their estimated date of delivery and delivered immediately before the older woman in the same hospital.
The ORs with 95% CIs were estimated throughout using unconditional logistic regression. ORs were adjusted for factors if there was a pre-existing hypothesis or evidence that the factors were potential confounders or mediators of the relationship between advanced maternal age and the outcome in question. To help examine the relative influence of the potential confounders and mediators on the association between maternal age and the outcome in question, models were adjusted in a hierarchical fashion: model 1 adjusted for sociodemographic factors; model 2 additionally adjusted for previous medical history; and model 3 additionally adjusted for relevant pregnancy related factors. ‘Missing’ was included as an extra category for variables that had ≥ 10% of missing data. Continuous variables were tested for evidence of departure from linearity by the addition of first-order fractional polynomials to the model and subsequent likelihood ratio testing. Continuous variables that showed evidence of non-linearity were treated and presented as categorical in the analysis, while those showing evidence of linearity were treated as continuous linear terms when adjusting for them in the analysis but presented as categorical for ease of interpretation. Plausible interactions were tested in the full regression model by the addition of interaction terms and subsequent likelihood ratio testing on removal, with p < 0.01 considered evidence of significant interaction to account for multiple testing.
Women who initially had a multiple pregnancy but then had fetal reduction were classified in the analysis according to the number of fetuses left after the reduction. Spontaneous first-trimester losses in women known initially to have a multiple pregnancy were classified in the analysis according to the post loss number of fetuses. Second-trimester losses in a multiple pregnancy were classified according to the pre-loss number of fetuses in the main analysis, but were not included when examining neonatal outcomes unless they occurred after 24 weeks. Logistic regression using robust SEs to allow for non-independence of neonates from multiple births was used when comparing neonatal outcomes.
Using the most recent national birth data,12,186,187 we anticipated identifying 406 women aged ≥ 48 years at their date of delivery and 812 comparison women. With these numbers of women the study would have had an estimated power of 80% at the 5% level of statistical significance to detect ORs of ≥ 1.5 and ≥ 2.0, assuming outcomes have an incidence of 40% and 5%, respectively. The actual number of older and comparison women identified during the study gave an estimated power of 80% at the 5% level of significance to detect ORs of ≥ 1.6 and ≥ 2.5, assuming the same outcome incidence levels.
Results
Maternal socioeconomic status
A substantial proportion of the sample did not have information on socioeconomic status (17% for cases and 13% for controls); thus, bias owing to missing data could not be ruled out. The results of multivariable regression analysis showed that, compared with controls, the cases were 1.51 (95% CI 1.18 to 1.94) times more likely to have missing socioeconomic information. Although not statistically significant, the odds of belonging to the routine/manual group was 1.17 (95% CI 0.94 to 1.45) times higher and belonging to an unemployed group was 1.22 (95% CI 0.92 to 1.61) times higher among women who suffered from severe maternal morbidity than among the controls (Table 25).
| Characteristic | Cases n = (%), N = 1144 | Controls n = (%), N = 2256 | uOR (95% CI) | aORa (95%CI) |
|---|---|---|---|---|
| Socioeconomic group | ||||
| Managerial/professional | 292 (25.5) | 567 (25.1) | 1.0 | 1.0 |
| Intermediate | 244 (21.3) | 482 (21.4) | 0.98 (0.80 to 1.21) | 1.17 (0.94 to 1.45) |
| Routine/manual | 273 (28.7) | 595 (26.4) | 0.89 (0.73 to 1.09) | 1.16 (0.93 to 1.45) |
| Unemployed | 142 (14.9) | 309 (13.7) | 0.89 (0.70 to 1.14) | 1.22 (0.92 to 1.61) |
| Missing | 193 (16.9) | 303 (13.4) | 1.24 (0.98 to 1.56) | 1.51 (1.18 to 1.94) |
| Ethnic group | ||||
| White | 827 (72.3) | 1796 (79.6) | 1.0 | 1.0 |
| Asian | 139 (12.2) | 197 (8.7) | 1.53 (1.22 to 1.93) | 1.57 (1.23 to 2.00) |
| Black | 108 (9.4) | 116 (5.1) | 2.02 (1.54 to 2.66) | 1.77 (1.32 to 2.36) |
| Other | 52 (4.6) | 69 (3.1) | 1.64 (1.13 to 2.37) | 1.50 (1.02 to 2.19) |
| Missing | 18 (1.6) | 78 (3.5) | 0.50 (0.30 to 0.84) | 0.51 (0.28 to 0.91) |
| Age (years) | ||||
| < 20 | 56 (4.9) | 139 (6.2) | 0.98 (0.70 to 1.39) | 1.08 (0.75 to 1.56) |
| 20–24 | 127 (11.1) | 449 (19.9) | 0.69 (0.54 to 0.89) | 0.71 (0.55 to 0.92) |
| 25–29 | 225 (19.7) | 549 (24.3) | 1.0 | 1.0 |
| 30–34 | 341 (29.8) | 622 (27.6) | 1.34 (1.09 to 1.64) | 1.33 (1.08 to 1.64) |
| ≥ 35 | 392 (34.3) | 479 (21.2) | 2.00 (1.63 to 2.45) | 1.98 (1.60 to 2.45) |
| Missing | 3 (0.3) | 18 (0.8) | 0.41 (0.12 to 1.39) | 2.66 (0.22 to 26.00) |
| Smoking status | ||||
| Non-smokers | 899 (78.6) | 1642 (72.8) | 1.0 | 1.0 |
| Smokers | 217 (19.0) | 553 (24.5) | 0.72 (0.60 to 0.86) | 0.89 (0.73 to 1.08) |
| Missing | 28 (2.5) | 61 (2.7) | 0.84 (0.53 to 1.32) | 0.95 (0.54 to 1.67) |
| BMI (kg/m2) | ||||
| Continuous (per kg/m2 increase in BMI) | 1.01 (1.00 to 1.03) | |||
| < 25 | 513 (44.8) | 1121 (49.7) | 1.0 | 1.0 |
| 25–29.9 | 297 (26.0) | 545 (24.2) | 1.19 (1.00 to 1.42) | 1.10 (0.92 to 1.32) |
| ≥ 30 | 206 (18.0) | 371 (16.5) | 1.21 (0.99 to 1.48) | 1.09 (0.88 to 1.34) |
| Missing | 128 (11.2) | 219 (9.7) | 1.28 (1.00 to 1.63) | 1.24 (0.95 to 1.61) |
| Coexisting medical conditionsb | ||||
| No | 1045 (91.4) | 2126 (94.2) | 1.0 | 1.0 |
| Yes | 93 (8.1) | 103 (4.6) | 1.84 (1.38 to 2.46) | 1.60 (1.18 to 2.17) |
| Missing | 6 (0.5) | 27 (1.2) | 0.45 (1.18 to 1.10) | 0.76 (0.17 to 3.41) |
| Parity | ||||
| Nulliparous | 403 (35.2) | 984 (43.6) | 1.0 | No significant effect on fit of model (excluded) |
| Multiparous | 738 (64.5) | 1250 (55.4) | 1.44 (1.24 to 1.67) | |
| Missing | 3 (0.3) | 22 (1.0) | 0.33 (0.10 to 1.12) | |
| Multiple pregnancy | ||||
| No | 1083 (94.7) | 2211 (98.0) | 1.0 | 1.0 |
| Yes | 59 (5.2) | 27 (1.2) | 4.46 (2.81 to 7.08) | 3.88 (2.42 to 6.22) |
| Missing | 2 (0.2) | 18 (0.8) | 0.23 (0.53 to 0.98) | 0.20 (0.02 to 2.54) |
| Past pregnancy conditionsc | ||||
| No | 1041 (91.0) | 2121 (94.0) | 1.0 | 1.0 |
| Yes | 94 (8.2) | 104 (4.6) | 1.81 (1.38 to 2.46) | 1.58 (1.16 to 2.14) |
| Missing | 9 (0.8) | 31 (1.4) | 0.59 (0.28 to 1.25) | 1.12 (0.36 to 3.54) |
Maternal ethnic group
After accounting for other factors and possible confounders sequentially, the fully adjusted model 4 showed that compared with white European women, the odds of severe maternal morbidity were 83% higher among black African women, 80% higher among black Caribbean women, 74% higher in Bangladeshi women, and 56% and 43% higher in the other non-white (non-Asian) and Pakistani groups, respectively (Table 26). There was remarkably little change in ORs with adjustment for other factors across the models suggesting that other sociodemographic and clinical factors had little or no confounding effects. Women with inadequate utilisation of antenatal care services were twice as likely to be at risk of severe morbidity as women who adequately utilised the services (aOR 1.97, 95% CI 0.96 to 4.04) and the odds of inadequate utilisation of antenatal was higher among black African (OR 4.46, 95% CI 1.47 to 11.48) and Caribbean (OR 4.35, 95% CI 0.49 to 18.19) women than among white European women. In this analysis, which included additional potential confounders, notably inadequate utilisation of antenatal care and had greater statistical power, there was no evidence of any association between maternal socioeconomic status and severe morbidity.
| Risk factors | Unadjusted, OR (95% CI) | Model 1, aOR (95% CI) | Model 2, aOR (95% CI) | Model 3, aOR (95% CI) | Model 4, aOR (95% CI) |
|---|---|---|---|---|---|
| Ethnicity | |||||
| White European | 1a | 1a | 1a | 1a | 1a |
| Indian | 0.97 (0.68 to 1.38) | 0.98 (0.69 to 1.40) | 0.95 (0.66 to 1.36) | 0.94 (0.66 to 1.35) | 1.00 (0.70 to 1.44) |
| Pakistani | 1.37 (1.03 to 1.81) | 1.38 (1.04 to 1.84) | 1.33 (0.99 to 1.77) | 1.32 (0.98 to 1.76) | 1.43 (1.07 to 1.92) |
| Bangladeshi | 1.73 (1.07 to 2.80) | 1.67 (1.02 to 2.74) | 1.61 (0.98 to 2.65) | 1.65 (1.00 to 2.72) | 1.74 (1.05 to 2.88) |
| Other Asian | 1.29 (0.90 to 1.85) | 1.32 (0.92 to 1.90) | 1.27 (0.88 to 1.83) | 1.28 (0.89 to 1.84) | 1.27 (0.87 to 1.84) |
| Black Caribbean | 1.79 (1.16 to 2.76) | 1.89 (1.21 to 2.94) | 1.84 (1.18 to 2.88) | 1.90 (1.21 to 2.97) | 1.80 (1.14 to 2.82) |
| Black African | 2.05 (1.58 to 2.65) | 1.90 (1.46 to 2.48) | 1.80 (1.38 to 2.35) | 1.83 (1.39 to 2.39) | 1.83 (1.39 to 2.40) |
| Other non-white (non-Asian)b | 1.59 (1.08 to 2.34) | 1.61 (1.09 to 2.38) | 1.56 (1.05 to 2.31) | 1.55 (1.04 to 2.30) | 1.56 (1.05 to 2.33) |
| Mixed | 1.33 (0.82 to 2.16) | 1.35 (0.82 to 2.23) | 1.37 (0.83 to 2.26) | 1.35 (0.82 to 2.23) | 1.28 (0.77 to 2.13) |
| Pregnancy-related factors | |||||
| Current pregnancy problems | |||||
| Anaemia | |||||
| No | 1a | 1a | 1a | 1a | |
| Yes | 1.80 (0.99 to 3.26) | 1.82 (1.01 to 3.31) | 1.83 (1.01 to 3.32) | 1.82 (1.00 to 3.32) | |
| Diabetes | |||||
| No | 1a | 1a | 1a | 1a | |
| Yes | 1.26 (0.87 to 1.83) | 1.26 (0.87 to 1.83) | 1.27 (0.87 to 1.85) | 1.20 (0.82 to 1.75) | |
| Previous pregnancy problems | |||||
| No | 1a | 1a | 1a | 1a | |
| Yes | 1.28 (1.09 to 1.50) | 1.28 (1.09 to 1.50) | 1.28 (1.09 to 1.50) | 1.27 (1.08 to 1.50) | |
| Pre-existing medical problems | |||||
| No | 1a | 1a | 1a | 1a | |
| Yes | 1.53 (1.33 to 1.75) | 1.55 (1.35 to 1.77) | 1.55 (1.35 to 1.77) | 1.54 (1.34 to 1.77) | |
| Parity | |||||
| Primiparous | 0.98 (0.86 to 1.12) | 0.98 (0.85 to 1.11) | 0.97 (0.85 to 1.11) | 1.00 (0.87 to 1.15) | |
| 1–3 | 1a | 1a | 1a | 1a | |
| > 3 | 1.79 (1.35 to 2.37) | 1.83 (1.38 to 2.42) | 1.82 (1.37 to 2.41) | 1.64 (1.23 to 2.20) | |
| Behavioural factors, demographic factors and BMI | |||||
| Smoking | |||||
| Non-smoker | 1a | 1a | 1a | ||
| Smoker | 0.84 (0.73 to 0.98) | 0.86 (0.74 to 1.01) | 0.88 (0.75 to 1.03) | ||
| Inadequate utilisation of antenatal care services | |||||
| No | 1a | 1a | 1a | ||
| Yes | 2.07 (1.02 to 4.23) | 2.04 (1.00 to 4.17) | 1.97 (0.96 to 4.04) | ||
| Occupational classification | |||||
| Managerial | 1a | 1a | |||
| Intermediate | 0.99 (0.83 to 1.18) | 1.04 (0.87 to 1.24) | |||
| Routine and manual | 0.88 (0.75 to 1.04) | 0.94 (0.79 to 1.11) | |||
| Unemployed | 0.91 (0.74 to 1.12) | 0.92 (0.74 to 1.15) | |||
| Age (years) | |||||
| < 20 | 1.63 (1.23 to 2.17) | ||||
| 20–34 | 1a | ||||
| ≥ 35 | 1.58 (1.37 to 1.82) | ||||
| BMIc (kg/m2) | |||||
| < 18.5 | 1.11 (0.77 to 1.60) | ||||
| 18.5–30 | 1a | ||||
| ≥ 30 | 1.03 (0.88 to 1.21) | ||||
Older maternal age
A total of 233 women of very advanced maternal age were notified to UKOSS and 454 comparison women. The median age of the older women was 49 years (range 48–61 years) while the median age of the comparison women was 31 years (range 16–46 years). Older women were significantly more likely than comparison women to be overweight or obese, to not smoke in pregnancy, have had previous uterine surgery not including previous caesarean section, have previous or pre-existing medical condition(s), be nulliparous, have a multiple pregnancy and have conceived following assisted conception (Table 27).
| Characteristic | n (%)a of older women (N = 233) | n (%)a of comparison women (N = 454) | uOR (95% CI) | p-value |
|---|---|---|---|---|
| Sociodemographic characteristics | ||||
| Ethnic group | ||||
| White | 165 (71) | 323 (71) | 1 | |
| Non-white | 67 (29) | 129 (29) | 1.02 (0.72 to 1.44) | 0.9259 |
| Marital status | ||||
| Married or cohabiting | 194 (85) | 375 (84) | 1 | |
| Single | 35 (15) | 71 (16) | 0.95 (0.61 to 1.48) | 0.8299 |
| Socioeconomic group | ||||
| Managerial and professional occupations | 91 (39) | 144 (32) | 1 | |
| Other | 103 (44) | 231 (51) | 0.71 (0.50 to 1.00) | 0.0511 |
| Missing | 39 (17) | 79 (17) | ||
| BMI (kg/m2) | ||||
| < 25 | 101 (44) | 260 (58) | 1 | |
| 25–29.9 | 75 (33) | 103 (23) | 1.87 (1.29 to 2.73) | 0.0011 |
| ≥ 30 | 52 (23) | 85 (19) | 1.57 (1.04 to 2.38) | 0.0318 |
| Smoking status | ||||
| Never/ex-smoker | 226 (99) | 407 (90) | 1 | |
| Smoked during pregnancy | 3 (1) | 45 (10) | 0.12 (0.04 to 0.39) | 0.0004 |
| Previous medical history | ||||
| Previous uterine surgery not including previous caesarean section | ||||
| No | 168 (74) | 418 (93) | 1 | |
| Yes | 60 (26) | 33 (7) | 4.52 (2.85 to 7.17) | < 0.0001 |
| Previous or pre-existing medical condition | ||||
| No | 129 (56) | 328 (72) | 1 | |
| Yes | 101 (44) | 126 (28) | 2.04 (1.46 to 2.84) | < 0.0001 |
| Pregnancy-related characteristics | ||||
| Parity | ||||
| 0 | 122 (53) | 200 (44) | 1 | |
| ≥ 1 | 108 (47) | 252 (56) | 0.7 (0.51 to 0.97) | 0.0299 |
| Previous caesarean section | ||||
| No | 179 (79) | 379 (84) | 1 | |
| Yes | 49 (21) | 72 (16) | 1.44 (0.96 to 2.16) | 0.0765 |
| Multiple pregnancy | ||||
| No | 189 (82) | 444 (98) | 1 | |
| Yes | 41 (18) | 10 (2) | 9.63 (4.73 to 19.63) | < 0.0001 |
| Conceived following assisted conception | ||||
| No | 50 (22) | 425 (96) | 1 | |
| Yes | 176 (78) | 19 (4) | 78.74 (45.13 to 137.38) | < 0.0001 |
Table 28 shows the pregnancy complications experienced by the older and comparison women. Unadjusted analysis suggests that older women were more likely than comparison women to have a range of complications including gestational hypertensive disorders, gestational diabetes, PPH, caesarean delivery, iatrogenic and spontaneous pre-term delivery and ITU admission. However, with the exception of gestational diabetes, caesarean delivery and ITU admission, these effects were attenuated and became non-significant after adjustment for confounding or mediating factors. The higher rate of multiple pregnancy and use of assisted conception in the older women explained most of this attenuation (see Table 28). There was evidence of significant interaction between caesarean delivery and parity: the raised odds of having a caesarean delivery were only apparent in nulliparous older women (aOR 9.90, 95% CI 3.64 to 26.92 in nulliparous women; aOR 0.71, 95% CI 0.31 to 1.66 in parous women). No other significant interactions were found. Among the older women who had a caesarean delivery, maternal age was the primary indication for 21% (36/175).
| Complication | n (%)a of older women (N = 233) | n (%)a of comparison women (N = 454) | uOR (95% CI) | Model 1 aOR (95% CI) | Model 2 aOR (95% CI) | Model 3 aOR (95% CI) |
|---|---|---|---|---|---|---|
| Any gestational hypertensive disorder | ||||||
| No | 196 (85) | 430 (95) | 1 | 1 | 1 | 1 |
| Yes | 34 (15) | 24 (5) | 3.11 (1.79 to 5.38) | 2.88 (1.63 to 5.09) | 2.84 (1.60 to 5.06) | 2.13 (0.75 to 6.02) |
| Any gestational hypertensive disorder managed by early delivery | ||||||
| No | 219 (95) | 442 (97) | 1 | 1 | 1 | 1 |
| Yes | 11 (5) | 12 (3) | 1.85 (0.80 to 4.26) | 1.72 (0.74 to 4.01) | 1.8 (0.77 to 4.22) | 1.28 (0.27 to 6.01) |
| Pregnancy-induced hypertension | ||||||
| No | 209 (91) | 440 (97) | 1 | 1 | 1 | 1 |
| Yes | 21 (9) | 14 (3) | 3.16 (1.57 to 6.33) | 2.85 (1.38 to 5.88) | 2.81 (1.35 to 5.84) | 2.82 (0.79 to 10.05) |
| Pre-eclampsia | ||||||
| No | 217 (94) | 444 (98) | 1 | 1 | 1 | 1 |
| Yes | 13 (6) | 10 (2) | 2.66 (1.15 to 6.16) | 2.55 (1.07 to 6.07) | 2.53 (1.05 to 6.09) | 1.16 (0.22 to 6.09) |
| Gestational diabetes | ||||||
| No | 188 (82) | 436 (96) | 1 | 1 | 1 | 1 |
| Yes | 42 (18) | 18 (4) | 5.41 (3.04 to 9.65) | 4.97 (2.73 to 9.04) | 4.78 (2.61 to 8.77) | 4.81 (1.93 to 12.00) |
| Gestational diabetes requiring insulin | ||||||
| No | 221 (96) | 452 (100) | 1 | 1 | 1 | 1 |
| Yes | 9 (4) | 2 (0) | 9.2 (1.97 to 42.96) | 8.12 (1.72 to 38.31) | 7.54 (1.57 to 36.17) | 3.64 (0.50 to 26.55) |
| Placenta praevia | ||||||
| No | 222 (97) | 453 (100) | ||||
| Yes | 8 (3) | 0 (0) | ||||
| Placental abruption | ||||||
| No | 226 (99) | 451 (100) | 1 | 1 | 1 | 1 |
| Yes | 3 (1) | 2 (0) | 2.99 (0.50 to 18.04) | 4.7 (0.65 to 34.09) | 4.31 (0.60 to 30.89)b | 1.2 (0.08 to 19.02)c |
| Diagnosed PPH | ||||||
| No | 169 (74) | 385 (85) | 1 | 1 | 1 | 1 |
| Yes | 59 (26) | 69 (15) | 1.95 (1.32 to 2.88) | 1.89 (1.26 to 2.84) | 1.74 (1.13 to 2.66)b | 2.03 (0.97 to 4.27)c |
| Diagnosed PPH requiring blood transfusion | ||||||
| No | 210 (94) | 441 (98) | 1 | 1 | 1 | 1 |
| Yes | 14 (6) | 8 (2) | 3.67 (1.52 to 8.90) | 3.53 (1.39 to 8.92) | 2.30 (0.87 to 6.05) | 4.33 (0.94 to 19.96) |
| Thrombotic event | ||||||
| No | 229 (100) | 453 (100) | ||||
| Yes | 0 (0) | 1 (0) | ||||
| Labour induced | ||||||
| No | 156 (69) | 321 (71) | 1 | 1 | 1 | 1 |
| Yes | 71 (31) | 133 (29) | 1.1 (0.78 to 1.55) | 1.18 (0.82 to 1.69) | 1.1 (0.75 to 1.61)b | 1.91 (1.03 to 3.54)c |
| Caesarean delivery | ||||||
| No | 50 (22) | 305 (67) | 1 | 1 | 1 | 1 |
| Yes | 178 (78) | 149 (33) | 7.29 (5.03 to 10.55) | 6.41 (4.39 to 9.37) | 5.9 (3.98 to 8.75)b | 2.78 (1.44 to 5.37)c |
| Gestational age at delivery (weeks) | ||||||
| Term (37+ weeks) | 176 (78) | 420 (93) | 1 | 1 | 1 | 1 |
| Iatrogenic pre-term (< 37 weeks) | 32 (14) | 17 (4) | 4.49 (2.43 to 8.30) | 4.49 (2.39 to 8.43) | 4.23 (2.19 to 8.18)b | 1.01 (0.30 to 3.45)c |
| Spontaneous pre-term (< 37 weeks) | 18 (8) | 17 (4) | 2.53 (1.27 to 5.02) | 2.44 (1.17 to 5.09) | 2.34 (1.09 to 5.00)b | 1.11 (0.28 to 4.45)c |
| Admitted to ITU | ||||||
| No | 224 (97) | 453 (100) | 1 | 1 | 1 | 1 |
| Yes | 6 (3) | 1 (0) | 12.13 (1.45 to 101.40) | 10.98 (1.28 to 94.01) | 10.96 (1.28 to 94.17) | 33.53 (2.73 to 412.24) |
Among the older women, a total of 268 fetuses survived beyond 24 weeks’ gestation (35 sets of twins, three sets of triplets and 189 singletons). Of these 268 fetuses known to be alive in utero beyond 24 weeks, three were stillborn antepartum; two of these were a set of twins, of which both were stillborn, and the other occurred in a women who initially had a triplet pregnancy but had already spontaneously lost one of the triplets in the second trimester before 24 weeks. A further two of the fetuses died shortly after birth following very pre-term delivery (< 28 weeks’ gestation), equating to a perinatal mortality rate of 18.7 per 1000 (95% CI 6.1 to 42.9 per 1000). Although this was more than double the national rate of 7.5 per 1000,95 the difference was not statistically significant (RR 2.5, 95% CI 1.0 to 5.9), noting the limited statistical power of this comparison.
The proportion of fetuses surviving beyond 24 weeks that had a congenital anomaly was similar between the older women and the comparison women (1.9%, 5/263 vs. 1.5%, 7/460; p = 0.702), as was the proportion that had other major complications such as respiratory distress syndrome and severe infection (2%, 4/205 vs. 3.8%, 13/344; p = 0.240). The proportion of fetuses that had a low birthweight (< 2500 g) was higher among those born to older women than comparison women (32%, 85/267 vs. 8%, 38/463; p < 0.001), although this difference disappeared after controlling for gestational age at delivery.
Conclusions and implications for policy and service provision
These national studies clearly demonstrate an increased risk of severe maternal morbidity among women of all ethnic minority backgrounds in the UK, except among women of Indian and mixed origins, and provide important insights into the independent association of inadequate utilisation of antenatal care, high parity and pregnancy in younger and older age with the odds of severe maternal morbidity. However, there is no clear association with maternal socioeconomic status after adjustment for confounding factors including inadequate utilisation of antenatal care.
As the population of ethnic minority groups in the UK continues to increase, it is important to focus on these ethnic disparities. The known risk factors for severe maternal morbidity explained very little of this disparity. There could be residual confounding in the analysis owing to factors that were not measured well (e.g. socioeconomic status) or not measured at all (e.g. the educational level of the women, cultural factors and social status). It is important to understand the role of these factors in increasing the risk of severe morbidity among pregnant women from diverse socioeconomic and ethnic backgrounds in the UK. This provides a focus for further research into possible pathways of prevention of severe maternal morbidity.
Policies have been developed by NICE for cardiovascular disease screening and prevention in which NICE recommends that certain ethnic groups that are at a higher risk of cardiovascular disease should be targeted for primary prevention and are considering measures to develop a UK-population-based risk scoring system, taking into account the ethnic differences in cardiovascular disease prevalence. 188 It may be time to consider a similar approach for care in pregnancy. While the ‘Maternity Matters’ guidance for service commissioning in England emphasised the need for maternity services to address the disproportionately higher risk of severe maternal morbidity among socially disadvantaged women, it does not provide any specific recommendations. 17 Our results suggest a continuing focus is needed on ensuring access to antenatal care among all pregnant women, including those from disadvantaged groups, as highlighted in 2010 NICE guidance. 189
This national cohort study clearly shows that women giving birth who are aged ≥ 48 years are at very high risk of both maternal and infant complications and adverse outcomes. Many of the increased risks in women of very advanced maternal age appear to be largely explained by the higher rate of multiple pregnancy or use of assisted conception in the older women, all of which are inextricably inter-related to older maternal age, with older age leading to a need for IVF if conception is to occur and age itself and IVF leading to an increased risk of multiple birth. These findings should be considered when counselling and managing women of very advanced maternal age. There may be a place for considering fetal reduction in women of very advanced age with multiple pregnancies although the long-term effects of fetal reduction on surviving infants is unclear. Organisations making recommendations regarding assisted conception including egg donation in older mothers, as well as single embryo transfer, should take these findings into account.
Chapter 8 Local versus external review of cases of severe maternal morbidity
Background
Methodical and detailed case review is commonly used as a strategy to improve health professionals’ care of pregnant women,190 not only through documenting the number and causes of morbidity and mortality, but also through identifying preventable factors. 3,9 Two approaches have been taken nationally to learning from adverse incidents in maternity care: external anonymised case review (confidential enquiries)2,3 and local (facility-based) reviews using tools such as root cause analysis. 168 Neither of these approaches has been applied systematically to investigate cases of near-miss maternal morbidity or compared with each other to assess the impact on local learning from adverse events. The aim of this workstream was to assess what types of severe maternal morbidity undergo local review in the UK and to compare the lessons identified for future care through external review (confidential enquiry) and local review of cases of severe maternal morbidity, in order to inform development of a strategy for reviewing ‘near-miss’ cases.
Research questions
-
What types of incidents trigger local reviews in the UK?
-
What is the quality of local guidance on conducting reviews of severe maternity incidents in the NHS?
-
What local approaches are being used to review near-miss maternal morbidity cases and how do these compare with an external case review approach (confidential enquiry)?
Methods
Conditions eliciting local reviews
All 211 consultant-led maternity units in the UK were contacted up to three times, via letter, e-mail and telephone, and asked to supply a copy of the list of incidents that triggered a local review as well as a copy of their maternity risk management strategy and/or their incident review procedure during 2012. Definitions for each type of incident were documented a priori. Conditions included sepsis, massive obstetric haemorrhage, abruption, cardiac arrest and severe blood loss. Varying thresholds were accepted for several categories, including ‘severe blood loss’, ‘low Apgar score’, ‘low cord pH’, ‘prolonged inpatient stay’ and ‘prolonged second/third stage’. The conditions included were classified by two researchers individually (AS and Olaa Mohamed-Ahmed) and disagreement resolved by discussion with a third reviewer if required (MK). Incidents that were listed by at least 5% of maternity units were reported under the following categories: maternal, neonatal or organisational incidents. The lists of incidents were compared with incidents recommended for review by the Royal College of Obstetricians and Gynaecologists (RCOG). 191
Protocols for local reviews
The Appraisal of Guidelines for Research and Evaluation (AGREE) II instrument was used to assess the quality of the incident review guidelines obtained from the mailing, using 23 items organised into six domains. 192 The domains were ‘scope and purpose’, ‘stakeholder involvement’, ‘rigour of development’, ‘clarity of presentation’, ‘applicability’ and ‘editorial independence’.
Each item was scored on a 7-point Likert scale ranging from 7 ‘strongly agree’ to 1 ‘strongly disagree’ with a mid-point of 4. An overall assessment of the quality of the guideline was made by calculating the mean average score from the 23 scores for each AGREE criteria. Any score of 0–2.9 was classified as ‘poor quality’, a score of 3–3.9 was ‘average quality’, a score of 4–4.9 was ‘good quality’ and a score of ≥ 5 was considered to be ‘high quality’.
Two researchers (AS and Olaa Mohamed-Ahmed) met to discuss and agree how best to apply each question within the AGREE II criteria to incident reporting within maternity services, prior to evaluating any guidelines. Each guideline was assessed independently by each researcher and if the score for any item differed by > 2 points, they met to discuss and resolve discrepancies in interpretation. Scores were revised accordingly, with a third researcher qualified in midwifery providing clarification on any outstanding issues. Analyses were based on the revised scores. Four additional issues were considered when appraising each guideline: the method(s) used for reviewing an incident, the types of professionals involved in the review, the methods for disseminating the outcomes and whether or not units had a process to audit changes in clinical practice arising from incidents.
Standardised domain quality scores for each guideline were calculated according to the AGREE II instrument standard methods. The possible range for standardised domain scores is 0–100%. Median domain scores with 95% CI and the proportion of guidelines scoring < 30%, 30–60% and > 60% were calculated. Scores for the lowest and highest scoring domains were compared with scores for other domains using the Wilcoxon matched pairs signed rank-sum test for non-parametric data.
External versus local reviews
Six sites were randomly selected following stratification from all NHS consultant-led maternity units in England [two large teaching hospital units (5000+ deliveries per year), two medium units (2–4999 deliveries per year) and two small units (1–1999 deliveries per year)] and on the basis of their Clinical Negligence Scheme for Trusts (CNST) level at the time of selection (one of each size with CNST level 1, the basic risk management level, and one of each size with CNST level 3, the highest risk management level).
Each unit provided a list of all cases of direct (obstetric) near-miss maternal morbidity that had triggered a local review assessment process during the previous 6 months. From these lists, six cases were randomly selected from each unit and full sets of clinical notes, together with their local review report, were requested. In total, 33 sets of anonymised clinical records were obtained and reviewed and the remaining three sets of notes were unavailable. The methodology used for and lessons identified from local reviews were described.
Eleven external reviewers, comprising five obstetricians, four midwives and two anaesthetists, assessed each set of anonymised medical records, using the confidential enquiry approach used by the MBRRACE-UK Confidential Enquiry into Maternal Deaths. 2,147 Two obstetricians, a midwife and two anaesthetists assessed the care of each woman. Five reviewers thus examined each woman’s care. Each primary assessor completed an independent review of the woman’s care, highlighting the lessons to be learned to improve care in the future. This was checked by a second assessor in the relevant specialty. External assessors were located throughout England and, to maintain anonymity, assessors were only asked to review the care of women who had been cared for outside their region. The assessment process and all individual findings were strictly confidential; all assessors were required to sign a confidentiality agreement.
Qualitative and quantitative approaches were used to compare the reviews. The reviews were read and reread, a coding frame was constructed and the data coded. Anticipated and emergent themes were then examined. A qualitative interpretive approach was taken, combining thematic analysis with constant comparison. 44,45 NVivo 9 was used to facilitate the analysis. Once the themes had been identified, the number of reviews identifying each theme was quantified and compared using a chi-squared or Fisher’s exact test as appropriate.
Results
Conditions eliciting local reviews
Of the 211 consultant-led maternity units in the UK, 71% (n = 150) provided a list of incidents that would trigger a local review. The conditions listed were highly variable, although those recommended by the RCOG were most frequently represented. No single incident or condition was recommended for review by every maternity unit.
The majority of units (> 90%) that responded included maternal and neonatal deaths, stillbirths, intensive care admissions, severe blood loss, shoulder dystocia, third- or fourth-degree tears, eclampsia, low Apgar scores and unplanned home birth (Table 29). The most commonly listed maternal conditions that had not been recommended for review by the RCOG included cord accidents (77%), cardiac arrest (69%) and sepsis (64%). Meconium aspiration (14%) and hypothermia on admission (9%) were often listed in addition to the other neonatal conditions recommended for review by the RCOG. A variety of organisational incidents featured on the trigger checklists including inadequate staffing levels (70%) and delay in access to theatre (61%).
| Maternal incidents | n (%) | Neonatal incidents | n (%) | Organisational incidents | n (%) |
|---|---|---|---|---|---|
| Severe blood lossa | 148 (99) | Stillbirtha | 144 (96) | Unplanned home birth including born before arrival and delivery outside warda | 138 (92) |
| Maternal deatha | 147 (98) | Term baby admitted to neonatal unita | 142 (95) | Medication errora | 124 (83) |
| ICU admissiona | 145 (97) | Neonatal deatha | 140 (93) | Retained swab or instrumenta | 124 (83) |
| Shoulder dystociaa | 143 (95) | Low Apgar scorea | 138 (92) | Unavailability or malfunctioning of equipment, facilities or cotsa | 124 (83) |
| Third-/fourth-degree tearsa | 141 (94) | Low cord pHa | 134 (89) | Hospital-acquired infectiona | 112 (75) |
| Eclampsiaa | 139 (93) | Undiagnosed fetal anomalya | 134 (89) | Unavailability of health recorda | 105 (70) |
| Return to theatrea | 129 (86) | Birth traumaa | 128 (85) | Inadequate staffing levels | 105 (70) |
| Undiagnosed breecha | 127 (85) | Neonatal seizures or encephalopathya | 100 (67) | Delay in response to call for assistancea | 101 (67) |
| Uterine rupturea | 127 (85) | Fetal laceration at caesarean sectiona | 95 (63) | Delay in access to theatre or > 30 minutes for category 1 caesarean section | 91 (61) |
| Readmission of mothera | 127 (85) | EUROCAT major congenital anomalya,b | 27 (18) | Delayed/missed diagnosis including cardiotocography | 67 (45) |
| Unsuccessful forceps/ventousea | 118 (79) | Meconium aspiration | 21 (14) | Antenatal misdiagnosis including undiagnosed small for gestational age | 67 (45) |
| Cord accident | 115 (77) | Hypothermia on admission | 14 (9) | Violation of local protocola | 65 (43) |
| Hysterectomy/laparotomya | 112 (75) | Incidents relating to anti-D immunoglobulin | 10 (7) | Transfers (in or ex utero transfer, in from community) | 65 (43) |
| Anaesthetic complicationsa | 106 (71) | Potential service user complainta | 55 (37) | ||
| Cardiac arrest | 103 (69) | Conflict over case managementa | 55 (37) | ||
| Sepsis | 96 (64) | Child protection incident | 48 (32) | ||
| Trauma to bladder or other organs | 95 (63) | Closure of unit or suspension of services | 39 (26) | ||
| Pulmonary embolisma | 91 (61) | Transfusion error | 29 (19) | ||
| Venous thromboembolisma | 83 (55) | Consent issues | 24 (16) | ||
| Prolonged second/third stage | 71 (47) | ‘Near-miss’ | 22 (15) | ||
| Perineal breakdown | 30 (20) | Confidentiality issues | 21 (14) | ||
| Pressure sore | 29 (19) | Identification error including incorrect labelling of specimens or baby | 19 (13) | ||
| Significant retention of urine | 22 (15) | ||||
| Placental abruption | 17 (11) | ||||
| Late booking or concealed pregnancy | 13 (9) | ||||
| Anaphylaxis | 12 (8) | ||||
| Prolonged inpatient stay | 11 (7) | ||||
| Untreated group B streptococci | 10 (7) | ||||
| AFE | 9 (6) | ||||
| HELLP syndrome | 8 (5) |
Protocols for local reviews
Of all 211 consultant-led maternity units in the UK, 70% (n = 148) provided an incident review protocol or risk management strategy. Trusts or health boards that had more than one unit (n = 22) all indicated that the same guideline was in use across all units. Thus, 120 guidelines applicable to 148 maternity units were included in the appraisal. Many of the guidelines received were maternity specific (n = 82, 68%) and the remainder applied to all incidents, irrespective of speciality, that occurred in a trust or board.
Scores for the ‘scope and purpose’ domain were significantly higher than scores for the other domains (p < 0.001). The median score for this domain was 86% and 112 of the 120 guidelines (93%) scored > 60%. Only 49 guidelines scored > 60% for the ‘stakeholder involvement’ domain, which includes being authored by a range of health professionals or consulting with staff. Scores for the ‘rigour of development’ domain were significantly less than for any other domain (p < 0.001). For the ‘clarity of presentation’ domain, 93% of guidelines scored > 60%. The median score for the ‘applicability’ domain was 56%, but only six guidelines scored < 30%. None of the guidelines referred to the independence of the guidelines from the funding body or any competing interests of the guideline development group members (see Methods, Conditions eliciting local reviews).
Root cause analysis was most commonly suggested as the methodology for reviewing serious maternity incidents (n = 97, 81%), with case review analysis at regular meetings for less-serious incidents. The guidelines for six units (4%) did not state the approach to be used for reviewing incidents. Other approaches to review incidents that were mentioned were significant event analysis (five units), trend analysis (four units) and one unit recommended choosing an approach from systems analysis with a contributory factor framework, brainstorming, the five whys, incident decision tree, fishbone diagram and gap analysis.
Most review guidelines required one or two designated people to review reports of all incidents, such as a risk midwife or manager. Other guidelines designated a team of people to review each incident including senior risk managers, lead clinicians and specialist midwives in risk (see Methods, Conditions eliciting local reviews). Incidents could be escalated to a supervisor of midwives, maternity risk management boards, adverse events committees, patient safety co-ordinators, relevant executive directors or arrangements made to have a committee with external professionals and an independent chairperson, as required.
Lessons learned from incident reviews were most commonly disseminated at meetings, in reports, newsletters and posters (n = 110, 92%), with individual feedback and support given to those involved in the incident. Other means of communication included e-mail, intranet, memos, via line managers, local and multidisciplinary forums, in the minutes of meetings and ‘message of the week’. Only 10 guidelines (8%) did not mention any means of communicating the findings of reviews with staff.
Many guidelines (n = 83, 69%) included details of a process to audit the impact of recommendations arising from incidents. A further 28 (23%) guidelines included mention of having an auditing process or a committee that would consider governance and assurance issues. It was not clear if the audit cycle around incidents would be completed. Only nine units that responded made no mention of audits.
External versus local reviews
The care of 33 women with near-miss maternal morbidities was assessed by the confidential enquiry panel. The majority of women (73%) had severe haemorrhage (> 1500 ml of blood loss).
Local reviews of the care of 28 of the women (85%) had been conducted and for the remainder a local review had not been conducted either because the incident had been assessed locally as not needing a review, or because cases were not selected for review owing to capacity issues. A formal report of the local review process and outcome had been written in only 11 cases (33%) and four of these local reviews used root cause analysis. For 12 cases (36%), a review group had discussed the incident and a summary of the outcome had been noted in a spreadsheet. For an additional five cases the documented local review comprised a timeline of events with brief notes on evidence of good care and if issues had occurred.
The categories of staff involved in conducting reviews varied between units. An individual midwife conducted four reviews, a group of midwives conducted two reviews, and five reviews (15%) involved both obstetricians and midwives. One of these five reviews included an anaesthetist and one included an obstetric trainee. It was not possible to identify the specialty or grade of reviewers in 17 local review reports. In five local reviews (15%) it was documented that the woman concerned had asked the review group to consider specific questions about her care and that she was later notified of the group’s conclusions and action plans.
In the local review reports, action plans had been written for 14 cases (42%). The conclusions from three local review reports (9%) included a recommendation to audit the subsequent change to clinical practice.
Costs of external reviews
The administrative time spent on organising, photocopying, anonymising and scanning each case and liaising with external assessors was a mean of 17 hours, which amounts to an average administrative cost of £300 per case. On average it took each external assessor 3 hours to read and report on each case. The cost of five external assessors’ time, based on estimated consultant and senior midwife salary costs,193 was £1800 per case. Thus, the total cost of conducting an external review into a near-miss case of maternal morbidity per case was estimated as £2100.
Comparison between local and external reviews
Many additional, detailed messages for care were identified in the external reviews (Table 30). Importantly, the external reviews highlighted aspects of good care in every case (n = 33, 100%) compared with only 55% (n = 18) of the local reviews that identified good care (p < 0.0005). The local reviews included individual disciplinary actions, for example four (12%) recommended individual supervisory action, which was not identified in any external reviews (p = 0.11). For 21% of the cases (n = 7), local review reports noted local factors affecting the situation, such as ‘staff were dealing with two emergencies simultaneously’ and ‘insufficient room for resuscitation’. External reviewers, making their assessment solely on the basis of the medical records, did not identify any local factors.
| Theme | Local reviews | External reviews |
|---|---|---|
| National guidelines (e.g. RCOG, NICE) or good practice points | ||
| Followed | 13 | 28 |
| Not followed | 11 | 24 |
| Senior review | ||
| Consultant involvement | 14 | 28 |
| Lack of consultant involvement | 7 | 12 |
| Communication | ||
| Good | ||
| with the patient and family | 2 | 19 |
| between staff members | 4 | 14 |
| Poor | ||
| with the patient and family | 3 | 9 |
| between staff members | 4 | 11 |
| Documentation | ||
| Some areas of good quality | 8 | 13 |
| Some areas of poor quality | 10 | 29 |
| Medication errors | 2 | 11 |
| Individual supervisory action | 4 | 0 |
| Delays in | ||
| Diagnosis | 5 | 22 |
| Obtaining a second opinion | 2 | 3 |
| Providing treatment (excluding drugs) | 7 | 9 |
| Administering drugs (e.g. antibiotics) | 3 | 5 |
| Requesting blood or clotting products | 0 | 2 |
| Gaining access to blood or clotting products | 0 | 2 |
| Transferring patients (e.g. to theatre or the labour ward) | 2 | 6 |
| Alternative suggestion for clinical approach | 0 | 16 |
Examples of themes identified
Overall, the lessons for care identified in the local reviews were briefer and less focused than those identified on external review. The following are examples of lessons identified from the local review of the care of a woman with a PPH and hysterectomy. Please note that quotations from external assessors are not attributed to maintain the confidentiality of the Confidential Enquiry process.
Syntometrine not indicated.
Incorrect dose of syntocinon.
Incorrect practice.
[Long] time scale between delivery of placenta and examination.
No notes from registrar.
No ongoing estimation of blood loss.
Guidelines followed once gynaecologist consultant arrived.
Incorrect estimation of blood loss.
Timely decision for hysterectomy.
Appropriate place for care.
In contrast, the external assessors’ reports into the same case gave more detailed comments:
Care provided to this lady appears to have been extremely well managed (NICE guidance: routine care for the healthy pregnant woman). 194
The multidisciplinary team appears to have responded quickly to the emergency situation.
Consultant obstetricians and anaesthetists were actively involved in the care.
In addition to the measures taken to control the haemorrhage, other procedures should have been considered at or before laparotomy to preserve the uterus. The notes do not reflect consideration of tying off the uterine arteries, calling for vascular surgical assistance or considering uterine artery embolisation (prevention and management of PPH). 195 I am unaware of the facilities available in the unit and if any of these measures could have been taken but potentially this would have prevented the need for hysterectomy.
The resuscitation was excellent, including ensuring active warming and temperature monitoring.
The medical notes are adequate but do not give a very clear picture of the events and the decision process at each stage. Lack of observation charts.
The lessons identified in a case of maternal sepsis illustrate the different focus observed among local compared with external reviews. The local review of the case noted the following points with respect to suggestions for local service improvements:
Delay in the administration of antibiotics within the ‘golden hour’.
Consultant obstetrician informed who discussed the incident with consultant on A&E [accident and emergency].
All pregnant women over 20 weeks’ gestation who attend A&E should be transferred to either the delivery suite or patient assessment unit (except those involved in major trauma when A&E should call the obstetrics and gynaecology team to attend A&E).
Feedback to A&E staff and include in communications diary.
Raise awareness with [ambulance service] advising that all women of ≥ 20 weeks should be brought straight to maternity.
In contrast, the external reviews of the same case focused more on specific aspects of clinical care and noted wider actions in the whole care pathway (including the GP):
There were delays in recognition and management of this woman’s sepsis.
Only two of the six ‘sepsis six bundle’ measures were implemented by A&E staff – bloods and oxygen therapy – and there was a significant delay in commencing IV antibiotics.
The ‘Surviving Sepsis’ campaign highlights the importance of early antibiotic therapy in the ‘golden hour’ after presentation.
Pregnancy should not be a reason to postpone antibiotic administration when sepsis is suspected and neither is it a contraindication to chest X-ray.
A MEWS [Modified Early Warning Score] of 7 should generate a more urgent medical assessment.
There may have been some delay in recognising the severity of her illness by her GP as it was documented she had been ill for 4–6 weeks.
There were several cases in which local reviews identified no lessons for care but for which external reviews considered that there were important lessons to be learned. For example, the following was noted from local assessment of the care of a woman with uterine inversion:
All appropriate actions had been undertaken and no reason could be found for it to have occurred and, therefore, no formal report was completed.
In contrast, the external reviewers noted clear lessons from reviewing the same woman’s care:
The management of this woman’s labour and delivery was complicated by the fact that she was not suitable for regional anaesthesia. There seems to be little planning for analgesia in labour or if anaesthesia were required at delivery (the option of central neuraxial blockade having been dismissed).
The anaesthetist, however, did not recommend any alternative options, nor did an anaesthetist review this woman in labour when pain management was clearly an issue. This led to a delay in the first stage of labour because there was reluctance to commence [Oxytocin (Syntocinon ® , Novartis)] (despite this being a primip[arous] induction) as this would strengthen contractions and consequently pain.
There was also a delay in the second stage (more than 4 hours) as although earlier delivery was considered it was thought a better option to allow this woman to continue pushing as vaginal examination was so difficult owing to her distress.
The alternative at this time was a general anaesthetic for a forceps delivery in theatre.
Uterine inversion occurred within 9 minutes of delivery of the baby, so almost certainly mismanagement of third stage.
I am sure delivery was challenging for all concerned. There was immediate recognition that uterine inversion had occurred and it was dealt with and managed appropriately.
Query whether the patient was debriefed or whether the significance for future pregnancies was discussed.
Conclusions and implications for practice
The three elements of this workstream have together identified that substantial variation exists in the local review of severe maternal morbidities, in terms of the definition and scope of incidents that trigger a review, the guidelines for conducting a review and the outputs and conclusions of reviews.
More importantly, it is clear that some units do not review individual cases of morbidity related to severe maternal sepsis, for example, despite this being of major national concern. The incidents featuring on the RCOG recommendations for incident reporting were the most commonly cited by individual centre trigger checklists, although a wide range of additional incident types were also reported. Local approaches have the advantage of being able to respond in a timely manner to incidents of emerging concern. Rates of maternal death due to sepsis are known to be of concern in the UK. 2 In response to this, almost two-thirds of units specifically reviewed the care of women with severe infective complications, despite sepsis not being listed among the conditions on the RCOG guidance. 191 Processes should exist for ongoing review and revision of the RCOG incident review trigger list, and addition of sepsis to this list would encourage local reviews to be conducted. Guidance on local serious incident review processes usually includes a list of conditions that require mandatory review. Guidance should also include how the list of conditions triggering a review should be maintained and adapted in the light of changing clinical and safety circumstances.
Consideration should be given to expanding stakeholder participation in guideline development. Although some guidelines were circulated to certain staff groups to obtain their views during development, service users, for example, were rarely consulted. Very few guidelines described facilitators or barriers to implementing their recommendations, or stipulated the resources necessary to conduct incident reviews (people, money and time). Only one guideline acknowledged that it may become necessary for reviewers to delegate other pressing duties in order to prioritise the review process (see Methods, Conditions eliciting local reviews). The independence of editorial involvement was often unclear and competing interests of guideline developers were rarely stated.
It was unclear in over one-quarter of guidelines whether or not changes in practice in response to review recommendations were audited or monitored, and such auditing is important to ensure that recommended changes are being implemented and are driving change. 168 Allied to this observation, very few of the local reviews we examined in detail advocated auditing the changes to care that were recommended.
Local and external reviews of the care of women who have had severe morbidities in pregnancy clearly add different perspectives. Both local and external review processes identified important messages to improve future care, although the number of specific messages identified was greater in the external reviews. The external reviews were more likely to comment on aspects of care that were considered good. Importantly, one-fifth of local reviews identified specific local service or situation-specific factors that impacted on women’s care; these lessons cannot be identified through external reviews alone. There was little evidence of multidisciplinary review at a local level, although it is possible that the review reports did not fully capture the multidisciplinary nature of discussions that may have occurred outside of the formal review meetings. The majority were conducted solely by midwives and only one involved an anaesthetist. Such differences may account for the greater number and scope of messages identified by the external review processes. It was also apparent that local review groups had a role to institute individual disciplinary procedures, when required, which may detract from the identification of generalised messages to improve clinical care.
However, the external review process is labour intensive, requires administrative support and, on average, we estimate it would cost £2100 per case. We were not able to capture costs for the local review processes. Although the costs appear high, if lessons are learned and implemented in practice, preventing future serious morbidity, the benefits would be potentially reaped in terms of future litigation costs prevented. These costs were estimated by the National Audit Office in 2013 as £700 out of the £3700 spent on average on each birth in England. 196 A further important research question would be to assess to what extent this cost is offset by savings from enhanced treatment through compliance with guidelines.
In this comparison of local and external reviews, we were unable to establish whether it was the external or the multiprofessional perspective that added to the lessons learned and, therefore, the apparent advantages of external reviews. Further evaluation is needed to establish whether or not there is added value to including an external perspective to local reviews once high-quality multidisciplinary local reviews are fully implemented.
Chapter 9 Quantifying the long-term impacts of peripartum hysterectomy
Background
Most studies of near-miss maternal morbidity, because of their retrospective hospital-based nature, report relatively short-term outcomes for women, for example admission to intensive care or the need for hysterectomy to control haemorrhage. 8,197,198 However, as evidenced by the study reported in Chapter 2, near-miss morbidities may have significant long-term impacts, both physically and psychologically, on parents, their infants and other children. Indeed, given that apparently normal birth may itself be associated with negative outcomes such as post-traumatic stress disorder (PTSD),199,200 it is possible that such outcomes would be even more evident in the situation of a severe life-threatening obstetric complication. One of the few studies in a developed country to have followed up women who experienced a severe obstetric morbidity along with a group of women who did not reported that a severe obstetric morbidity adversely affected the sexual function and general health of women along with increasing their health service utilisation 6–12 months post partum. 27 Further exploration of the long-term impacts is important to inform service provision and improve future care and outcomes. This workstream investigated the feasibility of long-term follow-up of affected women and their infants by conducting a survey of the experiences of a cohort of women who had a peripartum hysterectomy to control haemorrhage compared with women who did not.
Research questions
-
Is it feasible to follow-up an anonymous cohort of women who had a peripartum hysterectomy 7–8 years after the index birth?
-
What are the long-term health outcomes of women following a peripartum hysterectomy to control haemorrhage and how do they differ from the long-term health outcomes of women who gave birth but did not have a hysterectomy?
-
What is the additional health service usage following hospital discharge of women who had a peripartum hysterectomy to control haemorrhage?
-
How does the near-miss experience of a peripartum hysterectomy to control haemorrhage affect the women’s family and her relationships with her family?
-
Are there any aspects of the care experience that were particularly good or bad or could be improved?
Methods
A questionnaire study of women who have had a peripartum hysterectomy to control haemorrhage (cases) and women who gave birth but did not have a peripartum hysterectomy (controls) was undertaken.
Case and control identification and data collection
During a previous study,8 nominated clinicians in each obstetrician-led maternity unit in the UK reported information through UKOSS about all women (n = 315) who had a peripartum hysterectomy between February 2005 and February 2006. The clinicians also reported information on control women (n = 608), identified as the two women delivering immediately before a woman who had a hysterectomy during this time period. For ease of comparison with the previous study, we have continued to refer throughout to the cohort of women who underwent a hysterectomy as cases and the comparison group who did not have a hysterectomy as controls. All data requested were anonymous; no names, addresses, dates of birth, hospital or NHS numbers were sought. Cases and controls were allocated unique UKOSS identification numbers. The reporting clinicians were asked to keep their own record of this unique number and the patient identifiers. For the current study, we contacted the UKOSS reporting clinicians and asked them to post the following to the women who had a hysterectomy and their matched controls: a letter inviting the women to take part in the study, an information leaflet about the study and a questionnaire. Clinicians were also asked to inform us whether or not they had posted the study materials on to the women concerned. By including the UKOSS unique number for the women on the questionnaires we asked the clinicians to send to the women, we were able to monitor the response rate from women and compare the sociodemographic and index pregnancy characteristics of responders and non-responders. Women who returned a completed questionnaire were deemed to have consented to participate in the study.
The questionnaire, information leaflet and invitation letter sent to potential participants were developed in collaboration with the maternal near-miss surveillance programme advisory group made up of programme users, members of voluntary, user and special interest groups. The questionnaire asked women about a range of topics including their health and well-being; their care experience during and since the index pregnancy; their health service usage; questions about the women’s family including her baby (or babies) born in the index pregnancy; other pregnancies/births; stressful life events since the index pregnancy; and questions to provide sociodemographic information. When possible, standard questions from the National Maternity Survey were used. 201 The questionnaire included the Short Form questionnaire-12 items version 2 (SF-12v2),202 the Hospital Anxiety and Depression Scale (HADS)203 and the Post-traumatic Stress Disorder Symptom Scale Self-Report version (PSS-SR). 204
The SF-12v2 was used to assess women’s current general health/quality of life. It produces eight scale scores measuring eight domains of health (physical functioning, social functioning, role limitations due to physical problems, role limitations due to emotional problems, mental health, energy/vitality, pain and general health perception) and can yield two summary scores of physical and mental health. Scores range from 0 to 100, with higher scores representing better health performance in the scale. The HADS was used to assess current depression and anxiety symptoms in the women. It consists of a 7-item subscale that measures depression symptoms and a 7-item subscale that measures anxiety symptoms, with each item scored on a 4-point scale. Cut-off scores have been suggested for both scales to indicate whether someone is within the normal range (score 0–7) or in a mildly (score 8–10), moderately (score 11–14) or severely (score 15–21) disordered state. The PSS-SR was used to assess current PTSD in women in relation to their birth. The PSS-SR consists of 17 items corresponding directly to Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition diagnostic criteria for PTSD; the 17 items can be classified as belonging to one of three symptom clusters (re-experiencing, avoidance and hyper-arousal), which are scored on a 4-point scale of frequency. A diagnosis of PTSD is made when at least one re-experiencing, three avoidance and two hyperarousal symptoms are rated as occurring at least once a week or less/once in a while. In addition, a total score ranging from 0 to 51 is considered to be an indication of the severity of the disorder.
No identifiable personal information was requested; however, women were given the option of providing us with their contact details if they wanted to be sent the study results or wished to be contacted by a member of the research team to discuss any issues or concerns they might have had.
Study size
Assuming a response of 60% for cases and 50% for controls, we anticipated recruiting around 200 cases and 300 controls. Ayers and Pickering205 estimated the prevalence of PTSD 6 months post partum to be 1.5% among women who had planned a normal labour excluding those who suffered a perinatal or neonatal death. With 200 cases and 300 controls, this study would have had 80% power at the 5% level of significance to detect an increase from 1.5% to 7% in the proportion of cases with PTSD compared with controls. The prevalence of anxiety and depression symptoms of moderate to high severity among women in the UK has been estimated to be 9% and 12%, respectively,206 and postnatally up to 20% of women have depressive symptoms in the first 3 months after delivery. 207 With 200 cases and 300 controls, this study would have had 80% power at the 5% level of significance to detect an increase from 9% to 18% and an increase from 12% to 22% in the proportion of cases with anxiety and depression symptoms, respectively, compared with controls. The actual number of cases and controls who responded (30 cases and 48 controls) gave an estimated power of 80% at the 5% significance level to detect an increase from 1.5% to 25% in the proportion of cases with PTSD compared with controls. The actual number of cases and controls also gave an estimated power of 80% at the 5% significance level to detect an increase from 9% to 39% and an increase from 12% to 43% in the proportion of cases with anxiety and depression symptoms, respectively, compared with controls.
Statistical analysis
Descriptive statistics, including medians and proportions, were calculated. A chi-squared test, Fisher’s exact test or Wilcoxon rank-sum test, as appropriate, was used to compare the sociodemographic and index pregnancy characteristics of questionnaire responders and non-responders. These tests were also used, as appropriate, to compare respondents’ self-reported sociodemographic, social and obstetric characteristics, previous health, health outcomes, health-care usage, care experience and effect of the women’s birth experience on her family and relationships with her family according to whether or not she had a hysterectomy in the index pregnancy. Quantitative data analysis was performed using Stata 13 software. Free-text questionnaire responses were analysed using thematic content analysis using NVivo 10 software.
Ethics Committee and NHS Management Approvals
This study was approved on 4 July 2012 by the London Research Ethics Committee (reference 12/LO/0882). The Portfolio Adoption Form was submitted on 30 April 2012 and permission for the study to proceed using the Coordinated System for Permissions was granted on 3 May 2012. The application was submitted to NHS Research and Development Offices through the Coordinated System for Permissions on 17 May 2012. Notification that global governance reviews had been completed satisfactorily was received on 13 July 2012. In the 17 months between 13 July 2012 and 31 December 2013, the research team and Thames Valley Clinical Research Network staff attempted to acquire the required NHS Management permissions.
Results
Quantitative analysis
Response rate and sample characteristics
The 315 women who had a peripartum hysterectomy to control haemorrhage between February 2005 and February 2006 and the 608 matched controls identified in a previous study8 were reported by UKOSS clinicians working in 148 UK hospitals. After 17 months of seeking NHS management approval for the study, approval was obtained from 75% (111/148) of these hospitals. Study materials for 243 of the women who had a hysterectomy and 468 of the control women were sent out to the UKOSS clinicians. The clinicians reported sending on the study materials to a total of 408 women (145 women who had a hysterectomy and 263 control women) and reported not being able to post out the study materials to a total of 59 women (13 women who had a hysterectomy and 46 control women) for various reasons including not being able to identify or not having the address of the woman concerned (Table 31). Completed questionnaires were received from 78 women (30 women who had a hysterectomy and 48 control women) representing an overall response rate of 19% (21% for women who had a hysterectomy and 18% for control women). All the results must be interpreted with extreme caution given this low response rate and limited study power.
| Stage of the study, n (%) | Cases | Controls |
|---|---|---|
| Initial study population | 315 | 608 |
| Approvals obtained | 243 (77) | 468 (77) |
| Materials sent | 145 (60) | 263 (56) |
| Woman responded | 30 (21) | 48 (18) |
The mean time after the index birth that the questionnaire was completed was 8.1 years (range 7.4–8.6 years) for women who had a hysterectomy and 7.8 years (range 7.3–8.8 years) for control women. The women who had a hysterectomy that responded to the questionnaire were more likely than the women who had a hysterectomy who did not respond to be of white ethnicity [97% (29/30) vs. 75% (84/112); p = 0.009] and were more likely to have a lower BMI at the time of the index pregnancy [mean 24 kg/m2 (range 18–31 kg/m2) vs. mean 27 kg/m2 (range 18–44 kg/m2); p = 0.037]. No other significant differences were found between the women who had a hysterectomy who did and did not respond in terms of the following characteristics recorded at the time of the index pregnancy: maternal age, socioeconomic group, marital status, smoking status, current parity, mode of delivery, and whether or not the woman had a multiple pregnancy, experienced problems in her pregnancy, delivered prematurely, was admitted to ITU/HDU or experienced major morbidity (data not shown). The control women that responded to the questionnaire were more likely than the controls who did not respond to be of white ethnicity [98% (45/46) vs. 85% (176/206); p = 0.021] and at the time of the index pregnancy were more likely to be ≥ 35 years of age [40% (19/48) vs. 19% (40/215); p = 0.002], were more likely to have, or have partners who had, a managerial or professional occupation [52% (23/44) vs. 28% (52/188); p = 0.002], were more likely to have experienced major maternal morbidity [6% (3/48) vs. 1% (2/215), p = 0.044] and were less likely to have smoked during pregnancy [9% (4/46) vs. 26% (56/212); p = 0.01]. There were no statistically significant differences between the control women who did and did not respond in terms of the following characteristics recorded at the time of the index pregnancy: marital status, BMI, current parity, mode of delivery, and whether or not the women had a multiple pregnancy, experienced problems in her pregnancy, delivered prematurely or was admitted to ITU/HDU (data not shown).
Comparing the self-reported characteristics of the questionnaire respondents by whether or not the woman had a hysterectomy in the index pregnancy showed that the women who had a hysterectomy were more likely during the index pregnancy, labour or birth to have experienced other problems, to have had a caesarean delivery, to have delivered prematurely and their baby (or babies if multiple) was more likely to have been admitted to a neonatal unit; no other significant differences in obstetric, infant, sociodemographic or social characteristics were found (Table 32).
| Characteristic | n (%)a unless otherwise stated of cases (N = 30) | n (%)a unless otherwise stated of controls (N = 48) | p-value |
|---|---|---|---|
| Sociodemographic characteristics | |||
| Age (years) | |||
| < 35 | 4 (14) | 9 (20) | 0.468 |
| 35–39 | 5 (17) | 11 (25) | |
| ≥ 40 | 20 (69) | 24 (55) | |
| Median age in years (range) | 42 (31–49) | 40 (28–47) | 0.091 |
| Ethnic group | |||
| White | 28 (97) | 45 (98) | 1 |
| Non-white | 1 (4) | 1 (2) | |
| Age when left full-time education (years) | |||
| ≤ 16 | 10 (33) | 8 (17) | 0.086 |
| 17 or 18 | 12 (40) | 16 (33) | |
| ≥ 19 | 8 (27) | 24 (50) | |
| Socioeconomic group | |||
| Managerial and professional occupations | 12 (40) | 18 (38) | 0.825 |
| Other | 18 (60) | 30 (63) | |
| Experienced stressful life events since index pregnancy | |||
| No | 7 (23) | 12 (25) | 0.868 |
| Yes | 23 (77) | 36 (75) | |
| Median number of stressful life events experienced since index pregnancy (range) | 3 (1–8) | 1.5 (1–6) | 0.220 |
| Given enough help and support from partner/husband, other family members or friends in first year after index pregnancy | |||
| Yes, definitely, or to some extent | 29 (100) | 41 (87) | 0.077 |
| No | 0 (0) | 6 (13) | |
| Given enough help and support from partner/husband, other family members or friends in the last 12 months | |||
| Yes, definitely, or to some extent | 28 (93) | 44 (92) | 1 |
| No | 2 (7) | 4 (8) | |
| Obstetric and previous health characteristics | |||
| Parity | |||
| 1 | 7 (23) | 10 (21) | 0.061 |
| 2 | 10 (33) | 28 (58) | |
| ≥ 3 | 13 (43) | 10 (21) | |
| Median parity (range) | 2 (1–9) | 2 (1–5) | 0.214 |
| Experienced other problems during index pregnancy, labour or birth | |||
| No | 9 (30) | 26 (57) | 0.023 |
| Yes | 21 (70) | 20 (43) | |
| Caesarean delivery in index pregnancy | |||
| No | 10 (33) | 33 (69) | 0.002 |
| Yes | 20 (67) | 15 (31) | |
| Pre-term delivery (< 37 weeks) in index pregnancy | |||
| No | 16 (59) | 42 (98) | < 0.001 |
| Yes | 11 (41) | 1 (2) | |
| Baby (or babies) admitted to neonatal unit in index pregnancy | |||
| No | 12 (40) | 45 (94) | < 0.001 |
| Yes | 18 (60) | 3 (6) | |
| Index pregnancy baby (or babies) have long-term health problems now | |||
| No | 22 (73) | 43 (90) | 0.061 |
| Yes | 8 (27) | 5 (10) | |
| Woman had long-term health/well-being problems before index pregnancy | |||
| No | 22 (76) | 41 (87) | 0.201 |
| Yes | 7 (24) | 6 (13) | |
Health outcomes and health service usage
No significant differences in current health status as assessed by the SF-12v2, HADS and PSS-SR were found between the questionnaire respondents who had and had not had a hysterectomy in the index pregnancy (data not shown). However, the women who had a hysterectomy were more likely than those who had not to report experiencing the following health/well-being problems in the first year after the index birth: pain, depression, difficulties/pain during intercourse, severe tiredness/fatigue, ‘flash-backs’ to the labour or birth, menopausal symptoms and difficulties concentrating (Table 33). They were also more likely to report currently experiencing ‘flash-backs’ to the labour or birth and menopausal symptoms, although no significant differences were found in the proportion reporting consulting their GP since the index pregnancy about the reported health/well-being problems. Self-reported use of inpatient hospital services for health/well-being problems experienced since the index birth was also not significantly different between the women who had a hysterectomy and the control women [proportion reporting usage: 37% (11/30) vs. 19% (9/48); p = 0.078; median number of times used: 1 (range 1–3 times) vs. 1 (1–4 times); p = 0.263]. The same was true for self-reported use of day or out-patient services [proportion reporting usage: 54% (15/28) vs. 33% (15/46); p = 0.075]. The reasons for use of day or outpatient services were highly diverse.
| Health/well-being problem | n (%) of cases that experienced problem in the first year after index birth (N = 30) | n (%) of controls that experienced problem in the first year after index birth (N = 48) | p-value | n (%) of cases that experience problem now (N = 30) | n (%) of controls that experience problem now (N = 48) | p-value | n (%) of cases that talked to GP about problem (N = 30) | n (%) of controls that talked to GP about problem (N = 48) | p-value |
|---|---|---|---|---|---|---|---|---|---|
| The blues | 12 (40) | 12 (25) | 0.163 | 2 (7) | 4 (8) | 1 | 2 (7) | 4 (8) | 1 |
| Pain | 13 (43) | 9 (19) | 0.019 | 3 (10) | 5 (10) | 1 | 5 (17) | 5 (10) | 0.495 |
| Depression | 10 (33) | 4 (8) | 0.005 | 4 (13) | 9 (19) | 0.532 | 5 (17) | 9 (19) | 0.816 |
| Leaking of urine when you do not mean to (urinary incontinence) | 6 (20) | 8 (17) | 0.709 | 11 (37) | 11 (23) | 0.189 | 5 (17) | 5 (10) | 0.495 |
| Leaking of stools when you do not mean to (bowel incontinence) | 1 (3) | 0 (0) | 0.385 | 1 (3) | 2 (4) | 1 | 1 (3) | 2 (4) | 1 |
| Wound infection | 5 (17) | 6 (13) | 0.741 | 0 (0) | 1 (2) | 1 | 3 (10) | 6 (13) | 1 |
| Difficulties/pain during intercourse | 8 (27) | 3 (6) | 0.018 | 4 (13) | 4 (8) | 0.476 | 3 (10) | 1 (2) | 0.292 |
| Severe tiredness/fatigue | 10 (33) | 6 (13) | 0.027 | 5 (17) | 10 (21) | 0.65 | 4 (13) | 3 (6) | 0.287 |
| Anxiety/nerves | 8 (27) | 5 (10) | 0.061 | 5 (17) | 8 (17) | 1 | 4 (13) | 6 (13) | 1 |
| Sleep problems (not including being woken by baby/children) | 5 (17) | 2 (4) | 1 | 5 (17) | 12 (25) | 0.386 | 1 (3) | 4 (8) | 0.644 |
| Panic attacks | 2 (7) | 2 (4) | 0.636 | 1 (3) | 4 (8) | 0.644 | 1 (3) | 5 (10) | 0.397 |
| Flash-backs to the labour or birth | 10 (33) | 3 (6) | 0.002 | 5 (17) | 1 (2) | 0.029 | 2 (7) | 1 (2) | 0.555 |
| Menopausal symptoms (e.g. hot flushes) | 5 (17) | 0 (0) | 0.007 | 10 (33) | 6 (13) | 0.027 | 6 (20) | 3 (6) | 0.080 |
| Difficulties in concentrating | 7 (23) | 1 (2) | 0.004 | 7 (23) | 8 (17) | 0.467 | 0 (0) | 2 (4) | 0.520 |
The women’s family
When given several positive and negative statements about the effect their index birth experience had on their relationship with their family, women who had a hysterectomy were more likely than control women to strongly agree or agree that their birth experience had made it difficult to initially bond with their baby (or babies), but they were no more or less likely than control women to report, when applicable, that their birth experience had positively or negatively affected their relationship with their other children or their partner/husband. When asked when they first felt like their baby (or babies) from their index pregnancy really belonged to them, as another indication of bonding, women who had a hysterectomy were less likely than the control women to respond ‘during pregnancy’ or ‘at the birth’ [48% (14/29) vs. 89% (42/47); p < 0.001]. Women who had a hysterectomy were also less likely than control women to report first seeing, touching, holding or feeding their baby at the birth or the first day, were less likely to report having as much contact as they wanted with their baby shortly after birth and were less likely to report exclusively breastfeeding, although this difference in breastfeeding rates was only statistically significant in the first 6 weeks after birth (Table 34). The proportion of women reporting planning/wanting to have more children prior to the index labour/birth was similar between the women who had a hysterectomy and the control women [50% (15/30) vs. 52% (24/46); p = 0.853].
| n (%)a of cases (N = 30) | n (%)a of controls (N = 48) | p-value | |
|---|---|---|---|
| When first got to see baby | |||
| At the birth or first day | 23 (77) | 46 (100) | 0.001 |
| First week or more than a week | 7 (23) | 0 (0) | |
| When first got to touch baby | |||
| At the birth or first day | 16 (53) | 47 (100) | < 0.001 |
| First week or more than a week | 14 (47) | 0 (0) | |
| When first got to hold baby | |||
| At the birth or first day | 12 (40) | 46 (100) | < 0.001 |
| First week or more than a week | 18 (60) | 0 (0) | |
| When first got to feed baby | |||
| At the birth or first day | 10 (33) | 44 (94) | < 0.001 |
| First week or more than a week | 20 (67) | 3 (6) | |
| Shortly after gave birth, whether or not able to have as much contact as wanted with baby | |||
| Yes | 6 (21) | 37 (77) | < 0.001 |
| No | 23 (79) | 11 (23) | |
| How fed baby in the first week after birth | |||
| Breast milk only | 11 (39) | 28 (60) | 0.089 |
| Both breast and formula milk or formula milk only | 17 (61) | 19 (40) | |
| How fed baby in the first 6 weeks after birth | |||
| Breast milk only | 4 (14) | 19 (40) | 0.018 |
| Both breast and formula milk or formula milk only | 24 (86) | 28 (60) | |
| How fed baby in the 6 months after birth | |||
| Breast milk only | 3 (12) | 9 (19) | 0.519 |
| Both breast and formula milk or formula milk only | 23 (88) | 38 (81) | |
Experience during and after index pregnancy
When asked to describe their index birth experience using an adjective checklist, women who had a hysterectomy were more likely than those who had not to describe their birth experience as terrifying, traumatic, awful and upsetting (Table 35). They were also less likely to describe their birth experience as rewarding, happy or joyful. Of the women who had expectations about their index birth experience prior to their labour or birth, the proportion of women reporting their actual birth experience was ‘worse’ or ‘a lot worse’ than expected was higher, although not statistically significantly so, in the women who had a hysterectomy than the control women [74% (17/23) vs. 54% (21/39); p = 0.117].
| Adjective | n (%) of cases (N = 30) | n (%) of controls (N = 48) | p-value |
|---|---|---|---|
| Positive adjective | |||
| Straightforward | 4 (13) | 8 (17) | 0.691 |
| Rewarding | 5 (17) | 21 (44) | 0.014 |
| Wonderful | 4 (13) | 14 (29) | 0.106 |
| Perfect | 2 (7) | 4 (8) | 1 |
| Positive | 1 (3) | 9 (19) | 0.079 |
| Happy | 4 (13) | 16 (33) | 0.049 |
| Good | 3 (10) | 10 (21) | 0.212 |
| Joyful | 4 (13) | 19 (40) | 0.013 |
| Satisfying | 1 (3) | 4 (8) | 0.644 |
| Okay | 2 (7) | 8 (17) | 0.301 |
| Negative adjective | |||
| Terrifying | 13 (43) | 10 (21) | 0.034 |
| Traumatic | 22 (73) | 16 (33) | 0.001 |
| Unpleasant | 9 (30) | 6 (13) | 0.056 |
| Awful | 12 (40) | 4 (8) | 0.001 |
| Scary | 17 (57) | 23 (48) | 0.452 |
| Disappointing | 8 (27) | 5 (10) | 0.061 |
| Negative | 7 (23) | 4 (8) | 0.064 |
| Medicalised | 10 (33) | 9 (19) | 0.144 |
| Painful | 17 (57) | 25 (52) | 0.693 |
| Upsetting | 17 (57) | 9 (19) | 0.001 |
There was no significant difference between the questionnaire respondents who had and had not had a hysterectomy in terms of their rating of the overall quality of care they received in the labour/maternity ward, postnatal ward, ITU/HDU or first 6 weeks after the index birth following discharge from hospital. There was also no significant difference between the respondents who had and had not had a hysterectomy in terms of the proportion reporting not being given enough information about their own recovery after the index birth [22% (6/27) vs. 27% (12/45); p = 0.673]. When the women who had a hysterectomy were asked how well they felt the problems and procedures leading up to their hysterectomy were explained to them, 35% (9/26) responded ‘not very well’ or ‘not at all well’. There were no significant differences in the proportion of women reporting that they would have liked, but had not, talked to a doctor/midwife present during their labour/birth (p = 0.722), another doctor/midwife not present during their labour/birth (p = 0.515), a GP (p = 1.000), a health visitor (p = 1.000) or a counsellor/therapist (p = 0.099). The proportion of women reporting that they were not offered, but would have liked, the opportunity to look through their hospital notes since their index birth was similarly high among the women who had and had not had a hysterectomy [63% (19/30) vs. 60% (28/47); p = 0.742]. When asked whether or not they had heard of or found any useful support group(s) since their index birth, women who had a hysterectomy were more likely to report that they had not, but would have liked to have, found this [59% (17/29) vs. 23% (11/47); p = 0.002].
Qualitative analysis
Overall, 71% (55/78) of respondents to the questionnaire responded to the free-text question asking them if there was anything else they would like to say about their care during their index pregnancy, labour and birth or since birth. In addition, 23% (18/78) responded to the free-text question asking them if there was anything else they wanted to tell us. There were no significant differences in the characteristics of the women who did and did not respond to at least one of these free-text questions. The majority of the free-text responses were either entirely negative or a mixture of negative and positive in their tone and content. The content of the responses fell within a number of themes outlined below. In many themes, similar views were expressed in both cases and controls; comments from both groups of women are, therefore, included.
Theme: the staff providing care
The majority of women’s comments concerned the staff caring for them. Although some women expressed their views of the staff through single words such as ‘fantastic’ and ‘brilliant’, others wrote larger responses which fell within four main themes: the clinical care and competence of staff; the communication skills of and information provided by staff; listening skills/respect shown by staff; and the helpfulness/supportiveness of staff.
Subtheme: the clinical care and competence of staff
Some women felt that they received all the correct clinical care and their outcome was an unfortunate necessity:
I feel that everything that was done was the current management and unfortunately my hysterectomy was a necessity in order to save my life
Case, multiparous
By contrast, it was not uncommon for both women who had a hysterectomy and control women to be left questioning the clinical skill and competence of the staff caring for them having experienced an outcome they considered to be avoidable:
. . . they did a regular (every 2 weeks) internal scan. However they never looked carefully enough to realise the placenta [was] growing out of [my] womb and my baby and myself were in great danger . . . They could have avoided this happening to me and my family.
Case, multiparous
I also had a catheter fitted wrong which did not let urine pass and I ended up with tremendous pain in my kidneys. I could go on and on about the clinical errors!
Case, multiparous
I needed a C-section [caesarean section] in an emergency as my son got stuck – he was 10lb 1oz! – I really feel this outcome could have been predicted and the trauma of an emergency section could have been avoided. If I had been advised to have a planned section on the grounds of a big baby I would have taken that advice. But I went through an ‘induction’, labour and then rushed to theatre. Not a very pleasant experience.
Control, multiparous
I felt that too little was done to assess whether it was worth me labouring on naturally for almost 20 hours and making no progress – my baby was in a difficult position and I think medical intervention much earlier on would have been a better use of everybody’s time. I experienced a vaginal delivery but was exhausted/completely numb and my baby had to be ‘yanked out’! Not very natural!
Control, multiparous
Women from both groups expressed feeling traumatised having being given inadequate pain relief:
I could not move and I was in so much pain. My pain relief was stopped too early and it wasn’t until I was in so much pain I couldn’t cope that they gave pain relief back. I felt like I was being tortured.
Case, multiparous
During the section I felt pain so badly, I was crying out to my husband, then I felt my self ‘going’ and my husband said my face drained of colour and the lady gave me an injection.
Control, multiparous
Subtheme: the communication skills of and information provided by staff
Having a clear understanding of what was happening to them was very important to some of the women who experienced complications. Several women who had a hysterectomy described good communication with health professionals about this. For example, one woman reported how helpful she found discussions with her consultant, who clearly explained the possible consequences of her condition before she gave birth and 1 week after she gave birth talked her through exactly what had happened to her:
My consultant had full and frank discussions with my husband and me when the pregnancy abnormalities were detected. So we were forewarned that the birth was likely to be premature with a high risk of major bleeding and potential for high dependency/intensive care post delivery. About a week after the birth my consultant talked me through the pregnancy and birth from the medical side, explaining every event and decision in detail, sparing no information. This was really helpful to me as it meant I had no gaps in knowledge and enabled me to quickly understand and accept what happened.
Case, multiparous
Although one woman who had a hysterectomy did not understand what had happened to her, possibly because she was told but ‘too ill to take it in’, she describes how much her husband appreciated being given a detailed explanation by a member of staff:
There was a member of staff at the hospital who spent a good 20 minutes explaining to my husband about the hysterectomy I had (I was too ill). He was upset that I’d had to have it but she explained in great detail . . . It was an informal chat that helped my husband enormously and he mentioned many times how grateful he was for her taking the time. He was so relieved and it took a lot of worry away.
Case, multiparous
Some women in the control group were left unsure of what had happened to them and in need of further explanation:
I was induced at 42 weeks and yet my daughter was underweight and subsequently lost weight. I therefore stayed in hospital for 5–6 days following. I would have liked to understand why this was/why the health professionals were so cautious with my next pregnancy (a year later) as a result.
Control, multiparous
Four days after being induced left in private room with only paracetamol for pain and no information about what was happening to me.
Control, multiparous
Consistent with the quantitative results, several women from both groups also commented that they would have liked to have had the opportunity to look at their hospital notes.
It was also not uncommon for both women who had a hysterectomy and control women to report that their experience had been affected by the way in which staff communicated with them or their partner:
The only complaint I have and had at the time was with one particular nurse . . . This nurse was so unpleasant to me and others that I still think about her today. She was a very cruel lady and should not be nursing.
Case, multiparous
The first midwife was a bully and frightened me to such an extent that despite being fully dilated my contractions stopped. I felt I had no control over what was happening to me. The second midwife was excellent and positive.
Control, multiparous
My child was born underweight and the consultant/doctor who came to see me in the hospital asked ‘what have you done to make her so small?’!!
Control, multiparous
She [midwife] referred me to the hospital one evening when I was in pain – this turned out to be ‘Braxton Hicks’ but I was never made to feel as if I was a nuisance. After my baby was born and my placenta wasn’t delivered straight away I didn’t feel frightened that things weren’t going to plan. The midwives were very positive in their actions and I didn’t feel rushed.
Control, primiparous
Subtheme: listening skills/respect shown by staff
There were women who had a hysterectomy and control women who felt that staff did not properly listen to or respect their wishes, choices or concerns at various stages, which some found very upsetting:
After birth was in major pain and no one believed me for 6 hours until they did a ultrasound scan so was very upsetting and scary.
Case, multiparous
Baby was quite high and as I was induced they told me they had to break my water. I asked them not to as if I was a few more cm’s dilated it would be a very quick labour the same as my others. They said it was hospital policy to break my waters at this stage!! They held me down 3 nurses. I told them I didn’t want it done.
Case, multiparous
I was encouraged at prenatal classes to write a birth plan but this was ignored. The midwife at the birth did not want me to use my preferred birthing method (stool) and I didn’t get to use one until there was a shift change.
Control, multiparous
Baby had episodes of turning ‘blue’ – it was of great concern. Cord around neck at birth. I informed staff who didn’t seem concerned and didn’t even look/examine baby. No information/reassurance given.
Control, multiparous
Subtheme: supportiveness/helpfulness of staff
There were women who had a hysterectomy and control women who were very positive about the care and support they received from certain staff:
Excellent care from very caring staff pre and post surgery/birth.
Case, primiparous
I felt extremely looked after by people in the [hospital] during scans and regular check ups . . . Midwife present [at the birth] was very good and helpful – let us get on with it and gave assistance when/where necessary. She was brilliant.
Control, multiparous
Our midwife was wonderful – she had been the second midwife when our previous baby was stillborn. She saw my name on the list and offered to be with us. She just knew how to look after me and my mind. I wish I could see her again and tell her how truly wonderful she was.
Control, multiparous
However, many women reported feeling alone and unsupported by hospital staff shortly after giving birth, in the sense that they felt they received inadequate basic care themselves and/or insufficient assistance with the basic care of their baby. This was not limited to women who had a hysterectomy.
There were times when I had very bad bleeds and I was left on a few occasions to lie in my own body fluids for a number of hours.
Case, multiparous
After birth on the ward I was hardly bothered with when it came to being washed and teeth cleaned. I could not move and I was in so much pain.
Case, multiparous
My hospital care after my baby was born was sparse. The ward was very busy and as an older mum I seemed to be left to my own devices – I really didn’t know what to do with my baby – I found feeding her hard and I didn’t change her nappy until my husband came to see me later that day (a good 5/6 hours after she was born). I could have done with more support just after the birth.
Control, multiparous
Next morning I woke to find that I was saturated in blood, bed was sodden I had to ask for help and if the bed could be cleaned. Found picking up baby very difficult and so painful wasn’t offered any help in doing so, could feel stitches pulling every time. Not a good experience for a 1st time mum.
Control, primiparous
Establishing breastfeeding can be difficult for women, particularly after a severe complication. Only one of the women who had a hysterectomy commented about breastfeeding, describing an example of good breastfeeding support in ITU:
When I woke in intensive care [a nurse] called maternity to ask for my son to be brought to intensive care. While in intensive care a midwife helped me to express breast milk as they knew breast feeding was important to me.
Case, primiparous
However, it was not uncommon for control women to report receiving inadequate breastfeeding support:
Felt unsupported in breastfeeding. Midwife advised me to get formula even though baby was feeding well. Not given advice on support group for breastfeeding.
Control, primiparous
Following discharge from hospital, while some women were happy with the care they received others commented that they would have liked more support. This was not limited to women who had a hysterectomy.
I suffered the most awful postnatal depression and there is a distinct lack of mental health support. This needs to be addressed as I had suicidal thoughts often in the two-three years after the birth of my son.
Case, primiparous
I saw a private physio[therapist] afterwards to help with pelvic floor after weakness – would have liked some follow up care from NHS on this.
Control, multiparous
Theme: organisation/structure of care
Various aspects of the organisation/structure of care were commented on by several women, falling into four main themes: length of postnatal stay in hospital, staffing levels, continuity of care and setting/environment.
Subtheme: length of postnatal stay in hospital
While one control woman felt she was discharged too soon from hospital after giving birth, a few were happy to leave sooner, some because of poor care:
I was discharged after 6 hours, I feel it was too early.
Control, primiparous
I was then taken to [the ward], this care was awful, I was not feeling very well and had a bleed in my bed. I was made to change this myself whilst in a lot of pain. I was in this ward for 3 days. I was very pleased when I was allowed to go home.
Control, primiparous
Subtheme: continuity of care
There were several brief comments from both women who had a hysterectomy and control women suggesting that continuity of care could be improved. One woman expressed thoughts about treatment by different doctors:
The care I had was amazing in ICU and HDU. However, some of the treatment was chaotic – different doctors had different opinions on treatment. Luckily my midwife monitored me obsessively.
Case, multiparous
Subtheme: setting/environment
The experience of intrapartum or postpartum care of both groups of women was affected by features of the setting/environment:
I was moved to the critical care ward on the delivery suite where I was allowed to stay for 5 days until discharge, avoiding a busy ward.
Case, primiparous
After the birth I was put in the ward for mums whose baby’s were in [the special care baby unit] – not a great place to be as the only one with a baby in the room especially as some of the mums had very sick babies and I had not long lost a child.
Control, multiparous
Theme: long-term impact of birth experience
Several women included remarks about the extent to which their birth experience had affected them and/or their families. There were women from both groups whose birth experience does not seem to have caused adverse impacts:
I did not become a midwife because of my birth experience but I do not know if I would be a midwife today if that had not happened.
Case, multiparous
Although long and difficult, my labour resulted in a beautiful healthy baby and did not adversely affect me.
Control, multiparous
I didn’t want to talk to anyone at the [hospital] about my birth because at the time I just wanted to put the whole experience behind me and I had a beautiful baby girl which we had longed for. Looking back now I realise how lucky we were because things could have been a whole lot worse.
Control, multiparous
However, several of the women who had a hysterectomy commented on the long-lasting detrimental impacts of their birth experience on their own physical and/or mental health and some also spoke of the adverse effects on other family members:
It was a miracle we were both alive. As my surgeon said though I lost 10 years of my life. I spent 15 hours in theatre and a year to recover. Still physically and mentally affected by it. It had a great impact on my life and always will. I’m not the same person any more.
Case, multiparous
After the shock of losing my womb I felt very down and not like a complete woman any more. I would give anything to still have my womb and be having periods and then naturally gone into the menopause. Instead I went into the menopause at the age of 42. I feel old before my time and it still gets me down. My libido has all but vanished and this has put a strain on my marriage to a degree.
Case, multiparous
Mum had to give up work to look after me. I was bed bound for 6 weeks. I still had pain today.
Case, multiparous
One woman who had a hysterectomy reflected on how she would have liked to have had more children, while another commented that she had gone on to adopt a child to complete her family:
It is a shame the condition was not picked up earlier as I would have liked to have had more children.
Case, multiparous
We adopted a second son to complete our family.
Case, primiparous
Although able to have more children, one of the control women, who experienced a perineal tear and an emergency caesarean delivery, reflected that she needed months of physiotherapy, experienced nightmares about the experience and was unable to contemplate having more children for years after:
I was left with a 7 1/2 inch scar and extremely painful scar tissue (internally) which needed months of physiotherapy. I was unable to even think about having another baby for years and had continuous nightmares about my whole experience.
Control, multiparous
There were other control women who commented how their birth experience affected their approach to future pregnancies:
My experience made me request a home birth for my second child.
Control, multiparous
After my experience with the birth of my son in [hospital] it made me very upset and put me off having another baby at that hospital.
Control, primiparous
Conclusions and implications for policy and practice
All the conclusions about the results of this study must be tempered by the extremely low response, which was due to a combination of factors including difficulty in obtaining permissions to undertake the study, which was undoubtedly severely damaging and effectively renders this and similar studies unfeasible; reliance on third parties (reporting clinicians) to post questionnaires and the length of time since the index birth (averaging 8 years). Nevertheless, there were some indications of long-term morbidities following the initial hysterectomy. Women who had a peripartum hysterectomy to control haemorrhage were more likely than women who gave birth but did not have a hysterectomy to experience pain, depression, difficulties/pain during intercourse, severe tiredness/fatigue, ‘flash-backs’ to the labour or birth, menopausal symptoms and difficulties concentrating in the first year after their birth. They were also more likely to experience ‘flash-backs’ to the labour or birth and menopausal symptoms 7–8 years after the index birth. In addition, they were more likely to have difficulties bonding with their baby (or babies, if multiple) born in the index pregnancy and less likely to exclusively breastfeed this baby, at least in the first 6 weeks after birth.
We did not observe any significant differences in the sociodemographic, social or previous health characteristics of women who had and had not had a hysterectomy that could have accounted for the differences found in women’s health, infant bonding and breastfeeding rates between these groups of women. However, as expected, the women who had a hysterectomy were more likely during the index pregnancy, labour or birth to have experienced other problems, to have had a caesarean delivery, to have delivered prematurely and their baby (or babies, if multiple) was more likely to have been admitted to a neonatal unit, which could offer alternative explanations for some of the observed associations.
The study clearly has a number of limitations. The response was low, with small numbers of women completing the questionnaire, limiting the power of the study to detect real differences as statistically significant. Furthermore, there is a possibility of response bias, with the women who had a hysterectomy that responded to the questionnaire being more likely than the women who had a hysterectomy but did not respond to be of white ethnicity and more likely to have a lower BMI at the time of the index pregnancy. There was also evidence that the control women who responded to the questionnaire were more likely than the controls who did not respond to be of white ethnicity and at the time of the index pregnancy were more likely to be ≥ 35 years of age, more likely to have a higher socioeconomic status, more likely to have experienced major maternal morbidity and less likely to have smoked during pregnancy. Another limitation of our study is that our findings are based on self-report, which could have introduced bias based on women’s comprehension of questions, on their desire to respond appropriately and the degree to which they were able to accurately recall information.
Nonetheless, the qualitative analysis of the questionnaire responses highlighted a number of examples of good practice with regard to maternity care and examples of when care could have been improved, falling within the following themes: the clinical care and competence of staff; the communication skills of and information provided by staff; the listening skills/respect shown by staff; the supportiveness/helpfulness of staff; the length of postnatal stay in hospital; staffing levels; continuity of care; and the setting/environment. In common with the theme identified in the qualitative study reported in Chapter 2, a number of women identified a perceived need for counselling services. It is striking that many of these themes were also observed in the responses of the women who did not have a hysterectomy. In view of the clear messages for services identified in this chapter and Chapter 2, the qualitative study results were used as the basis for an investigation of experience-led commissioning (ELC) for maternity care. This is described in the following chapter.
Chapter 10 Taking forward women’s and their partners’ experiences: an investigation of experience-led commissioning for maternity care
Background
Experience-led commissioning is a commissioning process that provides a way of using patient experience as an integral part of clinical commissioning in the NHS. The aim of ELC is to put the experience of people, families and frontline teams at the centre of clinical commissioning by using ‘robust patient experience insights and co-design’ (Georgina Craig, designer of ELC methodology, personal communication) in a systematic way. ELC is relevant to the current movement within the NHS towards a more user-centred service, for which any modernisation of the NHS involves putting patients ‘at the centre of everything the NHS does’,208 by giving them more choice and control. A recent UK Government White Paper stated that this change will be achieved using the principle ‘no decisions about me without me’ and that services will be designed around users, rather than expecting users to fit into services. 209
The ELC combines a number of person-centred approaches (e.g. social marketing, social movement theory, Planning Alternative Tomorrows with Hope exercise, The Esther Project)210 and draws on the principles and practice of Experience Based Co-Design. 211–213 ELC brings Experience Based Co-Design principles and other person-centred approaches into commissioning and combines them with the use of the highest level of user qualitative evidence. In order to develop an ELC strategy, the relevant qualitative data are combined with local views and ‘traditional’ commissioning data sets (e.g. public health and service use) to better understand local need and experience. At the local level, qualitative data for ELC are gathered from a sample of local participants (including service users, frontline medical staff, GP commissioners and service providers). In addition to collecting local stories and to aid implementation of the ELC strategy, local co-design events also bring stakeholders together to respond to the insights and design solutions that build on local assets. This helps build a sense of ownership and commitment to the commissioning strategy, as well as momentum and energy for change.
It is clear from the outputs of the qualitative work conducted as part of this programme that benefit to patients will best be realised through changes to commissioned services. However, it is not clear how these data can be best used to inform commissioning processes. The aim of this workstream was to investigate the use of the ELC model for commissioning maternity services, informed by the qualitative data on women’s experiences of severe maternal morbidity, and compare this with a service commissioned through a standard process not informed by these data.
Research questions
-
What are participants’ views of the different commissioning processes?
-
How do commissioned models of maternity care differ with and without the ELC approach?
-
What are the costs of commissioning using the ELC model and how do they compare to standard commissioning costs?
Methods
Selection of intervention and comparison Clinical Commissioning Groups
To recruit a Clinical Commissioning Group (CCG) to apply ELC to maternity services commissioning, expressions of interest were sought from CCGs who were actively planning to recommission maternity services. We sought CCGs that were representative of the diversity of the UK population. CCGs who had previously applied ELC were excluded. Two CCGs, located in mixed urban/rural areas with a diverse multiethnic population expressed interest and these were randomly allocated as intervention and comparison groups. After allocation, the intervention CCG decided that it no longer wished to undertake the ELC process. We were unable at this stage to identify another CCG actively recommissioning maternity services and, therefore, the allocations were reversed and the intervention CCG became the comparison CCG and vice versa. Once the ELC intervention CCG had agreed to do the work, other local CCGs decided to work with them but with the ELC intervention CCG leading.
Evaluation
The evaluation focused on three aspects of the ELC project in particular: the development of a commissioning strategy for maternity services using ELC; the implementation of the ELC strategy for maternity services (including barriers and facilitators to implementation); and the cost implications of applying ELC for maternity services commissioning.
The evaluation followed a predominantly qualitative, comparative case study design. Here, we triangulated multiple sources of data including interviews with key stakeholders who were involved in the ELC for maternity services project (and a comparison group – a CCG cluster that did not use ELC for their maternity service commissioning). We undertook observations of events and included a documentary analysis. The cost implications analysis examined the costs associated with the development of a commissioning strategy, including staff/stakeholder’s time and resources, for both the ELC and comparison groups.
The aims of the evaluation were to:
-
investigate how ELC operates in a CCG for a health area where it has not been used previously (maternity services)
-
collect comparison group data (a CCG cluster that did not use ELC for their maternity service commissioning) in order to compare the commissioning processes and strategies between groups to establish what facilitated ELC might offer maternity services
-
document any therapeutic potential for mothers (and partners/family) who have had difficult experiences during maternity, as an outcome of their involvement in the ELC process
-
establish how the ELC programme works in maternity services
-
examine the cost implications of ELC for maternity services.
Sampling
The ELC interview participants were recruited predominantly from ELC events, but also via the ELC facilitators, both using convenience sampling. To examine the development stage of the ELC strategy, 19 key stakeholders involved in the ELC for maternity services project were invited to participate and agreed to be interviewed. Mothers, GP commissioners, health professionals, service providers, patient representatives, representatives from the Strategic Clinical Network and NHS England, and those delivering ELC were all recruited into the evaluation. All participants who were invited to participate in an interview agreed, with exception of one professional who did not respond to the e-mail invitation.
The ELC interview participants for the phase of the research that examined the implementation of the ELC strategy were recruited predominantly through researcher contacts developed from the first set of interviews and observations of ELC events, but also through the ELC staff and facilitators and members of the lead CCG. Ten key stakeholders (GP commissioners, health professionals, service providers, contracts team and those delivering ELC) who had been involved in the implementation of the ELC strategy were invited and agreed to be interviewed. Service users were not included in the sample as, at the time of the interviews, they had not been involved in the implementation.
We compared the ELC intervention group with the comparison group to shed light on the differences in commissioning processes, strategies and implementation to establish what ELC might offer maternity services (including a comparison of cost data). For the comparison group, a focus group (n = 4) and individual telephone interviews (n = 3) were conducted with staff who had been involved in maternity services commissioning for their CCG (including commissioners, clinical staff and staff involved in contracting).
Data collection
Interviews were conducted using a semistructured approach. For the strategy development phase of the research, ELC participants were interviewed by a member of the evaluation team at ELC events (n = 4), by telephone (n = 9) or by face-to-face interview elsewhere (in their home, at their workplace and at the University of Westminster, according to participant preference) (n = 6). The interview schedule aimed to elicit participant views and experiences of ELC. For the strategy implementation part of the research, interviews were conducted 5–6 months after the ELC strategy had been developed. Interviews were conducted by telephone (n = 8) or face to face in the workplace (n = 2). The interview schedule aimed to ascertain what (if any) steps towards implementing ELC recommendations had been made and barriers and facilitators to making these changes.
Research observations were conducted at 9 out of 13 of the ELC events and at other meetings including at three meetings in which the ELC team discussed their findings with the medical community and at two implementation planning meetings. The researcher kept a fieldwork journal with observations of ELC events and notes of key points from ELC e-mails and conversations. For the first two ELC events, journal entries were unstructured in order to gain a feel for the kind of information gathered. Once the data were reviewed, it was decided that it would be best to use a system for recording subsequent events to improve the rigour of observations. This system ensured all the relevant phenomena of interest were covered by the observations at each event. An amended version of the checklist structure recommended by Merriam214 was used. This checklist was modified based on observations of the first two ELC events to increase its relevance.
Representatives from the comparison group CCGs were interviewed using a focus group lasting approximately 2 hours and telephone interviews lasting between 30 and 48 minutes. The focus group/interviews were conducted using a semistructured approach. Questions aimed to establish the approach they had used to commissioning (including the extent of user and health professionals’ involvement), perceived advantages and disadvantages of this approach and ease of implementation of the strategy. In addition, in order to gain opinions of ELC resources, focus group participants were shown the ‘trigger film’ used at ELC events (see Box 4) and asked their opinions on the film.
The ELC documents and information were provided for the analysis by the ELC team and the lead CCG. Comparison group documents and information were provided by the CCG and from publicly available information.
Data analysis
Interviews and the focus group were typed up verbatim by a professional transcriber. The data were then analysed using thematic analysis. 215 The first researcher (AC) immersed herself in the data to develop an initial list of themes/codes, which was then debated with a second researcher (DR) to arrive at a final coding list. Data were inputted and explored in the qualitative data analysis software environment, NVivo 10. Typical quotations are used to illustrate findings. The role of the person making the quotation (e.g. user, GP) is not stated in order to preserve participant anonymity. Comparison group experiences of commissioning are used to supplement, enhance, clarify and contrast findings from the ELC intervention group. As the two groups were at different stages in commissioning it created difficulties in drawing out firmer conclusions from the comparison. Data from researcher observations of ELC events are used in particular to set the evaluation findings in context, to support, explain, critique and expand on the interview data and to highlight information that was not recorded in the interviews. For the ELC strategy implementation phase of the research, data from the follow-up interviews were used, but also supplemented with some reflections on moving forward from the initial set of interviews.
The documentary analysis examined the commissioning documents from the intervention and comparison groups for differences in their scope, focus, content and patient-centredness. To complement this paper analysis, key commissioning documents were analysed in NVivo 10, in which word frequency searches were conducted to generate word clouds. Other documents available to the research team were used as background information for the report and to set the evaluation findings in context.
Cost implication analysis
The cost implication analysis of ELC for maternity services set out to estimate the full cost of developing and implementing ELC, and to assess these costs set against the outcomes achieved as a result of the introduction of ELC. The estimates aimed to determine the marginal costs and costs relative to outcomes. At the time of reporting, only limited data for outcomes achieved were available. Therefore, this interim economic analysis looked at the development phase of the ELC strategy only and does not include an evaluation of the costs associated with implementation and the total costs incurred relative to outcomes.
Data for the cost analysis were collected through discussions with key stakeholders from the ELC facilitators and comparison group. They provided data on the specific costs involved and other resource input (e.g. staff/stakeholder time). In addition, the interviews with the ELC group (see Methods, Evaluation) collected information on the amount of time stakeholders had invested in the development of the ELC strategy to input into the cost estimates.
The ELC development activities took place between December 2013 and May 2014. Salary costs for NHS staff were estimated from publicly available sources: the Annual Personal Social Services Research Unit Unit Costs of Health and Social Care 2013,134 and the NHS Jobs website (www.jobs.nhs.uk). 216 An opportunity cost approach was taken to cost the time of service users. The national hourly minimum wage was assigned to their participation in stakeholder events. The majority of service users were on maternity leave and, as such, a stricter estimate of the cost of their time would depend on knowledge as to their employment role, whether they were employed full- or part-time and the terms of their maternity leave. It was not feasible within the scope of the present study to capture all of this information, so the national minimum wage, while likely an underestimate, was used. Costs were assessed at 2014 levels (using estimated inflation indices where necessary). Costs are reported as observed for the period of the development activities and are not extrapolated to annual costs.
Results
The intervention and comparison groups
Table 36 provides a comparison of the main characteristics of the ELC intervention and comparison groups. Although there are similarities, there are also important differences between the two groups. For example, the comparison group has longer-standing roles and more developed relationships than the intervention group. In addition, the groups were at different stages in their commissioning cycles. Note that although the national qualitative work undertaken to inform ELC collected experiences of women who experienced severe maternity morbidity, additional local data collected meant that a commissioning strategy for all maternity services was developed. The overall strategic commissioning aims are therefore comparable.
| Intervention group | Comparison group |
|---|---|
| Lead CCG working with three other local CCGs to develop a commissioning strategy for unexpected experiences of maternity care | Cluster of three local CCGs working together to develop a commissioning strategy for all maternity services |
| Work was conducted by the ELC support team, working with CCG GP commissioners and lead CCG women’s clinical lead | Work was conducted by CCG Children and Maternity Services commissioners who have clinical background in maternity/children’s services |
| Relationships between commissioners and providers were comparatively less well developed | CCG commissioners had well-developed and long-standing relationships with, and systems for working with, commissioning partners and providers (e.g. primary and secondary care management) |
| Adopted an entirely novel approach (ELC) to develop a new commissioning strategy | Updated and refreshed their existing maternity strategy, using national and local quantitative data and guidelines |
| Discussed prioritising and valuing user engagement in developing their commissioning strategy | Discussed prioritising and valuing user engagement in developing their commissioning strategy |
| User engagement: women and families were asked to share their experiences of birth and using maternity services, as part of the strategy development. This process began with a blank sheet of paper rather than a list of pre-defined questions. Women and families codesign the strategy with frontline health professionals | User engagement: women and families were asked to share their opinions of maternity services, and ongoing feedback (e.g. friends and family test) was reviewed as part of strategy development. In addition, women and families were asked to comment on the strategy once it had been developed. They were given specific options to choose from/asked about specific pathways |
| Frontline health professionals were asked about their experiences of delivering maternity services as part of the strategy development. Frontline health professionals codesigned the strategy with women and families | Frontline health professionals’ managers were specifically consulted during strategy development |
Participants’ views of the commissioning process
The experience-led commissioning process
Recruitment
The first step in developing the maternity services commissioning strategy was recruitment of key stakeholders by the lead CCG and the ELC team to ELC events and activities. This included mothers who had recently experienced an unexpected maternity event around the birth of their child and had used the local hospital, their families and relevant frontline health professionals. Many mothers were eager to be involved once they had heard about the project and some were even concerned that it was not publicised more widely to women through health professionals’ channels:
I was so delighted when I saw that poster, I jumped at the opportunity.
Public participant
I don’t think everyone knows about the initiative, so I think perhaps an improvement would be to let more people know so that more people could feed back on it . . . maybe a leaflet or a card with your discharge papers.
Public participant
The lead CCG was tasked with engaging with key professionals, commissioners and managers who would be responsible for decision making in the CCG, and developing and implementing the ELC-based strategy. This task proved to be complex and time-consuming:
We have the relationships with the communities, and that, you can’t replace that. So it does take time. It does take your time to get, build those relationships, guide and navigate this to the place where we know we need to get to. Talk to the seniors, talk to the grassroots, get everybody aligned to a place.
CCG participant
Participant engagement
It was generally agreed by participants that ELC achieved meaningful engagement, particularly with mothers. Engagement was thought to be helped when ELC events avoided medical terminology and acronyms, did not require expert/medical knowledge for women to participate, welcomed babies, were run at accessible locations (where mothers were, e.g. community locations) and times, encouraged ‘friendly’ atmospheres, and organised participant discussions in small groups:
I think it [ELC] also helps us focus [on] our marginalised groups as well and those groups that are often under heard and it encourages us to actually get out to those groups and not necessarily expect them to come to us, but us actually go to them.
CCG participant
I was a bit worried about coming, that it would all be grownups and I wouldn’t feel confident to speak up, but it was really relaxed, really enjoyable and set up in such a way, having the table facilitators, having a person at each table to do the writing and to keep everyone together, made it easier to speak up knowing that people weren’t going to be looking at you . . . A little bit worried that it might be full of professional speak and talking about, so the fact that it was sort of all done in layman’s terms made it very accessible.
Public participant
Meaningful engagement allowed local needs to be emphasised. Commissioners, for example, said that they had not previously realised that some areas for improvement identified by ELC were a problem, such as the negative experiences women were having on the postnatal ward. These insights were described as ‘eye opening’:
I was quite surprised at some things that women had told the ELC team were actually happening, and I didn’t know the postnatal ward had gone to such shambles, that women felt that.
CCG participant
The trigger film
Trigger films (Box 4) were shown at the beginning of a number of events to focus ELC participants on women’s lived experiences so as to facilitate patient-centred discussions, and these trigger films were popular. Participants felt able to emotionally connect with the narratives and described them as well-constructed, real, human, powerful, touching and emotional.
The trigger film is central to the ELC process; it shows a series of short video clips of real patients talking about their actual experiences of unexpected maternity events. It was developed for the ELC project from the data gathered as part of the qualitative research project described in Chapter 2.
It’s the fact that not only did they, these mothers experience what you’re experiencing, they actually said it in words that you understand so, and also you could sense what they were feeling in the film as well, so it was very well done. It touched me.
Public participant
Trigger films affected people in a number of ways:
-
Distinguished ELC events from work-based meetings, workshops and conferences by giving them a different, more affecting dimension.
-
Helped to focus people’s minds on why they were at the event.
-
Evoked a range of emotions.
-
Helped to illustrate how devastating it can be for women when childbirth goes wrong.
-
Put a human face to the work.
-
Increased understanding of what was important to women around birth.
Data from the comparison group suggested that trigger films need to be viewed in the right context and are of limited use without the facilitation that accompanies them as part of the ELC programme, supporting previous findings. 217 There was less of an emotional connection among clinicians in the comparison group and more concerns about the films emerged in this group. There were no such concerns for the ELC intervention group. Comparison group participants felt that:
-
Films highlighted issues of which they were mainly already aware.
-
They needed to know the whole story (e.g. what had gone wrong), not just snippets of information.
-
Emotional responses were limited among those with clinical background as they are frequently ‘desensitised’.
-
Films may be useful for commissioners who do not have a clinical background.
-
It may be more useful for providers to look at their own local patient experience data (e.g. from friends and family, complaints, incidents, family survey).
-
The focus was too specific (e.g. it’s about emergencies rather than all maternity care).
-
It may be useful to structure the film – antenatal, birth, postnatal – as this is now how things are structured with the new national maternity tariff.
-
The approach is negative and potentially frightening for women who had not had children.
Nevertheless, the researcher observed that the films did trigger discussions among these comparison group members, suggesting that expert facilitation here could result in new and useful perspectives too.
For mothers who had more recently been through a difficult experience related to the birth of their child, the films were particularly emotive and the importance of good facilitation in managing emotions was highlighted.
It was a bit uncomfortable being upset [at an ELC event], but then it was quite, in a way, I didn’t mind so much because at least then people know that there’s a human face to all the work they’re doing.
Public participant
The one event for which the trigger film had less impact (although women were still able to connect emotionally, see Engaging with ethnic minorities) was at the event attended by Somali women and other women from non-white ethnic backgrounds. Concerns were raised that the women in the film were of white ethnicity, yet there were people from various ethnic backgrounds in the room and indeed in the local area. In response to these concerns, more clips of women from ethnic minority backgrounds were included in subsequent films.
The majority of them was not even the same colour as me, but yet I felt what they were feeling.
Public participant
As I was watching it I actually thought, goodness me, we’ve got a room full of Asian and black women and there was no black faces on the video.
CCG participant
Speaking and discussion at events
Participants felt that barriers between women and health-care professionals (and also between different health professionals) had been broken down to some degree at ELC codesign events. Good facilitation, staff not wearing their uniforms and the way events were set up (e.g. lay friendly discussions) all contributed.
So that whole process weaves people together and it weaves people together in a way that seems to unite people and get people away from the, their entrenched camps, the baggage they came with.
CCG participant
Many participants said that the events allowed them to think differently and creatively about how to improve maternity services. Nevertheless, one participant commented that she had found some other attendees somewhat rigid in their approach to change, being stuck in mindsets about what was possible/practical to achieve for maternity services. However, ELC (and the facilitation in particular) was thought to provide a safe enough environment that participants could challenge and question any perceived lack of flexibility.
Opportunities to challenge, I think that’s been healthy.
CCG participant
You can’t say, oh we can’t do that. There’s always ways, possibly, or being more creative and being able to think out of the box and think, well, this isn’t good. Or, we could get better at doing it and really bringing minds together to be able to do that . . . There were a few people on the table that were a little bit more black and white and possibly couldn’t see change or something, and I was, I felt a little bit more I would be a bit, a lot more flexible.
CCG participant
Some participants felt that key issues in their minds had not been reflected in any of the projects/areas for change proposed out of the ELC process. Nevertheless, individuals were still able to take what they had learned and apply it to their own practice, even if it was not included in the final summary of commissioning insights.
If there’s serious physical problems they [women/families] want to know and they want to know straightaway. They don’t want people to beat around the bush . . . that’s a great piece of information for me. Is that brought into any of the projects that have been proposed? Not really.
CCG participant
There are things that we know that we don’t do that we should do, but I don’t think they’ve been shown in this report.
CCG participant
Engaging with ethnic minorities
The ELC intervention group is in an ethnically diverse area; therefore, it was particularly important that ELC engage with ethnic minorities, a group who are often thought to be difficult to access. One ELC event had high attendance comprising entirely members of ethnic minorities, particularly from the local Somali community. The success of the attendance at this event was due to local voluntary groups, which took time to assist with recruitment. Although some concerns were raised about a lack of interpreters and relative difficulties of tasks for women who may be illiterate in their own language, women assisted one another to be heard. In turn, this engagement with diversity also served to increase general awareness of the specific cultural issues in the local community.
I actually put it in my own time because I thought it was, the community needed to be heard . . . So that responsibility made me go out and spend that extra time to actually get them and come in and have their say.
Public participant
Some people said we didn’t have enough interpreters and we should have had a few more of the non-English speakers in different languages, etc. But I think that was just one odd person who raised that. But overall, as a third party who was just watching it and helping as required, I thought it actually went quite well. Because I thought even the Somali population . . . Were quite vocal and said what they wanted to say.
CCG participant
Even if English was your first language, it needs some literacy acumen to be able to process the question and then answer it, true to its form if you see what I mean?
Public participant
The woman’s experience
Women participating in ELC events and activities reported the most positive experiences of all participants. Many felt listened to and understood when talking about their maternity experiences (in a one-to-one interview or a group situation) and described the experience as ‘therapeutic’ and ‘cathartic’. In addition, they valued being part of a process that was doing something to change and improve things for other mums in the future.
I think that was, that was quite good as well actually, because there were other mums there and actually talking to them about, other people having similar experiences, or not even similar, just having difficult experiences, the same as I had a difficult experience and listening to the videos they had at the beginning and things I thought it was really brung home, so it was quite a good group therapy really.
Public participant
It’s nice to know that you’re being heard and it seems to be a . . . bit more than just I’m a patient representative on some kind of panel . . . It was just therapeutic to have been able to participate in something that’s hopefully going to lead to improvement, and it’s a more positive way of venting some of the anger and the upset that you feel towards the hospital.
Public participant
Reactions to feedback
The ELC identifies areas for improvement from a patient perspective and patient-centred findings were fed back to staff and also discussed at events. The majority of staff welcomed the feedback and perspectives on maternity services.
They [women/families] can tell us how it is on the end of the care that we deliver to them and some of that information has been tremendously helpful.
CCG participant
However, hearing problems and issues with the service professionals deliver can sometimes be challenging. When perceived as criticism, it can be demoralising for staff. Many of the lay attendees at events were conscious of how difficult it might be for staff. They valued the fact that staff had attended events and were prepared to listen to what was being said.
They [staff] were very open to hearing and clearly, clearly really enthusiastic about making things better and wanting to make change, which was brilliant. But they did get a little bit defensive, which is totally natural. And I just think it’s really important that their senior management team make them feel really supported through the whole process, because otherwise it could get quite demoralising.
CCG participant
Barriers and facilitators to developing the strategy
Participants discussed the key issues that had helped and hindered the development of the ELC strategy.
Many commissioners, health professionals and patient representatives interviewed were clearly committed to user engagement and understanding the women’s experiences (this was reflected in the ELC and comparisons groups’ narratives). This enthusiasm contributed to participants giving their time and energy to working with ELC to successfully develop a commissioning strategy, which for some meant working in their own time to get work done.
You can’t begin to commission a service if you don’t understand where the user’s coming from, it’s ridiculous, and commissioners that haven’t visited the units that they commission, I think that’s poor.
CCG participant
That’s in my own time, because I might be doing work in the evenings or, because there’s no other time to do things.
CCG participant
While this evaluation detected considerable commitment among commissioners to patient engagement and the ELC process, impressions were important too. Actions like commissioners leaving ELC events early could be seized upon by participants as demonstrating indifference.
I think the thing that I found a little disappointing, I had, I felt very positive about the meeting because I knew that the CCGs were going to be here and I knew that, you know, high level staff were going to be, the decision makers, the money people, were all going to be here, and the fact that they all left before lunch, it’s a little bit, were they really just paying lip service?
Public participant
Although commissioners were passionate about patient engagement and user narratives, they admitted that in the past they had found it difficult to know how to involve patients in commissioning processes. ELC was noted as providing a structure for gaining and using robust patient experience data in commissioning.
So the formal process of engaging with people so they will tell you their stories in ways they find acceptable, and that they can see that this is being professionally handled, that it is being valued, and that is going to influence the people who decide where services are placed.
CCG participant
I think that’s one of the biggest challenges, many of us that have a professional background believe in the power of narrative and what it can do, it’s actually that translation of narrative into robust commissioning processes that we haven’t quite actually buttoned down yet.
CCG participant
Challenges in engaging patients early on in the development of a commissioning strategy were also reported by the comparison group, who described a less focused approach to user engagement. However, they found this hard to manage and struggled to contend with the range of conflicting views without a specific process in place to do so. They found that users with strong personalities tended to dominate discussions, creating a perceived bias in the agenda. In addition, it was common for people to have personal agendas or come with apparent personal issues that had not been properly processed and which could use up valuable discussion time. With such complex dynamics, large groups were thought to easily become ‘unstable’ and ‘unfocused’ and, thus, had a tendency to go off track. Comparison group commissioners felt that users did not know what was wanted from them and so they became more challenging to engage. Finally, without the ELC facilitation process, users frequently seemed to have trouble articulating what they really wanted to happen. These kinds of issues led to concerns around representativeness and intractable challenges, thus discouraging more open-ended approaches. Therefore, the comparison group found it more effective to engage users by asking more specific questions, providing limited options for comments and asking what women thought of the commissioning strategy once it had been largely developed.
It sometimes, we’ve had groups where we’ve gone out and you’ve almost asked questions on those lines, what’s important about this? And actually you get, if there’s five people you get five, very different conflicting views. And maybe one of the people is slightly more confident than the rest and will almost over-ride and actually you have to manage that situation very, in a particular manner to prevent that from happening. But sometimes also that people may be bringing personal baggage into it that actually hasn’t been dealt with, so it’s about managing those situations and that can be quite difficult if it’s a large group as well and it can unstable.
Comparison CCG participant
Or you can get very little out of it, because you have, they don’t know what you want from them. Whereas if you give them scenarios or questions or options they can comment on them and then it tends to break the ice and they’re able to then contribute better.
Comparison CCG participant
Commissioners’ perceptions and experiences
There was a sense of satisfaction, and even pride, within the lead CCG that they had undertaken a process that was novel and innovative that could improve women’s and families’ experiences.
It was just water to someone who’d walked 40 miles across the desert. It was just so refreshing, necessary, vital in some ways, that we could see how right it was [hearing patient narratives].
CCG participant
I think, by [the end], we probably were a unique CCG and at least halfway achieving that, the voice of the patient shaping the services, which I don’t think many CCGs could be so proud to have been able to do. Because it’s a difficult thing to do.
CCG participant
However, using this novel approach also raised some questions and concerns for commissioners, such as the potential for ELC to require additional resources (time and costs); the impact of collecting negative feedback from ELC on their services; and the challenge of how to set commissioning outcomes for patient experience in a target driven organisation like the NHS. In addition, there were some concerns among commissioners regarding the role of women’s/families’ expectations in the ELC process. Some commissioners were concerned that women may develop unrealistic hopes and expectations compared with what the CCG was able to provide following ELC. Similarly, there were fears that women might be disappointed if identified changes were not in fact implemented.
It’s about not meeting their expectations so, for me as a patient, it’s far worse if . . . I’ve told them and they haven’t done anything about it. So I think it’s that reality check, that there are some things that we can fix, and there are some things that we can’t.
CCG participant
In contrast, during the ELC activities, women were particularly mindful of the budget restraints the NHS as a whole was under and commissioners from the comparison group also reported similar understanding among the patients they engaged with. ELC work found that there were similarities between what women and health professionals wanted for maternity services. One stakeholder believed that involving patients in the commissioning process actually gave them a better understanding and respect for NHS resource restraints.
I think the more that we can actually engage people in those discussions, the more understanding that we’ll actually have within the public about wise use of services as well as we go forward.
Public participant
Is experience-led commissioning different from the usual way of commissioning?
Most commissioners interviewed said ELC was entirely different from the way that commissioning was usually done, it was much more ‘innovative’ and ‘transformed’ the commissioning process. All commissioners had previous experiences of patient engagement within commissioning. One admitted that this had often just been informing patients once decisions had been made, or understanding patient experiences only through clinicians’ perspectives. Others had some experience of trying to involve patients earlier in the commissioning process. However, this was always in a much more limited way than ELC:
It was really the difference between cuneiform on a stone and reading Charles Dickens’ Great Expectations. It was that much of an advance.
CCG participant
The usual way of commissioning would be, we’d look at data, as in hard data, outcomes, we would not have probably even talked to any patients, but very, we’d only talk to patients probably at the end of the process of what we’ve designed. If we’re lucky we may talk to them, talk to the patient voice in any of this, through the clinical world. So the clinicians would bring to the table the experiences from their lens, what their patients that they’ve treated have gone through. Which is very different.
CCG participant
Nevertheless, two other interviewees had been involved in more meaningful patient engagement work. With the NHS push for more patient involvement in services, to one newer commissioner, ELC was felt to be the natural and obvious way to commission. However, it was agreed that ELC did take the level of patient involvement even further than they had done previously.
I’m quite a newbie to the commissioning and I was lucky that my first piece of work that I did was based from the NHS Institute’s model . . . go and ask them what they think. So I’ve kind of, to me there doesn’t seem to be a different way, or another way.
CCG participant
I’ve done lots of improvement work before, lots of patient involvement and engagement work, but this seems to take it on to the next level.
CCG participant
Others highlighted that the range of key stakeholders that ELC drew together was very unique in commissioning, which promoted greater equality between commissioners, frontline health professionals and patients. It also improved communication and relationship building between key stakeholders, which was particularly beneficial for the relationship between the CCG and providers. The comparison group also highlighted the importance of good communication in developing and implementing an effective commissioning strategy. All comparison group participants pointed to long-running, well-established and successful relationships with their partners, and agreed that effective communication and engagement between the range of parties involved in commissioning was one of the key elements to their effectiveness.
Generally there’s a very good relationship between the managers of the CCG and the [hospital], a very good relationship and understanding between the clinicians from the two areas. So we don’t tend, it’s not a shouty shouty type thing, it’s a very much an organisation, a very sensible, a very mature types of meetings we have where it’s very challenging in that we do challenge them to quite a degree, but it’s all done very professionally and because of that it’s very effective and very effective systems in place in terms of how it’s run.
Comparison CCG participant
Differences in commissioning documents
The ‘human’ and patient-centred elements of the ELC work, which was emphasised by the trigger films, also extended through the ELC documents. The documentary analysis found that ELC documents contained more ‘humanistic’ language, in contrast to comparison group documents, which were more technical in nature. For example, word clouds showed that high-frequency words that appeared in ELC documents included emotion, recovery, families, team and help. High-frequency words that appeared more in comparison group documents included review, clinic, provide, manage and ensure (Figures 7 and 8).
FIGURE 7.
Word cloud for ELC intervention group.

FIGURE 8.
Word cloud for comparison group.

The documentary analysis supported the finding that ELC took a more patient-centred approach but, more than this, included a deeper examination of patient experience, thus providing more detailed information than commissioners had expected.
‘Patient centredness’: recommendations outlined in documentation appeared to be more patient-centred in ELC (as opposed to comparison group) documents. In ELC documents, recommendations for change followed a lineage back to patient experiences. For example, recommendations to invest in joint training to ‘enable frontline teams to respond to (and to deeply understand) cultural differences, impact of non-verbal communication, and how to self-care and keep well’ linked to specific concerns raised by patients described earlier in the document. By contrast, a comparison group document did not evidence the view of patients when the reviewers concluded: ‘Both antenatal clinics were bright, welcoming and appeared very well organised with adequate facilities for specialist investigation and counselling’.
Language in documents: commissioning documents are written for commissioners, managers and staff to use, although commissioning documents are often publicly available. The attention to detail, context and use of lay language by ELC meant that ELC documents were likely to be easier for members of the public interested in commissioning to understand.
The approach: the documentary analysis highlighted differences between the approaches to commissioning between the two groups. The comparison group had taken a broad approach to examining the adequacy of structures, procedures, policies and pathways, and the functioning of different units in order to understand what areas are working and where improvements might be needed. A general outline is given, including statements on the safety of units, identifying which areas were working well and where no recommendations for change were required. On the other hand, ELC took a more ‘micro’ and less-broad approach, investigating patient and staff views directly about improvements needed and exploring problems and solutions in detail. Pathways were only examined if a salient problem had been highlighted. For example, both CCGs were ‘stretched’ by their birth rates and capacity; the comparison group looked at this issue by examining staffing levels, whereas ELC focused on investigating how being stretched impacted on staff ability to care for patients.
Level of detail: while comparison group documents tended to contain overarching statements of intent and targets, ELC documents focused more on specific processes, including a breakdown of the key issues involved and recommendations for improvement. The recommendations from the ELC approach are listed in Box 5. For example, in terms of improving patient information, the comparison group documents described targets (e.g. ‘to increase access to pre-conceptual care and health promotion to ensure best pregnancy outcome’). On the other hand, ELC documents noted that ‘clinician(s) may be overestimating women’s understanding of the consequences of diabetes’ and recommended that women who have had gestational diabetes and understand the consequences may be the best placed people to explain the impact to newly diagnosed mothers. However, the documentary analysis also found similarities between the two sets of documents in terms of the kinds of recommendations, targets and outcomes the CCGs wanted to see for maternity services. For example, both sets of documents highlight the importance of providing patients with continuity of care/a joined-up approach, flexibility and support particularly for vulnerable groups including ethnic minorities, as well as training for staff and improving care pathways/multiagency working.
-
Introduce story-based discharge planning.
-
Develop new and improved ways within existing service provision to connect women and community midwives and health visitors.
-
Create a nourishing, homely relaxing postnatal environment (postnatal ward).
-
Build on named midwife; create buddies (frontline maternity professional for every woman, including everyone from consultants down and GPs).
-
Improve emotional recovery (identifying PTSD).
-
Look at administrative burden on frontline maternity teams.
-
Improve staff well-being.
-
Support (hospital) to work on (organisational) cultural change.
-
Invest in joint training, education, learning and reflection for staff.
-
Build new capacity around emotional recovery among community midwives and health visitors (bereavement midwifery training).
-
Support the development of a volunteer strategy at [hospital] to deliver some of these initiatives (e.g. story-based discharge).
-
Build relationships between community midwives and health visitors.
-
Review health visitor and community midwifery alignment with GP practices.
-
Align story based discharge with existing improvement and streamlining work around postnatal discharge.
-
Join up commissioning.
-
Link up the [grant funding].
-
Link up with [lead CCG] 5-year strategic plan.
-
Set and measure new outcomes (contracting).
Does experience-led commissioning change commissioning intentions?
One question that has been asked of ELC is what effect does ELC have on commissioning intentions? That is, what do commissioners think they want to change and how does ELC alter these commissioning intentions, if at all? Commissioners found this a difficult question to consider. It was already known that maternity services for the intervention group were in need of improvement, but clear commissioning intentions about how to do this were not necessarily available. One commissioner admitted that without the ELC project, they may only have gone as far as to ‘tweak’ the current commissioning contract. They felt that using ELC had provided the CCG with more scope for reconsidering maternity services than they would otherwise had. Using ELC had meant that they looked at the area in a more substantial way, as well as attracting additional funding for implementing one of the changes identified:
I don’t think we’d have made any change. Personally, I think we’d have gone in, we’re in the middle of negotiating a contract, OK, I think we’d have gone in and tweaked a little bit of the contract. Our clinical lead would not have been as involved as she has been, which meant that [the lead CCG] would not have had the input that they have had into the contract. And secondly we would, because you know on the back of the maternity work we applied for the [grant], now that has got, attracted funding into [local hospital].
CCG participant
Another commissioner felt that ELC had raised some issues that he/she was already aware needed improving (e.g. direct access to ultrasound slots for GPs) and other issues that commissioners were less aware of (e.g. negative experiences on the postnatal ward and the need to increase communication between health visitors and midwives), resulting in some changes to commissioning intentions as a result of ELC. In addition, ELC had not picked up some clinical issues (e.g. reduction in caesarean section rates), but these were likely to be addressed through other targets such as the Commissioning for Quality and Innovation for the Trust.
These findings suggest that ELC did have some effect on commissioning intentions. This is in contrast to the comparison group who said that their patient engagement work had only tweaked or confirmed their commissioning strategy.
But a lot of what we did it didn’t change it reinforced, it was the smaller things that it was really helpful . . . But it was the little tweaky things rather than the big.
Comparison CCG participant
Cost implications
Background and limitations
It is important to view the cost findings in this report within the limitations of the evaluation. As outlined in Table 36, the ELC intervention group had undertaken a significant piece of work to develop a new commissioning strategy for maternity services, and the comparison group had updated and refreshed their existing strategy (this was subsequent to conducting a more significant piece of maternity work in earlier years). Research has found that commissioning activities often do not fit into neat commissioning cycles, meaning that finding a matching comparison group is a challenge. 218 Therefore, we have drawn limited conclusions in comparing the costs of these two different approaches.
Costs related to commissioning strategy development using experience-led commissioning
The estimation of the cost of development pulls together data from various sources. For the ELC strategy this included development of trigger films, activities undertaken by the ELC team to develop the strategy, and time invested by stakeholders associated with the CCG to facilitate the development of the ELC strategy (including meeting/activity attendance, networking).
The cost of researcher time to develop the trigger film and two films, not including the costs of the underpinning qualitative work, totalled £13,265.
Activities undertaken by the ELC support team to develop the strategy included desk work on programme development; gathering commissioning insights and facilitating codesign events; analysis of data; producing outputs; handover coaching for CCG to facilitate strategy implementation; and facilitator travel expenses. The costs of these activities totalled £56,900. A detailed breakdown of these costs is shown in Table 37. These costs would be lower (approximately £10,000 lower) if this approach were repeated with other CCGs for maternity services as some learning will have taken place.
| Activity | Cost estimate (£)a |
|---|---|
| Programme development and prototyping | |
| Desk work on programme development | 7500 |
| ELC programme prototyping | |
| Codesign one (current experience mapping) | 3750 |
| Codesign two (desired experience mapping) | 3750 |
| Codesign three (PATH) | 4500 |
| Codesign four (improvement contract co-design) | 4500 |
| Discovery interviews front-line caregivers, people, families (n = 10) | 3750 |
| Transcription of interviews (600 minutes) | 1500 |
| Analysis of interviews to identify commissioning improvement challenges | 8500 |
Triangulation and aggregation of all data to produce for CCG:
|
11,250 |
| Handover coaching for CCG implementation lead to ensure strategy moves swiftly into implementation (gratuity) | 0 |
| Project management/contribution overhead 10% | 4500 |
| Expenses (17 facilitator days at £200 each – includes travel and overnight) | 3400 |
| Total cost | 56,900 |
The cost for the time invested by stakeholder event participants was approximately £6800. This does not include time stakeholders spent at events that would have taken place regardless, such as continuing professional development events.
A significant cost of the development of the ELC strategy was attributed to networking within the CCG. A few stakeholders in managerial roles reported spending between 75 and 210 hours over the 6-month period in networking and communications. The estimated cost of these activities is considerable, approximately £26,900.
Within the limitations of this analysis, it was estimated that the total costs associated with developing the ELC strategy in maternity services in the ELC intervention group was > £103,000.
Costs related to commissioning strategy development for the comparison group
The comparison group found it challenging to provide extensive commissioning cost information retrospectively. The cost of stakeholder contributions to the development of a maternity commissioning strategy in the comparison group was estimated at < £6000. This includes time spent over a 3-month period by clinical leads/heads, managers, commissioners, communication and engagement lead, Chief Executive Officer and user, local authority and public health representatives. Additionally, the cost of venues for networking and communications necessary for the development of their strategy was estimated at £5000. As expected, given the differences in the commissioning work undertaken, this is significantly less than that observed for the ELC group, but it should be noted that these costs are estimates only.
Conclusions and implications for service commissioning
This study clearly shows that the experiences of women who have had severe morbidity in pregnancy can usefully be used as the basis for an ELC approach for maternity services. Although mothers frequently found the process therapeutic, some of the participants found listening to women’s experiences traumatic, emphasising the importance of good facilitation that not only anticipated but was able to manage strong emotions. The initial trigger film was generally effective in the ELC process, although not sufficiently ethnically diverse, and the balance of the film had to be adjusted during the ELC process. Regardless, the film provided an important way to represent the experiences of women who had severe complications and facilitated patient-focused discussions.
Participant views of the ELC process were largely positive, with the caveat that the CCG that was originally randomised to the ELC process subsequently declined to take part, which may infer less-positive views, and there was a clear perception that ELC led to greater engagement from both health professionals as well as users and a bridging of understanding between the two groups. The participants felt that the ELC process did lead to differences in the outcomes of the commissioning process compared with a standard commissioning approach and the documentary analysis supported this finding. The language of the commissioning strategy produced by the intervention group was more humanistic and less technical than that from the comparison group, and ELC recommendations were clearly linked back to patient experiences. The commissioning strategy from the comparison group tended to be more general, whereas that from the intervention group focused on a smaller number of specific areas in detail. However, there were also numerous similarities in the recommendations, targets and outcomes both CCGs included in their resulting strategies. Both strategies highlighted continuity of care, support for vulnerable groups, training for staff and improving multiagency working.
The costs of the ELC process were significantly greater than the costs of the standard process. At this stage, we cannot assess how these additional costs translate to change in services. Although there are differences in the resultant commissioning strategy for maternity services, implementation is still ongoing and, thus, further evaluation will be needed to determine whether the ELC process has resulted in a different maternity service and, in particular, whether or not the service developed is more responsive to the needs of women with severe morbidities and whether there are additional implementation costs or potential cost savings. It is also important to note that the costs of the process may decrease for other CCGs using this ELC model to commission maternity services, as many of the resources and processes have been developed and, therefore, the amount of staff time needed will be reduced. These factors will impact on the cost–benefit assessment, and thus this economic evaluation must be considered incomplete and interim at this stage. Further work is essential to assess the long-term outcomes of the commissioning strategy and the associated implementation costs, and how these compare with standard commissioning.
The findings from this evaluation support the following recommendations for future commissioning work in the NHS using ELC.
Developing a commissioning strategy using ELC:
-
Additional work is required in acknowledging and helping CCGs allocate the time/resources needed to align organisations to converting to a new way of commissioning from the outset.
-
Ensure the CCG is familiar with, and confident to deliver, the ELC process and plan of activities/event early on, so that it is clear to CCGs what needs to be done and when.
-
Ensure key providers feel fully engaged in the process and explore with them early on what the process means for them; help them relate the work to other strategic priorities.
-
Explore allowing CCGs to adapt some parts of the ELC process (e.g. working with ELC insights to develop a commissioning strategy), in order to meet the needs of different CCGs/populations more effectively.
-
Be aware that although ethnic minorities are included in the trigger films, local areas may have ethnic diversity that is not possible to include at a national level in trigger films.
-
Maintain balance of positive and negative experiences shown in trigger films, in consideration of the effect that negative experiences may have on patients and consequent group interactions in ELC.
-
Be aware that discussing negative emotional experiences for patients can be cathartic and empowering in an appropriately managed setting, but skilled facilitation is required to manage this important process.
Commissioning using ELC:
-
Commissioners could consider using ELC if they:
-
need a structure through which to engage with patients in a meaningful way
-
would like to give patients more influence over commissioning strategy content and development
-
want to build relationships and improve communication between key stakeholders within a health economy (e.g. CCG and providers, voluntary sector, patients)
-
require a transparent approach to commissioning decisions.
-
Further research/evaluation:
-
Link costs of developing the ELC strategy with outcomes (e.g. changes in practice, patient experience).
-
Future research could also investigate ELC outcomes numerically, looking more deeply into costs and other outcomes and over longer periods of time to allow the study of implementation. In particular, it is important to understand the longer-term impacts for implementation of the ELC strategy.
-
Develop a set of ‘benchmark’ costs for commissioning – for both new strategies and annual strategy refreshers – so that those seeking to innovate and test new methodologies can compare cost and benefits of different commissioning management methods.
-
The inclusion of a comparison group was useful to shed light on the commissioning processes, strategies and implementation to establish what ELC might offer maternity services. However, the inevitable differences between large organisations within the NHS mean that more ‘controlled’ comparisons would be difficult in this setting.
-
Undertake further work in order to explore ways of making ELC more understandable to those unfamiliar with the process.
Chapter 11 Discussion and conclusions
Many severe maternal morbidities are classified as adverse events and may be the subject of litigation claims. 196 They can have a life-long impact on surviving women and their families. Therefore, research is crucial both to improving safety within the NHS11 as well as outcomes for women and babies. The three aims of this programme were to implement a national programme of study of near-miss maternal morbidity to complement confidential enquiries into maternal deaths, to use mixed methodologies to improve the evidence base for disease prevention and treatment, and inform commissioning of maternity services, and to use the data to develop recommendations for best practice to prevent and manage near-miss maternal morbidities. Our User Advisory Group provided input at all stages of the component projects, including design, monitoring, analysis and dissemination. The series of studies clearly demonstrate the added value of research into maternal morbidity alongside research into maternal death: for each individual maternal death from one of these severe morbidities there were between 4 and 100 women who had severe illness but survived. In an average-sized maternity unit, this represents 0–4 affected women with each specific morbidity per year, emphasising the importance of collaborative research at a national or multinational level11,219 to inform management. This also provides a clear illustration of the difficulty of obtaining RCT evidence to guide treatment for these women. A study randomising only 100 women to two arms of a trial would require the participation of at least 100 units for 1 year or 33 units for 3 years. In this programme, we focused on using robust observational studies as a basis for addressing our key research questions.
Key findings and implications for practice or policy
The programme had seven objectives which have been used as the basis for the summary of the key findings below. This represents only a summary of key points; many more specific messages for care are reported in the publications about individual conditions and these should be referred to for further detail. 54,71,72,181,220,221
The incidence of the specific morbidities most commonly leading to maternal death in the UK
We examined the incidence of AFE, severe maternal sepsis, uterine rupture, HELLP syndrome and placenta accreta/increta/percreta. Because the studies conducted were national cohort or case–control studies, we were able to estimate incidence of each condition with a relatively high degree of precision. The incidences of these conditions ranged from 2 to 47 per 100,000 maternities and these estimates are useful in order to plan services as well as to inform the focus of labour ward skills and drills training programmes. In addition, as we collected national data, we were able to estimate incidences in specific population subgroups. Particular estimates that are of importance and of direct relevance to counselling and service delivery include those associated with the risk of uterine rupture and placenta accreta/increta/percreta. When estimated on a population basis, the incidence of uterine rupture in women who have had a previous caesarean delivery and who are planning a vaginal delivery is 1 in 500 maternities, lower than previous estimates. 75 This allows women to assess accurately the risk of this rare, but very serious, complication when deciding on their mode of delivery in these circumstances. Their decision-making can also take into account differences in incidence among women in spontaneous labour versus those who have labour induced or augmented. The incidence of uterine rupture among women with a previous caesarean section planning vaginal delivery who labour spontaneously is 1 in 800, compared with 1 in 330 among women who are induced or augmented. 181
The incidence of placenta accreta/increta/percreta can also be estimated among different subgroups of women. Women without a placenta praevia and who have not had a previous caesarean delivery have an estimated incidence of placenta accreta/increta/percreta of 1 in 33,000, women who had a previous caesarean delivery but who do not have a placenta praevia have an estimated incidence of 1 in 3300, and women who have had one or more previous caesarean deliveries and have a placenta praevia have an estimated incidence of placenta accreta/increta/percreta of 1 in 20. 72 It is thus clear that, in particular, the women who have had one or more previous caesarean deliveries and have a placenta praevia must be delivered in a unit that is equipped for the possibility of a placenta accreta/increta/percreta and associated haemorrhage, with appropriate delivery planning and the availability of a team to carry out a peripartum hysterectomy should this be necessary.
Recommendations
-
Clinicians should be aware of the frequency of these rare, but severe, complications and ensure the facilities and training are in place to manage women with these conditions when they occur.
-
Uterine rupture in women with a previous caesarean section planning vaginal delivery is less common than previously estimated and women should be advised of this when discussing their planned mode of delivery. The increased risk associated with induction or augmentation of labour should also be considered.
-
Women who have placenta praevia and have had a previous caesarean delivery are at high risk of placenta accreta/increta/percreta and delivery should be managed in accordance with this risk, which might require facilities to carry out a peripartum hysterectomy should this be necessary.
The contribution of existing risk factors to disease incidence and identifying steps which may be taken in clinical practice to address these factors to reduce incidence
We found several risk factors that were common to two or more severe maternal morbidities. In particular, older maternal age and interventions associated with delivery, that is, induction of labour and operative vaginal or caesarean delivery were commonly identified risk factors. This is of concern given current trends among the maternity population and maternity services, with a rising average maternal age at childbirth and rising caesarean delivery rates. 14,15 Actions supporting maternity settings in which women are at lower risk of intervention may therefore impact on the incidence of these maternal morbidities. 222,223 Multiple pregnancy and assisted reproductive techniques were also associated with particular conditions; rates of both are clearly interrelated and higher than previously. 15,224 Interestingly, in light of the findings in relation to maternal death, maternal obesity was surprisingly not consistently associated with the incidence of these severe maternal morbidities. Risks associated with maternal age and ethnicity are further discussed in subsequent sections (see The outcomes of the conditions for mother and infant and any groups in which outcomes differ and Factors that influence the risk of death and how these might be addressed to prevent death).
The significant risks associated with prior caesarean delivery and placenta praevia are highlighted above. In all the sources we examined, operative delivery was associated with raised odds of maternal sepsis. In our examination of data from the Grampian region of Scotland, women with sepsis who had a caesarean section were less likely than controls to receive antibiotics, highlighting the need for prophylaxis for every woman having a caesarean delivery. The association we observed between operative vaginal delivery and maternal sepsis emphasises the importance of strict aseptic technique and infection control measures in clinical practice.
Forty per cent of women with severe sepsis had pneumonia/respiratory infection as the source of sepsis. These results indicate that in addition to genital tract infection, respiratory infection is a major source of severe maternal sepsis irrespective of an influenza epidemic. This is borne out in the maternal deaths surveillance data for the UK, which also record a high proportion of deaths due to non-genital tract, and particularly respiratory, causes of sepsis. 2 Immediate implications are that in addition to precautions for genital tract sepsis, there is clearly an urgent need to improve timely recognition of severe respiratory tract infection in pregnant and recently pregnant women. The importance of early recognition was highlighted in a recent patient safety alert from NHS England225 and is illustrated in these vignettes extracted from the most recent UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity report:2
Two hours after delivery a woman became unwell on the postnatal ward feeling faint. Her oxygen saturation was low. She was reviewed by junior staff and found to be shocked, without evidence of major bleeding. Her temperature was never measured and sepsis was never considered. A diagnosis of haemorrhage was made and she was treated with fluids. She failed to improve and was taken to theatre for treatment of presumed haemorrhage where she had a cardiac arrest and could not be revived. At postmortem she was found to have overwhelming infection due to group A streptococcus.
Seven days after giving birth a woman became unwell at home with a fever. She was advised to attend the maternity unit immediately. On admission she was noted to be breathless with a rapid pulse and high temperature. She was seen quickly by the on call doctor. A diagnosis of severe infection was made and fluid resuscitation started immediately. Intravenous antibiotics were started within one hour of the diagnosis and she was transferred to the high dependency unit. She made a full recovery. Early recognition, clear advice and prompt treatment led to a good outcome without any further complications.
This first vignette also highlights another key finding with respect to risk and that is the rapid progression of disease severity associated with group A streptococcal infection, suspicion of which represents a clear red flag for urgent action. Rapid administration of antibiotics in cases of severe sepsis is significantly associated with decreased mortality in the non-pregnant population226 and rapid antibiotic administration is a cornerstone of many sepsis care bundles. 124,227 These should also apply to pregnancy and be appropriately tailored to any setting in which maternal sepsis may occur, including – in particular – freestanding midwifery units in light of the preponderance of group A streptococcal infection among women having normal vaginal deliveries. We also noted that women who are treated with antibiotics in the perinatal period are at significant risk of severe sepsis and, thus, there is a need to follow women up to ensure that treatment is effective. The different patterns of infection we observed in antenatal and postnatal women suggest that overall greater consideration needs to be given to the source of infection and, therefore, the most appropriate antibiotic to prescribe.
Recommendations
-
Older maternal age is associated with severe maternal morbidities and women should be aware of this if they are planning to delay childbearing.
-
Caesarean section delivery is associated with severe maternal morbidity in both current and future pregnancies. These risks, together with planned family size, need to be taken into account when planning mode of delivery.
-
Both primary and secondary care practitioners should remain aware that pregnant or recently pregnant women with suspected infection need closer attention than women who are not pregnant.
-
Antibiotic prescription does not necessarily prevent progression to severe sepsis and women should be followed up to ensure recovery.
-
The rapid progression to severe sepsis highlights the importance of early administration of high-dose i.v. antibiotics for anyone with suspected sepsis.
-
Signs of severe sepsis, particularly with confirmed or suspected group A streptococcal infection, should be regarded as an obstetric emergency and should be routinely included in obstetric emergency training courses.
-
Consideration could be given to a change of timing of prophylactic antibiotics to administration at time of decision for emergency caesarean section.
-
Vigilant infection control at vaginal delivery should be maintained.
-
Existing sepsis bundles should be used for any pregnant woman with suspected sepsis. There may be a place for tailored bundles in settings such as freestanding midwifery units, where some elements of standard bundles may not be immediately available.
How the conditions are managed and any variations in management, the impact that different management strategies or interventions have on outcomes and costs, and recommendations for best practice to improve outcomes for all women
Our interviews with women identified several circumstances for which variations in practice made an important difference to their experience and outcomes of care. Women who had an antenatal diagnosis of a condition that might be associated with early, emergency delivery (such as placenta praevia or HELLP syndrome), valued full explanations of what might happen in order to help them prepare and cope subsequently. For partners, frequent updates during the emergency helped them feel less isolated and anxious; partners also appreciated repeated explanations of events. Some women found transfer to a single room after discharge from critical care helpful to allow them to recover as well as adjust to new motherhood. Many valued intensive care outreach/follow-up where this was provided; however, this is not part of current maternity critical care guidance.
Specific management actions we investigated included the following.
Delayed delivery for HELLP/ELLP syndrome: we found no difference in outcomes for women in whom delivery was delayed by a short period, up to 48 hours, to those with planned immediate delivery. 54 Importantly, delay in delivery may be helpful to allow administration of steroids for fetal lung maturation. Notably, there was a high rate of eclampsia among both women with HELLP and ELLP syndromes and, therefore, magnesium sulphate could also be considered as prophylaxis. 228 HELLP/ELLP syndromes are covered in NICE clinical guideline 107, Hypertension in Pregnancy,229 although not in detail, and, therefore, these findings should be used to inform future revision.
Placental removal in women with placenta accreta/increta/percreta: women with placenta accreta/increta/percreta who had no attempt to remove any of their placenta with the aim of conserving their uterus or prior to hysterectomy, had reduced levels of haemorrhage and reduced need for blood transfusion, supporting recommendation of this practice. 230
Mode of delivery in women with prior caesarean section: as discussed above, we noted an increased risk of uterine rupture associated with trial of labour, but particularly if women were induced or their labour augmented. The risk of uterine rupture also increases with the number of previous caesarean deliveries, a short interval since the last caesarean section and labour induction and/or augmentation. These factors should be considered when counselling and managing the labour of women with a previous caesarean section. Current guidance231 recommends that women should be advised about the risks and benefits of planned vaginal birth after caesarean section when considering mode of delivery in subsequent pregnancies.
Second-line therapies for PPH: the economic evaluation we conducted based on observational data from the UKOSS study of management suggested that uterine compression sutures were the most cost-effective second-line strategy; however, further work is required to strengthen the fundamental inputs into the analytical model. The analytical techniques used here provide a useful model for the future of evidence synthesis using a decision-analytic model to conduct an economic evaluation using similar observational data in rare conditions where randomised trial evidence is lacking and never likely to be available.
We also used women’s experiences as the basis of an investigation of a new model for commissioning maternity services in order to improve outcomes of severe maternal morbidity. Although it is too early in the commissioning cycle to evaluate whether or not the commissioned service has made a difference to women’s outcomes, preliminary evaluation suggests that the ELC strategy may be more patient focused than the strategy developed using standard commissioning processes. Users, health professionals and commissioners felt that the ELC process was positive and led to greater engagement and dialogue between professional and users. The perspectives of each were recognised and valued. The participants felt that the process did lead to differences in the outcomes of the commissioning process compared with a standard commissioning approach, and examination of the documents produced suggested that patient experiences led directly to the recommendations produced. The commissioning strategy from the comparison group tended to be more technical in its language, noting that there were many similarities in the recommendations, targets and outcomes that both CCGs included in their resulting strategies. Both strategies highlighted continuity of care, support for vulnerable groups, training for staff and improving multiagency working. The costs of the ELC process were significantly greater than the costs of the standard process, but at this stage we cannot assess how these additional costs translate to change in services or improved outcomes.
Recommendations
-
Women who have a condition diagnosed antenatally that puts them at increased risk of an emergency delivery/life-threatening condition should be given an explanation of what might happen. This may need to be repeated on several occasions.
-
Partners should be given frequent updates during an emergency as well as both women and their partners receiving a subsequent full explanation of events.
-
Delay in delivery of up to 48 hours may be safely undertaken in women with HELLP syndrome in whom there is no fetal compromise and who remain clinically stable, and may assist in the delivery of antenatal steroids when these are indicated.
-
The number of previous caesarean sections and the time interval between the last delivery and conception should be taken into account when counselling women with previous caesarean deliveries about their mode of delivery in this pregnancy.
-
Uterine compression sutures are a more cost-effective second-line therapy for PPH than interventional radiology and guideline developers should be aware of this.
-
ELC may be used as a way to commission maternity services. The commissioned strategy appears more patient-focused and the process led to beneficial engagement of both user and health professional groups in commissioning services. Commissioners could consider using ELC if they:
-
need a structure through which to engage with patients in a meaningful way
-
would like to give patients more influence over commissioning strategy content and development
-
want to build relationships and improve communication between key stakeholders within a health economy (e.g. CCG and providers, voluntary sector, patients)
-
require a transparent approach to commissioning decisions.
-
The outcomes of the conditions for mother and infant and any groups in which outcomes differ
We focused on three different characteristics of women that can be associated with poor maternity outcomes. This work clearly demonstrated an increased risk of severe maternal morbidity among women from different ethnic minority groups and women of younger and older age. We did not, however, demonstrate any independent association with maternal socioeconomic status, after taking into account the effects of inadequate utilisation of antenatal care and high parity. This suggests that the most important need is to ensure that services are responsive to women of different ethnic and social groups, to ensure optimal utilisation of care. We experienced some challenges in obtaining wide representation from ethnic minority groups in the ELC process, but with specific events led from local community groups this was possible and this approach may provide a route for ensuring appropriate and responsive services. Recent NICE guidance189 has highlighted the importance of access to appropriate maternity services and this work further emphasises this.
The national cohort study of older women we undertook clearly shows that women giving birth who are aged ≥ 48 years are at very high risk of adverse outcomes for both mother and baby. Many of these increased risks appeared to be explained by the higher rate of multiple pregnancy or use of assisted conception in the older women. These findings should be considered when counselling and managing women of very advanced maternal age who are contemplating assisted reproduction. There may be a place for considering selective fetal reduction in women of very advanced age pregnant with multiple pregnancies. Organisations making recommendations regarding assisted conception including egg donation in older mothers, as well as single embryo transfer, should take these findings into account.
We investigated factors associated with poor outcome in women with AFE and showed that women who died or had permanent neurological injury were more likely to have a greater severity of disease at presentation, with rapidly progressive coagulopathy. Women who died or had permanent neurological disability were more likely to have had a hysterectomy and less likely to receive specific blood products. Consideration needs to be given to whether or not earlier treatments, including correction of coagulopathy, can reverse the cascade of deterioration that seems to be present with AFE and so improve survival in the most serious cases.
With regard to sepsis, we did note that there are major significant disparities in socioeconomic status and the risk of severe sepsis. The reasons behind this are unclear but highlight the importance of making women and their families fully aware of the symptoms and signs of sepsis associated with pregnancy (Box 6).
Sepsis can develop very quickly. Pregnant women or new mothers can appear relatively well and yet become seriously ill very quickly. Women and their families need to be aware of early warning signs. If you develop any of these symptoms you or your family should seek medical advice, or go to a maternity unit, quickly. If you think you or your partner has sepsis, getting rapid treatment with antibiotics may be life-saving.
THINK SEPSIS: SIGNS & SYMPTOMS TO WATCH OUT FOR:
-
High temperature (over 38.3 °C).
-
Chills and shivering.
-
Fast heartbeat.
-
Fast breathing, breathlessness.
-
Headache.
-
Extreme sleepiness.
Extract reproduced from Hinton on behalf of the MBRRACE-UK lay summary writing group. 232
Recommendations
-
Maternity services need to be responsive to women of different ethnic and social groups, to ensure optimal utilisation of care.
-
ELC may provide a route to fully engaging different social and ethnic groups in the commissioning of appropriate maternity services.
-
Older women are at considerably higher risk of pregnancy complications and this should be considered when counselling and managing women of very advanced maternal age, particularly in the context of assisted reproduction.
-
There may be a place for considering early fetal reduction in women of very advanced age with multiple pregnancies.
-
Recommendations regarding assisted conception including egg donation in older mothers, as well as single embryo transfer, should take into account the high risks of adverse pregnancy outcomes in older women with multiple pregnancies and who have undergone assisted reproduction.
-
Earlier treatments, including correction of coagulopathy, may reverse the cascade of deterioration that seems to be present with AFE and so improve survival in the most serious cases.
-
Women and their families should be fully aware of the symptoms and signs of sepsis associated with pregnancy.
Factors that influence the risk of death and how these might be addressed to prevent death
We found six factors to be associated with maternal death from direct pregnancy complications after adjustment: inadequate use of antenatal care; substance misuse; medical comorbidities; hypertensive disorders of pregnancy; previous pregnancy problems; and Indian ethnicity. Together, these contributed to 70% of the increased population attributable risk. Specific medical comorbidities, including asthma, autoimmune diseases, inflammatory/atopic disorders, mental health problems, essential hypertension, haematological disorders, musculoskeletal disorders and infections, were found to be associated with a higher risk of dying from the conditions included in this study. Medical comorbidities contributed 49% of the increased risk of fatality in the study population.
Maternal medical and mental health comorbidities have also been highlighted in the recent Confidential Enquiries into Maternal Death;2 nearly three-quarters of women who die during or shortly after pregnancy have medical comorbidities. 2 Additionally, although deaths from direct causes are significantly decreasing, there has been no decrease in indirect (medical) maternal deaths. 2 These studies together highlight the importance of identifying and appropriately managing maternal comorbidities. Although factors such as pre-existing medical conditions cannot be altered, their adverse consequences can potentially be mitigated through extra vigilance and proactive management. Although further research is required to investigate how best to provide pre-pregnancy counselling as well as multidisciplinary maternity care for these women, one of the most important implications is that women with medical comorbidities should be identified and fully assessed for their risks in pregnancy, and that health professionals should be aware of the associated risk of severe morbidity.
Uptake of antenatal care was found to be poorer among women with medical comorbidities in our study population, which could increase the adverse effects associated with these conditions. In our combined analysis after adjusting for medical comorbidities,233 the previously observed association of older maternal age and obesity with increased odds of dying160 was no longer evident, suggesting that this association may be mediated through medical comorbidities. Obesity nevertheless appeared to be a risk factor for sepsis mortality100 and ICU admission;122 however, it was evident from the missing data in the intensive care study that BMI was often not routinely recorded. Recording BMI and being aware of associated risks of obesity remains important. 73,123
Recommendations
-
Health professionals should be aware of the associated risk of dying from severe obstetric morbidity in women with medical comorbidities.
-
Women with medical comorbidities should be identified and fully assessed for their risks in pregnancy.
-
Inadequate uptake of antenatal care, substance misuse, hypertensive disorders of pregnancy, previous pregnancy problems and minority ethnicity are also associated with maternal death from direct pregnancy complications. The adverse consequences of these conditions could potentially be minimised through access to appropriate services, extra vigilance and proactive multidisciplinary management.
Whether or not an external confidential enquiry or local review approach can be used to investigate and improve the quality of care for affected women
We found that substantial variation exists in the local review of severe maternal morbidities, in terms of the definition and scope of incidents that trigger a review, the guidelines for conducting a review and the outputs and conclusions of reviews. External review (confidential enquiry) is, in contrast, carried out nationally using a standard approach. 2 Maternal sepsis cases, despite being of current major concern, are not reviewed in one-third of maternity units,234 and neither does the RCOG guidance recommend that they are reviewed. 191 Given that it was clear that local guidelines take a major steer from RCOG guidance, there is a case for adding maternal sepsis to the RCOG checklist of cases for review. It is also important that such trigger checklists are responsive at both a local and national level to emerging conditions of public health and/or patient safety concern. Processes should exist for ongoing review and revision of the incident review trigger list to respond to such concerns.
Stakeholder participation in local development of guidelines for serious incident investigation in maternity services could be widened. Some guidelines had been circulated to staff to obtain their views, but service users were rarely consulted. It was unclear in over one-quarter of guidelines whether changes in practice in response to review recommendations were audited or monitored. Such auditing is important to ensure recommended changes are being implemented and driving change. 168 This was reflected in the local reviews we examined as fewer than 1 in 10 of the local reviews we examined advocated auditing the changes to care that were recommended.
Local and external reviews of the care of women who have had severe morbidities in pregnancy clearly add different perspectives. Both local and external review processes identified important messages to improve future care, although the number of specific messages identified was greater in the external reviews. One-fifth of local reviews identified specific local service- or situation-specific factors which had an impact on women’s care and, importantly, these lessons cannot be identified through external reviews alone. There was little evidence of multidisciplinary review at a local level, which may account for the greater number and scope of messages identified by the external review processes. It was also apparent that local review groups had a role to institute individual disciplinary procedures, when required, and this may detract from identifying generalised and system-level messages to improve clinical care.
However, the external review process is labour intensive, requires administrative support and costs an average of £2100 per case. We were not able to capture costs for the local review processes. Although the costs appear high, if lessons are learned and implemented in practice preventing future serious morbidity, the benefits would be potentially reaped in terms of future litigation costs prevented.
Recommendations
-
Maternal sepsis should be added to both RCOG and local trigger checklists of cases, which should stimulate a local review.
-
Trigger checklists need to be responsive at both a local and national level to emerging conditions of public health and/or patient safety concern. Processes should exist for ongoing review and revision of the incident review trigger list to respond to such concerns.
-
Stakeholder participation in local development of guidelines for serious incident investigation in maternity services should be widened.
-
The implementation of recommendations from local reviews of the care of women with severe maternal morbidity should be audited to ensure that change has led to the desired improvement in outcomes.
-
Local reviews should be multidisciplinary including, as a minimum, obstetricians, midwives and anaesthetists, together with other professional groups such as physicians, GPs and health visitors, as appropriate.
-
At a local level, individual disciplinary procedures/recommendations should be separated from the incident review processes.
The longer-term impacts of near-miss maternal morbidity for women, their babies and families
All the women and their partners we interviewed had some long-term adverse consequences of their near-miss event. 46 For some, these did not have a major impact on the quality of their life and relationships going forward, for others their pregnancy or delivery experience continued to have a major impact often years after the event. Women often had no follow-up from hospital obstetric or midwifery staff. Primary care teams should routinely be made aware if a woman has a severe maternal morbidity so they can offer the support these women may need, both in coming to terms with the near miss and understanding what long-term impact this might have on their health and ability to have more children. In addition, primary care teams should be aware that these new mothers may be isolated from their peers and, therefore, support networks may be required. GPs should be alert for mental health problems developing and mindful of the impact that the near-miss experience can have on the whole family (including partner and other children) and be prepared to offer advice about future pregnancies.
All the partners/fathers we spoke to had been deeply affected by their partner’s life-threatening experiences. For some it had a profound impact on their long-term mental health. Long-term mental health problems in partners/fathers after a near-miss experience may have a big impact financially, practically and emotionally, and families may need additional support in this event. They often felt that counselling could have been beneficial, if it had been offered; however, clinicians should take into account that partners/fathers who experience mental health symptoms do not necessarily seek help.
Unfortunately, the challenges we had at several stages of the study, including obtaining NHS management approval to conduct the study, contributed to an extremely low response rate in our study of the long-term outcomes of women who had a peripartum hysterectomy. Nevertheless, the study suggested that women who had a peripartum hysterectomy to control haemorrhage were more likely than women who give birth but do not have a hysterectomy to experience pain, depression, difficulties/pain during intercourse, severe tiredness/fatigue, ‘flash-backs’ to the labour or birth, menopausal symptoms and difficulties concentrating in the first year after their birth; findings which were echoed in the interview study. They were also more likely to experience ‘flash-backs’ to the labour or birth and menopausal symptoms 7–8 years after the index birth. The qualitative analysis of the questionnaire responses highlighted a number of examples of good practice with regard to maternity care and examples for which care could have been improved. As in the interview study, a number of women identified a perceived need for counselling services.
Recommendations
-
When a woman has had a severe maternal morbidity, community midwives as well as her GP should be made aware of this when she is discharged from hospital.
-
Follow-up appointments with hospital obstetric and/or midwifery staff are helpful for women with severe maternal morbidities. However, women reported that they felt they needed these at varying times after the event; flexibility beyond the standard timing of 6 weeks post delivery would be helpful.
-
There should be a clear pathway for access to counselling services for women with severe maternal morbidities.
-
GPs should remain alert to the possibility of ongoing mental health problems in women who have had a severe pregnancy complication, as well as being aware that the experience may impact on the mental health of the woman’s partner.
Implications for future research
Pre-pregnancy
-
A key finding of this programme is the fact that maternal medical conditions are a significant risk for both morbidity and mortality, not only from indirect (medical), but also from direct (obstetric) morbidities. Pre-pregnancy assessment and advice could address many factors associated with morbidity, including optimising health and drug regimes in preparation for pregnancy. However, it is unclear how, and by whom, pre-pregnancy care is best delivered to access women with the wide spectrum of medical disorders that are associated with morbidity, while taking into account that up to 40% of pregnancies are unplanned. The outcomes, in terms of benefits for mothers and their babies, of pre-pregnancy counselling are not well established. Obtaining this evidence will be important for establishing appropriate services.
-
Allied to this, further research is needed to fully identify the pathways through which minority ethnicity is associated with severe maternal morbidity. This may include pre-pregnancy educational, cultural and social factors that provide a focus for further research into possible pathways of prevention of severe maternal morbidity.
During pregnancy
-
Research into these rare conditions can be difficult owing to issues of study power even with national collaborations such as UKOSS. Further studies, for example to investigate the role of early correction of coagulopathy in the prevention of mortality and severe morbidity from AFE, will require multinational collaborative studies, such as through the International Network of Obstetric Survey Systems (INOSS). 219
-
International comparative studies may be valuable to further investigate the impact of different management strategies. At the time this research was conducted, few comparable international studies existed because data collection was ongoing in many INOSS participating countries, the notable exception being the study completed from the Netherlands. 198 Comparison of the results of these studies with those recently published using similar methodology, such as those from the Nordic countries,235,236 may provide further information to guide optimal management.
-
Obstetric interventions, such as induction of labour and caesarean delivery, are associated with a number of severe morbidities and research to investigate methods to reduce intervention rates without increasing other adverse outcomes is important as a route to prevention of near-miss morbidity.
-
Further investigation is needed to establish the role of prophylactic antibiotics for the prevention of infection following operative vaginal delivery.
-
Some women found intensive care outreach services helpful and further studies are needed as to how this can be optimally provided to the maternity population to improve outcomes. There may also be a role for further investigation of the delivery of critical care to the maternity population. Although the critical care that women received appeared to be good, women did report difficulties in seeing and feeding their babies, and consideration should be given to their need to be a mother as well as a patient as part of their recovery from critical obstetric illness.
-
Economic evaluation of interventions in maternity care is as important as it is in other disciplines. This programme demonstrated that robust observational data can be used to conduct a cost–utility analysis and further studies of interventions for severe maternal morbidities could include similar approaches to ensure that both costs and outcomes are considered.
-
Future research should assess the efficacy of rapid antigen diagnostic tests for group A Streptococcus in obstetrics.
Following pregnancy
-
There has been little research on the long-term impact of traumatic birth and how best to help women. There is inconclusive evidence on the impact of debriefing programmes237 and this needs to be robustly evaluated.
-
Many of the women we interviewed reported symptoms associated with PTSD, which was also the case among women who had a peripartum hysterectomy. However, some women who had not had a severe pregnancy complication also reported similar symptoms. Further investigation of the role that severe pregnancy complications play as precipitating factors for PTSD is needed, alongside investigation of possible therapies to prevent traumatic flashbacks238 in both women who have, and those who have not, had severe pregnancy complications. Similar research is also needed for partners.
Serious incident reviews
-
Evidence-based, standard, national guidance on conducting local reviews of the care of women with severe maternal morbidity may be helpful to reduce variation in quality and outcomes of local reviews, and this should be evaluated in a prospective study.
-
Further evaluation is needed to establish whether or not there is added value to including an external perspective to local reviews once high-quality multidisciplinary local review processes are fully implemented.
-
The balance of cost/complexity versus benefit of local versus external reviews of the care of women with severe pregnancy morbidity needs to be fully established in a prospective study including audit of change in practice and outcomes.
Services
-
The cost–benefit of ELC needs to be fully evaluated, linking the costs of developing the commissioning strategy with outcomes such as changes in services and patient experience. The longer-term impacts of implementation of the ELC strategy need to be fully understood.
-
Costs of ELC may be reduced when the technique is used in other CCGs, as materials/processes developed in this study can be reused and do not need to be redeveloped. This requires further investigation.
-
Further research could explore ways of making ELC more understandable to those unfamiliar with the process.
Conclusions
Implementation of the findings of this research could prevent both future severe pregnancy complications as well as improving the outcome of pregnancy for women who have one of these severe morbidities. These ‘near-miss’ events represent only a small proportion of the women with complications, but improving care for women with the severest complications also benefits women with less-severe disease and, thus, the programme findings may have wider impacts. One of the clearest findings relates to the population of women with other medical and mental health problems in pregnancy and their risk of severe morbidity. Going forward, further research is needed into the means of preventing pregnancy complications as well as improving the outcomes of pregnancy complications in this group. With current trends in maternal age at childbirth as well as population trends in factors such as obesity, this group is likely to become more numerous. However, with the wide range of comorbidities experienced, detailed and nuanced research into models of pre-pregnancy, pregnancy and postnatal care and the impact on outcomes for women and their infants is clearly needed.
Acknowledgements
UKNeS coapplicant group: Marian Knight, Jennifer J Kurinczuk, Peter Brocklehurst, Alison Burton, Susan Sellers, Mervi Jokinen, Louise Locock, Dean Regier, Shona Golightly, Gwyneth Lewis, James Walker, Jenny Furniss, Maria Quigley, Sula Wiltshire, Jane Bell and Oliver Rivero-Arias.
The UKNeS Programme Manager, Melanie O’Connor, and Deputy Programme Manager, Anne Smith, have been pivotal in co-ordinating data collection for the component studies and in the preparation of the report. Programmers Patsy Spark and Phil Peirsegaele also played an essential role. We would also like to thank the other members of the admin team for their contributions, including previous programme managers Charlotte McClymont and Dominika Misztela.
We would also like to acknowledge the contribution of the User Advisory Group, the collaboration of clinicians contributing to the UKOSS, and the UKOSS Steering Committee, without whom this work would not have been possible. We would also like to acknowledge the following individuals who contributed to individual component studies: Zarko Alfirevic, Sohinee Bhattacharya, Jeffrey B Gould, David Harrison, Kim Hinshaw, Gilles Kayem, Henry C Lee, Nuala Lucas, Audrey Lyndon, Charlotte McClymont, Olaa Mohamed-Ahmed, Melanie O’Connor, Kathy Rowan, Patsy Spark and Derek J Tuffnell.
Contributions of authors
Marian Knight designed the programme, supervised the component studies and analyses, and wrote the first draft of the manuscript.
Colleen Acosta collected the data, coded the data, carried out the analysis and wrote the first draft of the articles which contribute to Chapter 4.
Peter Brocklehurst, Mervi Jokinen, Jennifer J Kurinczuk, Gwyneth Lewis, Maria Quigley and Susan Sellers contributed to the design of the studies and writing of the final report.
Anna Cheshire collected the data, coded the data, carried out the analysis and wrote the first draft report which contributes to Chapter 10.
Kathryn Fitzpatrick collected the data, coded the data, carried out the analyses, and wrote the first draft of articles which contribute to Chapters 3, 7 and 9.
Lisa Hinton collected the data, coded the data, carried out the analyses, wrote the first draft of the articles which contribute to Chapter 2, and also assisted with analysis for Chapters 8–10.
Bryn Kemp contributed to the analysis and writing of Chapter 8.
Anthea Lindquist collected the data, coded the data, carried out the analysis and wrote the first draft of an article which contributes to Chapter 7.
Louise Locock supervised the analysis in Chapter 2, advised on all qualitative aspects of the programme and contributed to the writing of the final report.
Manisha Nair coded the data, carried out the analysis and wrote the first draft of articles which contribute to Chapters 6 and 7.
Nishma Patel carried out the initial analysis for Chapter 5 and contributed to the writing of the final report.
Damien Ridge supervised Anna Cheshire and contributed to the writing of the final report.
Oliver Rivero-Arias completed the analysis for Chapter 5 and wrote the article which formed the basis of the chapter.
Anjali Shah collected the data, coded the data, carried out the analysis and wrote the first draft of the articles which contribute to Chapter 8.
Publications
The following publications have arisen from the work funded in this programme:
Acosta CD, Kurinczuk JJ, Lucas DN, Tuffnell DJ, Sellers S, Knight M, et al. Severe maternal sepsis in the UK, 2011–2012: a national case–control study. PLOS Med 2014;11:e1001672.
Acosta CD, Knight M, Lee HC, Kurinczuk JJ, Gould JB, Lyndon A. The continuum of maternal sepsis severity: incidence and risk factors in a population-based cohort study. PLOS ONE 2013;8:e67175.
Acosta CD, Bhattacharya S, Tuffnell D, Kurinczuk JJ, Knight M. Maternal sepsis: a Scottish population-based case–control study. BJOG 2012;119:474–83.
Acosta CD, Harrison DA, Rowan K, Lucas DN, Kurinczuk JJ, Knight M. Maternal morbidity and mortality from severe sepsis: a national cohort study. BMJ Open 2016; in press.
Fitzpatrick KE, Kurinczuk JJ, Alfirevic Z, Spark P, Brocklehurst P, Knight M. Uterine rupture by intended mode of delivery in the UK: a national case–control study. PLOS Med 2012;9:e1001184.
Fitzpatrick KE, Hinshaw K, Kurinczuk JJ, Knight M. Risk factors, management, and outcomes of hemolysis, elevated liver enzymes, and low platelets syndrome and elevated liver enzymes, low platelets syndrome. Obstet Gynecol 2014;123:618–27.
Fitzpatrick KE, Sellers S, Spark P, Kurinczuk JJ, Brocklehurst P, Knight M. Incidence and risk factors for placenta accreta/increta/percreta in the UK: a national case–control study. PLOS ONE 2012;7:e52893.
Fitzpatrick KE, Sellers S, Spark P, Kurinczuk JJ, Brocklehurst P, Knight M. The management and outcomes of placenta accreta, increta, and percreta in the UK: a population-based descriptive study. BJOG 2014;12:62–70.
Fitzpatrick KE, Tuffnell D, Kurinczuk JJ, Knight M. Incidence, risk factors, management and outcomes of amniotic-fluid embolism: a population-based cohort and nested case–control study. BJOG 2016;123:100–9.
Fitzpatrick KE, Tuffnell D, Kurinczuk JJ, Knight M. Pregnancy at very advanced maternal age: a UK population-based cohort study. BJOG 2016; in press.
Hinton L, Locock L, Knight M. Experiences of the quality of care of women with near-miss maternal morbidities in the UK. BJOG 2014;121(Suppl. 4):20–3.
Hinton L, Locock L, Knight M. Partner experiences of ‘near-miss’ events in pregnancy and childbirth in the UK: a qualitative study. PLOS ONE 2014;9:e91735.
Hinton L, Locock L, Knight M. Support for mothers and their families after life-threatening illness in pregnancy and childbirth: a qualitative study in primary care. Br J Gen Pract 2015;65:e563–9.
Hinton L, Locock L, Knight M. Maternal critical care: what can we learn from patient experience? A qualitative study. BMJ Open 2015;5:e006676.
Kayem G, Kurinczuk J, Lewis G, Golightly S, Brocklehurst P, Knight M. Risk factors for progression from severe maternal morbidity to death: a national cohort study. PLOS ONE 2011;6:e29077.
Knight M, Lewis G, Acosta CD, Kurinczuk JJ. Maternal near-miss case reviews: the UK approach. BJOG 2014;121(Suppl. 4):112–16.
Lindquist A, Knight M, Kurinczuk JJ. Variation in severe maternal morbidity according to socioeconomic position: a UK national case–control study. BMJ Open 2013;3:e002742.
Nair M, Kurinczuk JJ, Knight M. Ethnic variations in severe maternal morbidity in the UK – a case control study. PLOS ONE 2014;9:e95086.
Nair M, Kurinczuk JJ, Brocklehurst P, Sellers S, Lewis G, Knight M. Factors associated with maternal death from direct pregnancy complications: a UK national case–control study. BJOG 2015;122:653–62.
Shah A, Kemp B, Sellers S, Hinton L, O’Connor M, Brocklehurst P, et al. Towards optimising local reviews of severe incidents in maternity care: messages from a comparison of local and external reviews [published online ahead of print 24 March 2016]. BMJ Qual Saf 2016.
Shah A, Mohamed-Ahmed O, McClymont C, Knight M. Conditions triggering local incident reviews in UK hospital maternity units: a national survey. JRSM Open 2014;5:2054270414528898.
Shah A, Mohamed-Ahmed O, Peirsegaele P, McClymont C, Knight M. Incident reviews in UK maternity units: a systematic appraisal of the quality of local guidelines. BMC Pregnancy Childbirth 2015;15:58.
Data sharing statement
Data are available by request to the National Perinatal Epidemiology Unit Data Sharing Committee.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health.
References
- Lewis G. Why Mothers Die 2000–2002. London: RCOG; 2004.
- Knight M, Kenyon S, Brocklehurst P, Neilson J, Shakespeare J, Kurinczuk J, et al. Saving Lives, Improving Mothers’ Care – Lessons Learned to Inform Future Maternity Care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2009–12. Oxford: National Perinatal Epidemiology Unit, University of Oxford; 2014.
- Lewis GE. The Confidential Enquiry into Maternal and Child Health (CEMACH). Saving Mothers Lives: Reviewing Maternal Deaths to Make Childhood Safer – 2003–2005. London: CEMACH; 2007.
- Waterstone M, Bewley S, Wolfe C. Incidence and predictors of severe obstetric morbidity: case–control study. BMJ 2001;322:1089-93. http://dx.doi.org/10.1136/bmj.322.7294.1089.
- Filippi V, Ronsmans C, Gandaho T, Graham W, Alihonou E, Santos P. Women’s reports of severe (near-miss) obstetric complications in Benin. Stud Fam Plann 2000;31:309-24. http://dx.doi.org/10.1111/j.1728-4465.2000.00309.x.
- Pattinson RC, Hall M. Near misses: a useful adjunct to maternal death enquiries. Br Med Bull 2003;67:231-43. http://dx.doi.org/10.1093/bmb/ldg007.
- Say L, Pattinson RC, Gulmezoglu AM. WHO systematic review of maternal morbidity and mortality: the prevalence of severe acute maternal morbidity (near miss). Reprod Health 2004;1. http://dx.doi.org/10.1186/1742-4755-1-3.
- Knight M, Kurinczuk JJ, Spark P, Brocklehurst P. Cesarean delivery and peripartum hysterectomy. Obstet Gynecol 2008;111:97-105. http://dx.doi.org/10.1097/01.AOG.0000296658.83240.6d.
- Geller SE, Rosenberg D, Cox SM, Brown ML, Simonson L, Driscoll CA, et al. The continuum of maternal morbidity and mortality: factors associated with severity. Am J Obstet Gynecol 2004;191:939-44. http://dx.doi.org/10.1016/j.ajog.2004.05.099.
- Knight M, Kurinczuk JJ, Tuffnell D, Brocklehurst P. The UK Obstetric Surveillance System for rare disorders of pregnancy. BJOG 2005;112:263-5. http://dx.doi.org/10.1111/j.1471-0528.2005.00609.x.
- Knight M, Lindquist A. The UK Obstetric Surveillance System: impact on patient safety. Best Pract Res Clin Obstet Gynaecol 2013;27:621-30. http://dx.doi.org/10.1016/j.bpobgyn.2013.03.002.
- Live Births In England and Wales by Characteristics of Mother 1, 2013. London: ONS; 2014.
- Heslehurst N, Rankin J, Wilkinson JR, Summerbell CD. A nationally representative study of maternal obesity in England, UK: trends in incidence and demographic inequalities in 619 323 births, 1989–2007. Int J Obes 2010;34:420-8. http://dx.doi.org/10.1038/ijo.2009.250.
- Births in England and Wales by Parents’ Country of Birth 2013. Newport: ONS; 2014.
- Characteristics of Birth 2, England and Wales – 2013. London: ONS; 2014.
- Health Reform in England: Update and Commissioning Framework. London: Department of Health; 2006.
- Maternity Matters: Choice, Access and Continuity of Care in a Safe Service. London: Department of Health; 2007.
- PSA Delivery Agreement 18: Promote Better Health and Wellbeing for All. Norwich: Her Majesty’s Stationery Office; 2007.
- Tackling Health Inequalities: A Programme for Action. London: Department of Health; 2003.
- Kirkup B. The Report of the Morecambe Bay Investigation. London: The Stationery Office; 2015.
- NHS England . Maternity Review: Terms of Reference 2015. www.england.nhs.uk/wp-content/uploads/2015/03/maternity-rev-tor.pdf (accessed 13 April 2016).
- Baskett TF, O’Connell CM. Severe obstetric maternal morbidity: a 15-year population-based study. J Obstet Gynaecol 2005;25:7-9. http://dx.doi.org/10.1080/01674820400023408.
- Callaghan WM, Mackay AP, Berg CJ. Identification of severe maternal morbidity during delivery hospitalizations, United States, 1991–2003. Am J Obstet Gynecol 2008;199:133e1-8.
- Roberts CL, Cameron CA, Bell JC, Algert CS, Morris JM. Measuring maternal morbidity in routinely collected health data: development and validation of a maternal morbidity outcome indicator. Med Care 2008;46:786-94. http://dx.doi.org/10.1097/MLR.0b013e318178eae4.
- Wen SW, Huang L, Liston R, Heaman M, Baskett T, Rusen ID, et al. Severe maternal morbidity in Canada, 1991–2001. CMAJ 2005;173:759-64. http://dx.doi.org/10.1503/cmaj.045156.
- Knight M. Preeclampsia: increasing incidence but improved outcome?. Am J Hypertens 2008;21. http://dx.doi.org/10.1038/ajh.2008.33.
- Waterstone M, Wolfe C, Hooper R, Bewley S. Postnatal morbidity after childbirth and severe obstetric morbidity. BJOG 2003;110:128-33. http://dx.doi.org/10.1046/j.1471-0528.2003.02151.x.
- Knight M. Eclampsia in the United Kingdom 2005. BJOG 2007;114:1072-8. http://dx.doi.org/10.1111/j.1471-0528.2007.01423.x.
- Knight M. Antenatal pulmonary embolism: risk factors, management and outcomes. BJOG 2008;115:453-61. http://dx.doi.org/10.1111/j.1471-0528.2007.01622.x.
- Knight M, Nelson-Piercy C, Kurinczuk JJ, Spark P, Brocklehurst P. UK Obstetric Surveillance System . A prospective national study of acute fatty liver of pregnancy in the UK. Gut 2008;57:951-6. http://dx.doi.org/10.1136/gut.2008.148676.
- Knight M. behalf of UKOSS . Peripartum hysterectomy in the UK: management and outcomes of the associated haemorrhage. BJOG 2007;114:1380-7. http://dx.doi.org/10.1111/j.1471-0528.2007.01507.x.
- Knight M, Tuffnell D, Brocklehurst P, Spark P, Kurinczuk JJ. behalf of UKOSS . Incidence and risk factors for amniotic-fluid embolism. Obstet Gynecol 2010;115:910-17. http://dx.doi.org/10.1097/AOG.0b013e3181d9f629.
- Meisel ZF, Karlawish J. Narrative vs evidence-based medicine – and, not or. JAMA 2011;306:2022-3. http://dx.doi.org/10.1001/jama.2011.1648.
- Furuta M, Sandall J, Bick D. Women’s perceptions and experiences of severe maternal morbidity – a synthesis of qualitative studies using a meta-ethnographic approach. Midwifery 2014;30:158-69. http://dx.doi.org/10.1016/j.midw.2013.09.001.
- Thompson JF, Heal LJ, Roberts CL, Ellwood DA. Women’s breastfeeding experiences following a significant primary postpartum haemorrhage: a multicentre cohort study. Int Breastfeed J 2010;5. http://dx.doi.org/10.1186/1746-4358-5-5.
- Elmir R, Schmied V, Jackson D, Wilkes L. Between life and death: women’s experiences of coming close to death, and surviving a severe postpartum haemorrhage and emergency hysterectomy. Midwifery 2012;28:228-35. http://dx.doi.org/10.1016/j.midw.2010.11.008.
- De La Cruz C. A Mixed Method Study on the Peripartum Experience and Postpartum Effects of Emergency Hysterectomy Due To Postpartum Hemorrhage. Tampa, FL: University of South Florida; 2011.
- Snowdon C, Elbourne D, Forsey M, Alfirevic Z. Information-hungry and disempowered: a qualitative study of women and their partners’ experiences of severe postpartum haemorrhage. Midwifery 2012;28:791-9. http://dx.doi.org/10.1016/j.midw.2011.12.012.
- Fenech G, Thomson G. Tormented by ghosts from their past: a meta-synthesis to explore the psychosocial implications of a traumatic birth on maternal well-being. Midwifery 2014;30:185-93. http://dx.doi.org/10.1016/j.midw.2013.12.004.
- Kozhimannil K, Attanasio L, Joarnt L, McGovern P. Medically complex pregnancies and early breastfeeding behaviours: a retrospective analysis. PLOS ONE 2014;9. http://dx.doi.org/10.1371/journal.pone.0104820.
- DIPEx . The Research 2016. www.healthtalk.org/research/research-methods (accessed 11 April 2016).
- Patton MQ. Qualitative Evaluation and Research Methods. London: Sage; 1990.
- Coyne I. Sampling in qualitative research. Purposeful and theoretical sampling; merging or clear boundaries. J Adv Nurs 1997;26:623-30. http://dx.doi.org/10.1046/j.1365-2648.1997.t01-25-00999.x.
- Glaser BS, Strauss A. The Discovery of Grounded Theory. Chicago, IL: Aldine Publishing; 1967.
- Green JT, Thorogood N. Qualitative Methods in Health Research. London: Sage; 2004.
- Healthtalk.org . Conditions That Threaten Women’s Lives in Childbirth and Pregnancy n.d. www.healthtalk.org/peoples-experiences/pregnancy-children/conditions-threaten-womens-lives-childbirth-pregnancy/topics (accessed 13 April 2016).
- Henderson J, Redshaw M. Who is well after childbirth? Factors related to positive outcome. Birth 2013;40:1-9. http://dx.doi.org/10.1111/birt.12022.
- Desai SV, Law TJ, Needham DM. Long-term complications of critical care. Crit Care Med 2011;39:371-9. http://dx.doi.org/10.1097/CCM.0b013e3181fd66e5.
- Providing Equity of Critical and Maternal Care for the Critically Ill Pregnant or Recently Pregnant Woman. London: Royal College of Anaesthetists; 2011.
- Neligan PJ, Laffey JG. Clinical review: special populations – critical illness and pregnancy. Crit Care 2011;15. http://dx.doi.org/10.1186/cc10256.
- Kynoch K, Paxton J, Chang AM. ICU nurses’ experiences and perspectives of caring for obstetric patients in intensive care: a qualitative study. J Clin Nurs 2011;20:1768-75. http://dx.doi.org/10.1111/j.1365-2702.2010.03517.x.
- Campbell PT, Rudisill PT. Psychosocial needs of the critically ill obstetric patient: the nurse’s role. Crit Care Nurs Q 2006;29:77-80. http://dx.doi.org/10.1097/00002727-200601000-00008.
- Plaat F, Naik M. Critical care in pregnancy. Crit Care 2011;15. http://dx.doi.org/10.1186/cc10479.
- Fitzpatrick KE, Hinshaw K, Kurinczuk JJ, Knight M. Risk factors, management, and outcomes of hemolysis, elevated liver enzymes, and low platelets syndrome and elevated liver enzymes, low platelets syndrome. Obstet Gynecol 2014;123:618-27. http://dx.doi.org/10.1097/AOG.0000000000000140.
- Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol 2010;116:1302-9. http://dx.doi.org/10.1097/AOG.0b013e3181fdfb11.
- Conde-Agudelo A, Romero R. Amniotic fluid embolism: an evidence-based review. Am J Obstet Gynecol 2009;201.
- Knight M, Berg C, Brocklehurst P, Kramer M, Lewis G, Oats J, et al. Amniotic fluid embolism incidence, risk factors and outcomes: a review and recommendations. BMC Pregnancy Childbirth 2012;12. http://dx.doi.org/10.1186/1471-2393-12-7.
- Oyelese Y, Smulian JC. Placenta previa, placenta accreta, and vasa previa. Obstet Gynecol 2006;107:927-41. http://dx.doi.org/10.1097/01.AOG.0000207559.15715.98.
- Khong TY. The pathology of placenta accreta, a worldwide epidemic. J Clin Pathol 2008;61:1243-6. http://dx.doi.org/10.1136/jcp.2008.055202.
- Wu S, Kocherginsky M, Hibbard JU. Abnormal placentation: twenty-year analysis. Am J Obstet Gynecol 2005;192:1458-61. http://dx.doi.org/10.1016/j.ajog.2004.12.074.
- Miller DA, Chollet JA, Goodwin TM. Clinical risk factors for placenta previa-placenta accreta. Am J Obstet Gynecol 1997;177:210-14. http://dx.doi.org/10.1016/S0002-9378(97)70463-0.
- The National Sentinel Caesarean Section Audit Report. London: RCOG; 2001.
- Birth Statistics: Review of the Registrar General on Births and Patterns of Family Building in England and Wales. Newport: ONS; 2007.
- Weinstein L. Syndrome of hemolysis, elevated liver enzymes, and low platelet count: a severe consequence of hypertension in pregnancy. Am J Obstet Gynecol 1982;142:159-67. http://dx.doi.org/10.1097/00006254-198207000-00006.
- Sibai BM. The HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets): much ado about nothing?. Am J Obstet Gynecol 1990;162:311-16. http://dx.doi.org/10.1016/0002-9378(90)90376-I.
- Sibai BM. Diagnosis, controversies, and management of the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet Gynecol 2004;103:981-91. http://dx.doi.org/10.1097/01.AOG.0000126245.35811.2a.
- Haram K, Svendsen E, Abildgaard U. The HELLP syndrome: clinical issues and management. A Review. BMC Pregnancy Childbirth 2009;9. http://dx.doi.org/10.1186/1471-2393-9-8.
- Hofmeyr GJ, Say L, lmezoglu AM. WHO systematic review of maternal mortality and morbidity: the prevalence of uterine rupture. BJOG 2005;112:1221-8. http://dx.doi.org/10.1111/j.1471-0528.2005.00725.x.
- Caesarean Section. London: RCOG; 2004.
- Dodd JM, Crowther CA, Huertas E, Guise JM, Horey D. Planned elective repeat caesarean section versus planned vaginal birth for women with a previous caesarean birth. Cochrane Database Syst Rev 2004;4. http://dx.doi.org/10.1002/14651858.cd004224.pub2.
- Fitzpatrick K, Tuffnell D, Kurinczuk J, Knight M. Incidence, risk factors, management and outcomes of amniotic-fluid embolism: a population-based cohort and nested case–control study. BJOG 2016;123:100-9. http://dx.doi.org/10.1111/1471-0528.13300.
- Fitzpatrick KE, Sellers S, Spark P, Kurinczuk JJ, Brocklehurst P, Knight M. Incidence and risk factors for placenta accreta/increta/percreta in the UK: a national case–control study. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0052893.
- Knight M, Kurinczuk JJ, Spark P, Brocklehurst P. Extreme obesity in pregnancy in the United Kingdom. Obstet Gynecol 2010;115:989-97. http://dx.doi.org/10.1097/AOG.0b013e3181da8f09.
- Scott CA, Bewley S, Rudd A, Spark P, Kurinczuk JJ, Brocklehurst P, et al. Incidence, risk factors, management, and outcomes of stroke in pregnancy. Obstet Gynecol 2012;120:318-24. http://dx.doi.org/10.1097/AOG.0b013e31825f287c.
- Guise JM, Eden K, Emeis C, Denman MA, Marshall N, Fu RR, et al. Vaginal birth after cesarean: new insights. Evid Rep Technol Assess 2010;191:1-397. http://dx.doi.org/10.1097/aog.0b013e3181df925f.
- Key Population and Vital Statistics (2005). Newport: ONS; 2005.
- Vital Events Reference Tables (2008). Section 3: Births. Edinburgh: General Register Office for Scotland; 2009.
- Registrar General Annual Reports (2008) Section 3: Births. Belfast: NISRA; 2008.
- Birth Summary Tables, England and Wales 2012. Newport: ONS; 2013.
- Registrar General Annual Report 2011. Belfast: NISRA; 2012.
- Registrar General Annual Report 2010. Belfast: Nort NISRA; 2011.
- Registrar General Annual Report 2009. Belfast: NISRA; 2010.
- Key Population and Vital Statistics (2006). Newport: ONS; 2006.
- Key Population and Vital Statistics (2007). Newport: ONS; 2008.
- Vital Events Reference Tables (2009). Section 3: Births. Edinburgh: General Register Office for Scotland; 2010.
- Vital Events Reference Tables (2010). Section 3: Births. Edinburgh: General Register Office for Scotland; 2011.
- Vital Events Reference Tables (2011). Section 3: Births. Edinburgh: General Register Office for Scotland; 2012.
- Vital Events Reference Tables (2012). Section 3: Births. Edinburgh: General Register Office for Scotland; 2013.
- Registrar General Annual Reports (2012) Section 3: Births. Belfast: NISRA; 2013.
- Vital Events Reference Tables 2011. Edinburgh: General Register Office for Scotland; 2012.
- Birth Summary Tables, England and Wales 2011. Newport: ONS; 2012.
- Birth Summary Tables, England and Wales 2010. Newport: ONS; 2011.
- Vital Events Reference Tables 2010. Edinburgh: General Register Office for Scotland; 2011.
- Vital Events Reference Tables 2009. Edinburgh: General Register Office for Scotland; 2010.
- Perinatal Mortality 2008. London: CMACE; 2010.
- National Institute for Health and Care Excellence . NG25: Preterm Labour and Birth 2015 n.d. www.nice.org.uk/guidance/ng25 (accessed 13 April 2016).
- Garmi G, Salim R. Epidemiology, atiology, diagnosis, and management of placenta accreta. Obstet Gynecol Int 2012;2012. http://dx.doi.org/10.1155/2012/873929.
- Acosta CD, Bhattacharya S, Tuffnell D, Kurinczuk JJ, Knight M. Maternal sepsis: a Scottish population-based case–control study. BJOG 2012;119:474-83. http://dx.doi.org/10.1111/j.1471-0528.2011.03239.x.
- Acosta CD, Knight M, Lee HC, Kurinczuk JJ, Gould JB, Lyndon A. The continuum of maternal sepsis severity: incidence and risk factors in a population-based cohort study. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0067175.
- Centre for Maternal and Child Enquiries . Saving mothers’ lives: reviewing maternal deaths to make motherhood safer: 2006-2008. The eighth report of the confidential enquiries into maternal deaths in the United Kingdom. BJOG 2011;118:1-203.
- Schutte JM, Steegers EA, Schuitemaker NW, Santema JG, de Boer K, Pel M, et al. Rise in maternal mortality in the Netherlands. BJOG 2010;117:399-406. http://dx.doi.org/10.1111/j.1471-0528.2009.02382.x.
- Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367:1066-74. http://dx.doi.org/10.1016/S0140-6736(06)68397-9.
- Acosta CD, Knight M. Sepsis and maternal mortality. Curr Opin Obstet Gynecol 2013;25:109-16. http://dx.doi.org/10.1097/GCO.0b013e32835e0e82.
- Bhattacharya S, Townend J, Bhattacharya S. Recurrent miscarriage: are three miscarriages one too many? Analysis of a Scottish population-based database of 151,021 pregnancies. Eur J Obstet Gynecol Reprod Biol 2010;150:24-7. http://dx.doi.org/10.1016/j.ejogrb.2010.02.015.
- Humphrey T, Tucker JS. Rising rates of obstetric interventions: exploring the determinants of induction of labour. J Public Health 2009;31:88-94. http://dx.doi.org/10.1093/pubmed/fdn112.
- Bhattacharya S, Campbell DM. The incidence of severe complications of preeclampsia. Hypertens Pregnancy 2005;24:181-90. http://dx.doi.org/10.1081/PRG-200059873.
- Harrison DA, Brady AR, Rowan K. Case mix, outcome and length of stay for admissions to adult, general critical care units in England, Wales and Northern Ireland: the Intensive Care National Audit & Research Centre Case Mix Programme Database. Crit Care 2004;8:R99-111. http://dx.doi.org/10.1186/cc2834.
- The Information Centre . National Institutes of Health n.d. http://docdat.ic.nhs.uk/ (accessed 16 August 2012).
- Great Britain . National Health Service Act 2006 n.d. www.legislation.gov.uk/ukpga/2006/41/contents (accessed 13 April 2016).
- Office for National Statistics . Maternities: Office for National Statistics n.d. www.ons.gov.uk/ons/taxonomy/index.html?nscl=Maternities (accessed 6 May 2015).
- Northern Ireland Statistics and Research Agency . Births n.d. www.nisra.gov.uk/demography/default.asp8.htm (accessed 6 May 2015).
- Northern Ireland Statistics and Research Agency . Census 2001 Results n.d. www.nisra.gov.uk/census/2001/results.html (accessed 6 May 2015).
- Health & Social Care Information Centre . Hospital Episode Statistics n.d. www.hesonline.nhs.uk (accessed 6 May 2015).
- StatsWales . StatsWales n.d. https://statswales.wales.gov.uk/Catalogue/Health-and-Social-Care/NHS-Primary-and-Community-Activity/Maternity/Pre-2008–09 (accessed 6 May 2015).
- Black L. Incidence of Caesarean Sections Belfast: Northern Ireland Assembly; 2011 n.d. www.niassembly.gov.uk/globalassets/Documents/RaISe/Publications/2011/Health/14711.pdf (accessed 6 May 2015).
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003;348:1546-54. http://dx.doi.org/10.1056/NEJMoa022139.
- Padkin A, Goldfrad C, Brady AR, Young D, Black N, Rowan K. Epidemiology of severe sepsis occurring in the first 24 hrs in intensive care units in England, Wales, and Northern Ireland. Crit Care Med 2003;31:2332-8. http://dx.doi.org/10.1097/01.CCM.0000085141.75513.2B.
- Parker MM, Shelhamer JH, Bacharach SL, Green MV, Natanson C, Frederick TM, et al. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med 1984;100:483-90. http://dx.doi.org/10.7326/0003-4819-100-4-483.
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992;101:1644-55. http://dx.doi.org/10.1378/chest.101.6.1644.
- Comprehensive Critical Care: A Review of Adult Critical Care Services. London: Department of Health; 2000.
- Paruk F. Infection in obstetric critical care. Best Pract Res Clin Obstet Gynaecol 2008;22:865-83. http://dx.doi.org/10.1016/j.bpobgyn.2008.06.011.
- Knight M, Pierce M, Seppelt I, Kurinczuk JJ, Spark P, Brocklehurst P, et al. Influenza AH1N1v in pregnancy. BJOG 2011;118:1140-1. http://dx.doi.org/10.1111/j.1471-0528.2011.02958.x.
- Modder J, Fitsimons K. CMACE/RCOG Joint Guideline: Management of Women with Obesity in Pregnancy. London: RCOG; 2010.
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013;41:580-637. http://dx.doi.org/10.1097/CCM.0b013e31827e83af.
- Knight M. HTA – 13 96 07: ANODE: Prophylactic ANtibiotics for the Prevention of Infection Following Operative Delivery 2015. www.nets.nihr.ac.uk/projects/hta/139607 (accessed 8 May 2015).
- Foex BA. The problem of informed consent in emergency medicine research. Emerg Med J 2001;18:198-204. http://dx.doi.org/10.1136/emj.18.3.198.
- Phillips B, Ball C, Sackett D, Badenoch D, Straus S, Haynes B, et al. Oxford Centre for Evidence-Based Medicine Levels of Evidence Oxford: Centre for Evidence-Based Medicine 2001. www.cebm.net/index.aspx?o=1025 (accessed 21 January 2009).
- Postpartum Haemorrhage, Prevention and Management (Green-top Guideline No. 52). London: RCOG; 2009.
- Kayem G, Kurinczuk JJ, Alfirevic Z, Spark P, Brocklehurst P, Knight M. Specific second-line therapies for postpartum haemorrhage: a national cohort study. BJOG 2011;118:856-64. http://dx.doi.org/10.1111/j.1471-0528.2011.02921.x.
- Sekhon JS, Grieve RD. A matching method for improving covariate balance in cost-effectiveness analyses. Health Econ 2012;21:695-714. http://dx.doi.org/10.1002/hec.1748.
- Ades AE, Claxton K, Sculpher M. Evidence synthesis, parameter correlation and probabilistic sensitivity analysis. Health Econ 2006;15:373-81. http://dx.doi.org/10.1002/hec.1068.
- Sculpher MJ, Claxton K, Drummond M, McCabe C. Whither trial-based economic evaluation for health care decision making?. Health Econ 2006;15:677-87. http://dx.doi.org/10.1002/hec.1093.
- Grey A, Clarke PM, Wolstenholme J, Wordsworth S. Applied Methods of Cost-Effectiveness Analysis in Health Care. Oxford: Oxford University Press; 2010.
- Curtis L. Unit Costs of Health and Social Care. Canterbury: Personal Social Services Research Unit, University of Kent; 2013.
- EuroQol G. EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- Agency for Healthcare Research and Quality . Medical Expenditure Panel Survey Data Files 2003 n.d. http://meps.ahrq.gov/mepsweb/data_stats/download_data_files.jsp (accessed 13 April 2016).
- Agency for Healthcare Research and Quality . Medical Expenditure Panel Survey 2011 n.d. http://meps.ahrq.gov/mepsweb/ (accessed 13 April 2016).
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics 2000;17:479-500. http://dx.doi.org/10.2165/00019053-200017050-00006.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Social Value Judgments. Principles for the Development of NICE Guidance. London: NICE; 2008.
- Sculpher M. A cost–utility analysis of abdominal hysterectomy versus transcervical endometrial resection for the surgical treatment of menorrhagia. Int J Technol Assess Health Care 1998;14:302-19. http://dx.doi.org/10.1017/S0266462300012277.
- Agency for Healthcare Research and Quality . Medical Expenditure Panel Survey Data Files 2011 n.d. http://meps.ahrq.gov/mepsweb/data_stats/download_data_files.jsp (accessed 13 April 2016).
- Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. http://dx.doi.org/10.1097/00005650-199603000-00003.
- Grey AM, Rivero-Arias O, Clarke PM. Estimating the association between SF-12 responses and EQ-5D utility values by response mapping. Med Decis Making 2006;26:18-29. http://dx.doi.org/10.1177/0272989X05284108.
- Hammer GP, du Prel JB, Blettner M. Avoiding bias in observational studies: part 8 in a series of articles on evaluation of scientific publications. Dtsch Arztebl Int 2009;106:664-8.
- Kurinczuk JJ, Draper ES, Field DJ, Bevan C, Brocklehurst P, Grey R, et al. Experiences with maternal and perinatal death reviews in the UK – the MBRRACE-UK programme. BJOG 2014;121:41-6. http://dx.doi.org/10.1111/1471-0528.12820.
- Standard Occupational Classification. Newport: ONS; 2000.
- Ethnic Group Statistics: A Guide for the Collection and Classification of Ethnicity Data. Newport: ONS; 2003.
- Royston P. Multiple imputation of missing values: update. Stata J 2005;5:188-201.
- Marshall A, Altman DG, Royston P, Holder RL. Comparison of techniques for handling missing covariate data within prognostic modelling studies: a simulation study. BMC Med Res Methodol 2010;10. http://dx.doi.org/10.1186/1471-2288-10-7.
- Goffman D, Madden RC, Harrison EA, Merkatz IR, Chazotte C. Predictors of maternal mortality and near-miss maternal morbidity. J Perinatol 2007;27:597-601. http://dx.doi.org/10.1038/sj.jp.7211810.
- Mhyre JM, Bateman BT, Leffert LR. Influence of patient comorbidities on the risk of near-miss maternal morbidity or mortality. Anesthesiology 2011;115:963-72. http://dx.doi.org/10.1097/ALN.0b013e318233042d.
- Nair M, Kurinczuk JJ, Knight M. Ethnic variations in severe maternal morbidity in the UK – a case control study. PLOS ONE 2014;9. http://dx.doi.org/10.1371/journal.pone.0095086.
- Souza JP, Cecatti JG, Faundes A, Morais SS, Villar J, Carroli G, et al. Maternal near miss and maternal death in the World Health Organization’s 2005 global survey on maternal and perinatal health. Bull World Health Organ 2010;88:113-19. http://dx.doi.org/10.2471/BLT.08.057828.
- Kassebaum NJ, Bertozzi-Villa A, Coggeshall MS, Shackelford KA, Steiner C, Heuton KR, et al. Global, regional, and national levels and causes of maternal mortality during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:980-1004. http://dx.doi.org/10.1016/S0140-6736(14)60696-6.
- Gulmezoglu AM, Mathai M, Souza JP, d’Arcangues C, Mbizvo M. Misoprostol use in the community to reduce maternal death. Lancet 2010;376. http://dx.doi.org/10.1016/S0140-6736(10)61445-6.
- Knight M, Kurinczuk J, Spark P, Brocklehurst P. Inequalities in maternal health: a national cohort study of ethnic variation in severe maternal morbidities. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b542.
- World Health Organization . Global Database on Body Mass Index: World Health Organization; 2006 2013. http://apps.who.int/bmi/index.jsp?introPage=intro.html (accessed 11 June 2013).
- Kayem G, Kurinczuk J, Lewis G, Golightly S, Brocklehurst P, Knight M. Risk factors for progression from severe maternal morbidity to death: a national cohort study. PLOS ONE 2011;6. http://dx.doi.org/10.1371/journal.pone.0029077.
- Greenland S, Drescher K. Maximum likelihood estimation of the attributable fraction from logistic models. Biometrics 1993;49:865-72. http://dx.doi.org/10.2307/2532206.
- Adeoye IA, Onayade AA, Fatusi AO. Incidence, determinants and perinatal outcomes of near miss maternal morbidity in Ile-Ife Nigeria: a prospective case control study. BMC Pregnancy Childbirth 2013;13. http://dx.doi.org/10.1186/1471-2393-13-93.
- Clowse ME, Jamison M, Myers E, James AH. A national study of the complications of lupus in pregnancy. Am J Obstet Gynecol 2008;199:127e1-6.
- James AH, Bushnell CD, Jamison MG, Myers ER. Incidence and risk factors for stroke in pregnancy and the puerperium. Obstet Gynecol 2005;106:509-16. http://dx.doi.org/10.1097/01.AOG.0000172428.78411.b0.
- Jeng JS, Tang SC, Yip PK. Incidence and etiologies of stroke during pregnancy and puerperium as evidenced in Taiwanese women. Cerebrovasc Dis 2004;18:290-5. http://dx.doi.org/10.1159/000080354.
- Kadir RA, Lee CA, Sabin CA, Pollard D, Economides DL. Pregnancy in women with von Willebrand’s disease or factor XI deficiency. Br J Obstet Gynaecol 1998;105:314-21. http://dx.doi.org/10.1111/j.1471-0528.1998.tb10093.x.
- Villers MS, Jamison MG, De Castro LM, James AH. Morbidity associated with sickle cell disease in pregnancy. Am J Obstet Gynecol 2008;199.
- Beyond the Numbers: Reviewing Maternal Deaths and Complications to Make Pregnancy Safer. Geneva: World Health Organization; 2004.
- Oladapo OT, Sule-Odu AO, Olatunji AO, Daniel OJ. ‘Near-miss’ obstetric events and maternal deaths in Sagamu, Nigeria: a retrospective study. Reprod Health 2005;2. http://dx.doi.org/10.1186/1742-4755-2-9.
- Duley L. Maternal mortality associated with hypertensive disorders of pregnancy in Africa, Asia, Latin America and the Caribbean. Br J Obstet Gynaecol 1992;99:547-53. http://dx.doi.org/10.1111/j.1471-0528.1992.tb13818.x.
- Sachs BP, Brown DA, Driscoll SG, Schulman E, Acker D, Ransil BJ, et al. Maternal mortality in Massachusetts. Trends and prevention. N Engl J Med 1987;316:667-72. http://dx.doi.org/10.1056/NEJM198703123161105.
- Magee LA, Ornstein MP, von Dadelszen P. Fortnightly review: management of hypertension in pregnancy. BMJ 1999;318:1332-6. http://dx.doi.org/10.1136/bmj.318.7194.1332.
- Shen FR, Liu M, Zhang X, Yang W, Chen YG. Factors associated with maternal near-miss morbidity and mortality in Kowloon Hospital, Suzhou, China. Int J Gynaecol Obstet 2013;123:64-7. http://dx.doi.org/10.1016/j.ijgo.2013.06.011.
- Carroli G, Rooney C, Villar J. How effective is antenatal care in preventing maternal mortality and serious morbidity? An overview of the evidence. Paediatr Perinat Ep 2001;15:1-42. http://dx.doi.org/10.1046/j.1365-3016.2001.0150s1001.x.
- Danel I, Berg C, Johnson CH, Atrash H. Magnitude of maternal morbidity during labor and delivery: United States, 1993–1997. Am J Public Health 2003;93:631-4. http://dx.doi.org/10.2105/AJPH.93.4.631.
- Rochat RW, Koonin LM, Atrash HK, Jewett JF. Maternal mortality in the United States: report from the Maternal Mortality Collaborative. Obstet Gynecol 1988;72:91-7.
- Steinberg WM, Farine D. Maternal mortality in Ontario from 1970 to 1980. Obstet Gynecol 1985;66:510-12.
- Montan S. Increased risk in the elderly parturient. Curr Opin Obstet Gynecol 2007;19:110-12. http://dx.doi.org/10.1097/GCO.0b013e3280825603.
- Tackling Health Inequalities: 2007 Status Report on the Programme for Action. London: Department of Health; 2008.
- Hollowell J, Kurinczuk JJ, Brocklehurst P, Grey R. Social and ethnic inequalities in infant mortality: a perspective from the United Kingdom. Semin Perinatol 2011;35:240-4. http://dx.doi.org/10.1053/j.semperi.2011.02.021.
- Fitzpatrick KE, Kurinczuk JJ, Alfirevic Z, Spark P, Brocklehurst P, Knight M. Uterine rupture by intended mode of delivery in the UK: a national case–control study. PLOS Med 2012;9. http://dx.doi.org/10.1371/journal.pmed.1001184.
- Lindquist A, Knight M, Kurinczuk JJ. Variation in severe maternal morbidity according to socioeconomic position: a UK national case–control study. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2013-002742.
- Office for National Statistics . The National Statistics Socio-Economic Classification (NS-SEC Rebased on the SOC2010) n.d. www.ons.gov.uk/ons/guide-method/classifications/current-standard-classifications/soc2010/soc2010-volume-3-ns-sec--rebased-on-soc2010--user-manual/index.html (accessed 5 March 2015).
- Moser K, Stanfield KM, Leon DA. Birthweight and gestational age by ethnic group, England and Wales 2005: introducing new data on births. Health Stat Q 2008;39:22-31.
- Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol 1999;28:964-74. http://dx.doi.org/10.1093/ije/28.5.964.
- Vital Events Reference Tables 2013. Edinburgh: General Register Office for Scotland; 2014.
- Registrar General Annual Report 2013. Belfast: NISRA; 2014.
- NICE Clinical Guideline 67. Lipid Modification: Cardiovascular Risk Assessment and the Modification of Blood Lipids for the Primary and Secondary Prevention of Cardiovascular Disease. London: NICE; 2010.
- CG110: Pregnancy and Complex Social Factors: A Model for Service Provision for Pregnant Women with Complex Social Factors. London: NICE; 2010.
- Pattinson RC, Say L, Makin JD, Bastos MH. Critical incident audit and feedback to improve perinatal and maternal mortality and morbidity. Cochrane Database Syst Rev 2005;4. http://dx.doi.org/10.1002/14651858.cd002961.pub2.
- Improving Patient Safety: Risk Management for Maternity and Gynaecology (Clinical Governance Advice No. 2). London: RCOG; 2009.
- The AGREE Next Steps Consortium . Appraisal of Guidelines for Research &Amp; Evaluation II (AGREE II) Instrument 2009. www.agreetrust.org/wp-content/uploads/2013/10/AGREE-II-Users-Manual-and-23-item-Instrument_2009_UPDATE_2013.pdf (accessed 13 April 2016).
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: University of Kent; 2014.
- National Institute for Health and Care Excellence . CG62: Antenatal Care 2008 n.d. www.nice.org.uk/guidance/cg62 (accessed 15 April 2014).
- Royal College of Obstetricians and Gynaecologists . Green-Top Guideline 52: Postpartum Haemorrhage, Prevention and Management 2011. www.rcog.org.uk/womens-health/clinical-guidance/prevention-and-management-postpartum-haemorrhage-green-top-52 (accessed 12 May 2016).
- Maternity Services in England. London: The Stationery Office; 2013.
- Gasem T, Al Jama FE, Burshaid S, Rahman J, Al Suleiman SA, Rahman MS. Maternal and fetal outcome of pregnancy complicated by HELLP syndrome. J Matern Fetal Neonatal Med 2009;22:1140-3. http://dx.doi.org/10.3109/14767050903019627.
- Zwart JJ, Richters JM, Ory F, de Vries JIP, Bloemenkamp KWM, van Roosmalen J. Uterine rupture in the Netherlands: a nationwide population-based cohort study. BJOG 2009;116:1069-78. http://dx.doi.org/10.1111/j.1471-0528.2009.02136.x.
- Olde E, van der Hart O, Kleber R, van Son M. Posttraumatic stress following childbirth: a review. Clin Psychol Rev 2006;26:1-16. http://dx.doi.org/10.1016/j.cpr.2005.07.002.
- Goldbeck-Wood S. Post-traumatic stress disorder may follow childbirth. BMJ 1996;313. http://dx.doi.org/10.1136/bmj.313.7060.774.
- Redshaw M, Henderson J. Safely Delivered: A National Survey of Women’s Experience of Maternity Care 2014. Oxford: National Perinatal Epidemiology Unit; 2015.
- Ware JE, Turner-Bowker DM, Kosinkshi M, Gandek B. How to Score Version 2 of the SF-12 Health Survey. Lincoln, RI: QualityMetric; 2002.
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983;67:361-70. http://dx.doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. J Trauma Stress 1993;6:459-73. http://dx.doi.org/10.1002/jts.2490060405.
- Ayers S, Pickering AD. Do women get posttraumatic stress disorder as a result of childbirth? A prospective study of incidence. Birth 2001;28:111-18. http://dx.doi.org/10.1046/j.1523-536X.2001.00111.x.
- Psychiatric Morbidity among Adults living in Private Households, 2000: Summary Report. London: ONS; 2000.
- O’Hara MW, Wisner KL. Perinatal mental illness: definition, description and aetiology. Best Pract Res Clin Obstet Gynaecol 2014;28:3-12. http://dx.doi.org/10.1016/j.bpobgyn.2013.09.002.
- Department of Health . Complaints Figures Published by the Information Centre 2010. www.dh.gov.uk/en/MediaCentre/Statements/DH_118993 (accessed 10 October 2011).
- Equity and Excellence: Liberating the NHS. London: Department of Health; 2010.
- 360 Degree Feedback on Health Works End of Life Care Pledge and Design Event. London: GC Associates; 2011.
- NHS Institute for Innovation and Improvement . Experience Based Design 2011. www.institute.nhs.uk/quality_and_value/introduction/experience_based_design.html (accessed 11 October 2011).
- Bate P, Robert G. Towards more user-centric OD. Lessons from the field of experience-based design and a case study. J Appl Behav Sci 2007;43:41-66. http://dx.doi.org/10.1177/0021886306297014.
- Bate P, Robert G. Experience-based design: from redesigning the system around the patient to co-designing services with the patient. Qual Saf Health Care 2006;15:307-10. http://dx.doi.org/10.1136/qshc.2005.016527.
- Merriam SB. Qualitative Research And Case Study Applications In Education. San Francisco, CA: Jossey-Bass Publishers; 1998.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. http://dx.doi.org/10.1191/1478088706qp063oa.
- NHS . NHS Jobs n.d. www.jobs.nhs.uk (accessed May 2016).
- Cheshire A, Ridge D. Experience Led Commissioning for End of Life Care: Final Evaluation Report. London: University of Westminster; 2012.
- Smith J, Porter A, Shaw S, Rosen R, Blunt I, Mays N. Commissioning High-Quality Care for People with Long-Term Conditions. London: Nuffield Trust; 2013.
- Knight M. INOSS . The International Network of Obstetric Survey Systems (INOSS): benefits of multi-country studies of severe and uncommon maternal morbidities. Acta Obstet Gynecol Scand 2014;93:127-31. http://dx.doi.org/10.1111/aogs.12316.
- Acosta CD, Kurinczuk JJ, Lucas DN, Tuffnell DJ, Sellers S, Knight M, et al. Severe maternal sepsis in the UK, 2011–2012: a national case–control study. PLOS Med 2014;11. http://dx.doi.org/10.1371/journal.pmed.1001672.
- Fitzpatrick KE, Sellers S, Spark P, Kurinczuk JJ, Brocklehurst P, Knight M. The management and outcomes of placenta accreta, increta, and percreta in the UK: a population-based descriptive study. BJOG 2014;121:62-71. http://dx.doi.org/10.1111/1471-0528.12405.
- Brocklehurst P, Hardy P, Hollowell J, Linsell L, Macfarlane A, . Birthplace in England Collaborative Group . Perinatal and maternal outcomes by planned place of birth for healthy women with low risk pregnancies: the Birthplace in England national prospective cohort study. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d7400.
- National Institute for Health and Care Excellence . Intrapartum Care for Healthy Women and Their Babies 2014 n.d. www.nice.org.uk/guidance/cg190 (accessed 13 May 2015).
- Fertility Treatment in 2012: Trends and Figures. London: Human Fertilisation and Embryology Authority; 2014.
- NHS England . Patient Safety Alert: Resources to Support the Prompt Recognition of Sepsis and the Rapid Initiation of Treatment 2014. www.england.nhs.uk/wp-content/uploads/2014/09/psa-sepsis.pdf (accessed 11 September 2014).
- Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006;34:1589-96. http://dx.doi.org/10.1097/01.CCM.0000217961.75225.E9.
- The UK Sepsis Trust . Clinical Tools 2013. http://sepsistrust.org/clinical-toolkit/ (accessed 2 July 2014).
- Altman D, Carroli G, Duley L, Farrell B, Moodley J, Neilson J, et al. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet 2002;359:1877-90. http://dx.doi.org/10.1016/S0140-6736(02)08778-0.
- National Institute for Health and Care Excellence . CG107: Hypertension in Pregnancy 2010. www.nice.org.uk/guidance/CG107 (accessed 13 May 2015).
- Green-top Guideline 27: Placenta Praevia, Placenta Praevia Accreta and Vasa Praevia: Diagnosis and Management. London: RCOG; 2011.
- National Institute for Health and Care Excellence . CG132: Caesarean Section 2011. www.nice.org.uk/guidance/CG132 (accessed 13 May 2015).
- Hinton L. Saving Lives Improving Mothers’ Care Lay Summary 2014. Oxford: National Perinatal Epidemiology Unit; 2014.
- Nair M, Kurinczuk JJ, Brocklehurst P, Sellers S, Lewis G, Knight M. Factors associated with maternal death from direct pregnancy complications: a UK national case–control study. BJOG 2015;122:653-62. http://dx.doi.org/10.1111/1471-0528.13279.
- Shah A, Mohamed-Ahmed O, McClymont C, Knight M. Conditions triggering local incident reviews in UK hospital maternity units: a national survey. JRSM Open 2014;5. http://dx.doi.org/10.1177/2054270414528898.
- Colmorn LB, Petersen KB, Jakobsson M, Lindqvist PG, Klungsoyr K, Kallen K, et al. The Nordic Obstetric Surveillance Study: a study of complete uterine rupture, abnormally invasive placenta, peripartum hysterectomy, and severe blood loss at delivery. Acta Obstet Gynecol Scand 2015;94:734-44. http://dx.doi.org/10.1111/aogs.12639.
- Thurn L, Lindqvist PG, Jakobsson M, Colmorn LB, Klungsoyr K, Bjarnadottir RI, et al. Abnormally invasive placenta-prevalence, risk factors and antenatal suspicion: results from a large population-based pregnancy cohort study in the Nordic countries [published online ahead of print 29 July 2015]. BJOG 2015. http://dx.doi.org/10.1111/1471-0528.13547.
- Bastos MH, Furuta M, Small R, McKenzie-McHarg K, Bick D. Debriefing interventions for the prevention of psychological trauma in women following childbirth. Cochrane Database Syst Rev 2015;4. http://dx.doi.org/10.1002/14651858.cd007194.pub2.
- Holmes EA, James EL, Kilford EJ, Deeprose C. Key steps in developing a cognitive vaccine against traumatic flashbacks: visuospatial Tetris versus verbal Pub Quiz. PLOS ONE 2010;5. http://dx.doi.org/10.1371/journal.pone.0013706.
List of abbreviations
- A&E
- accident and emergency
- AFE
- amniotic fluid embolism
- AFLP
- acute fatty liver of pregnancy
- AGREE
- Appraisal of Guidelines for Research and Evaluation
- AMND
- Aberdeen Maternity and Neonatal Databank
- aOR
- adjusted odds ratio
- BMI
- body mass index
- CCG
- Clinical Commissioning Group
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CMACE
- Centre for Maternal and Child Enquiries
- CMP
- Case Mix Programme
- CNST
- Clinical Negligence Scheme for Trusts
- ELC
- experience-led commissioning
- ELLP
- elevated liver enzymes and low platelets syndrome
- EQ-5D-3L
- European Quality of Life-5 Dimensions-3 Level
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HDU
- high-dependency unit
- HELLP
- haemolysis, elevated liver enzymes and low platelets syndrome
- i.v.
- intravenous
- ICD-9
- International Classification of Disease, Ninth Edition
- ICER
- incremental cost-effectiveness ratio
- ICNARC
- Intensive Care National Audit & Research Centre
- ICU
- intensive care unit
- INOSS
- International Network of Obstetric Survey Systems
- ITU
- intensive therapy unit
- IVF
- in vitro fertilisation
- LR-test
- likelihood ratio test
- MBRRACE-UK
- Mothers and Babies: Reducing Risk through Audits and Confidential Enquiries across the UK
- MEPS
- Medical Expenditure Panel Survey
- NICE
- National Institute for Health and Care Excellence
- NICU
- neonatal intensive care unit
- NIHR
- National Institute for Health Research
- ONS
- Office for National Statistics
- OR
- odds ratio
- PAF
- population attributable fraction
- PPH
- postpartum haemorrhage
- PSA
- probabilistic sensitivity analysis
- PSS-SR
- Post-traumatic Stress Disorder Symptom Scale Self-Report version
- PTSD
- post-traumatic stress disorder
- QALY
- quality-adjusted life-year
- RCOG
- Royal College of Obstetricians and Gynaecologists
- RCT
- randomised controlled trial
- rFVIIa
- recombinant factor VIIa
- RR
- risk ratio
- SE
- standard error
- SF-12v2
- Short Form questionnaire-12 items version 2
- SIRS
- systemic inflammatory response syndrome
- UKOSS
- UK Obstetric Surveillance System
- uOR
- unadjusted odds ratio
- UTI
- urinary tract infection
- WTP
- willingness to pay