Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0407-10070. The contractual start date was in December 2008. The final report began editorial review in September 2014 and was accepted for publication in August 2015. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Ashley Blom reports grants from National Institute for Health research during the conduct of the study; and grants from Stryker, Orthimo and Azellon outside the programme.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Blom et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background to the RESTORE programme
Osteoarthritis of the hip and knee joints
Osteoarthritis is the most common form of joint disease and causes significant pain and disability in about 10% of people aged > 55 years in the UK. 1 The joints most frequently affected include joints in the hands, feet and spine, but osteoarthritis of the hips and knees is particularly likely to cause chronic pain and severe physical disability. These two conditions result in a huge health burden worldwide,2 particularly among older people, and are the main reason for the increasing utilisation of hip and knee joint replacements. 3
The severity of osteoarthritis of the hips or knees varies considerably. It is often relatively mild, only causing modest, variable discomfort and some restriction of activities without disrupting the person’s life in a major way. However, a significant minority of those who have these conditions develop more severe, progressive symptoms, which may result in their seeking professional help. Only about 50% of those with severe symptomatic lower limb osteoarthritis seek conventional medical help,4 some prefer to rely on help from everyday folk remedies, complementary or alternative medicine interventions, and many seek no help at all, considering their aches and pains and disability as an inevitable part of the ageing process and something that are not treatable medical problems. 5,6 However, the prevalence of these diseases is so high, that while many do not seek professional help, there are enough that do to result in a huge workload for general practitioners (GPs), rheumatologists and physical therapists. 7 In addition, osteoarthritis is increasing in prevalence as our population gets older and the number of people with a high body mass index (BMI) increases,8 and it is the major reason for lower limb joint replacement; thus, is a massive issue for orthopaedic service provision.
Who gets osteoarthritis of the hip and knee?
Osteoarthritis of the hip and knee are strongly age-related diseases. It is unusual for people to suffer from these before the age of 45 years and the prevalence then increases sharply with increasing age. 8 The three other main risk factors for osteoarthritis of the hip and knee are family history/genetic predisposition, previous injury to the joint and high BMI.
Given that age and high BMI are major risk factors for lower limb osteoarthritis, comorbidities related to age and obesity are very common in people receiving joint replacement for osteoarthritis. 9 Cardiovascular problems, sensory deficits (such as reduced sight or hearing) and diabetes are particularly common problems. 10
Treatment options for osteoarthritis of the hip and knee
In the UK, those seeking help for lower limb osteoarthritis generally start with their GP and most of the management of hip and knee disease occurs in primary care. There is no known treatment able to alter the progression of osteoarthritis, or anything that can cause an improvement in joint structure – there is no ‘cure’ available. However, there is a wide range of both non-surgical and surgical interventions that can reduce pain and improve function. Conservative options available through primary care include education and access to self-help packages, including behavioural change; local or systemic drug therapies; physical therapies; and orthotics. National Institute for Health and Care Excellence (NICE) and international guidelines recommend that everyone should be offered simple options to begin with, while recognising that this will be insufficient to manage the pain and disability in some. 11–14
If pharmacological and conservative treatments provide inadequate relief of symptoms, then total joint replacement is commonly recommended. The clinical effectiveness and cost-effectiveness of total joint replacement surgery as a treatment option for patients with advanced osteoarthritis is well established. 15,16 One of the challenges for patients and GPs dealing with osteoarthritis is who to refer to the surgeon and when surgical intervention is appropriate. There are no clear guidelines as to the severity of pain or disability that warrants moving from conservative management to surgery. 17 This difficulty is compounded by the knowledge that surgery is a dangerous, irreversible step that does not result in adequate relief of symptoms and restoration of function in everyone who undergoes it. 18
Total hip and knee replacement
Total hip and knee replacements are undertaken to relieve pain and improve function in people with advanced osteoarthritis of the hip or knee joint, whose symptoms are not controlled by conservative treatment. They are two of the most common elective NHS procedures with 75,366 primary total hip replacements (THRs) and 76,497 primary total knee replacements (TKRs) performed in England and Wales between April 2012 and March 2013. 3 The main disease resulting in this need for joint replacement is osteoarthritis, with about 91% of total hip joint replacements and 98% of total knee joint replacements being carried out for this indication. In England, the number of THRs conducted annually increased by 54% between 2001 and 2011 and the number of TKRs by 108% over the same period. 19,20 Projections for total hip and knee replacement provision for the UK and the USA have largely underestimated current need. 21,22 This continued increase in provision adds to the burden of health-care budgets to cope with financial pressures while keeping waiting lists to a minimum. 23 Therefore, it is important to ensure that any technological innovations in the management of total joint replacement in relation to decreasing pain and increasing function are a good use of medical and societal resources.
Patients’ experiences of hip and knee osteoarthritis, and of joint replacement
Qualitative studies have explored patients’ experiences of living with and managing osteoarthritis,5,24–27 decision-making about joint replacement,28 patient pathways to surgery4,29 and patient satisfaction with outcomes of joint replacement. 28,30 This body of research has highlighted the impact of lower limb osteoarthritis on individuals and the possibility that patients’ priorities are not always uppermost in current planning and delivery of treatments, including joint replacement. Research has also highlighted the importance of patient expectations in relation to the outcomes after joint replacement,31,32 the importance of what is said to patients about their treatment options5 and the fact that patients may not be satisfied with the outcome of their joint replacements, even if they tell the surgeon that they are doing well. 30 It is clear that we need to understand much more about what patients think about joint replacement and its outcomes, and improve the evidence base in this area. We also need to know the patient experience of joint replacement from pre-operative care to postoperative recovery.
The outcomes of hip and knee joint replacement
Traditionally, the success of a total hip or knee replacement was judged predominantly by the length of time the implant remained in situ. 33 The key issues for surgeons and prosthesis-producing companies were seen to be the design and fixation of a prosthesis that would last for ≥ 10 years, precluding the need for complex revision surgery. 34 However, owing to advances in surgical technique and prosthetics design, joint replacement now has good survivorship and post-surgical complication rates are low. 35,36 Over 95% of hip and knee replacements remain unrevised at 9 years after surgery. 3 Severe adverse events are uncommon, but complications such as dislocation,37 infection,38,39 fracture,40 thromboembolism41 and neurovascular damage42 may occur and can significantly impact on pain, function and quality of life (QoL). There is also a small mortality risk. 43,44
Attention has turned in recent years towards patient-reported outcomes in addition to technical outcomes. 45 Studies using patient-reported outcome measures (PROMs) suggest that mean pain and function scores after initial recovery from surgery are generally good. 46–48 However a significant minority of patients have persistent moderate or severe long-term pain and functional difficulties after joint replacement despite what appears to be otherwise successful surgery. 49–53 This fact has been obscured by the reporting of mean changes and averages for groups, ignoring the fact that although most people improve after hip or knee joint replacement, some improve more than others and some get little or no improvement or are worse after surgery than they were before it. 54 Pain and functional problems can impact on a patient’s usual activities and those suffering from chronic long-term pain may be unable to return to work or leisure activities, and require a higher level of care from relatives and friends. Long-term pain after joint replacement imposes a burden on service use and costs to the NHS for those seeking relief, and to patients, carers and society as a whole. It is a problem that has received little research or service attention in the past. 55,56
Patients living with osteoarthritis see function as key to their experience of illness. 24,57 Restoring function, alongside alleviation of pain, is a main aim of joint replacement. As noted, application of instruments such as the Western Ontario and McMaster Universities Arthritis Index (WOMAC) has suggested that there is an improvement in function in most (but not all) cases. 46,58,59 But the WOMAC, a self-report measure, like most other self-report measures of function, has no sound theoretical basis. 60 There are many alternatives, including observed measures of function, and more objective techniques such as pedometry or accelerometry,61 but very few data are available on what the most appropriate means of assessing function in the context of joint replacement might be.
Management of acute and chronic pain after joint replacement
Acute perioperative pain
The management of acute postoperative pain poses a significant challenge in all surgical specialties. It has been estimated that approximately 40% of surgical patients experience moderate or severe acute postoperative pain. 62 Poor management of pain can be distressing for patients and can significantly delay ambulation, lengthen hospital stay, increase the number of unanticipated hospital admissions and contribute to poor mental health. 63–65 Furthermore, severe acute postoperative pain is a risk factor for the development of chronic post-surgical pain. 66
Good perioperative pain management after joint replacement surgery allows early mobilisation and rehabilitation,67 which minimises risks of complications such as deep-vein thrombosis, pulmonary embolus, muscle and joint contractures, physical deconditioning, and chest infection. 68 Early mobilisation also facilitates early discharge with associated cost savings. 69 However, acute postoperative pain after joint replacement is often poorly managed70 and many methods of achieving perioperative pain relief, such as spinal or epidural anaesthetics and the use of opioids, can preclude early mobilisation. 71,72 Although there has been much interest in the management of perioperative pain, research has largely looked at the first 24–48 hours after surgery alone and the likely longer-term effects of good perioperative pain control have not been explored.
Chronic post-surgical pain
Chronic post-surgical pain is a significant problem after different types of surgery. It has been defined by the International Association for the Study of Pain (IASP) as ‘pain that has developed after surgery, and been present for at least three months, which is beyond the time for normal healing’. 73 Pain can be defined as chronic post-surgical pain if four criteria are fulfilled:74
-
pain developing after a surgical procedure
-
pain present for at least 2 months
-
other causes of pain have been excluded
-
the pain is not a continuation of a pre-existing condition.
However, application of such criteria is difficult for procedures such as joint replacement, for which the primary aim is relief of pre-existing pain and it can be very difficult to be sure whether or not the chronic problem is a continuation of a pre-existing one (e.g. through pain sensitisation). 75
There is considerable variation in the reported prevalence of chronic post-surgical pain, but it is clear that somewhere between 10% and 50% of patients experience the problem after different forms of surgery, including breast surgery, vasectomy, hernia repair and cardiac surgery. 76
Perioperative medical care and rehabilitation
In the UK, if a patient is considered for joint replacement for their osteoarthritis and they agree to the procedure being carried out, they will generally be asked to attend a pre-operative clinic for assessment of their general medical status and to make sure that the surgical team is clear on what operation will be performed. The medical screening at this clinic usually involves looking for major health problems that might preclude anaesthesia or surgery. The patient is also likely to be given some information about joint replacement, but this does not usually indicate what they should expect, in terms of pain and disability, after the immediate postoperative period. It is unusual for any action to be taken as a result of this screening, other than delaying the surgery if a major problem is uncovered.
Postoperatively, attention is paid to the wound, pain control and general health, and patients are usually encouraged to become active as soon as possible after the operation, with the help of a physiotherapist. In the UK, it is usual for patients to be discharged from hospital after about 3–5 days. Physiotherapists are almost always involved in postoperative mobilisation and provide general advice on what patients should do when they get home. The ability to climb a step or stair is often used as a criterion of a patient having reached a satisfactory functional status for hospital discharge. In contrast with some other countries, such as Germany, further rehabilitation is not generally available to UK NHS patients after their hip of knee joint replacement. Furthermore, there are no agreed standards or guidelines as to what should or should not be done perioperatively to optimise outcomes (relief of pain and improvement of function), partly because of the deficiencies in the evidence base. Therefore, it is not surprising that in the UK service provision before and after joint replacement is perceived to vary in level of provision and content. 77,78 Supply of aids and appliances as well as other occupational therapies are widely offered during the hospital stay. 79
The provision of adequate information and formal rehabilitation are potentially important adjuncts to joint replacement surgery that might both reduce the length of stay in hospitals and improve patient outcomes.
The rehabilitation process aims to support the patient in regaining pre-impairment levels of function and QoL, and reintegration into their social and personal environment. Ideally, for patients receiving joint replacement, rehabilitation should commence before surgery and be provided as appropriate throughout the different stages of recovery.
-
Pre-surgical interventions target optimisation of physical health and preparation for hospitalisation and recovery.
-
Occupational therapy and home modifications, provided pre- or postoperatively, help people perform activities of daily living (ADL) safely at home or at work.
-
Postoperative rehabilitation during the hospital stay focuses on regaining range of motion (ROM), functional independence in ADL and improving mobility.
-
Subsequent rehabilitation targets maintenance or improvements in muscle strength, joint range of movement, balance and co-ordination, mobility, and extended ADL.
According to the World Health Organization (WHO) International Classification of Functioning, Disability and Health (ICF) model, rehabilitation should be patient-centred and aim to maximise functional ability, facilitate activities and increase social participation. 80
Evidence on the effectiveness of different aspects of rehabilitation is limited. 81 Early systematic reviews considered interventions relating to pre-operative exercise,82 education83 and physiotherapy exercise. 84,85 Recommendations were limited by issues of study size and quality and, since the publication of these trials, more trials have been reported. There is no published systematic review of occupational therapy interventions in joint replacement.
The economic implications
In the context of scarce NHS resources, the evaluation of the cost-effectiveness of interventions to improve the outcomes of patients with osteoarthritis are increasingly pertinent. 86 Despite the well-established cost-effectiveness of providing total joint replacement surgery for advanced osteoarthritis, little is known about the long-term cost-effectiveness of interventions to improve outcomes after total joint replacement surgery. Economic evaluations of interventions targeting acute postoperative pain are generally truncated at point of hospital discharge or a few weeks after surgery. Evaluations in such studies are restricted to differences in costs of anaesthesia provided. 87–91 Other interventions focus on postoperative rehabilitation programmes but, generally, only the differences in costs in delivering treatment between arms is evaluated for the duration of the intervention. Longer-term studies to improve outcomes after surgery with a health economic evaluation have typically focused on delivery of physiotherapy treatments. 92,93 However, these have not included informal care costs and productivity losses which are needed to examine the broader impact on society. Only the latter, by including patient expenses, estimated costs beyond the health-care provider perspective. None have included informal care costs and productivity losses which would be needed to examine the broader impact on society.
Complex package of care
Ultimately, interventions within the joint replacement pathway should combine within a complex package of care with interaction between components. 94 Key areas in the development of a complex intervention relate to:
-
development
-
identifying the evidence base
-
identifying and developing theory
-
modelling process and outcomes
-
-
feasibility/piloting
-
testing procedures
-
estimating recruitment/retention
-
determining sample size
-
-
evaluation
-
assessing effectiveness
-
understanding change process
-
assessing cost-effectiveness
-
-
implementation
-
dissemination
-
surveillance and monitoring
-
long-term follow-up.
-
Development of a complex package of care requires input from diverse specialities and research methodologies. The authors of the Medical Research Council (MRC) guidelines recognise the importance of developmental studies before large-scale evaluations. 94
Overview of the ‘RESTORE’ programme
Recognising the presence of long-term pain and disability in many patients after total hip and knee replacement, we developed the REsearch STudies into the ORthopaedic Experience (RESTORE) programme of research. The aim of the RESTORE programme was to conduct research on methods to improve the experience and outcomes of people undergoing hip or knee replacement for osteoarthritis. As shown in Figure 1, the programme consisted of a series of interrelated work packages, all supported by appropriate patient and public involvement (PPI). Here we briefly overview the individual work packages.
FIGURE 1.
The RESTORE programme.

Patient and public involvement
Patient and public involvement has been a major feature of RESTORE. Meaningful patient involvement has supported all aspects of the programme. This comprised collaboration with Arthritis Care, patient representation on the RESTORE management group throughout its duration, establishment of a patient forum, employment of a PPI co-ordinator and having patient partners on each project steering group.
Since 2010, the patient forum, Patient Experience Partnership in Research (PEP-R), has supported RESTORE. Through facilitated group sessions, the patient forum provided input into refinement of patient recruitment materials, intervention development, readability of outcome assessment tools and dissemination of findings. Individual projects also had their own oversight groups, each of which included patients with an interest in joint replacement surgery to monitor the progress of the project. Patient involvement was carried out in line with INVOLVE’s guidance. We believe that this work might act as a good example of how to involve patients meaningfully and effectively in research studies in musculoskeletal disease.
Systematic literature reviews
Synthesis of evidence from previous research using systematic review methods and meta-analysis to:
-
assess the prevalence of long-term pain after total hip or knee replacement
-
identify methods used to measure chronic pain after TKR
-
identify predictors of long-term patient outcomes after total hip or knee replacement
-
evaluate the associations between comorbid conditions and patient outcomes after hip and knee replacement
-
evaluate the effectiveness of pre-surgical education and exercise interventions
-
support randomised controlled trials (RCTs) with reviews of perioperative pain management, occupational therapy and physiotherapy exercise.
Understanding the patient experience
To characterise and explore the patient pathway through total hip or knee replacement surgery in current routine NHS care.
Measuring functional outcomes in a cohort study of patients having joint replacement: the ADAPT study
In the Assessing Disability After Partial and Total joint replacement (ADAPT) longitudinal cohort study of patients with primary and revision hip and knee replacement, we compared the properties with responsiveness of a selection of commonly used outcome tools that assess function, examined how well they relate to the WHO ICF concepts, and explored the changes in the measures over time.
Randomised controlled trial of perioperative pain control: the APEX trial with full economic analysis and nested qualitative research
We examined the clinical effectiveness and cost-effectiveness of multimodal perioperative analgesia in total hip and knee replacement. The key intervention tested in the Arthroplasty Pain EXperience (APEX) RCT was an injection of local anaesthetic into the wound during total hip and knee replacement surgery to provide both short- and long-term pain relief. An economic evaluation was conducted to determine the cost-effectiveness of the intervention from a NHS and Personal Social Services (PSS) perspective. Data were also collected to allow a future economic evaluation from a societal perspective.
The APEX cohort study
Data from the APEX study provided the opportunity to assess the relationships between radiographic measures of osteoarthritis severity and patient-reported pain and function. We were also able to explore the associations between pre-operative patient factors and perioperative pain, and long-term patient outcomes. Pre-operative pressure pain thresholds (PPTs) were measured before surgery and we explored their value in predicting long-term pain after total hip and TKR.
Pain self-management: the SPIRAL study
In the Self-managing Pain In aRthritis and ArthropLasty (SPIRAL) study we conducted a pilot RCT to assess the feasibility of delivering a pain self-management course, run by Arthritis Care, to patients undergoing THR.
Occupational therapy: PROOF-THR
To assess the feasibility of occupational therapy provided before surgery, we conducted the Pilot Randomised controlled trial Of Occupational therapy For – Total Hip Replacement (PROOF-THR) pilot RCT in patients undergoing primary THR.
Physiotherapy exercise rehabilitation
We surveyed physiotherapy provision after total hip and knee replacement in large orthopaedic centres in England and Wales. We also conducted the Activity orientated REhabilitation following kNee Arthroplasty (ARENA) pilot RCT of an activity-orientated rehabilitation programme for patients undergoing primary TKR.
Economic analyses
We conducted a full economic analysis of the APEX RCT. In addition, for each of the pilot studies within the complex package of care development work stream, methods to collect resource-use data from a societal perspective were developed and assessed. These will facilitate full economic analyses in future definitive studies.
Complex package of care
Based on literature reviews, cohort and qualitative studies and RCTs we aim to provide recommendations to support the development of a complex package of care for patients receiving total hip and knee replacement.
Chapter 2 General systematic review methods: systematic reviews of long-term pain after hip and knee replacement, methods used to assess chronic pain and pre-operative predictors of long-term patient outcomes
Parts of this chapter have been reproduced from Beswick and colleagues. 18 This is an open-access article distributed under the terms of the Creative Commons Attribution Non-commercial License, which permits use, distribution, and reproduction in any medium, provided the original work is properly cited, the use is non commercial and is otherwise in compliance with the license. See: http://creativecommons.org/licenses/by-nc/2.0/ and http://creativecommons.org/licenses/by-nc/2.0/legalcode. Parts of this chapter have also been reproduced from Wylde and colleagues95 © 2013 The Authors. Arthritis Care & Research is published by Wiley Periodicals, Inc. on behalf of the American College of Rheumatology. This is an open access article under the terms of the Creative Commons Attribution NonCommercial License, which permits use, distribution, and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
Abstract
Background
We conducted systematic literature reviews on chronic pain after joint replacement, pre-operative predictors of patient outcomes and the impact of comorbidities.
Methods
Systematic reviews conducted in accordance with appropriate guidelines.
Results
About 7–23% of patients have moderate or severe pain after THR and about 10–34% after TKR.
There was extensive variation in pain outcome measures used in TKR research. Although there was an increase in use of patient-reported outcomes over time, few studies attempted to capture the incidence, character and impact of long-term pain.
Better pre-operative physical function and lower pain was associated with a better recovery in terms of joint specific pain and function. However, patients with poor physical function before surgery may have greater absolute improvement. Patients with depression before joint replacement had poorer long-term pain and functional outcomes. In patients receiving TKR, anxiety and poor general psychological health were associated with worse pain and functional outcomes. Across a broad range of BMI, patients benefited from joint replacement but those with highest levels may not achieve good functional and pain outcomes.
Although approximately 64% and 71% of patients receiving joint replacement have comorbidities, research on their relationship with long-term patient outcomes was limited. The impact of diabetes, previous heart disease and anaemia on the risk of post-surgical adverse events is recognised.
Conclusions
Systematic reviews identified the potential value of intervention before joint replacement and highlight the importance of appropriate assessment of long-term pain.
Systematic review methods
Comprehensive, systematic literature reviews are an essential prelude to developing interventions and trials. Systematic reviews aim to appraise evidence from published studies and have two broad objectives:
-
a synthesis of knowledge to guide decision-making
-
identification of deficits in the evidence base that merit further research.
A literature review can be considered systematic if the methods are sufficiently transparent and unbiased that it can be reproduced on the basis of:
-
sources of literature
-
how it was searched
-
why a study was included or excluded
-
which data were analysed and how
-
how study quality was assessed.
Numerous reviews of varying quality have been published on the care of patients receiving total hip or knee replacement. The first step in our systematic review was to identify previous systematic reviews. Reviews were updated or started anew depending on our assessment of the systematic nature of the methods used.
In the context of the RESTORE programme, we conducted systematic literature reviews of both cohort studies and evaluations of interventions in RCTs.
Reviews of cohort studies relate to assessment of chronic pain after hip or knee replacement, pre-operative determinants of patient centred outcomes, and comorbid conditions and patient outcomes after hip and knee replacement.
Reviews of interventions relate to pre-surgical exercise and education interventions, perioperative local anaesthetic infiltration, physiotherapy exercise after TKR, and occupational therapy in THR.
Methods and guidelines
Systematic reviews were conducted using methods based on those described in the Cochrane handbook of systematic reviews. 96 Guidelines appropriate to the study designs being reviewed were adhered to. In reviews of RCTs we referred to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines97 and in review of observational studies to Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines. 98 Composite PRISMA and MOOSE checklists for the eight systematic reviews conducted in the RESTORE programme are included in Appendices 1 and 2, respectively.
Each review was conducted following general structured methods as outlined here. Specific methods for each review are detailed in a separate table following the participants, interventions, comparisons, outcomes, and study design (PICOS) construct. 99
Identification of studies
Studies were identified by searching appropriate online databases with tailored search strategies. MEDLINE and EMBASE electronic databases were searched via the Ovid SP platform. Additionally, PsycINFO was searched via Ovid SP, Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCOhost and The Cochrane Library databases were searched if considered relevant to the topic. For the review of occupational therapy, Allied and Complementary Medicine Database (AMED) was searched via Ovid SP, Physiotherapy Evidence Database (PEDro), Education Resources Information Center (ERIC) via ProQuest, Center for International Rehabilitation Research Information and Exchange (CIRRIE) and OTDbase were also searched.
Search strategies as applied in MEDLINE and used in appropriate combinations are shown in Appendix 3. Searches were supplemented with hand-searching of reference lists from trials and review articles. Key articles were tracked in ISI Web of Science.
Criteria for including studies
Studies were included according to specific criteria described for each review. These covered patient inclusion, interventions, outcome measures and study type.
In evaluating the clinical effectiveness of interventions, we included studies that were RCTs with randomisation either at individual or cluster level. Because many relevant studies are not recent and conducted in diverse health-care settings, we also included studies with a quasi-randomised design (e.g. alternate allocation) but with no specific evidence of bias owing to allocation method. There were no language restrictions with the exception for our reviews of cohort studies looking at pre-surgical predictors and comorbidities.
Study selection
Bibliographic details of the articles identified were exported and managed in EndNote (Thomson Reuters, CA, USA) databases, where duplicates were removed. Titles and abstracts of articles were screened by one or two reviewers to exclude studies that were clearly not relevant. As recommended in the Cochrane handbook, studies were classified as potentially relevant if a reviewer had any doubts about relevance on the basis of title and abstract.
A final reading of potentially relevant articles and study selection based on defined eligibility criteria were carried out by two reviewers with further input of a relevant health-care professional, if required. Full papers for all potentially relevant studies were obtained electronically, from local libraries or through interlibrary loans. The progress of each review was recorded as a flow diagram.
Studies reported only as abstracts or for which we were unable to acquire full-text copies using interlibrary loans or e-mail contact with authors were excluded from the analyses. Reasons for exclusion at this stage were summarised in individual systematic review flow diagrams (see Figures 2, 4, 7, 8, 21, 37, 42 and 48).
Data extraction
Data were extracted from each paper by two reviewers or by one reviewer with data checked against source material by a second. For reviews of predictors and comorbidities, about 25% of articles were checked against source material by a second reviewer. Authors, colleagues and family helped to translate and interpret studies not published in English.
In reviews of observational cohort studies with no intervention reported, we extracted data relating to how representative the cohort is of the general population; variables and outcomes collected; methods of statistical analyses; as well as country, dates of data collection, and summaries of patient characteristics.
Data extracted from intervention studies included when and where the study was conducted; patient characteristics (mean age, percentage male/female, indication); inclusion criteria; description of interventions; timing, duration; health-care professionals providing care; and losses to follow-up and reasons. Results were recorded on piloted data extraction forms and Microsoft Excel® 2007 (Microsoft Corporation, Redmond, WA, USA) spreadsheets. If published reports did not contain the required data, authors were contacted. We also asked if any outcomes not reported in publications had been collected. If authors had provided information to other reviewers, then these data were included in our analyses and acknowledged appropriately. For continuous variables, means and standard deviations (SDs) were extracted. If outcomes were reported as means and confidence intervals (CIs), or medians and interquartile ranges (IQRs), appropriate conversions and estimations were used. 96
Data analysis
Outcome data were extracted to Microsoft Excel spreadsheets and analysed in Stata 12 (StataCorp LP, College Station, TX, USA) or RevMan 5 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark).
Studies were categorised according to our pre-specified criteria. If a sufficient number of intervention studies reported common outcomes, data were combined in meta-analyses. Combined outcomes were summarised as average mean difference (MD) if the outcome used a common measurement scale (e.g. length of hospital stay) or as average standardised mean difference (SMD) if different methods were used to assess a particular outcome. Generally we combined outcomes using random-effects meta-analysis96,100 and reported 95% CIs, p-values for the magnitude of effect and tests of heterogeneity. When possible, results are shown as forest plots.
When two interventions were reported with a shared control group, in meta-analysis the number for controls was halved.
Quality assessment
For observational cohort studies our quality assessment was based on the diversity of centres (registry, multiple centres, single centre or surgeon), and losses to follow-up.
For RCTs, the Cochrane risk-of-bias table was used to assess study quality. Bias was assessed independently by two reviewers or assessed by one and checked by a second, with disagreements resolved by discussion. Risk of bias was based on random sequence generation, allocation concealment, blinding of outcome assessment, completeness of outcome data, selective reporting and other potential sources. In the context of the studies we reviewed, completeness of outcome data collection and blinding of outcome assessment were considered the key issues relating to risk of bias.
Systematic review of the severity of long-term pain after total hip or knee replacement
Background
We aimed to identify studies reporting the proportion of people with significant long-term pain after total hip or knee replacement. Eligible studies reported prospective follow-up of consecutive, unselected patients who were representative of the primary total hip or knee replacement population. We limited follow-up to 3 months to 5 years as this reflects the time when pain48 and prosthesis-related outcomes101 can be considered optimal.
Methods
| General methods | As described in Systematic review methods |
| Databases and dates | MEDLINE and EMBASE from inception to 31 January 2011. Citations of key articles in ISI Web of Science and reference lists |
| Search strategy | Total hip or knee replacement/osteoarthritis/epidemiological study design/PROM. MEDLINE search strategy based on terms in Appendix 3 |
| Study design | Prospective follow-up of consecutive, unselected patients |
| Patients | Patients with primary total hip or knee replacement |
| Follow-up | 3 months to 5 years |
| Data extraction | Indication, pain outcome, baseline dates, country, study design, follow-up, how group selected, age, number of patients recruited, number who died and the number lost to follow-up |
| Outcomes | Patient-reported pain in the operated knee. Proportions of people with different severities of pain at follow-up were summarised as:
|
| Quality assessment | Representativeness of study population. Losses to follow-up |
Results
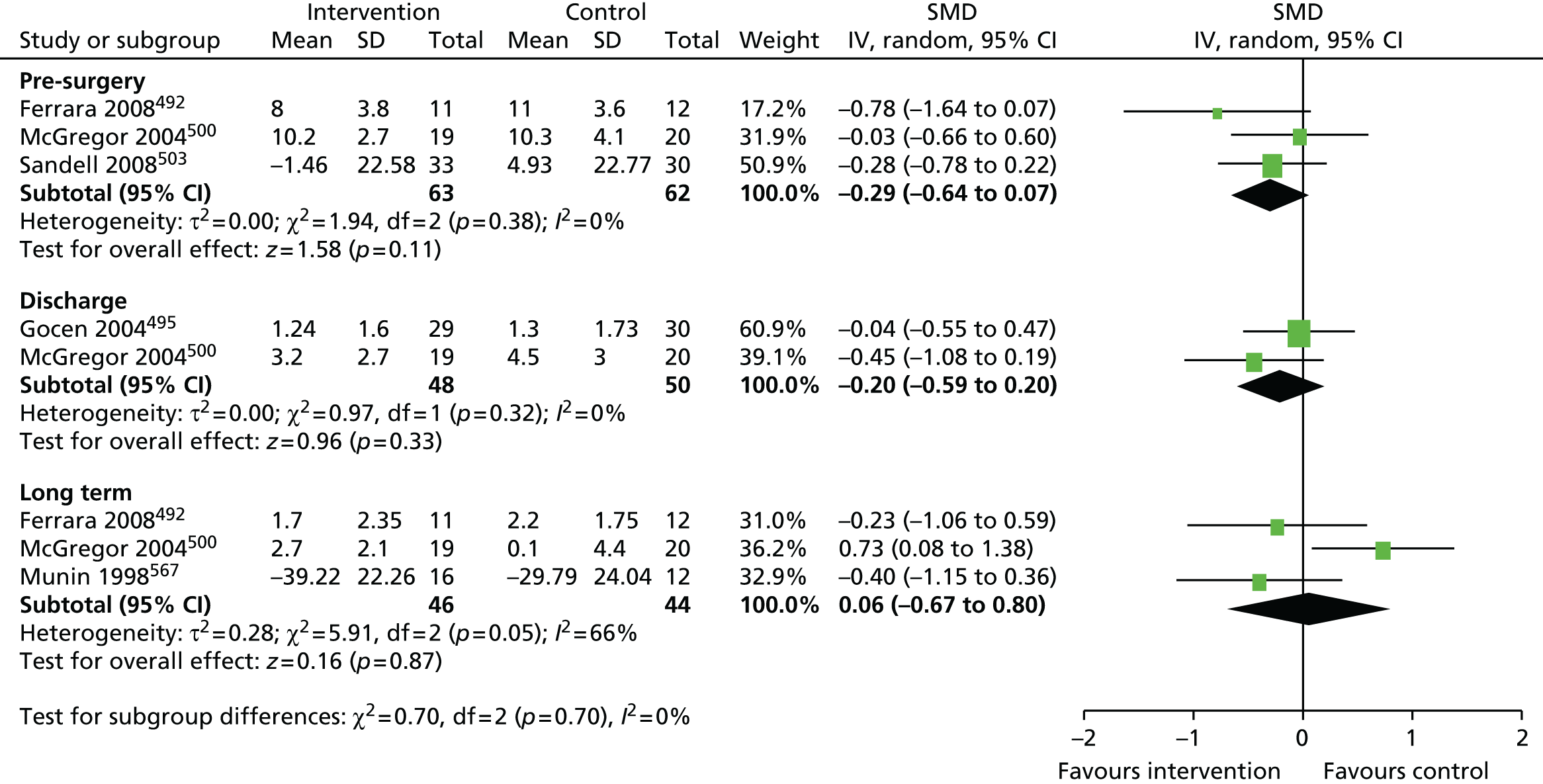
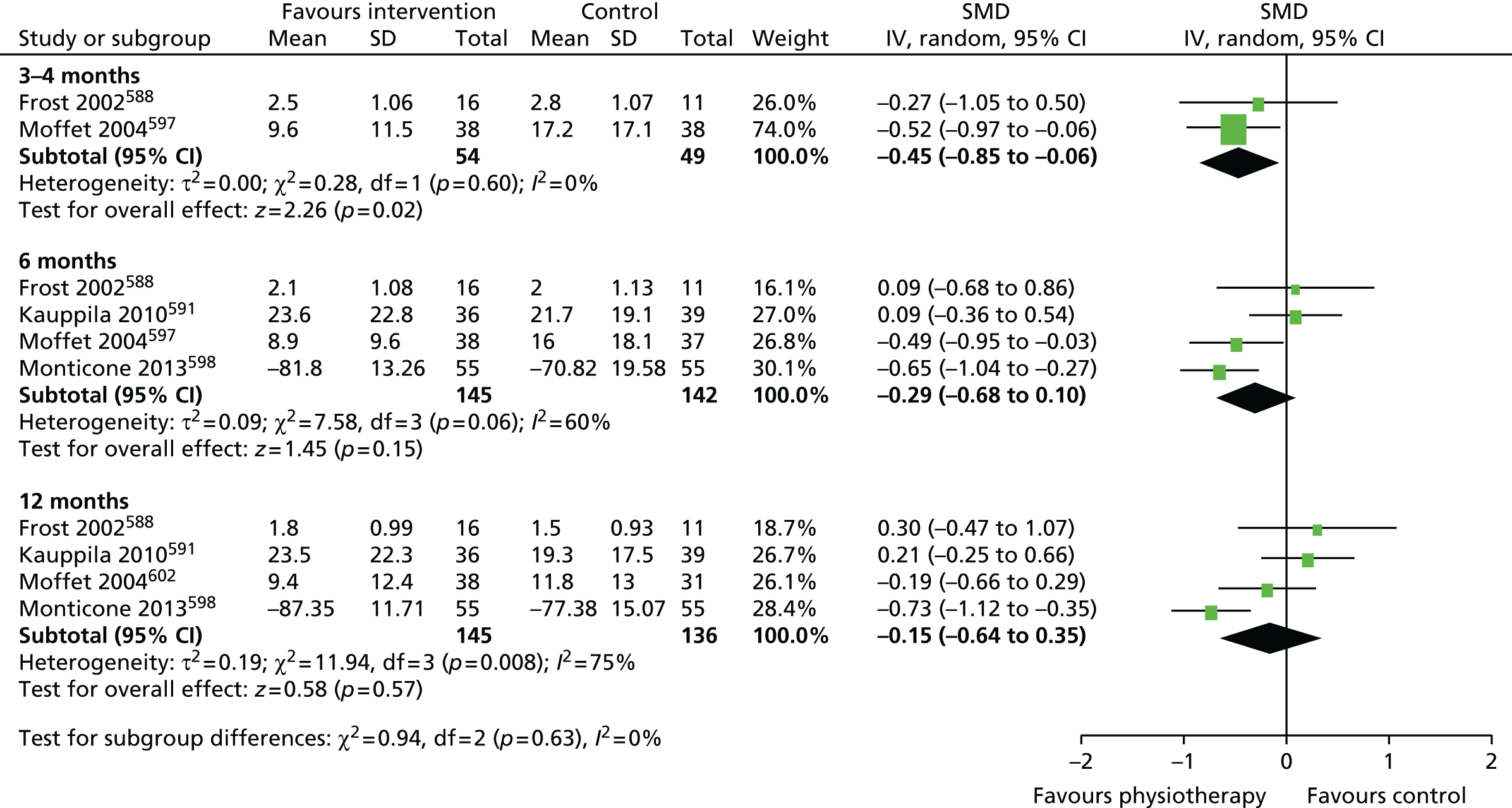
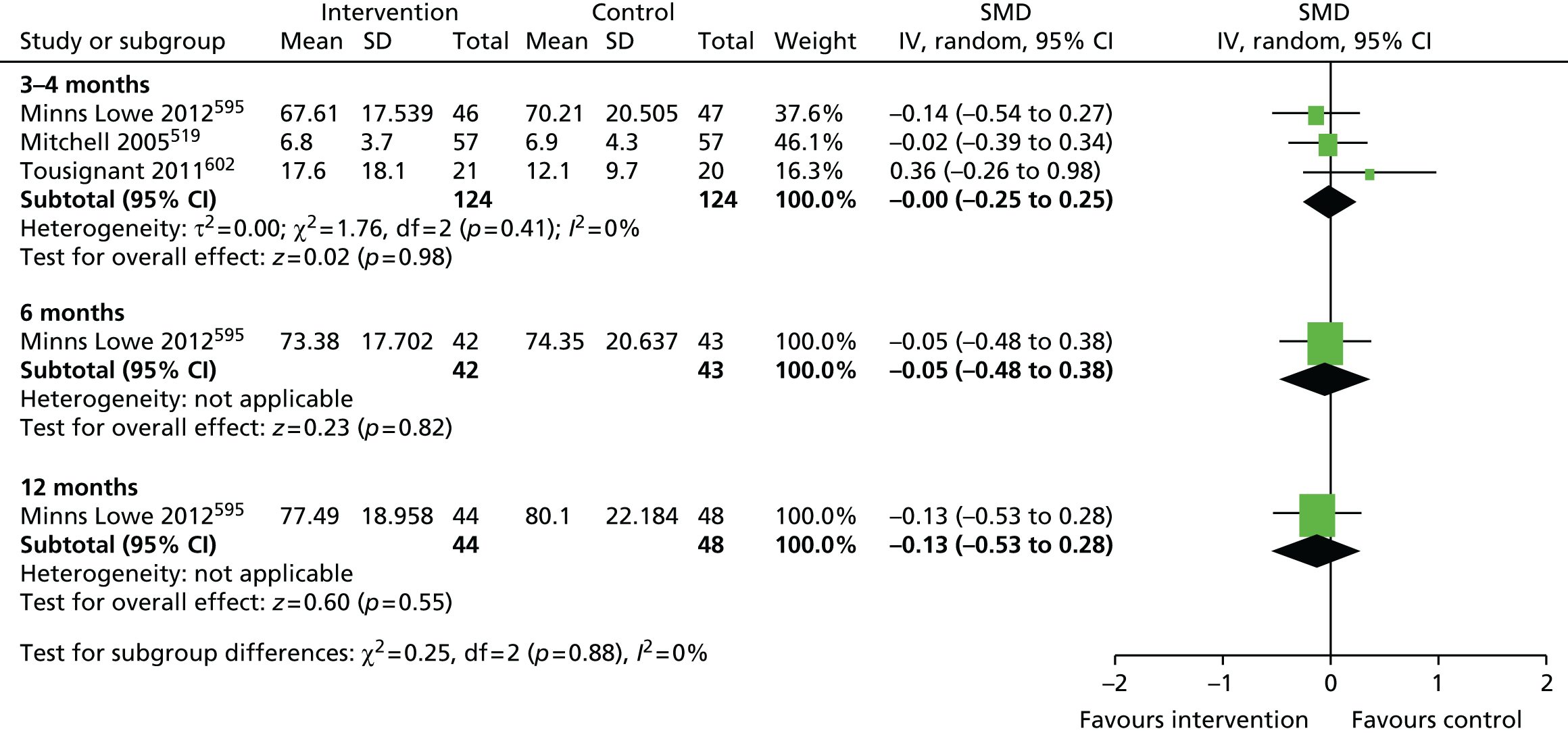
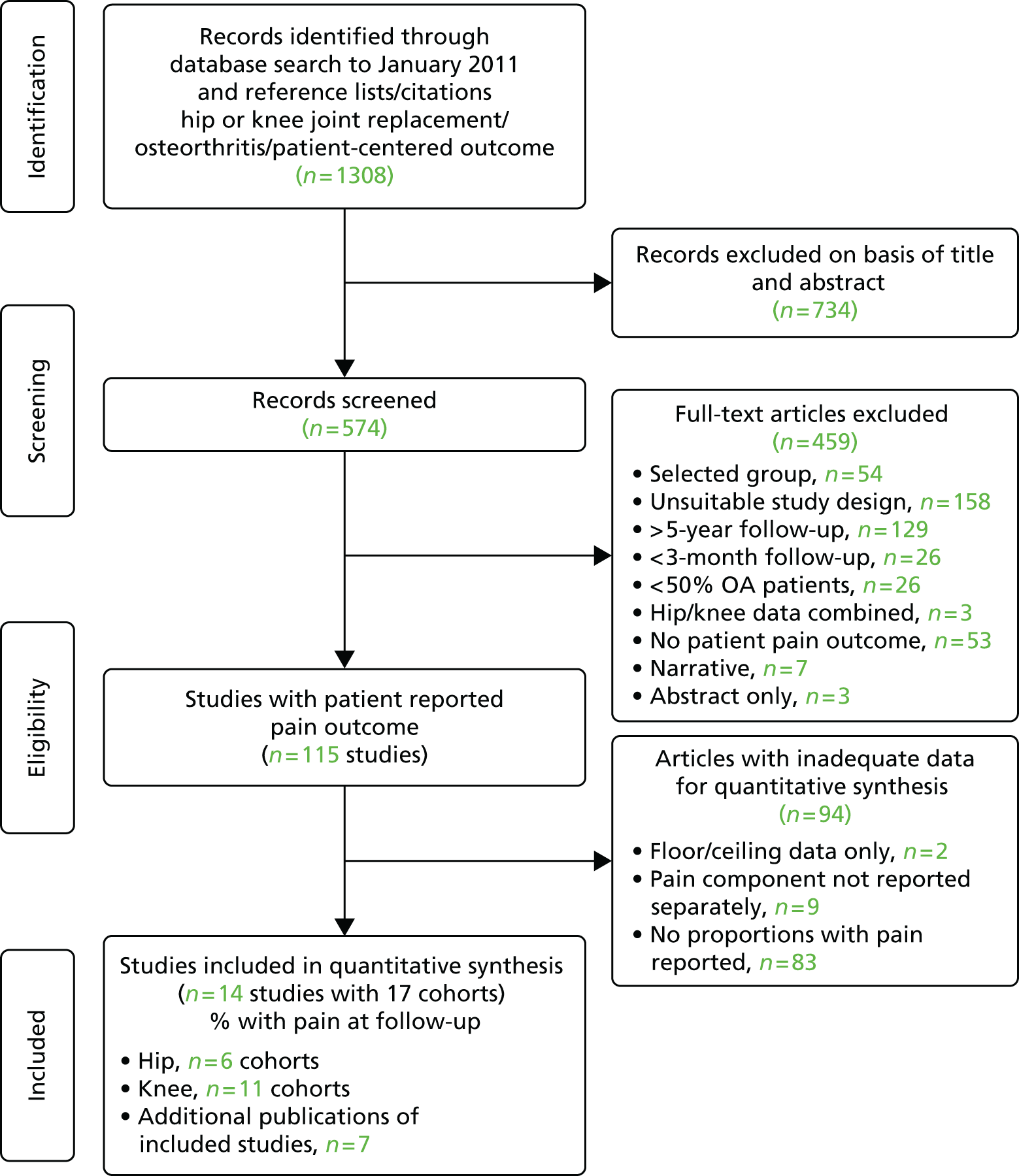
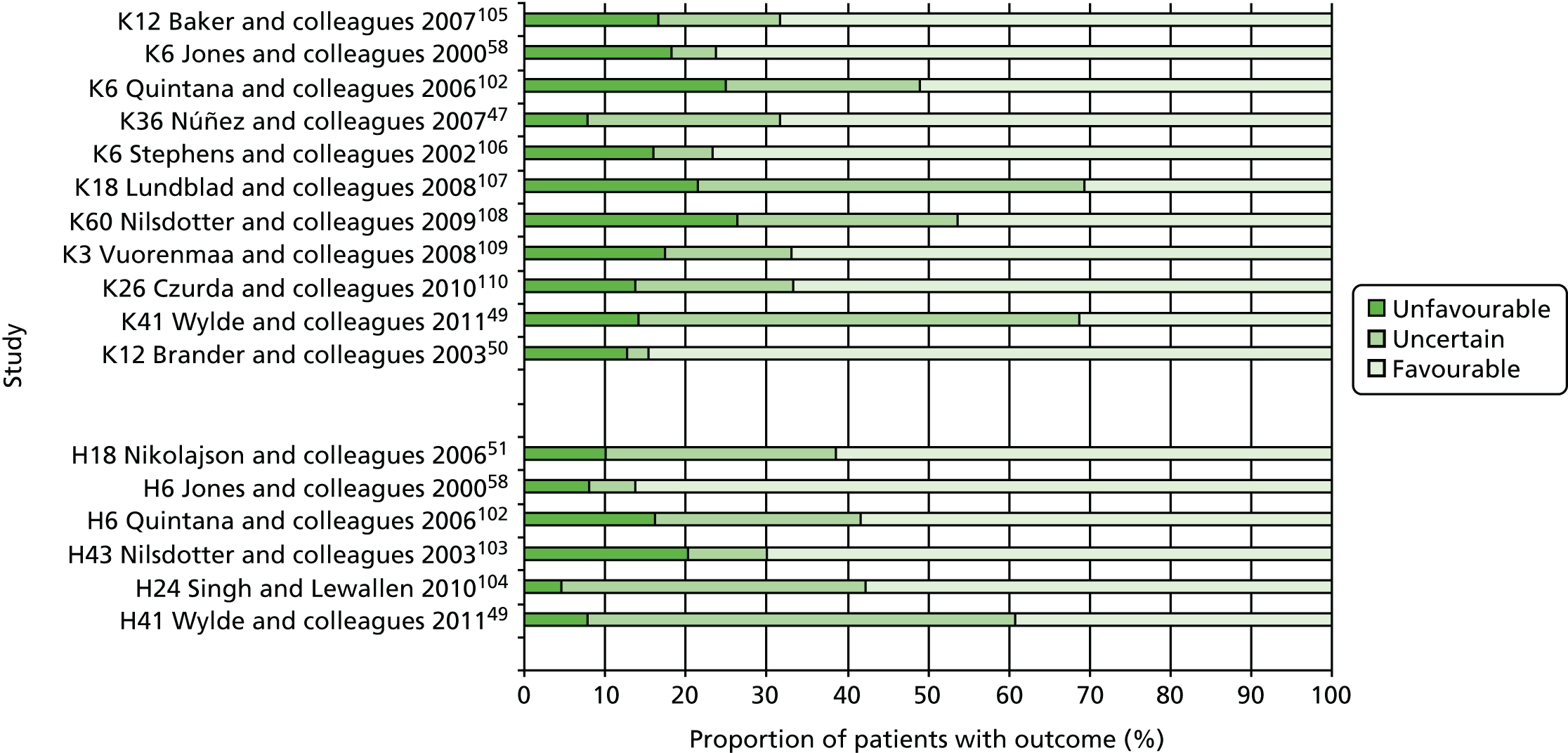
Review progress is summarised in Figure 2. Searches identified 1308 studies reporting patient-centred outcomes in patients with osteoarthritis. Of these, 115 studies were potentially eligible. After detailed evaluation, 14 articles describing 17 cohorts presented results classifiable as proportions of people with different extents of pain at follow-up. Six cohorts reported outcomes in hip replacement,49,51,58,102–104 and 11 in knee replacement patients. 47,49,50,58,102,105–110 The main reasons for exclusion at this stage were lack of a pain outcome separate from an overall outcome score or the presentation of results as means only. Patient and study characteristics are summarised briefly in Table 1 and in more detail with outcome data in Appendix 4.
FIGURE 2.
Systematic review of long-term pain after hip or knee replacement: flow diagram. OA, osteoarthritis.
| Author | Population | Pain outcome measure; follow-up time |
|---|---|---|
| THR | ||
| Nikolajson and colleagues 200651 | 1231 consecutive patients in a national joint registry | Authors’ own scale; 12–18 months |
| Jones and colleagues 200058 | Estimated 242 consecutive patients with hip replacement in health region | WOMAC pain; 6 months |
| Quintana and colleagues 2006102 | 784 consecutive patients scheduled for THR in seven teaching hospitals | WOMAC pain; 6 months |
| Nilsdotter and colleagues 2003103 | 92 consecutive patients with two surgical methods at single centre | WOMAC pain; mean 43 months |
| Singh and Lewallen 2010104 | 9154 consecutive patients from joint registry | Authors’ own scale; 24 and 60 months |
| Wylde and colleagues 201149 | 1401 consecutive patients at single centre | WOMAC pain; median 41 months |
| TKR | ||
| Baker and colleagues 2007105 | 9417 random sample of patients in joint registry | OKS pain; 12 months or latest available |
| Jones and colleagues 200058 | Estimated 292 patients in health region | WOMAC pain; 6 months |
| Quintana and colleagues 2006102 | 792 consecutive patients in seven centres | WOMAC pain; 6 months |
| Núñez and colleagues 200747 | 88 consecutive patients at a single centre | WOMAC pain; 36 months |
| Stephens and colleagues 2002106 | 68 patients aged ≥ 50 years | WOMAC pain; 6 months |
| Lundblad and colleagues 2008107 | 69 patients scheduled for knee replacement | VAS pain; 18 months |
| Nilsdotter and colleagues 2009108 | 102 responders to postal survey on waiting list for knee replacement | KOOS pain; 60 months |
| Vuorenmaa and colleagues 2008109 | 51 patients referred for knee replacement | VAS pain; 3 months |
| Czurda and colleagues 2010110 | 411 consecutive patients with computer assisted or conventional surgery | WOMAC pain; mean 26 months (range 18–42 months) |
| Wylde and colleagues 201149 | 1394 consecutive patients at single centre | WOMAC pain; median 28 months (range 14–43 months) |
| Brander and colleagues 200350 | 116 consecutive patients operated on by one surgeon | VAS pain; 12 months |
Studies ordered within hip and knee replacement groups by decreasing representativeness (multiple compared with single centre); and by increasing losses to follow-up.
Total hip replacement
Systematic searches identified six studies including a total of 13,031 patients. 49,51,58,102–104 Pain outcome assessments were based on the WOMAC pain scale or authors’ own methods.
One study was in patients recruited from a national joint registry. 51 Two studies were in multiple centres,58,102 and three were studies in single centres. 49,103,104 Cohorts were generally similar with regard to patient age (range of means or medians 65.0–73.0 years) and sex (range of percentage female 48.3–63%). Losses to follow-up ranged from 5.8% to 47.6%. We considered two markers of study quality: multiple compared with single centres and lower losses to follow-up.
Overall, an unfavourable pain outcome was seen in at least 4.8% and up to 20.5% of patients after THR (Figure 3). However these are likely to be underestimates as we do not have information on the outcomes in between 5.8% and 52.7% of patients.
FIGURE 3.
Systematic review of long-term pain after hip or knee replacement: proportion of patients with pain at follow-up. Preceding study author: H (hip) or K (knee) and months (follow-up). Studies ordered within hip and knee replacement groups by decreasing representativeness (multiple compared with single centre) and by increasing losses to follow-up.
Proportion of patients with outcome
Applying the conservative assumption that an equal proportion of patients with missing data had an unfavourable pain outcome, we estimate that at least 7–23% of patients experienced long-term pain after hip replacement. In three higher-quality, more representative studies conducted in multiple centres, this would reflect an unfavourable pain outcome in 9%,58 13%51 and 20%102 of patients, and in three studies with low losses to follow-up in 9%,58 13%51 and 23%103 of patients. Data from two studies considered more representative and with low losses to follow-up suggested that 9%58 to 13%51 of patients had an unfavourable pain outcome after THR.
Total knee replacement
Searches identified 11 studies including a total of 12,800 patients. 47,49,50,58,102,105–110 Pain outcome measures were based on the WOMAC and Knee Injury and Osteoarthritis Outcome Score (KOOS) pain scales, the Oxford Knee Score (OKS) pain dimension or pain measured on a visual analogue scale (VAS). One study was in patients recruited from a national joint registry. 105 Two studies were in patients from multiple centres,58,102 six studies were in patients treated at a single centre47,106–110 and one study reported all patients operated on by one surgeon. 50 Cohorts were generally similar with regard to patient age (range of means or medians 66–76 years) and sex (range of percentage female 54–86%), and the indication was osteoarthritis in ≥ 94% of patients when specified. Losses to follow-up ranged from 0% to 43.5%.
Overall, after TKR, an unfavourable pain outcome was seen in at least 8.0% and up to 26.5% of patients (see Figure 3). Assuming conservatively that the patients with missing data had similar pain outcomes, studies suggested that at least 10% to 34% of patients experience long-term pain after knee replacement. Applying this assumption in the higher-quality studies with potentially more representative populations, at least 19%,105 20%58 and 31%102 of patients had an unfavourable pain outcome after TKR. In four studies with low losses to follow-up, 10%,47 13%,105 17%106 and 20%58 of patients reported an unfavourable pain outcome at follow-up. In one study conducted in multiple centres with low losses to follow-up, 20% of patients reported an unfavourable pain outcome at follow-up. 58
Discussion
Well-conducted studies in representative populations of patients with primary total hip and knee replacement suggest that a significant proportion of people continue to have painful joints after surgery. Our analyses were limited by the small number of studies and different pain outcome measures. These precluded meta-analysis, calculation of a summary estimate and exploration of sources of heterogeneity.
The proportion of people with an unfavourable long-term pain outcome in studies ranged from about 7% to 23% after hip replacement, and 10% to 34% after knee replacement. In the best quality studies an unfavourable pain outcome was reported in ≥ 9% of patients after total hip and about 20% of patients after TKR.
Conclusion
For many people, total hip or knee replacement is effective in treating osteoarthritis pain. However, a significant proportion of people have painful joints after surgery.
Systematic review of methods used to assess chronic pain after total knee replacement
Background
Pain is a key outcome after TKR111 and our systematic review showed that 10–34% of patients report long-term pain. However, there is little guidance about which aspects of pain should be assessed. For clinical trials investigating efficacy of chronic pain treatments, the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) recommends that the assessment of pain should include an assessment of pain intensity, pain medication usage, pain quality and the temporal aspects of pain. 112 The aim of this review was to determine which outcome measures have been used to assess chronic pain after TKR by reviewing all original research articles published over a 10-year period.
Methods
| General methods | As described in Systematic review methods |
| Databases and dates | MEDLINE, EMBASE, PsycINFO, The Cochrane Library and CINAHL databases from 1 January 2002 to 22 November 2011 |
| Search strategy | Knee replacement/pain. MEDLINE search strategy based on terms in Appendix 3 |
| Study design | Study design filters were not applied. Empirical studies |
| Patients | Patients with knee replacement |
| Data extraction | Pain at ≥ 3 months |
| Outcomes | Study objective, study design, setting, country of the first author, number of study participants recruited, timings of assessments and outcome measures that contained pain items |
| Quality assessment | No assessment conducted |
Results
Characteristics of included studies
The review process and reasons for exclusion are summarised in Figure 4. A total of 8486 articles were identified in the literature searches and screened for potential eligibility by one reviewer. A second reviewer performed duplicate screening on a random 1000 articles but no relevant articles had been missed by the first reviewer. After screening, 1164 articles met the eligibility criteria for the review. Studies included in the review used a variable number of outcome measures that incorporated pain items (range 1–14), with 506 studies (43%) using two or more measures.
FIGURE 4.
Systematic review of methods used to assess chronic pain after TKR: flow diagram.

Multi-item tools
Overall, 54 different multi-item tools containing pain questions were used in the studies of TKR. Five multi-item tools were used in > 5% of the studies and these included the American Knee Society Score (AKSS),113 WOMAC,114 Hospital for Special Surgery Knee Score (HSS),115 Short Form questionnaire-36 items (SF-36)116 and OKS. 117 Details of the multi-item tools that were used in more than five studies and the number of items which assessed pain within each of these tools are provided in Table 2.
| Multi-item tool | Number of studies (%) that used tool | Number of items in tool | Number of items that assess pain |
|---|---|---|---|
| AKSS | 675 (58) | 10 | 1 |
| WOMAC | 267 (23) | 24 | 5 |
| HSS | 184 (16) | 7 | 2 |
| SF-36 | 165 (14) | 36 | 2 |
| OKS | 101 (9) | 12 | 5 |
| SF-12 | 54 (5) | 12 | 1 |
| KOOS | 26 (2) | 42 | 9 |
| EQ-5D | 25 (2) | 5 | 1 |
| Feller Patellar Score | 20 (2) | 4 | 1 |
| ADL Scale of the Knee Outcome Survey | 14 (1) | 17 | 1 |
| Lequesne Index | 11 (< 1) | 12 | 5 |
| Tegner and Lysholme score | 9 (< 1) | 8 | 1 |
| Total Knee Function Questionnaire | 9 (< 1) | 55 | 1 |
| NHP | 7 (< 1) | 45 | 8 |
| Self-Administered Patient Satisfaction Scale | 6 (< 1) | 4 | 1 |
| Stern and Insall Patellar Score | 6 (< 1) | 1 | 1 |
| Bristol Knee Score | 6 (< 1) | 9 | 1 |
| 15D | 6 (< 1) | 15 | 1 |
Geographical trends in the use of multi-item tools
The use of multi-item tools by countries that contributed > 50 articles to the review were compared (Figure 5). Nation-specific preferences for particular tools were apparent, with the AKSS being the most commonly used tool in studies from the USA, UK, Germany, South Korea and Australia. The HSS was most commonly used in studies from China, whereas the WOMAC was the most frequently used in Canadian studies.
FIGURE 5.
Systematic review of methods used to assess chronic pain after TKR: geographical trends in use of multi-item tools.

Temporal trends in the use of multi-item tools
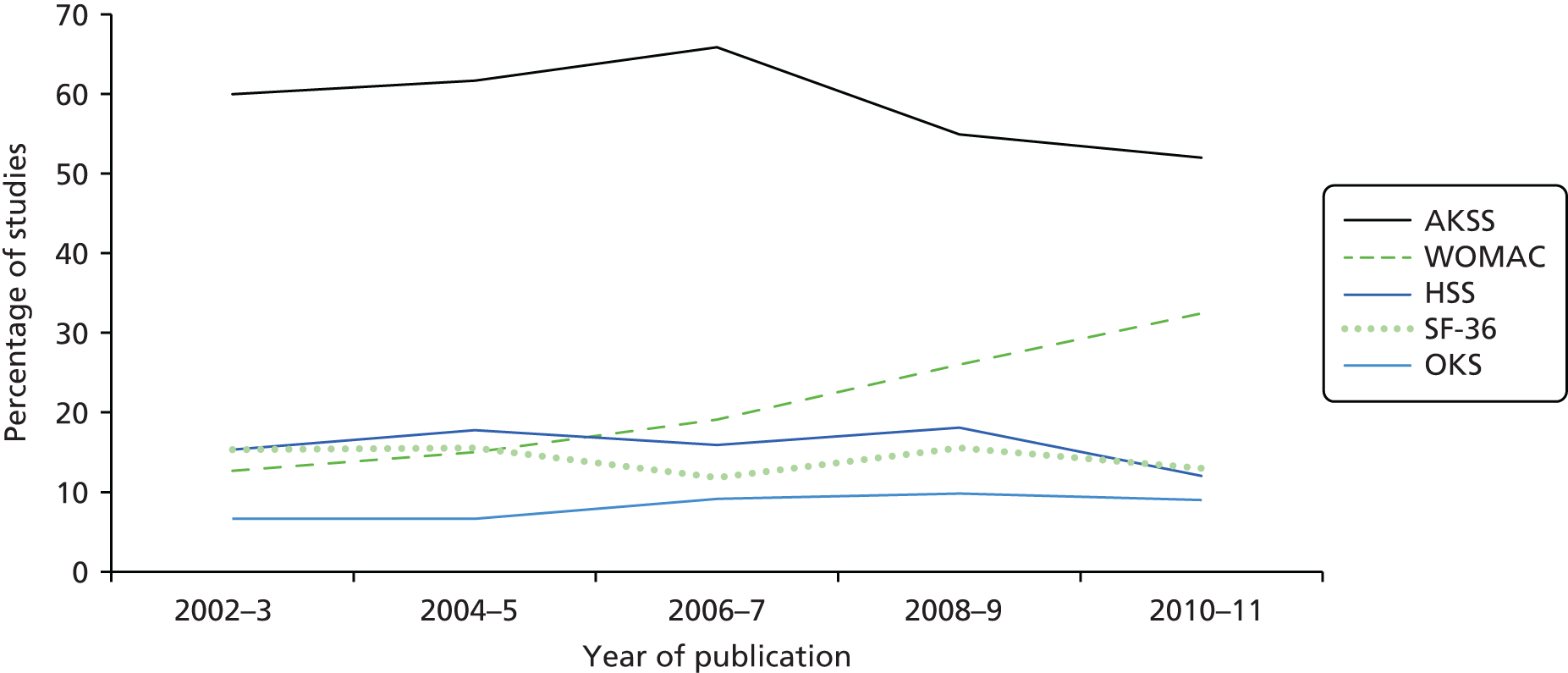
The percentage of studies using the five most commonly used multi-item tools over a 10-year period is displayed in Figure 6. From 2006 to 2011 there was a reduction in the proportion of studies that have used the AKSS, from 66% in 2006–7 to 52% in 2010–11. Over the same time period, there has been an increase in the proportion of studies that have used the WOMAC, from 19% in 2006–7 to 32% in 2010–11.
FIGURE 6.
Systematic review of methods used to assess chronic pain after TKR: temporal trends in the use of multi-item tools.
Single-item questions
Single-item questions were used 333 times to assess chronic pain after TKR. The aspects of pain assessed by the single-item questions, based on the framework provided by IMMPACT, are shown in Table 3. Pain severity was the most frequently assessed aspect of pain and the VAS was the most commonly used question format to assess pain severity.
| Pain domain | Examples of codes | Number (%) of single-item questions |
|---|---|---|
| Pain intensity | General pain intensity | 227 (68) |
| Average pain intensity | ||
| Worst pain intensity | ||
| Presence/absence of pain | ||
| Use of pain medication | Frequency of use | 8 (2) |
| Adherence | ||
| Decreased need | ||
| Pain quality | Location of pain (e.g. anterior knee pain) | 57 (17) |
| Temporal aspects of pain | Pain frequency | 33 (10) |
| Night pain | ||
| Constant pain | ||
| Intermittent pain | ||
| Physical functioning | Pain on walking | 98 (29) |
| Pain on climbing stairs | ||
| Pain during sports | ||
| Pain at rest | ||
| Emotional functioning | Unbearable pain | 5 (1.5) |
| Bothersome pain | ||
| Emotional well-being | ||
| Participant ratings of global improvement | Satisfaction with pain relief | 26 (8) |
| Reduction in pain from operation | ||
| Fulfilment of expectations |
Discussion
Numerous tools are available to assess general health and functional outcomes after TKR. 118 Despite a growing interest in investigating the burden, character and impact of long-term pain, we found that assessment has been inconsistent with extensive variation in the outcome measures used after TKR.
The AKSS is widely used in orthopaedic research,119,120 and was the most common method to assess long-term pain used in 58% of studies. The scale involves a clinician-conducted assessment and a composite score based on functional ability and measurements such as ROM and joint stability, and a single question about pain.
Our review showed a reduction over time in the use of the AKSS, accompanied by an increase in the use of the WOMAC. This may reflect increased awareness of the assessment of outcomes from a patient’s perspective. 121,122 There was also international variation in the use of multi-item tools.
Strengths of this review were the systematic and rigorous methods used to search and screen eligible articles, the wide inclusion criteria with diverse epidemiological and experimental study designs, and the inclusion of studies irrespective of language. Owing to the high volume of literature, it was not feasible to assess whether or not particular methods were used in studies of different quality.
Conclusion
Our systematic review shows that the assessment of long-term pain after TKR could be improved. Despite the availability of many validated pain-related instruments, few studies have attempted to capture the incidence, character and impact of chronic pain after TKR. Future research is needed to develop consensus and standardisation on which pain domains should be assessed after TKR.
Systematic review of pre-operative predictors of patient-centred outcomes after total hip and knee replacement
Background
Identification of pre-operative determinants and predictors of poor outcomes can guide the development of interventions and help target the provision of care. For factors that are determinants, the possibility exists that an intervention may alter the level of the factor and that this may lead to improved outcomes. Other variables cannot be changed by an intervention but may have value in predicting outcomes with care tailored for specific patient groups.
Associations between variables measured before joint replacement and post-surgical outcomes have been studied extensively. Establishment of a cohort study in an orthopaedic setting is relatively straightforward in the context of routine data collection and follow-up. However, it is important that analyses are conducted with robust statistical methods taking into account possible confounding factors.
The aim of this review was to identify high-quality systematic reviews and cohort studies that have assessed the predictive value of pre-surgical factors in relation to long-term post-surgical outcomes. In keeping with the themes of the RESTORE programme, we considered patient-reported outcomes.
Pre-surgical factors studied in detail were:
-
BMI
-
mental health status including anxiety and depression
-
pain
-
physical function.
Methods
| General methods | As described in Systematic review methods |
| Databases and dates | MEDLINE and EMBASE from inception to 15 October 2013 |
| Search strategy | Joint replacement/osteoarthritis/specified patient centred outcomes. MEDLINE strategy based on terms in Appendix 3 |
| Study design | Cohort studies with multivariable regression analysis or ANCOVA |
| Patients | Total hip or knee replacement. If both reported, included only if analysed separately and with at least 100 patients per analysis |
| Follow-up | At least 12 months |
| Data extraction | Date of publication, hip or knee, country, baseline dates, follow-up duration, pre-surgical measures |
| Outcomes | Patient-reported outcomes |
All titles and abstracts of articles published in 2010 were checked for inclusion by two reviewers experienced in orthopaedic research and systematic reviews. Comparison of inclusion decisions showed good agreement between reviewers. Subsequently titles and abstracts were checked by one reviewer but with oversampling in the event of uncertainty.
At an early stage, it became apparent that the literature base for the review was very large. We limited our inclusion to studies with ≥ 100 patients with at least 12 months’ follow-up and to studies published in English.
Quality assessment
A level of good quality of studies was implicit with study selection based on seven MINORS criteria:123
-
clear stated aim – multivariable analysis
-
inclusion of consecutive patients – cohort study
-
prospective collection of data – prospective
-
end points appropriate to aim of study – pain, function, satisfaction
-
unbiased assessment of end point – patient-reported outcomes
-
follow-up period appropriate to aim of study – at least 12 months
-
prospective calculation of study size – estimated at least 100.
For the eighth criterion we considered:
-
per cent follow-up of those at baseline (eligible) – > 80% good quality.
Furthermore we included one classification modified from the Newcastle-Ottawa quality assessment scale:124
-
representativeness of the cohort – registry or multiple centres good quality.
These last two classifications were used to assess quality of the studies relating to generalisability.
Results
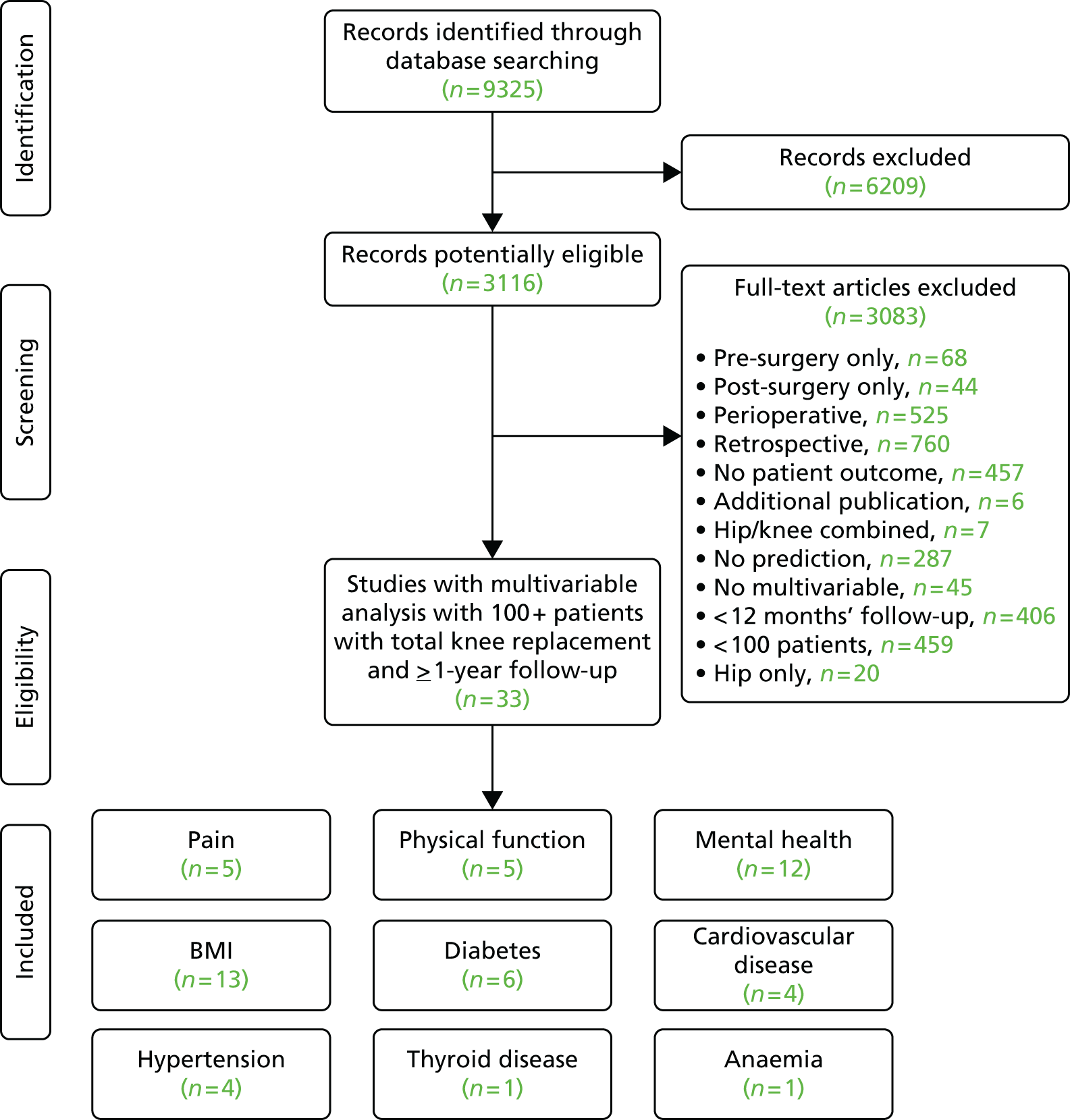
Review progress is summarised as flow diagrams for total hip and TKR in Figures 7 and 8. We identified one systematic review and 53 cohort studies that explored the relation between pre-surgical factors and long-term outcomes in multivariable analysis.
FIGURE 7.
Systematic review of pre-operative predictors of patient-centred outcomes after THR: flow diagram.

FIGURE 8.
Systematic review of pre-operative predictors of patient-centred outcomes after TKR: flow diagram.
Total hip replacement
Searches identified 26 studies reporting multivariable analyses including patients with THR. In this section we summarise results from 14 studies of pre-operative BMI, mental health, pain and physical function as predictors of long-term patient-reported outcomes. 31,103,104,125–135 Study characteristics are summarised in Appendix 5 with brief details in Table 4.
| Study | Number of patients; follow-up | Predictors | Outcome measures |
|---|---|---|---|
| Registry | |||
| Rolfson and colleagues 2009125 | 6158; 12 months | Mental health | VAS pain, satisfaction, EQ-5D domains |
| Multiple centres | |||
| Hajat and colleagues 2002126 | 3600; 12 months | Physical function | OHS |
| Jones and colleagues 2012127 | Estimated 167; 3 years | BMI | WOMAC |
| Judge and colleagues 201131 | 845; 12 months | Physical function | WOMAC |
| Judge and colleagues 2013128 | 1375; 60 months | BMI, mental health, physical function | OHS |
| Stevens and colleagues 2012129 | 653; 12 months | BMI | WOMAC, SF-36 |
| Single centre | |||
| Anakwe and colleagues 2011130 | 850; 12 months | Mental health, physical function | Satisfaction |
| Clement and colleagues 2011131 | 1312; 12 months | Mental health, physical function | OHS |
| Davis and colleagues 2011132 | 1095; 60 months | BMI | SF-36 |
| Gandhi and colleagues 2010133 | 636; 12 months and up to 72 months (mean 39 months) | BMI, mental health | WOMAC, SF-36 physical function |
| Garbuz and colleagues 2006134 and Xu and colleagues 2005136 | 147; 12 months | Pain, physical function | WOMAC pain |
| Moran and colleagues 2005135 | 687; minimum 18 months | BMI | SF-36 |
| Nilsdotter and colleagues 2003103 | 198; 12 months and at mean 43 months | BMI, mental health, pain | WOMAC function |
| Singh and Lewallen 2010104 | 5707; 24 months | BMI, mental health | Pain (five-response scale) |
Body mass index
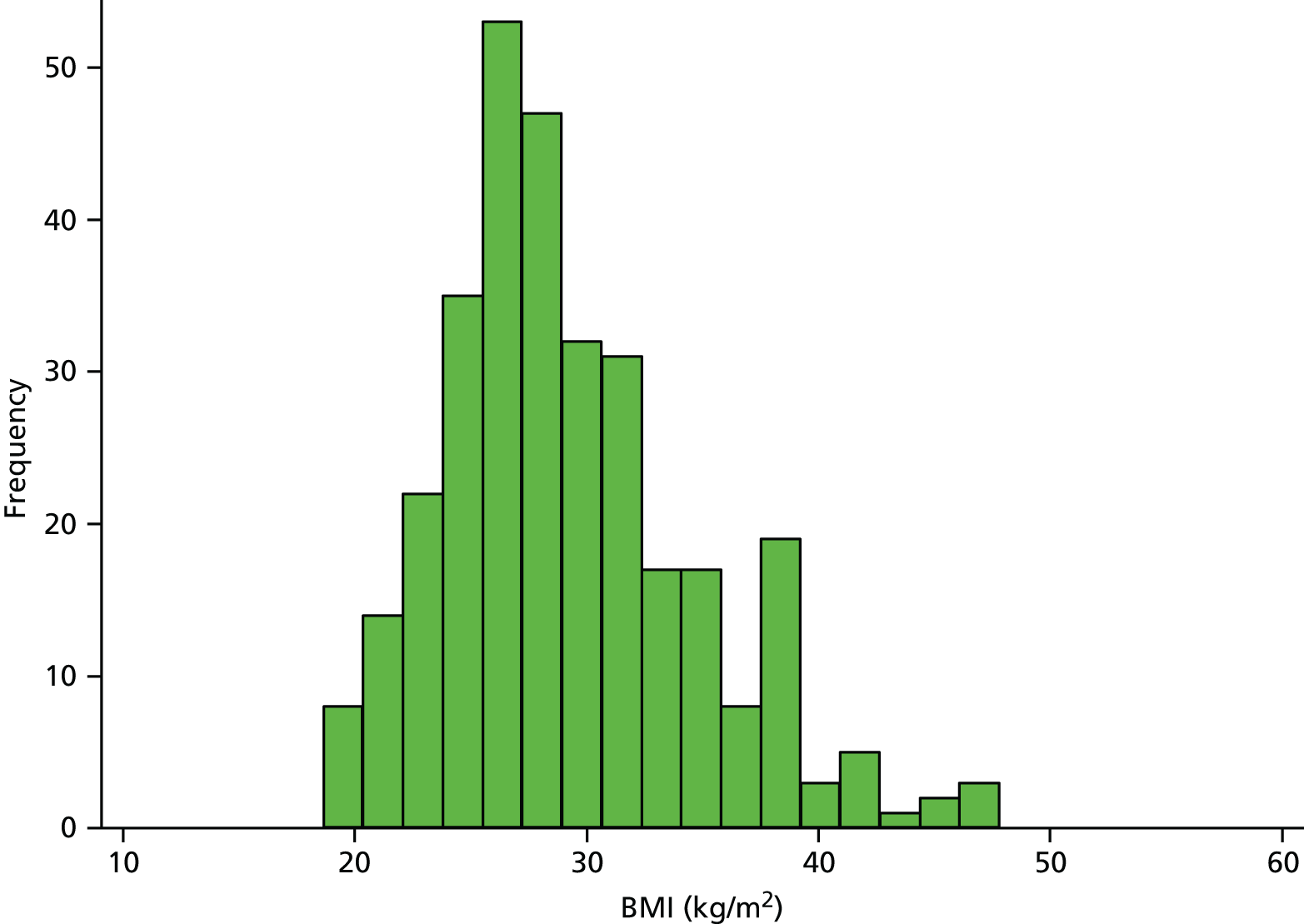
There is no clear evidence linking high BMI with the development of hip osteoarthritis,137 but people with higher BMI are more likely to require THR. For example, in a UK study including over 490,000 women, those with a BMI of > 30 kg/m2 had nearly 2.5 times the risk of requiring a THR of those with a BMI of < 22.5 kg/m. 138 Patients included in the National Joint Registry for England and Wales in 2012 had an average BMI of 28.71 kg/m2 (SD 5.29 kg/m2) and about 39% had a BMI of ≥ 30 kg/m2. 3 In the RESTORE APEX RCT, the mean BMI in 322 patients receiving THR was 29.1 kg/m2 (SD 5.5 kg/m2, range 18.6 to 47.8 kg/m2) and 4.3% of patients had a BMI of ≥ 40 kg/m2. The distribution of BMI of patients in the APEX cohort of patients with THR is shown in Figure 9.
FIGURE 9.
Distribution of BMI in the APEX THR cohort.
Our searches of MEDLINE and EMBASE considered long-term patient-reported outcomes after THR. Searches identified eight studies that specifically focused on the relationship between BMI and patient-reported outcomes at ≥ 12 months after THR. Three studies included patients from multiple centres,127–129 and five studies were conducted at a single centre. 103,104,132,133,135 Study details are summarised in Table 4. In studies with data that allowed estimation, rates of follow-up ranged from 6% to 38%.
Jones and colleagues followed up approximately 167 patients (72% eligible) from a Canadian health region 3 years after a THR. 127 The authors used WHO criteria to classify patients into groups of BMI (< 25 kg/m2, 25–29.9 kg/m2, 30–34.9 kg/m2, ≥ 35 kg/m2). In the cohort, 13.9% of patients had a BMI of ≥ 35 kg/m2. In an analysis with adjustment for age, sex, diabetes and cardiac disease, the authors reported that similar long-term WOMAC pain and function scores were achieved in patients with different levels of BMI. Considering data collected at 6 months, the authors noted that recovery of function and reduction of pain was slower in patients with a high BMI (≥ 35 kg/m2) than groups with lower BMI.
Judge and colleagues128 followed up 1375 patients (64% eligible) who had received a specific design of THR prosthesis at seven UK centres 5 years after surgery. The mean BMI of patients in the cohort followed up was 27.6 kg/m2 (SD 4.8 kg/m2) and it was treated as a continuous variable. In multivariable analyses including age, sex, primary diagnosis, occupation, comorbidities, health-related quality of life (HRQoL), hip ROM, surgical variables and Oxford Hip Score (OHS), there was a relationship between increasing BMI and poorer long-term function and pain as measured by the OHS. The authors considered the differences in function and pain associated with BMI to be small.
In a study at three orthopaedic centres in the Netherlands, Stevens and colleagues129 followed up 653 patients (77% eligible) 12 months after receiving a THR. The mean BMI was 27.0 kg/m2 (SD 4.1 kg/m2) and the authors defined three groups (< 25 kg/m2, 25–30 kg/m2, > 30 kg/m2). After adjusting analyses for age, sex, comorbidities and complications, increased BMI was associated with worse long-term function but the size of the effect was low, particularly in comparison to that of presence of comorbidities and complications.
Moran and colleagues135 followed up 687 patients (86% eligible) with THR at a single UK centre. The mean BMI in this cohort was 27.8 kg/m2 (SD 5 kg/m2) and the authors reported that only 9 out of 687 patients (1.3%) had a BMI of > 40 kg/m2 and that no conclusions could be drawn for these patients. After adjusting for sex, comorbidities, OHS and SF-36, BMI treated as a continuous variable was not a significant predictor for any SF-36 domains.
Nilsdotter and colleagues103 reported the follow-up of 198 patients (94% eligible) with THR from a single centre in Sweden at a mean of 3.6 years. The authors analysed BMI as a continuous variable but did not report mean or categorical values. Increasing BMI was associated with poorer long-term WOMAC function in univariate analysis. After adjustment for sex, comorbidities, WOMAC, SF-36 (including mental health), employment, marital status, contralateral osteoarthritis, need of walking assistance, walking distance, analgesic use and regional or widespread pain, BMI was not associated with long-term WOMAC function.
Davis and colleagues132 followed up 1095 patients (68% eligible) from a single UK centre 60 months after THR. The mean BMI in the cohort was 28 kg/m2 at baseline and 9.2% of patients had a BMI of ≥ 35 kg/m2. Patients were divided into groups according to their BMI (< 25 kg/m2, 25–29.9 kg/m2, 30–34.9 kg/m2, ≥ 35 kg/m2). In multivariable analysis with age, sex, pre-operative hip score, SF-36, comorbidities and consultant, increasing BMI predicted poorer long-term SF-36 physical function and bodily pain. However, the authors acknowledged that although absolute levels of long-term pain and function were poorer than in patients with low BMI, there were dramatic improvements in patient outcomes in those with high BMI.
Singh and Lewallen104 reported a 2-year follow-up of 5707 patients with THR (62% eligible) at a single US centre. Four per cent of patients had a BMI of ≥ 40 kg/m2 or greater. The authors divided patients into five groups according to their BMI (< 25 mg/m2, 25–29.9 mg/m2, 30–34.9 kg/m2, 35–39.9 kg/m2, ≥ 40 kg/m2). In an analysis adjusted for age, sex, comorbidities, depression, anxiety, operative diagnosis, distance from centre and implant design, patients with BMI of ≥ 35 kg/m2 reported significantly greater long-term moderate or severe pain than the lowest BMI group.
In a study from a single centre in Canada, Gandhi and colleagues133 followed up 636 patients (per cent eligible not reported) for an average of 3.3 years. The mean BMI in this cohort was 27.6 kg/m2 (SD 4.9 kg/m2) and was treated as a continuous variable in analyses. After adjusting for age, sex, comorbidities, WOMAC and SF-36 scores there was a non-significant trend for less improvement in WOMAC score with increasing BMI.
With the increasing levels of BMI in developed countries, it is important that patients and health-care providers are aware of any factors that influence the long-term outcome of THR. We identified eight studies that reported the association between BMI and a long-term patient-reported outcome in multivariable analysis. 103,104,127–129,132,133,135 If reported in the studies we identified, patients had broadly similar average BMIs to those reported in the National Joint Registry for England and Wales3 and the APEX cohort described in Chapters 6 and 7.
In three studies, the absolute long-term OHS,128 WOMAC function129 and a simple measure of pain severity104 were more favourable in lower BMI groups. In another study this was observed for WOMAC function in univariate but not multivariate analysis. 103 In two studies there were no long-term differences in WOMAC pain or function,127 WOMAC score or SF-36. 135 Further to the selection of studies according to specific quality criteria, studies had either one or no additional marker of quality based on centres studied and losses to follow-up. Differences in results of studies were not explained by issues relating to these additional markers of study quality.
In the four studies for which BMI was treated as a continuous variable in multivariable analysis, authors reported no strong association between BMI and long-term function,103,128,133,135 or pain. 128,135 Two studies reported changes in function which may be a more appropriate method of analysis as patients with higher BMI generally have poorer function before surgery. There were greater improvements in OHS128 and WOMAC133 in patients with lower BMI. Associations observed were not limited to studies according to additional markers of quality.
In four studies, authors focused on the relationship between categorical levels of BMI and patient-reported outcomes. There was some evidence that patients classified as obese (≥ 30 kg/m2) according to WHO classifications139 had poorer function129,132 or pain outcomes,104 but only one study had an additional marker of good quality. 127 In a fourth study with one additional marker of good quality, no association was noted between pre-operative BMI and long-term function or pain, although the authors noted a slower recovery in patients with high BMI. 127
Overall, the absolute levels of physical function and pain achieved after THR in patients with particularly high BMI may be somewhat lower than that achieved by other patients. However, there is a clear indication that many patients with high BMI benefit from THR with long-term improvements to physical function and reduction in long-term pain.
Pre-surgical mental health
The period between being placed on the waiting list and the day of surgery can be a time of distress for patients and is characterised by pain, poor physical function and uncertainty. Parsons and colleagues140 identified six major themes describing patients’ experiences of waiting for joint replacement: living and coping with pain; not being able to walk; coping with everyday activities; body image; help, advice and support; and the effect on family, friends and helpers.
Anxiety and depression are common in people with osteoarthritis. 141,142 In the APEX cohort of patients with osteoarthritis waiting for THR, pre-surgical anxiety and depression was identified using the Hospital Anxiety and Depression Scale (HADS) questionnaire. Definite or potential anxiety was reported by 33% of patients and definite or potential depression by 30% of patients.
One previous systematic review explored the relationship between pre-surgical anxiety and long-term patient outcomes in patients with THR. 143 Vissers and colleagues143 searched MEDLINE and EMBASE to January 2011 and identified nine studies including 8823 patients receiving THR. The authors reported that there was limited and conflicting evidence on the relationship between psychological factors and postoperative function and pain.
Our literature searches identified seven studies with ≥ 100 patients with THR followed up for ≥ 12 months with pre-surgical mental health included in multivariable analyses. One study reported data from a joint registry,125 one study included patients from multiple centres,128 and five studies collected data from patients treated at a single centre. 103,104,130,131,133 Study details are summarised in Table 4. In studies that reported the number of patients eligible, between 6% and 38% of patients were not followed up.
In a 12-month follow-up study of 6158 patients (92% eligible) from the Swedish Hip Arthroplasty Register, Rolfson and colleagues125 assessed the impact of the pre-operative European Quality of Life-5 Dimensions (EQ-5D) measure of anxiety and depression on long-term pain and satisfaction. In analysis of covariance adjusting for EQ-5D domains, comorbidities and age and sex, anxiety and depression were strong predictors of poor long-term pain relief and low patient satisfaction. Furthermore, in patients with persistent anxiety and depression, only 24% of patients showed improvement in the EQ-5D mobility dimension compared with 59% in those unaffected by high levels of anxiety or depression.
Judge and colleagues128 followed up 70% of 1375 patients eligible from seven UK centres 5 years after they had received a specific THR prosthesis. The multivariable model included the SF-36 mental health score, age, sex, primary diagnosis, occupation, comorbidities, HRQoL and pre-surgical OHS. Poorer mental health measured by the SF-36 mental health score was associated with a less favourable long-term patient outcome as measured by the OHS.
Anakwe and colleagues130 followed up 850 patients (94% eligible) 12 months after THR at a single UK centre. The Short Form questionnaire-12 items (SF-12) mental health component, diabetes, hypertension, history of depression, age, sex, SF-12 physical components, OHS, and musculoskeletal comorbidities were included in analyses. Although significant in univariate analyses, neither a history of depression nor the SF-12 mental health component predicted the level of long-term patient satisfaction in multivariable analysis.
Nilsdotter and colleagues103 followed up 198 patients with THR (94% eligible) at a single centre in Sweden after a mean of 3.6 years. Multivariable analysis included sex, comorbidities, WOMAC, SF-36 (including mental health), employment, marital status, contralateral osteoarthritis, need of walking assistance, walking distance, analgesic use and regional or widespread pain. In preliminary univariate analysis, pre-operative SF-36 mental health status was not a significant predictor of long-term WOMAC function and was not entered into multivariable analysis.
In a single-centre UK study, Clement and colleagues131 followed up 1312 patients with THR (per cent eligible not reported) at 12 months. In a multivariable analysis with age, deprivation, Charlson comorbidities, OHS, length of stay and SF-12 physical health, SF-12 mental health was a significant predictor of long-term change in OHS. Depression was the comorbidity with the strongest prediction of poor improvement in OHS.
Gandhi and colleagues133 followed up 636 patients (per cent eligible not reported) from a single centre in Canada for an average of 3.3 years. In multivariable analysis with age, sex, comorbidities, BMI and fixation (cemented or uncemented), SF-36 mental health was not associated with long-term changes in WOMAC score or SF-36 physical function.
In a single-centre US study, Singh and Lewallen104 followed up 5707 patients (62% eligible) 2 years after THR. Multivariable analyses included anxiety, depression, age, sex, comorbidities, operative diagnosis, distance from centre and implant design. Patients with depression but not anxiety [International Classification of Diseases (ICD) classifications] before surgery were more likely to report moderate to severe long-term pain at 2 years. A trend in a similar direction was not significant at 5 years.
All studies we included in the review reported multivariable analyses with inclusion of comorbidities and other factors in the statistical model. Authors examined outcomes in patients using generic measures of mental health (SF-12 mental health and SF-36 mental health), combined measures of anxiety and depression (EQ-5D anxiety/depression) and specific diagnoses of anxiety and depression (ICD code or clinical history).
In five studies in which generic mental health scores were measured before surgery using SF-36 or SF-12, results of multivariable analyses were inconsistent. 103,128,130,131,133 In two studies, patients with worse pre-surgical mental health scores had a poorer long-term outcome128 or less improvement measured by the OHS. 131 However, in two studies, SF-36 mental health scores measured before surgery did not predict long-term WOMAC function103 or change in overall WOMAC score. 133 Similarly pre-surgical SF-36 mental health did not predict long-term change in SF-36 physical function score. 133 In one study, the authors reported that long-term satisfaction was not predicted by pre-surgical SF-12 mental health score. 130 Inconsistencies between studies were not explained by differences in additional markers of quality.
Unlike the SF-36, which measures psychological distress and well-being, the mental health component of the EQ-5D relates specifically to anxiety and depression. In one study considered to be of good quality based on both additional markers, pre-operative anxiety/depression measured with the EQ-5D was associated with poorer long-term pain relief, satisfaction and mobility. 125 Three studies specifically reported outcomes in patients with anxiety or depression before surgery. In one study, patients with depression before surgery had poorer long-term pain outcomes but this was not the case for patients with anxiety. 104 In another study, depression before surgery was associated with poor improvement in OHS. 131 Both these studies were single centre and reported high losses to follow-up. In one study from a single centre but with low losses to follow-up, except in univariate analysis, patients with a history of depression did not report lower levels of long-term satisfaction after THR. 130
Overall, there was some evidence that patients with depression before THR may have poorer long-term outcomes but evidence for pre-surgical anxiety was weaker. For general mental health measures evidence was equivocal.
Pre-surgical pain
Pain is the principal indication for THR144 and the key patient expectations of surgery are good long-term functional and pain outcomes. 145,146 We identified two single-centre studies that had explored the relationship between pre-operative pain levels and long-term patient-reported outcomes after THR. 103,134 Details of studies are shown in Table 4.
In a study of 198 patients (94% eligible) from a single Swedish centre, Nilsdotter and colleagues103 reported a multivariable analysis with follow-up at a mean of 43 months. Increased levels of SF-36 bodily pain domain measured before surgery were associated with poorer long-term WOMAC function outcomes in a statistical model including age, sex, comorbidities, BMI and SF-36 physical function and mental health components.
Garbuz and colleagues134 followed up 147 patients (73% eligible) at a single Canadian centre 12 months after THR. Greater pre-operative pain measured with the WOMAC pain score was predictive of a poorer long-term WOMAC pain outcome in univariate analysis and after inclusion of age, sex and comorbidities in multivariable analysis.
Few studies have reported the relationship between pain levels before THR and long-term patient outcomes. In two studies with appropriate multivariable analyses, an increased level of pre-operative joint specific pain was associated with a greater risk of long-term pain and general pre-operative pain was predictive of poorer long-term functional outcome.
Pre-surgical physical function
As noted previously, the key expectations of patients undergoing total joint replacement are good long-term functional and pain outcomes. 145,146
Searches identified six studies that had followed up patients at 12 months and conducted multivariable analysis with pre-operative physical function included in the statistical model. Three studies were conducted in multiple centres,31,126,128 and three in single centres. 130,131,134 Characteristics of studies are summarised in Table 4.
In this classification we included four studies that reported overall WOMAC or OHS. As well as reflecting physical function these scores also include joint specific pain (OHS) or pain and stiffness (WOMAC).
Hajat and colleagues126 followed up 3600 patients (77% eligible) 12 months after THR at multiple UK centres. In multivariable analysis with age, sex, waiting time, comorbidities, housing and surgical factors, a worse pre-operative OHS was predictive of a worse long-term OHS.
In a European multicentre study, Judge and colleagues31 followed up 845 patients (64% eligible) with THR at 12 months. The authors included the WOMAC score, EQ-5D, age, sex, BMI, education, comorbidities and radiographic status in a multivariable model. A worse pre-operative WOMAC score was predictive of a poorer long-term outcome as judged by the Outcome Measures in Rheumatology-Osteoarthritis Research Society International (OMERACT-OARSI) rated good WOMAC response.
Judge and colleagues128 reported the 5-year follow-up of 1375 patients (70% eligible) who had received a specific THR prosthesis at seven UK centres. 128 In multivariable analyses including age, sex, BMI, primary diagnosis, occupation, comorbidities and HRQoL, the pre-operative OHS was the strongest determinant of long-term OHS.
Anakwe and colleagues130 reported a single UK centre study with 850 patients (94% eligible) followed up 12 months after THR. SF-12 physical and mental health components, diabetes, hypertension, history of depression, age, sex, OHS and musculoskeletal comorbidities were included in multivariable analysis. The SF-12 physical component score (PCS) measured before surgery did not predict long-term satisfaction. The pre-surgical OHS was associated with greater long-term dissatisfaction but only in univariate analysis.
In a study at a single Canadian centre, Garbuz and colleagues134 followed up 147 patients (73% eligible) 12 months after THR. In univariate analysis a 10-out-of-100-point difference in pre-surgical WOMAC function was associated with a 35% increase in long-term WOMAC function. In multivariable analysis age, sex, and comorbidity did not change the association.
Clement and colleagues131 reported 12-month follow-up of 1312 patients (per cent eligible not reported) with THR at a single UK centre. In a multivariable analysis with age, deprivation, comorbidities, length of stay and SF-12 physical and mental health, the pre-operative OHS was a strong predictor of long-term improvement in OHS.
The studies we identified suggested that better physical function before THR is associated with a better long-term functional outcome. This was apparent in one study with a specific functional measure134 and in four studies with a more general patient-reported outcome. 31,126,128,131 This observation was supported when considering studies with an additional marker of good quality. In one study with a more general measure of pre-operative physical function, there was no association with long-term satisfaction. 130
Total knee replacement
As shown in Figure 8, searches identified 33 studies reporting multivariable analyses including patients with TKR. In this section we summarise results from 22 studies of pre-operative BMI, mental health, pain and physical function as predictors of long-term patient-reported outcomes. 46,50,127,147–165 One study included important data in a second publication. 166 Details of studies are summarised in Appendix 6 with brief details in Table 5.
| Study | Number of patients; follow-up | Predictors | Outcome measures |
|---|---|---|---|
| Registry | |||
| Baker and colleagues 2012148 | 22,691; minimum 6 months | Mental health | OKS, EQ-5D |
| Franklin and colleagues 2008147 | 8050; 12 months | BMI, mental health | SF-12 PCS |
| Multiple centres | |||
| Alzahrani and colleagues 2011149 | 3177; 12 months | BMI | OKS, WOMAC |
| Cushnaghan and colleagues 2009151 | 259; mean 6 years | BMI | SF-36 PCS |
| Heck and colleagues 1998157 | 268; 24 months | Mental health, pain, physical function | SF-36 PCS |
| Jones and colleagues 2012127 | Estimated 209; 3 years | BMI | WOMAC |
| Lingard and colleagues 200446 | 741 at 1 year; 12 and 24 months | BMI, pain, physical function | WOMAC pain and function, SF-36 PCS |
| Lingard and colleagues 2007150 and Lingard and colleagues 200446 | 682; 12 and 24 months | Mental health | WOMAC pain and function |
| Merle-Vincent and colleagues 2011158 | 264; 24 months | Mental health | Satisfaction |
| Naylor and colleagues 2012154 | 146; 12 months | BMI | OKS |
| Papakostidou and colleagues 2012155 | 204; 12 months | BMI, mental health, pain | WOMAC |
| Perruccio and colleagues 2012152 | 435; mean 12.5 months | BMI | WOMAC |
| Singh and Lewallen 2013156 | 7139; 2 and 5 years | Mental health | Pain severity questionnaire |
| Sullivan and colleagues 2011153 | 120; 12 months | BMI, pain | WOMAC function and pain |
| Single centre | |||
| Ayers and colleagues 2005163 | 165; 12 months | Mental health, physical function | WOMAC physical function, SF-36 PCS |
| Brander and colleagues 200350 | 116 (149 TKRs); 12 months | Mental health | Pain VAS, McGill Pain Questionnaire |
| Clement and colleagues 2013164 | 966; 12 months | Mental health, physical function | OKS, Satisfaction |
| Deshmukh and colleagues 2002161 | 139; 12 months | BMI | NHP |
| Gandhi and colleagues 2010160 and Gandhi and colleagues 2010166 | 551; mean 3 years | BMI, mental health | WOMAC, SF-36 Role Physical, SF-36 Physical Function |
| Núñez, and colleagues 2009159 | 112; 7 years | BMI | WOMAC function and pain |
| Rajgopal and colleagues 2008162 | 550; 1 year | BMI | WOMAC |
| Scott and colleagues 2010165 | 1141; 12 months | Mental health, pain, physical function | Satisfaction |
Body mass index
There is a strong association between high BMI (≥ 30 kg/m2) and the development of knee osteoarthritis. 137 People with high BMI are also more likely to require a TKR than those with lower BMI. 138,167 For example, in a cohort of 315,495 people in Norway, men and women in the top quartile of BMI (≥ 27.3 kg/m2) had relative risks compared with the lowest quartile (< 21.6 kg/m2) of undergoing a TKR of 6.16 (95% CI 4.23 to 8.95; p < 0.0001) and 11.06 (95% CI 7.83 to 15.62; p < 0.0001), respectively. 167
Analyses of the National Joint Registry for England and Wales3 show that 56% of patients undergoing TKR in 2012 had a BMI of 30 kg/m2 or greater. In the RESTORE APEX RCT including 311 patients with TKR the mean BMI was 32.6 kg/m2 (SD 6.5 kg/m2, range 17.0–56.2 kg/m2). The distribution is shown in Figure 10. Thirteen per cent of patients had a BMI of 40 kg/m2 or greater.
FIGURE 10.
Distribution of BMI in the APEX TKR cohort.
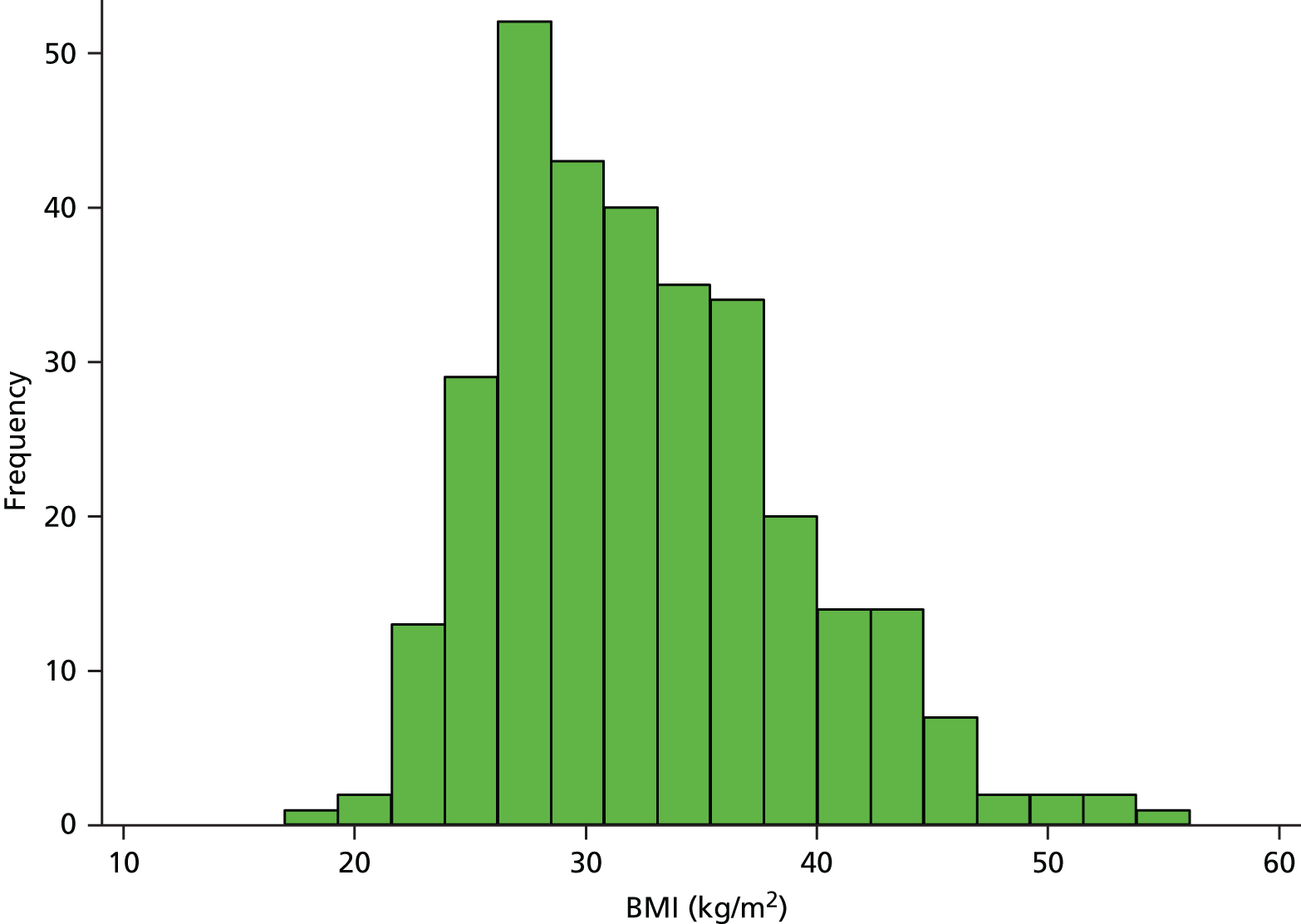
Although clearly of potential importance as a treatment for severe knee pain, irrespective of a patient’s BMI, concern has been expressed about the outcomes of TKR in patients considered to be obese. 137,168,169 Previous reviews have suggested that patients with a high BMI receiving TKR are at increased risk of deep infection,170–172 complications171–173 and need for revision surgery. 170,173 Long-term outcomes measured by surgeon assessed scores after TKR may be poorer in patients with a BMI of ≥ 40 kg/m2 but not in patients with BMI of 30–40 kg/m2. 173 No previous systematic review has considered the association of pre-operative BMI and long-term patient-reported outcomes.
Our systematic literature search identified 13 articles that met the inclusion criteria and were included in the review. 46,127,147,149,151–155,159–162 Details of these studies are summarised in Table 5. As a marker of generalisability, one study reported a registry analysis,147 eight studies included multiple centres,46,127,149,151–155 and four studies included patients from a single centre. 159–162
Body mass index as a predictor of joint specific pain and function
Four studies assessed the impact of BMI on joint specific PROMs. 154,159,162,166 These provided a composite measure of pain and functional limitations, specifically the OKS and total WOMAC score.
In the multicentre Australian study reported by Naylor and colleagues,154 146 patients (90% eligible) were followed up at 12 months after TKR. The mean BMI in this cohort was 32.8 kg/m2 (SD 5.7 kg/m2). In multivariable analysis including age, sex and knee range of movement, but not comorbidities, patients with higher BMI reported worse long-term OKS.
Gandhi and colleagues166 followed up a cohort of 677 patients with a total of 889 TKR operations (per cent eligible not reported) conducted by three surgeons in Canada 12 months after surgery. 166 The overall mean BMI in this cohort was not reported but the inclusion of patients with BMI of > 40 kg/m2 is implied in the metabolic syndrome groupings shown. After adjustment for age, sex, comorbidities and baseline WOMAC scores, BMI of > 30 kg/m2 was associated with poorer long-term WOMAC scores.
Patients in the Canadian study reported by Rajgopal and colleagues162 were treated with TKR by four surgeons at a single centre. At 12 months, 550 patients (per cent eligible not reported) were assessed with the WOMAC score. In this cohort, 12.5% of patients had a BMI of ≥ 40 kg/m2. BMI was analysed as a dichotomous variable < 40 or ≥ 40 kg/m2. In multivariable analysis with adjustment for age, sex, mental health, prior contralateral knee replacement, pre-surgical WOMAC score and comorbidities affecting gait, patients with a BMI of 40 kg/m2 had a poorer long-term WOMAC score.
In a single-centre Spanish study, Núñez and colleagues159 followed up 112 patients (77% eligible) 7 years after TKR. A total of 12.5% of patients in this study had a BMI of > 35 kg/m2. BMI was treated as a categorical variable with groups of < 35 kg/m2 and ≥ 35 kg/m2. In multivariable analyses with age, sex, comorbidities, sociodemographic and clinical characteristics, intraoperative variables, inpatient variables, postoperative clinical variables and pre-operative WOMAC scores, long-term WOMAC pain and function were poorest in patients with BMI of > 35 kg/m2.
Evidence on the association between BMI and long-term joint specific pain and function is largely based on analyses where patients with particularly high BMI levels are compared with those with lower levels. There was evidence that those with high BMI did not achieve the levels of joint specific physical function and pain control seen in those with a lower BMI. This was supported by the one study with an additional marker of quality (multiple centres). 154 However, this study did not include comorbidities in multivariable analysis. Across all studies, there was insufficient information to consider the overall range of BMI in relation to joint specific patient outcomes.
Body mass index as a predictor of WOMAC function scores
Six studies explored the impact of BMI on WOMAC function scores. 46,127,152,153,155,159
In a study including 860 patients (70% eligible at 2 years) from several countries, Lingard and colleagues46 reported follow-up at 12 and 24 months after TKR. BMI was treated as a continuous variable and the overall mean was 29.4 kg/m2 (SD 5.8 kg/m2). In multivariable analysis with age, sex, WOMAC, SF-36 mental health, knee flexion, working status, education, income, comorbidities and country, increasing pre-operative BMI was associated with poorer WOMAC function at 12-month follow-up, but not 24-month follow-up. The size of the effect was small compared with that observed for pre-operative WOMAC function score and number of comorbidities.
In a study in multiple Canadian centres, Jones and colleagues127 followed up an estimated 209 patients (72% eligible) at 3 years after TKR. A total of 19% of patients had a BMI of ≥ 35 kg/m2 before surgery. In multivariable analysis with age, sex and comorbidities, BMI was treated as a binary variable (< 35 kg/m2 or ≥ 35 kg/m2). Patients with BMI of ≥ 35 kg/m2 had a poorer long-term WOMAC function outcome.
In a study in multiple centres in Greece, Papakostidou and colleagues155 followed up 204 patients (90% eligible) 12 months after TKR. In this cohort, 52.9% of patients had BMI of ≥ 30 kg/m2. BMI was analysed as a binary variable. In multivariable analysis with age, sex, education, social support, place of residence and baseline status of knee, long-term WOMAC function levels were similar in patients with BMI under or over 30 kg/m2.
Perruccio and colleagues152 followed up 435 patients (88% eligible) at multiple Canadian centres at a mean of 12.5 months after TKR. In this cohort, 45.3% of patients had a BMI of ≥ 30 kg/m2. The authors included age, sex, education, comorbidities, other painful joints and WOMAC pain and function in multivariable analysis with BMI treated as a binary variable. Patients with BMI of < 30 kg/m2 and ≥ 30 kg/m2 reported similar long-term WOMAC function.
Sullivan and colleagues153 followed up 120 patients (per cent eligible not reported) from multiple Canadian centres 12 months after TKR. 153 Patients in this cohort had a mean BMI of 30.8 kg/m2. In multivariable analysis with age, sex, comorbidities, pain, function, surgery, pain catastrophising, pain-related fear of movement and depression, BMI was treated as a continuous variable. BMI did not predict long-term WOMAC function.
Núñez and colleagues159 followed up 112 patients (77% eligible) 7 years after TKR at a single centre in Spain. In multivariable analysis the authors included age, sex, comorbidities, sociodemographic and clinical characteristics, intraoperative variables, inpatient variables, postoperative clinical variables and pre-operative WOMAC scores. BMI was treated as a binary variable and those with BMI of ≥ 35 kg/m2 had worse long-term WOMAC function.
Overall, there was some suggestion from two studies that patients with more extreme levels of pre-operative BMI (≥ 35 kg/m2) had poorer long-term physical function. Only one of these studies had an additional marker of good quality. In two studies for which the analyses applied a division of less than or greater than 30 kg/m2, there was no apparent difference in functional outcome. 152,155 Both studies had two additional markers of study quality. In the two studies for which BMI was analysed as a continuous variable, there was either no association153 or a weak association46 between increasing levels of BMI and long-term WOMAC function.
Body mass index as a predictor of WOMAC pain scores
Six studies looked at whether or not BMI was a predictor of WOMAC pain at ≥ 12 months after TKR. 46,127,152,153,155,159
In a multicentre study from Canada, Jones and colleagues127 followed up an estimated 209 patients (72% eligible) at 3 years after TKR. A total of 19% of patients had BMI of ≥ 35 kg/m2. In multivariable analysis with adjustment for age, sex and comorbidities, BMI was treated as a binary variable (< 35 kg/m2 or ≥ 35 kg/m2). There was a borderline significant association between BMI and trajectory of pain recovery up to 3 years favouring patients with BMI of < 35 kg/m2.
Lingard and colleagues46 reported a multicentre study including 741 patients (86% eligible at 1 year) from the UK, USA and Australia with TKR followed up at 12 and 24 months. 46 The mean BMI was 29.4 kg/m2 (SD 5.8 kg/m2). In multivariable analysis, the authors included age, sex, WOMAC, SF-36 mental health, knee flexion, working status, education, income, comorbidities and country. With BMI treated as a continuous variable, there was no association between BMI and long-term WOMAC pain score at 12 months and 24 months.
Papakostidou and colleagues155 followed up 204 patients (90% eligible) from multiple centres in Greece 12 months after TKR. A total of 52.9% of patients had BMI of ≥ 30 kg/m2. In multivariable analysis with age, sex, education, social support, place of residence and baseline status of knee, BMI was treated as a binary variable. Long-term WOMAC pain levels were similar in patients with BMI under or over 30 kg/m2.
Perruccio and colleagues152 followed up 435 patients (88% eligible) at multiple Canadian centres at a mean of 12.5 months after TKR. A total of 45.3% of patients had a BMI of ≥ 30 kg/m2. The authors included age, sex, education, comorbidities, other painful joints and WOMAC pain and function in multivariable analysis with BMI treated as a categorical variable. There was no association between BMI and long-term WOMAC pain.
In a study of patients with TKR at multiple centres in Canada, Sullivan and colleagues153 followed up 120 patients (per cent eligible not reported) at 12 months after surgery. The mean BMI in this cohort was 30.8 kg/m2. The authors reported a multivariable analysis with BMI analysed as continuous variable and adjustment for pain, function, age, sex, comorbidities, surgery duration, surgeon, pain catastrophising, pain-related fear of movement and depression. In this analysis BMI was not a predictor of long-term WOMAC pain.
In a single-centre study from Spain, Núñez and colleagues159 followed up 112 patients (77% eligible) 7 years after TKR. The mean BMI in this cohort was 30.7 kg/m2. In multivariable analysis with age, sex, comorbidities, sociodemographic and clinical characteristics, intraoperative variables, inpatient variables, postoperative clinical variables and pre-operative WOMAC scores, patients with BMI of ≥ 35 kg/m2 had significantly worse WOMAC pain scores than patients with BMI of < 35 kg/m2. However, the analysis in this study was limited by the small number of patients in the high BMI group (n = 14).
In the six studies with pain measured using the WOMAC score, there was little to suggest that pre-operative BMI was a determinant of long-term pain with the possible exception of high body mass index (≥ 35 kg/m2). At these high levels of BMI, patients may be more likely to report long-term pain.
Body mass index as a predictor of general health outcomes
Three studies explored the influence of BMI on general health outcomes after TKR. 46,160,161 Outcomes reported were SF-36 domains or the Nottingham Health Profile (NHP).
Lingard and colleagues46 reported an international study including 860 patients (70% eligible) receiving TKR at multiple centres. The mean BMI was 29.4 kg/m2 (SD 5.8 kg/m2). Higher BMI was a predictor of poorer SF-36 physical function scores at 24 months but not at 12 months after surgery. Other variables included in this multivariable analysis were age, sex, patient-reported outcomes, mental health, knee flexion, working status, education, income, comorbidities and country.
Deshmukh and colleagues161 reported a UK study with 139 patients (77% eligible) treated by a single surgeon with follow-up at 12 months. In this study, the mean BMI was 28 kg/m2 (SD 4.5 kg/m2) and only two patients initially eligible had a BMI of ≥ 40 kg/m2. In multivariable analysis including age, sex, side of arthritis, comorbidities, baseline NHP and knee scores, BMI accounted for only a small percentage of the variation in long-term NHP scores.
In a single-centre study from Canada, Gandhi and colleagues160 followed up 551 patients (per cent eligible not reported) at a mean of 3 years after surgery. The mean BMI in patients in this study was 30.1 kg/m2 (SD 6.3 kg/m2). After adjustment for age, sex, ethnicity, education, comorbidities and SF-36 mental health status, BMI was not a predictor of long-term SF-36 role physical or physical function scores.
In summary, there is no clear evidence that BMI is a predictor of long-term general health outcomes after TKR. However, evidence is lacking at higher levels of BMI.
Body mass index as a predictor of change in patient-reported outcomes
Four studies examined the association between BMI and change in patient-reported outcomes from pre- to post TKR in multivariable statistical analysis. 147,149,151,162 In the two studies reporting eligibility, 53.4%147 and 60.6%151 of patients were not followed up. These high rates were largely explained by the study designs, a 1-year registry follow-up147 and a follow-up of patients placed on the waiting list for TKR after a mean of 6 years. 151
Franklin and colleagues147 reported a 12-month follow-up of US registry data including 8050 patients (46% eligible). No information on mean levels of BMI was included. BMI was analysed as a categorical variable with groups of < 30 kg/m2, 30–40 kg/m2 and > 40 kg/m2. In multivariable analysis with age, sex, mental health, physical health, diagnosis and quadriceps strength, patients with BMI of > 40 kg/m2 had less long-term functional gain as measured by the SF-12 PCS.
In the study in 3177 patients (per cent eligible not reported) from multiple centres reported by Alzahrani and colleagues,149 the mean BMI was 31 kg/m2 and was analysed as a continuous variable. 149 In a statistical model included age, sex and comorbidities, BMI was not a significant predictor of achieving a minimal clinical improvement on the OKS or WOMAC score at 12 months after surgery.
The study reported by Cushnaghan and colleagues151 included 259 patients (39% eligible) from three UK health districts followed up for a mean of 6 years after surgery. A total of 41.7% of patients followed up had BMI of ≥ 30 kg/m2 before surgery. Multivariable analysis included age, sex, SF-36, smoking habits, comorbidities, Kellgren and Lawrence grade, previous knee injury, other painful joints and Heberden’s nodes. The authors noted that patients with a BMI of ≥ 30 kg/m2 had a similar improvement in SF-36 PCSs compared with patients with BMI of < 30 kg/m2.
Rajgopal and colleagues162 followed up 550 patients (per cent eligible not reported) at a single centre 1 year after TKR. A total of 12.5% of patients had a BMI of ≥40 kg/m2. The authors included age, sex, mental health, prior contralateral surgery, WOMAC score and comorbidities in the multivariable model. There were no differences in long-term improvement in WOMAC function between patients with BMI of ≥40 kg/m2 compared with those with BMI of ≤ 40 kg/m2.
In summary, the evidence suggests that greater BMI is not associated with less improvement in PROMs after TKR, although there is the possibility from one study with no additional markers of good quality that patients with more extreme levels of BMI have a poorer improvement in general functional health. 147
Pre-surgical mental health
In their systematic review with searches of MEDLINE and EMBASE to January 2011, Vissers and colleagues143 identified 19 studies including 6274 patients receiving TKR. There was strong evidence that pre-operative pain catastrophising was associated with increased pain in the first year after surgery and that poorer pre-operative mental health was associated with worse long-term physical function and pain. The authors identified no strong evidence that pre-operative depression influenced functioning in the year after TKR.
In our systematic review, we identified 12 studies with ≥ 100 patients with TKR followed up for ≥ 12 months with pre-surgical mental health included in multivariable analyses. 50,147,148,150,155–158,160,163–165 Two studies reported data from joint registries,147,148 five studies included patients from multiple centres150,155–158 and five studies collected data from patients treated at a single centre. 50,160,163–165 Study details are summarised in Table 5.
In an analysis of the National Joint Registry for England and Wales, Baker and colleagues148 reported on 22,691 patients (55% eligible) with TKR followed up for at least 6 months and up to 12 months after surgery. The authors included age, OKS, EQ-5D, disability, general health, comorbidities, and surgical and hospital variables in a multivariable model. Pre-operative anxiety and depression measured using the EQ-5D were associated with poorer long-term improvement in OKS and EQ-5D.
Franklin and colleagues147 followed up 8050 patients (47% eligible) from a US joint registry 12 months after TKR. In multivariable analysis, the authors included SF-12 PCS, sex, age, BMI, osteoarthritis diagnosis and poor quadriceps strength in the statistical model. The pre-operative SF-36 mental component score was an independent predictor of poor long-term physical function measured with the SF-36 PCS.
Heck and colleagues157 followed up 268 patients (92% eligible) 24 months after TKR at multiple US centres. The authors conducted a multivariable analysis with age, ethnicity, sex, poverty, patient health status, WOMAC scales, SF-36 physical component, knee ROM, comorbidities, surgical factors and joint problems in the other knee. Poor pre-operative SF-36 mental health was associated with less improvement in the SF-36 PCS.
Lingard and Riddle150 followed up 628 patients (70% eligible at 2 years) who had received a specific TKR prosthesis at 12 international centres. 150 In a multivariable model with age, sex and comorbidities, patients with psychological distress identified using the SF-36 mental health component had worse WOMAC pain at 12 and 24 months than non-distressed patients. There was no strong evidence to support such a relationship with long-term WOMAC function although there was a trend in a similar a direction. The authors reported that there were no strong associations between pre-operative psychological distress and changes in function and pain over either follow-up period.
In the study of Merle-Vincent and colleagues,158 264 patients (87% eligible) with TKR at multiple centres in France were followed up after 24 months. In a multivariable analysis with age, sex, BMI, Lequesne index and joint space narrowing, there was no strong evidence that patients with feelings of depression before surgery were more dissatisfied with their TKR.
Papakostidou and colleagues155 followed up 204 patients (90% eligible) at two centres in Greece, 12 months after TKR. In a multivariable analysis with age, sex, BMI, education, social support, place of residence and baseline status of knee, the extent of pre-operative depressive symptoms, measured with the 10-Item Center for Epidemiologic Studies Short Depression Scale, were predictive of long-term VAS pain. The authors concluded that depressed mood had a strong positive correlation with long-term pain and functional limitation.
Singh and Lewallen156 followed up 7139 patients (65% eligible at 2 years) with TKR at multiple US centres 2 years after surgery. 156 Pain outcome was measured using a standardised Mayo Clinic questionnaire. In a multivariable analysis with age, sex, BMI, operative diagnosis and comorbidities, anxiety identified in the comorbidity assessment was an independent predictor of moderate to severe pain at 2 years, whereas depression was marginally not. The authors also followed patients up at 5 years but losses to follow-up were greater than at 2 years. There was evidence for an association between both pre-operative anxiety and depression and moderate to severe pain at this longer follow-up.
In a study in 165 patients (per cent eligible not reported) with TKR at a single US centre, Ayers and colleagues163 reported follow-up at 12 months. In multivariable analysis with age, sex, pre-operative function and comorbidities, poorer pre-operative SF-36 emotional health was associated with smaller improvements in SF-36 PCS and WOMAC physical function scores.
Brander and colleagues50 followed up 116 patients (per cent eligible not reported) at a single US centre at 12 months after TKR. In multivariable analysis with age, sex, other demographics and physiological, psychometric and pain variables, pre-operative depression and anxiety were associated with greater long-term VAS pain.
In their study of 966 patients (per cent eligible not reported) with TKR followed up for 12 months at a single UK centre, Clement and colleagues164 reported a multivariable analysis including age, sex, comorbidities, socioeconomic deprivation, OKS and the SF-12 components. Poorer pre-operative SF-36 mental health was associated with a poorer long-term improvement in OKS but was not related to satisfaction.
Gandhi and colleagues160 reported the follow-up of 551 patients (per cent eligible not reported) with TKR at a single Canadian centre at a mean of 3 years. In multivariable analysis including age, sex, ethnicity, BMI, education and comorbidity, patients with poorer pre-operative mental health according to the SF-36 had worse long-term WOMAC and SF-36 functional outcomes.
Scott and colleagues165 reported 12-month follow-up at a single UK centre of 1414 patients (87% eligible) who had received a specific TKR prosthesis. 165 In multivariable analysis with age, sex, SF-12 physical component, OKS and comorbidities, patients with depression or a poor SF-12 mental health status were more likely to be dissatisfied with their long-term outcome.
Studies investigating the relationship between pre-operative mental health and long-term patient outcomes used generic measures, specifically the SF-36 and SF-12 mental health components or more specific measures of anxiety or depression. For generic measures, poor mental health status before TKR was a predictor of long-term increased pain or poorer function in all seven studies that reported it. 147,150,157,160,163–165 There was a consistent suggestion in three studies that patients with anxiety had worse long-term pain or other patient outcomes. 50,148,156 In five studies, patients with depression or depressive symptoms had poorer long-term pain or functional outcomes. 50,148,155,156,158 Associations between aspects of pre-operative mental health and long-term patient outcomes were apparent in studies with one or two additional markers of good-study quality. For satisfaction as an outcome, the associations with pre-operative mental health measures were inconsistent.
Pre-surgical pain
We identified five studies with ≥ 100 patients with TKR followed up for ≥ 12 months with pre-surgical pain included in multivariable analyses. Four studies included patients from multiple centres,46,153,155,157 and one study was based on analyses of patients from a single centre. 165 Study details are summarised in Table 5.
Heck and colleagues157 followed up 268 patients (92% eligible) at multiple US centres 24 months after TKR. The authors conducted a multivariable analysis with the SF-36 mental health component, age, ethnicity, sex, poverty, patient health status, WOMAC scales, SF-36 physical component, knee ROM, comorbidities, surgical factors and joint problems in the other knee. In this analysis pre-operative WOMAC pain was not a predictor of the long-term SF-36 physical component.
Lingard and colleagues46 followed up 678 patients (79% eligible) from multiple international centres at 12 and 24 months after TKR. In multivariable analysis with age, sex, income, education, BMI, flexion, country, centre and comorbidities, one of the strongest determinants of long-term WOMAC pain was the pre-operative WOMAC pain score. The authors considered the difference in WOMAC pain scores to be clinically important.
In a study of 204 patients (90% eligible) from multiple centres in Greece, Papakostidou and colleagues155 reported follow-up at 12 months after TKR. In multivariable analysis including depression, sex, BMI, education, social support, age, place of residence and baseline status of knee, pre-operative WOMAC pain predicted long-term WOMAC pain.
Sullivan and colleagues153 followed up 120 patients (per cent eligible not reported) 12 months after TKR conducted at multiple Canadian centres. Greater pain catastrophising and pain-related fear of movement were predictors of poorer long-term WOMAC pain and function outcomes in multivariable analysis including age, sex, BMI, comorbidities, surgical factors, pain catastrophising, pain-related fear of movement and depression.
Scott and colleagues165 followed up 1414 patients (87% eligible) at a single UK centre 12 months after receiving a specific TKR prosthesis. Although significantly associated with long-term dissatisfaction in univariate analysis, there was no association between pre-operative OKS pain and dissatisfaction in a multivariable mode including OKS function, SF-12 physical and mental components, age, sex and comorbidities.
Studies comparing pre- and postoperative pain measures consistently showed that patients receiving TKR with higher levels of pre-operative pain had worse long-term pain. All studies had one or two additional markers of good quality. Associations with long-term physical function and satisfaction were inconsistent.
Pre-surgical physical function
We identified five studies with ≥ 100 patients with TKR followed up for ≥ 12 months with pre-surgical physical function included in multivariable analyses. 46,157,163–165 Details of studies are summarised in Table 5. Two studies were conducted in multiple centres46,157 and three studies in a single centre each. 163–165
Heck and colleagues157 followed up 268 patients (92% eligible) 24 months after TKR at multiple US centres. The authors included WOMAC function in a multivariable model with SF-36 mental health, age, ethnicity, sex, poverty, patient health status, SF-36, knee ROM, comorbidities, surgical factors and joint problems in the other knee. Patients with poorer WOMAC function had the greatest long-term improvement in SF-36 PCS. The authors noted that patients who were more likely to show improvement to general health had functional impairment at the time of surgery.
Lingard and colleagues46 followed up 678 patients (86% at 1 year) from multiple international centres at 12 and 24 months after TKR. The authors included age, sex, income, education, BMI, flexion, country, centre and comorbidities in multivariable analysis. Poor pre-operative WOMAC function was an independent predictor of poor long-term WOMAC function outcome.
In a study including 165 patients (per cent eligible not reported) with TKR from a single US centre, Ayers and colleagues163 reported follow-up at 12 months. The authors included pre-operative WOMAC physical function, age, sex and comorbidities in multivariable analysis. The model with increasing age and poorer physical function predicted a poorer SF-36 and WOMAC physical function outcome.
Clement and colleagues164 followed up 966 patients (per cent eligible not reported) for 12 months after TKR at a single UK centre. 164 Patients with a better OKS score before surgery had better long-term OKS but were more likely to be dissatisfied with their operation in multivariable analyses with age, sex, comorbidities, socioeconomic deprivation, SF-12 physical component and back pain.
Scott and colleagues165 reported a 12-month follow-up of 1414 patients (87% eligible) who had received a specific TKR prosthesis at a single UK centre. In multivariable analysis with SF-12 mental component, age, sex, depression and comorbidities, lower pre-operative OKS function was associated with greater satisfaction.
The relationship between pre-operative physical function and long-term patient outcomes after TKR was complex. In three studies there was a simple association between low pre-operative function before surgery and a poor long-term functional outcome. However, in another study patients with worse pre-operative function had a greater improvement in physical function. In two studies those with lower pre-operative function were more likely to be satisfied with their operation. Results of studies were consistent in those with one or two additional markers of good quality.
Discussion
Systematic review of cohort studies is a pragmatic exercise requiring specific inclusion criteria. In any population of people followed up before and after surgery the opportunity exists to carry out multivariable analyses. Inclusion of published analyses which may focus on the interests of researchers, editors, reviewers and readers is prone to bias.
For our reviews, we limited inclusion to studies with exclusively hip or knee replacement-based analyses. We included studies for which separate analyses were reported but not when data were combined as ‘joint replacement’. Studies were excluded if they had < 100 patients and a long-term outcome was classified as ≥ 12 months. Again this represents a pragmatic approach owing to the large number of studies reported in the literature.
In the context of study quality, proportion not followed up probably relates more to generalisability as multivariable analyses include only patients with variables measured before and after surgery (or some estimate).
Our overview of studies reporting long-term patient outcomes according to pre-operative BMI suggests that many patients with high BMI benefit from total hip and knee replacement with long-term improvements to physical function and reduction in long-term pain. However, there was some suggestion that the absolute levels of physical function and pain achieved in patients with especially high BMI may be somewhat lower.
Patients with depression before surgery may have poorer long-term pain and functional outcomes after total hip and knee replacement. For patients with anxiety or poor general psychological health, there was evidence for a relationship with worse pain and functional outcomes in patients receiving TKR but evidence in THR was equivocal.
Patients with better physical function and lower pain before total hip and knee replacement generally achieved a better recovery in terms of joint specific pain and function. Patients with poor physical function before surgery may have greater absolute improvement.
Conclusion
Longitudinal studies reporting the associations between pre-surgical factors and long-term patient outcomes after total hip or knee replacement suggest that interventions before surgery to optimise a patient’s physical function, pain levels and psychological health merit further study. In the context of advanced osteoarthritis for which conservative treatments have not controlled symptoms, an exercise and education intervention may aim to maintain functional levels or prevent further decline and facilitate recovery. Pain management and psychological counselling may also have a role in preparing patients for surgery and subsequent rehabilitation.
Systematic review of comorbid conditions and long-term patient-centred outcomes after total hip and knee replacement
Background
Multimorbidity is common in older people, with approximately 60% of people aged ≥ 65 years reporting two or more health conditions. 174 In a population aged ≥ 75 years, 60% reported one and 33.4% reported two or more health problems. 175 Among patients with advanced symptoms of osteoarthritis recruited into the APEX study, comorbid conditions were common; in patients receiving total hip and knee replacement, 64% and 71% of patients, respectively, reported at least one condition additional to osteoarthritis.
Details of prevalence of specific comorbid conditions in the APEX study are summarised in Table 6. In people receiving THR, commonly reported comorbid conditions were degenerative disc disease (24.7%), osteoporosis (15.0%), visual impairment (14.0%), upper gastrointestinal problems (14.7%), cardiovascular disease (12.0%), depression (13.7%), hearing impairment (8.3%), anxiety (11.7%) and diabetes (6.7%). In people receiving TKR, the commonly reported comorbid conditions were degenerative disc disease (22.9%), osteoporosis (18.5%), visual impairment (19.5%), upper gastrointestinal problems (18.2%), cardiovascular disease (20.2%), depression (10.8%), hearing impairment (15.2%), anxiety (9.4%) and diabetes (14.1%).
| Condition | THR | TKR | ||||
|---|---|---|---|---|---|---|
| Female | Male | All | Female | Male | All | |
| Angina | 3.9% | 4.1% | 4.0% | 8.5% | 11.8% | 10.1% |
| Congestive heart failure | 0.6% | 1.6% | 1.0% | 1.3% | 1.4% | 1.3% |
| Heart attack | 4.5% | 4.9% | 4.7% | 4.6% | 12.5% | 8.4% |
| Stroke or transient ischaemic attack | 2.8% | 4.9% | 3.7% | 7.2% | 9.7% | 8.4% |
| Peripheral vascular disease | 1.1% | 3.3% | 2.0% | 2.0% | 2.1% | 2.0% |
| Any cardiovascular disease | 8.4% | 17.2% | 12.0% | 16.3% | 24.3% | 20.2% |
| Degenerative disc disease | 28.1% | 19.7% | 24.7% | 23.5% | 22.2% | 22.9% |
| Asthma | 14.6% | 5.7% | 11.0% | 17.0% | 8.3% | 12.8% |
| Chronic obstructive pulmonary disease | 2.2% | 2.5% | 2.3% | 7.8% | 2.8% | 5.4% |
| Diabetes type 1 and 2 | 6.2% | 7.4% | 6.7% | 12.4% | 16.0% | 14.1% |
| Osteoporosis | 15.2% | 14.8% | 15.0% | 18.3% | 18.8% | 18.5% |
| Neurological diseases | 1.1% | 1.6% | 1.3% | 0.7% | 2.8% | 1.7% |
| Upper gastrointestinal disease | 14.0% | 15.6% | 14.7% | 23.5% | 12.5% | 18.2% |
| Depression | 18.0% | 7.4% | 13.7% | 13.7% | 7.6% | 10.8% |
| Anxiety or panic disorders | 12.9% | 9.8% | 11.7% | 12.4% | 6.3% | 9.4% |
| Visual impairment | 15.7% | 11.5% | 14.0% | 20.3% | 18.8% | 19.5% |
| Hearing impairment | 6.7% | 10.7% | 8.3% | 11.1% | 19.4% | 15.2% |
| Total number of patients | 178 | 122 | 300 | 153 | 144 | 297 |
Research on pre-surgical comorbidities has often focused on associations with adverse events. In a US study including > 950,000 patients with joint replacement, the in-hospital rate of serious postoperative adverse events including infection, non-healing wounds, pulmonary embolism and vascular complications was 2.6%. 36 In 260 patients with knee replacement, Kirschner and colleagues176 reported that 6% of patients had serious adverse events within 3 months of knee replacement. More general adverse events occurred in a further 26% of patients.
Adverse events are associated with lower patient satisfaction and poorer long-term HRQoL. In a study of 264 patients followed up 2 years after TKR, patients were more likely to be satisfied if they had no complications after surgery [odds ratio (OR) 6.6, 95% CI 1.8 to 24.7; p = 0.004]. 158 In another study of 112 patients followed up 7 years after TKR, the number of post-discharge complications was associated with poorer WOMAC pain (p < 0.001), stiffness (p = 0.018) and function (p = 0.042). 159 Furthermore, in a study of 1703 patients with TKR, 19% were dissatisfied with their outcome. 177 In a multivariable model, the OR for a patient being dissatisfied was 1.86 in the presence of a complication requiring hospital admission.
The majority of adverse events, after appropriate treatment, mobilisation and rehabilitation, have no serious effect on long-term recovery. For some patients the adverse events are more serious and the consequences severe. For example, patients with surgical site infections describe extreme pain, prolonged immobilisation, isolation and insecurity, and feelings of hopelessness. 178 If untreated with revision surgery, infection can result in severe pain, persistent dislocation and death. 179 Similarly, the consequences of pulmonary embolism are extremely serious, with a 3-month mortality rate of about 17.4%. 180 Deep-vein thrombosis is more common and, although treated effectively in most people with anticoagulant therapy, associated costs are substantial. In a Canadian study, average medical costs were CA$2503 [approximately £1615 (cost correct as of 2010)] with a further CA$2677 [approximately £1727 (cost correct as of 2010)] attributable to non-medical costs including loss of earnings, assistance and transport. 181 Khan and colleagues182 estimated that complications after non-cardiac surgery may lead to increases in hospital stays of 114%, with significant associated hospital costs.
Solomon and colleagues183 explored the predictors of adverse events (death, infection, pulmonary embolism, pneumonia, and myocardial infarction) within 3 months of knee replacement in a cohort of 9073 patients from 276 US hospitals. The 37% of patients with one or more comorbid health conditions had a 50% greater risk of an adverse event.
It is important to be aware of the increased risk of adverse events and poorer long-term patient outcomes in patients with additional comorbid conditions. Scores reflecting the extent of comorbidity are widely used to assess risk of death and adverse events in patients requiring joint replacement surgery. However, to guide care of patients receiving joint replacement it is also important to know if patients with specific comorbidities have different long-term patient-reported outcomes from those who are unaffected. Appropriate pre-surgical interventions with assessment and management of clinical conditions may help to reduce the incidence of adverse events and improve long-term patient outcomes after total hip and knee replacement.
Our aim was to review the evidence on the associations between comorbid conditions and long-term patient outcomes. When good-quality evidence was available on associations between comorbid conditions and post-surgical adverse events, we summarise this briefly.
Particular comorbid conditions prevalent in populations of patients receiving total hip or knee replacement and with potential for treatment were considered, specifically diabetes, cardiovascular disease, hypertension, thyroid disease and anaemia.
Methods
| General methods | As described in Systematic review methods |
| Databases and dates | MEDLINE and EMBASE from inception to 16 October 2013 |
| Search strategy | Joint replacement/specified patient centred outcomes. MEDLINE search strategy based on terms in Appendix 3. In addition, a series of specific searches linking total hip or knee replacement with specific comorbidities: diabetes, cardiovascular disease, hypertension, anaemia, thyroid disease |
| Study design | Cohort studies |
| Patients | Total hip or knee replacement |
| Follow-up | ≥ 12 months |
| Data extraction | Date of publication, hip or knee, country, baseline dates of study, follow-up duration, pre-surgical measures |
| Outcomes | Patient-reported outcomes |
| Quality assessment | Quality assessment related to generalisability as described in the systematic review of pre-operative predictors of patient-centred outcomes after total hip and knee replacement |
Results
Total hip replacement
The review flow diagram is shown in Figure 7. Searches identified five studies reporting analyses of specific comorbid conditions identified before surgery and long-term patient-reported outcomes. 127,130,166,184,185 Two of these studies reported separate analyses of different comorbidities in the same cohort. 184,185
Study characteristics are summarised in Appendix 7 with brief details in Table 7.
| Study | Number of patients; follow-up | Comorbidity | Outcome measures |
|---|---|---|---|
| Multiple centres | |||
| Cushnaghan and colleagues 2007184 Judge and colleagues 2012185 | 249; mean approximately 96 months | Hypertension | SF-36 PCS |
| Jones and colleagues 2012127 | Approximately 167 (231 eligible); 3 years | Cardiovascular disease, diabetes | WOMAC function and pain |
| Judge and colleagues 2012185 Cushnaghan and colleagues 2007184 | 249; mean approximately 96 months | Diabetes, thyroid disease | SF-36 |
| Single centre | |||
| Anakwe and colleagues 2011130 | 850; 12 months | Diabetes, hypertension | Satisfaction |
| Gandhi and colleagues 2010166 | 707; 12 months | Diabetes, hypertension | WOMAC |
Diabetes
In the APEX study, 6.7% of patients receiving a THR reported that they had type 1 or type 2 diabetes.
Searches identified four studies reporting long-term patient-reported outcomes in patients according to their pre-surgical diabetic status. Two studies each included patients from multiple centres,127,185 or a single centre. 130,166 The proportion of patients followed up varied from 6.3% to 49.9%, which was largely explained by lower follow-up rates in studies of longer duration.
In a study in two Canadian hospitals, Jones and colleagues127 followed up an estimated 167 patients (72% eligible) after THR. Diabetes mellitus was not a significant independent factor predicting recovery measured by WOMAC function or pain scores in a multivariable analysis including cardiac disease, age, sex, BMI, education, principal diagnosis, living arrangements, type of living accommodation, previous joint surgery, ambulatory status and number of comorbid conditions.
Judge and colleagues127 followed up 249 patients (50% eligible) from two UK health districts at a mean of 96 months. 185 In multivariable analysis with diabetes, hypertension, thyroid disease, age, sex, BMI, smoking habit, previous knee injury, Heberden’s nodes, number of painful joints and radiographic grade, the OR for no improvement in SF-36 physical function was 5.45 (95% CI 0.99 to 29.89) in patients with diabetes compared with non-diabetics. This was close to statistical significance. In another analysis of this cohort, the MD in change in SF-36 physical function was 25.8 points (95% CI 6.8 to 44.9 points) favouring patients with no diabetes. 184 However, both analyses were based on only eight patients with diabetes.
In a study at a single UK centre, Anakwe and colleagues130 followed up 850 patients (84% eligible) with THR for 12 months. Diabetes was not associated with long-term patient satisfaction in multivariable analyses including other comorbidities, age, sex, OHS, SF-12 physical and mental components and musculoskeletal comorbidities (p = 0.227).
Gandhi and colleagues166 followed up 707 patients (83% eligible) after a THR at a single Canadian centre. In a multivariable analysis with BMI, hypertension, hypercholesterolaemia, age, sex, WOMAC score, and cumulative illness rating scale, self-reported diabetic status did not predict the extent of WOMAC function improvement 12 months after THR (p = 0.46). However, in analyses considering the combination of medical conditions relating to metabolic syndrome (hypertension, obesity, hypercholesterolaemia and diabetes), presence of all four conditions was associated with a poorer long-term WOMAC score (p = 0.04).
The four studies we identified did not provide any conclusive evidence on the impact of diabetic status on long-term patient-reported outcomes. 127,130,166,185 This may be explained by good glycaemic management in patients with diabetes receiving THR in the cohorts we identified.
Patients with diabetes may be more likely to have complications after their THR. During the hospital stay, patients with diabetes may be at greater risk of stroke, pneumonia and requirement for a blood transfusion but not prosthetic joint infection. 186 In a literature search relating to infection including all studies reporting outcomes in patients according to diabetic status we identified 10 longitudinal studies with 376,138 patients in which 2487 deep infections were recorded. 186–195 As shown in Figure 11, the relative risk of developing a deep infection was 2.14 (95% CI 1.44 to 3.18) in patients with diabetes compared with non diabetics. We used a random-effects model owing to the high extent of heterogeneity (I2 = 74%). This was largely explained by one large study with follow-up only to hospital discharge. 186
FIGURE 11.
Summary plot of deep infection complications after THR by diabetic status. df, degrees of freedom; M–H, Mantel–Haenszel.

In an analysis of the US National hospital discharge survey including 43,215 patients receiving any orthopaedic surgery, diabetes was associated with an increased risk of inpatient mortality in univariate analysis but this was not statistically significant in multivariable analysis (OR 1.23; p = 0.18). 196
In a study of 16,317 patients with total hip or knee replacement, there was an increased risk of myocardial infarction and venous thromboembolism after joint replacement in patients with diabetes or hypertension. 197 Considering the cluster of hypertension, diabetes, obesity and dyslipidaemia that constitute metabolic syndrome, the risk of myocardial infarction was increased by 128% and the risk of venous thromboembolism more than tripled.
In a US cohort of 1,030,013 patients receiving total hip or knee replacement, patients with diabetes were compared by the level of glycaemic control. 198 In those patients with uncontrolled diabetes, the risk of surgical and systemic complications was significantly higher and mortality greater than in those with controlled diabetes. Danish hip registry data from 3278 patients also suggests increased risk of revision due to deep infection in patients with diabetes, particularly in those with short diabetes duration or diabetic complications probably reflecting poor blood glucose control. 194
Cardiovascular disease
In the APEX study, 12.0% of patients receiving THR reported a cardiovascular-related condition.
Our searches identified one study in multiple centres with multivariable analysis assessing patient-reported outcomes according to presence of cardiovascular disease. 127
In the study of Jones and colleagues127 at two Canadian centres, an estimated 167 patients (72% eligible) with THR were followed up at 3 years. In an analysis adjusted for age, sex, BMI, education, principal diagnosis, living arrangements, type of living accommodation, previous joint surgery, ambulatory status and number of comorbid conditions, patients with cardiac disease had worse long-term WOMAC pain (p = 0.014) and function (p = 0.012), and a slower recovery.
The mortality rate within 90 days after THR is about 1%199 and cardiovascular disease is generally the leading cause of death. 43 Research in patients receiving a THR with comorbid cardiovascular disease has mainly focused on adverse events after surgery.
Ackland and colleagues200 developed a risk index based on patient history of ischaemic heart disease, heart failure and cardiac risk factors. This method was able to stratify patients with elective orthopaedic surgery by risk of in-hospital adverse events. However, with an area under the receiver operating characteristic curve of 0.62 (95% CI 0.57 to 0.67), the predictive ability was modest.
Bozic and colleagues201 developed a risk calculator for 90-day mortality and prosthetic joint infection in patients with THR. The risk model included age, sex, ethnicity and socioeconomic status and 29 pre-operative comorbidities including cardiopulmonary conditions. The authors concluded that levels of risk could be used to counsel patients with heart disease before surgery. Sanders and colleagues202 explored the effect of time since a vascular event (stroke, myocardial infarction and unstable angina) on outcomes of total hip and knee replacement in 414,985 patients. The authors concluded that patients with a vascular event in the year before surgery were at greatest risk of death within 30 days of surgery.
In an analysis considering long-term outcomes, Singh and Lewallen203 noted a greater risk of periprosthetic fractures in patients with heart disease identified before THR.
Hypertension
In a US study including 53,252 Medicare patients with THR, 66% of patients had hypertension before surgery. 201
Our searches identified three studies that included hypertension before surgery in multivariable analysis for the prediction of long-term patient-reported outcomes. 130,166,185
Cushnaghan and colleagues151 reported a multivariable analysis in 249 patients (50% eligible) from two UK health districts followed up for 96 months after THR. Hypertension was not a significant predictor of change in SF-36 physical function score, MD in change 0.5 points (95% CI –6.5 to 7.6 points).
In a single-centre UK study with 850 patients (94% eligible) followed up for 12 months, Anakwe and colleagues130 compared levels of satisfaction with THR in patients with and without hypertension. A total of 8.7% of hypertensive patients were dissatisfied with their THR compared with 6.4% of those without hypertension. This did not reach the specified level of statistical significance (p < 0.10) for inclusion in multivariable analysis.
In their study of metabolic syndrome, Gandhi and colleagues166 followed up 707 patients (83% eligible) 12 months after THR at a single Canadian centre. In multivariable analysis with BMI, self-reported diabetic status, hypercholesterolaemia, age, sex, WOMAC and comorbidity score, hypertension was a significant predictor of lower overall WOMAC improvement at 1 year (p = 0.006).
Overall, there was limited evidence that patients with hypertension identified before surgery have poorer long-term patient outcomes. As described previously, its importance may be as part of the cluster of cardiovascular risk factors that constitute metabolic syndrome.
Considering adverse outcomes after THR, Bozic and colleagues201 reported that patients with hypertension had an increased risk of mortality and joint infection. In a multivariable analysis, hypertension was an independent variable included in a risk equation to identify patients at risk of joint infection and mortality.
Anaemia
We did not identify any studies reporting the association between pre-surgical anaemia and long-term patient-reported outcomes after THR. Searches identified one systematic review by Spahn204 looking at studies of perioperative anaemia and clinical outcomes. The author concluded that pre-operative anaemia in patients with hip and knee surgery was associated with increased need for blood transfusion, infection and death, poorer physical functioning and recovery, and longer hospital stay.
More recent analyses of registries and large cohort studies support this review. Jämsen and colleagues205 reported a multivariable analysis comparing mortality rates in 1998 patients with hip and knee replacement. After extensive adjustment for patient and clinical characteristics including other comorbidities, the hazard ratio for death was 1.47 (95% CI 1.08 to 1.99) in patients with pre-operative anaemia compared with those without. In another multivariable analysis including 15,722 patients reported by Greenky and colleagues,206 pre-operative anaemia was associated with a greater risk of prosthetic joint infection but not mortality. In a multivariable analysis including 40,919 patients with THR followed up for 90 days, Bozic and colleagues207 reported that patients with pre-operative anaemia had a greater risk of prosthetic joint infection (hazard ratio 1.36, 95% CI 1.15 to 1.62). Pre-operative anaemia was not associated with mortality within 90 days in multivariable analysis in this cohort.
O’Malley and colleagues208 identified factors associated with major complications after THR leading to delays in hospital discharge. In multivariable analysis including BMI, pre-operative bleeding disorder, comorbidities and surgical factors, pre-operative anaemia was associated with increased risk of major complications before discharge. Major complications resulted in a mean increase in hospital stay of 62 days.
In a prospective analysis of data from patients with THR at a single centre, Myers and colleagues209 noted a higher infection rate and need for transfusion in patients with pre-clinical anaemia on admission. Inpatient stay was, on average, 18 days in patients with anaemia compared with 11 days in patients with no pre-clinical anaemia.
Research on anaemia and THR has focused on early adverse events and death. Patients with anaemia may be at greater risk of infection and other major complications, are more likely to require blood transfusion and may have a substantially increased length of hospital stay.
Thyroid disease
In the Whickham survey of men with a median age of 58 years and women with a median age of 59 years, the prevalence of hypothyroidism was 1.3% and 9.3%, respectively. 210 This compares with the prevalence of treatment for hypothyroidism in people with severe osteoarthritis of 7.0% and 14.0% in men and women, respectively, in the APEX cohort.
Our searches identified one study with long-term patient outcomes after THR according to whether or not patients had thyroid disease. 185 Details of the study are summarised in Table 7.
In the study of Judge and colleagues,185 249 patients (50% eligible) with THR in two UK health districts were followed up for a mean of approximately 96 months. In univariate analysis, there was no suggestion of a difference in long-term SF-36 physical function in patients with thyroid disease compared with people without thyroid disease (OR for no improvement or worse SF-36 physical function 0.96, 95% CI 0.36 to 2.53). The OR for a meaningful improvement in SF-36 physical function score (at least 30 points) was 0.33 (95% CI 0.07 to 1.49), a non-significant trend favouring patients with no thyroid disease.
Total knee replacement
The review flow diagram is shown in Figure 8. Searches identified six studies that reported analyses of specific comorbid conditions identified before surgery and long-term patient-reported outcomes. 127,151,163–166 Study characteristics are summarised in Appendix 8 with brief details in Table 8.
| Study | Number of patients; follow-up | Comorbidity | Outcome measures |
|---|---|---|---|
| Multiple centres | |||
| Cushnaghan and colleagues 2009151 | 259; mean 6 years | Diabetes, hypertension, thyroid disease | SF-36 physical function |
| Jones and colleagues 2012127 | Estimated 209; 3 years | Cardiovascular disease, diabetes | WOMAC |
| Single centre | |||
| Ayers and colleagues 2005163 | 165; 12 months | Cardiovascular disease, diabetes | WOMAC function, SF-36 physical component |
| Clement and colleagues 2013164 | 966 (number eligible not specified) 12-month follow-up, losses to follow-up not described |
Diabetes, cardiovascular disease, hypertension, anaemia | OKS, satisfaction |
| Gandhi and colleagues 2010166 | 889 (approximately 1067 eligible); 12 months, 16.7% not followed up | Diabetes, hypertension | WOMAC |
| Scott and colleagues 2010165 | 1141 (1290 eligible); 12 months, 13.1% not followed up | Diabetes, cardiovascular disease, hypertension | Satisfaction |
Diabetes
Diabetes is a common comorbid condition in patients receiving TKR. In an Australian cohort of 1214 patients with knee replacement, the prevalence of diabetes was 17.0%. 211 Of 3,672,247 patients discharged from US hospitals after unilateral TKR, about 13% were diabetic. 212 In the APEX study, 14.1% of patients with TKR recruited into a UK RCT had type 1 or type 2 diabetes.
Six studies reported long-term patient outcomes according to pre-operative diabetic status. Of these, two were from multiple centres127,151 and four included patients from a single centre. 163–166
Cushnaghan and colleagues151 followed up 259 patients (39% eligible) from three UK health districts at a mean of 72 months after TKR. In multivariable analysis, a trend for greater improvement in SF-36 physical function in diabetic patients was not statistically significant (mean relative change 3.8, 95% CI –8.5 to 16.2). The analysis included only 16 patients with diabetes.
Jones and colleagues127 followed up an estimated 209 patients (72% eligible) after TKR at two Canadian centres. At 3 years, there was little to suggest that WOMAC pain and function differed according to diabetic status; however, the authors observed that pain and function scores worsened slightly after 6 months among diabetic patients but not in non-diabetics.
Ayers and colleagues163 followed up 165 patients (per cent eligible not reported) from a single US centre at 12 months after TKR. In multivariable analysis there was no association between diabetic status and long-term physical function measured by WOMAC and SF-36 scales.
In a study from a single UK centre with 1141 patients (87% eligible) followed up at 12 months, Scott and colleagues165 found no association between diabetic status and long-term satisfaction after TKR.
In a study from a single Canadian centre, Gandhi and colleagues166 followed up 889 patients (83% eligible) after TKR. Self-reported diabetic status showed a trend for poorer WOMAC score at 12 months but this was not statistically significant (p = 0.07). In analyses considering the combination of medical conditions relating to metabolic syndrome (hypertension, obesity, hypercholesterolaemia and diabetes), in the presence of all four conditions there was a trend for poorer long-term WOMAC score but this was not statistically significant (p = 0.08).
Clement and colleagues164 followed up 966 patients (per cent eligible not reported) at a single UK centre 12 months after TKR. In multivariable analysis, diabetes was not associated with a poorer long-term patient outcome (p = 0.47).
There is no strong evidence to suggest that long-term patient outcomes after TKR differ according to diabetic status; however, much research has focused on the risk of adverse events. In our literature search on the incidence of infection by diabetic status, we obtained data from 14 studies including 633,813 patients in which 3988 deep infections were recorded. 186,191,192,207,211,213–221 In a random-effects meta-analysis shown in Figure 12, the relative risk for infection in patients with diabetes compared with non-diabetics was 2.03 (95% CI 1.54 to 2.67).
FIGURE 12.
Summary plot of deep infection complication after TKR by diabetic status. df, degrees of freedom; M–H, Mantel–Haenszel.
In studies with more general orthopaedic inclusion criteria, patients with diabetes had an increased risk of myocardial infarction and venous thromboembolism. This was particularly evident in patients with the metabolic syndrome cluster of risk factors. 197 There was also a suggestion of an increased risk of inpatient mortality for patients with diabetes, but this was not statistically significant. 196
Cardiovascular disease
In the APEX study, 20.2% of patients receiving TKR reported a cardiovascular-related condition.
Our literature searches identified four studies reporting multivariable analysis of patient-reported outcomes according to presence of cardiovascular disease. One study was conducted in multiple centres127 while three studies included patients from a single centre. 163–165
Jones and colleagues127 followed up an estimated 209 patients (72% eligible) at 3 years after TKR at two centres. Cardiac disease before surgery was not a predictor of long-term WOMAC pain or function.
In the study of Ayers and colleagues,163 165 patients (per cent eligible not reported) with TKR at a single centre were followed up at 1 year. Only univariate analyses were reported for individual comorbid conditions. There was a lower improvement in the SF-36 physical component in patients with cardiovascular comorbidity than those without but the relationship was not strong. For WOMAC physical function, there was a suggestion that patients with cardiovascular comorbidity had a lesser improvement in physical function than those without.
Scott and colleagues165 followed up 1141 patients (87% eligible) with TKR at a single centre 12 months after surgery. In univariate analysis, there was little difference in long-term satisfaction in those with heart disease compared with those with no heart disease.
In the study of Clement and colleagues,164 966 patients (per cent eligible not reported) with TKR at a single centre were followed up for 12 months. Patients with a history of heart disease had a poorer improvement in OKS compared with those with no history of heart disease.
Overall, there was a suggestion that people with heart disease had somewhat poorer long-term improvement in pain and function, but this was only evident in two studies with no additional markers of good quality. Further research has studied the association between comorbid cardiac disease and adverse events.
Variables in the risk calculator for in-hospital adverse events described by Ackland and colleagues200 included specific cardiac diseases and risk factors but the discriminatory ability of the model was modest. Gill and colleagues222 looked at factors predicting mortality within 90 days of TKR in a cohort of 3048 patients, of whom 14 died. Patients with any major cardiovascular disease before surgery had a greater risk of death within 90 days of surgery. In the study of Sanders and colleagues,202 a greater risk of death within 30 days of surgery was observed in patients with a vascular event in the year preceding total hip and knee replacement.
Hypertension
Searches identified four studies reporting long-term patient outcomes according to whether or not patients had hypertension before surgery. Studies were in multiple151 or single centres. 164–166
In a study in three UK health districts, Cushnaghan and colleagues151 followed up 259 patients (39% eligible) from three centres at a mean of 72 months after TKR. In multivariable analysis, hypertension was not a significant predictor of change in SF-36 physical function score.
Gandhi and colleagues166 followed up 889 patients (83% eligible) after a TKR at a single Canadian centre. Similar WOMAC function improvement was observed at 1 year, irrespective of presence of hypertension. The authors noted a trend for poorer long-term WOMAC score in patients with the four conditions relating to metabolic syndrome but this was not statistically significant.
Scott and colleagues165 followed up 1141 patients (87% eligible) from a single UK centre 12 months after TKR. In univariate analysis, more patients with hypertension were dissatisfied with their outcome than those with no hypertension.
In a study at a single UK centre, Clement and colleagues164 followed up 966 patients (per cent eligible not reported) 12 months after TKR. There was no difference in change in OKS in patients with hypertension compared with those with no hypertension.
Considering adverse events, Bozic and colleagues223 followed up 83,011 patients with TKR 90 days after surgery. In multivariable analysis, hypertension identified pre-operatively was not associated with infection or mortality.
There was no clear evidence that patients with hypertension had worse long-term pain and function outcomes after TKR. As observed with diabetes, management of blood pressure may affect studies of the association between pre-operative hypertension and post-surgical outcomes. Most people with a diagnosis of hypertension will be treated before surgery; indeed management of undiagnosed hypertension may be required in the preparation for elective surgery.
Anaemia
We identified one study with patients from a single centre that reported long-term patient outcomes after TKR according to pre-operative anaemia status. 164
Clement and colleagues164 followed up 966 patients (per cent eligible not reported) 12 months after TKR at a single UK centre. There was a small difference in improvement in OKS between patients favouring those with no anaemia but the relationship was not strong.
The systematic review of Spahn204 considered clinical outcomes of patients with hip and knee surgery. Pre-operative anaemia was associated with post-surgical mortality and infection, increased need for blood transfusion, poorer physical functioning and recovery, and longer hospital stay.
More recently, Bozic and colleagues223 followed up 83,011 patients with TKR for 90 days after surgery. In multivariable analysis, pre-operative anaemia was associated with an increased risk of infection but not mortality.
Jämsen and colleagues205 reported an increased risk of death in 1998 patients receiving knee or hip replacement with pre-operative anaemia compared with those without. In a multivariable analysis including patients with total hip or knee replacement, Greenky and colleagues206 reported a greater risk of infection but not mortality.
In summary, there was no strong evidence relating pre-operative anaemia to long-term patient outcomes. However, by reducing adverse events and limiting hospital stay, strategies to manage anaemia before surgery may have substantial benefits for both patients and health-care providers.
Thyroid disease
Searches identified one study conducted in multiple centres reporting patient-reported outcomes in patients with or without thyroid disease who received a TKR. Cushnaghan and colleagues151 followed up 259 patients (39% eligible) in three English health districts at a mean of 72 months after TKR. In multivariable analysis, thyroid disease was not a significant predictor of change in the SF-36 physical component.
Discussion
Approximately 60–70% of patients receiving total hip and knee replacement report one or more comorbid condition and are at risk of an adverse event after surgery. Although this is valuable information in itself with the possibility of extra monitoring and care, preparatory strategies with treatment of individual conditions may prevent adverse events and improve long-term patient outcomes. Our systematic review of large prospective cohort studies investigating the relationship between pre-operative comorbidities and long-term patient outcomes highlighted some possible areas for intervention.
We did not confirm a relationship between diabetic status and long-term patient outcomes after total hip or knee replacement; however, patients with diabetes are at greater risk of early adverse events and are over twice as likely to have a deep infection after their total hip or knee replacement with potentially devastating consequences for patients and substantial health-care implications. Supported by the observation that patients with uncontrolled diabetes have poorer outcomes than those with better glycaemic control, optimisation of diabetes control before surgery may be of long-term benefit to patients receiving total hip or knee replacement and merits appropriate evaluation.
Although not consistently so, there was some suggestion that patients with cardiovascular disease had poorer patient outcomes after total hip and knee replacement. As with diabetes the impact of cardiovascular disease on patients may be an increased rate of adverse events, possibly limited to those patients with more recent occurrence of acute coronary syndromes.
Evidence on the importance of hypertension on long-term outcomes in patients with hip or knee replacement was equivocal. As a widely treated condition in older people, it is unlikely that the patients receiving joint replacement would have uncontrolled hypertension. No studies were found looking at outcomes by level of blood pressure control.
There was evidence from one study that diabetes and hypertension, in combination with other conditions (high BMI and hypercholesterolaemia) that make up metabolic syndrome may be associated with poorer long-term patient outcomes. 166 However, this was only statistically significant in patients with THR.
We identified no studies reporting long-term patient outcomes in patients according to presence of pre-operative anaemia. Patients with anaemia had increased need for perioperative blood transfusion and increased risk of early adverse events, which probably explains a substantially longer average hospital stay.
Further evidence is now required from intervention studies on whether or not improved management of conditions before surgery can improve long-term patient outcomes, possibly mediated through a reduction in adverse events. Encouragement for this approach in diabetes comes from two large studies. 194,198 In the US Nationwide Inpatient Sample of nearly 1 million patients with joint replacement, the risk of postoperative complications was increased in patients with uncontrolled diabetes. 198 This was supported by data from the Danish Hip Arthroplasty Registry suggesting that those with complications due to diabetes had a greater risk of revision, owing to deep infection. 194 However, an analysis of data from a US integrated health-care system did not report a poorer outcome in patients with HbA1c (glycated haemoglobin) ≥ 7%, a marker of poor diabetes control. 213
In this review, we focused on five specific comorbidities. Further studies are required to explore the associations between other conditions and long-term patient outcomes. For example, in the APEX cohort of patients receiving TKR, 19.5% of patients reported visual impairment and 15.2% reported hearing impairment. If there is evidence linking these to poor outcomes, this would indicate the potential value of appropriate screening and treatment.
In the UK, the Royal College of Anaesthetists and Association of Anaesthetists of Great Britain and Ireland provides patient guidance on preparation for surgery. 224 Recommendations include giving up or quitting smoking, reducing weight, dental check-up and GP check-up for long-standing medical problems. Numerous blood tests are routinely performed before surgery but their value in predicting adverse events225 and management of health conditions226,227 is uncertain. The best evidence on clinical effectiveness and cost-effectiveness of interventions and prognostic models comes from their evaluation in randomised trials and, ultimately, in systematic review and meta-analysis.
Conclusion
In specific clinical conditions, we found little research on patient-reported outcomes. In studies looking at long-term patient outcomes according to diabetic status, research was inconclusive. However, studies show that patients with diabetes, previous heart disease and anaemia are at greater risk of post-surgical adverse events and study of appropriate interventions through systematic review and RCTs is indicated.
Chapter 3 Patient and public involvement in the RESTORE programme
There has been ongoing PPI throughout the programme as follows.
Collaboration with Arthritis Care, a national charity supporting people with arthritis
The previous regional director (Pippa English-Penfold) and, subsequently, the Director of Service Development (Phil Baker) have taken part in programme meetings to determine the direction of work packages and the programme as a whole. We also held a mid-programme event at which Arthritis Care was represented and contributed. Within the SPIRAL study there has been active collaboration with Arthritis Care through the design and delivery of the ‘Challenging Pain’ and ‘Keep Challenging Pain’ interventions, pre- and post joint replacement. We have also attended Arthritis Care’s AGM in 2011 and presented an introduction to randomised trials and discussed SPIRAL with members of Arthritis Care.
Engagement with the Patient Experience Partnership in Research
In 2010 a patient group, the PEP-R group, was established to provide a forum through which patients could provide who contribute to the design and delivery of research in the University of Bristol’s Musculoskeletal Research Unit (MRU). This group currently comprises eight people who have musculoskeletal conditions, many of whom also have had joint replacements. Group members totalled 18 (nine women and nine men, aged 25–85 years), with a maximum of 11 members at any one time. The group meets for 2 hours every 6–8 weeks. In keeping with guidance from INVOLVE – the national advisory group that supports greater public involvement in NHS, public health and social care research228 – group members were reimbursed for their time and travel expenses.
Support and training for the patient partners was provided by a dedicated PPI co-ordinator who is a trained researcher with several years’ experience supporting patient partners in research. Support included structured training sessions and one-to-one meetings with patient partners. Training and learning is incorporated into PEP-R meetings. Researchers have described and discussed study design (e.g. randomised trials) in the context of studies. In response to group members’ feedback, sessions have also included discussion of epidemiology, statistics and qualitative methods. PEP-R members have also visited the MRU.
The co-ordinator liaised between research staff and patient partners. Researchers from the MRU work closely with the new ‘People and Research West of England’ partnership promoting and supporting service user involvement in research, and patient partners had access to events and the service user network. PEP-R group members are regularly provided with information about how their input has influenced the implementation of studies so that they can see their impact on the research, including verbal feedback on previously discussed projects at the start of every meeting and written feedback in the form of leaflets every few months.
Patient Experience Partnership in Research inputs into many studies within the unit and one of them was RESTORE. Researchers from RESTORE have attended eight meetings of the PEP-R group. The format of these sessions includes group discussion, presentations, card-sorting tasks and written answer sheets. It was important for researchers to have a written record of the session, so discussions are recorded on a flip chart or written sheets completed by group members. Material is sent out to group members in advance so that they have the chance to read it beforehand. They had the choice of providing their input verbally during a session, or by telephone or e-mail afterwards.
All of the work packages have been discussed and developed in collaboration with patient representatives and PEP-R. Patient representatives have provided input into patient recruitment and information literature, research processes, identifying outcomes of importance to patients, questionnaire design and dissemination and helped ensure that outputs of packages informed one another.
The work of the PEP-R group, including work on the RESTORE project and other projects was recognised in a University of Bristol’s engagement award 2014.
APEX
Patient representatives inputted into the refinement of patient information materials to ensure that they were clear. One issue with early recruitment materials in APEX was the inclusion of information about spinal anaesthesia. Through discussions with patients it became clear that this was confusing and potentially worrying. Therefore, this information was removed from the recruitment packs. PEP-R has learned about randomised trials through discussion of the APEX trial and of trials in general with researchers facilitating PEP-R sessions. Issues of blinding and sharing results with participants have been discussed with patient representatives and these discussions helped to inform the decision to tell participants in APEX about their allocation to intervention or control groups. Patient representatives advised on the appropriate terminology and the level and amount of information provided to participants when contacted to arrange their 12-month research follow-up visits. They confirmed that the proposed explanation of the visit made the need to undress for an examination explicit and favoured the less clinical term ‘surgical site’ as opposed to ‘wound’ or ‘scar’ with regard to the examination of the replaced joint. As a result, the research nurses were able to incorporate these changes into their subsequent interaction with the participants.
ADAPT
Patient representatives provided suggestions about how to frame the requirements of study participation to participants and in relation to questionnaires. In ADAPT, questionnaires were long and data were collected at three time points. This meant that data collection would involve patients visiting the hospital on three occasions each and also completing long questionnaires. Patient input helped in the presentation of the questionnaires to make them easier to read and complete; however, as these were validated instruments, changes could not be made to the questions or response options. Since study completion, patient representatives provided suggestions for feedback in leaflet form to study participants as well as suggestions of other ways of disseminating findings to the public. Their suggestions included providing a brief summary of the project as participants might have forgotten, using visual representations, signposting to other ways the information will be used and where participants could read about the study in greater detail, for example, giving links to journal articles.
SPIRAL
Patient representatives discussed the study in detail and provided their input into the intervention and study recruitment material, which led to refinements to all these aspects of the study. These discussions resulted in a change in the name of the study to SPIRAL and improving the formatting of the patient information sheet. They also discussed issues around non-participation in the study and made suggestions about how it could be made easier for patients to take part, including providing help with transport and holding the groups in local venues rather than hospitals. These suggestions will be used to guide the design of future research protocols. Since study completion, patient representatives provided suggestions for feedback in leaflet form to study participants.
ARENA
Patient representatives were asked to feedback on the development of this study. They provided input into the development of the exercise class in terms of patients’ requirements during the class and opinions of the exercises proposed. For example, they highlighted the importance of providing assistance with travel arrangements for those with transport difficulties, the inclusion of refreshment breaks and music during the class, and the additional of an exercise aimed to improve patients’ ability to get in and out of bed. They also emphasised the importance of study participants having details of person whom they could contact between classes. In response to this, a contact name and telephone number was available to all participants throughout the study. In addition, they provided the research team with reassurance that the introduction of an exercise class after knee replacement was a positive approach and they supported the concept of providing function-based exercises within a group setting. Following completion of the ARENA study, the patient representatives have supported further proposals to pursue a larger trial comparing the exercise class with usual care. They felt that providing further individualised treatment within the exercise class would be beneficial to patients. This has been included the design of a grant proposal to explore the exercise class in a future study.
PROOF-THR
Patient representatives discussed the protocol and study materials, leading to refinements in these. As a direct result of these discussions, the design of the main study compared with the pilot was changed by adding in a follow-up visit to check progress by the same occupational therapist (OT) who had visited them before surgery if the patient requested one. In addition, as a compromise for the patient input regarding follow-up, the OTs were given a pre-paid mobile phone each and the patients were given the number to call for verbal advice. This was actually used quite a lot and proved very popular with the patients. It was also suggested that the visiting OT give advice on benefits, but this is not standard OT practice as it is a complex area that requires specialist advice. Instead, helpful phone numbers were given to participants if requested.
Systematic reviews
The importance and value of systematic reviews has been discussed with the patient representatives. This has been particularly helpful in developing new projects with extensive discussions on how to follow-up results of reviews relating to comorbidities and rehabilitation.
Patient experience study
Patient representatives discussed this study in detail, leading to the addition of questions in the interview topic guide such as questions around when and why problems first developed with the joint. Other suggestions taken on board include adding an e-mail address to the back of the patient information booklet to enable potential participants to contact the research team via e-mail and the positive implications of including the Hip Disability and Osteoarthritis Outcome Score (HOOS) or KOOS questionnaires in the 12-month follow-up interview in addition to the pre-operation interview. Since study completion, the patient representatives have provided suggestions for feedback in leaflet form to study participants.
In addition, in January 2013 we held a dissemination event with members of PEP-R. Arranged as a ‘science café’, the event provided opportunity for discussion about all work carried out on the RESTORE programme.
Patient and public representation on steering groups
The overarching study group includes representation from a patient with experience of bilateral hip replacement. She has attended programme and work package meetings providing input into the development of questionnaires, suggestions for improvements to the studies and ensuring that the programme is relevant to patients (see Patient involvement in Patient Experience Partnership in Research: a personal view). In addition, the steering groups in the individual work packages APEX and the Patient Experience Study included patient and public representatives. Their role differed from those on the patient forum. Oversight groups monitored progress and conduct of the work package. These patient and public representatives discussed the study design, delivery and identifying avenues for dissemination and to ensure that study findings will be presented to a range of stakeholders. They were supported by the PPI co-ordinator.
Evaluation of patient and public involvement
We assessed patient involvement in the programme by collecting regular, systematic feedback from patient partners and researchers. This helped us refine our involvement processes as the programme progresses, as well as to provide advice on PPI activity to others within NHS and University sectors.
Eight patients, who were members of PEP-R at the time of the evaluation (November 2011), and 14 researchers completed a questionnaire examining the impact of the activity on themselves and the research. Group members described their interest and learning about the topics and research in general. They particularly valued feedback about how PEP-R’s input had shaped studies. Researchers identified the benefits of obtaining patients’ views on the importance, relevance and feasibility of their projects. They welcomed the opportunity to speak to an interested and knowledgeable group and stressed the importance of early involvement. A selection of comments are shown in Table 9.
| Group member | Comments |
|---|---|
| Group member E | We have been asked and listened to about our opinions, which has led to improved layout of information leaflets and questionnaires. Also the content and language used in leaflets and questionnaires |
| Group member A | Where funding has been granted, I am sure that PEP-R has contributed – our combined experience as patients has made a big contribution to the process. We know the research team listen carefully to our input |
| Group member C | Feedback from researchers also indicates that modifications to documentation, etc. have also been made |
| Researcher V | As a researcher you can have many ideas for research projects, but without consultation with patients, it can be difficult to know whether these issues are actually of importance to patients. I wanted to engage with PEP-R as it provided an opportunity to ensure that the research was of interest and relevance to patients |
| Researcher U | The ethics committee asked me about patient involvement . . . I picked up a feeling that my contact with the group more than satisfied this requirement. I also found many of the suggestions that were made by the group helped me to improve my study design |
Dissemination of research findings
Working with Arthritis Care and patient representatives, we have developed a dissemination strategy that addresses the needs of policy-makers, health professionals and service users. We envisage that this will be achieved by dissemination in reports, end of project feedback leaflets for research participants, peer-reviewed articles, conference presentations, lay summaries of findings in magazines and websites. Engagement with PEP-R will continue, particularly focusing on identification of dissemination strategies and working in partnership with researchers to develop plain English summaries of research.
Patient involvement in Patient Experience Partnership in Research: a personal view
Victoria Wells has been a patient-partner in RESTORE throughout its duration. She previously underwent joint replacement. This section outlines her experience of patient involvement in the programme and her views about the research.
I came from the operating table to sitting around the research table. I remember the first research meeting where everyone was introducing themselves and talking about RESTORE. Being part of that from a lay perspective gave the research extra grounding in experiences. It’s been good having nurses, patients, physios and surgeons working alongside researchers as they all bring different views, painting the whole picture. It’s very easy to have research that misses the patient and it’s good to have the patient there.
In RESTORE I’ve had individual conversations with the study leads, and researchers were able to approach me so that I could answer their questions. There were no barriers there and the researchers seemed not to be worried about asking me questions. They were able to ask me questions and were sensitive to the patient journey that I had had. As result of personal experience I’m able to put in real life experience into the research or make suggestions to approach other patient partners. I could also break down academic text into more readable forms, for example for patient leaflets. I was able to bring in some influence from ‘outside the box’ and it was also like being able to turn a box inside out.
With APEX my input was in how the study would be described to patients at the sensitive stage before surgery when people needed to know about their pain relief. My thoughts were listened at that early stage. I really remember coming to my second research meeting as we talked about information in the APEX trial and about the information that would eventually be disclosed to patients in the trial. Blinding was important, and so was the information about the treatment that patients got.
With ADAPT the questionnaires needed a lot of input before they went to patients, and that was important. With ARENA there were quite a few informal conversations for my input, although SPIRAL and ARENA the studies were patient-centred anyway. In the patient experience study I had quite a few conversations looking at the patient experience in the long-term, beyond the ward, and making the most of your joint replacement. I’ve had interesting conversations around the systematic review work, and there were informal discussions and I do feel like that work has taken my views onboard. With the health economics my engagement has also been informal and the cost-effectiveness side of the work is hugely important. With the informality it’s less like being interviewed and it’s more of a discussion and the team members are more relaxed and feel able to ask me questions, which is what you’d get in qualitative research because it would be a conversation and no pressure for a particular outcome because you can just explore the study and think about things in different ways. With the health economics and systematic reviews it’s been explained in ways that I can understand and made me think in a different way, and this is kind of hidden and useful.
Having contact with researchers hasn’t always been about having a specific outcome in mind, it’s about exploring a study that enables involvement, so that the thought processes are clear. Patient and public involvement doesn’t always need to be prescriptive, but this can only happen if you’ve built up a relationship over time and the involvement I have had I have built this up with the RESTORE team.
As a result of early involvement in RESTORE and my understanding of patient and public involvement I was able to advise on the creation of PEP-R (the Unit’s patient forum), which gave richer information from a collective of patients rather than an individual. I feel like it’s creating a minibus of people and we are passengers because no two experiences are the same. I’ve been involved in PEP-R but have also been able to hand over that baton to others, I was on the planning and steering group and was able to hand that over and that felt good.
Victoria Wells, University of Bristol, 2014, personal communication, reproduced with permission
Chapter 4 Understanding patient’s experiences of total hip and knee replacement: a qualitative study
Parts of this chapter have been reproduced with permission from Johnson EC, Horwood J, Gooberman-Hill R. Patients’ journeys through total joint replacement: patterns of medication use. Musculoskeletal Care 2014;12:92–102229 © 2013 John Wiley & Sons, Ltd; and reproduced with permission from Johnson and colleagues. 230
Abstract
Background
We aimed to characterise and explore the patient pathway through total hip or knee replacement surgery in current routine NHS care.
Methods
In a qualitative study, 34 patients receiving joint replacement were interviewed before surgery, 2–4 weeks, 6 and 12 months after surgery. Interviews elicited patients’ experiences of preparing for, undergoing and recovering from surgery. Analyses used a thematic approach or interpretive phenomenological analysis.
Results
Patients noted that delays to joint replacement in the NHS are common, which has implications for well-being. Patients’ experiences of time differ from the linear conceptualisation of time required to plan NHS services.
Undergoing surgery can increase feelings of vulnerability and alter a patient’s trust in their own body, the influence of interactions with others on confidence levels, and fears concerning the potential for causing harm to their new prosthesis. Patients rely extensively on, and value, both informal and formal support networks over the perioperative period. Transformation from a person living with osteoarthritis to a person recovering from a surgical intervention can lead to alterations in the assistance people receive from others.
When patients are not offered the support of health and social professionals, patients may feel distress and abandonment. Patient expectations for joint replacement surgery are complex and can be driven by previous personal experience, experiences of others and information provided by the hospital.
Conclusions
Our findings suggest important ways in which the provision and delivery of care and education for people undergoing joint replacement in routine NHS care can be refined and improved.
Background
Qualitative work has provided insight into the experience and impact of living with osteoarthritis including treatment options and surgery. 5,24–27 In relation to surgery, studies have explored pathways to surgery;4,29 decision-making about joint replacement;231 and patient satisfaction and outcome. 28,30 However, little research has explored how patients experience their journeys through joint replacement from pre-operative care to postoperative recovery. Our qualitative research addresses this gap in evidence by focusing on experiences of pre-operative circumstances and preparation, views about the hospital stay and the operation, as well as exploring longer-term recovery, rehabilitation and outcome in the year after surgery.
The research aimed to provide robust patient-centred evidence that could inform future design and delivery of health care for people undergoing joint replacement. We used an inductive approach to the work and the areas of literature that we draw on are those that became most relevant as data collection and analysis progressed. These were related to the wait for surgery, the experience of delay, confidence and expectations as well as the experience of time in the lead up to surgery.
Understanding the patient experience: total hip replacement
Waiting for hip replacement surgery
Within the NHS, waiting times for medical interventions are a recognised element of current health care,232 as patients passing through the system are provided with appointment dates for consultations and treatments by a system increasingly predicated on a discourse of patient choice. 233–235 A continual drive to reduce waiting times for intervention, to monitor and measure the passage of time, highlights the salience and relevance of a consideration of the temporal landscape within current health-care processes. The issue of waiting times is important given that a growing body of research highlights the detrimental impact that waiting for elective surgery can have. For example, patients awaiting hip surgery have previously reported experiencing significant increases in pain and physical disability,236 high levels of psychological distress237 and an overall deterioration in HRQoL. 238 Our exploration of patients’ experience of time aimed to provide in-depth understanding of the impact and implications of waiting for surgery in current NHS care.
The role of confidence during the journey through hip replacement
Confidence, which is concerned with a person’s judgement about their own, or others’, abilities and vulnerability,239 which can be defined as capable of being physically or emotionally wounded (www.merriam-webster.com/dictionary/vulnerable)240 are both concepts evident in the literature concerning the experience of older age. For example falls, which are common in this population,241,242 can result in reduced confidence and an enduring fear of falling. This can lead to people choosing to disengage from usual activities. 243 As osteoarthritis is associated with ageing, affecting 10% of people > 5 years of age in the UK,1 confidence and vulnerability may have particular relevance to the experiences of patients undergoing joint replacement. A subtheme ‘building confidence’ arose in a recent study,32 involving interviews with patients after they had undergone THR. This encompassed patients’ experiences of feeling fearful of falling and damaging their new hip and also related to confidence and use of walking aids after surgery. This builds on earlier work by Grant and colleagues244 which reported that with increasing confidence, patients who had undergone THR 4–6 months previously talked of slowly relinquishing their reliance on mobility aids. A metasynthesis of older adults’ lived experiences of discharge from hospital after undergoing orthopaedic intervention reports that patients’ confidence can be influenced by their perception of the expertise of staff and consistency of information received around the perioperative period. 245 This small body of work provides some initial understanding regarding the influence and relationship of elements of the orthopaedic surgical experience on patients’ confidence level and vulnerability. Our exploration of patients’ experiences aimed to build on and extend these insights by providing an in-depth understanding of the ways in which their confidence was affected by, and affected, their journey through hip replacement surgery.
The experience of support during the journey through knee replacement
Living with osteoarthritis and undergoing surgery brings about pain and functional limitations. We know from existing literature that at times of disability, informal care has a large part to play246 and recent work highlights the importance of informal support for people living with osteoarthritis. This shows that assistance from family and friends with everyday activities (such as help around the home) is valued140 and has positive implications for mental and physical health. 247,248 People with osteoarthritis also engage with more formal support, including contact with health professionals and social services. 249 However, patients may have little contact with health-care professionals after discharge from surgery, at which time family are particularly important in provision of support, including personal care. 32,250 The value of this support is well documented32,244 but can cause mixed reactions including gratitude and frustration towards family and concern about placing burden on others. 245,250,251 Importantly, previous research has not considered how patients’ relationships with others may change as they move from living with pain and limitations associated with osteoarthritis, through to postoperative recovery and to functional independence. Therefore, we conducted research to explore how undergoing and recovering from knee replacement surgery affects patients’ experiences and use of support networks.
An exploration of patients’ hopes and expectations for hip and knee replacement surgery
A large body of quantitative work has investigated patients’ expectations for recovery from elective orthopaedic surgery. 32,145,165,252–254 This evidence has highlighted the importance of considering the role and function of expectations around the perioperative period. For example, we have learned that patients’ pre-operative expectations for joint replacement are both important in their decision to have surgery252 and can help to predict outcomes. 253 We also know that realisation of patients’ pre-operative expectations after hip and knee replacement surgery are significant in influencing their reported outcomes and satisfaction. 145,165,254 This body of work has not provided detailed understanding of expectations for recovery from joint replacement from the patient perspective. A recent qualitative study,32 attempted to address this gap through an examination of the experiences of patients undergoing THR. The authors report that participants held high expectations of what having surgery ‘would do for them’ and suggest the value of patients having the opportunity to discuss their expectations of joint replacement in order to limit ‘false optimism’. This work illustrates the importance of using qualitative methods in order to gain novel insights into expectations of joint replacement surgery. However, McHugh and Luker32 report only on the expectations of hip replacement patients and interviews were undertaken 6–8 months after surgery, a time-point when participants’ recovery may have still been incomplete. Therefore, we were interested in gaining an in-depth understanding of patients’ expectations for recovery from both hip and knee replacement surgery, with a focus on the fulfilment of these expectations 12 months after surgery. We hoped to gain insight both into the processes by which patients’ expectations were formed and the reasons why their expectations were, or were not, met.
Methods for qualitative studies
Sampling and recruitment
Patients who were listed to undergo either total hip or knee replacement surgery in the Avon Orthopaedic Centre (AOC) were eligible to take part in the qualitative study. Between February 2011 and August 2012, study invitation packs were posted to 179 patients (111 hips and 68 knees). Of those who returned a reply slip to the research team expressing their agreement to be contacted about the study (n = 52), we purposively identified a sample of men and women who were a range of ages. These comprised 29 patients undergoing hip replacement and 10 undergoing knee replacement. The programme’s qualitative researcher contacted individuals in this sample to discuss the study in more detail and for any concerns to be addressed.
Of the 29 hip patients contacted, 24 agreed to meet with the researcher to take part in an initial interview. The remaining five were no longer eligible to take part (e.g. they had been recruited into an alternative study that precluded their inclusion, their operation date had been brought forward). All knee patients who were contacted agreed to meet with the researcher. These sample sizes ensured that data from the hip sample was at saturation point, such that no new themes were emerging from analysis by the end of data collection at the first data collection point. 255 The sample size for the knee cohort was determined as appropriate for the conduct of fine-grained interpretive phenomenological analysis. 256
Data collection
In-depth semistructured interviews were conducted with all participants after they had been placed on the surgical list for joint replacement surgery. We also aimed to interview participants 2–4 weeks, 6 and 12 months post surgery. All participants provided their written, informed consent to take part immediately prior to the initial interview. As the study was longitudinal, the researcher also sought participants’ verbal agreement to ongoing participation before each follow-up interview.
Initial interviews, which lasted between 65 and 135 minutes, took place at participants’ preferred location: either in their own homes (n = 29) or on University premises (n = 5). Follow-up interviews, which lasted from 20 to 90 minutes, largely took place over the telephone, other than when a participant requested a face-to-face interview in their own home (n = 6) or on University premises (n = 1). In addition, participants whose surgery was delayed by > 3 months from their original admission date (n = 3) were asked if they were willing to take part in an additional interview focusing specifically on their experience of delay. Two participants (one hip patient and one knee patient) agreed to this additional contact.
Interviews were carried out with 21 of the 24 hip patients and 8 of the 10 knee patients at the three follow-up points. Five participants did not take part in postoperative interviews: three because they chose not to have surgery, one because their medical circumstances precluded a follow-up interview, and one because the date of the surgery moved beyond the time constraints of this study.
The data collection time points and numbers of participants for hip and knee patients are shown in Tables 10 and 11.
| Time point number | Approximate time of interview in relation to surgery | Number of participants and interviews |
|---|---|---|
| Time point 1 | When on the waiting list for surgery | 24 participants |
| Time point 2 | 2–4 weeks after surgery | 21 participants (one had no operation and was not followed up, one had a delayed surgery date and was not contactable, one was interviewed about their delayed operation) |
| Time point 3 | 6 months after surgery | 21 participants |
| Time point 4 | 12 months after surgery | 21 participants |
| Additional time point | When a patient had a delayed surgery date | One participant |
| Total number of interviews | 88 interviews, with 24 participants |
| Time point number | Approximate time of interview in relation to surgery | Number of participants and interviews |
|---|---|---|
| Time point 1 | When on the waiting list for surgery | 10 participants |
| Time point 2 | 2–4 weeks after surgery | Eight participants (one did not have an operation and was not followed up, one had delayed operation) |
| Time point 3 | 6 months after surgery | Eight participants |
| Time point 4 | 12 months after surgery | Eight participants |
| Additional time point | When a patient had a delayed surgery date | One participant |
| Total number of interviews | 35 interviews, with 10 participants |
Interview procedure
Interview questions were framed by topic guides specific to each time point. They were informed by existing literature and developed through discussion with patient representatives. Core questions aimed to elicit participants’ experiences of preparing for, undergoing and recovering from surgery and additional probes were used to facilitate elaboration and to achieve depth. Pre-surgery interviews addressed pain and functional limitations, expectations, existing knowledge about surgery and its outcomes, and preferences for pre-operative management. Post-surgery interviews addressed pain, function, views on the care that patients have received as well as their future plans and hopes for/of rehabilitation and recovery. At the 6- and 12-month interviews, participants were asked to talk about their experiences of long-term recovery and adaptation. These interviews also revisited topics that arose from earlier interviews, including ongoing and missing support needs. Interviews exploring delay to surgery addressed participants’ views about delay and their experiences of support. Interviews were conducted by qualitative methodologists with social and behavioural science backgrounds and it was made clear to participants that the researchers were not clinical staff.
Interviews were audio-recorded, transcribed and anonymised, with the exception of two interviews which were recorded in note form owing to audio-recording equipment failure. Ethical approval was provided by NHS South West 1 REC (10/H0203/44).
Data analysis
Initially, we analysed data from all time points of patients undergoing hip replacement separately from the data from patients undergoing knee replacement. We used different analytic approaches with each data set. Once the analysis of the two data sets was completed, we brought the hip and knee data together and conducted analysis on the pre-operative and 12-month data together.
Hips: inductive thematic analysis
We used inductive thematic analysis for the data set of interviews with patients undergoing hip replacement. This was chosen as a means of exploring change over time as well as comparing and contrasting experiences in a relatively large data set which comprised 88 interviews by the time of completion. Analysis was iterative with data collection, with use of software to enable data organisation. Anonymised transcripts of audio-recordings and notes from interviews with patients undergoing hip replacement were imported into the qualitative data management software package ATLAS.ti® 6 (ATLAS.ti software; ATLAS.ti, Berlin, Germany). Initial analysis of transcripts began shortly after data collection started and was ongoing and iterative. Analysis informed further data collection such that early findings were used to refine the topic guides and identify questions to ask in future interviews. Transcripts from each participant were combined and treated as one single data set and were analysed using inductive thematic analysis. 257 A member of the research team first identified thematic codes which were grounded in the data. Next, through identifying connections between the codes, the research team clustered them into superordinate themes. To enhance analysis and enable team discussion and interpretation, double coding was conducted on a sample of four interview transcripts at each time point (total double coding on 16 interview transcripts). The double coding was conducted independently by members of the team who also had social and behavioural science backgrounds. The double-coding process was used as a means to stimulate close reading of the transcripts by the qualitative research team, who met regularly to discuss the codes and who worked to achieve a consensus about coding. Consensus, as agreement, was arrived at through discussion. To improve understanding of the whole data set, those aspects of data that appeared to contradict general experiences were identified and explored. The data from all patients, including those who did not have THR, were included in the analysis because their experiences of preparing for and waiting for surgery provide valuable insights.
Knees: interpretative phenomenological analysis
We used interpretative phenomenological analysis (IPA) to analyse data from knee replacement patients. This was chosen as a way to explore participants’ personal lived experiences and how they make sense of them in detail and depth with an emphasis on the detail of cases in a group of participants likely to be undergoing similar experiences. 256,258 IPA is an idiographic approach, focusing on the particular rather than the universal and starts with the detailed examination of case studies, which then tentatively progresses to more general statements about groups of individuals. 259 Taking this approach we were able to describe patients’ lived experiences and their process of preparing for, undergoing and recovering from surgery (i.e. how their experiences unfolded). The process of analysis was guided by a series of principles gleaned from the reflections of Smith and colleagues within research methods publications, e.g. Smith and Eatough258 and Smith and Osborn. 260
Hips and knees: inductive thematic analysis
After identifying salient themes relating to expectations in the hip replacement data and having conducted IPA with the knee replacement patient data, we were interested in also exploring these issues for knee patients. Therefore, we imported knee transcripts into ATLAS.ti and undertook thematic analysis on this data set, employing the procedures previously described to investigate these issues further. This enabled us to explore patients’ hopes and expectations of surgery across the longitudinal qualitative data set as a whole.
Results
As described in Methods for qualitative studies, 24 hip replacement patients and 10 knee replacement patients took part in the longitudinal qualitative study. Of the hip patients, 13 were men and 11 were women, with ages ranging from 52 to 82 years (Table 12). Of the knee replacement patients, six were men and four were women, with ages ranging from 61 to 78 years (Table 13).
| Pseudonym | Age (years) | Sex |
|---|---|---|
| Mr Bedford | 73 | Male |
| Mrs Burton | 70 | Female |
| Mr Day | 74 | Male |
| Mr Everett | 66 | Male |
| Mr Foreman | 61 | Male |
| Mr Golding | 62 | Male |
| Mr Granta | 82 | Male |
| Mrs Hardcastle | 71 | Female |
| Mr Higgs | 71 | Male |
| Mr Horton | 73 | Male |
| Mrs Kade | 73 | Female |
| Mrs King | 53 | Female |
| Mrs Lovell | 69 | Female |
| Mr McKenzie | 66 | Male |
| Mrs Noble | 74 | Female |
| Mrs O’Brian | 65 | Female |
| Mrs Quinn | 69 | Female |
| Mr Raynerb | 79 | Male |
| Mr Smith | 75 | Male |
| Mr Thomas | 58 | Male |
| Mr Upton | 52 | Male |
| Mrs Vickers | 80 | Female |
| Mrs Warburtonc | 77 | Female |
| Mrs Young | 72 | Female |
| Pseudonym | Age (years) | Sex |
|---|---|---|
| Mr Armstrong | 70 | Male |
| Mr Cook | 64 | Male |
| Mr Ostafewa | 78 | Male |
| Mrs French | 76 | Female |
| Mr Ings | 64 | Male |
| Mr Jackson | 68 | Male |
| Mrs Parkerb | 67 | Female |
| Mr Clark | 65 | Male |
| Mrs Evans | 74 | Female |
| Mrs Biggs | 61 | Female |
Waiting for hip replacement surgery
Within the hip data set we explored each participant’s journey from his or her initial referral to secondary care through to his or her final surgery date. As shown in Table 14, accounts revealed that participants took one of five main routes from referral to surgery.
| Pathway | Route from referral to surgery |
|---|---|
| Pathway A | No delay experienced (n = 12) in their secondary care pathway |
| Pathway B | Journey affected/delayed by hospital factors (n = 4). For example, earlier operations in the day over-running; unforeseen circumstances; administrator error and equipment failure |
| Pathway C | Journey affected/delayed by underlying health conditions (n = 5). These encompass both pre-existing conditions and those that were only diagnosed during pre-operative health screening. Appropriate pre- and perioperative management of a participant’s health result in both short- and long-term postponement of their operation |
| Pathway D | Journey affected/delayed by others health (n = 1). A patient chooses to postpone their surgery because of caring responsibilities/duties |
| Pathway E | Mixed pathways (n = 2). Participants experience more than one influence on their pathway. For example, initial postponement of their operation because of health issues and further cancellation owing to hospital factors |
The experience of waiting for surgery after entering secondary care and impact of delay and cancellation emerged from our analysis as salient issues for participants. We present a summary of experiences from two angles: (1) the psychosocial impact of waiting and (2) the conceptualisation and experience of ‘time’ during this period.
The psychosocial impact of waiting to undergo hip replacement
Two overarching themes relating to the psychosocial impact of waiting for THR were identified: emotional reactions and impact, and wider impact on social support networks (Box 1). The impact of waiting was influenced by the time that patients had spent waiting for surgery and their journeys through health care before surgery. These aspects of waiting for hip replacement are described in turn.
Yes, and can you recall how you felt on that morning, when you got that phone call saying, where are you?
To some extent I was taken a little bit in disbelief and quite devastated, to be perfectly honest. I’ve had operations before, but psychologically you build yourself up and think right, this is the day, it’s going to happen today, fine. But getting that phone call at . . . And yes, psychologically it knocked me for six. Probably for a couple of days I was obviously not very happy at all.
It’s frustrating for me because I can’t do anything.
Mr Rayner
Wider impact on social support networks
Because he definitely got it arranged for, he could come down when I was going in on the, this week, on the 25th. But it’s rearranging his work shifts, its rearranging things, whether he could get the time off to come down the next time.
Mrs Vickers
Yes, it was [a difficult time], not only for me, for the rest of my family as well, because they’d obviously geared everything up for everything to happen on that particular day.
What sort of alterations did they then have to make to their plans?
Transportation wise, my wife does some part time work, and she had to reorganise things so that we could make the trip on the Monday as well. It was probably more the fact that a phone call at that time, saying where are you, was a bit of a blow, to be perfectly honest.
Consequences of preceding pathway on current experience
I feel I wasted nearly a year of my life. Uh, not happy about it but uh, a certain amount of responsibility was mine, I went in and said I got sciatica. Um, I, most patients diagnose themselves in the first place but nobody questioned it. Nobody said ‘ah, hang on a minute it might be something else’. You know. Course it was not bad to start with, be fair it was not bad, but you know, it did not start full force, it’s gradually got more and more painful as time’s gone on.
Mr Horton
It’s been long enough now so. . . . Well we said we are ruling out most of this year. This year’s a non-entity.
Mr Smith
Psychological impact
Whether or not they experienced delay, participants all described emotional reactions to the experience of waiting.
Participants on pathways B, C and E who experienced postponement of their surgery date experienced a range of emotional reactions. Frustration and disappointment were frequently reported; however, some participants expressed understanding and acceptance of postponement. They tried to rationalise the news and described their gratitude when delay was a matter of weeks rather than months. The wait for surgery can also have more detrimental emotional consequences, for instance leading to a feeling of helplessness and utter desperation. Participants talked of feeling as if they were ‘left to linger’ in the secondary care system and forced to ‘live in limbo’.
Participants on pathway A without any delay or changes to their surgery date also described some disappointment at the length of time that they had had to wait for their operation. They recalled their dissatisfaction that their operations were not scheduled as soon as they had hoped and the disruption to their lives caused by this. The impact of delay on physical well-being and functioning also had some psychological effects. Mr Rayner’s experience provides a useful example. He experienced recurrent postponement of his operation owing to investigative but inconclusive tests for additional health problems. While waiting for THR he then experienced a rapid deterioration in his general health and functional well-being, and expressed ‘frustration’ at the situation.
Wider impact
Accounts illustrated how cancellation of operations could have wider impact, particularly affecting support networks. Participants explained how their friends and family also had to cope with and manage the participants’ own deteriorating health as well as share in their disappointment about a delay to the surgery date. Participants described how family and friends put their lives on hold and had to cope with the detrimental impact of ‘living in limbo’. Cancellation and delay also had a more practical impact as friends and family had to renegotiate their own plans to remain supportive during the perioperative period.
Consequences of preceding pathway on current experience
Participants’ views of their wait for surgery and the detrimental impact of the waiting period were influenced by their experience of time already spent living with pain and discomfort. Many participants described complex and sometimes slow journeys through health care from initial onset of their problems through to eventual referral to secondary care.
Two key factors were central in accounts of patients’ journeys through care. First, participants reported that they had initially delayed seeking advice and support from primary care practitioners. Reasons cited for this included a fear of the undesirable inevitable (i.e. surgery); other priorities (e.g. caring for a sick spouse); and choosing to ‘put up’ with pain and discomfort. Second, many participants reported the sense that referral from primary to secondary care had been delayed. Explanations provided for this delay included receiving an incorrect diagnosis; that their GP saw them as ‘too young’ to undergo THR; and that their GP strongly advocated alternative strategies (e.g. weight loss, use of pain relief). Participants’ accounts highlighted the interaction between frustration with management in primary care and subsequent impatience with the time spent waiting in secondary care.
Conceptualisation of time
Two overarching themes relating to conceptualisation of time were identified: unavoidable changes to use and passage of time in the lead-up to surgery, and time in the context of health care.
Unavoidable changes to use and passage of time in the lead-up to surgery
The progression of time from onset of osteoarthritis towards THR was marked by the experience of pain and patients made unavoidable changes to their use of time (Box 2).
The left hip, um the pain has been there for years, but not severe. I’ve felt it for years. Then when the right hip was done, yeah, it was definitely there then, that was in the end of 2004. Since then it’s progressed slowly, and then in the last year it’s got a lot worse.
Mrs Quinn
It’s made me feel very isolated because of not being able to get out and go and see people and do things . . . it does restrict your life really to the sofa. The more, the longer it goes on before the operation the more I’m sitting on the sofa.
Mrs Burton
It was um, anywhere from you know January 2010 to October 2011 or something like that you know. Uh, uh, we were working on the wrong lines . . . I feel I wasted nearly a year of my life.
Mr Horton
Well I can’t do anything for anybody really at the moment. Just stuck. I managed to get to my daughter’s wedding in April and my friends funeral in May. But apart from that I just don’t go anywhere. I just can’t do it. . . . I like going on coach trips and things but I haven’t been able to do anything you know.
Mrs Kade
Well I’m constantly aware, constantly aware of it. Well um, when you say totally, I suppose it’s, it governs your life, let’s put it like that.
Mr Bedford
The other night it was bad and I had to get up and move about, just to try and relieve it. I take paracetamol and sort of get back into bed and read . . . in the night-time everything is worse because it’s so quiet and dark and there’s nobody awake . . . it’s a long night. I get up and turn the television on . . . but I’ve been forcing myself not to do that, because then I’ll go and make a cup of coffee and then it sort of rolls in . . . I was waking up at three o’clock and not having any sleep until I went to bed the next night.
Mrs Young
Participants’ accounts highlighted how they had been living with pain for long periods of time and many had experienced deterioration and acceleration of their problems in the lead-up to surgery. As well as describing the long periods for which they had lived with pain, participants also described the experience of pain in terms of time. They described fleeting spasms (a ‘horrible twang’) that lasted seconds as well as constant, unrelenting pain or ‘throbbing ache’. Living a life in continuous pain with no respite appeared to provide the sense that time was drawn out during the lead-up to, and wait for, surgery.
Participants described making unavoidable and considerable changes to the way in which they spent their time because of pain and functional difficulties. When waiting for their operation, participants described withdrawal from their everyday activities and no longer actively engaging with life. For example, participants talked of inability to walk or stand for long because doing so resulted in ‘unbearable pain’. Many talked of giving up pastimes that had previously provided much pleasure, such as golf and gardening. Most of those who had been working had stopped doing so. Participants found themselves progressively unable to fill and enjoy their time as they once had done and, instead, described how they often found themselves ‘sitting down and doing nothing’ in their homes rather than actively engaging with life and ‘doing’. They talked about feelings of ‘lost time’ and a sense that time had slowed. The sense was in part due to the long process before diagnosis and then referral to secondary care. For some, such as Mr Horton, this was seen as a failure to diagnose the problem and working ‘along the wrong lines’.
In addition, the accounts of some participants indicated that day and night became conflated in the lead-up to surgery. While they would have previously been awake in the daytime, some reported resting and sleeping during the day to seek relief from the exhaustion of living in pain and due to side effects of pain medication. Participants also described pain onset or increasing intensity at night, which regularly woke them up or kept them awake. Unable to sleep well at night and sleeping during the day, participants experienced time slowing down as they waited for surgery and expressed distress, isolation and upset.
Time in the context of health care
Participants reported that their journeys through health care to arrive at surgery were lengthy, partly because they did not necessarily seek help for joint problems, but also because of slow processes of referral from primary to secondary care (Box 3). Such delays earlier in their journey through health care could influence the experience and impact of the wait for surgery once within the secondary care system.
I think the doctors [GPs] could have assessed the problem quicker no doubt . . . initially the first doctor I saw didn’t think I needed a hip operation. Four months after another doctor decided that maybe I should get checked out in [Hospital name] . . . That was a long wait. It would have been nice to have gone in maybe March or so and get an assessment and said this needs doing then. I’d have had it done by now . . . It’s been long enough now so . . . we’re ruling out most of this year. This year’s a non-entity.
Mr Smith
Anyway, psychologically preparing, yes it’s really thinking about um the actual day of going in, I guess. And there’s a lot of things to do in preparation for going in, reorganising things that you normally do every day, and that sort of thing . . . warning people that you cannot do this and you cannot do that, and yeah there’s a lot of that, takes up quite a lot of time.
Mrs Quinn
They give me exercises to do at home and I’ve been doing them religiously. Well they told us to do it twice a day. So I’ve been doing it twice a day . . . I would have been working today but I thought I’d take the day off. Give me a chance to get the shopping done, get myself packed, get myself in the right frame of mind to go.
Mr Golding
I wish it had happened a bit quicker but I suppose everybody wishes that. There seems to have been an awful long time from when he [anaesthetist] said to me it should be about six to eight weeks. It’s been a lot longer than that.
Mrs Noble
Well this actually you see, what happened was I was originally planned to go in this Saturday, then they said we want to call you forward to the Saturday before.
Did they explain the reasons for that?
No. No I think they had somebody who had dropped out. For some reason but I do not know. Um so yes I said ok but I had this funny feeling it would not happen. Um, I said to my wife I do not think it will happen . . . And I was right but um, yeah so they have had what they call a desktop conference i.e. I suppose across computers with the Consultant anaesthetist and they must decide on a plan and they’ll follow that plan through on Saturday.
Once in the secondary care system and before admission to hospital for surgery, participants’ time was also increasingly filled with activities relating to surgery. These included trips to hospital for pre-operative education, assessments and consultations, and tasks relating to psychological and physical preparation. These activities focused participants’ attention on, and heightened their awareness of, the upcoming date in their calendar and also meant that they had to arrange their other commitments and activities.
Participants also described how time in the lead-up to surgery did not always pass at a regular, steady pace. They felt that the date of their planned admission to hospital could appear closer or further away. Some participants felt that the date was approaching too quickly and this evoked anxiety and nervousness. Others were eager and impatient to have surgery and expectations about how long their wait would be were influenced by discussions with health-care professionals in secondary care. However, some were also unsure about how long they would have to wait and thought that the timing of their operation was not static, but would be changed. Participants described uncertainty and, in relation to their experience of time, how they had to put their lives on hold when waiting for surgery.
Participants also experienced changes to their admission date and this could be due to hospital factors, ill health or the option of a date change by choosing to change surgeon. However, the impact of these factors was sometimes complicated; for instance, one participant accepted the offer to change her surgeon because it would mean that she did not have as long to wait for her operation. The new date for her operation was then postponed because her glycaemic control for type 2 diabetes was not deemed adequate for surgery. A referral ‘back’ to primary care aimed to ensure support to achieve better glycaemic control, which was then followed by a wait to re-enter the secondary care system and to learn her surgery date.
The role of confidence during the journey through hip replacement
Within the longitudinal hip data set, we also explored how participants experienced confidence and vulnerability during their journeys through joint replacement surgery. Six themes were identified: participants’ changing trust in their bodies; feelings of vulnerability in relation to a surgical procedure; use and function of aids to be better safe than sorry; damage limitation and obeying the rules; awareness and fear of dislocation; and the influence of interactions with others on confidence.
Participants’ changing trust in their bodies
Participants’ accounts highlighted how their faith and trust in their body changed over the perioperative period (Box 4). During pre-operative interviews, participants described lack of confidence in their hip joints, describing their hips as ‘worn’, ‘damaged’ and no longer ‘strong’. Many had experienced the sense of their leg giving way beneath them, which meant that they felt that their bodies were letting them down and, as a result, participants spoke of feeling fearful of falling, ‘unsteady’ and ‘unsafe’; all of which impacted their daily lives. Before surgery, participants also spoke of feeling ‘frightened’ when using stairs without a rail and avoided certain movements to avoid putting too much pressure on their affected joint. Some participants also spoke of a sense of vulnerability in public areas, believing that they were unable to react quickly or effectively to situations (such as a physical attack).
I can still walk about which I’m glad that I can walk but as for sort of relying on it to uh, for movement, no I haven’t got a lot of confidence in it.
Mr Day (time point 1)
It gives me um, the stabbing pain and feels like it’s going to collapse.
Mr Smith (time point 1)
You know, you can’t go down and step into the garden and just dead head a few plants you know. Um. Try to use the walking sticks. Um I’m scared of using them because I don’t feel safe. I know I’ve got to practice with them but I’m scared of falling. I know with my luck if I fell it would be the other hip that went you know and then where would I be. So I sort of try and do what I can but think to myself no that’s really not safe. Don’t do that.
Mrs Kade (time point 1)
. . . and I think also just you know, you don’t feel so confident about doing things. You go out now and with all the crap you got going on out there, you know, you never know out there. I never worried about that, um, 2 years ago there was a big fight outside the house and I went out and broke it up and stopped half a dozen lads trying to kill a couple of others you know. Uh, went to court, did all my bit, you know, I wouldn’t do that now. I would feel vulnerable if I did that now, I would get hurt. Uh, because I don’t feel physically capable enough . . . I’m not able to move, I can’t run. Um, I certainly can’t change direction.
Mr Thomas (time point 1)
Postoperative experience: increased or changed confidence
I think things have worked out excellently. I believe – I feel um more confident in what I can do physically. Er, you know, doing jobs around and about. I feel er, in activity terms, I feel fitter, and I feel more capable of doing things er than I did before. So to me, you know, personally I think it’s exceeded what I probably expected.
Mr Thomas (time point 3)
Yeah I’m very confident. When you’re on two sticks, then you go down to – down to one stick, then you can have the confidence to – to walk without a stick initially. But then once you get that initial confidence over and say, ‘Oh I can do it with – do it without’, and then it just grows and grows and grows and it just happens, you just think of – of another hip, without the pain.
Mr McKenzie (time point 4)
Well I haven’t . . . I haven’t got the confidence in my leg I’ve had done. It’s because like seeing an X-ray to what they’ve done, I just fear that I don’t wanna damage it. D’you know what I mean . . . But I just haven’t got that confidence after this 12 months, I always fear that I don’t wanna fall down . . . I think it does take a bit of the quality of life away because you . . . for the moment you’re not . . . you’d like to do a lot more, and yet I haven’t got the confidence really with it. D’you know what I mean? Thinking about it, then will I be doing myself harm.
Mr Higgs (time point 4)
Um I’m um trying obviously to get myself sort of mentally and physically, you know, back to um how I was. Um but I’m um sort of struggling with the mental side of things at the moment . . . I do not know perhaps what my capabilities are now. Um so every now and again I sort of like push myself to um doing something, and I think, yes, I can do that, um and would have to go on to the next stage. Um I suppose where you have been physically impaired um in some respects, that you’re not quite sure what you can achieve now. Um so I’m having to do it in sort of small stages. And to say to myself, right, yes, you can do that, carry on to the – to the next thing.
Mr Foreman (time point 3)
After surgery, many participants described how they had quickly attained new or increased confidence in their body and prosthesis. They talked of feeling more physically capable and of how their leg no longer threatened to give way. Freedom from this, together with ability to engage in physical tasks that were previously difficult, enhanced trust and confidence in their bodies’ capabilities. Some participants spoke of their absolute confidence in their new hip joint and a belief that it would outlast them. However, at 12 months after their surgery, some participants said that they remained troubled by lack of confidence and faith in their body and the prosthesis. These participants described becoming more nervous of slipping over and falling since having surgery. They also spoke of a sense that the leg for which the hip joint was replaced felt weaker after surgery and uncertainty about the ability of their bodies. This had detrimental impact on their QoL.
Feelings of vulnerability in relation to a surgical procedure
Participants also articulated feelings of vulnerability in relation to the operation itself (Box 5). These feelings included concern about the body’s ability to withstand surgery and apprehension about the potential for surgery to go wrong (e.g. in relation to potential detrimental side effects of anaesthetic). Participants described concern about leaving hospital to return home, seeing hospital as a safe environment. Feelings of vulnerability were heighted for those participants who were concerned about returning home if they felt that there would be no one there to look after or support them. Some participants were also concerned about the potential for the failure of the prosthesis and the presence in their bodies of the materials from which the prosthesis was made.
I shouldn’t say this, but it worried me, I got myself in a – because they say that, you know, you’ve got to be in pretty good nick for operations, and it did worry me a bit, you know, whether my body’ll stick up to it.
Mr Higgs (time point 1)
Getting a bit scared now day by day. Yeah a little more scared.
What is it specifically that you feel a bit scared of or apprehensive about?
Having the operation . . . Just thinking what if it’s going to go wrong.
Um, but as to the operation yeah I am nervous. I hate hospitals anyway and I seem to be in and out in the last few years with various things being done so, you know, but previously there’s always been somebody in the house to look after me when I get home and there isn’t going to be anybody now. So I am a bit worried about that.
Mrs Burton (time point 1)
Postoperative concerns
I did, I felt quite sort of vulnerable and I didn’t want to be out of this sort of safe environment at that point . . . . I didn’t want anyone to think I was malingering and didn’t want to get on because that wasn’t true but there’s, it’s quite daunting when you leave that safe place.
Mrs Lovell (time point 2)
I always wonder if the joint will come out again. Because they do fail occasionally. There’s also been a bit of a scandal about um, wrong materials being used.
Mr Horton (time point 4)
Use and function of aids: better safe than sorry
Participants spoke about their use of, and the benefits of, walking aids over the perioperative period (Box 6). Before surgery, many used walking aids on a regular basis. They were used to maintain and boost confidence, offer reassurance, prevent falls and offer support in situations that evoked vulnerability. Use of aids peaked in the early weeks after surgery. At the 2- to 4-week postoperative interview, many participants continued to use walking aids, saying that this reduced the risk of harm to their new joint; this was in spite of feeling that they should be able to manage now without this ‘safety net’.
. . . but sometimes when I’ve been out walking; it feels as though the joint is going to give way . . . And of course you get excruciating pain when that happens and I suppose the stick has just helped as a little bit of a confidence booster, the fact that you’ve got something else that you’re not going to fall over with.
Mr Foreman (time point 1)
Use and function of aids after surgery
I can almost walk without a stick now but er, just to be on the safe side, I stay on it.
Mr Bedford (time point 2)
I have not used one [a stick] for months and months. Except when I’m on a rough walk, then I do because if you’re trudging through mud, the last walk I went on it was very, very muddy. Yes, I use one of those special walking sticks.
Mrs Quinn (time point 4)
At the time of the 6-month interview, most participants were no longer using walking aids; however, this was not universal. Some spoke of occasional use, for instance, when extra security was needed but others continued to use a walking stick regularly when outside, saying that aids were ‘just like a comfort blanket’ that provided reassurance.
Damage limitation and obeying the rules
After surgery, participants avoided and restricted movements and activities that could cause potential damage to their new joint (Box 7). They spoke of not wanting to ‘push their luck’ or to ‘push the joint to the limit, you know, before it’s settled’. They were nervous and described a need to remain respectful of their prosthesis and of the need to follow the postoperative restrictions and ‘rule book’. They talked of their ‘fear’ of ‘overdoing it’ and were careful and aware of their movements (e.g. standing for too long, twisting). Accounts suggested that these concerns and behaviours were driven both by a fear of harming their new joint and of not wanting to ‘undo any good that’s been done’. Some participants explained that after the initial postoperative weeks had passed they became more adventurous in the activities and movements that they engaged in. However, others remained apprehensive of particular activities (e.g. lifting, higher impact sports, dancing and gardening) and were careful when performing certain movements (e.g. bending to put tights on). This was related to a concern of not wanting to damage their new joint by placing strain on it.
I was dead scared to disobey any of the rules, I followed them religiously.
Mr Horton (time point 3)
I’m taking it really strictly and I follow the rules, I do not bend at the moment and I don’t lift anything, well nothing that might be heavy. Um so following all their rules, um move carefully, so at the moment I can’t say that anything is causing me any pain . . . I’m not allowed to bend or lift anything for 6 weeks. So I will go for 6 weeks before I try doing anything like bending or sort of going back to normal. Like sleeping, I don’t normally sleep on my back, but um I’ve been told that I have to stay on my back for 6 weeks, so I’ve been doing that . . . But I will follow exactly what they say until the 6 weeks are up.
Mr Golding (time point 2)
I don’t really want to get down on my hands and knees yet. I feel a little bit tremulous. But um that’s just me . . . I suppose once you’ve got something inside you that’s artificial and could go possibly wrong, it sort of er stops you. I don’t want to get down on hands and knees.
Mrs Hardcastle (time point 3)
I am always aware of it [new hip] because, as I say, I am, I um limit what I do because I don’t want, I won’t push myself. You know, I won’t [deep breath] run, jump and bend and I worry that I might do something to my hip. So I am aware all the time really.
Mrs King (time point 4)
Awareness and fear of dislocation
Before surgery, participants were mindful of the need to ‘look after’ their new joint to avoid dislocation. Concerns about dislocation peaked in the weeks after surgery (Box 8). At this time participants experienced occasions when they felt that their hip was about to dislocate and spoke of their apprehension. Awareness of the potential for dislocation was informed by the verbal information and written literature provided by the hospital. This was heightened by knowledge of other people’s experiences and previous personal experience. For some participants, concerns about potential dislocation became an enduring fear, continuing to influence their behaviour and activities in the long term.
This is the only thing I’m having trouble with getting used to, is that I know, once I’ve had it done, I mustn’t do that . . . Once they’ve done it, um I don’t want to – I’ll make damn sure I don’t dislocate it.
Mr Upton (time point 1)
Concerns after surgery
I think it was a couple of days after I came home, I was standing up and I was getting ready for bed, and I felt the head of the femur move. And I thought, ‘Oh my God, it’s going to dislocate.’ But it didn’t, it couldn’t have been in properly . . . I was a bit frightened.
Mrs O’Brian (time point 2)
I think what done me was I was talking to a gentleman who – who said um this friend he worked with had a new hip, and um he went back to work after three months, and like a silly fool, he lifted something heavy, and his joint come out. And it kind of done something to me, ‘cos I don’t want mine to do that.
Mr Higgs (time point 3)
And what sort of things have you been doing, or what have you done to get to that?
Well I think, if anything, it’s probably a question of what I haven’t been doing, I think, rather than what I have been doing. Um I haven’t been doing the sort of um – the wrong movements, um sort of crossing my legs and sort of twisting myself and doing whatever. Um by sticking to the rules, really the sort of not dislocating it bit, um that I came out with in my head, by sticking to that and not doing what I shouldn’t do, I think it’s helped it cure itself . . . I suppose, in a way, I was frightened to stop them, because I still felt that, you know, we were given this 12 weeks sort of time slot um about the fear of dislocation. But I think perhaps I was um so worried that I could still dislocate it, I made damn sure I wasn’t going to. Um and so even this time when I went in again, I was sort of pre-programmed, I didn’t have to be told what I had to do this time because I was still doing it with the other one.
The influence of interactions with others on confidence
Although interactions with others and knowledge about dislocation could reduce confidence, participants also described how their confidence could be increased through encounters (Box 9). For instance, some described how conversations with surgeons before surgery boosted their confidence in care and treatment that they would receive. Some spoke of the importance of education and information in order to feel informed about the operation and recovery. Health professionals also continued to bolster participants’ confidence in the weeks and months after surgery. In addition, observing the experiences of others who had had positive experiences of joint replacement enhanced confidence.
So actually going into the place, um again [name of Surgeon] said, ‘I’m going to come round to see you before I – you get wheeled off’, or whatever. And so, you know, I’ve got every confidence he’s going to come round and check I’m ready for it, and if I tell him, ‘I’ve got last minute thoughts and I don’t want you chopping my leg off’, um fine . . . well we had a joke when he first saw me, he said um, ‘I’ll try and make sure they’re both the same length when you come out’. Um to me, the human side of things is – is great.
Mr Upton (time point 1)
Um [clears throat] well prior to the operation there was a booklet. And there was a meeting, and er it was um – it was informative and comprehensive, and um I think that sort of everything that was mentioned fell into place. So as far as the information, there was plenty of information.
OK and did that information influence your recovery, do you think?
Yes it gave me a little bit more confidence. It’s er – it’s not knowing is which er chips away at the confidence, doesn’t it?
Um at first it felt a bit sort of um different. And er I sort of – I was afraid to sort of move my leg and, you know, you’re anticipating er – the anticipation of having the surgery, and you think, ‘Well what can I do? What am I able to do?’ But the physiotherapist, she sort of put me at ease and she um just told me what I can do gently to start with, and she helped me like move me leg, showed me which way I’ve got to move me legs and things, and what I’ve got to do.
Mr Day (time point 2)
I know several people. I actually work at a golf centre and, of course, a lot of the senior people have had hips and knees done. And yeah, they tell me how good the operations are. I know several people, actually . . . Well, they did say that one person didn’t feel any pain at all after he’d had . . . his knee, it was. They gave me a lot of confidence. Everybody I spoke to, they seemed to have had a good result . . . It does boost your confidence.
Mr Everett (time point 1)
Understanding the patient experience: total knee replacement
The experience of support during the journey through knee replacement
We were also interested in exploring how undergoing and recovering from knee replacement surgery alters patients’ experiences and use of their support networks. Using IPA259 we examined patients’ experience of knee replacement at all time points and identified three superordinate themes relating to the experience of support: (1) relationships with health professionals over the knee replacement journey; (2) implications for informal relationships and support networks; and (3) providing support to others.
Relationships with health professionals over the knee replacement journey
‘I’ve got faith in him’: trust and confidence in the surgical team
Participants who were undergoing knee replacement expressed considerable ‘trust’ and ‘faith’ in surgical teams. This seemed to relate to their experience of living with osteoarthritis, in which participants dealt with increasing pain and impaired mobility. By the time that they had reached secondary care, many felt that they had no choice but to rely on medical opinion and expertise, and that surgery was inevitable. This was rooted in previous positive encounters in consultations as well as experiences (their own and others) of successful outcomes after other types of surgery.
Contact with secondary care team
After having their operation, participants’ contact with secondary care health professionals shifted from the surgical team to a team of nurses, physiotherapists and auxiliaries. Relationships with health professionals also changed; participants wanted to receive support and guidance rather than the total control that they had wanted from surgical teams. After returning home, all participants had some contact, although often infrequent, with secondary care professionals – predominantly consultants and sometimes physiotherapists. They were ‘keen’ to receive follow-up appointments and ‘eager’ to obtain clinical opinion about aspects of the recovery process, for instance when they should stop using walking aids or return to leisure activities. These interactions bolstered confidence and offered reassurance.
‘You were sort of cut adrift’: unmet support needs during the recovery process
Participants also spoke of unmet support needs during the recovery process. For example, many felt that input from physiotherapists was received too late in the recovery process and that earlier involvement would have helped to reduce feelings of abandonment, enhanced motivation to exercise and facilitated earlier recovery. Postoperative aftercare in the community was also described as lacking. After discharge from hospital one participant, a widow, was not offered the support of a district nurse. Instead she described struggling with her own care, such as changing her surgical stockings, and had to implement her own support by paying for help. This participant felt that more formal support would have provided reassurance and reduced her feelings of isolation.
Differing perceptions of expertise: primary versus secondary care health professionals
Although confidence in secondary care health professionals was consistently high, participants trust in and willingness to seek support from primary health-care professionals was more mixed. Perceptions of support on offer and that received from primary care during the postoperative period was influenced by experience of care received before surgery. For example, some participants expressed dissatisfaction with primary care before surgery, describing their sense that care and advice had been inconsistent. When patients felt that primary care had not been helpful before their surgery, they were less likely to seek support actively from primary care on return home from hospital (Box 10).
He [has] done my brother’s leg, both legs, about 6 years before me, and he’s had – you know, brilliant. I asked for him. And he’s been there for a long time as well, he’s not a new chap.
Mr Jackson (time point 3)
Contact with secondary care team
They were caring . . . they’d be available any time um . . . the only trouble was then I wasn’t allowed to walk at all for that intervening more or less a week like, 4 days, 5 days . . . if I wanted to go to the loo I had to call for a nurse to come with a wheelchair to take me there.
Mr Armstrong (time point 2)
‘You were sort of cut adrift’: unmet support needs during the recovery process
I rang them week before last . . . he [physiotherapist] said ‘Of course we are very busy at the moment, but I will be in touch with you again, but there is a two to three week wait’ . . . But I was quite annoyed . . . I felt like saying to him well don’t bother. I mean they say I can drive within six to seven weeks . . . I wouldn’t even of had any physio by then . . . I know everybody is very busy and you’re only one of a number really aren’t you, but this physio thing really that did annoy me, because they stress about you having physio and making sure you bend your knee and all this and then nobody comes . . . it don’t make sense to me.
Mrs French (time point 2)
Differing perceptions of expertise: primary versus secondary care health professionals
I’m afraid my doctors are very poor at the moment . . . we’ve had the same doctor for about 40 years and he retired and at this practice we got now we get a different doctor every week, they seem to be coming and going. If you go to see anybody, they don’t know nothing about you . . . I had an experience with them, one doctor give me these tablets for pain killers, something I’d never tried before and then when I went back a couple of . . . I don’t know 3 or 4 weeks later I said ‘Oh your colleague gave me these’ and she said ‘He shouldn’t of never give you them’ and chucked them in the waste bin. I thought blimey they can’t even trust each other . . . So that’s put me off a bit.
Mr Jackson (time point 2)
Implications for informal relationships and support networks
Changes in level and type of assistance
There were changes in level and type of assistance provided and received at different points in the journey through knee replacement. Before surgery, participants described the importance of help provided by family and friends for everyday activities (such as fetching groceries and household chores). Immediately after surgery, the need for this kind of support sharply increased, with support needed for many more daily tasks, such as carrying a drink. Undergoing surgery also led to changes in participants’ roles in their relationships and family units. For example, one participant described how, when recovering from surgery, he was looked after by his children who were ‘running errands and things’ and felt that his wife treated him ‘like a baby’. These changes sometimes evoked negative emotions, including despondency and helplessness. However, as recovery from surgery progressed, the need for support and associated sense of helplessness reduced.
‘She’s always there you know when I want her’: the assumption that family will help
For participants who were married, help often came from spouses. Married participants (n = 7) who all lived with their spouses initially turned to their spouses for assistance during the journey through knee replacement. After surgery, in addition to spouses taking on increased responsibility for tasks relating to everyday living and functioning, they also assumed additional caring responsibilities, including help with personal care (e.g. helping to bathe). Some also played a role in medical aspects of the recovery process. For example, Mr Armstrong’s wife administered postoperative anticlotting injections and Mr Clark’s wife, a retired nurse, removed his stiches. Although many participants appeared comfortable in accepting that their spouse was occupying this novel role, others felt ‘awkward’ and embarrassed at asking spouses to undertake duties that they felt should be provided by paid professionals.
Use of extended informal support networks
Participants looked outside their immediate household to other family members (e.g. children, grandchildren, siblings) and friends to meet their postoperative support needs when they could not be fulfilled by a spouse. For example, several participants were the only driver in a household and this meant that friends and family were asked to drive on their behalf while postoperative restrictions were still in place (patients are currently told not to drive for 6–8 weeks after knee replacement). Participants who did not live with spouses asked friends and family for help, particularly in the early postoperative period.
‘I’m lucky’: willingness to accept help
Participants expressed mixed emotions about the help they received from their friends and family around the time of surgery. Several viewed such support as ‘helpful’ and ‘invaluable’ and felt fortunate to be ‘spoilt’ by friends and family who ‘rallied’ around them. Although surgery was often seen as a way of maintaining independence the time around surgery was a period when help and support from others was a necessity. Many participants craved their return to self-sufficiency and most participants did eventually regain the independence that they had sought (Box 11).
Well my wife has been working like a trooper you know [since discharged from hospital]. I mean, trouble is she won’t let me do stuff. . . . going to the shop. Um, you know getting a magazine, treating me like a baby. Um, I mean just doing extra. . . . I mean I cannot drive a car so, you know, I used to do virtually all the driving. Now my wife is doing all the driving.
Mr Cook (time point 2)
‘She’s always there you know when I want her’: the assumption that family will help
And where did you have that done, having the staples out?
Er now um [laughs] um my wife, [name], is a nurse. And um well she retired about a year ago. And um we thought rather than, you know, um – she knew the people in um – in the hospital, and they gave her a thing for taking them out, um she’s ever so good and ever so careful, rather than go into hospital. Um she took them out at home for me.
Use of extended informal support networks
What sort of things have they [friends and extended family members] been doing?
Lifts everywhere, my wife can’t drive . . . so to and from hospital for any appointments or to do the shopping, anything.
‘I’m lucky’: willingness to accept help
what sort of support have you had?
Well I’ve been lucky like that; the wife and the kids are pretty good, you know, I’ve had the support of them around me. I suppose if it was somebody living on their own it might be a bit different.
Yeah, what in particular are you thinking of?
Oh loneliness and getting to do things.
Providing support to others
Although not a shared experience, a striking feature in the accounts of some participants was the impact that knee replacement had on the support they provided to others and how caring responsibilities influenced their journey through joint replacement (Box 12). For example, Mrs Biggs, a widow and sole provider of support for her mother and brother-in-law, was particularly articulate about the reliance of others on her and the impact of surgery on this. Owing to her caring responsibilities, and despite limitations and pain imposed on her by osteoarthritis, she strived to maintain her role and not let others down. Undergoing surgery meant that Mrs Biggs temporarily passed her normal caring responsibilities onto her sister. Keenness and determination to decrease ‘burden on others’ as soon as possible and to return to her original role supporting others served to drive and motivate Mrs Biggs in her recovery from the operation. Successful knee replacement also meant that some participants felt able to assume a new role offering support to others, which they felt would have been impossible before their surgery.
are you where you thought you’d be six months ago in terms of recovery?
Um, yes I think I, because things moved so well after. Yeah, yeah. But I was determined any way that I wouldn’t be a burden to anyone.
No and does that make a difference do you think?
Yes, oh yeah . . . Well for me mentally it does because of course uh, you know I feel I got a lot of responsibility here to keep the home running and I don’t want uh, to feel any one else has to come in and um, you know take over from me . . . it certainly encouraged me to get going, yes, yes.
Yesterday, we got a little local, uh, pamphlet or whatever you’d like to call it down [town name] it was looking for volunteers actually to help families that might be in, were going through different kinds of troubles . . . so I’m going to ring them. And I wouldn’t have done that, well I would, you know – I’d, I’d, I couldn’t really move efficiently, you know. . . . I wouldn’t have done it 12 months ago. I wouldn’t have been physically able to just go out and be confident enough to walk.
Mrs Evans (time point 4)
Combined hip and knee data sets
An exploration of patients’ hopes and expectations for hip and knee replacement surgery
We were interested in learning more about participants’ expectations for recovery from hip and knee replacement surgery and how their expectations were met. We present here the findings of our analysis from the pre-operative and 12-month data sets.
Hopes and expectations relating to long-term pain after joint replacement
All participants hoped that hip or knee replacement would reduce their pain (Box 13). For most, this was a key motivating factor for their decision to have the operation. Some participants described awareness that long-term pain was a potential issue after surgery. However, some thought this might be mild while many hoped to achieve complete freedom from pain in their operated joint in the year after surgery. Expectations relating to pain were based on previous personal experience of undergoing joint replacement surgery, knowledge of others’ experiences – both successful and unsuccessful – and information resources provided by the hospital. Most participants also described a hope that they would be able to stop use of pain relief and anti-inflammatory medication in the year after surgery. However, some also thought that they would have to continue using medication to manage pain in other parts of their body. Participants also described their hopes for the positive benefits of reduced pain in the longer term after surgery, for instance improved mood and enhanced sleep quality.
Having the hip replacement, the motivation is to get rid of the pain.
Mrs Quinn (time point 1)
What about the pain, what are you hoping for?
Zero actually. I nearly, because I said that’s what happened with this. If it’s the same before when I had that one done it’s unbelievable.
I was telling him [a friend] about having mine done. He said ‘the one thing you’ll do when you wake up, he said you won’t have that friend with you’, and that’s why I call it a friend. Yeah, he said ‘you won’t have that annoying, nagging little thing that’s you know, grinding away quietly there’, he said. ‘It just won’t be there’ and he said that’s what he liked about it the most. He said ‘that sort of’ he said it was gone. He said it was completely not there. And [another friend] actually said the same as well. He said his pain just disappeared . . . He just said ‘my pain had gone away’. He said it was absolutely gorgeous.
Is that what you’re expecting or?
That’s what I would hope yes. You know, I won’t have that grumbling irritation, yeah.
So how else do you think your life will be different 6 months after your operation?
Well if I haven’t got pain I won’t be such a grumpy old git . . . because I’m sure I’m grumpy because I’m, I’m not, yeah I’m in pain. I’m not noticing it because you, yeah I’m saying I do affectively show it. But yeah I’m hope I’m not such a grumpy old sod. [Wife] will be able to tell you that probably better than me.
I read it in one of the leaflets that they [the hospital] gave me . . . you may still be in some pain. That’s one of the down, downsides of having a hip replacement, you may have one leg longer than the other, infection, still pain. It’s one of those risks. But hopefully that won’t be me.
Mrs King (time point 1)
After you’ve had the operation, would you expect to reduce or change the pain relief you have?
I expect to be reducing but I don’t expect to do without, because I have quite a bad neck . . . obviously the Co-codamol helps that as well, so I don’t expect to give up pain killers but I hope it will go down a bit.
Expectations relating to postoperative function
Before surgery participants described living with restrictions on movement and mobility, reliance on walking aids and inability to ‘do a lot physically nowadays’ (Box 14). They anticipated that joint replacement would confer better function and bring about a future in which they would be able to ‘get around easier’, walk further and with a ‘normal stride’, navigate stairs and steps with more confidence and ease, and achieve independence from walking aids. They also spoke of hoping to once again be able to kneel down, bend down and reach their toes and have the capability to return to riding a bike again.
And if we did speak in a year’s time, how do you think that life will have changed?
Well, I just hope that I can walk normally. And it would be nice to think I’d be pain free. This is the hip. And what more can you ask for? Hopefully to be able to do more or less what you did before, other than lifting.
Are you hoping to be doing some things in six months time, twelve months time that you are not now?
Well yeah I hoping to get out on the garden, kneeling down doing planting seeds, or weeding. At the moment I got to do it all standing up . . . Sit in the bath and have a bath. Kneeling in the bath, that’s a problem at the moment. I cannot kneel in the bath . . . I hope to be able to get up and down the stairs. At the moment I’m going up the stairs a stair at a time, not like the normal [banging on the table]. I go up one, rest up, one.
Well I’m hoping I can just walk out the door, I will be grateful just for that. I do not have big inspirations of climbing Mount Everest.
Mrs Young (time point 1)
I know I won’t be out Olympic sprinting but I accept that, I will hope that I will be um, far more capable than I am and that’s what I’m looking forward to.
Mr Thomas (time point 1)
Well everybody that I’ve spoken to has been very satisfied and uh, they’ve gone on well. As I say they’re walking again and walking with the group. So they can do what four or five miles I suppose, which is pretty good for anybody really. Um, and yes they’ve been very pleased . . . you think well if they can do it, I can do it as well.
Mrs Noble (time point 1)
Also a friend of mine, a lady up the road there, she’s had both of hers done and she’s walking now without sticks or anything.
Mr Bedford (time point 1)
Would you anticipate that you would be using any walking aids in the future?
I would always, unfortunately, have to use walking aids. It would be nice to think that I didn’t have to use them in the house. I always have to outside because I could have a fit at any time, and outside if you have a fit there tends to be things like concrete pavements and – and buses and things like that, that you might fall in front of.
Um, the surgeon when I saw him he said that its um its, what he was saying there was we’ll probably get your knee working like it did 10 years or 15 years ago. Don’t expect to go any better than that. And um he did tend to, so well that’s not too bad if I can get back to that.
Mr Armstrong (time point 1)
As a result of these changed functional abilities, participants anticipated that they would, in the year following surgery, be able to become more active and enhance their general level of health and fitness. However, several participants also acknowledged that there would continue to be some restrictions on their physical capabilities. For example, they spoke of how they would have to continue to avoid lifting heavy objects even in the long term and would also be unable to return to playing high-impact sports. They also believed that although walks to the local shops would become a future reality, hill trekking and climbing mountains would not. In addition, expectations relating to postoperative function were tempered by a sense that they may not achieve the level of function that they had before the onset of the problems with their hip or knee joint. This was informed by the sense that they continued to age and that some lived with problems in other parts of their body.
Like their expectations for pain, hopes for postoperative function were informed and shaped by their own and others’ experiences of undergoing similar medical interventions, in addition to information received around the time of surgery.
Expectations for changed engagement in social, work and life activities
Participants described expectations for participation in social and work activities after hip or knee replacement (Box 15). These expectations were related to anticipation of reduced pain and increased function and were particularly driven by observations of how well others had recovered from similar operations. For example, participants hoped to return to work and looked forward to attending social clubs again, meeting friends for lunch and other valued activities including golf, bowls, bell ringing and ballroom dancing. They also hoped to be able to take holidays once more, have day trips out and travel to see friends and family, both within the UK and abroad. Participants had to stop or limit these kinds of activities before surgery because of difficulties relating to their osteoarthritis.
Well when it started I thought that uh, it would be an advantage for me playing golf. Because as I say my mate who I play golf with for a long time, uh, he had both his knees done and he was getting bad. He had to pack up golf and he had his both done and he came back and he was playing all right. When I say playing all right he had no trouble with his knees. I thought this is fantastic you know.
Mr Ostafew (time point 1)
What are your motivations for having the operation?
Well to enjoy life I suppose like and, you know, not to sit back and think, ‘Well I’m finished for now.’ Because er the only time I’ll be finished for is when I’m underground, gone. Yeah that’s how I’m built inside. You know, I like get out and do something. And I might even do like little jobs again, because people – [I’m] in big demand.
I’ll go over and see my daughter and my, I’ve got three grandchildren over there [name of country] and then my life over there which I enjoy. I enjoy that over there . . . we’ll see how it works out. But that’s going too far ahead for me. I can only go so far at one time. I’m only gonna do one step at a time and see how I’ll, go into hospital first get that over and done with. Get up out of hospital, get up and going.
Mr McKenzie (time point 1)
I can’t get down to pick the ball up. My son’s got to come with me and pick the balls up for me so I can throw them down like you know [when playing the bowls]. I know it might sound a silly little thing but its, it’s an embarrassing sort of thing . . . it’s you know another little thing that I hope, in about 6 months time, when the season starts next year, I shall be able to go up and pick the balls up no problem.
Mr Day (time point 1)
I think um er you get – you do feel quite tired with the pain. And I think – I’m hoping that you get more energy so, you know, enthusiasm for things. I can’t say it’s completely gone or anything like that, but I think that you will – you will have more enthusiasm to do things. I haven’t actually stopped doing things much, but I realise that um some – some social things have gone down. I mean I still do all the activities um basically but um maybe with less enthusiasm. And so that will come back, and so quality of life.
What’s quality of life for you?
Well yes it’s, it’s feeling um enthusiasm for doing the things that you do. And being able to do things like babysitting grandchildren, like going visiting exhibitions like, you know, all the sort of things that would be nice to do, that maybe have been cut back.
Participants’ accounts also showed how they hoped that joint replacement would provide them with the chance to engage with life once again: ‘to be able to get out again’, to ‘go out and enjoy themselves’, ‘get on with their life’ and to ‘get back their quality of life’. For some, this also meant taking up new hobbies and interests such as joining a walking club and starting voluntary work. Many participants talked of their hope to be able to participate in these activities by certain points in time, for instance Christmas or their birthday. However, a few participants did not put time markers on when, and if, their goals would be achieved, talking of ‘just having to wait and see’ and ‘just depending on how I get on’.
Fulfilment of expectations relating to pain
Twelve months after surgery, 15 participants described complete relief from pain in their operated joint (Box 16). This group talked of being ‘absolutely over the moon’ with this outcome and of how they now ‘felt a hundred times better’. The operation had surpassed their expectations and they revealed how it was a ‘wonderful’ relief to be free of long-term pain. They described how it was ‘lovely’ to ‘move around without pain’; for example, how they were now able to bend down and walk without experiencing a ‘terrible pain’. As a result, as hoped, those in this group who were not living with pain in other parts of their body had halted their use of pain relief. However, 12 participants said that although they were free from the pre-operative intensity of pain arising from the grating of ‘bone on bone’, they continued to experience discomfort, soreness, tenderness, a dull ache or twinges in the area of their operated joint. They attributed this pain to a variety of causes. For example, some said that the discomfort was caused by their muscles tightening, while others thought that their recovery from surgery was not yet complete. Participants with ongoing problems were also able to identify triggers that intensified these sensations, such as exercise, ‘stretching themselves too much’, moving from sitting to standing, or standing in the same position for too long. Although living with ongoing discomfort, most members still said that they were ‘grateful’ for the treatment that they had. In keeping with attitudes to pain relief medication before surgery, participants in this group did not see pain to be ‘bad enough’ to warrant taking pain relief medication; however, two knee replacement participants described continued experience of a more ‘severe pain’ and they expressed frustration and unhappiness with this outcome. Again, as they did during the pre-operative interview, participants reflected on how previous experiences of joint replacement – their own and others’, in addition to information received around the time of surgery, played an important role in their expectations relating to pain after surgery.
No pain at all . . . no painkillers . . . I was on eight paracetamol a day I think it was, on the maximum and you couldn’t sleep at night because you could be comfortable in bed, so everything just hard work and just more complicated where I’ve got nothing like that now.
Mrs Noble (time point 4)
I don’t even think about it [the knee replacement] . . . I mean the only huge difference is, I’m not in any pain at all.
Mrs Evans (time point 4)
I’m very happy with it. I didn’t realise how good it would be to be free of pain, you know, as I am now like, you know. But um, yeah, I’ve been very happy with it.
Mr Day (time point 4)
I don’t normally think of it until it actually hurts . . . it’s when I’m in bed normally. Um not right away but in the middle of the night sort of thing you know . . . I put it down to me muscles, tightening up and you know, you can’t have things happen to your body and nothing you know, no come back on it.
Mrs Young (time point 4)
It’s uncomfortable then when I move. I sort of have – I’m like a little old lady again getting up, you know, I have to sort of push down on my arms to [deep breath] you know, like struggle to get – get up. But, you know, but I’d still rather be like that than have the pain I had before, definitely . . . before I had it done, yeah, that was – that was like a constant grinding, you know, when I walked it felt like it was – it was horrible. But this is like – I’m sure it’s muscles now.
Is this what you were expecting at this point to be sort of feeling?
I wasn’t expecting anything, to be honest . . . But I’m – I’m – I’m pleased if I’m not in that horrible pain. I can put up with the discomfort. Be nice not to wake up in the night like that but then, you know, suppose it’ll go in time . . . it’s more dull to what it was . . . like I say, it’s not pain, it’s not er, ‘Oh my God, you know, I’ve gotta go and take some tablets’. . . . And it’s not enough to warrant taking tablets.
Fulfilment of expectations relating to postoperative function
Participants spoke of how a reduction in the pain experience meant that at 12 months post operation they were able to move their body around with less difficulty and to walk further than they could before having joint replacement surgery (Box 17). They were also able to ride a bicycle, more confidently navigate steps and stairs and had experienced the anticipated independence from walking aids. However, this was not a universal outcome, with walking aids still used by some participants when they walked for any distance as they continued to offer reassurance, as they had done before surgery. Participants also talked of ways in which they continued to experience a lack of freedom and restrictions on the way in which they were able to move their body. For example, some highlighted how they were unable to run or experienced difficulties when bending down to the floor to, for example, pick up objects. Accounts also showed how participants continued to experience difficulties in walking up hills, how their walking pace had slowed and that they were unable to cope with longer walks, as they had hoped. Many of those who had undergone knee replacement surgery spoke of how they were now unable to kneel down. Nonetheless, these restrictions on movement, for most, did not seem to interfere with satisfaction with recovery and were perceived as ‘no major hindrance’. A perception among participants that general ageing also played a significant role in limiting the overall potential for movement and mobility can perhaps help to explain this view.
Are there any things you’re doing now that you could not do before the operation?
Um yeah I can – I can walk further . . . I’ve been up a ladder this week, and um there was no problem at all. Um so getting up and down stairs now, I can – I can do that naturally without sort of doing up one step at a time, if you know what I mean.
I used to take big strides when I walk but you can’t do that. Um but that’s no bother . . . Well I do have trouble at walking up a slope since I’ve had my leg done . . . but it will improve.
Mrs Young (time point 4)
And how are you finding the walking now?
Slowly, I walk quite slowly to be honest but I don’t know why I do, but I do but then my daughter said to me the other day ‘but mum, you don’t have to walk quickly, you’ve got all day to do it in, you don’t have to rush to the shops, just walk at a nice even pace’ . . . Looking at it logically she’s right, and then, but the thing is, when you get older you still think you’re 21 and you think your body should do what you did then and everything was at double speed wasn’t it, and then logic kicks in and you think no, no, that’s 50 years ago, just pace it a little bit . . . so I’m learning slowly.
So but um, walking any distance I’m better but I can’t. I mean we’ve left the walking group. We can’t. We tried it one day and we couldn’t keep up by miles. So I said to [walking group leader] ‘you’d better go on’ I said ‘we’ll catch the bus home’.
I am walking without sticks . . . I take the stick with me but very rarely do I need it and in actual fact I have found that walking with a stick makes my hip hurt so it is just an emergency thing really.
Mr Cook (time point 4)
Are there other things that you’re doing now that you couldn’t do before the operation?
Um hmm not – I feel happier carrying things, weights and sort of moving, yeah, just normal sort of things which I was reluctant to do before. Um I do feel safer up steps and ladders, because I didn’t realise how much you brace yourself through your legs, um I feel more confident with that . . . getting in and out of some cars and things, I find it easier, because I can bend my knee, you know.
Yeah and are there any things that you can’t do now?
I don’t kneel on it um at all. I haven’t knelt on it.
Fulfilment of expectations relating to engagement in social, work and life activities: ‘I’ve got my life back’
Participants talked of the ways in which their lives had positively changed since undergoing joint replacement surgery (Box 18). They described how they were now ‘more active’ and how life had become ‘more enjoyable’ as a result of having the operation. They spoke of having ‘a new lease of life’, of ‘making up for lost time’ and of how they were able to actively engage with more activities in each day now. As hoped, they had returned to many of the activities that they undertook prior to the onset of the difficulties with their hip or knee joint – a return to employment, social clubs, gardening, playing skittles, improved intimate relationships with partners and had already enjoyed holidays and trips to see family and friends. Planning for, and engaging in, these activities provided them with a psychological ‘lift’ and they talked of feeling ‘more positive’ and ‘optimistic’ about the future. However, for some participants, their pre-operative expectations to engage in particular social activities (e.g. taking holidays and games of golf) once they had recovered from their joint replacement surgery, were tempered, or had to be put on hold, because of other health conditions. In addition, a few participants who did not talk of additional health conditions also revealed that they continued to hold themselves back from undertaking the hobbies and activities that they had previously enjoyed and had hoped to return to (e.g. ballroom dancing). This was attributed to a lack of confidence in their new joint and concerns relating to falling.
I actually played nine holes um about a fortnight, three weeks ago, on a Sunday. We had a nice, sunny Sunday. Um, yeah, I got on with it all right. Um it’s my back which is the biggest sort of problem there but um, no, I coped with sort of nine holes, you know, on quite a long course, um without any problems at all really.
Mr Clark (time point 4)
I’ve been on holiday twice and I’m going on holiday twice again next year and I didn’t do anything like that for the last two year because I had too much pain to do it, and you know if we get a chance we go out for the day and so I really, I really have got a new lease of life completely.
Mrs Noble (time point 4)
I do my hand bells and, you know, we go out, um do little concerts. And er I play bridge during the wintertime and odd times during the summertime, um that’s Monday and Tuesday. Wednesday, if I don’t play golf, I go up the golf club for lunch and then go out to the women’s fellowship at church. So, you know, I’m out a lot.
Yeah and that’s quite a change from this time last year, isn’t it?
Yes um well I’m enjoying going out more, yes.
My life’s back, back to uh, normal. You know, huh, how can we put it. I suppose being an Englishman I’m always a bit sensitive about it. My sex life with my wife is now back to a much better position than it used to be. You know because sex became almost uh, impossible with my hip. But that is now, you know, back to a normal, sensible proportions. That has been a huge move forward.
Mr Thomas (time point 4)
How is it going with the skittle playing, are you still doing that?
Skittles, yeah, I played last night. [Laughs] Yeah, oh yeah, it’s improved it a bit . . . you know, once I couldn’t bend down and pick the balls up – um easy now.
And in terms of your recovery, how satisfied are you with it?
Oh satisfied 100%. I mean it’s, I was so crippled really beforehand. I couldn’t do things. I’ve got my life back again which, you know, is important to me . . . I do all the um – my activities at um – I go out quite a lot.
Unfortunately because of my neck we won’t be going on holiday abroad this year, I gave my holiday to my son that I’d booked because I can’t, don’t think I could cope with travelling by plane and that. We were going to the [name of county] but I don’t think I can cope with the travel at the moment.
Mr Golding (time point 4)
I mean if I want to I could go back dancing again but I’m a bit, I’m a little bit nervous about that so . . . and you see you’ve got the slippery floor at the dance and also I will, I wear flat shoes around the house, whereas if I went dances I would have a small heel on my shoe and that makes you a bit more unstable. And you can’t really go round the dance floor with a walking stick, so I’ll probably give that a miss but I think I’m doing as much, if not more this year than I thought I ever would be able to.
Mrs Noble (time point 4)
Discussion
Through employing a qualitative, longitudinal design, we have achieved a detailed understanding of a range of issues concerning the experience and impact of hip and knee replacement. Specifically, we have gained a comprehensive understanding of patients’ routes from referral to hip replacement surgery and have learned about the impact of waiting for surgery. This includes focus on patients’ psychosocial well-being and their conceptualisation of time. We have gained an understanding of how confidence influences, and is influenced by, experiences of undergoing and recovering from hip replacement surgery. We have also generated novel perspectives on the trajectories of support used by patients over the journey through knee replacement. We have also achieved an in-depth understanding of the nature of patients expectations for hip and knee replacement surgery, how these expectations are formed and the ways in which they are accomplished (or not) 12 months after surgery. All of these insights have been made possible by the study designs, which is one of the first to explore patients’ experiences of joint replacement in such detail from the pre- to 12-month postoperative period. Furthermore, a key strength of our work is the inductive nature of the approach, which ensured that the issues that we have addressed in this chapter are of particular salience and relevance to participants.
We have identified that delays to surgery are a common occurrence for patients in the NHS awaiting orthopaedic intervention. These changes to the date of surgery made by the system and patients’ changing perceptions while waiting for health care both have implications for patients’ well-being and this finding helps to explain views about health care. Our findings suggest that patients’ experiences of time in the lead up to surgery are complex and multidimensional and clearly differ from the linear conceptualisation of time that is required to plan NHS services. We have gained detailed and useful insights into how undergoing surgery can increase feelings of vulnerability and alter a patient’s trust in their own body, and the influence of interactions with others on confidence levels and the fears that patients have concerning the potential of causing harm to their new prosthesis. The research also highlights some of the strategies that patients engage in to limit this. We have learned that patients rely extensively on, and value, both informal and formal support networks over the perioperative period and that transformation from a person living with osteoarthritis to a person recovering from a surgical intervention can lead to alterations in the assistance participants received from others, including the source, type and level of assistance. However, when patients are not offered the support of health and social professionals over the perioperative period, for example to aid recovery, negative consequences can ensue (e.g. distress and feelings of abandonment). We have highlighted the complexity of patients’ expectations for joint replacement surgery and how these expectations can be driven by previous personal experience of undergoing joint replacement surgery, knowledge of others’ experiences – both successful and unsuccessful – and information resources provided by the hospital around the perioperative period. These insights will be useful in helping health-care professionals in educating, supporting and managing patients expectations to ensure that patients form realistic and achievable expectations for outcomes relating to pain, function and engagement with work, social and life activities.
Use of in-depth interviews facilitated a detailed exploration of participants’ experience of undergoing and recovering from joint replacement surgery. Follow-up interviews allowed for clarification of any issues raised in earlier interviews. They also facilitated the development of a closer researcher–participant rapport, which encouraged the disclosure of personal accounts, helping to generate novel insights and richer data. The use of topic guides allowed consistent exploration of salient issues across participants but also the opportunity for additional probing and reflection in order to facilitate examination of prominent and unanticipated issues. To ensure analytic rigour, analysis was conducted by a team of experienced qualitative methodologists with backgrounds in social and behavioural sciences. The analysis process included double coding, discussion and agreement to arrive at the final list of themes. Furthermore, we engaged in several other validation strategies: discussion of findings with patient representatives, reflexivity and seeking out and paying attention to negative cases. We do not claim that the experiences of the participants were representative of everyone awaiting hip and knee replacement surgery; however, the rigour of analysis helps to improve the credibility of findings. In addition, although the research was carried out with patients undergoing surgery at a single orthopaedic centre, men and women were included and the sample sizes were designed to accord with robust approaches. In the thematic approach used in the hips data set, we were confident that data saturation had been achieved. In the knees data set, use of IPA provided us with the opportunity to achieve depth in analysis and the data set size is within the norms of IPA methodology. The inclusion of patients from only one orthopaedic centre has the potential to affect transferability of findings, but the orthopaedic centre serves a diverse population in the region and it is likely that the results will resonate with the experiences of patients from other areas of the UK.
We took care in the design of the study to consider data collection approaches. Qualitative researchers have traditionally chosen to meet face to face with participants when carrying out in-depth interviews. However, research in the area now indicates that the mode of interview may have little impact on the number, character and depth of data generated during an interview. 261 However, we designed the study such that initial interviews took place in person to build rapport and consider it likely that this enabled the generation of even richer data during subsequent interviews that were conducted by telephone. The study had excellent retention; interviews were carried out with 21 out of the 24 hip patients and 8 out of the 10 knee patients at the three follow-up points.
Conclusion
Our findings suggest important ways in which the provision and delivery of care and education to people undergoing joint replacement in routine NHS care could be refined and improved. For example, patients can experience a range of emotional reactions if they experience delay and cancellation of their surgery date. Even without a delay, the wait for surgery alone can have detrimental physical and emotional consequences and cause wider psychosocial disruption. It is important that health professionals recognise these consequences, affirm patients’ experiences, identify those at increased risk and work towards minimising delay and cancellation of operation dates when possible. In addition, findings demonstrate the value of recognising the fluid and dynamic nature of time and broader temporal issues embedded in the perceptions, interpretations and experiences of patients in the lead up to joint replacement. Findings also highlight how patients appear to value the offer of postoperative physiotherapy shortly after surgery as well as longer-term follow-up in secondary care. The latter may be of particular value for those patients who experience complications after surgery or who are particularly troubled by a lack of confidence and faith in their prosthesis.
The findings of our analysis suggest the importance of future directions for work that concerns patients’ experiences of undergoing joint replacement surgery. For example, for some participants, concerns about potential dislocation became an enduring fear, something that influenced their behaviour and activities 1 year after surgery. This suggests the need to investigate the influence and impact of these concerns in the longer term and learn more about how best to support this group of patients. In addition, findings suggest the value of future work to address the specific impact of age, sex and cohabitation status on patients’ use of support networks around the perioperative period.
Chapter 5 Measuring functional outcomes in patients having hip and knee replacement: a cohort study
Parts of this chapter have been reproduced from Wylde and colleagues. 262 © 2012 Wylde et al.; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Some parts have also been reproduced with permission from Wylde V, Lenguerrand E, Brunton L, Dieppe P, Gooberman-Hill R, Mann C, et al. Does measuring the range of motion of the hip and knee add to the assessment of disability in people undergoing joint replacement? Orthop Traumatol Surg Res, vol. 100, pp. 183–6. 263 Copyright © 2014 Elsevier Masson SAS. All rights reserved. Parts of this chapter have also reproduced from Lenguerrand and colleagues264 © 2016 Lenguerrand et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background
In the ADAPT study we aimed to compare outcome measures over time in patients with hip or knee replacement and assess how well they measure impairment, activity limitation and participation.
Methods
Outcome measures were studied prospectively in 263 patients receiving joint replacement. Function was assessed prior to surgery and at 3 and 12 months using patient-completed questionnaires, clinician-administered tools and performance tests.
Results
A clinically significant improvement occurred in about 90% of patients with hip replacement and 70% of those with knee replacement. Patients with severe disease at the time of surgery were more likely to have substantial improvements in pain and functional ability.
Pain and function measures were highly correlated. People with anxiety or depression may assess themselves as being worse off than objective measures suggest. Measures of function may need adjustment for pain, psychological status, age and perhaps muscle strength to obtain a satisfactory picture of functional loss. Results suggested that physical function should be measured with both a PROM and a performance test. ROM is commonly assessed in clinical practice but did not correlate well with other measures of disease severity.
Conclusions
The ADAPT study highlighted the importance of different methods of assessing pain and function in patients receiving hip and knee replacement. Different pain characteristics predicted long-term pain in hip and knee replacement. Outcomes after joint replacement should be assessed with a patient-reported outcome and a functional test.
Background
In medical research it is conventional to use ‘outcome measures’ to assess change over time and the response to any intervention, such as a joint replacement. 265 Outcome measures are an artificial construct, as everyone’s life and health changes continuously and well-being is influenced by many factors other than a specific illness or its treatment. 266 Nevertheless, we need outcome measures at appropriate time points when comparing the value of different approaches to health care. Research suggests that most of the benefit that can accrue from a successful joint replacement has occurred by 12 months after the operation. 48 In addition, we need to measure the long-term costs of providing treatments, which allows us to assess whether or not incremental health benefits are worth the incremental costs required to provide them.
It is essential that patient-reported outcomes after joint replacement are continuously reviewed and monitored to improve practice and optimise the results of surgery. However, the use of many different outcome measures can lead to difficulty in applying evidence to clinical practice and renders comparisons across studies and meta-analyses problematic. 267 One recent systematic review found extensive variation in the outcome measures used in RCTs of joint replacement. 119
Measurement by clinician or patient report
The outcome after a hip or knee replacement can be assessed in different ways and can be classified according to who makes the judgement – a clinician, the patient alone, a ‘significant other’, or a mixture of two or more groups. In early studies, adverse events such as infection and prosthesis survival were the main issues of concern. 45 As prosthesis design and the control of adverse events improved, these issues become less important and attention turned towards clinician administered tools, such as the Harris Hip Score (HHS)268 and AKSS113 and more recently towards PROMs. 121 Clinician-administered tools have been widely criticised because of the recognised discordance between views of patients and clinicians. 269,270
Research studies in joint replacement frequently use PROMs assessing different domains. Patients rate their pain, function, HR-QoL, social participation, mental health and satisfaction with the outcome of health-care interventions. In England, following the report of Lord Darzi,121 PROMs are routinely collected after elective surgery. 271
General or specific measures
Outcome measures may be general reflecting overall pain, function and well-being or specific relating to an arthritic hip or knee. ‘Joint specific’ measures are used to assess the effectiveness of an intervention targeting a joint (such as joint replacement); however, it is important to find out if a patient’s general QoL has been affected.
It is generally agreed that we should assess pain and function when treating arthritis, as these are the two problems that bother people most. But it is not known what aspect of the pain (e.g. activity related pain, night pain or resting pain) and types of function (e.g. stair climbing, shopping, getting on a bus or playing golf) cause researchers endless problems. In addition, these often depend on issues such as culture and context.
There are many different outcome measures for use in assessment of health status and the response to interventions for people with arthritis and such instruments need to be validated before use. We need to be sure that they measure the outcome appropriately and that they are reproducible, responsive to change, consistent and acceptable to patients. Many researchers choose an instrument because it is widely used by others and this will help them to compare their results with those in the published literature.
Utilities
In health care, we attribute a measure of ‘utility’ to the time patients spend with a particular QoL profile. Utility is an economic term to describe the benefit that individuals derive from consuming goods or services. Because goods and services are scarce, individuals are faced with choices and their preferences are revealed by choosing to consume some goods and services over others. Individuals would rationally prefer goods and services that provide them with a higher utility level. In terms of health and health care, we consider that each patient has a given health profile that gives them a certain amount of health benefit or ‘utility’. Better health profiles are those for which patients have a higher QoL and, therefore, higher utility scores as well. We measure each individual’s health profile by asking patients to fill in generic HRQoL questionnaires. Such questionnaires can be filled in a myriad of ways, each corresponding to a different health profile. These are then sent for valuation to a random sample of individuals from the same population with a particular health profile. The weighted average of values for each health profile is the ‘utility score’ that a particular society has attributed to the specific health profiles.
Outcomes used in RESTORE
In Table 15, we summarise the key outcome measures used in the RESTORE programme.
| Name of measure | Mode of completion | General or joint specific | Health domain measured | Study used in |
|---|---|---|---|---|
| WOMAC114 | Self-complete | Joint specific | Joint pain, function and stiffness | SPIRAL, APEX, ADAPT, PROOF-THR |
| HOOS272 | Self-complete | Joint specific | Joint-related QoL, pain and function | Experience |
| KOOS273 | Self-complete | Joint specific | Joint-related QoL, pain and function | Experience, ARENA |
| OHS274 | Self-complete | Joint specific | Joint pain and function | PROOF-THR |
| OKS117 | Self-complete | Joint specific | Joint pain and function | |
| Pain Self-Efficacy Questionnaire275 | Self-complete | General | Self-efficacy for pain | SPIRAL, APEX |
| Brief COPE276 | Self-complete | General | Coping strategies | SPIRAL, APEX |
| Beliefs and Medicines Questionnaire277 | Self-complete | General | Beliefs about medicines | SPIRAL |
| EQ-5D278 | Self-complete | General | HR-QoL and utilities | SPIRAL, APEX, ARENA, PROOF-THR |
| Functional Comorbidity Index279 | Self-complete | General | Medical comorbidities | SPIRAL, APEX, ADAPT, ARENA |
| ICOAP280 | Self-complete | Joint specific | Joint pain | APEX |
| HADS281 | Self-complete | General | Depression and anxiety | APEX, ADAPT, PROOF-THR |
| Illness Perceptions Questionnaire-Revised282 | Self-complete | General | Illness perceptions | APEX |
| painDETECT283 | Self-complete | General | Neuropathic pain | APEX |
| Self-Administrated Patient Satisfaction Scale for Primary Hip and Knee Arthroplasty284 | Self-complete | Joint specific | Satisfaction with the outcome of joint replacement | ADAPT |
| Ab-IAP285 | Self-complete | General | Impairments, activity limitations and participation restrictions | ADAPT, ARENA, PROOF-THR |
| SF-12286 | Self-complete | General | HRQoL | ADAPT |
| MYMOP2287 | Patient-generated questionnaire completed by patient with assistance from researcher | Joint specific and general | Individualised symptoms and restricted ADL, well-being and medication usage | ADAPT, ARENA |
| HHS268 | Assessment by clinician | Joint specific | Joint pain, function, deformity and ROM | ADAPT |
| AKSS113 | Assessment by clinician | Joint specific | Joint pain, function, stability, ROM | ADAPT |
| Timed 20-metre walk288 | Completed by patient, assessed by clinician | General | Function (locomotion) | ADAPT |
| Timed get-up-and-go test289 | Completed by patient, assessed by clinician | General | Function (transfers and locomotion) | ADAPT |
| Sit-to-stand-to-sit290 | Completed by patient, assessed by clinician | General | Function (transfers) | ADAPT |
| Step test291 | Completed by patient, assessed by clinician | General | Function (ascending and descending stairs) | ADAPT |
| Balance test292 | Completed by patient, assessed by clinician | General | Function (balance) | ADAPT |
| Inertial sensor293 | Completed by patient, assessed by clinician | General | Function | ADAPT, PROOF-THR |
| LEFS294 | Self-complete | Specific | Function | ARENA |
| UCLA Activity Score295 | Self-complete | General | Activity level | ARENA |
| Activities-specific Balance Confidence Scale296 | Self-complete | General | Balance | ARENA |
| Self-efficacy for Rehabilitation297 | Self-complete | General | Self-efficacy for rehabilitation | ARENA |
| NEADL Questionnaire298 | Self-complete | General | Function | PROOF-THR |
| ICECAP-O299 | Self-complete | General | Well-being and utilities | PROOF-THR |
The main issues of concern to patients undergoing total hip or knee joint replacement include pain and functional problems that are related to the joint disease, as well as general QoL and satisfaction with the surgery. In this chapter we consider joint specific pain and function. Pain is a purely subjective domain, so that we are dependent on patient self-report to assess it. In contrast, function can be assessed in many different ways, which include patient report, observation of specific or general activities, measurement of certain activities and third party observations and perceptions.
The WHO introduced the ICF,80 which provides a theoretical framework on which to base the assessment of function. This framework splits function into three separate domains: impairment, activities limitations and participation restrictions. The value of this in the context of total hip or knee replacement can be illustrated by taking the example of climbing a step, a common problem for people considering a total hip or knee replacement. The impairments might include reduced joint movement, pain on movement and muscle weakness; the resulting activities limitations might be difficulty climbing stairs or difficulty getting onto a bus. Consequent participation restrictions might be inability to get to the shops or to go to stay with grandchildren because of the need to use stairs. Research has shown that the relationship between the impairment, activities limitations and participation restrictions domains of the ICF are not simple, with other factors such as self-efficacy and comorbidities acting as independent determinants of the relationships between these variables. 300
It has been recommended that a combination of outcome measures should be used to assess function after total hip or knee replacement. 301,302 However, there are many reasons not to use a wide number of measures with every patient, whether in clinical practice or research. These include patient fatigue and burden, time constraints of clinic and research appointments, and time taken to process and analyse multiple information sources. Therefore, there is a need for guidance about which outcome measures are the most useful in assessing function before and after total hip or knee replacement.
The aims of the ADAPT study were to compare the properties and responsiveness of a selection of commonly used measures that are either self-assessment tools or functional tests, to examine how well they relate to the ICF concepts of impairment, activities limitations and participation restrictions, and to explore the changes in the measures and domains of outcome over time.
Methods
The ADAPT study is a single-centre cohort study at the AOC. Based in the south-west of England, this is one of the largest elective orthopaedic units in the UK, with approximately 800 hip operations and 800 knee operations performed in 2011. 303 The ADAPT study was approved by Southwest 4 Research Ethics Committee (09/H0102/72) and all participants provided their informed, written consent to take part. The study was registered on the NIHR Clinical Research Network Portfolio (UKCRN ID 8311).
Inclusion/exclusion
Recruitment into the study began in February 2010 and finished in November 2011. Patients listed for one of the following operations were eligible: primary TKR, revision TKR, unicompartmental knee replacement, patellofemoral replacement, primary THR, revision THR or hip resurfacing. We included patients with different surgical procedures so that functional measures could be assessed across a range of people with diverse issues and degrees of functional loss.
Patients were excluded from the study if they lacked the capacity to provide informed consent. This was assessed by the research nurse in accordance with guidance from the integrated research application system, which is responsible for providing ethical approval in the UK and the Mental Capacity Act of 2005. 304 This decision was made by a research nurse if the patient met one of the following criteria: (1) they could not understand the information relevant to the decision to participate; (2) they were unable to retain the information about the study; (3) they were unable to use or weigh that information as part of the decision-making process; and (4) they were unable to communicate their decision about participation (whether by talking, using sign language or any other means). Another exclusion criterion was severe functional limitations such that the patient was unable to walk because this would prevent the patient from being able to attempt any of the functional tests. This was assessed in the discussion between the research nurse and the potential participant and was always a mutual decision by the researcher and the patient. Being unable to complete questionnaires in the English language was also an exclusion criterion because not all the validated questionnaires have been translated into other languages.
Participant recruitment
Potential participants were identified from the joint replacement waiting list by the code for the intended operation and sent a postal invitation. Patients who returned a reply form were telephoned by a research nurse to discuss participation. This included a full explanation of study involvement and a preliminary eligibility assessment by asking about the intended operation and assessing understanding of the information provided. Those that did not reply or who were missed from the initial postal invitation list owing to late scheduling of hospital appointments were approached by a research nurse when they attended the pre-operative assessment clinic. These patients were identified by daily checking of the clinic lists. If they were interested, eligibility was assessed and a full explanation of study involvement was provided. The first appointment was arranged then or patients were telephoned a few days later if they needed time to consider. Demographic data (age, sex and postcode) was recorded from all patients.
Assessment times
Participants attended a research appointment, lasting approximately 1 hour, at the AOC. Appointments were scheduled before surgery and then at 3 months and 1 year after surgery. Assessments were conducted at 3 months post operation to coincide with the standard clinical review, by which time most patients should have experienced a large improvement in pain and function. 48 Assessments were also conducted at 1 year post operation as outcomes can continue to improve up until this time point. 48 The inclusion of two postoperative assessments also allowed exploration of outcome trajectories and comparison of rates of improvement between different outcome domains (e.g. pain, function, participation).
At the initial pre-operative appointment, eligibility was confirmed, informed written consent was obtained and a questionnaire was given to participants to return by post before their operation. For the postoperative assessments, a questionnaire was sent out prior to the research appointment. At each time point, participants underwent a clinical assessment. These assessments were conducted by trained research nurses who followed standard operating procedures to ensure consistency and standardisation in data collection and who were assessed for competency in the examination procedures by a senior research nurse and orthopaedic surgeon. Risk assessments of the functional tests were undertaken and safe operating procedures specified. The data collected during the assessments were recorded by the research nurses on standardised proformas.
Selection of outcome measures
Table 16 provides a summary of the functional assessment measures used in the ADAPT study. The table also provides an overview of the ICF domains included within each functional assessment measure, with classification of self-completed PROMs and clinician-administered measures based on the results of an expert consensus study by Pollard and colleagues60 We also conducted a gait analysis using a single inertial sensor to derive motion parameters during ADL. 305
| Measure | ICF domains assessed | Mode of completion | Scoring | ||
|---|---|---|---|---|---|
| I | A | P | |||
| PROMsa | |||||
| WOMAC function subscale | – | ++ | +/– | Patient | 0–68 |
| Ab-IAP | ++ | ++ | ++ | Patient | I scale = 9–45, A scale = 17–85, P scale = 9–45 |
| SF-12 PCS | +/– | ++ | +/– | Patient | 0–100 |
| MYMOP2b | +/– | +/– | +/– | Patient with assistance from research nurse | 0–6 |
| Clinician-administered measuresc | |||||
| AKSS | + | ++ | – | Research nurse and patient | 0–100 |
| HHS | + | ++ | +/– | Research nurse and patient | 0–100 |
| Performance tests and motion analysisc | |||||
| Timed 20-metre walk | – | ++ | – | Research nurse and patient | Time, difficulty, motion parameters |
| Timed get-up-and-go test | – | ++ | – | Research nurse and patient | Time, difficulty, motion parameters |
| Sit-to-stand-to-sit test | – | ++ | – | Research nurse and patient | Completion, difficulty, motion parameters |
| Step tests | – | ++ | – | Research nurse and patient | Completion, difficulty, motion parameters |
| Single stance balance tests | – | ++ | – | Research nurse and patient | Completion, difficulty, motion parameters |
| Gait analysis parametersd | |||||
| Walking speed | ++ | – | – | Research nurse and patient | Metre/second |
| Walking cadence | ++ | – | – | Research nurse and patient | Step/minute |
| ROM pelvic obliquity | ++ | – | – | Research nurse and patient | Degree |
| Time to complete a step | ++ | – | – | Research nurse and patient | Second |
| Length of step | ++ | – | – | Research nurse and patient | Metre |
| Step Irregularities | ++ | – | – | Research nurse and patient | Variability in successive steps of the same leg |
| Asymmetries | ++ | – | – | Research nurse and patient | Ratio of asymmetry between steps time (seconds) of both legs |
There are a number of other measures that could have been included in this study such as the OHS and OKS,117,274 HSS,113 KOOS306 and NHP. 307 However, to avoid participation burden and fatigue only a selection of measures was chosen.
Patient-reported outcome measures
The following validated measures were used to provide disease-specific and generic self-reported measures of outcome.
The WOMAC function scale. 114 This disease-specific subscale, validated in osteoarthritis patients, consists of 17 questions assessing the extent of function limitations when performing a range of daily activities. Responses are provided on a 5-point Likert-type scale.
Aberdeen impairment, activity limitation and participation restriction measure (Ab-IAP). 285 This 35-item disease-specific measure, validated in osteoarthritis patients, uses the ICF framework to assess disability and produces scores for impairment, activities limitations and participation restrictions. Responses are provided on a 5-point Likert-type scale.
Short Form questionnaire-12 items. 116 This 12-item general health measure produces a PCS and mental component score scale. Responses are provided as binary options (yes/no) or on a Likert-type scale.
Measure Yourself Medical Outcome Profile 2 (MYMOP2). 287 This patient-generated instrument allows participants to generate and rate the severity of two symptoms that are concerning them and one activity important to them that is restricted by the symptoms. Participants also rate their general well-being, duration of symptom 1 and medication usage for symptom 1. At follow-up, participants are asked to rate the severity of the symptoms and degree of restriction of the activity that they identified at the first data collection point. Ratings are provided on scales of 0–6. The MYMOP2 was completed during research appointments by participants with the assistance of research nurses.
Participants also completed a number of other questionnaires to assess factors that have been found to influence outcomes after total hip or knee replacement (Table 17). At each assessment time, participants completed the HADS281 and the WOMAC pain and stiffness subscales. 114 Participants were also asked to rate how disabled they perceived themselves because of their joint problems and why, and to list three things that they were hoping for from their total hip or knee replacement. Pre-operatively, medical comorbidities were recorded using the Functional Comorbidity Index279 and information was collected about socioeconomic status (marital status, living arrangements, educational attainment, employment status), joints affected by arthritis and previous surgery on other joints. In the 1-year postoperative questionnaire, satisfaction with the outcome of surgery was assessed using the Self-Administered Patient Satisfaction Scale for Primary Hip and Knee Arthroplasty. 284
| Measure | Dimensions | Mode of completion | Scoring | Assessment times | ||
|---|---|---|---|---|---|---|
| Pre-operation | 3 month | 1 year | ||||
| WOMAC pain subscale | Joint pain | Patient | 0–20 | ✓ | ✓ | ✓ |
| WOMAC stiffness subscale | Joint stiffness | Patient | 0–8 | ✓ | ✓ | ✓ |
| HADS | Depression, anxiety | Patient | 0–21 | ✓ | ✓ | ✓ |
| SF-12 mental component score | Mental health | Patient | 0–100 | ✓ | ✓ | ✓ |
| Perceived level of disability | Function | Patient | 0–10 | ✓ | ✓ | ✓ |
| Three things hoping for from surgery | Expectations | Patient | Categorical | ✓ | ✓ | ✓ |
| Functional Comorbidities Index | Comorbidities | Patient | 0–18 | ✓ | ||
| Arthritis and surgery in other joints | Comorbidities | Patient | By joint/count | ✓ | ||
| Socioeconomic status | Socioeconomic | Patient | Categorical | ✓ | ||
| Self-Administered Patient Satisfaction Scale | Satisfaction | Patient | 0–100 | ✓ | ||
Clinician-administered measures
The HHS was completed with patients receiving hip replacement. 268 This assessment measure provides a total score of between 0 and 100 (worst to best) collected over four domains. Function, which includes limp, use of assistive devices, walking distance, managing stairs, using public transport, sitting comfortably and putting on shoes and socks, is weighted the most heavily and is assigned 47 points. Pain is assigned 44 points. The physical examination involves assessing deformity (4 points) and ROM (5 points).
The AKSS was completed with patients receiving knee replacement. 113 This assessment consists of a knee score and a function score, both with a total score ranging from 0 to 100 (worst to best). The knee score incorporates examiner’s rating of patients’ pain (50 points) and a clinical assessment of stability (25 points) and ROM (25 points), with deductions for flexion contracture, extension lag and misalignment. The function score consists of questions about walking distance (50 points) and stair climbing ability (50 points), with deductions for the use of walking aids.
Performance tests
Before performing each of these tasks, participants were asked if they thought that they would be able to perform the task and estimate how difficult they thought the test would be to perform on a 0–10 scale (no difficulty at all to impossible). After they had completed the test, they were asked to rate how difficult the task actually was to perform on the same 0–10 scale. The research nurse conducting the assessment also provided a rating of how difficult it appeared to be for the participant to perform the task. If participants were unwilling to attempt the test or the research nurse was unhappy to proceed because of safety concerns, the test was not performed. All tests were performed without the use of supportive aids except the timed 20-metre walk and are described in the order in which they were performed.
Timed 20-metre walk288
Participants were timed as they walked a 20-metre straight distance on level ground at their normal, comfortable speed. If the participant normally used a walking aid they were asked to try without it but, if they felt unable to do so, they completed the test using the walking aid. The recorded outcome was the time taken to complete the test.
Timed get-up-and-go test289
Participants sat on a height-adjustable chair such that a 90° angle was formed when the femur was horizontal and the tibia vertical with their feet shoulder width apart and their arms crossed against their chest. Participants were timed as they stood up from the chair without using their hands, walked at a normal pace past a marker 3 metres away, turn around, walked back and sat down again. The recorded outcome was whether or not participants were able to complete the activity and how long it took.
Sit-to-stand-to-sit290
Participants sat on a height-adjustable chair as described for the previous test. Participants then stood up, waited 2 seconds and sat down again without using their hands. The recorded outcome was whether or not participants were able to complete the activity.
Step test291
Participants stepped up onto a 20-cm-high block leading with the contralateral leg, waited 2 seconds and then stepped down from the block with the index leg leading, without using their arms. The test was then repeated with the index leg leading. If participants successfully completed this test, the test was repeated with a 30-cm-high block. The recorded outcome was whether or not participants were able to complete the activity.
Single stance balance test292
Participants stood with their feet together facing the research nurse and placed their palms gently on top of the research nurse’s palms. Participants then lifted their index leg and attempted to balance on their contralateral leg for 15 seconds. If the participant lost balance within 3 seconds, then the test was reattempted. If the participant lost balance before 15 seconds, the length of time was recorded. This test was then repeated while balancing on the index leg. If these tests were completed successfully, the participant repeated the tests with no stability support from the research nurse. The recorded outcome was whether or not participants were able to maintain the stance for 15 seconds.
Inertial sensor-based motion and gait analyses
Movement analysis by body-fixed inertial sensors containing accelerometers and gyroscopes enables the objective assessment of the translational and angular movements of body segments outside a gait laboratory. 293,308 We used a single 3 dimensional (3D) inertial sensor [41 × 63 × 24 mm; 39 g; Microstrain Inertia Link (Williston, VT)] containing accelerometers (± 5 g) and gyroscopes (± 300°/second) along the three orthogonal axes in frontal, sagittal and transverse plane and positioned centrally between both posterior superior iliac spines to measure trunk movements near the centre of gravity. Based on the 3D linear accelerations, angular rates and angular positions put out by the sensor and sent wirelessly to a computer at a 100 Hz sampling frequency via a Bluetooth® (Bluetooth SIG, Inc., Kirkland, WA) link, analysis algorithms calculated motion parameters such as step frequency, step asymmetry or trunk sway.
The inertial sensor was used to derive motion parameters from a battery of movement tasks which were clinically feasible to perform during a routine outpatient visit and which challenged the patient’s functional capacity in different ways: (1) locomotion (walking), (2) transfers (sit-to-stand-to-sit test, get-up-and-go test), (3) rising and descending (step test) and (4) balance tests (single-leg stance). The walk test309 and the step-test were repeated twice and the sit-to-stand-to-sit test was repeated three times to derive representative mean values or study possible effects of fatigue or warming up.
Data collection
Information on BMI, diagnosis, side of surgery, type of surgery and surgical approach was extracted from participants’ medical records.
Data recording
All data were entered into a password-protected database by research nurses or study administrators. The study was overseen by an independent Steering Committee which met every 6 months to discuss the progress of the study. Data monitoring, which involved double data entry and quality checks, was conducted every 3 months. All data were cleaned before data analysis was performed. Any inconsistencies were collegially discussed by an internal board of researchers involved in the data collection.
Sample size
This study involved exploratory analysis to compare different measures to assess function after total hip or knee replacement. Therefore, no formal sample size calculation was performed, although we aimed to recruit a sufficient number of patients to allow meaningful data analysis. We approached all patients listed for surgery with participating surgeons between February 2010 and November 2011. Previous longitudinal studies comparing measures of function in an orthopaedic population have included between 30 and 200 patients. 310–318
Analysis
The statistical methods used to analyse the data are described in each section of the results, which have been divided into three sections:
-
baseline demographic data
-
cross-sectional correlations of baseline data
-
analysis of change from longitudinal data including preliminary results from the gait analysis.
Results
Demography of the cohort
A total of 130 patients receiving hip replacement and 133 patients receiving knee replacement were recruited to the ADAPT study.
The patients listed for a hip surgery were planned to receive a primary THR (n = 78), revision THR (n = 44) or a hip resurfacing (n = 8). The 133 patients listed for a knee surgery were planned to receive a primary TKR (n = 51), revision TKR (n = 45), unicompartmental knee replacement (n = 32) or patellofemoral replacement (n = 5). The five patients awaiting patellofemoral joint replacement were excluded from the cross-sectional analysis owing to the isolated nature of their knee disease.
Not all data were available on all 258 patients and at each measurement point, so the subsequent analyses reported below are often on slightly smaller groups.
Patient demographics for the 249 participants with available pre-operative data (125 listed for hip replacement and 124 listed for knee replacement) are summarised in Table 18.
| Characteristic | Hip (n = 125) | Knee (n = 124) | |
|---|---|---|---|
| Surgery type (%) | Primary replacement | 65.6 | 40.3 |
| Revision surgery | 34.4 | 33.9 | |
| Unicompartmental surgery | – | 25.8 | |
| Sex (%) | Female | 50.4 | 51.6 |
| Age (years) | Median (25th, 75th)a | 64.4 (57.1, 72.5) | 68.3 (60.5, 73.9) |
| Painb | Mean (95% CI) | 53.9 (50.0 to 57.8) | 44.1 (40.7 to 47.4) |
| Psychological distressc (%) | 32.0 | 33.1 | |
| BMI, kg/m2 (%) | Median (25th, 75th)a | 27.0 (24.3, 30.4) | 30.4 (27.4, 34.5) |
| Overweight | 41.0 | 39.8 | |
| Obese | 26.8 | 52.7 | |
| Comorbidities (%) | 0 comorbidities | 46.0 | 36.0 |
| 1 comorbidity | 35.9 | 27.3 | |
| ≥ 2 comorbidities | 18.1 | 36.8 | |
| Arthritis (%) | 0 joint | 21.3 | 14.9 |
| 1 joint | 25.0 | 21.1 | |
| 2 joints | 19.7 | 14.1 | |
| 3 joints | 15.0 | 16.4 | |
| ≥ 4 joints | 19.0 | 33.5 | |
| Living alone (%) | 24.2 | 29.3 | |
| Education (%) | Normal leaving-school age or before | 54.2 | 61.1 |
| College | 23.0 | 25.1 | |
| University | 22.8 | 13.8 | |
| Working status (%) | Paid or volunteer activity | 46.4 | 30.7 |
| Retired | 48.0 | 62.9 | |
| Unemployed | 5.6 | 6.4 | |
Cross-sectional analysis of the different measures of function
Introduction
As noted above, one of the main aims of this study, within the overall programme was to improve our understanding of the best ways of measuring function before and after hip or knee joint replacement. In this section of the results, we describe the correlations between the different measures of function that we have data on at the baseline visit. We also describe the disparities/similarities in the associations between these measures and patient characteristics. Investigating these issues provides insight into how the outcomes as measured by these various tools can be interpreted and sheds insight into the comparability of the tests. The data should also aid those investigating disability caused by severe hip and knee pathology to make an informed choice of measurement tool.
Statistical analysis
The relationships between the different functional measures were assessed with correlation statistics. Spearman’s rank-order coefficients were used to assess correlations between continuous variables and point-biserial coefficients to assess correlations between continuous and dichotomous variables. These measures range from –1 to 1. The strength of correlation was interpreted as |0.00–0.25| = none–little, |0.26|–|0.49| = low, |0.50|–|0.69| = moderate, |0.70|–|0.89| = high, |0.90|–|1.00| = very high. Correlations between two binary measures were assessed with Cramér’s V-statistic, which ranges from 0 to 1. A value > 0.3 was considered very strong.
The association between participants’ characteristics and functional measures was investigated with linear regression for HHS, AKSS, WOMAC function, Aberdeen activity limitation subscale (Ab-A), Aberdeen participation restriction subscale (Ab-P) [transformed as root squared (score)], walking speed and get-up-and-go tests (transformed as 1/time). The step and balance tests produced dichotomous outcomes (able/unable to do test) and were investigated with a modified Poisson regression with robust variance estimation.
Individual patient characteristics were first considered in a univariate model. Factors that were found to be significant (p ≤ 0.05) were then investigated in a multivariate analysis to determine if their effects were confounded by other factors.
The analyses were conducted separately for hip and knee patients. Although few participants had missing information for one or more of the considered variables, missing data were addressed using a multiple imputation by chained equations approach. Ten imputations were generated and estimates were combined using Rubin’s rules. Statistical analyses were performed in Stata 12.1.
Results
The mean/median and range of scores for each of the functional measures are shown in Table 19. These data show that most participants had significant functional limitations, although the wide range of each of the measures suggests considerable variability.
| Outcome measure | Hip (n = 125) | Knee (n = 124) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Minimum | Maximum | Mean | SD | Minimum | Maximum | |
| Patient-reported measures (mean score) | ||||||||
| WOMACa function | 55.3 | 22.0 | 0.0 | 100.0 | 50.9 | 18.5 | 0.0 | 97.0 |
| Ab-Ab | 23.9 | 11.6 | 0.0 | 56.0 | 25.1 | 10.7 | 2.0 | 50.0 |
| Ab-Pc,d | 8.0 | 9.0 | 0.0 | 31.0 | 10.0 | 10.0 | 2.0 | 28.0 |
| Clinician-administered measures (mean score) | ||||||||
| HHSe | 54.0 | 17.5 | 23.2 | 97.0 | ||||
| AKSS-functionf | 53.7 | 15.4 | 0.0 | 90 | ||||
| AKSS pain, stability and ROMg | 43.1 | 15.2 | 10.0 | 82.4 | ||||
| Performance tests | ||||||||
| Walking speede (m/second) | 0.9 | 0.4 | 0.2 | 1.7 | 0.9 | 0.5 | 0.3 | 2.0 |
| Get-up-and-go test duratione (seconds) | 17.0 | 11.0 | 9.0 | 118.0 | 17.2 | 10.6 | 8.0 | 56.0 |
| Stepped 20-cm achievement (mean %) | 81.4 | 77.4 | ||||||
| Stepped 30-cm achievement (mean %) | 60.4 | 55.6 | ||||||
| Balance test achievement (mean %) | 46.6 | 33.9 | ||||||
Relationships between functional measures
Correlations between the different functional measures were all statistically significant, but some were much stronger than others (Table 20). The HHS correlated relatively well with PROMS and with walk time in patients with hip disease, but not so well with the other performance tests. The AKSS correlated poorly with all other types of functional measures in patients with knee disease. The highest correlations, in both hip and knee patients, were between the WOMAC and Ab-A scores – the two PROMs for disability; between the walking speed and the get-up-and-go test – the two timed tests; and between the balance test and 30-cm step test.
| Outcome measure | WOMAC | Ab-A | Ab-P | AKSS function | AKSS pain, stability and ROM | HHS | Walking speeda | Get-up-and-go testb | 20-cm step testc | 30-cm step testc | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hip | Ab-A | –0.87 | |||||||||
| Ab-P | –0.71 | 0.73 | |||||||||
| HHS | 0.71 | –0.73 | –0.67 | ||||||||
| Walking speed | 0.56 | –0.65 | –0.52 | 0.67 | |||||||
| Get-up-and-go test | –0.56 | 0.63 | 0.42 | –0.59 | –0.85 | ||||||
| 20-cm step test | 0.33 | –0.38 | –0.28 | 0.50 | 0.48 | –0.44 | |||||
| 30-cm step test | 0.37 | –0.49 | –0.28 | 0.48 | 0.60 | –0.50 | 0.58 | ||||
| Balance testd | 0.27 | –0.32 | –0.17e | 0.38 | 0.58 | –0.39 | 0.44 | 0.66 | |||
| Knee | Ab-A | –0.88 | |||||||||
| Ab-P | –0.63 | 0.69 | |||||||||
| AKSS function | 0.46 | –0.47 | –0.51 | ||||||||
| AKSS pain, stability and ROM | 0.40 | –0.33 | –0.37 | ||||||||
| Walking speed | 0.51 | –0.54 | –0.51 | 0.67 | 0.29 | ||||||
| Get-up-and-go test | –0.46 | 0.50 | 0.43 | –0.60 | –0.21 | –0.73 | |||||
| 20-cm step test | 0.25 | –0.28 | –0.27 | 0.50 | 0.27 | 0.46 | –0.60 | ||||
| 30-cm step test | 0.34 | –0.37 | –0.37 | 0.57 | 0.31 | 0.57 | –0.60 | 0.43 | |||
| Balance test | 0.22 | –0.29 | –0.26 | 0.33 | 0.21 | 0.27 | –0.29 | 0.31 | 0.61 | ||
Associations between patient characteristics and functional measures
Associations between patient characteristics and the different functional measures are shown in Tables 21 (hip) and 22 (knee). Pain was an important determinant of all measures of function in both patient groups. In contrast, age, sex and comorbidities discriminated between hip and knee patients as well as between the different methods of assessing disability. Sex affected most measures of disability in hip patients, but not in knee patients. Age affected the performance tests more than the PROMs or clinician-administered tests, whereas anxiety and depression had much more effect on the PROMs and clinician-administered measures than on the performance tests. BMI does not seem to be important and other comorbidities have more effect on tests of function in people with knee disease than those with hip disease.
| Hip | WOMAC functiona | Ab-Aa | Ab-Pa,b | HHSa | Walk (speed)a | Get-up-and-go testa,c | 20-cm step testd | 30-cm step testd | Balanced |
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | < 0.0001 | < 0.001 | < 0.05 e | < 0.0001 | |||||
| Sex | < 0.05 | < 0.05 | < 0.0001 | < 0.001 | < 0.01 | < 0.05 | |||
| Pain | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.05 | < 0.01 | < 0.05 |
| HADS | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.01 | < 0.05 | |||
| BMI | |||||||||
| Functional Comorbidity Index | < 0.05 | < 0.01 | < 0.05 | < 0.01 | |||||
| Arthritis | |||||||||
| Living alone | < 0.05 | < 0.05 | |||||||
| Education | < 0.05 | ||||||||
| Working status | < 0.01 | < 0.01 | < 0.05 | < 0.05 | < 0.01 |
| Knee | WOMAC functiona | Ab-Aa | Ab-Pa,b | AKSS-fa | AKSSa | Walking speeda | Get-up-and-go testa,c | 20-cm step testd | 30-cm step testd | Balanced |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | < 0.05 e | < 0.001 e | < 0.05 e | < 0.05 e | ||||||
| Sex | < 0.01 | |||||||||
| Pain | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.01 | < 0.05 | ||
| HADS | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.01 | < 0.05 | ||||
| BMI | ||||||||||
| Functional Comorbidity Index | < 0.01 | < 0.001 | < 0.05 | < 0.01 | < 0.05 | < 0.05 | < 0.05 | < 0.01 | ||
| Arthritis | ||||||||||
| Living alone | < 0.05 | < 0.05 | ||||||||
| Education | < 0.05 | < 0.05 | ||||||||
| Working status | < 0.001 | < 0.05 |
Discussion
This study compared different ways of assessing function in patients awaiting hip or knee replacement. Correlations were stronger within the same type of measures (PROMS, clinician-administered or performance test) than between approaches. Correlations that were usually < 0.9 imply that each of these measures describes a slightly different construct of function and that several of them would be needed to provide an accurate and exhaustive assessment of function. Nevertheless, the WOMAC function, Ab-A, HHS, walking test and the get-up-and-go tests had satisfactory convergence validity (correlation ≥ 0.3). This suggests that each of these measures can individually provide a reasonably comprehensive description of function if it is possible to conduct only one test. However, the AKSS and the balance test correlated poorly with most of the measures and should not be used alone. These findings are consistent with previous research which found moderate to strong (> 0.4) correlations with the WOMAC function and stair climbing, walking or the get-up-and-go test. 319–323 Moderate to strong correlations have also been reported pre-operatively between the WOMAC function and the HHS or its components,319,321,324 and small to moderate correlations have been found between the WOMAC function and the AKSS. 319,325,326 Our finding of a strong relationship between the Ab-A and WOMAC function score is not surprising as the Ab-A is based on several items of the WOMAC. 285 In this study, the Ab-A measure had slightly better correlations than the WOMAC function with all the other measures. This suggests that it may be the preferred tool for assessment of function in this population.
Previous inconclusive studies exploring the association between the HHS or AKSS and performance tests were based on moderate sample sizes, and mainly focused on associations between joint ROM and performance tests. 285,327–329 Our study highlighted that associations between patient characteristics and function differed according to the measurement approach used. For example, obesity was associated with poor AKSS but not with functional outcomes as measured any other way.
Responses to PROMs are influenced by factors including age, sex, mental health or socioeconomic characteristics. 322,330–333 Clinical assessments can also be influenced by patients’ characteristics; for instance, fat mass and bony structure affect the reliability and validity of extremity measurements,334 while age and vulnerability may influence communication with health professionals or interviewers. 335 Performance testing may not always assess ADL of relevance to an individual and may not take into account environmental or behavioural adaptations. 336 Tests are also likely to be confounded by factors such as sarcopenia, which in turn can be influenced by other patient characteristics such as activity or self-efficacy. 337
Although pain is a major determinant of function irrespective of measurement method, we found that psychological health influenced self-assessment more than performance-based methods. In addition, age affected performance measures, but not self-assessment. This has several implications. First, a causal investigation of function will be accurate, exhaustive and corroborative only if conducted simultaneously with several measures of function. Second, the investigation of any risk factor of function should be adjusted for the patient’s psychological status (if a self-assessment measure is used) or for patient age (if a performance test is used), and in both cases for pain. Third, any comparison of measures of function obtained with different measurement methods is flawed unless the effects of pain, age and psychological status are considered. Fourth, there is an age-related decline in function when measured objectively, but this is not evident on PROMs. Fifth, the effect of psychological factors on self-reported function, but not on objective measures, indicates that psychological status influences the perception of function more than the ability to do something; patients may be able to do more than they say they can do and may need encouragement to overcome anxiety. Finally, it seems that any assessment of function should be accompanied by pain assessment to obtain unconfounded assessment. The association of pain with function, even after taking into account the age and psychological status of the patients, confirms the lack of discriminant validity of currently used functional measures. This is to be expected with the clinician-completed HHS and AKSS, which include a pain component. It has also been observed previously between self-reported measures of pain function. 318 However, the association with the performance tests is more problematic as even the most ‘objective’ measures of function are confounded by pain.
These findings were obtained on patients from a single-centre orthopaedic unit; however, this is a large sample with a representative age range undergoing a diversity of procedures. The study also focused on a discrete number of assessment measures and did not include measures such as the OHS, OKS, KOOS or HOOS. Measures were selected to include a broad range of tools that could be administered at the same time alongside demographic information. Through this, we were able to compare effectively the measures and the influence of patient characteristics.
Conclusion
Our study shows that associations between patient characteristics and function differed according to the measurement approach used. Measures of pain and psychological health could be routinely used alongside self-report of activity limitations to enable appropriate adjustments. Performance-based tests are strongly influenced by age, possibly owing to age-related sarcopenia. If this is correct, for research purposes the inclusion of a simple muscle strength test, such as grip strength, alongside performance-based methods might aid interpretation of the findings.
Cross-sectional analysis of joint range of motion and its relevance to functional measures
Introduction
Range of motion is often routinely assessed in orthopaedic surgery. Measures of ROM are included in both the AKSS113 and HHS. 268 However, the relationship between ROM and function is contested, with some authors regarding ROM as a good determinant of function329 but others reporting poor correlations. 317,338 In view of ongoing use of ROM and continuing uncertainty about its relationship with function, this analysis of the ADAPT data was undertaken to investigate the relationship between ROM and our other measures of function. In this analysis we have also specifically looked at the different domains of function as described in the WHO ICF, that is, we have analysed the relationships between ROM and impairment, activities limitations and participation restriction separately.
Statistical analysis
Analyses were conducted separately for patients listed for hip and knee replacement. Spearman’s rank-order correlation coefficients were used to assess correlations between continuous variables. Point-biserial correlation coefficients were used to assess correlations between continuous and dichotomous variables. These correlation measures range from –1 to 1. The strength of correlation was interpreted as |0.00|–|0.25| = none–little, |0.26|–|0.49| = low, |0.50|–|0.69| = moderate, |0.70|–|0.89| = high, |0.90|–|1.00| = very high. Linear regression was conducted to adjust for the effect of demographic factors (age, sex, socioeconomic status, joints affected by arthritis, comorbidities and psychological status) on the relationship between WOMAC pain and self-report activity limitations. To adjust for the effect of demographic factors on the relationship between WOMAC pain and participation restrictions, the participation restrictions scale of the Aberdeen impairment, activity limitation and participation restriction measure (Ab-P) was transformed with a root square function to comply with the assumptions of the linear model.
To compare functional measures between patients with low and high active flexion, patients were dichotomised into those with low flexion (< 110° for knee patients and < 95° for hip patients) and those with high flexion (≥ 110° for knee patients and ≥ 95° for hip patients). This cut-off was chosen because 90° of hip and knee flexion is required when rising from sitting to standing in order for the centre of gravity in the sagittal plane to transfer from behind the midline (in sitting) to in front of the midline (in standing). Continuous variables were compared between these two groups using unpaired t-tests or Mann–Whitney U-tests for non-normally distributed variables. Categorical variables were compared using chi-squared tests. Statistical analysis was performed using Stata 12.
Results
Correlations between the measures of impairment (ROM and WOMAC pain) and measures of activity limitations [WOMAC function, activity limitations scale of the Aberdeen impairment, activity limitation and participation restriction measure (Ab-A), performance tests] are displayed in Table 23.
| Outcome measure | Self-report activity limitations | Function performance tests | Self-report participation restrictions | |||
|---|---|---|---|---|---|---|
| WOMAC function | Ab-A | 20-metre walk | Sit-to-stand-to-sit test | 20-cm step test | Ab-P | |
| Patients listed for hip replacement | ||||||
| Flexion | 0.29** | –0.35*** | –0.29** | 0.30*** | 0.34*** | –0.17 |
| Abduction + adduction | 0.29*** | –0.32*** | –0.36*** | 0.16 | 0.13 | –0.23* |
| Arc of rotation | 0.20* | –0.27** | –0.36*** | 0.11 | 0.25** | –0.17 |
| Pain | 0.80*** | –0.71*** | –0.44*** | 0.13 | 0.23** | –0.71*** |
| Patients listed for knee replacement | ||||||
| Active flexion | 0.43*** | –0.35*** | –0.38*** | 0.31*** | 0.31*** | –0.32*** |
| Active extension | –0.18 | 0.11 | 0.09 | –0.19* | –0.35*** | 0.06 |
| Pain | 0.78*** | –0.63*** | –0.32*** | 0.18* | 0.17 | –0.53*** |
Hip and knee ROM correlated weakly with self-report (Spearman’s rank-order correlation coefficients ranging from |0.11| to |0.43|) and observed activity limitations (|0.09| to |0.38|). In comparison, correlations between pain and self-report activity limitations were moderate to high (|0.63| to |0.80|) and remained so after adjustment for demographic factors (data not shown). However, correlations between pain and observed activity limitations were low (|0.13| to |0.44|). Correlations between individual WOMAC function items and ROM measurements were investigated to determine if ROM correlated with specific functions. All correlations were found to be low (|0.01| to |0.40|). The highest correlation in patients listed for hip replacement was between flexion and getting on/off toilet (–0.37) and in patients listed for knee replacement it was between flexion and getting in/out of a car (–0.40) and putting on socks/stockings (–0.40).
Relationship between measures of impairment and participation restrictions
Correlations between measures of impairment and participation restrictions (Ab-P) are displayed in Table 23. Hip and knee ROM correlated poorly with participation restrictions (|0.06| to |0.32|). In comparison, correlations between pain and participation restrictions were high in patients listed for hip replacement (–0.71) and moderate in patients listed for knee replacement (–0.53), and these correlations remained strong after adjustment for demographic factors.
Comparison of functional measures between patients with low and high active flexion
Patients listed for knee replacement with low flexion had significantly worse results on all measures of impairment, activity limitations and participation restrictions than patients with high flexion (Table 24). Patients listed for hip replacement with low flexion had significantly worse activity limitations than patients with high flexion.
| Outcome measure | Patients listed for knee replacement | Patients listed for hip replacement | ||||
|---|---|---|---|---|---|---|
| Low flexion (< 110°) (n = 54) | High flexion (≥ 110°) (n = 67) | p-value | Low flexion (< 95°) (n = 77) | High flexion (≥ 95°) (n = 48) | p-value | |
| Impairment measures | ||||||
| WOMAC pain score (mean, 95% CI) | 37 (32 to 42) | 50 (46 to 54) | < 0.0001 | 54 (49 to 58) | 54 (47 to 62) | 0.8873 |
| Activity limitation measures | ||||||
| WOMAC function score (mean, 95% CI) | 43 (38 to 47) | 58 (53 to 62) | < 0.0001 | 54 (49 to 59) | 59 (52 to 66) | 0.2548 |
| Ab-A score (mean, 95% CI) | 28 (26 to 31) | 22 (19 to 24) | 0.0007 | 25 (23 to 28) | 20 (17 to 24) | 0.0381 |
| 20-metre walk test time in seconds, median (25th, 75th centiles)a | 28 (22, 36) | 20 (17, 27) | 0.0002 | 23 (18, 30) | 10 (17, 25) | 0.3493 |
| Sit-to-stand-to-sit test (% completed) | 78 | 94 | 0.0009 | 84 | 97 | 0.048 |
| 20-cm step test (% completed) | 67 | 85 | 0.017 | 78 | 91 | 0.073 |
| Participation restriction measures | ||||||
| Ab-P, median (25th, 75th centiles) | 13 (8, 17) | 8 (5, 13) | 0.0013 | 8 (5, 16) | 8 (4, 12) | 0.2867 |
Discussion
The WHO ICF model offers a theoretical framework for describing and assessing disability. The data from this study show that in patients listed for joint replacement, there is a poor relationship between ROM and any of the disability measures used in this study, which contrasts with the strong relationship found between pain, activity limitations and participation restrictions. Previous studies have arrived at discordant conclusions about the relationship between function and ROM. Some reports suggest that ROM is an important determinant of function321,329 while others disagree. 317,338 Furthermore, it is suggested that ROM is important for some specific functions, or that a threshold of around 95–100° of flexion is required for adequate function. 338 Our data suggest that there may be such a threshold, but that ROM does not correlate with specific activities on the WOMAC function and modest restrictions of ROM are of little relevance to functional outcomes.
These findings are important for two reasons. First, commonly used methods of assessing patients’ disability, such as the AKSS and HHS, include ROM. Second, many orthopaedic surgeons often consider the achieved ROM of a replaced joint to be an important measure of surgical outcomes and discuss this with their patients. We suggest that as a measure of impairment, ROM is of little relevance to function and the only concern should be whether or not knee flexion is restricted to < 110° and, to a lesser extent, whether or not hip flexion is limited to < 95°.
Weaknesses of the study were the lack of randomisation of the order of the performance tests and inclusion of patients from only one specialist orthopaedic unit. However, by including patients listed for a range of joint replacement procedures, a diverse and varied sample was achieved. Strengths include the study’s relatively large size, the extent of and care taken with the measures of ROM and disability, and the good interobserver and intraobserver reliability for ROM.
Conclusion
These findings suggest that measuring ROM adds little value to assessment of impairment in patients undergoing joint replacement, unless hip or knee flexion is restricted to < 90° and, therefore, should not be used to assess disability in a pre-operative context.
Longitudinal analysis of changes in the different measures of function over time
Introduction
One of the main aims of the ADAPT study was to assess the responsiveness of various different measures of function and, in particular, to contrast the value of the three main different approaches (self-assessment, clinician-administered tools and functional tests) in assessing change after joint replacement. An important part of such an analysis is to assess which measures might have an important ceiling effect, that is which measures often reach their limits after joint replacement surgery, such that they cannot detect further improvement.
In this section we describe the changes seen in function between the pre-operative and 12-month postoperative assessment.
Statistical analysis
Analyses were conducted separately for patients listed for hip and knee replacement.
Change in function ability (as measured by the WOMAC function subscale, the SF-12 physical function subscale, the Ab-IAP, the MYMOP2 score, the get-up-and-go test, the timed 20-metre walk, the HHS and the AKSS) was defined as the 12-month postoperative score minus the pre-operative score. Patients were categorised into three groups: those with deteriorated function, those with unchanged function and those with improved function.
To determine if the individual changes were due to chance or not, we used the following approach. After transformation of the pre- and postoperative scores (using inverse, root square or logarithm function), the changes in physical function were normally distributed. Therefore, we used linear mixed models with random intercept and slope to regress the transformed outcomes on the time of assessment. 339 We then determined if the individual changes between pre- and post-surgery assessments were different from 0 using the 95% CI around each participant’s trajectories. This was derived from the post-estimation of the above models using fixed effects and subject-specific random effects. A 95% CI including 0 is not statistically significant, that is, the observed change is no different than a flat trajectory. This approach was preferred over the traditional relative change index measure as it does not depend on a deterministic external measure of test–retest reliability, which was not available for the studied scores. It also allows a better control of the regression to the mean effect by assuming that all scores are drawn from the same population distribution (shrinkage effect).
We also derived the minimum clinical important improvement (MCII) of each score, that is the improvement in functional score between two time points likely to be important from the patient’s perspective. We used an anchoring question about participant satisfaction with recreational activities at 12 months post surgery284 to dichotomise the ADAPT participants into two groups (very or somewhat satisfied vs. somewhat or very dissatisfied). We then calculated the cut-off point (MCII) on the distribution of score change, using a receiver operating characteristic curve analysis, to determine the threshold maximising the sensitivity and specificity. Patients experiencing an improvement greater or equal to this threshold were defined as having a clinically meaningful change in function.
We also determined the observed ceiling effect for each score at 12 months post operation. The ceiling effect was defined as the percentage of patients with a score equal to the highest possible score. For example, a patient with a WOMAC function subscale score of 100 [score ranges from 0 to 100 (worst to best)] has reached the ‘ceiling’ of the score and is considered to have the maximum possible functional ability. For ease of interpretation, the lowest values of scores ranging in the opposite direction to the WOMAC function scoring system such as the Ab-IAP measures [Ab-I 0–36, Ab-A 0–68, Ab-P 0–36; best to worst] or the MYMOP2 score [0–6 (best to worst)] were considered as the ‘maximum score’.
Results
This analysis was undertaken on patients who participated in both the baseline and 12-month assessments and provided information on at least one of the functional measures. Of the total cohort (n = 263, 130 hip replacement and 133 knee replacement patients), there were 104 hip replacement and 101 knee replacement patients who had this full data set and were included in these analyses.
Change in scores
The scores for the various functional measures, at baseline and 12 months post operation, are shown in Tables 25 and 26. Tables 27 and 28 also record the changes in each of the scores between baseline and 12 months.
| Outcome measure | Site | Pre-operative assessment | 12-month postoperative assessment | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | Median | n | Mean | SD | Median | ||
| WOMAC function | Hip | 103 | 56.3 | 22.3 | 56.7 | 103 | 88.9 | 15.3 | 95.6 |
| Knee | 101 | 51.9 | 18.4 | 50 | 101 | 75.2 | 23.1 | 83.8 | |
| SF-12 physical function subscale | Hip | 100 | 33.1 | 8.9 | 32.3 | 97 | 47.5 | 11.0 | 51.5 |
| Knee | 92 | 30.5 | 7.2 | 30.3 | 88 | 39.4 | 11.3 | 38.5 | |
| Ab-I | Hip | 94 | 17.9 | 6.6 | 18 | 98 | 4.5 | 5.1 | 3 |
| Knee | 94 | 19.6 | 5.4 | 20.0 | 99 | 10.2 | 7.4 | 8.0 | |
| Ab-A | Hip | 99 | 23 | 11.7 | 22 | 102 | 7 | 8.5 | 4 |
| Knee | 97 | 24.4 | 10.5 | 25.0 | 97 | 13.1 | 12.8 | 8.0 | |
| Ab-P | Hip | 100 | 9.6 | 7.1 | 8 | 102 | 2.8 | 4.7 | 1 |
| Knee | 96 | 11.0 | 6.0 | 10.0 | 95 | 5.5 | 5.8 | 3.0 | |
| MYMOP2 | Hip | 104 | 4.1 | 0.9 | 4.0 | 101 | 1.1 | 1.2 | 0.8 |
| Knee | 99 | 4.3 | 0.8 | 4.3 | 101 | 2.0 | 1.5 | 1.8 | |
| Get-up-and-go test time (duration in seconds to complete the test) | Hip | 96 | 20.8 | 14.8 | 17 | 97 | 13.7 | 6.0 | 12.0 |
| Knee | 90 | 19.4 | 8.8 | 17.0 | 96 | 16.3 | 9.1 | 13.0 | |
| 20-metre walk time (duration in seconds to walk a 20-metre distance) | Hip | 102 | 25.5 | 14.8 | 22 | 103 | 18.8 | 5.8 | 17 |
| Knee | 99 | 24.7 | 9.4 | 22.0 | 100 | 21.2 | 7.4 | 18.5 | |
| HHS | Hip | 103 | 53.5 | 16.8 | 54.8 | 102 | 86.9 | 15.9 | 93.1 |
| AKSS pain, stability and ROM | Knee | 98 | 44.1 | 14.9 | 45 | 99 | 73.1 | 20.6 | 81 |
| AKSS function | Knee | 100 | 55.9 | 13.1 | 52.5 | 101 | 66.2 | 19.5 | 65.0 |
| Outcome | Site | Pre-operative assessment | 12-month postoperative assessment | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Sit-to-stand-to-sit test | Hip | 95 | 91.4 | 99 | 96.1a |
| Knee | 90 | 89.1 | 95 | 94.1 | |
| Missing | Hip | 0 | 1 | ||
| Knee | 0 | 0 | |||
| 20-cm step test | Hip | 104 | 81.7 | 98 | 94.2 |
| Knee | 82 | 81.2 | 92 | 91.1 | |
| 30-cm step test | Hip | 64 | 61.5 | 84 | 82.4a |
| Knee | 40 | 40.0a | 71 | 70.3 | |
| Missing | Hip | 0 | 2 | ||
| Knee | 1 | 0 | |||
| Balance | Hip | 50 | 48.1 | 63 | 60.6 |
| Knee | 39 | 38.6 | 46 | 46.0a | |
| Missing | Hip | 0 | 0 | ||
| Knee | 0 | 1 | |||
| Get-up-and-go test | Hip | 96 | 92.3 | 98 | 94.2 |
| Knee | 93 | 92.1 | 96 | 95.1 | |
| 20-metre walk | Hip | 104 | 100.0 | 103 | 99.0 |
| Knee | 100 | 99.0 | 100 | 99.0 | |
Table 25 demonstrates that, as expected, scores on the function measures improve from baseline to 12 months. People in this cohort had better function after surgery than before they had the surgical intervention.
Table 26 shows similar results, suggesting that people’s function improves after surgery. However, a particular feature of these data is the number of participants who were able to complete certain tasks. Nearly everyone could complete most of the tests, both before and after surgery. However, two of the tests appear to be more discriminatory – the 30-cm step test and the balance test. As shown in Table 26, only 61.5% of people with hip disease could do the 30-cm step test prior to surgery, and this improved to 82.4% postoperatively; the equivalent figures for those with knee disease were 40.0% and 70.3%, respectively. The balance test was also difficult for patients with knee disease; only 38.6% of patients could perform the test pre-operatively and 46.0% postoperatively. Those with hip disease could do a little better – 48.1% were able to do it pre-operatively, and 60.6% postoperatively.
Change for better or worse and clinically important improvements
The above scores tell only a part of the story. They do not provide information about what proportion of people got better or worse, or whether or not the improvements were clinically, as well as statistically, important. In order to answer these questions, we investigated the following:
-
the number of patients with scores that indicated improvement, deterioration or no change in function between baseline and 12 months
-
the numbers of patients in the improved or deteriorated function categories whose changes were statistically significant
-
the proportion of patients for whom these changes reached clinical significance, using the anchoring satisfaction question.
The results of these analyses are shown in Tables 27 and 28. The data also allow us to further compare the degree of improvement following knee or hip replacement.
| Outcome | Hip | Knee | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Statistical changea | %MCIIb | Sample | Statistical changea | %MCIIb | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| WOMAC function | ||||||||||||
| Deterioration | 5 | 4.9 | 2 | 40.0 | 12 | 11.9 | 3 | 25.0 | ||||
| No change | 2 | 2.0 | 2 | 2.0 | ||||||||
| Improvement | 95 | 93.1 | 90 | 94.7 | 66 | 69.5 | 87 | 86.1 | 82 | 94.3 | 62 | 71.3 |
| Total | 102 | 100.0 | 92 | 90.2 | 66 | 64.7 | 101 | 100.0 | 85 | 84.2 | 62 | 61.4 |
| Unknown | 2 | 0 | ||||||||||
| SF-12 physical function | ||||||||||||
| Deterioration | 8 | 8.4 | 6 | 75.0 | 15 | 18.8 | 9 | 60.0 | ||||
| No change | 0 | 0.0 | 0 | 0.0 | ||||||||
| Improvement | 87 | 91.6 | 78 | 89.7 | 70 | 80.5 | 65 | 81.3 | ||||
| Total | 95 | 100.0 | 84 | 88.4 | 70 | 73.7 | 80 | 100.0 | 65 | 81.3 | 31 | 38.8 |
| Unknown | 9 | 21 | ||||||||||
| Ab-I | ||||||||||||
| Deterioration | 2 | 2.2 | 1 | 50.0 | 11 | 11.8 | 7 | 63.6 | ||||
| No change | 1 | 1.1 | 2 | 2.2 | ||||||||
| Improvement | 87 | 96.7 | 86 | 98.9 | 61 | 70.1 | 80 | 86.0 | 75 | 93.8 | 62 | 77.5 |
| Total | 90 | 100.0 | 87 | 96.7 | 61 | 67.8 | 93 | 100.0 | 82 | 88.2 | 62 | 66.7 |
| Unknown | 14 | 8 | ||||||||||
| Ab-A | ||||||||||||
| Deterioration | 6 | 6.2 | 4 | 66.7 | 15 | 16.0 | 7 | 46.7 | ||||
| No change | 1 | 1.0 | 2 | 2.1 | ||||||||
| Improvement | 90 | 92.8 | 87 | 96.7 | 80 | 88.9 | 77 | 81.9 | 68 | 88.3 | 66 | 85.7 |
| Total | 97 | 100.0 | 91 | 93.8 | 80 | 82.5 | 94 | 100.0 | 75 | 79.8 | 66 | 70.2 |
| Unknown | 7 | 7 | ||||||||||
| Ab-P | ||||||||||||
| Deterioration | 5 | 5.1 | 2 | 40.0 | 8 | 8.8 | 4 | 50.0 | ||||
| No change | 6 | 6.1 | 7 | 7.7 | ||||||||
| Improvement | 87 | 88.8 | 83 | 95.4 | 77 | 88.5 | 76 | 83.5 | 73 | 96.1 | 48 | 63.2 |
| Total | 98 | 100.0 | 85 | 86.7 | 77 | 78.6 | 91 | 100.0 | 77 | 84.6 | 48 | 52.8 |
| Unknown | 6 | 10 | ||||||||||
| MYMOP2 | ||||||||||||
| Deterioration | 3 | 3.0 | 1 | 33.3 | 8 | 8.1 | 5 | 62.5 | ||||
| No change | 0 | 0.0 | 1 | 1.0 | ||||||||
| Improvement | 98 | 97.0 | 95 | 96.9 | 77 | 78.6 | 90 | 90.9 | 84 | 93.3 | 61 | 67.8 |
| Total | 101 | 100.0 | 96 | 95.1 | 77 | 76.2 | 99 | 100.0 | 89 | 89.9 | 61 | 61.6 |
| Unknown | 3 | 2 | ||||||||||
| Get-up-and-go test time | ||||||||||||
| Deterioration | 10 | 11.0 | 8 | 80.0 | 18 | 20.2 | 12 | 66.7 | ||||
| No change | 3 | 3.3 | 9 | 10.1 | 9 | 100.0 | ||||||
| Improvement | 78 | 85.7 | 74 | 94.9 | 74 | 94.9 | 62 | 69.7 | 56 | 90.3 | 62 | 100.0 |
| Total | 91 | 100.0 | 82 | 90.1 | 74 | 81.3 | 89 | 100.0 | 68 | 76.4 | 71 | 79.8 |
| Unknown | 13 | 12 | ||||||||||
| Outcome | Hip | Knee | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Statistical changea | MCII ROC | Sample | Statistical changea | MCII ROC | |||||||
| n | % | n | % | n | %b | n | % | n | % | n | %b | |
| 20-metre walk time | ||||||||||||
| Deterioration | 15 | 14.9 | 12 | 80.0 | 19 | 19.4 | 15 | 79.0 | ||||
| No change | 10 | 9.9 | 12 | 12.2 | ||||||||
| Improvement | 76 | 75.3 | 67 | 88.2 | 67 | 88.2 | 67 | 68.4 | 57 | 85.1 | 47 | 70.2 |
| Total | 101 | 79 | 78.2 | 67 | 66.3 | 98 | 72 | 73.5 | 47 | 48.0 | ||
| Unknown | 3 | 3 | ||||||||||
| HHS | ||||||||||||
| Deterioration | 5 | 5.0 | 2 | 40.0 | ||||||||
| No change | 0 | 0.0 | ||||||||||
| Improvement | 96 | 95.1 | 91 | 94.8 | 86 | 89.6 | ||||||
| Total | 101 | 93 | 92.1 | 86 | 85.2 | |||||||
| Unknown | 3 | |||||||||||
| AKSS – knee score | ||||||||||||
| Deterioration | 10 | 10.4 | 6 | 60.0 | ||||||||
| No change | 0 | 0.0 | ||||||||||
| Improvement | 86 | 89.6 | 75 | 87.2 | 47 | 54.7 | ||||||
| Total | 96 | 81 | 84.4 | 47 | 49.0 | |||||||
| Unknown | 5 | |||||||||||
| AKSS – knee function | ||||||||||||
| Deterioration | 15 | 15.0 | 15 | 100.0 | ||||||||
| No change | 16 | 16.0 | ||||||||||
| Improvement | 69 | 69.0 | 69 | 100.0 | 56 | 81.16 | ||||||
| Total | 100 | 84 | 84.0 | 56 | 56 | |||||||
| Unknown | 1 | |||||||||||
As shown in Tables 27 and 28, there were a small number of people whose function did not change or deteriorate after surgery. Overall, each of the functional assessment methods is telling a similar story of a few people getting worse (but rarely significantly so) and most getting better, often significantly better. The walking time was more likely to show deterioration than other tests and arguably the ‘get-up-and-go test’ showed more differentiation between changes for the better or worse than other tests. Overall patients having hip surgery were more likely to improve functionally than those having knee replacement, with most of the measures used, although it is interesting to note that walking speed changes were very similar in both groups.
We also looked for ceiling effects on each of the functional measures at baseline and 12 months, as shown in Tables 29 and 30.
| Outcome | Hip | Knee | ||||
|---|---|---|---|---|---|---|
| Sample | Maximum scorea | Sample | Maximum scorea | |||
| N = 104 | n | % | N = 101 | n | % | |
| WOMAC function | ||||||
| Deterioration | 5 | 12 | ||||
| No change | 2 | 2 | 100.0 | 2 | 0 | 0.0 |
| Improvement | 95 | 23 | 24.2 | 87 | 10 | 11.5 |
| Total | 102 | 25 | 24.5 | 101 | 10 | 9.9 |
| Unknown | 2 | 0 | ||||
| SF-12 physical function | ||||||
| Deterioration | 8 | 15 | ||||
| No change | 0 | 0 | 0.0 | 0 | 0 | 0.0 |
| Improvement | 87 | 0 | 0.0 | 65 | 0 | 0.0 |
| Total | 95 | 0 | 0.0 | 80 | 0 | 0.0 |
| Unknown | 9 | 21 | ||||
| Ab-I | ||||||
| Deterioration | 2 | 11 | ||||
| No change | 1 | 0 | 0.0 | 2 | 0 | 0.0 |
| Improvement | 87 | 20 | 23.0 | 80 | 6 | 7.5 |
| Total | 90 | 20 | 22.2 | 93 | 6 | 6.5 |
| Unknown | 14 | 8 | ||||
| Ab-A | ||||||
| Deterioration | 6 | 15 | ||||
| No change | 1 | 1 | 100.0 | 2 | 0 | 0.0 |
| Improvement | 90 | 23 | 25.6 | 77 | 10 | 13.0 |
| Total | 97 | 24 | 24.7 | 94 | 10 | 10.6 |
| Unknown | 7 | 7 | ||||
| Ab-P | ||||||
| Deterioration | 5 | 8 | ||||
| No change | 6 | 3 | 50.0 | 7 | 0 | 0.0 |
| Improvement | 87 | 43 | 49.4 | 76 | 17 | 22.4 |
| Total | 98 | 52 | 46.9 | 91 | 17 | 16.8 |
| Unknown | 6 | 10 | ||||
| MYMOP2 | ||||||
| Deterioration | 3 | 8 | ||||
| No change | 0 | 0 | 0.0 | 1 | 0 | 0.0 |
| Improvement | 98 | 22 | 22.5 | 90 | 9 | 10.0 |
| Total | 101 | 22 | 21.8 | 99 | 9 | 8.9 |
| Unknown | 3 | 2 | ||||
| Outcome | Hip | Knee | ||||
|---|---|---|---|---|---|---|
| Sample | Maximum scorea | Sample | Maximum scorea | |||
| N = 104 | n | % | N = 101 | n | % | |
| Get-up-and-go test time | ||||||
| Deterioration | 10 | NA | NA | 18 | NA | NA |
| No change | 3 | NA | NA | 9 | NA | NA |
| Improvement | 78 | NA | NA | 62 | NA | NA |
| Total | 91 | NA | NA | 89 | NA | NA |
| Unknown | 13 | 12 | ||||
| 20-metre walk time | ||||||
| Deterioration | 15 | NA | NA | 19 | NA | NA |
| No change | 10 | NA | NA | 12 | NA | NA |
| Improvement | 76 | NA | NA | 67 | NA | NA |
| Total | 101 | NA | NA | 98 | NA | NA |
| Unknown | 3 | 3 | ||||
| HHS | ||||||
| Deterioration | 5 | |||||
| No change | 0 | 0 | 0.0 | |||
| Improvement | 96 | 4 | 4.2 | |||
| Total | 101 | 4 | 4.0 | |||
| Unknown | 3 | |||||
| AKSS – knee score | ||||||
| Deterioration | 10 | |||||
| No change | 0 | 0 | 0.0 | |||
| Improvement | 86 | 2.0 | ||||
| Total | 96 | 2 | 2.1 | |||
| Unknown | 5 | |||||
| AKSS – knee function | ||||||
| Deterioration | 15 | |||||
| No change | 16 | 0 | 0.0 | |||
| Improvement | 69 | 11.0 | ||||
| Total | 100 | 11 | 11.0 | |||
| Unknown | 1 | |||||
The striking finding here is that many of the self-assessment questionnaires that are routinely used to assess function in people with lower limb osteoarthritis show an important ceiling effect in response to joint replacement. The problem is particularly evident in the assessment of function after hip replacement; the WOMAC function subscale, SF-12 physical function measure and all three domains of the Aberdeen scale all reach a maximum score in between 20% and 50% of patients postoperatively. Rather fewer patients reached the maximum score after knee surgery, but the problem is a very real one for this intervention as well (between 8% and 22% of patients reaching the maximum on one or other of the scores). Timed tests such as the walk time or ‘get-up-and-go’ test, cannot, by definition, suffer from this problem and it is interesting to note that very few people achieved ‘top marks’ on either the HHS or AKSS.
Discussion
It is well known that, on average, people undergoing a hip or knee joint replacement get some functional benefit. 340 Our data support this, showing that, on average, there was a large improvement in the functional scores between baseline and 12 months after surgery. Our data also confirm findings from previous research that those undergoing hip surgery can, on average, expect more improvement in function that those having a knee replacement. 341,342
However, average scores obscure the fact that there can be big differences in the change and that some people may experience a decline in function. Our data suggest that very few people get worse, although quite a lot of those who improve are not achieving a level of improvement that can be called clinically, rather than statistically, significant. For example, if we examine the data in Table 27 carefully, they tell us that the WOMAC function score improved in 93% of people having hip replacement and 86% of those having knee replacement, and that this change was statistically significant in 90% and 82%, respectively. However, the change for the better was clinically significant in only some 70% of those having hip replacement and 61% of the knee replacement patients. Clearly, there is a need to be cautious in relation to the information given to people when they have a joint replacement regarding expected functional outcomes (as opposed to pain improvement).
The data do suggest that there are important differences in what is being assessed by self-report, clinician-administered tools and functional assessments, as was apparent in our cross-sectional data. An important finding in relation to that is the fact that the self-assessment tests often used by rheumatologists to detect change in response to non-surgical interventions (e.g. WOMAC and SF-12) show a big ceiling effect when used in the surgical setting.
Assessment of the trajectories of change
Introduction
The analysis presented in the previous section (see Longitudinal analysis of changes in the different measures of function over time) showed that the majority of participants experienced an improvement in function after joint replacement. To supplement and extend on this work, we undertook further analyses to investigate participants’ trajectories of recovery after joint replacement surgery, using data collected pre-operatively and at 3 months and 12 months after surgery.
First, we explored the trajectory of recovery, in terms of pain and function, in the first year postoperatively, with a particular focus on patients undergoing revision joint surgery. By including patients listed for different sort of surgical procedures, the ADAPT cohort study allowed us to investigate the specificities of pain and function changes following revision surgery. We then investigate how recovery of pain and function were interrelated. In these analyses, we used both a self-reported (WOMAC function) and objective measure of function (time to complete a 20-metre walk test) to identify any disparities in recovery pattern induced by assessment measure. Pain is a subjective experience and, therefore, we used the self-report WOMAC pain score for assessing pain severity. Finally, we present findings from a gait analysis study conducted on total primary hip replacement to shed light on the above self-reported versus objective findings comparison.
Statistical analysis
Analyses were conducted separately for patients undergoing hip and knee replacement surgery using Stata 13.1 (StataCorp LP, College Station, TX, USA).
Pain and function were analysed jointly using a multivariate linear mixed (MLM) regression with random intercepts and slopes. This approach allows the modelling of the longitudinal trajectory (patients’ trajectory) of each outcome measure and the assessment of the correlations within and between these outcome measures (correlation structure) in a single regression framework while providing unbiased estimations in a context of missing data (under the missing at random assumption). Postoperative change over time was modelled as two linear splines: one spline for the ‘immediate change’ occurring between the pre-operative assessment and the first postoperative assessment (3 months) and another spline for the ‘long-term change’ occurring between the two postoperative assessments (3 and 12 months). These changes were normally distributed for each outcome allowing the use of MLM regression. However, the postoperative function and pain scores were not normally distributed preventing the use of this modelling framework to compare participants’ scores at specific postoperative time points. Mann–Whitney U-tests were used for this purpose. The strength of correlation between parameters was interpreted as |0.00|–|0.25| = none–little, |0.26|–|0.49| = low, |0.50|–|0.69| = moderate, |0.70|–|0.89| = high, |0.90|–|1.00| = very high. p-values of ≤ 0.05 were considered statistically significant.
The MLM models were conducted on WOMAC pain and WOMAC function and replicated on WOMAC pain and time to complete the 20-metre walk test to investigate disparities in findings according to the method of functional assessment (self-reported vs. objective). The inverse of the 20-metre walk test completion time (Time–1) was derived to facilitate the comparison of the effects between outcomes (lower scores: worse function/pain score/time of test completion; higher scores: best function/pain score/time of test completion).
To assess if the pattern of changes differed by surgery type, patients were split into two groups: primary surgeries (including primary total hip and knee surgeries, knee unicompartmental and patellofemoral surgeries and revision surgeries). WOMAC pain, WOMAC function and Time–1 were modelled separately with univariable linear mixed (ULM) regressions. It was not possible to model all these outcomes in a single multivariate framework as the numbers of patients in the subgroups were not sufficient to fit a MLM (hip: revision, n = 43; primary, n = 80; knee: revision, n = 42; primary, n = 84). The ULM models were adjusted for the two time splines defined above, surgery type, their interaction and random effects on each of these parameters. Differences in the immediate- and long-term changes by surgery type (primary vs. revision) were tested using appropriate contrasts. A similar approach was used to investigate the influence of pre-operative pain/function score on the postoperative recovery pattern: patients were split into groups of high or low level of pre-operative pain using the pre-operative WOMAC pain median as a cut-off point (hip: median = 55; knee: median = 40); they were also split into groups of high or low level of pre-operative functional ability using the pre-operative WOMAC function and time to complete the 20-metre walk test medians as cut-off points (hip: median WOMAC function = 56, time to complete the 20-metre walk test = 22–1 seconds; knee: 50 and 22–1 seconds, respectively).
Results
A total of 123 hip replacement participants were considered. Of these patients, 80 (65%) had a primary THR and 43 (35%) had a revision hip replacement. They had a mean age of 65 years (SD 11 years) and BMI of 28 kg/m2 (SD 5 kg/m2). Half of them were female (n = 62) and retired or unemployed (n = 67) and 24% (n = 30) were living alone. Approximately 75% (n = 91) had osteoarthritis in at least one other joint.
Of the 123 hip replacement participants with a pre-operative assessment, 121 (98%) completed a WOMAC pain and function measure and 118 (96%) performed the timed 20-metre walk test. Of the 112 (91%) patients who participated in a 3-month assessment, all completed the WOMAC pain and function scores and 107 (87%) completed the 20-metre walk test. At 12 months, 110 (89%) hip patients were still in the study, 109 (89%) completed the WOMAC function score, and 108 (88%) completed the WOMAC pain score and the 20-metre walk test.
A total of 126 knee replacement participants were considered. Of these patients, 48 (38%) had a primary TKR, 42 (33%) a unicompartmental knee replacement, 5 (4%) a patellofemoral knee replacement and 42 (33%) a revision knee replacement. They had a mean age of 67 years (SD 10 years) and a BMI of 31 kg/m2 (SD 6 kg/m2). Approximately 55% (n = 69) were female, 66% (n = 83) were retired or unemployed and 29% (n = 37) were living alone. Approximately 83% (n = 104) had osteoarthritis in at least one other joint.
Of the 126 knee replacement participants with a pre-operative assessment, 123 (98%) completed the three outcome measures. Of the 115 (91%) patients who participated in a 3-month assessment, 113 (90%) completed the WOMAC function score and 114 (91%) the WOMAC pain score and the 20-metre walk test. At 12 months, 112 (89%) patients were still in the study, 111 (88%) completed the WOMAC scores and 102 (81%) the 20-metre walk test.
Pain and function measures at the different assessment points are presented in Table 31.
| Outcome measure | Hip | Knee | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | Median | 25th percentile | 75th percentile | p-valueb | n | % | Median | 25th percentile | 75th percentile | p-valueb | |||
| WOMAC function | Pre-operative | Total | 121 | 98.4 | 55.9 | 38.2 | 70.6 | 123 | 97.6 | 50.0 | 39.7 | 64.7 | ||
| Low level | 60 | 49.6 | 38.2 | 30.9 | 45.5 | < 0.001 | 59 | 48.0 | 39.7 | 27.9 | 42.9 | < 0.001 | ||
| High level | 61 | 50.4 | 70.6 | 63.3 | 82.4 | 64 | 52.0 | 64.7 | 55.9 | 72.8 | ||||
| 3 months | Total | 112 | 91.1 | 89.7 | 80.9 | 95.6 | 113 | 89.7 | 72.1 | 60.3 | 89.7 | |||
| Low level | 56 | 50.0 | 86.0 | 74.3 | 92.7 | < 0.01 | 52 | 46.0 | 65.9 | 44.1 | 75.0 | < 0.001 | ||
| High level | 56 | 50.0 | 91.4 | 85.3 | 97.1 | 61 | 54.0 | 83.8 | 69.1 | 94.1 | ||||
| 12 months | Total | 109 | 88.6 | 94.1 | 83.8 | 98.5 | 111 | 88.1 | 82.4 | 57.4 | 94.1 | |||
| Low level | 51 | 46.8 | 92.7 | 80.4 | 98.5 | NS | 54 | 48.7 | 64.7 | 39.7 | 85.3 | < 0.001 | ||
| High level | 58 | 53.2 | 95.6 | 87.5 | 100.0 | 57 | 51.4 | 91.2 | 73.5 | 95.6 | ||||
| Timed 20-metre walk | Pre-operative | Total | 118 | 95.9 | 22.0 | 18.0 | 28.0 | 123 | 97.6 | 22.0 | 18.0 | 30.0 | ||
| Low level | 58 | 49.2 | 29.0 | 25.0 | 35.0 | < 0.001 | 60 | 48.8 | 30.0 | 27.0 | 38.5 | < 0.001 | ||
| High level | 60 | 50.9 | 18.0 | 16.0 | 20.0 | 63 | 51.2 | 18.0 | 16.0 | 20.0 | ||||
| 3 months | Total | 107 | 87.0 | 18.0 | 16.0 | 22.0 | 114 | 90.5 | 20.0 | 18.0 | 26.0 | |||
| Low level | 50 | 46.7 | 20.0 | 18.0 | 23.0 | < 0.001 | 56 | 49.1 | 25.5 | 22.0 | 30.5 | < 0.001 | ||
| High level | 57 | 53.3 | 17.0 | 14.0 | 20.0 | 58 | 50.9 | 18.0 | 16.0 | 20.0 | ||||
| 12 months | Total | 108 | 87.8 | 17.0 | 15.0 | 21.0 | 102 | 81.0 | 18.5 | 16.0 | 24.0 | |||
| Low level | 50 | 46.3 | 20.0 | 17.0 | 23.0 | < 0.001 | 47 | 46.1 | 24.0 | 20.0 | 30.0 | < 0.001 | ||
| High level | 58 | 53.7 | 16.0 | 14.0 | 17.0 | 55 | 53.9 | 17.0 | 15.0 | 18.0 | ||||
| WOMAC pain | Pre-operative | Total | 121 | 98.4 | 55.0 | 35.0 | 70.0 | 123 | 97.6 | 40.0 | 30.0 | 60.0 | ||
| Low level | 66 | 54.6 | 40.0 | 30.0 | 50.0 | < 0.001 | 62 | 50.4 | 30.0 | 20.0 | 40.0 | < 0.001 | ||
| High level | 55 | 45.5 | 70.0 | 65.0 | 80.0 | 61 | 49.6 | 60.0 | 50.0 | 70.0 | ||||
| 3 months | Total | 112 | 91.1 | 95.0 | 80.0 | 100.0 | 114 | 90.5 | 75.0 | 50.0 | 90.0 | |||
| Low level | 61 | 54.5 | 95.0 | 75.0 | 100.0 | 0.010 | 57 | 50.0 | 55.0 | 40.0 | 80.0 | < 0.001 | ||
| High level | 51 | 45.5 | 100.0 | 90.0 | 100.0 | 57 | 50.0 | 80.0 | 70.0 | 90.0 | ||||
| 12 months | Total | 108 | 87.8 | 100.0 | 85.0 | 100.0 | 111 | 88.1 | 80.0 | 55.0 | 95.0 | |||
| Low level | 57 | 52.8 | 95.0 | 75.0 | 100.0 | NS | 56 | 50.5 | 62.5 | 45.0 | 85.0 | < 0.001 | ||
| High level | 51 | 47.2 | 100.0 | 90.0 | 100.0 | 55 | 49.6 | 90.0 | 75.0 | 100.0 | ||||
Hip replacement: change in function and pain
As expected, both function (Figure 13a) and pain scores (Figure 13b) improved after surgery. These improvements occurred mainly within the first 3 months following surgery [Table 32: +0.24 of WOMAC function point/day (p < 0.001); +0.28 WOMAC pain point/day (p < 0.001)]. There was no evidence of further changes after 3 months (see Table 32: WOMAC function and WOMAC pain, p-values = not significant). A similar pattern of recovery was observed with the objective measure of function (Figure 13c and Table 32) with a statistically significant mean immediate change (+0.002 seconds–1/month; p < 0.001) but no significant long-term mean change (p = 0.057). This latter effect is close to the 0.05 significance level, suggesting a marginal effect, but a larger sample would be required to provide a more definitive answer.
FIGURE 13.
Change in pain and function from pre-operative to 12 months after hip replacement. (a) WOMAC function; (b) WOMAC pain; and (c) 20-metre walk test.

| Hip | Self-reported functionb and painc | Objective functiond and painc | ||||
|---|---|---|---|---|---|---|
| Fixed effects | Coefficient | 95% CI | p-value | Coefficient | 95% CI | p-value |
| Pre-operative score: functione | 59.93 | 56.30 to 63.57 | < 0.001 | 0.05 | 0.04 to 0.05 | < 0.001 |
| Immediate Δ:f functiong,h | 0.24 | 0.20 to 0.28 | < 0.001 | 0.002 | 0.001 to 0.002 | < 0.001 |
| Long-term Δ:i functiong,h | 0.01 | –0.01 to 0.01 | NS | 0.00 | –0.00 to 0.00 | 0.057 |
| Preoperative score: paine | 59.74 | 56.20 to 63.28 | < 0.001 | 59.74 | 56.20 to 63.28 | < 0.001 |
| Immediate Δ: paing | 0.28 | 0.24 to 0.31 | < 0.001 | 0.28 | 0.24 to 0.31 | < 0.001 |
| Long-term Δ: paing | 0.00 | –0.01 to 0.01 | NS | 0.00 | –0.01 to 0.01 | NS |
| Random effects | SDj | 95% CI | SDj | 95% CI | ||
| Pre-operative score: function | 20.48 | 17.72 to 22.92 | 0.01 | 0.01 to 0.01 | ||
| Immediate Δ: function | 0.20 | 0.17 to 0.22 | 0.003 | 0.003 to 0.004 | ||
| Long-term Δ: function | 0.05 | 0.04 to 0.05 | 0.001 | 0.001 to 0.001 | ||
| Preoperative score: pain | 19.93 | 17.24 to 22.30 | 19.93 | 17.24 to 22.30 | ||
| Immediate Δ: pain | 0.20 | 0.17 to 0.22 | 0.20 | 0.17 to 0.22 | ||
| Long-term Δ: pain | 0.05 | 0.04 to 0.06 | 0.05 | 0.04 to 0.06 | ||
| Correlations | Correlationk | 95% CI | Correlationk | 95% CI | ||
| Preoperative function: preoperative pain | 0.78 | 0.71 to 0.85 | 0.39 | 0.24 to 0.54 | ||
| Preoperative function: immediate Δ function | –0.61 | –0.72 to –0.49 | –0.47 | –0.62 to –0.33 | ||
| Preoperative function: long-term Δ function | –0.12 | –0.31 to 0.06 | –0.12 | –0.31 to 0.07 | ||
| Immediate Δ function: long-term Δ function | –0.36 | –0.52 to –0.19 | –0.33 | –0.50 to –0.16 | ||
| Preoperative pain: immediate Δ pain | –0.56 | –0.69 to –0.44 | –0.56 | –0.69 to –0.44 | ||
| Preoperative pain: long-term Δ pain | 0.13 | –0.05 to 0.32 | 0.13 | –0.05 to 0.32 | ||
| Immediate Δ pain: long-term Δ pain | –0.56 | –0.69 to –0.43 | –0.56 | –0.69 to –0.43 | ||
| Preoperative function: immediate Δ pain | –0.45 | –0.60 to –0.31 | –0.17 | –0.35 to 0.01 | ||
| Preoperative function: long-term Δ pain | 0.06 | –0.13 to 0.25 | –0.04 | –0.23 to 0.15 | ||
| Preoperative pain: immediate Δ function | –0.36 | –0.52 to –0.20 | –0.45 | –0.60 to –0.30 | ||
| Preoperative pain: long-term Δ function | –0.10 | –0.29 to 0.09 | 0.22 | 0.03 to 0.40 | ||
| Immediate Δ function: immediate Δ pain | 0.76 | 0.68 to 0.84 | 0.49 | 0.34 to 0.63 | ||
| Immediate Δ function: long-term Δ pain | –0.38 | –0.54 to –0.21 | –0.14 | –0.33 to 0.05 | ||
| Immediate Δ pain: long-term Δ function | –0.25 | –0.43 to –0.07 | –0.15 | –0.34 to 0.04 | ||
| Long-term Δ function: long-term Δ pain | 0.64 | 0.53 to 0.75 | 0.05 | –0.14 to 0.25 | ||
The mean trajectories are derived from the fixed effects of linear mixed models regressing WOMAC pain, WOMAC function and Time–1 to perform the 20-metre walk test on the time of assessment parameterised as two linear splines (to assess immediate changes and long-term changes, see Table 32, footnotes f and i).
This overall pattern of change in self-reported function was observed in both primary and revision surgery patient groups (Figure 14a). However, the immediate change was twice as large (p < 0.001) for the primary (+0.28/day, 95% CI 0.24 to 0.33; p < 0.001) than for the revision (+0.15/day, 95% CI 0.10 to 0.20; p < 0.001) surgery group. No significant long-term improvement in function was observed after 3 months for either group. As a result of the different pace in immediate recovery, and despite similar levels of pre-operative WOMAC function scores, the median level of functional ability observed at 12 months post operation was higher in the primary surgery group (p = 0.01).
FIGURE 14.
Change in pain and function from pre-operative to 12 months after primary and revision hip replacement. (a) WOMAC function; (b) WOMAC pain; and (c) 20-metre walk test. The mean trajectories are derived from the fixed effects of linear mixed models regressing WOMAC pain, WOMAC function and Time–1 to perform the 20-metre walk test on the time of assessment parameterised as two linear splines (to assess immediate changes and long-term changes, see Table 32, footnotes f and i), the surgery type and their interactions.
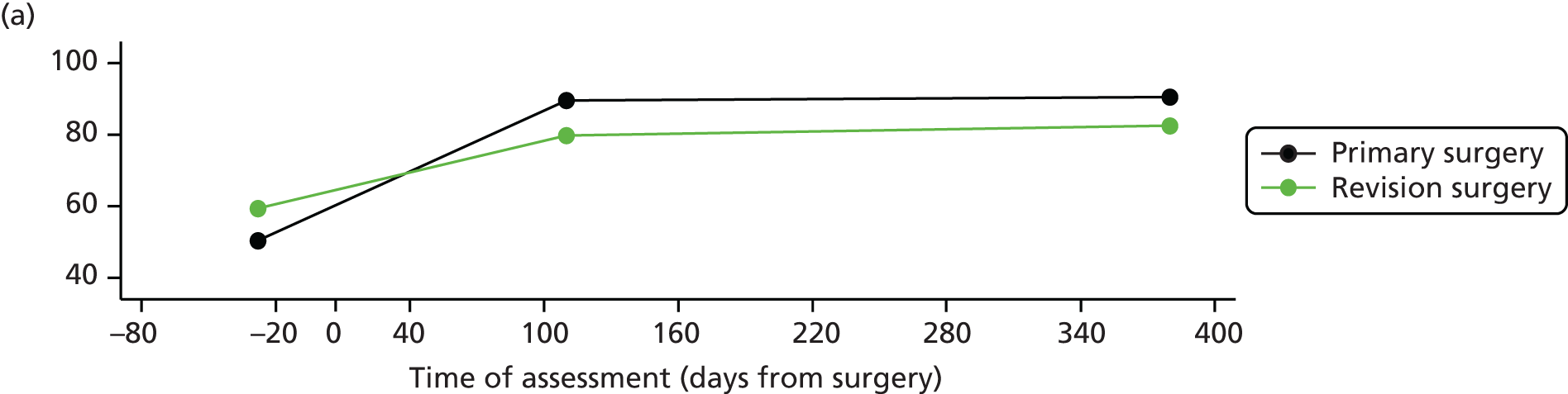

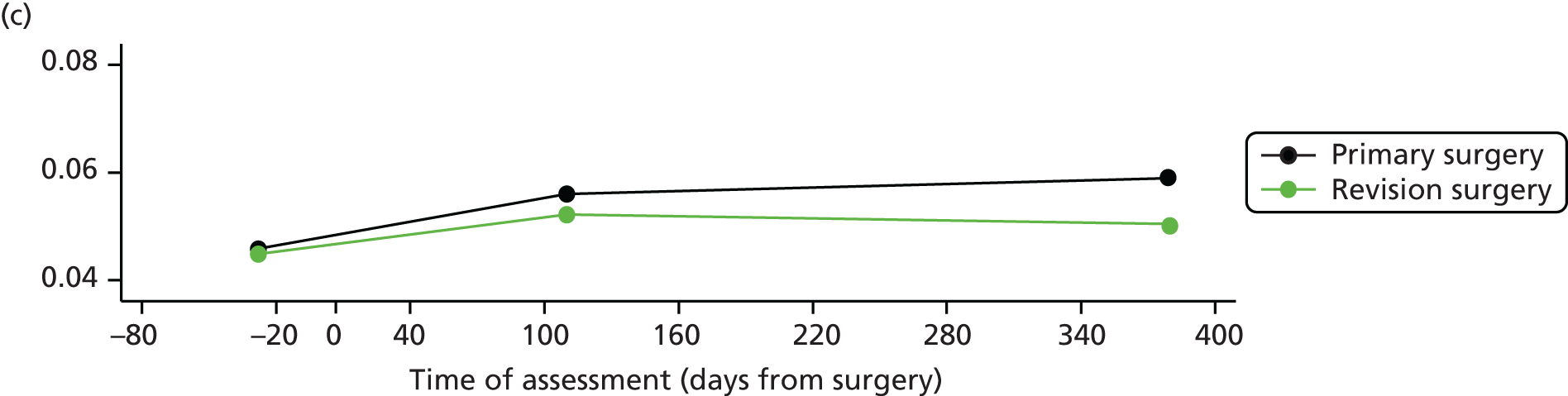
Similar results were observed for WOMAC pain (Figure 14b). Those patients listed for a primary surgery had more pain pre-operatively than those in the revision surgery group (p = 0.03), but their immediate improvement was twice as large (+0.33/day, 95% CI 0.29 to 0.38, vs. +0.17/day, 95% CI 0.11 to 0.22); long-term mean changes in pain were not significant for both groups. At 12 months, the primary surgery group had caught up with the revision group and had similar pain score levels (p = not significant).
In contrast, to the two other outcomes, the immediate improvements in walking time were similar for both surgical groups but their changes in objective function after 3 months were different (p = 0.05), being nearly flat for the revision group (p = not significant) whereas patients in the primary surgery group continued to experience an improvement in their function (p < 0.01). Both groups had the same pre-operative walking speed, but at 12 months the primary surgery group did better (p < 0.01).
Hip replacement: correlation structure between and within pain and function
Two sets of correlations are presented in Table 32, one relating to the joint MLM modelling of pain and self-reported function (‘self-reported model’) and another relating to the modelling of pain and objective measure of function (‘objective model’).
Participants were more likely to concomitantly report high/low level of pre-operative pain and functional disability (correlation: + 0.78), similar direction of immediate (+ 0.76) and long-term (+ 0.64) pain and function improvements. When an objective measure of function was considered, these correlations were weaker or non-existent (0.39, 0.49 and 0.05). With regard to the ‘functional improvement journey’, high pre-operative functional disability was correlated with large functional improvement within the first 3 months following surgery and those with more favourable pre-operative functional scores had smaller immediate functional gain (–0.61 in the ‘self-reported’ and –0.47 in the ‘objective’ models). This evidence is illustrated in Figure 15a and b. Participants in the low pre-operative function group had an immediate functional improvement nearly 2.5 times larger than those who were in the high function group (difference in slope between high/low groups: p < 0.0001 for both self-reported and objective function). As reported in Table 31, the two groups of patients had statistically significantly different levels of pre-operative function but had similar levels of functional ability 12 months after surgery (WOMAC function). A significant difference was still observed after surgery when an objective measure of function was considered but the gap had reduced (see Figure 15b).
FIGURE 15.
Change in pain and function from pre-operative to 12 months after hip replacement by pre-operative pain/function. (a) WOMAC function; (b) 20-metre walk test; and (c) WOMAC pain. The mean trajectories are derived from the fixed effects of linear mixed models regressing WOMAC pain, WOMAC function and Time–1 to perform the 20-metre walk test on the time of assessment parameterised as two linear splines (to assess immediate changes and long-term changes, see Table 32, footnotes f and i), the pre-operative status and their interactions. Pre-operative level of pain/function status are defined using median scores as cut-off threshold (median WOMAC pain = 55, WOMAC function = 56, time to complete the 20-metre walk test = 22–1 seconds).
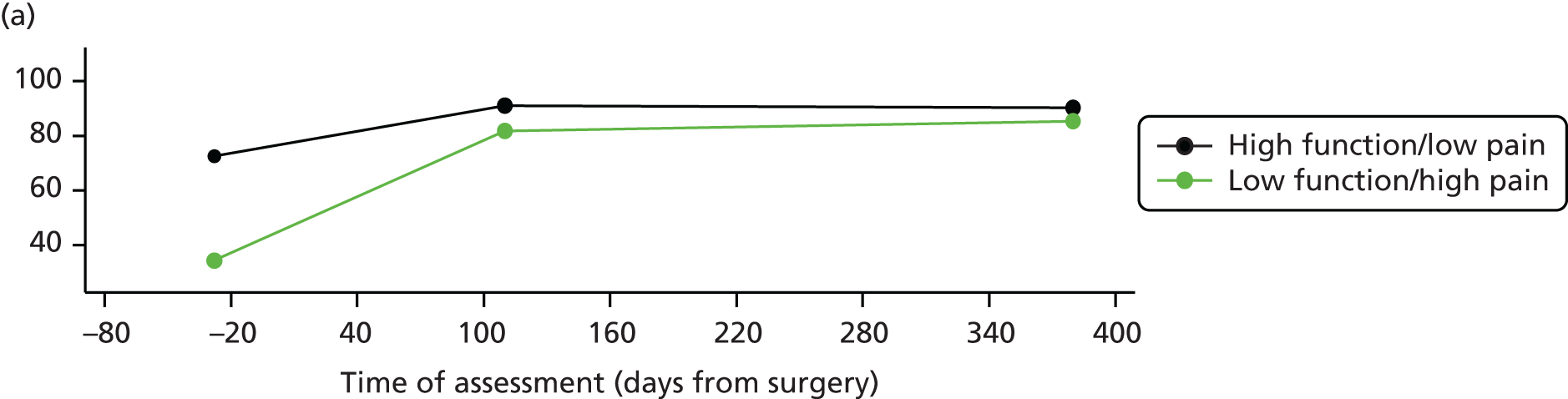
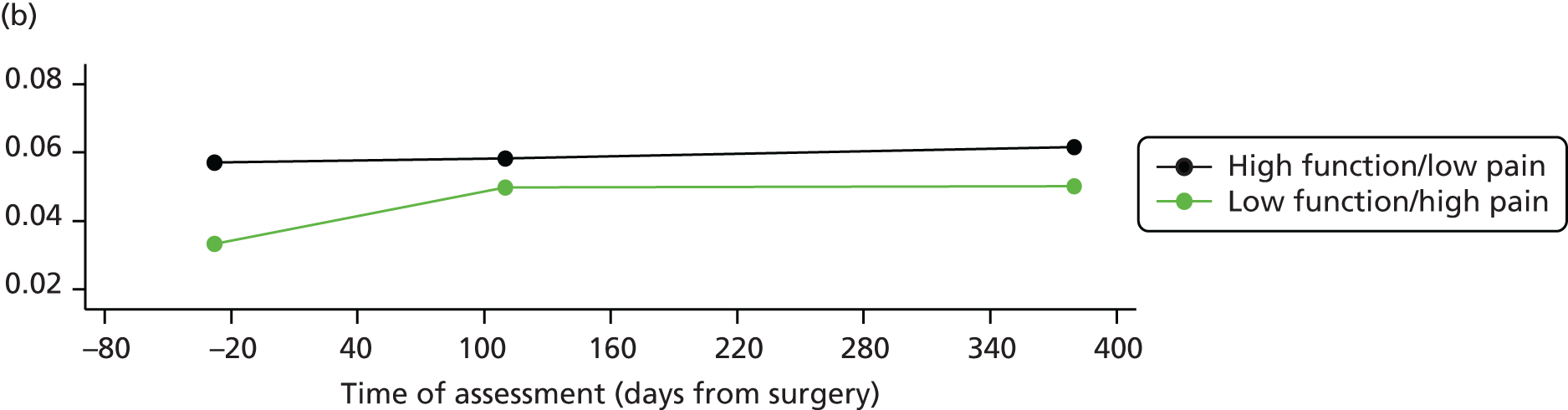
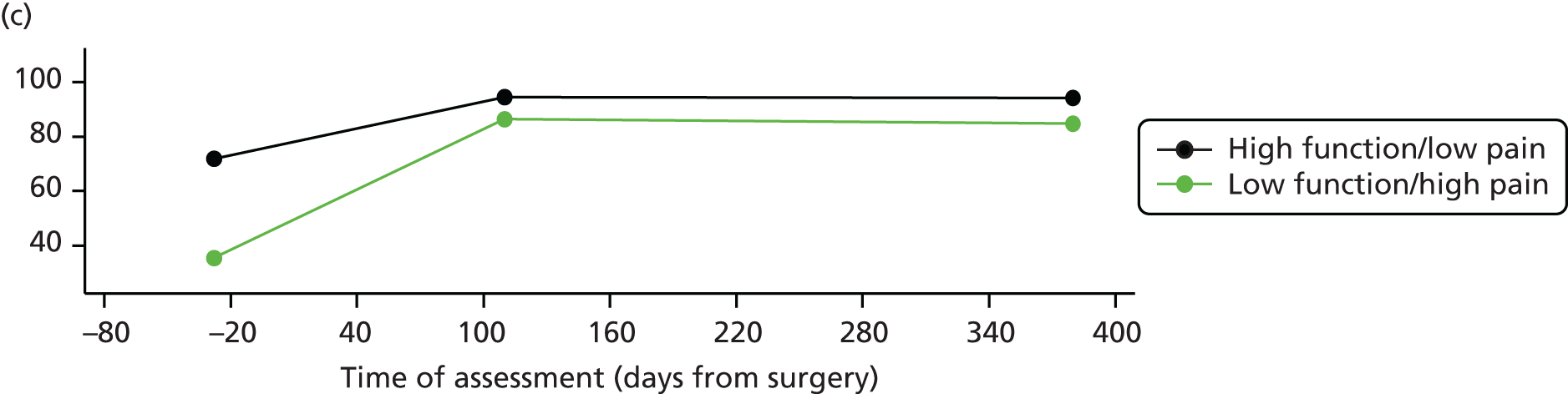
No relationship was observed between the pre-operative functional scores and long-term changes (see Table 32, correlations < 0.25). Large/small immediate functional improvements were correlated with small/large long-term functional improvement (–0.36 in the ‘self-reported’ and –0.33 in the ‘objective’ models).
With regard to the ‘functional improvement journey’, high pre-operative functional disability was correlated with large functional improvement within the first 3 months following surgery while those with more favourable pre-operative functional scores had smaller immediate functional gain (–0.61 in the ‘self-reported’ and –0.47 in the ‘objective’ models). This evidence is illustrated in Figure 15a and c. Participants in the low pre-operative function group had an immediate functional improvement nearly 2.5 times larger than those who were in the high function group (difference in slope between high/low groups: p < 0.0001 for both self-reported and objective function). As reported in Table 31, the two groups of patients had statistically different levels of pre-operative function but had similar levels of functional ability 12 months after surgery (WOMAC function). A significant difference was still observed after surgery when an objective measure of function was considered but the gap had reduced (see Figure 15b).
No relationship was observed between the pre-operative functional scores and long-term changes (see Table 32, correlations < 0.25). Large/small immediate functional improvements were correlated with small/large long-term functional improvement (–0.36 in the ‘self-reported’ and –0.33 in the ‘objective’ models).
With regard to the pain improvement trajectory, a similar picture was found, with evidence of relationships between the pre-operative scores and immediate changes (–0.56) and between the immediate- and long-term changes (–0.56). Participants with a high level of pre-operative pain had an immediate change that was twice as large as those in the low-pain group (Figure 15c, difference in slope between high/low groups: p < 0.001) and the pre-operative difference in pain severity was no longer present at 12 months postoperatively (see Table 31).
Pain and function were inter-related and appeared to influence each others ‘recovery journey’ as shown by the correlations between them. The patient-reported pre-operative level of functional ability was related to immediate change in pain (–0.45), with higher functional improvement for patients with worse pre-operative pain and lower pain improvement for those with better baseline functional ability. This relationship was not observed when function was objectively measured (objective model). Similarly, the level of pre-operative pain was related to the immediate changes in self-reported function (–0.36) as well as with immediate changes in objective function (–0.45). Pre-operative self-reported function did not seem to be correlated with long-term changes in pain and pre-operative pain did not seem to be correlated with long-term changes in self-reported function. Immediate self-reported functional improvement was correlated with long-term pain improvements (–0.38), with smaller long-term changes for those who had large immediate changes or larger long-term changes. Similarly, immediate improvement in pain was correlated with long-term improvement in perceived functional ability (–0.25). These relationships were not observed in the ‘objective model’.
Knee replacement: change in function and pain
Patients experienced an improvement in function (Figure 16a) and pain (Figure 16b) after their knee surgery. The improvements occurred mainly within the first 3 months following surgery (Table 33: + 0.18 of WOMAC function point/day, p < 0.001; + 0.21 WOMAC pain point/day, p < 0.001). There was no evidence of further changes after 3 months for WOMAC function (see Table 33: p = not significant) but there was some suggestion of further long-term improvement for pain (p = 0.051). Contrary to the self-reported measure of function, the time to complete the 20-metre walk test (Figure 16c and see Table 33) improved significantly until the 12-month assessment [immediate change + 0.001 seconds–1/month (p < 0.001); long-term change + 0.0002 seconds–1/month (p < 0.01)], with steeper improvement in the first 3 months (difference between the two slopes, p = 0.04).
FIGURE 16.
Change in pain and function from pre-operative to 12 months after knee replacement. (a) WOMAC function; (b) WOMAC pain; and (c) 20-metre walk test. The mean trajectories are derived from the fixed effects of linear mixed models regressing WOMAC pain, WOMAC function and Time–1 to perform the 20-metre walk test on the time of assessment parameterised as two linear splines (to assess immediate changes and long-term changes, see Table 32, footnotes f and i).
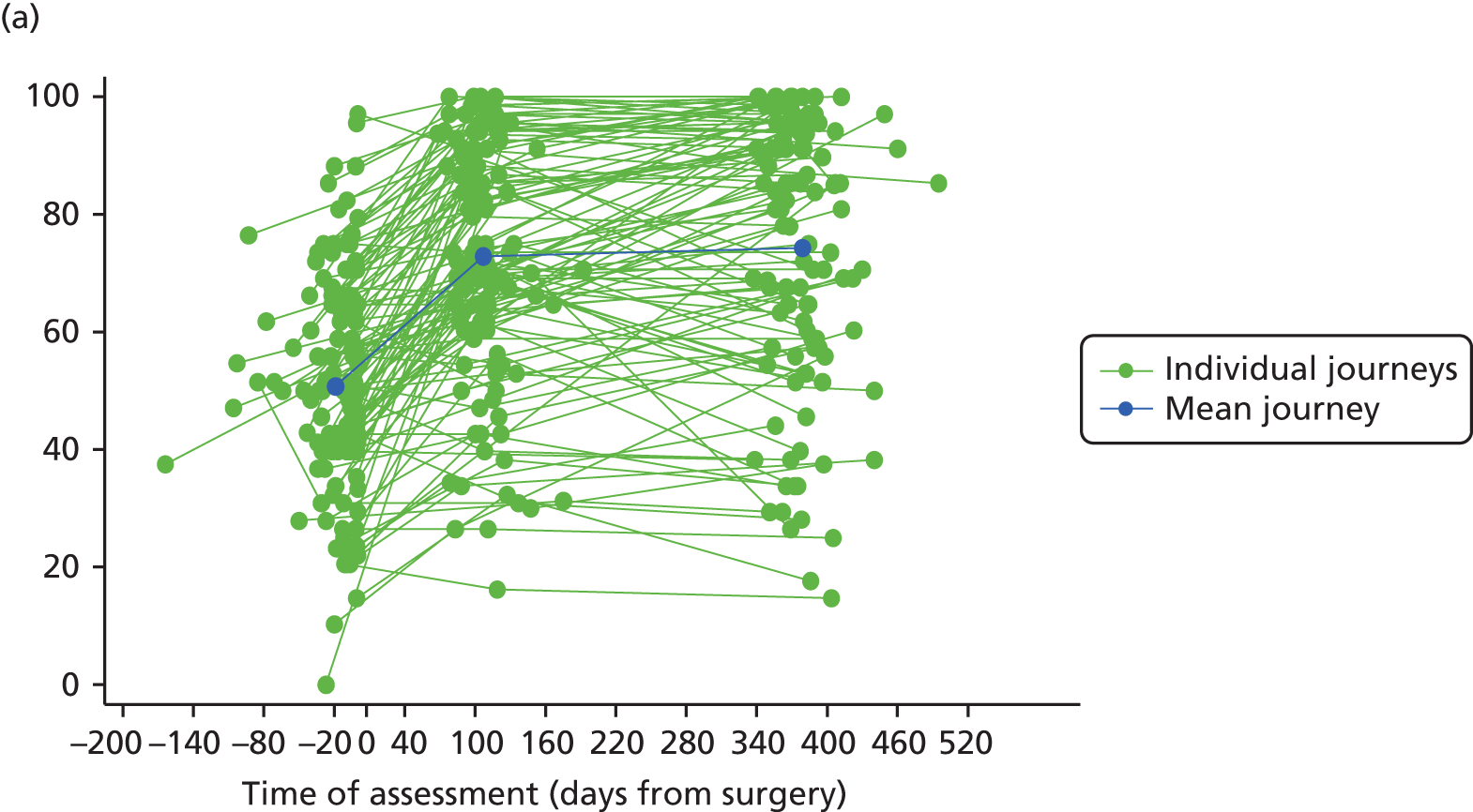
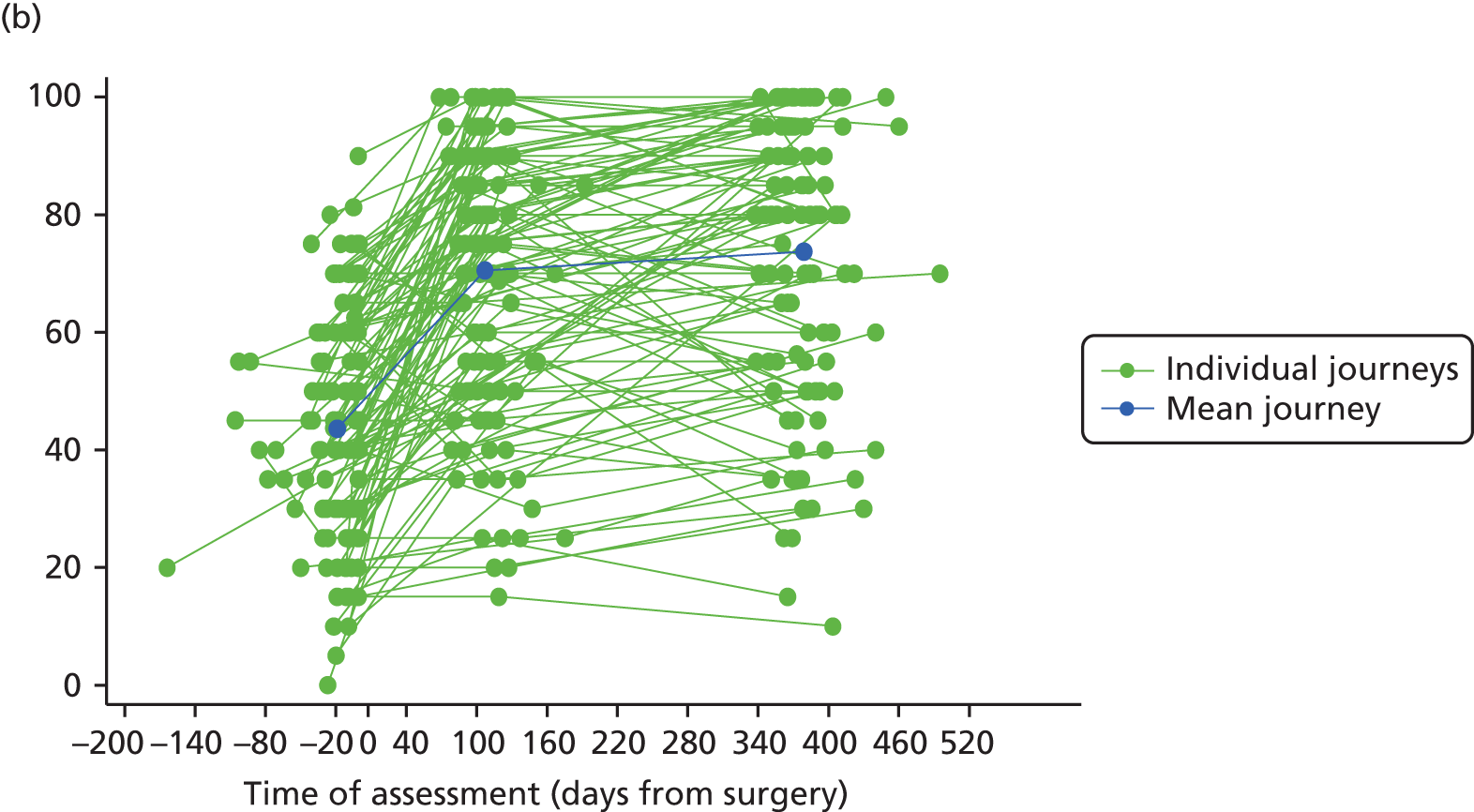

| Knee | Self-reported functionb and painc | Objective functiond and painc | ||||
|---|---|---|---|---|---|---|
| Fixed effects | Coefficient | 95% CI | p-value | Coefficient | 95% CI | p-value |
| Pre-operative score: functione | 54.07 | 50.86 to 57.28 | < 0.001 | 0.05 | 0.04 to 0.05 | < 0.001 |
| Immediate Δ:f functiong,h | 0.18 | 0.15 to 0.20 | < 0.001 | 0.001 | 0.000 to 0.002 | < 0.001 |
| Long-term Δ:i functiong,h | 0.01 | –0.00 to 0.02 | NS | 0.0002 | 0.0001 to 0.0004 | < 0.01 |
| Preoperative score: paine | 47.64 | 44.38 to 50.90 | < 0.001 | 47.64 | 44.38 to 50.90 | < 0.001 |
| Immediate Δ: paing | 0.21 | 0.18 to 0.25 | < 0.001 | 0.21 | 0.18 to 0.25 | < 0.001 |
| Long-term Δ: paing | 0.01 | –0.00 to 0.02 | 0.051 | 0.01 | –0.00 to 0.02 | 0.051 |
| Random effects | SDj | 95% CI | SDj | 95% CI | ||
| Pre-operative score: function | 18.25 | 15.80 to 20.40 | 0.01 | 0.01 to 0.02 | ||
| Immediate Δ: function | 0.15 | 0.13 to 0.17 | 0.003 | 0.003 to 0.004 | ||
| Long-term Δ: function | 0.05 | 0.05 to 0.06 | 0.001 | 0.001 to 0.001 | ||
| Preoperative score: pain | 18.48 | 16.00 to 20.67 | 18.48 | 16.00 to 20.67 | ||
| Immediate Δ: pain | 0.20 | 0.18 to 0.23 | 0.20 | 0.18 to 0.23 | ||
| Long-term Δ: pain | 0.06 | 0.05 to 0.07 | 0.06 | 0.05 to 0.07 | ||
| Correlations | Correlationk | 95% CI | Correlationk | 95% CI | ||
| Preoperative function: preoperative pain | 0.81 | 0.75 to 0.87 | 0.29 | 0.13 to 0.45 | ||
| Preoperative function: immediate Δ function | –0.26 | –0.43 to –0.09 | –0.31 | –0.48 to –0.15 | ||
| Preoperative function: long-term Δ function | –0.06 | –0.25 to 0.13 | –0.08 | –0.27 to 0.12 | ||
| Immediate Δ function: long-term Δ function | –0.19 | –0.37 to –0.01 | –0.49 | –0.64 to –0.35 | ||
| Preoperative pain: immediate Δ pain | –0.30 | –0.46 to –0.13 | –0.30 | –0.46 to –0.13 | ||
| Preoperative pain: long-term Δ pain | –0.01 | –0.20 to 0.18 | –0.01 | –0.20 to 0.18 | ||
| Immediate Δ pain: long-term Δ pain | –0.38 | –0.54 to –0.22 | –0.38 | –0.54 to –0.22 | ||
| Preoperative function: immediate Δ pain | –0.16 | –0.33 to 0.02 | 0.06 | –0.12 to 0.24 | ||
| Preoperative function: long-term Δ pain | 0.03 | –0.16 to 0.21 | 0.12 | –0.07 to 0.30 | ||
| Preoperative pain: immediate Δ function | –0.22 | –0.40 to –0.05 | –0.11 | –0.29 to 0.07 | ||
| Preoperative pain: long-term Δ function | –0.09 | –0.28 to 0.10 | 0.02 | –0.18 to 0.21 | ||
| Immediate Δ function: immediate Δ pain | 0.80 | 0.74 to 0.87 | 0.33 | 0.17 to 0.50 | ||
| Immediate Δ function: long-term Δ pain | –0.24 | –0.42 to –0.07 | –0.07 | –0.26 to 0.12 | ||
| Immediate Δ pain: long-term Δ function | –0.05 | –0.24 to 0.14 | –0.01 | –0.20 to 0.18 | ||
| Long-term Δ function: long-term Δ pain | 0.64 | 0.52 to 0.75 | –0.04 | –0.23 to 0.15 | ||
The differences in improvement patterns by surgical type group are presented in Figure 17. Patients in both the revision and primary groups experienced significant improvements within the first 3 months following their surgery in their subjective (Figure 17a; p < 0.0001 for both groups) and objective (Figure 17b, revision surgery, p = 0.02; other surgery, p = 0.01) measures of function. These immediate improvements in function were higher in the primary surgery group, the difference was not statistically significant (p > 0.05). Pain improved for both groups during the first 3 postoperative months (Figure 17c) but at a slower pace for the revision surgery group (+ 0.14/day, 95% CI 0.08 to 0.20; p < 0.0001) than for the primary surgery group (+ 0.25/day, 95% CI 0.21 to 0.29; p < 0.0001). No evidence of function or pain change was observed between 3 and 12 months, except in patients in the primary surgery group who experienced an improvement in their 20-metre walk test time completion (+ 0.0009 seconds–1/month; p = 0.01). Pre-operative levels of pain and function (subjective and objective) were similar in both surgery groups (p > 0.05), but at 12 months those who had a revision surgery had worse median scores (WOMAC function, p < 0.02; WOMAC pain, p < 0.01; 20-metre walk test time, p = 0.03).
FIGURE 17.
Change in pain and function from pre-operative to 12 months after primary and revision knee replacement. (a) WOMAC function; (b) 20-metre walk test; and (c) WOMAC pain. a, Other surgery includes primary, unicompartmental and patellofemoral procedures. The mean trajectories are derived from the fixed effects of linear mixed models regressing WOMAC pain, WOMAC function and Time–1 to perform the 20-metre walk test on the time of assessment parameterised as two linear splines (to assess immediate changes and long-term changes, see Table 33, footnotes f and i), the surgery type and their interactions.
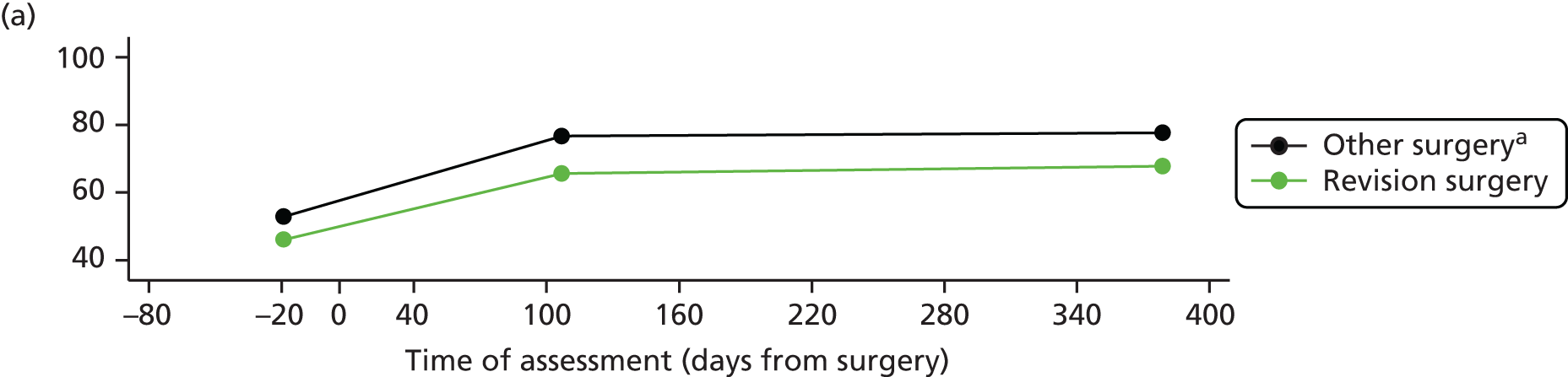
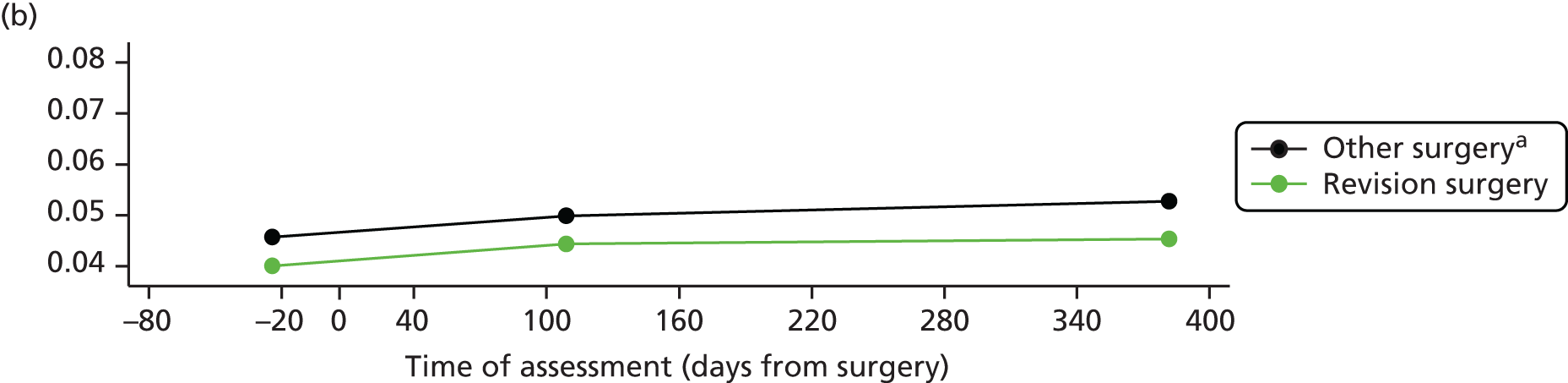
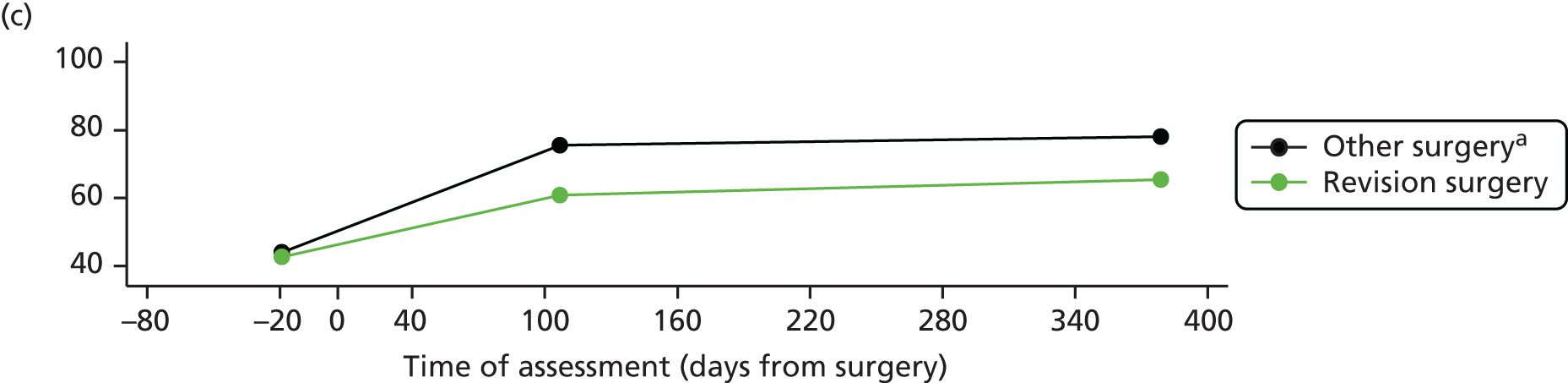
Knee replacement: correlation structure between and within pain and function
The correlation structures of the ‘self-reported’ and ‘objective’ models are presented in Table 33.
Participants were more likely to concomitantly report high/low level of pre-operative pain and functional disability (+ 0.81), similar direction of immediate (+ 0.80) and long-term (+ 0.64) pain and functional improvements. When an objective measure of function was considered, the corresponding correlations were much weaker or negligible (+ 0.29, + 0.33 and –0.04).
With regard to the ‘functional improvement journey’, low/high pre-operative function scores were correlated with high/low immediate improvement in function (self-reported model –0.26, objective model –0.31). In Figure 18a and b, we can notice steeper immediate improvements for those in the low pre-operative function group than in those in the high pre-operative function group (difference in slope between high/low groups: WOMAC function, p < 0.001; Time–1, p = 0.012).
FIGURE 18.
Change in pain and function from pre-operative to 12 months after knee replacement by pre-operative pain/function. (a) WOMAC function; (b) 20-metre walk test; and (c) WOMAC pain. The mean trajectories are derived from the fixed effects of linear mixed models regressing WOMAC pain, WOMAC function and Time–1 to perform the 20-metre walk test on the time of assessment parameterised as two linear splines (to assess immediate changes and long-term changes, see Table 33, footnotes f and i), the pre-operative status and their interactions. Pre-operative level of pain/function status are defined using median scores as cut-off threshold (median WOMAC pain = 40, WOMAC function = 50, time to complete the 20-metre walk test = 22–1 seconds).

The long-term changes in function did not seem to be related with the pre-operative scores (see Table 33). A weak relationship between the self-reported immediate- and long-term function change was observed (–0.19) whereas high/small immediate improvement in the 20-metre walk test completion time was correlated with small/high long-term improvement (–0.49).
With regard to the ‘pain improvement journey’, large immediate pain improvements were correlated with high pre-operative pain level and small immediate improvement with low pre-operative level of pain (–0.30). This is illustrated in Figure 18c, which shows that immediate improvement was steeper for patients in the low pre-operative pain group than for those in the high pre-operative pain group (difference in slope between high/low groups, p < 0.01).
The long-term pain change was not related to the pre-operative pain scores (see Table 33). Those with larger/smaller immediate pain improvements also had smaller/larger long-term pain improvement (–0.38).
With regard to the pain–function inter-relation, that is, the influence on each other’s ‘journey’, no evidence of relationship between the pre-operative function level and the postoperative changes in pain was observed. Pre-operative pain level was weakly correlated (–0.22) with immediate self-reported function change. This relation was not found with the objective measure of function. Long-term change in function appeared independent of the pre-operative pain severity. The self-reported immediate functional change was weakly correlated (–0.24) with long-term pain change, although this relationship was not observed in the objective model. The immediate pain change was not related to long-term change in function.
Assessment of function using accelerometry in patients receiving hip replacement
Patients were also invited to wear an inertial ambulatory motion sensor incorporating accelerometers and gyroscopes during the completion of the 20-metre walk test (see Inertial sensor-based motion and gait analyses). Participants were asked to walk along a straight flat corridor at their own preferred speed. Participants wore their own clothes and shoes but high-heeled shoes were not permitted. After crossing the finish line, one last step was allowed to establish a complete stop avoiding a significant slowdown within the 20 metres. The exact distance covered (20 metres + the last step) was measured and used for the following analyses. The test was conducted on the 36 patients listed for a primary THR without any history of previous lower limb joint surgery and with available longitudinal WOMAC function and ambulatory gait analysis data.
The collected measures are presented in Table 34 and longitudinal changes are reported in Figure 19.
| Outcome measure | Pre-operative | 3 months | 12 months | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n = 36 | Median | IQR | r | Median | IQR | r | Median | IQR | r |
| WOMAC function | 48.50 | 35.50–71.30 | 91.20 | 81.00–95.60 | 95.60 | 83.80–100.00 | |||
| Speed (metre/second) | 0.97 | 0.81–1.12 | 0.51a | 1.12 | 0.96–1.30 | 0.31 | 1.20 | 1.08–1.33 | 0.45a |
| Cadence (step/minute) | 106.00 | 98.40–113.90 | 0.31 | 110.50 | 102.30–117.30 | 0.27 | 112.70 | 107.30–119.30 | 0.37 |
| Step time (second) | 0.57 | 0.53–0.61 | –0.31 | 0.54 | 0.51–0.59 | –0.24 | 0.53 | 0.50–0.56 | –0.37a |
| Step length (metre) | 0.54 | 0.48–0.62 | 0.47a | 0.60 | 0.53–0.68 | 0.25 | 0.64 | 0.57–0.70 | 0.32 |
| ROM (degree) | 5.70 | 3.60–6.30 | 0.43a | 6.40 | 5.30–7.90 | 0.14 | 7.50 | 5.50–8.40 | 0.14 |
| Step irregularity (variability in successive steps of the same leg) | 0.05 | 0.03–0.07 | –0.06 | 0.05 | 0.03–0.06 | –0.26 | 0.04 | 0.03–0.06 | –0.39a |
| Step asymmetry (ratio of asymmetry between steps time (seconds) of both legs) | 4.75 | 1.78–7.82 | –0.11 | 2.92 | 1.58–7.05 | –0.06 | 2.67 | 0.88–5.51 | –0.33a |
FIGURE 19.
Change in patient-reported function and gait parameters from pre-operative to 12 months after hip replacement. (a) WOMAC function; (b) speed; (c) cadence; (d) ROM; (e) step time; (f) step length; (g) irregularities; and (h) asymmetries. The mean trajectories are derived from the fixed effects of linear mixed models regressing teach outcome on the time of assessment parameterised as two linear splines (to assess immediate changes and long-term changes, see Table 33, footnotes f and i).
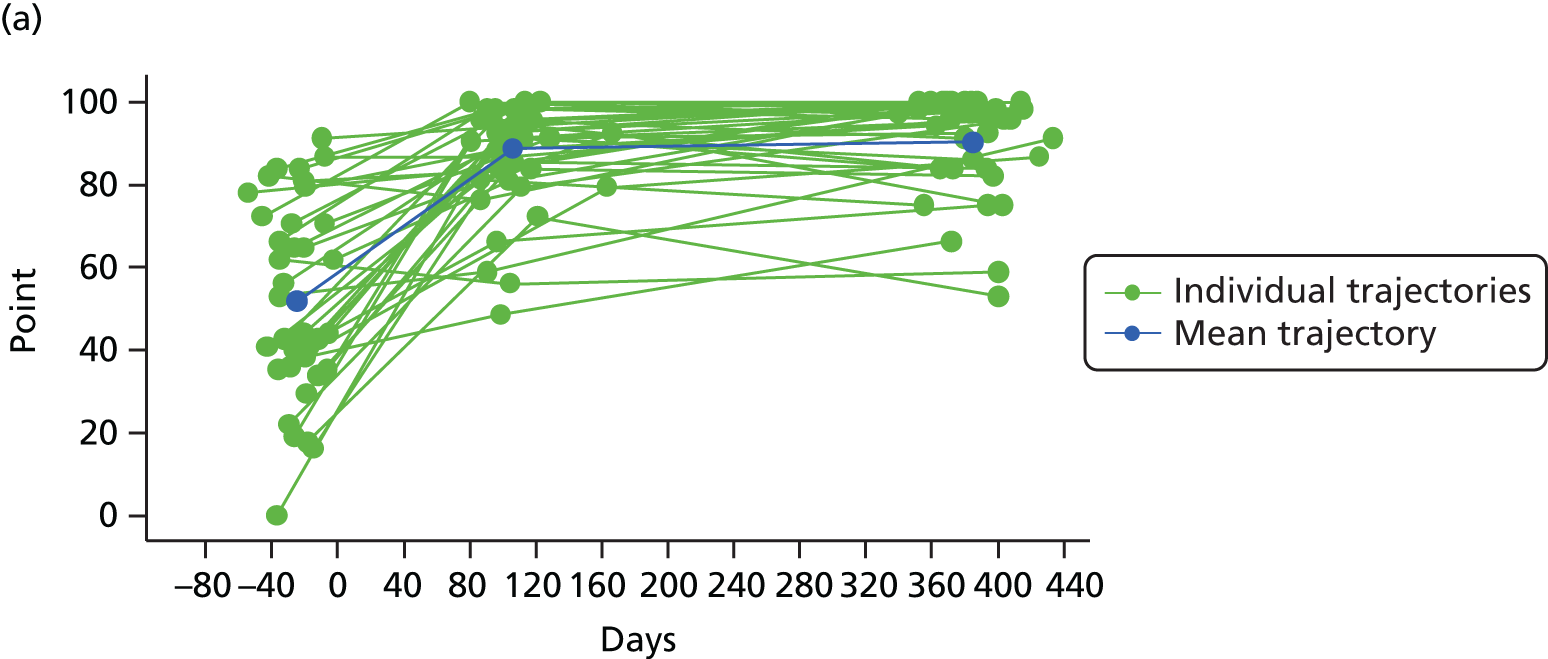
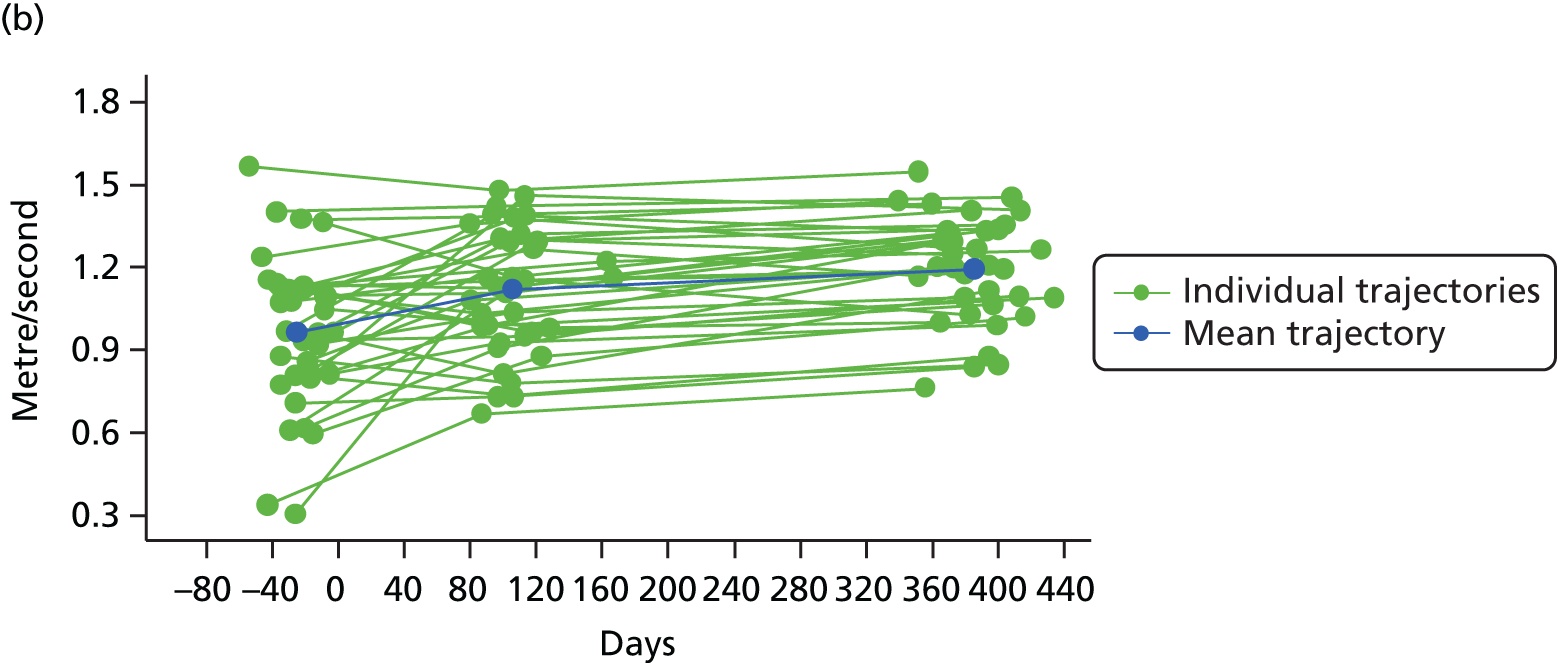
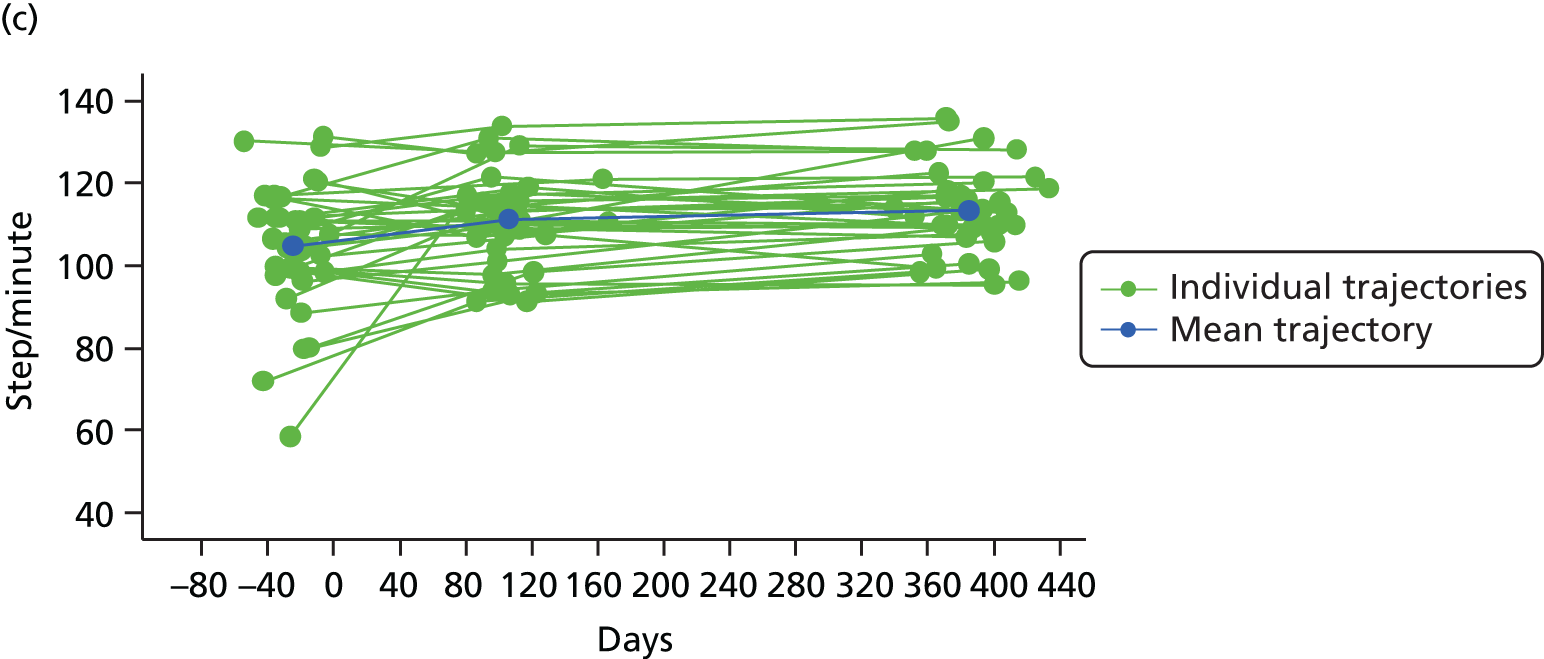
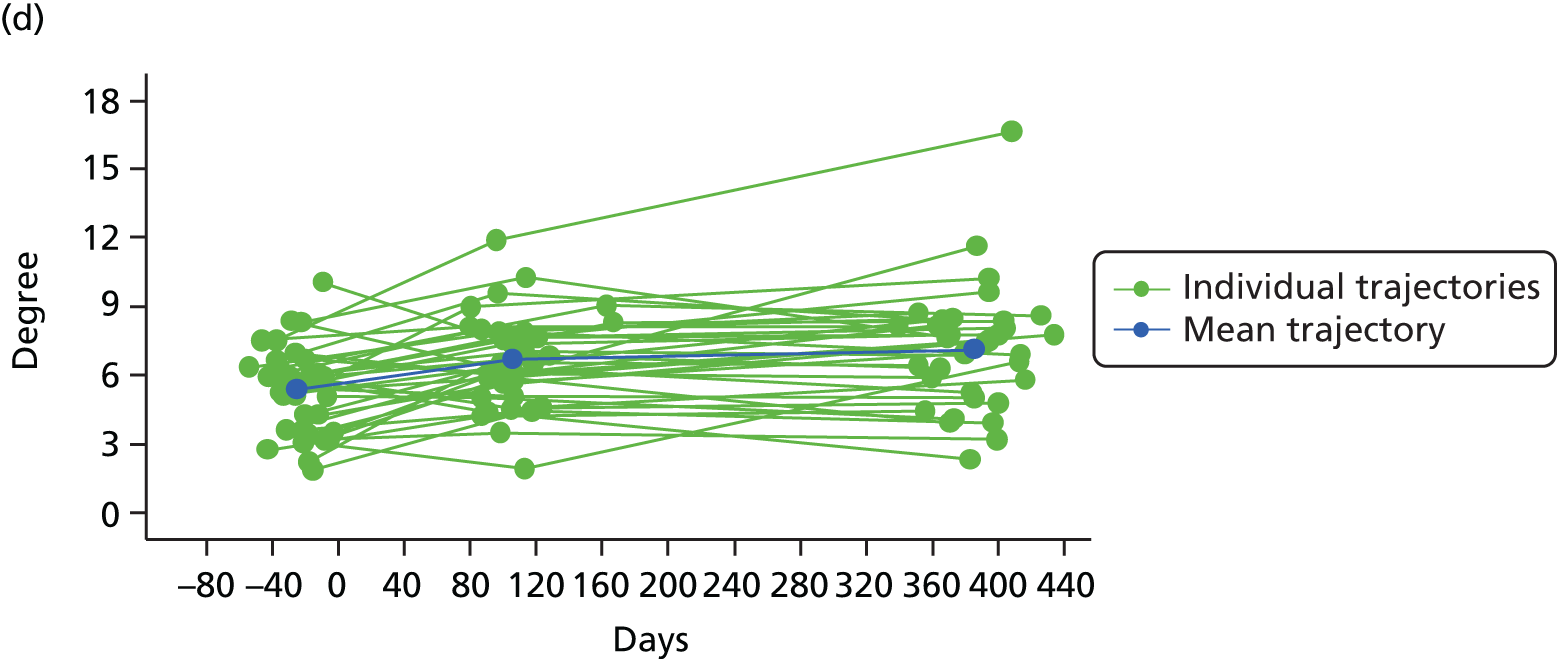
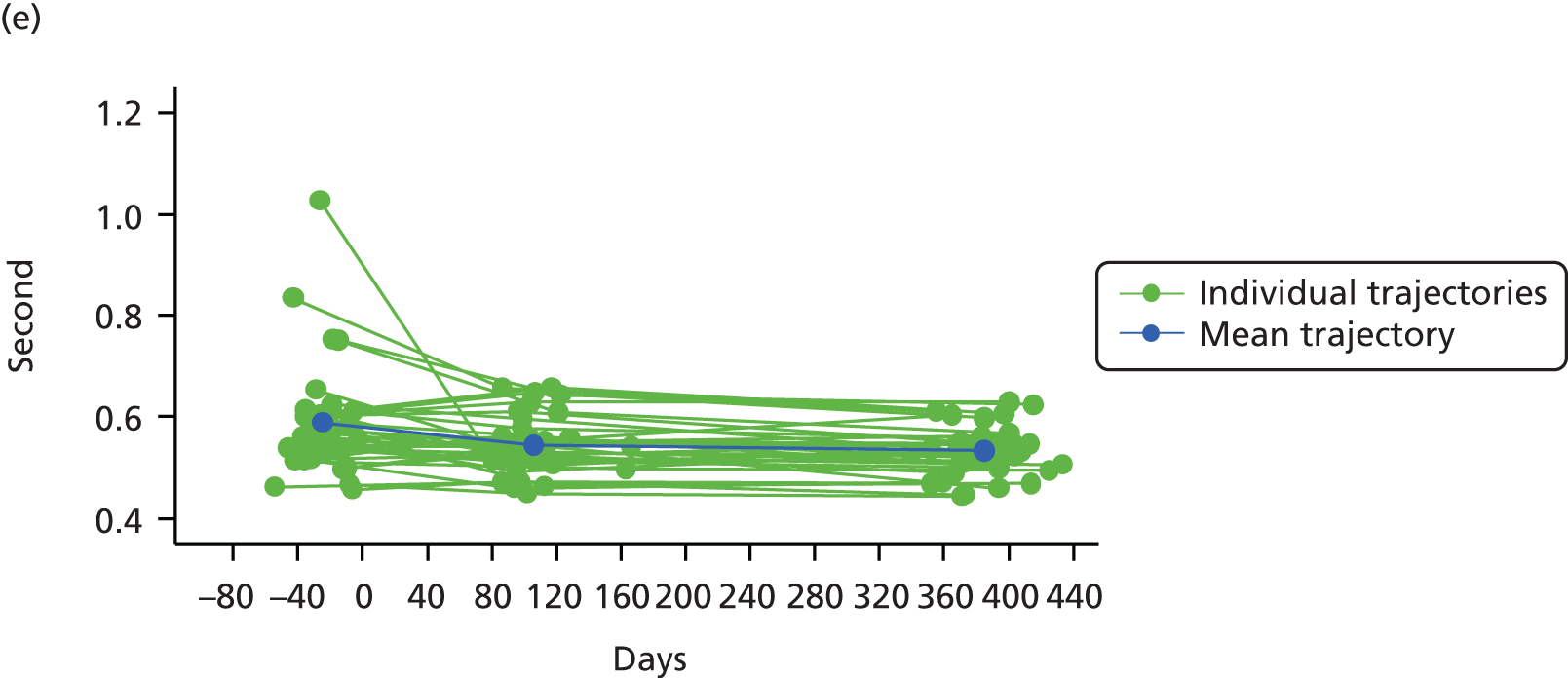
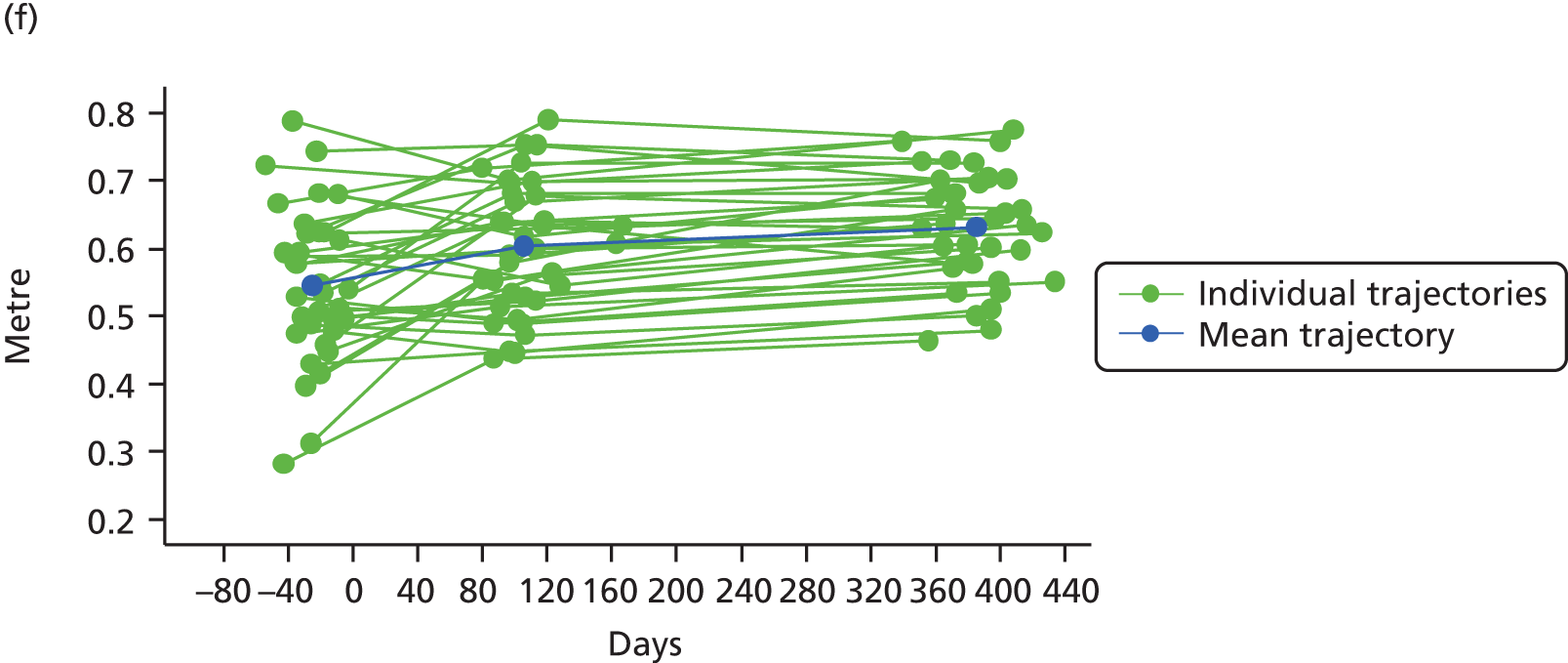
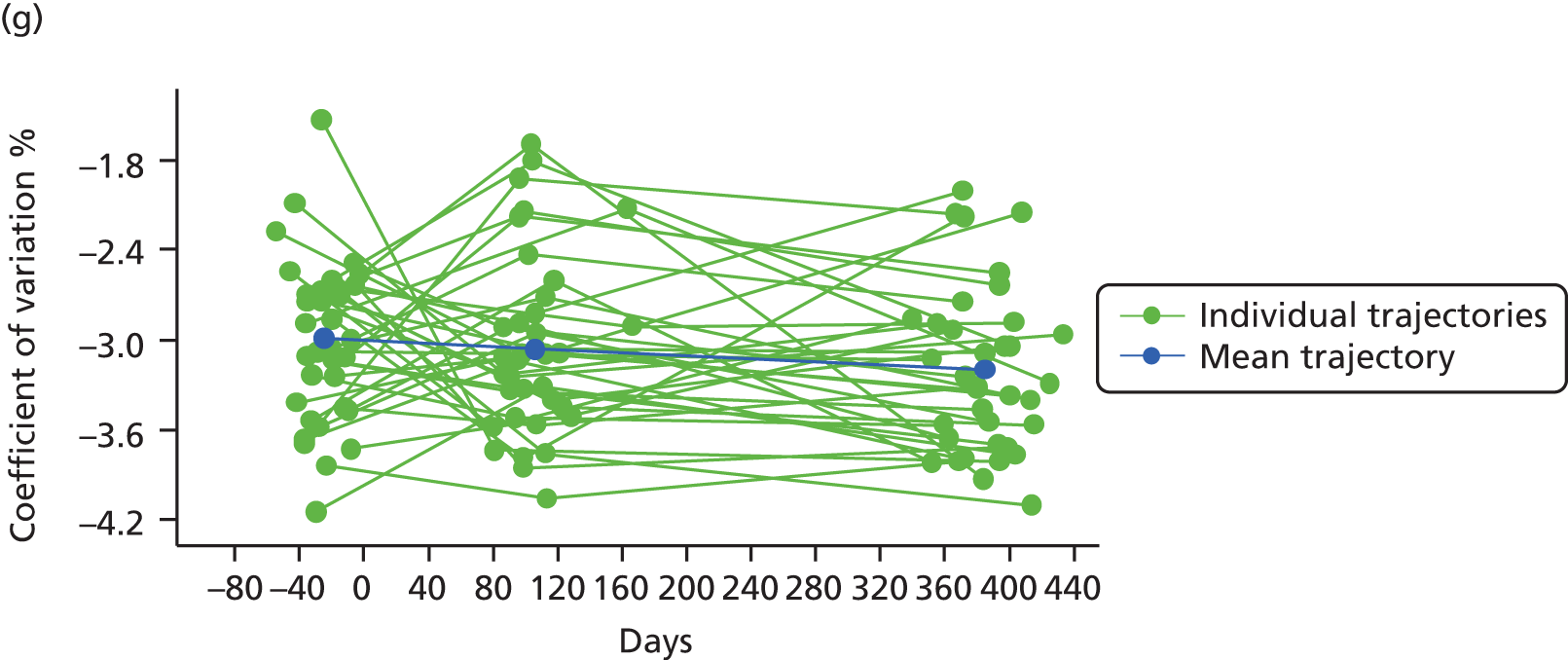
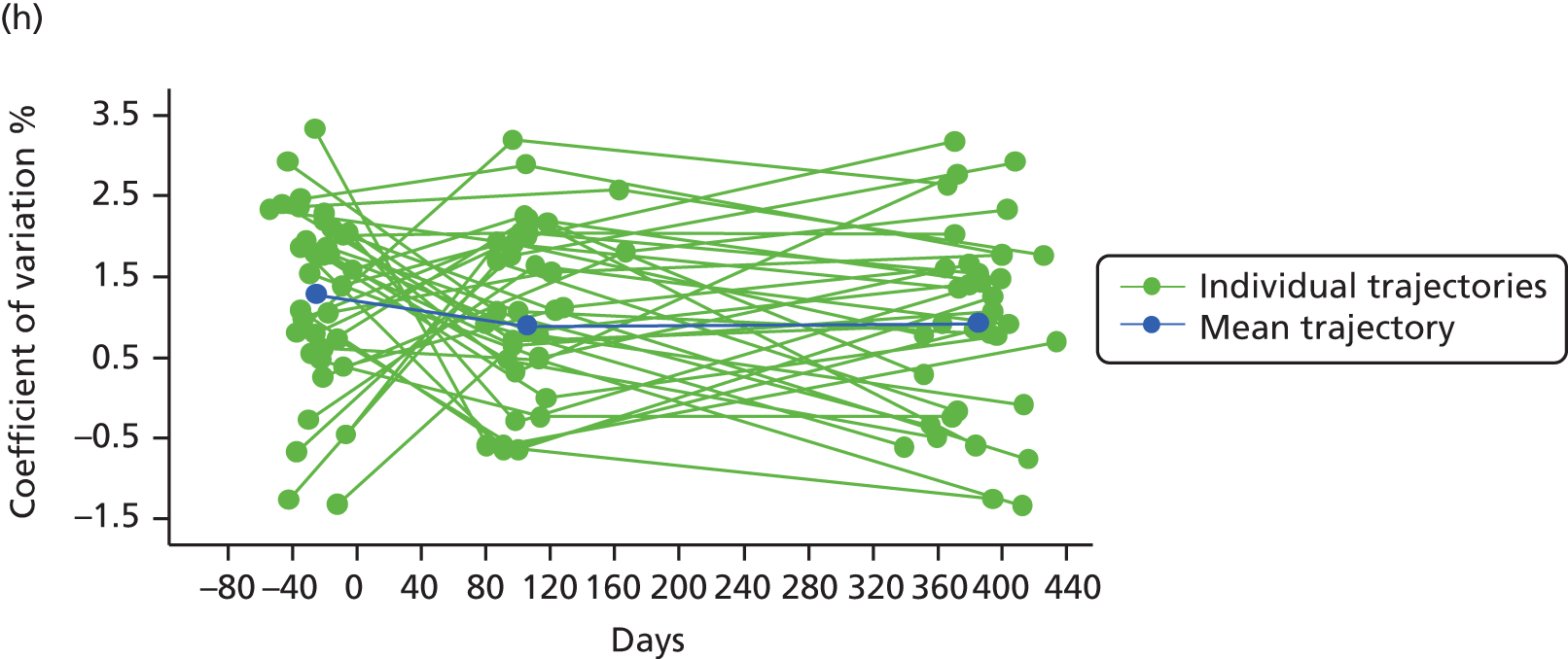
The 36 participants had the same pattern of WOMAC function recovery (see Figure 19) as the pattern observed in the overall sample (see Figure 13) – a large significant improvement within the first 3 months following the surgery (p < 0.0001) but no further improvement between 3 months and 12 months postoperatively (p > 0.05).
Pre-operatively, all the gait parameters had some weak to moderate correlations with WOMAC function (see Table 34). Apart from the ‘range of motion pelvic obliquity’ gait parameter, all the others maintained some weak correlations 12 months after the surgery. These findings suggest that the patient-reported measure partially reflect functional aspects captured by the gait analysis objective parameters.
Steps cadence and time to complete a step had the same course of longitudinal changes as the WOMAC function scores (see Figure 19).
Conversely, speed, ROM pelvic obliquity and step length continued to improve after the 3-month postoperative assessment (p < 0.0001).
The postoperative average changes in step irregularity and asymmetry were not statistically significant, suggesting that these aspects of function are not altered by THR surgery. However, there is a large heterogeneity in the patterns of individual changes (see Figure 19).
Discussion
These findings indicate that the pain and function trajectories in the first year following hip or knee surgery are similar, with most of the improvement occurring within the first 3 postoperative months. No clear indication of further improvement was observed after the 3 months postoperative assessment for those undergoing hip surgery. However, for those who had knee surgery, function measured objectively (20-metre walk test) continued to improve until 12 months post operation.
The absence of improvement after 3 months post operation could be viewed as an artefact resulting from the ceiling effect inherent to score bounded PROMs such as WOMAC limiting the ability to detect improvement for patients recovering very quickly. 343,344
However, the long-term mean changes associated with the objective function measures were marginal and much smaller than the one that occurred before 3 months and observed only among patients undergoing knee surgery. The gait analysis also revealed steeper slopes before 3 months and not all gait parameters had a statistically significant improvement beyond 3 months. Only residual changes might have to be expected after 3 months in proportion to those occurring before. The modest sample size of ADAPT limited our ability to adjust for factors known to be associated with the postoperative outcome such as age, sex, mental health and other comorbidities, and adjusted findings might have provided a slightly different picture. Our results are consistent with the existing literature. 48,341,342,345–350 Improvements in WOMAC physical function beyond 3 months were observed by Bachmeier and colleagues48 in patients who had undergone hip replacement. However, changes in WOMAC pain were marginal. For patients with knee replacement, changes beyond 3 months in both WOMAC function and pain were marginal. Heiberg and colleagues,347 in Norway, found a small but significant improvement after 3 months post operation among hip surgery patients for pain and subjective and objective measures of function [HOOS and 6-metre walk test (6MWT)]. Kennedy and colleagues348,349 found some further improvement between 3 and 6 months post knee or hip surgery but none thereafter using objective or PROMs measure of function [Lower Extremity Functional Scale (LEFS) and 6MWT] in a Canadian population. Halket and colleagues,341 in Canada, and Naylor and colleagues,350 in Australia, found hardly any improvement after 3 months in PROM measures of pain including WOMAC pain.
Interesting relationships have been found between the pre-operative score levels and postoperative changes. The pre-operative situation is negatively related to the immediate postoperative changes. The worse the situation before the surgery, the more likely the participants are to improve within the first few postoperative months; therefore, the better the pre-operative situation, the lower the immediate postoperative improvement. These findings can be induced by the ceiling effect inherent in the scoring system of PROMs in which those doing well have less room for improvement, magnifying the effect of those starting with lower scores. However, these negative correlations were also observed with the objective measure of function suggesting that more can be expected from the surgery when the patients have very poor pre-operative pain and function. Twelve months after their hip surgery, patients with poor pre-operative scores had caught up with those who had better pre-operative score. However, after their knee surgery, and despite a faster immediate improvement, participants with low function or high pain before their surgery still had significantly poorer function or pain level, even if the gap had reduced. This suggests that, even if there is a lot to expect from the joint surgery, any delay in the knee surgery might be associated with functional and/or pain degradation, which cannot be corrected by the operation. It is unlikely that these findings are driven by the revision/non-revision status of the knee participants. The prevalence of patients listed for a knee revision did not differ between high- and low-score groups; their pain or function pre-operative median scores did not differ from those listed for a first-time joint surgery.
For both groups, any intervention modifying the pre-operative pain level is likely to affect its course of immediate postoperative improvement, and the same is true for function. However, the relationship between pain and function differed between those having hip or knee surgery. For the hip patients, pain and function were interrelated whereas, for knee disease, no correlation between patients’ pre-operative score levels and postoperative changes was found. This could suggest that separate interventions specific to pain and function need to be designed for knee pre- and postoperative rehabilitation whereas more generic hip intervention could affect both domains simultaneously.
Our findings suggest that patients undergoing primary or revision surgery will experience an improvement in pain and function but the pattern of recovery will differ between these two types of surgery. Despite similar or better function and pain scores for patients undergoing a revision surgery than for those undergoing a primary joint surgery, a revision surgery does not seem to bring as much pain and functional improvement 12 months later. This finding is congruent with the existing literature351,352 and needs to be kept in mind when patients and clinicians discuss post-surgical expectations.
Finally, the pattern of functional recovery seems to be influenced by the method used to assess function, with significant long-term improvement observed in objective measure of function, but not with the self-reported measure. This could reflect the well-documented ceiling effect of the WOMAC function score. However, some of the gait analysis parameters, less subject to a ceiling effect, have a similar course of postoperative improvement as the self-reported measure of function. As the WOMAC function score is capturing information on several daily activities, this might also suggest that the potential loss of information induced by the use of a score-bounded instrument might not be as important as we think. It might also reflect the actual improvement pattern of some specific dimensions of function. Moreover, pain appears more correlated with the self-reported than with the objective measure of function. This might confirm previous work by Stratford and Kennedy353 which suggested an internal limitation of the WOMAC index scores: ‘activity overlap on the pain and function subscales plays a causal role in limiting the WOMAC physical function subscale’s ability to detect change’.
In all cases, these findings imply that it is valuable to use both self-reported and objective measures of function whenever possible. Doing so will capture a comprehensive longitudinal functional ability picture of patients undergoing joint replacement.
Discussion and conclusions
The ADAPT study aimed to investigate the different measures used to assess function in people undergoing hip or knee replacement and their responsiveness to the change resulting from joint replacement. The cohort included 263 people undergoing a mixture of hip and knee replacement, and primary and revision surgeries. This provided a mix of patients with a wide variety of different levels of disability, but the disadvantage of lacking homogeneity.
Our theoretical basis for the assessment of disability was the ICF, which differentiates between impairments, activities limitations and participation restrictions. We deliberately chose a number of different types of approach to functional assessment:
-
standard self-report measures widely used in rheumatology practice (the WOMAC and SF-12)
-
the Aberdeen measure, a recently developed self-assessment tool which differentiates between impairments, activities limitations and participation restrictions
-
clinician-administered tools widely used by orthopaedic practitioners (HHS and AKSS)
-
performance-based tests widely used by geriatricians (‘get-up-and-go test’, step tests, balance tests and walking time)
-
accelerometry tests, which are a recent development and have the promise of providing us with a more objective way or assessing function.
Measures were made immediately prior to surgery, at the standard 3-month follow-up visit and at 1 year.
Our key findings are:
-
There is no ‘right’ way to assess function in patients undergoing joint replacement.
We had hoped at the outset of this study that we might be able to conclude that some measures should be used, and others discarded, but the data do not support this. Arguably the ‘knee score’ component of the AKSS is of questionable value because it correlates poorly with other measures. Each of the different methods of assessing function appears to be measuring something a little different and is influenced by different covariates, so nothing is ‘right’ and nothing is ‘wrong’. The strongest correlations were between the different self-assessment measures and also between the different performance tests. However, the correlations between self-assessment measures and performance tests were much lower. This suggests that it might be wise to use one of each type of measure to obtain a satisfactory picture of the degree of functional loss in any individual patient. This is also confirmed by our comparisons of the longitudinal changes between patient-reported and performance test functional measures.
-
Self-assessment measures and functional tests are influenced by different factors.
We have shown that:
-
Pain affects every type of functional assessment measure.
-
Mental health status has a large influence on self-assessment measures but little effect on functional testing.
-
Age (and sex in the case of the hip replacement) affects laboratory tests of function but not self-assessment measures.
We interpret this as confirming previous research that suggests pain and function are inextricably linked in musculoskeletal disability, that people with anxiety or depression may assess themselves as being worse off than they objectively are and that the influence of age on functional tests may be mediated by sarcopenia (a hypothesis that requires further investigation).
The implication is that measures of function may need adjustment for pain, psychological status, age and perhaps muscle strength if we are to obtain a satisfactory picture of functional loss.
-
Range of joint motion is not a satisfactory surrogate measure for function.
It is relatively easy to assess the ROM of the hip or knee and this measure is commonly carried out in clinical practice. Health-care professionals and patients often assume that it provides a useful surrogate measure of osteoarthritis severity and/or functional problems. It constitutes an important part of both the HHS and the AKSS.
Our data indicate that ROM does not correlate well with other measures of disease severity and we would suggest that it should not be given any weight in patient assessment.
The ROM, within the ICF classification, is a measure of impairment. Pain is generally considered to be an impairment measure as well300 but in contrast to ROM correlates well with measures of activities limitations and participation restrictions. We would argue that it may be inappropriate to classify pain as an impairment measure in this context.
-
Function is improved 1 year after surgery in most, but not all, people.
Data on the outcomes of joint replacement are usually presented simply as the average difference in pain or function before and after surgery.
However, ‘averages’ do not tell us how many people might have got significantly (for them) better and, conversely, how many did not change or got worse. Our data are presented in such a way as to make these aspects of outcome totally explicit. They show that improvement that is both clinically and statistically significant will occur in some 90% of patients having a hip replacement and 70% of those having a knee replacement (an important difference between the two joint sites) and that, in contrast, some 5% of those having a hip replacement and 10% of those having a knee replacement will stay much the same or experience a deterioration in function 1 year after surgery. However, the degree of deterioration is rarely of clinical significance.
These data are important to patients and surgeons counselling them about the likely outcome of a joint replacement.
-
‘Ceiling effects’ are a major problem for many measures of function.
A possible limitation of a measure of function, and one that we were keen to explore, is that it reaches a ceiling, so that patients cannot improve further on that score, even if their clinical status does improve. Our data indicate that this is a significant problem for self-assessment measures such as WOMAC in the context of joint replacement and, to a lesser extent, for the clinician-administered HHS and AKSS. The longitudinal and gait analyses revealed that some ‘objective’ functional parameters were still improving after the surgery when the WOMAC function scores were reaching a plateau. However, other ‘objective’ gait parameters did have a similar pattern of improvement, suggesting that perhaps this ceiling effect is not necessarily as extensive as we might think and the WOMAC function score is still providing an acceptable reflection of functional change.
-
Walking speeds tell a different story.
We have put a reasonable amount of emphasis on the walking speed of patients in this study for three main reasons: first, it is a reasonably objective measure; second, it does not have a ceiling effect; and, third, it is a widely used surrogate measure of participation. 354–356 It also has some correlation with life expectancy. 357–362
Our data indicate that walking does not show such as good a response to joint replacement as most of the other measures used. Patients are more likely to be worse 1 year after surgery on their walking time than on other measures and the amount of improvement is rarely large. We believe that walking time is more dependent on other variables, many of which are age related, than the other measures.
-
Patients with hip and knee disease respond differently to joint replacement.
It is widely thought that people have a better outcome after a hip replacement than after a knee replacement, and our data support this idea. The likelihood of improvement and the amount of improvement is much greater for people having hip replacement than knee replacement, and there are subtle but important differences in the nature of the response and its determinants.
Patients and joint replacement surgeons need to consider hip and knee osteoarthritis as different diseases. Pain and function seem also to be differently inter-related over time between these two diseases. A more tailored course of intervention may be required for knee osteoarthritis to tackle pain and function, whereas an intervention tackling one of these domains is also likely to affect the other one for hip osteoarthritis.
-
The chances of a good response to joint replacement depend on the severity of the disease at the time of surgery.
Our data show this very clearly. This is not a new finding, but the ADAPT cohort does shed some new light on this important aspect of joint replacement.
Our findings suggest that we should think about the journey (the amount of change after surgery) and the destination (the ‘final’ point reached 1 year after surgery). Patients with very severe disease at the time of surgery are more likely to have a good journey (i.e. pain and functional ability will probably improve substantially), whether patients have hip or knee disease. But the destination differs for the two joint sites. Those with hip disease can have a similar good destination, irrespective of the starting point, whereas those with knee disease can never ‘catch up’ (i.e. have as good a final outcome or destination) if they start off with very severe disease at the time of surgery. This is an important finding with the possibility that we may be delaying surgery too long for many people with knee disease.
Finally, our findings show that patients listed for a revision surgery had slightly better pre-operative pain and similar functional ability than those listed for primary surgery. However, their postoperative gains do not seem to be as large as the improvement experienced by patients with primary joint surgery. Clinicians and patients should be aware of this to discuss and set the expectations from a revision surgery.
Chapter 6 Perioperative pain management with local anaesthetic infiltration in total hip and knee replacement: systematic review, randomised controlled trial, cost-effectiveness study, evaluation of nurse recruitment methods and qualitative study of views and experiences of trial participation and use of analgesics
Parts of this chapter have been reproduced with permission from Horwood and colleagues. 363 Crown Copyright © 2015 Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). Some parts have also been reproduced from Marques and colleagues364 © 2015 Marques et al. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated; and from Wylde and colleagues365 © 2011 Wylde et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In addition, some parts of this chapter have been reproduced from Marques and colleagues366 © 2014 Marques et al. ; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated; from Wylde and colleagues367 © 2015 International Association for the Study of Pain. This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 3.0 Licence; and from Mann C, Delgado D, Horwood J, Evaluation of internal peer-review to train nurses recruiting to a randomized controlled trial – Internal Peer-review for Recruitment Training in Trials (InterPReTiT), J Adv Nurs 2014, vol. 70, pp. 777– 90, with permission from John Wiley & Sons Ltd. © 2013 John Wiley & Sons Ltd. 368
Abstract
Background
We evaluated the clinical effectiveness and cost-effectiveness of perioperative local anaesthetic infiltration on long-term pain after joint replacement. We also studied the experience of trial involvement for health-care professionals and patients.
Methods
In the APEX RCTs, 322 patients receiving total hip and 316 patients receiving TKR were randomised to local anaesthetic infiltration or standard anaesthesia. All patients with TKR received a femoral nerve block (FNB). We also appraised existing research in a systematic review and conducted qualitative interviews with 24 patients and 15 health-care professionals about involvement in the APEX trials.
Results
In the APEX RCTs, local anaesthetic infiltration was associated with reduced pain 1 year after THR. In patients receiving TKR, there was no strong evidence that local anaesthetic infiltration reduced pain additional to that provided by FNB. From the NHS and PSS perspective, local anaesthetic infiltration is a cost-effective treatment option in THR.
Systematic review identified 36 RCTs. Local anaesthetic infiltration was effective in reducing short-term pain, particularly with addition of post-closure analgesia. In TKR, there was no evidence of benefit additional to a FNB.
Patients and health-care professionals recognised the importance of participating in RCTs.
Conclusions
Patients with THR should receive local anaesthetic infiltration, which is a cost-effective treatment option for the management of long-term pain. For patients receiving TKR, it may not provide additional benefit to FNB.
Background
Many patients undergoing total hip or knee replacement experience significant pain while in hospital. 70 In addition to the obvious benefits of reducing patient suffering and distress, good perioperative pain control has the added advantage of allowing early mobilisation and rehabilitation. 67 This minimises risks of complications such as deep-vein thrombosis, pulmonary embolus, muscle and joint contractures, physical deconditioning and chest infection. Early mobilisation allows early discharge, with short-term inpatient cost-savings for the NHS. Unfortunately, many of the traditional methods of achieving perioperative pain relief, such as spinal or epidural anaesthetics and the use of opioids, can preclude early mobilisation. 71,72
In diverse surgeries there is also evidence that increased levels of perioperative pain are associated with long-term pain, for example after breast surgery,369 inguinal hernia repair370 and thoracic surgery. 371 Large amounts of noxious input induced by surgery may contribute to the transition from acute to chronic pain through hyperexcitability and sensitisation of neurones within the central nervous system, leading to long-lasting amplification of pain signalling within the spinal cord. 66
Perioperative pain is managed with multimodal pain control strategies, with analgesics relieving pain with additive or synergistic effects. 372 Incorporation of high-volume local anaesthetic infiltration into the multimodal regimen has been used during different surgical procedures. In a systematic review of local anaesthetic infusion into wounds at the end of surgical procedures, Liu and colleagues373 identified studies dating from as early as 1983. The authors concluded that local anaesthetic infusion can improve analgesia, reduce opioid use and side effects, increase patient satisfaction and lead to reduced hospital stay. However, only one study included patients with TKR or THR. 374 Subsequently, more evaluations of local anaesthetic infiltration in joint replacement have been reported. 365
Aims
Our aims were to:
-
synthesise evidence from RCTs using systematic review and meta-analysis on the effectiveness of perioperative local anaesthetic infiltration for pain control in total hip and knee replacement
-
assess, in RCTs, the clinical effectiveness of local anaesthetic infiltration administered before wound closure as part of the multimodal regimen on the short- and long-term severity of joint pain after total hip or knee replacement
-
conduct an economic evaluation to determine the cost-effectiveness of local anaesthetic infiltration from a NHS and PSS perspective
-
identify and address training needs of nurses involved in patient recruitment to RCTs using embedded qualitative methods
-
explore, using qualitative methods, the experience of trial participation and surgery among patients and of trial involvement by health-care professionals.
Systematic review and meta-analysis of the effectiveness of perioperative local anaesthetic infiltration in total hip and knee replacement
Using systematic review methods and meta-analysis, our objective was to synthesise evidence from RCTs evaluating the effectiveness of perioperative local anaesthetic infiltration in reducing short- and long-term pain after hip and knee replacement. Secondary outcomes relate to opioid requirement, mobilisation and hospital stay.
Methods
| General methods | As described in Chapter 2, Systematic review methods |
| Databases and dates | MEDLINE, EMBASE and The Cochrane Library from inception to 11 December 2012. Citations of key articles in ISI Web of Science and reference lists |
| Search strategy | Hip or knee replacement/RCTs/anaesthesia and analgesia. MEDLINE search strategy based on terms in Appendix 3 |
| Study design | RCTs |
| Patients | Adults receiving primary total hip or knee replacement |
| Intervention | Local anaesthetic infiltration before wound closure. In addition, studies with intervention patients receiving additional delivery of analgesics through catheters and injections after wound closure. We excluded studies with interventions applied exclusively after wound closure |
| Controls | Patients receiving no local anaesthetic infiltration or placebo, or alternative analgesia regimens |
| Follow-up | Any time post operation |
| Data extraction | Country, baseline dates, participants (indication, age, sex), inclusion criteria, anaesthesia procedures common to randomised groups, intervention (including content of infiltrate, timing and volume), additional intervention group treatments, and control group treatment including placebo and alternative analgesia regimens |
| Outcomes | Pain at rest or during activity during hospital admission (24 and 48 hours after surgery); opioid consumption; mobilisation; length of hospital stay; and long-term pain and function. Complications were recorded and classified as serious (altered state of consciousness, atrial fibrillation, cardiac and hemodynamic changes requiring treatment, cardiac toxicity, central nervous system toxicity, dysarthria, dyspnoea, major surgical complications, pneumonia, pulmonary embolism, respiratory depression, seizures, swollen knee) or relating to deep infection. Vomiting and nausea |
| Quality assessment | Cochrane risk of bias: blind outcome assessment and losses to follow-up |
Statistical analyses
We conducted meta-analyses for pain at rest and during activity at 24 and 48 hours, length of hospital stay and complications. Follow-up times were approximated to the closest timing. When not specified, we assumed measurements were taken at rest. Analyses were carried out in Stata 12 and RevMan 5. Results are reported with 95% CIs and funnel plots were inspected to assess for small study effects. 375 Given the number of potential effect modifiers, we used random-effects models for all meta-analyses.
In meta-analysis, means and SD values of continuous variables, such as pain intensity, are required for intervention and control groups. Pain outcomes are sometimes reported as medians and IQRs owing to the recognised floor and ceiling effects of pain measures after successful pain management. This is less of an issue during early recovery. Kerr and Kohan376 presented distributions of pain intensity scores at rest and while walking on the first and second day after total hip or knee replacement. The proportion of people reporting no pain and, thus, reflecting a floor effect, ranged from 2% to 35% on days 1 and 2, and pain intensities showed near normal distributions.
When no measures of variance were reported, we contacted authors to obtain SDs. If necessary we estimated means and SDs from medians and IQRs96 from ranges using the method of Walter and Yao,377 or imputed values from the average per arm across studies.
As pain scores are reported on different scales we used the SMD as our measure of treatment effect in meta-analyses. 378 To help in the interpretation of these pooled estimates, we multiplied SMD values by the mean SD on the widely reported 100-point VAS scale for the outcome. As the use of this method is entirely dependent on the chosen ‘typical’ value,379 we used a mean SD calculated from control groups of all studies reporting the outcome. 96
For length of hospital stay, we compared means and medians in studies reporting both and examined individual-level data provided by some authors. 380,381 Distributions were right-skewed and followed a log-normal distribution. Some studies reported means and SDs directly. For studies that reported medians and IQRs, or ranges, we estimated means and SDs on the log scale and then back-transformed them to the natural (unlogged) scale. 382
Complications were compared between randomised groups using meta-analysis with summary statistics calculated as the Peto’s OR, the method of choice when event rates are low. 96,383
Analgesia regimen comparisons
Not all studies compared a local anaesthetic infiltration intervention with no intervention or placebo. Thus meta-analyses are reported separately for different regimen comparisons. These are summarised in Figure 20. Studies in patients with THR include comparisons A and B only. We report the combined comparison of groups (A + B) and further report A and B results separately. For studies in patients with TKR, we also report results from a combined meta-analysis across the first two subgroups (A + B), comparing local anaesthetic infiltration with or without further post-closure intervention against control. We further report all comparisons from A to E separately. Although we considered epidural analgesia as ‘usual care’ and, thus, included studies fort which this was used in comparisons A–E, we provide a summary of results.
FIGURE 20.
Systematic review of the effectiveness of perioperative local anaesthetic infiltration in total hip and knee replacement: possible anaesthesia regimens.

Heterogeneity and subgroup analyses
Heterogeneity within meta-analyses was studied using tau-squared and I2-statistics. 384 Sensitivity and subgroup analyses explored risk of bias in the study, use of additional analgesia delivered through a catheter or injection and inclusion of non-steroidal inflammatory agents or steroids in the infiltrate. We used meta-regression to quantify the differences in treatment effects between groups A and B.
Results
The review process is summarised in Figure 21. Searches identified 839 articles, of which 33 described 36 RCTs evaluating local anaesthetic infiltration in total hip or knee replacement. Characteristics of included studies are shown in Table 35 and a risk-of-bias assessment in Appendix 9. Thirteen studies were in patients undergoing THR385–396 or included a large majority of THR patients. 374 Twenty-three studies were in patients undergoing TKR. 380,381,386,397–414
FIGURE 21.
Systematic review of the effectiveness of perioperative local anaesthetic infiltration in total hip and knee replacement: flow diagram.
| Study; country (date) | Inclusion; total patients (intervention : control); mean age (intervention : control); percentage female (intervention : control) | Common treatment | Latest post-surgical follow-up; outcomes; losses to follow-up (intervention : control) | |
|---|---|---|---|---|
| Intervention treatment (volume of infiltrate) Further treatment (if given) |
Control | |||
| THR | ||||
| Aguirre and colleagues 2012;387 Switzerland (date not specified) | Minimally invasive; n = 76 (38 : 38); 58 : 58 years; 53% : 50% | Spinal anaesthesia, PCA morphine | 48 hours and to 3 months; i.v. morphine consumption, VAS pain at rest and with motion, electrocardiogram, skin inflammation or infection, satisfaction; 4 (2 : 2) lost to follow-up, three caused by catheter dislocation | |
| 20 ml of solution containing 60 mg of ropivacaine injected into wound before closure Further continuous infusion through catheter |
20 ml of placebo injection of saline Continuous infusion of saline through catheter |
|||
| Andersen and colleagues 2007;385 Denmark (2005–6) | OA, elective; n = 80 (40 : 40); 62 : 61 years; 90% : 85% | Spinal, postoperative oral oxycodone hydrochloride as required | 96 hours; VAS pain, length of stay, time to mobilisation, side effects and complications, motor block (Bromage scale); 5 (2 : 3) patients lost to follow-up | |
| 101.5 ml of solution containing 200 mg of ropivacaine, 30 mg of ketorolac (Toradol®, Roche) and 0.5 mg epinephrine infiltrated during surgery Further infiltrate through catheter intra-articularly 8 hours after surgery |
Epidural infusion | |||
| Andersen and colleagues 2007;388 Denmark (date not specified) | OA, uncemented, > 80 years; n = 37 (19 : 18); 62 : 64 years; 84% : 56% | Spinal anaesthesia, self-administered oral oxycodone as rescue medication | 6 weeks; VAS pain at rest and on leg raise up to 8 hours, WOMAC pain to day 4, WOMAC pain, stiffness and function after 1, 2, 4, 6 weeks, EQ-5D at 6 weeks, patient-controlled analgesic use to discharge, adverse events; 3 patients out of 10 not fitting inclusion criteria were identified retrospectively; no losses to follow-up | |
| 151.5-ml saline solution containing 300 mg of ropivacaine, 30 mg of ketorolac and 0.5 mg of adrenaline infiltrated during surgery Further infusion through catheter on day 1 |
Saline placebo infiltration Saline placebo infused through catheter on day 1 |
|||
| Bianconi and colleagues 2003;374 Italy (date not specified) | THR and TKR (78% THR), elective; n = 37 (18 : 19); 66 : 64 years; 79% : 83% | Spinal anaesthesia. Loading dose of i.v. morphine at end of surgery | 72 hours; VAS pain at 2, 4, 8, 12, 24, 48, 72 hours, opioid consumption (rescue medication), adverse events, length of hospital stay, patient satisfaction; no losses to follow-up | |
| 40 ml of saline containing 200 mg of ropivacaine (Naropin®, Astra Zeneca) infiltrated at end of surgery Further ropivacaine infusion through catheter for 55 hours after closure i.v. saline infusion for 24 h after surgery |
No placebo infiltration during surgery Saline infusion through catheter for 55 hours after closure i.v. morphine plus ketorolac infusion for 24 hours |
|||
| Busch and colleagues 2010;389 UK (2003–5) | OA, age < 80 years; n = 64 (32 : 32); 61 : 65 years; 50% : 54% | General or spinal anaesthesia, PCA morphine | 2 years; VAS at rest and activity, morphine consumption (PCA), VAS satisfaction, complications, HHS, WOMAC, length of hospital stay; no losses to follow-up | |
| 100 ml of saline solution containing 400 mg of ropivacaine (HCl®, Astra Zeneca), 30 mg of ketorolac (Toradol®, Astra Zeneca), 5 mg of morphine (Sabex) and 0.6 ml of epinephrine (1 : 1000) (Abbott Laboratories) infiltrated during surgery | No placebo infiltration | |||
| Dobie and colleagues 2012;390 UK (2006–7) | OA or RA; n = 96 (50 : 46); 67 : 67 years; 38% : 52% | Spinal, general, i.v. morphine after surgery as required | 6 days; VAS at 24 hours, morphine consumption, walking and stair test, mobilisation velocity and day, sit-to-stand test, home readiness, hospital stay, ILAS; 4 (4 : 0) patients did not receive intervention as planned. ITT results. Some data missing for one control | |
| 160 ml of saline solution containing 200 mg of levobupivacaine and adrenaline | No local infiltration | |||
| Lee and colleagues 2009;391 South Korea (2006–7); note: additional pre-emptive analgesia and epidural | 13% OA, 72% osteonecrosis; n = 60 (30 : 30); 51 : 55 years; 37% : 43% | General anaesthesia | 5 days; VAS pain, ambulation, doses of parenteral analgesia, time to straight leg raise, complications; no losses to follow-up described | |
| Pre-emptive analgesia with oral oxycodone and celecoxib. Epidural anaesthesia 90 ml of saline solution containing 5 mg of morphine, 40 mg of methylprednisolone and 6.8 mg of ropivacaine infiltrated during surgery postoperative oral oxycodone and paracetamol |
No pre-emptive analgesia No epidural No injection during surgery postoperative i.v. PCA and oral and injected analgesics as required |
|||
| Liu and colleagues 2011;392 China (2008–9) | OA, ASA I–III, < 80 years; n = 82 (41 : 41); 74 : 74 years; 75% : 77% | Spinal anaesthesia, PCA morphine | 15 days and 9 months (range 6–12 months) for infection; morphine use, VAS pain, surgical outcome, mobilisation (time to straight leg raise and 90 degree flexion); 2 (1 : 1) lost to follow-up | |
| 60 ml of saline solution containing 5 mg of morphine, 30 mg of bupivacaine, 1 ml of betamethasone and 0.5 ml of epinephrine infiltrated during surgery | 60 ml of saline infiltrated during surgery | |||
| Lu and Li 2010;393 China (date not specified) | Primary; n = 40 (20 : 20); no information on age and sex of patients | No description of common anaesthesia except PCA | 48 hours; VAS pain, use of PCA pump, adverse drug reactions; no losses to follow-up apparent | |
| COX-2 inhibitor before surgery 100-ml solution containing 0.15% of ropivacaine infiltrated at end of surgery COX-2 inhibitor after surgery |
No COX-2 inhibitor before surgery 100-ml saline placebo infiltrated at end of surgery No COX-2 inhibitor after surgery |
|||
| Lunn and colleagues 2011;394 Denmark (2009–10) | Age > 18 years; n = 120 (60 : 60); 67 : 67 years; 55% : 65% | Spinal with or without general. Multimodal oral analgesia | 8 hours and to discharge; VAS pain at rest and during walking and passive hip flexion, oxycodone consumption, complications; no losses to follow-up except ‘pain during walking’ with 18 (11 : 7) lost to follow-up | |
| 150-ml saline solution containing 0.2% ropivacaine (AstraZeneca) and 10 µg/ml of epinephrine infiltrated during surgery | 150 ml of saline placebo infiltrated during surgery | |||
| Murphy and colleagues 2012;395 Ireland (2009–10) | OA; n = 91 (45 : 46); 57 : 54 years; 49% : 38% | Spinal, PCA opioid analgesia | 72 hours; WOMAC pain, McGill Pain Questionnaire, VAS pain, morphine consumption, complications; 13 (6 : 7) lost to follow-up but some analyses used multilevel modelling to handle missing data | |
| 60 ml of saline containing 150 mg of levobupivacaine infiltrated during surgery | 60 ml of saline placebo | |||
| Parvataneni and colleagues Hip 2007;386 USA (2005–6) | OA; n = 71 (35 : 36); 64 : 61 years; 40% : 39% | Spinal anaesthesia with or without FNB | 3 months; VAS pain, total narcotic dose, functional recovery including time to straight leg raise, side effects of narcotic use, patient satisfaction; no losses to follow-up reported | |
| Intraoperative infiltration of 200–400 mg of bupivacaine, 4–10 mg of morphine sulphate, 300 µg of epinephrine, 40 mg of methylprednisolone acetate, 75 mg cefuroxime and 22 ml of saline. Total volume approximately 33 ml | No infiltration during surgery Post-surgical PCA |
|||
| Rikalainen-Salmi and colleagues 2012;396 Finland (2009–10) | OA, ASA I–III; n = 60 (30 : 30); 65 : 66 years (followed up); 66% : 61% (followed up) | Spinal, propofol if required, oxycodone (Oxanest®, Nycomed or OxyNorm®, Mundipharma) rescue medication | 8 weeks; NRS pain at rest and motion, oxycodone consumption, mobilisation, fulfilment of discharge criteria, satisfaction, adverse events and complications; 3 (1 : 2) lost to early follow-up; 7 (4 : 3) lost to long-term follow-up | |
| 101 ml of solution containing 125 mg of levobupivacaine (Chirocaine®, Abbott) and 30 mg of ketorolac (Toradol®, Roche) infiltrated during surgery 21 ml of solution containing 100 mg of levobupivacaine and 30 mg of ketorolac administered through catheter on morning of first postoperative day |
Intrathecal morphine (2 mg/ml, Nycomed) No placebo infiltration Sham catheter attached to skin with 21 ml of air administered on morning of first postoperative day (not inserted into joint) |
|||
| TKR | ||||
| Affas and colleagues 2011;397 Sweden (2007–8) | 77.5% OA, 22.5% RA, age > 18 years, ASA I–III, primary; n = 40 (20 : 20); 67 : 69 years; 45% : 60% | Spinal anaesthesia, PCA morphine | 24 hours; NRS pain intensity at rest and on movement, 24-hour morphine PCA consumption; no losses to follow-up. Missing data analysis reported | |
| 110 ml containing approximately 200 mg of ropivacaine (Narop®, Astra Zeneca), 20 mg of ketorolac (Toradol®, Roche) and 0.33 mg of epinephrine (Adrenalin®, NM Pharma) infiltrated during surgery Further intra-articular infiltration through catheter after surgery |
FNB (Narop®, Astra Zeneca) i.v. ketorolac (Toradol®, Roche) after surgery No placebo infiltration |
|||
| Andersen and colleagues 2010;398 Denmark (2007–8) | Age > 18 years; n = 49 (24 : 25); 67 : 69 years; 43% : 26% | Spinal anaesthesia, PCA morphine | 72 hours and to discharge, infection to 30 days; VAS/NRS pain, morphine requirement, side effects and complications, time to achieve discharge criteria, length of stay; 9 (3 : 6) patients lost to follow-up | |
| 151.5 ml of saline solution containing 300 mg of ropivacaine, 30 mg of ketorolac and 0.5 mg of epinephrine infiltrated during surgery Further continuous infusion through catheter after closure |
Epidural infusion of ropivacaine Postoperative i.v. ketorolac |
|||
| Busch and colleagues 2006;399 Canada (date not specified) | Age < 80 years; n = 64 (32 : 32); 66 : 70 years; 50% : 59% | General or spinal anaesthesia, PCA morphine | 6 weeks; VAS at rest and activity, morphine consumption (PCA), VAS satisfaction, complications, Knee Society Score, WOMAC, length of hospital stay; no losses to follow-up | |
| 100 ml of saline solution containing 400 mg of ropivacaine, 30 mg of ketorolac (Toradol®, Roche), 5 mg of epimorphine and 0.6 ml of epinephrine (1 : 1000) infiltrated during surgery | No placebo infiltration | |||
| Carli and colleagues 2010;400 Canada (2007–8) | OA, tricompartmental, cemented.; n = 40 (20 : 20); 71 : 71 years; 75% : 70% | Spinal, FNB and periarticular knee catheter inserted, PCA morphine | 6 weeks; morphine consumption, NRS pain at rest and walking, functional capacity, ability to walk 30 m, physical activity, SF-12, WOMAC; no losses to follow-up | |
| Solution of ropivacaine (0.2%), 1 ml of ketorolac (30 mg/ml), and 0.5 ml of epinephrine (1 mg/ml) with a total volume of 100 ml infiltrated during surgery Further infusion through catheter after closure |
Continuous FNB Saline injection Post-surgical infusion of saline |
|||
| Chen and colleagues 2012;401 China (2008) | OA, age < 76 years.; n = 81 (40 : 41); 66 : 65 years; 75% : 78% | Spinal anaesthesia, PCA morphine | 15 days and infection to 6 months; total morphine consumption, VAS pain at rest and motion, time to straight leg raise and 90 degree flexion, adverse events including delayed infection; 1 (0 : 1) patient lost to follow-up | |
| Intraoperative injection of a solution of magnesium sulphate (50 mg/kg) and 190 mg ropivacaine in normal saline to a volume of 100 ml | Intraoperative intra-articular injection of 100 ml normal saline | |||
| Essving and colleagues 2010;402 Sweden (2007–8) | OA, ASA I–III, 20–85 years; n = 48 (24 : 24); 72 : 70 years; 54% : 54% | General anaesthesia, PCA morphine | 3 months; PCA morphine consumption, VAS pain at rest and on knee flexion, time to home readiness, length of hospital stay, surgical outcome, functional outcome tests, OKS, EQ-5D, patient satisfaction, adverse events; 1 (0 : 1) patient lost to follow-up | |
| 116 ml of saline containing 300 mg of ropivacaine, 30 mg of ketorolac and 0.5 mg of epinephrine infiltrated during surgery. 50 ml of saline containing 100 mg of ropivacaine infiltrated before closure Further injection of mixture 21 hours after closure |
No placebo injections during surgery Post-surgical injection of saline at 21 hours |
|||
| Essving and colleagues 2011;403 Sweden (2009–10) | OA, ASA I–III, age 40–85 years; n = 50 (25 : 25); 71 : 71 years; 64% : 60% | Spinal anaesthesia, PCA morphine | 3 months; VAS pain, PCA morphine, verbal rating scale of satisfaction, functional tests, time to home readiness, OKS, EQ-5D, adverse events; 2 (0 : 2) patients lost to follow-up | |
| Spinal plus intrathecal saline Injection during surgery of 400 mg of ropivacaine (160 ml), 30 mg of ketorolac (1 ml) and 0.5 mg of epinephrine (5 ml) Further infiltrate through catheter on days 1 and 2 |
Spinal plus intrathecal morphine No injection during surgery Post-surgical infusion of saline through catheter |
|||
| Fu and colleagues 2009;404 China (2006–7) | OA, age < 80 years; n = 80 (40 : 40); 69 : 68 years; 75% : 78% | Spinal anaesthesia, PCA morphine | 15 days except ROM 90 days, infection 12 months; morphine consumption, VAS pain at rest and activity, ROM, time to straight leg raise, surgical outcomes, complications; no losses to follow-up. Missing data imputation described | |
| 60 ml of saline containing 5 mg of morphine, 30 mg of bupivacaine and 1 ml of betamethasone infiltrated during surgery | 60 ml of saline infiltrated during surgery | |||
| Fu and colleagues 2010;405 China (2008–9) | OA, age < 80 years; n = 100 (50 : 50); 68 : 67 years; 76% : 80% | Spinal anaesthesia, PCA morphine | 15 days except ROM at 90 days and infection to mean 7.5 months (range 6–9 months); VAS pain, morphine consumption (PCA and intramuscular), time to straight leg raise and 90 degree flexion, surgical outcomes, adverse reactions; no losses to follow-up | |
| Oral COX-2 inhibitor and tramadol 1 day before to 1 month after surgery 50 ml of saline containing 5 mg of morphine, 150 mg of ropivacaine, 0.5 ml of adrenaline and 1 ml of betamethasone infiltrated during surgery |
Oral placebo 1 day before to 1 month after surgery 50 ml of saline placebo infiltrated during surgery |
|||
| Han and colleagues 2007 interventions 1 and 2;406 Korea (2005–6); note: 2 intervention groups | Primary; n = (intervention 1 30 : intervention 2 30 : control 30); 69 : 68 : 67 years; 90% : 80% : 90% | Spinal and epidural anaesthesia, PCA morphine | 48 hours; incidence of booster PCA for 24 hours, amount of i.v. tramadol, VAS pain at rest and exercising, side effects, range of flexion; no losses to follow-up reported | |
| 50 ml saline solution containing 300 mg of ropivacaine, epinephrine (0.25 ml, 1 : 200,000) and 5 mg of morphine injected before wound closure 50 ml of saline solution containing 300 mg of ropivacaine and epinephrine (0.25 ml, 1 : 200,000) injected before wound closure |
50 ml of saline placebo | |||
| Koh and colleagues 2012;407 Korea (2008–9) | OA, unilateral; n = 101 (49 : 52); 70 : 70 years; 89% : 91% | FNB, spinal anaesthesia, PCA morphine | 7 days; VAS pain at rest (day 1) and on movement (days 4 and 7), PCA opioid consumption, use of rescue medication, pain compared with expectations, functional recovery (straight leg raise and flexion), satisfaction, side effects and complications, length of stay; 14 (4 : 10) did not receive treatment as planned. Results reported by ITT | |
| 50 ml of saline containing 300 mg of ropivacaine, 10 mg of morphine sulphate, 30 mg of ketorolac, epinephrine of 0.3 mg and 750 mg of cefuroxime injected/infiltrated during surgery | No placebo infiltration reported | |||
| Krenzel and colleagues 2009;408 USA (2007–8) | 96% OA elective.; n = 67 (35 : 32), one patient with staged bilateral TKR included twice; 67 : 65 years; 57% : 72% | FNB, spinal anaesthesia, PCA fentanyl | 24 hours; PCA fentanyl consumption, NRS pain, functional tests, time to straight leg raise, ambulation distance, surgical outcomes, adverse events; no losses to follow-up | |
| 20 ml infiltration of 100 mg of ropivacaine during surgery | 20 ml of saline placebo infiltrated during surgery | |||
| Mahadevan and colleagues 2012;409 UK (date not specified) | OA or RA, unilateral; n = 52 (26 : 26); 68 : 67 years; 54% : 58% | FNB, general anaesthesia, PCA morphine | 48 hours and to discharge; VAS pain, morphine consumption, active ROM, length of hospital stay; no losses to follow-up reported | |
| 25 ml of saline containing 0.375% levobupivacaine infiltrated during surgery | Sciatic nerve block No placebo infiltration reported |
|||
| Meftah and colleagues 2012;410 USA (2010–11) | Unilateral; n = 90 (45 : 45); 65 : 67 years; 64% : 64% | Pre-emptive analgesia | 3 days and to discharge, 6 months for infection, fracture and reoperation; pain at rest and ambulation, readiness for discharge; 1 (1 : 0) lost to all follow-up, 6 (4 : 2) lost to readiness for discharge follow-up | |
| 45.1 ml of saline solution containing 400–800 mg of marcaine, 8 mg of morphine sulphate, 0.3 mg of adrenaline, 750 mg of antibiotic and 40 mg of corticosteroids injected during surgery | FNB. PCA epidural No placebo injection reported |
|||
| Ng and colleagues 2012;411 China (2008–10); note: crossover design. Patients having both knees replaced | OA; n = 32 (16 : 16) surgeries but 16 patients only having two TKRs 3 months apart; 70 : 70 years; 88% : 88% | General anaesthesia, remifentanil infusion, PCA morphine | 3 days and to discharge; pain score at rest and motion, total morphine consumption, Knee Society Score, ROM, quadriceps power, satisfaction, adverse events and complications; no losses to follow-up reported | |
| 101.5 ml of saline solution containing 300 mg of ropivacaine, 1 mg of adrenaline and 40 mg of triamcinolone acetonide infiltrated during surgery Femoral catheter inserted and saline infused |
FNB Wound infiltration with 101.5 ml of saline |
|||
| Parvataneni and colleagues 2007;386 USA (2005–6) | OA; n = 60 (31 : 29); 69 : 71 years; 45% : 52% | Spinal anaesthesia with or without FNB | 3 months; VAS pain, total narcotic dose, functional recovery including time to straight leg raise, side effects of narcotic use, patient satisfaction; no losses to follow-up reported | |
| Intraoperative infiltration of 200–400 mg of bupivacaine, 4–10 mg of morphine sulphate 300 µg of epinephrine, 40 mg of methylprednisolone acetate, 75 mg of cefuroxime and 22 ml of saline. Total volume approximately 33 ml | No infiltration during surgery FNB at end of surgery Post-surgical PCA Effort to conceal allocation but no sham epidural |
|||
| Spreng and colleagues no i.v. injection 2010;412 Norway (2007–9) | Unilateral, non-cemented, no patella resurfacing, age > 17 years, ASA I–III; n = 68 (34 : 34); 67 : 66 years; 61% : 67% | Spinal. Propofol if indicated. PCA morphine | 72 hours and to discharge; VAS at rest and during knee flexion, morphine consumption, functional recovery, length of stay, satisfaction, mobilisation including walking distance, adverse events; 2 (1 : 1) lost to follow-up | |
| 150 ml of saline solution containing 150 mg of ropivacaine, 0.5 mg of epinephrine, 30 mg of ketorolac and 5 mg of morphine infiltrated during surgery Knee injected through catheter with ropivacaine and ketorolac solution after 22–24 hours i.v. injection with saline at 22–24 hours |
48 hours of epidural analgesia as soon as spinal started to wear off No wound infiltration during surgery. No injections through sham catheter. No sham epidurals |
|||
| Spreng and colleagues with i.v. injection 2010;412 Norway (2007–9) | Unilateral, non-cemented, no patella resurfacing, age > 17 years, ASA I–III.; n = 68 (34 : 34); 67 : 66 years; 61% : 67% | Spinal anaesthesia, propofol if indicated, PCA morphine | 72 hours and to discharge; VAS at rest and during knee flexion, morphine consumption, functional recovery, length of stay, satisfaction, mobilisation including walking distance, adverse events; 2 (1 : 1) lost to follow-up | |
| 150 ml of saline solution containing 150 mg of ropivacaine and 0.5 mg of epinephrine infiltrated during surgery In addition, i.v. injection of 1 ml of ketorolac (30 mg/ml) and 5 ml of morphine (1 mg/ml) Knee injected with saline at 22–24 hours (catheter) i.v. injection with ketorolac at 22–24 hours |
48 hours of epidural analgesia as soon as spinal anaesthetic started to wear off No wound infiltration during surgery. No injections through sham catheter |
|||
| Thorsell and colleagues 2010;381 Sweden (not specified) | OA or RA; n = 85 (46 : 39); 69 : 72 years (followed up); 81% : 73% (followed up) | Not specified, probable PCA | 4 days and to discharge; VAS pain, morphine consumption, satisfaction, mobilisation getting out of bed without assistance, walking with crutches), functional recovery, length of hospital stay; 21 (13 : 8) patients lost to follow-up data reported | |
| Spinal anaesthesia 156 ml of solution with 300 mg of ropivacaine, 0.5 mg of adrenaline and 30 mg of ketorolac infiltrated during surgery Further infiltrate through catheter intra-articularly on postoperative day 1 |
Spinal or epidural analgesia No placebo infiltration reported Postoperative pain relief with ropivacaine infusion through epidural catheter |
|||
| Toftdahl and colleagues 2007;380 Denmark (2005–6) | OA with planned spinal anaesthesia; n = 77 (40 : 37); 70 : 72 years; 63% : 60% | Spinal and after surgery immediate-release oxycodone and i.v. morphine if required | 4 days and to discharge; NRS pain, opioid consumption, mobilisation (able to walk > 3 metres, able to hold quadriceps tension for > 5 seconds), length of hospital stay, adverse events and complications; 4 (3 : 1) patients lost to follow-up | |
| 152 ml of solution containing 300 mg of ropivacaine, 30 mg of ketorolac and 0.5 mg of epinephrine infiltrated during surgery Further infiltrate through catheter intra-articularly on day of surgery and postoperative day 1 |
FNB prior to spinal anaesthesia No placebo infiltration Post-surgical continuous FNB |
|||
| Vendittoli and colleagues 2006;413 Canada (2003–4) | 95.2% OA; n = 42 (22 : 20); ages not specified; 73% : 70% | Spinal anaesthesia, PCA morphine | 5 days and to discharge; VAS pain at rest and during physiotherapy exercise, PCA morphine consumption, functional recovery, side effects; no losses to follow-up described | |
| 160 ml of solution containing, in total, 400 mg of ropivacaine (Naropin®, Astra Zeneca), 30 mg of ketorolac and 0.5 ml of adrenaline (1 : 1000) infiltrated during surgery Infiltrate through catheter intra-articularly on day 1 |
No placebo infiltration | |||
| Zhang and colleagues 2007;414 China (2006–7) | Unilateral; n = 60 (30 : 30); mean 68 years; 83% : 80% | PCA morphine | 72 hours; VAS pain at rest and activity, functional recovery; no losses to follow-up described | |
| 60 ml of solution containing 0.25% bupivacaine, epinephrine (1 : 200,000) and 10 mg of morphine infiltrated during surgery | No placebo injection | |||
Small study effects
Inspection of funnel plots for each meta-analysis gave no strong indication of publication bias or small study effects, but numbers of studies in individual analysis groups were small, such that assessment of asymmetry was difficult.
Total hip replacement
In 13 studies with 909 patients identified by searches, the mean number of patients with THR randomised was 70 (range 37–120). We assessed that 10 studies were at low risk of bias while three studies had unclear risk of bias owing to uncertainty about blinding of outcome assessments.
Results of the meta-analysis are summarised in Table 36.
| THR studies | n | Method | Pooled effect size (random effects) | 95% CI | p-value | I2 (%) | τ2 |
|---|---|---|---|---|---|---|---|
| (A + B) any local anaesthetic infiltration + usual anaesthesia vs. usual anaesthesia | |||||||
| Pain at rest at 24 hours | 12 | SMD | –0.605 | –1.051 to –0.160 | 0.0078 | 89 | 0.541 |
| Pain during activity at 24 hours | 9 | SMD | –0.848 | –1.450 to –0.246 | 0.0058 | 92 | 0.765 |
| Pain at rest at 48 hours | 11 | SMD | –0.285 | –0.520 to –0.050 | 0.018 | 58 | 0.09 |
| Pain during activity at 48 hours | 8 | SMD | –0.432 | –0.776 to –0.089 | 0.014 | 71 | 0.171 |
| Length of hospital stay | 9 | WMD | –0.829 | –1.540 to –0.118 | 0.022 | 84 | 0.866 |
| (A) local anaesthetic infiltration + usual analgesia vs. usual anaesthesia | |||||||
| Pain at rest 24 hours post operation | 7 | SMD | –0.633 | –1.208 to –0.059 | 0.031 | 90 | 0.529 |
| Pain during activity 24 hours post operation | 4 | SMD | –0.241 | –0.637 to 0.155 | 0.23 | 68 | 0.11 |
| Pain at rest 48 hours post operation | 6 | SMD | –0.134 | –0.348 to 0.080 | 0.22 | 19 | 0.014 |
| Pain during activity 48 hours post operation | 3 | SMD | –0.225 | –0.559 to 0.109 | 0.19 | 35 | 0.03 |
| Length of hospital stay | 5 | WMD | –0.257 | –0.622 to 0.108 | 0.17 | 14 | 0.029 |
| (B) local anaesthetic infiltration + post-closure analgesia + usual anaesthesia vs. usual anaesthesia | |||||||
| Pain at rest 24 hours post operation | 5 | SMD | –0.572 | –1.383 to 0.240 | 0.17 | 90 | 0.767 |
| Pain during activity 24 hours post operation | 5 | SMD | –1.378 | –2.499 to –0.257 | 0.016 | 94 | 1.525 |
| Pain at rest 48 hours post operation | 5 | SMD | –0.489 | –0.963 to –0.015 | 0.043 | 73 | 0.209 |
| Pain during activity 48 hours post operation | 5 | SMD | –0.599 | –1.158 to –0.040 | 0.036 | 80 | 0.319 |
| Length of hospital stay | 4 | WMD | –1.117 | –2.474 to 0.239 | 0.11 | 88 | 1.621 |
Pain
Data for meta-analysis were available for up to 12 studies depending on follow-up. 374,385–388,390–396 Overall, as shown in Table 36 and Figures 22 and 23, there was a reduction in pain at 24 and 48 hours at both rest and activity. For example, at rest at 24 hours, the average SMD favouring local anaesthetic infiltration was –0.61 (95% CI –1.05 to –0.16; p = 0.008) and during activity at 48 hours was –0.85 (95% CI –1.45 to –0.25; p = 0.006). This reflected reduced pain at 24 hours at rest by an estimated 12 points (95% CI 3 to 21 points p = 0.008) and during activity by 24 points (95% CI 7 to 42 points; p = 0.006) on a 100-point scale. Average effect sizes at 48 hours were smaller for pain at rest (SMD –0.29, 95% CI –0.52 to –0.05; p = 0.018) and during activity, (SMD –0.43, 95% CI –0.78 to –0.09; p = 0.014), corresponding to 5 and 10 points on a 100-point scale, respectively.
FIGURE 22.
Total hip replacement: 24-hour VAS pain score at rest and during activity by local anaesthetic infiltration grouping. SMD in pain at 24 hours post surgery. Nc, number in the control group; Ni, number in the intervention group. (a) Pain at rest; and (b) pain during activity.


FIGURE 23.
Total hip replacement: 48-hour VAS pain score at rest and during activity by local anaesthetic infiltration grouping. SMD in pain at 48 hours post surgery. Nc, number in the control group; Ni, number in the intervention group. (a) Pain at rest; and (b) pain during activity.

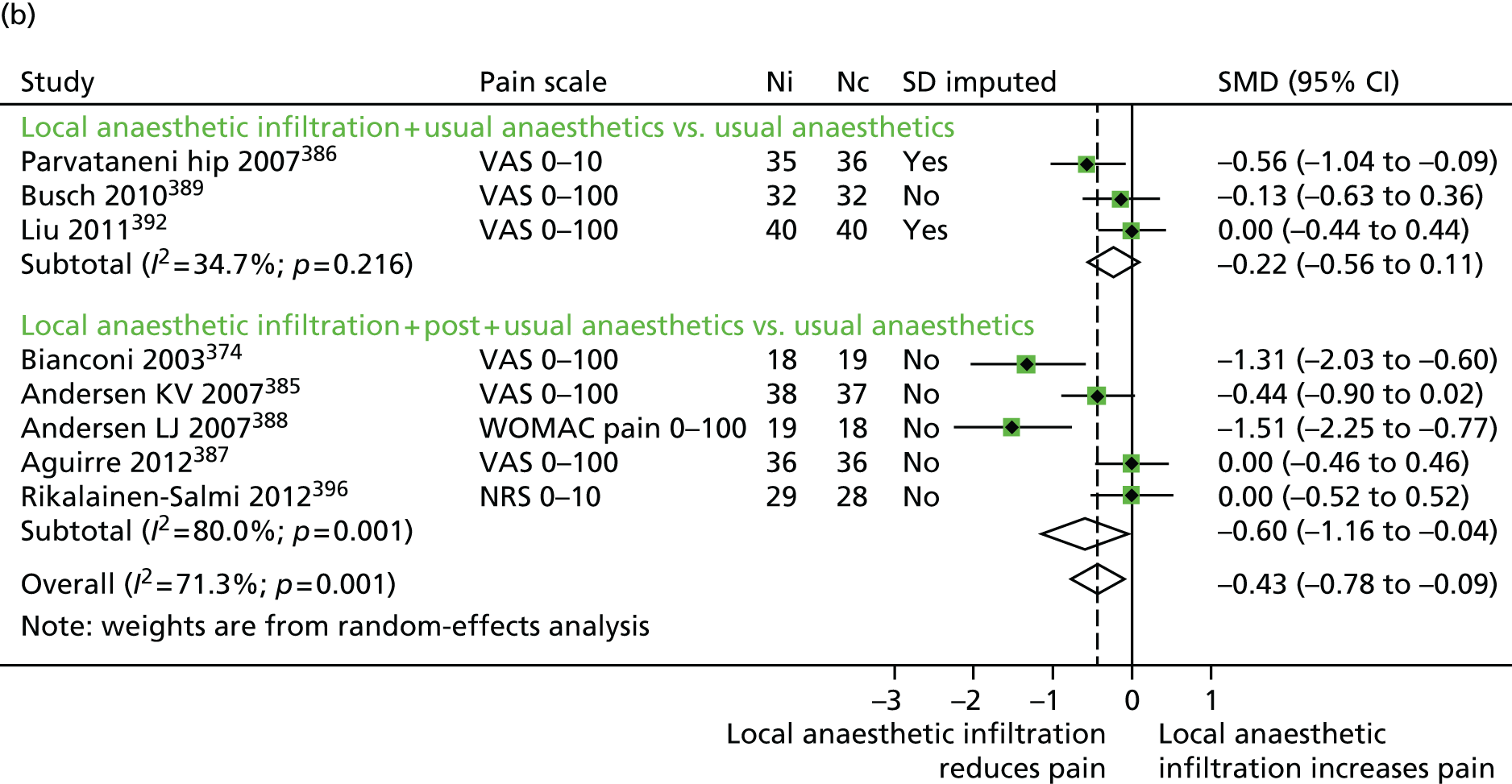
In seven studies, the comparison was between patients receiving local anaesthetic infiltration with no additional analgesia delivered through a catheter or injection (A group), and controls receiving no intervention or saline infiltration. 386,390–395 At rest at 24 hours, the local anaesthetic infiltration group reported reduced pain (average SMD –0.63, 95% CI –1.21 to –0.06; p = 0.031), equivalent to an estimated 12 points lower pain. There was no strong evidence that the intervention had an effect during activity or at 48 hours.
In five studies, the intervention group received local anaesthetic infiltration and further analgesia through a catheter or injection after wound closure (B group). 374,385,387,388,396 Pain was reduced on average at 24 hours during activity (SMD –1.38, 95% CI –2.5 to –0.26; p = 0.016), equivalent to a 40-point decrease, and at 48 hours at rest (SMD –0.49, 95% CI –0.96 to –0.02; p = 0.043) and during activity (SMD –0.6, 95% CI –1.16 to –0.04; p = 0.036) equivalent to 8- and 14-point decreases, respectively.
In one study, control patients received an epidural analgesia infusion. 385 Pain was lower for the duration of the epidural infusion but, at 48 hours, pain was higher in the control group than in the local anaesthetic infiltration group. In a study in which control patients received additional intrathecal morphine, there was no difference in pain outcomes at any time point. 396
Heterogeneity as expressed by the between-study variance (τ2) and the I2-statistic (see Table 36) was high and separate analysis for A and B groups did not appear to reduce this heterogeneity.
Considering nine studies with low risk of bias, the benefit for any local anaesthetic infiltration was still apparent. 374,386–388,390,392,394–396 Studies with low risk of bias had a marginally smaller reduction in pain after 24 hours at rest, averaging (SMD –0.49, 95% CI –0.89 to –0.09; p = 0.017), but during activity average pain reduction was greater (SMD –0.99, 95% CI –1.64 to –0.35; p = 0.003), corresponding to 28 points on a 100-point scale.
Opioid consumption
In 11 studies reporting an appropriate outcome, opioid consumption was reduced in local anaesthetic infiltration groups compared with controls. 374,385,387–390,392–396 The difference ranged from 12% to 92%. There was no suggestion of different effects in groups with or without additional post-closure analgesia through a catheter or injection. In the studies in which control patients received epidural385 or intrathecal analgesia,396 patients receiving local anaesthetic infiltration consumed 20% and 12% less morphine, respectively.
Mobilisation
Several different measures of mobilisation were reported. In three studies, patients receiving local anaesthetic infiltration with no additional postoperative component achieved a straight leg raise earlier than control patients. 386,391,392
More patients were able to walk during the first postoperative day in two studies in which further postoperative analgesia was provided through a catheter. 385,396 In one study with no additional analgesia, with the exception of those with adverse events, all patients were mobilised on the first postoperative day. 390 However, the mean walking speed over 6 metres was improved in intervention patients at a 2-day functional assessment.
In one study, 35% of patients receiving local anaesthetic infiltration were able to walk after 8 hours, compared with 87% of control patients receiving an epidural infusion. 385 In the study for which control patients received intrathecal morphine, 33% of these patients could walk further than 5 metres on the first postoperative day, compared with 71% of patients receiving local anaesthetic infiltration. 396
Length of hospital stay
As shown in Table 36 and Figure 24, patients receiving local anaesthetic infiltration spent an average of 0.83 fewer days (95% CI 0.12 to 1.54 fewer days; p = 0.022) in hospital than control patients. Benefit was mainly limited to local anaesthetic infiltration interventions with additional analgesia through a catheter (B group). Heterogeneity across studies was high (I2 = 84%), mainly in studies with additional postoperative analgesia.
FIGURE 24.
Total hip replacement: length of hospital stay by local anaesthetic infiltration grouping. Weighted mean difference in days. LoS, length of hospital stay. Nc, number in the control group; Ni, number in the intervention group.

When the comparison group received an epidural infusion, patients with local anaesthetic infiltration had on average a 2-day shorter hospital stay. In the study in which the comparison group received intrathecal morphine, there was no clear difference in discharge times.
Complications
The Peto’s OR for a major complication in patients with local anaesthetic infiltration compared with controls was 0.30 (95% CI 0.05 to 1.77; p = 0.18). This was based on only one major complication in 448 patients randomised to local anaesthetic infiltration and four major complications in 448 controls. Across all studies with 909 patients, five deep infections were reported, four in local anaesthetic infiltration patients and one in controls (Peto’s OR 3.47, 95% CI 0.58 to 20.81; p = 0.17). Four infections occurred in the 218 patients who received post-closure delivery of infiltrate through a catheter.
The incidence of vomiting (or vomiting and nausea if not reported separately) was reduced in patients receiving local anaesthetic infiltration in the five studies with data, (Peto’s OR 0.46, 95% CI 0.27 to 0.80; p = 0.006). There was only slight heterogeneity between studies.
Long-term outcomes
Five studies reported long-term outcomes. In the study of Andersen and colleagues,388 the median WOMAC pain scores at 6 weeks in intervention and control groups were 2 (range 0–50) and 7 (range 0–13), but this difference favouring intervention was not statistically significant (p = 0.07). At the 8-week follow-up, Rikalainen-Salmi and colleagues396 reported no significant differences in mobilisation or intensity or duration of pain. Parvataneni and colleagues386 reported that VAS pain scores 3 months after surgery were ‘comparable between groups’. Similarly Aguirre and colleagues387 reported no difference in analgesic consumption or pain during normal daily activities between groups at 3 months. In the study of Busch and colleagues,389 mean overall WOMAC scores at 2 years were more favourable in the intervention group (69.3, SD 23.5) than in the control group (76.6, SD 25.9) but this was not statistically significant (p = 0.24).
Total knee replacement
Searches identified 23 studies with 1439 patients with TKR randomised. The mean number of patients randomised was 63 (range 32–101). We assessed that 17 studies were at low risk of bias386,399–409,411–413 and that five studies had unclear risk of bias based on uncertainty about blinding of outcome assessments. 380,397,398,410,414 One study was assessed to be at high risk of bias owing to a large uneven loss to follow-up between randomised groups. 381
Results of the meta-analysis are summarised in Table 37.
| TKR studies | Number of studies | Method | Pooled effect size (random effects) | 95% CI | p-value | I2 (%) | τ 2 |
|---|---|---|---|---|---|---|---|
| (A + B) any local anaesthetic infiltration + usual anaesthesia vs. usual anaesthesia | |||||||
| Pain at rest at 24 hours | 12 | SMD | –0.398 | –0.576 to –0.219 | < 0.001 | 32 | 0.032 |
| Pain during activity at 24 hours | 12 | SMD | –0.453 | –0.671 to –0.235 | < 0.001 | 54 | 0.078 |
| Pain at rest at 48 hours | 12 | SMD | –0.325 | –0.546 to –0.103 | 0.0041 | 56 | 0.084 |
| Pain during activity at 48 hours | 11 | SMD | –0.273 | –0.500 to –0.046 | 0.018 | 56 | 0.081 |
| Length of hospital stay | 8 | WMD | –0.866 | –1.622 to –0.109 | 0.025 | 77 | 0.805 |
| (A) local anaesthetic infiltration with no additional post-wound closure analgesia + usual anaesthesia vs. usual anaesthesia | |||||||
| Pain at rest 24 hours post operation | 6 | SMD | –0.248 | –0.452 to –0.044 | 0.017 | 14 | 0.009 |
| Pain during activity 24 hours post operation | 6 | SMD | –0.283 | –0.470 to –0.096 | 0.0031 | 0 | 0 |
| Pain at rest 48 hours post operation | 6 | SMD | –0.155 | –0.458 to 0.148 | 0.32 | 61 | 0.086 |
| Pain during activity 48 hours post operation | 6 | SMD | –0.077 | –0.263 to 0.110 | 0.42 | 0 | 0 |
| Length of hospital stay | 1 | WMD | 0.092 | –0.890 to 1.073 | 0.85 | 100 | < 0.001 |
| (B) local anaesthetic infiltration + post-wound closure analgesia + usual anaesthesia vs. usual anaesthesia | |||||||
| Pain at rest 24 hours post operation | 6 | SMD | –0.587 | –0.829 to –0.346 | < 0.001 | 9 | 0.008 |
| Pain during activity 24 hours post operation | 6 | SMD | –0.693 | –1.152 to –0.234 | 0.0031 | 74 | 0.24 |
| Pain at rest 48 hours post operation | 6 | SMD | –0.52 | –0.778 to –0.262 | < 0.001 | 21 | 0.022 |
| Pain during activity 48 hours post operation | 5 | SMD | –0.594 | –0.997 to –0.191 | 0.0039 | 61 | 0.128 |
| Length of hospital stay | 7 | WMD | –1.023 | –1.822 to –0.224 | 0.012 | 76 | 0.761 |
| (C) local anaesthetic infiltration + FNB + usual anaesthesia vs. FNB + usual anaesthesia | |||||||
| Pain at rest 24 hours post operation | 3 | SMD | 0.253 | –0.514 to 1.021 | 0.52 | 81 | 0.37 |
| Pain during activity 24 hours post operation | 3 | SMD | 0 | –0.317 to 0.317 | 1 | 0 | 0 |
| Pain at rest 48 hours post operation | 2 | SMD | 0.254 | –0.429 to 0.937 | 0.47 | 67 | 0.166 |
| Pain during activity 48 hours post operation | 2 | SMD | –0.073 | –0.446 to 0.299 | 0.7 | 0 | 0 |
| Length of hospital stay | 2 | WMD | 0.07 | –0.838 to 0.978 | 0.88 | 0 | 0 |
| (D) local anaesthetic infiltration + usual anaesthesia vs. FNB + usual anaesthesia | |||||||
| Pain at rest 24 hours post operation | 3 | SMD | –0.241 | –0.604 to 0.122 | 0.19 | 44 | 0.046 |
| Pain during activity 24 hours post operation | 0 | ||||||
| Pain at rest 48 hours post operation | 1 | SMD | –0.18 | –0.571 to 0.211 | 0.37 | 100 | 0 |
| Pain during activity 48 hours post operation | 1 | SMD | 0.094 | –0.296 to 0.485 | 0.64 | 100 | 0 |
| Length of hospital stay | 1 | WMD | 1.52 | 0.054 to 2.986 | 0.042 | 100 | 0 |
| (E) local anaesthetic infiltration + post-wound closure analgesia + usual anaesthesia vs. FNB + usual anaesthesia | |||||||
| Pain at rest 24 hours post operation | 3 | SMD | –0.076 | –0.632 to 0.480 | 0.79 | 69 | 0.166 |
| Pain during activity 24 hours post operation | 3 | SMD | 0.159 | –0.869 to 1.187 | 0.76 | 90 | 0.741 |
| Pain at rest 48 hours post operation | 2 | SMD | 0.056 | –0.300 to 0.412 | 0.76 | 0 | 0 |
| Pain during activity 48 hours post operation | 2 | SMD | –0.202 | –1.034 to 0.631 | 0.63 | 75 | 0.275 |
| Length of hospital stay | 2 | WMD | –0.069 | –0.634 to 0.497 | 0.81 | 0 | 0 |
Pain
In patients receiving patients receiving local anaesthetic infiltration (A and B groups), pain at rest at 24 hours and during activity at 48 hours was reduced by SMD –0.40 (95% CI –0.58 to –0.22; p < 0.001) and –0.27 (95% CI –0.50 to –0.05; p = 0.018), respectively. This reflected reductions in pain at rest at 24 hours by an average of 10 points (95% CI 6 to 15 points; p < 0.001) and during activity at 48 hours by 8 points (95% CI 1.5 to 15 points; p = 0.018) on a 100-point scale.
Heterogeneity as expressed by the between-study variance (τ2) and the I2-statistic was moderate to low. Among studies with low risk of bias, heterogeneity reported was higher, possibly owing to variability across comparators and surgical characteristics.
Data were available for up to six trials comparing local anaesthetic infiltration with no additional post-closure analgesia (A group), with controls receiving no intervention or saline infiltration. 401,404–406,414 As shown in Table 37 and Figure 25, pain at 24 hours was reduced both at rest and during activity [SMDs –0.25 (95% CI –0.45 to –0.04; p = 0.017) and –0.28 (95% CI –0.47 to –0.10; p = 0.003), respectively]. In six trials where additional analgesia was delivered through a catheter or as an injection after wound closure (B group),398,402,403,412,413 reduction in pain at 24 hours was somewhat greater than controls at both rest and activity [SMDs –0.59 (95% CI –0.83 to –0.35; p < 0.001) and –0.69 (95% CI –1.15 to –0.23; p = 0.003)].
FIGURE 25.
Total knee replacement: 24-hour VAS pain score at rest and during activity by local anaesthetic infiltration grouping. SMD in pain at 24 hours post surgery. i.v., intravenous; Nc, number in the control group; Ni, number in the intervention group; NRS, numerical response scale. (a) Pain at rest; and (b) pain during activity.

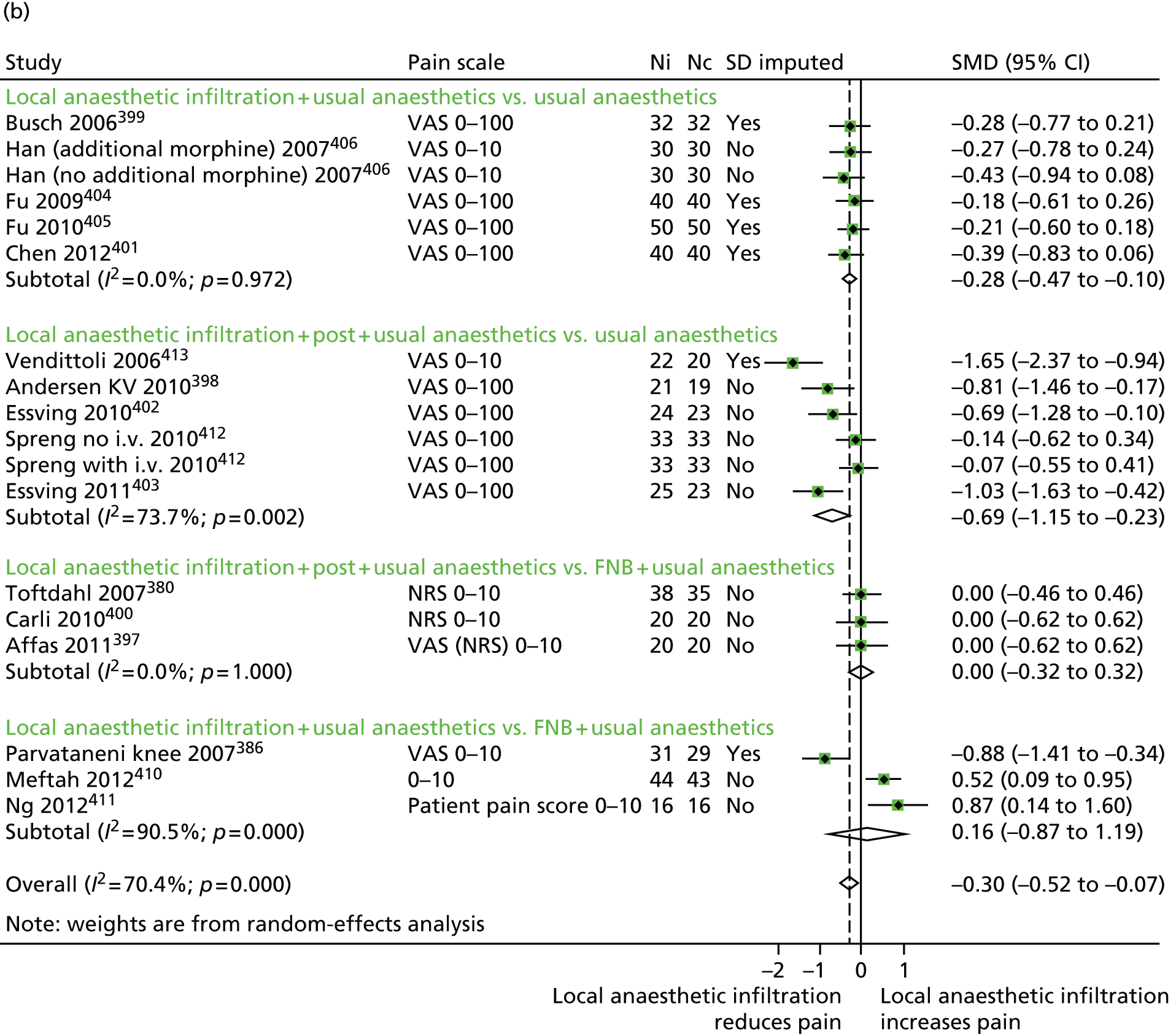
As shown in Figure 26, improvement in pain relief was sustained at 48 hours, but only if additional analgesia was provided postoperatively (B group), (SMD –0.52, 95% CI –0.78 to –0.26; p < 0.001) at rest and during activity (SMD –0.59, 95% CI –1.0 to –0.19; p = 0.004).
FIGURE 26.
Total knee replacement: 48-hour VAS pain score at rest and during activity by local anaesthetic infiltration grouping. SMD in pain at 48 hours post surgery. i.v., intravenous; Nc, number in the control group; Ni, number in the intervention group; NRS, numerical response scale. (a) Pain at rest; and (b) pain during activity.
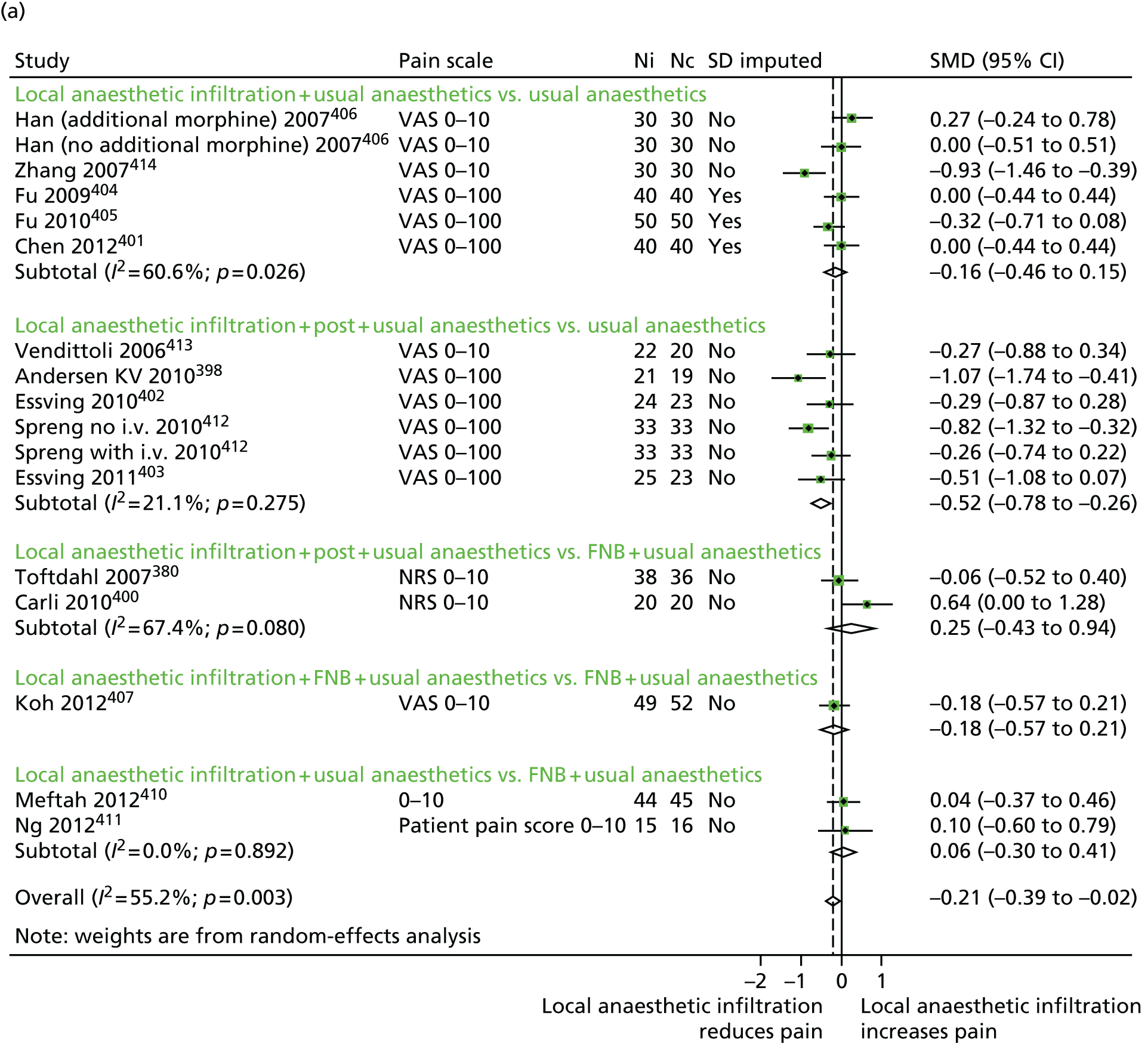
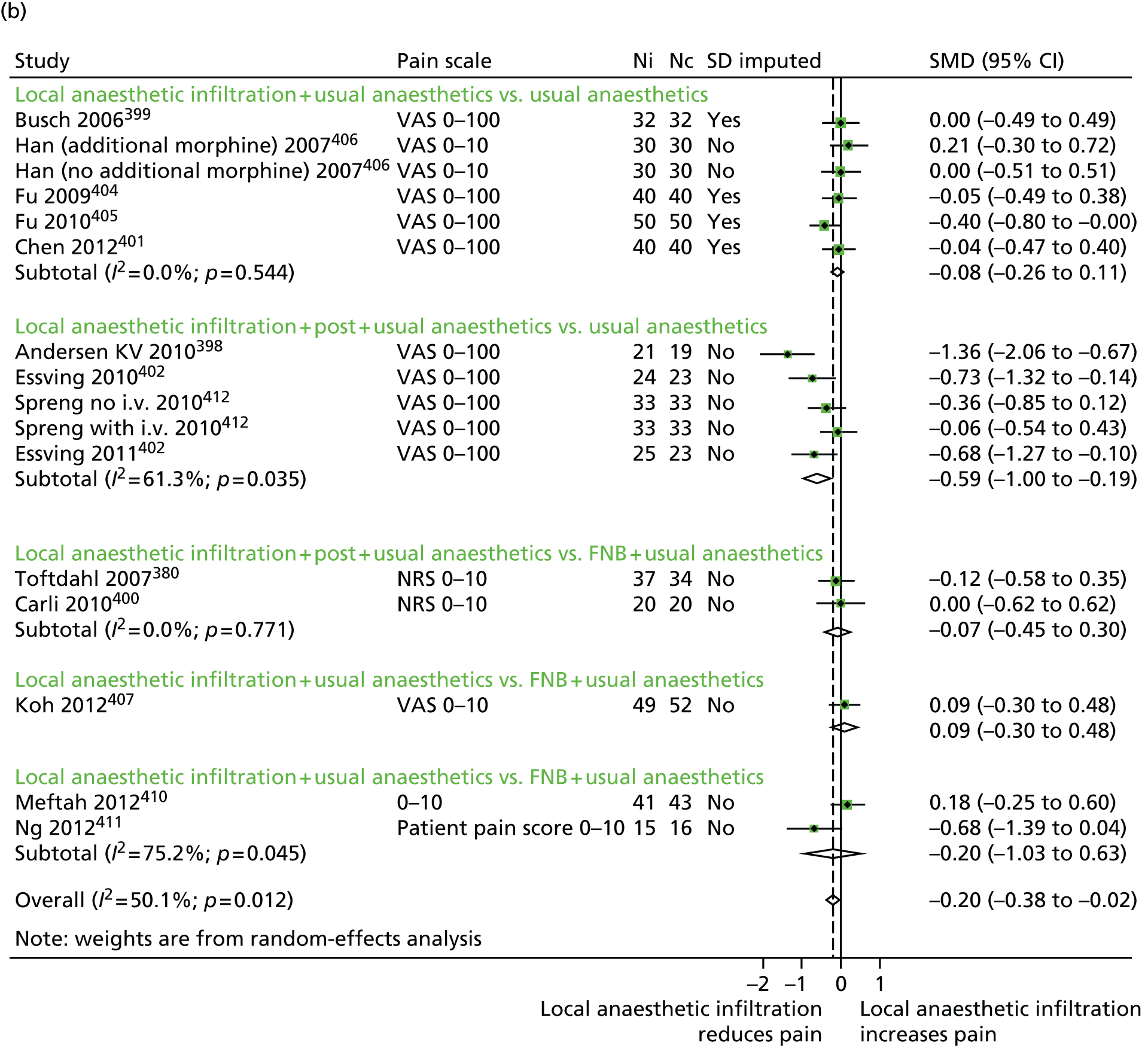
Heterogeneity as expressed by the between-study variance (τ2) and the I2-statistic was moderate to low.
Nine studies in TKR included a FNB analgesia regimen. 380,386,397,400,402,407–410 In three studies for which both randomised groups received a FNB (E),407–409 there was no evidence for added benefit of local anaesthetic infiltration in any outcome. In the six studies of a direct comparison of local anaesthetic infiltration with or without post-closure analgesia with a FNB in addition to usual analgesia (C and D),380,386,397,400,410,411 there was also no evidence of improved pain at any time point.
In eight comparisons between local anaesthetic infiltration with controls,381,398,399,402,403,412,413 additional ketorolac was included in the wound infiltrate. In seven comparisons with data, patients receiving additional analgesia in the infiltrate had lower pain than controls. 398,399,402,403,412,413 For example, pain was reduced on average at rest at 24 hours by SMD –0.68 (95% CI –0.94 to –0.42; p < 0.001) and during activity at 48 hours by SMD –0.59 (95% CI –1.01 to –0.17; p = 0.006), equivalent to a reduction of 17 and 30 points, respectively, on a 100-point scale compared with controls.
In four studies, control patients received either an epidural infusion,381,398,412 or intrathecal morphine. 403 Results of all studies supported a reduction in pain for patients receiving local anaesthetic infiltration compared with epidural or intrathecal morphine.
Opioid consumption
In all four studies reporting opioid consumption, this was reduced by 35–40% in the local anaesthetic infiltration group with no analgesia. 399,401,404,405 In three studies with additional post-closure analgesia opioid consumption was 32–52% lower than in the control groups. 402,403,413
In six studies for which the control group or both groups received FNB, there was little difference in opioid consumption between randomised groups. 380,386,397,400,407,408
In four studies for which patients receiving local anaesthetic infiltration with further post-closure analgesia were compared with patients receiving epidural anaesthesia, there was no consistent difference between groups. 381,398,412
Mobilisation
A range of mobilisation outcomes were reported in 19 studies. 380,381,386,398,400–410,412,413 Differences in outcome measures precluded meta-analysis.
In three studies, the time to achieve a straight leg raise was reduced by 44–50%,401,404,405 and in one study three times as many intervention patients could achieve a straight leg raise on the first postoperative day as controls. 386 In two studies in which FNB was given to all patients, the benefit for local anaesthetic infiltration was statistically significant; more patients receiving local anaesthetic infiltration were able to achieve a straight leg raise during the first postoperative day. 407,408
In four out of five studies, patients receiving local anaesthetic infiltration achieved better knee flexion. 405,406,409,413 Four studies reported ambulation as part of discharge-readiness criteria. 398,402,403,410 These criteria were met earlier in intervention patients in three studies,398,402,403 but were similar in one study in which control patients received a FNB. 410
Times to achieve diverse walking goals were reported in six studies. 380,381,400,408,412 In three studies, improvements were evident in intervention patients that received epidural analgesia381 or FNB compared with control groups. 380,400 Marginal improvements in walking outcomes for intervention patients compared with controls were reported in three studies. 408,412
One study each showed that intervention patients could get out of bed earlier than controls receiving epidural analgesia,381 and that the ability to hold quadriceps tension was improved compared with those receiving FNB. 380
Length of hospital stay
Data on length of hospital stay were available for eight studies in which patients were randomised to local anaesthetic infiltration or control. Results are summarised in Table 37 and Figure 27. There was some evidence that length of hospital stay was reduced in patients receiving local anaesthetic infiltration and additional post-closure delivery (B) by 1.0 day on average (95% CI 0.2 to 1.8 days; p = 0.012) compared with controls. In the one study (A) with no post-closure analgesia component, there was no difference in length of hospital stay.
FIGURE 27.
Total knee replacement: length of hospital stay by local anaesthetic infiltration grouping. Weighted mean differences in days. i.v., intravenous; LoS, length of stay; Nc, number in the control group; Ni, number in the intervention group.

In three studies for which the comparison group received FNB,380,400,410 there was no suggestion of a difference in length of hospital stay. In one study in which all randomised patients received a FNB the length of hospital stay was about 1.5 days shorter in the control patients who also received an additional sciatic nerve block. 409
Complications
Based on 11 reported events in 1439 patients, the odds of a major complication after TKR in patients receiving local anaesthetic infiltration compared with controls was 1.17 (95% CI 0.35 to 3.86; p = 0.80). Three deep infections were reported: two in the intervention group380,398 and one in the control group412 (Peto’s OR 1.85, 95% CI 0.19 to 17.83; p = 0.59). All infections occurred in patients receiving a catheter although in control patients nothing was injected through it.
Excluding one study in which intervention patients received additional morphine,406 the incidence of vomiting was lower in patients receiving local anaesthetic infiltration groups than in controls in eight studies (548 patients) with data (Peto’s OR 0.56, 95% CI 0.39 to 0.80). 401–406,412 Rates were similar whether or not additional analgesia was delivered through a catheter or injection. There was minor heterogeneity between studies.
Long-term outcomes
Five studies reported long-term patient outcomes measured at 6 weeks399,400 or 3 months. 386,402,403 Busch and colleagues399 found a non-significant difference in mean VAS pain scores at 6 weeks favouring the intervention group. Parvataneni and colleagues386 reported comparable pain scores between groups at the 3-month follow-up. In the studies of Essving and colleagues,402,403 there were no differences between median OKS at 3 months.
In a study comparing different approaches to perioperative pain control, Carli and colleagues400 reported an improved WOMAC score after 6 weeks in the control group who received FNB compared with the local anaesthetic infiltration group.
Discussion
Our results indicate that in patients with total hip and knee replacement, those receiving local anaesthetic infiltration experience less pain after 24 hours at rest and after 48 hours during activity than controls. For patients with THR, the reduction in pain was, on average, about 35% at rest at 24 hours and 28% at 48 hours during activity when compared with no infiltration analgesia or placebo. For patients with TKR, the estimated average reduction of pain was 26% at rest at 24 hours and 16% at 48 hours during activity for local anaesthetic infiltration when compared with no infiltration analgesia or placebo. In patients receiving TKR, inclusion of the non-steroidal anti-inflammatory agent ketorolac in the infiltrate seemed to enhance postoperative pain relief.
Local anaesthetic infiltration was associated with 0.83 and 0.87 fewer days in hospital for patients with THR and knee replacement, respectively, reduced opioid consumption, earlier mobilisation and fewer complications. The improvement in pain control and shorter hospital stay was greatest for patients receiving additional post-wound closure analgesia. However, this should be weighed against a suggestion of a higher risk of deep infection when delivered through an active catheter. Six infections occurred in 505 patients who received an active catheter (1.19%), compared with eight infections in all 2348 patients randomised (0.34%). Regarding other serious post-surgical complications, there was little evidence of differences between randomised groups.
In studies in patients with TKR comparing local anaesthetic infiltration against FNB, or using a FNB in both groups, there was no benefit for local anaesthetic infiltration. FNB is a well-established method of providing analgesia after TKR and is associated with reduced opioid requirement and, therefore, a reduction in the undesirable side effects of opiates, such as nausea and vomiting. However, after a FNB, there is decreased quadriceps function for a time and an increased risk of falls. 415,416
Our finding that mobilisation was consistently achieved earlier in patients receiving local anaesthetic infiltration may be a consequence of the reduced requirement for opioids, which may have contributed to the shorter average hospital stay. Opioid medication represents a key strategy in the management of post-surgical pain but its use can delay mobilisation and rehabilitation. 417 The majority of studies were concerned only with improving short-term outcomes, with only one study reporting outcomes beyond 3 months. 389 However, acute postoperative pain is an important risk factor for long-term pain418,419 and deserves appropriate consideration in future studies of perioperative pain control.
Our study evaluated the clinical effectiveness of local anaesthetic infiltration interventions and has a number of limitations. We noted a range of analgesia regimens, with different studies making different comparisons, particularly for TKR. Although meta-analyses performed were enhanced by extensive contact with authors, imputation was required for some measures of variability. The skewed nature of the hospital length of stay outcome required transformation of outcomes under assumptions of a log-normal distribution. 382 For opioid consumption and mobilisation outcomes, there was insufficient consistency in measures reported to conduct anything but a systematic narrative overview.
Conclusion
Our systematic review shows that local anaesthetic infiltration is effective in reducing short-term pain after TKR and THR when compared with no infiltration. It is enhanced with the addition of post-closure analgesia, although this needs to be considered in light of the infection risks associated with catheters. 420 In TKR, there may be no additional benefit if a FNB has already been sited.
The effect of local anaesthetic wound infiltration on chronic pain after total hip and knee replacement: APEX randomised controlled trials
Background
The need to evaluate the effectiveness of perioperative local anaesthetic infiltration in reducing long-term pain after joint replacement in appropriately powered RCTs is recognised. 421 The other components of the multimodal anaesthesia regimen were based on recommendations from the European Society of Regional Anaesthesia and Pain Therapy, PROcedure SPECific postoperative pain managemenT (PROSPECT), (procedure specific postoperative pain management) guidelines and, in patients receiving TKR, included FNB. 422
Aim
The aim of these two single-centre double-blind RCTs was to determine if using local anaesthetic infiltration, in addition to the standard anaesthetic regimen at the AOC, is clinically effective and cost-effective at reducing joint pain at 1 year after THR and TKR.
Patients and methods
The trials are registered as an International Standardised RCT (96095682). The protocol for the APEX trials was published in BMC Musculoskeletal Disorders. 365 The trials were approved by Southampton and South West Hampshire Research Ethics Committee B (09/H0504/94) and registered as a Clinical Trial of an Investigational Medicinal Product with the Medicine and Healthcare Regulatory Authority (18524/0215/001-0001) and EudraCT (2009-013817-93). The trials were overseen by a Data Monitoring Committee and Trial Steering Committee, which regularly reviewed safety data and monitored trial conduct. A Consolidated Standards of Reporting Trials (CONSORT) checklist is included in Appendix 10.
Patient recruitment
Patients were posted information about the study after they were listed for a joint replacement at the AOC. Eligible patients were then approached for recruitment by a research nurse when they attended a pre-operative assessment clinic. All participants provided informed written consent. Inclusion criteria included being listed for a primary THR or primary TKR for osteoarthritis and being willing and able to provide fully informed consent. Exclusion criteria included (1) any medical comorbidity that precludes spinal anaesthesia, regional blocks or the use of strong analgesics postoperatively, (2) severe dementia or psychiatric illness such that the patient was unable to complete the questionnaires or provide informed consent, (3) listed for simultaneous bilateral joint replacement, (4) having been in the APEX trial for a previous joint replacement and (5) being unable to understand English because not all the questionnaires have been translated and validated into other languages. In order to explore the problem of ascertainment bias (the patients enrolled in the trial not being representative of those undergoing arthroplasty), anonymised demographic data were recorded for all eligible patients.
Randomisation
Prior to surgery, patients were randomised to the standard care or the intervention group using an online computer-generated code (provided by the Bristol Randomised Trials Collaboration). Randomisation was stratified by operation site (THR/TKR) and minimised by baseline pain severity and surgical approach. The results of randomisation were concealed from the surgeons and anaesthetists until the beginning of the operation list so that knowledge of randomisation could not affect any clinical decisions they may have made. Trial participants and research nurses were blinded to the treatment allocation throughout the trial.
Intervention: total hip replacement
Standard care group
The standard anaesthetic care for THR patients was a spinal anaesthetic with 3 ml of 0.5% plain bupivacaine placed at the L3–4 or L4–5 interspace. Intraoperatively, the patient was either awake, sedated or under a light general anaesthetic. If there was intraoperative discomfort, then rescue analgesia in the form of intravenous (i.v.) fentanyl was titrated to effect. All participants were given 1 g of i.v. paracetamol 30 minutes before the end of the operation. In the recovery area immediately post operation, patients received (if no contraindications were present) 400 mg of ibuprofen administered orally. A patient-controlled analgesia (PCA) device was started containing 1 mg/ml morphine, a 1-mg bolus dose and a 5-minute lockout. If, on awakening, the patient was in pain with a rating of > 50 mm on a 100-mm pain VAS, a morphine bolus up to 0.2 mg/kg was administered as rescue analgesia. Each day during their hospital stay patients received a visit from a pain specialist nurse. Postoperative analgesia consisted of oral or i.v. paracetamol every 6 hours and, if no contraindications were present, 400 mg of oral ibuprofen every 8 hours. When the PCA was no longer needed, 30–60 mg of oral codeine phosphate every 6 hours, 50–100 mg of tramadol every 6 hours and 10–20 mg of morphine sulphate (Oramorph®, Boehringer Ingelheim) were prescribed as rescue analgesia.
Intervention group
The intervention group received the same anaesthetic and analgesic regime as the standard care group, plus an intraoperative local anaesthetic wound infiltration. The local anaesthetic mixture consisted of 60 ml of 0.25% bupivacaine with 1 in 200,000 adrenaline. If the patient weighed < 60 kg or was particularly frail, the volume of injectate was reduced to 50 ml, or lower if necessary. The surgeon injected the anaesthetic mixture into the joint capsule and short external rotators, fascia, fat and subcutaneous tissue.
Intervention: total knee replacement
Standard care group
In line with evidence-based guidance from PROSPECT, standard care consisted of a FNB and a spinal or general anaesthetic, depending on patient factors. 422 postoperative analgesia was provided as necessary and recorded.
Intervention group
The intervention group received the same anaesthetic regime, plus an intraoperative local anaesthetic infiltration, which consisted of 60 ml of 0.25% bupivacaine with 1 in 200,000 adrenaline. The local anaesthetic mixture was injected directly into the posterior capsule (25 ml), medial and lateral capsule (10 ml), fascia and muscle (10 ml) and subcutaneous tissues (15 ml), prior to wound closure.
Assessment times
Assessments are conducted pre-operatively, daily during the hospital stay and then at 3, 6 and 12 months postoperatively. Outcomes were assessed using self-report questionnaires, joint examinations, radiograph analysis, pressure algometry and extraction of data from hospital records.
Primary outcome measure
The primary outcome measure was the self-completed WOMAC pain scale114 at 12 months postoperatively. The five-item WOMAC pain scale is a widely used and validated measure of pain severity when performing daily activities. The questionnaire was completed with reference to the operated hip or knee. Scores were transformed onto a 0–100 scale, with lower scores indicating more severe pain.
Secondary outcome measures
Secondary outcomes were collected during the postoperative inpatient stay and at 3, 6 and 12 months postoperatively. Pain severity on admission to, and discharge from, the post-surgical recovery ward was rated on a 4-point Likert-type scale (none to severe pain). Pain severity for the remainder of the day of surgery was rated every 4 hours on a 0–10 scale (best to worst). On postoperative days 1–3, patients rated the severity of night pain, pain on movement and pain at rest on a 100-mm VAS. Satisfaction with pain relief and the occurrence of nausea and vomiting were also recorded daily during the inpatient stay. Length of hospital stay and postoperative analgesia use were extracted from medical records. At 3, 6 and 12 months postoperatively, patients completed the WOMAC function and Stiffness subscales114 and the Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). 280 The painDETECT questionnaire,283 a measure of neuropathic pain, was completed at 12 months postoperatively.
Pressure pain thresholds of the volar forearm were measured pre-operatively, at discharge from hospital and 12 months postoperatively using an algometer. Data on medical and surgical complications were recorded from hospital records during the inpatient stay, by a telephone call to patients at 3 months and by a comprehensive joint assessment by a research nurse at 12 months postoperatively. Cost-effectiveness was assessed through data on health services resource use collected from hospital records and patient self-report questionnaires. Preference-based HRQoL was measured by administering the European Quality of Life-5 Dimensions 3-Level version (EQ-5D-3L)278 at baseline and at all follow-up points. This provides a single index score for each patient’s health state profile.
Details of medical and surgical adverse events were recorded throughout the trial through review of medical records, self-report by patients and assessment by a research nurse.
Potential prognostic factors
Measures of possible effect modifiers included sociodemographic factors, Functional Comorbidity Index,279 Kellgren and Lawrence osteoarthritis grading scheme,423 HADS,281 Pain Self-Efficacy questionnaire,275 Illness Perceptions Questionnaire-Revised282 and Brief COPE. 276 Any imbalance between trial arms in factors that might influence the perception of pain and pattern of recovery was considered in sensitivity analyses.
Sample size calculation
A sample size of 300 patients in each trial provided 90% power to detect a difference of 0.5 SDs on the WOMAC pain scale with a two-sided 1% significance level, allowing for a 20% dropout rate. Previous research suggests SDs of around 17 on the WOMAC pain scale before surgery. 424 Hence, a difference between the treatment groups of 0.5 SDs equates to a difference of approximately 8–9 units on the WOMAC pain scale, which represents a minimally perceptible clinical improvement. 425
Statistical analysis
The hip and knee trials were analysed separately, undertaken in Stata 13.1, and reported in accordance with CONSORT guidelines.
Primary analyses
Following a predefined analysis plan agreed with the Trial Steering Committee,365 we used linear regressions to estimate between-group differences in mean WOMAC pain scores at 12 months postoperatively, adjusted for pre-operative WOMAC pain scores and surgical approach. All patients in their original assigned groups with available primary outcome data (THR, n = 281; TKR, n = 273) were included in the primary analyses [intention-to-treat complete cases (ITT-CC)].
Sensitivity analyses
Analyses were repeated on all the randomised 322 patients with THR and 316 patients with TKR using multiple imputation technique by chained equations (20 imputations for the THR trial, 25 imputations for the TKR trial) stratified by randomisation arm to handle missing outcomes [intention-to-treat imputed (ITT-imputed)]. 426 The analyses were also conducted on a per-protocol basis, excluding participants who did not receive their allocated intervention or did not have a primary outcome (participants who opted out, died or were lost to follow-up). Per-protocol analyses included 266 patients with THR and 259 patients with TKR [per protocol complete cases (PP)].
In addition, any potential imbalance in patients baseline characteristics were controlled for in the ITT-CC, ITT-imputed and PP analyses.
Post hoc analyses
To explore the impact of potential ceiling effect in the WOMAC pain score on the primary analyses, that is the robustness of the linear regression coefficient standard errors, CIs and p-values (via violations of the assumptions of homoscedasticity and normality), we conducted two further sensitivity analyses. First, we investigated transformations of WOMAC pain scores to model the primary outcomes as continuous variables in the linear regressions. Second, the scores were then modelled as a categorical variable using published threshold definitions [severe/extreme (0–50), moderate (51–75), mild (76–99), no (100) pain],49 and partial proportional odds regressions. 427 These categories relate to the original ordinal WOMAC pain scale (e.g. a patient who reports no pain for every item will score 100, and a patient who reports mild pain on all the items will score 75, etc.).
Secondary analyses
Drug intake, length of hospitalisation and patients’ complications after surgery were compared by treatment arm with the use of chi-squared or Fisher exact tests for categorical variables, t-tests or Wilcoxon Mann–Whitney U-tests for continuous variables. All analyses were performed on complete cases (patients with available outcome information) and the proportion of missing data is reported for each of these variables for information only.
Investigations of pain scores on days 1–3 after the operation were conducted using linear mixed models to account for repeated measurements within participants339 and using adequate transformation of the outcomes when required. The effect of the intervention on WOMAC pain scores at 3 months and 6 months post operation was also modelled using a mixed linear regression.
Period-specific intervention effects were modelled with interaction terms between the intervention and the assessment day/period parameters. The choice of modelling the raw or transformed continuous scores was based on the normality of the distribution of residuals assessed in a linear regression. When no suitable transformation was identified, the outcome was categorised and modelled with a non-linear regression.
For the THR trial, ICOAP and WOMAC Stiffness scores at 12 months post operation were dichotomised and modelled with a Poisson modified regression with robust variance estimation, a preferable approach to logistic regression when the outcome of interest has a high prevalence. 428 Investigations of the intervention effects on these outcomes at 3 and 6 months were conducted with an extension of this model accounting for the clustering of outcomes by patients. 429 For the TKR trial, ICOAP and WOMAC stiffness and function scores at 12 months post operation were modelled with linear regression and adequate transformation of the outcomes, when required. Investigations of the intervention effects on these outcomes at 3 and 6 months after surgery were conducted with a linear mixed model.
The WOMAC function scores at 12 months and then at 3 months and 6 months post operation were investigated with linear and linear mixed models in both THR and TKR trials. The painDETECT score at 12 months post operation was dichotomised and modelled with a Poisson modified regression with robust variance estimation in both trials.
The modelling strategy of the secondary outcomes was similar to the one described in the statistical analysis section of the main manuscript using an ‘intention-to-treat complete cases’ approach (THR, n = 281; TKR, n = 273), an ‘intention-to-treat imputed’ approach (n = 322 THR patients and n = 316 TKR patients) and a per-protocol approach (n = 266 THR patients and n = 259 TKR patients). Baseline analyses were adjusted for pre-operative WOMAC pain and surgical approach. Imbalanced baseline patients’ characteristics were then controlled for.
Results
Total hip replacement
Participants
Between November 2009 and February 2012, 630 eligible patients were approached about the trial. Of these patients, 322 (51%) were recruited and randomised: 163 to the intervention arm and 159 to the standard care arm (Figure 28). Primary outcome data were collected from 288 patients (87%). Baseline demographic and clinical characteristics were generally well balanced between the trial arms (Table 38). Differences between trial arms in sex, living arrangement and number of comorbidities were adjusted for in the analysis.
FIGURE 28.
Total hip replacement: APEX patient recruitment, randomisation and follow-up. Quest, in patient questionnaire; LtF, lost to follow-up.
| Characteristic | Intervention (n = 163) | Standard care group (n = 159) |
|---|---|---|
| Female sex, n (%) | 86 (53) | 103 (65) |
| Age (years), mean (SD) | 66.0 (11.4) | 66.4 (10.2) |
| BMI (kg/m2), mean (SD) | 28.9 (5.6) | 29.4 (5.4) |
| Living arrangement, n (%) | ||
| Live with someone | 110 (68) | 123 (77) |
| Live alone | 43 (26) | 32 (20) |
| Missing | 10 (6) | 4 (3) |
| Working status, n (%) | ||
| Paid employment/voluntary work | 47 (29) | 52 (33) |
| Retired | 104 (64) | 104 (65) |
| Missing | 12 (7) | 3 (2) |
| Education, n (%) | ||
| Compulsory age or before | 98 (60) | 108 (68) |
| College | 34 (21) | 30 (19) |
| University | 20 (12) | 17 (11) |
| Missing | 11 (7) | 4 (2) |
| Comorbidities, n (%) | ||
| 0 to 1 | 57 (35) | 53 (33) |
| 2 | 48 (30) | 36 (23) |
| 3 | 25 (15) | 36 (23) |
| ≥ 4 | 21 (13) | 28 (18) |
| Missing | 12 (7) | 6 (4) |
| Anxiety, n (%) | ||
| Definite | 26 (16) | 31 (19) |
| Potential | 26 (16) | 23 (15) |
| None | 100 (61) | 100 (63) |
| Missing | 11 (7) | 5 (3) |
| Depression | ||
| Definite, n (%) | 20 (12) | 25 (16) |
| Potential, n (%) | 25 (15) | 28 (18) |
| None, n (%) | 106 (65) | 101 (63) |
| Missing, n (%) | 12 (7) | 5 (3) |
| Self-efficacy, mean (SD) | 35.6 (13.7) | 34.2 (13.3) |
| WOMAC pain, mean (SD) | 43.4 (19.0) | 41.5 (17.9) |
| WOMAC function, mean (SD) | 43.7 (20.2) | 41.2 (17.2) |
| WOMAC stiffness, mean (SD) | 47.4 (24.9) | 42.6 (20.7) |
| Surgical approach, n (%) | ||
| Posterior | 151 (93) | 147 (92) |
| Lateral | 12 (7) | 12 (8) |
| Kellgren and Lawrence grade, n (%) | ||
| < 3 | 4 (3) | 8 (4) |
| ≥ 3 | 136 (83) | 128 (81) |
| Non-interpretable | 12 (7) | 12 (8) |
| Missing | 11 (7) | 11 (7) |
Primary outcome
As shown in Table 39, the majority of patients in both trial arms had excellent pain relief at 12 months after surgery, with a median WOMAC pain score in the intervention arm of 100 out of 100 (i.e. no pain) and in the standard care arm of 95 out of 100.
| Outcome | Intervention (n = 163) | Standard care (n = 159) |
|---|---|---|
| WOMAC pain at 12 months | ||
| Continuous score, by categorised pain level | ||
| Median (IQR) | 100 (10) | 95 (20) |
| None = 100 | 72 (44%) | 59 (37%) |
| Mild = 75–100 | 51 (31%) | 53 (33%) |
| Moderate = 50–75 | 16 (10%) | 16 (10%) |
| Severe = 0–50 | 2 (1%) | 12 (8%) |
| Missing data | 22 (14%) | 19 (12%) |
As shown in Table 40, the primary analysis found some evidence that patients in the intervention group had less pain at 12 months post operation than patients in the standard care group (coefficient 4.74, 95% CI 0.95 to 8.54; p = 0.015). This difference remained after further adjustments for baseline imbalances between groups, although it was less apparent in the adjusted intention to treat (ITT) with imputed data and per-protocol analyses.
| Model | ITT-CC | ITT-imputed | PP | |||
|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | |
| Baseline | 4.74 (0.95 to 8.54) | 0.015 | 5.35 (1.33 to 9.34) | 0.009 | 3.81 (–0.02 to 7.63) | 0.051 |
| Adjusted | 4.31 (0.63 to 7.98) | 0.022 | 4.36 (0.48 to 8.25) | 0.028 | 3.45 (–0.26 to 7.16) | 0.068 |
As described in the statistical methods, the assumptions of homoscedasticity and normality underlying the use of linear regression, our a priori primary analysis, were violated. Therefore, we performed a further secondary analysis of the primary outcome to test the robustness of the above findings by categorising the WOMAC pain score and using a partial proportional odds model. This method of analysis found similar results (Table 41), with evidence that patients in the intervention group were less likely to have severe pain at 12 months post operation than those in the standard care group (OR 10.19, 95% CI 2.10 to 49.55; p = 0.004). This finding remained after further adjustments for baseline imbalances between groups and was also observed in the ITT with imputed data and the per-protocol approaches.
| Groups compared | Baseline model | Adjusted model | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| (Moderate, mild or none) vs. reference = (severe) | ||||
| ITT-CC (n = 281) | 10.19 (2.10 to 49.55) | 0.004 | 10.78 (2.15 to 54.17) | 0.004 |
| ITT-imputed (n = 322) | 6.81 (1.81 to 25.68) | 0.005 | 6.26 (1.61 to 24.36) | 0.008 |
| PP (n = 266) | 8.93 (1.83 to 43.62) | 0.007 | 9.50 (1.88 to 47.76) | 0.006 |
| (Mild or none) vs. reference = (severe or moderate) | ||||
| ITT-CC (n = 281) | 1.72 (0.90 to 3.30) | 0.100 | 1.70 (0.86 to 3.33) | 0.125 |
| ITT-imputed (n = 322) | 1.76 (0.95 to 3.26) | 0.073 | 1.59 (0.84 to 3.02) | 0.157 |
| PP (n = 266) | 1.56 (0.81 to 3.02) | 0.186 | 1.54 (0.77 to 3.05) | 0.220 |
| (None) vs. reference = (severe, moderate or mild) | ||||
| ITT-CC (n = 281) | 1.40 (0.89 to 2.25) | 0.168 | 1.35 (0.82 to 2.22) | 0.243 |
| ITT-imputed (n = 322) | 1.42 (0.90 to 2.26) | 0.136 | 1.30 (0.80 to 2.10) | 0.290 |
| PP (n = 266) | 1.31 (0.80 to 2.14) | 0.277 | 1.27 (0.76 to 2.12) | 0.363 |
Secondary outcomes
Details of the analysis of secondary outcomes are shown in Table 42 and Appendices 11–28. There was no difference in any of the secondary outcomes except that patients in the intervention group reported less pain on postoperative night 2 (coefficient –0.81, 95% CI –1.40 to –0.23; p = 0.006) and less neuropathic pain at 12 months post operation (ITT adjusted model: relative risk 0.17, 95% CI 0.03 to 0.82; p = 0.028). Post-surgical superficial and deep wound infection rates were similar in the intervention group and standard care group (1.8% vs. 1.9%; p = 1.000).
| Model | ITT-CC | ITT-imputed | PP | |||
|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | |
| Pain over night (square root) | ||||||
| Day 1 | ||||||
| Baseline model | 0.29 (–0.29 to 0.87) | 0.325 | 0.25 (–0.36 to 0.85) | 0.419 | 0.36 (–0.22 to 0.95) | 0.223 |
| Adjusted model | 0.39 (–0.19 to 0.97) | 0.184 | 0.34 (–0.27 to 0.94) | 0.276 | 0.45 (–0.12 to 1.03) | 0.124 |
| Day 2 | ||||||
| Baseline model | –0.81 (–1.40 to 0.23) | 0.006 | –0.87 (–1.43 to 0.30) | 0.003 | –0.75 (–1.34 to 0.16) | 0.012 |
| Adjusted model | –0.71 (–1.30 to –0.13) | 0.016 | –0.78 (–1.35 to –0.20) | 0.008 | –0.66 (–1.25 to –0.08) | 0.025 |
| Day 3 | ||||||
| Baseline model | –0.29 (–0.89 to 0.31) | 0.347 | –0.40 (–0.98 to 0.18) | 0.173 | –0.09 (–0.70 to 0.51) | 0.765 |
| Adjusted model | –0.18 (–0.78 to 0.41) | 0.545 | –0.31 (–0.89 to 0.27) | 0.301 | 0.01 (–0.59 to 0.60) | 0.986 |
| Pain at rest (square root) | ||||||
| Day 1 | ||||||
| Baseline model | 0.12 (–0.36 to 0.60) | 0.621 | 0.12 (–0.35 to 0.59) | 0.619 | 0.15 (–0.33 to 0.63) | 0.546 |
| Adjusted model | 0.19 (–0.28 to 0.66) | 0.432 | 0.17 (–0.30 to 0.65) | 0.468 | 0.21 (–0.27 to 0.68) | 0.394 |
| Day 2 | ||||||
| Baseline model | –0.22 (–0.69 to 0.26) | 0.373 | –0.27 (–0.75 to 0.21) | 0.270 | –0.05 (–0.53 to 0.43) | 0.848 |
| Adjusted model | –0.15 (–0.62 to 0.33) | 0.546 | –0.21 (–0.70 to 0.27) | 0.381 | 0.01 (–0.46 to 0.49) | 0.962 |
| Day 3 | ||||||
| Baseline model | –0.34 (–0.83 to 0.15) | 0.171 | –0.45 (–0.92 to 0.02) | 0.062 | –0.21 (–0.71 to 0.28) | 0.391 |
| Adjusted model | –0.27 (–0.75 to 0.22) | 0.282 | –0.39 (–0.86 to 0.08) | 0.108 | –0.15 (–0.64 to 0.33) | 0.545 |
| Pain on movement | ||||||
| Day 1 | ||||||
| Baseline model | 1.52 (–3.90 to 6.95) | 0.582 | 1.25 (–4.20 to 6.69) | 0.653 | 1.26 (–4.21 to 6.74) | 0.651 |
| Adjusted model | 2.01 (–3.46 to 7.48) | 0.471 | 1.52 (–4.02 to 7.05) | 0.591 | 1.88 (–3.62 to 7.38) | 0.503 |
| Day 2 | ||||||
| Baseline model | 1.57 (–3.88 to 7.02) | 0.573 | 1.66 (–3.79 to 7.12) | 0.549 | 2.07 (–3.44 to 7.57) | 0.462 |
| Adjusted model | 2.07 (–3.43 to 7.56) | 0.461 | 1.93 (–3.61 to 7.48) | 0.494 | 2.69 (–2.83 to 8.21) | 0.339 |
| Day 3 | ||||||
| Baseline model | 0.01 (–5.56 to 5.58) | 0.997 | –0.47 (–5.88 to 4.95) | 0.866 | 0.27 (–5.35 to 5.89) | 0.925 |
| Adjusted model | 0.54 (–5.07 to 6.16) | 0.849 | –0.16 (–5.64 to 5.31) | 0.954 | 0.94 (–4.70 to 6.57) | 0.745 |
Total knee replacement
Participants
Between November 2009 and February 2012, 585 eligible patients were approached to take part in the trial. Of these patients, 316 (54%) were recruited and randomised: 157 to the intervention arm and 159 to the standard care arm (Figure 29). Primary outcome data were collected from 273 patients (86%). Baseline demographic and clinical characteristics were generally well balanced between the trial arms (Table 43). Differences between trial arms in working status, number of comorbidities, anxiety and depression were adjusted for in the analysis.
FIGURE 29.
Total knee replacement: APEX patient recruitment, randomisation and follow-up. LtF, lost to follow-up; Quest, in patient questionnaire. a, Withdrawn: one patient died, two opted out during the inpatient period; b, withdrawn: one patient was withdrawn for clinical reasons, one opted out during the inpatient period.
| Characteristic | Intervention group (n = 157) | Standard care group (n = 159) |
|---|---|---|
| Female sex, n (%) | 81 (52) | 86 (54) |
| Age (years), mean (SD) | 69.5 (9.4) | 68.7 (7.9) |
| BMI (kg/m2), mean (SD) | 32.4 (6.5) | 32.8 (6.5) |
| Living arrangement, n (%) | ||
| Live with someone | 112 (71) | 103 (65) |
| Live alone | 41 (26) | 44 (28) |
| Missing | 4 (3) | 12 (7) |
| Working status, n (%) | ||
| Paid employment/voluntary work | 41 (26) | 27 (17) |
| Retired | 111 (71) | 117 (74) |
| Missing | 5 (3) | 15 (9) |
| Education, n (%) | ||
| Compulsory age or before | 115 (73) | 109 (69) |
| College | 26 (17) | 25 (16) |
| University | 9 (6) | 10 (6) |
| Missing | 7 (4) | 15 (9) |
| Comorbidities, n (%) | ||
| 0 to 1 | 46 (29) | 39 (24) |
| 2 | 30 (19) | 41 (26) |
| 3 | 36 (23) | 25 (16) |
| ≥ 4 | 38 (24) | 43 (27) |
| Missing | 7 (5) | 11 (7) |
| Anxiety, n (%) | ||
| Definite | 33 (21) | 18 (11) |
| Potential | 24 (15) | 32 (20) |
| None | 93 (59) | 99 (62) |
| Missing | 7 (5) | 10 (6) |
| Depression | ||
| Definite, n (%) | 30 (19) | 16 (10) |
| Potential, n (%) | 29 (19) | 30 (19) |
| None, n (%) | 91 (58) | 104 (65) |
| Missing, n (%) | 7 (4) | 9 (6) |
| Self-efficacy, mean (SD) | 36.0 (13.4) | 37.3 (12.0) |
| WOMAC pain, mean (SD) | 42.5 (17.3) | 42.4 (16.1) |
| WOMAC function, mean (SD) | 46.1 (17.7) | 46.0 (17.9) |
| WOMAC stiffness, mean (SD) | 41.9 (21.0) | 41.1 (19.4) |
| Surgical approach, n (%) | ||
| Medial parapatellar | 122 (78) | 125 (79) |
| Subvastus | 35 (22) | 34 (21) |
| Kellgren and Lawrence grade, n (%) | ||
| < 3 | 133 (84) | 133 (84) |
| ≥ 3 | 1 (1) | 3 (2) |
| Non-interpretable | 1 (1) | 5 (3) |
| Missing | 22 (14) | 18 (11) |
Primary outcome
The majority of patients in both trial arms had good pain relief at 12 months after surgery, with a median WOMAC pain score in the intervention group of 90 (IQR 30) and in the standard care group of 85 (IQR 35) (Table 44).
| Outcome | Continuous score, by categorised pain level | |
|---|---|---|
| Intervention (n = 157) | Standard care (n = 159) | |
| WOMAC pain at 12 months | ||
| Median (IQR) | 90 (30) | 85 (35) |
| None = 100 | 45 (29%) | 36 (23%) |
| Mild = 75–100 | 44 (28%) | 44 (28%) |
| Moderate = 50–75 | 29 (18%) | 42 (26%) |
| Severe = 0–50 | 15 (10%) | 18 (11%) |
| Missing data | 24 (15%) | 19 (12%) |
The primary analysis revealed that there was no evidence that pain severity at 12 months after surgery was different between the intervention and standard care group (ITT-CC coefficient 3.83, 95% CI –0.83 to 8.49; p = 0.107; Table 45). This finding was consistently observed in the different approaches.
| Model | ITT-CC | ITT-imputed | PP | |||
|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | |
| Baseline | 3.83 (–0.83 to 8.49) | 0.107 | 3.33 (–1.21 to 7.88) | 0.146 | 4.21 (–0.66 to 9.09) | 0.090 |
| Adjusted | 4.14 (–0.51 to 8.80) | 0.081 | 4.16 (–0.37 to 8.69) | 0.082 | 4.60 (–0.28 to 9.50) | 0.064 |
Similar to the THR trial, the assumptions of the linear regression were violated. The partial odds model also revealed no difference in pain severity between the two groups with the exception that the per-protocol and ITT-CC analysis showed some evidence that the intervention reduced the number of patients with severe to moderate pain (Table 46).
| Groups compared | Baseline model | Adjusted model | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| (Moderate, mild or none) vs. reference = (severe) | ||||
| ITT-CC (n = 273) | 1.28 (0.60 to 2.72) | 0.515 | 1.27 (0.58 to 2.78) | 0.543 |
| ITT-imputed (n = 316) | 1.28 (0.63 to 2.61) | 0.497 | 1.34 (0.64 to 2.81) | 0.444 |
| PP (n = 259) | 1.28 (0.60 to 2.72) | 0.515 | 1.29 (0.59 to 2.81) | 0.527 |
| (Mild or none) vs. reference = (severe or moderate) | ||||
| ITT-CC (n = 273) | 1.61 (0.96 to 2.71) | 0.071 | 1.77 (1.02 to 3.07) | 0.042 |
| ITT-imputed (n = 316) | 1.48 (0.89 to 2.48) | 0.131 | 1.65 (0.95 to 2.86) | 0.077 |
| PP (n = 259) | 1.75 (1.03 to 2.97) | 0.039 | 1.94 (1.11 to 3.42) | 0.021 |
| (None) vs. reference = (severe, moderate or mild) | ||||
| ITT-CC (n = 273) | 1.41 (0.82 to 2.43) | 0.216 | 1.55 (0.87 to 2.75) | 0.135 |
| ITT-imputed (n = 316) | 1.39 (0.82 to 2.35) | 0.227 | 1.54 (0.88 to 2.69) | 0.133 |
| PP (n = 259) | 1.48 (0.85 to 2.59) | 0.168 | 1.64 (0.91 to 2.67) | 0.099 |
Secondary outcomes
Details of the analysis of secondary outcomes can be found in Table 47 and Appendices 11–28. There was some evidence that patients in the intervention group had fewer drugs administered on the recovery ward (98 vs. 118; p = 0.010) and reported less vomiting (26% vs. 39%; p = 0.025) and nausea (58% vs. 85%; p = 0.005) on postoperative day 1. There was no difference in any of the other secondary outcomes. Post-surgical superficial and deep wound infection rates were similar in the intervention group and standard care group (3.2% vs. 1.9%; p = 0.500).
| Model | ITT-CC | ITT-imputed | PP | |||
|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | |
| Pain over night | ||||||
| Day 1 | ||||||
| Baseline model | 3.81 (–3.07 to 10.69) | 0.278 | 4.12 (–2.87 to 11.12) | 0.248 | 5.46 (–1.50 to 12.41) | 0.124 |
| Adjusted model | 3.75 (–3.08 to 10.58) | 0.282 | 3.70 (–3.21 to 10.62) | 0.294 | 5.43 (–1.47 to 12.33) | 0.123 |
| Day 2 | ||||||
| Baseline model | 2.30 (–4.49 to 9.10) | 0.506 | 2.39 (–4.47 to 9.25) | 0.494 | 2.35 (–4.52 to 9.21) | 0.503 |
| Adjusted model | 2.20 (–4.54 to 8.95) | 0.522 | 1.97 (–4.85 to 8.80) | 0.571 | 2.26 (–4.56 to 9.08) | 0.516 |
| Day 3 | ||||||
| Baseline model | –3.55 (–10.58 to 3.49) | 0.323 | –2.13 (–9.14 to 4.89) | 0.552 | –3.77 (–10.87 to 3.33) | 0.298 |
| Adjusted model | –3.94 (–10.92 to 3.04) | 0.269 | –2.54 (–9.50 to 4.41) | 0.473 | –4.13 (–11.18 to 2.91) | 0.250 |
| Pain at rest | ||||||
| Day 1 | ||||||
| Baseline model | –2.53 (–7.62 to 2.56) | 0.330 | –2.39 (–7.74 to 2.95) | 0.379 | –1.89 (–7.12 to 3.34) | 0.478 |
| Adjusted model | –2.49 (–7.46 to 2.49) | 0.327 | –2.37 (–7.67 to 2.93) | 0.380 | –1.70 (–6.82 to 3.41) | 0.514 |
| Day 2 | ||||||
| Baseline model | 2.48 (–2.58 to 7.54) | 0.337 | 2.56 (–2.70 to 7.83) | 0.339 | 2.22 (–2.98 to 7.42) | 0.402 |
| Adjusted model | 2.52 (–2.42 to 7.46) | 0.318 | 2.59 (–2.58 to 7.76) | 0.325 | 2.41 (–2.67 to 7.50) | 0.352 |
| Day 3 | ||||||
| Baseline model | 0.63 (–4.56 to 5.83) | 0.811 | 0.73 (–4.47 to 5.94) | 0.782 | 0.06 (–5.27 to 5.38) | 0.983 |
| Adjusted model | 0.54 (–4.53 to 5.62) | 0.833 | 0.76 (–4.33 to 5.85) | 0.769 | 0.12 (–5.08 to 5.33) | 0.963 |
| Pain on movement | ||||||
| Day 1 | ||||||
| Baseline model | –1.08 (–6.64 to 4.47) | 0.702 | –1.06 (–6.88 to 4.77) | 0.721 | –0.69 (–6.41 to 5.03) | 0.814 |
| Adjusted model | –1.12 (–6.66 to 4.41) | 0.690 | –0.98 (–6.90 to 4.93) | 0.744 | –0.73 (–6.42 to 4.96) | 0.802 |
| Day 2 | ||||||
| Baseline model | 0.68 (–4.84 to 6.20) | 0.809 | 0.79 (–4.85 to 6.42) | 0.784 | 0.66 (–5.03 to 6.35) | 0.820 |
| Adjusted model | 0.64 (–4.87 to 6.15) | 0.818 | 0.86 (–4.86 to 6.58) | 0.768 | 0.62 (–5.04 to 6.28) | 0.830 |
| Day 3 | ||||||
| Baseline model | 1.00 (–4.65 to 6.65) | 0.729 | 1.07 (–4.86 to 6.99) | 0.724 | 0.58 (–5.23 to 6.39) | 0.845 |
| Adjusted model | 0.90 (–4.75 to 6.53) | 0.753 | 1.14 (–4.82 to 7.11) | 0.706 | 0.49 (–5.29 to 6.27) | 0.868 |
Comparison of the results of the hip and knee trial
Table 48 presents the effect of the intervention when all the participants of the THR and TKR trials are combined. The results are adjusted for pre-operative WOMAC pain scores, surgical approach and surgical site (hip or knee). Patients in the intervention group had a higher mean WOMAC score at 12 months after surgery than those in the standard care group (ITT-CC, between-group difference in mean WOMAC at 12 months: 4.10, 95% CI 1.08 to 7.12; p = 0.008; Table 48). This finding was consistently observed in the different approaches.
| Intervention effect | ITT-CC | ITT-imputed | PP | |||
|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | |
| Effect | 4.10 (1.08 to 7.12) | 0.008 | 4.28 (1.19 to 7.36) | 0.007 | 3.89 (0.77 to 7.00) | 0.014 |
The intervention effect observed in the hip patients was then contrasted with the intervention effect observed in the knee using an interaction term between the intervention effect (intervention or standard care) and site of surgery (hip or knee) in the above model. Despite the statistical significant effect observed in the hip group (ITT-CC, MD: 4.74; p = 0.015; see Table 40) and the non-significant effect observed in the knee group (ITT-CC, MD: 3.83; p = 0.107; see Table 45), these two effects were not statistically different (ITT-CC: p = 0.904; ITT-imputed: p = 0.657; and P: p = 0.682).
Discussion
These are the first reported RCTs powered to investigate the clinical effectiveness of local anaesthetic infiltration at reducing chronic pain at 12 months after THR and TKR. Administering local anaesthetic infiltration is easy and quick, with no increased morbidity or hospital stay. Our trials found evidence that local anaesthetic wound infiltration can reduce pain severity at 12 months after THR, but not TKR. The reasons for the observed difference in the effectiveness of the intervention in TKR and THR patients are unclear and require further research, but one contributing factor could be the use of FNB in both arms of the TKR trial.
Strengths of these trials include the long-term postoperative follow-up, the use of robust and validated outcome measures to assess pain, good rates of data collection for the primary outcome measure and use of an independent allocation system and blinding to minimise bias. Our sample population is representative of the population undergoing THR and TKR as a whole with a similar disease profile, sex mix and age range as reported by the National Joint Registry for England and Wales,3 and other national registries;430 thus, we believe our results to be generalisable. However, our trials do have weaknesses and it is important to note that the number of patients with severe chronic post-surgical pain in the THR trial was small, generating wide 95% CIs for the ORs in the secondary analysis of the primary outcome. These risks should be interpreted with caution. It is also important to keep in mind that the trials were not powered for the analyses of the primary outcome as categorical variables or to detect differences in treatment effect for the secondary outcomes. This may explain the lack of strong evidence for an intervention effect on acute postoperative pain in the two trials.
For many patients, THR and TKR are effective treatments for painful osteoarthritis, and additional interventions to improve pain relief are not required. However, a sizeable proportion of people report chronic post-surgical pain. 18 The findings of this trial suggest that local anaesthetic infiltration is beneficial for decreasing long-term pain in THR patients but not in TKR patients. In addition, we found some evidence that the intervention may reduce neuropathic pain at 12 months after THR, indicating that infiltration may benefit those patients experiencing severe and/or neuropathic pain in the long term, both of which can be difficult to treat once established. 431 Given that approximately 80,000 THR operations are performed annually in England, Wales and Northern Ireland,3 and 7–23% of patients are likely to develop severe chronic post-surgical pain,18 our findings suggest that routine use of local anaesthetic infiltration has the potential to improve pain outcomes for between 4600 and 15,300 patients every year in the NHS.
Conclusion
The double-blind APEX RCTs provide evidence that administering local anaesthetic infiltration before wound closure reduces chronic post-surgical pain at 1 year after THR. This suggests that the routine use of infiltration would be beneficial in improving long-term pain relief for THR patients. Findings from our TKR trial suggest that local anaesthetic infiltration would have little long-term benefit for TKR patients. However, it must be noted that the majority of patients had good or excellent long-term pain relief after both hip and knee replacement, regardless of whether they were randomised to local anaesthetic infiltration or not.
The cost-effectiveness of local anaesthetic wound infiltration on chronic pain after lower limb joint replacement: APEX randomised controlled trials
Introduction
The APEX economic evaluation assessed the cost-effectiveness of local anaesthetic wound infiltration in patients receiving (1) TKR and (2) THR, compared with usual care, at 1 year post surgery, from a NHS and PSS perspective. We also collected data allowing for a future economic evaluation from a societal perspective.
Methods
Resource use identification and collection
Collection of resource-use data was identical for both the hip and the knee trials. Resources used during the initial inpatient stay for joint replacement and subsequent inpatient stays and outpatient visits at Southmead Hospital during the 12 months of follow-up were extracted from medical records onto study designed proformas. Initial inpatient resource use included operating theatre time, perioperative local anaesthetic infiltration in the intervention group, time spent in recovery and number of days admitted to a ward after surgery. After initial hospital discharge, inpatient and outpatient resource-use data collected included the duration and reason for visit, ward details of inpatient admissions including day-case admissions, accident and emergency visits, and outpatient visits and specialty clinics attended during the 12-month follow-up period.
Use of PSS and other NHS resources was collected using patient-completed questionnaires, sent by post at 3, 6 and 12 months’ follow-up. Patient-reported data included secondary care visits at other hospitals; community-based health-care visits including GP, practice nurse, district nurse, community physiotherapist and OT contacts; medication use; and use of PSS, such as food at home and home care worker services, contacts with social workers, equipment provided to patients and changes made to patients’ homes during the follow-up period. We further collected private expenses, such as travel expenses, prescription costs, over-the-counter medications, privately paid equipment, changes made to the patient’s home, the burden of informal care and productivity losses through the collection of time-off work and leisure activities, and help from friends and relatives to allow for further future economic work.
We gave patients resource-use logs at hospital discharge at 3 and 6 months in order to facilitate their completion of these questionnaires. 432 Both the logs and the questionnaires were tailored to the type of joint replacement. Examples of the 3-month resource-use questionnaire and log are available online on the Data Instruments for Resource Use Measurement (DIRUM) database. 433,434
Valuation of resource use
Resources used during the initial hospital stay were valued using a microcosting approach, using unit costs obtained from the North Bristol Trust finance department. Estimates for time spent in theatre and recovery and admissions to hospital wards included staff time, overheads, consumables and medications. Unit costs for the local anaesthetic infiltration injection were provided by the Management and Procurement Department at Southmead Hospital.
Hospital resources used during the follow-up period were macrocosted and valued using Department of Health Reference costs435 for specialty outpatient clinic and tariffs for inpatient admissions. When tariffs could not be derived because of insufficient information, inpatient admissions were microcosted using the number of nights spent in the ward and a day ward cost estimate from the North Bristol Trust finance department.
Community-based resources and PSS were valued using Curtis’s Unit Costs of Health and Social Care. 436 Equipment and changes to patients’ homes, such as dressing aids, furniture raisers, walking aids and chair lifts, were financed by social services, but provided to patients – on loan – through OTs and physiotherapists at Southmead Hospital. We assumed a 2-year life period for all equipment and valued it as the fraction of equipment cost proportional to the duration of patient use. Unit costs were obtained from equipment suppliers to Southmead Hospital or online sources from other suppliers when procurement costs were not available.
Economic outcome measured
The primary health outcome for the APEX economic evaluation was the quality-adjusted life-year (QALY). A QALY is a measure of disease burden that weights survival by QoL. This generic measure allows for direct clinical effectiveness and cost-effectiveness comparisons between interventions across all patient groups and health conditions. NICE guidelines provide recommendations for the UK’s societal willingness to pay for one QALY gained (section 6.3, Decision-Making86) which allows for inferences about the absolute cost-effectiveness of interventions to be made. QALYs for the APEX trial were derived using the EQ-5D-3L questionnaire. 437 APEX patients completed the EQ-5D-3L at baseline, and at the 3-, 6- and 12-month follow-ups. The EQ-5D-3L questionnaire is a simple and quick tool developed by health economists to measure generic HRQoL with the purpose of estimating QALYs. It comprises five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each dimension has three levels: no problems, some problems or severe problems. Each of the possible 243 health states has been assigned a quality weight (utility) through a valuation survey of a sample of the UK population.
The preference-based quality weights are measured on a scale anchored at zero for death and 1 for the best imaginable health. Negative values for ‘health states worse than death’ are also possible. 438 The scale has cardinal properties, for example spending 1 year in a health state with a quality weight of 0.5 is equivalent to spending 6 months in perfect health (quality weight equal to 1). QALYs within APEX were derived for each trial arm, attributing the quality weights from the UK population to the patients’ answers to the EQ-5D-3L questionnaire,439 at baseline (pre-operative), and at the 3-, 6- and 12-month follow-ups. QALYs were then estimated using the area under the curve approach, which assumed a linear change between time points. 440
Data analysis
The economic evaluations in relation to total hip and TKR were conducted as two separate analyses; however, the same methodology was used for both analyses. The primary analysis for both of these evaluations was an ITT analysis and took a NHS and PSS perspective, in line with NICE guidelines. 86 Costs and QALYs were not discounted because of the 1-year duration of follow-up. All costs are reported in 2012–13 prices.
Costs were estimated by multiplying units of resource use by its unit cost (Table 49).
| Resource use | Unit cost | Assumption | Source |
|---|---|---|---|
| Initial inpatient admission | |||
| Theatre (per minute) | £14.22 | Includes implant cost, staff time, overheads, consumables, facilities | Mr Michael Iwasiuk, North Bristol NHS Trust Finance Department, 2014, personal communication |
| Injection of local anaesthesia infiltration | £2.00 | Box of bupivacaine with adrenaline 0.25%/1 in 200,000 is £20.00. One box contains 10 ampoules | Mrs Helen Wright, North Bristol NHS Trust Finance Department, 2014, personal communication |
| Recovery (per minute) | £3.84 | Includes staff time with overheads, consumables, facilities and medications administered during stay. Base cost per minute | Mr Michael Iwasiuk, North Bristol NHS Trust Finance Department, 2014, personal communication |
| Day in general orthopaedics ward: Frome, Severn, Kennett and Critical Care Unit | £311 | Includes staff time with overheads, consumables, facilities and medications administered during stay. Base cost per day | Mr Michael Iwasiuk, North Bristol NHS Trust Finance Department, 2014, personal communication |
| Day in other orthopaedics ward: Chew | £250 | Includes staff time with overheads, consumables, facilities and medications administered during stay. Base cost per day | Mr Michael Iwasiuk, North Bristol NHS Trust Finance Department, 2014, personal communication |
| Day in high-dependency unit | £1356 | Includes staff time with overheads, consumables, facilities and medications administered during stay. Base cost per day | Mr Michael Iwasiuk, North Bristol NHS Trust Finance Department, 2014, personal communication |
| Inpatient admissions following discharge from initial surgery | |||
| Revision surgery: TKR | £9439 | HB22 A Major Knee Procedures for Non-Trauma, with Major CC | NHS Reference Costs 2012–13: Non elective Long Stay435 |
| Revision surgery: THR | £8890 | HB12 A Major Hip Procedures for Non-Trauma, with Major CC | NHS Reference Costs 2012–13: Non elective Long Stay435 |
| Manipulation under anaesthetic: TKR | £2044 | HB24C Minor Knee Procedures for Non-Trauma, Category 2, without CC | NHS Reference Costs 2012–13: Non elective Long Stay435 |
| Infections | £4124 | Infections of Bones or Joints, with CC Score 5–8 | NHS Reference Costs 2012–13: Non elective Long Stay435 |
| Day case procedures: TKR | £655 | Day case: HB29Z Minimal Knee Procedures for Non-Trauma | NHS Reference Costs 2012–13: Non elective Day Case435 |
| Day case procedures: THR | £788 | Day case: HB19Z Minimal Hip Procedures for Non-Trauma | NHS Reference Costs 2012–13: Non elective Day Case435 |
| Nights in hospital for other admissions | £311 | Unit cost based on SMH cost per night in general orthopaedics ward | North Bristol NHS Trust Finance department |
| A&E and outpatient visits | |||
| A&E | £117 | Average of all accident and emergency visits | NHS Reference Costs 2012–13: Outpatient appointments: 180 Accident and Emergency435 |
| Trauma and orthopaedics: consultant led | £102 | Non-admitted face-to-face attendance, follow-up, consultant led | NHS Reference Costs 2012–13: Outpatient appointments: 110 Trauma and Orthopaedics435 |
| Trauma and orthopaedics: non-consultant led | £90 | Non-admitted face-to-face attendance, follow-up, non-consultant led | NHS Reference Costs 2012–13: Outpatient appointments: 110 Trauma and Orthopaedics435 |
| Physiotherapy: non-consultant led | £39 | Non-admitted face-to-face attendance, follow-up, non-consultant led | NHS Reference Costs 2012–13: Outpatient appointments: 650 Physiotherapy435 |
| General medicine: consultant led | £145 | Non-admitted face-to-face attendance, follow-up, consultant led | NHS Reference Costs 2012–13: Outpatient appointments: 300 General Medicine435 |
| Neurology: consultant led | £157 | Non-admitted face-to-face attendance, follow-up, consultant led | NHS Reference Costs 2012–13: Outpatient appointments: 400 Neurology435 |
| Respiratory: consultant led | £137 | Non-admitted face-to-face attendance, first appointment, consultant Led | NHS Reference Costs 2012–13: Outpatient appointments: 340 Respiratory Medicine435 |
| Pain management: consultant led | £136 | Non-admitted face-to-face attendance, follow-up, consultant led | NHS Reference Costs 2012–13: Outpatient appointments: 191 Pain management435 |
| Vascular: consultant led | £133 | Non-admitted face-to-face attendance, follow-up, consultant led | NHS Reference Costs 2012–13: Outpatient appointments: 107 Vascular surgery435 |
| Dermatology: consultant led | £95 | Non-admitted face-to-face attendance, follow-up, consultant led | NHS Reference Costs 2012–13: Outpatient appointments: 330 Dermatology435 |
| Haematology: consultant led | £209 | Non-admitted face-to-face attendance, follow-up, consultant led | NHS Reference Costs 2012–13: Outpatient appointments: 303 Clinical Haematology435 |
| Community-based health services | |||
| GP surgery visit | £45 | Base cost per patient contact with GP with qualifications, including direct care staff costs, lasting 11.7 minutes | PSSRU 2013: 10.8b General practitioner441 |
| GP home visit | £114 | Base cost per out of surgery visit with GP with qualifications, including direct care staff costs, lasting 23.4 minutes | PSSRU 2013: 10.8b General practitioner441 |
| Phoned GP for advice | £27 | Base cost per telephone consultation with GP with qualifications, including direct care staff costs, lasting 7.1 minutes | PSSRU 2013: 10.8b General practitioner441 |
| GP practice nurse visit | £13.43 | Based on 15.5 minutes per surgery consultation using the base cost (£52) of 1 hour of face-to-face contact with GP nurse with qualifications | PSSRU 2013: 10.6 Nurse (GP practice)441 |
| Phoned GP practice nurse for advice | £4 | Based on 6 minutes of GP nurse time using the base cost (£40) of 1 hour of GP nurse time with qualifications | PSSRU 2013: 10.6 Nurse (GP practice)441 |
| Repeat prescription (without seeing doctor) | £11.40 | Based on 3 minutes of GP time, using the base cost of 1 minute GP patient contact time (£3.80), with qualifications, including direct care staff costs | PSSRU 2013: 10.8b General practitioner441 |
| District nurse | £18.08 | Based on the assumption that the duration of a DN visit is the same as GP nurse visit (15.5 minutes) and using the base cost of 1 hour of community nurse visit (£70) with qualifications including travel | PSSRU 2013: 10.1 Community nurse441 |
| OT at home/GP surgery/clinic | £17 | Based on 30 minutes contact using the base cost (£34) of 1 hour of OT contact with qualifications | PSSRU 2013: 9.2 NHS community OT441 |
| Community physiotherapist at home/GP surgery/clinic | £17 | Based on 30 minutes contact using the base cost (£34) of 1 hour of physiotherapist contact with qualifications | PSSRU 2013: 9.1 Community physiotherapist |
| Prescriptions costs per consultation | £44.64 | Prescription costs per consultation (net ingredient cost) | PSSRU 2013:10.8b General practitioner |
| Social services | |||
| Home care worker (home help) provided by social services | £24 | Based on 1 hour of face-to-face weekday contact for independent sector home care provided for social services | PSSRU 2013: 11.6 |
| Food at home service (meals on wheels) | £3.14 | Based on one meal a day using the meals on wheels average weekly cost (2012/13) of £44, assuming two meals per day, 7 days a week | PSSRU 2013: 8.1.1 Community care package for older people: very low cost |
| Social worker visits | £113 | Based on a 30-minute visit using the base cost (£226) of 1 hour of face-to-face contact of social worker with qualifications | PSSRU 2013: 11.2 Social worker (adult services) |
| Social worker telephone calls | £39.50 | Based on a 30-minute telephone call using a base cost (£79) 1 hour of client related work of a social worker with qualifications | PSSRU 2013: 11.2 Social worker (adult services) |
| Home changes and equipment provided by social services | All unit costs for home changes and equipment are based on 3-month loan period, assuming a 24 months life span | ||
| Toilet seat or toilet raiser | £1.80 | Cost of equipment £14 | NRS price – equipment provider for Southmead Hospital (Mrs Catherine Hale, Head of Occupational Therapy at North Bristol NHS Trust, 2014, personal communication) |
| Dressing aids: socks, shoes, etc. | £1.25 | Cost of equipment £10 | NRS price – equipment provider for Southmead Hospital (Mrs Catherine Hale, Head of Occupational Therapy at North Bristol NHS Trust, 2014, personal communication) |
| Furniture raisers | £2.48 | Cost of equipment £20 | NRS price – equipment provider for Southmead Hospital (Mrs Catherine Hale, Head of Occupational Therapy at North Bristol NHS Trust, 2014, personal communication) |
| Perching stool | £6.00 | Cost of equipment £48 | NRS price – equipment provider for Southmead Hospital (Mrs Catherine Hale, Head of Occupational Therapy at North Bristol NHS Trust, 2014, personal communication) |
| Walker or trolley | £7.50 | Cost of equipment £60 | NRS price – equipment provider for Southmead Hospital (Mrs Catherine Hale, Head of Occupational Therapy at North Bristol NHS Trust, 2014, personal communication) |
| Crutches | £3.75 | Cost of equipment £30 | NRS price – equipment provider for Southmead Hospital (Mrs Catherine Hale, Head of Occupational Therapy at North Bristol NHS Trust, 2014, personal communication) |
| Commode | £5.69 | Cost of equipment £46 | NRS price – equipment provider for Southmead Hospital (Mrs Catherine Hale, Head of Occupational Therapy at North Bristol NHS Trust, 2014, personal communication) |
| Rails and hand grips | £2.85 | Cost of equipment £23 | NRS price – equipment provider for Southmead Hospital (Mrs Catherine Hale, Head of Occupational Therapy at North Bristol NHS Trust, 2014, personal communication) |
| Bath boards | £3.00 | Cost of equipment £24 | NRS price – equipment provider for Southmead Hospital (Mrs Catherine Hale, Head of Occupational Therapy at North Bristol NHS Trust, 2014, personal communication) |
| Hospital bed at home | £59.88 | Cost of equipment £479 | Google (Google Inc., Mountain View, CA, USA) search for procurement prices (cheaper range) |
| Bath lift | £44.75 | Cost of equipment £358 | Google search for procurement prices (cheaper range) |
| Chair and stair lift | £125.00 | Cost of equipment £1000 | Google search for procurement prices (cheaper range) |
The total cost for each individual patient for each of the 17 resource-use categories was calculated as the sum of the cost of the resource-use items. All available data were used to calculate means and SDs for resource use and costs for each category by trial arm. This enables comparisons between trial arms of absolute resource use and costs to aid decision-making. The cost categories were then grouped into initial inpatient stay costs, secondary care costs during the follow-up period, community-based health-care costs including medication, and PSS costs. The total cost for each individual patient for these four groups as well as total NHS costs and total NHS and PSS costs were calculated as above.
Mean and SDs for QALYS were calculated for each arm of the two trials.
Incremental costs for the four main cost groupings, and QALY differences between arms, were then estimated using ordinary least squares (OLS) regression, with robust standard errors adjusting for the APEX trial treatment group allocation and minimisation variables: baseline WOMAC pain score and surgical approach (see Equation 1). QALYs were further adjusted for baseline utility imbalances (see Equation 2). 443
Equation 1 for costs:
Equation 2 for QALYs:
where i = individual patient, j = surgery: TKR or THR, k = cost category, and error terms eijk and µijk are heteroskedasticity consistent (or robust) standard errors. 442 Baseline WOMAC pain score and surgical approach are the two variables used in the minimisation process for randomisation in both APEX trials.
Missing cost and QALY data were imputed using White’s chained equations for multiple imputation426 and Royston’s ‘ice’ command in Stata 13,444 to generate 20 complete data sets. This method uses regression techniques to estimate missing values, based on the values of available data. The 17 cost categories and four EQ-5D utility scores (baseline and three follow-up time points) were imputed jointly, by treatment group allocation, adjusting for trial minimisation variables and patient baseline characteristics [age, sex, BMI and dichotomous variables for education level (high vs. medium or low) and marital status (single vs. married or other)]. The imputation model was run for the hip and knee trial data separately and used predictive mean matching by trial arm. This avoided unrealistic utility scores or cost values. QALYs and grouped cost categories were then recalculated using the imputed values and incremental costs and QALYs with imputed data were then re-estimated using the models in Equations 1 and 2. A decision was made prior to the analysis that if the missing data were > 30%, then the primary analysis results would be based on the imputed data.
The incremental costs and QALYs for both trials were then examined for dominance, that is, when one of the arms cost less but had improved outcome compared with the other arm. If no arm was dominant then incremental cost-effectiveness ratios were estimated.
Incremental net monetary benefit (INMB) statistics for a range of societal willingness-to-pay thresholds were estimated. The net monetary benefit statistic is estimated by multiplying the QALY gained by the societal willingness to pay for QALY gained (λ) and then deducting the cost difference between the intervention and the control arm (see Equation 3).
Equation 3 for the INMB:
where LAI is the local anaesthetic infiltration arm and λ is the societal willingness to pay for a QALY threshold.
The INMB statistic quantifies whether or not society is willing to pay for incremental cost of providing the intervention. Positive INMB statistics indicate a cost-effective intervention, whereby society is willing to pay more for the health gain than the intervention costs, for a given willingness to pay threshold. We used the thresholds (λ) of £10,000 per QALY and NICE recommended £20,000 and £30,000 per QALY86 to estimate INMB statistics. In order to account for the uncertainty around the economic results, bootstrapped confidence intervals (BCIs) with 1000 replications were estimated for the adjusted costs, QALYs and the three INMB statistics. We further plotted bootstrapped cost and QALY estimates in cost-effectiveness planes. Cost-effectiveness acceptability curves (CEACs) illustrate the probability of the intervention being cost-effective, given a range of societal willingness-to-pay thresholds. All analyses were conducted using Stata 13.
Results
Tables 50 and 51 show the mean resource use and costs in total hip and total knee by trial arm.
| Resource | Intervention (N = 163) | Control (N = 159) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean resource use | SD | Mean cost (£) | SD (£) | n | Mean resource use | SD | Mean cost (£) | SD (£) | |
| Initial inpatient stay | ||||||||||
| Theatre time (minutes) | 148 | 99 | 29 | 1407 | 411 | 147 | 101 | 31.6 | 1441 | 449 |
| Recovery time (minutes) | 143 | 103 | 65 | 397 | 251 | 144 | 113 | 77.4 | 435 | 297 |
| Days in wards | 153 | 5.2 | 3.3 | 1597 | 1516 | 154 | 5.2 | 2.8 | 1553 | 886 |
| Secondary care after initial discharge | ||||||||||
| Inpatient admissions after initial dischargea | 115 | – | – | 341 | 1847 | 122 | – | – | 101 | 554 |
| Orthopaedics appointments | 142 | 1.96 | 1.2 | 199 | 121 | 146 | 1.97 | 1.4 | 201 | 138 |
| Physiotherapy appointments | 142 | 0.19 | 0.8 | 7 | 32 | 146 | 0.23 | 0.8 | 9 | 30 |
| A&E visits | 142 | 0.06 | 0.4 | 7 | 46 | 146 | 0.04 | 0.3 | 5 | 30 |
| Other appointments | 142 | 0.04 | 0.3 | 5 | 37 | 146 | 0.04 | 0.3 | 7 | 59 |
| Community-based resources | ||||||||||
| GP contacts | 107 | 1.90 | 3.3 | 61 | 113 | 108 | 2.66 | 4.5 | 83 | 145 |
| Nurse contacts | 110 | 1.60 | 4.1 | 25 | 70 | 114 | 1.24 | 2.7 | 18 | 41 |
| OT contacts | 113 | 0.04 | 0.4 | 1 | 7 | 116 | 0.08 | 0.5 | 1 | 8 |
| Community physiotherapist contacts | 109 | 0.25 | 1.1 | 4 | 19 | 113 | 0.58 | 1.8 | 10 | 30 |
| Prescriptions at GP consultation | 108 | 0.72 | 1.3 | 32 | 60 | 111 | 1.13 | 2.0 | 50 | 87 |
| Total NHS cost | 94 | 3768 | 1534 | 90 | 3818 | 1086 | ||||
| PSS | ||||||||||
| Home care worker (hours) | 139 | 1.11 | 8.1 | 27 | 195 | 144 | 5.36 | 56.3 | 129 | 1351 |
| Meals (food at home services) | 137 | 2.76 | 24.0 | 9 | 75 | 138 | 0.00 | 0.0 | 0 | 0 |
| Contacts with social worker | 138 | 0.05 | 0.5 | 4 | 36 | 144 | 0.13 | 1.1 | 7 | 59 |
| Home changesa | 161 | – | – | 1 | 3 | 158 | – | – | 2 | 5 |
| Total NHS + PSS cost | 90 | 3828 | 1618 | 88 | 3999 | 2088 | ||||
| Resource | Intervention (N = 157) | Control (N = 159) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean resource use | SD | Mean cost (£) | SD (£) | n | Mean resource use | SD | Mean cost (£) | SD (£) | |
| Initial inpatient stay | ||||||||||
| Theatre time (minutes) | 142 | 102 | 32 | 1449 | 453 | 145 | 103 | 32.9 | 1461 | 469 |
| Recovery time (minutes) | 140 | 94 | 44 | 359 | 169 | 136 | 104 | 69.1 | 398 | 265 |
| Days in wards | 147 | 5.9 | 3.9 | 1789 | 1224 | 149 | 5.2 | 2.9 | 1586 | 1034 |
| Secondary care after initial discharge | ||||||||||
| Inpatient admissions after initial dischargea | 103 | – | – | 104 | 533 | 110 | – | – | 296 | 907 |
| Orthopaedics appointments | 128 | 2.06 | 1.5 | 209 | 149 | 137 | 1.99 | 1.4 | 202 | 143 |
| Physiotherapy appointments | 128 | 0.44 | 2.1 | 17 | 82 | 137 | 0.40 | 1.3 | 16 | 50 |
| A&E visits | 128 | 0.16 | 0.7 | 18 | 84 | 137 | 0.18 | 1.2 | 20 | 145 |
| Other appointments | 128 | 0.00 | 137 | 0.03 | 0.2 | 4 | 34 | |||
| Community-based resources | ||||||||||
| GP contacts | 85 | 2.65 | 4.3 | 84 | 151 | 102 | 3.83 | 5.7 | 122 | 212 |
| Nurse contacts | 90 | 0.98 | 1.4 | 14 | 22 | 104 | 1.09 | 2.7 | 16 | 43 |
| OT contacts | 95 | 0.28 | 1.1 | 5 | 19 | 105 | 0.25 | 1.3 | 4 | 22 |
| Community physiotherapist contacts | 90 | 1.03 | 2.7 | 18 | 45 | 107 | 1.29 | 3.5 | 22 | 60 |
| Prescriptions at GP consultation (GP practice) | 87 | 1.30 | 2.7 | 58 | 121 | 105 | 1.74 | 3.7 | 78 | 165 |
| Total NHS cost | 72 | 3862 | 1310 | 82 | 4239 | 1803 | ||||
| PSS | ||||||||||
| Home care worker (hours) | 135 | 1.17 | 12.9 | 28 | 310 | 136 | 1.24 | 14.4 | 30 | 346 |
| Meals (food at home services) | 132 | 0.14 | 1.6 | 0 | 5 | 129 | 0.11 | 1.2 | 0 | 4 |
| Contacts with social worker | 133 | 0.11 | 1.1 | 4 | 45 | 134 | 0.14 | 1.6 | 7 | 84 |
| Home changesa | 157 | – | – | 3 | 13 | 158 | – | – | 1 | 4 |
| Total NHS + PSS cost | 72 | 3868 | 1309 | 79 | 4276 | 2076 | ||||
Available case categorical resource use and costs
For both total hip and knee replacement, the available case results do not suggest that administering local wound infiltration analgesia perioperatively increases operation time in theatre; however, local anaesthetic infiltration may reduce time in recovery by about 10 minutes. For THR, during the initial inpatient stay, the mean number of days spent in the ward was the same (5.2 days) for both trial arms. However, for TKR, the intervention arm had 5.9 mean number of ward days compared with 5.2 in the control arm.
In both THR and TKR, the intervention group seemed to have less community-based resource use, particularly in relation to GP contacts, than the control group.
For TKR, there were lower costs owing to hospital readmission for patients in the intervention group, whereas the reverse was true for patients receiving THR.
Personal Social Services costs contributed minorly to the overall costs of delivering treatment for both types of joint replacement.
All cost drivers for these trials display high variability, with large SDs around the categorical mean cost estimates.
Complete case costs
In the THR trial, 90 out of the 163 (55%) patients in the intervention arm and 88 out of the 159 (55%) patients in the control arm had complete NHS and PSS cost data. In the TKR trial, the corresponding figures were 72 out of 157 (46%) for the intervention arm and 79 out of 159 (50%) for the control arm patients. For the complete case analysis total unadjusted mean NHS and mean NHS + PSS cost were lower in the intervention group than in the control group for the two types of joint replacement.
Tables 52 and 53 report the economic evaluation results and differences between arms in outcomes with BCIs and totals by cost categories.
| Outcome or cost | Difference (intervention – control) | |||
|---|---|---|---|---|
| n | Mean | 95% CI | p-value | |
| QALYs | ||||
| QALY gain: available cases (unadjusted) | 216 | 0.071 | 0.018 to 0.124 | |
| QALY gain: available cases (adjusted)a | 216 | 0.064 | 0.018 to 0.110 | 0.007 |
| QALY gain: imputed data (adjusted)b | 322 | 0.056 | 0.015 to 0.097 | 0.008 |
| Cost (£) | ||||
| Initial inpatient stay | ||||
| Total of inpatient stay: available cases (unadjusted) | 274 | –127 | –367 to 114 | |
| Total of inpatient stay: available cases (adjusted)c | 273 | –123 | –364 to 118 | 0.32 |
| Total of inpatient stay: imputed data (adjusted)b | 322 | –24 | –345 to 297 | 0.88 |
| Secondary care after initial discharge | ||||
| Inpatient admissions after initial discharge: available cases (unadjusted) | 237 | 240 | –115 to 595 | |
| Inpatient admissions after initial discharge: available cases (adjusted)c | 236 | 251 | –114 to 617 | 0.18 |
| Inpatient admissions after initial discharge: imputed data (adjusted)b | 322 | 63 | –308 to 434 | 0.74 |
| Total outpatient visits: available cases (unadjusted) | 288 | –4 | –39 to 30 | |
| Total outpatient visits: available cases (adjusted)c | 287 | –2 | –36 to 32 | 0.92 |
| Total outpatient visits with: imputed data (adjusted)b | 322 | 2 | –36 to 39 | 0.94 |
| Total secondary care cost after initial discharge: available cases (unadjusted) | 232 | 239 | –138 to 617 | |
| Total secondary care cost after initial discharge: available cases (adjusted)c | 231 | 251 | –136 to 639 | 0.2 |
| Total secondary care cost after initial discharge: imputed data (adjusted)b | 322 | 64 | –314 to 443 | 0.74 |
| Community-based resources | ||||
| Total community-based costs: available cases (unadjusted) | 209 | –25 | –82 to 31 | |
| Total community-based costs: available cases (adjusted)c | 209 | –23 | –79 to 33 | 0.41 |
| Total community-based costs: imputed data (adjusted)b | 322 | –47 | –116 to 22 | 0.18 |
| Total NHS cost: available cases (unadjusted) | 184 | –50 | –436 to 335 | |
| Total NHS cost: available cases (adjusted)c | 184 | –43 | –427 to 341 | 0.83 |
| Total NHS cost: imputed data (adjusted)b | 322 | –7 | –535 to 521 | 0.98 |
| PSS | ||||
| Total PSS: available cases (unadjusted) | 264 | –100 | –343 to 142 | |
| Total PSS: available cases (adjusted)c | 263 | –83 | –289 to 123 | 0.43 |
| Total PSS: imputed data (adjusted)b | 322 | –167 | –421 to 88 | 0.2 |
| Total NHS + PSS cost: available cases (unadjusted) | 178 | –170 | –724 to 383 | |
| Total NHS + PSS cost: available cases (adjusted)c | 178 | –151 | –680 to 377 | 0.57 |
| Total NHS + PSS cost: imputed data (adjusted)b | 322 | –173 | –762 to 415 | 0.56 |
| RESULTS | ||||
| Mean QALY gain (adjusted, bootstrapped)b | 322 | 0.056 | 0.020 to 0.092 | 0.002 |
| Mean NHS + PSS cost difference (£) (adjusted, bootstrapped)b | 322 | –173 | –658 to 312 | 0.480 |
| INMB statistic: λ = £10,000 (adjusted, bootstrapped)b | 322 | £729 | £62 to £1397 | 0.032 |
| INMB statistic: λ = £20,000 (adjusted, bootstrapped)b | 322 | £1285 | £329 to £2242 | 0.009 |
| INMB statistic: λ = £30,000 (adjusted, bootstrapped)b | 322 | £1841 | £559 to £3122 | 0.005 |
| Outcome or cost | Difference (intervention – control) | |||
|---|---|---|---|---|
| n | Mean | 95% CI | p-value | |
| Outcome | ||||
| QALY gain: available cases (unadjusted) | 201 | 0.015 | –0.045 to 0.075 | |
| QALY gain: available cases (adjusted)a | 201 | 0.010 | –0.039 to 0.060 | 0.68 |
| QALY gain: imputed data (adjusted)b | 316 | 0.012 | –0.035 to 0.058 | 0.62 |
| Cost (£) | ||||
| Initial inpatient stay | ||||
| Total of inpatient stay: available cases (unadjusted) | 268 | 76 | –215 to 367 | |
| Total of inpatient stay: available cases (adjusted)c | 268 | 89 | –194 to 371 | 0.54 |
| Total of inpatient stay: imputed data (adjusted)b | 316 | 179 | –119 to 476 | 0.24 |
| Secondary care after initial discharge | ||||
| Inpatient admissions after initial discharge: available cases (unadjusted) | 213 | –191 | –391 to 8 | |
| Inpatient admissions after initial discharge: available cases (adjusted)c | 213 | –170 | –365 to 24 | 0.086 |
| Inpatient admissions after initial discharge: imputed data (adjusted)b | 316 | –278 | –517 to –38 | 0.024 |
| Total outpatient visits: available cases (unadjusted) | 265 | 2 | –46 to 50 | |
| Total outpatient visits: available cases (adjusted)c | 265 | 2 | –47 to 52 | 0.92 |
| Total outpatient visits with: imputed data (adjusted)b | 316 | 15 | –38 to 68 | 0.57 |
| Total secondary care cost after initial discharge: available cases (unadjusted) | 203 | –194 | –423 to 36 | |
| Total secondary care cost after initial discharge: available cases (adjusted)c | 203 | –165 | –391 to 61 | 0.15 |
| Total secondary care cost after initial discharge: imputed data (adjusted)b | 316 | –263 | –516 to –9 | 0.043 |
| Community-based resources | ||||
| Total community-based costs: available cases (unadjusted) | 177 | –69 | –176 to 37 | |
| Total community-based costs: available cases (adjusted)c | 177 | –65 | –181 to 50 | 0.27 |
| Total community-based costs: imputed data (adjusted)b | 316 | –51 | –153 to 51 | 0.32 |
| Total NHS cost: available cases (unadjusted) | 154 | –377 | –875 to 121 | |
| Total NHS cost: available cases (adjusted)c | 154 | –281 | –761 to 200 | 0.25 |
| Total NHS cost: imputed data (adjusted)b | 316 | –135 | –559 to 289 | 0.53 |
| PSS | ||||
| Total PSS: available cases (unadjusted) | 259 | –4 | –95 to 86 | |
| Total PSS: available cases (adjusted)c | 259 | –4 | –95 to 87 | 0.93 |
| Total PSS: imputed data (adjusted)b | 316 | 4 | –138 to 145 | 0.96 |
| Total NHS + PSS cost: available cases (unadjusted) | 151 | –408 | –962 to 146 | |
| Total NHS + PSS cost: available cases (adjusted)c | 151 | –329 | –851 to 193 | 0.21 |
| Total NHS + PSS cost – imputed data (adjusted)b | 316 | –131 | –595 to 332 | 0.58 |
| Results | ||||
| Mean QALY gain (adjusted, bootstrapped)b | 316 | 0.013 | –0.027 to 0.052 | 0.530 |
| Mean NHS + PSS cost difference (£) (adjusted, bootstrapped)b | 316 | –131 | –501 to £239 | 0.490 |
| INMB statistic: λ = £10,000 (adjusted, bootstrapped)b | 316 | £258 | –£362 to £879 | 0.410 |
| INMB statistic: λ = £20,000 (adjusted, bootstrapped)b | 316 | £386 | –£585 to £1356 | 0.440 |
| INMB statistic: λ = £30,000 (adjusted, bootstrapped)b | 316 | £513 | –£832 to £1857 | 0.450 |
In both the THR and the TKR analyses, differences in the imputed and adjusted NHS and NHS + PSS costs between the arms indicated that patients in the intervention group had lower mean costs than those in the control arm at 1 year.
For THR, differences between the arms in terms of NHS costs were very minor (–£7). In the intervention group, the mean cost per patient was £24 lower for the initial inpatient stay (95% BCI –£345 to £297; p = 0.88), £47 lower for community-based health-care costs (95% BCI –£116 to £22; p = 0.18) and £63 more for readmission costs (95% BCI –£308 to £434; p = 0.74) when compared with the control group. Mean PSS costs were lower in the intervention group by £167 per patient (95% BCI –£421 to £88; p = 0.2). This meant that the combined PSS + NHS mean cost per patient was £173 lower in the intervention group (95% BCI –£762 to £415; p = 0.56).
In contrast, for the intervention group receiving TKR, mean cost per patient for the initial inpatient stay was greater by £179 (95% BCI –£119 to £476; p = 0.24) and the mean cost per patient of readmissions was lower by £278 (95% BCI –£517 to –£38; p = 0.024). This meant an overall lower combined PSS + NHS mean cost of £131 per patient in the intervention group (95% BCI –£595 to £332; p = 0.58) compared with the control group.
For patients receiving THR, the adjusted and imputed 0.056 incremental QALY gain per patient (95% BCI 0.02 to 0.09; p = 0.002) for the intervention group was markedly higher than for those in the control group. This corresponded to patients in the intervention arm spending on average an estimated 20 more days in ‘perfect health’ than patients in the control arm. For the TKR patients, the estimated health benefit for the intervention arm was also positive but the findings were more uncertain, with a mean of 0.012 QALYs gained per patient and wider CIs crossing the null (95% BCI –0.027 to 0.052 QALYs; p = 0.53).
The cost and QALY results indicate that local anaesthetic infiltration is the dominant treatment option, that is, it is cost-saving and more effective than current clinical practice for both THR and TKR surgery.
The INMB statistics in relation to the THR study were positive, even at the more stringent willingness-to-pay threshold of £10,000 per QALY, resulting in a mean INMB of £729 (95% BCI £62 to £1397; p = 0.032). In the TKR analysis, our findings also indicate positive INMB statistics at all willingness-to-pay thresholds, but smaller absolute value statistics and more uncertainty around these estimates, with all BCIs crossing the null. Figures 30 and 31 plot the 1000 replications of the adjusted bootstrapped incremental cost-effectiveness estimates for THR and TKR in the cost-effectiveness plane and the corresponding CEACs.
FIGURE 30.
APEX cost-effectiveness plane and CEAC for THR. (a) Cost-effectiveness plane; and (b) CEAC. LAI, local anaesthetic infiltration.
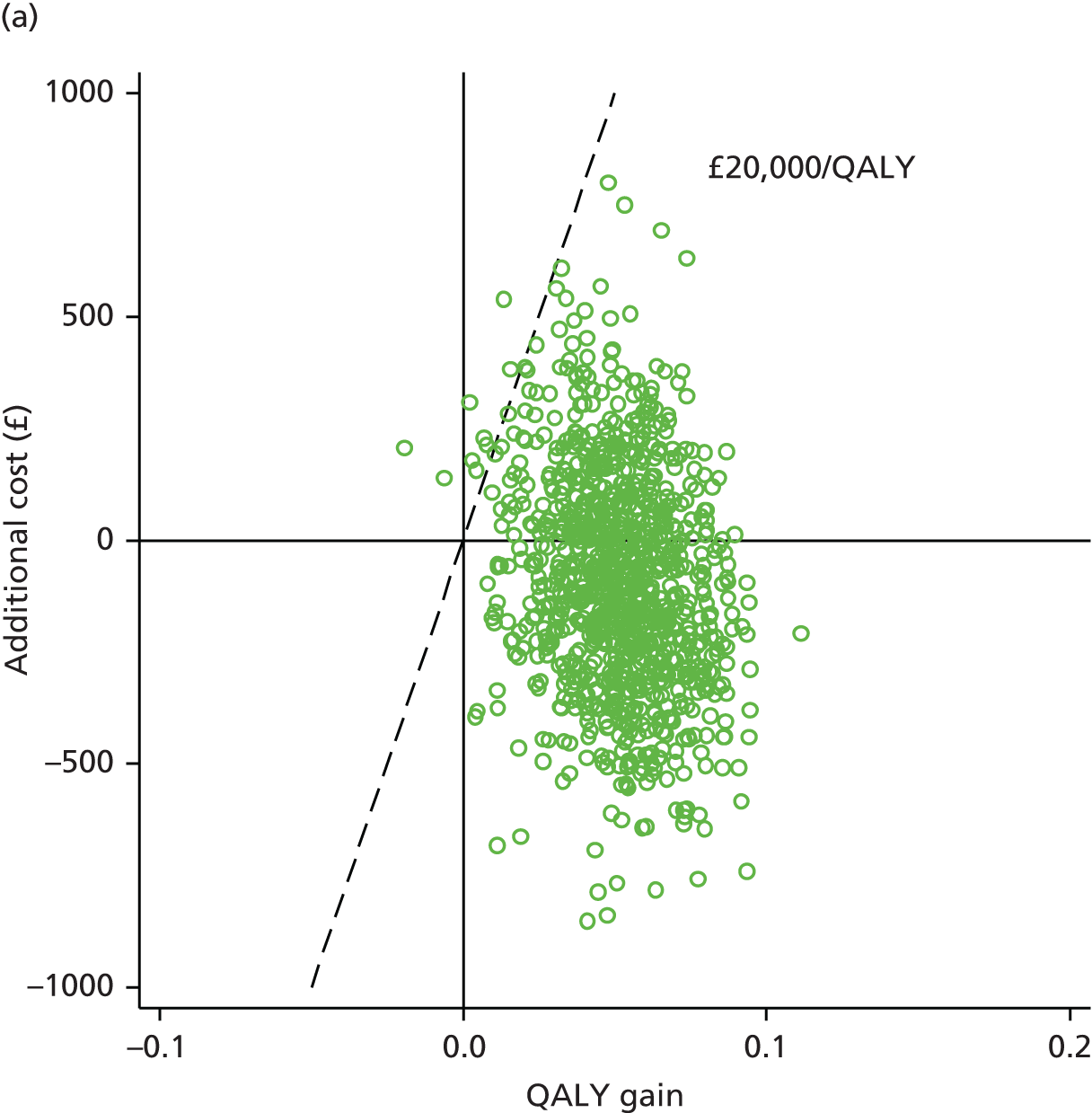
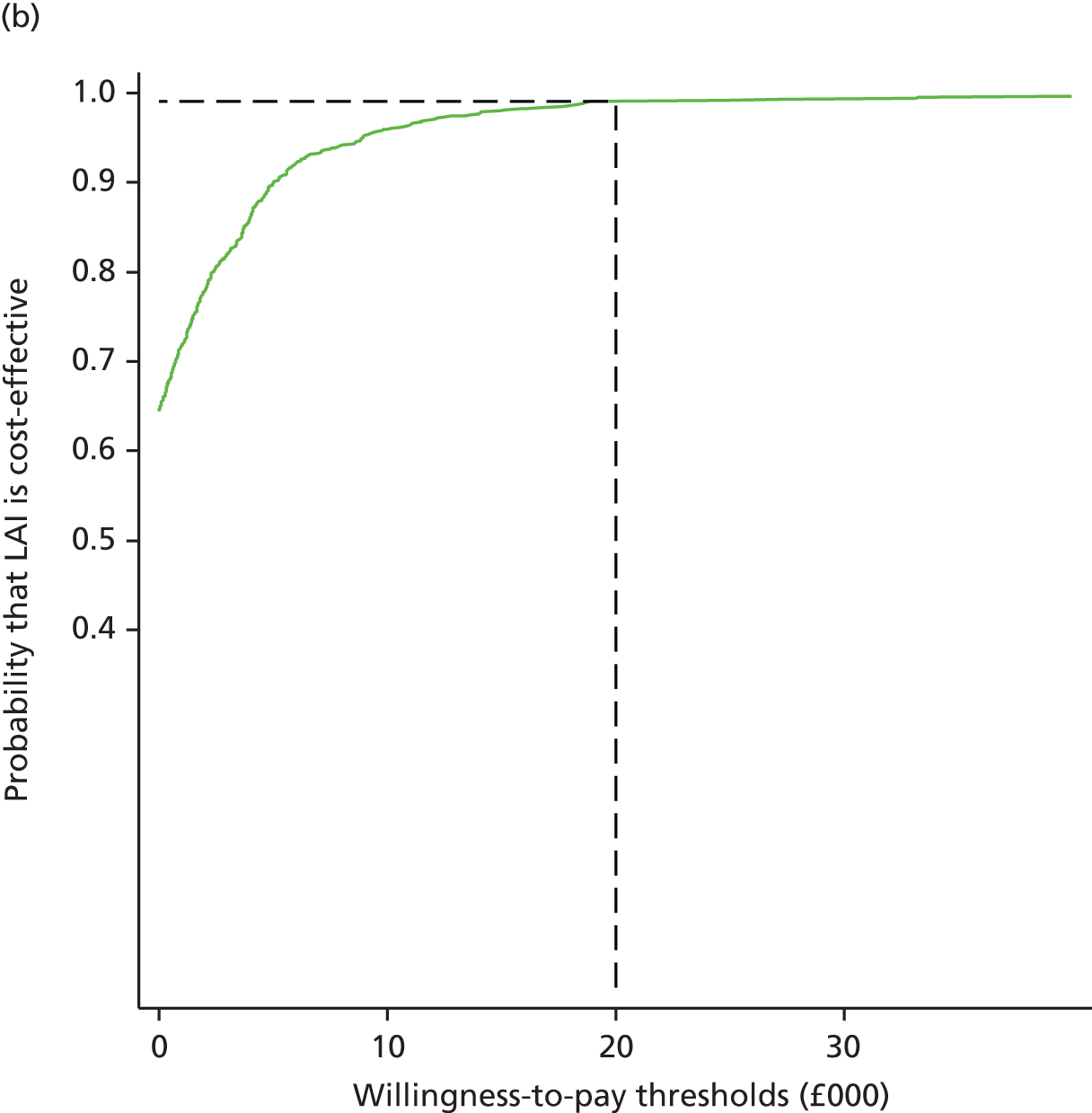
FIGURE 31.
APEX cost-effectiveness plane and CEAC for TKR. (a) Cost-effectiveness plane; and (b) CEAC. LAI, local anaesthetic infiltration.
Our findings are depicted in the cost-effectiveness planes for THR and TKR, with most estimates falling within the south-east quadrant of dominant strategies, more notably so for THR than for TKR. The CEAC shows the uncertainty around the economic results, with a probability of local anaesthetic infiltration being cost-effective in TKR only slightly > 60% at the £10,000 and £20,000 threshold, whereas the probability of it being cost-effective is > 99% in THR at £20,000 per QALY and > 96% at £10,000 per QALY.
Discussion
Our findings suggest that administering local anaesthetic infiltration before wound closure is a cost-effective treatment option, compared with current clinical analgesia regimens in joint replacement surgery. These findings are supported in THR surgery with large positive INMB statistics for the willingness-to-pay thresholds of £10,000, £20,000 and £30,000 and a probability of being cost-effective of > 96% at the lowest threshold. There is no statistical evidence for the positive INMB statistics for TKR surgery, the probability of being cost-effective is only slightly > 60% at £10,000 and £20,000, although results point to local anaesthetic infiltration being the dominant treatment option in both surgeries. Considering results from a NHS perspective only, the cost differences in favour of the intervention arm are greater for TKR than for THR.
Our study has limitations. The economic evaluation was carried out alongside the two APEX RCTs, which were powered to detect a difference in the primary clinical outcome between arms but not in the cost-effectiveness outcomes. Secondary economic analyses to include private expenses and productivity losses incurred during the follow-up period of these trials are planned but have not yet been conducted. Methodological uncertainties need to be explored in sensitivity analyses. For example, in the THR trial, PSS cost differences favouring the intervention arm were driven mainly by one control patient requiring many hours of home care provision. Excluding this patient would potentially lead to a decrease in net monetary benefits. Our bootstrapping methods will have under-represented the uncertainty of our results with smaller CIs; sensitivity analysis on multiple imputation and bootstrapping techniques would address this. Collection of resource-use data, particularly community-based resources and use of PSS, relied on patient-reported data from postal questionnaires completed at three follow-up points. This led to a substantial number of missing data and imputation was therefore needed. The imputed value estimates varied substantially from available case estimates. Such methodological uncertainties need also to be explored in sensitivity analyses.
Conclusion
The addition of local anaesthetic infiltration to the usual analgesia regimen from the perspective of the NHS and PSS is a cost-effective treatment option in primary THR. Given that the intervention was dominant, any uncertainty addressed through future analyses is unlikely to alter the intervention being cost-effective at current NICE thresholds. Our findings also indicate positive health benefits and cost savings in TKR, but with considerably more uncertainty around the cost-effectiveness result. Therefore, there is less evidence in favour of adopting local anaesthetic infiltration before wound closure in routine clinical practice for patients receiving TKR surgery.
Evaluation of patient recruitment
Sound recruitment processes are critical to the success of RCTs and ethical conduct mandates informed decision-making by participants. How trial information is explained is vital but communication and training can be inadequate.
In the early stages of the APEX RCTs, recruitment interviews between research nurses and potential participants were recorded and transcribed and used as the basis of a peer-review intervention to improve trial processes and recruitment. This study describes how this process we have named Internal Peer review for Recruitment Training in Trials (InterPReTiT)368 was developed and used to address the training needs of nurses recruiting to the APEX RCTs.
The aim of this study was to discuss the potential benefits, effectiveness and acceptability of this process as a universal method of training recruiters to trials. The discussion is informed by examination of the review forms the nurses completed over 3 months from 2009 to 2010 when they listened to audio-recordings of their recruitment interviews and by qualitative evaluation of the audio-recordings of the nurses’ discussions of these reviews.
Methods
Ethics approval was gained to record recruitment interviews to allow the peer-review process. The recruiting team consisted of four nurses working in clinical research roles. Before starting recruitment, the recruiting team familiarised themselves with the APEX trial protocols and research literature regarding best practice for trial recruitment.
Role-play was used to rehearse presenting study information using an interactive style. When recruitment began, the nurses gained consent from potential participants to audio-record using a digital recorder. It was explained to potential participants that the recruitment interview was being recorded for training purposes. Two potential participants declined to be recorded. In total, 53 recruitment interviews were recorded, including four interviews with individuals who declined trial participation.
Team members listened to their own, as well as their colleagues’, recruitment interviews and reviewed them using a standardised checklist to identify whether or not the trial aims, the trial arms, equipoise, trial involvement, voluntary participation, blinding and randomisation had been adequately explained. Free-text space was included to record patients’ questions, requests for extra information/clarification and any notable reactions. A roster ensured a different reviewer for each member of the team each week and meetings of the team were scheduled to discuss the reviews. The recruiting team met five times over a 12-week period during pilot phase of the trial.
The recruiting team completed 50 feedback forms, covering 35 recruitment interviews. Recruitment interviews were selected to be reviewed by more than one nurse because they were of particular interest. These included recruitment interviews for which potential participants ultimately declined trial participation and recruitment interviews when communicating the information had been challenging. When the first reviewer thought that a recruitment interview provided an example of good practice, it was recommended to the whole team to review.
Analysis
The findings presented derive from audio-recordings of three out of five peer-review meetings and from the review forms.
Review forms were scrutinised for instances of where the recruiting nurse had failed to convey items of trial information. When one recruitment interview was reviewed by more than one nurse, the review forms were compared to assess concordance across reviewers, both of the checklist items and of the free-text comments. Three out of the five review meetings were audio-recorded with the consent of the recruiting nurses and used in the evaluation. The first meeting was not recorded as we did not immediately recognise the value of doing so. The fifth meeting was not recorded as it had already been agreed at the previous meeting that the training process was complete. No outstanding concerns or uncertainties remained and the nurses now felt confident in their recruitment practice. Only two nurses attended the fifth meeting and nothing new arose from the discussion.
The three audio recordings of the review meetings were transcribed verbatim and analysed using Framework methodology. 445 This accommodates both predetermined themes and themes that arise inductively from the data. Each transcript was read and reread and then coded in duplicate so that salient content was integrated into the coding framework. The review forms were scrutinised for instances where the recruiting nurse had failed to convey one or more items of trial information and the free-text comments were integrated into the coding framework. The coding framework was developed from a review of the literature regarding recruitment difficulties for RCTs and from the need to check the content and delivery of trial information during the recruitment interviews. Certain codes including equipoise, understanding of randomisation and specific information about the trial process were included as predetermined themes in the coding framework. New codes arising from the data were added to these and amalgamated into a final overarching framework. New codes derived from the data, included the role of relatives and managing those who wished to decline.
The data were initially coded by one researcher who is a recruiting nurse who has qualitative research experience and also training in counselling skills. A second researcher who is an experienced qualitative researcher with a social and behavioural science background independently double coded the transcripts and no additional codes were identified.
Results
A flow diagram summarising the peer-review process in the APEX trials is shown in Figure 32.
FIGURE 32.
The peer-review process and evaluation.
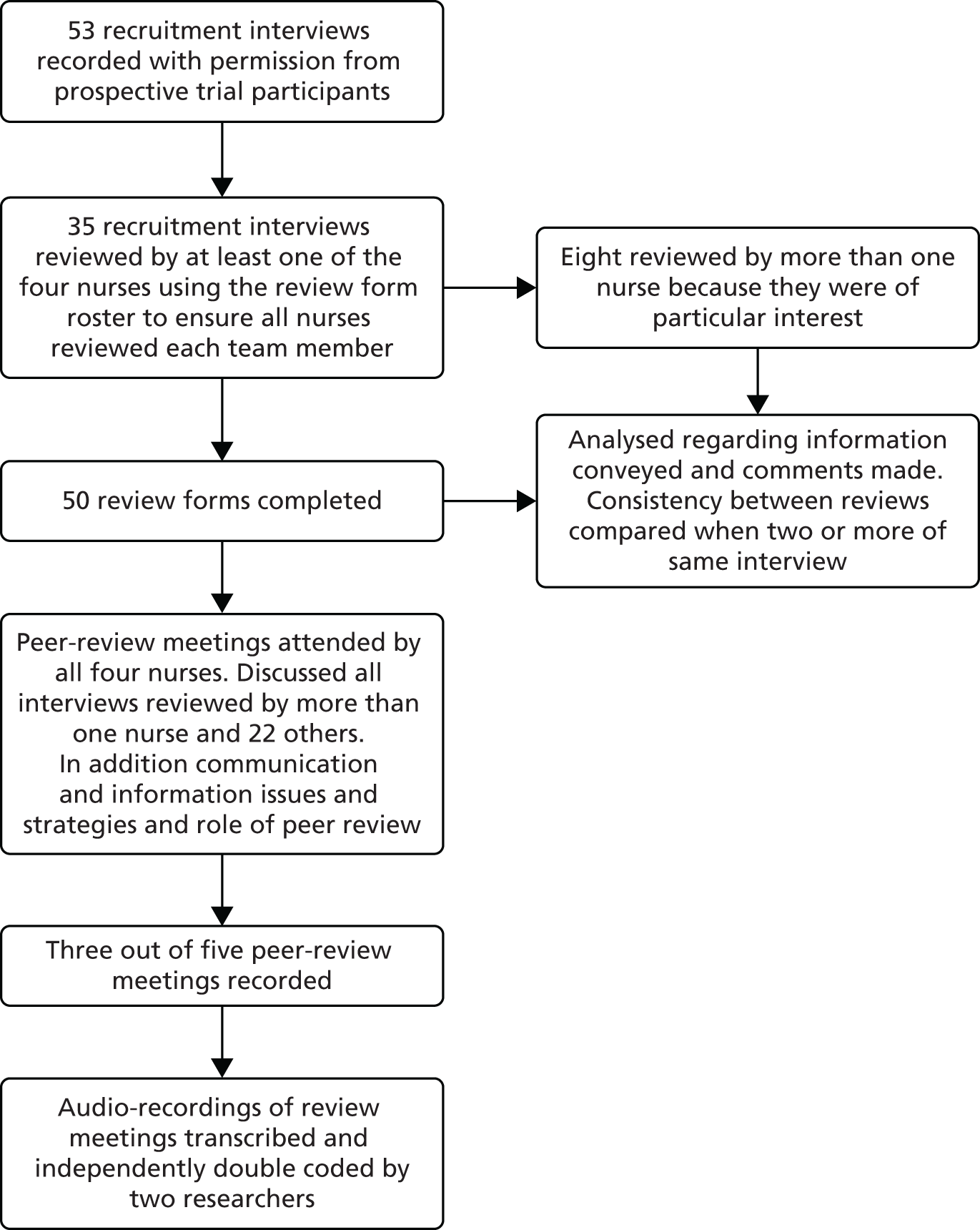
In the interviews that were reviewed by more than one nurse (n = 7), the inter-rater concordance was between 78% and 100% (mean 97%). There was universal agreement over which interviews demonstrated good practice and those which did not.
Interview durations ranged from 6 to 30 minutes, with a mean of 12.4 minutes. Review meetings lasted from 40 to 60 minutes.
Five main themes were identified from the qualitative analysis: provision of information, flexible communication of information, verifying participant understanding, ensuring voluntary participation and recruiters’ perceptions of the peer-review process. The results presented outline each theme and direct changes made to the design and conduct of the APEX trials as a consequence of the findings. Supporting data are presented in Table 54.
| Section A: provision of information | ||
|---|---|---|
| 1 | RN1 second meeting | She was given the message that whether or not she had a spinal was nothing to do with the study whereas what we’ve now found out is that if they’re a hip and they cannot have a spinal they can’t be in the study so if anyone who is for a hip is saying clearly I don’t want a spinal then they shouldn’t be recruited |
| 2 | RN3 second meeting | From reading the information sheet, she hadn’t grasped the trial because she didn’t think she was going to get an anaesthetic, she thought she was just going to get a local by the sounds of what she asked |
| 3 | RN4 third meeting | I think people are reading [the anaesthetic leaflet] and really taking a lot of notice of that and then maybe not giving perhaps quite so much attention to the information sheet |
| 4 | RN1 third meeting | The major benefit is that [the peer-review process] has made us all clarify exactly what the anaesthetic options are |
| Section B: flexible communication of information | ||
| 5 | RN2 second meeting | Patients are very different and you do have to tailor it and you try and make it conversational so it’s not so stilted and so artificial |
| 6 | RN4 third meeting | Most people have dental experience and it’s a good one to use, isn’t it, because people have that understanding |
| 7 | RN3 second meeting | If you have a planned kind of set routine to your patter, you know that you’re potentially going to cover every point but then if you make it fresh for each patient you might miss something |
| 8 | RN1 second meeting | I was . . . trying to keep in my head what I was trying to say and keep it clear and get to the end of it so you kind of override what the patient wants to say |
| 9 | RN2 second meeting | When it’s more participant led . . . you perhaps don’t give everything but you’ve still had a good consenting process, . . . they’re reassured and they actually understand it . . . and that’s come back that . . . they’ve got it |
| Section C: verifying participant understanding | ||
| 10 | RN4 second meeting | I found myself just repeating things just because I wasn’t really getting any feedback from her at all. And I found it really difficult because . . . there wasn’t really any verbal or any other sort of communication from them |
| 11 | RN1 second meeting | It’s checking comprehension isn’t it, which is different to asking if they’ve got any questions, because they might not have questions but they might have completely the wrong idea of the trial |
| 12 | RN3 second meeting | The patient told you their understanding of the study and you gap-filled . . . you could actually tell . . . from listening to it that she understood the study and she was consenting because she knew what was going to happen |
| 13 | RN2 second meeting | I think it’s doing that delicately without patronising. It’s sort of open questions but I know I find it difficult . . . how to get it from them, without patronising them or . . . putting them on the spot |
| Section D: ensuring voluntary participation | ||
| 14 | RN2 third meeting | I’ve said to a guy ‘you’re not quite sure or perhaps this isn’t for you’ and they’ve leapt on that with you know, enthusiasm, ‘yes, that’s quite right’ you know, and that having given them that door they’re out and down the corridor |
| 15 | RN1 fourth meeting | I thought . . . how we can break this up a bit more so that we get a bit more from her and make it possible for her to say no |
| 16 | RN4 second meeting | It is difficult because she had a daughter with her . . . You don’t know if she’s agreeing because, yes OK she is happy or is she agreeing because her daughter thinks it is a good idea? |
| 17 | RN3 fourth meeting | And it also shows . . . with the husband interjecting so much, how much it’s not just the patient you need to win over but the relative with them |
| Section E: recruiters’ perceptions of the peer-review process | ||
| 18 | RN4 second meeting | I just don’t feel very comfortable with tape-recording. I think it is a good way to learn . . . I think it does highlight your own strengths and weaknesses which is a good thing |
| 19 | RN3 second meeting | I was a bit like cringe . . . Oh my god, we’re going to record, we’re going to record and I’m slightly better with it now, but I can see the value of it and the learning experience from it |
| 20 | RN4 second meeting | I think once we knew that it was going to remain within this group that made me feel a bit more comfortable |
| 21 | RN2 third meeting | You want people to feel comfortable so that you can constructively say things about other people, you can reflect on yourself |
| 22 | RN1, RN2, RN4 third meeting | But it helps to increase trust, doesn’t it? In that we are all kind of working the same way |
| Reassurance as well, isn’t it | ||
| I think it’s brought us together more as a team | ||
| 23 | RN2 third meeting | You are more aware of reflecting on was that good, was that bad, why wasn’t it? . . . I think with any new study you develop your pattern and you often get stuck in that pattern. I think with this, I have definitely changed mine . . . and put things differently which I wouldn’t have done otherwise |
| 24 | RN4 third meeting | Wouldn’t you feel if you move onto another study that you’d want to do this |
Provision of information
To ensure that participants’ consent is fully informed, it is essential that trial information is conveyed accurately and consistently and in a manner that is clearly understood by potential participants. The analysis identified both faults in the communication of trial information and participants misunderstanding information.
From comparing recruitment interviews, the nurses identified that the information they were conveying regarding anaesthesia was not consistent between recruiters. It became apparent that the study protocol was unclear regarding how the standard anaesthetic technique for patients undergoing THR differed from that for patients undergoing TKR. Consequently, the diagram used to explain the different anaesthetic techniques to potential participants was incorrect regarding standard anaesthetic care (see Table 54, section A, quotation 1).
In addition, it became apparent that the description of the different anaesthetic techniques in the participant information sheet was frequently misunderstood and that many patients feared they might only receive a local anaesthetic during surgery (see Table 54, section A, quotation 2). This was partly because a separate information booklet about spinal anaesthesia produced by the Royal College of Anaesthetists in the UK was included with the study information. This deterred some participants from considering participation (see Table 54, section A, quotation 3).
Impact on trial design
A substantial amendment was submitted to the ethics committee to amend the participant information sheet, clarifying that the intervention anaesthetic was additional to the current standard anaesthetic. The explanatory diagram used in recruitment was amended to reflect the correct anaesthetic information. The protocol was also amended to remove the booklet about spinal anaesthesia from the study information pack sent to potential participants. Information routinely provided to patients receiving standard care did not include this booklet. The nurses were satisfied that they had now achieved clarity regarding the anaesthetic options (see Table 54, section A, quotation 4).
Flexible communication of information
The nurses recognised that ensuring information needs were properly met required a flexible, interactive, conversational style tailored to each patient, while still conveying standardised information (see Table 54, section B, quotation 5). Different ways of conveying key concepts were explored and the whole group subsequently adopted ones that were felt to be the most effective at communicating the concept because they drew on experiences familiar to the participant, for example explaining the intervention anaesthetic by likening it to the local anaesthetic used for dental work (see Table 54, section B, quotation 6).
In the early stages of recruitment, the recruiting nurses identified difficulty conveying all the necessary information while also making the recruitment interviews interactive and tailored to individual communication needs (see Table 54, section B, quotations 7 and 8). However, the nurses agreed that presenting all the information at once, using a script, made it harder for patients to comprehend and express questions or concerns and was less likely to elicit evidence of understanding (see Table 54, section B, quotation 9).
Impact on trial design
To allow for an interactive, tailored approach to engage participants and address their concerns and information needs, while still ensuring that all the information was conveyed, the use of a checklist instead of script was adopted.
Verifying patient understanding
In order to gain evidence that consent was informed, the nurses needed to stimulate sufficient interaction to address all potential concerns and questions and to confirm understanding. However, some patients were less responsive or did not ask questions (see Table 54, section C, quotations 10 and 11). Recruitment interviews were critiqued regarding helpful and unhelpful techniques to overcome this, such as using open questions, encouraging patients to interrupt, asking what understanding patients had of the study and asking them to summarise what the recruiting nurse had told them. The strategic use of open questions facilitated a participant-centred interaction in which potential gaps in understanding could be explored. However, the task of checking understanding needed to be accomplished respectfully and sensitively (see Table 54, section C, quotations 12 and 13).
Impact on trial design
Different ways of checking participants’ understanding were discussed and subsequently all the recruiters began by asking the potential participant what they had understood about the study from what they had read in the information leaflet. This conferred the advantages of confirming whether or not the patient had read the information leaflet, clarifying how much information they understood and recalled, and inviting the patient to take an active part in the discussion of the trial information from the outset.
Ensuring voluntary participation
Sometimes it was difficult to gauge potential participants’ understanding of the trial and whether or not they were genuinely willing to participate. The nurses realised that some patients may have difficulty in freely discussing their potential participation owing to anxiety about their intended operation, being out of their own environment and feeling somewhat intimidated by professional roles, or might simply find it difficult to say no. Sensitivity to both verbal and non-verbal cues prompted nurses to create opportunities for potential participants to decline (see Table 54, section D, quotations 14 and 15).
The role of participants’ relatives was also discussed as their presence could complicate assessment of truly informed consent. Relatives were often helpful in facilitating the patient to understand key trial information, but sometimes took it on themselves to decide about study involvement on behalf of the patient and had their own questions and concerns that needed addressing (see Table 54, section D, quotations 16 and 17). The nurses identified the need to deal respectfully with relatives’ questions and opinions while at the same time maintaining the focus on the potential participant’s wishes.
Recruitment interviews that ended without consent were felt to be particularly useful in the peer-review process because they provided an opportunity to review whether or not the recruiting nurse might have achieved a different result if the interaction had been managed differently or communication conveyed in a different way. They also provided an opportunity to evaluate the appropriate level of encouragement to participate and to check that if potential participants were reluctant to participate this was respected and handled appropriately. Reviewing these interactions provided an excellent opportunity to discuss the potential tension between maximising recruitment and ensuring that consent was truly voluntary and fully informed. The nurses were reassured that patients were being recruited appropriately and not subject to undue persuasion.
Recruiters’ perceptions of the peer-review process
The recruiting nurses also reflected on their experience of the peer-review process. Initially they were apprehensive about recording and hearing their own recruitment interviews, but recognised the potential benefits (see Table 54, section E, quotations 18 and 19). There was anxiety about feeling judged or being considered less effective than colleagues, but seeing it as a mutual learning experience contained within the nurse team helped to overcome reservations and was important to enable open discussion (see Table 54, section E, quotations 20 and 21).
Important perceived benefits of the peer-review process were reassurance about individual and team practice and increased team cohesion (see Table 54, section E, quotation 22). Other benefits included reflection on personal practice, which prompted changes in individual conduct of recruitment interviews (see Table 54, section E, quotation 23). Overall, the process was felt to be a valuable learning tool because it facilitated reflection and learning from others’ practice in a supportive and reassuring environment. All felt that overall practice and safeguards in recruitment were enhanced and that they would want to repeat this process in subsequent trials (see Table 54, section E, quotation 24).
Discussion
As recruitment to RCTs is pivotal to their success,446 it is crucial to equip recruiters adequately for their important role. In addition to a sound knowledge of protocol and ethical principles, those involved in recruitment require good interpersonal communication skills to respond flexibly to the specific information and comprehension needs of individual participants and to identify their concerns. Previous qualitative analysis of recordings of recruitment interviews447 has highlighted the need to improve communication techniques, in particular adequately explaining equipoise and the process of randomisation, in order to ensure fully informed consent. Acquisition of these skills has been identified as a key area to address to optimise recruitment,447,448 but there is as yet no reproducible process that has been demonstrated to be both effective and acceptable in a range of trials.
We have drawn on prior work conducted in the Prostate Testing for Cancer and Treatment (ProtecT) study, which sought to improve trial processes and recruitment to a multisite RCT using a variety of approaches, including using a peer-review process in which senior staff from one site reviewed the practice of another site. 449 Further work focused on using qualitative analysis of recruitment interviews, followed by feedback to recruiting staff in other trials to see if it could be used to improve recruitment. 450 However, difficulties relating to both logistics and acceptability limited effectiveness. de Salis and colleagues450 found that, because the analysis and feedback process had not been included in the initial study protocol of the trials, they were faced with governance issues which made it very difficult to implement peer review quickly enough to make a difference in trials currently under way. 450 They also found it difficult to get all members of the study team on board and to commit to the process, concluding that it was important to build it into the study protocol at the trial design stage. In addition, reluctance among recruiting nurses to take part in recording recruitment interviews that would be listened to and scrutinised by researchers outside the study team seriously hampered the process.
We aimed to overcome these difficulties. First, the initial APEX trial protocol specified that recordings of the recruitment interviews would be analysed during the pilot phase in order to ensure consistency of trial recruitment and assist with training needs of the study recruitment staff and this was included in all research governance applications. Embedding the peer-review process in the trial design from the outset ensured that all members of staff recruited to deliver the trial were made aware that recording recruitment interviews would be standard practice. Building this process into the protocol also means that the time and costs needed to conduct the peer-review process can be factored in when writing grant applications. Study performance gains, such as increased recruitment and acceptance of randomisation may potentially outweigh the cost of staff time to complete the process, but this needs to be formally evaluated in future work. In our study, the total time taken to listen to the recordings and complete review forms was 10.33 hours, based on an average of 12.42 minutes per recording and a total of 50 recruitment interviews reviewed. The total nurse time spent in review meetings was approximately 16 hours. Not all the interviews that were recorded were reviewed but having a good sample size allowed the purposive selection of interviews that were of particular value for the training process, because they provided examples of good practice, had been particularly challenging, or demonstrated the need for additional clarification about aspects of the trial. Furthermore, the length of time needed to complete the training process was not predetermined and the meetings concluded when consensus was reached among the nurses that no new learning was likely to be achieved and each felt competent in their recruitment practice. The period of time required for training is likely to differ according to the complexity of the trial and the intervention, and the experience of the recruiting nurses, but sufficient time needs to be given for at least two cycles of reviewing, evaluating and sharing practice, implementing new techniques or adjusting practice, and re-evaluating. Sufficient interviews need to be recorded to capture a good variety of interviews and circumstances and of participant interactions and responses.
Second, whereas de Salis and colleagues450 had been hampered by recruiting nurses’ reluctance to record recruitment interviews, we circumvented the nurses’ apprehension that others might scrutinise and appraise them by confining the peer review to the recruiting nurses. In addition, making the peer review exclusive to the recruiting nurse team enhanced team cohesion by helping to build trust and foster working relationships, benefiting both communication and efficiency.
Qualitative methods were used to evaluate the intervention from the points of view of both effectiveness and acceptability to the recruiting nurses. Effectiveness, in terms of benefits for the recruitment process, was evident in several areas. First, adherence to the protocol was improved. As a result of peer review, the recruiters increased their knowledge of the trial design, identified areas of the protocol that were ambiguous and clarified the eligibility criteria. Second, a barrier to recruitment was identified in the information that patients received prior to meeting the recruiting nurse and was subsequently addressed. Third, use of a checklist during review of recruitment recordings improved the consistency and completeness of information given verbally to potential participants. It would have been difficult to identify these issues using other quality control methods449 and, without this structured process during the initial pilot phase, problems may have continued for some time. Fourth, compliance with good clinical practice was enhanced by developing strategies to check participant understanding of trial information and key concepts, ensuring equipoise and gaining better evidence of informed voluntary consent, all of which can impact on trial recruitment and retention. 451 A formal trial of our methods could provide additional important evidence regarding effectiveness, as in this case we had no comparator and were unable to determine whether or not the intervention made a difference to recruitment rates. In future evaluations it would also be valuable to gain consent to analyse the recruitment interviews to unpack the processes at work and to directly compare patients’ and recruiting nurses’ perspectives of the same interview.
The acceptability of recruitment training by means of peer review was evident in the qualitative data. Our evaluation provided similar findings to other studies involving other health professionals and different contexts. Those involved in peer review as a training method often experience initial apprehension but later conclude that it is both supportive and valuable for improving practice. 452 Once initial apprehension was overcome, the recruiting nurses considered peer review to have been a very productive and acceptable training method. Each nurse within the team benefited from individual feedback and the opportunity to learn from other members of the team, which contributed to professional development in a way that was clearly recognised. Consideration needs to be given to how peer review is facilitated so that group members feel safe enough to share examples of practice that they feel are suboptimal. 453 The requisite skills and knowledge also need to be available to the group, either from the facilitator or from within the group as a whole. 452 In our case, one member had previous training in group facilitation and communication skills and others had extensive experience of trial recruitment.
Implications for nurses recruiting patients into clinical trials
Nurses recruiting to trials require additional training because they must obtain consent for an intervention that is administered for reasons other than anticipated benefit to the individual patient. Recruitment training should address both generic and study-specific skills as ‘each RCT has a unique – and uniquely complex – recruitment pathway and its own set of issues that need to be resolved’. 450 Although training those responsible for recruitment to a RCT is a crucial part of the set-up phase of a trial, it is often not done in a structured way. Our intervention provided a constructive forum to compare and critique ways of conveying the study information and become skilled at clearly articulating the trial design, which is vital to maximising recruitment. 454 From this process, we developed a set of recruitment competencies, specific to the APEX trial, that were then used in conjunction with the peer-review training method to train new members of the nurse recruiting team in a structured way. This was an important step towards formalising and evaluating recruitment training and in creating a process that could be applied to other trials. As a result of this work, the NHS sponsoring organisation adopted the principle of assessing the competencies of nurses recruiting to RCTs, creating a two-part competency framework based on the competencies developed in this study. The first part consists of generic competencies related to International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Good Clinical Practice principles. The second part consists of competencies specific to the individual trial, which must be specified and assessed by the principal investigator (PI) in order to delegate responsibility for recruitment to suitably experienced and qualified recruiters.
Conclusions
Sharing experience of recruitment and formal comparison of recruitment interviews in a creative and supportive environment can lead to the identification of best practice, improved communication skills and early awareness of issues and problems which might otherwise have an impact on recruitment for much longer.
Views and experiences of trial participation and use of analgesics
In addition to our longitudinal qualitative research focusing on experience of patients undergoing hip and knee replacement, we also conducted qualitative research with patients and health-care professionals who were involved in the APEX trials. This qualitative study aimed to describe and explore the experience of health-care professionals who were involved in the trials and patients’ experience of participation. The qualitative research with patients also aimed to explore patients’ experiences of joint replacement, with a focus on surgery and medication. Using qualitative methods in the context of controlled trials has long been advocated455 and such methods are valuable to improve understanding of the experiences of patients receiving, and staff delivering, a trial and intervention. 456,457
Background
Involvement in clinical trials
Previous research has highlighted weakness in the present orthopaedic literature, as studies are being given poor ratings in independent meta-analyses and the American Academy of Orthopaedic Surgeons evidence-based clinical guidelines. 458,459 This has led to a growing emphasis on the importance of orthopaedic evidence through higher-quality RCTs. 460
The success of a RCT is dependent on adequate recruitment and retention of patients within a timely manner. It has been suggested that 50% of RCTs fail to recruit their planned number of participants,451 which can undermine the power the study, lead to sampling bias and limit generalisability of results. Little is known about why patients may be motivated to take part in trials, or their experiences of taking part. Understanding these can be integral to successful recruitment and retention. 461 Trial success is equally dependent on the engagement of a large multidisciplinary clinical team and involves effective communication and attention to the study protocol. Less is known about clinical staff members’ experiences of trial involvement. Exploring the views of health-care professionals involved in a delivering RCTs and patients’ participating in the same trials enables the identification of factors that may enhance understanding of how to best deliver RCTs in orthopaedics.
Attitudes to analgesics
Patients living with osteoarthritis use medication including paracetamol, non-steroidal anti-inflammatory drugs and opioid medication to manage their condition. 26,27,462,463 Research has provided insights into how people living with osteoarthritis feel about and use pain relief, highlighting the complexity of adherence to these medications. 26,27 In addition, although persistent pain is the key indication for total hip or knee replacement, the need for pain management continues after surgery. 464,465 Over half of patients undergoing total hip or knee replacement report moderate to severe pain on the first day after surgery. 70 Chronic post-surgical pain is also common, with 10–34% of TKR patients and 7–23% of THR patients reporting long-term pain after their operation. 18 The pain associated with both osteoarthritis and total hip or knee replacement confirms the need for patients to receive appropriate intervention at all stages of the illness trajectory. Therefore, it is important to learn how undergoing total hip or knee replacement affects existing attitudes and behaviours with regard to pain medications.
Methods
Sampling and recruitment
During recruitment to the APEX trials, patients were asked if they were willing to be contacted about taking part in a qualitative interview. From those who agreed to contact we identified a sample of men and women who were a range of ages and comprised a balance of THR and TKR patients. The programme’s qualitative researcher telephoned individuals in this sample and asked if they were still interested in taking part in a qualitative interview. Of the 26 people contacted, 25 agreed to see the researcher to discuss study participation and interview; however, one of these subsequently withdrew from APEX and was no longer eligible to take part. Interviews were also conducted with a range of health-care professionals involved in the APEX trial. Health-care professionals were purposely sampled to include those involved in pre-assessment, surgery and recovery phase of the trial and contacted by telephone or e-mail. Fifteen health-care professionals took part in interviews. The samples are described at the start of Results, below. Data collection proceeded in parallel with analysis and continued until data saturation was reached such that no new insights were being achieved by the end of data collection. 255
Data collection
All participants provided their written, informed consent immediately prior to interview. Interview topic guides were used, informed by the literature. Patients were asked to describe their decision-making regarding trial participation, understanding and experience of the trial. In addition, patients were asked about their views on, and experience of, pain and pain relief as a way of contextualising their experiences of trial participation. Health-care professionals were asked to talk about their views and experiences of trial involvement.
Interviews with patients were conducted 2–4 weeks after surgery in participants’ own homes between April 2010 and January 2011 and lasted between 45 and 120 minutes. Health-care professional interviews were undertaken in hospital premises between March 2011 and June 2012 and lasted between 19 and 40 minutes. The research team comprised qualitative methodologists with backgrounds in social and behavioural sciences.
Data analysis
Interviews were audio-recorded, transcribed and anonymised. Interview transcripts were checked for accuracy and then imported into ATLAS.ti qualitative data analysis software, which aids the management and indexing of qualitative data. Analysis began shortly after data collection started and was ongoing and iterative. Thematic analysis,257 utilising a data-driven inductive approach,466 was used to scrutinise the data in order to identify and analyse patterns and themes of particular salience for participants and across the data set using constant comparison techniques. 467 Transcripts from patients and health-care professionals were analysed separately and, using an inductive approach, we assigned codes to all of the data. We developed a coding framework, which was refined as analysis progressed. The research team grouped the codes and coded material into superordinate themes by identifying connections between the codes and data. 259 To ensure robust analysis a subset of transcripts was independently double coded by other members of the research team and compared, any discrepancies were discussed within the research team and resolved in order to ensure robust analysis. We did not analyse the patient data according to the group allocation of participants within the randomised trial. This is because this nested qualitative study aimed to explore beliefs and behaviour relating to pain around the time of surgery and trial involvement. The trials aimed to study impact of additional analgesia on outcome at 12 months after surgery.
Results
The 24 patients who participated were 11 men and 13 women, aged 26–92 years. Fourteen were in the trial to undergo hip replacement and 10 were in the trial for knee replacement (Table 55). The 15 health-care professionals consisted of four men and 11 women, aged 28–56 years. They comprised three pre-operative clinical nurses, four ward nurses, four orthopaedic surgeons, two anaesthetists and two ward managers. We have not provided a table of participating health-care professionals so anonymity is not compromised. Patients were assigned pseudo initials and health-care professionals were assigned a number with their job role listed when we provide illustrative quotations.
| Pseudonym | Age (years) | Sex | Operated joint |
|---|---|---|---|
| Mrs A | 73 | Female | Hip |
| Mrs B | 65 | Female | Hip |
| Mrs C | 72 | Female | Knee |
| Mr D | 72 | Male | Knee |
| Mrs E | 79 | Female | Hip |
| Mr F | 67 | Male | Hip |
| Mrs G | 59 | Female | Hip |
| Mrs H | 73 | Female | Hip |
| Mr I | 53 | Male | Knee |
| Mrs J | 64 | Female | Knee |
| Mrs K | 63 | Female | Knee |
| Mrs L | 46 | Female | Knee |
| Mr M | 50 | Male | Hip |
| Mr N | 45 | Male | Hip |
| Mrs O | 52 | Female | Hip |
| Mrs P | 76 | Female | Knee |
| Mr Q | 75 | Male | Hip |
| Mr R | 26 | Male | Hip |
| Mr S | 76 | Male | Knee |
| Mr T | 74 | Male | Hip |
| Mrs U | 71 | Female | Knee |
| Mr V | 66 | Male | Knee |
| Mr W | 92 | Male | Hip |
| Mrs X | 77 | Female | Hip |
Findings from the interviews with health-care professionals
Health-care professionals views on trial involvement
Clinical staff said that they were happy to be involved in a trial that they understood as important, relevant to patient experiences and central to the clinical staff members’ professional practice (Table 56).
| Health-care professional | Quotation |
|---|---|
| S8, senior nurse | I think it’s really valuable because the more you can help people with pain, you know, it’s really - I think that’s one thing that the patients are frightened of, isn’t it, when they have surgery with anybody? I would be. . . . So, you know, the advances in pain relief and making sure they’re relatively pain-free, has got to be good |
| S9, trainee nurse practitioner | That’s really good. Because I mean that comes – you know, that rolls into what we’re doing as well, to what you’re doing, and everything is just contributing towards trying to keep the patient pain-free for longer, and to get them more mobile, and to get them out back into their own home, which is good |
| S10, surgeon | I thought that it was a good idea to give a little local anaesthetic, you know, so the patient um, they’re more comfortable when they wake up um and er the body gets time to acclimatise, so to speak, to the pain, rather than they wake up in serious pain. . . . I’ve never thought about doing an objective assessment, you know, um, you know, as qualitative as this |
Staff weighed up the perceived benefits and costs of being involved in the trial. Some clinical staff reported initial concerns relating to expectations of the research staff, the possibility of an increased workload and the logistics of recruiting patients in a busy clinic. These initial concerns were alleviated once they learned more about the study, with many commenting that they appreciated that the trial had been designed to have minimal impact on, and normalised into, their daily clinical practice (Table 57).
| Health-care professional | Quotation |
|---|---|
| S2, ward nurse | It was a little bit worrying and we’d think, ‘Oh gosh, you know, we’re all being watched.’ [Laughs.] You know, because you do think, oh research, are people going to be coming in, and are they going to be asking lots of questions? And I suppose sometimes there is a fear there of thinking, ‘Oh my gosh, you know, what is going to be expected?’ |
| S7, pre-operation clinic manager | Well it’s just like part of what happens now |
| S14, consultant anaesthetist | No I have no problem, I mean it’s a great opportunity to contribute to research . . . So there’s not much that we have to do there. OK, we had to just do a standardised anaesthetic and fill in the form, so I think you didn’t ask for too much |
| S12, consultant anaesthetist | I thought it was going to be OK because essentially it was using the same anaesthetic that we were already using for the knee, um for our primary knee replacements. So I didn’t think it would have much impact on what I did, in terms of the anaesthetic, so I was quite happy to go along with it |
Clinical staff noted that the research nurses played a central support role in clarifying procedural details and their flexibility in smoothing out any initial issues and reducing any burden of the trial on clinical staff was appreciated and welcomed (Table 58).
| Health-care professional | Quotation |
|---|---|
| S5, ward manager | Seeing someone every day is good. You know they are going to come up, if there’s any issues. Um. . . . So I think that’s worked well that they’re very visible |
| S6, ward manager | We haven’t really asked for as much help as they [research nurses] have offered. They have been here and given us a lot of information and they have again been offering staff any support they can possibly think of and um you know whether it was to do with filling out the forms or where we put the forms after we have completed them. You know what sort of support we need to give the patients. So yes they’ve been excellent really |
Health professionals’ views of patient benefit from trial participation
During the interview, some ward staff also commented on the benefits that they thought patients achieved by participating in a trial. These included patients gaining a better understanding of their situation through completing study questionnaires, an increased sense of security as patients felt that they received additional monitoring and satisfaction from involvement in health research (Table 59).
| Health-care professional | Quotation |
|---|---|
| S2, ward nurse | Oh they like it, because they’re involved. They feel like they’re involved and they’re participating in a trial, ‘I’m doing a study, I’m helping them do a study, looking at how we’re going to help patients in pain.’ So yeah, most – well all of them seem to be pleased about being on the trial |
| S3, senior ward nurse | I think it’s [trial participation] probably given the patients a lot more support and security maybe understanding in their own pain relief and to know they are being followed through as well |
| S6, ward manager | I think most of them [patients] seem very laid back about it. They have expressed absolutely no worries about it whatsoever and they seem very well informed. My feeling is that they probably think it’s quite exciting |
Findings from the interviews with patients
Trial participation
Patients reported several different reasons for trial participation. Altruistic reasons focusing on benefit to society were common; patients viewed their participation in trials as contributing to advancement of scientific knowledge. In addition, patients expressed gratitude to unknown members of previous generations who had participated in research that had contributed to improvements in medical care that they were currently receiving. This motivated them to participate in research to help future generations (Table 60).
| Health-care professional | Quotation |
|---|---|
| Mrs B | Well I just thought that anything that might help people in the future. You know if people don’t do things like that [then] improvements might take place but probably a lot more slowly. I think that it’s a good thing to do for the benefit of people who are having operations in the future really that’s why |
| Mrs G | I think, well unless you have guinea pigs how do you know how things are going to work? Yeah you don’t know if you were one of the people that got the stuff or if you were one of the people that didn’t but unless you have people to talk to and to find out about things how do you know? You’re never going to learn |
| Mrs Q | My attitude to that is things they know now somebody has done that in the past so if they learn more it’s going to be valuable to people in the future. I’m all for that. . . . Years ago they used to cut peoples legs off with saws, they’ve moved on quite a bit from that haven’t they |
Patients weighed up potential risks and benefits of trial participation and were motivated to take part in the trial if they saw few negative consequences in relation to being randomised to either of the trial arms. Patients said that APEX was seen as a low-risk trial to participate in owing to being randomised to standard care or standard care plus additional treatment. Patients did not feel like they would be ‘losing out on anything’ (Mrs L). The requirements of the trials in terms of data collection were not seen as a burden to the majority of patients. They considered the data collection methods and length of follow-up to be clear, acceptable and undemanding (Table 61).
| Health-care professional | Quotation |
|---|---|
| Mrs L | I thought well it’s not as though it’s going to be a new drug what you’re testing on her, it’s only going to be anaesthetic but it’s only going to be a trial if it works or it didn’t work. I mean it’s not as though I’m going to lose anything if it doesn’t work. I thought if it does work then I’ve gained a lot so it’s not as though it’s going to be a new drug you’re trying out if it’s only anaesthetic. So I thought well yeah, if it’s going to help me with the pain afterwards then yeah |
| Mrs K | It’s all self explanatory isn’t it really, no I don’t think so no, I understood and knew what it all entailed like. I mean it doesn’t really entail much apart from a few forms |
| Mrs E | Yeah, I thought, ‘Well, somebody got to do it’, I mean, all these things take time, and someone’s got to do it, and I thought, ‘Well, I don’t mind, I won’t be doing anything when I come home’. [Laughs.] Yeah, it’d be an interest anyway |
| Mr M | What harm can it do? I am sitting at home so I might as well, better than doing a crossword |
Patents were additionally motivated to participate in the trial by the hope that they may also have personal benefit from trial participation. Trial involvement brought with it the chance that patients might obtain the latest medical care, possibly receiving medical treatment that is not routinely available. Patients described the potential benefit of receiving more anaesthetic if they had been randomised into the intervention arm of the trial, while also demonstrating a good understanding of the process and the need for randomisation and blinding (Table 62).
| Health-care professional | Quotation |
|---|---|
| Mrs P | When she said they would inject into the scar before they send it up I thought ‘oh lovely a bit more pain, less pain’ [yes], but I don’t know whether I had it done so I suppose it is in my mind. I might have had it I don’t know |
| Mrs X | I just thought what a good thing um, you know, to have that extra help at that time, er to relieve pain, you know. So I thought it – yeah, I said to my daughter, ‘No, I will do that, because I think it’s really good’ |
Patients also reported a range of psychological benefits of trial participation. Patients described that a potential personal benefit from trial participation could be from increased surveillance by the researcher team. Even though trial participation may not directly improve their situation, the extra monitoring reassured patients. Patients also thought that completion of the questionnaires may have helped them to consider and better understand their pain and outcomes and helped quantity the patients’ pain experience (Table 63).
| Health-care professional | Quotation |
|---|---|
| Mr M | I think for a certain degree that I had some scepticism to a degree in my own mind that if there is a what if it went wrong, somebody else is looking from another corner at me, it may help to decipher why, when, where, what how it went wrong |
| Mrs P | Oh my pain going down? Yes because you don’t think do you. You think everyday ‘oh it is the same, oh I wish it would hurry up oh it is still there’. But it wasn’t you could tell and as like when I get up in the morning and I would go to the toilet, have my wash and come back in and then I would sit and fill it and I would think ‘oh it is a lot better today’ |
Patients who took part in the qualitative study nested within the APEX trials also reflected on their use of pain relief medication pre-surgery, during their hospital stay and while recovering from their operation at home. The analysis of the data relating to pain relief medication identified two superordinate themes relating to use of pain relief over these periods: shifting acceptability, and necessity and value. We also found that behaviour and beliefs relating to pain relief medication were influenced by external factors that are described after the two themes.
Summaries of patients’ experiences at different time points in their journeys through joint replacement are illustrated in Figure 33 and are summarised in Box 19. We present a description of the themes that describe how and why patients made their decisions about pain relief.
FIGURE 33.
Summary of patients’ pain relief experiences over the total hip or knee replacement journey.

-
Patients were living with chronic pain.
-
Patients had access to non-prescription medication and prescriptions for stronger pain relief.
-
However, use of pain relief medication was avoided and restricted.
I would take very, very rarely and only when the pain was unbearable . . . I could go a month or six weeks without because I don’t like taking tablets.
Mr N
Patterns of pain relief use during the hospital stay
-
Patients were faced with the threat of acute postoperative pain.
-
Many changed their use of pain relief during this period.
-
They were motivated and more willing to take full and regular doses of pain medication.
But I felt that I needed that, by golly that was needed without a doubt and I used to have one eye on the clock . . . especially as things started to wear off.
Mr M
Patterns of pain relief use while recovering at home
-
After discharge, patients initially took their medications as they had done in hospital.
-
However, within a few days of coming home, they returned to their pre-surgical pattern of medication use.
-
Patients cut back on their use of pain medication whether or not they were still in pain.
I was on regular medication [when I came home after total hip or knee replacement], I was obediently taking it, accepting my bag of drugs that I came out with thinking, maybe I won’t need all of that, but I did, and I went back to the GP, I did get some of the prescription replenished. But since then I’ve dropped the lower level medication down considerably.
Mrs O
Patients’ decisions about use of pain relief medication were influenced by their beliefs about the acceptability of medication use. This changed over time, with short-term use seen as acceptable but longer-term use seen as not acceptable. Patients’ willingness to use pain relief medication changed from their time before surgery, during their time in hospital and subsequently on their return home. Immediately after surgery, patients thought that recovery in the acute postoperative phase was time-limited and they started to reduce their use of pain medication. This was directly related to their wish to return to a situation of some normality in which they limited their use of pain relief. As time passed, long-term use was no longer acceptable. Decisions about medication use were also influenced by other factors including the acceptability of using pain medication compared with living with pain and associated limitations, concerns about side effects and beliefs about ‘light’ non-prescription medications compared with ‘heavy’ prescription medications.
| Health-care professional | Quotation |
|---|---|
| Mrs O | So over a sort of time scale, means to an end, I’m very compliant. But it’s the long-term aspect that I’m so defiant about. . . . I’m fine around accepting it when it’s short-term, short-term issue, and it’s, it’s deemed with a current problem. I can handle it then, yeah it’s becoming a long-term reliance that frightens me |
| Mrs P | I don’t like this feeling. . . . I would rather put up with the pain than be feeling nauseous because it is a horrible feeling. . . . So I have cut down to one now. I have got to stop because they are not doing me any good. They are stopping the pain but they are not making me eat |
| Mrs O | I was very, very reluctant to get on that, on that track as far as the heavier duty medication was concerned. So I was managing with over-the-counter stuff really. . . . I suppose you always needed to know that you had a margin to, to operate with. I don't know, yeah letting yourself down |
Patients described beliefs about the necessity and value of medication to help them manage their changing pain experience. Use of medication was in part a moral decision, based on notions about when it was right to take analgesia. Although many talked of experiencing chronic pain before their operation, most did not think the pain was ‘bad enough’ to justify use of pain relief medication. Patients first waited to see if they could manage without use of pain relief and only took it as a last resort to enable them to ‘live’ and function with pain. However, patients also reported that the intensity of pain and discomfort that they experienced immediately after surgery outweighed their concerns about certain medications, resulting in increased pain relief medication use. This view that medication was needed to facilitate coping with pain caused by surgery also influenced their decisions about medication in their early recovery period after discharge. Taking regular pain relief after their operation was also recognised by patients as an imperative component of their recovery process, an outlook that served to over-ride any long-held negative attitudes towards analgesia. Patients’ thoughts about the value of pain medication were also balanced against the intensity of their pain in the weeks after their operation. At 2–4 weeks post surgery, some patients had stopped taking pain medication. They explained that this was because they were no longer experiencing what they considered to be significant pain and, therefore, saw no real value or continued benefit in doing so.
| Health-care professional | Quotation |
|---|---|
| Mrs C | I felt well see if I could go around without taking them. . . . You know if you can manage without the tablets |
| Mrs G | And you can’t tell when the shooting pain’s going to come anyway so what was the point? |
| Mr M | Interviewer:And how did you feel when you woke up?Mr M:Rough, bloody rough, yes really rough, not right, and I was on a drip that I could control [PCA: morphine] but it was timed but I had to press the button to get any more, and it went to 2 minutes and 39 seconds because I counted 37, 39, yes, because I wanted that extra . . . I was a bit worried, especially with morphine, of becoming addicted to morphine because I have heard stories about people who are but I am fine |
| Mrs X | When I was in hospital, yes. And I had to struggle to get out of bed and, and take myself to the toilet. So in order to fortify myself, I had to, I knew I would have to take those painkillers, you see |
| Mrs E | I felt, ‘Oh, I don’t know what the pain’s about, so I’ll take . . . come off of them and see’, but I haven’t had any pain since. Touch wood. And then the next day, I thought, ‘I’m coming off this sickness one as well, because why am I taking it if I’m not being sick now’. So I came off that as well. So all I’m taking is my blood pressure tablets and two aspirin, that’s all I’m taking now |
Narratives illustrated the central role that health-care professionals can have in influencing patients’ views on and use of pain relief during their journey through total hip or knee replacement. Before patients had their operation, consultations with health-care professionals were useful in encouraging them to increase the effectiveness of their self-management by moving from ‘light’ pain relief to the ‘heavier’ prescription-only drugs. The influence of health-care professionals in patients’ use of pain relief increased when they went into hospital to have their operation. It was then that most no longer held firm to their long-standing beliefs about the acceptability, necessity and value of pain relief when ‘experts’ were directly on hand to provide pain management. Instead, they put their faith in health-care professionals to provide them with only those medications that were essential to help with their recovery. Accounts also revealed the heightened influence of health-care professionals over the ways in which patients used pain relief after their surgery. For example, the influence they had over decisions to carry on regularly using pain medications during their initial period of recovery at home.
| Health-care professional | Quotation |
|---|---|
| Mrs H | Pre-surgeryI was only taking paracetamol, four times a day, two four times a day. And then my doctor said ‘well you ought to be taking ibuprofen that will get the inflammation down’ so I was taking ibuprofen |
| Mr N | In hospitalAlways coming round with your tablets . . . and on their little rounds and anything from a couple of tablets at a time to about eight. You could have had a right cocktail sometimes. . . . I took whatever they gave me because . . . I, they know what they’re doing. I wouldn’t have dreamt of not taking any of them because it’s tried and tested so I had all the belief in it |
| Mr N | At homeInterviewer:So all the painkillers you’ve been taking since your operation, was that something that was discussed with you?Mr N:Yes. They explained what benefits . . . and obviously I’ve had to lower my guard on my tablet intake . . . and I go with the experience and knowledge of people telling me |
Discussion
Trial involvement
Both patients and health-care professionals reported that their initial interest in APEX was a result of the trial being perceived as an important area of research that was highly relevant to the patients’ experience of surgery. The data demonstrate the importance of addressing a question of substantial interest, relevance and value to health-care professionals and patients to get ‘buy-in’ from all. In addition, revealing the need to produce clear trial information for both patients’ and health-care professionals to explain the rational and importance of the RCT.
Patients and health-care professionals both stated weighing of perceived benefits and costs associated with involvement in APEX. The data demonstrate the need for minimal personal burden when undertaking a trial. 468 For health-care professionals, this was achieved by employing research nurses to ensure that the trial had minimal impact on daily clinical practice and also to be available to clarify procedural details to ensure the protocol was adhered to. Patients thought that participation in the trial had minimum costs to them and they felt that the possibility of randomisation to either trial arm, and the requirements of data collection, were acceptable. A limitation of the study is that we only captured the views of patients who agreed to take part in the trials and we were unable to speak with patients who declined to take part.
Patients motivations for trial involvement are multifaceted and complex,469 and include a range of personal and social elements. 470 Patients often stated altruistic reasons and expressed the desire to help others by contributing to the furthering of clinical knowledge. 471 However, only a minority of patients stated purely altruistic reasons, with many reporting a range of perceived physical and psychological benefits. 461 It is therefore vital during recruitment that time is spent on identifying and dispelling expectations that might not be met by the RCT.
Pain relief medication
Attitudes to pain relief medication are dynamic. Undergoing total hip or knee replacement has the potential to temporarily alter an individual’s view of the acceptability, necessity and value of pain relief medication. This alteration is related to views about the cause of pain (pain from intervention vs. pain from chronic condition) and interactions with health-care professionals. However, once initial recovery from surgery has begun, long-standing beliefs about the appropriate use of analgesia in the management of pain may again take prominence.
Health-care professionals appeared to exert considerable influence on patients’ beliefs about the essential nature of pain medication during the hospital stay. Although there is notable contact with health-care professionals during this period, in the pre-surgical period this contact is less frequent. After surgery, patients are discharged from hospital with pain medications, but contact with health-care professionals may be minimal, which means that contact before surgery might be particularly important. It was clear from this study that interactions between health-care professionals can provide crucial opportunities to discuss pain management. During these interactions, health-care professionals may wish to consider challenging or reinforcing patients’ beliefs about pain relief medication with a view to ensuring that patients’ pain is as well managed as possible.
Local anaesthetic anaesthesia: overall discussion and conclusions
Perioperative care should include appropriate multimodal anaesthesia supported by evidence from adequately powered RCTs. Systematic review identified 36 RCTs evaluating local anaesthetic infiltration in patients receiving THR and TKR. Few reported long-term post-surgical follow-up. Local anaesthetic infiltration was effective in reducing short-term pain when compared with no infiltration. Clinical effectiveness was enhanced with the addition of post-closure analgesia through drains that had been sited intraoperatively. However, there was some evidence to suggest that this was associated with an increased risk of infection. In patients receiving TKR, there was no evidence of additional benefit if a FNB had already been sited. FNBs affect knee extension and could delay postoperative mobilisation. Therefore, we need to compare local anaesthetic infiltration with FNB to see which is most effective and which allows earlier mobilisation and discharge.
In the APEX RCTs, local anaesthetic infiltration was associated with reduced long-term pain 1 year after THR. Findings in patients receiving TKR provided no strong evidence that local anaesthetic infiltration reduced long-term pain in addition to that provided by FNB. From the perspective of the NHS and PSS, local anaesthetic infiltration is a cost-effective treatment option in primary THR but evidence supporting its use in TKR was less strong.
Patients’ views on pain medication are dynamic and change in the perioperative period when they have more clinician contact and perceive a necessity to take analgesic to treat surgical pain. After surgery, clinician contact diminishes and patients tend to revert to traditional and long-standing beliefs about pain medication. The attitudes of patients to pain and pain medication throughout the patient journey need to be explored more fully, particularly with regard to whether or not patients’ needs are being addressed prior to surgery, and for those who have long-term pain.
In addition to the clinical results from the trial, we have learned valuable lessons about the running of trials from our qualitative work with patients, clinicians and researchers. Patients are willing to participate in a trial if they feel the question being addressed is important, if the participation burden is low and if there is a perceived or possible benefit for them. Clinicians are willing to participate if the appointment of research staff does not result in the trial impinging on clinical time.
Chapter 7 Exploring and understanding pain in the context of hip and knee replacement: a cohort study
Parts of this chapter have been reproduced from Wylde and colleagues472 © 2015 Wlyde V, Sayers A, Lenguerrand E, Gooberman-Hill, Pyke M, Beswick AD, et al. Preoperative widespread pain sensitization and chronic pain after hip and knee replacement: a cohort analysis. Pain 2015;156:47–54 and from Sayers and colleagues473 © 2016 The Authors. Arthritis Care & Research published by Wiley Periodicals, Inc. on behalf of the American College of Rheumatology. This is an open access article under the terms of the TODO: click through URL Creative Commons Attribution-Non Commercial-No Derivs License, which permits use and distribution in any medium, provided the original work is properly cited, the use is non-commercial and no modifications or adaptations are made.
Abstract
Background
The wealth of data collected in the APEX RCTs provided the opportunity for further analyses as a cohort study.
Methods
The APEX cohort study included 322 patients receiving a THR and 316 patients receiving a TKR. Radiographic measures of osteoarthritis severity were correlated with pre-operative WOMAC pain and function. Associations between measures of pain over time were explored. The association between pain and widespread pain sensitivity, measured using quantitative sensory testing (QST), was investigated.
Results
There was no relationship between the degree of radiographic damage and pain or function in patients waiting for THR. In patients waiting for TKR, those with the least severe radiographic damage reported more severe pain and poorer function.
Long-term pain after THR was predominantly associated with pain at rest during the pre-operative and acute postoperative period. In contrast, long-term pain after TKR was predominantly associated with the severity of pain on movement during the pre-operative period.
Pre-operative widespread pain sensitivity was not associated with change in pain severity from pre-operatively to 12 months postoperatively in patients with total hip or knee replacement.
Conclusions
There was an inverse association between radiographic severity of osteoarthritis and pain and function in patients waiting for TKR but no association in THR. Different pain characteristics predicted long-term pain in total hip and knee replacement. Pre-operative widespread pain sensitivity did not predict the amount of pain relief that patients experienced after joint replacement.
Background
As described in Chapter 6, between 2009 and 2012 322 patients undergoing THR and 316 undergoing TKR were recruited into the APEX trials.
The main purpose of the APEX trials was to assess the relationships between perioperative pain and patient outcomes at 12 months. However, the study also provides us with a large cohort of patients having hip and knee joint replacement surgery and the opportunity to investigate the associations between baseline characteristics, as well as the associations between baseline characteristics and outcomes at 1 year after surgery. The primary focus of these cohort analyses was on pain because we have comprehensive assessments of pre-operative pain, acute postoperative pain and chronic post-surgical pain.
Relationship between structural joint changes and pain and function
Background
Osteoarthritis can be defined on clinical terms, by radiographic evidences of changes, or on the basis of the pathology in the joint. Epidemiological studies of osteoarthritis have generally used radiographic assessment of joint damage to define the disease and the most widely used tool is the Kellgren and Lawrence X-ray grading system. 423
The relationship between radiographic evidence and pain is generally poor,474,475 although relatively few data have been published on this relationship in patients with osteoarthritis that is severe enough to result in them having joint replacement, as opposed to population- or service-based cohorts with milder forms of the disease. Furthermore, very few studies have looked at the separate relationships between pain and function and radiographic severity. Some recent data suggest that those patients coming to surgery who have milder radiographic changes respond less well to joint replacement than those with the most severe evidence of joint damage on a radiograph. 476,477
In the APEX study we have assessed all pre-operative radiographs available, using standard methods, and we are relating radiographic data to pre-operative pain and function data.
Methods
A single observer assessed all radiographs. The observer was a specialist registrar in trauma and orthopaedic surgery who had undergone two training sessions on the specific study research methods with one of the coPIs of the project who has extensive experience with radiographic changes in osteoarthritis. Standard proformas used to report the findings are shown in Appendices 29 and 30. After first checking that the radiograph was readable and reporting the presence on any existing implant, the Kellgren and Lawrence score423 was assessed using standard definitions of the five grades (0–4). We then assessed individual radiographic features (osteophyte, joint space narrowing, subchondral sclerosis and cysts) using the Altman and Gold atlas of individual radiographic features in osteoarthritis. 478 Finally, the pattern of osteoarthritis within the joint was reported. From these data, we then calculated a modified Kellgren and Lawrence grading, as previously reported by Dieppe and colleagues. 479 This divides the two most severe grades (3 and 4) into two subcategories each, based on the amount of joint space narrowing reported and the presence or absence of major subchondral bone remodelling. The purpose of this is to provide a finer differentiation of the severity of radiographic damage in severe osteoarthritis, which, on the traditional Kellgren and Lawrence score, will almost always be grade 3 or 4.
Inter-reader reliability was assessed by the main reader and his mentor assessing 30 random films selected from both the hip and knee cohorts independently, at the end of the study, and the κ-statistic was calculated.
One-way analyses of variance (ANOVAs) were used to analyse the differences between grades of modified Kellgren and Lawrence score WOMAC pain or function means. Tukey post hoc tests were conducted to identify which means differed from each other.
Results
A total of 322 hip patients and 316 knee patients were randomised in the APEX trials. The WOMAC pain scores were collected for each of these patients. The WOMAC function scores were available for only 304 hip and 296 knee participants. Data could not be obtained for 49 hip and 45 knee patients either because the pre-operative radiograph could not be found or because the quality of the film was too poor.
The tests of inter-reader reliability between the two observers for the modified Kellgren and Lawrence scores showed a moderately good level of agreement (κ = 0.55 for the hip and 0.48 for the knee).
The WOMAC pain and function scores are presented in Table 67. As can be seen, the modified Kellgren and Lawrence scoring resulted in four levels of osteoarthritis severity, with reasonable numbers in each category to relate to data on pain and other baseline variables. A small number of patients had little or no evidence of radiographic damage at the time of surgery (14 hip patients and five knee patients). This is both surprising and unexplained, but has been noted in other cohorts. 477,479
| Outcome WOMACa | Hip | Knee | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | mean | SD | F-test; p-valueb | n | mean | SD | F-test; p-valueb | ||
| Pain | mK&L < 3 | 14 | 37.1 | 8.9 | F(5,316) = 1.78; p = 0.116 | 5 | 38.0 | 23.1 | F(5,310) = 2.42; p = 0.036 |
| mK&L-3a | 35 | 42.1 | 21.4 | 14 | 30.0 | 12.9 | |||
| mK&L-3b | 71 | 46.5 | 18.3 | 109 | 40.8 | 17.0 | |||
| mK&L-4a | 64 | 43.8 | 16.5 | 96 | 44.3 | 17.0 | |||
| mK&L-4b | 89 | 42.0 | 19.6 | 47 | 44.8 | 14.9 | |||
| Not gradable | 49 | 37.4 | 18.1 | 45 | 44.3 | 16.2 | |||
| Function | mK&L < 3 | 12 | 32.6 | 16.7 | F(5,298) = 2.06; p = 0.070 | 4 | 52.6 | 12.1 | F(5,290) = 2.52; p = 0.030 |
| mK&L-3a | 34 | 45.2 | 16.8 | 10 | 27.9 | 11.8 | |||
| mK&L-3b | 68 | 46.8 | 17.6 | 104 | 45.4 | 16.7 | |||
| mK&L-4a | 59 | 43.3 | 19.2 | 92 | 47.2 | 18.6 | |||
| mK&L-4b | 86 | 40.5 | 18.8 | 45 | 48.5 | 19.1 | |||
| Not gradable | 45 | 39.1 | 20.5 | 41 | 46.2 | 16.7 | |||
No between-group differences in mean scores of self-reported pain and function were observed for the hip participants. These findings suggest the absence of relationship between the degree of radiographic damage and the severity of either pain or function in patients about to undergo a hip replacement for their advanced osteoarthritis.
However, at the knee joint we do see some interesting in different relationships. Participants in the modified Kellgren and Lawrence 3a group (those with the least severe radiographic evidence of osteoarthritis) had the worst pain and function scores. Their mean pain scores differed significantly (p < 0.05) from participants in the 4a, 4b and not-gradable groups, while their mean function score differed from each of the other groups. Participants in the other three groups (3b, 4a and 4b), who have the more severe damage on radiography, had comparable pain and function score levels – suggesting a possible threshold effect – but self-reported pain and function were significantly worse in those with the least severe radiographic evidence of osteoarthritis.
Discussion
Overall, the radiographic evidence of osteoarthritis changes that we observed are consistent with other cohort studies of people with severe hip or knee osteoarthritis. 477,479 The finding of no relationship between pain and radiographic severity at the hip is also consistent with previous data. 479 The striking and new finding from this study is the quite large differences between the severity of both self-reported pain and function in those with modest radiographic evidence of osteoarthritis changes at the knee compared with those with severe radiographic changes.
This key finding is at odds with the data from a similar cohort studied in a very similar way in Australia. 477 In that cohort, no relationship was found between pre-operative radiographic severity and baseline pain and function. However, the Australian health-care system is quite different from the NHS and there are long waiting lists for joint replacement;480 therefore, patients tend to come to surgery at a very late stage so one explanation for these differences could be the relative paucity of patients in the 3a modified Kellgren and Lawrence grade in the Australian cohort.
In our view, this finding makes sense in relation to decision-making regarding joint replacement. If a patient has significant pain and/or functional problems, and radiographic evidence of severe osteoarthritis changes, then surgery is likely to be recommended. However, if the radiographic changes are less severe, then the doctors will be more cautious, questioning whether or not the joint damage is the main cause of the pain. But if the patient is complaining of terrible pain and or functional problems, then the decision to operate is more likely to go ahead, even if the radiographic changes are mild. 481 However, it is interesting to note that we found no such relationship at the hip joint, emphasising the important difference between end-stage hip and knee osteoarthritis.
Pain
Background
Pain is the primary reason for patients electing to undergo joint replacement surgery and the expectations are that the surgery will provide pain relief. However, our work has shown that 7–23% of THR patients and 10–34% TKR patients experience chronic pain after surgery. 18 The difference in the prevalence of pain after THR and TKR is important and adds to the growing body of evidence that hip and knee osteoarthritis are different diseases. Within the APEX cohort, there are longitudinal pain data on both THR and TKR patients, which allows us to compare and contrast pain pathways with the aim of informing the clinical treatment of these diseases.
Within the surgical literature, there is a growing recognition of the importance of distinguishing between pain at rest and pain on movement due to differing mechanistic pathways and clinical implications, such as differential effectiveness of pharmacological therapies and impact on functional recovery. 482 The aim of this analysis was to compare and contrast the associations between pre-operative pain, acute post-surgical pain and chronic post-surgical pain after THR and TKR, focusing on the differences between pain at rest pain and pain on movement.
Methods
Exposures/mediators
The primary exposures of interest were:
-
pre-operative pain, measured using the WOMAC pain scale
-
acute postoperative pain measured on postoperative day 1, 2 and 3 using a VAS; the severity of pain on rest and pain on movement were rated
-
chronic post-surgical pain, measured using the WOMAC pain scale at 12 months after surgery.
Confounding variables
This analysis involved analysing data from the APEX trials as cohort data and, therefore, additional adjustment was required to control for confounding factors (as per any cohort study) and trial randomisation. Analyses were adjusted for sex and socioeconomic status, which consisted of employment status, cohabitation and educational attainment.
Statistical methods
Descriptive statistics
Population characteristics and outcome measures are reported as means, SDs and interquartile cut-off points for continuous measures, and as frequencies for categorical variables.
Traditional approach
Using linear (OLS) regression, the association between pre-operative pain, acute postoperative pain on movement and at rest, and chronic pain were investigated. Results are reported in natural units (per unit increase in the exposure) and the association with the outcome, 95% CIs, standard errors and p-values are also reported.
Pre- and postoperative WOMAC pain scores were transformed to a 0–100 scale and VAS scores were converted into a 10-point ordered response scale. Acute pain on movement and acute pain at rest scores were averaged across the nine measurement occasions.
Structural equation modelling approach
A structural equation modelling (SEM) framework was adopted for three reasons: (1) it provides a framework to conduct mediation analyses, that is, investigate the direct effect of pre-operative pain on chronic pain and indirectly via acute pain on movement or acute pain at rest, (2) it allows multi-item pain assessments to be investigated without simple aggregation of scores and (3) it can be estimated in the presence of missing data under the missing at random assumption using maximum likelihood with missing values. 483
Importantly, results from SEM are interpreted with respect to the latent constructs of pre-operative pain, acute pain on movement, acute pain at rest and chronic pain. The results are interpreted on the same scale as the scores were originally measured, that is the WOMAC pain scale (a 5-point scale), and the acute pain on movement or acute pain at rest are 10-point scales. Although using a SEM approach does not affect the interpretation of the association between pre-operative and chronic pain compared with the more traditional approach (as the scales are changed equally), the association between pre- and postoperative pains scores and VAS scores will be approximately 1/20th of the size, as the WOMAC is scored from 0 to 100 in the traditional approach.
Further analyses were conducted by grouping items in the pre-operative/postoperative WOMAC assessment more strongly associated with acute pain on movement and acute pain at rest. This subdivision enables the two main constructs of the WOMAC pain scale to be investigated simultaneously.
All analyses were investigated in THR and TKR patients separately. All analyses were conducted in Stata 13.1. Traditional analyses were conducted using the command and SEM models were estimated using the command using maximum likelihood with missing values. 483
Results
Descriptive data
Baseline characteristics of participants are provided in Table 68. Patients undergoing THR had a mean age of 66.2 years (SD 10.9 years), which was slightly younger than the mean of 69.1 years (SD 18.6 years) for TKR patients. A total of 321 THR and 316 TKR patients completed a pre-operative WOMAC pain scale and were included in the analyses. Pre-operative WOMAC pain scales contained very little missing information and pain levels were very similar between THR and TKR patients (Table 69).
| Location | Variable | n | Missing |
|---|---|---|---|
| Hip | Randomisation | ||
| Standard care | 159 | ||
| Intervention | 163 | 0 | |
| Sex | |||
| Male | 134 | ||
| Female | 188 | 0 | |
| Employment | |||
| Unemployed | 195 | ||
| Employed | 103 | 24 | |
| Retired | |||
| Not retired | 133 | ||
| Retired | 189 | 0 | |
| Cohabitation | |||
| Alone | 74 | ||
| Not alone | 232 | 16 | |
| Education | |||
| ≤ 16 years | 208 | ||
| > 16 years | 99 | 15 | |
| Knee | Randomisation | ||
| Standard care | 159 | ||
| Intervention | 157 | 0 | |
| Sex | |||
| Male | 150 | ||
| Female | 166 | 0 | |
| Employment | |||
| Unemployed | 220 | ||
| Employed | 66 | 30 | |
| Retired | |||
| Not retired | 98 | ||
| Retired | 218 | 0 | |
| Cohabitation | |||
| Alone | 84 | ||
| Not alone | 213 | 19 | |
| Education | |||
| ≤ 16 years | 224 | ||
| > 16 years | 69 | 23 | |
| Location | Time | Measure | n | n (complete) | % item response in missing | Mean | SD | 25th percentile | Median | 75th percentile |
|---|---|---|---|---|---|---|---|---|---|---|
| Hip | Pre operation | WOMAC | 321 | 320 | 80 | 44.04 | 20.41 | 30.0 | 45.0 | 55.0 |
| Acute | ||||||||||
| Move (VAS) | Day 1 | 301 | 271 | 58 | 5.76 | 2.33 | 4.0 | 5.7 | 7.7 | |
| Day 2 | 295 | 260 | 57 | 4.58 | 2.53 | 2.3 | 4.5 | 6.7 | ||
| Day 3 | 272 | 227 | 56 | 3.89 | 2.39 | 2.0 | 3.3 | 6.0 | ||
| Rest (VAS) | Day 1 | 301 | 283 | 54 | 3.14 | 1.99 | 1.7 | 2.7 | 4.3 | |
| Day 2 | 296 | 264 | 57 | 2.40 | 1.85 | 1.0 | 1.8 | 3.4 | ||
| Day 3 | 272 | 229 | 56 | 2.06 | 1.71 | 1.0 | 1.3 | 2.7 | ||
| Post operation | WOMAC | 283 | 279 | 80 | 89.04 | 16.85 | 85.0 | 95.0 | 100.0 | |
| Knee | Pre operation | WOMAC | 316 | 316 | 0 | 43.15 | 17.74 | 30.0 | 45.0 | 55.0 |
| Acute | ||||||||||
| Move (VAS) | Day 1 | 279 | 228 | 58 | 6.17 | 2.41 | 4.7 | 6.3 | 8.0 | |
| Day 2 | 284 | 259 | 56 | 5.87 | 2.39 | 4.2 | 6.0 | 7.7 | ||
| Day 3 | 260 | 217 | 59 | 4.70 | 2.48 | 2.7 | 5.0 | 6.3 | ||
| Rest (VAS) | Day 1 | 280 | 239 | 54 | 4.34 | 2.29 | 2.7 | 4.2 | 6.0 | |
| Day 2 | 284 | 264 | 55 | 3.97 | 2.36 | 2.0 | 4.0 | 5.7 | ||
| Day 3 | 260 | 223 | 57 | 3.04 | 2.12 | 1.0 | 2.7 | 4.3 | ||
| Post operation | WOMAC | 277 | 268 | 78 | 79.75 | 21.23 | 65.0 | 85.0 | 100.0 | |
During the acute post-surgical phase, VAS scales were well completed (90% and 87% for THR and TKR patients, respectively); however, lower completion rates were observed on postoperative day 3 (see Table 69). At 12 months postoperatively, 15% of patients with THR and 33% of patients with TKR reported severe/extreme pain, defined as WOMAC pain score of ≤ 50.
Traditional approach
Using simple linear regression, the association between pre-operative WOMAC (0/100) pain and chronic WOMAC (0/100) pain, adjusted for confounding factors, was investigated in THR and TKR patients (see Table 69). Pre-operative pain was significantly positively associated in both instances; however, the association in TKR patients was four times as large as in THR patients.
Acute pain on movement and acute pain at rest were also significantly positively associated with chronic pain in both THR and TKR patients. Owing to the traditional inverse coding of the WOMAC pain score (0 extreme pain, 100 no pain), this was an inverse association. The association between acute pain at rest and chronic pain was stronger than the association between acute pain on movement and chronic pain (Table 70).
| Region | Outcome | Model | Exposure | Adjusted | Beta | SE | 95% CI | p-value |
|---|---|---|---|---|---|---|---|---|
| OLS regression | ||||||||
| Hip | Postoperative WOMAC | 1 | Pre-operative WOMAC | Confounders | 0.105 | 0.05 | 0.006 to 0.204 | 0.0385 |
| 2 | Acute move | Confounders | –1.866 | 0.57 | –2.986 to –0.746 | 0.0012 | ||
| 3 | Acute rest | Confounders | –2.200 | 0.62 | –3.414 to –0.986 | 0.0004 | ||
| 4 | Pre-operative WOMAC | Confounders + acute move | 0.095 | 0.05 | –0.003 to 0.192 | 0.0575 | ||
| 5 | Pre-operative WOMAC | Confounders + acute rest | 0.092 | 0.05 | –0.005 to 0.190 | 0.0631 | ||
| 6 | Pre-operative WOMAC | Confounders + acute move + acute rest | 0.092 | 0.05 | –0.005 to 0.190 | 0.0636 | ||
| Knee | Postoperative WOMAC | 1 | Pre-operative WOMAC | Confounders | 0.434 | 0.07 | 0.295 to 0.574 | 0.0000 |
| 2 | Acute move | Confounders | –1.996 | 0.65 | –3.283 to –0.708 | 0.0025 | ||
| 3 | Acute rest | Confounders | –2.234 | 0.62 | –3.452 to –1.016 | 0.0004 | ||
| 4 | Pre-operative WOMAC | Confounders + acute move | 0.402 | 0.07 | 0.258 to 0.546 | 0.0000 | ||
| 5 | Pre-operative WOMAC | Confounders + acute rest | 0.389 | 0.07 | 0.245 to 0.534 | 0.0000 | ||
| 6 | Pre-operative WOMAC | Confounders + acute move + acute rest | 0.389 | 0.07 | 0.244 to 0.533 | 0.0000 | ||
| SEM (MLMV) | ||||||||
| Hip | 1LV postoperative WOMAC | 1 | Pre-operative WOMAC | Confounders | 0.169 | 0.07 | 0.038 to 0.300 | 0.0112 |
| 2 | Acute move | Confounders | 0.074 | 0.03 | 0.008 to 0.140 | 0.0289 | ||
| 3 | Acute rest | Confounders | 0.176 | 0.05 | 0.087 to 0.266 | 0.0001 | ||
| 4 | Pre-operative WOMAC | Confounders + acute move | 0.158 | 0.07 | 0.028 to 0.289 | 0.0174 | ||
| 5 | Pre-operative WOMAC | Confounders + acute rest | 0.141 | 0.07 | 0.013 to 0.268 | 0.0311 | ||
| 6 | Pre-operative WOMAC | Confounders + acute move + acute rest | 0.147 | 0.07 | 0.019 to 0.275 | 0.0247 | ||
| Knee | 1LV postoperative WOMAC | 1 | Pre-operative WOMAC | Confounders | 0.669 | 0.11 | 0.452 to 0.886 | 0.0000 |
| 2 | Acute move | Confounders | 0.138 | 0.04 | 0.052 to 0.224 | 0.0016 | ||
| 3 | Acute rest | Confounders | 0.218 | 0.06 | 0.104 to 0.331 | 0.0002 | ||
| 4 | Pre-operative WOMAC | Confounders + acute move | 0.622 | 0.11 | 0.403 to 0.841 | 0.0000 | ||
| 5 | Pre-operative WOMAC | Confounders + acute rest | 0.583 | 0.12 | 0.353 to 0.812 | 0.0000 | ||
| 6 | Pre-operative WOMAC | Confounders + acute move + acute rest | 0.567 | 0.13 | 0.305 to 0.830 | 0.0000 | ||
Adjusting the association between pre-operative and chronic pain for acute pain on movement and/or acute pain at rest resulted in minor attenuation of the association in THR patients, and a slightly stronger attenuation in TKR patients.
Structural equation modelling approach
Using the SEM approach resulted in a stronger association between pre-operative and 12-month WOMAC pain in both THR and TKR patients. The majority of the increase in the strength of association can be attributed to the more efficient use of data via the latent constructs as opposed to excluding those individuals with missing data (results not shown). Similarly, the associations between acute pain on movement, acute pain at rest and chronic pain have changed. In THR patients, the association between acute pain on movement and chronic pain has been attenuated (0.074 × 20 = |1.48| vs. |–1.86|), and the association between acute pain at rest and chronic pain has been enhanced (0.176 × 20 = |3.52| vs. |–2.2|). The pattern of change in TKR patients is different with the strength of association between acute pain at rest or acute pain on movement being greater than the traditional approach (see Table 70).
Furthermore, when the association between pre-operative pain and chronic pain is adjusted for acute pain, either on movement or at rest, there is stronger attenuation in the SEM approach, suggesting a stronger mediating effect of acute pain (see Table 70).
Mediation analysis
Single latent variable WOMAC
Using the WOMAC score as a single latent variable, the association between pre-operative pain, acute pain on movement/rest and chronic pain was investigated. Figure 34a and b illustrates the path models between latent variables for the THR and TKR patients, respectively. Arrows indicate the direction of effects. Coefficients are in the natural units of the measurement scales and p-values are based on z-distribution. Models are estimated using maximum likelihood allowing for missing values.
FIGURE 34.
Structural equation model of the association between latent variables pre-operative WOMAC (W–1), acute postoperative pain on movement (M), acute pain at rest (R) and postoperative WOMAC (W12). (a) Hip patients (n = 322); and (b) knee patients (n =316).

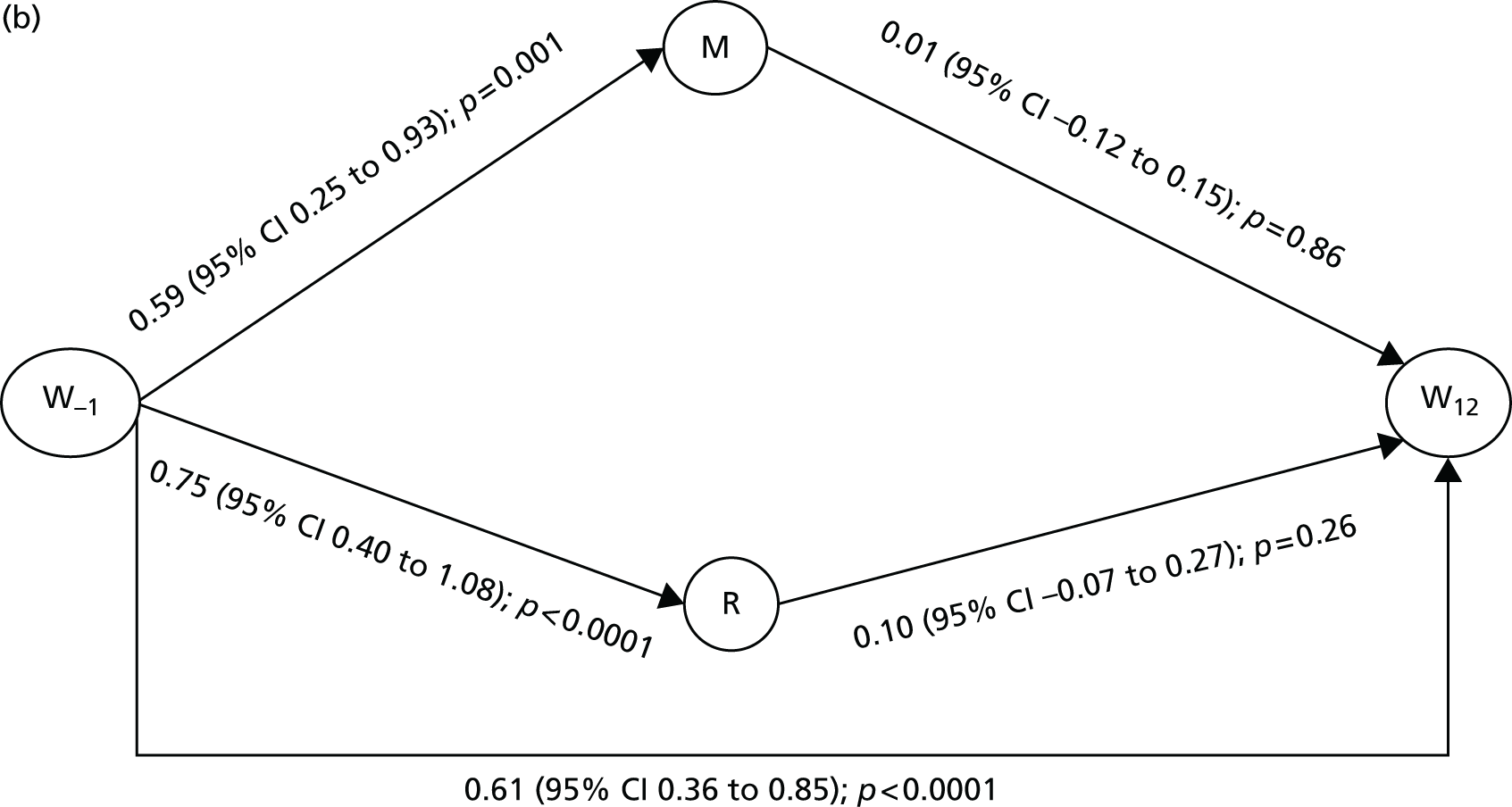
The WOMAC single latent variable models differ to those presented in Table 70, by allowing for pre-operative pain to influence acute pain on movement and acute pain at rest. In THR patients, the direct effect of pre-operative pain on chronic pain is nearly identical and only a minor increase in the standard error is observed. This model also highlights that the attenuation observed in model 6 compared with model 1 (see Table 70) is primarily as a result of the acute pain at rest pathway and not acute pain on movement.
The pattern of results in TKR patients is somewhat less clear. There is a strong association between pre-operative pain and both acute pain on movement and acute pain at rest. However, there is only a relatively weak effect of acute pain on movement and acute pain at rest on chronic pain.
Two latent variable WOMAC
Using the WOMAC score as a two latent variable (rest/movement) pain model pre- and postoperatively, the direct and indirect effects of pre-operative pain (rest/movement) were investigated with acute pain on movement/acute pain at rest and chronic pain (rest/movement). Figures 35 and 36 illustrate the path models between latent variables for the THR and TKR patients, respectively. Single-headed arrows indicate direction of effects, coefficients are in the natural units of the measurement scales, and p-values are based on z-distribution. Double-headed arrows indicate correlation coefficients. Models are estimated using maximum likelihood allowing for missing values.
FIGURE 35.
Structural equation model of the association between latent variables pre-operation WOMAC movement (Wm,–1), pre-operation WOMAC rest (Wr,–1), acute post-operation pain on movement (M), acute pain at rest (R), post-operation WOMAC movement (Wm,12) and post-operation WOMAC rest (Wr,12) in hip patients (n = 322).
FIGURE 36.
Structural equation model of the association between latent variables pre-operation WOMAC movement (Wm,–1), pre-operation WOMAC rest (Wr,–1), acute post-operation pain on movement (M), acute pain at rest (R), post-operation WOMAC movement (Wm,12) and post-operation WOMAC rest (Wr,12) in knee patients (n = 316).

In THR patients, the SEM approach clearly shows that the majority of the association previously seen in the single latent variable model between pre-operative and chronic pain is mediated directly via pre-operative pain at rest, with little or no effect of indirect pathways or directly via pre-operative pain on movement. However, an independent association of acute pain at rest is positively associated with chronic pain both on movement and at rest.
In TKR patients, the results are quite different and the two latent variable model highlights that the strongest association is between pre-operative pain on movement and chronic pain on movement. Similar to the THR patients, pre-operative pain at rest is also associated with chronic pain at rest, and acute pain at rest is also associated with chronic pain at rest, but not chronic pain on movement.
In Figure 35, the strong association between pre-operative pain and acute pain on movement/rest is clarified, demonstrating that pre-operative pain on movement is the strongest predictor of chronic pain at rest or on movement.
There was no evidence of significant indirect effects in either THR or TKR patients despite significant intermediate paths.
Discussion
Using a traditional OLS regression approach and a SEM approach, we have demonstrated that the associations between pain over time are different in THR and TKR patients, which has clinical implications for the treatment of painful hip and knee osteoarthritis. Furthermore, we have explored the unique constructs of pain on movement and pain at rest to gain further insights and further understand pain within the context of orthopaedic surgery.
Our study has highlighted the complex and unique relationships between pre-operative, acute postoperative and chronic post-surgical pain in patients undergoing THR and TKR. The different contributions of pain at rest and pain on movement were stark; chronic pain after THR was driven predominantly by pain on rest and chronic pain after TKR was driven predominantly by pain on movement. These findings allude to different patterns of pain mechanisms within hip and knee osteoarthritis and highlight the importance of future work to identify the sources and potential treatment options for these different pain mechanisms.
Pain sensitivity
Background
Understanding pain within the context of joint replacement surgery is one of the core themes of the RESTORE programme. In the previous analyses, we explored the associations between pre-operative, acute postoperative and chronic post-surgical pain after THR and TKR. In the analyses presented in this section, we are interested in further understanding why people develop chronic pain after joint replacement by exploring whether or not pre-operative pain sensitivity was a risk factor for developing this condition.
Previous research has been undertaken to identify risk factors for the development of chronic pain after joint replacement. However, this work has highlighted that very little of the variation in pain severity after joint replacement can be explained by pre-operative risk factors such age, sex, depression, joint pain and BMI. 52 This highlights the need to explore other pre-operative factors that could be used to identify patients at a high risk of chronic post-surgical pain. There is some preliminary research which suggests that pre-operative central pain sensitisation is associated with chronic pain after joint replacement. 107,484 Central pain sensitisation involves amplification in neuronal activity that occurs at a generalised level, leading to increased sensitivity to nociceptive input and reduced pain thresholds at sites distant to the painful area. It is now well established in the research literature that some patients with painful osteoarthritis have evidence of central pain sensitisation. 485 It is possible that patients with central pain sensitisation may be at a higher risk of experiencing chronic post-surgical pain after joint replacement because removal of the peripheral pain source may not reverse augmented central pain processing changes. Further research is needed to explore whether or not pre-operative central pain sensitisation, assessed using QST, contributes to the development of chronic pain after joint replacement.
The aim of these analyses was to determine if pre-operative PPTs measured at a pain-free body site are predictive of chronic post-surgical pain at 12 months after primary THR and TKR, independent of pre-operative pain.
Methods
Exposure
Quantitative sensory testing is a non-invasive method that measures participants’ responses to external stimuli of controlled intensity. Pre-operative PPTs were assessed at the volar forearm using a digital algometer (Somedic, Hörby, Sweden) with a 1-cm probe. The probe was held perpendicular to the skin and force applied at a constant rate of 10 kPa per second. The patient was instructed by the research nurse to say ‘stop’ when the sensation of pressure became the very first sensation of pain. Pressure algometry was repeated three times and between each reading the position of the algometer on the skin was altered very slightly to avoid sensitisation of the test area. The primary exposure is a standardised average of the three PPT measurements.
Outcome
The primary outcome for this analysis was the WOMAC pain score at 12 months after surgery. Total WOMAC pain scores were calculated as an average of all five items. As our previous analyses demonstrated the importance of distinguishing between movement pain and rest pain, we conducted further analysis with these subcomponents of the WOMAC pain score. Movement pain was calculated as an average of items 1, 2 and 4 and rest pain was calculated as an average of items 3 and 5.
Confounding variables
This analysis was adjusted for trial randomisation, age at recruitment, sex, cohabitation (living alone), employment status, educational attainment (after 16 years of age), height and weight.
Statistical methods
Descriptive statistics
Population characteristics and outcome measures are reported as means, SDs, interquartile cut-off points for continuous measures and as frequencies for categorical variables. In addition, the SD of the individual three PPTs was also calculated and summarised to indicate the variability of the QST measurements.
Cross-sectional/prospective analysis
Simple linear regression was used to investigate the association between average pre-operative pain (cross-sectional analysis) and postoperative pain (prospective analysis) and standardised PPTs. Three adjusted models were fitted: (1) minimally adjusted for sex and randomisation, (2) more fully adjusted, that is, model 1 and age, height, weight, cohabitation, employment and education, and (3) baseline adjusted, that is, model 2 and pre-operative pain score. The analyses were repeated using the average of all five WOMAC pain items, WOMAC items associated with movement pain and WOMAC items associated with rest pain. Results are interpreted per SD increase in PPT and its association with 1-unit change in pain response on the WOMAC pain scale either pre-operatively or postoperatively while holding all other factors constant.
Longitudinal analysis
Using a multilevel model, a longitudinal analysis of pain was assessed pre-operatively and at 12 months postoperatively. A multilevel approach allows simultaneous investigation of the effect of PPT on pre-operative pain and change in pain following surgery. This is subtly different from model 3 described in the prospective analysis, as the effect of PPT on pre-operative pain is not modelled. The effect of PPT on pre-operative pain is investigated by the inclusion of an interaction between the pre-operative measurement occasion and standardised PPT. Results are interpreted as per SD increase in PPT and its association with pre-operative pain. In addition, the effect of PPT on the change in pain response is also modelled by the inclusion of an interaction between standardised PPT and time. Results are interpreted as per SD increase in PPT and its association with change in reported pain from pre- to postoperative pain assessments while taking into account any effect of PPT on pre-operative pain.
To investigate the linearity of the PPT pain response on pre-operative pain and change in pain, two additional models were fitted. Using quintiles of pre-operative PPT, a longitudinal model was refitted with separate intercepts and a common slope. In addition, a fully stratified model of pain was fitted using five different intercepts and five different slopes. Models were compared using likelihood ratio tests.
All models are fitted using iterative generalised least squares in MLwiN (MLwiN, Centre for Multilevel Modelling, Bristol, UK) using the runmlwin command486 in Stata.
Results
Descriptive data
A total of 254 patients undergoing THR and 239 patients undergoing TKR had complete covariate information and were included in these analyses. Baseline characteristics of participants are provided in Table 71. Patients undergoing THR had a mean age of 66.5 years (SD 10 years), which was slightly younger than the mean of 69.1 years for the TKR patients.
| Patient characteristic | Hips | Knees |
|---|---|---|
| Randomised group | ||
| Standard care | 130 | 124 |
| Intervention | 124 | 115 |
| Sex | ||
| Male | 105 | 114 |
| Female | 149 | 125 |
| Employment | ||
| Unemployed | 163 | 183 |
| Employed | 91 | 56 |
| Retired | ||
| Not retired | 96 | 58 |
| Retired | 158 | 181 |
| Cohabitation | ||
| Alone | 53 | 70 |
| Not alone | 201 | 169 |
| Education | ||
| ≤ 16 years | 169 | 179 |
| > 16 years | 85 | 60 |
Pre-operative WOMAC pain scores were similar between patients undergoing THR and TKR, for both total pain scores and movement pain scores (Table 72). However, patients undergoing TKR had more severe pre-operative rest pain than those undergoing THR. WOMAC pain scores at 12 months after surgery were generally worse in patients undergoing TKR than in those undergoing THR, whether considering overall pain severity, movement pain or rest pain. The mean pre-operative PPT (kilopascals, kPa) for THR patients was 212 kPa (SD 98 kPa), which was similar to the mean PPT of 206 kPa (SD 103 kPa) for TKR patients.
| Joint replacement | Time | Measure | Mean | SD | 25th percentile | Median | 75th percentile |
|---|---|---|---|---|---|---|---|
| Hip | Pre operation | PPT mean | 212.17 | 97.68 | 137.7 | 193.3 | 266.0 |
| PPT SD | 38.42 | 31.86 | 16.7 | 29.2 | 53.4 | ||
| WOMAC | 3.28 | 0.74 | 2.8 | 3.2 | 3.8 | ||
| WOMAC move | 3.41 | 0.77 | 3.0 | 3.3 | 4.0 | ||
| WOMAC rest | 3.08 | 0.90 | 2.5 | 3.0 | 3.5 | ||
| Post operation | WOMAC | 1.43 | 0.67 | 1.0 | 1.2 | 1.6 | |
| WOMAC move | 1.45 | 0.71 | 1.0 | 1.0 | 1.7 | ||
| WOMAC rest | 1.40 | 0.70 | 1.0 | 1.0 | 1.5 | ||
| Knee | Pre operation | PPT mean | 205.65 | 102.62 | 132.0 | 185.7 | 253.0 |
| PPT SD | 33.90 | 27.47 | 16.2 | 27.4 | 41.5 | ||
| WOMAC | 3.27 | 0.65 | 2.8 | 3.2 | 3.6 | ||
| WOMAC move | 3.57 | 0.63 | 3.0 | 3.7 | 4.0 | ||
| WOMAC rest | 2.83 | 0.92 | 2.5 | 3.0 | 3.5 | ||
| Post operation | WOMAC | 1.74 | 0.83 | 1.0 | 1.4 | 2.2 | |
| WOMAC move | 1.85 | 0.90 | 1.0 | 1.7 | 2.3 | ||
| WOMAC rest | 1.59 | 0.83 | 1.0 | 1.0 | 2.0 |
Pain sensitivity and pre-operative pain severity
Total hip replacement
In both the minimally and fully adjusted linear regression models, pre-operative PPT was strongly associated with pre-operative pain severity (p = 0.002 and p = 0.001, respectively; Table 73). The same pattern of association was found using a linear mixed model (p = 0.001; Table 74). When the pre-operative WOMAC pain score was broken down in the subconstructs of movement pain and rest pain, PPT was significantly associated with movement pain but rest pain was not (see Tables 73 and 74).
| Model | Outcome | Model adjustments | Beta | SE | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Hip | ||||||
| Cross-Sectional | Pre-operative WOMAC | 1. Minimal | –0.144 | 0.05 | –0.235 to –0.054 | 0.002 |
| Pre-operative WOMAC Move | –0.183 | 0.05 | –0.278 to –0.088 | 0.000 | ||
| Pre-operative WOMAC Rest | –0.086 | 0.06 | –0.198 to 0.026 | 0.131 | ||
| Prospective | WOMAC 12 | –0.110 | 0.04 | –0.193 to –0.027 | 0.010 | |
| WOMAC 12 move | –0.131 | 0.04 | –0.219 to –0.043 | 0.004 | ||
| WOMAC 12 rest | –0.078 | 0.04 | –0.165 to 0.009 | 0.079 | ||
| Cross-Sectional | Pre-operative WOMAC | 2. Adjusted | –0.148 | 0.05 | –0.238 to –0.058 | 0.001 |
| Pre-operative WOMAC move | –0.187 | 0.05 | –0.281 to –0.092 | 0.000 | ||
| Pre-operative WOMAC rest | –0.091 | 0.06 | –0.203 to 0.021 | 0.111 | ||
| Prospective | WOMAC 12 | –0.104 | 0.04 | –0.187 to –0.020 | 0.015 | |
| WOMAC 12 move | –0.127 | 0.05 | –0.216 to –0.038 | 0.005 | ||
| WOMAC 12 rest | –0.068 | 0.04 | –0.155 to 0.019 | 0.126 | ||
| WOMAC 12 | 3. Adjusted + pre-operative pain | –0.091 | 0.04 | –0.176 to –0.006 | 0.036 | |
| WOMAC 12 move | –0.114 | 0.05 | –0.205 to –0.022 | 0.015 | ||
| WOMAC 12 rest | –0.059 | 0.04 | –0.147 to 0.028 | 0.181 | ||
| Knee | ||||||
| Cross-Sectional | Pre-operative WOMAC | 1. Minimal | –0.068 | 0.04 | –0.156 to 0.019 | 0.126 |
| Pre-operative WOMAC Move | –0.067 | 0.04 | –0.152 to 0.019 | 0.125 | ||
| Pre-operative WOMAC Rest | –0.071 | 0.06 | –0.193 to 0.052 | 0.258 | ||
| Prospective | WOMAC 12 | –0.063 | 0.06 | –0.174 to 0.047 | 0.259 | |
| WOMAC 12 move | –0.064 | 0.06 | –0.184 to 0.056 | 0.292 | ||
| WOMAC 12 rest | –0.062 | 0.06 | –0.173 to 0.049 | 0.271 | ||
| Cross-Sectional | Pre-operative WOMAC | 2. Adjusted | –0.088 | 0.04 | –0.175 to –0.001 | 0.047 |
| Pre-operative WOMAC move | –0.080 | 0.04 | –0.165 to 0.005 | 0.066 | ||
| Pre-operative WOMAC rest | –0.100 | 0.06 | –0.222 to 0.022 | 0.106 | ||
| Prospective | WOMAC 12 | –0.093 | 0.06 | –0.204 to 0.017 | 0.097 | |
| WOMAC 12 move | –0.097 | 0.06 | –0.217 to 0.023 | 0.114 | ||
| WOMAC 12 rest | –0.088 | 0.06 | –0.199 to 0.023 | 0.118 | ||
| WOMAC 12 | 3. Adjusted + pre-operative pain | –0.053 | 0.05 | –0.157 to 0.051 | 0.313 | |
| WOMAC 12 move | –0.062 | 0.06 | –0.177 to 0.054 | 0.293 | ||
| WOMAC 12 rest | –0.059 | 0.05 | –0.165 to 0.047 | 0.273 | ||
| Outcome | Beta | SE | 95% CI | p-value | Likelihood |
|---|---|---|---|---|---|
| Hip | |||||
| Pre-operative WOMAC pain | –0.157 | 0.05 | –0.250 to –0.065 | 0.001 | –507.2 |
| Change WOMAC pain | 0.047 | 0.06 | –0.071 to 0.164 | 0.44 | |
| Pre-operative WOMAC movement pain | –0.196 | 0.05 | –0.293 to –0.099 | 0.000 | –536.9 |
| Change WOMAC movement pain | 0.057 | 0.06 | –0.069 to 0.183 | 0.37 | |
| Pre-operative WOMAC rest pain | –0.101 | 0.06 | –0.215 to 0.013 | 0.083 | –572.9 |
| Change WOMAC rest pain | 0.031 | 0.07 | –0.103 to 0.164 | 0.65 | |
| Knee | |||||
| Pre-operative WOMAC pain | –0.087 | 0.04 | –0.173 to –0.002 | 0.045 | –491.8 |
| Change WOMAC pain | –0.013 | 0.05 | –0.119 to 0.093 | 0.81 | |
| Pre-operative WOMAC movement pain | –0.076 | 0.04 | –0.160 to 0.008 | 0.075 | –514.3 |
| Change WOMAC movement pain | –0.036 | 0.06 | –0.153 to 0.080 | 0.54 | |
| Pre-operative WOMAC rest pain | –0.108 | 0.06 | –0.226 to 0.010 | 0.074 | –577.2 |
| Change WOMAC rest pain | 0.022 | 0.06 | –0.104 to 0.148 | 0.74 | |
Total knee replacement
The patterns of associations were much weaker in TKR patients than in THR patients (see Table 73). In minimally adjusted models, there was no evidence of an association of PPT with total, movement or rest pain before surgery (p > 0.1). However, following more complete adjustment, the strength of the association increased to borderline significant for total pain severity (p = 0.047). Similarly, a weak association between PPT and pre-operative total pain severity (p = 0.045), but not with movement pain or rest pain, was found in the linear mixed model (see Table 74).
Pain sensitivity and 12-month postoperative pain severity
Total hip replacement
In the minimally and fully adjusted linear regression models, there was strong evidence of an association between pre-operative PPT and pain severity at 12 months following surgery (p = 0.01 and p = 0.015, respectively; see Table 73). These models showed that lower PPTs (higher pain sensitivity) were associated with more severe pain at 12 months following surgery. When the analyses were repeated with movement pain and rest pain, PPT was associated with movement pain but not rest pain at 12 months after surgery (see Table 73).
Total knee replacement
There was no evidence of an association between pre-operative PPT and pain severity at 12 months after surgery in any of the linear regression models (see Table 73). Similarly, further analysis found that PPT was not associated with rest pain or movement pain at 12 months post operation (see Table 73).
Pain sensitivity and change in pain severity
Total hip replacement
After adjusting for pre-operative pain severity, the associations in the linear regression models between PPT and 12-month pain severity were mildly attenuated; however, the association persisted (p = 0.036). Analysis was then undertaken to explore the association between pre-operative PPT and change in WOMAC pain scores from before surgery to 12 months after surgery (see Table 74). There was no evidence of an association between PPT and change in pain scores (p = 0.44). Similarly, no association was found between PPT and change in movement pain (p = 0.37) or rest pain (p = 0.65). Further analyses using PPT quintiles to explore the relationship between pre-operative PPT and change in pain scores showed similar results (data not shown).
Total knee replacement
There was no evidence of any association between PPT and change in pain score from pre-operative to 12 months after TKR surgery (p = 0.81; see Table 74). This finding was the same when pain severity was analysed as change in movement pain (p = 0.54) and rest pain (p = 0.74). Further analyses using PPT quintiles to explore the relationship between pre-operative PPT and change in pain scores showed similar results (data not shown).
Discussion
Using data from the APEX cohort study, we found a relationship between measures of before surgery central pain sensitisation and pre-operative pain severity in a large sample of patients with advanced hip and knee osteoarthritis. Our longitudinal study design also allowed us to demonstrate that there is a strong association between pre-operative PPTs and pain severity at 12 months after THR, but not after TKR. Uniquely, we have shown there is no evidence of effect modification of PPT on the efficacy of surgery in patients undergoing THR or TKR. This suggests that pre-operative pain sensitivity, assessed through measurement of forearm PPTs, does not influence the amount of pain relief that patients gain from joint replacement.
Chapter 8 Systematic review and meta-analysis of exercise and education interventions before total hip and knee replacement
Abstract
Background
We aimed to evaluate the clinical effectiveness of exercise and education in patients waiting for total hip or knee replacement.
Methods
We searched MEDLINE, EMBASE, CINAHL, PsycINFO, Cochrane and Web of Science databases from inception to March 2014. Searches covered hip and knee replacement, randomised trials and pre-surgery. Interventions targeted optimisation of pre-surgical health, preparation for in-hospital recovery and long-term health. Outcomes extracted in duplicate were combined in meta-analyses with additional data provided by authors. Study quality was assessed.
Results
Interventions targeting optimisation of pre-surgical health (n = 36) showed benefit compared with controls in physical function (SMD 0.28, 95% CI 0.16 to 0.40); pain (SMD 0.21, 95% CI 0.10 to 0.33); and anxiety (SMD 0.38, 95% CI 0.11 to 0.65). Benefit was mainly limited to THR and effect sizes were largely unaffected by study quality or exercise/education content.
In studies targeting in-hospital recovery (n = 27), post-surgical anxiety was lower in intervention patients (SMD 0.38, 95% CI 0.13 to 0.63) who also mobilised earlier.
Interventions targeting long-term outcomes (n = 18) showed no benefit.
Conclusions
Exercise and education before total hip and knee replacement can improve patients’ pre-surgical health and early recovery. Further research is required for knee replacement, intervention content and in relation to long-term outcomes.
Background
Previous systematic reviews of interventions before surgery have focused on the effectiveness of education83 and physiotherapy. 82 In this section we update these with a series of more recent reviews of RCTs using systematic review methods. Acknowledging the overarching aims of exercise and education before hip and knee replacement, we classified studies according to their primary objectives. Thus, interventions are targeting making improvements to one or more of pre-surgical physical and psychological health, preparedness for recovery in hospital, and long-term post-surgical outcomes.
Methods
| General methods | As described in Chapter 2, Systematic review methods |
| Databases and dates | MEDLINE, EMBASE, CINAHL, PsycINFO and The Cochrane Library from inception to 17 October 2013. Citations of key articles in ISI Web of Science and reference lists. Previous systematic reviews and meta-analyses were checked |
| Search strategy | Hip or knee replacement/RCT/pre-surgery. MEDLINE search strategy based on terms in Appendix 3 |
| Study design | RCTs with individual or cluster randomisation. Quasi-randomised designs (e.g. alternate allocation) |
| Patients | Adults waiting for total hip or knee replacement |
| Intervention | Non-pharmacological pre-surgical intervention with the aim of improving outcomes before or after joint replacement. Interventions between being placed on the waiting list and surgery:
|
| Controls | Usual care or minimal intervention |
| Follow-up | ≥ 3 months |
| Data extraction | Country, baseline dates, participants (indication, age, sex), inclusion and exclusion criteria, intervention and control group content, setting, timing, duration and intensity, follow-up time, losses to follow-up and reasons |
| Outcomes | Patient-reported physical function, pain and anxiety measured before surgery Patient-reported anxiety and pain, mobilisation measured in hospital or < 1 month after surgery, and length of hospital stay Patient-reported physical function and pain from 3 months after surgery (longest follow-up reported unless large loss to follow-up) |
| Quality assessment | Good, reasonable (e.g. non-blind follow-up with self-complete questionnaires), or possible bias (unequal or major loss to follow-up, or important baseline differences) |
Studies were classified into groups A, B and C independently by two reviewers with final decisions on classifications decided by consensus with input from other programme contributors.
Results
Review progress is summarised as a flow diagram in Figure 37. Searches identified 5073 articles. After screening and detailed evaluation, 48 articles describing 52 interventions met the inclusion criteria and study characteristics are summarised in Table 75. Studies reported interventions in patients before hip (n = 22),487–508 knee (n = 21),500,503,509–525 or either hip or knee replacement (n = 9). 526–534 Interventions focused on optimising pre-surgical health (n = 36), preparation for recovery in hospital (n = 27) and improving long-term outcomes (n = 18). Potential sources of bias are summarised in Appendix 8. Results of meta-analyses are summarised in Table 76.
FIGURE 37.
Systematic review and meta-analysis of exercise and education interventions before total hip and knee replacement: flow diagram.
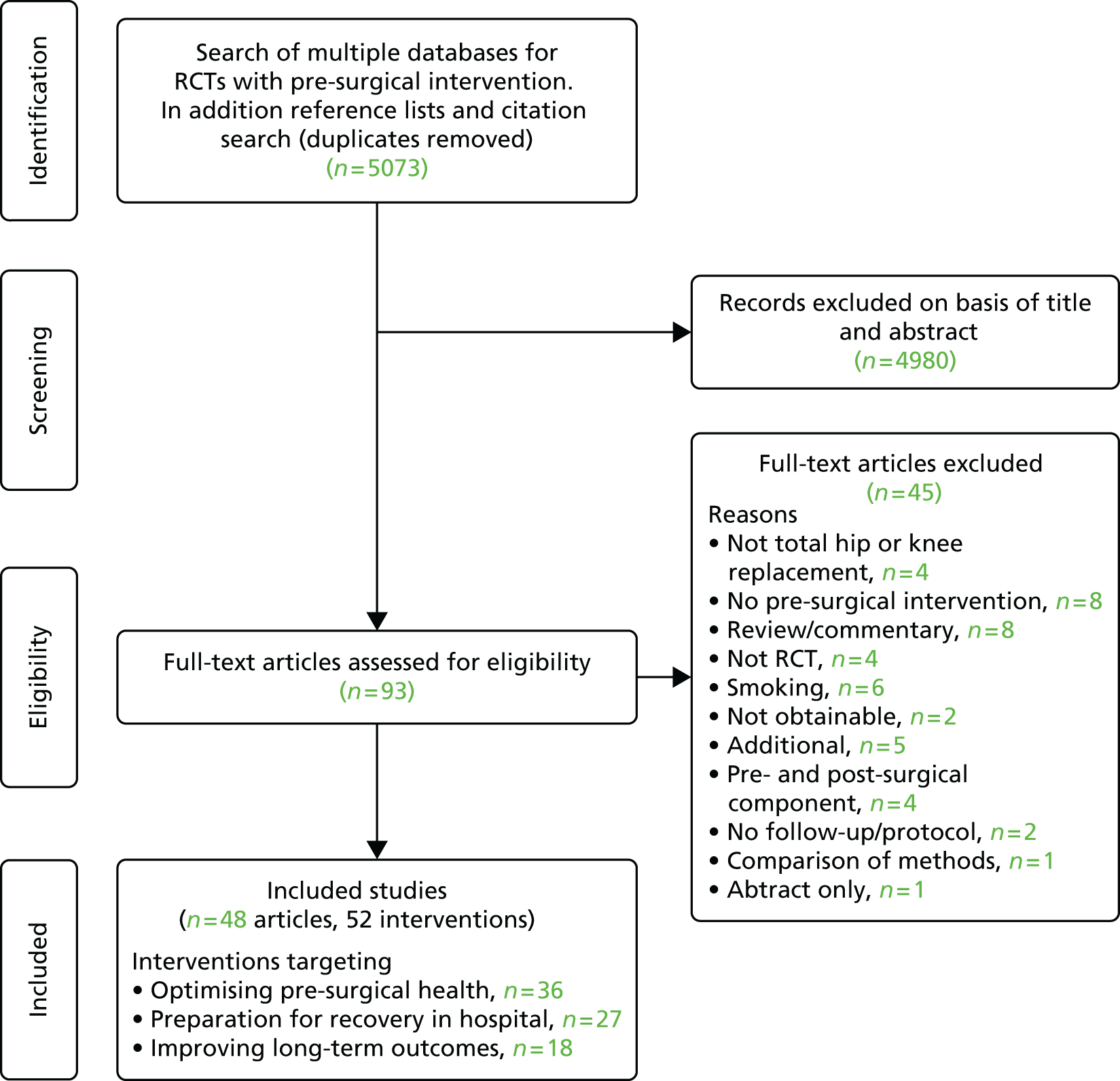
| Publication; location; date of study; focus of intervention (A = optimising health, B = hospital preparation, C = long term) | Hip or knee; indication; number randomised (intervention : control); mean age (years) (% female) | Primary focus of intervention; study setting; timing, duration and intensity; control group care | Follow-up interval; outcomes; losses to follow-up (intervention : control) |
|---|---|---|---|
| Aoki and colleagues 2009;509 Japan; 2004–5; A | Knee; severe knee osteoarthritis; n = 36 (17 : 19); 73.4 years (100%) | Exercise (stretching); home; from registration for surgery until admission; control: no intervention | After admission to hospital; VAS pain; no losses to follow-up |
| Beaupre and colleagues 2004;510 Canada; date not specified; A, B and C | Knee; non-inflammatory arthritis; n = 131 (65 : 66) – 16 patients (10 intervention, 6 control) did not have joint replacement: still eligible for review A. It is possible that the intervention was ‘highly effective’ in improving function and pain; 67 years (55%) | Multifactorial: exercise (gait re-education, functional, ROM, strengthening) and education; community physical therapy clinic group; three times per week for 4 weeks, 6 weeks before surgery; control: no intervention | Pre-operation, 3, 6 and 12 months after surgery; WOMAC, SF-36, LOS; 16 (10 : 6) patients cancelled surgery, six (4 : 2) patients lost to 12-month follow-up |
| Berge and colleagues 2004;487 UK; date not specified; A and C | Hip; osteoarthritis; n = 40 (19 : 21) – 4 intervention patients dropped out before programme; 71.3 years (67.5%) | Multifactorial: exercise (strengthening and stretching) and education with relaxation pain management programme; outpatient group; once or twice a week for 6 weeks, ≥ 6 months pre-surgery; control: no intervention | Follow-up 3 months after programme and minimum 1 year; NRS pain, AIMS; 3 (0 : 3) patients lost to first follow-up (surgery early). Overall 7 (1 : 6) lost to 1-year follow-up |
| Bitterli and colleagues 2009;488 Switzerland 2004–7; A and C | Hip; unilateral osteoarthritis or avascular necrosis; n = 80 (41 : 39); 66.8 years (38.8%) | Exercise (strengthening and balance); home; two times per day, 10 repetitions for 2–6 weeks; control: no intervention | Follow-up at 10 days, 4 months and 12 months after surgery; SF-36, WOMAC; (5 : 2) lost to pre-surgical follow-up; 18 (9 : 9) lost to 12-month follow-up |
| Bondy and colleagues 1999;526 USA; date not specified; A and B | Hip and knee; not specified; n = (65 : 69) – 200 patients randomised but baseline and pre-surgery questionnaires completed by 134 patients; 65.2 years (59.5%) | Education; home; < 3 weeks before surgery; control: pre-operative anaesthetist visit | No follow-up after surgery; STAI anxiety; of 200 randomised, 148 returned questionnaires of which 134 (67%) were completed sufficiently |
| Börjesson and colleagues 1996;511 Sweden; date not specified; A | Knee; unilateral medial osteoarthrosis I-III; n = 68 (34 : 34); 64 (50%) | Exercise (strengthening, ROM); hospital outpatient group; three times per week lasting 40 minutes for 5 weeks; control: no intervention | After 3 months (as close to end of intervention as possible), no follow-up after surgery; pain on walking using Borg scale; 0 losses to follow-up |
| Brown and colleagues 2012;512 USA; date not specified; A and C | Knee; osteoarthritis; n = 32(17 : 15); age and sex not described | Exercise; home and physical therapy clinic; partially supervised resistance and flexibility exercises and step training, training booklet; twice a week at home and once a week at physical therapy clinic for 50 minutes from 8 weeks before surgery; usual pre-operative care | 3 months; SF-36 (physical functioning and bodily pain); 14 (6 : 8) lost to follow-up |
| Butler and colleagues 1996;489 Canada; 1993–4; A and B | Hip; not specified; n = 80 (32 : 48); 62.6 years (51.3%) | Education; home; 4–6 weeks before surgery; control: mailed preadmission package only | To discharge; anxiety STAI, LOS; one patient died and no follow-up data from eight patients |
| aClode-Baker and colleagues 1997;490 UK; date not specified; B | Hip (76% primary); not specified; n = 78 (41 : 37) – 91 randomised, 13 operations cancelled or postponed; mean age not specified (66.7%) | Education; home; approximately 4 weeks before surgery; control patients seen routinely by nursing staff on admission | Day before surgery, days 1–7 after surgery and 8 days after discharge; NHP, Hamilton Anxiety and Depression, days to mobilisation, LOS, pain (descriptive ordinal scale); four (1 : 3) lost to postoperative anxiety follow-up |
| Cooil and Bithell 1997;491 UK; date not specified; B | Hip; not specified; n = 42 (21 : 21); 69 years (71.4%) | Education; hospital during admission, individual; day before surgery; control: information sheet but no further contact | Days 1 and 2 after operation; no outcomes relevant to review; no losses to follow-up reported |
| Crotty and colleagues 2009;527 Australia; 2005–6; A | Hip or knee; osteoarthritis; n = 152 (77 : 75); 67.5 years (60.5%) | Education; home, outpatient and community; individual and group; 5 weeks’ duration; control: no intervention | 6 months after intervention (11 patients in each group followed up mean 106 days after surgery); WOMAC; no losses to follow-up reported |
| aCrowe and Henderson 2003;528 Canada; date not specified; A and B | Hip or knee; osteoarthritis, rheumatoid arthritis; n = 133 (65 : 68); 70 years (80%) | Multifactorial: exercise (strengthening and endurance), education and occupational therapy; hospital outpatient, individual and group; one session soon after randomisation with additional physiotherapy and attendance at day care hospital; control: single standard pre-operative clinic visit | To hospital discharge; LOS, anxiety STAI, days to mobilisation; one patient did not receive surgery |
| aCuñádo Barrio and colleagues 1999;529 Spain; 1996–7; A and B | Hip or knee; not specified; n = 84 (42 : 42); 65 years (66.7%) | Education; hospital, individual; 2 days before the operation lasting 20 minutes; control: standard pre-operative programme | Pre-operative and 4 to 5 days post operation (as appropriate); anxiety STAI, mobilisation, LOS; eight (1 : 7) lost to follow-up |
| Daltroy and colleagues 1998;530 USA; 1985–7; B | Hip or knee; rheumatoid or osteoarthritis; n = 222, 2 × 2 factorial (58 : 58 : 52 : 54); 64 years (66%) | Education; hospital, individual; day before surgery; control: usual pre-operative preparation | Day 4 post operation; anxiety STAI, pain (Likert scale), LOS, no data were suitable for meta-analysis; data not available for six patients |
| D’Lima and colleagues 1996 (cardiovascular);513 USA; date not specified; A and C | Knee; rheumatoid or osteoarthritis; n = 20 (10 : 10); 70.6 years (35%) | Exercise (stretching, strengthening, cardiovascular conditioning); hospital, individualised; 45-minute sessions, three times a week for 18 weeks commencing 6 weeks before surgery; control: one meeting with physical therapist | Follow-up at 6 weeks and 1 week preoperation, and 3, 12, 24 and 48 weeks postoperatively; HSS; no losses to follow-up reported |
| D’Lima and colleagues 1996 (physical therapy);513 USA; date not specified; A and C | Knee; rheumatoid or osteoarthritis; n = 20 (10 : 10); 69.0 years (60%) | Exercise (strengthening, ROM); hospital, one-on-one programme; 45-minute sessions, three times a week for 18 weeks commencing 6 weeks before surgery; controls: one meeting with physical therapist | Follow-up at 6 weeks and 1 week preoperation, and 3, 12, 24 and 48 weeks postoperatively; HSS; no losses to follow-up reported |
| aDoering and colleagues 2000;492 Austria; date not specified; B | Hip; osteoarthritis; n = 100 (46 : 54); 59.6 years (38%) | Education; hospital, individual; day before surgery; control: no intervention | 1, 2 and 3 days post operation; STAI anxiety, VAS pain, LOS |
| Evgeniadis and colleagues 2008;514 Greece; 2006; A and C | Knee; idiopathic osteoarthritis; n = 48 (24 : 24); 68.3 years (76.3%) | Exercise (strengthening); home; 1 month before surgery for 3 weeks, three alternative days a week; controls: standard pre- and postoperative care | Day before surgery and 2, 6, 10 and 14 weeks after surgery; SF-36, ILAS; five (3 : 2) lost to follow-up |
| Ferrara and colleagues 2008;493 Italy; 2006–7; A and C | Hip; end-stage osteoarthritis; n = 23 (11 : 12); 63.4 years (60.9%) | Multifactorial: exercise (strengthening, stretching, cardiovascular) and occupational therapy; hospital, individual and group; 1 month prior to surgery, 60 minutes per day for 5 days per week; controls: no intervention | Day before surgery and 15 days, 4 weeks and 3 months after surgery; SF-36, WOMAC; two (0 : 2) patients lost to 3-month follow-up |
| Gilbey and colleagues 2003;494 Australia, 1997–9; A (C not included as post-surgical intervention) | Hip; osteoarthritis, post-traumatic and inflammatory arthritis, osteonecrosis, Paget’s disease; n = 68 (37 : 31) – a further eight patients withdrew before surgery; 65.2 years (61.8%) | Exercise (strengthening, ROM, gait re-education, hydrotherapy); clinic and home, group and individual; 8 weeks before surgery, two clinic- and two home-based sessions per week, clinic sessions lasted 1 hour; control group received in-hospital physical therapy | Week before surgery and 3, 12, and 24 weeks after surgery; WOMAC; two (2 : 0) chose to delay surgery |
| Giraudet-Le Quintrec and colleagues 2003;495 France; 1997–9; A and B | Hip; osteoarthritis; n = 100 (48 : 52); 63.5 years (56%) | Education; teaching hospital, small group (three to six patients); 2–6 weeks before surgery, one class lasting half day; controls received verbal information and leaflet | Follow-up 1–7 days post surgery; VAS pain, STAI anxiety, days to standing, LOS; one (0 : 1) patient did not complete STAI after surgery |
| Gocen and colleagues 2004;496 Turkey; date not specified; A, B and C | Hip; primary or secondary osteoarthritis; n = 59 (29 : 30); 51.3 years (35.6%) | Multifactorial: exercise (strengthening, stretching) and education; home and hospital; 8 weeks before surgery, three times per day; control: no intervention | Immediately pre-operative, at discharge, 3 months and 2 years; HHS, VAS pain, days to walking; one (1 : 0) not operated |
| Gstoettner and colleagues 2011;515 Austria; date not specified; A and C | Knee; severe unilateral osteoarthritis; n = 38 (18 : 20); 69.8 years (79%) | Exercise (strengthening, proprioception, balance, functional); home and hospital; 6 weeks before surgery, taught once per week for 45 minutes, then at home; control treatment received no intervention | Intervention patients followed up 6 weeks before surgery for WOMAC, 6 weeks after surgery; WOMAC; three (3 : 0) lost to follow-up |
| Heikkinen and colleagues 2008;516 Finland; 2005–6; B | Knee (study also included patients with shoulder arthroscopy); n = 59 (27 : 32) specifically knee; age and sex of knee patients not described | Education; home; website used on an average of 14 days before surgery (SD 19.1, range 1–121 days); control group received face-to-face education with nurse | 2 weeks postoperatively; no outcomes relevant to review; three (1 : 2) lost to follow-up but some of these may be shoulder arthroscopy patients |
| Hoogeboom and colleagues 2010;497 The Netherlands; 2007–8; A | Hip; end-stage osteoarthritis; n = 21 (10 : 11); 76 years (66.6%) | Exercise (strengthening, cardiovascular, function); outpatient and home, individual and group; 3–6 weeks before surgery, twice a week for 60 minutes; control group received usual pre- and postoperative care | Pre-operative and to discharge; HOOS; one (0 : 1) lost to follow-up |
| Huang and colleagues 2012;517 Taiwan; 2008–10; A and B | Knee; advanced osteoarthritis; n = 243 (126;117); 70.2 years (71.6%) | Exercise (strengthening) and education; clinic and home, group and individual; 2–4 weeks before surgery, 40-minute meeting in clinic followed by home-based programme; control: no intervention | To discharge and complications; LOS, VAS pain; no losses to follow-up reported |
| Johansson and colleagues 2007;498 Finland; 2003–4; B | Hip; elective; n = 123 (62 : 61); 62.4 years (51.2%) | Education; hospital pre-operative clinic, individual; 2 weeks before surgery, for up to 1 hour; control group received written education materials only | To discharge; LOS; 17 (7 : 10) lost to follow-up |
| Lewis and colleagues 2002;531 USA; date not specified; B | Hip or knee; not specified; n = 58 (29 : 29); 68.5 years (58.6%) | Education (interactive); pre-admission test centre, individual; controls were shown a non-interactive video | Days 1, 2 and 3 post operation; LOS; two (1 : 1) lost to follow-up |
| Lilja and colleagues 1998;499 Sweden; date not specified; B | Hip; consecutive patients waiting for THR; n = 50 (22 : 28) – further five patients excluded after randomisation; median 65 years (34%) | Education; hospital; day before surgery, 30 minutes; control: no intervention | Days 1, 2 and 3 post operation; VAS pain; no losses to follow-up further than one refused to participate after randomisation and four (3 : 1) withdrawn for medical reasons |
| Liu and Lu 2004;532 China; 2002; B | Hip and knee surgery (also arthroscopy); not specified but included tibia and fibula wounds and leg joint fusion; n = 74 years (39 : 35); 53.8 years (47.3%) | Education; location of intervention not specified, individual; time scale before surgery when education given not specified; traditional education | Time after post surgery not specified; LOS; no losses to follow-up reported |
| Mancuso and colleagues 2008 Hip;500 USA; 2001–3; A | Hip; 94.5% osteoarthritis; n = 177 (90 : 87); 70.5 years (55.9%) | Education; hospital, group; one class with additional 15-minute module; control patients received standard pre-operative class | Mean 4 days after class, no further follow-up; no relevant outcome |
| Mancuso and colleagues 2008 Knee;500 USA; 2001–3; A | Knee; 94.0% osteoarthritis; n = 143 (70 : 73); 71.5 years (57.3%) | Education; hospital, group; one class with additional 15-minute module; control patients received standard pre-operative class | Mean 4 days after class, no further follow-up; no relevant outcome |
| McDonald and colleagues 2001;533 USA; 1998–9; B | Hip and knee; osteoarthritis, primary and revision; n = 40 randomised, data on 31 reported (13 : 18); 74 years (74.2%) | Education; urban medical centre or home, group or individual; one session at pre-operative joint replacement class; control patients received a slide show on pain management and use of pain intensity scale | Postoperative days 1 and 2; McGill Pain Questionnaire-short form (measures sensory and effective pain and pain intensity); nine (7 : 2) lost to follow-up as some patients unable to complete questionnaires |
| McDonald and Molony 2004 (communication);518 USA; 2000–1; B | Knee; osteoarthritis; n = 26 (17 : 9); 71.8 years (63.4%) | Education; medical centres, group; one pre-operative class; comparison group received usual pre-operative class | Postoperative days 1 and 2, and 1 and 7 days post-hospital discharge by telephone; SF-MPQ affective pain, LOS; no losses to follow-up reported |
| McDonald and Molony 2004 (pain management);518 USA; 2000–1; B | Knee; osteoarthritis; n = 24 (15 : 9); 71.8 years (63.4%); | Education; medical centres, group; 10-minute film at pre-operative class; comparison group received usual pre-operative class | Postoperative days 1 and 2, and 1 and 7 days post-hospital discharge by telephone, no longer-term follow-up; SF-MPQ affective pain, LOS; no losses to follow-up reported |
| McGregor and colleagues 2004;501 UK; 1998–9; A, B and C | Hip; osteoarthritis; n = 39 (19 : 20); 71.9 years (42.9%) | Education; hospital and home, group; hip class 2–4 weeks before surgery; controls received standard care | 3 months postoperatively; WOMAC, LOS; four (4 : 0) patients lost to follow-up |
| McKay and colleagues 2012;519 Canada; 2010; A and C | Knee; osteoarthritis, primary; n = 22 (10 : 12); 61.9 years (59%) | Exercise (strengthening); research facility; 3 times a week for 6 weeks; control: non-specific upper-body strength training | Follow-up immediately before surgery and at 6 and 12 weeks after surgery; WOMAC; five (3 : 2) lost to 12-week follow-up |
| Mitchell and colleagues 2005;520 UK; 1999–2000; A (not C as intervention patients received additional postoperative care) | Knee; osteoarthritis; n = 114 (57 : 57) – 160 randomised but 45 (23 : 22) withdrew; 70.3 years (57.9%) | Exercise (gait re-education, ROM) and occupational therapy; home; 8 weeks before surgery, minimum of three pre-operative visits with up to 6 postoperative visits; control group received outpatient physiotherapy | Follow-up 12 weeks; no outcome reported before additional post-surgery physiotherapy; one (0 : 1) died postoperatively |
| Nuñez and colleagues 2006;521 Spain; 2001; A | Knee; osteoarthritis; n = 100 (51 : 49); 71.1 years (71%) | Multifactorial: exercise (strengthening, ROM, gait re-education, general) and education; hospital and home, groups of 12 patients, maximum; 3-month programme with 30-minute individual visits lasting in first week and at 3 months, and two group sessions of approximately 90 minutes in weeks 3 and 4; control group were seen individually twice by a physician | 9-month follow-up (after intervention); WOMAC, SF-36, self-reported HRQoL, number of GP visits and costs; 20 (8 : 12) lost to follow-up |
| Oosting and colleagues 2012;502 The Netherlands; date not specified; A and C | Hip; osteoarthritis, age > 65 years, frail; n = 30 (15 : 15); 78 years (80%) | Exercise (functional); outpatient and home; twice per week for 3–6 weeks and home four times per week; one physical therapist-led group session 3 weeks before surgery | 2–4 days before admission and up to 6 weeks after surgery; HOOS; 0 (0 : 2) lost to follow-up at pre-surgery assessment |
| Pellino and colleagues 1998;534 USA; 1995–6; A (surgery delayed in control group) | Hip and knee; elective orthopaedic surgery; n = 74 (39 : 35), 83 eligible; 53.8 years (50%) included in analysis | Education; learning centre, group and individual; one class; controls received traditional teaching at the hospital clinic | Up to 1 month follow-up after surgery; ability to complete perioperative care; seven patients did not complete questionnaires and two had surgery cancelled |
| Rooks and colleagues 2006 Hip;503 USA; 2001–3; A and C | Hip; osteoarthritis; n = 63 (32 : 31); 62 years (57.1%) | Exercise (strengthening, flexibility, cardiovascular, pool exercises); community fitness facility; three times per week for 6 weeks, 30–60 minutes; control: two education mailings and three telephone calls | Follow-up post intervention and at 8 and 26 weeks post surgery; WOMAC; 14 (7 : 7) lost to follow-up |
| Rooks and colleagues 2006 Knee;503 USA; 2001–3; A and C | Knee; osteoarthritis; n = 45 (22 : 23); 67.0 years (53.3%) | Exercise (strengthening, flexibility, cardiovascular, pool exercises); community fitness facility; three times per week for 6 weeks, 30–60 minutes each, increasing intensity from 4 to 6 weeks; control: two education mailings and three telephone calls | Follow-up post intervention and at 8 and 26 weeks post surgery; WOMAC; 16 (8 : 8) lost to follow-up |
| Sandell and colleagues 2008;504 UK; 2003; A and B | Hip; waiting time of 6 months or more; n = 89 (43 : 46); 68.2 years (65.1%) | Multifactorial: exercise (strengthening and gait), occupational therapy and pain management; outpatient clinic; 3–16 months before surgery; control: no intervention | Follow-up pre-surgery; AIMS2; 26 (10 : 16) lost to follow-up |
| Santavirta and colleagues 1994;505 Finland; 1989; B | Hip; n = 60 (27 : 33) – 73 patients randomised; 58 years (63%) | Education; during hospital admission; 10- to 60-minute session after admission; all patients received an 18-page illustrated patient guide | During admission and 2–3 months postoperatively; no relevant outcome; 13 (7 : 6) had operation postponed or dropped out for other reasons |
| Sjoling and colleagues 2003;522 Sweden; date not specified; B | Knee; osteoarthritis; n = 60 (30 : 30); 71 years (60%) | Education; hospital; day before surgery or 4 days pre-operatively; controls received standard information | 7–8 days postoperatively; state and trait anxiety, Daily Pain Index based on multiple VAS pain scores, LOS; no losses to follow-up (some data missing at time points) |
| Swank and colleagues 2011;523 USA; 2003–8; A | Knee; osteoarthritis and intractable pain; n = 71 (35 : 36); 62.8 years (65%) | Exercise (strengthening, stretching, walking, step training); hospital clinic and home, individual; commenced 4–8 weeks before surgery; control: no intervention | Week before surgery; VAS pain after functional tests; no losses to follow-up reported |
| Vukomanovic and colleagues 2008;506 Serbia; 2005–6; B and C | Hip; osteoarthritis; n = 45 (23 : 22); 58.2 years (66.7%) | Multifactorial: exercise (functional) and education; home; one education appointment with physiatrist, two practical classes with physiotherapist; controls received no education or physical therapy | Pre-operative period, immediate postoperative period and 15-month follow-up; VAS pain, OHS, time to walking, LOS, VAS sense of uncertainty; four (2 : 2) lost to 15-month follow-up, nine (5 : 4) total lost to follow-up |
| Weidenhielm and colleagues 1993;524 Sweden; date not specified; A and C | Knee (unicompartmental); osteoarthritis; n = 40 (20 : 20); 63.5 years (51.3%) | Exercise (strengthening); outpatient and home, group and individual; three times per week for 5 weeks; commenced 3 months before surgery; controls received no pre-operative therapy | Immediately before and 3 months after surgery; Pain (4 grade scale); one (1 : 0) had a heart attack and did not complete study |
| Wijgman and colleagues 1994;507 The Netherlands; 1991–2; A and B | Hip; primary coxarthrosis; n = 64 years (31 : 33); 65 years (75% female) | Multifactorial: exercise (strengthening, gait) and education; group; single 30-minute session, 2 days to 1 month before surgery; controls received standard care | Day 1 after surgery to 14 months; VAS pain, LOS, time to standing; 1 (1 : 0) lost to follow-up with surgical complication |
| Williamson and colleagues 2007;525 UK; 2004–6; A and C | Knee; osteoarthritis; n = 121 (60 : 61); 69.8 (52.9%) | Exercise (strengthening, balance); outpatient group; 1 hour once a week for 6 weeks; controls received an exercise and advice leaflet | 7 and 12 weeks after start of intervention and 3 months postoperatively; OKS, WOMAC, VAS pain, HADS; 9 (7 : 2) at 7 weeks, 42 (23 : 19) at 3 months post surgery |
| Wong and Wong 1985;508 Canada; 1982–3; B | Hip; elective; n = 98 (51 : 47); 66.7 years (68%) | Education; hospital; day of admission; controls received traditional pre-operative instruction | Six times daily for 4 days after surgery, no relevant outcomes; no losses to follow-up reported, three withdrawals with complications |
| Subgroup | Number of interventions | Number of patients | Method | Effect size (95% CI)a | p-value | Heterogeneity I2 (%) | Heterogeneity p-value |
|---|---|---|---|---|---|---|---|
| A. optimising pre-surgical health | |||||||
| Physical function before surgery | |||||||
| Overall | 19 | 1026 | SMD | 0.28 (0.16 to 0.40) | < 0.00001 | 0 | 0.48 |
| THR | 8 | 343 | SMD | 0.40 (0.18 to 0.61) | 0.0003 | 0 | 0.79 |
| TKR | 9 | 457 | SMD | 0.19 (–0.04 to 0.42) | 0.10 | 24 | 0.23 |
| Total hip or knee replacement | 2 | 226 | SMD | 0.31 (0.05 to 0.58) | 0.02 | 0 | 0.36 |
| Education-based interventions | 3 | 261 | SMD | 0.33 (0.09 to 0.58) | 0.007 | 0 | 0.60 |
| Exercise-based interventions | 12 | 494 | SMD | 0.26 (0.08 to 0.44) | 0.004 | 0 | 0.46 |
| Multifactorial interventions | 4 | 271 | SMD | 0.32 (–0.03 to 0.66) | 0.07 | 46 | 0.13 |
| Overall, higher-quality studies | 10 | 610 | SMD | 0.21 (0.04 to 0.38) | 0.02 | 8 | 0.37 |
| THR, higher-quality studies | 5 | 102 | SMD | 0.34 (0.06 to 0.62) | 0.02 | 0 | 0.50 |
| TKR, higher-quality studies | 4 | 256 | SMD | 0.07 (–0.25 to 0.39) | 0.65 | 35 | 0.20 |
| THR: high physiotherapy frequency/duration | 7 | 308 | SMD | 0.39 (0.16 to 0.62) | 0.0008 | 0 | 0.69 |
| TKR: high physiotherapy frequency/duration | 9 | 457 | SMD | 0.19 (–0.04 to 0.42) | 0.10 | 24 | 0.23 |
| THR: strengthening | 5 | 223 | SMD | 0.36 (0.09 to 0.62) | 0.009 | 0 | 0.52 |
| THR: cardiovascular exercise | 3 | 93 | SMD | 0.48 (0.03 to 0.93) | 0.04 | 11 | 0.32 |
| TKR: strengthening | 9 | 457 | SMD | 0.19 (–0.04 to 0.42) | 0.10 | 24 | 0.23 |
| Pain before surgery | |||||||
| Overall | 21 | 1375 | SMD | 0.21 (0.10 to 0.33) | 0.0004 | 13 | 0.29 |
| THR | 9 | 420 | SMD | 0.47 (0.28 to 0.67) | < 0.00001 | 0 | 0.68 |
| TKR | 11 | 803 | SMD | 0.09 (–0.05 to 0.23) | 0.22 | 0 | 0.74 |
| Total hip or knee replacement | 1 | 152 | 0.10 (–0.22 to 0.41) | ||||
| Overall, higher-quality studies | 16 | 1141 | SMD | 0.24 (0.09 to 0.38) | 0.001 | 25 | 0.17 |
| THR, higher-quality studies | 6 | 309 | SMD | 0.54 (0.31 to 0.77) | < 0.00001 | 0 | 0.77 |
| TKR, higher-quality studies | 9 | 680 | SMD | 0.10 (–0.05 to 0.25) | 0.21 | 0 | 0.57 |
| Education-based interventions | 3 | 286 | SMD | 0.20 (–0.03 to 0.44) | 0.09 | 0 | 0.38 |
| Exercise-based interventions | 13 | 597 | SMD | 0.23 (0.07 to 0.39) | 0.006 | 0 | 0.47 |
| Multifactorial interventions | 5 | 492 | SMD | 0.27 (–0.04 to 0.58) | 0.09 | 56 | 0.06 |
| Anxiety before surgery | |||||||
| Overall | 8 | 660 | SMD | 0.38 (0.11 to 0.65) | 0.006 | 65 | 0.005 |
| B. preparation for recovery in hospital | |||||||
| Anxiety shortly after surgery | |||||||
| Overall | 5 | 381 | SMD | 0.38 (0.13 to 0.63) | 0.003 | 32 | 0.21 |
| Pain shortly after surgery | |||||||
| Overall | 12 | 842 | SMD | 0.18 (–0.01 to 0.38) | 0.06 | 40 | 0.07 |
| THR | 6 | 385 | SMD | 0.13 (–0.07 to 0.33) | 0.21 | 0 | 0.56 |
| TKR | 4 | 352 | SMD | 0.14 (–0.29 to 0.57) | 0.52 | 58 | 0.52 |
| Total hip or knee replacement | 2 | 105 | SMD | 0.53 (–0.11 to 1.17) | 0.10 | 56 | 0.13 |
| Length of hospital stay | |||||||
| Overall | 10 | 997 | MD | –0.16 (–0.78 to 0.45) | 0.60 | 67 | 0.001 |
| THR | 5 | 372 | MD | 0.28 (–0.14 to 0.70) | 0.19 | 0 | 0.73 |
| TKR | 2 | 358 | MD | –0.93 (–1.29 to –0.57) | < 0.00001 | 0 | 0.41 |
| Total hip or knee replacement | 3 | 267 | MD | –0.41 (–3.43 to 2.61) | 0.79 | 67 | 0.05 |
| Time to mobilisation | |||||||
| Overall | 6 | 471 | MD | –0.17 (–0.30 to –0.04) | 0.009 | 0 | 0.68 |
| C. improving long-term outcomes | |||||||
| Long-term physical function | |||||||
| Overall | 15 | 577 | SMD | 0.08 (–0.09 to 0.26) | 0.35 | 9 | 0.35 |
| THR | 7 | 294 | SMD | 0.15 (–0.08 to 0.38) | 0.20 | 0 | 0.47 |
| TKR | 8 | 283 | SMD | 0.01 (–0.28 to 0.30) | 0.93 | 22 | 0.25 |
| Long-term pain | |||||||
| Overall | 10 | 414 | SMD | 0.03 (–0.16 to 0.23) | 0.73 | 0 | 0.80 |
| THR | 5 | 199 | SMD | –0.07 (–0.35 to 0.21) | 0.64 | 0 | 0.51 |
| TKR | 5 | 215 | SMD | 0.13 (–0.14 to 0.40) | 0.35 | 0 | 0.89 |
Optimising pre-surgical health
Of the 36 interventions (32 articles) targeting optimisation of pre-surgical health, 14 interventions were in patients waiting for THR,487–489,493–497,500–504,507 17 in patients waiting for TKR,500,503,509–515,517,519–521,523–525 and five in total hip and knee patients together. 526–529,534
Eighteen interventions were specifically exercise based,488,494,497,502,503,509,511–515,519,520,523–525 nine were education based,52,489,495,500,501,526,527,529 while nine were multifactorial with exercise and education components and, in some cases, an occupational therapy base. 487,493,496,504,507,510,517,521,528
Effect on pre-surgical physical function
Physical function before surgery was measured after 21 interventions using WOMAC (physical function or total), SF-36 physical function, HHS, HOOS, HSS, Arthritis Impact Measure Score 2 (AIMS2), AKSS or locally devised scores.
Data for meta-analysis were available for 19 interventions with 1026 participants. 488,493,494,496,497,501–503,510,513–515,519,521,525,527,534 In the random-effects meta-analysis shown in Table 76 and Figure 38, the average SMD was 0.28 (95% CI 0.16 to 0.40) favouring intervention. Inspection of the funnel plot did not suggest publication bias. Benefit for interventions was limited to eight studies with 343 patients waiting for THR (average SMD 0.40, 95% CI 0.18 to 0.61) and two studies with 226 hip and knee patients reported together. There was no heterogeneity across all studies or in those in patients waiting for hip replacement. In eight studies with 457 patients waiting for knee replacement, there was a non-significant trend for benefit (average SMD 0.19, 95% CI –0.04 to 0.42). In three education-based interventions with 261 patients, physical function was better in intervention patients (average SMD 0.33, 95% CI 0.09 to 0.58). Similarly, in 12 exercise-based interventions with 494 patients, interventions showed benefit (average SMD 0.26, 95% CI 0.08 to 0.44).
FIGURE 38.
Pre-surgical physical function outcome in studies targeting optimisation of pre-surgical health. df, degrees of freedom; IV, inverse variance. SF-36 physical function: Bitterli and colleagues. 488 WOMAC function: Ferrara and colleagues,493 Gilbey and colleagues,494 McGregor and colleagues,501 Rooks and colleagues,503 Beaupre and colleagues,510 Gstoettner and colleagues,515 (control values were baseline), McKay and colleagues,519 Nuñez and colleagues521 and Crotty and colleagues. 527 WOMAC total score: Williamson and colleagues. 525 HHS: Gocen and colleagues. 496 HOOS function in daily activities: Hoogeboom and colleagues497 and Oosting and colleagues. 502 HSS: D’Lima and colleagues. 513 SF-36 physical function: Evgeniadis and colleagues. 514 Locally devised score based on ability to perform pre- and postoperative care: Pellino and colleagues. 534

No heterogeneity was apparent. In four multifactorial interventions with 271 patients there was a trend for benefit (SMD 0.32, 95% CI –0.03 to 0.66). Considering studies separately in patients waiting for hip and knee replacement there was no suggestion that particular types of interventions were more effective.
In a sensitivity analysis with 10 interventions of good or reasonable quality with 610 patients, benefit was still apparent (SMD 0.21, 95% CI 0.04 to 0.38). Numbers in groups were small but benefit was mainly in patients waiting for hip replacement.
To evaluate physiotherapy content we analysed studies by intervention intensity (frequency and duration) and specific components. Higher intensity interventions showed benefit in seven studies with 308 patients waiting for hip replacement (SMD 0.39, 95% CI 0.16 to 0.62), and a trend for benefit in nine studies with 457 knee arthroplasty patients (SMD 0.19, 95% CI –0.04 to 0.42). However, numbers of studies with medium or low intensity exercise content were small.
In patients waiting for THR, five interventions focusing on strengthening showed benefit (SMD 0.36, 95% CI 0.09 to 0.62), as did three studies with cardiovascular exercise (SMD 0.48, 95% CI 0.03 to 0.93). Numbers of studies including other components targeting stretching, gait re-education, ROM, balance, endurance, flexibility, proprioception, pool-based exercises and functional exercises were too low to draw conclusions on content. There was no evidence to suggest that any interventions had an adverse effect on physical function.
In patients waiting for knee replacement, nine physiotherapy exercise interventions had a strengthening component. As shown in Table 76, there was a non-significant trend for benefit in improved physical function for interventions with a strengthening component compared with controls (SMD 0.19, 95% CI –0.04 to 0.42). As with studies in hip replacement, there were too few studies to draw conclusions on other exercise content.
Pain
Pain measured before surgery was reported for 25 interventions, with data suitable for meta-analysis in 21 interventions with 1375 patients. Interventions were associated with reduced pain (SMD 0.21, 95% CI 0.10 to 0.33), but this was limited to patients waiting for hip replacement (SMD 0.47, 95% CI 0.28 to 0.67). There was only slight heterogeneity and the funnel plot did not suggest publication bias (data not shown).
In 16 studies judged to be of good or reasonable methodological quality comprising 1141 patients,487,488,493–495,497,503,509–511,514,515,517,521,524,527 there was benefit for interventions (SMD 0.24, 95% CI 0.09 to 0.38), but this was statistically significant only in hip patients.
In 13 studies with 597 patients,488,494,497,502,503,509,511,514,515,519,524,525 exercise-based interventions showed benefit, SMD 0.23 (95% CI 0.07 to 0.39). In three education-based interventions495,501,527 and five multifactorial interventions,487,493,510,517,521 there were trends for benefit, with SMDs of 0.20 (95% CI –0.03 to 0.44) and 0.27 (95% CI –0.04 to 0.58). Numbers in some groups were small but all types of programme content showed benefit for patients waiting for THR.
Anxiety
Anxiety was reported pre-operatively for eight interventions, using the State–Trait Anxiety Inventory (STAI) in six studies,489,495,526,528,529 and HADS525 and Arthritis Impact Measure Score (AIMS) anxiety487 in one each. In eight studies487,489,495,525,526,528,529 with 660 patients, interventions were associated with lower anxiety with SMD 0.38 (95% CI 0.11 to 0.65). However, in quality assessment, five studies were classified as having risk of bias. 489,495,525,526,529
Preparation for recovery in hospital
In 27 studies (26 articles), a specific focus of the intervention was preparing patients for their hospital stay, including what to expect before, during and after surgery. 489–492,495,496,498,499,501,504–508,510,516–518,522,526,528–533 We identified 14 interventions in patients waiting for hip replacement,489–492,495,496,498,499,501,504–508 six interventions in patients waiting for knee replacement510,516–518,522 and seven intervention in patients waiting for either hip or knee replacement. 526,528–533 Nineteen interventions489,490,492,495,498,499,501,505,508,516,518,522,526,529–533 were primarily education based and eight were multifactorial with education and exercise content. 491,496,504,506,507,510,517,528
Post-surgical anxiety
Eight interventions reported anxiety outcomes within a month of surgery. 489,490,492,495,506,522,529,530 Five studies with 381 patients presented data suitable for the meta-analysis in Table 76 and Figure 39. 489,492,495,506,529 Four studies recorded anxiety using the STAI questionnaire during the hospital stay or the change from baseline and included either hip or hip and knee patients analysed together. 489,492,495,529 In a random-effects model, the MD was 2.97 (95% CI 0.64 to 5.30) in favour of reduced anxiety in the intervention group. One study reported sense of uncertainty on discharge using a VAS scale. 506 Inclusion of this in the meta-analysis and excluding one study with change scores gave an average SMD of 4.15 (95% CI 1.46 to 6.84).
FIGURE 39.
Post-surgical anxiety outcome in studies targeting preparation for recovery in hospital (MD). df, degrees of freedom; IV, inverse variance. STAI: Butler and colleagues,489 Doering and colleagues492 (from McDonald and colleagues83), Cuñádo Barrio and colleagues529 and Giraudet-Le Quintrec and colleagues495 (change score). VAS sense of uncertainty: Vukomanovic and colleagues;506 used as data were suitable for inclusion in meta-analysis. Authors also recorded anxiety, intervention group 0 (SD 0), control 22.25 (SD 34.64).
There was no evidence of heterogeneity and exclusion of one study with differing losses to follow-up between randomised groups did not affect the outcome. 529
Post-surgical pain
Fifteen interventions reported a pain outcome up to 1 month after surgery. For meta-analysis, we included the latest data at a fixed time point up to 1 month after surgery. If not available, we used data collected at discharge but such data may be affected by time in hospital. When possible, we used pain at movement in preference to pain at rest. Using these criteria, 12 interventions with 842 patients were included in the meta-analysis. Up to 1 month after surgery, pain was non-significantly lower in intervention groups than in controls (SMD 0.18, 95% CI –0.01 to 0.38). Similar trends were noted in hip and knee patients separately. There was no evidence of publication bias from inspection of the funnel plot but some heterogeneity was evident in trials including knee replacement patients (data not shown).
Results and heterogeneity were similar after exclusion of studies with possible bias owing to differences in patient characteristics, group follow-up rates or inclusion of data collected at discharge.
Length of hospital stay
Length of hospital stay was reported after 19 interventions with 997 patients. There was no statistically significant benefit for interventions (MD –0.16 days, 95% CI –0.78 to 0.45 days) reflecting a trend for lower length of hospital stay in intervention groups. The corresponding funnel plot did not suggest presence of publication bias. Heterogeneity (I2 = 67%) was not explained by one study with different follow-up rates in randomised groups. Although benefit for reduced length of stay was noted in knee replacement patients, this was based on two studies510,517 and was not supported by studies including both hip and knee patients. 528,529,531
Mobilisation after surgery
In six studies with 471 patients, time to mobilisation was reported. 495,496,506,507,528,529 Studies included only hip or hip and knee patients. Measures of mobilisation were time to walking in five studies495,496,506,528,529 and time to standing in one study. 507 In meta-analysis, time to mobilisation was shorter in the intervention groups (MD –0.17 days, 95% CI –0.30 to –0.04 days) and this was little changed if only time to walking was considered. Overall, there was no heterogeneity among studies.
Improving long-term outcomes
Nine pre-surgical interventions explicitly targeted improvement in long-term outcome after hip replacement487,488,493,496,501–503,506 and nine after knee replacement. 503,510,513–515,519,524,525 The focus of the intervention was exercise in 12 interventions, education in one intervention and multifactorial in five interventions.
Long-term physical function
All but three studies reported a functional outcome at 3 months or longer after surgery. 502,515,524 As shown in the meta-analysis in Table 76 and Figure 40, including 15 studies with 577 patients, there was no overall long-term benefit in intervention compared with control groups (SMD 0.08, 95% CI –0.09 to 0.26). Only moderate heterogeneity was apparent and there was no suggestion of publication bias.
FIGURE 40.
Physical function in studies targeting improvement of long-term outcomes. df, degrees of freedom; ILAS, Iowa Level of Assistance Scale; IV, inverse variance. WOMAC function: Ferrara and colleagues,493 McGregor and colleagues501 (supplied by author), Rooks and colleagues,503 McKay and colleagues519 and Beaupre and colleagues. 510 WOMAC total: Bitterli and colleagues. 488 OHS: Vukomanovic and colleagues. 506 OKS: Williamson and colleagues. 525 HHS: Gocen and colleagues. 496 AIMS: Berge and colleagues. 487 SDs based on data from Dawson and colleagues. 274 Hospital for special surgery knee rating scores: D’Lima and colleagues. 513 ILAS: Evgeniadis and colleagues. 514
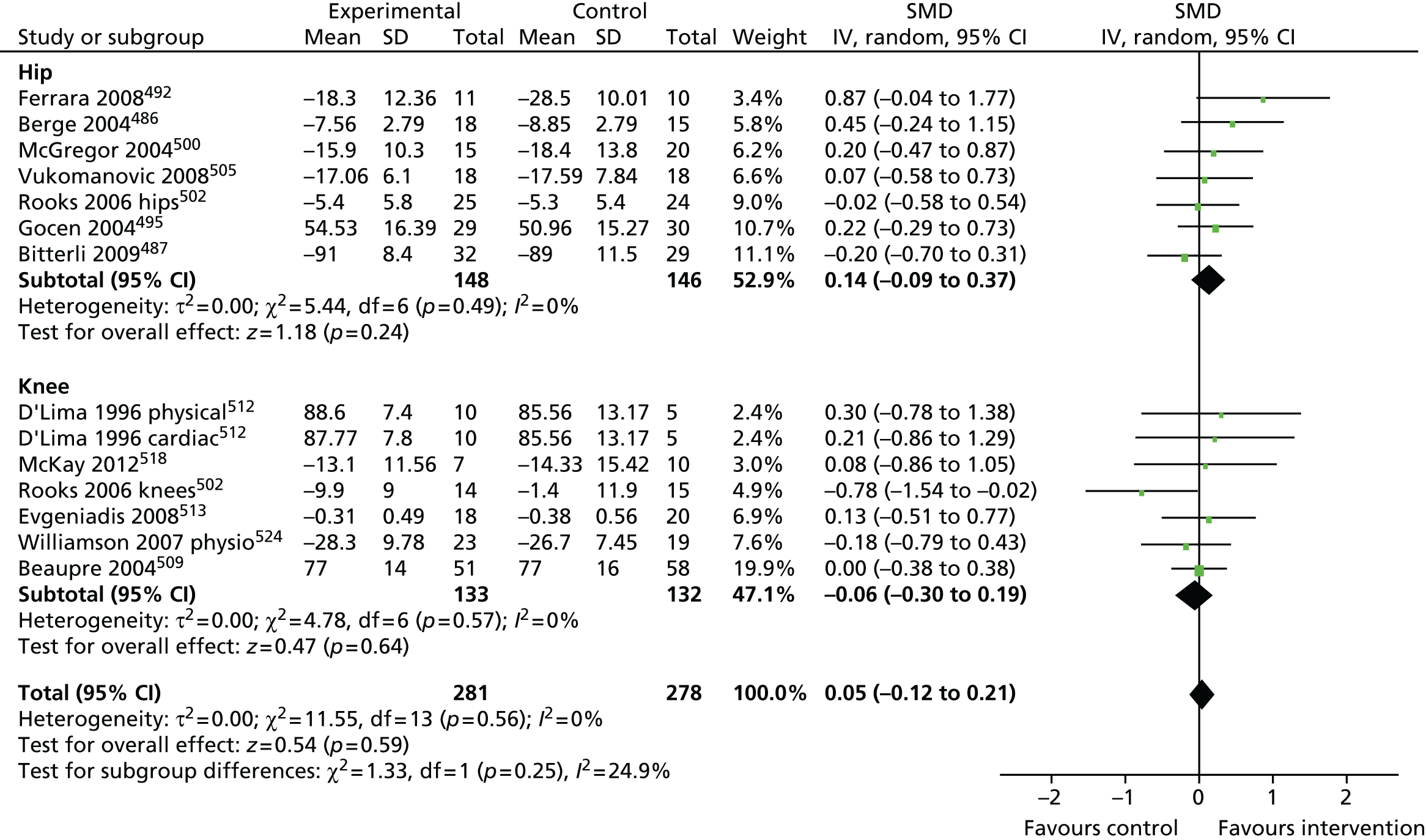
Long-term pain
As shown in Table 76, there was no benefit for reduced long-term pain after interventions in 10 studies with 414 patients (SMD 0.03, 95% CI –0.16 to 0.23). In addition, there was no heterogeneity among the studies.
Discussion
Before joint replacement, many interventions have been evaluated that aim to improve pre-surgical physical health, preparation for surgery and recovery, and achievement of good long-term outcomes. Studies were generally small and a minority reported long-term follow-up.
Evidence from studies considered to be of reasonable or good quality suggests that, in patients waiting for hip replacement, physical function can be enhanced and pain reduced before surgery. For patients with knee replacement, trends were apparent but were not statistically significant. There was some suggestion that interventions led to reduced anxiety before surgery but this was more convincing in patients followed up shortly after surgery in better-quality studies. Patients receiving interventions mobilised quicker after surgery but length of hospital stay did not differ significantly. For patients followed up after surgery, there was little to suggest that interventions had long-term benefit.
Interpretation of the effect size when using SMDs as the outcome can be difficult. Cohen interpreted effect sizes as ‘small’ (0.10), ‘medium’ (0.25) and ‘large’ (0.40). 535 Thus, interventions in patients waiting for hip replacement can be considered to show a medium to large effect on pre-surgical physical function and pain, depending on the study quality. The effect of interventions in hip and knee patients on reducing post-surgical anxiety was medium to large. Mobilisation was brought forward by about 4 hours in intervention groups, a potentially large effect in the context of recent studies with mean times to mobilisation of 48–72 hours.
The small number of interventions and the size of trials limited the analysis of long-term outcomes and there was little to suggest benefit. Differences in outcomes at long-term follow-up, particularly in small, underpowered studies, are likely to be overwhelmed by changes in physical function and pain that occur after hip or knee arthroplasty in the majority of patients. 18,48
Exercise provides the main focus of interventions aiming to improve pre-surgical physical function. However, the importance of specific exercise content was unclear. This may reflect the aims of pre-surgical physiotherapy exercise focusing on the maintenance of functional ability and prevention of decline, rather than post-surgical rehabilitation, which is substantially based on adjustment to physical changes associated with the prosthesis.
Conclusion
In randomised evaluations of pre-surgical exercise and education identified in our systematic review, there was a suggestion that physical function can be enhanced and pain reduced before surgery in patients waiting for hip replacement. Studies on patients with knee replacement did not provide strong evidence of benefit. Interventions were associated with reduced anxiety during the hospital admission and quicker mobilisation. The value of specific exercise content was unclear, which may reflect the aims of pre-surgical exercise to maintain functional ability and prevent decline whereas post-surgical rehabilitation is substantially based on adjustment to physical changes associated with the prosthesis.
Chapter 9 Clinical effectiveness and cost-effectiveness of a group-based pain self-management intervention for patients undergoing total hip replacement: feasibility study for a randomised controlled trial – SPIRAL
Parts of this chapter have been reproduced from Wylde and collegaues. 536 © 2014 Wylde et al. ; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited.
Abstract
Background
We conducted the SPIRAL study to evaluate the feasibility of a definitive RCT to assess the clinical effectiveness and cost-effectiveness of a group-based pain self-management course for patients undergoing THR.
Methods
Participants were randomised to attend a pain self-management course plus standard care or standard care only. The course consisted of two half-day sessions before surgery and one full-day session after surgery. Participants provided outcome and resource-use data prior to surgery and 1 month, 3 months and 6 months after surgery. Telephone interviews were conducted with non-participants to explore barriers to participation.
Results
Out of the 385 eligible patients with THR, 88 (23%) consented to participate. Common reasons for non-participation were views about the course and transport difficulties. Of 43 patients randomised to the intervention, 28 attended the pre-operative sessions and 11 attended the postoperative session. Participant satisfaction was high and feedback highlighted that patients enjoyed the group format. Retention of participants in the RCT was acceptable (83%) with high questionnaire return rates, with the exception of resource-use diaries.
Conclusions
Although participation in the group-based pain self-management course was low, those who attended provided positive feedback. The SPIRAL study highlights the importance of conducting feasibility work and evaluating the acceptability of an intervention prior to undertaking a full-scale RCT to assess the clinical effectiveness and cost-effectiveness of an intervention.
Background
Evidence is needed on the clinical effectiveness and cost-effectiveness of pain self-management programmes for patients undergoing THR. Previous studies of self-management programmes for patients with arthritis have faced challenges owing to low recruitment rates, poor uptake of the intervention and high attrition rates. 537–541 Therefore, prior to undertaking a RCT with an economic evaluation component of a pain self-management programme for patients undergoing THR, it is important to evaluate the feasibility of such a trial and the acceptability of the intervention to patients.
Feasibility and pilot work to explore trial processes can include testing trial procedures and data collection methods, randomisation processes, recruitment rates and attrition rates. 542 For example, attrition rates for self-management programmes have been found to be as high as 40–50%,540,541 and in one research study 29% of recruited patients attended none of the sessions on a 6-week self-management course. 537 Preliminary work prior to undertaking the definitive trial can often highlight unanticipated issues with trial design and conduct,543,544 which can then be addressed to maximise the success of intervention evaluation in a full-scale RCT. The importance of feasibility work to evaluate trial processes has been highlighted in a systematic review of cluster RCTs in primary care, which concluded that a number of reported issues with recruitment, adherence to trial protocol and data collection methods could have been pre-emptively identified and addressed through feasibility work. 545
In addition to testing trial processes, another objective of preliminary work prior to a full-scale RCT can be to test the acceptability of an intervention, particularly if the intervention is complex in nature. 546 Preliminary work to develop, refine and pilot complex interventions is recommended by the MRC. 94 Early evaluation of the acceptability of a complex intervention can highlight aspects of the intervention which can then be modified prior to a definitive trial. For example, feasibility work highlighted that booster sessions for a group-based cognitive–behavioural therapy intervention were poorly attended. 547 A feasibility study of a group-based acceptance and commitment therapy for chronic pain found that some patients found the sessions too long. 548 A pilot study of a complex intervention for diet and activity behaviour change in obese pregnant women found that increased flexibility of the timing of sessions was necessary to improve attendance and some activities, such as goal-setting, were better conducted individually rather than in a group setting to minimise perceptions of being judged. 549
Therefore, the aims of this study were twofold: (1) to evaluate the feasibility of conducting a RCT to assess the clinical effectiveness and cost-effectiveness of a group-based pain self-management course for patients undergoing THR, and (2) to assess the acceptability of the intervention. Specific objectives were to assess the feasibility of trial design and procedures, ascertain recruitment and retention rates, identify barriers to participation, develop resource-use data collection methods, assess questionnaire completion rates, and evaluate uptake and patient satisfaction with the course.
Patients and methods
The study was approved by the South West Central Bristol Research Ethics Committee (reference 11/SW/0056) and all participants provided their informed, written consent to participate. The trial was registered on the NIHR Clinical Research Network Portfolio (UKCRN ID 11270) and ISRCTN register (ISRCTN52305381).
Participant recruitment
Between June 2011 and June 2012, potential participants were identified from the joint replacement waiting list at one elective orthopaedic centre and sent a postal study invitation. Patients interested in participating in the study were asked to return a signed consent form and reply slip to the research team. Study recruitment materials were designed with input from patient representatives through the unit’s dedicated patient forum (PEP-R). 550 A researcher then contacted interested patients to ensure that they met the eligibility criteria and answer any questions they may have about the study. The inclusion criterion was being listed for a primary THR because of osteoarthritis. Exclusion criteria comprised lack of capacity or unwillingness to provide informed consent and inability to complete English-language questionnaires. In order to explore whether or not the patients enrolled in the study were representative of those undergoing joint replacement, basic demographic data on age and sex were recorded for all eligible patients who were approached about the study.
Telephone interviews with non-participants
To explore potential barriers to participating in the study, short, structured telephone interviews were conducted with patients who declined to participate in the study but returned a reply form giving permission for a researcher to telephone them to discuss why they decided not to participate. Reasons for non-participation were recorded on a standardised proforma by the researcher and entered into a Microsoft Excel spreadsheet.
Randomisation
Participants were randomised after recruitment using a computer-generated randomisation system (Minim). 551 Blinding of the research team or patients was not possible because the intervention involved attending a course. Participants were informed of the results of randomisation via a letter and those randomised to the intervention group were contacted by telephone to discuss course arrangements.
Assessment times
All participants completed postal questionnaires at baseline (after recruitment), prior to surgery and then at 1, 3 and 6 months after surgery. If no reply was received after 2 weeks then a single reminder was sent.
Questionnaires
At each assessment time, participants completed the following validated PROMs.
WOMAC114
This consists of 24 items and produces individual scores for hip pain, function and stiffness. Scores were transformed onto a 0–100 scale (worst to best).
Pain Self-Efficacy questionnaire275
This consists of 10 items regarding a person’s confidence in their ability to perform general activities, despite their pain. Scores range from 0 to 60, with a higher score reflecting stronger perceived self-efficacy.
Brief COPE276
This consists of 28 items about coping strategies and provides 14 distinct two-item subscales of coping reactions. Each subscale is scored from 1 to 4, with higher scores representing a greater reliance on a particular coping strategy.
Beliefs about Medicines Questionnaire – Specific277
This consists of 10 items assessing a person’s beliefs about their medication (limited to pain medication in this study). The questionnaire produces two subscales about the necessity of prescribed medication and concerns about potential adverse effects of prescribed medications, both of which are scored from 5 to 25, with a higher score indicating stronger beliefs.
European Quality of Life-5 Dimensions-5 Levels278
This consists of five questions with five levels each (from no problems to severe problems) which provides a standardised measure of general HRQoL across five dimensions.
Patients also completed questions about pain in other joints, fatigue and pain distress (both measured on a VAS), length of time spent on various exercises each week and current pain medication usage. In the baseline questionnaire, medical comorbidities were recorded using the Functional Co-morbidity Index279 and information was collected about socioeconomic status.
Resource use
The 3- and 6-month postoperative questionnaire included a full resource-use questionnaire to identify and measure NHS resources used including community-based doctor and nurse visits, physiotherapy and occupational therapy visits, secondary care inpatient and outpatient visits and medication, use of social services, patient expenses, informal care and productivity losses incurred in the period. Participants were given a pre-operative resource-use diary to record any resources used from randomisation until they had their surgery. They were asked to return the completed pre-operative diary to the research team when they returned their 1-month postoperative questionnaire. At 1 and 3 months post operation, patients were given a resource-use log to prospectively record their use of resources in the following period in order to aid them in the completion of the resource-use questions in the 3-month and 6-month questionnaires. 432 The aim of these questionnaires was not to formally evaluate the differences in costs and consequences of delivering a pain self-management course to patients undergoing THR, but to refine resource-use data collection methods. Therefore, analyses of these questionnaires focused on rates of missing data, which is a common issue with patient-completed postal resource-use questionnaires. 432
Intervention
The Challenging Pain and Keep Challenging Pain courses were delivered by Arthritis Care, a registered UK charity that has been delivering self-management courses since 1994. The courses were delivered by two lay trainers, who had experience of living with chronic pain. All the courses were held at the AOC, Southmead Hospital. Reimbursement of travel costs (mileage and parking fees) or a pre-paid taxi was offered to all participants who attended the courses.
Challenging Pain course
The pre-operative Challenging Pain course consisted of two sessions running over consecutive weeks, with each session lasting 2.5 hours. The 2-week course was developed from a longer 6-week course and has been evaluated by Arthritis Care. 552 The emphasis of the course was on pain management and introduced participants to a variety of cognitive pain management techniques, with the aim of providing coping skills to enable patients to manage their pain and its impact more effectively. Delivery involved a combination of presentations, group work, pair work, practical demonstrations and interactive sessions. The first session included introductions to conscious breathing, full-body relaxation, exercise, goal-setting and managing stress. The second session reviewed these topics and introduced pacing, medications and other therapies, guided imagery, managing negative thoughts and effective communication.
Postoperative Keep Challenging Pain course
All participants randomised to the intervention group were invited to attend an additional top-up Keep Challenging Pain course at between 6 weeks and 3 months post operation. This course was designed by Arthritis Care in conjugation with a physiotherapist specifically to be delivered to postoperative THR patients. This 5-hour session reviewed the pain management strategies that were introduced in the pre-operative course, provided advice on recovery after THR, reviewed goal-setting and problem solving, and included a practical exercise session lead by a registered physiotherapist.
Course evaluation
A short structured feedback questionnaire about the course was completed by participants at the end of the both the Challenging Pain and Keep Challenging Pain courses.
Surgery and postoperative physiotherapy
All participants received a primary THR using a posterior or anterolateral approach, at the discretion of the surgeon. Standard postoperative inpatient physiotherapy at the AOC consists of strengthening of the hip abductor muscle, flexor and extensor exercises, transfers to and from bed, and walking and stair climbing, with hydrotherapy and gym exercises if required. Outpatient physiotherapy is not routinely provided after discharge.
Sample size
No formal sample size calculation can be performed for a feasibility study. The average sample size for feasibility studies assessing trial design and the acceptability of interventions is approximately 60 patients. 553 A minimum of 80 patients (40 per arm) was deemed an appropriate sample size for this trial to allow an estimate of recruitment and retention rates and explore the acceptability of the intervention.
Analysis
In line with recommendations about good practice in the analysis and reporting of feasibility and pilot trials, analysis is descriptive and no comparisons of the outcomes between the two arms of the trial was conducted. 542 Descriptive statistics on recruitment rates, baseline patient characteristics, retention of participants and questionnaire return rates are presented as means and 95% CIs, medians and IQRs or percentages. Resource-use data collected from patient self-completed questionnaires were considered complete when the patient recorded enough data to allow costing using a national tariff. Completion rates were reported per question and aggregated per two economic perspectives – the NHS and PSS perspective – and a broader societal perspective. Data on reasons for non-participation in the trial were coded into themes by one researcher and these themes were then discussed and agreed with a second researcher. 445
Results
Recruitment rate and participants
Postal invitations were sent to 385 eligible patients and 88 patients consented to participate, giving a recruitment rate of 23%. A CONSORT flow diagram is shown in Figure 41.
FIGURE 41.
SPIRAL CONSORT flow diagram.
Baseline demographic and clinical characteristics of the participants are displayed in Table 77. Mean WOMAC scores indicated that patients had high levels of hip pain and functional limitations prior to surgery. Mean pain self-efficacy scores indicate the patient population were suitable for a pain management intervention. 275 Participants underwent THR surgery at a median of 12 weeks (IQR 8–15 weeks) after recruitment into the study. Non-participants had a similar median age (67 years, 95% CI 66 to 69 years) to participants but were more likely to be male (46% male).
| Patient characteristics | Overall (n = 88) | Allocated to intervention (n = 43) | Allocated to standard care (n = 45) |
|---|---|---|---|
| Mean age (95% CI) | 66 (64 to 68) | 65 (61 to 69) | 67 (64 to 70) |
| Female : male, % | 65 : 35 | 65 : 35 | 64 : 36 |
| Living alone, % | 19 | 18 | 20 |
| With college or university education, % | 35 | 32 | 39 |
| Retired, % | 60 | 61 | 59 |
| Mean WOMAC pain score (95% CI) | 38 (33 to 42) | 37 (31 to 43) | 38 (32 to 44) |
| Mean WOMAC function score (95% CI) | 37 (33 to 41) | 39 (33 to 45) | 35 (30 to 41) |
| Pain self-efficacy score (95% CI) | 32 (29 to 35) | 35 (30 to 39) | 30 (26 to 34) |
Reasons for non-participation
Brief telephone interviews were conducted with 57 (19%) non-participants. These patients had a mean age of 71 years (95% CI 68 to 74 years) and 37 were female. Patients gave 91 reasons for non-participation, most frequently relating to perceptions and views about the pain self-management course (Table 78). These reasons included previously attending pain self-management courses and finding them unhelpful, a perceived lack of need because pain was adequately managed, a dislike of group formats and concerns over difficulty in attending the course because of pain, age and/or other health conditions. The second most frequently given reason for non-participation concerned issues around travelling to the hospital to attend the course.
| Barriers to participation (number of patients) | Examples of reasons given |
|---|---|
| Thoughts about attending the course (25) | Difficult to sit and concentrate during workshops because of pain/age/other health conditions |
| Dislike of group session format | |
| Found previous pain management course unhelpful | |
| Can already manage pain | |
| Workshops would not be helpful as pain not too bad | |
| Difficult to attend because of other health conditions | |
| Would rather spend time doing other things | |
| Difficulty getting to hospital (22) | Unable to drive/use public transport owing to hip problems |
| Distance to hospital perceived as too far | |
| Would have to rely on family/friends for transport | |
| Limited mobility or uses wheelchair | |
| Other commitments (13) | Carer for family member |
| Employment | |
| Children | |
| Questionnaires (8) | Dislike of completing questionnaires |
| Difficult to complete because of other health conditions | |
| Lack of time because of other commitments | |
| Other hospital appointments (6) | Lack of time for additional visits to hospital |
| Inconvenient to make additional visits to hospital | |
| Feels already has enough knowledge (6) | Previous hip replacement |
| Knows people who have had hip replacement | |
| Has attended physiotherapy/exercise session | |
| Health-care related (6) | Operation may not be going ahead |
| Dissatisfied with co-ordination of care | |
| Other (5) | Emigrating |
| Recently widowed | |
| Has taken part in research before |
Retention
Fifteen patients were withdrawn from the study (17% of recruited participants): seven from the intervention group and eight from the standard care group (see Figure 41). Of the withdrawn patients, nine were withdrawn because they did not undergo surgery during the study period, five self-withdrew and one was withdrawn because they were recruited into another trial whose protocol precluded participation in two trials.
Intervention: attendance and acceptability
Pre-operative Challenging Pain course
Four pre-operative Challenging Pain courses were held, with between four and nine participants on each course. Out of the 43 participants randomised to the intervention group, 28 patients attended the pre-operative course (17 attended both workshops, 11 attended one workshop) at a median of 5 weeks (IQR 2–8 weeks) prior to surgery. Reasons for non-attendance are presented in Figure 41. Results from the course evaluation questionnaire are presented in Table 79. Free-text comments on the evaluation questionnaires frequently gave positive feedback on the group format of the workshop, which provided the opportunity to meet other people undergoing THR.
| Evaluation question | Challenging Pain course (n = 27) | Keep Challenging Pain course (n = 11) |
|---|---|---|
| Has the course been useful? (% yes) | 100 | 100 |
| Recommend for other THR patients? (% yes) | 100 | 100 |
| Mean usefulness (95% CI) | 7.3 (6.5 to 8.1) | 8.9 (8.4 to 9.5) |
| Mean satisfaction with content (95% CI) | 8.0 (7.2 to 8.7) | 9.0 (8.4 to 9.6) |
| Men satisfaction with delivery (95% CI) | 8.4 (7.7 to 9.0) | 9.0 (8.2 to 9.8) |
Postoperative Keep Challenging Pain course
Three postoperative Keep Challenging Pain course were held, with between two and five participants on each course. Out of the 43 participants randomised to the intervention group, 11 patients attended the postoperative course at a median of 9 weeks post operation (IQR 5–14 weeks). Reasons for non-attendance are presented in Figure 41. The results of the course evaluation questionnaire are presented in Table 79. Free-text comments on the evaluation questionnaires most frequently gave positive feedback on the physiotherapy session and the group format of the workshop.
Outcomes assessment and economic evaluation
The questionnaire return rates at each assessment time were high, ranging from 72% to 93% (Table 80). The rate of questionnaire return was similar between trial arms, with < 10% difference in return rates at each time point, except for the 3-month postoperative questionnaire, which was returned by more patients in the standard care arm than in the intervention arm (91% vs. 72%, respectively). Return rates for the pre-operative resource-use diaries were low, with only 35% of patients returning their diary.
| Time point | Median (25th, 75th percentile) time of completion | Intervention group, n/N (%) | Usual care group, n/N (%) | Overall, n/N (%) |
|---|---|---|---|---|
| Baseline | 10 weeks (5, 13) prior to surgery | 38/43 (88) | 42/45 (93) | 80/88 (91) |
| Preoperative | 1 week (0.5, 1.3) prior to surgery | 25/33 (76) | 29/34 (85) | 54/67 (81) |
| Pre-operative resource-use diary | Pre-operative to 1-month after surgery | 11/36 (31) | 15/38 (39) | 26/74 (35) |
| 1 month post operation | 4 weeks (3, 5) after surgery | 32/36 (89) | 35/38 (92) | 67/74 (91) |
| 3 months post operation | 13 weeks (13, 14) after surgery | 26/36 (72) | 34/37 (92) | 60/73 (82) |
| 6 months post operation | 26 weeks (26, 27) after surgery | 32/36 (89) | 32/37 (86) | 64/73 (88) |
Table 81 presents the completion rates of resource-use data in the 3- and 6-month postoperative questionnaires. For those who returned the resource-use questionnaire, completion rates for NHS resource-use questions were high for secondary-care resource use (> 90% completion on both arms) and medication use (> 80%), but less for community-based resources (65% and 66% for intervention and control arms, respectively). PSS data also had high completion rates (> 86% for all categories), particularly in the intervention group. When accounting for non-returners of follow-up questionnaires (10 in the intervention group and seven in the control group), completion rates were lower, with community-based resources being the lowest completed category. Overall, data for an economic evaluation from a NHS and PSS perspective were available for 33% of patients in the intervention group and 43% of patients in the standard care group. When considering other categories of resource use beyond health and social care, cost of travel was the least completed category. As a result, for an economic evaluation from a societal perspective, complete data were available only for 17% of patients in the intervention group and 19% of patients in the standard care group. The percentage of missing data was similar between trial arms, with the exception of four categories (social worker visits, time off usual activities, informal care time, and charities and support group visits), which had > 10% difference in completion rates between the trial arms. For these categories, patients in the intervention arm had higher completion rates than those in the standard care arm.
| Resource-use category | Intervention | Standard care | ||||
|---|---|---|---|---|---|---|
| Number complete | % of returners (n = 26) | % all (n = 36) | Number complete | % of returners (n = 29) | % all (n = 37) | |
| NHS resource use | ||||||
| Community-based visits | 17 | 65% | 47% | 19 | 66% | 51% |
| Hospital inpatient visits | 24 | 92% | 67% | 28 | 97% | 76% |
| Outpatient and A&E visits | 25 | 96% | 69% | 28 | 97% | 76% |
| Prescribed medications | 22 | 85% | 61% | 24 | 83% | 65% |
| PSS | ||||||
| Home care worker | 26 | 100% | 72% | 27 | 93% | 73% |
| Food at home services | 26 | 100% | 72% | 26 | 90% | 70% |
| Social worker visits | 26 | 100% | 72% | 25 | 86% | 68% |
| Home changes | 24 | 92% | 67% | 25 | 86% | 68% |
| NHS + PSS perspective | 12 | 46% | 33% | 16 | 55% | 43% |
| Other resources: productivity losses, informal care, private expenses and other | ||||||
| Time off work | 23 | 89% | 64% | 24 | 83% | 65% |
| Time off usual and leisure activities | 26 | 100% | 72% | 21 | 72% | 57% |
| Informal care time | 26 | 100% | 72% | 23 | 79% | 62% |
| Charities and support group visits | 26 | 100% | 72% | 25 | 86% | 68% |
| Privately paid therapies used | 23 | 89% | 64% | 25 | 86% | 68% |
| Travel costs | 13 | 50% | 36% | 14 | 48% | 38% |
| Over-the-counter medications | 25 | 96% | 69% | 27 | 93% | 73% |
| Societal perspective | 6 | 23% | 17% | 7 | 24% | 19% |
Discussion
This study looked at the feasibility of a RCT to evaluate the clinical effectiveness and cost-effectiveness of a group-based pain self-management intervention for patients undergoing THR and the acceptability of this intervention. Although feasibility studies are conducted to address trial design and methodology, a systematic review found that articles often include only a minimal discussion of the methodological findings and implications. 554 This feasibility study highlighted several methodological considerations that warrant further discussion.
Barriers to participation
Barriers to participation were explored using brief interviews with non-participants. These interviews identified that the most frequent reasons for non-participation were views and perceptions of the pain management course. These findings are in line with previous research, that identified that perceptions of the course and satisfaction with current self-management were reasons for non-participation in a trial of an arthritis self-management programme. 538 Difficulty in getting to the hospital was the second most frequent reason for non-participation, despite the offer of reimbursement of travel costs or a pre-paid taxi. Travel issues and the burden of additional appointments are commonly reported barriers to trial participation. 538,555 Future trials of group-based interventions may benefit from consideration of the location of the intervention. For example, interventions held in the community may have greater uptake than those delivered in a hospital, although trials of community-based group interventions also found that difficulties with travel is a common reason for non-participation. 538 Conducting these short interviews with non-participants identified a number of barriers to participation that could be addressed in further refinement work, highlighting the importance and value of conducting research with non-participants in feasibility studies.
Recruitment, retention and outcomes assessment
The recruitment rate for this trial was 23%, which is lower than the 42–79% recruitment rates reported in previous trials of pain self-management interventions for patients undergoing joint replacement. 487,527 However, other feasibility and pilot studies using a postal recruitment method have reported similarly low response rates. 547,556,557 Despite the low recruitment rate, retention of participants and questionnaire completion were high and similar between the trial arms, suggesting that randomisation and outcomes assessment were acceptable.
Recruitment into trials is known to be challenging and considerable research has been conducted into improving trial recruitment. Methods such as telephone reminders to non-responders, ‘opt-out’ recruitment strategies and financial incentives have been found to improve recruitment rates. 558 However, potential issues around coercion and undue influence can pose challenges to the implementation of these strategies. Financial incentives for research participation is a debated issue and ambiguities remain around what level of incentive constitutes undue influence, with little standardised guidance for Ethics Committees. 559 For example, we planned to offer participants free 1-year membership to Arthritis Care but the Ethics Committee perceived this as potentially coercive and asked for this offer to be removed from the study protocol. This demonstrates the challenges researchers can face in implementing measures to maximise recruitment into trials while remaining in-keeping with preferences of the NHS Research Ethics Committee.
Economic evaluation
The economic evaluation work highlighted the difficulty of collecting resource-use data from randomisation until surgery for this patient group. However, average waiting time for surgery in this patient group was 3 months and we would not expect the intervention to lead to behaviour change that would produce differences in cost drivers in the shorter term. In comparison with the pre-operative diaries, the postoperative resource-use questionnaires achieved good completion rates, allowing for a health and social care payer evaluation perspective to be taken. The completion rates could be further improved after imputation of community-based resource data. Although completion rates for a societal perspective were low, categories on productivity losses and informal carer time were well completed and can be of added value to a sensitivity analysis in a definite economic evaluation.
Acceptability of the intervention
In addition to assessing trial processes, this study evaluated the acceptability of the intervention. Feedback on the course was positive, suggesting that the course was acceptable and well received by those who attended. In particular, positive feedback was received on the group-based format, with patients commenting that they appreciated the opportunity to meet other people undergoing THR surgery. Studies evaluating group-based interventions in other clinical settings have also reported positive feedback on this format of intervention delivery. 549,557,560 Therefore, although the group format was a reason for non-participation for some patients, those who attended the course enjoyed the format and engagement with other patients. This highlights an issue affecting many trials: a potentially biased sample because of the self-selection of participants with a preference for the intervention. Differences in the characteristics of participants and non-participants are well known, with an underrepresentation of older people, women and ethnic minorities in clinical research. 561 Addressing willingness to participate owing to the nature of the intervention in feasibility work has the potential to lead to refinements in the intervention for a definitive trial and this knowledge has implications for the roll-out and uptake of interventions if subsequently implemented in clinical practice.
The Challenging Pain and Keep Challenging Pain courses were highly rated by participants, but attendance at the postoperative course was lower than at the pre-operative course. Reasons given for non-attendance were predominantly that people were unavailable on the dates set for the course. The logistics of scheduling group-based interventions is challenging, as many patients have limited availablity owing to other commitments. 538,549 Increasing flexibility in the scheduling of group-based interventions can be challenging, particularly within the financial constraints of a trial, but having the flexibility to run multiple courses is an important factor to consider when costing a trial.
Conclusion
Undertaking feasibility work for a RCT and evaluating the acceptability of an intervention can be a labour-intensive exercise. However, this study highlights the importance of conducting such work prior to undertaking a full-scale RCT to assess the clinical effectiveness and cost-effectiveness of an intervention. Several key messages can be taken from our experience. First, conducting brief telephone interviews with non-participants is an efficient and valuable method of collecting data on barriers to participation and we recommend including this as a core component of feasibility studies. These data can also provide insight into whether or not unwillingness to participate is due to the nature of the intervention, thereby providing early indications of potential issues in a definitive trial and with uptake of the intervention if implemented into clinical practice. Second, attempts to implement methods to improve patient recruitment need to be carefully designed in light of ethical considerations, such as the potential for inducements to be seen as coercion. Third, the logistical difficulties in scheduling groups and ensuring high attendance should not be underestimated and the potential to increase flexibility by running multiple courses should be considered when designing a budget for a trial. Finally, the ability of piloting resource-use questionnaires is a major advantage to improve the quality of the resource-use data available in the definitive economic evaluation.
Chapter 10 Occupational therapy in total hip replacement: systematic review and feasibility randomised controlled trial
Parts of this chapter have been reproduced with permission from Jepson P, Sands G, Beswick AD, Davis ET, Blom AW, Sackley CM. A feasibility randomised controlled trial of pre-operative occupational therapy to optimise recovery for patients undergoing primary total hip replacement for osteoarthritis (PROOF-THR). Clin Rehabil 2015;30:156–66. 562
Abstract
Background
Occupational therapy is routinely provided for patients with THR as part of the rehabilitation service but little is known about its clinical effectiveness.
Methods
We conducted a systematic review of the clinical effectiveness of occupational therapy interventions in patients receiving THR.
The PROOF-THR study evaluated the feasibility of a RCT of pre-surgical occupational therapy in patients waiting for THR. Primary objectives were to assess patient identification, recruitment and retention, acceptability of allocation and health resource use, and outcome measures.
Results
In patients receiving THR, the systematic review identified seven RCTs of occupational therapy, mainly combined with physiotherapy. There was a suggestion of improved function and reduced pain before surgery but this was not sustained after surgery. In the PROOF-THR study, 44 patients were randomised to pre-operative occupational therapy or usual care. Good recruitment rates, acceptability of randomisation of participants, successful intervention delivery, and reasonable attrition rates suggest a definitive trial would be feasible.
Conclusions
The successful recruitment and randomisation of participants and delivery of the intervention, plus the reasonable attrition rate, suggest that this trial design would be feasible to take forward into a definitive trial of occupational therapy provision before THR.
Background
Occupational therapy is routinely provided for patients with THR as part of the rehabilitation service but little is known about its effectiveness. The provision of compensatory equipment is a key aspect of occupational therapy practice but evidence is needed on its clinical effectiveness and to optimise its delivery. Economic pressures have had a dramatic impact on length of hospital stay following THR, such that reduction in length of stay has been a common outcome measure used to justify many pre-operative interventions. Hospitalisation for ≥ 10 days post surgery was common practice prior to 2000;563 however, now most patients are discharged after 4–5 days. 564 An important corollary of this reduced length of stay is the time available in hospital for recuperation, inpatient rehabilitation, education and discharge procedures. 565 Although some variation in practice does exist, it is usual practice in the UK for OTs to provide compensatory equipment, together with education on its use, in this short post-surgery/pre-discharge period. Pre-surgery home-based provision has been identified as desirable by patients and potentially assistive in functional rehabilitation. 566
To investigate evidence on the clinical effectiveness of a broad range of occupational therapy interventions in patients receiving THR, we conducted a systematic review of RCTs. We also conducted a feasibility study for a definitive randomised evaluation of the clinical effectiveness of a pre-surgery home-based occupational therapy intervention compared with hospital-based usual care.
Systematic review and meta-analysis of the effectiveness of occupational therapy interventions in total hip replacement
Background
Our aim was to identify RCTs evaluating occupational therapy interventions in isolation or as part of multifaceted interventions in patients receiving a THR. The key outcomes in occupational therapy relate to patient physical function, activity, social participation, HRQoL and pain, and prevention of dislocation.
Methods
| General methods | As described in Chapter 2, Systematic review methods |
| Databases and dates | MEDLINE, EMBASE, CINAHL, PsycINFO, AMED, PEDro, ERIC, CIRRIE, OTDbase and The Cochrane Library from inception to 24 June 2013. Citations of key articles in ISI Web of Science and reference lists |
| Search strategy | Hip replacement/RCT/occupational therapy. MEDLINE search strategy based on terms in Appendix 3 |
| Study design | RCTs with randomisation either at individual or cluster level. Quasi-randomised designs (e.g. alternate allocation) |
| Patients | Adults waiting for THR |
| Intervention | Occupational therapy |
| Controls | No occupational therapy intervention additional to usual care |
| Follow-up | Any post surgery |
| Data extraction | Country, baseline dates, participants (indication, age, sex), content of intervention and comparison group, length of follow-up, losses to follow-up |
| Potential outcomes | Patient-reported physical function |
| Patient-reported pain | |
| Limitations in self-care ADL | |
| Restrictions in extended or instrumental ADL | |
| Societal reintegration or discretionary activities | |
| Hip dislocation | |
| Adverse events including deep infection or joint revision surgery | |
| Quality assessment | Cochrane risk-of-bias table |
Results
Review progress is summarised as a flow diagram in Figure 42. Searches identified 4865 articles. Twenty-nine articles were reviewed in full. After screening and detailed evaluation, seven interventions met the inclusion criteria. 489,493,496,501,504,567,568 Study characteristics are summarised in Table 82. As our review focused on THR and the specific occupational therapy content provided to this patient group, we excluded two studies with both total hip and TKR patients with no separate outcome reporting. 528,569 Two studies were conducted in the UK and one each in Iceland, Turkey, the USA, Canada and Italy.
FIGURE 42.
Systematic review and meta-analysis of the effectiveness of occupational therapy interventions in THR: flow diagram
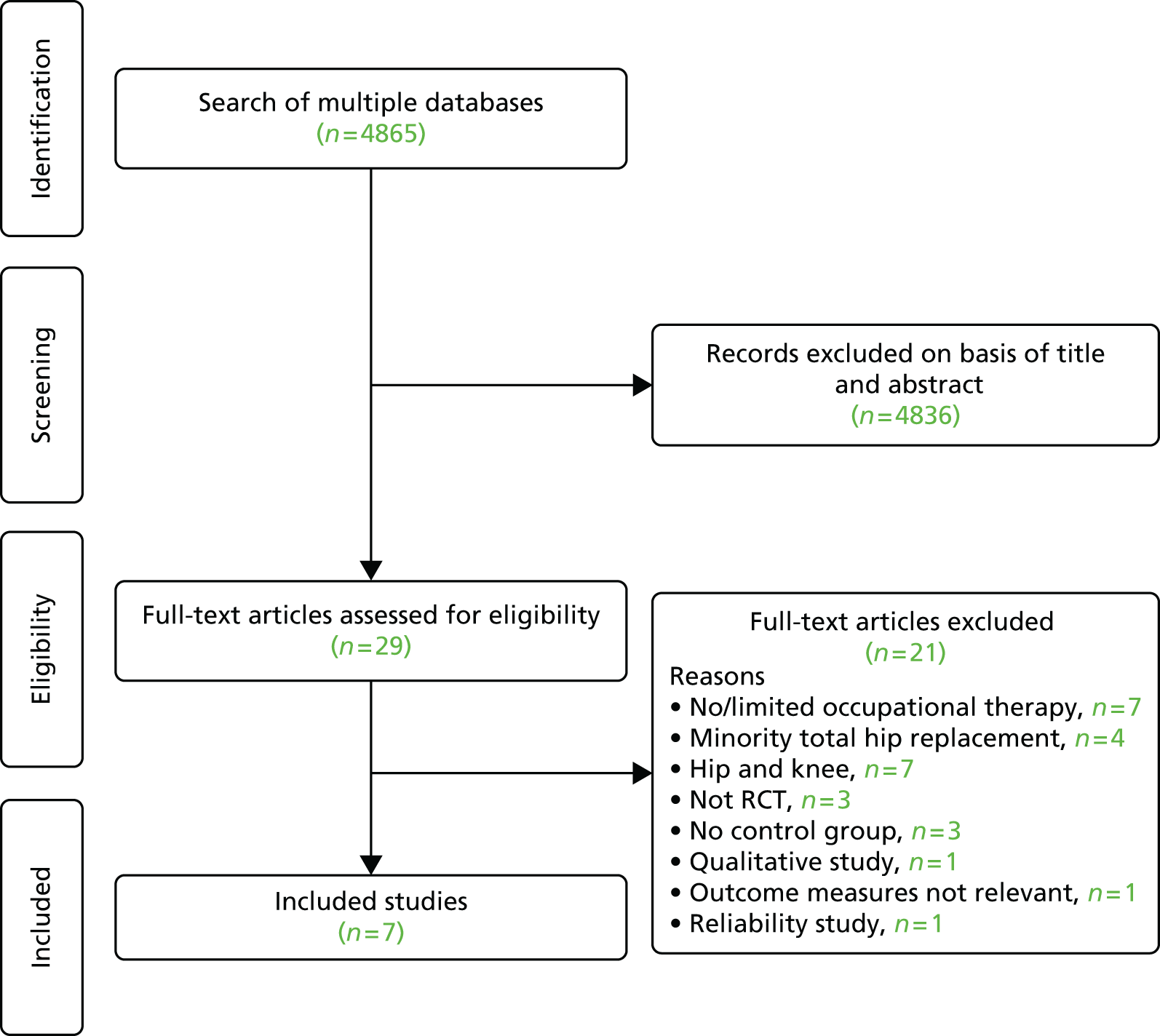
| Study; location; dates | Indication; number randomised (intervention : control); mean age (years) (% female) | Intervention; control | Outcomes; follow-up; losses to follow-up (intervention : control) |
|---|---|---|---|
| Butler and colleagues 1996;489 Canada; 1993–4 | Not specified; n = 80 (32 : 48); 62.6 (51.3) | Pre-admission education booklet; usual care – same information as in pre-admission booklet given following admission | State-Trait Anxiety Inventory, patient satisfaction questionnaire, exercise log and details of home adaptations, length of hospital stay; to discharge; one patient died and no follow-up data from eight patients |
| Ferrara and colleagues 2008;493 Italy; 2006–7 | End-stage osteoarthritis; n = 23 (11 : 12); 63.4 (60.9) | 5-day pre-admission intervention package 1 month before admission consisting of exercise, postural advice, advice on movement restrictions and prevention of prosthesis dislocation, use of devices (crutches, elevated toilet sets, bed raises, dressing/undressing adaptive devices), washing and bathing; no pre-admission intervention | WOMAC, SF-36, VAS (pain), Barthel, hip strength and range of movement; day before surgery, 15 days, 4 weeks, 3 months; two (0 : 2) patients lost to 3-month follow-up |
| Gocen and colleagues 2004;496 Turkey; not specified | Primary or secondary osteoarthritis; n = 59 (29 : 30) also one patient in intervention group who did not receive replacement; 51.3 (35.6) | Pre-operative exercises plus occupational therapy-based education class (movement restriction, use of adaptive devices, lifting and carrying, washing and dressing; no pre-operative intervention | HHS, VAS (pain), days to achieve functional milestones (walking, stairs, bed transfer, toilet transfer, chair transfer); day of discharge, 3 months, 2 years; no losses to follow-up |
| McGregor and colleagues 2004;501 UK; 1998–9 | Osteoarthritis; n = 39 (19 : 20); 71.9 (42.9) | Pre-admission hip class plus education booklet with information on surgery, rehabilitation, walking aids and home adaptations; usual care – no pre-operative advice | WOMAC, HHS, Barthel ADL, Positive-affect Negative-affect scale, Helplessness subscale of Rheumatology attitudes index, Cantril life satisfaction ladder, VAS (pain, fatigue and function), EQ-5D; day of admission, discharge, 3 months; four (4 : 0) patients lost to follow-up |
| Munin and colleagues 1998;567 USA; 1994–6 | High risk for requiring inpatient rehabilitation; n = 35 (19 : 16); 75 (85) | Phased postoperative rehabilitation starting day 3 post surgery; usual care – rehabilitation starting day 7 post surgery | Functional Status Index, SF-36, length of hospital stay, complications; 4 months; ITT analysis; nine (5 : 4) patients lost to follow-up |
| Sandell 2008;504 UK; 2003 | Waiting time of ≥ 6 months; n = 89 (43 : 46); 68.2 (65.1) | Preadmission multidisciplinary intervention by physiotherapist (exercises and gait improvement), nurse (additional advice). Pre-operative occupational therapy home assessment of functional constraints and provision of adaptive devices; no additional pre-operative treatment by OT or physiotherapist and standard advice from nurse | AIMS2, NHP; day of admission; 26 (10 : 16) lost to follow-up |
| Siggeirsdottir and colleagues 2005;568 Iceland; 1997–2000 | 90% osteoarthritis; n = 50 (27 : 23); 68 (52) | Pre-admission training and education programme. Post-discharge home physiotherapist, OT or nurse input as required; usual care – not specified | OHS, NHP, HHS Merle d’Aubigné and Postel score; 2, 4, 6 months; 2 (0 : 2) lost to pre-operative follow-up; three lost to 6-month follow-up (0 : 3) |
Participants
Studies included a total of 366 participants (range 23–89). The mean age of participants ranged from 51 to 75 years. The proportion of female patients in trials ranged from 36% to 85%.
Interventions
Five studies reported a pre-admission intervention with occupational therapy content compared with controls receiving usual care. Pre-surgical interventions were education with a booklet489 or a booklet and class501 or multidisciplinary with occupational therapy provided within an education and exercise programme. 493,496,504 One study evaluated a multidisciplinary intervention that provided occupational therapy as part of pre- and post-surgery education and home-based rehabilitation. 568 One study compared early rehabilitation including an occupational therapy session starting on post-surgical day 3 with similar post-surgery care commencing on post-surgical day 7. 567
Risk of bias
Potential sources of bias are summarised in Appendix 32. The main sources of possible bias were high losses to follow-up in three studies. 501,504,567 In one study, possible bias was identified as 123 patients with any hip replacement were randomised but only data from 80 patients with a primary hip replacement were analysed. 489
Outcomes
Results are summarised in Table 83. In analyses we only considered patient-reported physical function and pain.
| Outcome | Studies | Patients | Pooled effect size (95% CI) | p-value | I2 (%) |
|---|---|---|---|---|---|
| Patient-reported physical function | |||||
| Pre-surgery | 4 | 173 | –0.40 (–0.70 to –0.09) | 0.01 | 0 |
| Discharge | 1 | 39 | –0.24 (–0.87 to 0.39) | 0.45 | |
| Long term | 4 | 135 | –0.52 (–1.17 to 0.13) | 0.12 | 69 |
| Patient-reported physical function: low risk of bias | |||||
| Pre-surgery | 2 | 71 | –0.63 (–1.12 to –0.15) | 0.01 | 0 |
| Discharge | |||||
| Long term | 2 | 70 | –1.09 (–1.60 to –0.58) | < 0.0001 | 0 |
| Patient-reported pain | |||||
| Pre-surgery | 3 | 125 | –0.29 (–0.64 to 0.07) | 0.11 | 0 |
| Discharge | 2 | 98 | –0.20 (–0.59 to 0.20) | 0.33 | 0 |
| Long term | 3 | 90 | 0.06 (–0.67 to 0.80) | 0.87 | 66 |
| Patient-reported pain: low risk of bias | |||||
| Pre-surgery | 2 | 86 | –0.41 (–0.84 to 0.02) | 0.06 | 0 |
| Discharge | 1 | 59 | –0.04 (–0.55 to 0.47) | 0.89 | |
| Long term | 1 | 23 | –0.23 (–1.06 to 0.59) | 0.58 | |
| Length of hospital stay | |||||
| MD | 3 | 146 | –2.25 (–4.15 to –0.34) | 0.02 | 64 |
| Length of hospital stay: low risk of bias | |||||
| MD | 1 | 50 | –3.60 (–5.29 to –1.91) | < 0.0001 | |
| HRQoL | |||||
| Pre-surgery | 3 | 125 | –0.52 (–0.91 to –0.12) | 0.01 | 14 |
| Discharge | 1 | 39 | –0.06 (–0.68 to 0.57) | 0.86 | |
| Long term | 3 | 90 | –0.20 (–0.62 to 0.22) | 0.35 | 1 |
Physical function
Five studies assessed patient-reported physical function using functional domains of the OHS,568 SF-36,567 NHP,504 or WOMAC. 493,501 The results of meta-analyses are shown in Table 83 and Figure 43. Two studies included in the meta-analyses reported change scores. 504,567
FIGURE 43.
Patient-reported physical function. df, degrees of freedom; IV, inverse variance. WOMAC function: Ferrara and colleagues493 and McGregor and colleagues. 501 OHS function: Siggeirsdottir and colleagues. 568 NHP physical: Sandell and colleagues. 504 SF-36 physical function: Munin and colleagues. 567
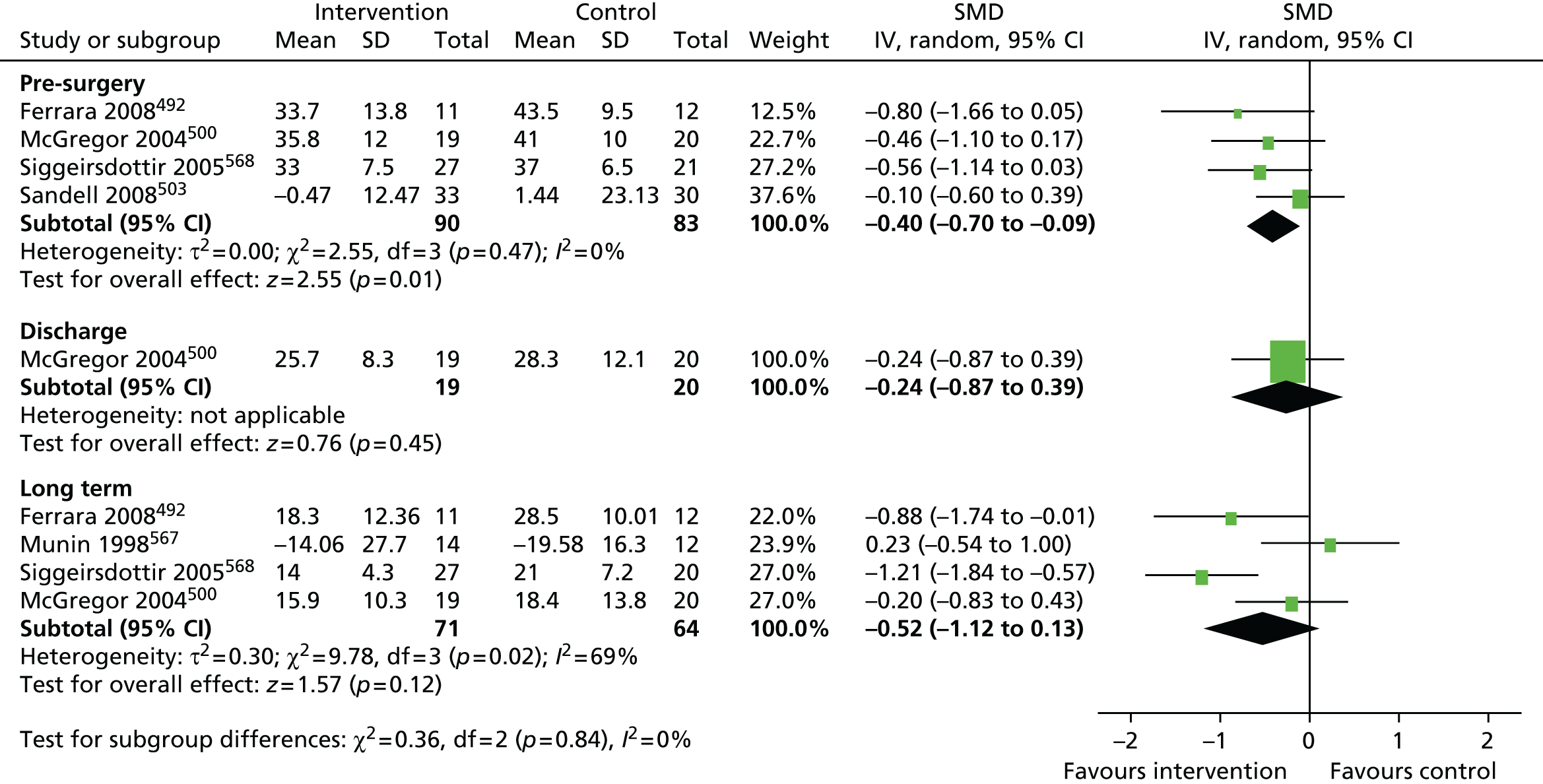
In the four studies with follow-up before surgery, physical function was better in patients who received an intervention with occupational therapy content (SMD –0.40, 95% CI –0.70 to –0.09; p = 0.01). 493,501,504,568 Benefit was apparent in the two studies with low or no reason to assume risk of bias. 493,568 In one study with follow-up at hospital discharge there was no suggestion of benefit501 but in four studies with longer-term follow-up there was a trend favouring occupational therapy interventions. 493,501,567,568 In two studies with low risk of bias or in which there was no reason to assume risk of bias, there was benefit for occupational therapy at long-term follow-up (SMD –1.09, 95% CI –1.60 to –0.58; p < 0.0001). 493,568
Pain
Pain outcomes were reported in five studies using a VAS pain scale,493,496,501 SF-36 bodily pain,567 NHP pain,504 or WOMAC pain score. 493,501 In the meta-analyses summarised in Table 83 and Figure 44, we used WOMAC pain scores in preference to VAS scores when available. One study reported a change score. 567
There was a trend in three studies for reduced pain in patients who received occupational therapy followed up before surgery,493,501,504 but only one study had a low risk of bias. 493 Studies were small and the limited evidence available from three studies did not support a long-term benefit. 493,501,567
Length of hospital stay
The length of stay or time to hospital discharge was reported in three studies. 489,567,568 The overall mean hospital stay was 10.1 days. As shown in Table 83 and Figure 45, patients receiving occupational therapy interventions spent a mean of 2.25 days fewer in hospital (95% CI –4.1 to –0.34 days; p = 0.02). Only one study was at low risk of bias and in this study the hospital stay was reduced further. 568
FIGURE 45.
Length of hospital stay. df, degrees of freedom; IV, inverse variance.

Health-related quality of life
Four studies reported a measure of HRQoL. These were SF-36 physical domains,493,504,567 and EQ-5D. 501 As shown in Table 83 and Figure 46, interventions with occupational therapy content before surgery showed benefit for improved HR-QoL but this was not sustained in the long term.
FIGURE 46.
Health-related quality of life. df, degrees of freedom; IV, inverse variance. SF-36 (physical composite score): Ferrara and colleagues. 493 EQ-5D McGregor and colleagues501 (data scaled from graph – original data not available). RAND-36 (role functioning physical): Munin and colleagues. 567 Arthritis impact measure score (satisfaction with life): Sandell and colleagues. 504
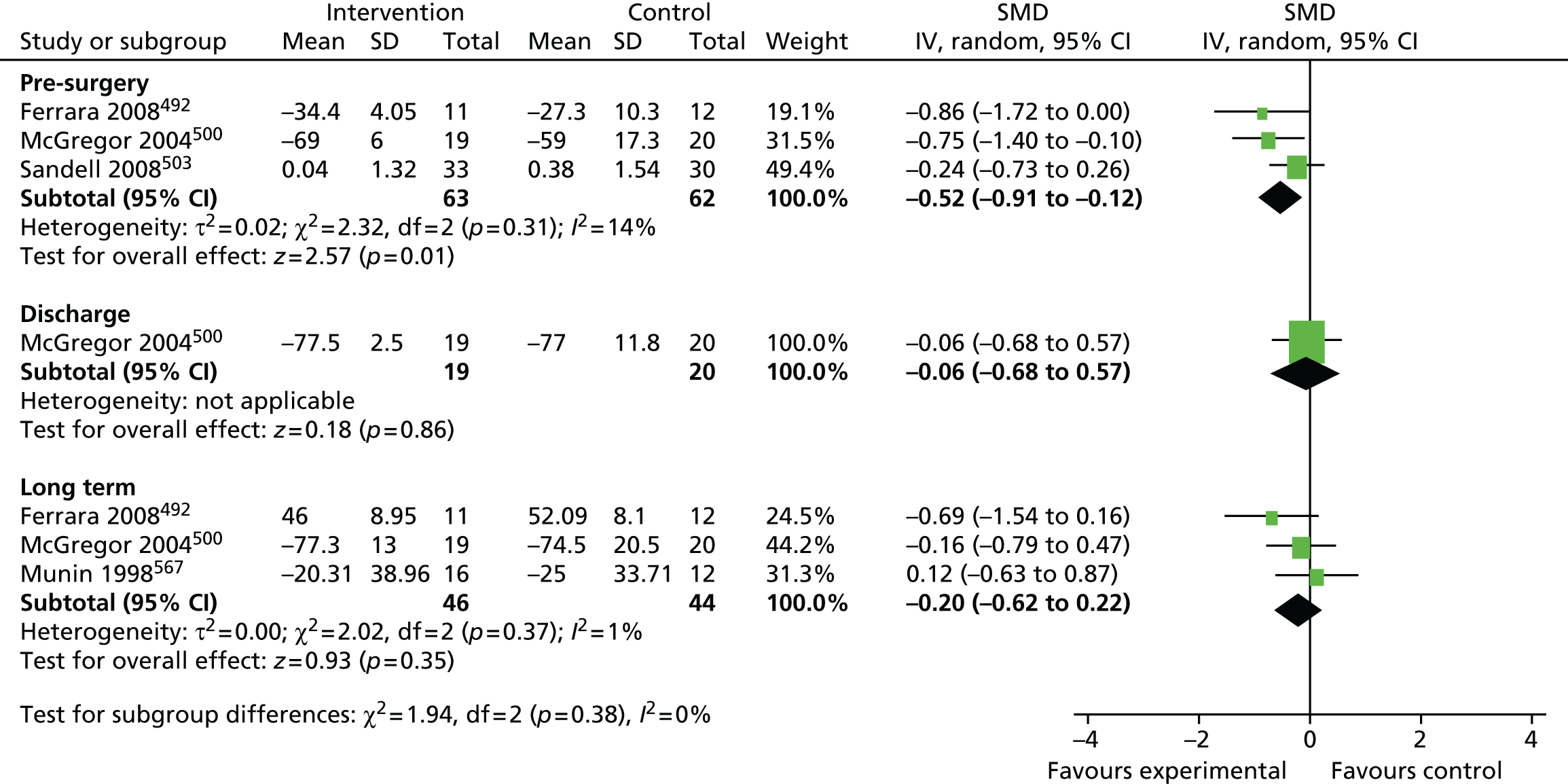
Other outcomes
Other key outcomes in studies of occupational therapy relate to prevention of dislocation (hip precautions) anxiety and social participation. These were infrequently reported. Dislocation was reported in one study with a total of only three events in 50 patients randomised. 568
One study reported a measure of anxiety. 489 After adjustment for sex, anxiety before surgery and at time of hospital discharge was lower in patients who received an intervention with pre-surgical education including occupational therapy content.
Social activity domains of the AIMS2 and NHP measured after the intervention but before surgery were reported in one study. 504 There was a statistically significant difference between groups in the NHP social isolation domain favouring the intervention. For the AIMS2 social activity domain, the effect was in the opposite direction, favouring the control group, but this was marginally not statistically significant.
Discussion
Few studies have evaluated the effectiveness of occupational therapy in patients receiving THR. In the seven studies that we identified, occupational therapy was mainly evaluated in combination with physiotherapy. Only in two studies was the occupational therapy component the principal focus of the intervention. In the study of Ferrara and colleagues,493 patients received a 5-day pre-admission occupational therapy-based intervention but only 23 patients were randomised. McGregor and colleagues501 evaluated a pre-admission educational hip class and booklet with a major focus on occupational therapy in 39 patients.
Overall, studies suggested a possible benefit for improved function and reduced pain before surgery, but this was not sustained after surgery. However, in three studies with data, there was a reduction in length of hospital stay of over 2 days when the overall average length of stay was 10 days.
Studies were generally small with little possibility of exploring key outcomes. A primary focus of occupational therapy in THR is the prevention of dislocation. This occurs in about 1% of patients treated by a posterior approach and about 4% of people treated with a lateral approach. 570 Only one study with three events in 50 patients randomised reported this outcome. OTs teach a range of hip precautions and provide equipment designed to help avoid extreme hip flexion, adduction and rotation with the aim of reducing the risk of dislocation,571,572 but recently their value in preventing dislocation has been questioned. 150,573,574 Recovery after hip replacement without traditional restrictions to movement may allow earlier rehabilitation.
Conclusion
The evidence base on the clinical effectiveness of occupational therapy in patients receiving THR is limited. The need for high-quality, appropriately powered randomised trials to evaluate aspects of occupational therapy in this population is indicated.
Occupational therapy: PROOF-THR
Background
We conducted a RCT to evaluate the feasibility of a randomised evaluation of a pre-surgical occupational therapy intervention in patients waiting for THR. The content of the intervention was based on evidence from literature review and discussions with the RESTORE steering group, experts in occupational therapy research and the PEP-R group. Control patients received usual care including occupational therapy after surgery as provided by the hospital.
The primary objectives were to assess rates of patient identification, recruitment and retention; acceptability of the intervention and control allocation; health resource use; and outcome measures. Secondary outcomes related to pain, functional activity and societal participation.
Methods
Study design and setting
We conducted a single-blind parallel-arm pilot RCT with randomisation at the level of the individual. Randomisation was stratified by hospital and age (< 65 years; ≥ 65 years). The feasibility study took place at the Royal Orthopaedic Hospital NHS Trust in Birmingham, which is a specialist orthopaedic hospital, and at Russells Hall Hospital, which is a general hospital and part of the Dudley Group NHS Foundation Trust. Participants were followed up for a period of 6 months after surgery and completed a series of quality-of-life questionnaires measuring function, societal participation and resource use.
Participants
Selection criteria
Inclusion criteria were:
-
patients listed for primary unilateral THR following review in orthopaedic clinic
-
osteoarthritis as the primary indication for surgery
-
no previous lower limb joint replacement surgery
-
no planned additional lower limb joint replacement surgery within 12 months
-
sufficient understanding of English to complete questionnaires (or proxy completion by representative who understood English).
Exclusion criteria were:
-
patients with inflammatory arthritis
-
primary indication for surgery was for pain relief only and no functional improvement is anticipated
-
patients who were unable to provide informed consent.
Recruitment
Research nurses screened the records of patients listed for a primary THR following assessment in participating orthopaedic assessment clinics. When eligibility was confirmed against the inclusion and exclusion criteria, the research nurse sent the potential participants an information study pack. The study pack contained the patient information leaflet, copy of the consent form and a letter of invitation to join the study. One week after posting of this information, potential participants were contacted by a member of the research team to ask if they would consider taking part in the study. Patients who expressed an interest in joining the study were approached by a member of the research team when they attended their pre-assessment clinic. The research nurses gave the potential participants time to discuss any issues or concerns they may have had prior to obtaining informed consent. Participants who did not use English as their first language were given a covering letter in their own language to invite them to take part. However, the assessments needed to be carried out using the English versions with the help of a relative or friend. Participants who were unable to do this were excluded as many of the outcome questionnaires were not validated in other languages. The patient’s GP was informed of the patient’s participation in the trial in writing, with the patient’s consent.
Randomisation
Participants were randomised between the two groups (1 : 1) using a random assignment computer algorithm. A block allocation sequence was used with stratification by hospital site and age (< 65 years; ≥ 65 years). The randomisation and sequence generation was performed by a statistician within the Primary Care – Clinical Research Trials Unit, based at the University of Birmingham.
Allocation concealment and blinding
Following randomisation and group allocation, a study OT was contacted by the randomisation team when a participant was allocated to receive the intervention and given the study ID of the participant. The OT then obtained participant contact details from the password-protected database and arranged a convenient time to deliver the intervention. Within the research team, group allocation was revealed to the treating therapist only. As is usual in non-pharmacological trials, the participants could not be blinded to their group assignment. All other investigators, and the trial statistician, were blind to the randomisation outcome and to all information indicating assignment.
Treatment as usual
Patients randomised to the control ‘treatment as usual’ arm of the study received the usual NHS care provided to all patients undergoing elective THR in the NHS trust where they received surgery. At both NHS trusts in this feasibility study, OTs provided compensatory equipment in hospital post surgery, which is usual UK practice. Both NHS trusts also provided a pre-surgery multidisciplinary education package.
Intervention
Patients randomised to the intervention arm of the study were visited prior to surgery by an OT, who assessed the individual needs of each participant and their home circumstances. The OT delivered an intervention package that included providing the compensatory devices required by the participant and educating them in how they should be used. In addition, the OT discussed with the participants any expectations and anxieties they (or their carer) may have had, gave explanations about the surgery, hospital stay and postoperative inpatient rehabilitation, and discussed in depth with the participant how they planned to manage when they returned home. This included liaising with other professionals as appropriate. In addition, the OT explained how the layout of the participant’s home may need temporary adaptation to reduce the risk of accidental dislocation. A structured home safety assessment was also performed by the OT, based on the Westmead Home Safety Assessment form. 575 In order to standardise the intervention effect, the OT delivered the intervention to all participants at 4 weeks prior to their individual planned admittance date for surgery.
Once the home-based intervention had been delivered, the participant received the usual care pathway of the trust providing their THR surgery. This included access to the pre-surgery multidisciplinary education session.
Outcomes
The primary outcomes address the feasibility issues with respect to a full-scale RCT:
-
Recruitment procedures and rates: recruitment procedures, identification of eligible patients, willingness of participants to be randomised, rates of recruitment and retention of participants in the trial.
-
Suitability of outcome measures: to refine the choice of outcome measures to be taken forward to the main trial.
-
Fidelity of the intervention: to investigate practicalities of OT compliance to the intervention delivery as designed in the protocol, the quality of the delivery and patient adherence with the intervention. An intervention study log will be completed which has been used successfully in previous studies.
-
Effect size and sample size: to provide data on the effect size to allow for an accurate estimate of the sample size required for the main trial.
-
Economic evaluation: to refine data collection required in order that an economic evaluation can be performed in the main trial.
Secondary outcomes
-
Pain.
-
Functional activity.
-
Societal participation.
Outcome measurement timing
Baseline assessments were completed before randomisation immediately after patients consented to participate. For follow-up assessments, a questionnaire pack with pre-paid return envelope was posted to all participants for completion at 4, 12 and 26 weeks post surgery. This questionnaire pack contained the following validated self-completed questionnaires:
-
WOMAC114
-
Ab-IAP285
-
HADS – anxiety subscale (HADS-A) and depression subscale (HADS-D)281
-
Nottingham Extended Activities of Daily Living scale (NEADL)298
-
EQ-5D-3L278
-
ICEpop CAPability measure for Older people (ICECAP-O). 299
At the final 26-week time point, the Client Service Receipt Inventory (CSRI)577 was used to record the frequency and duration with which participants used education, health and social care services and support over the duration of the assessment period. Records included accommodation and personal care/staff arrangements, and relevant informal care inputs. The CSRI allows systematic recording of service use in a manner commensurate with estimating the costs of support packages. Once the data were collected, all services and supports were listed, and a unit cost (per day, per hour, per contact, etc.) estimated for each one. When available, service costs were taken from publicly available sources for health such as the Department of Health NHS Reference Costs435 and social care costs from the PSS Research Unit health and social care unit costs. 441 In other cases, when services were unusual or likely to absorb a significant proportion of the total costs of support packages, service-specific unit costs were estimated using an equivalent methodology. 578 Each unit cost was multiplied by the frequency with which each participant uses each service to arrive a total care package cost for each person. This total can be disaggregated in various ways, for example, by perspective or by different groups of participants. This information was then be used in conjunction with the EQ-5D and the ICECAP-O results to establish the feasibility of conducting a cost per QALY analysis from the data collected.
Statistical analysis
The primary aims of this feasibility study were to identify effective recruitment procedures, suitability of outcome measures and the fidelity of the intervention. This was evaluated by assessment of recruitment rates and response rates to all the outcome measures at 4, 12 and 26 weeks following surgery in the intervention and control arms of the study.
Secondary outcome analyses of the data collected were by ITT with no interim analysis.
Results
The recruitment of participants for this study (n = 44) fell short of the initial target of 60. The CONSORT flow diagram in Figure 47 details the participant pathway through the study from the number of participants screened for inclusion in the study through to the number analysed.
FIGURE 47.
PROOF-THR CONSORT flow diagram.
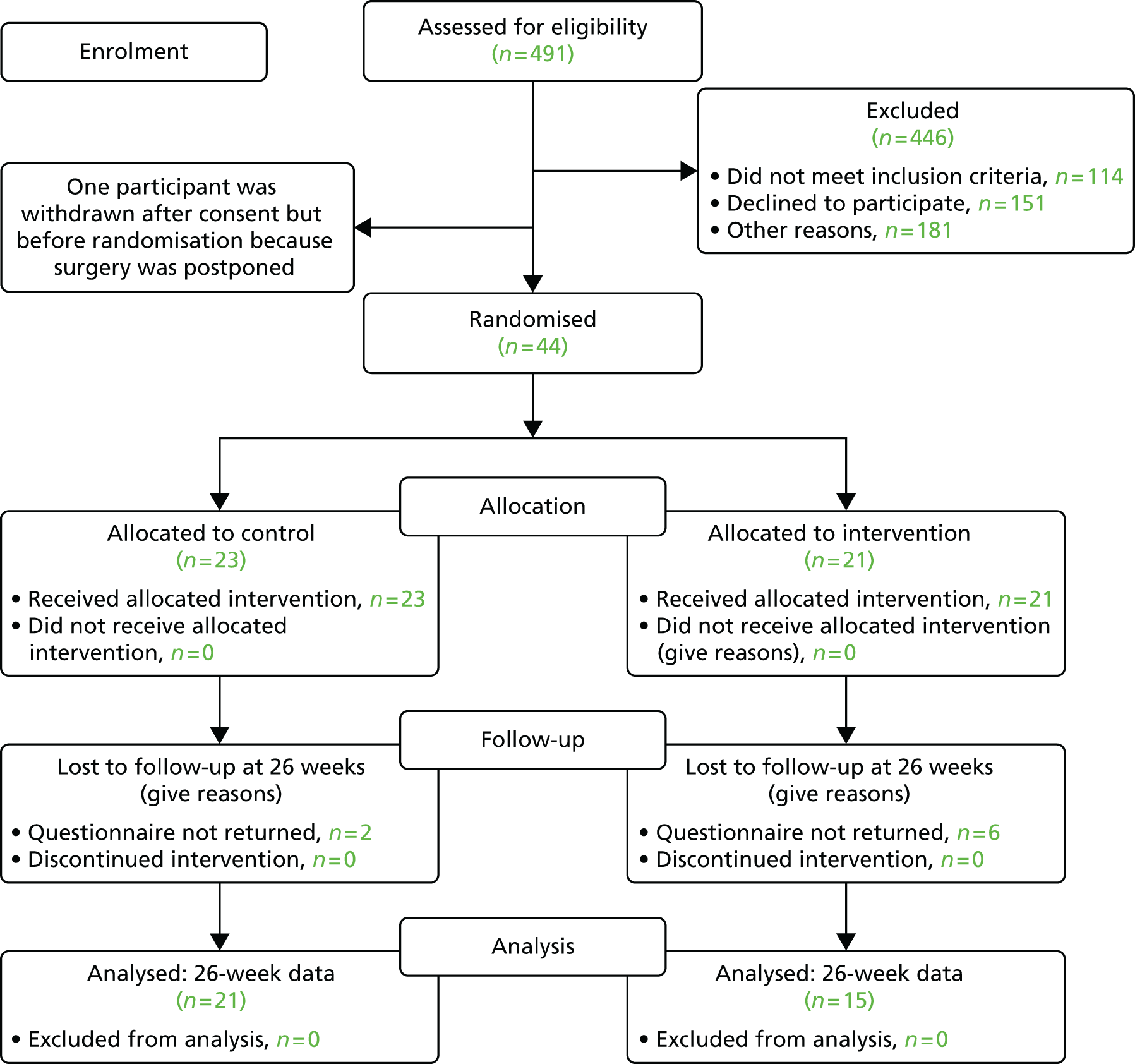
PROOF-THR descriptive statistics
Retention and missing data
Baseline demographic and clinical characteristics of the participants are shown in Table 84. The mean age at the time of surgery was 66 years (SD 10.8 years), with similar means observed in the intervention group, 67 years (SD 11.2 years), and standard care group, 65 years (SD 10.7 years). Overall, there were more male patients (54%), although the distribution of sex differed between the intervention (67% male, 33% female) and standard care (44% male, 56% female) groups. In total, 14% of the study participants lived alone, with 5% of these randomised to the intervention group and 22% allocated to standard care.
| Characteristic | Overall (n = 44) | Allocated to intervention (n = 21) | Allocated to standard care (n = 23) |
|---|---|---|---|
| Mean age at surgery (years) (SD) | 66 (10.8) | 67 (11.2) | 65 (10.7) |
| % female : male | 46:54 | 33:67 | 56:44 |
| % lives alone | 14 | 5 | 22 |
Forty-four participants completed the baseline questionnaire. The retention rates for each of the follow-up periods are presented in Table 85. The retention rate remained consistent throughout the study period, with 82% of the participants retained after 26 weeks for the follow-up questionnaire, although the CSRI return rate was slightly lower (70%) despite being sent alongside the 26-week questionnaire.
| Time point | Participants recruited, n | Data collected, n (%) | Lost to follow-up, % |
|---|---|---|---|
| Baseline | 44 | 44 (100) | 0 |
| 4 week | 44 | 37 (84) | 16 |
| 12 week | 44 | 37 (84) | 16 |
| 26 week | 44 | 36 (82) | 18 |
| CSRI | 44 | 31 (70) | 30 |
Questionnaire completion rates are presented in Table 86. Although the number of fully complete returned questionnaires was reasonable at baseline and at 26 weeks, completion rates were low in weeks 4 and 12, and for the CSRI questionnaire (< 50% complete).
| Time point/questionnaire | Number of completers | % of returners (n/N) | % all (n/N) |
|---|---|---|---|
| Baseline | 33 | 75 (33/44) | 75 (33/44) |
| 4 weeks | 13 | 35 (13/37) | 30 (13/44) |
| 12 weeks | 18 | 49 (18/37) | 41 (18/44) |
| 26 weeks | 23 | 64 (23/36) | 52 (23/44) |
| CSRI (26 weeks) | 15 | 48 (15/31) | 34 (15/44) |
Table 87 presents the distribution of missing (non-answered) questions for each scale used in the study and each time point. Missing data are most prevalent in the WOMAC (122 total missing) and NEADL (105 total missing), while a number of scales exhibit little in the way of missing data (e.g. HADS-A, HADS-D, Ab-P). Of the total missing data, the 4-week returned questionnaires are the greatest contributor, accounting for more than half of the missing WOMAC and NEADL data. Questionnaires with the least missing data were observed in the baseline (54 total missing) and 26-week (35 total missing) follow-ups.
| Questionnaire (number of questions) | Baseline missing | 4-week missing | 12-week missing | 26-week missing | Total missing |
|---|---|---|---|---|---|
| WOMAC (24) | 14 | 66 | 30 | 12 | 122 |
| OHS (12) | 3 | 8 | 2 | 1 | 14 |
| Ab-I (9) | 4 | 5 | 1 | 1 | 11 |
| Ab-A (17) | 9 | 26 | 21 | 3 | 59 |
| Ab-P (9) | 0 | 3 | 0 | 1 | 4 |
| NEADL (20) | 21 | 59 | 13 | 12 | 105 |
| HADS-A (7) | 2 | 2 | 0 | 0 | 4 |
| HADS-D (7) | 1 | 2 | 0 | 0 | 3 |
| EQ-5D (6) | 0 | 8 | 0 | 4 | 12 |
| ICECAP (5) | 0 | 6 | 0 | 1 | 7 |
| Total | 54 | 185 | 67 | 35 | 341 |
Descriptive statistics of scales
Tables 87–89 show the descriptive statistics for each of the outcome measures. Data for participants that had two or fewer missing questions per scale were filled using the mean of the data from the rest of the questions in that scale. Participants missing three or more questions per scale did not have a score calculated for that scale and were classed as missing (n = 6).
Table 88 presents the descriptive statistics for the functional section of the questionnaire comprising the WOMAC, OHS, Ab-IAP, NEADL, and HADS (-A and -D). The means for each scale grouped by allocation are also presented in Table 89. Data for each scale showed improvement at 4 weeks, apart from the NEADL scale, which showed improvement at 12 weeks. Improvement then continued to 26 weeks.
| Scale | Scale range | Baseline mean (SD) | 4-week mean (SD) | 12-week mean (SD) | 26-week mean (SD) |
|---|---|---|---|---|---|
| WOMAC | 0a–100 | 59.13 (17.47) | 31.38 (18.79) | 23.93 (19.09) | 13.31 (14.32) |
| OHS | 0–48a | 17.85 (7.32) | 27.80 (9.99) | 35.57 (9.69) | 40.43 (7.43) |
| Ab-I | 9a–45 | 31.62 (7.42) | 18.79 (7.60) | 16.21 (6.44) | 14.71 (5.51) |
| Ab-A | 17a–85 | 50.78 (14.15) | 33.61 (14.15) | 28.92 (12.57) | 25.24 (8.43) |
| Ab-P | 9a–45 | 21.57 (7.31) | 17.21 (6.65) | 13.43 (6.78) | 11.47 (3.63) |
| NEADL | 0–66a | 48.32 (12.46) | 44.20 (13.51) | 52.09 (17.34) | 59.63 (13.04) |
| HADS-A | 0a–21 | 6.63 (4.89) | 4.57 (4.32) | 3.27 (4.07) | 3.25 (3.61) |
| HADS-D | 0a–21 | 6.04 (3.59) | 4.46 (3.82) | 2.81 (3.44) | 2.28 (2.77) |
| Scale (scale range) | Baseline mean | 26-week mean | ||||
|---|---|---|---|---|---|---|
| Overall (SD) | Control (SD) | Intervention (SD) | Overall (SD) | Control (SD) | Intervention (SD) | |
| WOMAC (0a–100) | 59.13 (17.47) | 61.41 (18.32) | 56.50 (16.51) | 13.31 (14.32) | 15.67 (16.60) | 9.95 (9.86) |
| OHS (0–48a) | 17.85 (7.32) | 17.00 (6.28) | 18.79 (8.38) | 40.43 (7.43) | 39.03 (8.33) | 42.40 (5.63) |
| Ab-I (9a–45) | 31.62 (7.42) | 32.04 (6.15) | 31.13 (8.79) | 14.71 (5.51) | 15.46 (6.41) | 13.67 (3.89) |
| Ab-A (17a–85) | 50.78 (14.15) | 52.98 (13.82) | 48.48 (14.46) | 25.24 (8.43) | 27.00 (9.53) | 22.79 (6.09) |
| Ab-P (9a–45) | 21.57 (7.31) | 22.83 (6.36) | 20.19 (8.17) | 11.47 (3.63) | 11.57 (3.08) | 11.33 (4.41) |
| NEADL (0–66a) | 48.32 (12.46) | 49.26 (10.32) | 47.28 (14.67) | 59.63 (13.04) | 57.34 (16.18) | 62.53 (6.95) |
| HADS-A (0a–21) | 6.63 (4.89) | 6.56 (4.58) | 6.71 (5.33) | 3.25 (3.61) | 3.52 (3.66) | 2.87 (3.62) |
| HADS-D (0a–21) | 6.04 (3.59) | 5.64 (2.50) | 6.48 (4.51) | 2.28 (2.77) | 2.00 (2.00) | 2.67 (3.64) |
Tables 90 and 91 present the categorised HADS scores for anxiety and depression, respectively. The categories are as follows: low (0–7), borderline (8–10) and clinically significant (11–21). Percentages at both baseline and at 26 weeks are relatively consistent with those observed in the intervention and standard care groups, especially in the ‘low’ category. This is most apparent in the HADS-A data (see Table 90) where, at 26 weeks, the overall mean, mean intervention or mean standard care percentages are identical. Both the HADS-A and HADS-D mean data exhibit an increase in ‘low’ categorised responses from baseline to 26 weeks, in conjunction with a decrease in ‘borderline’ and ‘clinically significant’ responses.
| HADS-A score | Baseline mean (n = 44) | 26-week mean (n = 37) | ||||
|---|---|---|---|---|---|---|
| Overall (%) | Control (%) | Intervention (%) | Overall (%) | Control (%) | Intervention (%) | |
| Low (0–7) | 61 | 61 | 62 | 86 | 86 | 86 |
| Borderline (8–10) | 18 | 22 | 14 | 8 | 9 | 7 |
| Clinically significant (11–21) | 21 | 17 | 24 | 6 | 5 | 7 |
| HADS-D score | Baseline mean (n = 44) | 26-week mean (n = 37) | ||||
|---|---|---|---|---|---|---|
| Overall (%) | Control (%) | Intervention (%) | Overall (%) | Control (%) | Intervention (%) | |
| Low (0–7) | 75 | 82 | 67 | 97 | 100 | 93 |
| Borderline (8–10) | 14 | 9 | 19 | 0 | 0 | 0 |
| Clinically significant (11–21) | 11 | 9 | 14 | 3 | 0 | 7 |
Tables 92 and 93 present summaries of the EQ-5D and ICECAP-O scales from the health economics section of the questionnaire. All three of the scales used exhibit a mean increase in HRQoL, with high values observed at 26 weeks. A detailed breakdown of the answers to the EQ-5D and ICE-CAP-O questions is given in Appendices 33 and 34.
Resource-use data
Table 94 presents a summary of the number of items missing from the returned CSRI questionnaires. Missing data are most prevalent in the ‘medication’ and ‘friends/relatives help at home’ sections, while both the ‘friends/relatives time off work’ and ‘current work situation’ sections were mostly returned complete. Comparing the randomisation arms of the study, the greatest number of missing data was observed in the control group, in particular in the ‘medication’ and ‘friends/relatives help at home’ sections, which have a considerably higher number of missing values than the same sections in the intervention group.
| Question (number of possible responses per resource use) | Total missing responses | |
|---|---|---|
| Control | Intervention | |
| Hospital resource use (A&E, outpatient appointments, overnight stays) (3) | 12 | 13 |
| Service use (e.g. GP, physiotherapy) (16) | 16 | 11 |
| Medication (type and payment) (7) | 51 | 26 |
| Personal costs incurred for NHS/social services (e.g. transport, cleaning, child care) (5) | 23 | 16 |
| Time off work (5) | 4 | 1 |
| Friends/relatives help at home (how many hours of help needed for household tasks) (11) | 106 | 29 |
| Friends/relatives time off work (how many hours taken off work to provide help) (1) | 1 | 1 |
| Current work situation (7) | 3 | 0 |
| Total | 216 | 97 |
Discussion
This study looked at the feasibility of a pre-surgical occupational therapy intervention in patients waiting for THR. The primary objectives were to assess rates of patient identification, recruitment and retention; acceptability of the intervention and control allocation; health resource use; and outcome measures. Secondary outcomes related to pain, functional activity and societal participation.
Rates of patient identification
Participants were identified by research nurses screening patient notes in participating orthopaedic assessment clinics. Of the 491 patients screened, 332 patients (68%) were identified as eligible for inclusion in the trial. This represents a good rate of participant identification, with only 114 ineligible, mainly owing to previous or planned lower limb replacement (75%), which was an exclusion criterion.
Recruitment and retention
The recruitment rate for this trial was 22% of eligible patients, which is lower than the 48–85% recruitment rates reported in similar trials of rehabilitation regimes prior to or following THR surgery. 503,579,580 However, the recruitment rate may have been disproportionately influenced by the exclusion of patients enrolled in another conflicting trial (24% of the total excluded, n = 109). This is quite substantial as it is almost as many as the number of patients excluded because they did not meet the inclusion criteria (n = 114, 26%). In addition, of the 151 patients classified as ‘declined to participate’, there were 43 patients who expressed interest in the trial but were not randomised either because clinic appointments were brought forward, making it impractical to deliver the intervention, or because there was insufficient OT capacity to deliver the intervention. Therefore, the research nurses scheduled only 154 recruitment appointments, of which 20 were missed. In effect, this resulted in only 88 out of the 134 patients actually contacted refusing to participate. This makes the recruitment rate of patients contacted by the research nurses 34%.
Retention of participants in the study was high, with a follow-up questionnaire return rate of 82% at 26 weeks. This compares favourably with the 44–99% retention rates in clinical trials conducted between 1990 and 1999 reported by Davis and colleagues. 581
Of the returned follow-up questionnaires, 64% were fully complete with no missing answers. This is a slightly lower rate of completion than the 75% noted in other trials;582 however, it is not unusual for this type of trial to have some missing outcome data. 583 Through researcher contact with the participants involved with PROOF, it became apparent that some participants felt that the questionnaire pack was too long and repetitive. This was mainly due to this study comparing a number of outcome measures to determine which were most appropriate and useful for inclusion in a future definitive trial. Therefore, in a future trial, the questionnaire pack would probably be shorter, which may result in higher rates of response584 and fewer missing data.
Acceptability of the Intervention
There were no participant withdrawals after allocation and there was no crossover from the intervention to control arm. In addition, all participants allocated to the intervention arm received the occupational therapy intervention. This suggests that the content of the occupational therapy intervention was acceptable to the participants and easily deliverable by the therapists involved. Therefore, the content of the intervention in this feasibility study is suitable to be carried through to a definitive trial.
Health resource use
The health resource-use questionnaire (CSRI) had quite a poor rate of both return and completion, with only 34% of the 44 participants returning correctly completed forms. This would suggest that for a future definitive trial the CSRI form would need to be adapted perhaps to make it simpler to complete. Other methods that could be considered would be for participants to keep a diary of NHS appointments and other resource information or the sending of more frequent questionnaires to capture data rather than asking people to think back over 6 months.
Outcome measures
The outcome measures used in this trial varied in their level of completeness and number of missing data, which ranged from 7 to 122 over the trial period. The scales with the most missing questions across the four time points were WOMAC (122 missing items) and NEADL (105 missing items). The scales with the fewest missing data were HADS (7 missing items), ICEpop CAPability measure (ICECAP) (seven missing items), EQ-5D (12 missing items) and OHS (14 missing items).
Some of the questions in the outcome measures also conflict with the hip precautions clinicians generally offer patients after hip replacements, for example not to bend more than 90 degrees for 6 weeks after surgery. Therefore, this may suggest that the 4-week follow-up period is not suitable and can explain higher rates of missing data.
Power calculation
We used data from PROOF to conduct a power calculation for a more definitive RCT of pre-operative delivery of occupational therapy. Based on the OHS and assuming a power of 80%, α = 0.05 (two tailed), SD of 0.46 between arms at 26 weeks and attrition rate of 20%, we would require a total of 219 participants.
Conclusion
The PROOF-THR feasibility study has generated valuable insight into the feasibility and acceptability of an occupational therapy intervention for people having THR because of osteoarthritis. The recruitment of patients was negatively influenced by another conflicting trial taking place at the same site and, to a lesser extent, the practicalities of delivering the intervention when surgery times are very close to the pre-assessment clinic appointment. Therefore, to attain a better rate of recruitment in a future trial, ways to overcome these recruitment issues will need to be considered. The rates of follow-up were good, but there were high levels of missing data at some time points (4 weeks) and for some outcome measures (WOMAC, NEADL), which would suggest that these may need to be revised for future work. The CSRI form was not completed well by participants, so it may be necessary to use other methods of collecting health economic data in a definitive trial to accurately measure the cost-effectiveness of the intervention. However, with further strategies in place to facilitate recruitment and intervention delivery, it would be feasible to evaluate this intervention in a phase III definitive trial.
Chapter 11 Physiotherapy exercise after total knee replacement: systematic review, survey of provision and feasibility randomised controlled trial
Parts of this chapter have been reproduced with permission from Artz N, Dixon S, Wylde V, Beswick A, Blom A, Gooberman-Hill R. Physiotherapy provision following discharge after total hip and total knee replacement: a survey of current practice at high-volume NHS hospitals in England and Wales. Musculoskeletal Care 2012;11:31–8. 78 © 2012 John Wiley & Sons, Ltd. Some parts have also been reproduced from Artz and colleagues585 © 2015 Artz et al. ; licensee BioMed Central. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated. Some parts of this chapter have also been reproduced from Artz and colleagues. 586 This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 3.0 License (www.creativecommons.org/licenses/by-nc/3.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access page (https://us.sagepub.com/en-us/nam/open-access-at-sage).
Abstract
Background
Evidence on the clinical effectiveness of different aspects of post-discharge physiotherapy after hip and knee replacement is limited. We aimed to review existing research, survey current provision and assess the feasibility of a physiotherapy intervention.
Methods
In the light of other ongoing studies in THR we focused on TKR except in the survey of physiotherapy provision.
We conducted a systematic review of the clinical effectiveness of physiotherapy exercise in patients receiving TKR.
Physiotherapy services were surveyed at 24 high-volume orthopaedic centres in England and Wales.
In the ARENA study, the feasibility of a RCT evaluating a 6-week activity-orientated rehabilitation programme for patients with TKR was assessed.
Results
Systematic review and meta-analysis identified a few small studies suggesting that physiotherapy exercise can have short-term benefits for patients with TKR.
In the UK, physiotherapy is usually provided for patients with THR depending on clinical need. After TKR, group exercises focus on knee-specific strengthening, stretching and functional exercises.
In the ARENA study, we evaluated a 6-week group-based activity-orientated rehabilitation programme for patients with TKR. Of 124 eligible patients, 46 were randomised (37%). The main reasons for non-participation were travel related and the inability to commit to the intervention. The intervention was generally well received, and attendance was good (73%), with 84% of participants reporting that they were satisfied.
Conclusions
Our systematic review and survey suggest that research is required into effective provision of physiotherapy after TKR. The ARENA study suggests that a fully powered RCT of individualised and task-orientated exercise would be feasible.
Background
After TKR, current guidance recommends that in the immediate postoperative period patients have access to routine inpatient physiotherapy to improve mobility and functional independence prior to discharge. 587 However, following discharge from hospital after total joint replacement, physiotherapy service provision is perceived to vary widely between centres and there is little guidance on best practice.
In the light of other ongoing studies on THR, we focused on TKR except in the survey of physiotherapy provision, for which we also considered patients with THR.
We aimed to:
-
review evidence on the effectiveness of physiotherapy exercise interventions provided for patients with TKR after hospital discharge
-
survey current UK physiotherapy practice after total hip and knee replacement
-
carry out a small RCT to explore the feasibility of conducting a definitive trial of a group-based physiotherapy programme with individualised exercises targeted to individual patient aims.
Systematic review and meta-analysis of the effectiveness of physiotherapy exercise after total knee replacement
Background
As with all health technologies, evidence is required on the effectiveness of physiotherapy exercise after TKR. Our aim was to update a previous review84 and further explore the possible benefit of specific physiotherapy exercise modalities after TKR.
Methods
| General methods | As described in Chapter 2, Systematic review methods |
| Databases and dates | MEDLINE, EMBASE, CINAHL, PsycINFO and The Cochrane Library databases from inception to 4 October 2013. Previous systematic reviews. Citations in Web of Science and reference lists |
| Search strategy | Knee replacement/RCT/exercise, rehabilitation or physiotherapy. MEDLINE search strategy based on terms in Appendix 3 |
| Study design | RCTs with individual or cluster randomisation. Quasi-randomised designs |
| Patients | Adults with recent primary TKR |
| Intervention | Exercise-based or physiotherapy intervention after hospital discharge. Outpatient, community or home setting. Not electrical stimulation or acupuncture |
| Controls | Usual care or minimal intervention. Alternative formats of care |
| Follow-up | At least 3 months after surgery |
| Data extraction | Country, baseline dates, participants (indication, age, sex), inclusion and exclusion criteria, intervention and control group content, setting, timing, duration and intensity, follow-up time, losses to follow-up and reasons |
| Outcomes | Patient-reported disease-specific pain and function (e.g. WOMAC, KOOS, OKS, ILAS, VAS pain), the physiological outcome ROM and functional performance tests relating to walking |
| Quality assessment | Cochrane risk-of-bias table |
Results
Included studies
Review progress is summarised as a flow diagram in Figure 48. Searches for studies in knees and hips identified 1127 articles. After screening and detailed evaluation, 17 randomised trials met the inclusion criteria and the characteristics of the studies are presented in Table 95.
FIGURE 48.
Systematic review and meta-analysis of the clinical effectiveness of physiotherapy exercise after TKR: flow diagram.
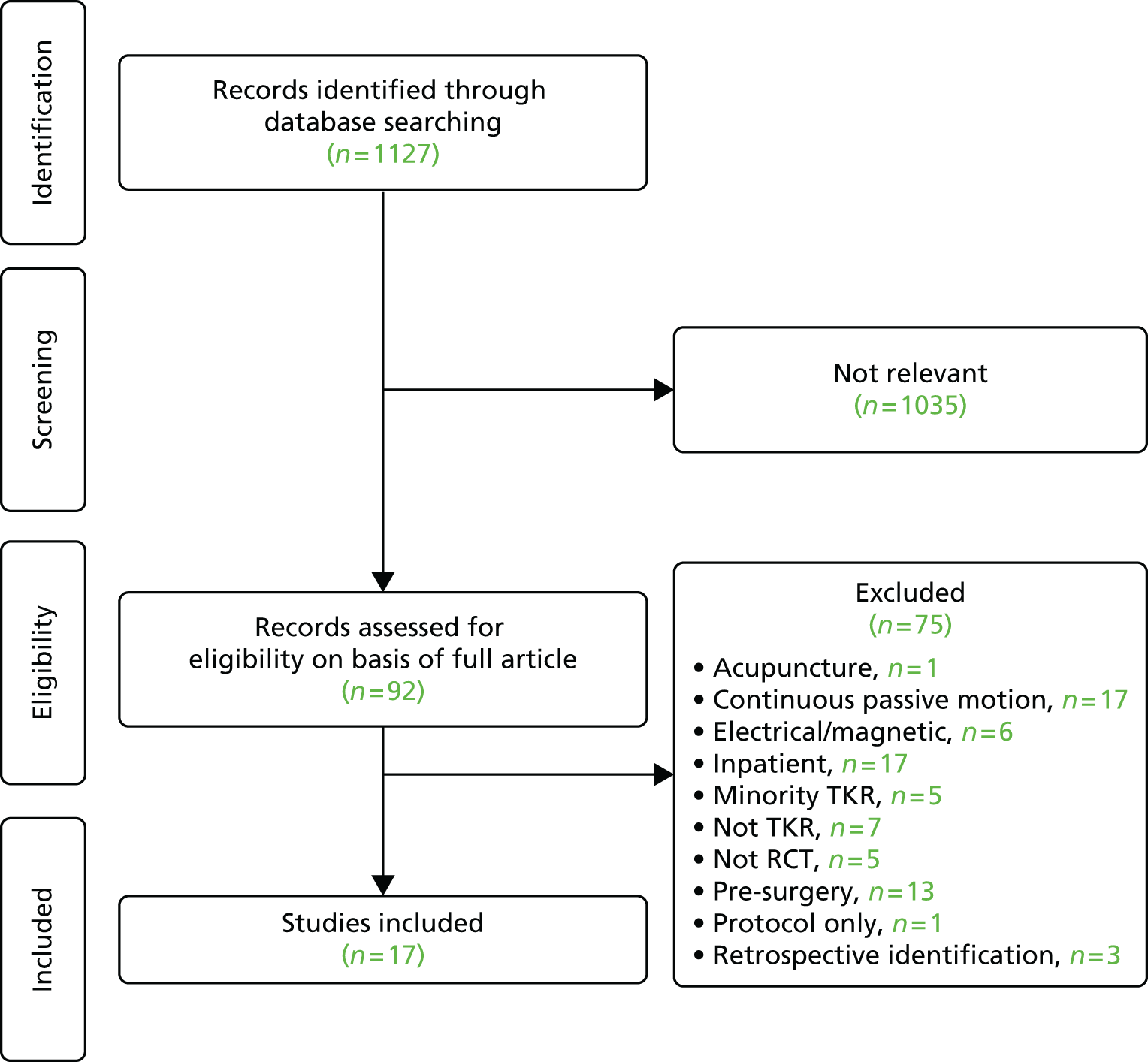
| Publication; location; date of study | Indication; number randomised (intervention : control); mean age (years) (% female) | Primary focus of intervention; study setting; intervention, health professional; timing, duration and intensity; control group care | Follow-up interval; outcomes; losses to follow-up (intervention : control) |
|---|---|---|---|
| Evgeniadis and colleagues 2008;514 Greece; 2006 | Osteoarthritis; n = 48 (24 : 24); 69 (56.3) | Strengthening; home; supervised exercise programme with emphasis on strengthening lower extremities; 8 weeks; controls received standard pre-operative and postoperative care | 6, 10 and 14 weeks after surgery; SF-36, ILAS, active ROM; 13 (9 : 4) not followed up |
| Frost and colleagues 2002;588 UK; 1995–6 | Osteoarthritis; n = 47 (23 : 24); 71.3 (48.9) | Functional exercise; home; warm-up exercise, chair rise, walking and leg lifts; number of visits and duration not specified; controls given instructions to continue exercises taught in hospital | 3, 6 and 12 months; VAS pain, ROM, leg extensor power, walking speed, gait speed; 20 (7 : 13) not followed up |
| Fung and colleagues 2012;589 Canada; 2009–10 | Not specified; n = 50 (27 : 23); 68.1 (66); | Balance and posture control additional to outpatient physiotherapy; outpatient department in rehabilitation hospital; Wii Fit™ (Nintendo Wii™; Nintendo of America, Redmond, WA, USA) gaming activities focused on multidirectional balance, and static and dynamic postural control; twice weekly for mean of about 8 weeks; all patients received twice-weekly outpatient physiotherapy; control patients also received 15 minutes of lower extremity strengthening and balance training exercises | Discharge from physiotherapy, estimate about 3 months; ROM, 2-minute walk test, NRS pain, LEFS, Activity-specific Balance Confidence Scale, length of rehabilitation, satisfaction; 0 lost to follow-up |
| Harmer and colleagues 2009;590 Australia; 2005–6 | Not specified; n = 102 (53 : 49); 68.3 (57) | Hydrotherapy compared with gym-based therapy; community pool; supervised classes in pool with walking forward and backward, stepping sideways, step-ups, jogging, jumping, kicking, knee ROM exercises, lunges and combined squats and upper extremity exercises; twice a week, 60-minute duration for 6 weeks; control patients received gym-based rehabilitation with ergometer cycling; walking on a treadmill; stair climbing; standing isometric, balance and knee ROM exercises at a bar; and sit-to-stand exercises | 8 and 26 weeks; WOMAC, VAS, 6-minute walk test, stair ascent, ROM, knee flexor and extensor, knee oedema; three (2 : 1) lost to 26-week follow-up |
| Kauppila and colleagues 2010;591 Finland; 2002–5 | Osteoarthritis; n = 86 (44 : 42); 70.6 (75.6); included 60–80 years | Multidisciplinary rehabilitation programme; university hospital outpatient department; week 1: physiotherapist assessment; three group sessions (45 minutes) with lower limb strengthening exercises, two pool gymnastic sessions (30 minutes) with lower limb stretching and mobility, and functional exercises focused on walking; lectures by social worker (60 minutes) and nutritionist (90 minutes); week 2: two lower limb strengthening exercise group sessions (45 minutes), three pool gymnastic sessions (45 minutes); orthopaedic surgeon lecture (45 minutes) and clinical assessment (15 minutes); daily supervised group stretching exercises (30 minutes); twice weekly supervised group Nordic walking (30 minutes); four group rehearsals of relaxation strategies (30 minutes); individualised exercise recommendations (40 minutes); two group sessions on coping strategies (90 minutes) and individual visit with psychologist – total 10 days at 2–4 months after surgery; control received an exercise programme to complete at home from 2 months after surgery | 2 months, 6 months, 12 months; WOMAC, 15-minute walk test, stair ascent/descent test, isometric strength, ROM; 11 (8 : 3) lost to 6- and 12-month follow-ups |
| Kramer and colleagues 2003;592 Canada; date not specified | Osteoarthritis; n = 160 (80 : 80); 68.4 (56.9) | Basic and advanced ROM and strengthening exercises; home- and clinic-based groups; attended outpatient physical therapy; therapists able to modify or add exercises, use therapeutic modalities, joint mobilisations or other measures as appropriate; between 2 and 12 weeks after surgery, two sessions per week for 1 hour per session; home-based rehabilitation only, received a telephone call once in weeks 2–6 and once in weeks 7–12 reminding them of the importance of exercise and to give advice | 12, 26 and 52 weeks; WOMAC, SF-36, AKSS, stair ascent and descent, 6-minute walk test; 26 (11 : 15) medical issues, withdrawn consent |
| Liebs and colleagues 2010;593 Germany; 2005–6 | Osteoarthritis or osteonecrosis; n = 159 (85 : 74); 69.8 (71.7) | Ergometer cycling (additional to standard programme); multiple hospitals; cycling with minimal resistance under guidance of a physical therapist, aim was to improve muscle coordination, proprioception and ROM; three times a week for at least 3 weeks, starting after the second postoperative week; controls received standard physiotherapy programme only | 3, 6, 12 and 24 months; WOMAC, SF-36 PCS, patient satisfaction; 24 (10 : 14) lost to follow-up at 3 months |
| Madsen and colleagues 2013;594 Denmark; 2010–11 | Osteoarthritis; n = 80 (40 : 40); 66.6 (41) | Group-based programme compared with home-based programme; physiotherapist-led strength endurance training, education, patient discussion, home exercises twice weekly with strength training, endurance training on exercise bike, walking, balance, training and muscle strength training; two sessions per week for 6 weeks starting 4–8 weeks after surgery, average of 10.5 sessions (range 4–12); home exercises with one or two planned visits with a local physiotherapist | 3 and 6 months; OKS, SF-36 physical function, EQ-5D, ROM, peak leg extensor power, balance test, 10-metre walk test, sit-to-stand tests, VAS pain during leg extensor power test; 10 (4 : 8) lost to follow-up |
| Minns Lowe and colleagues 2012;595 UK; 2006–9 | Osteoarthritis; n = 107; 94 (47 : 47) received surgery; 69.2 (58) | Home-based functional rehabilitation; home; two physiotherapist home visits within 2 weeks and at 6–8 weeks after discharge, assessment of function and rehabilitation progress on gait re-education, and use of walking aids; twice-daily exercise for 3 months: weight, partial knee bends/quarter squats, standing knee flexion and extension wall sits, heel and knee raises, step-overs and stretches; task training: getting in and out of a car, getting up from a chair at a table, walking outside and stairs; controls received usual physiotherapy treatment provided at the hospital without additional home visits | 3, 6 and 12 months; KOOS, OKS, leg extensor press power, 30 seconds timed sit-to-stand test, 10-metre timed walk test, ROM (provided by author) |
| Mitchell and colleagues 2005;520 UK; 1999–2000 | Osteoarthritis; n = 115 (57 : 58); 70.3 (57.9) | Home physiotherapy compared with outpatient group provision; up to six post-discharge home visits by community physiotherapist, patient assessment and individualised therapy relating to pain relief, knee flexion and extension, gait re-education, home and functional adaptations, reduction of swelling and mobilisation of soft tissues; before-surgery patients received 3 visits; controls received exercises and individual treatment 1–2 times a week | 12 weeks; WOMAC, SF-36, resource use and cost; one (0 : 1) lost to ITT analysis (45 patients withdrawn mainly pre-surgery) |
| Mockford and colleagues 2008;596 UK; date not specified | Osteoarthritis, rheumatoid arthritis; n = 143 (71 : 72); 70.2 (61.5) | Outpatient physiotherapy; outpatient department; 6 weeks starting within 3 weeks of hospital discharge; control received no outpatient physiotherapy following discharge, all patients were given a home exercise regime to follow on discharge | 3 months and 1 year; OKS, SF-12, Bartlett Patella Score, ROM, walking test; seven (4 : 3) not followed up |
| Moffet and colleagues 2004;597 Canada; 1997–9 | Osteoarthritis; n = 77 (38 : 39); 67.7 (59.7) | Intensive functional rehabilitation; rehabilitation institute; 12 physiotherapist-supervised sessions from 2 months after discharge with individualised home exercises, 60–90 minutes per week for 6–8 weeks; each session included warm-up, specific strengthening exercises, functional task-oriented exercises, endurance exercises and cool-down; ROM, pain and effusion monitored to optimise intervention; control group received usual care including possibility of supervised rehabilitation at home; all patients were taught a home exercise programme before hospital discharge | 4, 6, 12 months; WOMAC, SF-36, 6MWT; six (0 : 6) at 12 months |
| Monticone and colleagues 2013;598 Italy; 2010 | Osteoarthritis; n = 110 (55 : 55); 67 (64) | Home-based functional exercise programme; home; continuation of functional exercises provided in hospital, cognitive–behavioural intervention with home exercise book about the fear-avoidance model and management of kinesiophobia, monthly phone calls to strengthen adherence; twice-weekly 60-minute sessions for 6 months; no physiotherapy; advice to stay active | 6 and 12 months; KOOS ADL and pain, Tampa Scale for Kinesiophobia, NRS pain, SF-36; 0 losses to follow-up |
| Piqueras and colleagues 2013;599 Spain; 2008–10 | Osteoarthritis; n = 142 (72 : 70), 181 randomised but 142 completed baseline measures; 73.5 (72.4) | Outpatient and home-based telerehabilitation; five sessions under therapist supervision at rehabilitation department and five sessions at home; interactive virtual telerehabilitation, patients received information needed to perform exercises and remote therapist monitoring, therapy modified as rehabilitation evolved; system used wireless movement sensors, interactive software, a touch-screen computer and a web portal; daily 1-hour sessions for 10 days; conventional out-patient physical therapy, all randomised patients received a 2-week rehabilitation programme immediately after hospital discharge | 2 weeks after intervention and 3 months; ROM, muscle strength, walk speed, pain, WOMAC, timed up and go test; nine (4 : 5) lost to follow-up |
| Piva and colleagues 2010;600 USA; 2007–8 | Not specified; n = 43 (21 : 22); 68.5 (71.4) | Balance exercises (additional to supervised functional training programme); outpatient physical therapy department; additional balance exercises (agility and perturbation); control group received a supervised functional training programme without additional balance exercises; all patients received 12 sessions of functional training over 6 weeks; home exercises given to both groups at the end of the supervised programme | 2 months and 6 months; WOMAC, LEFS, timed chair rise test, gait speed; eight (3 : 5) lost to follow-up |
| Rajan and colleagues 2004;601 UK; 1998–9 | Monoarticular arthrosis; n = 120 (59 : 61); 68.5 (62.9) | Outpatient physiotherapy; outpatient; average 4–6 physiotherapy sessions; control group did not receive outpatient physiotherapy; all patients given a home exercise regime on discharge | 3 months, 6 months and 1 year; ROM; four (3 : 1) not followed up |
| Tousignant and colleagues 2011;602 Canada; date not specified | Not specified; n = 48 (24 : 24); 66 (not reported) | Functional rehabilitation; home; intervention group received telerehabilitation through high-speed internet. Progressive exercises to reduce disability and improve function in ADL. Family member or friend present to ensure safety; two sessions per week for 8 weeks; approximately 1 hour in duration; control group received usual home care services and outpatient rehabilitation over a 2-month period | 4 months; knee ROM, Berg balance scale, 30-second chair–stand test, WOMAC, timed get-up-and-go test, Tinetti test, functional autonomy measure (SMAF), SF-36; seven (3 : 4) not followed up |
Studies ranged in size from 43 to 160 patients (median 102 patients) and included a total of 1682 patients. When reported, the main diagnosis was osteoarthritis and the mean age in studies ranged from 66 to 73.5 years. The duration of follow-up ranged from 3 weeks to 24 months, but we describe data in our meta-analysis from 3 months onwards.
Intervention focus
The focus of the intervention was movement and exercise,591,596,601 exercises aimed at managing kinesiophobia,598 functional588,597 or strengthening exercise514 compared with minimal physiotherapy exercise as discussed in seven studies; home compared with outpatient provision as discussed in six studies;520,592,594,595,602 physiotherapy with additional balance589,600 or cycling components593 compared with standard physiotherapy as discussed in three studies; and pool-based compared with gym-based provision as discussed in one study. 590
Outcome measures
Outcomes reported in studies were classified as patient-reported physical function or pain, physiological tests and physical performance tests. The most frequently used physiological outcome was knee ROM measured with a universal goniometer. Measures of walking were the most widely reported performance outcomes.
Study quality
We completed a risk-of-bias assessment for each study and summarise these in Appendix 35. The main potential source of bias was from large and unequal losses to follow-up in six studies. 514,588,591–594 Two further studies were judged to be of reasonable quality with overall losses to follow-up of between 10% and 20%. 600,602 There was no suggestion of risk of bias in nine studies. 520,589,590,595–599,601
There was no clear evidence of publication bias from inspection of funnel plots. However, numbers of studies were small for several outcomes and in subgroup analyses.
Comparison of different physiotherapy interventions
Results for all comparisons are summarised in Table 96. Meta-analyses used random-effects models, an a priori decision based on the known variation in physiotherapy exercise content. Pooled effect sizes are SMDs except for ROM, for which MDs are reported.
| Follow-up | Studies | Patients | Pooled effect size (95% CI) | p-value | I2 (%) |
|---|---|---|---|---|---|
| Physiotherapy exercise compared with no intervention | |||||
| Physical function | |||||
| 3- to 4-month follow-up | 3 | 254 | –0.37 (–0.62 to –0.12) | 0.004 | 0 |
| 6-month follow-up | 3 | 260 | –0.43 (–0.95 to 0.08) | 0.10 | 76 |
| 12-month follow-up | 4 | 397 | –0.21 (–0.70 to 0.29) | 0.42 | 83 |
| Physical function in studies with low risk of bias | |||||
| 3- to 4-month follow-up | 2 | 119 | –0.35 (–0.62, –0.08) | 0.01 | 0 |
| 6-month follow-up | 2 | 185 | –0.64 (–1.15 to –0.13) | 0.01 | 65 |
| 12-month follow-up | 2 | 253 | –0.37 (–1.36 to 0.61) | 0.46 | 93 |
| Pain | |||||
| 3- to 4-month follow-up | 2 | 103 | –0.45 (–0.85 to –0.06) | 0.02 | 0 |
| 6-month follow-up | 4 | 287 | –0.29 (–0.68 to 0.10) | 0.15 | 60 |
| 12-month follow-up | 4 | 281 | –0.15 (–0.64 to 0.35) | 0.57 | 75 |
| Pain in studies with low risk of bias | |||||
| 3- to 4-month follow-up | 1 | 27 | –0.27 (–1.05 to 0.50) | 0.49 | |
| 6-month follow-up | 2 | 137 | –0.36 (–1.07 to 0.35) | 0.32 | 65 |
| 12-month follow-up | 1 | 110 | –0.73 (–1.12 to –0.35) | 0.0002 | |
| ROM extension | |||||
| 3- to 4-month follow-up | 2 | 178 | –4.14 (–7.10 to 1.18) | 0.006 | 82 |
| 6-month follow-up | 1 | 74 | 0.00 (–1.37 to 1.37) | 1.00 | |
| 12-month follow-up | 2 | 217 | 0.42 (–0.54 to 1.38) | 0.39 | 0 |
| ROM extension in studies with low risk of bias | |||||
| 3- to 4-month follow-up | 1 | 143 | –2.60 (–4.48 to –0.72) | 0.007 | |
| 6-month follow-up | 0 | ||||
| 12-month follow-up | 1 | 143 | 0.20 (–0.92 to 1.32) | 0.73 | |
| ROM flexion | |||||
| 3- to 4-month follow-up | 4 | 321 | –5.23 (–11.16 to 0.70) | 0.08 | 83 |
| 6-month follow-up | 3 | 217 | –4.06 (–6.67 to –1.46) | 0.02 | 0 |
| 12-month follow-up | 4 | 360 | –2.21 (–4.31 to –0.10) | 0.04 | 0 |
| ROM flexion in studies with low risk of bias | |||||
| 3- to 4-month follow-up | 1 | 116 | –2.00 (–4.78 to 0.78) | 0.16 | |
| 6-month follow-up | 1 | 116 | –5.00 (–8.14 to –1.86) | 0.002 | |
| 12-month follow-up | 2 | 259 | –2.38 (–4.80 to 0.05) | 0.05 | 0 |
| Walking | |||||
| Longest follow-up (all 12 months) | 3 | 169 | –0.17 (–0.48 to 0.13) | 0.27 | 0 |
| Home-based compared with outpatient delivery of physiotherapy exercise | |||||
| Physical function | |||||
| 3- to 4-month follow-up | 4 | 310 | –0.03 (–0.25 to 0.19) | 0.80 | 0 |
| 6-month follow-up | 2 | 150 | 0.05 (–0.27 to 0.38) | 0.74 | 0 |
| 12-month follow-up | 2 | 214 | 0.11 (–0.16 to 0.38) | 0.42 | 0 |
| Physical function in studies with low risk of bias | |||||
| 3- to 4-month follow-up | 2 | 199 | –0.15 (–0.43 to 0.13) | 0.29 | 0 |
| 6-month follow-up | 1 | 82 | 0.18 (–0.25 to 0.62) | 0.41 | |
| 12-month follow-up | 1 | 87 | 0.01 (–0.41 to 0.44) | 0.95 | |
| Pain | |||||
| 3- to 4-month follow-up | 3 | 248 | –0.00 (–0.25 to 0.25) | 0.98 | 0 |
| 6-month follow-up | 1 | 85 | –0.05 (–0.48 to 0.38) | 0.82 | |
| 12-month follow-up | 1 | 92 | –0.13 (–0.53 to 0.28) | 0.55 | |
| Pain in studies with low risk of bias | |||||
| 3- to 4-month follow-up | 2 | 207 | –0.07 (–0.35 to 0.20) | 0.59 | 0 |
| 6-month follow-up | 1 | 85 | –0.05 (–0.48 to 0.38) | 0.82 | |
| 12-month follow-up | 1 | 92 | –0.13 (–0.53 to 0.28) | 0.55 | |
| ROM extension | |||||
| 3- to 4-month follow-up | 3 | 261 | –0.21 (–0.46 to 0.05) | 0.11 | 6 |
| 6-month follow-up | 0 | ||||
| 12-month follow-up | 1 | 83 | –0.18 (–0.61 to 0.25) | 0.41 | |
| ROM extension in studies with low risk of bias | |||||
| 3- to 4-month follow-up | 3 | 261 | –0.21 (–0.46 to 0.05) | 0.11 | 6 |
| 6-month follow-up | 0 | ||||
| 12-month follow-up | 1 | 83 | –0.18 (–0.61 to 0.25) | 0.41 | |
| ROM flexion | |||||
| 3- to 4-month follow-up | 3 | 329 | –0.22 (–0.44 to –0.01) | 0.04 | 0 |
| 6-month follow-up | 1 | 68 | –0.18 (–0.65 to 0.30) | 0.47 | |
| 12-month follow-up | 2 | 202 | 0.07 (–0.21 to 0.35) | 0.61 | 0 |
| ROM flexion in studies with low risk of bias | |||||
| 3- to 4-month follow-up | 3 | 329 | –0.22 (–0.44 to –0.01) | 0.04 | 0 |
| 6-month follow-up | 1 | 68 | –0.18 (–0.65 to 0.30) | 0.47 | |
| 12-month follow-up | 1 | 83 | –0.05 (–0.48 to 0.38) | 0.81 | |
| Walking | |||||
| Longest follow-up (2 studies 12 months, 1 study 6 months) | 3 | 267 | –0.02 (–0.26 to 0.22) | 0.87 | 37 |
| Enhanced physiotherapy compared with control | |||||
| Physical function | |||||
| 3–4 months | 2 | 185 | 0.00 (–0.29 to 0.29) | 0.98 | 0 |
| 6 months | 2 | 171 | –0.16 (–0.46 to 0.15) | 0.31 | 0 |
| 12 months | 2 | 144 | 0.12 (–0.13 to 0.37) | 0.35 | 0 |
| Pain | |||||
| 3–4 months | 2 | 185 | –0.12 (–0.41 to 0.17) | 0.43 | 0 |
| 6 months | 2 | 171 | –0.17 (–0.47 to 0.13) | 0.27 | 0 |
| 12 months | 1 | 126 | 0.16 (–0.20 to 0.51) | 0.39 | |
Patient-reported physical function
Data were available at one or more time points for five studies that compared a physiotherapy intervention with a control that received minimal physiotherapy. 514,591,596–598 Studies reported WOMAC physical function, OKS, KOOS ADL scale or Iowa Level of Assistance Scale (ILAS) total score.
As shown in the meta-analysis in Table 96 and Figure 49, in three studies with 254 patients, physiotherapy exercise was associated with an improvement in physical function at 3–4 months (average SMD –0.37, 95% CI –0.62 to –0.12; p = 0.004). At 6 months there was a non-significant trend for benefit (SMD –0.43, 95%CI –0.95 to 0.08; p = 0.10), and little difference between groups at 12 months. Heterogeneity was high in studies reporting outcomes at six and 12 months and this was not explained by inclusion of studies with risk of bias. After exclusion of these studies with risk of bias, benefit was apparent at 3 and particularly at 6 months (SMD –0.64, –1.15 to –0.13; p = 0.01), but this was based on only two studies at each follow-up.
FIGURE 49.
Physiotherapy exercise compared with no intervention: physical function. df, degrees of freedom; IV, inverse variance. ILAS total: Evgeniadis and colleagues. 514 WOMAC function: Kauppila and colleagues591 and Moffet and colleagues. 597 OKS: Mockford and colleagues. 596 KOOS ADL: Monticone and colleagues. 598

Patient-reported pain
Four studies reported a pain outcome at one or more follow-up times. 588,591,597,598 Studies reported WOMAC pain, KOOS pain or OKS pain. As shown in Table 96 and Figure 50, in two studies with 103 patients a pain outcome was reported at 3–4 months with an average SMD of –0.45 (95% CI –0.85 to –0.06; p = 0.02) favouring physiotherapy exercise. There was a trend for benefit at 6 months in four studies with 287 patients (average SMD –0.29, 95% CI –0.68 to 0.10; p = 0.15). At the 12-month follow-up there was little to suggest benefit for patients receiving physiotherapy exercise compared with untreated controls in four studies with 281 patients. Heterogeneity was high at 6- and 12-month follow-ups. Only one study had low risk of bias at each of 3–4 and 12 months, precluding meta-analysis. At 6 months, two studies with low risk of bias maintained a trend for benefit but this did not approach conventional levels of statistical significance (average SMD –0.36, 95% CI –1.07 to 0.35; p = 0.32).
Range of motion
Range of motion extension data suitable for meta-analysis was available from three studies with 252 patients514,591,596 and ROM flexion from five studies with 396 patients. 514,588,591,596,601 As shown in Table 96 and Figure 51, there was little to suggest improved long-term ROM in patients receiving outpatient physiotherapy. Benefit was evident in only two studies with follow-up at 3 months after TKR (average MD –4.14, 95% CI –7.10 to –1.18; p = 0.006). 514,596
FIGURE 51.
Physiotherapy exercise compared with no intervention: ROM. df, degrees of freedom; IV, inverse variance.
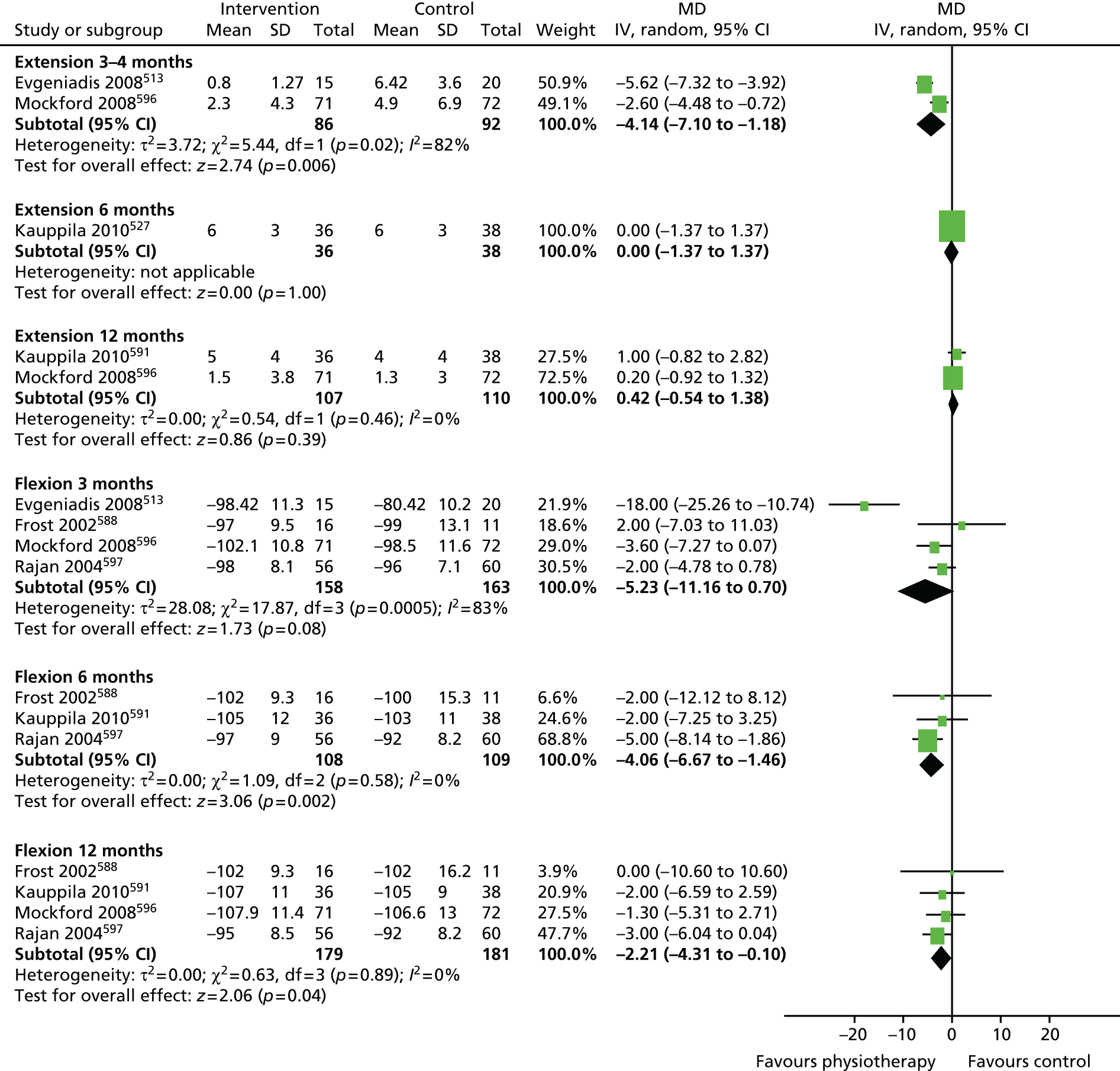
For ROM flexion there was no suggestion of short-term benefit from physiotherapy exercise. There was evidence of long-term benefit with average MD at 6 months of –4.06 (95% CI –6.67 to –1.46; p = 0.02) and at 12 months of –2.21 (95% CI –4.31 to –0.10; p = 0.04) in three588,591,597 and four studies,588,591,596,597 respectively. In studies with low risk of bias, the effect was still apparent although marginal at 12 months but this was based on only one study at 6 months601 and two studies at 12 months. 596,601
Physical performance
Measures of walking performance (metres walked in a set time, time to walk a specified distance and walking speed) were combined with attention paid to direction of effect. An improvement in walking performance in three studies was not significant588,591,597 (average SMD –0.17, 95% CI –0.48 to 0.13; p = 0.27). There was no heterogeneity across studies.
Patient-reported physical function
Physical function was measured using WOMAC, KOOS and OKS measures. Data were available at one or more time points for five studies with 437 patients. 520,592,594,595,602 As shown in Table 96 and Figure 52, there was no suggestion of a difference in functional outcome between home and outpatient provision at 3–4, 6 or 12 months. For example, at 3–4 months, the average SMD was –0.03 (95% CI –0.25 to 0.19; p = 0.80). No heterogeneity was apparent and consideration of higher-quality studies only marginally affected the outcome at 3–4 months in two studies (average SMD –0.15, 95%CI –0.43 to 0.13; p = 0.29), in favour of home-based rehabilitation.
FIGURE 52.
Home-based compared with outpatient physiotherapy exercise: physical function. df, degrees of freedom; IV, inverse variance. OKS: Madsen and colleagues. 594 KOOS ADL: Minns Lowe and colleagues. 603 WOMAC function: Kramer and colleagues,588 Mitchell and colleagues520 and Tousignant and colleagues. 602
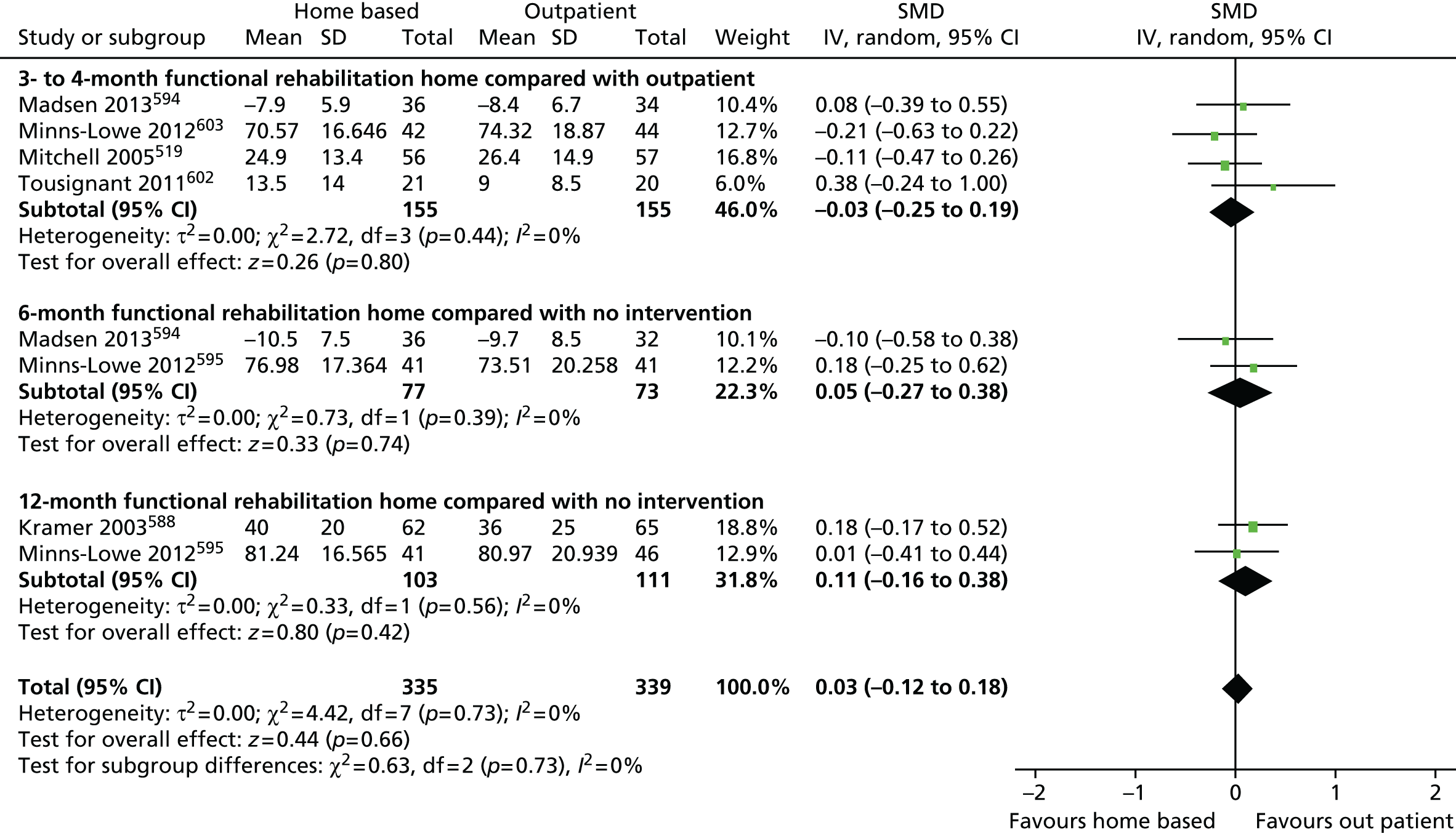
Patient-reported pain
Studies reported WOMAC pain, KOOS pain or VAS pain. Data were available at 3–4 months for three studies with 248 patients. 520,595,602 As shown in Table 96 and Figure 53, there was no difference in pain outcome between patients randomised to home-based or outpatient physiotherapy exercise (average SMD –0.00, 95% CI –0.25 to 0.25; p = 0.98). One study595 followed up 85 and 92 patients at 6 and 12 months and showed no benefit for reduced pain at either follow-up.
Range of motion
Range of motion extension was reported in three studies with 261 patients595,599,602 and ROM flexion in five studies with 448 patients. 594,595,599,602 Outcomes are summarised in Table 96 and Figure 54. There was no suggestion of a difference in ROM extension between randomised groups at any time point. For ROM flexion there was an improved ROM flexion at 3–4 months in patients who received home-based physiotherapy exercise compared with outpatient provision. This was maintained in studies with low risk of bias. There was no evidence for longer-term benefit in a small number of studies.
FIGURE 54.
Home-based compared with outpatient physiotherapy exercise: ROM. df, degrees of freedom; IV, inverse variance.

Physical performance
In three studies with 267 patients randomised there was no suggestion that walking performance differed between groups. 592,594,595
Pool-based physiotherapy
One study compared pool-based physiotherapy with gym-based physiotherapy exercise. 590 There was no difference in WOMAC physical function between randomised groups. Similarly, there was no difference for WOMAC pain between groups. For ROM extension and flexion, the authors reported that there were no group differences between pool-based and gym-based provision.
Additional physiotherapy components
One study with 159 patients evaluated addition of ergometer cycling to a general physiotherapy intervention. 593 There were no differences in pain outcome between randomised groups at any of the follow-up intervals from 3–4 months to 24 months.
Two studies evaluated addition of a balancing component to a general physiotherapy intervention with a total of 93 patients randomised. 589,600 Studies reported different follow-up times but individually there was no evidence for improvement in LEFS or WOMAC physical function. Similarly, numerical response scale pain and WOMAC pain were similar at all follow-up periods. Only one study that included additional balance training reported ROM extension and flexion at short-term follow-up. 589 There were no differences in either measure between intervention and control groups.
Discussion
Randomised controlled trials of physiotherapy and exercise interventions after TKR provide some evidence for short-term effectiveness. In the key analysis comparing patients who received a programme of physiotherapy exercise with those receiving no intervention, there were short-term benefits for physical function (SMD –0.37, 95% CI –0.62 to –0.12; p = 0.004), and pain (SMD –0.45, 95% CI –0.8 to –0.06; p = 0.02). However, these small-to-medium-sized effects,535 were based on only three studies with 254 patients514,596,597 and two studies with 103 patients randomised, respectively. 588,597
Facilitation of early recovery is an important objective of physiotherapy exercise-based rehabilitation. 604 However, physiotherapy should address patient expectations603 and the key expectations of patients undergoing knee replacement relate to long-term functional and pain outcomes. 145,146,605,606 No benefit, in terms of longer-term improvements in function or reduction in pain, were found in the RCTs of physiotherapy exercise that we identified.
Across the trials reporting the outcome, we observed benefit for physiotherapy exercise in studies with low risk of bias compared with controls for flexion only. ROM is a component of clinician-based outcome measures such as the Knee Society Clinical Rating System,113 which is a useful objective measure in clinical trials; however, it is a poor marker of implant success607,608 and does not influence patient satisfaction with their implant. 338
A measure of walking performance was included in over half of the studies we identified, but we were unable to confirm any possible benefit from physiotherapy exercise. In three studies, there was a trend for benefit, but this was not statistically significant. 588,591,597 Parent and Moffett609 evaluated a number of locomotor tests before and after TKR. They concluded that the 6-minute gait test is a simple and responsive measure of locomotor performance which, in conjunction with the WOMAC function difficulty subscale, provides accurate monitoring of early recovery. The need for measures of both gait and a patient-reported functional outcome was highlighted in the study of Lindemann and colleagues,610 in which the correlation between measures was low.
The PROMs included in our meta-analyses were chosen pragmatically based on published trial outcomes and reflect the move away from surgeon-assessed scores. 95 While WOMAC and other disease- and joint-specific PROMs can be used to describe the pattern of recovery after joint replacement,48 their sensitivity to change is affected by floor and ceiling effects. At 12 months after knee replacement, Roos and Toksvig-Larsen306 reported ceiling effects in 11% of patients for WOMAC function and 30% for WOMAC pain.
According to the ICF, the key measures of health relate to functional limitation, activity limitations and participation restriction. 80 In a review of outcome measures commonly used in joint arthroplasty rehabilitation research, Alviar and colleagues611 concluded that these issues are inadequately addressed. The Ab-IAP has been developed with the aim of assessing disability according to the ICF components. 285 The MyMOP2 focuses on management of symptoms but also includes assessment of a patient-specified activity restriction. 287 Use of such methods should allow assessment of an outcome pertaining to the patient’s pre-operative expectations.
There were insufficient studies with adequate patient numbers to provide conclusive evidence on different methods of provision. Physiotherapy exercise provided at home is an appealing approach with the possibility of wider acceptability and uptake. However, as previously estimated, equivalence or non-inferiority trials need large numbers of patients and have yet to be undertaken. Our meta-analysis included only 310 patients for the short-term physical function outcome and fewer for the key longer-term outcomes. Similar issues of study size affect interpretation of physiotherapy exercise provided in a hydrotherapy pool or enhanced with additional cycling and balancing components. This highlights the difficulty of developing a complex physiotherapy exercise intervention.
An important problem that home-based physiotherapy exercise may address is that uptake of rehabilitation is frequently low and that patients who do not attend are more likely to be those with poorer functional health. Optimising uptake and adherence to interventions is an important issue in rehabilitation. 612,613 In their systematic review of interventions for enhancing adherence with physiotherapy, McLean and colleagues612 found evidence of only short-term clinical effectiveness and cost-effectiveness of exercise adherence strategies. They concluded that a strategy to improve adherence to physiotherapy treatment should probably be multidimensional.
Some physiotherapy exercise will generally be provided to patients with TKR even if this comprises only advice following on from inpatient rehabilitation. Health-care professionals and policy-makers need to know what content and duration of physiotherapy exercise is necessary to improve short- and long-term outcomes and which patients are likely to benefit. Appropriate care can then be provided to each individual patient.
Despite the inclusion of 17 RCTs compared with six trials in the review of Minns Lowe and colleagues,84 our conclusion is similar, with a possible short-term benefit for physiotherapy exercise after TKR. Future studies should include credible evaluation of methods with well-designed and appropriately powered randomised trials with a focus on completeness of follow-up.
Physiotherapy: current provision
Background
To provide information about physiotherapy provision after THR and TKR, we conducted a survey of current post-discharge physiotherapy services provided to patients at high-volume orthopaedic centres in England and Wales.
Methods
The National Joint Registry for England and Wales 2010614 online database was screened for high-volume orthopaedic centres, defined as orthopaedic centres with > 500 hip or knee procedures,615 for inclusion in this survey. Twenty-four centres were identified and contacted. Fourteen centres were included in the survey of physiotherapy following THR and 23 centres in the survey of physiotherapy following TKR. The physiotherapy department at each orthopaedic centre was contacted by telephone by a member of the research team and an appropriate physiotherapy clinician was identified and requested to complete a short survey either over the telephone or via e-mail. The survey was conducted as a service evaluation with agreement from North Bristol NHS Trust.
Survey questionnaire
A short questionnaire, shown in Appendix 36, was developed to ascertain current physiotherapy delivered to patients after discharge from hospital following either primary THR or TKR. The questionnaire covered topics including routine physiotherapy pathways and referral processes following discharge, type of physiotherapy treatment provided, relevant precautions and information provided to patients. Survey information was collected on a Microsoft Word document then stored on a Microsoft Excel database for analysis. Data on hip and knee replacements were analysed separately. Orthopaedic centres participating were assigned a unique study number for anonymity and the respondent role was also recorded. Respondents were asked to confirm responses to the survey questionnaire via e-mail or post and make adjustments as necessary.
Analysis
Routine physiotherapy services provided at each centre were categorised into no routine physiotherapy (including provision of leaflets only), outpatient physiotherapy, home-based physiotherapy, or other (physiotherapy including telephone consultation and/or drop-in services). Outpatient physiotherapy was further categorised into one to one or group based. Treatment and exercises types were also categorised.
Results
Respondents from all of the 24 orthopaedic centres selected from the National Joint Registry completed the survey. The distribution of orthopaedic centres surveyed covered a large area of England and Wales with six in northern regions, four in southern regions, four in western regions, two in eastern regions, seven in the Midlands and one in South Wales. The job title of each respondent is shown in Appendix 37.
Physiotherapy following discharge after total hip replacement
None of the 14 centres surveyed referred patients to outpatient physiotherapy as a routine pathway of care following THR (Table 97). Three centres offered additional physiotherapy support either by telephone follow-up, drop-in service or a review appointment. One centre reported that the majority of patients were referred to outpatient physiotherapy at the patient’s local community hospital on the day of discharge. All centres reported that patients were referred to outpatient physiotherapy depending on clinical need. In one centre, patients were referred to outpatient physiotherapy only if they were on restricted weight bearing, progressing slowly with gait or exercise, of higher functional level and keen to return to physical exercise such as running or sport, had a leg length discrepancy, or had a foot drop following surgery.
| THR | TKR | |
|---|---|---|
| Total number of units | 14 | 23 |
| No additional routine physiotherapy input | 11 | 6 |
| Outpatient (1 : 1) physiotherapy | 0 | 5 |
| Group physiotherapy | 0 | 11 |
| Telephone consultation | 2a | 0 |
| Drop-in service | 1a | 0 |
| Review appointment/clinic | 1 | 1 |
Physiotherapy following discharge after total knee replacement
Sixteen out of the 23 centres surveyed referred patients to outpatient physiotherapy (see Table 97). Eleven of these centres referred direct to an exercise group after discharge and five referred patients directly to one-to-one outpatient physiotherapy. No physiotherapy treatment was recorded in six centres and one centre offered a short assessment within 2 weeks of discharge. Centres not providing routine outpatient physiotherapy stated that patients could be referred to one-to-one physiotherapy if a clinical need was identified. For example, in one centre, patients were referred to physiotherapy from the orthopaedic ward if they were not achieving full knee extension or 90° knee flexion, had evidence of poor quadriceps strength, required assistance to progress exercises and mobility, or if the patient was returning to hobbies or work that required a higher level of function. Difficulty achieving 90° knee flexion and evidence of poor quadriceps function were common criteria for provision of one-to-one outpatient physiotherapy.
Group physiotherapy following total knee replacement
All 11 orthopaedic centres providing group outpatient physiotherapy referred patients within 2 weeks of discharge from hospital. Group physiotherapy sessions varied in number and duration (20–60 minutes). Staffing of group sessions ranged between two and five physiotherapist practitioners working with at least one assistant or technician. One centre reported a mixed physiotherapy and occupational therapy group with eight members of staff in attendance. Types of treatment and exercise provided within group sessions are shown in Table 98.
| Strengthening | 11 |
| Stretching | 11 |
| Functional exercises | 9 |
| Task-related exercises | 3 |
| Cardiovascular exercise | 5 |
| Individualised exercise | 6 |
| One-to-one treatment | 6 |
One-to-one physiotherapy after discharge following total knee replacement
Most (four out of five) centres providing one-to-one physiotherapy saw patients within 2 weeks of discharge. The other centre referred patients at 6 weeks post operation to a single clinic for assessment and progression of gait re-education. At this centre, further physiotherapy was provided depending on patient needs. The treatments routinely delivered during one-to-one outpatient physiotherapy included specific knee joint exercises, functional exercises and advice. Manual therapy was used as required, often for restricted range of knee motion. No centres reported use of electrotherapy or acupuncture as a routine treatment. One centre indicated that patients with chronic pain had access to a pain management team.
Discussion
This survey of high-volume orthopaedic centres describes the routine physiotherapy services provided to patients following discharge after either THR or TKR. After THR, no high-volume orthopaedic centres offered routine physiotherapy unless patients were considered in clinical need of additional physiotherapy support. It has been suggested that physiotherapy provided after discharge is not necessary to achieve excellent short-term recovery. 616 The clinical reasoning around the decision not to provide additional physiotherapy following THR was not explored but may reflect the uncertain evidence supporting long-term benefits of physiotherapy after hip replacement,253 despite many patients continuing to have persistent muscle weakness and functional deficits compared with their age-matched peers592 up to 2 years after surgery.
In contrast to THR, most high-volume orthopaedic centres provide physiotherapy following TKR. Greater deficits in pain and function exist after TKR than THR,18,617 with patients reporting a greater need for physiotherapy after TKR. 618 In this survey, we found that 70% of centres routinely referred patients to outpatient services that included either one-to-one physiotherapy or an exercise group. Our findings are similar to that of previous research. 619 In addition, we found that 26% of centres did not offer routine physiotherapy after TKR – a proportion greater than that previously reported. 620 However, after TKR, centres in our survey not offering routine physiotherapy did refer on a needs basis.
Routine referral to supervised exercise groups was the most commonly reported physiotherapy treatment for patients after TKR. In patients with knee osteoarthritis, group physiotherapy can be a cost-effective way to deliver treatment without compromising effectiveness. 621,622 This approach allows patients with similar impairments to exercise within a supported environment621 and appears to be a favoured method of delivering physiotherapy after TKR.
Knee strengthening, stretching and functional exercises were the most common exercises reported in the physiotherapy groups, similar to that seen by Naylor and colleagues. 620 This inclusion of functional exercises is important as greater functional deficits are experience by dissatisfied patients254 and function-based exercises are of more benefit to patients after TKR. 84 Functional difficulties after TKR are observed in many patients, particularly with activities such as kneeling, squatting and gardening. 623,624 Including functional exercises in an exercise group may be a beneficial and cost-effective way to assist patients in returning to higher demanding activities and future research in this area is required.
This survey targeted high-volume orthopaedic centres and we report an excellent response rate. Although not a comprehensive survey of all orthopaedic centres in England and Wales, collecting information from these centres means the results are likely to represent the current trend in post-discharge physiotherapy provision and has allowed us to compare post-discharge physiotherapy between THR and TKR. Information was not gathered from smaller volume orthopaedic units, private or independent treatment centres limiting generalisability to all centres in England and Wales. However, future research should include such centres and gather information from other countries. Exploring the rationale behind provision of physiotherapy pathways was not investigated here; however, research is required to identify the factors that influence the physiotherapy service provision after THR and TKR.
Conclusion
The provision of physiotherapy following discharge after TKR is a more common practice than after THR for which ongoing physiotherapy is provided depending on clinical need. Group exercises are the favoured destination for patients following TKR in high-volume centres with focus on knee specific strengthening, stretching and functional exercises.
Physiotherapy: ARENA
Background
According to the WHO ICF model, rehabilitation should be patient centred and aim to maximise functional ability, facilitate activities and increase social participation. 80
Provision of rehabilitation following TKR should address patients’ individual preferences and needs,604 with exercises orientated towards activities that they consider important. This individualised approach is the basis of an exercise class developed for this study. Group physiotherapy gives a cost-effective way of delivering treatment without compromising its effectiveness625 and allows patients to participate in exercise within a supported environment in the company of peers with similar experiences and impairments. The inclusion of individualised exercise specific to each patient aims to increase self-efficacy and empower patients to take an active role as recommended by patient focus groups. 626 Higher levels of self-efficacy at 3 months have been associated with a greater level of functional activity at 9 months after joint replacement,627 and may enhance adherence to continued home exercise. Involving patients in the design of their rehabilitation may result in better long-term outcome and forms the basis of this function-based group intervention.
Our systematic review identified no particular format of post-discharge physiotherapy associated with improved long-term patient outcomes. Addressing individualisation and patient preferences as described in the MRC framework,94 we conducted a RCT to evaluate the feasibility of providing a 6-week postoperative activity-orientated rehabilitation programme for patients undergoing TKR. The objectives of the study were to determine uptake rates, reasons for non-attendance at classes, patient satisfaction with classes, patient-reported outcomes, and timing and suitability of the exercises and outcomes measures. The study also piloted a methodology to collect costs and outcomes to perform an economic evaluation.
Patients and methods
Ethics approval
Ethics approval for this study was provided by the South West – Cornwall and Plymouth Research Ethical Committee (NHS reference number 11/SW/0341), with study sponsorship and research and development approval provided from North Bristol NHS Trust (reference 2713). As a feasibility study of a RCT, the study was registered, UKCRN ID 12100.
Patient recruitment
Patients listed for primary TKR at the AOC were identified by a member of the clinical care team and invited to participate in this study. These patients were sent a study pack containing a patient information leaflet describing the nature and purpose of the study. When the patients attended their pre-assessment clinic, they were approached by a research nurse to discuss the study further and check eligibility. Patients wishing to participate in the study were asked to provide informed consent by signing the consent form. Patients not wishing to participate in the study were asked if they were willing to explain their reasons for not participating.
Inclusion criteria
Patients listed for primary TKR due to osteoarthritis.
Exclusion criteria
Patients listed for TKR for reasons other than osteoarthritis, patients listed for revision knee surgery including previous unicompartmental, tibial osteotomy or patellofemoral knee replacement surgery; patients unable to participate in exercise for any medical reason such as unstable cardiovascular or cardiorespiratory disease; patients with severe neurological disorders; patients unable to provide informed consent; and patients unable to understand written English, because not all the questionnaires have been validated in other languages.
Randomisation
Participants were allocated into either the intervention or usual care group, using Minim, by a member of administrative staff at the MRU not directly involved in the research study. Minimisation was used to ensure that each group had equal sex and age proportions. Patients were informed of the results of minimisation by telephone 2 weeks after their knee replacement by a member of the research team. Participants in the usual care group were instructed to continue with their current care and participants in the intervention group were invited to the AOC to participate in the exercise class. Prior to the exercise class, participants were asked to identify two functional goals that they would like to achieve following their knee replacement. Participants allocated to the exercise class were invited to bring a partner with them to the classes. Blinding of the research team or patients was not possible in this study because the intervention involved attending exercise classes run by the research physiotherapists.
Intervention: usual care
Usual physiotherapy care following TKR at the study centre consisted of a knee replacement booklet given out at a pre-operative education class. The booklet contained information about discharge planning, the pre-operative period, the operation day, early and later stage postoperative exercises, performing everyday functional activities, returning to work and hobbies, discharge goals, precautions, expectations and potential problems. Patients are advised on discharge to continue with the exercises in the booklet five times a day at home. Some patients could be referred to their local physiotherapy department for follow-up appointments and others may receive physiotherapy at home. This was at the discretion of the inpatient physiotherapy team or orthopaedic team. The patients may also be referred for physiotherapy by their GP or consultant.
Intervention: physiotherapy exercise class
The physiotherapy exercise class started 6 weeks after surgery and lasted for 6 weeks. Each class was 1 hour long and contained 14 separate 4-minute tasks-related exercise stations (Table 99) designed to address functional needs including muscle strength, balance, function and confidence. Participants were instructed to exercise at their own pace with a focus on performing quality movement rather than high quantity. In the first session, all the exercises were demonstrated to the participant by the physiotherapist and the two activity goals were discussed. These activity goals then formed the basis of individualised exercises incorporated into remaining exercise sessions. Two experienced physiotherapists supervised the class and participants were encouraged to discuss progression of exercise with the physiotherapists during the class. On completion of the class, participants were given a list of exercises, including their individual exercises, to continue with at home on a regular basis at their leisure. All travel and parking cost incurred by participants were refunded. Taxis were provided for patients unable to get to and from the class by public or personal transport.
| Exercise | Description | Task |
|---|---|---|
| Plinth exercises | This station is available for continuation of lower grade exercises such as ROM, quadriceps and hamstring strengthening and stretches. This can also be used for kicking practice and eccentric hamstrings | Maintain range and strength. Simulated kicking/swimming |
| Getting in/out of bed | Practise turning from back to side and to sitting. Then stand from sitting. Return to sitting then lying. Alternative exercise includes bridging then sit to stand. Plinth height can varied to increase level of difficulty | Getting in and out of bed. Sit-to-stand test |
| Balance tasks | Series of exercises including single-leg stance, wobble board, balance training, trampette, tandem walking. Exercises are progressed to include upper limb actions such as throwing and catching, reaching | Standing balance. Falls prevention |
| Treadmill | Practise straight line and inclined walking at various speeds | Walking on flat and uphill. Jogging |
| Squatting and crouching | Mini squat and semisquats. Progressing to full squat and crouching. Squats can be performed with the assistance of chairs, gym ball. Tasks can include cleaning floor, loading washing machine/dishwasher, DIY | Squatting down. Crouching down |
| Cycling | Static bike with variable levels of resistance | Cardiovascular fitness |
| Rowing and stepper machines | Optional cardiovascular station for those who may wish to return to gymnasium-based aerobic exercise machines | Gymnasium-based cardiovascular exercises |
| Individualised exercises (× 2) | Exercise designed specifically for individual patients | Individual task |
| Progressive kneeling | Kneeling at progressively increasing ranges of knee flexion on to cushioned or hard surfaces. Full kneeling and high kneeling. Kneeling to practise digging | Kneeling down. Gardening |
| Walking exercises | Walking over uneven surfaces. Walking carrying objects. Stepping into hoops over sticks | Walking. Falls prevention |
| Lunges | Mini lunges. Progressing depth of lunge. Lunge walking. Lunge to bowling or picking up objects | Picking up from floor. Bowling |
| Getting up from floor | Practise getting down and back up from a floor mat using chairs to assist | Getting off floor |
| Step up/down stairs | Stepping up and down on stairs of varying height | Stair ascent and descent |
Assessment times and outcome measures
All participants were asked to complete study questionnaires before surgery and at 2 weeks, 3 months and 6 months after surgery. In addition, participants were contacted at 6 weeks and 3 months after surgery to complete a MYMOP2 questionnaire. A study evaluation was completed by all participants at 3 months after surgery. Participants in the physiotherapy exercise class were also asked to provide feedback about exercise classes by completing an exercise class evaluation following the final exercise session.
Primary outcome measure
The main outcome measures for this study were the KOOS273 and the LEFS. 294 The KOOS is an extended version of the WOMAC and contains information about knee pain and stiffness, knee function, sports and recreation, and the LEFS is a 20-item scoring system to measure lower limb function.
Additional outcome measures
The study questionnaire also contained additional outcome measures to determine completion rates for the following: MYMOP2,287 University of California at Los Angeles (UCLA) Activity Score,295 Activities-specific Balance Confidence Scale,296 Self-efficacy for Rehabilitation,297 Ab-IAP,285 EQ-5D,278 satisfaction with knee replacement and rehabilitation, service and resource usage, and adherence to home exercise.
Study and exercise class evaluation
Participants were asked to complete a study evaluation at 3 months following surgery. This included questions about study documentation, appropriateness of study questionnaires and assessment times. Participants allocated to the physiotherapy exercise group were also asked to complete a class evaluation after completion of the exercise class. This included questions about the timing and duration of the exercise class, satisfaction with the class and exercises available and the opportunity to provide general comments on the class.
Reasons for non-attendance at classes
To assess adherence and any barriers to participation in the intervention, attendance and reasons for non-attendance at the exercise class were recorded.
Assessing non-participation
Ethical approval was provided to approach non-participants by telephone to ask their reasons for not taking part.
Statistical analysis
Descriptive statistics on study participants, recruitment and attrition rates, and physiotherapy outpatient referral rates were calculated. Participant feedback on the physiotherapy intervention was also explored. Analysis of return rates of study questionnaires and completion rates of outcome measures was calculated. Sample size calculations for future trials were calculated from selected outcome measures.
Results
Recruitment
A CONSORT flow diagram showing patient recruitment and retention in the study is shown in Figure 55. A total of 238 study packs were posted to potential participants, with 124 patients approached in the orthopaedic pre-assessment clinic. Of the 124 patients approached, 46 consented to participate in the study, giving a recruitment rate of 37.1%. Five patients were ineligible and one patient had surgery arranged outside the study period. Of the patients who declined to participate, 72 agreed to disclose their reasons for non-participation in this study. Baseline details of the 46 participants are summarised in Table 100 and were similar between both groups.
FIGURE 55.
ARENA CONSORT flow diagram. MUA, manipulation under anaesthetic.
| Characteristic | All | Intervention group (n = 23) | Usual care group (n = 23) |
|---|---|---|---|
| Mean age, years (range) | 68.6 (51–82) | 70.0 (57–81) | 67.2 (51–82) |
| Sex (male/female) | 22/24 | 11/12 | 11/12 |
| Laterality of implant (left/right) | 19/27 | 9/14 | 10/13 |
| Mean distance (miles) residing from hospital (range) | 8.1 (1.3–18.6) | 9.2 (1.7–17.7) | 7.1 (1.3–18.6) |
| Living alone, % | 27.7 | 17.3 | 38.1 |
| Retired, % | 71.3 | 82.6 | 60.0 |
| Additional joint pains, % | 85.7 | 100 | 71.4 |
| Back pain, % | 34.1 | 34.8 | 33.3 |
| Diabetes, % | 13.3 | 21.7 | 4.8 |
| Angina, % | 6.7 | 13.0 | 0.0 |
| Mean KOOS pain score (95% CI) | 42.4 (37.3 to 47.5) | 40.5 (33.4 to 47.6) | 44.5 (37.1 to 51.8) |
| Mean KOOS symptoms score (95% CI) | 42.2 (37.1 to 47.3) | 40.7 (33.6 to 47.8) | 43.9 (36.4 to 51.3) |
| Mean KOOS ADL score (95% CI) | 46.3 (40.7 to 51.8) | 41.8 (34.2 to 49.3) | 51.2 (43.3 to 59.1) |
| Mean KOOS sport/recreation score (SD) | 14.9 (20.9) | 12.2 (24.4) | 18.1 (15.8) |
| Mean KOOS QoL score (SD) | 15.6 (11.3) | 12.5 (12.6) | 18.4 (8.5) |
Reasons for non-participation
Seventy-two patients (23 male and 49 female) agreed to disclose their reasons for non-participation in this study. The average age of patients not participating was 70.1 years (range 50–91 years). Fifteen reasons for no participation were reported; the most frequent issues for not participating in the study were related to travelling distance, transportation and commitment to attend the exercise class if allocated. These reasons accounted for 54% of the reported reasons why patients did not wish to participate in the study. Other reasons included concerns around existing comorbidities, caring for partners or family members, resistance to completion of questionnaires, planned vacations following surgery, uncomfortable about exercising in groups and anxiety about the forthcoming surgery.
Post-discharge referral for additional physiotherapy
Overall, 53.7% of participants in this study were referred for outpatient physiotherapy after their TKR. In the usual care group, 52.6% of participants were referred for outpatient physiotherapy, compared with 57.1% in the physiotherapy intervention group.
Attendance at the exercise class
A total of 23 participants were randomised into the physiotherapy intervention. Of the 23 participants randomised, two participants were excluded prior to starting the exercise class, three participants did not attend, and one participant attended a single exercise before admission for a manipulation under anaesthetic for joint pain and stiffness. Of the three participants who did not attend, reasons for non-attendance were a complication of medical condition, readmission for manipulation under anaesthetic and one participant decided to take a vacation overseas. Overall, the attendance rate was 73%, with 13 participants attending all six exercise classes and four participants missing only one exercise class. The main non-medical reasons for non-attendance were that patients had arranged visits out of area on the day of the exercise class.
Evaluation of the exercise class
A total of 17 participants provided feedback on the exercise class. All participants felt that the duration of the session, at 1 hour, was the right amount of time to exercise. Three participants (18%) would have like to receive more than six sessions and one participant (6%) reported that the exercise class should have started sooner after surgery. Overall, all participants were satisfied with the range of exercises offered, with 15 (88%) participants reporting that they were very satisfied. The average usefulness of functional exercises and individual exercises within the class were scored as 9.6 out of 10 and 9.5 out of 10, respectively, on a numerical rating scale. All patients felt that the exercise class met their functional needs. For example:
coming to the class has been extremely useful. I felt the exercises were all relevant to everyday activities and really boosted my confidence on a day to day basis.
Pt042
there is no doubt that the sessions have expedited and enhanced my recovery. An extremely valuable experience.
Pt031
I think attending these sessions were very helpful emotionally and I felt better in that respect after the first session. Meeting other people to discuss problems with them and the staff was definitely a bonus.
Pt030
Return of study questionnaires and resource-use logs
Study questionnaire return rates were high at 2 weeks after surgery with a 95% return rate in both groups. At 3 months after surgery, the rate of the questionnaire return was lower in the usual care group with 70.0% (14/20) returning the study questionnaire, compared with 90.5% (19/21) for the intervention group. Two participants in the usual care group were contacted by telephone to complete the KOOS. At 6 months, the questionnaire return rate in the usual care and intervention group was 75% (15/20) and 100% (21/21), respectively. Resource-use log return rate at 3 months was 55% (11/20) in the usual care group compared with 90.5% (19/21) in the intervention group. A slight increase in return rate was observed at 6 months after surgery with resource-use log return rates of 75% (14/20) and 100% (21/21) in the usual care and intervention groups, respectively.
Completion of KOOS and LEFS outcome measures
Completion rates of the KOOS and LEFS are shown in Table 101. Completion of the KOOS subsections and the LEFS at 2 weeks after surgery was high for both intervention groups. At the 3- and 6-month stages, completion rates were lower in the usual care for all KOOS subsections and the LEFS compared with the intervention group. Completion of the KOOS and LEFS was generally less likely at the 3-month post-operation stage for both intervention groups.
| Outcome | 2 weeks after surgery | 3 months after surgery | 6 months after surgery | |||
|---|---|---|---|---|---|---|
| Return, n/N (%) | Completion, x/n (%) | Return, n/N (%) | Completion, x/n (%) | Return, n/N (%) | Completion, x/n (%) | |
| KOOS pain | ||||||
| Usual care | 19/20 (95) | 19/19 (100) | 14/20 (70) | 14/14 (100) | 15/20 (75) | 14/15 (93) |
| Physiotherapy | 20/21 (95) | 20/20 (100) | 19/21 (90) | 19/19 (100) | 21/21 (100) | 21/21 (100) |
| KOOS symptoms | ||||||
| Usual care | 19/20 (95) | 19/19 (100) | 14/20 (70) | 14/14 (100) | 15/20 (75) | 14/15 (93) |
| Physiotherapy | 20/21 (95) | 20/2 (100) | 19/21 (90) | 19/19 (100) | 21/21 (100) | 21/21 (100) |
| KOOS ADL | ||||||
| Usual care | 19/20 (95) | 19/19 (100) | 14/20 (70) | 14/14 (100) | 15/20 (75) | 15/15 (100) |
| Physiotherapy | 20/21 (95) | 20/20 (100) | 19/21 (90) | 19/19 (100) | 21/21 (100) | 21/21 (100) |
| KOOS sport recreation | ||||||
| Usual care | 19/20 (95) | 18/19 (95) | 14/20 (70) | 13/14 (93) | 15/20 (75) | 15/15 (100) |
| Physiotherapy | 20/21 (95) | 17/20 (85) | 19/21 (90) | 19/19 (100) | 21/21 (100) | 18/21 (86) |
| KOOS QoL | ||||||
| Usual care | 19/20 (95) | 19/19 (100) | 14/20 (70) | 13/14 (93) | 15/20 (75) | 14/15 (93) |
| Physiotherapy | 20/21 (95) | 20/20 (100) | 19/21 (90) | 19/19 (100) | 21/21 (100) | 21/21 (100) |
| LEFS | ||||||
| Usual care | 18/20 (90) | 17/18 (94) | 12/20 (60) | 11/12 (92) | 15/20 (75) | 14/15 (93) |
| Physiotherapy | 20/21 (95) | 19/20 (95) | 19/21 (91) | 19/19 (100) | 21/21 (100) | 19/21 (91) |
Completion of additional outcome measures
Completion rates of the additional outcome measures are shown in Table 102. Similarly to the LEFS and KOOS outcome measures, completion rates at 2 weeks were high in both groups. However, at 3 months after surgery, completion was poorer in the usual care group, with rates between 55% and 60%, than in the physiotherapy intervention group (76.2–90% completion). Similarly, at 6 months after surgery, completion rates were higher for the physiotherapy group than for the usual care group. Pain measured using a VAS demonstrated the poorest completion in both groups, with rates of 55% at 3 months and 50% at 6 months for the usual care group at 76.2% and 85.7%, respectively in the intervention group.
| Completion rates (%) of additional outcome measures | 2 weeks after surgery | 3 months after surgery | 6 months after surgery |
|---|---|---|---|
| ABC scale | |||
| Usual care | 17/20 (85%) | 12/20 (60%) | 15/20 (75%) |
| Physiotherapy | 20/21 (95%) | 19/21 (91%) | 21/21 (100%) |
| Pain (VAS) | |||
| Usual care | 17/20 (85%) | 11/20 (55% | 10/20 (50%) |
| Physiotherapy | 20/21 (95%) | 17/21 (76%) | 18/21 (86%) |
| UCLA activity score | |||
| Usual care | 18/20 (90%) | 12/20 (60%) | 15/20 (75%) |
| Physiotherapy | 20/21 (95%) | 19/21 (91%) | 20/21 (95%) |
| SER | |||
| Usual care | 18/20 (90%) | 12/20 (60%) | 14/20 (70%) |
| Physiotherapy | 20/21 (95%) | 19/21 (91%) | 20/21 (95%) |
| Ab-IAP | |||
| Usual care | 17/20 (85%) | 12/20 (60%) | 15/20 (75%) |
| Physiotherapy | 20/21 (95%) | 19/21 (91%) | 21/21 (100%) |
Completion of MYMOP2 questionnaires
Initial (6 weeks post operation) MYMOP2 scores were recorded for all participants that had surgery (41/41) and were not excluded from the study. At 3 months after surgery, the completion rate for the first follow-up MYMOP2 scores was 95% in the usual care group and 100% in the intervention group. At 6 months, the completion rates for the usual care and intervention groups were 85.7% and 95.2%, respectively.
Outcome data
Outcome scores for the KOOS subsections are shown in Table 103 and those for the LEFS and additional measures are shown in Table 104. Improvements in score are observed for all measures in both intervention groups from 2 weeks to 6 months after surgery. There is a trend for higher scores in the exercise group for all KOOS subsections and LEFS scores, but no statistical analysis has been carried due to the feasibility nature of this study.
| Outcome | Baseline surgery | 2 weeks after surgery | 3 months after surgery | 6 months after surgery |
|---|---|---|---|---|
| KOOS pain | ||||
| Usual care | 44.5 (36.7 to 52.2) | 52.8 (44.1 to 61.5) | 69.2 (57.9 to 80.5) | 70.9 (60.4 to 81.4) |
| Physiotherapy | 40.4 (33.3 to 47.5) | 46.7 (40.4 to 53.0) | 74.1 (64.6 to 83.8) | 78.6 (70.1 to 87.2) |
| KOOS symptoms | ||||
| Usual care | 43.9 (36.3 to 51.5) | 42.9 (37.8 to 48.0) | 54.8 (45.1 to 64.6) | 56.7 (48.3 to 65.1) |
| Physiotherapy | 40.6 (33.2 to 48.0) | 52.2 (45.7 to 58.8) | 59.6 (51.7 to 67.5) | 58.5 (51.6 to 65.3) |
| KOOS ADL | ||||
| Usual care | 51.1 (42.0 to 60.2) | 58.1 (48.3 to 68.0) | 76.1 (65.4 to 86.8) | 73.5 (63.4 to 83.7) |
| Physiotherapy | 40.9 (34.0 to 47.7) | 53.6 (43.5 to 63.8) | 81.2 (73.5 to 88.8) | 79.6 (71.1 to 88.3) |
| KOOS sport recreation | ||||
| Usual care | 18.0 (10.6 to 25.4) | 12.9 (7.0 to 18.8) | 27.9 (15.1 to 40.1) | 37.1 (24.5 to 49.7) |
| Physiotherapy | 12.2 (1.6 to 22.7) | 16.8 (2.8 to 29.5) | 39.2 (25.0 to 53.4) | 46.3 (34.8 to 57.8) |
| KOOS QoL | ||||
| Usual care | 19.0 (15.0 to 23.0) | 16.7 (13.1 to 18.8) | 36.1 (25.6 to 46.6) | 45.1 (34.2 to 56.0) |
| Physiotherapy | 12.4 (7.0 to 17.9) | 23.9 (15.6 to 32.1) | 52.4 (39.4 to 65.5) | 61.5 (52.6 to 70.5) |
| Outcome | Before surgery | 2 weeks after surgery | 3 months after surgery | 6 months after surgery |
|---|---|---|---|---|
| LEFS | ||||
| Usual care | 30.1 (24.01 to 36.23) | 48.8 (38.78 to 58.85) | 45.0 (35.32 to 54.68) | |
| Physiotherapy | 26.1 (20.32 to 31.89) | 55.8 (48.21 to 63.48) | 57.8 (49.54 to 66.15) | |
| ABC scale | ||||
| Usual care | 62.9 (51.9 to 74.0) | 60.5 (49.1 to 71.9) | 79.0 (66.7 to 91.3) | 80.6 (71.0 to 90.3) |
| Physiotherapy | 48.2 (37.7 to 58.6) | 43.7 (32.8 to 54.6) | 84.3 (77.1 to 91.7) | 84.1 (75.9 to 92.2) |
| Pain (VAS) | ||||
| Usual care | 6.0 (5.0 to 7.1) | 5.7 (4.6 to 6.8) | 3.6 (2.2 to 5.0) | 3.9 (1.7 to 6.0) |
| Physiotherapy | 7.3 (6.4 to 8.2) | 5.3 (4.1 to 6.5) | 3.5 (1.8 to 5.2) | 2.9 (1.3 to 4.6) |
| UCLA activity score | ||||
| Usual care | 4.4 (3.6 to 5.3) | 2.5 (2.2 to 2.9) | 4.3 (3.7 to 5.0) | 4.6 (3.7 to 5.6) |
| Physiotherapy | 3.5 (3.1 to 4.0) | 2.8 (2.4 to 3.1) | 4.9 (4.1 to 5.7) | 5.2 (4.4 to 5.9) |
| SER | ||||
| Usual care | 91.9 (78.7 to 105.0) | 91.2 (82.9 to 99.6) | 101.5 (86.8 to 116.2) | 103.8 (95.9 to 111.6) |
| Physiotherapy | 86.5 (71.0 to 101.9) | 87.3 (76.1 to 98.4) | 108.7 (102.4 to 115.0) | 110.7 (104.0 to 117.5) |
| Ab-IAP | ||||
| Usual care | 17.2 (13.9 to 20.4) | 20.8 (18.1 to 23.6) | 13.9 (10.0 to 17.8) | 15.4 (11.9 to 18.9) |
| Physiotherapy | 18.7 (15.4 to 21.9) | 19.5 (16.1 to 22.9) | 11.5 (9.5 to 13.5) | 10.9 (7.8 to 14.0) |
Sample size calculation
The minimal clinically important difference for the LEFS is nine scale points. 294 In our feasibility study, we observed a mean LEFS score of 45.0 points (SD 18.4 points). We have also allowed for a 39% missing data rate but we will implement measures in the definitive trial to improve LEFS completion rates. For the purposes of the sample size calculation we have assumed a similar SD for the LEFS at 12 months post operation, owing to the lack of published data on LEFS scores at this time point. We have also accounted for a missing data rate of 39%, although we will implement measures in the definitive trial to improve LEFS completion rates. Therefore, we have calculated that we will need to recruit a sample of 256 patients to allow us to detect a minimal clinically important difference on the LEFS between trial arms at 12 months post operation, assuming a power of 80%, a two-sided 5% significance level and accounting for up to 39% missing data or attrition.
Discussion
This study was designed to evaluate the feasibility of implementing a RCT comparing a physiotherapy exercise class with usual care for patients after TKR. We recruited 46 participants from 124 patients approached in an orthopaedic clinic, giving a recruitment rate of 37.1%. This rate is similar to that of a recent feasibility trial by Minns Lowe and colleagues595 with a recruitment rate of 34%, but lower than previous RCTs where recruitment rates range from 47% to 63%. 520,597 Recruitment into trials can be difficult and investigations to evaluate barriers in the recruitment process are important to optimise participant uptake. The main reasons for non-participation in our study were travel-related issues and the inability to commit to the intervention if allocated to this group. Issues with transportation have been highlighted in a previous feasibility study in which 43% of non-participation was due to travel and parking issues,595 indicating that provision of independent travel to study appointments could potentially increase recruitment rates. However, in our study participants were offered transport if allocated to the intervention group and reimbursement of travel costs. Greater emphasis on the provided travel arrangements in future trials may also be beneficial for patients considering participation in studies involving travelling to additional appointments.
Despite the burden of attending six additional physiotherapy appointments, the group physiotherapy exercise class was generally well received and attendance was high. The attendance rate of 73% is similar to that of other studies that involve repeated appointments for a physiotherapy intervention. 590 Harmer and colleagues compared land-based with water-based rehabilitation after TKR and reported an 81% attendance rate when participants attended more than eight treatment sessions. 590 Feedback from participants allocated to the intervention group in our study was generally positive and supportive of the exercise class, with 84% of participants reporting that they were satisfied. Participants reported that the class was of adequate duration and included exercises that met their needs. Although satisfaction following knee replacement is high,628 levels of physical function are lower in TKR patients,629 who often require more physiotherapy input than patients with other arthroplasties. 618 Several ongoing difficulties are reported after surgery. 618,630 Wright and colleagues630 reported that the most common functional complaints do not improve after knee replacement, the complaints include difficulty kneeling and crouching, and impaired ability to walk up and down stairs. Rogers and colleagues629 also found bending/stooping and walking 1 km a frequently reported difficulty after TKR. The aim of the exercise class was to provide patients with a series of different exercises, including kneeling, stairs and treadmill walking, to address these common difficulties and that can be transferable into normal daily life. Unfortunately, this study was not powered to determine whether or not such improvements can occur with the provision of our physiotherapy exercise class design and a larger-scale RCT is required.
It was also noted that the class was beneficial on an emotional level by increasing confidence and having regular contact with other patients, and access to professionals with expertise in rehabilitation was valued. Health professional support and engagement with peers is important for patients after joint replacement626 and can have a positive impact on functional attainment and QoL. 631 Group-based physiotherapy can be enjoyable for patients,628 allows patients to compare their progress with that of their peers and offers interaction between patients who have experienced knee replacement surgery. 628 Involving participants with designing their own rehabilitation was well received with the individualised exercise component scoring 9.5 out of 10 for usefulness. Thus, offering a one-to-one component, albeit in the initial stages of the class, and devising strategies to involve patients in their own rehabilitation is important to assist in adherence, empowerment and self-efficacy. 626,627 The concept of incorporating one-to-one physiotherapy within the group setting has been highlighted by Naylor and colleagues628 as potentially beneficial in allowing identification of persistent postoperative problems and by influencing adherence. Our study indicates that patients were satisfied with the nature of the exercise class and individualised component offered, suggesting that this is a feasible design for future large-scale RCTs.
In addition to developing an exercise intervention, we evaluated the use of a postal study questionnaire and resource-use log. Questionnaire return rates were found to be higher in the intervention group than the usual care group, particularly at 3 and 6 months after surgery. Additional telephone contact to collect the primary outcome measure increased the number KOOS scores recorded. Reasons for this low return rate in the usual care group were not explored in this study but this highlights the limitations of using postal questionnaires. The higher return rates in the intervention group are not unexpected and may reflect closer relationships developed between participants attending the group and the research team/physiotherapists compared with those not invited to attend the class. One participant in the usual care group quoted in the study evaluation document that ‘if I was in the group (referring to the exercise class) I would have probably filled out the forms’.
Another key part of this feasibility study was to investigate the use of different outcome measures for patients after TKR. It has been suggested that there is a need for newer measures to evaluate rehabilitation outcome in patients with TKR595 and part of this study was to look at the return and completion rates of study outcome measures. Of particular interest were the KOOS and LEFS. Although the return rates were higher in the physiotherapy intervention group than in the usual care group, the completion rates of those measures returned were similar between groups at both 3 and 6 months after surgery. Completion rates were lowest for the KOOS sport and recreation subsection at 6 months following surgery, particularly with questions related to running, jumping, kneeling and squatting, indicating that participants may have found these specific questions irrelevant and this should be a consideration for the use of this outcome measure in future trials.
Sample size
Using the LEFS as a functional outcome measure to generate a sample size calculation demonstrated that a total of 256 participants would be required to run a full-scale RCT powered at 80%. Using the LEFS as an outcome measure may provide a more feasible method of delivering a RCT comparing our physiotherapy intervention with usual care in a timely manner at our centre. The LEFS is a validated 20-item questionnaire that assesses lower limb functional impairment and difficulty in performing everyday tasks. The LEFS has been recommended as the outcome measure of choice to measure physical functioning in patients with knee osteoarthritis because of its good psychometric properties and minimal floor and ceiling effects,497 and has more recently been used in trials to compare physiotherapy interventions after TKR. 589,600 In the study by Fung and colleagues589 the LEFS was used as an outcome measure to compare the effects of additional Wii-Fit (Nintendo Wii™; Nintendo of America, Redmond, WA, USA) exercises with outpatient physiotherapy after TKR. The study included 50 participants and had a medium effect size. A sample size of 80 would be required for a fully powered trial. Other authors report varying sample sizes depending on outcome measures used. Minns Lowe and colleagues595 used the OKS to demonstrate that 521 participants would be required in each arm (90% power, level of significance 0.05) to run a RCT comparing usual care with home physiotherapy after TKR. Furthermore, Frost and colleagues588 reported that, depending on the outcome measure used, between 100 and 550 participants would be required to compare the effects of traditional and functional exercises in patients after TKR. Thus, the use of the LEFS in the present study would allow acceptable recruitment numbers and sufficient power to run a fully powered RCT comparing the physiotherapy group exercise with usual care.
Conclusion
The implementation of a RCT to compare a 6-week functional exercise group with usual care for patients after TKR is a feasible and acceptable design in a large orthopaedic centre. In order to run fully powered trial, a total of 256 participants would need to be recruited for the LEFS to be used as a primary outcome measure.
Physiotherapy exercise general discussion
We conducted a survey in 2011 of physiotherapy provision after total hip and knee replacement at high-volume orthopaedic centres in England and Wales. We found that physiotherapy following discharge after TKR was a more common practice than after THR, for which ongoing physiotherapy was provided depending on clinical need. Group exercises were the favoured destination for patients following TKR in high-volume centres with focus on knee-specific strengthening, stretching and functional exercises.
After THR, no high-volume orthopaedic centres offered routine physiotherapy unless patients were considered in clinical need of additional physiotherapy support.
In our studies of evaluations of physiotherapy exercise we focused on patients with TKR. We were aware of a systematic review in progress that was updating the review of physiotherapy exercise by Minns Lowe and colleagues,85 which is now published. 632 Although the authors concluded that published studies support some potential benefit for physiotherapy exercise in relation to patient function, walking and muscle strengthening, there is a need for further high-quality studies with adequate statistical power and long-term follow-up.
In our systematic review and meta-analysis of studies including patients with TKR, there was a suggestion that patients who received a programme of physiotherapy exercise achieved short-term improvements in physical function and pain compared with controls. However, this was based on a small number of studies with small numbers of patients randomised. Again based on limited evidence, there was no benefit suggested in relation to longer-term recovery.
There were insufficient studies with adequate patient numbers to provide conclusive evidence on different methods of provision. To evaluate the clinical effectiveness of home-based provision compared with physiotherapy exercise in an outpatient setting would require large studies, as the objective would be to show equivalence. On the basis of their pilot study, Minns Lowe and colleagues595 concluded that a more definitive evaluation of home-based compared with outpatient provision would require randomisation of 1271 patients with TKR.
In developing a new physiotherapy exercise intervention for evaluation in patients with TKR, we recognised the importance of individual patient concerns and the need to focus on activities that they consider important. This formed the basis of an individualised and task-orientated exercise class developed for evaluation in the ARENA study. Group physiotherapy is a cost-effective method of delivery and allows patients to participate in exercise within a supported environment in the company of other patients with similar problems. The inclusion of individualised exercises specific to each patient’s problems aimed to increase self-efficacy and to empower patients to take an active role in their rehabilitation.
We conducted a RCT to evaluate the feasibility of providing a 6-week postoperative activity-orientated rehabilitation programme for patients undergoing TKR. The group physiotherapy exercise class was generally well received and attendance was high, with 84% of participants reporting that they were satisfied. Participants reported that the class was of adequate duration and included exercises that met their needs. The main reasons for non-participation in the study were travel related and the inability to commit to the intervention.
In order to run a fully powered trial, we estimate that 256 participants would need to be recruited with the LEFS used as a primary outcome measure.
Chapter 12 Discussion and conclusions
The RESTORE programme
Well-conducted studies in representative populations of patients with total hip and knee joint replacement suggest that many people continue to have painful joints after surgery. The proportion of people with an unfavourable long-term pain outcome ranges from about 7% to 23% after hip replacement and 10% to 34% after knee replacement. Similarly, about 10% of patients with hip replacement and 30% with knee replacement do not have a long-term functional improvement that is clinically or statistically significant. The amount of improvement in walking performance is rarely large.
Improving the experience and outcome of joint replacement has the potential to impact on patients and services. The RESTORE programme was designed to deliver high-quality research focusing on understanding the experience of joint replacement and to identify ways to improve the experience and outcomes of people undergoing total hip or knee replacement for osteoarthritis.
We conducted research into care at key times in the patient pathway from being referred for total hip or knee replacement to the time after surgery when an optimal outcome should be achieved. We have used appropriate research methods to synthesise evidence from previous research, to explore the patient experience of surgery, to evaluate the clinical effectiveness, cost-effectiveness and acceptability of a perioperative pain management strategy, to compare the properties and responsiveness of outcome measures, and to assess the feasibility of new interventions before and after joint replacement. Approaches used were systematic reviews, cohort studies, fully powered RCTs and smaller feasibility randomised trials, a survey of current practice and qualitative studies. To ensure PPI throughout the programme, all studies were discussed and developed in collaboration with patient representatives and a patient forum.
Originally we had aimed to focus our ADAPT study on the development of new outcome measures relating to the pain and functional problems experienced by people with osteoarthritis, specifically ICOAP and Ab-IAP. Our patient group argued strongly that we should avoid using questionnaires that repeated themselves and we are aware of the fatigue experienced by study participants completing multiple questionnaires with associated poor-quality and incompleteness of data. Thus, in ADAPT, we studied key patient-reported outcomes, performance tests and movement analysis.
Another change from our original objectives relates to the design of a complex package of care supporting patients throughout the joint replacement pathway. We identified five possible elements: pre-surgical education, optimisation of pre-surgical health, occupational therapy and home modifications provided before surgery, physical therapy, and pain control. Preliminary systematic reviews by us as well as others did not identify evidence-based interventions to fulfil the specific elements. This indicated the need for feasibility studies as conducted in the RESTORE programme and ultimately fully powered RCTs. One of these, the ARENA randomised trial of physiotherapy exercise after TKR commenced in March 2015. 633
Ultimately, we aim to develop consensus statements and the conclusions of the RESTORE programme will be a cornerstone of these along with new studies based on these research findings and information from the National Joint Registry.
To draw together the conclusions from the separate research strands we now consider our findings in the context of research practice and the patient pathway from waiting for total hip or knee replacement through surgery and rehabilitation to long-term recovery.
Research practice
Numerous methods are available to assess general health and functional outcomes after hip and knee replacement. A limitation to several of our studies was the need for questionnaires to be completed in English by participants or carers. Validated translations of several questionnaires are now available and this should be a factor considered in future research.
In our ADAPT cohort study, we compared different measures used to assess function in people undergoing hip and knee replacement. The strongest correlations were between the different self-assessment measures and between the different performance tests. However, the correlations between self-assessment measures and performance tests were much lower, highlighting the importance of using both a self-assessment measure and a performance test to obtain a comprehensive assessment of function. Furthermore, associations between functional measures and other patient factors suggest that age, pain and psychological health should be considered as covariates in future analyses.
Ceiling effects were noted for self-assessment measures such as WOMAC and to a lesser extent for the clinician-administered scores. Some objective measures and gait analyses were still improving after surgery at a time when WOMAC function scores were reaching a plateau. However, other objective measures had a similar pattern of improvement, suggesting that the WOMAC function score provides a reasonable reflection of functional change.
Range of motion is commonly used to evaluate joint replacement outcome in clinical practice after hip and knee replacement and forms part of widely used outcome scores. In the ADAPT study, ROM did not correlate well with other measures of function and seemed to add little value to the assessment of functional impairment.
In our systematic review looking at pain outcome assessment in published research in TKR, we found that assessment has been inconsistent with extensive variation in measures used. The most commonly used outcome measure relied on clinician assessment with a single question about pain. Despite the availability of many validated pain-related instruments, few studies had attempted to capture the incidence, character and impact of chronic pain after TKR. Our review showed a reduction over time in the use of the AKSS, accompanied by an increase in the use of the WOMAC index, an established PROM. Future research is needed to develop consensus and standardisation on which pain domains should be assessed after TKR.
In evaluating the clinical effectiveness of interventions, RCTs and ultimately their systematic review and meta-analysis provide the most reliable evidence on the clinical effectiveness of interventions. In our APEX randomised trials investigating the clinical effectiveness and cost-effectiveness of wound infiltration anaesthesia in reducing chronic pain after hip and knee replacement, we achieved a recruitment rate of > 50%. Within the trials, research nurses used peer-review methods to support training and optimise recruitment practice. In qualitative interviews, patients and health-care professionals reported that they had weighed up the benefit and cost of involvement. They were interested in involvement in the APEX trials because they considered the trials to be important and relevant. Patients expressed their desire to help others by contributing to the furthering of clinical knowledge and many patients thought that they might benefit physically and psychologically from taking part.
Qualitative studies including APEX participants and health-care professionals also demonstrated the importance of clinical trials placing minimal burden on patients and health-care professionals. Health-care professionals wanted the trial to have minimal impact on daily clinical practice and patients wanted data collection and participation to be as easy as possible.
Waiting for total hip or knee replacement
In the APEX cohort we compared radiographic assessment of osteoarthritis severity with patient-reported function and pain severity. For patients with hip osteoarthritis, there was no strong association but in patients with knee osteoarthritis, those with less severe radiographic osteoarthritis had more severe pain and functional problems. This suggests that some patients may have pain that is driven predominantly by central pain sensitisation rather than peripheral changes within the joint.
Comparisons of recovery trajectories in hip and knee replacement show different inter-relationships between pain and function. In the APEX cohort, chronic pain after THR was driven predominantly by pain at rest while chronic pain after TKR was driven predominantly by pain on movement. These findings suggest different pain mechanisms within hip and knee osteoarthritis and highlight the importance of future work to identify the sources and potential treatment options for these different pain mechanisms.
Interviews with patients found that delays for surgery are a common occurrence in the NHS for patients awaiting orthopaedic intervention. Patients can experience a range of emotional reactions if their surgery is delayed or cancelled and the wait for surgery can have detrimental physical and emotional consequences. It is important that health professionals identify those at increased risk and work towards minimising delay and cancellation of operation dates where possible.
In our review of longitudinal studies, patients with better physical function and lower pain before total hip and knee replacement generally achieved a better recovery in terms of joint specific pain and function. Patients with poor physical function before surgery may have greater absolute improvement. This was also noted in the ADAPT study, in which patients with very severe disease at the time of surgery were more likely to have substantially improved long-term function. However, in patients receiving TKR, functional levels achieved were related to the levels of function before surgery. This suggests that delays to surgery with associated functional decline may lead to worse outcomes for patients with knee osteoarthritis.
In the longitudinal studies identified in the review, patients with depression before surgery had poorer long-term pain and functional outcomes. For patients receiving TKR, there was evidence of a relationship between anxiety and poor general psychological health before surgery and poorer long-term pain and functional outcomes. In the APEX cohort study, we found that pre-operative widespread pain sensitisation was not predictive of the amount of pain relief that patients gained from total hip or TKR.
Patients with a broad range of BMIs benefited from total hip and knee replacement but those with the highest BMIs may not achieve good long-term levels of function and pain control.
Our qualitative research, cohort study, feasibility trials and systematic review of longitudinal studies suggest that interventions before surgery to optimise a patient’s physical function, pain levels and psychological health merit further study. In the context of advanced osteoarthritis in which conservative treatments have not controlled symptoms, an exercise and education intervention may aim to maintain functional levels or prevent further decline. Alternatively, reduction of surgery waiting times may mean that patients do not experience further worsening of symptoms and thus poorer long-term functional outcomes.
In randomised evaluations of pre-surgical exercise and education identified in our systematic review, there was a suggestion that physical function can be enhanced and pain reduced before surgery in patients waiting for hip replacement. Studies in patients with knee replacement did not provide strong evidence for benefit. Interventions were associated with reduced anxiety during the hospital admission and quicker mobilisation. The value of specific exercise content was unclear, which may reflect the aims of pre-surgical exercise to maintain functional ability and prevent decline whereas post-surgical rehabilitation is substantially based on adjustment to physical changes associated with the prosthesis.
Pain management before surgery is important, particularly in the context of prolonged waiting times. In our feasibility randomised trial of a group-based pain self-management intervention for patients undergoing THR, the recruitment rate was 23%. Barriers to participation related to patient views of the course, satisfaction with current self-management and difficulty getting to the study centre. An issue in evaluation of pre-surgical interventions may be the perception of patients that conservative strategies have been exhausted and that only surgery can relieve pain. However, among those who attended, the group-based pain self-management intervention was acceptable and well received.
Conventionally, aids and home modifications to support independence are provided after surgery. Other aspects of occupational therapy, such as hip precautions, which aim to reduce the risk of dislocation, may be provided at pre-operative classes, in written material and reinforced during the hospital stay. In our systematic review (see Chapter 10), we found some evidence from a few small studies for benefit for occupational therapy in improving physical function, usually when provided in combination with physiotherapy exercise. Improvements to physical function and pain were only short-term. In the PROOF-THR study, we conducted a feasibility randomised trial of a pre-operative occupational therapy home visit with provision and instruction in use of compensatory devices, education and counselling. The successful recruitment, randomisation of participants and delivery of the intervention, plus the reasonable attrition rate, suggested that this trial design would be feasible to take forward into a large definitive trial.
Perioperative pain management
Our systematic review suggested that local anaesthetic infiltration before wound closure was effective in reducing short-term pain after total hip and knee replacement. Pain was further reduced with the addition of post-closure analgesia and, in TKR, with inclusion of ketorolac in the infiltrate. In TKR, there was little evidence to suggest additional benefit to that provided by FNB. Few studies explored the potential long-term impact of perioperative pain management with local anaesthetic infiltration.
Our APEX RCTs were fully powered evaluations including 322 patients receiving THR and 316 patients receiving TKR. Patients were randomised to an intervention of local anaesthetic wound infiltration or to a control group receiving usual care. In patients with THR, there was little benefit for local infiltration anaesthesia in reducing pain during the first 3 days after surgery. In our meta-analysis of previous studies, benefit for the specific regimen evaluated in the APEX trials was apparent only at rest at 24 hours with no benefit during activity or at 48 hours. Only when additional post-closure anaesthesia was provided through a catheter or injection was there benefit at 48 hours and during activity. Few previous studies reported long-term outcomes. In our APEX hip trial, local anaesthetic infiltration was associated with reduced incidence of long-term pain after THR.
In the APEX trial in patients with TKR, all patients had a FNB sited during surgery, a recognised method of providing analgesia after TKR. In the first 48 hours after surgery there was little suggestion of improvement in pain control in patients receiving local anaesthetic infiltration compared with controls. This was consistent with short-term results from systematic review in trials in which FNB was provided. There was no strong evidence for improved long-term pain control in patients receiving local anaesthetic infiltration in the APEX knee trial.
In the absence of FNB, our systematic review showed short-term benefit for local anaesthetic infiltration in patients receiving TKR, particularly if additional analgesia was provided through a catheter or injection after wound closure. In developing the protocol for the APEX trials, we decided against the use of extra delivery of anaesthesia. The use of catheters generally in health care is associated with a risk of infection. In the studies we reviewed, the rate of deep infection after hip or knee replacement was low and it is not possible to state conclusively that use of a catheter was associated with increased risk of infection. However, there were six infections in 505 patients who received an active catheter (1.19%), compared with a total of eight infections in all 2348 patients randomised (0.34%). Further cohort or registry studies may provide more definitive evidence on the safety of additional analgesia provided through catheters after wound closure.
From the perspective of the NHS and PSS, the addition of local anaesthetic infiltration to the usual analgesia regimen was a cost-effective treatment option in primary THR. Our findings also indicate positive health benefits and cost savings in TKR, but with considerably more uncertainty around the cost-effectiveness result.
Qualitative interviews with patients in the APEX study showed that the experience of joint replacement can temporarily alter patients’ views of the acceptability, necessity and value of pain relief medication around the time of surgery. Once recovery from surgery has started, then long-standing beliefs about appropriate use of pain relief medications may take prominence. This alteration is related to views about pain due to surgical intervention in contrast with interpretation of pain from living with long-term osteoarthritis. Importantly, views about pain medication use are influenced by interactions with health-care professionals.
Rehabilitation
Interviews highlighted how patients value the offer of postoperative physiotherapy shortly after surgery as well as longer-term follow-up in secondary care. The latter may be of particular value for those patients who experience complications after surgery or who lack confidence in their prosthesis.
In our survey of physiotherapy provision at high-volume orthopaedic centres in England and Wales, we found that physiotherapy following discharge after TKR was a more common practice than after THR. For patients following TKR, the focus was on knee-specific strengthening, stretching and functional exercises provided in a group setting.
In our systematic review and meta-analysis there was a suggestion that patients who received a programme of post-discharge physiotherapy exercise after TKR achieved short-term improvements in physical function and pain compared with controls (see Chapter 11). 585 However, this was based on a small number of studies with low numbers of patients randomised. There was no evidence, again from a few small studies, for better longer-term recovery in patients receiving physiotherapy exercise. Regarding provision at home, further research is needed to establish equivalence or additional benefit in comparison with that provided in an outpatient setting.
In the ARENA study, we assessed the feasibility of a randomised trial to evaluate a 6-week individualised and task-orientated exercise class. The inclusion of individualised exercises specific to each patient’s own concerns aimed to increase self-efficacy and to empower patients to take an active role in their rehabilitation. The group physiotherapy exercise class was generally well received and attendance and satisfaction were high. The main reasons for non-participation in the study were travel related and the inability to commit to the intervention. The ARENA study suggested that a fully powered RCT of individualised and task-orientated exercises would be feasible in patients with TKR.
Potential value of a complex package of care
In the RESTORE programme we have identified key time points when specific interventions merit evaluation in fully powered RCTs. Once there is evidence in place about the clinical effectiveness and cost-effectiveness of specific interventions, their value may be greatest if implemented as a complex intervention.
In the care of older people, a complex intervention can be regarded as ‘a combination of interdisciplinary teamwork for health and social problems’. 634 According to the UK MRC framework, a complex intervention has a number of interacting components, a number of behaviours and level of difficulty required by those delivering or receiving the intervention, a number of groups or organisational levels targeted, and a number and variability of outcomes. 94 A degree of flexibility or tailoring of the intervention can be incorporated. Some published interventions have covered both the pre- and post-surgical rehabilitation periods. For example, Gilbey and colleagues494 and Mitchell and colleagues520 demonstrated the feasibility of physiotherapy exercise in total hip and knee replacement patients, respectively.
Our research suggests potential value for a number of components for incorporation in a complex intervention:
-
pre-surgical education on the patient pathway, pain management, psychological counselling and advice or interventions relating to maintenance or improvement of physical function
-
pre-surgical health check to ensure appropriate management of health conditions
-
occupational therapy provided before surgery at home visit
-
evidence-based perioperative pain management
-
post-discharge physiotherapy with a patient choice element.
For patients, interventions embedded within a care pathway might be more acceptable than those provided exclusively before surgery when the perception is that little can be achieved, or after a care pathway when, for the majority of patients, a degree of improved function and pain relief have been achieved by the joint replacement itself.
Conclusions and recommendations
Future research is needed to develop consensus and standardisation on which pain domains should be assessed after TKR. For assessment of functional outcomes, researchers should consider a patient-reported outcome and a performance test.
Large-scale randomised evaluations in patients receiving total hip and knee replacements are feasible and their value is recognised by patients and health-care professionals. Health-care professionals want randomised trials to have minimal impact on daily clinical practice and patients want data collection and participation to be as easy as possible.
Local anaesthetic infiltration as evaluated in the APEX RCT is effective in reducing the incidence of long-term pain after THR and is also cost-effective. In TKR, there may be limited benefit for improved pain control if a FNB has already been sited.
We have shown that a group-based activity-orientated physiotherapy intervention is both feasible and acceptable to patients who have received a TKR. Further research is indicated to evaluate its clinical effectiveness and cost-effectiveness in an appropriately powered randomised evaluation.
Future research should evaluate the clinical effectiveness and cost-effectiveness of providing aids, home modifications and other aspects of occupational therapy to patients before their hospital admission for THR. We have shown this to be both feasible and acceptable to patients. Background research is required to support similar interventions in TKR.
Research should explore the possibility that optimising management of comorbid health conditions may improve outcomes for patients with total hip and knee replacement. In the first instance, this will require up-to-date systematic reviews of health management interventions and ultimately RCTs to evaluate the clinical effectiveness and cost-effectiveness of optimising the treatment of comorbid conditions. Provision of education, exercise and counselling before surgery may not be acceptable to a large proportion of patients waiting for total hip or knee replacement.
Ultimately, the optimal care for patients receiving total hip and knee replacement may be a complex intervention covering all stages of the patient pathway with the incorporation of patient choice and individualisation of therapies.
Acknowledgements
Management of the RESTORE programme
Ultimate responsibility for management of the programme lay with the PI, Professor Ashley Blom. Professor Paul Dieppe and Dr Rachael Gooberman-Hill were deputy PIs for the programme and provided significant input into the management, co-ordination and strategic development of all the work packages. Most of the staff recruitment was led by Dr Rachael Gooberman-Hill with input from North Bristol Research and Innovation into all NHS posts. The programme was co-ordinated by Dr Vikki Wylde with the help of Mrs Louise Hawkins. Three monthly programme meetings were held for all the coapplicants. The entire programme was hosted by North Bristol NHS Trust with the exception of the occupational therapy work package which was undertaken in two hospitals in the West Midlands. Subcontracts with the Universities of Bristol, Exeter, Birmingham and East Anglia facilitated shared working between the NHS and University academia. Individual work packages were led by Mr Andrew Beswick (systematic review), Dr Rachael Gooberman-Hill (qualitative studies and public/patient involvement), Professor Ashley Blom (APEX and ADAPT), Dr Vikki Wylde (pain management), Dr Neil Artz (physiotherapy), Professor Catherine Sackley (occupational therapy) and Dr Sian Noble (health economics). Assembly and writing of the final programme report was co-ordinated by Mr Andrew Beswick.
All trials were monitored regularly by the Research and Innovation department and APEX was inspected by the Medicines and Healthcare products Regulatory Agency as part of the routine inspection of North Bristol NHS Trust. Furthermore, all trials were monitored by independent Trial Steering Committees and APEX was additionally monitored by an independent data monitoring committee. Regular meetings were held for each separate trial by the research staff involved. In addition, we circulated monthly reports of progress in patient recruitment and outcomes.
Contributions of authors
Ashley W Blom (Professor, Orthopaedic Surgery) was the PI of this research programme and research lead for APEX and ADAPT. Contributed to all aspects of the research from inception to writing of the report.
Neil Artz (Lecturer, Physiotherapy) was the research lead for ARENA and the physiotherapy survey.
Andrew D Beswick (Research Fellow, Systematic Reviews) was a coapplicant on the grant application and research lead for the systematic reviews. Supervised design, conduct and analysis of studies within the research programme. Coordinated writing of the final report.
Amanda Burston (Research Assistant, PPI) contributed to all aspects of PPI.
Paul Dieppe (Professor, Health Services Research) was a coapplicant on the grant application and contributed to the research programme, particularly the ADAPT study, from inception to writing of the report.
Karen T Elvers (Research Associate, Systematic Reviews) contributed to the design, conduct and analysis of the systematic reviews.
Rachael Gooberman-Hill (Reader, Health Services Research) was a coapplicant on the grant application and research lead for the qualitative studies and PPI. Supervised design, conduct and analysis of studies within the research programme.
Jeremy Horwood (Senior Research Fellow, Qualitative Research) was a coapplicant on the grant application and contributed to the design, conduct and analysis of the qualitative studies.
Paul Jepson (Clinical Tutor in Physiotherapy, Physiotherapy) contributed to the design, conduct and analysis of the PROOF-THR study and occupational therapy systematic review.
Emma Johnson (Research Associate, Qualitative Research) contributed to the design, conduct and analysis of the qualitative studies.
Erik Lenguerrand (Research Fellow, Statistics) contributed to the statistical analysis of studies.
Elsa Marques (Research Associate, Health Economics) contributed to the design, conduct and analysis of the health economics work and systematic reviews.
Sian Noble (Senior Lecturer, Health Economics) was a coapplicant on the grant application and research lead for health economics.
Mark Pyke (Consultant Anaesthetist, Anaesthetics) was a coapplicant on the grant application and contributed to the design, conduct and interpretation of studies within the research programme.
Catherine Sackley (Professor, Rehabilitation) was a coapplicant on the grant application and research lead for PROOF-THR.
Gina Sands (Senior Research Associate, Rehabilitation) contributed to the conduct and analysis of the PROOF-THR study.
Adrian Sayers (Research Fellow, Statistics) contributed to the statistical analysis of data.
Victoria Wells (Former Patient) contributed patient and public perspective to all the research studies.
Vikki Wylde (Research Fellow, Health Services Research) was a coapplicant on the grant application, overall programme co-ordinator, research lead for SPIRAL and trial manager for APEX. Contributed to the design, conduct and analysis of studies within the research programme.
All authors contributed to the writing of the report.
Thanks to collaborators and contributors
Particular thanks go out to the patients who participated in the RESTORE RCTs and cohort studies, and to the patients and health-care professionals who we interviewed.
We would like to give special thanks for contributions to the RESTORE programme to Andy Judge, Anthony Sack, Avril Drummond, Bernd Grimm, Beverley Evanson, Caroline Roper, Catherine Minns Lowe, Cindy Mann, Constance Jamera, David Rea, Debbie Delgado, Diana Pratt, Ed Davies, Emma Clark, Emma Gendall, Hayley Jones, Ian Learmonth, James Berstock, Jennifer Tyler, Jon Tobias, Julia Edwards, Julie Bruce, Koye Odutola, Laura Miller, Leigh Morrison, Louise Hawkins, Luke Brunton, Mike Whitehouse, Natalija Stefanovich-Lawbuary, Nick Howells, Phil Baker, Pippa English-Penfold, Rachel Haynes, Samantha Dixon, Sara Brooks, Sharon Nolan, Sophie Whitcombe, Stijn Bolink, Sue Bowman, Sue Evans, Tim Peters, Toby Smith and Will Hollingsworth.
We would like to express particular thanks to the patient partners who have contributed to the PEP-R group and ultimately to the successful delivery of the RESTORE programme.
We acknowledge the support of the NIHR, through the Comprehensive Clinical Research Network.
Thanks also go to all the consultant orthopaedic surgeons and anaesthetists who contributed to the RESTORE programme, with special thanks to Gordon Bannister, John Church, Steve Eastaugh-Waring, Alan Gibson, Williams Harries, Paul Harvie, Katherine Jenkins, Michael Kelly, Nick Koehli, John Leigh, Sanchit Mehendale, Ronelle Mouton, Andrew Porteous and Jason Webb.
We would like to thank members of the APEX data monitoring committee (Paul Ewings, Neville Goodman and Adrian Taylor), APEX trial steering committee (Alan Montgomery, David Marsh, Julie Chappell and Cathy Stannard) and ADAPT steering committee (Shea Palmer and John Collins).
We would also like to thank Nigel Arden and the COAST programme team.
We are grateful for the support of Nicola Williams and colleagues at Research and Innovation, North Bristol NHS Trust, and also to the University of Bristol, University of Birmingham, University of East Anglia and the University of Exeter.
We would like to thank the authors of research studies who provided additional information for our systematic reviews: Aleksandra Vukomanovic, Alison Harmer, Alison McGregor, Ann Swank, Anna-Maija Kauppila, Brian Mockford, Carol Mancuso, Caroline Mitchell, Clare Sandell, Constant Busch, Daniel Rooks, Damien Bennett, Damien Byrne, Deborah McDonald, Gary Minto, Georgios Evgeniadis, Helen Frost, In Jun Koh, Janine-Sophie Giraudet LeQuintrec, Karen Toftdahl Bjørnholdt, Kirsi Johansson, Martin Thorsell, Michael Dewey, Michel Tousignant, Montserrat Núñez, Osamu Aoki, Per Essving, Per Wretenberg, Peiliang Fu, Rohan Rajan, Sara Piva, Simona Ferrante, Thomas Hoogeboom, Thoralf Leibs, Tim Ackland, Torben Bæk Hansen, Vera Fung, Yi ChenYun-Li Zhu and Yvette Bulthius.
Publications
APEX364,365,367,472,473
Wylde V, Gooberman-Hill R, Horwood J, Beswick A, Noble S, Brookes S, et al. The effect of local anaesthetic wound infiltration on chronic pain after lower limb joint replacement: a protocol for a double-blind randomised controlled trial. BMC Musculoskelet Disord; 2011;12:53.
Wylde V, Lenguerrand E, Gooberman-Hill R, Beswick AD, Marques E, Noble S, et al. Effect of local anaesthetic infiltration on chronic postsurgical pain after total hip and knee replacement: the APEX randomised controlled trials. Pain 2015;156:1161–70.
Wylde V, Sayers A, Lenguerrand E, Gooberman-Hill R, Pyke M, Beswick AD, et al. Preoperative widespread pain sensitization and chronic pain after hip and knee replacement: a cohort analysis. Pain 2015;156:47–54.
Marques E, Blom AW, Lenguerrand E, Wylde V, Noble SM. Local anaesthetic wound infiltration in addition to standard anaesthetic regimen in total hip and knee replacement: long-term cost-effectiveness analyses alongside the APEX randomised controlled trials. BMC Med 2015;13:151.
Sayers A, Wylde V, Lenguerrand E, Beswick AD, Gooberman-Hill R, Pyke M, et al. Rest pain and movement-evoked pain as unique constructs in hip and knee replacements. Arthritis Care Res 2016;68:237–45.
ADAPT262–265
Wylde V, Blom AW, Bolink S, Brunton L, Dieppe P, Gooberman-Hill R, et al. Assessing function in patients undergoing joint replacement: a study protocol for a cohort study. BMC Musculoskelet Disord 2012;13:220.
Wylde V, Lenguerrand E, Brunton L, Dieppe P, Gooberman-Hill R, Mann C, et al. Does measuring the range of motion of the hip and knee add to the assessment of disability in people undergoing joint replacement? Orthop Traumatol Surg Res 2014;100:183–6.
Bolink SAAN, Brunton LR, van Laarhoven S, Lipperts M, Heyligers IC, Blom AW, et al. Frontal plane pelvic motion during gait captures hip osteoarthritis related disability. Hip Int 2015;25:413–19.
Lenguerrand E, Wylde V, Gooberman-Hill R, Sayers A, Brunton L, Beswick AD, et al. Trajectories of Pain and Function after Primary Hip and Knee Arthroplasty: the ADAPT Cohort Study. PLOS ONE 2016;11:e0149306.
Patient experience229,230,363
Johnson E, Horwood J, Gooberman-Hill R. Patients’ journeys through total joint replacement: patterns of medication use. Musculoskeletal Care 2014;12:92–102.
Johnson EC, Horwood J, Gooberman-Hill R. Conceptualising time before surgery: the experience of patients waiting for hip replacement. Soc Sci Med 2014;116:126–33.
Horwood J, Johnson E, Gooberman-Hill R. Understanding involvement in surgical orthopaedic randomized controlled trials: a qualitative study of patient and health professional views and experiences. Int J Orthop Trauma Nurs 2016;20:3–12.
Systematic reviews18,95,366,585,635
Beswick A, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report pain after hip and knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2012;2:e000435.
Wylde V, Bruce J, Beswick A, Elvers K, Gooberman-Hill R. The assessment of chronic post-surgical pain after knee replacement: a systematic review. Arthritis Care Res 2013;65:1795–803.
Marques E, Jones H, Elvers K, Pyke M, Blom AW, Beswick A. Local anaesthetic infiltration for perioperative pain control in total hip and knee replacement: systematic review and meta-analyses of short- and long-term effectiveness. BMC Musculoskelet Disord 2014;15:220.
Jepson P, Beswick A, Smith TO, Sands G, Drummond A, Davis ET, et al. Assistive devices, hip precautions, environmental modifications and training to prevent dislocation and improve function after hip arthroplasty (protocol). Cochrane Database Syst Rev 2013;11:CD010815.
Artz N, Elvers KT, Lowe CM, Sackley C, Jepson P, Beswick AD. Effectiveness of physiotherapy exercise following total knee replacement: systematic review and meta-analysis. BMC Musculoskelet Disord 2015;16:15.
SPIRAL536
Wylde V, Marques E, Artz N, Blom AW, Gooberman-Hill. Effectiveness and cost-effectiveness of a group-based pain self-management intervention for patients undergoing total hip replacement: feasibility study for a randomised controlled trial. Trials 2014;15:176.
ARENA/physiotherapy survey78,586
Artz N, Dixon S, Wylde V, Beswick A, Blom AW, Gooberman-Hill R. Physiotherapy provision following discharge after total hip and total knee replacement: a survey of current practice at high volume NHS hospitals in England and Wales. Musculoskeletal Care 2013;11:31–8.
Artz N, Dixon S, Wylde V, Marques E, Beswick AD, Lenguerrand E, et al. Comparison of group-based outpatient physiotherapy with usual care after total knee replacement: a feasibility study for a randomized controlled trial. Clin Rehabil 2016;10:epub ahead of print 11 April.
PROOF-THR562
Jepson P, Sands G, Beswick AD, Davis ET, Blom AW, Sackley CM. A feasibility randomised controlled trial of pre-operative occupational therapy to optimise recovery for patients undergoing primary total hip replacement for osteoarthritis (PROOF-THR). Clin Rehabil 2015;30:156–66.
Research methods368
Mann C, Delgado D, Horwood J. Discussion and qualitative evaluation of internal peer-review to train nurses recruiting to a randomised controlled trial – internal peer-review for recruitment training in trials (InterPReTiT). J Adv Nurs 2014;70:777–90.
Health economic studies432
Marques E, Johnson EC, Gooberman-Hill R, Blom AW, Noble S. Using resource use logs to reduce the amount of missing data in economic evaluations alongside trials. Value Health 2013;16:195–201.
Patient and public involvement636
Gooberman-Hill R, Burston A, Clark E, Johnson E, Nolan S, Wells V, et al. Involving patients in research: considering good practice. Musculoskeletal Care 2013;11:187–90.
Others637–639
Wylde V, Jeffery A, Dieppe P, Gooberman-Hill R. The assessment of persistent pain after joint replacement. Osteoarthritis Cart 2012;20:102–5.
Brunton L, Wylde V, Dieppe P. Assessing the health status of people with arthritis: does the research help the clinician? The example of osteoarthritis of the knee. Rheumatology 2012;51:1143–4.
Marques E, Noble S, Blom AW, Hollingworth W. Disclosing total waiting times for joint replacement: Evidence from the English NHS using linked HES data. Health Econ 2014;23:806–20.
Conference presentations and posters
APEX
Wylde V, Lenguerrand E, Gooberman-Hill R, Beswick A, Marques E, Noble S, et al. The effect of intra-operative local wound infiltration on chronic pain after total hip replacement: a randomised controlled trial. Paris: OARSI conference; April 2014.
Wylde V, Lenguerrand E, Gooberman-Hill R, Beswick A, Marques E, Noble S, et al. The effect of local anaesthetic infiltration on chronic post-surgical pain after total hip and knee replacement: the APEX randomised controlled trials. Milan: European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis; March 2015.
ADAPT
Lenguerrand E, Wylde V, Brunton L, Gooberman-Hill R, Dieppe P, Blom AW. Measuring physical function among patients with lower limb joint impairment. Bristol: Population Health Early Career Researchers’ Event; June 2013.
Brunton L, Bolink S, Grimm B, Blom A. A novel gyrometer based assessment of the clinical orthopaedic single leg stance ‘trendelenburg test’. Glasgow: Second International Conference on Ambulatory Monitoring of Physical Activity and Movement; May 2011.
Bolink S, Brunton L, van Laarhoven, Lipperts M, Grimm B, Heyligers IC, et al. Inertial sensor based gait analysis: a clinical application in patients with osteoarthritis. Barcelona: Osteoarthritis Research Society International; April 2012.
Lenguerrand E, Wylde V, Brunton L, Gooberman-Hill R, Blom AW, Dieppe P. The need for caution in the selection and interpretation of measures of function for patients with severe hip and knee problems. Paris: Osteoarthritis Research Society International conference; April 2014.
Lenguerrand E, Wylde V, Sayers A, Beswick AD, Brunton L, Gooberman-Hill R, et al. Pain recovery after hip and knee replacement: a longitudinal study. Milan: European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis; March 2015.
Patient experience
Johnson E, Horwood J, Gooberman-Hill R. ‘I don’t like pill popping’: changes in patients’ attitudes and behaviours towards pain relief medication at the time of total joint replacement. Edinburgh: British Pain Society conference; June 2011.
Johnson E, Gooberman-Hill R, Horwood J. ‘I might as well, better than doing a crossword’: patients’ motivations for, and experience of, participation in an orthopaedic randomised controlled trial (RCT). Bristol: North Bristol Trust Research Day; November, 2011.
Johnson E, Gooberman-Hill R, Horwood J. ‘I might as well, better than doing a crossword’: patients’ motivations for, and experience of, participation in an orthopaedic randomised controlled trial (RCT). Bristol: Population Health Symposium; November 2011.
Johnson E, Horwood J, Gooberman-Hill R. Experiences of delay and cancellation in the secondary care pathway to total hip replacement: a qualitative study. Bristol: Population Health Annual Symposium; November 2012.
Johnson E, Horwood J, Gooberman-Hill R. Understanding the experience of time while waiting for hip replacement surgery. Liverpool: British Rheumatology Society Conference; April 2014.
Gooberman-Hill R, Horwood J, Johnson E. Waiting for hip replacement: patients’ experiences of time. Paris: Osteoarthritis Research Society International conference; April 2014.
Gooberman-Hill R, Horwood J, Johnson E. Psychological aspects of hip replacement: a longitudinal qualitative study of patients’ experiences. Milan: European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis; March 2015.
Systematic reviews
Beswick A, Dieppe P, Wylde V, Gooberman-Hill R, Blom A. What proportion of patients report pain after total hip or knee arthroplasty for osteoarthritis? A systematic review of prospective studies in unselected patients. Chicago, IL: American College of Rheumatology Annual Scientific Meeting; November 2011.
Elvers K, Stefanovich-Lawbuary N, Artz N, Gooberman-Hill R, Blom AW, Beswick A. Pre-surgical interventions to optimise functional health before hip and knee arthroplasty. A systematic review of randomised controlled trials. Berlin: European Federation of National Associations of Orthopaedics and Traumatology; May 2012.
Beswick A, Wylde V, Gooberman-Hill R, Blom AW, Dieppe P. What proportion of patients report long-term pain after total hip or knee arthroplasty for osteoarthritis? A systematic review of prospective studies in unselected patients. Berlin: European Federation of National Associations of Orthopaedics and Traumatology; May 2012.
Wylde V, Bruce J, Beswick A, Elvers K, Gooberman-Hill R. The assessment of chronic pain after knee replacement: a systematic review. Bournemouth: British Pain Society Annual Scientific Conference; April 2013.
Wylde V, Bruce J, Beswick A, Elvers K, Gooberman-Hill R. The assessment of chronic pain after knee replacement: a systematic review. Bristol: Population Health Early Career Researchers’ Event; June 2013.
Wylde V, Bruce J, Beswick A, Elvers K, Gooberman-Hill R. The assessment of chronic pain after knee replacement: a systematic review. Manchester: COMET conference; June 2013.
SPIRAL
Wylde V and Gooberman-Hill R. A pain management intervention for postoperative pain after hip replacement: barriers to trial participation. Bristol: Population Health Early Career Researchers Event; June 2012.
Wylde V and Gooberman-Hill R. A pain management intervention for postoperative pain after hip replacement: barriers to trial participation. Bristol: Population Health Annual Symposium; November 2012.
Wylde V, Marques E, Artz N, Blom AW, Gooberman-Hill R. Effectiveness and cost-effectiveness of a group-based pain self-management intervention for patients undergoing total hip replacement: feasibility study for a randomised controlled trial. Manchester: British Pain Society Annual Scientific Meeting; April 2014.
Wylde V, Marques E, Artz N, Blom AW, Gooberman-Hill R. Effectiveness and cost-effectiveness of a group-based pain self-management intervention for patients undergoing total hip replacement: feasibility study for a randomised controlled trial. Paris: Osteoarthritis Research Society International conference; April 2014.
ARENA/physiotherapy survey
Wylde V, Artz N, Dixon S, Marques E, Lenguerrand E, Blom AW, et al. Effectiveness and cost-effectiveness of a group-based outpatient physiotherapy intervention following knee replacement for osteoarthritis: feasibility study for a randomised controlled trial. Paris: Osteoarthritis Research Society International conference; April 2014.
Dixon S, Artz N, Wylde V, Beswick A, Blom AW, Gooberman-Hill R. Physiotherapy provision following discharge after total hip and total knee replacement: a survey of current practice at high volume NHS hospitals in England and Wales. Bristol: Population Health Early Career Researchers Event; June 2014.
Artz, N. Rehabilitation following total knee replacement. Bristol: Arthroplasty Care Practitioner’s Association annual conference; February 2013.
PROOF-THR
Jepson P, Hoppitt T, Beswick A, Wylde V, Sackley C. A pilot randomised controlled trial of pre-surgery occupational therapy intervention versus usual care to optimise recovery for patients undergoing primary total hip replacement for osteoarthritis. South West Society for Academic Primary Care Annual meeting, Devon: Society for Academic Primary Care Annual meeting, March 2012.
Jepson P, Sands G, Beswick A, Wylde V, Gravelle R, Sackley C. A pilot randomised controlled trial of occupational therapy to optimise recovery for patients undergoing primary total hip replacement for osteoarthritis (PROOF). Glasgow: Society for Research in Rehabilitation conference; June 2014.
Research methods
Gooberman-Hill R, Beswick A, Mann C, Johnson E, Horwood J, Blom A, et al. Enhancing the process of a clinical trial in osteoarthritis with embedded qualitative approaches. Belgium: Osteoarthritis Research Society International; September 2010.
Delgado D, Mann C. The importance of effective interpersonal and communication skills in order to maximise the buy-in of clinical staff: an experience of running a large RCT in a hospital setting. Harrogate: Annual Royal College of Nursing International Research Conference; May 2011.
Mann C, Gendall E, Morrison L, Delgado D. Peer review to enhance recruitment procedures in a large randomised controlled trial. Harrogate: Annual Royal College of Nursing International Research Conference; May 2011.
Mann C, Gendall E, Morrison L, Delgado D. Peer review to enhance recruitment procedures in a large randomised controlled trial (APEX). Bristol: North Bristol Trust Research Day; November 2011.
Lenguerrand E, Wylde V, Gooberman-Hill R, Noble S, Marques E, Dieppe P, et al. Trial data management: lessons from a large orthopaedic trial. Edinburgh: Second Clinical Trials Methodology Conference; November 2013.
Health economic methods
Noble S, Marques E, Johnson E, Blom A. The use of participant resource use logs in reducing the amount of missing data in resource use questionnaires. Toronto, ON: iHEA 8th World Congress on Health Economics; July 2011.
Noble S, Marques E, Johnson E, Blom A. The use of participant resource use logs in reducing the amount of missing data in resource use questionnaires. Bristol: MRC Clinical Trials Methodology Conference; October 2011.
Marques E, Johnson E, Gooberman-Hill R, Blom AW, Noble S. Using resource use logs to reduce the amount of missing data in economic evaluations alongside trials. Bristol: South West Health economists meeting; May 2012.
Patient and public involvement
Gooberman-Hill R, Burston A, Lenguerrand E, Clark E, Johnson E, PEP-R group. Patient involvement in clinical research: a qualitative evaluation of impact. Edinburgh: Second Clinical Trials Methodology Conference; November 2013.
Gooberman-Hill R, Burston A, Clark E, Johnson E, Nolan S, Wells V, et al. Evaluation of a patient involvement activity in musculoskeletal health research: impact on patients and researchers. Exeter: Consolidating the evidence base for public involvement in health services research event; November 2013.
Burston A, Gooberman-Hill R, Johnson E, Wells V, Clark E, Nolan S, et al. Patient involvement in musculoskeletal health research: a qualitative evaluation of impact. Liverpool: British Rheumatology Society Conference; April 2014.
Burston A. Panel member for workshop ‘Public involvement beyond health research’. Bristol: National Co-ordinating centre for Public Engagement Annual conference; November 2013.
Others
Marques E, Noble S, Blom A, Hollingworth W. Disclosing total waiting times: clinical pathways for patients awaiting total joint replacement. York: Health Economists Study Group; January 2011.
Marques E, Noble S, Blom A.W. and Hollingworth W. Hidden waiting times for total joint replacement: a study of linked HES data. Bristol: Population Health Symposium; November 2011.
Beswick A, Gooberman-Hill R, Horwood J, Wylde V, Marques E, Blom A, on behalf of the RESTORE study group. The RESTORE programme. Bristol: Population Health Symposium; September 2010.
Beswick A, Elvers K, Gooberman- Hill R, Smith A, Blom A. What is the evidence to guide surgical treatment of infected hip prostheses? Bath: Bath biomechanics symposium; September 2011.
RESTORE team. The RESTORE programme. Amsterdam: European Orthopaedic Research Society Conference; September 2012.
Gooberman-Hill, R. Arthritis Care working with The Musculoskeletal Research Unit. Bristol: Arthritis Care AGM; June 2011.
Horwood J, on behalf of the RESTORE group. The RESTORE programme. Bristol: WLCRN Musculoskeletal specialty meeting; October 2011.
Wylde V. Orthopaedic health services research at the Musculoskeletal Research Unit. Bristol: School of Clinical Sciences Showcase Event; May 2013.
Study feedback reports for patients
Study feedback reports for patients are included in Appendix 38.
Data sharing statement
Requests for research collaborations based on data collected in the RESTORE programme should be addressed to the programme corresponding author. Subject to ethical approval when required, and the appropriateness of analyses proposed, the authors are committed to the sharing of data, statistical code and research methods.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health.
References
- Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis 2001;60:91-7. http://dx.doi.org/10.1136/ard.60.2.91.
- Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ 2003;81:646-56.
- National Joint Registry for England and Wales: 10th Annual Report. Hemel Hempstead: NJR Centre; 2013.
- Thorstensson C, Gooberman-Hill R, Adamson J, Williams S, Dieppe P. Help-seeking behaviour among people living with chronic hip or knee pain in the community. BMC Musculoskelet Disord 2009;10. http://dx.doi.org/10.1186/1471-2474-10-153.
- Sanders C, Donovan J, Dieppe P. The significance and consequences of having painful and disabled joints in older age: co-existing accounts of normal and disrupted biographies. Sociol Health Illn 2002;24:227-53. http://dx.doi.org/10.1111/1467-9566.00292.
- Gignac MA, Davis AM, Hawker G, Wright JG, Mahomed N, Fortin PR, et al. ‘What do you expect? You’re just getting older’: a comparison of perceived osteoarthritis-related and aging-related health experiences in middle- and older-age adults. Arthritis Rheum 2006;55:905-12. http://dx.doi.org/10.1002/art.22338.
- Jordan K. What do general practitioners see? Musculoskeletal Matters Bulletin 1. Staffordshire: Arthritis Research UK Primary Care Centre; 2009.
- Neogi T, Zhang Y. Epidemiology of osteoarthritis. Rheum Dis Clin North Am 2013;39:1-19. http://dx.doi.org/10.1016/j.rdc.2012.10.004.
- Kadam UT, Jordan K, Croft PR. Clinical comorbidity in patients with osteoarthritis: a case–control study of general practice consulters in England and Wales. Ann Rheum Dis 2004;63:408-14. http://dx.doi.org/10.1136/ard.2003.007526.
- Reeuwijk KG, de Rooij M, van Dijk GM, Veenhof C, Steultjens MP, Dekker J. Osteoarthritis of the hip or knee: which coexisting disorders are disabling?. Clin Rheumatol 2010;29:739-47. http://dx.doi.org/10.1007/s10067-010-1392-8.
- Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cart 2008;16:137-62. http://dx.doi.org/10.1016/j.joca.2007.12.013.
- Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JWJ, Dieppe P, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the standing committee for international clinical studies including therapeutic trials (ESCISIT). Ann Rheum Dis 2003;62:1145-55. http://dx.doi.org/10.1136/ard.2003.011742.
- Zhang W, Doherty M, Arden N, Bannwarth B, Bijlsma J, Gunther K-P, et al. EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR standing committee for international clinical studies including therapeutics (ESCISIT). Ann Rheum Dis 2005;64:669-81. http://dx.doi.org/10.1136/ard.2004.028886.
- Osteoarthritis: National Clinical Guideline for Care and Management in Adults. London: NICE; 2008.
- Liang MH, Cullen KE, Larson MG, Thompson MS, Schwartz JA, Fossel AH, et al. Cost-effectiveness of total joint arthroplasty in osteoarthritis. Arthritis Rheum 1986;29:937-43. http://dx.doi.org/10.1002/art.1780290801.
- Chang RW, Pellisier JM, Hazen GB. A cost-effectiveness analysis of total hip arthroplasty for osteoarthritis of the hip. JAMA 1996;275:858-65. http://dx.doi.org/10.1001/jama.1996.03530350040032.
- Dreinhofer KE, Dieppe P, Sturmer T, Grober-Gratz D, Floren M, Gunther KP, et al. Indications for total hip replacement: comparison of assessments of orthopaedic surgeons and referring physicians. Ann Rheum Dis 2006;65:1346-50. http://dx.doi.org/10.1136/ard.2005.047811.
- Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2012;2. http://dx.doi.org/10.1136/bmjopen-2011-000435.
- Health and Social Care Information Centre . Hospital Episode Statistics, Main Procedures and Interventions: 4 Character Table 2001–02 2002. www.hscic.gov.uk/article/2021/Website-Search?productid=3651&q=4+character+table+2001-02&sort=Relevance&size=10&page=1&area=both#top (accessed 21 July 2016).
- Health and Social Care Information Centre . Hospital Episode Statistics, Main Procedures and Interventions: 4 Character Table 2011–12 2012. www.hscic.gov.uk/catalogue/PUB08288 (accessed 21 July 2016).
- Dixon T, Shaw M, Ebrahim S, Dieppe P. Trends in hip and knee joint replacement: socioeconomic inequalities and projections of need. Ann Rheum Dis 2004;63:825-30. http://dx.doi.org/10.1136/ard.2003.012724.
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007;89–A:780-5. http://dx.doi.org/10.2106/JBJS.F.00222.
- Torjesen I. NHS is unlikely to meet Nicholson challenge to deliver £20bn in efficiency savings, says King’s Fund. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e6496.
- Gooberman-Hill R, Woolhead G, Mackichan F, Ayis S, Williams S, Dieppe P, et al. Assessing chronic joint pain: lessons from a focus group study. Arthritis Rheum 2007;57:666-71. http://dx.doi.org/10.1002/art.22681.
- Gooberman-Hill R, French M, Dieppe P, Hawker G, Gooberman-Hill R, French M, et al. Expressing pain and fatigue: a new method of analysis to explore differences in osteoarthritis experience. Arthritis Rheum 2009;61:353-60. http://dx.doi.org/10.1002/art.24273.
- Sale JE, Gignac M, Hawker G. How ‘bad’ does the pain have to be? A qualitative study examining adherence to pain medication in older adults with osteoarthritis. Arthritis Rheum 2006;55:272-8. http://dx.doi.org/10.1002/art.21853.
- Milder TY, Lipworth WL, Williams KM, Ritchie JE, Day RO. ‘It looks after me’: how older patients make decisions about analgesics for osteoarthritis. Arthritis Care Res 2011;63:1280-6. http://dx.doi.org/10.1002/acr.20514.
- O’Neill T, Jinks C, Ong B. Decision-making regarding total knee replacement surgery: a qualitative meta-synthesis. BMC Health Serv Res 2007;7. http://dx.doi.org/10.1186/1472-6963-7-52.
- Sansom A, Donovan J, Sanders C, Dieppe P, Horwood J, Learmonth I, et al. Routes to total joint replacement surgery: patients’ and clinicians’ perceptions of need. Arthritis Care Res 2010;62:1252-7. http://dx.doi.org/10.1002/acr.20218.
- Woolhead GM, Donovan JL, Dieppe PA. Outcomes of total knee replacement: a qualitative study. Rheumatology 2005;44:1032-7. http://dx.doi.org/10.1093/rheumatology/keh674.
- Judge A, Cooper C, Arden NK, Williams S, Hobbs N, Dixon D, et al. Pre-operative expectation predicts 12-month postoperative outcome among patients undergoing primary total hip replacement in European orthopaedic centres. Osteoarthritis Cart 2011;19:659-67. http://dx.doi.org/10.1016/j.joca.2011.03.009.
- McHugh GA, Luker KA. Individuals’ expectations and challenges following total hip replacement: a qualitative study. Disabil Rehabil 2012;34:1351-7. http://dx.doi.org/10.3109/09638288.2011.644022.
- Aamodt A, Nordsletten L, Havelin LI, Indrekvam K, Utvåg SE, Hviding K. Documentation of hip prostheses used in Norway: A critical review of the literature from 1996–2000. Acta Orthop Scand 2004;75:663-76. http://dx.doi.org/10.1080/00016470410004021.
- Guidance on the Selection of Prostheses for Primary Total Hip Replacement. London: NICE; 2000.
- Havelin LI, Fenstad AM, Salomonsson R, Mehnert F, Furnes O, Overgaard S, et al. The Nordic arthroplasty register association: a unique collaboration between 3 national hip arthroplasty registries with 280,201 THRs. Acta Orthop 2009;80:393-401. http://dx.doi.org/10.3109/17453670903039544.
- Jain NB, Guller U, Pietrobon R, Bond TK, Higgins LD. Comorbidities increase complication rates in patients having arthroplasty. Clin Orthop Relat Res 2005;435:232-8. http://dx.doi.org/10.1097/01.blo.0000156479.97488.a2.
- Blom AW, Rogers M, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Dislocation following total hip replacement: the Avon Orthopaedic Centre experience. Ann R Coll Surg Engl 2008;90:658-62. http://dx.doi.org/10.1308/003588408X318156.
- Blom AW, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total hip arthroplasty: The Avon experience. J Bone Joint Surg Br 2003;85–B:956-9. http://dx.doi.org/10.1302/0301-620X.85B7.14095.
- Blom AW, Brown J, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total knee arthroplasty. J Bone Joint Surg Br 2004;86:688-91. http://dx.doi.org/10.1302/0301-620X.86B5.14887.
- Berry DJ. Epidemiology: hip and knee. Orthop Clin North Am 1999;30:183-90. http://dx.doi.org/10.1016/S0030-5898(05)70073-0.
- Lapidus L, Ponzer S, Pettersson H, de Bri E. Symptomatic venous thromboembolism and mortality in orthopaedic surgery – an observational study of 45 968 consecutive procedures. BMC Musculoskelet Disord 2013;14. http://dx.doi.org/10.1186/1471-2474-14-177.
- Smith TO, Blake V, Hing CB. Minimally invasive versus conventional exposure for total hip arthroplasty: a systematic review and meta-analysis of clinical and radiological outcomes. Int Orthop 2011;35:173-84. http://dx.doi.org/10.1007/s00264-010-1075-8.
- Berstock JR, Beswick AD, Lenguerrand E, Whitehouse MR, Blom AW. Mortality after total hip replacement surgery: a systematic review. Bone Joint Res 2014;3:175-82. http://dx.doi.org/10.1302/2046-3758.36.2000239.
- Parry MC, Smith AJ, Blom AW. Early death following primary total knee arthroplasty. J Bone Joint Surg Am 2011;93:948-53. http://dx.doi.org/10.2106/JBJS.J.00425.
- Wylde V, Blom AW. The failure of survivorship. J Bone Joint Surg Br 2011;93:569-70. http://dx.doi.org/10.1302/0301-620X.93B5.26687.
- Lingard EA, Katz JN, Wright EA, Sledge CB. Kinemax Outcomes Group . Predicting the outcome of total knee arthroplasty. J Bone Joint Surg Am 2004;86:2179-86.
- Núñez M, Núñez E, del Val JL, Ortega R, Segur JM, Hernández MV, et al. Health-related quality of life in patients with osteoarthritis after total knee replacement: factors influencing outcomes at 36 months of follow-up. Osteoarthritis Cart 2007;15:1001-7. http://dx.doi.org/10.1016/j.joca.2007.02.019.
- Bachmeier CJM, March LM, Cross MJ, Lapsley HM, Tribe KL, Courtenay BG, et al. A comparison of outcomes in osteoarthritis patients undergoing total hip and knee replacement surgery. Osteoarthritis Cart 2001;9:137-46. http://dx.doi.org/10.1053/joca.2000.0369.
- Wylde V, Hewlett S, Learmonth ID, Dieppe P. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain 2011;152:566-72. http://dx.doi.org/10.1016/j.pain.2010.11.023.
- Brander VA, Stulberg SD, Adams AD, Harden RN, Bruehl S, Stanos SP, et al. Predicting total knee replacement pain: a prospective, observational study. Clin Orthop Relat Res 2003;416:27-36. http://dx.doi.org/10.1097/01.blo.0000092983.12414.e9.
- Nikolajsen L, Brandsborg B, Lucht U, Jensen TS, Kehlet H. Chronic pain following total hip arthroplasty: a nationwide questionnaire study. Acta Anaesthesiol Scand 2006;50:495-500. http://dx.doi.org/10.1111/j.1399-6576.2006.00976.x.
- Judge A, Arden NK, Cooper C, Kassim Javaid M, Carr AJ, Field RE, et al. Predictors of outcomes of total knee replacement surgery. Rheumatology 2012;51:1804-13. http://dx.doi.org/10.1093/rheumatology/kes075.
- Hawker GA, Badley EM, Borkhoff CM, Croxford R, Davis AM, Dunn S, et al. Which patients are most likely to benefit from total joint arthroplasty?. Arthritis Rheum 2013;65:1243-52. http://dx.doi.org/10.1002/art.37901.
- Judge A, Cooper C, Williams C, Dreinhoefer K, Dieppe P. Patient-reported outcomes one year after primary hip replacement in a European collaborative cohort. Arthritis Care Res 2010;62:480-8. http://dx.doi.org/10.1002/acr.20038.
- Toms AD, Mandalia V, Haigh R, Hopwood B. The management of patients with painful total knee replacement. J Bone Joint Surg Br 2009;91:143-50. http://dx.doi.org/10.1302/0301-620X.91B2.20995.
- Kassam A, Dieppe P, Toms AD. An analysis of time and money spent on investigating painful total knee replacements. Br J Med Pract 2012;5.
- Hawker GA, Stewart L, French MR, Cibere J, Jordan JM, March L, et al. Understanding the pain experience in hip and knee osteoarthritis – an OARSI/OMERACT initiative. Osteoarthritis Cart 2008;16:415-22. http://dx.doi.org/10.1016/j.joca.2007.12.017.
- Jones CA, Voaklander DC, Johnston DWC, Suarez-Almazor ME. Health related quality of life outcomes after total hip and knee arthroplasties in a community based population. J Rheumatol 2000;27:1745-52.
- Bourne RB, Chesworth B, Davis A, Mahomed N, Charron K. Comparing patient outcomes after THA and TKA: is there a difference?. Clin Orthop Relat Res 2010;468:542-6. http://dx.doi.org/10.1007/s11999-009-1046-9.
- Pollard B, Johnston M, Dieppe P. What do osteoarthritis health outcome instruments measure? Impairment, activity limitation, or participation restriction?. J Rheumatol 2006;33:757-63.
- Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act 2008;5. http://dx.doi.org/10.1186/1479-5868-5-56.
- Dolin SJ, Cashman JN, Bland JM. Effectiveness of acute postoperative pain management: I. Evidence from published data. Br J Anaesth 2002;89:409-23. http://dx.doi.org/10.1093/bja/89.3.409.
- Duggleby W, Lander J. Cognitive status and postoperative pain: older adults. J Pain Symptom Manage 1994;9:19-27. http://dx.doi.org/10.1016/0885-3924(94)90142-2.
- Chung F, Ritchie E, Su J. Postoperative pain in ambulatory surgery. Anesth Analg 1997;85:808-16.
- Morrison RS, Magaziner J, McLaughlin MA, Orosz G, Silberzweig SB, Koval KJ, et al. The impact of postoperative pain on outcomes following hip fracture. Pain 2003;103:303-11. http://dx.doi.org/10.1016/S0304-3959(02)00458-X.
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet 2006;367:1618-25. http://dx.doi.org/10.1016/S0140-6736(06)68700-X.
- Wilmore DW, Kehlet H. Management of patients in fast track surgery. BMJ 2001;322:473-6. http://dx.doi.org/10.1136/bmj.322.7284.473.
- Thomas DC, Kreizman IJ, Melchiorre P, Ragnarsson KT. Rehabilitation of the patient with chronic critical illness. Crit Care Clin 2002;18:695-71. http://dx.doi.org/10.1016/S0749-0704(02)00011-8.
- Reilly KA, Beard DJ, Barker KL, Dodd CAF, Price AJ, Murray DW. Efficacy of an accelerated recovery protocol for Oxford unicompartmental knee arthroplasty – a randomised controlled trial. Knee 2005;12:351-7. http://dx.doi.org/10.1016/j.knee.2005.01.002.
- Wylde V, Rooker J, Halliday L, Blom A. Acute postoperative pain at rest after hip and knee arthroplasty: severity, sensory qualities and impact on sleep. Orthop Traumatol Surg Res 2011;97:139-44. http://dx.doi.org/10.1016/j.otsr.2010.12.003.
- Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, d’Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology 1999;91:8-15. http://dx.doi.org/10.1097/00000542-199907000-00006.
- Choi P, Bhandari M, Scott J, Douketis JD. Epidural analgesia for pain relief following hip or knee replacement. Cochrane Database Syst Rev 2003;3. http://dx.doi.org/10.1002/14651858.cd003071.
- International Association for the Study of Pain (IASP) . Classification of chronic pain. Pain 1986:1-226.
- Macrae W, Davies H, Crombie I. Epidemiology of Pain. Seattle: IASP Press; 1999.
- Wylde V. The Role of Pre-operative Pain Sensitisation in Chronic Pain after Total Knee Replacement. Bristol: University of Bristol; 2010.
- Macrae WA. Chronic pain after surgery. Br J Anaesth 2001;87:88-9. http://dx.doi.org/10.1093/bja/87.1.88.
- McMurray R, Heaton J, Sloper P, Nettleton S. Variations in the provision of occupational therapy for patients undergoing primary elective total hip replacement in the United Kingdom. Br J Occup Ther 2000;63:451-5. http://dx.doi.org/10.1177/030802260006300909.
- Artz N, Dixon S, Wylde V, Beswick A, Blom A, Gooberman-Hill R. Physiotherapy provision following discharge after total hip and total knee replacement: a survey of current practice at high-volume NHS hospitals in England and Wales. Musculoskeletal Care 2013;11:31-8. http://dx.doi.org/10.1002/msc.1027.
- Drummond A, Coole C, Brewin C, Sinclair E. Hip precautions following primary total hip replacement: a national survey of current occupational therapy practice. Brit J Occup Ther 2012;75:164-70. http://dx.doi.org/10.4276/030802212X13336366278059.
- International Classification of Functioning, Disability and Health. Geneva: WHO; 2001.
- Khan F, Ng L, Gonzalez S, Hale T, Turner-Stokes L. Multidisciplinary rehabilitation programmes following joint replacement at the hip and knee in chronic arthropathy. Cochrane Database Syst Rev 2008;2. http://dx.doi.org/10.1002/14651858.cd004957.pub3.
- Ackerman IN, Bennell KL. Does pre-operative physiotherapy improve outcomes from lower limb joint replacement surgery? A systematic review. Aust J Physiother 2004;50:25-30. http://dx.doi.org/10.1016/S0004-9514(14)60245-2.
- McDonald S, Hetrick SE, Green S. Pre-operative education for hip or knee replacement. Cochrane Database Syst Rev 2004;1. http://dx.doi.org/10.1002/14651858.cd003526.pub2.
- Minns Lowe CJ, Barker KL, Dewey M, Sackley CM. Effectiveness of physiotherapy exercise after knee arthroplasty for osteoarthritis: Systematic review and meta-analysis of randomised controlled trials. BMJ 2007;335:812-5. http://dx.doi.org/10.1136/bmj.39311.460093.BE.
- Minns Lowe C, Barker K, Dewey M, Sackley C. Effectiveness of physiotherapy exercise following hip arthroplasty for osteoarthritis: a systematic review of clinical trials. BMC Musculoskelet Disord 2009;10. http://dx.doi.org/10.1186/1471-2474-10-98.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Pitimana-aree S, Visalyaputra S, Komoltri C, Muangman S, Tiviraj S, Puangchan S, et al. An economic evaluation of bupivacaine plus fentanyl versus ropivacaine alone for patient-controlled epidural analgesia after total-knee replacement procedure: a double-blinded randomized study. Reg Anesth Pain Med 2005;30:446-51. http://dx.doi.org/10.1097/00115550-200509000-00005.
- Colwell CW, Morris BA. Patient-controlled analgesia compared with intramuscular injection of analgesics for the management of pain after an orthopaedic procedure. J Bone Joint Surg Am 1995;77:726-33.
- Gonano C, Leitgeb U, Sitzwohl C, Ihra G, Weinstabl C, Kettner SC. Spinal versus general anesthesia for orthopedic surgery: anesthesia drug and supply costs. Anesth Analg 2006;102:524-9. http://dx.doi.org/10.1213/01.ane.0000194292.81614.c6.
- Demeere JL, Merckx C, Demeere N. Cost minimisation and cost effectiveness in anaesthesia for total hip replacement surgery, in Belgium? A study comparing three general anaesthesia techniques. Acta Anaesthesiol Belg 2006;57:145-51.
- Strassels SA, Chen C, Carr DB. Postoperative analgesia: economics, resource use, and patient satisfaction in an urban teaching hospital. Anesth Analg 2002;94:130-7.
- Kauppila A-M, Sintonen H, Aronen P, Ohtonen P, Kyllonen E, Arokoski JPA. Economic evaluation of multidisciplinary rehabilitation after primary total knee arthroplasty based on a randomized controlled trial. Arthritis Care Res 2011;63:335-41.
- Tribe KL, Lapsley HM, Cross MJ, Courtenay BG, Brooks PM, March LM. Selection of patients for inpatient rehabilitation or direct home discharge following total joint replacement surgery: a comparison of health status and out-of-pocket expenditure of patients undergoing hip and knee arthroplasty for osteoarthritis. Chronic Illness 2005;1:289-302. http://dx.doi.org/10.1177/17423953050010041101.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337.
- Wylde V, Bruce J, Beswick A, Elvers K, Gooberman-Hill R. The assessment of chronic post-surgical pain after knee replacement: A systematic review. Arthritis Care Res 2013;65:1795-803. http://dx.doi.org/10.1002/acr.22050.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 2011. http://handbook.cochrane.org/ (accessed 21 July 2016).
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006-12. http://dx.doi.org/10.1016/j.jclinepi.2009.06.005.
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 2000;283:2008-12. http://dx.doi.org/10.1001/jama.283.15.2008.
- Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well-built clinical question: a key to evidence-based decisions. ACP J Club 1995;123:A12-3.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Contr Clin Trials 1986;7:177-88. http://dx.doi.org/10.1016/0197-2456(86)90046-2.
- Roberts VI, Esler CNA, Harper WM. A 15-year follow-up study of 4606 primary total knee replacements. J Bone Joint Surg Br 2007;89–B:1452-6. http://dx.doi.org/10.1302/0301-620X.89B11.19783.
- Quintana JM, Escobar A, Arostegui I, Bilbao A, Azkarate J, Goenaga JI, et al. Health-related quality of life and appropriateness of knee or hip joint replacement. Arch Int Med 2006;166:220-6. http://dx.doi.org/10.1001/archinte.166.2.220.
- Nilsdotter AK, Petersson IF, Roos EM, Lohmander LS. Predictors of patient relevant outcome after total hip replacement for osteoarthritis: A prospective study. Ann Rheum Dis 2003;62:923-30. http://dx.doi.org/10.1136/ard.62.10.923.
- Singh J, Lewallen D. Predictors of pain and use of pain medications following primary Total Hip Arthroplasty (THA): 5,707 THAs at 2-years and 3,289 THAs at 5-years. BMC Musculoskelet Disord 2010;11. http://dx.doi.org/10.1186/1471-2474-11-90.
- Baker PN, van der Meulen JH, Lewsey J, Gregg PJ. The role of pain and function in determining patient satisfaction after total knee replacement. Data from the National Joint Registry for England and Wales. J Bone Joint Surg Br 2007;89–B:893-900. http://dx.doi.org/10.1302/0301-620X.89B7.19091.
- Stephens MAP, Druley JA, Zautra AJ. Older adults’ recovery from surgery for osteoarthritis of the knee: psychosocial resources and constraints as predictors of outcomes. Health Psychol 2002;21:377-83. http://dx.doi.org/10.1037/0278-6133.21.4.377.
- Lundblad H, Kreicbergs A, Jansson KA. Prediction of persistent pain after total knee replacement for osteoarthritis. J Bone Joint Surg Br 2008;90–B:166-71. http://dx.doi.org/10.1302/0301-620X.90B2.19640.
- Nilsdotter AK, Toksvig-Larsen S, Roos EM. Knee arthroplasty: are patients’ expectations fulfilled? A prospective study of pain and function in 102 patients with 5-year follow-up. Acta Orthop 2009;80:55-61. http://dx.doi.org/10.1080/17453670902805007.
- Vuorenmaa M, Ylinen J, Kiviranta I, Intke A, Kautiainen HJ, Mälkiä E, et al. Changes in pain and physical function during waiting time and 3 months after knee joint arthroplasty. J Rehabil Med 2008;40:570-5. http://dx.doi.org/10.2340/16501977-0213.
- Czurda T, Fennema P, Baumgartner M, Ritschl P. The association between component malalignment and postoperative pain following navigation-assisted total knee arthroplasty: results of a cohort/nested case–control study. Knee Surg Sports Traumatol Arthrosc 2010;18:863-9. http://dx.doi.org/10.1007/s00167-009-0990-y.
- Bellamy N, Kirwan J, Boers M, Brooks P, Strand V, Tugwell P, et al. Recommendations for a core set of outcome measures for future phase III clinical trials in knee, hip, and hand osteoarthritis. Consensus development at OMERACT III. J Rheumatol 1997;24:799-802.
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005;113:9-19. http://dx.doi.org/10.1016/j.pain.2004.09.012.
- Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res 1989;248:13-4. http://dx.doi.org/10.1097/00003086-198911000-00004.
- Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833-40.
- Insall JN, Ranawat CS, Aglietti P, Shine J. A comparison of four models of total knee-replacement prostheses. J Bone Joint Surg Am 1976;58:754-65.
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473-83. http://dx.doi.org/10.1097/00005650-199206000-00002.
- Dawson J, Fitzpatrick R, Murray D, Carr A. Questionnaire on the perceptions of patients about total knee replacement. J Bone Joint Surg Br 1998;80:63-9. http://dx.doi.org/10.1302/0301-620X.80B1.7859.
- Garratt AM, Brealey S, Gillespie WJ. Patient-assessed health instruments for the knee: a structured review. Rheumatology 2004;43:1414-23. http://dx.doi.org/10.1093/rheumatology/keh362.
- Riddle DL, Stratford PW, Bowman DH. Findings of extensive variation in the types of outcome measures used in hip and knee replacement clinical trials: a systematic review. Arthritis Rheum 2008;59:876-83. http://dx.doi.org/10.1002/art.23706.
- Kelly JC, Glynn RW, O’Briain DE, Felle P, McCabe JP. The 100 classic papers of orthopaedic surgery: a bibliometric analysis. J Bone Joint Surg Br 2010;92:1338-43. http://dx.doi.org/10.1302/0301-620X.92B10.24867.
- Darzi A. High Quality Care for All: NHS Next Stage Review Final Report. London: Department of Health; 2008.
- Jha AK, Orav EJ, Zheng J, Epstein AM. Patients’ perception of hospital care in the United States. NEJM 2008;359:1921-31. http://dx.doi.org/10.1056/NEJMsa0804116.
- Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg 2003;73:712-6. http://dx.doi.org/10.1046/j.1445-2197.2003.02748.x.
- Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses 2013. www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed 20 May 2015).
- Rolfson O, Dahlberg LE, Nilsson J-A, Malchau H, Garellick G. Variables determining outcome in total hip replacement surgery. J Bone Joint Surg Br 2009;91–B:157-61. http://dx.doi.org/10.1302/0301-620X.91B2.20765.
- Hajat S, Fitzpatrick R, Morris R, Reeves B, Rigge M, Williams O, et al. Does waiting for total hip replacement matter? Prospective cohort study. J Health Serv Res Policy 2002;7:19-25. http://dx.doi.org/10.1258/1355819021927638.
- Jones CA, Cox V, Jhangri GS, Suarez-Almazor ME. Delineating the impact of obesity and its relationship on recovery after total joint arthroplasties. Osteoarthritis Cart 2012;20:511-18. http://dx.doi.org/10.1016/j.joca.2012.02.637.
- Judge A, Arden NK, Batra RN, Thomas G, Beard D, Javaid MK, et al. The association of patient characteristics and surgical variables on symptoms of pain and function over 5 years following primary hip-replacement surgery: A prospective cohort study. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2012-002453.
- Stevens M, Paans N, Wagenmakers R, van Beveren J, van Raay JJAM, van der Meer K, et al. The influence of overweight/obesity on patient-perceived physical functioning and health-related quality of life after primary total hip arthroplasty. Obesity Surgery 2012;22:523-9. http://dx.doi.org/10.1007/s11695-011-0483-1.
- Anakwe RE, Jenkins PJ, Moran M. Predicting dissatisfaction after total hip arthroplasty: a study of 850 patients. J Arthroplasty 2011;26:209-13. http://dx.doi.org/10.1016/j.arth.2010.03.013.
- Clement ND, Muzammil A, Macdonald D, Howie CR, Biant LC. Socioeconomic status affects the early outcome of total hip replacement. J Bone Joint Surg Br 2011;93:464-9. http://dx.doi.org/10.1302/0301-620X.93B4.25717.
- Davis AM, Wood AM, Keenan ACM, Brenkel IJ, Ballantyne JA. Does body mass index affect clinical outcome post-operatively and at five years after primary unilateral total hip replacement performed for osteoarthritis? A multivariate analysis of prospective data. J Bone Joint Surg Br 2011;93:1178-82. http://dx.doi.org/10.1302/0301-620X.93B9.26873.
- Gandhi R, Dhotar H, Davey JR, Mahomed NN. Predicting the longer-term outcomes of total hip replacement. J Rheumatol 2010;37:2573-7. http://dx.doi.org/10.3899/jrheum.100149.
- Garbuz DS, Xu M, Duncan CP, Masri BA, Sobolev B. Delays worsen quality of life outcome of primary total hip arthroplasty. Clin Orthop Relat Res 2006;447:79-84. http://dx.doi.org/10.1097/01.blo.0000203477.19421.ed.
- Moran M, Walmsley P, Gray A, Brenkel IJ. Does body mass index affect the early outcome of primary total hip arthroplasty?. J Arthroplasty 2005;20:866-9. http://dx.doi.org/10.1016/j.arth.2005.02.008.
- Xu M, Garbuz DS, Kuramoto L, Sobolev B. Classifying health-related quality of life outcomes of total hip arthroplasty. BMC Musculoskelet Disord 2005;6. http://dx.doi.org/10.1186/1471-2474-6-48.
- Workgroup of the American association of hip and knee surgeons evidence based committee . Obesity and total joint arthroplasty: a literature based review. J Arthroplasty 2013;28:714-21. http://dx.doi.org/10.1016/j.arth.2013.02.011.
- Liu B, Balkwill A, Banks E, Cooper C, Green J, Beral V. Relationship of height, weight and body mass index to the risk of hip and knee replacements in middle-aged women. Rheumatology 2007;46:861-7. http://dx.doi.org/10.1093/rheumatology/kel434.
- Obesity and Overweight Fact sheet No 311 Updated March 2013. Geneva: WHO; 2013.
- Parsons GE, Godfrey H, Jester RF. Living with severe osteoarthritis while awaiting hip and knee joint replacement surgery. Musculoskeletal Care 2009;7:121-35. http://dx.doi.org/10.1002/msc.145.
- Axford J, Butt A, Heron C, Hammond J, Morgan J, Alavi A, et al. Prevalence of anxiety and depression in osteoarthritis: use of the Hospital Anxiety and Depression Scale as a screening tool. Clin Rheumatol 2010;29:1277-83. http://dx.doi.org/10.1007/s10067-010-1547-7.
- Gleicher Y, Croxford R, Hochman J, Hawker G. A prospective study of mental health care for comorbid depressed mood in older adults with painful osteoarthritis. BMC Psychiatry 2011;11. http://dx.doi.org/10.1186/1471-244X-11-147.
- Vissers MM, Bussmann JB, Verhaar JAN, Busschbach JJV, Bierma-Zeinstra SMA, Reijman M. Psychological factors affecting the outcome of total hip and knee arthroplasty: a systematic review. Semin Arthritis Rheum 2012;41:576-88. http://dx.doi.org/10.1016/j.semarthrit.2011.07.003.
- Crawford RW, Murray DW. Total hip replacement: indications for surgery and risk factors for failure. Ann Rheum Dis 1997;56:455-7. http://dx.doi.org/10.1136/ard.56.8.455.
- Scott CE, Bugler KE, Clement ND, MacDonald D, Howie CR, Biant LC. Patient expectations of arthroplasty of the hip and knee. J Bone Joint Surg Br 2012;94:974-81. http://dx.doi.org/10.1302/0301-620X.94B7.28219.
- Gonzalez Saenz de Tejada M, Escobar A, Herrera C, Garcia L, Aizpuru F, Sarasqueta C. Patient expectations and health-related quality of life outcomes following total joint replacement. Value Health 2010;13:447-54. http://dx.doi.org/10.1111/j.1524-4733.2009.00685.x.
- Franklin PD, Li W, Ayers DC. Functional outcome after total knee replacement varies with patient attributes. Clin Orthop Relat Res 2008;466:2597-604. http://dx.doi.org/10.1007/s11999-008-0428-8.
- Baker PN, Deehan DJ, Lees D, Jameson S, Avery PJ, Gregg PJ, et al. The effect of surgical factors on early patient-reported outcome measures (PROMS) following total knee replacement. J Bone Joint Surg Br 2012;94 B:1058-66.
- Alzahrani K, Gandhi R, Debeer J, Petruccelli D, Mahomed N. Prevalence of clinically significant improvement following total knee replacement. J Rheumatol 2011;38:753-9. http://dx.doi.org/10.3899/jrheum.100233.
- Lingard EA, Riddle DL. Impact of psychological distress on pain and function following knee arthroplasty. J Bone Joint Surg Am 2007;89:1161-9. http://dx.doi.org/10.2106/JBJS.F.00914.
- Cushnaghan J, Bennett J, Reading I, Croft P, Byng P, Cox K, et al. Long-term outcome following total knee arthroplasty: a controlled longitudinal study. Ann Rheum Dis 2009;68:642-7. http://dx.doi.org/10.1136/ard.2008.093229.
- Perruccio AV, Power JD, Evans HMK, Mahomed SR, Gandhi R, Mahomed NN, et al. Multiple joint involvement in total knee replacement for osteoarthritis: Effects on patient-reported outcomes. Arthritis Care Res 2012;64:838-46. http://dx.doi.org/10.1002/acr.21629.
- Sullivan M, Tanzer M, Reardon G, Amirault D, Dunbar M, Stanish W. The role of presurgical expectancies in predicting pain and function one year following total knee arthroplasty. Pain 2011;152:2287-93. http://dx.doi.org/10.1016/j.pain.2011.06.014.
- Naylor JM, Yeo AE, Mittal R, Ko VW, Harris IA. Improvements in knee range and symptomatic and functional behavior after knee arthroplasty based on preoperative restriction in range. J Arthroplasty 2012;27:1100-5. http://dx.doi.org/10.1016/j.arth.2011.09.023.
- Papakostidou I, Dailiana ZH, Papapolychroniou T, Liaropoulos L, Zintzaras E, Karachalios TS, et al. Factors affecting the quality of life after total knee arthroplasties: a prospective study. BMC Musculoskelet Disord 2012;13. http://dx.doi.org/10.1186/1471-2474-13-116.
- Singh JA, Lewallen DG. Medical and psychological comorbidity predicts poor pain outcomes after total knee arthroplasty. Rheumatology 2013;52:916-23. http://dx.doi.org/10.1093/rheumatology/kes402.
- Heck DA, Robinson RL, Partridge CM, Lubitz RM, Freund DA. Patient outcomes after knee replacement. Clin Orthop Relat Res 1998;356:93-110. http://dx.doi.org/10.1097/00003086-199811000-00015.
- Merle-Vincent F, Couris CM, Schott AM, Conrozier T, Piperno M, Mathieu P, et al. Factors predicting patient satisfaction 2 years after total knee arthroplasty for osteoarthritis. Joint Bone Spine 2011;78:383-6. http://dx.doi.org/10.1016/j.jbspin.2010.11.013.
- Núñez M, Lozano L, Núñez E, Segur JM, Sastre S, Maculé F, et al. Total knee replacement and health-related quality of life: factors influencing long-term outcomes. Arthritis Rheum 2009;61:1062-9. http://dx.doi.org/10.1002/art.24644.
- Gandhi R, Dhotar H, Razak F, Tso P, Davey JR, Mahomed NN. Predicting the longer term outcomes of total knee arthroplasty. Knee 2010;17:15-8. http://dx.doi.org/10.1016/j.knee.2009.06.003.
- Deshmukh RG, Hayes JH, Pinder IM. Does body weight influence outcome after total knee arthroplasty? A 1-year analysis. J Arthroplasty 2002;17:315-19. http://dx.doi.org/10.1054/arth.2002.30776.
- Rajgopal V, Bourne RB, Chesworth BM, MacDonald SJ, McCalden RW, Rorabeck CH. The impact of morbid obesity on patient outcomes after total knee arthroplasty. J Arthroplasty 2008;23:795-800. http://dx.doi.org/10.1016/j.arth.2007.08.005.
- Ayers DC, Franklin PD, Ploutz-Snyder R, Boisvert CB. Total knee replacement outcome and coexisting physical and emotional illness. Clin Orthop Relat Res 2005;440:157-61. http://dx.doi.org/10.1097/01.blo.0000185447.43622.93.
- Clement ND, Jenkins PJ, MacDonald D, Nie YX, Patton JT, Breusch SJ, et al. Socioeconomic status affects the Oxford knee score and short-form 12 score following total knee replacement. Bone Joint J 2013;95–B:52-8. http://dx.doi.org/10.1302/0301-620X.95B1.29749.
- Scott CE, Howie CR, MacDonald D, Biant LC. Predicting dissatisfaction following total knee replacement: a prospective study of 1217 patients. J Bone Joint Surg Br 2010;92:1253-8. http://dx.doi.org/10.1302/0301-620X.92B9.24394.
- Gandhi R, Razak F, Davey JR, Mahomed NN. Metabolic syndrome and the functional outcomes of hip and knee arthroplasty. J Rheumatol 2010;37:1917-22. http://dx.doi.org/10.3899/jrheum.091242.
- Apold H, Meyer H, Nordsletten L, Furnes O, Baste V, Flugsrud G. Risk factors for knee replacement due to primary osteoarthritis, a population based, prospective cohort study of 315,495 individuals. BMC Musculoskelet Disord 2014;15. http://dx.doi.org/10.1186/1471-2474-15-217.
- Yeung E, Thornton-Bott P, Walter WL. Patient obesity: A growing concern of successful total knee arthroplasty. Semin Arthroplasty 2010;21:87-91. http://dx.doi.org/10.1053/j.sart.2010.01.001.
- Gillespie GN, Porteous AJ. Obesity and knee arthroplasty. Knee 2007;14:81-6. http://dx.doi.org/10.1016/j.knee.2006.11.004.
- Kerkhoffs GM, Servien E, Dunn W, Dahm D, Bramer JA, Haverkamp D. The influence of obesity on the complication rate and outcome of total knee arthroplasty: a meta-analysis and systematic literature review. J Bone Joint Surg Am 2012;94:1839-44. http://dx.doi.org/10.2106/JBJS.K.00820.
- Samson AJ, Mercer GE, Campbell DG. Total knee replacement in the morbidly obese: a literature review. ANZ J Surg 2010;80:595-9. http://dx.doi.org/10.1111/j.1445-2197.2010.05396.x.
- Dowsey MM, Choong PF. Early outcomes and complications following joint arthroplasty in obese patients: a review of the published reports. ANZ J Surg 2008;78:439-44. http://dx.doi.org/10.1111/j.1445-2197.2008.04554.x.
- McElroy MJ, Pivec R, Issa K, Harwin SF, Mont MA. The effects of obesity and morbid obesity on outcomes in TKA. J Knee Surg 2013;26:83-8. http://dx.doi.org/10.1055/s-0033-1341407.
- Kirchberger I, Meisinger C, Heier M, Zimmermann AK, Thorand B, Autenrieth CS, et al. Patterns of multimorbidity in the aged population. Results from the KORA-Age study. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0030556.
- Williams ID, O’Doherty LJ, Mitchell GK, Williams KE. Identifying unmet needs in older patients--nurse-GP collaboration in general practice. Aust Fam Physician 2007;36:772-6.
- Kirschner S, Lutzner J, Gunther K-P, Eberlein-Gonska M, Krummenauer F. Adverse events in total knee arthroplasty: Results of a physician independent survey in 260 patients. Patient Saf Surg 2010;4. http://dx.doi.org/10.1186/1754-9493-4-12.
- Bourne R, Chesworth B, Davis A, Mahomed N, Charron K. Patient satisfaction after total knee arthroplasty: Who is satisfied and who is not?. Clin Orthop Relat Res 2010;468:57-63. http://dx.doi.org/10.1007/s11999-009-1119-9.
- Andersson AE, Bergh I, Karlsson J, Nilsson K. Patients’ experiences of acquiring a deep surgical site infection: An interview study. Am J Infect Control 2010;38:711-17. http://dx.doi.org/10.1016/j.ajic.2010.03.017.
- Hunter G, Dandy D. The natural history of the patient with an infected total hip replacement. J Bone Joint Surg Br 1977;59–B:293-7.
- Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the international cooperative pulmonary embolism registry (ICOPER). Lancet 1999;353:1386-9. http://dx.doi.org/10.1016/S0140-6736(98)07534-5.
- Guanella R, Ducruet T, Johri M, Miron MJ, Roussin A, Desmarais S, et al. Economic burden and cost determinants of deep vein thrombosis during 2 years following diagnosis: a prospective evaluation. J Thrombosis Haemostas 2011;9:2397-405. http://dx.doi.org/10.1111/j.1538-7836.2011.04516.x.
- Khan NA, Quan H, Bugar JM, Lemaire JB, Brant R, Ghali WA. Association of postoperative complications with hospital costs and length of stay in a tertiary care center. J Gen Intern Med 2006;21:177-80. http://dx.doi.org/10.1007/s11606-006-0254-1.
- Solomon DH, Chibnik LB, Losina E, Huang J, Fossel AH, Husni E, et al. Development of a preliminary index that predicts adverse events after total knee replacement. Arthritis Rheum 2006;54:1536-42. http://dx.doi.org/10.1002/art.21772.
- Cushnaghan J, Coggon D, Reading I, Croft P, Byng P, Cox K, et al. Long-term outcome following total hip arthroplasty: a controlled longitudinal study. Arthritis Rheum 2007;57:1375-80. http://dx.doi.org/10.1002/art.23101.
- Judge A, Javaid MK, Arden NK, Cushnaghan J, Reading I, Croft P, et al. Clinical tool to identify patients who are most likely to achieve long-term improvement in physical function after total hip arthroplasty. Arthritis Care Res 2012;64:881-9. http://dx.doi.org/10.1002/acr.21594.
- Bolognesi MP, Marchant MH, Viens NA, Cook C, Pietrobon R, Vail TP. The impact of diabetes on perioperative patient outcomes after total hip and total knee arthroplasty in the United States. J Arthroplasty 2008;23:92-8. http://dx.doi.org/10.1016/j.arth.2008.05.012.
- Chan PKH, Brenkel IJ, Aderinto J. The outcome of total hip arthroplasty in diabetes mellitus. Br J Diabetes Vasc Dis 2005;5:146-9. http://dx.doi.org/10.1177/14746514050050030601.
- Choong PFM, Dowsey MM, Carr D, Daffy J, Stanley P. Risk factors associated with acute hip prosthetic joint infections and outcome of treatment with a rifampin based regimen. Acta Orthopaedica 2007;78:755-65. http://dx.doi.org/10.1080/17453670710014527.
- Cordero-Ampuero J, de Dios M. What are the risk factors for infection in hemiarthroplasties and total hip arthroplasties?. Clin Orthop Relat Res 2010;468:3268-77. http://dx.doi.org/10.1007/s11999-010-1411-8.
- Dowsey MM, Choong PFM. Obesity is a major risk factor for prosthetic infection after primary hip arthroplasty. Clin Orthop Relat Res 2008;466:153-8. http://dx.doi.org/10.1007/s11999-007-0016-3.
- Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty 2012;27:726-9. http://dx.doi.org/10.1016/j.arth.2011.09.013.
- Namba RS, Paxton L, Fithian DC, Stone ML. Obesity and perioperative morbidity in total hip and total knee arthroplasty patients. J Arthroplasty 2005;20:46-50. http://dx.doi.org/10.1016/j.arth.2005.04.023.
- Namba RS, Inacio MCS, Paxton EW. Risk factors associated with surgical site infection in 30,491 primary total hip replacements. J Bone Joint Surg Br 2012;94:1330-8. http://dx.doi.org/10.1302/0301-620X.94B10.29184.
- Pedersen AB, Mehnert F, Johnsen SP, Sorensen HT. Risk of revision of a total hip replacement in patients with diabetes mellitus: a population-based follow up study. J Bone Joint Surg Br 2010;92:929-34. http://dx.doi.org/10.1302/0301-620X.92B7.24461.
- Vannini P, Ciavarella A, Olmi R, Flammini M, Moroni A, Galuppi V, et al. Diabetes as pro-infective risk factor in total hip replacement. Acta Diabetologica Latina 1984;21:275-80. http://dx.doi.org/10.1007/BF02642901.
- Bhattacharyya T, Iorio R, Healy WL. Rate of and risk factors for acute inpatient mortality after orthopaedic surgery. J Bone Joint Surg Am 2002;84–A:562-72.
- Dy CJ, Wilkinson JD, Tamariz L, Scully SP. Influence of preoperative cardiovascular risk factor clusters on complications of total joint arthroplasty. Am J Orthoped 2011;40:560-5.
- Marchant MH, Viens NA, Cook C, Vail TP, Bolognesi MP. The impact of glycemic control and diabetes mellitus on perioperative outcomes after total joint arthroplasty. J Bone Joint Surg Am 2009;91:1621-9. http://dx.doi.org/10.2106/JBJS.H.00116.
- Blom A, Pattison G, Whitehouse S, Taylor A, Bannister G. Early death following primary total hip arthroplasty: 1,727 procedures with mechanical thrombo-prophylaxis. Acta Orthop 2006;77:347-50. http://dx.doi.org/10.1080/17453670610046244.
- Ackland GL, Harris S, Ziabari Y, Grocott M, Mythen M, SOuRCe Investigators. Revised cardiac risk index and postoperative morbidity after elective orthopaedic surgery: a prospective cohort study. Br J Anaesth 2010;105:744-52. http://dx.doi.org/10.1093/bja/aeq245.
- Bozic KJ, Ong K, Lau E, Berry DJ, Vail TP, Kurtz SM, et al. Estimating risk in Medicare patients with THA: an electronic risk calculator for periprosthetic joint infection and mortality. Clin Orthop Relat Res 2013;471:574-83. http://dx.doi.org/10.1007/s11999-012-2605-z.
- Sanders RD, Bottle A, Jameson SS, Mozid A, Aylin P, Edger L, et al. Independent preoperative predictors of outcomes in orthopedic and vascular surgery: the influence of time interval between an acute coronary syndrome or stroke and the operation. Ann Surg 2012;255:901-7. http://dx.doi.org/10.1097/SLA.0b013e31824c438d.
- Singh JA, Lewallen DG. Peptic ulcer disease and heart disease are associated with periprosthetic fractures after total hip replacement. Acta Orthopaedica 2012;83:353-9. http://dx.doi.org/10.3109/17453674.2012.717844.
- Spahn DR. Anemia and patient blood management in hip and knee surgery: a systematic review of the literature. Anesthesiology 2010;113:482-95. http://dx.doi.org/10.1097/ALN.0b013e3181e08e97.
- Jämsen E, Puolakka T, Eskelinen A, Jantti P, Kalliovalkama J, Nieminen J, et al. Predictors of mortality following primary hip and knee replacement in the aged. A single-center analysis of 1,998 primary hip and knee replacements for primary osteoarthritis. Acta Orthop 2013;84:44-53. http://dx.doi.org/10.3109/17453674.2012.752691.
- Greenky M, Gandhi K, Pulido L, Restrepo C, Parvizi J. Preoperative anemia in total joint arthroplasty: is it associated with periprosthetic joint infection?. Clin Orthop Relat Res 2012;470:2695-701. http://dx.doi.org/10.1007/s11999-012-2435-z.
- Bozic KJ, Lau E, Kurtz S, Ong K, Rubash H, Vail TP, et al. Patient-related risk factors for periprosthetic joint infection and postoperative mortality following total hip arthroplasty in Medicare patients. J Bone Joint Surg Am 2012;94:794-800. http://dx.doi.org/10.2106/JBJS.K.00072.
- O’Malley NT, Fleming FJ, Gunzler DD, Messing SP, Kates SL. Factors independently associated with complications and length of stay after hip arthroplasty: analysis of the national surgical quality improvement program. J Arthroplasty 2012;27:1832-7. http://dx.doi.org/10.1016/j.arth.2012.04.025.
- Myers E, O’Grady P, Dolan AM. The influence of preclinical anaemia on outcome following total hip replacement. Arch Orthop Trauma Surg 2004;124:699-701. http://dx.doi.org/10.1007/s00402-004-0754-6.
- Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol 1995;43:55-68. http://dx.doi.org/10.1111/j.1365-2265.1995.tb01894.x.
- Dowsey MM, Choong PFM. Obese diabetic patients are at substantial risk for deep infection after primary TKA. Clin Orthop Relat Res 2009;467:1577-81. http://dx.doi.org/10.1007/s11999-008-0551-6.
- Memtsoudis SG, Gonzalez Della Valle A, Besculides MC, Gaber L, Sculco TP. In-hospital complications and mortality of unilateral, bilateral, and revision TKA: based on an estimate of 4,159,661 discharges. Clin Orthop Relat Res 2008;466:2617-27. http://dx.doi.org/10.1007/s11999-008-0402-5.
- Adams AL, Paxton EW, Wang JQ, Johnson ES, Bayliss EA, Ferrara A, et al. Surgical outcomes of total knee replacement according to diabetes status and glycemic control, 2001 to 2009. J Bone Joint Surg Am 2013;95:481-7. http://dx.doi.org/10.2106/JBJS.L.00109.
- Asensio A, Antolin FJ, Sanchez-Garcia JM, Hidalgo O, Hernandez-Navarrete MJ, Bishopberger C, et al. Timing of DVT prophylaxis and risk of postoperative knee prosthesis infection. Orthopedics 2010;33. http://dx.doi.org/10.3928/01477447-20100924-12.
- Chesney D, Sales J, Elton R, Brenkel IJ. Infection after knee arthroplasty a prospective study of 1509 cases. J Arthroplasty 2008;23:355-9. http://dx.doi.org/10.1016/j.arth.2007.05.052.
- Fan JCH, Hung HH, Fung KY. Infection in primary total knee replacement. Hong Kong Med J 2008;14:40-5.
- Galat DD, McGovern SC, Larson DR, Harrington JR, Hanssen AD, Clarke HD, et al. Surgical treatment of early wound complications following primary total knee arthroplasty. J Bone Joint Surg Am 2009;91:48-54. http://dx.doi.org/10.2106/JBJS.G.01371.
- Jämsen E, Nevalainen P, Kalliovalkama J, Moilanen T. Preoperative hyperglycemia predicts infected total knee replacement. Eur J Intern Med 2010;21:196-201. http://dx.doi.org/10.1016/j.ejim.2010.02.006.
- Meding JB, Reddleman K, Keating ME, Klay A, Ritter MA, Faris PM, et al. Total knee replacement in patients with diabetes mellitus. Clin Orthop Relat Res 2003:208-16. http://dx.doi.org/10.1097/01.blo.0000093002.90435.56.
- Moon HK, Han CD, Yang IH, Cha BS. Factors affecting outcome after total knee arthroplasty in patients with diabetes mellitus. Yonsei Med J 2008;49:129-37. http://dx.doi.org/10.3349/ymj.2008.49.1.129.
- Namba RS, Chen Y, Paxton EW, Slipchenko T, Fithian DC. Outcomes of routine use of antibiotic-loaded cement in primary total knee arthroplasty. J Arthroplasty 2009;24:44-7. http://dx.doi.org/10.1016/j.arth.2009.05.007.
- Gill GS, Mills D, Joshi AB. Mortality following primary total knee arthroplasty. J Bone Joint Surg Am 2003;85–A:432-5.
- Bozic KJ, Lau E, Kurtz S, Ong K, Berry DJ. Patient-related risk factors for postoperative mortality and periprosthetic joint infection in medicare patients undergoing TKA. Clin Orthop Relat Res 2012;470:130-7. http://dx.doi.org/10.1007/s11999-011-2043-3.
- You and Your Anaesthetic. London: The Royal College of Anaesthetists and The Association of Anaesthetists of Great Britain and Ireland; 2008.
- Fritsch G, Flamm M, Hepner DL, Panisch S, Seer J, Soennichsen A. Abnormal pre-operative tests, pathologic findings of medical history, and their predictive value for perioperative complications. Acta Anaesthesiol Scand 2012;56:339-50. http://dx.doi.org/10.1111/j.1399-6576.2011.02593.x.
- Bryson GL, Wyand A, Bragg PR. Preoperative testing is inconsistent with published guidelines and rarely changes management. Can J Anaesth 2006;53:236-41. http://dx.doi.org/10.1007/BF03022208.
- Czoski-Murray C, Lloyd Jones M, McCabe C, Claxton K, Oluboyede Y, Roberts J, et al. What is the value of routinely testing full blood count, electrolytes and urea, and pulmonary function tests before elective surgery in patients with no apparent clinical indication and in subgroups of patients with common comorbidities: a systematic review of the clinical and cost-effective literature. Health Technol Assess 2012;16. http://dx.doi.org/10.3310/hta16500.
- Briefing Notes for Researchers: Involving the Public in NHS, Public Health and Social Care Research. Eastleigh: INVOLVE; 2012.
- Johnson EC, Horwood J, Gooberman-Hill R. Patients’ journeys through total joint replacement: patterns of medication use. Musculoskeletal Care 2014;12:92-102. http://dx.doi.org/10.1002/msc.1062.
- Johnson EC, Horwood J, Gooberman-Hill R. Conceptualising time before surgery: the experience of patients waiting for hip replacement. Soc Sci Med 2014;116:126-33. http://dx.doi.org/10.1016/j.socscimed.2014.06.037.
- Hudak PL, Frankel RM, Braddock C, Nisenbaum R, Luca P, McKeever C, et al. Do patients’ communication behaviors provide insight into their preferences for participation in decision making?. Med Decis Making 2008;28:385-93. http://dx.doi.org/10.1177/0272989X07312712.
- Harrison A, Appleby J. Reducing waiting times for hospital treatment: lessons from the English NHS. J Health Serv Res Policy 2009;14:168-73. http://dx.doi.org/10.1258/jhsrp.2008.008118.
- Greener I. Towards a history of choice in UK health policy. Sociol Health Illn 2009;31:309-24. http://dx.doi.org/10.1111/j.1467-9566.2008.01135.x.
- Fotaki M. Patient choice and equity in the British National Health Service: towards developing an alternative framework. Sociol Health Illn 2010;32:898-913. http://dx.doi.org/10.1111/j.1467-9566.2010.01254.x.
- Department of Health/Payment by Results team . Choice Framework 2012. www.nhs.uk/choiceintheNHS/Rightsandpledges/NHSConstitution/Documents/2013/choice-framework-2013–14.pdf (accessed 12 November 2013).
- Davis AM, Agnidis Z, Badley E, Davey JR, Gafni A, Gollish J, et al. Waiting for hip revision surgery: the impact on patient disability. Can J Surg 2008;51:92-6.
- Ackerman IN, Graves SE, Wicks IP, Bennell KL, Osborne RH, Ackerman IN, et al. Severely compromised quality of life in women and those of lower socioeconomic status waiting for joint replacement surgery. Arthritis Rheum 2005;53:653-8. http://dx.doi.org/10.1002/art.21439.
- Ackerman IN, Bennell KL, Osborne RH, Ackerman IN, Bennell KL, Osborne RH. Decline in Health-Related Quality of Life reported by more than half of those waiting for joint replacement surgery: a prospective cohort study. BMC Musculoskelet Disord 2011;12. http://dx.doi.org/10.1186/1471-2474-12-108.
- Carol Craig, Centre for Confidence and Well-being . Centre for Confidence and Well-Being 2014. www.centreforconfidence.co.uk/flourishing-lives.php?p=cGlkPTU3MQ== (accessed 30 June 2015).
- Merriam–Webster, Incorporated . Merriam–Webster Dictionary n.d. www.merriam-webster.com/dictionary/vulnerable (accessed 1 April 2016).
- Tromp AM, Pluijm SM, Smit JH, Deeg DJ, Bouter LM, Lips P. Fall-risk screening test: a prospective study on predictors for falls in community-dwelling elderly. J Clin Epidemiol 2001;54:837-44. http://dx.doi.org/10.1016/S0895-4356(01)00349-3.
- Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing 2006;35:ii37-ii41. http://dx.doi.org/10.1093/ageing/afl084.
- Ziden L, Scherman MH, Wenestam CG. The break remains – elderly people’s experiences of a hip fracture 1 year after discharge. Disabil Rehabil 2010;32:103-13. http://dx.doi.org/10.3109/09638280903009263.
- Grant S, St John W, Patterson E. Recovery from total hip replacement surgery: ‘it’s not just physical’. Qual Health Res 2009;19:1612-20. http://dx.doi.org/10.1177/1049732309350683.
- Perry MA, Hudson HS, Meys S, Norrie O, Ralph T, Warner S. Older adults’ experiences regarding discharge from hospital following orthopaedic intervention: a metasynthesis. Disabil Rehabil 2012;34:267-78. http://dx.doi.org/10.3109/09638288.2011.603016.
- Gooberman-Hill R, Ebrahim S. Informal care at times of change in health and mobility: a qualitative study. Age Ageing 2006;35:261-6. http://dx.doi.org/10.1093/ageing/afj065.
- Sherman AM. Social relations and depressive symptoms in older adults with knee osteoarthritis. Soc Sci Med 2003;56:247-57. http://dx.doi.org/10.1016/S0277-9536(02)00023-0.
- Evers AW, Kraaimaat FW, Geenen R, Bijlsma JW. Psychosocial predictors of functional change in recently diagnosed rheumatoid arthritis patients. Behav Res Ther 1998;36:179-93. http://dx.doi.org/10.1016/S0005-7967(98)00019-9.
- Gustafsson BÅ, Heikkilä K, Ekman S-L, Ponzer S. In the hands of formal carers: Older patients’ experiences of care across the perioperative period for joint replacement surgery. Int J Orthop Trauma Nurs 2010;14:96-108. http://dx.doi.org/10.1016/j.ijotn.2010.01.002.
- Perry MA, Hudson S, Ardis K. ‘If I didn’t have anybody, what would I have done?’: Experiences of older adults and their discharge home after lower limb orthopaedic surgery. J Rehabil Med 2011;43:916-22. http://dx.doi.org/10.2340/16501977-0874.
- Heine J, Koch S, Goldie P. Patients’ experiences of readiness for discharge following a total hip replacement. Aust J Physiother 2004;50:227-33. http://dx.doi.org/10.1016/S0004-9514(14)60112-4.
- Mancuso CA, Jout J, Salvati EA, Sculco TP. Fulfillment of patients’ expectations for total hip arthroplasty. J Bone Joint Surg Am 2009;91:2073-8. http://dx.doi.org/10.2106/JBJS.H.01802.
- Mahomed NN, Liang MH, Cook EF, Daltroy LH, Fortin PR, Fossel AH, et al. The importance of patient expectations in predicting functional outcomes after total joint arthroplasty. J Rhematol 2002;29:1273-9.
- Noble PC, Conditt MA, Cook KF, Mathis KB. Patient expectations affect satisfaction with total knee arthroplasty. Clin Orthop Relat Res 2006;452:35-43. http://dx.doi.org/10.1097/01.blo.0000238825.63648.1e.
- Sandelowski M. Qualitative analysis: What it is and how to begin. Res Nurs Health 1995;18:371-5. http://dx.doi.org/10.1002/nur.4770180411.
- Smith JA. Reflecting on the development of interpretative phenomenological analysis and its contribution to qualitative research in psychology. Qual Res Psychol 2004;1:39-54.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. http://dx.doi.org/10.1191/1478088706qp063oa.
- Smith JA, Eatough V, Breakwell GM, Hammond S, Fife-Schaw C, Smith JA. Research Methods in Psychology. London: Sage; 2006.
- Smith JA, Osborn M, Breakwell GM. Doing Social Psychology Research. Oxford: The BPS and Blackwell Publishing Ltd; 2004.
- Smith JA, Osborn M, Smith JA. Qualitative Psychology: A Practical Guide to Research Methods. London: Sage; 2008.
- Sturges JE, Hanrahan KJ. Comparing telephone and face-to-face qualitative interviewing: a research note. Qualitative Research 2004;4:107-18. http://dx.doi.org/10.1177/1468794104041110.
- Wylde V, Blom AW, Bolink S, Brunton L, Dieppe P, Gooberman-Hill R, et al. Assessing function in patients undergoing joint replacement: a study protocol for a cohort study. BMC Musculoskelet Disord 2012;13. http://dx.doi.org/10.1186/1471-2474-13-220.
- Wylde V, Lenguerrand E, Brunton L, Dieppe P, Gooberman-Hill R, Mann C, et al. Does measuring the range of motion of the hip and knee add to the assessment of disability in people undergoing joint replacement?. Orthop Traumatol Surg Res 2014;100:183-6. http://dx.doi.org/10.1016/j.otsr.2013.09.016.
- Lenguerrand E, Wylde V, Gooberman-Hill R, Sayers A, Brunton L, Beswick AD, et al. Trajectories of Pain and Function after Primary Hip and Knee Arthroplasty: The ADAPT Cohort Study. PLOS ONE 2016;11. http://dx.doi.org/10.1371/journal.pone.0149306.
- Bourne RB. Measuring tools for functional outcomes in total knee arthroplasty. Clin Orthop Relat Res 2008;466:2634-8. http://dx.doi.org/10.1007/s11999-008-0468-0.
- Paterson C, Baarts C, Launso L, Verhoef MJ. Evaluating complex health interventions: a critical analysis of the ‘outcomes’ concept. BMC Compl Alternative Med 2009;9. http://dx.doi.org/10.1186/1472-6882-9-18.
- Clarke M. Standardising outcomes for clinical trials and systematic reviews. Trials 2007;8. http://dx.doi.org/10.1186/1745-6215-8-39.
- Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am 1969;51:737-55.
- Hewlett SA. Patients and clinicians have different perspectives on outcomes in arthritis. J Rheumatol 2003;30:877-9.
- Gioe TJ, Pomeroy D, Suthers K, Singh JA. Can patients help with long-term total knee arthroplasty surveillance? Comparison of the American Knee Society Score self-report and surgeon assessment. Rheumatology 2009;48:160-4. http://dx.doi.org/10.1093/rheumatology/ken439.
- Guidance on the Routine Collection of Patient Reported Outcome Measures (PROMs). London: Department of Health; 2009.
- Nilsdotter AK, Lohmander LS, Klassbo M, Roos EM. Hip disability and osteoarthritis outcome score (HOOS) – validity and responsiveness in total hip replacement. BMC Musculoskelet Disord 2003;4. http://dx.doi.org/10.1186/1471-2474-4-10.
- Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS) – development of a self-administered outcome measure. J Orthop Sports Phys Ther 1998;28:88-96. http://dx.doi.org/10.2519/jospt.1998.28.2.88.
- Dawson J, Fitzpatrick R, Carr A, Murray D. Questionnaire on the perceptions of patients about total hip replacement. J Bone Joint Surg Br 1996;78:185-90.
- Nicholas MK. The pain self-efficacy questionnaire: taking pain into account. Eur J Pain 2007;11:153-63. http://dx.doi.org/10.1016/j.ejpain.2005.12.008.
- Carver CS. You want to measure coping but your protocol’s too long: consider the brief COPE. Int J Behav Med 1997;4:92-100. http://dx.doi.org/10.1207/s15327558ijbm0401_6.
- Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res 1999;47:555-67. http://dx.doi.org/10.1016/S0022-3999(99)00057-4.
- Williams A, Kind P, Hopkins A. Measure of the Quality of Life: The Uses To Which They May Be Put. London: Royal College of Physicians of London; 1992.
- Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol 2005;58:595-602. http://dx.doi.org/10.1016/j.jclinepi.2004.10.018.
- Hawker GA, Davis AM, French MR, Cibere J, Jordan JM, March L, et al. Development and preliminary psychometric testing of a new OA pain measure – an OARSI/OMERACT initiative. Osteoarthritis Cart 2008;16:409-14. http://dx.doi.org/10.1016/j.joca.2007.12.015.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. http://dx.doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Moss-Morris R, Weinman J, Petrie K, Horne R, Cameron L, Buick D. The revised illness perception questionnaire (IPQ-R). Psychol &Amp; Health 2002;17:1-16. http://dx.doi.org/10.1080/08870440290001494.
- Freynhagen R, Baron R, Gockel U, Tolle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22:1911-20. http://dx.doi.org/10.1185/030079906X132488.
- Mahomed N, Gandhi R, Daltroy L, Katz JN. The self-administered patient satisfaction scale for primary hip and knee arthroplasty. Arthritis 2011;2011. http://dx.doi.org/10.1155/2011/591253.
- Pollard B, Dixon D, Dieppe P, Johnston M. Measuring the ICF components of impairment, activity limitation and participation restriction: an item analysis using classical test theory and item response theory. Health Qual Life Outcomes 2009;7. http://dx.doi.org/10.1186/1477-7525-7-41.
- Ware J, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. http://dx.doi.org/10.1097/00005650-199603000-00003.
- Paterson C. Measuring outcomes in primary care: a patient generated measure, MYMOP, compared with the SF-36 health survey. BMJ 1996;312:1016-20. http://dx.doi.org/10.1136/bmj.312.7037.1016.
- Senden R, Grimm B, Heyligers IC, Savelberg HH, Meijer K. Acceleration-based gait test for healthy subjects: reliability and reference data. Gait Posture 2009;30:192-6. http://dx.doi.org/10.1016/j.gaitpost.2009.04.008.
- Podsiadlo D, Richardson S. The timed ‘Up & Go’: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142-8. http://dx.doi.org/10.1111/j.1532-5415.1991.tb01616.x.
- Janssen WG, Bussmann HB, Stam HJ. Determinants of the sit-to-stand movement: a review. Phys Ther 2002;82:866-79.
- van den Dikkenberg N, Meijer OG, van der Slikke RM, van Lummel RC, van Dieen JH, Pijls B, et al. Measuring functional abilities of patients with knee problems: rationale and construction of the DynaPort knee test. Knee Surg Sports Traumatol Arthrosc 2002;10:204-12. http://dx.doi.org/10.1007/s00167-002-0279-x.
- Hardcastle P, Nade S. The significance of the Trendelenburg test. J Bone Joint Surg Br 1985;67:741-6.
- Senden R, Grimm B, Meijer K, Savelberg H, Heyligers IC. The importance to including objective functional outcomes in the clinical follow up of total knee arthroplasty patients. Knee 2011;18:306-11. http://dx.doi.org/10.1016/j.knee.2010.07.008.
- Binkley JM, Stratford PW, Lott SA, Riddle DL. The lower extremity functional scale (LEFS): scale development, measurement properties, and clinical application. North American Orthopaedic Rehabilitation Research Network. Phys Ther 1999;79:371-83.
- Amstutz HC, Thomas BJ, Jinnah R, Kim W, Grogan T, Yale C. Treatment of primary osteoarthritis of the hip. A comparison of total joint and surface replacement arthroplasty. J Bone Joint Surg Am 1984;66:228-41.
- Powell LE, Myers AM. The activities-specific balance confidence (ABC) scale. J Gerontol A Biol Sci Med Sci 1995;50A:M28-34. http://dx.doi.org/10.1093/gerona/50A.1.M28.
- Waldrop D, Lightsey OR, Ethington CA, Woemmel CA, Coke AL. Self-efficacy, optimism, health competence, and recovery from orthopedic surgery. J Couns Psychol 2001;48:233-8. http://dx.doi.org/10.1037/0022-0167.48.2.233.
- Nouri F, Lincoln N. An extended activities of daily living index for stroke patients. Clin Rehabil 1987;1:301-5. http://dx.doi.org/10.1177/026921558700100409.
- Coast J, Peters TJ, Natarajan L, Sproston K, Flynn T. An assessment of the construct validity of the descriptive system for the ICECAP capability measure for older people. Qual Life Res 2008;17:967-76. http://dx.doi.org/10.1007/s11136-008-9372-z.
- Pollard B, Johnston M, Dieppe P. Exploring the relationships between international classification of functioning, disability and health (ICF) constructs of impairment, activity limitation and participation Restriction in people with osteoarthritis prior to joint replacement. BMC Musculoskelet Disord 2011;12. http://dx.doi.org/10.1186/1471-2474-12-97.
- Kennedy D, Stratford PW, Pagura SM, Walsh M, Woodhouse LJ. Comparison of gender and group differences in self-report and physical performance measures in total hip and knee arthroplasty candidates. J Arthroplasty 2002;17:70-7. http://dx.doi.org/10.1054/arth.2002.29324.
- Wright AA, Cook CE, Baxter GD, Garcia J, Abbott JH. Relationship between the western Ontario and McMaster Universities osteoarthritis index physical function subscale and physical performance measures in patients with hip osteoarthritis. Arch Phys Med Rehabil 2010;91:1558-64. http://dx.doi.org/10.1016/j.apmr.2010.07.016.
- National Joint Registry for England and Wales: 8th Annual Report. Hemel Hempstead: NJR Centre; 2011.
- Mental Capacity Act 2005. London: The Stationary Office; 2005.
- Bolink SA, van Laarhoven SN, Lipperts M, Heyligers IC, Grimm B. Inertial sensor motion analysis of gait, sit-stand transfers and step-up transfers: differentiating knee patients from healthy controls. Physiol Meas 2012;33:1947-58. http://dx.doi.org/10.1088/0967-3334/33/11/1947.
- Roos E, Toksvig-Larsen S. Knee injury and osteoarthritis outcome score (KOOS) – validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes 2003;1. http://dx.doi.org/10.1186/1477-7525-1-17.
- Hunt SM, McEwen J. The development of a subjective health indicator. Sociol Health Illn 1980;2:231-46. http://dx.doi.org/10.1111/1467-9566.ep11340686.
- Ornetti P, Maillefert JF, Laroche D, Morisset C, Dougados M, Gossec L. Gait analysis as a quantifiable outcome measure in hip or knee osteoarthritis: a systematic review. Joint Bone Spine 2010;77:421-5. http://dx.doi.org/10.1016/j.jbspin.2009.12.009.
- Reininga IH, Stevens M, Wagenmakers R, Boerboom AL, Groothoff JW, Bulstra SK, et al. Compensatory trunk movements in patients with hip osteoarthritis: accuracy and reproducibility of a body-fixed sensor-based assessment. Am J Phys Med Rehabil 2011;90:681-7. http://dx.doi.org/10.1097/PHM.0b013e31820f955e.
- van den Akker-Scheek I, Zijlstra W, Groothoff JW, Bulstra SK, Stevens M. Physical functioning before and after total hip arthroplasty: perception and performance. Phys Ther 2008;88:712-19. http://dx.doi.org/10.2522/ptj.20060301.
- Stratford PW, Kennedy DM. Performance measures were necessary to obtain a complete picture of osteoarthritic patients. J Clin Epidemiol 2006;59:160-7. http://dx.doi.org/10.1016/j.jclinepi.2005.07.012.
- Gandhi R, Tsvetkov D, Davey JR, Syed KA, Mahomed NN. Relationship between self-reported and performance-based tests in a hip and knee joint replacement population. Clin Rheumatol 2009;28:253-7. http://dx.doi.org/10.1007/s10067-008-1021-y.
- Boardman DL, Dorey F, Thomas BJ, Lieberman JR. The accuracy of assessing total hip arthroplasty outcomes: a prospective correlation study of walking ability and 2 validated measurement devices. J Arthroplasty 2000;15:200-4. http://dx.doi.org/10.1016/S0883-5403(00)90242-0.
- Kennedy DM, Stratford PW, Wessel J, Gollish JD, Penney D. Assessing stability and change of four performance measures: a longitudinal study evaluating outcome following total hip and knee arthroplasty. BMC Musculoskelet Disord 2005;6. http://dx.doi.org/10.1186/1471-2474-6-3.
- Stevens-Lapsley JE, Schenkman ML, Dayton MR. Comparison of self-reported knee injury and osteoarthritis outcome score to performance measures in patients after total knee arthroplasty. PM&Amp;R 2011;3:541-9. http://dx.doi.org/10.1016/j.pmrj.2011.03.002.
- Stratford PW, Kennedy DM, Riddle DL. New study design evaluated the validity of measures to assess change after hip or knee arthroplasty. J Clin Epidemiol 2009;62:347-52. http://dx.doi.org/10.1016/j.jclinepi.2008.06.008.
- Mizner RL, Petterson SC, Clements KE, Zeni JA, Irrgang JJ, Snyder-Mackler L. Measuring functional improvement after total knee arthroplasty requires both performance-based and patient-report assessments: a longitudinal analysis of outcomes. J Arthroplasty 2011;26:728-37. http://dx.doi.org/10.1016/j.arth.2010.06.004.
- Terwee CB, van der Slikke RM, van Lummel RC, Benink RJ, Meijers WG, de Vet HC. Self-reported physical functioning was more influenced by pain than performance-based physical functioning in knee-osteoarthritis patients. J Clin Epidemiol 2006;59:724-31. http://dx.doi.org/10.1016/j.jclinepi.2005.11.019.
- Bream E, Black N. What is the relationship between patients’ and clinicians’ reports of the outcomes of elective surgery?. J Health Serv Res Policy 2009;14:174-82. http://dx.doi.org/10.1258/jhsrp.2009.008115.
- Kauppila AM, Kyllonen E, Mikkonen P, Ohtonen P, Laine V, Siira P, et al. Disability in end-stage knee osteoarthritis. Disabil Rehabil 2009;31:370-80. http://dx.doi.org/10.1080/09638280801976159.
- Lin YC, Davey RC, Cochrane T. Tests for physical function of the elderly with knee and hip osteoarthritis. Scand J Med Sci Sports 2001;11:280-6. http://dx.doi.org/10.1034/j.1600-0838.2001.110505.x.
- Swinkels A, Allain TJ. Physical performance tests, self-reported outcomes, and accidental falls before and after total knee arthroplasty: An exploratory study. Physiother Theory Pract 2013;26:432-42. http://dx.doi.org/10.3109/09593985.2012.755590.
- Unnanuntana A, Mait JE, Shaffer AD, Lane JM, Mancuso CA. Performance-based tests and self-reported questionnaires provide distinct information for the preoperative evaluation of total hip arthroplasty patients. J Arthroplasty 2012;27:770-5e1.
- McGrory BJ, Freiberg AA, Shinar AA, Harris WH. Correlation of measured range of hip motion following total hip arthroplasty and responses to a questionnaire. J Arthroplasty 1996;11:565-71. http://dx.doi.org/10.1016/S0883-5403(96)80111-2.
- Ghanem E, Pawasarat I, Lindsay A, May L, Azzam K, Joshi A, et al. Limitations of the knee society score in evaluating outcomes following revision total knee arthroplasty. J Bone Joint Surg Am 2010;92:2445-51. http://dx.doi.org/10.2106/JBJS.I.00252.
- Lingard EA, Katz JN, Wright RJ, Wright EA, Sledge CB. Validity and responsiveness of the knee society clinical rating system in comparison with the SF-36 and WOMAC. J Bone Joint Surg Am 2001;83–A:1856-64.
- Jacobs CA, Christensen CP. Correlations between knee society function scores and functional force measures. Clin Orthop Relat Res 2009;467:2414-19. http://dx.doi.org/10.1007/s11999-009-0811-0.
- Shields RK, Enloe LJ, Evans RE, Smith KB, Steckel SD. Reliability, validity, and responsiveness of functional tests in patients with total joint replacement. Phys Ther 1995;75:169-76.
- Steultjens MP, Dekker J, van Baar ME, Oostendorp RA, Bijlsma JW. Range of joint motion and disability in patients with osteoarthritis of the knee or hip. Rheumatology 2000;39:955-61. http://dx.doi.org/10.1093/rheumatology/39.9.955.
- Cress ME, Schechtman KB, Mulrow CD, Fiatarone MA, Gerety MB, Buchner DM. Relationship between physical performance and self-perceived physical function. J Am Geriatr Soc 1995;43:93-101. http://dx.doi.org/10.1111/j.1532-5415.1995.tb06372.x.
- Dowd JB, Todd M. Does self-reported health bias the measurement of health inequalities in U.S. adults? Evidence using anchoring vignettes from the health and retirement study. J Gerontol B Psychol Sci Soc Sci 2011;66:478-89. http://dx.doi.org/10.1093/geronb/gbr050.
- Louie GH, Ward MM. Association of measured physical performance and demographic and health characteristics with self-reported physical function: implications for the interpretation of self-reported limitations. Health Qual Life Outcomes 2010;8. http://dx.doi.org/10.1186/1477-7525-8-84.
- Wright AA, Hegedus EJ, Baxter GD, Abbott JH. Measurement of function in hip osteoarthritis: developing a standardized approach for physical performance measures. Physiother Theory Pract 2011;27:253-62. http://dx.doi.org/10.3109/09593985.2010.491150.
- Gajdosik RL, Bohannon RW. Clinical measurement of range of motion. Review of goniometry emphasizing reliability and validity. Phys Ther 1987;67:1867-72.
- Williams SL, Haskard KB, DiMatteo MR. The therapeutic effects of the physician-older patient relationship: effective communication with vulnerable older patients. Clin Interv Aging 2007;2:453-67.
- Jordan KP, Wilkie R, Muller S, Myers H, Nicholls E. Arthritis Res Campaign Natl P . Measurement of change in function and disability in osteoarthritis: current approaches and future challenges. Curr Opin Rheumatol 2009;21:525-30. http://dx.doi.org/10.1097/BOR.0b013e32832e45fc.
- Clark DO. Age, socioeconomic status, and exercise self-efficacy. Gerontologist 1996;36:157-64. http://dx.doi.org/10.1093/geront/36.2.157.
- Miner AL, Lingard EA, Wright EA, Sledge CB, Katz JN. Knee range of motion after total knee arthroplasty: how important is this as an outcome measure?. J Arthroplasty 2003;18:286-94. http://dx.doi.org/10.1054/arth.2003.50046.
- Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata Volume II: Categorical Responses, Counts, and Survival. College Station, TX: Stata Press; 2012.
- Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty: a qualitative and systematic review of the literature. J Bone Joint Surg Am 2004;86–A:963-74.
- Halket A, Stratford PW, Kennedy DM, Woodhouse LJ. Using hierarchical linear modeling to explore predictors of pain after total hip and knee arthroplasty as a consequence of osteoarthritis. J Arthroplasty 2010;25:254-62. http://dx.doi.org/10.1016/j.arth.2009.01.007.
- Kennedy DM, Stratford PW, Hanna SE, Wessel J, Gollish JD. Modeling early recovery of physical function following hip and knee arthroplasty. BMC Musculoskelet Disord 2006;7. http://dx.doi.org/10.1186/1471-2474-7-100.
- Escobar A, Quintana JM, Bilbao A, Arostegui I, Lafuente I, Vidaurreta I. Responsiveness and clinically important differences for the WOMAC and SF-36 after total knee replacement. Osteoarthritis Cart 2007;15:273-80. http://dx.doi.org/10.1016/j.joca.2006.09.001.
- Quintana JM, Escobar A, Bilbao A, Arostegui I, Lafuente I, Vidaurreta I. Responsiveness and clinically important differences for the WOMAC and SF-36 after hip joint replacement. Osteoarthritis Cart 2005;13:1076-83. http://dx.doi.org/10.1016/j.joca.2005.06.012.
- Davis AM, Perruccio AV, Ibrahim S, Hogg-Johnson S, Wong R, Streiner DL, et al. The trajectory of recovery and the inter-relationships of symptoms, activity and participation in the first year following total hip and knee replacement. Osteoarthritis Cart 2011;19:1413-21. http://dx.doi.org/10.1016/j.joca.2011.08.007.
- Fitzgerald JD, Orav EJ, Lee TH, Marcantonio ER, Poss R, Goldman L, et al. Patient quality of life during the 12 months following joint replacement surgery. Arthritis Rheum 2004;51:100-9. http://dx.doi.org/10.1002/art.20090.
- Heiberg KE, Ekeland A, Bruun-Olsen V, Mengshoel AM. Recovery and prediction of physical functioning outcomes during the first year after total hip arthroplasty. Arch Phys Med Rehabil 2013;94:1352-9. http://dx.doi.org/10.1016/j.apmr.2013.01.017.
- Kennedy DM, Stratford PW, Riddle DL, Hanna SE, Gollish JD. Assessing recovery and establishing prognosis following total knee arthroplasty. Phys Ther 2008;88:22-3. http://dx.doi.org/10.2522/ptj.20070051.
- Kennedy DM, Stratford PW, Robarts S, Gollish JD. Using outcome measure results to facilitate clinical decisions the first year after total hip arthroplasty. J Orthop Sports Phys Ther 2011;41:232-9. http://dx.doi.org/10.2519/jospt.2011.3516.
- Naylor JM, Harmer AR, Heard RC, Harris IA. Patterns of recovery following knee and hip replacement in an Australian cohort. Aust Health Rev 2009;33:124-35. http://dx.doi.org/10.1071/AH090124.
- Baker P, Cowling P, Kurtz S, Jameson S, Gregg P, Deehan D. Reason for revision influences early patient outcomes after aseptic knee revision. Clin Orthop Relat Res 2012;470:2244-52. http://dx.doi.org/10.1007/s11999-012-2278-7.
- Field RE, Cronin MD, Singh PJ. The Oxford hip scores for primary and revision hip replacement. J Bone Joint Surg Br 2005;87:618-22. http://dx.doi.org/10.1302/0301-620X.87B5.15390.
- Stratford PW, Kennedy DM. Does parallel item content on WOMAC’s pain and function subscales limit its ability to detect change in functional status?. BMC Musculoskelet Disord 2004;5. http://dx.doi.org/10.1186/1471-2474-5-17.
- Ekstrom H, Dahlin-Ivanoff S, Elmstahl S. Effects of walking speed and results of timed get-up-and-go tests on quality of life and social participation in elderly individuals with a history of osteoporosis-related fractures. J Aging Health 2011;23:1379-99. http://dx.doi.org/10.1177/0898264311418504.
- Desrosiers J, Noreau L, Rochette A, Bourbonnais D, Bravo G, Bourget A. Predictors of long-term participation after stroke. Disabil Rehabil 2006;28:221-30. http://dx.doi.org/10.1080/09638280500158372.
- Hamzat TK, Kobiri A. Effects of walking with a cane on balance and social participation among community-dwelling post-stroke individuals. Eur J Phys Rehabil Med 2008;44:121-6.
- Fujita K, Takahashi H, Miura C, Ohkubo T, Sato Y, Ugajin T, et al. Walking and mortality in Japan: the Miyagi cohort study. J Epidemiol 2004;14:S26-32. http://dx.doi.org/10.2188/jea.14.S26.
- Kujala UM, Kaprio J, Sarna S, Koskenvuo M. Relationship of leisure-time physical activity and mortality: the Finnish twin cohort. JAMA 1998;279:440-4. http://dx.doi.org/10.1001/jama.279.6.440.
- LaCroix AZ, Leveille SG, Hecht JA, Grothaus LC, Wagner EH. Does walking decrease the risk of cardiovascular disease hospitalizations and death in older adults?. J Am Geriatr Soc 1996;44:113-20. http://dx.doi.org/10.1111/j.1532-5415.1996.tb02425.x.
- Lee IM, Rexrode KM, Cook NR, Manson JE, Buring JE. Physical activity and coronary heart disease in women: is ‘no pain, no gain’ passe?. JAMA 2001;285:1447-54. http://dx.doi.org/10.1001/jama.285.11.1447.
- Morioka S. A cohort study on the relationship between lifestyles and total mortality. Nihon Koshu Eisei Zasshi 1996;43:469-78.
- Seki N. Relationships between walking hours, sleeping hours, meaningfulness of life (ikigai) and mortality in the elderly: prospective cohort study. Nihon Eiseigaku Zasshi 2001;56:535-40. http://dx.doi.org/10.1265/jjh.56.535.
- Horwood J, Johnson E, Gooberman-Hill R. Understanding involvement in surgical orthopaedic randomized controlled trials: a qualitative study of patient and health professional views and experiences. Int J Orthop Trauma Nurs 2016;20:3-12. http://dx.doi.org/10.1016/j.ijotn.2015.05.002.
- Marques E, Blom AW, Lenguerrand E, Wylde V, Noble SM. Local anaesthetic wound infiltration in addition to standard anaesthetic regimen in total hip and knee replacement: long-term cost-effectiveness analyses alongside the APEX randomised controlled trials. BMC Medicine 2015;15. http://dx.doi.org/10.1186/s12916-015-0389-1.
- Wylde V, Gooberman-Hill R, Horwood J, Beswick A, Noble S, Brookes S, et al. The effect of local anaesthetic wound infiltration on chronic pain after lower limb joint replacement: a protocol for a double-blind randomised controlled trial. BMC Musculoskelet Disord 2011;12. http://dx.doi.org/10.1186/1471-2474-12-53.
- Marques E, Jones H, Elvers K, Pyke M, Blom A, Beswick A. Local anaesthetic infiltration for peri-operative pain control in total hip and knee replacement: systematic review and meta-analyses of short- and long-term effectiveness. BMC Musculoskelet Disord 2014;15. http://dx.doi.org/10.1186/1471-2474-15-220.
- Wylde V, Lenguerrand E, Gooberman-Hill R, Beswick AD, Marques E, Noble S, et al. Effect of local anaesthetic infiltration on chronic postsurgical pain after total hip and knee replacement: the APEX randomised controlled trials. Pain 2015;156:1161-70.
- Mann C, Delgado D, Horwood J. Evaluation of internal peer-review to train nurses recruiting to a randomized controlled trial – Internal Peer-review for Recruitment Training in Trials (InterPReTiT). J Adv Nurs 2014;70:777-90. http://dx.doi.org/10.1111/jan.12254.
- Caffo O, Amichetti M, Ferro A, Lucenti A, Valduga F, Galligioni E. Pain and quality of life after surgery for breast cancer. Breast Cancer Res Treat 2003;80:39-48. http://dx.doi.org/10.1023/A:1024435101619.
- Poobalan AS, Bruce J, King PM, Chambers WA, Krukowski ZH, Smith WC. Chronic pain and quality of life following open inguinal hernia repair. Br J Surg 2001;88:1122-6. http://dx.doi.org/10.1046/j.0007-1323.2001.01828.x.
- Perttunen K, Tasmuth T, Kalso E. Chronic pain after thoracic surgery: a follow-up study. Acta Anaesthesiol Scand 1999;43:563-7. http://dx.doi.org/10.1034/j.1399-6576.1999.430513.x.
- Jin F, Chung F. Multimodal analgesia for postoperative pain control. J Clin Anesth 2001;13:524-39. http://dx.doi.org/10.1016/S0952-8180(01)00320-8.
- Liu SS, Richman JM, Thirlby RC, Wu CL. Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: A quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg 2006;203:914-32. http://dx.doi.org/10.1016/j.jamcollsurg.2006.08.007.
- Bianconi M, Ferraro L, Traina GC, Zanoli G, Antonelli T, Guberti A, et al. Pharmacokinetics and efficacy of ropivacaine continuous wound instillation after joint replacement surgery. Br J Anaesth 2003;91:830-5. http://dx.doi.org/10.1093/bja/aeg277.
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d4002.
- Kerr DR, Kohan L. Local infiltration analgesia: a technique for the control of acute postoperative pain following knee and hip surgery: a case study of 325 patients. Acta Orthop 2008;79:174-83. http://dx.doi.org/10.1080/17453670710014950.
- Walter SD, Yao X. Effect sizes can be calculated for studies reporting ranges for outcome variables in systematic reviews. J Clin Epidemiol 2007;60:849-52. http://dx.doi.org/10.1016/j.jclinepi.2006.11.003.
- Hedges LV, Olkin I. Statistical Methods for Meta-analysis. San Diego: Academic Press; 1985.
- Scholten RJPM, de Beurs E, Bouter LM. From effect size into number needed to treat. Lancet 1999;354. http://dx.doi.org/10.1016/S0140-6736(05)77952-6.
- Toftdahl K, Nikolajsen L, Haraldsted V, Madsen F, Tonnesen EK, Soballe K. Comparison of peri- and intraarticular analgesia with femoral nerve block after total knee arthroplasty: a randomized clinical trial. Acta Orthop 2007;78:172-9. http://dx.doi.org/10.1080/17453670710013645.
- Thorsell M, Holst P, Hyldahl HC, Weidenhielm L. Pain control after total knee arthroplasty: a prospective study comparing local infiltration anesthesia and epidural anesthesia. Orthopedics 2010;33:75-80. http://dx.doi.org/10.3928/01477447-20100104-13.
- Higgins JP, White IR, Anzures-Cabrera J. Meta-analysis of skewed data: combining results reported on log-transformed or raw scales. Stat Med 2008;27:6072-92. http://dx.doi.org/10.1002/sim.3427.
- Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med 2007;26:53-77. http://dx.doi.org/10.1002/sim.2528.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. http://dx.doi.org/10.1002/sim.1186.
- Andersen KV, Pfeiffer-Jensen M, Haraldsted V, Soballe K, Andersen KV, Pfeiffer-Jensen M, et al. Reduced hospital stay and narcotic consumption, and improved mobilization with local and intraarticular infiltration after hip arthroplasty: a randomized clinical trial of an intraarticular technique versus epidural infusion in 80 patients. Acta Orthop 2007;78:180-6. http://dx.doi.org/10.1080/17453670710013654.
- Parvataneni HK, Shah VP, Howard H, Cole N, Ranawat AS, Ranawat CS. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections. A prospective randomized study. J Arthroplasty 2007;22:33-8. http://dx.doi.org/10.1016/j.arth.2007.03.034.
- Aguirre J, Baulig B, Dora C, Ekatodramis G, Votta-Velis G, Ruland P, et al. Continuous epicapsular ropivacaine 0.3% infusion after minimally invasive hip arthroplasty: a prospective, randomized, double-blinded, placebo-controlled study comparing continuous wound infusion with morphine patient-controlled analgesia. Anesth Analg 2012;114:456-61. http://dx.doi.org/10.1213/ANE.0b013e318239dc64.
- Andersen LJ, Poulsen T, Krogh B, Nielsen T, Andersen LJ, Poulsen T, et al. Postoperative analgesia in total hip arthroplasty: a randomized double-blinded, placebo-controlled study on peroperative and postoperative ropivacaine, ketorolac, and adrenaline wound infiltration. Acta Orthop 2007;78:187-92. http://dx.doi.org/10.1080/17453670710013663.
- Busch CA, Whitehouse MR, Shore BJ, MacDonald SJ, McCalden RW, Bourne RB. The efficacy of periarticular multimodal drug infiltration in total hip arthroplasty. Clin Orthop Relat Res 2010;468:2152-9. http://dx.doi.org/10.1007/s11999-009-1198-7.
- Dobie I, Bennett D, Spence DJ, Murray JM, Beverland DE. Periarticular local anesthesia does not improve pain or mobility after THA. Clin Orthop Relat Res 2012;470:1958-65. http://dx.doi.org/10.1007/s11999-012-2241-7.
- Lee K-J, Min B-W, Bae K-C, Cho C-H, Kwon D-H. Efficacy of multimodal pain control protocol in the setting of total hip arthroplasty. Clin Orthop Surg 2009;1:155-60. http://dx.doi.org/10.4055/cios.2009.1.3.155.
- Liu W, Cong R, Li X, Wu Y, Wu H. Reduced opioid consumption and improved early rehabilitation with local and intraarticular cocktail analgesic injection in total hip arthroplasty: a randomized controlled clinical trial. Pain Med 2011;12:387-93. http://dx.doi.org/10.1111/j.1526-4637.2010.01043.x.
- Lu ZD, Li P. Analgesic effect of periarticular Ropivacaine infiltration and cyclooxygenase-2 inhibitor following total hip arthroplasty. J Clin Rehabil Tissue Engineering Res 2010;14:7991-4.
- Lunn TH, Husted H, Solgaard S, Kristensen BB, Otte KS, Kjersgaard AG, et al. Intraoperative local infiltration analgesia for early analgesia after total hip arthroplasty: a randomized, double-blind, placebo-controlled trial. Reg Anesth Pain Med 2011;36:424-9. http://dx.doi.org/10.1097/AAP.0b013e3182186866.
- Murphy TP, Byrne DP, Curtin P, Baker JF, Mulhall KJ. Can a periarticular levobupivacaine injection reduce postoperative opiate consumption during primary hip arthroplasty?. Clin Orthop Relat Res 2012;470:1151-7. http://dx.doi.org/10.1007/s11999-011-2108-3.
- Rikalainen-Salmi R, Forster JG, Makela K, Virolainen P, Leino KA, Pitkanen MT, et al. Local infiltration analgesia with levobupivacaine compared with intrathecal morphine in total hip arthroplasty patients. Acta Anaesthesiol Scand 2012;56:695-70. http://dx.doi.org/10.1111/j.1399-6576.2012.02667.x.
- Affas F, Nygårds E-B, Stiller C-O, Wretenberg P, Olofsson C. Pain control after total knee arthroplasty: a randomized trial comparing local infiltration anesthesia and continuous femoral block. Acta Orthop 2011;82:441-7. http://dx.doi.org/10.3109/17453674.2011.581264.
- Andersen KV, Bak M, Christensen BV, Harazuk J, Pedersen NA, Soballe K. A randomized, controlled trial comparing local infiltration analgesia with epidural infusion for total knee arthroplasty. Acta Orthop 2010;81:606-10. http://dx.doi.org/10.3109/17453674.2010.519165.
- Busch CA, Shore BJ, Bhandari R, Ganapathy S, MacDonald SJ, Bourne RB, et al. Efficacy of periarticular multimodal drug injection in total knee arthroplasty. A randomized trial. J Bone Joint Surg Am 2006;88:959-63. http://dx.doi.org/10.2106/JBJS.E.00344.
- Carli F, Clemente A, Asenjo JF, Kim DJ, Mistraletti G, Gomarasca M, et al. Analgesia and functional outcome after total knee arthroplasty: periarticular infiltration vs continuous femoral nerve block. Br J Anaesth 2010;105:185-95. http://dx.doi.org/10.1093/bja/aeq112.
- Chen Y, Zhang Y, Zhu YL, Fu P. Efficacy and safety of an intra-operative intra-articular magnesium/ropivacaine injection for pain control following total knee arthroplasty. J Int Med Res 2012;40:2032-40. http://dx.doi.org/10.1177/030006051204000548.
- Essving P, Axelsson K, Kjellberg J, Wallgren O, Gupta A, Lundin A. Reduced morphine consumption and pain intensity with local infiltration analgesia (LIA) following total knee arthroplasty: a randomized double-blind study involving 48 patients. Acta Orthop 2010;81:354-60. http://dx.doi.org/10.3109/17453674.2010.487241.
- Essving P, Axelsson K, Aberg E, Spannar H, Gupta A, Lundin A. Local infiltration analgesia versus intrathecal morphine for postoperative pain management after total knee arthroplasty: a randomized controlled trial. Anesth Analg 2011;113:926-33. http://dx.doi.org/10.1213/ANE.0b013e3182288deb.
- Fu P, Wu Y, Wu H, Li X, Qian Q, Zhu Y. Efficacy of intra-articular cocktail analgesic injection in total knee arthroplasty – a randomized controlled trial. Knee 2009;16:280-4. http://dx.doi.org/10.1016/j.knee.2008.12.012.
- Fu PL, Xiao J, Zhu YL, Wu HS, Li XH, Wu YL, et al. Efficacy of a multimodal analgesia protocol in total knee arthroplasty: a randomized, controlled trial. J Int Med Res 2010;38:1404-12. http://dx.doi.org/10.1177/147323001003800422.
- Han CD, Lee DH, Yang IH. Intra-synovial ropivacaine and morphine for pain relief after total knee arthroplasty: a prospective, randomized, double blind study. Yonsei Med J 2007;48:295-300. http://dx.doi.org/10.3349/ymj.2007.48.2.295.
- Koh IJ, Kang YG, Chang CB, Do SH, Seong SC, Kim TK. Does periarticular injection have additional pain relieving effects during contemporary multimodal pain control protocols for TKA?: A randomised, controlled study. Knee 2012;19:253-9. http://dx.doi.org/10.1016/j.knee.2011.03.007.
- Krenzel BA, Cook C, Martin GN, Vail TP, Attarian DE, Bolognesi MP. Posterior capsular injections of ropivacaine during total knee arthroplasty: a randomized, double-blind, placebo-controlled study. J Arthroplasty 2009;24:138-43. http://dx.doi.org/10.1016/j.arth.2009.03.014.
- Mahadevan D, Walter RP, Minto G, Gale TC, McAllen CJ, Oldman M. Combined femoral and sciatic nerve block vs combined femoral and periarticular infiltration in total knee arthroplasty. A randomized controlled trial. J Arthroplasty 2012;27:1806-11. http://dx.doi.org/10.1016/j.arth.2012.05.018.
- Meftah M, Wong AC, Nawabi DH, Yun RJ, Ranawat AS, Ranawat CS. Pain management after total knee arthroplasty using a multimodal approach. Orthopedics 2012;35:e660-4. http://dx.doi.org/10.3928/01477447-20120426-19.
- Ng FY, Ng JKF, Chiu KY, Yan CH, Chan CW. Multimodal periarticular injection vs continuous femoral nerve block after total knee arthroplasty. A prospective, crossover, randomized clinical trial. J Arthroplasty 2012;27:1234-8. http://dx.doi.org/10.1016/j.arth.2011.12.021.
- Spreng UJ, Dahl V, Hjall A, Fagerland MW, Ræder J. High-volume local infiltration analgesia combined with intravenous or local ketorolac+morphine compared with epidural analgesia after total knee arthroplasty. Br J Anaesth 2010;105:675-82. http://dx.doi.org/10.1093/bja/aeq232.
- Vendittoli PA, Makinen P, Drolet P, Lavigne M, Fallaha M, Guertin MC, et al. A multimodal analgesia protocol for total knee arthroplasty. A randomized, controlled study. J Bone Joint Surg Am 2006;88:282-9. http://dx.doi.org/10.2106/JBJS.E.00173.
- Zhang J, Jiang Y, Shao JJ, Shen H, Wang Q, Zhang XL. Effect of periarticular multimodal drug injection on pain after total knee arthroplasty. Chinese J Clin Rehabil 2007;11:8678-82.
- Auroy Y, Benhamou D, Bargues L, Ecoffey C, Falissard B, Mercier FJ, et al. Major complications of regional anesthesia in France: the SOS Regional Anesthesia Hotline Service. Anesthesiology 2002;97:1274-80. http://dx.doi.org/10.1097/00000542-200211000-00034.
- Sharma S, Iorio R, Specht L, Davies-Lepie S, Healy W. Complications of femoral nerve block for total knee arthroplasty. Clin Orthop Relat Res 2010;468:135-40. http://dx.doi.org/10.1007/s11999-009-1025-1.
- Tang R, Evans H, Chaput A, Kim C, Tang R, Evans H, et al. Multimodal analgesia for hip arthroplasty. Orthop Clin North Am 2009;40:377-87. http://dx.doi.org/10.1016/j.ocl.2009.04.001.
- Perkins FM, Kehlet H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology 2000;93:1123-33. http://dx.doi.org/10.1097/00000542-200010000-00038.
- Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth 2008;101:77-86. http://dx.doi.org/10.1093/bja/aen099.
- Gupta A. Wound infiltration with local anaesthetics in ambulatory surgery. Curr Opin Anaesthesiol 2010;23:708-13. http://dx.doi.org/10.1097/ACO.0b013e32833f0dd7.
- Gibbs DM, Green TP, Esler CN. The local infiltration of analgesia following total knee replacement: a review of current literature. J Bone Joint Surg Br 2012;94:1154-9. http://dx.doi.org/10.1302/0301-620X.94B9.28611.
- Neugebauer EA, Wilkinson RC, Kehlet H, Schug SA. PROSPECT: a practical method for formulating evidence-based expert recommendations for the management of postoperative pain. Surg Endosc 2007;21:1047-53. http://dx.doi.org/10.1007/s00464-006-9186-4.
- Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16:494-502. http://dx.doi.org/10.1136/ard.16.4.494.
- Tubach F, Ravaud P, Baron G, Falissard B, Logeart I, Bellamy N, et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis 2005;64:29-33. http://dx.doi.org/10.1136/ard.2004.022905.
- Ehrich EW, Davies GM, Watson DJ, Bolognese JA, Seidenberg BC, Bellamy N. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol 2000;27:2635-41.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 2011;30:377-99. http://dx.doi.org/10.1002/sim.4067.
- Hardin J, Hilbe J. Generalized Linear Models and Extensions. College Station, TX: Stata Press; 2007.
- Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702-6. http://dx.doi.org/10.1093/aje/kwh090.
- Zou G, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res 2013;22:661-70. http://dx.doi.org/10.1177/0962280211427759.
- Garellick G, Karrholm J, Rogmark C, Rolfson O, Herberts P. Swedish Hip Arthroplasty Register Annual Report. Göteborg: Centre of Registers Region Västra Götaland; 2011.
- O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med 2009;122:S22-32. http://dx.doi.org/10.1016/j.amjmed.2009.04.007.
- Marques E, Johnson EC, Gooberman-Hill R, Blom AW, Noble S. Using resource use logs to reduce the amount of missing data in economic evaluations alongside trials. Value Health 2013;16:195-201. http://dx.doi.org/10.1016/j.jval.2012.09.008.
- Noble S. APEX – Hip Replacement Participant Resource Use Log: 0 to 3 Months 2012. www.dirum.org/instruments/details/54 (accessed 21 July 2016).
- Noble S. APEX – 3-Month Postoperative Hip Questionnaire 2012. www.dirum.org/instruments/details/55 (accessed 21 July 2016).
- Reference Costs 2011–2012. London: Department of Health; n.d.
- Curtis L. Unit Costs of Health and Social Care 2013. Canterbury: University of Kent; 2014.
- EuroQol Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- Patrick DL, Starks HE, Cain KC, Uhlmann RF, Pearlman RA. Measuring preferences for health states worse than death. Med Decis Making 1994;14:9-18. http://dx.doi.org/10.1177/0272989X9401400102.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQol: Results From a UK General Population Survey. York: University of York, Centre for Health Economics; 1995.
- Edwards RT, Hounsome B, Russell D, Russell I, Williams N. QALY calculation alongside randomised controlled trials: from the torch to the traffic light. Paris: Franco-British Meeting in Health Economics CES/HESG; 2004.
- Curtis L. Unit Costs of Health and Social Care. Canterbury: University of Kent; 2012.
- White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica 1980;48:817-38. http://dx.doi.org/10.2307/1912934.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. http://dx.doi.org/10.1002/hec.944.
- Royston P. Multiple imputation of missing values: update. Stata J 2005;5:188-201.
- Ritchie J, Spencer L, Bryman A, Burgess RG. Analyzing Qualitative Data. London: Routledge; 1994.
- Watson JM, Torgerson DJ. Increasing recruitment to randomised trials: a review of randomised controlled trials. BMC Med Res Methodol 2006;6. http://dx.doi.org/10.1186/1471-2288-6-34.
- Wade J, Donovan JL, Lane JA, Neal DE, Hamdy FC. It’s not just what you say, it’s also how you say it: opening the ‘black box’ of informed consent appointments in randomised controlled trials. Soc Sci Med 2009;68:2018-28. http://dx.doi.org/10.1016/j.socscimed.2009.02.023.
- Robinson EJ, Kerr CE, Stevens AJ, Lilford RJ, Braunholtz DA, Edwards SJ, et al. Lay public’s understanding of equipoise and randomisation in randomised controlled trials. Health Technol Assess 2005;9. http://dx.doi.org/10.3310/hta9080.
- Lane JA, Wade J, Down L, Bonnington S, Holding PN, Lennon T, et al. A peer review intervention for monitoring and evaluating sites (PRIME) that improved randomized controlled trial conduct and performance. J Clin Epidemiol 2011;64:628-36. http://dx.doi.org/10.1016/j.jclinepi.2010.10.003.
- de Salis I, Tomlin Z, Toerien M, Donovan J. Using qualitative research methods to improve recruitment to randomized controlled trials: the Quartet study. J Health Serv Res Policy 2008;13:92-6. http://dx.doi.org/10.1258/jhsrp.2008.008028.
- Fletcher B, Gheorghe A, Moore D, Wilson S, Damery S. Improving the recruitment activity of clinicians in randomised controlled trials: a systematic review. BMJ Open 2012;2. http://dx.doi.org/10.1136/bmjopen-2011-000496.
- Rout A, Roberts P. Peer review in nursing and midwifery: a literature review. J Clin Nurs 2008;17:427-42.
- Cameron N, McMillan R. Enhancing communication skills by peer review of consultation videos. Education for Primary Care 2006;17:40-8. http://dx.doi.org/10.1080/1475990X.2006.11493509.
- Paramasivan S, Huddart R, Hall E, Lewis R, Birtle A, Donovan JL. Key issues in recruitment to randomised controlled trials with very different interventions: a qualitative investigation of recruitment to the SPARE trial (CRUK/07/011). Trials 2011;12. http://dx.doi.org/10.1186/1745-6215-12-78.
- Weaver T, Renton A, Tyrer P, Ritchie J. Combining qualitative studies with randomised controlled trials is often useful. BMJ 1996;313. http://dx.doi.org/10.1136/bmj.313.7057.629.
- Donovan J, Little P, Mills N, Smith M, Brindle L, Jacoby A, et al. Quality improvement report. Improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. BMJ 2002;325:766-70. http://dx.doi.org/10.1136/bmj.325.7367.766.
- Lewin S, Glenton C, Oxman AD. Use of qualitative methods alongside randomised controlled trials of complex healthcare interventions: methodological study. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b3496.
- Bhandari M, Devereaux PJ, Swiontkowski MF, Tornetta P, Obremskey W, Koval KJ, et al. Internal fixation compared with arthroplasty for displaced fractures of the femoral neck. A meta-analysis. J Bone Joint Surg Am 2003;85–A:1673-81.
- Garrett WE, Swiontkowski MF, Weinstein JN, Callaghan J, Rosier RN, Berry DJ, et al. American board of orthopaedic surgery practice of the orthopaedic surgeon: part-II, certification examination case mix. J Bone Joint Surg Am 2006;88:660-7. http://dx.doi.org/10.2106/JBJS.E.01208.
- Swiontkowski MF, Agel J. Lessons learned from benchmark orthopaedic trials. J Bone Joint Surg Am 2012;94:11-4. http://dx.doi.org/10.2106/JBJS.K.01622.
- Sikweyiya Y, Jewkes R. Potential motivations for and perceived risks in research participation: ethics in health research. Qual Health Res 2013;23:999-1009. http://dx.doi.org/10.1177/1049732313490076.
- Gooberman-Hill R, Heathcote C, Reid CM, Horwood J, Beswick AD, Williams S, et al. Professional experience guides opioid prescribing for chronic joint pain in primary care. Fam Pract 2011;28:102-9. http://dx.doi.org/10.1093/fampra/cmq083.
- The Care and Management of Osteoarthritis in Adults. London: NICE; 2008.
- Joelsson M, Olsson LE, Jakobsson E. Patients’ experience of pain and pain relief following hip replacement surgery. J Clin Nurs 2010;19:2832-8. http://dx.doi.org/10.1111/j.1365-2702.2010.03215.x.
- Niemi-Murola L, Poyhia R, Onkinen K, Rhen B, Makela A, Niemi TT. Patient satisfaction with postoperative pain management--effect of preoperative factors. Pain Manag Nurs 2007;8:122-9. http://dx.doi.org/10.1016/j.pmn.2007.05.003.
- Boyatzis R. Transforming Qualitative Information: Thematic Analysis and Code Development. Thousand Oaks, CA: Sage; 1998.
- Charmaz K. Constructing Grounded Theory: A Practical Guide Through Qualitative Analysis. London: Sage; 2006.
- Jerosch-Herold C, Shepstone L, Vaughan S, Barrett B, Larson D, Chojnowski A. A questionnaire-based survey of participants’ decisions regarding recruitment and retention in a randomised controlled trial – lessons learnt from the SCoRD trial. Contemp Clin Trials 2011;32:363-8. http://dx.doi.org/10.1016/j.cct.2011.01.014.
- Hallowell N, Cooke S, Crawford G, Lucassen A, Parker M, Snowdon C. An investigation of patients’ motivations for their participation in genetics-related research. J Med Ethics 2010;36:37-45. http://dx.doi.org/10.1136/jme.2009.029264.
- Locock L, Smith L. Personal benefit, or benefiting others? Deciding whether to take part in clinical trials. Clin Trials 2011;8:85-93. http://dx.doi.org/10.1177/1740774510392257.
- McCann S, Campbell M, Entwistle V. Reasons for participating in randomised controlled trials: conditional altruism and considerations for self. Trials 2010;11. http://dx.doi.org/10.1186/1745-6215-11-31.
- Wylde V, Sayers A, Lenguerrand E, Gooberman-Hill R, Pyke M, Beswick AD, et al. Preoperative widespread pain sensitization and chronic pain after hip and knee replacement: a cohort analysis. Pain 2015;156:47-54. http://dx.doi.org/10.1016/j.pain.0000000000000002.
- Sayers A, Wylde V, Lenguerrand E, Beswick AD, Gooberman-Hill R, Pyke M, et al. Rest pain and movement-evoked pain as unique constructs in hip and knee replacements. Arthritis Care Res 2016;68:237-45. http://dx.doi.org/10.1002/acr.22656.
- Bedson J, Croft P. The discordance between clinical and radiographic knee osteoarthritis: A systematic search and summary of the literature. BMC Musculoskelet Disord 2008;9. http://dx.doi.org/10.1186/1471-2474-9-116.
- Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol 2000;27:1513-7.
- Valdes AM, Doherty SA, Zhang W, Muir KR, Maciewicz RA, Doherty M. Inverse relationship between preoperative radiographic severity and postoperative pain in patients with osteoarthritis who have undergone total joint arthroplasty. Semin Arthritis Rheum 2012;41:568-75. http://dx.doi.org/10.1016/j.semarthrit.2011.07.002.
- Dowsey MM, Nikpour M, Dieppe P, Choong PF. Associations between pre-operative radiographic changes and outcomes after total knee joint replacement for osteoarthritis. Osteoarthritis Cart 2012;20:1095-102. http://dx.doi.org/10.1016/j.joca.2012.05.015.
- Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cart 2007;15:A1-56.
- Dieppe P, Judge A, Williams S, Ikwueke I, Guenther K-P, Floeren M, et al. Variations in the pre-operative status of patients coming to primary hip replacement for osteoarthritis in European orthopaedic centres. BMC Musculoskelet Disord 2009;10. http://dx.doi.org/10.1186/1471-2474-10-19.
- Australian Hospital Statistics 2012–13. Elective Surgery Waiting Times. Canberra, ACT: Australian Institute of Health and Welfare; 2013.
- Gooberman-Hill R, Sansom A, Sanders C, Dieppe P, Horwood J, Learmonth I, et al. Unstated factors in orthopaedic decision-making: a qualitative study. BMC Musculoskelet Disord 2010;11. http://dx.doi.org/10.1186/1471-2474-11-213.
- Srikandarajah S, Gilron I. Systematic review of movement-evoked pain versus pain at rest in postsurgical clinical trials and meta-analyses: a fundamental distinction requiring standardized measurement. Pain 2011;152:1734-9. http://dx.doi.org/10.1016/j.pain.2011.02.008.
- Stata 13 Structural Equation Modeling Reference Manual. College Station: TX: Stata Press; 2013.
- Wylde V, Palmer S, Learmonth ID, Dieppe P. The association between pre-operative pain sensitisation and chronic pain after knee replacement: an exploratory study. Osteoarthritis Cart 2013;21:1253-6. http://dx.doi.org/10.1016/j.joca.2013.05.008.
- Suokas AK, Walsh DA, McWilliams DF, Condon L, Moreton B, Wylde V, et al. Quantitative sensory testing in painful osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cart 2012;20:1075-85. http://dx.doi.org/10.1016/j.joca.2012.06.009.
- Leckie G, Charlton C. runmlwin: a Program to Run the MLwiN Multilevel Modeling Software from within Stata. J Stat Softw 2013;52:1-40.
- Berge DJ, Dolin SJ, Williams AC, Harman R. Pre-operative and postoperative effect of a pain management programme prior to total hip replacement: a randomized controlled trial. Pain 2004;110:33-9. http://dx.doi.org/10.1016/j.pain.2004.03.002.
- Bitterli R, Sieben JM, Hartmann M, de Bruin ED. Pre-operative, sensory–motor training for patients undergoing total hip replacement: a randomised controlled trial. Phys Med Rehab Kuror 2009;19:193-201. http://dx.doi.org/10.1055/s-0029-1220725.
- Butler GS, Hurley CA, Buchanan KL, Smith-VanHorne J. Prehospital education: effectiveness with total hip replacement surgery patients. Patient Educ Couns 1996;29:189-97. http://dx.doi.org/10.1016/0738-3991(96)00883-X.
- Clode-Baker EG, Draper E, Raymond N, Haslam C, Gregg P. Preparing patients for total hip replacement. A randomized controlled trial of a preoperative educational intervention. J Health Psychol 1997;2:107-14. http://dx.doi.org/10.1177/135910539700200111.
- Cooil J, Bithell C. Pre-operative education for patients undergoing total hip replacement: a comparison of two methods. Physiother Theory Pract 1997;13:163-73. http://dx.doi.org/10.3109/09593989709036459.
- Doering S, Katzlberger F, Rumpold G, Roessler S, Hofstoetter B, Schatz DS, et al. Videotape preparation of patients before hip replacement surgery reduces stress. Psychosom Med 2000;62:365-73. http://dx.doi.org/10.1097/00006842-200005000-00010.
- Ferrara PE, Rabini A, Aprile I, Maggi L, Piazzini DB, Logroscino G, et al. Effect of pre-operative physiotherapy in patients with end-stage osteoarthritis undergoing hip arthroplasty. Clin Rehabil 2008;22:977-86. http://dx.doi.org/10.1177/0269215508094714.
- Gilbey HJ, Ackland TR, Wang AW, Morton AR, Trouchet T, Tapper J. Exercise improves early functional recovery after total hip arthroplasty. Clin Orthop Relat Res 2003:193-200. http://dx.doi.org/10.1097/00003086-200303000-00025.
- Giraudet-Le Quintrec J-S, Coste J, Vastel L, Pacault V, Jeanne L, Lamas J-P, et al. Positive effect of patient education for hip surgery: a randomized trial. Clin Orthop Relat Res 2003:112-20. http://dx.doi.org/10.1097/01.blo.0000079268.91782.bc.
- Gocen Z, Sen A, Unver B, Karatosun V, Gunal I. The effect of preoperative physiotherapy and education on the outcome of total hip replacement: a prospective randomized controlled trial. Clin Rehabil 2004;18:353-8. http://dx.doi.org/10.1191/0269215504cr758oa.
- Hoogeboom TJ, Dronkers JJ, van den Ende CHM, Oosting E, van Meeteren NLU. Preoperative therapeutic exercise in frail elderly scheduled for total hip replacement: a randomized pilot trial. Clin Rehabil 2010;24:901-10. http://dx.doi.org/10.1177/0269215510371427.
- Johansson K, Salantera S, Katajisto J. Empowering orthopaedic patients through preadmission education: results from a clinical study. Patient Educ Couns 2007;66:84-91. http://dx.doi.org/10.1016/j.pec.2006.10.011.
- Lilja Y, Rydn S, Fridlund B. Effects of extended preoperative information on perioperative stress: an anaesthetic nurse intervention for patients with breast cancer and total hip replacement. Intensive Crit Care Nurs 1998;14:276-82. http://dx.doi.org/10.1016/S0964-3397(98)80688-5.
- Mancuso CA, Graziano S, Briskie LM, Peterson MGE, Pellicci PM, Salvati EA, et al. Randomized trials to modify patients’ preoperative expectations of hip and knee arthroplasties. Clin Orthop Relat Res 2008;466:424-31. http://dx.doi.org/10.1007/s11999-007-0052-z.
- McGregor AH, Rylands H, Owen A, Dore CJ, Hughes SPF. Does preoperative hip rehabilitation advice improve recovery and patient satisfaction?. J Arthroplasty 2004;19:464-8. http://dx.doi.org/10.1016/j.arth.2003.12.074.
- Oosting E, Jans MP, Dronkers JJ, Naber RH, Dronkers-Landman CM, Appelman-de Vries SM, et al. Preoperative home-based physical therapy versus usual care to improve functional health of frail older adults scheduled for elective total hip arthroplasty: a pilot randomized controlled trial. Arch Phys Med Rehabil 2012;93:610-6. http://dx.doi.org/10.1016/j.apmr.2011.11.006.
- Rooks DS, Huang J, Bierbaum BE, Bolus SA, Rubano J, Connolly CE, et al. Effect of preoperative exercise on measures of functional status in men and women undergoing total hip and knee arthroplasty. Arthritis Rheum 2006;55:700-8. http://dx.doi.org/10.1002/art.22223.
- Sandell C-L. A multidisciplinary assessment and intervention for patients awaiting total hip replacement to improve their quality of life. J Orthop Nurs 2008;12:26-34. http://dx.doi.org/10.1016/j.joon.2007.11.002.
- Santavirta N, Lillqvist G, Sarvimaki A, Honkanen V, Konttinen YT, Santavirta S. Teaching of patients undergoing total hip replacement surgery. Int J Nurs Stud 1994;31:135-42. http://dx.doi.org/10.1016/0020-7489(94)90040-X.
- Vukomanovic A, Popovic Z, Durovic A, Krstic L. The effects of short-term preoperative physical therapy and education on early functional recovery of patients younger than 70 undergoing total hip arthroplasty. Vojnosanit Pregl 2008;65:291-7. http://dx.doi.org/10.2298/VSP0804291V.
- Wijgman AJ, Dekkers GH, Waltje E, Krekels T, Arens HJ. No positive effect of preoperative exercise therapy and teaching in patients to be subjected to hip arthroplasty. Ned Tijdschr Genees 1994;138:949-52.
- Wong J, Wong S. A randomized controlled trial of a new approach to preoperative teaching and patient compliance. Int J Nurs Stud 1985;22:105-15. http://dx.doi.org/10.1016/0020-7489(85)90021-5.
- Aoki O, Tsumura N, Kimura A, Okuyama S, Takikawa S, Hirata S. Home stretching exercise is effective for improving knee range of motion and gait in patients with knee osteoarthritis. J Phys Ther Sci 2009;21:113-19. http://dx.doi.org/10.1589/jpts.21.113.
- Beaupre LA, Lier D, Davies DM, Johnston DBC. The effect of a preoperative exercise and education program on functional recovery, health related quality of life, and health service utilization following primary total knee arthroplasty. J Rheumatol 2004;31:1166-73.
- Börjesson M, Robertson E, Weidenhielm L, Mattsson E, Olsson E. Physiotherapy in knee osteoarthrosis: effect on pain and walking. Physiother Res Int 1996;1:89-97. http://dx.doi.org/10.1002/pri.6120010205.
- Brown K, Topp R, Brosky JA, Lajoie AS, Brown K, Topp R, et al. Prehabilitation and quality of life three months after total knee arthroplasty: a pilot study. Perceptual &Amp; Motor Skills 2012;115:765-74. http://dx.doi.org/10.2466/15.06.10.PMS.115.6.765-774.
- D’Lima DD, Colwell CW, Morris BA, Hardwick ME, Kozin F. The effect of preoperative exercise on total knee replacement outcomes. Clin Orthop Relat Res 1996:174-82. http://dx.doi.org/10.1097/00003086-199605000-00020.
- Evgeniadis G, Beneka A, Malliou P, Mavromoustakos S, Godolias G. Effects of pre- or postoperative therapeutic exercise on the quality of life, before and after total knee arthroplasty for osteoarthritis. J Back Musculoskelet 2008;21:161-9.
- Gstoettner M, Raschner C, Dirnberger E, Leimser H, Krismer M. Preoperative proprioceptive training in patients with total knee arthroplasty. Knee 2011;18:265-70. http://dx.doi.org/10.1016/j.knee.2010.05.012.
- Heikkinen K, Helena LK, Taina N, Anne K, Sanna S. A comparison of two educational interventions for the cognitive empowerment of ambulatory orthopaedic surgery patients. Patient Educ Couns 2008;73:272-9. http://dx.doi.org/10.1016/j.pec.2008.06.015.
- Huang SW, Chen PH, Chou YH. Effects of a preoperative simplified home rehabilitation education program on length of stay of total knee arthroplasty patients. Orthop Traumatol Surg Res 2012;98:259-64. http://dx.doi.org/10.1016/j.otsr.2011.12.004.
- McDonald DD, Molony SL. Postoperative pain communication skills for older adults. West J Nurs Res 2004;26:836-52. http://dx.doi.org/10.1177/0193945904269292.
- McKay C, Prapavessis H, Doherty T. The effect of a prehabilitation exercise program on quadriceps strength for patients undergoing total knee arthroplasty: a randomized controlled pilot study. PM&Amp;R 2012;4:647-56. http://dx.doi.org/10.1016/j.pmrj.2012.04.012.
- Mitchell C, Walker J, Walters S, Morgan AB, Binns T, Mathers N. Costs and effectiveness of pre- and postoperative home physiotherapy for total knee replacement: randomized controlled trial. J Eval Clin Pract 2005;11:283-92. http://dx.doi.org/10.1111/j.1365-2753.2005.00535.x.
- Nuñez M, Nuñez E, Segur JM, Macule F, Quinto L, Hernandez MV, et al. The effect of an educational program to improve health-related quality of life in patients with osteoarthritis on waiting list for total knee replacement: a randomized study. Osteoarthritis Cart 2006;14:279-85. http://dx.doi.org/10.1016/j.joca.2005.10.002.
- Sjoling M, Nordahl G, Olofsson N, Asplund K. The impact of preoperative information on state anxiety, postoperative pain and satisfaction with pain management. Patient Educ Couns 2003;51:169-76. http://dx.doi.org/10.1016/S0738-3991(02)00191-X.
- Swank AM, Kachelman JB, Bibeau W, Quesada PM, Nyland J, Malkani A, et al. Prehabilitation before total knee arthroplasty increases strength and function in older adults with severe osteoarthritis. J Strength Cond Res 2011;25:318-25. http://dx.doi.org/10.1519/JSC.0b013e318202e431.
- Weidenhielm L, Mattsson E, Brostrom LA, Wersall-Robertsson E. Effect of preoperative physiotherapy in unicompartmental prosthetic knee replacement. Scand J Rehabil Med 1993;25:33-9.
- Williamson L, Wyatt MR, Yein K, Melton JT. Severe knee osteoarthritis: a randomized controlled trial of acupuncture, physiotherapy (supervised exercise) and standard management for patients awaiting knee replacement. Rheumatology 2007;46:1445-9. http://dx.doi.org/10.1093/rheumatology/kem119.
- Bondy LR, Sims N, Schroeder DR, Offord KP, Narr BJ. The effect of anesthetic patient education on preoperative patient anxiety. Reg Anesth Pain Med 1999;24:158-64. http://dx.doi.org/10.1016/s1098-7339(99)90078-0.
- Crotty M, Prendergast J, Baftersby MW, Rowett D, Graves SE, Leach G, et al. Self-management and peer support among people with arthritis on a hospital joint replacement waiting list: a randomised controlled trial. Osteoarthritis Cart 2009;17:1428-33. http://dx.doi.org/10.1016/j.joca.2009.05.010.
- Crowe J, Henderson J. Pre-arthroplasty rehabilitation is effective in reducing hospital stay. Can J Occup Ther 2003;70:88-96. http://dx.doi.org/10.1177/000841740307000204.
- Cuñádo Barrio A, Legarre Gil MJ, Ruiz Castón J, Silveira de la Torre JS, Caballero Martínez L, García López F. The effect of a structured, individualized ‘nursing visit’ on the anxiety of surgical patients. A randomized clinical study. Enfermería Clínica 1999;9:98-104.
- Daltroy LH, Morlino CI, Eaton HM, Poss R, Liang MH. Preoperative education for total hip and knee replacement patients. Arthritis Care Res 1998;11:469-78. http://dx.doi.org/10.1002/art.1790110607.
- Lewis C, Gunta K, Wong D. Patient knowledge, behaviour, and satisfaction with the use of a preoperative DVD. Orthop Nurs 2002;21:41-3.
- Liu W, Lu M. Empowerment model of preoperative education for orthopaedic patients. Chin J Clin Rehab 2004;8:840-1.
- McDonald DD, Freeland M, Thomas G, Moore J. Testing a preoperative pain management intervention for elders. Res Nurs Health 2001;24:402-9. http://dx.doi.org/10.1002/nur.1040.
- Pellino T, Tluczek A, Collins M, Trimborn S, Norwick H, Engelke ZK, et al. Increasing self-efficacy through empowerment: preoperative education for orthopaedic patients. Orthop Nurs 1998;17:48-51. http://dx.doi.org/10.1097/00006416-199807000-00009.
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988.
- Wylde V, Marques E, Artz N, Blom A, Gooberman-Hill R. Effectiveness and cost-effectiveness of a group-based pain self-management intervention for patients undergoing total hip replacement: feasibility study for a randomized controlled trial. Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-176.
- Buszewicz M, Rait G, Griffin M, Nazareth I, Patel A, Atkinson A, et al. Self management of arthritis in primary care: randomised controlled trial. BMJ 2006;333. http://dx.doi.org/10.1136/bmj.38965.375718.80.
- Ackerman IN, Buchbinder R, Osborne RH. Factors limiting participation in arthritis self-management programmes: an exploration of barriers and patient preferences within a randomized controlled trial. Rheumatology 2013;52:472-9. http://dx.doi.org/10.1093/rheumatology/kes295.
- Ackerman IN, Buchbinder R, Osborne RH. Challenges in evaluating an Arthritis Self-Management Program for people with hip and knee osteoarthritis in real-world clinical settings. J Rheumatol 2012;39:1047-55. http://dx.doi.org/10.3899/jrheum.111358.
- Newman S, Steed L, Mulligan K. Self-management interventions for chronic illness. Lancet 2004;364:1523-37. http://dx.doi.org/10.1016/S0140-6736(04)17277-2.
- Bruce B, Lorig K, Laurent D. Participation in patient self-management programs. Arthritis Rheum 2007;57:851-4. http://dx.doi.org/10.1002/art.22776.
- Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004;10:307-12. http://dx.doi.org/10.1111/j. 2002.384.doc.x.
- McMurran M, Cox WM, Whitham D, Hedges L. The addition of a goal-based motivational interview to treatment as usual to enhance engagement and reduce dropouts in a personality disorder treatment service: results of a feasibility study for a randomized controlled trial. Trials 2013;14. http://dx.doi.org/10.1186/1745-6215-14-50.
- Farrin A, Russell I, Torgerson D, Underwood M. Differential recruitment in a cluster randomized trial in primary care: the experience of the UK back pain, exercise, active management and manipulation (UK BEAM) feasibility study. Clin Trials 2005;2:119-24. http://dx.doi.org/10.1191/1740774505cn073oa.
- Eldridge SM, Ashby D, Feder GS, Rudnicka AR, Ukoumunne OC. Lessons for cluster randomized trials in the twenty-first century: a systematic review of trials in primary care. Clin Trials 2004;1:80-9. http://dx.doi.org/10.1191/1740774504cn006rr.
- Lancaster GA, Campbell MJ, Eldridge S, Farrin A, Marchant M, Muller S, et al. Trials in primary care: statistical issues in the design, conduct and evaluation of complex interventions. Stat Methods Med Res 2010;19:349-77. http://dx.doi.org/10.1177/0962280209359883.
- Cramer H, Salisbury C, Conrad J, Eldred J, Araya R. Group cognitive behavioural therapy for women with depression: pilot and feasibility study for a randomised controlled trial using mixed methods. BMC Psychiatry 2011;11. http://dx.doi.org/10.1186/1471-244X-11-82.
- McCracken LM, Sato A, Wainwright D, House W, Taylor GJ. A feasibility study of brief group-based acceptance and commitment therapy for chronic pain in general practice: recruitment, attendance, and patient views. Prim Health Care Res Dev 2013;15:312-23. http://dx.doi.org/10.1017/S1463423613000273.
- Poston L, Briley AL, Barr S, Bell R, Croker H, Coxon K, et al. Developing a complex intervention for diet and activity behaviour change in obese pregnant women (the UPBEAT trial); assessment of behavioural change and process evaluation in a pilot randomised controlled trial. BMC Pregnancy Childbirth 2013;13. http://dx.doi.org/10.1186/1471-2393-13-148.
- Gooberman-Hill R, Burston A. Improving Musculoskeletal Research in North Bristol Through Public Participation 2013.
- Evans S, Royston P, Day S. Minim: Allocation by Minimisation in Clinical Trials 2004. www-users.york.ac.uk/∼mb55/guide/minim.htm (accessed 21 July 2016).
- Gondwe R, Andrea-Jones S, Cowlard J, Taylor M, Yaylor A, Undy B. Evaluate the effectiveness of a new self-management programme for patients with chronic pain. Ann Rheum Dis 2008;67.
- Billingham SA, Whitehead AL, Julious SA. An audit of sample sizes for pilot and feasibility trials being undertaken in the United Kingdom registered in the United Kingdom Clinical Research Network database. BMC Med Res Methodol 2013;13. http://dx.doi.org/10.1186/1471-2288-13-104.
- Shanyinde M, Pickering RM, Weatherall M. Questions asked and answered in pilot and feasibility randomized controlled trials. BMC Med Res Methodol 2011;11. http://dx.doi.org/10.1186/1471-2288-11-117.
- Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol 1999;52:1143-56. http://dx.doi.org/10.1016/S0895-4356(99)00141-9.
- Lansdown H, Howard K, Brealey S, MacPherson H. Acupuncture for pain and osteoarthritis of the knee: a pilot study for an open parallel-arm randomised controlled trial. BMC Musculoskelet Disord 2009;10. http://dx.doi.org/10.1186/1471-2474-10-130.
- Stafford L, Foley E, Judd F, Gibson P, Kiropoulos L, Couper J. Mindfulness-based cognitive group therapy for women with breast and gynecologic cancer: a pilot study to determine effectiveness and feasibility. Support Care Cancer 2013;21:3009-19. http://dx.doi.org/10.1007/s00520-013-1880-x.
- Treweek S, Lockhart P, Pitkethly M, Cook JA, Kjeldstrom M, Johansen M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2012-002360.
- Klitzman R. How IRBs view and make decisions about coercion and undue influence. J Med Ethics 2013;39:224-9. http://dx.doi.org/10.1136/medethics-2011-100439.
- Chambers SK, Foley E, Galt E, Ferguson M, Clutton S. Mindfulness groups for men with advanced prostate cancer: a pilot study to assess feasibility and effectiveness and the role of peer support. Support Care Cancer 2012;20:1183-92. http://dx.doi.org/10.1007/s00520-011-1195-8.
- Wang OJ, Krumholz HM. Clinical trial participation: are we studying the patients we are trying to treat?. Eur J Heart Fail 2009;11:1021-2. http://dx.doi.org/10.1093/eurjhf/hfp137.
- Jepson P, Sands G, Beswick AD, Davis ET, Blom AW, Sackley CM. A feasibility randomised controlled trial of pre-operative occupational therapy to optimise recovery for patients undergoing primary total hip replacement for osteoarthritis (PROOF-THR). Clin Rehabil 2015;30:156-66. http://dx.doi.org/10.1177/0269215515576811.
- Hip Replacements: An Update. London: National Audit Office; 2003.
- NHS Choices . Hip Replacement – Recovery 2014. www.nhs.uk/Conditions/Hip-replacement/Pages/Recovery.aspx (accessed 7 April 2014).
- Westby MD, Kennedy D, Jones D, Jones A, Doyle-Waters MM, Backman C. Post-acute physiotherapy for primary total knee arthroplasty. Cochrane Database Syst Rev 2008;2. http://dx.doi.org/10.1002/14651858.cd007099.
- Orpen N, Harris J. Patients’ perceptions of preoperative home-based occupational therapy and/or physiotherapy interventions prior to total hip replacement. Brit J Occup Ther 2010;73:461-9. http://dx.doi.org/10.4276/030802210X12865330218267.
- Munin MC, Rudy TE, Glynn NW, Crossett LS, Rubash HE. Early inpatient rehabilitation after elective hip and knee arthroplasty. JAMA 1998;279:847-52. http://dx.doi.org/10.1001/jama.279.11.847.
- Siggeirsdottir K, Olafsson O, Jonsson H, Iwarsson S, Gudnason V, Jonsson BY. Short hospital stay augmented with education and home-based rehabilitation improves function and quality of life after hip replacement: randomized study of 50 patients with 6 months of follow-up. Acta Orthop 2005;76:555-62. http://dx.doi.org/10.1080/17453670510041565.
- Weaver FM, Hughes SL, Almagor O, Wixson R, Manheim L, Fulton B, et al. Comparison of two home care protocols for total joint replacement. J Am Geriatr Soc 2003;51:523-8. http://dx.doi.org/10.1046/j.1532-5415.2003.51162.x.
- Jolles BM, Bogoch ER. Posterior versus lateral surgical approach for total hip arthroplasty in adults with osteoarthritis. Cochrane Database Syst Rev 2006;3. http://dx.doi.org/10.1002/14651858.cd003828.pub3.
- Mahoney CR, Pellicci PM. Complications in primary total hip arthroplasty: avoidance and management of dislocations. Instr Course Lect 2003;52:247-55.
- Lucas B. Total hip and total knee replacement: postoperative nursing management. Br J Nurs 2008;17:1410-14. http://dx.doi.org/10.12968/bjon.2008.17.22.31866.
- Peak EL, Parvizi J, Ciminiello M, Purtill JJ, Sharkey PF, Hozack WJ, et al. The role of patient restrictions in reducing the prevalence of early dislocation following total hip arthroplasty. A randomized, prospective study. J Bone Joint Surg Am 2005;87:247-53. http://dx.doi.org/10.2106/JBJS.C.01513.
- Restrepo C, Mortazavi SM, Brothers J, Parvizi J, Rothman RH. Hip dislocation: are hip precautions necessary in anterior approaches?. Clin Orthop Relat Res 2011;469:417-22. http://dx.doi.org/10.1007/s11999-010-1668-y.
- Clemson L, Roland M, Cumming R. Occupational therapy assessment of potential hazards in the homes of elderly people: an inter-rater reliability study. Aust Occup Therap J 1992;39:23-6. http://dx.doi.org/10.1111/j.1440-1630.1992.tb01753.x.
- Murray DW, Fitzpatrick R, Rogers K, Pandit H, Beard DJ, Carr AJ, et al. The use of the Oxford hip and knee scores. J Bone Joint Surg Br 2007;89:1010-14. http://dx.doi.org/10.1302/0301-620X.89B8.19424.
- Beecham JK, Knapp MRJ, Thornicroft G, Brewin C, Wing JK. Measuring Mental Health Needs. London: Gaskell; 1992.
- Beecham J, Knapp M. The Economics of Mental Health Care. Aldershot: Arena; 1995.
- Jan MH, Hung JY, Lin JC, Wang SF, Liu TK, Tang PF. Effects of a home program on strength, walking speed, and function after total hip replacement. Arch Phys Med Rehabil 2004;85:1943-51. http://dx.doi.org/10.1016/j.apmr.2004.02.011.
- Larsen K, Sørensen OG, Hansen TB, Thomsen PB, Søballe K. Accelerated perioperative care and rehabilitation intervention for hip and knee replacement is effective: a randomized clinical trial involving 87 patients with 3 months of follow-up. Acta Orthop 2008;79:149-59. http://dx.doi.org/10.1080/17453670710014923.
- Davis LL, Broome ME, Cox RP. Maximizing retention in community-based clinical trials. J Nurs Scholarsh 2002;34:47-53. http://dx.doi.org/10.1111/j.1547-5069.2002.00047.x.
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319:670-4. http://dx.doi.org/10.1136/bmj.319.7211.670.
- Wood AM, White IR, Thompson SG. Are missing outcome data adequately handled? A review of published randomized controlled trials in major medical journals. Clin Trials 2004;1:368-76. http://dx.doi.org/10.1191/1740774504cn032oa.
- Edwards P, Roberts I, Clarke M, DiGuiseppi C, Pratap S, Wentz R, et al. Increasing response rates to postal questionnaires: systematic review. BMJ 2002;324. http://dx.doi.org/10.1136/bmj.324.7347.1183.
- Artz N, Elvers KT, Lowe CM, Sackley C, Jepson P, Beswick AD. Effectiveness of physiotherapy exercise following total knee replacement: systematic review and meta-analysis. BMC Musculoskelet Disord 2015;16. http://dx.doi.org/10.1186/s12891-015-0469-6.
- Artz N, Dixon S, Wylde V, Marques E, Beswick AD, Lenguerrand E, et al. Comparison of group-based outpatient physiotherapy with usual care after total knee replacement: a feasibility study for a randomized controlled trial. Clin Rehabil 2016;10. http://dx.doi.org/10.1177/0269215516642503.
- Knee Replacement: A Guide to Good Practice. London: British Orthopaedic Association; 1999.
- Frost H, Lamb SE, Robertson S. A randomized controlled trial of exercise to improve mobility and function after elective knee arthroplasty. Feasibility, results and methodological difficulties. Clin Rehabil 2002;16:200-9. http://dx.doi.org/10.1191/0269215502cr483oa.
- Fung V, Ho A, Shaffer J, Chung E, Gomez M. Use of Nintendo Wii Fit in the rehabilitation of outpatients following total knee replacement: a preliminary randomised controlled trial. Physiotherapy 2012;98:183-8. http://dx.doi.org/10.1016/j.physio.2012.04.001.
- Harmer AR, Naylor JM, Crosbie J, Russell T. Land-based versus water-based rehabilitation following total knee replacement: a randomized, single-blind trial. Arthritis Rheum 2009;61:184-91. http://dx.doi.org/10.1002/art.24420.
- Kauppila AM, Kyllonen E, Ohtonen P, Hamalainen M, Mikkonen P, Laine V, et al. Multidisciplinary rehabilitation after primary total knee arthroplasty: a randomized controlled study of its effects on functional capacity and quality of life. Clin Rehabil 2010;24:398-411. http://dx.doi.org/10.1177/0269215509346089.
- Kramer JF, Speechley M, Bourne R, Rorabeck C, Vaz M. Comparison of clinic- and home-based rehabilitation programs after total knee arthroplasty. Clin Orthop Relat Res 2003;410:225-34. http://dx.doi.org/10.1097/01.blo.0000063600.67412.11.
- Liebs TR, Herzberg W, Ruther W, Haasters J, Russlies M, Hassenpflug J. Ergometer cycling after hip or knee replacement surgery: a randomized controlled trial. J Bone Joint Surg Am 2010;92:814-22. http://dx.doi.org/10.2106/JBJS.H.01359.
- Madsen M, Larsen K, Madsen IK, Soe H, Hansen TB. Late group-based rehabilitation has no advantages compared with supervised home-exercises after total knee arthroplasty. Dan Med J 2013;60.
- Minns Lowe CJ, Barker KL, Holder R, Sackley CM. Comparison of postdischarge physiotherapy versus usual care following primary total knee arthroplasty for osteoarthritis: an exploratory pilot randomized clinical trial. Clin Rehabil 2012;26:629-41. http://dx.doi.org/10.1177/0269215511427749.
- Mockford BJ, Thompson NW, Humphreys P, Beverland DE. Does a standard outpatient physiotherapy regime improve the range of knee motion after primary total knee arthroplasty?. J Arthroplasty 2008;23:1110-14. http://dx.doi.org/10.1016/j.arth.2007.08.023.
- Moffet H, Collet J-P, Shapiro SH, Paradis G, Marquis F, Roy L. Effectiveness of intensive rehabilitation on functional ability and quality of life after first total knee arthroplasty: a single-blind randomized controlled trial. Arch Phys Med Rehabil 2004;85:546-56. http://dx.doi.org/10.1016/j.apmr.2003.08.080.
- Monticone M, Ferrante S, Rocca B, Salvaderi S, Fiorentini R, Restelli M, et al. Home-based functional exercises aimed at managing kinesiophobia contribute to improving disability and quality of life of patients undergoing total knee arthroplasty: a randomized controlled trial. Arch Phys Med Rehabil 2013;94:231-9. http://dx.doi.org/10.1016/j.apmr.2012.10.003.
- Piqueras M, Marco E, Coll M, Escalada F, Ballester A, Cinca C, et al. Effectiveness of an interactive virtual telerehabilitation system in patients after total knee arthoplasty: a randomized controlled trial. J Rehabil Med 2013;45:392-6. http://dx.doi.org/10.2340/16501977-1119.
- Piva SR, Gil AB, Almeida GJM, DiGioia AM, Levison TJ, Fitzgerald GK. A balance exercise program appears to improve function for patients with total knee arthroplasty: a randomized clinical trial. Phys Ther 2010;90:880-94. http://dx.doi.org/10.2522/ptj.20090150.
- Rajan RA, Pack Y, Jackson H, Gillies C, Asirvatham R. No need for outpatient physiotherapy following total knee arthroplasty: a randomized trial of 120 patients. Acta Orthop Scand 2004;75:71-3. http://dx.doi.org/10.1080/00016470410001708140.
- Tousignant M, Moffet H, Boissy P, Corriveau H, Cabana F, Marquis F. A randomized controlled trial of home telerehabilitation for post-knee arthroplasty. J Telemed Telecare 2011;17:195-8. http://dx.doi.org/10.1258/jtt.2010.100602.
- Barron CJ, Klaber Moffett JA, Potter M. Patient expectations of physiotherapy: Definitions, concepts, and theories. Physiother Theory Pract 2007;23:37-46. http://dx.doi.org/10.1080/09593980601147843.
- Westby MD, Brittain A, Backman CL. Expert consensus on best practices for post-acute rehabilitation after total hip and knee arthroplasty: a Canada and United States Delphi study. Arthritis Care Res 2014;66:411-23. http://dx.doi.org/10.1002/acr.22164.
- Muniesa JM, Marco E, Tejero M, Boza R, Duarte E, Escalada F, et al. Analysis of the expectations of elderly patients before undergoing total knee replacement. Arch Gerontol Geriatr 2010;51:e83-7. http://dx.doi.org/10.1016/j.archger.2010.01.003.
- Mancuso CA, Sculco TP, Wickiewicz TL, Jones EC, Robbins L, Warren RF, et al. Patients’ expectations of knee surgery. J Bone Joint Surg Am 2001;83:1005-12.
- Tew M, Forster IW, Wallace WA. Effect of total knee arthroplasty on maximal flexion. Clin Orthop Relat Res 1989:168-74. http://dx.doi.org/10.1097/00003086-198910000-00027.
- Park KK, Chang CB, Kang YG, Seong SC, Kim TK. Correlation of maximum flexion with clinical outcome after total knee replacement in Asian patients. J Bone Joint Surg Br 2007;89:604-8. http://dx.doi.org/10.1302/0301-620X.89B5.18117.
- Parent E, Moffet H. Comparative responsiveness of locomotor tests and questionnaires used to follow early recovery after total knee arthroplasty. Arch Phys Med Rehabil 2002;83:70-8. http://dx.doi.org/10.1053/apmr.2002.27337.
- Lindemann U, Becker C, Unnewehr I, Muche R, Aminin K, Dejnabadi H, et al. Gait analysis and WOMAC are complementary in assessing functional outcome in total hip replacement. Clin Rehabil 2006;20:413-20. http://dx.doi.org/10.1191/0269215506cr958oa.
- Alviar MJ, Olver J, Brand C, Hale T, Khan F. Do patient-reported outcome measures used in assessing outcomes in rehabilitation after hip and knee arthroplasty capture issues relevant to patients? Results of a systematic review and ICF linking process. J Rehabil Med 2011;43:374-81. http://dx.doi.org/10.2340/16501977-0801.
- McLean SM, Burton M, Bradley L, Littlewood C. Interventions for enhancing adherence with physiotherapy: A systematic review. Manual Ther 2010;15:514-21. http://dx.doi.org/10.1016/j.math.2010.05.012.
- Beswick A, Rees K, Griebsch I, Taylor F, Burke M, West R, et al. Provision, uptake and cost of cardiac rehabilitation programmes: improving services to under-represented groups. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8410.
- National Joint Registry for England and Wales: 7th Annual Report. Hemel Hempstead: National Joint Registry Centre; 2010.
- Judge A, Chard J, Learmonth I, Dieppe P. The effects of surgical volumes and training centre status on outcomes following total joint replacement: analysis of the Hospital Episode Statistics for England. J Public Health 2006;28:116-24. http://dx.doi.org/10.1093/pubmed/fdl003.
- Hunt GR, Crealey G, Murthy BVS, Hall GM, Constantine P, O’Brien S, et al. The consequences of early discharge after hip arthroplasty for patient outcomes and health care costs: comparison of three centres with differing durations of stay. Clin Rehabil 2009;23:1067-77. http://dx.doi.org/10.1177/0269215509339000.
- Salmon P, Hall GM, Peerbhoy D, Shenkin A, Parker C. Recovery from hip and knee arthroplasty: Patients’ perspective on pain, function, quality of life, and well-being up to 6 months postoperatively. Arch Phys Med Rehabil 2001;82:360-6. http://dx.doi.org/10.1053/apmr.2001.21522.
- de Beer J, Petruccelli D, Adili A, Piccirillo L, Wismer D, Winemaker M. Patient perspective survey of total hip vs total knee arthroplasty surgery. J Arthroplasty 2012;27:865-9.e5. http://dx.doi.org/10.1016/j.arth.2011.12.031.
- Lingard EA, Berven S, Katz JN, Kinemax Outcomes G. Management and care of patients undergoing total knee arthroplasty: variations across different health care settings. Arthritis Care Res 2000;13:129-36. http://dx.doi.org/10.1002/1529-0131(200006)13:3%3C129::AID-ANR1%3E3.0.CO;2-6.
- Naylor J, Harmer A, Fransen M, Crosbie J, Innes L. Status of physiotherapy rehabilitation after total knee replacement in Australia. Physiother Res Int 2006;11:35-47. http://dx.doi.org/10.1002/pri.40.
- Hurley MV, Walsh N, Bhavnani V, Britten N, Stevenson F. Health beliefs before and after participation on an exercised-based rehabilitation programme for chronic knee pain: doing is believing. BMC Musculoskelet Disord 2010;11. http://dx.doi.org/10.1186/1471-2474-11-31.
- Zhang W, Nuki G, Moskowitz RW, Abramson S, Altman RD, Arden NK, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cart 2010;18:476-99. http://dx.doi.org/10.1016/j.joca.2010.01.013.
- Weiss JM, Noble PC, Conditt MA, Kohl HW, Roberts S, Cook KF, et al. What functional activities are important to patients with knee replacements?. Clin Orthop Relat Res 2002:172-88. http://dx.doi.org/10.1097/00003086-200211000-00030.
- Noble PC, Gordon MJ, Weiss JM, Reddix RN, Conditt MA, Mathis KB. Does total knee replacement restore normal knee function?. Clin Orthop Relat Res 2005:157-65. http://dx.doi.org/10.1097/01.blo.0000150130.03519.fb.
- Hurley MV, Walsh NE, Mitchell HL, Pimm TJ, Williamson E, Jones RH, et al. Economic evaluation of a rehabilitation program integrating exercise, self-management, and active coping strategies for chronic knee pain. Arthritis Rheum 2007;57:1220-9. http://dx.doi.org/10.1002/art.23011.
- Westby M, Backman C. Patient and health professional views on rehabilitation practices and outcomes following total hip and knee arthroplasty for osteoarthritis: a focus group study. BMC Health Serv Res 2010;10. http://dx.doi.org/10.1186/1472-6963-10-119.
- Orbell S, Johnston M, Rowley D, Davey P, Espley A. Self-efficacy and goal importance in the prediction of physical disability in people following hospitalization: a prospective study. Br J Health Psychol 2001;6:25-40. http://dx.doi.org/10.1348/135910701169034.
- Naylor JM, Mittal R, Carroll K, Harris IA. Introductory insights into patient preferences for outpatient rehabilitation after knee replacement: implications for practice and future research. J Eval Clin Pract 2012;18:586-92. http://dx.doi.org/10.1111/j.1365-2753.2010.01619.x.
- Rogers KD, Blyth FM, March LM, Jorm L. A nested case–control analysis of self-reported physical functioning after total knee replacement surgery in the 45 and Up Study Cohort. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2012-002291.
- Wright JG, Santaguida PL, Young N, Hawker GA, Schemitsch E, Owen JL. Patient preferences before and after total knee arthroplasty. J Clin Epidemiol 2010;63:774-82. http://dx.doi.org/10.1016/j.jclinepi.2009.08.022.
- Young NL, Cheah D, Waddell JP, Wright JG. Patient characteristics that affect the outcome of total hip arthroplasty: a review. Can J Surg 1998;41:188-95.
- Minns Lowe CJ, Davies L, Sackley CM, Barker KL. Effectiveness of land-based physiotherapy exercise following hospital discharge following hip arthroplasty for osteoarthritis: an updated systematic review. Physiotherapy 2015;101:252-65. http://dx.doi.org/10.1016/j.physio.2014.12.003.
- Wylde V, Artz N, Marques E, Lenguerrand E, Dixon S, Beswick AD, et al. Effectiveness and cost-effectiveness of outpatient physiotherapy after knee replacement for osteoarthritis: study protocol for a randomised controlled trial. Trials 2016;17. http://dx.doi.org/10.1186/s13063-016-1418-x.
- Beswick AD, Rees K, Dieppe P, Ayis S, Gooberman-Hill R, Horwood J, et al. Complex interventions to improve physical function and maintain independent living in elderly people: a systematic review and meta-analysis. Lancet 2008;371:725-35. http://dx.doi.org/10.1016/S0140-6736(08)60342-6.
- Jepson P, Beswick A, Smith TO, Sands G, Drummond A, Davis ET, et al. Assistive devices, hip precautions, environmental modifications and training to prevent dislocation and improve function after hip arthroplasty. Cochrane Database Syst Rev 2013;11. http://dx.doi.org/10.1002/14651858.cd010815.
- Gooberman-Hill R, Burston A, Clark E, Johnson E, Nolan S, Wells V, et al. Involving patients in research: considering good practice. Musculoskeletal Care 2013;11:187-90.
- Marques E, Noble S, Blom AW, Hollingworth W. Disclosing total waiting times for joint replacement: evidence from the English NHS using linked HES data. Health Econ 2014;23:806-20. http://dx.doi.org/10.1002/hec.2954.
- Wylde V, Jeffery A, Dieppe P, Gooberman-Hill R. The assessment of persistent pain after joint replacement. Osteoarthritis Cart 2012;20:102-5. http://dx.doi.org/10.1016/j.joca.2011.11.011.
- Brunton LR, Wylde V, Dieppe PA. Assessing the health status of people with arthritis: example of osteoarthritis of the knee. Rheumatology 2012;51:1143-4. http://dx.doi.org/10.1093/rheumatology/ker301.
Appendix 1 PRISMA checklist
Relating to Chapters 6, 8, 10 and 11
| Section/topic | Number | Checklist item | Reported in section |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both | Yes |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number | Abstracts reflect overall report including systematic reviews |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | All yes |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to PICOS | All yes |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g. web address), and, if available, provide registration information including registration number | Reviews commenced before registration became widely adopted excepting Review 11a which is registered as a Cochrane review |
| Eligibility criteria | 6 | Specify study characteristics (e.g. PICOS, length of follow-up) and report characteristics (e.g. years considered, language, publication status) used as criteria for eligibility, giving rationale | All yes |
| Information sources | 7 | Describe all information sources (e.g. databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | All yes |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | Appendix 3 |
| Study selection | 9 | State the process for selecting studies (i.e. screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | All yes |
| Data collection process | 10 | Describe method of data extraction from reports (e.g. piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | All yes |
| Data items | 11 | List and define all variables for which data were sought (e.g. PICOS, funding sources) and any assumptions and simplifications made | All yes |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis | All yes |
| Summary measures | 13 | State the principal summary measures (e.g. risk ratio, difference in means) | All yes |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g. I2) for each meta-analysis | All yes |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g. publication bias, selective reporting within studies) | All yes |
| Additional analyses | 16 | Describe methods of additional analyses (e.g. sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified | All yes |
| Results | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | All yes |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g. study size, PICOS, follow-up period) and provide the citations | All yes |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12) | All yes |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and CIs, ideally with a forest plot | Where appropriate |
| Synthesis of results | 21 | Present results of each meta-analysis done, including CIs and measures of consistency | All yes |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15) | All yes |
| Additional analysis | 23 | Give results of additional analyses, if done [e.g. sensitivity or subgroup analyses, meta-regression (see Item 16)] | Where appropriate |
| Discussion | |||
| Summary of evidence | 24 | Summarise the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g. health-care providers, users, and policy-makers) | All yes |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g. risk of bias), and at review-level (e.g. incomplete retrieval of identified research, reporting bias) | All yes |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research | All yes |
| Funding | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g. supply of data); role of funders for the systematic review | As for the whole report |
Appendix 2 MOOSE checklist
Relating to Chapter 2
| Recommendation | Reported |
|---|---|
| Reporting of background should include | |
| Problem definition | All yes |
| Hypothesis statement | Aim described for all |
| Description of study outcome(s) | All yes |
| Type of exposure or intervention used | All yes |
| Type of study designs used | All yes |
| Study population | All yes |
| Reporting of search strategy should include | |
| Qualifications of searchers (e.g. librarians and investigators) | In the context of the overall programme |
| Search strategy, including time period included in the synthesis and keywords | Appendix 3 |
| Effort to include all available studies, including contact with authors | Where appropriate. We did not request new analyses of longitudinal studies |
| Databases and registries searched | All yes |
| Search software used, name and version, including special features used (e.g. explosion) | All yes |
| Use of hand searching (e.g. reference lists of obtained articles) | All yes |
| List of citations located and those excluded, including justification | Reasons for exclusion summarised in Review flow diagrams. Details of individual reasons for study exclusion available from report authors |
| Method of addressing articles published in languages other than English | Described in each review methods section |
| Method of handling abstracts and unpublished studies | Generally excluded |
| Description of any contact with authors | Authors of studies with appropriate data but with specific missing information were contacted by e-mail |
| Reporting of methods should include | |
| Description of relevance or appropriateness of studies assembled for assessing the hypothesis to be tested | All yes |
| Rationale for the selection and coding of data (e.g. sound clinical principles or convenience) | Where appropriate |
| Documentation of how data were classified and coded (e.g. multiple raters, blinding, and interrater reliability) | Where appropriate |
| Assessment of confounding (e.g. comparability of cases and controls in studies where appropriate) | Not relevant |
| Assessment of study quality, including blinding of quality assessors; stratification or regression on possible predictors of study results | Cohort generalisability |
| Assessment of heterogeneity | In relation to methods of assessment |
| Description of statistical methods (eg, complete description of fixed or random-effects models, justification of whether the chosen models account for predictors of study results, dose–response models, or cumulative meta-analysis) in sufficient detail to be replicated | Where applicable described in methods sections |
| Provision of appropriate tables and graphics | All yes |
| Reporting of results should include | |
| Graphic summarising individual study estimates and overall estimate | Where applicable included in results sections |
| Table giving descriptive information for each study included | All yes |
| Results of sensitivity testing (eg, subgroup analysis) | In narrative reviews considering different measures |
| Indication of statistical uncertainty of findings | All yes |
| Reporting of discussion should include | |
| Quantitative assessment of bias (e.g. publication bias) | Risk of bias limited by strict inclusion criteria |
| Justification for exclusion (e.g. exclusion of non–English-language citations) | As feasible in individual reviews |
| Assessment of quality of included studies | If appropriate, good-quality studies identified according to inclusion criteria |
| Reporting of conclusions should include | |
| Consideration of alternative explanations for observed results | All yes |
| Generalisation of the conclusions (i.e. appropriate for the data presented and within the domain of the literature review) | All yes |
| Guidelines for future research | All yes |
| Disclosure of funding source | As for overall programme |
Appendix 3 Systematic review search strategies as applied in MEDLINE via Ovid SP
Total hip or total knee replacement terms
-
Arthroplasty, Replacement, Knee/ or Arthroplasty, Replacement, Hip/
-
exp Arthroplasty, Replacement, Hip/ or exp Hip Prosthesis/ or hip replacement.mp.
-
exp Arthroplasty, Replacement, Knee/ or exp Knee Prosthesis/ or knee replacement.mp.
-
hip prosthesis.mp. or exp Hip Prosthesis/
-
knee prosthesis.mp. or exp Knee Prosthesis/
-
total hip.tw.
-
total knee.tw.
-
hip implant.mp.
-
knee implant.mp.
-
(knee$ adj5 (arthroplast$ or replacement$ or implant$ or prothes$)).mp.
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10
Osteoarthritis
-
osteoarthriti$.mp. or Osteoarthritis, Hip/ or Osteoarthritis/ or Osteoarthritis, Knee/
Epidemiological study design
-
survey.mp. or exp Data Collection/
-
randomized controlled trial.mp. or exp Randomized Controlled Trials/
-
prospective study.mp. or exp Prospective Studies/
-
observational study.mp.
-
Comparative Study/
-
exp EPIDEMIOLOGY/ or epidemiology.mp.
-
longitudinal study.mp. or exp Longitudinal Studies/
-
case control study.mp. or exp Case-Control Studies/
-
evaluation study.mp. or exp Evaluation Studies/
-
follow up study.mp. or exp Follow-Up Studies/
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or10
Key patient-reported pain outcome measures
-
WOMAC.mp.
-
western ontario.mp.
-
american knee.mp.
-
aks.mp.
-
arthritis impact.mp.
-
oxford hip.mp.
-
oxford knee.mp.
-
hoos.mp.
-
koos.mp.
-
lequesne.mp.
-
self appraisal.mp.
-
vas.mp.
-
visual analogue.mp.
-
osteoarthritis outcome score.mp.
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14
Chronic pain
-
exp Pain/ or exp Complex Regional Pain Syndromes/ or exp Pain Clinics/ or exp Pain, Postoperative/ or exp Pain, Intractable/ or exp Pain Measurement/ or exp Patellofemoral Pain Syndrome/ or exp Pain, Referred/ or pain.mp. or exp Pain Threshold/ or exp Pain Perception/
-
(pain adj5 (chronic or persistent or long-term)).mp.
-
analgesi$.mp.
-
ache$.mp.
-
discomfort$.mp.
-
outcome$.mp.
-
neuropath$.mp.
-
1 or 2 or 3 or 4 or 5 or 6 or 7
Patient-reported outcome measures (hip, knee, osteoarthritis, generic)
-
Arthritis Impact Measurement Scale.tw.
-
“Quality of Life”/ or Sickness Impact Profile/ or Assessment of Quality of Life.mp.
-
Osteoarthritis Treatment Satisfaction Questionnaire.tw.
-
Euroqol.tw.
-
eq5d.mp.
-
eq-5d.mp.
-
(Hip and knee questionnaire).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
Health Status Questionnaire.mp.
-
(Knee Injury and Osteoarthritis Outcome Score).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
McMaster Toronto Arthritis Patient Preference Disability Questionnaire.mp.
-
McGill Pain.mp.
-
Patient-based Measure of the Severity of Osteoarthritis of the Knee.mp.
-
Nottingham health profile.mp.
-
Oxford knee score.mp.
-
Osteoarthritis Pain Assessment.mp.
-
Rand 36-Item.mp.
-
sf12.tw.
-
sf-12.tw.
-
MOS short.tw.
-
sf36.tw.
-
sf-36.tw.
-
Arthoplasty Outcome Evaluation Questionnaire.tw.
-
Visual analog.tw.
-
World Health Organization Quality of Life assessment instrument.tw.
-
(Western Ontario and McMaster Universities Arthritis Index).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
Hip disability.mp. and Osteoarthritis Outcome Score.tw. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
-
International Knee Documentation Committee Subjective Knee Form.tw.
-
bristol knee score.mp.
-
Short Musculoskeletal Function Assessment.tw.
-
Lequesne.mp.
-
New zealand score.mp.
-
modems.tw.
-
mood adjective.tw.
-
MACL.tw.
-
arthritis impact.tw.
-
KOOS.tw.
-
HOOS.tw.
-
osteoarthitis outcome score.tw.
-
mactar.tw.
-
NHP.tw.
-
Nottingham health.tw.
-
OHS.tw.
-
OKS.tw.
-
Oxford hip score.tw.
-
short form.tw.
-
vas.tw.
-
womac.mp.
-
western ontario.tw.
-
IKD.tw.
-
International knee.tw.
-
smfa.tw.
-
short musculoskeletal.tw.
-
satisfaction.tw.
-
function score.tw.
-
(activit$ adj8 daily living).tw.
-
ADL.tw.
-
QOL.tw.
-
likert.tw.
-
depression.tw.
-
anxiety.tw.
-
PROM.tw.
-
patient reported.tw.
-
visual analog$.tw.
-
expectation.mp. or EXPECTATION/
-
satisfaction.mp. or SATISFACTION/ or PATIENT SATISFACTION/
-
depressive symptoms.tw.
-
POSTOPERATIVE PAIN/
-
POSTOPERATIVE COMPLICATION/
-
psychological distress.tw.
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69
Pre-surgical exercise and education
-
exp preoperative care/
-
preoperative period.mp. or Preoperative Period/
-
pre-surg$.tw.
-
presurg$.tw.
-
before surg$.tw.
-
pre-operat$.tw.
-
preoperat$.tw.
-
1 or 2 or 3 or 4 or 5 or 6 or 7
Anaesthsia and analgesia in joint replacement
-
Anesthetics, Local/ or local anaesthetic.mp.
-
Anesthetics, Local/ or Anesthesia, Local/ or Local anaesthesia.mp.
-
Anesthetics/ or Anesthesia/ or anaesthesia.mp. or Anesthetics, Local/ or Anesthesia, Local/
-
anesthesia.mp.
-
anaesthetic.mp.
-
amides.mp. or Amides/
-
(“Huneke neural therapy” or “ Neural therapy of Huneke” or benzocaine or bensokain or “ Aminobenzoic Acid” or “ Aminobenzoate” or bupivacain* or buvacaina or sensorcaine or marcain* or svedocain* or levobupivacaine or carticain* or articain* or dibucaine or cinchocaine or Cincain or Nupercain* or Sovcaine or etidocaine or duranest or “ W19053” or “ W 19053” or “ W-19053” or Lidocaine or Lignocaine or Octocaine or Xylesthesin or Xylocaine or Dalcaine or Xylocitin or Xyloneural or Mepivacain* or Carbocaine or Polocaine or isocaine or isogaine or Scandicain* or prilocaine or Propitocaine or Tetracaine or Tetrakain or Amethocaine or Dicaine or Pantocaine or Pontocaine or Trimecaine or Mesocaine or ropivacaine).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
-
1 or 2 or 3 or 4 or 5 or 6 or 7
Perioperative wound infiltration
-
(incision or port* or (surg* and wound)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
-
acetabular.mp.
-
infiltration.mp.
-
wound infiltration.mp.
-
wound catheter.mp.
-
peri-articular.mp.
-
periarticular.mp.
-
intraarticular.mp.
-
intra-articular.mp.
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9
Randomised controlled trial
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
randomized.ab.
-
placebo.ab.
-
randomly.ab.
-
trial.ab.
-
groups.ab.
-
(animals not (humans and animals)).sh.
-
1 or 2 or 3 or 4 or 5 or 6 or 7
-
9 not 8
Randomised controlled trial terms for physiotherapy systematic review
-
Randomized Controlled Trials as Topic/
-
randomized controlled trial/
-
random allocation/
-
double blind method/
-
single blind method/
-
clinical trial/
-
clinical trial, phase i.pt.
-
clinical trial, phase ii.pt.
-
clinical trial, phase iii.pt.
-
clinical trial, phase iv.pt.
-
randomized controlled trial.pt.
-
multicenter study.pt.
-
clinical trial.pt.
-
exp clinical trials as topic/
-
(clinical adj trial$).tw.
-
((singl$ or doubl$ or treb$ or tripl$) adj (blind$3 or mask$3)).tw.
-
Placebos/
-
placebo$.tw.
-
randomly allocated.tw.
-
(allocated adj2 random$).tw.
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20
Occupational therapy
-
occupational therapy.sh.
-
self help devices.sh.
-
splints.sh.
-
(occupational adj1 therap$).ti,ab.
-
splint$.ti,ab.
-
((assist$ or help$) adj5 (device$ or technolog$)).ti,ab.
-
((sel$ or home$) adj5 (care$ or manage$)).ti,ab.
-
((environment$ or home$ or domestic$ or house$) adj5 adapt$).ti,ab.
-
((daily or domestic$ or house$ or home$) adj5 (activit$ or task$ or skill$ or chore$)).ti,ab.
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9
Physiotherapy
-
physical therapy techniques/ or cryotherapy/ or electric stimulation therapy/ or transcutaneous electric nerve stimulation/ or hydrotherapy/
-
exercise movement techniques/ or exercise/ or exercise therapy/ or walking/
-
rehabilitation/ or “activities of daily living”/ or early ambulation/
-
Postoperative Care/
-
Ambulatory Care/
-
Rehabilitation Centers/
-
Home Care Services/
-
(physiotherap$ or physio therap$ or pt).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
therap$.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
rehab$.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10
Appendix 4 Systematic review of long-term pain after hip or knee replacement: study characteristics
| Author; country; date of baseline | Population; study design; losses to follow-up | Study type; mean age (SD) (years); % female; indication | Pain outcome measure | Number of patients with: | ||
|---|---|---|---|---|---|---|
| Favourable outcome | Uncertain outcome | Unfavourable outcome | ||||
| THR | ||||||
| Nikolajson and colleagues 2006;51 Denmark; 2003 | 1231 consecutive patients in a national joint registry with 94% of hip replacements recorded. 93.6% response rate to postal questionnaire; 5.9% lost to follow-up | Registry; 71.6 (SD 8.7); % female not reported; 100% degenerative hip arthritis, operation through a posterolateral surgical approach | Authors’ own scale of presence of hip pain and impact on daily life; self-completed; 12- to 18-month follow-up | 754 (hip pain not present) | 4 died, 117 lost to follow-up, 62 bilateral or further operation, 167 hip pain present with no/mild impact on daily life | 127 (pain with moderate, severe or very severe impact on daily life) |
| Jones and colleagues 2000;58 Canada; 1995–7 | 242 consecutive patients listed for and received joint replacement in health region; 5.8% lost to follow-up or died (estimated as not reported for hip and knee separately) | Multiple centres; 68.2 (SD 11.1); 60%; 94% osteoarthritis | WOMAC pain; self-completed; losses to follow-up estimated proportionately as not reported for hip and knee separately; 6-month follow-up | 208 (no pain/mild pain defined as more than a 10-point gain on the 100 point WOMAC pain dimension) | 14 lost to follow-up (estimated) | 20 (moderate/severe pain defined as a gain of less than 10 points on the 100-point WOMAC pain dimension) |
| Quintana and colleagues 2006;102 Spain; 1999–2000 | 784 consecutive patients willing to participate and with complete pre-surgical data scheduled to undergo THR in seven teaching hospitals. 82.4% response; prospective; 25.5% lost to follow-up | Multiple centres; 69.2 (SD 9.2); 48.3%; 94% osteoarthritis | WOMAC pain; self-completed (postal); 6-month follow-up | 456 (patients reporting improvement in pain greater than minimal clinical important difference 24.55/100) | 200 lost to follow-up | 128 (patients reporting no improvement in pain greater than minimal clinical important difference 24.55/100) |
| Nilsdotter and colleagues 2003;103 Sweden; 1995–8 | 219 consecutive patients with 2 surgical methods at single department of orthopaedics. For proportion with pain at follow-up n = 92; 5.9% lost to follow-up | Single centre; 71 (range 50–92); 55%; 100% osteoarthritis | WOMAC pain; self-completed; favourable/unfavourable estimates based on extrapolation of partial follow-up; mean 43-month follow-up | 153 (pain improved by more than 10/100 units reflecting detectable clinical improvement) | 8 died, 13 lost to follow-up | 45 (pain improved by < 10/100 units reflecting no detectable clinical improvement) |
| Singh and Lewallen 2010;104 USA; 1993–2005 | 9154 consecutive patients from joint registry sent postal questionnaire or completed at outpatient clinic or telephone; mean age of patients followed up 65.0 years (SD 13); 37.7% lost to follow-up | Single centre; 65.0 (SD 13.3); 51%; 87% osteoarthritis | Single question: how much pain do you have in your operated hip? None, mild, moderate or severe; 24-month follow-up (also 60-month with greater losses to follow-up) | 5272 (none or mild pain) | 3447 lost to follow-up | 435 (moderate or severe pain) |
| Wylde and colleagues 2011;49 UK; 2004–6 | 1401 consecutive patients on an orthopaedic centre database with postal follow-up; 47.6% lost to follow-up | Single centre; median 73 (range 65–78); 63%; majority osteoarthritis | WOMAC pain; median 41-month follow-up (range 35–48 months) | 818 (no pain for the past 3 months or mild persistent pain in replaced hip) | 71 died, 1 revision, 667 lost to follow-up | 114 (moderate or severe persistent pain for 3 months in replaced hip, WOMAC 0–75/100) |
| TKR | ||||||
| Baker and colleagues 2007;105 UK; 2003 | 9417 questionnaire follow-up of random sample of patients in joint registry; 14.9% lost to follow-up | Registry; 70.7 (range 25–98); 57% (estimate); 96% osteoarthritis | OKS pain dimension; self-completed postal questionnaire; 12-month follow-up or latest available | 6427 (did not report persistent knee pain) | 1407 lost to follow-up or died | 1583 (reported persistent knee pain) |
| Jones and colleagues 2000;58 Canada; 1995–7 | n = 292, approximately 81% of consecutive patients listed for and who subsequently received joint replacement in health region; 5.8% lost to follow-up or died (estimated as not reported for hip and knee separately) | Multiple centres; 69.2 (SD 9.2); 59%; 94% osteoarthritis | WOMAC pain; self completed; losses to follow-up estimated proportionately as not reported for hip and knee separately; 6-month follow-up | 222 (no pain/mild pain defined as more than a 10-point gain on the WOMAC pain dimension) | 16 lost to follow-up or died (estimated) | 54 (moderate/severe pain defined as a gain of < 10 points on the WOMAC pain dimension) |
| Quintana and colleagues 2006;102 Spain; 1999–2000 | 792 consecutive patients scheduled to undergo TKR in seven teaching hospitals willing to participate and with complete pre-surgical data (83.4% response); 24.1% lost to follow-up | Multiple centres; 71.9; 73%; 100% osteoarthritis | WOMAC pain; self-completed; 6-month follow-up | 402 (patients reporting improvement in pain greater than minimal clinical important difference 22.6/100) | 191 lost to follow-up | 199 (patients reporting no improvement in pain greater than minimal clinical important difference 22.6/100) |
| Núñez and colleagues 2007;47 Spain; 2000–1 | 88 consecutive patients at a single tertiary care centre 8.0% lost to follow-up | Multiple centres; 74.8 (SD 5.6); 81%; 100% osteoarthritis | WOMAC pain; self-completed; 36-month follow-up | 60 (improvement in postoperative pain scores) | 1 died, 7 lost to follow-up, 13 contralateral or other surgery | 7 (no improvement in postoperative pain scores) |
| Stephens and colleagues 2002;106 USA; date not specified | 68 patients referred for and received knee replacement aged ≥ 50 years; 7.4% lost to follow-up | Single centre; 67.4 (SD 8.1) followed up; 54% followed up; 100% osteoarthritis | WOMAC pain; self-completed (postal); 6-month follow-up | 52 (decrease in pain) | 5 lost to follow-up | 11 (no change or increase in pain) |
| Lundblad and colleagues 2008;107 Sweden; date not specified | 69 patients scheduled for knee replacement; 10.1% lost to follow-up (including deaths) | Single centre; 68 (range 40–80); 50.7%; 100% osteoarthritis | VAS pain; self-completed postal; 18-month follow-up | 21 (no pain at rest or with movement) | 7 lost to follow-up or died, 26 pain with movement | 15 (pain at rest and movement) |
| Nilsdotter and colleagues 2009;108 Sweden; 1999–2001 | 102 responders to postal survey on waiting list for knee replacement; 12.7% lost to follow-up | Single centre; 71 (SD 8); 61.8%; 100% osteoarthritis | KOOS pain compared with pre-operatively; self-completed postal; 60-month follow-up | 47 (much less or less pain than pre-operatively) | 9 died, 13 lost to follow-up, 6 operated bilaterally | 27 (similar or more pain than pre-operatively) |
| Vuorenmaa and colleagues 2008;109 Finland; date not specified | 51 patients referred for knee replacement; 11.8% lost to follow-up | Single centre; 70 (SD 5); 86%; 100% osteoarthritis | VAS pain; self-completed; pain calculated from 20% followed up had moderate or severe pain (defined as score of > 30 on a 100-mm pain VAS); 3-month follow-up | 34 (none or mild pain) | 1 died, 6 lost to follow-up, 1 infection | 9 (moderate or severe pain) |
| Czurda and colleagues 2010;110 Austria; 2003–5 | 411 consecutive patients with computer assisted or conventional surgery with at least 18 months follow-up; 13.4% lost to follow-up | Single centre; 75–76 (range 45–96); 76%; 100% osteoarthritis | WOMAC pain; telephone interview; mean 26-month follow-up (range 18–42 months) | 273 (no report of painful knees – no moderate or worse response in any WOMAC pain dimension) | 2 died, 55 lost to follow-up, 24 infection, trauma, reoperation, poor general condition | 57 (painful knees – moderate or worse response in any WOMAC pain dimension) |
| Wylde and colleagues 2011;49 UK; 2004–6 | 1394 consecutive patients on an orthopaedic centre database; 45.3% lost to follow-up | Single centre; median 73 (range 28–96); 59%; Majority osteoarthritis | WOMAC pain; self-completed postal questionnaire; median 28-month follow-up (range 14–43) | 433 (no pain for the past 3 months or mild persistent pain in replaced hip) | 62 died, 4 revision, 696 lost to follow-up | 199 (moderate or severe persistent pain for 3 months in replaced hip, WOMAC 0–75/100) |
| Brander and colleagues 2003;50 USA 1998–2000 | 116 consecutive patients (1 surgeon); 0% lost to follow-up | Single surgeon; 66 (SD 10.5); 55.2%; 94% osteoarthritis | VAS pain; self-completed questionnaire; 12-month follow-up | 98 (no significant pain, VAS score of ≤ 40) | 1 died, 2 revision or dislocation | 15 (significant pain, VAS score of > 40) |
Appendix 5 Systematic review of pre-operative predictors of patient-centred outcomes after total hip replacement: study characteristics
| Study; country; dates | Number of patients; follow-up | Statistical analysis and variables in analysis | Predictor | Outcome measures |
|---|---|---|---|---|
| Registry | ||||
| Rolfson and colleagues 2009;125 Sweden; 2002–5 | n = 6, 158 (approximately 92% of eligible); 12 months, estimated 8% not followed up | ANCOVA with EQ-5D anxiety and depression, age, sex, comorbidities, VAS pain, EQ-5D | Mental health | VAS pain, satisfaction, EQ-5D domains |
| Multiple centres | ||||
| Hajat and colleagues 2002;126 UK; 1996–7 | n = 3600 (4657 eligible); 12 months, 22.7% not followed up | Multiple regression analysis with OHS, waiting time, comorbidities, age, sex, housing, surgical factors | Physical function | OHS |
| Jones and colleagues 2012;127 Canada; 1995–7 | n = approximately 167 (231 eligible); 3 years, 28% not followed up | Linear mixed model with BMI (< 25, 25–29.9, 30–34.9, ≥ 35 kg/m2), age, sex, diabetes and cardiac disease | BMI | WOMAC |
| Judge and colleagues 2011;31 Europe; dates not specified | n = 845 (1327 eligible); 12-month follow-up, 36.3% with no baseline or follow-up data | Ordered logistic regression modelling with WOMAC score, EQ-5D, age, sex, BMI, education, living arrangements, ambulatory status, comorbidities and radiographic status | Physical function | WOMAC |
| Judge and colleagues 2013;128 UK; 1999–2002 | n = 1375 patients with 1431 THR at baseline; 60 months, 20% lost to 1-year follow-up, 30% lost to 5-year follow-up | Repeated measures linear regression with BMI as a continuous variable, age, sex, primary diagnosis, occupation, specific comorbidities, SF-36, OHS, ROM, surgical variables | BMI, mental health physical function | OHS |
| Stevens and colleagues 2012;129 Netherlands; 2005–7 | n = 653 (848 eligible); 12 months, 23% lost to follow-up | Linear regression (structural equation model) with BMI (< 25, 25–30, > 30 kg/m2), age, sex, comorbidities and complications | BMI | WOMAC, SF-36 |
| Single centre | ||||
| Anakwe and colleagues 2011;130 UK; 2003–8 | Osteoarthritis estimated 98%; n = 850 (907 eligible); 12 months, 6.3% not followed up | Multivariate binary logistic regression with SF-12 mental health component, diabetes, hypertension, history of depression, age, sex, SF-12 physical components, OHS, musculoskeletal comorbidities | Mental health, physical function | Satisfaction |
| Clement and colleagues 2011;131 UK; 2006–8 | Osteoarthritis; n = 1312 patients with 1359 THR followed up; 12 months, no loss to follow-up information | Ordinal logistic regression with SF-12 mental health, age, deprivation, Charlson comorbidities including depression, OHS, length of stay and SF-12 physical health | Mental health, physical function | OHS |
| Davis and colleagues 2011;132 UK; 1998–2005 | n = 1095 (1617 at baseline); 60 months, 32% not followed up | Multiple regression linear analysis with BMI (< 25, 25–29.9, 30–34.9, ≥ 35 kg/m2), age, sex, pre-operative HHS, SF-36, comorbidities, consultant | BMI | SF-36 |
| Gandhi and colleagues 2010;133 Canada; 1998–2005 | n = 636 with pre- and postoperative data; 12 months and up to 72 months (mean 39 months), loss to follow-up not described | Multivariable longitudinal regression with BMI as a continuous variable, age, sex, comorbidities, WOMAC, SF-36 | BMI, mental health | WOMAC, SF-36 physical function |
| Garbuz and colleagues 2006;134 also Xu and colleagues 2005;136 Canada; 2001–3 | n = 147 (total 201 eligible); 12 months, 27% lost to follow-up | Log-linear regression with WOMAC pain, age, sex and comorbidities | Pain, physical function | WOMAC pain |
| Moran and colleagues 2005;135 UK; 1998–2000 | n = 687 (800 eligible); minimum 18 months, 14% lost to follow-up | Multiple linear regression analysis with BMI as a continuous variable, sex, comorbidities, OHS, SF-36 | BMI | SF-36 |
| Nilsdotter and colleagues 2003;103 Sweden; 1995–8 | n = 198 (total 219 recruited); 12 month and at mean 43-month follow-up, 6% lost to follow-up | Stepwise multivariate logistic regression with BMI as a continuous variable, age, sex, comorbidity, WOMAC, SF-36 (including mental health), employment, marital status, contralateral osteoarthritis, need of walking assistance, walking distance, analgesic use, regional or widespread pain | BMI, mental health, pain | WOMAC function |
| Singh and Lewallen 2010;104 USA; 1993–2005 | n = 5707 (9154 at baseline); 24 months (and longer), 38% of patients not followed up at 2 years | Multivariable-adjusted logistic regression analyses with BMI (< 25, 25–29.9, 30–34.9, 35–39.9, ≥ 40 kg/m2), age, sex, Deyo-Charlson, ASA, depression, anxiety, operative diagnosis, distance from centre and implant design | BMI, mental health | Pain (5-item response scale) |
Appendix 6 Systematic review of pre-operative predictors of patient-centred outcomes after total knee replacement: study characteristics
| Study; country; dates | Number of patients; follow-up | Statistical analysis and variables in analysis | Outcome measures | |
|---|---|---|---|---|
| Registry | ||||
| Baker and colleagues 2012;148 UK; 2008–11 | n = 22,691 (40,925 eligible); minimum 6-month follow-up, 44.6% not followed up | Stepwise multiple regression analysis with EQ-5D depression and anxiety, age, OKS, EQ-5D, comorbidities, disability, general health, ASA grade, and surgical and hospital variables | Mental health | OKS, EQ-5D |
| Franklin and colleagues 2008;147 USA; 2000–5 | n = 8050 (17,270 eligible); follow-up at 12 months, 53.4% not followed up | Multivariate mixture models with BMI analysed as < 30, 30–40, > 40 kg/m2, sex, age, SF-12 MCS and PCS, osteoarthritis diagnosis and poor quadriceps strength | BMI, mental health | SF-12 PCS |
| Multiple centres | ||||
| Alzahrani and colleagues 2011;149 Canada; 1998–2007 | n = 3,177; follow-up at 12 months, losses to follow-up not described | Multivariable logistic regression modelling with BMI analysed as a continuous variable, age, sex and comorbidities | BMI | OKS (2720 patients), total WOMAC (457 patients) |
| Cushnaghan and colleagues 2009;151 UK; 1995–7 | n = 259 (657 eligible but not all had TKR); mean follow-up of 6 years, approximately 60.6% not followed up | Linear regression with BMI analysed as < 25, 25 to < 30, ≥ 30 kg/m2, age, sex, SF-36 PCS, smoking habits, comorbidities, Kellgren and Lawrence grade, previous knee injury, other painful joints and Heberden’s nodes | BMI | SF-36 PCS |
| Heck and colleagues 1998;157 USA; 1992–3 | n = 268 (291 eligible); 24-month follow-up, 7.9% not followed up | Stepwise logistic regression model with SF-36 mental health, age, ethnicity, sex, poverty, patient health status, WOMAC scales, SF-36, knee ROM, comorbidities, surgical factors and joint problems in the other knee | Mental health, pain, physical function | SF-36 physical component |
| Jones and colleagues 2012;127 Canada; 1995–7 | n = approximately 209 (289 eligible); follow-up at 3 years, approximately 27.7% not followed up | Linear mixed modelling with BMI analysed as binary variable (30–34.9 and ≥ 35 kg/m2), age, sex and comorbidities | BMI | WOMAC |
| Lingard and colleagues 2004;46 UK, USA, Australia; 1997–8 | n = 741 at 1 year, 678 at 2 years (860 eligible); follow-up at 12 and 24 months, 13.8% and 21.2% lost to follow-up at 12 and 24 months | Hierarchical model with BMI analysed as a continuous variable, age, sex, PROMs score, mental health, knee flexion, working status, education, income, comorbidities and country | BMI, pain, physical function | WOMAC pain and function, SF-36 PCS |
| Lingard and colleagues 2007;150 also Lingard and colleagues 2004;46 UK, USA, Australia; 1997–8 | n = 682 (974 eligible); follow-up at 12 and 24 months, 30.0% not followed up at 2 years | General linear model with SF-36 mental distress, age, sex, and comorbidities | Mental health | WOMAC pain and function scores |
| Merle-Vincent and colleagues 2011;158 France; dates not specified | n = 264 (299 eligible); 24-month follow-up, 13.3% not followed up | Multivariate logistic regression with feelings of depression, age, sex, BMI, Lequesne index and joint space narrowing | Mental health | Satisfaction |
| Naylor and colleagues 2012;154 Australia; 2008–9 | n = 146 (191 eligible); follow-up at 12 months, 23.6% not followed up | Mixed model with BMI analysed as continuous variable, age and sex as covariates. Performed separately for pre-operative flexion and extension variables | BMI | OKS |
| Papakostidou and colleagues 2012;155 Greece; dates not specified | n = 204 (224 eligible); follow-up at 12 months, 9.8% not followed up | General linear model multivariable analysis with BMI analysed as binary variable (under and over 30 kg/m2), sex, education, social support, age, place of residence, baseline status of knee | BMI, mental health, pain | WOMAC |
| Perruccio and colleagues 2012;152 Canada; 2006–8 | n = 435 (494 eligible); mean follow-up at 12.5 months, 11.9% not followed up | Linear regression with BMI analysed as 25–29 kg/m2 (overweight) and > 30 kg/m2 (obese), age, sex, education, comorbidity count, other painful joints and pain/function | BMI | WOMAC |
| Singh and Lewallen 2013;156 USA; 1993–2005 | n = 7139 (approximately 10,980 eligible); follow-up at 2 and 5 years, 35% and 43% lost to follow-up, respectively | Multivariable adjusted model with age, sex, BMI, ASA class, distance from medical centre, operative diagnosis, implant fixation (cement status), six Deyo-Charlson comorbidity categories, anxiety and depression | Mental health | Pain severity questionnaire |
| Sullivan and colleagues 2011;153 Canada; dates not specified | n = 120 (number eligible not specified); follow-up at 12 months, loss to follow-up not described | Multiple regression with BMI analysed as continuous variable, pain, function, age, sex, comorbidities, surgery duration, surgeon, pain catastrophising, pain-related fear of movement and depression | BMI, pain | WOMAC function and pain |
| Single centre | ||||
| Ayers and colleagues 2005;163 USA; dates not specified | n = 165 (number eligible not specified); 12 months, loss to follow-up not described | Blocked multiple regression analysis with SF-36 mental health, age, sex, comorbidities, SF-36 physical component and WOMAC physical function | Mental health, physical function | WOMAC physical function, SF-36 physical component |
| Brander and colleagues 2003;50 USA; 1998–2000 | n = 116 consecutive patients with 149 TKRs (no information on eligibility); follow-up at 12 months, no information on losses to follow-up | Multiple regression analysis with anxiety (STAI) and depression (Beck Depression Inventory), age, sex, BMI, physiologic, psychometric and heightened pain | Mental health | Pain VAS and McGill questionnaire-SF scores |
| Clement and colleagues 2013;164 UK; 2007–9 | n = 966 (number eligible not specified); 12-month follow-up, losses to follow-up not described | Multivariate linear and bivariate regression analyses with SF-36 mental health component, age, sex, comorbidities, socioeconomic deprivation, OKS and SF-12 physical component | Mental health, physical function | OKS, satisfaction |
| Deshmukh and colleagues 2002;161 UK; 1992–5 | n = 139 (180 eligible); follow-up at 12 months, 22.8% not followed up | Hierarchical multiple regression analysis with BMI analysed as continuous variable, age, sex, side of arthritis, comorbidities, NHP and AKSS scores | BMI | NHP |
| Gandhi and colleagues 2010;160 Gandhi and colleagues 2010;166 Canada; 1998–2005 | n = 551 (number eligible not specified); mean follow-up 3 years, loss to follow-up not described | Multivariable longitudinal regression model with BMI analysed as continuous variable, age, sex, ethnicity, education, comorbidities and SF-36 mental health | BMI, mental health | Total WOMAC score, SF-36 Role Physical, SF-36 Physical Function |
| Núñez; and colleagues 2009;159 Spain; 2000 | n = 112 (146 eligible); follow-up at 7 years, 23.3% not followed up | Explanatory multiple linear regression models with BMI analysed as a binary variable (< 35 and ≥ 35 kg/m2), age, sex, comorbidities, sociodemographic and clinical characteristics, intraoperative variables, inpatient variables, postoperative clinical variables and pre-operative WOMAC scores | BMI | WOMAC function and pain |
| Rajgopal and colleagues 2008;162 Canada; 1987–2004 | n = 550 (number eligible not specified); follow-up at 1 year, loss to follow-up not described | Linear regression model with BMI analysed as binary variable (< 40 or ≥ 40 kg/m2), age, sex, mental health, prior contralateral TKR, WOMAC score and presence of comorbidity affecting gait | BMI | WOMAC |
| Scott and colleagues 2010;165 UK; 2006–8 | n = 1141 (1290 eligible); 12 months, 13.1% not followed up | Multiple ordinal logistic regression with depression, SF-12 MCS, age, sex, SF-12 PCS, OKS and comorbidities | Mental health, pain, physical function | Satisfaction |
Appendix 7 Systematic review of comorbid conditions and long-term patient-centred outcomes after total hip replacement: study characteristics
| Study; country; dates | Number of patients; follow-up | Statistical analysis and variables in analysis | Comorbidity | Outcome measures |
|---|---|---|---|---|
| Multiple centres | ||||
| Cushnaghan and colleagues 2007;184 Judge and colleagues 2012;185 UK; 1993–5; two health districts | Osteoarthritis; n = 249 (643 eligible); mean follow-up approximately 96 months, 49.9% not followed up | Logistic regression modelling; diabetes, hypertension, thyroid disease, age, sex, BMI, smoking habit, previous knee injury, Heberden’s nodes, number of painful joints and radiographic grade | Hypertension | SF-36 PCS |
| Jones and colleagues 2012;127 Canada; 1995–7 | n = approximately 167 (231 eligible); 3 years, 28% not followed up | Linear mixed modelling; comorbidity including diabetes and cardiac disease, age, sex, BMI, education, principal diagnosis, living arrangements, type of living accommodation, previous joint surgery, ambulatory status and number of comorbid conditions | Diabetes, cardiovascular disease | WOMAC function and pain |
| Judge and colleagues 2012;185 Cushnaghan and colleagues 2007;184 UK; 1993–5 | Osteoarthritis; n = 249 (643 eligible); mean follow-up approximately 96 months, 49.9% not followed up | Logistic regression modelling; diabetes, hypertension, thyroid disease, age, sex, BMI, smoking habit, previous knee injury, Heberden’s nodes, number of painful joints and radiographic grade | Diabetes, thyroid disease | SF-36 |
| Single centre | ||||
| Anakwe and colleagues 2011;130 UK; 2003–8 | Osteoarthritis estimated 98%; n = 850 (907 eligible); 12 months, 6.3% not followed up | Multivariate binary logistic regression; diabetes, hypertension, history of depression, age, sex, SF-12 physical and mental components, OHS and musculoskeletal comorbidities | Diabetes, hypertension | Satisfaction |
| Gandhi and colleagues 2010;166 Canada; 1998–2006 | Osteoarthritis; n = 707 (approximately 850 eligible); 12 months, 16.7% not followed up | Linear regression; number of metabolic syndrome factors (BMI of > 30 kg/m2, diabetes, hypertension and hypercholesterolaemia), age, sex, BMI, WOMAC and Cumulative Illness Rating Scale | Diabetes, hypertension | WOMAC |
Appendix 8 Systematic review of comorbid conditions and long-term patient-centred outcomes after total knee replacement: study characteristics
| Study; country; dates | Number of patients; follow-up | Statistical analysis and variables in analysis | Comorbidity | Outcome measures |
|---|---|---|---|---|
| Multiple centres | ||||
| Cushnaghan and colleagues 2009;151 UK; 1995–7 | n = 259 (657 eligible but not all had TKR); mean follow-up of 6 years, approximately 60.6% not followed up | Linear regression; diabetes, hypertension, thyroid disease, age, sex, BMI, smoking habit, previous knee injury, Heberden’s nodes, number of painful joints, and radiographic grade | Diabetes, hypertension, thyroid disease | SF-36 physical function |
| Jones and colleagues 2012;127 Canada; 1995–7 | n = approximately 209 (289 eligible); follow-up at 3 years, approximately 27.7% not followed up | Linear mixed modelling; comorbidity including cardiac disease and diabetes, age, sex, BMI, education, principal diagnosis, living arrangements, type of living accommodation, previous joint surgery, ambulatory status and number of comorbid conditions | Diabetes, cardiovascular disease | WOMAC |
| Single centre | ||||
| Ayers and colleagues 2005;163 USA; not specified | n = 165 (number eligible not specified); 12 months, loss to follow-up not described | Blocked multiple regression analyses with cardiovascular disease, pulmonary disease, lower extremity (non-arthritis), rheumatoid, endocrine disease, age, sex, SF-36 physical and mental health, components and WOMAC physical component | Diabetes, cardiovascular disease | WOMAC function, SF-36 physical component |
| Clement and colleagues 2013;164 UK; 2007–9 | n = 966 (number eligible not specified); 12-month follow-up, losses to follow-up not described | Multivariate linear and bivariate regression analyses with SF-36 mental health component, age, sex, comorbidities, socioeconomic deprivation, OKS and SF-12 physical component | Diabetes, cardiovascular disease, hypertension, anaemia | OKS Satisfaction |
| Gandhi and colleagues 2010;166 Canada; 1998–2006 | n = 889 (approximately 1067 eligible); 12 months, 16.7% not followed up | Linear regression; number of metabolic syndrome risk factors (BMI of > 30 kg/m2; diabetes, hypertension and hypercholesterolaemia), age, sex, BMI, WOMAC and Cumulative Illness Rating Scale | Diabetes, hypertension | WOMAC |
| Scott and colleagues 2010;165 UK; 2006–8 | n = 1141 (1,290 eligible); 12 months, 13.1% not followed up | Multiple ordinal logistic regression; heart disease, hypertension, lung disease, vascular disease, neurological problems, diabetes, stomach ulcer kidney disease, liver disease, anaemia and depression | Diabetes, cardiovascular disease, hypertension | Satisfaction |
Appendix 9 Local anaesthetic infiltration in total knee and hip replacement: Cochrane risk-of-bias table
| Study | Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel | Blinding of outcome assessment (detection bias) | Incomplete outcome data addressed (attrition bias) | Lack of selective reporting (reporting bias) | Lack of other sources of bias | Power calculation reported | Our evaluation |
|---|---|---|---|---|---|---|---|---|---|
| THR | |||||||||
| Andersen KV and colleagues 2007385 | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | Unclear: no reason to assume bias |
| Lee and colleagues 2009391 | ∼ | ∼ | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ | Unclear: no reason to assume bias |
| Lu and Li 2010393 | ∼ | ∼ | ∼ | ∼ | ∼ | ∼ | ∼ | ✗ | Unclear: no reason to assume bias |
| Aguirre and colleagues 2012387 | ∼ | ∼ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Andersen LJ and colleagues 2007388 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ∼ | ✓ | Low risk of bias |
| Bianconi and colleagues 2003374 | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Busch and colleagues 2010389 | ✓ | ∼ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Dobie and colleagues 2012390 | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Liu and colleagues 2011392 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Lunn and colleagues 2011394 | ✓ | ✓ | ✓ | ✓ | ∼ | ✓ | ✓ | ✓ | Low risk of bias (except pain during activity with 18 patients unable to complete test) |
| Murphy and colleagues 2012395 | ∼ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Parvataneni and colleagues 2007386 | ∼ | ∼ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Rikalainen-Salmi and colleagues 2012396 | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| TKR | |||||||||
| Affas and colleagues 2011397 | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | Unclear: no reason to assume bias |
| Andersen KV and colleagues 2010398 | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ∼ | ✓ | Unclear: no reason to assume bias (small difference in reasons for losses to follow-up) |
| Meftah and colleagues 2012410 | ∼ | ∼ | ✗ | ∼ | ✓ | ✓ | ✓ | ✗ | Unclear: no reason to assume bias |
| Toftdahl and colleagues 2007380 | ∼ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | Unclear: no reason to assume bias |
| Zhang and colleagues 2007414 | ∼ | ∼ | ✗ | ∼ | ✓ | ✓ | ✓ | ✓ | Unclear: no reason to assume bias |
| Busch and colleagues 2006399 | ✓ | ∼ | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | Low risk of bias |
| Carli and colleagues 2010400 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Chen and colleagues 2012401 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Essving and colleagues 2010402 | ✓ | ∼ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Essving and colleagues 2011403 | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ∼ | ✓ | Low risk of bias |
| Fu and colleagues 2009404 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Fu and colleagues 2010405 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | Low risk of bias |
| Han and colleagues 2007406 | ✓ | ∼ | ∼ | ✓ | ✓ | ✓ | ✓ | ✗ | Low risk of bias |
| Han and colleagues 2007406 | ✓ | ∼ | ∼ | ✓ | ✓ | ✓ | ✓ | ✗ | Low risk of bias |
| Koh and colleagues 2012407 | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Krenzel and colleagues 2009408 | ∼ | ∼ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Mahadevan and colleagues 2012409 | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Ng and colleagues 2012411 | ✓ | ∼ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Parvataneni and colleagues 2007386 | ∼ | ∼ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Spreng and colleagues (no i.v. injection) 2010412 | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Spreng and colleagues (with i.v. injection) 2010412 | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Vendittoli and colleagues 2006413 | ✓ | ∼ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Thorsell and colleagues 2010381 | ∼ | ∼ | ✗ | ∼ | ✗ | ✓ | ✓ | ✗ | Possible bias (large uneven losses to follow-up; allocation to groups on basis of date of birth) |
Appendix 10 CONSORT 2010 checklist of information for APEX randomised controlled trial
| Section/topic | Item | Checklist item | Reported |
|---|---|---|---|
| Title and abstract | |||
| 1a | Identification as a randomised trial in the title | Yes | |
| 1b | Structured summary of trial design, methods, results, and conclusions (for specific guidance see CONSORT for abstracts) | Yes | |
| Introduction | |||
| Background and objectives | 2a | Scientific background and explanation of rationale | Yes |
| 2b | Specific objectives or hypotheses | Yes | |
| Methods | |||
| Trial design | 3a | Description of trial design (such as parallel, factorial) including allocation ratio | Yes |
| 3b | Important changes to methods after trial commencement (such as eligibility criteria), with reasons | N/A | |
| Participants | 4a | Eligibility criteria for participants | Yes |
| 4b | Settings and locations where the data were collected | Yes | |
| Interventions | 5 | The interventions for each group with sufficient details to allow replication, including how and when they were actually administered | Yes |
| Outcomes | 6a | Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed | Yes |
| 6b | Any changes to trial outcomes after the trial commenced, with reasons | Statistical analysis as described | |
| Sample size | 7a | How sample size was determined | Yes |
| 7b | When applicable, explanation of any interim analyses and stopping guidelines | N/A | |
| Randomisation: | Yes | ||
| Sequence generation | 8a | Method used to generate the random allocation sequence | Yes |
| 8b | Type of randomisation; details of any restriction (such as blocking and block size) | Yes | |
| Allocation concealment mechanism | 9 | Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned | Yes |
| Implementation | 10 | Who generated the random allocation sequence, who enrolled participants, and who assigned participants to interventions | Yes |
| Blinding | 11a | If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how | Yes |
| 11b | If relevant, description of the similarity of interventions | N/A | |
| Statistical methods | 12a | Statistical methods used to compare groups for primary and secondary outcomes | Yes |
| 12b | Methods for additional analyses, such as subgroup analyses and adjusted analyses | Yes | |
| Results | |||
| Participant flow (a diagram is strongly recommended) | 13a | For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analysed for the primary outcome | Yes |
| 13b | For each group, losses and exclusions after randomisation, together with reasons | Yes | |
| Recruitment | 14a | Dates defining the periods of recruitment and follow-up | Yes |
| 14b | Why the trial ended or was stopped | N/A | |
| Baseline data | 15 | A table showing baseline demographic and clinical characteristics for each group | Yes |
| Numbers analysed | 16 | For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups | Yes |
| Outcomes and estimation | 17a | For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% CI) | Yes |
| 17b | For binary outcomes, presentation of both absolute and relative effect sizes is recommended | N/A | |
| Ancillary analyses | 18 | Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory | Yes |
| Harms | 19 | All important harms or unintended effects in each group (for specific guidance see CONSORT for harms) | Yes |
| Discussion | |||
| Limitations | 20 | Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses | Yes |
| Generalisability | 21 | Generalisability (external validity, applicability) of the trial findings | Yes |
| Interpretation | 22 | Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence | Yes |
| Other information | |||
| Registration | 23 | Registration number and name of trial registry | Yes |
| Protocol | 24 | Where the full trial protocol can be accessed, if available | Yes |
| Funding | 25 | Sources of funding and other support (such as supply of drugs), role of funders | Yes |
Appendix 11 Pain on admission to the recovery ward, on discharge from the recovery ward and during the day of surgery for the APEX THR and TKR trial
| Pain outcome | Hips | Knees | ||||
|---|---|---|---|---|---|---|
| Intervention, (N = 163), n (%) | Standard care, (N = 159), n (%) | p-valuesa,b | Intervention, (N = 157), n (%) | Standard care, (N = 159), n (%) | p-valuesa,b | |
| Pain on admission to the recovery ward | 0.169 | 0.062 | ||||
| No pain | 115 (70.6) | 111 (69.8) | 108 (68.8) | 86 (54.1) | ||
| Mild pain | 9 (5.5) | 3 (1.9) | 7 (4.5) | 11 (6.9) | ||
| Moderate pain | 4 (2.5) | 10 (6.3) | 8 (5.1) | 16 (10.1) | ||
| Severe pain | 4 (2.5) | 2 (1.3) | 12 (7.6) | 18 (11.3) | ||
| Missing | 31 (19) | 33 (20.8) | 22 (14) | 28 (17.6) | ||
| Pain on discharge from the recovery ward | 0.759 | 0.097 | ||||
| No pain | 92 (56.4) | 87 (54.7) | 93 (59.2) | 78 (49.1) | ||
| Mild pain | 29 (17.8) | 25 (15.7) | 34 (21.7) | 31 (19.5) | ||
| Moderate pain | 4 (2.5) | 6 (3.8) | 6 (3.8) | 13 (8.2) | ||
| Severe pain | 0 (0) | 0 (0) | 1 (0.6) | 5 (3.1) | ||
| Missing | 38 (23.3) | 41 (25.8) | 23 (14.6) | 32 (20.1) | ||
| Pain during day of surgery | 0.515 | 0.325 | ||||
| No pain | 26 (16) | 28 (17.6) | 37 (23.6) | 30 (18.9) | ||
| Mild pain | 65 (39.9) | 51 (32.1) | 41 (26.1) | 36 (22.6) | ||
| Moderate pain | 44 (27) | 43 (27) | 44 (28) | 61 (38.4) | ||
| Severe pain | 8 (4.9) | 11 (6.9) | 14 (8.9) | 16 (10.1) | ||
| Missing | 20 (12.3) | 26 (16.4) | 21 (13.4) | 16 (10.1) | ||
Appendix 12 Length of stay for the APEX THR and TKR trial
| Length of hospital stay | Hips | Knees | ||||
|---|---|---|---|---|---|---|
| Intervention, (n = 163) | Standard care, (n = 159) | p-value1,2a,b | Intervention, (n = 157) | Standard care, (n = 159) | p-value1,2a,b | |
| Median days (25th, 75th) | 4 (4, 5) | 5 (4, 6) | 0.2476 | 4 (4, 6) | 5 (4, 6) | 0.5864 |
| Missing, n (%) | 10 (6.1) | 8 (5.0) | 14 (8.9) | 12 (7.6) | ||
Appendix 13 Postoperative inpatient pain scores for the APEX THR and TKR trial
| Postoperative day | Pain outcome | Hips | Knees | ||
|---|---|---|---|---|---|
| Intervention, (n = 163) | Standard care, (n = 159) | Intervention, (n = 157) | Standard care, (n = 159) | ||
| Pain at night | |||||
| Day 1 | Median (IQRa) | 35 (50) | 36 (45) | 48 (55) | 42 (55) |
| Missing number (%) | 17 (10.4) | 12 (7.5) | 28 (17.8) | 18 (11.3) | |
| Day 2 | Median (IQRa) | 12 (22) | 23 (33) | 45 (55) | 39 (49) |
| Missing number (%) | 18 (11.0) | 13 (8.2) | 23 (14.7) | 15 (9.4) | |
| Day 3 | Median (IQRa) | 8 (20) | 11 (28) | 24 (37) | 32 (47) |
| Missing number (%)b | 22 (14.2) | 17 (11.1) | 29 (18.8) | 25 (16.0) | |
| Pain at rest | |||||
| Day 1 | Median (IQRa) | 23 (28) | 26 (30) | 37 (34) | 44 (32) |
| Missing number (%) | 16 (9.8) | 10 (6.3) | 26 (16.7) | 17 (10.7) | |
| Day 2 | Median (IQRa) | 12 (26) | 16 (24) | 35 (53) | 35 (37) |
| Missing number (%) | 17 (10.4) | 13 (8.2) | 22 (14.0) | 15 (9.4) | |
| Day 3 | Median (IQRa) | 7 (18) | 13 (18) | 21 (28) | 21 (36) |
| Missing number (%)b | 23 (14.8) | 17 (11.1) | 29 (18.8) | 25 (16.0) | |
| Pain on movement | |||||
| Day 1 | Median (IQRa) | 52 (34) | 56 (37) | 58 (37) | 62 (26) |
| Missing number (%) | 16 (9.8) | 10 (6.3) | 26 (16.6) | 17 (10.7) | |
| Day 2 | Median (IQRa) | 39 (41) | 42 (40) | 56 (37) | 57 (36) |
| Missing number (%) | 18 (11.0) | 13 (8.2) | 22 (14.0) | 15 (9.4) | |
| Day 3 | Median (IQRa) | 28 (44) | 32 (37) | 42 (39) | 46 (40) |
| Missing number (%)b | 23 (14.8) | 17 (11.1) | 29 (18.8) | 25 (16.0) | |
Appendix 14 Drugs and side effects during recovery for the APEX THR and TKR trial
| Drugs and side effects | Hips | Knees | |||||
|---|---|---|---|---|---|---|---|
| Intervention, (n = 163) | Standard care, (n = 159) | p-valuea,b | Intervention, (n = 157) | Standard care, (n = 159) | p-valuea,b | ||
| Drugs | |||||||
| Drugs administered | Number (%) | 109 (66.9) | 100 (62.9) | 0.176 | 98 (62.4) | 118 (74.2) | 0.010 |
| Missing | Number (%) | 11 (6.8) | 4 (2.5) | 12 (7.6) | 13 (8.2) | ||
| Strong opioids (units) | Median (IQR) | 3 (8) | 4.5 (9) | 0.4503 | 3 (6) | 3 (15) | 0.441c |
| Side effects | |||||||
| Nausea and vomiting | Number (%) | 80 (49.1) | 88 (55.4) | 0.429 | 79 (50.3) | 87 (54.7) | 0.417 |
| Missing number (%) | 10 (6.1) | 4 (2.5) | 12 (7.6) | 12 (7.6) | |||
| Antiemetics | Number (%) | 19 (11.7) | 20 (12.6) | 0.916 | 16 (10.2) | 21 (13.2) | 0.391 |
| Missing number (%) | 10 (6.1) | 4 (2.5) | 12 (7.6) | 13 (8.2) | |||
| Repeat femoral block | Number (%) | 0 (0.0) | 0 (0.0) | N/A | 2 (1.3) | 3 (1.9) | 0.909 |
| Missing number (%) | 10 (6.1) | 4 (2.5) | 12 (7.6) | 12 (7.6) | |||
| Sign of toxicity | Number (%) | 1 (0.6) | 0 (0.0) | 0.497 | 1 (0.6) | 0 (0.0) | 0.313 |
| Missing number (%) | 10 (6.1) | 4 (2.5) | 12 (7.6) | 12 (7.6) | |||
Appendix 15 Prevalence of vomiting, nausea and analgesia intake during the first 48 hours after recovery for the APEX THR and TKR trial
| Opioid intake, vomiting and nausea | Hips | Knees | |||||
|---|---|---|---|---|---|---|---|
| Intervention (n = 163) | Standard care (n = 159) | p-valuea,b | Intervention (n = 157) | Standard care (n = 159) | p-valuea,b | ||
| Opioid intake | |||||||
| Strongc | Median (IQR) | 97 (88) | 101 (105) | 0.381d | 120 (99) | 130 (115) | 0.324d |
| Missing number (%) | 10 (6.1) | 12 (7.6) | 13 (8.3) | 13 (8.2) | |||
| Weakc | Median (IQR) | 0 (0) | 0 (0) | 0.625d | 0 (0) | 0 (0) | 0.433d |
| Missing number (%) | 10 (6.1) | 12 (7.6) | 13 (8.3) | 13 (8.2) | |||
| Vomiting | |||||||
| Day 1 | Number (%) | 47 (33.3) | 51 (35.4) | 0.711 | 32 (26.0) | 51 (39.2) | 0.025 |
| Missing number (%) | 22 (13.5) | 15 (9.4) | 34 (21.7) | 29 (18.2) | |||
| Day 2 | Number (%) | 28 (17.2) | 33 (20.8) | 0.392 | 19 (15.1) | 29 (20.4) | 0.255 |
| Missing number (%) | 24 (14.7) | 24 (15.1) | 31 (19.8) | 17 (10.7) | |||
| Day 3 | Number (%) | 10 (8.9) | 10 (8.3) | 0.888 | 9 (8.0) | 14 (12.6) | 0.261 |
| Missing number (%) | 42 (27.1) | 33 (21.6) | 45 (28.7) | 48 (30.2) | |||
| Nausea | |||||||
| Day 1 | Number (%) | 71 (50.4) | 100 (69.4) | 0.001 | 58 (47.5) | 85 (64.9) | 0.005 |
| Missing number (%) | 15 (9.4) | 22 (13.5) | 35 (22.3) | 28 (17.6) | |||
| Day 2 | Number (%) | 67 (48.6) | 77 (56.6) | 0.181 | 52 (41.6) | 72 (51.1) | 0.123 |
| Missing number (%) | 25 (15.3) | 23 (14.5) | 32 (20.4) | 18 (11.3) | |||
| Day 3 | Number (%) | 38 (33.6) | 46 (38.3) | 0.455 | 36 (32.1) | 44 (39.6) | 0.243 |
| Missing number (%) | 42 (27.1) | 33 (21.6) | 45 (28.7) | 48 (30.2) | |||
Appendix 16 Intention-to-treat and per-protocol analysesa of the effect of the intervention on pain (WOMAC) at 3 and 6 months after surgery for the APEX THR and TKR trial
| Analysis model | ITT-CC | ITT-imputed | PP | |||
|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | |
| Hips | ||||||
| 3 months | ||||||
| Unadjusted | 2.92 (–1.12 to 6.97) | 0.156 | 3.38 (–0.76 to 7.53) | 0.110 | 2.78 (–1.40 to 6.97) | 0.192 |
| Adjusted | 1.71 (–2.32 to 5.74) | 0.405 | 2.05 (–2.00 to 6.10) | 0.321 | 1.49 (–2.57 to 5.56) | 0.472 |
| 6 months | ||||||
| Unadjusted | 1.87 (–2.15 to 5.88) | 0.362 | 2.84 (–1.00 to 6.67) | 0.147 | 1.25 (–2.91 to 5.40) | 0.556 |
| Adjusted | 0.61 (–3.31 to 4.53) | 0.761 | 1.51 (–2.24 to 5.26) | 0.431 | –0.08 (–4.13 to 3.97) | 0.968 |
| Knees | ||||||
| 3 months | ||||||
| Unadjusted | –0.52 (–4.85 to 3.79) | 0.813 | –0.80 (–5.12 to 3.52) | 0.716 | –0.26 (–4.72 to 4.20) | 0.91 |
| Adjusted | –0.32 (–4.52 to 3.88) | 0.881 | –0.42 (–4.62 to 3.79) | 0.846 | –0.26 (–4.61 to 4.09) | 0.907 |
| 6 months | ||||||
| Unadjusted | 4.10 (–0.22 to 8.43) | 0.063 | 4.37 (0.11 to 8.63) | 0.044 | 4.34 (–0.15 to 8.83) | 0.058 |
| Adjusted | 4.26 (0.04 to 8.48) | 0.048 | 4.75 (0.61 to 8.90) | 0.025 | 4.30 (–0.08 to 8.68) | 0.055 |
Appendix 17 Intention-to-treat and per-protocol analysesa of the effect of the intervention on pain (WOMAC) at 3 and 6 months after surgery for the APEX THR trial
| Analysis model | ITT-CC | ITT-imputed | PP | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| 3 months post operation | ||||||
| (Moderate, mild or none) vs. reference = (severe) | ||||||
| Baseline | 2.36 (0.54 to 10.37) | 0.255 | 2.67 (0.60 to 11.92) | 0.198 | 2.47 (0.52 to 11.78) | 0.257 |
| Adjusted | 1.86 (0.43 to 8.00) | 0.406 | 2.01 (0.46 to 8.81) | 0.357 | 1.88 (0.41 to 8.74) | 0.418 |
| (Mild or none) vs. reference = (severe or moderate) | ||||||
| Baseline | 2.63 (0.95 to 7.29) | 0.064 | 2.85 (1.02 to 7.94) | 0.045 | 2.72 (0.90 to 8.24) | 0.076 |
| Adjusted | 2.14 (0.79 to 5.79) | 0.134 | 2.24 (0.83 to 6.08) | 0.112 | 2.19 (0.75 to 6.42) | 0.153 |
| (None) vs. reference = (severe, moderate or mild) | ||||||
| Baseline | 2.61 (1.03 to 6.65) | 0.044 | 2.61 (1.04 to 6.52) | 0.040 | 2.84 (1.02 to 7.93) | 0.046 |
| Adjusted | 2.05 (0.83 to 5.10) | 0.122 | 2.04 (0.84 to 4.94) | 0.113 | 2.15 (0.80 to 5.81) | 0.130 |
| 6 months post operation | ||||||
| (Moderate, mild or none) vs. reference = (severe) | ||||||
| Baseline | 6.52 (1.21 to 35.29) | 0.030 | 7.19 (1.40 to 36.97) | 0.018 | 5.66 (0.94 to 33.87) | 0.058 |
| Adjusted | 5.04 (0.93 to 27.22) | 0.06 | 5.32 (1.05 to 26.85) | 0.043 | 4.26 (0.72 to 25.27) | 0.111 |
| (Mild or none) vs. reference = (severe or moderate) | ||||||
| Baseline | 2.28 (0.79 to 6.57) | 0.128 | 2.50 (0.90 to 6.94) | 0.079 | 2.26 (0.72 to 7.13) | 0.165 |
| Adjusted | 1.74 (0.61 to 4.94) | 0.297 | 1.92 (0.70 to 5.28) | 0.206 | 1.69 (0.55 to 5.22) | 0.361 |
| (None) vs. reference = (severe, moderate or mild) | ||||||
| Baseline | 1.16 (0.47 to 2.86) | 0.740 | 1.22 (0.50 to 2.99) | 0.658 | 1.18 (0.44 to 3.16) | 0.737 |
| Adjusted | 0.93 (0.38 to 2.23) | 0.863 | 0.98 (0.41 to 2.33) | 0.958 | 0.92 (0.35 to 2.39) | 0.858 |
Appendix 18 Intention-to-treat and per-protocol analysesa of the effect of the intervention on pain (WOMAC) at 3 and 6 months after surgery for the APEX TKR trial
| Analysis model | ITT-CC | ITT-imputed | PP | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| 3 months post operation | ||||||
| (Moderate, mild or none) vs. reference = (severe) | ||||||
| Baseline | 1.12 (0.35 to 3.60) | 0.850 | 1.09 (0.37 to 3.26) | 0.873 | 1.19 (0.36 to 4.01) | 0.774 |
| Adjusted | 1.01 (0.32 to 3.19) | 0.989 | 1.22 (0.41 to 3.63) | 0.723 | 1.03 (0.31 to 3.42) | 0.957 |
| (Mild or none) vs. reference = (severe or moderate) | ||||||
| Baseline | 0.86 (0.32 to 2.32) | 0.770 | 0.79 (0.31 to 2.02) | 0.621 | 0.86 (0.31 to 2.40) | 0.772 |
| Adjusted | 0.86 (0.32 to 2.27) | 0.760 | 0.89 (0.35 to 2.26) | 0.798 | 0.82 (0.30 to 2.26) | 0.700 |
| (None) vs. reference = (severe, moderate or mild) | ||||||
| Baseline | 1.45 (0.33 to 6.39) | 0.623 | 1.77 (0.44 to 7.13) | 0.425 | 1.47 (0.31 to 6.94) | 0.628 |
| Adjusted | 1.58 (0.36 to 6.87) | 0.540 | 1.98 (0.49 to 7.93) | 0.335 | 1.57 (0.33 to 7.33) | 0.570 |
| 6 months post operation | ||||||
| (Moderate, mild or none) vs. reference = (severe) | ||||||
| Baseline | 2.95 (0.81 to 10.71) | 0.100 | 2.80 (0.85 to 9.29) | 0.091 | 3.08 (0.81 to 11.68) | 0.097 |
| Adjusted | 2.48 (0.70 to 8.82) | 0.161 | 2.73 (0.82 to 9.09) | 0.101 | 2.55 (0.69 to 9.44) | 0.162 |
| (Mild or none) vs. reference = (severe or moderate) | ||||||
| Baseline | 1.83 (0.68 to 4.95) | 0.235 | 1.84 (0.72 to 4.72) | 0.205 | 1.92 (0.68 to 5.40) | 0.217 |
| Adjusted | 1.70 (0.64 to 4.52) | 0.289 | 1.98 (0.78 to 5.05) | 0.151 | 1.71 (0.62 to 4.73) | 0.303 |
| (None) vs. reference = (severe, moderate or mild) | ||||||
| Baseline | 1.92 (0.58 to 6.29) | 0.283 | 1.98 (0.63 to 6.23) | 0.242 | 1.68 (0.49 to 5.75) | 0.408 |
| Adjusted | 2.03 (0.63 to 6.55) | 0.235 | 2.42 (0.76 to 7.66) | 0.134 | 1.72 (0.51 to 5.81) | 0.380 |
Appendix 19 Intention-to-treat and per-protocol analyses of the effect of the intervention on pain (ICOAP)a at 3, 6 and 12 months after surgery in the APEX THR trial
| Analysis model | ITT-CC | ITT-imputed | PP | |||
|---|---|---|---|---|---|---|
| RR (95% CI) | p-value | RR (95% CI) | p-value | RR (95% CI) | p-value | |
| 3 monthsb | (n = 259) | (n = 322) | (n = 246) | |||
| Baseline | 0.58 (0.30 to 1.15) | 0.120 | 0.56 (0.29 to 1.08) | 0.086 | 0.59 (0.30 to 1.15) | 0.122 |
| Adjusted | 0.66 (0.34 to 1.29) | 0.222 | 0.65 (0.34 to 1.25) | 0.195 | 0.66 (0.34 to 1.30) | 0.233 |
| 6 monthsb | (n = 251) | (n = 322) | (n = 239) | |||
| Baseline | 0.77 (0.37 to 1.62) | 0.495 | 0.69 (0.35 to 1.38) | 0.295 | 0.77 (0.35 to 1.68) | 0.515 |
| Adjusted | 0.88 (0.41 to 1.86) | 0.731 | 0.80 (0.40 to 1.60) | 0.525 | 0.88 (0.40 to 1.92) | 0.742 |
| 12 monthsc | (n = 265) | (n = 322) | (n = 251) | |||
| Baseline | 0.40 (0.18 to 0.86) | 0.020 | 0.49 (0.25 to 0.96) | 0.038 | 0.42 (0.19 to 0.92) | 0.031 |
| Adjusted | 0.44 (0.21 to 0.96) | 0.038 | 0.58 (0.30 to 1.14) | 0.115 | 0.47 (0.22 to 1.03) | 0.059 |
Appendix 20 Intention-to-treat and per-protocol analyses of the effect of the intervention on pain (ICOAP)a at 3, 6 and 12 months after surgery in the APEX TKR trial
| Analysis model | ITT-CC | ITT-imputed | PP | |||
|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | |
| 3 monthsb | (n = 242) | (n = 316) | (n = 231) | |||
| Baseline | 0.11 (–0.50 to 0.72) | 0.725 | 0.13 (–0.44 to 0.71) | 0.649 | 0.08 (–0.54 to 0.70) | 0.801 |
| Adjusted | 0.12 (–0.48 to 0.72) | 0.697 | 0.10 (–0.49 to 0.68) | 0.746 | 0.11 (–0.49 to 0.72) | 0.711 |
| 6 monthsb | (n = 231) | (n = 316) | (n = 218) | |||
| Baseline | –0.46 (–1.08 to 0.15) | 0.140 | –0.38 (–0.98 to 0.21) | 0.205 | –0.47 (–1.10 to 0.16) | 0.144 |
| Adjusted | –0.45 (–1.06 to 0.16) | 0.146 | –0.42 (–1.01 to 0.16) | 0.158 | –0.45 (–1.07 to 0.17) | 0.159 |
| 12 monthsc | (n = 257) | (n = 316) | (n = 243) | |||
| Baseline | –0.15 (–0.76 to 0.48) | 0.646 | –0.06 (–0.68 to 0.57) | 0.855 | –0.16 (–0.81 to 0.50) | 0.632 |
| Adjusted | –0.29 (–0.90 to 0.34) | 0.380 | –0.20 (–0.81 to 0.42) | 0.529 | –0.30 (–0.95 to 0.35) | 0.364 |
Appendix 21 Intention-to-treat and per-protocol analyses of the effect of the intervention on function (WOMAC)a at 3, 6 and 12 months after surgery in the APEX THR trial
| Analysis model | ITT-CC | ITT-imputed | PP | |||
|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | |
| 3 monthsb | (n = 257) | (n = 322) | (n = 244) | |||
| Baseline | 636.04 (71.40 to 1200.68) | 0.027 | 632.0 (40.01 to 1223.99) | 0.036 | 614.4 (42.3 to 1186.5) | 0.035 |
| Adjusted | 487.9 (–59.29 to 1035.1) | 0.081 | 472.8 (–102.7 to 1048.3) | 0.107 | 443.9 (–108.0 to 995.9) | 0.115 |
| 6 monthsb | (n = 254) | (n = 322) | (n = 241) | |||
| Baseline | 326.6 (–239.28 to 892.40) | 0.258 | 425.9 (–141.8 to 993.5) | 0.141 | 265.1 (–308.0 to 838.2) | 0.365 |
| Adjusted | 188.2 (–361.7 to 738.1) | 0.502 | 266.7 (–286.5 to 819.9) | 0.344 | 106.6 (–447.5 to 660.7) | 0.706 |
| 12 monthsc | (n = 266) | (n = 322) | (n = 252) | |||
| Baseline | 500.6 (–80.6 to 1081.9) | 0.091 | 623.4 (46.1 to 1200.7) | 0.034 | 400.7 (–192.2 to 993.6) | 0.184 |
| Adjusted | 459.4 (–97.3 to 1016.13) | 0.105 | 499.4 (–58.61 to 1057.5) | 0.079 | 363.2 (–201.3 to 930.7) | 0.209 |
Appendix 22 Intention-to-treat and per-protocol analyses of the effect of the intervention on function (WOMAC) at 3, 6 and 12 months after surgery in the APEX TKR trial
| Analysis model | ITT-CC | ITT-imputed | PP | |||
|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | |
| 3 monthsa | (n = 233) | (n = 316) | (n = 223) | |||
| Baseline | 0.19 (–4.12 to 4.50) | 0.931 | –0.68 (–4.99 to 3.64) | 0.759 | 0.60 (–3.86 to 5.07) | 0.790 |
| Adjusted | 0.71 (–3.44 to 4.86) | 0.738 | 0.04 (–4.13 to 4.21) | 0.985 | 0.97 (–3.32 to 5.26) | 0.658 |
| 6 monthsa | (n = 227) | (n = 316) | (n = 215) | |||
| Baseline | 2.54 (–1.79 to 6.88) | 0.250 | 1.76 (–2.56 to 6.09) | 0.425 | 2.82 (–1.67 to 7.32) | 0.218 |
| Adjusted | 2.97 (–1.21 to 7.15) | 0.164 | 2.48 (–1.72 to 6.67) | 0.247 | 3.11 (–1.21 to 7.44) | 0.159 |
| 12 monthsb | (n = 258) | (n = 316) | (n = 246) | |||
| Baseline | 1.20 (–3.24 to 5.65) | 0.594 | 1.81 (–3.38 to 7.00) | 0.491 | 1.22 (–3.41 to 5.86) | 0.604 |
| Adjusted | 1.88 (–2.49 to 6.24) | 0.398 | 3.03 (–2.22 to 8.28) | 0.256 | 1.85 (–2.69 to 6.40) | 0.422 |
Appendix 23 Intention-to-treat and per-protocol analyses of the effect of the intervention on stiffness (WOMAC)a at 3, 6 and 12 months after surgery for the APEX THR trial
| Analysis model | ITT-CC | ITT-imputed | PP | |||
|---|---|---|---|---|---|---|
| RR (95% CI) | p-value | RR (95% CI) | p-value | RR (95% CI) | p-value | |
| 3 monthsb | (n = 258) | (n = 322) | (n = 245) | |||
| Baseline | 0.87 (0.50 to 1.53) | 0.634 | 0.80 (0.46 to 1.37) | 0.414 | 0.97 (0.55 to 1.72) | 0.920 |
| Adjusted | 0.96 (0.55 to 1.67) | 0.881 | 0.86 (0.51 to 1.45) | 0.566 | 1.08 (0.62 to 1.90) | 0.784 |
| 6 monthsb | (n = 246) | (n = 322) | (n = 233) | |||
| Baseline | 0.99 (0.45 to 2.19) | 0.990 | 0.96 (0.46 to 2.00) | 0.921 | 1.01 (0.44 to 2.30) | 0.983 |
| Adjusted | 1.03 (0.48 to 2.21) | 0.942 | 1.03 (0.50 to 2.13) | 0.927 | 1.05 (0.47 to 2.34) | 0.907 |
| 12 monthsc | (n = 266) | (n = 322) | (n = 252) | |||
| Baseline | 0.60 (0.29 to 1.24) | 0.167 | 0.55 (0.29 to 1.04) | 0.068 | 0.68 (0.33 to 1.44) | 0.318 |
| Adjusted | 0.64 (0.31 to 1.35) | 0.246 | 0.64 (0.34 to 1.21) | 0.173 | 0.73 (0.34 to 1.56) | 0.414 |
Appendix 24 Intention-to-treat and per-protocol analyses of the effect of the intervention on stiffness (WOMAC) at 3, 6 and 12 months after surgery in the APEX TKR trial
| Analysis model | ITT-CC | ITT-imputed | PP | |||
|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | Coefficient (95% CI) | p-value | |
| 3 monthsa | (n = 237) | (n = 316) | (n = 227) | |||
| Baseline | 2.94 (–2.05 to 7.94) | 0.248 | 1.23 (–3.85 to 6.30) | 0.635 | 2.89 (–2.24 to 8.02) | 0.270 |
| Adjusted | 3.53 (–1.44 to 8.49) | 0.164 | 2.10 (–3.04 to 7.24) | 0.423 | 3.20 (–1.91 to 8.30) | 0.219 |
| 6 monthsa | (n = 228) | (n = 316) | (n = 216) | |||
| Baseline | 4.03 (–1.02 to 9.08) | 0.117 | 3.22 (–2.06 to 8.52) | 0.234 | 3.68 (–1.52 to 8.89) | 0.166 |
| Adjusted | 4.54 (–0.47 to 9.55) | 0.076 | 4.09 (–1.18 to 9.36) | 0.128 | 3.93 (–1.24 to 9.09) | 0.136 |
| 12 monthsb | (n = 256) | (n = 316) | (n = 242) | |||
| Baseline | 1.78 (–3.21 to 6.78) | 0.483 | 1.81 (–3.38 to 7.00) | 0.491 | 1.77 (–3.40 to 6.94) | 0.500 |
| Adjusted | 2.70 (–2.37 to 7.77) | 0.296 | 3.03 (–2.22 to 8.28) | 0.256 | 2.63 (–2.63 to 7.89) | 0.325 |
Appendix 25 Intention-to-treat and per-protocol analyses of the effect of the intervention on neuropathic pain as measured by painDETECT (< 13, ≥ 13) at 12 months after surgerya in the APEX THR trial
| Analysis model | ITT-CC (n = 267) | ITT-imputed (n = 322) | PP (n = 253) | |||
|---|---|---|---|---|---|---|
| RR (95% CI) | p-value | RR (95% CI) | p-value | RR (95% CI) | p-value | |
| Baseline | 0.17 (0.04 to 0.76) | 0.021 | 0.21 (0.05 to 0.83) | 0.027 | 0.17 (0.04 to 0.77) | 0.022 |
| Adjusted | 0.17 (0.03 to 0.82) | 0.028 | 0.22 (0.05 to 0.90) | 0.035 | 0.16 (0.03 to 0.83) | 0.030 |
Appendix 26 Intention-to-treat and per-protocol analyses of the effect of the intervention on neuropathic pain as measured by painDETECT (< 13, ≥ 13) at 12 months after surgerya in the APEX TKR trial
| Analysis model | ITT-CC (n = 254) | ITT-imputed (n = 316) | PP (n = 241) | |||
|---|---|---|---|---|---|---|
| RR (95% CI) | p-value | RR (95% CI) | p-value | RR (95% CI) | p-value | |
| Baseline | 0.92 (0.60 to 1.42) | 0.715 | 0.99 (0.67 to 1.47) | 0.973 | 0.94 (0.60 to 1.47) | 0.792 |
| Adjusted | 0.95 (0.61 to 1.49) | 0.834 | 1.05 (0.69 to 1.61) | 0.796 | 0.98 (0.62 to 1.53) | 0.918 |
Appendix 27 Patient complications and serious adverse events in the APEX THR trial
| Patient complications and SAE | Intervention (N = 163) | Standard care (N = 159) | p-valuea |
|---|---|---|---|
| Deceased patient, n (%) | 0 (0.0) | 6 (3.8) | N/A |
| Patient with an infection, n (%)b | 3 (1.8) | 3 (1.9) | 1.000 |
| Patients with SAE, n (%) | 41 (25.2) | 55 (34.6) | 0.090 |
| Patients by count of SAEs, n (%) | 0.611 | ||
| 1 | 29 (17.8) | 44 (27.7) | |
| 2 | 10 (6.1) | 9 (5.7) | |
| 3 | 2 (1.2) | 2 (1.3) | |
| Total SAEs, n | 55 | 68 | |
| Expedited SAEs, % | 85.5 | 82.4 | 0.640 |
Appendix 28 Patient complications and serious adverse events for the APEX TKR trial
| Patient complications and SAE | Intervention (N = 157) | Standard care (N = 159) | p-valuea |
|---|---|---|---|
| Deceased patient, n (%) | 3 (1.9) | 1 (0.6) | 0.369 |
| Patient with an infection, n (%)b | 5 (3.2) | 3 (1.9) | 0.500 |
| Patients with SAE, n (%) | 63 (40.1) | 58 (36.5) | 0.505 |
| Patients by count of SAEs, n (%) | 0.685 | ||
| 1 | 48 (30.6) | 48 (30.2) | |
| 2 | 11 (7.0) | 8 (5.0) | |
| 3 | 4 (2.6) | 2 (1.3) | |
| Total SAEs, n | 82 | 69 | |
| Expedited SAEs, % | 85.4 | 82.9 | 72 |
Appendix 29 Pre-operative hip X-ray form
Appendix 30 Pre-operative knee X-ray form
Appendix 31 Pre-surgical exercise and educational interventions before total hip and knee replacement: Cochrane risk-of-bias table
| Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data addressed (attrition bias) | Lack of selective reporting (reporting bias) | Lack of other sources of bias | Our evaluation | |
|---|---|---|---|---|---|---|---|
| Aoki and colleagues 2009509 | ∼ | ∼ | ✓ | ✓ | ✓ | ✓ | Low risk of bias (allocated by odd or even last digit of hospital number; no losses to follow-up described) |
| Beaupre and colleagues 2004510 | ✓ | ✓ | ✓ | ∼ | ✓ | ✓ | Unclear: no reason to assume bias (10 intervention and six control patients excluded from analyses as they did not have joint replacement eligible for Review A. It is possible that the intervention was ‘highly effective’ in improving function and pain) |
| Berge and colleagues 2004487 | ✓ | ∼ | ✓ | ∼ | ✓ | ✓ | Unclear: no reason to assume bias (two control group patients decided to delay joint replacement and were excluded from analyses; three control patients had operation early and lost to follow-up) |
| Bitterli and colleagues 2009488 | ✓ | ✓ | ✓ | ∼ | ✓ | ∼ | Unclear: no reason to assume bias (two intervention group patients had joint replacement brought forward. 9/41 intervention and 9/39 control patients lost to follow-up) |
| Bondy and colleagues 1999526 | ✓ | ∼ | ✓ | ✗ | ✓ | ✓ | Possible bias (6/17 intervention and 8/15 control patients lost to follow-up) |
| Börjesson and colleagues 1996511 | ∼ | ∼ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Brown and colleagues 2012512 | ∼ | ✓ | ✓ | ✗ | ✓ | ✓ | Possible bias (6/17 intervention and 8/15 control patients lost to follow-up) |
| Butler and colleagues 1996489 and from McDonald and colleagues 200483 | ∼ | ✓ | ✓ | ✗ | ✓ | ✓ | Possible bias (data analysed for primary replacement but all hip replacement patients randomised; no follow-up of 8/80 patients) |
| Clode-Baker and colleagues 1997490 and from McDonald and colleagues 200483 | ✓ | ∼ | ✓ | ✗ | ✓ | ✓ | Possible bias (no details on distribution between randomised groups of 13 patients with cancelled operations) |
| Cooil and Bithell 1997491 | ✓ | ∼ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Crotty and colleagues 2009527 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Crowe and Henderson 2003528 | ✓ | ✓ | ✓ | ∼ | ✓ | ✓ | Unclear: no reason to assume bias (5/65 losses to follow-up – discharge destination – in intervention but none in control groups, other losses not shown) |
| Cuñado Barrio and colleagues 1999529 | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | Possible bias (1/42 intervention and 7/42 control patients lost to follow-up) |
| Daltroy and colleagues 1998530 | ✓ | ∼ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| D’Lima and colleagues 1996 cardiac513 | ✓ | ∼ | ∼ | ✗ | ✓ | ✗ | Possible bias (small numbers of patients: baseline demographic differences) |
| D’Lima and colleagues 1996 physical therapy513 | ✓ | ∼ | ∼ | ✗ | ✓ | ✗ | Possible bias (small numbers of patients: baseline demographic differences) |
| Doering and colleagues 2000492 | ∼ | ∼ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Evgeniadis and colleagues 2008514 | ✓ | ✓ | ✓ | ∼ | ✓ | ✓ | Unclear: no reason to assume bias (5/48 patients lost to follow-up) |
| Ferrara and colleagues 2008493 | ✓ | ∼ | ✓ | ∼ | ✓ | ✓ | Low risk of bias (0/11 and 2/12 control patients lost to 3-month follow-up) |
| Gilbey and colleagues 2003494 | ∼ | ✓ | ∼ | ✓ | ✓ | ∼ | Unclear: no reason to assume bias (alternate allocation within blocks of patient age, 2/37 intervention patients chose to delay surgery) |
| Giraudet Le Quintrec and colleagues 2003495 | ✓ | ✓ | ✓ | ✓ | ✓ | ∼ | Low risk of bias. Possible bias for anxiety outcome (imbalance in baseline anxiety between groups) |
| Gocen and colleagues 2004496 | ✓ | ∼ | ✓ | ✓ | ✓ | ✗ | Possible bias (intervention patients significantly younger than controls) |
| Gstoettner and colleagues 2011515 | ✓ | ✓ | ∼ | ✓ | ✓ | ✗ | Possible bias (control group baseline pain and function values used for comparison with post-intervention pre-surgical outcome) |
| Heikkinen and colleagues 2008516 | ✓ | ∼ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Hoogeboom and colleagues 2010497 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias (one control patient lost to follow-up unexplained) |
| Huang and colleagues 2012517 | ∼ | ∼ | ∼ | ✓ | ✓ | ✓ | Unclear: no reason to assume bias (randomly allocated by chart number) |
| Johansson and colleagues 2007498 | ✓ | ✓ | ✓ | ∼ | ✓ | ✓ | Unclear: no reason to assume bias (length of stay data from hospital notes, 18/123 lost to follow-up) |
| Lewis and colleagues 2002531 | ∼ | ∼ | ∼ | ✓ | ✓ | ✓ | Unclear: no reason to assume bias |
| Lilja and colleagues 1998499 | ∼ | ∼ | ✓ | ∼ | ✓ | ✓ | Unclear: no reason to assume bias (one patient refused to participate after randomisation and 3/22 intervention and 1/28 control patients withdrawn for medical reasons |
| Liu and Lu 2004532 | ∼ | ∼ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Mancuso and colleagues 2008 hips500 | ∼ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias (class was unit of randomisation) |
| Mancuso and colleagues 2008 knees500 | ∼ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias (class was unit of randomisation) |
| McDonald and colleagues 2001533 | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | Possible bias (7/20 intervention and 2/20 control patients lost to follow-up) |
| McDonald and Molony 2004 communication518 | ✓ | ✓ | ✓ | ✓ | ✓ | ∼ | Unclear: no reason to assume bias (recruitment to control group stopped early) |
| McDonald and Molony 2004 pain management518 | ✓ | ✓ | ✓ | ✓ | ✓ | ∼ | Unclear: no reason to assume bias (recruitment to control group stopped early) |
| McGregor and colleagues 2004501 | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | Possible bias (4/19 intervention patients lost to review) |
| McKay and colleagues 2012519 | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ | Possible bias (baseline differences in WOMAC pain and function, 5/22 lost to 12-week follow-up) |
| Mitchell and colleagues 2005520 | ✓ | ✓ | ✓ | ∼ | ✓ | ✓ | Unclear: no reason to assume bias (45/160 withdrawals including 24 patients with surgery cancelled). No evidence that study withdrawal varied by group or WOMAC scores |
| Nuñez and colleagues 2006521 | ✓ | ✓ | ✓ | ∼ | ✓ | ✓ | Low risk of bias (20% loss to follow-up but no evidence that the loss of patients was related to baseline characteristics or to group) |
| Oosting and colleagues 2012502 | ✓ | ✓ | ✓ | ∼ | ✓ | ✓ | Low risk of bias (0/15 and 2/15 lost to pre-surgical follow-up) |
| Pellino and colleagues 1998534 | ✓ | ✓ | ✓ | ∼ | ✓ | ✗ | Possible bias (recruitment into intervention quicker than control group, surgery delayed more frequently in control group) |
| Rooks and colleagues 2006 hips503 | ✓ | ✓ | ✓ | ∼ | ✓ | ✗ | Possible bias (14/63 lost to follow-up, dropouts had a higher level of function than completers) |
| Rooks and colleagues 2006 knees503 | ✓ | ✓ | ✓ | ∼ | ✓ | ✓ | Low risk of bias (16/45 lost to follow-up but baseline characteristics similar) |
| Sandell 2008504 | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | Possible bias (22/89 patients had surgery before follow-up) |
| Santavirta and colleagues 1994505 and McDonald and colleagues 200483 | ∼ | ∼ | ✗ | ∼ | ✓ | ✓ | Unclear: no reason to assume bias (postponement or other loss to follow-up similar in intervention 7/27 and control 6/33) |
| Sjöling and colleagues 2003522 | ∼ | ∼ | ✓ | ✓ | ✓ | ✓ | Low risk of bias (alternate allocation after first chosen at random) |
| Swank and colleagues 2011523 | ✓ | ✓ | ∼ | ✓ | ✓ | ✓ | Unclear: no reason to assume bias (outcomes on patients who completed both testing sessions) |
| Vukomanovic and colleagues 2008506 | ✓ | ✓ | ✓ | ∼ | ✓ | ✓ | Low risk of bias (5/23 intervention and 4/22 lost to longer-term follow-up) |
| Weidenhielm and colleagues 1993524 | ∼ | ∼ | ∼ | ✓ | ✓ | ✓ | Unclear: no reason to assume bias (allocation by drawing lots) |
| Wijgman and colleagues1994507 and McDonald and colleagues 200483 | ∼ | ∼ | ∼ | ✓ | ∼ | ✓ | Unclear: no reason to assume bias |
| Williamson and colleagues 2007525 | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | Possible bias (before surgery there were 7/60 intervention and 2/61 control patients lost to follow-up, large losses to follow-up after surgery) |
| Wong and Wong 1985508 | ∼ | ∼ | ✓ | ✗ | ✓ | ✓ | Low risk of bias |
Appendix 32 Occupational therapy in total hip replacement: Cochrane risk-of-bias table
| Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data addressed (attrition bias) | Lack of selective reporting (reporting bias) | Lack of other sources of bias | Our evaluation | |
|---|---|---|---|---|---|---|---|
| Butler and colleagues 1996489 and McDonald and colleagues 200483 | ∼ | ✓ | ✓ | ✗ | ✓ | ✗ | Possible bias (data analysed for primary replacement but all hip replacement patients randomised; no follow-up of 8/80 patients) |
| Ferrara and colleagues 2008493 | ✓ | ∼ | ✓ | ∼ | ✓ | ✓ | Low risk of bias in short term. Unclear: no reason to assume bias in long-term (small study with 3/12 control patients lost to follow-up) |
| Gocen and colleagues 2004496 | ✓ | ∼ | ✓ | ✓ | ✓ | ✗ | Unclear: no reason to assume bias (intervention patients were significantly younger than control patients) |
| McGregor and colleagues 2004501 | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | Possible bias (4/19 intervention patients were lost to review) |
| Munin and colleagues 1998567 | ✓ | ✓ | ∼ | ✗ | ✓ | ✓ | Possible bias (9/35 patients lost to follow-up) |
| Sandell 2008504 | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | Possible bias (large loss to follow-up mainly owing to 22 patients having surgery before follow-up) |
| Siggeirsdottir and colleagues 2005568 | ✓ | ✓ | ✓ | ∼ | ✓ | ✓ | Low risk of bias (3/23 controls lost to 6-month follow-up) |
Appendix 33 PROOF-THR detailed summary of European Quality of Life-5 Dimensions scale
| Topic | Baseline (n = 44) | 4 weeks (n = 36) | 12 weeks (n = 37) | 26 weeks (n = 36) |
|---|---|---|---|---|
| Mobility | ||||
| I have no problems in walking about | 0% | 44% | 68% | 72% |
| I have some problems in walking about | 100% | 56% | 32% | 28% |
| I am confined to bed | 0% | 0% | 0% | 0% |
| Self-care | ||||
| I have no problems with self-care | 36% | 60% | 76% | 94% |
| I have some problems washing or dressing myself | 64% | 40% | 21% | 6% |
| I am unable to wash or dress myself | 0% | 0% | 3% | 0% |
| Usual activities | ||||
| I have no problems with performing my usual activities | 14% | 31% | 54% | 74% |
| I have some problems with performing my usual activities | 77% | 47% | 41% | 26% |
| I am unable to perform my usual activities | 9% | 22% | 5% | 0% |
| Pain/discomfort | ||||
| I have no pain or discomfort | 0% | 29% | 43% | 60% |
| I have moderate pain or discomfort | 57% | 66% | 54% | 40% |
| I have extreme pain or discomfort | 43% | 6% | 3% | 0% |
| Anxiety/depression | ||||
| I am not anxious or depressed | 66% | 71% | 84% | 78% |
| I am moderately anxious or depressed | 34% | 29% | 13% | 22% |
| I am extremely anxious or depressed | 0% | 0% | 3% | 0% |
Appendix 34 PROOF-THR detailed summary of ICECAP scale (baseline data)
| Topic | Baseline (n = 44) | 4 weeks (n = 36) | 12 weeks (n = 37) | 26 weeks (n = 36) |
|---|---|---|---|---|
| Love and friendship | ||||
| I cannot have any of the love and friendship that I want | 5% | 3% | 3% | 3% |
| I can have a little of the love and friendship that I want | 9% | 8% | 5% | 3% |
| I can have a lot of the love and friendship that I want | 23% | 40% | 22% | 19% |
| I can have all the love and friendship that I want | 64% | 49% | 70% | 75% |
| Thinking about the future | ||||
| I can only think about the future with a lot of concern | 9% | 6% | 5% | 3% |
| I can only think about the future with some concern | 11% | 11% | 14% | 11% |
| I can think about the future with only a little concern | 55% | 44% | 38% | 43% |
| I can think about the future without any concern | 25% | 39% | 43% | 43% |
| Doing things that make you feel valued | ||||
| I am unable to do any of the things that make me feel valued | 5% | 3% | 2% | 0% |
| I am able to do a few of the things that make me feel valued | 23% | 17% | 11% | 6% |
| I am able to do many of the things that make me feel valued | 48% | 54% | 38% | 36% |
| I am able to do all of the things that make me feel valued | 25% | 26% | 49% | 58% |
| Enjoyment and pleasure | ||||
| I cannot have any of the enjoyment and pleasure that I want | 2% | 3% | 3% | 0% |
| I can have a little of the enjoyment and pleasure that I want | 43% | 36% | 16% | 5% |
| I can have a lot of the enjoyment and pleasure that I want | 39% | 36% | 46% | 42% |
| I can have all of the enjoyment and pleasure that I want | 16% | 25% | 35% | 53% |
| Independence | ||||
| I am unable to be at all independent | 2% | 3% | 3% | 0% |
| I am able to be independent in a few things | 23% | 13% | 5% | 3% |
| I am able to be independent in many things | 45% | 65% | 43% | 36% |
| I am able to be completely independent | 30% | 19% | 49% | 61% |
Appendix 35 Physiotherapy interventions after total knee replacement: Cochrane risk-of-bias table
| Random sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of outcome assessment (detection bias) | Incomplete outcome data addressed (attrition bias) | Lack of selective reporting (reporting bias) | Lack of other sources of bias | Our evaluation | |
|---|---|---|---|---|---|---|---|
| Evgeniadis and colleagues 2008514 | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | Possible bias (9/24 intervention and 4/24 control patients lost to follow-up |
| Frost and colleagues 2002588 | ✓ | ∼ | ✓ | ✗ | ✓ | ✓ | Possible bias (7/23 intervention and 13/24 control patients lost to follow-up) |
| Fung and colleagues 2012589 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Harmer and colleagues 2009590 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias (small losses to follow-up) |
| Kauppila and colleagues 2010591 | ✓ | ∼ | ✗ | ✗ | ✓ | ✗ | Possible risk of bias (8/44 intervention and 3/42 control patients lost to follow-up, owing to uneven losses to follow-up, baseline differences in prevalence of comorbidities and WOMAC score) |
| Kramer and colleagues 2003592 and Minns Lowe 200784 | ∼ | ∼ | ✓ | ✗ | ✓ | ✓ | Possible risk of bias (‘medical issue’ losses to follow-up differed between groups, 7.5% in clinic and 15% in home-based groups) |
| Liebs and colleagues 2010593 | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | Possible risk of bias (10/85 intervention and 14/74 control patients lost to follow-up) |
| Madsen and colleagues 2013594 | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | Possible risk of bias (4/40 intervention and 8/40 control patients lost to follow-up) |
| Minns Lowe and colleagues 2012595 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias (low losses to follow-up at 12 months) |
| Mitchell and colleagues 2005520 | ✓ | ✓ | ✓ | ✓ | ✓ | ∼ | Low risk of bias (randomisation before surgery with pre-surgical intervention component). Surgery cancelled for 24 patients |
| Mockford and colleagues 2008596 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias (4.7% patients excluded from analysis as lost to follow-up) |
| Moffet and colleagues 2004597 | ✓ | ✓ | ✓ | ∼ | ✓ | ✓ | Low risk of bias. Possible risk of bias for 12 month outcomes (uneven loss to follow-up) |
| Monticone and colleagues 2013598 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Piqueras and colleagues 2013599 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Piva and colleagues 2010600 | ✓ | ✓ | ✓ | ∼ | ✓ | ✓ | Unclear: no reason to assume bias (3/21 intervention and 5/22 control patients lost to follow-up) |
| Rajan and colleagues 2004601 | ✓ | ∼ | ✓ | ✓ | ✓ | ✓ | Low risk of bias |
| Tousignant and colleagues 2011602 | ✓ | ✓ | ✓ | ∼ | ✓ | ∼ | Unclear: no reason to assume bias (3/24 randomised to control withdrew owing to knowledge of group allocation. 3/24 intervention and 4/24 control patients lost to follow-up) |
Appendix 36 Telephone survey questionnaire for current provision of physiotherapy following discharge after total hip replacement and total knee replacement
Appendix 37 Job title of the respondent at each of the twenty-four orthopaedic centres surveyed
| Orthopaedic unit | Procedure surveyed (THR/TKR) | Position of staff member surveyed |
|---|---|---|
| 1 | Both | Clinical therapies manager |
| 2 | Both | Orthopaedic therapy team lead |
| 3 | Both | Clinical lead |
| 4 | Both | Extended scope physiotherapy practitioner |
| 5 | Both | Clinical lead |
| 6 | Both | Team lead |
| 7 | Both | Therapy lead |
| 8 | Both | Senior physiotherapist |
| 9 | Both | Clinical lead |
| 10 | TKR only | Senior physiotherapist |
| 11 | Both | Clinical lead |
| 12 | Both | Senior physiotherapist |
| 13 | Both | Clinical lead |
| 14 | Both | Extended scope physiotherapy practitioner |
| 15 | TKR only | Senior orthopaedic physiotherapist |
| 16 | TKR only | Senior OT |
| 17 | TKR only | Senior physiotherapist |
| 18 | TKR only | Senior physiotherapist |
| 19 | TKR only | Senior physiotherapist |
| 20 | TKR only | Clinical lead |
| 21 | TKR only | Clinical lead |
| 22 | TKR only | Senior outpatient physiotherapist |
| 23 | TKR only | Senior orthopaedic physiotherapist |
| 24 | THR only | Outpatient manager |
Appendix 38 Study feedback reports for patients
List of abbreviations
- 3D
- three-dimensional
- 6MWT
- 6-metre walk test
- Ab-A
- Aberdeen activity limitation subscale
- Ab-I
- Aberdeen impairment subscale
- Ab-IAP
- Aberdeen impairment, activity limitation and participation restriction measure
- Ab-P
- Aberdeen participation restriction subscale
- ADAPT
- Assessing Disability After Partial and Total joint replacement
- ADL
- activities of daily living
- AIMS
- Arthritis Impact Measure Score
- AIMS2
- Arthritis Impact Measure Score 2
- AKSS
- American Knee Society Score
- AMED
- Allied and Complementary Medicine Database
- ANOVA
- analysis of variance
- AOC
- Avon Orthopaedic Centre
- APEX
- Arthroplasty Pain EXperience
- ARENA
- Activity orientated REhabilitation following kNee Arthroplasty
- BCI
- bootstrapped confidence interval
- BMI
- body mass index
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CIRRIE
- Center for International Rehabilitation Research Information and Exchange
- CONSORT
- Consolidated Standards of Reporting Trials
- CSRI
- Client Service Receipt Inventory
- DIRUM
- Data Instruments for Resource Use Measurement
- EQ-5D
- European Quality of Life-5 Dimensions
- EQ-5D-3L
- European Quality of Life-5 Dimensions 3-Level version
- ERIC
- Education Resources Information Center
- FNB
- femoral nerve block
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HADS-A
- Hospital Anxiety and Depression Scale – anxiety subscale
- HADS-D
- Hospital Anxiety and Depression Scale – depression subscale
- HHS
- Harris Hip Score
- HOOS
- Hip Disability and Osteoarthritis Outcome Score
- HRQoL
- health-related quality of life
- HSS
- Hospital for Special Surgery Knee Score
- i.v.
- intravenous
- IASP
- International Association for the Study of Pain
- ICD
- International Classification of Diseases
- ICECAP
- ICEpop CAPability measure
- ICECAP-O
- ICEpop CAPability measure for Older people
- ICF
- International Classification of Functioning, Disability and Health
- ICOAP
- Measure of Intermittent and Constant Osteoarthritis Pain
- ILAS
- Iowa Level of Assistance Scale
- IMMPACT
- Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials
- INMB
- incremental net monetary benefit
- IPA
- interpretative phenomenological analysis
- IQR
- interquartile range
- ITT
- intention to treat
- ITT-CC
- intention-to-treat complete cases
- ITT-imputed
- intention-to-treat imputed
- KOOS
- Knee Injury and Osteoarthritis Outcome Score
- LEFS
- Lower Extremity Functional Scale
- MCII
- minimum clinical important improvement
- MD
- mean difference
- MLM
- multivariate linear mixed
- MOOSE
- Meta-analysis of Observational Studies in Epidemiology
- MRC
- Medical Research Council
- MRU
- Musculoskeletal Research Unit
- MYMOP2
- Measure Yourself Medical Outcome Profile 2
- NEADL
- Nottingham Extended Activities of Daily Living
- NHP
- Nottingham Health Profile
- NICE
- National Institute for Health and Care Excellence
- OHS
- Oxford Hip Score
- OKS
- Oxford Knee Score
- OLS
- ordinary least squares
- OR
- odds ratio
- OT
- occupational therapist
- PCA
- patient-controlled analgesia
- PCS
- physical component score
- PEDro
- Physiotherapy Evidence Database
- PEP-R
- Patient Experience Partnership in Research
- PI
- principal investigator
- PICOS
- participants, interventions, comparisons, outcomes, and study design
- PP
- per protocol complete cases
- PPI
- patient and public involvement
- PPT
- pressure pain threshold
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROM
- patient-reported outcome measure
- PROOF-THR
- Pilot Randomised controlled trial Of Occupational therapy For–Total Hip Replacement
- PROSPECT
- PROcedure SPECific postoperative pain managemenT
- PSS
- Personal Social Services
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- QST
- quantitative sensory testing
- RCT
- randomised controlled trial
- RESTORE
- REsearch STudies into the ORthopaedic Experience
- ROM
- range of motion
- SD
- standard deviation
- SEM
- structural equation modelling
- SF-12
- Short Form questionnaire-12 items
- SF-36
- Short Form questionnaire-36 items
- SMD
- standardised mean difference
- SPIRAL
- Self-managing Pain In aRthritis and ArthropLasty
- STAI
- State–Trait Anxiety Inventory
- THR
- total hip replacement
- TKR
- total knee replacement
- UCLA
- University of California at Los Angeles
- ULM
- univariable linear mixed
- VAS
- visual analogue scale
- WHO
- World Health Organization
- WOMAC
- Western Ontario and McMaster Universities Arthritis Index