Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0407-10177. The contractual start date was in September 2008. The final report began editorial review in April 2014 and was accepted for publication in April 2016. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Hywel Williams reports membership of National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Commissioning Board and NIHR Journals Library Board. Hywel Williams is Deputy Director of the NIHR programme and chairperson of the HTA Commissioning Board. On 1 January 2016 he became Programme Director for the HTA programme. Sandra Lawton reports personal fees from Genus Pharmaceuticals and Almirall Pharmaceuticals outside the submitted work. Jo Leonardi-Bee reports grants from F. Hoffmann-La Roche outside the submitted work. Anthony D Ormerod reports personal fees from Amgen, grants from Abbvie, Jansen, Merck, Novartis and Pfizer and non-financial support from Jansen outside the submitted work. Jochen Schmitt reports personal fees from AbbVie and Novartis and grants from Novartis, Pfizer, Merck Sharp & Dohme Ltd and ALK-Abelló outside the submitted work. Eric Simpson reports grants from National Institutes of Health K23 (National Institutes of Health Patient-Oriented Research Career Development Award) during the conduct of the study and outside the submitted work. James Mason has been involved as a university-employed health economist on several other funded dermatological studies, but none presents a conflict with the content or findings presented in this report. Lester Firkins reports membership of NIHR Journals Library Board, James Lind Alliance and patient and public involvement reference group. Sally Crowe reports personal fees from the University of Nottingham during the conduct of the study. Nicholas Evans reports being a trustee of the Psoriasis Association. John Norrie reports grants from University of Aberdeen and from University of Glasgow during the conduct of the study, and is a member of the NIHR Journals Library Editorial Group.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2016. This work was produced by Thomas et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Navigating this report
This report is a broad programme of related activities looking at the prevention and treatment of skin disease. It covers five distinct topic areas (Table a and Figure a).
Key milestones for each work programme are outlined, along with a summary of additional activities that were incorporated into the programme of activities during the funding period.
All amendments and additions to the programme award were approved by NIHR prior to implementation.
| Key outputs | Included in original plan | Amendments/additions to original plan |
|---|---|---|
| Eczema prevention work programme (see Chapter 1) | ||
| Overview of systematic reviews | ✓ | None |
| Defining a new case of eczema | Additional | Additional systematic review conducted to establish how to capture the primary outcome of eczema incidence in the BEEP trial |
| Pilot work | ✓ | Follow-up extended from 6 months to 2 years and additional mechanistic and patient preference work included |
| Proposal for a RCT to determine whether or not emollients can prevent eczema development | ✓ | The BEEP trial funded by NIHR HTA |
| Patient resources/guidelines updated | ✓ | Information used to inform existing guidelines and patient information resources rather than creating new ones |
| Eczema treatment work programme (see Chapter 2) | ||
| Scoping systematic review of eczema treatments | ✓ | Published as separate NIHR report |
| The GREAT database | ✓ | Expanded to include RCTs published prior to 2000 and systematic reviews published since 2000 |
| Validation of the GREAT database | Additional | Validation study to establish the sensitivity and specificity of the GREAT database in identifying all published RCT evidence |
| Systematic review of selective outcome reporting in eczema trials | Additional | Spin-off methodological paper demonstrating the utility of the GREAT database for informing methodological projects |
| HOME initiative | Additional | International consensus initiative to establish a core outcome set for future eczema trials |
| Eczema PSP | ✓ | None |
| CLOTHing for the relief of Eczema Symptoms – a study proposal | ✓ | CLOTHES trial funded by NIHR HTA |
| Scoping systematic review of eczema treatments | ✓ | Information used to inform existing guidelines and patient information resources rather than creating new ones |
| Vitiligo work programme (see Chapter 3) | ||
| Update of Cochrane systematic review | ✓ | Updated in 2010 and 2014 |
| Vitiligo PSP | ✓ | None |
| Systematic review of outcome measures | Additional | Systematic review of outcome measures used in published vitiligo RCTs and online survey to establish what is important for patients and clinicians |
| Gaining consensus over outcomes for use in future vitiligo trials | Additional | An international activity designed to established core outcome domains to be included in future vitiligo trials |
| Validation of patient-reported treatment response | Additional | Patient survey and online discussion groups to establish face validity of patient-report definition of treatment success |
| Pilot RCT of light-therapy devices used at home | ✓ | None |
| Survey of UK clinical practice | ✓ | None |
| Proposal for a trial light therapy and topical corticosteroids for the treatment of vitiligo (HI-Light) | ✓ | HI-Light trial funded by NIHR HTA as a result of prioritisation setting partnership |
| Patient resources/guidelines updated | ✓ | Information used to inform existing guidelines and patient information resources rather than creating new ones |
| Squamous cell skin cancer work programme (see Chapter 4) | ||
| Systematic review of SCC treatments: RCTs | ✓ | None |
| Systematic review of SCC treatments: observational studies | Additional | Additional review of observational studies added to the programme since the Cochrane review of RCTs found just one RCT on the topic |
| Identification of potential research topics and development of a clinical trial scenario | Additional | Survey of dermatologists, plastic surgeons, radiologists and other clinicians involved in SCC management to establish the most important treatment uncertainties to be addressed by research |
| An analysis of SCC treated over a 1-year period | ✓ | None |
| Qualitative feasibility study | ✓ | None |
| Treatment uncertainties for high risk SCC – a trial proposal | ✓ | None |
| Patient resources/guidelines updated (decision aids removed from planned activities) | ✓ | Information used to inform existing guidelines and patient information resources rather than creating new ones |
| Pyoderma gangrenosum work programme (see Chapter 5) | ||
| A RCT of prednisolone vs. ciclosporin in the treatment of PG: STOP GAP trial | ✓ | None |
| Treatment response of patients receiving topical treatments for PG: a prospective cohort observational study | ✓ | None |
| Capacity development | ||
| Dermatology patient panel established and trained | ✓ | None |
| Industry liaison project | ✓ | Removed from programme as this aspect is now provided by NIHR Clinical Research Networks |
| Three researchers trained to PhD level | ✓ | |
| Training fellowships for Specialist Registrars and Dermatology nurses supported by UKDCTN | ✓ | Additional fellowships developed for staff and associate specialist and GPs |
FIGURE a.
Gantt chart showing delivery of the work packages. Time range: September 2008 to March 2014. a, Awarded funding for proposed RCT. CLOTHES, CLOTHing for the relief of Eczema Symptoms.
Chapter 1 Eczema prevention work programme
Abstract
Introduction
Although eczema is a very common condition and its prevalence is on the increase, its prevention has received little attention. We sought to pull together various strands of best evidence to provide the building blocks to inform the design of primary research in this area.
Methods
The approaches used were as follows.
-
An overview of systematic reviews of interventions for primary prevention of eczema.
-
A systematic review of how new cases of eczema have been defined in previous primary prevention studies.
-
A pilot randomised controlled trial (RCT) to test the feasibility of conducting a trial of emollients to prevent eczema.
-
A parent survey to determine emollient preferences, together with biophysical studies, to make sure that the chosen emollients improve barrier function.
-
An application for a full-scale RCT of emollients for eczema prevention.
Results
-
Our overview of seven systematic reviews (comprising 39 RCTs and 11,897 participants) failed to find clear evidence that any of the commonly perceived strategies for eczema prevention work.
-
The review of how new cases of eczema are defined in trials found that many definitions rely on the presence of signs or symptoms over a long period of time (up to 1 year), which is not appropriate for defining new cases of eczema.
-
Our pilot RCT included 124 families and confirmed that recruitment and retention was feasible and that contamination was minimal.
-
Our surveys and biophysical studies identified two emollients as being acceptable to parents and effective in skin barrier protection.
Conclusion
This work has provided the key building blocks for justifying and refining the design of a new approach to eczema prevention by enhancing the skin barrier from birth. Our application to the National Institute Health Research (NIHR) Health Technology Assessment (HTA) programme for a full-scale trial has been successful. 1
Content
| Details | Comment |
|---|---|
| Introduction | Briefly summarises eczema epidemiology and pathology |
| Overview of systematic reviews | An analysis of all the studies which have tried different methods to prevent eczema developing |
| Defining a new case of eczema: a systematic review | A review of how previous studies have diagnosed new cases of eczema in infants given that guidelines usually require signs or symptoms to be present for long time periods |
| Pilot work for a randomised controlled trial to determine whether or not emollients can prevent eczema development (BEEP) | A trial to assess how feasible a study examining skin barrier enhancement (using an emollient) would be to perform and how best to perform it |
| Proposal for a randomised controlled trial to determine whether emollients can prevent eczema development | A proposal for a large scale trial assessing whether emollients can prevent eczema development. A funding application to the NIHR HTA funding stream |
| Patient and public involvement | A summary of the contributions made by patients and how this influenced the work |
| Summary and conclusions |
Introduction
Terminology
The World Allergy Organization (WAO) suggests that the phenotype of atopic eczema (AE) should be referred to as ‘eczema’ unless specific immunoglobulin E (IgE) antibodies are demonstrated. Furthermore, the terms ‘eczema’ and ‘dermatitis’ are synonymous, thus ‘eczema’ is typically used in this report.
Characteristics and burden of eczema
Eczema2 is a very common skin problem affecting 16–30% of children in the UK and around 20% of children worldwide. 3,4 Global surveys have shown that eczema is on the increase but it is not clear why. 5 Eczema usually starts in infancy and around 40% of cases persist into adulthood, especially those with early and widespread disease. 6 Although all skin areas can be affected, eczema often starts on the cheeks and limbs and then settles in the skin creases. Reliable diagnostic criteria have been developed that emphasise flexural involvement, early onset and dry skin. 7 Constant scratching results in skin damage and causes a vicious itch–scratch cycle. Scratching can result in bleeding, secondary bacterial infection and sleep loss to the child and family. Damage from scratching may lead to autoreactivity developing against skin components, which can lead to disease chronicity. 8 Eczema is also associated with attention deficit hyperactivity disorder, perhaps as a consequence of severe disease in early life. 9 The stigma associated with a visible skin disease adversely affects the quality of life (QoL) of the child and family, yet it is often trivialised as ‘only a skin disease’. The family impact of caring for a child with moderate or severe eczema is greater than that caring for children with type 1 diabetes mellitus, mainly owing to sleep deprivation, employment loss, time to care for eczema and financial costs. 10 In the World Health Organization 2010 Global Burden of Disease survey,11 eczema was the most common reason for disability-adjusted life-years. 11 Eczema results in a high economic burden,12 with overall costs comparable to asthma. 13 Families often incur additional costs for special clothing and creams. 10,14 A systematic review of 59 studies estimated that direct costs of eczema treatment in the USA could be as high as $3.8B per year. 14 Eczema is a chronic condition accounting for the highest number of new general practitioner (GP) consultations in England for a skin complaint. 15 Moderate to severe eczema often requires referral to secondary care. Guidelines for children with eczema were produced by the National Institute for Health and Care Excellence (NICE) in 2007. 16
Relationship of eczema to other allergic diseases
Children with eczema, especially severe eczema, are at increased risk of also developing other allergic IgE-mediated diseases including food allergies, allergic asthma and allergic rhinitis (hay fever). Together, these are the most common chronic diseases of childhood and represent a major financial burden to the NHS, with direct costs estimated at over £1B per annum in 2004. 17 Eczema is often the first manifestation of the so-called ‘atopic march’, in which a child progresses from eczema to asthma and allergic rhinitis later in life (Figure 1). 18,19 Eczema is strongly associated with peanut allergy and sensitisation to other foods such as milk, eggs, soy, wheat and fish. 20 Population-based cohort studies reveal that around one in three children with eczema go on to develop asthma, especially allergic asthma, in later life. 21 Allergic rhinitis is usually the last of the allergic diseases to appear and is about three times more common in children with eczema in early life. 22 Around half of UK school children with eczema also suffer from allergic rhinitis. 23
FIGURE 1.
Atopic march: allergic diseases tend to develop in a specific order.
Causes of eczema
Eczema is a complex disease caused by the interplay of multiple genetic and environmental factors. The early onset of disease, the rising prevalence and increased incidence of eczema in smaller families,24 as well as in those from a higher socioeconomic background and in those migrating to Western countries from countries with a low prevalence of eczema, suggest that environmental factors operating early in life play a critical role in determining disease expression. 5,25 The ‘hygiene hypothesis’ has been proposed to explain why allergic diseases are more prevalent in developed societies. This hypothesis suggests that a lack of stimulation of the developing immune system by microbes prevents its full maturation. However, experimental evidence for this hypothesis is still conflicting. 26,27 Environmental risk factor studies have also shown conflicting results and have not, to date, led to useful preventative strategies. 28–31 Eczema is highly heritable and shows strong familial clustering. 32
Although genetically determined variation in cutaneous and systemic inflammation are important in eczema predisposition,33 common mutations in the gene encoding filaggrin, a key skin barrier protein, represent the strongest known genetic risk factor for eczema. 34,35 A meta-analysis of 24 studies demonstrated that loss-of-function mutations in the filaggrin gene are found in approximately 9% of the white European population; these individuals have a measurable reduction in their skin barrier function and a striking threefold increased risk of AE. 36
Overview of systematic reviews
The following text is adapted with permission from John Wiley & Sons Ltd from Foisy M, Boyle RJ, Chalmers JR, Simpson EL, Williams HC. Overview of reviews. The prevention of eczema in infants and children: an overview of Cochrane and non-Cochrane reviews. Evid Based Child Health 2011;6:1322–39. 37
Summary
What was already known about this topic?
-
Many published studies have evaluated different interventions aimed at preventing eczema. Often, these have been incorporated into systematic reviews on the topic.
-
However, there is no single source of information that compares all of the different interventions; thus, it is difficult to make comparisons and clinical recommendations.
What did this study add?
-
This overview of systematic reviews presented the available evidence from Cochrane and non-Cochrane reviews on seven interventions for preventing eczema.
-
There was no clear evidence that any of the interventions reviewed can reduce the incidence of eczema.
-
In a subgroup of high-risk infants, there was a significant reduction in the incidence of eczema in those given prebiotics compared with no prebiotics.
-
A similar reduction in eczema incidence was seen in high-risk infants who were exclusively breastfed for 6 months compared with those given non-breast milk liquids and/or solid food at 3–6 months, but this effect was not sustained past the age of 2 years.
-
There were very few published data on the prevention of truly AE, that is, eczema associated with IgE sensitisation; data on probiotics versus no probiotics showed no significant difference.
-
Adverse event data were available for infants only. The only adverse event to emerge from the review was that more infants receiving probiotics were spitting up (reflux/regurgitation) at 1 and 2 months of age than those not receiving probiotics.
-
We were not able compare breastfeeding versus infant formula alone or the avoidance of pets and other aeroallergens from birth owing to an absence of published systematic reviews dealing with these topics that met the inclusion criteria.
Introduction
Successful prevention of eczema may be possible by either modifying environmental risk factor(s) or compensating for genetic variations that lead to eczema development. Many systematic reviews have been published that have assessed interventions for eczema prevention but, more often than not, these examine prevention of all allergies including asthma and seasonal allergic rhinitis. Although some systematic reviews have dealt with eczema alone, these tend to cover just one intervention, making it difficult to draw any comparative data or conclusions across all of the commonly tried prevention strategies. The purpose of this overview is to present the most up-to-date evidence from Cochrane and non-Cochrane reviews on pharmacological and non-pharmacological interventions for preventing eczema in infants and children in order to ensure that any attempts at devising new preventative strategies build on previous work rather than inadvertently duplicating it. 38
Several interventions to prevent eczema have been proposed and tested, primarily based on allergen avoidance, but despite this there are no clear, evidence-based guidelines for eczema prevention. 39 To date, interventions to modify the infant’s exposure to dietary antigens pre- or postnatally have been the major focus of eczema prevention research on the basis that these might prevent food sensitisation and, thereby, promote intestinal and skin health. Such interventions include the promotion of exclusive breastfeeding for a defined period of time, maternal dietary antigen avoidance and the use of hydrolysed protein formula (in which the allergenic properties of cow’s milk are reduced by breaking down the proteins in the milk) or soy formula for those infants who are not exclusively breastfed.
More recent studies have attempted to capitalise on the theoretical link between eczema and intestinal health, such as differences in intestinal microbiota composition and possible altered intestinal permeability and inflammatory markers in people with eczema. 40,41 The presence of an altered intestinal milieu in infants at risk for developing eczema has been shown in many, but not all, studies, and is therefore an area of ongoing controversy and research. 42,43 Dietary interventions such as prebiotic and probiotic supplementation may modify early intestinal microbiota development. 41,44 Some studies have also demonstrated that maternal supplementation with omega-3 polyunsaturated fatty acids during pregnancy may decrease the risk of infant eczema. 45
Other approaches for eczema prevention have focussed on T helper cell type 1 (Th1) or regulatory T cell type immune responses, which have been found to be altered in eczema. 46 The close association between eczema and allergic sensitisation has also led some investigators to test the effects of avoiding specific aeroallergens such as pet allergens and house dust mites.
Promotion of skin barrier function by use of emollients in early life is another approach that has been suggested,47 based on the observations that skin barrier dysfunction may play a role in initiating eczema. In 2006, Palmer et al. 34 showed an association between genetic mutations in the gene encoding the skin barrier protein filaggrin and the development of eczema, a finding that has now been replicated in > 20 studies. 36 Several lines of evidence support a skin barrier approach to eczema prevention including data showing that barrier dysfunction precedes presentation of eczema in infants with a filaggrin gene mutation48 and an early case–control study found an association between use of petroleum ointment and the development of eczema. 49 It is also possible that protecting the barrier from birth may reduce IgE sensitisation and the subsequent development of other allergic disease. 50
Methods
Systematic reviews were identified using the 2010 UK NHS Evidence Skin Disorders Annual Evidence Updates Mapping Exercise on Atopic Eczema, which had previously identified all systematic reviews on eczema prevention published between January 2000 and August 2010.
To ensure trials were only counted once, only one review on each topic was included. The criteria for inclusion were:
-
data on participants between 0 and 18 years of age available separately from any adult data presented
-
search date for the review had been conducted no more than 5 years ago
-
contained RCTs only (with the exception of breastfeeding and pet avoidance because RCTs are not ethical)
-
contained data to enable calculation of a risk ratio (RR) and confidence interval (CI).
If a Cochrane review was published that met the inclusion criteria, this was automatically selected. If no Cochrane review was published for an intervention, then the most up-to-date non-Cochrane review that met the inclusion criteria was included instead.
Two reviewers (MF, JRC) independently assessed the eligibility of each potential review based on the inclusion criteria listed above. Both reviewers agreed on the final set of included reviews.
Two reviewers (ELS, JRC) independently assessed the methodological quality of each included review using the Assessing the Methodological Quality of Systematic Reviews (AMSTAR) tool. 51 Both reviewers evaluated whether or not each review satisfied the eleven different criteria of the AMSTAR tool. In addition to recording which AMSTAR criteria were fulfilled, the reviewers also made a subjective assessment of overall quality of each review by assigning an overall rating of ‘good’, ‘fair’ or ‘poor’ based on their global evaluation of the review and informed by the AMSTAR criteria. Discrepancies between reviewers were resolved by a third reviewer (MF).
The primary outcome measures were pre-specified as:52
-
development of eczema (clinical phenotype)
-
development of AE (IgE sensitisation).
Secondary outcomes were pre-specified as:
-
any atopy/IgE sensitisation
-
eczema severity
-
time to development of eczema
-
QoL
-
health-care utilisation
-
adverse events.
The main analysis included all ages and all risk levels of developing eczema. Two subgroup analyses were also pre-specified:
-
infants (≤ 2 years of age) and children (> 2 to 18 years of age)
-
high-risk participants (family history of allergic disease) and participants not selected for risk.
One reviewer (MF) extracted the following information from each of the included reviews: inclusion criteria (including population, intervention, comparisons and outcomes), methodological quality of included trials and numeric results. Numeric results were extracted for aggregate data (all ages and risk levels combined) as well as for our pre-specified subgroups examining different ages and risk levels. When trials or reviews documented outcomes for the same patients at both infancy and childhood, we included only the childhood data in the aggregate data. Numeric results were extracted from the published reviews, and RevMan5 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) was used for all statistical analyses. A second reviewer (JRC) independently verified accuracy of numeric results and discrepancies were resolved through discussion.
All data contained in the included reviews were dichotomous; therefore, all data in this overview were summarised using RRs with 95% CIs. RRs describe the probability of the event in the treatment group compared with the probability of the event in the control group, and are interpreted as statistically significant if the 95% CIs do not touch unity. A random-effects model was used for all outcomes in order to provide the most conservative estimate.
For all pooled effect estimates, the accompanying I2-values were reported and represent the degree of statistical heterogeneity between the trials. An I2-value close to 0% indicates minimal or no heterogeneity of trials, whereas an I2-value of ≥ 50% indicates substantial heterogeneity. 53
Results
Results of the search
Seven systematic reviews were included, containing 39 relevant trials (11,897 participants) (see Appendix 1). Six were Cochrane reviews covering the following interventions for preventing eczema: maternal dietary antigen avoidance,54 optimal duration of exclusive breastfeeding,55 hydrolysed protein formula,56 prebiotics,57 probiotics58 and soy formula. 59 One non-Cochrane review on omega-3 and -6 fatty acid supplementation was also included. 60 The included Cochrane review on optimal duration of exclusive breastfeeding compared prolonged exclusive breastfeeding with the introduction of complementary solid or liquid foods following a short period of exclusive breastfeeding. It did not compare breastfeeding with infant formula alone. Therefore, we sought to include a review on this topic, but none met our inclusion criteria. Similarly, none of the non-Cochrane reviews examining the avoidance of pets or other aeroallergens satisfied the inclusion criteria, so we were unable to assess these interventions.
Eczema
There was no strong evidence that any of the seven interventions reviewed can prevent the development of eczema (Table 1). However, there was weak evidence that some of the interventions may reduce the risk of developing eczema in some subgroups.
| Comparison | Participants (trials) | RR (95% CI) | I2 (%) |
|---|---|---|---|
| Exclusive breastfeeding for at least 6 months vs. introduction of solids at 3–6 months | 3731 (2) | 0.75 (0.42 to 1.32) | 61 |
| Hydrolysed formula vs. cow’s milk formula (prolonged feeding) | 1478 (8) | 0.87 (0.70 to 1.08) | 0 |
| Extensively hydrolysed formula vs. cow’s milk formula (prolonged feeding) | 912 (3) | 0.84 (0.58 to 1.23) | 19 |
| Partially hydrolysed formula vs. cow’s milk formula (prolonged feeding) | 823 (7) | 0.92 (0.72 to 1.17) | 0 |
| Extensively hydrolysed formula vs. partially hydrolysed formula (prolonged feeding) | 1061 (4) | 0.88 (0.73 to 1.05) | 0 |
| Hydrolysed formula vs. human milk (early short-term feeding) | 09 (1) | 0.48 (0.05 to 4.41) | – |
| Hydrolysed formula vs. cow’s milk formula (early short-term feeding) | 77 (1) | 0.34 (0.04 to 3.15) | – |
| Soy formula vs. cow’s milk formula | 744 (3) | 1.23 (0.99 to 1.53) | 0 |
| Maternal antigen avoidance vs. standard diet | 360 (3) | 0.95 (0.63 to 1.44) | 21 |
| Omega-3 fatty acid supplementation vs. placebo | 664 (3) | 1.10 (0.78 to 1.54) | 45 |
| Omega-6 fatty acid supplementation vs. placebo | 259 (2) | 0.80 (0.56 to 1.16) | 0 |
| Prebiotic vs. no prebiotic | 432 (2) | 0.79 (0.21 to 2.94) | 80 |
| Prebiotic vs. other prebiotica | 150 (1) | 0.22 (0.07 to 0.76)b | – |
| Probiotic vs. no probioticc | 1492 (6) | 0.85 (0.66 to 1.08) | 46 |
Exclusive breastfeeding for at least 6 months compared with introduction of non-breast milk liquids and/or solid food at 3–6 months did not significantly decrease the overall incidence of eczema. However, in a subgroup analysis of high-risk infants, exclusive breastfeeding for 6 months significantly decreased the risk of developing eczema by 60% (RR 0.40, 95% CI 0.21 to 0.78), an effect that was not significant beyond 2 years of age or in infants not selected for risk of developing allergic disease.
When prebiotics were compared with no prebiotics in infants not selected for risk, there was no overall effect on eczema prevention. However, in high-risk infants, use of prebiotics was found to significantly decrease risk of developing eczema by 58% (RR 0.42, 95% CI 0.21 to 0.84). One trial61 comparing different types of prebiotics found that a combination of polydextrose, galacto-oligosaccharide and lactulose compared with polydextrose and galacto-oligosaccharide alone significantly decreased the incidence of eczema by 78% in infants not selected for risk of developing allergic disease (RR 0.22, 95% CI 0.07 to 0.76).
Compared with cow’s milk formula, there was no clear benefit of prolonged feeding of hydrolysed milk formula (all types) or subgroup analyses of partially or extensively hydrolysed milk formula. Prolonged feeding with extensively versus partially hydrolysed milk formula showed no significant difference. Early short-term feeding of hydrolysed milk formula (all types) showed no benefit over human milk or cow’s milk formula. Soy milk formula versus cow’s milk formula also showed no significant benefit. Maternal antigen avoidance was no better than standard diet, omega-3 or -6 fatty acid supplementation was no better than placebo, and there was also no effect of probiotics compared with no probiotics. None of the above subgroup analyses was significant based on either age or risk level.
Atopic eczema
Although we pre-specified AE associated with IgE sensitisation as one of our two primary outcomes, only one comparison – probiotics versus no probiotics – provided data examining this outcome. There was no significant difference in the overall incidence of AE, nor in subgroup analyses of high-risk infants and infants not selected for risk.
Atopy
Data were available for three different comparisons that measured atopic sensitisation using skin prick tests to various allergens: exclusive breastfeeding for at least 6 months versus introduction of solids at 3–6 months, maternal dietary antigen avoidance versus standard diet and omega-3 fatty acid supplementation versus placebo. No significant differences were identified.
Adverse events
The only data available on adverse events were from infants, based on nine different comparisons of interventions. The only significant difference identified in adverse events was in the comparison of probiotics versus no probiotics. Parents reported that significantly more infants receiving probiotics were spitting up (reflux/regurgitation) at 1 and 2 months of age (RR 1.88, 95% CI 1.03 to 3.45, and RR 1.69, 95% CI 1.02 to 2.80); however, premature infants receiving probiotics experienced a 65% reduction in necrotising enterocolitis and/or death (RR 0.35, 95% CI 0.15 to 0.83).
Discussion
Quality of the evidence
Significant numbers of trials and participants have been included in this overview, allowing reasonably robust conclusions to be drawn about the effectiveness of different interventions. A particular problem in some included reviews was the heterogeneity in the nature of the interventions (e.g. partially vs. extensively hydrolysed formula, and whey vs. casein formula) and population (e.g. early, late and prolonged formula feeding; exclusively breastfed, mixed breastfed or exclusively formula-fed; and high or normal risk for allergic disease) which makes it difficult to perform meta-analyses in such populations. Trials of prebiotic and probiotic interventions also suffered from large heterogeneity in the interventions and it should be noted that several probiotic trials have been published since the last systematic review on this subject,62–70 warranting an updated review.
The quality of the systematic reviews included in this overview was high: six reviews were Cochrane reviews and all seven reviews in this overview addressed most of the AMSTAR51 systematic review quality criteria and received overall ratings of ‘good’ (all included reviews are detailed in Appendix 1).
Overall completeness and applicability of evidence
Many of the interventions of interest that we identified prior to conducting this overview were captured in Cochrane and non-Cochrane systematic reviews. Comparisons examining exclusive breastfeeding for at least 6 months, hydrolysed formula, omega-3 fatty acids and probiotics contained significant numbers of trials and participants and were thus powered to detect important treatment effects. However, analyses of soy formula, maternal antigen avoidance and prebiotics included smaller numbers of participants and studies, so may have missed important treatment effects on the incidence of eczema.
Other areas for which more systematic reviews and trials are needed are interventions to promote normal immune development and interventions to correct skin barrier dysfunction. The latter is particularly important in view of the growing body of evidence for abnormalities of skin barrier function in eczema. Common loss-of-function mutations in the gene encoding the epidermal barrier protein filaggrin are a major predisposing factor for eczema34 and skin barrier dysfunction can be detected prior to eczema development. 48
There is a need for more complete reporting of adverse events within systematic reviews of interventions for eczema prevention. In addition, greater numbers of trials must report the effects of these interventions on eczema prevalence beyond infancy, particularly in normal-risk infants.
Limitations
This overview was undertaken using a consensus-based process informed by The Cochrane Collaboration expertise in preventing bias and provides a thorough review of several interventions for eczema prevention. A particular strength of the overview was collaboration with the authors of the 2010 NHS Evidence Skin Disorders Annual Evidence Update on Atopic Eczema, which ensured a thorough literature search and access to all published systematic reviews of interventions for preventing eczema. All analyses were defined a priori, such as the inclusion of reviews of non-randomised studies for two interventions (breastfeeding and pet avoidance) where RCTs are ethically difficult to perform.
The strength of some conclusions was limited by small numbers of trials or participants included in the relevant reviews and the analysis of probiotic effects was limited by the recent publication of many relevant trials62–70 that have not yet been incorporated into a Cochrane systematic review. A more recent meta-analysis incorporating many of these trials71 found that probiotic supplementation significantly reduces risk of developing eczema in infancy (RR 0.79, 95% CI 0.67 to 0.92), a finding supported by subsequent reviews. 72
It was not possible to extract data from the published systematic reviews on breastfeeding versus no breastfeeding and pet exposure in a form that could be incorporated in this overview. The systematic review of breastfeeding versus no breastfeeding73 did find a significant protective effect against eczema [odds ratio (OR) 0.70, 95% CI 0.50 to 0.99]; however, when one controversial trial was excluded from the analysis, this comparison became non-significant (OR 0.84, 95% CI 0.64 to 1.09). The review on pet exposure74 found that, in longitudinal studies, exposure to cats (OR 0.76, 95% CI 0.62 to 0.92), dogs (OR 0.68, 95% CI 0.53 to 0.87), or ‘any furry pets’ (OR 0.79, 95% CI 0.74 to 0.84) was associated with reduced risk of developing eczema. However, one trial noted that when adjustments were made for avoidance behaviour in participants allergic to pets, the protective effect of cats became non-significant.
We identified only one review which reported specifically on ‘atopic eczema’ as defined by the WAO recommendations as an outcome rather than ‘all eczema’. 2 Additionally, although we pre-specified AE associated with IgE sensitisation as one of our two primary outcomes, only one comparison – probiotics versus no probiotics – provided data examining this outcome. There was no significant difference in the overall incidence of AE, nor in subgroup analyses of high-risk infants and infants not selected for risk.
It is worth noting that very few of the systematic reviews evaluated atopy/allergic sensitisation as an outcome, despite it being reported in many of the included RCTs. Although this may be justified because atopy/allergic sensitisation is not a widely recognised disease entity, the primary prevention of atopy/allergic sensitisation may be associated with prevention of later onset allergic disease and is therefore worth including in future systematic reviews in this area. In addition, no data were identified for four of our pre-specified secondary outcomes: eczema severity, time to development of eczema, QoL and health-care utilisation. All are important parameters to measure in future trials and systematic reviews; for example, there is little gain in preventing eczema incidence if the absolute number of severe cases (which disproportionately account for most health-care costs) remains unchanged.
Agreements and disagreements with other studies or reviews
The results of this overview are broadly consistent with those of other published trials and reviews of RCTs, but are less in agreement with reviews and practice guidelines that include ‘all allergic disease’ or ‘allergy’ as primary outcomes or that include significant numbers of non-randomised trials. For example, a recent German taskforce on allergy prevention recommended exclusive breastfeeding for 4 months or, if not possible, the use of a hydrolysed formula for high-risk infants and general avoidance of pets. 75 These differences might stem from the inclusion of other allergy outcomes and a large number of non-randomised studies in the German taskforce recommendations. A recent RCT of hydrolysed formula for preventing allergic disease in high-risk infants supports our conclusion that further research is needed before recommending this intervention to prevent eczema. 76
Implications for clinical practice
This overview of systematic reviews on eczema prevention has not found any clear evidence that any of the main interventions reviewed can reduce the incidence of eczema. That does not mean to say that some interventions do not work; new, larger, well-conducted trials may indeed show a modest benefit in time. However, the current evidence is simply not strong enough to influence practice recommendations. Some interventions, such as the use of soy instead of cow’s milk, are unlikely to show a clinically useful benefit based on the lower 95% CIs and the pooled effect estimates. It may be considered that some of the interventions are unlikely to do much harm; however, it should be considered that adverse events were poorly reported in many of the included trials.
It is also worth noting that we have not been able to address some of the most commonly asked questions by parents (e.g. whether or not exclusive breastfeeding or owning a pet prevents eczema) owing to lack of appropriate data. This may partially reflect the ethical difficulties of conducting trials in these areas. Furthermore, some interventions such as ‘probiotics’ are simply too heterogeneous to consider as one intervention – some may work but some may be completely ineffective. This overview has found that the possible benefit of some interventions (such as exclusive breastfeeding or prebiotics) may only be present in infants born to families at high risk for allergic disease and that the magnitude of risk reduction is larger than the RRs from unselected populations. Again, caution has to be expressed as these results were based on only one trial each with significant limitations.
Implications for research
These findings indicate that there is little justification for further study of some proposed interventions for eczema prevention, such as omega-3 fatty acid supplementation and use of soy milk instead of cow’s milk. Other interventions, such as hydrolysed formula, prebiotics and probiotics, have shown inconclusive results and are worthy of further study. New interventions designed to enhance skin barrier function, such as intensive use of emollients, water softeners or avoidance of alkaline soaps, should also be explored and a separate overview of reviews examining interventions for preventing asthma and other allergic diseases is warranted.
Defining a new case of eczema: a systematic review
The following text is adapted from J Allergy Clin Immunol, vol. 130, iss. 1, Simpson EL, Keck LE, Chalmers JR, Williams HC, How should an incident case of atopic dermatitis be defined? A systematic review of primary prevention studies; pp. 137–44 (see www.sciencedirect.com/science/article/pii/S0091674912002680),77 copyright 2012 with permission from Elsevier.
Summary
What was already known about this topic?
-
There are many published definitions for the diagnosis of AE. Many require the presence of signs or symptoms over a long period of time as a diagnostic criterion, but this is not appropriate for defining new cases.
-
Interventions that delay rather than prevent the onset of eczema could still have a significant public health impact. Identification of such interventions requires more precise determination of the date of onset of eczema than is offered by cumulative incidence rates.
What did this study add?
-
This study was a systematic review of how incident cases of eczema have been defined in previous primary prevention studies.
-
Many different definitions have been used to define a new case of AE, with the Hanifin and Rajka criteria the most commonly applied. Many trials used a definition that was unique to that particular study.
-
The Hanifin and Rajka criteria include presence of signs or symptoms over a long period of time as one of the major criteria, but only two studies stated how they dealt with this in relation to definition a new cases.
-
We propose a minor modification to the UK Working Party Criteria to define an incident case to reduce the timescale from 1 year to 4 weeks for the presence for the presence of an itchy skin condition.
Introduction
Research into the prevention of eczema has been hampered by inconsistency in study methodology, especially with regard to methods for defining high-risk populations and disease outcomes. 78–80 In addition, although there are well-established validated definitions for diagnosing established eczema, there are no standardised definitions for defining an incident case, as is needed for longitudinal birth cohort studies or interventional prevention studies.
The problem is not easy to resolve as many definitions of eczema include having signs or symptoms over a long period of time as a diagnostic criterion, which is clearly unsuitable for defining an incident case. Although some prevention studies measure cumulative incidence rates at 1 or 2 years, a more precise determination of the date of onset of eczema is especially important when evaluating prevention strategies that may only delay the onset of disease. Identifying strategies that delay, rather than prevent, the disease onset would still have a significant public health impact, given the high prevalence of eczema and the finding that the earlier onset disease predicts a more severe disease course. 81 We sought to conduct a systematic review of how new cases of eczema have been defined in previous primary prevention studies of eczema, using systematic review methodology.
Methods
Included studies
Prospective, interventional, prevention trials published after 1980, which specified eczema as an outcome, were eligible. Studies whose primary outcome was not eczema, such as asthma prevention trials, but had eczema as a secondary outcome, were included. There were no age or language restrictions. Observational cohort studies were excluded as these studies most often use cumulative incidence as an outcome. Current validated definitions for the diagnosis of eczema are suitable for the measurement of cumulative incidence with a maximum precision of 1 year. We aimed to investigate incident case definitions that would capture cases as they occur in a primary prevention trial, enabling a time of disease onset to be determined.
Information sources
Studies were identified by searching electronic databases, scanning reference lists of articles and eczema reviews, and consultation with experts in the field. The search was applied to MEDLINE (1980–2012), Cochrane (1980–2012) and the last database search was run on 5 January 2011 (see Appendix 2). Multiple articles were identified through hand-searching, predominantly of reviews identified in search results, reviewing the literature and from the NHS Evidence mapping exercise of systematic reviews for eczema prevention. 82
Study selection
All database search results were entered into RefWorks (ProQuest LLC, MI, USA) in which duplicates were removed. Screening for eligibility was performed independently in a standardised manner by two reviewers (ELS and LEK) based on titles and abstracts. Discrepancies between reviewers were resolved by consensus. Full-text articles for all studies past screening were obtained. Full-text copies of studies with seemingly eligible titles but without abstracts were automatically included and scrutinised further for eligibility. All full-text articles were assessed for eligibility by one reviewer prior to data collection. Of note, if multiple publications of a larger longitudinal trial were identified, they were counted as only one definition unless the definitions significantly differed between publications, in which case they were kept separate.
Data collection process/items
Data collection was performed by one reviewer using a data extraction database created for the study. Information extracted from each trial included the type of intervention and the eczema definition used. One author (LEK) extracted the above data from the included studies and a second reviewer (ELS) double-checked all verbatim definitions that were considered to lack a true definition of eczema. Disagreements were resolved by discussion between the two reviewers and consultation with a third arbitrating author.
Summary measures
The primary outcome measured was the presence of any specified definition for eczema.
Results
Study selection
The study selection procedure is summarised in the flow chart in Figure 2. A total of 108 articles met the criteria for inclusion. Seven of these were multiple publications from the same longitudinal study, so this definition was counted only once, resulting in 102 distinct studies for inclusion in the analysis.
FIGURE 2.
Study flow chart for showing screening process for screening studies related to defining new cases of eczema. AD, atopic dermatitis.

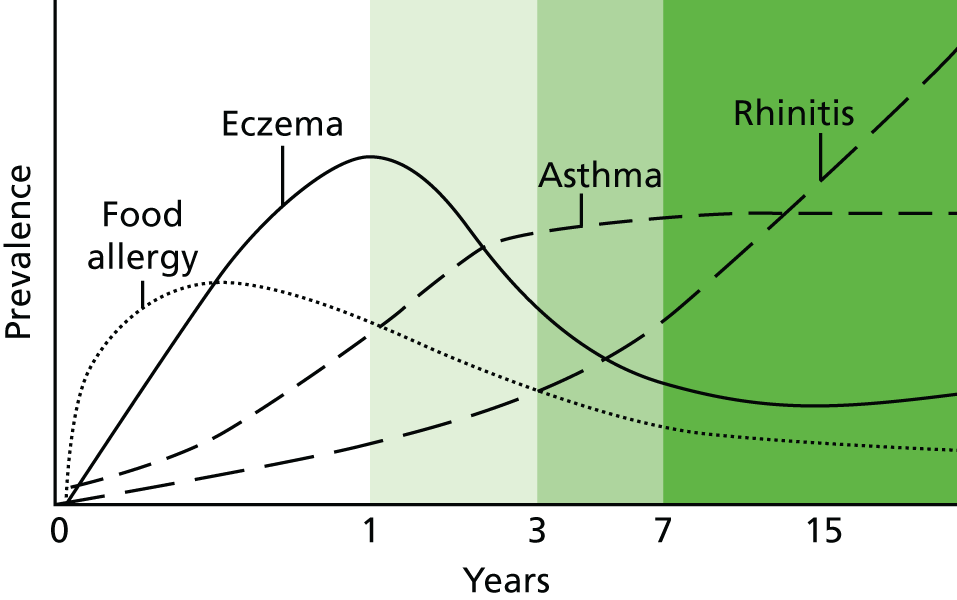
More than 80% of the included 102 studies evaluated a dietary intervention on either the mother or the infant, with infant formula as the most common eczema prevention strategy used. The other interventions were non-dietary allergen avoidance, vaccination and an emollient intervention (Figure 3).
FIGURE 3.
Interventions assessed in the 102 included studies relating to defining new cases of eczema.
Definitions of eczema
Of the 102 studies selected for further analysis of eczema definition, only 75 (73.5%) included some form of a description of the criteria used to diagnose eczema. The other 27 articles mentioned either a diagnosis made by a questionnaire that was unavailable for review (one study), a general morphological description of eczema (four studies), a physician or investigator diagnosis of ‘eczema’ without further elaboration of specified criteria (20 studies), or no description at all (two studies). 77
Of the 75 studies with reported disease criteria, the Hanifin and Rajka criteria were the most commonly used disease criteria (28 studies; Table 2). Of those studies that used the Hanifin and Rajka criteria (which includes chronic or chronically relapsing dermatitis as one of its four major diagnostic criteria), only two studies specified how they dealt with the anomaly of disease chronicity in relation to definition a new case. 84,85 The study by Laitinen et al. 85 required visible eczema to be present for at least 4 weeks at the 6- and 12-month visits and for at least 8 weeks at the 24- and 48-month visits. Arslanoglu et al. 84 required ‘symptoms’ to be present for at least 4 weeks to meet criteria for eczema.
| Name of definition | Studies, n (%) | Definition | Reference number |
|---|---|---|---|
| Hanifin–Rajka | 28 (27) | Must have three or more basic features: pruritus, typical morphology and distribution, chronic or chronically relapsing, personal or family history of atopy plus three minor criteria. See source for full definition83 | 45,47,64,65,67,68,84–106 |
| Unique | 21 (21) | Variable | 54,107–125 |
| ISAAC | 4 (4) | Has your child had this itchy rash at any time in the last 12 months? Has this itchy rash at any time affected any of the following: the folds of the elbows, behind the knees, in front of the ankles, under the buttocks or around the neck, ears or eyes?126 | 127–130 |
| UK Working Party | 7 (7) | Must have an itchy skin condition in the last 12 months. Plus three or more of onset below age 2,a history of flexural involvement, history of generally dry skin, personal history of other atopic disease,b visible flexural dermatitis as per photographic protocol131 | 63,66,69,132–134 |
| Hanifin and Lobitz | 5 (5) | Must have each of the following: pruritus, typical morphology and distribution, tendency towards chronic or chronically relapsing dermatitis. Plus two or more of the following features: personal or family history of atopic disease, immediate skin test reactivity, white dermographism and/or delayed blanch to cholinergic agents, anterior subcapsular cataracts; or four or more features listed in the reference Hanifin and Lobitz135 | 136–140 |
| Seymour | 4 (4) | The criteria used in selecting infants with atopic dermatitis were the presence of at least two major, or one major and one minor, feature from the following lists. Major features included family history of atopic disease (asthma, seasonal rhinitis, or atopic dermatitis), evidence of pruritic dermatitis, typical facial or extensor, eczematous or lichenified dermatitis. Minor features included xerosis/ichthyosis/hyperlinear palms, perifollicular accentuation, chronic scalp scaling, periauricular fissures141 | 70,142–144 |
| Halken | 4 (4) | AE was diagnosed if physical examination revealed areas of scaly, erythematous and itchy eczematous rash, primarily of the face, scalp and flexural folds. Only eczema with at least two locations in typical areas relapsing with a duration of at least 3 months was recorded145 | 145–148 |
| Moore | 2 (2) | Eczematous skin lesions were classified as 0 (normal skin); 1 (dry skin, cradle cap, mild perioral erythema); 2 (some or all of these features with, in addition, an area of skin, usually on the face or behind the ears, that was red, scaly, cracked, or weeping); 3 (as 2 but more extensive lesions, usually on the face, trunk, and limbs). Grades 2 and 3 were regarded as eczema149 | 149,150 |
| None | 27 (26) | 106,151–174 |
Disease definitions cited in eczema prevention studies
A disease definition that was unique to that particular study (21 studies) was the second most commonly used disease definition. These reports did not cite a specific source and none described a scientific method or even more detailed empirical reasoning for justifying the choice of a novel definition of an incident case.
On closer examination of the 21 definitions that were unique to an individual study, most definitions included pruritus, the presence of visible eczema, and disease distribution requirements. Only 50% of these definitions had a time requirement, which ranged from requiring ‘chronic disease’ to 4 weeks of eczema needing to be present (data not shown).
Discussion
Main findings
We found a large degree of variability in the methods used to define an incident case of eczema in relevant prevention studies, with one-quarter of studies failing to report any form of definition whatsoever. Of the studies reporting some form of definition of an incident case, many used a definition that was unique to that particular study, rendering comparisons between studies very difficult. In addition, most studies used eczema incident case definitions without strict time requirements. Combined, these findings demonstrate an urgent need for a standardised, valid and repeatable definition of an incident case of eczema in order to improve the ability to compare outcomes between studies and to allow more informative meta-analyses of prevention studies. Although no previous studies have examined incident case definitions for eczema, our results are consistent with the larger problem of the lack of standardised disease outcome measures in eczema research. For example, Schmitt et al. 175 found 20 different scoring systems for measuring the severity of eczema. Chamlin et al. 78 found 30 different definitions for defining an individual as high risk for developing eczema. In 2010 an international group called the Harmonising Outcome Measures for Eczema (HOME) initiative (see Chapter 2, Harmonising Outcome Measures for Eczema initiative) began the process of creating an accepted core group of outcome measures for eczema research. 176
Problems with existing definitions
The two most commonly used validated criteria found in our review were the Hanifin and Rajka criteria and the UK Working Party refinement of the Hanifin and Rajka criteria. Despite the potential usefulness of these criteria in reliably identifying cases of established eczema, as would be used for determining cumulative incidence over time in cohort studies,177 they were not designed for defining an incident case in prospective prevention studies. By defining an incident case in prevention trials, as opposed to a cumulative incidence, incidence rates can be more accurately calculated and a more accurate date of disease onset can be established. In order to qualify as a case of eczema using the Hanifin and Rajka criteria, at least three out of four of the following major criteria need to be fulfilled:
-
the presence of eczema
-
typical distribution
-
pruritus
-
a relapsing and remitting course.
The definition of ‘relapsing and remitting’ is not further defined and is left to the discretion of the investigator. The UK criteria state that a child must have an itchy skin condition in the past 12 months. These time requirements, although appropriate for diagnosing established cases of eczema, become problematic when diagnosing new onset eczema during the course of a prospective study.
Defining an incident case of eczema is not simply a question of noting the first time an eczematous rash appears in an infant because previous studies have shown that many forms of transient eczematous rashes occur often in infants, even in those children who do not eventually develop true eczema. 178 Thus, there is an urgent need for a standardised definition of an incident case of eczema that offers a satisfactory trade-off between overinclusion of transient eczematous eruptions of irritant and other aetiologies and overexclusion of genuine milder short-lived forms of eczema that still represent a health-care problem.
Proposed solution
Until more sophisticated validation studies can be performed, we suggest a modification of the UK Working Party criteria for eczema, adapted for prospective observational or interventional studies. This modification specifies a time frame that the eczema must be present in order to be considered as a case of eczema and allows for a diagnosis to be made even if the rash is treated early in its course. We propose the following definition, based on empirical reasoning considering the requirements of such a definition, and informed by previous studies that signal the best markers of true eczema. 178
A history of an itchy skin condition, which is either continuous or intermittent, lasting at least 4 weeks, plus three or more of the following:
-
a history of a rash in the skin creases (folds of elbows, behind the knees, fronts of ankles or around the neck), or on the extensor aspects of the forearms or lower legs
-
a personal history of asthma or hay fever or a history of atopic disease in a first-degree relative
-
a history of generally dry skin since birth
-
visible flexural dermatitis and/or visible dermatitis on the forearms or lower legs with absence of axillary involvement as defined by our online photographic protocol. 179,180
Visual confirmation of eczema diagnosis by a clinician, dermatology nurse or a research nurse suitably trained in recognising the symptoms of eczema is recommended. Clearly it is important to add the proviso that any infant fulfilling these criteria but who, on examination by a suitably trained health professional, is deemed to have a different skin disease, will be classified as not having eczema.
There are several benefits of our proposed definition, the strongest being that it is based on a current eczema definition that has undergone extensive scientific development assessing validity, repeatability and applicability. The only change added to the UK Working Party definition is the addition of a specified time requirement of 4 weeks. This time requirement should exclude most transient eczematous rashes that are typically irritant in nature and usually of little medical consequence. The use of an established eczema definition for incident eczema that is derived from one used for prevalent eczema allows for consistency in defining the public health burden of disease when assessed using different study designs. Another benefit of this proposed definition is that it does not allow a definition of eczema to be made based on the presence of facial eczema alone. Halkjaer et al. 178 found that 40% of children with facial eczema do not eventuate into chronic eczema. Finally, this definition allows for early treatment intervention during the course of a prospective study and does not require the disease to be untreated for a full 4 weeks if anti-inflammatory therapy is needed. Therefore, if a child develops significant eczema in the classic locations, it would be unethical to withhold treatment. Treatment can begin immediately if needed and, provided some degree of symptoms last for a 4-week period, a diagnosis of eczema will still be captured using this definition.
Very mild cases of new eczema treated immediately resulting in complete clearance will not be captured by such an approach, although it is debatable how many of these case are true eczema and it is probably wise to treat such new very mild cases with emollients alone until the disease declares itself. This approach is consistent with the recommendation to avoid diagnosing asthma in an infant based on a single episode of wheezing.
Particular strengths of this study include its systematic approach to the review of the literature and extensive searching of reference lists for prevention studies not found on the initial search. The findings are particularly relevant given the renewed interest in eczema prevention research and prevention by the National Eczema Association in the United States. 181
With regard to limitations, it is possible that other unpublished definitions of incident cases exist but these would not be detected by our search. In addition, the proposed eczema definition has not yet undergone extensive validity testing in prospective studies in the field. However, this limitation has to be tempered with the alternative practice to date, which has been to use unsuitable definitions or an array of poorly defined or completely undefined definitions. Our proposed definition is meant to be a starting point and we encourage those undertaking or designing new prospective studies of eczema to include it along with their preferred definitions so that knowledge of its utility and validity can be built up.
Pilot work for a randomised controlled trial to determine whether or not emollients can prevent eczema development (BEEP)
The following text is adapted from Simpson EL, Chalmers JR, Hanifin JM, Thomas KS, Cork MJ, McLean WH, Brown SJ, Chen Z, Chen Y, Williams HC. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. Journal of Allergy and Clinical Immunology 2014;134:818–23. 182 Crown Copyright © 2014 Published by Elsevier Inc. on behalf of the American Academy of Allergy, Asthma & Immunology. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/3.0/).
Summary
What was already known about this topic?
-
Studies showing an association between loss-of-function filaggrin gene mutations and AE have generated interest in the potential for enhancing the skin barrier to prevent the development of eczema.
-
Emollients can reduce the incidence of flares of existing eczema and there is some evidence from small pilot studies that emollients may have a preventative effect against development of eczema.
-
A definitive RCT is needed but pilot work is required before this can go ahead.
What did this study add?
-
Parents appear to be willing to participate in an emollient prevention trial. Those in the intervention group adhered to the emollient regimen and those in the control group were prepared to refrain from using emollients.
-
We were able to establish that the liquid-paraffin based emollients, Doublebase® gel (Dermal Laboratories Ltd) and Diprobase® cream (Bayer Plc) are popular with parents and do not have any detrimental effects on skin barrier function.
-
This pilot RCT provides the first signal from a RCT that daily full body emollient application from birth can prevent eczema.
-
Caution should be shown in reading too much into the finding of reduced eczema at 6 months in this study because it might simply represent suppression of very mild eczema by the continued use of emollients.
-
The results of the pilot study and emollient studies were used to support a successful bid to the NIHR HTA to conduct a definitive RCT of emollients for the prevention of eczema.
Introduction
Importance of the skin barrier
Although previous eczema research has focused on the role of the immune system in atopic inflammation, the strong association between filaggrin mutations and AE (as well as atopic asthma and allergic rhinitis) has generated interest in the potential role of the skin barrier as the key early event leading to eczema development.
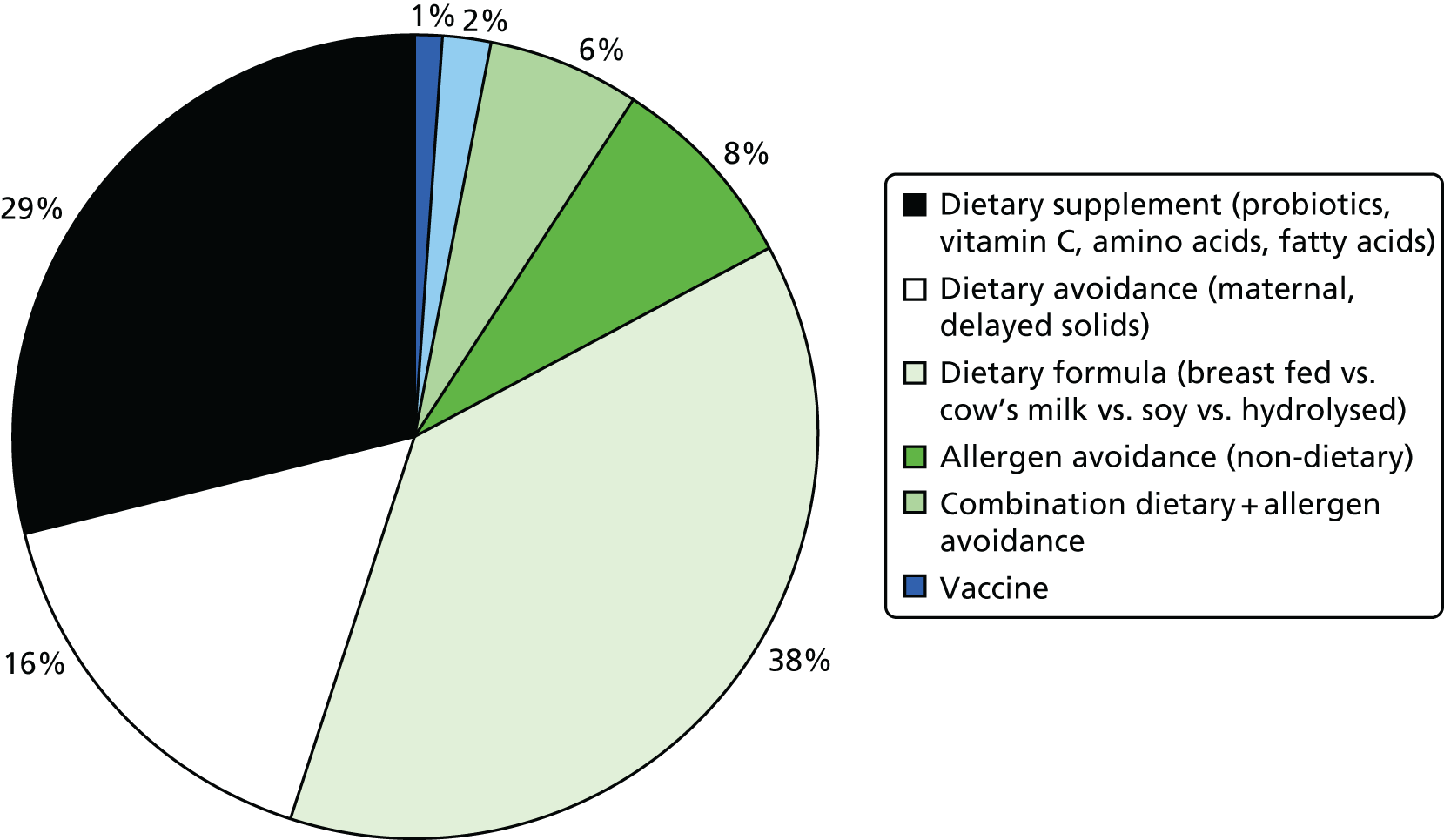
Dry skin is very common in eczema,183 even in the absence of known filaggrin loss-of-function mutations. A defective skin barrier allows water to be lost from the skin, resulting in a generally dry skin – one of the first abnormalities to be noticed in babies who eventually develop eczema, which supports the notion that the primary event in the development of eczema and atopy is a dysfunctional skin barrier. The skin barrier not only keeps useful things like water in, but also helps to keep out potentially harmful things such as irritants, bacteria and allergens. The use of harsh soap and detergents can raise the pH of the outer layers of the skin and disturb the fine balance of enzymes, proteins, lipids and micro-organisms on the skin surface. 184 A rise in pH leads to further breakdown of the skin barrier and is therefore a common pathway through which genetic and environmental factors influence skin barrier function. 184–186 Skin irritation from soaps and other wash products is worse in children with a pre-existing skin barrier defect (Figure 4).
FIGURE 4.
Model of skin barrier function. In healthy skin, the corneodesmosomes (iron rods) are intact throughout the stratum corneum. At the surface, the corneodesmosomes start to break down as part of the normal desquamation process, analogous to iron rods rusting (a). In an individual who is genetically predisposed to eczema, premature breakdown of the corneodesmosomes leads to enhanced desquamation, analogous to having rusty iron rods all the way down through the brick wall (b). If the iron rods are already weakened, an environmental agent, such as soap, can corrode them much more easily. The brick wall starts falling apart (c) and allows the penetration of allergens (d). Reprinted from J Allergy Clin Immunol, vol. 118, iss. 1, Cork MJ, Robinson DA, Vasilopoulos Y, Ferguson A, Moustafa M, MacGowan A, et al. New perspectives on epidermal barrier dysfunction in atopic dermatitis: gene–environment interactions, pp. 3–21, copyright 2006 with permission from Elsevier. 187
It is also possible that the skin is the primary organ for development of allergic sensitisation. Animal studies have suggested that IgE sensitisation may occur via the skin. 188 Even though allergens are too large to penetrate the skin directly, the defective skin barrier makes it easier for allergens to interact with skin cells such as Langerhans cells, which are responsible for initiating sensitisation. 189 The observation that mutations in the gene coding for the skin barrier protein filaggrin are associated with peanut allergy independently of eczema,190 and that the use of creams containing peanut oil on the skin during the first 6 months of life may be a linked to developing a peanut allergy,191 further support the notion that the skin might be a primary route of sensitisation for food allergies. If true, then the skin barrier is a target for prevention of not only eczema, but also for food allergy and progression to asthma and allergic rhinitis in the atopic march.
This evidence provides the basis for our proposal, to perform a primary prevention trial designed to enhance the skin barrier, by using emollients from birth, in an effort to prevent eczema and associated allergic diseases. It is unclear how long skin barrier enhancement needs to occur in order to produce possible lifelong benefits, but as allergic diseases occur in the first few months of life, and most sensitisation occurs in the first year of life, we have opted for a 12-month emollient intervention period.
Emollients and the skin barrier
Emollient (moisturiser) therapy improves the skin barrier function. An emollient improves skin hydration by trapping in water. Emollients have also been shown to play a role in preventing irritant occupational hand eczema. 192 Emollients have been shown in premature babies to reduce the incidence of skin inflammation,193 to reduce flares of eczema (secondary prevention) and to decrease the need for topical steroids. 194 Not all emollients are the same, as they vary in their consistency from greasy paraffin derivatives to lighter, water-based, creams.
Primary prevention and the NHS
Primary prevention is a highly desirable goal in a chronic disease such as eczema with no cure. Parents with experience of eczema are often anxious to know whether or not their future children will develop eczema and what they can do minimise the risk. 195 If primary prevention of eczema using a strategy of early skin barrier enhancement with simple low-cost emollients works, it would represent a significant cost saving for the NHS through reduced treatment and appointment costs, especially in those cases persisting into adulthood. Further cost savings would result if early skin barrier enhancement prevents sensitisation and associated food allergy, asthma or allergic rhinitis. Even if the frequency of eczema cannot be significantly reduced, a reduction in the severity distribution of eczema could reduce the distress to patients, the number of consultations in primary care and subsequent referrals to secondary care.
Other emollient prevention studies
An early case–control study conducted in Kenya and published in 1991 suggested that petroleum had a protective effect against the development of eczema,49 but this study has not been followed up with a definitive RCT. One RCT from Bangladesh has shown that barrier enhancement from sunflower oil and Aquaphor ointment may reduce serious infections in preterm babies. 196 A small Japanese pilot study197 [International Clinical Trials Registry Platform study identifier (ID): UMIN000004544] of 70 patients looking at emollients as a prevention strategy for eczema reported a significant difference between eczema onset between the proactive and reactive groups, and that this demonstrated that emollient use in early life can protect high-risk babies from developing eczema. Another small, short-term pilot study conducted in Japan randomised 71 babies at high risk of atopic disease to skin care instructions (including emollients) versus no instructions and found no difference in diagnosed eczema at 6 months. However, the group did show that positive reaction to skin prick tests was lower in the intervention group. 198 An open-label pilot study of emollient therapy from birth showed only 15% of high-risk infants developed eczema against an expected rate of 30–50%. 47 This study also showed that emollient therapy was a safe and acceptable intervention.
We are not aware of any other definitive trials under way to evaluate the prevention of eczema through barrier enhancement after searching trial registries (World Health Organization meta-register from inception to 18 December 2013). We did find one commercial study (n = 400) taking place in the USA and Canada (NCT01577628) that is evaluating a cosmetic moisturiser containing shea butter, paraffin, waxes and vegetable oils (Lipikar Balm AP, Cosmétique Active International) for the prevention of eczema. 199
This section describes the pilot work that has been conducted in order to design a large definitive RCT of emollient therapy from birth as an eczema prevention strategy. A pilot RCT was carried out to determine the feasibility of a large RCT, followed by a parent preference ranking exercise of emollients and mechanistic studies to look at the effects of emollients on the skin barrier to inform the choice of emollient(s) in the main RCT.
Pilot randomised controlled trial
Methods
The full protocol is available. 200
Study design
This was a multicentre, multinational (UK and USA) two-arm parallel group, assessor-blind, randomised, controlled, pilot trial of 6 months’ duration. The intervention started within the first 3 weeks of birth.
Participants
Infants at high risk of developing eczema, defined as having a parent or full sibling that has (or had) doctor-diagnosed eczema, asthma or allergic rhinitis, were included. Infants needed to be in overall good health and the mother at least 16 years of age at delivery and capable of giving informed consent. If the mother had taken Lactobacillus rhamnosus supplements during pregnancy, they were excluded. Babies were excluded if they were born prior to 37 weeks’ gestation, or if they had a major congenital anomaly, hydrops fetalis, any immunodeficiency, a severe genetic skin disorder or a serious skin condition that would make the use of emollients inadvisable.
Intervention
Skin care advice
Both the intervention and the control groups were given an infant skin care advice booklet that reflected current guidelines. 201 The guidance advises parents to (1) to avoid soap and bubble bath, (2) use a mild, fragrance-free synthetic cleanser that has been designed specifically for babies, (3) avoid bath oils and additives, (4) use a mild, fragrance-free shampoo designed specifically for babies and avoid washing the suds over the baby’s body and (5) avoid using baby wipes when possible (see Appendix 3).
Emollients
Parents in the intervention group were offered a choice of three different emollients of different viscosities: an oil, a gel/cream and an ointment.
In the UK, the choices were sunflower seed oil (William Hodgson and Co.), Doublebase gel, which contains liquid paraffin and isopropyl myristate, or 50 : 50 white soft paraffin/liquid paraffin (commonly known as ’50 : 50’ ointment).
In the USA, Cetaphil® Cream (Galderma Laboratories) was offered instead of Doublebase gel and Aquaphor® Healing Ointment (Eucerin®) instead of 50 : 50 ointment (Table 3). Research nurses provided samples to help parents make their choice and changing emollient was permitted during the study. Sunflower seed oil offered parents a ‘natural’ product and was chosen over olive oil because of evidence that olive oil can harm the skin barrier owing to the high levels of oleic acid. 202 Therefore, a sunflower seed oil with low oleic acid content (25%) and high linoleic acid content (64%) was used in this study. Doublebase gel or Cetaphil cream were offered because they are popular with parents in eczema clinics. The 50 : 50 ointment or Aquaphor Healing Ointment were offered because some parents prefer a heavier emollient. None of the emollients offered contain sodium lauryl sulfate because this emulsifier has been shown to adversely affect the skin barrier. 203
| Emollient type | UK | USA |
|---|---|---|
| Oil | Sunflower seed oil | |
| Cream/gel | Doublebase gel | Cetaphil cream |
| Ointment | 50 : 50 ointment | Aquaphor Healing Ointment |
Parents were asked to apply the emollient to the baby’s entire body surface, with the exception of the scalp and nappy area if preferred, starting as soon as possible after birth (within a maximum of 3 weeks) and continuing until the infant was 6 months of age. Instructions were to apply the emollient in gentle downwards strokes in the direction of the hair growth at least once a day and always after a bath. The concomitant use of probiotic supplements containing L. rhamnosus was discouraged during the study.
Outcomes
The primary outcome for this pilot study was the proportion of eligible families who approached the study team who were willing to be randomised.
Secondary outcomes were:
-
proportion of families eligible for the trial
-
proportion of families accepting the initial invitation to participate
-
percentage of early withdrawals
-
cumulative incidence of eczema at 6 months
-
age at onset of eczema and the proportion which are transient cases
-
incidence of emollient-related adverse events
-
proportion of families who found the interventions acceptable
-
reported adherence with intervention
-
amount of contamination in the control group as a result of increased awareness
-
success of blinding of the assessor to the allocation status.
Filaggrin gene mutation testing was performed by the laboratory of Irwin McLean evaluating for the four most common mutations (R501X, R2447X, 2282del4, S3247X) using Taqman® allelic discrimination assays (Thermo Fisher Scientific Inc., Waltham, MA, USA), as described previously. 204
An adaptation of the validated UK Working Party Criteria131 was used to measure new cases of eczema (see Defining a new case of eczema: a systematic review). These adaptations were a reduction in the duration of presence of symptoms from 1 year to 4 weeks and reflect the distribution pattern of the signs and symptoms of eczema in this young age group.
A history of an itchy skin condition for at least 2 days a week for the past 4 weeks plus three or more of:
-
A history of a rash in the skin creases (folds of elbows, behind the knees, fronts of ankles or around the neck), forearms or lower legs. Symptoms must have been present for at least 1 month either continuously or intermittently unless a topical anti-inflammatory therapy for symptom relief was required. If so, symptoms may have been present for a shorter duration.
-
A personal history of asthma or hay fever (seasonal allergic rhinitis) or a history of atopic disease in a first-degree relative.
-
A history of a generally dry skin since birth.
-
Visible flexural eczema as defined by a photographic protocol and/or visible eczema on the forearms or lower legs with absence of axillary involvement.
Visit schedule and randomisation
The duration of this trial was 6 months. Methods of identifying suitable families differed between the UK and the USA. In the UK, families were usually identified and screened during pregnancy. After the family had made contact with the study team and initial eligibility checks had been carried out by the co-ordinating centre, the research nurse carried out the screening and consent visit, usually at the family home. The baseline visit including randomisation then took place within 3 weeks of delivery, usually as a home visit. In the USA, families were identified by research nurses visiting the postnatal wards each day and approaching parents about the study directly. After giving parents time to consider the study, the research nurse returned to the family to take consent and randomise.
Infants were randomised in a 1 : 1 ratio using random block sizes to either the intervention or control group using a central web-based computer generated internet randomisation service provided by the Nottingham Clinical Trials Unit (NCTU). The allocation list was held by the NCTU and concealed from trial investigators and other trial staff. Allocation was only released to the research nurse by telephone once eligible participants’ details were irrevocably entered into the online database by the co-ordinating centre staff. Randomisation was stratified by the recruiting research nurse. In the case of multiple births, the first born was the index child.
The research nurse contacted parents by telephone at 10 days and 6 weeks, with a face-to-face visit at 12 weeks (usually at home in the UK and as a clinic visit in the USA). This was then followed by a further telephone call at 18 weeks and the final contact was a clinic visit at 24 weeks for an assessment by the dermatologist or dermatology specialist nurse, who conducted a blinded assessment of the skin. In addition to these scheduled contact points, parents were encouraged to contact the research nurse if they had any concerns about the child’s skin. If parents reported symptoms of eczema, then an unscheduled visit to the hospital to see the dermatologist was arranged so that presence of eczema could be confirmed.
Recruitment and setting
Recruitment took place in the UK and the USA between May 2010 and May 2011. In the UK, research nurses were based in three acute NHS hospital trusts (Nottingham University Hospitals, Derby Hospitals and United Lincolnshire Hospitals) and one GP surgery (The Surgery @ Wheatbridge, Chesterfield). In the USA, the study recruited in Oregon Health & Science University Hospital (Portland, OR, USA).
In Nottingham, a research nurse was able to work solely on this study, whereas for the other centres, the research nurses were involved in a number of different trials. Because this was a feasibility study, in the UK, participating families were recruited via a number of different routes in order to test the success of different strategies including invitation letters from GP, posters, leaflets, advertising and by involving health visitors and community and hospital midwives.
Blinding
It is not possible to blind parents in a trial of daily emollient application and it was anticipated that parents would want to discuss their baby’s skin with the nurse. Therefore, the research nurses who conducted the follow-up visits were not blinded to treatment allocation either. An independent outcome assessor who was blinded to treatment allocation performed the skin examinations and diagnosis of eczema. This was usually a dermatologist or a dermatology nurse specialist, although at the primary care site, this was a GP. The effectiveness of blinding was examined by asking the independent assessor to report whether or not they were aware of which group the family was in when they conducted the skin examination and, if unblinding had occurred, the reason why. The statistician was blinded to treatment group until the analysis was complete.
Approvals
The study was given ethical approval by the Nottingham Research Ethics Committee (REC) in the UK (reference 09/H0407/43) and the Institutional Review Board at Oregon Health and Science University in the USA, and approved by all participating institutions. The trial was registered at Current Controlled Trials (ISRCTN84854178) and ClinicalTrials.gov (NCT01142999).
Sample size
This was a pilot study and, therefore, not powered to establish clinical effectiveness of the intervention. The sample size was dictated by the time and resource available. One hundred families would provide an estimate within 10 percentage points for a 95% CI of the proportion willing to be randomised, assuming between 40% and 60% were willing to be randomised.
Data analysis
The primary clinical end point was the week 24 cumulative incidence of doctor-diagnosed eczema. Infants were classed as having developed eczema if either the investigator or another medically qualified person (e.g. a GP) judged that the infant had eczema on examination at any point during the 24-week intervention period.
The primary clinical end point analysis was conducted on an intention-to-treat (ITT) basis using a complete case approach. Multiple sensitivity analyses were performed for the primary clinical end point.
-
Missing data were imputed for infants with missing week 24 skin examination data because they were either lost to follow-up or withdrew during the 24-week intervention period (for reasons other than developed eczema). The following imputations were performed:
-
Multiple imputation. First, a logistic regression model was created which included the strongest known predictors for eczema derived from a review of the literature. This model included filaggrin status, centre, family history of atopic disease and the assigned intervention group. Variables were then selected for the imputation based on the smallest Akaike information criterion.
-
All those with missing data assumed to have developed eczema.
-
All those with missing data assumed to have NOT developed eczema.
-
All those with missing data in emollient arm assumed to have developed eczema and all those in the control arm assumed to have NOT developed eczema (worst case).
-
All those with missing data in control arm assumed to have developed eczema and all those in the emollient arm assumed to have NOT developed eczema (best case).
-
-
Excluding any non-confirmed cases of eczema (i.e. not diagnosed by a study investigator).
Results
The combined results for the UK and USA participants are presented here for each outcome. However, owing to methodological differences between the UK and the USA, particularly around recruitment methods and emollient choices, the UK data (used to inform the design of the main UK-based RCT) are also presented separately.
Baseline characteristics
The baseline characteristics were broadly balanced across the two groups for eczema risk factors apart from filaggrin mutation status (Table 4).
| Characteristic | Total (n) | Control, n (%) | Emollient, n (%) |
|---|---|---|---|
| One parent with eczema | 122 | 59 (51.0) | 33 (52.0) |
| Both parents with eczema | 122 | 59 (9.0) | 63 (5.0) |
| Any FLG mutation | 95 | 8 (17.8) | 13 (26.0) |
| FLG heterozygous | 95 | 6 (13.3) | 13 (26.0) |
| FLG homozygous or compound heterozygous | 95 | 2 (4.4) | 0 (0.0) |
| Normal delivery | 121 | 42 (71.2) | 47 (75.8) |
| Delivery by caesarean | 121 | 17 (28.8) | 15 (24.2) |
| Multiple births | 124 | 0 (0.0) | 1 (1.6) |
Willingness to participate
The primary outcome of this pilot RCT was to determine the proportion of families willing to participate in a randomised trial of emollients for the prevention of eczema. A total of 295 families met the eligibility criteria, of which 124 (42%) were willing to be randomised, 64 to the intervention group and 60 to the control group as shown in Figure 5. Recruitment took place in five centres (Table 5).
FIGURE 5.
Patient flow in UK and US study populations (combined) for pilot emollient study.
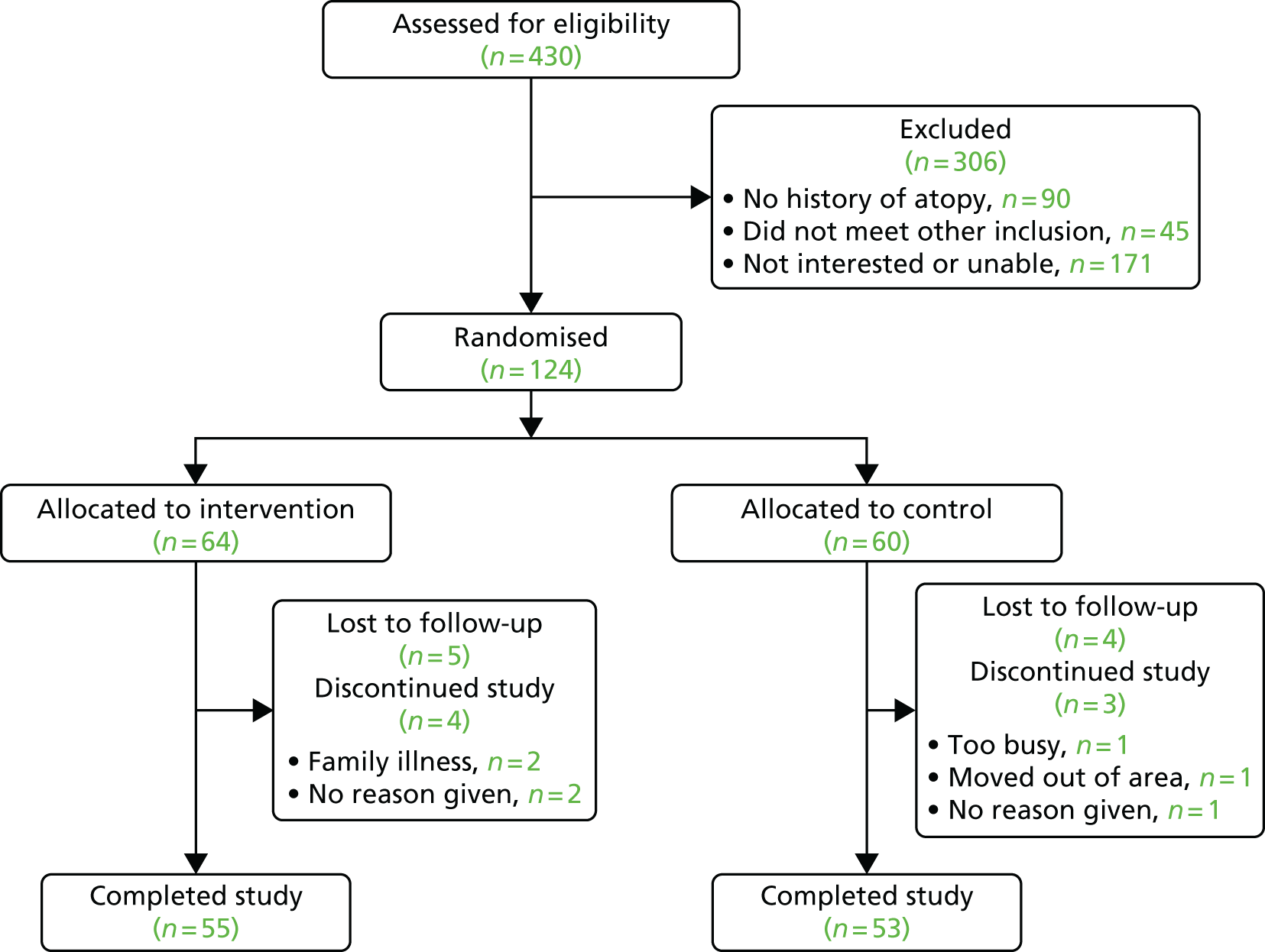
| Country | Centre | Setting | Control | Emollient | Total |
|---|---|---|---|---|---|
| UK | Chesterfield | General practice | 2 | 0 | 2 |
| Derby | Hospital | 10 | 15 | 25 | |
| Nottingham | 21 | 20 | 41 | ||
| Lincoln | 4 | 6 | 10 | ||
| USA | Portland, OR | 23 | 23 | 46 | |
| Total | 60 | 64 | 124 | ||
In the UK, willingness to participate was higher than the overall study average. One hundred and sixty-eight families enquired about the trial, of which 121 were eligible after screening during pregnancy (Figure 6). Three families were subsequently not eligible owing to post-delivery exclusions. Of the 120 eligible families, 78 (65%) were randomised. Six families (5%) declined to participate after the baby had been born, despite agreeing to participate during pregnancy. The reasons given were that wider family circumstances meant it was no longer practical to take part or that they had changed their mind about being willing to be randomly allocated to either group.
FIGURE 6.
Patient flow in UK pilot emollient study.
The most common reason for excluding families at screening in the UK was that the baby was due to be born after the end of the planned recruitment period (n = 13), but this was only an issue towards the later part of the study as the deadline for randomisation approached. Seven (4%) families in the UK were excluded at screening because of a lack of family history of atopy.
Withdrawal rates
A total of 16 participants withdrew from the trial (12.9%); nine from the intervention arm and seven from the control arm. Nine out of the 16 withdrawals were lost to follow-up.
In the UK, the withdrawal rate was lower than the overall rate, with six (8%) participants withdrawing from the trial. Two participants were lost to follow-up after moving away from the area, three withdrew from the trial because of family circumstances or illness and one declined to give a reason for leaving the trial.
Incidence of eczema at 6 months
A statistically significant protective effect was seen with the use of daily emollient on the cumulative incidence of eczema at 6 months of age (Figure 7), with a relative risk reduction of 50% (RR of 0.50, 95% CI 0.28 to 0.90; p = 0.017). Sensitivity analysis using a multiple imputation approach for missing data from the 16 participants who withdrew or were lost to follow-up showed a similar protective effect of emollient therapy as the complete case results with an OR 0.31 (95% CI 0.13 to 0.75; p = 0.01). No effect modification was seen when results were stratified by filaggrin mutation status.
FIGURE 7.
Cumulative incidence of eczema at 6 months in pilot emollient study (RR 0.50, 95% CI 0.28 to 0.90; p = 0.017).

Incidence of emollient-related adverse events
Three superficial cutaneous infections occurred in each group, all considered mild in nature. There were no reports of irritant or allergic contact eczema.
Acceptability of the intervention
To inform the decision regarding which emollient(s) to include in the main RCT, parents were offered a choice of emollient in this pilot. The most popular choice in both the UK and the USA was the cream/gel formulation, chosen by 43 (67.2%) of families (Table 6). Only a small number opted for the ointment, with just under one-quarter choosing the oil. The percentages choosing each type of emollient were very similar in the USA and the UK (data not shown).
| Emollient type | UK | USA | n (%) |
|---|---|---|---|
| Oil | Sunflower seed oil | 15 (23.4) | |
| Cream/gel | Doublebase gel | Cetaphil cream | 43 (67.2) |
| Ointment | 50 : 50 ointment | Aquaphor Healing Ointment | 6 (9.4) |
| Total | 64 (100) | ||
The main reasons that the parents in the UK gave for choosing Doublebase gel were that it absorbed well and was not too greasy. Families chose sunflower seed oil mainly because it was a ‘natural product’ or because they also wanted to use if for baby massage. Some preferred the heavier feel of the 50 : 50 ointment. All reported that the emollient was ‘acceptable’ and none of the families withdrew from the study because they did not like the emollient.
Reported adherence with intervention
Parents reported good adherence to the intervention, with > 75% of parents in both countries applying the emollient daily throughout the intervention period (Table 7).
| Emollient use | Week 6, n (%) | Week 12, n (%) | Week 18, n (%) | Week 24, n (%) |
|---|---|---|---|---|
| None | 0 (0.0) | 0(0.0) | 1 (1.8) | 0 (0.0) |
| 1–2 days per week | 1 (1.9) | 0 (0.0) | 1 (1.8) | 3 (5.6) |
| 3–4 days per week | 4 (7.4) | 5 (9.1) | 5 (8.8) | 5 (9.3) |
| 5–6 days per week | 7 (13.0) | 8 (14.6) | 7 (12.3) | 2 (3.7) |
| Every day | 42 (77.8) | 42 (76.4) | 43 (75.4) | 44 (81.5) |
| Total | 54 | 55 | 57 | 54 |
Contamination of the control group
The amount of emollient use by the control group during the 6-month intervention period was measured by asking parents whether or not they had regularly applied anything to their baby’s skin and extracting any emollients from the answers given. To reflect the nature of the intervention, contamination was defined as regular, generalised application of emollient for reasons other than the treatment of cradle cap, nappy rash or eczema. Eight control group families used emollients in a way that met this definition, making the overall contamination rate for the trial 8 out of 60 (13%).
In the UK, the contamination rate of the control group was lower, with only 3 out of 37 (8%) meeting our definition. None reported using emollient bath oils other than for the treatment of eczema. A high proportion of families reported using moisturisers in the weeks immediately following delivery and prior to being randomised into the trial (33/78, 42%). This was mainly due to community midwives and health visitors promoting the use of moisturisers for newborn baby dry skin.
Success of blinding of the assessor to the allocation status
A total of five skin examinations were carried out by an assessor who was aware which group the infant was in (all in the UK). In four of these cases, the infant had already been diagnosed with eczema and the only person available to perform the skin examination was already aware of this, having previously treated the infant. One family was not able to bring the child into hospital for an independent assessment so the research nurse, who was aware of the treatment allocation, visited the family home to conduct the skin examination.
Recruitment
In the UK, a number of different recruitment strategies were tested in order to establish the most effective methods for recruiting and retaining families for the main trial (Table 8). The highest number of enquiries was from families who had received a letter from their GP. Participating surgeries invited all pregnant women of > 12 weeks’ gestation who were registered with their practice. The 59 enquiries were generated from 935 letters sent out by 21 GP surgeries. A high proportion of enquiries also came from parents who had seen posters about the study in hospital, usually in the antenatal waiting rooms. Several other recruitment methods produced a smaller number of enquiries including newspaper advertising, hospital and community midwives, health visitors and antenatal classes.
| Where families found out about the study | Number of enquiries generated (%) |
|---|---|
| Received invitation from GP | 59 (35.1) |
| Responded to poster in the hospital | 34 (20.2) |
| Responded to advert in local newspaper | 18 (10.7) |
| Identified in a dermatology clinic | 8 (4.8) |
| Responded to poster in a public place | 7 (4.2) |
| Midwife or health visitor mentioned study | 7 (4.2) |
| Publicity via eczema patient support group | 6 (3.6) |
| Responded to poster in GP surgery | 3 (1.8) |
| Study mentioned in an antenatal clinic or class | 3 (1.8) |
| Picked up leaflet in hospital | 2 (1.2) |
| GP mentioned study | 2 (1.2) |
| Other | 3 (1.8) |
| Not known | 16 (9.5) |
| Total number of enquiries | 168 (100.0) |
In the USA, a different approach was taken to recruitment and a single method was employed. The research nurses visited the post-natal wards each day and approached mothers about the study. After being given sufficient time to consider their participation, they were screened and randomised while in hospital.
Discussion
Implications for the main randomised controlled trial: feasibility outcomes
A high proportion of families were willing to participate in this study, indicating that a trial of emollients for the prevention of eczema in babies is feasible. In the UK, screening took place during pregnancy but randomisation took place after the baby was born because of concerns that families might have changed their mind about participating in the interim leading to a high withdrawal rate. The results showed that the number of families who declined to participate after the baby was born despite having previously consented to take part at screening during pregnancy was low. However, when combined with the need to exclude babies who do not meet post-delivery eligibility criteria, randomisation prior to delivery in the main RCT would result in an unacceptably high post-randomisation drop-out rate. Therefore, randomising after delivery remains a better option. The retention rate in the study was high, but it is clear that for the main RCT all efforts must be taken to keep in contact with families who move out of the area.
Among the UK recruiting centres, the highest recruitment rates were reported in those supported by a nurse who was solely dedicated to this particular study. The employment of dedicated research nurses in the main RCT should therefore be considered. The number of willing and eligible families per GP surgery is too low to consider GP surgeries as recruiting centres; they should instead be involved as patient identification centres (PICs). Pregnant women should be targeted when they visit for antenatal care as this covers all surrounding practices.
The purpose of including several recruitment strategies in the UK was to identify methods that would be transferable to a large national RCT. To judge the effectiveness of each strategy, the number of enquiries generated was considered alongside the time and costs involved. Displaying posters in the hospital is cheap and takes very little time. In this trial, posters in the antenatal clinic waiting rooms proved an effective recruitment strategy. Virtually all pregnant women attend the hospital antenatal clinic for scans during pregnancy and are likely to be thinking about the baby during these visits. Another effective approach was to ask research-active GP practices to search their databases for pregnant women and send out postal invitations with the study team contact details. This was done in conjunction with the Primary Care Research Network (PCRN) East Midlands and South Yorkshire. Although there are administrative costs associated with this process, practices that work with PCRN East Midlands and South Yorkshire are well rehearsed in conducting this type of recruitment activity and it proved to be the most successful way of recruiting pregnant women for this trial.
The time needed by staff at the co-ordinating centre to field the enquiries from families responding to posters and invitation letters should not be underestimated. To minimise the time spent screening ineligible families, all adverts, leaflets, posters and invitation letters included the statement that at least one family member had to have (or have had in the past), eczema, asthma or hay fever. To ensure this simple message was not lost, the only other eligibility criteria included in the publicity material was the need to be pregnant (or have very recently delivered, depending on the location of the poster). This approach was successful, with only a few enquiries received from families without a history of atopy. In fact, the most common reason for ineligibility was that the baby’s due date was past the end of the scheduled recruitment period. This was unavoidable when parents approached the study team towards the end of the recruitment period, but this reduction of eligible families as the trial nears the end does need to be taken into account in future trial planning.
Media advertising in newspapers generated a reasonable number of enquiries but it is costly compared with other recruitment methods and did not result in a sufficiently high number of enquiries to justify the costs involved. Asking midwives and health visitors to inform families about the study did not prove to be an effective recruitment strategy. Despite the study nurses spending time explaining the study, the feedback from the midwives and health visitors was that they did not have the time in their current workload to pass on this information to families.
At one centre, research midwives were able hand out leaflets to all women in hospital after delivery, but this was clearly not a good time to approach parents in the UK as this generated very few enquiries. Recruiting during pregnancy allowed families more time to consider the study properly, rather than immediately post delivery, which can be a hectic time with lots of new information being given to parents. In fact, in the USA, there was a higher withdrawal rate and lower adherence when mothers were recruited during the immediate postnatal period.
The recruitment strategies tested in the UK required the families to be sufficiently motivated to actively contact the study team and this may have also contributed to the high retention rate. Actively recruiting families, as took place in the USA centre, would also have large cost implications for the main RCT because it is time-consuming and requires a research nurse to spend long periods of time visiting antenatal and postnatal departments to speak directly to families.
Occasionally, families in the UK did contact the study team after their baby was born, usually because they had seen a poster. Providing it was possible to randomise them within 3 weeks of delivery, they were eligible to participate. However, because this was reactive, it was less efficient for research nurses in planning their workloads, so efforts should remain focused on recruitment during pregnancy for the main RCT.
This pilot RCT provided information on whether or not parents would be prepared to adhere to the intervention of once-a-day all-over body application of emollient to their baby for the first 6 months of life. The high adherence rate, positive feedback from parents regarding acceptability of the emollient, and the fact that no withdrawals were owing to the emollient, strongly suggest that a large RCT of this intervention is feasible. Some parents reported that their baby became upset when the emollient was applied in the early weeks but they were prepared to continue with the intervention as this was probably due to the baby being undressed rather than the application of the emollient itself. Adding this to the skin care guidance would probably help parents as they would know this is a normal response in the early weeks. There was a clear signal from the choices made by parents and reasons given that a cream/gel formulation should be offered in the main RCT.
There was concern that simply finding out about the potential for emollients to prevent eczema would mean parents applying emollients to their baby regardless of which group they are in. Emollients are easy to obtain without prescription and are relatively cheap and so a high level of contamination in the control group was a real possibility. Two approaches were taken to minimise contamination of the control group: (1) presenting a balanced view in the participant information leaflet and explaining that it was not yet know if the intervention was effective and (2) minimising resentful demoralisation by avoiding the term ‘control group’ and instead referring to the intervention and control groups as the emollient group and the skin care group, respectively. The reported level of contamination was low, meaning that it should be possible to include a control group who are asked to refrain from using emollients in the main RCT.
The low levels of unblinding seen here suggest that in this model it is possible to include a blinded assessment of eczema status by including independent assessors. However, this proved to be an inefficient way to conduct the study. Even in centres where there were two or three staff available to perform the skin examinations, it was often very difficult and time-consuming for the research nurses to co-ordinate a time that suited busy families and dermatologists. Therefore, although blinding of the outcome by using an independent assessor was possible, a better option for the larger main RCT would be blinded research nurses performing the skin examination and diagnosis of eczema at the family home. This would require an increase in staffing levels at the co-ordinating centre to carry out interim contact with families to ensure that the research nurses remain blinded.
Differences in the design of the pilot and main randomised controlled trial
As the design of the main RCT has been refined, differences between the pilot RCT and main RCT have inevitably arisen. The main differences are a longer intervention period (12 months rather than 6 months), follow-up to 2 years rather than 6 months to ensure true eczema is being measured, and less frequent contact with families to ensure the study is pragmatic and to protect the blinding of the research nurses to carry out the skin examination for the primary outcome at 2 years. These may adversely affect the retention rates so measures need to be in place to compensate for this. In addition, the pilot RCT families have been followed up to 2 years of age, so analysis of these data will be helpful in anticipating any problems.
Although recruitment methods were adequate, the subsequent main RCT should reflect trends such as the use of software designed to operate on mobile devices (‘apps’), the increased ownership of smartphones and the use of media outlets other than newspapers such as websites and online groups aimed at pregnant women.
Incidence of eczema: clinical outcomes
This pilot RCT provides the first randomised evidence that daily full-body emollient application from birth can prevent eczema. The results were in line with a previously published open-label pilot study that showed a reduction in incidence of eczema. 47 The emollient therapy did not cause significant adverse effects.
Emollients are first-line therapy for the management of established eczema. The exact mechanisms by which emollients exert their effects are not completely understood, but it is possible that emollients correct subclinical skin barrier dysfunction by improving skin hydration and, thus, preventing skin dryness and cracking. This may be enough to stop irritants and allergens, which can initiate inflammation and an allergic response. In addition, both human and mouse studies suggest skin barrier improvement may prevent IgE sensitisation191,205,206 and, if so, this approach may also prevent allergic asthma and food allergy.
Strengths of this pilot are the RCT design, blinded outcome assessment and external validity of studying the intervention in two countries and several centres. The major limitation is that the primary objective was to assess feasibility and so the study was not powered to demonstrate clinical effectiveness. This focus on feasibility also meant that we included only a relatively short follow-up period of 6 months, although families are being followed up by postal questionnaire. Long-term follow up is crucial for any future RCTs of this promising prevention strategy. Although the study was broadly balanced with respect to most baseline factors, there were more infants with filaggrin mutations in the emollient group than the control group. However, this is highly unlikely to explain the treatment effect; in fact, any effect would be to minimise the effect of the intervention because it is thought that those with mutations are more likely to develop eczema.
Future research will require RCTs of much larger cohorts of infants to confirm these results and to determine whether or not filaggrin genotype modifies the effect of therapy.
Conclusion
This pilot study has shown that a RCT of emollients for the prevention of eczema in babies is feasible and that emollients as an intervention to prevent eczema look promising. These results have been used to inform the design of a definitive RCT (see Proposal for a randomised controlled trial to determine whether or not emollients can prevent eczema development).
Choosing an emollient for the main randomised controlled trial
Introduction
The intervention in any prevention study needs to be highly acceptable to participants, otherwise there is a high risk of poor adherence and low-quality data. Therefore, parental acceptability of the emollient is a particularly important issue for the main RCT. The pilot RCT clearly showed that parents preferred a cream/gel emollient rather than an oil or ointment. There is a lack of evidence regarding the effect of many of the commonly used emollients on the skin barrier, although it is reported that some emollients, including aqueous cream and olive oil, can adversely affect the skin barrier. 202,203 To inform the decision on which emollient(s) should be included in a large definitive RCT of emollients for preventing eczema, further data on the emollients were required.
Therefore, we undertook a UK parent preference study of potentially suitable emollients and the two most popular emollients were taken forward to undergo a series of tests to assess their effect on skin barrier function.
Choice of emollients to be included in the preference study
To avoid emollients with ingredients known to be potentially harmful, we excluded emollients that contained the emulsifier sodium lauryl sulfate, as this has been shown to damage the skin barrier. 203 We also avoided emollients that contain parabens or protein residue that could result in sensitisation (oat, soya or plant oils) which excluded some popular emollients, for example Aveeno® (Johnson & Johnson Ltd) as it contains oatmeal. We excluded spray emollients as they contain propellant, the effects of which on the skin barrier are not understood, and formulations containing special additives, such as ceramides, because they are relatively expensive. The following four emollients were chosen: Doublebase gel, Diprobase cream, Oilatum® Junior cream (Stiefel, GlaxoSmithKline Consumer Healthcare), and Diprobase ointment (Bayer Plc). Diprobase cream and Oilatum Junior cream both contain the emulsifier cetostearyl alcohol, which is a commonly found in emollients, and its inclusion in the main RCT would permit some degree of generalisation across other emollients. Doublebase gel, which was popular in the pilot RCT, contains isopropyl myristate as the emulsifier. Diprobase ointment absorbs quite well relative to other ointments so was included.
Parent emollient preference study
Methods
A group of twenty parents who had taken part in the pilot RCT were given small samples of Doublebase gel, Diprobase cream, Oilatum Junior cream and Diprobase ointment (in a tube) and asked to rank them in order of preference. The two most popular emollients would then be taken forward into the mechanistic studies.
Results
Nineteen out of 20 (95%) parents responded with their preferences. The most popular two choices were Doublebase gel and Diprobase cream (Table 9) and so these would be taken forward to the skin barrier study.
| Rank | Emollient | Number of parents ranking emollient as first choice |
|---|---|---|
| 1 | Doublebase gel | 7 |
| 2 | Diprobase cream | 5 |
| 3 | Oilatum Junior cream | 4 |
| 4 | Diprobase ointment (in tube) | 3 |
Effects of emollients on the skin barrier function
Methods
Doublebase gel and Diprobase cream were chosen for comparison based on the parent preference data. Two cohorts of volunteers with a previous history of eczema (having a predisposition to a defective skin barrier, but no active signs of eczema for 6 months) were recruited. The first cohort comprising 18 volunteers (14 were female and the mean age of all 18 volunteers was 29 years ± 8 years) treated the volar side of one forearm with two fingertip units of Doublebase gel, and the other forearm with no treatment (untreated control), twice daily for 4 weeks (the site was determined at random for each subject). The clinical (dryness) and biophysical properties of the test sites were determined before and after the 4-week treatment regimen to ascertain the effect of the emollient. The biophysical parameters included transepidermal water loss (TEWL) to determine skin barrier function, capacitance (an indirect measure of stratum corneum hydration) and skin surface pH. In addition, the restoration of TEWL following tape-stripping was measured as an indicator of the effect of the emollients on skin barrier recovery. The second cohort, comprising 19 volunteers (14 were female and the mean age of all 19 volunteers was 33 years ± 2 years), undertook a similar regimen using Diprobase cream instead of Doublebase gel.
Results
A 4-week treatment regimen with either Doublebase gel or Diprobase cream did not affect skin barrier function (as indicated by unchanged TEWL) or estimated stratum corneum thickness. Skin barrier recovery following tape-stripping was unaffected by either treatment. Hydration was significantly improved following treatment with both emollients. Measurements were collected 24 hours after the final application, a necessity for determination of TEWL (primary measure). The sites treated with Doublebase gel were on average 42% ± 5.1% more hydrated than the control sites, whereas for Diprobase cream the increase was 14% ± 4.8%. It is possible that the difference in hydration could be due to a difference in the duration of the test product is on the skin.
Skin treated with the emollients had a statistically significant increase in skin surface pH, with a mean change of 0.4 ± 0.08 for Doublebase gel and 0.1 ± 0.05 for Diprobase cream, compared with the change in pH of untreated skin, 0.1 ± 0.07 and –0.08 ± 0.05, respectively. Ideally, any topical product for treating or preventing eczema should not affect pH, as skin pH is known to be increased during exacerbations and is associated with skin barrier breakdown. However, the other biophysical parameters indicated no evidence of clinically significant skin barrier breakdown.
In summary, neither Doublebase gel or Diprobase cream damaged the skin barrier. The results demonstrate that either Doublebase gel or Diprobase cream, and by inference other simple emollients formulated with isopropyl myristate or cetostearyl alcohol, are suitable interventions for an eczema prevention RCT.
Summary of emollient choice studies
These studies allowed us to identify two emollients, Doublebase gel and Diprobase cream, that are popular with parents and do not damage the skin barrier and so can be recommended for use in the main RCT.
Summary of pilot studies
We have carried out a series of studies to enable a definitive RCT of emollients for the prevention of eczema to take place.
A pilot RCT, which demonstrated that parents were willing to participate, willing to adhere to the emollient regimen and found the intervention acceptable. Parents in the control group were prepared to refrain from using emollients. The pilot study also provided useful insights into the best ways to recruit to a main RCT:
-
Although not powered to detect a clinical difference, the pilot study showed a 50% reduction in the chances of developing eczema in the emollient group (RR of 0.50, 95% CI 0.28 to 0.90; p = 0.017).
-
A parent preference study of emollients showed that Doublebase gel and Diprobase cream were the most popular choices with parents.
-
The effects of these two emollients were then assessed for their effects on skin barrier function and tests showed that they are suitable for inclusion in the main RCT.
The definitive RCT was funded by the NIHR HTA1 and started in November 2014.
Proposal for a randomised controlled trial to determine whether or not emollients can prevent eczema development
Introduction
There is a growing interest in skin barrier enhancement as a means of preventing and treating skin disease, and the window of opportunity to randomise a population to a ‘no emollient’ group will probably only last for around 5 years. Uncontrolled studies claiming various benefits of expensive ‘barrier enhancers’ such as those containing ceramide may make it difficult for parents to agree to being randomised into a study that includes a group with no active intervention. The proposed definitive trial will provide clear and straightforward guidance for health professionals about whether or not they should be recommending or prescribing emollients for infants at risk of developing eczema. Long-term adherence to emollient use can be a problem; a study that produces a clear answer may strongly influence prescribing habits and usage. Dissemination to parents and relevant health-care professionals (HCPs) including midwives, health visitors, GPs and public health specialists will be a key trial output.
Aims and objectives
The aim of this RCT is to test the hypothesis that daily skin barrier enhancement with emollients in babies at high risk of allergic disease will reduce eczema development.
The primary objective is to determine whether or not enhancing the skin barrier by daily emollient application from birth for the first year of life can prevent the onset of eczema in high-risk infants, when compared with a control group who are not told to apply daily emollients.
Our secondary objectives are to determine whether or not:
-
any eczema preventative effect is sustained into later childhood
-
eczema severity differs between the two groups
-
the intervention can prevent the onset of other allergic diseases (asthma and allergic rhinitis)
-
if effective, the intervention is cost-effective for the NHS.
Trial design
This is a pragmatic, parallel group, multicentre, assessor-blind randomised controlled primary prevention trial (Figure 8). Participation in the main part of the trial will be 2 years in duration (from within 3 weeks of birth until approximately the child’s second birthday). This will comprise a 1-year intervention phase, followed by assessment of the primary outcome at 2 years. The children will then move into the long-term follow up part of the trial, in which they will be assessed by questionnaire until they are 5 years old.
FIGURE 8.
Overview of design for RCT to determine whether or not emollients can prevent eczema development.

Participants and setting
Participants
Participants will be newborn babies at high risk of developing eczema (on the basis of having a parent or sibling with current or previous doctor-diagnosed asthma, eczema or allergic rhinitis). Family history of atopic disease is still the most consistent, most common and strongest risk factor for predicting the development of eczema; the risk of a child developing eczema increases 1.5–4 times when there is a family history of atopic disease. 207,208 Recruiting centres will be chosen to ensure a representative range of socioeconomic and ethnic group mixes to help with external validity.
Setting
This multicentre trial will be primarily research nurse-led and will take place in a total of eight centres. There will be four main study sites (in Nottingham, Sheffield, Bristol and London), each with a part-time trial-funded research nurse to recruit approximately 1000 families (250 per centre) over 2 years. This is comparable with the recruitment rates achieved in the pilot study.
Approximately 30 centres in the UK expressed an interest in being involved with this trial, but to keep the trial co-ordination manageable, we plan to only include a further four centres with Medicines for Children Research Network (MCRN) and Comprehensive Local Research Network (CLRN) research nurses to recruit the remaining 300 families. However, this high level of interest does mean that if recruitment is slower than expected, we are able to add in further centres. We have an excellent track record of recruiting into national trials in collaboration with CLRN/MCRN nurses.
We will recruit a cohort of pregnant women from the community for this prevention study. Our pilot study recruited successfully, ahead of target and allowed us to refine our recruitment strategies. We will work with the PCRN to identify GP surgeries to send invitation letters to pregnant women in their practice. We will display posters advertising the trial in hospitals (particularly in antenatal areas and delivery suites) and advertise in local media.
Eligibility criteria
Inclusion criteria
-
Participant (i.e. the newborn baby) must have a parent or sibling with a history of eczema, allergic rhinitis or asthma.
-
Infant in overall good health.
-
Mother at least 16 years of age at delivery and capable of giving informed consent.
Exclusion criteria
-
Preterm birth (defined as birth prior to 37 weeks’ gestation).
-
Sibling already randomised to this trial.
-
Major congenital anomaly.
-
Significant inflammatory skin disease at birth (except seborrhoeic eczema).
-
Any immunodeficiency disorder or severe genetic skin disorder.
-
Any condition that would make the use of emollients inadvisable or not possible.
Randomisation and blinding
The randomisation schedule will be based on a computer-generated pseudo-random code using random permuted blocks of randomly varying size, created by the NCTU in accordance with their standard operating procedure (SOP) and held on a secure University of Nottingham server. The trial administrator will carry out the randomisation via a web-based system and alert the family as to which group they are in.
The research nurse will remain blinded throughout the trial, in order to carry out the blinded skin examination at 2 years, but the family will be unblinded throughout.
Intervention
The intervention is daily application of emollient to the baby’s entire body surface area (BSA), including the face, for the first year of life. The scalp and nappy area can be avoided if preferred. Our pilot study has shown that parents found this intervention acceptable, were willing to adhere to this regimen and readily adopted it into their daily routine.
Parents will be offered a choice of two emollients: Doublebase gel or Diprobase cream. This choice has not been influenced to any degree by the manufacturers. They have been selected because our pilot work showed that parents like these emollients and they are not harmful to the skin barrier. Furthermore, they are easy to obtain and so lead to generalisable results. Both are readily available with or without prescription, they are commonly used in the NHS for treating eczema and are not expensive ‘niche’ products. There are many other emollients with a similar formulation.
Control treatment
There is no placebo for the emollient in this trial. Instead, parents in the control group will not be advised to use emollients. It is simply not possible to apply any product to the skin that is sufficiently similar in appearance to an emollient but that lacks any moisturising properties. This means that parents in the intervention and control groups are unblinded, which is why our primary outcome measure is an objective, assessor-blinded outcome to minimise information bias.
Skin care advice: both groups
As in the pilot study, parents in the intervention and the control groups will receive general advice on best practice skin care for their baby in line with the NICE guidance on Routine Postnatal Care of Women and their Babies. 201 This will include avoiding harsh detergents, bubble bath and fragranced baby wipes and ensuring that wash products are mild, unperfumed synthetic detergents formulated specially for babies. Frequency of bathing and washing will be left to the discretion of the parents. Data on washing practices, products used and bathing frequency will be collected and a multivariate regression analysis based on the whole sample will be considered in order to investigate a possible interactive effect between those washing products and emollient.
Outcomes
Primary outcome
The primary outcome will be the proportion of infants with a diagnosis of eczema measured by 1-year period prevalence of eczema when the child reaches 2 years of age. Rationale: we will use standard diagnostic criteria for those < 4 years of age (the UK refinement of the Hanifin and Rajka diagnostic criteria)7 which are: must have an itchy skin condition in the last 12 months, plus at least three of:
-
history of flexural involvement
-
history of a generally dry skin
-
history of atopic disease in a first-degree relative
-
visible dermatitis as per photographic protocol. 209 These robust and validated criteria are applicable to a range of ethnic groups.
They are commonly used to confirm diagnosis of eczema in treatment trials and are the most widely used criteria for epidemiological studies. 177 To reflect the chronicity of eczema that may vary across seasons, the criteria refer to signs and symptoms present over the past year. A 1-year period prevalence at age 2 years has been chosen in order to exclude transient eczematous rashes that are common in the first year of life, most of which are probably irritant (saliva and nappy) and not true AE.
Secondary outcomes
-
Parental-reported 2-year cumulative incidence of eczema according to the UK refinement of the Hanifin and Rajka diagnostic criteria for eczema, not including the skin examination. Rationale: even though eczema in the first year will contain more ‘noise’, an incident measure may still be appropriate to reflect the ‘force’ of incidence as opposed to prevalent disease which may reflect factors of disease chronicity as well as incidence.
-
Point prevalence of eczema on skin examination (visible eczema as per photographic protocol) at 2 years. Rationale: a blinded outcome measure that takes less than 1 minute to perform and might reflect more persistent disease.
-
Time to onset of eczema (parental report of doctor diagnosis) and first topical corticosteroid prescription. Rationale: to determine whether or not emollients can delay the onset of eczema.
-
Severity of eczema; if the child has been diagnosed with eczema, the Eczema Area and Severity Index (EASI) will be carried out by the research nurse at 2 years and Patient-Oriented Eczema Measure (POEM) scale completed by the parents at each contact point. Rationale: any reduction in severity would mean significant health benefits and would reduce NHS costs.
-
Two-year cumulative incidence of parental-reported wheezing. 177 Rationale: early wheezing has been shown to be a predictor of allergies in later life.
-
A within-trial cost-effectiveness analysis will be conducted from the perspective of the NHS at 2 years, with a family perspective captured separately. Longer-term analysis will be conducted using economic modelling as appropriate. Health-care resource use and costs will be collected regularly throughout the trial and used to inform this analysis. Costs associated with both the emollient (estimated to be £60 per child) and other NHS care for the treatment of eczema will be collected. For the family perspective, time off school and work will be monitored, along with expenditure on skin care products and other eczema-related items. Rationale: to obtain an understanding of whether or not the proposed intervention offers value for money in the NHS and for families.
-
Effect of the intervention on QoL will be assessed using the following scales:
-
parental/main carer health-related QoL at baseline and when the child is 2 years old, measured using the European Quality of Life-5 Dimensions-3 Levels (EQ-5D-3L)
-
Infant’s Dermatitis Quality of Life Index (IDQOL) at 2 years old. 210 The IDQOL has been developed for use in children < 4 years and has previously been used in economic evaluations of interventions for eczema. 211
-
an eczema-specific instrument for capturing utility in children with eczema at 2 years212 – this scale produces a utility score and has four health domains (activities, mood, comfort and sleep). The difference in utility between the two groups will be estimated by taking account of the difference in the number of children that develop eczema and any difference in eczema severity (children do not have eczema at the point of randomisation). Subsequently quality-adjusted life-year (QALY) estimates will inform the longer-term economic model based analysis. Rationale: to see whether or not any benefits in disease frequency are accompanied by improvements in QoL for the child and families.
-
-
Incidence of possible adverse reactions to the emollient including skin infections, occlusion, folliculitis and slipping owing to emollient use. Rationale: although extremely safe, a precise estimate of possible adverse effects is essential to balance benefits and harms.
Saliva samples will be collected from all participants for DNA extraction to allow genotyping for the most prevalent loss-of-function mutations in the gene encoding filaggrin. This will allow a stratified subgroup analysis to test the hypothesis that individuals with one or more filaggrin null mutations show the most significant reduction in AE incidence as a result of intensive emollient therapy.
Tertiary (long-term) outcomes
Long-term follow-up is important for a chronic disease like eczema. The main part of the trial, which includes the primary and all secondary outcomes, assesses the infants up to the age of 2 years. These data will be analysed and reported when complete. Children will continue to be followed up by questionnaire at 3, 4 and 5 years old to determine:
-
1-year period prevalence of doctor-diagnosed eczema and parental-reported eczema using the UK diagnostic criteria (without the physical signs for symptoms in the last year) at 3, 4 and 5 years old
-
incidence of allergic rhinitis and asthma assessed by parental report of doctor diagnosis and parental questionnaire at 5 years old
-
severity of eczema at 3, 4 and 5 years old (POEM score)
-
parental/main carer health-related QoL when the child is 3, 4 and 5 years old using EQ-5D-3L.
Study schedule and data collection
Screening, consent and randomisation
Screening will mostly take place during the third trimester of pregnancy, when consent will be obtained. Once the family have notified the research nurse that the baby has been born safely, or the research nurse has contacted the maternity unit to establish this, and eligibility is confirmed, randomisation will take place. To ensure the research nurses remain blinded, the trial administrator will notify parents which group they have been assigned to. The emollient will then be posted to parents along with a booklet and a web link to a video demonstrating how to apply the emollient correctly. There is a need to start the intervention soon after birth (within a maximum 3 weeks of delivery) because it is thought that the stratum corneum is not mature at birth and because imbalances in the immune system [Th1 vs. T helper cell type 2 (Th2) shift] probably occur quite early at a critical period of development of the thymus gland. 213,214
Screening after delivery
If parents become aware of the trial after delivery, then providing they can start within 3 weeks of birth, they will be accepted onto the trial.
Assessment and follow up to 2 years old
Parents will be contacted by e-mail with secure web links for completion of questionnaires at 3, 6 and 18 months (or by post if no internet). Missing data and non-responders will be prompted by repeat e-mails and post, with resource intensive telephone calls as the last option. They will receive a telephone call from the trial administrator at 1 year and a home visit by the research nurse at 2 years. We will use the Medical Research Information Service data to get updates on the child’s health status. Parents in both groups will be advised that if the baby develops a skin problem at any point that they are concerned about then they should seek medical care. Any treatment of eczema or other skin problems will be through the child’s GP. At the 2-year visit, parents will be reminded not to reveal their allocation status to the research nurse until after the key skin examination and questions have been entered for the primary outcome. Research nurses will be asked to confirm their blinding status at the time of assessment. Table 10 shows details of the data collection schedule.
| Activity | During pregnancy | Within 3 weeks of delivery | Follow-up (main study) | Long-term follow-up | |||||
|---|---|---|---|---|---|---|---|---|---|
| Initial approach by parents | Screening visit | Baseline (day 0) | 1–2 weeks | 3 and 6 months | 1 year | 18 months | 2 years | Years 3, 4 and 5 | |
| Check eligibility | Telephone call and e-mail | Home visit | Telephone call | ||||||
| Informed consent | Home visit | ||||||||
| Randomisation (day 0) | Telephone call | ||||||||
| Supply emollients and skin care advice | Telephone call | ||||||||
| Check started study correctly | |||||||||
| Parental report of eczema symptoms and doctor diagnosis of eczema | Telephone call | Home visit | ✗ | ||||||
| POEM severity score (if have eczema) | Telephone call | Home visit | |||||||
| QoL questionnaires | Telephone call | Home visit | ✗ | ||||||
| Health service utilisation | Telephone call | Home visit | ✗ | ||||||
| Collect data on bathing and skin care habits and products | Telephone call | Home visit | ✗ | ||||||
| Collect data on other possible eczema prevention interventions | Home visit | ||||||||
| Check adherence (emollient group) contamination (control group) | Telephone call | Home visit | |||||||
| Wheezing questionnaire | Home visit | ||||||||
| Adverse events | Telephone call | Home visit | |||||||
| Stop interventiona | Telephone call | ||||||||
| Diagnosis of eczema by blinded research nurse (diagnostic criteria including skin exam) primary outcome | Home visit | ||||||||
| EASI severity score carried out by blinded research nurse (if have eczema) | Home visit | ||||||||
| Parental report of asthma and allergic rhinitis symptoms and doctor diagnosis | ✗b | ||||||||
Long-term follow-up (when the child is 3, 4 and 5 years of age)
The long-term follow-up after the main trial of children at 3, 4 and 5 years of age will be conducted by web-based and postal questionnaire directly to the parents to keep the costs of this data collection to a minimum, while still obtaining key data on long-term outcomes.
Adherence
Parents in the intervention group will be asked at each contact how many containers of emollient they have used and also to report whether or not they have applied the emollient every day, most days, approximately every other day, once or twice a week or less than once a week.
Health economics
Within-trial analysis
Patient-specific data collected during the trial will be used. The incremental cost per eczema case prevented, incremental cost per one point less improvement in IDQOL and incremental cost per QALY will be estimated. As cost data are inherently skewed, bootstrapping will be used to estimate CIs around the mean differences in costs and benefits. 215 An incremental cost-effectiveness analysis will be performed using accepted methods216 with data reported in a disaggregated way. Analysis of uncertainty will follow recommended practice [with results presented as cost-effectiveness acceptability curves (CEACs)].
Longer-term model-based analysis
If emollient intervention is found to be effective at 2 years, a longer-term economic model will be developed to model the economic costs and benefits of the intervention for a single birth cohort from birth to 16 years, in order to better inform policy recommendations.
An economic evaluation conducted alongside a trial is likely to provide a conservative estimate of cost-effectiveness because it captures only the short-term costs and benefits. Taking a longer time perspective is particularly important for preventative interventions, as, although the costs are incurred early (within this study in the first year of life) the benefits (if found) are likely to be felt over a number of years and are likely to be underestimated in a within-trial analysis.
Using a combination of trial data, published data and expert opinion, a decision analytic model will be developed, taking a NHS perspective. The model will be developed in accordance with current practice and guidelines. 215,217 Having identified the potential management pathway for the intervention, we will develop and verify the model structure (this will probably use a Markov chain approach). Future costs and benefits will be discounted in line with recommendations. Analysis of uncertainty will be undertaken using probabilistic sensitivity analysis with results presented as CEACs.
Statistical analysis
Analyses will be performed using the ITT approach. Patterns of missing data will be checked before analysis and multiple imputations for missing outcomes will be considered when it is apparent. To test efficacy of the intervention, difference in the primary outcome measured by the prevalence of eczema in the past year at 2 years between the two arms will be compared using a 2 × 2 chi-squared statistic. Sensitivity analysis for missing data treatment in the outcome will be conducted.
Analysis of secondary outcomes will follow the same ITT principle, missing data handling procedures and descriptive analyses. Different methods will be used to test efficacy of the intervention for different type of outcomes. The incidence of eczema or wheezing or atopy between trial arms will be compared by RR and tested using statistic based on Poisson distribution. The t-test will be used for continuous outcomes such as the severity score and some QoL measures. The Kaplan–Meier function and log-rank test will be used for the time to onset of eczema. For the severity scores, a mixed model for repeated measures will be used to test the mean difference and change trend over time in the two arms.
Baseline measurements including family history of eczema, maternal or paternal inheritance, sibling eczema, pet exposure and filaggrin genotype mutations will be included as covariates in the primary and secondary outcome analyses under two conditions.
-
If the baseline variables significantly differ between the two study groups, they will be included in conditional logistic regression.
-
If missing outcomes show an association with baseline status and/or follow-up measures, we will include baseline as well as follow-up data in repeated analyses in which such missing data are automatically handled by the computation procedure for repeated measures that are available in statistical software packages without losing statistical efficiency.
We will investigate whether or not post-randomisation variables differ between the intervention and control groups. Variables found to show a statistically significant difference (p < 0.05) between study groups will be included in multiple logistic regression analysis in which the treatment efficacy will be tested with adjustment for those variables. It is estimated that the multiple logistic regression analysis can easily incorporate the four expected covariates (use of emollients by the control group, breastfeeding, house dust mite reduction measures and probiotic use) without loss of statistical efficiency and power. If post-randomisation variables do not differ significantly between the study groups, then a simple chi-squared test will be used for testing the treatment efficacy.
Detailed descriptive analysis of adverse effects or side effects will be conducted. Information on treatment adherence will be summarised and possible impact on intervention effect in the primary and some secondary outcomes will be investigated when it becomes necessary.
To investigate possible interactive effects between different washing products and practices (such as use of bubble baths) with emollient on the outcomes, we shall first describe the types and percentage usage of different washing products and practices will be described, and compared between the two groups. A new covariate to reflect such usage, either as dichotomous measure or an ordinal category, would be derived based on findings of descriptive analysis. The derived measure will be included in a multivariate logistic model, together with the treatment indicator to see to what extent other wash product usage might impact on the effect size of the treatment effect.
In order to test the hypothesis that individuals with one or more filaggrin null mutations show the most significant reduction in AE incidence following intensive emollient therapy, we will undertake a planned subgroup analysis to examine the effect of filaggrin mutations on the primary outcome and some secondary outcomes in multivariate regression analysis. We shall use Stata/SE 11.2 (StataCorp LP, College Station, TX, USA) for the analyses.
Sample size
There are data available on which to base an accurate estimation of the control group event rate; however, data on which to base the expected effect size are much more limited. A modified ITT analysis (complete cases only) in our pilot study showed a 50% reduction [43.4% developed eczema in the control group compared with 21.8% in the emollient group (OR 0.36, 95% CI 0.16 to 0.84; p < 0.0001)].
We have opted to assume a fairly conservative expected control event rate of 30%, rather than the approximately 40% seen in our pilot study, as this is more in line with the published data in this high-risk population. Our anticipated effect size is lower than that observed in our pilot study for five reasons: (1) the shorter intervention period and close contacts with participants in the pilot study might have enhanced treatment concordance, which would not be maintained in a national study with a longer (12-month) duration of the intervention; (2) eczema was only assessed at 6 months in the pilot, which might have included more transient cases; (3) it is possible that the halving of eczema in the pilot study was simply due to a direct treatment effect of emollients concealing mild eczema, which would not occur in a longer-term assessment at 2 years of age, when daily emollient application ceased at 1 year; (4) short-term efficacy studies typically overestimate treatment effects when tested in large-scale national trials with more heterogeneous populations and less research team contact; and (5) our participants will come from a slightly more diverse socioeconomic and ethnic background than in our pilot study.
Based on a control event rate of 30% and a conservative expected relative reduction of 30%, that is, a rate of 21% in the treatment group, with 90% power and allowing for 20% attrition, a total sample size of 1282 babies is required (Table 11). The calculation was performed using Stata/SE 11.2.
| Control event rate (%) | Emollient event rate (%) | Relative reduction (%) | Absolute reduction (i.e. point difference) | Number per arm | Total | Total with 20% attrition |
|---|---|---|---|---|---|---|
| 25 | 21 | 16 | 4 | 2374 | 4748 | 5935 |
| 30 | 21 | 30 | 9 | 513 | 1026 | 1282 |
| 35 | 21 | 40 | 14 | 229 | 458 | 572 |
| 40 | 21 | 48 | 19 | 132 | 264 | 330 |
Patient and public involvement
Throughout the development of this proposal, we have taken advice from parents of children with eczema. We asked all parents for their feedback and opinion as part of the pilot study, as well as asking a smaller panel of parents and eczema support groups about specific design issues as the project has developed such as increasing the length of the intervention from 6 months to 1 year, including allergy tests and reducing the number of face-to-face contacts. We have also had input from the Centre of Evidence Based Dermatology patient panel in a workshop session dedicated to this project. Our parents have helped write the plain English summary for this application and will continue to play a vital role throughout the life cycle of this trial; we will include a parent on the Trial Steering Committee (TSC) and they will also help us to write and review any documents and advice that will be given to parents, as they did for the pilot study.
Ethical arrangements
Ethics
We do not anticipate any issue with obtaining ethical approval for this study; the pilot study, which was very similar in design, was approved with no major issues. Ethical and trust/primary care trust approvals will be obtained before the study commences. There are a number of exclusion criteria that need to be established before randomisation, which will necessitate checking the records of the infant and mother; consent will be obtained to do this.
Risks and anticipated benefits
Emollients are widely used in babies in children and are generally very safe. However, it is possible the child may have a local skin reaction to the emollient or that occlusion may lead to folliculitis or skin infections. Babies will have slippery skin for a short time after the emollient is applied so parents/carers need to take extra care when handling the baby at this time. Parents will be made aware of these risks in the participant information and during the consent process, and incidence will be monitored throughout the trial.
We cannot guarantee any benefit as we do not know if this intervention is effective.
Informed consent
Written informed consent will be obtained from one of the parents before any study related procedure is undertaken.
Research governance
The University of Nottingham will sponsor this trial, which will be run according to good clinical practice and the University SOPs. An independent TSC and Data Monitoring Committee (DMC) will oversee the trial as described in the application form.
Confidentiality of data
All participant data will be handled and stored in accordance with the University of Nottingham SOPs and the Data Protection Act. 218 Archiving of the data shall be for a minimum of 7 years after the date of any related publication.
Potential sources of bias
We have thought carefully about measures to reduce potential for bias in this randomised trial. Selection bias will be reduced by allocation concealment; a web-based randomisation system will be used into which all data needed for randomisation are irrevocably entered prior to randomisation. Both groups will receive equal contact to reduce performance bias. To minimise information bias, we will use an outcome assessor (research nurse) who is blinded to treatment allocation for the primary outcome measure. Attrition bias will be reduced by regular contacts with participants and by doing an ITT analysis. We will register the trial to prevent any selective outcome reporting bias and we shall prevent publication bias by guaranteeing that all results will be published in the public domain regardless of the direction of results.
Contamination of the control group is clearly a potential risk for this study and the effect would be to diminish the estimation of the true magnitude of potential benefit. In an individually randomised study, the patient information leaflet could, even if written in a neutral and balanced way, promote the perception in the control group that emollients from birth are ‘good’ for preventing eczema. However, our pilot study of 124 families has allowed us to assess potential sources of contamination. We were open and honest with parents and explained that we truly do not know whether or not emollients are beneficial. We showed that the number of parents in the control group who frequently applied emollients to their baby was very low (8%) and that it is possible to have a relatively clean control group in this type of trial. We plan to repeat the approaches that were successful in the pilot and to introduce additional measures to help mitigate against any potential contamination. We will actively monitor the rate and degree of contamination throughout the trial. As indicated in our stopping criteria, if emollient use in controls exceeds 25% at 6 months, we will discuss the implications with the Data Monitoring and Ethics Committee and if necessary stop the trial on their advice through the TSC.
Project timetable
The first 6 months will be for study set-up, then recruitment and intervention from months 7 to 30, follow-up from months 19 to 54, analysis and first report generation for year 2 outcomes at months 55 to 60. Longer-term eczema, asthma and allergic rhinitis questionnaire outcomes at 5 years and final report will be carried out at in months 61 to 96 (Table 12).
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | ||
|---|---|---|---|---|---|---|
| Set-up and approvals | ||||||
| Appoint staff and training | ||||||
| Recruitment and intervention phase | ||||||
| Follow-up, data collection and management | ||||||
| Analysis, write-up and dissemination | ||||||
Patient and public involvement
Patient and public involvement (PPI) was an absolutely key component for this work package, particularly in the pilot RCT work. We sought the opinion on the design of the pilot RCT from the National Eczema Society and from several members of the Centre of Evidence Based Dermatology patient panel in a dedicated workshop. The pilot RCT was designed to get essential advice from parents and carers with regard to the components and interventions to be included in a full-scale RCT. As described in more detail in the sections above, the key collaboration with patients and carers for this work package included allowing parents in the pilot RCT to choose between three types of emollients (an oil, a cream/gel formulation and a grease-based product) and establishing their reasons for the choice they made. A subsequent, more detailed, study with 20 parents was carried out to establish their preferences for different emollients and further advice on the design of the definitive RCT was sought from a panel of parents who had taken part in the pilot RCT.
Patient and public involvement was a key aspect in determining the following outcomes of this work programme.
-
Confirming the prioritisation of the topic of eczema prevention by the willingness and enthusiasm to participate in the pilot RCT, with good retention of participants and low usage of emollients in the control group.
-
Establishing that a cream/gel based emollient was widely accepted over other emollient types in a way that could be incorporated into the busy routine of looking after a growing infant.
-
Identifying that two emollients (Doublebase gel and Diprobase cream) were preferred over others in the parental preference study.
-
A smaller panel of parents who took part in the pilot study have advised the team on a number of changes from the pilot to the main Barrier Enhancement for Eczema Prevention (BEEP) RCT application:
-
increasing the length of the intervention from 6 months in the pilot to 1 year
-
including skin prick tests
-
reducing the number of face-to-face contacts with the nurses
-
helping with the lay summary for the main BEEP grant application.
-
Parents will continue to play a vital role throughout the life cycle of this trial; we will include a parent on the TSC and they will also help us to write and review any documents and advice that will be given to parents, as they did for the pilot study.
Summary and conclusions
Why is this research package important?
Too little attention has been paid to the possible prevention of eczema, which is so much more desirable than allocating large resources to treating children with established disease and using treatments that only provide symptomatic control. This work package has provided useful evidence to help inform parents and policy-makers when making decisions about eczema prevention. To date, none of the currently tested interventions has been shown to prevent eczema (with the possible exception of some strains of probiotics).
The work package has provided the research community with useful basic building blocks for identifying uncertainties and for designing and conducting prevention studies in eczema, which this research team is now taking forward. For example, in our systematic review of how incident (new) cases of eczema were defined, in the 102 studies that met our inclusion criteria, the disease was either not defined at all, or an inappropriate definition was used that required the disease to have been present for some time; by definition, such a case is not a new one. There were almost as many disease definitions as there were studies, making comparisons between studies almost impossible, thereby limiting the value of such research. The same mistakes were repeated time after time because previously nobody had systematically reviewed the area in its entirety in order to learn and progress.
Much research in the area of eczema prevention has been conducted in isolation and without taking heed of previous research as summarised in systematic reviews and their overviews. There is a great potential for progress on eczema prevention globally if there was greater international co-ordination in designing and registering all relevant primary and secondary research and learning from the errors observed in the past. This could be achieved without sacrificing the desirable independence of various research groups from different countries around the world.
Undertaking a pilot trial prior to embarking on a large scale RCT of emollients was absolutely essential. It was important to establish whether or not busy parents would wish to get involved in a study that required them apply emollients daily to apparently healthy babies for 6 months. Furthermore, there was concern that parents in the no-treatment control group would start applying emollients anyway because we had alerted them to the potential of emollients preventing eczema. It was also crucial to explore parental choices about emollients and to make sure that those chosen did actually do something to the skin barrier. As it turned out, our pilot RCT showed that parents were willing to participate in such a study, with good concordance with the intervention and with little evidence of contamination in the control group. Our surveys of parents identified two popular emollients which, when subsequently tested, showed satisfactory evidence of skin barrier enhancement, reducing the risk of testing ineffective interventions in the definitive study. The fact that the pilot RCT showed a halving in the risk of developing eczema at 6 months needs to be tempered with caution as the study was not powered to show clinical benefit and such a result may simply reflect a tendency of the emollients to mask very mild eczema. Nevertheless, the signal has provided a powerful boost to justifying a definitive study, which started in November 2014.
Implications for clinical practice
Many guidelines recommend practices for the prevention of eczema development in children. For example, the guidelines produced by the American Academy of Allergy, Asthma and Immunology219 recommend interventions, such as exclusive breastfeeding for the first 4–6 months and a hydrolysed formula milk rather than whole cow’s milk for those who are not breastfed. However, we did not find any strong evidence to support such a stance and some evidence indicates that such approaches may actually increase the risk of some allergic diseases such as peanut allergy. 220 Although it is always tempting to give proactive advice to parents with personal or family experience of allergic disease wishing to prevent eczema and associated allergies in a future child, our review suggests that there is no clear evidence that the commonly tried strategies, such as dietary interventions or avoidance of allergens, achieve this. Breastfeeding is clearly best for babies in terms of prevention of infections, nutrition and bonding, and mothers should be encouraged to initiate and sustain breastfeeding when possible. Those many mothers who cannot or do not wish to breastfeed should not feel pressure to do so because of the argument that breastfeeding reduces the risk of eczema, as the evidence does not support this. Parents should feel free to do what is best for their child in terms of breastfeeding, use of milk substitutes and introduction of solids, with appropriate HCP support.
Some evidence has emerged since our overview of prevention reviews, suggesting that administration of probiotics could prevent eczema. 221 However, most of these studies come from one group in Scandinavia and their generalisability to a UK setting remains unclear, especially with regard to the best strain of probiotics, whether or not they should be given during pregnancy as well as early life and for how long. Until new primary research in the UK clarifies the role of probiotics for preventing eczema, their use cannot be routinely recommended even though their adverse event profile appears relatively benign.
Implications for research
Systematic reviews of eczema prevention
Systematic reviews of eczema prevention continue to be done to a high standard by groups such as those working in The Cochrane Collaboration. Nonetheless, our overview of systematic reviews for the prevention of eczema should ideally be regularly updated so that HCPs, patients, policy-makers and guideline developers continue to have a document in which they can examine all of the commonly tried eczema prevention strategies. Those conducting systematic reviews and overviews of such reviews need to take heed of the large variation in the way dietary interventions, allergen avoidance and probiotics delivery can vary between studies. Care should be taken in judging whether or not such studies are sufficiently similar to combine in a meta-analysis. The ideal standard comparator in future systematic reviews should be normal care (no treatment). In the absence of a ‘no-treatment’ group, it is difficult to say whether or not a lack of significant difference between the effectiveness of dietary interventions is owing to neither being effective or both being effective. Systematic reviews need to use clear definitions of eczema and, when possible, undertake subgroup analyses for those with true atopy (i.e. eczema plus sensitisation to common allergens). Long-term outcomes, including disease severity, and other allergic diseases such as asthma need to be included as important outcomes in such systematic reviews. Furthermore, future systematic reviews on this topic should register the review plan on the Prospective Register of Systematic Reviews (PROSPERO) database222 in order to reduce unwanted duplication of research.
Future randomised controlled trials of eczema prevention
The key implications of the work reported here for future RCTs of eczema prevention are straightforward.
-
Ensure that the population targeted is clearly identified as being high risk (family history of allergic disease) or normal risk (general population).
-
Ensure that the intervention is sufficiently well described to permit others to replicate the study. The study should clearly define whether the intervention was given prenatally, postnatally or both.
-
The comparator for a prevention study should ideally be no treatment to clearly establish the effectiveness of any prevention strategy.
-
An incident case definition should not include lengthy disease chronicity as one of its diagnostic criteria.
-
Care should be taken in diagnosing eczema confidently in the first year of life because many transient forms of irritant and seborrhoeic eczema can occur in that period.
-
Outcomes for prevention studies should ideally be measured up to 5 years after birth as initial reductions in eczema incidence may be lost over time.
-
Eczema severity should also be measured in such studies in order to ensure that the fraction of eczema cases prevented are the clinically important ones.
International collaboration on a ‘family’ of eczema prevention studies
We encourage others working on eczema/allergic disease prevention studies to contact this team so that learning may be shared. This applies especially to interventions designed to prevent eczema through barrier enhancement from birth, so that common outcomes can be used to permit meta-analysis within the context of systematic reviews, or prospective meta-analysis if appropriate.
Future research recommendations
In addition to the specific lessons learned from reviewing the evidence of eczema prevention studies and the pilot work discussed above, we recommend four research topics in no particular order:
-
An overview of all eczema prevention systematic reviews should ideally be undertaken every 5 years so that policy can be informed by new research as it emerges.
-
A set of core outcome measures for all future eczema prevention studies should be agreed internationally.
-
Although we do not recommend further research into dietary interventions such as breastfeeding, avoidance of allergens and delayed introduction of solids, which have now been researched for 40 years with no clear evidence of benefit, there is plenty of scope for more independent studies of probiotics for prevention of eczema. These should clearly specify the strain used and allow separate analysis of whether or not administration in the prenatal period offers additional benefit to administration to infants in the postnatal period.
-
There are also many other important aspects of research needed in eczema prevention studies using emollients such as comparing more refined ‘designer’ skin barrier enhancers with cheaper petrolatum-based emollients, altering the duration and intensity of emollients in early life, combining emollients with additional recommendations on bathing and education within a complex intervention delivered to individual families or at a community level and, finally, conducting studies in both high-risk (families with allergic disease) and low-risk general populations.
Chapter 2 Eczema treatment work programme
Abstract
Introduction
Eczema [AE or atopic dermatitis (AD)] is the most common global cause of disability-adjusted life-years lost owing to non-fatal skin diseases.
Methods
We sought to add value to the field of eczema research by:
-
systematically reviewing all possible treatments of eczema in an overarching review223
-
developing a database of all eczema RCTs
-
developing core outcome sets for eczema trials
-
prioritising future research in partnership with patients
-
submitting an application for a new clinical trial.
Results
Our systematic review included 287 new RCTs in eight treatment categories. Our database of eczema RCTs contains > 500 trials displayed in a user-friendly searchable interface. Uses have included an evaluation of protocol registration and outcome reporting bias in eczema trials. Our core outcome set project identified the four domains of symptoms, signs, QoL and long-term control. The research priority setting partnership (PSP) identified 14 main uncertainties for future eczema research, including greater emphasis on non-pharmacological interventions.
Conclusion
This work programme has provided an international platform for mapping the evidence for eczema treatments and prioritising future research. Our application to the NIHR HTA programme for a national trial on specialised clothing for eczema has been successful.
Content
This chapter describes a range of complementary projects relating to the treatment of eczema, for which some navigation may be helpful for the reader.
As details of the epidemiology, prevalence and impact of eczema have been described in the earlier chapter of eczema prevention (see Chapter 1), these details have not been included again here.
| Details | Comment |
|---|---|
| Scoping systematic review of eczema treatments | This chapter provides an overview of a large overarching scoping systematic review of all eczema RCTs and systematic reviews. The full report is published as a separate report223 |
| The Global Resource of EczemA Trials database | Describes the development of an online, searchable database that includes all RCTs and systematic reviews of eczema treatments. This resource is intended to reduce duplication of effort in searching for research evidence and should be particularly helpful for systematic reviewers and guideline writers |
| Validation of the Global Resource of EczemA Trials database | Summary of validation work to establish the sensitivity and specificity of the GREAT database for identifying all relevant evidence |
| Systematic review of selective outcome reporting in eczema trials | A study looking at the difference between outcomes reported in trials summarised in the GREAT database compared with outcomes listed in trial protocols |
| Harmonising Outcome Measures for Eczema initiative | An international initiative to establish a core outcome set for use in future eczema trials |
| Eczema priority setting partnership | A James Lind Alliance PSP to establish the most important treatment uncertainties as identified by patients and clinicians |
| CLOTHing for the relief of Eczema Symptoms (CLOTHES) trial: a study proposal | Proposal for a multicentre RCT of silk therapeutic clothing for the management of eczema. A funding application to the NIHR HTA funding stream |
| Patient and public involvement | A summary of the input made by patients and how this effected the work within this chapter |
| Summary and conclusions |
Scoping systematic review of eczema treatments
The following text is adapted from Hoare et al. 224 under the UK government’s non-commercial licence for public sector information.
Summary
What was already known about this topic?
-
There have been many systematic reviews of eczema treatments published, mostly for single interventions, as well as an NIHR HTA systematic review of eczema treatments in 2000. 224
-
Since 2000, there has been no similar overarching systematic review of eczema treatments.
What did this study add?
-
An up-to-date summary of the RCT evidence for or against eczema treatments.
-
Clear signposting of eczema treatments which require more research, with specific recommendations about future research and how this could be prioritised.
-
An overview of the quality of reporting and risk of bias in eczema treatment trial reports.
Introduction
Eczema, also referred to as ‘atopic eczema’ or ‘atopic dermatitis’, is a chronic inflammatory skin condition characterised by an itchy red rash. Eczema affects around 20% of UK children and 5% of UK adults and its prevalence is increasing. Eczema is a complex disease caused by a combination of genetic and environmental influences.
Objectives
This review aimed to scope and summarise current RCTs of eczema to inform evidence-based clinical practice and to identify possible research gaps for the future. The review is an update of a similar review published in 2000 by the NIHR HTA programme. The new information in this update places current treatment options in the context of best-quality evidence. The review was conducted as part of this NIHR Programme Grants for Applied Research award (RP-PG-0407-10177) and is published in full as a separate report. 223
Methods
Only RCTs of treatments for eczema were included, as other forms of evidence are associated with higher risks of bias. In order to be included in this review, the following key elements were required: a full report of the trial; availability of data relevant to the therapeutic management of eczema; mention of randomisation; comparison of two or more treatments in people; and data that had been collected prospectively. Participants of all ages were included. Diagnosis of eczema could be according to published diagnostic criteria, or as diagnosed by a clinician.
Outcomes
The outcomes assessed included change in patient-rated symptoms, global severity as rated by patients or physicians, change in composite rating scales (both named and unnamed), QoL and adverse events.
The following electronic databases were searched: MEDLINE (end of 1999 to 31 August 2013); EMBASE (end of 1999 to 31 August 2013); The Cochrane Controlled Trials Register and the Cochrane Skin Group Specialised Trials Register; Latin American and Caribbean Health Sciences (LILACS) database; Allied and Complementary Medicine Database (AMED); and Cumulative Index to Nursing and Allied Health Literature (CINAHL). Disease terms for AE (as a text word and medical subject heading term, if possible) were combined with a search for RCTs. A manual filtering process was undertaken to assess whether or not a reference fitted the review’s inclusion criteria. When doubt existed from the abstract or title, the full paper was requested and excluded studies were recorded by one reviewer and checked by a second reviewer in case of uncertainty. All papers were catalogued on an Endnote database (X6; Thomson Reuters, CA, USA).
There were no language restrictions; non-English papers were screened for eligibility by international colleagues and data were fully abstracted if eligible.
Results
Main findings
In addition to the 254 RCTs identified in the original 2000 review, this update includes an additional 287 new RCTs, making 541 RCTs in total covering 92 different interventions for treating AE.
Trial reporting was generally poor, with ‘unclear’ categories dominating the assessments: randomisation method (2% high, 36% low and 62% unclear risk of bias), allocation concealment (3% high, 15% low and 82% unclear risk of bias) and blinding or masking of the intervention (17% high, 26% low and 57% unclear risk of bias). Only 22 (8%) of trials were considered to be at low risk of bias for all three quality criterion.
Fourteen interventions or treatment approaches were felt to have reasonable evidence of benefit. These include the use of topical corticosteroids and topical calcineurin inhibitors, both for the treatment of active AE, and as intermittent proactive (maintenance) therapy for the prevention of AE flares. Other interventions including AtopiclairTM emollient, ultraviolet (UV) light therapy, azathioprine and ciclosporin (Neoral®, Novartis Pharmaceuticals), all had reasonable evidence of benefit compared with placebo/vehicle. Similarly, RCT evidence suggested that education may be beneficial, although the exact components of a successful programme are unclear.
There was evidence of no clinically useful benefit for the following treatment comparisons: topical corticosteroids containing antibiotics for non-infected eczema; emollient with furfuryl palmitate in children with unspecified eczema severity; cipamfylline cream, in adults with eczema on the arms of unspecified severity; Mycobacterium vaccae vaccine in children with moderate to severe eczema; probiotics for treating established eczema in children whose disease severity was not clearly described; ion exchange water softening devices, in children with moderate to severe eczema; and dietary supplements rich in linoleic acid, such as evening primrose oil and borage oil in children and adults with eczema of unspecified severity.
The trial evidence was not clear enough to make recommendations for the use of emollients to reduce severity of eczema and prevent flares or to reduce the need for other eczema treatments; topical corticosteroids in combination with antibiotics for infected eczema; wet wraps on top of topical corticosteroids; antiseptic bath additives; topical antifungals; other topical treatments such as WBI-1001 cream; topical coal tar; topical vitamin B12; vitreoscilla filiformis lysate cream; oral treatments including antihistamines, prednisolone; methotrexate; montelukast; mycophenolate mofetil; immunotherapy (desensitisation); omalizumab; mepolizumab; oral pimecrolimus; oral naltrexone; autologous blood therapy; tandospirone citrate; full spectrum light therapy; excimer laser; intravenous immunoglobulin, specialised clothing (silk or synthetic fibres with or without antibiotics); environmental interventions such as house dust mite reduction; staying in a different climate; different approaches to organisation of care such as additional visits to the doctors or nurse led clinics; support groups; E-Health management; prebiotics, dietary restrictions and synbiotics; complementary therapies such as Chinese herbal treatment; hypnotherapy; massage therapy; aromatherapy; acupuncture; acupressure; other herbal treatments; psychological therapies such as stress reduction techniques and biofeedback; and balneotherapy (salt baths).
There was a complete absence of RCT evidence for dilution of topical corticosteroids, order of application of topical corticosteroids and emollients, impregnated bandages (zinc or ichthammol paste bandages), modified bathing habits (non-antiseptic bath additives, soap avoidance, frequency of bathing) and the role of routine allergy testing.
Changes in the evidence base since the previous review in 2000
Topical calcineurin inhibitors, educational interventions, oral azathioprine and Atopiclair emollient have entered the category of ‘reasonable evidence of benefit’ since the previous review in 2000.
Some interventions have now been tested sufficiently to suggest that they are not clinically useful. These include topical corticosteroids containing antibiotics for eczema that is not overtly infected, probiotics, ion-exchange water softeners and supplements rich in linoleic acid.
As in the previous review, many dietary, non-pharmacological, complementary and other topical or systemic interventions have been investigated in small and generally poorly reported trials, resulting in inconclusive findings.
Clinical relevance of the new evidence
Patients and setting
The population of eczema patients included in the published trials is generally skewed towards patients with moderate or severe disease, as most of the included trials recruited participants through secondary care. For some interventions, such as systemic treatments and light therapy, this may be appropriate. However, for the more commonly used topical interventions such as emollients, topical corticosteroids and bath products, it is important to also test the interventions in patients with mild disease in a primary care setting in order to improve the external validity of trial evidence.
There has been some improvement in the length of RCTs, with many trials of topical corticosteroids and calcineurin inhibitors lasting 6 months to 1 year. Pharmaceutical companies tend to perform placebo-controlled studies, but unfortunately these do not give information on how new treatments compare with existing treatments. For example, topical tacrolimus (Protopic®; Astellas Pharma) and pimecrolimus have now been tested in a total of 30 placebo controlled studies, the ethics of which is questionable. However, it is encouraging that some trials are now using ‘standard care’ as a control intervention, which will make it easier to assess the clinical relevance of the study results.
Outcomes
There has been a modest improvement in the number of trials, including patient-reported outcome measures (PROMs), although the results were often not well reported. The move towards more trials using the same core outcome sets as encouraged by the HOME initiative (www.homeforeczema.org)225 can only be beneficial for future clinical interpretation and evidence syntheses.
Limitations of this review
Although there is always a chance that a mainly electronic search of reference databases will miss certain RCTs owing to misclassification, we have attempted to mitigate this risk by searching both the main bibliographic databases (MEDLINE and EMBASE) and several smaller, specialist, databases (CINAHL, AMED and LILACS). Blinding of the review team to the authors of the trials was not practically possible, which may have resulted in bias when summarising qualitative aspects of the results. However, we used a standardised approach to summarising the data and in the assessment of risk of bias, and we attempted to make clear distinctions between what the studies found and our own interpretation of study findings. Given the very wide scope of this review and heterogeneous nature of participants, interventions and outcomes, it has not been possible to undertake detailed meta-analysis for single interventions. These will hopefully be conducted in much narrower intervention-specific Cochrane systematic reviews. As with all systematic reviews, the evidence presented will become out of date quite rapidly for some topics and readers are directed to our Global Resource of EczemA Trials (GREAT) database226 for newly published RCTs of eczema.
The decision to include only RCTs could imply that there is no research at all for treatments without RCTs. This is, of course, not the case and many of the treatments that are lacking in RCT evidence have been studied using uncontrolled designs. Rare treatment adverse effects could also have been missed.
Our classification of treatment options into categories such as ‘evidence of benefit to support’ is not the same as a positive recommendation for widespread use or otherwise, as that is the remit of guideline developers and depends on factors such as magnitude of benefit, adverse effects, how the treatment compares with existing active treatments, as well as factors such as availability and cost.
Discussion
Implications for research
Primary research
Although not unique to eczema, perhaps the biggest priority for future research is to better understand why researchers across the world continue to conduct small, poorly planned, unregistered and poorly reported trials. In addition, there is a lack of clinical trials conducted in a primary care setting, where most patients are seen, and the questions being investigated all too often fail to reflect the most pressing questions for clinicians and patients.
Our recent James Lind Alliance PSP (see Eczema priority setting partnership) identified the most important treatment uncertainties as judged by patients and clinicians. When set in the context of the updated evidence base from the review, the following areas identified from the PSP seem to be most pressing.
Priority areas with no current RCT evidence are:
-
What role might allergy tests play in treating AE?
-
What is the best way for people with AE to wash?
-
Which should be applied first when treating AE – emollients or topical corticosteroids? Priority areas with limited RCT evidence.
-
What is the best and safest way of using topical corticosteroids for AE?
-
What is the long-term safety of applying topical steroids to the skin for AE?
-
Which emollient is the most effective and safe in treating AE?
-
What is the best psychological treatment for itching/scratching in AE?
-
What are the best and safest ‘natural’ products to apply to the skin?
-
How much does avoidance of irritants and allergens help people with AE?
-
What is the role of diet in treating AE (exclusion diets and nutritional supplements)?
-
Which is more effective in the management of AE: education programmes, GP care, nurse-led care or dermatology-led care of multidisciplinary teams?
-
Which is safer and more effective in treating AE: topical corticosteroids or calcineurin inhibitors (especially for proactive flare prevention)?
-
How effective are interventions to reduce skin infections in the management of AE?
-
What is the best and safest way of using drugs that suppress the immune system (particularly in children)?
Nevertheless, some important topics have already been picked up by NIHR funding bodies and large pragmatic trials are currently under way in the UK evaluating the role of topical and oral antibiotics for the treatment of infected eczema [Children with Eczema Antibiotic Management (CREAM)] study [UK Clinical Research Network (UKCRN) ID 11233], silk clothing for the management of moderate to severe eczema (UKCRN ID 15132) and the role of bath emollients in the management of eczema [Bath Additives in the Treatment of Childhood Eczema (BATHE)227].
Secondary research
Several Cochrane reviews of eczema have either been completed or are in progress, which will provide a more detailed and in-depth analysis of specific interventions. 223 Overviews of existing systematic reviews are also needed, as is the application of mixed treatment comparisons for understanding more about the relative value of treatments that have not yet been compared in head-to-head trials.
Methodological research
The greatest methodological challenge is in the field of outcome measures. Despite significant progress from international consensus in identifying the four core outcome domains of symptoms, clinical signs, QoL and long-term control, there is much remaining work to be done in identifying and developing appropriate instruments for these domains and for establishing suitable tools for use in routine clinical practice.
Implications for health care
The evidence base of RCTs for eczema has accelerated since the HTA systematic review conducted in 2000 and many commissioners, guideline developers, HCPs and patients can now refer to this report for a rapid summary of relevant evidence to guide everyday decisions in the treatment of eczema. In addition to the established approach for treating eczema flares with topical corticosteroids, evidence supports the value of a proactive approach for maintaining eczema remission, through the use of twice weekly topical corticosteroids, topical tacrolimus or pimecrolimus. Educational approaches have also emerged as a potentially promising intervention, although further work is needed to establish the most important components of the intervention and the most cost-effective ways of delivering education in different health settings.
Equally important is the understanding that some interventions now have sufficient evidence to suggest little or no benefit for eczema patients. These include the use of topical corticosteroids containing antibiotics when used for the management of non-infected eczema, probiotics, ion-exchange water softeners and supplements rich in linoleic acid (e.g. borage oil, evening primrose oil).
The Global Resource of EczemA Trials database
The following text is adapted from Nankervis et al. 228 © 2011 Nankervis et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Summary
What was already known about this topic?
-
Searching for RCTs is a lengthy and detailed process that is repeated by researchers around the world and uses up research time.
-
There are hundreds of RCTs on eczema treatment across many different journals in many different languages.
What did this study add?
-
A freely available online database holding key information on RCTs of eczema treatments, including interventions compared, numbers randomised, inclusion and exclusion criteria, and outcomes.
-
The ability to browse the GREAT database by treatment category or treatment and search specifically using keywords across the whole database, or within a specific field.
-
Provision of a resource that can prevent duplication of effort in searching for RCTs and systematic reviews of eczema treatments. The GREAT database will be particularly useful for people writing clinical guidelines or patient information resources, systematic reviewers and health researchers interested in eczema treatments, and for HCPs and patients seeking easy access to research evidence.
Introduction
Eczema research is conducted all over the world and > 70 systematic reviews have been published on treatments for eczema, covering a large number of eczema treatment areas. Although the coverage and breadth of topic areas in these systematic reviews is encouraging, some interventions such as full spectrum light therapy and excimer laser (Talos®, Wavelight Laser Technology AG, Erlangen, Germany) treatment are currently not included in any form of systematic review, and many existing systematic reviews are out of date. 229
Searching for published RCTs and systematic reviews can be a laborious task involving the examination of hundreds and sometimes thousands of individual references online followed by scrutiny of full-paper copies of studies and their associated citation lists in order to obtain a few papers of interest. 230 Often the use of a very sensitive search strategy is necessary in order to be as inclusive as possible, as is the case when conducting a Cochrane systematic review. 231 The more sensitive the search, the more complete the research; however, this adds to the number of references which need to be manually filtered, thereby increasing the time taken to complete a project.
A resource that brings together all RCTs on eczema treatment using a highly sensitive, comprehensive search has the potential to significantly shorten the length of many eczema research projects. Just as some countries such as the UK host national reference collections of entities such as plant species in the National Collection of Dahlias, our idea is to host a similar reference collection of eczema RCTs that is freely accessible, comprehensive, easily searched and complete that can be used for international eczema research, in the hope that it will lead to improvement in patient care.
The GREAT database brings together information on all RCTs of eczema treatments published. The database currently holds records of all RCTs published and indexed up to August 2013 and will be updated on an ongoing basis.
Methods
Construction and content
To be included in the GREAT database, a trial has to fulfil the following broad criteria.
-
The trial report must include a clear mention of randomisation.
-
The participants must have established eczema either diagnosed by a clinician or using the Hanifin and Rajka criteria232 or the UK Working Party criteria,131 or criteria very close to these.
-
There must be prospective allocation of people with eczema to two or more interventions.
-
The trial must measure at least one efficacy outcome.
Search strategy
To ensure that as many RCTs as possible were identified, we searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library and the Cochrane Skin Group Specialised Register, which includes hand-searching of dermatology conference proceedings, as well as MEDLINE (via Ovid), EMBASE (via Ovid) (see Appendix 4), LILACS, AMED and CINAHL databases from the beginning of 2000 to the end of 2010.
The searches for MEDLINE and EMBASE were based on the Cochrane highly sensitive search strategy for RCTs,231 combined with a list of known terms for eczema, intended to be as comprehensive as possible.
Results
The GREAT database was constructed with ease of use as its primary objective. The structure of the database allows the user as much, or as little, specificity as they wish. If a particular treatment area (such as non-pharmacological treatments) is of interest, there is a browse facility in which treatments and treatment categories are listed and linked to all appropriate trials.
Clicking on a treatment category brings up a list of all treatments in the category and clicking on one of the treatments brings up a list of all trials in which that treatment is reported. Selecting a trial will give access to the trial information (Figure 9). If a particular trial or other details, such as a specified outcome measure, is of interest the search page allows the trial details to be accessed directly using a term of interest, such as ‘double blind’ and quickly have access to a list of trials whose records contain the term (Figure 10). For each trial, the database gives the citation for the published report and where possible a link out to the PubMed or journal record for the trial.
FIGURE 9.
The GREAT database hierarchy is available on drop-down menus. © University of Nottingham and NUH NHS Trust 2013. Reproduced with permission. 226
FIGURE 10.
The GREAT database search facility. © University of Nottingham and NUH NHS Trust 2013. Reproduced with permission. 226
For trials from 2000 onwards, the database provides information on the trial in a clear and simple format. There is basic information about the interventions, study design and methodology (such as use of blinding and ITT methods), number of participants randomised, withdrawals, outcomes, authors’ conclusions, sponsorship, conflicts of interest and quality of reporting (Figure 11).
FIGURE 11.
The GREAT database: individual trial information. © University of Nottingham and NUH NHS Trust 2013. Reproduced with permission. 226

All levels of specificity are catered for: a search for all trials published within certain years, using a truncation (*) of a word for multiple possible terms (e.g. blind* to retrieve blind, blinding, blinded). If it is common for a term to be spelled more than one way, then a ‘wildcard’ (?) can be used in place of the part of the word that may be different (e.g. ?dema for oedema or oedema). It is also possible to do more complicated searches using the ‘OR’ and ‘AND’ terms and by restricting the search to a certain field or group of fields for one or more terms.
The GREAT database website scores 93% for accessibility using the LIDA instrument, a tool which assesses accessibility by looking at page setup, access restrictions, amount of outdated code and compatibility with NHS directives. 83 The website is already being accessed in the UK as well as around the world in countries such as Sudan, Germany, Ireland, USA, Poland, France and Spain. 233
Discussion
The GREAT database has been created to speed up eczema research. The collective time saved by research groups around the world can now be used to make strides in optimising the treatment of eczema, in order to further benefit people with eczema. This up-to-date resource fills a gap which no other resource covers in such detail. The information provided aims to allow users of the GREAT database to quickly and easily decide if the trial is of interest to them. The complete citation(s) and link(s) to PubMed, if possible, for each trial are given to facilitate obtaining the full paper for further scrutiny.
The database will also result in health gains, as knowledge locked in obscure RCTs is liberated freely to HCPs working in clinic, or indeed to patients who wish to investigate the evidence for a particular treatment. The database will continue to be expanded in order to secure its place as the number one resource of RCTs on eczema treatment both now and in the future. The only other database that contains records of RCTs of eczema (as well as RCTs on all other medical subjects) and is updated regularly is Clinical Trials. The Clinical Trials database gives details of RCTs and controlled clinical trials on eczema treatment taken from MEDLINE, EMBASE and other databases along with the Cochrane Groups’ specialised registers, which include citations found by hand searching. The Cochrane Skin Group Specialised Register has been collecting full paper copies of controlled clinical trials and RCTs for 15 years.
The information given on each trial in Clinical Trials often includes the abstract and may allow the user to retrieve the full paper. The Clinical Trials database gives a very thorough list of published and unpublished reports of trials and is useful for those interested in controlled clinical trials as well as RCTs, but it does not give any extracted information. By comparison, the GREAT database gives enough information to usually save having to retrieve the full paper for every single eczema trial. In addition, if only interested in eczema, skills in database searching would be required to be sure to obtain a specific and fully comprehensive list of only eczema trials from the Clinical Trials database.
Possible future uses of the GREAT database
For those in need of instant access to published RCTs on eczema treatments, the GREAT database now meets this need. For trials published prior to 2000, when extracted data are not available in the GREAT database record, help is at hand from the NIHR HTA systematic review on treatments for eczema. 224 This provides full citations with some relevant and useful information. There is, however, still significant effort involved in wading through this document for each trial, with no facility to quickly search for your area or trial of interest. One way to extend the usefulness of this resource in the future will be to provide extracted information for RCTs before 2000 through the GREAT database.
The GREAT database would quickly lose some of its usefulness if there were no subsequent searches added. Although there will be regular update searches for the GREAT database until late 2014, it is the aspiration of the Centre of Evidence Based Dermatology to continue the work of keeping the information in the database up to date past this point and well into the future. This will encourage and facilitate other research groups around the world to prepare and maintain similar international trial collections for other important skin diseases such as psoriasis, melanoma and vitiligo.
Validation of the Global Resource of EczemA Trials database
The following text is adapted from Nankervis et al. 234 © Nankervis et al. ; licensee BioMed Central. 2015. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Summary
What was already known about this topic?
-
The GREAT database holds detailed information about RCTs of eczema treatments.
What did this study add?
-
The GREAT database has a fairly high level of sensitivity.
-
The sensitivity of the GREAT database can be improved further, most probably through dual-filtering of references.
-
The GREAT database is a valuable resource for the eczema community, although researchers conducting specific systematic reviews will still need to employ hand-searching techniques and direct contact with triallists for unpublished data.
Introduction
The GREAT database is a collection of records for RCTs of eczema treatment produced from a highly sensitive search of six reference databases. 228 The aim of this database is to save research time by:
-
creating only one database to search for trials of eczema treatment
-
providing enough pertinent information about each trial to allow systematic reviewers and guideline writers to decide whether or not the trial fits their inclusion criteria without having to obtain and scrutinise the full papers for all possible trials themselves.
If the potential users of the GREAT database are not convinced that it will provide a comprehensive list of all eczema trials, then they are far less likely to use it as their main database and will conduct their own searches. This will not save research time or help to prevent research wastage as was the original intention.
In order to encourage potential users of the GREAT database to use this resource instead of searching bibliographic databases for themselves, an evaluation of the GREAT database is needed. This study is a validation of the GREAT database compared with the searches done for Cochrane and non-Cochrane systematic reviews.
Objectives
The objective of this study was to assess the sensitivity of the GREAT database in comparison with the studies found in published systematic reviews of eczema treatment. Sensitivity is defined as the number of relevant RCTs identified in the GREAT database, divided by the total number of relevant RCTs in the included systematic reviews.
Methods
All methods described below were undertaken by two authors (HN and AD or ED), independently, with any discrepancies being resolved by another author acting as an arbiter (JI).
As there is no obvious ‘gold standard’ resource of trials of eczema treatments, systematic reviews were considered the ‘gold standard’ and the GREAT database was the ‘comparator’.
It was decided, a priori, to restrict the comparison of the GREAT database to being against all published Cochrane reviews and five non-Cochrane reviews of eczema treatments. 235–239 The latter were those with the most recent search end dates. Reviews also had to have a full list of citations for the included studies and sufficient details of the review’s inclusion criteria to allow a fair comparison with the GREAT database.
Identifying systematic reviews of eczema treatments
The mapping of systematic reviews of AE housed at the Centre of Evidence Based Dermatology240,241 has records of reviews from 2000 onwards. This map of systematic reviews was mined independently by two authors for all Cochrane systematic reviews and non-Cochrane reviews involving eczema treatments. Any disagreements about which systematic reviews to include as per the pre-specified inclusion criteria were resolved by the arbiter before the list was finalised.
Identifying included and excluded trials from systematic reviews
Two authors independently scrutinised the included systematic reviews to obtain the list of citations of included studies. Any disagreements between the two authors about the included and excluded studies were resolved by the arbiter. Excluded studies were defined as those cited as such in the review text or a table(s). Any trials not in English and not in the GREAT database had the appropriate data extracted by a researcher with the required language skills, if further information was required.
Identifying trials from the Global Resource of EczemA Trials database
Two authors independently attempted to find all the included and excluded trials previously identified from the systematic reviews, using the search facility and exploration of the GREAT database via the browse and filter facilities. When an included or excluded trial was not found in the GREAT database, the reasons for this were explored and recorded.
The reported inclusion criteria for each systematic review were used to identify any additional trials in the GREAT database that could have been eligible for inclusion in the review, but were not in the list of included studies of that review. When a trial was identified in the GREAT database that was not in the included studies list of one or more of the systematic reviews the reasons for this were explored, consulting the full trial paper and GREAT if necessary. Any disagreements between the two author’s results were resolved by the independent arbiter (JI) before the results were analysed.
Results
Summary of results
A search of the mapping of eczema systematic reviews240 revealed 59 eczema treatment systematic reviews published since 2000. Of these, six were Cochrane reviews242–247 and were included in the study. Five non-Cochrane systematic reviews235–239 with the most recent search end date and which matched all other inclusion criteria were included in the study and 48 were excluded. Details of the excluded reviews are available in the map of systematic reviews of AE. 240
The six Cochrane reviews included 72 RCTs on eczema treatments and no other studies. All of the reviews searched The Cochrane Library, MEDLINE, and EMBASE; three searched the AMED;243,244,246 one searched the CINHAL;246 two searched LILACS;243,244 three searched PsycINFO;243–245 and two searched the ISI Web of Science. 243,244 Four242,243,245,246 of the six reviews searched various other databases and repositories such as ongoing trials databases, the Food and Drug Administration, European Medicines Agency, and Chinese databases. The Cochrane reviews included in this study covered topical calcineurin inhibitors, antibacterials, dietary interventions, Chinese herbal medicine, psychological and educational interventions and probiotics.
The five non-Cochrane reviews included 33 RCTs on eczema treatments out of a total of 85 studies that included RCTS, cohort studies and controlled trials on other dermatoses. Four reviews searched The Cochrane Library,235–237,239 four searched MEDLINE,235–238 three searched EMBASE,235–237 one searched Current Contents,238 one searched HomInform238 and two searched PubMed. 236,238 Four235,237–239 out of five of the reviews undertook manual searching of the reference lists of included trials, reviews and other sources such as textbooks and guidelines. The non-Cochrane reviews included in this study covered homeopathy, azathioprine, topical calcineurin inhibitors and topical corticosteroids.
Assessment of the trials missing from the GREAT database
The GREAT database did not contain all the included trials for four237,242–244 of the 11235–239,242–247 included systematic reviews. There were 17 out of 105 trials in total (Table 13) that were not found in the GREAT database. The reasons for this are discussed below.
| Review | Included trials | Included trials that could be found in the GREAT database | Reasons for trials missing from the GREAT database | Sensitivity of the GREAT database | |
|---|---|---|---|---|---|
| Captured trials (%) | Captured trials plus those missed in error (%) | ||||
| Cochrane reviews | |||||
| Ashcroft et al. 2007242 | 31 | 23/31 | Unpublished trial records obtained from a pharmaceutical company (n = 5). A report of the same trial was present, but the citation for the included study was not (n = 2). Not included in the GREAT database in error (n = 1) | 72 | 90 |
| Bath-Hextall et al. 2008247 | 9 | 9/9 | No trials missing from the GREAT database | 100 | 100 |
| Birnie et al. 2008243 | 21 | 16/21 | Randomisation not explicitly reported and other exclusion criteria fulfilled (n = 3). Trial was not aiming to ‘treat’ the eczema, only reduce bacterial numbers (n = 1). Not included in the GREAT database in error (n = 1) | 76 | 95 |
| Boyle et al. 2008244 | 12 | 11/12 | Not included in the GREAT database in error (n = 1) | 92 | 92 |
| Ersser et al. 2007245 | 5 | 5/5 | No trials missing from the GREAT database | 100 | 100 |
| Zhang et al. 2005246 | 4 | 4/4 | No trials missing from the GREAT database | 100 | 100 |
| Total | 72 | 58 | 14 trials missing the GREAT database | 81 | 93 |
| Non-Cochrane reviews | |||||
| Schram et al. 2011235 | 2 | 2/2 | No trials missing from the GREAT database | 100 | 100 |
| Yin et al. 2011236 | 4 | 4/4 | No trials missing from the GREAT database | 100 | 100 |
| Svensson et al. 2011237 | 17 | 14/17 | Two Japanese Phase II RCTs which fit GREAT inclusion criteria reported only within a review, omitted from the GREAT database in error (n = 2) Abstract which did not present information about results (n = 1) |
82 | 82 |
| Simonart et al. 2011238 | 1 | 1/1 | No trials missing from the GREAT database | 100 | 100 |
| Schmitt et al. 2011239 | 9 | 9/9 | No trials missing from the GREAT database | 100 | 100 |
| Totals | 33 | 30 | 3/33 trials missing from the GREAT database | 91 | 91 |
| Overall totals | 105 | 88 | 17/105 trials missing from the GREAT database | 84 | 93 |
Three trials248–250 did not fit the GREAT database inclusion criteria primarily owing to not giving an adequate description of randomisation having occurred.
One of the trials did not fulfil the GREAT inclusion criteria as the trial was not aiming to treat eczema, only to try to reduce bacterial numbers. 251
One of the trials was not included in the GREAT database as it has only been published as a conference abstract, which did not report results from the trial. 252 Although abstracts of trials are added to the GREAT database if there is no full trial report found, this abstract was not added as it was not reporting the completed trial results.
Five trials identified through a pharmaceutical company trials database253 as being unpublished did not contain enough information in the trial records provided to be certain whether or not these trials appear in the GREAT database (Novartis Pharmaceuticals Corporation, Basel, Switzerland, 2005 and 2006).
Seven trials out of 88 (8%) were identified from systematic reviews and fitted the GREAT inclusion criteria, but were not present in the GREAT database. 252,254–258 Two trials were included in a review paper,257 two were additional citations for a trial that was already included in the GREAT database,254,255 and one252 was published as a conference abstract and did not report trial results.
Trials found in the Global Resource of EczemA Trials database that potentially should have been included in systematic reviews
For one of the included Cochrane reviews on antistaphylococcal interventions,243 three trials233,259,260 were identified using the GREAT database that do not appear as included studies or on the excluded studies list for the review. This study’s authors are reasonably sure from the trial reports and the reported inclusion criteria of the review, that the three trials should have been included in this review. It is possible that the review authors managed to gather additional information about these trials which resulted in them being excluded; however, as the trials are not listed as excluded studies when they were so close to inclusion, this is less likely.
Strengths and weaknesses
Using published systematic reviews to validate the database helps potential users to make informed decisions for ‘real world’ uses of the GREAT database. The disadvantage with comparing systematic reviews against the GREAT database is apparent when the inclusion criteria of the review and the database differ, leading to an apparent lowering of the sensitivity of the database.
Many treatments that the GREAT database covers have not been assessed at all during this validation as only a selection of systematic reviews were used. It is clear from the results of this validation work that the sensitivity of the database differs depending on the treatment being reviewed.
Reviewing the GREAT database against all the Cochrane eczema treatment systematic reviews available has ensured that a complete comparison of the reviews with a very rigorous methodology. The main weakness with this comparison is that authors of Cochrane reviews are expected to contact trial authors for information not present in the trial report. The GREAT database methodology does not call for this to happen owing to practical constraints such as time.
Discussion
Six trials that were included in systematic reviews were not included in the GREAT database as they had not been published. For all eczema trials to be included in the GREAT database, they must be published. Efforts to get all clinical trials registered and published, such as the all trials campaign,261 are helping to bring this aim forward. When adequate information about trials was available, 5 out of 105 (5%) trials had been missed owing to errors in the current searching and filtering processes for the GREAT database (Table 14). This validation study identified two trials254,255 as missing, but these were actually dual publications for a trial that was included in the GREAT database under a different citation. This is something that will need to be addressed in the future if confidence in the GREAT database is to be secured. Greater efforts will be made in future to link all reports and abstracts from the same trial to the same trial record, in order to prevent bias being introduced by multiple reporting of the same trial data.
| Details | Number of trials, n/N (%) |
|---|---|
| Proportion of trials missing from the GREAT database | 17/105 (16) |
| Proportion of trials missing in error from the GREAT database (two of these were included, but under a different citation) | 7/105 (7) |
| Proportion of trials truly missing from the GREAT database in error (two of these were only cited in a review paper)a | 5/105 (5) |
Five of the missing trials248–252 most likely do not appear in the GREAT database owing to differences between the inclusion criteria of the review and the GREAT database, or their interpretation. The inclusion criteria for the GREAT database are broad and were designed to encompass all the RCTs of eczema treatment in relation to clinical benefits and harms.
There is room to improve the validity of the database by giving a more detailed explanation of the way in which the inclusion criteria are interpreted. Although only a small sample of systematic reviews were used for this validation, research into whether or not the current level of detail about inclusion criteria reported in systematic reviews allows for an adequate replication of the review by others could assist future research.
Conclusions
Implications for the future development of the Global Resource of EczemA Trials database
One of the most obvious areas for improvement of the database highlighted by the validation is dual-filtering of references in order to reduce errors of omission of eczema treatment RCTs. This is when two authors independently assess each reference against the inclusion criteria and then any references for which the two authors disagree are resolved, involving a third author if necessary. Dual-filtering has recently become part of the GREAT database methodology and should further improve the sensitivity of the database.
When relevant additional research questions are being addressed, such as bacterial load, then the GREAT database does not currently cater for these. Other possible additions to the GREAT database could be the interrogation of unpublished trials databases, searching of additional specialist databases, such as PsycINFO or Chinese databases, or adding all citations found for each trial, including leaving citations for conference abstracts in the database. Widening the inclusion criteria of the GREAT database beyond its current scope and whether or not to add to the process of searching and filtering for the GREAT database requires careful consideration as to its feasibility, impact and resource use.
Implications for the use of the Global Resource of EczemA Trials database
The sensitivity of the GREAT database is fairly high, but there is room for improvement. The level of true errors of omission owing to faults in the filtering process for the GREAT database was low (5%) but needs to be improved to reassure users as to the utility of the database.
It is clear that, currently, for many research questions and when broader inclusion criteria than those for the GREAT database are needed, additional specific searches of specialist databases, such as PsycINFO, and unpublished trials registers are needed in addition to searching the GREAT database.
It is important to be aware that the GREAT database offers much more than a standard bibliographic database, which only provides citations and abstracts of articles. The potential time savings resulting from the GREAT database cannot be quantified within the scope of this validation study and should also be assessed in order to measure the potential benefits of this resource. The GREAT database is also a resource that is specifically designed to be accessible for all the potential users, such as guideline writers, patient information development, methodologists, health-care practitioners and patients.
The GREAT database is sufficiently sensitive and is a resource that is quick and simple to interrogate for most users, particularly for those looking for the best evidence for specific treatments that have not been included in a full systematic review, or for people looking for new trial evidence published after the systematic review search end date. The more the GREAT database is used and added to on an iterative basis, the better it will be.
Systematic review of selective outcome reporting in eczema trials
The following text is adapted with permission from Macmillan Publishers Ltd: J Invest Dermatol. Nankervis H, Baibergenova A, Williams HC, Thomas, KS. Prospective registration and outcome-reporting bias in randomised controlled trials of eczema treatments: a systematic review; 132, 2727–34. Copyright 2011. 262
Summary
What was already known about this topic?
-
Authors of RCTs are now compelled to register a protocol of the trial in one of the publicly available trials registration databases, preferably before the trial starts.
-
Selective outcome reporting bias, when outcomes originally planned are either not reported or altered when reported in the final published trial report.
What did this study add?
-
Not many RCTs of eczema treatments had been registered at all and even fewer had been properly registered (before trial recruitment began).
-
Of those that were registered, it was difficult to assess outcome reporting bias using trial registration records, as they mostly lack the required detail about the primary or other outcomes.
-
Only 5% of the eczema RCTs examined were registered a priori, gave sufficient detail about the primary outcome to judge adherence to the original registration record, and reported the primary outcome in line with the original registration (or included an explanation as to why this had changed).
Introduction
As previously discussed, the GREAT database can be used to expedite other studies using eczema treatment RCTs. This is particularly true of systematic reviews and RCT methodology papers, for which having all the eczema treatment RCTs together allows authors to quickly obtain a collection of trials that fall into the study inclusion criteria. The study described below is an example of the GREAT database being used as the basis for a methodological study. 262 This project was conducted over a period of several months as part of a Women’s Dermatological Society mentorship award. This project would not have been possible within the time frame of the award had the trials not already been identified and available in the GREAT database.
Outcome reporting bias in a clinical study is defined as selective reporting of a subset of study findings based on the significance and direction of the results. Selective reporting of outcomes has been well recognised in general medicine trials for which approximately 40% to 62% of studies have been found to have at least one primary outcome that was changed, introduced or omitted. 263 The effects of such distortion of what was planned in a study are potentially serious for clinical practice. In their review of the effects of reporting outcome bias in systematic reviews, Kirkham et al. 264 found that out of 42 meta-analyses with a statistically significant result, eight (19%) became non-significant after adjustment for outcome reporting bias and 11 (26%) would have overestimated the treatment effect by ≥ 20%. The problem of reporting outcome bias has not been studied among dermatology RCTs.
One of the main ways of reducing selective reporting outcome bias is to require all trial investigators to register details of their trial in a publicly accessible register before the trial starts recruitment. Those reading the final trial report can then go back to the original trial registration to check if what was highlighted as the primary outcome measure in the published report corresponded to that in the original registration. In 2004, the International committee of Medical Journal Editors (ICMJE) initiated a policy requiring investigators to register their trials into a clinical trials registry before participant enrolment begins as a condition of publication in one of their journals. 265 This policy came into effect in July 2005. A number of dermatology journals followed this policy including the British Journal of Dermatology and the Journal of Investigative Dermatology in 2005. 266 A number of trial registers sprang up in the early 1990s containing different core items, culminating in the World Health Organization hosting an International Clinical Trial Registry Platform of approved trial registries267 that fulfilled key quality criteria and collected a minimum set of key study domains. Around the same time, dermatology journals also encouraged authors to report all of the important study features included in the Consolidated Standards of Reporting Trials (CONSORT) Statement. 268
This study sought to assess the extent to which outcome reporting bias is evident in the field of dermatology, using published RCTs of eczema treatments in people with eczema (AD) as an example. The study had three objectives:
-
To assess the proportion of eczema treatment RCTs registered.
-
To see whether or not the lack of registration was associated with differences in risk of bias, sample size and funding source.
-
To assess the level of possible outcome reporting bias of the primary outcome in eczema treatment trials.
Our hypotheses were that:
-
less than half of eczema RCTs would have been registered
-
quality of reporting of trials without pre-registration would be poorer
-
even in the trials that were properly registered, selective reporting outcome bias may still exist, that is, there may be discrepancies between the primary outcome that was registered and the primary outcome reported in the published trial report.
Methods
A systematic search for RCTs of eczema treatments that had been published since January 2007 was conducted. For all identified trials, the World Health Organization International Clinical Trial Registry Platform was searched for proof of approved trial registration.
Searching for published eczema trials
We used a convenience sample of all published eczema trials that have been captured in the GREAT database. 226 This open-access resource brings together information on all RCTs of eczema treatments published since the beginning of 2000 in order to facilitate future methodological research and systematic reviews. To identify randomised trials to include in the GREAT database the following resources are searched: MEDLINE, EMBASE and AMED (via Ovid), CINAHL (via EBSCOhost) and LILACS. The search strategy for MEDLINE uses the following subject terms: eczema, AE, AD, infantile eczema, childhood eczema, neurodermatitis and besnier’s Prurigo, combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE (sensitivity maximising version, 2008 revision). 269 Search strategies for the other resources are modelled on the MEDLINE strategy. The Cochrane Library270 and the Cochrane Skin Group Specialised register are also searched using the same eczema terms. The search runs from the beginning of 2000 and is updated regularly.
Inclusion criteria for the present study
Allowing 1.5 years from the ICMJE policy of trial registration coming into effect, we included all eczema treatment RCTs contained in the GREAT database that had been published between 1 January 2007 and 31 July 2011. Whether or not these trials contained safety data was not relevant to this study and so did not form part of the inclusion or exclusion criteria. We excluded RCTs that were published in abstract form only. If both an abstract and a full paper were found, only the full paper was included. (Details of all trials included in this study can be found in the full publication.)262
Identifying trial registration
If the trial registration ID number was stated in the final publication, we used this number to find the trial registration. When this number was not stated, we tried to obtain the registration record using the World Health Organization International Clinical Trial Registry Platform267 which searches a number of different trial registries throughout the world (Box 1). Two people searched for trial registration independently (AB and HN) using a combination of key words: ‘eczema’ or ‘atopic dermatitis’ plus keywords describing the trial’s interventions such as ‘pimecrolimus’ or ‘probiotics’. All registration records that met the search criteria were reviewed to find the one that matched the published trial using key information supplied in the record [e.g. the name of principal investigator (PI), funding source and trial design]. As a rule, we did not spend more than 15–20 minutes looking for each record, on the grounds that trial registration should be easy and quick to identify.
Australian New Zealand Clinical Trials Registry.
Chinese Clinical Trial Register.
Clinical Research Information Service, Republic of Korea.
ClinicalTrials.gov.
Clinical Trials Registry – India.
Cuban Public Registry of Clinical Trials.
German Clinical Trials Register.
Iranian Registry of Clinical Trials.
ISRCTN.org.
Japan Primary Registries Network.
Pan African Clinical Trial Registry.
Sri Lanka Clinical Trials Registry.
The Netherlands National Trial Register.
Distinguishing between ‘registered’ and ‘properly registered’ trials
All eczema treatment trials in the sample from the GREAT database, registered on the World Health Organization International Clinical Trial Registry Platform were included in the number of ‘registered’ trials. This provides the overall level of trial registration for eczema trials, regardless of whether or not they fulfilled the guidelines for trial registration correctly. In addition, we identified a ‘properly registered’ subset of these trials which could be used to assess outcome reporting bias. We defined ‘properly registered’ trials as being those trials for which the primary outcome was explicitly stated in the registration document and for which the trial was registered no later than the end of the study (last patient visit). In cases when the start of participant enrolment was stated but an end date was not recorded, we considered a trial to be properly registered if the registration was lodged within 12 months of the study start date. Only ‘properly registered’ trials were included in the evaluation of outcome reporting bias.
To take into account the amendments and possible changes that could have taken place after initial trial registration but prior to data lock, we used the website provided on the World Health Organization trial registration webpage to go to the source record for each of the ‘properly registered’ trials in the primary register and looked for additional information or updates regarding the trial.
Comparison between registered and non-registered trials
We compared registered and non-registered trials with respect to several characteristics including number of participants randomised, funding and quality of reporting. The quality of reporting was assessed using The Cochrane Collaboration’s Risk of Bias Tool272 in four different domains: randomisation method, allocation concealment, blinding (of participants, personnel and outcome assessors), and completeness of outcome data. Each trial was assessed for high, low or unclear risk of bias for each of these domains using the revised terms suggested in the updated version of the Cochrane Handbook272 to improve interpretation of the risk of bias.
Comparison of primary outcome between publication and registration among properly registered trials
For each properly registered trial we reviewed and compared primary outcomes reported in the publication with those stated in the registration. Both the primary outcome and the time frame for analysis were recorded. We chose to limit the review of outcome bias to the primary outcome for this first study owing to the importance of primary outcomes in determining the success or otherwise of a study and because detailed analysis of secondary outcomes was beyond the scope of our available resources.
We defined a discrepancy as follows:
-
when the primary outcome in the published report was different from that in the registration
-
when the time frame for assessing the primary outcome in the published report was different from the registration.
Sample size calculation
Sample size was determined by the number of available trials that had been published since the introduction of the ICMJE guidelines. We also estimated that we would need around 100 studies in order to estimate the proportion of registered studies (which we hypothesised would be around 50%) to within 10 percentage points. All eczema RCTs published in the last 4.5 years were included.
Data assessment
The following comparisons were made:
-
The proportion of trials registered.
-
The proportion of trials properly registered versus the proportion registered in any form.
-
The proportion of registered trials with the registration number stated in the final publication.
-
The average sample size of registered trials versus non-registered trials.
-
The proportion of trials that declared a funding source.
-
The proportion of registered versus non-registered trials with a placebo comparator.
-
The number of trials with a low/unclear/high risk of bias for each of the four domains for registered trials versus non-registered trials.
-
Number of properly registered trials with a missing time frame for the primary outcome.
-
Number of properly registered trials with a vague and/or unclear primary outcome.
Statistical analysis
We used frequency and percentages for categorical variables, and mean with standard deviation (SD) or median and interquartile ranges (IQRs) for continuous variables, as appropriate. Proportions were compared using chi-squared tests and Fisher exact tests where appropriate. Trends over time were assessed using chi-squared tests for trend. Continuous variables were compared using t-tests and Mann–Whitney U-tests for non-parametric data. A value of p < 0.05 (two tailed) was considered statistically significant. All statistics were calculated using Statistical Package for the Social Sciences (SPSS) version 16 (SPSS Inc., Chicago, IL, USA) except chi-squared tests for trend, which were calculated using StatsDirect (StatsDirect, Altrincham, UK). The protocol for this study was posted on the Centre of Evidence Based Dermatology, University of Nottingham, website273 at the beginning of the study in September 2011, and prior to data analysis.
Results
A total of 122 eczema RCTs were published between 1 January 2007 and 31 July 2011 and included in the GREAT database (Figure 12). Twelve studies were excluded because they had published abstracts, but it was not possible to identify a full trial publication. Two publications came from the same study with a later publication being a follow-up of the original trial. 274 Only the first publication, describing the original trial was included in this study.
FIGURE 12.
The GREAT database search for RCTs.

Levels of registration of eczema treatment trials
Of the remaining 109 included studies, only 37 (34%) had been registered in any form, as shown in the study flow chart in Figure 12. In 9 out of these 37 trials, the trial registration was more than a year after the study had been completed and published (the longest time interval was almost 3 years after study completion) and so could not be defined as ‘properly registered’. Only 20 (54%) out of the 37 registered studies indicated their trial registration number in the final trial publication.
Among the 37 studies with a registered protocol, 18 studies (49%) had ‘properly registered’ according to our definition. Although there has been an increase in trial registration from 2007 onwards, the proportion of ‘properly registered’ trials was still low, reaching a maximum of 29% (4/14) in the first half of 2011. The increasing trend in the proportion of registered eczema treatment trials was significant (p = 0.003) (Figure 13).
FIGURE 13.
Registry of eczema RCT trials.
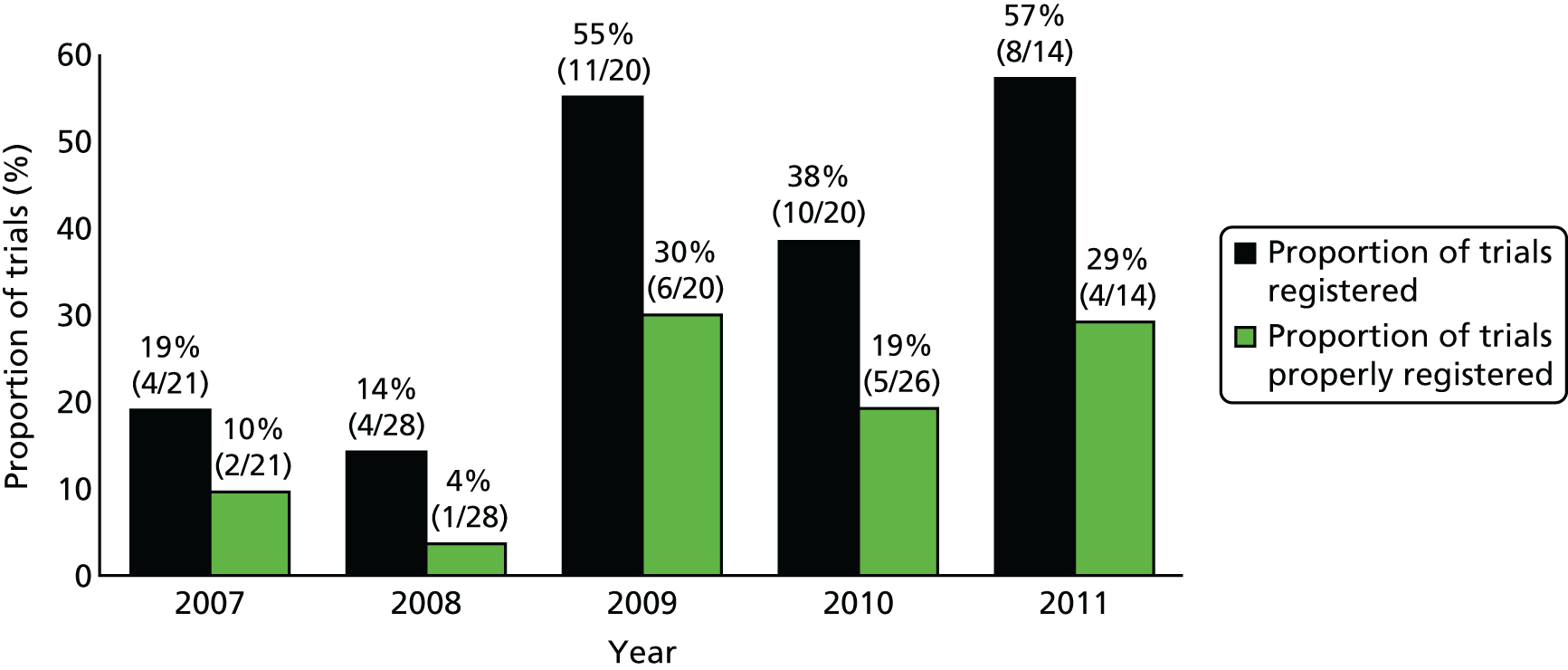
Differences between trials with and without registration
Although registered trials had a larger median sample size of 70 people (IQR 37.5–192) compared with 60 people (IQR 30–104.5) for trials without registration, this difference was not statistically significant (p = 0.405). Non-registered trials were significantly less likely to specify their funding source in the subsequent publication than registered trials (3% vs. 30%; p = 0.001) and a post hoc analysis looking at choice of comparator showed that non-registered trials were no more likely to include a placebo comparator than registered trials (70% vs. 57%; p = 0.188).
Overall, the differences in the estimated risk of bias were not statistically significant, with the exception of allocation concealment domain, for which registered trials scored marginally better: 24% (9/37) were at low risk of bias compared with 8% (6/72) for non-registered trials (p = 0.04; Figure 14).
FIGURE 14.
Risk of bias for eczema RCT trials. (a) Proportion of trials at risk of bias: registered trials; and (b) proportion of trials at risk of bias: non-registered trials.
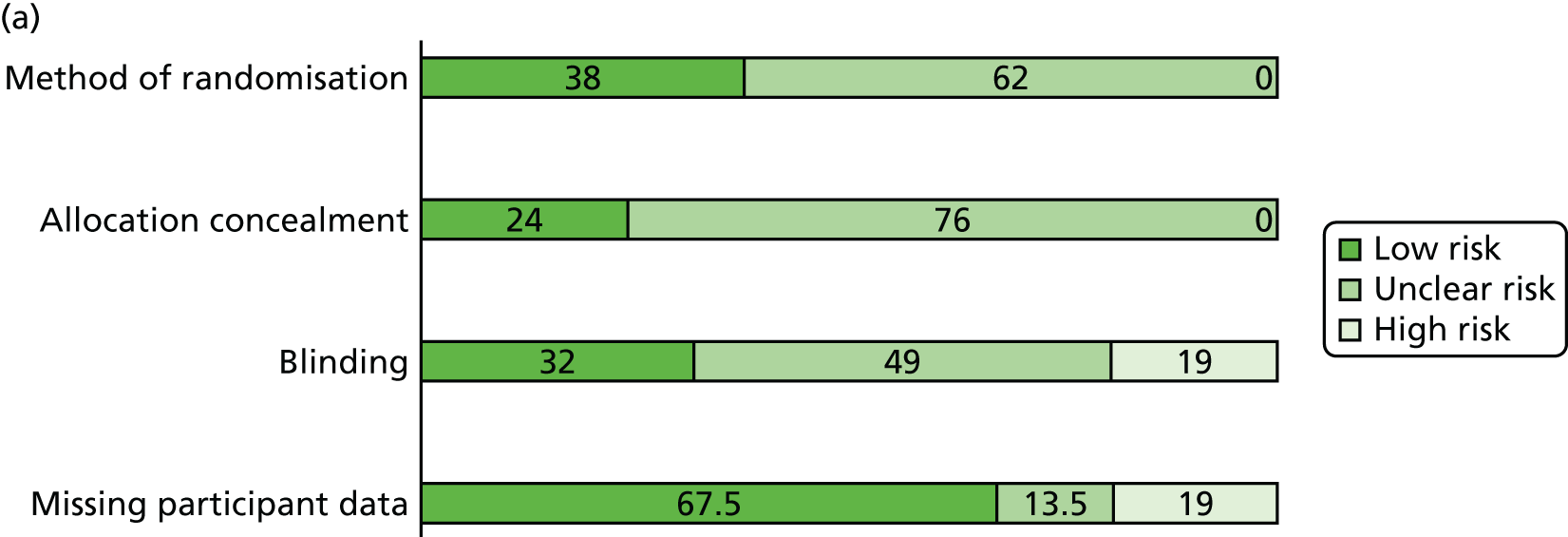
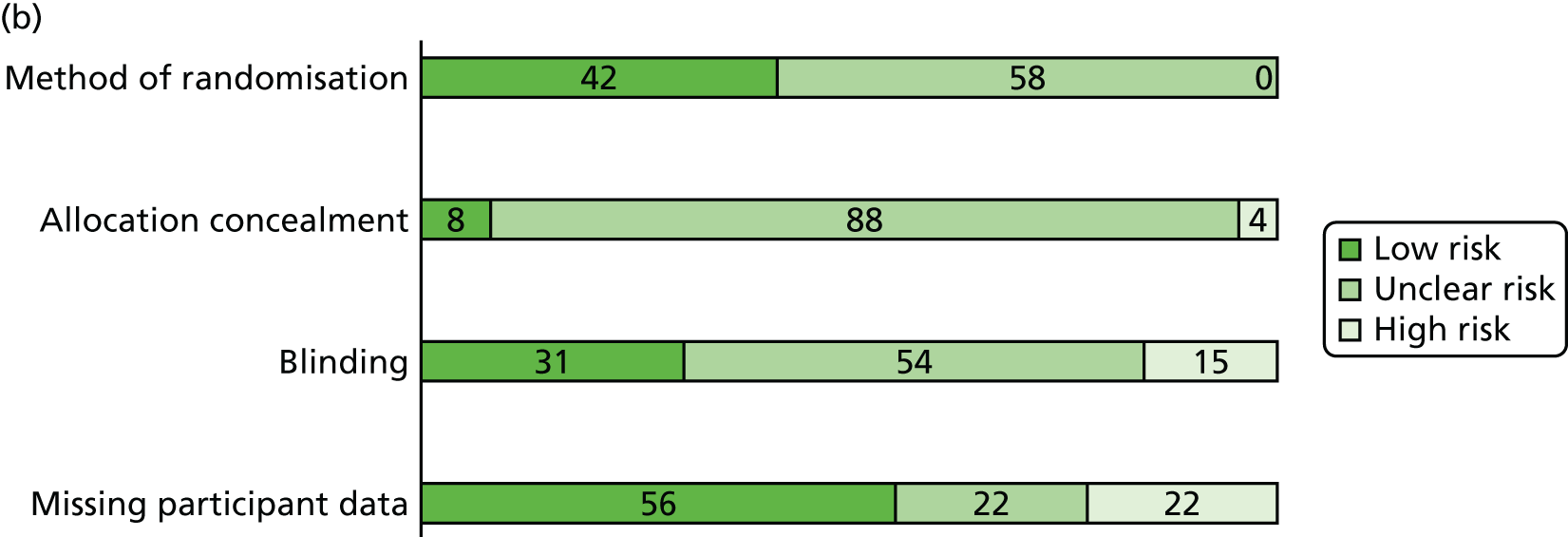
Outcome reporting in properly registered trials and their registration records
The most common discrepancy was missing time frame information for measurement of the primary outcome: in 8 out of 18 trials, registration records did not state the timing of the primary outcome measurement. In addition, the description of the primary outcome was often vague and unclear, which occurred in 5 out of the 18 ‘properly registered’ trials (Table 15). For example, a primary outcome might be described as ‘efficacy in treating exacerbations’ or ‘stable remission’, without further detail as to how this was to be measured or assessed.
| Reference | Primary outcome | Statistical significance of results | Comments | |||
|---|---|---|---|---|---|---|
| Publication | Registration | |||||
| Assessment | Time frame | Assessment | Time frame | |||
| Hon et al. 2007275 | Changes in SCORAD index and CDLQI | 12 weeks | Changes in SCORAD index | 12 weeks | SCORAD index: NSS, CDLQI: SS (p = 0.008) | Introduction of a new outcome that showed SS results |
| Murrell et al. 2007276 | Proportion of patients with IGA score = 0/1 | 6 weeks | Changes in IGA scores | Not stated | SS (p < 0.001) | Missing time frame in protocol |
| Peserico et al. 2008277 | Time to relapse of AD | 16 weeks | Time to relapse of AD | 16 weeks | SS (p < 0.0001) | |
| Huang et al. 2009278 | Change in EASI score | 3 months | Change in EASI score | 3 months | SS (p = 0.004) | |
| Moore et al. 2009279 | Change in SCORAD index | 4 weeks | Change in SCORAD index | Not stated | SS (p < 0.001) | Missing time frame in protocol |
| Ruer-Mulard et al. 2009280 | Time to relapse (confirmed by IGA and pruritus score) | 16 weeks | Time to relapse confirmed by IGA and pruritus score | Not stated | NSS | Missing time frame in protocol |
| Wirén et al. 2009194 | Time to relapse of AD confirmed by ADSI | 26 weeks | Compatibility of the skin with the new formulation; effect of maintenance therapy with an emollient cream on the possible recurrence of AE | Compatibility study: 3 weeks; maintenance study: up to 6 months | Time to relapse: SS (p < 0.01) | Vague definition of primary outcome and time frame in protocol |
| Hoeger et al. 2009281 | Proportion of IGA = 0/1 | Day 43 | Proportion of IGA = 0 or 1 (clear or almost clear) | Not stated | SS (p < 0.001) | Missing time frame in protocol |
| Gambichler et al. 2009282 | Change in SASSAD | 6 weeks | Change in SCORAD index | Not stated | NSS | Change in eczema score; missing time frame |
| van der Aa 2010274 | Change in SCORAD index | 12 weeks | Decrease in SCORAD index > 25% from baseline | 12 weeks | NSS | |
| Tzaneva et al. 2010283 | Length of remission | 12 months | Length of remission | 12 months | SS (p = 0.012) | |
| Foelster Holst et al. 2010284 | Proportion of IGA = 0/1 | 4 weeks | Measure efficacy in treating acute exacerbation | 4 weeks | NSS | Vague definition of outcome in protocol |
| Schmitt et al. 2010285 | Proportion of patients with stable remission (SCORAD index improvement ≥ 50%) and no flare (SCORAD index ≥ 75% of baseline) | 18 weeks | Stable remission in both treatment groups | Not stated | SS (p = 0.031) | Vague definition of outcome in protocol; Missing time frame |
| Brenninkmeijer et al. 2010286 | Change in PAIS | 10 weeks treatment plus 6 months follow-up | Change in PAIS | Not stated | At 10 weeks: NSS; follow-up: SS (p < 0.05) | Missing time frame |
| Armstrong et al. 2011287 | Change in POEM score | 12 weeks | Change in POEM score | 12 weeks | SS (p = 0.0043) | |
| Bangert et al. 2011288 | 1. Reduction in EASI, 2. reduction in number of leucocytes in skin biopsies | 3 weeks | Determining whether pimecrolimus cream has an effect on the cellular and molecular profile of AD skin | Not stated | 1. EASI: p-value not provided, 2. number of CD45+ cells in biopsy SS difference (p = 0.047) | Vague definition of primary outcome; Missing time frame |
| Frankel et al. 2011289 | Change in IGA and TLSS | 4 weeks | Improvement and maintenance of PGA, TLSS, subjective AD control | 26 weeks | NSS | Vague definition of outcome; different time frame |
| Thomas et al. 2011290 | Change in SASSAD | 12 weeks | Change in SASSAD | 12 weeks | NS | |
Assessment of outcome reporting bias among properly registered trials
The lack of detail and clarity of reporting for the outcomes of registered trials both from the registration record and the published trial report makes the objective of assessing outcome reporting bias in eczema treatment trials difficult. One study introduced a new statistically significant primary outcome that was not present in the registration record (original primary outcome was not significant),275 one study changed the scale measuring eczema severity from Severity Scoring of Atopic Dermatitis (SCORAD) index to Six Area, Six Sign Atopic Dermatitis (SASSAD),282 and one study changed the time frame for assessment of the primary outcome from 26 weeks to 4 weeks. 289 None of these changes resulted in a significant result being reported.
Discussion
Main findings
Our study has shown that the proportion of published eczema RCTs registered on an approved trial registry has increased from 19% in 2007 to 57% in 2011. The fact that over half of the RCTs published in 2011 were registered is cause for some optimism. However, the proportion of trials that were ‘properly registered’ (lodging details of the proposed trial design prior to the end of the study, or within 12 months of the recorded trial start date and indicating the primary outcome in the registration) was considerably lower. Taking into account that our definition of ‘properly registered’ was a very generous one, this is a particularly sobering result. Overall, out of the 109 eczema RCTs examined, only five trials were registered a priori, gave sufficient detail about the primary outcome to judge adherence to the original registration record and reported the primary outcome in line with the original registration (or included an explanation as to why this had changed). That only 5% of recent eczema trials were registered correctly and with enough detail to assess outcome reporting bias for the primary outcome, coupled with the observation that non-registered or incompletely registered studies fail to highlight the shortcomings of such omissions, is bad science and a potential waste of resources. 38,291
Even when investigators had pre-registered their trial, 46% failed to include details of their trial registration in the published trial report, suggesting that investigators and journal editors do not yet appreciate the importance of such information in their trial reports. The revised version of the CONSORT statement for guidance on the reporting of RCTs now explicitly recommends that details of trial registration be reported fully and this should improve trial reporting in the future. 268
The fact that some investigators chose to pre-register their trial could be an indicator of trial quality and this was explored using the four key domains known to be associated with high risk of bias. 272 In this sample of eczema RCTs, there was a suggestion that trial quality might be improved in registered trials, but this was only significant for the domain of allocation concealment and is possibly limited by the modest sample size of our survey.
With regard to the hypothesis that eczema trials are subject to outcome reporting bias, we found some evidence of discrepancies between trial registration and trial reports; however, these were generally as a result of unclear and non-specific trial registration information rather than clear signs of biased reporting. In general, the number of correctly registered trials was so low that firm conclusions are difficult. Greater efforts could certainly be made to provide more detail regarding the primary outcome and its timing within trial registry records. It is hoped that reviews such as this one will be helpful in informing triallists of the importance and relevance of detailed information being lodged in the trial registries. It is also important that changes to the registration record subsequent to trial commencement are fed into the trial registries in a timely fashion, in order to maintain transparency throughout. The concept of threaded publications, enabling the tracking of clinical research studies from inception and the linking of all resulting publications including the raw data when these are available, is another emerging tool to ensure consistency between what was planned and what was done. 292
Comparison with other studies
Overall, the rates for registration of eczema RCTs were lower than those reported for other surveys of general medical journals and larger specialities. Mathieu et al. 293 looked at RCTs indexed in 2008 in 10 general medicine and specialty journals (cardiology, rheumatology and gastroenterology) and out of the 323 included trials, 45.5% were adequately registered, 13.9% trials were registered after the completion of study and 12% were registered with no or unclear description of the primary outcome.
A systematic review of studies examining the impact of selective outcome reporting in RCTs identified five studies and compared the trial publication with trial registration record. It found that between 40% and 62% of trials had at least one primary outcome that had been changed, introduced or omitted. 263 These rates are higher than the rates found in the current study, which might be a reflection of improvements in trial reporting subsequent to the introduction of the ICMJE policy. Alternatively, it is possible that higher rates of outcome reporting bias would have been found if more of the eczema trials had been registered, thus allowing a comparison of the published results with the trial registration. It is conceivable that, to date, only the better quality eczema trials have been registered appropriately.
Strengths and limitations of our study
This study used the GREAT database to identify and assess all eczema trials published over the last four and a half years. Using this global collection of appraised eczema trials allowed for the speedy completion of this review and the easy identification of all relevant RCTs that had been identified through a sensitive search of several bibliographic databases. Including all trials on a particular topic, rather than just those reported in specific journals or as a random sample of published trials, meant that our study is less open to selection bias. Eczema is one of the most common skin conditions and is commonly researched. More than 250 RCTs of eczema treatments have been published in the last 10 years alone. 226 It is possible that for other common skin conditions, the proportion of trials registered and possible outcome reporting bias may be different, which makes the results of this study difficult to generalise to the field of dermatology research as a whole.
Our definition of a properly registered trial as one that was registered before the end of the study was a generous one, given that the ICMJE requires that all clinical trials need to be registered in an approved publicly accessible clinical trial register before patient recruitment begins. It is likely that the bottom line figure of only 5% of all recent eczema trials that have been properly registered would have been even worse if this more stringent definition had been applied, although in many cases it was impossible for us to tell when recruitment began and when the study ended. On the point of timing, it is worth noting that some triallists registered their trials after the study had been completed, which defeats the whole purpose of prospective trial registration as a tool to prevent inappropriate post hoc analysis. Our sample size was relatively small, which affected the overall precision of our estimates of ‘properly registered’ RCTs.
Finally, this review limited its scope to an evaluation of the primary outcomes assessed in trials. Others have reported considerable outcome reporting bias for secondary outcomes, particularly in relation to the preferential reporting of statistically significant secondary outcomes. 294
Implications of our findings
The introduction of mandatory trial as required by the Journal of Investigative Dermatology is a great opportunity to improve the quality and truthfulness of trial reporting, which will in turn lead to better clinical decision-making by reducing the prevalence of spurious and misleading results and reduction in research wastage. 38 Although publication bias of whole studies is relatively well known, the phenomena of selective outcome reporting bias within studies is possibly less well understood within the dermatology clinical community. 295
Ideally, deviations from the trial registration record should be described in the published articles so that readers can interpret the results in full knowledge of the changes made. For the full benefits of trial registration to be realised, it is important that all investigators, funders, journal editors, peer reviewers, readers and the public play an active role in making full use of trial registration information and highlighting the need for transparent trial reporting. Journal referees and readers of clinical trials especially should make more use of scrutinising trial registries to note if a clinical trial has truly been registered prospectively and that the outcomes reported in the paper are consistent with what was planned. Those conducting systematic reviews are also in a good position to check on and comment on trial registration of included trials and to assess whether or not selective outcome reporting bias was likely to have occurred.
Conclusion
Adequate trial registration for eczema RCTs is poor. Registration of all trials in a publicly accessible database is a critical step towards ensuring the transparent reporting of clinical trial results that affect health care.
Harmonising Outcome Measures for Eczema initiative
The following text is adapted with permission from the following papers: Schmitt et al. 176 © 2010 The Authors. BJD © 2010 British Association of Dermatologists; and Schmitt et al. 296 © 2012 John Wiley & Sons A/S. The following text is also adapted with permission from the following papers: Schmitt et al. 297 This article was published in J Allergy Clin Immunol, vol. 134, Schmitt J, Spuls PI, Thomas KS, Simpson E, Furue M, Deckert S, et al. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. pp. 800–7. © American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. 2014. Chalmers et al. 298 © 2014 The Authors. British Journal of Dermatology published by John Wiley & Sons Ltd on behalf of British Association of Dermatologists (BAD). This is an open access article under the terms of Creative Commons Attribution-NonCommercial License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
Summary
What was already known about this topic?
-
The high number of scales used to measure the severity of eczema means that it is difficult to compare the results of different trials in a meta-analysis.
-
The aim of HOME is to recommend a set of core outcome measures to be included in all eczema trials.
What did this study add?
-
Two working meetings were held at which a combination of formal presentations and nominal group techniques were used to progress the HOME project.
-
Outcome measures must meet the Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT) filter to be included in the core set. The filter comprises truth (validity), discrimination (sensitivity to change and responsiveness) and feasibility (ease of use, costs, time to perform and interpret).
-
The four domains of clinical signs, symptoms, a long-term measure that captures flares and remissions and health-related quality of life (HRQoL) should all be measured by the core outcome set.
-
The clinical signs of eczema should be assessed by an investigator and both the intensity and extent of signs should be measured.
-
The following four clinical signs of eczema are essential for the scale to achieve content validity: erythema, excoriation, oedema/papulation and lichenification.
-
Consensus was achieved that EASI be recommended as the core outcome measure for the clinical signs of eczema.
Background to the Harmonising Outcome Measures for Eczema project
There are currently > 20 different instruments used to measure the severity of AE in clinical trials. 175,299 Because these instruments assess different aspects of AE in different ways, trials cannot be readily compared and meta-analyses are often not possible. Consequently, clinical decision-making is limited by a lack of standardisation and validation of outcome measures. The global, multiprofessional HOME aims to standardise and validate a core set of outcome measurements for AE,176 as others have done for osteoarthritis,300 childhood asthma,301 juvenile systemic lupus erythematosus and juvenile dermatomyositis. 302
In 2007, a systematic review critically appraised the validity, reliability and ease of use of all published measures to assess the severity of AE. 175 This review concluded that only three of the 20 named scales (SCORAD index,303 EASI304 and POEM305) had been adequately validated such that their use can be recommend. 175 Although proactive treatment of flare prevention is currently a relevant strategy given that AE is a chronic disease,176 a recent systematic review highlighted there is no clear definition and validated measure of disease flares. 306 The corollary of a lack of high-quality outcomes research is that current clinical trials face the possibility of overestimating, underestimating or completely missing the true effect of an intervention for AE.
An international initiative was therefore launched to aid the standardisation of outcome measures for AE. The first stage was to conduct an online Delphi consensus exercise to suggest a preliminary core set of outcome domains that should be used in every clinical trial and in clinical record keeping. 307 An outcome ‘domain’ is defined as a distinct aspect of disease such as QoL, clinical signs, symptoms, or cost and, typically, each outcome domain can be measured by different instruments and/or scales. Our Delphi consensus exercise involved 46 individuals representing four stakeholder groups (consumers, clinical experts, regulatory agency representatives and journal editors) from 13 countries and four continents. 307 There was consensus from this Delphi exercise that measures for the domains symptoms, physician-assessed clinical signs and long-term control of flares should be assessed in every future AE trial, but there was no consensus whether or not HRQoL should be included in the core set of domains. 307
Following on from this Delphi exercise, Hywel Williams and Jochen Schmitt set up an international and multiperspective group called the HOME initiative225 with the overall purpose to derive core outcome measures for AE research and clinical record keeping through collaborative research. Membership of HOME is free and open to anyone interested in AE outcomes research.
The first meeting of the HOME initiative was a short exploratory workshop (HOME I) held during the International Symposium on Atopic Dermatitis/New Trends in Allergy meeting in Munich in 2010. 176 There was overwhelming support at this workshop among the international community to address AE outcomes research collaboratively as an open group and it was agreed that a second meeting would be arranged in which the initial findings of the Delphi exercise could be built on.
Harmonising Outcome Measures for Eczema II meeting
The second working meeting (HOME II) took place in July 2011 and the aims were to:
-
refine or confirm the preliminary core set of outcome domains for AE trials derived from the Delphi procedure
-
define minimum criteria for the recommendation of instruments to measure specific core outcome measures in clinical trials of interventions for AE
-
prioritise research into outcome measures for AE.
Methods
Study design
This 2-day face-to-face meeting was held in June 2011 and was hosted by Dr Phyllis Spuls from the Academic Medical Center in Amsterdam. It was an international multiprofessional consensus study involving patient representatives, clinicians, methodologists and pharmaceutical industry representatives. The HOME II meeting planning group consisted of Phyllis Spuls, Jochen Schmitt and Hywel Williams.
In contrast to our previous online Delphi exercise,307 to ensure the task was manageable, the group focused exclusively on outcome measures for clinical trials in this study meeting. Core outcome measures for record keeping in clinical practice are equally important and we plan to address these in the future.
Study participants
We invited participants from the Delphi consensus study,307 HOME I meeting attendees176 and clinicians and methodological experts who had expressed interest in HOME or have recently published in the field of AE outcomes research. We also invited individuals who were involved in the development of specific AE outcomes measurement175 as well as representatives from the pharmaceutical industry. We invited Professor Maarten Boers as an external expert, who, as a founding member of the OMERACT group, has been involved in outcomes research in rheumatology since 1992. 308 The meeting was free of charge for all participants and travel expenses for some of the patient representatives and researchers were covered by patient associations or academic institutions.
Study procedures
There was a strong focus on evidence-based information before consensus voting. Group techniques and modified Delphi exercises were applied as described previously by the OMERACT group. 308,309 Scientific information was provided in the form of presentations and handouts of key publications. Patient representatives additionally received plain-language information material before the meeting that had been developed by one of the consumers (Rosemary Humphreys) and an organiser (HW). To allow structured and effective group discussions we divided the panel into two groups, assigned one moderator and one rapporteur to each group, and applied nominal group technique methods. For consensus voting, an anonymous electronic key pad voting system was used. For further details concerning the study procedures please refer to the HOME website. 225
Statistical analysis
Based on his previous experiences from OMERACT meetings, Maarten Boers proposed the following definition of consensus (consensus is reached if < 30% of the voters disagree). None of the participants disagreed with this definition. There was 100% agreement that the whole group of participants voted as one without dividing it into stakeholder groups (as done in the previously published online Delphi exercise307). For the purposes of sensitivity analyses, information on stakeholder groups (i.e. clinicians, consumers, others including pharmaceutical industry representatives and methodologists) was collected. Participants were asked to nominate which stakeholder group they were most closely related to.
For each question counts and percentages of participants who ‘agreed’, were ‘undecided’ and ‘disagreed’ are presented.
Results
A total of 43 individuals from 10 countries participated in the HOME II consensus study. The panel comprised an approximately equal number of males (n = 23; 53%) and females (n = 20; 47%). The majority of participants (n = 30; 71%) were from Europe (UK, n = 10; the Netherlands, n = 10; Germany, n = 5; France, n = 3; and Sweden, n = 2). The panel included six participants from Japan, three from Brazil, two from Israel, one from the USA and one from Australia. Clinical experts were the largest stakeholder group (n = 29; 67%). The panel included five consumers, five methodologists and one pharmaceutical industry representative.
Refinement of the preliminary core set of outcome domains for atopic eczema trials
During group discussions both groups agreed that four domains should be included into the core set of outcome domains for AE trials: clinical signs, symptoms, a long-term measure that captures flares and remissions and HRQoL. It was noted that long-term disease control could be viewed as a derivative function of clinical signs and symptoms over time and not an independent item of source data. Some group members favoured generic HRQoL measures, whereas others favoured disease-specific QoL measures.
Both groups discussed the role of biomarkers, coping behaviour and treatment utilisation as outcome domains for AE trials. Both groups agreed that biomarkers should not be added to the core outcome domains for the time being as taking blood from patients might hamper recruitment to clinical trials. This decision did not preclude the inclusion of biomarkers in the future, as the disadvantages may be outweighed by advancements in identification. Neither group favoured the inclusion of coping behaviour nor treatment utilisation into the core set of outcome domains.
The results of consensus voting on refinement of core outcome domains for clinical trials to investigate the efficacy of interventions for AE reflected the discussions and are summarised in Table 16.
| Question | Total votes | n (%) | ||
|---|---|---|---|---|
| Agree | Undecided | Disagree | ||
| Do you agree on ‘clinical signs’, ‘symptoms’ and ‘long-term control of flares’ as core domains? | 40 | 32 (80) | 3 (8) | 5 (13) |
| Do you agree to add ‘QoL’ as core domain? | 41 | 31 (76) | 6 (15) | 4 (10) |
| Should ‘coping’ be considered for inclusion as core domain? | 41 | 4 (10) | 8 (20) | 29 (71) |
| Should ‘biomarkers’ be considered for inclusion as core domain | 41 | 4 (10) | 5 (12) | 32 (78) |
| Should ‘treatment utilisation’ be considered for inclusion as core domain? | 41 | 6 (15) | 9 (22) | 26 (63) |
There was broad consensus that clinical signs, symptoms, long-term control of flares and QoL should be used as core outcome domains for future AE trials. Each stakeholder group agreed on this core set of outcome domains. Agreement among the group of consumers was 100% (n = 5).
There was also consensus that biomarkers, treatment utilisation and compliance should not be added to the core set of outcome domains at the moment. Interestingly, three out of five consumers favoured the inclusion of biomarkers into the core set of outcome domains.
Definition of quality requirements to recommend outcome measures
A series of presentations was given to the panel on instruments for measuring outcomes in eczema trials. Jochen Schmitt presented a systematic review on named outcome measures for AE, in which reliability, validity, sensitivity to change and acceptability were used as criteria for recommendation of measurements. 175
Maarten Boers presented the ‘OMERACT filter,310 which defines three criteria necessary for outcome measures to be recommended:
-
truth – validity
-
discrimination – sensitivity to change, responsiveness
-
feasibility – ease of use, costs, time to perform and interpret.
Group discussion indicated that the adoption of the OMERACT filter was generally favoured by the panel. Consensus voting resulted in absolute agreement (100%) of the panel, that necessary criteria to recommend an outcome measure for AE trials are truth, discrimination and feasibility as defined previously by the OMERACT group. 310
Prioritisation of future atopic eczema outcomes research issues
Group discussions were used to identify important AE outcomes research topics. The following research topics were considered important by both groups: identification of adequate measurements for the four core outcome domains, measurement of quality of care, collapsing core outcome domains/conceptual mapping, biomarkers and definitions of disease severity (i.e. how does ‘mild’, ‘moderate’ and ‘severe’ AE translate into a score and vice versa). Additional issues indicated as important by members of one group were measurement of coping behaviour, self-efficacy, empowerment, illness perception, patient satisfaction and corticosteroid phobia. Definition of incident AE in prevention trials and definition of subtypes of AE were additional suggested issues for the future outcomes research agenda.
The results of consensus voting about the prioritisation of future AE outcomes research are summarised in Table 17. The majority of panel members wanted to identify adequate measurements for the core outcome domains clinical signs, symptoms and long-term control of flares. About half of the panel also considered the identification of adequate measurements for HRQoL and how to define the severity of AE to be important. A minority prioritised the identification of biomarkers and measures for the domain ‘quality of care’ and the development of methods to explore whether or not several different core outcome domains could be further collapsed into one composite score (see Table 17).
| AE outcomes research topic | Highest priority, n (%) | Not highest priority, n (%) |
|---|---|---|
| Identify/validate measures for core outcomes domain ‘clinical signs’ | 28 (80) | 7 (20) |
| Identify/validate measures for core outcomes domain ‘symptoms’ | 24 (69) | 11 (31) |
| Identify/validate measures for core outcomes domain ‘long-term control of flares’ | 30 (86) | 5 (14) |
| Identify/validate measures for core outcomes domain ‘QoL’ | 17 (49) | 18 (51) |
| Identify/validate measures for domain ‘quality of care’ | 6 (17) | 29 (83) |
| Identify/validate adequate biomarkers | 4 (11) | 31 (89) |
| Collapsing core domains and conceptual mapping | 9 (26) | 26 (74) |
| Definition of disease severity (i.e. mild, moderate, severe AE) | 20 (57) | 15 (43) |
Discussion
Forty-three individuals from five continents representing clinicians, patients, methodologists and the pharmaceutical industry participated in the HOME II consensus study.
The previously described307 core outcome domains of symptoms, clinical signs and long-term control of flares were confirmed by the panel. An additional core outcome domain of HRQoL was identified with broad consensus. It thus appears that the four core outcome domains of symptoms, clinical signs, long-term control of flares and QoL should be measured consistently in future AE trials. Therefore, it is clear that more than one of the currently available instruments will need to be included in the core outcome set as none cover all four domains (Table 18).
| Score | Clinical signs | Symptoms | Long-term measure of disease control |
|---|---|---|---|
| ADAM | ✓ | ✓ | |
| ADASI | ✓ | ✓ | |
| ADSI | ✓ | ||
| BCSS | ✓ | ||
| EASI | ✓ | ||
| FSSS | ✓ | ✓ | |
| IGADA | ✓ | ||
| Leicester index | ✓ | ||
| NESS | ✓ | ✓ | ✓ |
| OSAAD | ✓ | ||
| POEM | ✓ | ||
| RL score | ✓ | ✓ | ✓ |
| SA-EASI | ✓ | ✓ | |
| SASSAD | ✓ | ||
| SCORAD index | ✓ | ✓ | |
| SIS | ✓ | ||
| SSS | ✓ | ✓ | |
| TBSA | ✓ | ||
| TIS | |||
| WAZ-S | ✓ | ✓ |
The OMERACT filter310 as a quality requirement to recommend outcome measures by the HOME group has been adapted with full consensus of the HOME II panel. In order to be recommended, outcome measures need to be valid, to discriminate between different disease states and need to be feasible in terms of ease of use, costs, performance and interpretation.
The HOME initiative will continuously review new and emerging outcome measures as they are published, such as the EASIdig (EASI score established from the digital images), a recently published digital tool for the digital assessment of severity and extent of AE, that has been shown to correlate with SCORAD index and EASI scores. 311
The panel highlighted the need to design and/or validate adequate instruments that meet the OMERACT filter to measure the four core outcome domains symptoms, clinical signs, long-term control and QoL.
Study strengths and limitations
Following systematic research,175,299,306,312,313 an international online Delphi consensus study on core outcome domains307 and an exploratory meeting (HOME I) that resulted in the formation of the HOME group,176 the study presented here was the first extended 2-day meeting in which patient representatives, clinicians, methodologists and pharmaceutical industry representatives with an interest in AE outcomes research from various parts of the world met, worked together and formed an active working group. A significant strength of HOME is the close links with the OMERACT group, a similar initiative in rheumatology that started in 1992. 308 Maarten Boers, a founding member of OMERACT, guided us throughout the HOME II consensus study and proposed the use of the OMERACT filter,310 which has been successfully used in rheumatology. The HOME group also has strong links with other interdisciplinary outcome research initiatives included in Core Outcome Measures in Effectiveness Trials (COMET). 314
Although individuals from all areas of the world were invited, colleagues from Africa, China or India were not able to attend this particular meeting and only one US invitee was able to attend. In addition, a more equal representation of some stakeholders groups such as the regulators, pharmaceutical industry and patients with a range of disease severity or in remission could enhance the representativeness of our findings and recommendations. We held the third HOME meeting (HOME III) in the USA specifically to attract a wider range of participants (see www.homeforeczema.org225 for details).
Harmonising Outcome Measures for Eczema III meeting
The third meeting of the HOME initiative was held in San Diego, CA, from 6 April to 7 April 2013 (HOME III). The aims of the HOME III meeting were specifically to:
-
discuss and interpret new research since the previous HOME meeting (HOME II) on the four core outcome domains that should be assessed in every clinical trial investigating interventions for AE, that is, signs, symptoms, long-term control and QoL
-
decide which instrument(s) should be used to measure clinical signs of AE
-
prioritise areas for further research in the four core domains.
Methods
Study design
A combination of formal presentations and nominal group techniques were used at the meeting. Consensus was achieved through an iterative and cumulative process over the 2 days. The decision was taken prior to the meeting that members would need to be present in order to vote to ensure that decisions were based on the data presented and the subsequent discussions.
Each session began with presentations of relevant research, followed by whole group and breakout group discussions. Voting was anonymised by using electronic handsets and TurningPoint© (Turning Technologies, Belfast, UK) software to analyse the results in real time. Voting took place as one panel; all stakeholders were included in every vote. The previously agreed consensus rule296 was applied here; that is consensus is reached when < 30% of the voters disagree.
Study participants
All members of HOME were invited to participate. There were a total of 56 attendees from around the world: North America (n = 32), Europe (n = 18), Japan (n = 4) and South America (n = 2). These included a mixture of prior HOME meeting attendees and new members. Details of the breakdown by stakeholder group can be found in Table 19. Most attendees had been involved with designing or recruiting into clinical trials (86%) and approximately half had used EASI and/or SCORAD index.
| Stakeholder group | n (%) |
|---|---|
| Dermatologists | 31 (55) |
| Patient/care provider/patient representative | 9 (16) |
| Pharmaceutical industry representative | 7 (13) |
| Methodologists (non-clinical) | 5 (9) |
| Nurses | 2 (3.5) |
| Other | 2 (3.5) |
| Total | 56 (100) |
The meeting received no sponsorship from any commercial organisation. With the exception of patient representatives, attendees covered their own travel and accommodation costs.
Patient representatives from France, UK and USA attended the meeting. A plain English summary was produced and circulated prior to the meeting and they had time before the meeting to ask any questions about the process and content. The patient representatives were then present at all sessions and participated in the discussion and voting.
Results
Session 1: introduction
Professor Hywel Williams (UK) opened the meeting by presenting the background to HOME and highlighted that core outcomes are an essential part of good clinical research to compare outcomes of studies across the world and in time. He then summarised the work undertaken by HOME members to date and explained the HOME roadmap, which describes the steps needed to progress each HOME work programme, that is identify all instruments, establish the extent and quality of testing, determine which instruments should be shortlisted and carry out validation studies as required. He summarised the progress of the rheumatology outcomes group (OMERACT) over the previous 20 years and the group were reminded of the need to put aside preferences and allegiances and work together as a global community for the benefit of patients.
Professor Williams also stressed that the use of a core outcome does not exclude other outcomes being measured in a clinical trial, nor does it imply that the core outcome has to be a primary outcome. A core outcome is simply the minimum set that should be measured and reported in all future eczema trials so results can be compared and that researchers are free to include other outcomes as appropriate.
Dr Jasvinder Singh (USA) from the OMERACT group was invited to attend the meeting to act as an external advisor and to keep the meeting focused and fair. Dr Singh explained why OMERACT was needed, highlighting the similarities between the situation in rheumatoid arthritis research 20 years ago and the situation with eczema now. An important message was that in the interests of achieving consensus the group should accept that the outcome of the consensus may not be perfect but they should ask themselves the question ‘is it preferable to the status quo?’. The core outcomes can be the primary or secondary outcome but researchers should be aware that, if it is not the primary outcome, there is a danger that the study may be underpowered for this outcome measure. The core set can be updated to reflect new findings about disease or if a new stakeholder group is added. The measures included in the core set can also be different for children and adults.
Professor Kim Thomas (UK) highlighted that core outcomes need to be feasible in all settings and trial designs. The core outcome set should (1) include at least one objective measure because not all interventions/trial settings can be blinded, (2) be relatively quick and easy to perform with minimal training required, (3) perform well and (4) measure things of importance to patients and clinicians. Discussions highlighted that there was a variation in how a representative site was determined.
Dr Eric Simpson (USA) explained that the Investigator Global Assessment (IGA) is required by regulatory agencies to be performed in clinical trials and presented data from this review showing that there is huge variation on how IGA is conducted and called for standardisation. In the discussion that followed, the group felt that although not a core domain, the HOME initiative should be involved with helping to standardise and better validate the IGA.
Session 2: signs domain
Professor Jochen Schmitt (Germany) opened the session by reminding the group that it had previously been agreed that signs assessed by a score should be included as a core outcome. He stated the objectives of the session were to achieve consensus on content validity and on which instrument to use to assess clinical signs of AE in future clinical trials.
Dr Mandy Schram (the Netherlands) explained that the TIS score315 is quick and easy to administer because it includes only erythema, oedema and excoriation, each measured at one representative site. Therefore, because of the simplicity of the scale, work had been done to look at the responsiveness of TIS based on previously published trials and was presented here.
Professor Jochen Schmitt (Germany) and Stephanie Deckert (Germany) presented the results of a systematic review assessing the measurement properties of sixteen AE sign scales. Each included scale was evaluated using pre-defined criteria including the OMERACT filter of truth, discrimination and feasibility310 and the quality of the methodological studies using the COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) checklist. 316 The performance of each scale was then categorised as follows.
-
Outcome measure meets all requirements to be recommended for use.
-
Outcome measure meets two or more quality items, but performance in all other required quality items is unclear, so that the outcome measure has the potential to be recommended in the future depending on the results of further validation studies.
-
Outcome measure has low quality in at least one required quality criteria (≥ 1 rating of ‘minus’) and, therefore, is not recommended to be used any more.
-
Outcome measure has (almost) not been validated. Its performance in all or most relevant quality items is unclear, so that it is not recommended to be used until further validation studies clarify its quality.
The two scales that had been the most extensively validated were SCORAD index303 and EASI317 (Table 20). Both scales have adequate content and construct validity, are responsive and internally consistent and have acceptable interobserver reliability (Table 21). Floor and ceiling effects are unlikely to be an issue with either scale. 330 Intraobserver reliability is unclear for SCORAD index but adequate for EASI. Both scales measure the intensity and extent of the signs. Two other scales, TIS315 and SASSAD,326 fulfil some of the methodological quality criteria but further consensus on content validity is required. The simple scoring system327 has reasonable content validity but very little validation work has been carried out on it. Further details of the methods and results of this systematic review are published elsewhere. 330 The conclusion from the systematic review was that EASI and SCORAD were shown to be the most valid and reliable instruments to assess clinical signs of AD.
| Measurement property (name) | Content validity | Construct validity | Internal consistency | Intraobserver reliability | Interobserver reliability | Sensitivity to change | Floor or ceiling effects | Interpretability | Acceptability | Recommendationa |
|---|---|---|---|---|---|---|---|---|---|---|
| ADAM318 | – | NR | + | NR | +/– | NR | NR | NR | NR | C |
| ADASI319 | +/– | NR | NR | NR | NR | NR | NR | NR | + | D |
| ADQ320 | – | +/– | NR | NR | NR | NR | NR | NR | + | C |
| BCSS321 | – | – | NR | NR | + | NR | NR | NR | NR | C |
| EASI322 | + | + | + | + | +/– | + | NR | NR | NR | B |
| OSAAD323 | – | +/– | NR | NR | + | NR | + | NR | – | C |
| POEM305 | – | – | + | + | NA | + | NR | NR | + | C |
| PO-SCORAD index324 | +/– | +/– | NR | NR | NA | NR | NR | NR | + | D |
| SA-EASI325 | + | +/– | NR | NR | NA | NR | NR | NR | NR | D |
| SASSAD326 | +/– | + | NR | NR | +/– | NR | + | NR | + | – |
| SCORAD index303 | + | + | +/– | NR | + | + | + | + | +/– | B |
| SSS327 | – | NR | NR | NR | NR | NR | + | NR | NR | C |
| TIS315 | +/– | +/– | +/– | NR | +/– | NR | + | + | + | – |
| W-AZS328 | – | NR | NR | NR | NR | NR | NR | NR | NR | C |
| Unnamed scale329 | +/– | NR | NR | NR | + | NR | NR | NR | + | D |
| Unnamed scale329 | +/– | NR | NR | NR | + | NR | NR | NR | + | D |
| Measurement property (name) | Measurement property (description) | Inclusion in OMERACT filter | Criteria for adequate rating (+) | Criteria for intermediate rating (+/−) | Criteria for inadequate rating (−) |
|---|---|---|---|---|---|
| Content validity | Extent to which the domain ‘signs’ is comprehensively represented by the items in the instrument | Truth | Clinicians OR patients were involved in item selection; clinicians AND patients consider ≥ 90% of items as representative for the domain AE signs | Clinicians OR patients were involved in item selection; clinicians AND patients consider at 70% to 89% of items as representative for the domain AE signs | Neither clinicians nor patients were involved in item selection; clinicians OR patients consider < 70% of items as representative for the domain AE signs |
| Construct validity | Extent to which an instrument truly measures the construct ‘atopic eczema signs’ | Truth | Two different instruments that aim to measure signs of AE show high correlation (i.e. factor loading/correlation coefficient > 0.70); OR specific hypotheses were formulated AND ≥ 75% of the results are in accordance with these hypotheses | Two different instruments that aim to measure signs of AE show high correlation (i.e. factor loading/correlation coefficient 0.60–0.69) | Two different instruments that aim to measure signs of AE do not show high correlation (i.e. factor loading/correlation coefficient < 0.60); AND/OR specific hypotheses were formulated BUT < 75% of hypotheses were confirmed, despite adequate design and methods |
| Internal consistency | Extent to which items in the scale ‘signs’ are interrelated (measure the same construct) | Discrimination | Factor analyses performed on adequate sample size (seven times the number of items and > 100) AND Cronbach α calculated per dimension AND Cronbach α of 0.70–0.95 | No factor analysis OR doubtful design or method | Cronbach α < 0.70 or > 0.95, despite adequate design and methods |
| Test–retest reliability/intrarater variability | Extent to which repeated measurement by the same investigator provides identical results | Discrimination | Correlation coefficient > 0.90 OR percentage variation < 5% OR coefficient of variation < 10% | Correlation coefficient 0.80–0.90 OR percentage variation 5% to 10% OR coefficient of variation 10% to 20% | Correlation coefficient < 8.80. Percentage variation > 10% OR coefficient of variation > 20% |
| Interobserver reliability | Extent to which application of the instrument by different investigators provides identical results | Discrimination | Intraclass correlation > 0.80 OR weighted κ > 0.60 OR coefficient of variation < 20% OR ANOVA (percentage variance explained by observer) < 10% | Intraclass correlation 0.60–0.80 OR weighted κ 0.40–0.60 OR coefficient of variation 20% to 30% OR ANOVA (percentage variance explained by observer) 10% to 20% | Intraclass correlation < 0.60 OR weighted κ > 0.4 OR coefficient of variation > 30% OR ANOVA (percentage variance explained by observer) > 20% |
| Sensitivity to change/responsiveness | Ability of questionnaire to detect clinically important changes over time | Discrimination | MIC defined; MIC > SDC | MIC defined, but doubtful design or method; MIC > SDC | MIC not defined; MIC ≤ SDC |
| Interpretability | Degree to which one can assign qualitative meaning to quantitative scores (i.e. ranges for clear/almost clear, mild, moderate and severe eczema) | Feasibility | Ranges of the scale have been defined that represent ‘clear/almost clear,’ ‘mild,’ ‘moderate,’ and ‘severe’ AE/AE signs AND MIC defined | Mean and SD scores presented of ≥ 3 relevant subgroups of patients but no clear ranges in scale for qualitative meaning of severity of signs defined OR no MIC defined | No information found on interpretation |
| Floor or ceiling effects | Number of respondents who achieved the lowest or highest possible score | Not required by OMERACT filter | ≤ 15% of the respondents (of validation study but not in a given RCT) achieved the highest or lowest possible scores | Doubtful design or method | > 15% of the respondents (of validation study but not in a given RCT) achieved the highest or lowest possible scores despite adequate design and method |
| Acceptability/ease of use | Degree to which the score can be applied easily, given constraints of time and money | Feasibility | Time to administer < 7 minutes AND score can be used without charge AND no specific tools needed | Time to administer 7–10 minutes AND score can be used without charge AND no specific tools needed | Time to administer > 10 minutes OR score cannot be used without charge OR specific tool needed |
The main differences between the two scales were then discussed: (1) the use of a representative site in SCORAD index, whereas EASI measures the intensity of lesions at multiple body parts and (2) SCORAD index relies on the assumption that the extent of AD has a linear relationship with severity, which has been shown not to be the case in all patients. 331
Professor Schmitt also described a previous study175 that showed both intensity and extent of disease should be measured. There followed an in-depth discussion on the signs domain for which named scales were put aside and a significant amount of time was dedicated to discussing which signs are essential to reflect the construct of the disease and should therefore be included in the core outcome. With reference to the book Measurement In Medicine – A Practical Guide,332 Professor Schmitt explained that when the conceptual framework was considered, signs were a formative model, because the items of instrument form the construct (domain) to be measured. Therefore, content validity, defined as the degree to which the domain ‘signs’ are comprehensively represented by the items in the instrument, is crucial. In other words, the items in the instrument all need to be relevant and all relevant items need to be included.
The main points arising from the discussion are summarised in Box 2.
The three signs that have previously been shown to be independent predictors of patient-rated disease severity (excoriations, erythema and oedema/papulation) were all considered to be important signs.
In addition, lichenification should also be considered for inclusion because it often more prominent in darker skin types and reflects the chronic relapsing nature of the disease.
The relative important of other signs were discussed including crusting/oozing, xerosis/dryness, blanching and flaking.
It was generally felt that there should be some measure of area involvement in the scale.
Discussions suggested that it would be appropriate for the core outcome ‘clinical signs’ to be an investigator-assessed objective measure to reduce information bias and because the other three domains (symptoms, QoL and long-term control) are primarily patient reported. index Discussions highlighted that it is important to remember that patient versions of scales are not the same as investigator versions (e.g. EASI and SA-EASI325 or SCORAD index and patient-oriented SCORAD index333) and should not be used interchangeably. However, only core outcome measures were under discussion at this meeting and investigators are free to also include any other scales including patient-reported signs scores in addition to the core outcome measure which will be the investigator version.
SA, self-administered.
In the subsequent voting session, consensus was achieved on the following points (Table 22) for details of voting results).
-
The core outcome measure for the signs domain should include erythema, excoriation, oedema/papulation and lichenification as a minimum to achieve content validity.
-
Both the intensity and extent of each clinical sign in the core set should be measured.
-
No other signs are essential to be included, so further individual signs were not voted on.
| Signs domain | Yes (%) | No (%) | Unsure (%) |
|---|---|---|---|
| Should this domain measure intensity of clinical signs? | 93 | 2 | 4 |
| Should this domain measure extent of clinical signs? | 89 | 4 | 7 |
| Should this domain measure both intensity and extent of clinical signs? | 96 | 4 | 0 |
| Should signs be assessed by an investigator? | 91 | 2 | 7 |
| Is it essential that erythema is included in the domain ‘clinical signs’? | 95 | 2 | 2 |
| Is it essential that excoriation is included in the domain ‘clinical signs’? | 81 | 7 | 12 |
| Is it essential that oedema/papulation is included in the domain ‘clinical signs’? | 84 | 5 | 11 |
| Is it essential that lichenification is included in the domain ‘clinical signs’? | 79 | 7 | 14 |
| Are there any other signs that you think are essential to be included? | 17 | 83 | – |
Only once there was consensus on important criterion of content validity did the discussion move on to which of the named scales identified in the systematic review met the criteria for content validity AND standards for validity in other areas. Professor Schmitt referred back to the systematic review results to confirm that objective SCORAD index and EASI both include the four signs voted as essential for inclusion (erythema, excoriations, oedema/papulation and lichenification) and, therefore, it was agreed that there was no need to create an entirely new scale and these two scales would be shortlisted for consideration. Objective SCORAD index was considered from this point onwards (rather than SCORAD index) because the purpose here was to identify a scale to measure only the domain of eczema signs. Objective SCORAD index measures signs only whereas SCORAD index is a composite measure which measures both signs and symptoms. Professor Schmitt reiterated both objective SCORAD index and EASI had construct validity and both are freely available at no cost. He also noted the eczema community is very fortunate to have two good scales to choose from to measure clinical signs.
The properties of each scale were then described to the group (Table 23).
| Objective SCORAD index (presented by Professor Jochen Schmitt) | EASI (presented by Dr Eric Simpson) |
|---|---|
| A representative site is selected for each sign | An average score for different areas of the body for each of the essential signs is used (four signs and four body sites) |
| Measures six signs: the four agreed essential signs plus oozing/crusting and dryness | Measures only the four agreed essential signs |
| Gives more weight to intensity than extent | Each sign and extent are equally weighted |
Nominal group techniques were then used to achieve consensus on which scale to recommend for inclusion in the core outcome set. The group split into five smaller groups with mixed nationalities in each to discuss the advantages and disadvantages of two scales. Each of the small groups reached independent conclusions and voted on their preferred scale; all five groups independently voted in favour of EASI.
The conclusions and reasons why EASI was favoured over objective SCORAD index were then shared with the group as a whole. One of the major concerns with objective SCORAD index was the selection of a representative site, whereas this is not an issue for EASI. In addition, because objective SCORAD index also measures oozing/crusting and dryness, extra data are being collected, whereas EASI includes only the four signs agreed as essential. The groups felt that extent is not given enough importance in objective SCORAD index but it does have sufficient weighting in the EASI scale and, lastly, EASI distinguishes between different body areas and this could be important for different age groups and possibly for future treatments which may target particular body sites.
Professor Schmitt then reminded the whole group that the aim of HOME is to recommend just one scale per domain for the core outcome set (as agreed at the HOME II meeting) and so the whole group proceeded to vote on whether objective SCORAD index or EASI should be recommended for inclusion in the core outcome set (Table 24).
| Question | EASI (%) | Objective SCORAD index (%) | Unsure (%) |
|---|---|---|---|
| Which of these instruments should be included in the core set? | 90 | 7 | 2 |
| Yes | No | Unsure | |
| Do we add an asterisk to our recommendation that objective SCORAD index be measured in addition to EASI in future clinical trials where possible? | 57 | 36 | 7 |
The result of the vote was 90% in favour of EASI to be recommended as the core outcome measure for the clinical signs of eczema (7% in favour of objective SCORAD index and 2% were unsure).
It was proposed that HOME recommend including both objective SCORAD index and EASI for a period of time to collect comparative data on the two scales, but this was not supported as 36% voted against. However, researchers are free to include other scales as appropriate to suit their trials.
Session 3: quality-of-life domain
Dr Shehla Admani (USA) presented a focused review of the nine most commonly used QoL measurement tools in AE trials and the properties of each instrument.
Dr Christian Apfelbacher (Germany) proposed a protocol to systematically assess measurement properties of eczema-specific measures of HRQoL and identify outcome measures for eczema-specific QoL. This will follow similar methods to the signs systematic review330 and will be completed ahead of the HOME IV meeting.
There followed a discussion on the QoL domain. It is clear that HOME should liaise with the American Association of Dermatology regarding systematic reviews of HRQoL being conducted. The use of Patient Reported Outcomes Measurement Information System should be considered334 but internationalisation of the terminology used would be required. Generic scales can perform as well as specific scales with regard to sensitivity to change but are often not popular with patients as they can be lengthy to complete. The fact that different scales may perform better for specific age groups was highlighted and that work on the QoL domain should include significant patient input.
Session 4: symptoms domain
Dr Phyllis Spuls (the Netherlands) opened the session by distinguishing between signs and symptoms stating it is not necessarily the nature of the sign or symptom which defines it, but who observes it. A symptom is any feature which is observed by the patient whereas a sign is observed by other people.
Dr Spuls showed that symptoms were reported in three-quarters of trials published between January 2000 and May 2012, with itch and sleep loss being the most commonly reported. Other symptoms that were reported less frequently included stinging, burning, pain, rash and dryness. SCORAD index was the most commonly used named scale to measure symptoms but most trials that used SCORAD index (92%) did not report the symptoms separately to the overall composite score.
Dr Laura von Kobyletzki (Sweden) presented the results of a web-based survey asking patients and parents/carers in 31 countries to rate the importance of a list of physical signs and symptoms. From the 831 responses (65.5% female, age range < 5 to > 66 years), itch and pain were the highest rated, with hot/inflamed skin, amount of skin affected by eczema, eczema on visible sites (e.g. hands and face), sensitive sites, bleeding, weeping, cracks and sleep difficulties were also considered important. Therefore, more than just itch and sleep loss should be considered when evaluating outcome measures for eczema symptoms.
Dr Norito Katoh (Japan) presented a study showing that there was significant correlation between the visual analogue scale (VAS) and the verbal rating scale in 949 Japanese patients with itchy skin diseases. Each category of verbal rating scale (no pruritus, mild, moderate, severe, very severe) differed significantly from the other categories based on the VAS scoring. Although not directly leading to HOME objectives, this is an example of when useful work on eczema outcomes is related to HOME.
In the subsequent discussion on the symptoms domain, it was agreed that the next steps towards a consensus on symptoms outcome measure should follow the HOME roadmap. This will involve (1) finalising the important constructs in the symptoms domain from patient’s perspective by extending the survey presented by Dr von Kobyletzki to non-western, non-white skinned patients and (2) systematic review of the validation studies on currently available instruments and assessment of the quality of the scales. The overlap with QoL should be considered carefully throughout. Input from patients raised several important issues: (1) the effects of sleep loss are important and generic sleep loss questions may not detect the real impact of AE on sleep, (2) fatigue is a different symptom to sleep loss and (3) different symptoms are important to individuals of different ages.
It was then agreed by voting that itch and sleep loss were not sufficient on their own to reflect the construct of the disease; other symptoms should also be considered for inclusion. The detailed voting results can be found in Table 25.
| Symptoms domain | Yes (%) | No (%) | Unsure (%) |
|---|---|---|---|
| Should symptoms include items in addition to itch and sleep loss? | 78 | 18 | 5 |
Session 5: long-term control domain
Professor Thomas began by presenting some issues that are particular to this domain. She emphasised that the HOME group needs to agree on the definition of ‘long-term control’ and determine whether or not it is truly a separate domain or simply a repeated measurement of other core outcomes. There are several options for measuring long-term control, including flares, escalation of treatment, assessment of well-controlled weeks (WCW) and the accessing of health resources, but the outcome measure needs to suit trials of all durations and reflect that eczema is a chronic disease. There are parallels with asthma where researchers have recently published a consensus on using ‘escalation of therapy’ as an indicator of control. 335
Professor Thomas presented data from a systematic review on definition of flares. Out of the 26 included studies, 21 different flare definitions were used. Flares (as currently defined) may not be a good contender for a core outcome measure for the HOME long-term control domain, particularly for trials with long follow-up or minimal patient contact as there are difficulties in collecting the data required.
Professor Thomas presented results of a validation study to test the previously published definition of flare based on ‘escalation of therapy due to worsening of disease’. This is similar to flare definitions used in the asthma literature and is an intuitively understood concept that correlated moderately well with other eczema severity scales (POEM,305 TIS315 and SASSAD). 326 However, capturing flares in this way does require completion of daily diaries which is resource intensive and so may not be suitable for all settings.
The final presentation from Professor Thomas focused on assessment of WCW and how well this performs in capturing long-term control. Although the concept of WCW is intuitive and well understood, the data collection and management is complex and resource intensive so may not be suitable in all trial settings. Nevertheless, similar to flares, WCW correlated moderately well with other eczema severity scales (POEM, TIS and SASSAD).
The relative merits of the two broad approaches to measuring long-term control of eczema were discussed and are summarised in Table 26.
| Measure of flares/WCW | Repeated serial measurements of the other three domains (signs, symptoms and QoL) |
|---|---|
| Measurement of flares should be a patient-reported outcome because a flare is a significant event for patients and so they are well placed to determine when a flare has occurred. Trials often do not have enough clinic visits to enable flares to be measured by the investigator | This could be attractive and efficient way of measuring long-term control if the other domains are already being captured in the trial |
| There is variability between patients in how they define a flare so individual flare definitions may be needed in a trial. It should be remembered that patients can have poor control without a flare | Scales would need to be completed frequently enough to capture the fluctuations in the eczema which may impact on the feasibility of this approach |
| A definition of a flare (including the end of a flare) needs to be agreed on if it is to be used | The EASI scale only captures the eczema at that moment, so to capture signs over the long term, frequent trial visits with the investigator would be required |
| Parents can sometimes get confused about whether or not questions on WCW refer to the eczema or the child’s behaviour | There are many ways these data can be analysed to measure long-term control (e.g. mixed models, fixed effect models) |
| Need to determine whether or not the floor effect is important because trials are measuring a benefit | Does not reflect any changes in treatment |
| Existing trial data, in which a mixture of daily, weekly and monthly data have been collected, should be used to establish the frequency of data collection needed |
There is great variation in how people define long-term control including stable disease over a period of months, lack of need for escalation of treatment/rescue therapy, number and duration of flares, duration of the trial (long term) and repeated measurement of the other three core outcome domains. It was agreed by voting that the next stage is to conduct a systematic review to establish how long-term control has been captured in other trials (and possibly other long-term prospective studies) followed by a systematic review of validation studies if there are any. Work on addressing some of the other questions can then begin. Voting suggested that long-term control is a unique concept that requires its own outcome tool but there was mixed opinion regarding whether or not long-term control should be completed exclusively by patients and how often long-term control should be measured. However, it was agreed this domain should apply to trials of > 3 months duration. Details of the voting results can be found in Table 27.
| Long-term control domain | Yes (%) | No (%) | Unsure (%) | |||
|---|---|---|---|---|---|---|
| Is a systematic review of long-term control outcomes required as the next step? | 68 | 18 | 15 | |||
| Is long-term control a unique concept that requires its own outcome tool? | 68 | 18 | 15 | |||
| Should ‘long-term control’ be completed exclusively by patients? | 33 | 56 | 11 | |||
| Daily | Weekly | Monthly | Depends on trial design | Unsure | ||
| How often should long-term control ideally be measured by patients? (%) | 16 | 42 | 11 | 16 | 16 | |
| 1 month | 2 months | 3 months | 6 months | 12 months | ||
| In trials that purport to measure long-term control, what is the minimum recommended duration? (%) | 3 | 0 | 41 | 38 | 18 | |
Discussion
The HOME III was a productive meeting that built on the consensus from the previous HOME II meeting that the four core domains to be measured are signs, symptoms, long-term control and QoL. At this meeting, consensus was achieved that EASI be recommended as the core outcome measure for the clinical signs of eczema. This does not preclude the use of other signs scales in a trial, the core outcome is simply the minimum that should be recorded and reported trial data can be combined in the future. Progress was also made towards the goal of recommending core outcome measures for the remaining three domains of symptoms, long-term control and QoL.
The first stage of the process towards recommending core outcomes for measuring the clinical signs of eczema was to determine the important criterion of content validity, that is which signs of eczema are essential to reflect the construct of the disease and, therefore, must be included in the core outcome measure. It was agreed by consensus that the four essential signs were excoriations, erythema, oedema/papulation and lichenification. A significant proportion of the meeting time was dedicated to this stage of the process and only once consensus was achieved on content validity did the discussion move on to named scales. Only two scales identified in the systematic review measured all four essential criteria and met the standards for validity in other areas: objective SCORAD index and EASI. These two scales were therefore taken forward for further consideration as the core outcome measure and discussion in small, mixed-nationality groups focused on the differences between the two scales such as the use of ‘representative lesions’ and percentage area of body involvement. Eventually a vote was held on which would be recommended as the core outcome. In other words, the process did not start with deciding which scale was best; it began with deciding what essential signs the core outcome needed to measure and then identifying which scales measured these essential signs and performed well enough to be considered as a core outcome.
Given that the meeting was held in the USA, there was potential for the outcome to be biased in favour of signs scales commonly used in the USA. However, discussions were open and frank and all present at the meeting agreed from the outset to put aside any allegiances to allow progress towards consensus. Presenters and facilitators were from all over the world (Europe, n = 8; USA, n = 3; and Japan, n = 1) and when discussing in breakout groups, a mix of nationalities were in each group. We also used keypads so that voting was anonymous. The final vote on clinical signs was overwhelmingly in favour of EASI.
The long-term control domain proved to be particularly tricky. It was immediately apparent that the concept of long-term control of eczema means very different things to different people and it was clear that the group needed to go back a step to clarify this before progress could be made towards recommending an outcome measure. There is also a significant body of work to be done in both the symptoms and QoL domains, starting with systematically identifying all available tools and quality rating the tools. These three domains will be the focus of the next HOME meeting.
Consensus statement
Identification of the best instrument to measure clinical signs of atopic eczema in clinical trials
The HOME roadmap (Figure 15) has been developed as guidance on how to identify appropriate instruments to measure each of the four core outcome domains of AE. This section describes in detail how we applied the HOME roadmap for the core domain ‘clinical signs of atopic eczema’. Instruments for other domains have not yet been agreed by the HOME initiative.
FIGURE 15.
The HOME roadmap. a, For trials the scope should generally be global.
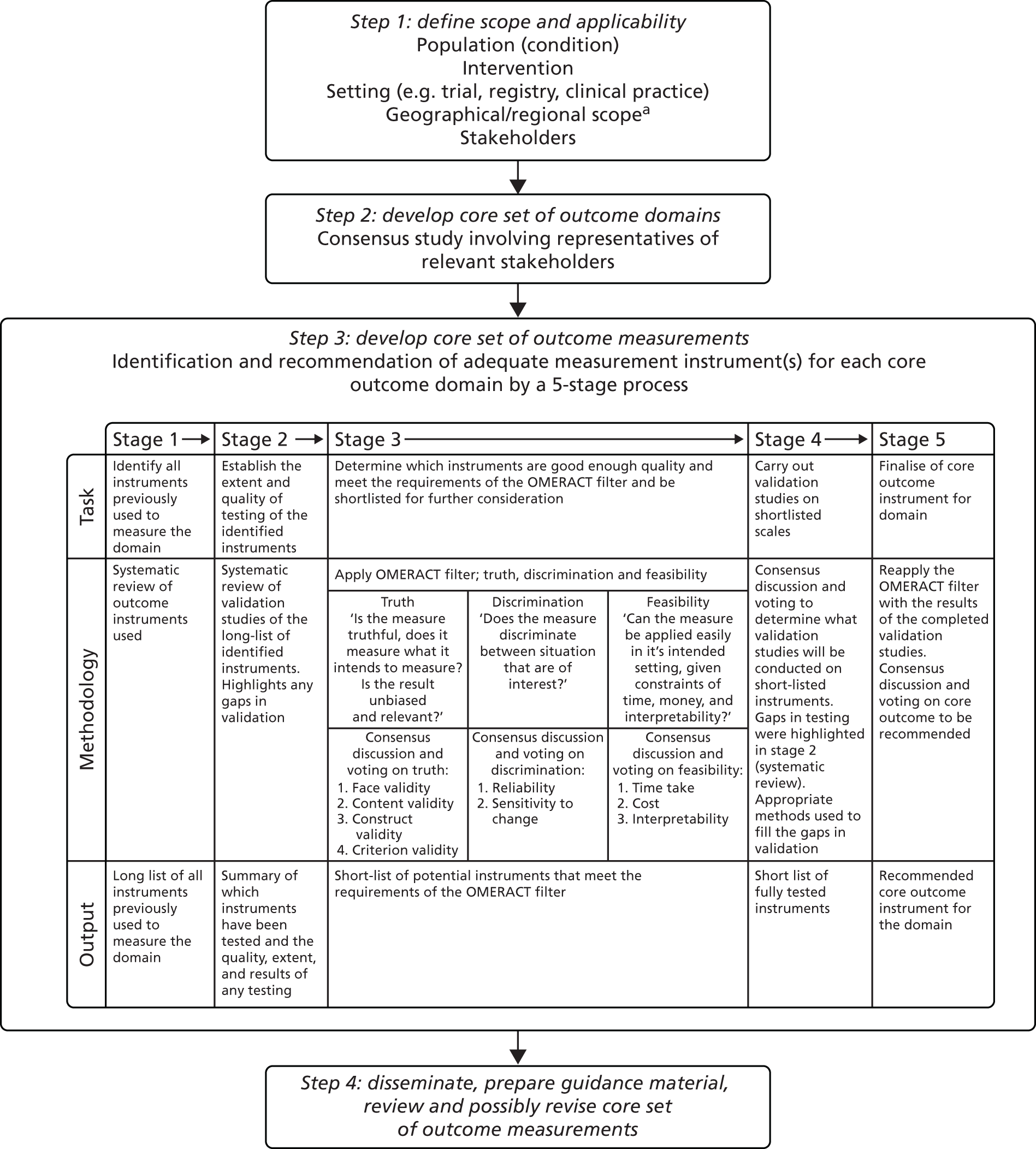
In 2007, a systematic review175 identified 20 named instruments to assess the severity of AE. Another systematic review336 summarised outcome measures in all 791 clinical trials on interventions for AE published in 1985–2010 and identified SCORAD index303 and EASI as the two most widely used instruments to measure clinical signs of AE.
To establish the extent and quality of testing of the identified instruments, we conducted a systematic review330 of inauguration and validation studies on all published instruments to measure clinical signs of AE. Out of the 16 different instruments identified to assess clinical signs of AE, only the EASI and the SCORAD index were identified as valid and reliable.
Based on predefined quality criteria also suggested by other outcomes, research initiatives such as COSMIN,337 the EASI has adequate validity, responsiveness, internal consistency, intraobserver reliability and intermediate interobserver reliability. The SCORAD index has adequate validity, responsiveness, interobserver reliability and unclear intraobserver reliability. 330 The SASSAD326 severity score and the TIS315 score fulfil some quality criteria, but the performance in other required measurement properties is unclear. 330 However, both SASSAD and TIS only have intermediate content validity (SASSAD does not capture oedema/population and TIS does not include BSA involved), which is a disadvantage compared with EASI and SCORAD index. 330 The clinical signs of AE are assessed by a physician in the EASI, SCORAD index, SASSAD and TIS. The POEM305 scale is a reliable and responsive instrument to assess the signs and symptoms of AE by the patient, but was not developed as a tool to capture objective AE signs. 330
At the HOME III meeting in San Diego on 6–7 April 2013,338 the international panel of 56 individuals from 10 countries including Asia, Europe, South America and the USA representing stakeholders such as consumers, dermatologists, nurses, methodologists and pharmaceutical industry specified the content required to adequately and completely assess ‘clinical signs’ of AE.
There was broad consensus that the instrument used should be scored by a physician and rate both the intensity and extent of the AE signs. Intensity should be assessed for the following signs: erythema, excoriation, oedema/papulation and lichenification.
The panel further reached consensus that there are no other essential signs to be included in an instrument. As highlighted by the systematic review (stage 2), only the objective SCORAD index and EASI include the four essential signs and performed adequately in validation studies. 17 Using a nominal group technique involving small group work, followed by whole panel discussion, the EASI was identified as being preferable to the SCORAD index to be used for the assessment of clinical signs. The main reasons for this choice were that (1) it only includes the four essential signs, thus avoiding redundancy; (2) assessment of severity of AE signs is assessed at multiple body sites, rather than at a single representative site for each sign, which can be problematic when using the SCORAD index; and (3) that the extent of AE lesions has sufficient weighting (50% of total score) in the EASI, but is not given enough importance in SCORAD index (approximately 20% of total score). In the final consensus voting patients, physicians, methodologists and pharmaceutical industry representatives agreed to recommend EASI as the core outcome for the assessment of clinical signs to be used in all future AE trials. In the final voting, 90% of the panel indicated that the EASI should be included into the core set of outcomes domains to measure clinical signs of AE, 7% voted for the objective SCORAD index, and 2% were unsure. 322
Recommendation
Conducting an iterative process of systematic reviews and international consensus sessions involving a range of stakeholders including patients, we followed the HOME roadmap and identified the EASI322 as the core outcome instrument to measure the core domain ‘clinical signs of atopic eczema’ in all future trials investigating the interventions for AE.
Conclusion
All investigators, pharmaceutical industry and regulatory authorities are asked to comply with this consensus and to use at least the EASI in all future AE trials to enable evidence-based decision-making and scientific communication in the future.
Eczema priority setting partnership
The following text is adapted with permission from Batchelor et al. 195 © 2012 The Authors. BJD © 2012 British Association of Dermatologists.
Summary
What was already known about this topic?
-
There is uncertainty regarding the effectiveness of many of the commonly used treatments for eczema.
-
Eczema research to date has largely been driven by the priorities of the pharmaceutical industry and academia.
What did this study add?
-
By means of an explicit process, we identified the top 14 uncertainties in eczema treatment that are important to patients who have the disease, their carers and the HCPs who treat them.
-
These prioritised treatment uncertainties can be used to guide researchers and funding bodies in their decisions regarding future investment of time and resources in eczema research.
Introduction
Given the large number of treatment uncertainties highlighted in our systematic review of eczema treatment, it is important that some form of prioritisation occurs. Traditionally, much of the research agenda has been set by the pharmaceutical industry and academics, but the priorities of these groups may be very different from those of patients and HCPs. 339 Therefore, we formed a partnership with the James Lind Alliance to identify and prioritise eczema treatment uncertainties that are of importance to patients who have the disease, their carers and the HCPs who treat them. The James Lind Alliance, funded by the NIHR and the Medical Research Council (MRC), facilitates co-operation between patients and clinicians to identify important treatment uncertainties, thereby influencing the prioritisation of future research. 340
The objectives of this eczema PSP were: (1) for patients and carers and HCPs to identify eczema treatment uncertainties; (2) to agree a prioritised list of those uncertainties; (3) to publicise the results of the PSP; and (4) to submit the results to research commissioning bodies.
Methods
The five stages of the PSP, which are outlined in Box 3 took place between April 2011 and January 2012. We adopted the methods outlined by the James Lind Alliance339 to meet the needs of this particular PSP and to overcome some of the issues encountered during the previous vitiligo PSP, while remaining true to the James Lind Alliance’s ethos of transparency, accountability and inclusivity. In particular, we sought to ensure that the treatment uncertainties that are important to patients and HCPs were prioritised at an earlier stage, rather than relying on consensus developed at the final workshop. The workshop was then used to ensure adequate engagement with all relevant stakeholders in the development of possible research questions that could address the identified treatment uncertainties.
The PSP was co-ordinated from the Centre for Evidence Based Dermatology in Nottingham, with oversight by a representative of the James Lind Alliance (Sally Crowe). A Steering Group was formed to manage the PSP process. This Steering Group comprised an independent chairperson (SC), four patients/carers (including a representative from the National Eczema Society), four HCPs (two dermatologists, a dermatology nurse specialist and a GP) and three researchers/administrators at the Centre for Evidence Based Dermatology at the University of Nottingham.
Stage 1: initiation – identification of potential partner organisations and individuals, ensuring representation of patients, carers and HCPs.
Stage 2: consultation – participants invited to submit up to five eczema treatment uncertainties.
Stage 3: collation – any non-questions or uncertainties not related to eczema or its treatment removed. Unique questions combined into ‘indicative uncertainties’ and reworded into standard question format.
Stage 4: ranking – participants asked to select top 10 indicative uncertainties.
Stage 5: workshop – to formulate research questions based on shared (patient/carer and HCPs) uncertainties.
Stage 1: initiation
We identified potential partner organisations and individuals through a process of peer knowledge and consultation, using the Steering Group members’ networks and through the James Lind Alliance’s existing register of affiliates. We were careful to ensure representation of the following groups: people who have had eczema; carers of people who have had eczema; and doctors, nurses and professionals allied to medicine with clinical experience of eczema.
Organisations/groups approached at this stage and who agreed to participate included National Eczema Society; Nottingham Support Group for Carers of Children with Eczema; Skin Care Campaign; BAD; British Dermatological Nursing Group; Queen’s Nursing Institute; Royal College of Nursing; Royal Pharmaceutical Society; UK Dermatology Clinical Trials Network (UKDCTN); the HOME Initiative; MCRN; Cochrane Skin Group; Primary Care Dermatology Society; Society for Academic Primary Care Dermatology Research Specialist Interest Group; and Royal College of General Practitioners. Potential partners were informed about the PSP, invited to participate and encouraged to affiliate with the James Lind Alliance.
The James Lind Alliance and Steering Group assessed organisations for any potential conflict of interest that might have led to unacceptable bias if they were to participate in the PSP. 341 Parties in which a potential conflict of interest was identified were not invited to participate.
Stage 2: consultation – collection of treatment uncertainties
The aim of this stage was to collect treatment uncertainties using the following question: ‘What question(s) about eczema treatments would you like to see answered by research?’ To enable people from a wide geographical area to take part, we collected data using online and paper surveys. 342 This methodology has the additional advantage of avoiding interviewer bias343 and interview effects. 19 We carried out the online survey (SurveyMonkey®, SurveyMonkey Inc., Palo Alto, CA, USA) and sent a paper copy to participants on request. For practical and ethical reasons, we asked the carers of children with eczema to complete a survey on their child’s behalf. As eczema is such a common condition, if a participant reported that they were both a HCP and a patient or carer, then they were categorised as being a HCP for the summary statistics.
Participants submitted a maximum of five treatment uncertainties. The survey questionnaire was designed to capture each participant’s uncertainty about the effects of treatments for eczema. The survey text and accompanying information sheet were checked for readability and ease of understanding by lay members of the Steering Group. We provided broad treatment categories (e.g. topical, systemic, non-drug treatments) in order to guide participants in submitting uncertainties. Completion of the survey was considered to imply consent to participation in the prioritisation process. Any uncertainties registered on Database of Uncertainties about the Effects of Treatments (DUETs) but not represented in the list of uncertainties generated through the survey, were added to the list of uncertainties.
The survey was advertised through a combination of direct e-mails and newsletters to members of the partner organisations and through links on relevant websites. Individuals with eczema who had been in contact with the Centre for Evidence Based Dermatology and the James Lind Alliance were contacted directly and invited to participate.
Stage 3: collation of treatment uncertainties
The aim of this stage was to review the treatment uncertainties gathered in the consultation stage and to create a list of ‘indicative uncertainties’ (i.e. those that represent a number of similar uncertainties submitted in the survey). We first removed any non-questions (e.g. statements or comments), uncertainties not related to treatment of eczema (e.g. aetiology) or uncertainties not relating to eczema. We then reviewed the remaining uncertainties and, when appropriate, combined any similar uncertainties to create ‘indicative uncertainties’, which we refined into a standard format. 344 An example of several treatment uncertainties and the indicative uncertainty into which they were refined is given in Box 4. Any indicative uncertainties that arose from single treatment uncertainties were removed prior to the ranking stage.
-
What are the long-term health effects of using steroid creams compared with the long-term effects of leaving the skin untreated?
-
Aren’t I causing long-term damage to my skin by constantly using steroid creams?
-
How long is too long when using hydrocortisone?
-
I am worried about the long-term use of steroids on the skin. Should I be worried?
-
How appropriate is prolonged steroid use?
Examples of the underlying questions that informed the typical indicative uncertainty
The process of collating the treatment uncertainties was conducted by a single researcher (TC) in the first instance. The full list and wording of the indicative uncertainties was then checked by members of the Steering Group (ensuring that each indicative uncertainty had been approved by at least one patient and one HCP). Consensus was sought from all members of the Steering Group if differences in opinion were apparent. Once the list of indicative uncertainties had been approved, uncertainties that could be resolved with by published systematic reviews345 were removed from the process.
Stage 4: ranking
The aim of this stage was to rank the indicative uncertainties into those that both patients and HCPs felt were important. The list of indicative uncertainties was converted into a draft survey, which was user tested by members of the Steering Group and representatives of the UKDCTN patient panel. We sent a link to the online survey (or paper copy) to all those who responded to the first survey outlined in stage 2, asking participants to select a maximum of 10 uncertainties that they considered to be the most important.
Results of the ranking exercise were then reviewed by the Steering Group and an agreed priority list established based on rank order when ordered by frequency of votes. In the event of a large difference between the number of patients and HCPs taking part, it was agreed that the ranked lists would be examined both separately and combined for all participants.
Stage 5: workshop to develop research questions
The aim of the workshop was to review the results of the prioritisation exercise and to start to develop research questions on the basis of the prioritised uncertainties.
A selection of participants, drawn from partner organisations and individuals that had contributed to the prioritisation process, were invited to take part. In addition to these patients and HCPs, researchers with experience in the design and conduct of applied clinical research were also invited to attend for this final stage, as it was felt that their expertise would be crucial in the development of well-formulated and feasible research questions. Participants were asked to complete a declaration of interests, including disclosure of relationships with for-profit organisations.
Participants were divided into four discussion groups. Each group was led by an independent facilitator and comprised broadly equal numbers of patients, HCPs and researchers. Group members received summary information about each indicative uncertainty, including details of the original wording of the treatment uncertainties (as submitted by the participants during the consultation stage) in order to provide contextual information about the topic when developing potential research questions. Each of the groups was asked to address up to four treatment uncertainties and it was anticipated that each broad area of uncertainty would potentially result in several focussed research questions.
A standard pro forma, based on a participants, intervention, comparator, outcome (PICO) format, was used to guide discussions.
Results
Consultation and collation stages
A total of 493 participants (341 patients/carers; 132 HCPs; 20 unclear) submitted a total of 1070 uncertainties in the initial consultation stage. After removal of submissions that were either non-questions, uncertainties not related to treatment of eczema or uncertainties not relating to eczema, 718 uncertainties remained (including 61 from the UK DUETs – 55 from systematic reviews and 6 from patients). These were represented by 65 indicative uncertainties, across 17 treatment categories. Thirteen out of the 65 indicative uncertainties were removed because only one person submitted them, therefore 52 indicative uncertainties progressed to the ranking stage. The Steering Group agreed that this was a suitably representative and manageably sized list to take forward to the next stage of the process.
Ranking stage
A total of 514 individuals participated in this survey, of whom 399 (78%) were patients/carers and 106 (21%) were HCPs and 9 were unclear (1%). The Steering Group noted the imbalance between patient/carer and clinician respondents and although this was felt to reflect an achievement in ensuring patient/carer participation, they also recognised the importance of representing the views of HCPs adequately. Consequently, the ranked uncertainties were examined as both a single combined list and for the two groups separately.
Four uncertainties featured in the top 10 lists for both patients/carers and HCPs and these were immediately prioritised as being important (hereafter referred to as ‘shared priorities’). The remaining uncertainties from the two top 10 lists were assessed. Some uncertainties were combined at this stage as they were very similar and the Steering Group wanted to ensure that individual topics did not dominate the final shortlist at the expense of other important topics. Three uncertainties relating to diet were combined in this way from the patients’ list to form the single indicative uncertainty: ‘What is the role of diet in treating eczema: exclusion diets and nutritional supplements?’ Two uncertainties relating to education were combined from the HCPs’ list to create the uncertainty: ‘Which is more effective in the management of eczema: education programmes, GP care, nurse-led care, dermatologist-led care or multidisciplinary care?’ After these revisions had been made, the next five highest ranked uncertainties from the patients/carers’ priorities and the HCPs’ priorities were added to the four shared priorities, giving a shortlist of 14 priority area (for future research) (Table 28).
| Uncertainty | Suggested research question(s) |
|---|---|
| What is the best and safest way of using topical steroids for eczema: frequency of application, potency, length of time, alternating with other topical treatments, and age limits for treatment? | In patients with eczema seen in primary care does a regular ‘weekend’ therapy of topical steroids lead to better management than a reactive approach? |
| Does an early aggressive treatment policy lead to better outcomes than standard practice? | |
| Does inducing remission with a stronger/longer duration of topical steroids have a long-term impact on disease exacerbations? | |
| What is the long-term safety of applying steroids to the skin for eczema? | |
| What is the long-term safety of applying steroids to the skin for eczema? | What are the specific long-term side effects of applying steroids to the skin, as opposed to non-treatment or under treatment? |
| What role might food allergy tests play in treating eczema? | What is known already about the accuracy of food allergy tests and about which foods should be tested in eczema patients? (This should include background to the prevalence of food allergies in eczema patients) |
| What is the predictive value of different food allergy tests? | |
| What role might food allergy tests play in the treatment of eczema in the 0- to 5-year age range? | |
| Which emollient is the most effective and safe in treating eczema? | What determines patient choice and use of emollients and does choice affect outcome? |
| What is the optimal amount of emollient to use? | |
| What are the most harmful and beneficial ingredients of an emollient? |
Workshop and resulting research questions
The 1-day workshop took place in January 2012 and involved 40 participants (11 patients, 17 HCPs, 7 researchers, 4 facilitators and 1 observer). Following brief presentations about the prioritisation process and the aims of the day, participants went into their groups to discuss the priority areas.
Overall, 6 out of the 14 prioritised uncertainties were discussed during the day and these resulted in 13 possible research questions. Details of the research questions arising from the four shared priority topics were given most consideration and these are listed in Table 28. The proposed research questions are intended to be a broad reflection of how the priority topics might usefully be interpreted and addressed, as viewed by the workshop participants on the day. They are not intended to be exhaustive in their scope or to be fully developed in terms of detail and breadth.
Discussion
Our prioritisation process has demonstrated numerous uncertainties surrounding many commonly used eczema treatments. Such widespread uncertainty surely has an impact on the consistency of information given by HCPs and patients’ confidence in, and adherence to, this advice. 340 Many of the uncertainties have been highlighted as research gaps in previous guidelines and our PSP process has confirmed them as being important to both patients and HCPs.
Interestingly, a number of themes are evident across the prioritised uncertainties and they are reflected in the research recommendations in recent guidelines. For example, clinical trials are needed to assess the optimum treatment regimens for topical corticosteroids and to assess their long-term safety. 346 The effectiveness of education programmes for patients and HCPs, designed to promote understanding of and adherence to treatments, is another recurring theme across the PSP and recent guidelines. 346–348
It is also interesting that similar themes (long-term safety and education programmes) have featured prominently in other PSPs convened by the James Lind Alliance (Sally Crowe, Crowe Associates, 2012, personal communication).
To date, the James Lind Alliance have conducted eight PSPs, covering a wide range of health conditions. Given the team’s experience of running the vitiligo PSP in 2009/10,344 we were keen to innovate and expand the remit of the PSP. This was achieved by moving beyond the simple generation of prioritised treatment uncertainties, to helping with the generation of subsequent research questions by means of consensus and open engagement with service users at the final workshop. Such early engagement should ensure that subsequent research studies are based on clinical need and may help to facilitate early implementation of research findings. 349
The concept of agreeing the priority topics in advance of the workshop (on the basis of broad consensus achieved during the earlier stages of the PSP) worked well and resulted in a list of priority topics that had considerable breadth while still being generated through a transparent and democratic process.
It is deeply concerning that despite > 500 RCTs of eczema treatments having been published,224,228 some of the most fundamental questions about the long-term management of eczema remain unanswered. All the uncertainties identified by this PSP have been added to the UK DUETs350 to provide reference for funding bodies and researchers. The challenge now is to utilise existing and new partnerships, such as those developed during this process, to undertake high-quality, pragmatic trials that reflect the priorities of people who live with or treat eczema on a daily basis.
CLOTHing for the relief of Eczema Symptoms (CLOTHES) trial: a study proposal
The following text is adapted from Harrison et al. 351 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Summary
What was already known about this topic?
-
Silk clothing for eczema has been available on prescription in the UK since 2009. It is thought that the clothing may help eczema by maintaining a constant humidity next to the skin, reducing the number of bacteria on the skin and by the prevention of scratching. However, the existing trial evidence to support its use is limited to small trials with inconclusive findings.
What did this study add?
-
This trial assesses the clinical effectiveness and cost-effectiveness of silk therapeutic clothing for the long-term management of eczema in children.
Introduction
A recent update of the Cochrane review of interventions to reduce Staphylococcus aureus in the management of AE352 included a section on antimicrobial clothing. The review found two small RCTs of silk clothing,353,354 but these were too small (22 and 30 participants, respectively) and of too limited a duration (12 and 4 weeks, respectively) to be able to inform clinical practice. The NIHR HTA systematic review of treatments for eczema224 has now been updated as described in the first section of this report (see Chapter 1), but no additional RCTs on the use of silk clothing have been identified.
On the basis of the existing trial data, it is not possible to establish if silk clothing provides measurable benefits for patients with AE. If shown to be beneficial, then the effects may be due to several factors: (1) silk clothing may enhance hydration of the stratum corneum and also allow sweat to evaporate, (2) the fine fibres are comfortable to wear and less likely to induce itch (the itch threshold is much lower in those with eczema), (3) the clothes have antimicrobial properties that may decrease colonisation with S. aureus and (4) they may simply protect the skin from the damaging effects of scratching when worn, thereby interfering with the itch–scratch–itch cycle.
Despite the limited trial evidence, these garments are currently available on prescription in the UK and their popularity among patients is growing. A large, well-designed, pragmatic trial is needed to see whether or not such clothing offers a genuine benefit to children with eczema. The proposed trial has been designed to assess the clinical effectiveness and cost-effectiveness of silk clothing in a large, multicentre, pragmatic RCT (CLOTHES trial351) in order to inform clinical decision-making within the NHS. The proposal and feasibility work that follows was developed as part of this eczema treatment work package and was submitted to the NIHR HTA Board in 2013 as a result of a commissioning call on this topic and was eventually funded with recruitment starting in December 2013.
Aims and objectives
-
To assess whether or not silk therapeutic clothing, when used in addition to standard eczema care, reduces eczema severity in children over a period of 6 months.
-
To estimate the likely cost-effectiveness of silk therapeutic clothing from a NHS (base-case) and a family perspective.
Trial design
Three hundred children with eczema (AD) will be randomised into a pragmatic, observer-blind, parallel-group RCT to evaluate the addition of silk therapeutic clothing (medical device) to standard eczema care. Recruitment will take place in five UK centres (Nottingham, London, Cambridge, Isle of Wight and Portsmouth), with potential participants being identified in secondary care, primary care or by direct advertising. Additional sites have been identified as a contingency if recruitment is slow.
Children will be allocated to receive standard care plus silk therapeutic clothing (three sets of long sleeved tops and leggings, or bodysuits for infants), or standard care alone. Primary outcome for the RCT (eczema severity) will be assessed at 6 months by research nurses blinded to allocation status. An objective outcome measure has been chosen to capture disease control rather than a PROM as it will not be possible to blind participants to their treatment allocation and there is considerable potential for detection bias in a trial such as this in which prior belief in the intervention may be high.
After 6 months, participants in the control group will be given silk clothing. Two months later (8 months after recruitment), both groups will be asked to complete a questionnaire recording eczema symptoms, QoL and satisfaction with the clothing (including adherence, tolerability and durability) (Table 30).
Qualitative work will take place in parallel to the trial in order to identify factors that influence the use of silk clothing and to identify potential barriers to implementation. This will take the form of nested focus group discussions with a selection of parents/carers and children from the trial (once they have completed the primary outcome) and parallel structured interviews with commissioners and HCPs responsible for prescribing the garments.
An online survey, which was live for 3 weeks in January/February 2012, resulted in > 350 responses that have been used to inform the design of the CLOTHES trial. The survey has informed us as to:
-
how and when the clothing should be worn
-
barriers to involvement in the study (including whether or not participants in the control group should be given an opportunity to use the silk clothing after the primary outcome at 6 months)
-
current use of wet and dry wrapping in normal care
-
frequency of washing the clothing
-
information that parents would like to receive in the trial information sheets
-
willingness to take part in the trial.
Pilot and feasibility work
An internal pilot RCT will be conducted over the first 6 months of trial recruitment. Progress with recruitment and retention will be monitored monthly by the Trial Management Group. If progress is below target, strategies will be implemented to improve progress. We have also pre-specified the following stop/go criteria to be assessed by the TSC at 6 months (target recruitment = 75 participants by 6 months) (Table 29).
| Criteria to be assessed at 6 months | Proposed action |
|---|---|
| ≥ 90% of target recruitment and retention | Continue with main trial as planned |
| 70–89% of target recruitment and retention | Continue with main trial. Implement strategies for improvement |
| 50–69% of target recruitment and retention | Urgent measures required, discuss plans with TSC and NIHR HTA |
| < 50% of target recruitment and retention | Stop trial unless good reason for delay and rectifiable solution can be readily implemented |
Adherence in wearing the clothing will be assessed in the intervention group only and will act as a trigger for concern if participants report using the clothing less than 50% of the time.
Setting and target population
Target population
Children (aged 6 months to 15 years) with moderate or severe eczema (AD) and who live within travelling distance of a recruiting centre will be included in the trial. Presence of eczema will be confirmed using the UK Diagnostic Criteria for Atopic Eczema131 and eczema severity judged using the Nottingham Eczema Severity Scale. 355
Setting
Participants will be recruited from five recruiting centres (approximately 60–70 participants per centre over 18 months of recruitment). Recruitment is expected to increase gradually over the first 6 months of the trial to allow for staggered site set-up. Target recruitment across all centres: 0 to 3 months (10 participants per month); 3 to 6 months (15 participants per month); 7 to 18 months (18–20 participants per month).
Inclusion/exclusions
Exclusion criteria include the inability to give informed consent; having taken systemic medication or light therapy within the previous 3 months (because such treatments have long-lasting effects); taken oral steroids or started a new treatment regimen within the last month; used wet/dry wraps ≥ 5 times in the last month (because the use of these would prevent the silk clothing from coming into contact with the skin); currently using silk clothing for their eczema and unwilling to stop using the clothing during the trial; and currently taking part in another clinical trial. If a family has more than one child who meets the eligibility criteria, then they will be asked to choose just one child who would be willing and able to take part.
Randomisation and blinding
The randomisation schedule (stratified by centre and by child’s age: < 2 years, 2–5 years and > 5 years), will be generated by computer using a randomly varying block size. The randomisation schedule will be generated and held by the NCTU and allocations released by e-mail to the co-ordinating centres once the research nurse has irrevocably entered participant information onto an online randomisation website. This will conceal allocation to treatment from the research nurses and participants at the baseline visit. Packaging, labelling and distribution of clothing will be performed by staff at the co-ordinating centre and sent to participants by first-class post. Although it will not be possible to blind participants to their treatment allocation, efforts will be made to minimise expectation bias by emphasising in the trial literature that the evidence supporting the use of silk clothing for eczema is currently limited and that we do not yet know if this clothing offers any benefit over standard care.
The primary outcome for this trial will be assessed using a validated eczema severity scale, conducted by trained research nurses who are blinded to treatment allocation throughout the trial. Assessments will take place every 2 months in clinic and the same research nurse will conduct all assessments for each participant.
Every effort will be made to ensure that research nurses remain blinded throughout the trial. Participants will be reminded in their clinic appointment letters not to wear the clothing when they attend the clinic or to mention the clothing in any way when talking to the research nurses. All questions relating to the acceptability and use of the clothing will be completed by either postal or online questionnaire and telephone contact with participants will be made by trial administrators from the co-ordinating centre whenever possible. If the research nurses become unblinded, this will be recorded and used to inform a sensitivity analysis. A similar approach was successfully used in the HTA-funded Softened Water Eczema Trial in which the nurses were inadvertently unblinded in just 3.5% of cases (11/316). 356
Packaging, labelling and distribution of clothing will be performed by staff at the co-ordinating centre and sent to participants by first-class post. Although it will not be possible to blind participants to their treatment allocation, efforts will be made to minimise expectation bias by emphasising in the trial literature that the evidence supporting the use of silk clothing for eczema is currently limited and that we do not yet know if this clothing offers any benefit over standard care.
As two brands of silk garment are currently available on prescription in the UK [DermaSilk™ (Espère Healthcare Ltd, Shefford, UK) and DreamSkin™ (Dreamskin Health Ltd, Hatfield, UK)], participants will be randomly allocated to these two brands as they enter the trial.
Trial interventions
Intervention group: silk clothing plus standard care
The medical device under investigation is a knitted, sericin-free silk therapeutic garment with a Conformité Européene mark for use in eczema (DermaSilk and DreamSkin). Sericin is a protein that coats the outside of silk fibres and has the potential to cause allergic reactions. Medical-grade silk (such as silk used for stitches during operations) has the sericin removed for this reason. The silk clothing will be worn at night and, when possible, during the day. Both brands will be included in the trial in order to limit commercial advantage to one particular company. Participants will receive three sets of pyjamas (long-sleeved vest and leggings or all-in-one body suits depending on the age of the child) (Figure 16). This will give sufficient garments to allow for washing between uses. The same washing instructions will be provided for both brands of clothing (40 °C machine wash or hand wash, normal washing powder can be used). Adherence in wearing the garments will be collected using participants’ diaries and follow-up questionnaire.
FIGURE 16.
Child wearing silk clothing.
The clothing will have product labels removed and be supplied using trial-specific packaging prior to distribution from the co-ordinating centre. Clothing will be replaced as required during the 6-month RCT (e.g. because they are worn out or because the child has grown). Once the trial follow-up is complete at 6 months, the garments will not be replaced. Two months after the end of the trial, parents will be surveyed about continued use of clothing and willingness of GPs to prescribe them. It will be explained to participants at this stage that it is not possible for the trial team to recommend use of the garments or to insist that their GPs prescribe them.
Participants allocated to the silk clothing will continue to use their standard eczema care (as described in Control group: standard care).
Control group: standard care
Standard advice about what clothing to use for a child with eczema will be provided (avoid wool and wear cool, loose, clothing – especially cotton and linen), but specific products will not be recommended. All participants (active and control groups) will continue with their standard eczema care in line with NICE guidance. 357 No efforts will be made to intervene or change a child’s standard eczema care unless the research nurse thinks that the skin may be infected. In this case, the research nurse will contact the child’s GP surgery by telephone and arrange for appropriate treatment of the infection to be initiated.
Participants will be asked not to start new eczema treatments (other than antibiotics for infection) unless medically indicated and will be discouraged from routinely using wet or dry wrap dressings. Any changes to a child’s treatment regimen, or use of wet/dry wrapping will be recorded, but the child will remain in the trial.
If a child is currently using ‘specialist’ cotton clothing (e.g. special sleep suits with built-in mittens), the use of these garments will be recorded, but will not be grounds for exclusion. However, participants in the control group will be asked to refrain from using prescription clothing (including silk and garments used for wet wrapping) during the trial. Once the primary outcome has been collected at 6 months, children in the control group will be given a supply of silk clothing.
Outcomes
Outcome measures chosen are in line with the four domain recommendations made by the HOME initiative.
Primary outcome
-
The primary end point is eczema severity measured by the objective EASI358 at baseline, 2, 4 and 6 months. Assessments will be made by research nurses who have been trained in using the EASI tool and who are blinded to group allocation. The same research nurse will assess the skin at each time point for each participant in order to minimise inter-observer variability.
Secondary outcomes
-
Global assessment of the eczema, assessed by research nurses (IGA) and by participants [Participant Global Assessment (PGA)] at baseline, 2, 4 and 6 months.
-
Three-Item Severity at baseline, 2, 4 and 6 months, assessed by the research nurses and used to assess eczema severity. TIS is an objective eczema scale that has been recommended for use in eczema trials. 175,315 Given the inability to blind treatment allocation for participants and potential high prior belief in the effectiveness of clothing for the relief of eczema, we feel that an additional objective outcome measure of eczema severity is warranted.
-
Use of topical treatments: number of days when emollient, topical steroids, topical calcineurin inhibitors and wet wrap bandaging is used throughout the trial.
-
Eczema symptoms using the POEM scale weekly. 305 The POEM scale is a well-validated tool that is recommended by NICE for use in monitoring treatment success in normal practice. 357 Although this outcome is necessarily subjective in nature (making it unsuitable for the primary outcome), it is an important measure of disease severity that captures treatment effects more generally (including sleep disturbance, itching, bleeding, oozing, flaking and dryness).
-
Number of skin infections: defined as suspected skin infections that require antibiotic or antiviral treatment.
-
Cost-effectiveness and cost–utility analyses will be presented from the perspective of the NHS (base case) and the family. Costs will include assessment of the durability of silk clothing and the need for replacement items, as well as other NHS and family items of resource use. (see Measurement of costs and outcomes to estimate cost-effectiveness).
-
Health-related QoL at baseline, 6 and 8 months: Dermatitis Family Impact (DFI)359 will be used to assess the impact on the whole family, as this scale can be applied to all participants regardless of their age. EQ-5D-3L360 will provide a utility measure for the main carer and age-appropriate utility measures will provide an eczema-specific utility score212 and a general health utility score [Children’s Health Utility Index 9 dimensions (CHU-9D)]. 361 Utility measurement in young children can be particularly problematic and there is a lack of validated scales that can be used in this context. For children < 5 years, it will not be possible to assess health utility directly on the CHU-9D. For these children, utility scores will be modelled based on eczema severity scores. This approach has been successfully used by the study team in a previous eczema trial. 356
-
Adherence, acceptability and tolerability of the garments as assessed by children and parents/carers in participant diaries [for the intervention group during the 6 month RCT and by questionnaire at 8 months (for both groups)]. These issues will also be explored in more detail through focus group discussions with trial participants.
It is unlikely that silk clothing will result in any adverse device effects and, as such, the collection of adverse effects will be limited to an assessment of the number of skin infections as outlined above.
The number of children with mutations in the gene encoding for filaggrin will also be mapped and used to inform a planned subgroup analysis, based on the assumption that children with at least one loss-of-function mutation in the gene encoding for filaggrin will be more likely to respond favourably to the silk clothing.
| Outcomes collected | Month 0 | Month 2 | Month 4 | Month 6 | Month 8 |
|---|---|---|---|---|---|
| Informed consent for main study | ✓ | ||||
| Informed consent for genetic study (optional) | ✓ | ||||
| Eligibility checks | ✓ | ||||
| Eczema severity (NESS) | ✓ | ||||
| Supply garments | ✓, intervention | ✓, control | |||
| Demographics | ✓ | ||||
| Collect saliva sample (optional) | ✓ | ||||
| Issue diary | ✓ | ✓ | ✓ | ✓ | |
| EASI and TIS | ✓ | ✓ | ✓ | ✓ | |
| IGA and PGA | ✓ | ✓ | ✓ | ✓ | |
| Topical treatment usage | ✓ | ✓ | ✓ | ✓ | |
| Medication for skin infection | ✓ | ✓ | ✓ | ✓ | |
| Use of wet and dry wraps | ✓ | ✓ | ✓ | ✓ | |
| Online questionnaire (weekly) including POEM, topical treatment use and use or wet and dry wraps | ✓ | ✓ | ✓ | ✓ | |
| Final online questionnaire | ✓ | ||||
| DFI | ✓ | ✓ | |||
| EQ-5D-3L of parent | ✓ | ✓ | |||
| Child utility scales (AD-QoL, CHU-9D) | ✓ | ✓ | |||
| Number of infections | ✓ | ✓ | ✓ | ✓ | |
| Serious adverse events | ✓ | ✓ | ✓ | ||
| NHS and family resource use | ✓ | ✓, diary | ✓, diary | ✓, diary | ✓ |
| Adherence | Questionnaire | Questionnaire | Questionnaire | Questionnaire | |
| Durability of clothing | ✓ | ✓ | |||
| Acceptability (parent and child) | ✓ | ✓ | |||
| Replace garments if required (intervention group only) | ✓ | ✓ |
Qualitative study
Qualitative work has already been used to inform the design of the proposed trial using an online patient survey and workshop discussions with member of the Patient Panel at the Centre of Evidence Based Dermatology. This initial work will be supplemented by an evaluative study during the trial that will be used to inform our implementation and dissemination strategy.
The purpose of the evaluative study is to elicit detailed information from (1) parents and children about factors that influence use of silk clothing and (2) clinicians’ and commissioners’ views on factors that influence their decisions to offer silk clothing in the management of children with eczema. At the end of the trial this information will be used to provide a more complete understanding of how silk garments are used and what factors might prevent or encourage their use. This work will also help by suggesting possible explanations as to why the treatments work or do not work, depending on the outcome of the effectiveness trial.
Three data sets will be collected using a combination of nested focus groups with parents and children from the trial and structured telephone interviews with clinicians and commissioners unrelated to the trial. Focus groups have been chosen instead of structured interviews for the trial participants as we are keen to gather the views of the children who have worn the clothing during the trial. Purposive sampling will be used to ensure, when possible, variance in ethnicity and social class within groups. Focus groups will involve children in the intervention group and their parents when the intervention period has been completed. Parent–child pairs will attend their groups concurrently at local venues.
-
Parent focus groups × 3–4. Will have a maximum of eight participants, be audio taped and last up to 1.5 hours. Participants will be encouraged to debate their views freely. A topic guide will be used to guide conversation.
-
Child focus groups × 2. Will be moderated by an experienced children’s practitioner assisted by a note-taking observer and last up to 1.5 hours. Up to eight children aged ≥ 5 years will be recruited to each group and age appropriate activities will be used, for example talking and listening, stories and dramas, games, or painting and drawing. Artefacts (explained by children) and moderator notes will be collected. Parents or carers will meet the moderator and be invited to ask any questions they may have at the beginning of their child’s focus group. Towards the end of the session, parents and children will join together with the researchers for refreshments and debriefing. Children will be given a badge, a certificate and a small ‘goody bag’ to thank them for their participation.
-
Telephone interviews. Up to 20 clinicians and members of area prescribing committees will be purposefully recruited. The interview schedule will be pre-tested and will be sent to participants prior to the interview. Interviews will be planned at a convenient time, last for 20–30 minutes and, with permission, will be audio-taped with written notes recorded.
Data from the parent and child focus groups and interviews will be analysed separately using a manual process of content analysis. Audio tapes will be transcribed verbatim and transcripts, notes and artefacts (with child explanations) will be reviewed in detail, coding the substance of the data. Theoretical codes will be added beside each code as a reminder of any thoughts or questions attached to the codes which will be compared with the aim of identifying similarities and differences among incidence in the data. Codes will be condensed into higher levels of abstraction to form categories, mid-point indicators that depict the essential relationships between data and theory. Categories will be continuously reviewed to ensure that they cover variations within the data. Care will be taken through the data analysis process to avoid losing the qualitative richness of the data. To ensure data integrity at least two members of the research team will be involved in the analysis process.
Measurement of costs and outcomes to estimate cost-effectiveness
The objective of the economic evaluation is to estimate the cost-effectiveness of usual care with silk therapeutic clothing compared with usual care without silk therapeutic clothing for the management of eczema in children from a NHS and family perspective, over the 6-month trial period. The family perspective will be captured (including time off school/work) and reported separately.
We will identify and measure the resources used as part of the intervention (those that would be incurred if the intervention were part of routine care) through the thorough recording of this information during the trial. We will also identify and measure other NHS costs that may change as a result of the intervention, including visits to health professionals and medication usage. In line with previous work in this clinical area,356 these will be collected via patient diaries to be completed by the families in both arms of the study. Related resources used by the family will be collected in a similar manner. Appropriate unit costs362 will subsequently be assigned to each item of resource use for a standard price year. The incremental cost of usual care with silk therapeutic clothing can then be estimated by comparing the mean cost in each arm of the study.
The primary measures of clinical effectiveness for the cost-effectiveness analysis will be the difference in number of participants who achieved ‘treatment success’ (defined as those with at least a 50% improvement in EASI at 6 months compared with baseline). Secondary analyses will be conducted using continuous data from the EASI scale, the DFI scale, eczema specific utility measures212 and generic measures of health utility as measured using the EQ-5D-3L (for the main carer) and the CHU-9D for children. 361 A cost–utility analysis in which effectiveness is measured in terms of the QALYs for child and main carer will be undertaken [using linear interpolation and area under the curve (AUC) with baseline adjustment363].
The above will enable both the incremental cost and incremental effect of usual care with silk therapeutic clothing compared with usual care without silk therapeutic clothing to be estimated. If one of these options were shown to be less costly and more effective than the other, then this would suggest that it ‘dominates’ the other and represents a cost-effective use of scarce resources. Alternatively, the incremental cost-effectiveness ratio (ICER) associated with usual care with silk therapeutic clothing will be estimated and assessed in relation to a range of cost-effectiveness thresholds, for example £20,000–30,000 per QALY is recommended by NICE. 364 The associated level of uncertainty will also be characterised by estimating the CEACs. Sensitivity analysis will also be undertaken to assess the robustness of conclusions to changes in key assumptions.
Statistical analysis and sample size
All analyses will be carried out in accordance with CONSORT guidelines using Stata/SE 11.2. Appropriate descriptive statistics will be used to compare randomised participants with those who were eligible and not randomised and to compare the randomised arms at baseline. The primary approach to analysis will be ITT without imputation.
The primary analysis of EASI will be performed using a multilevel model framework, with observations at 2, 4 and 6 months (level 1) nested within participants (level 2) and including baseline EASI and the stratification factors (site and age group) as covariates
Supportive analyses of the primary end point, analyses of secondary and safety end points, planned subgroup analyses, sensitivity analyses including imputation of missing outcome data and accounting for varying adherence with allocation, and any further exploratory analyses will be conducted using appropriate multivariable regression models and documented in the statistical analysis plan (SAP), which will be finalised prior to unblinding of the study. Any changes in the planned statistical methods will be documented in the trial report.
Three hundred participants provides 90% power, at the 5% significance level (two-tailed) to detect a difference of around three points between the groups in mean EASI scores over 2, 4 and 6 months using a repeated measures analysis of covariance, assuming a SD of 13, a correlation between EASI scores at different time points of 0.6 and loss to follow-up of 10%.
A three-point improvement in EASI represents a small, but still clinically meaningful, difference between groups and we are keen to ensure that the study is sufficiently powered to detect this magnitude of difference as it is unlikely that a trial like this will be done again. A small treatment response could still be worthwhile to the NHS as this non-pharmacological treatment is assumed to have no adverse effects and because eczema affects so many people. It is also likely that a relatively small response on the objective primary outcome will be reflected in larger, more clinically meaningful, treatment effects in the patient-reported outcomes.
Patient and public involvement
Like all other aspects of this Programme Grant, patients and carers were extensively involved in this work programme. Patient representatives were involved in commenting on the language structure, layout and content of earlier versions of the overarching systematic review of eczema treatments and the GREAT database, but their strongest involvement has been with the HOME core outcomes project, the PSP and the CLOTHES silk clothing trial. Several patients from the UK, France, the Netherlands and Brazil have contributed in person to the development of core outcome sets for future clinical trials of eczema (HOME) project, where they were instrumental in ensuring adequate representation of patient reported outcomes, such as QoL. By definition, the PSP involved many HCPs, patients and carers who worked together to identify priority areas for future research.
The newly funded clinical trial on silk therapeutic clothing (CLOTHES) has involved patients and carers throughout the preliminary work in advising on the practicalities of participating in such a trial. A preliminary discussion group session was held as part of a training day for the Centre of Evidence Based Dermatology’s patient panel. Seven members of the panel with experience of eczema joined the workshop to discuss (1) the types of clothing to be included in the proposed trial; (2) how to communicate benefits and potential risks of using the clothing, in order to reduce expectation bias; (3) possible barriers to involvement and information that participants might want to receive prior to taking part in the trial; and (4) the importance of re-packaging the clothing and being clear about how and when the clothing should be worn.
This discussion group session was followed by an online survey in January/February 2012, which resulted in > 350 responses for people with experience of living with eczema (either as a patient or as a parent/guardian). The survey was used to inform: (1) how and when the clothing should be worn (at night, and during the day whenever possible); (2) barriers to involvement in the study (including whether or not participants in the control group should be given an opportunity to use the silk clothing after the primary outcome at 6 months); (3) current use of wet and dry wrapping in normal care (children using wet wraps ≥ 5 times per month were excluded); (4) frequency of washing the clothing (to inform guidance documents); (5) information that parents would like to receive in the trial information sheets (including travel commitments, availability of appointments outside of school hours and clear understanding of why the trial is needed); and (6) willingness to take part in the trial (to inform recruitment rates).
Patient representatives continue to work as part of the CLOTHES trial development group and have helped by commenting on patient information sheets, and by piloting the data collection tools (especially the online weekly diary). Two families have contributed by engaging in media interviews, which has resulted in a lot of interest and good recruitment.
More formal support for the CLOTHES trial has come from patient support groups, such as the National Eczema Society and the Nottingham Support Group for Carers of the Children with Eczema. These groups have been key supporters throughout the entire work programme, and have been central in facilitating patient involvement, boosting recruitment and disseminating the results of our research.
Summary and conclusions
Why is this research package important?
This work package is simply a large piece of connected work that exceeded our expectations in terms of delivery and impact. First, it is worth revisiting what we set out to do and what we eventually did. The eczema treatment work programme in our original Programme Grants for Applied Research application included the:
-
updated systematic review of eczema treatments
-
an international collection of all RCTs on eczema
-
a James Lind Alliance prioritisation process has for determining future eczema research priorities
-
information resources for patients.
We have delivered each of these objectives with the exception of patient information resources, mainly because resources were already being produced by existing bodies such as NHS Choices, which we have contributed to rather than duplicating effort in creating new resources. Part of our aspiration to develop patient information resources was to develop patient decision aids for some key decisions in the care of eczema patients such as choice of systemic therapies, but there was little support from our patient and public colleagues on the need for such an aid, mainly because the choices were very wide rather than dichotomous and because the evidence to inform the risks and benefits with alternative treatment choices was so poor. So with the agreement of the NIHR programme, this aspect was dropped.
Two substantial projects were added to this work programme: (1) the work on developing core outcome sets for future eczema trials (HOME initiative225) and (2) a full-scale trial application and protocol for a pragmatic national randomised controlled clinical trial on the potential benefit or otherwise of silk clothing for children with eczema, which has since been funded by the NIHR HTA programme. The latter clinical trial, which started recruiting in December 2013, indicates the value of the NIHR in offering a range of funding opportunities that link well to each other so that the value of funding in one programme benefits the other in a joined up way. The choice of primary outcome for this trial was also amended prior to start of recruitment so that eczema severity could be measured using the approved instrument as defined by the HOME group (see HOME III meeting).
All of the projects within this work programme have traceable impacts that have led to important benefits for patients and the NHS. Briefly, the main impacts at the time of writing this report are as follows.
Systematic review: used by NICE to inform decision as to whether or not an update is needed for the NICE Guidance on the treatment of eczema in children (Catherine White, NICE, 2016, personal communication). Hywel Williams is an advisor or author on these guideline groups.
The GREAT database: funding bodies such as the NIHR HTA programme are already using the GREAT database when searching for relevant evidence on eczema treatments. Spin-off projects that use the database to draw attention to design and reporting faults such as the one on quantifying selective reporting outcome bias are likely to increase.
The HOME: the Dutch dermatological society AD guidelines group have invited our team to their guidelines development meeting in the Spring of 2014, as they wish to incorporate our core outcome measure findings. Other colleagues such as Dr Jerry Tan in Canada have been successful in learning from the eczema core outcomes initiative to win funds from the US National Institute of Research to conduct a similar core outcomes project for acne. The road map of work developed by the HOME group has been highlighted as a beacon by the COMET group,365 who has included presentations about the work at their annual meetings.
Eczema PSP: the results of the eczema PSP were sent to the NIHR Efficacy and Mechanism Evaluation programme and other funders and resulted in a specific call for applications on the topic of skin disease. The NIHR HTA programme has also since commissioned a trial on bath emollients for eczema and a systematic review on educational interventions.
CLOTHES: feasibility work around the use of patient reported outcomes and how they relate to other outcomes such as EASI is being used to inform the design of other NIHR HTA eczema trials such as bath emollients (Dr Miriam Santer, University of Southampton, 2012, personal communication).
Implications for clinical practice
Most of the implications for clinical practice for this work programme emerge from the updated overarching systematic review and are summarised in detail in the accompanying report. 223 The yield of 287 new RCTs covering 92 different treatments provides a much firmer evidence base for supporting or refuting currently used eczema treatments and for guiding commissioners, health-care providers, HCPs and patients with regard to those that are currently not used on the NHS. We have found sufficient evidence to support the use of topical corticosteroids and calcineurin inhibitors for treating eczema flares reactively, and for the prevention of flares by using them proactively for two consecutive days each week. There is now also reasonable evidence to support educational interventions, UV light and systemic therapies such as azathioprine. Our review also concluded that some treatments such as evening primrose/borage oil, probiotics, topical corticosteroid combinations, topical corticosteroids more than once daily and ion exchange water softeners have no useful benefit for people with established eczema. We also found that the evidence base to support the use of many commonly used treatments such as antiseptic bath additives, emollients, antihistamines, wet wrap bandages and traditional Chinese herbs was simply insufficient and needs to be addressed by future primary research. Finally, we identified no good quality clinical trial evidence on some aspects of eczema treatment such as how often to bathe and whether or not to avoid soaps, the use of diluted topical corticosteroid combinations and the practice of allergy testing followed by avoidance of potential allergens. This is not to say that widely practised customs, such as avoiding the use of soap or the use of allergy tests in eczema patients should be abandoned, but simply that such topics might be looked at more critically in the future through appropriate research.
The systematic review may itself become a useful tool for HCPs to use when discussing treatment options for people with eczema and will hopefully be widely used by guideline developers. Similarly, the GREAT database on eczema RCTs will be used widely and bookmarked by HCPs who can quickly find trials on a particular treatment category from the easily searchable interface.
Implications for research
The overarching systematic review has included a detailed section on future primary, secondary and methodological research (see Scoping systematic review of eczema treatments). Overall, there is much to be done to ensure that all future trials and evidence syntheses prospectively register their study protocol and that they report all of the relevant information using well-established reporting guidance such as CONSORT and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). How best to engage the international research community and journal editors in such an initiative would be a useful research project in itself. In addition to highlighting the specific interventions that require new primary research, the review has highlighted the paucity of clinical trials carried out in primary care, which is where most people with eczema in the UK are treated.
The GREAT database lends itself as a platform for supporting new primary research (checking for existing trials when applying for funding), new evidence syntheses (to check for overlap with existing systematic reviews) and for supporting funding bodies in checking for existing research on different treatments. As the example of the study on selective reporting outcome bias demonstrated, the GREAT database is also a powerful tool for methodological research and can be used for researching important issues such as the appropriate analysis of end points and sample size issues for informing future trials.
The core outcomes project (HOME) now has a life of its own. Even though good progress has been achieved over the last 4 years in identifying the four core domains, and for selecting the most suitable instrument for measuring clinical signs, research into determining the best instruments for measuring eczema symptoms and eczema-associated QoL is still needed, as does the whole concept of what is meant by a ‘flare’ of eczema. Research is also needed to see how existing eczema severity measures compare with each other and good-quality training protocols and support are needed to ensure that they are used as intended. Research into eczema outcomes that can be used in routine clinical practice is also needed so that clinical outcomes for different health-care providers can be compared and benchmarked to current best practice.
The eczema PSP is, by definition, a means for patients, carers and HCPs to identify future research priorities. The main priorities that emerged from that exercise included a clearer understanding of the optimum ways of using topical corticosteroids (especially in terms of long-term safety), the role that food allergy might play in determining eczema treatment and the use of emollients.
Future research recommendations
Each project as described in this report has already identified specific research recommendations and topics for further research. The future research agenda for eczema clinical trials is a simple one. Such trials need to:
-
be undertaken only in the context of existing evidence as summarised in our systematic review and GREAT database
-
be registered before recruitment starts
-
be well designed and large enough to find clinically important effects if they are present
-
use core outcomes in order to permit subsequent evidence synthesis
-
be published and reported fully according to CONSORT guidance
-
involve patients so that the most relevant questions and outcomes are included.
Chapter 3 Vitiligo work programme
Abstract
Introduction
Vitiligo is a common skin disorder characterised by loss of pigment. High-quality research into management options is lacking.
Objective
To improve the evidence base for vitiligo treatment by building and systematically reviewing what is known, prioritising future research and undertaking a pilot RCT with view to undertaking a definitive study.
Methods
Various methods were used including systematic reviews, a James Lind Alliance PSP, consensus and validation exercises and an external pilot RCT.
Results
Our Cochrane review identified 38 new RCTs have been reported since the 2006 review. 366 The quality of evidence to support commonly used treatments is still low. The PSP, involving 302 patients and 142 clinicians, identified 10 research priorities. A systematic review of vitiligo outcome measures highlighted disparity between what is traditionally measured (percentage repigmentation) and what is meaningful to patients (acceptability of treatment response), resulting in an international initiative to identify core domains for future vitiligo trials. A pilot RCT of handheld narrowband UV light B (NB-UVB) therapy used at home recruited 29 vitiligo participants in 3 months, demonstrating feasibility. The pilot study also resulted in the production of a training digital versatile disc (DVD) and manual that are now available for NHS and public use.
Conclusions
This work has delivered key components lacking in this field including an appraisal of existing treatment evidence, identifying priorities for future research, developing better outcomes measures and demonstrating feasibility of a novel intervention. A funding application to the HTA programme for a national trial on handheld NB-UVB was successful.
Content
This chapter describes a range of complementary projects relating to the treatment of vitiligo, for which some navigation may be helpful for the reader.
| Details | Comment |
|---|---|
| Update of Cochrane systematic review | A summary of the Cochrane review (2010) of Vitiligo treatment RCTs367 |
| Vitiligo priority setting partnership | An activity which assessed what questions were considered most important to patients and clinicians |
| Systematic review of outcome measures | A dual approached which reviewed published outcome measures and the views of patients and clinicians on these outcomes |
| Gaining consensus over outcomes for use in future vitiligo trials | An international activity designed to established core outcome domains to be included in future vitiligo trials |
| Validation of patient-reported treatment response | An activity which developed a patient-rated outcome measure |
| Pilot randomised controlled trial of light therapy devices used at home | A study which assessed the feasibility of treating vitiligo using handheld UV light devices |
| Survey of UK clinical practice | Surveyed the opinions of clinicians regarding vitiligo treatments |
| Proposal for a trial light therapy and topical corticosteroids for the treatment of vitiligo (HI-Light) | A feasibility study assessing the use of UV light for the treatment of vitiligo in the home |
| Patient and public involvement | Summary of how the project has been informed by and developed in partnership with patients |
| Summary and conclusions |
Update of Cochrane systematic review
The following text is adapted with permission from Whitton ME, Pinart M, Batchelor J, Lushey C, Leonardi-Bee J, González U. Interventions for vitiligo. Cochrane Database Syst Rev 2010;1:CD003263. 367 Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Summary
What was already known about this topic?
-
A Cochrane systematic review of interventions for vitiligo, published in 2006, identified only 19 eligible RCTs, covering eight broad treatment categories. Most were small and of relatively poor quality.
-
The review found some evidence to support existing therapies for vitiligo, but the usefulness of the findings were confounded by the different designs and outcome measurements as well as the lack of QoL measures and adverse effect reporting in the source studies.
What did this study add?
-
There has been a marked increase in the number of RCTs assessing interventions for vitiligo; 38 new studies were identified for inclusion in this updated review.
-
The quality of design of vitiligo RCTs has improved somewhat over the past few years, but many RCTs are still poorly designed and reported.
-
In vitiligo RCTs, outcomes are assessed in many different ways, making intertrial comparisons very difficult and meta-analysis almost impossible. Future RCTs should aim to focus on a core set of outcomes, ideally including patient-centred outcomes.
-
Light therapies, either alone or in combination with other treatments, were the most commonly assessed interventions. However, studies are needed to establish the dosage, safety and long-term efficacy of NB-UVB monotherapy in treating vitiligo.
-
Combination therapies, particularly those that include a light source, show some promise in treating vitiligo, but larger studies are needed.
-
Topical tacrolimus appears to have a similar effect to topical corticosteroids, but with a better safety profile and warrants further study to establish whether or not this intervention is a viable, cost-effective alternative.
-
Randomised controlled trials assessing psychological interventions and cosmetic camouflage are lacking.
Introduction
Vitiligo is a disease that causes patchy loss of pigment from areas of skin. It is thought that this is due to the damage or destruction of melanocytes (pigment-producing cells). Vitiligo patches can appear anywhere on the skin, but commonly affected sites include the areas around mucosal surfaces, the genitals and any sun-exposed areas, such as the face and hands. Most people develop the disease before the age of 20 years368 and global prevalence estimates are typically in the range 0.5–2% (but regional prevalence estimates may vary according to cultural and social differences). 369
Causes
There are several hypotheses for the cause of vitiligo, none of which fully explains the disease. Several factors, including autoimmune, biochemical, genetic, neuronal and environmental, may interact to contribute to its development. 370–372 Although evidence indicates an important role for genetic factors,373–375 it also seems that triggers such as trauma to the skin, hormonal changes and psychological distress may need to be present for the disease to become apparent. 376 A number of observations suggest that vitiligo is an autoimmune disease, such as its association with other autoimmune diseases (e.g. pernicious anaemia, thyroid disorders, diabetes mellitus and Addison’s disease377) and the presence of antibodies directed against melanocytes in affected people.
Impact
Although neither life-threatening nor symptomatic (except that depigmented patches burn easily when exposed to the sun), vitiligo can be cosmetically and psychologically devastating,378 resulting in reduced self-esteem,379 poor body image,380 difficulties in sexual relationships381 and feelings of social isolation and stigmatisation. 382 Some people with vitiligo experience discrimination in employment, particularly in jobs where they have to deal with the public. 383 The effect of vitiligo on QoL is often overlooked,384 although more recent studies have started to address this. 385
Typical treatments
As yet, there is no cure for vitiligo nor any effective method of stopping its spread. Several interventions have been used in the treatment of vitiligo, including pharmacological agents (e.g. topical, intralesional or systemic corticosteroids; topical and systemic immunomodulators; topical calcineurin inhibitors; and vitamin D analogues); various forms of phototherapy [e.g. UV light A (UVA), NB-UVB and broadband UV light B (UVB), psoralen and ultraviolet light A (PUVA), various forms of laser, and monochromatic excimer light]; surgical procedures (grafting, melanocyte transplantation, micropigmentation); cosmetic measures (depigmentation, cosmetic camouflage, fake tan); complementary therapies; and psychological interventions. Many published studies describe combination therapies, usually combining a light source with another form of treatment.
Why it was important to do this review
When the original systematic review was published in 2006,366 there were only 19 eligible published RCTs and no systematic review covering all available interventions for vitiligo. The 2010 update367 was needed to assess new interventions, to highlight the gaps in research and the need for better designed and powered studies, thus informing clinical decisions and future research priorities. 344 The findings of the 2010 update are summarised in this chapter.
Having published the updated review in 2010, we have also embarked on an additional update, the initial findings of which are included in this chapter, although formal analysis has not yet been completed.
Methods
Criteria for study selection
The review included all RCTs of interventions for vitiligo, including participants of all ages and with any type of vitiligo.
Primary outcomes of interest were:
-
QoL, measured using a validated tool
-
percentage repigmentation of vitiliginous skin.
Secondary outcomes of interest were:
-
cessation of spread of vitiligo or stabilisation of the disease, defined as:
-
no increase in the size of individual vitiligo patches measured objectively
-
no new lesions appearing, despite no improvement in existing patches resulting from treatment
-
-
long-term permanence of repigmentation resulting from treatment (at least 2 years of follow-up)
-
adverse effects.
Search methods for identification of studies
Full details of the search strategies for this review can be found in the full version, accessible from The Cochrane Library. 367
Electronic searches for published trials were carried out in the following databases: Cochrane Skin Group Specialised Register; CENTRAL in The Cochrane Library; MEDLINE; EMBASE; PsycINFO; CINAHL; LILACS; AMED and ISI Web of Knowledge.
Ongoing trials were identified using a number of online registers. The bibliographies of all included and excluded studies and reviews were also checked to identify further trials.
For all search methods, studies in other languages were included if they met the criteria and could be translated into English.
Data collection
Two of the reviewers checked the titles and abstracts identified in the search, independently assessed the full text of all studies of possible relevance and decided which trials met the inclusion criteria. Any disagreements were resolved by discussion and a decision made by consensus.
One reviewer extracted data from all the new studies, which were also allocated randomly to four of the other reviewers who independently extracted data. One of three reviewers checked the data extraction forms for discrepancies, ensuring that they did not check their own forms. When discrepancies could not be solved by reference to the text of the studies, differences were resolved by consensus.
At least two reviewers independently assessed risk of bias for the new studies identified in the updated search. The following aspects of studies were considered:
-
the method of generation of the randomisation sequence
-
the method of allocation concealment
-
who was blinded or not blinded
-
how many participants dropped out of the study overall and whether or not participants were analysed in the groups to which they were originally randomised (ITT).
Data analysis
Details of the SAP can be found in the full version of the review. 367
We expressed the results as RR with 95% CIs for dichotomous outcomes and difference in means with 95% CI for continuous outcomes.
When there were multiple intervention groups within a trial, pairwise comparisons were made of similar active interventions versus no treatment, placebo or another active intervention. No crossover trials were identified in the review. Internally-controlled trials were analysed using appropriate techniques for paired designs (e.g. for continuous outcomes using Wilcoxon signed-rank test or paired t-test; or for dichotomous data using McNemar’s test) when available and were not pooled with studies of other designs. However, when paired data could not be extracted from the papers, we presented the data using non-paired methods. If participant dropout led to missing data, we conducted an ITT analysis. Trial authors of studies were contacted to provide missing statistics such as SD. Statistical heterogeneity was assessed using I2. Data were synthesised using meta-analysis techniques if I2 was < 80%.
Publication bias was not tested as insufficient data were available for similar types of interventions.
Data synthesis
For studies with a similar type of active intervention, a meta-analysis was performed to calculate a weighted treatment effect across trials, using a random-effects (DerSimonian and Laird) model. When it was not possible to perform a meta-analysis, the data were summarised for each trial. If raw data could not be extracted, we extracted the results from appropriate statistical analyses presented in the paper and reported these in the review. We considered p < 0.05 as statistically significant. Data relating to adverse effects were described qualitatively as reported in each trial. Subgroup analyses were not conducted as no substantial heterogeneity (I2 > 50%) existed between studies for the primary outcome.
Insufficient numbers of studies were included in the meta-analysis for sensitivity analyses to be feasible.
Results
Randomised controlled trials included in this review
A total of 57 studies (3139 participants) met the inclusion criteria (19 studies from the original review, 38 new studies). Of these, 18 were ‘within-participant, left/right comparison studies’, one was a ‘left/right comparison study’, three were ‘within-participant studies’ and the rest were parallel group studies. All included studies allocated either participants or bilaterally symmetrical lesions to treatment groups in a random manner. Twenty-nine of the studies were placebo controlled and the rest used active comparators. The number of participants in each study varied from 8 to 596. The majority of studies (n = 38) recruited < 50 participants and most (n = 40) included participants with symmetrical vitiligo. All but one of the studies386 included both male and female participants and three studies recruited only children. 387–389 Most of the studies were carried out in Asia (n = 26) or Europe (n = 19), with fewer undertaken in the Americas (n = 10) or Africa (n = 2). 367
A total of seven studies were excluded. 390–396 The reasons are documented in the full version of the review (see ‘Characteristics of excluded studies’ table367). In addition, 13 studies were omitted on the basis that they were awaiting classification and five were ongoing at the time of the review. 367
Interventions
This update found many studies assessing interventions not described in the original review.
-
New topical interventions included pimecrolimus,397,398 tacalcitol,399–401 5-fluorouracil (5-FU),402 topical lactic acid,403 catalase/dismutase404 and an antioxidant and mitochondrial stimulating cream. 405
-
New oral interventions included Zengse pill with oral cobamamide plus topical psoralea tincture,406 Polypodium leucotomos,407,408 levamisole (Ergamisol®, Janssen Pharmaceutica),409 minipulses of betamethasone,410 azathioprine,411 oral antioxidants412 and phenylalanine. 405
-
New light therapies included monochromatic excimer light,400,413 broadband UVB410,414 and erbium-doped yttrium aluminium garnet laser. 402
-
New surgical interventions included minipunch and split skin grafts415 and transplantation of autologous melanocytes. 416,417
-
There was also one new study assessing psychological interventions. 418
The use of a light source, either as monotherapy or in combination with oral or topical photoactive chemicals (i.e. psoralens or khellin), or with other interventions, is the most common method used in practice. 419 Light therapies were also the most common intervention assessed in the included studies (42 studies, 13 of which were in the original review and 29 new studies). Two new studies assessed topical steroids as monotherapy. 404,420
Outcomes
All studies assessed repigmentation as an outcome. No two studies used exactly the same method of measuring the amount of repigmentation, but the majority rated the percentage repigmentation in categories (e.g. 0–25%, 25–50%, 50–75%, > 75%). Many authors made an attempt to define the amount of repigmentation that was considered ‘successful’ but opinions varied considerably. Repigmentation was usually assessed visually by a clinician, using photographs of the lesions. However, some studies used planimetry (tracing lesions on transparent paper) and some analysed photographs of lesions using morphometry analysis.
Small numbers of studies measured cessation of spread of vitiligo and/or improvement in QoL, the latter using Skindex-29, the Dermatology Life Quality Index (DLQI), or the Children’s Dermatology Life Quality Index (CDLQI). The duration of the studies varied widely, from 3 weeks to 3 years. Within these periods, some studies continued treatment until an optimal response was achieved.
Risk of bias in included studies
To assess risk of bias in the included studies, we considered the generation of the randomisation sequence, allocation concealment, blinding and losses to follow-up. The method of generation of the randomisation sequence was deemed adequate in 32 of the studies.
Although some studies were described as ‘double-blind’ or implied double-blinding, not all of them provided details as to how blinding was maintained. Many studies were within-participant comparisons of different interventions so it was not possible for the participants (and sometimes the clinicians) to be blinded. Blinding of participants was also not possible in some other studies because two different modalities of intervention were being assessed (e.g. oral vs. topical interventions). In seven studies, participants, clinicians and assessors were all blinded. In 14 studies, blinding of participants, clinicians or outcome assessors was not stated. 367
Overall, 16.6% of all study participants included in the review were lost to follow-up. In 23 of the included studies, data were analysed on an ITT basis. This was either because there were no losses to follow-up (16 studies) or because data from dropouts were included in an explicit ITT analysis. For two of the studies it was unclear whether or not an ITT analysis had been performed. 410,421
Effects of interventions
Not all of the 57 included studies reported on the outcomes of interest for this review. In general, the extent of variation in the ways in which vitiligo was measured, as well as the number of combination interventions assessed, prevented data pooling. Analyses for this review were therefore based primarily on individual studies, with the exception of one meta-analysis.
Many of the trials had control arms in which none of the participants improved. This resulted in large RRs with very large associated 95% CIs, exacerbated by the relatively small participant numbers in many of the trials. Analyses for this review were therefore based primarily on individual studies, with the exception of one meta-analysis of two studies assessing the combination of tacrolimus ointment and 308-nm excimer laser367 (Figure 17).
FIGURE 17.
Analyses reporting statistically significant treatment differences on the outcome of percentage repigmentation > 75%. df, degrees of freedom; M–H, Mantel–Haenszel.
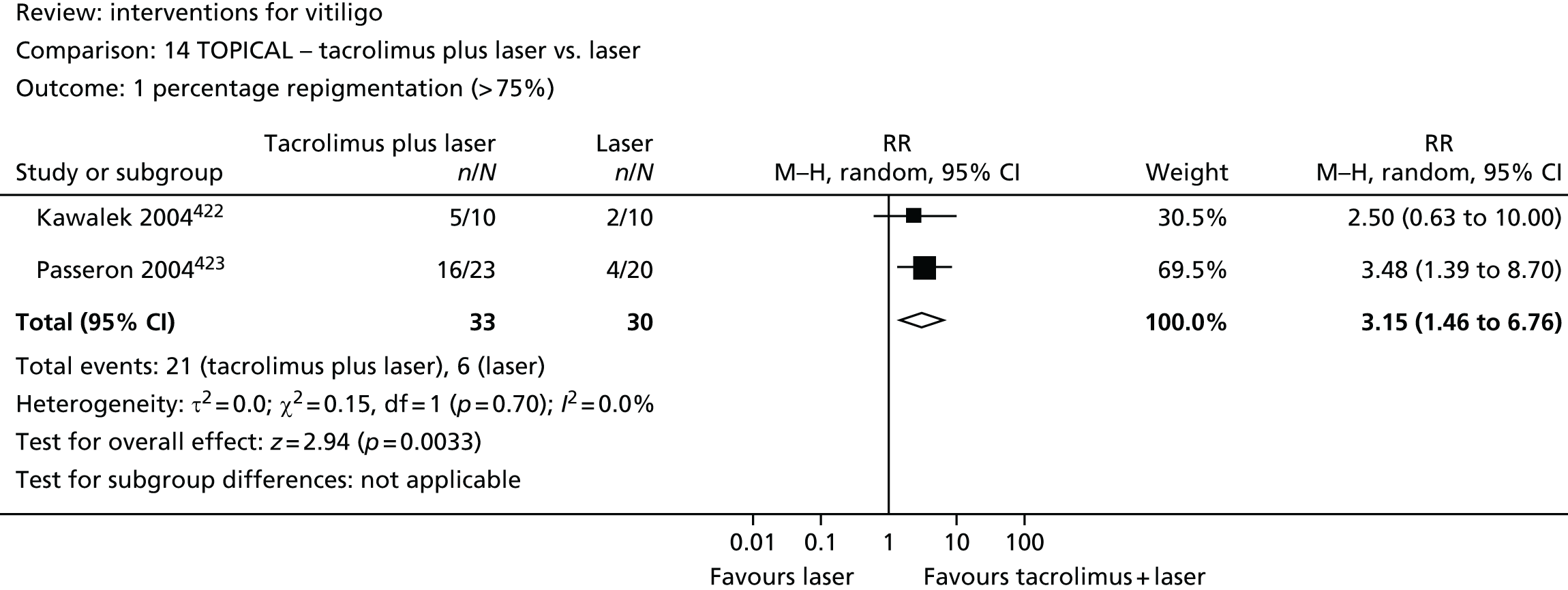
Primary outcomes
Quality of life
Four studies assessed the primary outcome of QoL. Two were assessed this using DLQI49,62 and two407,424 used Skindex-29; however, we were not able to perform statistical analysis on these data.
Percentage of repigmentation > 75%
Many of the studies in this review assessed this primary outcome; however, not all of the studies used objective methods of assessing repigmentation and so the data could not be analysed. Fifteen analyses from studies comparing various interventions showed a statistically significant difference between the proportions of participants achieving > 75% repigmentation (see Figure 17 and original publication367).
All of these analyses compared one active intervention with another active comparator, with the exception of one,425 which compared oral Ginkgo biloba with placebo and showed a greater proportion of participants achieving repigmentation when they received G. biloba. From the studies assessing two active interventions, the only topical monotherapy that had evidence of superior rates of repigmentation compared with another active intervention was clobetasol propionate (Dermovate®, GlaxoSmithKline), which was shown to be superior to psoralen with sunlight exposure (PUVAsol). 387 The majority of analyses showing statistically significant differences were from studies that assessed combination interventions, which generally included some form of light treatment; however, this was not always the case.
Secondary outcomes
Cessation of spread of vitiligo
Six studies assessed the secondary outcome of cessation of spread of vitiligo. 397,409,425–428 Only one study showed superiority of an active intervention over placebo;428 participants receiving oral G. biloba were more than twice as likely to achieve cessation of spread than those receiving placebo.
Long-term permanence of repigmentation
None of the studies was able to demonstrate long-term repigmentation as specified in our secondary outcomes (i.e. at 2 years’ follow-up).
Adverse effects
Adverse effects were reported in 40 out of the 57 studies in the updated review. Most of the studies evaluating topical or intralesional corticosteroids reported adverse effects including atrophy, telangiectasia, acneiform papules and hypertrichosis, with the exception of the fluticasone propionate (Cutivate®, GlaxoSmithKline) study,429 which detected no evidence of atrophy. The use of topical calcipotriol (Dovonex®, Leo Pharma) or tacalcitol alone or in combination led to erythema, itching and irritation. The main adverse effects reported in participants treated with topical tacrolimus were erythema, blistering or burning sensations at the treatment site, although in some studies the more severe adverse effects may have been due to combination with other interventions, such as the laser. 422 There were only two studies that assessed topical pimecrolimus. Although the one that assessed it as monotherapy397 reported no adverse effects, the one that combined topical pimecrolimus with NB-UVB398 reported self-limiting erythema and itching which could also be attributed to the additional use of NB-UVB light.
Regarding adverse effects with oral treatments, nausea occurred in participants treated with L-phenylalanine, G. biloba and oral minipulses of betamethasone combined with PUVA. Participants treated with oral minipulses of betamethasone alone or combined with light therapies (PUVA or NB-UVB) also experienced weight gain, but only when oral minipulses of betamethasone was combined with light therapies. Some of those receiving P. leucotomos or oral placebo also reported mild gastrointestinal complaints. 407 Similar adverse effects were seen when using combination therapy with antioxidant and mitochondrial stimulating cream405 and also with Zengse pill with or without oral cobamamide plus topical psoralea tincture. 406
Adverse effects with light therapies included nausea in participants treated with PUVA (owing to use of oral psoralen) and erythema in participants treated with either PUVA or NB-UVB. The use of NB-UVB led to transient itching and dryness of the skin in several studies. When sunlight was combined either with trimethylpsoralen or 8-methoxypsoralen, half of the participants reported nausea, itching, dizziness, headaches, eye discomfort and vague gastrointestinal symptoms. 430 Studies assessing lasers showed many adverse effects such as burning and/or stinging as well as moderate to severe erythema.
Surgical interventions such as punch grafts or minigrafts sometimes led to adverse effects such as cobblestoning, scarring, graft depigmentation and graft displacement. Suction blister grafts or split skin grafts led mainly to the Koebner phenomenon (which can itself exacerbate vitiligo) and hypopigmentation or, to a lesser extent, hyperpigmentation, scarring and infection at both donor and recipient sites. With regard to melanocyte transplantation, the only adverse effect reported was bacterial infection at the recipient site.
Discussion
Implications for practice
Owing to the small numbers of participants and heterogeneity of design of the studies in this review, it is difficult to make firm recommendations for clinical practice. However, a number of implications may be considered:
-
There is moderate evidence for the use of topical corticosteroids, although long-term use is likely to lead to adverse effects. Topical non-steroidal immunomodulators may be a promising alternative, although the theoretical risk of skin cancer must be considered for long-term combination use with phototherapy.
-
Most of the studies that assessed combination interventions employed light therapies (UVA, PUVA or UVB). In general, combination interventions were superior to monotherapies.
-
There is limited evidence for the benefit of oral G. biloba.
-
There is limited evidence for the benefit of split-thickness skin grafts plus PUVAsol. Surgical therapies can be effective for small areas in people with stable disease (bearing in mind the risk of adverse effects with suction blister grafts).
-
There is a lack of formal evidence supporting the use of cosmetic camouflage, depigmentation (in severe cases) or psychological interventions.
Implications for research
Since the original review was published in 2006, there has been an increase in the number and quality of RCTs assessing interventions for vitiligo. However, it is still not clear which are the best interventions for this condition. Until the causes of vitiligo are better understood, treatments will continue to be based on the many theories that exist for this disease. Although there has been a noticeable increase in basic scientific research, more investment is needed so that new discoveries in experimental models can be systematically advanced to preclinical and clinical stages.
Summarised below are the recommendations that emerged from this update of the review. Many of the recommendations relate to the design and reporting of future clinical trials for vitiligo and are explored in more detail in a separate publication. 431
-
Standardised methodologies for describing and classifying vitiligo and for assessing the effect of interventions need to be developed and used by trial investigators.
-
Study design should take into account variations in participant features, including skin colour, patient age or duration of the disease, extent and type of vitiligo, as well as site of affected areas.
-
In order to assess the efficacy of an intervention, trials should last for at least 1 year.
-
The following outcomes should be considered in the design of future studies:
-
cessation of spread of vitiligo (ideally with 2-year follow-up)
-
patient-centred outcomes (e.g. HRQoL measures).
-
-
Studies are needed to establish the dosage, safety and long-term efficacy of NB-UVB monotherapy in treating vitiligo.
-
Topical tacrolimus appears to have a similar effect to topical corticosteroids, but with a better safety profile; however, this finding must be replicated in further studies to establish it as a viable, cost-effective alternative.
-
The combination of two active interventions (especially those incorporating some form of phototherapy) have shown promise and require further investigation.
-
A greater number of well-conducted RCTs of complementary or psychological interventions are required.
-
Studies are needed on the use of cosmetic camouflage, particularly with regard to the long-term effect on QoL.
Update the review beyond 2010
In addition to the review commissioned as part of this Programme Grant, we have also undertaken a further update of the Cochrane review432 and the results should be available in 2014. We have identified a further 39 RCTs for this latest update, giving a total of 96 RCTs to be included in the review. Many of these (n = 41) are within-participant studies and most have < 50 participants.
New interventions assessed by RCTs in this update include transplantation of autologous cultured or non-cultured melanocytes, autologous non-cultured hair follicle outer root sheath cell suspension, autologous non-cultured epidermal cell suspension, carbon dioxide laser, helium neon laser, broadband UVA, tetrahydrocucurminoid cream, and alpha-tocopherol (vitamin E) plus NB-UVB. As in the previous update, many of the new studies assess interventions in combination, usually including a form of light therapy. Nine of the new RCTs assess surgical interventions, reflecting an increasing interest in this area of treatment. Just over half of the studies report our primary outcome of > 75% repigmentation and more than half report adverse events.
There has been a huge increase in the number of vitiligo trials in recent years, which is reflected in the large number of registered ongoing studies (n = 44). Two of these studies are investigating afamelanotide (Scenesse®, Clinuvel), which was one of the top 10 research uncertainties identified in the vitiligo PSP (see Vitiligo Priority Setting Partnership).
Vitiligo priority setting partnership
Summary
What was already known about this topic?
The pharmaceutical and medical technology industries and academia play an essential role in developing new treatments.
-
However, it is increasingly recognised that patients and HCPs have a key role to play in identifying important areas for research.
-
The priorities of industry and academics are not necessarily the same as those of patients and clinicians.
-
It is essential, therefore, that researchers and funding bodies are aware of the needs of patients and clinicians.
What did this study add?
The research areas identified provide direction for future research activity by highlighting the questions of importance to patients and HCPs.
Introduction
There are many treatment options for vitiligo but there is a lack of high-quality information regarding relative efficacy on which to base treatment decisions. 367 The aim of the vitiligo PSP was to identify the most important uncertainties surrounding the treatment of this condition and to steer future research to questions of importance to both people living with the disease and the clinicians who treat them.
Methods
The vitiligo PSP was co-ordinated at the Centre of Evidence Based Dermatology with numerous stakeholders from professional organisations and patients’ support groups. The vitiligo PSP adopted the methods advocated by the James Lind Alliance,367,433 which were refined to meet the needs of this particular partnership. 433 The vitiligo PSP had five stages.
Stage 1: initiation (January 2009 to February 2009)
The aim of this stage was to establish the vitiligo PSP by raising awareness and identifying and engaging potential stakeholders. The following professional bodies and patient support groups were approached during this stage: BAD; UKDCTN; NHS Evidence-Skin Disorders; Cochrane Skin Group; British Dermatological Nursing Group; Changing Faces; British Red Cross Camouflage Service; Skin Care Campaign; Primary Care Dermatology Society; Vitiligo European Task Force; and British Association of Skin Camouflage and Vitiligo Society. Individual researchers, dermatologists, specialist nurses and psychologists with a special interest in vitiligo were also informed. Our research group, called the Steering Group, included 12 members with knowledge and interest in vitiligo.
Stage 2: consultation (March 2009 to August 2009)
The aim of this stage was to collect treatment uncertainties. An online and paper survey was undertaken that encouraged patients and clinicians to submit their questions about the treatment of vitiligo. Paper copies of the questionnaire were sent to the Vitiligo Society (n = 1268) and to BAD (n = 835). E-mails were sent to members of the UKDCTN (n = 500) and details of the project (with links to the online survey) were advertised on the websites and in the newsletters of the relevant organisations listed above. Additional treatment uncertainties were identified from existing sources of current evidence: updated Cochrane systematic review367 and the BAD guidelines for diagnosis and management of vitiligo. 434
Stage 3: collation (August 2009 to December 2009)
Submitted uncertainties were reviewed by members of the Steering Group. Questions on the aetiology, natural history and prevention of the disease (uncertainties not related to treatment) were excluded at this stage. The treatment uncertainties were then collated into groups representing an area of uncertainty. A structured format was then used to refine these uncertainties in order to aid comparison of different questions in the next stage (Table 31). For example, the refined uncertainty ‘How effective is UVB light therapy when combined with creams or ointments in treating vitiligo?’ includes submitted uncertainties on combination of NB-UVB with a number of different topical agents (such as corticosteroids, calcineurin inhibitors and vitamin D analogues).
| Type of question | Format of question |
|---|---|
| Effectiveness of a single treatment | How effective is (treatment X) in treating vitiligo? |
| One treatment compared with another | Which treatment is more effective in treating vitiligo: (treatment X or treatment Y)? |
| One treatment combined with another | How effective is (treatment X) when combined with (treatment Y) in treating vitiligo? |
| Management of the disease, rather than ‘treatment’ (e.g. camouflage or psychological interventions) | How much does (treatment X) help patients with vitiligo? |
| Speculative treatments not yet on the market (e.g. gene therapy, stem cell therapy) | What role might (treatment X) play in the treatment of vitiligo? |
Stage 4: ranking (January 2010 to February 2010)
The aim of the ranking exercise was to identify the 25 uncertainties that the PSP felt were the most important priorities for future research. The ranking exercise was open to all people who gave contact details during the consultation. It was also advertised via websites and newsletters of relevant organisations, as per the consultation stage. In addition, advertisements and articles were placed in The Voice magazine (GV Media Group Ltd, London) for black and ethnic minorities, the British Dermatological Nursing Group magazine435 (British Dermatological Nursing Group, Belfast) and the bulletin of The Primary Care Dermatology Society435 (The Primary Care Dermatology Society, Hatfield) to target specific groups that had been under-represented during the consultation stage.
Participants were asked to vote for up to three uncertainties they felt were most important (three individual votes), either online436 or using paper questionnaires. The order in which uncertainties appeared on the survey was randomised in order to guard against response bias.
Stage 5: final prioritisation workshop (March 2010)
The aim of this final stage was to identify the top 10 most important treatment uncertainties for vitiligo by creating consensus through a face-to-face workshop of HCPs and patients. Selected participants of previous stages of the vitiligo PSP attended this workshop. Efforts were made to ensure that equal numbers of patients and HCPs attended. The workshop was a full-day event, held at the London offices of BAD on 25 March 2010. Further details of the methods used during the vitiligo PSP are outlined in the James Lind Alliance guidebook. 437
Ethics
This project was approved by the Medical School REC, University of Nottingham, UK (ethics reference number G/2/2009).
Statistical methods
We aimed for a minimum of 100 participants in the consultation and the ranking exercise and for 20 participants for the final prioritisation workshop. This sample size was estimated on the basis of previous James Lind Alliance PSPs438 and determined by the time frame available for the vitiligo PSP. Data from all stages were stored and analysed in Microsoft Access® 2007 (Microsoft Corporation, Redmond, WA, USA) and processed by the Steering Group members.
Results
Of the 2303 surveys circulated, 461 (20%) were returned. The response rate for members of the Vitiligo Society was 24% (307/1268) and for BAD/UKDCTN members was 14% (119/835). Sixty-six per cent of responses (302/461) were from patients, 31% (142/461) were from HCPs, and 3% (17/461) were from other sources. More women responded than men (53% women, 30% men, 17% did not specify) and the majority were aged 30–60 years (8% were aged < 30 years, 50% were aged 30–60 years, 25% were aged > 60 years and 17% did not specify).
Overall, 1427 questions were submitted, of which 660 specifically related to the treatment of vitiligo and went forward to the next stage. Of these, 31% (206/660) were from HCPs, 49% (320/660) were from patients and 20% (134/660) did not specify their background. An additional 58 treatment uncertainties were identified from the BAD guidelines and the updated Cochrane systematic review. The resulting 718 uncertainties were refined into 93 treatment uncertainties, which were used for the ranking exercise.
For the ranking exercise, 230 people responded (patients, 72%; HCPs, 23%; did not specify, 5%), submitting 638 individual votes. Nineteen paper voters were excluded as they submitted more than three votes. The number of votes per uncertainty ranged from 0 to 49 (median 5). The demographic characteristics of participants in the ranking exercise were broadly similar to those in the consultation stage (63% were women and 55% were aged between 30 and 60 years). Of those who specified their ethnicity (n = 127), 42% were white and 12.6% were from black and ethnic minorities.
As more patients participated in the ranking exercise than HCPs, the Steering Group considered the ranked priorities of patients and HCPs separately when formulating a list of 23 uncertainties for discussion at the final prioritisation workshop (Table 32).
| Rank | Indicative uncertainty |
|---|---|
| 1 | How effective are systemic immunosuppressants in treating vitiligo? |
| 2 | How much do psychological interventions (such as counselling) help people with vitiligo? |
| 3 | Which treatment is more effective for vitiligo: light therapy or calcineurin inhibitors (e.g. tacrolimus, pimecrolimus)? |
| 4 | How effective is UVB light therapy when combined with creams or ointments (e.g. steroid creams) in treating vitiligo? |
| 5 | What role might gene therapy play in the treatment of vitiligo? |
| 6 | How effective are hormones or hormone related substances that stimulate pigment cells (MSH analogues, such as afamelanotide) in treating vitiligo? |
| 7 | Which treatment is more effective for vitiligo: calcineurin inhibitors (e.g. tacrolimus, pimecrolimus) or steroid creams/ointments? |
| 8 | Which treatment is more effective for vitiligo: steroid creams/ointments or light therapy? |
| 9 | How effective is the addition of psychological interventions to patients using cosmetic camouflage for improving their QoL? |
| 10 | How effective is pseudocatalase cream (combined with brief exposure to UVB) in treating vitiligo? |
| 11 | How effective is piperine cream in treating vitiligo? |
| 12 | What role might stem cell therapy play in treating vitiligo? |
| 13 | How effective is the transplant of pigment cells (autologous melanocyte transplantation) in treating vitiligo? |
| 14 | Which treatment is more effective for vitiligo: PUVA or UVB therapy? |
| 15 | Which treatment is more effective for improving the QoL of patients with vitiligo: cosmetic camouflage or a treatment to aid repigmentation? |
| 16 | What role do vitamins and supplements play in the management of vitiligo? |
| 17 | How much does depigmentation help patients with vitiligo? |
| 18 | What role does diet play in the management of vitiligo? |
| 19 | How effective is natural sunlight in achieving repigmentation of the skin in patients with vitiligo? |
| 20 | What role might lifestyle (e.g. stress, smoking) play in the management of vitiligo? |
| 21 | How effective are steroid injections into the skin in treating vitiligo? |
| 22 | How effective is ViTiX® (Laboratoire Dermatologique ACM, Clichy, France) in treating vitiligo? |
| 23 | How effective are homeopathic remedies in treating vitiligo? |
The final workshop was attended by 47 people (21 patients or their representatives and 16 HCPs). Feedback following the workshop showed that all attendees were either very satisfied or satisfied with the top 10 uncertainties identified on the day.
Important recurring themes for researchers to consider when developing future trials also emerged and are summarised in Box 5. These themes covered general issues that were relevant to all therapeutic interventions for vitiligo.
Which treatments are effective and safe for children?
Do treatment success rates differ according to the site(s) affected, or the gender/age/ethnicity/skin phototypes of patients?
What are the long-term outcomes of treatments for vitiligo (especially side effects)?
What is the optimal duration and optimal timing for treatments of vitiligo?
What is the optimal maintenance regimen in order to prevent relapse?
Interventions for segmental vitiligo.
Discussion
Vitiligo has traditionally been given a relatively low priority in the dermatology research agenda, as shown by the number and quality of studies on vitiligo to date. 367 The updated systematic review367 is helpful in identifying many important research gaps for clinical trials, but these have largely come from the research community and may not reflect the issues most pertinent to patients and clinicians. At present, the only intervention licensed for use in vitiligo in the UK is cosmetic camouflage and there is an urgent need for RCTs of interventions for vitiligo.
Implications for research
The identified uncertainties provide a steer for future research activity by guiding researchers and funding bodies to questions of importance to patients and HCPs. At this point it is important to remember that the vitiligo PSP aimed to identify treatment uncertainties. These are then used as reference to inform future research questions as developed by individual research teams. It is entirely possible that one treatment uncertainty will result in several related research questions.
Reflections on the process
It is possible to offer recommendations for future PSPs, based on the key challenges faced during this process.
-
Information about the existing research evidence for the different treatments should be presented in a patient-friendly format at the beginning of the workshop. This would allow all participants to engage in the process more effectively, regardless of their background, experience or levels of expertise.
-
Most of the uncertainties (during consultation) were broad and non-specific, or did not specify the comparator, the duration of treatment or the population, whereas ones from the research community were often very focused. For the purposes of priority setting, the uncertainties should be reasonably broad so that all issues are easily understood. These broad uncertainties will necessarily be developed into more detailed research questions by individual research groups. It is encouraging that some funding bodies are now reflecting this by issuing ‘broad’ themed calls in targeted areas.
Impact of the priority setting partnership
The topics prioritised here cover a broad range of interventions including topical therapies, light therapy, psychological support and new and emerging therapies. These research suggestions have been distributed to relevant funding bodies in the UK and will be used to inform future research funding priorities. The NIHR HTA funding stream was proactive in picking up these research priorities and put out a commissioned call designed to address two of the suggested topic areas (uncertainties 4 and 8 as listed in Table 32). This has led to a proposed trial assessing the use of handheld NB-UVB units, with and without topical corticosteroid, which is due to start in June 2014 [see Proposal for a trial light therapy and topical corticosteroids for the treatment of vitiligo (HI-Light)]. Another important research priority on psychological interventions for the management of vitiligo has been picked up by a group of trainee dermatologists who are currently working up a project proposal through the UKDCTN’s Trainee Network.
This PSP has also stimulated industry partners to look again at this disease and new research programmes have been started looking at the role of afamelanotide (a hormone that is thought to stimulate pigmentation in the skin) for the treatment of vitiligo.
Based on our findings, we recommend the following:
-
More research on the effectiveness and safety of systemic immunosuppressants for the treatment of vitiligo such as methotrexate (Maxtrex®, Pharmacia) and ciclosporin. Research in this field would potentially contribute to our knowledge about the aetiology of the disease, which is believed to have a strong autoimmune component372,377 (which would be addressed in uncertainty 1 as listed in Table 32).
-
Evaluation of currently available and widely used treatments such as topical corticosteroids and calcineurin inhibitors in a head-to-head RCT (which would be addressed in uncertainty 7 as listed in Table 32).
-
Evaluation of the clinical effectiveness and safety of NB-UVB. More detailed information is needed to answer questions such as ‘should the first line treatment for vitiligo be topical agents (topical steroids or calcineurin inhibitors) or more systemic intervention such as NB-UVB’. A factorial trial design could evaluate all three treatment options in one trial (which would be addressed in uncertainties 3 and 8 as listed in Table 32).
-
Evaluation of psychological interventions by conducting a systematic review of the current literature and substantial pilot and exploratory qualitative work prior to progressing to a full RCT. Together with active treatments, psychological interventions are believed to be of great importance. More evidence is needed to establish the role of psychological support as monotherapy (which would be addressed in uncertainty 2 as listed in Table 32) as well as in combination with other treatments for vitiligo (which would be addressed in uncertainty 9 as listed in Table 32).
-
Evaluation of innovative treatments such as afamelanotide and pseudocatalase, which seem to be important and promising to both clinicians and patients (which would be addressed in uncertainties 6 and 10 as listed in Table 32).
-
Evaluation of NB-UVB combination therapies with topical agents, which also reflects the current research trend, shown by the Cochrane systematic review, that combination treatments seem to be more effective than monotherapies367 (which would be addressed in uncertainty 4 as listed in Table 32).
-
More research into the pathophysiology and the aetiology of the disease based on the interest expressed by clinicians and patients on exploration of potential effectiveness of gene therapy and stem cells (which would be addressed in uncertainties 5 and 12 as listed in Table 32).
In recommending the above, we are not commenting on the legitimacy of the interventions that have been prioritised, but reporting what clinicians and patients identified as important research topics in order to meet their needs.
Systematic review of outcome measures
The following text is adapted with permission from Eleftheriadou et al. 439 © 2012 The Authors. BJD © 2012 British Association of Dermatologists.
Summary
What was already known about this topic?
-
There is a lack of consensus as to the best outcome measures for use in the vitiligo research, which makes it difficult to compare results of RCTs and to perform a meta-analysis.
-
Patients’ views are rarely considered on this issue.
What did this study add?
-
This study provided a new perspective by combining a survey of the views of patients, clinicians and researchers with data published in RCTs.
-
Although repigmentation was measured in almost all trials, it was measured using 48 different scales in 54 trials.
-
In the absence of an internationally agreed core outcome set, we propose that repigmentation, cosmetic acceptability, global assessment of disease, QoL, maintenance of repigmentation, stabilisation of vitiligo and side effects should be included in all future vitiligo trials.
-
Further work is required to establish how best to measure these outcomes.
Introduction
There a lack of consensus in the definition and methods of assessment of vitiligo. 440 The updated Cochrane systematic review published in 2010 concluded that the majority of studies differ greatly in the ways in which vitiligo is measured and in the myriad combinations of interventions assessed. The heterogeneity of studies in the review made it impossible to combine trial results in meta-analyses. 431 Although a new international consensus definition and classification of vitiligo has been proposed, consensus over measurement of disease response is still lacking. 441 Over a period of 43 years, 68 different single or combination interventions have been evaluated in 57 RCTs. 367
In clinical trials, selecting outcomes that are relevant to patients and to those making decisions about health care309 is crucial. 442 Vitiligo is a cosmetically and psychologically devastating disease causing great distress, embarrassment and difficulties in relationships. 378 Therefore, one would expect that patients’ views on the effectiveness of treatments would be considered important. However, patient-centred outcomes have rarely been included in vitiligo trials, despite previous recommendations for inclusion in studies on vitiligo367 and other dermatological conditions. 443
The main aim of this study was to extract and report the outcomes reported in published RCTs of vitiligo treatments and to compare these to the outcomes that patients and clinicians considered to be important. This is a first step towards developing recommendations for outcomes to be used in future trials of treatments for vitiligo.
Methods
Systematic review of outcome measures in randomised controlled trials of treatments for vitiligo
A review was conducted of the outcome measures used in RCTs published in English and included in the updated Cochrane systematic review published in 2010. 367 Studies published in other languages were included if they met the criteria and could be translated into English. The review included RCTs published up to November 2009. All of the outcomes reported in the eligible RCTs were retrieved and the scales and measures used to monitor these were collated. Information on outcome assessors was also collected.
Two researchers extracted the data from each eligible study using a standardised pro forma. In the event of any discrepancies, a third researcher adjudicated.
Survey of the most desirable outcomes for vitiligo identified by patients and clinicians
A survey was conducted as part of the previously described vitiligo PSP. 344 Patients and HCPs were asked about their views on what outcomes should be measured in future vitiligo trials. The survey was open for submissions between January and March 2009.
Ethics
The survey was approved by the Medical School REC, University of Nottingham, UK (ethics reference number G/2/2009).
Statistical methods
Data were stored and analysed in Microsoft Excel® 2007 (Microsoft Corporation, Redmond, WA, USA).
Results
Systematic review of outcome measures in randomised controlled trials of treatments for vitiligo
A total of 57 RCTs were evaluated, three of which were excluded as published English translations were not available. Only 22.2% (12/54) of trials had clearly stated the primary outcome measures in the abstract or the main text. The majority of these trials defined the primary outcome as repigmentation (10/12; 83.3%). Other primary outcomes included the size of target lesions (1/12; 8.3%) and the number of new vitiliginous lesions (1/12; 8.3%).
In total, 25 different outcomes were reported in 54 RCTs on the treatment of vitiligo (Table 33). The most frequently reported outcome was repigmentation which was measured in 96.3% (52/54) of trials, followed by side effects (85.2%; 46/54), number of treatment sessions or time (31.5%; 17/54) and cumulative dose to reach repigmentation (22.2%; 12/54).
| Outcome | Trials (%) | References |
|---|---|---|
| Repigmentation | 52 (96.3) | Anbar et al. 2008,402 Arca et al. 2006,386 Asawanonda et al. 2008,414 Barman et al. 2004,426 Bhatnagar et al. 2007,444 Casacci et al. 2007,413 Cestari et al. 2001,445 Czajkowski 2004,416 Dawid et al. 2006,397 Dell’Anna et al. 2007,412 El Mofty et al. 2006,446 Ermis et al. 2001,447 Esfandiarpour et al. 2009,398 Farah et al. 1967,421 Goldinger et al. 2007,448 Hamzavi et al. 2004,449 Hofer et al. 2005,450 Kandil 1974,451 Kawalek et al. 2004,422 Khalid et al. 1995,387 Khandpur et al. 2005,415 Kumaran et al. 2006,420 Leone et al. 2006,399 Lepe et al. 2003,389 Lim-Ong et al. 2005,427 Lu-yan et al. 2006,400 Mehrabi and Pandya 2006,452 Middelkamp-Hup et al. 2007,407 Navarro et al. 2002,453 Ozdemir et al. 2002,454 Parsad et al. 1998,455 Parsad et al. 2003,425 Passeron et al. 2004,423 Pathak et al. 1984,430 Procaccini et al. 1995,456 Radmanesh and Saedi 2006,411 Rath et al. 2008,410 Reyes et al. 2006,408 Rodríguez-Martin et al. 2009,401 Rojas-Urdaneta and Poleo-Romero 2007,405 Ruíz Maldonado and Tamayo Sánchez 1975,388 Sanclemente et al. 2008,404 Sassi et al. 2008,424 Schallreuter et al. 2002,457 Siddiqui et al. 1994,428 Shi et al. 2008,406 Tegta et al. 2006,458 Tjioe et al. 2002,459 van Geel et al. 2004,417 Vasistha and Singh 1979,460 Yones et al. 2007,461 Westerhof et al. 1999429 |
| Side effects and harms (including blood parameters for monitoring purposed only) | 46 (85.2) | Anbar et al. 2008,402 Asawanonda et al. 2008,414 Barman et al. 2004,426 Bhatnagar et al. 2007,444 Casacci et al. 2007,413 Cestari et al. 2001,445 Czajkowski 2004,416 Dawid et al. 2006,397 Dell’Anna et al. 2007,412 Ermis et al. 2001,447 Esfandiarpour et al. 2009,398 Hamzavi et al. 2004,449 Hofer et al. 2005,450 Kandil 1974,451 Kawalek et al. 2004,422 Khalid et al. 1995,387 Khandpur et al. 2005,415 Kumaran et al. 2006,420 Leone et al. 2006,399 Lepe et al. 2003,389 Lim-Ong et al. 2005,427 Lu-yan et al. 2006,400 Mehrabi and Pandya 2006,452 Middelkamp-Hup et al. 2007,407 Navarro et al. 2002,453 Ozdemir et al. 2002,454 Parsad et al. 1998,455 Parsad et al. 2003,425 Passeron et al. 2004,423 Pathak et al. 1984,430 Radmanesh and Saedi 2006,411 Rath et al. 2008,410 Reyes et al. 2006,408 Rodríguez-Martin et al. 2009,401 Rojas-Urdaneta and Poleo-Romero 2007,405 Ruíz Maldonado and Tamayo Sánchez 1975,388 Sanclemente et al. 2008,404 Sassi et al. 2008,424 Siddiqui et al. 1994,428 Shi et al. 2008,406 Tegta et al. 2006,458 Tjioe et al. 2002,459 van Geel et al. 2004,417 Vasistha and Singh 1979,460 Yones et al. 2007,461 Westerhof et al. 1999429 |
| Number of treatment sessions/time to reach repigmentation | 17 (31.5) | Anbar et al. 2008,402 Asawanonda et al. 2008,414 Bhatnagar et al. 2007,444 Casacci et al. 2007,413 Dell’Anna et al. 2007,412 Ermis et al. 2001,447 Hamzavi et al. 2004,449 Hofer et al. 2005,450 Kawalek et al. 2004,422 Kumaran et al. 2006,420 Leone et al. 2006,399 Lu-yan et al. 2006,400 Radmanesh and Saedi 2006,411 Rodriguez-Martin et al. 2009,401 Tegta et al. 2006,458 Yones et al. 2007461 |
| Cumulative dose | 12 (22.2) | Asawanonda et al. 2008,414 Casacci et al. 2007,413 Cestari et al. 2001,445 Ermis et al. 2001,447 Goldinger et al. 2007,448 Hofer et al. 2005,450 Lim-Ong et al. 2005,427 Lu-yan et al. 2006,400 Middelkamp-Hup et al. 2007,407 Passeron et al. 2004,423 Procaccini et al. 1995,456 Tjioe et al. 2002459 |
| Pattern of repigmentation | 9 (16.7) | Anbar and Stern 2008,402 Casacci et al. 2007,413 Cestari et al. 2001,445 Esfandiarpour et al. 2009,398 Kumaran et al. 2006,420 Radmanesh and Saedi 2006,411 Rodríguez-Martin et al. 2009,401 van Geel et al. 2004,417 Yones et al. 2007461 |
| Cessation of spreading/disease activity | 7 (13.0) | Dawid et al. 2006,397 Dell’Anna et al. 2007,412 Lim-Ong et al. 2005,427 Papadopoulos et al. 2004,418 Parsad et al. 2003,425 Rath et al. 2008,410 Siddiqui et al. 1994428 |
| QoL | 5 (9.3) | Agarwal et al. 2005,409 Middelkamp-Hup et al. 2007,407 Papadopoulos et al. 2004,418 Sassi et al. 2008,424 Yones et al. 2007461 |
| Patient global assessment | 4 (7.4) | Hamzavi et al. 2004,449 Middelkamp-Hup et al. 2007,407 Rodriguez-Martin et al. 2009,401 Yones et al. 2007461 |
| Satisfaction with the treatment | 4 (7.4) | Hofer et al. 2005,450 Mehrabi and Pandya 2006,452 Passeron et al. 2004,423 Westerhof et al. 1999429 |
| Colour matching | 3 (5.6) | Bhatnagar et al. 2007,444 Tegta et al. 2006,458 Yones et al. 2007461 |
| Clinician global assessment | 3 (5.6) | Hamzavi et al. 2004,449 Middelkamp-Hup et al. 2007,407 Sassi et al. 2008424 |
| Histological samples | 3 (5.6) | Rojas-Urdaneta and Poleo-Romero 2007,405 Navarro et al. 2008,453 Westerhof et al. 1999429 |
| Presence of cytokines, proliferation and CD expression of T-lymphocytes | 3 (5.6) | Middelkamp-Hup et al. 2007,407 Reyes et al. 2006,408 Shi et al. 2008406 |
| Stability of gained repigmentation | 2 (3.7) | Kumaran et al. 2006,420 Lim-Ong et al. 2005427 |
| Tolerability of treatment | 1 (1.9) | Passeron et al. 2004423 |
| Concentration of epidermal hydrogen peroxide | 1 (1.9) | Schallreuter et al. 2002457 |
| Change in colour | 1 (1.9) | Sanclemente et al. 2008404 |
| Cosmetic acceptability of the results | 1 (1.9) | Barman et al. 2004426 |
| Stress | 1 (1.9) | Papadopoulos et al. 2004418 |
| Body image | 1 (1.9) | Papadopoulos et al. 2004418 |
| Self-esteem | 1 (1.9) | Papadopoulos et al. 2004418 |
| General health | 1 (1.9) | Papadopoulos et al. 2004418 |
| Response to treatment in light vs. dark skin types | 1 (1.9) | Middelkamp-Hup et al. 2007407 |
| Catalase activity, reactive oxygen species, saturation of lipids | 1 (1.9) | Dell’Anna et al. 2007412 |
| Time to suction blister formation and duration of cell cultures | 1 (1.9) | Czajkowski 2004416 |
Repigmentation
Although it was the most frequently reported outcome in the RCTs, repigmentation was measured using a great variety of scales (Table 34) including grades, scores (e.g. 0–4), categories (e.g. poor to excellent, partial to complete), quartiles and other quintiles (e.g. 0–24, 25–50, 51–74, 75–100), percentages (e.g. 0–40, 40–60, 60–100) and mean difference in lesion size. Five trials (9.3%) used more than one scale to measure repigmentation.
| Repigmentation scale | Details | Assessment |
|---|---|---|
| Grades | ||
| 0–3 | None (0); regular (1); good (2); excellent (3) | By patient445 |
| None (0); < 25% (1); 25–50% (2); 51–100% (3) | Clinically445 | |
| None (0); minimal follicular (1+); follicular (2+); > 50% follicular/confluent (3+) | Clinically, digital images457 | |
| None (0); < 50% (moderate) (1); 50–80% (good) (2); > 80% (excellent) (3) | Clinically399 | |
| 0–4 | No change (0); 1–25% (1); 26–50% (2); 51–75% (3); 76–100% (4) | Digital images414 |
| The article presented only: initial, 0–25%; complete, 75–100% | Clinically447 | |
| No change (0); poor, 1–25% (1); moderate, 26–50% (2); good, 51–75% (3); excellent, 76–100% (4) | Clinically, photographs413 | |
| No response (0); minimal, 1–24% (1); moderate, 25–50% (2); marked, 50–75% (3); complete, 100% (4) | Clinically, photographs422 | |
| 0–5 | 0% (0); 1–5% (1); 6–25% (2); 26–50% (3); 51–75% (4); 76–100% (5) | Clinically, digital images450 |
| 0–11 | None (0); ≥ 2 mm (1); 2.1–4 mm (3); 4.1–6 mm (5); 6.1–8 mm (7); 8.1–10 mm (9); > 10mm (11) | Clinically453 |
| VASI | Quantitative parametric score | Clinically449 |
| % quartiles | Response: minimal/none, 0–24%; moderate/mild, 25–50%; marked/moderate, 50–75%; excellent/marked or complete response, 76–100% | Clinically,410,420,444 paper tracing,420 digital images420,444 |
| Response: poor/minimal, 0–24%; moderate, 25–49%; good, 50–74%; excellent/complete, 76–100% | Clinically,398,400,401,423 digital images386,400 | |
| 1 = 0–25%; 2 = 25–50%; 3 = 50–75%; 4 = 75–100% | Clinically and photographs456 | |
| Minimal (25%); moderate (50%); marked (75%) or complete | Clinically, photographs, paper tracing, written description and measurement425,455 | |
| None, 0%; poor/minimal response, 1–25%; moderate/mild response, 26–50%; good/moderate response, 51–75%; marked/excellent response, 76–100% | Clinically,387,389,458 digital images using morphometry,389 photographs and planimetry461 | |
| Tertiles/quantiles | ||
| % | Response: minimal, 0–24%; mild/moderate, 25–49% or 50%; moderate/marked/complete, 50 or 51–100% | Clinically,48,386 digital images386 |
| Mild or minimal or poor response, 0–24 or 25%; moderate or good response, 25 or 26–75%; marked or excellent response, 76–100% | Clinically and planimetry,402,411 digital photographs and morphometry analysis427 | |
| No response; beginning, 1–25%; good, 25–90%; complete cure, 90–100% | Clinically, photographs451 | |
| Absent, 0%; moderate,1–49%; good, 50–75%; excellent, 76–100% | Clinically, photographs412 | |
| None, 0–50%; good, 51–100% | Clinically, patient421 | |
| None, 0%; significant and satisfying, 75%; complete, 100% | Clinically429 | |
| Ineffective, worse or no difference; improved, < 50%; marked, > 50%; cured, 100% | Clinically406 | |
| Deterioration; stable, no changes; partial, 25–40%; incomplete, 40–60%; good, > 60%428 | Clinically, photographs | |
| Failure, 0%; poor, 0–40%; moderate, 40–60%; good/very good, > 60%446 | ||
| Poor, < 30%; fair, 31–50%; good, 51–75%; very good, 76–90%; excellent, 91–100%415 | ||
| Reduction of SA | Pigment spread (millimetres)426 | |
| Mean size of lesions | Mean size of lesions (centimetres)445 | |
| Increase in pigmentation of surface area | 0, absence of repigmentation; 1, 3 mm; 3, 3.1–5 mm; 5, 5.1–7 mm; 7, 7.1–9 mm; 9, 9.1–11 mm; 11, > 11mm405 | Clinically and acetate sheets to mark the edges |
| % | ||
| Surface repigmentation | Mean %: 0–100% | Clinically389,404,429,445,448,459 digital images389,404,407,417,445,448,452,459 digital morphometry404 transparent sheets tracing417 |
| Size of target lesions | 0–100% | Planimetry397 |
| Change of affected skin | 0–100% | Clinically,386,388,411 photographs388 and tracing411 |
| Success | Only 100% repigmentation | Clinically416 |
| Categories | ||
| Categories | Response: none; fair, slight improvement; good, increase/decrease in size of pigmented spots/lesions; excellent, considerable increase/decrease in size of pigmented spots/lesions | Clinically460 |
| Clearance of lesions | Clear/not clear | UV photographs424 |
| Reduction ≥ 75% of overall lesions | Reduction of at least 75%/no reduction of at least 75% | UV photographs424 |
| Number of new lesions | Number of new lesions | Clinically409 |
In total, repigmentation was measured using 48 different scales in 54 eligible trials. Although 29.6% (16/54) of the trials used quartiles, 14 different scales were created including differences in the definition of quartiles and the names of the corresponding categories. For example, Kumaran et al. 420 and Bhatnagar et al. 444 both used the ‘0–24%, 25–50%, 50–75%, 76–100%’ quartiles, but Kumaran et al. 420 reported moderate improvement at 25–50% repigmentation and Bhatnagar et al. 444 at 50–75% repigmentation of vitiliginous lesions. The definition of excellent repigmentation or success varied from trial to trial and included values from ‘any repigmentation’ to 100% repigmentation of vitiliginous lesions.
Trials assessed repigmentation by clinical assessment only (17/54; 31.5%) or in combination with other methods, such as digital images, paper tracing or planimetry (29/54; 53.7%). Six trials (11.1%) used only image-based methods such as digital images, planimetry and image analysis of reflected UV photographs in assessing repigmentation. Only 3.7% trials (2/54) incorporated patient-assessed repigmentation.
Pattern of repigmentation and colour matching
Pattern of repigmentation was reported in 16.7% (9/54) of trials. This was usually briefly mentioned in the discussion section or the results section only. Perifollicular and peripheral/perifollicular patterns were mentioned in 3.7% (2/54) and 9.3% (5/54) of the trials, respectively. A total of 4% of the trials reported all patterns (perifollicular, marginal and diffuse) of repigmentation (Table 35). Change in colour and colour matching of vitiliginous lesions was assessed in 5.6% (3/54) and 1.9% (1/54) trials, respectively. These were assessed by clinicians only,444,458 a combination of clinical assessment and photographs461 or by using digital images. 404 Patient assessment of colour matching was not included in any of the trials (see Table 35). Response to the treatment in light versus dark skin types was reported in one trial (1.9%) and was measured clinically.
| Outcome | Details | Method of assessment/assessor | Trials |
|---|---|---|---|
| Depigmentation | Percentiles 1 = 1–25%; 2 = 26–50%; 3 = 51–75%; 4 = 76–100%414 | Digital images | 1 |
| Pattern of repigmentation | Follicular, peripheral, marginal, diffuse38,42,53,57,62,63,96,177,405 | Clinically,398,401,402,411,413,420,445,461 planimetry402,411 and photographs398,401,413,417,445 | 9 |
| Colour matching of newly repigmented lesion to the surrounding normal skin | Darker, same, lighter444,458 | Clinically | 2 |
| Excellent, not excellent (no further details) | Clinically and photographs | 1 | |
| Change in colour | Commission Internationale de L'Eclairage L, a, b system461 | Digital images | 1 |
| QoL | DLQI409,418,461 | Patients | 3 |
| Skindex-29407,424 | Patients | 2 | |
| WHOQOL-BREF questionnaire for QoL409 | Patients | 1 | |
| General health | General health questionnaire418 | Patients | 1 |
| Body image | Body image dysphoria: situational inventory of body image dysphoria; body image feelings: body image automatic thoughts questionnaire418 | Patients | 1 |
| Patients | |||
| Self-esteem | Rosenberg self-esteem scale418 | Patients | 1 |
| Stress | Perceived stress scale418 | Patients | 1 |
| Cessation of spreading of vitiligo/disease activity | Not clear425 | Clinically, photographs, planimetry, written description and measurement | 1 |
| Not clear (one sentence in the discussion section)410 | Not clear | 1 | |
| VIDA score115,449 | Patients | 2 | |
| No scale418 | Photographs | 1 | |
| Positive, stable, deteriorating428 | Clinically and photographs | 1 | |
| Binary scale: stabilised/not stabilised412 | Clinically and photographs | 1 | |
| Clinical global assessment | Very severe; severe; more or less severe; not so severe407 | Clinically | 1 |
| Complete improvement (100%); very much improved (76–99%); much improved (51–75%); improved (26–50%); minimal change (1–25%); no change449 | Clinically | 1 | |
| Anchored horizontal VAS424 | Clinically | 1 | |
| Patient global assessment | VAS: –5 to +5401 | Patients | 1 |
| VAS: 0 to 1047,62 | Patients | 2 | |
| Complete improvement (100%); very much improved (76–99%); much improved (51–75%); improved (26–50%); minimal change (1–25%); no change449 | Patients | 1 | |
| Satisfaction with the treatment | VAS: 0 to 10450 | Patients | 1 |
| Questionnaire (no further details)452 | Patients | 1 | |
| Opinion on treatment/side effects questionnaire (no further details)429 | Patients | 1 | |
| Opinion on treatment efficacy and degree of satisfaction questionnaire. Scale: poor; good; excellent423 | Patients | 1 | |
| Cosmetic acceptability of the results | Satisfied/unsatisfied426 | Patients | 1 |
| Tolerability of the treatment | VAS423 | Patients | 1 |
| Response to treatment in light vs. dark skin types | Descriptive407 | Clinically | 1 |
| Stability of gained regimentation and development of new lesions | Within 1 year after treatment. Scale: not clear427 | Not clear | 1 |
| Within 20 weeks after treatment. Categories: maintained repigmentation; continued to improve; pigmentation faded; development of new lesions420 | Not clear | 1 |
Cessation of disease activity
Seven RCTs (13.0%) measured the cessation of spreading of vitiligo during treatment period; only four (7.4%) stated the scale used.
Stability of gained repigmentation
Two trials (3.7%) assessed stability of gained repigmentation and development of new lesions. The timescales of the assessment of this outcome were 1 year and 20 weeks after the completion of the treatment.
Patients’ opinion on treatment efficacy
Only 16.7% of studies (9/54) reported patients’ opinions regarding treatment efficacy and degree of satisfaction with the treatment including cosmetic acceptability of the results. In particular, 7.4% of trials (4/54) assessed patient satisfaction with the treatment by asking them directly using a questionnaire or using a VAS. However, details of which questions were asked were generally lacking. Cosmetic acceptability of the results and patient global assessment were monitored in 1.9% (1/54) and 7.4% (4/54), respectively. The latter was measured in three different ways (see Table 35). Tolerability of the treatment was briefly reported in one trial (1.9%).
Quality of life
Five trials (9.3%) assessed QoL of vitiligo patients. Different questionnaires were used DLQI (3/54; 5.6%), Skindex-29 (2/54; 3.7%) and World Health Organization QoL questionnaire (1/54; 1.9%).
General health, stress, body image and clinician global assessment
One trial17 reported several patient-centred outcomes: stress, body image, self-esteem and general patient’s health. Clinician global assessment was measured using three different scales in three trials (5.5%).
Side effects and harms
Various side effects and harms, such as erythema, blistering, graft failure, Koebner phenomenon and hyperpigmentation around vitiliginous lesions, were reported in 46 out of the 54 eligible trials (85.2%). In nine trials, parameters such as full blood count, kidney and liver function tests were measured for the purpose of monitoring possible treatment side effects only and, therefore, were included in this category of outcomes. Side effects and harms were generally reported briefly in the results or discussion section of the article with limited information on frequency or severity.
Specific blood, skin parameters and other outcomes
Histopathology of vitiliginous lesions (3/54; 5.6%), presence of cytokines, proliferation and CD expression of T-lymphocytes (3/54; 5.6%), concentration of epidermal hydrogen peroxide (1/54; 1.9%), and catalase activity and lipid saturation (1/54; 1.9%) were measured as an indicator of effectiveness of compared treatment.
Number of treatments to gain repigmentation, cumulative dose and time to suction blister formation were sometimes reported in trials when relevant to delivery of the intervention (see Table 35).
Survey of the most desirable outcomes for vitiligo identified by patients and clinicians
Eighty-seven per cent (401/461) of vitiligo PSP participants suggested at least one outcome (response rate 87%). In total, 438 suggestions were made and 23.5% (103/438) of these were excluded as non-relevant, such as questions on inheritance and trigger factors for vitiligo.
Of the remaining 335 suggestions, 68% of responses were from patients, 25% from clinicians and 7% did not specify. More women responded than men (53% women; 30% men and 17% did not specify) and most were aged 30–60 years (8%, < 30 years; 50%, 30–60 years; 25%, > 60 years; 17% did not specify).
The most popular outcome among all responders was repigmentation of vitiliginous lesions (68%), followed by cessation of spreading of the disease (15%), QoL (6%), maintenance of repigmentation (4%) and other outcomes such as camouflage effectiveness and depigmentation.
Repigmentation of vitiliginous lesions
Statements such as ‘cosmetically acceptable repigmentation rather than percentage’, ‘normal-looking skin’, ‘90–100% repigmentation’ and ‘quick and long lasting (at least 2 years)’ were made by survey responders. Repigmentation on visible areas such as face and hands was also considered important (Figure 18).
FIGURE 18.
Desirable outcomes for (a) clinicians; and (b) patients.
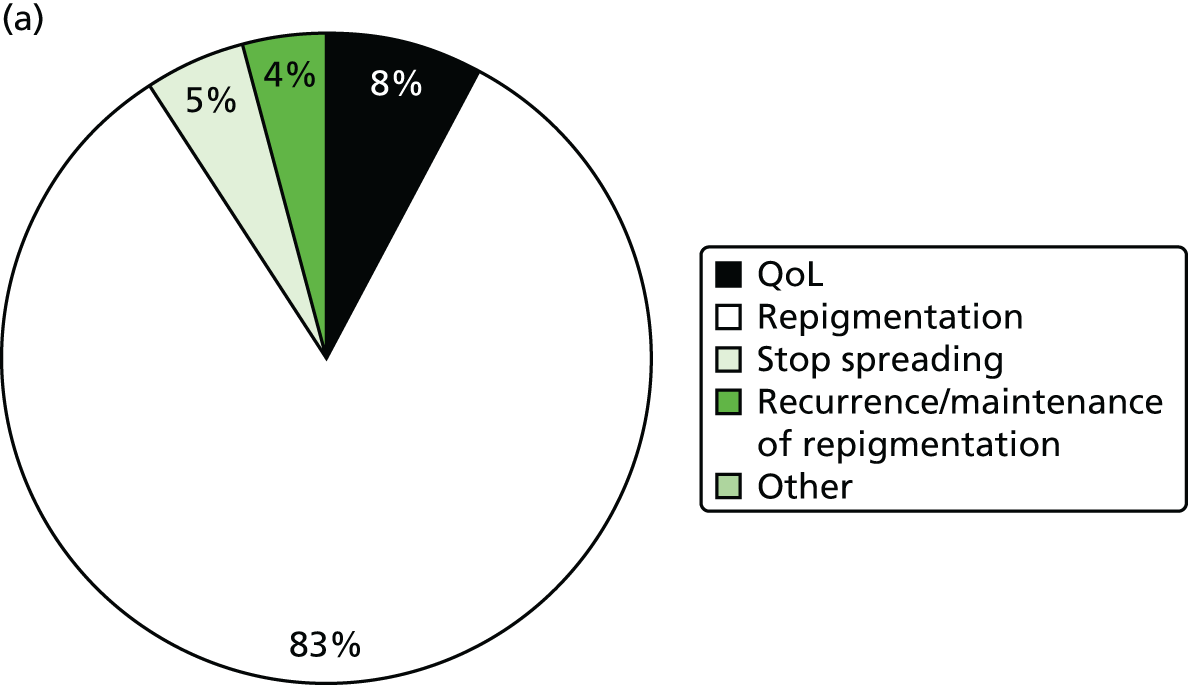

Cessation of spread of vitiligo
Nineteen per cent of patients found cessation of the disease important compared with only 5% of clinicians (see Figure 18).
Quality of life
Nearly equal percentages of patients (9%) and clinicians (8%) found the QoL of vitiligo patients to be an important outcome. Statements such as ‘to reduce the stress and embarrassment, psychological impact, support, coping strategies and self-confidence, psychological support for children with vitiligo’ were reported (see Figure 18).
Other submissions included depigmentation and camouflage improvement.
Provisional recommendations for a vitiligo core outcome set
We believe that the findings of this review and survey are a good starting point for creating international consensus over a core outcome set for vitiligo trials.
In the interim, we propose that the following outcomes should be considered in future trials of vitiligo treatments (Table 36).
| Proposed outcomes | Example of scale | Assessment method | Comments |
|---|---|---|---|
| Repigmentation | % quartiles: 0–24%; 25–49%; 50–74%; 75–100% | Clinician with digital or UV photographs | The method of assessment will depend on its availability and appropriate training of personnel |
| Cosmetically acceptable repigmentation | VAS (bad, fair, good, excellent) | Patient | This will take account of the colour match of the newly repigmented lesions to the surrounding normal skin including hyperpigmentation around the lesions if applicable |
| Global assessment of the disease | VAS: complete improvement; very much improved; much improved; improved; minimal change; no change | Patient and clinician | A unified, combined, scale should be used by both patients and clinicians, which would be quick and easy to use in both clinical setting and research environment |
| QoL | Skindex-29 | Patient | More research is needed to determine the best QoL scale |
| Maintenance of gained repigmentation | VAS: complete maintenance; partial maintenance; return to pre-treatment; progression from pre-treatment | Patient and clinician | It is well known that repigmentation of vitiliginous lesions can take months and that depigmentation can recur. Therefore, it is important to assess maintenance of gained repigmentation when weighting the treatment benefits against the harms |
| Cessation of spread of the disease | VIDA score397,427,462 | Patient and clinician with digital photography | Cessation of spread of the disease is an important outcome owing to the unpredictable nature of vitiligo, which can be devastating and distressing for patients. Stabilisation of the disease until repigmentation occurs was reported as a realistic measure of outcome431 |
| Side effects and harms | Descriptive – inclusive of patient’s perspective | Patient and clinician | Side effects and harms of an intervention should be clearly reported in the results section with relevant frequencies |
Discussion
Relevant and reliable outcomes play a crucial role in the correct interpretation and comparison of the results of different treatment modalities. 299 Lately, it has been increasingly recognised that outcome measures in trials should be relevant to patients and clinicians;309 however, no attempts have been made, either by researchers or by pharmaceutical companies, to standardise outcome measures for the treatment of vitiligo. Initial attempts have been made to create a consensus in the way that vitiligo is assessed. An innovative scoring tool was proposed in 2007 by the Vitiligo European Task Force;440 however, this had not been used in any of the RCTs included in this review. This scoring system combines the three dimensions of the disease: extent, staging and spreading, and takes into consideration the presence of white hair and the body sites of vitiliginous lesions. 440
It has been reported that 85% of research funding is wasted across all aspects of the research cycle. 38,314 Three of the four sources of waste are closely related to the outcomes used in trials: important outcomes are not assessed, published research fails to set the study in the context with all previous similar research and > 50% of planned study outcomes are not reported. 38
The majority of the trials in the systematic review reported repigmentation, but this was measured using 48 different scales. In total, 25 different outcomes were reported in 54 trials. Patients and clinicians put greatest emphasis on repigmentation, QoL, cessation of spread of the disease and maintenance of gained repigmentation. However, cosmetically acceptable repigmentation as assessed by patients, rather than percentage of repigmentation per se, should be measured when possible. Our further work to develop a patient-rated outcome measure of treatment success has shown that the phrase ‘cosmetic acceptability’ was not favoured by patients, as it seems impersonal and implies that vitiligo is merely a cosmetic problem. Our work has suggested that the ‘noticeability’ of the vitiligo is useful surrogate term for this concept (validation of patient-reported treatment response).
Recommendations for future vitiligo trial design
Guidelines for designing and reporting clinical trials on vitiligo have suggested that patient-centred outcomes should be incorporated into the design of future trials of vitiligo treatments, along with QoL, percentage of repigmentation, permanence of gained repigmentation and the arrest of the progression of the disease. 431 The results of this study support such a recommendation.
Although QoL has been reported in only 9% of trials, it is considered an important outcome from a patient’s point of view and was also proposed as a primary outcome in the guidelines for designing and reporting clinical trials for vitiligo. 431
To overcome the issue of different body sites responding differently to treatments, it has been suggested that studies perform stratified analyses based on body sites. 431 Similarly, revised international classifications for the stability of disease recommends that disease stability (or activity) be assessed for individual lesions rather than a person overall, as this may fluctuate by site of involvement. 441
Strengths and weaknesses of the study
The survey responders included patients with vitiligo, their parents/guardians, dermatologists and other HCPs with an interest in vitiligo. As a result, the proposed outcomes reflect a broad spectrum of views.
A convenience sample of RCTs included in the updated Cochrane systematic review was chosen based on resources and time limitations. It is possible that more recent trials have been more consistent in including patient-reported outcomes and QoL scales in line with recent recommendations. 431
Implications for research
To our knowledge, this study is the first to systematically summarise outcomes reported in RCTs of vitiligo treatments and to compare these outcomes with perspectives from patients and clinicians. It is encouraging that repigmentation appears to be a good candidate for a core outcome domain in future vitiligo trials. However, it is still unclear how this (and other outcomes) should be measured and there is no consensus over what constitutes a treatment success. Future research is needed to address how best to capture treatment success from a patient’s perspective.
An international consensus initiative to define an agreed set of core outcomes for use in future clinical trials of vitiligo treatments is now under way. Further details of this project are reported in Gaining consensus over outcomes for use in future vitiligo trials.
Gaining consensus over outcomes for use in future vitiligo trials
The following text is adapted with permission from Eleftheriadou V, Thomas K, van Geel N, Hamzavi I, Lim H, Suzuki T, et al. Developing a core outcome set for vitiligo clinical trials: international e-Delphi consensus. Pigment Cell and Melanoma Research 2015. 463 © 2015 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Summary
What was already known about this topic?
-
Systematic reviews of intervention trials are hampered by a lack of consistent reporting of trial outcomes.
-
Development of core outcome sets can help to prevent variation in practice and can facilitate pooled analysis of data across trials.
-
An internationally agreed core outcome set for vitiligo trials is not currently available.
What did this study add?
-
International agreement has been reached over the core outcome domains to be included in future vitiligo trials.
-
Future trials should include:
-
repigmentation
-
side effects
-
maintenance of gained repigmentation as essential core outcomes.
-
-
A further four items were recommended for inclusion, but were not considered to be essential:
-
cosmetic acceptability of the results
-
QoL
-
cessation of spreading of vitiligo
-
tolerability or burden of treatment.
-
-
Further work is needed to establish how best to measure these core domains.
Introduction
The previous chapters of this report have outlined the heterogeneity of current vitiligo RCTs, the importance of including patients’ view when assessing treatment success and provided a provisional list of what UK-based patients and HCPs felt were important aspects to measure in future vitiligo trials.
The current study aimed to develop international consensus over a core outcome set for vitiligo trials that is acceptable to HCPs, patients, researchers and regulatory bodies.
The work was conducted as part of an international collaboration co-ordinated through the International Federation of Pigment Cell Societies (IFPCS) and co-ordinated from the Centre of Evidence Based Dermatology at the University of Nottingham.
Methods
Study design
An international web-based electronic Delphi (e-Delphi) consensus exercise was conducted between January 2013 and September 2013. A total of 101 self-selecting participants took part from 25 countries. Participants included HCPs with an interest in vitiligo (dermatologists, n = 51; nurse, n = 1); patients with vitiligo or their carers (n = 32); and others interested stakeholders (researcher, n = 1; representatives of regulatory agencies, n = 2; journal editors, n = 14).
The e-Delphi process included three rounds, each taking approximately 2–4 weeks to complete. The first round was conducted using a questionnaire distributed by e-mail, but subsequent rounds were conducted using Survey Monkey software. Reminders were sent by e-mail 7–10 days prior to the submission deadline.
Identification of participants
Potential participants were identified through the IFPCS. Representatives of each subgroup of the IFPCS, that is, Asian, Japanese, European and Pan-American societies, contacted their local patient support groups in order to identify patients willing to participate in this process. In addition, representatives identified regulatory bodies and journal editors and invited them to participate.
All communication took place electronically and participants were given clear deadlines for taking part in each round of the three rounds. No financial incentives were provided to any of the participants and completion of round one was taken as consent to participate.
Participants were strongly encouraged to continue participation throughout all three rounds, as this was important for maintaining the validity of the consensus approach.
Preparation of the initial survey
A list of possible outcomes to be capture in vitiligo trials was compiled on the basis of our systematic review of outcomes measures reported (see Systematic review of outcome measures) and from the patient and clinician surveys reported in Survey of UK clinical practice. In addition, demographic information about the participants was captured including country and experience of vitiligo (patient, parent/guardian, dermatologist, editor, regulatory body, researcher). Copies of the survey questions used are appended (see Appendix 5).
Outcomes included in the initial survey were:
-
repigmentation
-
cosmetically acceptable repigmentation
-
global assessment of the disease
-
QoL
-
maintenance of gained repigmentation
-
cessation of spread
-
side effects and harms
-
self-esteem
-
psychological impact.
Consensus criteria
Each participant was asked to rate the importance of the individual items in the questionnaire using a five-point Likert scale (very important, important, less important, not important and not sure).
In the first round, participants were also asked to list additional outcomes they considered to be potentially relevant, but which had not been included on the original list.
Consensus was defined as having being achieved if at least 75% of participants in any two categories of responders (HCPs/patients/others) were in agreement. Responses from each round were collated and analysed by VE prior to circulation of the subsequent round.
For round 2, the additional outcomes identified during round one were added as additional questions and outcomes for which there was consensus that the item was ‘not important’ as a core outcome were removed. Details of items removed at each stage were provided and participants had the opportunity to agree or disagree with that decision.
Participants received feedback on the group’s opinion for each domain (modal response and percentages for each category) and were then asked to answer the questionnaire again in the light of this feedback, with the aim of moving closer to consensus.
For the final round, participants were presented with the selected core outcome domains from round two and were asked to identify which of the outcomes were ‘essential’ in the core outcome set and which were ‘recommended’.
-
Essential: must be relevant to all interventions for vitiligo and reported in all clinical trials for the treatment of vitiligo.
-
Recommended: should be reported if relevant to the intervention being tested and trial design used.
Free-text answers were summarised and organised thematically.
Sample size and justification
We initially aimed to involve approximately 12 participants from each stakeholder group (clinicians, patients and regulatory bodies/journal editors), resulting in a total sample size of 34 participants. However, the final sample size was dictated by the number of responses to the initial e-mail invitation. No-one who expressed an interest in taking part was prevented from doing so.
Results
Overall 101 participants took part in the e-Delphi process and 81 out of 101 (80.2%) completed all three rounds. A full list of all participants is included in the acknowledgements section of this report. The response rates for each round are summarised in Table 37.
| Stakeholder group | Round 1, N = 101 | Round 2, N = 87 | Round 3, N = 81 |
|---|---|---|---|
| Dermatologist, n | 51 | 43 | 42 |
| Patients/carers, n | 32 | 26 | 21 |
| Other (journal editors, regulatory authorities, nurses, researchers), n | 18 | 18 | 18 |
Following round one, three outcomes were removed from the proposed outcome domains as they failed to achieve 75% of the vote for at least two of the stakeholder groups. Excluded items were self-esteem, psychological impact and global assessment of disease. Two new items were added for consideration in round two: tolerability or burden of treatment and economic impact.
Following round two, a list of seven core outcome domains were confirmed as being important for inclusion in future vitiligo trials, these included repigmentation, cosmetic acceptability of the results, QoL, maintenance of gained repigmentation, cessation of spreading of vitiligo, side effects and harms of treatment, and tolerability or burden of treatment. Only economic impact of treatment was removed following round two.
Following round three there was consensus that three items were essential and should be included in all future vitiligo trials. These were: (1) repigmentation, (2) side effects and (3) maintenance of gained repigmentation. A further four items were recommended for inclusion, but were not considered to be essential. These were: (1) cosmetic acceptability of the results, (2) QoL, (3) cessation of spreading of vitiligo and (4) tolerability or burden of treatment.
Full details of responses from each stakeholder group are summarised in Table 38.
| Domain (%) | Patients, n = 21, response rate = 81% | Dermatologists, n = 42, response rate = 95% | Editors, regulatory representatives, researchers and nurses, n = 18, response rate = 100% | All participants, n = 81, response rate = 93% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Essential | Recommended | Not sure | Essential | Recommended | Not sure | Essential | Recommended | Not sure | Essential | Recommended | Not sure | |
| Repigmentation | 95 | 5 | 0 | 100 | 0 | 0 | 94 | 6 | 0 | 98 | 2.5 | 0 |
| Cosmetically acceptable repigmentation | 33 | 67 | 0 | 51 | 44 | 5 | 50 | 44 | 6 | 47 | 51 | 2 |
| QoL | 71 | 19 | 10 | 58 | 39 | 3 | 22 | 72 | 6 | 53 | 42 | 5 |
| Maintenance of gained repigmentation | 81 | 19 | 0 | 49 | 46 | 5 | 67 | 33 | 0 | 61 | 36 | 3 |
| Cessation of spreading of vitiligo | 71 | 24 | 5 | 70 | 30 | 0 | 61 | 33 | 6 | 68 | 28 | 4 |
| Side effects and harms | 67 | 33 | 0 | 88 | 12 | 0 | 50 | 44 | 6 | 74 | 25 | 1 |
| Tolerability/burden of treatment | 48 | 48 | 4 | 54 | 46 | 0 | 33 | 62 | 6 | 47 | 51 | 2 |
In response to free-text questions, the only additional comments were that depigmentation should also be considered when relevant (n = 1) and that the next steps will be to evaluate relevant instruments for capturing these domains (n = 1).
Discussion
This study represents the first attempt to establish an internationally agreed core outcome set for use in vitiligo clinical trials. The methods used followed agreed standards for consensus development, using guidance outlined by the HOME initiative296 and the Core Outcome Measures for Effectiveness Trials initiative. 314
In confirming a set of core outcome domains for use in future vitiligo trials, it is hoped that future studies will focus on outcomes that are relevant and important to both patients and HCPs; thus, improving the relevance and interpretability of future research findings.
Further work is now needed to clarify the definition and timing of these core outcome domains, prior to moving on to establishing a standardised scale to measure each outcome. For example, some authors advocate a minimum of 12 months’ follow-up after the end of treatment,367,431 but this may not be possible for all trials.
The work described in Validation of patient-reported treatment response would suggest that the term ‘cosmetically acceptable repigmentation’ is poorly understood by patients and this may have influenced its placing in the final list of required outcome domains as being recommended, but not essential.
Implications for research
The identification of core outcome domains is just the first step in developing an agreed core outcome set for vitiligo trials. Work is now required to establish the best way of defining each of these outcomes (including appropriate time frames) and to identify the most suitable outcome instruments that are standardised and well validated. In defining recommended instruments for capturing each of the seven domains, work will now take place following the roadmap outlined by the HOME initiative and using guidance by the COSMIN initiative. 464
All seven outcome domains will be collected in the HI-Light trial [see Proposal for a trial light therapy and topical corticosteroids for the treatment of vitiligo (HI-Light)], although it is possible that the specific instruments selected will not be compatible with those selected through future iterations of this international initiative. Nevertheless, by working closely with groups around the world, it is to be hoped that agreement over provisional instruments can be defined quite quickly while further validation work is under way.
This study represents an important first step for improving the quality of future vitiligo trials. Through consensus over a core outcome set, the ability to use trial findings in meta-analyses will be improved, thus facilitating better use of trial data and allowing systematic reviewers to combine data to generate clinically relevant findings.
Validation of patient-reported treatment response
The following text is adapted from Tour et al. 465 © 2014 Tour et al. ; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Summary
What was already known about this topic?
-
Patient-rates outcome measures are not used widely in vitiligo trials.
-
The most commonly used outcome measure in vitiligo trials to date has been percentage repigmentation; however, from the point of view of people with vitiligo, this is not a very meaningful measure.
-
Previous work has suggested that a measure of cosmetic acceptability of the treatment response might be more meaningful to people with vitiligo.
What did this study add?
-
People with vitiligo do not like the term ‘cosmetic acceptability’, as it is impersonal and suggests that vitiligo is merely a cosmetic problem. How noticeable the vitiligo is after treatment is a more meaningful concept.
-
Using an online survey and online discussion groups, we have developed a patient-rated outcome measure to assess the ‘noticeability’ of vitiligo after treatment.
Introduction
Vitiligo is a chronic depigmenting skin disease characterised by loss of skin colour in patches. 367 It affects people of all ages, ethnic groups and skin types and approximately 0.5–1% of the world’s population,367,439 although estimates are higher in countries and cultures where the stigma of the skin disease may be higher. 367
There is no cure for vitiligo but there are many treatments available to manage it. These include various topical and oral preparations, light therapy, surgical procedures, psychological therapies and complementary therapies. 367 The only licensed treatment for vitiligo in the UK is cosmetic camouflage,466 although many other treatments are used in clinical practice.
Physical symptoms in vitiligo are usually mild but the unpredictable nature of the disease and its tendency to progress in the majority of cases can be psychologically467 and cosmetically overwhelming. 367,439,468 Living with vitiligo can be a continuous struggle, with the psychological characteristics of each individual determining their ability to adjust to and cope with disfigurement. 469
Although clinical studies have assessed many treatments for vitiligo, the heterogeneity of these studies makes comparison of the effectiveness of treatments – alone or combined – very difficult. 367,431,439 The updated Cochrane systematic review of interventions for vitiligo published in 2010,367 and other reviews, have highlighted problems such as variance in design and a lack of standardised outcome measures and scales used in clinical trials. 344,367,431,439 There is a pressing need to develop core outcome measures so that effectiveness of treatments can be compared and combined more easily across trials. 439
It is important that outcomes used in trials are relevant to patients as well as clinicians. 439 Repigmentation tends to be the most frequent outcome measure used, although scales used vary from study to study. 367,439 Vitiligo results in patches of depigmented skin thus, intuitively, it would seem to be a simple matter of recording treatment success or failure based on changes in the amount of repigmented skin. However, the following aspects make this assessment difficult.
-
Vitiligo often affects multiple anatomical sites and lesions vary in size and shape, making physical measurement of lesions on all affected areas difficult and time-consuming.
-
When repigmentation of the vitiligo patches does occur, it can often be uneven, with pigmented spots within the white areas (perifollicular) or of a different skin tone to a patient’s normal skin colour. This can reduce the cosmetic acceptability of the treatment response and a simple assessment of percentage of repigmentation may fail to capture this aspect.
-
Highly visible sites such as the hands and face are associated with higher psychological morbidity, again leading to a potential reduction in the acceptability of treatment response.
Patient-assessed repigmentation is not commonly used as an outcome measure, whereas clinical assessment and objective methods such as software analysis of digital images are more widely used. 439 What might be considered an improvement by clinicians in percentage terms may not be perceived as such by patients. Recommendations have been made for the inclusion of patient views in the effectiveness of treatment owing to the psychological impact of the disease367,431 although, to date, these have rarely been put into practice. 439
The aim of the study was to develop and provide preliminary data on the face validity of a PROM on the acceptability of treatment response.
Specific objectives were:
-
to conduct patient surveys and online discussion groups to establish the most appropriate form of wording and scale to use
-
to ensure that the wording of the question assessing satisfaction with treatment response is relevant to, and easily understood by, vitiligo patients (face validity).
In addition to improving our understanding of what constitutes a successful treatment response from a patient perspective, it is anticipated that the resulting outcome measure will be useful for use in future vitiligo trials and clinical practice. The results will also be used to inform an ongoing international initiative to establish a core outcome set for use in vitiligo trials470 to enable effective comparisons across trials and to allow recommendations of effective treatments to become clearer.
Methods
This project was conducted in two stages. First, an online survey was used to identify which aspects of treatment response are important to patients. Then, the online survey was followed by three separate online discussion groups, in which the results of the survey were explored with patients and consensus was reached regarding the most appropriate form of wording for the proposed PROM.
The project was approved by the Medical School REC at the University of Nottingham (ethics reference number LTg15082013 SoM Dermatol).
Online survey
Participants
Participants were recruited from an existing mailing list held at the Centre of Evidence Based Dermatology at the University of Nottingham. This list consisted of individuals who had participated in a previous Vitiligo PSP344 and those who had contacted us expressing interest in being involved in vitiligo research. In addition, participants were recruited through the UK Vitiligo Society Facebook Page and details of the survey were ‘Tweeted’ under the UKDCTN Twitter (Twitter, Inc., San Francisco, CA, USA; www.twitter.com) account. Participants included both those who had sought treatment for their vitiligo and those who had not, and included parents/carers of children with vitiligo as well as those with vitiligo themselves. Efforts were made to ensure broad representation across all age and ethnic groups. Although recruitment was targeted largely at participants in the UK, there were no exclusions based on country of residence and details of nationality were recorded.
Survey distribution
The survey was created using a secure internet-based survey system (SurveyMonkey) and consisted of 14 questions. It was estimated that the survey would take around 5 minutes for participants to complete. No incentives were offered for participation.
Details of the survey were sent to 188 potential participants from our existing mailing list. Potential participants were sent information about the purpose of the research, together with a web link to the survey itself. The survey was open from 29 July 2013 until the 19 August 2013. Two reminders were e-mailed to all on the mailing list and additional posts were placed on the Vitiligo Society’s Facebook (Facebook, Inc., Menlo Park, CA, USA; www.facebook.com) page and the UKDCTN Twitter feed in order to broaden recruitment.
Data collection included demographic details, the extent of the vitiligo and previous treatments used, opinions on what a ‘cosmetically acceptable response’ to treatment meant to the participant and whether or not they felt it was the same as ‘satisfaction with the result’. A selection of 11 words and phrases to describe treatment response were also presented, from which participants chose the most meaningful to them (see Table 40).
Images were used to judge both a ‘worthwhile’ treatment response and minimum level of treatment response that a participant was willing to accept. Participants were also asked to look at a series of head shots. The images featured a young boy with dark skin with a vitiliginous lesion. Using image manipulation software (Adobe® Photoshop CS2; Adobe Systems Incorporated, San Jose, CA, USA) the lesion is gradually removed from the sequential images to simulate repigmentation at different intensities. Participants were asked to indicate the degree of repigmentation that they considered worthwhile after 9 months of treatment, followed by the minimum level of repigmentation they would be prepared to accept.
Online discussion groups
Participants
Survey participants who indicated interest in further involvement in research were invited to participate in online discussion groups. All participants received a £10 Amazon (Amazon.com, Inc., Bellevue, WA, USA) e-voucher.
Invitations were sent by e-mail, with an information sheet attached. In total, 57 initial invitations were sent. Reminder e-mails and further invitations were sent, if necessary, to ensure that 6–8 people were confirmed for each of the three discussion groups. 471 To aid participation, two groups were held in the evening.
To ensure familiarity with the concepts involved and the context in which the patient assessment of treatment response would be placed, confirmed participants were sent reading material on clinical research methods and primary outcome measures. Participants were also advised to read information on vitiligo from the NHS Choices web pages472 and to watch a short video explaining clinical trials from the MRC website. 225
Hosting the online discussion groups
Online discussion groups were used in favour of face-to-face focus group discussions in order to facilitate engagement with a broad range of participants from throughout the UK. 473–475
The online discussion groups were hosted in a private chat room based within the Vitiligo Society’s web pages. All participants followed an e-mail link, registered for the group and, once approved by the moderator (ST), were given access to the chat room for the time of their online discussion. Prior to the groups taking place, participants were sent information about the objectives of the groups and about consent to participate. Participants gave consent at time of registration and were encouraged to use an alias if they wished to remain anonymous.
Each group lasted for approximately 90 minutes to allow adequate time for discussion while avoiding participant fatigue. Participants were able to type text and send emoticons as in a standard chat room. Groups were facilitated by two to three members of the research team.
The discussion groups were semistructured; a list of prompts was prepared in advance and these were inserted into the discussion thread at relevant time points. This ensured similarity between the questions asked of each group. A selection of the prompts used is shown in Box 6. Page links were created for images and inserted into the discussion thread at relevant times during the discussion to allow participants to view images of vitiligo before and after treatment and a selection of measurement scales.
The survey results showed that the three main areas of importance to people with regard to judging treatment success were (in order of frequency):
-
colour match between their vitiligo and normal skin (i.e. how well it blends in)
-
how noticeable the vitiligo is
-
a reduction in the size of the white patches.
Which of these do you think is the most important if we are trying to capture a measure of treatment success?
Prompt 2 (wording of questions about how noticeable vitiligo is)Let’s try some example questions that ask about how noticeable your vitiligo is. These questions can be used at the end of a trial to ask people about how successful their treatment is. What do people think about these possible questions? Does the wording seem right?
-
Q1 How noticeable do you feel your vitiligo is, compared with the start of treatment?
-
Q2 How successful do you feel the treatment has been, in terms of how noticeable the vitiligo is?
-
Q3 How satisfied are you with how noticeable the vitiligo is?
-
Q4 Compared to before treatment, how noticeable is your vitiligo now?
If some areas of vitiligo respond well to treatment and some do not, do you think that this question is useful to measure how noticeable the vitiligo is on all body sites affected? Or do you think that the question is only useful for assessing individual patches of vitiligo?
Adequate time was allowed after insertion of each prompt to allow participants to respond and discuss with each other freely. The facilitators guided discussion with the aim of trying to achieve consensus and summarised the discussion findings at various intervals to check that all participants were in agreement.
At the end of the discussion group, a copy of the entire discussion thread was downloaded and saved.
Sample size and participant selection
The sample size for this study was dictated by the time and resources available. However, we aimed to include at least 100 participants in the survey and 18–20 participants in the discussion groups in order to gather a broad selection of views.
For the discussion groups, purposive sampling was used to ensure diversity in terms of ethnicity and age within the groups. Using Statistical Product and Service Solutions (SPSS) 21 software (IBM Corporation, Armonk, NY, USA), potential participants who had responded to the online survey were split into three groups: parents/carers of those with vitiligo (n = 13), people with vitiligo aged 17–45 years (n = 45) and those aged ≥ 46 years (n = 76). Participants’ ages were taken into consideration when forming the discussion groups given the potentially greater familiarity with technology and ‘text speak’ in younger participants. 474 Invitations were sent to all parents/carers, plus a random selection from other age groups. All potential participants from non-white ethnic backgrounds were invited, as well as a random selection of those from white backgrounds. This was to enable discussion of treatment for vitiligo in the context of different skin types.
Statistical analysis
Survey results were analysed using SPSS statistics software. Results were presented descriptively and responses to open questions were analysed thematically by a researcher (ST) and checked by a second researcher (JB) for agreement. This allowed for comparison between themes emerging from open and closed questions to be made, as well as allowing for ranking of themes by popularity overall for use in the discussion groups.
Because the main objective of the discussion groups was to seek consensus regarding the most appropriate wording of the question to ask people about the response of vitiligo to treatment, the results were not formally coded in the manner of a typical discussion group. Instead, consensus reached during each discussion group was summarised at the end of each session and presented back to the participants of the group for approval. These are summarised below (see Summary of areas of consensus), with examples of key comments made by participants. Two researchers independently checked the copy of each discussion group thread to ensure that all relevant points had been adequately identified and extracted.
Although more formal methods of consensus development (e.g. Delphi and Nominal Group Technique) are the focus of much academic consideration, informal consensus groups such as those employed here are commonly employed in health-care settings. 476 To counter some of the criticisms that informal mechanisms for reaching consensus lack ‘control’, ‘focus’ and ‘scientific credibility’,476 here data are handled and analysed systematically following an adapted version of template analysis. 477,478 Template analysis utilises a hierarchical model to organise text in order to aid interpretation. In this case, each point of consensus was taken as a higher-level organising code within which to summarise and consider the group discussion. Therefore, for each point of agreement, the discussion that led towards this was considered and coded to reflect those factors that contributed towards the consensus and those that were a barrier. This process was completed for each discussion group with a final template constructed to include all statements for which agreement spanned the different groups. This mode of analysis provides greater depth in understanding consensus, both mapping when agreement occurred and charting the process and factors which generated it.
Results
Online survey
Participants
In total, 202 responses were received. Of these, 165 (82%) were included in analyses and, of these, 154 (76%) were fully completed surveys (Figure 19).
FIGURE 19.
Participant flow diagram for vitiligo survey.
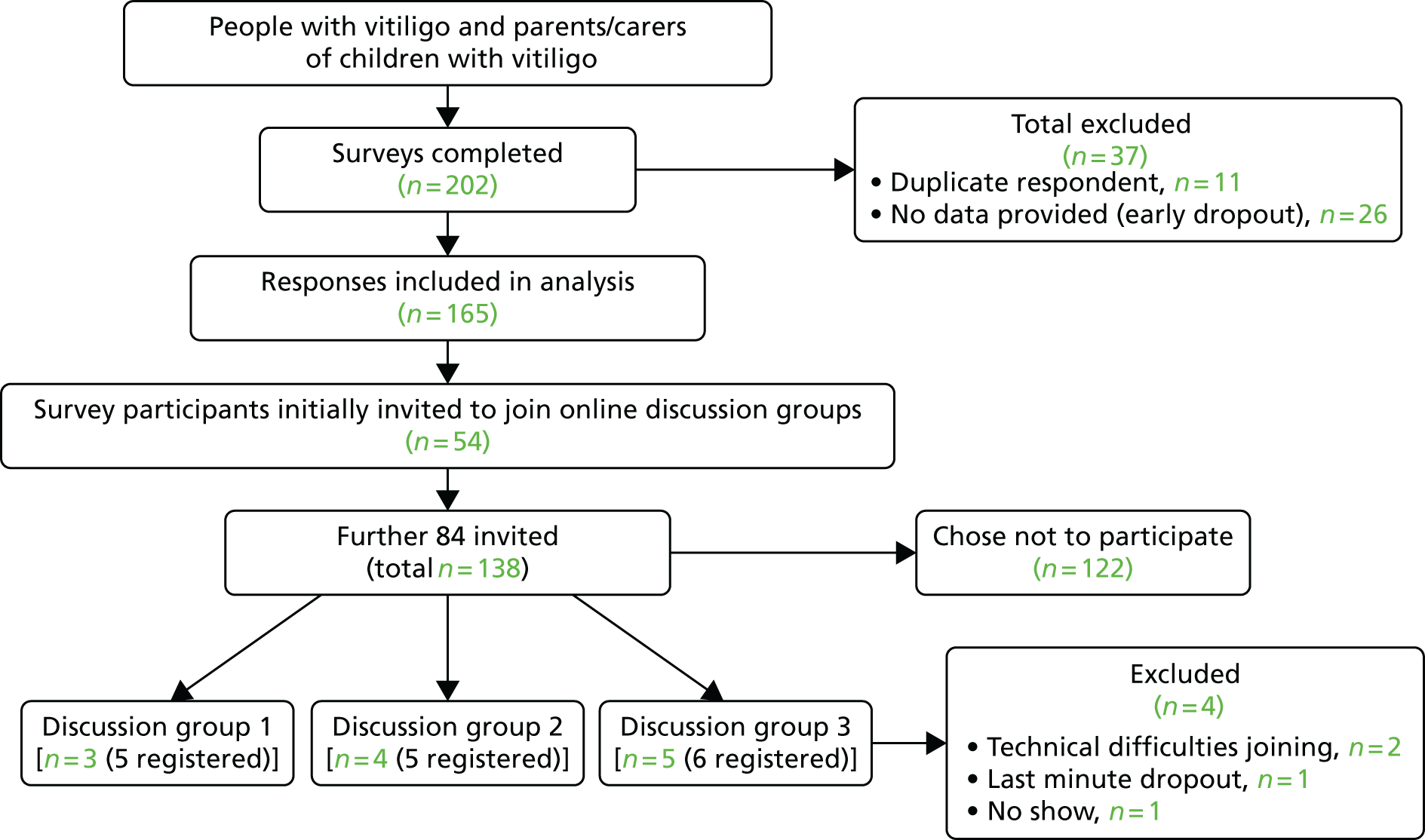
Responses were excluded for the following reasons:
-
If the survey had been completed from the same internet protocol (IP) address more than once:
-
the first completely filled survey was included and the rest excluded.
-
if multiple surveys were completed fully, only the first was included.
-
-
The same two rules applied for duplicate e-mail addresses given.
-
If the survey had not been completed past question 1, it was excluded.
The only exception to these exclusion criteria was when e-mail addresses and demographic responses indicated that two different individuals had responded from the same IP address, so both sets of responses were included.
Baseline characteristics of the survey participants are summarised in Table 39.
| Characteristic | Online survey (N = 165), n (%) | Online discussion groups (N = 12), n (%) |
|---|---|---|
| Responses completed on behalf of | ||
| Themselves | 149 (90.3) | 12 (100) |
| Other | ||
| Total number of responses for those responding on behalf of others | 14 (8.5) | 0 (0) |
| Child with vitiligo | 13 (92.9) | 0 (0) |
| Spouse with vitiligo | 1 (7.1) | 0 (0) |
| Themselves and other(s) | 2 (1.2) | 0 (0) |
| Unknown | 0 (0) | 0 (0) |
| Age (years) | ||
| < 5 | 1 (0.6) | 0 (0) |
| 5–16 | 10 (6.1) | 1 (8.3) |
| 17–30 | 17 (10.3) | 2 (16.7) |
| 31–45 | 43 (26) | 3 (25) |
| 46–65 | 55 (33.3) | 3 (25 |
| > 65 | 29 (17.6) | 3 (25) |
| Unknown | 10 (6.1) | 0 (0) |
| Ethnicity | ||
| White British | 117 (70.9) | 9 (75) |
| White Irish | 1 (0.6) | 0 (0) |
| Other white background | 15 (9.1) | 0 (0) |
| Any other mixed background | 1 (0.6) | 0 (0) |
| Indian/British Indian | 9 (5.5) | 1 (8.3) |
| Pakistani/British Pakistani | 1 (0.6) | 0 (0) |
| Bangladeshi/British Bangladeshi | 2 (1.2) | 1 (8.3) |
| Caribbean/British Caribbean | 1 (0.6) | 1 (8.3) |
| African/British African | 1 (0.6) | 0 (0) |
| Other | 7 (4.2) | 0 (0) |
| Unknown | 10 (6.1) | 0 (0) |
| Country of residence | ||
| UK | 135 (81.8) | 11 (91.7) |
| USA | 12 (7.3) | 1 (8.3) |
| Europe (excluding UK) | 3 (1.8) | 0 (0) |
| Australia | 1 (0.6) | 0 (0) |
| Asia | 1 (0.6) | 0 (0) |
| Dual: UK and other | 2 (1.2) | 0 (0) |
| Unknown | 11 (6.7) | 0 (0) |
| Duration of diagnosis(years) | ||
| 6–12 months | 3 (1.9) | 0 (0) |
| 1–2 years | 3 (1.9) | 0 (0) |
| 2–5 years | 12 (7.7) | 2 (16.7) |
| 5–10 years | 26 (16.8) | 1 (8.3) |
| > 10 years | 111 (71.6) | 9 (75) |
| Percentage of skin affected (estimate) | ||
| 0–10 | 37 (23.3) | 1 (8.3) |
| 10–25 | 49 (30.8) | 4 (33.3) |
| 25–50 | 33 (20.8) | 5 (41.7) |
| 50–80 | 24 (15.1) | 2 (16.7) |
| > 80 | 16 (10.1) | 0 (0) |
| Unknown | 6 (3.6) | 0 (0) |
| Area(s) of skin affecteda | ||
| Face/neck | 129 (78.2) | 10 (83.3) |
| Body | 110 (66.7) | 7 (58.3) |
| Arms | 113 (68.5) | 8 (66.7) |
| Legs | 109 (66.1) | 8 (66.7) |
| Hands | 127 (77) | 10 (83.3) |
| Feet | 113 (68.5) | 9 (75) |
| I’d rather not say | 2 (1.2) | 0 (0) |
| Other (responses included: under arms, genitalia and hair) | 35 (21.2) | 2 (16.7) |
| Treatments used previouslya | ||
| No treatment | 57 (34.5) | 2 (16.7) |
| Topical corticosteroid | 41 (24.8) | 3 (25) |
| Tacrolimus | 46 (27.9) | 6 (50) |
| Pimecrolimus (Elidel®, Meda Pharmaceuticals Ltd) | 6 (3.6) | 0 (0) |
| Vitamin D derived cream or ointment (e.g. calcipotriol) | 5 (3) | 2 (16.7) |
| UVB | 33 (20) | 5 (41.7) |
| PUVA | 20 (12.1) | 2 (16.7) |
| Not sure | 1 (0.6) | 0 (0) |
| Other (responses Included diet changes and alternative therapies) | 28 (17) | 3 (25) |
The majority of participants were aged between 31 and 65 years of age and had had vitiligo for > 10 years. One hundred and thirty three (80.6%) of those completing the survey were from white ethnic backgrounds and 135 (81.8%) were from the UK.
Survey results
When thinking about repigmentation of vitiligo after treatment, what does a ‘cosmetically acceptable result’ mean to you?
There were 143 responses to this open question. Multiple themes per response were allowed, yielding a total of 237 items of information coded from the 143 responses. The three most common themes related to the concept of the skin returning to normal and the vitiligo patches being less visible or noticeable. Reduction in the size of the lesion was ranked fourth and was mentioned in just 12.2% of responses. The main themes to emerge are summarised in Table 40.
| Theme | Responses (%) |
|---|---|
| Blends well with skin | 45 (19.0) |
| Less noticeable | 35 (14.8) |
| Skin back to normal | 31(13.1) |
| Reduction in white patches | 29 (12.2) |
| Confident/comfortable | 25 (10.5) |
| Repigment visible sites | 19 (8.0) |
| Any improvement | 17 (7.2) |
| Mostly repigmented | 14 (5.9) |
| Cosmetics | 9 (3.8) |
| Unaffected by tanning | 5 (2.1) |
| Means nothing | 4 (1.7) |
| Lasting repigmentation | 3 (1.3) |
| Completely depigmented | 1(0.4) |
Six responses were not relevant to the question, such as ‘I have given up on treatment after various unsuccessful attempts’. Four respondents (1.7%) stated specifically that a ‘cosmetically acceptable result’ was not meaningful to them and not an encouraging phrase.
The list below gives some possible words or phrases used to describe treatment results in vitiligo. Please tell us the words/phrases that best reflect how you would judge whether or not a vitiligo treatment has worked (please tick up to THREE options).
This question received 157 responses. The most popular words/phrases are summarised in Table 41. Eighteen responses (4.3%) were given under the category ‘Other’ and most were not relevant to treatment response, such as ‘Never had treatment’.
| Words/phrases | Number of votes (%) |
|---|---|
| Good colour match between treated vitiligo patches and normal skin | 72 (17.0) |
| Skin is back to normal | 66 (15.6) |
| Feel better about appearance of skin | 58 (13.7) |
| Reduction in area of skin affected by vitiligo | 48 (11.3) |
| Even pattern of repigmentation | 43 (10.2) |
| Cosmetically acceptable result | 26 (6.1) |
| Satisfied with result | 23 (5.4) |
| Worth continuing with treatment | 21 (5.0) |
| Other | 18 (4.3) |
| Worthwhile result | 17 (4.0) |
| Result of treatment is acceptable | 9 (2.1) |
When thinking about the repigmentation of vitiligo after treatment, do you think that ‘cosmetic acceptability of result’ and ‘satisfaction with the result’ mean the same thing?
In total, 159 responses were given for this question, with 88 (55.3%) answering ‘No’, 46 (28.9%) answering ‘Yes’ and 25 (15.7%) answering ‘Not sure’.
An open comment box allowed respondents to give further details, which suggested that participants felt that ‘cosmetic acceptability’ was a medical view or that of someone else and that ‘satisfaction with the result’ was a more personal and patient-led view. An example response was ‘The second statement suggests that the person is happy with the result whereas the first statement sounds more medical . . .’. In addition, negative views about the term ‘cosmetic acceptability’ were given, such as ‘rather vague’, ‘impersonal’ and ‘implies vitiligo only affects skin’.
Please give us any other suggestions on the questions we should ask people about the result of vitiligo treatment.
The main theme that emerged was asking questions regarding psychological factors (36 responses; 34.6%) such as individual feelings, confidence and comfort in wearing fewer clothes. The next emerging themes were details about the treatment (19 responses; 18.3%) and the duration of the improvement (14 responses; 13.5%). Other results are summarised in Table 42.
| Theme | Number of responses (%) |
|---|---|
| Psychological | 36 (34.6) |
| Treatment details | 19 (18.3) |
| Improvement duration | 14 (13.5) |
| Was the treatment worth the results | 8 (7.7) |
| Adverse effects | 6 (5.8) |
| Satisfaction | 6 (5.8) |
| Back to normal | 4 (3.8) |
| Has colour returned | 3 (2.9) |
| Reduction in white patches | 3 (2.9) |
| Sun protection | 2 (1.9) |
| Would they do it again | 2 (1.9) |
| Make it simple | 1 (1.0) |
After 9 months of vitiligo treatment, which of the pictures below shows a level of treatment response that you feel would be worthwhile to you? Please choose the letter associated with the chosen image.
The 158 responses are summarised in Table 43. Of these, 126 (79.7%) indicated that the three most repigmented images (representing 80%, 95% and 100% repigmentation) represented a worthwhile treatment response, of whom 57 (36.1%) stated that the image corresponding to approximately 90% repigmentation would be worthwhile and 51 (32.3%) stated that image corresponding to full repigmentation would be a worthwhile response after 9 months of treatment.
| Treatment response image (approximate % repigmentation) | Worthwhile treatment response, number of votes (%) | Minimum treatment response acceptable, number of votes (%) |
|---|---|---|
| 20 | 2 (1.3) | 8 (5.1) |
| 30 | 0 (0) | 8 (5.1) |
| 40 | 3 (1.9) | 7 (4.5) |
| 50 | 7 (4.4) | 17 (10.9) |
| 60 | 8 (5.1) | 16 (10.3) |
| 70 | 12 (7.6) | 26 (16.7) |
| 80 | 18 (11.4) | 43 (27.6) |
| 95 | 57 (36.1) | 22 (14.1) |
| 100 | 51 (32.3) | 9 (5.8) |
After 9 months of vitiligo treatment, which of the pictures below represents the MINIMUM treatment response that you would be prepared to accept?’
The 156 responses are summarised in Table 43. Of these, 43 (27.6%) stated that the image corresponding to approximately 80% repigmentation would be the minimum treatment response they would be prepared to accept, 26 (16.7%) chose the image corresponding to approximately 70% repigmentation and 9 (5.8%) indicated that they would not accept anything less than complete repigmentation.
Key messages to be explored in discussion groups
The survey revealed some key messages, which were taken forward to the online discussion groups for further exploration.
The first of these was that there were three main areas of importance to people when judging treatment success, namely colour match between their vitiligo and normal skin (i.e. how well it blends in), how noticeable the vitiligo is and a reduction in the size of the white patches.
Another key message for further exploration was that the majority of respondents said they would consider ≥ 80% repigmentation to be a worthwhile treatment response after 9 months and that the minimum they would be prepared to accept would be 70% repigmentation.
Online discussion groups
Three online discussion groups were held, involving a total of 12 participants (n = 4, n = 3 and n = 5). Participants ranged in age from 16 to > 65 years and had been affected by vitiligo for between 4 and 27 years. An additional four participants were registered to join the groups but did not participate because they had technical difficulties in accessing the chat room or were unavailable at the last minute. A total of 50 pages (approximately 16,000 words) of text were obtained from the three groups and analysed as described above.
Summary of areas of consensus
All three groups achieved consensus both within and between the individual discussion groups in several areas. Points for which there was consensus across the groups included:
-
The most important concept when asking about success of treatment response is ‘how noticeable the vitiligo is after treatment’.
-
A scale with five response options (both words and numbers) is the best scale to use when answering the question ‘Compared to before treatment, how noticeable is the vitiligo now?’:
-
more noticeable (1)
-
as noticeable (2)
-
slightly less noticeable (3)
-
a lot less noticeable (4)
-
no longer noticeable (5).
-
-
A score of 4 or 5 on the above five-point scale would represent a successful treatment response.
-
The question should only be used to assess individual vitiligo lesions, rather than all areas affected by vitiligo.
These areas of consensus are discussed in more detail below.
In response to the question regarding the most important concept when judging treatment success, all three groups were unanimous that the most important concept was how ‘noticeable’ the vitiligo is after treatment. Although some participants initially felt that other concepts were important, after further discussion with other participants, consensus was soon reached with minimal input from the facilitators. Moreover, several participants commented that the ‘noticeability’ of the vitiligo was a useful ‘catch-all’ phrase that covered elements of the other two concepts (colour match/blending and a decrease in size of the lesions). For example:
Of the three you have written, I think 1 and 3 are covered by 2.
I would say, most noticeable first, as 1 and 3 determine this.
Participants in all groups acknowledged that for people with paler skin tones, ‘noticeability’ may be less of an issue than for people with darker skin:
I am lucky in that I have very fair skin so my condition is not that easy to notice . . . but I imagine it is a big issue if it can be seen.
Do you think that if your vitiligo was in visible areas, that how noticeable it was would be the most important to you?
Yes.
Having established that the ‘noticeability’ of the vitiligo was the most important concept, participants then decided on the best wording of the question to ask trial participants in order to establish the ‘noticeability’. Prompts used for this discussion are shown in Box 6 (prompt 2).
There was agreement that the wording of Q3 in prompt 2 was confusing, as it was counterintuitive to be asked about ‘satisfaction’ with noticeable vitiligo:
Because noticeable to me, denotes it is noticeable – which is a negative and yet I am being asked how satisfied I am – which is a bit confusing.
There was rapid development of consensus in the first two groups that Q4 (‘Compared to before treatment, how noticeable is your vitiligo now?’) was the most appropriate and easy to understand, and the third group agreed with this:
Love it – uncomplicated and to the point.
When groups were asked about the best scale with which to measure responses to the question, a preference was expressed for a linear scale as opposed to a scale made up of images such as pictograms or emoticons:
So generally we prefer a line scale like number 4 rather than images?
I think a numerical scale feels more accurate.
However, participants felt that the linear scale needed to contain a reasonable number of choices:
I think it’s sometimes more difficult to make a judgement when there are fewer parameters.
Although the first group expressed a provisional preference for a linear scale with 10 divisions, time was limited for discussion of this point and so the discussion was developed further in groups 2 and 3. The subsequent groups felt that a five-point scale with adjectival markers was best:
So coming back to our original question with the five responses, do you think we need more than five?
That looks OK to me.
Think the five covers everything.
The groups were unanimous that a ‘successful’ treatment would need to score at least a ‘4’ on this scale [a lot less noticeable (4) or no longer noticeable (5)].
Having agreed on the question ‘Compared to before treatment, how noticeable is your vitiligo now?’ in group 1, further discussion with the participants in groups 2 and 3 showed support for the response options shown in the left-hand column of Table 44, as part of the agreed five-point scale. The final wording of two responses was amended slightly after the discussion groups had been completed (so that all responses consistently included the word ‘noticeable’). The amended wording, shown in the right-hand column of Table 44, was circulated among all participants and there was unanimous support for it.
| Wording agreed during discussion groups | Amended wording, approved after discussion group by participants |
|---|---|
| Worse than before (1) | More noticeable (1) |
| About the same (2) | As noticeable (2) |
| Slightly less noticeable (3) | Slightly less noticeable (3) |
| A lot less noticeable (4) | A lot less noticeable (4) |
| Hardly noticeable (5) | No longer noticeable (5) |
In particular, participants expressed a preference for having both numbers and words on the scale and for the option of ticking a box to give an answer. Participants in all groups agreed that the question and scale were suitable when assessing vitiligo lesions that have partially repigmented but which, owing to hyperpigmentation or uneven repigmentation, are actually more noticeable after treatment. Below is a summary of a discussion when participants in one group were shown some ‘before-and-after’ images that included hyperpigmentation (Figure 20).
FIGURE 20.
A set of ‘before-and-after’ images showing hyperpigmentation used in the online discussion groups.
That is interesting. There is a reduction in vitiligo area but the patchiness makes it look more obvious. In spite of the partial repigmentation, I would answer 1.
On balance I think I would say 2, because the area near to the eye has responded well but the chin seems more noticeable now that it is not such a large block.
To me it is more blotchy so 1.
So if this was your vitiligo, would you say that treatment was successful or unsuccessful?
Unsuccessful I think.
Partly successful, but if I had to opt for successful or unsuccessful, I would go for unsuccessful as it has made the vitiligo more obvious.
Unsuccessful.
In the final discussion group, participants were asked to comment specifically on whether the question could be used to ask about all areas affected by vitiligo or whether it was best used to assess specific patches (see prompt 3 in Box 6). Participants were unanimous that the question should be specific to target areas and that this was particularly important for visible sites:
The question must be specific to certain areas.
Depends on whether some areas means face and hands and arms.
Different areas react differently to treatment.
So people are saying that the question needs to be specific to treated areas?
Yes, if you clearly identified the areas of treatment.
If you are treating some random patches, then the second question is better.
Has to be specific to area treated.
If you can repigment hands and face, any measure of success will be high. If you repigment anywhere else, it’s debatable.
The third group was also asked about the suitability of the question when the trial participant is a child. Generally there was support for the use of the question, with the parent/carer answering, with input from the child if appropriate:
I feel that parents could answer for their child as they would be fully aware of the child’s feelings.
I think the questions would be suitable for children, although their responses might be a bit more optimistic than adults.
For a child, I think noticeability will be determined by their peers – a parent may or may not have good insight into this.
Discussion
Summary of main findings
This work has provided valuable insight into how patients evaluate treatment success and has laid the foundation for creating a validated patient-rated outcome measure for use in future trials of vitiligo treatments.
Although the concept of a ‘cosmetically acceptable result’ had previously been identified as an important measure of treatment success among people with vitiligo,439 our initial survey work showed that this term was rather unhelpful to patients, who felt that it was vague, impersonal and rather ‘medical’, or it implied that vitiligo was just something to be covered up (using cosmetic camouflage).
There was good agreement between the open and closed responses to survey questions. Common themes including ‘blends well with skin’, ‘less noticeable’ and ‘reduction in white patches’, were mentioned most frequently in response to an open question, whereas the most popular phrases in response to a more closed question were ‘good colour match’, ‘reduction in area of skin affected’ and ‘even pattern of repigmentation’.
‘Feel better about appearance of skin’ was another popular theme and although this is a highly important concept, we did not pursue it further in the discussion groups because psychological response to treatment was beyond the remit of this study. Specific validated scales to assess the impact of vitiligo on psychological well-being have been described elsewhere479,480 as have QoL scales regarding physical appearance and cosmetic products. 481
Another main theme to emerge from the survey results was that many respondents equated a cosmetically acceptable result with ‘skin is back to normal’. Although this is, of course, the ideal result for people with vitiligo, the likelihood of vitiliginous skin fully returning to normal after treatment is low. In addition, responses to a question about the skin being ‘back to normal’ would be in a binary ‘yes/no’ form and would not allow for a scale of more gradual increments, which is more likely to be useful when measuring the partial repigmentation expected after treatment. ‘Skin back to normal’ would also be covered by the top rating on any scale used to judge treatment success. For these reasons, we decided not to pursue this theme further during the discussion groups, focusing instead on the other three key themes that had emerged (colour match between their vitiligo and normal skin, how noticeable the vitiligo is and a reduction in the size of the white patches).
Responses to survey questions about the minimum level of repigmentation considered to be worthwhile after a 9-month period of treatment showed that people with vitiligo generally hope for very high degrees of repigmentation; nearly 80% of respondents said they would consider ≥ 80% repigmentation to be worthwhile and 64% said that the minimum they would be prepared to accept would be ≥ 70% repigmentation. This was helpful in guiding our understanding of what patients might consider to be a clinically meaningful treatment response and corresponds with the quartile of > 75% repigmentation being taken to represent treatment success in many previous vitiligo trials. 439
The online discussion groups used in the second stage of this work were very successful and popular among the participants. Owing to the widespread availability of internet access and increasing familiarity with online means of communication, online discussion groups are emerging as a useful medium for conducting health research. 473,474 The study also reflects an increasing use of electronic communication to support group decision-making and consensus-making. 475
It was striking that the members of the online discussion groups quickly reached consensus in a number of areas. The first area of consensus was that the ‘noticeability’ of vitiligo was the most important concept when assessing treatment success and that the ‘noticeability’ of vitiligo is a useful ‘catch-all’ concept that reflects the other two main concepts to emerge from the survey (colour match/blending and a decrease in size of the lesions).
Consensus was also reached rapidly regarding the use of a five-point scale of responses, including words and numbers, when answering the question: ‘Compared to before treatment, how noticeable is the vitiligo now?’. Participants were happy that this scale was suitable for assessing lesions with different percentages and patterns of repigmentation and there was strong consensus that a score of 4 or 5 on the scale equated with treatment success. Participants also agreed unanimously that if individual vitiligo lesions are treated, the noticeability of the lesions should be assessed individually, as opposed to assessing noticeability of vitiligo as a global measure for all affected areas of skin.
Strengths and limitations of the study
Limitations of this research include the fact that the participants involved in the survey and discussion groups were almost entirely based in the UK. It is possible that the views of people with vitiligo in other countries may be quite different from those in the UK and this may limit the external validity of the patient-rated measure. Another limitation was the absence of parents/carers of children with vitiligo from the discussion groups. We tried hard to recruit such individuals to the groups, by offering to host the groups at times that would be convenient for them, but none of the parents/carers who had participated in the survey were willing, or able, to join the discussion groups. We did, however, obtain positive feedback from parents/carers about the outcome measure after the discussion groups had taken place.
Given the potentially disproportionate impact of vitiligo on people with darker skin types, it was possibly disappointing that only 4.2% of study participants were from black and ethnic groups. However, this figure is representative of the mix of ethnicities in the UK population.
Implications for research
This work has demonstrated, for the first time, that percentage repigmentation may not be the best measure of vitiligo treatment success from a patient’s perspective. Instead, how noticeable the vitiligo patches are is a key concept for patients. Greater awareness of patients’ perspectives in judging treatment response in future clinical trials is essential. Additional work is now required to validate this measure further, in particular with respect to construct validity. We will ensure that these findings are incorporated into future international discussions regarding the most appropriate core outcome measure for inclusion in future vitiligo trials.
Pilot randomised controlled trial of light therapy devices used at home
The following text is adapted from Eleftheriadou et al. 482 © 2014 Eleftheriadou et al. ; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Summary
What was already known about this topic?
-
Narrowband UVB is a recommended treatment option for vitiligo, with evidence indicating particular value for phototherapy as part of combination treatment approaches.
-
Handheld NB-UVB units offer the potential to reduce resource utilisation and increase accessibility to this treatment.
-
Patients with vitiligo are currently buying handheld NB-UVB units and using them at home unsupervised, but there are currently no studies evaluating these devices for use in this patient population.
What did this study add?
-
The results of this pilot trial suggest that a national multicentre RCT involving home handheld phototherapy devices is both feasible and acceptable to patients and clinicians.
-
A number of practical recommendations for the design of a full RCT have been derived from the findings of this pilot trial.
-
Preliminary data suggest a good safety and tolerability profile for the handheld NB-UVB device, with demonstrated efficacy.
Introduction
Recommended treatment approaches for vitiligo
The BAD clinical guidelines for the management of vitiligo recommend NB-UVB (311–12 nm), tacrolimus and topical steroids to treat the condition. 434 The Cochrane systematic review update indicated that combination interventions, which mostly included a form of phototherapy, were superior to monotherapies. 367 However, it also highlighted the lack of good-quality evidence supporting light, and other therapies, for vitiligo. 367
In 2013, a new European Guideline for vitiligo was published, recommending early treatment of small lesions of recent onset and childhood vitiligo with a combination of phototherapy and topical agents. 483
Phototherapy for the treatment of vitiligo
Narrowband UVB is typically available in secondary care, requiring regular visits to the hospital and usually involving whole-body cabinets suitable for extensive vitiligo. 434 However, there are various devices available on the market for the delivery of NB-UVB, including hand and feet units and handheld units. The choice of device is usually based on the size and location of the lesions and the percentage of the body surface affected. 484 Handheld NB-UVB units are portable and lightweight devices, which are suitable for the treatment of small areas of the skin. Benefits of using handheld devices at home include reduction in the number of hospital visits, sparing of uninvolved skin, fewer costs for patients (such as travelling costs) and ability to treat an early stage of the disease, when the intervention might be more effective. 485 Although there are currently no studies evaluating handheld NB-UVB devices for vitiligo, trials and patient experience using these devices for home treatment of scalp psoriasis showed that they are effective, well-tolerated, easy to use and safe. 485,486
The effectiveness and safety of NB-UVB for the treatment of vitiligo was identified during the vitiligo PSP (see Vitiligo Priority Setting Partnership) as an important research topic for both patients and clinicians. 344 Feedback from patients (via the Vitiligo Society UK and Vitiligo Support International) suggests that patients are currently buying handheld NB-UVB units and using them at home unsupervised. If handheld devices prove to be effective and safe for the treatment of vitiligo at home, this could be an important addition to the treatment options available to patients with focal or early vitiligo.
Objectives
The main aim of this pilot trial was to determine the feasibility of conducting a national, multicentre, RCT to determine the effectiveness and safety of home handheld NB-UVB phototherapy units for early and/or focal vitiligo.
Primary objective
-
To establish the proportion of eligible participants and their willingness to be randomised to home NB-UVB.
Secondary objectives
-
To prepare a training package for participants explaining how to use the intervention and how to deal with possible side effects.
-
To establish possible short-term side effects and whether or not the device is suitable for home use with limited medical supervision.
-
To establish participants’ adherence to and satisfaction with the treatment.
-
To test the outcomes for the main RCT including repigmentation, cessation of spreading of the disease, impact on QoL, global improvement in vitiligo and Patient Benefit Index (PBI).
Methods
Approvals
This trial was approved by the National Research Ethics Service committee of East Midlands (REC reference: 11/EM/0331) and registered with ClinicalTrials.gov (ISRCTN: NCT01478945).
Trial design
This was a feasibility, double-blind, multicentre, parallel-group, sham device-controlled RCT of handheld NB-UVB phototherapy for the treatment of vitiligo at home. The acronym for this trial was HI-Light (Home Intervention of Light therapy) trial for vitiligo. The overall duration of the trial was 7 months, comprising a 3-month recruitment period (1 March 2012 to 31 May 2012) and a 4-month treatment period (until 31 September 2012).
Participants were recruited at the Queen’s Medical Centre and NHS Treatment Centre in Nottingham, and at Leicester Royal Infirmary in Leicester. PCRNs in both Leicester and Nottingham were involved. King’s Mill Hospital, Mansfield, and local GP practices (Nottingham and Leicester) were used as PICs along with direct advertising to participants through the Vitiligo Society UK and the Centre of Evidence Based Dermatology.
Randomisation and blinding
Participants were randomly allocated to active treatment or sham device group (2 : 1 ratio). In the active treatment group, participants received either a Dermfix 1000MX (Androv Limited, Buckinghamshire, UK) or Waldmann NB-UVB 109 (H. Waldmann GmbH & Co. KG, Villingen-Schwenningen, Germany) handheld device (1 : 1 ratio). The randomisation was based on a computer-generated pseudo-random code, using random permuted blocks of randomly varying size, created by the NCTU in accordance with their SOP and held on a secure University of Nottingham server. The randomisation was stratified by the recruiting site (Nottingham and Leicester). Participants, research nurses, dermatologists and independent outcome assessors were blinded. Only the trial administrator and the data manager at the NCTU were aware of participants’ allocation.
Participants and settings
The PIs of both recruiting centres compiled a list of prospective vitiligo patients (September 2011 to January 2012). PICs ran searches in their databases to identify individuals at least 5 years of age with a diagnosis of vitiligo. A dedicated website436 was available for the purpose of this trial. Individuals of at least 5 years of age with non-segmental spreading or stable vitiligo (confirmed by a dermatologist), affecting < 25% of their BSA, were included in the trial. No medical treatment for vitiligo in the previous 2 weeks or during the trial were permitted. For each participant, up to three vitiliginous lesions were chosen, preferably on three different anatomical areas. Exclusion criteria were segmental or universal vitiligo, previous history of skin cancer, recent/concurrent radiotherapy, photosensitivity, use of immunosuppressive or photosensitive drugs, pregnant or lactating women, major medical comorbidities and vitiligo limited to the genitalia.
All participants (or their parent/legal guardian if a participant was < 16 years) provided written informed consent before they entered the trial. In addition, a second consent form was signed on completion of the NB-UVB training session.
Outcomes
The primary outcome measure for this pilot trial was the proportion of eligible participants who were willing to be randomised.
The secondary outcomes were as follows.
-
Proportion of identified individuals expressing interest in the trial and fulfilling eligibility criteria.
-
Withdrawal rates and missing data.
-
Proportion of participants adhering to, and satisfied with, the treatment. Adherence was monitored by reviewing participants’ treatment diaries.
-
Incidence of NB-UVB short-term adverse events – erythema (grade 1–4), pruritus, perilesional hyperpigmentation, hypersensitivity reactions, cold sores, dry skin.
-
Proportion of participants and assessors for whom the blinding of the allocated group (active/sham device group) was maintained.
-
A number of efficacy outcome measures for the main large trial were also tested:
-
Repigmentation: lesions were traced using Convatec transparencies at baseline and week 16 visits. Lesions were measured by using the ImageJ 1.47d (National Institutes of Health, New York, NY, USA).
-
Cessation of spreading of vitiligo during the trial period.
-
Impact on the QoL of participants: DLQI487 and CDLQI488 on baseline and week 16 visits.
-
Global improvement in vitiligo: five-point Likert scale (much worse, a bit worse, no change, a bit better and much better) at week 16 visit.
-
PBI489 at baseline and week 16 visit.
-
Colour match of newly repigmented vitiliginous lesions (bad, fair or excellent) at week 16 visit.
-
Trial procedures
An age-appropriate information sheet was sent to each potential participant and telephone contact established. Pre-screening was conducted via telephone. Eligible individuals attended an initial 1.5- to 2-hour trial visit in hospital, which included a training session on how to complete the treatment diary, adjust treatment time according to the erythema response and what to do in case of short-term side effects. Hospital visits thereafter were on the following day [minimum erythemal dose (MED) results read] and at weeks 8 and 16 (final face-to-face visit) (Figure 21).
FIGURE 21.
Flow chart of the pilot HI-Light trial configuration.
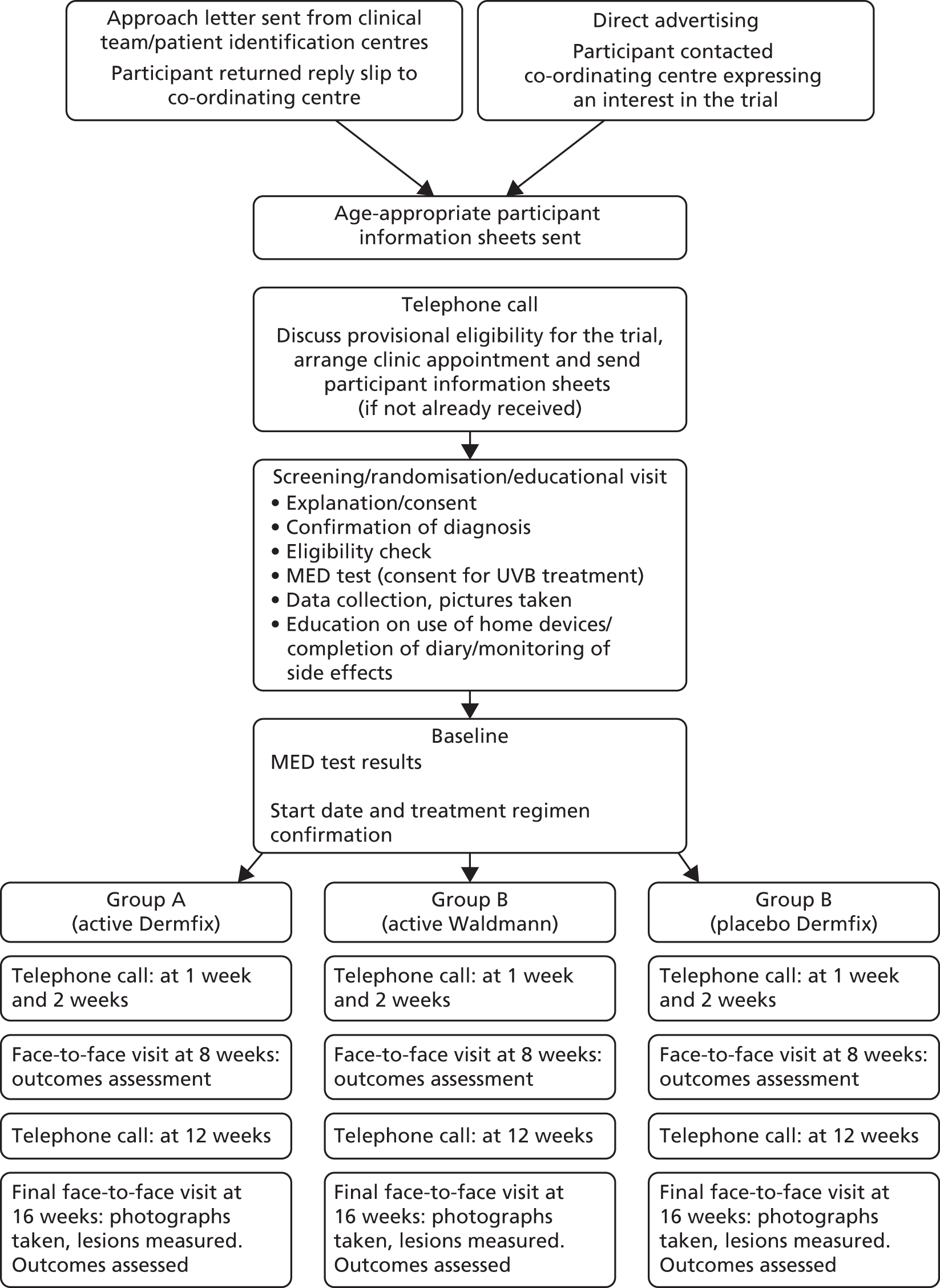
Interventions
In the active group, two different handheld NB-UVB devices were evaluated: Dermfix 1000 NB-UVB and Waldmann NB-UVB 109. The sham device was identical to the active device, Dermfix 1000, with the only difference being that a special plastic cover which blocked the emission of NB-UVB rays. Output of each of the handheld devices was tested before and after the trial.
The treatment was self-administered by the participant or administered by their parent/legal guardian on alternate days. Each participant received a personalised treatment plan according to his/her Fitzpatrick skin type (Table 45). For each participant, skin types, as determined by MED test and dermatologist opinion, were compared with each other to determine whether or not the MED test would be required for the future national RCT.
| Skin type | Exposure time (seconds) | |||
|---|---|---|---|---|
| Duration of first treatment | +20% of first treatment | –20% of first treatment | Maximum | |
| I | 15 | +3 | –3 | 180 |
| II | 20 | +4 | –4 | 240 |
| III | 25 | +5 | –5 | 300 |
| IV | 30 | +6 | –6 | 360 |
| V | 30 | +6 | –6 | 360 |
| VI | 30 | +6 | –6 | 360 |
Statistical analysis
Demographic characteristics of the participants, measures of adherence to the treatment plan and all other outcome data, including outcomes for the main RCT, were summarised by descriptive statistics or frequency tables and stratified by active/sham groups. No formal statistical analyses were performed on outcome measures as this was a pilot study to determine feasibility of a definitive trial. All analyses were performed using Stata SE 11 and Microsoft Excel 2007.
Sample size
This was a pilot study, for which no formal statistically based sample size estimate was applicable. A minimum of 21 participants from two recruitment sites was deemed appropriate to compare the devices and to measure recruitment rates for each site.
Results
Recruitment and eligibility
In total, 97 people expressed an interest in this pilot trial (Figure 22).
-
Forty-eight invitation letters were sent to vitiligo patients who attended dermatology departments at the Queen’s Medical Centre/NHS Treatment Centre in Nottingham (31 patients) and the Leicester Royal Infirmary (17 patients). Completed reply slips were returned by 38 (79.2%) patients [29/31 (93.5%) from Nottingham and 9/17 (52.9%) from Leicester].
-
Sixty-seven invitation letters were sent from two GP surgeries (one in Leicester and one in Nottingham). From these, 28 (41.8%) completed reply slips were received. Six additional GP practices expressed an interest in taking part, but were not deemed to be required for this pilot trial.
-
The Vitiligo Society UK sent 74 invitation letters to members in Nottingham, Leicester and Birmingham. Fourteen patients (18.9%) were interested in the trial.
-
A total of 248 patients held on a mailing list at the Centre of Evidence Based Dermatology were also informed about the trial.
FIGURE 22.
Overview of recruitment strategy for pilot HI-Light trial.
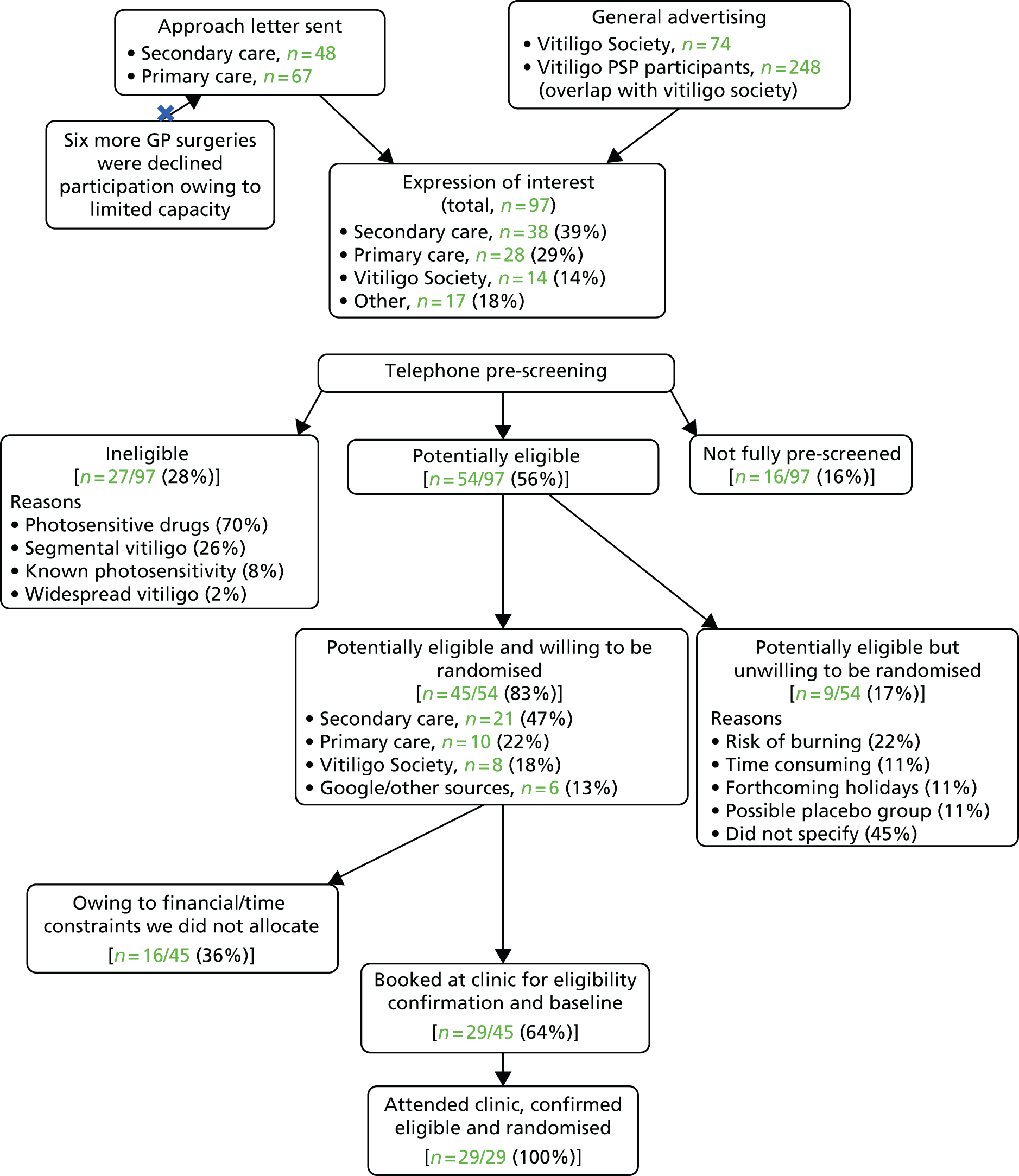
In total, 55.6% (54/97) of people who expressed interest in the trial were successfully pre-screened by telephone and provisionally met the trial eligibility criteria. Of these, 83.3% (45/54) were willing to attend the baseline visit and be randomised (see Figure 22).
Owing to time and financial constraints, only 29 out of 45 potential participants were booked to attend a baseline hospital visit on a ‘first-come, first-served’ basis. All 29 potential participants who attended the baseline visit were confirmed as being eligible and were subsequently randomised into the pilot trial (29/29; 100%) (see Figure 22). Thus, 29 participants with 84 representative lesions were randomised in the pilot trial. Owing to the small sample size, there were some discrepancies between groups in the patients’ baseline demographics, such as a greater mean age in the sham group (Table 46).
| Baseline characteristics | All groups | Active group | Sham group |
|---|---|---|---|
| Number of participants | 29 | 19 | 10 |
| Adult | 23 | 14 | 9 |
| Child | 6 | 5 | 1 |
| Age range (years) | 5–71 | 5–71 | 13–51 |
| Age (years), mean ± SD | 31.7 ± 17.9 | 27.63 ± 18.6 | 39.4 ± 13.5 |
| Adults | 38.6 ± 14.8 | 38 ± 15.8 | 42.3 ± 0.85 |
| Children | 10.25 ± 3.5 | 9.9 ± 3.6 | 13 ± 0.0 |
| Sex (female/male) | 15/14 | 10/9 | 5/5 |
| Ethnicity, n/N (%) | |||
| White British | 20/29 (69.0) | 12/19 (64.0) | 8/10 (80.0) |
| Mixed | 1/29 (3.5) | 1/19 (5.0) | 0 (0.0) |
| Black/black Caribbean | 2/29 (7.0) | 2/19 (11.0) | 0 (0.0) |
| Indian | 2/29 (7.0) | 1/19 (5.0) | 1/10 (10.0) |
| Pakistani | 1/29 (3.5) | 1/19 (5.0) | 0 (0.0) |
| Asian | 2/29 (7.0) | 1/19 (5.0) | 1/10 (10.0) |
| Other ethnic group | 1/29 (3.5) | 1/19 (5.0) | None |
| Duration of vitiligo (years) | |||
| Mean ± SD | 12.28 ± 9.67 | 11.36 ± 10.12 | 14.01 ± 8.5 |
| Minimum | 2 | 2 | 5 |
| Maximum | 33 | 33 | 28 |
| Status of vitiligo | |||
| Stable, n | 6 | 3 | 3 |
| Spreading, n | 19 | 14 | 5 |
| Repigmenting, n | 4 | 2 | 2 |
| % surface area covered by vitiligo | |||
| Mean ± SD | 8.83 ± 6.19 | 9.84 ± 5.96 | 6.9 ± 6.17 |
| Minimum | 2 | 3 | 2 |
| Maximum | 25 | 25 | 21 |
| Number of lesions selected for treatment | 84 | 56 | 28 |
Withdrawals
Three participants (10.3%) withdrew from the treatment (two patients from the active group and one from sham group). The reasons for withdrawals were that the treatment was too time-consuming (3.4%; 1/29) and the lack of improvement in vitiligo (6.9%; 2/29). Only one (3.4%) participant was lost to follow-up (Figure 23).
FIGURE 23.
Flow diagrams of randomised participants for pilot HI-Light trial.

Missing data
All participants, except one who was lost to follow-up, completed the end of study questionnaire in full (28/29; 96.6%). The DLQI and PBI questionnaires at baseline and week 16 were completed by 96.6% (28/29) of participants but, at baseline, one participant did not complete the questionnaires. One missing diary and week 16 questionnaire (3.4%; 1/29) belonged to the patient lost to follow-up. For analysis of cessation of spread, 50 out of the 58 treated lesions at baseline were available for analysis. The research team made every effort possible to contact and find the patient, including reaching out to his regular GP. Unfortunately, neither the diary nor the device was recovered.
Adherence
All but one treatment diary (28/29; 96.6%) were returned and analysed. Out of the 29 participants, 25 (86.2%) completed the 4-month treatment course and 21 of these (84.0%) administered phototherapy at home according to their instructions. Common mistakes were no treatment time reduction (6/25; 24.0%) or incorrect time reduction (1/25; 4.0%) following a missed treatment session. These minor deviations from the treatment schedule did not result in serious side effects.
Satisfaction with the treatment
Participants were asked whether or not they would use the device again and whether or not they would recommend the treatment to others. Participants in the active group were more likely to be satisfied with their treatment than participants allocated to the sham group (31.5% vs. 20%). The majority of participants in both groups said they would use the handheld device again and recommend it to others (67.9% and 64.2%, respectively).
Both active devices (Dermfix and Waldmann) received the following positive comments: easy to use, portable, compact, convenient to operate at home, no need of coming to the hospital, easy to perform and flexible. Additional positive comments on the Dermfix device related to the suitability of its curved shape for treating vitiligo patches.
Side effects
In the active treatment group, erythema grade 1 and 2 was reported in 27.6% (8/29) and 13.8% (4/29) of participants, respectively. Only one patient reported erythema grade 3 (3.4%). Other side effects included pruritus (2/29; 6.9%), hyperpigmentation around the lesions (3/29; 10.3%), dry skin (3/29; 10.3%) and herpes labialis (cold sores) (1/29; 3.4%). In the sham group, two patients reported erythema grade 1 (6.9%).
Repigmentation
In the active treatment group, the mean (SD) size of lesions decreased from 15.4 cm2 (20.2 cm2) at baseline to 14.4 cm2 (18 cm2) at week 16. In the sham group, the mean size increased from 20.5 cm2 (19.4 cm2) to 21 cm2 (20.9 cm2) in the same time frame.
High-grade repigmentation (defined as 75–100%) was noted in two participants in the active treatment group (11.8%), compared with none in the sham group. In the active treatment group, 74.4% (29/39) of all lesions showed some repigmentation compared with 39.1% (9/23) of lesions in the sham device group (Tables 47–49).
| Number of participants | Repigmentation compared with baseline after 16 weeks of treatment, n (%) | |||||
|---|---|---|---|---|---|---|
| 0 or worse | 1–24 | 25–49 | 50–74 | 75–100 | Total | |
| Active group (N = 17) | 6 (35) | 8 (47) | 1 (6) | 0 (0) | 2 (12) | 17 |
| Sham group (N = 10) | 4 (40) | 6 (60) | 0 (0) | 0 (0) | 0 (0) | 10 |
| Total | 10 (37) | 14 (52) | 1 (4) | 0 (0) | 2 (7) | 27 |
| Anatomical site | |||||
|---|---|---|---|---|---|
| Face/neck | Trunk | Upper limbs | Lower limbs | Hands/feet | |
| Number of participantsa | 6 | 6 | 13 | 11 | 3 |
| Repigmentation, n (%) | |||||
| Negative: 0% | 2 (33) | 3 (50) | 3 (22) | 2 (18) | 0 (0) |
| 1–24% | 1 (17) | 2 (33) | 8 (62) | 7 (64) | 2 (67) |
| 25–49% | 1 (17) | 0 (0) | 1 (8) | 2 (18) | 1 (33) |
| 50–74% | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 75–100% | 2 (33) | 1 (17) | 1 (8) | 0 (0) | 0 (0) |
| Baseline: mean lesion size (cm2 ± SD) | 75.4 ± 7.4 | 13 ± 15.9 | 11.2 ± 10.9 | 21 ± 29.1 | 18.9 ± 25.7 |
| 16 week: mean lesion size (cm2 ± SD) | 47.4 ± 4.4 | 13.7 ± 17.4 | 10.3 ± 9.6 | 20.6 ± 25.8 | 22.6 ± 26.6 |
| Anatomical site | |||||
|---|---|---|---|---|---|
| Face/neck | Trunk | Upper limbs | Lower limbs | Hands/feet | |
| Number of participantsa | 5 | 1 | 8 | 5 | 4 |
| Repigmentation, n (%) | |||||
| Negative: 0% | 3 (60) | 0 (0) | 3 (37) | 5 (100) | 2 (50) |
| 1–24% | 1 (20) | 1 (100) | 5 (63) | 0 (0) | 2 (50) |
| 25–49% | 1 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 50–74% | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 75–100% | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Baseline: mean lesion size (cm2 ± SD) | 10.7 ± 5.6 | 34.8 | 22.6 ± 18.4 | 22.6 ± 24 | 20.9 ± 8.8 |
| 16 week: mean lesion size (cm2 ± SD) | 11.3 ± 7 | 28.2 | 23.2 ± 21.6 | 26.1 ± 27.2 | 20.7 ± 6.4 |
Cessation of spreading of vitiligo
In the active group, 44.0% of lesions (22/50) remained stable throughout the trial compared with 46.4% (13/28) of lesions in the sham group. Twenty-two per cent (11/50) and 22% (4/18) of stable lesions started repigmenting in the active and the sham group, respectively.
Quality of life
Treatment had little impact on QoL. In the active treatment group, mean (SD) DLQI scores were 2.8 (3.2) at baseline and 3.2 (2.3) at week 16, compared with 3.8 (3.2) and 3.7 (3.8) in the sham group, respectively. Data from the CDLQI were not analysed owing to insufficient numbers of children in the trial.
Global improvement in vitiligo
Three patients (16.7%) in the active treatment group rated their vitiligo as being ‘much better’ compared with none in the sham group. Based on ratings by research nurses and independent outcome assessors, vitiligo was considered to be ‘much better’ in 23.5% (4/17) patients in the active treatment group compared with 0% in the sham group.
Benefit evaluation in vitiligo
There was no difference between the active and sham groups in the PBI index, with both groups achieving a score of approximately 1 (‘slight benefit’); actual mean (SD) values were 0.92 (1.2) and 0.91 (0.99), respectively.
Colour match of vitiliginous patches
In the active treatment group, around one-third of newly repigmented lesions were rated as good to excellent by patients (16/53, 30.2%), research nurses (16/50, 32.0%) and independent assessors (12/50, 24.0%). There were no reports of good or excellent newly repigmented lesions in the sham group.
Success of blinding
At the end of the trial, 70.4% (19/27) of participants and 59.3% (16/27) of research nurses guessed treatment allocation correctly. The main reasons for unblinding of the research nurses were erythema (3/10; 30.0%) and improvement in vitiligo (6/10, 60.0%) in active groups, and lack of treatment response (6/6; 100.0%) in sham group.
Minimum erythema dose/skin typing
The starting dose, as determined by the MED test, was the same as that determined by a dermatologist’s estimation of skin type for half of the group (16/29; 55.2%). In 20.7% (6/29) of participants, the MED test results showed that their skin was more sensitive to NB-UVB light than predicted by a dermatologist. In 24.1% (7/29) of participants, the MED results were higher than the ones predicted by dermatologists allowing them to be prescribed a higher dose of NB-UVB.
Discussion
The HI-Light pilot trial was the first trial evaluating the use of handheld phototherapy at home and testing the feasibility of conducting the first national multicentre RCT on home handheld phototherapy for vitiligo. Although this was not an efficacy trial, this pilot trial was a crucial preliminary step to support a grant application for a national multicentre RCT and to develop a training package on home-targeted phototherapy for vitiligo.
Summary of main findings
Ease of recruitment into the trial was very encouraging. The target number of participants was exceeded and the recruitment period lasted only 3 months instead of 6 months. Telephone pre-screening of potential participants prior to the hospital visit proved to be very successful and helped to minimise the use of resources associated with hospital visits.
Only 10% of participants withdrew from the trial, compared with the 20% previously reported in a trial on hospital phototherapy. 427 The findings suggest that patients with vitiligo are very keen to take part in trials on home phototherapy using handheld devices.
High levels of adherence to the treatment plan and the report of only one case of erythema grade 3 in this trial indicate that the training session and materials on how to self-administer home phototherapy were adequate and sufficiently easy to follow. Erythema grades 1 and 2 are generally considered acceptable, and sought-after, responses to light therapy. Surprisingly, two patients in the sham group also reported erythema grade 1. The most likely explanation for this might be confusion of erythema grade 1 with erythema caused by normal warming up of the device during treatment.
Treatment effects on patient-centred outcomes such as satisfaction and QoL were not extensive, but these must be interpreted with caution given the limited duration of this pilot trial. In the active treatment groups, 75% of lesions showed some degree of repigmentation. In a clinical setting, this is usually an indication to continue phototherapy treatment. 2
Strengths and limitations
The main limitations of this trial were the short treatment period and the small sample size, although it was not designed to be an efficacy trial. Nevertheless, 4 months was adequate to capture initial treatment response in some patients.
Although the secondary care recruitment was excellent, this is likely to be lower in centres where research nurse support is inadequate.
The unsuitability of the ConvaTec transparencies for skin mapping of vitiliginous lesions was a potential source of measurement error in the repigmentation measurements. As this pilot trial did not seek to answer efficacy questions on home handheld phototherapy, the above did not affect the validity of the results.
Recommendations for future trials
Based on the results described here, the following recommendations can be made.
Recommendation 1: recruitment through primary care
Primary care is likely to be the main source of potential participants (especially for early and limited disease). In order to reduce the burden on clinic space and availability of investigators, it is recommended that potential participants are pre-screened over the telephone.
Recommendation 2: skin mapping using transparencies
Skin mapping captures the three-dimensional character of vitiliginous lesions and does not require standardisation, specific body positioning or expensive equipment. It is also easy to replicate and relatively cheap. However, the pattern of repigmentation (perifollicular or diffuse) and the colour match of newly repigmented lesions made it difficult to clearly identify the edges of the vitiliginous area. Thus, it is important to identify a suitable (very thin, flexible and not shiny) transparency, which could also be scanned into a digital image without the need for manual transfer. Good lighting in the clinic room and contouring of the vitiliginous lesion with a surgical skin mapping pen are recommended if this method is being used.
Recommendation 3: minimum erythemal dose testing
Minimum erythemal dose testing is recommended for future trials on home handheld phototherapy and is necessary to ensure patients’ safety and appropriate NB-UVB dose administration.
Recommendation 4: training digital versatile disc
A training manual and a DVD on how to use handheld devices at home has been produced in order to standardise this intervention and ensure consistency in the training provided the training package developed for this trial proved to be comprehensive, well understood by participants and safe.
Recommendation 5: success of blinding
Although it was possible to mask treatment allocation, NB-UVB light therapy blinding is likely to be compromised and should therefore be planned for. Including active treatments in all groups could help to avoid unblinding resulting from differential treatment response and may be appropriate in some cases.
Implications for research
The results of this pilot trial suggest that a national multicentre RCT involving home handheld phototherapy devices is both feasible and acceptable to patients and clinicians. It would address an important area of unmet need, potentially providing a useful treatment strategy for patients with limited/early disease and assist future research into the treatment of vitiligo, based on the topics of importance for patients and clinicians. 344
Survey of UK clinical practice
Summary
What was already known about this topic?
-
The most commonly used first-line treatments for vitiligo are topical corticosteroids, calcineurin inhibitors and NB-UVB. However, there is no clear guidance on how best to use these interventions and there is wide variation in practice.
What did this study add?
-
This study provided insight from clinician members of the UKDCTN to inform the design of a planned national RCT of topical interventions for vitiligo.
Introduction
Although the only licensed treatment for the management of vitiligo in the UK is cosmetic camouflage, several treatments are recommended for use in clinical guidelines and are commonly used in clinical practice (Clinical Knowledge Summary,490 BAD guidelines434 and European guidelines483). The most commonly used first-line treatments for vitiligo are potent or superpotent topical corticosteroids, calcineurin inhibitors and NB-UVB. However, the relatively poor evidence base for vitiligo treatments367 means that it is unclear how best to use these interventions and there is wide variation in practice.
Prior to embarking on a national RCT of treatments for vitiligo, we sought to characterise current practice in the UK, with a view to defining the most appropriate choice of topical interventions and dosing regimens for the trial. Secondary objectives were to gather clinicians’ views on aspects of the design of the proposed trial and to identify potential barriers to recruitment.
Methods
Recruitment
An online survey was conducted among clinician members of the UKDCTN. This is a collaborative group of HCPs, patients and researchers with an interest in research into the treatment and prevention of skin disease.
Survey design
The survey was created using a secure internet based survey system (SurveyMonkey) and was live for 4 weeks in August 2012. Two reminders were sent by e-mail prior to closing the survey. Ethical approval was not required for this study.
Those receiving the survey were provided with background information about how the research question had been identified344 and an outline of the proposed trial design. In addition, respondents were given brief information about current guidelines on the treatment of vitiligo and were reminded that current clinical trial evidence does not support the use of any particular topical corticosteroid over another. Survey questions are shown in Box 7.
Q1. What topical corticosteroid (if any) would you normally use for vitiligo in the following situations?
Adults (excluding sensitive sites, e.g. axillae, face).
Sensitive sites in adults (e.g. axillae, face).
Children > 5 years (excluding sensitive sites).
Sensitive sites in children (e.g. axillae, face).
Q2. The proposed trial would recruit adults and children > 5 years (including those with vitiligo on the face). Do you agree that fluticasone propionate 0.05% (Cutivate), is an appropriate topical corticosteroid?
If ‘No’ or ‘Maybe’, give details.
Q3. The proposed regimen for the fluticasone propionate is once daily application for first 4 weeks, followed by 1 week on treatment/1 week off treatment for the remaining 8 months. Do you agree this is appropriate?
If ‘No’ or ‘Maybe’, give details.
Q4. The proposed duration of treatment with fluticasone propionate is 9 months. Do you think this is appropriate?
If ‘No’ or ‘Maybe’, give details.
Q5. Does your department have experience of recruiting patients into RCTs?
Q6. If there is anything about the proposed trial that would make it difficult for you to take part, please give details.
Q7. Would you be willing (as a local investigator) to randomise patients with vitiligo into the proposed trial, assessing treatment with a handheld NB-UVB device, or topical corticosteroid, or both treatments in combination?
Q8. Approximately how many patients with limited vitiligo (< 10% BSA) do you see per month?
Q9. Do you have access to a database to enable identification of patients with vitiligo?
Q10–12. Details of clinician’s place of work (if interested in being a recruitment site).
Q13. Do you have support from a comprehensive local research networks research nurse (minimum 40% full-time equivalent), who can help with recruitment into clinical trials?
Q14. Does your department include nurses who are trained in phototherapy?
Q15. Do you have a medical photography service at your hospital?
Q16. Is it your usual practice to take photographs of patients with vitiligo, in order to monitor treatment response?
Q17. Any other comments on the proposed trial.
The first part of the survey gathered information about UK clinicians’ current practice in the treatment of vitiligo and sought their opinions on the most appropriate topical corticosteroid to use in the trial and how frequently it should be used.
The second part of the survey sought clinicians’ views on the proposed interventions to be used in the planned national RCT. 436 Respondents were asked about the proposed treatment regimen for the trial of fluticasone propionate applied once daily for 1 month and then once daily for alternate weeks thereafter. Respondents were also asked if they would theoretically be happy to randomise patients into a trial comparing handheld NB-UVB light units, both with and without topical corticosteroid.
Further questions in the final part of the survey were designed to identify potential recruiting sites for the main RCT. Questions covered the respondents’ experience of clinical trials and whether or not they had access to a research nurse in their departments. Respondents were also asked about the number of patients with vitiligo seen in their clinics, whether or not such patients could be identified via databases or similar records and whether or not they used medical photography services to document treatment response. We also sought opinions about the proposed trial and asked if there were any potential barriers to recruitment at the respondents’ respective centres.
Results
The UKDCTN had around 480 clinician members at the time of the survey and 95 (20%) of these clinician members responded to the survey.
The preferred topical corticosteroids for the treatment of vitiligo are summarised in Table 50. The main findings were that for non-sensitive sites in adults, potent or superpotent topical corticosteroids were favoured (most commonly mometasone furoate or clobetasol propionate), but for sensitive sites in adults (e.g. the axillae and face) a moderate potency topical corticosteroid was generally chosen. Some respondents specified a short exposure regimen for sensitive sites, such as application on alternate weeks. For children, most clinicians preferred a potent topical corticosteroid on non-sensitive sites and moderate potency topical corticosteroids for sensitive sites (although 18.9% did not use topical treatments at all on sensitive sites in children). Small numbers of respondents specified that they preferred to use topical calcineurin inhibitors (e.g. tacrolimus, pimecrolimus) instead of topical corticosteroids (see Table 50).
| Category | Treatment | Number of participants (%) | |||
|---|---|---|---|---|---|
| Adults | Children | ||||
| Excluding sensitive sites | Sensitive sites | Excluding sensitive sites | Sensitive sites | ||
| Superpotent | Clobetasol propionate | 43 (45) | 4 (4) | – | – |
| Not specified | – | – | 9 (9) | 2 (2) | |
| Potent | Mometasone furoate | 38 (40) | 10 (10) | 41 (43) | 4 (4) |
| Betamethasone | 10 (11) | 4 (4) | – | 1 (1) | |
| Fluticasone | 4 (4) | 7 (7) | 7 (7) | 3 (3) | |
| Fluocinolone acetonide | 1 (1) | 7 (7) | 2 (2) | 1 (1) | |
| Not specified | 3 (3) | 1 (1) | 1 (1) | 2 (2) | |
| Moderate potency | Clobetasone butyrate | 1 (1) | 44 (46) | 11 (12) | 31 (33) |
| Betnovate RD | – | – | 2 (2) | 1 (1) | |
| Mild potency | Hydrocortisone | – | 9 (9) | – | – |
| Not specified | – | – | 2 (2) | 23 (24) | |
| Topical calcineurin inhibitors | Tacrolimus | – | 7 (7) | 3 (3) | 8 (8) |
| Pimecrolimus | – | – | 1 (1) | 2 (2) | |
| None | NA | – | 9 (9) | 5 (5) | 18 (19) |
| Don’t know | NA | – | – | – | 2 (2) |
Responses to the question about the proposed use of fluticasone propionate as the topical corticosteroid in the forthcoming trial (Q2) showed that the majority of respondents (68%) were in support, 29% said ‘maybe’ and 3% said ‘no’. Reasons given for not saying ‘Yes’ included ‘not on face/skin folds’ (8%), ‘unsure about use in children’ (8%), ‘not potent enough’ (5%) and ‘prefer more commonly used steroid’ (4%). Sixty-three respondents (68%) were in support of using fluticasone propionate daily for 1 month and then on alternate weeks for 8 months in the trial (Q3), with 24% saying ‘maybe’ and 8% saying ‘no’. Reasons for not saying ‘yes’ included ‘not on sensitive sites’ (7%), ‘continuous use better’ (7%) and ‘never used this regimen/what is the evidence for this topical corticosteroid or this regimen?’ (6%). Regarding use of the fluticasone propionate for a total of 9 months in the trial (Q4), 58% were in support of this, 32% said ‘maybe’ and 8% said ‘no’. Reasons for not saying ‘yes’ included ‘too long/6 months better’ (12%), ‘what if treatment fails?/depends on response’ (10%), ‘what is the evidence for this?’ (6%), ‘need option to stop if side effects’ (4%) and ‘too short’ (3%). Regarding the responses to questions to aid potential site identification in the forthcoming trial (Q5–16), 58% respondents said that they would be happy to recruit participants into a trial assessing the proposed interventions (Q7), with 24% saying ‘maybe’ and 15% saying ‘no’. Potential barriers to recruitment (Q6) mentioned by respondents included ‘lack of staff’ (17%), ‘lack of time’ (11%) and ‘lack of vitiligo patients’ (8%). In response to the same question, 27% respondents specifically said that they saw no potential barriers to recruitment in their department.
Only 18% of respondents said that they had access to a database (Q9/11) that would enable them to identify patients with vitiligo who had previously attended their department, 36% said they could search through previous clinic letters and 12% said they could identify patients prospectively. The number of patients with vitiligo affecting < 10% of their BSA seen per month (Q8) ranged from ‘less than 1’ (33%), ‘1–2’ (36%), ‘3–4’ (12%), ‘5–10’ (8%), to ‘more than 10’ (5%). More than three-quarters of respondents (79%) had previous experience of recruiting into clinical trials (Q5) and 43% had access to a 40% full-time equivalent research nurse to help with recruitment (Q13). The majority of departments (93%) had nurses trained in phototherapy and 88% had access to medical photography, with 73% taking baseline photographs of vitiligo prior to commencing treatment.
Discussion
This survey has been extremely useful in informing the ongoing work to prepare for the proposed multicentre RCT [see Proposal for a trial light therapy and topical corticosteroids for the treatment of vitiligo (HI-Light)].
With respect to which topical corticosteroid to use in the trial, the survey revealed that although many clinicians use a superpotent topical corticosteroid to treat vitiligo in non-sensitive sites in adults (as advised by clinical guidelines), there was broad support for the use of a potent topical corticosteroid in the trial and, in particular, for the proposed treatment regimen (daily for 1 month then alternate weeks). This was particularly reassuring, given that a large number of respondents tend to use only moderate potency topical corticosteroids to treat vitiligo in sensitive sites in adults and moderate or mild potency preparations in children.
The vast majority of respondents who used potent topical corticosteroids prescribed mometasone furoate (Elocon®, Merck Sharp & Dohme Ltd) and some respondents said they were not familiar with using fluticasone propionate. As a result, the trial development group has subsequently decided to use mometasone furoate as the topical corticosteroids in the national trial. Consideration was also given as to whether or not to use two different topical corticosteroids for sensitive and non-sensitive sites, given that many respondents use moderate potency topical corticosteroids when treating the latter. However, the decision was made to use only mometasone furoate in the trial for simplicity and to improve adherence. To allow for the tendency of many clinicians to use a lower potency topical corticosteroid in sensitive sites, the trial development group agreed that the mometasone furoate should be applied on alternate weeks, as it was thought that this would improve the acceptability of the intervention among potential recruiting clinicians. In recognition of concerns over potential lack of flexibility in the dosing regimen, the trial protocol will specify that treatment with topical corticosteroids should be stopped early if full repigmentation is achieved before the 9-month end point, or if significant adverse events occur due to use of topical corticosteroids.
The number of vitiligo patients seen by a given dermatology department is variable. This could be, in part, attributable to the ethnic demographics of the community in which a clinic serves. Further work to identity potential recruiting sites for the RCT will need to focus on departments where more vitiligo patients attend. It is possible that departments that do not currently see significant numbers of vitiligo patients could still be effective recruiting sites, if eligible patients in the community (who have not yet been referred to secondary care owing to perceived lack of treatment options) can be identified effectively through the use of GP surgeries as PICs.
Although virtually all departments have some nursing staff trained in phototherapy, only a minority of these nurses will be able to recruit patients to the trial, owing to the large amount of time needed (2–3 days per week). However, if the trial proved that the handheld NB-UVB units are efficacious and cost-effective in treating early vitiligo, it is hoped that they may be adopted as part of the phototherapy service offered by dermatology departments. Involvement of the phototherapy nurses at recruitment sites will hopefully aid subsequent adoption of the intervention into clinical practice.
The identification by this survey of the widespread use of medical photographs to assess vitiligo treatment response has also been useful in guiding the future trial. High-quality medical photographs will be taken of the vitiligo lesions at baseline and after 9 months of treatment.
The trial development group were greatly encouraged by the survey respondents’ support for the proposed trial. Many respondents said that they did not perceive any particular barriers to their participation in the trial. The most frequently mentioned barriers to participation (lack of time and staff) can hopefully be addressed by effective use of resources offered by the NIHR Comprehensive Research Network in supporting NIHR portfolio trials.
Strengths and limitations
Although this survey has been useful in informing the design and conduct of a future trial, it is possible that the views and current practice described by the survey responders is not representative of practice throughout the UK. In particular, the UKDCTN is a predominantly secondary care organisation and the views of clinicians treating patients in a primary care setting may not be reflected in these responses.
Nevertheless, the subsequent trial will be conducted in secondary care (as light therapy is not currently supported in a primary care setting). As such, it is hoped that the results reflect the views of the clinicians who are most likely to be contributing to delivery of the proposed trial.
Conclusion
The data obtained through this survey have been used to inform the trial design and conduct for a national trial of vitiligo treatments. In particular, the findings have guided the choice of topical corticosteroid and dosing regimen; the need for flexibility in the treatment schedule; reassurance as to the use of medical photographs to monitor treatment response in normal clinical practice; and aspects of recruitment site identification. This will help to ensure that the trial runs smoothly and assesses interventions that are of relevance to clinicians who treat vitiligo patients. This will aid the adoption of trial results into clinical practice if the interventions are found to be effective.
Proposal for a trial light therapy and topical corticosteroids for the treatment of vitiligo (HI-Light)
Summary
What was already known about this topic?
-
The updated Cochrane review described earlier identified that combination treatments appear to be more effective than monotherapies for vitiligo.
-
Good-quality data on the safety and efficacy of combined use of two of the recommended first-line treatments, topical corticosteroids and NB-UVB, are sparse.
-
Various preliminary assessments, described in this chapter, have generated recommendations for the design of a national RCT investigating NB-UVB and topical corticosteroids for vitiligo.
What did this study add?
-
The proposed study will provide high-quality data on the efficacy and safety of these two treatments.
-
By focusing on established priority research topics, utilising validated patient-centred outcome measures and incorporating the extensive input obtained from clinicians and experience gained from the pilot trial, this study has the potential to set a high standard for future trials of treatments for vitiligo.
Introduction
As discussed previously in this chapter (see Update of Cochrane systematic review), topical corticosteroids and NB-UVB are among the recommended first-line treatment options for vitiligo434,483 and evidence suggests that combination treatments may be more effective than monotherapies. 367
This section summarises the design of a proposed national RCT of NB-UVB light therapy and topical corticosteroids for the treatment of patients with early and limited vitiligo. This funding proposal has subsequently been approved for funding by the NIHR HTA Board. 491 The design of this trial has been informed by the findings presented throughout this chapter, in particular the results of the pilot RCT of handheld NB-UVB devices and the survey of UK clinical practice (see Pilot randomised controlled trial of light therapy devices used at home).
Two studies have previously assessed the combination of UV light and topical corticosteroids in treating vitiligo. 427,429 Other studies have used a combination of these two treatments with other interventions (e.g. surgical treatments such as skin grafting) or combination treatments (including other light sources such as laser) with topical corticosteroids, but these combinations are sufficiently dissimilar to the proposed interventions in our trial to limit their relevance. The latest ongoing update of the Cochrane review has not identified any other studies assessing the specific combination of UV light and topical corticosteroids.
Previous studies carried out to assess the effect of a combination of topical corticosteroids and UV light had considerable limitations. A trial conducted by Westerhof et al. 429 compared the combination of UVA light therapy and fluticasone propionate with monotherapy, and showed evidence of improved pigmentation with combination treatment. However, UVA is less widely used in recent years owing to increased carcinogenicity relative to UVB. Lim-Ong et al. 427 showed that a combination of clobetasol propionate and NB-UVB produced improved repigmentation relative to monotherapy, but this trial recruited only 25 patients, five of whom did not complete the study. Another study by Kroon et al. 492 in Amsterdam assessing NB-UVB both alone and in combination with fluticasone propionate has recently been completed but final data are not yet available. This trial used full-body NB-UVB cabinets, as opposed to the handheld devices to be used in this trial and aimed to recruit around 50 patients.
Aims and objectives
-
To assess the effectiveness of home NB-UVB and topical corticosteroids for the treatment of patients with early and limited non-segmental vitiligo – used as monotherapy and in combination for up to 9 months.
-
To assess whether or not any response is maintained once treatment is stopped.
-
To assess the cost-effectiveness of the interventions.
Trial design
The proposed trial is a three-arm, double-blind, RCT comparing potent topical corticosteroid to home light therapy using handheld devices and a combination of the two (Figure 24). Participants will receive up to 9 months of treatment. The primary outcome will be assessed by participants at the end of this treatment phase. Follow-up will be for 12 months after end of treatment (21 months from randomisation).
FIGURE 24.
Proposed design of the HI-Light RCT. TCS, topical corticosteroid (mometasone furoate 0.1%).
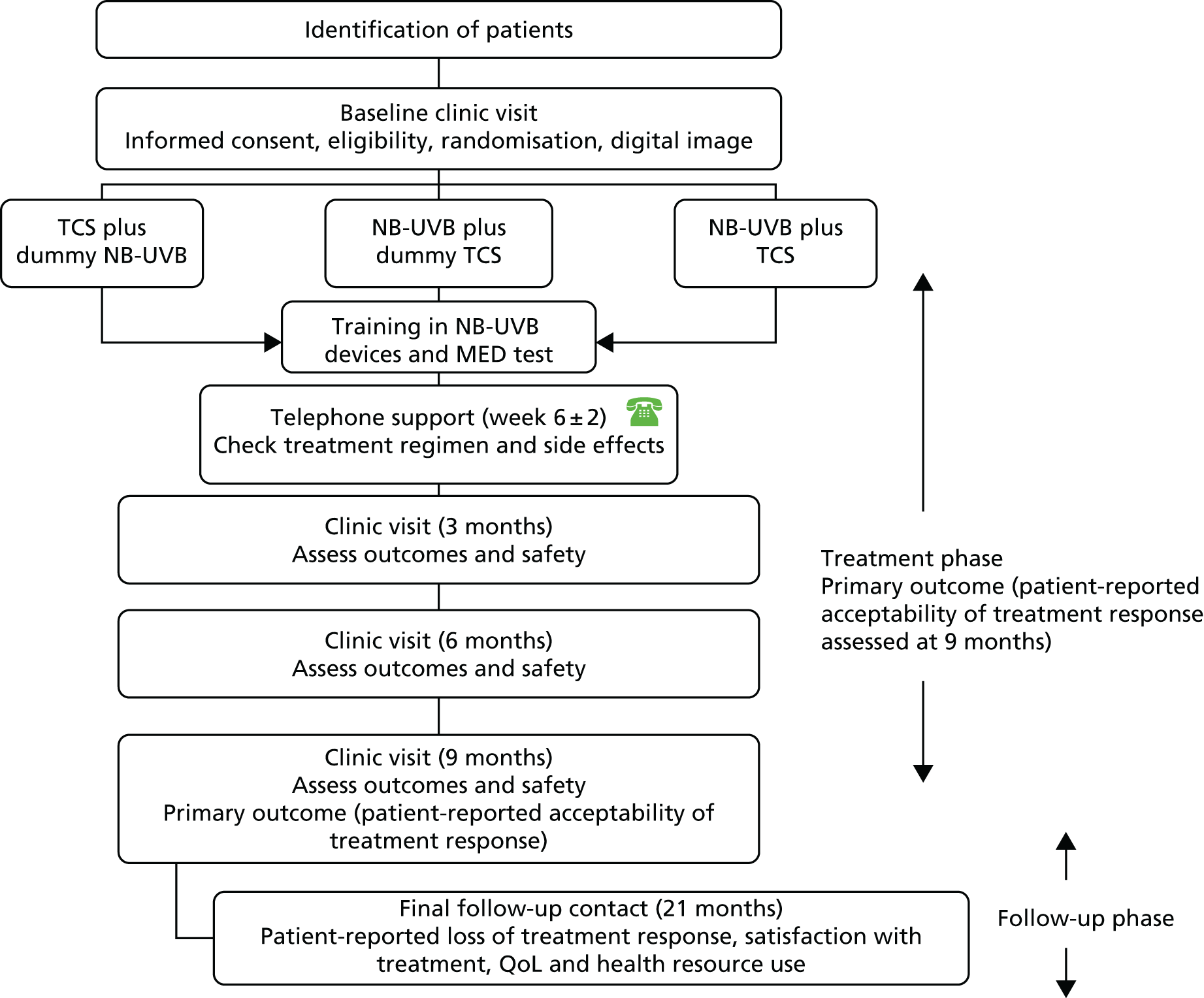
Treatment allocation will be masked from participants, research nurses, PIs and statisticians by using sham NB-UVB devices and a placebo for topical corticosteroids. Only the dispensing pharmacist will be aware of the allocation. The allocation will be held on a secure web server at the trial co-ordinating centre.
Setting and target population
Target population
Participants will be children (at least 5 years of age) and adults with early and limited non-segmental vitiligo. Limited vitiligo is defined as vitiligo affecting < 10% of the BSA. Early vitiligo is defined as having at least one patch that has appeared or spread in the last 12 months.
Setting
Recruitment and delivery of the intervention will be provided in secondary care, but participants will also be identified through targeted mail shots from GP practices (with the help of the PCRN) and by direct advertising (as successfully piloted). All treatments will be self-administered at home. Recruitment will take place in 10–12 UK hospitals. Site selection will be based on availability of a research nurse (minimum 40% full-time equivalent); a dermatologist who is willing to act as PI for the site; availability of phototherapy services that can support delivery of the intervention; availability of medical photography services; and proven track record of recruiting into other NIHR portfolio trials. These centres will be identified with the assistance of the UKDCTN and the NIHR Dermatology Specialty Group.
Eligibility
Inclusion criteria
-
Patients with a diagnosis of non-segmental vitiligo, as confirmed by a dermatologist or dermatology nurse.
-
Vitiligo limited to < 10% of the BSA and at least one lesion that has appeared or spread in the last 12 months.
-
At least 5 years old (as the child will need to be mature enough to sit still during treatment and to keep eyes closed if treatment is on the face).
-
Able and willing to give informed consent (or parental consent in the case of children).
Exclusion criteria:
-
Other types of vitiligo (e.g. segmental or universal vitiligo).
-
Patients with vitiligo limited to areas of the body for which NB-UVB light therapy or potent topical corticosteroids would be inappropriate (e.g. around the genitals).
-
History of radiotherapy use.
-
Pregnant or lactating women.
-
Current use of immunosuppressive or strongly photosensitising drugs.
-
Previous history of skin cancer.
-
Known photosensitivity.
-
Any major medical comorbidity that would limit the patient or carer’s ability to administer the intervention safely at home (e.g. severe behavioural difficulties).
Only one participant will be recruited per family. If more than one person is eligible for entry into the trial, the choice of who should take part will be made by the family members.
Randomisation and blinding
The randomisation schedule (stratified by centre and age: 5–16 years or > 16 years) will be generated by a computer using a randomly varying block size. The randomisation schedule will be generated and held by the NCTU and will be released to the central pharmacy, who will package and label the study interventions prior to distribution to the local clinical trials pharmacies. Participants will be allocated to interventions once the research nurse has irrevocably entered participant information onto the online randomisation website maintained by NCTU.
The primary outcome will be assessed by trial participants at the end of the 9-month treatment phase.
Blinding will be achieved using sham NB-UVB devices (as tested in our pilot RCT, see Pilot randomised controlled trial of light therapy devices used at home) and sham vehicle for the topical corticosteroids. Although every effort will be made to maintain the masking of trial interventions, it is possible that this may be compromised in several ways (Table 51).
| Person | Blinding status | Comments |
|---|---|---|
| Pharmacy and medical physics department staff | Open | Responsible for packaging and labelling the interventions prior to allocation to participants |
| Participants | Masked | Blinding may be compromised if participants allocated to NB-UVB develop side effects such as erythema or tanning of the surrounding skin |
| Research nurses (outcome assessors for secondary outcomes) | Masked | May be compromised for participants allocated to NB-UVB if signs of erythema or tanning around the lesion are present during clinic visits |
| PIs | Masked | May be compromised for participants allocated to NB-UVB if severe side effects are experienced that require medical attention. In order to limit this, staff at the co-ordinating centre will be trained to provide standardised advice in response to the most commonly experienced, mild adverse reactions (e.g. mild burning, cold sores and dry skin) |
| Trial management staff at co-ordinating centre | Masked | May be compromised as will be the main point of contact for trial participants in order to protect blinding of the research nurses |
| Statistician | Masked | Unlikely to be compromised |
| Health economist | Masked | Unlikely to be compromised |
As there is a relatively high risk that participants allocated to NB-UVB therapy may be able to guess their treatment allocation, additional measures will be taken in order to limit the impact of this in the trial results. These include: (1) patient preference for the trial interventions will be assessed at baseline and used to inform the analysis (see Statistical analysis), (2) on exit from the trial, participants (and research nurses) will be asked which treatment they thought they had received and why and (3) information for potential participants will emphasise that all participants receive at least one active treatment for their vitiligo, thus reducing potential expectation bias.
Trial interventions
Participants will be allocated to one of three treatment groups.
-
Group A: handheld NB-UVB light plus placebo topical corticosteroid.
-
Group B: potent topical corticosteroid (mometasone furoate 0.1%) plus sham NB-UVB.
-
Group C: handheld NB-UVB plus potent topical corticosteroid (mometasone furoate 0.1%).
Participants will be encouraged to choose for themselves which vitiligo patches they wish to treat and this will be recorded at baseline. All treatments will be self-administered at home.
Trial delivery
Delivery of intervention
Trial interventions will be dispensed from local clinical trials pharmacies. The quantity of topical corticosteroid prescribed will be flexible depending on the number of vitiligo patches involved. Further prescriptions will be issued every 3 months.
Training in the use of the NB-UVB devices will be supported through existing phototherapy services. However, these devices are not commonly used in the NHS at this time and so it is likely that specific training will be required in order to train site personnel in how to use these devices safely. As a result, all research nurses and local phototherapy teams will be trained in the use of the devices and delivery of training to trial participants will be flexible according to the needs of individual site. A training DVD and information booklet will be given to participants on entry into the trial. This will standardise training across all sites and will facilitate use of the devices in the NHS should the trial prove them to be effective.
In line with best clinical practice, participants will be given an MED test prior to starting light treatment, in order to establish the most appropriate starting dose. This test will be conducted at the baseline visit and participants will return the following day for reading of the test and clarification of the treatment regimen.
Safety testing and quality control of the light therapy devices
Experience from our pilot trial suggests that output of the handheld NB-UVB devices varies by approximately 10% of the mean (although one outlier varied by as much as 30%). In order to minimise this variation, we will use just one device (Dermfix) and all units will be tested centrally prior to distribution to recruiting sites. Variation in output can be due to the quality of the bulb and to variations in the power supply. If, after changing the bulb, a device is found to fall outside a pre-specified level of acceptable output (normal range for these devices is currently being established), then the device will be returned to the manufacturer and a replacement device acquired. Further testing of the devices is currently under way to characterise changes in their output more accurately.
At the end of the treatment period, all devices will be returned to the co-ordinating centre for retesting.
Role of principal investigator and other dermatologists at site
Although participants will usually be seen by a research nurse at the trial visits, the local dermatologists (PI or coinvestigators) will be responsible for confirmation of the diagnosis, issuing of the prescription and for any medical care required as a result of adverse reactions to the study interventions.
Role of the research nurse
Research nurses will assist with the identification and recruitment of participants, provide clinical assessments of treatment response at follow-up visits, deliver training in use of the trial interventions, provide general advice on minimising side effects and conduct data entry and administrative duties.
Outcome measures
Primary outcome
The primary outcome will be patient-reported treatment success for a target lesion at 9 months. The target lesion will be defined by participants on entry into the trial as being a lesion that has appeared or spread in the last year (in line with the eligibility criteria for determining ‘early’ vitiligo) and which the participant would most like to see an improvement in.
Treatment success will be measured using the following question: ‘Compared to before treatment, how noticeable is the vitiligo now?
-
More noticeable (1)
-
As noticeable (2)
-
Slightly less noticeable (3)
-
A lot less noticeable (4)
-
No longer noticeable (5)’.
In order to judge this, participants will be shown the baseline images of their vitiligo. Treatment success is defined as participants who report a treatment response that results in their vitiligo being either ‘a lot less noticeable’ or ‘no longer noticeable’ as defined in the work described in Validation of patient-reported treatment response.
Secondary outcomes
Secondary outcomes will be as follows:
-
Participant-reported treatment success at 9 months for different body sites (face and neck, hands and feet, limbs and trunk).
-
Percentage repigmentation at 9 months compared with baseline at a single target lesion. This will be measured by the research nurse, who will be blinded to the treatment allocation. Site and timing of onset of treatment response will be assessed at 3, 6 and 9 months.
-
Treatment usage and adherence, assessed at 3, 6 and 9 months.
-
QoL at 9 and 21 months. Tools for assessing QoL will be Vitiligo-Specific Quality-of-Life Instrument (a recently validated scale to assess the impact of vitiligo on QoL in adults);361 Skindex-29 (for adults, consists of 3 domains: symptoms, emotions and functioning431); European Quality of Life-5 Dimensions (EQ-5D) (provides health utilities for adults360,493) and CHU-9D for children. 361
-
Adverse device effects and adverse reactions during treatment.
-
Within-trial cost-analysis from a NHS perspective.
Follow-up phase only
Proportion of participants who report a loss of treatment response (for any treated lesion) at the end of the no-intervention follow-up phase.
Summary of data collection schedule
Participants will be pre-screened and, if broadly eligible, will attend a hospital clinic for the baseline assessment (including informed consent, eligibility, baseline characteristics, randomisation and education in the use of the trial interventions).
At 6 weeks (± 2 weeks), participants will be telephoned in order to check both that treatments are being used appropriately and for adverse reactions. Throughout treatment, patients will complete a diary that records treatment adherence and adverse reactions. Subsequent clinic visits will take place at 3, 6 and 9 months (Table 52). For participants who are unable to return to clinic for assessment of the primary outcome at 9 months, a questionnaire will be sent for self-completion at home in order to minimise missing data. Participants will be sent questionnaires (online or paper) to capture maintenance of treatment success, repigmentation, QoL and health resource use during the follow-up phase.
| Outcome collected | Pre-screen | 0 months | 0 + 1 day | Months | 9 months (primary outcome) | 21 months | ||
|---|---|---|---|---|---|---|---|---|
| 1.5 | 3 | 6 | ||||||
| Eligibility checks | ✓ | ✓ | ||||||
| Consent and randomisation | ✓ | |||||||
| Baseline characteristics | ✓ | |||||||
| Digital images | ✓ | |||||||
| Training in use of intervention | ✓ | ✓ | ||||||
| MED test (and results) | ✓ | (✓) | ||||||
| Telephone support | ✓ | |||||||
| Tracing of target lesion by research nurse | ✓ | ✓ | ✓ | ✓ | ||||
| Acceptability of response assessed by participants – global response and up to three individual vitiligo patches | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Site and timing of onset of treatment | ✓ | ✓ | ✓ | |||||
| QoL (VitiQoL, Skindex-29, EQ-5D for adults, CHU-9D for children) | ✓ | ✓ | ✓ | |||||
| Adverse reactions | ✓, diary | ✓, diary | ✓, diary | |||||
| Treatment usage and adherence | ✓, diary | ✓, diary | ✓, diary | |||||
| Health resource use | ✓ | ✓, diary | ✓, diary | ✓, diary | ✓ | |||
Cost and outcomes for cost-effectiveness analysis
The within-trial economic evaluation will primarily seek to estimate the incremental cost-effectiveness of (1) active handheld NB-UVB light, (2) active handheld NB-UVB with potent topical corticosteroids and (3) potent topical corticosteroids from a NHS perspective. In those seeking active treatment for vitiligo, potent topical corticosteroids alone represents standard care.
An incremental cost analysis will be conducted from a NHS perspective capturing the intervention resource use and other health resource use at baseline, 3, 6, 9 months and end of follow-up. In addition to a NHS perspective, but presented separately, we will record and estimate the out-of-pocket and time costs of treatment for patients and parents. Intervention and wider health-care costs will be estimated during the trial through patient questionnaires and medical records. These will be valued using published unit costs or patient-reported estimates for a common price year.
We will use the EQ-5D-3L for adults and CHU-9D for children to estimate the QALYs over the study period using linear interpolation and AUC analysis with baseline adjustment. 363 The interventions will be ranked from least to most costly, dominated interventions (i.e. those that are more expensive and less effective) or those subject to extended dominance [i.e. less effective and have a higher ICER will be excluded before recalculating the ICERs which will be assessed in relation to a range of cost-effectiveness thresholds (different levels of willingness to pay for health benefits)]. Decision uncertainty will be presented via CEACs based on non-parametric bootstrapping of cost and effect pairs. This will provide robust trial evidence to inform decision-makers about the likely cost-effectiveness of interventions for vitiligo in particular about whether or not NB-UVB light is more cost-effective than topical corticosteroid alone and about whether or not combination treatment offers greater value for money than either treatment alone.
Statistical analysis
An ITT analysis will be performed. For the primary outcome, number and percentage of patients achieving ‘treatment success’ will be reported for each treatment arm at different time points while on treatment and at the end of follow-up. Outliers and patterns of missing data will be checked prior to data analysis. For the efficacy of combination treatment over the monotherapies, we will use chi-squared statistic for the overall difference in success rates of the three treatment groups at 9 months. We will also use logistic regression analysis in which specific treatment effects are compared, with adjustment for baseline covariates. To examine possible variability of the outcomes at different body sites, a two-level logistic regression model will be used in which body sites are level one units and nested within individuals at level two. Analyses of secondary outcomes will also be by ITT. Methods of statistical analyses will be determined by the type of outcome measure. Binary outcomes will be described by rate or proportion and tested by chi-squared statistic. Continuous outcome will be described by average or median depending on the distribution of the variable and tested by analysis of variance or a non-parametric method for three groups. When possible, confounding of the treatment effects is identified and requires correction, generalised linear models will be considered for multivariate analysis of secondary outcomes according to their data type. Time to onset of treatment response will be analysed by survival time statistics such as Kaplan–Meier estimator and log-rank tests. Proportional hazard model will be considered if multivariate analysis is required for the time related variables. For all analyses, differences between the three treatment arms are the key interest. Treatment effects on status of lesion (active or inactive, old or new) will be investigated as appropriate.
Sample size
The research literature with which to inform our sample size is limited. However, based on evidence of previous trials of light therapy in combination with topical steroids427,429 and of topical corticosteroid as monotherapy404 we have calculated the sample size assuming a range of different treatment responses.
These estimates assume a chi-squared test for a contingency table [degrees of freedom (df) = 2 and Cramer’s correlation coefficient for contingency table] with test power at 0.90 and alpha level = 0.05/3 = 0.017 to adjust for multiple comparisons, and loss to follow-up of 15%. The calculation was performed in the package Power Analysis for Sample Size version 11 (NCSS, Kaysville, UT, USA).
In order to be conservative in our sample size estimate, we aim to recruit 440 participants into the trial (Table 53). This assumes a success rate of 40% for NB-UVB + topical corticosteroid; 20% for NB-UVB (+ sham topical corticosteroid); and 10% for topical corticosteroid (+ sham NB-UVB).
| Scenarios for % success | Effect size | n | n with loss to follow-up |
|---|---|---|---|
| NB-UVB + TCS: 50% | 0.220 | 330 | 388 |
| NB-UVB + sham TCS: 30% | |||
| Sham NB-UVB + TCS: 15% | |||
| NB-UVB + TCS: 60% | 0.277 | 210 | 234 |
| NB-UVB + sham TCS: 30% | |||
| Sham NB-UVB + TCS: 15% | |||
| NB-UVB + TCS: 40% | 0.206 | 375 | 441 |
| NB-UVB + sham TCS: 20% | |||
| Sham NB-UVB + TCS: 10% |
Patient and public involvement
Vitiligo was included as a work programme as a result of direct lobbying by patients over the need for better-quality research evidence on which to base treatment decisions. Having led the first Cochrane review of interventions for vitiligo in 2006, Mrs Maxine Whitton, a vitiligo sufferer and advocate of the need for high-quality trials of vitiligo treatments, joined the team as a patient researcher and patient lead for the vitiligo work programme.
The patient support group the ‘Vitiligo Society’ has been instrumental in advertising and promoting our work to its members, as well as providing access to private chat rooms in order to facilitate patient involvement in this research. They will be key partners in helping to continue to disseminate the findings and impact of this work in the future.
Many members of the public have contributed to this work package, most notably to the sections ‘vitiligo PSP’, ‘validation of patient-reported treatment response’ and ‘pilot HI-Light trial’. Having involvement and support from patients at every stage of this work package has ensured that the most important questions are now being addressed, that outcomes are measured is such a way as to be meaningful to patients with vitiligo, and has ensured that the proposed interventions are acceptable to patients and the clinicians who treat them.
In relation to the development of the main HI-Light trial that will commence in 2014, the work described in this report has had a direct impact in the following ways.
-
Ensuring that the most important question is being addressed: the future trial will address two areas of treatment uncertainty that were identified as being of high priority for future research in the PSP. This project involved 302 patients and 142 clinicians who worked together to identify shared priority topics.
-
Ensuring the right outcomes are measured: during the initial scoping survey conducted as part of the PSP patients were asked what outcomes they felt should be measured in future clinical trials of vitiligo treatments. This work highlighted the discrepancy between what is usually measured in vitiligo trials and what patients want to see from vitiligo treatments. In particular, the acceptability of treatment response and QoL were highlighted as being important.
-
Helping to establish how to measure ‘acceptable treatment response’: a second patient survey was conducted involving 202 patients, this time to establish the exact wording to be used when eliciting patient-reported treatment response. This was followed by three online discussion groups involving a total of 12 patients to reach consensus over the wording of the question, possible response options and to define what constitutes a clinically meaningful response. Additional work is ongoing to further validate the scale, in which up to 100 patients and 20 clinicians are being asked to rate a series of clinical photographs using the newly developed scale. This work is being used to inform the choice of the primary outcome for the main trial.
-
Ensuring the treatment is acceptable to patients and that they are able to follow the treatment protocol at home: the pilot RCT was used to inform this aspect of the trial. Twenty-nine participants used the home NB-UVB devices for 4 months and provided feedback at the end of the trial on how suitable the training material was, any barriers to involvement in the trial, and recommendations for the best way of supporting participants in the future RCT. Of the participants who took part in the pilot trial, 80% said that they would be prepared take part in future research of this kind and we subsequently produced a training DVD on how to use home light therapy to supplement the written and oral training provided during the pilot trial. Three patients (two adults and one child) contributed to this training DVD in which they talked about their experience of living with vitiligo and taking part in the trial. This DVD is now freely available. 273
-
Ensuring that the treatment protocol reflects normal practice: we feel that PPI is not just about meaningful engagement with patients, but also includes appropriate engagement with the clinical community responsible for the treatment of patients with vitiligo. In order to ensure that the proposed treatment regimens for the HI-Light trial are in line with current practice and are acceptable to clinicians, a survey of members of the UKDCTN was conducted (including dermatologists, GPs and dermatology nurses). This survey was used to gauge the level of enthusiasm for the trial and to ensure that the proposed choice of topical corticosteroid and dosing regimen were acceptable. This work was important to ensure support for the trial by potential recruiting clinicians and to ensure that the results of the trial are more likely to be adopted into practice.
The relationship that our team has built up over the last 5 years with patients, clinicians and patient support groups means that we now have an active network of > 400 supporters who regularly receive updates on progress with our vitiligo research. This broad advisory group will support the delivery of the national HI-Light trial by advertising the trial among relevant groups and helping with dissemination of the trial findings. Early engagement with all key stakeholders means that the results will be widely disseminated, thus encouraging early adoption of the trial findings.
Summary and conclusions
Why is this work programme important?
This Programme Grant included vitiligo as a topic as it is an under-researched area that can have a disproportionate and devastating effect on people with dark skin from UK black and ethnic minority populations. In line with the NIHR adding value agenda, the work started with a comprehensive review of the current position of vitiligo research, which helped to identify some of the reasons why vitiligo treatment has not progressed over many years, despite an increasing number of vitiligo trials. These include a lack of satisfactory core outcome measures, a paucity of evidence regarding commonly used treatments and disparity between clinicians and patients as to what constitutes a good treatment response. The PSP brought together patients and HCPs to narrow down the most urgent priorities for future research. Furthermore, our work on outcome measure has prompted the establishment of an international ‘vitiligo core outcomes working group’ and VE is now helping to lead this work through further international e-Delphi surveys and consensus meetings.
The vitiligo work programme has delivered all that it set out to do and more. In particular, the feasibility work was extended to include an additional systematic review of outcome measures used in vitiligo trials and a patient survey to establish what is most important to patients. This work highlighted the apparent disparity between what has traditionally been used to measure treatment response (percentage repigmentation) and what patients feel is most important to them (how noticeable the vitiligo is). Having decided on the most important treatment uncertainty that the programme should help to address, initial feasibility work was modified to include a pilot RCT of NB-UVB light therapy using handheld units at home. This innovative pilot trial has been used to inform a successful funding application to the NIHR HTA programme.
The full-scale trial involving 440 patients with early and limited vitiligo will be by far the largest vitiligo trial conducted in the world to date and is the first to test the use of home NB-UVB-light therapy in combination with topical corticosteroids. The design of this definitive trial has been critically informed by the studies described in this report (from ensuring that the right question was asked in the first place, through to defining the outcomes, trial design and conduct) and the results will be used to inform clinical practice.
Implications for clinical practice
Many patients with vitiligo are never seen by a specialist or offered any active treatment by their GP. The NICE Clinical Knowledge Summary guidelines for vitiligo494 were developed on the basis of the 2010 Cochrane systematic review and recommend the use of potent topical corticosteroids for first-line therapy in adults and referral to secondary care for children requiring treatment, or for patients who are unresponsive to first-line therapy.
Other clinical guidelines that have been informed by the Cochrane systematic review include patient information sites such as the NHS Choices vitiligo module472 (which was newly developed as a result of the Cochrane review), the BAD guidelines for the management of vitiligo,434 European guidelines for the management of vitiligo483 and guidelines on designing and reporting vitiligo trials. 431
Although it is disappointing that the Cochrane systematic review found mainly small, inconclusive, trials covering a host of different interventions, some clinical implications can be drawn:
-
There is moderate evidence for the use of topical corticosteroids, although long-term use is likely to lead to adverse effects. Topical non-steroidal immunomodulators, such as tacrolimus, may be a promising alternative, but the theoretical risk of skin cancer must be considered for long-term combination use with phototherapy.
-
Most of the studies that assessed combination interventions employed light therapies (UVA, PUVA or UVB). In general, combination interventions were superior to monotherapies.
-
There is limited evidence for the benefit of oral G. biloba.
-
There is limited evidence for the benefit of split-thickness skin grafts plus PUVAsol. Surgical therapies can be effective for small areas in people with stable disease (bearing in mind the risk of adverse effects with suction blister grafts).
-
There is a lack of formal evidence supporting the use of cosmetic camouflage, depigmentation (in severe cases) or psychological interventions.
In relation to light therapy provision in the UK, handheld units are not currently widely used, although some centres now use them for the management of scalp psoriasis (Dr Robert Dawe, Ninewells Hospital and Medical School, 2012, personal communication). This means that for the moment at least, light therapy for vitiligo patients in the UK is generally limited to people with severe and widespread disease who receive treatment in a hospital setting using full-body units. Travelling to hospital for treatment 2–3 times per week is often impractical for many patients and there are concerns about long-term safety, particularly in children.
Handheld NB-UVB units are widely available for purchase over the internet and are licensed for use in vitiligo. Many patients are choosing to purchase these devices for themselves and are using them in an unsupervised manner. We would always advise that patients seek medical advice when using light therapy devices and the DVD produced for training in the use of home NB-UVB is freely available as a training resource should clinicians wish to access it. 273
The results of the HI-Light trial will inform clinical practice and allow evidence-based guideline development for the use of topical steroids and home-based handheld light therapy in vitiligo. The evidence base is particularly scant for children and so the inclusion of both adults and children in the HI-Light trial will be an important advance.
In the meantime, we would encourage clinicians to use current guidelines to inform treatment decisions and to judge treatment success using a simple patient-reported outcome. Our preliminary work in defining such an outcome suggests that the following wording is meaningful to patients:
‘Compared to before treatment, how noticeable is the vitiligo now?
-
More noticeable (1)
-
As noticeable (2)
-
Slightly less noticeable (3)
-
A lot less noticeable (4)
-
No longer noticeable (5)’.
Implications for research
This is the largest coherent programme of vitiligo research to date and has helped to stimulate debate and interest in vitiligo research internationally. In particular, the work on outcome measures has resulted in the establishment of an international consensus partnership to develop a core set of outcomes for future research and has served to highlight the possible disconnect between what is typically measured in vitiligo trials and what is meaningful to patients.
In the 2010 Cochrane review, just two vitiligo trials had been conducted in the UK, and so we are delighted that, for the first time, the NIHR are to fund a large, multicentre, pragmatic trial of first-line therapy for vitiligo. The study protocol is summarised in this report and will be made freely available to other researchers wishing to design similar studies.
In publishing the list of priority topics for further research, this programme has provided a focus for future research activity and trials are already under way that tackle some of the priority topic areas. For example, two studies are currently listed in trial registries investigating afamelanotide and the UKDCTN issued a themed finding call in 2013 for pump-priming funds to support the development of a trial into one of the top 10 priority topics. This award went to a group of dermatology trainees for a proposal to develop a trial of psychological interventions for vitiligo.
Future research recommendations
Developments in basic science and genetics mean that new treatments for vitiligo are on the horizon. The work of the Programme Grant will allow meaningful standardised clinical trials to assess efficacy and inform clinical practice for vitiligo sufferers. In the meantime, priority should now be given to addressing the topics identified through the PSP and in achieving international consensus over core outcomes. In this way it will be possible to combine the results of future trials in meta-analyses and, thus, inform clinical decision-making.
Particular recommendations for future research arising from the PSP include:
-
More research on the effectiveness and safety of systemic immunosuppressants for the treatment of vitiligo such as methotrexate and ciclosporin. Research in this field would potentially contribute to our knowledge about the aetiology of the disease, which is believed to have a strong autoimmune component.
-
Evaluation of currently available and widely used treatments such as topical corticosteroids and calcineurin inhibitors in a head-to-head RCT.
-
Evaluation of psychological interventions by conducting a systematic review of the current literature and substantial pilot/and exploratory qualitative work prior to progressing to a full RCT. Together with active treatments, psychological interventions are believed to be of great importance. More evidence is needed to establish the role of psychological support as monotherapy, as well as in combination with other treatments for vitiligo.
-
Further evaluation of pseudocatalase as a treatment modality.
-
More research into the pathophysiology and the aetiology of the disease based on the interest expressed by clinicians and patients on exploration of potential effectiveness of gene therapy and stem cells.
Future trials should include a treatment period of at least 9–12 months and include a period of follow-up after treatment is stopped in order to ascertain maintenance of repigmentation. Outcomes of importance to patients include acceptability of treatment response, cessation of spread, maintenance of repigmentation, QoL and side effects of treatment.
Research into interventions that are suitable for children and are of for use on sensitive areas of the body (such as the face) are particularly lacking.
Chapter 4 Squamous cell skin cancer work programme
Abstract
Introduction
Evidence regarding the treatment of squamous cell skin cancer (SCC) is scarce. We sought to evaluate the existing evidence for SCC treatment options and to gather opinions and background data to inform the design of a large pragmatic RCT.
Methods
The approaches taken were:
-
systematic reviews of the treatment of SCC using evidence from (1) RCTs and (2) observational studies
-
an online survey of clinicians to establish research priorities
-
feasibility studies to inform the design of a pragmatic RCT of high-risk SCC, including (1) a histological audit of SCCs treated over a 1-year period and (2) questionnaires and a focus group.
Results
The reviews of SCC treatments identified only one RCT comparing recurrence between intervention groups and 118 observational studies, covering seven treatment modalities.
The online survey of clinicians identified optimal excision margins and the role of adjuvant radiotherapy (ART) as key areas of uncertainty in the management of high-risk SCCs.
The questionnaires and focus group revealed a lack of knowledge about SCC among patients and provided insight that will inform the development of participant information resources.
The histological audit provided data on the numbers, types and 5-year outcomes of SCCs that are crucial to plan recruitment and guide statistical powering of the proposed definitive study.
Conclusions
The outputs of this work programme are key to informing the design of a RCT, which will compare conventional excision with a wide surgical margin and Mohs micrographic surgery (MMS), and ART versus no ART in high-risk SCCs.
Content
This chapter describes work that has been done as the basis for developing a proposal for a RCT that will address the management of SCC. It includes an in-depth appraisal of the current evidence base for SCC treatments and also describes background exploratory and feasibility projects that have been integral to this work programme.
| Details | Comment |
|---|---|
| Introduction | An overview of the burden of SCC, its causes, risk factors and an introduction to treatment |
| Systematic review of squamous cell skin cancer treatments: randomised controlled trials | A Cochrane systematic review of RCTs that have evaluated the effectiveness of treatments for primary SCC |
| Systematic review of squamous cell skin cancer treatments: observational studies | An appraisal of the evidence base of the effectiveness of SCC treatments from studies other than RCTs |
| Identification of potential research topics and development of a clinical trial scenario | Description of a multidisciplinary survey of clinicians to identify clinically important research topics and collaborations which have been key during development of the trial proposal |
| An analysis of squamous cell skin cancer treated over a 1-year period | A study evaluating the types of SCCs submitted to the histopathology department over the course of 1 year and an assessment of treatment outcomes in patients within 5 years of treatment |
| Qualitative feasibility study | Questionnaire and focus group study to examine the hypothetical willingness of potential participants to take part in the proposed RCT and an exploration of possible barriers to recruitment into the trial |
| Treatment uncertainties for high-risk squamous cell skin cancer: a trial proposal | Proposal for a two stage trial of wide local surgical excision with a 10-mm margin vs. MMS, and ART vs. no ART for patients with high-risk SSC |
| Patient and public involvement | Description of the ways in which patients have played an important role throughout the lifespan of the project |
| Summary and conclusions | An overview of how this work is having an impact on SCC guideline development and implications for clinical practice and future SCC research |
Introduction
Squamous cell skin cancer is the second most common type of non-melanoma skin cancer (NMSC) after basal cell skin cancer (BCC) and originates from keratinocytes in the epidermis. It may occur on any part of the body where there are squamous cells, either de novo in the absence of a precursor lesion, or from an area of pre-existing actinic keratosis or Bowen’s disease.
Invasive SCC is characterised by the presence of malignant cells in the dermis which have breached the epidermal basement membrane. Connective tissue, nerves and blood vessels may be involved, and there is also the potential for the tumour to cause local tissue destruction, to metastasise to regional lymph nodes or distant organs and, rarely, to cause death.
The tumour may present clinically as a smooth or hyperkeratotic enlarging plaque, nodule or ulcer and may be associated with pain, pruritus or bleeding when traumatised. Induration, the limits of which may not be sharply defined, can spread beyond the extent of clinically apparent tumour. Invasive SCC has the potential to recur and metastasise to regional lymph nodes or distant organs and, if left untreated or inadequately treated, may cause extensive local tissue destruction.
There were 99,549 new cases of NMSC registered in the UK during 2010. 495 Based on an approximate ratio of one SCC to four BCCs, this equates to approximately 20,000 cases of SCC per annum. However, the true incidence of SCC is not known and this figure is likely to be a significant underestimate; recording of NMSC is known to be incomplete, with a lack of robust published data by cancer registries around the world. 496,497 On a global scale, local studies indicate that Australia has the greatest burden of SCC, with a reported age-standardised incidence of 387 per 100,000 of the population. 498 Older people have a higher incidence of the disease than younger people and it also more commonly affects men than women. 499–502
Causes and risk factors
Chronic exposure to UV radiation is the most important factor contributing to the development of SCC. 503 This is reflected by the increased prevalence of SCC at sites on the body that are generally unprotected from sunlight, such as the head and neck. However, exposure to other carcinogens such as arsenic, tobacco, psoralens and ionising radiation may also increase SCC risk. 504–508 Infection with some types of human papillomavirus has also been in implicated in the aetiology of SCC. 509,510
Overall, risk of SCC is a combination of environmental factors and an individual’s response to these. People with fair skin that sunburns readily and with red or blonde hair are at greatest risk of developing SCC. 511 Some genetic conditions such as albinism, xeroderma pigmentosum and recessive dystrophic epidermolysis bullosa may also predispose those affected to the early development of SCCs. Impairment of the immune system is an important risk factor. This is exemplified by the greatly increased risk of developing SCC in solid organ transplant recipients when compared with the general population, with reversal of the usual BCC to SCC ratio. 512–515
Treatments
The aims of treatment of SCC are to completely remove or destroy the tumour and to minimise functional and cosmetic impairment. Management of SCC is facilitated by the stratification of patients based on the American Joint Committee on Cancer (AJCC) staging system. 516 This takes into account tumour features associated with a worse prognosis (tumour diameter > 2 cm; tumour depth > 2 mm; Clark’s level of IV or more; location on the ear, lip and non-sun-exposed sites; the presence of perineural invasion; and poorly differentiated or undifferentiated histology), together with the nodal and metastatic status of the patient.
Several different treatment modalities are used to manage SCC; however, the mainstay treatment for the majority of SCCs, and recommended in current guidelines, is surgical excision. 517 This is performed either by conventional excision with a margin of normal-looking skin, or MMS, in which sequential layers of tumour-containing skin are excised and examined microscopically until all tumour has been removed. Some SCCs are amenable to treatment with radiotherapy, which may also sometimes be used as an adjunct to initial surgical treatment. For small, well-defined, SCCs considered to be at low-risk of recurrence or metastasis, curettage and cryotherapy or electrodesiccation may also represent treatment options if performed by experienced practitioners.
Systematic review of squamous cell skin cancer treatments: randomised controlled trials
The following text is adapted with permission from Lansbury L, Leonardi-Bee J, Perkins W, Goodacre T, Tweed JA, Bath-Hextall FJ. Interventions for non-metastatic squamous cell carcinoma of the skin. Cochrane Database Syst Rev 2010;4:CD007869. 518 Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Summary
What was already known about this topic?
-
Cutaneous SCC is the second most common cancer worldwide.
-
There are several treatment options for SCC available.
-
Current management guidelines are based predominantly on evidence from case series.
What did this study add?
-
As a basis for appraisal of the current evidence base of the effectiveness of different treatments for cutaneous SCC, this review assessed the evidence available from RCTs.
-
The one eligible published RCT compared time with recurrence in patients treated with adjuvant chemotherapy versus no adjuvant chemotherapy after primary surgery. There was no significant difference in outcome between the two study arms.
-
There are no RCTs that directly compare the effectiveness of different treatments for the types of SCC that are seen routinely in clinical practice.
Introduction
Why it was important to do this review
The burden of SCC to both individuals and to the health-care system is only likely to grow owing to the increased proportion of elderly people in the population. More than 80% of NMSCs occur in people aged ≥ 60 years and with an increasingly ageing population, a 50% increase in NMSC workload for UK dermatologists by 2030 has been predicted. 519 Consequently, a consistent and practical approach to the management of SCC will become imperative in future years. Current SCC management guidelines are largely based on evidence from case series and there have been no systematic reviews previously conducted in this area. Therefore, this review was a starting point from which to appraise the current evidence base of the effectiveness of different treatments, identify where more evidence appraisal is required and to help direct researchers towards future research requirements.
Objective of the review
The objective of this review was to assess the evidence available from RCTs for the effectiveness of treatments used in the management of cutaneous SCC.
Methods
Details of the search strategies for this review can be found in the full publication, accessible from The Cochrane Library. 518 The review included all RCTs of interventions for primary, non-metastatic SCC of the skin, which included adult participants with one or more histologically proven eligible cutaneous SCCs.
Interventions included surgery (excisional surgery or MMS); destructive treatments (curettage and cautery, cryosurgery, photodynamic therapy, laser therapy, radiotherapy); topical therapy; intralesional treatments; and chemotherapy. Adjuvant treatments were also included as interventions.
Primary outcomes of interest were:
-
time to recurrence (local, regional and distant) after apparently successful initial treatment
-
QoL.
Secondary outcomes of interest were:
-
early treatment failure within 6 months confirmed histologically
-
number of adverse events by the end of treatment
-
cosmetic appearance as assessed by (1) the participant or (2) the clinician
-
discomfort to the participant during and after treatment
-
death.
Search methods for identification of studies
An electronic search was performed for relevant published trials in the following databases: The Cochrane Skin Group Specialised Register, CENTRAL in The Cochrane Library, MEDLINE, EMBASE, PsycINFO, AMED and LILACS. In addition, we searched the following trials registers for ongoing trials: the metaRegister of Controlled Trials (www.controlledtrials.com); the US National Institutes of Health ongoing trials registry (www.clinicaltrials.gov); the Australian and New Zealand Clinical Trials Registry (www.anzctr.org.au); the World Health Organisation International Clinical Trials Registry platform (www.who.int/trialsearch); and the Ongoing Skin Trials Register (www.nottingham.ac.uk/ongoingskintrials).
Bibliographies of published studies and key review articles were scanned in order to identify additional relevant trials. Translation of studies into English was carried out if necessary.
Data collection
Two reviewers checked the titles and abstracts identified from the searches and independently assessed the full text of potentially relevant studies to decide if they met the inclusion criteria. Any disagreements were resolved by discussion and a consensus decision was made.
Data for each study were independently extracted by two authors, with resolution of discrepancies by a third author. A risk-of-bias assessment was done based on the following components:
-
the method of generation of the randomisation sequence
-
method of allocation concealment
-
blinding of participants, clinicians and outcome assessors
-
loss of participants to follow-up in each arm and if analysis was on an ITT basis
-
the degree of certainty that participants had SCC
-
baseline comparison of the study arms in terms of disease severity.
Data analysis
Details of the statistical plan may be found in the full version of the review. 518 Results were expressed as hazard ratios (HR) with 95% CIs for time-to-event outcomes.
Results
Randomised controlled trials included in this review
Fourteen possible RCTs were identified for inclusion, of which 12 were fully published papers520–531 and two were abstracts. 532,533 However, only one RCT met the inclusion criteria for this review. 520 Two other trials were also identified, one had just been completed but was excluded as the author informed us that there were no participants with cutaneous SCC [Continuous Hyperfractionated Accelerated Radiotherapy Schedule Weekend-less (CHARTWEL] trial]. 534 No published results were available at the time from the other ongoing trial (TROG 05.01). 535
The included RCT520 was a single-centre, parallel-group, study from the USA that included 65 evaluable male and female patients with pathologically confirmed aggressive skin SCC. The primary outcome was time to tumour recurrence or development of a second primary tumour. A total of 31 participants received adjuvant 13-cis-retinoic acid and interferon alpha (IFN-α) after surgical treatment, whereas the 34 participants in the control arm received no adjuvant chemotherapy after primary surgical treatment.
Excluded studies
Fourteen potentially eligible RCTs were excluded from the review, the details of which are documented in the full version of the review. 518
Risk of bias in included study
To assess the risk of bias in the included study, we considered the generation and concealment of the randomisation sequence, blinding, losses to follow-up, the degree of certainty that participants had SCC and baseline severity of disease. The randomisation method was described as permuted block randomisation within strata but details about the adequacy of concealment of allocation were not available. Neither clinicians nor participants were blinded to allocation owing to the nature of the intervention. Analysis of the primary outcome was carried out on all evaluable participants apart from one who withdrew consent immediately after randomisation. Histological confirmation of SCC was obtained for all participants and there were no clinically relevant or demographic baseline differences between participants in each trial arm.
Effects of interventions
Primary outcomes
In the one included study, no statistically significant difference between participants who received adjuvant chemotherapy and those who did not was found in the time to recurrence (HR 1.08, 95% CI 0.43 to 2.72) (Figure 25).
FIGURE 25.
Forest plot comparing time to recurrence between 13-cis-retinoic acid plus IFN-α treatment arm and control arm.

The included trial did not compare QoL between the treatment-arm and control-arm participants.
Secondary outcomes
The most frequently reported adverse events among participants in the treatment arm of the included trial were dry skin, fatigue and generalised lip and eye reactions. However, a total number of adverse events could not be determined, as it was possible that participants may have experienced more than one adverse event each.
No treatment-related deaths were reported in the included trial. There were two non-treatment-related deaths during follow-up: one in the treatment arm and one in the control arm.
No data were available for the other secondary outcomes of early treatment failure, aesthetic appearance and discomfort to participants.
Update of the review
In 2014 this systematic review was marked as stable in The Cochrane Library as no additional eligible RCTs had been published since the original systematic review was conducted.
Discussion
Only one RCT met the inclusion criteria for this systematic review, comparing time to recurrence between a group of SCC patients receiving adjuvant 13-cis-retinoic acid and IFN-α after surgery with a group who did not receive additional chemotherapy. The risk of bias in the included study was unclear across several of the domains assessed. Furthermore, it may have been underpowered to detect a significant difference between the two groups owing to difficulties accruing a sufficient number of patients with the aggressive types of SCCs that were being evaluated in the trial. A large number of potential studies were retrieved in the database search. However, the majority could not be considered for inclusion in the review, as they were either uncontrolled case series (which we have evaluated in a separate systematic review, see Systematic review of squamous cell skin cancer treatments: observational studies) or they were RCTs which only included BCCs or non-invasive SCCs such as actinic keratosis or Bowen’s disease.
Therefore, the review has highlighted the lack of evidence from RCTs regarding the effectiveness of treatments for non-metastatic SCCs and those that are less aggressive than the cases assessed in the included trial. It is, however, the less aggressive SCCs that are most commonly seen in clinical practice and for which the evidence base for treatments is currently lacking.
Implications for clinical practice
Owing to the lack of RCTs, coupled with the limitations (including small sample size) in the one included trial, it was not possible to make specific recommendations for clinical practice from this review.
Implications for research
Invasive SCC is a common NMSC yet its management has not been investigated in the form of rigorous RCTs to the same extent as BCC or intraepithelial neoplasia. Gaps in the evidence base which may be usefully investigated by future RCTs are discussed further in our systematic review of observational studies of SCCs treatments and in our survey of clinicians, in which specific trial proposals are suggested (see Treatment uncertainties for high-risk squamous cell skin cancer: a trial proposal). In addition, a number of general suggestions have arisen from this systematic review regarding future research, which are summarised below.
-
Primary invasive SCCs should be studied separately from other types of NMSCs and non-invasive tumours.
-
Standardised outcome measures would improve consistency across studies and make their findings easier to compare.
-
Outcomes that should be assessed include 5-year recurrence, QoL, safety and tolerability profiles, cosmetic appearance, and cost implications.
-
Tumour prognostic features such as diameter, depth, histology and site should be taken into consideration when designing trials and analysing the results.
-
Studies should be adequately powered and multicentre trials undertaken if accrual rates are likely to be low.
-
The management of the patient with SCC may be multidisciplinary; therefore, collaboration between specialities should be encouraged.
The management of people at particularly high risk of developing SCC, such as those who are immunosuppressed or with a predisposing genetic condition, has not been addressed in this review. It is, however, an important issue and an area worthy of separate review.
Systematic review of squamous cell skin cancer treatments: observational studies
The following text is adapted from Lansbury et al. 536 This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 3.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/3.0/.
Summary
What was already known about this topic?
-
Squamous cell skin cancer is the second most common type of skin cancer, with the potential to recur, metastasise and lead to death.
-
Surgical excision is currently the preferred treatment for SCC, but no RCTs have directly compared different treatment modalities for the types of SCCs seen in routine clinical practice.
What did this study add?
-
This systematic review assessed the evidence base for the different treatment modalities and included evidence from observational studies.
-
Accurate comparisons of estimates of treatment effects were not possible from the current evidence and the significance of apparent differences between treatments should be interpreted cautiously.
-
The current evidence base for SCC treatments is extremely limited and there is a need for well-designed comparative studies to help stratify patients and optimise their clinical management.
Introduction
In general, the treatment of SCC has not been thoroughly or rigorously studied. In 2010, we published a Cochrane systematic review of treatment of primary non-metastatic SCC,518 which found only one RCT520 that compared recurrence between groups receiving either adjuvant 13-cis retinoic acid and IFN-α after initial surgery, or no adjuvant therapy (see Systematic review of squamous cell skin cancer treatments: randomised controlled trials). The review did not identify any RCTs that assessed the effectiveness of different interventions used commonly in clinical practice in the UK. 518
This current review looked at non-RCT studies that have assessed the effectiveness of common treatment modalities used in everyday practice for SCC. The aim was to provide an overview of the current evidence base, to highlight areas in which the evidence base requires strengthening, and to stimulate future research in the field.
Methods
The systematic review was conducted according to the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidance. 537 Details of the protocol for this systematic review are registered on PROSPERO and can be accessed online. 538
Search strategies
We searched the MEDLINE (1948 onwards) and EMBASE (1980 onwards) databases to December 2012 for relevant studies using search criteria for observational studies based on Scottish Intercollegiate Guideline Network (SIGN) filters. 121 We checked the bibliographies of included studies and recent review articles for additional articles that were relevant. Owing to the large number of studies and limited accuracy of translation, only studies published in English were retrieved.
Inclusion and exclusion criteria
We included all studies, other than RCTs, reporting surgical excision, MMS, radiotherapy (external radiotherapy, brachytherapy and ART), laser irradiation, photodynamic therapy, cryotherapy, curettage and electrodesiccation, topical treatments or other chemotherapy as treatment of previously untreated invasive SCC, which was non-metastatic at presentation. Based on our previous Cochrane systematic review, the main outcomes of interest were recurrence of SCC during follow-up from 1 month to 10 years after treatment and QoL, with secondary outcomes of initial response to treatment, cosmetic appearance or death due to disease. We excluded studies for which we were unable to extract data for primary non-metastatic SCC. These included studies containing data for mixed populations of SCC and BCC, previously treated and untreated SCCs, or primary and metastatic SCCs. Studies for which separate data were not reported for different treatment modalities were also excluded. Owing to the large number of studies, studies reporting outcomes after surgical excision and MMS were only included if there were ≥ 20 eligible participants, unless they were restricted to a specific anatomical location, such as periorbital or auricular.
Study selection and data extraction
Three review authors (LL, FB-H, JL-B) independently checked the titles and/or abstracts of studies that potentially met the inclusion criteria. Studies which clearly did not refer to treatment of SCC of the skin were excluded. The full text was obtained for those studies that potentially fulfilled the inclusion criteria or for which the scope was unclear. Any disagreements were resolved through discussion between the authors.
Data were extracted independently by two reviewers (Louise Lansbury and Joanna Browne/Alemayehu Amberbir) and entered onto a standardised, pre-piloted data extraction form for assessment of study quality and evidence synthesis. A third author (JL-B, FB-H) resolved any discrepancies.
Quality of reporting and risk of bias
The relative quality of the reporting of each study was evaluated, using a self-developed tool539 based on criteria suggested by Albrecht for reporting case series and case reports (see web appendix 3 of the published paper518).
Case series and open-label studies were scored for the number of reporting quality items present and arbitrarily rated as being of poor (score 0–3), intermediate (4–7) or good quality (8–10). For those studies in which pharmaceutical preparations were an integral part of the treatment modality, we also recorded the declaration of sponsorship by a pharmaceutical manufacturer.
Data analysis
For each study, raw proportions were calculated using the number of events divided by the total number of people in the study. The variances of the raw proportions were stabilised using the Freeman–Tukey variant of the arcsine square root transformation. 540 Pooled analyses were conducted on the transformed quantity using a random effect model, to allow for heterogeneity resulting from inherent biases within the studies. Analyses were conducted using StatsDirect Version 2 (StatsDirect Ltd, Cheshire, UK). We were not able to directly allow for differences in length of follow-up using time to event as an outcome measure owing to the lack of such data in the papers. However, when possible, we performed subgroup analysis for which we compared the outcomes in those studies in which the mean follow-up was given as < 2 years, between 2 and 5 years and > 5 years.
To examine the effect of removing studies with greatest potential for risk of bias, we conducted a sensitivity analysis, if possible, by repeating the analysis with data from selected papers meeting at least three of the following criteria: ≥ 50 SCCs reported; mean follow-up > 3 years; recurrence type specified; scoring 8–10 on our reporting quality assessment. Adverse events and cosmetic appearance outcomes were described qualitatively.
Results
The searches identified 3826 publications after removal of duplicates. Of these, 451 were potentially eligible based on their titles; however, evaluation of the abstracts identified 161 records that were not relevant to the review. A total of 290 full-text articles were assessed for eligibility, of which 172 were excluded, mainly owing to lack of separable primary SCC data, leaving 118 that were included in the review (Figure 26).
FIGURE 26.
A PRISMA flow chart of studies.
There were 106 non-comparative studies and 12 single-case reports, which were included owing to a lack of more robust study designs for particular interventions. Four studies reported outcomes for more than one treatment modality. (Full details of the studies, including details of the methodology, types of SCC included and quality of reporting can be found in web appendix 3 of the published paper. 518)
A summary of the risk of bias for the studies is presented in Figure 27. Forty-eight per cent of studies were evaluated as having collected data retrospectively and 36% prospectively; the remaining studies could not be evaluated with regard to the design owing to insufficient information in the publications. Overall, 41% of studies were assessed as being at high or unclear risk of attrition bias because the analyses did not account for losses to follow-up. Selection of a specific treatment modality on the basis of tumour or patient characteristics was assessed as presenting a high risk of bias in 54% of the studies, with low risk in 15%. Risk of bias relating to selection could not be assessed in the remaining 31% of included studies owing to insufficient reporting in the publications.
FIGURE 27.
Risk-of-bias assessment for included studies.
Overall, we classified 13% of the case series as being of poor reporting quality, 56% as intermediate quality and 30% as high quality. Of 24 studies in which topical or systemic treatments were reported, seven (29%) received some form of sponsorship from a pharmaceutical company but did not specifically declare sponsor involvement in the design, results and analysis of the study.
Surgical excision
We included 12 studies (1144 patients). Local recurrence during follow-up after surgical excision ranged from 0% to 15%,541–552 with an estimated overall pooled recurrence of 5.4% (95% CI 2.5 to 9.1; I2 = 81%) (Figure 28).
FIGURE 28.
Surgical excision: local recurrence.
Duration of follow-up varied between the studies. One study had a mean follow-up period of < 2 years (16 months) with local recurrence of 1.8% of surgically excised SCCs of the head and neck region. 545 In those studies with mean follow-up of between 2 and 5 years (736 patients), recurrence ranged from 0% to 13.0%, with a pooled estimate of recurrence of 5.0% (95% CI 2.3 to 8.3; I2 = 62%). 541–543,546–548,550,552 One of the 12 studies had a minimum follow-up period of 5 years and reported no local recurrences of 86 surgically excised SCCs at various sites. 544 Three studies reported recurrence of 4.8% for eyelid SCCs,552 10.5% for trunk and extremity SCCs549 and 15.3% for SCCs of the pinna551 (Figure 29), but did not specify for how long patients were followed.
FIGURE 29.
Surgical excision: local recurrence – ear location.
The SCCs located in the ear region were associated with highest recurrence rates. Three studies (n = 261) in which SCCs of the pinna were surgically excised gave a pooled average local recurrence of 14.1% (95% CI 10.2 to 18.5; I2 = 0%),547,550,551 compared with a significantly lower pooled average of 3.2% (95% CI 1.5 to 5.5; I2 = 57%) for the studies (n = 916) in which SCCs at other sites were included (Figures 30 and 31). 541–546,548,549,552 A non-significant tendency for increased local recurrence with increasing SCC diameter was noted in one series, with local recurrences in 12.2% (95% CI 4.6% to 24.7%) of the 49 lesions < 10 mm in diameter, 14.3% (95% CI 7.8% to 23.2%) of the 91 lesions 10–30 mm in diameter, 21.7% (95% CI 7.4% to 43.7%) of 23 lesions 30–40 mm in diameter, and 42.8% (95% CI 9.9% to 81.6%) of the seven tumours > 40 mm in diameter. 551
FIGURE 30.
Surgical excision: local recurrence – non-ear location.
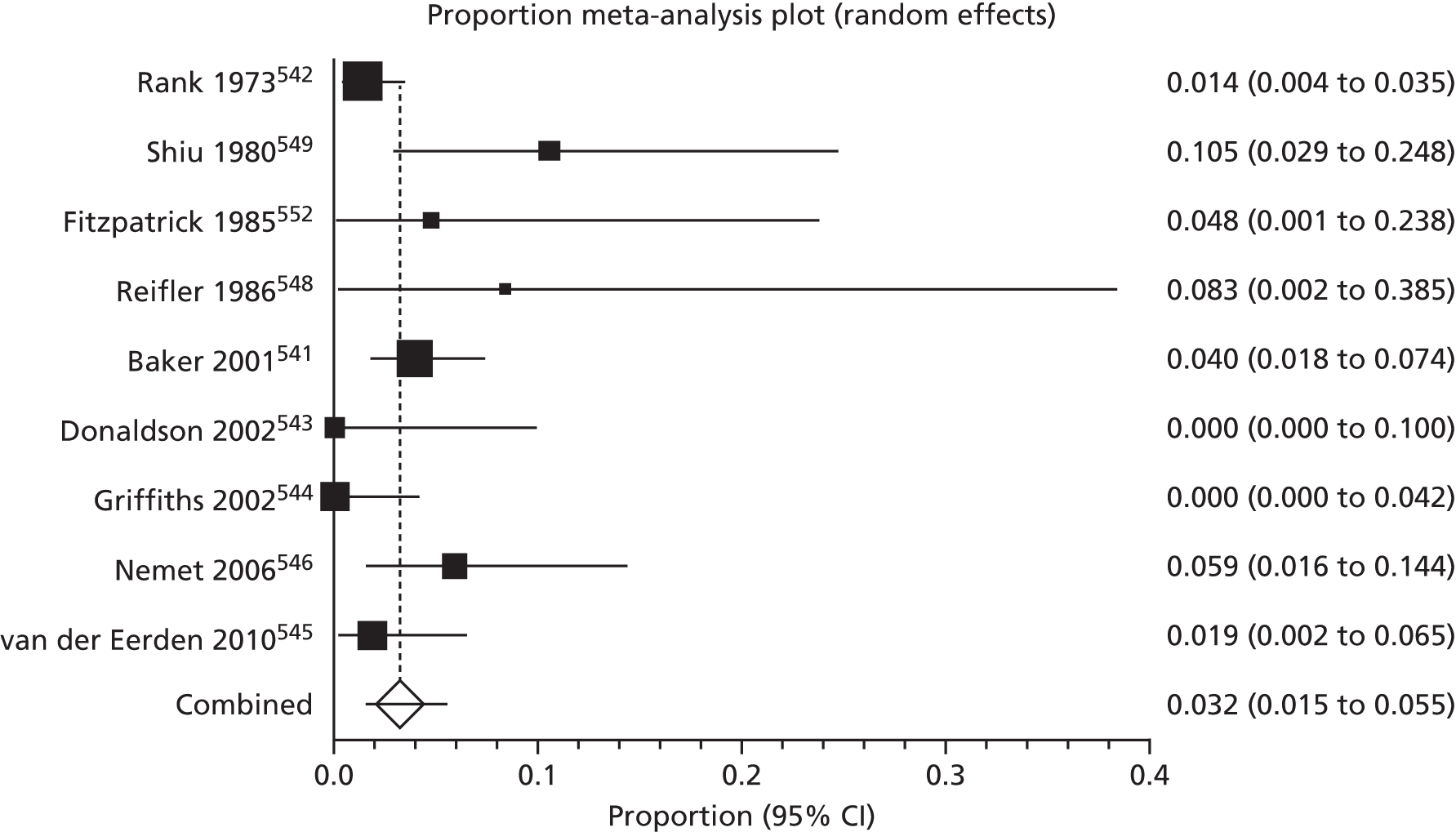
FIGURE 31.
Surgical excision: regional recurrence.
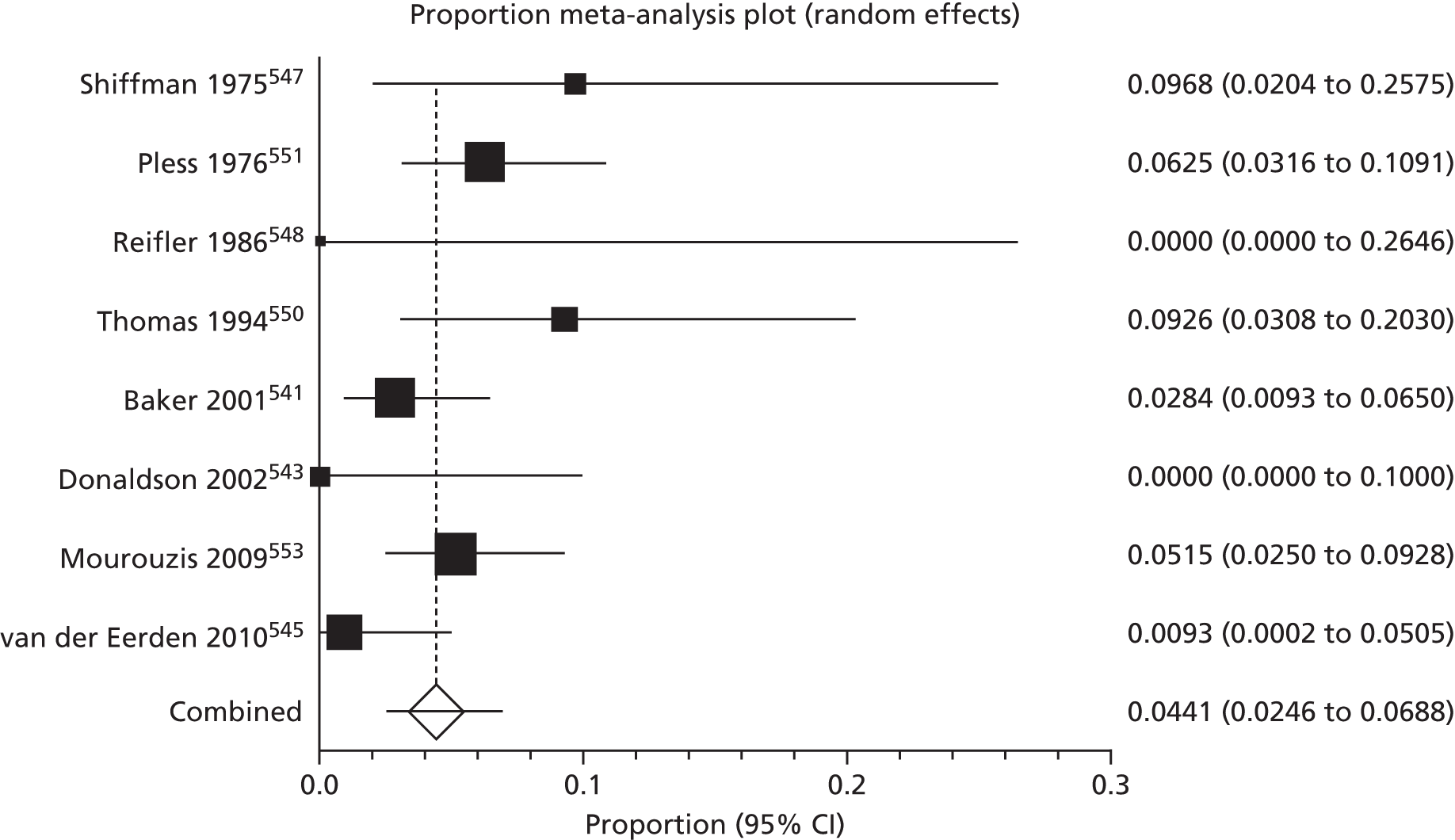
Sensitivity analysis of the four papers meeting our criteria for studies at lowest risk of bias had no significant effect on local recurrence (4.2%, 95% CI 0.6% to 10.8%; I2 = 81.4%). 544–546,550
Recurrence in regional lymph nodes after surgical excision of SCC was reported in eight series (comprising 786 patients), ranging from 0% to 9.7%,541,543,545,547,548,550,551,553 with pooled average recurrence of 4.4% (95% CI 2.4 to 6.9; I2 = 50%) (see Figure 31). Sensitivity analysis in which only the three papers considered at lowest risk of bias were included had no significant effect on regional recurrence (4.6%, 95% CI 1.3% to 10.0%; I2 = 72%). 545,550,553
One study (108 patients)545 had a mean duration of follow-up of < 2 years, with 0.1% recurrence (95% CI 0% to 5.1%). In four studies,541,543,548,553 specified mean duration of follow-up was between 2 and 5 years with pooled average recurrence of 3.6% (95% CI 1.9% to 5.9%; I2 = 11%). None of the studies had mean follow-up of > 5 years and in three papers follow-up duration was either not specified or given as a broad range. 547,550,551
The pooled average regional recurrence in those series in which only SCCs located around the ear were treated was 7.7% (95% CI 4.8% to 11.2%; I2 = 0%),547,550,551 which was substantially greater than the pooled average regional recurrence of 2.9% (95% CI 1.4% to 5.0%; I2 = 27%) for the five remaining studies which included other head and neck locations541,543,545,548,553 (Figures 32 and 33).
FIGURE 32.
Surgical excision: regional recurrence – ear location.
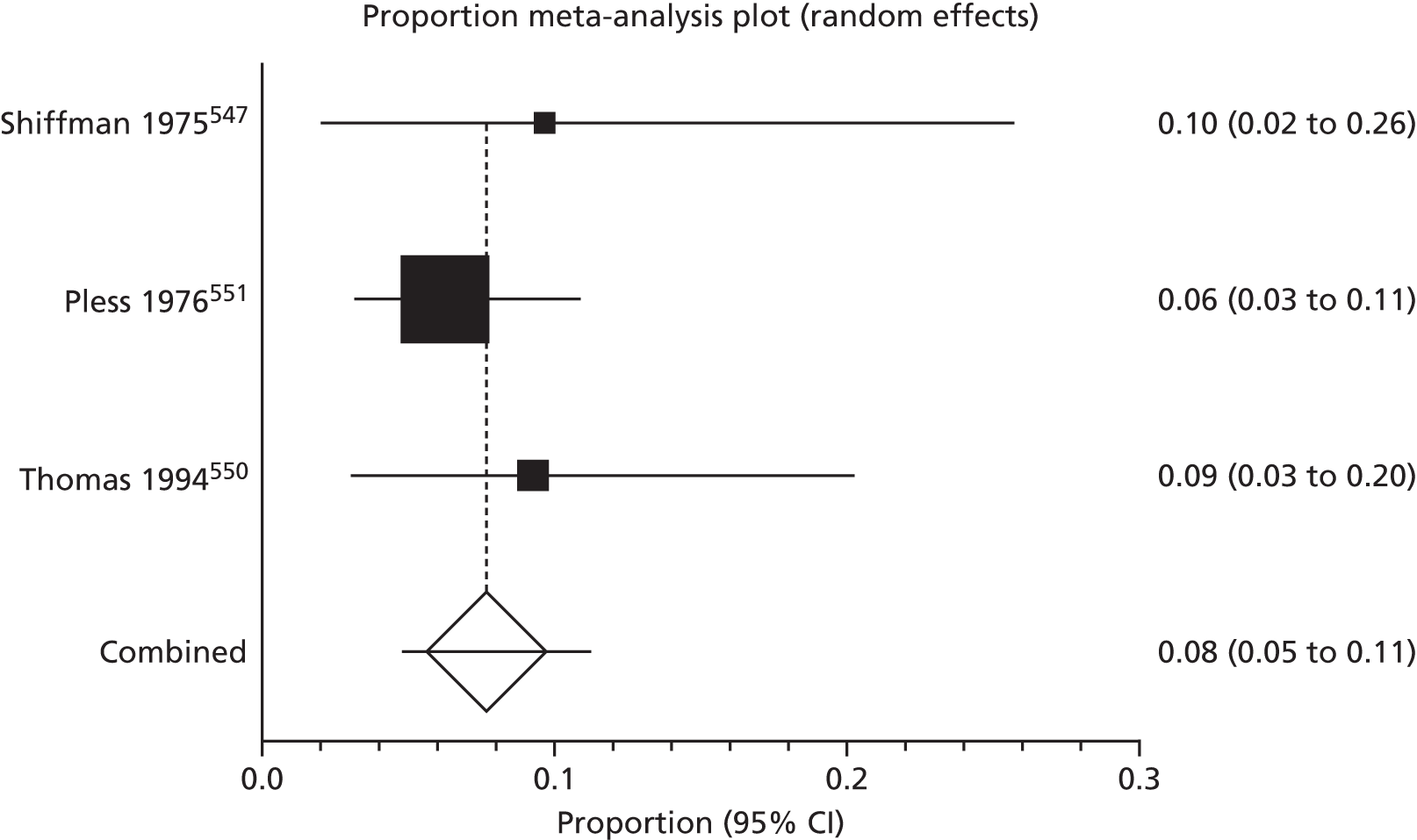
FIGURE 33.
Surgical excision: regional recurrence – non-ear location.
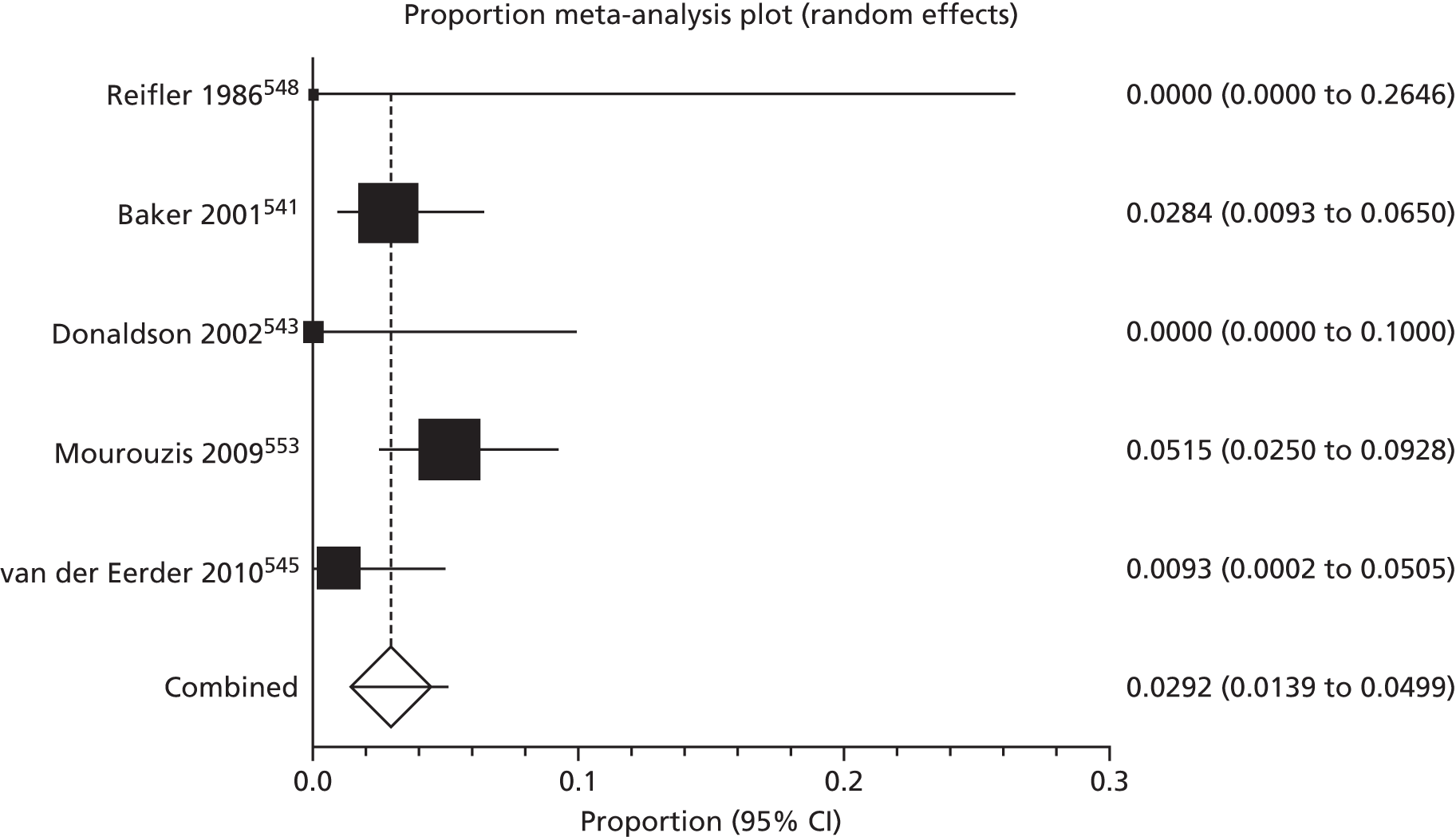
We found two studies that reported distant metastases after surgical excision. Of 211 patients with SCCs at various sites who were followed up for at least a year, only one developed distant metastasis. 554 There were no distant metastases in any of 35 patients with periocular SCC during a mean follow-up period of 31.1 months. 543
In four articles (comprising 146 patients), recurrence was reported but not defined as being local, regional or distant. Two of these studies had mean follow-up periods > 5 years, with pooled average recurrence of 5.4% (95% CI 0.7% to 27.6%). 555,556 There were no reported recurrences in the study in which mean follow-up was < 5 years. 557
The fourth study included 13 patients with stage I or II SCC of the external ear, with a relatively high recurrence of 61.5% (95% CI 31.6% to 86.1%) during follow-up which ranged from 6 months to 20 years. 558
From analysis of eight studies (485 patients) with primary SCC, deaths attributable to disease ranged from 0% to 8.1% during follow-up, with a pooled average of 4.1% (95% CI 1.7% to 7.6%; I2 = 58%)541,543,544,547–550,556 (Figure 34).
FIGURE 34.
Surgical excision: deaths from disease.

Three studies in which the follow-up period was specified as between 2 and 5 years541,543,548 had a significantly lower pooled average of 0.8% (95% CI 0% to 2.5%; I2 = 0%) than the two studies with follow-up of > 5 years from which the pooled average percentage of patients dying from their disease was 8.6% (95% CI 4.7% to 13.6%). 544,556 In three papers, duration of follow-up was not specified or was given as a range only. 547,549,550 No deaths were reported in either of the two included studies in which SCCs of the eyelid were surgically excised. 543,548
Incompleteness of surgical excision was reported in 11 studies (comprising 2343 excisions). Overall, the pooled average estimate of incomplete excisions was 8.8% (95% CI 5.3% to 13.0%; I2 = 89%)541,544,546,550,553,555,559–563 (see Appendix 6 and Figure 35). Definitions of incomplete excision within the studies were not consistent.
FIGURE 35.
Surgical excision: incomplete excision.
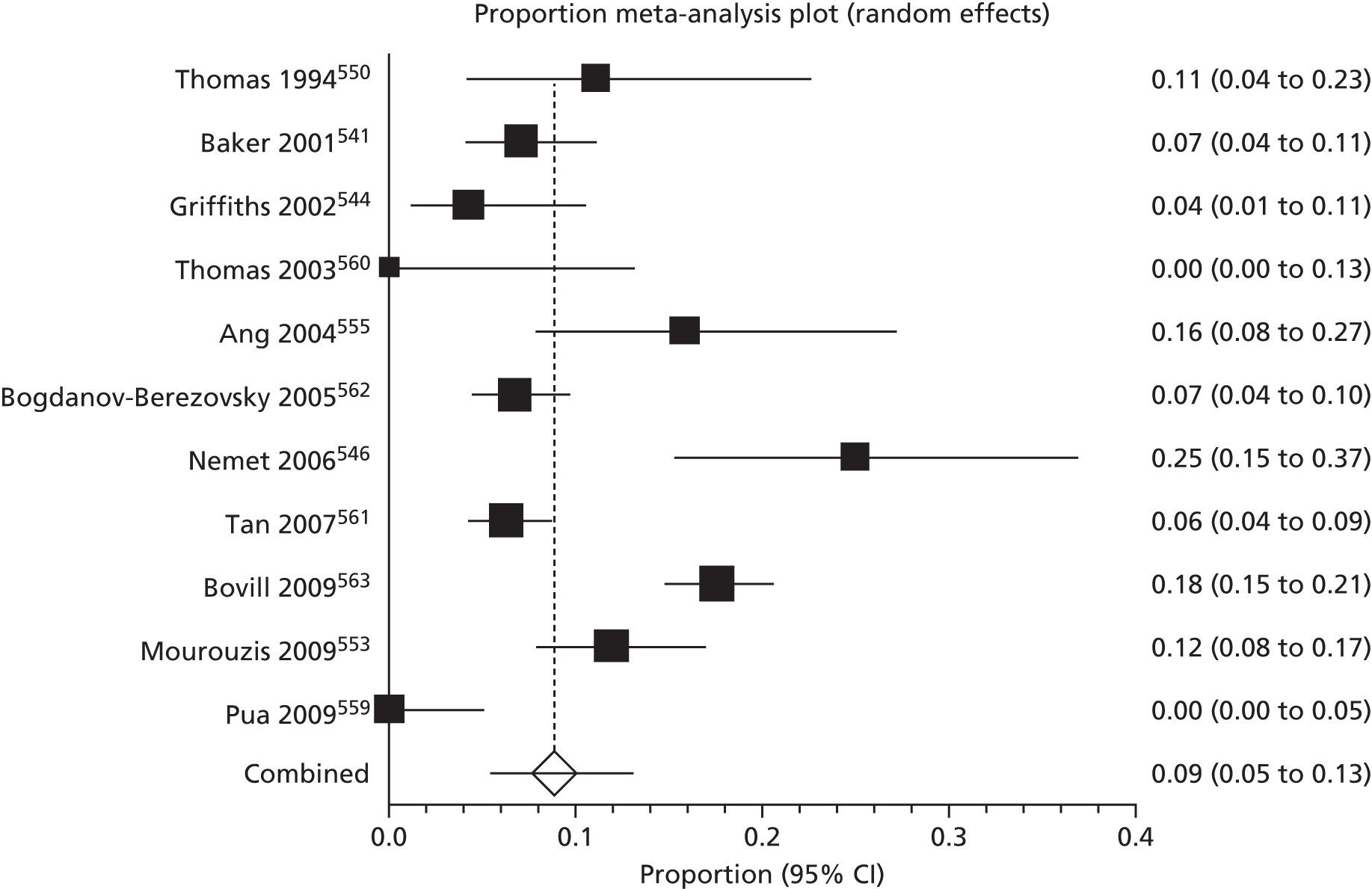
These were based on the presence of tumour cells at the surgical margin,553,559,561,562 the presence of residual tumour at or within 1 mm of the lateral or deep margins of the excised specimen,555 tumour within one microscopic high-power field (0.5 mm)560 and the presence of tumour at or ‘close to’ the margin of the resected specimen. 563 There was variation in the excision margins employed. In one prospective study, margins of 2 mm to > 10 mm were used,561 with 6.2% of tumours being incompletely excised (95% CI 4.2% to 8.8%). In a further prospective series in which none of the SCCs was incompletely excised, excision margins were based on the clinical diagnosis and surgeon’s preference. 560 The other studies assessing incomplete excision were retrospective reviews and in those in which the excision margin was specified, margins between 3 mm and 6 mm were used. 546,553,555,559,562 The highest percentage of incompletely excised tumours were observed after excision of periorbital lesions with a 5-mm margin, with 25% being incompletely excised (95% CI 15.3% to 37.0%). 546
None of the included studies reported SCC specific QoL, cosmetic appearance or adverse event data.
Summary: surgical excision
-
Twelve studies, mostly retrospective case series of limited quality and with follow-up periods which varied between studies.
-
Local recurrence varied owing to different time points when assessed, with average recurrence of 5.4% (95% CI 2.5% to 9.1%; 12 studies, n = 1144).
-
Regional recurrence average estimate 4.4% (95% CI 2.4% to 6.9%; eight studies, n = 786).
-
Ear location significantly associated with local and regional recurrence.
-
Unspecified recurrence average 5.4% (95% CI 0.7% to 27.6%; two studies, n = 113).
-
Death from disease average 4.1% (95% CI 1.7% to 7.6%; eight studies, n = 485).
-
Increased proportion of deaths attributable to disease in studies with follow-up > 5 years compared with follow-up between 2 and 5 years 8.6% (95% CI 4.7% to 13.6%; two studies, n = 149) versus 0.8% (95% CI 0.1% to 2.5%; three studies, n = 223), respectively.
-
Incomplete excision average 8.8% (95% CI 5.4% to 13.0%; 11 studies, n = 2343).
Mohs micrographic surgery
Sixteen studies reported relevant outcomes after MMS. 545,558,564–577 In a seminal series of papers, Mohs reported 5-year cure rates for previously untreated SCCs of 95.7% for SCC of the trunk and extremities;578 96.6% for the ear;579 97.8% for facial, scalp and neck SCCs;578 98.5% for eyelid SCCs;580 and 98.8% for SCCs of the nose. 578 These rates translated to a pooled 5-year cure rate 97.4% for the 2133 SCCs at all sites (95% CI 96.2% to 98.3%; I2 = 48%).
Ten studies reported local recurrence,545,564–572 ranging from 0% (95% CI 0% to 36.9%) in one small study including eight periorbital SCCs,564 up to 5.7% (95% CI 1.9% to 12.9%) in a study of auricular SCCs. 571 For the 10 studies (comprising 1572 participants), the pooled average local recurrence was 3.0% (95% CI 2.2% to 3.9%; I2 = 0%) (see Appendix 7 and Figure 36). Sensitivity analysis, including only the six studies meeting the pre-specified criteria, had no significant impact on local recurrence (2.7%, 95% CI 1.9% to 3.7%; I2 = 0%). 545,565,567–569,572
FIGURE 36.
Mohs micrographic surgery: local recurrence.
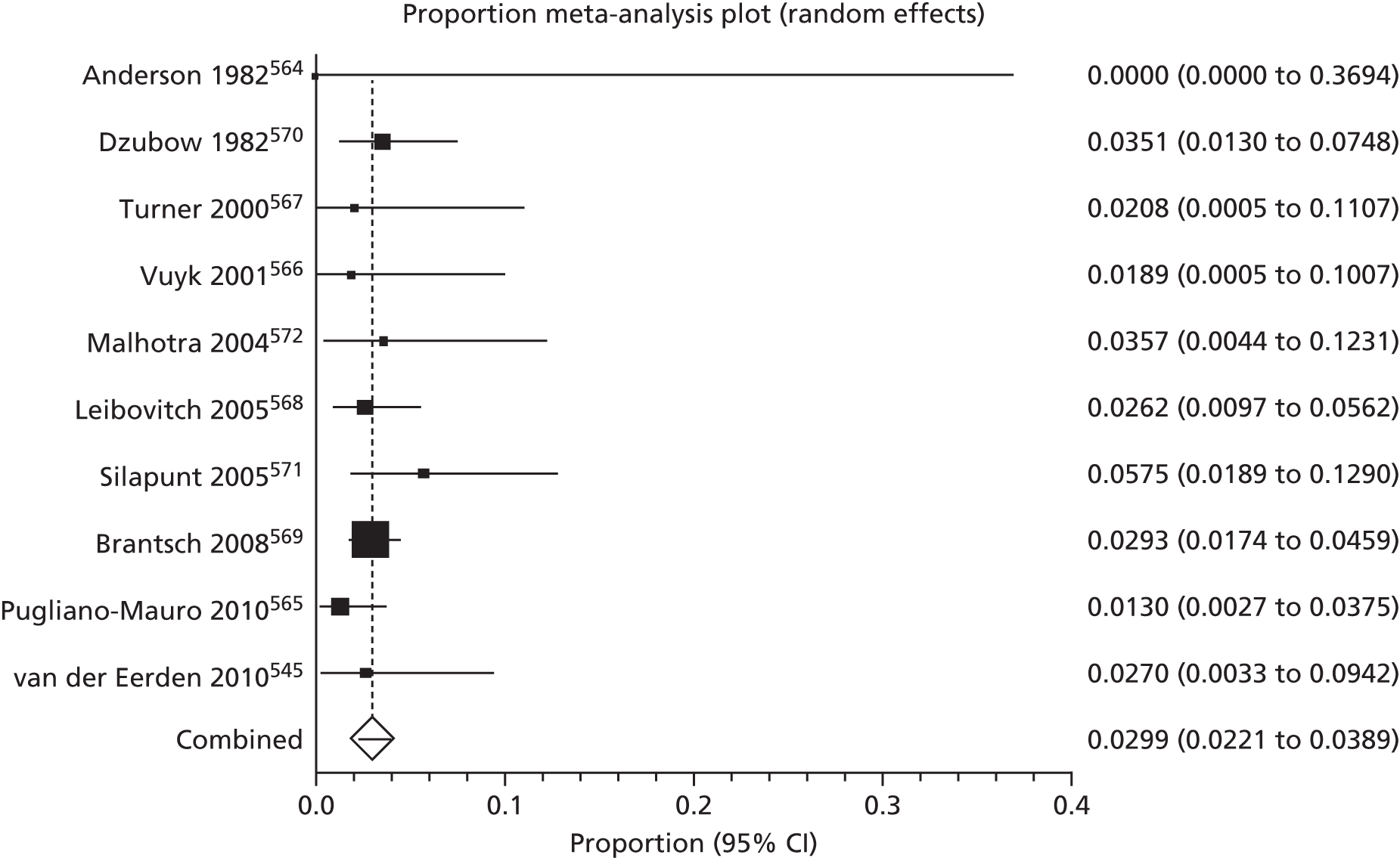
Recurrence in the one study with specified mean follow-up of < 2 years570 was 3.5% (95% CI 1.3% to 7.5%), which did not differ significantly from the average recurrence of 2.8% (95% CI 2.0% to 3.9%; I2 = 0%) in seven studies with mean follow-up of between 2 and 5 years,545,564–567,569,571 and 3.1% (95% CI 1.4% to 5.4%) in the two studies with mean follow-up of > 5 years. 568,572
Six studies reported recurrence in the regional lymph nodes after treatment with MMS. 545,564,565,567,569,573 On pooled analysis (comprising 1162 patients) the average regional recurrence was 4.2% (95% CI 2.3% to 6.6%; I2 = 56%) (Figure 37). There was no significant impact on regional recurrence in the sensitivity analysis, which included only four studies meeting the criteria,545,565,567,569 with average recurrence of 3.2% (95% CI 1.9% to 5.0%, I2 = 29%).
FIGURE 37.
Mohs micrographic surgery: regional recurrence.

Specified mean follow-up was between 2 and 5 years in five studies,545,564,565,567,569 with pooled regional recurrence of 3.4% (95% CI 1.8% to 5.3%; I2 = 34%). None of the studies had mean follow-up of > 5 years.
One study reported no distant metastases during at least 5 years of follow-up in 229 patients treated with MMS. 568 In a case series of 87 auricular SCCs, no distant metastases were reported during a mean follow-up period of 34.6 months. 571 One smaller series of 48 SCCs treated by MMS observed one patient with distant metastasis during a mean follow-up of 3.4 years,567 and a further series including eight patients with periocular SCC also noted one patient with metastases to the lung;564 however, the authors presumed that this patient had subclinical spread of tumour prior to treatment with MMS as there was no evidence of local recurrence.
Five studies (766 patients) did not define recurrence as being local, regional or distant558,574–577 with a pooled average unspecified recurrence of 4.7% (95% CI 0.7% to 11.7%; I2 = 81%) (Figure 38). The highest proportion of unspecified recurrences was seen in a small series of 16 external ear SCCs during follow-up of between 6 months and 20 years, in which 31.2% of tumours recurred (95% CI 11.7% to 58.7%). 558
FIGURE 38.
Mohs micrographic surgery: unspecified recurrence.

Three studies (735 patients) specified mean duration of follow-up as being between 2 and 5 years. 575–577 For these studies, the average unspecified recurrence was 2.2% (95% CI 0.3% to 5.4%; I2 = 61%). The remaining studies558,574 did not specify the mean duration of follow-up.
Four studies with mean follow-up of between 2 and 5 years reported death attributable to SCC,564,565,569,571 with an average of 1.1% (95% CI 0.2% to 2.6%; I2 = 49%) of the 941 eligible patients dying from disease on pooled analysis (Figure 39). One of the included studies reported a relatively high proportion of deaths compared with the other studies. 564 It was a small series of eight patients with periocular SCCs, one of whom developed regional metastases and lung metastases without evidence of local recurrence, indicating that the tumour had spread subclinically prior to treatment.
FIGURE 39.
Mohs micrographic surgery: deaths attributable to disease.
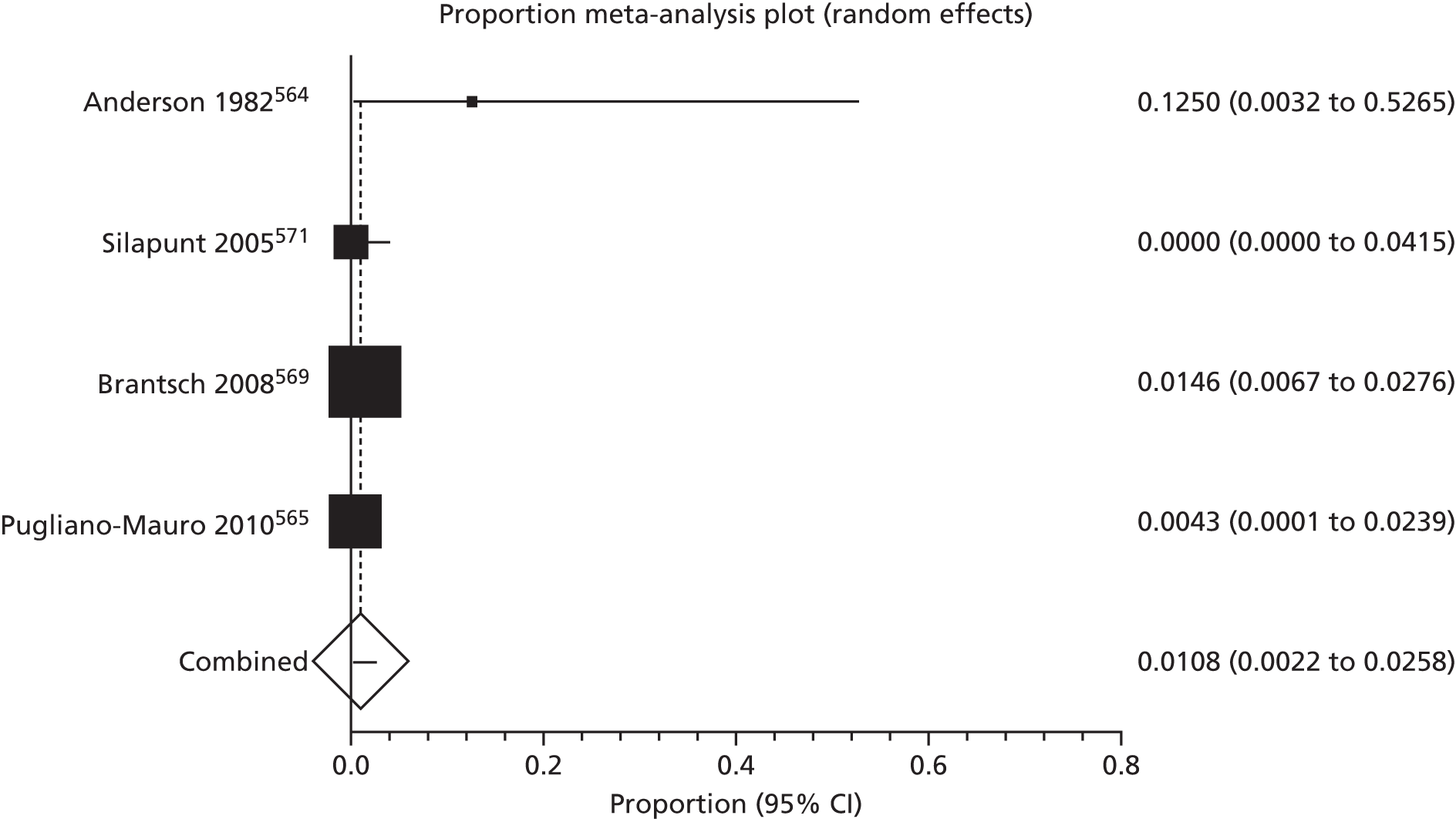
None of the included studies reported separate SCC data for QoL, cosmetic outcomes or adverse events.
Summary: Mohs micrographic surgery
-
Sixteen case series, prospective and retrospective.
-
Local recurrence average 3.0% (95% CI 2.2% to 3.9%; 10 studies, n = 1572).
-
Regional recurrence average 4.2% (95% CI 2.3% to 6.6%; six studies, n = 1162).
-
Unspecified recurrence average 4.7% (95% CI 0.7% to 11.7%; five studies, n = 766).
-
Death from disease average 1.1% (95% CI 0.2% to 2.6%; four studies, n = 941).
External radiotherapy
We found 14, mostly retrospective, studies in which a total of 1018 primary SCCs were treated with external radiotherapy. 542,547,554,581–591 Seven studies (comprising 761 patients) reported local recurrence after external radiotherapy, with pooled average local recurrence of 6.4% (95% CI 3.0% to 11.0%; I2 = 76%)542,547,581,582,584,585,591 (see Appendix 8 and Figure 40). Three studies were included in the sensitivity analysis,582,585,591 with no significant effect on local recurrence (7.3%, 95% CI 2.1% to 15.4%; I2 = 87%).
FIGURE 40.
External radiotherapy: local recurrence.
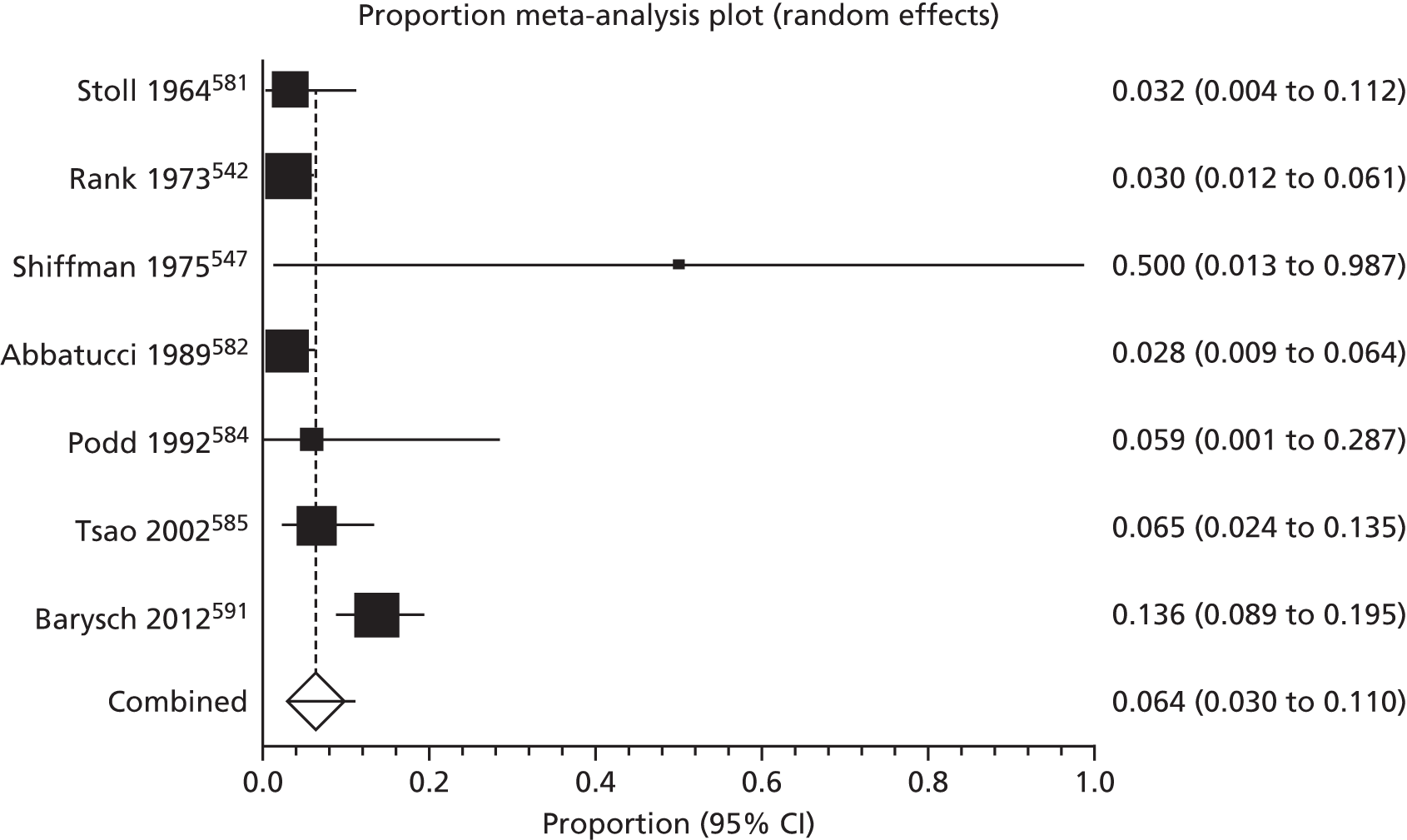
From the four studies for which the mean follow-up period was between 2 and 5 years,542,582,585,591 the pooled average recurrence was 6.1% (95% CI 2.2% to 11.7%; I2 = 85%). None of the studies had mean follow-up of > 5 years and in three studies duration of follow-up was not specified or was given as a broad range. 547,581,584
In one study, location in the ear and scalp region was found to be significantly associated with relapse of tumour compared with other sites (p = 0.025). 591 Age and tumour size were also significantly correlated with risk of relapse in this study (p = 0.012 and p < 0.0001, respectively), with a trend towards better outcome with well-differentiated tumours, although statistical significance was not reached (p = 0.1). Two studies (with 155 patients in total) assessed only nasal SCCs, with a pooled average local recurrence of 5.6% (95% CI 2.6% to 9.7%). 581,585 In a further two small studies (19 patients), which included only SCCs of the pinna, the pooled average local recurrence was 20.3% (95% CI 0.0% to 64.6%),547,584 although the wide CI suggests this is not significantly different from recurrence of nasal SCCs.
Regional lymph node failure was also reported in three studies (comprising 272 patients in total),547,585,591 giving an average regional recurrence of 2.6% on pooled analysis (95% CI 0.04% to 8.9%; I2 = 70%) (Figure 41). In both larger studies,585,591 which included patients with SCCs of the nose and at various sites, respectively, the mean duration of follow-up was between 2 and 5 years. In the third study, there were only two eligible patients with SCC of the pinna, one of whom developed metastasis. 547 Excluding this study from the analysis had no impact on the outcome.
FIGURE 41.
External radiotherapy: regional recurrence.
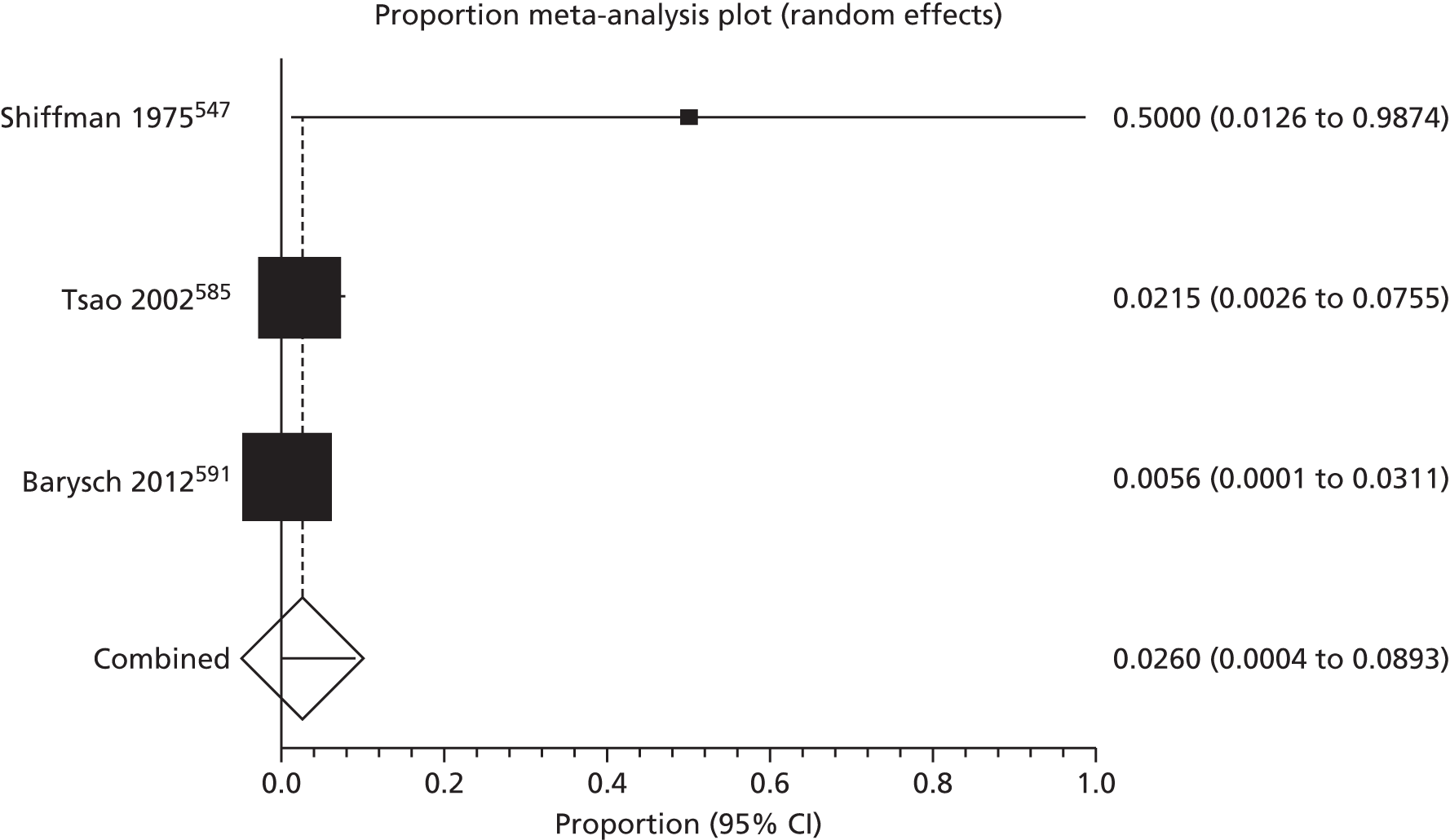
One study reported locoregional recurrence after either local radiotherapy alone, or after local radiotherapy plus radiotherapy to first echelon lymph nodes. 586 Overall recurrence in the 37 SCCs treated with local radiotherapy alone was 30.0% (95% CI 15.9% to 47.0%), ranging from 14.3% (95% CI 0.3% to 57.9%), for the seven tumours classified as T2, to 29.2% (95% CI 12.6% to 51.1%) of the 24 T3 tumours, up to 50% (95% CI 11.8% to 88.2%) for the six T4 tumours. However, with wide overlapping CIs, statistical significance cannot be inferred from these differences. For the five T4 tumours that were treated with local radiotherapy plus nodal radiotherapy, there was one recurrence (20%, 95% CI 0.5% to 71.6%).
Recurrence was not defined as local, regional or distant in a further six studies. 554,583,587–590 Pooled data from the 220 treated SCCs from the studies gave an average recurrence of 4.8% (95% CI 0.6% to 12.8%; I2 = 70%) (Figure 42).
FIGURE 42.
External radiotherapy: unspecified recurrence.
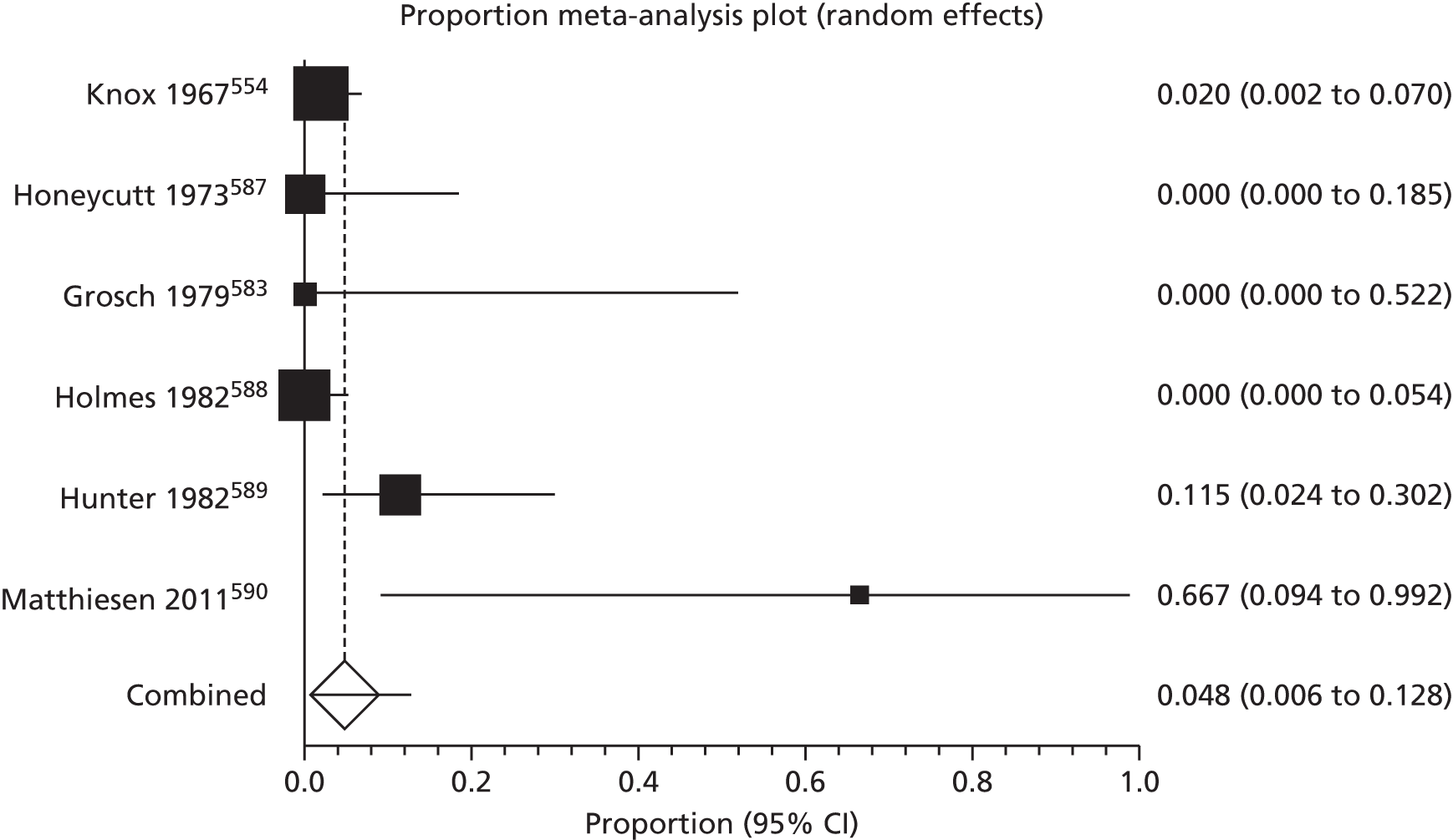
Two of the studies583,590 had a mean duration of follow-up of < 2 years with pooled recurrence of 27.2% (95% CI 2.0% to 89%). However, the number of patients was very small (n = 5) and, in one of the studies,590 only T4 tumours were treated with recurrence in two out of three patients. Average recurrence in the two studies with specified mean duration of follow-up of between 2 and 5 years was 6.1% (44 patients, 95% CI 0% to 22.6%). 587,589 There were no studies in which the mean follow-up period was > 5 years, with unspecified mean follow-up duration in the remaining two studies. 554,588
We found five studies including 191 patients that reported deaths as a result of SCC,547,585,588–590 with an average of 9.1% of patients dying from their disease on pooled analysis (95% CI 1.4% to 22.8%; I2 = 79%)23,67,70–72 (Figure 43). The greatest proportion of deaths was observed in a study of advanced T4 tumours in which two of three patients with eligible SCCs died (66%, 95% CI 9.4% to 99.1%)590 during a mean follow-up period of 14 months. For studies with mean duration of follow-up between 2 and 5 years, the average recurrence was 4.8% (119 patients, 95% CI 1.6% to 9.8%). 585,589 None of the studies had mean duration of follow-up > 5 years.
FIGURE 43.
External radiotherapy: deaths from disease.
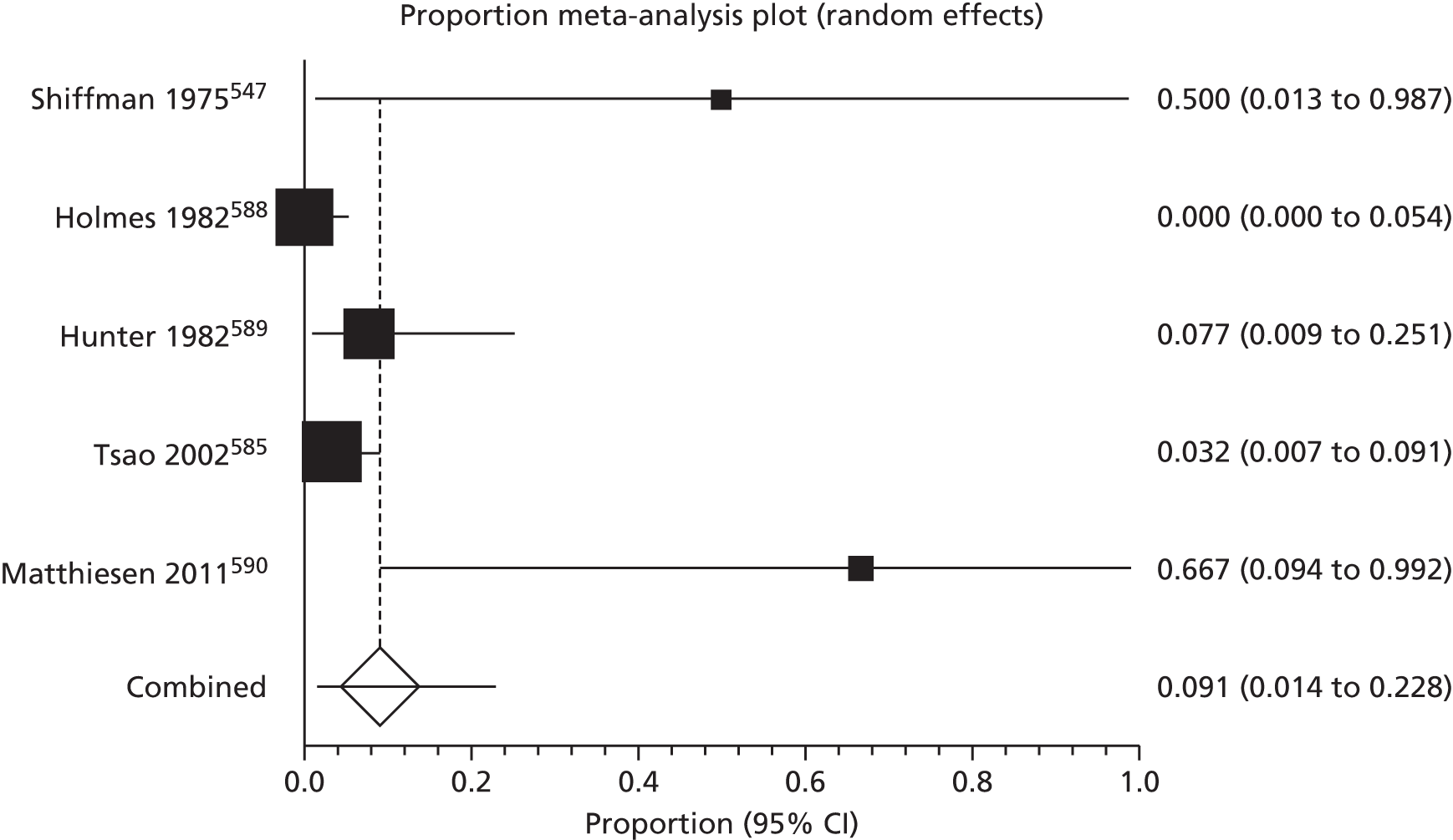
The SCC-specific data for cosmetic appearance and adverse events were not available from any of the included studies.
Summary: external radiotherapy
-
One prospective and 13 retrospective series.
-
Variation between studies for radiation source and length of follow-up.
-
Local recurrence average 6.4% (95% CI 3.0 to 11.0, 7 studies; n = 761).
-
Regional recurrence average 2.6% (95% CI 0.04 to 8.9, 3 studies; n = 272).
-
Unspecified recurrence average 4.8% (95% CI 0.6 to 12.8, 6 studies; n = 220).
-
Death from disease average 9.1% (95% CI 1.4 to 22.8, 5 studies; n = 191).
Brachytherapy
Six studies (comprising 88 SCCs) reported recurrence after brachytherapy592–597 (see Appendix 9 and Figure 44), giving a pooled average local recurrence of 5.2% (95% CI 1.6% to 10.5%; I2 = 0%). Of these, four were prospective reports (35 SCCs)592,594,595,597 and two (53 SCCs) were retrospective,593,596 with varying follow-up periods from an average of 9.6 months595 up to a median of 55 months. 593
FIGURE 44.
Brachytherapy: regional recurrence.
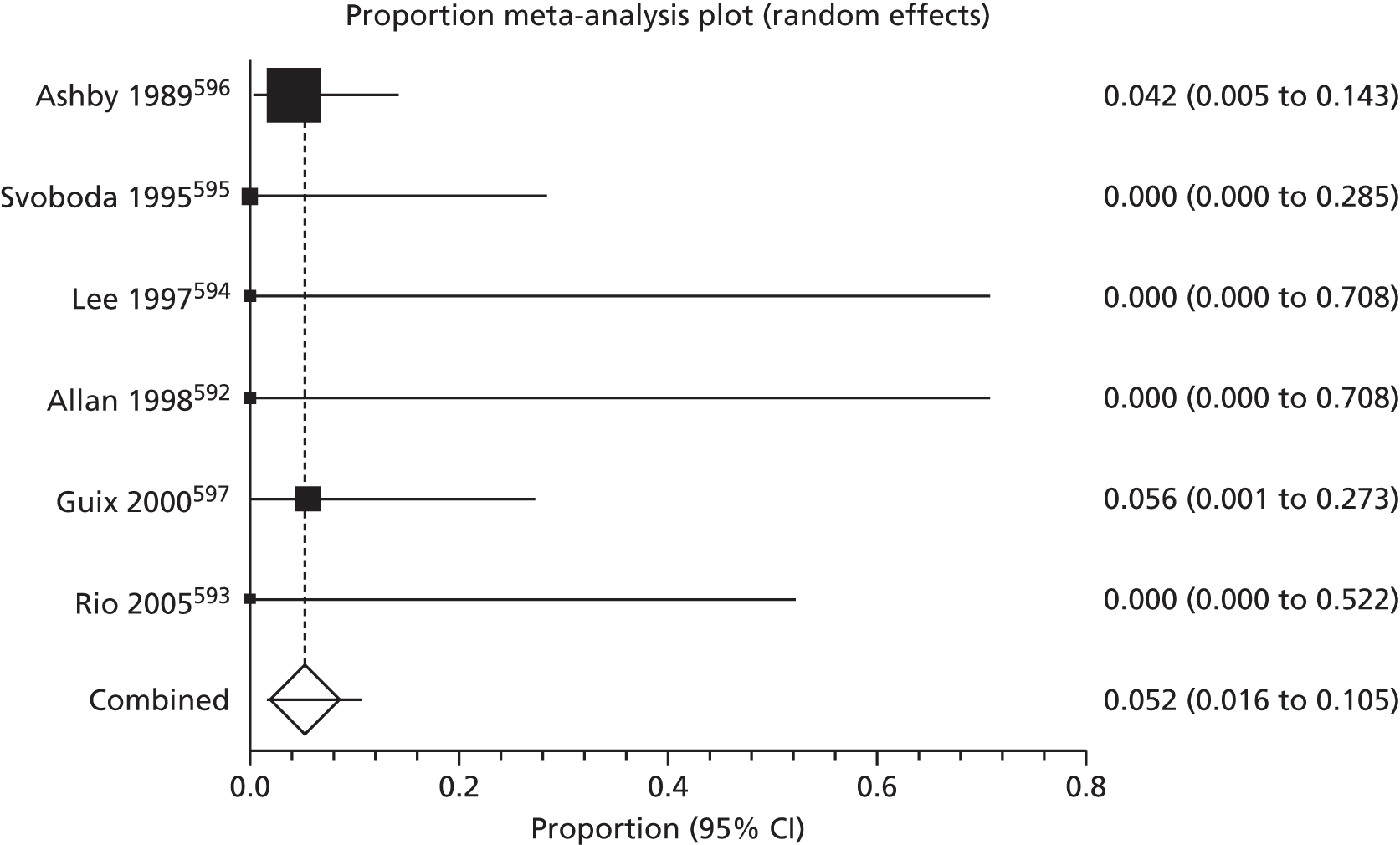
Four studies had no recurrences during follow-up. 592–595 In the largest study, a retrospective review in which 48 SCCs at various sites were treated with a superficial radon mould, there were two local recurrences of hand and scalp SCCs at 10 and 6 months, respectively. 596 The other reported SCC recurrence occurred 23 months after high-dose rate brachytherapy with a 192Ir surface mould and was a 4-cm tumour located on the frontal area. 597 No patients in this study developed regional or distant metastases after treatment.
One study reported that 4 out of 48 (8.3%) SCCs treated with a radon mould persisted after initial treatment and required treatment by other methods to ablate the lesions. 596 The study authors attributed their high failure rate to the inclusion of tumours with a high volume or, when the thickness was > 4 mm, to inappropriately treating with brachytherapy.
None of the included studies reported on deaths attributable to disease. Furthermore, SCC-specific data for cosmetic appearance and adverse events were not available from any of the included studies.
Summary: brachytherapy
-
Four prospective, two retrospective studies.
-
Variable methods of application and radiation and generally short follow-up periods.
-
Generally small numbers of patients.
-
Local recurrence average 5.2% (95% CI 1.6% to 10.5%; six studies, n = 88).
-
No regional or distant metastases or deaths attributable to disease reported.
Adjuvant radiotherapy
We included nine studies in which ART was used with surgery to treat previously untreated SCCs which were non-metastatic at presentation. (See Appendix 10 for details of these studies and pooled outcome data.)
The ART was administered for perineural invasion in five retrospective studies (comprising 22 patients). 598–602 In one of these studies,600 local recurrence occurred in two out of six patients with asymptomatic perineural invasion in nerve branches of 0.4 mm diameter. All excised SCCs had clear surgical margins of at least 3 mm. One of these patients also had regional metastasis and the other had distant metastasis after treatment. Metastasis to the skull 1 year after treatment was reported in one patient with symptomatic perineural invasion affecting the supraorbital nerve in a further series. 599 In the other three studies, two of which included patients with asymptomatic perineural invasion in unnamed nerves598,601 and one in which there was involvement of named cranial nerves,602 there were no reports of recurrence following treatment during follow-up ranging from 10.4 months to 104.8 months.
Four studies (47 patients) reported outcomes after ART for SCCs other than those with perineural invasion. These included patients with pinna SCCs,547 trunk and extremity SCCs603,604 and those with aggressive SCCs post cardiothoracic transplant. 605 The basis on which patients were selected to receive ART as opposed to surgical monotherapy was not clearly identified in any of the studies. Three of the included studies were retrospective547,604,605 and the other was a prospective assessment of ART to draining lymph nodes in a group of patients with trunk and extremity SCCs (50% of which developed in an area of erythema ab igne). 603 ART was administered to the draining regional lymph nodes in both included studies of trunk and extremity SCC. 603,604 The irradiation field was not specified in the other studies. 547,605 Three out of the four studies reported recurrence after treatment during follow-up ranging from < 1 year to > 3 years. Three patients out of 26 (12%) developed local recurrence 6–12 months after treatment in the included prospective study, with regional recurrence in one patient. No distant metastases were reported during follow-up of up to 12 months. 603 Local recurrence was also reported in two out of six patients who developed SCC after cardiothoracic transplantation, one of whom also developed regional recurrence. A further patient in this series also had a ‘systemic’ relapse despite local control of their SCC. 605
One study reported two deaths (of four eligible patients) attributable to SCC at 6 and 11 months post treatment for perineural invasion involving named cranial nerves. Both patients had intracranial disease extending through a peripheral foramen but had refused an intracranial operation. 602 No deaths attributable to SCC after ART treatment for perineural invasion were reported in any of the remaining three studies (16 patients). 598,600,601
Three studies (comprising 21 patients) addressing ART of other SCCs had data on patient deaths, with one reporting the death of three patients out of six who had post cardiothoracic transplant SCCs between 8 months and 54 months after diagnosis. 605 No deaths were reported in the other studies,547,604 which included patients with trunk and extremity SCCs, and those with SCC of the pinna (pooled data are presented in Appendix 10).
In one study, initial failure of wide local excision and ART to control disease locally was reported in one patient (out of six),605 who died 15 months after treatment.
Mild erythema, dry and moist desquamation and alopecia of hair-bearing areas in the irradiated field after ART were the most commonly reported adverse events in included studies. 598,599,603 Single adverse events recorded were wound infection and serous otitis media,598 self-limiting mucositis, radiation dermatitis and residual mild xerostomia,601 and reactive lymphoedema of the leg. 603
Summary: adjuvant radiotherapy
Five small retrospective studies:
-
Local recurrence average 18.2% (95% CI 3.8% to 39.8%, five studies;598–602 n = 22).
-
Regional recurrence average 8.3% (95% CI 1.1% to 21.4%, five studies;598–602 n = 22).
-
Distant metastasis average 11.5% (95% CI 2.4% to 26.1%, five studies;598–602 n = 22).
-
Death from disease average 11.1% (95% CI 0.4% to 33.1%, four studies;598,600–602 n = 20).
One prospective,603 three retrospective small studies:547,604,605
-
Local recurrence average 11.1% (95% CI 2.4% to 25.0%, four studies;547,603–605 n = 47).
-
Regional recurrence average 8.5% (95% CI 2.5% to 17.6%, four studies;547,603–605 n = 47).
-
Distant metastasis average 3.2% (95% CI 0.1% to 10.4%, four studies;547,603–605 n = 47).
-
Death from disease average 13.9% (95% CI 0.04% to 50.2%, three studies;547,604,605 n = 21).
Curettage and electrodesiccation
Details of the included studies are shown in Appendix 11. Only one small retrospective study of 15 patients with SCC of the pinna described local and regional recurrence separately after treatment by curettage and electrodesiccation. 547 Of the 15 patients included, three had local recurrence (20%), of whom one (7%) developed regional disease and two died as a result of their disease.
Seven studies (comprising 1131 patients) that included SCCs from various sites reported on recurrence after curettage and electrodesiccation, but did not specify the nature of the recurrence. 554,557,587,606–609 On pooled analysis, average recurrence was 1.7% (95% CI 0.6% to 3.4%; I2 = 59%) (Figure 45). We did not perform a sensitivity analysis as none of the studies met the criteria for this.
FIGURE 45.
Curettage and electrodesiccation: unspecified recurrence. a, triple cycle of curettage and electrodesiccation; and b, double cycle of curettage and electrodesiccation.
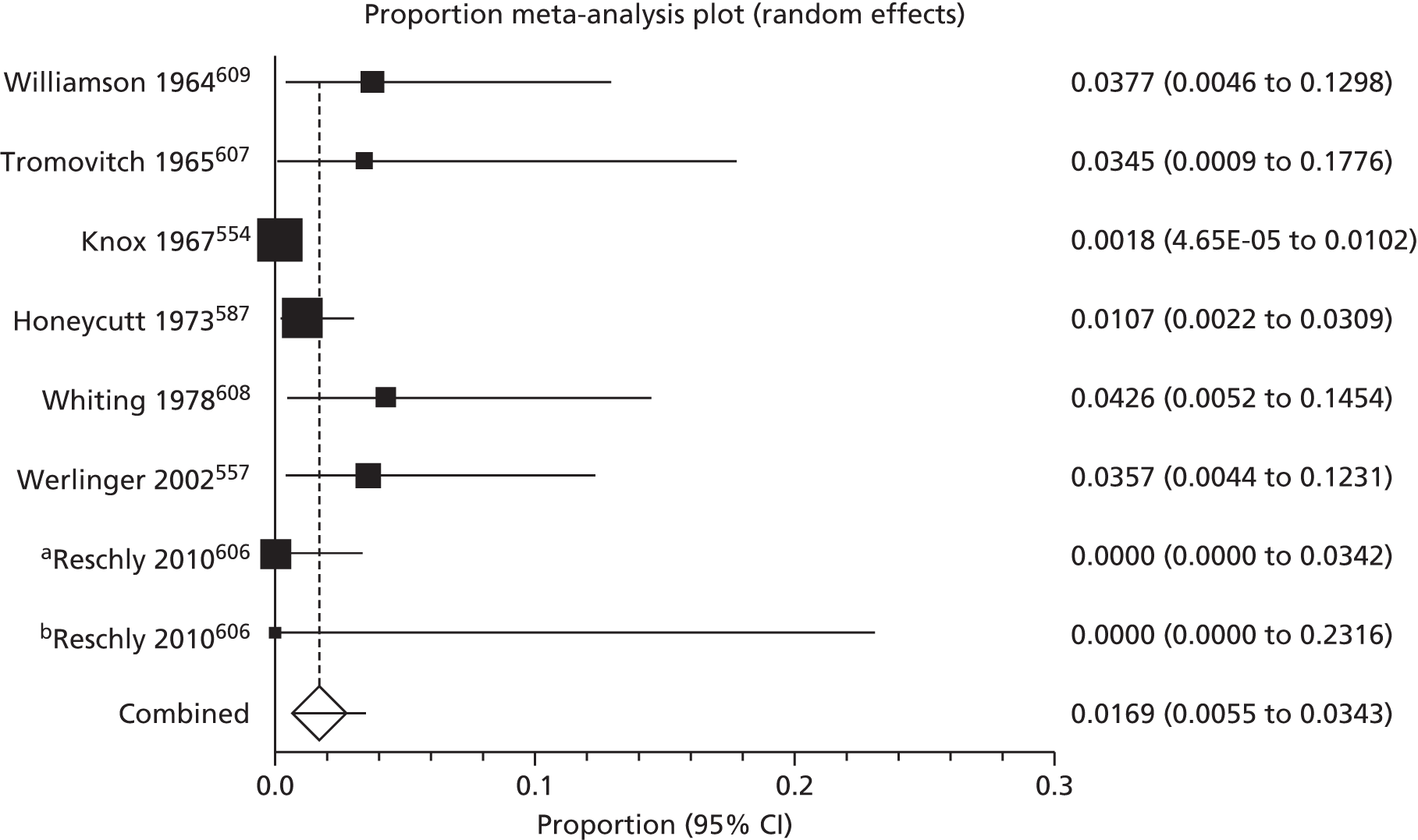
For the two studies557,609 with specified mean follow-up periods of between 2 and 5 years, the pooled recurrence was 4.5% (109 patients, 95% CI 1.4% to 9.0%). Only one study607 had a mean follow-up of > 5 years, with recurrence in 1 out of 29 patients (3.4%, 95% CI 0% to 17.8%). The remaining studies did not specify mean duration of follow-up.
Most of the treated SCCs in these series were small, with a total of 91% having a diameter of < 2 cm in the studies in which data about diameter were provided. 554,587,606,609 Increased lesion size as a significant prognostic feature was observed in one study and recurrence in the 17 SCCs > 2 cm was 11.8% (95% CI 1.4% to 36.4%) compared with 0.4% (95% CI 0.0% to 2.1%) in the 264 SCCs < 2 cm. 587 One study separated results according to the number of treatment cycles used with no recurrences after either two or three cycles. 606 Two studies specified the number of cycles of electrodesiccation as either double607 or triple,608 but this information was not reported for the remaining studies.
Cosmetic outcome was reported in just one of the included studies (41 patients)608 and rated as ‘good’ in 29% of SCCs, ‘satisfactory’ in 54% or ‘poor’ in 17%, although no definition of each of these terms was provided and it was unclear how soon after treatment the assessment of cosmesis was made.
None of the included studies reported adverse event data.
Summary: curettage and electrodesiccation
-
Eight retrospective series of variable follow-up periods.
-
Treated SCCs mostly < 2 cm diameter.
-
Unspecified recurrence average 1.7% (95% CI 0.5% to 3.4%; seven studies, n = 1131).
-
A total of 20% recurrence after curettage and electrodesiccation of pinna SCC (one study, n = 20).
-
Lesion size of > 2 cm shows significantly greater average recurrence than those sized < 2 cm: 11.8% (95% CI 1.4% to 36.4%, 17 SCCs) versus 0.4% (95% CI 0.0% to 2.1%; 264 SCCs, one study).
Cryotherapy
There were eight studies (comprising 273 patients) that described recurrence after cryotherapy. 610–617 Only one of these reported a case of recurrence after cryotherapy,617 from a study population of 34 patients with SCCs at any site who were treated with a double freeze–thaw cycle using liquid nitrogen. Data from the 273 patients in the eight studies gave a pooled average recurrence of 0.8% (95% CI 0.1% to 2.2%; I2 = 0%) (Figure 46). (See Appendix 12 for details of prognostic features of the SCCs that were included.) Sensitivity analysis was not conducted as only one study met our pre-specified criteria,616 with no reported recurrences in 53 patients (0% recurrence, 95% CI 0% to 6.7%).
FIGURE 46.
Cryotherapy: unspecified recurrence.
In five studies,610,611,613,615,616 the mean duration of follow-up was between 2 and 5 years, with pooled average recurrence of 0.4% (221 patients) (95% CI 0% to 1.7%; I2 = 0%). None had mean follow-up of > 5 years and for three studies,612,614,617 follow-up was given as a range only.
An overall cure rate of 97% was reported after either a single or double freeze–thaw cycle with liquid nitrogen in a retrospective series of 563 SCCs at any site that were treated over a 23-year period. 618 The authors did not define ‘cure’, so this rate may include lesions that failed to respond to the initial treatment in addition to those which recurred. The duration of follow-up was not specified.
Failure to respond to initial treatment was reported in one patient out of 34 (3%) in one prospective series. 617 A double freeze–thaw cycle was used to treat the original SCC, a 5-mm lesion on the scalp, which showed little clinical response despite a second course of cryotherapy 2 months after the initial treatment.
None of the studies in which cosmetic appearance and adverse events were reported separated results obtained for SCCs and BCCs treated by cryotherapy, but presented results for NMSCs as a whole.
Summary: cryotherapy
-
Six prospective and three retrospective series with variable follow-up periods.
-
Mostly < 2 cm diameter, low-risk lesions.
-
Recurrence average 0.8% (95% CI 0.1% to 2.2%; eight studies, n = 273).
Photodynamic therapy
There were 14 small prospective studies (comprising 297 patients) which evaluated the response of SCCs to photodynamic therapy619–632 (see Appendix 13). Three studies separated SCCs according to level or depth of invasion619,622,625 and one was a non-randomised two-arm comparison of topical photodynamic therapy either with or without a 5% glycolic acid penetration enhancer. 624 On pooled analysis, an average of 72.0% of treated lesions appeared to respond completely to treatment (95% CI 61.5% to 81.4%; I2 = 71%) (Figure 47). Five studies specified that histological assessment of at least some of the treated areas was done to confirm apparent clinical response. 620,622,629,631,632
FIGURE 47.
Photodynamic therapy: apparent complete response. a, elevated; b, early invasive; c, nodular; d, superficial; e, no glycolic acid; f, plus glycolic acid; g, invasive; and h, microinvasive.

In eight of the included studies, SCCs that had apparently completely responded to photodynamic therapy initially were observed for recurrence. 621–623,625,629–632 Pooled recurrence data from these studies (119 SCCs) gave an odds of recurrence of 26.4% (95% CI 12.3% to 43.7%; I2 = 72%) (Figure 48). The results are summarised in Appendix 13. Mean duration of follow-up ranged from 6 months (at which time the trial was abandoned owing to recurrence in more than 50% of lesions)629 to 38 months. 621
FIGURE 48.
Recurrence after apparent complete response of SCCs following photodynamic therapy. a, ‘Superficial’ SCCs; b, ‘nodular’ SCCs; c, ‘microinvasive’ SCCs; and d, ‘invasive’ SCCs.
One study evaluated cosmetic appearance on a scale of 1–4 (excellent to poor) at two time points (3 months and 24 months) after treatment,625 with high agreement between patient and investigator scores for both. At 3 months, 4% of 46 microinvasive- (Clark level II) and invasive-treated (Clark level III/IV) SCCs were assessed as having ‘excellent’ cosmetic appearance, with 48% ‘good’, 44% ‘fair’ and 4% ‘poor’. By 24 months, of 31 assessable treated lesions, 6% were rated as being of ‘excellent’ cosmetic appearance, 36% ‘good’, 48% ‘fair’ and 10% ‘poor’. Tumour thickness, depth of dermal penetration and the degree of cell atypia were found by the authors to be univariate predictors of outcome (Kruskal–Wallis test; p < 0.01). One smaller study also evaluated cosmetic appearance on a scale of 1–4 (very good to poor), with five treated lesions (55.6%, nine patients) being assessed as having very good appearance, three (33%) as good and one (11%) as fair. 632 None was deemed have poor appearance in this study. Two further studies described ‘very satisfactory cosmetic results’624 or ‘very good’ with no scar formation and only transient residual hypo- or hyperpigmentation. 621
Separate SCC data for adverse events were not available from the included studies.
Summary: photodynamic therapy
-
Fourteen small prospective case series.
-
Histological confirmation of apparent initial clinical response sought in 5 out of 14 studies.
-
Follow-up for recurrence in 8 out of 14 studies.
-
Apparent initial complete response average 72.0% (95% CI 61.5% to 81.4%; 14 studies, n = 297).
-
Recurrence after apparent initial complete response average 26.4% (95% CI 12.3% to 43.7%; eight studies, n = 119).
Treatments with less robust data
Laser therapy
One retrospective study examined the treatment of 86 facial SCCs (excluding eyelid carcinomas) with neodymium laser irradiation at a total dose ranging from 118 J to 3520 J. 633 Patients were followed for a mean follow-up period of 8.2 years (range 5–11 years). Out of a total of 3275 patients (all NMSCs) treated by neodymium laser during the study inclusion period, 438 (13.4%) were not followed up during the first 5 years. Overall, there were four recurrences in the remaining 86 SCC patients (4.7%). Of the 48 tumours < 1 cm in diameter, one recurred (2.1%, 95% CI 0.05% to 11.1%) compared with 6.4% of the 31 tumours sized between 1 and 2 cm in diameter (95% CI 0.8% to 21.4%), and 14.2% (95% CI 0.4% to 57.9%) for the seven SCCs > 2 cm in diameter. However, with wide and overlapping CIs, these differences were not statistically significant.
Death from disease was not reported in this study.
One year post treatment, 65% of treated areas was assessed as having ‘good’ cosmetic appearance (lesion not visible) and 35% as ‘acceptable’ (slightly visible scarring, redness or depigmentation) by a clinician. By year 3, 74% of areas were graded as having good appearance and the remainder were acceptable.
Most of the observed effects in this study occurred in the first few days post radiation, most commonly reactive hyperaemia, oedema and slight soreness which were mild in severity and transient. No systemic adverse events were noted.
Topical imiquimod
We found nine papers reporting the use of topical imiquimod to treat SCCs eligible for this review. There was one prospective case series that included three patients with four SCCs,634 one retrospective case series in which there was one eligible patient with SCC635 and the remainder were case reports of one or two patients. 636–642
Outcomes after treatment for these studies are summarised in Table 54.
| Study | Type of study (number of eligible patients) | Dose | Initial response | Follow-up | Recurrence | Adverse events | Cosmesis |
|---|---|---|---|---|---|---|---|
| Peris et al. 2006634 | Open-label trial [three patients/four SCCs (temple, inner canthus, leg, forehead), all unsuitable surgical candidates] | o.d./5× per week/8–12 weeks | 4/4 complete clinical regression. No histologically evident tumour on post-treatment biopsies | Mean 25 months (range 24–27 months) | None | Erythema (3/3); erosion (2/3); pruritus (3/3); burning (1/3); hypopigmentation (1/3); ulceration (1/3). No systemic adverse events | – |
| Ross et al. 2010635 | Retrospective case series (one SCC of upper eyelid) | 5×/week initially, decreased to 2×/week owing to irritation and chemical conjunctivitis | Complete clinical regression at 3 months (not confirmed histologically) | 6 months | None in 6 months | Skin irritation and chemical conjunctivitis resolved when frequency of application decreased | – |
| Eklind et al. 2003636 | Case reports (two renal transplant patients – temple and sternum) | Self-applied 3× per week/12 weeks | Part 1. No evidence of SCC on 6 month biopsy | 6 and 8 months | None at 6/8 months | Part 1. Some scaling and scar at initial site at 16 weeks | – |
| Part 2. ‘Free of cancer’ at 8 months | Part 2. Encrusted and inflammatory erythema 5 weeks post treatment. Erythema at week 12 gradually subsiding | ||||||
| Flórez et al. 2004637 | Case report (SCC of leg, surgery refused) | Under occlusion every other day for 8 hours/8 weeks | No histological evidence of SCC in excised residual papule at 2 months | 12 months | None | Local erythema, superficial erosive changes, discomfort. No systemic adverse events | – |
| Konstantopoulou et al. 2006638 | Case report (one patient/three SCCs foot and lower leg, surgery refused, radiotherapy considered poor option) | 3× per week for 8–12 hours initially then increased to 5× per week/19 weeks or no clinical evidence of residual tumour at sites showing response | Complete clinical response in 2/3 SCCs at 2 weeks with no histological evidence of invasive SCC on biopsy. One SCC failed to respond (excised) | 16 months | No recurrence in two SCCs showing complete response initially | N/A | N/A |
| Martín-García 2005639 | Case report (nasal SCC, surgery refused) | Daily/2 weeks then 5×/week. Total duration 12 weeks | Complete clinical disappearance confirmed histologically 2 weeks post treatment | 1 year | No local or regional recurrence | N/A | N/A |
| Nouri et al. 2003640 | Case report (invasive superficial SCC of nasal tip, other treatments refused) | Self-applied o.d./6 weeks total (2 week break owing to irritation) | No visible or histological SCC 1 month post treatment | 4 weeks | N/A | Irritation and crusting midway through treatment necessitating treatment break. No visible erythema post treatment | ‘Cosmetically pleasing’ – no fibrosis, scarring, discolouration, residual erythema |
| Oster-Schmidt 2004642 | Case reports (two patients with ear lobe and upper leg SCCs unsuitable for surgery) | o.d./5× per week for 2 weeks | Histological clearance on 3-month post-treatment biopsy for both SCCs | 21 months and 8 months | No clinical evidence of recurrence at 21 months or 8 months (patient died of unrelated cause) | No adverse events | ‘Remarkable improved cosmetic result’ |
| Oster-Schmidt and Dirschka 2005641 | Case report (one patient, SCC of back of hand, other treatments refused) | o.d. for 4 weeks initially, repeated at 6 months | Histological clearance 4 weeks after initial course | 4 years | No recurrence | Oedema and mild burning. No systemic adverse events | ‘Excellent’ |
Post-treatment complete response was observed in all patients in eight of the studies (comprising 12 patients),634–637,639–642 with histological confirmation of clearance in all but one study. 635 One case report of topical imiquimod use observed no response in one of three foot and lower leg SCCs in the same patient. 638 All the studies apart from one640 followed patients for recurrence for varying periods ranging from 6 months to 4 years, with no reported recurrences. None of the studies reported on deaths attributable to disease.
Skin irritation was commonly reported,634–637,640,641 with chemical conjunctivitis reported in one patient with periocular SCC. 635 No systemic adverse events were reported.
5-Fluorouracil
We found four studies in which single-agent 5-FU was used to treat eligible SCCs, two of which related to intralesional treatment643,644 and two to topical administration of 5-FU645,646 (Table 55).
| Study | Type of study (number of eligible patients) | Dose | Initial response | Follow-up | Recurrence | Adverse events | Cosmesis |
|---|---|---|---|---|---|---|---|
| Intralesional 5-FU | |||||||
| Kraus et al. 1998643 | Prospective multicentre open label pilot (23 evaluable patients with SCCs confined to upper half of reticular dermis) | Intratumoral FU/adrenaline at 1 ml (30 mg)/lesion/week at weekly intervals for up to 6 treatments. Mean cumulative dose 3.7 ml (range 0.6–6 ml) | 22/23 histologically confirmed clearance | 16 weeks (treated area) completely excised | Not assessed | 19/23 (82.6%) superficial erosions, 9/23 (39.1%) necrosis, clearing several weeks after last treatment. Localised temporary alopecia around treated scalp lesions No clinically sig systemic reactions or adverse events | Clinician assessed – 91% ‘good’ to ‘excellent’. Patient assessed – 100% ‘good’ to ‘excellent’ |
| Morse et al. 2003644 | Case report, SCC nasolabial fold | Intralesional 5-FU. 0.8–2.4 ml once per week for 8 weeks. Total dose 12.8 ml | No residual SCC after eighth injection | 5 months | None | N/A | N/A |
| Topical 5-FU | |||||||
| Hamouda et al. 2001645 | Prospective cohort of XP patients with multiple facial SCCs (n = 10) | b.i.d. topical application. Mean treatment duration 6 months (2–36) | 7/10 superficial regression. Out of five patients biopsied post treatment, one had no residual tumour but four had persistent tumour in deep dermal layer | Every 2 months. Mean not specified | Not assessed | Well-tolerated, some cases of pruritus with erythema | 8/10 crust disappearance and tumour decrease. 4/10 improved QoL |
| Litwin et al. 1972646 | Prospective cohort (33 patients with 53 SCCs) | Topical 5-FU (5%,10% or 20%), od or bd. Average treatment time 10.2 weeks (range 5–37 weeks). 79.2% had one course, 17% two courses and 3.8% three courses | 42/53 (79%) complete post-treatment regression (64% confirmed histologically). 8/53 (15%) partial regression. 3/53 (6%) progression of SCC | Average 23.2 months (range 3–48 months) | None in those free of disease at least 1 year after completion of treatment | Pain in lesions overlying cartilage | – |
There was one prospective multicentre pilot study (23 patients) which evaluated intratumoral 5-FU, with histologically confirmed clearance in 22 patients (96%) at 16 weeks post treatment. 643 Recurrence was not assessed. A case report of intralesional 5-FU reported no recurrence 5 months after treatment. 644
One series of 33 patients with 53 SCCs reported complete post-treatment regression of tumour in 42 SCCs (79%) treated with up to three courses of 5%, 10% or 20% topical 5-FU, of which 27 (64%) were confirmed histologically. 646 The remaining SCCs regressed partially (15%) or progressed (6%). No recurrences were observed in those who were disease-free at least 1 year after treatment. Another series which only included patients with xeroderma pigmentosum reported superficial regression in 7 out of 10 patients with multiple SCCs, although the number of lesions assessed was not specified. 645 Residual tumour remained in the deep dermal layer in 4 out of 5 patients biopsied and recurrence was not assessed. Four out of the 10 patients reported improved QoL, although this was not formally assessed.
None of the studies reported deaths attributable to SCC.
Cosmetic outcome was reported in one study of intralesional 5-FU, with physicians rating cosmetic outcome as good to excellent in 91% of cases, slightly lower than the 100% good to excellent rating of patients. 643 This study reported superficial erosions in 19 out of the 23 (83%) patients and necrosis in 9 out of the 23 (39%), which cleared after several weeks, plus local temporary alopecia around scalp lesions. No systemic adverse events were noted in any of the studies.
Interferon
There were four case series that reported outcomes after intralesional administration of IFN at varying total doses,647–650 the details of which are summarised in Table 56. The largest prospective multicentre series reported histologically confirmed clearance in 24 out of 27 (89%) SCCs in actinically damaged skin, but did not assess recurrence as the site was excised after 18 weeks. 647 A small prospective series observed histologically confirmed clearance at 3 months in all three included patients with lower leg SCCs but, again, recurrence was not assessed. 648 One case series649 reported recurrence of an ear SCC 4 years after treatment with human natural leucocyte IFN in one of 24 patients, although it was unclear how many patients had appeared to respond initially to treatment and what became of those who failed to show a complete response. No recurrence was seen after 23 months in the one patient with an ear SCC who was included in a series of NMSCs treated with intralesional IFN. 650
| Study | 3Type of study | Dose | Initial response | Follow-up | Recurrence | Adverse events | Cosmesis |
|---|---|---|---|---|---|---|---|
| Edwards et al. 1992647 | Prospective multicentre open label trial, 27 SCCs in actinically damaged skin | Intralesional IFN-α2b, as many injections of 1.5 million units as required to blanch tumour and small margin of normal-looking skin 3× per week/nine treatments | 24/27 (88.9%) histological clearance | 18 weeks (site excised) | Not assessed | 65% (of all 48 in trial) had > more than one AE – myalgias, headache, fever. Rigors, flu-like symptoms. 10% severe AE causing interruption of daily activity but none dangerous or long-lasting. 14.6% mildly increasing LFTs. 6.2% decreasing granulocyte count. 4.2% decreasing platelet count | Patient assessed: 76.9% excellent, 15.4% very good, 3.8% good, 3.8% satisfactory, 0% poor. Clinician assessed: 76.9% excellent, 15.4% very good, 7.7% good, 0% satisfactory, 0% poor |
| Wickramasinghe et al. 1989648 | Prospective series, threw patients with SCC, lower leg | Intralesional recombinant IFN-α2 0.9 million units 3× per week/3 weeks | 3/3 complete clinical response confirmed histologically | 3 months | Not assessed | Transient local discomfort at site. Depressive mood in 1 out of total of 19 patients in series | N/A |
| Ikić et al. 1995649 | Retrospective series, 28 patients with eligible SCCs | Human natural leucocyte IFN 400,000–1.2 million units/12–13 applications/3–6 weeks. Total 5.6–21.6 million units | Complete response in 32/52 patients (all SCCs in series). Unclear if remainder were partial/non-responders and what became of them | Unclear | 1/24 (ear SCC at 4 years) | N/A | N/A |
| Recombinant IFN-α2c 2–5 million units/20 applications/4 weeks. Total 40–100 million units | Initial response not reported for recombinant IFN treated | N/A | 0/4 over 3–7 years | N/A | N/A | ||
| Kim et al. 2004650 | Case series including one patient with ear SCC | Intralesional IFN-α2b 2 million units/3× per week/3 weeks. Total 18 million units | N/A | 23 months | No recurrence | Influenza-like symptoms, short-term neurological effects (dizziness, paraesthesia, weakness, confusion, dysarthria, short-term memory loss). Depression at higher doses, transiently ↑ LFTs, reversible dose-related bone marrow suppression | N/A |
None of the included studies reported on deaths attributable to SCC.
One study evaluated cosmetic outcome, with both patients and clinicians rating the appearance as excellent or very good for 93% of lesions treated, and the remainder being rated as either good or satisfactory. 647
Adverse events were described in three studies,647,648,650 with flu-like symptoms and transient derangement of liver function being the most commonly reported events. Depression of mood and reversible dose-related bone marrow suppression were also reported. Severe adverse events causing disruption of daily activity were reported in 10% of all 48 patients treated in one study, although none was dangerous or lasting. 647
Retinoids
Oral 13-cis-retinoic acid (0.3–0.5 mg/kg/day) was administered with calcitriol (1,25-dihydroxyvitamin D3) (0.5–1 µg/day) for 3 to 14 months in a prospective series which included six patients who, between them, had 27 previously untreated histologically proven SCCs at various sites and who were selected on the basis of being unsuitable for standard local therapy owing to the multiplicity of their lesions and their location. 651 Treatment was stopped at 3 months in one of the six patients owing to lack of response. One patient had ‘complete regression’ (assessed by clinical reduction in lesion size but not assessed histologically) of their three SCCs at 15 months, and the remaining four patients had partial reduction in tumour size of between 30% and 85%, although it was unclear at what time point this response was assessed and some patients had remained on treatment. All patients treated had mild skin and mucosal reactions, with more pronounced inflammation and crusting of the scalp in three male patients which improved with antibiotic ointment. Two patients also had a transient slight increase in serum triglycerides and two others had a transient increase in urine calcium, all of which resolved when the dose was decreased. No SCC-related deaths were reported.
There was one case report of the use of single-agent oral isotretinoin given at a dose of 2 mg/kg/day for 6 months to a patient with multiple cutaneous SCCs of the legs. 652 Although the number of treated lesions was not specified accurately, one lesion of ‘approximately’ 20 SCCs remained after 6 months and was reported as a keratoacanthoma when examined microscopically after excision. None of the regressed lesions recurred during the 36 months after treatment, although three new SCCs arose in previously unaffected areas. There was no mention of adverse events in this study.
Other treatments
Cetuximab
We found one case report of the use of cetuximab (a monoclonal antibody that binds to the epidermal growth factor receptor) in combination with γ-irradiation to treat a large unresectable 12-cm SCC of the temple. 653 Cetuximab was given 24 hours pre-irradiation at a dose of 400 mg/m2 and in 200-mg/m2 infusions at weekly intervals throughout the irradiation (total radiation dose 45 Gy). By 4 weeks post treatment the tumour was regressing and, although a histologically confirmed tumour was still present at 8 months, it had decreased in size to 0.2 × 1.0 cm and was excised surgically, with no evidence of further spread 14 months after treatment. The treatment was well-tolerated with a follicular-pustular exanthema that healed quickly with corticosteroid therapy.
Combination systemic treatments
Treatment of eligible SCCs with various combinations of drugs was described in five studies. 654–658 These were generally small case series with only a small number of patients with eligible SCCs, or case reports, and are summarised in Table 57. In all of the studies definitive initial treatment with surgery or radiotherapy was not possible. Different chemotherapy regimens and modes of administration were used in each study, with follow-up ranging from 8 months to > 7 years. One study reported limb salvage in two patients after hyperthermic limb perfusion with chemotherapy, with amputation in a third patient with progressive disease after treatment. 655 Of three patients with SCCs of disfiguring size who were treated with neoadjuvant chemotherapy prior to surgery, complete response was seen in two patients, although one of them had a local recurrence of tumour after 8 months, and no response was seen in one patient who died from their disease 10 months after treatment. 656 No recurrences or metastasis were reported in the remaining studies, although these were all single case reports. 654,657,658 All of the studies reported adverse events related to chemotherapy. Two treatment related deaths were reported: one of 15 patients had multiorgan failure after hyperthermic isolated limb perfusion655 and one of 14 died from a pulmonary infection superimposed on lung fibrosis following neoadjuvant chemotherapy. 656
| Study | Type of study (number of eligible patients) | Treatment details | Outcome | Follow-up | Adverse events |
|---|---|---|---|---|---|
| Fujisawa et al. 2006654 | Case report (76 year old, non-metastatic SCC of cheek, complete resection too difficult) | 4 mg/m2/day of i.v. cisplatin on days 1–5, plus 400 mg/m2/day of 5-FU for 7 days, with concurrent external beam radiotherapy 2 Gy/day 5× per week, total dose 64 Gy | No recurrence or metastasis during follow-up | 5 years | Mild grade 1 myelosuppression. Ulcer resolving within 3 months |
| Olieman et al. 1999655 | Prospective series (three patients with locally advanced eligible SCCs of limbs, curative resection not possible without severe mutilation or impaired function) | Hyperthermic isolated limb perfusion – subcutaneous rIFNγ 0.2 mg OD for 2 days prior to 90-minute infusion of 0.2 mg IFNγ plus 3 mg (arm) or 4 mg (leg) of TNFγ and 10–13 mg/l of melphalan under 39–40°C hyperthermic conditions with excision at 6–8 weeks, if possible | 1/3 complete response (no viable tumour cells); 1/3 partial response; 1/3 local progressive disease and regional disease at 2 months post treatment (then unavailable for follow-up). Limb salvage in two patients with complete or partial response. Amputation in patient with progressive disease | Mean 43 months for 2 patients available for follow-up | Multiorgan failure, deep infection, septic shock and death in 1/15 patients. 1/15 superficial wound infection |
| Sadek et al. 1990656 | Prospective series (three patients with eligible SCCs of disfiguring size) | Neoadjuvant chemotherapy: 100 mg/m2 of cisplatin day 1, 650 mg/m2 of 5-FU by continuous i.v. infusion during 5 days, i.v. 15 mg of bleomycin on day 1 then 16 mg/m2/day of continuous i.v. infusion during 5 days. Repeated every 3–4 weeks for 2–3 cycles. Followed by surgery or IFN (not specified when in relation to chemotherapy) | 2/3 complete response, 1/3 no change (DoD at 10 months). Local recurrence after apparent CR in 1/2 at 8 months | 8, 10 and 22 + months | Pulmonary infection superimposed on fibrotic lung and death in 1/14 patients. Nausea and vomiting in all patients. Grade 3/4 haematological abnormalities in 4/14 patients. Transient trophic and pigmented bleomycin related skin changes |
| Sheen et al. 2003657 | Case report (SCC big toe, amputation refused) | Intra-arterial 50 mg/day infusion of MTX for 8 days plus 6 mg of i.m. folinic acid 6 hourly for 8 days, then intermittent arterial infusion of 50 mg of MTX weekly until wound healed | Complete response 2 months after start of treatment (mass disappeared). No recurrence during follow-up | 7 years 3 months | Generalised skin rash and grade 1 itch |
| Tantranond et al. 1992658 | Case report (SCC of pinna, surgery not indicated as bone involvement, radiotherapy doses prohibitive) | Topical 5-FU plus 60 mg/m2 of i.v. cisplatin on day 1 plus i.v. 5-FU on days 1–4 plus 50 mg b.i.d. of oral isotretinoin. Six cycles in total every 28 days | No evidence of residual SCC after fifth course. No recurrence during follow-up | 2.5 years | Isotretinoin discontinued after 60 days due to severe cheilitis |
Discussion
This is the first systematic review assessing the effectiveness of all treatment modalities for primary non-metastatic SCC and giving an overview of current evidence from non-randomised studies.
Caution needs to be exercised when comparing outcomes after different treatment modalities owing to the limitations of the included studies. Surgery with a predefined excision margin is the treatment of choice for the majority of cutaneous SCCs, with MMS being recommended for SCCs considered to be higher risk or in cosmetically-sensitive areas. Our pooled analysis suggests lower local recurrence rates and deaths attributable to disease after MMS than surgical excision, despite the fact that tumours treated by this method are likely to be higher risk, although there have been no RCTs to directly compare the two treatments. The pooled analysis indicated that regional recurrence was of a similar magnitude for both treatment modalities; this may be suggestive of subclinical spread of some higher-risk tumours treated with MMS to regional lymph nodes at the time of treatment. Overlapping CIs for average effect estimates for the different treatments suggest that apparent differences between treatments may not be significant. This is in agreement with finding of Chren et al. 659 who conducted a large prospective cohort study of all primary NMSCs and reported no significant difference in hazard of recurrence between surgically excised tumours and those treated with MMS.
In our pooled analysis of external radiotherapy, average local recurrence was slightly higher than that seen after conventional surgical excision, although the differences are probably not significant with overlapping CIs. Interestingly, average regional recurrence was lower, although these data were generated from just two studies,585,591 with other studies not specifying whether the recurrences they reported were local or regional failures, so the true significance of this is unclear. The lower local recurrence rates from the studies in which brachytherapy was used may be a reflection of the more superficial, lower risk nature of the included SCCs treated by this method, although patients numbers were generally small and limited follow-up of only a few months in some of the studies may be inadequate to detect later recurrences. The greater rates of recurrence, metastasis and death from disease observed with ART after surgical excision is in accordance with other studies,660 although numbers in included studies were small and for non-perineural invasion SCCs the reasons justifying the use of ART was not always clear. The results may therefore be a reflection of selection of those SCCs with a particular poor prognosis and the identification of prognostic factors which may benefit from ART remains an area of uncertainty and one in which prospective studies are required.
Lowest recurrence rates were observed after cryotherapy and curettage and electrodesiccation, respectively, but the majority of SCCs included in these analyses were small and considered to be low-risk lesions. The evidence is poor to advocate the use of these techniques in lesions considered at higher risk of recurrence and recurrent SCCs.
Based on our results, the use of photodynamic therapy to treat invasive SCCs cannot be advocated. Few studies confirmed histological clearance in apparently completely responsive SCCs and, in those that attempted to do so, residual tumour remained in a number of biopsies. Furthermore, more than one-quarter of those tumours that had appeared to completely respond to photodynamic therapy initially recurred during follow-up.
Not all those with SCC are amenable to surgical treatment or radiotherapy and some people are susceptible to multiple SCCs as a result of a genetic or immune predisposition. Such groups pose particular therapeutic challenges and there is a growing need for effective topical or systemic agents that could be used in these cases. The current evidence supporting the use of these agents to treat primary SCCs is largely anecdotal, based on single case reports or very small numbers of eligible patients in open-label trials with limited follow-up and generally lacking recurrence data. Nonetheless, it is an interesting area for further development as new insights into the pathogenesis and targeted therapies emerge.
Although we included QoL as one of our outcomes, none of the studies included in this review measured this. PROMs have great potential to improve the quality of health services by providing validated evidence of health from the patient’s perspective. Two recent systematic reviews of PROMs in skin cancer showed that there have been limited evaluations of PROMs specifically designed for patients with NMSCs and, furthermore, that the questionnaires developed so far are not perfect for assessment of QoL in these particular patients. 661,662 Nevertheless, the incorporation of patient-reported outcomes will undoubtedly be important in the development of future clinical trials comparing treatments for SCC and should be able to capture QoL issues which are important to patients with this condition, including detailed assessment of cosmetic and functional outcomes at specific time points.
Strengths and limitations of this systematic review
Although we tried to be as thorough as possible in our literature search, it is inevitable that we have failed to find relevant studies. Observational studies, and especially case series, are less easy to identify from searching literature databases than RCTs. Usually they are not identifiable from the title and are less consistently indexed according to study design in bibliographic databases. There is undoubtedly also an element of publication bias with these types of studies.
We have not addressed treatment of recurrent SCCs and tumours known to be metastatic at presentation. Many studies have been excluded as they included previously treated relapsed tumours without separation of data from non-recurrent tumours. Such recurrent tumours may have different features to those which have not been treated previously and they may, therefore, be more likely to recur or be resistant to treatment.
Similarly, we did not specifically search for studies relating solely to the management of SCC in solid organ transplant recipients, although some of the studies did include such patients. Cutaneous SCC is an important cause of morbidity in this group of patients, associated with the likelihood of multiple tumours and with a potentially more aggressive clinical course. 663 Therefore, it is perhaps a subject suitable for separate consideration and beyond the scope of this more general review.
We found that mean follow-up periods were generally poorly reported and few studies reported mean follow-up of > 5 years, so assessment of recurrence according to duration of follow-up was limited in our review. When possible, we did subgroup analyses to compare outcomes in those studies with mean follow-up periods of < 2 years, between 2 and 5 years, and > 5 years. Our main finding on subgroup analysis was that the proportion of deaths attributable to SCC was significantly greater for studies with a mean duration of follow-up > 5 years after conventional surgical excision compared with those with shorter follow-up. Between 70% and 90% of recurrences and metastases occur within the first 2 years after treatment573,664,665 and 95% within 5 years,665,666 so the results from our analysis are probably representative of the true recurrence up to 5 years of follow-up.
Bias and quality of reporting
Validated tools for assessing the risk of bias in non-randomised studies are limited, making the evaluation of study quality less objective than for RCTs. There is little in the literature which specifically addresses the assessment of case series, so we have based our evaluation on a modified risk-of-bias assessment tool from The Cochrane Collaboration,272 together with suggestions drawn up by Albrecht et al. 667 for improving the quality of case series. The latter are based on a few published articles668–670 and Albrecht’s own experience of systematic reviews of case series and reports. 667
Most of the included studies were of limited methodological quality and prone to bias (see Figure 27), with variable patient mixes in terms of prognostic factors, overall disease severity and duration of follow-up. Recruitment bias with selection of particular treatment modalities based on tumour or patient characteristics is a serious consideration for case series. Bias was positively identified or there was an unclear risk in 85% of the studies in this review, making it impossible to directly compare the effectiveness of different treatments. In 41% of studies, losses to follow-up were either incompletely reported or were not mentioned at all, in which case it was difficult to assess the risk of attrition bias.
Stratification of risk
A limited number of studies stratified outcomes according to particular prognostic indicators, although in the majority of studies it was not possible to stratify results from data provided. Ear location as a poor prognostic feature is supported by our pooled analysis of data from studies in which ear and other locations were considered separately. We did not do a pooled analysis of other features considered to confer high risk owing to different reporting methods in the studies in which these factors were considered. Increased risk of recurrence with tumours > 2 cm was noted in some of the included studies,544,551,573 although this finding was not supported by Mourouzis et al. ,553 with 60% of metastases originating in SCCs < 2 cm, nor by Dzubow et al. ,570 who found a trend towards significance with tumours > 5 cm in diameter. Several studies showed the importance of SCC depth as a risk factor for recurrence. No metastases were observed in SCCs < 2 mm in depth by Mourouzis et al. ,553 in accordance with Brantsch et al. 569 who reported a significantly increased risk of metastases for SCCs > 2 mm thick. Griffiths et al. 544 also reported a significant difference in thickness between SCCs in patients who died of their disease and those who did not. Poor differentiation was noted to be an adverse prognostic feature in two of the included studies,553,573 with the presence of perineural invasion being significantly associated with a worse outcome in one of the series. 573
There are currently no accurate prognostic models to stratify SCC patients and to help guide clinical decisions, leading to a lack of uniformity in the management of the tumour. In 2010, the AJCC updated the staging system for SCC, incorporating high-risk features into the Tumour Classification. 671 Although an improvement on the previous classification, it is not without criticism. 672–674 Alternative staging systems have been proposed in an attempt to stratify the large group of heterogeneous T2 tumours according to their prognostic features666,673 and, although further validation work is required, could be useful tools when designing future clinical trials.
Identification of potential research topics and development of a clinical trial scenario
Summary
What was already known about this topic?
-
There are no RCTs comparing the effectiveness of different treatments for the forms of SCC commonly seen in clinical practice.
What did this study add?
-
Clinicians from various specialties identified clinically important areas of uncertainty relating to the management of SCC and suggested topics for future research.
-
Optimal excision margins, the role of radiotherapy and optimal follow-up of patients with SCC were the areas of uncertainty which were of particular concern for clinicians.
-
The suggested research topics have contributed towards the identification of a potential future RCT.
-
The National Cancer Research Institute (NCRI) non-melanoma subgroup of the melanoma clinical studies group (CSG) is helping to develop a trial proposal for submission to funding bodies, based on discussions prompted by this study.
Introduction
The evidence base for the efficacy of treatments for SCC is poor and largely based on case series. Currently, no published data from RCTs are available comparing treatments for the types of SCC that are seen in routine clinical practice. 518,536 Given the enormous service burden of treating non-melanomas, the need for large, prospective studies to compare different treatments has been recognised as a research priority in the UK. 517,675 The development of a proposal for a RCT addressing current uncertainties in the management of SCC is one of the primary objectives of our work.
A number of gaps in the evidence have been outlined in the systematic review (see Systematic review of squamous cell skin cancer treatments: randomised controlled trials). To supplement these findings, we sought to approach clinicians who are treating SCC regularly and invite them to identify the most important uncertainties in SCC treatment and which research areas they would like to see addressed in a RCT. The treatment of SCC is undertaken by a wide range of clinicians, dermatologists, plastic surgeons, clinical oncologists and surgeons of several disciplines such as general; ear, nose and throat; maxillofacial; and ocular surgery. It is not recommended that GPs should definitively treat SCCs themselves. 675 However, they have an important role to play in diagnosing and urgently referring cases of suspected SCC to a specialist member of the local skin cancer multidisciplinary team or specialist skin cancer multidisciplinary team (SSMDT). For complicated cases and SCCs that are considered to be at high risk of recurring or metastasising, a multidisciplinary approach to management may be required.
We therefore sought to canvas a broad range of clinicians, with the following aims:
-
To gain an overview of current SCC treatment practices.
-
To invite clinicians to suggest potential research topics on SCC treatment, as a guide for future discussions regarding the development of a RCT trial proposal.
-
To offer clinicians the opportunity to express their interest in taking part in a future clinical trial.
-
To develop a scenario for a RCT of SCC treatments based on the concerns of the clinicians surveyed and previously identified gaps in the evidence.
Methods
The vast majority of SCCs are treated by dermatologists, plastic surgeons or clinical oncologists. Prominent professional bodies for these specialties were identified as points of distribution for the survey, namely:
-
the British Society for Dermatological Surgery (BSDS), representing dermatologists who carry out skin surgery
-
the British Association of Plastic Reconstructive and Aesthetic Surgeons (BAPRAS), the membership of which is mainly plastic surgeons but with some members from other surgical specialties
-
the skin site-orientated e-network (SOeN) of the Royal College of Radiologists (RCR), a subgroup of clinical oncologists with an interest in skin cancer
-
the UKDCTN, a mixed group of dermatologists, GPs, clinical nurse specialists in dermatology and patients, all of whom have an interest in promoting research in dermatology.
The survey was developed in an electronic format using online software (SurveyMonkey). Four different versions were generated, one for each of the targeted professional bodies. Although all four surveys sought answers to similar questions, each one was developed with one or more members from each of the groups to ensure that the questions were relevant to that particular group (see Appendix 14 for an example copy). Approval of each survey was obtained from the president of the BSDS, the Executive Committee of BAPRAS and the President of the RCR. Surveys were piloted by members of the Centre of Evidence Based Dermatology to test the functionality of the online format. Each survey was presented over seven pages, with one to four questionnaire items per page. Respondents were able to review and change their answers before submitting.
Between January and March 2010, each professional organisation sent an e-mailed invitation letter and web link to the corresponding electronic survey to members on their distribution list (BSDS, BAPRAS, UKDCTN) or posted it on the specialist group website (SOeN). The invitation letter explained the purpose of the work, approximately how long the survey would take to complete and reassured participants of the anonymity of their responses. For the UKDCTN survey, potential participants were asked not to complete the survey if they had already taken part via one of the other organisations surveyed. The survey could only be accessed once from a particular IP address, in an attempt to prevent duplicate entries. Completion of the survey was voluntary, with no incentives offered. Surveys remained open for responses for 3 weeks, a reminder being sent to members via each organisation 1 week prior to closure. Responses were analysed from both completed surveys and those that were terminated early (i.e. the respondent did not complete all the questionnaire pages). Raw data were exported into an Excel spreadsheet to allow simple statistical analysis and generation of graphical representations. Free-text responses were analysed manually. Response rates were calculated by dividing the number of people who were sent the link to the survey by the number who started it, multiplied by 100. Completion rates were calculated by dividing the number who started the survey by the number who completed it, multiplied by 100. A Checklist for Reporting Results of Internet E-Surveys was used for reporting the results of the survey. 676
The ideas identified for research topics were discussed at meetings of the NCRI non-melanoma subgroup of the melanoma CSG, a multidisciplinary group of dermatologists, plastic surgeons, clinical oncologists, medical oncologists, statisticians and lay members having an interest in promoting research in the field of NMSC. By iterative discussions, a scenario for a RCT was established, ahead of further development into a proposal for submission to funding bodies.
Results
Response rates for each of the professional organisations are shown in Table 58.
| Organisation | Number receiving survey | Number starting survey (response rate %) | Number completing survey (completion rate %) |
|---|---|---|---|
| BSDS | 250 | 70 (28.0) | 63 (90.0) |
| BAPRAS | 851 | 138 (16.2) | 110 (79.7) |
| SOeN | 249 | 6 (2.4) | 5 (83.3) |
| UKDCTN | 470 | 92 (19.6) | 77 (83.7) |
| Overall | 1820 | 306 (16.8) | 255 (83.3) |
Professional capacity
More than three-quarters of those who responded to the survey were consultants in dermatology, plastic surgery or clinical oncology (Figure 49) and 82.4% of respondents had been in clinical practice for > 5 years (Figure 50).
FIGURE 49.
Professional capacity of survey respondents (n = 302).
FIGURE 50.
Length of clinical practice of respondents (n = 205).
Treatment practices
Based on data from respondents who were able to give a numerical estimate, the average number of SCCs treated annually by members of each organisation is shown in Table 59. A further 41 respondents gave a range for number of SCCs treated, four were not certain and unable to estimate, one was retired and one was no longer doing skin cancer clinics.
| Organisation | Mean number of SCC treated per annum (range; median; mode) |
|---|---|
| BSDS (n = 49) | 65 (12–400; 50; 100) |
| BAPRAS (n = 92) | 76 (0–500; 50; 50) |
| UKDCTN (n = 66) | 47 (2–200; 30; 50) |
| SOeN (n = 4) | 26 (8–50; 22.5; mode not applicable) |
The majority of BSDS, BAPRAS and UKDCTN respondents treat primary invasive non-metastatic SCC by attempted single excision with a predetermined margin (98.6%, 98.3% and 92.8% of respondents from these groups, respectively). Fewer respondents reported being able to offer MMS (17.4%, 11.1% and 9.6%, respectively). All the clinical oncologists use either radiotherapy alone or as an adjuvant postoperatively and one also uses chemoradiation (although the numbers who responded to the SOeN survey were only very small so this should not be regarded as representative of the practice of all clinical oncologists). Other treatments sometimes used by members of the BSDS and UKDCTN include curettage and cautery (some specified for lesions that appear to be low risk, or in the very old and frail), cryotherapy and topical cytotoxic agents. Radiotherapy was also reported to be a treatment used by a few members of these groups, although presumably after discussion with colleagues in their skin cancer specialist multidisciplinary team (MDT) and referral to a clinical oncologist.
Biopsies
Most respondents from the BSDS, BAPRAS and UKDCTN reported that biopsies of suspected SCCs are either rarely done or are performed in about 25–50% of cases (Figure 51). In contrast, all six of the clinical oncologists who responded stated that they always biopsy pre-treatment.
FIGURE 51.
Pre-treatment biopsy rates by professional group.
Follow-up
Almost two-thirds of respondents reported that they would follow-up a patient with a SCC considered to be high risk for between 2 and 5 years (Figure 52), compared to only 26% who would follow-up for < 2 years. In contrast, patients with ‘low-risk’ SCCs would be followed up for < 1 year by 57% of respondents, with a further quarter following them up for 1–2 years (Figure 53).
FIGURE 52.
Follow-up of ‘high-risk’ lesions (n = 275).
FIGURE 53.
Follow-up of ‘low-risk’ lesions (n = 267).
Research topics
One of the aims of this survey was to identify areas relating to the management of SCC for which clinicians feel there is a need for further research, in order to guide the development of ideas for clinical trials. Based on the research categories proposed in the questionnaires, optimisation of surgical treatment, the role of radiotherapy and the role of newer agents were the areas in which there was greatest interest (Table 60).
| Research category | Number of responses to ‘need for a clinical trial’ (%) | Total | |
|---|---|---|---|
| Yes | No | ||
| Optimising surgery | 181 (75.7) | 58 (24.3) | 239 |
| Role of radiotherapy | 157 (71.0) | 64 (29.0) | 221 |
| Role of chemotherapy | 108 (54.3) | 91 (45.7) | 199 |
| Role of newer agents | 174 (79.4) | 45/219 (20.5) | 219 |
| Other | 28 (40.0) | 42 (60.0) | 70 |
There were some differences in research area priorities between the groups. Optimisation of surgery and the role of radiotherapy were considered the most important areas for research by BSDS members (78.7% and 75.8%, respectively). For BAPRAS and UKDCTN members, the role of newer agents (86.7% and 83.9%, respectively) and the optimisation of surgery (78.8% and 71.6%, respectively) were the top two areas.
In addition, respondents were invited to submit free-text suggestions for specific research questions and other comments relating to a potential clinical trial. Several broad categories could be derived from the responses and the numbers of responses in each category were counted. Some respondents entered free text that could be split into more than one category, so each free-text comment was subdivided as necessary. The categories identified and distribution of respondents’ comments are listed in Table 61.
| Research topic | BSDS (N = 26), n (%) | BAPRAS (N = 31), n (%) | UKDCTN (N = 18), n (%) | RCR (N = 3), n (%) | Total (N = 78), n (%) |
|---|---|---|---|---|---|
| Excision margins | 8 (30.8) | 12 (38.7) | 4 (22.2) | – | 24 (30.8) |
| Role of radiotherapy | 8 (30.8) | 6 (19.4) | 3 (16.7) | 3 (100.0) | 20 (25.6) |
| Follow-up | 7 (26.9) | 7 (22.6) | 4 (22.2) | – | 18 (23.1) |
| Prognostic model | 1 (3.8) | 7 (22.6) | 2 (11.1) | – | 10 (12.8) |
| Mohs vs. excision | 3 (11.5) | 2 (6.4) | – | – | 5 (6.4) |
| New agents | 1 (3.8) | 2 (6.4) | 2 (11.1) | – | 5 (6.4) |
| Topical agents | 1 (3.8) | 2 (6.4) | 1 (5.6) | – | 4 (5.1) |
| Curettage vs. surgery for low-risk SCC | 3 (11.5) | – | 1 (5.6) | – | 4 (5.1) |
| Chemotherapy/electrochemotherapy | – | 3 (9.8) | – | – | 3 (3.8) |
| Treatment of immune suppressed/transplant | 2 (8.0) | 1 (3.2) | – | – | 3 (3.8) |
| Early diagnosis techniques | – | 1 (3.2) | 2 (11.1) | – | 3 (3.8) |
| Imaging techniques | 1 (3.8) | 2 (6.4) | – | – | 3 (3.8) |
| Photodynamic therapy | – | 2 (6.4) | – | – | 2 (2.6) |
| Sentinel lymph node biopsy | – | 2 (6.4) | – | – | 2 (2.6) |
Free-text replies tended to be general identification of topics for possible research rather than specific ideas for clinical trials. Nonetheless, the top three areas of uncertainty expressed by clinicians and those for which it was felt there was a need for more research were excision margins, the role of radiotherapy for high-risk SCCs or incompletely excised SCCs and optimal follow-up regimes. There was also recognition of the current lack of a prognostic model on which to base treatment and follow-up. Although the need for more research into the role of other treatments such as chemotherapy and newer therapies was generally considered to be a research priority (see Table 60), there was a lack of research questions relating to specific agents in the free-text replies.
Outcomes
The survey also sought to identify which outcomes were considered to be of greatest importance to clinicians after treatment of SCC (Table 62).
| Outcome | Very important, n (%) | Important, n (%) | Fairly important, n (%) | Not important, n (%) | Total, n |
|---|---|---|---|---|---|
| Survival | 223 (86.1) | 27 (10.4) | 7 (2.7) | 2 (0.8) | 259 |
| Regional recurrence | 224 (85.8) | 34 (13.0) | 2 (0.8) | 1 (0.4) | 261 |
| Local recurrence | 206 (78.9) | 50 (19.2) | 5 (1.9) | 0 (0.0) | 261 |
| QoL | 126 (48.6) | 107 (41.3) | 23 (8.9) | 3 (1.2) | 259 |
| Persistent ulceration | 108 (42.4) | 101 (39.6) | 41 (16.1) | 5 (1.9) | 255 |
| Persistent pain | 103 (40.4) | 99 (38.8) | 47 (18.4) | 6 (2.4) | 255 |
| Acceptability to patient | 77 (29.6) | 128 (49.2) | 53 (20.4) | 2 (0.8) | 260 |
| Disfigurement | 72 (27.8) | 157 (60.6) | 29 (11.2) | 1 (0.4) | 259 |
| Contracture | 38 (15.2) | 132 (52.8) | 74 (29.6) | 6 (2.4) | 250 |
| Pain of procedure | 20 (7.8) | 95 (37.3) | 100 (39.2) | 40 (15.7) | 255 |
Almost all of the short- and long-term outcomes suggested (with the exception of pain of procedure) were considered to be ‘very important’ or ‘important’ by the majority of those who responded to the question. However, survival and local and regional recurrence were the three outcomes considered to be of greatest importance.
Theoretical willingness to recruit into a future randomised controlled trial for squamous cell skin cancer
Clinicians were asked if they would be prepared to recruit their patients into either a future full-scale RCT of SCC treatments or into a feasibility study. The results are summarised for each professional group in Table 63.
| Potential willingness to recruit patients | BSDS (N = 61), n (%) | BAPRAS (N = 98), n (%) | UKDCTN (N = 74), n (%) | RCR (N = 4), n (%) | Total (N = 237), n (%) |
|---|---|---|---|---|---|
| Yes, full-scale RCT | 32 (52.4) | 38 (38.8) | 31 (41.9) | 4 (100.0) | 105 (44.3) |
| Yes, but feasibility study only | 2 (3.3) | 2 (2.0) | 1 (1.4) | – | 5 (2.1) |
| No | 5 (8.2) | 28 (28.6) | 16 (21.6) | – | 49 (20.7) |
| Maybe | 22 (36.1) | 30 (30.6) | 26 (35.1) | – | 78 (32.9) |
Development of a scenario for a clinical trial
The results of the clinicians’ research priorities were presented to a multidisciplinary group of clinicians, statisticians and a patient representative from the NCRI non-melanoma subgroup of the melanoma CSG. Further to these discussions and considering the evidence gaps identified in the systematic reviews (see Systematic review of squamous cell skin cancer treatments: observational studies), a scenario for a two-stage clinical trial was drawn up based on surgical excision margins and the role of ART after surgery for high-risk SCCs. This scenario was then used as the basis for the development of feasibility studies, which will be discussed later in this chapter.
Discussion
Surveys are a useful research tool to elicit practice patterns, behaviours and concerns of physicians. 677 The purpose of this survey was to gain an overview of how cutaneous SCCs are currently being treated across the UK and to help identify potential topics for a RCT based on what clinicians consider to be important areas of uncertainty in the management of SCC. We did not seek to assess adherence to current clinical guidelines.
The response rate varied across the professional organisations from 2.4% from the SOeN of the RCR up to 28% for the BSDS membership. The low rate seen with the SOeN may be a reflection of the survey having been posted on a webpage with restricted access and would have required members to specifically log on to the site before gaining access to the survey. As the survey was not targeted specifically at clinicians with a particular interest in skin cancer, it is likely that only those with such an interest will have responded; therefore, there will be an element of self-selection bias. This is reflected in the average number of SCCs treated by the respondents over a 1-year period (see Table 59). However, we feel that any conclusions from the survey are valid, as we are primarily interested in the views and opinions of clinicians who are treating SCC regularly.
Current treatment practices
Several treatment options are available for managing SCCs and guidance for clinicians is given in multiprofessional guidelines, based on whether the tumour is considered to be at low or high risk of recurrence and/or metastasis. 517 From these guidelines, surgical excision is generally the treatment of choice for the majority of SCCs and was undertaken by the vast majority of specialists in this survey, other than clinical oncologists who use radiotherapy. Thirty-three of the 255 respondents (12.9%) were able to offer MMS, a treatment which may be considered for high-risk tumours and those in functionally sensitive areas. Other treatments such as cryotherapy, and curettage and cautery, which the guidelines state may be indicated for small, low-risk tumours, were also used by respondents although less frequently than surgical options. Topical cytotoxics were also used by 19 respondents (7.4%), although their use is not recommended in the guidelines; evidence in support of their value is very limited and based mostly on single-case reports.
Over three-quarters of respondents from BSDS, BAPRAS and UKDCTN replied that they either rarely biopsied before treatment or only biopsied between 25% and 50% of suspected SCCs, as surgical excision in itself gives a definitive diagnosis. In contrast, the clinical oncologists always biopsied suspected SCCs before radiotherapy. Between 32% and 47% of SCCs may be incorrectly diagnosed prior to surgery678,679 and it has been suggested that pre-treatment biopsies may be required in a greater number of cases so that excision may be expedited. Furthermore, lack of histology from destructive treatments such as cryosurgery or curettage and cautery may contribute to the under-reporting of SCCs that is recognised in the UK. 680 However, it has been estimated that if pre-treatment biopsies were carried out for every skin tumour, there would be a sevenfold increase in the number of tumours assessed,678 which could be prohibitively costly and unfeasible.
Identification of research topic and trial scenario
Our appraisal of the evidence conducted as part of this project concluded that the evidence base for the effectiveness of SCC treatments is poor (see Systematic review of squamous cell skin cancer treatments: randomised controlled trials). In the absence of evidence from RCTs, this survey has provided useful cross-speciality information about the kind of trials that clinicians would find valuable to guide practice in the treatment of SCC. It is encouraging that there is interest in the field, with nearly half of the clinicians surveyed expressing their provisional expression of willingness to take part in future trial work. This is, however, likely to be an overestimate, and willingness to actually recruit patients is likely to depend on the research question being addressed by the trial, which at this stage was not known and the facilities available at their centre. Particular areas of uncertainty identified by the respondents related to optimal excision margins, the role of ART in the management of higher-risk SCCs and follow-up regimes for SCC patients. There was also interest in the role of newer therapies to treat SCCs, comparison of MMS with standard excision and also concern about the lack of a prognostic model on which to base treatment decisions. These topics reflect the gaps in the evidence which we found in our systematic reviews518,536 and indicate that there is potential need for well-designed trials in this area.
Following multidisciplinary discussions of the survey results and taking the current evidence base into account, the trial scenario that is being put forward will address the management of high-risk SCCs and will incorporate four of the topics raised by clinicians in two stages of randomisation. In the first three-arm stage, two excision margins of different sizes will be compared with a third MMS arm. In the second stage, ART after excision will be compared with no ART for the SCCs deemed to be at highest risk of recurrence, based on the presence of pre-defined histological features.
Given the lack of previous RCTs, this large-scale prospective study will offer the first randomised comparison of standard surgery with MMS for SCCs and will also allow comparison of excision margins to assess whether the currently recommended 6-mm excision margin for high-risk SCCs is adequate or whether outcomes may be significantly improved by taking a larger margin. SCCs that recur after previous treatment have a much higher overall recurrence rate of 25% when compared with the rate of 5.2% for primary tumours;665 therefore, adequate treatment of the original tumour is imperative. A margin of normal-looking skin around the clinically apparent tumour is taken during treatment with the aim of eradicating subclinical tumour extensions. Nevertheless, despite apparently adequate clearance of the tumour, recurrences do occur. The recommendation in current UK guidelines517 of 4-mm and 6-mm excision margins for well-defined low-risk tumours and high-risk tumours, respectively, is based on one prospective study assessing subclinical extension of tumour and the surgical margins that would be required to clear 95% of tumours. 681 However, recurrence of a tumour after excision was not an outcome of this study. The Australian Cancer Network Guidelines682 also recommend a 4-mm excision margin for ‘favourable lesions’, for example well-differentiated lesions < 2 cm in diameter, but to obtain similar rates of local control for tumours > 2 cm, margins up to 10 mm are recommended with even wider margins for very large lesions. However, there are no large-scale prospective studies with long-term follow-up that have compared the adequacy of different excision margins in the treatment of SCC. An audit of plastic surgeons’ adherence to the BAD guidelines 2 years after their publication revealed that there was a lack of consensus regarding excision margins in this group and that the current recommended margins were frequently not followed. 683,684 Indeed, margins around lesions in the head and neck region were often smaller than the recommendations, with larger margins than recommended being taken on the trunk and limbs.
Mohs micrographic surgery, in which sequential layers of skin are mapped, excised and examined microscopically until complete clearance of tumour has been achieved, has the advantage of being relatively tissue-sparing compared with standard surgery. Furthermore, it offers the potential to follow subclinical extensions of tumour and perineural involvement to complete excision. However, Mohs requires more resources than excision and is more costly,685,686 and a large prospective cohort study has reported that outcomes after MMS for NMSCs are similar to those after excision. 659 A RCT of MMS versus excision has been conducted for BCCs, revealing similar 5-year recurrence rates for the two treatments; the investigators concluded that surgical excision is probably sufficient for most cases of primary BCC. 687 However, there have been no randomised studies comparing Mohs and surgical excision for SCC, so the proposed trial will be the first of its kind to be conducted.
In the second stage of the proposed trial scenario, participants with the highest-risk SCCs would be randomised after surgery to receive ART or no ART. Current BAD guidelines517 recommend radiotherapy to treat non-resectable tumours with well-defined margins, but do not give any specific recommendation regarding which patients should be considered for ART. The Cancer Council of Australia guidelines682 recommend consideration of ART for SCCs with any of the following features: T4, rapidly growing, recurrent, close excisional margin < 5 mm, perineural invasion, lymphovascular invasion, in-transit metastases or regional lymph node involvement. Incomplete excision and extensive perineural invasion of a large nerve are considered to be indications for ART in the National Comprehensive Cancer Network guidelines. 688 However, there have been no RCTs comparing surgery alone to surgery plus ART, and a systematic review660 concluded that current data were insufficient to identify high-risk features in which ART may be beneficial. Furthermore, those treated with surgery plus radiotherapy were significantly more likely to develop regional and distant metastases than those treated with surgery alone. This was attributed to the fact that very few cases in the surgery plus radiotherapy data set had documentation of clear surgical margins and also that there may have been selection bias in that those with more advanced stages of disease were selected to receive ART. The proposed trial would be the first randomised study to assess the role of ART in high-risk SCCs and, together with data from SCCs which are only included in the first randomisation stage, should also provide useful data on which a prognostic model can be developed to promote appropriate targeting of interventions.
Implications for future research
This survey has allowed identification of areas of treatment uncertainty that are important to clinicians. Taken together with evidence gaps which were highlighted in appraisal of the evidence base (see Systematic review of squamous cell skin cancer treatments: randomised controlled trials), this has allowed us to develop a scenario for a potential RCT. In order to develop the scenario further and in readiness for submission of the trial proposal to funding bodies, feasibility work has been undertaken and is discussed in subsequent sections of this chapter.
There was also interest from clinicians in other potential research topics, such as the role of new chemotherapeutic agents. Although these are not part of the main proposal, they could form the basis of trials of SCC treatments in the future.
An analysis of squamous cell skin cancer treated over a 1-year period
Summary
What was already known about this topic?
-
Squamous cell skin cancer is the second most common type of skin cancer and typically affects older people.
-
Squamous cell skin cancers have the potential to recur, metastasise and may lead to death.
-
Early identification of SCCs considered to be at higher recurrence of recurrence and/or metastasis is important, based on the presence of particular clinical and histological features.
-
There is currently no consistent prognostic model for SCCs.
What did this study add?
-
Over the course of 1 year, 375 cutaneous SCCs from 357 patients were submitted to the histopathology department of a regional centre serving a population of 1,070,000 people.
-
Recurrence from SCC is rare; within 5 years of treatment, local recurrence occurred in 6.2% of SCCs and regional recurrence in 3.3%.
-
Within 5 years of treatment, 44% of the study population had died, although SCC-specific mortality was low (2.7%).
-
Baseline outcome data will be used to guide statistical powering of the proposed trial.
-
Using various scenarios for classification of high-risk status, the number of patients who would potentially be eligible to be recruited into the proposed trial has been estimated and could be extrapolated to other regional centres in the UK.
Introduction
We aimed to gain an overview of the numbers, types and baseline 5-year outcomes of SCCs that were being treated across specialties at a regional centre each year. This would facilitate the assessment of likely numbers and demographics of patients potentially eligible for recruitment into the proposed RCT and help to guide its statistical powering. Only patients with ‘high-risk’ SCCs will be recruited into the trial, which will be in two stages. The first stage will compare outcomes between different surgical excision margins and MMS and the second will evaluate the effect of ART compared with no ART. We therefore assessed SCCs in terms of the presence of prognostic features according to the most recent AJCC classification of SCCs and also by the prognostic criteria recommended by the Royal College of Pathologists (RCPath) which would mandate referral to the skin cancer MDT to examine if there was a relationship between particular prognostic features and outcomes.
Methods
An anonymised web-based Access database was created to collect retrospective data on SCCs submitted to the Histopathology Department at Queen’s Medical Centre, Nottingham, between 1 April 2006 and 31 March 2007. Primary cutaneous SCCs were identified by Systematized Nomenclature of Medicine codes M80703, 80713, 80743, 80753 and 80513, and histopathology data from the data set recorded in the appropriate fields.
Features of the tumour including anatomic location, diameter, depth of invasion, Clark level, differentiation, histological growth pattern, perineural invasion and vascular invasion were recorded. In addition, excised tumours were classified according to T classification based on the sixth and seventh editions of the AJCC staging criteria. 689 T classification in the sixth edition690 was based on tumour diameter and did not take into account additional high-risk pathological features (T1 < 2 cm, T2 = 2–5 cm, T3 > 5 cm), whereas, in the seventh edition,691 T2 SCCs are either those with a diameter of > 2 cm or those ≤ 2 cm in diameter but which also have two or more additional high-risk features (> 2 mm deep, Clark level IV or more, the presence of perineural invasion, located on the ear or non-hair-bearing lip, or being poorly differentiated or undifferentiated). As data on depth and level of invasion were routinely available only for excised SCCs, only these samples were given a T classification. Treatment of tumour and clearance of lateral and deep surgical margins were recorded when possible. Non-invasive SCCs, actinic keratosis and Bowen’s disease were not included in the analysis. Tumours were excluded if they were in anogenital locations or mucosal.
Any SCCs identified were linked to the clinical record database via hospital identification numbers. Data on recurrences (local or to regional lymph nodes), distant metastases and death (either attributable to SCC or from another cause), occurring within 5 years of treatment were recorded on the research database by the dermatology department. Deaths that were recorded as attributable to SCC were checked by the dermatologist from the patient’s case records. For patients with more than one SCC treated over the course of the year, the SCC with the greatest number of adverse prognostic features was selected for analysis of potential trial participant numbers.
We analysed baseline demographic variables, as well as clinical and histopathological data, using descriptive statistics and frequency tabulation in SPSS 21. Statistical significance of differences between variables was assessed by chi-squared test probability or Fisher’s test with two-tailed p-value if the number of observed outcomes was fewer than five. Differences between means of continuous variables were assessed using the independent samples t-test. p-values of ≤ 0.05 were considered to be statistically significant. Outcome frequencies were based on excised tumours for which data were available on local recurrence, regional recurrence, distant metastases and death.
Written permission to conduct the study was obtained from the Cancer and Associated Specialties Directorate Clinical Director of Nottingham University Hospitals NHS Trust.
Results
The initial pathology database search identified 431 primary invasive SCCs that had been submitted over the 12-month period. Ten of these were excluded on further review as they were sited in anogenital, mucosal or non-cutaneous locations. Out of the 421 SCCs remaining, 298 (70.8%) were recorded as being excision, 5 (1.2%) as incisional biopsies, 61 (14.5%) as punch biopsies, 3 (0.7%) as shavings, 34 (8.1%) as curettings, 5 (1.2%) as widening of a previous excision and 15 (3.6%) were not specified. After exclusion of SCCs in which a preceding biopsy was matched with an excision specimen, 375 SCCs remained for inclusion in the analysis. There were no significant differences between the demographics of patients who had excision and those for whom another type of specimen was submitted but with no matching excision specimen [male 66.7% for excisions vs. 61.7% for others (χ2 = 0.686; p = 0.407); mean age 76.0 years (SD 11.89 years) versus 75.6 years (SD 10.19 years) (independent samples t-test = 0.279, df = 373; p = 0.780)].
Demographics of population
In total, there were 357 patients with one or more SCC treated between April 2006 and March 2007. The characteristics of the study population are summarised in Table 64.
| Characteristic | N = 357 |
|---|---|
| Gender, n (%) patients | |
| Male | 231 (64.7) |
| Female | 126 (35.3) |
| Age (years) | Mean 76 (range 34–99) |
| Number of SCCs, n (%) patients | |
| 1 | 343 (96.1) |
| 2 | 10 (2.8) |
| 3 | 4 (1.1) |
An independent samples t-test did not reveal a statistically significant difference between the mean ages of males and females in the study population (t = 0.814, df = 355; p = 0.416). Most patients (343, 96.1%) had a single SCC over the 12-month period, although 14 (3.9%) had at least one other SCC at a different anatomical site treated during this period.
Characteristics of tumours
Tumour characteristics are summarised in Table 65.
| Characteristic | N = 375 |
|---|---|
| Location, n (%) | |
| Head and neck | 231 (61.6) |
| Trunk | 23 (6.1) |
| Upper limb | 53 (14.1) |
| Lower limb | 63 (16.8) |
| Not specified | 5 (1.3) |
| Tumour diameter | |
| Mean (mm) | 18.1 |
| Median (mm) | 12.0 |
| ≤ 2 cm, n (%) | 162 (43.2) |
| > 2 cm, n (%) | 33 (8.8) |
| Not specified, n (%) | 180 (48.0) |
| Tumour depth | |
| Mean (mm) | 4.9 |
| Median (mm) | 3.0 |
| ≤ 2 mm, n (%) | 90 (24.0) |
| 2.1 to ≤ 4 mm, n (%) | 124 (33.1) |
| > 4 mm, n (%) | 64 (17.1) |
| Not specified, n (%) | 97 (25.8) |
| Level of invasion, n (%) | |
| Papillary dermis | 5 (1.3) |
| Upper reticular dermis | 14 (3.7) |
| Mid reticular dermis | 87 (23.2) |
| Deep reticular dermis | 113 (30.1) |
| Subcutaneous | 54 (14.4) |
| Not specified | 102 (27.2) |
| Differentiation, n (%) | |
| Well/moderately | 212 (56.5) |
| Poor/undifferentiated | 88 (23.5) |
| Not specified | 75 (20) |
| Histological type, n (%) | |
| Classic/no special type | 245 (65.3) |
| Acantholytic | 7 (1.9) |
| Spindle cell | 1 (0.3) |
| Desmoplastic | 3 (0.8) |
| Not specified | 119 (31.7) |
| Perineural invasion, n (%) | |
| Present | 16 (4.3) |
| Not present | 268 (71.5) |
| Not specified | 91 (24.3) |
| Vascular invasion, n (%) | |
| Present | 6 (1.6) |
| Not present | 278 (74.1) |
| Not specified | 91 (24.3) |
There were significant differences in the anatomical location of SCCs between men and women (Figure 54), with men more likely to have a SCC located in the head and neck region (p < 0.0001) and with location on the leg being significantly greater in females (p = 0.006). Of the SCCs that were located in the head and neck region, men were significantly more likely to have a SCC in the ear area (p < 0.0001), whereas women were more likely to have them on the cheek (p = 0.04), lip (p = 0.05) or neck (p = 0.03). Although a greater percentage of SCCs in males were located on the scalp (21.2% in males vs. 9.6% in females), this did not quite achieve statistical significance (p = 0.0687) (Figure 55).
FIGURE 54.
Anatomical distribution of SCCs in males and females (%).
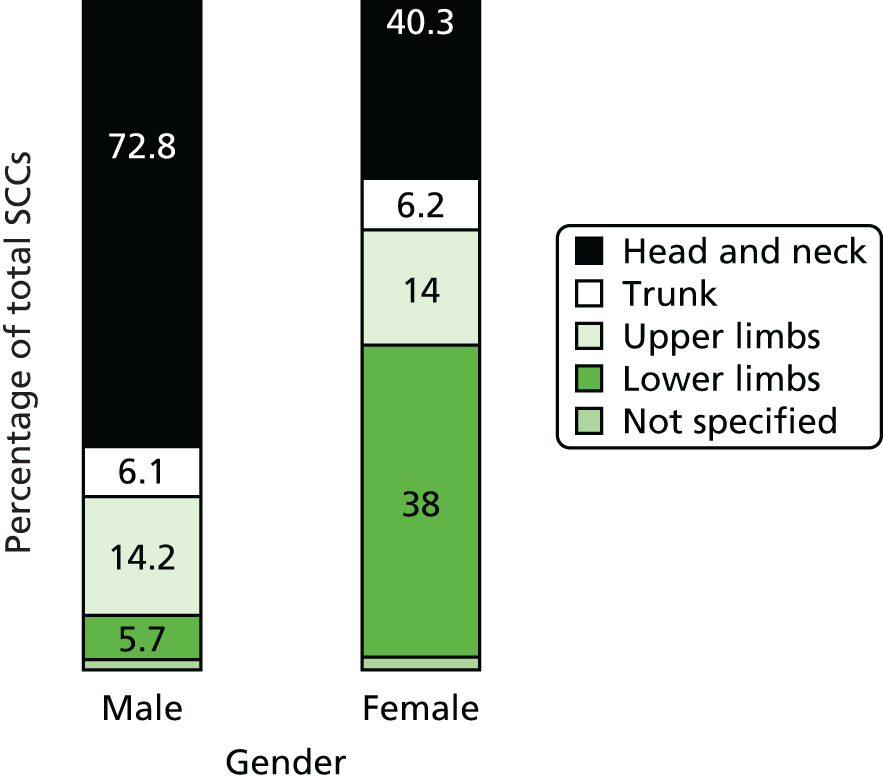
FIGURE 55.
Distribution of head and neck SCCs (%) by gender. Green, male; and blue, female.
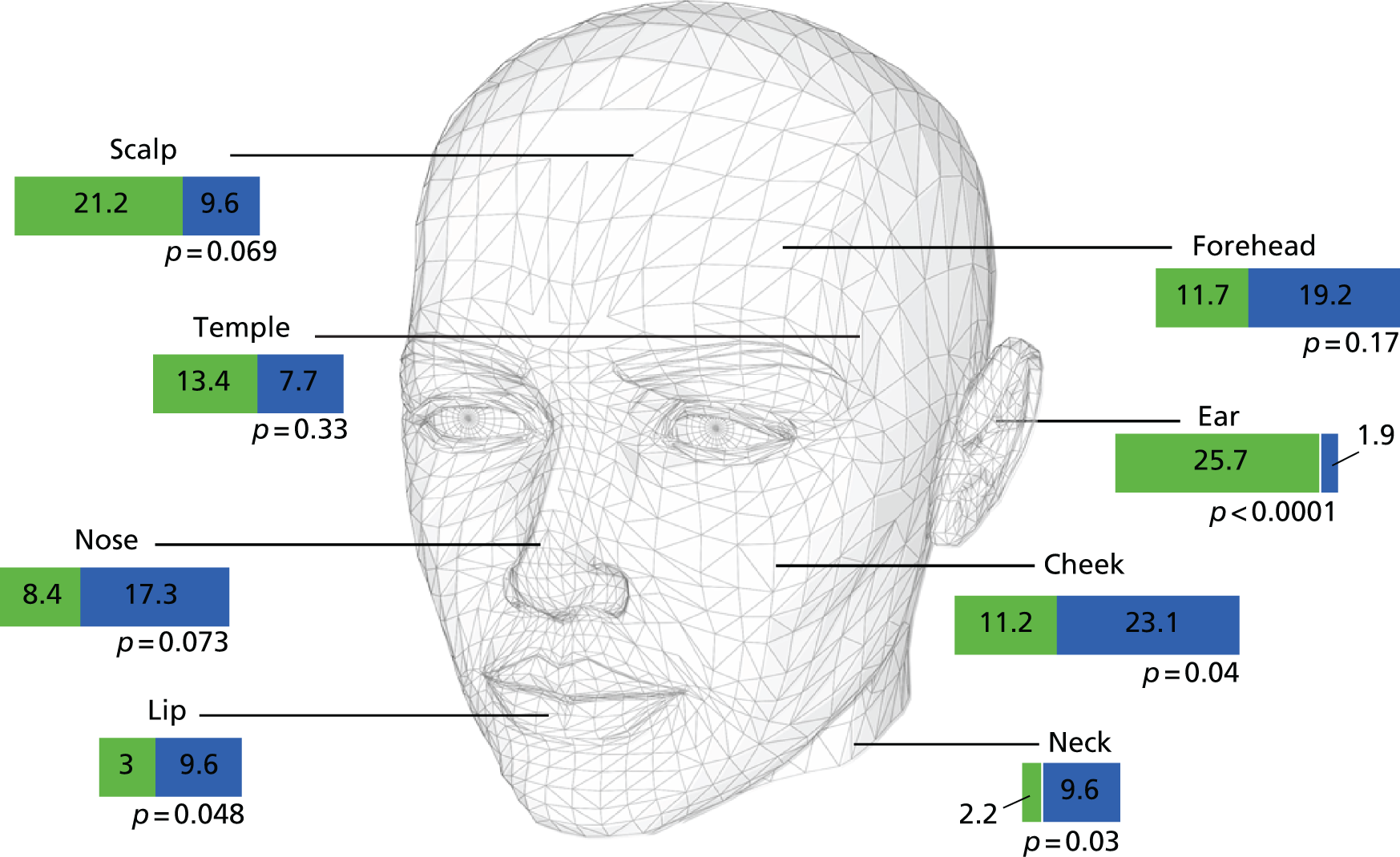
Treatment modality was usually not recorded on the histopathology database (362/421, 86%). Excisional surgery was recorded as the treatment modality for 57/421 (13.5%) of SCCs and MMS in 2/421 (0.5%).
Narrow peripheral histological clearance margins (< 1 mm) were found in 6 out of 213 (2.8%) of excisions for which these data were available, compared with narrow deep histological margins in 49 out of 214 (22.9%) of excised SCCs, a difference which was statistically significant (Fisher’s test p = 0.001). Out of the 204 excisions for which data were available for both margins, 4 (2.0%) had both narrow peripheral and deep margins.
Outcome analysis
Outcome data were available for 351 out of 375 (93.6%) of SCCs in total, and for 276 out of 294 (93.9%) of excisions in 265 patients, the results for which are summarised in Table 66.
| Outcome | n/N (%) with outcome |
|---|---|
| Local recurrence | 17/276 (6.2) |
| Regional recurrence | 9/276 (3.3) |
| Distant metastases | 0/265 (0) |
| All-cause mortality | 116/265 (43.8) |
| SCC-attributable death | 4/265 (1.5) |
Figure 56 is a flow chart in which the number and types of recurrences are broken down according to patients’ mortality status at 5 years, for both the entire SCC data set (all specimen types) and for excision only. Overall mortality over the 5 years was high given the mean age of the study population, with 149 out of the total 337 patients having died during the study period; 140 (94.0%) of these died from an unrelated or unknown cause.
FIGURE 56.
Flow chart of outcomes for SCCs (all specimen types in black, excisions only in green).
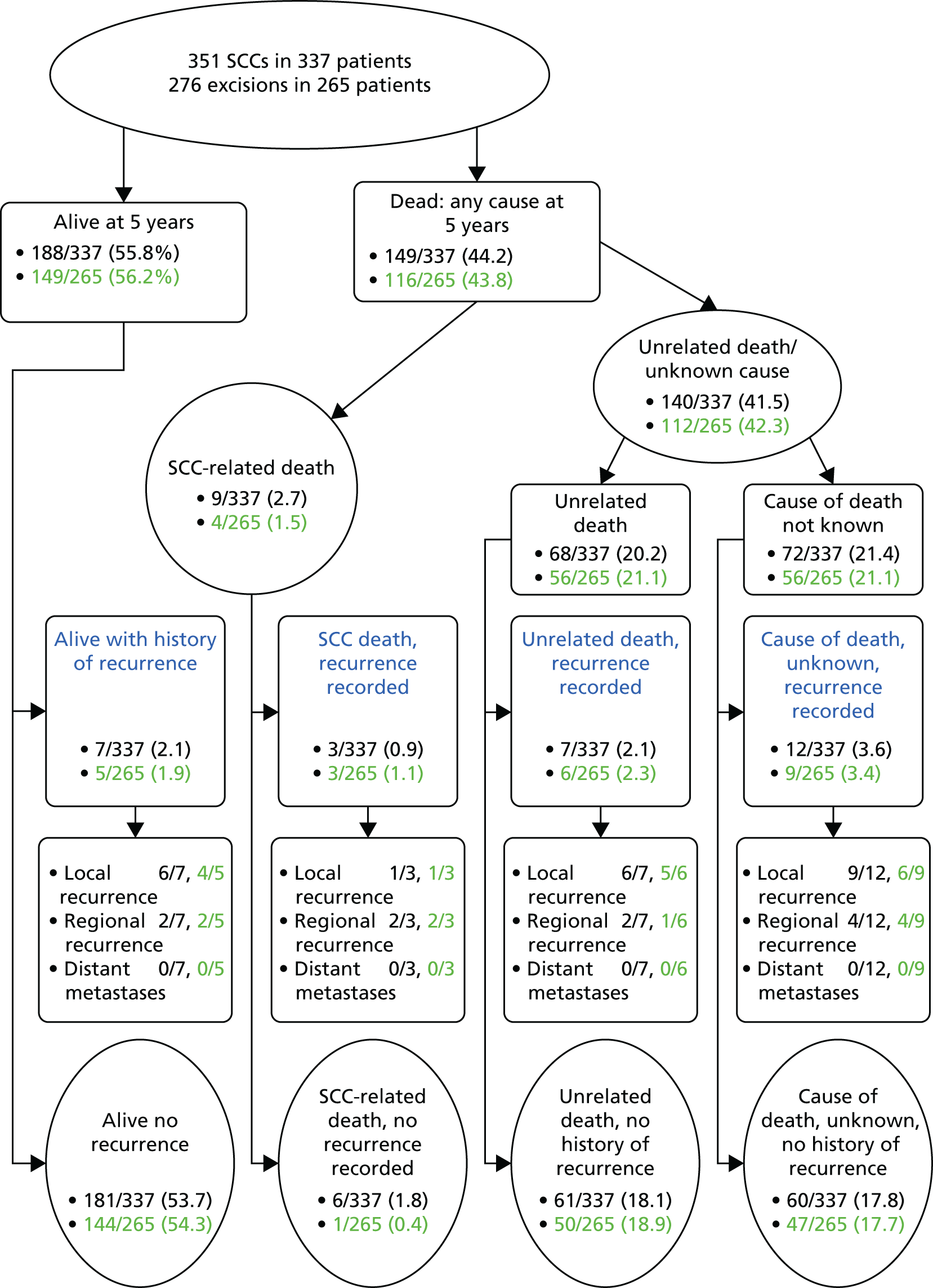
Tumour classification based upon American Joint Committee on Cancer staging criteria
The sixth and seventh editions of the AJCC T classifications are summarised for comparison in Table 67a and Table 67b, respectively. The number of SCCs and patients who would be classified as T2 is upgraded when compared with the earlier sixth edition, based on diameter of > 2 cm, or having a diameter of < 2 cm with two or more of the following features: depth of > 2 mm; Clark level IV or more; perineural invasion; poorly differentiated or undifferentiated; and located on ear or hair-bearing lip.
| T classification | n (%) excised SCCs (N = 294) | n (%) patients (N = 275) |
|---|---|---|
| T1 < 2 cm diameter | 153 (52) | 142 (51.6) |
| T2 = 2–5 cm | 23 (7.8) | 22 (8) |
| T3 > 5 cm | 10 (3.4) | 8 (2.9) |
| Not classifiable from data available | 108 (36.7) | 103 (37.4) |
| T classification | n (%) excised SCCs (N = 294) | n (%) patients (N = 275) |
|---|---|---|
| T1 < 2 cm with fewer than two high-risk features | 35 (11.9) | 31 (11.3) |
| T2 > 2 cm with fewer than two high-risk features OR tumour any size with two or more high-risk features | 151 (51.4) | 141 (51.3) |
| T3 based on invasion of maxilla, mandible, orbit, temporal bone rather than size | – | – |
| Not classifiable from data available | 108 (36.7) | 103 (37.4) |
Moreover, out of the 186 SCCs with sufficient data to enable T classification, 118 (63.4%) were < 2 cm in diameter and would be classified as T2 only when histopathology data were available based on the presence of two or more additional high-risk features.
High-risk features as per Royal College of Pathologists criteria
Table 68 shows a summary of the excised SCCs and patients with high-risk status, based on the presence of high-risk features as defined by the RCPath.
| Pathological feature | n (%) patients (N = 275) |
|---|---|
| Depth > 4 mm | 58 (21.1) |
| Clark level V | 46 (16.7) |
| Poorly differentiated/undifferentiated | 77 (28.0) |
| High-grade histological type | 9 (3.3) |
| Perineural invasion | 15 (5.5) |
| Vascular invasion | 4 (1.5) |
A total of 151 (51.4%) excised SCCs in 142 (51.6%) patients had one or more high-risk pathology features (Table 69). There were an additional 33 excised SCCs (11.2%) in 30 (10.9%) patients that were > 2 cm in diameter, but in which no other high-risk pathological features were recorded. Twelve SCCs (in 12 patients) and one SCC (in one patient) were located in the ear-region or on the lip, respectively, but were < 2 cm in diameter and had no other high-risk features recorded.
| Number of high-risk pathological features | n (%) excisions (N = 294) | n (%) patients (N = 275) |
|---|---|---|
| 0 | 143 (48.6) | 133 (48.4) |
| 1 | 98 (33.3) | 93 (33.8) |
| 2 | 35 (11.9) | 32 (11.6) |
| 3 | 17 (5.8) | 16 (5.8) |
| 4 | 1 (0.3) | 1 (0.4) |
Subgroup analyses were performed to compare outcomes between the AJCC classification and the cumulative number of RCPath high-risk pathological features (Table 70). No statistically significant differences in outcomes were detected when SCCs classified as either T1 or T2 were compared. There were statistically significant differences for all-cause mortality between patients with no high-risk pathology features and those with two features (Fisher’s test p = 0.0091), and those whose SCCs were classified as T2 compared with those with no high-risk features (Fisher’s test p = 0.0365). Patients with two high-risk features were significantly more likely to have died due to SCC-related disease than those with no high-risk features (p = 0.0418). Similarly, patients with T2 SCCs were significantly more likely to have died from a non-SCC-related cause of death within 5 years than those with no high-risk pathological features (Fisher’s test p = 0.0510), and those with two high-risk features were more likely to have died from another cause at 5 years than those with no high-risk features (p = 0.0508). Patients with no high-risk features were also significantly more likely to be alive with no recurrence at 5 years than those with two high-risk features (Fisher’s test p = 0.0173).
| Outcome | AJCC, n/N (%) with outcome | Cumulative number of RCPath high-risk criteria, n/N (%) with outcome | |||||
|---|---|---|---|---|---|---|---|
| T1 | T2 | None | 1 | 2 | 3 | 4 | |
| Local recurrence (SCC as unit of analysis) | 1/34 (2.9) | 13/141 (9.2) | 5/111 (4.5) | 4/93 (4.3) | 4/29 (13.8) | 1/12 (8.3) | 0/1 (0.0) |
| Regional recurrence (SCC as unit of analysis) | 0/34 (0.0) | 7/141 (5.0) | 1/111 (0.9) | 4/93 (4.3) | 1/29 (3.4) | 0/12 (0.0) | 0/1 (0.0) |
| Distant metastases (patient as unit of analysis) | 0/33 (0.0) | 0/135 (0.0) | 0/107 (0.0) | 0/88 (0.0) | 0/28 (0.0) | 0/12 (0.0) | 0/1 (0.0) |
| All-cause death | 12/33 (36.4) | 67/135 (49.6) | 38/107 (35.5) | 43/88 (48.9) | 18/28 (64.3) | 6/12 (50) | 1/1 (100.0) |
| Death attributable to SCC | 0/33 (0.0) | 2/135 (1.5) | 0/107 (0.0) | 2/88 (2.3) | 2/28 (7.1) | 0/12 (0.0) | 0/1 (0.0) |
| Unrelated death/cause not known | 12/33 (36.4) | 65/135 (48.1) | 38/107 (35.5) | 41/88 (46.6) | 16/28 (57.1) | 6/12 (50) | 1/1 (100.0) |
| Alive and no recurrence at 5 years | 20/33 (60.1) | 65/135 (48.1) | 67/107 (62.6) | 43/88 (48.9) | 10/28 (35.7) | 5/12 (58.3) | 0/1 (0.0) |
Summary of number of patients potentially eligible for the proposed trial
The number of patients having SCCs excised with features that may make them eligible for randomisation into the proposed RCT will depend on the final eligibility criteria that are decided upon in the trial protocol in order to define ‘high-risk’ SCCs, but various scenarios are summarised in Table 71 based upon the number of excised SCCs having these features.
| Scenario for eligibility | n (%) patients (N = 275) |
|---|---|
| (A) T2 SCC (based on seventh edition AJCC classification) | 141 (51.3) |
| (B) One or more RCPath-defined high-risk pathology feature (any diameter, any location) | 142 (51.6) |
| (C) Two or more RCPath-defined high-risk pathology features (any diameter, any location) | 47 (17.1) |
| In addition to (B) and (C) above, the following may also be eligible for inclusion | |
| > 2 cm diameter alone, but having no other RCPath-defined high-risk features and not located at ear or lip | 6 (2.2) |
| > 2 cm diameter alone, with one other RCPath-defined high-risk features and not located at ear or lip | 9 (3.3) |
| Ear location only but no other high-risk features and < 2 cm | 13 (4.7) |
| Ear location only with one other high-risk feature and < 2 cm | 7 (2.5) |
| Lip location only but no other high-risk features and < 2 cm | 1 (0.4) |
In the second stage of the proposed trial, eligible patients will be further randomised to receive either ART or no ART. Eligibility for further randomisation is anticipated to be those patients with tumours that are > 2 cm in diameter, > 2 mm deep or Clark level V, and are poorly or undifferentiated, plus those with perineural invasion. Based on these criteria, the number of eligible patients who would potentially be eligible for the second stage of randomisation is summarised in Table 72.
| High-risk pathological features | n (%) patients | n (%) patients also having PNI |
|---|---|---|
| Diameter, depth and differentiation | 4/353 (1.1) | 0/353 (0.0) |
| Diameter, depth or Clark V, and differentiation | 7/353 (2.0) | 2/353 (0.6) |
| Numbers with just two of these features | ||
| Diameter and depth | 14/353 (4.0) | 1/353 (0.3) |
| Diameter and differentiation | 10/353 (2.8) | 2/353 (0.6) |
| Diameter and subcutaneous | 11/350 (3.1) | 3/350 (0.8) |
| Depth and differentiation | 43/353 (12.2) | 3/353 (0.8) |
| Depth and subcutaneous | 18/349 (5.1) | 3/349 (0.8) |
| Subcutaneous and differentiation | 29/267 (8.2) | 6/267 (2.2) |
| PNI alone | 16/353 (4.5) | – |
Discussion
A total of 421 non-metastatic SCCs were submitted over the 1-year study period to the histopathology laboratory, which serves a population of approximately 1,070,000 people under the auspices of the Nottinghamshire Locality of the East Midlands Cancer Network. With a mean age at presentation of 76 years, ranging from 34 to 99 years, older people in this sample were more commonly affected than the young and about twice as many men were affected as women. The preponderance for SCC to develop on the sun-exposed areas of the head and neck in both sexes and the excess of tumours located on the legs in females and the ears in men is consistent with the findings from other studies499,692 and is likely to be a reflection of the different clothing and hairstyles and exposure of skin from receding hair.
Although a common tumour, recurrences of SCC are fortunately rare. However, a small subset do go on to have local recurrence after treatment or experience spread to the regional lymph nodes or distant organs, and some may die as a direct result of their disease. In the present study, 6.2% of SCCs recurred locally during the 5 years after treatment. This was similar to the 5.4% local recurrence seen after conventional excision which we recently reported in our systematic review and pooled analysis of case series of treatments for SCC536 and the 4.6% local recurrence reported in a large 10-year prospective cohort study of 985 patients. 693 Metastasis to regional lymph nodes occurred in 3.3% of excised SCCs. This was comparable with the 1.9–2.6% figure for nodal metastasis recently reported over a 10-year study period in a retrospective study of 6164 patients666 and with other studies in which nodal metastases of 3.7% and 4% have been reported569,693 and the 4.4% after surgical excision in our pooled analysis of case series. 536 Overall mortality from any cause was high with 43.8% of the study population having died over the 5 years, although this is not entirely surprising given the advanced age of the group. However, deaths that were attributable to SCC were rare, with 2.7% of our total population dying as a result of their disease. This was similar to the 1.5% and 2.1% figures reported in other studies569,693 and slightly less than the 4.1% we found on pooled analysis of case series of surgical excision, which may be partly explained by possible misrecording of deaths as being due to SCC in what were mostly retrospective studies. Inaccurate death certification has been recognised as a particular problem for NMSC, so these data should be interpreted cautiously. 694 In the four patients with excision in whom death was attributed to SCC, two were reported to have also had regional metastases and one also had local recurrence.
We did not assess outcomes after different treatment modalities in this study, as most were treated by surgical excision and there were inadequate numbers which were known to have been treated by other modalities. It is also possible that SCCs that were treated by destructive modalities such as cryotherapy or curettage and electrodesiccation did not have any pathology recorded on the database. A recent prospective study of consecutive non-melanoma cancers found that recurrence rates were similar after excision and MMS, even when the conventional risk factors for recurrence were adjusted for. 659 This was supported by our recent systematic review and pooled analysis of treatments for SCC in which there was significant overlap of CIs between different treatment modalities. 536
Although only a small percentage of SCCs recur, it is important to identify those that are at greatest risk of recurring at an early stage. Currently the definition of ‘high-risk SCC’ is very variable but the development of a prognostic model is an important step towards targeting the most appropriate treatments to those who are most likely to benefit from them, for example ART and nodal staging. In the most recent AJCC classification (AJCC7), primary SCCs are classified as being T1 or T2, with higher-risk T2 tumours being those > 2 cm in horizontal diameter or < 2 cm but with two or more additional characteristics associated with poor prognosis, features which were not incorporated into previous editions: depth of > 2 mm; Clark level IV or more; perineural invasion; poorly differentiated or undifferentiated; and primary site on ear or hair-bearing lip. 689 This has resulted in a significant increase in the number of SCCs that have been upgraded to T2 tumours from 8% to > 50%. The new classification was designed to stratify patients more accurately than the previous version and, in general, represents an improvement. Nonetheless, it is not without criticism; it omits several variables associated with high-risk disease, such as host immunosuppression, previously treated tumours and the presence of chronic inflammation or location in burns and scars, and there is also some confusion regarding the precise lip location (hair-bearing or non-hair-bearing) as defined in the AJCC manual. 674 An alternative tumour staging system has been proposed in an attempt to offer better prognostic stratification of AJCC7 T2 tumours, in which T1 tumours have no risk factors but are upgraded to T2a in the presence of either perineural invasion or poor/undifferentiated or invasion beyond subcutaneous fat, T2b tumours have 2–3 risk factors and T3 tumours have bone invasion or have all four risk factors (> 2 cm diameter, perineural invasion, poorly differentiated and invasion beyond subcutaneous fat). 673 This proposed alternative may be useful for future studies to define high-risk tumours, although it remains to be validated.
The high-risk features used in the AJCC7 are not completely identical to those defined by NICE guidelines as being high-risk for the purposes of MDT referral and patient management and treatment. In the NICE guidelines, tumours > 4 mm in depth and those extending into subcutaneous tissue (Clark level V) are considered to be high-risk. 675 The BAD multiprofessional guidelines also adopt these criteria in their stratification of low- and high-risk SCCs695 and they are adopted as high-risk features in the RCPath minimum data set. 696 Currently, there is limited evidence that the presence of lymphovascular invasion as an independent risk-factor for metastasis and death,553,697 but its presence is listed as a high-risk pathological feature in the RCPath minimum data set. Similarly, desmoplastic, acantholytic, spindle, metaplastic, sarcomatoid, adenosquamous growth patterns and SCCs with an adjacent area of Bowen’s disease are considered to be high-risk features in the RCPath and National Clinical Guidelines. 695,696
In view of the lack of consistency in defining a high-risk patient with SCC, we conducted analyses based on both the current AJCC7 classification and on the presence of high-risk features specified by the RCPath in order to compare potential numbers of eligible SCC for the proposed trials using various eligibility criteria scenarios for high-risk scenarios. In our univariate analysis, we found an association between local recurrence and the presence of perineural invasion, which is consistent with other studies. 673,698 Local recurrence was also associated with the presence of vascular invasion in our study. Poorly differentiated and undifferentiated tumours were significantly associated with increased mortality at 5 years from any cause and patients with such tumours were less likely to be alive with no history of recurrence at 5 years. Other studies in which multivariate analysis has been conducted have found an association between metastasis and tumour size and depth. 673,699 When we compared outcomes after classifying tumours as T2 according to the AJCC7 system and according to the cumulative number of high-risk pathological features, patients with T2-classified SCCs and also those with at least two high-risk features were significantly less likely to be alive and recurrence free at 5 years than those with no high-risk pathology features. However, we did not find statistically significant differences for locoregional recurrence or distant metastases.
Further elucidation of the inter-relationship between the various prognostic features and outcomes will require large prospective studies to be conducted. The main objective of the current analysis was to determine the types and numbers of SCC that are treated in order to guide the design of such a study. We only studied outcomes in patients known to have had excisions; excluded patients had other types of specimens submitted to pathology and may have received other forms of treatment. The lack of data about treatment received prevented us from comparing outcomes after different treatment modalities. Another limitation is that we did not examine the impact of the patients’ immune status on outcomes, as these data were generally not recorded on the pathology database. Nevertheless, this would not affect the assessment of the number of potentially eligible SCCs for entry into the trial, as immunosuppressed patients would not be excluded.
Implications for future research
The current study forms part of the feasibility work for a future RCT into management of high-risk SCCs. It has allowed us to estimate the approximate number of ‘high-risk’ SCC patients in one UK centre based on different scenarios for tumour eligibility. We envisage that these results may be extrapolated to other centres in the UK, based on the size of the population served, in order to calculate the number of centres that would be required to participate in the trial. The number of participants needed to be recruited will, in turn, be based on powering calculations, which will be informed by the number of those who experienced adverse outcomes during the 5 years after treatment.
Qualitative feasibility study
Summary
What was already known about this topic?
-
Recruitment into RCTs can be challenging, with fewer than one-third reaching their recruitment target.
-
Recruitment of older people into clinical trials is particularly challenging.
-
Barriers to participation in clinical trials may be protocol, patient or physician related.
-
Suboptimal recruitment into clinical trials may result in misleading conclusions being drawn and limit the external validity of the trial.
What did this study add?
-
Participants generally had poor understanding of SCC even though all had experienced treatment of the condition.
-
Understanding of the processes of clinical research and randomisation was poor among participants.
-
Overall participants did not regard the proposed RCT as being unfeasible.
-
Randomisation to one of the surgical arms in the first stage of the proposed trial was not overly concerning for participants, although willingness to be randomised would depend if their SCC was located in a cosmetically or functionally sensitive site.
-
Generally participants would be more reluctant to be randomised into the ART versus no radiotherapy arm of the second stage of the proposed trial. Concerns about receiving radiation were cited as the main reason for this.
-
Potential participants in a future RCT would want information about the trial to be provided in a variety of formats.
-
The randomisation process will require thorough explanation and may require additional time and staff input to ensure that participants are thoroughly cognisant of the process and to optimise their willingness to be randomised.
-
The concept of clinical equipoise will need reinforcing in order to overcome triallists’ and participants’ potential preference for one treatment arm over the other.
Introduction
Squamous cell skin cancer is a common cancer; however, the evidence base for the effectiveness of different treatments is poor, with no RCTs comparing treatments for the types of SCCs seen in everyday clinical practice518 and a lack of consensus regarding the use of some treatment modalities such as ART and sentinel lymph node biopsy. As a result, there is wide variation in management practices. Furthermore, there is currently no prognostic model to guide decisions about which treatments or combination of treatments are most appropriate for optimal patient management. One of our objectives in this project is to develop a proposal for a RCT that will address two areas of management uncertainty for high-risk SCCs, optimal excision margins and the role of ART, which were identified as research gaps in our appraisal of the evidence and as priorities for research in our survey of clinicians.
It is recognised that recruitment into multicentre RCTs can be difficult, with fewer than one-third reaching their recruitment target and more than half requiring extension. 700 Apart from the funding and ethical implications, this may lead to a type II error, in which it is concluded incorrectly that there is no significant difference between the treatment arms. In addition, the incidence of NMSCs increases with age, with approximately 80% occurring in people > 60 years,519 but the recruitment of older patients into clinical trials can be particularly challenging. It is reported that only one-quarter to one-third of potentially eligible older people are enrolled into trials,701 which may significantly impact on external validity. On analysis of our own retrospective SCC case series data, we found an average age at presentation of 75 years (see An analysis of squamous cell skin cancer treated over a 1-year period). The RCT we are proposing is therefore going to require successful recruitment from a largely elderly population. As there have been no previous RCTs specifically addressing this type of skin cancer, it is important to examine the beliefs and experiences of a population representative of potential RCT participants. This will help to identify possible barriers and drivers to recruitment, thus facilitating the design of the trial and assessment of the resources required.
Aims
-
To evaluate potential barriers to successful recruitment into a proposed future two-stage RCT of SCC surgery and ART.
-
To assess likely willingness of patients to be randomised into the proposed RCT.
-
To explore current understanding of their condition and clinical research generally in people previously treated for SCC, with a view to developing appropriate participant information resources for the proposed RCT.
Methods
Patients with cutaneous SCC who had been treated by one of the consultant dermatologists (WP) at Nottingham University Hospitals NHS Trust between 1 January 2012 and 31 December 2012 were sent a letter from the clinical care team inviting them to take part in the study, along with a participant information sheet (see Appendix 15) explaining the purpose of the study. Private patients were excluded. Consent to take part was implied by return of a reply slip allowing the research team to contact them.
A postal questionnaire with open and closed questions relating to the design of the proposed RCT and hypothetical willingness to be randomised to each stage was designed, with workshop input from members of the UKDCTN Patient Panel, who also piloted the questionnaire. The questionnaire was sent to identified potential participants who were asked to return their completed questionnaire within 14 days of receipt (see Appendix 16).
The questionnaire proposed a focus group, designed to explore patients’ attitudes to research and their condition generally, and to discuss issues around the trial itself in greater depth. Respondents who expressed an interest in such a group were telephoned by the research team and invited to take part. Seven participants consented to take part; therefore, one focus group was held and facilitated by two researchers (Louise Lansbury and Paul Leighton). Discussion was based around a broad topic guide, which included willingness to take part in clinical research generally, willingness to participate in each stage of the two-stage trial being proposed and possible barriers to taking part in research. Participants’ use of information resources and knowledge of their condition was probed to ascertain the needs of participants in the proposed RCT in terms of information provision. The focus group lasted approximately 90 minutes and was recorded using digital recording equipment and transcribed in full.
Data from the questionnaires and focus group were analysed using a Thematic Framework Analysis approach. 702,703 An initial a priori thematic framework was constructed from the literature on clinical treatment and clinical trial recruitment, containing themes on knowledge of SCC, treatment experiences, attitudes towards research and understanding of randomisation. The framework was a simple model constructed selectively to address the research question and reflecting the straightforward purpose of this research. Subtopics were amended if there was an excess of data or if no data were captured for a particular theme. A thematic map was generated to reflect the content of the focus group discussion and questionnaire responses and to generate insight into recruitment to the proposed trial (Figure 57). Data from the questionnaires and focus groups were coded, indexed and charted onto the thematic framework for interpretation according to the research objectives. Coding was ‘broad brush’ and largely descriptive to reflect the straightforward research aims.
FIGURE 57.
Thematic framework of factors influencing willingness to participate in a two-stage trial of surgery and ART for SCC.
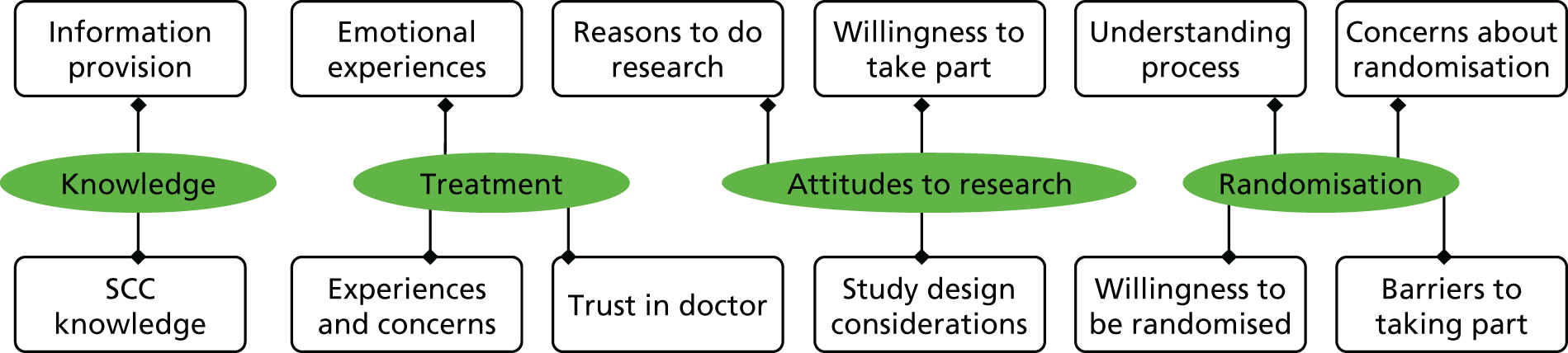
Demographic data were analysed using SPSS 21 statistical software.
Ethical approval for the study was granted by the Proportionate Review Sub-Committee of the National Research Ethics Service Committee West Midlands –Coventry and Warwickshire (REC reference 13/WM/0051). Research and innovation approval was given by the Nottingham University Hospitals NHS Trust in its capacity as a PIC, and the study was included on the NIHR Clinical Research Network Portfolio.
Results
Fifty-nine patients were identified as being potentially eligible for the study, having had surgical excision of a SCC within the specified 12-month period and were sent letters inviting them to participate. Thirty reply slips (51%) were returned, including one informing the team that the patient had subsequently passed away, so questionnaires were posted to 29 potential participants. Completed questionnaires were returned for 24 participants (83%).
Nineteen men (79%) and five women (21%), with a mean age of 72.5 years (SD 8.98 years), took part in the study. Three-quarters (n = 18) of participants were retired and the remainder were either in full- or part-time employment (n = 3 participants), self-employed (n = 1) or not working due to ill health (n = 2). Educational status varied among respondents, with seven (29%) having professional and/or postgraduate qualifications, two (8%) holding a university undergraduate degree, two (8%) having ‘A’ levels (Advanced levels), three (13%) having ‘O’ levels (Ordinary levels) or equivalent, one (4%) having a School Certificate, 8 (33%) having no formal qualifications, and one (4%) not specifying highest educational attainment.
A total of seven participants also agreed to take part in the focus group (six men, one woman), with a mean age of 70.4 years (SD 9.22 years).
The thematic map was organised according to four overarching themes:
-
knowledge of the condition
-
experiences of treatment
-
attitudes towards research
-
attitudes towards randomisation.
Patient knowledge of the condition
Two main areas of knowledge of SCC were identified during the focus group discussion:
-
knowledge of SCC itself, including existing knowledge of causes, risk factors and prognosis
-
information resources used to get information, their adequacy and requirements for provision of information resources for the proposed trial.
Knowledge of squamous cell skin cancer
Overall participants in the focus group did not feel well-informed about their condition, even though some of them had received treatment for multiple skin cancers. There was little pre-existing knowledge of SCC prior to diagnosis, with some participants previously never having heard of it as a discrete type of skin cancer. Several participants recognised sun exposure as a major risk factor and there was some speculation that the reason that males are more commonly affected than females may be because females using sun protection measures more than men.
. . . the reason that there’s more men here, that they get it more than women. Is it because women wear a lot of make-up on their faces.
Participant (male, 64 years)
Presumably women are more eager to use the sun creams. I’m thinking of my young daughter and wife, is that they’re all very keen to protect their skin whereas men don’t seem to bother as much.
Participant (male, 79 years)
Most participants had at some time attempted to rationalise why they had developed the cancer. Exposure of a cut to aluminium and wire, exposure to grinding dust, radiation treatment, a specific sunburn event, and long-term medication use were cited as potential causes of individual skin cancers. Knowledge of prognosis was variable with some participants not being aware that some SCCs can recur or that there is an increased risk of developing new skin cancers elsewhere. The more ‘experienced’ skin cancer patients, who had a history of more than one skin cancer, felt quite confident that they would know how to recognise a new skin cancer.
Information provision
Although participants generally had access to information leaflets that were given to them in clinic explaining the condition and its treatment, some could not remember much about them, while others found the leaflets were quite useful and interesting. However, there was general agreement that they could have been much better informed about SCC. Some felt that they had been given the leaflets and told to go away and read them, but would like to have had more explanation from the clinicians treating them, but there was acknowledgement of the workload and time constraints of the medical staff and potential to compromise time to treatment, which was a concern to participants.
I got just a leaflet . . . They never explained. They said ‘Take that, read that’. I suppose they were busy doing other things.
It sounds like the doctors could talk to you a bit more?
Yeah, I think so. Especially those that are performing surgery on you or putting you under the knife.
That’s going to eat into the time they’ve got and we’ve said we want quicker treatment.
The internet was another source of information used by those with access to learn more about their condition and, for some, was the main source of information. This was done independently and they had not been recommended particular sites by the clinicians.
Another potential source of information was the skin cancer specialist nurse and, although some participants had been given details of this service during their treatment, none had actually accessed the facility after treatment. One participant also admitted that they were attending the focus group as they felt very poorly informed and were hoping to learn more about SCC from the session.
Participants indicated in the discussion that if they were invited to take part in the proposed RCT, they would like to have a choice of formats through which participant information is provided. It was felt that a website dedicated to the trial would be useful, with details of the trial itself and the research team, with the proviso that any written information, either web-based or in leaflet form, should be easy to understand and the language not too technical. Some participants expressed that they would also want to a face-to-face discussion about the trial, but the concern about taking up too much of the clinician’s time was again raised. There was general agreement that access to either a member of the research team or a specialist nurse in order to discuss the trial itself or their own clinical care would be satisfactory and would lessen the burden on the clinician.
Experiences of treatment
Three areas were identified regarding treatment experiences:
-
experiences and concerns about diagnosis, referral and treatment
-
emotional experience
-
trust in the clinicians.
Experiences and concerns about diagnosis, referral and treatment
Although all participants had experienced surgical excision of their skin cancer, there was some variation in their overall experiences and satisfaction with the service. This was most evident with initial diagnosis and referral, whereby delayed diagnosis by their GP was reported by a couple of participants, which resulted in late referral and the feeling of mistreatment of the condition:
The delays for me were treatment by the doctor [GP], who I felt was mistreating me and I complained and finally convinced a senior doctor that I had to be referred to dermatology.
Participant (male, 79 years)
My main concern was with the identification of the SCC as this was not identified by my GP and it was several weeks before I was referred to hospital and the appropriate action taken. By the time it was dealt with the wound had grown and resulted in two operations to ensure complete removal.
Questionnaire respondent (male, 60 years)
The concern was raised that GPs should be better educated to recognise skin cancers at an early stage and to make rapid referrals to the specialist secondary care team. Timeliness of treatment was important to participants and, although none expressed dissatisfaction with the 2-week wait rule, some were frustrated that the system could not be bypassed if they subsequently developed new skin cancers. The idea of a specialist ‘walk-in’ treatment day centre attracted some support during the focus group discussion.
Overwhelmingly, the most important treatment outcome for focus group participants was complete removal of their SCC and minimising the chances of it recurring:
When you find out you’re going to have an op, all you want to do is make sure the cancer is taken away completely. I don’t care how big it is, how deep, but just make sure you get it all away. That was my attitude to surgery.
Participant (male, 79 years)
The most important outcomes of treatment were considered to be removal of the cancer (10/24) and minimising the risk of recurrence (11/24); for the remaining three respondents, both outcomes were equally important.
Assurance that the cancer was removed was of greater importance than the size of the surgical wound itself; nevertheless, concerns were expressed about donor skin graft sites, which may prove to be more problematic than the recipient site.
Emotional experience
Anxiety, fear and the need for reassurance that the SCC has been treated adequately were all experienced by participants, indicating that SCC is a condition that is viewed as being as serious as other forms of cancer and not merely a trivial inconvenience to those affected by it:
When you hear that ‘c’ word, you naturally assume the worst, whether it’s a small cancer or a big cancer, you know, you naturally assume the worst and, I can’t think of anything to say – I want to be on this earth as long as possible . . . Anxiety, fear, I experienced all that. When they told me I thought my world had collapsed . . . But the fear and anxiety when you’re waiting to have to go and have it done was horrible, I wouldn’t want anybody to go through that. I thought my world was coming to an end.
Participant (male, 62 years)
Trust in the clinicians
The concept of trust in the treating physician underpinned much of the discussion in the focus group. Implicit faith in the knowledge and skills of the specialist was voiced by some of the participants, with unquestioning acceptance that the treatment they were receiving was in their best interest:
I trust the doctor; I trust doctors because that’s their job you know. I asked the surgeon ‘Did you get it out?’ and he says ‘I’m doing them every day, I think I got it all, I’ve cut more of it away but I think I got it all out.’ So I trust him . . . So I believe, I believe in my surgeon, and my life was in his hands.
Participant (male, 62 years)
I mean, you just go and he said ‘We’ll have to cut it out’ and you just say ‘Well, all right, just get on with it.’ I can’t tell them what to do. You just trust them.
Participant (male, 81 years)
On the other hand, some challenged the belief that the surgeon could confidently say that all the cancerous tissue had been removed.
The surgeon knows; he’s doing them all them every so when he cuts you open he can see roughly, I’m sure he can see, roughly what’s there and what he can get out.
I’d be interested to know the answer to that – can he? . . . Can the surgeon tell when he’s chopping away?
No, I don’t think he can.
Having confidence in the treating physician as a pre-requisite to participating in the proposed trial was raised in the questionnaire responses:
I would want to have faith in the surgeon/consultant giving the advice and/or operating.
Questionnaire respondent (male, 84 years)
Providing information to potential participants about the team involved in the trial may, therefore, be an important strategy to increase trust in those who are going to be administering the treatment arms, thereby encouraging participation.
Attitudes to research
Four main areas were identified relating to attitudes towards research:
-
Reasons for participating in research.
-
Study design considerations.
-
Willingness to participate in the proposed RCT.
-
Barriers to participating in clinical research.
Reasons for participating in research
During the focus group, both personal and more general reasons for taking part in clinical research were discussed. There was a general feeling that clinical research is important in this country. An altruistic sense of giving some benefit to others and giving something back for treatment received were common themes:
I love the thought of helping others if possible.
Participant (male, 62 years)
As for research, I’d be delighted to give something back to the Treatment Centre; they’ve treated me so well . . .
Participant (male,67 years)
I’m willing to go the extra yard to help others.
Participant (male, 80 years)
Any kind of research is for the benefit of us all, not just ourselves here but for everybody and it is quite important.
Participant (male, 79 years)
The idea was also expressed that in addition to helping others, discovering more about a disease and advancing treatment, the participant themselves may also benefit from taking part in research:
As I believe skin cancer will return to me, I would like to be involved in any research.
Questionnaire respondent (male, 62 years)
Study design considerations
The nature of the interventions in the arms of a RCT was considered to be an important factor that may influence the decision about whether or not to participate. Further to discussion about trials involving surgery and those involving new drugs, participants generally felt more comfortable with the idea of taking part in surgical trials:
I suppose there is a history, not necessarily in this area, but other researchers, where they have gone ahead and introduced drugs which have later on proved to be not quite what they thought. I suppose thalidomide is a name that comes to mind, but perhaps more drugs than – I think here we’re talking more surgery.
Participant (male, 67 years)
. . . I think it comes from the things you hear on the news – how somebody died I don’t know how many years ago from taking a tablet from research. So yeah, I would definitely be more comfortable with surgery.
Participant (female, 64 years)
Some participants were less sceptical than others about taking part in drug trials, although the idea was expressed that wariness about taking part in such trials may be tempered if they were terminally ill:
If I was terminally ill I would take anything; if it didn’t help me perhaps it would help someone else in the future, but yeah, if I was terminally ill that might be different.
Participant (female, 64 years)
The idea that participation in a clinical trial may result in getting a new treatment or in closer monitoring of their condition had been considered as an attractive reason to take part and some agreed that presentation of the trial in such a way may increase their willingness to participate, although this was not an issue for everyone.
Willingness to participate in the proposed randomised controlled trial
There was general agreement in the focus group discussion that, in principle, participation in the first surgical stage of the proposed trial would not be overly concerning, although reservations were expressed about having larger margins for SCCs located on the face (particularly in younger or female patients) or periorificially, where function could be compromised.
I think it could depend on who you are, how old you are, where it is as to how much . . . If you’re a young woman you might prefer as little as possible.
I think the site of this is important too. Obviously if it’s on the face you wouldn’t be volunteering for 10 mm if it wasn’t necessary, and it hasn’t been proved necessary yet.
I think if it’s near the eye or any other opening, if you think it’s going to affect the working of the eye by pulling the nerves or damaging the nerves or whatever then I would think a bit more carefully about this . . . but if it’s on your shoulder or whatever, then you’d take a bit more.
I’d go for that. If it’s on your face then go for as little as possible . . .
Problematic skin grafts following surgery had been experienced by some of the focus group participants and were discussed as a factor that would impact negatively on willingness to take part in a trial involving larger excision margins.
Willingness to participate in the second stage of the proposed RCT, in which ART after surgical excision will be compared with no ART, was more reserved. Several participants both in the focus group and questionnaire respondents expressed fear over having additional radiotherapy, with possible side effects and ‘doubts whether radiotherapy really works – too random kill or cure’ (male, 60 years) being raised as reasons for reluctance to participate.
One participant who had previously had radiotherapy as part of a trial was very suspicious that his skin cancer had been caused by the radiotherapy and has subsequently been left ‘very frightened of all forms of it’ (male, 80 years). The psychological impact of being in the ART arm of the proposed RCT was also raised as an issue in the focus group discussion, with the suggestion that the disease would be perceived as being more serious by the participant:
I think it would definitely take over your life and it also becomes in your head more serious . . . up to 6 weeks, you know, in your head, it’s more serious.
Participant (female, 64 years)
Barriers to participation
Other than the specific reasons indicated above regarding willingness to take part in the individual stages of the RCT, more general barriers to recruitment were identified in the focus group and questionnaires. Some of these were age-related: extra visits to hospital, feeling worn-out after hospital visits, lack of concentration, concern over the future and pre-existing deafness were some potential barriers that were of concern to individuals. The inconvenience of possible extra hospital visits in terms of time commitments, transport, travel and parking expenses was an important consideration for some. The retired focus group participants discussed that time may less of an issue for them but did feel that parking and travel expenses should be reimbursed for participants.
Attitudes to randomisation
Three areas relating to randomisation in RCTs were identified:
-
Understanding of the randomisation process.
-
Concerns about randomisation.
-
Hypothetical willingness to be randomised in the proposed RCT.
Understanding randomisation
The concept of randomisation was an area of confusion, with some focus group participants never having heard of the process and others having vague ideas about why and how randomisation is done:
Is that when they pick from all angles, all walks of life . . .?
I would imagine it’s a bit like Ernie that picks a number out and that’s the one you get.
. . . I think sometimes you use a control, so sometimes you give a placebo where you get no treatment at all.
Concerns about randomisation
Concerns about randomisation raised by respondents to the questionnaire (who had been given a brief outline of the randomisation process in the participant information sheet), related to misunderstanding of the purpose of randomisation, lack of equipoise and the perceived threat to the optimal care of the patient:
People might meet this situation as a life-or-death predicament and would therefore want the optimal treatment and not be randomised so they take pot-luck – whether or not radiotherapy is offered when it is something they might need – not to be withheld therefore.
Questionnaire respondent (male, 62 years)
Fear of not getting the treatment which is most effective for their SCC.
Questionnaire respondent (female, 64 years)
My hesitation arises out of the obvious concern that the randomised treatment selection will not be the optimum treatment for me . . . although I appreciate that the study is in fact an attempt to establish optimisation.
Questionnaire respondent (male, 76 years)
Interestingly, one participant felt that the uncertainty of the effectiveness of the different treatment arms in the RCT would actually discourage them from wanting to take part.
Further to explanation of the randomisation process in the focus group, the consensus was that randomisation should not be seen as threat to the best care of the participant, although there was still some concern about not getting the usual treatment for a condition and about delays to treatment which may be incurred during the research process:
Maybe if I wasn’t getting something that somebody normally got for what I have, so that I was actually not getting it, then I think . . . mm, is this the right thing to do?
Participant (female, 64 years)
If you had a very fast growing cancer like my wife, when you could almost sit at the table and watch it grow, then you’d want it going into fairly quickly.
Participant (male, 80 years)
Hypothetical willingness to be randomised into proposed trial
Questionnaire participants were asked whether or not they would hypothetically be prepared to be randomised into one or both stages of the proposed trial. Seven out of the 24 (29%) questionnaire respondents indicated that they would definitely be willing to be randomised to both stages of the proposed RCT and a further 5 (21%) indicated that they would probably be willing to be randomised to both stages. Two participants (8%) said they definitely would not want to randomised to either stage and another two (8%) were not sure. A further three (13%) respondents indicated that they would only want to be randomised for the first surgical stage of the trial and an additional two (8%) would probably be willing to be randomised to the first stage only. One respondent (4%) indicated willingness to be randomised to the second ART stage only. Therefore, in total, 17 (71%) participants indicated definite or probable hypothetical willingness to be randomised into the first stage of the RCT and 13 (54%) into the second stage.
However, in response to questions about preference for one treatment arm over the other for each of the stages of the trial, six of those who claimed they would definitely or probably be willing to be randomised indicated a strong preference for one of the treatment arms. Four of these respondents favoured a more radical approach, with preference for the larger surgical excision margin and ART.
Sounds to me like a better chance of removing everything.
Questionnaire respondent (male, 68 years)
Gives more chance of removing cancer (10-mm margin).
Questionnaire respondent (male, 86 years)
Again I feel it will help in complete recovery (ART).
Questionnaire respondent (male, 86 years)
Cosmetic reasons were given for preference for a smaller margin in the remaining two participants.
Discussion
This study has identified some previously recognised factors that may influence recruitment into clinical trials generally but, to our knowledge, is the first that has specifically addressed recruitment into a trial for cutaneous SCC. By recognising misunderstandings and concerns about clinical research generally, and the specific trial proposed, appropriate strategies can be devised to overcome these and to enhance recruitment.
We found that specific knowledge about cutaneous SCC was generally poor, with confusion about the causes of the condition and its prognosis, even among those who had a history of previous skin cancers. This confirms the findings of two other studies that have assessed the knowledge of patients with skin cancer, although neither of these related specifically to SCC. 516,704 In terms of recruitment into the proposed trial, a lack of patient knowledge about SCC in itself should not be a barrier as potential participants will receive patient information resources providing background information about SCC, including its natural history, the treatments options and possible implications of the different treatment arms. The way that such information is presented will be key to enhancing the understanding of potential participants. It should be clear, use language that is appropriate to a lay person and available in a choice of formats. The need for the provision of high-quality information that is appropriate to the needs of the patient at that point in their diagnosis, and repeated over time, has been highlighted previously. 675 Receiving a diagnosis of skin cancer induces anxiety and fear in those affected and the lack of recall of information given at initial diagnosis may be a reflection of the emotional state of the patient at the time. Skin cancer guidance advises that information provided should therefore be repeated over time and should be available in other formats such as audiotapes of consultations, videos or other specialised materials, if appropriate. 675 This was raised as an option that potential participants would like to have.
There was general recognition of the need for rapid diagnosis and treatment of SCC, and an overwhelming view that the most important outcome of treatment should be to completely remove the tumour and to minimise the risk of it recurring. Although generally not a life-threatening condition, those affected want minimal delays in referral and treatment. Compromised timeliness of treatment caused by the research process itself would, therefore, not be tolerated by potential participants. However, delays previously experienced by participants in this study were related to diagnosis of SCC rather than the treatment received after referral to the specialist. Current recommendations are that patients with suspected cutaneous SCC should be urgently referred to a specialist and seen within 2 weeks of referral. 675,705 Perceived delays in referral by the GP owing to misdiagnosis caused frustration and enhanced anxiety for those who had had such an experience and raised concern that some GPs may not be adequately educated to diagnose suspected SCC. The ability of GPs to diagnose skin cancer varies widely706,707 and although several studies have evaluated the impact of educational interventions on improving diagnostic accuracy among primary health-care providers, these tend to be isolated interventions and have generally not been rigorously evaluated. 708 Educational interventions have been found to be of variable effectiveness706,709 and modifying and maintaining clinical practice in the long term can be challenging. 710 Studies addressing educational interventions with outcomes that focus on performance changes are therefore required, but are outside the remit of the current study and the RCT we are proposing.
Understanding of the clinical research process and how and why randomisation is done was lacking among participants in this study. Nonetheless, there was consensus that clinical research is important and that it helps moves treatment forward. A sense of altruism was identified as a positive driver to participate in research. In addition, there was some feeling that having a terminal illness as opposed to less advanced disease in an individual may enhance this altruistic attitude. Other studies of willingness to take part in clinical trials in patients with metastatic disease as opposed to primary disease have confirmed this. 711,712 Conversely, reasons to participate may also be in the self-interest of the individual, based on the belief that the treatment they receive may be an advantage to them personally and that by helping to move the evidence base forward, they may themselves benefit from any advances should they require treatment for the same condition in the future.
Misunderstanding of the concept of randomisation, or the perceived advantage of one treatment arm over another, may be seen as threats to optimal care and may fuel uncertainty and additional anxiety that may compromise successful trial recruitment. Even though questionnaire respondents in this study had been given information about the nature of the randomisation process and its purpose, uncertainties and concerns about randomisation were evident from their responses, underpinning the need for clear, accurate provision of such information for potential trial participants. However, the idea of randomisation is difficult for lay people to accept and their interpretation may differ from that of medical professionals, so there may be a need for further discussions with potential recruits in order that they are informed sufficiently to be able to give informed consent. 713 Furthermore, provision of additional information has been shown to sway attitudes positively towards participation in those initially dissenting from taking part in cancer trials and careful in-depth discussion may resolve some of the concerns about randomisation. 714
Patient preference for one treatment arm over the other was expressed by some participants in this study. This included some of those who claimed that they would be hypothetically willing to be randomised into one or both stages of the trial, although the perceived benefit of one of the treatment arm is evidently going to influence their final decision whether or not to take part and risk receiving their non-preferred treatment. This attitude is a recognised barrier to recruitment715 and one that will need to be probed and challenged by researchers and clinicians who are presenting the trial and taking part in discussions with potential participants.
Several studies have found that the clinician introducing the trial to potential participants has the greatest influence on their decision to take part or not and that lack of confidence in the doctor will negatively influence this decision. 716 The role of trust in the knowledge and advice given by the clinician has been seen to be of importance to participants in this study. The level of this trust is therefore likely to have a positive impact on patients’ willingness to take part in the proposed trial. On the other hand, some patients may feel that the process of randomisation may compromise the doctor–patient relationship and undermine trust in the doctor, preferring instead for the clinician to make the any treatment decisions for them. Trust as a disincentive to participate in trials has also been reported elsewhere. 717 Clinical equipoise in individual clinicians recruiting participants into trials is of fundamental importance to a trial’s success and lack of equipoise may be a particular issue in trials involving surgical interventions. 718,719 Additional training of clinicians consenting participants may therefore be necessary to help overcome this. Another approach would be to run a parallel, non-randomised preference arm alongside the main RCT. 718
General willingness to participate in clinical research may be moderated by factors relating specifically to the trial in question. Regarding the proposed SCC trial, participation in the surgical stage was seen as less of a threat than, for example, taking part in a trial involving a new drug or in a trial in which there was the possibility of being randomised to a placebo arm, which has been recognised as a disincentive to participate in trials. 720,721 Although almost three-quarters of participants claimed they would definitely or probably be willing to be randomised into the first surgical stage, the decision about whether or not to participate may be complicated by potential cosmetic implications, particularly for younger, female, patients and for SCCs located on the face or in a functionally sensitive area (e.g. around the eye). Overall, hypothetical willingness to be randomised into the second stage of the proposed trial involving ART was less enthusiastic, with 54% claiming definite or probable willingness to be randomised. Concerns about receiving radiation were cited as the major barrier to participation in those who said they would not be willing to be randomised. In contrast, of the participants who did claim to be potentially willing to be randomised, those who expressed a preference for one of the treatment arms said they had a strong preference to receive the ART as they felt there was a better chance of it clearing the cancer. The proportion of potential participants who are hypothetically willing to be randomised is therefore likely to be an overestimate of the actual numbers, which will need to be taken into account when designing the trial. Other patient-related factors were identified as barriers to the proposed trial and primarily related to participation in the second radiotherapy stage. Some saw the extra hospital visits that would be required as part of the radiotherapy regime as an inconvenience in terms of time, transport and cost, so the provision of additional costs for reimbursement of trial-incurred expenses should be considered as a strategy to encourage participation. Advanced age, physical frailty, the presence of comorbidities and uncertainty about the future were also identified as reasons why some people may be reluctant to become involved in research. Age-related factors are recognised as a significant barrier to recruitment into trials701,722 yet, in the proposed trial, the population pool from which recruits will be drawn will be largely > 70 years of age. Exclusion criteria related to comorbid conditions and previous malignancies may therefore need to be modified in the protocol if older and more frail patients are to be included in the research and for the trial results to be externally valid. Extra resources may additionally be required for research personnel to spend time with older participants to explain the protocol and to obtain informed consent.
Strengths and limitations of the study
Evaluation of the understanding and concerns about clinical research generally and the specific trial being developed provides an insight into potential barriers that may affect the willingness to participate in such a trial and the opportunity to incorporate strategies in the protocol to overcome these barriers.
A limitation of this study is that all participants had previously had surgical treatment of a cutaneous SCC and, therefore, had more insight and expectations of treatment than people presenting for the first time and invited to participate in the proposed trial. However, as none of them had had ART after surgery, this insight would be less of an issue for the second stage of the proposed trial. An advantage of having participants with prior treatment experience is that it allows for evaluation of the needs for the provision of information resources and, given the lack of a patient support group for this condition, has supplied a pool of volunteers who are theoretically willing to review patient materials produced.
People who returned their questionnaires and took part in the focus group may be more motivated to take part in research generally; therefore, the numbers who declared hypothetical willingness to be randomised may not be a true reflection of the population pool generally and needs to be interpreted with caution. Although more males than females participated in this study, this was not intentional and reflects the demographics of the population that is affected by this condition.
Implications for research
This study has allowed us to evaluate potential barriers to participating in the proposed skin cancer trial in a population representative of those who would be eligible to take part in the trial itself. Importantly, those who participated in this study did not reject the proposed trial as being unfeasible.
Actual numbers who are willing to participate in each stage will not be known until the trial is under way and the suggestion from this study is that eligible participants are less likely to want to be randomised into the second stage of the trial. The incorporation into the protocol of an initial pilot phase to assess recruitment may therefore be prudent, with an alternative strategy such as a parallel non-randomised arm if participants express a strong preference for one treatment over the other or if recruitment does not reach target.
Understanding the needs and concerns of potential participants will allow the development of appropriate information resources for the trial, which will be of crucial importance to help potential participants make an informed decision about whether or not to take part in the trial.
Treatment uncertainties for high-risk squamous cell skin cancer: a trial proposal
Summary
What was already known about this topic?
-
There have been no RCTs that have directly compared the effectiveness of different treatments for SCC.
-
Treatment variability exists among clinicians who manage SCCs.
-
Locoregional recurrence occurs in approximately 5% of SCCs, but prognosis is poor once a SCC has metastasised.
-
A risk stratification model is required so that treatment decisions are targeted most appropriately.
What did this study add?
-
This proposed multicentre, multidisciplinary study will be the first RCT to address clinically important management uncertainties for commonly encountered primary, non-metastatic SCCs.
-
The proposed RCT will directly compare outcomes for high-risk SCCs treated with either conventional excision with a wide surgical margin or MMS and will provide data on the adequacy of clinical and histological margins.
-
The proposed RCT will evaluate the role of ART in treating the highest-risk SCCs.
-
This trial has the potential to generate high-quality data that will contribute towards the development of a risk-stratification model and assist management decision-making.
-
The results of the proposed trial will feed into clinical management guidelines and inform future research needs.
Introduction
As discussed previously in this chapter, there has been a lack of research comparing the effectiveness of SCC treatments and no RCTs have compared different treatments for the primary, non-metastatic, SCCs that are seen most commonly in everyday clinical practice.
The clinician survey that we conducted, and that is reported earlier in this chapter, identified areas of management uncertainty across specialities. This, together with the results of the systematic reviews we conducted, has identified topics which have formed the basis of the proposal for this RCT. Following extensive multidisciplinary discussions, a two-stage trial is being proposed that has been prioritised for further development by the NCRI melanoma CSG and which will be submitted for a funding application on the near future. In the first stage, T2 SCCs will be randomised to be excised with a either a 10-mm surgical excision margin or MMS. Furthermore, as there is currently a lack of evidence regarding the effectiveness of ART in treating SCCs, patients with SCCs that are identified as being at higher-risk of relapse (T2b) will be further randomised to receive ART or no radiotherapy in the second stage of the trial. It is also envisaged that the trial will provide useful data about the types of SCCs that are likely to respond better to the interventions being applied, which will help with the development of a prognostic model and will help clinicians to direct particular therapies most appropriately.
The protocol for this trial is still under development with support from the NCRI non-melanoma subgroup of the melanoma CSG and has not yet been submitted for funding. This section is therefore an outline of the proposal and so details may be subject to change and will be finalised after further discussion within the group.
Aims and objectives
-
To assess whether or not there is a difference in the rate and timing of locoregional relapse between patients with high-risk T2 SCCs that have been excised with a 10-mm surgical margin and those treated with MMS.
-
To assess whether or not there is a difference in the rate and timing of locoregional relapse in patients who are treated with post-operative radiotherapy and those who are treated by excision with a 10-mm margin or MMS alone.
-
To develop a prognostic model for treatment of patients with high-risk SCC.
Trial design
The proposed trial is a pragmatic, multicentre, two-stage, non-blinded, RCT comparing excision with a 10-mm surgical margins with MMS and comparing ART after surgery with no ART (Figure 58). The primary outcome will be time to first locoregional recurrence within 3 years of treatment, although participants will be followed up for 5 years after the end of treatment for assessment of late outcomes associated with radiotherapy.
FIGURE 58.
Proposed outline of SCC trial.

Owing to the nature of the interventions, treatment allocation will not be masked from the participants or PIs.
Setting and target population
Target population
Participants will be adults (at least 18 years of age) with primary invasive, non-metastatic, high-risk SCC of the skin. The Brigham and Women’s Hospital tumour staging criteria723 will be used to identify patients eligible for entry into the trial. Patients having a T2 SCC with at least one risk factor (tumour diameter ≥ 2 cm, poorly differentiated, perineural invasion of a nerve of calibre ≥ 0.1 mm, or invasion beyond subcutaneous fat ≥ 4 mm deep) will be eligible to be randomised into the first surgical stage of the trial. Only patients randomised during the first stage of the trial, and who have 2 or 3 of the above risk factors (i.e. T2b SCCs), will be eligible to be randomised into the second radiotherapy versus no radiotherapy stage of the trial. Therefore, all participants will take part in the first stage of the trial and patients who have not been randomised in the first stage will not be eligible to participate.
Setting
Recruitment and delivery of the interventions will be provided in secondary care, with identification of potential participants by the skin cancer MDT. The trial will be a multicentre trial and site selection will be based on (1) the availability of a dermatologist or plastic surgeon who is willing to be PI for the site, (2) the availability of a clinical oncology service that can administer the ART intervention and (3) proven track record of recruiting into other clinical trials.
Eligibility
Inclusion criteria: first stage (10-mm excision versus Mohs micrographic surgery)
-
Patients with a diagnosis of primary, invasive, non-metastatic, cutaneous SCC confirmed on diagnostic biopsy.
-
The SCC is staged as T2 and has at least one high-risk factor, based on the Brigham and Women’s Hospital staging criteria.
-
A 10-mm surgical margin is achievable at the site of the eligible SCC.
-
The patient is able and willing to give informed consent.
Exclusion criteria: first stage
-
Recurrent, previously treated, SCC.
-
Surgery contraindicated (e.g. coagulopathy).
-
Pregnant or lactating women.
Inclusion criteria: second stage (ART versus no ART)
-
The patient was randomised to excision with a 10-mm margin or Mohs in the first stage of the trial.
-
The SCC is staged as T2b and has two or three high-risk factors, based on the Brigham and Women’s Hospital staging criteria.
-
The patient is able and willing to give informed consent.
Exclusion criteria: second stage
-
The patient was not randomised to one of the surgical arms in the first stage of the trial.
-
History of prior radiotherapy for skin cancer or other conditions.
-
Radiotherapy contraindicated – location on back of hand or lower leg.
Only one eligible SCC per patient will be randomised.
Randomisation and blinding
The randomisation schedule will be generated by computer using a randomly varying block size and will be generated and held by the Clinical Trials Unit administering the trial. The trial administrator will carry out the randomisation via a web-based system and alert the surgeon as to which group they are in. The second stage of randomisation will take place after surgical excision and when histology is known in order to identify those SCCs meeting the additional layer of eligibility criteria. Only excised SCCs with histological confirmation of clearance of ≥ 1 mm from the peripheral margin and fascial plane deeper than the level of invasion for deep margin will be eligible for randomisation.
There will be no blinding of participants or PIs throughout the trial. Assessors of photographs of cosmetic appearance will be blinded as to participants’ identity, interventions received and time since treatment.
Interventions
Surgery
In the first stage of the trial, the intervention will be standard surgical excision with either a 10-mm margin of normal-looking skin around the SCC, or MMS to microscopic clearance of tumour. At presentation, all potentially eligible SCCs will have a diagnostic biopsy. SCCs with at least one high-risk factor (T2) will be eligible to be entered into the trial. Delivery of the MMS will be by a consultant dermatologist or plastic surgeon who has undergone a period of additional training in the technique.
Adjuvant radiotherapy
Adjuvant radiotherapy will be administered by a clinical oncologist. The exact regime has yet to be finalised but the total recommended dose is 45–55 Gy in 2–5 Gy fractions over the course of several weeks and should be given within 12 weeks of surgical excision. The treated area will include a pre-defined margin of normal-looking skin.
The follow-up of participants who receive only surgery and those who also have ART will be identical.
Outcome measures
Primary outcomes
Primary outcome will be time to locoregional recurrence from initial randomisation up to 3 years after treatment. A standard definition of local recurrence will be drawn up to distinguish it from metachronous (new primary) tumours.
Secondary outcomes
The secondary outcomes will be as follows:
-
Time to distant metastases within 3 years of initial randomisation.
-
Time to tumour-related death within 3 years of initial randomisation.
-
Overall disease-free survival (time from randomisation to death from any cause).
-
Completeness of surgical excision by measurement of histologically clear margin.
-
Number of Mohs layers required to clearance of tumour.
-
Quality of life at baseline and at each follow-up consultation after completion of treatment. The tool for assessing QoL will be the Skin Cancer Index,724 which is a validated disease-specific tool for patients with NMSC.
-
Cosmetic appearance of treated area assessed photographically at baseline (post surgery but before radiotherapy if applicable) and 2 and 5 years post treatment by three assessors blinded to participant identity, treatment allocation and year of follow-up.
-
Within-trial cost analysis from a NHS perspective.
Study schedule and data collection
Screening for the first stage of the trial will take place on identification of a high-risk SCC as defined in the trial protocol. This will either be on the initial visit to the skin cancer clinic after referral from primary care or, for SCCs only identified as being high-risk after histology results are available and, therefore, eligible for randomisation, on the first clinic visit after initial surgery. Randomisation into the second stage will take place when full histological classification after surgery is available which will allow for identification of the highest-risk SCCs as defined in the protocol. Participants potentially eligible for randomisation will be discussed in the skin cancer MDT. Participants randomised to receive ART will be treated no more than 12 weeks after their initial surgery.
Participants will be followed up on a 3-monthly basis until first recurrence or for 3 years after initial randomisation, with a final assessment at 5 years to allow for assessment of late outcomes associated with radiotherapy. As this is a pragmatic trial, there will be a window of flexibility for the 3-monthly visits. Participants will be given information sheets advising them how to self-examine the treated area, local skin and lymph nodes, and will have access to the research team between clinic visits should they have concerns about recurrences or new tumours.
Histological confirmation of suspected relapses should be sought when possible. When histology is not readily obtainable, radiological or photographic evidence should be recorded of the relapse. Management of relapses will be at the discretion of the treating clinician in discussion with the SSMDT. Management of distant metastases may involve the enrolment of the patient in other clinical trials appropriate to that scenario. The proposed study schedule is summarised in Table 73.
| Identification high-risk SCC | Day 0a | Within 2 weeks | First post-surgery visit/identification highest-risk SCCs | 2–12 weeks post surgery | Follow-up as per protocol up to 5 years | 5 years | |
|---|---|---|---|---|---|---|---|
| Informed consent and counsellinga | ✓ | ||||||
| Randomisation to first stage (surgical) | ✓ | ||||||
| Surgical intervention | ✓ | ||||||
| Outcome measurement: margin clearance/number of Mohs layers | ✓ | ||||||
| Baseline QoL | ✓ | ||||||
| Baseline photography, clinician and participant rating for cosmetic appearance assessment | ✓ | ||||||
| Informed consent and counsellingb if applicable | ✓ | ||||||
| Randomisation to second stage if applicable (ART) | ✓ | ||||||
| ART intervention | ✓ | ||||||
| Outcome assessment (time to locoregional recurrence, distant metastases, SCC-related death | ✓ | ||||||
| QoL assessment | ✓ (6 months, 2 years) | ||||||
| Photographic, clinician and participant rating for cosmetic outcome assessment | ✓ (2 years) | ✓ | |||||
| Overall survival | ✓ |
Health economics
An economic evaluation will be conducted alongside the trial and will be incorporated into the protocol. A health economist with experience of cost analysis will be on the Trial Development Team.
Statistical analysis and sample size
Sample size will be calculated using the baseline recurrence rates established as part of the 5-year outcome assessment work which was conducted earlier in this project and from recurrence rates in the systematic review of observational studies. 536 The sample size will be calculated by a statistician with experience of cancer trials who will be a member of the Trial Development Team. All analyses will be documented in the SAP, which will be finalised prior to database lock. This will also include methods to deal with missing data and sensitivity and subgroup analyses when appropriate.
Ethical arrangements
The main ethical issues are:
-
that eligible patients should be aware of the uncertainty regarding the best approach to the management of high-risk primary SCCs
-
that trial participation must not delay the pathway to the definitive treatment of SCC.
The key members of the SSMDT managing SCC, typically dermatologists, clinical oncologists and plastic surgeons, will be local investigators. Approval by the REC and local Research and Development team will be obtained before investigators enrol participants. Clinicians will retain responsibility to take immediate action to protect the health and interest of individual participants.
Risk and anticipated benefits for participants
Surgery is the current mainstay of treatment for patients with SCC of the skin and is generally safe; however, there is a small risk of excessive bleeding and infection. Some tumours, particularly those that are large or in cosmetically complex areas may require a flap or graft for repair. Furthermore, patients who are unable to lie down because of a comorbid condition may not tolerate MMS, which is a potentially lengthy procedure.
Radiotherapy is an established treatment modality for cutaneous SCC, either on its own or as adjuvant therapy, and is generally well-tolerated in this context. As multiple treatment sessions are required, patient convenience may be compromised. Ionising radiation is also associated with a small increased risk of cutaneous carcinoma within the treatment field. Atrophy, hypopigmentation, alopecia and telangiectases are also commonly seen late cutaneous sequelae of radiotherapy, which may be unacceptable for younger patients. Owing to the risk of radionecrosis, radiotherapy is not advisable for lesions overlying bone or cartilage.
Participants will be made aware of the risks in the participant information resources as well as when they are counselled for informed consent. Incidence of adverse events will be monitored throughout the trial.
Potential benefits to participants cannot be guaranteed, although all participants will have surgical excision to manage their primary disease. Participants who experience emotional distress as a result of participating in the trial will be offered details of a counselling service.
Informed consent
The nature and purpose of both stages of the trial will be explained to potential participants when they are first approached to take part. However, they will be required to give their written informed consent separately for each stage of the trial, if applicable. All participants will give their written informed consent prior to randomisation to one of the two surgical arms. Participants who are then eligible to take part in the second stage of the trial on the basis of their high-risk pathology will give written informed consent prior to randomisation to receive ART or no ART. A trained member of the research team will counsel participants about the reasons that the trial is being conducted, potential risks associated with the interventions and the purpose of randomisation. Participants will have time to consider whether or not they wish to give their informed consent and will have access to a member of the research team to discuss further if required. Participants’ rights to decline trial participation without giving a reason will be respected.
Informing participants of possible benefits and risks
Participant information leaflets (PILs) will be prepared in line with current guidelines and will be informed by the results of the feasibility study which was undertaken as part of this project. These will contain information about the trial, how the trial may affect patients, and outline likely benefits and risks to participants. The trial will also have a dedicated website containing this information and details of the research team.
Research governance
The trial will be run in accordance with the sponsor’s SOPs and managed through a Clinical Trial Unit with expertise in cancer trials. An independent TSC will be established prior to initiation of the trial, which will oversee the conduct of the trial. A DMC will be set up to ensure participant safety throughout the trial.
Confidentiality of data
All participants’ data will be handled and stored in accordance with the sponsor’s SOPs and the Data Protection Act. 218 Trial documentation will be retained using secure archiving facilities for 7 years.
Trial regulation requirements
As the trial involves radiation, a Medical Physics Expert (a registered clinical scientist registered with the Health Professions Council) will be involved with writing the ethics application and a study contact.
The trial will be registered on an approved trial registry prior to the start of recruitment and the protocol and analysis plan will be published in full.
Patient and public involvement
In contrast to some chronic skin diseases, there is no patient support group for people who are affected by cutaneous SCC. Nevertheless, we have aimed to involve patients and stakeholders throughout the work package whenever possible. Early engagement with all key stakeholders will help to encourage the wide dissemination of the results of the final study, leading to early adoption of the trial findings and impact on guidelines and policies.
During the process of identifying a topic for future research in the form of a RCT, we engaged with clinicians from various specialties that are involved in the day-to-day management of patients affected by SCC. Their helpful comments and suggestions led to the identification of potential RCTs and have provided a pool of potential collaborators for the trial itself.
Patients themselves have had, and will continue to have, a vital role throughout the life of the trial. A former skin cancer patient was a coauthor on our Cochrane Systematic Review,518 providing valuable input from a lay person’s perspective. Furthermore, as we have taken the trial proposal forward towards a funding application in collaboration with the NCRI non-melanoma subgroup of the melanoma CSG, we have received insightful comments on the proposal from the patient representative who sits on that group. However, it has been in the feasibility study in which patients have had the greatest impact on our work. Evaluation of the feasibility of the proposed trial from the perspective of potential participants was considered crucial in guiding the design of the proposed RCT and to aid the development of participant resources. In addition, the early identification of possible barriers to recruitment was important in order to develop strategies to optimise recruitment, particularly from a target population which is predominantly elderly. The feasibility study took the form of a questionnaire and focus group. We sought the opinion of members of the Centre for Evidenced Based Dermatology patient panel, including some who had a history of skin cancer themselves, in an interactive workshop session in which the questionnaire was refined and delivery of the questionnaire and focus group was discussed. Patients who had been treated for SCC over the course of the previous 12 months and who were representative of the population from which participants in the proposed RCT will be recruited took part in the feasibility study. Key points were identified from this work; these have already informed the proposed RCT and will continue to do so as participant resources are developed for the trial.
-
Receiving a diagnosis of SCC induces anxiety and patients may struggle to recall information given at the initial consultation. Repeating information and the use of non-written formats such as videos and audiotapes may help overcome this. The availability of participant information resources in a variety of formats is desirable.
-
Information resources must be in plain language that is easily understood by patients from a range of educational backgrounds.
-
The concept of randomisation is poorly understood and will need thorough explanation if recruitment to the trial is to be optimised. This may require additional staff and time resources to ensure participants understand and are comfortable with its principles.
-
Randomisation to one of the surgical arms of the trials would generally not be problematic for feasibility studies participants, unless the SCC was located in a cosmetically or functionally sensitive area. Fewer participants would feel comfortable about being randomised to receive ART or not, mainly owing to their perceived fear of receiving radiation. This could have an impact on the successful recruitment into the second stage of the proposed RCT and is being taken into account when writing participant information sheets. The degree of risk of radiation when used in this context must be carefully explained and the notion of clinical equipoise reinforced.
-
In view of the advanced age of potential participants in the proposed RCT, exclusion criteria in terms of existing comorbidities should not be too rigid and extra staff and time input may be required to allay the concerns of older patients.
-
Financial arrangements should be in place to cover the costs of extra hospital visits that may be incurred by participants randomised to receive ART.
-
Importantly, potential participants did not reject the proposed RCT as being unfeasible from a patient’s perspective.
This programme of work has shown that patients with SCC would like to know more about their condition, but also that there is interest in helping with future research. It will be important to include a patient representative on the TSC. Furthermore, we now have a group of patients from the focus group who are willing to review documents and other resources produced for participants in the trial.
Summary and conclusions
Why is this research programme important?
Squamous cell skin cancer is the second most common type of NMSC, with approximately 20,000 new cases of SCC diagnosed in the UK each year; however, this is likely to be a significant underestimate. Although the prognosis is generally good after surgery, between 3% and 6% of patients experience locoregional recurrence and an estimated 550 deaths occur annually in the UK as a result of NMSC, the majority of which are attributable to SCC. The incidence of SCC is increasing worldwide, yet the evidence base for its treatment is poor.
This SCC work programme commenced with a Cochrane Systematic Review of treatments for primary non-metastatic SCC that highlighted the need for well-designed randomised studies in order to improve the evidence base for the management of this very common condition. A second review of observational studies was undertaken to comprehensively assess the currently available next best evidence from non-randomised studies (overwhelmingly case series). 536 This review, now published in the British Medical Journal536 and has been used to inform the SIGN guidelines725 for the treatment of SCC.
An online survey of clinicians was undertaken to gain an overview of current SCC treatment practices, to understand which outcomes clinicians considered to be of greatest importance, to offer clinicians the opportunity to suggest potential trials and to gauge their interest in taking part in a future trial. Two key areas of uncertainty relating to the management of high-risk SCC were identified: optimal excision margins and the role of ART. After iterative discussions with multidisciplinary colleagues at the NCRI non-melanoma subgroup of the melanoma CSG, a proposal for a pragmatic two-stage RCTs addressing these areas of uncertainty has been agreed on and has been incorporated into the melanoma CSG’s strategic plan. This will be the first RCT to include patients with the forms of SCC that are seen in routine clinical practice and to compare the effectiveness of different management strategies for high-risk SCCs.
Implications for clinical practice
The current mainstay of treatment for SCC is surgery, either with conventional excision of tumour alone with a margin of normal-looking skin, or MMS. Other treatments in the current management guidelines517 include radiotherapy, either alone or as an adjunct to surgery, curettage and cautery, or cryotherapy. Treatment choice is based on tumour and patient characteristics. However, treatment practices such as excision margins and ART are not standardised and vary according to individual clinicians and, in some cases, the availability of facilities for particular treatments.
The clinician survey and evidence appraisal conducted as part of this Programme Grant indicated that there are areas of management uncertainty that are not addressed in current guidelines. The proposed RCT will provide much needed evidence to inform future guideline development regarding excision margins, the use of MMS and the role of ART in treating high-risk SCCs. Stratification of patients according to their risk will guide clinical practice and will assist with clinical decision-making regarding the most appropriate treatment for individual patients.
During the course of the Programme Grant, the following guidelines have made use of the work:
Implications for research
This is the largest programme of dedicated clinical research into SCC treatment to date and has stimulated interest in SCC research.
The identification of gaps in the evidence and areas of treatment uncertainty has resulted in the development of a proposal for the first RCT of its kind for this very common cancer. Important research questions will be addressed in this trial:
-
Adequacy of excision margins in surgical excision of high-risk SCCs.
-
Effectiveness of Mohs surgery compared with surgical excision.
-
The role of ART.
The results of the analysis of SCCs submitted to histopathology have given an indication of the number and types of high-risk SCCs eligible for inclusion in the RCT and the frequency of outcomes in patients within 5 years after treatment, which is informing development of the protocol.
Patients feel that the proposed RCT is feasible from the perspective of potential participants. However, they identified that poor understanding of the nature and purpose of randomisation and perceived lack of equipoise regarding the treatment arms could be major barriers to successful recruitment, and will need to be taken into account when presenting the trial to participants. Furthermore, their information requirements will be taken into account when producing participant resources for the trial to ensure that these are delivered in the most appropriate formats.
Future research recommendations
Given the current lack of studies of good methodological quality, there is certainly scope for the development of further RCTs in the future. Our clinician survey has indicated that, apart from the areas of uncertainty that will be investigated in our proposed trial, there is also interest in the role of newer agents such as cetuximab to treat SCC and the optimisation of follow-up schedules for SCCs according to their risk of recurrence. Patient-reported outcomes and QoL measures should be integral to future SCC research. In addition, as the population becomes increasingly aged and the burden of SCC on health service provision continues to grow, evaluation of cost-effectiveness of different treatments will be an important consideration.
Patients affected by SCC clearly deserve to receive treatments that are both appropriate for them and supported by good-quality evidence. However, SCC does not behave in the same way as other skin cancers, such as BCC, and the evidence base for treatments is currently very poor. The work of this Programme Grant has helped to address this issue, with a view to informing guidelines and future clinical decision-making and by generating a step-change in data needed to effectively plan for a definitive national RCT.
Chapter 5 Pyoderma gangrenosum work programme
Abstract
Introduction
Good-quality data to inform practice on the management of pyoderma gangrenosum (PG) are lacking. Anecdotal evidence and retrospective case series indicate that prednisolone and ciclosporin are among the most commonly used oral treatments.
Methods
The Study of Treatments fOr Pyoderma GAngrenosum Patients (STOP GAP)727 trial comprised two parts:
-
A multicentre, parallel-group, observer-blind RCT of ciclosporin (4 mg/kg/day) versus prednisolone (0.75 mg/kg/day).
-
A prospective observational cohort study of patients treated with topical therapy.
Results
A total of 121 patients out of a planned 140 were enrolled into the RCT (n = 59 prednisolone and n = 62 ciclosporin). There was no evidence of a difference between ciclosporin and prednisolone in the velocity of healing over 6 weeks, adjusted mean difference 0.00 cm2/day (95% CI –0.20 to 0.21 cm2/day; p = 0.975). Median time to healing was 134 days for ciclosporin compared with 112 days for prednisolone. In both groups, < 50% of lesions had healed by 6 months and almost 30% had a recurrence. Approximately two-thirds of patients experienced adverse reactions, with more serious infections occurring in the prednisolone group and more hypertension and renal dysfunction occurring with ciclosporin.
Sixty-six patients, with generally smaller lesions, were enrolled into the observational study of topical therapy, 74.2% of whom received the superpotent corticosteroid, clobetasol propionate 0.05%. By 6 months, 43.8% of ulcers had healed on topical therapy alone and the median time to healing was 45.5 days (IQR 29.5–160.0 days). Baseline ulcer size was a significant predictor of time to healing (hazard ratio 0.94, 95% CI 0.88 to 1.00; p = 0.043) and 14.8% patients had a recurrence. One-third of patients required systemic therapy.
Conclusions
Ciclosporin and prednisolone demonstrated similar efficacy in treating PG, although neither treatment was associated with a good healing rate. Many patients with limited PG can probably be managed effectively with topical therapy, thereby avoiding the adverse reactions associated with systemic treatments. The choice of systemic treatment is probably down to patient choice, guided by the likely adverse event profile.
Content
This chapter describes two complementary studies relating to the treatment of PG as outlined below.
| Details | Comment |
|---|---|
| A randomised controlled trial of prednisolone versus ciclosporin in the treatment of pyoderma gangrenosum: STOP GAP trial | Contains details of a national multicentre trial which compared prednisolone and ciclosporin for the treatment of PG |
| Treatment response of patients receiving topical treatments for pyoderma gangrenosum: a prospective cohort observational study | An observational study that was run in parallel with the RCT described in the previous chapter, which monitored the response of patients receiving topical treatments |
| Patient and public involvement | A summary of how patients and practising clinicians have contributed to the design, conduct and development of this work programme |
| Summary and conclusions |
A randomised controlled trial of prednisolone versus ciclosporin in the treatment of pyoderma gangrenosum: STOP GAP trial
The following text is adapted from Ormerod et al. 727 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
Summary
What was already known about this topic?
-
Pyoderma gangrenosum is a severe, painful ulcerative condition of the skin, with a significant mortality.
-
The evidence base for current treatments is very weak, with a single published RCT involving 30 participants.
-
In more severe cases, prednisolone has historically been the main systemic therapy, but many clinicians have since switched to another systemic therapy, ciclosporin, in the belief it is more effective and has fewer side effects.
-
Both prednisolone and ciclosporin have significant predictable side effects.
What did this study add?
-
The hypothesis that ciclosporin is more effective than prednisolone was not supported by our trial evidence. Both agents were of similar efficacy and neither were outstanding, with only about 50% of ulcers being healed by 6 months.
-
We found that adverse events were very common (around two-thirds of patients) in both groups and the nature of the adverse event profile (serious infections with prednisolone and hypertension and renal dysfunction with ciclosporin) may help to inform which treatment could be considered according to underlying patient risk factors.
-
Recurrence of PG is common – approximately one-third of cases will suffer a further episode within 2–3 years.
-
Current therapeutic strategies are inadequate and further research into more effective treatment options is required.
Introduction
Pyoderma gangrenosum is an inflammatory ulcerative skin disease, which is frequently painful and often occurs in association with conditions such as inflammatory bowel disease (IBD), arthritis or haematological malignancy. 728 A 2012 retrospective cohort study in the UK has reported an adjusted incidence rate (standardised to the European standard population) of 0.63 per 100,000 person-years. 729 The development of PG was associated with a threefold increased risk of death compared with general population controls, and a 72% increased mortality over controls with IBD.
There are currently no national or international guidelines covering the management of PG. Patient information issued by BAD describes topical and systemic treatment options, as well as lesser used options such as intravenous steroids or biologics. Topical treatments for PG include potent steroid preparations or calcineurin inhibitors and commonly prescribed systemic treatments comprise antibiotics, steroids and immunosupressants. 730 Only one RCT in patients with PG is reported in the literature. 731 This small study of 30 patients compared the anti-tumour necrosis factor (TNF)-α monoclonal antibody infliximab (5 mg/kg) to placebo. Significantly more patients in the infliximab group demonstrated clinical improvement at 2 weeks than placebo (the primary endpoint; 46% vs. 6%, respectively; p = 0.025). However, owing to its cost, infliximab is not currently considered a first-line treatment for PG.
Consistent with the lack of good-quality RCT evidence, systematic reviews of treatments for PG have primarily relied on anecdotal reports or retrospective case series. 732 Based on the available evidence, systemic corticosteroids such as prednisolone are generally considered to be the most predictable, effective, medications for PG when delivered in adequate doses. 733 However, retrospective data also lend support to the use of ciclosporin. A number of case reports document complete remission of steroid refractory, IBD-related PG lesions with ciclosporin. 734,735 Complete response was reported for all participants in a study of five patients receiving 4–5 mg/kg/day of oral ciclosporin734 and 11 patients receiving the drug at an initial concentration of 4 mg/kg/day intravenously. 735 Other case series have reported encouraging proportions of patients, with a range of underlying diseases, achieving complete responses to ciclosporin (10 out of 11736 and 3 out of 7737). Vidal et al. 738 and Vidal and Alomar739 performed a review of 26 cases of classical PG. A total of 3–6 mg/kg/day of ciclosporin was used in 22 of these patients, with 51 episodes between them. Among these episodes, a complete response was recorded for 96% and a partial response for 3%. The second most commonly used treatment was prednisone, used in 15 patients and 26 episodes. Complete responses were recorded in 61% episodes, partial responses for 26% and no response for 11%. However, data from these studies are challenging to interpret as the majority of patients were receiving concomitant steroids rather than ciclosporin alone.
Given the complete absence of high-quality evidence on the first-line treatment of PG, a RCT (STOP GAP) was conducted in order to test the hypothesis that ciclosporin was superior to prednisolone in the treatment of PG. 727
Methods
A summary of the trial methods is presented here. For further details, please consult the trial protocol740 or the trial website. 741
Trial design and oversight
The STOP GAP trial was a multicentre, parallel-group, observer-blind, RCT to compare the efficacy and safety of ciclosporin with prednisolone. It was a pragmatic trial that reflected current practice as far as possible. Patients were assessed at baseline, 2 weeks, 6 weeks and when the ulcer had healed (maximum of 6 months). Appropriate national ethics and regulatory approvals (ethics: 09/H0903/5, Medicines and Healthcare Products Regulatory Agency: 19162/0213/001, EudraCT: 2008–008291–14) were obtained and all participants gave written informed consent. The trial was co-ordinated from the NCTU at the University of Nottingham. Oversight of the trial was performed by monthly Trial Management Group meetings and an independent TSC that met twice a year. All data issues, including safety, were overseen by progress reports presented to an independent DMC. The trial was registered at Controlled-Trials.com (ISRCTN35898459) prior to start of recruitment.
Participants
Recruitment took place at 39 hospitals in the UK and inclusion and exclusion criteria are listed in Box 8.
Pyoderma gangrenosum as diagnosed by the recruiting dermatologist. (An ulcerative lesion may have mixed aetiology, but provided the investigator has confidence that a clinical diagnosis of PG is appropriate then they are eligible. Other contributing factors and atypical features will be captured in the case report form.)
Must have a measurable ulceration (e.g. not pustular PG).
Aged > 18 years.
Able to provide written, informed consent.
Exclusion criteriaGranulomatous PG – this condition is very rare and may respond differently to treatment.
Ciclosporin or prednisolone or intravenous immunoglobulin therapy in the previous month.
Already participating in another clinical trial.
Pregnant, lactating or at risk of pregnancy.
Hypersensitivity to prednisolone or ciclosporin.
Biopsy consistent with a different diagnosis.
Biopsies will be used to exclude alternative aetiologies (e.g. malignancy, granulomatous PG, arteritis) rather than to confirm the diagnosis of PG, as histology is supportive rather than pathognomonic. Ideally, the biopsy will be a 1.5-cm rectangular biopsy taken through the edge of the ulcer and left to granulate and heal by secondary intention. Alternatively, two separate punch biopsies done at the edge of the ulcer and at the extending margin may be used. It is not normal practice to await histological confirmation before initiating therapy, so patients will be randomised prior to receiving histological results. If the histology indicates an alternative aetiology, the participant will be excluded at that time.
Clinically significant renal impairment that would result in the investigator not normally treating with either study drug.
Any pre-treatment investigations, the results of which would prompt the investigator not to use either study drug.
A diagnosis of malignancy or pre-malignant disease where treatments might interfere with ongoing therapy or might cause harm (e.g. history of lymphoma, multiple lymphoma, leukaemia, CIN, systemic cytotoxic therapy).
The patient has a concurrent medical condition that means the investigator would not normally treat the patient with either of the study drugs (e.g. a degree of hypertension that would not lead to using either of the study drugs, advanced heart failure, poorly controlled diabetes, history of peptic ulcer, malignancy in previous years).
Administration of a live vaccine (Bacillus Calmette–Guérin, measles, mumps, rubella, yellow fever, oral polio, oral typhoid) within the last 2 weeks.
The patient is currently taking rosuvastatin (Crestor®, AstraZenica) for the treatment of hypercholesterolaemia, as this is contraindicated when taking ciclosporin.
Participants were asked not to use any topical therapy (e.g. corticosteroids or calcineurin inhibitors) after randomisation. Patients who required first-line topical therapy were invited to enter a parallel observational study (see Treatment response of patients receiving topical treatments for pyoderma gangrenosum: a prospective cohort observational study).
Interventions
Participants were randomised to receive either 0.75 mg/kg/day of oral prednisolone in a single dose or 4 mg/kg/day of ciclosporin in two divided doses. The dose of study drug could be adjusted (up or down) according to normal practice, but clinicians were encouraged not to alter the dose until week two if possible. The maximum increase permitted per day was 1 mg/kg/day for prednisolone and 5 mg/kg/day for ciclosporin.
A change to the protocol was made in August 2011 after recruitment of 82 participants. A patient with a very high body mass index who was randomised to prednisolone experienced bowel perforation on a dose of 110 mg/day. As a result of this serious adverse event, ceiling doses of 75 mg/day of prednisolone and 400 mg/day of ciclosporin were implemented from thereon, regardless of body weight.
Randomisation and blinding
Participants were randomised (1 : 1) to treatment allocation using a computer-generated pseudorandom list, using permuted blocks of randomly varying size between two and six (using the ralloc add-on for Stata, Stata Corporation, Texas, USA). Randomisation was stratified by lesion size (≥ 20 cm2 vs. lesions < 20 cm2) and presence or absence of underlying systemic disease. For the purposes of randomisation, lesion size was estimated based on the maximum longitudinal length and maximum perpendicular length and converted to approximate area by the formula (Equation 1), which approximates to an ellipse.
Treatment allocation was concealed until interventions were all assigned and recruitment, data collection, data cleaning and analysis using sham treatment codes were complete except for the purpose of DMC analyses.
This was an observer-blind study. The primary outcome (velocity of healing) and global treatment response were assessed from digital images of the target lesion by assessors blind to the allocated treatment; however, clinicians and participants were aware of their treatment allocation. Full blinding was not possible owing to logistic and methodological difficulties in blinding treatment allocation. For example, the two drugs require different dosing regimens and different arrangements for monitoring of side effects. Blinding the trial interventions using placebo medications was beyond the scope of this pragmatic trial and may have had a detrimental effect on treatment adherence resulting from need for additional tablets. Nevertheless, treatment allocation was only revealed to the recruiting physician once participants’ details and key stratification variables had been irrevocably entered by the physician onto the web-based randomisation system maintained by NCTU.
Assessments
Clinic visits were conducted by a dermatologist and took place at baseline, week 2, week 6 (primary outcome) and when the ulcer had healed (up to a maximum of 6 months post randomisation). Clinic visits consisted of standard clinical tests, medical history taking, assessment of side effects, measurement of the ulcer and clinician’s evaluation of the target lesion. A digital image of the target lesion was also taken at baseline, week 6 and final visit. The same clinician saw the participants at each clinic visit whenever possible.
Participants assessed the severity of their PG and QoL at baseline, 6 weeks and on healing (or 6 months if not healed) (see Appendices 17 and 18 for baseline CRF and week 6 CRF). In addition, they completed a study diary that captured daily pain scores and use of analgesics for the first 6 weeks, plus impact on daily activities, use of dressings, adverse events and the use of health services throughout the trial (see Appendix 19). Adherence to trial medication was assessed using patient diaries. These data were categorised as using medication every day, most days, some days or never.
At the end of the trial, investigators obtained hospital records of those for whom the target lesion had healed to ascertain the recurrence of PG and time to recurrence.
Digital images were used to assess the blinded outcomes of velocity of healing and global treatment response. If digital images were not available, then physical measurements of the lesion taken during clinic visits and global response by the treating clinician were used.
A standardised template was photographed alongside the target ulcer in order to calibrate the image in the image analysis software (Figure 59). Images were stored electronically and transferred in an anonymised fashion to the co-ordinating centre at NCTU. Each image was loaded into the image analysis software and the circumference of the lesion was manually drawn by two trained assessors (Bryony Elliot and Joanne Perdue) using Verge Videometry VEV MD software (Vista Medical, Winnipeg, MB, Canada).
FIGURE 59.
Pyoderma gangrenosum ulcer measurement using image analysis software.
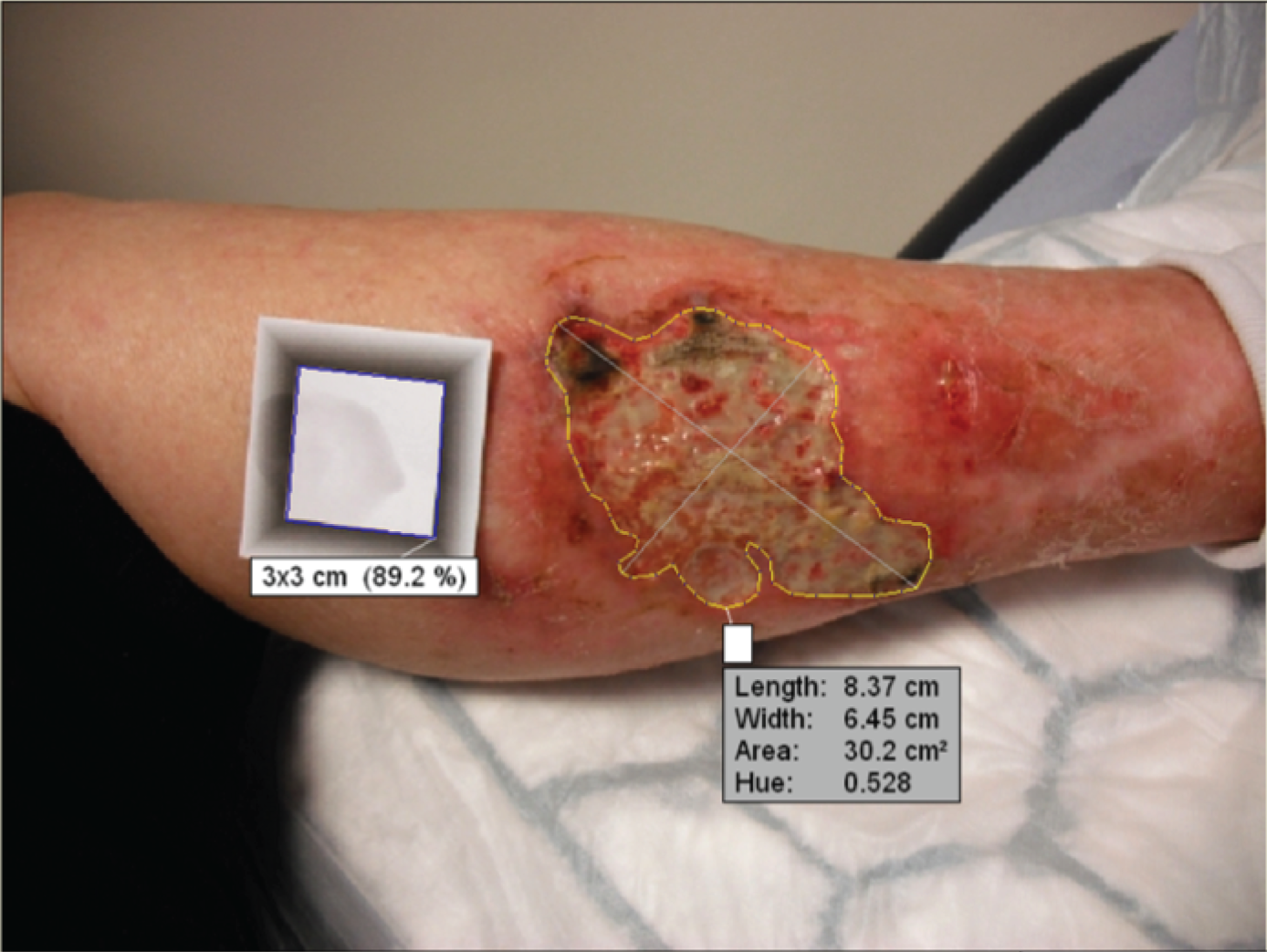
For the global treatment response, an independent dermatologist (Suyin Ong) scored patients’ treatment response using a pair of images from baseline and final visit.
Quality control of digital image assessments
All images were independently reviewed by two dermatologists to ensure that the lesions were consistent with a diagnosis of PG and that the measurements taken by the trained assessors were an accurate representation of the ulcer size. In cases when discrepancies were observed, the rules in Table 74 were applied.
| Image assessments | Action |
|---|---|
| Both measurements map the lesion appropriately and measurements agree within a ratio of 1 : 1.1 | Use mean of two measurements |
| Both measurements map the lesion appropriately but measurements disagree by a ratio of > 1 : 1.1 | Two dermatologists to remeasure and use the mean of the new measurements |
| One measurement has mapped the lesion incorrectly (e.g. when healed areas have mistakenly been included owing to residual erythema or surface changes) | Discard the image that was mapped incorrectly and use the second measurement |
| Both measurements have mapped the lesion incorrectly | Dermatologists to redraw the measurement and use this for analysis |
Outcomes
Primary outcome
Velocity of healing at 6 weeks measured used the formula below (Equation 2). Date X is the earliest of either the date at which the lesion stopped requiring dressings if this occurred prior to 6 week visit, or the date of the 6 week visit. Healing was captured for a single target lesion per patient. If multiple lesions were present, the lesion that could be photographed on a single plane (i.e. not around the curvature of a limb) was designated the target lesion.
Velocity of healing was chosen for the primary outcome as it has been shown in previous studies to be a good predictor of healing in patients with leg ulcers742,743 and because blinded assessment was possible using digital images and independent assessors. Being able to assess velocity of healing at 6 weeks also minimised the risk of missing data. Nevertheless, it was always our intention that time to healing be considered the most important secondary outcome, as it is more clinically relevant and is easier to interpret. Time to healing also gives an indication of the duration of treatment and, therefore, the potential for cumulative drug toxicity.
Secondary outcomes
Assessed by participants based on the time at which sterile dressings were no longer required for the wound and confirmed using digital photography at the first opportunity. If the date the lesion stopped requiring dressings was not recorded, the date of the clinic visit was used.
A seven-point Likert scale ranging from completely clear to worse (assessed by clinicians, participants and by digital images).
This is a previously published PG assessment scale including erythema and border elevation. 744 Erythema and border elevation are each scored from zero to four (representing none to very severe). Resolution of inflammation was taken to have been achieved if both items were scored as zero (none) as per the original scale. 744 This score was recorded by clinicians for each clinic visit and participants by completing a postal-return questionnaire. In response to feedback from patients during development of the trial, an additional question probing the degree of exudate was also included. This information is presented separately and was not incorporated into the ‘resolution of inflammation’ score.
For the first 6 weeks, participants reported daily pain severity in their study diary on a scale from zero to four (none, mild, moderate, severe or extreme) and whether or not painkillers were taken that day.
Assessed at baseline, 6 weeks and 6 months (or healed), using validated questionnaires (DLQI,487 EQ-5D-3L745 and EQ-5D VAS360). DLQI is a disease-specific QoL measure including 10 questions each with four levels (0–3) scored from 0 (no effect) to 30 (extremely large effect on QoL). EQ-5D-3L is a generic QoL measure with five domains each scored at three levels; findings are mapped onto societal health state preference values referenced to scores of 0 (dead) and 1 (perfect health). Negative scores are possible for some heath states considered worse than death.
Costs and health service resource use were compared from a health service perspective. Patient diaries were used to capture health service contacts related to the treatment of PG. These were then returned to the trial team during scheduled contacts (at 6 weeks and 6 months). National unit costs for 2012 were applied to resource use providing a cost of care for each patient during follow-up, unit costs included outpatient visits (£139), community nurse visits (£39), practice nurse contacts (£14), GP consultations (£43) and GP home visits (£110). 362 Hospital consultations were calculated by identifying the most common Healthcare Resource Group (HRG) codes for PG hospital admissions from national Hospital Episode Statistics data and calculating a weighted average per diem (£323/day) for these codes using national reference costs. 746 Ciclosporin and prednisolone treatment costs were estimated in two steps. The product of the daily dose prescribed and treatment duration were used to calculate a total quantity of treatment (mg). National prescribing data were accessed to calculate the average cost by weight of these drugs given the current national prescribing pattern. 747 These average costs were then applied to the weight of active drug provided and the patient drug cost added to their cost of care.
A recurrence was defined as the occurrence of a further episode of PG (at any site) that appeared after the target lesion was confirmed as being healed by a physician or nurse. The period of follow-up available varied depending on the time at which the participant was randomised into the trial.
Treatment failures were defined as being participants who withdrew (or were withdrawn) from their randomised treatment because of treatment intolerance or worsening of the PG, or those whose target lesion remained unhealed after 6 months of follow-up.
Defined as adverse events that were possibly, probably or definitely related to the study medication.
Sample size
This was a superiority trial, with prednisolone as the control intervention. In order to provide 80% power (5% level of significance) to detect a difference in means of 0.5 SDs in the primary outcome of velocity of healing at 6 weeks, the total target sample size was 140 participants, assuming a loss to follow-up of 10%.
Statistical analysis
The primary analysis was conducted according to the ITT principle. The ITT population was defined as all randomised patients, excluding those whose later diagnosis was determined to be something other than PG. All patients with available data at both the baseline and the 6-week visit were included in the primary analysis. By way of sensitivity analyses, if neither a digital image, nor physical measurements taken during clinic visits were available at 6 weeks, multiple imputation was used based on the assumption that the data were missing at random. We also used the date of visit at which the lesion was declared as healed, in the event that the date the lesion stopped requiring dressings was not available. Differences between treatment groups for the primary outcome at 6 weeks were analysed using a linear regression model.
Secondary outcomes were analysed as follows: Cox regression models for the time to healing of the target lesion and the time to recurrence; linear regression models for DLQI, EQ-5D and EQ-VAS scores (adjusted for baseline values) and for self-reported pain (which were summarised using AUC); proportional odds models for the categorical secondary outcomes, including global assessment of improvement; and logistic regression models for the resolution of inflammation (by clinician and patient). Comparisons between the average number of times painkillers were used between the treatment groups were made using an adjusted non-parametric test. Analyses were adjusted for the stratification variables of lesion size and presence or absence of underlying autoimmune disease. In addition, sensitivity analysis of the primary outcome further adjusted for additional baseline variables including age, sex, weight, size of recruiting centre and geographical region.
Other sensitivity analyses were conducted in which participants who switched randomised treatments or who received both trial drugs in combination during the period of the trial were either excluded from the analysis for the primary outcome of velocity of healing, or included for the secondary outcome of time to healing analysis but censored at the time of change. Resource and cost data were highly skewed and, thus, parameter uncertainty was estimated by method of bootstrap using 5000 replications. All statistical analyses were conducted with the use of SAS software, version 9.2 (SAS Institute Inc., Cary, NC, USA) and R version 2.10.1 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Study population
Recruitment took place from June 2009 to November 2012.
Out of 499 patients screened, 121 were eligible for the trial and gave written informed consent (86% of target). Fifty-nine patients were assigned to the prednisolone group and 62 to the ciclosporin group (Figure 60). Of these patients, nine (six in the prednisolone group, three in the ciclosporin group) were subsequently found not to have PG and so were withdrawn after randomisation; making the analysable population 112 participants. In addition, there were two losses to follow-up in each arm before the primary end point at 6 weeks was reached. Two-thirds of participants (75/112; 67.0%) were recruited from dermatology clinics and the remainder from a wide range of other disciplines including gastroenterology, general medicine, surgery, rheumatology and tissue viability. The median number of participants per recruiting centre was three (minimum one and maximum 20).
FIGURE 60.
The STOP GAP CONSORT flow diagram. a, Number of patients who had information on whether or not the lesion had healed at any point during the study up to 6 months after randomisation (main secondary outcome of time to healing).

Baseline characteristics of the participants were well-balanced between the groups (Table 75). On entry into the trial, methotrexate was being taken by one patient in each group, azathioprine was being taken by three patients in the ciclosporin group and one in the prednisolone group, and tetracycline was being taken by three patients in the prednisolone group.
| Characteristics | Ciclosporin (N = 59) | Prednisolone (N = 53) |
|---|---|---|
| Demographics | ||
| Age (years), mean (SD) | 57.2 (16.9) | 51.3 (15.2) |
| Sex, n female (%) | 42 (71.2) | 31 (58.5) |
| Ethnicity, n white (%) | 55 (93.2) | 53 (100) |
| Weight (kg) | ||
| Mean (SD) | 88.4 (24.5) | 93.2 (27.2) |
| Minimum, maximum | 50.0, 171.0 | 50.6, 151.0 |
| Medical history | ||
| Underlying comorbidities, n (%) | ||
| Crohn’s disease | 5 (8.5) | 3 (5.7) |
| Ulcerative colitis | 7 (11.9) | 8 (15.1) |
| Rheumatoid arthritis | 4 (6.8) | 4 (7.5) |
| Other inflammatory arthritis | 3 (5.1) | 3 (5.7) |
| Monoclonal gammopathy | 0 (0.0) | 0 (0.0) |
| Myeloma | 0 (0.0) | 0 (0.0) |
| Haematological malignancy | 0 (0.0) | 0 (0.0) |
| Other malignancy | 4 (6.8) | 0 (0.0) |
| Diabetes | 4 (6.8) | 9 (17.0) |
| Renal impairment | 2 (3.4) | 0 (0.0) |
| Epilepsy | 0 (0.0) | 1 (1.9) |
| Characteristics of PG | ||
| Type of PG, n (%) | ||
| Classical | 50 (84.7) | 47 (88.7) |
| Cribriform | 4 (6.8) | 2 (3.8) |
| Peristomal | 2 (3.4) | 2 (3.8) |
| Bullous | 0 (0.0) | 1 (1.9) |
| Unsure | 3 (5.1) | 1 (1.9) |
| Previous episode of PG, n (%) | 17 (28.0) | 14 (26.4) |
| Area of target lesion (cm2), median (Q1; Q3) | 9.1 (3.6; 24.7) | 8.1 (2.4; 20.2) |
| Location of lesion, n (%) | ||
| Upper limb | 2 (3.4) | 1 (1.9) |
| Lower limb | 41 (69.5) | 34 (64.2) |
| Other | 16 (27.1) | 18 (34.0) |
| Number of lesions | ||
| Number | 59 | 51 |
| Mean (SD) | 2.2 (1.8) | 2.6 (2.4) |
| Minimum, maximum | (1, 10) | (1, 12) |
| Erythema, n (%) | ||
| None | 4 (6.8) | 2 (3.8) |
| Slight | 2 (3.4) | 3 (5.7) |
| Moderate | 21 (35.6) | 15 (28.3) |
| Severe | 22 (37.3) | 17 (32.1) |
| Very severe | 10 (16.9) | 16 (30.2) |
| Border elevation, n (%) | ||
| None | 4 (6.8) | 1 (1.9) |
| Slight | 24 (40.7) | 29 (54.7) |
| Moderate | 19 (32.2) | 17 (32.1) |
| Severe | 8 (13.6) | 5 (9.4) |
| Very severe | 4 (6.8) | 1 (1.9) |
| Exudate, n (%) | ||
| None | 3 (5.1) | 1 (1.9) |
| Slight | 7 (11.9) | 9 (17.0) |
| Moderate | 32 (54.2) | 27 (50.9) |
| Severe | 12 (20.3) | 3 (5.7) |
| Very severe | 5 (8.5) | 13 (24.5) |
During the trial, 16 out of 112 (14.3%) participants either switched to the alternative trial drug or received the two drugs concurrently (n = 8, 15.1%, for prednisolone vs. n = 8, 13.6%, for ciclosporin). Of these events, five occurred prior to the primary outcome assessment at 6 weeks (one in the prednisolone group and four in the ciclosporin group).
Data on adherence to study medication were available from 68 out of 112 (60.7%) participants. Of these, 36 out of 37 (97.3%) in the ciclosporin group and 29 out of 31 (93.5%) in the prednisolone group took their treatment every day in the first 6 weeks of the trial.
Primary outcome
In total, 108 participants (96.4%) had data at both baseline and week 6. Of these, 86 (79.6%) had blinded outcome data on the basis of digital images. For 22 participants (20.4%), velocity of healing was assessed on the basis of ‘unblinded’ physical measurements taken by investigators during clinic visits, as digital images were either unavailable or of insufficient quality to allow assessment.
For participants with data at both baseline and 6 weeks, the median (Q1; Q3) lesion area at baseline was 9.1 cm2 (4.5 cm2; 24.7 cm2) in the ciclosporin group versus 6.8 cm2 (2.4 cm2; 20.2 cm2) in the prednisolone group. The median (Q1; Q3) changes in lesion area from baseline to week 6 were –1.7 cm2 (–6.9 cm2; 0.7 cm2) and –2.0 cm2 (–5.7 cm2; 0.4 cm2), respectively. The calculated unadjusted mean for the velocity of healing at 6 weeks was –0.21 cm2/day (SD 1.00 cm2/day) for ciclosporin and –0.14 cm2/day (SD 0.42 cm2/day) for prednisolone.
The primary analysis showed no significant difference between the two treatments for velocity of healing at 6 weeks (Table 76).
| Primary outcome | |||||
|---|---|---|---|---|---|
| Velocity of healing at 6 weeks (cm2/day) | Mean (SD) | Mean difference (ciclosporin – prednisolone) | Adjusted mean differencea | 95% CI | p-value |
| Ciclosporin (n = 57) | –0.213 (0.998) | –0.074 | 0.003 | –0.204 to 0.211 | 0.975 |
| Prednisolone (n = 51) | –0.139 (0.417) | ||||
| Secondary outcomes | |||||
| Time to healingb | Number healed by 6 months (%)a | Median time to healing in days (IQR) | Hazard ratio for healinga | 95% CI | p-value |
| Ciclosporin (n = 59) | 28 (47.5) | 134.0 (60.0–183.0) | 0.94 | 0.55 to 1.63 | 0.839 |
| Prednisolone (n = 53) | 25 (47.2) | 112.0 (46.0–182.0) | |||
| Time to recurrence | Number with PG recurrence (%)c | Median time to recurrence in days (IQR) | Hazard ratio for healinga | 95% CI | p-value |
| Ciclosporin (n = 27) | 8 (29.6) | 582.0 (172.0–932.0) | 1.43 | 0.50 to 4.07 | 0.501 |
| Prednisolone (n = 25) | 7 (28.0) | 612.0 (148.0–934.0) | |||
Sensitivity analyses for velocity of healing
Similar results were observed for the sensitivity analyses in which missing data were imputed (adjusted mean difference: 0.001 cm2/day, 95% CI –0.204 to 0.206; p = 0.994) and, separately, after adjusting for additional baseline covariates (adjusted mean difference: –0.100 cm2/day, 95% CI –0.328 to 0.127; p = 0.382).
Excluding the five patients (who either swapped to the alternative trial drug or used both drugs in combination prior to the 6-week visit) did not change the overall treatment effect (adjusted mean difference: –0.036, 95% CI –0.211 to 0.139; p = 0.685).
Secondary outcomes
All secondary outcomes were consistent with the primary outcome in showing no significant difference between the two treatments.
Time to healing
At 6 weeks, nine participants (15.3%) in the ciclosporin group and 11 (20.8%) in the prednisolone group had healed. By 6 months, the proportion healed had increased to 47.5% (n = 28) and 47.2% (n = 25) in the ciclosporin and prednisolone groups, respectively. The median time to healing was 134 days for ciclosporin compared with 112 days for prednisolone. The Cox regression model for time to healing showed no significant difference between the interventions (see Table 76 and Figure 61).
FIGURE 61.
Kaplan–Meier plot showing time to healing of ulcer by treatment group for RCT participants.

Sensitivity analyses for time to healing
Adjusting for additional baseline covariates was consistent with the main result (HR 1.01, 95% CI 0.57 to 1.79; p = 0.985).
Sixteen participants swapped to the alternative trial drug or used both drugs in combination during the follow-up period (n = 8 randomised to ciclosporin and n = 8 to prednisolone). Sensitivity analysis excluding these participants was also consistent with the main result (HR 0.861, 95% CI 0.49 to 1.52; p = 0.604).
Global assessment of efficacy
There were no significant differences between the treatments in global assessments of efficacy at final visit, whether based on data from physicians, patients or blinded assessments using digital images (Figures 62–64).
FIGURE 62.
Global treatment response (assessed by clinician). p = 0.3285; OR: 1.457 (95% CI 0.685 to 3.098).
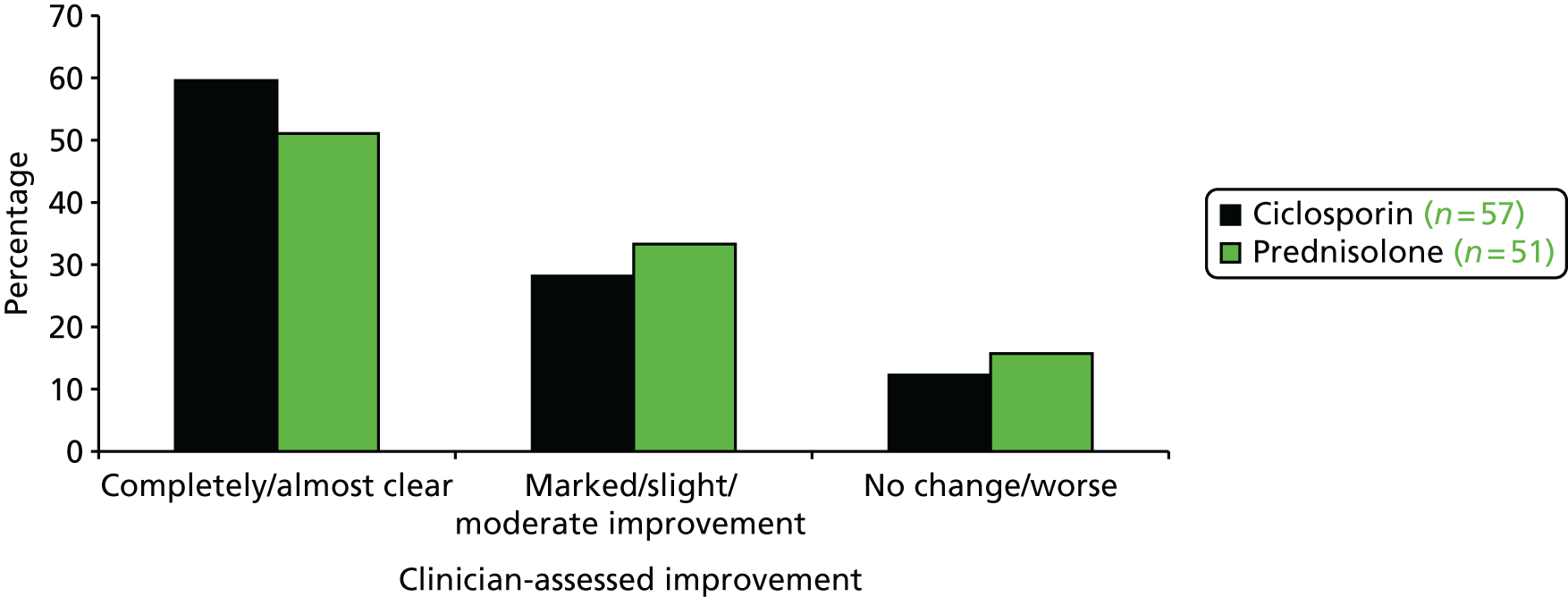
FIGURE 63.
Global treatment response (assessed by patient). p = 0.6702; OR: 0.814 (95% CI 0.315 to 2.103).
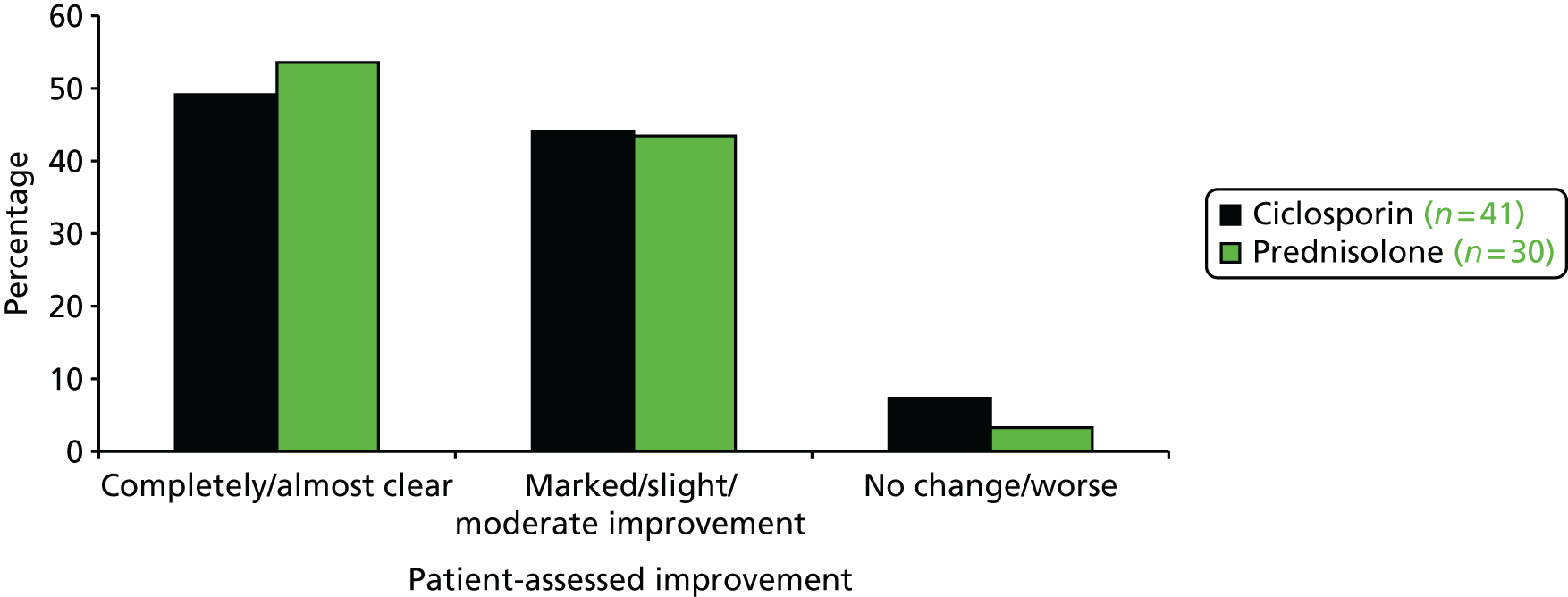
FIGURE 64.
Global treatment response (assessed by independent clinician from digital images). p = 0.4199; OR: 1.393 (95% CI 0.623 to 3.114).
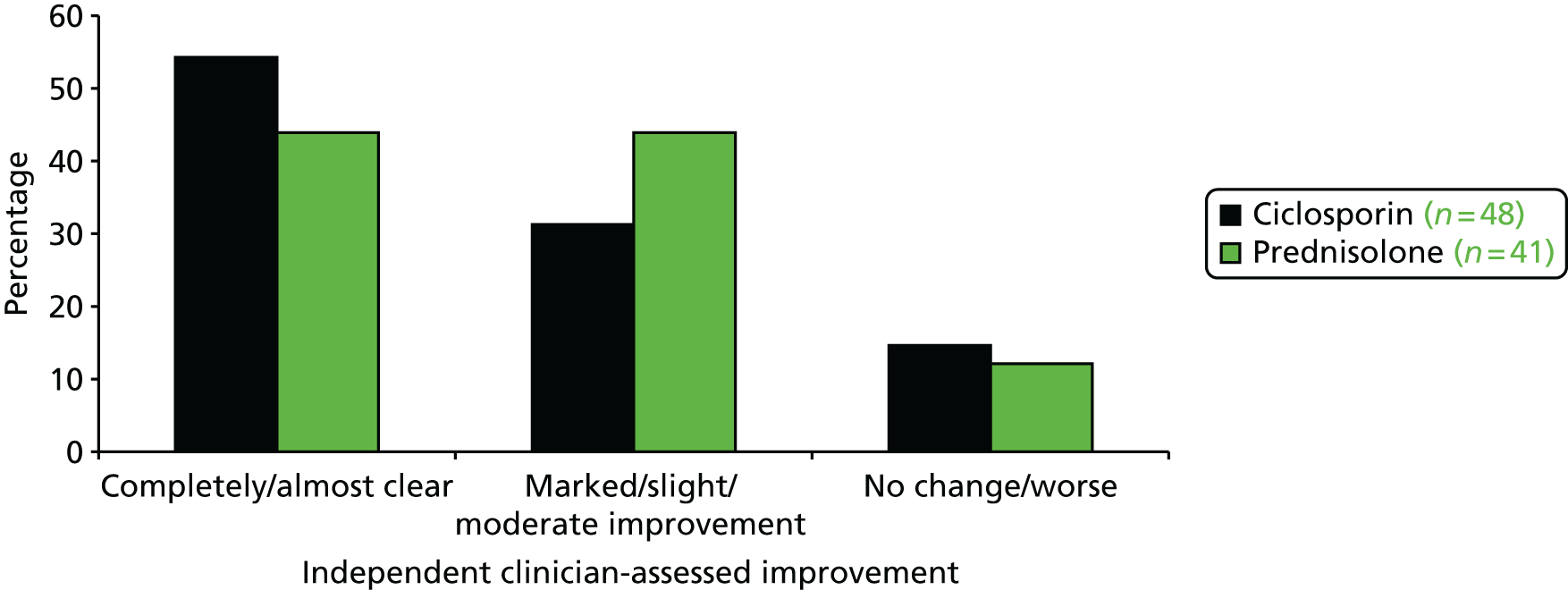
Resolution of inflammation
Full details of inflammation assessment at baseline (including change in exudate) are tabulated in Table 77. There were no between-group differences in the resolution of inflammation as assessed by clinicians at either 6 weeks or final visit (Tables 77–79).
| Parameter | Ciclosporin, n (%) | Oral prednisolone, n (%) |
|---|---|---|
| Erythema | N = 56 | N = 51 |
| Worse | 8 (14.3) | 6 (11.8) |
| Same | 13 (23.2) | 13 (25.5) |
| Improved | 35 (62.5) | 32 (62.7) |
| Border elevation | N = 57 | N = 51 |
| Worse | 7 (12.3) | 7 (13.7) |
| Same | 16 (28.1) | 11 (21.6) |
| Improved | 34 (59.6) | 33 (64.7) |
| Exudate | N = 57 | N = 51 |
| Worse | 6 (10.5) | 6 (11.8) |
| Same | 16 (28.1) | 14 (27.5) |
| Improved | 35 (61.4) | 31 (60.8) |
| Parameter | Ciclosporin, n (%) | Oral prednisolone, n (%) |
|---|---|---|
| Erythema | N = 57 | N = 51 |
| Worse | 6 (10.5) | 3 (5.9) |
| Same | 11 (19.3) | 10 (19.6) |
| Improved | 40 (70.2) | 38 (74.5) |
| Border elevation | N = 57 | N = 51) |
| Worse | 2 (3.5) | 8 (15.7 |
| Same | 15 (26.3) | 9 (17.6) |
| Improved | 40 (70.2) | 34 (66.7) |
| Exudate | N = 57 | N = 51 |
| Worse | 5 (8.8) | 4 (7.8) |
| Same | 7 (12.3) | 8 (15.7) |
| Improved | 45 (78.9) | 39 (76.5) |
| Treatment | n | Week 6, n (%) | ORb | 95% CI | p-value |
|---|---|---|---|---|---|
| Ciclosporin | 56 | 5 (8.9) | 1.03 | 0.27 to 3.97 | 0.964 |
| Prednisolone | 51 | 6 (11.8) | |||
| Treatment | n | Final visit (up to 6 months), n (%) | ORb | 95% CI | p-value |
| Ciclosporin | 57 | 10 (17.5) | 1.11 | 0.39 to 3.12 | 0.849 |
| Prednisolone | 51 | 10 (19.6) |
Self-reported pain
The mean self-reported pain score reduced from 1.92 (SD 1.06) in week 1 to 1.26 (SD 1.15) by week 6. There was no difference between ciclosporin and prednisolone groups in AUC for the average weekly pain scores over the first 6 weeks (Table 80).
| Time points | Ciclosporin | Prednisolone | Mean difference (ciclosporin – prednisolone) | Adjusted mean differencea | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Pain scores (range 0 to 4) (high scores = worse) | ||||||
| Week 1 | ||||||
| n | 47 | 38 | ||||
| Mean (SD) | 1.98 (1.0) | 1.84 (1.2) | ||||
| Week 2 | ||||||
| n | 46 | 37 | ||||
| Mean (SD) | 1.74 (1.1) | 1.69 (1.3) | ||||
| Week 3 | ||||||
| n | 46 | 36 | ||||
| Mean (SD) | 1.59 (1.0) | 1.48 (1.2) | ||||
| Week 4 | ||||||
| n | 45 | 35 | ||||
| Mean (SD) | 1.34 (1.2) | 1.50 (1.2) | ||||
| Week 5 | ||||||
| n | 46 | 34 | ||||
| Mean (SD) | 1.22 (1.1) | 1.49 (1.3) | ||||
| Week 6 | ||||||
| n | 45 | 32 | ||||
| Mean (SD) | 1.10 (1.0) | 1.49 (1.3) | ||||
| AUC weeks 1–6 (range 0–20) | ||||||
| n | 45 | 32 | ||||
| Mean (SD) | 7.5 (4.8) | 7.9 (5.6) | –0.40 | –0.48 | –2.82 to 1.87 | 0.685 |
| DLQI (range 0 to 30) (high score = worse) | ||||||
| Baseline | ||||||
| n | 58 | 53 | ||||
| Mean (SD) | 10.3 (7.3) | 13.2 (9.0) | ||||
| 6 weeks | ||||||
| n | 43 | 38 | ||||
| Mean (SD) | 6.2 (6.1) | 9.1 (8.2) | ||||
| Final visit | ||||||
| n | 38 | 28 | –1.5 | –0.45 | –3.46 to 2.56 | 0.767 |
| Mean (SD) | 4.8 (6.8) | 6.3 (7.6) | ||||
| EQ-5D-3L (range –0.594 to 1.000) (low scores = worse) | ||||||
| Baseline | ||||||
| n | 56 | 52 | ||||
| Mean (SD) | 0.51 (0.35) | 0.44 (0.38) | ||||
| 6 weeks | ||||||
| n | 45 | 40 | ||||
| Mean (SD) | 0.65 (0.30) | 0.54 (0.38) | ||||
| Final visit | ||||||
| n | 42 | 27 | 0.13 | 0.13 | –0.02 to 0.28 | 0.095 |
| Mean (SD) | 0.76 (0.30) | 0.63 (0.41) | ||||
| EQ-5D VAS (range 0 to 100) (low scores = worse) | ||||||
| Baseline | ||||||
| n | 57 | 53 | ||||
| Mean (SD) | 62.6 (22.2) | 61.4 (21.5) | ||||
| 6 weeks | ||||||
| n | 45 | 41 | ||||
| Mean (SD) | 70.9 (16.0) | 66.2 (25.1) | ||||
| Final visit | ||||||
| n | 41 | 29 | 2.6 | 0.48 | –9.32 to 10.29 | 0.922 |
| Mean (SD) | 73.2 (20.5) | 70.6 (22.3) | ||||
The median (Q1; Q3) number of days on which painkillers were used in the first 6 weeks was 14.0 days (0.0 days; 38.0 days) in the ciclosporin group and 20.5 days (1.0 days; 40.0 days) in the prednisolone group, with a non-significant treatment effect (p = 0.782).
Health-related quality of life
All HRQoL scores improved during the period of the trial. No significant between-group differences were identified in DLQI, EQ-5D or EQ-5D VAS (see Table 80).
Time to recurrence
Analysis of recurrence was based on 52 out of 53 (98%) of the lesions that had healed by 6 months. Follow-up for these patients ranged from 0 to 40.3 months, depending on when they were recruited into the trial. Of those receiving ciclosporin, eight patients (29.6%) had a recurrence, compared with seven (28.0%) of the prednisolone-treated patients. There was no significant treatment effect in the time to the first recurrence (see Table 76).
Number of treatment failures
Treatment failure was documented in approximately half of the patients in each group (29/59, 49.2%, in the ciclosporin group and 26/53, 49.1%, in the prednisolone group; p = 0.88).
Adverse reactions
Overall, 40 (67.8%) of participants in the ciclosporin group and 35 (66.0%) in the prednisolone group experienced at least one adverse reaction. Specific events that occurred in at least 3% of patients in either treatment group are presented in Table 81 (see Appendix 20 full details of all adverse reactions).
| Upper-level classification | Lower-level classification | Ciclosporin (N = 59), n (%) | Prednisolone (N = 53), n (%) |
|---|---|---|---|
| Blood and the lymphatic system disorders | Anaemia | 2 (3.4) | 0 (0.00) |
| Leucocytosis | 0 (0.0) | 5 (9.4) | |
| Endocrine disorders | Diabetes | 0 (0.0) | 3 (5.7) |
| Metabolism and nutrition disorders | Hyperglycaemia | 0 (0.0) | 5 (9.4) |
| Nervous system disorders | Tremor | 5 (8.5) | 2 (3.8) |
| Headache | 5 (8.5) | 0 (0.0) | |
| Paraesthesia | 2 (3.4) | 0 (0.0) | |
| Euphoria | 0 (0.0) | 3 (5.7) | |
| Depression | 1 (1.7) | 2 (3.8) | |
| Gastrointestinal disorders | Nausea | 12 (20.3) | 1 (1.9) |
| Vomiting | 4 (6.8) | 0 (0.0) | |
| Diarrhoea | 2 (3.4) | 0 (0.0) | |
| Candidiasis | 1 (1.7) | 2 (3.8) | |
| Cardiovascular disorders | Hypertension | 10 (16.9) | 4 (7.5) |
| Oedema | 0 (0.0) | 2 (3.8) | |
| Hepatobiliary disorders | Hepatic dysfunction | 2 (3.4) | 1 (1.9) |
| Skin and subcutaneous tissue disorders | Hypertrichosis | 2 (3.4) | 0 (0.0) |
| Musculoskeletal, connective tissue and bone disorders | Muscle cramps | 2 (3.4) | 0 (0.0) |
| Myalgia | 2 (3.4) | 1 (1.9) | |
| Arthralgia | 2 (3.4) | 0 (0.0) | |
| Renal and urinary disorders | Renal dysfunction | 18 (30.5) | 1 (1.9) |
| General disorders | Serious infection (requiring hospitalisation or parenteral antibiotic) | 0 (0.0) | 6 (11.3) |
| Other infection | 4 (6.8) | 5 (9.4) | |
| Fatigue | 2 (3.4) | 4 (7.5) | |
| Weight increase | 1 (1.7) | 4 (7.5) |
Adverse reactions differed between the treatments as would be expected based on each drug’s recognised tolerability profile. Differences of note include 5.7% patients developing diabetes and 9.4% developing hyperglycaemia in the prednisolone group versus none for either condition in the ciclosporin group. A higher number of participants in the prednisolone group developed a serious infection (11.3%), with no occurrence in the ciclosporin group, and disorders of the lymphatic system (9.4%), although this was less prevalent in the ciclosporin group (3.4%). Headache was reported by 8.5% patients in the ciclosporin group but by none treated with prednisolone. Nausea, vomiting and diarrhoea were all more common in the ciclosporin group (20.3%, 6.8% and 3.4%, respectively) than in the prednisolone group (1.9%, 0.0% and 0.0%, respectively). Renal dysfunction was also notably more common in the ciclosporin than the prednisolone group (30.5% vs. 1.9%, respectively).
There were nine serious adverse reactions (SARs) recorded throughout the trial: two in the ciclosporin group and seven in the prednisolone group. The SARs in the ciclosporin-treated patients were a ruptured abdominal aortic aneurysm and a case of acute kidney injury with elevated serum creatinine (212 µmol/l). Both of these events were considered ‘possibly related’ to study treatment. In the prednisolone group, the SARs were one case of bowel perforation (probably related), five serious infections (requiring hospitalisation or parenteral antibiotic, two probably related and three possibly related) and one other infection (possibly related). One of the serious infections (septicaemia Gram-negative bacilli) resulted in death.
Cost analysis
Use of resources and costs were similar when comparing groups with two exceptions (Table 82). The cost of treatment drugs was significantly higher for the ciclosporin group, as would be anticipated. There was a non-significant increase in time in hospital in the prednisolone group. Of the six patients with > 10 days admission during the study, five received prednisolone (54, 48, 46, 38 and 16 days) and one received ciclosporin (14 days).
| Resource use and costs | Ciclosporin, mean (SD) | Predisolone, mean (SD) | Ciclosporin – predisolone, mean (95% CI) |
|---|---|---|---|
| NHS contacts (complete: 0–24 weeks) | |||
| GP clinic visits | 3.32 (8.33) | 1.62 (2.50) | 1.70 (–1.10 to 4.49) |
| GP home visits | 0.00 (0.00) | 0.21 (0.68) | –0.21 (–0.45 to 0.04) |
| Practice nurse visits | 6.71 (14.23) | 7.17 (16.38) | –0.46 (–7.95 to 7.02) |
| District nurse visits | 4.66 (13.13) | 7.62 (28.95) | –2.96 (–14.30 to 8.37) |
| Outpatient visits | 9.87 (15.51) | 6.14 (10.35) | 3.73 (–2.48 to 9.94) |
| Inpatient days | 0.29 (1.25) | 4.41 (13.32) | –4.12 (–8.99 to 0.74) |
| Drug cost (£) (0–24 weeks) | 1105 (564) | 202 (120) | 903 (755 to 1050) |
| Care cost (£) (0–8 weeks) | 1050 (1705) | 2138 (4393) | –1088 (–2567 to 392) |
| Care cost (£) (9–24 weeks) | 767 (1446) | 1448 (3332) | –681 (–1885 to 524) |
Discussion
Given the lack of good-quality published data relating to the management of PG, there is a clear need for trials that are robust in design and relevant to clinical practice. On this basis, the STOP GAP trial has, for the first time that we are aware of, compared two of the most commonly used treatments in a RCT setting. Patients were recruited from a range of centres around the UK in order to ensure that the sample was representative. The trial procedure was designed to reflect normal clinical practice as closely as possible, with dosing adjusted according to clinician opinion. The data collected in this trial included assessments by clinicians and patients as well as independent analysis of digital photographs; thus, providing both objective and subjective measures of treatment success.
Since starting the STOP GAP trial, an expert opinion consensus document considering safety, efficacy and cost placed prednisolone as preferred treatment and ciclosporin as second-ranked therapy among the many suggested interventions. 740 Nonetheless, prior to the design of this trial, various studies had reported high proportions of patients with PG achieving complete responses with ciclosporin treatment734–737 which lead the STOP GAP trial to test the hypothesis that ciclosporin was superior to prednisolone for the treatment of PG. However, the study revealed no difference between the two treatments across a range of efficacy outcomes and there were narrow CIs around those lack of differences, suggesting that the study was large enough to exclude clinically important differences that might have been missed. Perhaps the most important finding was that contrary to the commonly held impression that these drugs are very efficacious in PG, fewer than half of the ulcers were healed by either treatment after prolonged therapy. The large number of patients who switched treatments or added in topical medications during the trial further reflects this generally poor treatment response.
In this study, approximately two-thirds of patients reported adverse reactions in both treatment groups, 12% of whom experienced at least one serious event (two randomised to ciclosporin and seven randomised to prednisolone). This information is important given that fewer than half of the participants achieved complete healing. Though the overall rates of adverse reactions were almost identical in the two groups, the side effects observed differed in line with the known side effect profiles of these drugs. More SARs including infections were reported in the prednisolone group than the ciclosporin group. It is worth considering that the median time to healing of almost 4 months indicates that patients would need to be exposed to the treatment-associated risks over long periods of time.
Analysis of resource use and cost data appears to support the clinical findings in that these provide no strong rationale for ranking one treatment before another; informed decision-making should reflect awareness of the side effect profiles and patient preference.
Initial pilot work for the STOP GAP trial included discussions with patients as to the most important outcomes to be included in PG trials. As a result, the degree of exudation was added to the PG severity assessment scale proposed by Foss et al. 744 and pain was recorded daily for the first 6 weeks. Although patients reported pain as being the most important symptom associated with PG, the pain scores reported here were relatively low (approximate mean of 1 on a 0–5 scale). It is possible that these scores were confounded by concurrent use of painkilling medication.
Study limitations
The study was observer-blind rather than double-blind in design owing to logistical and methodological difficulties in blinding treatment allocation within the resources available to the trial. However, every effort was made to capture the primary outcome in a blinded fashion and all secondary analyses were supportive of this main analysis, suggesting that minimal bias was introduced by this approach. Power to explore the impact on QoL was limited owing to missing data from postal questionnaires and so a full cost-effectiveness analysis was not possible and data were presented descriptively in order to guide clinically decision-making.
Given the lack of a placebo or no treatment third arm in this study, it is possible that neither drug is effective in treating PG. However, such a notion is not consistent with clinical experiences of some cases of rapid healing once systemic therapy is introduced. The costs, risks and logistics of developing appropriate placebos for two potent active systemic treatments with flexible dosing precluded the use of placebos or overencapsulation in our study.
The eventual sample size of 121 patients was slightly smaller than the 140 that had been planned. Funding for the study was available for a 5-year period, by the end of which the recruitment target had nearly been reached and the decision was made to close the trial on the scheduled end date.
Generalisability
This was a pragmatic RCT that recruited in multiple secondary hospitals throughout the UK. As such, it is likely that the study has relatively good external validity and the patients recruited into this trial are reflective of the kinds of patients who commonly present with PG in the UK.
Clinical conclusions
The results from this trial suggest that the outcome for patients with PG requiring systemic therapy is likely to be similar whether prednisolone or ciclosporin is chosen as the first agent. However, there are differences in side effect profiles, which should be considered on a case-by-case basis to select the optimal treatment. For example, a person with a previous history of infections, such as recurrent cellulitis or infections associated with joint prostheses, might be more suitable for ciclosporin and a person with previous hypertension or borderline renal impairment might be more suitable for prednisolone.
Based on the data from this study, it might be expected that with ciclosporin or prednisolone monotherapy, approximately one in six patients should be healed at 6 weeks. The median time to healing is closer to 4 months and over half of patients may not achieve resolution even after 6 months of treatment. This knowledge may help clinicians to measure treatment response, to manage patients’ expectations and to provide a baseline comparison for future cohorts or observational studies. The fact that almost 30% of patients experienced a recurrence indicates that this is a long-term condition, for which follow-up of patients and possible preventative maintenance therapy may be required.
Patient information resources on PG can now be updated on the basis of these trial findings in order to provide better-quality information to patients with this painful and debilitating condition.
Treatment response of patients receiving topical treatments for pyoderma gangrenosum: a prospective cohort observational study
The following text is adapted with permission from Thomas et al. 748 This article was published in J Am Acad Dermatol, Thomas KS, Ormerod AD, Craig FE, Greenlaw N, Norie J, Mitchell E, et al. , Clinical outcomes and response of patients applying topical therapy for pyoderma gangrenosum: a prospective cohort study, Copyright Elsevier (2016).
What is already known
-
Pyoderma gangrenosum is a severe acute and relapsing condition with a significant mortality.
-
The evidence base for treatment is largely anecdotal.
-
The natural history of the disease has not been studied.
-
Milder cases can be treated with topical therapies, including corticosteroids and tacrolimus, but it is not known how well such milder cases respond in routine clinical practice.
What this study adds
-
The most commonly used topical therapy in the UK is the superpotent topical corticosteroid, clobetasol propionate 0.05%.
-
Topical therapies used as monotherapy are likely to be effective in healing mild PG in approximately half of cases when treated over a 6-month period, with a median time to healing of 1.5 months.
-
In patients treated topically, approximately one-third are likely to require escalation of therapy to systemic treatments such as prednisolone or ciclosporin.
-
Pyoderma gangrenosum has a substantial impact on HRQoL comparable to that of chronic heart disease.
-
The initial size of the ulcer is a predictor of time to healing and should be adjusted for in future studies and when considering choice of therapy.
Introduction
Pyoderma gangrenosum is a rare, painful ulcerative inflammatory dermatosis that is associated with considerable morbidity728,749 and a reported threefold increased risk of death. 729 Given that mortality in PG exceeds that of underlying comorbid disease, it may be inferred that the disease itself and/or the therapeutic interventions used for its treatment are responsible for the increased risk of death in affected patients.
The current literature on managing this condition is based almost entirely on case reports and retrospective case series, but suggests that the most commonly used treatments are systemic. 732 A study of 21 patients with PG has indicated that systemic treatments (particularly prednisolone and ciclosporin) appear to be most effective in multilesional and disseminated forms of PG, whereas topical tacrolimus is useful in localised cases. 750 Along with potent topical steroids, tacrolimus is one of the most commonly used topical treatments for PG. 727,732 Although studies of topical treatments in PG are very limited in their number and size, some encouraging data are available to support the efficacy of tacrolimus in this setting. For example, in a study of 23 patients with peristomal PG, lesions were completely healed in 7 out of 11 patients treated with topical tacrolimus 0.3% [in a carmellose sodium paste (Orabase™; Convatec Ltd, Uxbridge, UK)] compared with 5 out of 13 treated with clobetasol propionate 0.05%. 751 In five patients who had failed to respond adequately to multiple systemic and topical treatments for peristomal PG, the addition of topical tacrolimus was associated with healing within 6 weeks. 751
Given the risks associated with systemic therapy and the evidence of increased mortality among patients with PG, we included a parallel observational cohort study alongside the STOP GAP RCT (see A randomised controlled trial of prednisolone versus ciclosporin in the treatment of pyoderma gangrenosum: STOP GAP trial),740 to investigate the efficacy of topical therapy as a first-line treatment approach. The concurrent running of these trials was planned in order to optimise recruitment for both studies and patients who were ineligible for one study on the basis of their treatment requirements were considered for the other, whenever appropriate.
Ethics and regulatory approvals were obtained (ethics: 09/H0903/5, Medicines and Healthcare Products Regulatory Agency: 19162/0213/001) and all participants gave written informed consent. Oversight of the study was performed by monthly Trial Management Group meetings and a Trial Steering Group which met twice a year. All data issues, including safety, were overseen by progress reports presented to an independent DMC.
Methods
Trial design
This was a prospective cohort study of patients with a clinical diagnosis of PG in whom topical therapy was indicated. Patients with more severe PG were included in this cohort if they were ineligible for the parallel STOP GAP RCT of systemic therapies as described in the previous section (see A randomised controlled trial of prednisolone versus ciclosporin in the treatment of pyoderma gangrenosum: STOP GAP trial).
Participants were enrolled for a period of up to 6 months or until the target PG ulcer had healed. Follow-up clinic visits were in accordance with normal practice at 2 weeks, 6 weeks and 6 months (or healed if sooner). Medications were prescribed as per local practice at the recruiting hospital, and concurrent systemic therapies for the management of PG were prohibited.
Outcomes were in accordance with those of the STOP GAP RCT with the exception of blinded outcome assessments using digital images, safety outcomes and health resource use. Using the same outcome measures and instruments to capture treatment response meant that informal comparison of outcomes from the two studies was possible.
Study objectives
Given the need to provide clinically relevant guidance on the management of PG, this observational trial aimed to address the following questions:
-
What is the typical treatment response for patients for whom topical therapy is indicated (patients with mild PG and/or systemic therapy is not appropriate)?
-
What proportion of participants require escalation of treatment to systemic medication?
-
What is the impact of PG on patient-reported morbidity and QoL?
-
What baseline factors predict treatment response?
Participants
Recruitment took place in secondary care hospitals throughout the UK. Potential participants were identified from both inpatient and outpatient clinics covering a range of specialties (dermatology, rheumatology, gastroenterology and general medicine).
Patients who were screened for the STOP GAP RCT but who were either ineligible or did not wish to take part were screened for inclusion into this cohort study.
Participants were all aged ≥ 18 years and had a clinical diagnosis of PG (confirmed by the recruiting dermatologist) and at least one measurable ulcer (target lesion). For cases where there was doubt over the clinical diagnosis, an expert panel confirmed or refuted the diagnosis.
Eligibility criteria for the cohort study were the same as for the STOP GAP RCT (see A randomised controlled trial of prednisolone versus ciclosporin in the treatment of pyoderma gangrenosum: STOP GAP trial), with the exception of criteria relating to the suitability of prescribing ciclosporin or prednisolone (e.g. pregnancy, sensitivity to the trial drugs or concurrent medical conditions leading to contraindication of the trial drugs).
Treatment with systemic therapies for the management of underlying comorbidities was permitted.
Interventions
Patients received topically applied interventions for the treatment of PG. The investigator was free to use whichever topical therapy and dosage regimen they preferred according to local practice. Systemic therapies for the treatment of PG were prohibited. If escalation to systemic treatment was required, participants were screened for inclusion in the STOP GAP RCT. If eligible, they were withdrawn from the observational cohort study and reconsented for inclusion in the RCT.
Assessments and outcomes
Participants were followed for a maximum of 6 months. Study visits took place at 2 weeks, 6 weeks and 6 months (or at time of healing if sooner). Other unscheduled consultations took place as per normal practice.
The following outcomes were assessed: (1) velocity of healing at 6 weeks (primary outcome in line with RCT primary outcome); (2) proportion healed by 6 weeks and 6 months; (3) time to healing; (4) global assessment of improvement (assessed by clinician and patient) at 6 weeks and final visit; (5) inflammation assessment assessed by clinician at baseline, 6 weeks and final visit;744 (6) pain in the first 6 weeks; and (7) QoL assessed at baseline and final visit (EQ-5D745 and DLQI487).
Healing was defined as the point at which dressings were no longer required, was reported by the patients, and a clinic visit was arranged to confirm healing as soon as possible thereafter. In cases when the date on which dressings were stopped was unavailable, then healing was assumed to have taken place on the day that the ulcer was confirmed as healed by the recruiting clinician. Pain scores, use of pain medication and use of dressings were collected using daily diaries. Pain was assessed on a scale from zero to four for the first 6 weeks of treatment.
Measures taken to control bias
This was an open study with no control group. In order to mitigate the risk of bias, consecutive participants were enrolled into the study and followed up prospectively. Outcomes were assessed using standard methods and clinicians’ and patients’ views were compared when appropriate. Every effort was made to maintain follow-up of all participants.
Sample size
This was a pragmatic observational cohort study running alongside the STOP GAP RCT740 and its size was determined by the rate of recruitment to the RCT. No formal sample size calculation was performed, as this was a descriptive study without formal between-treatment comparisons.
Statistical analysis
All participants who received at least one topical medication for their PG were included in the analysis. Pre-defined populations were:
-
all participants who received at least one topical medication (full data set)
-
all participants who received clobetasol propionate 0.05%
-
all participants who received a topical calcineurin inhibitor (tacrolimus or pimecrolimus).
All data are presented descriptively and presented in summary data tables. To facilitate comparison, data relating to participants of the RCT are included alongside those of the topical therapy cohort, but no formal comparisons have been made – either between treatment groups or between the RCT and observational study.
If a participant received more than one topical medication they were included in all relevant study populations. Participants who withdrew owing to lack of treatment response or who started a systemic medication during the period of the study were classed as treatment failures for the topical medication.
Exploratory analyses adjusting for lesion size at baseline, presence of underlying autoimmune disease, age, weight, sex and size of recruiting centre were conducted to determine possible factors associated with treatment response. Linear regression models were used for continuous outcomes, logistic regression for binary outcomes and Cox proportional hazards for time to event outcomes.
All patients with available data at both the baseline and the 6-week visit were included in the primary analysis. By way of sensitivity analyses, multiple imputation for the primary outcome was used based on the assumption that the data were missing at random. We also used the date of visit at which the lesion was declared as healed, in the event that the date the lesion stopped requiring dressings was not available.
Results
Participants and treatment allocation
Recruitment took place in 28 secondary care hospitals between June 2009 and November 2012.
A total of 67 participants were enrolled in the study, but one was subsequently excluded from the analysis having received oral prednisolone (Figure 65).
FIGURE 65.
Participant flow observational study. a, Could be receiving more than one treatment; b, number of patients who had information on whether or not the lesion had healed at any point during the study up to 6 months after randomisation (main secondary outcome of time to healing).
Forty-nine (74.2%) participants received clobetasol propionate 0.05%, 10 (15.2%) received tacrolimus 0.03% or 0.1% and eight received other topical interventions including other topical corticosteroids (n = 6), fludroxycortide impregnated tape (Haelan® Tape, Typharm Ltd) (n = 1) and lymecycline (Tetralysal® 300, Galderma) (n = 1). One participant received both clobetasol propionate and tacrolimus and was therefore included in both subgroups. Five participants in the clobetasol propionate group were taking concurrent anti-inflammatory/immune modifying medications for the treatment of other conditions including azathioprine (n = 2), tetracyclines (n = 2) and anti-TNF (n = 1).
The reason for choosing systemic or topical therapy was recorded and responses broadly classified as topical treatment failure (for those opting for systemic therapy) (n = 47), features of the disease (n = 43) and patient’s preference (n = 6).
Details of demographic and baseline characteristics are summarised in Table 83. The majority of participants were identified through dermatology services (47; 71.2%); others were referred from gastroenterology (7; 10.6%), rheumatology (1; 1.5%), general medicine (2.0; 3%) and other sources (9; 13.6%). Baseline characteristics for participants in the cohort study of topical treatments were broadly similar to those enrolled in the parallel RCT with the exception that the lesion sizes were generally smaller (see Table 83).
| Characteristics | RCT | Cohort study | Cohort subgroups | |
|---|---|---|---|---|
| N = 112 | N = 66 | Clobetasol propionate, N = 49 | Tacrolimus, N = 10 | |
| Demographics | ||||
| Age (years), mean (SD) | 54.4 (16.3) | 57.3 (17.3) | 57.5 (17.9) | 53.0 (13.0) |
| Sex, n female (%) | 73 (65.2) | 44 (66.7) | 34 (69.4) | 6 (60.0) |
| Ethnicity, n white (%) | 108 (96.4) | 64 (97.0) | 47 (95.9) | 10 (100.0) |
| Weight (kg), mean (SD) | 90.7 (25.8) | 80.4 (20.3) | 77.8 (17.2) | 86.2 (29.7) |
| Medical history | ||||
| Underlying comorbidities, n (%) | ||||
| Crohn’s disease | 8 (7.1) | 6 (9.1) | 2 (4.1) | 2 (20.0) |
| Ulcerative colitis | 15 (13.4) | 8 (12.1) | 7 (14.3) | 1 (10.0) |
| Rheumatoid arthritis | 8 (7.1) | 2 (3.0) | 2 (4.1) | 0 (0.0) |
| Other inflammatory arthritis | 6 (5.4) | 5 (7.6) | 3 (6.1) | 2 (20.0) |
| Monoclonal gammopathy | 0 (0.0) | 1 (1.5) | 1 (2.0) | 0 (0.0) |
| Myeloma | 0 (0.0) | 1 (1.5) | 1 (2.0) | 0 (0.0) |
| Haematological malignancy | 0 (0.0) | 1 (1.5) | 1 (2.0) | 0 (0.0) |
| Other malignancy | 4 (3.6) | 6 (9.1) | 5 (10.2) | 0 (0.0) |
| Diabetes | 13 (11.6) | 7 (10.6) | 5 (10.2) | 2 (20.0) |
| Renal impairment | 2 (1.8) | 3 (4.5) | 2 (4.1) | 0 (0.0) |
| Epilepsy | 1 (0.9) | 1 (1.5) | 1 (2.0) | 0 (0.0) |
| Characteristics of PG | ||||
| Type of PG, n (%) | ||||
| Classical | 97 (86.6) | 55 (83.3) | 43 (87.8) | 9 (90.0) |
| Cribriform | 6 (5.4) | 1 (1.5) | 0 (0.0) | 0 (0.0) |
| Peristomal | 4 (3.6) | 6 (9.1) | 3 (6.1) | 1 (10.0) |
| Bullous | 1 (0.9) | 2 (3.0) | 2 (4.1) | 0 (0.0) |
| Unsure | 4 (3.6) | 2 (3.0) | 1 (2.0) | 0 (0.0) |
| Previous episode of PG, n (%) | 31 (27.7) | 18 (27.3) | 12 (24.5) | 3 (30.0) |
| Area of target lesion (cm2) | ||||
| n | 112 | 65 | 48 | 10 |
| Median (Q1; Q3) | 9.0 (3.2; 24.4) | 4.7 (2.4; 11.0) | 4.4 (1.6; 10.5) | 6.8 (2.8; 11.0) |
| Location of lesion: n (%) | ||||
| Upper limb | 3 (2.7) | 7 (10.6) | 6 (12.2) | 0 (0.0) |
| Lower limb | 75 67.0) | 39 (59.1) | 29 (59.2) | 6 (60.0) |
| Other | 34 (30.4) | 20 (30.3) | 14 (28.6) | 4 (40.0) |
| Number of lesions | ||||
| n | 110 | 65 | 48 | 10 |
| Mean (SD) | 2.4 (2.1) | 1.6 (1.2) | 1.6 (1.1) | 1.8 (1.1) |
| Erythema, n (%) | ||||
| N | 112 | 66 | 49 | 10 |
| None | 6 (5.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Slight | 5 (4.5) | 9 (13.6) | 10 (20.4) | 1 (10.0) |
| Moderate | 36 (32.1) | 10 (15.2) | 15 (30.6) | 8 (80.0) |
| Severe | 39 (34.8) | 32 (48.5) | 16 (32.7) | 1 (10.0) |
| Very severe | 26 (23.2) | 15 (22.7) | 8 (16.3) | 0 (0.0) |
| Border elevation, n (%) | ||||
| N | 112 | 65 | 49 | 10 |
| None | 5 (4.5) | 14 (21.5) | 6 (12.2) | 0 (0.0) |
| Slight | 53 (47.3) | 23 (35.4) | 24 (49.0) | 1 (10.0) |
| Moderate | 36 (32.1) | 23 (35.4) | 17 (34.7) | 8 (80.0) |
| Severe | 13 (11.6) | 4 (6.2) | 1 (2.0) | 1 (10.0) |
| Very severe | 5 (4.5) | 1 (1.5) | 1 (2.0) | 0 (0.0) |
| Exudate, n (%) | ||||
| N | 112 | 66 | 49 | 10 |
| None | 4 (3.6) | 8 (12.1) | 9 (18.4) | 0 (0.0) |
| Slight | 16 (14.3) | 13 (19.7) | 12 (24.5) | 1 (10.0) |
| Moderate | 59 (52.7) | 27 (40.9) | 22 (44.9) | 8 (80.0) |
| Severe | 15 (13.4) | 11 (16.7) | 4 (8.2) | 1 (10.0) |
| Very severe | 18 (16.1) | 7 (10.6) | 2 (4.1) | 0 (0.0) |
Adherence to medication
Only 12 out of 66 (18.2%) participants provided data on adherence to their prescribed treatments at the end of the study. Nevertheless, the levels of treatment response achieved would suggest that the participants were using their medications broadly as prescribed.
Treatment response
Details of the clinical outcomes are summarised in Table 84.
| Outcomes | Subgroups | |||
|---|---|---|---|---|
| RCT participants, n = 112 | All cohort participants, n = 66 | Clobetasol propionate, n = 49 | Tacrolimus, n = 10 | |
| Velocity of healinga | n = 108 | n = 54 | n = 37 | n = 10 |
| Mean (SD), cm2/day | –0.2 (0.8) | –0.1 (0.3) | –0.1 (0.2) | –0.1 (0.1) |
| % healed by final visit (up to 6 months) | n = 112 | n = 64 | n = 47 | n = 10 |
| n (%) | 53 (47.3) | 28 (43.8) | 20 (42.6) | 5 (50.0) |
| Time to healing (days) | n = 112 | n = 64 | n = 47 | n = 10 |
| Median (Q1; Q3) | 113.5 (54.0; 182.5) | 45.5 (29.5; 160.0) | 43.0 (22.0; 145.0) | 54.5 (43.0; 161.0) |
| Area of lesion (cm2)a | n = 108 | n = 55 | n = 38 | n = 10 |
| Baseline, median (Q1; Q3) | 9.0 (3.2; 24.8) | 5.9 (1.8; 13.6) | 6.4 (1.6; 14.0) | 6.8 (2.8; 11.0) |
| Final visit, median (Q1; Q3) | 0.0 (0.0; 8.1) | 0.0 (0.0; 9.0) | 0.0 (0.0; 9.0) | 1.2 (0.0; 3.5) |
| Mean change from baseline at final visit (SD) | –9.1 (51.1) | –4.2 (11.5) | –4.0 (11.9) | –3.9 (6.0) |
| Median change (Q1; Q3) | –5.0 (–15.8; –1.5) | –3.4 (–8.7; –0.3) | –1.7 (–7.4; –0.2) | –3.3 (–8.5; –0.3) |
| Resolution of inflammationb | n = 107 | n = 54 | n = 49 | n = 10 |
| 6 weeks, n (%) | 11 (10.3) | 8 (14.8) | 6 (16.2) | 0 (0.0) |
| n = 108 | n = 55 | n = 38 | n = 10 | |
| Final visit, n (%) | 20 (18.5) | 12 (21.8) | 10 (26.3) | 1 (10.0) |
| AUC for weekly pain in first 6 weeks (range 0–20) | n = 77 | n = 37 | n = 24 | n = 7 |
| Mean (SD) | 7.6 (5.2) | 5.4 (5.2) | 5.6 (5.2) | 7.3 (6.3) |
| DLQI (range 0–30) | n = 111 | n = 66 | n = 49 | n = 10 |
| Baseline, mean (SD) | 11.7 (8.2) | 8.4 (6.0) | 8.5 (6.0) | 8.8 (4.6) |
| n = 66 | n = 49 | n = 32 | n = 10 | |
| Final visit, mean (SD) | 5.5 (7.2) | 6.2 (6.8) | 7.6 (7.5) | 4.6 (5.4) |
| EQ-5D (range –0.594 to 1) | n = 108 | n = 66 | n = 49 | n = 10 |
| Baseline, mean (SD) | 0.48 (0.4) | 0.59 (0.3) | 0.60 (0.3) | 0.51 (0.3) |
| n = 69 | n = 51 | n = 34 | n = 10 | |
| Final visit, mean (SD) | 0.71 (0.4) | 0.69 (0.3) | 0.65 (0.3) | 0.73 (0.3) |
| EQ-5D VAS (range 0–100) | n = 110 | n = 66 | n = 49 | n = 10 |
| Baseline, mean (SD) | 62.0 (21.8) | 67.0 (20.4) | 65.6 (21.9) | 64.4 (15.9) |
| n = 70 | n = 50 | n = 33 | n = 10 | |
| Final visit, mean (SD) | 72.1 (21.2) | 73.6 (20.5) | 69.3 (22.2) | 78.2 (13.1) |
| Recurrence (in those who had healed by 6 months)c | n = 52 | n = 27 | n = 19 | n = 5 |
| n (%) | 15 (28.8) | 4 (14.8) | 4 (21.1) | 0 (0.0) |
Velocity of healing was –0.1 cm2 per day on average (SD 0.3 cm2 per day). This is approximately half that observed in the RCT patients receiving systemic therapy, but the method of assessment was different for the two studies (physical measurements by clinician versus planimetry from digital images) and so direct comparison is difficult. The mean change from baseline in area of the lesion at the final visit was –4.2 cm2 (SD 11.5 cm2), with similar changes reported in the clobetasol and tacrolimus subgroups [–4.0 cm2 (SD 11.9 cm2) and –3.9 cm2 (SD 6.0 cm2), respectively].
Overall, 28 (42.4%) participants healed on topical therapy alone within the 6-month study period. Twenty two (33.3%) required systemic therapy and, of these, 13 (59.1%) went on to be enrolled into the STOP GAP RCT (see Figure 65). For those that entered into the RCT, eight (61.5%) healed by 6 months, with 3 out of the 13 (23.1%) healing by 6 weeks.
Ulcers healed in a median duration of 45.5 days, with wide variation (IQR 29.5–160.0 days) (see Table 84 and Figures 66 and 67). Cox proportional hazards model suggested that size of initial lesion was an important predictive factor in determining time to healing (HR 0.94, 95% CI 0.88 to 1.00; p = 0.043). Presence of underlying autoimmune disease was not predictive (HR 0.90, 95% CI 0.41 to 1.95; p = 0.786).
FIGURE 66.
Kaplan–Meier plot of time to healing (all participants) in observational cohort study.
FIGURE 67.
Kaplan–Meier plot of time to healing in observational cohort study.
Global disease severity as reported by clinicians and patients is summarised in Figures 68 and 69. Self-reported pain gradually reduced during the first 6 weeks of treatment and QoL scores improved for both disease-specific (DLQI) and general health status (EQ-5D) questionnaires (see Table 84). No covariates were predictive of scores at final visit for any of these outcomes, other than baseline scores for DLQI and EQ-5D VAS (DLQI estimate –0.47, 95% CI –0.77 to –0.17; p = 0.003). The EQ-5D VAS estimate was –0.40 (95% CI –0.65 to –0.15; p = 0.003).
FIGURE 68.
Global treatment response at final visit (clinician assessed).
FIGURE 69.
Global treatment response at final visit (patient assessed).

Recurrence
Of the 28 participants whose ulcer had healed, 27 had recurrence data available (minimum follow-up from time of healing 5.5 months; maximum follow-up 37.2 months). Overall 4 out of 27 (14.8%) participants had a recurrence subsequent to their initial episode.
Discussion
Main findings
This prospective cohort study of patients receiving topical therapy for the treatment of their PG lends support to previous suggestions that many patients with limited PG can be managed effectively with topical therapy alone. For almost half of the participants, healing was achieved within the 6-month study window and most of these had healed within 2 months. This is similar to the proportions healed in the STOP GAP RCT, for which, again, roughly half of the ulcers had healed by 6 months. Care should be taken when comparing healing rates between those participants in the RCT and those taking part in the observational study as those in the RCT had more severe disease, demonstrated by the increased number of ulcers, larger ulcer size at baseline, greater impact on QoL and longer time to healing. Of those who failed to heal on topical therapy, one-third subsequently received systemic therapy, which suggests that not all patients can be adequately treated with topical therapy alone.
The most important predictor of time to healing was size of the ulcer at presentation. This is consistent with our previous case note audit.
Given the increased mortality risk for patients with PG compared with patients with IBD and apparently healthy individuals,729 it is important to evaluate the role of topical therapies for the management of PG further. Similar concerns about increased mortality and morbidity in bullous pemphigoid patients that could be partly due to systemic therapies such as prednisolone led to a RCT by Joly et al. ,752 who found that mortality was reduced in those treated with potent topical steroids rather than systemic steroids. The lessons from pemphigoid raises the question of whether or not topical therapies should be the first line treatment for patients with PG.
The potential impact of PG on patients’ QoL life is high. Baseline EQ-5D scores of 0.59 (cohort study) and 0.48 (RCT) are comparable to patients with mild to severe heart failure; where EQ-5D scores of 0.78 (SD 0.18) to 0.51 (SD 0.21), respectively, have been reported. 753
One of the objectives of this observational study was to maintain contact with potential trial participants in order to improve recruitment into the RCT. In this regard, the parallel cohort study was extremely effective and resulted in an additional 13 out of 121 (10.7%) patients being enrolled into the RCT. For trials of rare conditions, when the evidence base is limited, the added complexities and expense of running a parallel observational study of this kind can often be warranted. 754
Strengths and limitations
This multicentre study included 66 participants, which is much larger than any of the previously published prospective cohort studies of PG patients published to date. 750,751,755 Clinicians enrolling participants into the study prescribed topical medication in line with current local practice and allocations were not randomised. As a result, it is not possible to make formal comparison of different topical treatments such as corticosteroids versus tacrolimus. Data on subgroups of patients are presented for interest, but should be interpreted cautiously. There is a hint that tacrolimus may be an effective treatment for PG, but further evaluation in comparison to potent or superpotent corticosteroids is required. Very little is known about the natural history of PG if left untreated. In the absence of a placebo control arm, it is not possible to say whether or not the lesions would have healed without intervention, although clinical experience would suggest that this is unlikely.
Generalisability
This was a pragmatic study that sought to reflect current practice in the UK. For a rare condition such as PG it was necessary to recruit in many hospitals and this aids the generalisability of the study results. Nevertheless, this cohort of patients was recruited alongside a RCT of systemic treatments for PG and this may have impacted on the type of patients agreeing to take part. On the whole, patients with more severe disease were randomised into the RCT and those with milder disease entered the cohort study.
Clinical conclusions
The possibility that mild PG may be controlled effectively using topical agents without incurring the side effect profile associated with systemic treatments is a very important finding for guiding clinical practice. Care should be taken when comparing the results of the cohort of patients participating in the topical treatment study with those from treated with systemic treatments in the RCT as those in the RCT were a different population with larger presenting ulcers. The importance of ulcer size on presentation in determining treatment response and the relatively high recurrence rates seen in this population of people with mild disease are also important clinical findings that will assist clinicians in optimising the management of their patients with PG, and in managing patients expectations with regard to the potential effectiveness of treatments.
Patient and public involvement
Development of the STOP GAP trial and parallel observational study was informed by pilot work conducted prior to the start of this NIHR Programme Grant [funded through a research grant from the British Skin Foundation (reference S317)]. This initial feasibility work was useful in establishing the importance of the topic to the dermatology community, for informing the study design and ensuring that the results of the trial reflected normal care as far as possible, thus, facilitating implementation of trial findings.
For a complex trial of a rare skin condition such as this it was extremely important to engage with HCPs responsible for treating patients with PG, as well as patients themselves. For this section, we therefore consider patient and public involvement to include both patients with PG and the health-care practitioners who provide their routine care.
Involving health-care professionals in the development of the STOP GAP trial
The STOP GAP trial was conceived and developed through the UKDCTN. This is a collaborative group of dermatologists, nurses, patients and researchers with a shared interest in the treatment and prevention of skin disease. The UKDCTN was established by Professor Hywel Williams in 2002 with the specific remit of improving the evidence base for rare and uncommon skin conditions, something that could only be achieved through large collaborative trials, such as the STOP GAP trial.
During the early stages of development, the STOP GAP trial was informed by four surveys including a survey of the membership of the BAD, members of the UKDCTN and members of gastroenterology and rheumatology professional groups. These surveys were used to inform the following aspects:
-
Prioritisation of the research question: research into the treatment of PG was one of the top 10 research areas identified by members of the BAD, and was voted on by the membership of the UKDCTN as a priority topic for further development by the group.
-
Choice of interventions: ciclosporin and prednisolone were identified as the most appropriate systemic treatments to compare head to head, and topical corticosteroids and calcineurin inhibitors (e.g. tacrolimus) were identified as the most appropriate topical agents. Guidance was also provided on appropriate doses and treatment regimen to be used.
-
Trial design: including duration of follow-up, choice of outcome measures, site selection and anticipated number of patients seen each year.
In addition to the surveys, a case note review was undertaken in 10 UK secondary care hospitals. 756 This retrospective case note review involving 136 cases of PG is one of the largest reviews of its kind and was important in establishing many aspects of the trial design (Table 85).
| Results from case note review | Impact on trial design | |
|---|---|---|
| Choice of interventions | Most commonly used systemic therapies: prednisolone and ciclosporin | Prednisolone and ciclosporin compared head-to-head in RCT |
| Recruitment rates | Mean incidence per hospital: six patients per year | 40–50 recruiting centres required (assuming 1 patient eligible and willing to be enrolled per centre per year) |
| Eligibility criteria | Biopsy taken in 52.7% of cases | Biopsy not required for diagnosis of PG as part of eligibility criteria |
| 14% had diabetes | Diabetes removed as a reason for exclusion | |
| Stratification variables | Most important predictor of treatment response: size of lesion on presentation | Randomisation stratified by size of lesion at baseline |
| Duration of trial | Median time to healing: 5 months | Time to healing assessed over 6 months instead of 4 months as originally planned |
| Outcomes | Recurrence proportion: 18% | Recurrence included as secondary outcome variable |
Patient involvement in the STOP GAP trial
Patients have been involved in the development of the STOP GAP trial from the outset and have played a key role throughout. The trial design was informed by a focus group involving three PG patients and structured interviews with a further two patients (one as an inpatient and one by telephone).
As a result of this work, changes were made to the study design including:
-
greater emphasis on the importance of capturing pain as an outcome measure
-
details of discharge from the wound was added to the PG severity assessment tool. Patients were generally very supportive of the trial and were keen to see research into the condition.
In addition, a prospective feasibility study in three UK centres recruited patients with either PG or leg ulcers and followed them up for a period of 4 weeks. One aspect of the feasibility study was to evaluate the suitability of asking patients to document healing between clinic visits by taking photographs of their PG lesion at home. This approach proved to be time-consuming and difficult for patients, which resulted in poor-quality images and missing data. As a result, it was decided that images taken during clinic visits was a more suitable approach.
Three PG patients agreed to assist the trial team by reviewing the trial documentation and information sheets, prior to the start of the trial. In addition, a patient joined the Trial Steering Group and his role was a lay member.
Participants have all received a copy of the study results and are being encouraged to actively disseminate these findings among their families, friends and health-care providers.
Summary and conclusions
Why is this research programme important?
Despite the severity of the symptoms associated with PG, and high associated mortality, the evidence base for treatment of the condition is weak. A single, small RCT has provided evidence of the efficacy of infliximab versus placebo in treating PG,749 but good-quality data relating to first-line treatment options are currently lacking. Without such data, management of PG cannot be standardised or optimised, especially in the absence of national or international guidelines.
The two studies described here from the PG work programme have generated data that are highly relevant to clinical practice. In the absence of guidelines, treatment decisions for cases of PG may be based on indirect comparisons of data between studies; any level of discrepancy in the designs intervention, control and outcomes can make such comparisons inappropriate. Small studies of ciclosporin for the treatment of PG have reported high proportions of patients achieving complete response,734–737 but no direct comparison has been performed between this and the other commonly used systemic treatment – prednisolone. By comparing these two drugs in a RCT setting, we have generated robust, head-to-head data disproving the hypothesis that ciclosporin is superior to prednisolone for the treatment of PG. The narrow CIs around our finding of ‘no difference’ exclude the possibility that we could have missed clinically important differences owing to sampling error. The study incorporated both objective and subjective measures of treatment success, in order to allow a thorough comparison of the treatments. Furthermore, although the adverse reaction profiles of the two drugs were generally as expected, the overall levels of efficacy were surprisingly low. The generally held conception that these systemic drugs are effective treatment options for PG is called into question by the low 6-month healing rates, as well as the propensity of patients to switch treatments or add in topical therapies during the RCT.
By virtue of its observational design, the prospective cohort study generated fewer robust data than the RCT. However, it was important both in promoting recruitment into the randomised study (particularly useful given the low prevalence of PG) and in generating clinically relevant data that complement the findings of the RCT. In the absence of management guidelines for PG, this study provided an opportunity to examine how the condition is being managed in practice and how effective current topical treatment strategies are. By conducting the two studies in parallel, we ensured that, as far as possible, any patient with PG who was willing to take part could be included. Furthermore, certain similarities in the design of the RCT and cohort study mean that, to some extent, data may be considered together in order to generate a more complete picture of this poorly studied disease area.
Implications for clinical practice
Despite published reports of particularly high response rates when treated with ciclosporin,734–737 the RCT reported here has provided a robust demonstration that ciclosporin monotherapy is not superior to prednisolone in promoting healing in PG. Clinicians can use this information to guide their treatment choice, which may, as a result, become more dependent on issues of safety and tolerability as well as cost. However, clinicians may also be influenced by the surprisingly low overall healing rates reported in this study. Judgement will need to be made on a patient-by-patient basis relating to the risk–benefit profiles of these systemic treatments, particularly bearing in mind the findings of the cohort study, where topical treatments were found to be effective as monotherapy in healing PG over 6 months in around half of cases. Caution is urged in comparing data between the two studies; it is important to consider, for example, that patients in the cohort study generally had milder disease than those in the RCT. Nonetheless, clinicians may wish to bear in mind that although the RCT called into question the general efficacy of systemic treatments for PG, data from the cohort study have indicated that topical therapies may be effective in many mild cases, thus offering the potential to avoid the adverse reactions that can be associated with systemic therapies, or at least a strategy whereby topical are tried first and systemic are used if topical fail.
As well as addressing a clinically important question, the STOP GAP trial has provided important opportunities for the dermatology clinical community to learn and share expertise in the diagnosis and management of PG. Such opportunities are invaluable to junior dermatologists in particular, who may only see one or two patients with PG per year. The top recruiting centres have also now been identified as specialist centres for the clinical care of PG patients under new NHS commissioning arrangements.
Implications for research
The lack of a clear difference in the efficacy of two of the most commonly used systemic treatments for PG, as well as the low healing rates reported in both treatment groups, suggests that more direct comparative studies should be performed to establish whether or not there are better systemic treatment options. Well-designed RCTs comparing agents such as dapsone, tetracyclines and colchicines, as well as anti-TNF therapies, would be valuable in generating an evidence base on which guidelines may be developed. Supported by the findings of the cohort study of topical therapies, there is also good rationale a direct comparison of topical treatments such as clobetasol against systemic treatments such as oral prednisolone in future RCTs. It would be interesting to examine the effects of topical treatments in more severe cases of PG, as these cases were generally enrolled in the RCT of systemic treatments rather than the cohort study. Formal assessment of the use of combinations of systemic or topical and systemic therapies, as well as aspects of general care such as limb elevation and dressings, would also inform practice.
Recruiting patients to studies of rare conditions such as PG can be challenging. Based on the positive experience of recruiting patients through the UKDCTN for the STOP GAP trial, the use of such collaborative networks – particularly on an international scale – would be recommended for future trials. Such an initiative is now being led by members of our team to establish an International Federation of Dermatology Clinical Trial Networks757 to encourage the sharing of research protocols for international trials.
The pragmatic approach to patient enrolment used in this programme also helped to promote recruitment and ensured that the findings were representative of real-life practice.
The importance of baseline ulcer size in determining treatment response was confirmed by this study and future trials may therefore benefit from adjustment for this variable at baseline. In contrast, the presence of underlying inflammatory disease was not found to be a strong predictor of treatment response.
The decision to use velocity of healing at 6 weeks as the primary outcome measure for the STOP GAP trial was a pragmatic one based on the need for a blinded outcome that could be collected for all randomised patients with minimal loss to follow-up. In this regard the outcome worked relatively well. However, it is a difficult outcome to interpret clinically and we would recommend that time to healing or proportion healed at 6 months should also be included as important outcomes in future trials. The event rates and variability of the various outcome measures used in our two studies also provide critical information for sample size calculations for future studies.
Patients reported pain as being the most important symptom in determining morbidity during pilot work, but this proved to be difficult to collect reliably and was relatively unresponsive to change, probably owing to confounding factors such as use of painkillers. Further work is needed to establish a valid and reliable PROM for PG and future trials may seek to explore the efficacy of different pain relieving medications.
Future research recommendations
-
The results of the two STOP GAP studies would suggest that a trial of topical therapies, such as clobetasone propionate, against systemic therapy for all patients with PG would be an important next step. Such a study could be non-inferiority in design provided that serious adverse events were less in the topical therapy group.
-
If topical corticosteroids are shown to be non-inferior to oral steroids but with gains in reduced serious adverse effects, then a RCT comparing topical corticosteroids against topical tacrolimus may also be worthwhile to see which is superior.
-
There is a suggestion from the current literature that combination therapy may be more effective than monotherapy. A trial of ciclosporin plus prednisolone versus either treatment alone may be useful in informing practice.
-
The relatively poor treatment response for all current PG treatments would suggest that other treatments should be considered and evaluated. Possible therapeutic options that have been discussed in the literature as potentially promising options include dapsone, tetracyclines and colchicines, as well as anti-TNF therapies.
-
The newly established specialist services for PG in the UK may provide an opportunity for monitoring treatment response routinely. Such an approach could usefully inform the evidence base for PG, particularly in relation to drug safety, the identification of possible predictors of response (thus informing a stratified treatment approach), disease morbidity and costs.
-
As with many rare diseases, robust outcome measures for capturing treatment response are lacking for PG. Further work on development of valid and reliable outcome tools for use in future PG trials and clinical record-keeping would be helpful.
Chapter 6 Conclusions
Impact of this research and dissemination
Delivering and demonstrating genuine impact within the NHS and beyond is challenging even in a 5-year Programme Grant, as incorporation of evidence into clinical practice can take several years even in the presence of clear findings. 758 It should also be noted that one of our deliverables with the most robust clinical findings, the STOP GAP RCT on PG, has taken the entire 5 years of the Programme Grant to complete. Much of the purpose of this Programme Grant was to generate new research priorities and to develop existing research ideas through feasibility work so that the risks associated with full-scale studies can be identified and minimised. Definitive national research rests on a pyramid of interlocking pieces of information that must be completed before ‘big research’ that can directly inform clinical practice can flourish.
Yet, within this Programme Grant, there is already ample evidence of impact both in terms of breadth (e.g. international adoption of core outcome sets for eczema trials) and reach (e.g. use of the GREAT database to inform the development of the American Academy of Dermatology guidelines on AD307). The detailed impact of each work package has already been summarised in the relevant chapters, but a broad summary of the main impacts across the five work package is shown in Table 86.
| Work stream | Guidelines informed | Further funded research | Other impact |
|---|---|---|---|
| Eczema prevention | Barrier enhancement with emollients for the prevention of eczema (BEEP trial). Funded by NIHR HTA1 A functional mechanistic study of the effect of emollients on the structure and function of the skin barrier. Funded by Nottingham University Hospitals NHS Trust Pump-priming fund759 |
New definition of an incident case | |
| Eczema treatment | NHS Choices eczema module760 NICE quality standards for eczema357 SIGN guideline on management of AE in primary care347 Map of Medicine care pathway Clinical knowledge summaries eczema module761 Royal College of Paediatric and child health allergy treatment pathway762 Emollients, education and QoL: the Royal College of Paediatrics and Child Health care pathway for children with eczema763 American Academy of Dermatology AD guidelines764 Dutch AD guideline765 |
CLOTHing for the relief of Eczema Symptoms (CLOTHES trial). Funded by NIHR HTA | New core outcome sets for eczema treatment research Hosting the International Symposium of Atopic Dermatitis in Nottingham, May 2014 |
| Vitiligo | European guidelines for the management of vitiligo483 BAD guidelines for the management of vitiligo434 Clinical Knowledge Summary module for vitiligo490 NHS Choices vitiligo module472 |
Home interventions and light therapy for vitiligo (HI-Light Vitiligo Trial). Funded by NIHR HTA491 | New definition of outcomes that reflects patient’s concerns New focus on delivery of NB-UVB light therapy at home rather than in a hospital setting |
| SCC | SIGN guidelines for the management of high risk squamous cell carcinoma NICE skin cancer evidence update766 NHS Choices skin cancer module767–769 |
Full application in preparation | |
| PG | UpToDate®: Schadt770 | Top recruiting centres in STOP GAP trial identified as lead treatment centres for NHS specialised commissioning services for PG. Within 6 months of publication, the results of the study had been translated into at least seven languages including Hebrew, Portuguese and Japanese |
Three overarching messages for further research
Perhaps the most important conclusion from our research is to underscore the importance of the research cycle of systematically reviewing what is already known, prioritising the uncertainties that are found, reducing further unknowns through qualitative and quantitative feasibility work and then proceeding to full-scale national assessments, as shown in Figure 70.
FIGURE 70.
Stages of the research cycle.
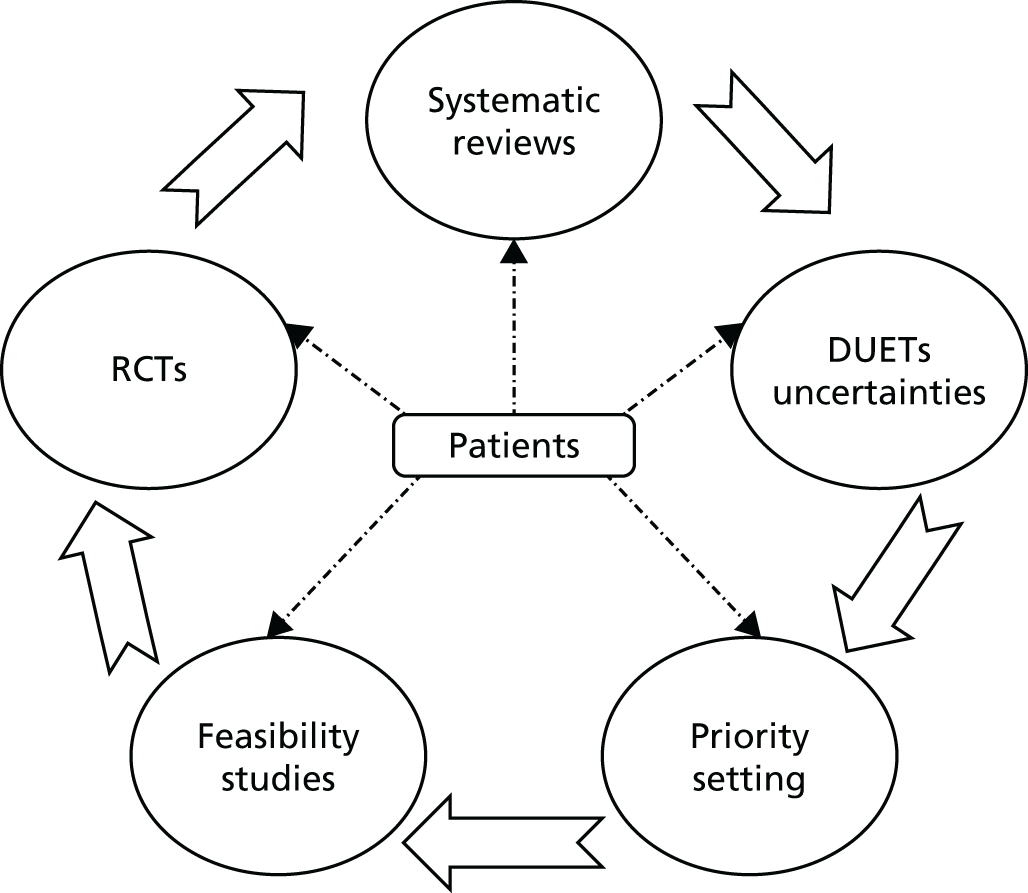
Many people refer to the research cycle in scholarly articles and on funding websites, and following it may sound like an obvious and desirable way to proceed but making it really work is another matter. As both the vitiligo and eczema systematic reviews have shown, most RCTs in dermatology have not addressed questions that seem to matter most to patients. There are often serious flaws in study designs and many are far too small to draw conclusions from, resulting in many potentially useful therapies being discarded through a common lack of understanding that ‘no evidence of an effect’ is not the same as ‘evidence of no effect’. 5 Most of the RCTs we encountered have not been reported fully, thereby preventing them from contributing to their ‘second life’ in the form of meta-analysis within systematic reviews. It would seem there is a culture of deliberately bypassing the research cycle in dermatology, which is probably owing to a widespread lack of awareness of clinical research methodology by the dermatology research community, funders of dermatology research, journal editors and national dermatology societies.
Our first summative message is a simple one: fewer rather than more studies need to be done. Trials that are funded should be bigger and better, so that more robust conclusions can be drawn. New research should be identified and prioritised within the context of existing research and larger studies should be undertaken through national or international collaboration.
As one of our patient collaborators commented having led the update of the Cochrane systematic review on vitiligo:
It is time to stop the endless cycle of small, inconclusive trials – we need fewer, but better quality trials that address topics that have been prioritised as being important.
Mrs Maxine Whitton, MBE (Member of the Most Excellent Order of the British Empire) (University of Nottingham, 2014, personal communication)
Study protocols and final study findings need to be reported completely and honestly in order to minimise the pernicious effects of distorting the scientific record through publication bias, selective reporting outcome bias and framing biases produced by spin.
Research wastage caused by the continual production of small, poorly designed and poorly reported studies in dermatology needs to be discouraged by all involved in research including researchers, funders, journal editors and patients/public. Instead we recommend following the basic premises of the research cycle: identifying uncertainties from systematic reviews, prioritising topics with stakeholders, reducing the risks associated with a full-scale trial through feasibility work and then conducting large collaborative pragmatic studies that best inform everyday practice in the NHS and other health-care systems.
Our second overarching research message is that there is a lack of high-quality dermatology research in primary care where, paradoxically, most skin care is delivered in the UK. This mismatch or inverse research care law (which states that where the research need is greatest the quantity and quality of research is least224) is nicely illustrated in Figure 71 that one of our researchers (Helen Nankervis) drew during her final reflections on this work at a dissemination event. Thankfully, this important deficiency in primary care research into skin diseases is now being filled by emerging groups such as the Society for Academic Primary Care Special Interest Group for Dermatology. 771
FIGURE 71.
Diagram representing the lack of eczema research in primary care, where most people are treated.
Our third message is that skin disease represents a substantial disease burden throughout the world. Thanks to the Global Burden of Disease initiative, it is now much clearer that skin disease contributes to a substantial burden of disease worldwide. A detailed analysis of skin data from the 2010 Global Burden of Disease surveys showed that skin conditions were the second to eleventh leading cause of years lived with disability when explored at the country level and, at a global level, skin disease was the fourth most common reason for non-fatal disease burden. 11 A further analysis of how skin disease burden in the 2010 surveys was matched by research funding from the US National Institutes of Health indicated that some diseases such as dermatitis and NMSC were well matched, whereas others such as cellulitis, pressure ulcers, urticaria, acne, viral and fungal diseases, scabies, and melanoma were less well represented. 772 A similar pattern of under-representation was evident for systematic reviews published by The Cochrane Collaboration. Measurement of skin disease burden using disability-adjusted life-year metrics allows comparison with other diseases. 768 Disease burden is an important but not exclusive component for guiding research funding. Further annual updates and refinements of the models used to asses global burden of disease are ongoing and serve as an important resource for prioritising future skin disease research.
Strengths and limitations of this research programme
Flexibility and joined up thinking throughout the National Institute for Health Research
One of the key aspects of the Programme Grants for Applied Research scheme is flexibility, so that effort can be vied between different work packages depending on need and time. We appreciated the way that the NIHR staff engaged in a discussion about the best way of publishing our systematic review of AE treatments, which is now published as a stand-alone report. 223 This review was an update of the NIHR HTA review conducted by some of the authors in the year 2000 and publishing our update as a separate report allowed the use of hyperlinks to the previous report, which saves readers from flicking from one report to another. This is a good example of the family of NIHR programmes working well together. Another example of linkage between the NIHR funding schemes was the success in obtaining funding for three large national trials through the NIHR HTA programme as a result of the prioritisation and pilot work conducted within the Programme Grant.
How do we perform according to the National Institute for Health Research ‘adding value’ agenda?
The NIHR adding value agenda is based on a landmark paper by Chalmers and Glasziou38 on the four stages where research wastage occurs (Figure 72), which was published after our Programme Grant started.
FIGURE 72.
The four principal stages of avoidable research waste. Reprinted from Lancet, vol. 374, Chalmers I, Glasziou P, Avoidable waste in the production and reporting of research evidence, pp. 86–9, Copyright (2009), with permission from Elsevier. 38
Therefore, it is worthwhile to reflect to see how our Programme Grant fulfils this agenda.
Stage 1: ensuring research questions are relevant to clinicians and patients
The eczema prevention work programme started with an overview of existing systematic reviews, which confirmed the need to explore new approaches for disease prevention, such as the use of emollient form birth in high-risk groups. This work programme also undertook a systematic review of definitions of an incident case of eczema and came up with a practical suggestion for defining incident cases of eczema that is now being used in the main BEEP trial. The eczema treatment work programme was based largely around a wide scoping systematic review, which was followed by a PSP exercise involving patients, clinicians and other stakeholders under the guidance of the James Lind Alliance. Further work in the eczema treatment work programme involving patients and clinicians and methodologists identified the core outcome domains that need to be measured in eczema trials and a roadmap on how to develop such core outcomes for clinical research:
Not having a defined and comparable outcome measure for a particular condition is a bit like watching a football match in which the rules have not been defined. No-one then knows who has won and why.
Lester Firkins OBE (Officer of the Most Excellent Order of the British Empire), Chair of the James Lind Alliance and Chair of our Executive Group, 2014, personal communication
The vitiligo work programme began by updating a Cochrane systematic review and was also followed by a James Lind Alliance PSP that resulted in a number of prioritised topics including the one taken forward by the group on handheld UV devices for home use:
The prioritisation process gave a wide variety of people the chance to submit and rate questions. I feel this was an excellent approach to take as it allowed us the chance to submit those burning questions that we would like concrete answers to.
Participant of the PSP
The SCC work programme started with a Cochrane systematic review and because only one RCT meeting the specified criteria was found, it then progressed to the next level of useable evidence, that is cohort and other observational studies that provided critical information for informing the planning of a definitive RCT. To prioritise the most urgent research questions for SCC, stakeholders (including dermatologists, plastic surgeons and radiographers) were surveyed. The resulting suggestions were further developed and prioritised by the NCRI non-melanoma CSG with view to a full RCT application in the summer of 2014.
The PG work programme did not start with a Cochrane review, mainly because the study had to begin immediately if it was to be completed within the 5-year time frame. The choice of treatments was prioritised by surveys of the UKDCTN and through its prioritisation panel and at steering group meetings. Systematic reviews and prioritisation exercises have thus been a strong theme of this entire Programme Grant. 773
Stage 2: ensuring that design and methods are appropriate to the questions posed
We used a mixture of qualitative and quantitative research methods that best fitted the questions posed and reported those methods fully according to recommended reporting guidelines such as PRISMA for systematic reviews and CONSORT 2010 for RCTs. 774 Steps were taken in designing trials to reduce the impact of bias. Selection bias was minimised by using computer generated randomisation sequences and concealed treatment allocation through the NCTU. Performance bias was minimised by dealing with treatment groups as similarly as possible and information bias was minimised by using blinded outcome assessment (e.g. digital photographs of pyoderma ulcers that were read by independent observers in the STOP GAP trial). All analyses were conducted according to the ITT principle to reduce attrition bias. All of our RCTs have been pragmatic in design in order to ensure good generalisability in readiness for when the results are applied to everyday clinical practice in the NHS.
Stage 3: ensuring accessible and full publication
All work emanating from this Programme Grant, regardless of whether or not the results have been encouraging, has been published fully and honestly in this publicly accessible report and in the accompanying peer-reviewed journal articles (see Acknowledgments, Publications). The team has deliberately targeted and paid for open access journals when possible.
Stage 4: ensuring reports are unbiased and useable
All of the trials and systematic review published in this report have been preceded by a published protocol so that readers can check whether or not all specified outcomes were reported faithfully. Particular care was taken to ensure that interventions referred to in our studies have been described fully and in such a way that others could replicate the intervention in clinical practice. 769 Thus, the correct method for using the handheld NB-UVB unit has been summarised in a specially produced training DVD that involved substantial input from patients and which is now freely available on the resources section of the Centre of Evidence-Based Dermatology website. 775
It is clear that we have fulfilled the ‘adding value’ research agenda very well, not because we have followed the Chalmers and Glasziou’s38 recommendations slavishly, but because we were already very much engaged with such principles. It is difficult to argue why new research should happen without pausing to see what research has been done, or that an appropriate design should be used for certain types of research question. Such principles should be regarded as mandatory aspects of clinical research rather than ‘adding value’.
Have we done too much?
A critical business person might look at Table 87, which shows what was planned and what was delivered, and comment that we have been unwise for overdelivering so heavily, as this could have compromised our ability to deliver the original objectives. However, the potential impact of any additional work undertaken was carefully reviewed by the Executive Group prior to implementation in order to guard against this.
| Type of output | Planned | Actual |
|---|---|---|
| Systematic reviews | 4 | 8 |
| PSPs | 2 | 3 |
| Pilot RCTs | 1 | 2 |
| Feasibility studies | 2 | 3 |
| Full-scale RCT | 1 | 1 |
| Funding proposals for RCTs | 3 | 4 |
| Core outcome initiatives | 0 | 2 |
| Industry liaison project | 1 | 0 |
It is important to acknowledge that overcommitting to too many offers of research collaborations is a hazard within a Programme Grant and is one that could potentially result in an implosion of the central themes if too much peripheral work takes over. We have taken the view throughout our Programme Grant that we would exploit any relevant opportunities that arise that do not incur costs or risks above that which could be covered within the flexible nature of this Programme Grant. Thus, the opportunity to front the development of international core outcome sets for eczema and vitiligo trials was something that fell nicely into our remit as the work was so relevant to our work programme on eczema treatment and vitiligo. Opportunities to ensure that the results of our research were included in the NHS Map of Medicine, patient information resources (NHS Choices) and on clinical knowledge summaries for GPs also came our way in a timely fashion and were better placed to meet national information needs than creating similar resources on our own institutional website.
The group did say ‘no’ to a number of projects that did not fit in terms of theme and resources. Therefore, we do not view this Programme Grant as ‘overdelivering’. We have instead used our budget and resources wisely in order to take advantage of the changing needs for research over time by collaborating with colleagues for good dividends and for very little additional outlay – perhaps a better definition of ‘adding value’.
Lessons learned that can be shared with others applying for Programme Grants for Applied Research
An important aspect of a successful programme of work is to share lessons learned with others who wish to undertake similar programmes of work. Our main ‘lessons learned’ were presented at a final dissemination event on 11 February 2014 and are summarised in Box 9.
-
Start early when applying for a Programme Grant – putting together a large coherent programme with multiple partners can take a considerable time.
-
Undertake preliminary work through a development grant or other means.
-
Collaborate with the best people possible and outside your own institution.
-
Plan your research so that each work programme overlaps rather than entirely sequential research that is dependent on critical pinch points that can delay or sabotage subsequent work.
-
Allow plenty of time and ensure adequate funding for each of the work programmes and components.
-
Invest a lot of time in integrating new staff and in training patient and public members – time which will be rewarded as the work progresses.
-
Communicate with your funder and host NHS Trust regularly about key decisions and outputs – we found quarterly reports particularly useful for this.
-
Provide opportunities for shared learning across the individual work packages.
-
Publish as much as possible as the work progresses and be careful to retain Crown Copyright so that all published material can be included in the final published report.
-
Allow plenty of time (6 months) for drafting, proofreading and producing the final report in a format that can be understood by a wide readership.
The critical importance of social capital and developing people
Activities of our patient panel
The Centre of Evidence Based Dermatology has always had a strong record of patient and carer involvement in its research. This Programme Grant allowed us to establish and train a specialist patient panel with an interest in skin research, which has transformed much of our activity in this area.
We began by recruiting patients and carers into the panel who were already taking part in our work and then advertised the panel on our website and those of relevant patient support groups and charities. Five years on, the panel is well developed and has > 20 active members (both patients and carers) affected by a variety of skin disorders. Members of the panel have been involved in a wide range of Programme Grant research activities including:
-
joining Steering Groups for PSPs
-
undertaking consumer reviews for the Cochrane Skin Group
-
helping with the design of patient related materials such as surveys and information leaflets
-
giving feedback on the design of clinical trials
-
being coapplicants on grant applications and members of study teams
-
involvement in core outcome measure projects
-
helping with dissemination and implementation activities.
A full log of all activities and projects contributed to by members of our patient panel is appended (see Appendix 21). In order to ensure that panel members were well supported in their activities, annual training days were held with an average of 12 members attending each one. The days were split into presentations to inform and educate panel members and workshop sessions to assist with the development of relevant research projects. Areas covered include the design of clinical trials, Cochrane systematic reviews, jargon busting, clinical research and evidence-based medicine. We keep in contact with panel members via a regular newsletter updating them on the progress of projects and activities.
The many benefits of the patient panel to our research are obvious; we have a significant number of engaged and trained patients/carers affected by a wide variety of skin disorders who are involved in an extensive range of research activities. The commitment of our panel members is illustrated by their length of involvement in the panel and the variety of activities they are increasingly taking part in. Members can benefit from participation in the Patient Panel in a number of ways. This includes learning new skills and the satisfaction of knowing they are helping others in their situation – the latter being the most common reason stated for joining the panel. Direct health benefits for panel members have been stated as coping better with their condition, an increased confidence in dealing with HCPs and an increased understanding of their disease.
The importance of a shared vision and purpose
Many have asked us why our Programme Grant has been successful in terms delivering our key objectives plus 50% more within time and within budget. In addition to strong programme management and the flexibility afforded by the nature of the Programme Grants for Applied Research, a key factor has been the genuine engagement of all involved, whether they were doctor of philosophy (PhD) students, members of the patient panel or those working in support services such as finance and research and development. The vision of what each work programme was trying to achieve was clear to all who engaged with us and it was a vision that engendered a sense of a common and worthwhile purpose. Such engagement did not happen by chance, but as a result of an active and planned process which needed time, effort and preparation. Examples include providing good information to existing staff about the new Programme Grant and how the new work would be mutually beneficial, annual away-day events for the whole team to learn how to work together in groups, being prepared to travel to other locations where our collaborators were based, and by setting aside time to train and work with our patient panel.
Rather than rushing straight onto the next research programme in the traditional ‘butterfly behaviour of researchers, moving onto the next flower well before the previous one has been fully exploited’ highlighted by the late Allessandro Liberati,776 the team held two outward-facing events during the lifetime of the project – one after 3 years and one at the end. These meetings were used to mark closure of different aspects of the programme, whereupon all collaborators, and others interested in programme grants, were invited to celebrate and learn.
At the final closure meeting, each participant was given a mug, a notebook and bag with our logo (Figure 73), and the chief investigator even composed a song for the occasion.
FIGURE 73.
Items given to participants of final meeting.

The close-down event provided a moment to pause, reflect and achieve closure on an important chapter of our lives and it was very well received. We conclude that building and maintaining social capital around a shared vision and working closely with patients is the key to delivering successful clinical research. Good clinical research starts with people and ends with people.
The development of new research skill and capacity in the area of skin disease, especially in previously neglected areas of dermatology such as vitiligo and cutaneous SCC, is also a key feature of our Programme Grant. Three PhDs will be awarded as a result of the work, which means that three more skilled people are able to contribute to the NIHR effort in the form of mature and independent researchers. We have already summarised aspects of personal development of individual researchers and patients in our special Programme Grant final booklet,777 but it is worth reflecting for a moment at some of the quotes in that booklet from the various people involved (Box 10).
To be able to read of the REAL discoveries that have been made is wonderful. To my mind, these researchers are exemplars in the way they use funders’ support to get meaningful and always transparent benefits.
Lester Firkins, Independent Chair of the Executive Group
I think patient involvement can really ‘humanise’ research so that patient consideration can be built into the objective, process and outcome of the studies. And at the end, I believe this is the most important part of any research.
Amina Ahmed, member of our patient panel
All in all I found it an amazingly positive experience, and it was hugely encouraging to meet so many specialists with a strong commitment to eczema research and treatment.
Tim Burton, member of our patient panel
it taught me to look for the things that are NOT there as well as those that are.
Helen Nankervis, Research Associate
My opinion is that everyone should experience research. It is an eye opener – it completely changes your mind-set and the way you see everything around you.
Viktoria Eleftheriadou, Research Associate
Although this programme is coming to an end, we are just part way through the journey towards eczema prevention, and exciting times lie ahead as we undertake a large trial of emollients that has been funded as a result of the work done here.
Joanne Chalmers, Research Fellow
For a disease which has been so under-researched, it is gratifying to see the impact our work is starting to have on redressing this situation.
Louise Lansbury, Research Associate
It is very difficult to recruit sufficient patients to studies of rare and serious diseases such as pyoderma gangrenosum. STOP GAP has achieved impressive recruitment to produce evidence, which will guide clinicians in offering patients treatment that is as safe and as effective as possible.
Dr Nick Levell, STOP GAP trial recruiting clinician
Thank you very much for providing me with this opportunity to learn about evidence-based medicine with you and your UK Dermatology Clinical Trials Network team. This is certainly one of the most important skills I have gained in dermatology training and I am very grateful to you.
Dr Suyin Ong, UKDCTN Specialist Registrar Fellow (2010–12)
Finally, it is evident from the huge collaborative efforts involved in this Programme Grant that many contacts have been formed that will help to spin off other research initiatives. Quite apart from the NIHR HTA-funded trials that were sparked off wholly by this programme of work, new groups are forming, such as a national network for supporting trials into epidermolysis bullosa – a rare but potentially devastating skin condition where the skin fails to heal after minor friction. We have also shared our experience in developing clinical trials by forming an International Federation of Dermatology Clinical Trials Networks. 757 The experience of delivering the successful Programme Grant has given the team the confidence to work more closely with the local clinical trials unit to support a research methodology fellowship application and also to apply for and win funds from the University of Nottingham to expand senior capacity that will enable our Centre of Evidence Based Dermatology to achieve a step change in its ability to deliver NHS-focused research of the highest calibre.
Acknowledgements
We would like to acknowledge the following individuals, organisations and institutions for their contributions to this programme grant. The work described in the report has been a huge collaborative effort for the benefit of patients with skin disease. We are very grateful to all those who have given up their time and resources to make this work a success.
Further details regarding specific contributions can be found in the relevant published papers.
Programme Grant Executive Group
Hywel Williams.
Kim Thomas.
Nicholas Evans.
Lester Firkins.
Jane Ravenscroft.
Andrew Nunn.
Barbara Maston/Bryony Elliot.
Programme Grant Steering Group
Fiona Bath-Hextall.
Joanne Chalmers.
Sally Crowe.
Finola Delamere.
Viktoria Eleftheriadou.
Nicholas Evans.
Louise Lansbury.
Carron Layfield.
Jo Leonardi-Bee.
James Mason.
Eleanor Mitchell.
Helen Nankervis.
John Norrie.
Andrew Nunn.
Anthony Ormerod.
Rameshbhai Patel.
William Perkins.
Kim Thomas.
Maxine Whitton.
Hywel Williams.
Patient Support Groups
National Association for Crohn’s and Colitis.
National Eczema Society.
Nottingham Support Group for Carers of Children with Eczema.
The Karen Clifford Skin Cancer Charity.
Vitiligo Society.
Patient contributors
Amina Ahmed.
Alexandra Barto-Smith.
Julie Block.
Tim Burton.
Jo Clayton.
Anne Collier.
Margaret Cox.
Roger Dainty.
Adrian Day.
Rachel Fletcher.
Joanne Foster.
Fred Fredriksen.
Marjorie Howard.
Carolyn Hughes.
Rosmary Humphreys.
Sara Kuppuswami.
Saravanapriya Loganathan.
Deborah Mason.
Stephanie Merhand.
Louise Morgan.
Kaspar Mossman.
Kirsteen Murray.
Paul Mussell.
Colette O’Sullivan.
Jo Parris.
Anjna Rani.
Jenni Rishworth.
Amanda Roberts.
Lisa Sharples.
Stephen Shippard.
Jason Simons.
Derek Stewart.
Piyada Tedstone.
Jack Tweed.
Tom Volkman.
Ebony Vassell.
Jasmin Vassell.
Jo Wedd.
Maxine Whitton.
Jennifer Viles.
Collaborating institutions
Nottingham University Hospitals NHS Trust.
University of Nottingham.
Birmingham Clinical Trials Unit.
British Association of Dermatologists.
British Dermatological Nursing Group.
British Skin Foundation.
British Society for Gastroenterology.
British Society for Rheumatologists.
Centre for Healthcare Randomised Trials.
Cochrane Skin Group.
European Wound Management Association.
Imperial College London.
James Lind Alliance.
Leg Ulcer Forum.
Lindsay Leg Club Foundation.
Medical Faculty Carl Gustav Carus, TU Dresden.
Medical Research Council Clinical Trials Unit.
NHS Choices.
NHS Direct.
Nottingham Clinical Trials Unit.
Nottingham County Teaching primary care trust.
Oregon Health and Science University.
Patient UK website.
Rare Disease UK.
Research Design Service (North East region and Trent region).
Robertson Centre for Biostatitics.
Tissue Viability Society.
UK Dermatology Clinical Trials Network.
University of Aberdeen.
University of Bristol.
University of Dundee.
University of Durham.
University of East Anglia.
University of Sheffield.
University of Southampton.
Welsh Wound Network.
West Hertfordshire Hospitals NHS Trust.
Wound Alliance.
Wounds UK.
National Institute for Health Research Clinical Research Network support
Birmingham and Black Country CLRN.
Central and East London CLRN.
Cheshire and Merseyside CLRN.
County Durham and Tees Valley CLRN.
Clinical Research Centre Cymru Research Networks.
Cumbria and Lancashire CLRN.
Essex and Hertfordshire CLRN.
Greater Manchester CLRN.
Hampshire and Isle of Wight CLRN.
Kent and Medway CLRN.
Leicestershire, Northants and Rutland CLRN.
London (north-west) CLRN.
London (south) CLRN.
Norfolk and Suffolk CLRN.
North and east Yorkshire and north Lincolnshire CLRN.
Northumberland, Tyne and Wear CLRN.
Peninsula CLRN.
Scottish PCRN.
South Yorkshire CLRN.
Surrey and Sussex CLRN.
Thames Valley CLRN.
Trent CLRN.
Western CLRN.
West Anglia CLRN.
West Midlands (north) CLRN.
West Midlands (south) CLRN.
West Yorkshire CLRN.
Contributors to eczema prevention work programme
Lorne Becker.
Robert Boyle.
Sara Brown.
Linda Campbell.
Christine Carocci.
Joanne Chalmers.
Lisa Charlesworth.
Yiyi Chen.
Zunqiu Chen.
Mike Cork.
Simon Danby.
Sue Davies-Jones.
Matthew French.
Douglas Grindlay.
Jon Hanifin.
Sandra Lawton.
Matthew Leighton.
Troy Lubianski.
Irwin McLean.
Alan Maplethorpe.
Alan Montgomery.
Johanna Perdue.
Matt Ridd.
Tracy Sach.
Daniel Simpkins.
Eric Simpson.
Kim Thomas.
Jim Thornton.
Hywel Williams.
Min Yang.
BEEP trial pilot recruiting sites
-
Nottingham University Hospitals NHS Trust: Hywel Williams (PI), Sue Davies-Jones, Jo Llewellyn, Sandra Lawton, Ruth Murphy and Jane Ravenscroft.
-
Derby Hospitals NHS Foundation Trust: Adam Ferguson (PI), Vanessa Unsworth, Nicola Watson, Coral Smith and Ruth Ballington.
-
United Lincolnshire Hospitals NHS Trust: Krisztina Scharrer (PI), Kristina Ewing, Sarah Booker, Alison Raynor, Mandy Roper and Andrew Dainty.
-
The Research Institute at Wheatbridge, Chesterfield: Mark Loveland (PI) and Sian Le-Beau.
-
Oregon Health & Science University, Portland, OR, USA: Eric Simpson (PI), Shahan Baig-Lewis, Gretchen Barron, Lori Kelly, Lindsey Severson and Susan Tofte.
Contributors to eczema treatment work programme
Christian Apfelbacher.
Patricia Atkinson.
Małgorzata Baławp.
Akerke Baibergenora.
Sebastien Barbarot.
Tomi Beck.
Katja Boehm.
Maarten Boers.
Robert Boyle.
Joanne Chalmers.
Tessa Clarke.
Martin-Clavijo.
June Cody.
Kathyn Cowan.
Ching Ching-Chi.
Ivan Chromej.
Kathryn Cowan.
Finola Delamere.
Alida DePase.
Magdalene Dohil.
Liz Doney.
Gordon Dooley.
Lawrence Eichenfield.
Viktoria Eleftheriadou.
Alexandra Erven.
Sam Feng.
Mark Fenton.
Alireza Firooz.
Masutaka Furue.
Masaki Futamura.
Sherman Gu.
Mary Glover.
Deanne Hewson.
Joseph Jabbar.
Euiseok Kim.
Gudula Kirtschig.
Louise Lansbury.
Sandra Lawton.
Alan Mablethorpe.
Agustin Martin-Clavijo.
Margaret McPhee.
Fiona Meredith.
Helen Nankervis.
Yukihiro Ohya.
Tom Platts-Mills.
Emma Pynn (née Smith).
Liv Reinar.
Amanda Roberts.
Evelien Roekevisch.
Natasha Rogers.
Lesley Rushton.
Matt Ridd.
Tracey Sach.
Jordan Samuels.
Jochen Schmitt.
Mandy Schram.
Eric Simpson.
Jasvinder Singh.
Sherie Smith.
Phyllis Spuls.
Kyle Tang.
Kim Thomas.
Gu X.
Margaret Whittingham.
Maxine Whitton.
Hywel Williams.
Contributors to vitiligo work programme
Perways Akram.
Anton Alexandroff.
Patricia Atkinson.
Jonathan Batchelor.
Joanne Chalmers.
Lisa Charlesworth.
Kathryn Cowan.
Sue Davies-Jones.
Robert Dawe.
Shelley Dowey.
Lelia Duley.
Viktoria Eleftheriadou.
Richard Farley.
Mark Fenton.
Fred Fredriksen.
Joanne Llewellyn.
Claire Lushey.
Samir Mehta.
Alan Montgomery.
Johanna Perdue.
Jane Ravenscroft.
Andy Rogers.
Tracey Sach.
Miriam Santer.
Catherine Shelley.
Kim Thomas.
Selina Tour.
Jennifer Viles.
Graham Watson.
Diane Whitham.
Maxine Whitton.
Hywel Williams.
Adrian Yong.
Susan Yule.
HI-Light pilot recruiting sites
King’s Mill Hospital, Mansfield: PIC.
Leicester Royal Infirmary: Anton Alexandroff (PI), Catherine Shelley Nottingham NHS Treatment Centre (Circle Partnership UK): Jonathan Batchelor (PI), Susan Davies-Jones, Viktoria Eleftheriadou and Joanne Llewellyn.
Nottingham University Hospitals NHS Trust: Jane Ravenscroft (PI), Susan Davies-Jones, Viktoria Eleftheriadou and Joanne Llewellyn.
Contributors to squamous cell carcinoma work programme
Alemayehu Amberbir.
Jane Barrett.
Fiona Bath-Hextall.
Joanna Browne.
Lynette Chadwick.
An-Wen Chan.
Chih-Mei Chen.
Mary-Margaret Chren.
Karen Corrall.
Carsten Flohr.
Katherine Fowkes.
Timothy Goodacre.
Teenah Handiside.
Louise Lansbury.
Pat Lawton.
Iain Leach.
Paul Leighton.
Jo Leonardi-Bee.
George Lynham.
Alan Maplethorpe.
Philippa Middleton.
Dedee Murrell.
Johanna Perdue.
William Perkins.
Jack Tweed.
National Cancer Research Institute non-melanoma subgroup of the melanoma clinical studies group
Catherine Harwood.
Charlotte Proby.
Pat Lawton.
John Lear.
Jerry Marsden.
Jenny Nobes.
Keith Wheatley.
Neil Steven.
Steve Nicholson.
Marc Moncrieff.
Carie Corner.
Contributors to pyoderma gangrenosum work programme
STOP GAP Trial Steering Committee – independent members (Trial Steering Committee)
John Ingram.
Calum Lyon.
Sarah Meredith.
Paul Mussell.
Frank Powell.
Daniel Wallach.
STOP GAP Trial Management Group
Julie Barnes.
Fiona Craig.
Kath Foster.
Nicola Greenlaw.
Ellie Harrison.
Alan Maplethorpe.
James Mason.
Eleanor Mitchell.
John Norrie.
Tony Ormerod.
Aisha Shafayat.
Daniel Simpkins.
Kim Thomas.
Diane Whitham.
Hywel Williams.
STOP GAP Data Monitoring Committee
Angela Crook.
Alison McDonald.
Julie Schofield.
STOP GAP trial recruiting centres
Aberdeen Royal Infirmary, NHS Grampian: Anthony Ormerod (PI), Fiona Craig and Linda Lawson.
Aneurin Bevan Health Board: Alex Anstey (PI), Catherine Watkins, Sarah Mitchell, Richard Goodwin and Cilla Benge.
Barts and The London NHS Trust: Frances Lawlor (PI).
Basildon and Thurrock University Hospitals NHS Foundation Trust: Gosia Skibinska, (PI), N Ariffin, Janice Armitt, Nhlanhla Mguni, Maxwell Masuku, Kerry Goodsell and Linda Johnson.
Betsi Cadwaladr University Health Board: Diane Williamson (PI), Richard Williams, Ewa Turczanska, Alison Devine, Angela Steen, Val Loftus, and Corrina Marsden.
Brighton and Sussex University Hospitals NHS Trust: Paul Farrant (PI), Mary Flowerdew, Wendy Harman, Lindsay Atkinson, Jessie Felton and Claudia deGiovanni.
Cardiff & Vale University Health Board: John Ingram (PI), Girish Patel, Mabs Chowdhury, Richard Motley, Anne Thomas, Colin Long, Anew Morris, Vincent Piguet, Manju Kalavala and Ru Katugampla.
Chesterfield Royal Hospital NHS Foundation Trust: Francisca Ezughah (PI), Graham Colver, Amanda Whileman and Amanda Gascoigne.
City Hospitals Sunderland NHS Foundation Trust: Catherine Blasdale (PI), Stephanie Lateo, Neil Rajan, Anne Thomson and Sivakumar Natarajan.
County Durham and Darlington NHS Foundation Trust: Shyamal Wahie (PI), Therese Sripathy, Maneesha Vatve, Vrinda Bajaj, Anne Thomson, Keith Freeman and Mary Carr.
Derby Hospitals NHS Foundation Trust: Adam Ferguson (PI) and Katherine Riches.
East Kent Hospitals University NHS Foundation Trust: Susannah Baron (PI), Claire Fuller, Anthea Potter, Laura Brockway, Emilia Duarte-Williamson, Ashley Cooper and Susan Thompson.
Guys’ and St Thomas’ NHS Foundation Trust: Catherine Smith (PI), Gemma Minifie, Naomi Hare, Kate Thornberry, Shika Gupta and Sinead Langan.
Harrogate and District NHS Foundation Trust: Alison Layton (PI), Angela Wray, Benjamin Walker, Gayle Law and Elizabeth Marshall.
Hull & East Yorkshire Hospitals NHS Trust: Shernaz Walton (PI), Katherine Ashton, Angela Oswald, Deborah Graham, Peter Jones and Vanessa Smith.
Hywel Dda Health Board: Debbie Shipley (PI), Claire Duggan, Sarah Jones, Carol Thomas, Sally-Ann Rolls and Emma Veysey.
Newcastle Upon Tyne Hospitals NHS Foundation Trust: Simon Meggitt (PI).
NHS Lanarkshire Monklands Hospital: Christopher Evans (PI), Suzanne Clements, Gayle Moreland and Margaret Nisbet.
Norfolk and Norwich University Hospitals NHS Foundation Trust: Nick Levell (PI), Kevin Lee, Pariyawan Rakvit, George Millington, Karen Banks-Dunnell, Natasha Chetty, Clive Grattan, Syed Shah and Donna Butcher.
North Cumbria University Hospitals NHS Trust: Marinela Nik (PI), Kathleen Gilbanks and Neil Cox.
Northern Devon Healthcare NHS Trust: Karen Davies (PI) and Nick Lawton.
Nottingham University Hospitals NHS Trust: John English (PI), Ruth Murphy, William Perkins, Hywel Williams, Sheelagh Littlewood, Jan Bong, Moona Malik, Jonathan Batchelor, Catriona Wootton, Sue Davies-Jones, Joanne Llewellyn, Suzanne Cheng, Maulina Sharma, Janet Angus, Sandeep Varma and Stuart Cohen.
Nottingham NHS Treatment Centre (Circle Partnership UK): John English (PI), Ruth Murphy, William Perkins, Hywel Williams, Sheelagh Littlewood, Jan Bong, Moona Malik, Jonathan Batchelor, Catriona Wootton, Sue Davies-Jones, Joanne Llewellyn, Suzanne Cheng, Maulina Sharma, Janet Angus, Sandeep Varma and Stuart Cohen.
Oxford University Hospitals NHS Trust: Graham Ogg (PI), Susan Burge, Vanessa Venning, Susan Cooper, Tess McPherson and Lisa Matter.
Raigmore Hospital, NHS Highland: James Vestey (PI), Paula Martin, Sue Ross and Charlotte Barr.
Royal Berkshire NHS Foundation Trust: Daron Seukeran (PI), Helena Malhomme (PI), Jennie King, Janet Dua and Karen Wilmott.
Royal Devon & Exeter NHS Foundation Trust: Christopher Bower (PI) and Robert James.
South London Healthcare NHS Trust: Anna Chapman (PI), Natalie Miller, Yana Estfan and Gwendoline Reeves.
Taunton and Somerset NHS Foundation Trust: Rachel Wachsmuth (PI) and Victoria Lewis.
Sandwell and West Birmingham Hospitals NHS Trust: Shireen Velangi (PI), Weronika Szczecinska and Tinomuda Shumba.
Sherwood Forest Hospitals NHS Foundation Trust: Jane Ravenscroft (PI), John English, Jan Bong, Azaharry Yaakub and Hong Trinh.
South Devon Healthcare NHS Foundation Trust: Alison Clegg (PI), Jill Adams, Sarah Burns and Tessa Frost.
The Royal Liverpool and Broadgreen University Hospitals NHS Trust: Hazel Bell (PI), Richard Azurdia, Maeve Walsh, Caroline Angit, Kok Ngan, Anea Young, Julie Murgaza, Paula Taylor and Hamish Hunter.
University Hospitals Birmingham NHS Foundation Trust: Agustin Martin-Clavijo (PI), Renuga Raghavenan, Lucy Evriviades and Helen Lewis.
University Hospitals Bristol NHS Foundation Trust: Giles Dunnill (PI), Adam Bray and David De Berker.
University Hospitals of Leicester NHS Trust: Graham Johnston (PI), John McKenna, Catherine Shelley, Mohammad Ghazavi and Alison Hill.
Weston Area Health NHS Trust: Maggie Kirkup (PI), Glenn Saunders, Hugh Lloyd-Jones, Dawn Simmons and Donna Cotterill.
Whipps Cross University Hospital NHS Trust: Anthony Bewley (PI), Michael Galivo, Jane Watts, Karen Gibbon and Anshoo Sahota.
York Teaching Hospital NHS Foundation Trust: Calum Lyon (PI), Jill Green and Julia Stainforth.
Contributions of authors
Kim S Thomas (Professor of Applied Dermatology Research) substantially contributed to the design, methodology, data collection and analysis for all of the work programmes. She was the Programme Manager and a member of the Executive Group and Steering Group. She contributed to the writing, revision and approval of the final report.
Jonathan M Batchelor (Consultant Dermatologist) substantially contributed to the design, methodology, data collection and analysis of the vitiligo work programme and eczema PSP. He was a member of the Steering Group and contributed to the writing, revision and approval of the final report.
Fiona Bath-Hextall (Associate Professor) substantially contributed to the design, methodology, data collection and analysis of the SCC work programme. She was a member of the Steering Group and contributed to the writing, revision and approval of the final report.
Joanne R Chalmers (Research Fellow) substantially contributed to the design, methodology, data collection and analysis of the eczema prevention and eczema treatment work programmes. She was a member of the Steering Group and contributed to the writing, revision and approval of the final report.
Tessa Clarke (Senior Trials Development Manager) substantially contributed to the design, methodology, data collection and analysis of the eczema treatment and vitiligo work programmes. She contributed to the writing, revision and approval of the final report.
Sally Crowe (James Lind Alliance facilitator) substantially contributed to the design, methodology, data collection and analysis of the PSPs. She was a member of the Steering Group and contributed to the revision and approval of the final report.
Finola M Delamere (Managing Editor of the Cochrane Skin Group) substantially contributed to the design, methodology and analysis of the eczema treatments work package. She was a member of the Steering Group and contributed to the revision and approval of the final report.
Viktoria Eleftheriadou (Research Associate) substantially contributed to the design, methodology, data collection and analysis of the vitiligo work programme. She was a member of the Steering Group and contributed to the writing, revision and approval of the final report.
Nicholas Evans (Director of Business Development) substantially contributed to oversight of all of the work programmes. He was a member of the Executive Group and Steering Group and contributed to the revision and approval of the final report.
Lester Firkins (Chairperson of the James Lind Alliance) substantially contributed to oversight of all of the work programmes and was the chairperson of the Executive Group and a member of the Steering Group. He contributed to the revision and approval of the final report.
Nicola Greenlaw (Medical Statistician) substantially contributed to the design, methodology, and analysis of the PG work programme. She was a member of the Steering Group and contributed to the writing, revision and approval of the final report.
Louise Lansbury (Research Associate) substantially contributed to the design, methodology, data collection and analysis of the SCC work programme. She was a member of the Steering Group and contributed to the writing, revision and approval of the final report.
Sandra Lawton (Nurse Consultant) substantially contributed to the design, methodology, data collection and analysis of the eczema treatment work programme. She was a member of the Steering Group and contributed to the revision and approval of the final report.
Carron Layfield (UK Dermatology Clinical Trials Manager) substantially contributed to leading the patient and public involvement aspects of the work. She was a member of the Steering Group and contributed to the writing, revision and approval of the final report.
Jo Leonardi-Bee (Associate Professor in Medical Statistics) substantially contributed to the design, methodology, data collection and analysis of the SCC work programme. She was a member of the Steering Group and contributed to the writing, revision and approval of the final report.
James Mason (Professor of Health Economics) substantially contributed to the design, methodology, data collection and analysis of the PG work programme. He was a member of the Steering Group and contributed to the writing, revision and approval of the final report.
Eleanor Mitchell (Senior Trial Manager) substantially contributed to the design, methodology and data collection for the PG work programme. She was a member of the Steering Group and contributed to the writing, revision and approval of the final report.
Helen Nankervis (Research Associate) substantially contributed to the design, methodology, data collection and analysis of the eczema treatment work programme. She was a member of the Steering Group and contributed to the writing, revision and approval of the final report.
John Norrie (Professor of Medical Statistics) substantially contributed to the design, methodology, data collection and analysis of the PG work programme. He was a member of the Steering Group and contributed to the revision and approval of the final report.
Andrew Nunn (Professor of Medical Statistics) substantially contributed to oversight of all of the work programmes. He was a member of the Executive Group and Steering Group and contributed to the revision and approval of the final report.
Anthony D Ormerod (Consultant Dermatologist and Professor of Dermatology) substantially contributed to the design, methodology, data collection and analysis of the PG work programme. He was a member of the Steering Group and Lead Clinician for the STOP GAP trial. He also contributed to the writing, revision and approval of the final report.
Ramesh Patel (GP and Commissioner) contributed to oversight of all of the work programmes, was a member of the Steering Group and contributed to the revision and approval of the final report.
William Perkins (Consultant Dermatologist) substantially contributed to the design, methodology, data collection and analysis of the SCC work programme. He was a member of the Steering Group and contributed to the revision and approval of the final report.
Jane C Ravenscroft (Consultant Dermatologist) substantially contributed to oversight of all of the work programmes and substantially contributed to the design, methodology, data collection and analysis of the vitiligo work programme. She was a member of the Executive Group and Steering Group and contributed to the revision and approval of the final report.
Jochen Schmitt (Consultant Dermatologist and Health Services Researcher) substantially contributed to the design, methodology, data collection and analysis of the HOME project, and contributed to the revision and approval of the final report.
Eric Simpson (Consultant Dermatologist and Associate Professor) substantially contributed to the design, methodology and data collection for the eczema prevention work programme and some aspects of the eczema treatment work programme. He contributed to the writing, revision and approval of the final report.
Maxine E Whitton (Patient Researcher) substantially contributed to the design, methodology, data collection and analysis of the vitiligo work programme. She was the patient lead for the vitiligo work programme, a member of the Steering Group and contributed to the writing, revision and approval of the final report.
Hywel C Williams (Professor of Dermato-epidemiology and Consultant Dermatologist) substantially contributed to the design, methodology, data collection and analysis for all of the work programmes. He was the Chief Investigator for the programme, a member of the Executive Group and Steering Group and contributed to the writing, revision and approval of the final report.
Editorial support
Natasha Rogers (Post-Doctoral Research Associate/Authors’ Editor) worked with the writing team, contributed to the writing and revision of the content, and compiled the final report. Editorial assistance also provided by Becky Fox-Spencer (Independent Medical Writer).
Publications
Eczema prevention
Simpson EL, Chalmers JR, Hanifin JM, Thomas KS, Cork MJ, McLean WH, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol 2014;134:818–23.
Chalmers JR, Williams HC. Introducing the BEEP Study (Barrier Enhancement for Eczema Prevention). Exchange. London: National Eczema Society; December 2009: number 135.
Foisy M, Boyle RJ, Chalmers JR, Simpson EL, Williams HC. Overview of Reviews The prevention of eczema in infants and children: an overview of Cochrane and non-Cochrane reviews. Evid Based Child Health 2011;6:1322–39.
Lawton S. Understanding skin care and skin barrier function in infants. Nurs Child Young People 2013;25:28–33.
Simpson EL, Keck LE, Chalmers JR, Williams HC. How should an incident case of atopic dermatitis be defined? A systematic review of primary prevention studies. J Allergy Clin Immunol 2012;130:137–44.
Williams HC, Chalmers JR, Simpson EL. Prevention of atopic dermatitis. F1000 Med Rep 2012;4:24.
Eczema treatment
Batchelor J. Unanswered Questions in the Treatment of Eczema – A Step Forward. Press release for BAD Annual Conference, 3–5 July 2012.
Batchelor JM, Ridd MJ, Clarke T, Ahmed A, Cox M, Crowe S, et al. The eczema PSP: a collaboration between patients, carers, clinicians and researchers to identify and prioritize important research questions for the treatment of eczema. Br J Dermatol 2013;168:577–82.
Boyle RJ, Chalmers JR. Probiotics and Prebiotics for the Treatment and Prevention of Eczema. Exchange. London: National Eczema Society; September 2011; edition 141.
Calderon MA, Boyle RJ, Nankervis H, García Núñez I, Williams HC, Durham S. Specific allergen immunotherapy for the treatment of atopic eczema (Protocol). Cochrane Database Syst Rev 2010;10:CD008774.
Chalmers JR, Schmitt J, Apfelbacher C, Dohil M, Eichenfield LF, Simpson EL, et al. Report from the third international consensus meeting to harmonise core outcome measures for atopic eczema/dermatitis clinical trials (HOME). Br J Dermatol 2014;171:1318–25.
Futamura M, Thomas KS, Grindlay DJ, Doney EJ, Torley D, Williams HC. Mapping systematic reviews on atopic eczema – an essential resource for dermatology professionals and researchers. PLOS ONE 2013;8:e58484.
Lushey C. Setting Priorities and Reducing Uncertainties for People with Skin Disease (SPRUSD). Dispatches. London: The Vitiligo Society; March 2009: number 50.
Nankervis H, Baibergenova A, Williams HC, Thomas KS. Prospective registration and outcome-reporting bias in randomized controlled trials of eczema treatments: a systematic review. J Invest Dermatol 2012;132:2727–34.
Nankervis H, Maplethorpe A, Williams HC. Mapping randomized controlled trials of treatments for eczema – the GREAT database (the Global Resource of EczemA Trials: a collection of key data on randomized controlled trials of treatments for eczema from 2000 to 2010). BMC Dermatol 2011;11:10.
Nankervis H, Thomas KS, Delamere FM, Barbarot S, Rogers NK, Williams HC. Scoping systematic review of treatments for eczema. Programme Grants Appl Res 2016;4(7).
Nankervis H, Thomas KS, Delamere FM, Barbarot S, Smith S, Rogers NK, Williams HC. What is the evidence-base for atopic eczema treatments? A summary of published randomised controlled trials [published online ahead of print August 22 2016]. Br J Dermatol 2016.
Nankervis H, Smith EV, Boyle RJ, Rushton L, Williams HC, Hewson DM, Platts-Mills T. House dust mite reduction and avoidance measures for treating eczema. Cochrane Database Syst Rev 2010;3:CD008426.
Nankervis H, Devine A, Williams HC, Ingram JR, Doney E, Delamere F, et al. Validation of the global resource of eczema trials (GREAT database). BMC Dermatol 2015;15:4.
Nankervis H. Systematic Review of Eczema Treatments. Exchange. London: National Eczema Society; August 2013.
Schmitt J, Langan S, Deckert S, Svensson A, von Kobyletzki L, Thomas K, et al. Assessment of clinical signs of atopic dermatitis: A systematic review and recommendation. J Allergy Clin Immunol 2013;132:1337–47.
Schmitt J, Spuls P, Boers M, Thomas K, Chalmers J, Roekevisch E, et al. Towards global consensus on outcome measures for atopic eczema research: results of the HOME II meeting. Allergy 2012;67:1111–17.
Schmitt J, Williams H, HOME Development Group. Harmonising Outcome Measures for Eczema (HOME). Report from the First International Consensus Meeting (HOME 1), Munich, Germany; 24 July 2010. Br J Dermatol 2010;163:1166–8.
Schmitt J, Spuls PI, Thomas KS, Simpson E, Furue M, Deckert S, et al. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol 2014;134:800–7.
Schmitt J, Apfelbacher C, Spuls PI, Thomas KS, Simpson EL, Furue M, et al. The Harmonizing Outcome Measures for Eczema (HOME) roadmap: a methodological framework to develop core sets of outcome measurements in dermatology. J Invest Dermatol 2015;135:24–30.
Simpson E. Are epidermal defects the key initiating factors in the development of atopic dermatitis? Br J Dermatol 2010;163:1147–8.
Harrison E, Haines R, Cowdell F, Sach T, Dean T, Pollock I, et al. A multi-centre, parallel group superiority trial of silk therapeutic clothing compared to standard care for the management of eczema in children (CLOTHES Trial): study protocol for a randomised controlled trial. Trials 2015;16:390.
Thomas KS. What Questions Do You Have About the Treatment of Eczema? Time to Have Your Say. Exchange. London: National Eczema Society; December 2010; number 138.
Thomas KS, Williams HC. A New Programme of Independent Eczema Research. Exchange. London: National Eczema Society; March 2009; number 132.
Thomas KS. EASI does it: a comparison of four eczema severity scales. Br J Dermatol 2015;173:316–17.
Vitiligo
Eleftheriadou V, Thomas K, van Geel N, Hamzavi I, Lim H, Suzuki T, et al. Developing core outcome set for vitiligo clinical trials: international e-Delphi consensus. Pigment Cell Melanoma Res 2015;28:363–9.
Tour SK, Thomas KS, Walker D, Leighton P, Yong ASW, Batchelor JM. Survey and online discussion groups to develop a patient-rated outcome measure on acceptability of treatment response in vitiligo. BMC Dermatol 2014;14:10.
Eleftheriadou V, Thomas K, Ravenscroft J, Whitton M, Batchelor J, Williams H. Feasibility, double-blind, randomised, placebo-controlled, multi-centre trial of hand-held NB-UVB phototherapy for the treatment of vitiligo at home (HI-Light trial: Home Intervention of Light therapy). Trials 2014;15:51.
Crowe S. Research Priorities for Vitiligo - an Opportunity in 2009. How Can You be Involved? Dispatches. London: The Vitiligo Society; November 2008: number 49.
Eleftheriadou V. Future horizons in vitiligo research: focusing on the recommendations of the Cochrane systematic review ‘Interventions for vitiligo’ 2010. Br J Dermatol 2013;169(Suppl. 3):67–70.
Eleftheriadou V, Thomas KS, Batchelor JM, Ravenscroft JC, Whitton ME. Core outcomes in vitiligo trials: progress and challenges. Clin Invest 2013;3:417–19.
Eleftheriadou V, Thomas KS, Whitton ME, Batchelor JM, Ravenscroft JC. Which outcomes should we measure in vitiligo? Results of a systematic review and a survey among patients and clinicians on outcomes in vitiligo trials. Br J Dermatol 2012;167:804–14.
Eleftheriadou V, Whitton ME, Gawkrodger DJ, Batchelor J, Corne J, Lamb B, et al. Future research into the treatment of vitiligo: where should our priorities lie? Results of the vitiligo PSP. Br J Dermatol 2011;164:530–6.
Eleftheriadou V, Whitton M. Top Ten Uncertainties for the Treatment of Vitiligo. Dispatches. London: The Vitiligo Society; March 2011: number 56.
Eleftheriadou V, Whitton ME. Vitiligo Project: The TOP 10 Uncertainties for the Treatment of Vitiligo Defined. Dispatches. London: The Vitiligo Society; July 2010.
Eleftheriadou V. Have Your Say in Research into Vitiligo (Advert only). Bulletin of Primary Care Dermatology Society. London: The Vitiligo Society; 2009:9.
Eleftheriadou V, Thomas K. Have your say in research in to vitiligo. Dermatol Nurs 2009;8:56–7.
Eleftheriadou V. SPRUSD UPDATE: A Big Thank You to all Those Who Completed the Survey. Dispatches. London: The Vitiligo Society; November 2009: number 52.
Gawkrodger DJ, Ormerod AD, Shaw L, Mauri-Sole I, Whitton ME, Watts MJ, et al. Vitiligo: concise evidence based guidelines on diagnosis and management. Postgrad Med J 2010;86:466–71.
González U, Whitton M, Eleftheriadou V, Pinart M, Batchelor J, Leonardi-Bee J. Guidelines for designing and reporting clinical trials in vitiligo. Arch Dermatol 2011;147:1428–36.
Teovska Mitrevska N, Eleftheriadou V, Guarneri F. Quality of life in vitiligo patients. Dermatol Ther 2012;25(Suppl. 1):28–31.
Taieb A, Alomar A, Böhm M, Dell'anna ML, De Pase A, Eleftheriadou V, et al. Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol 2013;168:5–19.
Whitton ME, Pinart M, Batchelor J, Lushey C, Leonardi-Bee J, González U. Interventions for vitiligo. Cochrane Database Syst Rev 2010;1:CD003263.
Whitton M. The Cochrane Systematic Review of Interventions for Vitiligo: Why it is Important. Dispatches. London: The Vitiligo Society; November 2009: number 52.
Squamous cell carcinoma
Lansbury L, Leonardi-Bee J, Perkins W, Goodacre T, Tweed JA, Bath-Hextall FJ. Interventions for non-metastatic squamous cell carcinoma of the skin. Cochrane Database Syst Rev 2010;4:CD007869.
Lansbury L, Leonardi-Bee J, Perkins W, Goodacre T, Tweed JA. Interventions for non-metastatic squamous cell carcinoma of the skin (Protocol). Cochrane Database Syst Rev 2009;3:CD007869.
Lansbury L, Bath-Hextall F, Perkins W, Stanton W, Leonardi-Bee J. Interventions for non-metastatic squamous cell carcinoma of the skin: systematic review and pooled analysis of observational studies. BMJ 2013;347:f6153.
Lansbury L, Leonardi-Bee J, Perkins W, Bath-Hextall F. Interventions for Non-metastatic Squamous Cell Carcinoma of the Skin: A Systematic Review and Pooled Analysis of Observational Studies. Review for Scottish Intercollegiate Guidelines Network (SIGN) meeting. Edinburgh; December 2012.
Pyoderma gangrenosum
Craig FF, Thomas KS, Mitchell EJ, Williams HC, Norrie J, Mason JM, et al. UK Dermatology Clinical Trials Network's STOP GAP trial (a multicentre trial of prednisolone versus ciclosporin for pyoderma gangrenosum): protocol for a randomised controlled trial. Trials 2012;13:51.
Mitchell E. RCT of treatments for pyoderma gangrenosum: time to get involved. Wounds UK 2010;6:27–32.
Mitchell E. First Major Scientific Study into Rare Inflammatory Skin Condition. Press release, July 2012.
Mitchell E. Do you see patients with pyoderma gangrenosum? Leg Ulcer Forum Journal 2010;24:46–8.
Thomas KS, Ormerod AD, Craig FE, Greenlaw N, Norie J, Mitchell E, et al. Clinical outcomes and response of patients applying topical therapy for pyoderma gangrenosum: a prospective cohort study. J Am Acad Dermatol 2016;75:940–9.
Ormerod AD, Thomas KS, Craig FE, Mitchell E, Greenlaw N, Norrie J, et al. Comparison of the two most commonly used treatments for pyoderma gangrenosum: results of the STOP GAP randomised controlled trial. BMJ 2015;350:h2958.
Wilkes S, Williams HC, Ormerod AD, Craig FE, Greenlaw N, Norrie J, et al. Is speed of healing a good predictor of eventual healing of pyoderma gangrenosum? J Am Acad Dermatol 2016;75:1216–20.
Other
Editorial in the National Eczemas Society Members’ Magazine. Exchange. London: National Eczema Society; June 2011: edition 140 (4).
Layfield C, Clarke T, Thomas KS, Williams HC. Developing a network in a neglected area of clinical research: the UK Dermatology Clinical Trials Network. Clinical Investigation 2011;1:943-9.
Ridd M, Thomas KS, Wallace P, O’Sullivan F. Dermatology research in primary care: why, what and how? Br J Gen Pract 2011;61:89–90.
Viles J. BAD Guidelines Published. Dispatches. London: The Vitiligo Society; March 2009: number 50.
Data sharing statement
We support initiatives to encourage data sharing. Requests for sharing of data can be directed to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health.
References
- NIHR Evaluation, Trials and Studies Coordinating Centre . A Randomised Controlled Trial to Determine Whether Skin Barrier Enhancement With Emollients Can Prevent Eczema in High Risk Children n.d. www.nets.nihr.ac.uk/projects/hta/126712 (accessed 4 October 2016).
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the WAO, October 2003. J Allergy Clin Immunol 2004;113:832-6. http://dx.doi.org/10.1016/j.jaci.2003.12.591.
- Malik G, Tagiyeva N, Aucott L, McNeill G, Turner SW. Changing trends in asthma in 9–12 year olds between 1964 and 2009. Arch Dis Child 2011;96:227-31. http://dx.doi.org/10.1136/adc.2010.189175.
- Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI. ISAAC Phase Three Study Group . Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol 2009;124:1251-8.e23. http://dx.doi.org/10.1016/j.jaci.2009.10.009.
- Williams H, Stewart A, von Mutius E, Cookson W, Anderson HR. International Study of Asthma and Allergies in Childhood (ISAAC) Phase One and Three Study Groups . Is eczema really on the increase worldwide?. J Allergy Clin Immunol 2008;121:947-54.e15. http://dx.doi.org/10.1016/j.jaci.2007.11.004.
- Williams HC, Wuthrich B, Williams HC. Atopic Dermatits. Cambridge: Cambridge University Press; 2000.
- Williams HC, Burney PG, Pembroke AC, Hay RJ. The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. III. Independent hospital validation. Br J Dermatol 1994;131:406-16. http://dx.doi.org/10.1111/j.1365-2133.1994.tb08532.x.
- Tang TS, Bieber T, Williams HC. Does ‘autoreactivity’ play a role in atopic dermatitis?. J Allergy Clin Immunol 2012;129:1209-15.e2. http://dx.doi.org/10.1016/j.jaci.2012.02.002.
- Schmitt J, Buske-Kirschbaum A, Roessner V. Is atopic disease a risk factor for attention-deficit/hyperactivity disorder? A systematic review. Allergy 2010;65:1506-24. http://dx.doi.org/10.1111/j.1398-9995.2010.02449.x.
- Kemp AS. Cost of illness of atopic dermatitis in children: a societal perspective. Pharmaco Economics 2003;21:105-13. http://dx.doi.org/10.2165/00019053-200321020-00003.
- Hay RJ, Johns NE, Williams HC, Bolliger IW, Dellavalle RP, Margolis DJ, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol 2014;134:1527-34. http://dx.doi.org/10.1038/jid.2013.446.
- Jenner N, Campbell J, Marks R. Morbidity and cost of atopic eczema in Australia. Australas J Dermatol 2004;45:16-22. http://dx.doi.org/10.1111/j.1440-0960.2004.00046.x.
- Verboom P, Hakkaart-Van L, Sturkenboom M, De Zeeuw R, Menke H, Rutten F. The cost of atopic dermatitis in the Netherlands: an international comparison. Br J Dermatol 2002;147:716-24. http://dx.doi.org/10.1046/j.1365-2133.2002.04964.x.
- Mancini AJ, Kaulback K, Chamlin SL. The socioeconomic impact of atopic dermatitis in the United States: a systematic review. Pediatr Dermatol 2008;25:1-6. http://dx.doi.org/10.1111/j.1525-1470.2007.00572.x.
- Kerr OA, Tidman MJ, Walker JJ, Aldridge RD, Benton EC. The profile of dermatological problems in primary care. Clin Exp Dermatol 2010;35:380-3. http://dx.doi.org/10.1111/j.1365-2230.2009.03586.x.
- NICE . NICE Guideline: Management Of Atopic Eczema In Children From Birth Up To The Age Of 12 Years 2007. www.nice.org.uk/guidance/cg57 (accessed 25 July 2016).
- Gupta R, Sheikh A, Strachan DP, Anderson HR. Burden of allergic disease in the UK: secondary analyses of national databases. Clin Exp Allergy 2004;34:520-6. http://dx.doi.org/10.1111/j.1365-2222.2004.1935.x.
- Punekar YS, Sheikh A. Establishing the sequential progression of multiple allergic diagnoses in a UK birth cohort using the General Practice Research Database. Clin Exp Allergy 2009;39:1889-95. http://dx.doi.org/10.1111/j.1365-2222.2009.03366.x.
- Zheng T, Yu J, Oh MH, Zhu Z. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol Res 2011;3:67-73. http://dx.doi.org/10.4168/aair.2011.3.2.67.
- Bergmann MM, Caubet JC, Boguniewicz M, Eigenmann PA. Evaluation of food allergy in patients with atopic dermatitis. J Allergy Clin Immunol Pract 2013;1:22-8. http://dx.doi.org/10.1016/j.jaip.2012.11.005.
- van der Hulst AE, Klip H, Brand PL. Risk of developing asthma in young children with atopic eczema: a systematic review. J Allergy Clin Immunol 2007;120:565-9. http://dx.doi.org/10.1016/j.jaci.2007.05.042.
- von Kobyletzki LB, Bornehag CG, Hasselgren M, Larsson M, Lindstrom CB, Svensson A. Eczema in early childhood is strongly associated with the development of asthma and rhinitis in a prospective cohort. BMC Dermatol 2012;12. http://dx.doi.org/10.1186/1471-5945-12-11.
- Austin JB, Kaur B, Anderson HR, Burr M, Harkins LS, Strachan DP, et al. Hay fever, eczema, and wheeze: a nationwide UK study (ISAAC, international study of asthma and allergies in childhood). Arch Dis Child 1999;81:225-30. http://dx.doi.org/10.1136/adc.81.3.225.
- Williams HC, Strachan DP, Hay RJ. Childhood eczema: disease of the advantaged?. BMJ 1994;308:1132-5. http://dx.doi.org/10.1136/bmj.308.6937.1132.
- Simpson CR, Newton J, Hippisley-Cox J, Sheikh A. Trends in the epidemiology and prescribing of medication for eczema in England. J R Soc Med 2009;102:108-17. http://dx.doi.org/10.1258/jrsm.2009.080211.
- Flohr C, Pascoe D, Williams HC. Atopic dermatitis and the ‘hygiene hypothesis’: too clean to be true?. Br J Dermatol 2005;152:202-16. http://dx.doi.org/10.1111/j.1365-2133.2004.06436.x.
- Flohr C, Yeo L. Atopic dermatitis and the hygiene hypothesis revisited. Curr Probl Dermatol 2011;41:1-34. http://dx.doi.org/10.1159/000323290.
- Bowatte G, Lodge C, Lowe AJ, Erbas B, Perret J, Abramson MJ, et al. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta-analysis of birth cohort studies. Allergy 2015;70:245-56. http://dx.doi.org/10.1111/all.12561.
- Campbell BE, Lodge CJ, Lowe AJ, Burgess JA, Matheson MC, Dharmage SC. Exposure to ‘farming’ and objective markers of atopy: a systematic review and meta-analysis. Clin Exp Allergy 2015;45:744-57. http://dx.doi.org/10.1111/cea.12429.
- Pelucchi C, Galeone C, Bach JF, La Vecchia C, Chatenoud L. Pet exposure and risk of atopic dermatitis at the pediatric age: a meta-analysis of birth cohort studies. J Allergy Clin Immunol 2013;132:616-22.e7. http://dx.doi.org/10.1016/j.jaci.2013.04.009.
- Mendell MJ, Mirer AG, Cheung K, Tong M, Douwes J. Respiratory and allergic health effects of dampness, mold, and dampness-related agents: a review of the epidemiologic evidence. Environ Health Perspect 2011;119:748-56. http://dx.doi.org/10.1289/ehp.1002410.
- Ellinghaus D, Baurecht H, Esparza-Gordillo J, Rodríguez E, Matanovic A, Marenholz I, et al. High-density genotyping study identifies four new susceptibility loci for atopic dermatitis. Nat Genet 2013;45:808-12. http://dx.doi.org/10.1038/ng.2642.
- Brown SJ, McLean WH. Eczema genetics: current state of knowledge and future goals. J Invest Dermatol 2009;129:543-52. http://dx.doi.org/10.1038/jid.2008.413.
- Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet 2006;38:441-6. http://dx.doi.org/10.1038/ng1767.
- Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med 2011;365:1315-27. http://dx.doi.org/10.1056/NEJMra1011040.
- Rodríguez E, Baurecht H, Herberich E, Wagenpfeil S, Brown SJ, Cordell HJ, et al. Meta-analysis of filaggrin polymorphisms in eczema and asthma: robust risk factors in atopic disease. J Allergy Clin Immunol 2009;123:1361-70.e7. http://dx.doi.org/10.1016/j.jaci.2009.03.036.
- Foisy M, Boyle RJ, Chalmers JR, Simpson EL, Williams HC. Overview of reviews. The prevention of eczema in infants and children: an overview of Cochrane and non-Cochrane reviews. Evid-Based Child Health 2011;6:1322-39. http://dx.doi.org/10.1002/ebch.827.
- Chalmers I, Glasziou P. Avoidable waste in the production and reporting of research evidence. Lancet 2009;374:86-9. http://dx.doi.org/10.1016/S0140-6736(09)60329-9.
- Thygarajan A, Burks AW. American Academy of Pediatrics recommendations on the effects of early nutritional interventions on the development of atopic disease. Curr Opin Pediatr 2008;20:698-702. http://dx.doi.org/10.1097/MOP.0b013e3283154f88.
- Rosenfeldt V, Benfeldt E, Nielsen SD, Michaelsen KF, Jeppesen DL, Valerius NH, et al. Effect of probiotic Lactobacillus strains in children with atopic dermatitis. J Allergy Clin Immunol 2003;111:389-95. http://dx.doi.org/10.1067/mai.2003.389.
- Kalliomäki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol 2001;107:129-34. http://dx.doi.org/10.1067/mai.2001.111237.
- Adlerberth I, Strachan DP, Matricardi PM, Ahrné S, Orfei L, Aberg N, et al. Gut microbiota and development of atopic eczema in 3 European birth cohorts. J Allergy Clin Immunol 2007;120:343-50. http://dx.doi.org/10.1016/j.jaci.2007.05.018.
- Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, et al. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut 2007;56:661-7. http://dx.doi.org/10.1136/gut.2006.100164.
- Björkstén B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol 2001;108:516-20. http://dx.doi.org/10.1067/mai.2001.118130.
- Furuhjelm C, Warstedt K, Larsson J, Fredriksson M, Böttcher MF, Fälth-Magnusson K, et al. Fish oil supplementation in pregnancy and lactation may decrease the risk of infant allergy. Acta Paediatr 2009;98:1461-7. http://dx.doi.org/10.1111/j.1651-2227.2009.01355.x.
- Warner JA, Miles EA, Jones AC, Quint DJ, Colwell BM, Warner JO. Is deficiency of interferon gamma production by allergen triggered cord blood cells a predictor of atopic eczema?. Clin Exp Allergy 1994;24:423-30. http://dx.doi.org/10.1111/j.1365-2222.1994.tb00930.x.
- Simpson EL, Berry TM, Brown PA, Hanifin JM. A pilot study of emollient therapy for the primary prevention of atopic dermatitis. J Am Acad Dermatol 2010;63:587-93. http://dx.doi.org/10.1016/j.jaad.2009.11.011.
- Flohr C, England K, Radulovic S, McLean WH, Campbel LE, Barker J, et al. Filaggrin loss-of-function mutations are associated with early-onset eczema, eczema severity and transepidermal water loss at 3 months of age. Br J Dermatol 2010;163:1333-6. http://dx.doi.org/10.1111/j.1365-2133.2010.10068.x.
- Macharia WM, Anabwani GM, Owili DM. Effects of skin contactants on evolution of atopic dermatitis in children: a case control study. Trop Doct 1991;21:104-6.
- Flohr C, Perkin M, Logan K, Marrs T, Radulovic S, Campbell LE, et al. Atopic dermatitis and disease severity are the main risk factors for food sensitization in exclusively breastfed infants. J Invest Dermatol 2014;134:345-50. http://dx.doi.org/10.1038/jid.2013.298.
- Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007;7. http://dx.doi.org/10.1186/1471-2288-7-10.
- Johansson SG, Hourihane JO, Bousquet J, Bruijnzeel-Koomen C, Dreborg S, Haahtela T, et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy 2001;56:813-24. http://dx.doi.org/10.1034/j.1398-9995.2001.t01-1-00001.x.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. London: The Cochrane Collaboration; 2008.
- Kramer MS, Chalmers B, Hodnett ED, Sevkovskaya Z, Dzikovich I, Shapiro S, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA 2001;285:413-20. http://dx.doi.org/10.1001/jama.285.4.413.
- Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev 2002;1. http://dx.doi.org/10.1002/14651858.cd003517.
- Osborn DA, Sinn J. Formulas containing hydrolysed protein for prevention of allergy and food intolerance in infants. Cochrane Database Syst Rev 2006;4. http://dx.doi.org/10.1002/14651858.cd003664.pub2.
- Osborn DA, Sinn JK. Prebiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst Rev 2007;4. http://dx.doi.org/10.1002/14651858.cd006474.pub2.
- Osborn DA, Sinn JK. Probiotics in infants for prevention of allergic disease and food hypersensitivity. Cochrane Database Syst Rev 2007;4. http://dx.doi.org/10.1002/14651858.cd006475.pub2.
- Osborn DA, Sinn J. Soy formula for prevention of allergy and food intolerance in infants. Cochrane Database Syst Rev 2006;4. http://dx.doi.org/10.1002/14651858.cd003741.pub4.
- Anandan C, Nurmatov U, Sheikh A. Omega 3 and 6 oils for primary prevention of allergic disease: systematic review and meta-analysis. Allergy 2009;64:840-8. http://dx.doi.org/10.1111/j.1398-9995.2009.02042.x.
- Ziegler E, Vanderhoof JA, Petschow B, Mitmesser SH, Stolz SI, Harris CL, et al. Term infants fed formula supplemented with selected blends of prebiotics grow normally and have soft stools similar to those reported for breast-fed infants. J Pediatr Gastroenterol Nutr 2007;44:359-64. http://dx.doi.org/10.1097/MPG.0b013e31802fca8c.
- Boyle RJ, Ismail IH, Kivivuori S, Licciardi PV, Robins-Browne RM, Mah LJ, et al. Lactobacillus GG treatment during pregnancy for the prevention of eczema: a randomized controlled trial. Allergy 2011;66:509-16. http://dx.doi.org/10.1111/j.1398-9995.2010.02507.x.
- Dotterud CK, Storrø O, Johnsen R, Oien T. Probiotics in pregnant women to prevent allergic disease: a randomized, double-blind trial. Br J Dermatol 2010;163:616-23. http://dx.doi.org/10.1111/j.1365-2133.2010.09889.x.
- Huurre A, Laitinen K, Rautava S, Korkeamäki M, Isolauri E. Impact of maternal atopy and probiotic supplementation during pregnancy on infant sensitization: a double-blind placebo-controlled study. Clin Exp Allergy 2008;38:1342-8. http://dx.doi.org/10.1111/j.1365-2222.2008.03008.x.
- Kim JY, Kwon JH, Ahn SH, Lee SI, Han YS, Choi YO, et al. Effect of probiotic mix (Bifidobacterium bifidum, Bifidobacterium lactis, Lactobacillus acidophilus) in the primary prevention of eczema: a double-blind, randomized, placebo-controlled trial. Pediatr Allergy Immunol 2010;21:e386-93. http://dx.doi.org/10.1111/j.1399-3038.2009.00958.x.
- Kopp MV, Hennemuth I, Heinzmann A, Urbanek R. Randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: no clinical effects of Lactobacillus GG supplementation. Pediatrics 2008;121:e850-6. http://dx.doi.org/10.1542/peds.2007-1492.
- Niers L, Martín R, Rijkers G, Sengers F, Timmerman H, van Uden N, et al. The effects of selected probiotic strains on the development of eczema (the PandA study). Allergy 2009;64:1349-58. http://dx.doi.org/10.1111/j.1398-9995.2009.02021.x.
- West CE, Hammarström ML, Hernell O. Probiotics during weaning reduce the incidence of eczema. Pediatr Allergy Immunol 2009;20:430-7. http://dx.doi.org/10.1111/j.1399-3038.2009.00745.x.
- Wickens K, Black PN, Stanley TV, Mitchell E, Fitzharris P, Tannock GW, et al. A differential effect of 2 probiotics in the prevention of eczema and atopy: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol 2008;122:788-94. http://dx.doi.org/10.1016/j.jaci.2008.07.011.
- Soh SE, Aw M, Gerez I, Chong YS, Rauff M, Ng YP, et al. Probiotic supplementation in the first 6 months of life in at risk Asian infants – effects on eczema and atopic sensitization at the age of 1 year. Clin Exp Allergy 2009;39:571-8. http://dx.doi.org/10.1111/j.1365-2222.2008.03133.x.
- Tang ML, Lahtinen SJ, Boyle RJ. Probiotics and prebiotics: clinical effects in allergic disease. Curr Opin Pediatr 2010;22:626-34. http://dx.doi.org/10.1097/mop.0b013e32833d9728.
- Foolad N, Brezinski EA, Chase EP, Armstrong AW. Effect of nutrient supplementation on atopic dermatitis in children: a systematic review of probiotics, prebiotics, formula, and fatty acids. JAMA Dermatol 2013;149:350-5. http://dx.doi.org/10.1001/jamadermatol.2013.1495.
- Yang YW, Tsai CL, Lu CY. Exclusive breastfeeding and incident atopic dermatitis in childhood: a systematic review and meta-analysis of prospective cohort studies. Br J Dermatol 2009;161:373-83. http://dx.doi.org/10.1111/j.1365-2133.2009.09049.x.
- Langan SM, Flohr C, Williams HC. The role of furry pets in eczema: a systematic review. Arch Dermatol 2007;143:1570-7. http://dx.doi.org/10.1001/archderm.143.12.1570.
- Muche-Borowski C, Kopp M, Reese I, Sitter H, Werfel T, Schäfer T. Allergy prevention. Dtsch Arztebl Int 2009;106:625-31.
- Lowe AJ, Hosking CS, Bennett CM, Allen KJ, Axelrad C, Carlin JB, et al. Effect of a partially hydrolyzed whey infant formula at weaning on risk of allergic disease in high-risk children: a randomized controlled trial. J Allergy Clin Immunol 2011;128:360-5.e4. http://dx.doi.org/10.1016/j.jaci.2010.05.006.
- Simpson EL, Keck LE, Chalmers JR, Williams HC. How should an incident case of atopic dermatitis be defined? A systematic review of primary prevention studies. J Allergy Clin Immunol 2012;130:137-44. http://dx.doi.org/10.1016/j.jaci.2012.01.075.
- Chamlin SL, Kaulback K, Mancini AJ. What is “high risk?” a systematic review of atopy risk and implications for primary prevention. Pediatr Dermatol 2009;26:247-56. http://dx.doi.org/10.1111/j.1525-1470.2008.00807.x.
- Williams HC. Prevention of atopic eczema: a dream not so far away?. Arch Dermatol 2002;138:391-2. http://dx.doi.org/10.1001/archderm.138.3.391.
- Williams HC. Two “positive” studies of probiotics for atopic dermatitis: or are they?. Arch Dermatol 2006;142:1201-3. http://dx.doi.org/10.1001/archderm.142.9.1201.
- Rystedt I. Prognostic factors in atopic dermatitis. Acta Derm Venereol 1985;65:206-13.
- Shams K, Grindlay DJ, Williams HC. What’s new in atopic eczema? An analysis of systematic reviews published in 2009–10. Clin Exp Dermatol 2011;36:573-7. http://dx.doi.org/10.1111/j.1365-2230.2011.04078.x.
- Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol 1980;60:44-7.
- Arslanoglu S, Moro GE, Schmitt J, Tandoi L, Rizzardi S, Boehm G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J Nutr 2008;138:1091-5.
- Laitinen K, Kalliomäki M, Poussa T, Lagström H, Isolauri E. Evaluation of diet and growth in children with and without atopic eczema: follow-up study from birth to 4 years. Br J Nutr 2005;94:565-74. http://dx.doi.org/10.1079/BJN20051503.
- Bruno G, Milita O, Ferrara M, Nisini R, Cantani A, Businco L. Prevention of atopic diseases in high risk babies (long-term follow-up). Allergy Proc 1993;14:181-6. http://dx.doi.org/10.2500/108854193778878682.
- Chandra RK. Five-year follow-up of high-risk infants with family history of allergy who were exclusively breast-fed or fed partial whey hydrolysate, soy, and conventional cow’s milk formulas. J Pediatr Gastroenterol Nutr 1997;24:380-8. http://dx.doi.org/10.1097/00005176-199704000-00005.
- Chirico G, Gasparoni A, Ciardelli L, De Amici M, Colombo A, Rondini G. Immunogenicity and antigenicity of a partially hydrolyzed cow’s milk infant formula. Allergy 1997;52:82-8. http://dx.doi.org/10.1111/j.1398-9995.1997.tb02549.x.
- de Jong MH, Scharp-van der Linden VT, Aalberse RC, Oosting J, Tijssen JG, de Groot CJ. Randomised controlled trial of brief neonatal exposure to cows’ milk on the development of atopy. Arch Dis Child 1998;79:126-30. http://dx.doi.org/10.1136/adc.79.2.126.
- de Seta L, Siani P, Cirillo G, Di Gruttola M, Cimaduomo L, Coletta S. The prevention of allergic diseases with a hypoallergenic formula: a follow-up at 24 months. The preliminary results. Pediatr Med Chir 1994;16:251-4.
- Denburg JA, Hatfield HM, Cyr MM, Hayes L, Holt PG, Sehmi R, et al. Fish oil supplementation in pregnancy modifies neonatal progenitors at birth in infants at risk of atopy. Pediatr Res 2005;57:276-81. http://dx.doi.org/10.1203/01.PDR.0000148279.72611.1D.
- Dunstan JA, Mori TA, Barden A, Beilin LJ, Taylor AL, Holt PG, et al. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: a randomized, controlled trial. J Allergy Clin Immunol 2003;112:1178-84. http://dx.doi.org/10.1016/j.jaci.2003.09.009.
- Exl BM, Deland U, Secretin MC, Preysch U, Wall M, Shmerling DH. Improved general health status in an unselected infant population following an allergen-reduced dietary intervention programme: the ZUFF-STUDY-PROGRAMME. Part II: infant growth and health status to age 6 months. ZUg-FrauenFeld. Eur J Nutr 2000;39:145-56. http://dx.doi.org/10.1007/s003940070018.
- Fälth-Magnusson K, Kjellman NI. Allergy prevention by maternal elimination diet during late pregnancy – a 5-year follow-up of a randomized study. J Allergy Clin Immunol 1992;89:709-13. http://dx.doi.org/10.1016/0091-6749(92)90378-F.
- Grüber C, van Stuijvenberg M, Mosca F, Moro G, Chirico G, Braegger CP, et al. Reduced occurrence of early atopic dermatitis because of immunoactive prebiotics among low-atopy-risk infants. J Allergy Clin Immunol 2010;126:791-7. http://dx.doi.org/10.1016/j.jaci.2010.07.022.
- Hattevig G, Sigurs N, Kjellman B. Effects of maternal dietary avoidance during lactation on allergy in children at 10 years of age. Acta Paediatr 1999;88:7-12. http://dx.doi.org/10.1111/j.1651-2227.1999.tb01259.x.
- Hoppu U, Rinne M, Salo-Väänänen P, Lampi AM, Piironen V, Isolauri E. Vitamin C in breast milk may reduce the risk of atopy in the infant. Eur J Clin Nutr 2005;59:123-8. http://dx.doi.org/10.1038/sj.ejcn.1602048.
- Kajosaari M. Atopy prophylaxis in high-risk infants. Prospective 5-year follow-up study of children with six months exclusive breastfeeding and solid food elimination. Adv Exp Med Biol 1991;310:453-8. http://dx.doi.org/10.1007/978-1-4615-3838-7_58.
- Kitz R, Rose MA, Schönborn H, Zielen S, Böhles HJ. Impact of early dietary gamma-linolenic acid supplementation on atopic eczema in infancy. Pediatr Allergy Immunol 2006;17:112-17. http://dx.doi.org/10.1111/j.1399-3038.2005.00369.x.
- Matejek N, Schwamberger H, Bohles H. The influence of breast feeding on the development of atopic dermatitis. Exclusive breast feeding versus initial short term feeding of a partial hydrolysate followed by breast milk. Nutrition Research 1998;18:1389-93. http://dx.doi.org/10.1016/S0271-5317(98)00117-1.
- Moro G, Arslanoglu S, Stahl B, Jelinek J, Wahn U, Boehm G. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch Dis Child 2006;91:814-19. http://dx.doi.org/10.1136/adc.2006.098251.
- Pöysä L, Korppi M, Remes K, Juntunen-Backman K. Atopy in childhood and diet in infancy. A nine-year follow-up study. I. Clinical manifestations. Allergy Proc 1991;12:107-11. http://dx.doi.org/10.2500/108854191779011800.
- Sigurs N, Hattevig G, Kjellman B. Maternal avoidance of eggs, cow’s milk, and fish during lactation: effect on allergic manifestations, skin-prick tests, and specific IgE antibodies in children at age 4 years. Pediatrics 1992;89:735-9.
- Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol 2007;119:184-91. http://dx.doi.org/10.1016/j.jaci.2006.08.036.
- von Berg A, Koletzko S, Grübl A, Filipiak-Pittroff B, Wichmann HE, Bauer CP, et al. The effect of hydrolyzed cow’s milk formula for allergy prevention in the first year of life: the German Infant Nutritional Intervention Study, a randomized double-blind trial. J Allergy Clin Immunol 2003;111:533-40. http://dx.doi.org/10.1067/mai.2003.101.
- D’Agata A, Betta P, Sciacca P, Morano C, Praticò G, Curreri R, et al. Role of dietary prevention in newborns at risk for atopy. Results of a follow-up study. Pediatr Med Chir 1996;18:469-72.
- Almqvist C, Garden F, Xuan W, Mihrshahi S, Leeder SR, Oddy W, et al. Omega-3 and omega-6 fatty acid exposure from early life does not affect atopy and asthma at age 5 years. J Allergy Clin Immunol 2007;119:1438-44. http://dx.doi.org/10.1016/j.jaci.2007.01.046.
- Arshad SH, Matthews S, Gant C, Hide DW. Effect of allergen avoidance on development of allergic disorders in infancy. Lancet 1992;339:1493-7. http://dx.doi.org/10.1016/0140-6736(92)91260-F.
- Businco L, Cantani A, Meglio P, Bruno G. Prevention of atopy: results of a long-term (7 months to 8 years) follow-up. Ann Allergy 1987;59:183-6.
- Chan YH, Shek LP, Aw M, Quak SH, Lee BW. Use of hypoallergenic formula in the prevention of atopic disease among Asian children. J Paediatr Child Health 2002;38:84-8. http://dx.doi.org/10.1046/j.1440-1754.2002.00725.x.
- Chandra RK, Puri S, Cheema PS. Predictive value of cord blood IgE in the development of atopic disease and role of breast-feeding in its prevention. Clin Allergy 1985;15:517-22. http://dx.doi.org/10.1111/j.1365-2222.1985.tb02304.x.
- Chandra RK, Singh G, Shridhara B. Effect of feeding whey hydrolysate, soy and conventional cow milk formulas on incidence of atopic disease in high risk infants. Ann Allergy 1989;63:102-6.
- Chandra RK, Puri S, Suraiya C, Cheema PS. Influence of maternal food antigen avoidance during pregnancy and lactation on incidence of atopic eczema in infants. Clin Allergy 1986;16:563-9. http://dx.doi.org/10.1111/j.1365-2222.1986.tb01995.x.
- de Jong MH, Scharp-Van Der Linden VT, Aalberse R, Heymans HS, Brunekreef B. The effect of brief neonatal exposure to cows’ milk on atopic symptoms up to age 5. Arch Dis Child 2002;86:365-9. http://dx.doi.org/10.1136/adc.86.5.365.
- Filipiak B, Zutavern A, Koletzko S, von Berg A, Brockow I, Grübl A, et al. Solid food introduction in relation to eczema: results from a four-year prospective birth cohort study. J Pediatr 2007;151:352-8. http://dx.doi.org/10.1016/j.jpeds.2007.05.018.
- Herrmann ME, Dannemann A, Grüters A, Radisch B, Dudenhausen JW, Bergmann R, et al. Prospective study of the atopy preventive effect of maternal avoidance of milk and eggs during pregnancy and lactation. Eur J Pediatr 1996;155:770-4. http://dx.doi.org/10.1007/BF02002904.
- Kalliomäki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 2001;357:1076-9. http://dx.doi.org/10.1016/S0140-6736(00)04259-8.
- Lucas A, Brooke OG, Morley R, Cole TJ, Bamford MF. Early diet of preterm infants and development of allergic or atopic disease: randomised prospective study. BMJ 1990;300:837-40. http://dx.doi.org/10.1136/bmj.300.6728.837.
- Marks GB, Mihrshahi S, Kemp AS, Tovey ER, Webb K, Almqvist C, et al. Prevention of asthma during the first 5 years of life: a randomized controlled trial. J Allergy Clin Immunol 2006;118:53-61. http://dx.doi.org/10.1016/j.jaci.2006.04.004.
- Rautava S, Kalliomäki M, Isolauri E. Probiotics during pregnancy and breast-feeding might confer immunomodulatory protection against atopic disease in the infant. J Allergy Clin Immunol 2002;109:119-21. http://dx.doi.org/10.1067/mai.2002.120273.
- Schoetzau A, Filipiak-Pittroff B, Franke K, Koletzko S, Von Berg A, Gruebl A, et al. Effect of exclusive breast-feeding and early solid food avoidance on the incidence of atopic dermatitis in high-risk infants at 1 year of age. Pediatr Allergy Immunol 2002;13:234-42. http://dx.doi.org/10.1034/j.1399-3038.2002.01050.x.
- Szajewska H, Mrukowicz JZ, Stoińska B, Prochowska A. Extensively and partially hydrolysed preterm formulas in the prevention of allergic diseases in preterm infants: a randomized, double-blind trial. Acta Paediatr 2004;93:1159-65. http://dx.doi.org/10.1111/j.1651-2227.2004.tb02742.x.
- van den Berg A, van Zwol A, Moll HA, Fetter WP, van Elburg RM. Glutamine-enriched enteral nutrition in very low-birth-weight infants: effect on the incidence of allergic and infectious diseases in the first year of life. Arch Pediatr Adolesc Med 2007;161:1095-101. http://dx.doi.org/10.1001/archpedi.161.11.1095.
- Vandenplas Y, Hauser B, Van den Borre C, Sacre L, Dab I. Effect of a whey hydrolysate prophylaxis of atopic disease. Ann Allergy 1992;68:419-24.
- Willems R, Duchateau J, Magrez P, Denis R, Casimir G. Influence of hypoallergenic milk formula on the incidence of early allergic manifestations in infants predisposed to atopic diseases. Ann Allergy 1993;71:147-50.
- Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet 1998;351:1225-32.
- Dunder T, Tapiainen T, Pokka T, Uhari M. Infections in child day care centers and later development of asthma, allergic rhinitis, and atopic dermatitis: prospective follow-up survey 12 years after controlled randomized hygiene intervention. Arch Pediatr Adolesc Med 2007;161:972-7. http://dx.doi.org/10.1001/archpedi.161.10.972.
- Koopman LP, van Strien RT, Kerkhof M, Wijga A, Smit HA, de Jongste JC, et al. Placebo-controlled trial of house dust mite-impermeable mattress covers: effect on symptoms in early childhood. Am J Respir Crit Care Med 2002;166:307-13. http://dx.doi.org/10.1164/rccm.2106026.
- Laubereau B, Brockow I, Zirngibl A, Koletzko S, Gruebl A, von Berg A, et al. Effect of breast-feeding on the development of atopic dermatitis during the first 3 years of life – results from the GINI-birth cohort study. J Pediatr 2004;144:602-7. http://dx.doi.org/10.1016/j.jpeds.2003.12.029.
- Nilsson L, Kjellman NI, Björkstén B. A randomized controlled trial of the effect of pertussis vaccines on atopic disease. Arch Pediatr Adolesc Med 1998;152:734-8. http://dx.doi.org/10.1001/archpedi.152.8.734.
- Williams H, Burney P, Hay R, Archer CB, Shipley MJ, Hunter JJ, et al. The U.K. Working Party’s diagnostic criteria for atopic dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. Br J Dermatol 1994;131:383-96. http://dx.doi.org/10.1111/j.1365-2133.1994.tb08530.x.
- Kuitunen M, Kukkonen K, Juntunen-Backman K, Korpela R, Poussa T, Tuure T, et al. Probiotics prevent IgE-associated allergy until age 5 years in cesarean-delivered children but not in the total cohort. J Allergy Clin Immunol 2009;123:335-41. http://dx.doi.org/10.1016/j.jaci.2008.11.019.
- Kukkonen K, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, et al. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol 2007;119:192-8. http://dx.doi.org/10.1016/j.jaci.2006.09.009.
- van Gool CJ, Thijs C, Henquet CJ, van Houwelingen AC, Dagnelie PC, Schrander J, et al. Gamma-linolenic acid supplementation for prophylaxis of atopic dermatitis – a randomized controlled trial in infants at high familial risk. Am J Clin Nutr 2003;77:943-51.
- Hanifin JM, Lobitz WC. Newer concepts of atopic dermatitis. Arch Dermatol 1977;113:663-70. http://dx.doi.org/10.1001/archderm.1977.01640050123023.
- Fälth-Magnusson K, Kjellman NI. Development of atopic disease in babies whose mothers were receiving exclusion diet during pregnancy – a randomized study. J Allergy Clin Immunol 1987;80:868-75. http://dx.doi.org/10.1016/S0091-6749(87)80279-8.
- Zeiger RS, Heller S. Development of nasal basophilic cells and nasal eosinophils from age 4 months through 4 years in children of atopic parents. J Allergy Clin Immunol 1993;91:723-34. http://dx.doi.org/10.1016/0091-6749(93)90191-H.
- Shohet L, Shahar E, Davidson S. Breast feeding as prophylaxis for atopic eczema: a controlled study of 368 cases. Acta Paediatr Hung 1985;26:35-9.
- Hattevig G, Kjellman B, Sigurs N, Björkstén B, Kjellman NI. Effect of maternal avoidance of eggs, cow’s milk and fish during lactation upon allergic manifestations in infants. Clin Exp Allergy 1989;19:27-32. http://dx.doi.org/10.1111/j.1365-2222.1989.tb02339.x.
- Zeiger RS, Heller S, Mellon MH, Forsythe AB, O’Connor RD, Hamburger RN, et al. Effect of combined maternal and infant food-allergen avoidance on development of atopy in early infancy: a randomized study. J Allergy Clin Immunol 1989;84:72-89. http://dx.doi.org/10.1016/0091-6749(89)90181-4.
- Seymore J, Keswick B, Hanifin JM, Jordan P, Milligan M. Clinical effects of diaper types on skin of normal infants and infants with atopic dermatitis. J Am Acad Dermatol 1987;17:988-97. http://dx.doi.org/10.1016/S0190-9622(87)70288-6.
- Abrahamsson TR, Jakobsson T, Böttcher MF, Fredrikson M, Jenmalm MC, Björkstén B, et al. Probiotics in prevention of IgE-associated eczema: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol 2007;119:1174-80. http://dx.doi.org/10.1016/j.jaci.2007.01.007.
- Odelram H, Vanto T, Jacobsen L, Kjellman NI. Whey hydrolysate compared with cow’s milk-based formula for weaning at about 6 months of age in high allergy-risk infants: effects on atopic disease and sensitization. Allergy 1996;51:192-5. http://dx.doi.org/10.1111/j.1398-9995.1996.tb00063.x.
- Böttcher MF, Abrahamsson TR, Fredriksson M, Jakobsson T, Björkstén B. Low breast milk TGF-beta2 is induced by Lactobacillus reuteri supplementation and associates with reduced risk of sensitization during infancy. Pediatr Allergy Immunol 2008;19:497-504. http://dx.doi.org/10.1111/j.1399-3038.2007.00687.x.
- Halken S, Høst A, Hansen LG, Osterballe O. Effect of an allergy prevention programme on incidence of atopic symptoms in infancy. A prospective study of 159 “high-risk” infants. Allergy 1992;47:545-53. http://dx.doi.org/10.1111/j.1398-9995.1992.tb00680.x.
- Halken S, Hansen KS, Jacobsen HP, Estmann A, Faelling AE, Hansen LG, et al. Comparison of a partially hydrolyzed infant formula with two extensively hydrolyzed formulas for allergy prevention: a prospective, randomized study. Pediatr Allergy Immunol 2000;11:149-61. http://dx.doi.org/10.1034/j.1399-3038.2000.00081.x.
- Halken S, Høst A, Hansen LG, Osterballe O. Preventive effect of feeding high-risk infants a casein hydrolysate formula or an ultrafiltrated whey hydrolysate formula. A prospective, randomized, comparative clinical study. Pediatr Allergy Immunol 1993;4:173-81. http://dx.doi.org/10.1111/j.1399-3038.1993.tb00088.x.
- Nentwich I, Michková E, Nevoral J, Urbanek R, Szépfalusi Z. Cow’s milk-specific cellular and humoral immune responses and atopy skin symptoms in infants from atopic families fed a partially (pHF) or extensively (eHF) hydrolyzed infant formula. Allergy 2001;56:1144-56. http://dx.doi.org/10.1111/j.1398-9995.2001x.00926.x.
- Moore WJ, Midwinter RE, Morris AF, Colley JR, Soothill JF. Infant feeding and subsequent risk of atopic eczema. Arch Dis Child 1985;60:722-6. http://dx.doi.org/10.1136/adc.60.8.722.
- Marini A, Agosti M, Motta G, Mosca F. Effects of a dietary and environmental prevention programme on the incidence of allergic symptoms in high atopic risk infants: three years’ follow-up. Acta Paediatr Suppl 1996;414:1-21. http://dx.doi.org/10.1111/j.1651-2227.1996.tb14267.x.
- Burr ML, Limb ES, Maguire MJ, Amarah L, Eldridge BA, Layzell JC, et al. Infant feeding, wheezing, and allergy: a prospective study. Arch Dis Child 1993;68:724-8. http://dx.doi.org/10.1136/adc.68.6.724.
- Burr ML, Miskelly FG, Butland BK, Merrett TG, Vaughan-Williams E. Environmental factors and symptoms in infants at high risk of allergy. J Epidemiol Community Health 1989;43:125-32. http://dx.doi.org/10.1136/jech.43.2.125.
- Businco L, Marchetti F, Pellegrini G, Cantani A, Perlini R. Prevention of atopic disease in “at-risk newborns” by prolonged breast-feeding. Ann Allergy 1983;51:296-9.
- Custovic A, Simpson BM, Simpson A, Kissen P, Woodcock A. NAC Manchester Asthma and Allergy Study Group. Effect of environmental manipulation in pregnancy and early life on respiratory symptoms and atopy during first year of life: a randomised trial. Lancet 2001;358:188-93. http://dx.doi.org/10.1016/S0140-6736(01)05406-X.
- Hide DW, Matthews S, Matthews L, Stevens M, Ridout S, Twiselton R, et al. Effect of allergen avoidance in infancy on allergic manifestations at age two years. J Allergy Clin Immunol 1994;93:842-6. http://dx.doi.org/10.1016/0091-6749(94)90375-1.
- Hide DW, Matthews S, Tariq S, Arshad SH. Allergen avoidance in infancy and allergy at 4 years of age. Allergy 1996;51:89-93. http://dx.doi.org/10.1111/j.1398-9995.1996.tb00040.x.
- Horak F, Matthews S, Ihorst G, Arshad SH, Frischer T, Kuehr J, et al. Effect of mite-impermeable mattress encasings and an educational package on the development of allergies in a multinational randomized, controlled birth-cohort study – 24 months results of the Study of Prevention of Allergy in Children in Europe. Clin Exp Allergy 2004;34:1220-5. http://dx.doi.org/10.1111/j.1365-2222.2004.02024.x.
- Kjellman NI, Johansson SG. Soy versus cow’s milk in infants with a biparental history of atopic disease: development of atopic disease and immunoglobulins from birth to 4 years of age. Clin Allergy 1979;9:347-58. http://dx.doi.org/10.1111/j.1365-2222.1979.tb02493.x.
- Lauritzen L, Kjaer TM, Fruekilde MB, Michaelsen KF, Frøkiaer H. Fish oil supplementation of lactating mothers affects cytokine production in 2 1/2-year-old children. Lipids 2005;40:669-76. http://dx.doi.org/10.1007/s11745-005-1429-6.
- Lilja G, Dannaeus A, Foucard T, Grafflonnevig V, Johansson SGO, Oman H. Effects of maternal diet during late pregnancy and lactation on the development of atopic diseases in infants up to 18 months of age – in vivo results. Clin Exp Allergy 1989;19:473-9. http://dx.doi.org/10.1111/j.1365-2222.1989.tb02416.x.
- Lindfors AT, Danielsson L, Enocksson E, Johansson SG, Westin S. Allergic symptoms up to 4–6 years of age in children given cow milk neonatally. A prospective study. Allergy 1992;47:207-11. http://dx.doi.org/10.1111/j.1398-9995.1992.tb00652.x.
- Lovegrove JA, Hampton SM, Morgan JB. The immunological and long-term atopic outcome of infants born to women following a milk-free diet during late pregnancy and lactation: a pilot study. Br J Nutr 1994;71:223-38. http://dx.doi.org/10.1079/BJN19940129.
- Mallet E, Henocq A. Long-term prevention of allergic diseases by using protein hydrolysate formula in at-risk infants. J Pediatr 1992;121:S95-100. http://dx.doi.org/10.1016/S0022-3476(05)81415-5.
- Miskelly FG, Burr ML, Vaughan-Williams E, Fehily AM, Butland BK, Merrett TG. Infant feeding and allergy. Arch Dis Child 1988;63:388-93. http://dx.doi.org/10.1136/adc.63.4.388.
- Oldaeus G, Anjou K, Björkstén B, Moran JR, Kjellman NI. Extensively and partially hydrolysed infant formulas for allergy prophylaxis. Arch Dis Child 1997;77:4-10. http://dx.doi.org/10.1136/adc.77.1.4.
- Porch MC, Shahane AD, Leiva LE, Elston RC, Sorensen RU. Influence of breast milk, soy or two hydrolyzed formulas on the development of allergic manifestations in infants at risk. Nutr Res 1998;18:1413-24. http://dx.doi.org/10.1016/S0271-5317(98)00119-5.
- Saarinen KM, Juntunen-Backman K, Järvenpää AL, Kuitunen P, Lope L, Renlund M, et al. Supplementary feeding in maternity hospitals and the risk of cow’s milk allergy: A prospective study of 6209 infants. J Allergy Clin Immunol 1999;104:457-61. http://dx.doi.org/10.1016/S0091-6749(99)70393-3.
- Schmitz J, Digeon B, Chastang C, Dupouy D, Leroux B, Robillard P, et al. Effects of brief early exposure to partially hydrolyzed and whole cow milk proteins. J Pediatr 1992;121:S85-9. http://dx.doi.org/10.1016/S0022-3476(05)81413-1.
- Shao J, Sheng J, Dong W, Li YZ, Yu SC. Effects of feeding intervention on development of eczema in atopy high-risk infants: an 18-month follow-up study. Zhonghua Er Ke Za Zhi 2006;44:684-7.
- Steenhuis TJ, van Aalderen WM, Bloksma N, Nijkamp FP, van der Laag J, van Loveren H, et al. Bacille-Calmette-Guerin vaccination and the development of allergic disease in children: a randomized, prospective, single-blind study. Clin Exp Allergy 2008;38:79-85.
- Tsai YT, Chou CC, Hsieh KH. The effect of hypoallergenic formula on the occurrence of allergic diseases in high risk infants. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi 1991;32:137-44.
- van den Berg A, van Elburg RM, Twisk JWR, Fetter WPF. Glutamine-enriched enteral nutrition in very low birth weight infants. Design of a double-blind randomised controlled trial ISRCTN73254583. BMC Pediatr 2004;4. http://dx.doi.org/10.1186/1471-2431-4-17.
- Vandenplas Y, Hauser B, Van den Borre C, Clybouw C, Mahler T, Hachimi-Idrissi S, et al. The long-term effect of a partial whey hydrolysate formula on the prophylaxis of atopic disease. Eur J Pediatr 1995;154:488-94. http://dx.doi.org/10.1007/BF02029362.
- von Berg A, Koletzko S, Filipiak-Pittroff B, Laubereau B, Grübl A, Wichmann HE, et al. Certain hydrolyzed formulas reduce the incidence of atopic dermatitis but not that of asthma: three-year results of the German Infant Nutritional Intervention Study. J Allergy Clin Immunol 2007;119:718-25. http://dx.doi.org/10.1016/j.jaci.2006.11.017.
- Schmitt J, Langan S, Williams HC. European Dermato-Epidemiology Network . What are the best outcome measurements for atopic eczema? A systematic review. J Allergy Clin Immunol 2007;120:1389-98. http://dx.doi.org/10.1016/j.jaci.2007.08.011.
- Schmitt J, Williams H. Harmonising Outcome Measures for Eczema (HOME). Report from the First International Consensus Meeting (HOME 1), Munich, Germany; 24 July 2010. Br J Dermatol 2010;163:1166-8. http://dx.doi.org/10.1111/j.1365-2133.2010.10054.x.
- Brenninkmeijer EE, Schram ME, Leeflang MM, Bos JD, Spuls PI. Diagnostic criteria for atopic dermatitis: a systematic review. Br J Dermatol 2008;158:754-65. http://dx.doi.org/10.1111/j.1365-2133.2007.08412.x.
- Halkjaer LB, Loland L, Buchvald FF, Agner T, Skov L, Strand M, et al. Development of atopic dermatitis during the first 3 years of life: the Copenhagen prospective study on asthma in childhood cohort study in high-risk children. Arch Dermatol 2006;142:561-6. http://dx.doi.org/10.1001/archderm.142.5.561.
- Williams HC, Forsdyke H, Boodoo G, Hay RJ, Burney PG. A protocol for recording the sign of flexural dermatitis in children. Br J Dermatol 1995;133:941-9. http://dx.doi.org/10.1111/j.1365-2133.1995.tb06930.x.
- Williams H, Flohr C. So How Do I Define Atopic Eczema? A Practical Manual for Researchers Wishing to Define Atopic Eczema n.d. http://www.nottingham.ac.uk/dermatology/eczema/Section3-2.html (accessed 22 July 2016).
- National Eczema Association n.d. http://nationaleczema.org/research/nea-funded-research/ (accessed 8 July 2016).
- Simpson EL, Chalmers JR, Hanifin JM, Thomas KS, Cork MJ, McLean WH, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol 2014;134:818-23. http://dx.doi.org/10.1016/j.jaci.2014.08.005.
- Uehara M, Miyauchi H. The morphologic characteristics of dry skin in atopic dermatitis. Arch Dermatol 1984;120:1186-90. http://dx.doi.org/10.1001/archderm.1984.01650450068021.
- Cork MJ, Danby S. Aqueous cream damages the skin barrier. Br J Dermatol 2011;164:1179-80. http://dx.doi.org/10.1111/j.1365-2133.2011.10390.x.
- Hachem JP, Houben E, Crumrine D, Man MQ, Schurer N, Roelandt T, et al. Serine protease signaling of epidermal permeability barrier homeostasis. J Invest Dermatol 2006;126:2074-86. http://dx.doi.org/10.1038/sj.jid.5700351.
- Cork MJ. The importance of skin barrier function. J Dermatolog Treat 1997;8:7-13. http://dx.doi.org/10.3109/09546639709160948.
- Cork MJ, Robinson DA, Vasilopoulos Y, Ferguson A, Moustafa M, MacGowan A, et al. New perspectives on epidermal barrier dysfunction in atopic dermatitis: gene–environment interactions. J Allergy Clin Immunol 2006;118:3-21. http://dx.doi.org/10.1016/j.jaci.2006.04.042.
- De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization?. J Invest Dermatol 2012;132:949-63. http://dx.doi.org/10.1038/jid.2011.435.
- Nakajima S, Igyártó BZ, Honda T, Egawa G, Otsuka A, Hara-Chikuma M, et al. Langerhans cells are critical in epicutaneous sensitization with protein antigen via thymic stromal lymphopoietin receptor signaling. J Allergy Clin Immunol 2012;129:1048-55.e6. http://dx.doi.org/10.1016/j.jaci.2012.01.063.
- Brown SJ, Relton CL, Liao H, Zhao Y, Sandilands A, Wilson IJ, et al. Filaggrin null mutations and childhood atopic eczema: a population-based case-control study. J Allergy Clin Immunol 2008;121:940-46.e3. http://dx.doi.org/10.1016/j.jaci.2008.01.013.
- Lack G, Fox D, Northstone K, Golding J. Avon Longitudinal Study of Parents and Children Study Team . Factors associated with the development of peanut allergy in childhood. N Engl J Med 2003;348:977-85. http://dx.doi.org/10.1056/NEJMoa013536.
- Williams C, Wilkinson SM, McShane P, Lewis J, Pennington D, Pierce S, et al. A double-blind, randomized study to assess the effectiveness of different moisturizers in preventing dermatitis induced by hand washing to simulate healthcare use. Br J Dermatol 2010;162:1088-92. http://dx.doi.org/10.1111/j.1365-2133.2010.09643.x.
- Edwards WH, Conner JM, Soll RF. Vermont Oxford Network Neonatal Skin Care Study Group . The effect of prophylactic ointment therapy on nosocomial sepsis rates and skin integrity in infants with birth weights of 501 to 1000 g. Pediatrics 2004;113:1195-203. http://dx.doi.org/10.1542/peds.113.5.1195.
- Wirén K, Nohlgård C, Nyberg F, Holm L, Svensson M, Johannesson A, et al. Treatment with a barrier-strengthening moisturizing cream delays relapse of atopic dermatitis: a prospective and randomized controlled clinical trial. J Eur Acad Dermatol Venereol 2009;23:1267-72. http://dx.doi.org/10.1111/j.1468-3083.2009.03303.x.
- Batchelor JM, Ridd MJ, Clarke T, Ahmed A, Cox M, Crowe S, et al. The Eczema Priority Setting Partnership: a collaboration between patients, carers, clinicians and researchers to identify and prioritize important research questions for the treatment of eczema. Br J Dermatol 2013;168:577-82. http://dx.doi.org/10.1111/bjd.12040.
- Darmstadt GL, Saha SK, Ahmed AS, Ahmed S, Chowdhury MA, Law PA, et al. Effect of skin barrier therapy on neonatal mortality rates in preterm infants in Bangladesh: a randomized, controlled, clinical trial. Pediatrics 2008;121:522-9. http://dx.doi.org/10.1542/peds.2007-0213.
- Horimukai K, Morita K, Narita M, Kondo M, Kitazawa H, Nozaki M, et al. Application of moisturizer to neonates prevents development of atopic dermatitis. J Allergy Clin Immunol 2014;134:824-30.e6. http://dx.doi.org/10.1016/j.jaci.2014.07.060.
- Kataoka Y, Yoshida N, Nishino H, Maeda N, Sarumaru T, Kijima A, et al. Can skin care from neonatal period prevent the onset of atopic dermatitis?. Allergo J 2010;19.
- ClinicalTrials.gov . Daily Use of Lipikar Balm AP From Birth in Infants at High Risk of Developing Atopic Dermatitis n.d. https://clinicaltrials.gov/ct2/show/NCT01577628 (accessed 3 October 2016).
- Centre of Evidence Based Dermatology . Barrier Enhancement for Eczema Prevention (BEEP) Feasibility Study n.d. www.nottingham.ac.uk/research/groups/cebd/projects/1eczema/beep-feasibility-study.aspx (accessed 8 July 2016).
- Routine Postnatal Care of Women and Their Babies. London: NICE; 2006.
- Danby SG, AlEnezi T, Sultan A, Lavender T, Chittock J, Brown K, et al. Effect of olive and sunflower seed oil on the adult skin barrier: implications for neonatal skin care. Pediatr Dermatol 2013;30:42-50. http://dx.doi.org/10.1111/j.1525-1470.2012.01865.x.
- Danby SG, Al-Enezi T, Sultan A, Chittock J, Kennedy K, Cork MJ. The effect of aqueous cream BP on the skin barrier in volunteers with a previous history of atopic dermatitis. Br J Dermatol 2011;165:329-34. http://dx.doi.org/10.1111/j.1365-2133.2011.10395.x.
- Sandilands A, Terron-Kwiatkowski A, Hull PR, O’Regan GM, Clayton TH, Watson RM, et al. Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat Genet 2007;39:650-4. http://dx.doi.org/10.1038/ng2020.
- Fallon PG, Sasaki T, Sandilands A, Campbell LE, Saunders SP, Mangan NE, et al. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat Genet 2009;41:602-8. http://dx.doi.org/10.1038/ng.358.
- Brown SJ, Asai Y, Cordell HJ, Campbell LE, Zhao Y, Liao H, et al. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J Allergy Clin Immunol 2011;127:661-7. http://dx.doi.org/10.1016/j.jaci.2011.01.031.
- Bohme M, Wickman M, Lennart Nordvall S, Svartengren M, Wahlgren CF. Family history and risk of atopic dermatitis in children up to 4 years. Clin Exp Allergy 2003;33:1226-31. http://dx.doi.org/10.1046/j.1365-2222.2003.01749.x.
- Dold S, Wjst M, von Mutius E, Reitmeir P, Stiepel E. Genetic risk for asthma, allergic rhinitis, and atopic dermatitis. Arch Dis Child 1992;67:1018-22. http://dx.doi.org/10.1136/adc.67.8.1018.
- Williams HC, Flohr C. So How Do I Define Atopic Eczema? n.d. www.nottingham.ac.uk/~mzzfaq/dermatology/eczema/ (accessed 8 July 2016).
- Lewis-Jones S. Quality of life and childhood atopic dermatitis: the misery of living with childhood eczema. Int J Clin Pract 2006;60:984-92. http://dx.doi.org/10.1111/j.1742-1241.2006.01047.x.
- Schuttelaar ML, Vermeulen KM, Coenraads PJ. Costs and cost-effectiveness analysis of treatment in children with eczema by nurse practitioner vs. dermatologist: results of a randomized, controlled trial and a review of international costs. Br J Dermatol 2011;165:600-11. http://dx.doi.org/10.1111/j.1365-2133.2011.10470.x.
- Stevens KJ, Brazier JE, McKenna SP, Doward LC, Cork MJ. The development of a preference-based measure of health in children with atopic dermatitis. Br J Dermatol 2005;153:372-7. http://dx.doi.org/10.1111/j.1365-2133.2005.06736.x.
- van der Velden VH, Laan MP, Baert MR, de Waal Malefyt R, Neijens HJ, Savelkoul HF. Selective development of a strong Th2 cytokine profile in high-risk children who develop atopy: risk factors and regulatory role of IFN-gamma, IL-4 and IL-10. Clin Exp Allergy 2001;31:997-1006. http://dx.doi.org/10.1046/j.1365-2222.2001.01176.x.
- Casaca VI, Illi S, Suttner K, Schleich I, Ballenberger N, Klucker E, et al. TBX21 and HLX1 polymorphisms influence cytokine secretion at birth. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0031069.
- Briggs A, Gray A. The distribution of health care costs and their statistical analysis for economic evaluation. J Health Serv Res Policy 1998;3:233-45.
- Drummond MF, Sculpher MJ, O’Brien BJ, Stoddart GL, Torrance GW. Methods For The Economic Evaluation Of Health Care Programmes. New York, NY: Oxford University Press; 2005.
- Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Data Protection Act. London: The Stationery Office; 1998.
- The American Academy of Allergy, Asthma & Immunology . Prevention of Allergies and Asthma in Children: Tips to Remember 2013 n.d. www.aaaai.org/conditions-and-treatments/library/at-a-glance/prevention-of-allergies-and-asthma-in-children.aspx (accessed 25 July 2016).
- Paton J, Kljakovic M, Ciszek K, Ding P. Infant Feeding Practices and Nut Allergy over Time in Australian School Entrant Children. Int J Pediatr 2012;2012. http://dx.doi.org/10.1155/2012/675724.
- Pelucchi C, Chatenoud L, Turati F, Galeone C, Moja L, Bach JF, et al. Probiotics supplementation during pregnancy or infancy for the prevention of atopic dermatitis: a meta-analysis. Epidemiology 2012;23:402-14. http://dx.doi.org/10.1097/EDE.0b013e31824d5da2.
- Prospective Register of Systematic Reviews (PROSPERO) Database n.d. www.crd.york.ac.uk/PROSPERO (accessed 1 January 2014).
- Nankervis H, Thomas KS, Delamere FM, Barbarot S, Rogers NK, Williams HC. Scoping systematic review of treatments for eczema. Programme Grants Appl Res 2016;4.
- Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technol Assess 2000;4.
- Harmonizing Outcome Measures for Eczema n.d. www.homeforeczema.org (accessed 8 July 2016).
- Global Resource for Eczema Trials n.d. www.greatdatabase.org.uk (accessed 8 July 2016).
- NIHR Evaluation, Trials and Studies Coordinating Centre . BATHE (Bath Emollients for Treatment of CHildhood Eczema) n.d. www.nets.nihr.ac.uk/projects/hta/1115301 (accessed 6 October 2016).
- Nankervis H, Maplethorpe A, Williams HC. Mapping randomized controlled trials of treatments for eczema – the GREAT database (the Global Resource of Eczema Trials: a collection of key data on randomized controlled trials of treatments for eczema from 2000 to 2010). BMC Dermatology 2011;11. http://dx.doi.org/10.1186/1471-5945-11-10.
- Garritty C, Tsertsvadze A, Tricco AC, Sampson M, Moher D. Updating systematic reviews: an international survey. PLOS ONE 2010;5. http://dx.doi.org/10.1371/journal.pone.0009914.
- Betrán AP, Say L, Gülmezoglu AM, Allen T, Hampson L. Effectiveness of different databases in identifying studies for systematic reviews: experience from the WHO systematic review of maternal morbidity and mortality. BMC Med Res Methodol 2005;5. http://dx.doi.org/10.1186/1471-2288-5-6.
- Lefebvre C, Manheimer E, Glanville J, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. London: The Cochrane Collaboration; 2008.
- Hanifin J, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl 1980;92:44-7.
- Hung SH, Lin YT, Chu CY, Lee CC, Liang TC, Yang YH, et al. Staphylococcus colonization in atopic dermatitis treated with fluticasone or tacrolimus with or without antibiotics. Ann Allergy Asthma Immunol 2007;98:51-6. http://dx.doi.org/10.1016/S1081-1206(10)60859-9.
- Nankervis H, Devine A, Williams HC, Ingram JR, Doney E, Delamere F, et al. Validation of the Global resource of Eczema Trials (GREAT) database. BMC Dermatology 2015;15. http://dx.doi.org/10.1186/s12895-015-0024-z.
- Schram ME, Borgonjen RJ, Bik CM, van der Schroeff JG, van Everdingen JJ, Spuls PI. Off-Label Working and Project Group of Dutch Society of Dermatology and Venereology . Off-label use of azathioprine in dermatology: a systematic review. Arch Dermatol 2011;147:474-88. http://dx.doi.org/10.1001/archdermatol.2011.79.
- Yin Z, Xu J, Luo D. Efficacy and tolerance of tacrolimus and pimecrolimus for atopic dermatitis: a meta-analysis. J Biomed Res 2011;25:385-91. http://dx.doi.org/10.1016/S1674-8301(11)60051-1.
- Svensson A, Chambers C, Ganemo A, Mitchell SA. A systematic review of tacrolimus ointment compared with corticosteroids in the treatment of atopic dermatitis. Curr Med Res Opin 2011;27:1395-406. http://dx.doi.org/10.1185/03007995.2011.582483.
- Simonart T, Kabagabo C, De Maertelaer V. Homoeopathic remedies in dermatology: a systematic review of controlled clinical trials. Br J Dermatol 2011;165:897-905. http://dx.doi.org/10.1111/j.1365-2133.2011.10457.x.
- Schmitt J, von Kobyletzki L, Svensson A, Apfelbacher C. Efficacy and tolerability of proactive treatment with topical corticosteroids and calcineurin inhibitors for atopic eczema: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol 2011;164:415-28. http://dx.doi.org/10.1111/j.1365-2133.2010.10030.x.
- Centre of Evidence Based Dermatology . Mapping of Systematic Reviews on Atopic Eczema n.d. www.nottingham.ac.uk/research/groups/cebd/resources/index.aspx (accessed 25 July 2016).
- Futamura M, Thomas KS, Grindlay DJ, Doney EJ, Torley D, Williams HC. Mapping systematic reviews on atopic eczema – an essential resource for dermatology professionals and researchers. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0058484.
- Ashcroft DM, Chen LC, Garside R, Stein K, Williams HC. Topical pimecrolimus for eczema. Cochrane Database Syst Rev 2007;4. http://dx.doi.org/10.1002/14651858.cd005500.pub2.
- Birnie AJ, Bath-Hextall FJ, Ravenscroft JC, Williams HC. Interventions to reduce Staphylococcus aureus in the management of atopic eczema. Cochrane Database Syst Rev 2008;3. http://dx.doi.org/10.1002/14651858.cd003871.pub2.
- Boyle RJ, Bath-Hextall FJ, Leonardi-Bee J, Murrell DF, Tang ML. Probiotics for treating eczema. Cochrane Database Syst Rev 2008;4. http://dx.doi.org/10.1002/14651858.cd006135.pub2.
- Ersser SJ, Latter S, Sibley A, Satherley PA, Welbourne S. Psychological and educational interventions for atopic eczema in children. Cochrane Database Syst Rev 2007;3. http://dx.doi.org/10.1002/14651858.cd004054.pub2.
- Zhang W, Leonard T, Bath-Hextall F, Chambers CA, Lee C, Humphreys R, et al. Chinese herbal medicine for atopic eczema. Cochrane Database Syst Rev 2005;2.
- Bath-Hextall F, Delamere FM, Williams HC. Dietary exclusions for established atopic eczema. Cochrane Database Syst Rev 2008;1. http://dx.doi.org/10.1002/14651858.cd005203.pub2.
- Fattah AA, El-Shiemy S, Faris R, Tadros SS. A comparative clinical evaluation of a new topical steroid ‘halcinonide’ and hydrocortisone in steroid-responsive dermatoses. J Int Med Res 1976;4:228-31.
- Nilsson EJ, Henning CG, Magnusson J. Topical corticosteroids and Staphylococcus aureus in atopic dermatitis. J Am Acad Dermatol 1992;27:29-34. http://dx.doi.org/10.1016/0190-9622(92)70151-5.
- Leyden JJ, Kligman AM. The case for steroid – antibiotic combinations. Br J Dermatol 1977;96:179-87. http://dx.doi.org/10.1111/j.1365-2133.1977.tb12541.x.
- Boguniewicz M, Sampson H, Leung SB, Harbeck R, Leung DY. Effects of cefuroxime axetil on Staphylococcus aureus colonization and superantigen production in atopic dermatitis. J Allergy Clin Immunol 2001;108:651-2. http://dx.doi.org/10.1067/mai.2001.118598.
- Breneman D, Fleischer A, Abramovits W, Farber H. Tacrolimus ointment with and without topical steroids in patients with moderate to severe atopic dermatitis. J Am Acad Dermatol 2006;54.
- Novartis Pharmaceuticals Corporation . Clinical Trials Results Database n.d. www.novctrd.com/ctrdWebApp/clinicaltrialrepository/public/login.jsp (accessed 17 August 2016).
- Staab D, Kaufmann R, Bräutigam M, Wahn U. CASM981CDE04-Study Group . Treatment of infants with atopic eczema with pimecrolimus cream 1% improves parents’ quality of life: a multicenter, randomized trial. Pediatr Allergy Immunol 2005;16:527-33. http://dx.doi.org/10.1111/j.1399-3038.2005.00306.x.
- Thaci D, Foelster Holst R, Hoger P, Kaufmann R, Brautigam M, Weidinger S. Pimecrolimus cream 1% is effective in the treatment of atopic eczema in infants irrespective of clinical score. J Eur Acad Dermatol Venereol 2003;17.
- Kirjavainen PV, Salminen SJ, Isolauri E. Probiotic bacteria in the management of atopic disease: underscoring the importance of viability. J Pediatr Gastroenterol Nutr 2003;36:223-7. http://dx.doi.org/10.1097/00005176-200302000-00012.
- Nakagawa H. Comparison of the efficacy and safety of 0.1% tacrolimus ointment with topical corticosteroids in adult patients with atopic dermatitis: review of randomised, double-blind clinical studies conducted in Japan. Clin Drug Investig 2006;26:235-46. http://dx.doi.org/10.2165/00044011-200626050-00001.
- Siegfried EKN, Molina C, Kianifard F, Abrams K. Safety and efficacy of early intervention with pimecrolimus cream 1% combined with corticosteroids for major flares in infants and children with atopic dermatitis. J Dermatol Treat 2006;17:143-50. http://dx.doi.org/10.1080/09546630600647297.
- Ravenscroft JC, Layton AM, Eady EA, Murtagh MS, Coates P, Walker M, et al. Short-term effects of topical fusidic acid or mupirocin on the prevalence of fusidic acid resistant (FusR) Staphylococcus aureus in atopic eczema. Br J Dermatol 2003;148:1010-17. http://dx.doi.org/10.1046/j.1365-2133.2003.05292.x.
- Larsen FS, Simonsen L, Melgaard A, Wendicke K, Henriksen AS. An efficient new formulation of fusidic acid and betamethasone 17-valerate (fucicort lipid cream) for treatment of clinically infected atopic dermatitis. Acta Derm Venereol 2007;87:62-8. http://dx.doi.org/10.2340/00015555-0174.
- Sense About Science . Alltrials 2014. www.alltrials.net/ (accessed 25 July 2016).
- Nankervis H, Baibergenova A, Williams HC, Thomas KS. Prospective registration and outcome-reporting bias in randomized controlled trials of eczema treatments: a systematic review. J Invest Dermatol 2011;132:2727-34. http://dx.doi.org/10.1038/jid.2012.231.
- Dwan K, Altman DG, Arnaiz JA, Bloom J, Chan AW, Cronin E, et al. Systematic review of the empirical evidence of study publication bias and outcome reporting bias. PLOS ONE 2008;3. http://dx.doi.org/10.1371/journal.pone.0003081.
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c365.
- DeAngelis CD, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. JAMA 2004;292:1363-4. http://dx.doi.org/10.1001/jama.292.11.1363.
- Ormerod AD, Williams HC. Compulsory registration of clinical trials. Br J Dermatol 2005;152:859-60. http://dx.doi.org/10.1111/j.1365-2133.2005.06727.x.
- World Health Organization . International Clinical Trial Registry Platform n.d. www.who.int/ictrp/en (accessed 23 November 2011).
- CONSORT . The CONSORT Statement n.d. www.consort-statement.org (accessed 23 November 2011).
- Lefebvre C, Manheimer E, Glanville J, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. London: The Cochrane Collaboration; 2011.
- The Cochrane Library n.d. www.thecochranelibrary.com (accessed 10 July 2016).
- World Health Organization . Data Providers n.d. www.who.int/ictrp/search/data_providers/en/index.html (accessed 12 August 2011).
- Higgins JPT, Altman DG, Higgins JP, Green S, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1. London: The Cochrane Collection; 2011.
- University of Nottingham . Centre of Evidence Based Dermatology n.d. www.nottingham.ac.uk/dermatology (accessed 11 July 2016).
- van der Aa LB, Heymans HS, van Aalderen WM, Sillevis Smitt JH, Knol J, Ben Amor K, et al. Effect of a new synbiotic mixture on atopic dermatitis in infants: a randomized-controlled trial. Clin Exp Allergy 2010;40:795-804. http://dx.doi.org/10.1111/j.1365-2222.2010.03465.x.
- Hon KL, Leung TF, Ng PC, Lam MC, Kam WY, Wong KY, et al. Efficacy and tolerability of a Chinese herbal medicine concoction for treatment of atopic dermatitis: a randomized, double-blind, placebo-controlled study. Br J Dermatol 2007;157:357-63. http://dx.doi.org/10.1111/j.1365-2133.2007.07941.x.
- Murrell DF, Calvieri S, Ortonne JP, Ho VC, Weise-Riccardi S, Barbier N, et al. A randomized controlled trial of pimecrolimus cream 1% in adolescents and adults with head and neck atopic dermatitis and intolerant of, or dependent on, topical corticosteroids. Br J Dermatol 2007;157:954-9. http://dx.doi.org/10.1111/j.1365-2133.2007.08192.x.
- Peserico A, Städtler G, Sebastian M, Fernandez RS, Vick K, Bieber T. Reduction of relapses of atopic dermatitis with methylprednisolone aceponate cream twice weekly in addition to maintenance treatment with emollient: a multicentre, randomized, double-blind, controlled study. Br J Dermatol 2008;158:801-7. http://dx.doi.org/10.1111/j.1365-2133.2008.08436.x.
- Huang JT, Abrams M, Tlougan B, Rademaker A, Paller AS. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics 2009;123:e808-14. http://dx.doi.org/10.1542/peds.2008-2217.
- Moore EJ, Williams A, Manias E, Varigos G, Donath S. Eczema workshops reduce severity of childhood atopic eczema. Australas J Dermatol 2009;50:100-6. http://dx.doi.org/10.1111/j.1440-0960.2009.00515.x.
- Ruer-Mulard M, Aberer W, Gunstone A, Kekki OM, López Estebaranz JL, Vertruyen A, et al. Twice-daily versus once-daily applications of pimecrolimus cream 1% for the prevention of disease relapse in pediatric patients with atopic dermatitis. Pediatr Dermatol 2009;26:551-8. http://dx.doi.org/10.1111/j.1525-1470.2009.00981.x.
- Hoeger PH, Lee KH, Jautova J, Wohlrab J, Guettner A, Mizutani G, et al. The treatment of facial atopic dermatitis in children who are intolerant of, or dependent on, topical corticosteroids: a randomized, controlled clinical trial. Br J Dermatol 2009;160:415-22. http://dx.doi.org/10.1111/j.1365-2133.2008.08928.x.
- Gambichler T, Othlinghaus N, Tomi NS, Holland-Letz T, Boms S, Skrygan M, et al. Medium-dose ultraviolet (UV) A1 vs. narrowband UVB phototherapy in atopic eczema: a randomized crossover study. Br J Dermatol 2009;160:652-8. http://dx.doi.org/10.1111/j.1365-2133.2008.08984.x.
- Tzaneva S, Kittler H, Holzer G, Reljic D, Weber M, Hönigsmann H, et al. 5-Methoxypsoralen plus ultraviolet (UV) A is superior to medium-dose UVA1 in the treatment of severe atopic dermatitis: a randomized crossover trial. Br J Dermatol 2010;162:655-60. http://dx.doi.org/10.1111/j.1365-2133.2009.09514.x.
- Foelster Holst R, Reitamo S, Yankova R, Worm M, Kadurina M, Thaci D, et al. The novel protease inhibitor SRD441 ointment is not effective in the treatment of adult subjects with atopic dermatitis: results of a randomized, vehicle-controlled study. Allergy 2010;65:1594-9. http://dx.doi.org/10.1111/j.1398-9995.2010.02417.x.
- Schmitt J, Schäkel K, Fölster-Holst R, Bauer A, Oertel R, Augustin M, et al. Prednisolone vs. ciclosporin for severe adult eczema. An investigator-initiated double-blind placebo-controlled multicentre trial. Br J Dermatol 2010;162:661-8. http://dx.doi.org/10.1111/j.1365-2133.2009.09561.x.
- Brenninkmeijer EE, Spuls PI, Lindeboom R, van der Wal AC, Bos JD, Wolkerstorfer A. Excimer laser vs. clobetasol propionate 0.05% ointment in prurigo form of atopic dermatitis: a randomized controlled trial, a pilot. Br J Dermatol 2010;163:823-31. http://dx.doi.org/10.1111/j.1365-2133.2010.09858.x.
- Armstrong AW, Kim RH, Idriss NZ, Larsen LN, Lio PA. Online video improves clinical outcomes in adults with atopic dermatitis: a randomized controlled trial. J Am Acad Dermatol 2011;64:502-7. http://dx.doi.org/10.1016/j.jaad.2010.01.051.
- Bangert C, Strober BE, Cork M, Ortonne JP, Luger T, Bieber T, et al. Clinical and cytological effects of pimecrolimus cream 1% after resolution of active atopic dermatitis lesions by topical corticosteroids: a randomized controlled trial. Dermatology 2011;222:36-48. http://dx.doi.org/10.1159/000321711.
- Frankel A, Sohn A, Patel RV, Lebwohl M. Bilateral comparison study of pimecrolimus cream 1% and a ceramide-hyaluronic acid emollient foam in the treatment of patients with atopic dermatitis. J Drugs Dermatol 2011;10:666-72.
- Thomas KS, Dean T, O’Leary C, Sach TH, Koller K, Frost A, et al. SWET Trial Team . A randomised controlled trial of ion-exchange water softeners for the treatment of eczema in children. PLOS Med 2011;8. http://dx.doi.org/10.1371/journal.pmed.1000395.
- Chalmers I. Underreporting research is scientific misconduct. JAMA 1990;263:1405-8. http://dx.doi.org/10.1001/jama.1990.03440100121018.
- Faure H, Hrynaszkiewicz I. The ISRCTN Register: achievements and challenges 8 years on. J Evid Based Med 2011;4:188-92. http://dx.doi.org/10.1111/j.1756-5391.2011.01138.x.
- Mathieu S, Boutron I, Moher D, Altman DG, Ravaud P. Comparison of registered and published primary outcomes in randomized controlled trials. JAMA 2009;302:977-84. http://dx.doi.org/10.1001/jama.2009.1242.
- Chan AW, Hróbjartsson A, Haahr MT, Gøtzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291:2457-65. http://dx.doi.org/10.1001/jama.291.20.2457.
- Chan AW, Altman DG. Identifying outcome reporting bias in randomised trials on PubMed: review of publications and survey of authors. BMJ 2005;330. http://dx.doi.org/10.1136/bmj.38356.424606.8F.
- Schmitt J, Spuls P, Boers M, Thomas K, Chalmers J, Roekevisch E, et al. Towards global consensus on outcome measures for atopic eczema research: results of the HOME II meeting. Allergy 2012;67:1111-17. http://dx.doi.org/10.1111/j.1398-9995.2012.02874.x.
- Schmitt J, Spuls PI, Thomas KS, Simpson E, Furue M, Deckert S, et al. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol 2014;134:800-7. http://dx.doi.org/10.1016/j.jaci.2014.07.043.
- Chalmers JR, Schmitt J, Apfelbacher C, Dohil M, Eichenfield LF, Simpson EL, et al. Report from the third international consensus meeting to harmonise core outcome measures for atopic eczema/dermatitis clinical trials (HOME). Br J Dermatol 2014;171:1318-25. http://dx.doi.org/10.1111/bjd.13237.
- Charman C, Chambers C, Williams H. Measuring atopic dermatitis severity in randomized controlled clinical trials: what exactly are we measuring?. J Invest Dermatol 2003;120:932-41. http://dx.doi.org/10.1046/j.1523-1747.2003.12251.x.
- Bellamy N, Kirwan J, Boers M, Brooks P, Strand V, Tugwell P, et al. Recommendations for a core set of outcome measures for future phase III clinical trials in knee, hip, and hand osteoarthritis. Consensus development at OMERACT III. J Rheumatol 1997;24:799-802.
- Sinha IP, Gallagher R, Williamson PR, Smyth RL. Development of a core outcome set for clinical trials in childhood asthma: a survey of clinicians, parents, and young people. Trials 2012;13. http://dx.doi.org/10.1186/1745-6215-13-103.
- Ruperto N, Ravelli A, Murray KJ, Lovell DJ, Andersson-Gare B, Feldman BM, et al. Preliminary core sets of measures for disease activity and damage assessment in juvenile systemic lupus erythematosus and juvenile dermatomyositis. Rheumatology 2003;42:1452-9. http://dx.doi.org/10.1093/rheumatology/keg403.
- Severity scoring of atopic dermatitis: the SCORAD index . Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 1993;186:23-31. http://dx.doi.org/10.1159/000247298.
- Silverberg NB. A pilot trial of dermoscopy as a rapid assessment tool in pediatric dermatoses. Cutis 2011;87:148-54.
- Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol 2004;140:1513-19. http://dx.doi.org/10.1001/archderm.140.12.1513.
- Langan SM, Thomas KS, Williams HC. What is meant by a ‘flare’ in atopic dermatitis? A systematic review and proposal. Arch Dermatol 2006;142:1190-6. http://dx.doi.org/10.1001/archderm.142.9.1190.
- Schmitt J, Langan S, Stamm T, Williams HC. Harmonizing Outcome Measurements in Eczema (HOME) Delphi panel . Core outcome domains for controlled trials and clinical recordkeeping in eczema: international multiperspective Delphi consensus process. J Invest Dermatol 2011;131:623-30. http://dx.doi.org/10.1038/jid.2010.303.
- Tugwell P, Boers M. OMERACT conference on outcome measures in rheumatoid arthritis clinical trials: introduction. J Rheumatol 1993;20:528-30.
- Tugwell P, Boers M, Brooks P, Simon L, Strand V, Idzerda L. OMERACT: An international initiative to improve outcome measurement in rheumatology. Trials 2007;8. http://dx.doi.org/10.1186/1745-6215-8-38.
- Boers M, Brooks P, Strand CV, Tugwell P. The OMERACT filter for Outcome Measures in Rheumatology. J Rheumatol 1998;25:198-9.
- Tremp M, Knafla I, Burg G, Wüthrich B, Schmid-Grendelmeier P. ’EASIdig’ – a digital tool to document disease activity in atopic dermatitis. Dermatology 2011;223:68-73. http://dx.doi.org/10.1159/000330333.
- Charman C, Williams H. Outcome measures of disease severity in atopic eczema. Arch Dermatol 2000;136:763-9. http://dx.doi.org/10.1001/archderm.136.6.763.
- Charman CR, Venn AJ, Williams HC. Measurement of body surface area involvement in atopic eczema: an impossible task?. Br J Dermatol 1999;140:109-11. http://dx.doi.org/10.1046/j.1365-2133.1999.02617.x.
- Williamson P, Clarke M. The COMET (Core Outcome Measures in Effectiveness Trials) Initiative: Its Role in Improving Cochrane Reviews. Cochrane Database Syst Rev 2012;5. http://dx.doi.org/10.1002/14651858.ed000041.
- Wolkerstorfer A, de Waard van der Spek FB, Glazenburg EJ, Mulder PG, Oranje AP. Scoring the severity of atopic dermatitis: three item severity score as a rough system for daily practice and as a pre-screening tool for studies. Acta Derm Venereol 1999;79:356-9. http://dx.doi.org/10.1080/000155599750010256.
- Terwee CB, Bot SD, de Boer MR, van der Windt DA, Knol DL, Dekker J, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol 2007;60:34-42. http://dx.doi.org/10.1016/j.jclinepi.2006.03.012.
- Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol 2001;10:11-8. http://dx.doi.org/10.1034/j.1600-0625.2001.100102.x.
- Charman DP, Varigos GA. Grades of severity and the validation of an atopic dermatitis assessment measure (ADAM). J Outcome Meas 1999;3:162-75.
- Bahmer FA. ADASI score: atopic dermatitis area and severity index. Acta Derm Venereol Suppl 1992;176:32-3.
- Carel K, Bratton DL, Miyazawa N, Gyorkos E, Kelsay K, Bender B, et al. The Atopic Dermatitis Quickscore (ADQ): validation of a new parent-administered atopic dermatitis scoring tool. Ann Allergy Asthma Immunol 2008;101:500-7. http://dx.doi.org/10.1016/S1081-1206(10)60289-X.
- Verwimp JJ, Bindels JG, Barents M, Heymans HS. Symptomatology and growth in infants with cow’s milk protein intolerance using two different whey-protein hydrolysate based formulas in a Primary Health Care setting. Eur J Clin Nutr 1995;49:39-48.
- Tofte SJ, Graeber M, Cherill R, Omoto M, Thurston M, Hanifin JM. Eczema area and severity index (EASI): a new tool to evaluate atopic dermatitis. J Eur Acad Dermatol Venereol 1998;11. http://dx.doi.org/10.1016/S0926-9959(98)95291-6.
- Sugarman JL, Fluhr JW, Fowler AJ, Bruckner T, Diepgen TL, Williams ML. The objective severity assessment of atopic dermatitis score: an objective measure using permeability barrier function and stratum corneum hydration with computer-assisted estimates for extent of disease. Arch Dermatol 2003;139:1417-22. http://dx.doi.org/10.1001/archderm.139.11.1417.
- Vourc’h-Jourdain M, Barbarot S, Taieb A, Diepgen T, Ambonati M, Durosier V, et al. Patient-oriented SCORAD: a self-assessment score in atopic dermatitis. A preliminary feasibility study. Dermatology 2009;218:246-51. http://dx.doi.org/10.1159/000193997.
- Housman TS, Patel MJ, Camacho F, Feldman SR, Fleischer AB, Balkrishnan R. Use of the Self-Administered Eczema Area and Severity Index by parent caregivers: results of a validation study. Br J Dermatol 2002;147:1192-8. http://dx.doi.org/10.1046/j.1365-2133.2002.05031.x.
- Berth-Jones J. Six area, six sign atopic dermatitis (SASSAD) severity score: a simple system for monitoring disease activity in atopic dermatitis. Br J Dermatol 1996;135:25-30. http://dx.doi.org/10.1111/j.1365-2133.1996.tb00706.x.
- Costa C, Rilliet A, Nicolet M, Saurat JH. Scoring atopic dermatitis: the simpler the better?. Acta Derm Venereol 1989;69:41-5.
- Silny W, Czarnecka-Operacz M, Silny P. The new scoring system for evaluation of skin inflammation extent and severity in patients with atopic dermatitis. Acta Dermatovenerol Croat 2005;13:219-24.
- Murao Y, Mae H, Konishi T, Gao X, Tanizawa T. Semiquantitative scoring in children under treatment for atopic dermatitis. Pediatr Int 2004;46:330-6. http://dx.doi.org/10.1111/j.1442-200x.2004.01885.x.
- Schmitt J, Langan S, Deckert S, Svensson A, von Kobyletzki L, Thomas K, et al. Harmonising Outcome Measures for Atopic Dermatitis (HOME) Initiative . Assessment of clinical signs of atopic dermatitis: a systematic review and recommendation. J Allergy Clin Immunol 2013;132:1337-47. http://dx.doi.org/10.1016/j.jaci.2013.07.008.
- Charman CR, Venn AJ, Williams H, Bigby M. Measuring atopic eczema severity visually: which variables are most important to patients?. Arch Dermatol 2005;141:1146-51. http://dx.doi.org/10.1001/archderm.141.9.1146.
- de Vet HC, Terwee CB, Mokklink LB, Knol DL. Measurement in Medicine – A Practical Guide. New York, NY: Cambridge University Press; 2011.
- Stalder JF, Barbarot S, Wollenberg A, Holm EA, De Raeve L, Seidenari S, et al. Patient-Oriented SCORAD (PO-SCORAD): a new self-assessment scale in atopic dermatitis validated in Europe. Allergy 2011;66:1114-21. http://dx.doi.org/10.1111/j.1398-9995.2011.02577.x.
- Health Measures . Patient Reported Outcomes Measurement Information System n.d. www.nihpromis.org (accessed 8 July 2016).
- Fuhlbrigge A, Peden D, Apter AJ, Boushey HA, Camargo CA, Gern J, et al. Asthma outcomes: exacerbations. J Allergy Clin Immunol 2012;129:34-48. http://dx.doi.org/10.1016/j.jaci.2011.12.983.
- Rehal B, Armstrong AW, Armstrong A. Health outcome measures in atopic dermatitis: a systematic review of trends in disease severity and quality-of-life instruments 1985–2010. PLOS ONE 2011;6. http://dx.doi.org/10.1371/journal.pone.0017520.
- Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol 2010;63:737-45. http://dx.doi.org/10.1016/j.jclinepi.2010.02.006.
- Minutes of the HOME III Meeting, 2013, 6–7 April 2013; San Diego, USA. University of Nottingham: Nottingham; 2013.
- Wollenberg A, Reitamo S, Girolomoni G, Lahfa M, Ruzicka T, Healy E, et al. Proactive treatment of atopic dermatitis in adults with 0.1% tacrolimus ointment. Allergy 2008;63:742-50. http://dx.doi.org/10.1111/j.1398-9995.2008.01683.x.
- Chalmers I. Well informed uncertainties about the effects of treatments. BMJ 2004;328:475-6. http://dx.doi.org/10.1136/bmj.328.7438.475.
- Buckley B, Grant AM, Firkins L, Greene AC, Frankau J. Working together to identify research questions. Continence UK 2007;1:76-81.
- Levine K, Aldridge A. Surveying the Social World: Principles and Practice in Survey Research. Buckingham: Open University Press; 2001.
- Grey D. Doing Research in the Real World. London: Sage Publications; 2004.
- Eleftheriadou V, Whitton ME, Gawkrodger DJ, Batchelor J, Corne J, Lamb B, et al. Future research into the treatment of vitiligo: where should our priorities lie? Results of the vitiligo priority setting partnership. Br J Dermatol 2011;164:530-6. http://dx.doi.org/10.1111/j.1365-2133.2010.10160.x.
- Schnopp C, Holtmann C, Stock S, Remling R, Fölster-Holst R, Ring J, et al. Topical steroids under wet-wrap dressings in atopic dermatitis – a vehicle-controlled trial. Dermatology 2002;204:56-9. http://dx.doi.org/10.1159/000051811.
- Beattie PE, Lewis-Jones MS. A pilot study on the use of wet wraps in infants with moderate atopic eczema. Clin Exp Dermatol 2004;29:348-53. http://dx.doi.org/10.1111/j.1365-2230.2004.01583.x.
- Management of Atopic Eczema in Primary Care. Edinburgh: SIGN; 2011.
- Thaçi D, Reitamo S, Gonzalez Ensenat MA, Moss C, Boccaletti V, Cainelli T, et al. Proactive disease management with 0.03% tacrolimus ointment for children with atopic dermatitis: results of a randomized, multicentre, comparative study. Br J Dermatol 2008;159:1348-56. http://dx.doi.org/10.1111/j.1365-2133.2008.08813.x.
- Eccles MP, Armstrong D, Baker R, Cleary K, Davies H, Davies S, et al. An implementation research agenda. Implement Sci 2009;4. http://dx.doi.org/10.1186/1748-5908-4-18.
- Breneman D, Fleischer AB, Abramovits W, Zeichner J, Gold MH, Kirsner RS, et al. Intermittent therapy for flare prevention and long-term disease control in stabilized atopic dermatitis: a randomized comparison of 3-times-weekly applications of tacrolimus ointment versus vehicle. J Am Acad Dermatol 2008;58:990-9. http://dx.doi.org/10.1016/j.jaad.2008.02.008.
- Harrison E, Haines R, Cowdell F, Sach T, Dean T, Pollock I, et al. A multi-centre, parallel group superiority trial of silk therapeutic clothing compared to standard care for the management of eczema in children (CLOTHES Trial): study protocol for a randomised controlled trial. Trials 2015;16. http://dx.doi.org/10.1186/s13063-015-0921-9.
- Bath-Hextall F, Birnie AJ, Ravenscroft JC, Williams H. Interventions to reduce Staphylococcus aureus in the management of atopic eczema: an updated Cochrane review. Br J Dermatol 2010;163:12-26. http://dx.doi.org/10.1111/j.1365-2133.2010.09743.x.
- Koller DY, Halmerbauer G, Böck A, Engstler G. Action of a silk fabric treated with AEGIS in children with atopic dermatitis: a 3-month trial. Pediatr Allergy Immunol 2007;18:335-8. http://dx.doi.org/10.1111/j.1399-3038.2006.00511.x.
- Stinco G, Piccirillo F, Valent F. A randomized double-blind study to investigate the clinical efficacy of adding a non-migrating antimicrobial to a special silk fabric in the treatment of atopic dermatitis. Dermatology 2008;217:191-5. http://dx.doi.org/10.1159/000141648.
- Emerson RM, Charman CR, Williams HC. The Nottingham Eczema Severity Score: preliminary refinement of the Rajka and Langeland grading. Br J Dermatol 2000;142:288-97. http://dx.doi.org/10.1046/j.1365-2133.2000.03300.x.
- Thomas KS, Koller K, Dean T, O’Leary CJ, Sach TH, Frost A, et al. A multicentre randomised controlled trial and economic evaluation of ion-exchange water softeners for the treatment of eczema in children: the Softened Water Eczema Trial (SWET). Health Technol Assess 2011;15. http://dx.doi.org/10.3310/hta15080.
- NICE Clinical Guideline 57: Management of Atopic Eczema in Children from Birth up to the Age of 12 Years. Department of Health; 2007.
- Barbier N, Paul C, Luger T, Allen R, De Prost Y, Papp K, et al. Validation of the Eczema Area and Severity Index for atopic dermatitis in a cohort of 1550 patients from the pimecrolimus cream 1% randomized controlled clinical trials programme. Br J Dermatol 2004;150:96-102. http://dx.doi.org/10.1111/j.1365-2133.2004.05696.x.
- Lawson V, Lewis-Jones MS, Finlay AY, Reid P, Owens RG. The family impact of childhood atopic dermatitis: the Dermatitis Family Impact Questionnaire. Br J Dermatol 1998;138:107-13. http://dx.doi.org/10.1046/j.1365-2133.1998.02034.x.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. http://dx.doi.org/10.1016/0168-8510(96)00822-6.
- Ratcliffe J, Stevens K, Flynn T, Brazier J, Sawyer M. An assessment of the construct validity of the CHU9D in the Australian adolescent general population. Qual Life Res 2012;21:717-25. http://dx.doi.org/10.1007/s11136-011-9971-y.
- Curtis L. Unit Costs of Health and Social Care. Canterbury: Personal Social Services Research Unit, University of Kent; 2010.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. http://dx.doi.org/10.1002/hec.944.
- Guide to the Methods of Technology Appraisal. London: NICE Publications; 2008.
- Core Outcomes Measures in Effectiveness Trials (COMET) Initiative n.d. www.comet-initiative.org (accessed 8 July 2016).
- Whitton ME, Ashcroft DM, Barrett CW, Gonzalez U. Interventions for vitiligo. Cochrane Database Syst Rev 2006;1. http://dx.doi.org/10.1002/14651858.cd003263.pub3.
- Whitton ME, Pinart M, Batchelor J, Lushey C, Leonardi-Bee J, González U. Interventions for vitiligo. Cochrane Database Syst Rev 2010;1. http://dx.doi.org/10.1002/14651858.cd003263.pub4.
- Lerner AB. On the etiology of vitiligo and gray hair. Am J Med 1971;51:141-7. http://dx.doi.org/10.1016/0002-9343(71)90232-4.
- Krüger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol 2012;51:1206-12. http://dx.doi.org/10.1111/j.1365-4632.2011.05377.x.
- Schallreuter KU, Bahadoran P, Picardo M, . Vitiligo pathogenesis: autoimmune disease, genetic defect, excessive reactive oxygen species, calcium imbalance, or what else?. Exp Dermatol 2008;17:139-40. http://dx.doi.org/10.1111/j.1600-0625.2007.00666.x.
- Spritz RA. The genetics of generalized vitiligo and associated autoimmune diseases. Pigment Cell Res 2007;20:271-8. http://dx.doi.org/10.1111/j.1600-0749.2007.00384.x.
- Westerhof W, d’Ischia M. Vitiligo puzzle: the pieces fall in place. Pigment Cell Res 2007;20:345-59. http://dx.doi.org/10.1111/j.1600-0749.2007.00399.x.
- Bhatia PS, Mohan L, Pandey ON, Singh KK, Arora SK, Mukhija RD. Genetic nature of vitiligo. J Dermatol Sci 1992;4:180-4. http://dx.doi.org/10.1016/0923-1811(92)90017-6.
- Fain PR, Gowan K, LaBerge GS, Alkhateeb A, Stetler GL, Talbert J, et al. A genomewide screen for generalized vitiligo: confirmation of AIS1 on chromosome 1p31 and evidence for additional susceptibility loci. Am J Hum Genet 2003;72:1560-4. http://dx.doi.org/10.1086/375451.
- Spritz RA, Gowan K, Bennett DC, Fain PR. Novel vitiligo susceptibility loci on chromosomes 7 (AIS2) and 8 (AIS3), confirmation of SLEV1 on chromosome 17, and their roles in an autoimmune diathesis. Am J Hum Genet 2004;74:188-91. http://dx.doi.org/10.1086/381134.
- Al’Abadie MS, Kent GG, Gawkrodger DJ. The relationship between stress and the onset and exacerbation of psoriasis and other skin conditions. Br J Dermatol 1994;130:199-203. http://dx.doi.org/10.1111/j.1365-2133.1994.tb02900.x.
- Rezaei N, Gavalas NG, Weetman AP, Kemp EH. Autoimmunity as an aetiological factor in vitiligo. J Eur Acad Dermatol Venereol 2007;21:865-76. http://dx.doi.org/10.1111/j.1468-3083.2007.02228.x.
- Lerner AB, Nordlund JJ. Vitiligo. What is it? Is it important?. JAMA 1978;239:1183-7. http://dx.doi.org/10.1001/jama.1978.03280390079031.
- Papadopoulos L, Bor R, Legg C. Coping with the disfiguring effects of vitiligo: a preliminary investigation into the effects of cognitive-behavioural therapy. Br J Med Psychol 1999;72:385-96. http://dx.doi.org/10.1348/000711299160077.
- Porter J, Beuf AH, Nordlund JJ, Lerner AB. Psychological reaction to chronic skin disorders: a study of patients with vitiligo. Gen Hosp Psychiatry 1979;1:73-7. http://dx.doi.org/10.1016/0163-8343(79)90081-1.
- Porter JR, Beuf AH, Lerner AB, Nordlund JJ. The effect of vitiligo on sexual relationships. J Am Acad Dermatol 1990;22:221-2. http://dx.doi.org/10.1016/0190-9622(90)70028-G.
- Parsad D, Dogra S, Kanwar AJ. Quality of life in patients with vitiligo. Health Qual Life Outcomes 2003;1. http://dx.doi.org/10.1186/1477-7525-1-58.
- Porter J, Beuf AH, Lerner A, Nordlund J. Response to cosmetic disfigurement: patients with vitiligo. Cutis 1987;39:493-4.
- Kent G, Al’Abadie M. Psychologic effects of vitiligo: a critical incident analysis. J Am Acad Dermatol 1996;35:895-8. http://dx.doi.org/10.1016/S0190-9622(96)90112-7.
- Ongenae K, Dierckxsens L, Brochez L, van Geel N, Naeyaert JM. Quality of life and stigmatization profile in a cohort of vitiligo patients and effect of the use of camouflage. Dermatology 2005;210:279-85. http://dx.doi.org/10.1159/000084751.
- Arca E, Taştan HB, Erbil AH, Sezer E, Koç E, Kurumlu Z. Narrow-band ultraviolet B as monotherapy and in combination with topical calcipotriol in the treatment of vitiligo. J Dermatol 2006;33:338-43. http://dx.doi.org/10.1111/j.1346-8138.2006.00079.x.
- Khalid M, Mujtaba G, Haroon TS. Comparison of 0.05% clobetasol propionate cream and topical Puvasol in childhood vitiligo. Int J Dermatol 1995;34:203-5. http://dx.doi.org/10.1111/j.1365-4362.1995.tb01570.x.
- Ruíz Maldonado R, Tamayo Sánchez L. 4–5-8 trimethylpsoralen in vitiligo. Controlled study of its therapeutic and toxic effect in children. Actas Dermosifiliogr 1975;66:513-26.
- Lepe V, Moncada B, Castanedo-Cazares JP, Torres-Alvarez MB, Ortiz CA, Torres-Rubalcava AB. A double-blind randomized trial of 0.1% tacrolimus vs 0.05% clobetasol for the treatment of childhood vitiligo. Arch Dermatol 2003;139:581-5. http://dx.doi.org/10.1001/archderm.139.5.581.
- Babu A, Thappa DM, Jaisankar TJ. Punch grafting versus suction blister epidermal grafting in the treatment of stable lip vitiligo. Dermatol Surg 2008;34:166-78. http://dx.doi.org/10.1097/00042728-200802000-00005.
- El Mofty M, Mostafa W, Esmat S, Youssef R, Azzam O, Hunter N, et al. Narrow band Ultraviolet B 311 nm in the treatment of vitiligo: two right-left comparison studies. Photodermatol Photoimmunol Photomed 2006;22:6-11. http://dx.doi.org/10.1111/j.1600-0781.2006.00189.x.
- Mofty ME, Zaher H, Esmat S, Youssef R, Shahin Z, Bassioni D, et al. PUVA and PUVB in vitiligo – are they equally effective?. Photodermatol Photoimmunol Photomed 2001;17:159-63. http://dx.doi.org/10.1034/j.1600-0781.2001.170403.x.
- Godse KV. Comparison of two diluents of 1% methoxsalen in the treatment of vitiligo. Indian J Dermatol Venereol Leprol 2008;74. http://dx.doi.org/10.4103/0378-6323.41405.
- Rondon-Lugo AJ, Weiss E, Perez RP, Fuenmajor ME, Rothe J, Bricano L. [Double blind comparative study between melagenina and placebo in the treatment of vitiligo. Dermatologia Venezolana 1987;25:45-8.
- El-Zawahry M, Soliman M, El-Ansary M, El-Zawahry MB, Aziz EA, Rady M. A clinical and immunological study of the effect of the different modalities of treatment on vitiligo patients. J Pan-Arab League Dermatol 1997;8:63-75.
- Ghosh SK, Roy AK. Comparative evaluation of different regimens in the treatment of vitiligo. Indian J Dermatol 1994;39:69-72.
- Dawid M, Veensalu M, Grassberger M, Wolff K. Efficacy and safety of pimecrolimus cream 1% in adult patients with vitiligo: results of a randomized, double-blind, vehicle-controlled study. J Dtsch Dermatol Ges 2006;4:942-6. http://dx.doi.org/10.1111/j.1610-0387.2006.06124.x.
- Esfandiarpour I, Ekhlasi A, Farajzadeh S, Shamsadini S. The efficacy of pimecrolimus 1% cream plus narrow-band ultraviolet B in the treatment of vitiligo: a double-blind, placebo-controlled clinical trial. J Dermatolog Treat 2009;20:14-8. http://dx.doi.org/10.1080/09546630802155057.
- Leone G, Pacifico A, Iacovelli P, Paro Vidolin A, Picardo M. Tacalcitol and narrow-band phototherapy in patients with vitiligo. Clin Exp Dermatol 2006;31:200-5. http://dx.doi.org/10.1111/j.1365-2230.2005.02037.x.
- Lu-yan T, Wen-wen F, Lei-hong X, Yi J, Zhi-zhong Z. Topical tacalcitol and 308-nm monochromatic excimer light: a synergistic combination for the treatment of vitiligo. Photodermatol Photoimmunol Photomed 2006;22:310-14. http://dx.doi.org/10.1111/j.1600-0781.2006.00250.x.
- Rodríguez-Martín M, García Bustínduy M, Sáez Rodríguez M, Noda Cabrera A. Randomized, double-blind clinical trial to evaluate the efficacy of topical tacalcitol and sunlight exposure in the treatment of adult nonsegmental vitiligo. Br J Dermatol 2009;160:409-14. http://dx.doi.org/10.1111/j.1365-2133.2008.08906.x.
- Anbar TS, Westerhof W, Abdel-Rahman AT, Ewis AA, El-Khayyat MA. Effect of one session of ER:YAG laser ablation plus topical 5Fluorouracil on the outcome of short-term NB-UVB phototherapy in the treatment of non-segmental vitiligo: a left-right comparative study. Photodermatol Photoimmunol Photomed 2008;24:322-9. http://dx.doi.org/10.1111/j.1600-0781.2008.00385.x.
- Sharquie KE, Abdulla MS. Treatment of vitiligo with topical 15% lactic acid solution in combination with ultra violet-A. Saudi Med J 2005;26:1013-15.
- Sanclemente G, Garcia JJ, Zuleta JJ, Diehl C, Correa C, Falabella R. A double-blind, randomized trial of 0.05% betamethasone vs. topical catalase/dismutase superoxide in vitiligo. J Eur Acad Dermatol Venereol 2008;22:1359-64. http://dx.doi.org/10.1111/j.1468-3083.2008.02839.x.
- Rojas-Urdaneta JE, Poleo-Romero AG. Evaluation of an antioxidant and mitochondria-stimulating cream formula on the skin of patients with stable common vitiligo. Invest Clin 2007;48:21-3.
- Shi N, Chen YJ, Wang J, NI H. Clinical observation on the effect of Zengse Pill in treating patients with vitiligo of qi-stagnancy and blood-stasis syndrome type. Chin J Integr Med 2008;14:303-6. http://dx.doi.org/10.1007/s11655-008-0303-1.
- Middelkamp-Hup MA, Bos JD, Rius-Diaz F, Gonzalez S, Westerhof W. Treatment of vitiligo vulgaris with narrow-band UVB and oral Polypodium leucotomos extract: a randomized double-blind placebo-controlled study. J Eur Acad Dermatol Venereol 2007;21:942-50. http://dx.doi.org/10.1111/j.1468-3083.2006.02132.x.
- Reyes E, Jaén P, de las Heras E, Carrión F, Alvarez-Mon M, de Eusebio E, et al. Systemic immunomodulatory effects of Polypodium leucotomos as an adjuvant to PUVA therapy in generalized vitiligo: A pilot study. J Dermatol Sci 2006;41:213-16. http://dx.doi.org/10.1016/j.jdermsci.2005.12.006.
- Agarwal S, Ramam M, Sharma VK, Khandpur S, Pal H, Pandey RM. A randomized placebo-controlled double-blind study of levamisole in the treatment of limited and slowly spreading vitiligo. Br J Dermatol 2005;153:163-6. http://dx.doi.org/10.1111/j.1365-2133.2005.06556.x.
- Rath N, Kar HK, Sabhnani S. An open labeled, comparative clinical study on efficacy and tolerability of oral minipulse of steroid (OMP) alone, OMP with PUVA and broad / narrow band UVB phototherapy in progressive vitiligo. Indian J Dermatol Venereol Leprol 2008;74:357-60. http://dx.doi.org/10.4103/0378-6323.42905.
- Radmanesh M, Saedi K. The efficacy of combined PUVA and low-dose azathioprine for early and enhanced repigmentation in vitiligo patients. J Dermatolog Treat 2006;17:151-3. http://dx.doi.org/10.1080/09546630600791442.
- Dell’Anna ML, Mastrofrancesco A, Sala R, Venturini M, Ottaviani M, Vidolin AP, et al. Antioxidants and narrow band-UVB in the treatment of vitiligo: a double-blind placebo controlled trial. Clin Exp Dermatol 2007;32:631-6. http://dx.doi.org/10.1111/j.1365-2230.2007.02514.x.
- Casacci M, Thomas P, Pacifico A, Bonnevalle A, Paro Vidolin A, Leone G. Comparison between 308-nm monochromatic excimer light and narrowband UVB phototherapy (311–13 nm) in the treatment of vitiligo – a multicentre controlled study. J Eur Acad Dermatol Venereol 2007;21:956-63. http://dx.doi.org/10.1111/j.1468-3083.2007.02151.x.
- Asawanonda P, Kijluakiat J, Korkij W, Sindhupak W. Targeted broadband ultraviolet b phototherapy produces similar responses to targeted narrowband ultraviolet B phototherapy for vitiligo: a randomized, double-blind study. Acta Derm Venereol 2008;88:376-81.
- Khandpur S, Sharma VK, Manchanda Y. Comparison of minipunch grafting versus split-skin grafting in chronic stable vitiligo. Dermatol Surg 2005;31:436-41. http://dx.doi.org/10.1097/00042728-200504000-00010.
- Czajkowski R. Comparison of melanocytes transplantation methods for the treatment of vitiligo. Dermatol Surg 2004;30:1400-5.
- van Geel N, Ongenae K, De Mil M, Haeghen YV, Vervaet C, Naeyaert JM. Double-blind placebo-controlled study of autologous transplanted epidermal cell suspensions for repigmenting vitiligo. Arch Dermatol 2004;140:1203-8. http://dx.doi.org/10.1001/archderm.140.10.1203.
- Papadopoulos L, Walker C, Anthis L. Living with vitiligo: a controlled investigation into the effects of group cognitive-behavioural and person-centred therapies. Dermatol Psychosom 2004;5:172-7. http://dx.doi.org/10.1159/000083091.
- Morison WL, Hann SK, Nordlund JJ, Lerner AB. Vitiligo: A Monograph on the Basic and Clinical Science. Oxford: Blackwell Science Ltd; 2000.
- Kumaran MS, Kaur I, Kumar B. Effect of topical calcipotriol, betamethasone dipropionate and their combination in the treatment of localized vitiligo. J Eur Acad Dermatol Venereol 2006;20:269-73. http://dx.doi.org/10.1111/j.1468-3083.2006.01420.x.
- Farah FS, Kurban AK, Chaglassian HT. The treatment of vitiligo with psoralens and triamcinolone by mouth. Br J Dermatol 1967;79:89-91. http://dx.doi.org/10.1111/j.1365-2133.1967.tb11461.x.
- Kawalek AZ, Spencer JM, Phelps RG. Combined excimer laser and topical tacrolimus for the treatment of vitiligo: a pilot study. Dermatol Surg 2004;30:130-5. http://dx.doi.org/10.1097/00042728-200402000-00002.
- Passeron T, Ostovari N, Zakaria W, Fontas E, Larrouy JC, Lacour JP, et al. Topical tacrolimus and the 308-nm excimer laser: a synergistic combination for the treatment of vitiligo. Arch Dermatol 2004;140:1065-9. http://dx.doi.org/10.1001/archderm.140.9.1065.
- Sassi F, Cazzaniga S, Tessari G, Chatenoud L, Reseghetti A, Marchesi L, et al. Randomized controlled trial comparing the effectiveness of 308-nm excimer laser alone or in combination with topical hydrocortisone 17-butyrate cream in the treatment of vitiligo of the face and neck. Br J Dermatol 2008;159:1186-91. http://dx.doi.org/10.1111/j.1365-2133.2008.08793.x.
- Parsad D, Pandhi R, Juneja A. Effectiveness of oral Ginkgo biloba in treating limited, slowly spreading vitiligo. Clin Exp Dermatol 2003;28:285-7. http://dx.doi.org/10.1046/j.1365-2230.2003.01207.x.
- Barman KD, Khaitan BK, Verma KK. A comparative study of punch grafting followed by topical corticosteroid versus punch grafting followed by PUVA therapy in stable vitiligo. Dermatol Surg 2004;30:49-53.
- Lim-Ong M, Leveriza R, Ong B, Frez M. Comparison between narrow-band UVB with topical corticosteroid and narrow-band UVB with placebo in the treatment of vitiligo: a randomized controlled trial. J Phillipine Dermatol Soc 2005;14:17-22.
- Siddiqui AH, Stolk LM, Bhaggoe R, Hu R, Schutgens RB, Westerhof W. L-phenylalanine and UVA irradiation in the treatment of vitiligo. Dermatology 1994;188:215-18. http://dx.doi.org/10.1159/000247142.
- Westerhof W, Nieuweboer-Krobotova L, Mulder PG, Glazenburg EJ. Left-right comparison study of the combination of fluticasone propionate and UV-A vs. either fluticasone propionate or UV-A alone for the long-term treatment of vitiligo. Arch Dermatol 1999;135:1061-6. http://dx.doi.org/10.1001/archderm.135.9.1061.
- Pathak MA, Mosher DB, Fitzpatrick TB. Safety and therapeutic effectiveness of 8-methoxypsoralen, 4,5’,8-trimethylpsoralen, and psoralen in vitiligo. Natl Cancer Inst Monogr 1984;66:165-73.
- González U, Whitton M, Eleftheriadou V, Pinart M, Batchelor J, Leonardi-Bee J. Guidelines for designing and reporting clinical trials in vitiligo. Arch Dermatol 2011;147:1428-36. http://dx.doi.org/10.1001/archdermatol.2011.235.
- Whitton ME, Pinart M, Batchelor J, Leonardi-Bee J, González U, Jiyad Z, et al. Interventions for vitiligo. Cochrane Database Syst Rev 2015;2. http://dx.doi.org/10.1002/14651858.CD003263.pub5.
- The James Lind Alliance Guidebook. Oxford: James Lind Alliance; 2010.
- Gawkrodger DJ, Ormerod AD, Shaw L, Mauri-Sole I, Whitton ME, Watts MJ, et al. Guideline for the diagnosis and management of vitiligo. Br J Dermatol 2008;159:1051-76. http://dx.doi.org/10.1111/j.1365-2133.2008.08881.x.
- Eleftheriadou V, Thomas K. Have your say in research into vitiligo (Advert only). Dermatol Nurs 2009;8:56-7.
- University of Nottingham . Centre of Evidence Based Dermatology n.d. www.vitiligostudy.org.uk (accessed 8 July 2016).
- James Lind Alliance . JLA Guidebook n.d. www.jlaguidebook.org (accessed 8 July 2016).
- Elwyn G, Crowe S, Fenton M, Firkins L, Versnel J, Walker S, et al. Identifying and prioritizing uncertainties: patient and clinician engagement in the identification of research questions. J Eval Clin Pract 2010;16:627-31. http://dx.doi.org/10.1111/j.1365-2753.2009.01262.x.
- Eleftheriadou V, Thomas KS, Whitton ME, Batchelor JM, Ravenscroft JC. Which outcomes should we measure in vitiligo? Results of a systematic review and a survey among patients and clinicians on outcomes in vitiligo trials. Br J Dermatol 2012;167:804-14. http://dx.doi.org/10.1111/j.1365-2133.2012.11056.x.
- Taïeb A, Picardo M. VETF Members. The definition and assessment of vitiligo: a consensus report of the Vitiligo European Task Force. Pigment Cell Res 2007;20:27-35. http://dx.doi.org/10.1111/j.1600-0749.2006.00355.x.
- Ezzedine K, Lim HW, Suzuki T, Katayama I, Hamzavi I, Lan CC, et al. Revised classification/nomenclature of vitiligo and related issues: the Vitiligo Global Issues Consensus Conference. Pigment Cell Melanoma Res 2012;25:E1-13. http://dx.doi.org/10.1111/j.1755-148X.2012.00997.x.
- Sinha I, Jones L, Smyth RL, Williamson PR. A systematic review of studies that aim to determine which outcomes to measure in clinical trials in children. PLOS Med 2008;5. http://dx.doi.org/10.1371/journal.pmed.0050096.
- Townshend AP, Chen CM, Williams HC. How prominent are patient-reported outcomes in clinical trials of dermatological treatments?. Br J Dermatol 2008;159:1152-9. http://dx.doi.org/10.1111/j.1365-2133.2008.08799.x.
- Bhatnagar A, Kanwar AJ, Parsad D, De D. Comparison of systemic PUVA and NB-UVB in the treatment of vitiligo: an open prospective study. J Eur Acad Dermatol Venereol 2007;21:638-42. http://dx.doi.org/10.1111/j.1468-3083.2006.02035.x.
- Cestrai TF, Correia R, Dias MCS, Albaneze R, Fernandez EI. Comparative study of two psoralens in topical phototherapy for vitiligo. An Bras Dermatol 2001;76:683-92.
- El Mofty M, Mostafa W, Youssef R, El-Fangary M, Elramly AZ, Mahgoub D, et al. Ultraviolet A in vitiligo. Photodermatol Photoimmunol Photomed 2006;22:214-6. http://dx.doi.org/10.1111/j.1600-0781.2006.00229.x.
- Ermis O, Alpsoy E, Cetin L, Yilmaz E. Is the efficacy of psoralen plus ultraviolet A therapy for vitiligo enhanced by concurrent topical calcipotriol? A placebo-controlled double-blind study. Br J Dermatol 2001;145:472-5. http://dx.doi.org/10.1046/j.1365-2133.2001.04286.x.
- Goldinger SM, Dummer R, Schmid P, Burg G, Seifert B, Läuchli S. Combination of 308-nm xenon chloride excimer laser and topical calcipotriol in vitiligo. J Eur Acad Dermatol Venereol 2007;21:504-8. http://dx.doi.org/10.1111/j.1468-3083.2006.02016.x.
- Hamzavi I, Jain H, McLean D, Shapiro J, Zeng H, Lui H. Parametric modeling of narrowband UV-B phototherapy for vitiligo using a novel quantitative tool: the Vitiligo Area Scoring Index. Arch Dermatol 2004;140:677-83. http://dx.doi.org/10.1001/archderm.140.6.677.
- Hofer A, Hassan AS, Legat FJ, Kerl H, Wolf P. Optimal weekly frequency of 308-nm excimer laser treatment in vitiligo patients. Br J Dermatol 2005;152:981-5. http://dx.doi.org/10.1111/j.1365-2133.2004.06321.x.
- Kandil E. Treatment of vitiligo with 0–1 per cent betamethasone 17-valerate in isopropyl alcohol – a double-blind trial. Br J Dermatol 1974;91:457-60. http://dx.doi.org/10.1111/j.1365-2133.1974.tb13086.x.
- Mehrabi D, Pandya AG. A randomized, placebo-controlled, double-blind trial comparing narrowband UV-B Plus 0.1% tacrolimus ointment with narrowband UV-B plus placebo in the treatment of generalized vitiligo. Arch Dermatol 2006;142:927-9. http://dx.doi.org/10.1001/archderm.142.7.927.
- Navarro JR, Canales AA, Ponce S. Autologous skin minigraft and ingestion of 8-methoxypsoralen in patients with stable vitiligo vulgaris. Dermatologia Rev Mex 2002;42:260-7.
- Ozdemir M, Cetinkale O, Wolf R, Kotoğyan A, Mat C, Tüzün B, et al. Comparison of two surgical approaches for treating vitiligo: a preliminary study. Int J Dermatol 2002;41:135-8. http://dx.doi.org/10.1046/j.1365-4362.2002.01391.x.
- Parsad D, Saini R, Verma N. Combination of PUVAsol and topical calcipotriol in vitiligo. Dermatology 1998;197:167-70. http://dx.doi.org/10.1159/000017991.
- Procaccini E, Riccio G, Monfrecola G. Ineffectiveness of topical khellin in photochemotherapy of vitiligo. J Dermatol Treat 1995;6:117-20. http://dx.doi.org/10.3109/09546639509097164.
- Schallreuter KU, Moore J, Behrens-Williams S, Panske A, Harari M. Rapid initiation of repigmentation in vitiligo with Dead Sea climatotherapy in combination with pseudocatalase (PC-KUS). Int J Dermatol 2002;41:482-7. http://dx.doi.org/10.1046/j.1365-4362.2002.01463.x.
- Tegta GR, Parsad D, Majumdar S, Kumar B. Efficacy of autologous transplantation of noncultured epidermal suspension in two different dilutions in the treatment of vitiligo. Int J Dermatol 2006;45:106-10. http://dx.doi.org/10.1111/j.1365-4632.2004.02403.x.
- Tjioe M, Gerritsen MJ, Juhlin L, van de Kerkhof PC. Treatment of vitiligo vulgaris with narrow band UVB (311 nm) for one year and the effect of addition of folic acid and vitamin B12. Acta Derm Venereol 2002;82:369-72. http://dx.doi.org/10.1080/000155502320624113.
- Vasistha LK, Singh G. Vitiligo and intralesional steroids. Indian J Med Res 1979;69:308-11.
- Yones SS, Palmer RA, Garibaldinos TM, Hawk JL. Randomized double-blind trial of treatment of vitiligo: efficacy of psoralen-UV-A therapy vs Narrowband-UV-B therapy. Arch Dermatol 2007;143:578-84. http://dx.doi.org/10.1001/archderm.143.5.578.
- Bhor U, Pande S. Scoring systems in dermatology. Indian J Dermatol Venereol Leprol 2006;72:315-21. http://dx.doi.org/10.4103/0378-6323.26722.
- Eleftheriadou V, Thomas K, van Geel N, Hamzavi I, Lim H, Suzuki T, et al. Developing a core outcome set for vitiligo clinical trials: international e-Delphi consensus. Pigment Cell and Melanoma Research 2015;28:363-9. http://dx.doi.org/10.1111/pcmr.12354.
- Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, et al. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res 2010;19:539-49. http://dx.doi.org/10.1007/s11136-010-9606-8.
- Tour SK, Thomas KS, Walker D, Leighton P, Yong ASW, Batchelor JM. Survey and online discussion groups to develop a patient-rated outcome measure on acceptability of treatment response in vitiligo. BMC Dermatology 2014;14. http://dx.doi.org/10.1186/1471-5945-14-10.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2013.
- Dertlioğlu SB, Cicek D, Balci DD, Halisdemir N. Dermatology life quality index scores in children with vitiligo: comparison with atopic dermatitis and healthy control subjects. Int J Dermatol 2013;52:96-101. http://dx.doi.org/10.1111/j.1365-4632.2012.05616.x.
- Hann SK, Chun WH, Park YK. Clinical characteristics of progressive vitiligo. Int J Dermatol 1997;36:353-5. http://dx.doi.org/10.1046/j.1365-4362.1997.00162.x.
- Thompson AR, Kent G, Smith JA. Living with vitiligo: dealing with difference. Br J Health Psychol 2002;7:213-25. http://dx.doi.org/10.1348/135910702169457.
- COMET – Core Outcome Measures in Effectiveness Trials 2013. www.comet-initiative.org/studies/details/357 (accessed 22 July 2016).
- McNally NJ, Phillips DR, Williams HC. Focus groups in dermatology. Clin Exp Dermatol 1998;23:195-200. http://dx.doi.org/10.1046/j.1365-2230.1998.00381.x.
- NHS Choices . Vitiligo 2014. www.nhs.uk/conditions/vitiligo/Pages/Introduction.aspx (accessed 22 July 2016).
- Walker DM. The internet as a medium for health service research. Part 1. Nurse Res 2013;20:18-21. http://dx.doi.org/10.7748/nr2013.03.20.4.18.e294.
- Walker DM. The internet as a medium for health services research. Part 2. Nurse Res 2013;20:33-7. http://dx.doi.org/10.7748/nr2013.05.20.5.33.e295.
- Baltes BB, Dickson MW, Sherman MP, Bauer CC, LaGanke JS. Computer-mediated communication and group decision making: a meta-analysis. Organ Behav Hum Dec Process 2002;87:156-79. http://dx.doi.org/10.1006/obhd.2001.2961.
- Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CF, Askham J, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess 1998;2.
- Brooks J, King N. Qualitative Psychology in the Real World: The Utility of Template Analysis n.d.
- King N, Symon G, Cassell C. Qualitative Organizational Research: Core Methods and Current Challenges. London: Sage; 2012.
- Lilly E, Lu PD, Borovicka JH, Victorson D, Kwasny MJ, West DP, et al. Development and validation of a vitiligo-specific quality-of-life instrument (VitiQoL). J Am Acad Dermatol 2013;69:e11-8. http://dx.doi.org/10.1016/j.jaad.2012.01.038.
- Krishna GS, Ramam M, Mehta M, Sreenivas V, Sharma VK, Khandpur S. Vitiligo impact scale: an instrument to assess the psychosocial burden of vitiligo. Indian J Dermatol Venereol Leprol 2013;79:205-10. http://dx.doi.org/10.4103/0378-6323.107637.
- Beresniak A, de Linares Y, Krueger GG, Talarico S, Tsutani K, Duru G, et al. Validation of a new international quality-of-life instrument specific to cosmetics and physical appearance: BeautyQoL questionnaire. Arch Dermatol 2012;148:1275-82. http://dx.doi.org/10.1001/archdermatol.2012.2696.
- Eleftheriadou V, Thomas K, Ravenscroft J, Whitton M, Batchelor J, Williams H. Feasibility, double-blind, randomised, placebo-controlled, multi-centre trial of hand-held NB-UVB phototherapy for the treatment of vitiligo at home (HI-Light trial: Home Intervention of Light therapy). Trials 2014;15. http://dx.doi.org/10.1186/1745-6215-15-51.
- Taieb A, Alomar A, Böhm M, Dell’anna ML, De Pase A, Eleftheriadou V, et al. Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol 2013;168:5-19. http://dx.doi.org/10.1111/j.1365-2133.2012.11197.x.
- Ibbotson SH, Bilsland D, Cox NH, Dawe RS, Diffey B, Edwards C, et al. An update and guidance on narrowband ultraviolet B phototherapy: a British Photodermatology Group Workshop Report. Br J Dermatol 2004;151:283-97. http://dx.doi.org/10.1111/j.1365-2133.2004.06128.x.
- Mysore V. Targeted phototherapy. Indian J Dermatol Venereol Leprol 2009;75:119-25. http://dx.doi.org/10.4103/0378-6323.48655.
- Caccialanza M, Piccinno R, Cappio F, Rozza M, Mainardi L. Phototherapy of psoriasis of the scalp. Results in 21 patients treated with a special portable ultraviolet rays lamp. G Ital Dermatol Venereol 1989;124:LXI-LXV.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994;19:210-16. http://dx.doi.org/10.1111/j.1365-2230.1994.tb01167.x.
- Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol 1995;132:942-9. http://dx.doi.org/10.1111/j.1365-2133.1995.tb16953.x.
- Augustin M, Gajur AI, Reich C, Rustenbach SJ, Schaefer I. Benefit evaluation in vitiligo treatment: development and validation of a patient-defined outcome questionnaire. Dermatology 2008;217:101-6. http://dx.doi.org/10.1159/000128992.
- NICE . Clinical Knowledge Summary – Vitiligo n.d. http://cks.nice.org.uk/vitiligo (accessed 25 July 2016).
- NIHR Evaluation, Trials and Studies Coordinating Centre . Home Interventions and Light Therapy for the Treatment of Vitiligo (HI-Light Vitiligo Trial) n.d. www.nets.nihr.ac.uk/projects/hta/122402 (accessed 6 October 2016).
- ClinicalTrials.gov . Effect of Fluticasone Proprionate 0.05% on Narrow Band UV-B in Active Vitiligo n.d. www.clinicaltrials.gov/show/NCT01246921 (accessed 18 July 2016).
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- NICE . Vitiligo n.d. http://cks.nice.org.uk/vitiligo (accessed 3 October 2016).
- Cancer Research UK. Skin Cancer Statistics n.d. www.cancerresearchuk.org/cancer-info/cancerstats/types/skin/ (accessed 22 July 2016).
- Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol 2012;166:1069-80. http://dx.doi.org/10.1111/j.1365-2133.2012.10830.x.
- van der Geer S, Siemerink M, Reijers HA, Verhaegh ME, Ostertag JU, Neumann HA, et al. The incidence of skin cancer in dermatology. Clin Exp Dermatol 2013;38:724-9. http://dx.doi.org/10.1111/ced.12080.
- Staples MP, Elwood M, Burton RC, Williams JL, Marks R, Giles GG. Non-melanoma skin cancer in Australia: the 2002 national survey and trends since 1985. Med J Aust 2006;184:6-10.
- Buettner PG, Raasch BA. Incidence rates of skin cancer in Townsville, Australia. Int J Cancer 1998;78:587-93. http://dx.doi.org/10.1002/(SICI)1097-0215(19981123)78:5<587::AID-IJC10>3.0.CO;2-E.
- Marks R. Squamous cell carcinoma. Lancet 1996;347:735-8. http://dx.doi.org/10.1016/S0140-6736(96)90081-1.
- Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol 1994;30:774-8. http://dx.doi.org/10.1016/S0190-9622(08)81509-5.
- Sober AJ. Diagnosis and management of skin cancer. Cancer 1983;51:2448-52. http://dx.doi.org/10.1002/1097-0142(19830615)51:12+<2448::AID-CNCR2820511311>3.0.CO;2-L.
- Rosso S, Zanetti R, Martinez C, Tormo MJ, Schraub S, Sancho-Garnier H, et al. The multicentre south European study ‘Helios’. II: Different sun exposure patterns in the aetiology of basal cell and squamous cell carcinomas of the skin. Br J Cancer 1996;73:1447-54. http://dx.doi.org/10.1038/bjc.1996.275.
- De Hertog SA, Wensveen CA, Bastiaens MT, Kielich CJ, Berkhout MJ, Westendorp RG, et al. Relation between smoking and skin cancer. J Clin Oncol 2001;19:231-8.
- Katz KA, Marcil I, Stern RS. Incidence and risk factors associated with a second squamous cell carcinoma or basal cell carcinoma in psoralen + ultraviolet a light-treated psoriasis patients. J Invest Dermatol 2002;118:1038-43. http://dx.doi.org/10.1046/j.1523-1747.2002.01769.x.
- Lim JL, Stern RS. High levels of ultraviolet B exposure increase the risk of non-melanoma skin cancer in psoralen and ultraviolet A-treated patients. J Invest Dermatol 2005;124:505-13. http://dx.doi.org/10.1111/j.0022-202X.2005.23618.x.
- Nijsten TE, Stern RS. The increased risk of skin cancer is persistent after discontinuation of psoralen+ultraviolet A: a cohort study. J Invest Dermatol 2003;121:252-8. http://dx.doi.org/10.1046/j.1523-1747.2003.12350.x.
- Karagas MR, Nelson HH, Zens MS, Linet M, Stukel TA, Spencer S, et al. Squamous cell and basal cell carcinoma of the skin in relation to radiation therapy and potential modification of risk by sun exposure. Epidemiology 2007;18:776-84. http://dx.doi.org/10.1097/EDE.0b013e3181567ebe.
- Karagas MR, Nelson HH, Sehr P, Waterboer T, Stukel TA, Andrew A, et al. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst 2006;98:389-95. http://dx.doi.org/10.1093/jnci/djj092.
- Harwood CA, Surentheran T, Sasieni P, Proby CM, Bordea C, Leigh IM, et al. Increased risk of skin cancer associated with the presence of epidermodysplasia verruciformis human papillomavirus types in normal skin. Br J Dermatol 2004;150:949-57. http://dx.doi.org/10.1111/j.1365-2133.2004.05847.x.
- Gallagher RP, Hill GB, Bajdik CD, Coldman AJ, Fincham S, McLean DI, et al. Sunlight exposure, pigmentation factors, and risk of nonmelanocytic skin cancer. II. Squamous cell carcinoma. Arch Dermatol 1995;131:164-9. http://dx.doi.org/10.1001/archderm.1995.01690140048007.
- Moloney FJ, Comber H, O’Lorcain P, O’Kelly P, Conlon PJ, Murphy GM. A population-based study of skin cancer incidence and prevalence in renal transplant recipients. Br J Dermatol 2006;154:498-504. http://dx.doi.org/10.1111/j.1365-2133.2005.07021.x.
- Wisgerhof HC, van der Geest LG, de Fijter JW, Haasnoot GW, Claas FH, le Cessie S, et al. Incidence of cancer in kidney-transplant recipients: a long-term cohort study in a single center. Cancer Epidemiol 2011;35:105-11. http://dx.doi.org/10.1016/j.canep.2010.07.002.
- Jensen AO, Svaerke C, Farkas D, Pedersen L, Kragballe K, Sorensen HT. Skin cancer risk among solid organ recipients: a nationwide cohort study in Denmark. Acta Derm Venereol 2010;90:474-9. http://dx.doi.org/10.2340/00015555-0919.
- Alam M, Brown RN, Silber DH, Mullen GM, Feldman DS, Oren RM, et al. Cardiac Transplant Research Database Group . Increased incidence and mortality associated with skin cancers after cardiac transplant. Am J Transplant 2011;11:1488-97. http://dx.doi.org/10.1111/j.1600-6143.2011.03598.x.
- Bath-Hextall F, Jenkinson C, Kumar A, Leonardi-Bee J, Perkins W, Cox K, et al. Longitudinal, mixed method study to look at the experiences and knowledge of non melanoma skin cancer from diagnosis to one year. BMC Dermatol 2013;13. http://dx.doi.org/10.1186/1471-5945-13-13.
- Motley R, Kersey P, Lawrence C. Royal College of Radiologists Faculty of Clinical Oncology . Multiprofessional guidelines for the management of the patient with primary cutaneous squamous cell carcinoma. Br J Dermatol 2002;146:18-25. http://dx.doi.org/10.1046/j.0007-0963.2001.04615.x.
- Lansbury L, Leonardi-Bee J, Perkins W, Goodacre T, Tweed JA, Bath-Hextall FJ. Interventions for non-metastatic squamous cell carcinoma of the skin. Cochrane Database Syst Rev 2010;4. http://dx.doi.org/10.1002/14651858.cd007869.pub2.
- Diffey BL, Langtry JA. Skin cancer incidence and the ageing population. Br J Dermatol 2005;153:679-80. http://dx.doi.org/10.1111/j.1365-2133.2005.06799.x.
- Brewster AM, Lee JJ, Clayman GL, Clifford JL, Reyes MJ, Zhou X, et al. Randomized trial of adjuvant 13-cis-retinoic acid and interferon alfa for patients with aggressive skin squamous cell carcinoma. J Clin Oncol 2007;25:1974-8. http://dx.doi.org/10.1200/JCO.2006.05.9873.
- Brandt MG, Moore CC, Jordan K. Randomized control trial of fluorescence-guided surgical excision of nonmelanotic cutaneous malignancies. J Otolaryngol 2007;36:148-55. http://dx.doi.org/10.2310/7070.2007.0020.
- Cham BE, Daunter B, Evans RA. Topical treatment of malignant and premalignant skin lesions by very low concentrations of a standard mixture (BEC) of solasodine glycosides. Cancer Lett 1991;59:183-92. http://dx.doi.org/10.1016/0304-3835(91)90140-D.
- Coates AS, Tattersall MH, Swanson C, Hedley D, Fox RM, Raghavan D. Combination therapy with methotrexate and 5-fluorouracil: a prospective randomized clinical trial of order of administration. J Clin Oncol 1984;2:756-61.
- Haas CD, Coltman CA, Gottlieb JA, Haut A, Luce JK, Talley RW, et al. Phase II evaluation of bleomycin. A Southwest oncology Group study. Cancer 1976;38:8-12. http://dx.doi.org/10.1002/1097-0142(197607)38:1<8::AID-CNCR2820380103>3.0.CO;2-4.
- Healy JB. The use of topical 5-fluorouracil in the treatment of skin tumours – preliminary report. J Ir Med Assoc 1969;62:41-6.
- Landthaler M, Braun-Falco O. Use of the TDF factor in soft roentgen radiotherapy. Hautarzt 1989;40:774-7.
- Lui H, Hobbs L, Tope WD, Lee PK, Elmets C, Provost N, et al. Photodynamic therapy of multiple nonmelanoma skin cancers with verteporfin and red light-emitting diodes: two-year results evaluating tumor response and cosmetic outcomes. Arch Dermatol 2004;140:26-32. http://dx.doi.org/10.1001/archderm.140.1.26.
- Moseley HS, Sasaki T, McConnell DB, Merhoff GC, Wilson WL, Grage TB, et al. A randomized pilot study comparing two regimens in the treatment of squamous cell carcinoma. J Surg Oncol 1976;8:35-42. http://dx.doi.org/10.1002/jso.2930080106.
- Anon . Bleomycin in advanced squamous cell carcinoma: a random controlled trial. Report of Medical Research Council Working Party on Bleomycin. Br Med J 1976;1:188-90. http://dx.doi.org/10.1136/bmj.1.6003.188.
- Perez CA, Pajak T, Emami B, Hornback NB, Tupchong L, Rubin P. Randomized phase III study comparing irradiation and hyperthermia with irradiation alone in superficial measurable tumors. Final report by the Radiation Therapy Oncology Group. Am J Clin Oncol 1991;14:133-41. http://dx.doi.org/10.1097/00000421-199104000-00008.
- Seyss R. On the practice of soft ray and proximity radiotherapy in skin and lip carcinomas. Wien Med Wochenschr 1968;118:34-6.
- Eedy D. Immunomodulators in the treatment of skin cancer. Barcelona: Proceedings of the the European Academy of Dermatology and Venereology, 12th Congress; 15–18 October 2003. J Eur Acad Dermatol Venereol 2003;17.
- Radny P, Garbe C, Bruckner-Tuderman L, Nashan D. Selective electrochemical tumour ablation (SECTA) with intralesional bleomycin. Rome: Proceedings of the European Association of Dermato-Oncology, 3rd meeting; 23–5 June 2006. J Invest Dermatol 2006;126.
- ClinicalTrials.gov . Radiation Therapy in Treating Patients With Head and Neck Cancer n.d. http://clinicaltrials.gov/show/NCT00021125.
- ClinicalTrials.gov . Post-Operative Concurrent Chemo-Radiotherapy Versus Post-Operative Radiotherapy for Cancer of the Head and Neck n.d. https://clinicaltrials.gov/ct2/show/NCT00193895 (accessed 3 October 2016).
- Lansbury L, Bath-Hextall F, Perkins W, Stanton W, Leonardi-Bee J. Interventions for non-metastatic squamous cell carcinoma of the skin: systematic review and pooled analysis of observational studies. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f6153.
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. http://dx.doi.org/10.1001/jama.283.15.2008.
- Lansbury L, Bath-Hextall F, Leonardi-Bee J, Perkins W. Effectiveness of Interventions for Primary, Non-Metastatic Squamous Cell Carcinoma of the Skin: A Systematic Review of Observational Studies 2011. www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42011001450 (accessed 3 October 2016).
- Williams H, Bigby M, Diepgen T, Herxheimer A, Naldi L, Rzany B. Evidence-Based Dermatology. Oxford: Blackwell Publishing; 2008.
- Stuart A, Ord JK. Kendall’s Advanced Theory of Statistics. London: Edward Arnold; 1994.
- Baker NJ, Webb AA, Macpherson D. Surgical management of cutaneous squamous cell carcinoma of the head and neck. Br J Oral Maxillofac Surg 2001;39:87-90. http://dx.doi.org/10.1054/bjom.2000.0584.
- Rank B. Surgery and skin cancer. Ann R Coll Surg Engl 1973;52:148-64.
- Donaldson MJ, Sullivan TJ, Whitehead KJ, Williamson RM. Squamous cell carcinoma of the eyelids. Br J Ophthalmol 2002;86:1161-5. http://dx.doi.org/10.1136/bjo.86.10.1161.
- Griffiths RW, Feeley K, Suvarna SK. Audit of clinical and histological prognostic factors in primary invasive squamous cell carcinoma of the skin: assessment in a minimum 5 year follow-up study after conventional excisional surgery. Br J Plast Surg 2002;55:287-92. http://dx.doi.org/10.1054/bjps.2002.3833.
- van der Eerden PA, Prins ME, Lohuis PJ, Balm FA, Vuyk HD. Eighteen years of experience in Mohs micrographic surgery and conventional excision for nonmelanoma skin cancer treated by a single facial plastic surgeon and pathologist. Laryngoscope 2010;120:2378-84. http://dx.doi.org/10.1002/lary.21139.
- Nemet AY, Deckel Y, Martin PA, Kourt G, Chilov M, Sharma V, et al. Management of periocular basal and squamous cell carcinoma: a series of 485 cases. Am J Ophthalmol 2006;142:293-7. http://dx.doi.org/10.1016/j.ajo.2006.03.055.
- Shiffman NJ. Squamous cell carcinomas of the skin of the pinna. Can J Surg 1975;18:279-83.
- Reifler DM, Hornblass A. Surgical management of squamous cell carcinoma of the lid. Ophthal Plast Reconstr Surg 1986;2:75-82. http://dx.doi.org/10.1097/00002341-198601050-00004.
- Shiu MH, Chu F, Fortner JG. Treatment of regionally advanced epidermoid carcinoma of the extremity and trunk. Surg Gynecol Obstet 1980;150:558-62.
- Thomas SS, Matthews RN. Squamous cell carcinoma of the pinna: a 6-year study. Br J Plast Surg 1994;47:81-5. http://dx.doi.org/10.1016/0007-1226(94)90163-5.
- Pless J. Carcinoma of the external ear. Scand J Plast Reconstr Surg 1976;10:147-51. http://dx.doi.org/10.3109/02844317609105202.
- Fitzpatrick PJ, Harwood AA. Acute epithelioma – an aggressive squamous cell carcinoma of the skin. Am J Clin Oncol 1985;8:468-71. http://dx.doi.org/10.1097/00000421-198512000-00004.
- Mourouzis C, Boynton A, Grant J, Umar T, Wilson A, Macpheson D, et al. Cutaneous head and neck SCCs and risk of nodal metastasis - UK experience. J Craniomaxillofac Surg 2009;37:443-7. http://dx.doi.org/10.1016/j.jcms.2009.07.007.
- Knox JM, Freeman RG, Duncan WC, Heaton CL. Treatment of skin cancer. South Med J 1967;60:241-6. http://dx.doi.org/10.1097/00007611-196703000-00004.
- Ang P, Tan AW, Goh CL. Comparison of completely versus incompletely excised cutaneous squamous cell carcinomas. Ann Acad Med Singap 2004;33:68-70.
- Friedman HI, Cooper PH, Wanebo HJ. Prognostic and therapeutic use of microstaging of cutaneous squamous cell carcinoma of the trunk and extremities. Cancer 1985;56:1099-105. http://dx.doi.org/10.1002/1097-0142(19850901)56:5<1099::AID-CNCR2820560524>3.0.CO;2-R.
- Werlinger KD, Upton G, Moore AY. Recurrence rates of primary nonmelanoma skin cancers treated by surgical excision compared to electrodesiccation-curettage in a private dermatological practice. Dermatol Surg 2002;28:1138-42.
- Yoon M, Chougule P, Dufresne R, Wanebo HJ. Localized carcinoma of the external ear is an unrecognized aggressive disease with a high propensity for local regional recurrence. Am J Surg 1992;164:574-7. http://dx.doi.org/10.1016/S0002-9610(05)80709-3.
- Pua VS, Huilgol S, Hill D. Evaluation of the treatment of non-melanoma skin cancers by surgical excision. Australas J Dermatol 2009;50:171-5. http://dx.doi.org/10.1111/j.1440-0960.2009.00531.x.
- Thomas DJ, King AR, Peat BG. Excision margins for nonmelanotic skin cancer. Plast Reconstr Surg 2003;112:57-63. http://dx.doi.org/10.1097/01.PRS.0000067479.77859.31.
- Tan PY, Ek E, Su S, Giorlando F, Dieu T. Incomplete excision of squamous cell carcinoma of the skin: a prospective observational study. Plast Reconstr Surg 2007;120:910-16. http://dx.doi.org/10.1097/01.prs.0000277655.89728.9f.
- Bogdanov-Berezovsky A, Cohen AD, Glesinger R, Cagnano E, Rosenberg L. Risk factors for incomplete excision of squamous cell carcinomas. J Dermatolog Treat 2005;16:341-4. http://dx.doi.org/10.1080/09546630500424649.
- Bovill ES, Cullen KW, Barrett W, Banwell PE. Clinical and histological findings in re-excision of incompletely excised cutaneous squamous cell carcinoma. J Plast Reconstr Aesthet Surg 2009;62:457-61. http://dx.doi.org/10.1016/j.bjps.2007.11.041.
- Anderson RL. Results in eyelid malignancies treated with the Mohs fresh-tissue technique. Trans New Orleans Acad Ophthalmol 1982;30:380-91.
- Pugliano-Mauro M, Goldman G. Mohs surgery is effective for high-risk cutaneous squamous cell carcinoma. Dermatol Surg 2010;36:1544-53. http://dx.doi.org/10.1111/j.1524-4725.2010.01576.x.
- Vuyk HD, Lohuis PJ. Mohs micrographic surgery for facial skin cancer. Clin Otolaryngol Allied Sci 2001;26:265-73. http://dx.doi.org/10.1046/j.1365-2273.2001.00484.x.
- Turner RJ, Leonard N, Malcolm AJ, Lawrence CM, Dahl MG. A retrospective study of outcome of Mohs’ micrographic surgery for cutaneous squamous cell carcinoma using formalin fixed sections. Br J Dermatol 2000;142:752-7. http://dx.doi.org/10.1046/j.1365-2133.2000.03422.x.
- Leibovitch I, Huilgol SC, Selva D, Hill D, Richards S, Paver R. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia I. Experience over 10 years. J Am Acad Dermatol 2005;53:253-60. http://dx.doi.org/10.1016/j.jaad.2005.02.059.
- Brantsch KD, Meisner C, Schönfisch B, Trilling B, Wehner-Caroli J, Röcken M, et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol 2008;9:713-20. http://dx.doi.org/10.1016/S1470-2045(08)70178-5.
- Dzubow LM, Rigel DS, Robins P. Risk factors for local recurrence of primary cutaneous squamous cell carcinomas. Treatment by microscopically controlled excision. Arch Dermatol 1982;118:900-2. http://dx.doi.org/10.1001/archderm.1982.01650230028021.
- Silapunt S, Peterson SR, Goldberg LH. Squamous cell carcinoma of the auricle and Mohs micrographic surgery. Dermatol Surg 2005;31:1423-7.
- Malhotra R, Huilgol SC, Huynh NT, Selva D. The Australian Mohs database: periocular squamous cell carcinoma. Ophthalmology 2004;111:617-23. http://dx.doi.org/10.1016/j.ophtha.2003.07.020.
- Cherpelis BS, Marcusen C, Lang PG. Prognostic factors for metastasis in squamous cell carcinoma of the skin. Dermatol Surg 2002;28:268-73.
- Tomsick RS, Menn H. Squamous cell carcinoma of the fingers treated with chemosurgery. South Med J 1984;77:1124-6. http://dx.doi.org/10.1097/00007611-198409000-00017.
- Mohs FE. Chemosurgery for skin cancer: fixed tissue and fresh tissue techniques. Arch Dermatol 1976;112:211-15. http://dx.doi.org/10.1001/archderm.1976.01630260033010.
- Skaria AM. Recurrence of basosquamous carcinoma after Mohs micrographic surgery. Dermatology 2010;221:352-5. http://dx.doi.org/10.1159/000320127.
- Thomas CJ, Wood GC, Marks VJ. Mohs micrographic surgery in the treatment of rare aggressive cutaneous tumors: the Geisinger experience. Dermatol Surg 2007;33:333-9.
- Mohs FE. Chemosurgery: microscopically controlled surgery for skin cancer – past, present and future. J Dermatol Surg Oncol 1978;4:41-54. http://dx.doi.org/10.1111/j.1524-4725.1978.tb00379.x.
- Mohs F, Larson P, Iriondo M. Micrographic surgery for the microscopically controlled excision of carcinoma of the external ear. J Am Acad Dermatol 1988;19:729-37. http://dx.doi.org/10.1016/S0190-9622(88)70229-7.
- Mohs FE. Micrographic surgery for the microscopically controlled excision of eyelid cancers. Arch Ophthalmol 1986;104:901-9. http://dx.doi.org/10.1001/archopht.1986.01050180135046.
- Stoll HL, Milgrom H, Traenkle HL. Results of roentgen therapy of carcinoma of the nose. Arch Dermatol 1964;90:577-80. http://dx.doi.org/10.1001/archderm.1964.01600060043007.
- Abbatucci JS, Boulier N, Laforge T, Lozier JC. Radiation therapy of skin carcinomas: results of a hypofractionated irradiation schedule in 675 cases followed more than 2 years. Radiother Oncol 1989;14:113-19. http://dx.doi.org/10.1016/0167-8140(89)90055-8.
- Grosch E, Lambert HE. The treatment of difficult cutaneous basal and squamous cell carcinomata with electrons. Br J Radiol 1979;52:472-7. http://dx.doi.org/10.1259/0007-1285-52-618-472.
- Podd TJ. Treatment of lower limb basal cell and squamous cell carcinomas with radiotherapy. Clin Oncol 1992;4:44-5. http://dx.doi.org/10.1016/S0936-6555(05)80773-3.
- Tsao MN, Tsang RW, Liu FF, Panzarella T, Rotstein L. Radiotherapy management for squamous cell carcinoma of the nasal skin: the Princess Margaret Hospital experience. Int J Radiat Oncol Biol Phys 2002;52:973-9. http://dx.doi.org/10.1016/S0360-3016(01)02752-3.
- Kwan W, Wilson D, Moravan V. Radiotherapy for locally advanced basal cell and squamous cell carcinomas of the skin. Int J Radiat Oncol Biol Phys 2004;60:406-11. http://dx.doi.org/10.1016/j.ijrobp.2004.03.006.
- Honeycutt WM, Jansen GT. Treatment of squamous cell carcinoma of the skin. Arch Dermatol 1973;108:670-2. http://dx.doi.org/10.1001/archderm.1973.01620260020006.
- Holmes ME, Bomford CK. The use of a short distance cobalt unit in the treatment of primary skin tumours. Br J Radiol 1982;55:225-8. http://dx.doi.org/10.1259/0007-1285-55-651-225.
- Hunter RD, Pereira DT, Pointon RC. Megavoltage electron beam therapy in the treatment of basal and squamous cell carcinomata of the pinna. Clin Radiol 1982;33:341-5. http://dx.doi.org/10.1016/S0009-9260(82)80285-7.
- Matthiesen C, Thompson JS, Forest C, Ahmad S, Herman T, Bogardus C. The role of radiotherapy for T4 non-melanoma skin carcinoma. J Med Imaging Radiat Oncol 2011;55:407-16. http://dx.doi.org/10.1111/j.1754-9485.2011.02277.x.
- Barysch MJ, Eggman N, Beyeler M, Panizzon RG, Seifert B, Dummer R. Long-term recurrence rate of large and difficult to treat cutaneous squamous cell carcinomas after superficial radiothrapy. Dermatology 2012;224:59-65. http://dx.doi.org/10.1159/000337027.
- Allan E, Stanton A, Pye D, Collins C, Perry L, Filby M, et al. Fractionated high dose rate brachytherapy moulds – a precise treatment for carcinoma of the pinna. Radiother Oncol 1998;48:277-81. http://dx.doi.org/10.1016/S0167-8140(98)00059-0.
- Rio E, Bardet E, Ferron C, Peuvrel P, Supiot S, Campion L, et al. Interstitial brachytherapy of periorificial skin carcinomas of the face: a retrospective study of 97 cases. Int J Radiat Oncol Biol Phys 2005;63:753-7. http://dx.doi.org/10.1016/j.ijrobp.2005.03.027.
- Lee JD, Park KK, Lee MG, Kim EH, Rhim KJ, Lee JT, et al. Radionuclide therapy of skin cancers and Bowen’s disease using a specially designed skin patch. J Nucl Med 1997;38:697-702.
- Svoboda VH, Kovarik J, Morris F. High dose-rate microselectron molds in the treatment of skin tumors. Int J Radiat Oncol Biol Phys 1995;31:967-72. http://dx.doi.org/10.1016/0360-3016(94)00485-4.
- Ashby MA, Pacella JA, Degroot R, Ainslie J. Use of a radon mould technique for skin cancer: results from the Peter MacCallum Cancer Institute (1975–84). Br J Radiol 1989;62:608-12. http://dx.doi.org/10.1259/0007-1285-62-739-608.
- Guix B, Finestres F, Tello J, Palma C, Martinez A, Guix J, et al. Treatment of skin carcinomas of the face by high-dose-rate brachytherapy and custom-made surface molds. Int J Radiat Oncol Biol Phys 2000;47:95-102. http://dx.doi.org/10.1016/S0360-3016(99)00547-7.
- Barrett TL, Greenway HT, Massullo V, Carlson C. Treatment of basal cell carcinoma and squamous cell carcinoma with perineural invasion. Adv Dermatol 1993;8:277-304.
- Cottel WI. Perineural invasion by squamous-cell carcinoma. J Dermatol Surg Oncol 1982;8:589-600. http://dx.doi.org/10.1111/j.1524-4725.1982.tb00317.x.
- DeAmbrosis K, De’Ambrosis B. Nonmelanoma skin cancer with perineural invasion: report of outcomes of a case series. Dermatol Surg 2010;36:133-8. http://dx.doi.org/10.1111/j.1524-4725.2009.01367.x.
- Geist DE, Garcia-Moliner M, Fitzek MM, Cho H, Rogers GS. Perineural invasion of cutaneous squamous cell carcinoma and basal cell carcinoma: raising awareness and optimizing management. Dermatol Surg 2008;34:1642-51. http://dx.doi.org/10.1111/j.1524-4725.2008.34341.x.
- Osguthorpe JD, Abel CG, Lang P, Hochman M. Neurotropic cutaneous tumors of the head and neck. Arch Otolaryngol Head Neck Surg 1997;123:871-6. http://dx.doi.org/10.1001/archotol.1997.01900080105013.
- Khan NA, Akhtar S, Kharadi MY, Andrabi WH, Darzi MA. Role of elective irradiation to drainage sites in squamous cell carcinoma of the skin trunk and extremities. JK Pract 1999;6:35-8.
- Lifeso RM, Rooney RJ, el-Shaker M. Post-traumatic squamous-cell carcinoma. J Bone Joint Surg Am 1990;72:12-8.
- Veness MJ, Quinn DI, Ong CS, Keogh AM, Macdonald PS, Cooper SG, et al. Aggressive cutaneous malignancies following cardiothoracic transplantation: the Australian experience. Cancer 1999;85:1758-64. http://dx.doi.org/10.1002/(SICI)1097-0142(19990415)85:8<1758::AID-CNCR16>3.0.CO;2-F.
- Reschly MJ, Shenefelt PD. Controversies in skin surgery: electrodessication and curettage versus excision for low-risk, small, well-differentiated squamous cell carcinomas. J Drugs Dermatol 2010;9:773-6.
- Tromovitch TA. Skin cancer; treatment by curettage and desiccation. Calif Med 1965;103:107-8.
- Whiting DA. Skin tumours in white South Africans. Part V. Treatment of skin tumours. S Afr Med J 1978;53:166-70.
- Williamson GS, Jackson R. Treatment of squamous cell carcinoma of the skin by electrodesiccation and curettage. Can Med Assoc J 1964;90:408-13.
- Kuflik EG. Cryosurgery for skin cancer: 30-year experience and cure rates. Dermatol Surg 2004;30:297-300. http://dx.doi.org/10.1097/00042728-200402002-00011.
- Fraunfelder FT, Zacarian SA, Limmer BL, Wingfield D. Cryosurgery for malignancies of the eyelid. Ophthalmology 1980;87:461-5. http://dx.doi.org/10.1016/S0161-6420(80)35207-X.
- Kuflik EG. Cryosurgical treatment for large malignancies on the upper extremities. J Dermatol Surg Oncol 1986;12:575-7. http://dx.doi.org/10.1111/j.1524-4725.1986.tb01954.x.
- Fontana AM, Muti E. Results with cryotherapy in skin tumours. Panminerva Med 1975;17:384-9.
- Nordin P, Stenquist B. Five-year results of curettage-cryosurgery for 100 consecutive auricular non-melanoma skin cancers. J Laryngol Otol 2002;116:893-8. http://dx.doi.org/10.1258/00222150260369390.
- Peikert JM. Prospective trial of curettage and cryosurgery in the management of non-facial, superficial, and minimally invasive basal and squamous cell carcinoma. Int J Dermatol 2011;50:1135-8. http://dx.doi.org/10.1111/j.1365-4632.2011.04969.x.
- Lindemalm-Lundstam B, Dalenbäck J. Prospective follow-up after curettage-cryosurgery for scalp and face skin cancers. Br J Dermatol 2009;161:568-76. http://dx.doi.org/10.1111/j.1365-2133.2009.09310.x.
- Holt PJ. Cryotherapy for skin cancer: results over a 5-year period using liquid nitrogen spray cryosurgery. Br J Dermatol 1988;119:231-40. http://dx.doi.org/10.1111/j.1365-2133.1988.tb03205.x.
- Graham GF, Clark LC. Statistical analysis in cryosurgery of skin cancer. Clin Dermatol 1990;8:101-7. http://dx.doi.org/10.1016/0738-081X(90)90072-9.
- Kennedy JC, Pottier RH, Pross DC. Photodynamic therapy with endogenous protoporphyrin IX: basic principles and present clinical experience. J Photochem Photobiol B, Biol 1990;6:143-8. http://dx.doi.org/10.1016/1011-1344(90)85083-9.
- Lui H, Salasche S, Kollias N, Wimberly J, Flotte T, McLean D, et al. Photodynamic therapy of nonmelanoma skin cancer with topical aminolevulinic acid: a clinical and histologic study. Arch Dermatol 1995;131:737-8. http://dx.doi.org/10.1001/archderm.131.6.737.
- Baptista J, Martinez C, Leite L, Cochito M. Our PDT experience in the treatment of non-melanoma skin cancer over the last 7 years. J Eur Acad Dermatol Venereol 2006;20:693-7. http://dx.doi.org/10.1111/j.1468-3083.2006.01574.x.
- Calzavara-Pinton PG. Repetitive photodynamic therapy with topical delta-aminolaevulinic acid as an appropriate approach to the routine treatment of superficial non-melanoma skin tumours. J Photochem Photobiol B, Biol 1995;29:53-7. http://dx.doi.org/10.1016/1011-1344(95)90253-8.
- Fink-Puches R, Soyer HP, Hofer A, Kerl H, Wolf P. Long-term follow-up and histological changes of superficial nonmelanoma skin cancers treated with topical delta-aminolevulinic acid photodynamic therapy. Arch Dermatol 1998;134:821-6. http://dx.doi.org/10.1001/archderm.134.7.821.
- Ziolkowski P, Osiecka BJ, Oremek G, Siewinski M, Symonowicz K, Saleh Y, et al. Enhancement of photodynamic therapy by use of aminolevulinic acid/glycolic acid drug mixture. J Exp Ther Oncol 2004;4:121-9.
- Calzavara-Pinton PG, Venturini M, Sala R, Capezzera R, Parrinello G, Specchia C, et al. Methylaminolaevulinate-based photodynamic therapy of Bowen’s disease and squamous cell carcinoma. Br J Dermatol 2008;159:137-44. http://dx.doi.org/10.1111/j.1365-2133.2008.08593.x.
- Haddad R, Nesher E, Weiss J, Skornick Y, Kashtan H. Photodynamic therapy for Bowen’s disease and squamous cell carcinoma of the skin. Photodiagnosis Photodyn Ther 2004;1:225-30. http://dx.doi.org/10.1016/S1572-1000(04)00048-1.
- Fritsch C, Goerz G, Ruzicka T. Photodynamic therapy in dermatology. Arch Dermatol 1998;134:207-14. http://dx.doi.org/10.1001/archderm.134.2.207.
- Harth Y, Hirshowitz B, Kaplan B. Modified topical photodynamic therapy of superficial skin tumors, utilizing aminolevulinic acid, penetration enhancers, red light, and hyperthermia. Dermatol Surg 1998;24:723-6. http://dx.doi.org/10.1111/j.1524-4725.1998.tb04240.x.
- Pennington DG, Waner M, Knox A. Photodynamic therapy for multiple skin cancers. Plast Reconstr Surg 1988;82:1067-71. http://dx.doi.org/10.1097/00006534-198812000-00021.
- Wolf P, Rieger E, Kerl H. Topical photodynamic therapy with endogenous porphyrins after application of 5-aminolevulinic acid. An alternative treatment modality for solar keratoses, superficial squamous cell carcinomas, and basal cell carcinomas?. J Am Acad Dermatol 1993;28:17-21. http://dx.doi.org/10.1016/0190-9622(93)70002-b.
- Feyh J, Goetz A, Müller W, Königsberger R, Kastenbauer E. Photodynamic therapy in head and neck surgery. J Photochem Photobiol B, Biol 1990;7:353-8. http://dx.doi.org/10.1016/1011-1344(90)85168-V.
- Kübler AC, Haase T, Staff C, Kahle B, Rheinwald M, Mühling J. Photodynamic therapy of primary nonmelanomatous skin tumours of the head and neck. Lasers Surg Med 1999;25:60-8. http://dx.doi.org/10.1002/(SICI)1096-9101(1999)25:1<60::AID-LSM8>3.0.CO;2-X.
- Moskalik K, Kozlow A, Demin E, Boiko E. Powerful neodymium laser radiation for the treatment of facial carcinoma: 5 year follow-up data. Eur J Dermatol 2010;20:738-42.
- Peris K, Micantonio T, Fargnoli MC, Lozzi GP, Chimenti S. Imiquimod 5% cream in the treatment of Bowen’s disease and invasive squamous cell carcinoma. J Am Acad Dermatol 2006;55:324-7. http://dx.doi.org/10.1016/j.jaad.2006.04.004.
- Ross AH, Kennedy CT, Collins C, Harrad RA. The use of imiquimod in the treatment of periocular tumours. Orbit 2010;29:83-7. http://dx.doi.org/10.3109/01676830903294909.
- Eklind J, Tartler U, Maschke J, Lidbrink P, Hengge UR. Imiquimod to treat different cancers of the epidermis. Dermatol Surg 2003;29:890-6.
- Flórez A, Feal C, de la Torre C, Cruces M. Invasive squamous cell carcinoma treated with imiquimod 5% cream. Acta Derm Venereol 2004;84:227-8.
- Konstantopoulou M, Lord MG, Macfarlane AW. Treatment of invasive squamous cell carcinoma with 5-percent imiquimod cream. Dermatol Online J 2006;12.
- Martín-García RF. Imiquimod: an effective alternative for the treatment of invasive cutaneous squamous cell carcinoma. Dermatol Surg 2005;31:371-4. http://dx.doi.org/10.1097/00042728-200503000-00024.
- Nouri K, O’Connell C, Rivas MP. Imiquimod for the treatment of Bowen’s disease and invasive squamous cell carcinoma. J Drugs Dermatol 2003;2:669-73.
- Oster-Schmidt C, Dirschka T. Therapy of cutaneous squamous cell carcinoma in two retirement home residents. J Dtsch Dermatol Ges 2005;3:705-8.
- Oster-Schmidt C. Two cases of squamous cell carcinoma treated with topical imiquimod 5%. J Eur Acad Dermatol Venereol 2004;18:93-5. http://dx.doi.org/10.1111/j.1468-3083.2004.00852.x.
- Kraus S, Miller BH, Swinehart JM, Shavin JS, Georgouras KE, Jenner DA, et al. Intratumoral chemotherapy with fluorouracil/epinephrine injectable gel: a nonsurgical treatment of cutaneous squamous cell carcinoma. J Am Acad Dermatol 1998;38:438-42. http://dx.doi.org/10.1016/S0190-9622(98)70502-X.
- Morse LG, Kendrick C, Hooper D, Ward H, Parry E. Treatment of squamous cell carcinoma with intralesional 5-Fluorouracil. Dermatol Surg 2003;29:1150-3.
- Hamouda B, Jamila Z, Najet R, Slim T, Rafiaa N, Noureddine B, et al. Topical 5-fluorouracil to treat multiple or unresectable facial squamous cell carcinomas in xeroderma pigmentosum. J Am Acad Dermatol 2001;44. http://dx.doi.org/10.1067/mjd.2001.113476.
- Litwin MS, Ryan RF, Ichinose H, Reed RR, Kremetz ET. Proceedings: Use of 5 fluorouracil in the topical therapy of skin cancer: a review of 157 patients. Proc Natl Cancer Conf 1972;7:549-61.
- Edwards L, Berman B, Rapini RP, Whiting DA, Tyring S, Greenway HT, et al. Treatment of cutaneous squamous cell carcinomas by intralesional interferon alfa-2b therapy. Arch Dermatol 1992;128:1486-9. http://dx.doi.org/10.1001/archderm.1992.01680210064008.
- Wickramasinghe L, Hindson TC, Wacks H. Treatment of neoplastic skin lesions with intralesional interferon. J Am Acad Dermatol 1989;20:71-4. http://dx.doi.org/10.1016/S0190-9622(89)70009-8.
- Ikić D, Padovan I, Pipić N, Cajkovac V, Kusić Z, Daković N, et al. Interferon reduces recurrences of basal cell and squamous cell cancers. Int J Dermatol 1995;34:58-60. http://dx.doi.org/10.1111/j.1365-4362.1995.tb04382.x.
- Kim KH, Yavel RM, Gross VL, Brody N. Intralesional interferon alpha-2b in the treatment of basal cell carcinoma and squamous cell carcinoma: revisited. Dermatol Surg 2004;30:116-20. http://dx.doi.org/10.1111/j.1524-4725.2004.30020.x.
- Skopinska M, Majeski S, Bollag W, Jablonska S. Calcitrol and isotretinoin combined therapy for precancerous and cancerous skin lesions. J Dermatolog Treat 1997;8:5-10. http://dx.doi.org/10.3109/09546639709160501.
- Levine N, Miller RC, Meyskens FL. Oral isotretinoin therapy. Use in a patient with multiple cutaneous squamous cell carcinomas and keratoacanthomas. Arch Dermatol 1984;120:1215-17. http://dx.doi.org/10.1001/archderm.1984.01650450097029.
- Göppner D, Nekwasil S, Franke I, Gollnick H, Leverkus M. Successful combination therapy of a locally advanced squamous cell carcinoma of the skin with cetuximab and γ-irradiation. J Dtsch Dermatol Ges 2010;8:826-8. http://dx.doi.org/10.1111/j.1610-0387.2010.07526.x.
- Fujisawa Y, Umebayashi Y, Ichikawa E, Kawachi Y, Otsuka F. Chemoradiation using low-dose cisplatin and 5-fluorouracil in locally advanced squamous cell carcinoma of the skin: a report of two cases. J Am Acad Dermatol 2006;55:81-5. http://dx.doi.org/10.1016/j.jaad.2005.12.035.
- Olieman AF, Liénard D, Eggermont AM, Kroon BB, Lejeune FJ, Hoekstra HJ, et al. Hyperthermic isolated limb perfusion with tumor necrosis factor alpha, interferon gamma, and melphalan for locally advanced nonmelanoma skin tumors of the extremities: a multicenter study. Arch Surg 1999;134:303-7. http://dx.doi.org/10.1001/archsurg.134.3.303.
- Sadek H, Azli N, Wendling JL, Cvitkovic E, Rahal M, Mamelle G, et al. Treatment of advanced squamous cell carcinoma of the skin with cisplatin, 5-fluorouracil, and bleomycin. Cancer 1990;66:1692-6. http://dx.doi.org/10.1002/1097-0142(19901015)66:8<1692::AID-CNCR2820660807>3.0.CO;2-Y.
- Sheen YS, Sheen MC, Sheu HM, Yang SF, Wang YW. Squamous cell carcinoma of the big toe successfully treated by intra-arterial infusion with methotrexate. Dermatol Surg 2003;29:982-3.
- Tantranond P, Balducci L, Karam F, Wang TY, Parker M, Hescock H, et al. Alternative management of cutaneous squamous cell carcinoma in an elderly man: report of a case and review of the literature. J Am Geriatr Soc 1992;40:510-12. http://dx.doi.org/10.1111/j.1532-5415.1992.tb02021.x.
- Chren MM, Linos E, Torres JS, Stuart SE, Parvataneni R, Boscardin WJ. Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol 2013;133:1188-96. http://dx.doi.org/10.1038/jid.2012.403.
- Jambusaria-Pahlajani A, Miller CJ, Quon H, Smith N, Klein RQ, Schmults CD. Surgical monotherapy versus surgery plus adjuvant radiotherapy in high-risk cutaneous squamous cell carcinoma: a systematic review of outcomes. Dermatol Surg 2009;35:574-85. http://dx.doi.org/10.1111/j.1524-4725.2009.01095.x.
- Gibbons E, Casañas i Comabella C, Fitzpatrick R. A structured review of patient-reported outcome measures for patients with skin cancer, 2013. Br J Dermatol 2013;168:1176-86. http://dx.doi.org/10.1111/bjd.12310.
- Bates AS, Davis CR, Takwale A, Knepil GJ. Patient-reported outcome measures in nonmelanoma skin cancer of the face: a systematic review. Br J Dermatol 2013;168:1187-94. http://dx.doi.org/10.1111/bjd.12269.
- Zwald FO, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part II. Management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol 2011;65:263-79. http://dx.doi.org/10.1016/j.jaad.2010.11.063.
- Dinehart SM, Pollack SV. Metastases from squamous cell carcinoma of the skin and lip. An analysis of twenty-seven cases. J Am Acad Dermatol 1989;21:241-8. http://dx.doi.org/10.1016/S0190-9622(89)70168-7.
- Rowe DE, Carroll RJ, Day CL. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol 1992;26:976-90. http://dx.doi.org/10.1016/0190-9622(92)70144-5.
- Brougham ND, Dennett ER, Cameron R, Tan ST. The incidence of metastasis from cutaneous squamous cell carcinoma and the impact of its risk factors. J Surg Oncol 2012;106:811-15. http://dx.doi.org/10.1002/jso.23155.
- Albrecht J, Meves A, Bigby M. A survey of case reports and case series of therapeutic interventions in the Archives of Dermatology. Int J Dermatol 2009;48:592-7. http://dx.doi.org/10.1111/j.1365-4632.2009.04031.x.
- Moses LE. The series of consecutive cases as a device for assessing outcomes of intervention. N Engl J Med 1984;311:705-10. http://dx.doi.org/10.1056/NEJM198409133111104.
- Abel U. Erkenntnisgewinn mittels nichtrandomisierter Therapiestudien-gaining understanding through nonrandomized therapeutic studies. Ellipse 1999;15:48-5.
- Jenicek M. Clinical Case Reporting in Evidence-based Medicine. London: Hodder Arnold Publication; 2001.
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. New York, NY: Springer-Verlag New York; 2010.
- Breuninger H, Brantsch K, Eigentler T, Häfner HM. Comparison and evaluation of the current staging of cutaneous carcinomas. J Dtsch Dermatol Ges 2012;10:579-86. http://dx.doi.org/10.1111/j.1610-0387.2012.07896.x.
- Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, Hwang WT, Gelfand JM, Whalen FM, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol 2013;149:402-10. http://dx.doi.org/10.1001/jamadermatol.2013.2456.
- Buethe D, Warner C, Miedler J, Cockerell CJ. Focus Issue on Squamous Cell Carcinoma: Practical Concerns Regarding the 7th Edition AJCC Staging Guidelines. J Skin Cancer 2011;2011. http://dx.doi.org/10.1155/2011/156391.
- Improving Outcomes for People with Skin Tumours Including Melanoma: The Manual. London: NICE; 2006.
- Eysenbach G. Improving the quality of Web surveys: the Checklist for Reporting Results of Internet E-Surveys (CHERRIES). J Med Internet Res 2004;6. http://dx.doi.org/10.2196/jmir.6.3.e34.
- Kellerman SE, Herold J. Physician response to surveys. A review of the literature. Am J Prev Med 2001;20:61-7. http://dx.doi.org/10.1016/S0749-3797(00)00258-0.
- Brown SJ, Lawrence CM. The management of skin malignancy: to what extent should we rely on clinical diagnosis?. Br J Dermatol 2006;155:100-3. http://dx.doi.org/10.1111/j.1365-2133.2006.07307.x.
- Ashby MA, Smith J, Ainslie J, McEwan L. Treatment of nonmelanoma skin cancer at a large Australian center. Cancer 1989;63:1863-71. http://dx.doi.org/10.1002/1097-0142(19900501)63:9<1863::AID-CNCR2820630934>3.0.CO;2-4.
- Alecu M, Ursaciuc C, Hãlãlãu F, Coman G, Merlevede W, Waelkens E, et al. Photodynamic treatment of basal cell carcinoma and squamous cell carcinoma with hypericin. Anticancer Res 1998;18:4651-4.
- Brodland DG, Zitelli JA. Surgical margins for excision of primary cutaneous squamous cell carcinoma. J Am Acad Dermatol 1992;27:241-8. http://dx.doi.org/10.1016/0190-9622(92)70178-I.
- Basal Cell Carcinoma, Squamous Cell Carcinoma (and Related Lesions) – A Guide to Clinical Management in Australia. Sydney, Australia; 2008.
- Hemington-Gorse SJ, Staiano JJ, Dhital SK, Fahmy FS, McGeorge DD, Juma AM. Adherence to the multiprofessional guidelines for the management of primary cutaneous squamous cell carcinoma: a re-audit of UK plastic surgeons. Eur J Plast Surg 2006;29:157-61. http://dx.doi.org/10.1007/s00238-006-0071-0.
- Staiano JJ, Juma AM, Dhital SK, McGeorge DD. Excision margin for cutaneous squamous cell carcinoma: is it standardised?. Eur J Plast Surg 2004;27:135-9. http://dx.doi.org/10.1007/s00238-004-0634-x.
- Essers BA, Dirksen CD, Nieman FH, Smeets NW, Krekels GA, Prins MH, et al. Cost-effectiveness of Mohs Micrographic Surgery vs Surgical Excision for Basal Cell Carcinoma of the Face. Arch Dermatol 2006;142:187-94.
- Wilson LS, Pregenzer M, Basu R, Bertenthal D, Torres J, Asgari M, et al. Fee comparisons of treatments for nonmelanoma skin cancer in a private practice academic setting. Dermatol Surg 2012;38:570-84. http://dx.doi.org/10.1111/j.1524-4725.2011.02231.x.
- Mosterd K, Krekels GA, Nieman FH, Ostertag JU, Essers BA, Dirksen CD, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol 2008;9:1149-56. http://dx.doi.org/10.1016/S1470-2045(08)70260-2.
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology . Basal Cell and Squamous Cell Skin Cancers 2013. www.nccn.org/professionals/physician_gls/PDF/nmsc.pdf (accessed March 2013).
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4. http://dx.doi.org/10.1245/s10434-010-0985-4.
- Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DC, et al. AJCC Cancer Staging Manual. New York, NY: Springer; 2002.
- Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. New York, NY: Springer; 2011.
- Brewster DH, Bhatti LA, Inglis JH, Nairn ER, Doherty VR. Recent trends in incidence of nonmelanoma skin cancers in the East of Scotland, 1992–2003. Br J Dermatol 2007;156:1295-300. http://dx.doi.org/10.1111/j.1365-2133.2007.07892.x.
- Schmults CD, Karia PS, Carter JB, Han J, Qureshi AA. Factors predictive of recurrence and death from cutaneous squamous cell carcinoma: a 10-year, single-institution cohort study. JAMA Dermatol 2013;149:541-7. http://dx.doi.org/10.1001/jamadermatol.2013.2139.
- Weinstock MA, Bogaars HA, Ashley M, Litle V, Bilodeau E, Kimmel S. Inaccuracies in certification of nonmelanoma skin cancer deaths. Am J Public Health 1992;82:278-81. http://dx.doi.org/10.2105/AJPH.82.2.278.
- Motley RJ, Preston PW, Lawrence CM. Multi-Professional Guidelines for the Management of the Patient With Primary Cutaneous Squamous Cell Carcinoma BAD 2008. www.bad.org.uk/library-media%5Cdocuments%5CSCC_2009.pdf (accessed November 2016).
- Slater D, Walsh M. Dataset for the Histological Reporting of Primary Cutaneous Squamous Cell Carcinoma and Regional Lymph Nodes (2nd Edition). London: RCPath; 2012.
- Moore BA, Weber RS, Prieto V, El-Naggar A, Holsinger FC, Zhou X, et al. Lymph node metastases from cutaneous squamous cell carcinoma of the head and neck. Laryngoscope 2005;115:1561-7. http://dx.doi.org/10.1097/01.mlg.0000173202.56739.9f.
- Veness MJ, Palme CE, Morgan GJ. High-risk cutaneous squamous cell carcinoma of the head and neck: results from 266 treated patients with metastatic lymph node disease. Cancer 2006;106:2389-96. http://dx.doi.org/10.1002/cncr.21898.
- Kyrgidis A, Tzellos TG, Kechagias N, Patrikidou A, Xirou P, Kitikidou K, et al. Cutaneous squamous cell carcinoma (SCC) of the head and neck: risk factors of overall and recurrence-free survival. Eur J Cancer 2010;46:1563-72. http://dx.doi.org/10.1016/j.ejca.2010.02.046.
- Watson JM, Torgerson DJ. Increasing recruitment to randomised trials: a review of randomised controlled trials. BMC Med Res Methodol 2006;6. http://dx.doi.org/10.1186/1471-2288-6-34.
- Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol 2005;23:3112-24. http://dx.doi.org/10.1200/JCO.2005.00.141.
- Pope C, Ziebland S, Mays N. Qualitative research in health care. Analysing qualitative data. BMJ 2000;320:114-16. http://dx.doi.org/10.1136/bmj.320.7227.114.
- Ritchie J, Spencer L, Bryman A, Burgess R. Analysing Qualitative Data. London: Routledge; 1993.
- Wright L, Bramwell R. A qualitative study of older people’s perceptions of skin cancer. Health Educ J 2001;60:256-64. http://dx.doi.org/10.1177/001789690106000307.
- Referral Guidelines for Suspected Cancer. NICE; 2005.
- Bedlow AJ, Cliff S, Melia J, Moss SM, Seyan R, Harland CC. Impact of skin cancer education on general practitioners’ diagnostic skills. Clin Exp Dermatol 2000;25:115-18. http://dx.doi.org/10.1046/j.1365-2230.2000.00590.x.
- Chen SC, Bravata DM, Weil E, Olkin I. A comparison of dermatologists’ and primary care physicians’ accuracy in diagnosing melanoma: a systematic review. Arch Dermatol 2001;137:1627-34. http://dx.doi.org/10.1001/archderm.137.12.1627.
- Goulart JM, Quigley EA, Dusza S, Jewell ST, Alexander G, Asgari MM, et al. Skin cancer education for primary care physicians: a systematic review of published evaluated interventions. J Gen Intern Med 2011;26:1027-35. http://dx.doi.org/10.1007/s11606-011-1692-y.
- Shariff Z, Roshan A, Williams AM, Platt AJ. 2-Week wait referrals in suspected skin cancer: does an instructional module for general practitioners improve diagnostic accuracy?. Surgeon 2010;8:247-51. http://dx.doi.org/10.1016/j.surge.2010.03.004.
- Girgis A, Sanson-Fisher RW, Howe C, Raffan B. A skin cancer training programme: evaluation of a postgraduate training for family doctors. Med Educ 1995;29:364-71. http://dx.doi.org/10.1111/j.1365-2923.1995.tb00027.x.
- Catt S, Langridge C, Fallowfield L, Talbot DC, Jenkins V. Reasons given by patients for participating, or not, in Phase 1 cancer trials. Eur J Cancer 2011;47:1490-7. http://dx.doi.org/10.1016/j.ejca.2011.02.020.
- Garcea G, Lloyd T, Steward WP, Dennison AR, Berry DP. Differences in attitudes between patients with primary colorectal cancer and patients with secondary colorectal cancer: is it reflected in their willingness to participate in drug trials?. Eur J Cancer Care (Engl) 2005;14:166-70. http://dx.doi.org/10.1111/j.1365-2354.2005.00535.x.
- Featherstone K, Donovan JL. Random allocation or allocation at random? Patients’ perspectives of participation in a randomised controlled trial. BMJ 1998;317:1177-80. http://dx.doi.org/10.1136/bmj.317.7167.1177.
- Jenkins V, Farewell D, Batt L, Maughan T, Branston L, Langridge C, et al. The attitudes of 1066 patients with cancer towards participation in randomised clinical trials. Br J Cancer 2010;103:1801-7. http://dx.doi.org/10.1038/sj.bjc.6606004.
- Kaur G, Hutchison I, Mehanna H, Williamson P, Shaw R, Tudur Smith C. Barriers to recruitment for surgical trials in head and neck oncology: a survey of trial investigators. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2013-002625.
- Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol 1999;52:1143-56. http://dx.doi.org/10.1016/S0895-4356(99)00141-9.
- Jenkins V, Fallowfield L. Reasons for accepting or declining to participate in randomized clinical trials for cancer therapy. Br J Cancer 2000;82:1783-8. http://dx.doi.org/10.1054/bjoc.2000.1142.
- McCulloch P, Taylor I, Sasako M, Lovett B, Griffin D. Randomised trials in surgery: problems and possible solutions. BMJ 2002;324:1448-51. http://dx.doi.org/10.1136/bmj.324.7351.1448.
- Hamilton DW, de Salis I, Donovan JL, Birchall M. The recruitment of patients to trials in head and neck cancer: a qualitative study of the EaStER trial of treatments for early laryngeal cancer. Eur Arch Otorhinolaryngol 2013;270:2333-7. http://dx.doi.org/10.1007/s00405-013-2349-8.
- Locock L, Smith L. Personal benefit, or benefiting others? Deciding whether to take part in clinical trials. Clin Trials 2011;8:85-93. http://dx.doi.org/10.1177/1740774510392257.
- Jenkins V, Farewell V, Farewell D, Darmanin J, Wagstaff J, Langridge C, et al. TTT Steering committee. Drivers and barriers to patient participation in RCTs. Br J Cancer 2013;108:1402-7. http://dx.doi.org/10.1038/bjc.2013.113.
- McMurdo ME, Roberts H, Parker S, Wyatt N, May H, Goodman C, et al. Improving recruitment of older people to research through good practice. Age Ageing 2011;40:659-65. http://dx.doi.org/10.1093/ageing/afr115.
- Karia PS, Jambusaria-Pahlajani A, Harrington DP, Murphy GF, Qureshi AA, Schmults CD. Evaluation of American Joint Committee on Cancer, International Union Against Cancer, and Brigham and Women’s Hospital tumor staging for cutaneous squamous cell carcinoma. J Clin Oncol 2013;32:327-34. http://dx.doi.org/10.1200/JCO.2012.48.5326.
- Rhee JS, Matthews BA, Neuburg M, Logan BR, Burzynski M, Nattinger AB. Validation of a quality-of-life instrument for patients with nonmelanoma skin cancer. Arch Facial Plast Surg 2006;8:314-18. http://dx.doi.org/10.1001/archfaci.8.5.314.
- Management of Primary Cutaneous Squamous Cell Carcinoma. Edinburgh: SIGN; 2014.
- NICE . Improving Outcomes for People With Skin Tumours, Including Melanoma: Evidence Update October 2011 2011. http://arms.evidence.nhs.uk/resources/hub/517121/attachment (accessed 22 July 2016).
- Ormerod AD, Thomas KS, Craig FE, Mitchell E, Greenlaw N, Norrie J, et al. Comparison of the two most commonly used treatments for pyoderma gangrenosum: results of the STOP GAP randomised controlled trial. BMJ 2015;350. http://dx.doi.org/10.1136/bmj.h2958.
- Binus AM, Qureshi AA, Li VW, Winterfield LS. Pyoderma gangrenosum: a retrospective review of patient characteristics, comorbidities and therapy in 103 patients. Br J Dermatol 2011;165:1244-50. http://dx.doi.org/10.1111/j.1365-2133.2011.10565.x.
- Langan SM, Groves RW, Card TR, Gulliford MC. Incidence, mortality, and disease associations of pyoderma gangrenosum in the United Kingdom: a retrospective cohort study. J Invest Dermatol 2012;132:2166-70. http://dx.doi.org/10.1038/jid.2012.130.
- Patient Information Leaflet on Pyoderma Gangrenosum. London: BAD; 2013.
- Brooklyn TN, Dunnill MG, Shetty A, Bowden JJ, Williams JD, Griffiths CE, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut 2006;55:505-9. http://dx.doi.org/10.1136/gut.2005.074815.
- Reichrath J, Bens G, Bonowitz A, Tilgen W. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol 2005;53:273-83. http://dx.doi.org/10.1016/j.jaad.2004.10.006.
- Ruocco E, Sangiuliano S, Gravina AG, Miranda A, Nicoletti G. Pyoderma gangrenosum: an updated review. J Eur Acad Dermatol Venereol 2009;23:1008-17. http://dx.doi.org/10.1111/j.1468-3083.2009.03199.x.
- Capella GL, Frigerio E, Fracchiolla C, Altomare G. The simultaneous treatment of inflammatory bowel diseases and associated pyoderma gangrenosum with oral cyclosporin A. Scand J Gastroenterol 1999;34:220-1.
- Friedman S, Marion JF, Scherl E, Rubin PH, Present DH. Intravenous cyclosporine in refractory pyoderma gangrenosum complicating inflammatory bowel disease. Inflamm Bowel Dis 2001;7:1-7. http://dx.doi.org/10.1097/00054725-200102000-00001.
- Matis WL, Ellis CN, Griffiths CE, Lazarus GS. Treatment of pyoderma gangrenosum with cyclosporine. Arch Dermatol 1992;128:1060-4. http://dx.doi.org/10.1001/archderm.1992.01680180054005.
- Elgart G, Stover P, Larson K, Sutter C, Scheibner S, Davis B, et al. Treatment of pyoderma gangrenosum with cyclosporine: results in seven patients. J Am Acad Dermatol 1991;24:83-6. http://dx.doi.org/10.1016/0190-9622(91)70016-U.
- Vidal D, Puig L, Gilaberte M, Alomar A. Review of 26 cases of classical pyoderma gangrenosum: clinical and therapeutic features. J Dermatolog Treat 2004;15:146-52. http://dx.doi.org/10.1080/09546630410031909.
- Vidal D, Alomar A. Successful treatment of periostomal pyoderma gangrenosum using topical tacrolimus. Br J Dermatol 2004;150:387-8. http://dx.doi.org/10.1111/j.1365-2133.2003.05804.x.
- Craig FF, Thomas KS, Mitchell EJ, Williams HC, Norrie J, Mason JM, et al. UK Dermatology Clinical Trials Network’s STOP GAP trial (a multicentre trial of prednisolone versus ciclosporin for pyoderma gangrenosum): protocol for a randomised controlled trial. Trials 2012;13. http://dx.doi.org/10.1186/1745-6215-13-51.
- University of Nottingham . Centre of Evidence Based Dermatology: Pyoderma Gangrenosum Study (STOP GAP) n.d. www.stopgaptrial.co.uk (accessed 22 July 2016).
- Kantor J, Margolis DJ. A multicentre study of percentage change in venous leg ulcer area as a prognostic index of healing at 24 weeks. Br J Dermatol 2000;142:960-4. http://dx.doi.org/10.1046/j.1365-2133.2000.03478.x.
- Cardinal M, Phillips T, Eisenbud DE, Harding K, Mansbridge J, Armstrong DG. Nonlinear modeling of venous leg ulcer healing rates. BMC Dermatol 2009;9. http://dx.doi.org/10.1186/1471-5945-9-2.
- Foss CE, Clark AR, Inabinet R, Camacho F, Jorizzo JL. An open-label pilot study of alefacept for the treatment of pyoderma gangrenosum. J Eur Acad Dermatol Venereol 2008;22:943-9. http://dx.doi.org/10.1111/j.1468-3083.2008.02680.x.
- Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ 1998;316:736-41. http://dx.doi.org/10.1136/bmj.316.7133.736.
- NHS Reference Costs: Financial Year 2011 to 2012. London: Department of Health; 2012.
- Health and Social Care Information Centre . Prescription Cost Analysis – England, 2012 n.d. www.hscic.gov.uk/catalogue/PUB10610 (accessed 22 July 2016).
- Thomas KS, Ormerod AD, Craig FE, Greenlaw N, Norie J, Mitchell E, et al. Clinical outcomes and response of patients applying topical therapy for pyoderma gangrenosum: a prospective cohort study. J Am Acad Dermatol 2016;75:940-9. http://dx.doi.org/10.1016/j.jaad.2016.06.016.
- Brooklyn T, Dunnill G, Probert C. Diagnosis and treatment of pyoderma gangrenosum. BMJ 2006;333:181-4. http://dx.doi.org/10.1136/bmj.333.7560.181.
- Marzano AV, Trevisan V, Lazzari R, Crosti C. Pyoderma gangrenosum: study of 21 patients and proposal of a ‘clinicotherapeutic’ classification. J Dermatolog Treat 2011;22:254-60. http://dx.doi.org/10.3109/09546631003686069.
- Lyon CC, Stapleton M, Smith AJ, Mendelsohn S, Beck MH, Griffiths CE. Topical tacrolimus in the management of peristomal pyoderma gangrenosum. J Dermatolog Treat 2001;12:13-7. http://dx.doi.org/10.1080/095466301750163518.
- Joly P, Roujeau JC, Benichou J, Delaporte E, D’Incan M, Dreno B, et al. A comparison of two regimens of topical corticosteroids in the treatment of patients with bullous pemphigoid: a multicenter randomized study. J Invest Dermatol 2009;129:1681-7. http://dx.doi.org/10.1038/jid.2008.412.
- Dyer MT, Goldsmith KA, Sharples LS, Buxton MJ. A review of health utilities using the EQ-5D in studies of cardiovascular disease. Health Qual Life Outcomes 2010;8. http://dx.doi.org/10.1186/1477-7525-8-13.
- UK Dermatology Clinical Trials Network’s STOP GAP Trial Team . Recruitment into Trials of Rare Conditions – Experiences from the STOP GAP Trial n.d.
- Rice SA, Woo PN, El-Omar E, Keenan RA, Ormerod AD. Topical tacrolimus 0.1% ointment for treatment of cutaneous Crohn’s Disease. BMC Res Notes 2013;6. http://dx.doi.org/10.1186/1756-0500-6-19.
- Craig F, Thomas K, Layfield C, Ingram J, Muller S, Ismail MA, et al. Management of pyoderma gangrenosum by UK dermatologists: a pilot study to inform a trial. Br J Dermatol 2009;161:4-5. http://dx.doi.org/10.1111/j.1365-2133.2009.09123.x.
- International Federation of Dermatology Clinical Trial Networks . Global Collaboration in Dermatology Research n.d. www.ifdctn.org/ifdctn/index.aspx (accessed 22 July 2016).
- Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8060.
- Danby SG, Chalmers J, Brown K, Williams HC, Cork MJ. A functional mechanistic study of the effect of emollients on the structure and function of the skin barrier. Br J Dermatol 2016. http://dx.doi.org/10.1111/bjd.14684.
- NHS Choices . Atopic Eczema - Treatment 2014. www.nhs.uk/Conditions/Eczema-(atopic)/Pages/Treatment.aspx (accessed 25 July 2016).
- NICE Clinical Knowledge Summaries . Eczema - Atopic n.d. http://cks.nice.org.uk/eczema-atopic (accessed 25 July 2016).
- Eczema Care Pathway. London: Royal College of Paediatrics and Child Health; 2011.
- Cox H, Lloyd K, Williams H, Arkwright PD, Brown T, Clark C, et al. Emollients, education and quality of life: the RCPCH care pathway for children with eczema. Arch Dis Child 2011;96:i19-24. http://dx.doi.org/10.1136/archdischild-2011-300695.
- Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol 2014;71:116-32. http://dx.doi.org/10.1016/j.jaad.2014.03.023.
- Dirven-Meijer PC, De Kock CA, Nonneman MGM, Van Sleeuwen D, De Witt-de Jong AWF, Burgers JS, et al. NHG Guideline Eczema. Huisarts Wet 2014;57:240-52.
- NICE . Improving Outcomes for People With Skin Tumours Including Melanoma Relating to the Management of Low Risk Basal Cell Carcinomas (BCCs) in the Community n.d. www.nice.org.uk/guidance/csg8 (accessed 25 July 2016).
- NHS Choices . Skin Cancer Module n.d. www.nhs.uk/conditions/Cancer-of-the-skin/Pages/Introduction.aspx (accessed 25 July 2016).
- Karimkhani C, Boyers LN, Prescott L, Welch V, Delamere FM, Nasser M, et al. Global burden of skin disease as reflected in Cochrane Database of Systematic Reviews. JAMA Dermatol 2014;150:945-51. http://dx.doi.org/10.1001/jamadermatol.2014.709.
- McCulloch P, Altman DG, Campbell WB, Flum DR, Glasziou P, Marshall JC, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet 2009;374:1105-12. http://dx.doi.org/10.1016/S0140-6736(09)61116-8.
- Schadt C. Pyoderma Gangrenosum: Treatment and Prognosis n.d. www.uptodate.com/contents/pyoderma-gangrenosum-treatment-and-prognosis (accessed 22 July 2016).
- Society for Academic Primary Care Special Interest Group for Dermatology n.d. www.sapc.ac.uk/special-interest-group/dermatology (accessed 3 October 2016).
- Karimkhani C, Boyers LN, Margolis DJ, Naghavi M, Hay RJ, Williams HC, et al. Comparing cutaneous research funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases with 2010 Global Burden of Disease results. PLOS ONE 2014;9. http://dx.doi.org/10.1371/journal.pone.0102122.
- Chalmers I, Bracken MB, Djulbegovic B, Garattini S, Grant J, Gülmezoglu AM, et al. How to increase value and reduce waste when research priorities are set. Lancet 2014;383:156-65. http://dx.doi.org/10.1016/S0140-6736(13)62229-1.
- Equator Network . Enhancing the QUAlity and Transparency Of Health Research n.d. www.equator-network.org (accessed 17 February 2014).
- University of Nottingham . Centre of Evidence-Based Dermatology: Training DVD on Use of Hand-Held, NB-UVB Light at Home n.d. www.nottingham.ac.uk/research/groups/cebd/videos/training-vitiligo.aspx (accessed 17 February 2014).
- Magrini N, Smith R. Alessandro Liberati. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e1101.
- University of Nottingham . Prevention and Treatment of Skin Disease: Setting Priorities and Reducing Uncertainties n.d. www.nottingham.ac.uk/research/groups/cebd/documents/annual-reports/uon-prevention-and-treatment-aw-forweb.pdf (accessed 25 July 2016).
- Cork MJ, Elliot P. Causes and treatment of childhood eczema. Exchange (Quarterly Journal of the National Eczema Society) 2002;105:14-5.
Appendix 1 Characteristics of included Cochrane and non-Cochrane reviews
| Review topic, type of review | Review title, authors, last assessed as up to date | Number of studies, pooled sample size (range) | Population | Intervention | Comparison | Outcomes for which data are reported |
|---|---|---|---|---|---|---|
| Breastfeeding, Cochrane review | Optimal duration of exclusive breastfeeding, Kramer MS, Kakuma R, 200655 | 22, 10,168, (26–3483) | Lactating mothers and their healthy, term, singleton infants | Exclusive breastfeeding for at least 3 months | Continued exclusive breastfeeding or mixed breastfeeding (other liquid or solid foods) | Child outcomes: growth, infections, morbidity, mortality, micronutrient status, neuromotor and cognitive development, asthma, AE, other allergic diseases, diabetes, blood pressure and subsequent chronic diseases. Maternal outcomes: postpartum weight loss, duration of lactational amenorrhoea and chronic diseases |
| Diet, non-Cochrane review | Omega 3 and 6 oils for primary prevention of allergic disease: systematic review and meta-analysis, Anandan C, Nurmatov U, Sheikh A, 200860 | 6, 1337, (65–516) | Pregnant or lactating women and/or infants without an existing allergic condition, at high and low risk of developing eczema/AD, allergic rhinitis, asthma and/or other allergic disorders | Omega 3 and omega 6 fatty acid supplementation either alone or in combination | Placebo | Eczema/AD, asthma, allergic rhinitis, food allergy, skin prick tests, total IgE levels, disease severity, lung function, neonatal cytokines, plasma fatty acids |
| Formula, Cochrane review | Formula containing hydrolysed protein for prevention of, allergy and food intolerance in infants, Osborn DA, Sinn JKH, 200656 | 18, 7680, (16–5317) | Infants in the first 6 months of life without clinical evidence of allergy | Early short-term hydrolysed formula or prolonged use of a hydrolysed formula (any type) | Human milk or cow’s milk | Primary: all allergic diseases and food intolerance. Secondary: asthma, AD/eczema, allergic rhinitis, cow’s milk or soy protein allergy or intolerance, food allergy or intolerance, urticaria and anaphylaxis. Harms: growth, cost and infant feed refusal |
| Formula, Cochrane review | Soy formula for prevention of allergy and food intolerance in infants, Osborn DA, Sinn J, 200659 | 3, 772, (50–487) | Infants in the first 6 months of life without clinical evidence of, allergy or food intolerance | Soy formula | Human milk, cow’s milk or hydrolysed formula | Primary: all allergic diseases and food intolerance. Secondary: growth and cost |
| Maternal dietary exclusions, Cochrane review | Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child, Kramer MS, Kakuma R, 201255 | All studies: 4, prevention only: 3, all studies: 417 (17–210), prevention only:, 400 (26–210) | Pregnant or lactating women at high risk for giving birth to an atopic child based on history of atopic disease (eczema, asthma or hay fever), and lactating mothers of infants with established AE | Diet with exclusion or reduced quantity of potentially antigenic foods (cow’s milk, egg, peanuts, fish and chocolate) | Standard diet | Primary: occurrence and severity of atopic disease in the child. Secondary: nutritional status of the mother and fetus, other pregnancy outcomes, positive skin prick test to ingested antigens and cord blood IgE levels |
| Prebiotics, Cochrane review | Prebiotics in infants for prevention of allergic disease and food hypersensitivity, Osborn DA, Sinn JKH, 200757 | 7, 837, (30–259) | Infants in the first 6 months of life without clinical evidence of, allergic disease or food hypersensitivity, with and without risk factors for allergic disease and food hypersensitivity | Any prebiotics added to human milk or infant formula | Control (placebo or no treatment) or a different prebiotic | Primary: all allergic diseases and food hypersensitivity. Secondary: asthma, dermatitis/eczema, allergic rhinitis, cow’s milk or soy protein hypersensitivity or allergy, food allergy, urticaria and anaphylaxis. Harms: growth, costs, infant feed refusal and infection |
| Probiotics, Cochrane review | Probiotics in infants for prevention of allergic disease and food hypersensitivity, Osborn DA, Sinn JK, 200758 | 12, 2974, (62–1223) | Enterally fed infants in the first 6 months of life without clinical, evidence of allergic disease or food hypersensitivity, with and without risk factors for allergy and food hypersensitivity | Any probiotics added to human milk or infant formula, with or without added prebiotics | Control (placebo or no treatment) or a different probiotic | Primary: all allergic diseases and food hypersensitivity. Secondary: asthma, dermatitis/eczema, allergic rhinitis, cow’s milk or soy protein hypersensitivity or allergy, food allergy, urticaria and anaphylaxis. Harms: growth, cost, infant feed refusal and infection with probiotic bacteria |
| Comparison | Number of participants (trials) | RR (95% CI) | I 2 |
|---|---|---|---|
| Exclusive breastfeeding for at least 6 months vs. introduction of solids at 3–6 months | 3731 (2) | 0.75 (0.42 to 1.32) | 61% |
| Hydrolysed formula vs. cow’s milk formula (prolonged feeding) | 1478 (8) | 0.87 (0.70 to 1.08) | 0% |
| Extensively hydrolysed formula vs. cow’s milk formula (prolonged feeding) | 912 (3) | 0.84 (0.58 to 1.23) | 19% |
| Partially hydrolysed formula vs. cow’s milk formula (prolonged feeding) | 823 (7) | 0.92 (0.72 to 1.17) | 0% |
| Extensively hydrolysed formula vs. partially hydrolysed formula (prolonged feeding) | 1061 (4) | 0.88 (0.73 to 1.05) | 0% |
| Hydrolysed formula vs. human milk (early short-term feeding) | 90 (1) | 0.48 (0.05 to 4.41) | – |
| Hydrolysed formula vs. cow’s milk formula (early short-term feeding) | 77 (1) | 0.34 (0.04 to 3.15) | – |
| Soy formula vs. cow’s milk formula | 744 (3) | 1.23 (0.99 to 1.53) | 0% |
| Maternal antigen avoidance vs. standard diet | 360 (3) | 0.95 (0.63 to 1.44) | 21% |
| Omega 3 fatty acid supplementation vs. placebo | 664 (3) | 1.10 (0.78 to 1.54) | 45% |
| Omega 6 fatty acid supplementation vs. placebo | 259 (2) | 0.80 (0.56 to 1.16) | 0% |
| Prebiotic vs. no prebiotic | 432 (2) | 0.79 (0.21 to 2.94) | 80% |
| Prebiotic vs. other prebiotica | 150 (1) | 0.22 (0.07 to 0.76)b | – |
| Probiotic vs. no probioticc | 1492 (6) | 0.85 (0.66 to 1.08) | 46% |
| Risk level | Comparison | Number of participants (trials) | RR (95% CI) | I 2 |
|---|---|---|---|---|
| High risk | Exclusive breastfeeding for at least 6 months vs. introduction of solids at 3–6 months | 135 (1) | 0.40 (0.21 to 0.78)a | – |
| Hydrolysed formula vs. cow’s milk formula (prolonged feeding) | 2558 (8) | 0.86 (0.70 to 1.06) | 0% | |
| Extensively hydrolysed formula vs. cow’s milk formula (prolonged feeding) | 1726 (3) | 0.83 (0.58 to 1.21) | 19% | |
| Partially hydrolysed formula vs. cow’s milk formula (prolonged feeding) | 1361 (7) | 0.90 (0.71 to 1.15) | 0% | |
| Extensively hydrolysed formula vs. partially hydrolysed formula (prolonged feeding) | 1865 (4) | 0.89 (0.74 to 1.07) | 0% | |
| Soy formula vs. cow’s milk formula | 461 (1) | 1.20 (0.95 to 1.52) | – | |
| Maternal antigen avoidance vs. standard diet | 360 (3) | 0.95 (0.63 to 1.44) | 21% | |
| Omega 3 fatty acid supplementation vs. placebo | 83 (1) | 1.49 (0.84 to 2.63) | – | |
| Omega 6 fatty acid supplementation vs. placebo | 259 (2) | 0.80 (0.56 to 1.16) | 0% | |
| Prebiotic vs. no prebiotic | 206 (1) | 0.42 (0.21 to 0.84)b | – | |
| Probiotic vs. no probiotic | 1420 (4) | 0.86 (0.66 to 1.12) | 58% | |
| Not selected for risk | Exclusive vs. mixed breastfeeding for 3–7 months | 3483 (1) | 1.00 (0.60 to 1.69) | – |
| Prebiotic vs. no prebiotic | 226 (1) | 1.62 (0.62 to 4.26) | – | |
| Prebiotic vs. other prebioticc | 150 (1) | 0.22 (0.07 to 0.76)d | – | |
| Probiotic vs. no probiotic | 72 (1) | 0.63 (0.21 to 1.89) | – |
| Risk level | Comparison | Number of participants (trials) | RR (95% CI) | I 2 |
|---|---|---|---|---|
| High risk | Exclusive breastfeeding for at least 6 months vs. introduction of solids at 3–6 months | 113 (1) | 0.97 (0.50 to 1.89) | – |
| Hydrolysed formula vs. cow’s milk formula (prolonged feeding) | 950 (2) | 0.74 (0.40 to 1.38) | 40% | |
| Extensively hydrolysed formula vs. cow’s milk formula (prolonged feeding) | 651 (1) | 0.86 (0.63 to 1.17) | – | |
| Partially hydrolysed formula vs. cow’s milk formula (prolonged feeding) | 510 (2) | 0.75 (0.37 to 1.51) | 46% | |
| Extensively hydrolysed formula vs. partially hydrolysed formula (prolonged feeding) | 661 (1) | 0.92 (0.67 to 1.26) | – | |
| Soy formula vs. cow’s milk formula | 283 (2) | 1.51 (0.74 to 3.10) | 18% | |
| Omega 3 fatty acid supplementation vs. placebo | 516 (1) | 0.85 (0.62 to 1.18) | – | |
| Not selected for risk | Hydrolysed formula vs. human milk (early short-term feeding) | 90 (1) | 0.48 (0.05 to 4.41) | – |
| Hydrolysed formula vs. cow’s milk formula (early short-term feeding) | 77 (1) | 0.34 (0.04 to 3.15) | – | |
| Omega 3 fatty acid supplementation vs. placebo | 65 (1) | 1.24 (0.79 to 1.95) | – |
| Comparison | Outcome | Number of participants (trials) | RR (95% CI) | I 2 |
|---|---|---|---|---|
| Exclusive breastfeeding for at least 6 months vs. introduction of solids at 3–6 months | Positive skin prick test (unspecified) at 6 years | 331 (1) | 0.99 (0.73 to 1.35) | – |
| Maternal antigen avoidance vs. standard diet | Positive skin prick test to egg at 2 years | 335 (2) | 0.95 (0.52 to 1.74) | 0% |
| Positive skin prick test to milk at 2 years | 335 (2) | 0.86 (0.16 to 4.59) | 13% | |
| Omega 3 fatty acid supplementation vs. placebo | Positive skin prick test for ‘any atopy’a | 560 (2) | 0.92 (0.76 to 1.11) | NR |
| Positive skin prick test to house dust mites | 560 (2) | 1.04 (0.81 to 1.34) | NR |
| Comparison | Adverse event | Number of participants (trials) | RR (95% CI) | I 2 |
|---|---|---|---|---|
| Exclusive breastfeeding for at least 6 months vs. introduction of solids at 3–6 months | Death in first 12 months | 3483 (1) | 2.30 (0.21 to 25.37) | – |
| Hydrolysed formula vs. cow’s milk formula (prolonged feeding) | Feeding problems | 141 (1) | 2.18 (0.49 to 9.68) | – |
| Refusal to drink formula | 46 (1) | 7.62 (0.43 to 133.78) | – | |
| Extensively hydrolysed formula vs. cow’s milk formula (prolonged feeding) | Feeding problems | 96 (1) | 1.38 (0.24 to 7.89) | – |
| Partially hydrolysed formula vs. cow’s milk formula (prolonged feeding) | Feeding problems | 91 (1) | 3.07 (0.65 to 14.40) | – |
| Extensively hydrolysed vs. partially hydrolysed formula (prolonged feeding) | Feeding problems | 95 (1) | 0.45 (0.12 to 1.69) | – |
| Maternal antigen avoidance vs. standard diet | Preterm birth | 236 (2) | 10.06 (0.52 to 192.26) | NA |
| Prebiotic vs. no prebiotic | Feed intolerance leading to formula discontinuation | 226 (1) | 1.81 (0.82 to 3.99) | – |
| Prebiotic vs. other prebiotica | Feed intolerance leading to formula discontinuation | 150 (1) | 1.73 (0.82 to 3.67) | – |
| Probiotic vs. no probiotic | Sepsis | 367 (1) | 0.63 (0.39 to 1.04) | – |
| Spitting up at 1 month of age | 188 (1) | 1.88 (1.03 to 3.45)b | – | |
| Spitting up at 2 months of age | 188 (1) | 1.69 (1.02 to 2.80)b | – | |
| Gastrointestinal problems at 1 month of age | 188 (1) | 1.47 (0.63 to 3.43) | – | |
| Gastrointestinal problems at 2 months of age | 188 (1) | 0.59 (0.22 to 1.55) | – | |
| Gastrointestinal problems in first 12 months | 188 (1) | 0.93 (0.54 to 1.60) | – | |
| Necrotising enterocolitis and/or death | 145 (1) | 0.35 (0.15 to 0.83)c | – |
Appendix 2 Search strings for MEDLINE and The Cochrane Library databases
MEDLINE
-
exp Dermatitis, Atopic/
-
exp Asthma/ep, et
-
(eczem$ or (atop$ adj3 dematit$)).mp. [mp=title, original title, abstract, name of substance word, subject heading word]
-
2 and 3
-
1 or 4
-
exp Cohort Studies/
-
exp Incidence/
-
6 and 5
-
5 and 7
-
8 or 9
-
exp Primary Prevention/
-
5 and 11
-
10 or 12
-
limit 13 to (“all infant (birth to 23 months)” or “preschool child (2 to 5 years)” or “child (6 to 12 years)”)
-
limit 14 to yr=“1980 - current“
The Cochrane Library
-
(eczem$ or (atop$ adj3 dematit$)).mp. [mp=title, original title, abstract, mesh headings, heading words, keyword]
-
(cohort$ or longitud$ or prospect$ or follow$ or incidence).mp.
-
1 and 2
-
((eczem$ or (atop$ adj3 dematit$)) adj7 prevent$).mp. [mp=title, original title, abstract, mesh headings, heading words, keyword]
-
4 or 3
Appendix 3 Skin care guidelines for intervention group
The diagrams on page 410 have been reproduced with permission from Cork and Elliot. 778
Appendix 4 EMBASE search strategy
-
random$.mp.
-
factorial$.mp.
-
(crossover$ or cross-over$).mp.
-
placebo$.mp. or PLACEBO/
-
(doubl$ adj blind$).mp.
-
(singl$ adj blind$).mp.
-
(assign$ or allocat$).mp.
-
volunteer$.mp. or VOLUNTEER/
-
Crossover Procedure/
-
Double Blind Procedure/
-
Randomized Controlled Trial/
-
Single Blind Procedure/
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12
-
exp Dermatitis, Atopic/
-
atopic dermatitis.mp.
-
atopic eczema.mp.
-
exp NEURODERMATITIS/
-
neurodermatitis.mp.
-
infantile eczema.mp.
-
childhood eczema.mp.
-
(besnier$ and prurigo).mp.
-
eczema.mp. or exp Eczema/
-
21 or 17 or 20 or 15 or 14 or 22 or 18 or 16 or 19
-
23 and 13
Appendix 5 Questionnaire on international outcomes
Round 1: international outcomes consensus for vitiligo – e-Delphi exercise
(1) In your opinion how import are the following outcomes to be included in the core outcomes set for future vitiligo trials?
| Outcome | Very important | Important | Less important | Not important | Not sure | |
|---|---|---|---|---|---|---|
| 1. | Repigmentation | |||||
| 2. | Cosmetically acceptability of the results | |||||
| 3. | Global assessment of the disease/overall impact of the disease | |||||
| 4. | Quality of life | |||||
| 5. | Maintenance of gained repigmentation | |||||
| 6. | Cessation of spreading of vitiligo | |||||
| 7. | Side effects and harms | |||||
| 8. | Self-esteem | |||||
| 9. | Psychological impact |
(2) Are there any additional outcomes that you perceive to be important and should be included in the core outcomes set for vitiligo? (Please list)
| 1. | |
| 2. | |
| 3. |
(3) Do you have any further comments about the core outcomes set for future vitiligo trials?
(4) Please indicate if you are:
-
Patient with vitiligo (or carer)
-
Dermatologist
-
Nurses
-
General Practitioners
-
Representative of regulatory body (which one?)
-
Journal editor (which one?)
-
Other (please specify)
Round 2: international outcomes consensus for vitiligo – e-Delphi exercise
(1) In your opinion how import are the following outcomes to be included in the core outcomes set for future vitiligo trials?
| Outcome | Very important | Important | Less important | Not important | Not sure | |
|---|---|---|---|---|---|---|
| 1. | Repigmentation | |||||
| 2. | Cosmetically acceptability of the results (including time required to achieve it) | |||||
| 3. | Quality of life | |||||
| 4. | Maintenance of gained repigmentation | |||||
| 5. | Cessation of spreading of vitiligo | |||||
| 6. | Side effects and harms | |||||
| 7. | Tolerability/burden of treatment | |||||
| 8. | Economic impact of treatment |
(2) Do you have any further comments about the core outcomes set for future vitiligo trials?
(3) Please indicate if you are:
-
Patient with vitiligo (or carer)
-
Dermatologist
-
Nurse
-
General Practitioner
-
Representative of regulatory body (which one?)
-
Journal editor (which one?)
-
Other (please specify)
(4) Please indicate which country you represent:
Appendix 6 Incomplete excision after surgical excision
Summary of studies reporting completeness of SCC excision
| First author, year | n | Site | Proportion incompletely excised (95% CI) | Prognostic features | Excision margin |
|---|---|---|---|---|---|
| Ang 2004555 | 63 | All | 0.16 (0.08 to 027) | Mean diameter 19.7mm | R; 4- to 6-mm margin |
| Baker 2001541 | 227 | Head and neck | 0.07 (0.04 to 0.11) | No data | R; margin NS |
| Bogdanov-Berezovsky 2005562 | 369 | All | 0.07 (0.04 to 0.10) | Complete excised: diameter 1.1 cm; thickness 0.6 cm; differentiation: 84.6% well; moderate 13.6%; poor 5.6% Incomplete excised: diameter 0.9 cm; depth 0.4 cm; differentiation: well 72.2%; moderate 22.2%; poor 5.6% |
R; 3- to 6-mm margin |
| Bovill 2009563 | 676 | All | 0.18 (0.15 to 0.21) | Re-excision cohort: mean diameter 17.2 mm; mean thickness 6.02 mm; differentiation: well 43%; moderate 42%; poor 15%; PNI 3.6% | R; margin NS |
| Griffiths 2002544 | 86 | All | 0.04 (0.01 to 0.11) | Median thickness 3.1 mm. Mean diameter 13 mm (surviving pts) vs. 20 mm (DoD) 3.2% immunocompromised | R; mean margin 7.2 mm (surviving) vs. 6.3 mm (DoD) |
| Mourouzis 2009553 | 218 | Head and neck | 0.12 (0.08 to 0.17) | Diameter: 63%< 2 cm; 33.8% 2–4 cm; 3.2% > 4 cm. Differentiation: 78.2% well; 21.8% poor. PNI in 2.3%; PVI in 0.5% | R; 5-mm margin |
| Nemet 2006546 | 68 | Periocular | 0.25 (0.15 to 0.37) | Differentiation: 86.8% well; 13.2% moderate | R; 5-mm margin |
| Pua 2009559 | 69 | All | 0.00 (0.00 to 0.05) | Diameter: < 1 cm to ≥ 2cm | R; 4 mm (‘wider’ for larger tumours) |
| Tan 2007561 | 480 | All | 0.06 (0.04 to 0.09) | Diameter: 52.3% < 1 cm; 40.8% < 2 cm; 33% > 2cm | P; margin 2 mm to > 10mm |
| Thomas 1994550 | 54 | Pinna | 0.11 (0.04 to 0.23) | 52% stage I; 37% stage II; 0% stage III; 11% stage IV. Different: 68.5% well; 9.3% moderate; 7.4% poor | R; wedge excision to complex surgical procedure |
| Thomas 2003560 | 26 | All | 0.00 (0.00 to 0.13) | Average diameter: 16.9 mm 26% > 10 mm. Differentiation: 57.8% well; 38.5% moderate; 3.8% poor | P; margin based on diagnosis and surgeon’s preference |
Appendix 7 Recurrence and death from disease after Mohs micrographic surgery
Summary of studies reporting local recurrence, regional recurrence, distant metastases, unspecified recurrence or death from disease after excision of squamous cell skin cancer by Mohs micrographic surgery
| First author, year | n | Site | Follow-up (range) | Proportion with outcome (95% CI) | Prognostic features | ||||
|---|---|---|---|---|---|---|---|---|---|
| LR | RR | DM | UR | DoD | |||||
| Anderson 1982564 | 8 | Eyelid | Average 36 months (1–57) | 0.00 (0.00 to 0.37) | 0.12 (0.00 to 0.53) | 0.12 (0.00 to 0.53) | – | 0.12 (0.00 to 0.53) | No details |
| Brantsch 2008569 | 615 | All | Mean 43 months (1–165) | 0.03 (0.02 to 0.04) | 0.04 (0.03 to 0.06) | – | – | 0.01 (0.01 to 0.03) | Median diameter 15 mm: mean thickness 3 mm: differentiation – 53% good; 22% moderate; 25% poor; desmoplasia 8%: Immunosuppression 5% |
| Cherpelis 2002573 | 186 | Various | 6 months to 10 years | – | 0.08 (0.04 to 0.12) | – | – | – | Diameter: 20%> 20 mm: 28% Clark level V: 9.5% poorly differentiated: 4% PNI |
| Dzubow 1982570 | 171 | All | Mean 18.6 months (1–136) | 0.04 (0.01 to 0.07) | – | – | – | – | Diameter: 18.7% 1–9 mm; 68.7% 10–49 mm; 12.6% > 50mm |
| Leibovitch 2005568 | 229 | All | 5 years | 0.03 (0.01 to 0.06) | – | 0.00 (0.00 to 0.02) | – | – | Diameter: 25.6% < 1 cm; 46.2% 1–1.9 cm; 15.4% 2–2.9 cm; 6.6% 3–3.9 cm; 2.3% 4–4.9 cm; 1% 5–5.9 cm; 0.5% 6–7.9 cm; 0.1% 8–10 cm. Differentiation: 34.8% well; 36.4% moderate; 6% poor; 10.4% acantholytic |
| Malhotra 2004572 | 56 | Periocular | Mean 77.3 months | 0.04 (0.00 to 0.12) | – | – | – | – | Diameter: 57% 0–0.9 cm; 38% 1–1.9 cm; 5% 2–2.9 cm. PNI in 4.3% |
| Mohs 1976575 | 615 | Various | Up to 5 years | – | – | – | 0.01 (0.00 to 0.02) | – | No data |
| Pugliano-Mauro 2010565 | 231 | High risk | Mean 3.9 years | 0.01 (0.00 to 0.04) | 0.02 (0.00 to 0.04) | – | – | 0.00 (0.00 to 0.02) | ‘High risk’. Diameter average 1.5 cm ± 0.7 cm. Immunosuppressed: 20% |
| Silapunt 2005571 | 87 | Auricle | 2 years | 0.06 (0.02 to 0.13) | – | 0.00 (0.00 to 0.04) | – | 0.00 (0.00 to 0.04) | Average surface area: 3.04 cm2 |
| Skaria 2010576 | 54 | Not specified | Mean 59.6 months | – | – | – | 0.02 (0.00 to 0.10) | – | No data |
| Thomas 2007577 | 66 | Various | Mean 45 months | – | –. | – | 0.04 (0.01 to 0.13) | – | Mean size: 3.9 cm2 |
| Tomsick 1984574 | 15 | Fingers | 5 months to 7 years | – | – | – | 0.00 (0.00 to 0.22) | – | 3 patients with SCC in area of chronic radiodermatitis |
| Turner 2000567 | 48 | All | Mean 3.4 years | 0.02 (0.00 to 0.11) | 0.04 (0.01 to 0.14) | 0.02 (0.00 to 0.11) | – | – | Median diameter 15 mm (3–40 mm). Median depth 2 mm (0.4–25 mm). Differentiation: grade 1 – 37.2%; grade 2 – 44.2%; grade 3 – 14%; grade 4 – 4.6%. Vascular invasion – 16% |
| Van der Eerden 2010545 | 74 | Head and neck | Mean 24 months | 0.03 (0.00 to 0.09) | 0.01 (0.00 to 0.07) | – | – | – | No data |
| Vuyk 2001566 | 53 | Head and neck | Mean 33 months | 0.02 (0.00 to 0.10) | – | – | – | – | Poorly to well differentiated |
| Yoon 1992558 | 16 | External ear | 6 months to 20 years | – | – | – | 0.31 (0.11 to 0.59) | – | Stages 0–IV (only data for stages I–II included) |
Appendix 8 Recurrence and death after external radiotherapy
Summary of studies reporting local recurrence, regional recurrence, unspecified recurrence or death from disease after treatment of squamous cell skin cancer with external radiotherapy
| First author, year | n | Site | RT dose | Follow-up | Proportion with outcome (95% CI) | Prognostic features | |||
|---|---|---|---|---|---|---|---|---|---|
| LR | RR | UR | DoD | ||||||
| Abbatucci 1989582 | 179 | Face | Superficial, 0.5–1-cm peripheral margin, 1-mm deep margin. Most 30.6 Gy, three fractions, 14 days | Minimum 2 years | 0.03 (0.01 to 0.06) | – | – | – | Diameter < 1.6 cm to ≥ 4 cm |
| Barysch 2012591 | 177 | All (head and neck 87%) | Superficial Beryllium-windowed soft X-rays | Mean 4.9 years (SD 4.7, 95% CI 4.2 to 5.6) | 0.14 (0.10 to 0.20) | 0.01 (0.00 to 0.03) | – | – | Mean area 3.5cm2. Differentiation: 66.7% good; 22.4% moderate; 10.9% poor. |
| Grosch 1979583 | 5 | Head and hand | 6–10 meV electron beam. Total dose 4000–6000 R, 10–20 fractions, 14–28 days | Mean 15 months (6–33 months) | – | – | 0.00 (0.00 to 0.52) | – | No data |
| Holmes 1982588 | 67 | Various | Short distance Cobalt unit. 5000–5500 cGy, 10–15 fractions, 2–3 weeks | 2–8 years | – | – | 0.00 (0.00 to 0.05) | 0.00 (0.00 to 0.05) | No data |
| Honeycutt 1973587 | 18 | Various | X-rays, total dose 4500R, 9 or 15 fractions | 4 years (4–8 years) | – | – | 0.00 (0.00 to 0.18) | – | 39% < 2 cm; 61% > 2-cm diameter |
| Hunter 1982589 | 26 | Pinna | 10meV electron beam. Total dose 4500–5500 cGy, 8–15 daily fractions | Average 44 months (12–136 months) | – | – | 0.11 (0.02 to 0.30) | 0.08 (0.01 to 0.25) | Mean duration 26 months (1–186 months) |
| Knox 1967554 | 101 | All | Total dose 4000–5000 R, 500 R or 1000 R every other day, up to 10 fractions | > 1 year | – | – | 0.02 (0.00 to 0.07) | – | 79% < 2 cm; 21% > 2-cm diameter |
| Kwan 2004586 | 37 | Various | Orthovolt X-rays, or electrons, or megavoltage photons, or combination of electrons and photons. Total dose < 4000 cGy-> 6000 cGy, 5–25 fractions | Median 42 months (1.4–97.1 months) | 0.30 (0.16 to 0.47) (‘locoregional recurrence’) | – | – | – | T2 – 19%; T3 – 65%; T4 – 16% |
| Matthiesen 2011590 | 3 | Cheek/forehead | 3D conformal RT with 6 mV photons or intensity modulated RT with 6-mV photons. Total dose 7425–7980 cGy, 33–35 fractions | Mean 14.3 months (4–36 months) | – | – | 0.67 (0.09 to 0.99) | 0.67 (0.09 to 0.99) | All T4. Volumes: 126 cm3, 175 cm3, 341 cm3. Bone involvement in one |
| Podd 1992584 | 17 | Lower leg | Photon based. Dose not specified | Not specified | 0.06 (0.00 to 0.29) | – | – | – | No data |
| Rank 1973542 | 231 | Not spec | No details | 2 years | 0.03 (0.01 to 0.06) | – | – | – | No data |
| Shiffman 1975547 | 2 | Pinna | No details | < 1 year to > 3 years | 0.5 (0.01 to 0.99) | 0.5 (0.01 to 0.99) | – | 0.5 (0.01 to 0.99) | One SCC 2–4 cm (no data for second) |
| Stoll 1964581 | 62 | Nose | Roentgen therapy 4000, up to 8000 R in 300 R to 500 R fractions over up to 26 days | < 6 months to 144 months | 0.03 (0.00 to 0.11) | – | – | – | Diameter: < 0.5 cm 33.9%; 0.5–1 cm 56.4%; 1.5–2.5 cm 4.8%; > 2.5 cm 4.8% |
| Tsao 2002585 | 93 | Nose | Orthovoltage (81%); electrons (14%), megavoltage X-rays (4%); high-energy photons (1%) | Median 2.9 years (0.2–10.4 years) | 0.06 (0.02 to 0.14) | 0.02 (0.00 to 0.08) | – | 0.03 (0.01 to 0.09) | T1 64%; T2 11.7%; T3 0%; T4 7.4%; Tx 17%. Five immunosuppressed patients |
Appendix 9 Local recurrence after brachytherapy
Summary of studies reporting local recurrence after treatment of squamous cell skin cancer by brachytherapy
| First author, year | n | Site | Brachytherapy modality and dose | Follow-up | Proportion with LR (95% CI) | Prognostic features | Study design |
|---|---|---|---|---|---|---|---|
| Allan 1998592 | 3 | Pinna | HDR Microselectron® (Nucletron International, Munich, Germany)192Ir plane or mould. 42.5–45 Gy/eight fractions | Minimum 18 months | 0.00 (0.00 to 0.71) | Confined to skin of pinna | P |
| Ashby 1989596 | 48 | Any (head and neck 33%) | Radon mould 35–40 Gy. Overall treatment time 6 days 20 hours | 45.3 months (1–146 months) | 0.04 (0.01 to 0.14) | Median tumour volume 1099 mm3 (16–6300 mm3). Differentiation: 84% well; 16% moderate | R |
| Guix 2000597 | 18 | Facial | HDR custom-made 192Ir surface mould 60–65 Gy/33–36 fractions (< 4 cm diameter) or boosted to 75–80 Gy after 3-week pause (> 4 cm diameter) | Minimum 12 months | 0.06 (0.00 to 0.27) | Diameter (all SCCs): < 2 cm 23.5%; 2–5 cm 73.5%; 5–8 cm 3%. PNI: 5.8%. Lymphatic invasion 14.7% | P |
| Lee 1997594 | 3 | Scalp, neck and face | 166Ho impregnated patch for total 30 minutes to 1 hour. 50 Gy | 8–20 months | 0.00 (0.00 to 0.71) | Selected superficial tumours only | P |
| Rio 2005593 | 5 | Facial | Interstitial brachytherapy with 192Ir wires. Average dose 50–65 Gy, mean implantation time 79 hours | Median 55 months (6–132 months) | 0.00 (0.00 to 0.52) | Lip carcinomas excluded | R |
| Svodoba 1995595 | 11 | Any | HDR 192Ir after loaded moulds. 12–50 Gy/1–15 fractions | Average 9.6 months (5–22 months) | 0.00 (0.00 to 0.28) | Area < 0.5cm2 to > 6.1cm2 | P |
Appendix 10 Adjunctive radiotherapy studies and pooled estimates of outcomes after adjuvant radiotherapy
Summary of studies reporting local recurrence, regional recurrence, distant metastases or death from disease after surgical treatment of squamous cell skin cancer plus adjuvant radiotherapy
| First author, year | n | Reasons for ART | Surgical treatment | Site of ART (local/regional) | Dose of ART | Follow-up |
|---|---|---|---|---|---|---|
| Barrett 1993598 | 3 | Head and neck PNI (asymptomatic) | Surgical excision or MMS | Not specified | Mean 51.7 Gy/18–22 fractions | Mean 28.3 months (18–37 months) |
| Cottel 1982599 | 2 | Head and neck PNI both symptomatic (infraorbital and supraorbital nerves) – ‘most difficult cases’ selected for ART | MMS | Primary site and course of involved cranial nerve | 4600–5000 rads (200 rads/day over 4.5–6 weeks) | Mean 30 months (24–36 months) for ART |
| DeAmbrosis 2010600 | 6 | Head and neck PNI, nerve diameter 0.15–0.4 mm (all asymptomatic) but indications for RT inconsistent | Excision | Not specified | Not specified | Mean 104.8 months (44–218 months) |
| Geist 2008601 | 7 | Head and neck PNI (all incidental) | MMS | Tumour bed and first echelon lymphatics and course of involved nerve | Mean dose 57.9 Gy (52–66 Gy)/20–33 fractions | Mean 10.4 months (4–20 months) |
| Khan 1999603 | 26 | No specific reasons. Prospective cohort with SCC > 2 cm diameter | Excision | Elective irradiation of draining lymph nodes | Total 45 Gy/20 fractions | Up to 12 months |
| Lifeso 1990604 | 11 | Unclear | Amputation or WLE | Regional lymph nodes | 4500 rads/20 fractions/4 weeks | Mean 37 months (24–86 months) |
| Osguthorpe 1997602 | 4 | Head and neck PNI supraorbital, infraorbital and buccal nerves – regional lymphatic or perivascular spread, neural spread on multiple nerves from primary tumour site, extension through bony foramen so requiring extended resection | MMS with or without intracranial clearance | Not specified | Mean 56.2 Gy (50–65 Gy) | 49.5 months (6–99 months) |
| Shiffman 1975547 | 4 | Not specified | Surgery | Not specified | Not specified | < 1 year to > 3 years |
| Veness 1999605 | 6 | Cardiothoracic transplant patients who developed ‘aggressive cutaneous malignancies’ but not specified how patients were selected to have ART | WLE | Not specified | Mean dose 52 Gy | Mean 25.8 months (8–54 months) |
| Intervention | Local recurrence (95% CI/I2) (%) | Regional recurrence (95% CI/I2) (%) | Distant metastases (95% CI/I2) (%) | Died from disease (95% CI/I2) (%) |
|---|---|---|---|---|
| ART for PNIa | 18.2 (3.9 to 39.8/37) (n = 22) | 8.3 (1.1 to 21.4/0)(n = 22) | 11.5 (2.4 to 26.1/1)(n = 22) | 11.1 (0.4 to 33.1/45)(n = 20) |
| ART for other types of SCCb | 11.1 (2.4 to 25.0/35) (n = 47) | 8.5 (2.5 to 17.6/0)(n = 47) | 3.2 (0.1 to 10.4/9)(n = 47) | 13.9 (0.05 to 50.2/74) (n = 21) |
Appendix 11 Recurrence after curettage and electrodesiccation
Summary of studies reporting squamous cell skin cancer recurrence after treatment by curettage and electrodesiccation
| First author, year | n | Site | Follow-up | Proportion with recurrence (95% CI) | Prognostic features | P/R |
|---|---|---|---|---|---|---|
| Knox 1967554 | 545 | Various | > 1 year | 0.00 (0.00 to 0.01) | Diameter: 91% < 2 cm; 9% > 2cm | R |
| Honeycutt 1973587 | 281 | Various | 4–8 years | 0.01 (0.00 to 0.03) | Diameter: 94% < 2 cm; 6% > 2cm | R |
| Reschly 2010606 | 120 | Exposed body surface excluding lip and ear | 13–33 months | 0.00 (0.00 to 0.03) [triple cycle] 0.00 (0.00 to 0.23) [double cycle] | Diameter: < 2 cm | R |
| Shiffman 1975547 | 15 | Pinna | < 1 year to > 3 years | 0.20 (LR) 0.07 (RR) | Diameter: < 2 cm 59.6%; 2–4 cm 28.8%; > 4 cm 3.8%. Invasion of cartilage 21.1% | R |
| Tromovitch 1965607 | 29 | NS | Average 6.8 years (minimum 5 years) | 0.03 (0.00 to 0.18) | No data | R |
| Werlinger 2002557 | 56 | Various | Mean 4.1 years | 0.04 (0.00 to 0.12) | No separate EDC data | R |
| Whiting 1978608 | 47 | No data | 6–12 months then ‘thereafter as necessary’ | 0.04 (0.01 to 0.14) | No data | R |
| Williamson 1964609 | 53 | Various | 5 years | 0.04 (0.00 to 0.13) | Diameter: < 2 cm 60.4%; > 2 cm 39.6% | R |
Appendix 12 Included cryotherapy studies
Summary of studies reporting SCC recurrence after treatment by cryotherapy
| First author, year | n | Site | Follow-up | Proportion with recurrence (95% CI) | Prognostic features | P/R |
|---|---|---|---|---|---|---|
| Fontana 1975613 | 7 | Unspecified | 32 months to 5 years | 0.00 (0.00 to 0.41) | No data | R |
| Fraunfelder 1980611 | 21 | Periocular | Average 21.6 months | 0.00 (0.00 to 0.16) | Diameter: < 10 mm 71%; 29% 11–20+mm | P |
| Graham 1990618 | 563 | All | Unspecified | ‘Cure’ (not defined) 97.3% | Diameter: 25% < 5 mm; 56.4% 6–12 mm; 15.5% 13–24 mm; 3.1% > 24 mm | R |
| Holt 1988617 | 34 | All | Range 6 months to 5.5 years | 0.03 (0.00 to 015) | Diameter: > 2 cm 14.7%. Indeterminate margins, tethering, deeply invasive and SCCs of external ear involving underlying cartilage excluded | P |
| Kuflik 1986612 | 5 | Arms and hands | Range 12 months to 6 years | 0.00 (0.00 to 0.52) | Diameter: all 2–5cm | P |
| Kuflik 2004610 | 134 | All | 5 years | 0.00 (0.00 to 0.03) | Diameter: 3–5 mm. ‘Only cases amenable to cryotherapy were treated’ | R |
| Lindemalm-Lundstam 2009616 | 53 | Face and scalp | Mean 42 months | 0.00 (0.00 to 0.07) | Diameter: mean 8 mm (range 1–76 mm). Lesions in area circumscribed by upper lip and nasolabial folds excluded | P |
| Nordin 2002614 | 13 | External ear | Up to 10 years (8/13 at least 5 years) | 0.00 (0.00 to 0.25) | Diameter: mean 18 mm (range 5–70 mm) | P |
| Peikert 2011615 | 6 | Below neck | 5 years | 0.00 (0.00 to 0.46) | Diameter: all < 2 cm. No invasion beyond papillary dermis. Spindle cell and poorly differentiated SCCs excluded | P |
Appendix 13 Photodynamic therapy regimes
Summary of photodynamic therapy regimes used in studies reporting outcomes after SCC treatment by photodynamic therapy
| Study | n | Photosensitiser/occlusion time | Light source, irradiance, dose | n treatments | Prognostic features |
|---|---|---|---|---|---|
| Topical photosensitiser | |||||
| Baptista 2006621 | 4 | Topical 20% ALA/4–6 hours | 630 nm for 1000 seconds at 100 mW/cm2. Total dose 100 J/cm2 | Up to 5 | No data |
| Calzavara-Pinton 1995622 | 18 | Topical 20% ALA/6–8 hours | 630 nm for 10–15 minutes until slight pain or burning stopped at 100 mW/cm.2 Total dose 60–80 J/cm2 | Every other day until area eroded without clinically evident tumour, or stopped when no further improvement after 2 further treatments | Diameter: superficial SCCs median 18 mm (range 12–45 mm); nodular median 15 mm (range 5–25 mm) |
| Calzavara-Pinton 2008625 | 71 | Topical 160 mg/g MAL/3 hours | 635 nm ± 18 nm at 37 J/cm2 at irradiance of 86 mW/cm2 | 2, 7 days apart | Diameter: mean 20 mm (range 15–30 mm). Depth: 56% Clark level II; 44% Clark level III/IV |
| Fink-Puchs 1998623 | 35 | Topical 20% ALA oil-in-water/4 hours | Either unfiltered full-spectrum visible light OR filtered light of > 515 nm, or > 570 nm or > 610 nm for 5–30 minutes. Median total light dose 61 J/cm2 | 1 | Diameter: 1.6–6 cm. Depth: confined to papillary dermis |
| Fritsch 1998627 | 36 | Topical 20% ALA/4–6 hours | Incoherent light source 570–750 nm for 20 minutes. 80 mW/cm2 for superficial SCCs up to 150 mW/cm2 for nodular and exulcerated SCCs. 96–180 J/cm2 | Maximum of 3 (1 month apart) | Diameter: 0.5–3.1 cm. 28 described as ‘superficial’ (not defined) |
| Haddad 2004626 | 43 | Topical 20% ALA/16 hours | Non-laser light at 580–720 nm and 1250–1600 nm for 10 minutes at 100 J/cm2 | 1–3 | Diameter: mean 1.4 ± 0.8 cm (range 1–3 cm) |
| Harth 1998628 | 5 | Modified topical 20% ALA plus 2% EDTA and 2% DMSO/12 hours | Red light (585–720 nm) at 150 mW/cm2 and near infrared at 1.25–1.6 mm) at 50 mW/cm2 for 10–15 minutes | 1–3 | ‘Superficial’ |
| Kennedy 1990619 | 8 | Topical 20% ALA/3–6 hours | Filtered light > 600 nm at 150–300 mW/cm2 for 3.5–30 minutes. Total dose 15–150 mW/cm2 | Repeated weekly for 2 elevated SCCs | 6 early invasive, 2 elevated |
| Lui 1995620 | 2 | Topical 20% ALA/3 hours | Red light at 19–44 mW/cm.2 Total dose 100 J/cm2 | 1 | Diameter: > 5mm |
| Wolf 1993630 | 6 | Topical 20% ALA oil in water/4–8 hours | Unfiltered light at 100 mW/cm2 for 15 minutes, or red light at 100 mW/cm2 for 30 minutes. Total dose 90 J/cm2 | Unclear | Diameter: 1–6 cm. Depth: all early invasive |
| Ziokolwski 2004624 | 23 | Group 1: topical 20% ALA + 5% DMSO + 5% EDTA 4 hours occlusion Group 2: topical 20% ALA + 5% DMSO + 5% EDTA + glycolic acid 4 hours occlusion |
650 nm ± 30 nm light at 100 mW/cm.2 Total dose 85–87.6 J/cm2 | Up to 3 sessions | Diameter: group 1 – 2–7 mm; group 2 – 2–9 mm |
| Systemic photosensitiser | |||||
| Feyh 1990631 | 5 | Systemic haematoporphyrin derivative 2 hours prior to PDT. Dose not specified | 630 nm laser light at 100 mw/cm.2 Dose 100 J/cm2 | – | All T1 |
| Kubler 1999632 | 9 | Meta-tetrahydroxy-phenylchlorin (mTHPC) at 0.15mg/kg i.v. 96 hours pre-PDT | 652 nm red light at 100 mW/cm.2 Total dose 20 J/cm2 | – | No data |
| Pennington 1988629 | 32 | Systemic haematoporphyrin derivative 5mg/kg i.v. bolus 3 days prior to PDT | 630 nm coherent light at 30 J/cm2 | – | Estimated maximal thickness < 1cm |
Appendix 14 Example survey
Appendix 15 Participant information sheet
Appendix 16 Incomplete excision after surgical excision
Appendix 17 Baseline case report form
Appendix 18 Week 6 case report form
Appendix 19 Patient diary
Appendix 20 The STOP GAP trial: all patients with at least one adverse reaction
| Condition | Total (N = 112), n (%) | Ciclosporin (N = 59), n (%) | Prednisolone (N = 53), n (%) |
|---|---|---|---|
| Any | 75 (66.96) | 40 (67.80) | 35 (66.04) |
| Blood and the lymphatic system disorders | |||
| Any | 7 (6.25) | 2 (3.39) | 5 (9.43) |
| Anaemia | 2 (1.79) | 2 (3.39) | 0 (0.00) |
| Thrombocytopenia | 1 (0.89) | 1 (1.69) | 0 (0.00) |
| Leucocytosis | 5 (4.46) | 0 (0.00) | 5 (9.43) |
| Other blood and the lymphatic system disorder | 2 (1.79) | 0 (0.00) | 2 (3.77) |
| Endocrine disorders | |||
| Any | 5 (4.46) | 1 (1.69) | 4 (7.55) |
| Menstrual disturbances | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Gynaecomastia | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Cushings syndrome | 1 (0.89) | 0 (0.00) | 1 (1.89) |
| Hirsutism | 1 (0.89) | 1 (1.69) | 0 (0.00) |
| Diabetes | 3 (2.68) | 0 (0.00) | 3 (5.66) |
| Other endocrine disorder | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Metabolism and nutrition disorders | |||
| Any | 6 (5.36) | 1 (1.69) | 5 (9.43) |
| Hyperlipidaemia | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Hyperuricaemia | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Hyperkalaemia | 1 (0.89) | 1 (1.69) | 0 (0.00) |
| Hypomagnesaemia | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Hyperglycaemia | 5 (4.46) | 0 (0.00) | 5 (9.43) |
| Alcohol intolerance | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Other metabolism and nutrition disorder | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Nervous system disorders | |||
| Any | 20 (17.86) | 12 (20.34) | 8 (15.09) |
| Tremor | 7 (6.25) | 5 (8.47) | 2 (3.77) |
| Headache | 5 (4.46) | 5 (8.47) | 0 (0.00) |
| Paraethesia | 2 (1.79) | 2 (3.39) | 0 (0.00) |
| Euphoria | 3 (2.68) | 0 (0.00) | 3 (5.66) |
| Mood swings | 1 (0.89) | 0 (0.00) | 1 (1.89) |
| Depression | 3 (2.68) | 1 (1.69) | 2 (3.77) |
| Personality changes | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Insomnia | 1 (0.89) | 0 (0.00) | 1 (1.89) |
| Psychosis | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Seizures | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Vertigo | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Other nervous system disorder | 5 (4.46) | 2 (3.39) | 3 (5.66) |
| Gastrointestinal disorders | |||
| Any | 28 (25.00) | 17 (28.81) | 11 (20.75) |
| Anorexia | 1 (0.89) | 1 (1.69) | 0 (0.00) |
| Nausea | 13 (11.61) | 12 (20.34) | 1 (1.89) |
| Vomiting | 4 (3.57) | 4 (6.78) | 0 (0.00) |
| Abdominal pain | 2 (1.79) | 1 (1.69) | 1 (1.89) |
| Diarrhoea | 2 (1.79) | 2 (3.39) | 0 (0.00) |
| Gingival hyperplasia | 1 (0.89) | 1 (1.69) | 0 (0.00) |
| Dyspepsia | 2 (1.79) | 1 (1.69) | 1 (1.89) |
| Peptic ulceration | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Abdominal distension | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Oesophageal ulceration | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Candidiasis | 3 (2.68) | 1 (1.69) | 2 (3.77) |
| Pancreatitis | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Perforation of bowel | 1 (0.89) | 0 (0.00) | 1 (1.89) |
| Gastrointestinal blood loss | 2 (1.79) | 1 (1.69) | 1 (1.89) |
| Other gastrointestinal disorder | 9 (8.04) | 3 (5.08) | 6 (11.32) |
| Cardiovascular disorders | |||
| Any | 15 (13.39) | 10 (16.95) | 5 (9.43) |
| Hypertension | 14 (12.50) | 10 (16.95) | 4 (7.55) |
| Hypotension | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Congestive cardiac failure | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Oedema | 2 (1.79) | 0 (0.00) | 2 (3.77) |
| Other cardiovascular disorder | 1 (0.89) | 1 (1.69) | 0 (0.00) |
| Hepatobiliary disorders | |||
| Any | 3 (2.68) | 2 (3.39) | 1 (1.89) |
| Hepatic dysfunction | 3 (2.68) | 2 (3.39) | 1 (1.89) |
| Other hepatobiliary disorder | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Skin and subcutaneous tissue disorders | |||
| Any | 7 (6.25) | 4 (6.78) | 3 (5.66) |
| Hypertrichosis | 2 (1.79) | 2 (3.39) | 0 (0.00) |
| Allergic rashes | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Bruising, petechiae and ecchymosis | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Skin atrophy | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Telangiectasia | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Acne | 1 (0.89) | 0 (0.00) | 1 (1.89) |
| Burning sensation | 1 (0.89) | 1 (1.69) | 0 (0.00) |
| Pruritus | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Erythema | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Facial flushing | 2 (1.79) | 1 (1.69) | 1 (1.89) |
| Folliculitis | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Other skin and subcutaneous tissue disorder | 2 (1.79) | 1 (1.69) | 1 (1.89) |
| Musculoskeletal, connective tissue and bone disorders | |||
| Any | 9 (8.04) | 7 (11.86) | 2 (3.77) |
| Muscle cramps | 2 (1.79) | 2 (3.39) | 0 (0.00) |
| Myalgia | 3 (2.68) | 2 (3.39) | 1 (1.89) |
| Myopathy | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Osteoporosis | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Avascular osteonecrosis | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Arthralgia | 2 (1.79) | 2 (3.39) | 0 (0.00) |
| Arthritis | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Tendon rupture | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Other musculoskeletal, connective tissue and bone disorder | 2 (1.79) | 1 (1.69) | 1 (1.89) |
| Renal and urinary disorders | |||
| Any | 22 (19.64) | 19 (32.20) | 3 (5.66) |
| Renal dysfunction | 19 (16.96) | 18 (30.51) | 1 (1.89) |
| Other renal and urinary disorder | 3 (2.68) | 1 (1.69) | 2 (3.77) |
| General disorders | |||
| Any | 26 (23.21) | 8 (13.56) | 18 (33.96) |
| Serious infection – requiring hospitalisation or parenteral antibiotic | 6 (5.36) | 0 (0.00) | 6 (11.32) |
| Other infection | 9 (8.04) | 4 (6.78) | 5 (9.43) |
| Thromboembolism | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Fatigue | 6 (5.36) | 2 (3.39) | 4 (7.55) |
| Weight increase | 5 (4.46) | 1 (1.69) | 4 (7.55) |
| Cancer | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Other general disorder | 1 (0.89) | 1 (1.69) | 0 (0.00) |
| Allergy | |||
| Any | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Anaphylactic reaction | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Allergic reactions (not rashes) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Rhinitis | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Other allergy | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Ophthalmic | |||
| Any | 1 (0.89) | 0 (0.00) | 1 (1.89) |
| Increased intra-ocular pressure | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Glaucoma | 1 (0.89) | 0 (0.00) | 1 (1.89) |
| Papilloedema | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Cataracts | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Corneal or scleral thinning | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Exophthalmos | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Death | |||
| Any | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Death | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Other side effects | |||
| Any | 3 (2.68) | 2 (3.39) | 1 (1.89) |
| Other | 3 (2.68) | 2 (3.39) | 1 (1.89) |
Appendix 21 Log of activities by members of our patient panel
| Date | Activity | Panel members |
|---|---|---|
| July 2009 | Comment and review on design of BEEP eczema prevention pilot study (SPRUSD) | Amanda Roberts |
| October 2009 | Eczema Treatment Decision Aid Focus Group (SPRUSD) | Amina Ahmed |
| October 2009 onwards | Involvement in SCC Cochrane review and project (SPRUSD) | Jack Tweed |
| November 2009 | Initial Patient Panel Training Event (introduction to clinical research, workshops on BEEP pilot, CLOTHES study and vitiligo PSP) | 18 attendees |
| December 2009 | Comment and review on design and content of young person’s survey for a UKDCTN acne prevention study | Jenni Rishworth and Jo Clayton |
| December 2009 onwards | Membership of UKDCTN Steering and Executive Committees as PPI representatives | Jo Clayton and Amanda Roberts |
| December 2009 onwards | Cochrane consumer reviewer | Carolyn Hughes and Anjna Rani |
| January 2010 | Update on Cochrane Systematic Review on interventions for vitiligo published (SPRUSD) | Maxine Whitton |
| March 2010 | Attendance at Cochrane Consumer event | Carolyn Hughes |
| March 2010 | Participation in final priority setting meeting for vitiligo PSP (SPRUSD) | Lisa Sharples and Maxine Whitton |
| May 2010 | Comment and review on design and content of a carer survey for the FLAME infected eczema study (application for an NIHR HTA commissioned call, unsuccessful) | Kirsteen Murray and Amina Ahmed |
| May 2010 | Attendance at Annual Evidence Based Update meeting on eczema (Amanda Roberts participated as a patient representative in expert Q&A panel) | Marjorie Howard, Amina Ahmed, Amanda Roberts, Anjna Rani |
| June 2010 | Comment and review of lay summary of SWET eczema study results (NIHR HTA funded study) | Carolyn Hughes and Amina Ahmed |
| June 2010 | Second patient panel training event (guest speaker Derek Stewart, jargon busting and trial design, workshop on prioritising eczema research) | Nine attendees |
| June 2010 | Coauthorship of article on CEBD Patient Panel in Involve Summer Newsletter | Amanda Roberts and Jason Simons |
| September 2010 | Speaker at first Vitiligo World Congress, Milan | Maxine Whitton |
| October 2010 onwards | Membership of Eczema Treatments PSP Steering Group (SPRUSD) | Amina Ahmed, Amanda Roberts, Marjorie Howard and Anjna Rani |
| November 2010 | Attendance at Involve Patient Involvement Conference | Anjna Rani |
| January 2011 onwards | Membership of Trial Management Committee for CREAM infected eczema study (NIHR HTA commissioned call funded study) | Amanda Roberts |
| March 2011 | Third patient panel training event (Cochrane reviews, other activities to get involved in, guest speaker from Skcin, workshop on patient decision aids) | 11 attendees |
| March 2011 onwards | Cochrane consumer reviewer | Jo Clayton, Colette O Sullivan, Amanda Roberts |
| May 2011 | Attendance at Annual Evidence Based Update meeting on psoriasis (participated as a patient representative in expert Q&A panel) | Jason Simons |
| July 2011 onwards | Membership of NIHR Dermatology Specialty Group as PPI representatives | Jason Simons and Collette O’Sullivan |
| October 2011 onwards | Involvement in Nottingham University Hospitals Trust Research fund as lay reviewers | Anjna Rani, Jo Clayton, Amanda Roberts |
| October 2011 | Assistance with training session and feedback on handheld UV devices for vitiligo HI-Light pilot study (SPRUSD) | Jo Clayton |
| November 2011 | Fourth patient panel training event (SPRUSD open meeting – feedback on project to date) | 10 members attended |
| January 2012 | Participation in final priority setting meeting for eczema treatments PSP (SPRUSD) | Amina Ahmed, Amanda Roberts, Tim Burton, Anjna Rani |
| January 2012 onwards | Membership of All-Party Parliamentary group on skin | Maxine Whitton |
| March 2012 | Attendance at Cochrane Skin Group Annual Meeting | Anjna Rani and Maxine Whitton |
| March 2012 | Submission of poster abstract on CEBD Patient Panel for Involve Annual Meeting (abstract accepted) | Amanda Roberts |
| April 2012 | Coapplicant on eczema CLOTHES study (NIHR HTA commissioned call funded study) | Amina Ahmed |
| May 2012 onwards | Membership of Trial development group for UKDCTN hELP study on erosive lichen planus (funded by NIHR Clinical Fellowship) | Jo Clayton |
| May 2012 | Fifth patient panel training event held (Lifecycle of a clinical trial, Cochrane terminology, What is Evidence-based medicine, workshops on SCC, BATHE and Involve CEBD Patient Panel poster) | 12 attendees |
| June 2012 | Coauthorship of a chapter for Evidence-Based Dermatology (Third Edition) ‘The role of Patient and Public Involvement in Evidence Based Dermatology) | Amanda Roberts, Anjna Rani, Colette O’Sullivan, Jason Simons |
| September 2012 | Production of NIHR DSG PPI video | Jason Simons and Colette O’Sullivan |
| October 2012 onwards | Lay membership of NICE quality standards eczema in children topic expert group | Amanda Roberts |
| November 2012 | Attendance at Involve 2012 Annual meeting and Poster Presentation | Amanda Roberts |
| November 2012 | Membership of BATHE eczema study TSC (NIHR HTA commissioned call funded study) | Amanda Roberts |
| January 2013 | Coapplicant on HI-Light Study (NIHR HTA commissioned call funded study) | Maxine Whitton |
| February 2013 onwards | PPI input into HOME eczema project (SPRUSD) | Tim Burton |
| April 2013 | Attendance at HOME III meeting, San Diego (SPRUSD) | Tim Burton |
| April 2013 | Sixth patient panel training event held (CEBD communications event) | 9 attendees |
| May 2013 | Membership of NETSCC PPI reference group | Amanda Roberts |
| June 2013 onwards | Involvement in ALPHA hand eczema study (comment and review on application to NIHR HTA commissioned call; will join TSC if project is funded) | Amanda Roberts |
| September 2013 | Membership of TSC for COMET eczema study (NIHR RfPB funded study) | Amina Ahmed |
| January 2014 | Assisted with media interviews for the CLOTHES trial | Amina Ahmed and her son |
| February 2014 | Attendance at final dissemination meeting | Eight attendees |
Glossary
- Abstracted
- Essential information from published trial reports collected and summarised separately.
- Allergen
- A substance that elicits an immune response in certain people.
- Allergy
- An excessive immune response to a substance.
- Allocation
- Assignment to a treatment group within a clinical trial.
- Atopy
- Predisposition to mount an excessive immune response.
- Bias
- Factors that may alter the outcome of a study.
- Blind
- When treatment allocation is unknown by the people taking part and the researchers conducting the trial. A trial can be double blind (in which neither the participants nor the investigators know the treatment allocation), or single blind (in which only one of these groups knows the treatment allocation).
- Calcineurin inhibitors
- Non-steroidal treatments that block a chemical that activates inflammation.
- Corticosteroids
- A group of anti-inflammatory medications.
- Cutaneous
- Relating to, or affecting, the skin.
- Emollient
- A non-cosmetic moisturiser designed to prevent and treat dry skin.
- Erythema
- Redness of the skin.
- Excoriation
- Destruction or removal of skin from scratching.
- Feasibility study
- Preliminary work undertaken to assess the viability of a project.
- Flexural
- Relating to the parts of the skin that come into contact with each other when a joint bends.
- Forest plot
- Graphical representation of information from the individual studies that went into the meta-analysis to show the overall effect of the question of interest.
- Immunoglobulin
- A protein in the blood stream used by the immune system to identify harmful substances or organisms.
- Immunosuppressant
- A drug that reduces the ability of the immune system to fight infection.
- Induration
- Skin thickening and swelling.
- Intention-to-treat analysis
- An assessment of participants according to their initial treatment assigned regardless of other factors (such as whether they dropped out or switched treatments).
- Koebner phenomenon
- Development of lesions on the skin after trauma.
- Lesions
- Areas of skin affected by physical signs of skin disease.
- Lichenification
- Thickening of the skin causing normal lines in the skin to seem more prominent.
- Meta-analysis
- Combining the results of several studies to estimate the average treatment effect.
- Outcome
- A planned measurement described in the protocol.
- Perifollicular
- Surrounding a hair follicle.
- Phenotype
- Observable characteristics of an organism.
- Pilot study
- A small study that is conducted prior to a full clinical study to test the feasibility of conducting a large-scale study.
- Placebo
- An inert product that is similar in size, shape and taste to the active treatment.
- Planimetry
- Physical measurement or mapping of an area.
- Priority setting partnership
- A collaboration of health-care professionals, patients and other relevant partners to agree on the most important treatment uncertainties for a given medical condition.
- Prospective study
- A study whereby data are collected for participants as they progress through a study.
- Pruritus
- Itching.
- Randomised controlled trial
- A way to compare treatments. Participants are randomly assigned to either receive the treatment being assessed or an alternative treatment, which may be a placebo or no treatment.
- Random sequence generation
- Ensuring that there is an equal probability of being assigned to a control or treatment group according to a pre-defined list.
- Rhinitis
- Inflammation inside the nose caused by an allergy.
- Sensitisation
- Exposure to an allergen for the first time, causing the immune system to create specific antibodies for the allergen.
- Skin barrier
- The protection that the skin provides against the environment.
- Stratum corneum
- The outer of layer of skin that forms the skin barrier.
- Systematic review
- A way of looking for, and summarising, research evidence in a structured and systematic way to ensure that all available evidence is included in the review.
- Systemic (treatment)
- Medication that affects the whole body – this can be either oral treatments or, in some cases, physical treatments such as ultraviolet light.
- T helper cell type 1/2
- White blood cells.
- Topical
- Applied to the body, usually the skin.
- Transepidermal water loss
- Total quantity of water lost through the skin.
List of abbreviations
- 5-FU
- 5-fluorouracil
- AD
- atopic dermatitis
- AE
- atopic eczema
- AJCC
- American Joint Committee on Cancer
- AMED
- Allied and Complementary Medicine Database
- AMSTAR
- Assessing the Methodological Quality of Systematic Reviews
- ART
- adjuvant radiotherapy
- AUC
- area under the curve
- BAD
- British Association of Dermatologists
- BAPRAS
- British Association of Plastic Reconstructive and Aesthetic Surgeons
- BATHE
- Bath Additives in the Treatment of Childhood Eczema
- BCC
- basal cell skin cancer
- BEEP
- Barrier Enhancement for Eczema Prevention
- BSA
- body surface area
- BSDS
- British Society for Dermatological Surgery
- CDLQI
- Children’s Dermatology Life Quality Index
- CEAC
- cost-effectiveness acceptability curve
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CHU-9D
- Children’s Health Utility Index 9 dimensions
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CLRN
- Comprehensive Local Research Network
- COMET
- Core Outcome Measures in Effectiveness Trials
- CONSORT
- Consolidated Standards of Reporting Trials
- COSMIN
- COnsensus-based Standards for the selection of health Measurement INstruments
- CREAM
- Children with Eczema Antibiotic Management
- CSG
- clinical studies group
- df
- degrees of freedom
- DFI
- Dermatitis Family Impact
- DLQI
- Dermatology Life Quality Index
- DMC
- Data Monitoring Committee
- DUET
- Database of Uncertainties about the Effects of Treatment
- DVD
- digital versatile disc
- EASI
- Eczema Area and Severity Index
- e-Delphi
- electronic Delphi
- EQ-5D
- European Quality of Life-5 Dimensions
- EQ-5D-3L
- European Quality of Life-5 Dimensions-3 Levels
- GP
- general practitioner
- GREAT
- Global Resource of EczemA Trials
- HCP
- health-care professional
- HI-Light
- Home Intervention of Light therapy
- HOME
- Harmonising Outcome Measures for Eczema
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- IBD
- inflammatory bowel disease
- ICER
- incremental cost-effectiveness ratio
- ICMJE
- International Committee of Medical Journal Editors
- ID
- identifier
- IDQOL
- Infant’s Dermatitis Quality of Life Index
- IFN
- interferon
- IFNα
- interferon alpha
- IFPCS
- International Federation of Pigment Cell Societies
- IGA
- Investigator Global Assessment
- IgE
- immunoglobulin E
- IP
- internet protocol
- IQR
- interquartile range
- ITT
- intention to treat
- LILACS
- Latin American and Caribbean Health Sciences
- MCRN
- Medicines for Children Research Network
- MDT
- multidisciplinary team
- MED
- minimum erythemal dose
- MMS
- Mohs micrographic surgery
- MRC
- Medical Research Council
- NB-UVB
- narrowband ultraviolet light B
- NCRI
- National Cancer Research Institute
- NCTU
- Nottingham Clinical Trials Unit
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMSC
- non-melanoma skin cancer
- OMERACT
- Outcome Measures in Rheumatoid Arthritis Clinical Trials
- OR
- odds ratio
- PBI
- Patient Benefit Index
- PCRN
- Primary Care Research Network
- PG
- pyoderma gangrenosum
- PGA
- Participant Global Assessment
- PhD
- doctor of philosophy
- PI
- principal investigator
- PIC
- patient identification centre
- PIL
- patient information leaflet
- POEM
- Patient-Oriented Eczema Measure
- PPI
- patient and public involvement
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROM
- patient-reported outcome measure
- PROSPERO
- Prospective Register of Systematic Reviews
- PSP
- priority setting partnership
- PUVA
- psoralen and ultraviolet light A
- PUVAsol
- psoralen with sunlight exposure
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCPath
- Royal College of Pathologists
- RCR
- Royal College of Radiologists
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RR
- risk ratio
- SAP
- statistical analysis plan
- SAR
- serious adverse reaction
- SASSAD
- Six Area, Six Sign Atopic Dermatitis
- SCC
- squamous cell skin cancer
- SCORAD
- Severity Scoring of Atopic Dermatitis
- SD
- standard deviation
- SIGN
- Scottish Intercollegiate Guideline Network
- SOeN
- site-orientated e-network
- SOP
- standard operating procedure
- SPSS
- Statistical Package for the Social Sciences (prior to version 19)
- SPSS
- Statistical Product and Service Solutions (from version 19 onwards)
- SSMDT
- specialist skin cancer multidisciplinary team
- STOP GAP
- Study of Treatments fOr Pyoderma GAngrenosum Patients
- TEWL
- transepidermal water loss
- Th1
- T helper cell type 1
- Th2
- T helper cell type 2
- TIS
- Three-Item Severity
- TNF
- tumour necrosis factor
- TSC
- Trial Steering Committee
- UKCRN
- UK Clinical Research Network
- UKDCTN
- UK Dermatology Clinical Trials Network
- UV
- ultraviolet
- UVA
- ultraviolet light A
- UVB
- ultraviolet light B
- VAS
- visual analogue scale
- WAO
- World Allergy Organization
- WCW
- well-controlled week