Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0606-1083. The contractual start date was in August 2007. The final report began editorial review in February 2014 and was accepted for publication in November 2015. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Bob Woods reports that Bangor University has received royalties from the sale of therapy manuals in the UK and the USA. Ian Russell reports that Swansea University has received funds from University College London for lectures and staff mentoring.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Orrell et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Dementia is very common in old age and the frequency of dementia increases with age, from around 5% in those aged > 65 years to around 20% in those aged > 85 years. 1,2 In the UK there are 850,000 people living with dementia3 which leads to progressive intellectual deterioration, increasing disability and social exclusion. 4 This has an enormous social and economic impact on health and social care services, and on family carers. There is little high-quality research on the effectiveness of psychological and social interventions, and there is an urgent need to find more useful interventions to help reduce the impact of dementia on people with dementia, carers and society. Drug treatments have an important role in dementia care, but in the UK they are limited to people with Alzheimer’s disease, with mild to moderately severe dementia, have a limited impact on the illness, are not suitable for all patients and cost approximately £1000 per year. 5 There is increasing recognition that psychological and social interventions may have comparable value6 and may be preferable (e.g. when medication may have intolerable side effects). 7
In the UK there is recognition that psychological therapies for older people should be more widely available, and the National Service Framework for Older People4 states that ‘treatment for dementia always involves using non-pharmacological management strategies such as mental stimulation’ (contains public sector information licensed under the Open Government Licence v3.0). However, the delivery of such therapies has been generally unstandardised, and many evaluations of psychological treatments have been either small or of poor methodological quality, or both. A number of systematic reviews of psychosocial interventions are now available,8–10 as well as a number of Cochrane reviews on interventions with a cognitive focus. 11,12 Cognitive stimulation therapy (CST) is an evidence-based approach which has been shown to be beneficial to cognitive function and quality of life, and also cost-effective. 6,13 Indeed, the recent draft National Institute for Health and Care Excellence (NICE) guidelines on dementia14 recommend that all people with mild to moderate dementia should be ‘given the opportunity to participate in a structured group cognitive stimulation programme’.
In the UK, reminiscence work with people with dementia has an extensive history;15,16 it involves enjoyable activities that tap into early memories and encourage communication and well-being. However, its popularity has not led to a corresponding body of evidence on its effects. The Remembering Yesterday, Caring Today (RYCT) trial platform suggested that it was useful to involve family carers in reminiscence groups with people with dementia, and a recent meta-analysis of outcomes for family caregivers confirms that involving people with dementia and their carers together is more effective than working with carers only. 10 Over the last decade, the needs of carers have remained a high priority, with a national strategy published in 1999. 17 Being a family carer is stressful and a recent study showed that one in three carers had a mental illness. 18 The carers of people with dementia experience greater strain and distress than the carers of other older people. 19 The Expert Patients Programme for people with long-term conditions aims to increase their confidence, improve their quality of life and better manage their conditions. 20 In a 2006 White Paper21 the government pledged funding for the creation of an Expert Carer Programme, which would include training to develop carers’ skills in addition to self-care skills of people with dementia. The progressive decline and the changing nature of dementia over time mean that family carers’ needs will change. 22 There is an evidence base for cognitive–behavioural packages being the predominant approach for psychoeducation, stress and behaviour management, with principles and basic components that could be disseminated for use by non-therapists. For example, in caring for a person with dementia, sessions may include coping with the psychological and behavioural symptoms of dementia.
In the last few years there has also been an increasing emphasis on maintaining older people with dementia at home, rather than admitting them to hospital, to help to maintain their independence and quality of life. The document Everybody’s Business: Integrated Mental Health Services for Older Adults23 advised that community mental health teams (CMHTs) for older people needed to have some provision for 24-hour home-based crisis support, and Raising the Standard: Specialist Services for Older People with Mental Illness24 highlighted the need for alternatives to inpatient care. A 2005 review25 noted the lack of evidence for alternatives to acute psychiatric admissions for older people and another study from the same year indicated that home treatment teams (HTTs)/crisis teams are effective at reducing admissions for those aged < 65 years. 26 There are suggestions that this approach may also reduce admissions for those aged > 65 years with mental health problems,27,28 including people with dementia.
Aims and objectives
The aim of this research programme is to prevent excess disability, promote social inclusion, improve health outcomes and enhance the quality of life of people with dementia and their carers. The aim was achieved by a rigorous 5-year programme of psychosocial research building on our existing work in cognitive stimulation, reminiscence work and carer support, and also by a new initiative developing intensive home support to manage crises at home and prevent admission to hospital for people with dementia.
Cognitive stimulation therapy is an evidence-based approach which has been shown to be beneficial to cognitive function and quality of life, and also cost-effective. As the degree of cognitive benefit from CST is similar to that from cholinesterase inhibitors, longer-term CST may have an impact on long-term care.
Reminiscence work with people with dementia taps into early memories and encourages communication and well-being, and a recent meta-analysis indicated that involving people with dementia and their carers is more effective than working with carers only. Our trial platform successfully developed a manual for joint reminiscence, RYCT, and suggests that RYCT improves the caring relationship and benefits both people with dementia and carers. Our experience from the Befriending and Costs of Caring (BECCA) programme29 showed that many ex-carers are motivated to support others at an earlier stage in their role as a family carer, through mentoring and teaching.
There is some evidence that HTTs may reduce admissions for people with dementia; however, better evidence for their effectiveness is required before wider implementation is considered.
This research programme provides essential evidence to clarify the role of each of these interventions in helping to support people at home, reducing hospital and care home admissions, and improving the quality of life of people with dementia and their carers.
The three projects are (1) cognitive stimulation groups for people with dementia to improve their cognition and quality of life, (2) a new initiative called the Expert Carer Programme, which trains ex-carers to help new carers of people with dementia and was undertaken alongside reminiscence groups for people with dementia and their carers which help to maintain quality of life and improve their relationships, and (3) the development of intensive home support to help manage crises at home and prevent admission to hospital for people with dementia.
Each of the three projects completed a number of components of the pathway through development of theory, modelling, feasibility and evaluation to dissemination and implementation, as illustrated in the Medical Research Council (MRC) framework for complex interventions. 30
All of these approaches were carefully evaluated to look at their potential benefits to people with dementia and their carers. We have also produced training manuals which will be made widely available to help other services implement the same approaches.
Objectives
-
To develop a model to identify the most promising interventions and components for an effective home treatment package (HTP) for dementia.
-
To carry out systematic reviews in the areas of home treatment for dementia and to update the Cochrane review on reality orientation/CST for dementia.
-
To develop a HTP for dementia and a package for carer supporters.
-
To carry out a pilot study for (a) the reminiscence and carer programmes separately and in combination, and (b) the effectiveness of maintenance CST (MCST) with donepezil.
-
To provide definitive randomised controlled trials (RCTs) for MCST, RYCT and the Carer Supporter Programme (CSP).
-
To conduct in-depth field testing of the HTP for dementia.
-
To conduct a post-RCT surveillance study of MCST in practice, including minimal outcome measures and qualitative approaches.
-
To provide economic evaluations for the MCST and CSP/RYCT interventions.
-
To involve users, carers and the voluntary sector, and to develop a model of user/carer involvement that can be widely used.
-
To develop training and manuals for all three interventions.
-
To disseminate service models, training programmes, tools and outcomes.
This report outlines the work undertaken for each project and individual aims and objectives for each study are given in their respective chapters. The development of the interventions focuses on the involvement of service users and key stakeholders.
User and carer involvement
User and carer involvement is now central to research and development (R&D) strategy in health and social care nationally, and their involvement is required as a condition of funding. 31 User involvement and collaboration has been found to improve the quality, depth and utility of research. 32,33 We developed a strategy for user and carer involvement as part of the SHIELD (Support At Home – Interventions to Enhance Life in Dementia) research programme. 34 The strategy encompassed principles of good involvement practice (i.e. clarity and transparency, respect, diversity, flexibility and accessibility) and meaningful involvement. 35 Service users and carers were involved in consensus workshops, focus groups and consultation processes for developing interventions to ensure their relevance and acceptability. User involvement can help develop more theoretically coherent and evidence-based interventions, which are more likely to be practical, generalisable and meaningful for potential users. 36 Carers were also involved in the recruitment of staff and provision of training and as part of the SHIELD research programme steering and monitoring committees.
Ethics arrangements and research governance for the SHIELD research programme
All projects obtained ethics approval from a NHS Research Ethics Committee and local R&D approvals for all sites involved in the research. Amendments to protocols were made and approvals were sought from ethics committees as needed throughout the research programme. The trial was sponsored by University College London and North East London Foundation Trust (NELFT). Our Programme Steering Committee consisted of an independent chairperson and external committee members comprised interested clinicians, academics, voluntary sector staff and service users, as well as the grantholders and key individuals from the research programme (see Acknowledgements). 37 A Data Monitoring and Ethics Committee was created as a subcommittee of the Programme Steering Committee. This group consisted of an independent academic as chairperson, an independent statistician and a family carer37 (see Acknowledgements).
Consent
Informed consent was sought whenever appropriate. Participants were at various stages of dementia ranging from mild to moderate, to severe. Some participants were competent to give informed consent for participation, provided that appropriate care was taken in explaining the research and sufficient time was allowed for them to reach a decision. However, those people with more advanced dementia were also included and, in these situations, the provisions of the Mental Capacity Act were followed. 38,39 It was helpful for a family member or other supporter to be involved, and we aimed to ensure that this was done whenever possible. It was made clear to all that no disadvantage would accrue if they chose not to participate. In seeking consent, we followed current guidance from the British Psychological Society40 on the evaluation of capacity; thus, consent was regarded as a continuing process rather than a definitive, and willingness to participate was continually checked through discussion with participants during the assessments. When a participant’s level of impairment was more severe or increased so that they were no longer able to provide informed consent, the provisions of the Mental Capacity Act were followed. 38,39 The initial giving of informed consent provided a clear indication of the person’s likely perspective on continuing at this point, and the family caregiver was consulted in this regard. If a participant with dementia became distressed during the assessments, the assessments were discontinued.
Adverse events
Prospective participants were fully informed of the potential risks and benefits of the projects they were recruited to. A reporting procedure was in place to ensure that serious adverse events (SAEs) were reported to the chief investigator (MO). 41 On becoming aware of an adverse event involving a participant or carer, the research programme co-ordinator (JH) assessed its seriousness. A SAE was defined in the trial as an untoward occurrence experienced by either a participant or a carer which resulted in death; was life-threatening; required hospitalisation or prolongation of existing hospitalisation; resulted in persistent or significant disability or incapacity; was otherwise considered medically significant by the investigator; or was within the scope of the Protection of Vulnerable Adults protocol. 41 A reporting form was submitted to the chief investigator, who assessed whether or not the SAE was related to the conduct of the trial and was unexpected. If SAEs were judged to be related and unexpected, they were reported to the Multicentre Research Ethics Committee and the trial Data Monitoring and Ethics Committee.
Changes from the planned protocol
National Institute for Health Research (NIHR) funding for SHIELD was received in August 2007 and the research programme commenced in February 2008. The original scope of the programme was to undertake four trials examining the effectiveness of MCST programmes, the implementation of MCST in practice, RYCT and the CSP, and a HTP for dementia. Time and budgetary constraints meant that we were advised by the NIHR review team not to complete a trial of the home treatment intervention. This will now be tested in a further NIHR-funded trial. All studies followed the MRC guidance for developing and evaluating complex interventions. 30
Chapter 2 Maintenance cognitive stimulation therapy
Background
Cognitive ‘training’, cognitive ‘stimulation’ and cognitive ‘rehabilitation’ have on occasion been used almost interchangeably, but Clare and Woods42 have proposed clear definitions for all of these terms. Cognitive stimulation has been defined as the engagement in a range of activities and discussions (usually in a group) aimed at the general enhancement of cognitive and social functioning. 42 CST6 is a therapeutic non-pharmacological treatment for dementia that aims to optimise cognitive function based on the notion of cerebral plasticity. A large multicentre RCT of CST found that it improved cognition and quality of life,6 and was cost-effective. 13 Indeed, the 2007 draft NICE/Social Care Institute for Excellence guideline on dementia14 recommended that all people with mild-to-moderate dementia should have the opportunity to take part in a structured programme of cognitive stimulation groups.
As CST is only a brief intervention (a 14-session programme over 7 weeks), it is necessary to investigate if its benefits can be extended over a longer period of time. This has been investigated as a pilot study43 in which the 16 additional weekly sessions led to a significant improvement in cognitive function for those receiving MCST, compared with those receiving CST only. The programme grant funded a full-scale trial of MCST over 24 weeks.
The MSCT programme included an update of the Cochrane review on reality orientation/cognitive stimulation, explored the long-term effects of a MCST programme versus CST for dementia and compared the effectiveness of two different training approaches with care staff from a range of dementia care settings. The cognitive stimulation groups for people with dementia aimed to improve cognition and quality of life. The training package comprises a workbook, a digital versatile disc (DVD) and training seminars.
Work package 1: update of the Cochrane review on cognitive stimulation therapy
A systematic review and meta-analysis evaluating the effectiveness and impact of cognitive stimulation in dementia
A systematic review and meta-analysis evaluating the effectiveness and impact of cognitive stimulation in dementia was conducted with the Cochrane Dementia and Cognitive Improvement Group (CDCIG), based in Oxford, UK. 44 The review followed the Specialised Register of the CDCIG, called ALOIS (Action in Language, Organisations and Information Systems). This yielded 94 studies, of which 15 were RCTs meeting the inclusion criteria. The analysis included 718 participants (407 receiving cognitive stimulation and 311 in control groups).
Objectives
-
To evaluate the effectiveness and impact of cognitive stimulation interventions aimed at improving cognition for people with dementia, including any negative effects.
-
To indicate the nature and quality of the evidence available on this topic.
-
To assist in establishing the appropriateness of offering cognitive stimulation interventions to people with dementia and identifying the factors associated with their efficacy.
Review methods
Protocol and registration
The protocol was registered with The Cochrane Library and can be found online at http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD005562.pub2/pdf.
Criteria for considering studies for this review
We selected RCTs examining the effect of cognitive stimulation for dementia if they had been published and written in English, peer reviewed and presented in a journal article. Authors were contacted for missing data, such as details of randomisation, means and standard deviations (SDs).
Search methods for identification of studies
The search methods included a combination of the search terms cognitive stimulation, reality orientation, memory therapy, memory groups, memory support, memory stimulation, global stimulation and cognitive psychostimulation, which were used to search ALOIS on 6 December 2011. The studies were identified from the following databases:
-
health-care databases: MEDLINE, EMBASE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO and Latin American and Caribbean Health Sciences Literature (LILACS)
-
trial registers: metaRegister of Controlled Trials, Umin (University Hospital Medical Information Network) Japan Trial Register and World Health Organization portal [which covers ClinicalTrials.gov, International Standard Randomised Controlled Trial Number (ISRCTN), Chinese Clinical Trials Register, German Clinical Trials Register, Iranian Registry of Clinical Trials and the Netherlands National Trials Register, plus others]
-
The Cochrane Library’s Central Register of Controlled Trials
-
a number of grey literature sources: ISI Web of Knowledge Conference Proceedings, Index to Theses, Australasian Digital Theses.
Additional searches in each of the sources listed above to cover the time frame from the last searches performed for the Specialised Register to December 2011 were run to ensure that the search for the review was as up to date as possible. A total of 670 references were retrieved from the December 2011 update search. After deduplication and a first assessment, authors were left with 94 references to further assess for inclusion, exclusion or discarding.
Participants
Participants were any age with a diagnosis of dementia (Alzheimer’s disease, vascular dementia or mixed Alzheimer’s and vascular dementia, other types of dementia), including all severity levels of dementia, indicated through group mean scores, range of scores or individual scores on a standardised scale, such as the Mini-Mental State Examination (MMSE)45 or Clinical Dementia Rating (CDR). 46 The participants could receive the intervention in a variety of settings (own home, outpatient setting, day care setting or residential setting). We documented when participants were receiving concurrent treatment with acetylcholinesterase inhibitors (AChEIs).
Interventions
Participants attended regular therapy sessions (involving a group or family caregiver) for a minimum period of 4 weeks. The intervention needed to describe a cognitive stimulation intervention targeting cognitive and social functioning, offering generalised cognitive practice. These may also be described as reality orientation groups, sessions or classes. The intervention needed to be compared with ‘no treatment’, ‘standard treatment’ or placebo.
Outcome measures
These assessed the short- (immediately after the intervention) and medium-term (follow-up 1 month to 1 year after the intervention finished) impact on the intervention on the person with dementia, the family caregiver or both. For the person with dementia, outcome measures needed to evaluate performance on at least one cognitive measure and/or include the assessment of any of the following variables: mood, well-being, activities of daily living (ADLs), behaviour, neuropsychiatric symptoms and social engagement. Rates of attrition and reasons for withdrawal were noted. Family caregiver outcomes, such as self-reported well-being, depression and anxiety, burden, strain and coping, and satisfaction with the intervention, were considered.
Data collection and analysis
Searches were conducted as detailed above to identify all relevant published studies. The date and time of each search, together with details of the version of the database used, were recorded. Additional information was sought, as outlined above, and hard copies of articles were obtained.
Quality assessment
Two reviewers independently screened the identified RCTs for inclusion and the final list of included studies was reached by consensus. Trials not meeting the criteria were excluded. The studies were assessed against a checklist of quality requirements using the Cochrane approach:
-
grade A – ‘low risk’: adequate concealment (randomisation; concealed allocation)
-
grade B – ‘unclear risk’: ‘randomised’, but methods uncertain
-
grade C – ‘high risk’: inadequate concealment of allocation or no randomisation, or both.
Only trials with a grade A or B ranking were included in the review. Again, the reviewers worked independently to ascertain which studies met the quality criteria, and consensus was reached through discussion. Attempts were made to obtain additional information from the study authors when needed.
Data extraction
Descriptive characteristics (such as quality of randomisation and blinding) and study results were extracted, recorded and entered into RevMan 5.1 (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark). Additionally, letters and e-mails were sent to some authors of controlled trials asking for essential and additional information (statistics, sources of bias and details of randomisation). The summary statistics required for each trial and each outcome for continuous data were the mean change from baseline, the standard error (SE) of the mean change and the number of patients for each treatment group at each assessment. When changes from baseline were not reported, we extracted the mean, SD and number of patients for each treatment group at each time point, if available. We calculated the required summary statistics from the baseline and assessment time treatment group means and SDs, assuming in this case a zero correlation between the measurements at baseline and assessment time. This conservative approach was chosen, as it is preferable in a meta-analysis. For binary data, the numbers in each treatment group and the numbers experiencing the outcome of interest were sought. The baseline assessment was defined as the latest available assessment prior to randomisation, but no longer than 2 months prior. For each outcome measure, data were sought on every patient randomised. To allow an intention-to-treat analysis, the data were sought irrespective of compliance, or whether or not the patient was subsequently deemed ineligible or otherwise excluded from treatment or follow-up. Discussion between the two reviewers and the other authors was used to resolve any queries.
Data analyses
RevMan 5.1 was used for the meta-analysis. Analyses were adjusted to the random-effects model, owing to the heterogeneity of trials. Because trials used different tests to measure the same outcomes, the measure of the treatment difference for any outcome that we used was the weighted mean difference when the pooled trials used the same rating scale or test, and the standardised mean difference (SMD) (the absolute mean difference divided by the SD) when different rating scales or tests were used. For binary outcomes, such as clinical improvement or no clinical improvement, the odds ratio was used to measure treatment effect. A weighted estimate of the typical treatment effect across trials was calculated. Overall estimates of the treatment difference were employed, presenting the overall estimate from a fixed-effects model and performing a test for heterogeneity using a standard chi-squared statistic. The reviewers achieved consensus on the interpretation of the statistical analyses, seeking specialist statistical advice from the CDCIG as required. Non-randomised studies were described in tabular form and the reviewers discussed and reached consensus on the presentation of the findings in the background to the review.
Results
Studies included in the review
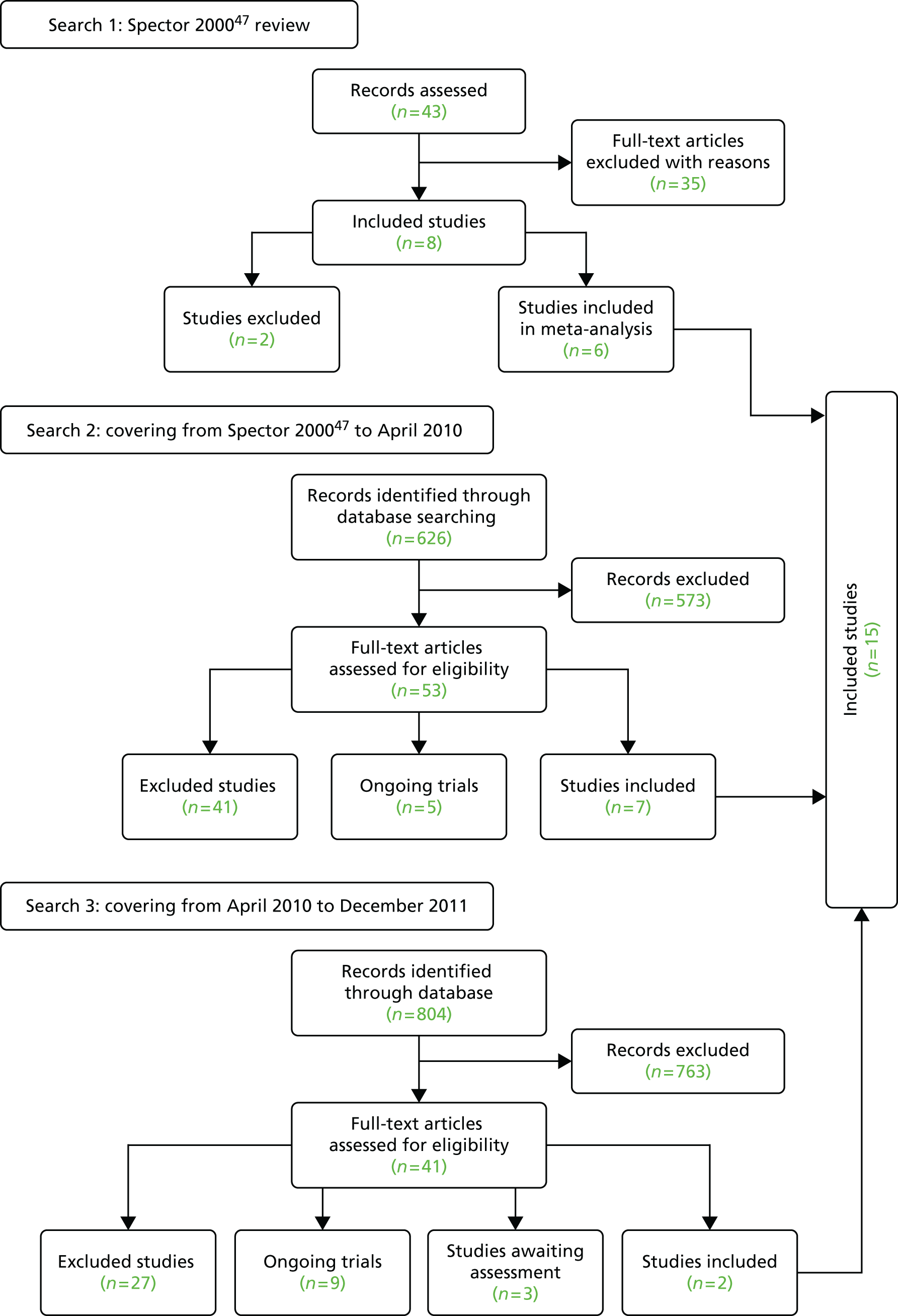
Ninety-four studies were identified since the last review through the literature search. 47 Two reviewers independently assessed eligibility. Of the 94 references, nine studies met the inclusion criteria48–55 and were included in the analysis. Three recent studies were left awaiting classification. 56–58 Our previous review47 included eight studies in the meta-analysis and six of these met the criteria for inclusion in this updated review. 59–64 Two studies from our previous review were excluded this time, as the data needed for the meta analysis were not available. 65,66 Therefore, a total of 15 studies was included in the analysis (Figure 1).
FIGURE 1.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart of the review and selection process of studies.
Quality of included studies
The quality of each study was assessed according to the four criteria outlined in the Cochrane Collaboration Handbook:67 selection bias, performance bias, attrition bias and detection bias. The details of biases and descriptions of studies can be seen in Table 1. Performance bias was difficult to evaluate. With psychological interventions, unlike drug trials, it is impossible to totally blind patients and staff to treatment. Patients are often aware that they are being treated preferentially, staff involved may have different expectations of treatment groups and independent assessors may be given clues from patients during the assessments. There may also be ‘contamination’ between groups, in terms of groups not being held in separate rooms and staff bringing ideas from one group to another.
| Study ID | Intervention | Content of therapy | Alternative activity | Allocation concealment | Attrition bias (dropouts) | Detection bias |
|---|---|---|---|---|---|---|
| Baines et al.,59 1987 | 30 minutes, five times per week for 4 weeks | RO board, multisensory stimulation | Reminiscence therapy/no treatment | No details | 0/15 dropouts | Assessment by independent psychologist and staff not involved in therapy No details of assessors |
| Baldelli et al.,60 1993 | 60 minutes, three times per week for 3 months | No details | No treatment (TAU) | No details | 0/23 | No details of assessors |
| Baldelli et al.,48 2002 | 60 minutes, five times per week for 1 month | No details | Physical therapy programme | No details | 0/87 | No details of assessors |
| Bottino et al.,49 2005 | 90 minutes, once per week for 5 months | Temporal and spatial orientation, discussion of interesting themes, reminiscence activities, naming people, daily activities, planning use of calendars and clocks | AChEIs only | Randomised blocks design | 0/13 | Assessment by a blind and independent assessor |
| Breuil et al.,61 1994 | 60 minutes, twice per week for 5 weeks | Drawing, associated words, object naming, categorising objects | No treatment | No details | 5/61 dropouts | Assessment by a psychologist unaware of group allocation |
| Buschert et al.,50 2011 | 120 minutes, once per week for 6 months | Multicomponent cognitive group intervention: for Alzheimer’s disease group emphasis on cognitive stimulation (for mild cognitive impairment group more emphasis on cognitive training) | Pencil-and-paper exercises for self-study and monthly meetings | Blocked randomisation procedure | No attrition n = 35 | Cognitive assessments made by an assessor blind to group allocation |
| Chapman et al.,51 2004 | 90 minutes, once per week for 8 weeks | Current events; discussion of hobbies and activities; education regarding Alzheimer’s disease; life story work; links with daily life encouraged | AChEIs only | SAS procedure | 6/54 | Assessment by a psychologist unaware of group allocation |
| Coen et al.,52 2011 | 45 minutes, twice per week for 7 weeks | Cognitive stimulation | No treatment | Computerised randomisation and random number tables were used | No attrition n = 27 | Tests administered by staff blind to group membership. Not clear if staff ratings were made by staff who were blinded |
| Ferrario et al.,62 1991 | 60 minutes, five times per week for 21 weeks | No details | No treatment | No details | 2/21 dropouts | No details of assessors |
| Onder et al.,53 2005 | 30 minutes, three times per week for 25 weeks | Current information, topics of general interest, historical events and famous people, attention, memory and visuospatial | AChEIs only | Computerised block randomisation procedure | 19/156 | Assessment by a psychologist unaware of group allocation |
| Requena et al.,54 2006 | 45 minutes, five times per week for 24 months | Orientation, body awareness, family and society, caring for oneself, reminiscing, household tips, animals, people and objects | AChEIs only No treatment |
Registration order procedure | 10/50 | Assessment by a psychologist unaware of group allocation |
| Spector et al.,55 2001 | 45 minutes, two/three times per week for 7 weeks | Orientation, categorising objects, sounds, number, physical and word games, current events | No treatment | Drawing names from a sealed container | 8/35 | Assessment by a researcher blinded to group allocation |
| Spector et al.,47 2000 | 45 minutes, twice per week for 7 weeks (14 sessions) | Orientation, categorising objects, sounds, number, physical and word games, current events | No treatment | Drawing names from a sealed container | 34/201 | Assessment by a researcher blinded to group allocation |
| Wallis et al.,63 1983 | 30 minutes, five times per week for 3 months | Repetition of orientation information (e.g. time, place, weather), charts, pictures, touching objects and material | Diversional occupational therapy (group and individual activities) | Drawing from a hat, consecutive allocation | 22/60 dropouts | Assessment by a senior nurse or occupational therapist unaware of group allocation |
| Woods, 197964 | 30 minutes, five times per week for 20 weeks | Daily personal diary, group activities (dominoes, spelling, bingo), naming objects, reading RO board | ‘Social therapy’ (various group activities) | Drawing from a hat | 4/18 dropouts | Mixture: some assessments blind, some others not |
The latter effect would be reduced with clear therapeutic protocols, the existence of which was not mentioned in any of the studies, although during a face-to-face meeting with Professor Woods he stated that ‘Checks were made to ensure compliance with the therapeutic protocol’ (Bangor University, 2008, personal communication).
In relation to contamination, Wallis et al. 63 and Baines et al. 59 both stated that staff were unaware of the allocation of patients to groups, as they were removed from the setting for treatment, and several other studies described the groups being run in a separate or specific room. 6,55,62,64 Most studies took steps to ensure that at least part of the assessment of outcomes was carried out by assessors blinded to treatment allocation. Only three studies48,60,62 did not report the blinding of assessors. Of course, even independent assessors may be given clues from participants during the assessments, but this was not reported as an issue in the studies reviewed here. Using independent assessors works well for evaluating change in cognition or self-reported mood, well-being and quality of life. Ratings of day-to-day behaviour and function are typically carried out by care staff, who may be more difficult to keep blind to group allocation, unless the group sessions are carried out in a separate location to which all participants are taken. Whether or not the patients were blind to treatment is a controversial issue, depending on how much information was given to them and their level of comprehension.
Several studies reported48,62–64 that the reality orientation groups were held in separate areas, reducing the chance of contamination. Spector et al. 6,55 said that the groups were run in a separate and specific room for the programme. The other studies did not provide information regarding where groups were held. 49,51,53
All of the studies reported on attrition to the programme, although the intention-to-treat analysis plan was described in only two. 6,51 Given the nature of the condition and the age of the participants, attrition in several studies was remarkably small, with zero attrition recorded in six studies48–50,52,59,60 out of 180 participants. The largest attrition rate was reported in the study by Wallis et al. ,63 in which there was 39% attrition in the group of participants with dementia. In this study, patients who attended < 20% of the group sessions were eliminated from the study. Requena et al. 54 reported 32% attrition, but this was over a 2-year period. The two largest studies had rates of 19%59 and 17%6 over periods of 6 and 2 months, respectively.
Detailed treatment protocols were hard to find from the authors of the included studies, so the extent to which the cognitive stimulation was delivered as intended could be questioned. Some recent studies described that staff received training and/or supervision in running the groups. Chapman et al. 51 described weekly meetings held to ensure that their treatment programme was implemented as designed:
Sub-groups were led by a licensed speech-language pathologist and three masters level speech-language pathology students; all underwent two hour training before the groups started; weekly meetings to ensure the programme was implemented as designed.
Onder et al. 53 described how family caregivers were trained by a multidisciplinary team and given a manual and specific schedule for each session. No records were made, however, of how often caregivers delivered the sessions or how closely the manual was followed. The only available data from an early study came from Professor Woods, who stated during a face-to-face meeting that ‘A sample of sessions were tape-recorded and rated to ensure compliance with the therapeutic protocol’ (Bangor University, 2008, personal communication).
Meta-analysis
Data from the included studies were entered into ‘Metaview’ (the Cochrane term for meta-analysis). Data were identified, included and pooled from the 15 included RCTs,6,48–55,59–64 with a total of 718 participants (407 in experimental groups and 311 in control groups). Analyses were adjusted to the random-effects model, owing to the heterogeneity of trials, and SMDs, because trials used different measures to assess the same outcomes.
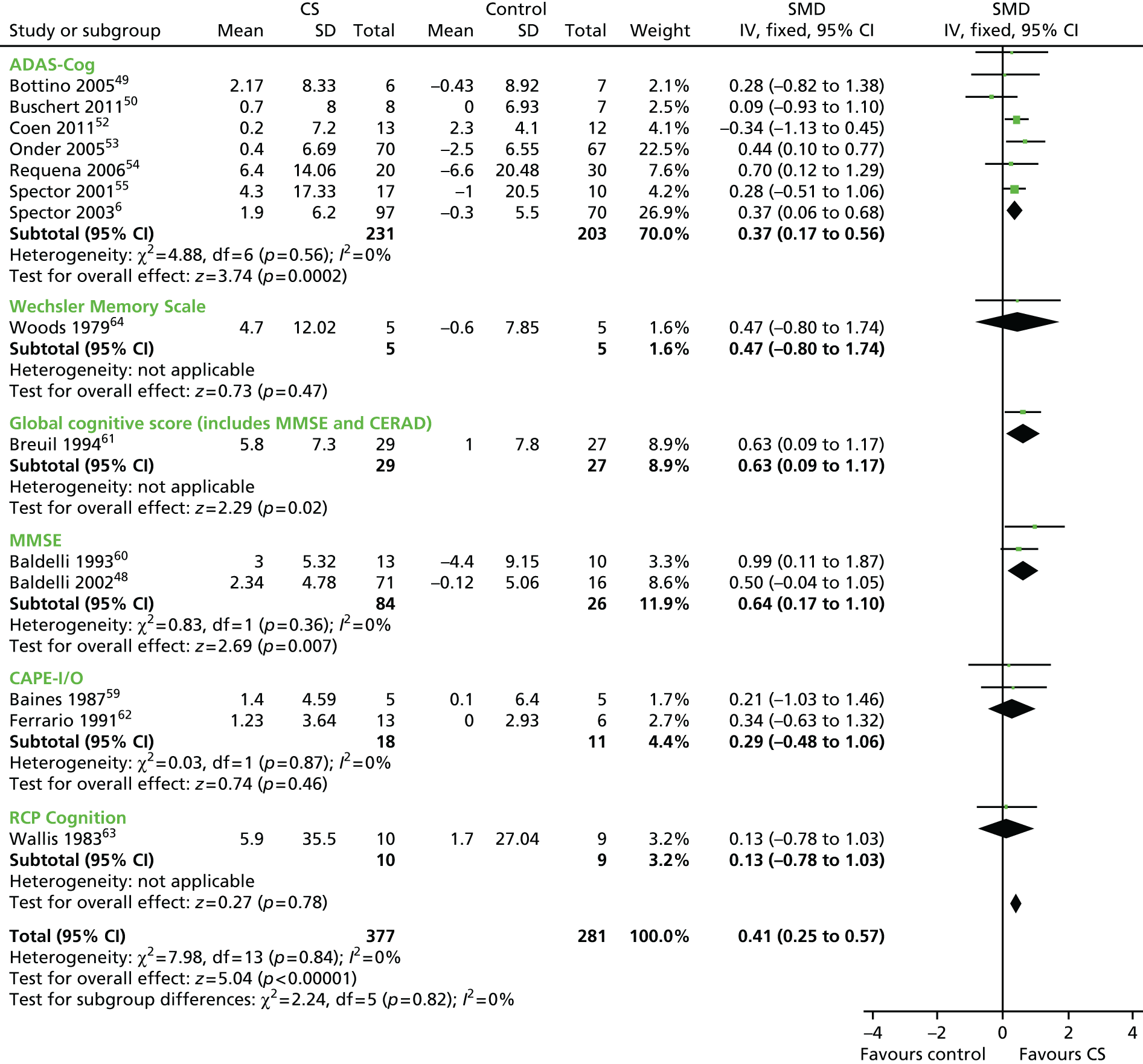
In order to evaluate the effect of cognitive stimulation on cognitive function, 14 RCTs that included useable data were included in the analysis (n = 658; 377 received treatment and 281 received no treatment or placebo). In comparison with the control groups at the post-treatment assessment, cognitive stimulation was associated with significant improvements on all measures of cognition. The overall results in the cognitive section were significantly in favour of treatment (Figure 2). The overall effect size (SMD) was 0.41 [95% confidence interval (CI) 0.25 to 0.57]. The results were strongly weighted by Breuil et al. 61 (n = 61), Onder et al. 53 (n = 137) and Spector et al. 6 (n = 201), the largest studies. The largest effect sizes can be seen at the 12-month point in Requena’s et al. 54 study [SMD 0.70 on the Alzheimer’s Disease Assessment Scale – Cognitive Subscale (ADAS-Cog)] and the Baldelli et al. 60 study (SMD 0.99 on the MMSE), both of which offered above-average duration of exposure to cognitive stimulation. Other studies with longer exposure62 had below-average effect size and other studies which offer only 10 hours of exposure had above-average effect size (0.63), showing that these effects require replication, as the CIs are broad and cross zero.
FIGURE 2.
Forest plot of comparison for CS vs. no CS: outcome – cognition. ADAS-Cog, Alzheimer’s Disease Co-Operative Study–Cognitive Subscale; CAPE I/O, Clifton Assessment Procedures for the Elderly – Information/Orientation scale; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; CI, confidence interval; CS, cognitive stimulation; df, degrees of freedom; IV, inverse variance; RCP, Royal College of Physicians.
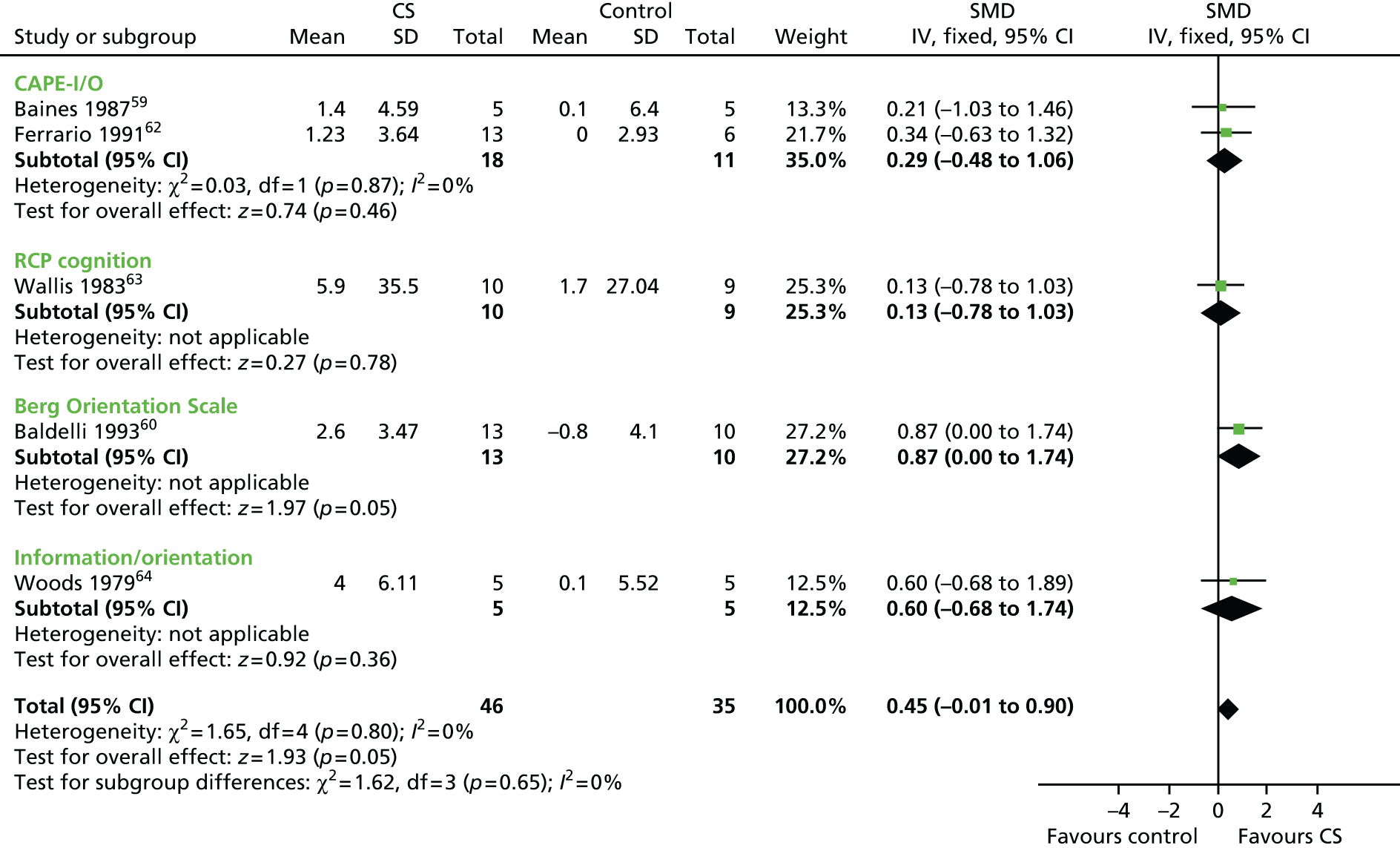
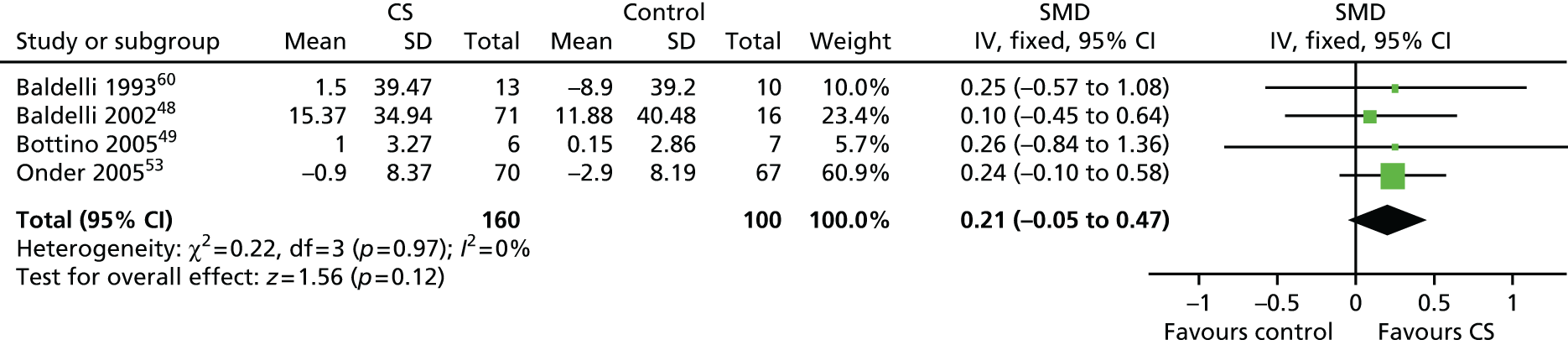
To evaluate the effectiveness of cognitive stimulation on behaviour, three separate meta-analyses were conducted. One focused on outcome measures seen as a problem, the second focused on ADLs and the third focused on general behaviour outcomes. Three studies used behavioural outcome measures seen as a problem (Figure 3) including 166 participants, showing that no difference was apparent related to cognitive stimulation (SMD –0.14, 95% CI –0.44 to 0.17; z = 0.86; p = 0.39). The ADL measure results also did not achieve significance (Figure 4), including four studies, involving 260 participants. There was no benefit identified associated with cognitive stimulation (SMD 0.21, 95% CI –0.05 to 0.47; z = 1.56; p = 0.12). The third behaviour analysis (Figure 5) (general behaviour) showed a similar picture, with no difference emerging. Eight studies reported data on relevant scales, including 416 participants (SMD 0.13, 95% CI –0.07 to 0.32; z = 1.30; p = 0.20).
FIGURE 3.
Forest plot of comparison for CS vs. no CS: outcome – ADAS-Cog. CS, cognitive stimulation; df, degrees of freedom; IV, inverse variance.
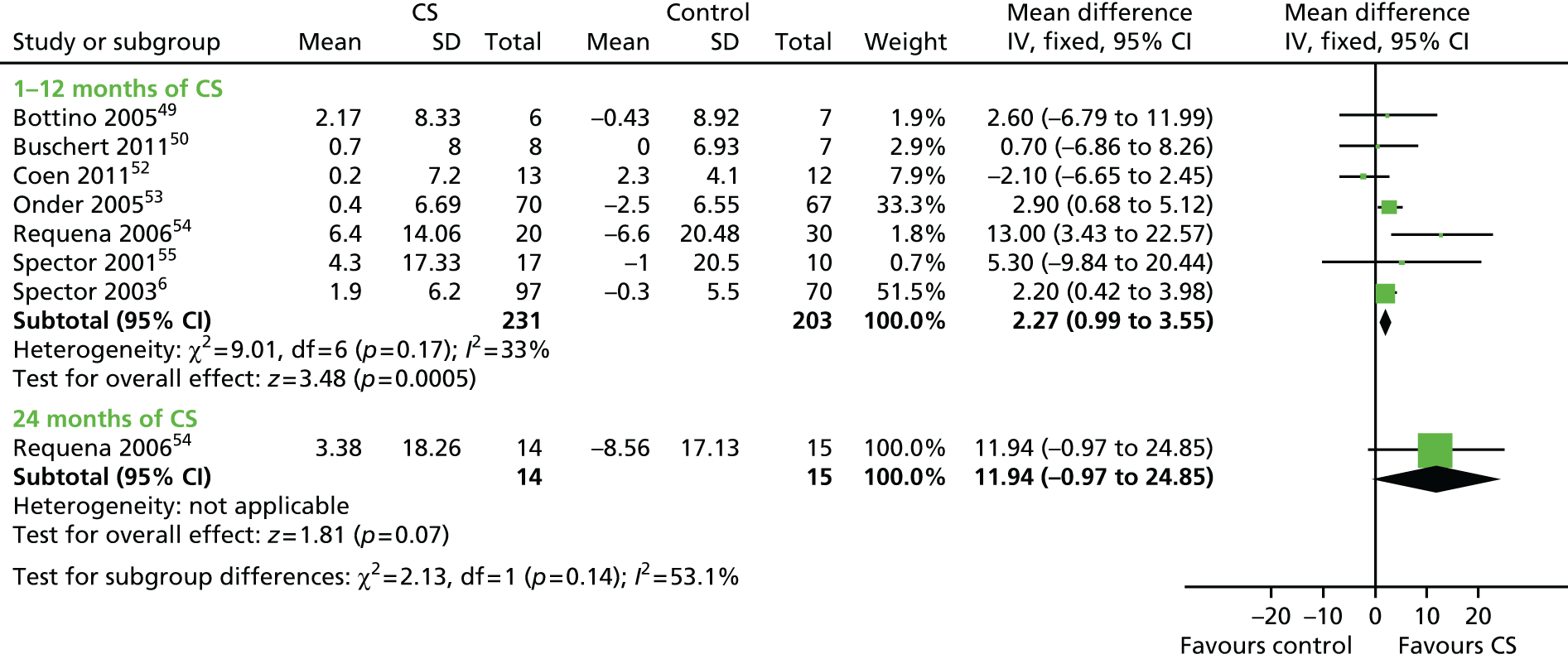
FIGURE 4.
Forest plot of comparison for CS vs. no CS: outcome – MMSE. CS, cognitive stimulation; df, degrees of freedom; IV, inverse variance.

FIGURE 5.
Forest plot of comparison for CS vs. no CS: outcome – other cognitive measure (information/orientation). CAPE-I/O, Clifton Assessment Procedures for the Elderly – Information/Orientation scale; CS, cognitive stimulation; df, degrees of freedom; IV, inverse variance; RCP, Royal College of Physicians.
Five studies, involving 201 participants, used a self-report measure of mood (the Geriatric Depression Scale or the Montgomery–Åsberg Depression Rating Scale) (Figure 6). Cognitive stimulation is not associated with a clear improvement in mood (SMD 0.22, 95% CI –0.09 to 0.53; z = 1.42; p = 0.16). This is of similar magnitude to the finding of proxy report of mood and anxiety, where the SMD is close to zero (SMD 0.05, 95% CI –0.21 to 0.31) (Figure 7).
FIGURE 6.
Forest plot of comparison for CS vs. no CS: post treatment, outcome – communication and social interaction. CS, cognitive stimulation; df, degrees of freedom; IV, inverse variance; MOSES, Multidimensional Observation Scale for Elderly Subjects.
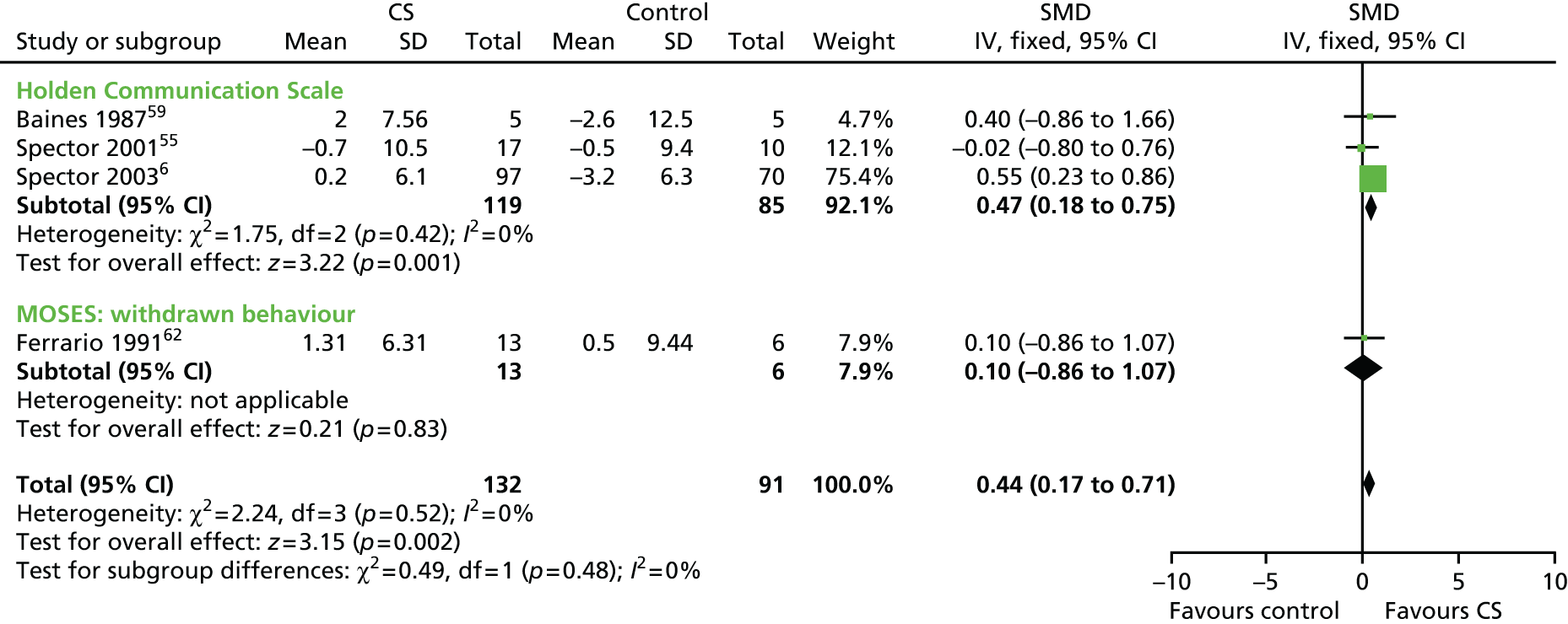
FIGURE 7.
Forest plot of comparison for CS vs. no CS: outcome – GDS. CS, cognitive stimulation; df, degrees of freedom; IV, inverse variance.

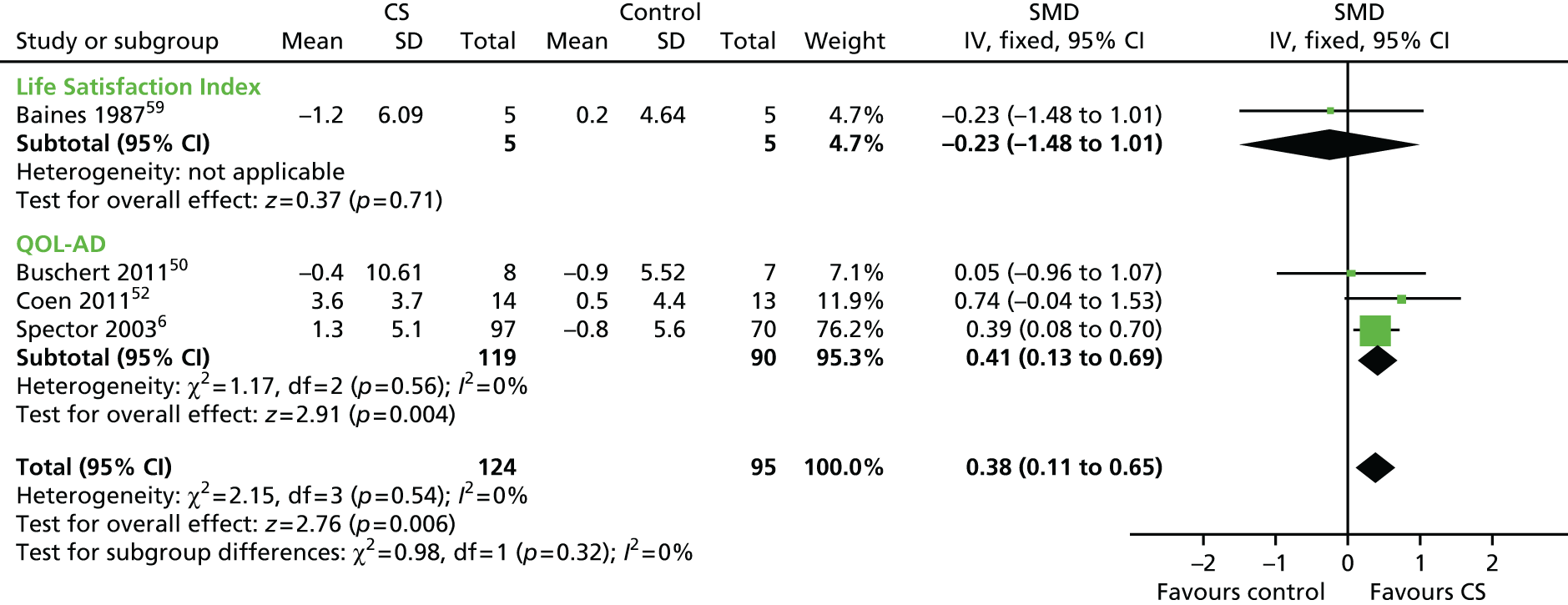
Four studies included self report well-being and quality-of-life measures (n = 219) (Figure 8). The analysis showed a significant improvement on this outcome measures following treatment, compared with control groups. The SMD was 0.38 (95% CI 0.11 to 0.65; z = 2.76; p = 0.006).
FIGURE 8.
Forest plot of comparison for CS vs. no CS: outcome – quality of life. CS, cognitive stimulation; df, degrees of freedom; IV, inverse variance; QOL-AD, Quality of Life – Alzheimer’s Disease.
Follow-up
Owing to the great variety in follow-up data, the analyses were divided in two sections: short- and long-term follow-up. Short-term follow-up analysis included Baines et al. 59 and Wallis et al. ,63 who had a 1-month follow-up, and Baldelli et al. ,60 who reported data from a 3-month follow-up. Long-term follow-up data included Chapman et al. ,51 who reported useable data only from a 10-month follow-up, a much longer period in the context of the progression of dementia. For cognitive measures (Figure 9), the three studies with short-term follow-up reported data for 52 participants. The significant advantage for cognitive stimulation on cognitive measures seen immediately post treatment remained at this point (SMD 0.57, 95% CI 0.01 to 1.14; z = 2.00; p = 0.05). For the 54 participants included by Chapman et al. 51 (Figure 10), there was no significant effect on either the MMSE (SMD 0.18) or the ADAS-Cog (SMD 0.12) at the 10-month follow-up. No other significant results were found in the other outcome measures at either the short- or the long-term follow-up analysis.
FIGURE 9.
Forest plot of comparison for CS vs. no CS: post treatment, outcome – mood, staff-reported. CS, cognitive stimulation; df, degrees of freedom; IV, inverse variance; RAID, Rating of Anxiety in Dementia.
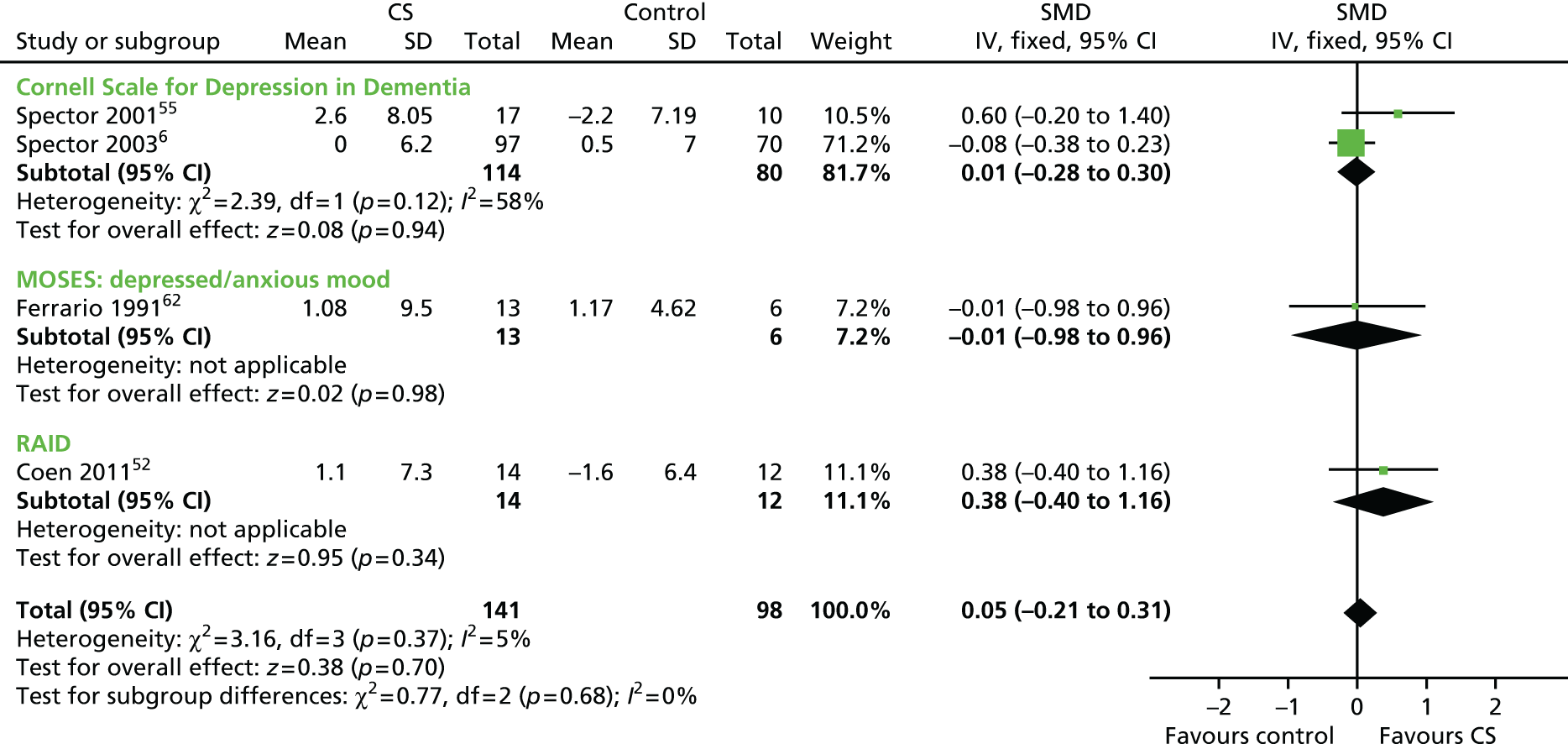
FIGURE 10.
Forest plot of comparison for CS vs. no CS: outcome – ADLs. CS, cognitive stimulation; df, degrees of freedom; IV, inverse variance.
Discussion
The results of this updated meta-analysis of 15 studies with a total of 718 participants (407 receiving treatment and 311 controls) provide strong evidence of the benefits of cognitive stimulation for dementia on cognitive function. Moreover, they provide positive evidence that benefits can be extended to quality of life and self-reported well-being outcome measures. This review did not show a clear relationship between either the amount/frequency or the length of intervention and benefits to cognition, and the studies that showed most benefit were both shorter (Spector et al. 6 and Breuil et al. 61 having 7 and 5 weeks, respectively) and longer (Onder et al. ,53 with 25 weeks). The MMSE was of a similar size to the SMD (1.30) of the Spector et al. 6 and Onder et al. 53 studies. Because MMSE scores decline, on average, by 2 to 4 points per year on the MMSE for dementia,68 the benefits of cognitive stimulation might equate to a 6-month delay in the usual cognitive deterioration.
Recent reviews of psychosocial interventions for dementia are in line with the findings of this review and give strong recommendation for the use of cognitive stimulation for dementia. 8,69 Moreover, the 2011 World Alzheimer’s Report1 offered some evidence in relation to the benefits of AChEI medication and cognitive stimulation. This review also offered some evidence in relation to AChEI medication. Five out of the 15 included studies report data whereby all of the participants were prescribed AChEI medication in combination with cognitive stimulation. The additional effect of cognitive stimulation, over and above the medication (in four studies providing post-treatment data), was 3.18 points on the ADAS-Cog, compared with the overall finding (from seven RCTs) of 2.27 points. This supports the proposition that cognitive stimulation is effective irrespective of whether or not AChEIs are prescribed, and any effects are in addition to those associated with the medication.
Conclusions
Our review consolidates the growing evidence that cognitive stimulation improves cognitive function in people with dementia. It also indicates that cognitive stimulation benefits not only cognition but also self-reported well-being and quality of life, as has been reported by qualitative studies and people with dementia for a long time. 70 These benefits are over and above any antidementia medication effects.
Work package 2: development of the maintenance cognitive stimulation therapy programme
This section reports the development of the MCST programme manual. We followed the MRC guidelines for the development and evaluation of complex interventions30 using a systematic review of the literature44 and the original CST trial programme6 to produce a full draft of a manual for a MCST programme. As in the main CST programme, maintenance sessions focus on ‘themes’, with a primary emphasis on cognitive stimulation, while incorporating the process of reminiscence therapy and multisensory stimulation. Group names and songs, a ‘reality orientation board’ and introductory exercises provided continuity between sessions.
Objectives
-
To identify, from the Cochrane review studies, the interventions that have shown to be effective and have had an impact at improving cognition and quality of life of people with dementia, including any negative effects.
-
To indicate the nature and quality of the interventions and identify key themes and elements.
-
To develop, from the analysed interventions and current CST training manual, 24 weekly sessions of MCST.
-
To develop a MCST training package for care staff based on the existing CST manual. This comprised a manual workbook, a DVD and training seminars.
Development of maintenance cognitive stimulation therapy sessions and workbook
We selected all relevant and effective interventions from the CST Cochrane review. We contacted study authors with the aim of obtaining additional information, hard copies of the intervention programme manuals and the manuals translated into English. The research team developed a database identifying key themes from the initial CST manual71 guiding principles and sessions, and included the 16 sessions developed for the MCST pilot project. A draft manual (version I) was produced based on the results.
The results from the analysis of the cognitive stimulation manuals, including the original papers, manuals and table database, were presented in a consensus workshop (comprising key academics, research staff and clinical staff involved in CST practice) and used to validate and review a subsequent draft of the MCST manual (version II), which included 24 maintenance sessions and was produced using the results of the consensus workshop. The MCST manual (version II) was discussed in focus groups with care staff (three groups), carers (three groups) and people with dementia (three groups) to review key themes, feasibility and potential modifications. The revised MCST manual was further modified in consultation with the attendees of the consensus workshop and key contributors to other aspects of process (e.g. a sample of care staff, carers, clinical staff and leading experts on CST).
The next draft of the MCST manual (version III) was produced to publication quality and was used in the development of a revised version of the CST training package compromising the revised manual, a CST/DVD including extra maintenance sessions, a PowerPoint® 2003 (Microsoft Corporation, Redmond, WA, USA) presentation, methods of evaluation and adherence. We developed the training package in consultation with trainers from Dementia UK.
Focus groups: a comparison of the views of people with dementia, staff and family carers
We included qualitative testing of the intervention through focus groups, developing a consultation process with people with dementia, family carers and staff. The aim was to identify improvements for the draft version of the MCST manual. Focus groups were the natural choice, as the aim was to gain comprehensive views of the key stakeholders with regard to the MCST programme. A key strength of focus groups is their ability to ‘tune in’ to the attitudes and perceptions of users regarding services.
Method
Sample
Focus groups were undertaken separately with three user groups that consisted of key stakeholders in the project. Three focus groups were carried out with people with dementia, three groups were carried out with staff and three groups were carried out with family carers of people with dementia. In total, 17 people with dementia, 13 staff and 18 family carers took part in separate focus groups. In the people with dementia groups, there were eight men (47%) and nine women (53%). Their mean age was 78 years, they all scored mild to moderate on the CDR scale46 and were able and willing to consent to participate in the focus groups. The staff groups consisted of three men (23%) and 10 women (77%), with a mean age of 36 years. All were permanent, paid staff, with at least 1 month’s employment, from residential homes, day centres or day hospitals. Their main duties involved caring for people with dementia. The family carers consisted of six men (33%) and 12 women (67%), with a mean age of 53 years. All were former or current carers of a person with dementia and had at least monthly contact during their time as a family carer.
Purposive sampling, for example sex, length of caring experience and dementia type, was used to ensure a wide range of participants. The centres selected were typical in size, organisational structure and management of others in the area. When people were recruited via local carers’ organisations (Uniting Carers for Dementia and Alzheimer’s Society), permission was also obtained from the management committees of these groups.
Procedure
We used the Noticeable Problems Checklist72 to screen potential participants who were then approached for their consent and further screening using the CDR scale. Participants with a CDR score of mild to moderate were included, provided that they did not meet the exclusion criteria (diagnosis of severe learning disability and/or diagnosis of depression, anxiety or other mental or physical illness adversely affecting their ability to participate in focus groups) and were willing to take part in the discussion groups. Seventeen participants met the inclusion criteria and agreed to take part. Members of staff who were working directly with people with dementia in the different centres approached were invited to take part in the groups. Eighteen family caregivers were recruited through Uniting Carers, Dementia UK and the Alzheimer’s Society. Participants were reminded that they could leave the group at any time.
Two researchers conducted self-contained, hourly focus groups. The sessions included a brief presentation about the overall project. One facilitator led the interview, with the second person actively listening and seeking clarification, to ensure the adequacy and accuracy of content as appropriate. 73 The second facilitator also took substantive and methodological field notes during and immediately after the focus groups, as recommended by Burgess. 74 A semistructured interview schedule based on the CST empirical literature was used as cues for open questions. The groups focused on the 24 themes developed for the MCST programme and the cognitive stimulation definition by Clare et al. 11
Design
Focus groups were chosen in preference to individual interviews, as they are useful for stimulating discussion, generating ideas to explore topics in depth, gaining insight and obtaining rich data. 75 A focus group interview schedule was constructed and developed to provide a framework for the discussions, which was piloted and adapted. The participants were encouraged to express their opinions and discuss issues through a series of open questions, which covered various aspects of mental stimulation activities, beginning with a presentation of the programme on a DVD and followed by open questions designed to elicit the activities and themes that participants particularly enjoyed and factors that contributed to the programme activities being stimulating and meaningful. Questions included ‘what do you think about use it or lose it/mental stimulation?’ and ‘could you tell us a bit about what sort of things you do that you find as being mental stimulating for you and you enjoy doing them?’. Pictures and materials used in the different themes and activities were used to help stimulate discussion in the participant groups. All groups used the same schedule, and participants were asked their opinions of what activities/themes they thought would be successful among people with dementia.
Analyses
The focus group interviews were tape-recorded and transcribed. The facilitators then reviewed the transcripts. The notes taken by the second facilitator were consulted to clarify the context of the discussion (e.g. benefits of being part of a group that was expressed by people with dementia focus groups). We used an inductive (data driven) thematic analysis approach76 to code and analyse the data. Inductive analysis allows themes to emerge from the data and is useful when the intent is exploratory and descriptive, as in our case. Consistent with various qualitative research methods, the focus group inquiry allows participants the freedom to provide information that does not necessarily fit with any expectation/hypotheses going into the research. This openness to new and unexpected information allows measurement designers to more fully ‘ground’ the content of new patient-reported outcomes in the concerns and issues that participants think are relevant. 77
To develop a thematic codebook, researchers immersed themselves in the transcripts and rereading to gain deeper understanding of and familiarity with the content. One transcript was revisited and analysed into exclusive in vivo categories; that is, one theme was applied to one unit of meaning and the words used by the participant were adopted as the label of the theme. These themes were then applied to the remaining transcripts using a method of constant comparison to refine themes within the coding manual. During this process themes became more conceptual, with some original themes split into subthemes and others linked under one category depending on the emphasis placed on each theme within the transcripts. The resulting coding manual provided a meaningful way of understanding the views of the participants and assessing similarities and differences across the different groups (people with dementia, staff and family caregivers). Using the final codebook, all transcripts were coded independently by both researchers and then compared to reach 100% consensus.
Results
Thematic analysis revealed themes relating to perceptions and opinions of ‘mental stimulation/use it or lose it’, ‘examples of mental stimulating activities of daily life‘, ‘factors influencing successfulness and unsuccessfulness of a mental stimulation activity’ and ‘opinions and perceptions of specific themes of the presented maintenance CST programme’. Patterns of themes were found among the different groups (people with dementia, family caregivers and members of staff).
In the quotations below, PWD refers to the group ‘people with dementia’, FC refers to the group ‘family caregivers’, SC refers to the group ‘staff caregivers’, FG1 and FG2 refer to the focus group numbers and the digits refer to the line numbers in the transcript.
Mental stimulation: ‘use it or lose it’
This included discussing their opinions regarding mentally stimulating activities, ‘use it or lose it’, in terms of their views and beliefs about the effects of keeping the brain active. All participants showed general agreement about the usefulness of keeping the brain active.
People with dementia expressed the view that keeping the brain active was very important and a way of relieving frustration. They thought that it was essential for a healthy life and preserving their mental abilities, and that engaging in activities would keep the brain going. Some family carers expressed the view that the need for mental stimulation was universal and could bring neurological (building connections in the brain) and mental (helping with mood, anxiety, depression) benefits to everyone, and was important for promoting a healthy lifestyle and well-being. Staff and family carers also thought that there were added benefits to people with dementia in terms of increasing confidence, giving a sense of achievement, satisfaction, retaining skills and enjoyment.
No participants with dementia expressed negative views about the value of ‘use it or lose it’; however, there were concerns about the importance of keeping the brain active from members of staff and family carers groups who gave examples of individuals for whom the idea of ‘use it or lose it’ did not apply. Famous writers and politicians were given as examples of people who maintained a mentally stimulating, active lifestyle but nevertheless developed dementia. Some family caregivers expressed concerns that mental stimulation programmes may result in people with dementia losing confidence, experiencing anxiety or a sense of inferiority if confronted with their own cognitive deficits and difficulties as a result of undertaking a challenging mental activity.
Perceived stimulation in everyday life
Listening to music, singing and dancing, reading, painting, drawing, cooking and knitting were highlighted as being important for people with dementia. Factors that made an activity important included being interesting, being enjoyable, having a relaxing effect and helping to pass the time. Reading was a popular activity for most people with dementia as it raised their confidence and helped to increase interaction and participation when part of a group. In particular, talking and listening to others appeared enjoyable and were highly valued among people with dementia. They thought that talking to another person, to a pet or to the TV maintained their links with important current and past relationships and helped to reduce feelings of isolation.
Being part of a group helps considerably. I think being left on your own is not as effective as being part of a group. I belong to an African Caribbean group and I find that very stimulating. We talk on a number of things what we have done and why we did it. I think talking is very important.
PWD: FG1; 175–179
Family caregivers perceived that activities involving music (e.g. listening to music, singing, tapping and clapping) were those that people with dementia enjoyed the most.
Sounds and music are very important to stimulate something that’s there already so they recognise . . .
FC: FG2; 183–184
Those in the family caregivers group also spoke of having the opportunity to enjoy dinner together and to reminisce together by looking at photographs or sharing memories.
They learned a lot about others, everyone was telling their old past stories of how they went to school in shared shoes and things like this as a family. And we got so much information and it really stimulated them.
FC: FG2; 232–238
Staff caregivers thought that their planned activities were the most valuable stimulating activities for people with dementia. Staff participants identified reminiscence as an activity people with dementia enjoyed and often engaged in. However, there appeared to be little understanding of its value, and concern that it did not relate to the present.
I think we have to be careful with reminiscence, for me one of the best bits of this therapy is the variety of activities that you are going to do, otherwise it can get very repetitive as you tend to do things that you know work well, like reminiscence. You have to set goals within every session . . .
SC: FG1; 60–66
Factors influencing success of a cognitive stimulation programme
The perceptions of what made a CST programme successful were based on the person’s values and beliefs, with their interests and routines reinforcing a sense of identity and sense of belonging. Staff and family caregivers appreciated that the philosophy of the programme should be person centred and that enjoyment was a measure of what made programme activities successful.
People with dementia stated that basic human courtesies were important: being kind, trying to make others happy and not underestimating participants’ abilities.
Nothing that involves cruelty. As long as there’s kindness you can’t fault it.
PWD: FG1; 366
Being able to discuss, learn and make contributions was also mentioned.
I think it is very important to learn new things, you don’t stop learning ‘till you die.
PWD; FG1; 669–672
Other factors highly valued in the groups were activities that included reminiscence as an aid to orientation and activities that were provided in a multisensory way. Activities involving discussion and sharing opinions among the group were highly valued, as were challenging activities and quizzes requiring right or wrong answers.
Some people will remember more things than others you know. But it’s good for the brain . . .
PWD: FG1; 576–579
In contrast, family caregivers and staff participant groups stated that activities in the programme should not be based on right or wrong answers, so that people with dementia did not feel under pressure. ‘Playing it safe’ was perceived as being very important and this helped people feel more secure.
You have to adapt to the person and never ask them to do anything they can’t do because they have a sense of self and it will give them a sense of inferiority or inadequacy.
FC: FG2; 90–94
Most staff acknowledged that it was important to identify participants’ individual preferences, skills and abilities, as this affected their level of engagement in activities, whereas some recognised the need to adapt activities to a participant’s capabilities as a way of providing choice and contributing to their well-being. Some family carers stressed the importance of providing this programme to people only in the mild-to-moderate stages of dementia, as they thought that it would not be appropriate for people in more advanced stages.
The group participants should be of [a] similar level of dementia.
SC; FG2; 176
They also thought that group participants should be chosen based on having similar abilities and interests so that the group could run successfully and be stimulating and enjoyable. Both staff and family carer groups noted that attention needed to be paid to each participant’s level of hearing and vision, as they thought that high levels of impairment in either would limit a person’s participation.
You have to think about the personality dynamics within each group.
SC: FG2; 109
Staff and family caregivers also indicated that the group facilitator’s skills, knowledge, understanding of dementia and attitudes towards participants was also key to running the group effectively. The need for several facilitators was mentioned, as was limiting the number of group participants. Appropriate equipment was identified as another key factor to the success of this programme.
I think the size of the group has to be pretty small as an important factor.
FC: FG2; 388–391
Presented themes
A total of 19 themes was presented to the focus groups of people with dementia, family caregivers and staff. Fourteen themes were from the existing CST programme. Five new themes were developed from the literature review and the pilot MCST study (Table 2).
| Maintenance session | Version 2 | Version 3 | People with dementia | Staff | Family carers |
|---|---|---|---|---|---|
| New session themes | |||||
| Useful tips | 11 24 | 11 24 | Excellent: discussion (learn and teach) and reminiscence | Excellent: discussion (learn and teach) and reminiscence | Good: some concerns about healthy tips |
| Visual clips | 13 | 13 | Not interested | Good theme | Very good, discussion, reminiscence and multisensorial |
| Thinking cards | 12 22 | 12 | Very good | Mixed opinions, down on list | Mixed opinions |
| Art discussion | 14 | 14 | Mixed emotions | Mixed emotions: will generate discussion | Very good, discussion |
| Using objects | 10 | 10 22 | Very good | Excellent theme: discussion and reminiscence | Excellent theme: discussion and reminiscence |
| CST session themes | |||||
| Physical games | 8 | 8 | Very good | Very good | Very good |
| Sounds | 7 | 7 | Very good | Very good, music and multisensorial | Very good, music and multisensorial |
| Childhood | 1 23 | 1 23 | Good, enjoyable | Very good: multisensorial and reminiscence | Very good: multisensorial and reminiscence |
| Food | 3 17 | 3 17 | Very good | Very good: multisensorial | Very good: multisensorial |
| Current affairs | 2 21 | 2 | Very interesting | Mixed emotions: needs to include reminiscence | Mixed emotions, not for everyone. Needs to include reminiscence |
| Faces/scenes | 15 | 15 | Very successful (discussion and reminisce) | Very good theme: discussion | Very good theme: discussion, recognition |
| Associated words | 18 | 18 | Very good | Very good at the right level | Very good |
| Being creative | 4 | 4 | Fun | Good theme, some dangers (not for everyone) | Very good: multisensorial |
| Categorising objects | 9 | 9 | Mixed emotions | Very successful (discussion and reminisce) | Very successful (discussion and reminisce) group cohesiveness |
| Orientation | 19 | 19 | Very good | Very good: discussion and reminiscence | Very good: discussion and reminiscence |
| Using money | 20 | 20 | Very good | Mixed emotions, sensitive topic | No good topic |
| Number games | 5 | 5 | Not popular | Mixed opinions, some dangers (not for everyone) | Mixed emotions |
| Word games | 16 | 16 21 | Excellent activities | Very popular, good | Good activity, pay attention to presentation |
| Quiz | 6 | 6 | Excellent theme | Very popular, good | Good |
The five new themes
People with dementia rated useful tips, thinking cards and using objects as very positive themes. They stated that these themes were good for learning, hearing other people’s opinions and giving their own opinions in the group. They thought that the themes could help the group cohesiveness and would trigger conversation. Family caregivers and staff groups also thought that useful tips and using objects were good themes for the session and highly valued the involvement of reminiscence in the activities as an aid to orientation. Some staff expressed concern that the thinking cards theme would not work but others challenged that it would. Some family caregivers thought that the proposed activities for this theme would not be appropriate, as some people did not like ‘closing your eyes and imagining’ and might feel uncomfortable with this. Other carers liked this theme and thought that the questions were a good way of stimulating conversation and possibly helping group cohesion.
People with dementia rated visual clips discussion and art discussion as neutral. Although they liked the idea of group discussion, they did not feel enthusiastic about the topics presented. Staff and family caregivers groups rated these themes positively, as visual prompts were highly valued and they liked the idea of promoting discussion. Some staff had successfully run these types of activities previously with people with dementia and advised that materials be chosen appropriately.
Existing cognitive stimulation therapy themes
Participants were asked to rate and organise the presented themes as very positive, neutral or negative, and to rank them in order of those themes perceived as most and least successful. My life (childhood and occupations), food and orientation were perceived as positive themes; they were applicable to everyone, helped people to keep in touch with themselves and were multisensorial. Quizzes and word games were rated highly among people with dementia, who thought that they were very stimulating and helped the brain to keep working and ‘ticking together’. Family caregivers and staff also rated these two themes very highly. Physical games, sounds, faces and scenes, categorising objects, associated words and being creative were also rated positively by all groups, as these were good for stimulating recognition, reminiscence and discussion, offered multisensorial stimulation and promoted keeping healthy and active.
Number games was the only theme rated very low by people with dementia, who said that, as numbers were not something they related to very well and were a bit meaningless to them, this was not their preferred choice of activity. Family caregivers and staff shared similar views, as their previous experience indicated that number games required more one-to-one work with people with dementia, and were frustrating and pointless unless the numbers were related to pricing.
Using money and current affairs were rated very low by the family caregivers and staff groups but rated highly by people with dementia. Some family caregiver and staff participants stated that people with dementia did not often relate to current affairs (owing to the disease) and it would be meaningless to present topics about news unless reminiscence was used as a context for the information. However, people with dementia expressed a great interest in current affairs and stated that they loved reading newspapers. People with dementia also stated that they would enjoy talking about the value of money, and there were spontaneous comments about the value of money and the cost of bus journeys in the past and the present. In contrast, family caregivers and staff thought that money was too complicated and not a good theme, as it could be a very sensitive topic for some people with dementia.
Discussion
We used a novel approach to refine an existing psychological intervention programme that investigated the opinions, types of activities and qualities which make a cognitive stimulation programme more successful for people with dementia. An advantage of using focus groups with people with dementia is that sharing experiences may trigger recall. A possible disadvantage is that they rely on verbal communication and short-term memory, both of which are often impaired in people with the condition. 78
Opinions about mental stimulation programme and key factors for success
People with dementia thought that keeping the brain active was essential and they acknowledged that this helped with their memory losses and difficulties. This finding supports Barnett’s79 statement that bereavement with memory losses is a major theme for people with dementia, who value the opportunity to be listened to. They also value being in a group. As indicated in other studies, socialising is an important activity for older people in care. 80–82 People with dementia valued quality social interactions, especially when being part of a group, and feeling a sense of belonging, reflecting Kitwood’s83 theory, which claims that positive interactions reinforce the personhood of those with dementia.
Conversely, staff and family carers expressed mixed opinions about the effectiveness of keeping the brain active; they cited examples of public figures who had developed dementia and said that the focus should be on the factors that would make a programme successful or unsuccessful for people with dementia. The main factors to consider when planning a CST group were grouped into participant characteristics (level of dementia, sensory impairments, personality, interests, life history), facilitator characteristics (knowledge about dementia, group skill and personality), group size and materials (multisensorial prompts, age appropriate).
Limitations
Staff focus groups included managers or senior carers, the presence of whom may have affected the opinions expressed by other staff members. Participants sometimes appeared reluctant to give personal examples, perhaps inhibited by the stigma of sharing information in a group, and this may be a disadvantage of using focus groups. We selected a thematic analysis, as it is a method for identifying, analysing and reporting patterns (themes) within data. Although thematic analysis is used widely, there is a lack of consensus regarding its precise methodology. 84
Conclusion
Findings from the user focus groups regarding MCST programmes support the 2006 NICE guidelines on dementia85 that state that all people with mild to moderate dementia should be ‘given the opportunity to participate in a structured group cognitive stimulation programme’. Positive agreement was found among 14 themes and suggestions were made for the five remaining themes. These results were used to revise the manual for the MCST programme.
Work package 3: maintenance cognitive stimulation therapy for dementia – a single-blind, multicentre, randomised controlled trial of maintenance cognitive stimulation therapy versus cognitive stimulation therapy for dementia
Introduction
This section describes the study protocol for a pragmatic RCT of CST versus CST followed by a 24-week MCST programme undertaken with people experiencing mild to moderate dementia. This research programme aims to provide essential evidence to clarify the role of long-term CST interventions alone and in combination with cholinesterase inhibitors, and to assess the cost-effectiveness of this long-term vision.
Methods
Design
The design was a single-blind, multicentre RCT of CST groups for dementia versus MCST groups (Figure 11). After the completion of the initial CST programme (twice-weekly 45-minute sessions for 7 weeks), participants were randomly allocated to the treatment group (maintenance sessions weekly for 24 weeks) or the control group (usual care). Data were collected at baseline (baseline 0), after completion of the 7-week CST programme (baseline 1), and at 3- (follow-up 1) and 6-month follow-ups (follow-up 2). It was calculated that a sample size of 230 participants was needed at baseline 1 to detect an effect size of 0.39 on the ADAS-Cog,86 with power of 80% using a 5% significance level. Ethics approval was obtained through the Multicentre Research Ethics Committee (reference number 08/H0702/68). The clinical trial was registered as ISRCTN26286067.
FIGURE 11.
Flow diagram of the trial: trial and randomisation stages.

Participants
The participants were people meeting the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV)87 criteria for dementia graded as mild to moderate on the CDR scale,46 with the ability to communicate, hear and see well enough to participate in the group, with no major physical illness or disability, and without a learning disability. Half of the sample was recruited from care homes and half was recruited from community settings, including CMHTs, day centres and voluntary organisations in London, Essex and Bedfordshire. Of the 21 centres initially contacted, one refused to participate and two were excluded owing to a lack of participants.
Randomisation
The randomisation process in this trial was undertaken in two stages: randomisation 1 and randomisation 2. Figure 11 sets out the two-stage randomisation process. The allocation ratio at randomisation 1 stage was 1 : 1; into either group A or group B, with both groups receiving 7 weeks of CST.
The allocation ratio at randomisation 2 stage was 1 : 1; into either the control group or the treatment group.
The sample was stratified to ensure that equal numbers of participants taking cholinesterase inhibitors were randomised into either the MCST or the control group. The North Wales Organisation for Randomised Trials in Health (NWORTH) was responsible for undertaking the remote randomisation. NWORTH is accredited as a Clinical Trials Unit by the UK Clinical Research Collaboration and funded as part of the Clinical Research Collaboration Cymru, notably for Health Technology Assessment trials.
Blinding
The participants could not be blinded to group allocation owing to the nature of the intervention. The researchers involved in collecting participant consent and in the interviews were not involved in the randomisation process and were blinded to treatment group allocation at follow-up. Our experience in the previous CST trial, as shared by those conducting similar projects, is that participants may occasionally and inadvertently inform researchers of the treatment they are receiving. We aimed to reduce this effect by giving explicit reminders to participants before the assessment visit and by the use of self-report measures whenever feasible. On completion of the two follow-up assessments, the assessors recorded their impression of which arm of the trial each participant belonged to and their confidence in that prediction. This enabled us to test whether or not the inadvertent loss of blinding leads to bias, and to adjust for any bias that was detected.
Intervention
Cognitive stimulation therapy is an intervention for people with mild to moderate dementia, designed following extensive evaluation of the available research, and is an evidence-based treatment. 55 The programme consists of 14 45-minute sessions that are run twice-weekly. Each session incorporates the use of a ‘reality orientation board’, displaying both personal and orientation information, including the group name (as chosen by participants). The guiding principles of CST involve using new ideas, thoughts and associations; using orientation (but sensitively and implicitly); a focus on opinions rather than facts; using reminiscence as an aid to the here-and-now; providing triggers to aid recall; the creation of continuity and consistency between sessions; focus on implicit (rather than explicit) learning; stimulating language; stimulating executive functioning; and being person centred (treating people as unique individuals with their own personalities and preferences). The CST programme aims to create an environment in which people have fun, learn, strengthen their abilities and improve their relationships to other group members, thus maintaining their social and cognitive skills at their optimum ability.
The MCST programme is an evidence-based maintenance group therapy programme for people with dementia. It comprises 24 sessions of MCST, based on the theoretical concepts of reality orientation/cognitive stimulation and grounded in the original CST programme. The aim is to create an evidence-based maintenance group therapy programme for people with dementia, based on the same principles as those of the CST programme. A summary of the CST and MCST programme can be found in Table 3.
| CST session | Main activity | MCST session |
|---|---|---|
| 1 | Physical games | 8 |
| 2 | Sound | 7 |
| 3 | My life | 1 and 23 |
| 4 | Food | 3 and 17 |
| 5 | Current affairs | 2 |
| 6 | Faces/scenes | 15 |
| 7 | Associated words, discussion | 18 |
| 8 | Being creative | 4 |
| 9 | Categorising objects | 9 |
| 10 | Orientation | 19 |
| 11 | Using money (clip adverts II) | 20 |
| 12 | Number game | 5 |
| 13 | Word game | 16 and 21 |
| 14 | Team games, quiz | 6 |
| NEW | Useful tips | 11 and 24 |
| NEW | ‘Golden Expression’ cards | 12 |
| NEW | Art discussion | 14 |
| NEW | Visual clips discussion | 13 |
| NEW | Using objects | 10 and 22 |
Recruitment and training of facilitators
Each CST and MCST group had two facilitators, one from the research team and a cofacilitator who was a member of staff from the recruited centre (e.g. a care home). The facilitators had at least 1 year of experience in dementia care. The main facilitators often had a background in mental health nursing, occupational therapy or clinical psychology, as well as experience in dementia care and group facilitation skills. The use of two facilitators for each group enabled effective debriefing and reflection to occur at the end of each session. All facilitators attended 1-day CST training developed by one of the CST pioneers (AS) as part of the dissemination strategy. The training provided detailed background and description of CST, and used learning methods including group observation, role-playing and small group exercises.
Usual care
The participants allocated to the control group received treatment as usual (TAU). This can vary between and within centres, and may change over time but, in principle, the interventions offered to this group were also available to those in the active treatment groups. Therefore, the trial examined the additional effects of MCST. Our approach to costing the services and interventions received allowed us to monitor whether or not the TAU group had been receiving similar therapeutic interventions. The use of antidementia medication was recorded as part of the costing information collected. It was possible that participants in the TAU group were involved in some form of cognitive stimulation work during the 24 weeks of the study period. However, it is very unlikely that such a structured approach to CST was offered in any of the centres. It is this systematic group-based approach that was the focus of this evaluation.
Ethics arrangements
Risks and anticipated benefits for trial participants
There appears to be no documented harmful side effects of participating in CST groups, and no serious adverse reactions were apparent in the CST study. The participants in the groups consistently reported benefits, including enjoyment and feelings of validation and self-worth. 88 Participants’ inclination to continue meeting following the sessions gave an indication of how valuable they found these benefits. All adverse events were recorded and reports as per the SHIELD adverse events policy. 41
Consent
All recruited participants were in the mild-to-moderate stages of dementia, and were able to give informed consent for participation. Whenever possible, a family member or other supporter was included. It was made clear to participants and family caregivers that no disadvantage would occur if they chose not to participate. In seeking consent, we followed current guidance from the British Psychological Society40 on evaluation of capacity. If a participant’s level of impairment increased, so that they were no longer able to provide informed consent, the provisions of the Mental Capacity Act 200538,39 were followed.
Outcome measures
Primary and secondary measures were completed at baseline (time 0), after the 7 weeks of the CST programme (first follow-up, time 1), 3 months after beginning of the maintenance groups (second follow-up, time 2) and 6 months after the beginning of the maintenance groups (third follow-up and primary end point, time 3).
Primary outcome measures
-
Cognition was measured using the ADAS-Cog. 86 The ADAS-Cog consists of 11 tasks measuring the disturbances of memory, language, praxis, attention and other cognitive abilities, which are often referred to as the core symptoms of Alzheimer’s disease.
-
Quality of life was measured using the Quality of Life – Alzheimer’s disease (QOL-AD) scale. 89 The QOL-AD covers 13 domains of quality of life. It has good internal consistency, validity and reliability, and its use is recommended by the European consensus on outcome measures for psychosocial interventions in dementia. 90
Secondary outcomes
-
Cognition was measured using the MMSE,45 a brief, widely used test of cognitive function that has good reliability and validity.
-
Communication was assessed using the Holden Communication Scale. 91 This scale is completed by staff or family caregivers and covers a range of social behaviour and communication variables.
-
Depression was assessed using the Cornell Scale for Depression in Dementia. 92 This scale rates depression in five broad categories using information from interviews with staff and participants. Good reliability and validity have been demonstrated.
-
Anxiety is assessed using the Rating Anxiety in Dementia scale. 93 This rates anxiety in four main categories and uses information from interviews with staff and participants. It has good validity and reliability.
-
Behaviour was assessed using the Neuropsychiatric Inventory (NPI). 94 The NPI assesses 10 behavioural disturbances occurring in dementia patients. It has good validity and reliability.
-
Activities of daily living were assessed using the Alzheimer’s Disease Co-operative Study – Activities of Daily Living Inventory (ADCS-ADL). 95 The ADCS-ADL is a structured questionnaire originally created to assess functional capacity over the range of dementia severity. The sensitivity and reliability of the scale have been established. 95
-
Family caregiver health was assessed using the Short Form-12 Health Survey (SF-12). 96 This scale measures generic health concepts relevant across age, disease and treatment groups. The SF-1296 comprises eight concepts commonly represented in health surveys. It is a self-administered measure that provides a comprehensive, psychometrically sound and efficient way to measure health from the patient’s point of view by scoring standardised responses to standard questions. This measure was used only when a family caregiver was available from the community sample participants.
-
Costs were assessed using the validated Client Services Receipt Inventory (CSRI),97 adapted for this study. Used extensively in studies of mental health and dementia,13 the CSRI gathers comprehensive data on accommodation, medication and services received. In this trial the data covered the previous 3 months (at baseline and after treatment) or the 3 and 6 months’ follow-up points. Unit costs were then attached to services and support received, based on nationally relevant estimates of long-run marginal opportunity costs. Two quality-of-life measures were also included for cost–utility analyses. The EuroQol-5 Dimensions (EQ-5D)98 is a standardised instrument for use as a measure of health-related quality of life. It contains a three-level coding system for five dimensions. The instrument includes a global rating of current health using a visual analogue scale (VAS). The Dementia Quality of Life (DEMQOL)99 uses self-rated reports of quality of life administered by a trained interviewer; there is also a separate scale for family caregiver or members of staff reports, called the DEMQOL-Proxy. It covers five domains of quality of life. The DEMQOL has high internal consistency and acceptable inter-rater reliability, and indicates concurrent validity through moderate associations with the QOL-AD and DEMQOL. 99
Analyses
The assessments were scored and data were entered into MACRO™ (version 3.0.84, Infermed, Elsevier, London, UK), Infermed’s electronic data capture system that produces a fully auditable trail for data from input to extraction for analysis. For cleaning and analysis purposes, SPSS (Statistical Product and Service Solutions; version 20, IBM Corporation, Armonk, NY, USA) syntax was written to extract the relevant data from MACRO. No changes were made to the SPSS files. The randomisation was stratified according to use of AChEIs and living situation (i.e. living in the community or in care homes). A paired t-test was applied to the data to establish if there was a difference between the pre and post scores on the various outcome measures. This analysis was then followed by a repeated measure linear model to allow various variables to be taken into account. The model was fitted using post score as the dependent variable, with living situation, marital status, sex and AChEI medication as factors and age as a covariate. Finally, a two-sample independent t-test was conducted to compare the Spector et al. 6 control group results with the results seen in this group.
Complete-case data analysis was used initially to establish the results, followed by the analysis with imputations. When individual data points were missing within a scale, data were imputed by using scale/subscale means according to the validated rules for the measures. When an outcome measure total score was missing, it was imputed using a multiple imputation regression model comprising treatment group allocation, marital status, ethnicity, centre, age, sex, AChEIs, living situation (i.e. care home or community) and other outcome measures scores. The model used was a forward step model; that is, baseline outcome scores (baseline 0 and baseline 1) were used to help predict follow-up 1 scores, and then both of these were used to predict follow-up 2 scores. No data were imputed for those cases in which all assessments were missing. There were no participants missing for follow-up 1 who returned for follow-up 2.
Primary analyses used an intention-to-treat basis, analysing participants according to the group to which they were randomised and using all data. Multilevel modelling was used to address clustering within randomised groups. An analysis of covariance (ANCOVA) adjusted for baseline differences in outcome variables. Analyses considered the evaluation 6 months after the second randomisation as the primary end point. Secondary analyses considered the effects at 3 months. Age, sex, AChEI use and baseline scale scores were entered as covariates, and ‘centre’ was entered as a random factor. A secondary analysis included a trial platform for the MCST/AChEIs effectiveness that used the same model as for the complete analysis including an interaction term between AChEIs and the treatment group to identify any effect between the two factors for cognition (MMSE and ADAS-Cog) and quality of life (QOL-AD).
Results
Eighteen centres took part in the study trial (nine care homes and nine community), comprising 354 participants who were screened and 272 who participated in the CST groups. The reasons for exclusion included failure to meet the inclusion criteria (n = 42) or refusal to participate (n = 37). A total of 36 participants were lost between first and second randomisation (baseline 0 and baseline 1), leaving 236 participants randomised to the MCST or usual care group. The reasons for withdrawal can be found in the Consolidated Standards of Reporting Trials (CONSORT) diagram (Figure 12). Overall retention was good. At 6-month follow-up (follow-up 2) (primary end point), 199 participants (84%) remained in the study and there were 218 (92%) at 3-month follow-up (follow-up 1). The withdrawal rate was similar in both arms of the trial and the response rate, excluding deaths, was 89% at follow-up 2 and 96% at follow-up 1. During phase 1 trial CST mean attendance was 10 sessions and during phase 2 trial MCST mean attendance was 18 sessions.
FIGURE 12.
The CONSORT diagram of participants through the trial. At phase 1, the mean CST attendance was 10 sessions and at phase 2 the mean MCST attendance was 18 sessions.
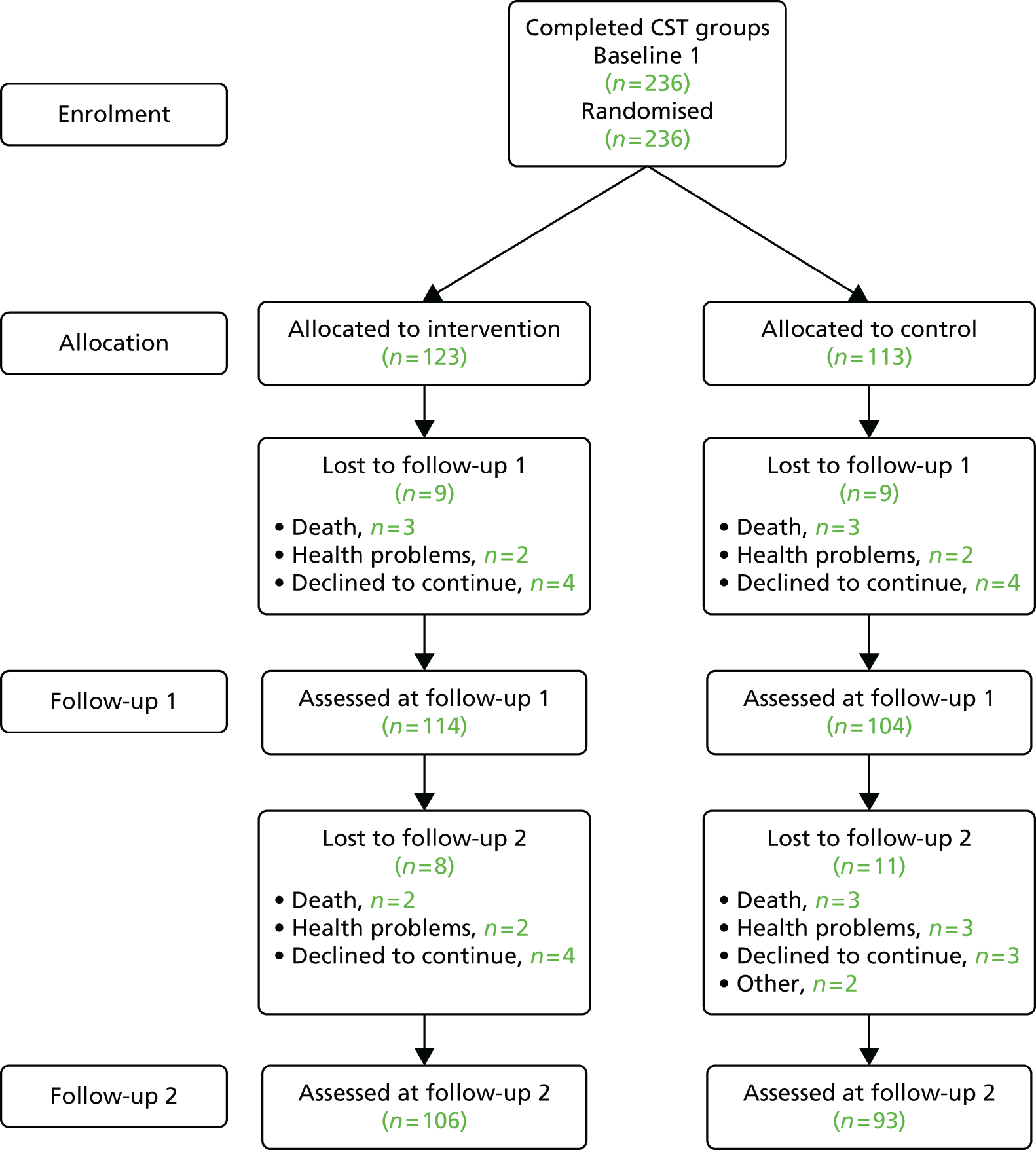
A total of 82 (31%) participants were receiving AChEIs, only 16 (14%) of whom resided in care homes. Table 4 compares community and care home participant characteristics in terms of age, sex and AChEIs, and provides information about the total participant group. Most of the sample had moderate dementia, with a mean MMSE score of 16.8 (SD 5.5) and a mean ADAS-Cog score of 34.3 (SD 12.9). The community group were less cognitively impaired at baseline (MMSE 18.9, SD 5.7) and had a higher mean ADAS-Cog of 30.5 (SD 13.1); this compared with a mean MMSE of 16.2 (SD 5.1) and ADAS-Cog of 40.6 (SD 11.4) for the care home sample. The total sample scored in the mid-range on the QOL-AD (mean 36.3, SD 12.9) and DEMQOL (mean 93.4, SD 11.4). Mean scores were in the mid-range on the measures of dependency (ADCS-ADL 42.3, SD 17.7) and behavioural symptoms (NPI 16.0, SD 12.9). The average attendance for the CST programme was 10.3 sessions (range 0–14 sessions) and 81% of participants attended seven or more sessions of the programme.
| Characteristic | Community | Residential | All |
|---|---|---|---|
| Number of participants | 159 | 113 | 272 |
| Number (%) of prescribed AChEIs | 67 (42) | 16 (14) | 82 (31) |
| Age (years), mean (SD) | 81.6 (7.6) | 85.7 (8.5) | 82.6 (8.1) |
| Sex (female), n (%) | 96 (80) | 81 (72) | 177 (61) |
Of the 236 participants randomised into the trial at second randomisation, 123 were allocated to the intervention MCST group and 113 were allocated to usual care. Table 5 shows that the groups appeared well matched in terms of demographic and clinical measures. The mean age was 83 years and most participants were white and female. A total of 135 participants (57.2%) were living in the community and 101 (42.8%) were living in care homes. One-third was taking AChEI medication (32%).
| Characteristic | Intervention (N = 123) | Control (N = 113) |
|---|---|---|
| Age (years), mean (SD) | 82.66 (7.9) | 83.47 (7.2) |
| Sex (female), n (%) | 80 (65) | 70 (62) |
| Ethnicity (white), n (%) | 111 (90) | 104 (92) |
| Marital status (widow), n (%) | 54 (44) | 57 (50) |
| Dementia diagnosis (Alzheimer’s disease), n (%) | 38 (31) | 35 (31) |
| On AChEIs (yes), n (%) | 42 (34) | 34 (30) |
| Accommodation (care home), n (%) | 51 (41) | 50 (44) |
| ADAS-Cog score, mean (SD) | 31.12 (14.6) | 33.21 (13) |
| QOL-AD score, mean (SD) | 36.12 (4.8) | 36.51 (5.7) |
| MMSE score, mean (SD) | 17.79 (5.6) | 17.79 (5.4) |
| DEMQOL score, mean (SD) | 94.81 (10.9) | 95.08 (11.7) |
| NPI score, mean (SD) | 13.84 (12.9) | 11.34 (9.1) |
| ADCS-ADL score, mean (SD) | 42.67 (17.2) | 41.51 (18.1) |
| QOL-AD-Proxy score, mean (SD) | 33.69 (5.9) | 33.33 (4.9) |
| DEMQOL-Proxy score, mean (SD) | 102.19 (13.5) | 102.25 (11.2) |
| Cornell score, mean (SD) | 4.40 (4.2) | 3.78 (3.6) |
| RAID score, mean (SD) | 4.59 (3.8) | 4.49 (3.7) |
Predictors of change in cognition and quality of life between baseline and follow-up
A repeated measure linear model explored the impact of other variables. The model was fitted for age, living situation (community/care home), sex, marital status and taking AChEIs, and using post score as the dependent variable. As a result of fitting the models in this way, the results showed that both age and sex were important factors for the effectiveness of CST. For MMSE, age was a significant predictor of effectiveness of CST, with older participants appearing to benefit more. At the mean age of 82 years there was little difference between the pre and post score, but participants older than this appeared to benefit more, with a possible increase in MMSE score. For ADAS-Cog, age again was a significant predictor of the effectiveness of CST, with older participants benefiting more, in the same way as with the MMSE. Sex proved to be a significant variable in the complete-case study analysis, and showed a strong correlation with cognitive improvement, with female ADAS-Cog scores improving more than male scores.
Living situation was also shown to be an important variable for some of the staff-completed outcome measures (Table 6). For the NPI, a decrease in score was seen for the community sample [18.08 (SE 2.25) to 13.89 (SE 2.24)], while there was a small increase in NPI score for the care-home-based participants [11.35 (SE 2.48) to 13.42 (SE 2.48)]. This indicates a benefit for the community sample.
| Measure | Estimated mean before CST (SE) | Estimated marginal mean after CST (SE) | F-value | p-value | Other variable significant in the model |
|---|---|---|---|---|---|
| MMSE | 15.8 (0.99) | 18.5 (0.89) | 20.7 | < 0.001 | Age F = 5.5; p = 0.019 |
| ADAS-Cog | 35.0 (2.0) | 30.6 (2.3) | 16.8 | < 0.001 | Age F = 12.5; p < 0.001 |
| QOL-AD | 35.7 (0.9) | 36.3 (0.9) | 0.001 | 0.97 | None |
| DEMQOL | 93.4 (2.0) | 92.4 (1.9) | 8.38 | 0.004 | None |
| ADCS-ADL | 44.0 (2.8) | 44.6 (2.8) | 0.24 | 0.32 | Age F = 8.64; p = 0.004 |
| NPI | 14.7 (2.2) | 13.6 (2.2) | 4.11 | 0.044 | Type F = 6.25; p = 0.013 |
| QOL-AD-Proxy | 33.3 (1.0) | 32.8 (1.0) | 2.91 | 0.089 | None |
| DEMQOL-Proxy | 96.7 (3.4) | 100.6 (3.2) | 27.24 | < 0.001 | Type F = 8.39; p = 0.004 |
For the DEMQOL (proxy), both community- and care-home-based participants saw a mean increase. However, the care home increase [94.2 (SE 3.61) to 100.87 (SE 3.39)] was larger than the community increase [99.32 (SE 3.47) to 100.26 (SE 3.26)]. In fact, this can be viewed as the community sample remaining steady while the care home sample were brought into alignment with what was observed in the community.
For the imputed proxy EQ-5D utility values, age was seen as a significant factor, with older participants appearing to benefit more. For the proxy EQ-5D VAS, the community sample showed a mean increase from 60.19 (SE 3.63) to 67.52 (SE 3.36), while, conversely, the care home sample showed a small mean decrease from 65.47 (SE 3.29) to 62.72 (SE 3.04). In summary, we have identified benefits for the community sample on NPI scores and proxy EQ-5D VAS, and there is a benefit for care home sample for the DEMQOL-Proxy.
Change when comparing the results with those of a similar control group
Independent sample t-tests were used to compare the complete-case data set results with the Spector et al. 6 control group, which had a mean age of 84.7 years and a 3 : 1 ratio of female to male. For the ADAS-Cog, the Spector et al. 6 control group showed a mean reduction of 0.3, while the CST group showed a mean reduction of 2.72. This gives a mean difference of 2.42 [t = 2.27, degrees of freedom (df) = 240; p = 0.024], with CIs of 0.33 to 4.51. For the MMSE, the Spector et al. 6 control group reduced by an average of 0.4 points, while the CST group saw a mean increase of 0.9 points. This gave a mean difference of 1.3 points (t = 2.76, df = 293; p = 0.006), with a CI of 0.38 to 2.22 points (Table 7). At follow-up, the CST group had demonstrated significantly better results than the Spector et al. 6 control group on both MMSE and ADAS-Cog. There was no difference between the CST group and the Spector et al. 6 control group on the QOL-AD.
| Measure | Spector et al.6 control group, mean change (SD) [n] | Current study, mean change (SD) [n] | Values |
|---|---|---|---|
| MMSE | –0.4 (3.5) [70] | 0.93 (3.3) [225] | t = 2.76; p = 0.006 |
| ADAS-Cog | –0.3 (5.5) [70] | –2.72 (8.3) [172] | t = 2.26; p = 0.024 |
| QOL-AD | –0.8 (5.6) [70] | –0.08 (4.9) [225] | t = 0.92; p = 0.357 |
Using an adjusted ANCOVA comparing groups (treatment and controls), Table 8 shows the analysis of the primary end point (follow-up 2) and secondary end point (follow-up 1). At 6-month follow-up the treatment group had significantly higher scores on self-rated quality of life measured by the QOL-AD than the usual care group (p = 0.04).
| Outcome measure | Primary end point follow-up 2 (6-month follow-up) | Secondary end point follow-up 1 (3-month follow-up) | ||||||
|---|---|---|---|---|---|---|---|---|
| Treatment, mean (SE) | Control, mean (SE) | Group difference, mean (SE) | Between-group difference (p-value) | Treatment, mean (SE) | Control, mean (SE) | Group difference, mean (SE) | Between-group difference (p-value) | |
| ADAS-Cog score | 35.94 (2.79) | 35.29 (2.85) | 0.19 (1.61) | 0.91 | 35.32 (2.56) | 34.47 (2.59) | –0.85 (1.29) | 0.16 |
| QOL-AD score | 35.62 (1.43) | 33.84 (1.53) | –1.78 (0.91) | 0.04 | 34.29 (1.03) | 33.97 (1.04) | –0.32 (0.61) | 0.70 |
| MMSE score | 16.34 (1.21) | 15.49 (1.25) | –0.85 (0.58) | 0.17 | 16.09 (0.88) | 15.79 (0.91) | –0.30 (0.52) | 0.52 |
| DEMQOL score | 89.13 (3.55) | 88.83 (3.56) | –0.30 (1.52) | 0.86 | 89.85 (2.34) | 90.71 (2.38) | 0.86 (1.31) | 0.55 |
| NPI score | 18.76 (3.78) | 20.35 (3.94) | 1.58 (2.16) | 0.44 | 14.71 (2.84) | 16.18 (2.76) | 1.47 (1.55) | 0.25 |
| ADCS-ADL score | 43.29 (2.88) | 42.35 (2.87) | –0.94 (1.51) | 0.48 | 43.58 (2.32) | 40.94 (2.32) | –2.64 (1.30) | 0.05 |
| QOL-AD-Proxy | 34.12 (1.41) | 34.05 (1.41) | –0.07 (0.74) | 0.93 | 33.93 (1.05) | 32.40 (1.07) | –1.53 (0.59) | 0.008 |
| DEMQOL-Proxy | 97.75 (3.23) | 96.61 (3.21) | –1.13 (1.71) | 0.47 | 101.36 (2.67) | 98.12 (2.67) | –3.24 (1.50) | 0.04 |
Cognition on the MMSE also favoured the MCST group but this was not significant. Centre emerged as a significant covariate in relation to the ADAS-Cog, Cornell and Rating Anxiety In Dementia scales. At 3 months’ follow-up, the MCST group had significantly higher scores than the usual care group on proxy-rated quality of life on both the QOL-AD (p = 0.008) and the DEMQOL (p = 0.04), as well as ADLs as measured by the ADCS-ADL (p = 0.05).
The MCST-AChEIs platform adjusted results at 6- and 3-month follow-up showed that the group receiving MCST plus AChEIs scored highest on the MMSE (see Table 3), than those receiving MCST only, those not receiving MCST and those not taking AChEIs. There is a significant interaction term between treatment group and taking AChEIs. Post hoc testing indicated that there was a significant difference between the MCST plus AChEIs group and the usual care and AChEIs group (p = 0.025). No significant interactions were found on any of the other outcome measures.
Numbers needed to treat
The number needed to treat is the calculation of the number of people who need to be treated in a particular intervention for one favourable outcome to be achieved. The number needed to treat calculation uses the proportion of participants who benefit in each treatment group. A number needed to treat analysis using QOL-AD change was used between baseline and follow-up 2, as this was the primary end point. An improvement of one SD on the QOL-AD (large effect size) was taken to indicate major clinical benefit, and 0.5 of a SD change (moderate effect size) was taken to indicate moderate clinical benefit. When calculating a large size effect (1 SD), the proportion benefiting was 0.18 (17/96) for the treatment group compared with 0.07 (6/81) for the control group and, therefore, 9.7 people needed to be treated for 1 to benefit (95% CI 5.0 to 129.9 people). When calculating a moderate size effect (0.5 SD), the proportion benefiting was 42 out of 96 (0.44) for the treatment group compared with 16 out of 81 (0.20) of the control group and, therefore, 4.2 people needed to be treated for 1 to benefit (95% CI 2.7 to 9.2 people).
Health economics
Methods
The main economic analysis compared MCST with usual care from a health and social care perspective. Secondary analyses also included the costs of unpaid carer time (societal perspective) and the cost of MCST as if it had been provided by health-care assistants rather than by the research team (‘in-practice’ implementation). A subgroup analysis also compared individuals receiving only MCST with individuals using an AChEI in addition. Health and social care services used by participants and inputs from unpaid carers were captured by the CSRI97 and completed with family carers or centre care workers. When possible, unit costs for the services were drawn from the Personal Social Services Research Unit compendium for 2011. 100 We used a 3.5% discount rate (recommended by Her Majesty’s Treasury) for items providing benefit for more than 1 year, such as equipment or adaptations. Medication costs were obtained from the British National Formulary. 101
The economic analysis estimated incremental costs and incremental effects and their CIs with seemingly unrelated regression methods with 1000 bootstrap replications. The information obtained through the economic analysis has led to the calculation of incremental cost-effectiveness ratios (ICERs) and to plotting cost-effectiveness acceptability curves (CEACs), showing the probability that MCST is a cost-effective addition to usual care against a series of hypothetical willingness-to-pay (WTP) values.
Results
Looking at the primary outcomes in the health and social care perspective (Table 9), the mean cost for each 1-point difference on QOL-AD was £266. Looking at the CEAC for this outcome, the probability that MCST would be seen as more cost-effective than usual care alone would be 90% at a WTP of about £1400 (Figure 13). There are no established WTP thresholds for QOL-AD against which to compare this finding, but a cost of only £1400 for a 1-point difference on a 40-point scale is modest. Based on previous studies,44 the effect size of ‘standard’ CST on the QOL-AD scale is around 0.4 SD, which represents a modest increment. In the current study the difference at follow-up for MCST was estimated to be 1.78 points (SD 0.34 points). A 2-point difference in QOL-AD can be considered to be clinically significant and a cost of £2800 to achieve such a result may be attractive to decision-makers. When the outcome was measured in terms of ADAS-Cog, the probability that MCST would be seen as cost-effective was low across all values of WTP (Figure 14).
| Outcome measure | Incremental cost (£ 2010/11), mean (95% bootstrap CI) | Incremental effect, mean (95% bootstrap CI) | ICER |
|---|---|---|---|
| 1–6 months | |||
| ADAS-Cog | 473.89 (–315.45 to 1263.23) | –0.65 (–4.08 to 2.77) | Usual care dominant |
| QOL-AD | 473.46 (–315.61 to 1262.53) | 1.78 (–0.39 to 3.95) | 266 |
| MMSE | 474.01 (–316.15 to 1264.17) | 0.85 (–0.48 to 2.18) | 558 |
| ADCS-ADL | 471.57 (–317.67 to 1260.81) | 0.95 (–2.50 to 4.39) | 498 |
| QOL-AD-Proxy | 472.70 (–314.60 to 1260.01) | 0.07 (–1.63 to 1.76) | 7050 |
| DEMQOL-Proxy | 472.31 (–338.46 to 1283.07) | 1.13 (–2.48 to 4.74) | 419 |
| QALY (EQ-5D) | 474.81 (–314.38 to 1263.99) | 0.0013 (–0.0200 to 0.0223) | 365,276 |
| QALY (Proxy EQ-5D) | 473.60 (–315.48 to 1262.68) | 0.0176 (–0.0050 to 0.0403) | 26,835 |
| QALY (DEMQOL) | 518.39 (–346.60 to 1383.39) | 0.0039 (–0.0092 to 0.0170) | 132,539 |
| QALY (DEMQOL-Proxy) | 401.52 (–441.99 to 1245.04) | 0.0062 (–0.0049 to 0.0173) | 64,785 |
| 1–3 months | |||
| ADAS-Cog | 366.38 (–150.51 to 883.26) | –0.86 (–4.14 to 2.43) | Usual care dominant |
| QOL-AD | 366.22 (–150.20 to 882.63) | 0.32 (–1.17 to 1.81) | 1139 |
| MMSE | 366.30 (–150.22 to 882.81) | 0.30 (–0.80 to 1.40) | 1223 |
| ADCS-ADL | 366.35 (–150.38 to 883.08) | 2.64 (–0.36 to 5.65) | 139 |
| QOL-AD-Proxy | 365.85 (–150.07 to 881.76) | 1.53 (0.27 to 2.79) | 240 |
| DEMQOL-Proxy | 367.96 (–151.88 to 887.81) | 3.24 (–0.02 to 6.50) | 114 |
| QALY (EQ-5D) | 366.40 (–150.80 to 883.59) | –0.0037 (–0.0117 to 0.0043) | Usual care dominant |
| QALY (Proxy EQ-5D) | 365.74 (–150.45 to 881.92) | 0.0077 (–2.94 × 10–6 to 0.0155) | 47,339 |
| QALY (DEMQOL) | 387.08 (–192.12 to 966.27) | 0.0014 (–0.0030 to 0.0058) | 276,791 |
| QALY (DEMQOL-Proxy) | 358.43 (–169.61 to 886.45) | 0.0034 (–0.0003 to 0.0070) | 105,904 |
FIGURE 13.
Cost-effectiveness acceptability curve: MCST vs. usual care – 6 months, health and social care perspective, with effectiveness measured on the QOL-AD scale.
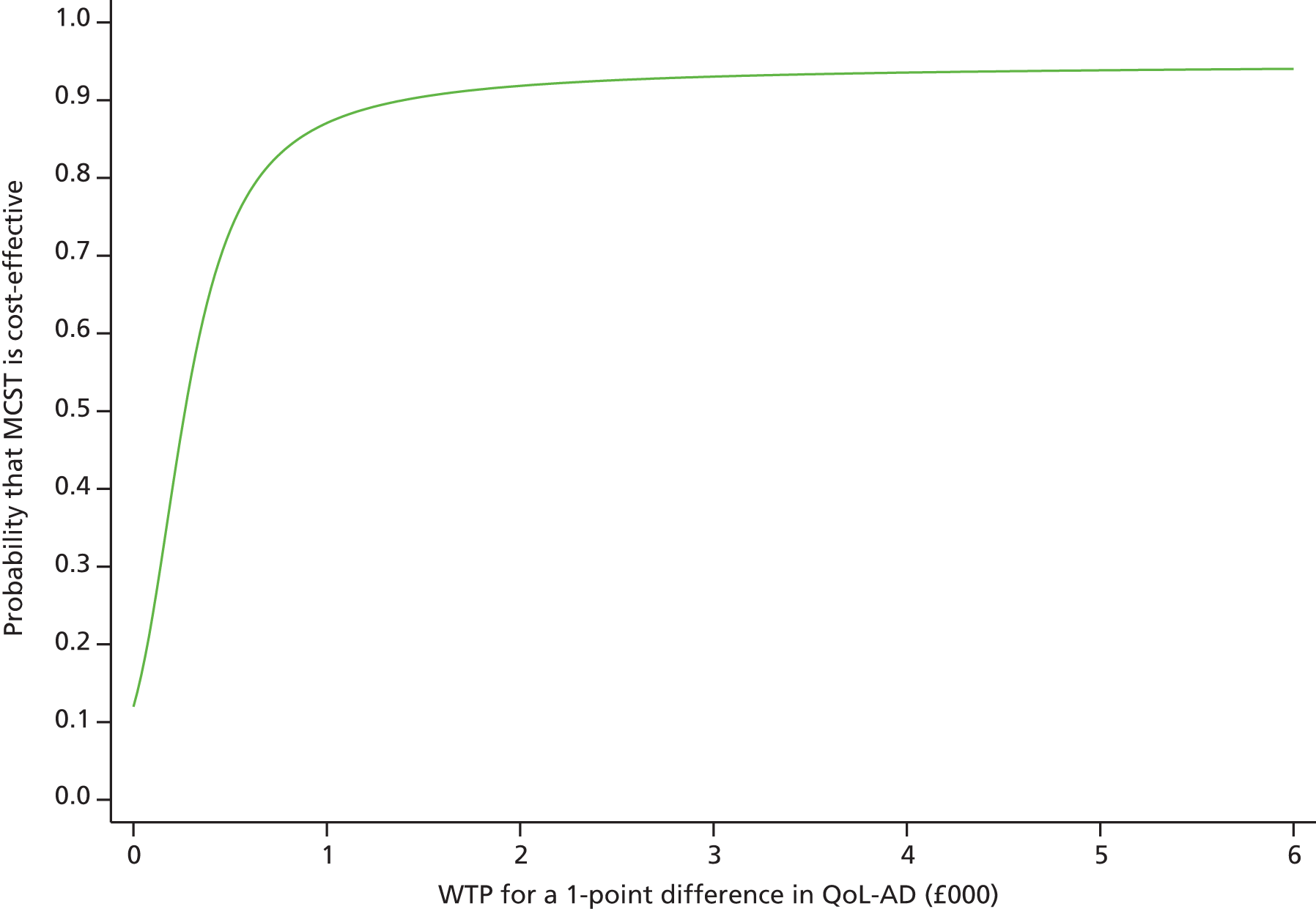
FIGURE 14.
Cost-effectiveness acceptability curve: MCST vs. usual care – 6 months, health and social care perspective, with effectiveness measured on the ADAS-Cog.
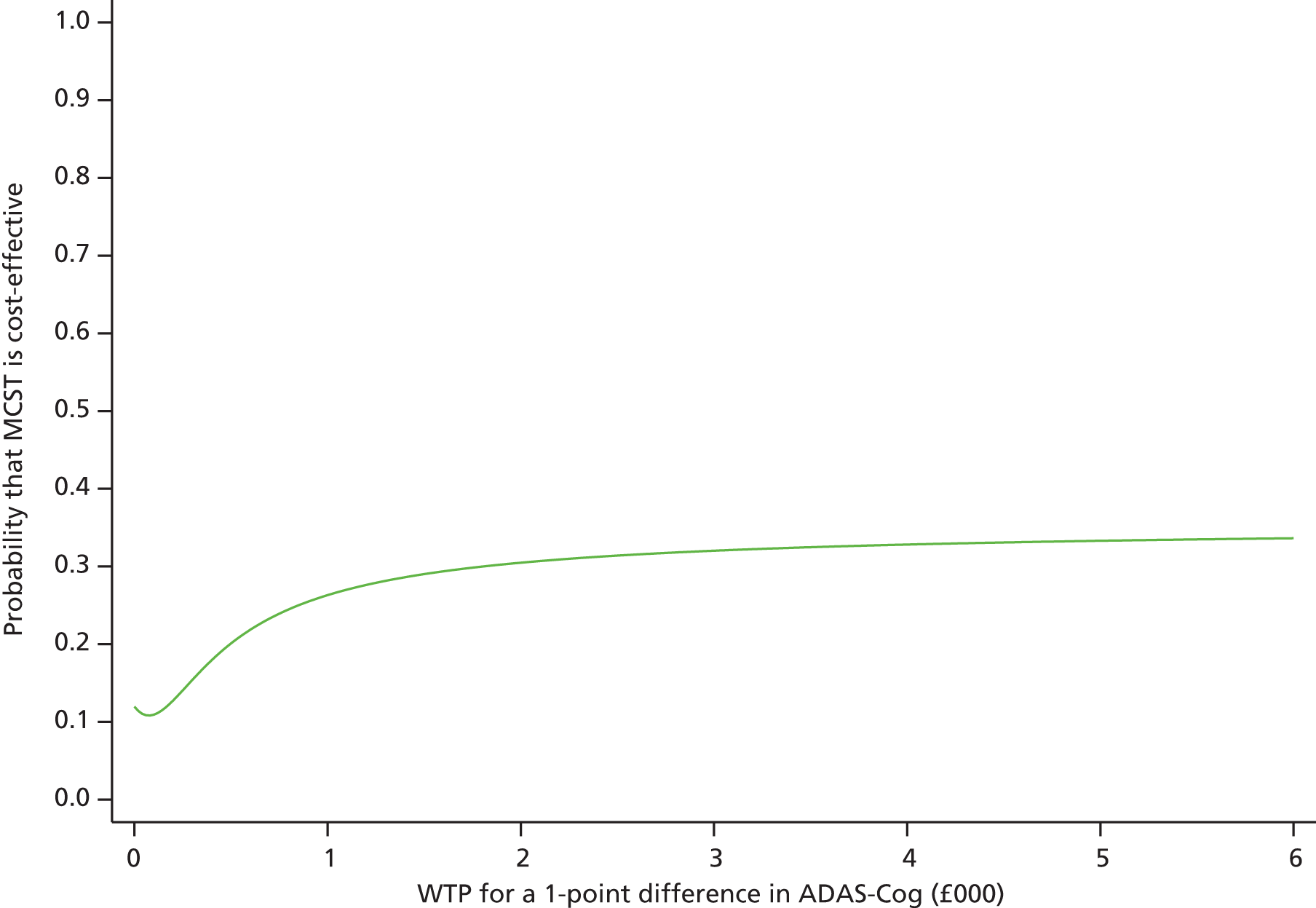
Secondary economic analyses indicated that MCST was more cost-effective than usual care over the shorter period of 3 months when outcomes were measured in terms of proxy-rated quality of life (using QOL-AD and DEMQOL) and ADLs (using ADCS-ADL), but less cost-effective than usual care on all other outcomes, including quality-adjusted life-years (QALYs).
In sensitivity analyses we broadened the cost measure to include unpaid care and support, conducting the evaluation from a societal perspective. The cost-effectiveness case for adding MCST to usual care was, again, mixed. Subgroup analyses found that the combination of MCST and AChEI was more cost-effective than AChEI and usual care by reference to a number of outcomes, including cost per QALY.
Discussion
The benefits of CST on cognitive function are now well documented and the results of this study provide additional evidence for the effectiveness of the programme developed by Spector et al. 6 The study showed that CST has a generalised cognitive benefit for people with dementia evident in comparisons of change scores, and in comparisons with the changes shown by the control group from the previous trial. Unlike the Spector et al. 6 study and the recent Cochrane review,44 this study found a positive change in behaviour following the CST intervention as measured by the NPI. There was also a significant improvement in quality of life as measured by the DEMQOL and EQ-5D (VAS) (for both participant and proxy-rated versions), but no significant change was found using the QOL-AD. Previous studies have identified the need for quality-of-life measures in dementia that are able to detect any changes in quality of life in response to both interventions and the progression of the disease,102 so that they can be used to establish the benefits of treatment for people with dementia. In this analysis, the DEMQOL seemed to be a more sensitive instrument than the QOL-AD for measuring change in quality of life in dementia. 103 Conversely, the two measures may be measuring different aspects of quality of life. These findings need to be explored further in future trials.
Predictors of success: who benefits most?
The benefits of CST were found to be independent of the use of AChEIs, which is in line with the Cochrane review findings and other studies. 44,49,51,53 In line with the earlier study,6,104 greater improvements in cognition were associated with female sex. Men might be more reluctant to communicate as they are usually in the minority in most groups, with women outnumbering men in the groups by a ratio of 4 : 1. 6,104 It may be that the sex majority dictates the style in which the groups are run, for example more ‘talking’ in female-dominated groups and more ‘doing’ in male-dominated groups. Further work is needed to explore these sex differences in response to CST and to develop interventions more geared towards the attributes of men.
The greater effect for the older people in this study is an unexpected finding. It may suggest that these older residents are experiencing more excess disability, showing impairment beyond that resulting directly from the dementia. It may be that they receive less stimulation in general than the slightly younger participants, and so benefit more from the new intervention. The findings of differences in outcomes between those living in the community and those living in care homes relate to all measures completed by a proxy: a family member or a member of staff. Their ratings may be affected systematically by different factors (e.g. family carer ratings are typically influenced by their level of strain; staff in care homes may change or have limited contact with residents on which to base their judgements). Further work is needed to explore these differences in perspectives and in the changes reported.
Maintenance cognitive stimulation therapy
This is the first major investigation to compare the short- and long-term impacts of CST for people with dementia. The investigation indicates that after standard CST (7 weeks, twice weekly), 24 weeks of MCST sessions both improves quality of life for people with dementia at 6-month follow-up and benefits quality of life and ADLs at 3-month follow-up, in comparison with the usual care control group. Cognitive stimulation programmes for dementia have a significant positive effect on cognition;44,105 however, there are concerns that the benefits may only persist for a limited period of time. 44 These results indicate that a lower intensity input of once-weekly CST sessions can continue to be effective after the initial twice-weekly CST programme is completed. In comparison with the first baseline before CST, cognitive function in the MCST group had decreased by only 0.35 points on the MMSE, whereas cognitive function in the usual care (CST) group had decreased by 1.65 points at follow-up 2. The ADAS-Cog showed a similar pattern of results. On the basis that MMSE scores in mild-to-moderate dementia are expected to decrease by 2–4 points per year,106 these results suggest that, relative to no treatment, the MCST programme continues to have a protective effect on cognition. Other studies have also found that a longer-term cognitive stimulation intervention can be effective in reducing cognitive decline in dementia. 107,108
However, benefits to cognition alone may not be sufficient to justify an extensive programme of intervention unless they are accompanied by other benefits, and a few studies have explored the long-term impact of psychosocial interventions on quality of life. 109 This study indicates that the MCST programme improves quality of life and ADLs. As CST alone improves both cognition and quality of life,6,105 this provides a strong case for an ongoing programme of CST being supported in health and social care settings.
The MCST-AChEIs subanalysis results confirm the results of similar studies,6 suggesting that the additional effects of cognitive stimulation on cognition are over and above those of medication alone. In addition, the Cochrane review showed that type of control condition (e.g. usual care or social activities) made no difference to outcome, demonstrating that cognitive stimulation, rather than social activities, was responsible for the improvements in outcome.
Previous studies have shown that CST is effective in improving cognition and quality of life,6 and is cost-effective. 13 CST is endorsed in NICE clinical guidelines. 14
Findings from this new trial suggest that, although outcome gains were modest over 6 months, the continuation of CST appeared cost-effective in terms of self-rated quality of life, cognition measured on the MMSE and proxy-rated QALYs. MCST in combination with AChEIs offered cost-effectiveness gains when cognition was measured as the outcome.
The external validity of this pragmatic trial is high, as the sample came from a wide variety of settings. However, participants were almost exclusively white British and, therefore, solid conclusions on the applicability or effectiveness of this intervention to people of other ethnic or cultural groups cannot be made. Having said this, we recently successfully adapted and ran a local CST group in Hindi, and CST programmes have been run successfully in 20 countries.
Limitations of study
The SDs for the cognitive measures were higher than those in the original Spector study,6 suggesting that a larger sample may be needed to achieve significance differences in cognition after MCST relative to CST alone. Proxy measures (e.g. the ADCS-ADL and NPI) were rated by the family carer or staff members who were aware of participant group allocation, and so this might have introduced detection bias into the ratings. The subgroup of people on AChEIs might also have received extra psychiatric services during the trial. However, comparisons of the AChEIs-only group and MCST plus AChEIs group data confirm that the effects of the MCST programme are above and beyond those of medication alone.
Conclusion
This project provides further evidence that CST benefits cognition and quality of life for people with dementia, and suggests that the benefits are in addition to the effects of antidementia medication. CST appears to be more beneficial for women and people older than the average age of those in the study. This provides good evidence for the clinical effectiveness and cost-effectiveness of continuing CST beyond the initial programme and shows that quality of life and ADLs continue to benefit from extended CST.
Work package 4: maintenance cognitive stimulation therapy in practice
Cognitive stimulation therapy has been shown to improve cognition and quality of life, but little is known about the best way of ensuring implementation of CST in care settings. A recent pilot study found that one-third of people who attended CST training went on to run CST in practice, but staff identified a lack of support as a key reason for the lack of implementation. 110
The research project was divided into three studies. The Staff Training and Outreach (STANDOUT) and Monitoring and Outreach (MONOU) studies assessed the effects of outreach support on uptake of CST in practice, as well as looking at staff outcome measures and adherence to the programme. The observational study recruited people with dementia to complete minimal outcome measures before and after CST, and after the delivery of the MCST programme.
Methods
Design
The STANDOUT and MONOU studies differed in how staff members were recruited into the research and in their previous exposure to CST. Staff members in the STANDOUT study were new to CST, having had no prior experience of the programme, whereas staff recruited into the MONOU study had either previously attended CST training or purchased the CST ‘Making a difference’ manual. The observational study looked at the effects of CST and MCST on people with dementia in the practice context delivered by staff members. The three studies together provided evidence on both the most effective way of facilitating the implementation of CST, and uptake and effectiveness of the approach in a clinical context (Table 10). Ethics approval was obtained for the project through the Multi-centre Research Ethics Committee (reference number RP-PG-0606-1083). The clinical trial was registered as ISRCTN28793457.
| Title | STANDOUT trial | MONOU trial | Observational study |
|---|---|---|---|
| Aim | To assess the effectiveness of staff training and outreach support | To assess the implementation in practice of CST and outreach support | To assess the effectiveness of CST in practice |
| Participants | Dementia care staff | Dementia care staff | People with dementia |
| Experience | No previous CST experience or training | Previously received CST manual/training | Staff have varying levels of experience |
| Number | 175 | 66 | 89 |
| Resources | CST manual, MCST manual and DVD | CST manual, MCST manual and DVD | CST manual, MCST manual and DVD |
| Training | Yes | Variable | Variable |
| Outreach | 50% | 50% | Variable |
| Assessment time frame | Baseline, 6 and 12 months | Baseline, 6 and 12 months | Before and after CST (0–7 weeks or 0–14 weeks) and MCST (31 or 38 weeks) |
STANDOUT trial
The design was a pragmatic, multicentre, single-blind, two-treatment-arm RCT. The STANDOUT trial sample comprised dementia care staff from specialist and non-specialist dementia care settings. All participants received the training package as TAU, consisting of a CST training day, a training DVD, the CST ‘Making a difference’ manual and the MCST ‘Making a difference 2′ manual, and cluster randomised by centre prior to the training day into the intervention group or to TAU. The intervention group consisted of a local co-ordinator, e-mail support and an online forum. Each staff member was expected to complete three questionnaires, the first before the training day and the others at 6 and 12 months thereafter (Figure 15).
FIGURE 15.
Flow diagram of the STANDOUT trial and assessment schedule.
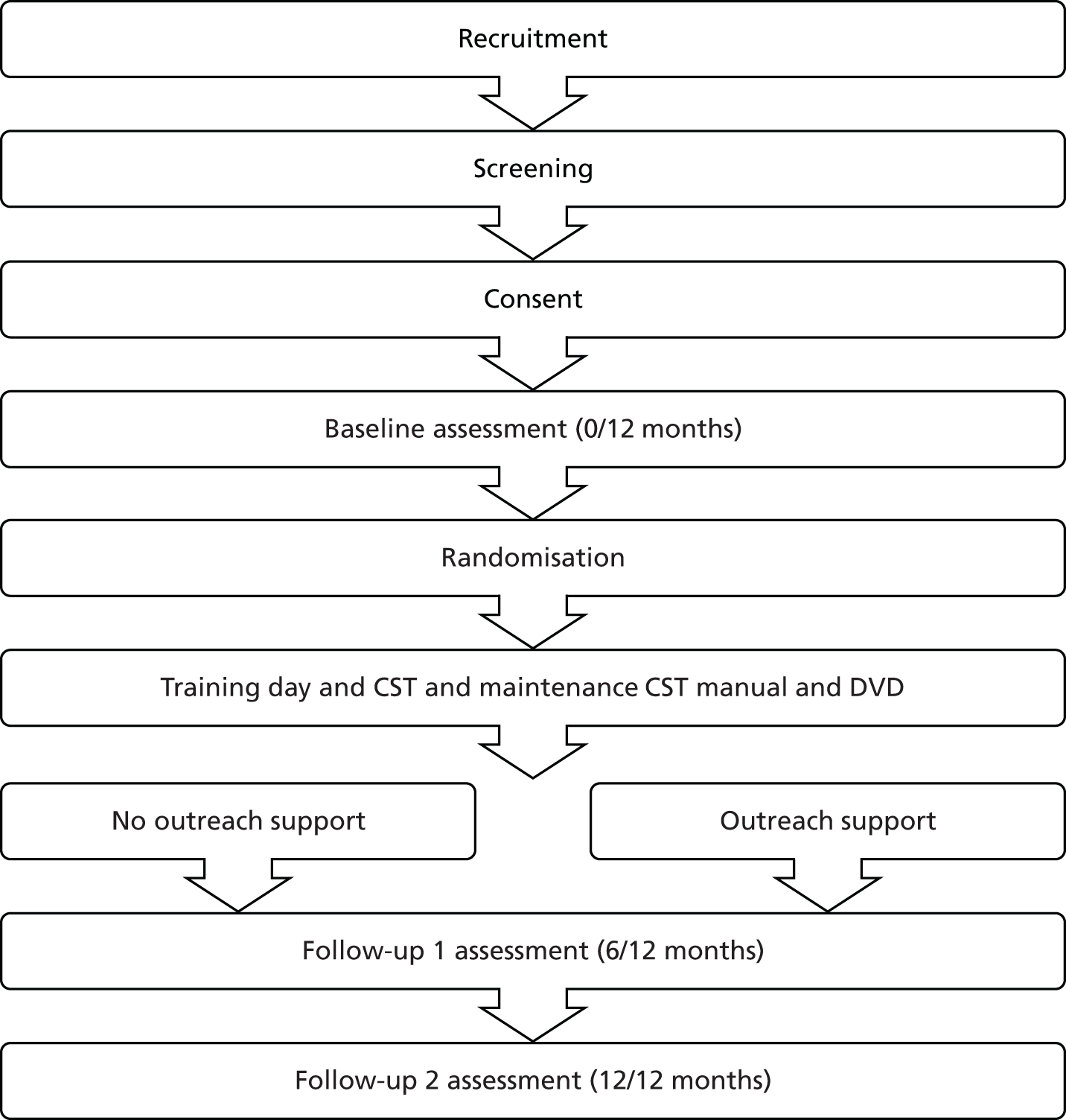
Recruitment
Those recruited to the trial included staff from care homes, day centres and NHS trusts from various locations across the UK. The trial was advertised through the Journal of Dementia Care, the National Care Forum and the UK Clinical Research Network study portfolio, and referrals were made through the commercial CST training day. The researcher also followed up individuals who, prior to the start of the research, had expressed an interest in CST or in attending a CST training day. Using the inclusion criteria, the researcher was then able to assess all expressions of interest from individuals, centres and trusts.
Participants
Staff members were screened to ensure that they met the inclusion criteria: (1) having adequate written and spoken English; (2) being able to complete online assessments at three time points; (3) having at least two other team members to run groups with; (4) reaching agreement with management to have 2 hours per week set aside to run the CST groups, and 1 hour following on from this for the 24-week MCST programme; and (5) being able to provide between five and eight people with mild-to-moderate dementia who were willing to participate and met the inclusion criteria (described in the observational study). Attempts were made to recruit a minimum of three staff members per centre for the logistical reason of being able to consistently run the groups. Originally, 40 centres were considered feasible to recruit the 120 staff members required for the STANDOUT trial; however, in total 173 staff members across 50 centres were recruited into the study. This was because there was a higher demand for receiving the CST training than for entering the MONOU study and, as the analyses for the STANDOUT and MONOU studies were combined, statisticians on the SHIELD programme advised that this would not compromise the integrity of the trial, as there had always been the intention to analyse the results from both the STANDOUT and MONOU trials together. Working on the premise of a 15% attritition rate, the sample size provided sufficient numbers for (1) the staff training outreach support versus no outreach support, and (2) monitoring and outreach versus no outreach support to estimate effect size and the feasibility of the trial. The attrition rate was an estimation based on previous research conducted in CST. 111
Training package
The CST ‘Making a difference’ and MCST ‘Making a difference 2′ manuals, along with the staff training DVD, were distributed to all staff members participating in the study. The staff training DVD comprises an introduction by Dr Aimee Spector, a table listing the order of the CST and MCST sessions, and key principles. There is also video footage of each CST and MCST session run with people with dementia for staff to observe and questions after each clip to encourage reflective learning and discussion. All staff members attended a CST training day. The training comprised different perspectives of dementia, main psychosocial approaches for dementia, development and evaluation of CST, session themes and examples of activities for sessions, key principles, planning of sessions and the setting up of a group and reflective learning of issues that arise when implementing and running groups. The training used learning methods, such as role-play, and small group exercises and the DVD was used when time was spent reflecting on the sessions and critically appraising how the session had been run. At the end of the training day, time was taken to emphasise the importance of completing the attendance and adherence forms after each completed session; these forms would then be collected by the researcher at the end of the research time.
Randomisation
Cluster randomisation occurred prior to staff attending the CST training day to ensure that staff members from the same centre received the same level of support. The allocation ratio for randomisation was 1 : 1, into either the intervention or TAU. NWORTH was responsible for undertaking the remote randomisation.
Treatment as usual
Staff members within centres randomised to the control group attempted to deliver the CST as usual without the additional outreach support. The training package offered to the intervention group was also available to those in the control group. Therefore, the trial examined the additional effects of the outreach support.
Intervention
A pilot study conducted, prior to this research being undertaken, identified that outreach support should consist of (1) an online forum, (2) e-mail support and (3) local supervision. 110 The online forum was an online discussion site. It was accessible using a username and password. The first time a person attempted to enter the site, an e-mail was sent to the researcher for their approval to ensure that the person in question had been randomised to the intervention group. The use of login details allowed a record to be kept of the number of people accessing the service and how many times they did so. Staff members were able to write up a variety of messages, such as comments on sessions, asking questions and asking for advice. The same researcher who had extensive experience of CST and experience of running groups delivered the e-mail support and local supervision, unless the centre was able to provide their own person to deliver the supervision. Both services were made available as often as was needed. The role of the local supervisor was to help to resolve practical issues that were encountered in attempting to run CST groups. The supervisors recorded all the support given.
Primary outcome measure
The primary outcome measure was the total number of sessions attended over the duration of the CST and MCST programme. This was determined by the total number of sessions run multiplied by the average number of people at each. This was recorded by staff using the monitoring progress form located in the ‘Making a difference’ manual112 that included recording who was in attendance, and rating level of interest, communication, enjoyment and mood on a scale of 1–5 (i.e. 1 = no interest, 2 = little interest shown, 3 = some interest shown, 4 = interest shown and 5 = great interest shown). This measure was completed at the end of each session from the start of the CST programme to the end of intervention delivery, until the MCST groups had been completed or the groups had been discontinued.
Secondary outcome measures
-
Adherence to the CST and MCST programme was measured using an adherence list designed for the trial and was based on 18 key principles developed as part of the MCST programme. 112 The adherence records were reviewed by a member of administration to mark whether or not staff adhered to the key principles as laid out in the ‘Making a difference 2′ manual and to highlight any similar issues that were arising across centres. Any ambuiguity in participants’ responses was discussed with a researcher until consensus was reached regarding whether or not the participant was adhering to the key principles. Job satisfaction was measured using the Minnesota Satisfaction Questionnaire. 113 This consists of 100 questions and comprises 20 dimensions with five items per scale, using a 5-point Likert rating scale. The measure has adequate internal reliability.
-
Staff members’ approach to dementia was measured using the Approaches to Dementia Questionnaire (ADQ). 114 The ADQ has 19 statements about the person with dementia and the care that they receive. The scale has high validity and good reliability using Cronbach’s α for its person-centeredness and hopefulness subscales.
-
Knowledge was measured using the Dementia Knowledge – 20 scale. 115 This consists of 20 questions for which there are five possible answers. The scale has sufficient reliability and was administered at baseline and final follow-up only.
-
Perceived sense of competence was measured using the Sense of Competence in Dementia care – Staff questionnaire. 116 It comprises 17 items categorised into four subscales: professionalism, building relationships, care challenges and sustaining personhood. The scale has good internal consistency.
-
Learning characteristics of staff were measured using the brief version of the Learning Transfer System Inventory (LTSI). 117 The constructs of the LTSI are validated using common factor analysis. 118 The brief form comprises 16 questions that are categorised into four major groups: trainee characteristics, motivation, work environment and ability. All of the items use 5-point Likert-type scales from 1 (strongly disagree) to 5 (strongly agree). 110
-
Barriers to change in the workplace were measured using the Barriers to Change Questionnaire. 119 It comprises 19 questions focusing on institutional constraints, support from colleagues, philosophical opposition, client dissatisfaction, interference and positive factors, and allows the addition of any further comments.
-
The emotional and behavioural responses relating to challenging behaviour presented by the person with dementia were measured by the Controllability Beliefs Scale. 120 The scale has 15 items based on a 5-point scale. Higher scores indicate higher staff member belief in the level of control demonstrated by the person with dementia. The scale has good internal reliability.
Consent
Ethics and local R&D approvals were obtained and all staff members provided informed consent to participate in the trial. Consent was also sought from a manager in each centre recruited.
Blinding
Although staff members could not be blinded to their allocation, the majority of the assessment data was completed online and independently of the research team. To maintain anonymity throughout the trial, an administrator on the SHIELD programme team assigned an identification number to all participants who completed the survey. The researcher administering the outreach support had the contact details of the staff members but was unaware of their individual code, and hence they were blinded to identifying the staff members. The staff members were aware of their code and it was emphasised that it was not to be discussed with the research team member. A reminder was also included in the e-mail that the administrator sent to the participants asking them to complete the follow-up assessments.
MONOU trial
The design was a pragmatic, multicentre, single-blind Phase IV trial. The participants were staff members from centres that had previously purchased the CST ‘Making a difference’ manual or had attended a CST training course. Once enrolled in the research, all staff received the free MCST ‘Making a difference 2′ manual and DVD. It was recorded whether participants had the manual only or manual and training before centres were cluster randomised into outreach support or TAU (Figure 16). The time points for completing the questionnaire were the same as for the STANDOUT trial. However, the participants also completed a retrospective questionnaire on their use of CST in practice prior to the research.
FIGURE 16.
Flow diagram of the MONOU trial and assessment schedule.

Recruitment
Recruitment of staff members for the MONOU study was created from records of purchased CST manuals via Hawker publications and a database of attendees generated from previously run CST training days. Staff members were then contacted to determine if they were interested in participating in the study. A CST poster also advertised the research on the CST website (www.cstdementia.com), on the SHIELD website (www.ucl.ac.uk/shield) and through the Journal of Dementia Care. In addition to this, as the project was a UK Clinical Research Network portfolio-adopted study, NHS trusts nationwide could approach the research team for their eligibility to participate in the research to be assessed.
Participants
The participants were dementia care staff who had the CST manual or had attended the CST training day and were able to implement the CST programme once or twice weekly followed by the MCST programme. The screening and inclusion criteria matched those for the STANDOUT study, except a minimum of one staff member could be recruited per centre; because of this it was estimated that between 40 and 120 centres were required to recruit 120 staff members. This figure also accounts for a 15% (240 × 0.75 = 180) attrition rate. However, in total 68 staff members were recruited across 13 centres. This was due to difficulty in identifying and recruiting suitable participants.
Randomisation
Once staff had completed their baseline assessment, it was recorded who had the manual or training, and randomisation occurred. This was created by dividing into two clusters, taking into consideration centre size and type of previous training (manual vs. manual and training); staff were then independently randomised to receive the intervention or TAU by remote e-mail service to NWORTH. Owing to differing numbers in each centre, an effort was made to keep similar numbers in the control and experimental groups, with an allocation ratio for randomisation of 1 : 1, into either the intervention or the TAU group.
Treatment as usual
Sites randomised to the control group were expected to deliver the CST as usual. Each centre randomised into TAU received a monthly telephone call from a member of administration to see what stage they were at in the delivery of the programme, but they were not given any advice. The trial examined the additional effects of the outreach support and long-term effect in practice.
Intervention
The outreach support options were identical to those in the STANDOUT study, with one difference: to emulate CST in practice, staff members identified the local supervisor, and, if this was not possible, it was recorded and the staff members were then offered the researcher’s services. All of the staff members randomised to receive outreach support were encouraged to use all options available to them, but this was not compulsory. Staff members were monitored to measure their usage of the outreach support options. A monthly telephone call was also made by the researcher to each centre in receipt of outreach to determine what stage of the programme they were at and, if advice was sought, this was recorded accordingly.
Primary outcome measure
Attendance as the primary outcome was identical to that in the STANDOUT trial. If the response to a person attending the session was left blank and no reason was given for this, the researcher assumed that the person had not attended the session. If a person was introduced part way through the programme, the sessions that had not been offered to them were excluded from the total number of sessions considered, as the person’s non-attendance at these sessions had not been because of a refusal to attend but because the sessions had been unavailable by the time they joined.
Secondary outcome measures
The secondary outcome measures were identical to those in the STANDOUT trial.
Consent
Informed consent was gained from each staff member and a member of management, and this was identical to the STANDOUT trial. Ethics and local R&D approvals were obtained.
Blinding
The procedure for blinding was identical to that in the STANDOUT trial.
Analyses
In total, 300 individuals, 28 centres and 12 trusts expressed interest or were approached to participate in the research. Overall, 241 staff members were recruited across 63 centres in both the STANDOUT and MONOU trials. All of the staff members consented and completed the baseline assessment before randomisation. Four people were randomised by association, after randomisation had occurred but before the centre was aware of whether or not they were in receipt of the intervention. In total there were 115 (48%) people not in receipt of the intervention across 28 centres and 126 (52%) who were in receipt of intervention across 35 centres. The majority of staff members were female (88%) and white (71%). Generally, staff had between 1 and 10 years of experience (43%), with 41% having a qualification up to diploma/degree level. Most sites were dementia specialist settings (64%), with 33% being care home settings (Table 11).
| Staff demographics | Frequency, n (%) |
|---|---|
| Sex (female) | 212 (88) |
| Ethnicity (white) | 170 (71) |
| Age (45–54 years) | 56 (23) |
| Level of experience (1–10 years) | 103 (43) |
| Qualification (diploma/degree) | 99 (41) |
| Dementia setting (care home) | 80 (33) |
| Specialist dementia setting (yes) | 155 (64) |
A combined analysis of the results from the STANDOUT trial (175 participants) and the MONOU trial (66 participants) was carried out. The primary outcome was the total number of sessions attended for each centre (total number of sessions run × average number of people at each) per centre, assuming an intracluster correlation of p < 0.05.
For the group receiving the intervention of outreach support, 18 (51%) out of 35 centres went on to deliver the CST programme, whereas in the TAU group 12 (43%) out of 28 centres delivered the programme (Table 12).
| Intervention | Number of centres (n) | No CST, n (%) | Number of CST programmes run, n (%) |
|---|---|---|---|
| Outreach support | 35 | 17 (49) | 18 (51) |
| No outreach support | 28 | 16 (57) | 12 (43) |
A chi-squared statistic suggests that there is not a statistically significant difference in the proportion of CST groups run in the intervention group and the number of CST groups run in the TAU group (p = 0.458).
Leading on from the CST programme, 12 (67%) out of the 18 centres in the intervention group went on to deliver the MCST and 8 (67%) out of 12 centres in the TAU group delivered the MCST programme (Table 13).
| Intervention | No CST (n) | Number of CST programmes only, n (%) | Number of MCST programmes run, n (%) |
|---|---|---|---|
| Outreach support | 17 | 6 (33) | 12 (67) |
| No outreach support | 16 | 4 (33) | 8 (67) |
A chi-squared statistic suggests there is a statistically significant difference, with more MCST groups run in the intervention group than in the TAU group (p = 0.011).
Staff at each centre running the CST and MCST programmes marked the attendance of the people with dementia after each session was completed. This information was then collected at the end of the programme and the researcher entered it into a Microsoft Excel® 2003 spreadsheet (Microsoft Corporation, Redmond, WA, USA). By calculating the primary outcome of total number of sessions attended for each centre, the researcher was able to group the centres by the average number of people attending during the programme. Centres unable to run the programme scored zero, centres considered to have delivered CST poorly scored < 41 overall (and so had, on average, fewer than three group members) and centres considered to have delivered CST OK scored between 42 and 69, reflecting that, on average, there were three or four group members. Finally, CST was considered good if a centre scored ≥ 70, demonstrating that, on average, in these centres there were ≥ 5 group members over the duration of the programme (Table 14).
| Intervention and rating of CST | No groups | CST poor | CST OK | CST good | Total |
|---|---|---|---|---|---|
| Intervention | |||||
| No | 16 | 0 | 3 | 9 | 28 |
| Yes | 18 | 0 | 5 | 12 | 35 |
| Total | 34 (33)a | 0 | 8 | 21 | 63 |
A chi-squared statistic suggests that there is no statistical significance between the centres that were in receipt of the intervention and those that were not, and the delivery of the programme as determined by the number of average attendees across the CST programme (p = 0.87; 2 df). However, Table 14 does not reflect one centre that ran the programme in the intervention group, as the attendance and adherence booklet was mislaid, so for the purposes of this table the centre has been included in the intervention ‘no groups’ column.
From the centres that followed the CST programme with the MCST programme, the primary outcome of total number of sessions attended enabled the researcher to group the centres according to the average number of people who attended across the delivery of the programme (24 sessions). A score of 0 was assigned to centres in which groups were not run; centres in which MCST was considered to have been delivered poorly (a score of < 71 indicating fewer than three group members) were assigned a score of 1. Centres with between 3 and fewer than 5 people, on average scoring between 72 and 119, were given a score of 2 and those centres with ≥ 5 group members, as demonstrated by a score of > 120, were assigned a score of 3 (Table 15).
| Intervention and rating of MCST | No MCST groups | MCST poor | MCST OK | MCST good | Total |
|---|---|---|---|---|---|
| Intervention | |||||
| No | 21 | 0 | 5 | 2 | 28 |
| Yes | 22 | 2 | 5 | 6 | 35 |
| Total | 43 | 2 | 10 | 8 | 63 |
A chi-squared statistic suggests that there is no statistically significant difference between the centres in receipt of the intervention and the average number of attendees in the delivery of the MCST programme (p = 0.35; 3 df).
A comparison was also carried out to look at the different pathways by which participants entered the trial, either ‘new’ to CST (STANDOUT) or with previous experience (MONOU). Of the centres recruited into the STANDOUT trial, 17 out of 50 went on to deliver the CST programme. The MONOU trial had a total of 13 centres and, of these, 12 went on to deliver the CST programme (Table 16).
| Trial | No CST groups | CST poor | CST OK | CST good | Total |
|---|---|---|---|---|---|
| STANDOUT | 33 | 0 | 4 | 13 | 50 |
| MONOU | 1 | 0 | 4 | 8 | 13 |
| Total | 34 | 0 | 8 | 21 | 63 |
A chi-squared statistic suggests that there is a statistically significant difference, in that more CST groups were run in the MONOU group than in the STANDOUT group (p = 0.001; 1 df).
This comparison was also considered for the centres within the two strands of the trial that went on to deliver the MCST programme. Within the STANDOUT trial, 9 of the 17 centres continued with the MCST programme, whereas in the MONOU trial 11 of the 13 centres went on to deliver the MCST programme (Table 17).
| Trial | No groups | MCST poor | MCST OK | MCST good | Total |
|---|---|---|---|---|---|
| STANDOUT | 41 | 1 | 4 | 4 | 50 |
| MONOU | 2 | 1 | 6 | 4 | 13 |
| Total | 43 | 2 | 10 | 8 | 63 |
A chi-squared statistic suggests there is a statistically significant difference, in that more MCST groups were run in the centres recruited into the MONOU trial than in those recruited into the STANDOUT trial (p = 0.000; 3 df).
The researcher recorded outreach support each time it was accessed by a staff member in the intervention group. In total, three centres signed up to the online forum, the e-mail support was accessed twice and local supervision in the form of a telephone call was used 15 times. However, within this staff members initiated three of the telephone calls, whereas the remaining 12 were the monthly follow-up support telephone calls in which staff members were asked if they needed support. It was also noted that within the MONOU trial two of the trusts (Kent and North Staffordshire) had a support network already set up that they accessed in monthly meetings across the duration of their involvement in the research.
Secondary outcome measures
To determine if the centres running the CST programme were adhering to the key principles, one-third of the CST adherence records were randomly chosen. The adherence questions were devised with the CST key principles in mind (Table 18). There were five CST records chosen from the centres in receipt of the intervention and five records from centres in the TAU group; these comprised six centres in the STANDOUT trial and four centres in the MONOU trial.
| Number | Adherence question | Key principle |
|---|---|---|
| 1 | Was the session pitched at the right level for all group members? | Mental stimulation |
| 2 | Were people encouraged to think of new ideas during the session? | New ideas, thoughts and associations |
| 3 | Was time spent on the date, time, weather and feelings of group members? | Using orientation, but sensitively and implicitly |
| 4 | Did the discussion focus on opinion over fact? | Opinion rather than facts |
| 5 | Were past experiences used to bring people in to the here and now? | Using reminiscence as an aid to the here and now |
| 6 | Did you follow the session structure? | Continuity and consistency between sessions |
| 7 | Were indirect questions used during the session? | Implicit (rather than explicit) learning |
| 8 | Was everyone encouraged to participate in the session? | Stimulating language |
| 9 | Did anyone struggle to join in with the session? | Stimulating executive functioning |
| 10 | Were the individual needs of each group member met? | Person centred |
| 11 | Was respect shown between group members and the facilitators? | Respect |
| 12 | Did everyone equally contribute to the session? | Involvement |
| 13 | Was every opinion valued within the group? | Inclusion |
| 14 | Were group members given the choice of activities for the session? | Choice |
| 15 | Did people seem to enjoy the session? | Fun |
| 16 | Was everyone given enough time to contribute to the session? | Maximising potential |
| 17 | Is there a good relationship between group members? | Building/strengthening relationships |
When the responses were recorded, any adherence questions left blank were considered incorrect and for any adherence sheets missing these were deducted from the overall score that the centre obtained. The self-reported adherence score of not adhering to the key principle across the delivery of the CST programme ranged from 11% to 24%. The three main key principles that were not adhered to were: (14) were group members given the choice of activities for the session, (9) did anyone struggle to join in with the session and (7) were indirect questions used during the session. However, when key principle 17 was reviewed by the researcher there was either no comment or it was stated that they were following the session structure. So, it was considered that the question was ambiguous and needs further clarification in the future. With regard to key principle 9, when the centre highlighted that people were struggling within the centre it generally was a result of one or two group members being more impaired than the others or being unwell on the day of the session. It was expected that people’s participation over the time frame of the programme might vary and it is arguably a positive sign that the staff were able reflect on the session and identify participants struggling during the session, as one would hope that this would enable the staff to then cater to the individual needs of each participant and to keep the group as inclusive as possible. Key principle 7 identified that there was a lack of indirect questions used when delivering the programme. The use of indirect questions is important to ensure that people do not feel put on the spot, so it is useful to understand that this is a principle that staff find difficult to adhere to.
An ANCOVA was applied to the secondary outcomes measures including job satisfaction, approaches to dementia, dementia knowledge, responses relating to challenging behaviour, perceived sense of competence and learning characteristics, as well as barriers to change. Each measure was calculated factoring in sex, age, frequency in the delivery of CST, type of centre, whether or not it was a specialist dementia setting, how the participant was recruited into the project (MONOU/STANDOUT) and, finally, if they were in receipt of the intervention.
A one-way between-group ANCOVA was conducted to compare the effectiveness of outreach support as the intervention versus TAU on participants’ job satisfaction, approaches to dementia, dementia knowledge, responses relating to challenging behaviour, perceived sense of competence and learning characteristics, as well as barriers to change. The independent variable was the type of intervention (outreach support or no outreach support) and the dependent variables consisted of scores on the secondary outcomes at 6 and 12 months. Participants’ scores at baseline in these measures were used as the covariate in the analysis. Preliminary checks were carried out to ensure that there was no violation of the assumptions of normality, linearity, homogeneity of variances, homogeneity of regression slopes and reliable measurement of the covariates.
Job satisfaction as measured by the Minnesota Satisfaction Questionnaire113 evaluated job satisfaction using a score ranging from 100 to 500, with a higher score indicating a higher sense of satisfaction. In general, job satisfaction increased for both the control and intervention groups at follow-up 1, but decreased at follow-up 2 to lower than the baseline score. After adjusting for baseline scores, job satisfaction showed no statistical difference at follow-up 1 (p = 0.72) or follow-up 2 (p = 0.99).
Approach to dementia was measured using the ADQ114 and looked at the attitude of the staff members, with a low score suggesting a negative attitude and a higher score indicating a positive attitude (19–95). The score in both the control and intervention groups increased slightly over the duration of the research. However, there was no significant difference for approaches to dementia on the hope subscale at follow-up 1 (p = 0.82) or follow-up 2 (p = 0.97), or person-centred subscale at follow-up 1 (p = 0.18) or follow-up 2 (p = 0.95).
Perceived barriers to change, including institutional constraints, support from colleagues, philosophical opposition, interference and additional factors, were measured using the Barriers to Change Questionnaire,119 with a higher score indicating more perceived barriers (0–80). Overall, the score decreased at follow-up 1, but then increased at follow-up 2. For barriers to change there was no significant difference at either time point, follow-up 1 (p = 0.54) or follow-up 2 (p = 0.96).
To look at training transfer, the brief LTSI117 was used looking at learning characteristics, motivation, work environment and ability/enabling, with a higher score (16–80) indicating a more positive transfer of learning. The scores improved at follow-up 1 compared with baseline and, although they decreased at follow-up 2, still remained higher than the baseline score. For transfer of learning there was no significant difference at follow-up 1 (p = 0.1) or follow-up 2 (p = 0.34) when comparing the intervention and control groups.
To determine the level of control that the staff member considered the person with dementia to have over their own behaviour, the Controllability Beliefs Scale120 was used, with a higher score indicating a higher level of control (15–75). The challenging behaviour scale was split into two subscales: high control and low control. Over the duration of the study, both the intervention and the control groups attributed a lower controllability rating, although neither follow-up 1 (p = 0.56) nor follow-up 2 (p = 0.84) showed a significant difference. In addition, although the lower controllability score increased at follow-up 1 and decreased at follow-up 2 but remained higher than baseline, the low control score was not statistically significant at follow-up 1 (p = 0.20) or at follow-up 2 (p = 0.65).
To understand the level of dementia knowledge, the Dementia Knowledge – 20 scale115 was used at baseline and follow-up 2 only. Within the scale there are two subdomains, dementia core knowledge and dementia care knowledge, with a higher score demonstrating a higher level of dementia knowledge (0–20); however, this was low at baseline, it remained low at final follow-up and there was no significant difference at this time point (p = 0.72).
Sense of competence was measured using the Sense of Competence in Dementia care – Staff questionnaire,116 with a higher score demonstrating a higher perceived rating of competence (17–68). Sense of competence continually increased at follow-up 1 and follow-up 2 and, although not reaching significance at follow-up 1 (p = 0.61), showed statistical difference at follow-up 2 (p = 0.05), indicating that sense of competence significantly increased at final follow-up compared with the control group (Table 19).
| Measure | Subscale | Intervention | Baseline mean score (SE) | Follow-up 1 mean score (SE) | Significance | Follow-up 2 mean score (SE) | Significance |
|---|---|---|---|---|---|---|---|
| ADQ | Hope | Y | 31.5 (4.20) | 31.3 (1.51) | 0.82 | 32.2 (1.68) | 0.97 |
| N | 30.8 (5.30) | 31.1 (1.26) | 32.2 (1.40) | ||||
| Person centred | Y | 18.3 (3.64) | 19.8 (1.54) | 0.18 | 19.1 (1.55) | 0.95 | |
| N | 17.6 (3.28) | 18.5 (1.30) | 19 (1.30) | ||||
| BARCQ | Y | 33.9 (17.25) | 26.6 (4.79) | 0.54 | 31.9 (6.92) | 0.96 | |
| N | 35.5 (17.32) | 28.5 (3.96) | 32.1 (5.68) | ||||
| LTSI | Y | 56 (7.20) | 61 (2.76) | 0.1 | 57.8 (3.21) | 0.34 | |
| N | 55.6 (6.58) | 58.1 (2.29) | 59.8 (2.65) | ||||
| CBS | High control | Y | 42.1 (7.48) | 40.4 (2.37) | 0.56 | 26.7 (1.45) | 0.84 |
| N | 41.4 (6.82) | 41.3 (1.97) | 26.5 (1.19) | ||||
| Low control | Y | 11.6 (4.05) | 16.6 (1.90) | 0.2 | 14.5 (0.67) | 0.65 | |
| N | 11.9 (3.96) | 15 (1.56) | 14.3 (0.54) | ||||
| DK-20 | Y | 3.30 (0.12) | N/A | N/A | 2.97 (0.23) | 0.82 | |
| N | 3.15 (0.10) | N/A | 2.89 (0.26) | ||||
| MSQ | Y | 374.7 (53.07) | 395.6 (12.93) | 0.72 | 362.7 (22.48) | 0.99 | |
| N | 373.2 (49.04) | 392.2 (15.66) | 362.5 (19.20) | ||||
| SCID-S | Y | 54.5 (7.81) | 58.3 (2.27) | 0.61 | 61.4 (2.44) | 0.05 | |
| N | 55.4 (7.92) | 57.6 (1.87) | 58.2 (2.00) |
Observational study of cognitive stimulation therapy in practice
The design was a multicentre, longitudinal observational study with people with dementia. Sites that were running or in the process of setting up CST groups completed minimal outcome measures at three time points with people with dementia participating in the CST and MCST programme (Figure 17). The measures were completed before the group started (baseline), at 7 or 14 weeks depending on how they implemented the CST programme (once or twice weekly) and after the MCST at 31 or 38 weeks. However, this end time point did differ when taking into consideration staff and group member availability and holidays. The intervention of CST was routinely offered in the care setting. The aim of this study was to determine whether or not groups were running in practice and to determine if the positive findings for cognition and quality of life for the person with dementia found in previous CST research5 could be demonstrated in practice.
FIGURE 17.
Flow diagram of observational study and assessment schedule.
Recruitment
Centres that were starting or running CST groups were approached and the staff were asked to complete measures of cognition and quality of life with the people with dementia taking part in the groups. If the staff were unfamiliar with the measures then the researcher agreed to complete the follow-up time points with the people with dementia. The centre type, level of staff experience and training were all recorded. The centres were given the MCST manual and staff training DVD. The recruited participants had a confirmed diagnosis of mild to moderate dementia.
Participants
Centres that were currently running or setting up CST groups were approached to participate in the observational study. It was explained that they would be expected to run the CST and MCST programme. Eleven centres were recruited, with some running more than one programme, providing us with 89 people with dementia. To participate, the people with dementia had to have a score of between 0.5 and 2 on the CDR scale,121 a diagnosis of dementia, adequate spoken and written English, the ability to participate in a ‘meaningful’ conversation and the ability to remain in a group for 45 minutes. The participants also needed to have adequate eyesight and hearing, be able and willing to give informed consent and have the ability to complete a cognitive and quality-of-life measure at three intervals over 1 year. Once between five and eight people with dementia in a centre gave informed consent to complete the minimal outcome measures with a staff member or researcher, the centre was recruited in to the study and a letter explaining their participation in the research was sent to the people with dementia’s general practitioners (GPs). The interviews with people with dementia were carried out by a researcher or staff member who was trained to undertake the assessment and had training in Good Clinical Practice and taking informed consent.
Training package
The staff members in the centres had the CST manual or had previously attended CST training which included the CST manual. To participate in the research programme, in addition to the resources they already had the staff at the centre received the MCST manual and DVD.
Randomisation
No randomisation was necessary, as this was a naturalistic study of CST in practice, so people with dementia who were about to start the CST programme were approached and informed, and their consent was obtained.
Outcome measures
The primary and secondary outcome measures for people with dementia were completed at baseline (time 0) prior to the CST programme starting, and then post CST groups (time 1) (at 7 or 14 weeks depending on whether groups were implemented once or twice weekly). The final follow-up was completed after the MCST had been completed or the programme had ceased running (time 2). Sociodemographic information was collected about the person with dementia, including age, sex, ethnicity, diagnosis of dementia, type of diagnosis and medication. Medication was recorded at each follow-up to mark any differences for the duration of their participation in the trial.
-
The primary outcome was cognition as measured by the MMSE. 45 The MMSE has a score of up to 30 points and is widely used as a brief indicator of level of cognitive impairment. It has good reliability and validity.
-
The secondary outcome measure was quality of life as measured by the QOL-AD. 89 The QOL-AD is a 13-item scale measuring different aspects of life. The scale totals 52 points and higher scores indicate better quality of life. It has good internal consistency, validity and reliability. 122
Consent
Ethics and local R&D approvals were obtained and all participants provided informed consent to participate in the trial. The British Psychological Society’s guidance on the evaluation of capacity was adhered to, as it was in the MCST trial. 111
Blinding
Blinding was unnecessary for the staff members and researchers, as each person with dementia had the opportunity to participate in the CST and MCST programme and the staff members or researcher were completing the assessment at each time point.
Analyses
Analysis was a pre–post analysis based on intention to treat, in that all collected data made available by the person with dementia were included, regardless of whether or not that person completed the programme. Imputation methods such as last observation carried forward are of limited use in dementia, as there is the expectation of gradual decline and that participants will be lost through illness or death. A linear regression model was used when there were missing data to predict the missing value and impute the total when possible. The sample size calculation accounted for the number of people expected to be available at the study end point. All participants were in receipt of the CST and MCST programme. Analysis took into account the evaluation at 24 weeks after CST as the primary end point. The secondary analysis considered the effects immediately following the CST programme. Age, sex, AChEI and baseline scores on the two scales being measured were entered as covariates, together with ‘centre’ entered as a random factor.
Ethics arrangements
Risks and anticipated benefits for trial participants
As per the previous study, no documented harmful side effects from participating in either CST training or the running of the CST and MCST programme were apparent and any SAEs were reported to the chief investigator.
Results
The majority of the sample was female (57%) and white (94%), with a mean age of 80 years. Just over half were diagnosed with Alzheimer’s disease (51%), with 17% diagnosed with Alzheimer’s and vascular dementia, 16% diagnosed with vascular only and the remaining 16% diagnosed with another type of dementia or unknown. Approximately two-thirds were on dementia medication (62%), with 35% accessing the CST groups through a memory clinic (35%), although overall 91% were in a specialist dementia setting (Table 20).
| Demographic | Frequency |
|---|---|
| Sex, n (%) | |
| Female | 51 (57) |
| Ethnicity, n (%) | |
| White | 84 (94) |
| Age in years (mean) | |
| 48–92 | 80 |
| Type of dementia, n (%) | |
| Alzheimer’s | 45 (51) |
| Alzheimer’s and vascular | 15 (17) |
| Vascular | 14 (16) |
| AChEIs, n (%) | |
| Yes | 55 (62) |
| Type of centre, n (%) | |
| Memory clinic | 31 (35) |
| Specialist dementia setting, n (%) | |
| Yes | 81 (91) |
If the CST programme was adhered to twice weekly and followed up immediately with the MCST programme, a person would be expected to participate for 7 months and 21 days. For the centres that delivered the CST twice weekly, the amount of time spent in the trial (from baseline to final follow-up assessment) ranged from 7 months and 23 days to 9 months and 6 days. When the CST programme was run once weekly and followed up with the MCST programme, a person would be expected to participate for 9 months and 14 days. The amount of time participants spent in the trial ranged from 8 months and 27 days to 12 months and 29 days. The variation in time was accounted for by practical issues such as time constraints, lack of staffing, transport difficulties or lack of group members.
Cognition
Cognition as measured by the MMSE45 remained stable over the time frame of the CST and MCST programme. At baseline, 89 participants ranged in score from 7 to 30, with a mean score of 21.2. At follow-up 1, 62 participants ranged in score from 10 to 29, with a mean score of 22. At follow-up 2, 55 participants ranged in score from 4 to 30, with a mean score of 21.4 (Table 21).
| Time point | MMSE score | |||
|---|---|---|---|---|
| Range | Mean | SD | ||
| Lower | Upper | |||
| Baseline | 7 | 30 | 21.2 | 4.6 |
| Follow-up 1 | 10 | 29 | 22 | 4.8 |
| Follow-up 2 | 4 | 30 | 21.4 | 5.6 |
A paired sample t-test was conducted to evaluate the impact of CST on cognition at baseline and final follow-up scores. There was no statistical significance difference in MMSE scores from baseline (mean = 21.36, SD 4.9) to final follow up (mean = 21.42, SD 5.68) [t = –0.138, p = 0.891 (two-tailed)]. The mean increase in the MMSE scores was 0.06 with a 95% CI ranging from –0.85 to 0.74. The eta-squared statistic (–0.14) indicated no effect size (Table 22). A paired sample t-test was also conducted between baseline and follow-up 1; however, there was no statistical significance between time points (p = 0.16).
| Paired sample statistics | Mean | SD | SE |
|---|---|---|---|
| MMSE baseline | 21.36 | 4.9 | 0.66 |
| MMSE follow-up 2 | 21.42 | 5.68 | 0.77 |
Quality of life
Quality of life as measured by the QOL-AD 89 increased slightly over the time frame of the CST and MCST programme. At baseline, 89 participants ranged in score from 0 to 49, with a mean score of 35.7. At follow-up 1, 62 participants ranged in score from 24 to 49, with a mean score of 36.8. At follow-up 2, 56 participants ranged in score from 25 to 47, with a mean score of 36.7 (Table 23).
| Time point | QOL-AD score | |||
|---|---|---|---|---|
| Range | Mean | SD | ||
| Lower | Upper | |||
| Baseline | 0 | 49 | 35.7 | 7.7 |
| Follow-up 1 | 24 | 49 | 36.8 | 5.6 |
| Follow-up 2 | 25 | 47 | 36.7 | 5.3 |
A paired sample t-test was conducted to evaluate the impact of CST on quality of life at baseline and final follow-up score. There was no statistical significance difference in QOL-AD scores from baseline (mean 36.34, SD 7.63) to final follow-up (mean 36.7, SD 5.3) [t = –0.43, p = 0.667 (two-tailed)]. The mean increase in the QOL-AD scores was 0.39 with a 95% CI ranging from –2.21 to 1.43. The eta-squared statistic (–0.00) indicated no effect size (Table 24).
| Paired sample statistics | Mean | SD | SE |
|---|---|---|---|
| QOL-AD baseline | 36.34 | 7.64 | 1.02 |
| QOL-AD follow-up 2 | 36.73 | 5.30 | 0.71 |
Discussion
This project pragmatically evaluated the effectiveness of staff training and outreach support by increasing the delivery of CST in practice by outreach support intervention in both new (STANDOUT trial) and experienced (MONOU trial) CST practitioners. The MONOU trial provided a naturalistic evaluation of the benefits of manual only versus manual and training in CST implementation, and both the STANDOUT and MONOU trial identified staff and situational factors that impeded or facilitated CST implementation. It also allowed the research to demonstrate, on a large scale, the knowledge, views and understanding, and approach of staff members to dementia in a variety of care settings nationwide with the secondary outcome measures.
As outreach support was rarely accessed, it may seem unsurprising that there was no statistical difference in the delivery of the CST programme between the intervention and TAU groups. However, this study shows similar uptake of CST (one in three) to that in the previously conducted pilot study110 after 1-day training for the STANDOUT trial. There was an increase in both the intervention group (51%) and the TAU group (43%), compared with the 33% reported in the pilot study. This indicated that the outreach support increases the uptake of CST; however, it may be that research involvement inflated this figure also, as when the centre enrolled in the study they agreed to intend to implement the programme. One might expect this initial effort to have dwindled by the time of implementing the MCST programme but this study demonstrates that, with the additional outreach support, the centres were more likely to run MCST following on from the original programme.
The secondary outcome measures provided a useful overview of staff perceptions and knowledge before, during and after the research study, and there was an indication that the intervention improved staff sense of competence. However, it is important to consider the situational factors that impeded the running of the CST and MCST programme. In particular, staff left posts because of restructuring, left their current post or retired, or there was a lack of staff or a lack of suitable participants when considering the inclusion criteria or the time frame of the study. There was a higher than expected dropout rate that could be attributed to these reasons; however, another reason was that staff who did not run the programme felt that it was unnecessary to complete the questionnaire or staff did not have enough time to complete it. On reflection the questionnaire could have been shortened in attempt to maintain a higher retention rate.
In relation to the observational study, it provided an evaluation of long-term cognitive and quality-of-life benefits of CST and MCST in practice. It is the first study to measure outcome measures with people with dementia when only staff members are delivering CST and MCST as part of usual care. Although there were no statistically significant differences from baseline to final follow-up in either cognition or quality of life, the scores remained stable over the time frame of the programme. This is useful as previous research has suggested a decrease of 2–4 points on the MMSE over time frame of a year and a half. 123
Although this study looks at groups in practice, and this was reflected in the time frame of the follow-ups, it would be useful to conduct a trial in which staff members in centres emulated the time frame the researchers followed in previous work111 so that it would be possible to make a direct comparison.
Conclusions
In conclusion, the project has attempted to demonstrate the potential benefits of offering outreach support to dementia care staff across a variety of settings. There is no statistical difference between the intervention and TAU groups in running CST. However, those in the intervention group were more likely than those in TAU to go on to run MCST. Positively, in the delivery of CST no groups were considered poor, with all in the OK–good range (≥ 3 group members) and the majority in the latter category. However, this rating did diminish slightly for the MCST programme, for which the majority were in the OK range. There were more groups run among the staff recruited as part of the MONOU trial, and this could be attributed to the staff’s prior experience in delivering CST making them more adept at running the programme.
There was no significant difference in secondary outcome measures when comparing the intervention group with TAU, apart from the staff members in the intervention group at final follow-up considering themselves more competent. It is a positive finding that staff in receipt of outreach support consider themselves more competent, although it would also be useful to develop a measure to determine if this is evident in practice.
With regard to the observational study, there may not have been an improvement in cognition and quality of life over the duration of the programme; however, maybe just as importantly, there was no deterioration in scores either. This is important as the CST originally designed to be run twice weekly was also implemented once weekly and this did not affect the cognition and quality-of-life scores. A future trial would be useful to control the frequency in delivery of the CST, the time frame of completing the assessment time points and the rigorousness of the inclusion criteria required to participate in the programme.
Overall, this study has made a positive step towards demonstrating the benefits of offering outreach support to staff in delivering CST as part of their usual practice. Although the support may not be able to overcome the wider factors that might impede the successful delivery of the programme, such as organisational change, it does appear to build on the staff members’ perceived levels of competence and increase the delivery of the MCST programme following on from the original programme.
Focus groups about the experience and effect of maintenance cognitive stimulation therapy
Three focus groups were undertaken with people with dementia taking part in the observational study and the staff facilitating these sessions. The results were originally written up by Priyanka Chauhan, a BSc (Bachelor of Science) psychology student supervised by Professor Martin Orrell, entitled ‘The experience and effect of Maintenance Cognitive Stimulation Therapy (maintenance CST): The perspective of people with dementia and maintenance CST group facilitators’. However, the focus groups were co-run and supported by a researcher (Amy Streater) as part of the SHIELD programme.
Aim
The study explored the experiences and effects of participating in the MCST programme by conducting semistructured interviews with people with dementia and CST facilitators. It also aimed to explore the mechanisms of change and compare the findings with those of a previously conducted study110 on the experiences of CST to determine if the MCST continued the perceived benefits identified from the original programme.
Sample
Ten people with dementia who had completed the CST programme and were part way through the MCST programme were recruited from an East London community day centre for older people. The mean age was 84 years and the sample was made up of four male and six female participants. There was a mean baseline MMSE45 score of 16, indicating a moderate level of impairment. All had completed the CST programme and were up to the sixth MCST session.
Five staff members were recruited that had previously acted as a cofacilitator or main facilitator in running the CST programme and were in the process of running the MCST programme with the people with dementia recruited into the study. The day centre was purposefully chosen as it had previously been enrolled in the MCST research project111 and staff had gone on to run their own groups following on from the research, it was considered that the staff were experienced enough to provide an in-depth experience of the MCST programme. The staff members either had attended the CST training day or had the CST ‘Making a difference’ manual. All staff members were female and between them they had an average of 11 years of dementia care experience.
Inclusion criteria
People with dementia were initially approached if they had completed the CST programme and regularly attended the MCST programme that was currently under way at the centre. However, they also needed to (1) have met the DSM-IV124 criteria for dementia, (2) have scored between 10 and 24 on the MMSE,45 (3) be able to adequately communicate, (4) see and hear well enough to participate in the group, (5) not present behaviour or have a physical illness that could limit their participation, and (6) not have a diagnosis of a learning disability. These inclusion criteria were used to ensure that people could adequately participate and provide useful information in the focus group.
The staff members were considered eligible to participate in the focus group if they had participated in the running of the CST and MCST programme and met with the service users accessing CST at least twice weekly; this was considered sufficient time for staff to be familiar enough with the service users that they could comment on everyday effects that might arise from the MCST programme.
Procedure
Managers were approached 1 month before the focus groups were undertaken to gauge their level of interest and identify potential participants from both staff and people with dementia. A week prior to the focus groups being undertaken, a researcher approached the staff and people with dementia and went through the information and consent form, allowing any questions to be asked. The researcher went through the information sheet and consent form again on the day of the focus group and discussed this with the participant for a second time before informed consent was obtained. Consent was obtained in accordance with the British Psychological Society40 guidelines and ongoing consent was adhered to; participants were reminded that they could withdraw at any time and no adverse consequences would result from this.
Materials
A discussion guide was devised, based on the findings from a previous study,110 to guide the conversation. This allowed a reliable comparison to be made between the person’s MCST experience and the original CST programme. Questions were created with the aim of understanding any mechanisms of change that might be occurring; this was in relation to a person-centred approach (PCA). 125 Swaab’s theory of stimulating underused cognitive ability126 and the biopsychosocial model127 were explored. All focus groups were audio-taped and field notes were taken by a cofacilitator to document any useful non-verbal communication and interaction of group members that might have been lost in the transcription stage of the data analysis.
Data analysis
The data analysis followed the stages of framework analysis128 and followed the principles of thematic content analysis. 129 Each focus group transcript was analysed with the following steps: (1) immersion in the data; (2) categorising the data to identify a thematic framework; (3) coding; and (4) interpretation and association. In the first stage it consisted of the researcher familiarising himself or herself with the general ideas generated from the focus group discussion that were identified from reading the transcripts. In stage 2, ‘open coding’130 was used to categorise the data into general themes, followed by themes more representative of the data. To ensure the validity of the themes, a second researcher also analysed the transcripts so that a final set of themes was agreed by both researchers. The third stage involved the coding of the transcripts in accordance with the thematic framework and this was then followed by the final stage of interpreting the findings in accordance with the existing literature.
Results
After the data were analysed, two main themes emerged across the three focus groups. The first was ‘positive experiences of being in the group’ including the subthemes ‘enjoyable company’, ‘positive feelings’ and ‘benefits of a smaller group’. The second theme identified was ‘cognitive stimulation and cognitive benefits’, with the subthemes ‘importance of and improvement in communication’, ‘importance of variety’ and ‘improvements in memory’.
In the following quotations, to clarify who was speaking, P will be used to identify a person with dementia and S will be used to identify a staff member.
Positive experiences of being in the group
People with dementia emphasised the building of relations resulting from their participation in the programme and time spent with others as key reasons that they enjoyed the sessions. They openly expressed their enjoyment and appreciation of interacting with others, including the staff.
It’s nice to have a group, it feels like you’ve got friends.
P
Current affairs was discussed as being the most enjoyable activity (see Importance of conversation); however, people with dementia were receptive to all of the activities in the programme and emphasised that the company of others was the primary reason that the sessions were enjoyable.
I’m never bothered about what they are going to discuss, I can be a listener as well as a talker . . . when I’m with people the atmosphere does me.
P
People with dementia often reported feelings of happiness and feeling lifted and more relaxed. Some also mentioned that they could recall the MCST session later when they were at home, demonstrating lasting effects beyond the group itself.
It’s nice, when you get indoors you’re relaxed and in your mind you can see it all over again. You’re doing it all over again in your mind, you relive it.
P
Another repeated positive feeling was that of being valued and comfortable.
Nobody’s taking the quiet mick out of anybody.
P
Staff members also noted that the group members felt a sense of inclusion and that the attendees highlighted the group they attended as ‘theirs’.
They ask each other ‘Which group are you going?’ and he says ‘No, no, no, I’m going to my own group, this is my own group’.
S
Staff members felt that the larger group size usually used for activities in the day centre (group size of 12) would not generate the positive feelings demonstrated by the CST group members. Having a group size of between five and eight people facilitated the positive feelings. People with dementia were also aware of the difference in numbers.
She’s allowed to say what she wants to say. I think because it’s a smaller and it’s a happy group as we say.
S
When we amongst a lot of others (common areas), it’s not the same.
P
However, the point was made that a balance had to be struck in terms of group size so that groups did not get too small with the potential to bring in new members.
We’ve been so small we haven’t diversified a lot. I’d like to see us know get more people in here so discussions become more broad and diversify.
P
Cognitive stimulation and cognitive benefits
Both staff members and people with dementia believed that cognitive stimulation occurred through discussion. Discussion is one form of cognitive stimulation with the MCST programme, as it helps to facilitate the learning of new information and allows people to express their opinions. All participants rated a session theme entitled ‘Current affairs’ the most enjoyable.
It is nice to hear other people’s opinion, you think to yourself, ‘Oh I never thought of that’. It livens you up.
P
You can develop some sort of discussion and people will join in it, irrespective whether they like it or not, and that gives a feeling that they appreciate you and don’t want to decline what you’re saying.
P
Staff members noticed improvements in people with dementia’s ways of expressing themselves and identified an increase in their willingness to communicate and participate in the MCST group.
She never used to say much, but in the group she’s got loads to say, I just have to sit back and listen to her, just going on and on and she won’t stop you know.
S
She’s thinking about things and more confident about expressing her opinions.
S
The staff members clearly felt that cognitive stimulation occurred owing to the range of activities and discussion topics available and raised the idea that the variety of sessions would be key to the continuing success of the MCST programme. The service users also emphasised the importance of flexibility and choice within the programme that would not be possible without there being a variety of sessions.
You’ve got different sections to it, you’ve got the reminiscence you know, you’ve got the newspapers, you’ve got the activities so even if there is something that they are not interested in the activities, but you might find that they would have a lovely discussion during the newspaper reading. There is always something for someone.
S
They [staff] allow a free discussion . . . it creates a good atmosphere, it gives people confidence then to talk.
P
Both service users and staff reported that the sessions aided recall and led to specific improvements in memory, such as the repetition of the group song or orientation to the current time and place.
They didn’t even know the road that the day centre was on . . . now as soon as we sit down . . . they can tell you the resource centre, [road name], even the postcode now.
S
I’m using it more . . . I was becoming really as if I had no memory at all. Since coming here it’s revived it all.
P
Discussion
This study aimed to explore the experiences and effects of the MCST programme for people with dementia and the perceptions of the facilitators. It also looked to identify possible mechanisms for change and to see if the findings of this study were comparable with the themes identified in the CST programme,110 to see if the MCST programme sustains the positive experiences and effects when it follows on from CST. The identified themes indicate that there is a similarity between the experiences and effects of CST and those of the MCST programme, and each theme ties into at least one of the mechanisms of change explored: Kitwood’s PCA,125 Swaab’s theory of stimulating underused cognitive abilities126 and the biopsychosocial model. 127
Comparison with Spector et al.110 CST findings
The first theme identified was ‘positive experience of being in the group’, which was identified in the previous study. Within this theme, ‘positive feeling’ was also found in the 2011 study and within this were feelings of enjoyment, relaxation and something to look forward to. The second subtheme, ‘enjoyable company’, corresponds to the identified subtheme in the previous study of ‘supportive and non-threatening environment’, as both recognise the positive effects of relationship building between group members and with the facilitators. A difference in the main theme between this study and that by Spector et al. 110 is that the latter did not find the subtheme of ‘benefits of a smaller group’ and this study did not report the service users gaining and remaining confident for the remainder of the day after CST. However, the increase in confidence for people with dementia had been identified through their family caregivers and, as this demographic was not used in this study, it cannot be assumed that this is different for the MCST programme. However, it is important to note that staff members noticed that for some people with dementia their increased confidence outside the group could be considered evidence of their enhanced confidence (see Importance of and improvement in communication).
Two of the subthemes, ‘cognitive stimulation and cognitive benefits’ and ‘importance of and improvement in communication’, tally with the subtheme ‘positive experience of being in the group’ as identified in the 2010 study. Within this were the subthemes ‘finding it easier to talk’ and ‘sharing a diagnosis’, which can be considered reasons for improvement in communication. An increase of confidence in communication ties in with the first theme of ‘positive experience of being in the group’, as comfort in a smaller group size, valued contributions and friendship can lead to a heightened confidence in expressing oneself. The corresponding subthemes demonstrate that the opportunity that is created in CST to discuss, give and receive opinions is a factor in the perceived success of the CST and MCST programme. It would seem unlikely that conversation would have been an important element without having ‘positive feelings’ and the ‘benefits of a smaller group’. The importance placed on opinion links into the CST key principles of opinion over fact and implicit learning, both of which fall under ‘positive person work’ (PCA). Placing value on what people say rather than on the factual content or accuracy of what they say increases their confidence in expressing their opinion. The subtheme ‘importance of variety’, under the main theme of ‘cognitive stimulation and cognitive benefits’, did not correspond to the Spector et al. 110 study, yet it was a key point of agreement in this study (see Mechanisms of change); however, this might be have been emphasised owing to the extended duration of the MCST programme.
Both a general and a specific improvement in memory were identified in Spector et al. 110 and in this study. It could be argued that ‘improvement in memory’ resulted from the use of reality orientation,131 as a result of the reality orientation board used for every CST and MCST session and the repetitiveness of the implicit information processing (group name, song, location) as well as the session structure as presented in both the CST and MCST manual. This can be related to the subtheme of ‘positive experience of being in the group’, as one might expect that, without an environment conducive to participation and enjoyment, the likelihood of someone becoming actively involved in the discussion and activities might decrease and, in turn, lead to a negative impact on their memory and cognition.
Mechanisms of change
An analysis of the mechanisms of change was necessary to develop an understanding of what is effective in relation to CST. Two of the themes emerged in this study, ‘benefits of a smaller group’ and ‘importance of variety’, that can be considered mechanisms of change. As both the Spector et al. 110 and this study identified ‘positive experience of being in a group’ as a theme, it is apparent that both the CST and MCST programmes created an environment that facilitated ‘positive feelings’ of value and inclusion as well as the building of relationships. This theme is evidence of the benefits of using a PCA. 125 A PCA places emphasis on seeing the person with dementia as a person and an individual, rather than defining them according to their diagnosis.
The ‘benefits of a smaller group’ subtheme appears to fit well as part of a PCA125 as it encourages more in-depth relationships, something one might expect to be harder with a larger group . The possibility of developing friendships in a small group might encourage the active participation of each group member and increase the cognitive benefits of CST. 126 So, although a larger group might adhere to the same principles, it may not demonstrate the same benefits for the group members.
The variety of activities and current affairs to be discussed at the beginning of each session follows another key principle of CST: choice. Allowing choice reiterates that the group belongs to the group members and increases the chance of catering to each person’s needs and interests, further facilitating the use of a PCA. The flexibility of variety is in keeping with the biopsychosocial model,127 which states that treatment should consider the fixed biological factors as well as the changeable psychological and social factors when working with a person with dementia. This model works with CST as it identifies the fixed factors that are reflected in the inclusion criteria of the therapy of scoring 10–24 on the MMSE,45 as well as the tractable factors such as sensory impairment when it is necessary for the person with dementia to see and hear well enough to participate in the programme. 6 The biopsychosocial model does link in with the inclusion criteria used for participation in the programme but it is more strongly related to the psychological factors as recognised in the model. CST as a psychosocial intervention creates a social support network through the group format of the therapy leading to improvements in social relations and uses a multisensory stimulation through the session themes and materials to encourage implicit mental stimulation (psychological tractable factor). An understanding of the person’s history, personality and beliefs before starting CST emphasises a PCA as this can be considered when running discussion or activities. The variety offered by CST also allows for personal psychology and individual differences to be taken into account when creating sessions that can cater for a range of interests.
Limitations
The study focused on the opinions generated from the people with dementia participating in the programme as well as those of the staff members facilitating the groups. As the staff members were familiar with the service users, it was not considered necessary to include family members. However, when conducting the focus group, staff did not feel comfortable commenting on the possible after-effects of MCST. Staff might also be unaware of the person with dementia’s feelings about the group, whereas the service user might be more open with a family member about their experiences. So, focus groups run with family members might be useful when considering the after-effects and a more in-depth understanding of the perceptions of the MCST programme.
Owing to the limited time frame of the study the focus groups were conducted after the sixth session of MCST. The full maintenance programme is run over 24 weeks so the opinions expressed were after one-third of the programme had been delivered. It could be considered that focus groups conducted at the end of the MCST programme would be more beneficial in getting a truer account of the experience of the programme, as in the study the people with dementia could discuss the experience of the first six sessions only. This point might not be as relevant to the staff as they were able to draw on their previous experience of the MCST programme, having delivered it prior to the current group.
As there was a time constraint on collecting the data, only one centre could be recruited into the study. This might have biased the results as the feedback on the MCST programme was specific to the delivery of it in that particular centre. There was also a small number of participants (10 people with dementia and five staff members) and so the amount of feedback was limited to a small sample size. However, the same thoughts about the programme were mentioned across the three groups, so it might be that more participants would not necessarily generate any additional themes but rather would strengthen those already identified in this study.
Conclusion
This study used focus groups to explore the experience and effect of MCST with people with dementia and staff members, and in addition looked at the mechanisms of change and compared the findings with those of a previously conducted study on service users’, family members’ and staff members’ experience of CST. 110 This study identified that the experience of MCST closely matched the experience of CST and that MCST appears to preserve the perceived benefits across the duration of the programme. The main themes identified were ‘positive experience of being in the group’ and ‘cognitive stimulation and cognitive benefits’, and both of these can be indirectly related to one of the mechanisms of change (PCA and benefits of a small group). Further research is required to look further at the mechanisms of change identified in both quantitative and qualitative methods and relate this to CST and MCST to determine what is most important in attempting to further understand and increase the benefits for people with dementia.
Chapter 3 Peer support and joint reminiscence for people with dementia and their carers
Work package 1: development of a Carer Supporter Programme training package
The CSP (RYCT) trains experienced carers to support newer carers of people with dementia; reminiscence groups are also held jointly for people with dementia and their carers to maintain quality of life and improve relationships.
Our intervention was developed from the BECCA trial29 of a carer support intervention in which trained volunteer lay workers befriended family carers of people with dementia for companionship and conversation. 29 Supporters did not necessarily have personal experience of caring for a relative with dementia, but the most successful volunteers were those who were ex-carers. Building on the BECCA work, we focused on the involvement of family carers in the development of a peer-support programme in which newer family carers are matched with and then supported by current or former family carers of people with dementia who provide a listening ear, conversation and companionship, moral support and signposting to useful organisations and services.
In developing the CSP package, we followed consensus methods involving principles of good practice (i.e. clarity and transparency, respect, diversity, flexibility and accessibility) and meaningful involvement. 35 Service users (people with dementia and their family carers) were involved in the development of the intervention using two consultation methods, which are not commonly participated in by service users. 33 As shown in Figure 18, these were a modified Delphi process and consensus conference to develop the content of the intervention (study 1) and a consultation to develop and refine information and consent documents (study 2).
FIGURE 18.
Model of consensus methods used in the development of SHIELD CSP (study 1, modified Delphi and consensus conference). DeNDRon, Dementias and Neurodegenerative Diseases Network.
Study 1: modified Delphi and consensus conference
Aim
To develop a peer-support intervention from the carer perspective in consultation with service users.
Design
A combination of a modified Delphi process132,133 and consensus conference134 was used to explore the details of the peer-support programme from the carer and volunteer perspective. A combination was used to allow participants to respond personally and privately, but also to provide an opportunity to discuss their ideas and concerns.
Participants
Service users and stakeholders were approached through organisations with an interest in dementia, family carers, peer support/voluntary work, NHS organisations and universities. Twenty-five people expressed interest in taking part and completed round 1 of the modified Delphi process. Of these, 21 attended the consensus conference. The delegates comprised eight current and former carers, seven members of voluntary organisations supporting carers and people with dementia or representing volunteers, three clinical health professionals, an academic specialising in the area and two others (did not specify). Round 2 was sent to the original 25 stakeholders as well as to an additional eight who had become involved as the project progressed. In round 2 five questionnaires were returned from three former family carers (one of whom had also worked with carers), one current family carer and one representative of a charity for people with dementia and their carers.
Method
Three weeks before the conference, a round 1 Delphi questionnaire was sent along with copies of the raft participant information sheets and related recruitment materials to provide context to the proposed peer-support intervention. Delegates were required to complete the questionnaire before attending the conference and either post it back to the research team or hand it to the team on the day. The questions concerned the intervention name, the role of the peer supporter, the content and duration of training, desirable peer-supporter characteristics, the criteria to be used to match peer supporters with family carers (matching criteria), support for peer supporters and monitoring the matches for research purposes. These items were developed by the research team with reference to the service user feedback for BECCA, along with ideas and concepts trialled by other research groups in their work with peer supporters. 135–137
During the conference, round 1 data were analysed and the results were presented for discussion. The delegates were split into smaller groups, with careful consideration given to creating heterogeneous groups, as these can be the most productive when aiming to explore uncertainties and develop ideas. 133 Each group was given a question from round 1 to focus on, facilitated by a member of the research team who made notes on a flip-chart. These notes were used to feed back to the larger group and were also a record of the discussions held.
After the conference, the round 1 results were analysed in more detail with particular focus on identifying areas in which consensus had not been reached. Each question was scored on a six-point Likert-type scale measuring strength of consensus (where 1 = unimportant/unsuitable/unfeasible and 6 = important/suitable/feasible). The delegates were also asked to rate their most preferred and least preferred options. The responses to the question items were summarised as medians, with higher medians indicating higher importance/suitability, as these are more robust than means and provide a better indication of distribution of consensus as opposed to central tendency. 133 Percentages were also calculated for most preferred and least preferred options. By using these two response formats, it was possible both to understand the extent of overall consensus (through most and least preferred options) and to assess the strength of consensus by analysing the medians for each item. The scores from all of the delegates were given equal weighting, regardless of perceived ‘expertise’, meaning that the responses of service users were equal to those of other delegates working professionally in the area.
Items with the lowest medians (1 and 2) were excluded from round 2. Items with highest medians (5 and 6) were also excluded because consensus was that these items should clearly be incorporated. Items with median scores of between 3 and 4 were included in the round 2 questionnaire along with clarifying information. This information was based on identified areas of confusion and disagreement from notes taken during the conference. For instance, the difference between ‘giving advice’ and ‘sharing experiences’ was raised as an issue during the conference as it was not clear as to how they differed in reality. An item had been included on ‘giving advice’ to raise debate. ‘Advice giving’ had been explicitly placed outside the remit of the BECCA befriending volunteers, given the potential legal ramifications of providing advice. However, carers would often state that they would ‘value the advice’ of a more experienced peer. Definitions and further information were provided in round 2 to set out some distinction. A summary of the consensus conference discussions held were incorporated into round 2 and more space for open-ended responses allowed delegates to clearly outline their views, reducing the need for a third round. Thematic analysis was carried out for all open-ended responses. The analysis was inductive (data driven) with themes defined as specific patterns of interest within the participants’ responses. 138 A third round was found to be unnecessary as disparate views had been resolved at the end of round 2. The results from rounds 1 and 2 (including the thematic analysis of round 2 comments) are presented together to illustrate the delegates’ contribution to the programme through the consensus process.
Results
Name of intervention and intervention providers
The peer-support programme was planned at a time when the Expert Patient Programme was being evaluated, and the parallel initiative for ‘Expert Carers’ was in development. The term ‘expert carer’ has been used in different ways,139 but preliminary conversations with stakeholders made it clear that even the most experienced family carers were uncomfortable with the ‘expert’ label. The programme was given the working title ‘Experienced Carer Programme’, in which peers would be referred to as ‘experienced carers’. As carers provide support to other carers, it was essential that service users were involved in naming the intervention. The delegates were presented with a number of alternative names to describe the peer supporters and were asked for further suggestions. The options included expert carer, mentor, buddy, experienced carer and carer supporter. Round 1 indicated that delegates thought that peers providing the intervention preferred the terms experienced carer and carer supporter (both medians = 5). Experienced carer was the most preferred option (n = 7 of 12 responses; 58.3%), whereas buddy was the least preferred option (n = 8 of 13 responses; 61.5%). The conference delegates also thought that experienced carer and carer supporter would be the preferred terms for people receiving the intervention and both received a median of 5, with experienced carer ranked as most preferred (n = 6 of 10 responses; 60%), followed by carer supporter (n = 4 of 10 responses; 40%).
To reach an effective consensus (and allow time for discussion of the content of the intervention), a nominal group technique was used. 140 The delegates were asked to vote on potential names, including those presented in the round 1 questionnaire and a series of names suggested by the delegates during the nominal group. Two rounds of voting took place. In the second round carer supporter was most popular with 15 votes, whereas experienced carer received eight votes. Carer supporter was preferred, as it more accurately described the role of the volunteer, and former carers objected to the term experienced carer, as ‘experienced’ was a difficult term to define. On this basis, the intervention was renamed the SHIELD CSP.
The role of the carer supporter
The delegates were also consulted on the types of support the carer supporters should provide to help to build the newer carers’ confidence in their caring role. The median scores were ≥ 5 for all items other than specific training (median = 4) (Table 25). In round 1, ‘providing encouragement and moral support’ was rated as most important (n = 4 of 8 responses; 50%). The delegates were less certain about the importance of carer supporters providing specific training or completing exercises from a ‘toolkit’ with the new carer. This was echoed in discussions during the conference focusing on the importance of building an open and flexible relationship based on trust, companionship and encouragement.
| Role of carer supporter | Median | Interquartile range |
|---|---|---|
| Providing encouragement and moral supporta | 6 | 0 |
| Listening to carers’ experiences | 6 | 0 |
| Signposting to services and/or resources | 6 | 1 |
| Encouraging carer self-care | 6 | 1 |
| Talking about common challenges of caring for a person with dementia | 5 | 2 |
| Supporting problem-solving | 5 | 2 |
| Chatting about life outside/beyond caring | 5 | 2 |
| Meeting with both the carer and person with dementia | 5 | 2 |
| Specific training tasks/exercises to compete with newer carerb | 4 | 1 |
In round 2, respondents were asked more specifically about the use of a ‘toolkit’ consisting of optional exercises that carer supporters could use during their meetings with carers to aid communication,136 such as asking newer carers to talk about their role as carer and the obligations they may feel, the history of their relationship with the person they care for, their social circle to identify ‘helpful others’ and any future concerns. The results indicated that a toolkit was not popular (median = 2). Thematic analysis of open-ended responses from former family carers revealed that that the role of the carer supporters should be to meet the needs of the carer. This could be as simple as being there to listen, which was actually seen as being the most important aspect of the role:
Not having someone to talk to is one of worst aspects of caring for someone with dementia. To have opportunity to talk with someone else who is caring, or has cared, is very valuable.
ID [identification data] 2
As such, a toolkit would not help to build the relationship and, for this reason, specific tasks were not incorporated into the SHIELD CSP intervention model at this stage but were discussed further with family carers during the pilot training for carer supporters once the trial had started.
Training content, duration and techniques
The carer supporter training intended to provide a mix of good practice guidelines and support skills. The research team suggested specific training topics to achieve this, such as information about dementia, services and resources, supporting self-care, problem-solving and the use of ‘standard scripts’ to introduce specific topics or tasks. In round 1 all items but one received a median score of > 5, and were seen as being important aspects of training (Table 26). Responses from both the round 1 questionnaire and the consensus conference discussions indicated that, although it was important to be an emotionally supportive peer, carer supporters should also have a strong knowledge base about dementia and dementia services/resources. In addition, delegates thought it was very important for carer supporters to be are aware of the boundaries of their role and to be trained to be able to say no to inappropriate requests. The use of ‘standard scripts’ to introduce specified topics or to help carer supporters ‘break the ice’ at initial meetings received a median of 4. Comments on the questionnaires, along with discussion during the conference itself, revealed that delegates were unsure of what standard scripts were.
| Training | Median | Interquartile range |
|---|---|---|
| Topics for volunteer training (n = 20) | ||
| Information about dementiaa | 6 | 0 |
| Information about services and resourcesb | 6 | 0 |
| Supporting self-careb | 6 | 1 |
| Supporting problem-solvingb | 5 | 1 |
| Standard scripts to introduce specified topics or tasks/exercises (n = 19)c | 4 | 3 |
| Training techniques (n = 19) | ||
| Telling one’s own story of caringd | 6 | 2 |
| Additional readinge | 5 | 2 |
| Video/DVD examples of recommended discussion topics/exercisesf | 6 | 1 |
Round 2 provided more explanation for this term ‘standard scripts’ and opinions were sought on whether or not they would be useful. Opinion remained divided, with a range of scores between 1 and 4 (median = 1). Some thought that standard scripts could be a very valuable tool, particularly one former carer who had also worked with family carers:
A standard script is extremely useful as a prompt to gaining all the required information. It is very easy to miss something important when listening to a carer’s issues that may prove useful in working with that person.
ID4
Others had concerns, such as one former carer who wrote:
If needing to resort to using standard scripts it could send out message about the lack of volunteers’ skills, confidence and above all sincerity. It is the sincerity and empathy that are so important.
ID2
Again, owing to the lack of consensus, it was decided that the use of standard scripts would be raised as a topic for discussion during pilot training sessions.
Regarding the duration of the training programme, 19 (of 20) valid responses were received, of which 14 (73.7%) confirmed that six 2-hour modules were adequate; delegates also highlighted the need for ongoing support and training. A range of techniques was planned for the training sessions, including short lectures, discussions, illustrative examples and role-play, all of which had all worked effectively in the BECCA training module. For the SHIELD CSP, other training techniques suggested for inclusion were telling one’s own story of caring, additional reading and video/DVD examples. Only these three techniques were included in round 1 because they had not been used in BECCA. Telling one’s own story and video/DVD examples were both seen as very suitable (both medians = 6), whereas additional reading received a median score of 5. After discussions during the conference it was seen as least suitable, as delegates raised queries regarding its feasibility. This item was, therefore, retained for round 2 but opinions continued to differ with scores ranging from 2 to 6 (median = 4), indicating that participants thought that it had limited feasibility. As a former carer wrote in round 2:
Some [carer supporters] may be interested in becoming more knowledgeable by additional reading, some may lose confidence if they feel they require and are required to have additional knowledge before being considered as a supporter . . . For example, I am a book and research-oriented person; I know other former carers who are excellent carers but not interested in serious study.
ID1
Another round 2 question asked, if additional reading was not feasible, whether or not it would be appropriate for other material to be requested. It was unanimously agreed that this was the case. A current carer summarised:
. . . not all carer supporters would like to do all recommended tasks. The information should be available with details of resources and no compulsion.
ID3
Carer supporter characteristics
To identify the type of carer supporter who could be most helpful to the newer carer, a list of characteristics was presented for evaluation (Table 27). All delegates agreed that the ability to listen was absolutely essential and this prompted the strongest response received from all items on the questionnaire, with all 19 responses giving this a score of 6. A tolerant attitude (median 6) and being knowledgeable about dementia (median 6) were also found to be essential. Advice-giving continued to be out of favour (median 4) but, surprisingly, the sharing of experiences during meetings was also unpopular (median 4), even though one of the perceived values of an experienced carer is that they have common or shared experiences. Delegates thought that some carers may feel that the carer supporter was ‘taking over’, with the envisaged worst consequence being that experiences may result in the carer supporter ‘off-loading’, thus adding to the family carers’ burdens. Conference discussions revealed that delegates were unclear about the difference between using experience to guide discussion and sharing personal experiences. Therefore, in round 2, the concepts of using and sharing experience were defined more clearly. Using personal experiences was described as carer supporters using their experiences to assist carers in problem-solving or signposting to useful resources but not to give advice on courses of action. Sharing personal experiences was described as carer supporters talking about their own personal caregiving journey. It was also made clear that training would explicitly cover the importance of not ‘off-loading’. However, consensus was not reached as both approaches received a range of scores (median = 4), perhaps indicating that the difference was not so clear. Thematic analysis revealed that, despite the varying scores, all delegates thought that sharing experiences was vital as the basis of the SHIELD CSP regardless of whether the question concerned sharing or using experience. As one former carer wrote:
Sharing experiences, exchanging information evoke a spirit of kinship among fellow carers and begins the learning curve of dementia care and coping strategies and other life experiences of living with dementia.
ID3
| Carer supporter characteristics | Median | Interquartile range |
|---|---|---|
| Ability to listena | 6 | 0 |
| Tolerant attitude | 6 | 0 |
| Keen to give advice | 4 | 2 |
| Keen to share their experienceb | 4 | 2 |
| Keen to use their experienceb | 5 | 2 |
| Knowledgeable about dementia | 6 | 0 |
However, all had concerns about the risks associated with allowing carer supporters to share their experiences, perhaps leading to these mixed views. In particular, a representative of a dementia charity wrote:
After 17 years working with carers of people with dementia it is my experience that very few are able to be objective about their own experience. If some time has passed . . . they may offer out of date information, or allow a bad personal experience to colour the conversation.
ID5
Comments from delegates stated that they thought that giving advice should be avoided. Although delegates emphasised that using and sharing experiences could be useful in terms of signposting carers to useful resources or simply conveying empathy, all thought that this would need to be very carefully considered before being implemented. This discussion was taken forward to the pilot training.
Matching criteria
For the SHIELD CSP intervention to reach its full potential, newer carers needed to feel some warm and genuine connection with the carer supporters. Previous research suggests peers and those they support be matched on a set of criteria associated with personal constructs and characteristics. 8 However, experience from the BECCA programme29 highlighted the need for flexibility in making matches. To ensure balance between theory and practice, and to generate ideas concerning the most important characteristics for people to have in common, potential matching criteria were explored in round 1. Psychological health (i.e. feelings of burden or mood state) was considered to be essential (median 5), along with view of caregiving (i.e. as burden, challenge to face, problems to solve) (median 5). Being matched on marital status (median 2) or employment status (median 1) were considered unimportant (Table 28), although during the conference discussions the former was seen as an important criterion. Other matching criteria such as sex, age, relationship to care recipient and geographical location did not result in a firm consensus. One reason for this, suggested during the conference, was that certain criteria may be important to some but not to all (e.g. matching according to sex). Further discussion during the conference led to the proposal of hobbies, religious or ethnic origin, and type of dementia and age of onset as matching criteria.
| Matching | Delphi round | |||
|---|---|---|---|---|
| 1 | 2 | |||
| Median | Interquartile range | Median | Interquartile range | |
| Sex | 4 | 2 | 5 | 2 |
| Marital status | 2 | 2 | ||
| Employment status (including retirement)a | 1 | 3 | ||
| Age | 4 | 1 | 4 | 1.5 |
| Education | 3 | 3 | ||
| View of caregiving (e.g. as burden, challenge to face, problems to solve) | 5 | 3 | ||
| Psychological health (e.g. sense of burden, mood state)b | 5 | 3 | ||
| Relationship to care recipient | 4 | 2 | ||
| Geographical location | 4 | 2 | 3 | 4.5 |
| Religious/spiritual views | 5 | 3 | ||
| Cultural/ethnic background | 6 | 1 | ||
| Interests/hobbies | 2 | 3.5 | ||
| Relationship to care recipient | 3 | 4 | ||
| Type of dementia | 2 | 4 | ||
| Age of onset | 6 | 3 | ||
In round 2, these criteria were explored further and consensus was reached that, where possible, carers and supporters should have the option to be matched according to sex, religious/spiritual views, cultural or ethnic background and, to a greater extent, relative’s age at onset of dementia. How sensitively such differences in experience could be viewed was highlighted by a former carer:
If you have cared for someone with young onset, as I did, you are apt to get a bit irritated with someone who cared for someone in their 80s. It’s not the same, as we share that sense of loss yes, but not that of an ‘out of turn’ experience which is so distressing where young family and all the other related issues come into play.
ID2
Delegates thought that matching according to interests and hobbies and type of dementia was not important. Indeed, some thought that different interests could stimulate conversation and interest, and, with good training, type of dementia would not be important. As one former carer wrote:
Supporter is providing companionship and moral support to someone who is tired, worn out and isolated. The support is for the carer, not the patient.
ID1
There was less agreement on geographical location and relationship to relative. Despite consensus for the majority of criteria, the decision was made to ask participants and carer supporters about the characteristics that they would like to be matched on, as the point of matching was to achieve a warm and supportive relationship. Indeed, this was found to be the most practical approach during the subsequent pilot trial of the SHIELD CSP. Feedback from the organisers of the scheme indicated that matches were not made according to a list of objective criteria, but on a judgement made by the managers as to whether or not a pair would get on well. Thus, the criteria were seen to be useful in identifying and avoiding potential areas of conflict, but were not used as the basis for making matches.
Ongoing support for carer supporters
Carer supporters needed to feel supported in their role, and delegates were consulted on the best methods to achieve this. The research team suggested group meetings, individual face-to-face contact and additional training, which were all approved by the delegates (median 6 for all). Additional comments from both round 1 and conference discussions confirmed that local co-ordinators should provide ongoing support, with more frequent contact encouraged during the first 3 months of the 10-month intervention. In terms of the frequency of contact, once per month was thought to be most appropriate (n = 10 of 19; 52.6%), although during the discussions some suggested an ‘open door’ policy of support, which has since been adopted.
Monitoring matches for research purposes
The frequency and nature of contact between carer supporters and carers needed to be monitored to assess how much intervention time the participants received. In round 1, delegates were consulted on the best ways of accurately monitoring contacts while avoiding burdening the carer supporters. Methods such as checklists, telephone contacts and diaries were suggested. Of these, regular telephone calls from the local co-ordinator to the volunteer were judged as most feasible (median 6), whereas checklists were second (median 5). Comments during the conference warned against time-consuming and burdensome procedures, which may explain the lack of consensus on completing a diary (median 4). The decision was made for carer supporters to complete a checklist of items, suggested during the conference, which would be administered by a co-ordinator during regular telephone meetings.
Study 2: informed consent document consultation
Aim
These documents were necessary to enable participants to decide whether or not to take part in the research trial with valid informed consent. The aim of the consultation was to ensure the appropriateness and suitability of the consent documents (recruitment leaflet and information booklets for carers and their relatives with dementia) and improve readability of the documents.
Design
To manage time constraints and reduce the potential burden on readers, postal consultations were used. The materials for consultation had been adapted from the BECCA study,29 so some materials were able to be reused whereas others had to be newly developed. This is a novel approach to developing informed consent documents, which has logistical and practical advantages over existing methods.
Participants
As carers and their relatives with dementia were involved in the SHIELD CSP, both groups took part in the reader consultations. The consultations were anonymous and the packs were sent to readers by post. Family carers were contacted through the Uniting Carers network of the charity Dementia UK. People with dementia were contacted through the East Anglia and North Thames hubs of the Dementias and Neurodegenerative Diseases Network (DeNDRoN), and were members of the Patient and Public Involvement Forum. In both cases, the research team sent consultation packs to identified gatekeepers at each organisation who then forwarded the packs to interested members of their participant involvement panels.
Method
Before beginning consultations, the research team incorporated the outcomes of the modified Delphi process and consensus conference into the draft materials. Although the focus of the reader consultations was on the recruitment leaflet and information booklets, draft consent forms were also included in the consultation to show respondents what participants would be expected to decide on and, therefore, the type of information the booklets should contain. Carers were asked to read the recruitment leaflet, the information booklet for family carers and the consent form. People with dementia were asked to read the information booklet for people with memory problems and the corresponding consent form. The respondents were then asked to complete feedback forms that asked about the clarity of information about the research study, what commitment would be required for taking part, information about right to withdraw from the research study and the layout of the booklet. The respondents rated the clarity of information on a 5-point Likert-type scale (where 1 = no/not clear, 3 = partially clear and 5 = yes/very clear). The respondents were also asked about the overall quality of each document on a similar scale (where 1 = low, 3 = moderate and 5 = high). Space was also provided for respondents to write other suggestions and comments. In total, 12 carer consultation packs and 12 person with dementia consultations packs were distributed, with 6 and 11 packs returned, respectively.
Results
Median scores and interquartile ranges were calculated. Responses with median scores of 1–4 were taken as being unclear or not as clear as possible, and actions were taken to amend these areas of concern. Ease of readability and grade level of the leaflet and information booklets were also assessed using the Flesch–Kincaid tests available in Microsoft Word® 2003 (Microsoft Corporation, Redmond, WA, USA). 141 The Flesch–Kincaid Reading Ease test is based on the average number of words per sentence and the number of syllables, taking into account the number of words about people in the passage, and the number of sentences addressed to an audience. A score of 90–100 is considered easily understandable by an 11-year-old, a score of 60–70 is considered easily understandable by 13- to 15-year-olds and a score of ≤ 30 is considered easily understandable by graduates. 142 The Flesch–Kincaid Grade Level score rates text in terms of US school years: a score of 8.0 means that an eighth-grade student (aged about 13 years) would be able to understand the information, which is the recommended level of readability for standard documents for the general population. 143
Recruitment leaflet
Readers thought that the recruitment leaflet was very clear and that the layout was easy to read (median 5), and they understood what the study was about (median 5). The details of the study were clear (median 5). Readers were less certain about the type of participants the study aimed to recruit (median 4.5). The overall quality score reflected these issues. The leaflet was rated as being high quality, but with some potential shortcomings and room for improvement (median 4.5). Written comments helped to develop the accessibility of the leaflet. For example, feedback indicated that there was too much white/blank space; headings could be larger for more impact and enhance the leaflet’s readability. Finally, it was suggested that black-and-white printing can be harsh on the eye and that glossy paper might soften the initial starkness. To address these concerns, the text font was increased in size to reduce the amount of white space and increase the impact of the headings, and glossy paper was used. Through the consultation process, the readability of the leaflet increased slightly from 58.7 to 58.9 post consultation, whereas the grade level dropped from 9.2 to 9.1.
Family carer information booklet
Several elements of the booklet were rated very highly, and overall it was seen as being very clear and detailed (Table 29). Importantly, the information concerning withdrawal and implications for participating in the intervention were also clear. However, comments focused on layout and design rather than on content, with some readers referring to the booklets as having a ‘daunting format’ and ‘rather clinical’. The panel suggested more breaks between sections to make the booklet more user-friendly, and the research team also added pictures to make the booklet less formal and more inviting. Before the consultation, the information booklet had a Flesch–Kincaid score of 58.8 and a grade level of 10.4. After the suggested changes were made, the Flesch–Kincaid score increased to 61.5 and grade level to 9.7.
| Question | Median | Interquartile range |
|---|---|---|
| Is the layout of the booklet easy to read? | 4.5 | 3 |
| Is it clear what the study is about? | 5 | 1.25 |
| Does it provide enough detail about the study to make an informed decision about whether or not to participate? | 5 | 1.25 |
| Is it clear as to what will happen to the participant at each stage of research? | 5 | 1.25 |
| Does it provide enough information about what the participant is committing to by consenting to take part? | 5 | 0.50 |
| Is it clear that the participants can withdraw from the study at any point without affecting the care they receive from health or social services or their legal rights? | 5 | 0.25 |
| Is it clear that if the participant does withdraw from the research interviews, they will no longer be able to receive any of the interventions? | 5 | 0.50 |
| How would you rate the overall quality of the booklet?a | 4.5 | 3.25 |
Information booklet for people with memory problems
The booklet was rated ‘clear’ (median 4) but only the information regarding withdrawal from the study was rated ‘very clear’ (median 5). Comments to improve the layout included presenting the booklet as an invitation to participate rather than an extension of the family carer booklet, and to use the word ‘informal’ wherever possible to reduce the anxiety associated with official documents. It was also highlighted that, in a booklet for people with dementia, the purpose of the study should appear at the beginning of the booklet to help people retain the information. Comments indicated that the purpose and role of carer supporters were not clear enough, and further clarification was required about what would happen if the person with memory problems did not want to take part, and whether or not the carer could still have access to the planned interventions (Table 30).
| Question | Median | Interquartile range |
|---|---|---|
| Is the layout of the booklet easy to read? | 4 | 2 |
| Is it clear what the study is about? | 4 | 2 |
| Does it provide enough detail about the study to make an informed decision about whether or not to participate? | 4 | 1 |
| Is it clear as to what will happen to the participant at each stage of research? | 4 | 2 |
| Does it provide enough information about what the participant is committing to by consenting to take part? | 4 | 2 |
| Is it clear that the participants can withdraw from the study at any point without affecting the care they receive from health or social services or their legal rights? | 5 | 0 |
| How would you rate the overall quality of the booklet?a | 4 | 1.25 |
As a result, the information booklet was made less formal and was styled more as an invitation. The interviews were described as informal and the purpose of the study was presented first. A larger font was used, and the role of a carer supporter was clarified along with reassurance that the carer could still be involved in the study even if the person with dementia declined involvement. Before the consultation, the Flesch–Kincaid score for the information booklet was 60.8, which increased to 63.7. In addition, grade level decreased from 9.2 to 8.5.
Using the documents in practice
These documents were used in the pilot trial of SHIELD CSP resulting in a further round of amendments in response to comments and concerns raised by participants during the pilot. Tables 31 and 32 show the improvements made as a result of the reader consultations, but also show that there was very little additional improvement made as a result of participant feedback during the pilot.
| Type of document | Original | Post reader consultation | Post pilot |
|---|---|---|---|
| Recruitment leaflet | 58.7 | 58.9 | 58.5 |
| Family carer information booklet | 58.8 | 61.5 | 61.6 |
| Information booklet for people with memory problems | 60.8 | 63.7 | 63.7 |
| Type of document | Original | Post reader consultation | Post pilot |
|---|---|---|---|
| Recruitment leaflet | 9.2 | 9.1 | 9.2 |
| Family carer information booklet | 10.4 | 9.7 | 9.6 |
| Information booklet for people with memory problems | 9.2 | 8.5 | 8.5 |
It was noted that readers in the consultation reviewed the documents at an abstract level for meaning, flow and format, whereas participants in the pilot trial reviewed the documents at the practical level to clarify the information and weigh up the personal implications of participating. Both types of consultation are important in developing consent documents, although the latter has little impact on the readability of documents.
Service users contributed to the development of the intervention in a unique way. Not only was the name of the intervention changed as a result of their input, but their personal experiences provided insight into the important elements of role of the carer supporter and identified important issues about carer supporters adding burden to the carers participating in the trial. The service users went on to also propose practical ways to manage the matches to reduce the likelihood of this happening. This contribution was vital, and highlights the importance of involving service users in the development of complex interventions, particularly when the intervention itself relies on peer support.
Limitations and implications for service user involvement
The consultation methods proved fruitful for developing the SHIELD CSP. The research team strove to create an environment in which service users would feel empowered, which seemed to be the case for study 1 but less the case for study 2. Anonymised methods of contributing, which may assume a level of expertise or even comfort with the research process, are adequate and suitable ways to involve service users in research when service users will have varying access to different levels of education and other resources. Service users met face to face in study 1 but not in study 2, and it may be that they value methods more that allow them to debate ideas with others. This may also serve to empower them and to ensure that their contribution is experienced as, and seen to be, meaningful.
Conclusions
Often service users are consulted so as to improve a research grant, research methodology or therapeutic intervention, but rarely have the opportunity to help determine specific research topics. Constraints on research processes and resources may explain why much service user involvement is consultative. 143 The current report highlights ways in which service users can still be involved meaningfully while also highlighting the negative impact that some methods can have on power relationships between researchers and service users.
The contribution made by service users makes it more likely that the finally developed intervention will have high levels of acceptability. This is likely to increase adherence and enhance uptake, both of which are important in psychosocial intervention trials, and have been a challenge for many trials evaluating interventions for family carers of people with dementia.
Work packages 2 and 3: exploratory trial and randomised controlled trial of peer support and joint reminiscence for people with dementia and their family carers – a factorial randomised controlled trial
Introduction
The peer-support intervention, namely the CSP, was based on self-efficacy theory and developed through a review of the existing evidence base and consultation with key stakeholders (work package 1). Self-efficacy is a person’s belief in their ability to perform competently and capably in given situations. 144 A previous trial of befriending for family carers of people with dementia (the BECCA trial)29 found no significant benefit of listening support by trained volunteer lay workers, compared with TAU. However, the most ‘successful’ volunteer supporters were former family carers, which raises the possibility that peer supporters may have advantages over general befriending volunteers.
Joint reminiscence has recently been evaluated in the REMCARE (REMiniscence groups for people with dementia and their family CAREgivers) trial145 and did not identify any significant benefits for people with dementia or their carers. Indeed, there was evidence of increased anxiety for the family carers. In the REMCARE trial, the main focus was on the person with dementia. In contrast, the current study includes a greater carer focus. Furthermore, it was hoped that the combination of one-to-one peer support and reminiscence would enhance the RYCT intervention by both encouraging the use of reminiscence and improving communication techniques in the home.
The 2 × 2 factorial multisite RCT assessed both effectiveness and cost-effectiveness of (1) one-to-one peer support for carers and (2) group reminiscence (alone and in combination) for people with dementia and their carers, compared with usual care. Factorial design allows for the combination of treatments, and a 2 × 2 factorial design is in effect ‘two trials in one’. There is an assumption that there will be no interaction between the interventions such that two main effects and one interaction term form the main analysis. The three null hypotheses are:
-
there is no effect of CSP compared with TAU
-
there is no effect of RYCT compared with TAU
-
there is no interaction between RYCT and CSP.
Methods
Design
A 2 × 2 factorial, pragmatic, multisite RCT design was used. Participant carers were randomised individually to carer support (CSP) or TAU after consent and baseline data collection, and then rerandomised on a group basis to joint reminiscence therapy (RYCT) or TAU. This gave the four ‘cells’ of CSP, RYCT, combined CSP-RYCT and TAU only. All participants continued to receive usual care. To ensure enough participants to run viable RYCT groups, randomisations between TAU, RYCT, CSP and combined CSP/RYCT were in the proportions 1 : 2 : 1 : 2. Stratification variables were used for each stage of randomisation. At first randomisation, kinship within the dyad (spouses vs. offspring) and locality were used as stratification variables. At second randomisation (2 : 1 reminiscence vs. no reminiscence), the first allocation was added as a stratification variable to keep the four arms in balance. Follow-up data were collected 5 and 12 months after the first randomisation, with the main end point at 12 months.
In accordance with MRC guidance on complex interventions,32 there was a feasibility phase prior to expansion to full trial. The pilot trial ran in two London boroughs with the focus of the first site directed towards appropriateness and acceptability of procedures, the second site focusing on logistics and timing of the interventions in relation to the timetable for recruitment and research interviewing procedures. Monitoring methods were developed in accordance with the draft checklist, and the outcomes of these were systematically recorded alongside the recording of the implications for the main trial. Examples of the monitoring methods used, outcomes of the monitoring process, and decisions made on the potential pooling of pilot and full trial data have been described by Charlesworth et al. 146 As the changes between the feasibility and full trial were minimal, the feasibility phase was considered an internal pilot and data were carried forward to the full trial.
Randomisation procedure
Figure 19 shows the flow diagram for the SHIELD CSP-RYCT trial. Administrators at each site entered participants’ variables into a remote web-based randomisation, which then allocated them in equal proportions between CSP and TAU on an individual basis. Once adequate dyads had been randomised within each site (target 24, range 16–30), they were entered on a group basis into a second randomisation between RYCT and TAU. The (unblinded) administrator at each site then informed carers of their allocation by letter and liaised with the RYCT facilitator and/or CSP co-ordinator, as appropriate.
FIGURE 19.
Flow diagram of the SHIELD CSP-RYCT trial.
Blinding
Owing to the nature of the interventions, it was not possible to blind participants or providers to their allocated intervention. In this trial, for example, both the CSP co-ordinator and the RYCT facilitator needed to know which carers were allocated to the combined interventions. However, the research interviewers who assessed outcomes were blinded, mainly by their being denied access to the web-based management system. The research interviewers recorded their perception of participants’ allocations for use as a covariate in statistical analysis.
Ethics arrangements and research governance
Ethics approval was given by the Outer North East London Research Ethics Committee (09/H0701/54). The clinical trial was registered as ISRCTN37956201. As per the previous studies, a reporting procedure was in place to ensure that SAEs were reported to the chief investigator. The consent procedures were described in the trial protocol. 147 Three formal groups met regularly to manage the trial within the SHIELD programme: the Project Management Group, the Programme Steering Committee and the Data Monitoring and Ethics Committee.
Setting
The trial ran in community settings in England (four boroughs in North East London plus sites in Norfolk, Northamptonshire and Berkshire).
Participants
Eligibility criteria
All participants were adult (aged ≥ 18 years) carers for a relative or close friend living at home in the community with dementia as defined by DSM-IV criteria. 124 Carers were excluded if they or the person they cared for had a learning disability or a non-progressive brain injury. Carers with a diagnosed terminal illness were also excluded, as were those already taking part in another psychosocial intervention study.
Recruitment procedures
Recruitment took place in memory clinics, in outpatient clinics, with community psychiatric nurses, admiral nurses, psychiatrists and GPs, and through carers’ registers, local media and online carer support forums and websites. ‘Direct’ recruitment strategies included the use of leaflets, flyers, posters and oral presentations at carer events. Strategies for newer and ‘hard-to-reach’ carers included invitations in local papers and newsletters. Indirect recruitment strategies involved engaging with community health, social services and voluntary sector staff who acted as ‘gatekeepers’ between the research team and potential participants. Gatekeepers were briefed on eligibility criteria and provided with recruitment literature. The contact details of potential participants were passed to the research team only with the consent of those potential participants. At some sites, participants were recruited from research registers and research network databases, where family carers and people with dementia had given their permission to be approached about potentially relevant research. Interested carers were contacted by researchers who provided further information about the trial and gave the opportunity for the carers to ask questions. For those meeting the eligibility criteria, arrangements were made with them for a face-to-face interview, with times and venues organised to accommodate the carer’s needs and preferences.
Sample size
Sample size calculations were based on the BECCA29 and REMCARE148 trials. These predicted effect sizes, defined as average effect per participant divided by population SD, of 0.42 for CSP and 0.35 for RYCT. In a 2 × 2 factorial design using a 2 : 1 allocation ratio in favour of groups receiving RYCT, a completed sample of 240 dyads would yield power of > 90% to detect both main effects using a significance level of 5%. This design would also yield power of > 80% to detect interaction between CSP and RYCT equivalent to an effect size of 0.4, using an analogous definition. As both the REMCARE trial platform and BECCA retained some 80% of participants, the aim was to recruit 300 dyads in 13 rounds of 24 dyads to yield a final sample of 240 dyads.
Interventions
Each intervention was organised and provided independently of the research assessments.
The focus of this intervention was on peer support for family carers by family carers. The participant carers allocated to this intervention were contacted by a local carer supporter co-ordinator who met to discuss the peer-support programme and to consider an appropriate match from a pool of trained carer supporter volunteers with experience of caring for a relative with dementia. The carer supporter co-ordinator then facilitated a first meeting between the supporter and supportee. The target number of meetings for the carer support intervention was for 12 weekly meetings, each lasting 1 hour, followed by fortnightly meetings for the next 5 months. The meetings took place in the carer’s own home or in a public venue such as a café. Carer supporters were encouraged to provide a listening ear, encouragement and morale support. They could also signpost the carer to resources and services but were explicitly requested not to offer tangible support, ‘sitting’ for the person with dementia or direct advice. Meetings were arranged to include or exclude the person with dementia according to the family carer’s preference.
For dyads allocated to this intervention, both the family carer and the person with dementia were invited to attend a local reminiscence group. Twelve weekly sessions, each lasting 2 hours, covered themes ‘across the lifespan’, following Schweitzer and Bruce’s RYCT programme. 149 Each session explored its theme using multisensory triggers and activities, including group discussions, small group activity, handling objects, enacting or improvisation and singing songs. Each session was led by two experienced facilitators, supported by a team, including volunteers, health and social care staff and trainees, to facilitate small group discussion and activities, and engage the people with dementia. During four of the sessions, the family carers met separately from the main group for approximately 45 minutes with the aim of developing listening and communication skills, and considering how the activities and strategies used in the sessions could be carried over into the home environment. The RYCT intervention ran in community settings such as church halls, with transport provided if needed. After the 12 weekly sessions, monthly reunion sessions took place over a further 7 months using previously successful themes or new themes, depending on the preferences of the group. All members of the RYCT team attended a training day led by one of the original RYCT programme authors.
When participants were offered both contact with a carer supporter and the opportunity to attend the RYCT programme, the carer supporter was asked to attend the RYCT sessions ahead of individual meetings with the carer. These carer supporters were also invited to an additional 2-hour training session on the topic of reminiscence at home, to enable them to better support the family carer in implementing the strategies and advice provided during the RYCT carers’ sessions. The aim of this intervention was to extend the benefits of RYCT through the carer supporters bringing knowledge of the care dyad to the group, and encouraging reminiscence in the family carer’s home.
Treatment as usual
All participants continued to receive the usual care available in their area. All trial participants were given a list of useful local resources, tailored to their area.
Intervention adherence and cost
The MRC Framework for Developing and Evaluating Complex Interventions32 recommends that researchers monitor the extent to which interventions have been delivered to and received by the participants. 150 Measures were designed specifically for this trial to capture:
-
treatment delivery – the extent to which the intervention provider adhered to the treatment protocol, the absence of any other intervention and the quality of the intervention
-
treatment receipt – the extent to which the participant received the intervention, the intensity of the intervention received and the satisfaction with that intervention.
To allow calculation of an estimation of the cost per dyad per session, the following information was collected during the interventions. For CSP, information was collated by the carer supporter manager on Microsoft Excel spreadsheets. These reported the time all carer supporters spent each month on travel and training and on providing support to family carers. The spreadsheets for each site also included claims for the volunteers’ expenses and time spent by the carer supporter co-ordinator on directly supporting each carer supporter. The family carer’s identification data were recorded, as were the carer supporter’s identification data. For RYCT groups the information recorded was team membership, including professional background and grade (Agenda for Change band or similar), team members’ travel (mode and mileage) to the sessions, team attendance at each session and the number of hours of staff time utilised per session including travelling time. The external facilitator was usually allocated 1 day per session and other team members were allocated between 3 and 5 hours per session. The programme ‘overheads’ which included the 1-day and 2-hour training sessions and attendance by the facilitator and team members, the overarching administrative support, the costs for the venue, refreshments and other materials required for each programme such as a camera, photographs and RYCT manuals.
Participant data-collection procedures
Once the carer and the person with dementia had consented to take part in the research, baseline data were collected, with follow-up at 5 and 12 months post randomisation. For the family carer, psychometric questionnaires were either completed with the researcher or self-completed, depending on the carer’s preference. The questionnaire for the person with dementia was always completed with the researcher.
To maximise data collection, variations in methods were made. For instance, some carers were interviewed over several appointments and some carers completed the questionnaires by themselves and used the included stamped addressed envelope to return them. Most carers were interviewed in their own homes; however, neutral venues were organised for those who preferred an alternative location (e.g. NHS settings). At follow-up, psychometric self-completion questionnaires were posted to participants along with the covering letter confirming time and date of the follow-up interview to allow carers to complete the questionnaire prior to the interview if they wished.
Measures
Demographic information was collected from family carers for both the carer and the person with dementia, including age, sex, kinship, education and living situation. The nature of the carers’ social network was assessed using the Practitioner Assessment of Network Type. 151 The cognitive status of the person with dementia was measured using the MMSE45 and an interviewer rating of the global functioning of persons with dementia was made using the CDR scale. 46 Information on use of health, social care, voluntary sector and other relevant services for both the carer and person with dementia was collected through interviews with carers, using the CSRI (see Resource use and cost measures). 97
Primary outcomes
The primary outcome was the family carers’ health-related quality of life, measured by the validated and widely used mental component summary (MCS-12) of the UK SF-12. 152 The SF-12 measures general health status from the perspective of the participant and, in addition to the MCS-12, also allows for the generation of a second subscore, the physical component summary (PCS-12). The SF-12 includes eight concepts commonly represented in health surveys: physical functioning, role functioning physical, bodily pain, general health, vitality, social functioning, role functioning emotional and mental health. A higher score is indicative of better mental and physical health. Reliability is 0.74 for the MCS-12 and 0.78 for the PCS-12. 152 Validity is 0.97 for the MCS-12 and 0.67 for the PCS-12. 96
The primary outcome for the person with dementia was quality of life as measured by the QOL-AD. 103 The measure assesses 13 items, namely physical health, energy, mood, living situation, memory, family, marriage, friends, chores, fun, money, self and life as a whole. The 13 items are summed to generate a total score of between 13 and 52. Responses range from poor (1), fair and good to excellent (4), with higher scores indicative of better quality of life. It has good internal consistency, validity and reliability. 89 The QOL-AD proxy was also completed by the carer, which is identical in structure and content to the person with dementia version.
Secondary outcomes for family carers
-
Health-related quality of life The EQ-5D98 comprises five items and a VAS. The measure includes five dimensions of quality of life (mobility, self-care, usual activities, pain/discomfort and anxiety/depression), with each dimension scored on a three-level scale ranging from 1, ‘no problem’, to 3, ‘extreme problem’, and is used as the basis for calculating utility scores. It also includes a VAS on which respondents are asked to rate their current health, ranging from 0 points (worst possible health state) to 100 points (best possible health state).
-
Anxiety and depression The Hospital Anxiety and Depression Scale (HADS)153 comprises 14 items, with seven evaluating anxiety (e.g. ‘worrying thoughts go through my mind’) and seven assessing depression (e.g. ‘I have lost interest in my appearance’). The HADS is scored on a 4-point scale ranging from 0 to 3, with a higher score indicating higher levels of anxiety and depression. The scale also measures the severity of anxiety and depression; the score for each subscale can range from 0 to 21. Cut-off points have been used to indicate caseness, whereby a score of ≤ 7 indicates a non-case for both subscales, a score of 8–10 indicates a doubtful case and a score of ≥ 11 indicates a definite case. The HADS subscales have been found to have good reliability and validity in a range of contexts and populations. 153,154 Scores for the entire scale (emotional distress) were also calculated, ranging from 0 to 42, with higher scores indicating more distress.
-
Emotional loneliness This was measured using the 2-item Loneliness Scale. 155 The questions used are ‘over the past 7 days, how much have you felt distressed by feeling lonely/feeling lonely even when you are with people?’ Both items are rated on a 5-point scale from ‘not at all’ (0) to ‘extremely’ (4). The scores of the items are summed to create a total loneliness score, with a range of between 0 and 8.
-
Caregiver distress This was measured using the caregiver distress scale of the NPI. 94 The NPI evaluates the frequency and severity of 10 behavioural disturbances that can occur in dementia (delusions, hallucinations, dysphoria, anxiety, agitation/aggression, euphoria, disinhibition, irritability/lability, apathy and aberrant motor activity) and the associated distress experienced by the carer, rated from 0 (no distress) to 5 (extreme or very severe distress) for each domain. The distress score total is calculated by the sum of all the individual domain scores, with a range of between 0 and 60.
-
Positive affect This was measured using the positive affect scale from the Positive and Negative Affect Schedule. 156 Participants rate the extent to which they have felt each of 10 positive mood states (e.g. determined) within a specific time frame along a five-point scale ranging through ‘very slightly or not at all’ (1), ‘a little’, ‘moderately’, ‘quite a bit’ and ‘extremely’ (5). Scores range between 10 and 50, with higher scores indicative of greater positivity.
-
Positive aspects of caring These were measured using the four-item positive aspects subscale from the Carers of Older People in Europe (COPE) index. 157 Responses are recorded from the categories never, sometimes, often and always (or not applicable).
-
Personal growth The 3-item version Personal Growth Index was used. 158 Respondents report the degree to which they agree or disagree with statements indicating feelings of personal growth on a six-point scale from 1 (strongly disagree) to 6 (strongly agree). The Index score is obtained by summation of the three item scores, ranging between 3 and 18. Higher scores are indicative of higher personal growth.
-
Relationship quality The Quality of Caregiver–Patient Relationship (QCPR)159 comprises 14 items measuring expressed emotion along two dimensions: level of absence of criticism and conflict, and level of warmth. Responses are scored on five-point Likert scales ranging from ‘totally disagree’ to ‘totally agree’, with the criticism and conflict subscale items reverse coded so that a higher score reflects a better relationship. Possible scores range between 14 and 70 and scores are dichotomised, with scores of > 42 denoting a good relationship and those of ≤ 42 denoting a poor relationship.
Secondary outcomes for people with dementia
Secondary outcomes for people with dementia included the HADS, QCPR and EQ-5D, as also used with the family carers. In addition, family carers gave a rating of the person with dementia’s functional capacity in ADLs using the ADCS-ADL. 95 The inventory comprises 23 items covering physical and mental functioning and independence in self-care within the last 4 weeks. Each item consists of a series of hierarchical questions designed to determine a person’s ability to perform one of the ADLs, ranging from total independence to total inability. The total score is calculated by summation of item scores, ranging between 0 and 78. Lower values are indicative of greater disability. The inventory has good reliability. 95
Quality of life was also assessed using the DEMQOL,99 both the person with dementia version and the carer-completed proxy version. The DEMQOL covers the domains of health, well-being, cognitive functioning, social relationships and self-concept. Items are rated on a 4-point scale ranging from 1 (a lot) to 4 (not at all) and summed to produce a total score. A higher score is indicative of better quality of life. The DEMQOL comprises 28 items and scores range between 28 and 112, whereas the DEMQOL-Proxy comprises 31 items and scores range between 31 and 124. It has high internal consistency (0.87) and acceptable inter-rater reliability (intraclass correlation coefficient 0.84).
Measuring potential mechanisms action
Modelling of the stress process in family carers of people with dementia has indicated the value of psychological and social resources in influencing carer outcomes. We therefore included measures of coping,160,161 self-efficacy (Revised Scale for Caregiving Self-Efficacy),162 self-efficacy in responding to challenging behaviours163 and social support. 164
Identifying resource use
The CSRI97 adapted for use in this study gathered information on health and social care services used by participants with dementia and by their family carers. For the purposes of the trial the CSRI was completed three times, on each occasion asking about service use retrospectively over the previous 3 months: at randomisation to the trial (baseline), at 5 months after the first randomisation (after 3 months of weekly intervention) and at 12-month follow-up. Carers identified services used by participants with dementia covering the categories of accommodation, hospital services, community services, equipment and adaptations, day services and medication use. Carers also provided records of their own medication use and identified any other services used which fell in one of the other aforementioned categories. Unpaid family carer inputs were also collected.
Data management and statistical analysis
Data were entered into MACRO for clinical trials. Data audits were carried out throughout the trial to check ongoing data integrity. These audits were performed on a randomly selected 10% sample of data for each site at each time point, to ensure that the data entered into the MACRO database were consistent with the hard-copy paper questionnaires. Once data had been entered into the MACRO database and had been screened and cleaned for any inconsistencies or errors, they were then transferred into SPSS. Further accuracy checks were then carried out, such as manually checking for out-of-range values and scoring.
Handling missing data
When individual items were missing within scales or subscales, data were imputed before the calculation of the scale or subscale score. Pro-rating within measures was undertaken at the 20% missing item level (e.g. for a 5-item score if there was one item missing this was completed with the mean of the other items). Multiple imputations at time points were conducted; however, no imputation was completed for a dyad if all measures were missing at a time point.
Effectiveness analysis
As recommended for the analysis of a factorial design, an ‘at the margins’ analysis was carried out for the two main effects of CSP versus not CSP (alone or in combination with RYCT) and RYCT versus not RYCT (alone or in combination with CSP). The interaction term was also included in the analysis. A multilevel ANCOVA was used with 12 months’ follow-up data as the dependent variable and with baseline score as the covariable. Group allocation was treated as fixed effects, together with sex and dyad relationship. The effects of locality (including personnel) and time were treated as random effects and the covariance structure of this was assessed. A sensitivity analysis was performed to test whether or not plausible changes in key variables, such as carers’ ages and relationships to their relatives with dementia, affected findings.
Intervention costs
Costs of the CSP intervention included staff costs and training for the volunteer carer supporters, plus volunteers’ time and expenses. Each site employed a carer supporter co-ordinator for 1 day per week who was part of either a NHS or a voluntary sector organisation. CSP intervention costs were allocated to each family carer/dyad according to the amount of time the carer supporter spent supporting each family carer/dyad, plus the costs of reimbursing volunteers’ travel. The unit cost for providing the carer supporter training included trainer costs, costs of the venue, materials and refreshments, and cost of producing a specially developed DVD. A manual (a 70-page spiral-bound book) was posted to each trainee carer supporter and its development was undertaken during the staff time already identified. Time and expenses data were sorted so that entries for each family carer/dyad were listed together and three ‘time’ totals calculated over the period they received the intervention: (1) carer supporter time spent training, including travel; (2) time spent by the carer supporter supporting the family carer, including travel; and (3) ‘other’ time, mainly when the carer supporter was in contact with the carer supporter co-ordinator (direct support). Expenses and time were also totalled by family carer/dyad over the period they received the intervention. Three values were used for volunteer time: £0 when a public sector cost perspective was taken and two values for when a societal perspective was employed – a replacement cost (health-care assistant) and an opportunity cost (minimum wage). Public sector costs based on the service-level agreements constituted the ‘overarching’ costs associated with activities that allowed the intervention (carer support) to be implemented: recruiting, organising, training and supporting the carer supporter.
For the RYCT intervention, staff costs were the major component and a unit cost (per hour) was estimated for each team member employed by a health or social care agency or voluntary organisation. This included the mean/median salary for that professional group/grade, additional salary on-costs such as employers’ National Insurance and superannuation contributions, and a proportion of the salary to cover direct and indirect overheads. Using this cost per hour, a cost per session for each staff member was estimated and their travel costs were added. Each time a particular member of staff was present at one of the sessions, their unique staff ‘cost per session’ was included, allowing a total cost for any given session to be calculated. Dyad transport costs were calculated separately and included the reimbursed transport costs for each dyad attending at each session. The cost per dyad per session was calculated by dividing the total cost per session by the number of dyads attending that session. The cost per session per dyad is mainly driven by the number of dyads attending each session, but also by the number of team members present. In turn, these figures were totalled for each participating dyad to arrive at a cost per programme per dyad that varied depending on which sessions they attended. Thus, a unique cost for each dyad was calculated which reflected how much of the intervention they received.
Costs of resource use
Unit costs were obtained so as to reflect long-run marginal opportunity costs, drawn where possible from the Personal Social Services Research Unit compendium for 2011. 100 We used a 3.5% discount rate (as recommended by Her Majesty’s Treasury) for items that provide a benefit for > 1 year, such as equipment or adaptations. Medication costs were obtained from the British National Formulary database. 101 Costs for equipment and adaptations to homes were obtained from market sources. Although it was possible to find unit costs in 2011 prices for most items, for a very small number of services we used the Consumer Price Index inflation rate to adjust costs to 2011 price levels.
The costs of unpaid family carer inputs were calculated in three ways, following the approach used for volunteers. For the public sector perspective, a value of £0 per hour was employed. For the societal perspective, the opportunity cost approach assumed that the unpaid carer would be able to find employment with a wage rate equal to the national minimum wage (which was the hourly cost used in this calculation) and the replacement cost was estimated as the hourly cost of a health-care assistant, under the assumption that a care worker would need to be hired to provide care if the unpaid family carer was unable to do so.
Cost-effectiveness analyses
The primary economic evaluation estimated the incremental cost-effectiveness of:
-
those in the sample receiving CSP compared with those who did not
-
those in the sample receiving RYCT compared with those who did not
-
CSP and RYCT combined compared with TAU.
For the primary analysis we adopted a societal perspective, with the main outcome measures being the MCS-12 score of the SF-12 for the carer and QOL-AD for the person with dementia. The secondary analyses adopted both a health and social care perspective and a societal perspective, with effectiveness measured by the carer PCS-12 and QALYs estimated using EQ-5D for carers, and both DEMQOL- and DEMQOL-Proxy-based QALYs for the person with dementia. As these analyses have many possible combinations of outcomes, we followed the standard economic evaluation approach of extended dominance. There were four potential results from each intervention group comparison. For instance:
-
the intervention is less costly and more effective (has better outcomes) than usual care (TAU), in which case the decision-maker would be likely to be attracted to the intervention
-
the intervention is more costly and less effective than usual care (TAU), in which case it would be unlikely that the decision-maker would consider the intervention over TAU
-
the intervention is less costly but less effective than TAU
-
the intervention is more costly and more effective than TAU.
Results (1) and (2) are scenarios that exhibit strong dominance, and the recommendations made to the decision-maker are typically straightforward. For results (3) and (4), however, the decision is not as straightforward and decision-makers will need to weigh the potential outcome differences against the cost differences before deciding whether or not to implement the new intervention. For these instances, we estimated ICERs, defined as ICER = ΔC/ΔE, where ΔC denotes the difference in mean cost between the interventions being compared and ΔE denotes the difference in the outcome measure between the two interventions. These assist in providing a cost per unit of outcome for the outcome measure of interest. Incremental costs and effects, and their CIs, were estimated with seemingly unrelated regression methods with 1000 bootstrap replications. We plotted CEACs when necessary to show the probability that an intervention is cost-effective relative to its comparator against a series of hypothetical WTP values.
One sensitivity analysis recalculated carer costs (used under the societal perspective) by adopting a replacement cost approach. In this case the hourly cost of providing care was set to be equal to the hourly cost of hiring a health-care assistant to provide care for the person with dementia: a replacement cost approach.
Results
Participant flow
A summary of participant flow throughout the trial is provided in the CONSORT diagram (Figure 20). In total, the research team received 640 expressions of interest from carers and 639 were screened for eligibility. Of these, 292 family carers consented into the trial, with the remaining 347 carers excluded from the trial. Of these, 177 carers were excluded for primary eligibility reasons, with the most frequent reasons including that researchers were unable to contact the family carer (n = 66), time constraints (n = 33) and that the person with dementia had recently passed away (n = 12). It is not possible to determine whether or not those 170 who declined involvement would have been eligible for the trial. The reasons for exclusion are shown in Figure 20. No demographic or psychosocial information was collected about potential participants prior to their giving written informed consent. As a result, it is not possible to determine whether or not those who decided to take part in the research differ from those carers who decided not to take part in the research or were excluded from the trial.
FIGURE 20.
The CONSORT summary of participant flow.
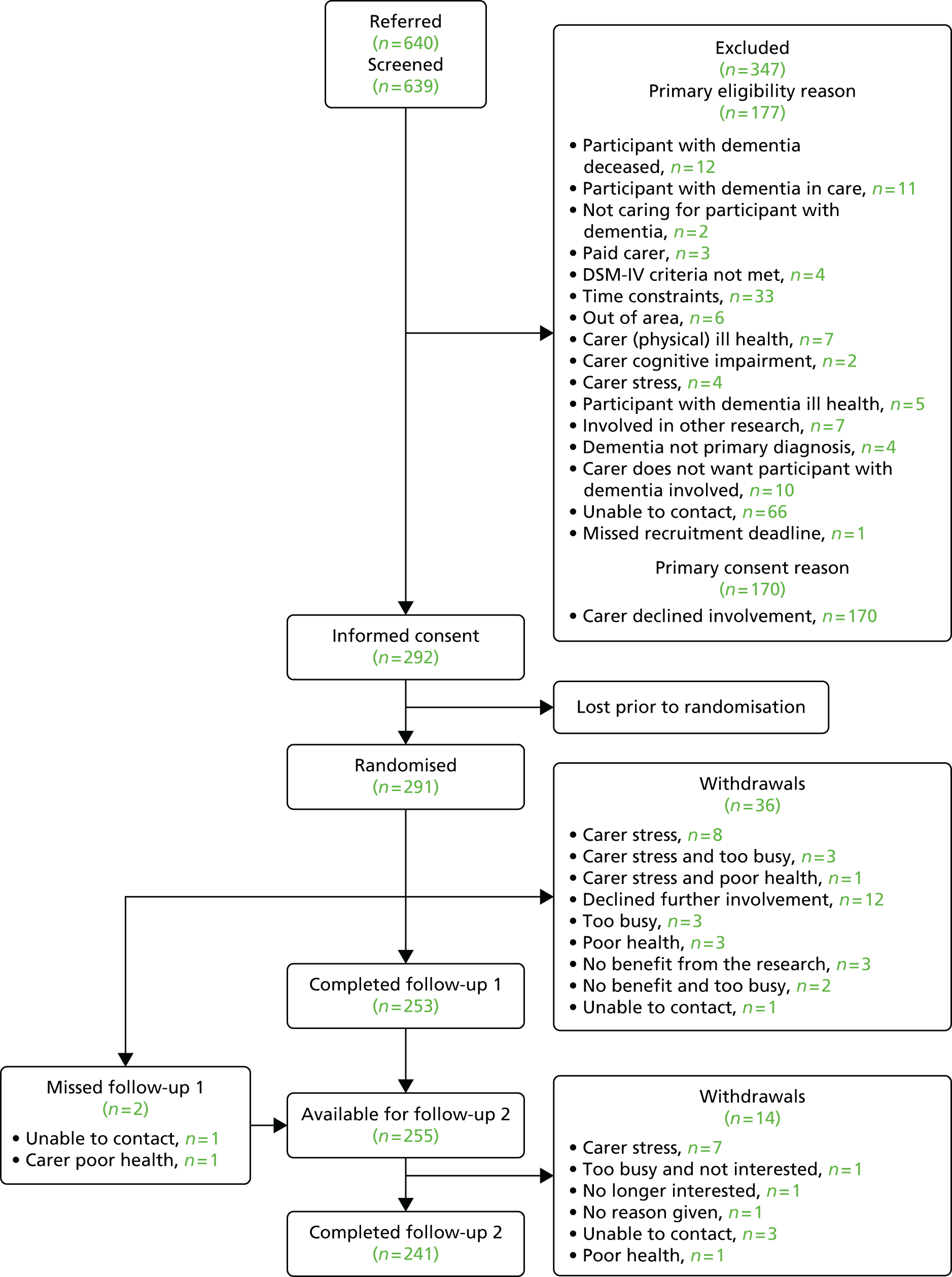
Of the 292 family carers who gave informed consent to take part in the research, one withdrew before randomisation. A total of 291 carers completed the baseline assessment and were randomised between January 2010 and March 2012. Of the 291 dyads randomised, only 289 were included in the final analysis. Two dyads were removed as there were no data recorded at any time point throughout the trial.
Follow-up interviews were carried out as soon as could be arranged after the interview due date, this being 5 and 12 months post randomisation. A total of 253 family carers completed the first follow-up (5 months post randomisation), whereas 36 dyads withdrew before the first follow-up, mainly as a result of stress, poor health and time constraints. Two of the dyads were not available to complete the first follow-up assessment, but were available to complete the second follow-up assessment. In total, 241 family carers completed the final follow-up (12 months post randomisation) giving an overall 12-month retention rate of 83%. However, loss to follow-up was slightly greater in the TAU group than in the intervention groups (Table 33).
| Group | Baseline (n) | Follow-up 1 (n) | Follow-up 2, n (%) |
|---|---|---|---|
| CSP | 48 | 42 | 42 (88) |
| CSP/RYCT | 97 | 84 | 80 (83) |
| TAU | 47 | 39 | 36 (77) |
| RYCT | 97 | 90 | 83 (86) |
| Total | 289 | 255 | 241 |
Baseline characteristics
Demographic information
Demographic information for family carers and people with dementia, for the total sample and by allocated group, can be seen in Tables 34 and 35. Of the 289 family carers who took part in the research, over two-thirds (68%) were female (68%) and their mean age was 67 years. Family carers were predominantly white British (89%) and married or cohabiting (86%). Approximately two-thirds were spousal carers and had school-only education. The mean duration of caring was > 4 years, with > 2 years since diagnosis. The people with dementia had a mean age of 80 years. Just over half of the people with dementia were female (54%). The majority were white British (89%) and married or cohabiting (69%), and 8 out of 10 were residing with their carer. Only 2 out of 10 (21%) people with dementia had completed further education. Alzheimer’s disease was the most common dementia diagnosis, followed by vascular dementia, although a significant proportion had no specific diagnosis.
| Characteristic | Level | Total (N = 289), n (%) | CSP (N = 48), n (%) | CSP/RYCT (N = 97), n (%) | TAU (N = 47), n (%) | RYCT (N = 97), n (%) | p-value |
|---|---|---|---|---|---|---|---|
| Sex | Female | 197 (68.17) | 29 (60.42) | 66 (68.04) | 30 (63.83) | 72 (74.23) | 0.34 |
| Ethnicity | White British | 258 (89.27) | 45 (93.75) | 84 (86.60) | 39 (82.98) | 90 (92.78) | 0.18 |
| Other | 31 (10.73) | 3 (6.25) | 13 (13.40) | 8 (17.02) | 7 (7.22) | ||
| Marital status | Married/cohabiting/civil partnership | 248 (85.81) | 44 (91.67) | 85 (87.63) | 37 (78.72) | 82 (84.54) | 0.31 |
| Relationship to relative with dementia | Spouse/partner | 183 (63.32) | 32 (66.67) | 60 (61.86) | 29 (61.70) | 62 (63.92) | 0.96 |
| Live with relative | Yes | 230 (79.58) | 39 (81.25) | 78 (80.41) | 40 (85.11) | 73 (75.26) | 0.55 |
| Highest level of education | School leaver (aged 14–16 years) | 179 (61.94) | 37 (77.08) | 60 (61.86) | 18 (38.30) | 64 (65.98) | 0.002 |
| Further/higher education | 100 (34.60) | 10 (20.83) | 33 (34.02) | 26 (55.32) | 31 (31.96) | ||
| Age (years), mean (SD) | 66.68 (12.30) | 69.04 (10.54) | 65.84 (12.43) | 66.81 (14.66) | 66.30 (11.76) | 0.48 | |
| Months since diagnosis, mean (SD) | 31.25 (26.31) | 29.64 (29.64) | 20.65 (30.85) | 25.53 (29.83) | 26.08 (33.21) | 0.94 | |
| Months of caring, mean (SD) | 52.52 (38.00) | 58.81 (58.81) | 38.08 (51.24) | 42.28 (52.04) | 36.17 (50.94) | 0.43 | |
| PANT social network assignment | Family dependent | 10 (20.83) | 34 (35.41) | 9 (19.15) | 30 (31.58) | 0.06 | |
| Locally integrated | 13 (27.10) | 32 (33.33) | 16 (34.04) | 29 (30.53) | |||
| Local self-contained | 11 (22.72) | 19 (19.79) | 12 (25.53) | 21 (22.11) | |||
| Wider community focused | 4 (8.33) | 4 (4.17) | 8 (17.02) | 8 (8.42) | |||
| Private | 10 (20.83) | 7 (7.29) | 2 (4.26) | 7 (7.37) |
| Characteristic | Level | Total (N = 289), n (%) | CSP (N = 48), n (%) | CSP/RYCT (N = 97), n (%) | TAU (N = 47), n (%) | RYCT (N = 97), n (%) |
|---|---|---|---|---|---|---|
| Sex | Female | 153 (53.68) | 27 (56.25) | 49 (50.52) | 29 (61.70) | 48 (49.48) |
| Ethnicity | White British | 253 (87.54) | 46 (95.83) | 81 (92.05) | 37 (78.72) | 89 (91.75) |
| Marital status | Married/cohabiting/civil partnership | 196 (69.01) | 34 (70.83) | 68 (70.10) | 29 (61.70) | 65 (67.01) |
| Living situation | Living alone | 45 (15.57) | 6 (12.50) | 11 (11.34) | 8 (17.02) | 20 (20.62) |
| Cohabiting with partner | 194 (68.31) | 35 (72.92) | 65 (67.01) | 29 (61.70) | 65 (67.01) | |
| Living in the community with relatives, friends/other people | 45 (15.57) | 7 (14.58) | 21 (21.65) | 7 (14.89) | 10 (10.31) | |
| Highest level of education | School leaver (aged 14–16 years) | 216 (77.98) | 36 (75.0) | 72 (74.23) | 33 (70.21) | 75 (77.32) |
| Further/higher education | 61 (21.11) | 10 (20.83) | 22 (22.68) | 11 (23.40) | 18 (18.56) | |
| Type of dementia | Alzheimer’s disease | 134 (46.37) | 28 (58.33) | 40 (41.24) | 20 (42.55) | 46 (47.42) |
| Vascular dementia | 49 (16.96) | 7 (14.58) | 20 (20.62) | 5 (10.64) | 17 (17.53) | |
| Other/not known | 106 (36.70) | 13 (27.08) | 37 (38.14) | 22 (46.81) | 34 (35.05) | |
| Age (years), mean (SD) | 79.59 (7.87) | 79.79 (8.19) | 79.34 (7.54) | 79.49 (7.31) | 79.77 (8.36) | |
| CDR | 0.5 | 2 (4.17) | 8 (8.25) | 6 (12.77) | 13 (13.40) | |
| 1 | 24 (50.0) | 49 (50.52) | 26 (55.32) | 48 (49.48) | ||
| 2 | 15 (31.25) | 31 (31.96) | 10 (21.28) | 19 (19.59) | ||
| 3 | 5 (10.42) | 7 (7.22) | 2 (4.26) | 4 (4.12) |
There were no significant differences between allocated groups in terms of demographics with the exception of education for family carers, as those in the TAU group had achieved higher levels of education. There was an indication that ethnicity varied between the groups, but there was greater variation between localities, with the proportion of white British carers ranging between 71.7% in the London Borough of Waltham Forest and 100% in areas such as Norfolk. These differences reflected the ethnic differences of the local populations from which the participants were drawn.
Baseline psychometric measures
Scores on psychometric measures at baseline are shown in Tables 36 and 37. The groups are mainly equivalent for people with dementia, with the exception of MMSE score where the TAU group was less impaired than other groups. For the family carers, the groups were also equivalent at baseline, with the exception of EQ-5D utility scores (higher in the RYCT/CSP group), positive affectivity score (higher in the TAU group) and personal growth (higher in the TAU group).
| Outcome measure | CSP (n = 48), mean (SD) | CSP/RYCT (n = 97), mean (SD) | TAU (n = 47), mean (SD) | RYCT (n = 97), mean (SD) |
|---|---|---|---|---|
| SF-12 version 1 (UK) | ||||
| MCS-12 | 38.42 (4.54) | 38.28 (4.24) | 39.90 (6.01) | 39.12 (5.27) |
| PCS-12 | 30.33 (6.51) | 30.07 (6.32) | 32.24 (7.15) | 31.59 (6.71) |
| EQ-5D | ||||
| Your own health state today | 74.44 (20.99) | 74.27 (19.82) | 63.64 (19.89) | 68.1 (20.9) |
| Utility | 0.76 (0.19) | 0.83 (0.18) | 0.73 (0.26) | 0.75 (0.25) |
| HADS | ||||
| Anxiety | 6.86 (4.09) | 6.29 (4.41) | 6.51 (5.09) | 6.68 (4.31) |
| Depression | 5.13 (3.71) | 5.41 (3.71) | 5.15 (4.46) | 6.48 (4.72) |
| Total (20%) | 11.99 (7.06) | 11.70 (7.67) | 11.66 (8.81) | 13.17 (8.28) |
| PANAS | ||||
| Affectivity (20%) | 31.77 (7.69) | 30.53 (6.81) | 34.05 (7.96) | 29.43 (7.36) |
| COPE index | ||||
| Positive aspects of caring (20%) | 12.74 (2.16) | 12.57 (2.42) | 12.72 (2.30) | 12.63 (2.20) |
| NPI | ||||
| Carer distress | 13.46 (8.91) | 10.98 (8.63) | 11.52 (8.64) | 13.37 (13.46) |
| PGI | ||||
| Personal growth | 14.46 (3.14) | 13.47 (3.36) | 15.02 (3.10) | 13.98 (2.91) |
| QCPR | ||||
| Warmth (20%) | 32.40 (4.94) | 31.34 (5.60) | 33.50 (5.00) | 31.57 (5.39) |
| Lack of criticism and conflict (20%) | 21.17 (5.05) | 20.67 (4.61) | 21.42 (4.27) | 21.36 (4.82) |
| QCPR total (20%) | 53.56 (9.22) | 52.01 (9.56) | 54.92 (8.43) | 52.93 (9.28) |
| Loneliness | 2.21 (2.04) | 2.41 (2.49) | 1.89 (2.36) | 2.41 (2.40) |
| Outcome measure | CSP (n = 48), mean (SD) | CSP/RYCT (n = 97), mean (SD) | TAU (n = 47), mean (SD) | RYCT (n = 97), mean (SD) |
|---|---|---|---|---|
| QOL-AD | ||||
| Self-report | 37.07 (4.75) | 35.60 (5.82) | 37.55 (5.94) | 36.72 (5.50) |
| Proxy | 30.91 (6.01) | 29.99 (5.89) | 32.16 (6.81) | 30.66 (5.35) |
| DEMQOL | ||||
| Self-report | 93.39 (12.37) | 90.55 (13.38) | 92.44 (11.19) | 92.11 (12.33) |
| Proxy | 89.29 (15.62) | 94.38 (14.14) | 93.50 (15.82) | 93.75 (13.31) |
| EQ-5D | ||||
| Utility value | 0.74 (0.30) | 0.73 (0.27) | 0.82 (0.23) | 0.72 (0.27) |
| Own health state today | 76.22 (18.08) | 70.41 (19.42) | 76.42 (16.02) | 69.60 (21.19) |
| HADS | ||||
| Anxiety | 3.76 (3.23) | 5.01 (3.64) | 4.27 (3.18) | 4.52 (3.53) |
| Depression | 4.73 (3.85) | 5.62 (3.80) | 4.74 (4.08) | 5.37 (3.83) |
| Total | 8.49 (6.27) | 10.63 (6.32) | 9.00 (6.28) | 9.89 (6.27) |
| MMSE | 16.34 (6.37) | 17.53 (6.35) | 19.74 (5.37) | 16.32 (7.03) |
| ADCS-ADL total | 42.00 (16.39) | 41.16 (18.14) | 44.25 (19.35) | 42.17 (17.60) |
| NPI total | 23.43 (17.70) | 21.40 (16.94) | 22.45 (17.17) | 28.13 (22.34) |
| QCPR | ||||
| Warmth | 35.10 (3.12) | 34.85 (3.46) | 35.73 (2.84) | 35.62 (3.22) |
| Criticism | 23.42 (3.75) | 22.58 (4.10) | 22.90 (4.18) | 22.74 (4.14) |
| Total | 58.52 (6.11) | 57.43 (6.56) | 58.63 (5.96) | 58.36 (6.41) |
Completers versus non-completers
A comparison of baseline characteristics using Fisher’s exact test and Mann–Whitney U-test of those dyads who completed final follow-up with those who withdrew before final follow-up did not indicate any significant differences. The baseline characteristics that were considered included carer sex (p = 0.74), age (p = 0.12), ethnicity (p = 0.67), marital status (p = 0.62), carer/care recipient relationship type (p = 0.37), living situation (p = 0.12), level of education (p = 0.76), type of dementia (p = 0.53), length of time spent caring (p = 0.75) and length of time since the person with dementia was diagnosed (p = 0.56).
Intervention uptake and adherence
In the 49 dyads allocated to the CSP intervention there was a 76% uptake of the intervention, in the 97 dyads allocated to the RYCT intervention there was a 61% uptake of the intervention and in the 97 dyads allocated to the combined intervention there was a 82% uptake of at least one of the interventions.
Effectiveness results
The results of the primary intention-to-treat analysis for family carers (SF-12 MCS-12 at 12 months) and all secondary outcome analyses are shown in Tables 38 and 39. There was no indication of benefit of either peer support or reminiscence intervention over control on any of the outcome measures. There was no indication of a significant interaction between the interventions.
| Outcome measure | CSP vs. no CSP | RYCT vs. no RYCT | Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Complete case | df, F-value; p-value | Mean difference | CSP mean (SE) | No-CSP mean (SE) | df, F-value; p-value | Mean difference | RYCT mean (SE) | No-RYCT mean (SE) | df, F-value; p-value | |
| Multiple imputation | df range, F-value range; p-value range | Pooled mean difference | CSP mean (SE) | TAU mean (SE) | df range, F-value range; p-value range | Pooled mean difference | RYCT mean (SE) | TAU mean (SE) | df range, F-value range; p-value range | |
| MCS-12 | Complete case | F(1,228) = 0.52; p = 0.47 | 0.52 | 41.56 (3.41) | 41.05 (3.46) | F(1,228) = 0.02; p = 0.89 | 0.10 | 41.36 (3.40) | 41.25 (3.48) | F(1,228) = 0.11; p = 0.74 |
| Imputation | F(1,228) = 0.52 (0.52–0.52); p = 0.47 (0.47–0.47) | 0.52 | 41.56 (3.41) | 41.05 (3.46) | F(1,228) = 0.02 (0.02–0.02); p = 0.89 (0.89–0.89) | 0.10 | 41.36 (3.40) | 41.25 (3.48) | F(1,228) = 0.11 (0.11–0.11); p = 0.74 (0.74–0.74) | |
| PCS-12 | Complete case | F(1,228) = 0.52; p = 0.47 | 0.61 | 43.86 (3.50) | 43.25 (3.55) | F(1,228) = 0.20; p = 0.65 | –0.43 | 43.34 (3.48) | 43.77 (3.56) | F(1,228) = 0.002; p = 0.98 |
| Imputation | F(1,228) = 0.52 (0.52–0.52); p = 0.47 (0.47–0.47) | 0.61 | 43.86 (3.50) | 43.25 (3.55) | F(1,228) = 0.20 (0.20–0.20); p = 0.65 (0.65–0.65) | –0.43 | 43.34 (3.48) | 43.77 (3.56) | F(1,228) = 0.002 (0.002–0.002); p = 0.98 | |
| EQ-5D VAS | Completed case | F(1,222) = 1.01; p = 0.32 | 1.38 | 70.72 (1.70) | 69.34 (1.73) | F(1,222) = 0.03; p = 0.87 | 0.27 | 70.17 (1.46) | 69.90 (1.94) | F(1,222) = 0.9 (0.98); p = 0.34 |
| Imputation | F(1,228) = 0.32 (0.12–0.86); p = 0.57 (0.36–0.73) | 0.38 | 69.73 (1.75) | 69.35 (1.78) | F(1,228) = 0.19 (0.11–0.46); p = 0.66 (0.5–0.74) | 0.92 | 70.00 (1.50) | 69.08 (1.99) | F(1,228) = 1.22 (1.08–1.39); p = 0.27 (0.24–0.3) | |
| EQ-5D utility | Complete case | F(1,222) = 2.36; p = 0.13 | 0.05 | 0.77 (0.03) | 0.72 (0.03) | F(1,222) = 0.42; p = 0.52 | –0.03 | 0.73 (0.02) | 0.76 (0.03) | F(1,222) = 0.07; p = 0.79 |
| Imputation | F(1,228) = 3.21 (2.61–3.9); p = 0.07 (0.05–0.11) | 0.06 | 0.77 (0.03) | 0.71 (0.03) | F(1,228) = 0.58 (0.42–0.85); p = 0.45 (0.36–0.52) | –0.03 | 0.72 (0.03) | 0.75 (0.03) | F(1,228) = 0.15 (0.12–0.24); p = 0.7 (0.62–0.73) | |
| HADS anxiety | Complete case | F(1,225) = 1.51; p = 0.22 | –0.39 | 6.83 (0.36) | 7.22 (0.37) | F(1,225) = 0.44; p = 0.51 | 0.34 | 7.19 (0.31) | 6.85 (0.41) | F(1,225) = 0.78; p = 0.38 |
| Imputation | F(1,228) = 1.66 (0.9–1.71); p = 0.2 (0.19–0.34) | –0.35 | 6.91 (0.37) | 7.26 (0.37) | F(1,228) = 0.25 (0.2–0.64); p = 0.62 (0.43–0.66) | 0.30 | 7.23 (0.31) | 6.93 (0.42) | F(1,228) = 1.12 (0.65–1.32); p = 0.29 (0.25–0.42) | |
| HADS depression | Complete case | F(1,225) = 0.04; p = 0.84 | –0.03 | 5.93 (0.34) | 5.96 (0.34) | F(1,225) = 0.01; p = 0.94 | –0.03 | 5.93 (0.29) | 5.96 (0.39) | F(1,225) = 0.14; p = 0.71) |
| Imputation | F(1,228) = 0.02 (0.01–0.05); p = 0.9 (0.83–0.94) | 0.01 | 5.99 (0.33) | 5.97 (0.34) | F(1,228) = 0.04 (0.001–0.09); p = 0.84 (0.76–0.97) | –0.07 | 5.95 (0.29) | 6.02 (0.38) | F(1,228) = 0.2 (0.12–0.25); p = 0.65 (0.61–0.73) | |
| HADS total (20%) | Complete case | F(1,225) = 0.61; p = 0.44 | –0.42 | 12.77 (0.64) | 13.19 (0.65) | F(1,225) = 0.08; p = 0.78 | 0.26 | 13.11 (0.55) | 12.85 (0.73) | F(1,225) = 0.37; p = 0.54 |
| Imputation | F(1,228) = 0.62 (0.32–0.69); p = 0.43 (0.41–0.57) | –0.35 | 12.90 (0.64) | 13.25 (0.65) | F(1,228) = 0.02 (0.01–0.14); p = 0.9 (0.7–0.94) | 0.18 | 13.17 (0.55) | 12.99 (0.73) | F(1,228) = 0.55 (0.3–0.67); p = 0.46 (0.41–0.59) | |
| PANAS positive affectivity (20%) | Original | F(1,224) = 0.09; p = 0.77 | 0.23 | 30.59 (0.56) | 30.37 (0.57) | F(1,224) = 2.06; p = 0.15 | 1.08 | 31.02 (0.48) | 29.94 (0.64) | F(1,224) = 0.01; p = 0.93 |
| Imputed | F(1,228) = 0.05 (0.01–0.07); p = 0.82 (0.79–0.93) | 0.16 | 30.47 (0.56) | 30.30 (0.57) | F(1,228) = 1.59 (1.27–1.92); p = 0.21 (0.17–0.26) | 0.94 | 30.85 (0.48) | 29.92 (0.65) | F(1,228) = 0.01 (0–0.09); p = 0.93 (0.77–0.99) | |
| COPE positive aspects of caring (20%) | Original | F(1,204) = 0.15; p = 0.7 | –0.12 | 12.14 (0.20) | 12.26 (0.21) | F(1,204) = 0.2; p = 0.65 | 0.11 | 12.26 (0.17) | 12.14 (0.24) | F(1,204) = 0.07; p = 0.79 |
| Imputed | F(1,228) = 0.13 (0–0.24); p = 0.72 (0.62–0.99) | 0.01 | 12.13 (0.20) | 12.11 (0.22) | F(1,228) = 0.12 (0–0.35); p = 0.73 (0.55–0.99) | 0.08 | 12.16 (0.17) | 12.08 (0.24) | F(1,228) = 0.01 (0–0.19); p = 0.93 (0.66–0.99) | |
| Carer distress (NPI) | Original | F(1,191) = 2.46; p = 0.12 | 2.44 | 11.94 (0.88) | 9.51 (0.90) | F(1,191) = 0.12; p = 0.73 | –0.29 | 10.58 (0.73) | 10.87 (1.02) | F(1,191) = 2.71; p = 0.1 |
| Imputed | F(1,228) = 2.73 (1.02–3.08); p = 0.1 (0.08–0.31) | 1.82 | 12.63 (0.95) | 10.82 (0.96) | F(1,228) = 0.05 (0.01–0.11); p = 0.82 (0.74–0.92) | –0.09 | 11.68 (0.88) | 11.77 (1.00) | F(1,228) = 0.72 (0.09–1.42); p = 0.4 (0.23–0.76) | |
| Personal growth (PGI) | Original | F(1,223) = 3.77; p = 0.05 | –0.40 | 12.03 (0.22) | 12.43 (0.23) | F(1,223) = 1.29; p = 0.26 | 0.36 | 12.41 (0.19) | 12.05 (0.26) | F(1,223) = 1.46; p = 0.23 |
| Imputed | F(1,228) = 4.03 (3.4–5.57); p = 0.05 (0.02–0.07) | –0.41 | 12.03 (0.23) | 12.44 (0.23) | F(1,228) = 1.41 (1.1 to 1.62); p = 0.24 (0.2 to 0.3) | 0.37 | 12.42 (0.19) | 12.05 (0.26) | F(1,228) = 1.43 (1.11–2.08); p = 0.23 (0.15–0.29) | |
| QCPR warmth | Original | F(1,204) = 0.05; p = 0.82 | 0.09 | 31.36 (0.46) | 31.27 (0.49) | F(1,204) = 1.87; p = 0.17 | 0.82 | 31.72 (0.39) | 30.91 (0.55) | F(1,204) = 0.16; p = 0.69 |
| Imputed | F(1,228) = 0.43 (0.01–0.65); p = 0.51 (0.42–0.93) | 0.26 | 31.32 (0.46) | 31.06 (0.49) | F(1,228) = 2.26 (1.42–3.05); p = 0.13 (0.08–0.23) | 0.89 | 31.63 (0.40) | 30.74 (0.53) | F(1,228) = 0.1 (0.01–0.44); p = 0.76 (0.51–0.92) | |
| QCPR absence of criticism and conflict (20%) | Original | F(1,204) = 2.1; p = 0.15 | 0.78 | 22.23 (0.40) | 21.45 (0.43) | F(1,204) = 1.71; p = 0.19 | 0.70 | 22.19 (0.34) | 21.49 (0.48) | F(1,204) = 0.001; p = 0.97 |
| Imputed | F(1,228) = 3.92 (3.2–4.87); p = 0.05 (0.03–0.07) | 1.11 | 22.08 (0.41) | 20.97 (0.44) | F(1,228) = 3.58 (1.91–4.71); p = 0.06 (0.03–0.17) | 0.98 | 22.02 (0.37) | 21.04 (0.49) | F(1,228) = 0.1 (0.003–1.78); p = 0.76 (0.18–0.96) | |
| QCPR total (20%) | Original | F(1,204) = 0.96; p = 0.33 | 0.90 | 53.60 (0.73) | 52.70 (0.78) | F(1,204) = 2.39; p = 0.12 | 1.49 | 53.89 (0.62) | 52.40 (0.87) | F(1,204) = 0.06; p = 0.81 |
| Imputed | F(1,228) = 2.17 (1.32–3.08); p = 0.14 (0.08–0.25) | 1.42 | 53.41 (0.73) | 52.00 (0.76) | F(1,228) = 3.9 (3.19–4.54); p = 0.05 (0.03–0.08) | 1.87 | 53.64 (0.63) | 51.77 (0.84) | F(1,228) = 0.04 (0.002–0.53); p = 0.84 (0.47–0.97) | |
| Loneliness | Original | F(1,224) = 0.26; p = 0.61 | 0.17 | 2.68 (0.19) | 2.51 (0.19) | F(1,224) = 1.02; p = 0.31 | –0.24 | 2.47 (0.16) | 2.71 (0.21) | F(1,224) = 0.42; p = 0.52 |
| Imputed | F(1,228) = 0.24 (0.19–0.45); p = 0.63 (0.5–0.66) | 0.19 | 2.72 (0.19) | 2.53 (0.19) | F(1,228) = 1.25 (0.91–1.54); p = 0.26 (0.22–0.34) | –0.27 | 2.49 (0.16) | 2.76 (0.22) | F(1,228) = 0.71 (0.47–0.89); p = 0.4 (0.35–0.49) | |
| Outcome measure | CSP vs. no CSP | RYCT vs. no RYCT | Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Complete case | df, F-value, p-value | Mean difference | Mean CSP (SE) | Mean no CSP (SE) | df, F-value, p-value | Mean difference | Mean RYCT (SE) | Mean no RYCT (SE) | df, F-value, p-value | |
| Multiple imputation | df range, F-value range; p-value range | Pooled mean difference | Mean CSP (SE) | Mean TAU (SE) | df range, F-value range; p-value range | Pooled mean difference | Mean RYCT (SE) | Mean TAU (SE) | df range, F-value range; p-value range | |
| QOL-AD self-report | Original | F(1,125) = 0.004; p = 0.95 | –0.08 | 37.85 (0.63) | 37.93 (0.63) | F(1,125) = 0.43; p = 0.52 | 0.51 | 38.14 (0.53) | 37.63 (0.72) | F(1,125) = 0.43; p = 0.51 |
| Imputed | F(1,226) = 0.23 (0.06–0.77); p = 0.63 (0.38–0.8) | –0.16 | 36.43 (0.72) | 36.59 (0.82) | F(1,226) = 1.5 (0.02–4.69); p = 0.22 (0.03–0.9) | 0.70 | 36.86 (0.86) | 36.16 (0.74) | F(1,226) = 0.06 (0.01–1.7); p = 0.06 (0.01–1.7) | |
| QOL-AD proxy | Original | F(1,204) = 0.5; p = 0.48 | –0.23 | 28.83 (0.46) | 29.05 (0.50) | F(1,204) = 0.06; p = 0.81 | –0.31 | 28.78 (0.38) | 29.10 (0.57) | F(1,204) = 5.91; p = 0.02 |
| Imputed | F(1,226) = 0.22 (0.15–1.1); p = 0.64 (0.3–0.69) | –0.12 | 28.51 (0.45) | 28.63 (0.53) | F(1,226) = 0.003 (0–0.21); p = 0.95 (0.65–0.99) | –0.20 | 28.47 (0.40) | 28.67 (0.56) | F(1,226) = 4.47 (2.46–5.94); p = 0.04 (0.02–0.12) | |
| DEMQOL self-report | Original | F(1,123) = 4.39; p = 0.04 | 2.51 | 96.64 (1.26) | 94.13 (1.27) | F(1,123) = 0.02; p = 0.88 | –0.37 | 95.20 (1.06) | 95.57 (1.43) | F(1,123) = 2.2; p = 0.14 |
| Imputed | F(1,226) = 7.39 (5.02–9.7); p = 0.01 (0.002–0.03) | 2.67 | 94.04 (1.33) | 91.37 (1.92) | F(1,226) = 1 (0.12–2.57); p = 0.32, (0.11–0.73) | –1.38 | 92.01 (1.39) | 93.39 (1.93) | F(1,226) = 1.63 (0.25–5.76); p = 0.2 (0.02–0.62) | |
| DEMQOL-Proxy | Original | F(1,202) = 2.29; p = 0.13 | –3.12 | 92.38 (1.08) | 95.51 (1.17) | F(1,202) = 0.06; p = 0.81 | 0.17 | 94.03 (0.89) | 93.86 (1.32) | F(1,202) = 3.03; p = 0.08 |
| Imputed | F(1,226) = 2.62 (1.44–3.04); p = 0.11 (0.08–0.23) | –2.79 | 92.22 (1.21) | 95.00 (1.14) | F(1,226) = 0.02 (0.001 to 0.34); p = 0.9 (0.56 to 0.97) | –0.18 | 93.52 (0.95) | 93.70 (1.37) | F(1,226) = 1.67 (0.84–3.61); p = 0.2 (0.06–0.36) | |
| Anxiety | Original | F(1,119) = 0.23; p = 0.63 | 0.14 | 3.27 (0.41) | 3.13 (0.40) | F(1,119) = 0.92; p = 0.34 | 0.55 | 3.48 (0.33) | 2.93 (0.46) | F(1,119) = 3.95; p = 0.05 |
| Imputed | F(1,226) = 0.16 (0.001–0.46); p = 0.69 (0.5–0.98) | 0.07 | 3.90 (0.34) | 3.84 (0.35) | F(1,226) = 2.42 (1.15–3.56); p = 0.12 (0.06–0.28) | 0.65 | 4.20 (0.31) | 3.55 (0.37) | F(1,226) = 0.53, (0.08–1.1); p = 0.47 (0.3–0.77) | |
| Depression | Original | F(1,119) = 0.33; p = 0.57 | –0.26 | 3.85 (0.42) | 4.11 (0.41) | F(1,119) = 0.001; p = 0.97 | 0.02 | 4.00 (0.34) | 3.97 (0.47) | F(1,119) = 0.03; p = 0.87 |
| Imputed | F(1,226) = 0.1 (0.001–0.93); p = 0.75 (0.34–0.98) | 0.05 | 5.32 (0.50) | 5.27 (0.53) | F(1,226) = 0.02 (0.002–1.03); p = 0.88 (0.31–0.96) | 0.15 | 5.37 (0.49) | 5.22 (0.51) | F(1,226) = 0.09 (0.002–1.53); p = 0.76 (0.22–0.97) | |
| HADS total | Original | F(1,119) = 0.27; p = 0.6 | –0.05 | 7.19 (0.70) | 7.24 (0.68) | F(1,119) = 0.24; p = 0.63 | 0.49 | 7.46 (0.57) | 6.97 (0.78) | F(1,119) = 1.57; p = 0.21 |
| Imputed | F(1,226) = 0.15 (0.003–1.05); p = 0.69 (0.31–0.96) | 0.07 | 9.24 (0.63) | 9.17 (0.72) | F(1,226) = 0.8 (0.38–2.61); p = 0.37 (0.11–0.54) | 0.73 | 9.57 (0.61) | 8.84 (0.70) | F(1,226) = 0.17 (0.13–1.56); p = 0.68 (0.21–0.72) | |
| ADCS-ADL total | Original | F(1,158) = 0.09; p = 0.76 | –2.42 | 40.56 (1.30) | 42.98 (1.41) | F(1,158) = 3.55; p = 0.06 | –3.84 | 39.85 (1.08) | 43.69 (1.57) | F(1,158) = 5.63; p = 0.02 |
| Imputed | F(1,226) = 0.46 (0.002–2.22); p = 0.5 (0.14–0.96) | –2.48 | 35.30 (1.47) | 37.77 (2.14) | F(1,226) = 3.59 (1.52–4.04); p = 0.06 (0.05–0.22) | –3.23 | 34.92 (1.44) | 38.15 (2.07) | F(1,226) = 3.33 (2.27–3.8); p = 0.07 (0.05–0.13) | |
| QCPR total | Original | F(1,123) = 0.26; p = 0.61 | 0.83 | 60.51 (0.84) | 59.68 (0.81) | F(1,123) = 2.07; p = 0.15 | –0.29 | 59.95 (0.69) | 60.24 (0.93) | F(1,123) = 0.18; p = 0.67 |
| Imputed | F(1,226) = 0.41 (0.01–3.35); p = 0.52 (0.07–0.92) | –0.19 | 57.30 (1.40) | 57.49 (0.71) | F(1,226) = 6.78 (3.02–10.98); p = 0.01 (0.001–0.08) | –1.26 | 56.77 (0.95) | 58.03 (1.02) | F(1,226) = 0.94 (0.03–2.2); p = 0.33 (0.14–0.85) | |
| Warmth | Original | F(1,122) = 0.002; p = 0.96 | 0.15 | 35.56 (0.49) | 35.41 (0.48) | F(1,122) = 0.54; p = 0.46 | –0.89 | 35.04 (0.40) | 35.93 (0.54) | F(1,122) = 0.46; p = 0.5 |
| Imputed | F(1,226) = 0.94 (0.6–3.48); p = 0.33 (0.06–0.44) | –0.17 | 34.27 (0.69) | 34.44 (0.45) | F(1,226) = 0.5 (0.19–1.49); p = 0.48, (0.22–0.66) | –1.38 | 33.67 (0.59) | 35.05 (0.48) | F(1,226) = 0.54 (0.02–1.32); p = 0.46 (0.25–0.9) | |
| Criticism | Original | F(1,122) = 0.66; p = 0.42 | 0.21 | 24.65 (0.52) | 24.45 (0.51) | F(1,122) = 0.08; p = 0.78 | 0.49 | 24.80 (0.43) | 24.30 (0.58) | F(1,122) = 0.01; p = 0.94 |
| Imputed | F(1,226) = 1.47 (0.12–3.36); p = 0.23 (0.07–0.73) | –0.25 | 22.86 (1.02) | 23.11 (0.47) | F(1,226) = 1.38 (0.44–3.93); p = 0.24 (0.05–0.51) | 0.12 | 23.04 (0.70) | 22.93 (0.82) | F(1,226) = 0.03 (0.001–1.42); p = 0.86 (0.23–0.98) | |
There was some indication of impact on the person with dementia. There was a significant benefit to quality of life as measured by the DEMQOL (p = 0.04) for those allocated to the CSP intervention. The interaction term was significant for daily functioning (p = 0.02), quality of life as measured by the QOL-AD proxy (p = 0.02) and anxiety (p = 0.05), indicating that the assumption of independence between the two interventions was not valid.
Intervention
Costs
Table 40 reports the average costs per dyad for the CSP and RYCT interventions. The costs vary depending on the perspective chosen, as there are carer inputs to the delivery of the interventions.
| Intervention | Perspective and cost assumption | Cost per dyad (£) | ||
|---|---|---|---|---|
| Mean | Median | Range | ||
| CSP | Health and social care | 2136 | 1143 | 32–12,249 |
| Societal; replacement cost | 2837 | 1817 | 36–14,489 | |
| Societal; opportunity cost | 2339 | 1390 | 33–12,782 | |
| RYCT (12 week) | Health and social care | 1661 | 1704 | 0–5419 |
| Societal; replacement cost | 2165 | 2300 | 0–6382 | |
| Societal; opportunity cost | 1785 | 1839 | 0–5652 | |
| RYCT reunion sessions | Health and social care | 551 | 454 | 0–1854 |
| Societal; replacement cost | 770 | 771 | 0–2146 | |
| Societal; opportunity cost | 604 | 583 | 0–1924 | |
Costs of health and social care services
For the pre-baseline period, most of the health and social care-related costs were made up by community care services, with an average of £1171 for a 3-month period, whereas (care-related) accommodation costs contributed to a much smaller extent (£88). However, at final follow-up, the average quarterly expenditure for residential care had increased to £1335. Hospital service use typically increased with time, with costs averaging £301 at baseline and £410 at second follow-up. Costs of day-care services and medications barely altered over time, averaging within the £200–300 range at both baseline and final follow-up. For the overall 12-month period, community services remained the highest contributor to total health and social care costs (£4686), followed by accommodation costs (£3225) and hospital services (£1640). The smallest cost component was adaptations and equipment (£269).
Comparing total health and social care expenditures for the person with dementia with those of the family carer at baseline, the cost for the former was about 15 times higher (£2170 vs. £141). This gap was wider at second follow-up (£3475 vs. £154: 23 times higher). For the total 12-month period, the average health and social care costs were £12,497 for the person with dementia and £614 for the family carer.
The only adjusted differences in the distributions of costs between groups found to be significant at the 5% confidence level were residential and accommodation costs at first follow-up and for the whole 12-month period, for family carer at baseline and for some of the cost aggregates, which included intervention-related costs.
There was no difference in frequency of service use for either family carers or people with dementia between intervention groups or TAU, although accommodation showed the most variance between groups (p-values ranged between 0.10 and 0.15 depending on the time point). At baseline, a small percentage of people with dementia used care accommodation and this percentage increased over time, so that by final follow-up > 25% of some intervention groups generated accommodation costs. About two-thirds of the people with dementia used hospital services at baseline, declining to around half at final follow-up. At baseline, 95% of people with dementia made use of some sort of community service, this percentage falling to 85% at final follow-up. At baseline, day services were used by half of the sample of people with dementia, with this percentage remaining fairly stable between groups and time points. Every person with dementia was receiving some form of unpaid family care at baseline, given the study design, with this percentage remaining at ≥ 90% at final follow-up.
At baseline, the percentage of carers using health and social care services ranged between 38% and 46%, depending on the treatment group, with this percentage declining slightly at final follow-up. About two-thirds of carers reported using medication, whereas for the people with dementia the mean was about 90%.
Table 41 displays the incremental costs, incremental effects (both measured over 12 months) and ICERs for the two primary outcomes: MCS-12 for carers and QOL-AD for people with dementia. Results are shown from three perspectives: two societal perspectives using (in turn) opportunity cost and replacement cost approaches to value unpaid care, and a health and social care perspective, which does not include unpaid care. This analytic strategy was repeated for the secondary outcomes: PCS-12 for carers and QALYs for people with dementia derived in turn from the EQ-5D, DEMQOL and DEMQOL-Proxy measures. These results are shown in Table 42.
| Variable | CSP vs. not CSP | RYCT vs. not RYCT | CSP/RYCT combined vs. TAU | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Incremental cost (£, 2010/11), mean (95% bootstrapped CI) | Incremental effect, mean (95% bootstrapped CI) | ICER (£) | Incremental cost (£ 2010/11), mean (95% bootstrapped CI) | Incremental effect, mean (95% bootstrapped CI) | ICER (£) | Incremental cost (£ 2010/11), mean (95% bootstrapped CI) | Incremental effect, mean (95% bootstrapped CI) | ICER (£) | |
| Societal perspective: opportunity cost scenario (0–12 months) | |||||||||
| MCS-12 | 3957 (–1105 to 9019) | 0.46 (–1.39 to 2.31) | 8601 | 1492 (–4423 to 7408) | –1.07 (–2.93 to 0.78) | Not-RYCT dominant | 7509 (–347 to 15,365) | –1.16 (–3.90 to 1.59) | TAU dominant |
| QOL-AD | 3973 (–1082 to 9028) | –0.03 (–1.66 to 1.60) | Not-CSP dominant | 1495 (–4417 to 7408) | 0.67 (–1.54 to 2.89) | 2220 | 7520 (–318 to 15,358) | 0.50 (–2.45 to 3.45) | 15,015 |
| Societal perspective: replacement cost scenario (0–12 months) | |||||||||
| MCS-12 | 3543 (–11,230 to 18,315) | 0.46 (–1.39 to 2.31) | 7666 | –7423 (–23,647 to 8801) | –1.07 (–2.93 to 0.79) | 6914 | 739 (–26,122 to 27,600) | –1.15 (–3.90 to 1.59) | TAU dominant |
| QOL-AD | 3436 (–11,319 to 18,191) | –0.04 (–1.67 to 1.59) | Not-CSP dominant | –7431 (–23,659 to 8797) | 0.67 (–1.55 to 2.89) | RYCT dominant | 651 (–26,222 to 27,524) | 0.49 (–2.46 to 3.45) | 1325 |
| Health and social care perspective (12 months) | |||||||||
| MCS-12 | 2887.9 (–850.9 to 6626.7) | 0.46 (–1.39 to 2.31) | 6312 | 4356 (230 to 8482) | –1.08 (–2.93 to 0.78) | Not-RYCT dominant | 8543 (4152 to 12,994) | –1.16 (–3.90 to 1.58) | TAU dominant |
| QOL-AD | 2888 (–845 to 6620) | –0.04 (–1.67 to 1.59) | Not-CSP dominant | 4356 (234 to 8478) | 0.67 (–1.56 to 2.89) | 6517 | 8543 (4157 to 12,928) | 0.49 (–2.47 to 3.45) | 17,428 |
| Variable | CSP vs. not CSP | RYCT vs. not RYCT | CSP/RYCT combined vs. TAU | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Incremental cost (£ 2010/11), mean (95% bootstrapped CI) | Incremental effect, mean (95% bootstrapped CI) | ICER (£) | Incremental cost (£ 2010/11), mean (95% bootstrapped CI) | Incremental effect, mean (95% bootstrapped CI) | ICER (£) | Incremental cost (£ 2010/11), mean (95% bootstrapped CI) | Incremental effect, mean (95% bootstrapped CI) | ICER (£) | |
| Societal perspective: opportunity cost scenario (0–12 months) | |||||||||
| PCS-12 | 3969 (–1088 to 9026) | 0.81 (–1.07 to 2.68) | 4920 | 1495 (–4422 to 7411) | –1.26 (–3.21 to 0.68) | Not-RYCT dominant | 7517 (–333 to 15,368) | –0.85 (–3.39 to 1.69) | TAU dominant |
| QALY (EQ-5D) | 3966 (–1090 to 9022) | 0.02 (–0.01 to 0.05) | 208,400 | 1494 (–4424 to 7412) | –0.03 (–0.06 to 0.00) | Not-RYCT dominant | 7515 (–338 to 15,369) | –0.01 (–0.05 to 0.03) | TAU dominant |
| QALY (DEMQOL) | 3956 (–1112 to 9024) | 0.01 (–0.01 to 0.03) | 292,435 | 1492 (–4427 to 7412) | –0.01 (–0.04 to 0.02) | Not-RYCT dominant | 7508 (–359 to 15,376) | 0.00 (–0.04 to 0.04) | 183,580,636 |
| QALY (DEMQOL-Proxy) | 3994 (–1060 to 9048) | 0.01 (–0.01 to 0.03) | 636,633 | 1499 (–4408 to 7406) | 0.01 (–0.01 to 0.03) | 141,665 | 7534 (–283 to 15,351) | 0.02 (–0.01 to 0.06) | 306,490 |
| Societal perspective: replacement cost scenario (0–12 months) | |||||||||
| PCS-12 | 3610 (–11,165 to 18,386) | 0.81 (–1.07 to 2.68) | 4477 | –7418 (–23,647 to 8811) | –1.26 (–3.21 to 0.68) | 5865a | 794 (–26,059 to 27,647) | –0.85 (–3.39 to 1.69) | TAU dominant |
| QALY (EQ-5D) | 3445 (–11,361 to 18,251) | 0.02 (–0.01 to 0.05) | 178,979 | –7431 (–23,667 to 8806) | –0.03 (–0.06 to 0.00) | 247,968a | 659 (–26,226 to 27,583) | –0.01 (–0.05 to 0.03) | TAU dominant |
| QALY (DEMQOL) | 3486 (–11,331 to 18,303) | 0.01 (–0.01 to 0.03) | 258,113 | –7427 (–23,658 to 8804) | –0.01 (–0.04 to 0.02) | 737,676a | 692 (–26,218 to 27,602) | 0.00 (–0.04 to 0.04) | 35,308,661 |
| QALY (DEMQOL-Proxy) | 3669 (–11,077 to 18,416) | 0.01 (–0.01 to 0.03) | 595,549 | –7414 (–23,635 to 8807) | 0.0105 (–0.01 to 0.03) | RYCT dominant | 843 (–25,946 to 27,631) | 0.0243 (–0.01 to 0.05) | 34,624 |
| Health and social care perspective (0–12 months) | |||||||||
| PCS-12 | 2886 (–850 to 6623) | 0.81 (–1.07 to 2.68) | 3578 | 4356 (233 to 8479) | –1.26 (–3.21 to 0.68) | Not-RYCT dominant | 8541 (4153 to 12,929) | –0.85 (–3.39 to 1.69) | TAU dominant |
| QALY (EQ-5D) | 2889 (–844 to 6622) | 0.02 (–0.01 to 0.05) | 151,027 | 4356 (229 to 8482) | –0.03 (–0.06 to 0.00) | Not-RYCT dominant | 8544 (4151 to 12,938) | –0.01 (–0.05 to 0.03) | TAU dominant |
| QALY (DEMQOL) | 2887 (–849 to 6622) | 0.01 (–0.01 to 0.03) | 213,369 | 4357 (230 to 8482) | –0.01 (–0.04 to 0.02) | Not-RYCT dominant | 8541 (4155 to 12,928) | 0.00 (–0.04 to 0.04) | 182,898,801 |
| QALY (DEMQOL-Proxy) | 2880 (–857 to 6618) | 0.0063 (–0.0126 to 0.0251) | 457,823 | 4358 (238 to 8479) | 0.0106 (–0.0076 to 0.0288) | 411,373 | 8533 (4146 to 12,920) | 0.0246 (–0.0062 to 0.0555) | 346,568 |
For carers, none of CSP, RYCT or CSP/RYCT combined appears to be cost-effective compared with its respective comparator, whatever the study perspective.
Looking at the primary outcome for carers (MCS-12) and taking the primary perspective (societal with opportunity cost values), both RYCT and CSP/RYCT combined are dominated; that is, they are less effective and have higher costs than their respective comparators. CSP alone had marginally better outcomes and higher costs (although neither was significantly different), with the resultant ICER suggesting that the cost for a 1-point improvement on the MCS-12 is £8601. There is no external threshold with which to compare this ICER, but it looks high given that MCS-12 scores can range from 0 to 100.
The findings are the same under a health and social care perspective: RYCT and CSP/RYCT combined are dominated – indeed, the combined intervention is significantly more costly than TAU – and CSP has a high ICER.
Recalculating carer costs using a replacement cost approach, CSP still has a high ICER and CSP/RYCT combined is dominated. RYCT significantly reduces costs but has worse outcomes than its comparator (the ICER is £6914 for each 1-point worsening on the MCS-12). Given that neither the cost nor the outcome difference in this case is significant – and that the ‘cost reduction’ is the imputed value of carer time, and therefore not easily transferable as a ‘saving’ for use elsewhere in the system – it is unlikely that the decision-maker would choose RYCT in these circumstances.
The two secondary outcomes for carers were PCS-12 and QALYs generated by the EQ-5D. Looking first at PCS-12 and taking the primary perspective (societal, opportunity cost assumption), both RYCT and CSP/RYCT combined are dominated by their comparators. CSP has higher costs but better outcomes than not CSP (although neither achieves significance) and the ICER appears high at £4920, although there are no established thresholds for PCS-12 against which to compare this figure.
The findings are similar under a health and social care perspective: RYCT and CSP/RYCT combined are both dominated, and in fact each has significantly higher cost than its comparator, whereas CSP has an ICER value (£3578) likely to be considered high.
Under a societal perspective with replacement cost values for carer time, CSP/RYCT combined is dominated by its comparator (TAU). RYCT has lower costs but worse outcomes (although neither is significant). In a similar vein to the earlier argument, this finding is unlikely to encourage the decision-maker to choose RYCT over non-RYCT because the differences are not significant, and the ‘saving’ is in the ascribed value of carer time that is not easily transferred to another use in the care system. CSP has slightly better effectiveness on PCS-12 than not CSP but also higher costs (although neither is significant) and the ICER appears high.
Using QALY as an outcome measure, whether measured using EQ-5D (for carers) or DEMQOL or DEMQOL-Proxy (for people with dementia), is helpful in cost-effectiveness analyses because it is then possible to compare an estimated ICER value (when adopting a health and social care perspective) with thresholds recommended by NICE, usually quoted as £20,000 per QALY gained.
From both a societal perspective (opportunity costs) and health and social care perspectives the findings are the same: CSP has (non-significantly) higher costs and (non-significantly) lower costs than not CSP (with ICERs considerably greater than the NICE threshold) and both RYCT and CSP/RYCT combined are dominated by their comparators. (The QALY difference for RYCT is small but statistically significant, and under a health and social care perspective RYCT also has significantly higher costs.)
From a societal perspective (replacement costs), CSP is again unlikely to be seen as cost-effective given its high ICER. RYCT generates significantly lower QALYs than not RYCT but also reduces costs, and for the reasons noted earlier is unlikely to be seen as the preferred option. CSP/RYCT combined is dominated by TAU.
The primary outcome measure for people with dementia was QOL-AD. CSP is dominated by not CSP when adopting any of the perspectives and costing approaches. RYCT had marginally but not significantly better effectiveness on this measure, higher cost from the societal perspective with opportunity cost and also the health and social care perspective (with cost difference being significant), and marginally but not significantly lower cost from the societal perspective with replacement cost assumptions. There are no established thresholds against which to compare ICER values for QOL-AD. The CEAC (Figure 21) suggests that RYCT is cost-effective across a wide range of WTP values. For comparison, the CEAC in Figure 22 is for the same comparison but now from a health and social care perspective: RYCT is not cost-effective. CSP/RYCT combined has high ICER values under two perspectives and, under the other (societal with replacement costs), the estimated CEAC (Figure 23) does not suggest that it would be seen as cost-effective.
FIGURE 21.
Cost-effectiveness acceptability curve for RYCT, using QOL-AD as outcome, from a societal perspective (replacement cost assumption).
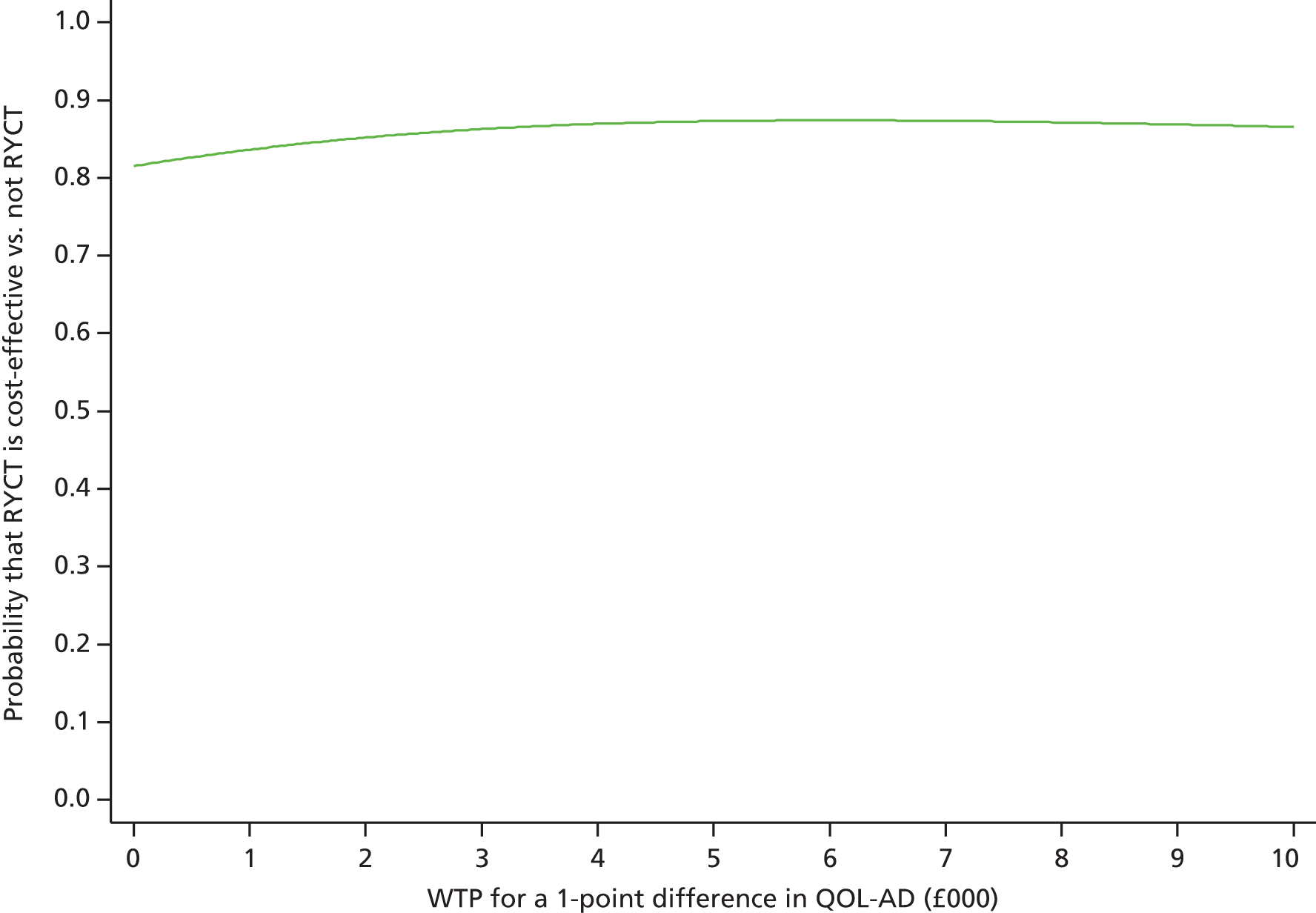
FIGURE 22.
Cost-effectiveness acceptability curve for RYCT, using QOL-AD as outcome, from a health and social care perspective.
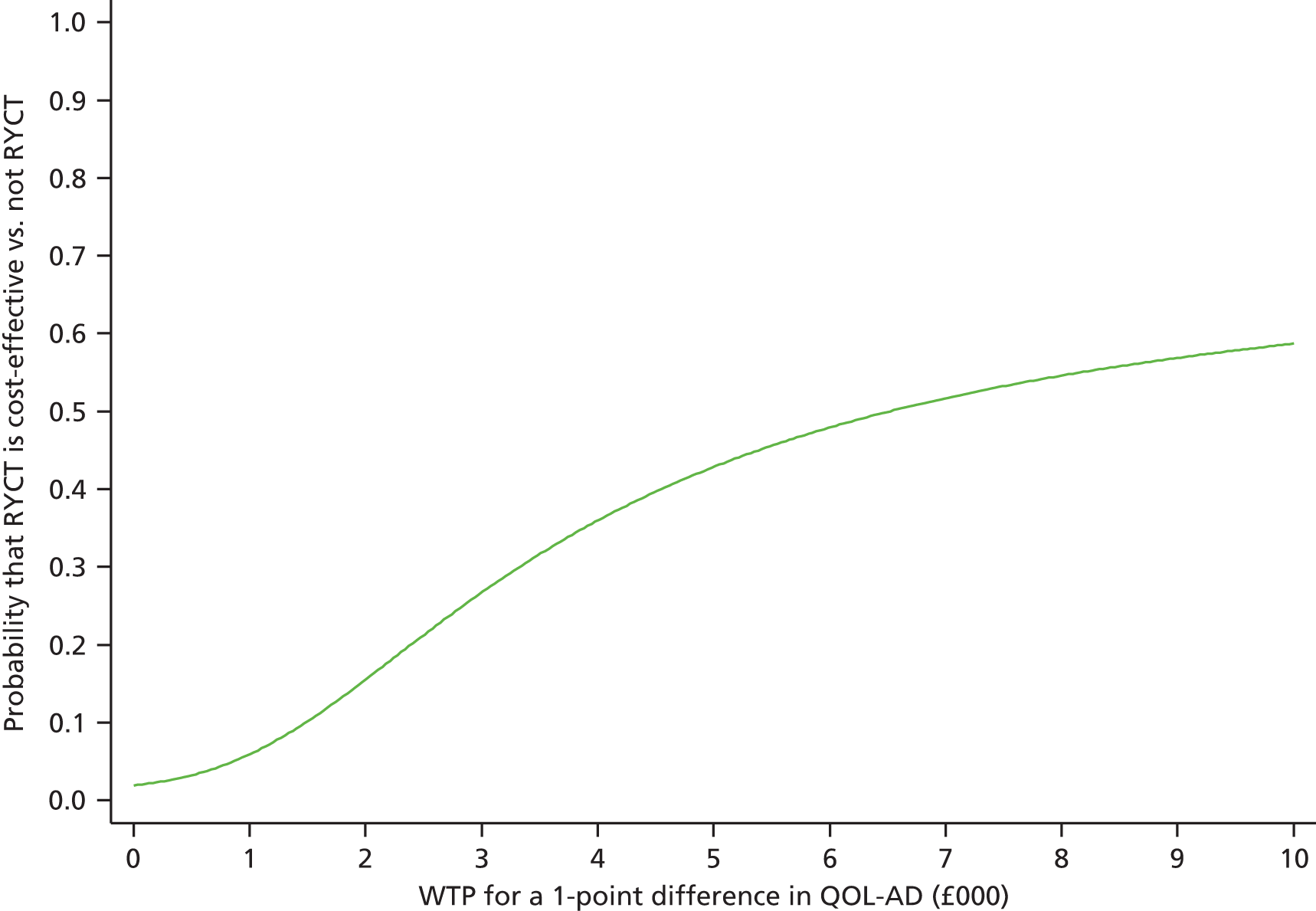
FIGURE 23.
Cost-effectiveness acceptability curve for CSP-RYCT combined, using QOL-AD as outcome, from a societal perspective (opportunity cost).
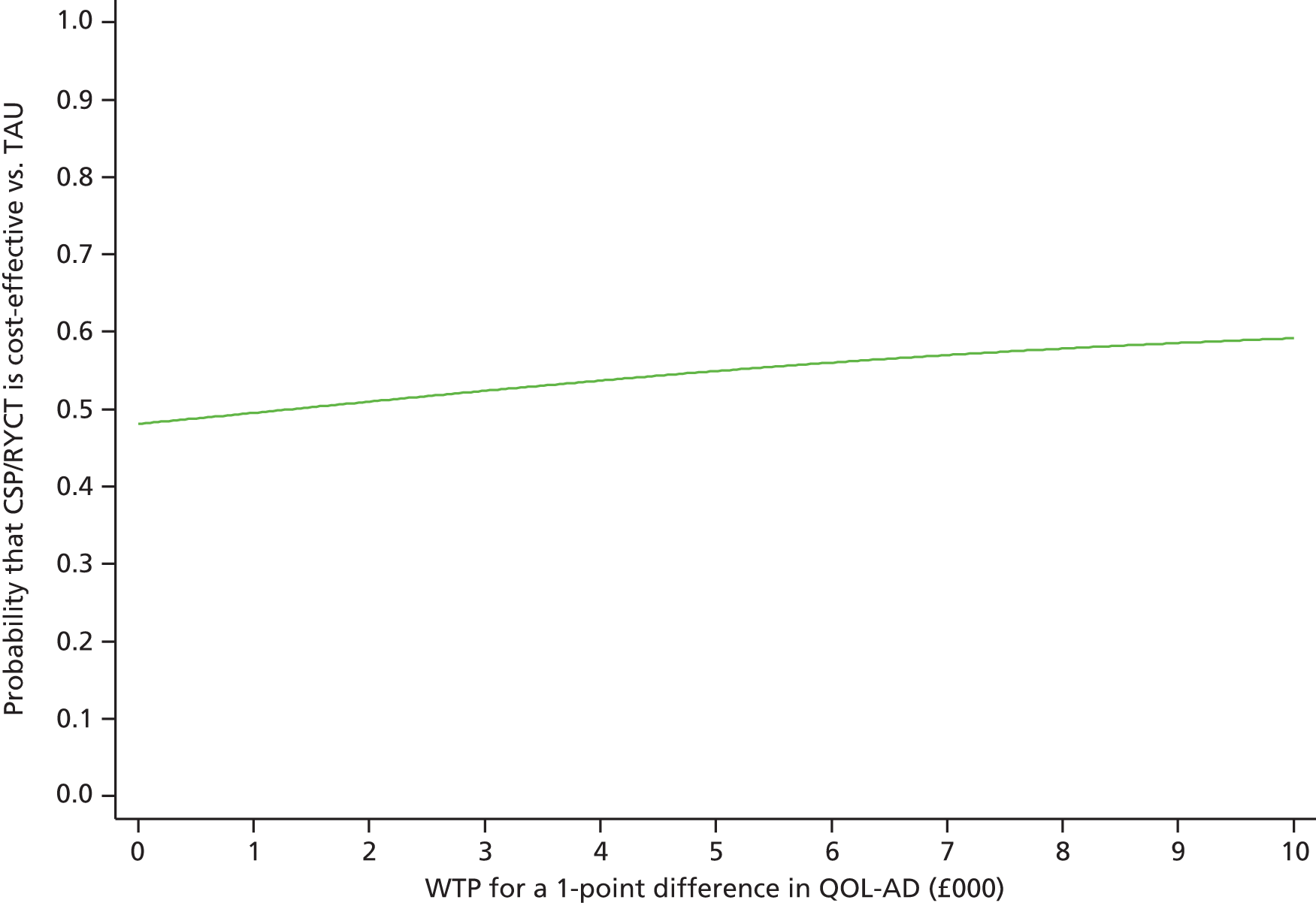
The secondary outcomes for people with dementia were QALYs measured by DEMQOL and DEMQOL-Proxy. From a societal perspective (opportunity costs), CSP is not cost-effective by reference to either measure, as ICER values are all considerably above the NICE threshold. RYCT is dominated by its comparator using DEMQOL, has a high ICER (£141,665 per QALY) using DEMQOL-Proxy and is not cost-effective. CSP/RYCT combined is not cost-effective, as the ICERs are again considerably above the NICE threshold.
From a societal perspective with replacement costs, CSP is not cost-effective. RYCT is not cost-effective using DEMQOL, but dominates not RYCT using DEMQOL-Proxy and is cost-effective. CSP/RYCT combined has a very ICER using DEMQOL and, although the ICER is much closer to the NICE threshold using DEMQOL-Proxy (although the comparison is not so relevant given the perspective), the CEAC (Figure 24) does not suggest cost-effectiveness as the curve barely gets above the 0.5 probability level, even at high WTP values.
FIGURE 24.
Cost-effectiveness acceptability curve for CSP-RYCT combined, using QALYs from DEMQOL-Proxy as outcome, from a societal perspective (replacement cost).
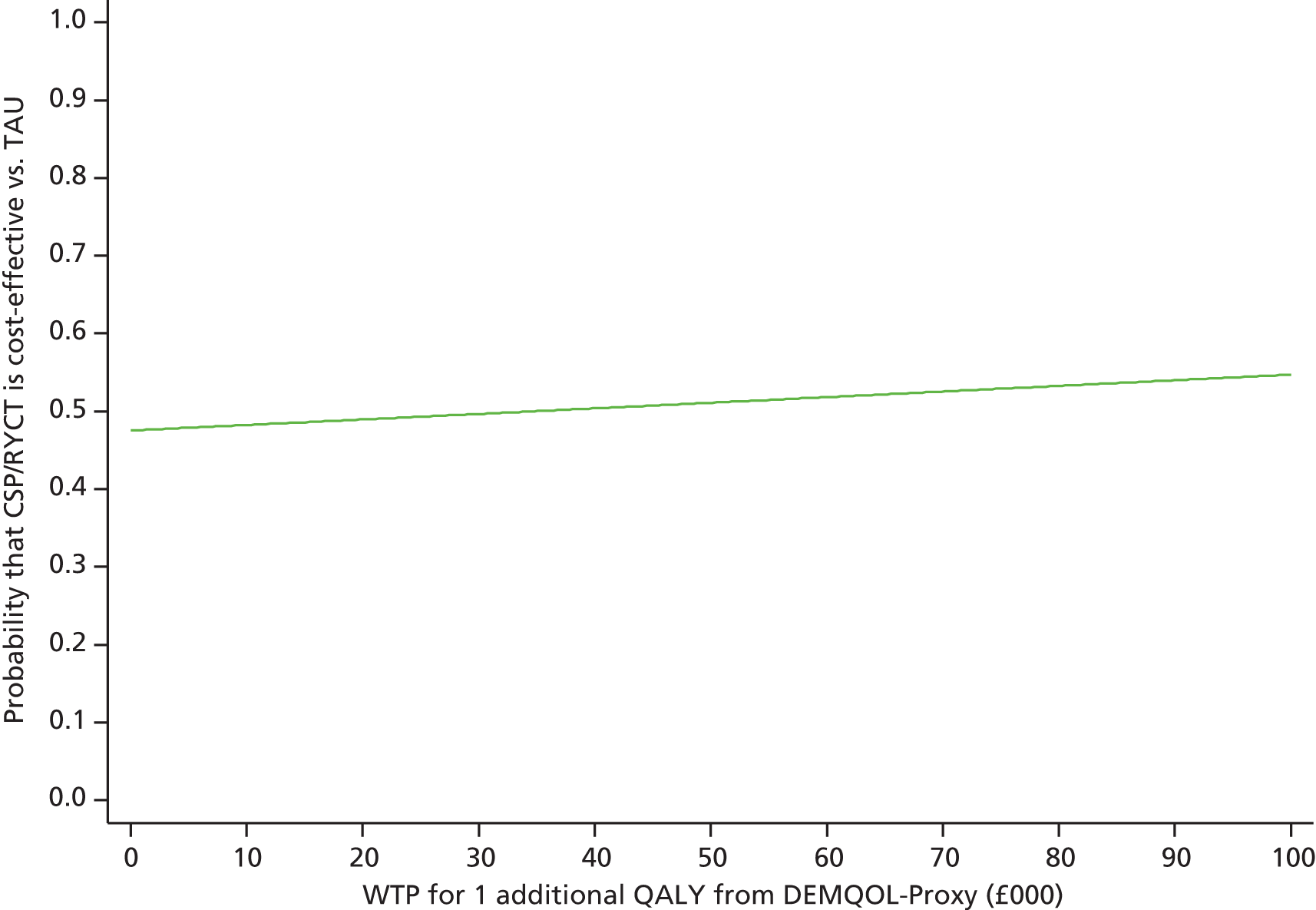
From a health and social care perspective, none of the interventions is cost-effective.
Bringing these cost-effectiveness results together:
-
CSP is not cost-effective whether considering outcomes for carers or outcomes for people with dementia.
-
RYCT is not cost-effective when considering outcomes for carers or most outcomes for people with dementia. It is cost-effective when looking at the QOL-AD measure and from a societal perspective with replacement costs, but this was not the primary perspective chosen for the analysis before the trial began.
-
CSP-RYCT combined is not cost-effective when considering outcomes for carers or outcomes for people with dementia.
Discussion
In this trial we had made some adaptations to both interventions to increase the awareness of intervention providers to the needs of family carers. These changes led to greater acceptability of the one-to-one support intervention, as 76% carers offered peer support took up this offer, much higher than in the BECCA befriending intervention.
Overall, the groups were well matched at baseline. However, given the significantly higher MMSE score for the TAU group at baseline, a sensitivity analysis was carried out in which the MMSE score was taken into account. The results continued to show a significant benefit to the person with dementia on quality of life for those whose family carers had CSP.
The overall recruitment and retention met targets, and differences in group sizes were anticipated owing to the 2 : 1 allocation ratio in favour of RYCT. The group sizes varied from those expected owing to differential withdrawal rates between the groups. Although the overall retention rate was 83%, only 77% of those allocated to usual care were available at follow-up.
The intention-to-treat analysis of primary and secondary outcomes at the primary end point (12 months post randomisation) showed no benefit to family carers of either intervention. In contrast, people with dementia whose family carers were in the CSP arm of the trial reported significantly higher quality of life than those who were not in receipt of one-to-one peer support, and the interaction between CSP and RYCT was significant for a number of outcome variables for the person with dementia. The finding of increased anxiety for those carers receiving reminiscence was not replicated.
With one exception, the economic evaluation did not find CSP, RYCT or the combined intervention to be cost-effective. The exception was that RYCT was more cost-effective than its comparator when looking at the QOL-AD measure for people with dementia. But this was only when adopting a societal perspective with replacement costs, an approach that attaches quite a high value to the unpaid time of family carers, and which was not the primary perspective chosen a priori for the economic analysis. Overall, therefore, we did not find evidence that the interventions were cost-effective.
The results of this trial should be considered generalisable within the UK as participants were drawn from a wide range of community settings including those already well embedded in services and also new users of health and social care services for people with dementia and family carers. There was a higher proportion of non-white carers and people with dementia than was recruited to either BECCA or REMCARE.
The factorial trial design presents challenges to the interpretation of results for a number of reasons. First, by allocating participants to carer support (CSP), reminiscence (RYCT), a combination of the two (CSP/RYCT) or usual care, and by reporting the baseline characteristics of these four groups, we give the impression of a four-arm trial in which three interventions (CSP, RYCT and CSP/RYCT) are each compared with TAU. Reporting the baseline scores for each of these four groups highlights some potential differences between the intervention groups and usual care (TAU). However, in the analysis, the only place in which the TAU group is used is in the cost-effectiveness calculations, when CSP/RYCT is compared with TAU. In all other analyses the four groups are collapsed into two. For the CSP analyses, those allocated to receive carer support either with or without RYCT are compared with those who are not allocated to receive carer support (i.e. including those in the RYCT arm) and, for the RYCT analyses, those allocated to receive RYCT either with or without carer support are compared with those who are not allocated to receive RYCT (i.e. including those in the CSP arm). Second, when carrying out the statistical analysis there is an assumption that the two interventions do not interact. In this trial, however, we were anticipating added benefit to those carers and people with dementia who were allocated to the combined CSP/RYCT intervention. Indeed, the interaction terms were significant for a number of the outcome variables for the person with dementia, indicating that the main effects (in this case non-significant) should be interpreted with caution.
The data collected in this trial include a comprehensive set of measures on coping and social support, the secondary analysis of which will increase our understanding of the interplay of these variables. In particular, by collecting information from both family carers and people with dementia we have the opportunity to carry out analyses at the level of the dyad. This is something that has been long called for, but as yet rarely executed in the field of dementia care. Multilevel analyses are becoming increasingly popular in other fields in which interactions within the dyad influence the outcomes of each member of that dyad.
Conclusions
There is no indication of benefit to carers of providing one-to-one peer support or group reminiscence sessions. However, there is an indication that group reminiscence has the potential to be effective in maintaining the quality of life of people with dementia, but not cost-effective as the cost per QALY is considerably above the NICE threshold. Using a factorial design requires the assumption that interventions are independent of each other, but the finding of significant interactions between the two main effects meant that this assumption of independence was violated. However, the study was not adequately powered for an analysis of each intervention with TAU.
For more information on the SHIELD carer supporter manual, please contact the R&D office at NELFT or e-mail SHIELD@nelft.nhs.uk.
Chapter 4 Home treatment programme: development of a home treatment package for people with dementia and their family caregivers
Background
There is growing recognition of the need to provide alternatives to hospital admission for older people in both general and psychiatric settings, particularly for those with dementia,165 in whom crises often lead to reduced quality of life and admission to either hospital or a care home. A recent study showed that 42% of individuals aged > 70 years with unplanned admission into an acute hospital had dementia, rising to 48% in those aged > 80 years. 166 People with dementia can be difficult to discharge from hospital and often have longer stays than those without. 166 Older people with mental health problems were generally excluded by the home treatment services developed in the wake of the National Service Framework for Mental Health,167 and those with mental illness by the intermediate care services established following the recommendations of the National Service Framework for Older People. 4 Studies of HTTs for physically ill older people have shown that these services help to reduce the number of acute admissions and length of hospital stay. 168,169
Objective
The HTP will develop a model of intensive home support to help manage crises at home and prevent admission to hospital for people with dementia.
Work package 1: Cochrane review/systematic reviews
A systematic review and meta-analysis evaluating the effectiveness and impact of case/care management approaches to home support for people with dementia
A systematic review and meta-analysis evaluating the effectiveness and impact of case/care management approaches to home support for people with dementia was conducted with the Cochrane Collaboration Cognitive Impairment and Dementia group, based in Oxford, UK. 169,170 Case management can be defined as a strategy for organising and co-ordinating care services at the level of the individual patient, with the aim of providing long-term care for people with dementia as an alternative to admission to a care home or hospital. The review followed the Specialised Register of the CDCIG, called ALOIS. This yielded 139 studies, of which 11 were RCTs meeting the inclusion criteria. An updated search in February 2012 identified a further two studies which were also included. The analysis comprised 9615 subjects.
Objectives
Primary objective
To evaluate the effectiveness of case management approaches to home support for people with dementia from the perspective of the different people involved (patients, carers and staff), compared with other forms of treatment including TAU, standard community treatment and other non-case management interventions, on delaying institutionalisation, improving quality of life and/or reducing the number of hospital admissions.
Secondary objective
To study whether or not other potential mediating variables affect case management outcomes (e.g. key structural and organisational features of case management interventions and also the methodological characteristics of studies).
Review methods
Protocol and registration
The protocol was registered with The Cochrane Library (http://archie.cochrane.org).
Criteria for considering studies for this review
Randomised controlled trials of case management interventions for people with dementia who lived in the community and their carers were included, if they had been published and written in English, peer reviewed and presented in a journal article. Authors were contacted for missing data, such as details of randomisation, means and SDs.
Search methods for identification of studies
The search methods included a combination of the search terms patient care management, case management, intensive case management, care management, managed care programs, care co-ordination, care pathway and managed care, which were used to search ALOIS, Specialised Register of the CDCIG and EPOC (Effective Practice and Organisation of Care) register between November and December 2008, and was updated in February 2012. The studies were identified from the Specialised Register of the CDCIG. This register contains records from the following major health-care databases: The Cochrane Library, MEDLINE, EMBASE, PsycINFO, CINAHL and LILACS, as well as ongoing clinical trial databases and other grey literature sources for the most recent records. We contacted the first authors of important identified RCTs that were potentially suitable for inclusion to request additional information on related new, unpublished or in-press studies that had not been identified in the main search. We also cross-checked the reference lists and citation reports of trials and relevant systematic reviews as identified by the above methods. Additional searches were made of the following sources: Web of Science [including Science Citation Index Expanded (SCI-EXPANDED) and Social Science Citation Index] and the Campbell Collaboration/SORO database. We also searched the Specialized Register of the Cochrane Effective Practice of Care Group using the search terms dementia OR demented OR Alzheimer in any fields. A total of 10,440 references were retrieved from the November 2008 initial search. After deduplication and a first assessment, the authors were left with 145 references to further assess for inclusion and exclusion or discard.
Participants
Participants were any age and sex with a diagnosis of dementia (Alzheimer’s disease, vascular dementia or mixed Alzheimer’s and vascular dementia, other types of dementia) who lived in the community (excluding people in institutions receiving 24 hours of care), and their carers. Studies which focused on patients only or both patient and carer dyads were included, whereas those which focused exclusively on carers were not included.
Intervention
Participants received any case or care management intervention delivered in the community (not in hospital/residential care settings). Interventions were screened to ensure that they predominantly focused on the planning and co-ordination of services. The intervention needed to be compared with TAU, standard community treatment, other non-case management or waiting list controls.
Outcome measures
Outcomes related to either patients or to patient–carer dyads and were grouped into short- (6 months), medium- (10–18 months) and long-term (24–36 months) time points. For the person with dementia, the outcome measures needed to record levels of institutionalisation (admissions to hospital or care homes and length of stay), mortality, quality of life and assessment of any of the following variables: cognition, behaviour, mood, ADLs, service use and cost. Family caregiver outcomes such as self-reported quality of life, burden or distress, depression and anxiety, social support and satisfaction with the intervention were considered. Cost and attrition were recorded when available. Studies which focused exclusively on carer outcomes were not included.
Data collection and analysis
Searches were conducted as detailed above to identify all relevant published studies. The date and time of each search, together with details of the version of the database used, were recorded. Additional information was sought, as outlined above, and hard copies of articles were obtained.
Quality assessment
Three reviewers independently screened the identified RCTs for inclusion and the final list of included studies was reached by consensus. Trials not meeting the criteria were excluded. The studies were assessed against a checklist of quality requirements using the Cochrane approach (see Figure 22), as follows.
-
Grade A: ‘low risk’ – adequate concealment (randomisation; concealed allocation).
-
Grade B: ‘unclear risk’ – ‘randomised’, but methods uncertain.
-
Grade C: ‘high risk’ – inadequate concealment of allocation or no randomisation, or both.
No studies were excluded because of poor quality in the review. Again, the reviewers worked independently to ascertain which studies met the quality criteria, and consensus was reached through discussion. Attempts were made to obtain additional information from the study authors when needed.
Data extraction
Descriptive characteristics (such as quality of randomisation and blinding) and study results were extracted, recorded and entered into RevMan 5.1. Additionally, letters and e-mails were sent to some authors of controlled trials asking for essential and additional information (e.g. statistics, sources of bias and details of randomisation). The summary statistics required for each trial and each outcome for continuous data were the mean change from baseline (if reported), the SD of the mean change and the number of patients for each treatment group at each assessment. The baseline assessment was defined as the latest available assessment prior to randomisation, but no longer than 2 months to after randomisation. Categorical responses were extracted for categorical outcome data (e.g. admitted to hospital/not admitted). For the meta-analysis of follow-up data, the most frequently assessed time points were combined across trials (3, 6, 12, 18 and 36 months). To allow an intention-to-treat analysis, the data were sought irrespective of compliance, whether or not the patient was subsequently deemed ineligible or otherwise excluded from treatment or follow-up. If intention-to-treat data were not available in the publication, ‘on-treatment’ data were sought (i.e. the data of those who completed the trial). Discussion between the three reviewers and the other authors was used to resolve any queries.
Data analysis
RevMan 5.1 was used for the meta-analysis. The meta-analysis required the combination of data from trials using the same rating scale/test or a different rating scale/test to assess an outcome. The measure of the treatment difference for any outcome was the weighted mean difference when the pooled trials used the same scale and the SMD, the absolute mean difference divided by the SD, when different rating scales were used. Summary statistics (n, mean and SD) were required for each rating scale at all assessment points, for both treatment groups in each trial for change from baseline. For continuous data (or ordinal data approximating a normal distribution), the mean change from baseline, the SD and the number of patients for each treatment group at all assessments were analysed. For binary outcomes, such as clinical improvement or no clinical improvement, the odds ratio was used to measure treatment effect. The treatment differences from both fixed- and random-effects models were examined and a test for heterogeneity was performed using a standard chi-squared statistic and an I2-statistic. When a significant degree of heterogeneity was present, a random-effects model was preferred. The reviewers achieved consensus on the interpretation of the statistical analyses, seeking specialist statistical advice from the CDCIG as required. Non-randomised studies were described in tabular form and the reviewers discussed and reached consensus on the presentation of the findings in the background to the review.
Studies included in the review
One hundred and forty-five studies were identified through the literature search. Three reviewers independently assessed eligibility. Of the 145 references, 11 studies met the inclusion criteria and were included in the analysis. The trial search co-ordinator (AS) carried out the initial screening of these and 99 references were inspected by two reviewers. Two further trials were identified as eligible for inclusion in the review. Therefore, a total of 13 studies was included in the analysis (Figure 25).
FIGURE 25.
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis flow chart of the review and selection process of studies.
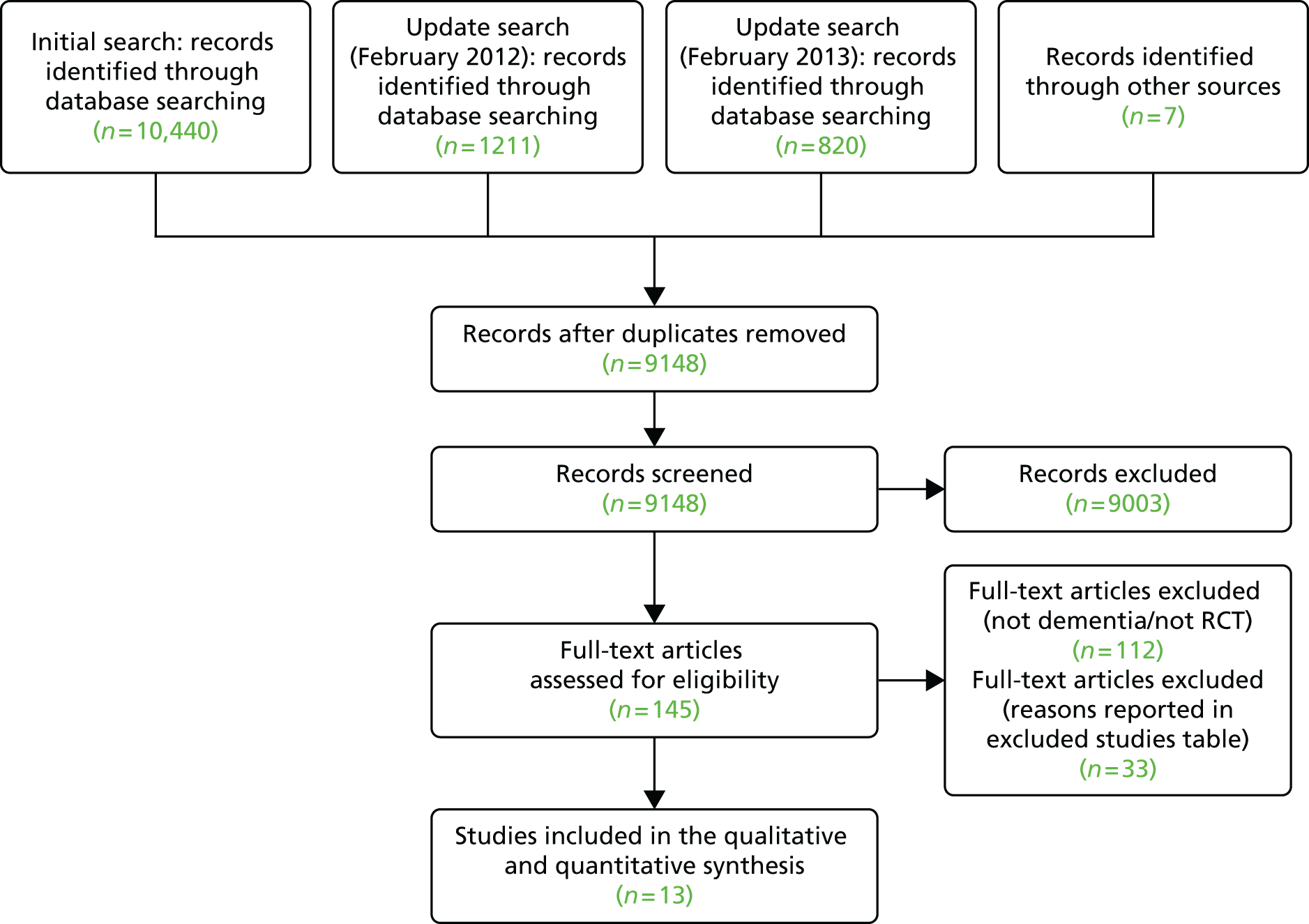
Quality of included studies
The quality of each study was assessed according to the four criteria outlined in the Cochrane Collaboration Handbook:171 selection bias, performance bias, attrition bias and detection bias. Details of biases and description of studies can be seen in Figure 26. Performance bias was difficult to evaluate. With psychological interventions, unlike drug trials, it is impossible to totally blind patients and staff to treatment. Patients are often aware that they are being treated preferentially, staff involved may have different expectations of treatment groups and independent assessors may be given clues from patients during the assessments. There may also be ‘contamination’ between groups, in terms of groups not being held in separate rooms and staff bringing ideas from one group to another.
FIGURE 26.
Risk of bias for all studies.
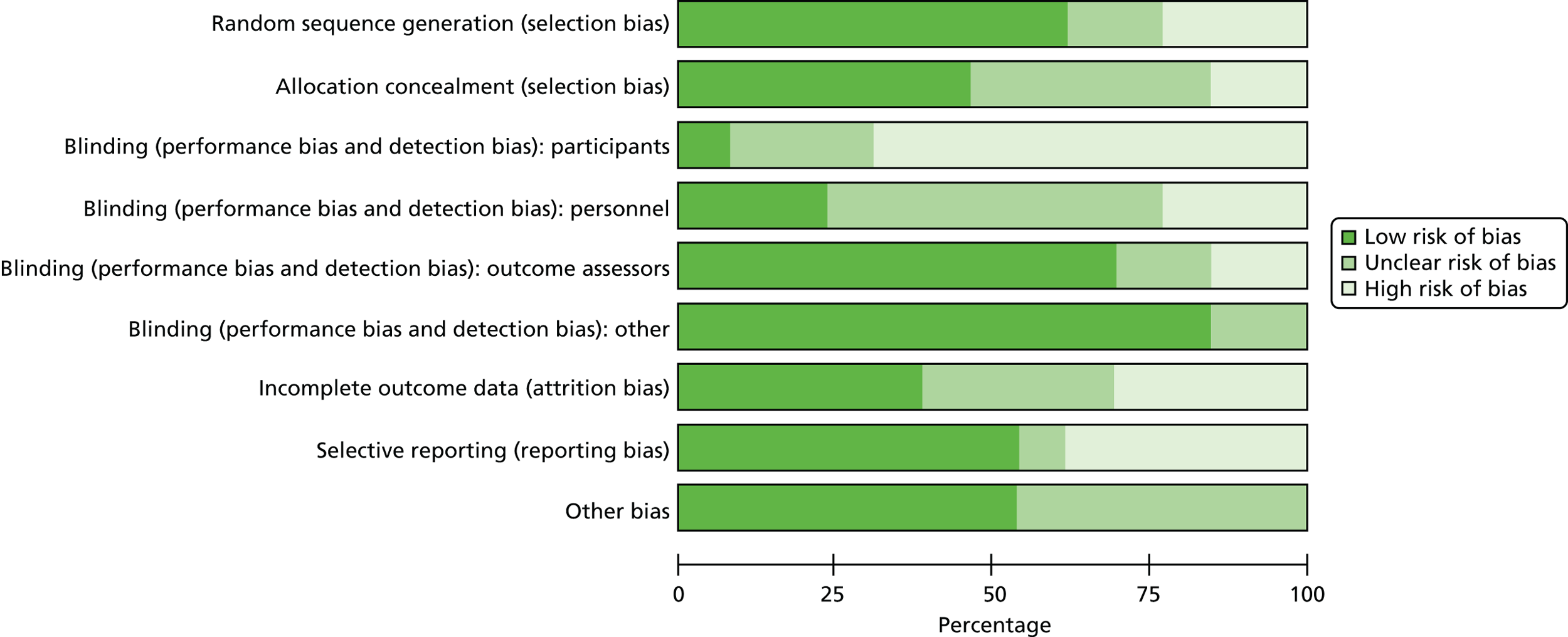
Results
We included 13 trials (9615 participants) in this review, although interventions varied somewhat. Four trials provided data on admissions to either residential or nursing homes, and results significantly favoured the case management group at 6 months (n = 5561, four RCTs; odds ratio 0.83, 95% CI 0.70 to 0.99; I2 = 0%; p = 0.04) but not for other time points (Figure 27). We detected a reduction in hospital admissions (mean number of nights) in the intervention group at both 6 (n = 341, three RCTs; mean difference 0.63, 95% CI 0.40 to 0.86; I2 = 40%; p = 0.00001) and 12 months (n = 575, six RCTs; mean difference –1.94, 95% CI –3.68 to 0.60; I2 = 99%; p = 0.03). Ten trials recorded deaths but there were no significant differences between groups at 4–6, 12, 18–24 or 36 months’ follow-up.
FIGURE 27.
Institutionalisation. Studies listed can be found in the Cochrane review. M–H, Mantel–Haenszel.
Eight trials assessed carer burden and benefit for the case management group was found at 6 months (n = 4726, five RCTs; SMD –0.07, 95% CI –0.12 to –0.01; I2 = 2%; p = 0.02). Three of the trials measuring carer depression at 18 months showed greater improvement in the case management group (n = 2888, three RCTs; SMD –0.08, 95% CI –0.16 to –0.01; I2 = 0%; p = 0.03).
Three trials assessed quality of life of patients or carers with various scales at different time points. There was no clear evidence that case management improved quality of life for either group. Data on carer well-being and at 6 months found a significant improvement was shown in the case management group (n = 203, one RCT; SMD –2.53, 95% CI –5.20 to 0.13; p = 0.03).
Six trials provided data on behaviour problems, which significantly favoured case management at 10–12 (n = 433, five RCTs; SMD –0.18, 95% CI –0.39 to 0.03; I2 = 18%; p = 0.05) and 18 months (n = 255, three RCTs; SMD –0.29, 95% CI –0.53 to –0.04; I2 = 0%; p = 0.02) (Figure 28). There was no evidence of benefit to patient depression (three trials), functional abilities (three trials), cognition (six trials), carer distress (three trials) or carer mood (five trials) at any time point. The use of services varied, with the intervention group receiving significantly more community services.
FIGURE 28.
Challenging behaviour. Studies listed can be found in the Cochrane review. IV, inverse variance.
Discussion
All but three of the RCTs had a duration of ≥ 12 months but only six trials lasted for ≥ 18 months. The studies included in this review came from a variety of countries and contexts (from the USA, Canada, Finland, the Netherlands, Hong Kong, India and the UK); were set in primary care practices, dementia resource centres, memory clinics outpatient clinics, self-referrals, day centres and GP practices; and were administered by case managers from a range of professional groups.
This review provides some evidence for the benefits of case management in terms of (1) reduced hospital length of stay at 6 and 12 months, (2) reduced behaviour disturbance at 12 (five trials) and 18 months (three trials), (3) reduced carer burden (three trials) and (4) improved carer well-being (only one trial). There are signs that although case management, as intended, involves a higher use of community services, this may be offset by a lower use of acute services and hospitalisations, but on the evidence available it is not clear how it may affect overall health-care costs. In most of the studies, case management was just one aspect of a broader programme of care, making it difficult to study the specific effects in detail. This review indicates that there is evidence to show that case management is beneficial and effective for both the person with dementia and their carer. However, there was heterogeneity of the interventions, outcomes measured and time points across the 13 included trials. Further work is needed to identify what aspects of case management (or service models) are associated with most improved outcomes.
The finding that case management for people with dementia reduces admission to long-term care in the short term (< 12 months) appears to be one of the most consistent in the literature. Case management reduced behavioural problems in people with dementia in both the short- and the long-term follow-up, but did not appear to improve patient outcomes such as patient quality of life, cognition and depression. In addition, hospital admissions and mortality were unaffected.
Case management also reduced carer burden in both the long and the short term. Interestingly, case management did not benefit carer’s mood or distress. There was very limited evidence reported on use of services and service use costs, and indications that case management leads to higher use of community-based services. However, there was little indication that case management affected health-care use. Consequently, service use costs appeared to be higher in the case management intervention groups.
In this review, case management focused on the planning and co-ordination of services required to meet the identified needs of the person with dementia, although the forms of case management differed. The core tasks of assessment, care planning and implementation/management were common to all but one trial, but there was considerable variation in their delivery. Most studies used face-to-face contact to deliver case management but one used solely telephone contact. Intensity of the case management varied, frequency of contact between the case managers and the patients/carers varied from 1 to 2 or more contacts per month, and caseload size ranged between 13 and 100 patients. Length of intervention varied between 4 months and 2 years. However, given the limited data available for the long-term effects of case management, it is difficult to conclude whether these observed effects are due to the duration or frequency of the intervention or other mediating variables. This highlights the need for consistency in the choice of outcome measures in the studies.
The case management interventions described across the studies had varying objectives and goals. In many studies the case management interventions were specifically targeted at predetermined outcomes (e.g. carer burden or institutionalisation), so it is possible that other beneficial effects of the interventions were not measured. There was appreciable variability in the case management approaches, content of case management interventions, target populations, degree of control and influence over allocation of care resources, and intensity and duration. As the case management interventions varied considerably, this made it difficult to link outcomes to the specific components of the interventions. The effect sizes may not be independent and so a more powerful multilevel analysis might have assisted in the disentangling of the effects.
Only two of the studies reported data on use of prescribed medications, and there were not enough data to draw reliable conclusions about whether or not certain prescribed medications have an influence over the effectiveness of case management interventions. This could be considered in future research studies.
Access to forms of case management or other services were present in some of the control groups for studies included in this review. The use of case management was measured among the control groups in this study, and there was a reported significant difference in the numbers receiving case management in the interventional and control arms, but there may be some degree of contamination in the results. One study reported that the control group also had access to the standard home care programme. It was noted in the Newcomer study that control group cases may have been exposed to comparable benefits, such as case management and community care benefits, if they were participating in the Medicaid programmes. For this reason, the demonstration programmes were encouraged not to seek or accept applications from those receiving Medicaid. However, there were still around 7% of participants each in the treatment and control groups who were Medicaid recipients. However, statistical controls were put in place to adjust for the potential effect of Medicaid participation. Our results did not illuminate any particular effects of these differences in control conditions on the outcomes.
The Newcomer trial172,173 was problematic during the analyses, as it used two models of case management, with one that offered a higher community service reimbursement and more access to the case manager. For most outcomes we used the results of the two models combined. In addition, this study had very large sample sizes in comparison with the other studies and so heavily weighted the results in the meta analyses. Consequently, we conducted the analyses with and without this study, and for some outcomes we found that the results were no longer significant if this study was removed (e.g. institutionalisation).
The exclusion of studies for this review was usually because they were not RCTs, did not focus on people with dementia or did not meet the criteria for a case management intervention. We defined case management as any intervention delivered in the community predominantly focused on the planning and co-ordination of services to meet the needs of the person with dementia.
The quality of the included studies is variable but most were free of selection bias owing to the use of adequate methods for random sequence generation and allocation concealment. However, all of the studies included in the review were subject to some level of performance bias, where either the participants or the case managers or both were unblinded. However, 9 out of the 13 studies had blinded outcome assessors and the others were either high risk or unclear but, overall, there was a low risk of detection bias. There were huge variations in the sample sizes within studies. One study had 8095 participants and another study had only 81 participants. Most studies had between 100 and 200 participants. Attrition bias was low overall across the studies.
For each outcome measure, to allow an intention-to-treat analysis, the data were sought irrespective of compliance, whether or not the patient was subsequently deemed ineligible or otherwise excluded from treatment or follow-up. If intention-to-treat data were not available in the publication, ‘on-treatment’ data were sought (i.e. the data of those who completed the trial). Data on adverse effects of attrition were noted. Reporting bias on the whole was classified as low risk in this review but four studies were rated at high risk of reporting bias as they did not report data on all of the outcomes specified in the study.
Future studies should use process measures to demonstrate the extent to which case management is delivered and provided. Process evaluations are particularly important for interpreting outcomes and for understanding how an intervention is implemented across multiple sites. Although 7 of the 13 studies reported using standardised protocols, the use of well-developed manuals and protocols should be more widespread, as they can help to ensure the transparency, replicability and integrity of this complex intervention. This highlights the need for greater consistency in process level and quality-of-care indicators (which systematically describe how the interventions are implemented). These could include the number of people with a care plan and how it is often monitored, reviewed and updated; the number of times the person is visited, followed up or telephoned by the case manager; and the number of telephone calls or contacts that the case manager makes contacts on behalf of the person with dementia or carer. Future studies should consider including quality measures such as these to help to ascertain the active ingredients of case management by relating these to outcomes. These process indicators are not necessarily good outcomes, as the acid test of an outcome measure is the extent to which you can value it in its own right (i.e. it is not a proxy). In general, however, the overall quality of the included studies is reasonable, particularly in the more recent studies that have more detailed information on case management components and delivery, participants’ information, inclusion/exclusion criteria, the use of consistent outcome measures and increased sample sizes.
Case managers delivering the intervention were from a range of professional backgrounds (nurses, social workers, occupational therapists and psychiatrists) and were based in a variety of settings including primary care and dementia resource centres. The training that the case managers received to deliver case management also ranged considerably between the trials in terms of both the mode of provision of training and the content. Only three trials reported on provision of dementia training for their case managers and several of the studies did not report any details on training for the case managers. The case manager was responsible for the co-ordination of services and treatment between organisations and agencies. It would appear that in only three of the studies the case managers were taking responsibility for managing the wider care network. In many other studies they appeared to be more focused on co-ordinating the work of their own service alone, which represents a narrower focus of case management responsibility. Such differences in case manager involvement and range and breadth of responsibilities taken by case managers are likely to be critical determinants of variations in outcome.
Studies included a high variability of patients and carers. This variability reflected the severity of dementia. Five studies included both mild and moderate severity. Three studies included predominantly moderate and four studies included mostly mild dementia. Within this review, there was no indication that the severity of the dementia had any relationship to the efficacy of the intervention. However, further work may be necessary to ascertain whether the severity of dementia or other subgroups are more or less likely to benefit from case management.
A number of trials reported participants with significant comorbidities whereas others excluded those with physical comorbidities. There was perhaps less variability in social characteristics and only one study reported that a high proportion of patients were socioeconomically disadvantaged.
Two previous reviews have been completed in this area. Pimouguet et al. 174 reviewed 12 trials, seven of which we included. As in our review, Pimouguet et al. 174 noted the effects of delaying institutionalisation for people with dementia, but also concluded that there was not sufficient evidence to draw conclusions about the effects of case management on costs and resource utilisation. The most recent review by Somme et al. 175 included six studies, five of which were included in this review. Somme et al. 175 concluded that more effective case management related to both better integration between the health and social service organisations, and the intensity of the case management. Olazarán et al. 69 reviewed 179 RCTs across 26 categories of non-pharmacological interventions. They concluded that there was grade B evidence for multicomponent interventions for people with dementia and their carers in relation to cognition, ADLs, behaviour, mood for person with dementia and carer, quality of life of person with dementia and carer, and carer psychological well-being. Other systematic reviews on efficacy of non-pharmacological interventions have also shown positive benefits for the person with dementia and the carer,8 although they have not considered the efficacy of case management specifically within the reviews.
Finally, consideration should be given to the possibility of publication bias in this review. Trials that do not produce positive findings appear less likely to be published and this can lead to a risk of a biased set of studies included in systematic reviews. However, there is likely to be very low risk of publication bias for this review, as our comprehensive search strategy did not restrict searches to peer-reviewed journals only; for example, the Jansen et al. 176 study was a PhD (Doctor of Philosophy) conducted in the Netherlands and was included as a trial in this review. We cannot rule out the possibility that we have missed unpublished trials with negative results but, overall, the risk of publication bias in this review was low. In future, the publishing of trials based on their results should be less of a problem as many trials are required to be registered with a recognised clinical controlled trials register and many trial protocols are being published.
Managing crises for people with dementia: a systematic review
Introduction
People with dementia are at increased risk of acute admission to hospital and generally have poorer outcomes than those without dementia. There is an increasing body of evidence reporting on physical health problems among people with dementia, including falls, fractures, seizures, infections and pneumonia, which are highly associated with hospital admissions. Psychiatric factors, particularly behavioural problems, are also a key risk factor for hospital admissions and many people admitted to hospital with dementia enter institutional care on discharge, rather than returning to their own homes.
Aims
To conduct a systematic review and meta analysis of literature of the factors leading to hospital admission for people with dementia in comparison with (1) people without dementia acutely admitted and (2) people with dementia remaining in the community.
Methods
Types of papers included in the review
Controlled comparison studies, which included cohort studies, epidemiological studies, case–control studies, systematic literature reviews and descriptive studies, were eligible for inclusion in this review.
Types of risk factors
The following categories of risk factors for people with dementia associated with hospitalisation were considered for inclusion in this review: physical problems, psychiatric problems, carer factors and environmental factors.
Types of comparison groups
In this review we included two sets of group comparison data:
-
risk factor profiles in people admitted to hospital comparing those with dementia and those without
-
risk factor profiles in people with dementia comparing people admitted to hospital with people in the community.
Types of outcome measures
The outcomes in this review included the following:
-
number of patients admitted to hospital
-
total number of admissions to hospital
-
time to hospitalisation.
Types of participants
The inclusion criteria for the studies were that the samples contained:
-
people aged ≥ 60 years
-
people with a dementia diagnosis of any type.
Search methods for identification of studies
The NHS electronic library records were searched, which contained records from the following major health-care databases: MEDLINE, EMBASE, PsycINFO, CINAHL, Web of Science and PubMed for the period 1999 to 30 June 2010.
The search terms were old, elder, aged, memory problems, memory disorders, cognition, cognitive disorders, dementia, Alzheimer’s, vascular, fronto-temporal, predictors, causes, crisis, indicators, risk profiles, risk factors, model, risk assessment, clinical indicators, prediction, trends, forecasting, probability, prevalence, emergency, hospital, psychiatric, hospitalisation, patient admission, patient transfer, patient readmission, institutionalisation, admissions, nursing home, care home, long term care.
Reference lists of key papers were checked and relevant systematic reviews were identified by the above methods.
Data collection and analysis
Selection of studies
Titles and abstracts of citations obtained from the search were examined independently by two researchers and any obviously irrelevant articles were discarded. The full texts of those studies deemed potentially relevant were obtained. When it was not possible to accept or reject based on title and abstract alone, the full text of the citation was obtained for further evaluation. The assessment of eligibility criteria was undertaken from the full text by two reviewers (Sandeep Toot and Mike Devine). In addition, the third reviewer (Martin Orrell) independently reviewed the selected studies and agreement was reached on which papers should be included.
Assessment of validity
For all references, the studies were assigned a level of evidence according to the Centre for Evidence Based Medicine (CEBM; www.cebm.net/levels_of_evidence.asp#levels) guidelines. Levels of evidence ranged from 1 to 5, with lower numbers indicating higher quality. Studies rated between levels 1 and 4 were included in this review. Two reviewers (Sandeep Toot and Mike Devine) assigned levels of evidence to each study independently and, in cases of disagreement, discussed the studies until a conclusion was agreed. Grades of recommendation for risk factors across the studies were then assigned an overall grade of evidence from A to D according to the CEBM criteria (grade A represents consistent level 1 studies, indicating the best-quality evidence, and grade D is the lowest level of evidence, representing level 5 evidence or troublingly inconsistent or inconclusive studies at any level). In addition, the third reviewer (MO) independently reviewed the assigned levels of evidence and grades of recommendation for the studies and agreement was reached on the assignments and gradings.
Results
Included studies
In total, 2938 references were identified through the searches, of which 2765 were excluded by reference to title and abstract alone, as they did not cover risk factors associated with crisis. A further 69 papers were excluded on the basis that (1) they did not include people with dementia aged > 60 years in their sample, or (2) they did not explicitly report risk factors associated with crisis involving people with dementia. Of the remaining 104 papers, four were systematic reviews, for which the reference lists were checked. However, no new papers were identified. Ninety papers were excluded on the basis that they reported risk factors associated with crisis for people with dementia leading to nursing home placement. Ten studies were included in this review and assigned to level 2 or 4 according to the CEBM guidelines, comprising nine prognostic cohort studies166,177–184 and one case–control study185 (Table 43).
| Study (year) and country | Level of evidence CEBM | Description of study sample | Type of admission | Study period | Type of study |
|---|---|---|---|---|---|
| Nourhashémi et al.,178 (2001) France | 4 | Dementia group: Alzheimer’s disease Non-dementia group: elderly controls |
Dementia group: Alzheimer’s acute care unit Non-dementia group: emergency general hospital admissions |
4-month study (January–April 1997) | Prognostic cohort study (prospective) |
| Malone et al.,183 (2009) USA | 4 | Dementia group: Alzheimer’s disease Non-dementia group: matched elderly controls |
Acute hospital admissions | 6 years (retrospective study 2000–6) Information collected via a health insurance and pharmacy database |
Prognostic cohort study |
| Natalwala et al.,182 (2008) UK | 4 | Dementia group: unspecified dementia, Alzheimer’s disease and vascular dementia Non-dementia group: healthy elderly controls |
General hospital admissions (emergency, elective, planned and day care admissions) | 5-year retrospective study (2002–7) | Prognostic cohort study |
| Tuppin et al.,184 (2009)a France | 4 | Dementia group: all types of dementia including Alzheimer’s disease Non-dementia group: elderly controls aged > 60 years |
All types of general hospital admissions | Retrospective information extraction from a national health insurance database (2007) | Prognostic cohort study |
| Carter and Porell,181 (2005) USA | 4 | Dementia group: Alzheimer’s disease and related dementias Non-dementia group: elderly controls |
Any type of acute hospitalisation (ambulatory care-sensitive conditions: preventable hospital admissions) | Retrospective 3-year prognostic cohort study (1991–3) | Prognostic cohort study |
| Sampson et al.,166 (2009) UK | 2 | Dementia group: any type of dementia diagnosis Non-dementia group: elderly controls aged > 70 years |
Unplanned acute general hospital admissions | Longitudinal study over 6 months (June–December 2007) | Prognostic cohort study (prospective) |
| Albert et al.,177 (1999) USA | 4 | Dementia group: Alzheimer’s disease Non-dementia group: elderly controls |
Acute general hospital admissions | Retrospective study over 21 months (January 1996–September 1997) | Prognostic cohort study |
| Orrell and Bebbington,185 (1995) UK | 4 | Dementia group: all types of dementia | Day patients or inpatients to a psychogeriatric assessment unit | 6 months prior to admission were investigated but for this review we will only consider 3 months preceding the index date | Case–control study |
| Ibach et al.,180 (2004) | 4 | Dementia group: dementia with frontotemporal lobar degeneration | Admissions to a geriatric psychiatry hospital | Not given | Prognostic cohort study |
| Andrieu et al.,179 (2002) | 2 | Dementia group: Alzheimer’s disease | All types of acute general hospital admissions (general, psychiatric, specialised unit) | 12-month study | Prognostic cohort study (prospective) |
Studies comparing hospital admissions for people with or without dementia
The risk factors reported below were all categorised into broader themes by the research team. The key themes and risk factors can be found in Table 44.
| Primary reason system | Subcategory (where specified) | Study (year) | Level of evidence CEBM | Proportion of all admissions (dementia group) | Proportion of all admissions (non-dementia group) | RR (95% CI; p-value) of given factor being primary cause of admission |
|---|---|---|---|---|---|---|
| Orthopaedics | Falls/injury | Nourhashémi et al.178 (2001) | 4 | 22/118 | 698/6891 | 1.84 (1.25 to 2.70; 0.002) |
| Fractures (all) | Malone et al.183 (2009) | 4 | 955/5396 | 428/5396 | 2.23 (2.00 to 2.48; < 0.001)a | |
| Hip fracture | Natalwala et al.182 (2008) | 4 | 167/2561 | 835/53,123 | 4.15 (3.53 to 4.87; < 0.001) | |
| General (including falls/fractures) | Tuppin et al.184 (2009)b | 4 | 20,855/263,985 | 4595/63,985 | 1.10 (1.07 to 1.13; < 0001) | |
| Respiratory | Infection | Carter and Porell181 (2005) | 4 | 110/1782 | 123/2195 | 1.10 (0.86 to 1.41; 0.450) |
| Sampson et al.166 (2009) | 2 | 62/262 | 29/355 | 2.90 (1.92 to 4.37; < 0.001) | ||
| Natalwala et al.182 (2008) | 4 | 331/2561 | 1614/53,123 | 4.25 (3.81 to 4.76; < 0.001) | ||
| General (including infection) | Tuppin et al.184 (2009)b | 4 | 16,103/263,985 | 2168/63,985 | 1.80 (1.72 to 1.88; < 0.001) | |
| Urology/renal | Infection | Carter and Porell181 (2005) | 4 | 51/1782 | 47/2195 | 1.34 (0.90 to 1.98; 0.146) |
| Sampson et al.166 (2009) | 2 | 37/262 | 17/355 | 2.95 (1.70 to 5.12; < 0.001) | ||
| Natalwala et al.182 (2008) | 4 | 116/2561 | 440/53,123 | 5.47 (4.47 to 6.68; < 0.001) | ||
| General (including infection) | Tuppin et al.184 (2009)b | 4 | 33,262/263,985 | 6718/63,985 | 1.20 (1.17 to 1.23; < 0.001) | |
| Gastrointestinal | Infection | Carter and Porell181 (2005) | 4 | 80/1782 | 66/2195 | 1.49 (1.08 to 2.06; 0.015) |
| General (including infection) | Nourhashémi et al.178 (2001) | 4 | 17/118 | 676/6891 | 1.47 (0.94 to 2.29; 0.090) | |
| Tuppin et al.184 (2009)b | 4 | 17,951/263,985 | 4834/63,985 | 0.90 (0.87 to 0.93; < 0.001) | ||
| Cardiology | General | Tuppin et al.184 (2009)b | 4 | 15,839/263,985 | 3199/63,985 | 1.20 (1.16 to 1.25; < 0.001) |
| Acute cardiac syndrome | Sampson et al.166 (2009) | 2 | 12/262 | 43/355 | 0.38 (0.20 to 0.70; 0.002) | |
| Cardiovascular | Nourhashémi et al.178 (2001) | 4 | 11/118 | 1073/6891 | 0.60 (0.34 to 1.05; 0.076) | |
| Neurology | Tuppin et al.184 (2009)b | 4 | 39,334/263,985 | 1799/63,985 | 5.30 (5.06 to 5.55; < 0.001) | |
| Nourhashémi et al.178 (2001) | 4 | 9/118 | 1072/6891 | 0.49 (0.26 to 0.92; 0.027) | ||
| Psychiatric | General | Tuppin et al.184 (2009)b | 4 | 16,631/263,985 | 443/63,985 | 9.10 (8.28 to 10.00; < 0.001) |
| Behavioural disturbance | Nourhashémi et al.178 (2001) | 4 | 31/118 | 93/6891 | 19.47 (13.53 to 28.00; < 0.001) | |
| Other | Syncope/collapse | Natalwala et al.182 (2008) | 4 | 102/2561 | 777/53,123 | 2.72 (2.22 to 3.33; < 0.001) |
| Fever | Nourhashémi et al.178 (2001) | 4 | 13/118 | 951/6891 | 0.80 (0.48 to 1.34; 0.394) | |
| Infection (general) | Albert et al.177 (1999) | 4 | 29/70 | 52/191 | 1.52 (1.06 to 2.19; 0.024) | |
| Dehydration | Natalwala et al.182 (2008) | 4 | 79/2561 | 147/53,123 | 11.15 (8.51 to 14.61; < 0.001) |
For risk factors within the following four physical health-related themes, relative risks (RRs) were either directly extracted or calculated by the research team from the relevant studies. The data were then pooled in meta analyses and presented in forest plots for each theme.
Four prognostic cohort studies178,182–184 reported that people with dementia are at greater risk of an orthopaedic crisis (e.g. falls and all types of fractures) resulting in hospital admission than people without dementia. The RR for dementia inpatients versus non-dementia inpatients was 1.19 (95% CI 1.16 to 1.22; p < 0.001) (Figure 29).
FIGURE 29.
Orthopaedics meta-analysis and summary statistics.
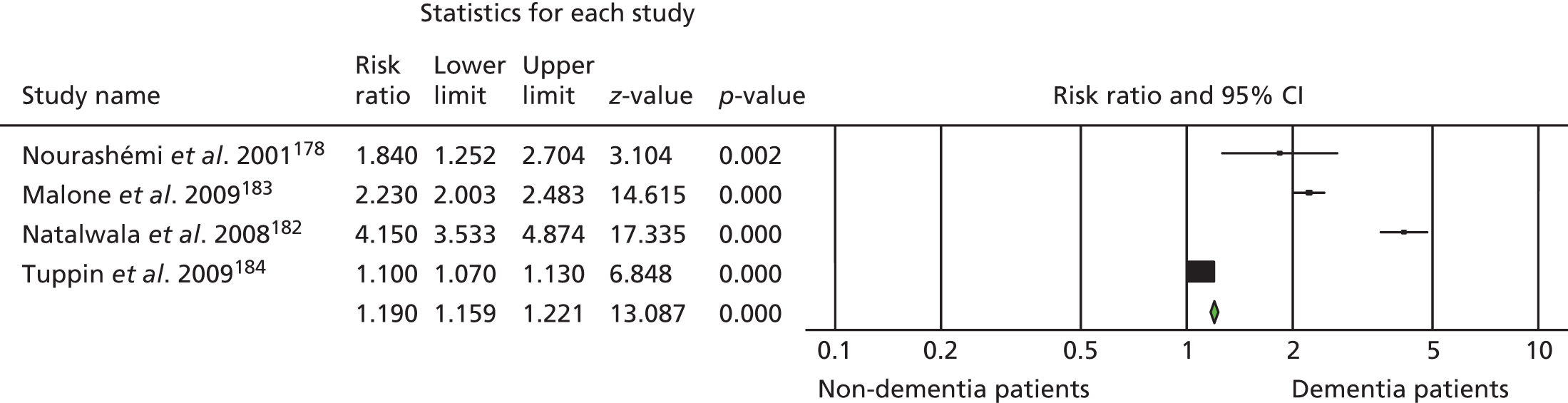
Four prognostic cohort studies166,181,182,184 investigated respiratory crises resulting in hospital admission. All but one of these reported that people with dementia are at greater risk of respiratory crisis resulting in hospitalisation than people without dementia (see Table 44). The RR for dementia inpatients compared with non-dementia inpatients was 2.00 (95% CI 1.92 to 2.08; p < 0.001) (Figure 30).
FIGURE 30.
Respiratory meta-analysis and summary statistics.
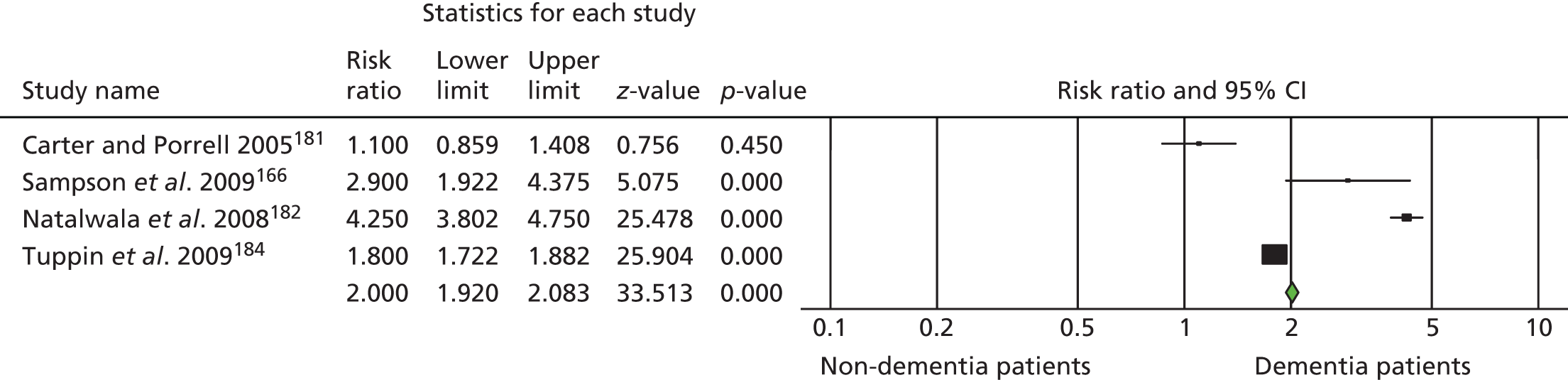
Four prognostic cohort studies166,181,182,184 reported results on urological/renal factors leading to hospital admission. All four reported that people with dementia are at greater risk than people without dementia of experiencing a urological crisis resulting in hospital admission (see Table 44). All except the Carter and Porell181 study reported statistically significant findings and, according to the meta-analysis, the RR for dementia inpatients compared with non-dementia inpatients was 1.23 (95% CI 1.20 to 1.26; p < 0.001) (Figure 31).
FIGURE 31.
Urology/renal meta-analysis and summary tables.
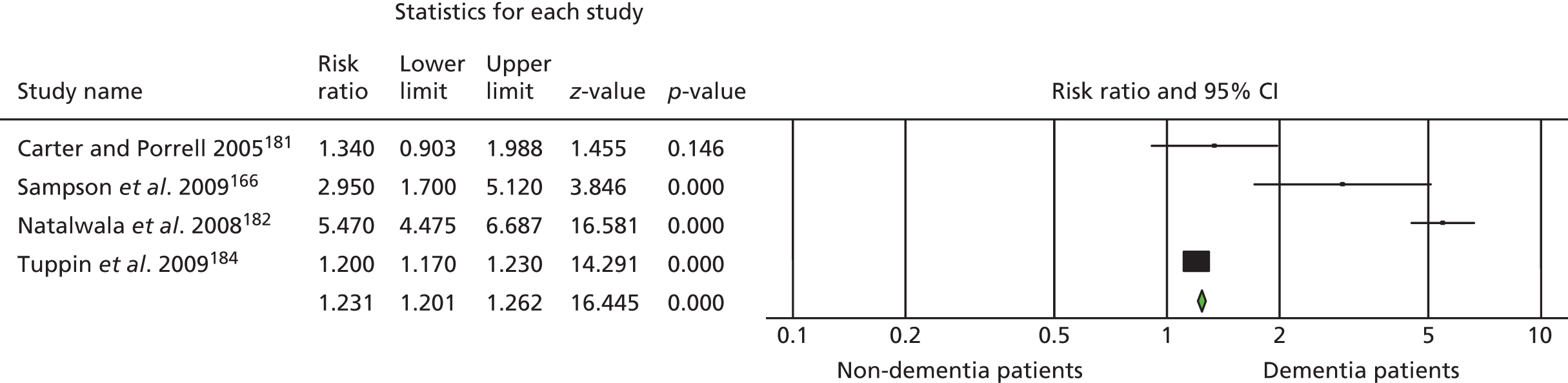
Three prognostic cohort studies178,181,184 informed the meta-analysis for gastrointestinal factors leading to hospitalisation, but there was no consistent pattern. Nourhashémi et al. 178 and Carter and Porell181 reported that people with dementia were more at risk of experiencing a gastrointestinal crisis resulting in hospitalisation than people without dementia. However, the evidence in the Nourhashémi et al. 178 study was not statistically significant. In contrast, Tuppin et al. 184 found that people with dementia had a lower risk of experiencing a gastrointestinal crisis resulting in admission than those without dementia (see Table 44). A meta-analysis gave a lower RR of 0.91 (95% CI 0.88 to 0.94; p < 0.001) for dementia versus non-dementia inpatients (Figure 32).
FIGURE 32.
Gastrointestinal meta-analysis and summary statistics.
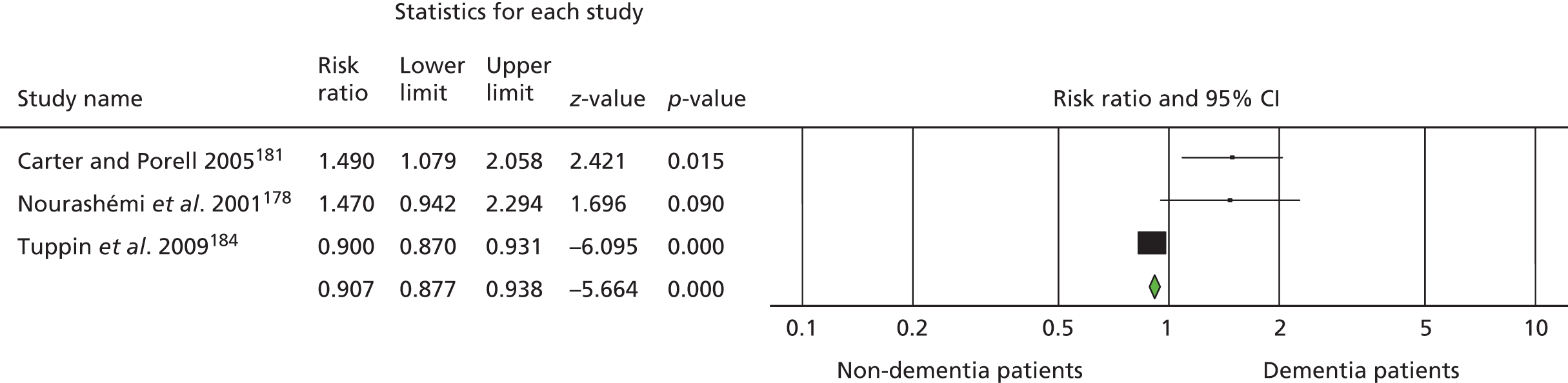
For risk factors within the following physical health-related themes, RR were again either directly extracted or calculated by the research team from the relevant studies. However, reported data were not pooled by meta-analysis for these factors owing to potential heterogeneity between risk factors included within these themes.
Three prognostic cohort studies166,178,184 reported data on cardiological factors leading to hospital admission (see Table 44). Tuppin et al. 184 reported that people with dementia had a significantly higher risk than those without dementia of acute hospitalisation because of general cardiological factors (RR 1.20, 95% CI 1.16 to 1.25; p < 0.001). In contrast, Sampson et al. 166 reported that people with dementia had a significantly lower risk than those without dementia of experiencing a crisis leading to hospitalisation due to acute cardiac syndrome (RR 0.38, 95% CI 0.20 to 0.70; p = 0.002). Similarly, Nourhashémi et al. 178 reported that people with dementia had a lower risk than those without dementia of experiencing a cardiovascular crisis resulting in hospital admission (RR 0.60, 95% CI 0.34 to 1.05; p = 0.076).
Nourhashémi et al. 178 and Tuppin et al. 184 reported contrasting evidence on neurological crises resulting in hospital admissions. Tuppin et al. 184 found that people with dementia had a much higher risk than those without of being hospitalised secondary to a neurological problem (RR 5.30, 95% CI 5.06 to 5.55; p < 0.001), whereas Nourhashémi et al. 178 found that people with dementia had a significantly lower risk of having a neurological crisis leading to hospital admission (RR 0.49, 95% CI 0.26 to 0.92; p = 0.027).
Several other physical health-related factors were reported across the studies. Natalwala et al. 182 reported a significantly higher risk of people with dementia being admitted to hospital due to syncope/collapse (RR 2.72, 95% 2.22 to 3.33; p < 0.001) and due to dehydration (RR 11.15, 95% CI 8.51 to 14.61; p < 0.001) compared with people without dementia. Nourhashémi et al. 178 found that people with dementia had a lower risk than people without dementia of experiencing fever leading to admission, but this finding was not statistically significant (RR 0.80, 95% CI 0.48 to 1.34; p = 0.394). Finally, Albert et al. 177 reported that people with dementia had a significantly higher risk than those without of being hospitalised due to infection (all types) (RR 1.52, 95% CI 1.06 to 2.19; p = 0.024).
Two prognostic cohort studies reported statistically significant findings relating to hospital admissions caused by a psychiatric crisis. Tuppin et al. 184 found that people with dementia had a much higher risk than of those without psychiatric crisis resulting in hospitalisation (RR 9.10, 95% CI 8.28 to 10.00; p < 0.001). Similarly, Nourhashémi et al. 178 found that people with dementia had a much higher risk of behavioural disturbance resulting in hospital admission (RR 19.47, 95% CI 13.53 to 28.00; p < 0.001).
Comparing people with dementia admitted versus not admitted
In the following analyses of people with dementia, RR refers to the probability of being admitted with a given risk factor relative to the probability of being admitted without this risk factor. In effect, this gives an estimate of the ‘admission risk multiplier’ conferred by the given risk factor for people with dementia (see Table 44). It was not possible to complete meta-analyses on these risk factors owing to either potential heterogeneity between risk factors included within these themes or lack of studies.
Three prognostic cohort studies177,179,180 reported data on behavioural problems in people with dementia. Ibach et al. 180 found that the presence of behavioural disturbance placed people with fronto-temporal dementia at a slightly higher risk of being admitted to hospital, but their finding was not statistically significant (RR 1.15, 95% CI 0.90 to 1.49; p = 0.277). They also reported that neither depressive symptoms (RR 0.75, 95% CI 0.45 to 1.26; p = 0.273) nor speech disturbances (RR 0.20, 95% CI 0.03 to 1.21; p = 0.088) were major precipitants of hospitalisation among people with fronto-temporal dementia. Similarly, Albert et al. 177 found that the presence of agitation placed people with dementia at a slightly higher risk of admission, but again this was not statistically significant (RR 1.30, 95% CI 0.81 to 2.10; p = 0.280). Andrieu et al. 179 reported specific behavioural problems which placed people with dementia at greater risk of being hospitalised, namely night-time agitation (RR 1.45, 95% CI 0.61 to 3.44; p = 0.400), wandering (RR 1.36, 95% CI 0.74 to 2.52; p = 0.325) and sleep disorder (RR 1.89, 95% CI 1.01 to 3.53; p = 0.046). Of these, sleep disorder was the only significant factor.
One case–control study185 considered the association of life events (environmental and social factors) with risk of admission for people with dementia living in the community and found that changes in both routine (RR 1.55, 95% CI 1.18 to 2.03; p = 0.002) and environment (RR 1.57, 95% CI 1.20 to 2.11; p = 0.001) significantly increased their risk of hospital admission.
Andrieu et al. 179 reported that increased dependency problems in several ADLs was associated with a higher risk of hospitalisation for people with dementia: bathing (RR 2.86, 95% CI 1.60 to 5.13; p < 0.001), dressing (RR 1.96, 95% CI 1.07 to 3.60; p = 0.030), toileting (RR 2.15, 95% CI 1.15 to 4.02; p = 0.017) and eating (RR 2.22, 95% CI 1.17 to 4.23; p = 0.015) all placed people with dementia at a significantly higher risk of being admitted to hospital. Continence dependency in people with dementia also placed them at greater risk of hospitalisation, but this finding was not statistically significant (RR 1.24, 95% CI 0.58 to 2.64; p = 0.578).
Finally, Andrieu et al. 179 reported that falls placed people with dementia at a significantly higher risk of being hospitalised (RR 1.76, 95% CI 1.08 to 3.64; p = 0.028).
Discussion
This is the first review to systematically assess the physical and psychiatric risk factors for admission for people with dementia. In particular, people with dementia were more likely to have either orthopaedic (e.g. falls/fractures) or respiratory/urological (e.g. infections) precipitants of admission than those without dementia. Most of the published work to date has focused on general hospital admissions, and the causes of psychiatric admission in people with dementia remain a relatively neglected area of study. Only two studies180,185 specifically investigated the causes of psychiatric admission, of which one focused only on cases of fronto-temporal dementia, whereas the other also included admissions to day hospital facilities. 185 However, one study178 included admissions to a dementia-specific unit among its analysis of all admissions, and three further studies167,179,184 included some analysis of the influence of psychiatric and behavioural disturbances on admission to acute general hospitals. As expected, psychiatric and behavioural disturbance was found to increase the risk of admission for people with dementia in relation to those without, and sleep disturbance emerged as a particular risk factor for admission. Furthermore, the finding that disruption to the social and environmental milieu often precipitated admission for people with dementia185 provides one of several possible explanations for subsequent behavioural and psychological disturbances. The link between acute physical illness and behavioural change in people with dementia has long been appreciated, and yet psychiatric and general medical services are not always as well integrated as they might be to equip them to deal with these problems.
People with dementia had higher risks of acute admission for respiratory and urological indications (likely to include a high proportion of respiratory and urinary tract infections), and for all-cause infections. Potentially preventable hospital admissions, such as those for urinary tract infections, were 78% more common in people with dementia. 186 There are several potential explanations for this finding. First, people with dementia might be more prone to infection, secondary to factors such as reduced mobility, inadequate fluid intake and the impaired performance of daily living tasks concerning personal hygiene. Second, people with dementia might have reduced or delayed help-seeking behaviour, through either reduced recognition of symptoms or impaired communication skills. Consequently, an acute infection might not be diagnosed in a person with dementia until the physical symptoms become severe, or secondary behavioural or psychological symptoms of delirium develop and, even then, carers or health-care staff might misattribute behavioural disturbance to a direct effect of the dementia rather than seek an underlying physical health cause. Therefore, physical health conditions that can be controlled in the general population through effective community management and treatment, also known as ‘ambulatory care sensitive’ conditions, are more likely to result in acute hospital admission for people with dementia. This highlights the importance of a more holistic approach to providing community dementia care, encompassing the cognitive, behavioural, psychological and physical needs of people with dementia and providing responsive care packages and health education to both people with dementia and their carers.
The need for proper community assessment and supportive interventions for people with dementia is further highlighted by the findings that they are at consistently higher risk than the general population of sustaining falls and fractures necessitating acute hospital admission, and that they are more likely to have significant difficulties with ADLs, including bathing, dressing, toileting and eating. In some cases, an acute decline in ADL abilities might be the result of concomitant physical illness. However, physical health can decline as a result of reduced mobility, poor nutritional intake or impaired personal hygiene. Furthermore, patients with dementia may be admitted to acute hospitals as a result of their social circumstances, often when they have been unable to care for themselves and without appropriate support. For example, neglect and poor provision of care at home could lead to problems such as malnutrition, urinary tract infections, chest infections, falls and fractures.
The UK National Audit Office187 reported how people with dementia receive poorer-quality care in acute hospitals and how the lack of skill of acute hospital staff in caring for people with dementia can have adverse effects on the symptoms of dementia. The National Audit of Dementia Care in General Hospitals188 found that two-thirds of hospital staff felt that they had received insufficient training in dementia care. Given what we now know about the poor outcomes of hospital admission for people with dementia, unnecessary acute hospital admissions must be avoided, and this requires the provision of early community assessment, recognition of problems and appropriate interventions by multidisciplinary teams, including medical staff, social workers, occupational therapists, nurses and trained care staff.
One limitation of this review is that several risk factors could not be analysed using a meta-analysis either owing to potential heterogeneity within risk factors (e.g. cardiovascular, psychiatric and neurological disorders) or because there were insufficient studies for a meta-analysis of the identified risk factors (e.g. social/environmental factors and ADLs). However, two studies166,178 concluded that people with dementia were less likely than those without to be admitted for an acute cardiac syndrome. This might reflect a lower recognition of acute angina or myocardial infarction in those with dementia, owing to reduced reporting of specific symptoms or a tendency to interpret any accompanying confusion as part of the established clinical syndrome of dementia.
Conclusion
Many acute hospital admissions for people with dementia are potentially preventable. Poor outcomes of acute hospital admission for people with dementia have been widely documented; therefore, efforts need to be focused on developing responsive and integrated community services. People with dementia are at increased risk of hospitalisation owing to behavioural and psychological disturbances, and many older people’s HTTs tend to focus on prevention and management of psychiatric crises and reducing psychiatric admissions. This review highlights the need for recognition of the physical health risks in these patients, including a high index of suspicion for conditions such as urinary tract and respiratory infections, and a low threshold for early treatment in the community. This approach could potentially prevent acute admission to general hospitals and reduce the associated risk of delirium, so that mental state and behaviour remain more stable. This highlights the importance of integrated working between services for older people’s mental health, primary care, social welfare, intermediate care and hospital liaison.
The effectiveness of crisis resolution/home treatment teams for older people with mental health problems: a systematic review and scoping exercise
Aims
Our aim was to conduct a systematic review of evidence to evaluate the effectiveness of crisis resolution/home treatment approaches to support older people with mental health problems at home compared with other forms of treatment and to scope home treatment services for older people with mental health problems, produce a typology of such services and review the typology in the context of policy and research findings.
Methods: literature review
The method used was a literature review; controlled comparison studies, including RCTs, controlled before-and-after studies and interrupted time series were eligible for inclusion in this review. Observational studies, theoretical papers and government frameworks and policies were also reviewed.
Types of interventions
Experimental intervention
Any crisis resolution/home treatment intervention for older people with a diagnosed mental health problem.
Control
For controlled studies, control groups included TAU, standard community treatment and waiting list controls.
Types of participants
The inclusion criteria for participants in the studies included in the review were those aged ≥ 65 years, diagnosed with a mental health condition and living in the community (excluding 24-hour care institutions).
Types of outcome measures
Primary outcomes included the following: number of admissions, length of hospital stay, maintenance of community residence and patient quality of life. Secondary outcome measures included cognition, use of medication, ADLs, patient/carer satisfaction, service use, health and social care costs and mortality.
Search methods for identification of studies
Electronic searches: we searched the Specialised Register of the CDCIG, which included records from The Cochrane Library, MEDLINE, EMBASE, PsycINFO, CINAHL and LILACS, and many ongoing clinical trial databases and grey literature sources. We also searched Web of Science and the Campbell Collaboration/SORO database.
Data collection and analysis
Selection of papers: titles and abstracts of citations obtained from the search were examined and irrelevant articles were discarded. The full texts of studies were obtained and assessed against our inclusion criteria. Attempts were made to obtain additional information from the study authors when necessary. A second reviewer independently reviewed the selected studies and agreement was reached on papers for inclusion.
Assessment of validity
The studies (primary research) were assigned a level of evidence according to the CEBM guidelines (www.cebm.net/levels_of_evidence.asp#levels). Levels of evidence ranged from 1 to 5, with lower numbers indicating higher quality. Studies rated between levels 1 and 4 were included in this review.
Procedure
Both reviewers assigned levels of evidence to each study independently and, in cases of disagreement, discussed the studies until a conclusion was agreed. The outcomes across the studies were then assigned an overall grade of evidence from A to D according to the CEBM criteria. Grade A represents consistent level 1 studies indicating the best evidence; grade B represents consistent level 2 or 3 studies; grade C represents evidence from level 4 studies or extrapolations from level 2 or 3 studies; and grade D evidence is the lowest level and represents level 5 evidence or troublingly inconsistent or inconclusive studies of any level.
Methods: scoping exercise
From consultations with colleagues, a review of policy and research literature, plus a scoping exercise we sought to describe the diversity of models of HTTs identifying three principal models. Five crisis resolution home treatment teams (CRHTTs) for older people across the UK were visited to identify what kinds of crises take place, key attributes associated with crises, models of service provision, areas of need and key themes to be included in a HTP. Recent case examples were discussed. The services had common aims: to provide a rapid response to older adults presenting in mental health crisis, to offer assessment and treatment at home as an alternative to hospital admission wherever possible and to facilitate timely discharge from psychiatric inpatient units.
Results: literature review
There were 8222 references found, of which 8185 were excluded by reference to title and abstract alone as they either did not cover crisis interventions or did not include people with mental health problems, and 27 were excluded as they did not consider older people in the sample. Ten references were included: three cohort studies,170,189,190 one descriptive study,27 one survey-related research study,191 two theoretical papers192,193 and three government policy documents. 23,194,195
Cohort studies and descriptive study
Three cohort studies and one descriptive study were included in the review (Table 45). All were assigned level 4 evidence according to the CEBM guidelines. Dibben et al. 190 studied the impact of a CRHTT 6 months before and 6 months after the service was implemented. Participants in this study were older people with mental health problems. There were 65 participants in the pre-CRHTT (comparison) group and 102 in the post-CRHTT (intervention) group.
| Study reference (level of evidence) and type of study | Description of intervention and participants (n) | Description of control group | Number of follow-ups and follow-up points | Results: control group | Results: intervention group | Summary of results |
|---|---|---|---|---|---|---|
| Ratna 1982170 (4); cohort study | Community-orientated old age psychiatry service; providing intensive 24-hour crisis support in the community Intervention group, n = 142 Sainsbury et al. 1965:196 Chichester comparison group, n = 216 Sainsbury et al. 1965:196 Salisbury comparison group, n = 121 |
Two retrospective cohorts from the Sainsbury et al. 1965196 study in which there was no crisis service | Referral (intervention group, n = 142; comparison group,a n = 337) Follow up 1: 24 months (intervention group, n = 142; comparison group,b n = 119) |
Total number of admissions, n (%) Comparison groupa at referral, n = 132 (39) Comparison groupb at follow-up, n = 71 (60) Average length of hospital stay (mean stay in days) Comparison groupa at follow-up, n = 101 Maintenance of community residence, n (%) Comparison groupb at follow-up, n = 42 (35) Mortality rates (%) Comparison groupb at follow-up, 44% |
Total number of admissions, n (%)
|
Reduction in numbers of hospital admissions (at referral and follow-up) Reduction in length of hospital stay at follow-up Increase in proportion of people remaining in community at follow up Improvement in mortality rates at follow-up |
| Doyle and Varian 1994189 (4); cohort study | Crisis intervention service operating through the CMHT Intervention group, n = 70 |
The 24-hour community orientated old age psychiatry service as described in Ratna 1982170 | Referral Follow-up 1: 36 months |
Refer to Ratna 1982170 | Total number of admissions, n, %
|
No difference at referral compared with Ratna 1982170 Lower proportion of people remaining in community at follow-up than in Ratna 1982170 No difference in mortality rates at follow-up compared with Ratna 1982170 |
| Dibben et al., 2008190 (4); before-and-after cohort study | CRHTT Pre CRHTT, n = 65 Post CRHTT, n = 102 |
6 months’ pre CRHTT data were collected | Data were collected 6 months pre CRHTT and 6 months post CRHTT | Total number of admissions: n = 65, 100% Average length of hospital stay: mean (SD) days: 49 (45.62) |
Total number of admissions: n = 70, 69% Average length of hospital stay: mean (SD) days: 53 (46.40) |
Reduction in number of hospital admissions (statistically significant) No difference in length of hospital stay |
| Richman et al., 200327 (4); prospective descriptive study | Outreach support team based within a day hospital, providing support in crisis Waiting list for an inpatient bed, n = 40 |
No comparison group | Referral 3 months |
n/a Assumption of 100% admission rate for crises before introduction of intervention |
Total number of admissions, n (%)
|
Reduction in number of hospital admissions |
Ratna170 investigated the efficacy of a 24-hour community-orientated old age psychiatric crisis service in North London, UK. The study concerned 142 older patients presenting in mental health crisis who had been referred to the service over a 6-month period in 1976 and followed up for 2 years. The data were compared with retrospective data from a study196 of two other psychiatric services, one a community service in Chichester (n = 216) and the other a hospital-centred service in Salisbury (n = 121), neither of which provided a crisis service. For the purpose of this review, the data from the two services in the Sainsbury et al. 196 study were combined and included as one comparison group.
Doyle and Varian189 described a crisis intervention service operating between 9 a.m. and 5 p.m. on weekdays. Participants in this study were 70 older people with mental health problems, followed up for 3 years from referral (1989–92). The findings were compared with data from the Ratna170 study.
A prospective descriptive study27 described the activity and outcomes of an outreach support team based in a day hospital for older people with mental illness. This service operated 12 hours per day, 7 days per week. Routine clinical, demographic and service use information was described but no attempt was made to draw a causal relationship between the activities of the outreach support team and subsequent maintenance in the community. The participants were 40 older people with mental health problems. There was no comparison group.
Primary outcomes
Number of admissions
There is grade C evidence of a reduction in number of hospital admissions when older people with mental health problems are referred to crisis intervention services at a point when they would otherwise have been admitted to hospital.
Dibben et al. 190 found a statistically significant reduction in admissions 6 months after the introduction of the CRHTT. Sixty-nine per cent of referrals (n = 70) were admitted in the intervention group, compared with 100% (n = 65) in the comparison group (see Table 45). Ratna170 also found that a lower percentage of patients were admitted to hospital on referral to the crisis service (29%; n = 41) than in the combined comparison group (39%; n = 32). At 2-year follow-up, 34% (n = 48) of those in the crisis intervention service had been admitted, compared with 60% (n = 1) in the merged comparison group. Doyle and Varian189 found that, on referral, there was almost no difference in the percentage of the sample admitted to hospital between the 9 a.m. and 5 p.m. service (31%; n = 22), and Ratna’s170 24-hours service group (29%; n = 41) (see Table 45). Richman et al. 27 found that 25% (n = 10) of patients referred to the crisis service were admitted to hospital at the point of referral or within the 3 months’ follow-up.
Length of stay
There was grade D evidence to suggest that there may be a reduction in the length of hospital stay with a crisis intervention service. Dibben et al. 190 found no difference in length of hospital stay 6 months after the introduction of the CRHTT, as the average length of stay was 53 days in the intervention group compared with 49 days in the comparison group (see Table 45). Ratna170 found that, over 2 years’ follow-up, the average length of stay in hospital was shorter for all patients referred to the crisis service (22 days) than for those in the combined comparison group (101 days). The data in the Ratna170 paper were converted to give the average length of stay for all participants in the cohorts, including those who were not admitted. Doyle and Varian189 stated that the mean length of stay in hospital for patients admitted via the 9 a.m.–5 p.m. crisis service was 49 days within the 3-year follow-up. The authors compared this with Ratna’s170 24-hour crisis service, where mean length of stay of only those admitted to hospital via the crisis service was 91 days. However, Doyle and Varian189 did not state number of patients admitted to hospital over the follow-up period and it was not, therefore, possible to compare average length of stay for all patients in the cohort with Ratna’s intervention and combined comparison groups.
Maintenance of community residence
There was grade D evidence to suggest that crisis interventions help maintain community living for older people with mental health problems. Ratna170 found that a higher percentage of people remained at home after 2 years’ follow-up in the 24-hours crisis service group (49%; n = 69) than in the overall comparison group (35%; n = 42) (see Table 45). Doyle and Varian189 stated that 31% (n = 22) were living at home at 3 years’ follow-up (see Table 45).
Secondary outcomes
Mortality
There was grade D evidence for impact on mortality in older people with mental health problems referred to a crisis intervention service. Ratna170 found, after 2 years’ follow-up, that mortality rates were lower for patients in the crisis service group (29%) than for those in the overall comparison group (44%) (see Table 45), and Doyle and Varian189 found a 30% mortality rate in their 9 a.m.–5 p.m. crisis service group after 3 years’ follow-up (see Table 45).
Use of services
There was grade D evidence for impact on use of services for older people with mental health problems referred to a crisis intervention service. Doyle and Varian189 reported that 22% of patients received domiciliary follow-up and 9% attended a day hospital/centre, and compared data with the Ratna170 study, in which 61% of patients received domiciliary follow-up and 32% attended a day hospital/centre.
Service use costs
There was grade D evidence regarding service use costs in a crisis intervention service for older people with mental health problems. Only one study considered this outcome. Richman et al. 27 found that the cost per patient per month in an outreach support team was £823, which was lower than the £1814 cost per patient per month on an inpatient ward. However, these were staff costs only and did not reflect total costs of the services. This cannot therefore be considered a detailed cost analysis.
Results: scoping exercise
We visited all five mental health home treatment services for older people identified across England in 2009 (Table 46). We compared and contrasted the three service models that these services followed in practice.
| Generic home treatment | Specialist home treatment | Intermediate care | ||||
|---|---|---|---|---|---|---|
| Team 1 | Team 2 | Team 3 | ||||
| Team composition | Multidisciplinary plus psychiatrist sessions | Multidisciplinary | Multidisciplinary | Multidisciplinary | Multidisciplinary | |
| Specialist older people’s staff | Lead nurse plus three staff | All staff | All staff | All staff | All staff | |
| Dementia referrals | Initially excluded, now accepted | Accepted | Accepted | Accepted (team is dementia specific) | Accepted | |
| Hours of operation Monday–Friday | 24/7 | 24/7 | 9 a.m.–7 p.m. | 9 a.m.–5 p.m. | 8 a.m.–6 p.m. | |
| Hours of operation bank holidays and weekends | 24/7 | 24/7 | 9 a.m.–5 p.m. | Closed | 9 a.m.–5 p.m. | |
| New referrals assessed at weekends | Y | Y | Y | N | N | |
| Telephone advice overnight | Y | Y (existing clients only) | N | N | N | |
| New referrals accepted overnight | Y (A&E only) | N | N | N | N | |
| Eligibility criteria | Admission imminent | Admission imminent | Admission within days/weeks | Admission within days/weeks or potential care transition | Admission within days/weeks or potential care transition | |
| Assessment | Within 1 hour | Within 1 hour | Within 24 hours | Within 24 hours | Within 24 hours | |
| Gatekeeping role | Y | Y (except for new referrals overnight) | N | N | N | |
| Focus | Crisis intervention | Crisis intervention | Crisis prevention | Crisis prevention | Crisis prevention | |
| Inpatient discharge facilitated | Y | Y | Y | Y | Y | |
| Local integrated older people’s CMHT | Y | Y | N (CPNs only) | Y | Y | |
Generic home treatment team
This team operated 24/7, offering immediate assessment (within 1 hour) to all patients meeting the referral criteria, which included the need for immediate psychiatric admission in the absence of intensive home treatment. The team performed a gatekeeping role for inpatient admissions, with all proposed admissions being referred to the team first to see whether or not home treatment would be possible.
Specialist older adults home treatment team
This service was fully functional between 8 a.m. and 8 p.m. on weekdays and between 9 a.m. and 5 p.m. at weekends and on bank holidays, and offered an overnight on-call telephone service to existing clients but did not take new referrals or carry out new assessments overnight. This team considered itself a crisis resolution team and the referral criteria included that a psychiatric inpatient admission would be immediately warranted in the absence of intensive home treatment. The team performed a gatekeeping role for inpatient admissions during its hours of full operation and carried out next-day assessments on the ward for all patients admitted outside these hours to assess their suitability for home treatment.
Intermediate care
Three of the teams we visited could be described as intermediate care teams for older adults with mental illness. These teams did not operate an overnight service but two operated extended hours on weekdays and weekends. Consequently, none of these three teams provided a gatekeeping role for inpatient admissions. Rather, they sought to avert the development of crises by encouraging early referral of patients whose mental health was deteriorating, particularly where such deterioration would otherwise be likely to result within days or weeks in either admission to a psychiatric hospital or a transition of care, especially involving a change of accommodation. The assessment of all new referrals took place as soon as possible and, in any case, within 24 hours of receipt.
Night-time presentations
Overnight presentations requiring immediate psychiatric admission are rare in those aged > 65 years. The generic HTT was the only service we visited that was fully operational on a 24/7 basis and saw an average of just two older people presenting at night per month. Our own local audit of acute psychiatric admissions found that fewer than 8% of older adults admitted over a 1-year period had presented in crisis overnight (between 8 p.m. and 8 a.m.), and three-quarters of these night-time admissions were under the Mental Health Act,197 suggesting that home treatment was probably inappropriate at that point in time.
Discussion
This review found insufficient evidence for the efficacy of CRHTTs in supporting older people with mental health problems to remain at home, as a result of a lack of properly designed research studies. Owing to the lack of consistent and good-quality evidence, it is not possible to say whether or not crisis resolution services for older people with mental health problems reduce length of stay in hospital from the three studies which considered this outcome. In particular, we were not able to calculate the average length of stay for the entire cohort in the Doyle and Varian189 study. The level of recommendation for maintenance of community residence was grade D. Only two studies looked at this outcome. 170,189 It is possible that the 24-hours crisis service can sustain people at home for longer, but this apparent finding might also have arisen as a result of changes in service provision, such as the shift towards care/nursing home placements, with long-term hospital beds becoming increasingly rare. The effects of crisis resolution services on mortality rates, service use and service costs were all given an overall grade D of recommendation. This was due to weakness in the study design, contrasting results, inappropriate comparison groups or lack of data.
From our scoping exercise of HTTs we were able to establish some key themes. Although we visited only five teams across the UK, these services were representative of the three models found in the Cooper et al. 191 UK survey. The home treatment services had varying definitions of crisis. Comparison of efficacy between different models is made difficult by variations in aims and referral criteria between different services. In addition, broad outcomes such as reduction in hospital admissions may be of limited utility in this instance, as baseline admission threshold varies widely between geographical areas on the basis of availability of inpatient and community resources and clinician preference, among other considerations.
Work package 2: development of a home treatment package for people with dementia and their family caregivers
Development of the home treatment manual
This section reports the development of the home treatment manual and advisory protocol to support people with dementia and their carers during crises to help avoid care home and hospital admissions. Crises in dementia may be precipitated by a number of interacting factors that lead to a point at which the individual and their carer cannot cope, or may arise from a single traumatic event. Contributing factors include carer burden, behavioural and psychological characteristics, physical health problems, social factors related to the person with dementia and their environment. 27,181,198–205 We followed the MRC guidelines for the development and evaluation of complex interventions30 using a systematic review of the literature and the scoping exercise of HTTs. 206
Focus groups
Aim
The aims of the focus groups were to identify factors precipitating crises and to identify interventions that may help manage crises for people with dementia living at home and their carers.
Method
Design
Focus groups were selected as they provide an ideal method for exploring people’s own meaning and understanding of a health-related problem through promoted discussion. 207 Focus groups provide an opportunity to observe the processes through which meaning is constructed and negotiated in the social context of the group itself77,138 and allow the in-depth exploration of topics to provide rich data sources. 75 Focus groups capture the participants’ views and perspectives and show them that their views are important and taken seriously. 73 The findings from these focus groups have been reported in accordance with the Consolidated Criteria for Reporting Qualitative Research (COREQ) 32-item checklist. 208
Participants
Focus groups were conducted with the following stakeholder groups: people with dementia (three groups), family carers (three groups) and staff from HTTs and older people’s CMHTs (three groups). These three groups ensured that the views of relevant stakeholders were included in the development of the home treatment advisory protocol. There were between four and eight participants in each focus group. The groups were purposively sampled to ensure a rich and meaningful source of data so that there was variation within our stakeholder groups in order to maximise the validity of findings. Key older people’s HTT and CMHT staff were identified from NELFT and Kent and Medway NHS and Social Care Partnership Trust. Eighteen people with mild-to-moderate dementia (female, n = 10, 56%; male, n = 8, 44%) living in the community, and who were willing and able to consent, were recruited from two day hospitals and a day centre within NELFT. Fifteen family carers (female, n = 9, 60%; male, n = 6, 40%) who lived locally were recruited via voluntary sector organisations such as Dementia UK and local carer support groups. We included both experienced carers (provided care for at least 2 years) and new carers (provided care for < 1 year), and carers from a range of ethnicities and age groups. In addition, the carers we approached had different types of relationships with their relatives including spouses, children and nieces/nephews. The family carers were providing or had previously provided care for a minimum of 4 hours per week for a relative with dementia. Nineteen health-care professionals (female, n = 11, 58%; male, n = 8, 42%) who work with people with dementia at times of crisis, including occupational therapists, Admiral Nurses, day hospital managers, HTT managers, psychologists and psychiatric nurses, were recruited into the groups. The groups were of mixed sex whenever possible. Ethics and local R&D approvals were obtained (Research Ethics Committee reference number 10/H0701/20). Informed consent was sought from all participants who met the inclusion criteria.
Procedure
Three focus groups lasting 60–90 minutes were held for each of the three different stakeholder groups. The sessions commenced with a brief presentation about the project overall. An explanation of the focus group ‘ground rules’ was given and the participants were given the opportunity to ask questions before being asked to focus on the topics for discussion. Each focus group was presented with the following definition: ‘A crisis is defined as an urgent demand for immediate psychiatric intervention for a patient living in the community’. 170 The participants were asked their opinions on the definition and were then asked to focus on causes of crisis involving people with dementia and their carers. Later, the participants were asked to discuss which interventions and forms of support they thought would help during a crisis or help to prevent a crisis. A framework was used to construct and develop the focus group discussions. The participants were asked to express their opinions and discuss issues through using a series of open questions. The questions included in the framework were:
-
What sort of issues do you think could lead to a crisis situation? What about in the context of people who are having memory problems? (Prompts from the evidence base provided if necessary.)
-
What kind of support do you think would be helpful in a crisis?
-
Do you think any of the following would be helpful in a crisis? (Interventions from the evidence base.)
Analyses
Two researchers conducted the focus groups. One acted as the moderator by facilitating the group, keeping the discussion going and encouraging all group members to participate fully. The second researcher actively listened, sought clarification and ensured the accuracy of content as needed during the interview,73 as well as recording field notes during and immediately after the focus groups. 74 Participants gave permission for the focus groups to be audio tape-recorded and these were transcribed verbatim. The data were qualitatively analysed to identify broad similarities and differences, using a data-driven inductive thematic analysis76,209 and ‘long table’ approach to code and analyse information. Thematic analysis is useful for summarising key features of a large body of data, as well as highlighting similarities and differences across the data set. 209 This can generate unanticipated insights, which is consistent with focus groups inquiry that allows participants the freedom to provide information that does not necessarily fit with any expectation going into the research. Inductive thematic analysis searches for themes that emerge directly from the data as being important to the description of the phenomenon. 210 This approach complemented our research questions, which were designed to be exploratory to extract descriptive data.
Two researchers independently analysed the focus group transcripts as a method of quality control and validation. In addition, both researchers read the focus group transcripts twice to familiarise themselves with the data. Initial codes were generated by coding interesting features within the transcripts in a systematic and rigorous way across the entire data set, collating data relevant to each code. The codes were then collated into potential themes, gathering all data relevant to each theme. The themes were then applied to all of the transcripts again to assess the applicability, and were reviewed and refined as necessary, generating a ‘thematic’ map of analysis. The themes were continually reviewed through ongoing analysis, and clear definitions and names were finally applied to the agreed themes. A codebook was produced, which highlighted the views of the three stakeholder groups, allowing us to assess the similarities and differences between them.
Tables 47 and 48 present the themes derived from this study according to category and stakeholder group for causes of crisis and interventions that could help to prevent or manage a crisis. The numbers of counts illustrate the number of times the theme was discussed by participants in each stakeholder group. There were five categories relating to themes for causes of crisis: (1) carer related; (2) environmental; (3) vulnerability; (4) behavioural/psychological; and (5) physical health (see Table 47). There were four categories relating to themes for interventions: (1) home environment; (2) carer related; (3) professional health-care support; and (4) social and home care support (see Table 48).
| Stakeholder group | Carer-related factors | Environmental factors | Vulnerability factors | Behavioural/psychological factors | Physical health factors |
|---|---|---|---|---|---|
| People with dementia | Family carer absent (5) Family carer death (1) |
Hazards relating to daily living tasks in the home (16) Physical hazards around the home (5) Too much activity/stimulus in the home (2) Outdoor safety (1) |
Outdoor safety (6) Inability to identify potential risks (5) Changes in environment (3) Social isolation (2) Reluctance in calling for/refusing to call for help/assistance (2) Person with dementia is being abused (1) Inability to manage finances/bills (1) Non-adherence to medication routine (1) |
Severity of memory impairment (9) Anxiety symptoms (2) Depressive symptoms (1) Poor ability to communicate effectively (1) |
Falls (5) |
| Carers | Family carer burden (16) Family carer’s mental health (14) Limited family carer awareness and understanding of dementia (6) Family carer is unable to access support services (4) Family carer absent (4) Family carer’s physical health (2) Family carer is abusing person with dementia (2) Carer refusing help or assistance (1) Family carer is not actively involved in the care planning process (1) |
Poor inadequate community services (8) Hazards relating to daily living tasks in the home (5) Physical hazards around the home (4) Lack of co-ordinated health and social support services (4) Unable to access essential amenities (2) Outdoor safety (1) Unplanned absence of care staff (1) |
Inability to identify potential risks (7) Reluctance or refusing to access help (3) Refusing/forgetting to take medication or taking too many medicines (1) Non-adherence to medication routine (1) Declining support services (1) |
Severity of memory impairment (8) Wandering (8) Physical aggression (5) Verbal aggressions (2) Poor inability to communicate effectively (2) Sleep disturbances (2) Hallucinations (1) Depressive symptoms (1) Repetitive speech (1) |
Incontinence (4) Infections (4) Falls (3) Immobility/difficulty in walking (3) Pain (1) |
| HCPs | Family carer’s mental health (9) Limited family carer awareness (5) Family carer burden (3) Family carer absence (3) Carers refusing help (1) Family carer is abusing person with dementia (1) Family carer’s physical health (1) |
Poor/inadequate community services (17) Changes in the home environment (6) Lack of co-ordinated health and social support services (6) Unsuitably trained paid care staff (5) Changes in family and relationships (4) Hazards relating to daily living tasks (3) Lack of supportive neighbours/friends in the local area (3) Unplanned absence of paid care staff (3) Lack of activities in the home for person with dementia (2) Different types of weather/seasons (1) Living alone (1) Reduced driving ability (1) Physical hazards around the home (1) Unable to access essential amenities (1) |
Very poor eating/drinking (6) Declining support services (4) Reluctance or refusing to access help (3) Inability to identify potential risks (3) Person with dementia is being abused (3) Social isolation for person with dementia (2) Non-adherence to medication routine (2) Inability to manage finances/bills (2) Severe neglect of personal hygiene/personal care (1) Outdoor safety (1) |
Severity of memory impairment (6) Wandering (4) Verbal aggression (3) Hallucinations (2) Sleep disturbance/excessive night-time activity (2) Depressive symptoms (2) Paranoia/suspicious symptoms (1) Poor ability to communicate (1) Disinhibition (1) Physical aggression (1) |
Immobility/difficulty in walking (3) Incontinence (3) Infections (3) Falls (2) Chronic diseases (2) Delirium (1) Constipation (1) Medication side effects (1) |
| Stakeholder group | Home environment | Carer related | Professional health-care support | Social and home care support |
|---|---|---|---|---|
| People with dementia | Supportive friends/neighbours in local area (11) Communication equipment (11) Equipment/adapted furniture/rails/ramps around the home (9) Prompts/cues/reminders around the home (8) Presence of a family carer (8) Maintaining a routine of daily living tasks (4) Administering/monitoring medication (1) Specialist assistive technology (1) |
Family carer education (1) | Easy access to A&E services in hospital (4) Specialist assessments by a MDT member of staff (2) |
Provision of home care services (6) Access to emergency services (3) Flexible provision of services (1) |
| Carers | Specialist assistive technology (9) Equipment/adapted furniture/rails/ramps around the home (7) Engaging in purposeful activities around the home (3) Communications equipment (2) Administering medication (1) Supportive friends/neighbours in the local area (1) |
Family carer education and training (10) Involvement in family carer support groups (4) Direct payments (3) Emergency access to respite in the home (3) Accessing the internet for advice and support (1) Planning care and support services with the carer (1) Counselling for family carers (1) Advice about financial/legal matters (1) Emergency access to respite in a residential care home (1) |
One main point of contact (5) Admiral Nurse (5) Implementation of a co-ordinated care plan (5) Medication reviews (4) Access to HCPs 24 hours per day (4) Telephone helpline (2) Support/specialist training for HCPs (2) Signposting (2) Daily visits by HTT (2) Easy access to a day hospital (1) Easier/better access to GPs (1) Referrals being made earlier to support services (1) |
Provision of home care services (9) Specialist training for home care staff (4) Provision of day care/centre services (3) Centralised database (1) Flexible provision of services (1) Immediate/emergency provision of care (1) Access to emergency services (1) |
| HCPs | Specialist assistive technology (4) Equipment/adapted furniture/rails/ramps around the home (1) Communication equipment (1) Engaging in purposeful activities around the home (1) |
Emergency access to respite in the home (9) Family carer education and training (7) Planning care and support services with the family carer (3) Accessing internet for advice and support (1) Involvement with carer support groups (1) |
HCPs available for longer hours (6) Physical health checks (5) One main point of contact (5) Implementation of a co-ordinated care plan (5) Specialist assessments by MDT (5) Support/specialist training for HCPs (4) Easy access to GP (3) Daily visits by HTT (3) Signposting (3) Telephone helpline (2) Access to Admiral Nurse (2) Referrals being made earlier to support services (2) Access to specialist safeguarding adult teams (2) Involving person with dementia in planning of their care (1) Medication review (1) Provision of stimulating activities to person with dementia (1) |
Provision of home care services (4) Easy access to emergency services (1) Specialist training for home care staff (1) Provision of day care/centre services (1) Immediate/emergency provision of care (1) Centralised database (1) |
Results
Causes of crisis
People with dementia thought that risks and hazards were the key factors in precipitating crises, including hazards in daily living tasks in the home such as leaving the gas on or taps running, cooking and fire hazards:
I thought I had forgotten to turn the fire off and you wouldn’t see how quickly I ran to get home.
Physical health-related factors were rarely mentioned by people with dementia, but falls were a serious concern and physical hazards around the home could lead to a crisis:
[M]aybe living alone is a crisis and I end up dead . . . I fall everywhere! Bathroom, kitchen, sitting room.
Outdoor safety was frequently mentioned, including road safety, forgetting keys and accidents in the garden:
Being involved in an accident . . . on the roads . . . accident in the garden . . . At work!
People with dementia were concerned about their inability to identify potential risks, such as opening the front door at night without knowing who was on the other side and letting strangers or burglars into the home:
I shouldn’t have opened it [the front door]. You know it was about 11 o’clock . . . Without knowing who is on the other side.
Carers were also worried about people with dementia’s inability to identify risks and hazards:
My dad will bring in anyone from the street . . . to our house, which is a bit of a worry.
Similarly, carers had concerns about household hazards, such as leaving on the electric cooker, leaving taps running or leaving the gas on, and physical hazards leading to accidents in the shower or kitchen. Carers thought that a key factor in causing crises was family carer burden due to excessive caring commitments and their own mental health:
I live a little way away and I’ve got four children; and that’s hard in itself. I have to look after her as well. Some days, I might be having a crisis before I have even seen her.
Male carers felt distressed and uncomfortable about taking on intimate tasks to which they were not accustomed, such as looking after their wives’ personal hygiene, as illustrated by this quotation from a discussion between two male carers:
. . . the crisis I had was with incontinence and being a man, I didn’t like the idea, even with your wife this is a personal thing, especially to a woman it’s very important, even to my wife, married all those years.
Some carers reported feeling very depressed. One carer, whose wife became very emotional and cried for long periods of time when her memory was rapidly deteriorating, reported:
You say to yourself, I wish I was dead.
Staff also acknowledged that the family carer’s mental health could lead to a crisis. People with dementia, on the other hand, rarely mentioned carer-related factors but were aware that the absence of a family carer could potentially lead to a crisis, particularly for those who relied heavily on their carer in their everyday lives.
People with dementia, carers and staff often thought that the severity of the person’s memory impairment led to a crisis situation; one person with dementia stated:
[S]aying the wrong things at the wrong time . . . putting your foot right in it!
One carer described how their relative’s memory impairment led to significant problems:
. . . asking them [neighbours] to call the police to get this woman [person with dementia’s wife] out of his house.
Behavioural problems, including wandering behaviour and physical aggression, were often a cause of great concern to carers. One carer described their concerns about wandering behaviour:
[M]orning or night . . . she was missing and I woke up at 6 and she was right down the front at the water’s edge.
Physical health-related factors were not mentioned as often in any of the groups, but incontinence, falls and infections were discussed at length, and very often the ensuing crisis was quite intense and traumatic for both the carer and the person with dementia. Staff reported that people with dementia with poor eating/drinking patterns could find themselves in a crisis resulting in hospital admission:
. . . get admitted to hospital because it’s weight loss because they are refusing food.
Poor and inadequate community services, including poor continuity of care, was a leading cause of crisis according to staff and carers. Staff reported that a crisis could often arise as a result of physical problems not being fully investigated or health-care professionals not acting quickly enough or calling in support services. Staff reported that:
. . . a lot of the time GPs don’t act quickly enough on call, or in support services . . . social services are quite slow to see people.
One carer described NHS continuing care services as:
[A] hospital ward with no staff. That’s what it feels like! You are on your own. There is nobody there!
Staff thought that the introduction of new home care staff could be confusing and upsetting for people with dementia. One member of staff reported a recent example and added:
[T]here was an incident today. Home care staff changed and everything was chaotic.
Additionally, staff thought that unsuitably trained home care staff and people with dementia declining support services often triggered a crisis. Staff mentioned that home environment changes (e.g. adaptations) could cause confusion for people with dementia. Staff were concerned about changes in the family (e.g. relatives getting married or having children), and the family carer having limited awareness about dementia could lead to arguments between carers and people with dementia and, subsequently, to crises.
Interventions in a crisis
People with dementia thought that prompts/cues/reminders around the home, such as lists and notes, could be very useful to help them to avoid various crisis situations. Communications equipment (people with dementia), such as having a telephone, and equipment/adaptations (people with dementia and carers) were thought to help prevent crises. People with dementia said:
You could use your phone if you had a bad fall. If you had a mobile you could take around with you
and:
I have a gas fire . . . so now I have had it disconnected. I’ve got my central heating, so I’ll be warm . . . so I’m safer!
Family carers and staff highlighted the value of assistive technology such as gas detectors, personal safety alarms, alerts/pagers and movement detectors. One carer said:
If you are worried about somebody getting out of bed, or getting out of a chair, like I was, they are absolutely brilliant. It actually gave me a bit more freedom as well . . .
People with dementia primarily thought that having a network of supportive friends/neighbours in the local area and having a family carer were most useful in helping to prevent a crisis and also during a time of crisis:
My neighbour, she watches my lights go on and off, so she knows when I go to bed when it’s dark.
People with dementia, carers and staff all thought that home care services were a very useful intervention during a time of crisis. As one carer said:
It’s been a great help to me, because my wife goes to the cinema and goes out walking. The home care worker cooks, cleans and she is a real bubbly person . . . I know that I can go out and feel secure that she is in good hands.
Carers also highlighted the importance of providing home care staff who have specialist training in working with people with dementia and their relatives. Staff also advocated emergency access to respite in the home:
Instant home-based respite. Somebody sitting in the day and somebody overnight.
People with dementia highlighted some professional health-care support interventions that could help them in a crisis, but their focus was on having easier access to accident and emergency services in hospital and the emergency services. One carer described an out-of-hours doctor service:
I found that actually every time I rang them about myself or my wife, I got a response and they came out to see me.
Staff also stated that there was a need for health-care professionals to be available for extended hours.
Staff also suggested regular physical health checks, stating that many crises were unrelated to a mental health condition, whereas carers valued medication reviews. Staff thought multidisciplinary team assessments were valuable in preventing and managing a crisis. Carers particularly valued having an Admiral Nurse:
Thank God for the Admiral Nurses. They are the ones to tell you everything.
Carers and staff thought that the implementation of a care plan and having one main point of contact (known to both the person with dementia and the carer) were helpful interventions in a crisis:
Someone you can rely on that you trust you can get hold of. I know not everybody is available 24 hours a day.
Staff said ‘with people with dementia it’s important to have some kind of continuity’ and stressed the value of ‘familiarity and to know what that person needs’.
Staff and carers stressed the importance of providing family carers with education and training in dementia. Carers wanted general courses on first aid, moving and handling, and information on coping strategies to help prevent a crisis:
A first aid course . . . would teach me the basics what to do with a scald, what to do with a fire burn, because they are the household things I am going to be dealing with.
Carers also valued having access to and attending family carer support groups to prevent crises.
Staff, on the other hand, stressed the importance of a more tailored approach:
Every client is different. Information that you give to that person, like individual care for the client or carer, you need to gear it to the right level and their understanding.
Discussion
We explored the views of a diverse range of stakeholders involved in crises with people with dementia. People with dementia worried about the risks and increased vulnerability associated with their declining cognition but wanted support to allow them to remain at home safely. However, rather than expecting a lot of input from professionals, they valued informal support such as local support from family, friends and neighbours, notes and reminders, mobile phones, and aids and adaptations around the home to help manage risks related to gas, electric, cooking and fire. Family carers also had concerns about vulnerability and safety, and valued home adaptations and specialist assistive technology as being especially useful to them and their relative with dementia during times of crisis. Staff also mentioned that specialist assistive technology should be more widely available for families in crisis.
Family carers had a broad understanding of the range of factors precipitating crises but their main concerns related to increased carer burden, poor carer mental health and lack of support from other family members or other services. Carers valued education and training as being useful to help prevent, and to help them cope during, times of crisis.
Strengths and weaknesses of study
All groups found it easier to discuss causes of crisis than to identify interventions to help. The three key stakeholder groups from a range of backgrounds, settings and disciplines allowed us to achieve theoretical validity and theoretical saturation as well as a comprehensive and rich data set. However, some professional groups were absent from our study, particularly those from outside mental health services such as GPs and social services. People with dementia required more frequent prompts from the group facilitator than the other stakeholder groups and, despite encouragement, some participants contributed little to the discussion. Hearing impairment led to some difficulties but the research team had anticipated this and ensured that there was additional staff support available when needed.
Clinical and policy implications
Crises faced by people with dementia and their families are complicated and distressing. 192 Interventions need to be flexible and tailored to both the individual person’s needs and their crisis situation. 211 The findings from this study support the involvement of services users and carers in service planning.
Conclusion
People with dementia and family carers have much to offer in their understanding of the most important causes and the most useful interventions in times of crisis. Although health-care professionals often emphasised more costly and intensive interventions (such as extended-hours services and multidisciplinary interventions), people with dementia often preferred support from family and friends, notes and reminders, and home adaptations to reduce risks.
Identifying and managing crisis for people with dementia and their carers
Methods
Aim
Our aim was to conduct a consultation and consensus exercise with a stakeholder network, through undertaking an online survey with academics, practitioners, voluntary sector and service users, to identify the primary causes of crises, what interventions are most likely to be useful in a crisis (immediate) and what are most likely to prevent a crisis (preventative) in dementia, and to provide the basis for development of the home treatment manual.
Design
An online survey was used with a network of key stakeholders to identify the primary causes of crises and interventions that are useful for managing or preventing a crisis for people with dementia and their carers. The online survey was part of a modified Delphi process132,133 to develop a model of home treatment for the SHIELD research programme. The online questionnaire was designed using results of a literature review and analysis of focus groups that explored the causes of crisis in dementia. 212 The results of the survey were subsequently included in a manual of home treatment interventions. The effectiveness of using the manual will be evaluated in a larger RCT.
Participants
A network of key stakeholders included academic experts in the field of dementia, health and social care practitioners, medical practitioners, emergency services, professional bodies, voluntary sector staff, home care agency staff, people with dementia and family carers.
Procedure
Online survey
An online survey was designed using SurveyMonkey (Palo Alto, CA, USA; www.surveymonkey.com). The respondents were asked to provide their opinions on the importance and frequency of the causes of crisis, and the effectiveness and usefulness of identified interventions, and to suggest other factors that they felt had not been covered.
Structure of the questionnaire
The thematic analysis of the focus group transcripts led to the identification of five categories of the causes of crisis: (1) behavioural/psychological; (2) physical health; (3) vulnerability; (4) family carer; and (5) environment. Each category comprised a list of factors that could lead to a crisis for people with dementia and their carers (Box 1). Participants were asked to choose the 50% from the selection that they thought were most likely to result in a crisis. The questionnaire also allowed for comments relating to causes that might not have been included.
-
Anxiety symptoms (e.g. constant worrying, irritability, agitation).
-
Delusions (false beliefs).
-
Depressive symptoms (e.g. suicidal thoughts, low mood).
-
Disinhibition (e.g. overfamiliarity, inappropriate comments).
-
Hallucinations (e.g. seeing and/or hearing things that are not there).
-
Physical aggression (e.g. hitting out, throwing things).
-
Poor ability to communicate effectively.
-
Repetitive speech and actions.
-
Severity of memory impairment (disorientation, forgetfulness).
-
Sleep disturbance/excessive night-time activity.
-
Sudden and unexplained changes in mood (e.g. crying).
-
Suspicious/paranoid ideas (persecutory beliefs/accusatory thoughts).
-
Verbal aggression (e.g. shouting, threatening and abusive comments).
-
Wandering (e.g. wandering excessively around the home/outdoors, night-time walking).
-
Alcohol problems.
-
Chronic diseases (e.g. heart conditions, chest problems, diabetes).
-
Constipation.
-
Delirium (confusional state – sudden onset).
-
Falls.
-
Immobility/difficulty walking.
-
Incontinence.
-
Infections (e.g. urinary tract infection, chest infection).
-
Medication side effects.
-
Pain.
-
Declining support services (e.g. care package).
-
Inability to identify potential risks (e.g. leaving the front door open, bogus callers).
-
Inability to manage finance/bills.
-
Non-adherence to medication routine.
-
Outdoor safety (road awareness, getting lost).
-
Person with dementia/memory problems is being abused (e.g. physically, verbally, emotionally, sexually, financially).
-
Reluctance/refusal to call for help or assistance.
-
Severe neglect of personal hygiene/personal care.
-
Social isolation.
-
Very poor eating/drinking.
-
Death of the family carer.
-
Family carer burden (e.g. stress, workload).
-
Family carer is abusing the person with dementia/memory problems.
-
Family carer is being abused.
-
Family carer is experiencing financial difficulties.
-
Family carer is not actively involved in the care planning process.
-
Family carer is unable to access support services (e.g. home care services, respite).
-
Family carer mental health (depression, anxiety).
-
Family carer refusing help or assistance.
-
Family carer’s physical health.
-
Limited family carer awareness and understanding of dementia/memory problems.
-
Sudden absence of family carer (e.g. hospitalisation).
-
Changes in family and relationships.
-
Changes in the home environment.
-
Hazards related to daily living tasks in the home.
-
Lack of activities in the home for the person with dementia/memory problems.
-
Lack of co-ordination between health and social support services.
-
Lack of supportive neighbours/friends.
-
Living alone.
-
Physical hazards around the home.
-
Poor/inadequate community services.
-
Reduced driving ability.
-
Too much activity/stimulus in the home.
-
Unable to access essential amenities.
-
Unsuitably trained paid care staff.
-
Unplanned absence of paid care staff.
The thematic analysis of the focus group transcripts led to four categories of interventions being identified: (1) professional health-care support; (2) social home care support; (3) family carer support; and (4) home living environment. Each category comprised a list of interventions and respondents were asked to indicate whether or not those interventions were likely to be useful in a crisis and/or preventing a crisis.
Dissemination of the questionnaire
The questionnaire was available in both online and paper versions for completion between November 2010 and January 2011. Dissemination was via professional organisations, NHS trusts, DeNDRoNs (health-care professionals), direct/personal e-mail correspondence with published academics, Dementia UK (carers), participants of the focus groups (carers and health-care professionals) and day centres/hospitals through one-to-one meetings with people with dementia following consultation with service managers.
Ethics considerations
Ethics approval for the research was obtained from the local ethics committee for the consultation and consensus activities as part of the SHIELD home treatment programme (Research Ethics Committee reference number 10/H0701/20). Prior to starting the questionnaire, respondents were asked to tick a box indicating their understanding that they were completing the questionnaire as part of a research project and consenting to their participation, and anonymised responses being used in the results. The research questionnaire was anonymous and participants had the option to leave their contact details on an anonymously linked page to be eligible for entry into a prize draw for a £150 shopping voucher. All questions were mandatory and the questionnaire could be accessed online at www.surveymonkey.com/s/9THNMRW.
The participants attending the consensus conference all signed consent forms giving permission for their participation and for their anonymised responses to be used in the results.
Statistical analysis
A chi-squared test was used to examine if there were differences in the response rates between the four groups. As the respondents included some groups with frequency counts of fewer than five, Fisher’s exact test was used in addition to the chi-squared test. When relevant, the Fisher’s exact p-value is quoted.
Results
Responses
A total of 719 respondents completed the questionnaire, comprising 20 academics (3%), 562 health sector (78%), 54 family carers (8%), 23 social care sector (3%), 16 emergency services (2%), 12 voluntary sector (2%), four people with dementia (1%) and 28 others (4%).
Of the respondents, 627 (87%) were female and 620 (86%) reported having been involved in a crisis involving people with dementia and their carers. A total of 711 (99%) questionnaires were completed online, whereas four (1%) were completed by hand and returned by post, and four (1%) were completed by hand by the researcher through interviewing people with dementia.
The personal demographic information was used to group the types of participants into the following four categories for analysis: 395 (55%) physical health practitioners, 227 (32%) mental health practitioners, 72 (10%) consumers (people with dementia, family carers and voluntary sector) and 25 (4%) academics.
The top five causes of crisis, ranked by each of the four demographic groups, are listed in Table 49. When a ranking of 1–5 is given, a low ranking indicates that the factor was selected most frequently in that domain as being more likely to cause a crisis.
| Domain | Mental health (N = 227), n (%) | Physical health (N = 395), n (%) | Consumer (N = 72), n (%) | Academic (N = 25), n (%) | Total (N = 719), n (%) | p-value |
|---|---|---|---|---|---|---|
| Behavioural/psychological | ||||||
| Wandering | 198 (87)2 | 331 (84)1 | 51 (71)1 | 21 (84)3 | 601 (84)1 | 0.02 |
| Physical aggression | 206 (91)1 | 304 (77)2 | 46 (64)4 | 23 (92)1 | 579 (81)2 | 0.0001 |
| Sleep disturbance | 182 (80)3 | 281 (71)3 | 51 (71)1 | 20 (80)4 | 534 (74)3 | 0.07 |
| Verbal aggression | 170 (75)4 | 273 (69)4 | 45 (63)5 | 22 (88)2 | 510 (71)4 | 0.04 |
| Suspicious/paranoid ideas | 164 (72)5 | 234 (59)5 | 39 (54) | 16 (64)5 | 453 (63)5 | 0.004 |
| Anxiety symptoms | 117 (52) | 232 (59) | 41 (57) | 16 (64)5 | 406 (57) | 0.3 |
| Severity of memory impairment | 68 (30) | 181 (46) | 47 (65)3 | 7 (28) | 303 (42) | 0.0001 |
| Physical health | ||||||
| Falls | 205 (90)2 | 345 (87)1 | 62 (86)1 | 23 (92)1 | 635 (88)1 | 0.6 |
| Infection | 208 (92)1 | 321 (81)2 | 61 (85)2 | 21 (84)2 | 611 (85)2 | 0.004 |
| Delirium | 195 (86)3 | 281 (71)3 | 49 (68)3 | 18 (72)3 | 543 (76)3 | 0.0001 |
| Immobility | 102 (45)4 | 202 (51)4 | 30 (42)5 | 11 (44)5 | 345 (48)4 | 0.3 |
| Incontinence | 88 (39) | 190 (48)5 | 44 (61)4 | 16 (64)4 | 335 (47)5 | 0.006 |
| Medication side effects | 93 (41)5 | 169 (43) | 28 (39) | 6 (24) | 296 (41) | 0.3 |
| Alcohol problems | 52 (23) | 73 (18) | 11 (15) | 11 (44)5 | 147 (20) | 0.02 |
| Vulnerability | ||||||
| Inability to identify potential risks | 173 (76)1 | 298 (75)1 | 55 (76)1 | 20 (80)1 | 546 (76)1 | 1.0 |
| Very poor eating and drinking | 170 (75)3 | 267 (68)2 | 43 (60)3 | 18 (60)3 | 495 (69)2 | 0.04 |
| Person with dementia is being abused | 173 (76)1 | 245 (62)3 | 44 (61)2 | 14 (56)4 | 476 (66)3 | 0.001 |
| Declining support services | 140 (61)4 | 220 (56)4 | 41 (57)4 | 13 (52)5 | 414 (58)4 | 0.5 |
| Outdoor safety | 129 (57)5 | 188 (48) | 35 (49)5 | 13 (52)5 | 365 (51)5 | 0.2 |
| Severe neglect of personal hygiene | 120 (53) | 194 (49)5 | 29 (40) | 17 (68)2 | 360 (50) | 0.1 |
| Family carer | ||||||
| Family carer burden | 182 (80)2 | 314 (80)1 | 62 (86)1 | 19 (76)2 | 577 (80)1 | 0.6 |
| Sudden absence of family carer | 184 (81)1 | 296 (75)3 | 53 (74)2 | 20 (80)1 | 553 (77)2 | 0.3 |
| Family carer’s physical health | 165 (73)*3 | 301 (76)2 | 49 (68)3 | 18 (72)4 | 533 (74)3 | 0.4 |
| Death of the family carer | 160 (71)4 | 268 (68)4 | 43 (60) | 17 (68)5 | 488 (68)4 | 0.4 |
| Family carer mental health | 146 (64)5 | 235 (60)5 | 46 (64)4 | 19 (76)2 | 446 (62)5 | 0.3 |
| Family carer unable to access services | 110 (49) | 207 (52) | 46 (64)4 | 12 (48) | 375 (52) | 0.1 |
| Environment | ||||||
| Physical hazards around the home | 162 (71)3 | 315 (80)1 | 46 (64)4 | 16 (64) | 539 (75)1 | 0.005 |
| Hazards related to daily living tasks | 172 (76)1 | 264 (67)3 | 44 (61)5 | 18 (72)2 | 498 (69)2 | 0.04 |
| Living alone | 136 (60) | 286 (72)2 | 55 (76)1 | 15 (60) | 492 (68)3 | 0.004 |
| Unable to access essential amenities | 164 (72)2 | 261 (66)4 | 43 (60) | 18 (72)2 | 486 (68)4 | 0.2 |
| Changes in the home environment | 161 (71)4 | 254 (64)5 | 48 (67)2 | 19 (76)1 | 482 (67)5 | 0.3 |
| Inadequate community services | 138 (61) | 238 (60) | 44 (61)5 | 15 (60) | 435 (61) | 1.0 |
| Unsuitably trained care staff | 139 (61)5 | 230 (58) | 45 (63) | 18 (72)2 | 432 (60) | 0.5 |
| Lack of co-ordination between health/social services | 108 (48) | 248 (63) | 48 (67)2 | 18 (72)2 | 422 (59) | 0.0005 |
There were significant differences in the top five rankings of crisis in the behavioural/psychological factors domain. Wandering (p = 0.02) was the most commonly selected factor across the four groups (84%). Although ‘wandering’ was ranked as their second choice, more mental health practitioners (87%) chose it as a risk factor than consumers (71%), who ranked ‘wandering’ and ‘sleep disturbance’ equally in first place. Consumers (i.e. people with dementia, carers and the voluntary sector) (64%) ranked ‘physical aggression’ (p < 0.0001) in fourth place, compared with 91% of mental health practitioners and 92% of academics who ranked it first. Sixty-five per cent of consumers selected ‘severity of memory impairment’ (p = 0.004) as a top-five cause of crisis, compared with < 30% of academics and mental health practitioners. In addition, more mental health practitioners (72%) chose ‘suspicious and paranoid behaviour’ (p = 0.004) than consumers (54%), who did not rank it in their top five responses.
There were significant differences in the top five rankings of crisis in the physical health factors domain. More mental health practitioners (86%) selected ‘delirium’ (p = 0.00009) than did consumers (68%). In contrast, more consumers (61%) selected ‘incontinence’ (p = 0.006) than did mental health practitioners (39%). Academics considered ‘alcohol problems’ (p = 0.02) a more significant cause of crisis (44%), compared with the other three groups, particularly consumers (15%).
There were significant differences in the top five rankings of crisis in the vulnerability domain. More mental health practitioners selected ‘very poor eating and drinking’ (p = 0.04) than did consumers and academics (60%). In addition, 76% of mental health practitioners selected ‘person with dementia is being abused’ (p = 0.001), compared with < 56% of academics and 61% of consumers.
There appeared more consensus across the four groups’ rankings of family carer factors that could cause a crisis, with no significant differences observed. Interestingly, ‘death of family carer’ did not feature in the top five rankings of consumers.
There were significant differences in the top five rankings of crisis in the environment domain. More physical health practitioners selected ‘physical hazards around the home’ (p = 0.005) as a top cause of crisis than did consumers and academics. In addition, 76% of mental health practitioners considered ‘hazards related to daily living tasks’ (p = 0.04) more likely to cause a crisis, compared with 61% of consumers. Seventy-six per cent of consumers (76%) selected ‘living alone’ (p = 0.004) as a top five cause of crisis, compared with 60% of mental health practitioners and 68% of academics.
The list of interventions for each of the four categories (professional health-care support, social home care support, family carer support and home living environment) that all respondents thought were most likely to be useful in a crisis and those most likely to prevent a crisis is shown in Table 50. Where a ranking of 1–5 is given, a low ranking indicates the intervention was selected most frequently in that category as being useful or preventative in a crisis.
| Intervention by category | Mental health (N = 227) | Physical health (N = 395) | Consumer (N = 72) | Academic (N = 25) | Total (N = 719) | All (N = 719) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Useful, n (%) | Prevent, n (%) | Useful, n (%) | Prevent, n (%) | Useful, n (%) | Prevent, n (%) | Useful, n (%) | Prevent, n (%) | Useful, n (%) | p-value | Prevent, n (%) | p-value | |
| Professional health care | ||||||||||||
| Professionals available 24 hours a day | 158 (70)1 | 94 (41) | 301 (76)1 | 199 (50) | 48 (67)1 | 39 (54) | 15 (60)1 | 10 (40) | 522 (73)1 | 0.07 | 342 (48) | 0.1 |
| Accident and emergency services | 142 (63)4 | 44 (19) | 272 (69)2 | 85 (22) | 48 (67)1 | 15 (21) | 14 (56)2 | 4 (16) | 476 (66)2 | 0.3 | 148 (21) | 0.9 |
| Health-care professionals’ longer hours | 155 (68)2 | 94 (42) | 261 (66)3 | 217 (55) | 44 (61)5 | 29 (40) | 11 (44) | 14 (56) | 471 (66)3 | 0.1 | 356 (50) | 0.006 |
| Safeguarding adults team | 143 (63)3 | 120 (53) | 236 (60)5 | 261 (66) | 44 (61)5 | 30 (42) | 14 (56)2 | 11 (44) | 437 (61)4 | 0.8 | 422 (59) | 0.00004 |
| Telephone helpline | 123 (54)5 | 108 (48) | 246 (62)4 | 179 (45) | 45 (63)4 | 34 (47) | 13 (52)5 | 12 (48) | 427 (59)5 | 0.2 | 333 (46) | 1.0 |
| One point of contact | 123 (54)5 | 137 (60) | 217 (55) | 252 (64) | 47 (65)3 | 44 (61) | 14 (56)2 | 17 (68) | 401 (56) | 0.4 | 450 (63) | 0.8 |
| Specialist training for health-care staff | 89 (39) | 200 (88)2 | 128 (32) | 351 (89)4 | 26 (36) | 62 (86)1 | 5 (20) | 20 (80)3 | 248 (35) | 0.1 | 633 (88)3 | 0.5 |
| Specialist multidisciplinary assessments | 88 (39) | 194 (86)5 | 107 (27) | 353 (89)2 | 21 (29) | 58 (81) | 7 (28) | 18 (72) | 223 (31) | 0.03 | 623 (87)4 | 0.02 |
| Co-ordinated care plan | 94 (41) | 203 (89)1 | 98 (25) | 353 (89)2 | 18 (25) | 62 (86)1 | 4 (16) | 22 (88)1 | 214 (30) | 0.00007 | 640 (89)2 | 0.8 |
| Day hospital | 72 (32) | 154 (68) | 110 (28) | 289 (73) | 27 (38) | 42 (58) | 3 (12) | 19 (76)5 | 212 (30) | 0.07 | 504 (70) | 0.06 |
| Medication review | 71 (31) | 162 (71) | 79 (20) | 323 (82) | 18 (25) | 49 (68) | 3 (12) | 20 (80)3 | 171 (24) | 0.008 | 554 (77) | 0.005 |
| Physical health checks | 45 (20) | 184 (81) | 61 (15) | 329 (83) | 15 (21) | 56 (78)5 | 6 (24) | 18 (72) | 127 (18) | 0.3 | 587 (82) | 0.4 |
| Person with dementia involved in care planning | 42 (19) | 188 (83) | 62 (15) | 340 (86)5 | 20 (28) | 48 (67) | 3 (12) | 19 (76)5 | 127 (18) | 0.1 | 595 (83) | 0.001 |
| Provision of purposeful activities | 38 (17) | 197 (87)4 | 50 (13) | 337 (85) | 13 (18) | 59 (82)4 | 3 (12) | 17 (68) | 104 (15) | 0.4 | 610 (85)5 | 0.1 |
| Earlier referrals to support services | 18 (8) | 198 (87)3 | 37 (9) | 369 (93)1 | 9 (13) | 62 (86)1 | 0 (0) | 22 (88)1 | 64 (9) | 0.3 | 651 (91)1 | 0.02 |
| Social home care | ||||||||||||
| Emergency provision of care | 187 (82)1 | 115 (51) | 340 (86)1 | 185 (47) | 62 (86)1 | 36 (50) | 21 (84)1 | 8 (32) | 610 (85)1 | 0.6 | 344 (48) | 0.3 |
| Emergency services | 186 (82)2 | 43 (19) | 335 (85)2 | 69 (18) | 61 (85)2 | 12 (17) | 21 (84)1 | 3 (12) | 603 (84)2 | 0.8 | 127 (18) | 0.9 |
| Centralised database of people and their needs | 153 (67)3 | 120 (53)5 | 271 (69)3 | 242 (61)5 | 49 (68)3 | 46 (64)5 | 15 (60)3 | 14 (56)5 | 488 (68)3 | 0.8 | 422 (59)5 | 0.2 |
| Flexible provision of services | 151 (67)4 | 185 (82)4 | 262 (66)4 | 291 (74)4 | 44 (61)4 | 56 (78)4 | 14 (56) | 17 (68)4 | 471 (66)4 | 0.6 | 549 (76)4 | 0.1 |
| Specialist training for home care staff | 98 (43)5 | 215 (95)2 | 124 (31) | 376 (95)1 | 30 (42)5 | 67 (93)1 | 8 (32)5 | 20 (80)3 | 260 (36)5 | 0.02 | 678 (94)1 | 0.04 |
| Home care services | 80 (35) | 217 (96)1 | 143 (36)5 | 362 (92)3 | 25 (35) | 65 (90)2 | 8 (32)5 | 22 (88)1 | 256 (36) | 1.0 | 666 (93)2 | 0.1 |
| Day care services | 49 (22) | 203 (89)3 | 74 (19) | 366 (92)2 | 12 (17) | 62 (86)3 | 0 (0) | 22 (88)1 | 135 (19) | 0.03 | 653 (91)3 | 0.2 |
| Family carer | ||||||||||||
| Respite in a residential care home | 188 (83)2 | 101 (45) | 333 (84)2 | 174 (44) | 57 (79)2 | 27 (38) | 20 (80)1 | 9 (36) | 598 (83)2 | 0.6 | 311 (43) | 0.6 |
| Respite in a day centre/day hospital | 182 (80)3 | 109 (48) | 322 (82)3 | 175 (44) | 57 (79)2 | 29 (40) | 19 (76)3 | 8 (32) | 580 (81)3 | 0.8 | 321 (45) | 0.4 |
| Counselling | 56 (25)4 | 190 (84)4 | 116 (29)4 | 348 (88)4 | 22 (31)4 | 60 (83)4 | 4 (16)5 | 19 (76)5 | 198 (28)4 | 0.3 | 617 (86)4 | 0.2 |
| Family carer’s education | 53 (23)5 | 216 (95)1 | 108 (27)5 | 374 (95)1 | 22 (31)4 | 68 (94)1 | 5 (20)4 | 20 (80)3 | 188 (26)5 | 0.5 | 678 (94)1 | 0.05 |
| Carer support groups | 41 (18) | 197 (87)3 | 76 (19) | 355 (90)3 | 16 (22) | 61 (85)3 | 1 (4) | 21 (84)1 | 134 (19) | 0.2 | 634 (88)3 | 0.4 |
| Planning care with family carer | 46 (20) | 213 (94)2 | 58 (15) | 373 (94)2 | 11 (15) | 67 (93)2 | 3 (12) | 19 (76)5 | 118 (16) | 0.3 | 672 (94)2 | 0.02 |
| Advice about financial matters | 23 (10) | 171 (75)5 | 56 (14) | 346 (88)5 | 8 (11) | 57 (79)5 | 1 (4) | 21 (84)1 | 88 (12) | 0.3 | 595 (83)5 | 0.001 |
| Home living environment | ||||||||||||
| Communication equipment | 173 (76)1 | 122 (54) | 296 (75)1 | 215 (54) | 50 (69)1 | 36 (50) | 11 (44)3 | 13 (52) | 530 (74)1 | 0.008 | 386 (54) | 0.9 |
| Presence of family carer | 161 (71)2 | 200 (88)3 | 244 (62)2 | 354 (90)5 | 44 (61)3 | 68 (94)1 | 16 (64)1 | 23 (92)1 | 465 (65)2 | 0.1 | 645 (90)3 | 0.5 |
| Supportive neighbours/friends | 142 (63)3 | 196 (86)4 | 224 (57)3 | 331 (84) | 45 (63)2 | 56 (78)5 | 13 (52)2 | 19 (76) | 424 (59)3 | 0.4 | 602 (84) | 0.2 |
| Specialist assistive technology | 90 (40)4 | 188 (83) | 114 (29)4 | 339 (86) | 20 (28)4 | 56 (78)5 | 3 (12)4 | 18 (72) | 227 (32)4 | 0.004 | 601 (84) | 0.1 |
| Administering/monitoring medication | 62 (27)5 | 204 (90)1 | 66 (17)5 | 366 (93)2 | 11 (15)5 | 62 (86)3 | 3 (12)4 | 21 (84)3 | 142 (20)5 | 0.008 | 653 (91)2 | 0.1 |
| Routine of daily living tasks | 34 (15) | 204 (90)1 | 45 (11) | 368 (93)1 | 4 (6) | 67 (93)2 | 1 (4) | 23 (92)1 | 84 (12) | 0.1 | 662 (92)1 | 0.5 |
| Home equipment/adaptations | 24 (11) | 196 (86)4 | 47 (12) | 357 (90)3 | 8 (11) | 62 (86)3 | 2 (8) | 21 (84)3 | 81 (11) | 0.9 | 636 (89)4 | 0.3 |
| Prompts/cues around the home | 24 (11) | 186 (82) | 47 (12) | 357 (90)3 | 6 (8) | 52 (72) | 3 (12) | 20 (80)5 | 80 (11) | 0.1 | 615 (86)5 | 0.0001 |
| Engaging in purposeful activities | 27 (12) | 186 (82) | 35 (9) | 336 (85) | 7 (10) | 55 (76) | 0 (0) | 20 (80)5 | 69 (10) | 0.9 | 597 (83) | 0.3 |
Useful interventions
There appeared to be more consensus across the four groups’ top five rankings of professional health-care support factors that could useful in a crisis, with no significant differences observed. Consumers ranked ‘easy access to accident and emergency services in a hospital’ top of their selection, compared with mental health practitioners, who ranked it third.
There were significant differences in the rankings of the top five immediate interventions in the social home care domain. More mental health practitioners (43%) selected ‘specialist training for home care staff’ (p = 0.02) than did physical health practitioners (31%). Moreover, this factor did not feature in the top five rankings of physical health practitioners.
There appeared to be more consensus across the four groups’ rankings of family carer factors that could useful in a crisis, with no significant differences observed.
There were significant differences in the rankings of the top five immediate interventions in the home living environment domain. More mental health practitioners (76%) selected ‘communication equipment’ (p = 0.008) than did academics (44%). Similarly, fewer academics (12%) selected ‘specialist assistive technology’ (p = 0.004) than mental health practitioners (40%), physical health practitioners (29%) and consumers (28%). Moreover, 27% of mental health practitioners selected ‘administering/monitoring medication’ (p = 0.008), in comparison with lower numbers of physical health practitioners (17%), consumers (15%) and academics (12%).
Preventative interventions
There were significant differences in the top five rankings of preventative interventions in the professional health-care support domain. ‘Referrals made earlier to support services’ (p = 0.02) was the top-ranked factor across the four groups, although more physical health practitioners (94%) selected this factor than consumers (86%). In addition, 86% of physical health practitioners selected ‘specialist assessments by a member of the multidisciplinary team’ (p = 0.02) as a preventative intervention, compared with 72% of academics.
There were significant differences in the top five rankings of preventative interventions in the social home care domain. Fewer academics (80%) selected ‘specialist training for home care staff’ than the other three groups (> 93%).
There were significant differences in the top five rankings of preventative interventions in the family carer domain. Fewer academics (80%) selected ‘family carers education/training’ (p = 0.05) compared with the other three groups (94%). Similarly, fewer academics (76%) chose ‘planning care and support services with the family carer’ (p = 0.02), compared with the other three groups (93%). Again, academics’ (84%) views differed from those in the other three groups (> 93%). Academics’ (84%) views also differed in their joint top ranking of ‘advice about financial/legal matters’ (p = 0.001) from those of mental health practitioners (76%), who ranked it fifth.
There were significant differences in the rankings of the top five preventative interventions in the home living environment domain. More physical health practitioners (90%) selected ‘prompts/cues/reminders placed around the home’ (p = 0.0001) than did the other groups, particularly consumers (72%).
Discussion
This is the first quantitative study that has compared the views of people involved in the management of crisis and dementia. The results suggest differences of opinion between professionals, consumers and academics as to what can cause a crisis and what interventions can be useful and/or prevent a crisis for people with dementia and their carers. Consumers’ rankings of the causes of crisis differed more frequently than those of the other three groups. Academics’ views also differed, particularly regarding interventions that could be useful in a crisis. These variances are important to recognise, particularly in the development of policy and strategies aimed at avoiding crises and managing them effectively.
Consumers’ views differed in three of the five domains of crisis, which suggests that they may interpret and manage crisis situations differently from health-care professionals. This divergence of opinion reflects previous studies’ findings that people with dementia and family carers do not always hold similar views on the provision of health-care services. 213,214
There is increased policy emphasis on involving people with dementia and family carers in planning the care that they receive, as nearly half report little or no involvement in the decisions and choices made about the support services they receive. 215 The results of this study suggest the need to listen more closely to the views of people with dementia and their families about their needs and choices when planning care interventions, particularly at times of crisis, as these may not reflect the priorities as assessed by health-care professionals. The findings also highlight the importance of government policy and practice standards about providing client-centred care that meets the needs and preferences of people with dementia. 216
Government policies and guidelines highlight the need for health-care practitioners to work more collaboratively in understanding and supporting people with dementia. 217 Interestingly, the results from this study suggest that there were no significant differences between mental and physical health practitioners’ views on the causes of crisis.
Conversely, the results also show that consumers may not always interpret or place significance on factors associated with crisis, given their lower ranking of abuse, neglect and alcohol consumption, which in turn has implications on safety and mortality. This could suggest the need for health-care professionals to explore these issues with consumers and develop an educative role while recognising the sensitivities that individuals may feel about disclosing this type of information.
The findings support the relevance and importance of family carer-related factors as a contributor to crisis. In contrast, Toot et al. 212 found that people with dementia did not consider issues such as family carer burden or carer absence as significant, which suggests that people with dementia and family carers have different views about family carer-related crisis.
The provision of timely and immediate interventions is critical in resolving crisis in dementia, which is often complicated by comorbidity and safety issues. In comparison, the provision of preventative interventions is important, as the degenerative and complex nature of dementia and its impact on families mean that the support and care provided should offer stability and be sustainable in the longer term. The results of this study demonstrate that interventions selected as being useful in a crisis were, in most cases, less frequently selected as being useful to prevent a crisis, as illustrated by the significant differences between how the interventions were ranked.
Overall, there was more consensus in how the groups ranked the top five interventions that could be useful in a crisis. The results reflect the literature that identifies that people with dementia and their carers viewed being able to access accident and emergency services in a crisis situation as important. 212 Unfortunately, people with dementia are more likely to experience negative outcomes following a hospital admission, such as longer stays, increased confusion, infection and disorientation. 217 Given that the number of avoidable hospital admissions is rising,218 health-care practitioners could have an educative role in enabling consumers to be aware of the range of primary and secondary care services available that were traditionally associated with accident and emergency services in acute hospitals.
The timing of when specialist assessments and opinions are sought from other professionals could indicate differences in practice, with mental health practitioners considering this to be useful in a crisis and physical health practitioners ranking it as being preventative. The implications for training all staff in the range of potential services and assessments that could promote well-being and support for people with dementia and their carers need to be considered.
The use of assistive technology in the management of dementia is widely reported in the literature; however, different opinions regarding its efficacy as a useful and/or preventative intervention were observed in the results. The disparity between mental health practitioners’ and academics’ views could suggest a difference between the clinical and practical applications of assistive technology and the published evidence supporting its use as an immediate or preventative intervention.
The findings also challenge the use of prompts and cues as an effective preventative intervention for people with dementia, with consumers ranking this lower than the other three groups. This is of particular interest when comparing advice published by charities and carer support groups of the importance of this type of intervention. Equally, understanding the different views between people with dementia and family carers about the use of prompts and cues could be important, especially if this is valued by people with dementia and not by family carers.
The study has also shown the usefulness of using online surveys to obtain opinions from a wide range of respondents and to reach a broad sample of participants. The use of online surveys is an affordable and effective way of consulting with relevant stakeholders and has been shown to have no consistent differences to postal surveys in response effects. 219 Although the use of online surveys is advantageous in offering accessibility and anonymity,220 there is a risk of attracting inappropriate or hoax respondents owing to its ease of access. This risk was counteracted by sending the invitation and access to the survey through known carer, organisational and professional networks.
Implications for practice
The results provide a useful insight into the perspectives of consumers and could further develop assessment processes, for example robust risk assessments that also capture individuals’ unmet needs. Risk and unmet needs assessments should explore carers’ perceptions about what they feel they could or could not cope with, which in turn could influence carer education and support strategies as part of a preventative care plan. Health-care professionals may also benefit from understanding a wider range of consumer-related issues that could affect the cause and management of crisis, including understanding perceptions about incontinence, and potentially low awareness of types of abuse, which could form part of a risk management strategy. Supporting both carers and staff to understand how government policy is reconfiguring services to provide support in the home and that accident and emergency is not the only option in a crisis may also have quality-of-life benefits for the person with dementia. Equally, policy-makers may wish to consider consumers’ views about accessing services in a crisis and that further education and promotion of alternative routes to seeking support should be considered.
The findings could serve as a point of reference for practitioners and managers, supporting supervision and decision-making tools around care planning and risk management. The findings also have potential implications for the provision of interventions, particularly in situations in which consumers’ views differ; therefore, practitioners may need to understand the different viewpoints and be equipped to negotiate between parties.
The research shows differences and similarities between how groups of professional groups perceive and interpret crisis situations. Developing a workforce education plan that increases the awareness of dementia and the key issues related to crisis, interventions and the roles of the multidisciplinary teams in health, social care and the voluntary sectors could increase competence and increase collaboration in practice.
Plans have already been developed to progress the findings from this research into a manual of interventions to support the common causes of crisis in dementia. It is anticipated that this resource will be developed and used in practice to support practitioners, carers and people with dementia living at home.
Implications for research
The study demonstrates that differences of opinion exist between the stakeholders involved in the management of crisis situations. The findings show that the needs of consumers differ at times from the views of health-care professionals and academics.
Further research could be undertaken with a larger sample of people with dementia and carers, to gain a greater understanding of their perspectives on crisis. This could also be supported by a qualitative study to explore thoughts and feelings about crisis to enrich the research findings. Consideration could also be given to exploring the different views held by people with dementia and carers in more detail, as this study highlighted differences between the two groups in the literature which are not analysed in this research.
As academics are significant contributors to the evidence base on dementia, further research could be undertaken with a larger sample of this group to gain a greater understanding of their perspectives on crisis. A qualitative study could be used to explore their views on the use of alcohol as a cause of crisis, in addition to preventative interventions such as family carer support and assistive technology.
Further research is required about the delivery of home interventions in a time of crisis and potential challenges to their successful implementation. The use of care management as a method of preventing and managing crisis situations could be explored further to assist in the development of best practice treatment models.
Limitations
The use of online surveys may introduce bias owing to the non-representative nature of the internet population. 220 However, the large response received would indicate that an increasing number of people now have access to the internet, including family carers. The option of completing paper versions of the survey was taken by a small sample of people with dementia, with assistance from a researcher. There was a significantly higher number of respondents in the physical health group than in the mental health group and this positively reflects the increasing contribution of the physical health sector in caring for people with dementia, which has been previously acknowledged. 216 Future work could usefully explore further the perspectives of the relatively under-represented groups in the present study, for example people with dementia.
Conclusion
The study aimed to compare stakeholders’ views of what causes a crisis for people with dementia and their carers in addition to identifying the interventions that are preventative and useful in a managing a crisis.
Views differed between the groups and could vary depending on the factors being presented. Consumers’ rankings of causes of crisis differed more frequently than those of other three groups. Academics’ views also differed, particularly regarding interventions to support a crisis. These variances are important and highlight the need for further research to understand the perspectives of others when planning and managing support for people with dementia and their family carers.
This study identified the top five responses and could be extended to explore all the factors that were presented. A larger sample of people with dementia and academics may provide more comparable data to contribute to understanding crisis in dementia. Investigating the different views of people with dementia and their carers would also be important.
This was a large-scale study that generated 99% of its responses online, demonstrating the potential of using technology to generate opinions from a wide range of participants.
Development of the home treatment protocol
Manual development
A manual of home treatment interventions was drafted, incorporating the results of the systematic reviews, scoping visits to HTTs, focus groups with HTT and older persons’ mental health services staff, family caregivers and people with dementia, and the results of the online questionnaires sent out to the stakeholder network. The home treatment manual included a home treatment advisory protocol for assessing people with dementia and their family carers at times of crises, and incorporates the Threshold Assessment Grid risk assessment, the Camberwell Assessment of Need for the Elderly (CANE) and a care planning tool with case examples. From the findings of the online questionnaire we were able to develop a table of interventions that were incorporated into the home treatment manual (draft 1) and included the four categories of professional health care, home living environment, social home care and family care support. In the table, the interventions were presented in two distinct formats: a list of those interventions preventing a crisis and a list of those considered most useful in a crisis.
Consensus conference
A consensus conference was organised for consultation on the home treatment manual (draft 1) and the home treatment advisory protocol. Invitations were sent to 99 people from our database of key stakeholders, which included service users (people with dementia and family carers), practitioners, voluntary organisations, independent sector and professional bodies. The consensus conference consisted of a series of short presentations on the development of the manual and a review of the existing evidence about crisis interventions; a presentation on HTTs in Kent was also given. Participants were then divided up into small working groups. Each working group was assigned one of the categories – behavioural/psychological, physical health, vulnerability, family carer and environment – and asked to select the top five interventions for all the factors in each of these categories. Participants selected the top five interventions that were most likely to be useful in a crisis (immediate) and those most likely to prevent a crisis (preventative) from the table of interventions generated through the online stakeholder survey.
Following the selection of the top five interventions, participants then applied the home treatment advisory protocol to case vignettes and identified relevant interventions as part of the care planning process. Participants had the choice of selecting interventions from the top five and the fuller table of interventions identified as being most likely to be useful in a crisis (immediate) and those most likely to prevent a crisis (preventative), or to identify additional interventions if these were thought to be more appropriate. The feedback from the consensus conference was used to develop a glossary of interventions and was incorporated into a further version of the home treatment manual (draft 2).
There were 23 participants who attended the consensus conference on 6 April 2011. Of these, there were three (13.0%) service users (family carers), one (4.3%) voluntary organisation representative, 15 (65.2%) practitioners, one (4.3%) representative from the independent sector and three (13.0%) representatives from professional bodies, who were divided into five small working groups. In addition, e-mail consultation was undertaken with 13 academics and clinicians on the stakeholder database. Each working group was assigned one of the categories – behavioural/psychological, physical health, vulnerability, family carer, environment – and selected five interventions that were most likely to be useful in a crisis (immediate) and most likely to prevent a crisis (preventative) for each of the factors listed. These responses have been amalgamated into a glossary of interventions, which was incorporated into the second draft of the home treatment manual. Following the selection of the top five interventions, participants then applied the home treatment advisory protocol to case vignettes and identified relevant interventions as part of the care planning process. Of the 10 case vignettes that were completed, all of the care plans were completed using both immediate or preventative interventions selected from either the top five interventions identified for that factor or the fuller list of interventions. The feedback from the participants was unanimous in that the top five interventions selected were relevant and appropriate for meeting the needs identified in the case vignettes.
Case review workshops
To test the initial suitability and application of using the home treatment manual (version 2) and advisory protocol, five case review workshops were held in Manchester, Hertfordshire and East London. There were 45 health-care practitioners who participated in these sessions aimed at reviewing the content of draft version 2 of the home treatment manual and applying the advisory protocol to clinical situations that they had managed. The workshops generated both individual and group feedback on the use of the home treatment manual in addition to 40 completed cases using the home treatment advisory protocols. Feedback and suggestions from the case review workshops were used to draft the home treatment manual (version 3). Participants were generally positive about using the manual but had reservations about some aspects. This included being unclear about the relationship between the need (CANE) and risk (Threshold Assessment Grid) assessments, and how to interpret the scores and prioritise the unmet needs. It was agreed that needs should be prioritised as immediate/high/moderate/low. Participants also had difficulty matching the unmet need to the cause of crisis, so the bracketed examples were reintroduced, for example chronic diseases (e.g. heart conditions, chest problems, diabetes) and inability to identify potential risks (e.g. leaving the front door open, being unable to identify bogus callers). There was some sensitivity around wording, such as the use of the term ‘wandering’ and the phrase ‘family carer is abusing the person with dementia/memory problems’, and the term ‘abusing’ was replaced with neglecting. The care plan within the advisory protocol was thought not to be detailed enough and this was renamed as a care planning tool. Navigating through the manual was difficult for those unfamiliar with it and the introduction of tabs or colour coding was suggested, which we adopted for the glossary in the manual.
Ratification and consensus on the home treatment manual
Consultation to validate and ratify the home treatment manual (version 3) was undertaken with 50 people from our database of practitioners, family carers and experts in home treatment. All were sent a copy of the manual. The participants were asked to focus attention on sections 1 and 2 of the home treatment manual and to provide feedback on the relevance of the contents and its applicability to practice in managing or preventing crisis situations in dementia. They could comment on the whole manual if they preferred and were asked to test the practicality and applicability of using it by applying the home treatment advisory protocol to one of the case vignettes in the manual.
The questions asked were:
-
Are you happy with the manual in its current form?
-
Was the manual easy to read and follow?
-
Was there anything you didn’t like about the manual?
-
Do you think anything is missing from the manual?
-
Is there anything you would change in the manual?
-
Any other comments?
Feedback received on the home treatment manual
In total there were 15 respondents, who consisted of one family carer, an occupational therapy practice officer and 13 practitioners: nurses occupational therapists, a clinical psychologist, a service manager and a consultant psychiatrist. About half of those consulted read the complete manual and three read the manual and applied the vignettes. Although there were general concerns about the length of the home treatment manual, there was consensus on the manual being informative, easy to read and use, relevant to practice, comprehensive and suitable for use.
The following is a selection of comments obtained through validation of the home treatment manual:
The manual contains some valid points and offers excellent advice around crisis situations and managing crisis situations.
Respondent 4
I have worked in a crisis team for over 2 years and to see the ‘process’ formalised and based on evidence is excellent.
Respondent 5
This manual will probably help in auditing the effectiveness of crisis teams, something which we found quite difficult.
Respondent 6
. . . I think the manual is useful as it provides a very structured approach without being overly prescriptive medicalised or complicated. I think it is a very practical manual but still very person centred . . .
Respondent 11
I think this is an excellent piece of work and look forward to the next phase.
Respondent 10
What a fantastic piece of work this is, I can really see some benefit in how we could roll some of this out within physical health care services, especially in our Community Inpatient Services, where we see quite a bit of dementia . . .
Respondent 12
I like the document. It is a time-consuming read as it is detailed and at times complex . . . However, it is relevant and supports good practice . . .
Respondent 13
Following the consultation, changes were made to the home treatment manual, which included reducing its length, changing the wording when requested (e.g. psychiatric/mental health, vignettes/’case examples’ or scenario, wandering/purposeful walking) and adding in a quick access guide and a list of abbreviations and acronyms. The manual was modified and version 4 of the home treatment manual was drafted.
Discussion
This study demonstrates the usefulness of involving a broad range of stakeholders in the shaping of care approaches for dementia. From our consultation we have been able to determine what practitioners in mental and physical health and services users consider to be the key causes of crisis in dementia, and what interventions they judge as most helpful in managing or preventing the crisis situation. Our consultation exercises during the consensus conference allowed us to build on the results of the online survey and link the most relevant interventions to specific factors that cause crisis in dementia. Immediate interventions are critical in resolving crises in dementia, which are often complicated and multifactorial situations owing to comorbidity and safety issues, whereas preventative interventions are important as the degenerative and complex nature of dementia and its impact on families mean that the support and care provided should offer stability and be sustainable in the longer term. Overall there is a general agreement about what factors are most likely to result in crisis and what is helpful in managing the situation. The involvement of service users (both people with dementia and family carers) has been key in this process, as the relevance and appropriateness of the interventions offered increase the likelihood of acceptance of and compliance with care planning.
The case review workshops and consensus work enabled the manual to be further improved to prepare it to a level at which it was ready for field testing in clinical practice. Although there were concerns about the manual’s length, and the additional work involved, it was regarded as easy to use and practical in clinical situations.
Limitations
There was a lower number than expected of people who attended the consensus conference and this is reflective of the demands on people’s time and commitments in the workplace. The NHS is currently undergoing significant change and the organisations and staff invited identified difficulties in finding the time to attend, as shown by the numbers of requests to consult electronically. Nevertheless, those participants who did attend offered a useful contribution and allowed us to complete the consultation exercises.
Implications for practice
Our study has led to the development of a glossary of interventions to help practitioners with care planning and targeting interventions to meet the common causes of crisis in dementia. Developing a systematic and structured approach to managing and preventing crisis in dementia has allowed us to establish a model of good practice for home treatment interventions. The glossary of interventions has been incorporated into our manual of home treatment interventions, which received a positive initial response by the practitioners and service users involved in the consensus process and was judged relevant and appropriate to clinical practice. Using the glossary may help practitioners by providing a systematic approach to identifying appropriate interventions that can help to resolve the relevant issues contributing to a crisis situation. The prompt and effective resolution of the problems contributing to crisis may, in turn, lead to the avoidance of unnecessary or inappropriate hospital admissions and delays in care home placement. Potential benefits are, therefore, indicated for people with dementia and family carers with the wider implementation of the home treatment manual in practice.
Implications for research
Before the home treatment manual can be introduced into clinical practice, further feasibility testing of the manual is needed. There is also a need to evaluate the effectiveness of implementing the home treatment manual and the application of the glossary of interventions in practice, which should be undertaken in a rigorous and robustly designed clinical trial. The study does show the positive advantage of using online surveys to reach and consult with a wide range of stakeholders, and offers potential opportunities for future research.
Conclusion
The inclusion of service users is vital in developing interventions in dementia that will be considered relevant and preference based. Furthermore, the development of a glossary of interventions linked to the main causes of crisis in dementia has the potential for improving patient outcomes. Further research is, however, needed to show the effectiveness of applying the glossary of interventions in practice. Our use of an online survey was effective in allowing us to consult with a broad range of stakeholders and is a medium that should be explored further for use in research. The results of this study were used to further develop the home treatment manual and produce the version for field testing.
Work package 3: feasibility study of using the home treatment manual and advisory protocol in practice
Aim
The feasibility study was used to assess the practicality and applicability of using the home treatment manual (version 4) and advisory protocol in practice to assess its potential for use in a large-scale clinical trial.
Objectives
We tested aspects of feasibility, tolerability and efficacy of using the home treatment manual and advisory protocol for people with dementia who are experiencing a crisis situation.
Our objectives were:
-
to assess if the home treatment manual and advisory protocol can be delivered as intended
-
to test the procedures for recruitment to and the retention and delivery of the intervention.
Sample
We recruited 17 mental health keyworkers who support people with dementia either approaching or in crisis situations who would use the home treatment manual with their clinical caseload. The practitioners were recruited from HTTs and CMHTs in Lancashire Care Trust and NELFT. The home treatment advisory protocol was applied to 21 clinical cases of people with dementia–caregiver dyads. The clinical cases were either new referrals to the older person’s mental health service, or existing cases, who had been identified as approaching or experiencing a crisis situation.
Intervention
The home treatment manual is intended for use for people with dementia and their family carer who require home treatment interventions to avert or reduce the need for admission to hospital. It contains guidance on assessment and the delivery of interventions to manage or reduce the risk of crisis in dementia. A training day was provided on the use of the home treatment manual and advisory protocol, and supervision structures were agreed before the feasibility study commenced. The clinical research staff provided supervision to the teams through telephone and e-mail contact. The home treatment advisory protocol was applied in accordance with the home treatment manual with 21 clinical cases of people with dementia–caregiver dyads. The home treatment manual and advisory protocol were implemented on initial assessment following referral to the service, or when the risk of hospital admission was identified for those people with dementia who were known to the service.
Consent
Permission was obtained from the service managers for the clinical area and from the mental health keyworkers for their participation in the study.
Data collected
We collected data from the home treatment advisory protocol, which were applied at two time points: at initial assessment and on evaluation. The data included the following clinical outcomes for the person with dementia: the number of unmet needs, the level of risk determined and the number of admissions to hospital or to care homes. All of this information was collected from the completed advisory protocols. All information was anonymised and used to evaluate practitioners’ ability to use the manual and advisory protocol with clinical cases. These data included the following clinical outcomes for the person with dementia: the number of unmet needs, the level of risk determined and any admissions to hospital or to care homes. All of the information was available from the advisory protocol.
Feedback was collected from the mental health keyworkers about their use of the manual through completion of the Adherence to Protocol Questionnaire and an individually completed questionnaire. This included minimal demographic information about the mental health keyworker, such as their profession, their place of work and the number of clinical cases in which they applied the advisory protocol. A group discussion was also to be held with mental health keyworkers to explore their attitudes and opinions about using the home treatment manual and advisory protocol.
Results
Of the 17 mental health keyworkers trained to implement the home treatment manual and advisory protocol, only 10 practitioners from Lancashire Care used the home treatment manual and advisory protocol; they were based in CMHTs (n = 3) and HTTs (n = 7). The reasons for the remaining seven practitioners not using the home treatment manual and advisory protocol were change of role/promotion (n = 2), did not care co-ordinate/admininstrate (n = 1), declined (n = 1), medium-/long-term sickness (n = 2) and unknown (n = 1).
Of the 21 home treatment advisory protocols collected, there were completed forms for the Threshold Assessment Grid, with 21 out of 21 completed with 100% accuracy; the CANE, with 21 out of 21 completed with 100% accuracy; the care planning tool, with 21 out of 21 completed with 100% accuracy; and the discharge care planning tool, which had 16 out of 21 completed.
Only 2 out of 10 practitioners completed the Adherence to Protocol Questionnaire and 8 out of 10 practitioners provided feedback using the questionnaire, two of whom completed the questionnaire jointly. The comments are given in Table 51. The reasons for the remaining two practitioners not providing feedback were sick leave (n = 1) and annual leave (n = 1). The group feedback discussion was unable to be held as, despite three attempts to schedule these groups, a quorum of at least three participants could not be achieved.
| Question | Rating | Comments |
|---|---|---|
| The home treatment manual and advisory protocol are suitable for purpose in their current form | Strongly agree (n = 1) Agree (n = 4) Disagree (n = 2) |
CANE useful (n = 2) Time-consuming (n = 5) Coding-related difficulties (n = 3) |
| The home treatment manual is easy to read, follow and apply | Agree (n = 6) Don’t know (n = 1) |
It is thorough and well thought out. It is good to have a tool to use that is comprehensive and standardised – it reinforces our current practice |
| Do you think anything is missing from the manual? | No (n = 5) Yes (n = 1) Missing (n = 1) |
The CANE doesn’t cover sleep problems, also needs a section for ‘other’
I found that the terminology relating to the codes was broad enough to encompass all the identified actions on the care plan |
| Is there anything you would change in the manual? | No (n = 1) Yes (n = 5) Missing (n = 1) |
Manual is too long (n = 3) Easier reference systems (n = 2) Need an ‘idiot’s’ step-by-step guide to completing the documents (n = 2) |
| Other comments I can see the value of the manual and protocol for new or inexperienced staff in order to standardise practice. However, in order for it to be workable, it needs to be used regularly by these practitioners so that it becomes second nature to use For those professionals who are inexperienced with working with people with dementia, then the manual provides a guide of helpful interventions to consider |
Conclusion
In general, people found the manual and advisory protocol useful; however, most did state that they found it a time-consuming process because it was novel and they were doing it in addition to their current care planning procedures. Some participants were not able to complete the discharge care planning tool and stated that this was because the service users were in their care for > 8 weeks, which was the time frame for the feasibility study.
Recommendations to the manual were made and it was thought by some that the detailed description of the development process at the front of the manual was not required. A number of participants stated that they would like a smaller, more ‘simplified’ manual, with improved reference systems and a ‘step-by-step’ flow chart to guide them through the process.
The manual and advisory protocol were acknowledged as useful, especially for staff inexperienced in working with people with dementia in crisis. However, it was felt that if this process was to be implemented, an electronic or online format would be easier to use.
For more information on the SHIELD home treatment advisory protocol and guidance, please contact the R&D office at NELFT or e-mail SHIELD@nelft.nhs.uk.
Chapter 5 Conclusions and recommendations for future research
Conclusions
There is an urgent need for useful and effective interventions to help to reduce the impact of dementia on patients, carers and society. The aim of our research programme was to prevent excess disability, promote social inclusion, improve health outcomes and enhance quality of life for people with dementia and their carers. The aim was achieved by our rigorous 5-year programme of psychosocial research, which comprised three projects: (1) MCST groups for people with dementia to improve their cognition and quality of life; (2) an expert carer programme that trained ex-carers to help new carers of people with dementia and that were undertaken alongside reminiscence groups for people with dementia and their carers to help maintain quality of life and improve their relationships; and (3) developing intensive home support to help manage crises at home and prevent admission to hospital for people with dementia. We used mixed-methods approaches, all of which were carefully evaluated for their potential benefits to people with dementia and their carers.
The objectives for the project were achieved in that each of the three projects completed a number of components of the pathway, through the development of theory, modelling, feasibility and evaluation to dissemination and implementation, as illustrated in the MRC framework for complex interventions. 30 We developed a strategy for user and carer involvement as part of the SHIELD research programme and we have also produced training manuals, which will be made widely available to help other services implement the same approaches.
We updated the Cochrane review on reality orientation/CST for dementia and developed a package for carer supporters. Initial pilot studies were conducted for the MCST and the CSP to ensure that the detailed design, methods and procedures were robust and fit for purpose. We then conducted definitive RCTs for MCST, RYCT and the CSP, along with economic evaluations for the MCST and CSP/RYCT interventions. Continuing MCST improved quality of life, improved cognition for those taking AChEIs and is cost-effective. Moreover, our results support other work indicating that drug and psychosocial interventions may potentially work better together than either intervention alone. Although the post-RCT surveillance observational study of MCST in practice did not find a noticeable improvement in cognition or quality of life at follow-up 8 months later, it is encouraging that neither declined over time. However, many participants in the observational study had only mild cognitive impairment and, therefore, may have been too high functioning to benefit cognitively from CST. The CSP/RYCT study did not find any particular benefits for family carers, although quality of life expressed in terms of QALYs derived from the EQ-5D showed a limited but significant reduction for people participating to RYCT. It could be the case that the structure of the RYCT programme was putting an excessive strain on the carers’ side. However, both CSP and RYCT appeared to improve quality of life for people with dementia. RYCT has the potential to be both effective and cost-effective in maintaining the quality of life of people with dementia, but the cost per QALY would be far beyond the NICE-accepted price window. Using a factorial design assumes that interventions are independent of each other, but for people with dementia we found that there were significant interactions.
We carried out systematic reviews in the areas of home treatment for dementia to identify the most promising interventions and components for an effective HTP for dementia. The finding that case management for people with dementia reduces admission to long-term care is consistent with the related literature. Case management also reduced behavioural problems in people with dementia. On the evidence available it is not clear how it may affect overall health-care costs. People with dementia and family carers have much to offer in their understanding of the causes and best interventions in times of crisis. Staff suggested more costly and intensive interventions, whereas carers liked education and support, and people with dementia appreciated support from family, as well as home adaptations and technology to reduce risks. The consensus methods and field testing enabled the production of an easy-to-use HTP to help staff working in crisis teams prevent admissions for people with dementia. The HTP requires evaluation in a full-scale multicentre trial.
The new wave of complex interventions shows great potential for benefit for people with dementia. Alongside this research into psychosocial interventions, further advances in methodology will be required, particularly in relation to process evaluations and implementation. Recent funding rounds by the NIHR and Economic and Social Research Council should help the UK to remain at the forefront of dementia care research with the potential to improve the lives of millions of people with dementia across the world.
Recommendations for future research
Maintenance cognitive stimulation therapy
-
Cognitive stimulation therapy has the potential to improve cognition and well-being in many people with dementia in addition to any potential benefits from antidementia medication. Future research and practice need to investigate the use of CST delivered by family carers and for those in other cultural and ethnic minority groups.
-
Maintenance cognitive stimulation therapy, originally designed to be run twice weekly, was implemented once weekly and this did not affect the cognition and quality-of-life scores. A future trial would be useful to control the frequency of CST delivery, the time frame of completing the assessment time points and the rigorousness of the inclusion criteria required to participate in the programme.
-
Further research is required to look further at the mechanisms of change identified in both quantitative and qualitative methods and relate this to CST and MCST to determine what is most important in attempting to further understand and increase the benefits for the person with dementia.
Carer Supporter Yesterday, Caring Today
-
The data collected in this trial include a comprehensive set of measures on coping and social support, the secondary analysis of which will increase our understanding of the interplay of these variables.
-
By collecting information from both family carers and people with dementia, we have the opportunity to carry out analyses at the level of the dyad. This is something that has been long called for, but as yet rarely executed, in the field of dementia care.
-
Multilevel analyses are becoming increasingly popular in other fields in which interactions within the dyad influence the outcomes of each member of that dyad. Future studies using a factorial design should be adequately powered for an analysis of each intervention with TAU.
Home treatment programme
-
Further research into crisis resolution interventions for older people with mental health problems should be based on sound theory, so that a robust evidence base can be created to drive future policy development.
-
Further research is required about the delivery of interventions at home during a time of crisis and potential challenges to their successful implementation. The use of care management as a method of preventing and managing crisis situations through a co-ordinated and planned response could be explored further to assist in the development of best practice treatment models.
-
Future research is needed to understand how carers make decisions when dealing with crises and how these findings can be incorporated into providing appropriate and acceptable crisis interventions.
-
Our use of an online survey was effective in allowing us to consult with a broad range of stakeholders and is a medium that should be explored further for use in research.
-
There is a need for a RCT to establish the efficacy of crisis resolution/home treatment services for older people with mental health problems.
Acknowledgements
The SHIELD MCST programme (ISRCTN26286067); the evaluation and comparison of the effectiveness of staff training in CST and its implementation in practice (ISRCTN28793457); the Carer Supporter/RYCT programme (ISRCTN37956201) and the SHIELD Home Treatment Programme are all part of the SHIELD project (application number RP-PG-0606-1083), which is funded by the NIHR Programme Grants for Applied Research funding scheme held by NELFT. The grantholders are Professor Orrell (University College London), Professor Woods (Bangor), Professor Challis (Manchester), Professor Moniz-Cook (Hull), Professor Russell (Swansea), Professor Knapp (London School of Economics and King’s College London) and Dr Charlesworth (University College London). Professor Orrell is the chief investigator for SHIELD. The web-based randomisation system and MACRO databases were developed in collaboration with NWORTH. This report presents independent research commissioned by the NIHR under its Programme Grants for Applied Research scheme (RP-PG-0606-1083). The sponsor of this research programme is NELFT.
The MCST in practice manual is published by Hawker Publications and is available at www.careinfo.org/products-page/books/making-a-difference-2/.
For the CSP/RYCT trial, the site principal investigators are Georgina Charlesworth (NELFT), Sue Rey (Northamptonshire Healthcare NHS Foundation Trust), Fiona Poland (University of East Anglia for the Norfolk and Waveney Mental Health NHS Foundation Trust site), Margaret Fox (University of East Anglia) and Gwen Bonner (Berkshire Healthcare NHS Foundation Trust).
The following voluntary organisations are also involved in the provision of the carer supporter element of the programme: in North East London – Age Concern Havering, Redbridge Respite Care Association, Carers of Barking and Dagenham, and Waltham Forest Carers Association; and in Norfolk – Age UK Norfolk.
Additional sources of funding for each site:
-
North East London – Central and East London Clinical Research Network (CEL1042)
-
Northampton – Leicestershire, Northamptonshire, and Rutland Clinical Research Network and Thames Valley DeNDRoN
-
Norwich – Norfolk and Suffolk Health Innovation and Education Cluster and East Anglia DeNDRoN
-
Berkshire – Thames Valley Clinical Research Network and Thames Valley DeNDRoN.
For the MCST trial, the site principal investigator was Dr Helen Donovan, Consultant Clinical Psychologist, Bedfordshire and Luton Mental Health and Social Care Partnership NHS Trust.
We would like to thank:
-
Mr John Brouder, Chief Executive, NELFT
-
all of the people with dementia and family carers who took part in the various research activities undertaken as part of the SHIELD research programme
-
care home and day centre staff
-
Bedfordshire and Luton Mental Health and Social Care Partnership NHS Trust
-
NELFT
-
East London NHS Foundation Trust
-
Kent and Medway NHS and Social Care Partnership Trust
-
Lancashire Care NHS Foundation Trust
-
Camden and Islington NHS Foundation Trust
-
CDCIG for help with the Cochrane Reviews
-
Joanne Knowles and U Hla Htay – consumer reviewers
-
DeNDRoN
-
Central and East London Research Network
-
Mental Health Research Network
-
Uniting Carers, Dementia UK
-
Alzheimer’s Society Redbridge
-
Dementia Resource Centre
-
Redbridge Respite Care Association
-
Age Concern Havering.
Administrative staff in North East London NHS Foundation Trust
Cathy Forbes.
Professional organisations that helped to disseminate surveys
Royal College of Nursing.
College of Occupational Therapy.
Programme Steering Committee
Professor James Lindesay (chairperson), Professor of Psychiatry for the Elderly, Leicester University.
Dr Vincent Kirchner, Consultant Psychiatrist, Older Persons’ Mental Health Services, Camden.
Professor Jan Oyebode, Professor of Dementia Care, University of Bradford.
Rachel Thompson, Dementia Project Lead, Royal College of Nursing.
Elayne Dunn, ‘CHAT’ (Cognitive Help and Therapy), Sussex.
Dr Graham Stokes, Director of Dementia Care, BUPA Care Services.
David Prothero, Family Carer, Uniting Carers, Dementia UK.
Data Monitoring and Ethics Committee
Professor Jill Manthorpe (chairperson), Professor of Social Work, King’s College London.
Dr Zoe Hoare, Trial Statistician, NWORTH.
Jennifer Hellier, Independent Statistician, King’s College London.
David Prothero, Carer, Uniting Carers for Dementia, Dementia UK.
North Wales Organisation for Randomised Trials in Health
Paul MacDonald.
David Hunnisett.
Research team
Professor Fiona Poland, Professor of Social Research Methodology, University of East Anglia.
Professor Jennifer Beecham, Professor of Health and Social Care Economics, London School of Economics.
Dr Siobhan Reilly, Senior Lecturer, Lancaster University.
Dr Claudia Miranda, Universidad de Valparaíso, Chile.
Dr Aimee Spector, Senior Lecturer, University College London.
Dr Karen Burnell, Lecturer, University of Portsmouth.
Amriptal Rehill, Health Economist, London School of Economics.
Reem Malouf, Statistician, University of Oxford.
Priyanka Chauhan, PhD student, University College London.
North East London NHS Foundation Trust
Dr Jennifer Wenborn, Programme Manager.
Dr Mike Devine, Consultant Psychiatrist.
Linda Smith, Clinical Researcher.
Caroline O’Haire, Clinical Researcher.
Ritchard Ledgerd, Clinical Researcher.
Nadia Crellin, Research Assistant.
Alex Feast, Research Assistant.
James Sinclair, Research Assistant.
Nina Melunsky, Research Assistant.
Elisa Aguirre, Research Assistant.
Amy Streater, Research Assistant.
Lauren Yates, Research Assistant.
Keir Yong, Research Assistant.
Deepak Sankhla, Research Assistant.
Sandra Nolle, Trial Administrator.
Contributions of authors
Professor Martin Orrell (Professor of Ageing and Mental Health at University College London and Honorary Consultant Old Age Psychiatrist and Director of Research and Development at NELFT) was the chief investigator for this project overall. He was the chief investigator for the development of the SHIELD research programme and initiated the proposal that led to this work. He led the team at University College London and NELFT, participating in the design and conduct of all stages of the research and report preparation.
Dr Juanita Hoe (Senior Clinical Research Associate at University College London) was the Programme Co-ordinator for the SHIELD Research Programme. She participated in the design and conduct of all stages of the research and report preparation, other than the grant preparation, and co-ordinated the input of the London elements of the research.
Dr Georgina Charlesworth (Lecturer at University College London) led on the SHIELD CSP/RYCT programme and participated in the design and conduct of all stages of the research and report preparation.
Professor Ian Russell (Professor of Clinical Trials at University of Swansea) was the methodological advisor for the SHIELD research programme. He participated in the design and conduct of all stages of the research and report preparation.
Professor David Challis (Professor of Community Care Research/Director of Personal Social Services Research Unit at University of Manchester) contributed expertise in case management and participated in the design and conduct of all stages of the research and report preparation.
Professor Esme Moniz-Cook (Consultant Clinical Psychologist, Humber Mental Health Teaching NHS Trust) provided input into the CST and staff training as well as the HTP study.
Professor Martin Knapp (Professor of Social Policy at London School of Economics) led on health economics and evaluating the cost-effectiveness of the trial interventions. He participated in the design and conduct of all stages of the research and report preparation.
Professor Bob Woods (Professor of Clinical Psychology of the Elderly at Bangor University) led on the updated review of CST and participated in the design and conduct of all stages of the research and report preparation.
Dr Zoe Hoare (Senior Statistician and Research Fellow at Bangor University) was the Trial Statistician.
Dr Elisa Aguirre (a PhD student at University College London and NELFT researcher) was involved in the MCST programme.
Sandeep Toot (a PhD student at University College London and NELFT researcher) was involved in the development and delivery of the HTP programme.
Amy Streater (a PhD student at University College London and NELFT researcher) developed and carried out the MCST in practice programme.
Nadia Crellin (a PhD student at University College London and NELFT researcher) was involved in the CSP/RYCT programme.
Dr Chris Whitaker (Senior Statistician at NWORTH Clinical Trials Unit, Bangor University) supervised the analysis of the data.
Francesco d’Amico (Health Economist at London School of Economics) was involved in cost data analysis.
Amritpal Rehill (Health Economist at London School of Economics) was involved in cost data analysis.
Data sharing statement
Anonymised open-access copies of the SHIELD trial databases are available via NWORTH.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health.
References
- Prince M, Wimo A, Guerchet M, Ali G, Wu YT, Prina M. World Alzheimer Report 2011 – The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends. London: Alzheimer’s Disease International; 2011.
- Jorm AF, Korten AE, Henderson AS. The prevalence of dementia: a quantitative integration of the literature. Acta Psychiatr Scand 1987;76:465-79. https://doi.org/10.1111/j.1600-0447.1987.tb02906.x.
- Luengo-Fernandez R, Leal J, Gray A. The Prevalence, Economic Cost and Research Funding of Dementia Compared with Other Major Diseases. Cambridge: Alzheimer’s Research Trust; 2010.
- National Service Framework for Older People. London: Department of Health; 2001.
- Kaduszkiewicz H, Zimmerman T, Beck-Bornholdt H, van den Busche H. Cholinesterase inhibitors for patients with Alzheimers disease: systematic review of randomised clinical trials. BMJ 2005;331:321-3. https://doi.org/10.1136/bmj.331.7512.321.
- Spector A, Thorgrimsen L, Woods B, Royan L, Davies S, Butterworth M, et al. A randomised controlled trial investigating the effectiveness of an evidence-based cognitive stimulation therapy programme for people with dementia. Br J Psychiatry 2003;183:248-54. https://doi.org/10.1192/bjp.183.3.248.
- McShane R, Keene J, Gedling K, Fairburn C, Jacoby R, Hope T. Do neuroleptic drugs hasten cognitive decline in dementia? Prospective study with necropsy follow up. BMJ 1997;314:266-70. https://doi.org/10.1136/bmj.314.7076.266.
- Livingston G, Johnston K, Katona C, Paton J, Lyketsos CG. Old Age Task Force of the World Federation of Biological Psychiatry . Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. Am J Psychiatry 2005;162:1996-2021. https://doi.org/10.1176/appi.ajp.162.11.1996.
- Woods RT, Qizilbash N. Evidence-Based Dementia Practice. Oxford: Blackwell; 2002.
- Brodaty H, Green A, Koschera A. Meta-analysis of psychosocial interventions for caregivers of people with dementia. J Am Geriatr Soc 2003;51:657-64. https://doi.org/10.1034/j.1600-0579.2003.00210.x.
- Clare L, Woods RT, Moniz Cook ED, Orrell M, Spector A. Cognitive rehabilitation and cognitive training for early-stage Alzheimer’s disease and vascular dementia. Cochrane Database Syst Rev 2003;4. https://doi.org/10.1002/14651858.cd003260.
- Spector A, Orrell M, Davies S, Woods B. Reality Orientation for Dementia: A Review of the Evidence of Effectiveness. Oxford: The Cochrane Library; 1998.
- Knapp M, Thorgrimsen L, Patel A, Spector A, Hallam A, Woods B, et al. Cognitive stimulation therapy for people with dementia: cost-effectiveness analysis. Br J Psychiatry 2006;188:574-80. https://doi.org/10.1192/bjp.bp.105.010561.
- Dementia: A NICE-SCIE Guideline on Supporting People With Dementia and Their Carers in Health and Social Care. Leicester: British Psychological Society; 2007.
- Gibson F. The Past in the Present: Using Reminiscence in Health and Social Care. Baltimore, MD: Health Professions Press; 2004.
- Woods RT, McKiernan F, Haight BK, Webster J. The Art and Science of Reminiscing: Theory, Research, Methods and Applications. Washington, DC: Taylor & Francis; 1995.
- Caring about Carers: A National Strategy for Carers. London: Department of Health; 1999.
- Singleton N, Aye Maung N, Cowie A, Sparks J, Bumpstead R, Meltzer H. Mental Health of Carers. London: The Stationery Office; 2002.
- Eagles JM, Craig A, Rawlinson F, Restall DB, Beattie JA, Besson JA. The psychological well-being of supporters of the demented elderly. Br J Psychiatry 1987;150:293-8. https://doi.org/10.1192/bjp.150.3.293.
- Our Health, Our Care, Our Say: A New Direction for Community Services. London: Department of Health; 2006.
- Progress on Policy: The Expert Patients Programme. London: Department of Health; 2006.
- Aneshensel C, Pearlin LI, Mullan JT, Zarit SH, Whitlatch CI. Profiles in Caregiving. The Unexpected Career. San Diego, CA: Academic Press; 1995.
- Everybody’s Business: Integrated Mental Health Services for Older Adults – A Service Development Guide. London: Department of Health; 2005.
- Pinner G, Hillam J, Branton T, Ramakrishnan A. Raising the Standard: Specialist Services for Older People with Mental Illness. Royal College of Psychiatrists; 2006.
- Draper B, Low LF. What is the effectiveness of acute hospital treatment of older people with mental disorders?. Int Psychogeriatr 2005;17:539-55. https://doi.org/10.1017/S1041610205001663.
- Johnson S, Nolan F, Pilling S, Sandor A, Hoult J, McKenzie N, et al. Randomised controlled trial of acute mental health care by a crisis resolution team: the north Islington crisis study. BMJ 2005;331. https://doi.org/10.1136/bmj.38519.678148.8F.
- Richman A, Wilson K, Scally L, Edwards P, Wood J. Service innovations: an outreach support team for older people with mental illness – crisis intervention. Psychiatric Bull 2003;27:348-51. https://doi.org/10.1192/pb.27.9.348.
- McNab L, Smith B, Minardi HA. A new service in the intermediate care of older adults with mental health problems. Nurs Older People 2006;18:22-6. https://doi.org/10.7748/nop2006.04.18.3.22.c2418.
- Charlesworth G, Shepstone L, Wilson E, Reynolds S, Mugford M, Price D, et al. Befriending carers of people with dementia: randomised controlled trial. BMJ 2008;336:1295-7. https://doi.org/10.1136/bmj.39549.548831.AE.
- A Framework for the Development and Evaluation of RCTs for Complex Interventions to Improve Health. London: MRC; 2003.
- Research Governance: Framework for Health and Social Care. London: Department of Health; 2005.
- Craig N, Dieppe P, Macintyre S, Michie S, Nazareth I, Pettigrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Nilsen ES, Myrhaug HT, Johansen M, Oliver S, Oxman AD. Methods of consumer involvement in developing healthcare policy and research, clinical practice guidelines and patient information material. Cochrane Database Syst Rev 2006;3. https://doi.org/10.1002/14651858.cd004563.pub2.
- Poland F, Charlesworth G, Hoe J. SHIELD User and Carer Strategy 2008:1-6.
- Guidance for Service User Involvement in the Mental Health Research Network. London: SURGE (part of the UK Mental Health Research Network); 2005.
- Glasby J, Beresford P. Who knows best? Evidence based practice and service user contribution. Crit Soc Pol 2006;28:268-84. https://doi.org/10.1177/0261018306059775.
- Hoe J. Research Governance – Clinical Trial Monitoring for the SHIELD Project 2010:1-7.
- Mental Capacity Act 2005. Code of Practice. London: The Stationery Office; 2007.
- Mental Capacity Act 2005. London: The Stationery Office; 2005.
- Conducting Research with People not Having the Capacity to Consent to their Participation. A Practical Guide for Researchers. Leicester: British Psychological Society; 2008.
- Hoe J. SHIELD Research Programme Adverse Events Procedure 2009:1-10.
- Clare L, Woods RT. Cognitive training and cognitive rehabilitation for people with early-stage Alzheimer’s disease: a review. Neuropsychol Rehabil 2004;14:385-401. https://doi.org/10.1080/09602010443000074.
- Orrell M, Spector A, Thorgrimsen L, Woods B. A pilot study examining the effectiveness of maintenance cognitive stimulation therapy (MCST) for people with dementia. Int J Geriatr Psychiatry 2005;20:446-51. https://doi.org/10.1002/gps.1304.
- Woods B, Aguirre E, Spector AE, Orrell M. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev 2012;2. https://doi.org/10.1002/14651858.cd005562.pub2.
- Folstein MF, Folstein SE, McHugh PR. ‘Mini Mental State’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. https://doi.org/10.1016/0022-3956(75)90026-6.
- Hughes C, Berg L, Danziger W, Coben L, Martin R. A new clinical scale for the staging of dementia. Br J Psychiatry 1982;140:566-72. https://doi.org/10.1192/bjp.140.6.566.
- Spector A, Orrell M, Davies S, Woods RT. Reality orientation for dementia. Cochrane Database Syst Rev 2000;4. https://doi.org/10.1002/14651858.cd001119.pub2.
- Baldelli MV, Boiardi R, Fabbo A, Pradelli JM, Neri M. The role of reality orientation therapy in restorative care of elderly patients with dementia plus stroke in the subacute nursing home setting. Arch Gerontol Geriatr Suppl 2002;8:15-22. https://doi.org/10.1016/S0167-4943(02)00098-5.
- Bottino CM, Carvalho IA, Alvarez AM, Avila R, Zukauskas PR, Bustamante SE, et al. Cognitive rehabilitation combined with drug treatment in Alzheimer’s disease patients: a pilot study. Clin Rehabil 2005;19:861-9. https://doi.org/10.1191/0269215505cr911oa.
- Buschert VC, Friese U, Teipel SJ, Schneider P, Merensky W, Rujescu D, et al. Effects of a newly developed cognitive intervention in amnestic mild cognitive impairment and mild Alzheimer’s disease: a pilot study. J Alzheimers Dis 2011;25:679-94.
- Chapman SB, Weiner MF, Rackley A, Hynan LS, Zientz J. Effects of cognitive-communication stimulation for Alzheimer’s disease patients treated with donepezil. J Speech Lang Hear Res 2004;47:1149-63. https://doi.org/10.1044/1092-4388(2004/085).
- Coen RF, Flynn B, Rigney E, O’Connor E, Fitzgerald L, Murray C, et al. Efficacy of a cognitive stimulation therapy programme for people with dementia. Ir J Psychol Med 2011;28. https://doi.org/10.1017/S0790966700012131.
- Onder G, Zanetti, O, Giacobini E, Frisoni GB, Bartorelli L, . Reality orientation therapy combined with cholinesterase inhibitors in Alzheimer’s disease: randomised controlled trial. Br J Psychiatry 2005;187:450-5. https://doi.org/10.1192/bjp.187.5.450.
- Requena C, Maestú F, Campo P, Fernández A, Ortiz T. Effects of cholinergic drugs and cognitive training on dementia: 2-year follow-up. Dement Geriatr Cogn Disord 2006;22:339-45. https://doi.org/10.1159/000095600.
- Spector A, Orrell M, Davies S, Woods B. Can reality orientation be rehabilitated? Development and piloting of an evidence-based programme of cognition-based therapies for people with dementia. Neuropsychol Rehabil 2001;11:377-97.
- Buettner LL, Fitzsimmons S, Atav S, Sink K. Cognitive stimulation for apathy in probable early-stage Alzheimer’s. J Aging Res 2011;2011. https://doi.org/10.4061/2011/480890.
- Fernandez-Calvo B, Contador I, Serna A, de Lucena VM, Ramos F. The effect of an individual or group intervention format in cognitive stimulation of patients with Alzheimer’s disease. Revista De Psicopatología Y Psicología Clínica 2010;15:115-23. https://doi.org/10.5944/rppc.vol.15.num.2.2010.4090.
- Niu YX, Tan JP, Guan JQ, Zhang ZQ, Wang LN. Cognitive stimulation therapy in the treatment of neuropsychiatric symptoms in Alzheimer’s disease: a randomized controlled trial. Clin Rehabil 2010;24:1002-11. https://doi.org/10.1177/0269215510376004.
- Baines S, Saxby P, Ehlert K. Reality orientation and reminiscence therapy. A controlled cross-over study of elderly confused people. Br J Psychiatry 1987;151:222-31. https://doi.org/10.1192/bjp.151.2.222.
- Baldelli MV, Pirani A, Motta M, Abati E, Mariani E, Manzi V. Effects of reality orientation therapy on elderly patients in the community. Arch Gerontol Geriatr 1993;17:211-18. https://doi.org/10.1016/0167-4943(93)90052-J.
- Breuil V, De Rotrou J, Forette F, Tortrat D, Ganansia Ganem A, Frambourt A, et al. Cognitive stimulation of patients with dementia: preliminary results. Int J Geriatr Psychiatry 1994;9:3-17. https://doi.org/10.1002/gps.930090306.
- Ferrario E, Cappa G, Molaschi M, Rocco M, Fabris F. Reality orientation therapy in institutionalized elderly patients: preliminary results. Arch Gerontol Geriatr 1991;12:139-42.
- Wallis GG, Baldwin M, Higginbotham P. Reality orientation therapy – a controlled trial. Br J Med Psychol 1983;56:271-7. https://doi.org/10.1111/j.2044-8341.1983.tb01556.x.
- Woods RT. Reality orientation and staff attention: a controlled study. Br J Psychiatry 1979;134:502-7. https://doi.org/10.1192/bjp.134.5.502.
- Gerber GJ, Prince PN, Snider HG, Atchison K, Dubois L, Kilgour JA. Group activity and cognitive improvement among patients with Alzheimer’s disease. Hosp Community Psychiatry 1991;42:843-5. https://doi.org/10.1176/ps.42.8.843.
- Hanley IG, McGuire RJ, Boyd WD. Reality orientation and dementia: a controlled trial of two approaches. Br J Psychiatry 1981;138:10-4. https://doi.org/10.1192/bjp.138.1.10.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions v5.1.0 2011.
- Mohs R, Bloom FE, Kupfer DJ. Psychopharmacology. 4th Generation of Progress. New York, NY: American College of Neuropsychopharmacology; 2000.
- Olazarán J, Reisberg B, Clare L, Cruz I, Peña-Casanova J, Del Ser T, et al. Nonpharmacological therapies in Alzheimer’s disease: a systematic review of efficacy. Dement Geriatr Cogn Disord 2010;30:161-78. https://doi.org/10.1159/000316119.
- Spector A, Orrell M, Woods B. Cognitive stimulation therapy (CST): effects on different areas of cognitive function for people with dementia. Int J Geriatr Psychiatry 2010;25:1253-8. https://doi.org/10.1002/gps.2464.
- Spector A, Thorgrimsen L, Woods B, Orrell M. Making a Difference: An Evidence-Based Group Programme to Offer Cognitive Stimulation Therapy (CST) to People with Dementia: Manual for Group Leaders. London: Hawker Publications; 2006.
- Levin E. Noticeable Problems Checklist. London: National Institute for Social Work; 1989.
- Morgan DL. Focus Groups as Qualitative Research. Thousand Oaks, CA: Sage; 1997.
- Burgess R. In the Field: An Introduction to Field Research. London: Allen and Unwin; 1984.
- Bowling A. Research Methods in Health, Investigating Health and Health Services. Buckingham: Open University Press; 1997.
- Boyatzis RE. Transforming Qualitative Information: Thematic Analysis and Code Development. London: Sage; 1998.
- Sofaer S. Qualitative methods: what are they and why use them?. Health Serv Res 1999;34:1101-18.
- Murphy C, Killick J, Allan K. Hearing the User’s Voice: Encouraging People with Dementia to Reflect on their Experiences of Services. Stirling: Dementia Services; 2001.
- Barnett E. Including the Person with Dementia in Designing and Delivering Care: ‘I Need to Be Me!’. London: Jessica Kingsley; 2000.
- Atwal A, Owen S, Davies R. Struggling for occupational satisfaction: older people in care homes. Br J Occupational Therapy 2003;66:118-24. https://doi.org/10.1177/030802260306600306.
- Cummings SM. Predictors of psychological well-being among assisted-living residents. Health Soc Work 2002;27:293-302. https://doi.org/10.1093/hsw/27.4.293.
- Jonas-Simpson C, Mitchell GJ, Fisher A, Jones G, Linscott J. The experience of being listened to: a qualitative study of older adults in long-term care settings. J Gerontol Nurs 2006;32:46-53. https://doi.org/10.3928/0098-9134-20060101-15.
- Kitwood T. Dementia Reconsidered: The Person Comes First. Buckingham: Open University Press; 1997.
- Tuckett AG. Applying thematic analysis theory to practice: a researcher’s experience. Contemp Nurse 2005;19:75-87. https://doi.org/10.5172/conu.19.1-2.75.
- Dementia: Supporting People with Dementia and their Carers in Health and Social Care. London: NICE; 2006.
- Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry 1984;141:1356-64. https://doi.org/10.1176/ajp.141.11.1356.
- Diagnostic and Statistical Manual of Mental Disorders. Arlington, VA: American Psychiatric Association; 2000.
- Gadner C. The Impact of Cognitive Stimulation Therapy Groups on People With Dementia: Views from Participants, their Carers and Group Facilitators. London: University College London; 2009.
- Logsdon RG, Gibbons LE, McCurry SM, Teri L. Quality of life in Alzheimer’s disease: patient and caregiver reports. J Ment Health Aging 1999;5:21-32.
- Moniz-Cook E, Vernooij-Dassen M, Woods R, Verhey F, Chattat R, De Vugt M, et al. A European consensus on outcome measures for psychosocial intervention research in dementia care. Aging Ment Health 2008;12:14-29. https://doi.org/10.1080/13607860801919850.
- Holden UP, Woods RT. Positive Approaches to Dementia Care. Edinburgh: Churchill Livingstone; 1995.
- Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell Scale for Depression in Dementia. Biol Psychiatry 1988;23:271-84. https://doi.org/10.1016/0006-3223(88)90038-8.
- Shankar KK, Walker M, Frost D, Orrell MW. The development of a valid and reliable scale for rating anxiety in dementia (RAID). Aging Ment Health 1999;3:39-4. https://doi.org/10.1080/13607869956424.
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308-14. https://doi.org/10.1212/WNL.44.12.2308.
- Galasko D, Bennet D, Sano M, Ernesto C, Thomas R, Grundman M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. The Alzheimer Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997;11:S33-9. https://doi.org/10.1097/00002093-199700112-00005.
- Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. https://doi.org/10.1097/00005650-199603000-00003.
- Beecham J, Knapp M, Thornicroft G, Brewin C, Wing J. Measuring Mental Health Needs. London: Gaskell; 1992.
- EuroQoL Group . EuroQoL: a new facility for the measurement of health related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Smith SC, Lamping DL, Banerjee S, Harwood A, Foley B, Smith P, et al. Measurement of health related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology. Health Technol Assess 2005;9. https://doi.org/10.3310/hta9100.
- Curtis L. Unit Costs of Health and Social Care 2011. Canterbury: Personal Social Services Research Unit, University of Kent; 2011.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2010.
- Hoe J, Hancock G, Livingston G, Woods B, Challis D, Orrell M. Changes in the quality of life of people with dementia living in care homes. Alzheimer Dis Assoc Disord 2009;23:285-90. https://doi.org/10.1097/WAD.0b013e318194fc1e.
- Logsdon RG, Gibbons LE, McCurry SM, Teri L. Assessing quality of life in older adults with cognitive impairment. Psychosom Med 2002;64:510-19. https://doi.org/10.1097/00006842-200205000-00016.
- Woods B, Thorgrimsen L, Spector A, Royan L, Orrell M. Improved quality of life and cognitive stimulation therapy in dementia. Aging Ment Health 2006;10:219-26. https://doi.org/10.1080/13607860500431652.
- Aguirre E, Hoare Z, Streater A, Spector A, Woods B, Hoe J, et al. Cognitive stimulation therapy (CST) for people with dementia – who benefits most?. Int J Geriatr Psychiatry 2013;28:284-90. https://doi.org/10.1002/gps.3823.
- Clark CM, Sheppard L, Fillenbaum GG, Galasko D, Morris JC, Koss E, et al. Variability in annual Mini-Mental State Examination score in patients with probable Alzheimer disease: a clinical perspective of data from the Consortium to Establish a Registry for Alzheimer’s Disease. Arch Neurol 1999;56:857-62. https://doi.org/10.1001/archneur.56.7.857.
- Zanetti O, Frisoni GB, De Leo D, Dello Buono M, Bianchetti A, Trabucchi M. Reality orientation therapy in Alzheimer disease: useful or not? A controlled study. Alzheimer Dis Assoc Disord 1995;9:132-8. https://doi.org/10.1097/00002093-199509030-00003.
- Metitieri T, Zanetti O, Geroldi C, Frisoni GB, De Leo D, Dello Buono M, et al. Reality orientation therapy to delay outcomes of progression in patients with dementia. A retrospective study. Clin Rehabil 2001;15:471-8. https://doi.org/10.1191/026921501680425199.
- Cooper C, Mukadam N, Katona C, Lyketsos CG, Blazer D, Ames D, et al. A systematic review of the effectiveness of non-pharmacologic interventions to improve quality of life and well-being in people with dementia. Am J Geriatr Psychiatry 2013;21:173-83. https://doi.org/10.1016/j.jagp.2012.10.018.
- Spector A, Orrell M, Aguirre E. Translating research into practice: a pilot study examining the use of cognitive stimulation therapy (CST) after a one-day training course. Nonpharmacol Ther Dement 2011;1:63-72.
- Aguirre E, Spector A, Hoe J, Russell IT, Knapp M, Woods RT, et al. Maintenance cognitive stimulation therapy (CST) for dementia: a single-blind, multi-centre, randomized controlled trial of maintenance CST vs. CST for dementia. Trials 2010;11. https://doi.org/10.1186/1745-6215-11-46.
- Aguirre E, Spector A, Streater A, Hoe J, Woods B, Orrell M. Making a Difference 2. London: Hawker Publications; 2012.
- Weiss DJ, Dawis RV, England GW, Lofquist LH, Lofquist LH, Dawis RV. Application of the Theory of Work Adjustment to Rehabilitation and Counseling. Minneapolis, MN: Work Adjustment Project Industrial Relations Center, University of Minnesota; 1967.
- Lintern T, Woods B. Approaches to Dementia Questionnaire. Bangor: University of Wales; 1996.
- Shanahan N, Orrell M, Schepers AK, Spector A. The development and evaluation of the DK-20: a knowledge of dementia measure. Int Psychogeriatr 2013;25:1899-907. https://doi.org/10.1017/S1041610213001142.
- Schepers AK, Orrell M, Shanahan N, Spector A. Sense of competence in dementia care staff (SCIDS) scale: development, reliability, and validity. Int Psychogeriatr 2012;24:1153-62. https://doi.org/10.1017/S104161021100247X.
- Holton EF, Bates RA, Ruona WEA. Development of a generalized learning transfer system inventory. Hum Res Dev Quarterly 2000;11:333-60. https://doi.org/10.1002/1532-1096(200024)11:4<333::AID-HRDQ2>3.0.CO;2-P.
- Holton EF, Bates R, Leimbach M, Torraco R. Proceedings of the 1997 Academy of Human Resource Development Annual Meeting. Atlanta, GA: Academy of Human Resource Development; 1997.
- Corrigan PW, Kwartarini WY, Pramana W. Staff perception of barriers to behavior therapy at a psychiatric hospital. Behav Modif 1992;16:132-44. https://doi.org/10.1177/01454455920161007.
- Dagnan D, Grant F, McDonnell A. Understanding challenging behaviour in older people; the development of the controllability Beliefs Scale. Behav Cogn Psychother 2004;32:501-6. https://doi.org/10.1017/S1352465804001675.
- Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993;43:2412-14. https://doi.org/10.1212/WNL.43.11.2412-a.
- Thorgrimsen L, Selwood A, Spector A, Royan L, de Madariaga Lopez M, Woods RT, et al. Whose quality of life is it anyway? The validity and reliability of the Quality of Life-Alzheimer’s Disease (QoL-AD) scale. Alzheimer Dis Assoc Disord 2003;17:201-8. https://doi.org/10.1097/00002093-200310000-00002.
- Hensel A, Angermeyer MC, Riedel-Heller SG. Measuring cognitive change in older adults: reliable change indices for the Mini-Mental State Examination. J Neurol Neurosurg Psychiatr 2007;78:1298-303. https://doi.org/10.1136/jnnp.2006.109074.
- Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1994.
- Kitwood T, Miesen BML, Jones GMM. Care-Giving in Dementia: Research and Applications. London: Routledge; 1997.
- Swaab DF. Brain ageing and Alzheimer’s disease: ‘wear and tear’ versus ‘use it or lose it’. Neurobiol Aging 1991;12:317-24. https://doi.org/10.1016/0197-4580(91)90008-8.
- Spector A, Orrell M. Using a biopsychosocial model of dementia as a tool to guide clinical practice. Int Psychogeriatr 2010;22:957-65. https://doi.org/10.1017/S1041610210000840.
- Ritchie J, Spencer L, Bryman A, Burgess RG. Analysing Qualitative Data. 1994.
- Burnard P. A method of analysing interview transcripts in qualitative research. Nurse Educ Today 1991;11:461-6. https://doi.org/10.1016/0260-6917(91)90009-Y.
- Berg BL. Qualitative Research Methods for the Social Sciences. Boston, MA: Allyn and Bacon; 1989.
- Taulbee LR, Folsom JC. Reality orientation for geriatric patients. Hosp Community Psychiatry 1966;17:133-5. https://doi.org/10.1176/ps.17.5.133.
- Fink A, Kosecoff J, Chassin M, Brook RH. Consensus methods: characteristics and guidelines for use. Am J Public Health 1984;74:979-83. https://doi.org/10.2105/AJPH.74.9.979.
- Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CF, Askham J, et al. Consensus development methods, and their use in clinical guideline development. Health Technol Assess 1998;2.
- Perry S, Kalberer JT. The NIH consensus-development program and the assessment of health-care technologies: the first two years. N Engl J Med 1980;303:169-72. https://doi.org/10.1056/NEJM198007173030334.
- Pickett-Schenk SA, Bennett C, Cook JA, Steigman P, Lippincott R, Villagracia I, et al. Changes in caregiving satisfaction and information needs among relatives of adults with mental illness: results of a randomized evaluation of a family-led education intervention. Am J Orthopsychiatry 2006;76:545-53. https://doi.org/10.1037/0002-9432.76.4.545.
- Pillemer K, Suitor J. Peer support for Alzheimer’s caregivers: is it enough to make a difference?. Res Aging 2002;24:171-92. https://doi.org/10.1177/0164027502242001.
- Toseland RW, Smith GC. Effectiveness of individual counseling by professional and peer helpers for family caregivers of the elderly. Psychol Aging 1990;5:256-63. https://doi.org/10.1037/0882-7974.5.2.256.
- Yardley L, Murray M, Marks DF, Yardley L, Murray M. Qualitative Analysis of Talk and Text. Research Methods for Clinical and Health Psychology. London: Sage; 2004.
- Hare P, Newbronner E. Looking After Me: Evaluating a New Self-Management Course for Carers. Report for the Expert Patient Programme and the Long-Term Medical Conditions Alliance 2004.
- Delbecq A, Van de Ven A. A group process model for problem identification and program planning. J Appl Behav Sci 1971;7:467-92. https://doi.org/10.1177/002188637100700404.
- Flesch R. A new readability yardstick. J Appl Psychol 1948;32:221-33. https://doi.org/10.1037/h0057532.
- Caygill L, Summerfield P, Ormrod J. Assessing the readability of mental health leaflets. J Community Nurs 2000;14:18-20.
- Staniszewska S, Jones N, Newburn M, Marshall S. User involvement in the development of a research bid: barriers, enablers and impacts. Health Expect 2007;10:173-83. https://doi.org/10.1111/j.1369-7625.2007.00436.x.
- Bandura A. Self-Efficacy: The Exercise of Control. New York, NY: WH Freeman and Company; 1997.
- Woods R, Bruce E, Edwards R, Elvish R, Hoare Z, Hounsome B, et al. REMCARE: Reminiscence groups for people with dementia and their family caregivers effectiveness and cost-effectiveness pragmatic multicentre randomised trial. Health Technol Assess 2012;16. https://doi.org/10.3310/hta16480.
- Charlesworth G, Burnell K, Hoe J, Orrell M, Russell I. Acceptance checklist for clinical effectiveness pilot trials: a systematic approach. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-78.
- Charlesworth G, Burnell K, Beecham J, Hoare Z, Hoe J, Wenborn J, et al. Peer support for family carers of people with dementia, alone or in combination with group reminiscence in a factorial design: study protocol for a randomised controlled trial. Trials 2011;12. https://doi.org/10.1186/1745-6215-12-205.
- Woods RT, Bruce E, Edwards RT, Hounsome B, Keady J, Moniz-Cook ED, et al. Reminiscence groups for people with dementia and their family carers: pragmatic eight-centre randomised trial of joint reminiscence and maintenance versus usual treatment: a protocol. Trials 2009;10. https://doi.org/10.1186/1745-6215-10-64.
- Schweitzer P, Bruce E. Remembering Yesterday, Caring Today – Reminiscence in Dementia Care: A Guide to Good Practice. London: Jessica Kingsley Publishers; 2008.
- Lichstein KL, Riedel BW, Grieve R. Fair tests of clinical trials: a treatment implementation model. Adv Behav Res Ther 1994;16:1-29. https://doi.org/10.1016/0146-6402(94)90001-9.
- Wenger GC. Support Networks of Older People: A Guide for Practitioners. Bangor: Centre for Social Policy Research and Development, University of Wales; 1994.
- Jenkinson C, Layte R. Development and testing of the UK SF-12 (short form health survey). J Health Serv Res Policy 1997;2:14-8.
- Snaith RP. The Hospital Anxiety And Depression Scale. Health Qual Life Outcomes 2003;1. https://doi.org/10.1186/1477-7525-1-29.
- Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002;52:69-77. https://doi.org/10.1016/S0022-3999(01)00296-3.
- Stroebe W, Stroebe M, Abakoumkin G, Schut H. The role of loneliness and social support in adjustment to loss: a test of attachment versus stress theory. J Pers Soc Psychol 1996;70:1241-9. https://doi.org/10.1037/0022-3514.70.6.1241.
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 1988;54:1063-70. https://doi.org/10.1037/0022-3514.54.6.1063.
- McKee KJ, Philp I, Lamura G, Prouskas C, Oberg B, Krevers B, et al. The COPE index – a first stage assessment of negative impact, positive value and quality of support of caregiving in informal carers of older people. Aging Ment Health 2003;7:39-52. https://doi.org/10.1080/1360786021000006956.
- Ryff CD, Keyes CL. The structure of psychological well-being revisited. J Pers Soc Psychol 1995;69:719-27. https://doi.org/10.1037/0022-3514.69.4.719.
- Spruytte N, Van Audenhove C, Lammertyn F, Storms G. The quality of the caregiving relationship in informal care for older adults with dementia and chronic psychiatric patients. Psychol Psychother 2002;75:295-311. https://doi.org/10.1348/147608302320365208.
- Carver CS. You want to measure coping but your protocol’s too long: consider the brief COPE. Int J Behav Med 1997;4:92-100. https://doi.org/10.1207/s15327558ijbm0401_6.
- Cooper C, Katona C, Livingston G. Validity and reliability of the brief COPE in carers of people with dementia: the LASER-AD Study. J Nerv Ment Dis 2008;196:838-43. https://doi.org/10.1097/NMD.0b013e31818b504c.
- Steffen AM, McKibbin C, Zeiss AM, Gallagher-Thompson D, Bandura A. The revised scale for caregiving self-efficacy: reliability and validity studies. J Gerontol B Psychol Sci Soc Sci 2002;57:P74-86. https://doi.org/10.1093/geronb/57.1.P74.
- Crellin N, Charlesworth G, Orrell M. Measuring family caregiver efficacy for managing behavioural and psychological symptoms in dementia: a psychometric evaluation. Int Psychogeriatr 2014;26:93-103.
- Newsom JT, Rook KS, Nishishiba M, Sorkin DH, Mahan TL. Understanding the relative importance of positive and negative social exchanges: examining specific domains and appraisals. J Gerontol B Psychol Sci Soc Sci 2005;60:P304-12. https://doi.org/10.1093/geronb/60.6.P304.
- Identifying Alternatives to Hospital for People with Dementia: Report of Findings. London: National Audit Office; 2007.
- Sampson EL, Blanchard MR, Jones L, Tookman A, King M. Dementia in the acute hospital: prospective cohort study of prevalence and mortality. Br J Psychiatry 2009;195:61-6. https://doi.org/10.1192/bjp.bp.108.055335.
- National Service Framework for Mental Health: Modern Standards and Service Models. London: Department of Health; 1999.
- Martin F, Oyewole A, Moloney A. A randomized controlled trial of a high support hospital discharge team for elderly people. Age Ageing 1994;23:228-34. https://doi.org/10.1093/ageing/23.3.228.
- McCusker J, Verdon J. Do geriatric interventions reduce emergency department visits? A systematic review. J Gerontol A Biol Sci Med Sci 2006;61:53-62. https://doi.org/10.1093/gerona/61.1.53.
- Ratna L. Crisis intervention in psychogeriatrics: a two-year follow-up study. Br J Psychiatry 1982;141:296-301. https://doi.org/10.1192/bjp.141.3.296.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] 2011. www.cochrane-handbook.org (accessed 7 November 2012).
- Newcomer R, Spitalny M, Fox P, Yordi C. Effects of the Medicare Alzheimer’s Disease Demonstration on the use of community-based services. Health Serv Res 1999;34:645-67.
- Newcomer R, Miller R, Clay T, Fox P. Effects of the Medicare Alzheimer’s disease demonstration on Medicare expenditures. Health Care Financ Rev 1999;20:45-6.
- Pimouguet C, Lavaud T, Dartigues JF, Helmer C. Dementia case management effectiveness on health care costs and resource utilization: a systematic review of randomized controlled trials. J Nutr Health Aging 2010;14:669-76. https://doi.org/10.1007/s12603-010-0314-4.
- Somme D, Trouve H, Dramé M, Gagnon D, Couturier Y, Saint-Jean O. Analysis of case management programs for patients with dementia: a systematic review. Alzheimers Dement 2012;8:426-36. https://doi.org/10.1016/j.jalz.2011.06.004.
- Jansen AP, van Hout HP, Nijpels G, Rijmen F, Dröes RM, Pot AM, et al. Effectiveness of case management among older adults with early symptoms of dementia and their primary informal caregivers: a randomized clinical trial. Int J Nurs Stud 2011;48:933-43. https://doi.org/10.1016/j.ijnurstu.2011.02.004.
- Albert SM, Costa R, Merchant C, Small S, Jenders RA, Stern Y. Hospitalization and Alzheimer’s disease: results from a community-based study. J Gerontol A Biol Sci Med Sci 1999;54:M267-71. https://doi.org/10.1093/gerona/54.5.M267.
- Nourhashémi F, Andrieu S, Sastres N, Ducassé JL, Lauque D, Sinclair AJ, et al. Descriptive analysis of emergency hospital admissions of patients with Alzheimer disease. Alzheimer Dis Assoc Disord 2001;15:21-5. https://doi.org/10.1097/00002093-200101000-00003.
- Andrieu S, Reynish E, Nourhashemi F, Shakespeare A, Moulias S, Ousset PJ, et al. Predictive factors of acute hospitalization in 134 patients with Alzheimer’s disease: a one year prospective study. Int J Geriatr Psychiatry 2002;17:422-6. https://doi.org/10.1002/gps.624.
- Ibach B, Poljansky S, Barta W, Koller M, Wittmann M, Hajak G. Working Group Geriatric Psychiatry Germany . Patterns of referring of patients with frontotemporal lobar degeneration to psychiatric in- and out-patient services. Results from a prospective multicentre study. Dement Geriatr Cogn Disord 2004;17:269-73. https://doi.org/10.1159/000077152.
- Carter MW, Porell FW. Vulnerable populations at risk of potentially avoidable hospitalizations: the case of nursing home residents with Alzheimer’s disease. Am J Alzheimers Dis Other Demen 2005;20:349-58. https://doi.org/10.1177/153331750502000605.
- Natalwala A, Potluri R, Uppal H, Heun R. Reasons for hospital admissions in dementia patients in Birmingham, UK, during 2002–7. Dement Geriatr Cogn Disord 2008;26:499-505. https://doi.org/10.1159/000171044.
- Malone D, Mucha L, McLaughlin T, Sklar A, Harley C. Increased risk of serious co-morbidities in a cohort of AD patients compared to a similar non-AD cohort. Alzheimers Dement 2009;5. https://doi.org/10.1016/j.jalz.2009.04.381.
- Tuppin P, Kusnik-Joinville O, Weill A, Ricordeau P, Allemand H. Primary health care use and reasons for hospital admissions in dementia patients in France: database study for 2007. Dement Geriatr Cogn Disord 2009;28:225-32. https://doi.org/10.1159/000238394.
- Orrell M, Bebbington P. Life events and senile dementia. I. Admission, deterioration and social environment change. Psychol Med 1995;25:373-86. https://doi.org/10.1017/S0033291700036278.
- Phelan EA, Borson S, Grothaus L, Balch S, Larson EB. Association of incident dementia with hospitalizations. JAMA 2012;307:165-72. https://doi.org/10.1001/jama.2011.1964.
- Improving Services and Support for People with Dementia. London: National Audit Office; 2007.
- Report of the National Audit of Dementia Care in General Hospitals. London: Royal College of Psychiatrists; 2011.
- Doyle H, Varian J. Crisis intervention in psychogeriatrics: a round the clock commitment?. Int J Geriatr Psychiatry 1994;9:65-72. https://doi.org/10.1002/gps.930090114.
- Dibben C, Saeed H, Stagias K, Khandaker GM, Rubinsztein JS. Crisis resolution and home treatment teams for older people with mental illness. Psychiatr Bull 2008;32:268-70. https://doi.org/10.1192/pb.bp.107.018218.
- Cooper C, Regan C, Tandy AR, Johnson S, Livingston G. Acute mental health care for older people by crisis resolution teams in England. Int J Geriatr Psychiatry 2007;22:263-5. https://doi.org/10.1002/gps.1650.
- Michon A. Crisis intervention in dementia. Psychol Neuropsychiatr Vieil 2006;4:121-5.
- Parker J. Crisis intervention: a practice model for people who have dementia and their carers. Pract Soc Work Action 2007;19:115-26. https://doi.org/10.1080/09503150701393635.
- The Mental Health Policy Implementation Guide. London: Department of Health; 2001.
- Securing Mental Health for Older Adults. London: Department of Health; 2004.
- Sainsbury P, Costain WR, Grad J. Psychiatric Disorders in the Aged. Manchester: Geigy; 1965.
- Mental Health Act 2007. London: The Stationery Office; 2007.
- Yaffe K, Fox P, Newcomer R, Sands L, Lindquist K, Dane K, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA 2002;287:2090-7. https://doi.org/10.1001/jama.287.16.2090.
- Gaugler JE, Kane RL, Newcomer R. Resilience and transitions from dementia caregiving. J Gerontol B Psychol Sci Soc Sci 2007;62:P38-44. https://doi.org/10.1093/geronb/62.1.P38.
- Smith GE, O’Brien PC, Ivnik RJ, Kokmen E, Tangalos EG. Prospective analysis of risk factors for nursing home placement of dementia patients. Neurology 2001;57:1467-73. https://doi.org/10.1212/WNL.57.8.1467.
- Spitznagel MB, Tremont G, Duncan Davis J, Foster SM. Psychosocial predictors of dementia caregiver desire to institutionalise: caregiver, care recipient, and family relationship factors. J Geriatr Psychiatry Neurol 2006;19. https://doi.org/10.1177/0891988705284713.
- Kim JM, Shin IS, Jeong SJ, Gormley N, Yoon JS. Predictors of institutionalization in patients with dementia in Korea. Int J Geriatr Psychiatry 2002;17:101-6. https://doi.org/10.1002/gps.494.
- Banerjee S, Murray J, Foley B, Atkins L, Schneider J, Mann A. Predictors of institutionalisation in people with dementia. J Neurol Neurosurg Psychiatr 2003;74:1315-16. https://doi.org/10.1136/jnnp.74.9.1315.
- Luppa M, Luck T, Brähler E, König HH, Riedel-Heller SG. Prediction of institutionalisation in dementia. A systematic review. Dement Geriatr Cogn Disord 2008;26:65-78. https://doi.org/10.1159/000144027.
- Pasquini M, Leys D, Rousseaux M, Pasquier F, Hénon H. Influence of cognitive impairment on the institutionalisation rate 3 years after a stroke. J Neurol Neurosurg Psychiatr 2007;78:56-9. https://doi.org/10.1136/jnnp.2006.102533.
- Toot S, Devine M, Orrell M. The effectiveness of crisis resolution/home treatment teams for older people with mental health problems: a systematic review and scoping exercise. Int J Geriatr Psychiatry 2011;26:1221-30. https://doi.org/10.1002/gps.2686.
- Wilkinson S. Focus groups in health research: exploring the meanings of health and illness. J Health Psychol 1998;3:329-48. https://doi.org/10.1177/135910539800300304.
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349-57. https://doi.org/10.1093/intqhc/mzm042.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Fereday J, Muir-Cochrane E. Demonstrating rigor using thematic analysis: a hybrid approach of inductive and deductive coding and theme development. Int J Qual Methods 2006;5.
- Parker J, Bradley G. Transforming Social Work Practice in Assessment, Planning Intervention and Review. Exeter: Learning Matters; 2003.
- Toot S, Hoe J, Ledgerd R, Burnell K, Devine M, Orrell M. Causes of crises and appropriate interventions: the views of people with dementia, carers and healthcare professionals. Aging Ment Health 2013;17:328-35. https://doi.org/10.1080/13607863.2012.732037.
- Denson LA, Winefield HR, Beilby JJ. Discharge planning for long term care needs: the values and priorities of older people, their younger relatives and health professionals. Scand J Caring Sci 2012;27. https://doi.org/10.1111/j.1471-6712.2012.00987.x.
- Harmer BJ, Orrell M. What is meaningful activity for people with dementia living in care homes? A comparison of the views of older people with dementia, staff and family carers. Aging Ment Health 2008;12:548-58. https://doi.org/10.1080/13607860802343019.
- Skills for Care and Dementia UK . Dementia: Workers &Amp; Carers Together. A Guide for Social Care Workers on Supporting Family And Friends Carers of People With Dementia 2012. www.dementiauk.org/assets/files/what_we_do/uniting_carers/Dementia_Workers_and_Carers_Together.pdf (accessed 13 December 2013).
- Living Well with Dementia – The National Dementia Strategy: Joint Commissioning Framework for Dementia. London: Department of Health; 2009.
- Sörensen S, Duberstein P, Gill D, Pinquart M. Dementia care: mental health effects, intervention strategies, and clinical implications. Lancet Neurol 2006;5:961-73. https://doi.org/10.1016/S1474-4422(06)70599-3.
- Care Quality Commission . Care Quality Commission Care Update Issue 2: March 2013 2013. www.cqc.org.uk/sites/default/files/media/documents/cqc_care_update_issue_2.pdf (accessed 12 January 2013).
- Rowe G, Poortinga W, Pidgeon N. A comparison of responses to internet and postal surveys in a public engagement context. Sci Commun 2006;27:352-75. https://doi.org/10.1177/1075547005284668.
- Eysenbach G, Wyatt J. Using the internet for surveys and health research. J Med Internet Res 2002;4. https://doi.org/10.2196/jmir.4.2.e13.
List of abbreviations
- AChEI
- acetylcholinesterase inhibitor
- ADAS-Cog
- Alzheimer’s Disease Assessment Scale – Cognitive Subscale
- ADCS-ADL
- Alzheimer’s Disease Co-Operative Study – Activities of Daily Living Inventory
- ADL
- activity of daily living
- ADQ
- Approaches to Dementia Questionnaire
- ALOIS
- Action in Language, Organisations and Information Systems
- ANCOVA
- analysis of covariance
- BECCA
- Befriending and Costs Of Caring
- CANE
- Camberwell Assessment of Need for the Elderly
- CDCIG
- Cochrane Dementia and Cognitive Improvement Group
- CDR
- Clinical Dementia Rating
- CEAC
- cost-effectiveness acceptability curve
- CEBM
- Centre for Evidence Based Medicine
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CMHT
- community mental health team
- CONSORT
- Consolidated Standards of Reporting Trials
- COPE
- Carers of Older People in Europe
- CRHTT
- crisis resolution home treatment team
- CSP
- Carer Supporter Programme
- CSRI
- Client Services Receipt Inventory
- CST
- cognitive stimulation therapy
- DEMQOL
- Dementia Quality of Life
- DeNDRoN
- Dementias and Neurodegenerative Diseases Network
- df
- degree of freedom
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition
- DVD
- digital versatile disc
- EQ-5D
- EuroQoL-5 Dimensions
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HTP
- home treatment package
- HTT
- home treatment team
- ICER
- incremental cost-effectiveness ratio
- ISRCTN
- International Standard Randomised Controlled Trial Number
- LILACS
- Latin American and Caribbean Health Sciences Literature
- LTSI
- Learning Transfer System Inventory
- MCS-12
- mental component summary
- MCST
- maintenance cognitive stimulation therapy
- MMSE
- Mini-Mental State Examination
- MONOU
- Monitoring and Outreach
- MRC
- Medical Research Council
- NELFT
- North East London NHS Foundation Trust
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NPI
- Neuropsychiatric Inventory
- NWORTH
- North Wales Organisation for Randomised Trials in Health
- PCA
- person-centred approach
- PCS-12
- physical component summary
- PhD
- Doctor of Philosophy
- QALY
- quality-adjusted life-year
- QCPR
- Quality of Caregiver–Patient Relationship
- QOL-AD
- Quality of Life – Alzheimer’s Disease
- R&D
- research and development
- RCT
- randomised controlled trial
- REMCARE
- REMiniscence groups for people with dementia and their family CAREgivers
- RR
- relative risk
- RYCT
- Remembering Yesterday, Caring Today
- SAE
- serious adverse event
- SD
- standard deviation
- SE
- standard error
- SF-12
- Short Form-12 Health Survey
- SHIELD
- Support At Home – Interventions to Enhance Life in Dementia
- SMD
- standardised mean difference
- SPSS
- Statistical Product and Service Solutions
- STANDOUT
- Staff Training and Outreach
- TAU
- treatment as usual
- VAS
- visual analogue scale
- WTP
- willingness to pay