Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0407-10452. The contractual start date was in August 2008. The final report began editorial review in December 2015 and was accepted for publication in September 2016. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Lucilla Poston has received payment from International Life Sciences Institute Europe as reimbursement of expenses incurred in attending a workshop on obese pregnancy and long-term outcomes and was paid as a member of the Tate and Lyle Research Advisory Group 2007–10, before submission of this work. Lucilla Poston also reports a research grant from Abbott Nutrition, outside the submitted work. Thomas AB Sanders reports personal consultancy fees from the Natural Hydration Council, Heinz Foods, Archer Daniels Midland, the Global Dairy Platform and GlaxoSmithKline, outside the submitted work, and is a trustee and scientific governor for the British Nutrition Foundation, outside the submitted work. Keith M Godfrey reports reimbursement of travel and accommodation expenses from the Nestlé Nutrition Institute, outside the submitted work; research grants from Abbott Nutrition and Nestec, outside the submitted work; and patents pending for phenotype prediction, predictive use of 5‘-C-phosphate-G-3’ (CPG) methylation and maternal nutrition composition, outside the submitted work. During the period of research reported here, Jane Sandall was a member of the National Institute for Health Research (NIHR) Programme Grants for Applied Research core group of methodological experts (2011–15), and of the NIHR Health Services and Delivery Research Programme Commissioning Board (2012–15) and Stephen C Robson was a Medical Research Council/NIHR Efficacy and Mechanism Evaluation board member (2012–15).
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Poston et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 The UK Pregnancies Better Eating and Activity Trial phase 1 – development phase: involvement of patients and providers in development of the UK Pregnancies Better Eating and Activity Trial intervention
This programme of work was designed to develop a behavioural intervention combining dietary and physical activity (PA) advice to improve insulin sensitivity, and thereby reduce gestational diabetes mellitus (GDM) and large-for-gestational age (LGA) deliveries, in obese pregnant women. The programme followed the UK’s Medical Research Council framework for the development and evaluation of complex interventions, which is delineated by three phases. 1
-
Development phase: to determine, following a review of the relevant literature, the best approach and method of delivery of the proposed intervention, develop and standardise the content and delivery method, and assess feasibility and acceptability to women and providers with a view to optimising the intervention for use in a pilot trial.
-
Pilot: to undertake a pilot trial to establish the efficacy of the intervention in changing dietary and PA behaviours, and to evaluate all practical aspects of delivering the intervention.
-
Randomised controlled trial (RCT): to undertake a RCT to determine whether or not the intervention reduces GDM and LGA deliveries in obese pregnant women.
The aim of phase 1 was to determine the best approach and method of delivery for the proposed intervention, develop and standardise content and delivery method, and assess feasibility and acceptability to women and providers with a view to optimising the intervention for use in a pilot trial (phase 2).
Objectives and design
To assess appropriateness, acceptability and delivery of the intervention in target groups
To understand likely motivations for, and barriers to, changes in diet and activity in pregnancy by developing a quantitative measure of knowledge and attitudes about healthy eating and activity.
At the onset of this programme there were no pre-existing validated instruments to assess attitudes towards diet and PA in obese pregnant women. Initial work was undertaken to explore the relative clinical effectiveness of different behaviour change strategies used in previous studies of healthy lifestyle interventions for pregnant women. All relevant studies had focused on the reduction of gestational weight gain (GWG) as the primary end point. None was targeting insulin resistance, which was the focus of this programme. Ten controlled trials of interventions that aimed to reduce GWG through changes in diet or PA were reviewed. Meta-analysis showed that, overall, diet and PA change was effective in reducing GWG, but there was considerable heterogeneity in outcomes. 2 The analysis showed that sample characteristics and aspects of intervention design, content, delivery and evaluation differed between studies, and that these were likely to explain the variation between studies in effectiveness. A common issue was the failure to evaluate changes in behaviour or its psychological determinants, as well as inadequate detail of the intervention content. These were likely to have contributed to difficulty in identification of the processes by which weight change was effected. Because of this, it was difficult to discern active intervention ingredients. The study concluded that behaviour-based GWG reduction interventions should be more systematically designed, evaluated and reported to build on insights from behavioural science. This important conclusion reinforced our intention to systematically develop an evidenced-based intervention based on known theory, and our decision to pilot the intervention to evaluate all aspects of the intervention, particularly to evaluate change in behaviour, acceptability and its psychological determinants.
A questionnaire to measure attitudes towards diet and PA in pregnancy was then developed, based on psychological models of determinants of health behaviour, previous literature in pregnant women and interviews conducted with pregnant women (see Appendix 1). The questionnaire comprised the following constructs: attitudes to healthy behaviours, social norms, intention to change behaviour, knowledge regarding nutritional and PA recommendations and motivations for food choices. The outcomes of a study using this questionnaire were published as below by Gardner B, Croker H, Barr S, Briley A, Poston L, Wardle J; UPBEAT Trial. Psychological predictors of dietary intentions in pregnancy. J Hum Nutr Diet 2012;4:345–53. 3 Minor formatting edits have been made to the published text, which is reproduced with permission.
Psychological predictors of dietary intentions in pregnancy
Abstract
Background: Consuming a healthy diet in pregnancy has the potential to improve obstetric outcome, including minimising the risk of macrosomia. Effective promotion of dietary change depends on identifying and targeting determinants of gestational diet. The present study aimed to model psychological predictors of intentions to reduce the intake of high-fat and high-sugar foods, and increase fruit and vegetable (F&V) consumption, among pregnant women.
Methods: One hundred and three pregnant women completed questionnaire measures of intentions to modify the consumption of the target foods, current intake, perceived vulnerability to, and severity of, adverse outcomes of non-healthful consumption of these foods (i.e. ‘threat’), benefits of dietary change to mother and baby, barriers to dietary changes and social approval for dietary change (‘subjective norms’). A cross-sectional design was used. Logistic regression analyses were undertaken to model dietary change intentions.
Results: Participants who reported excessive current intake of high-fat and high-sugar foods were more likely to commit to reducing the intake of these foods. The perceived benefits for mother and baby enhanced the mothers’ intentions to eat more F&V and fewer high-fat foods and marginally significantly increased their intentions to reduce the consumption of high-sugar foods. There were no effects of threat, barriers or subjective norms.
Conclusions: Lack of effects for barriers, threat and subjective norms may indicate that pregnant women discount barriers to health-promoting behaviour, understand the threat posed by unhealthy eating and perceive social approval from others. Dietary change interventions for pregnant women should emphasise likely positive outcomes for both mother and child.
Introduction
Research in gestational diet and nutrition has traditionally focused on preventing nutritional deficiencies in the maternal diet and ensuring adequate neonatal growth. 4 There is, however, growing interest in the potential for dietary changes among pregnant women with GDM or obesity to minimise the risk of macrosomia and associated adverse outcomes, including the risk of obesity in the child in later life. 4–6 Maternal glucose is the main substrate for the growing fetus and, consequently, maternal hyperglycaemia may result in fetal hyperinsulinaemia, thereby increasing growth rate and thus the risk of macrosomia. 7 Maternal insulin sensitivity reduces with advancing pregnancy. 8 Certain dietary interventions might, theoretically, reduce insulin resistance among pregnant women. 9 Reducing the consumption of foods rich in carbohydrates that release glucose rapidly into the bloodstream [i.e. high-glycaemic index (GI) foods] can promote glycaemic control and improve insulin sensitivity by manipulating the type of carbohydrate-rich foods and drinks consumed rather than necessarily restricting the quantity of carbohydrates per se. 7,10 A recent review found some evidence to support the use of low-GI diets, defined by the consumption of carbohydrates that release glucose gradually, in women with GDM, although it is less clear whether or not they could also benefit non-diabetic women. 7 Two intervention trials in non-diabetic women have yielded promising results, with reduced delivery rates of babies who are LGA (i.e. > 90th birthweight percentile) being observed among women who ate a low-GI diet relative to those consuming a usual diet. 11,12 Notably, both studies included women with a range of body weights, suggesting that dietary glycaemic control may benefit both normal and overweight women, although there are some concerns over ensuring adequate fetal growth, particularly in normal-weight women. 13 Another study investigating overweight and obese pregnant women found that a low-glycaemic load (GL) diet (i.e. foods with low values on an index that accounts for both GI value and carbohydrate content) improved cardiovascular risk factors, lengthened pregnancy duration and increased infant head circumference. 14 Further clinical trials are planned or are under way that aim to explore the impact of such diets on pregnancy outcome in obese women, these include the UK Pregnancies: Better Eating and Activity Trial [UPBEAT; International Standard Randomised Controlled Trial Number (ISRCTN) 89971375], described in this programme, and another in women who have had a previous macrosomic pregnancy. 15
Reducing the GI relies on changing dietary behaviour in pregnant women. Changing pregnant women’s food choices will be aided by identifying and targeting the determinants of diet in pregnancy. Behaviour change is often portrayed as a consequence of changes in psychological variables: modifying knowledge, attitudes and beliefs in turn influences intentions16 and ultimately alters behaviour. 17 However, interventions designed to improve diet in pregnancy, including those designed specifically to lower the GI or GL,12,14 have neglected psychological changes. 2,18 Identifying psychological determinants of dietary decisions in pregnancy would assist intervention development in two respects. First, psychological variables represent potential targets for intervention. Second, assessing changes on these variables can aid our understanding of why diet has or has not changed in response to an intervention. 17 However, little empirical evidence is available regarding the psychological determinants of dietary behaviour in pregnancy.
Pregnancy has been portrayed as a transitional life event that is psychologically characterised by heightened awareness of (and responsiveness to) threats to the health of either mother or child. 19 Risk perceptions may therefore underpin motivation to take health-protective action in pregnancy. The health belief model (HBM)20 proposes that responses to risk are underpinned by two psychological dimensions: threat perception and behavioural evaluation. 21 Threat perceptions are a function of perceived susceptibility to, and perceived severity of, a threat (e.g. GWG or its associated health complications), and behavioural evaluations refer to the expected benefits of, and barriers to, taking action (e.g. consuming a nutritionally balanced diet) to avert the threat. The HBM predicts that protective behaviour will be elicited where the individual perceives themselves as vulnerable to a serious threat, the action is deemed beneficial and there are few barriers to taking action. Although originally proposed as direct determinants of health behaviour, subsequent research has indicated that the HBM constructs largely influence action indirectly via the formation of intentions, which subsequently determine behavioural responses. 16
As determinants of intention, the threat perceptions and cost–benefit analyses proposed by the HBM compete with social influences in guiding health motivation in pregnancy. 22 For example, intentions to consume a healthy diet in pregnancy are likely to be a function not only of the subjective utility of a nutritionally balanced diet for averting health threats, but also expected (dis)approval from relevant others for consuming such a diet. 22 Expectations of friends, family and health-care professionals may therefore contribute to dietary decisions in pregnancy independently of a subjective threat and behaviour evaluations.
Dietary change focused on a reduced intake of saturated fat and high-sugar foods, as well as increased F&V consumption, has been proposed as an approach for reducing insulin resistance in pregnancy,9 and is consistent with the UK national guidance for healthy eating in pregnancy. 23 The present study assessed pregnant women’s attitudes and motivations concerning these food types, and examined which psychological variables predicted intentions to consume healthier quantities of these foods over the remainder of the pregnancy term. Our analysis draws on variables from the HBM,20 augmented by a measure of perceived social approval (i.e. ‘subjective norms’). 22 To isolate the unique influence of psychological variables on behaviour change intentions, we controlled for the current intake of each of the three foods, as well as the pregnancy-related personal characteristics that may influence dietary intentions or behaviour [pre-pregnancy body mass index (BMI), gestational age and parity]. 24,25
Materials and methods
Participants and procedures
Participants were approached, in person, by a researcher at antenatal clinics and given a paper questionnaire (together with a prepaid envelope) for return (see Appendix 1). One hundred and three pregnant women returned completed questionnaires. Data were collected from one hospital outpatient clinic and five community clinics in south-east England (n = 92) and one hospital outpatient clinic in north-east England (n = 11). Response rate data were not available. A cross-sectional design was used. Ethics approval for the study was obtained from a NHS Research Ethics Committee (reference number 09/H0802/5).
Measures
Personal characteristics
Participants completed self-report measures of parity, gestational age (in weeks), height (m/cm or ft/in) and pre-pregnancy weight (kg or st). Height and weight measures were used to compute pre-pregnancy BMI values. For sample description purposes, participants also indicated their ethnicity and highest educational qualification.
Psychological variables
Unless otherwise indicated, psychological variables were measured using single items in the form of statements with which participants indicated (dis)agreement on a five-point scale (1 = strongly disagree to 5 = strongly agree). Scores on multi-item scales represented the mean of all component items. Measures related to three dietary behaviours in pregnancy: eating (more) F&V, eating (less) high-fat food and eating (less) high-sugar food.
Current behaviour was estimated based on the perceived adequacy of current dietary intake [‘Do you think the amount of (F&V/high-fat food/high-sugar food) you eat is much too little/too little/about right/too much/much too much?’]. Participants who reported eating ‘too much’ F&V or ‘too little’ high-fat or high-sugar food were excluded from analyses; this removed nine participants (7%) from the high-fat and high-sugar food analyses, respectively, and four participants (3%) from the F&V analyses. Remaining values were dichotomised to denote deficient current health behaviour (‘much too little’ or ‘too little’ F&V, ‘too much’ or ‘much too much’ high-fat or high-sugar foods, coded as 0), or adequate current behaviour (‘about right’, coded 1).
Intention items followed a stem: ‘Over the rest of your pregnancy, do you intend to eat much less/eat a little less/ not change/eat a little more/eat much more [F&V, high-sugar foods, high-fat foods]?’. Participants intending to eat less healthily (i.e. less F&V, more high-sugar food or more high-fat food, one participant per behaviour, 1%) were excluded from respective analyses. Intention scores were transformed into binary values to represent no intended change (coded 0) or an intention to consume a healthier diet (coded 1, i.e. more F&V, less high-fat food and less high-sugar food).
Perceived vulnerability and perceived severity were each measured using two items. Items related to adverse health outcomes for mother and baby separately [e.g. vulnerability, ‘Eating too few F&V could cause problems for (me/my baby)’; severity, ‘I would worry about (my health/my baby’s health) if I ate too few F&V’]. For each behaviour, all four items were consistently highly intercorrelated (minimum a = 0.91) and so were combined into a composite threat measure.
Perceived benefits related to positive outcomes for mother [e.g. ‘Eating (more F&V/less high-fat foods/less high-sugar foods) than I do now would . . . make me look better/make me feel better/prevent me putting on too much extra weight’] and baby (‘. . . be good for my baby/reduce my chances of having a baby that is too big’). Items were adapted from previous research. 26,27 For each behaviour, the five items were combined into a reliable composite index (minimum a = 0.82).
Perceived barriers items were combined into behaviour-specific indices. Barriers to consuming F&V related to difficulty of access and preparation, and cost (e.g. ‘It costs too much to eat more F&V’). Barriers to reducing fat consumption related to high-fat foods being easy to cook, satisfying cravings and helping deal with stress. Barriers to reducing sugar consumption related to using high-sugar foods to satisfy cravings and helping deal with stress.
For each behaviour, subjective norm items, which focused on expected approval from family and health-care professionals, respectively (e.g. ‘My family would approve of me eating fewer high-fat foods during pregnancy’), were combined because of strong inter-item correlations (minimum, r = 0.66; minimum, a = 0.80).
Statistical analysis
Data were analysed using predictive analytics software (PASW Statistics version 18.0; SPSS Inc., Chicago, IL, USA). Multiple logistic regressions were run to explore psychological predictors of intentions to consume a healthier diet, controlling for personal characteristics (pre-pregnancy BMI, gestational age, parity) and current behaviour. For ease of interpretation, psychological variables (threat, benefits, barriers, subjective norms) were dichotomised, using a median split to separate lower (below the median value) and higher scores (at or above the median).
Cases with missing intention values were excluded from the analyses to ensure that dependent variable scores were observed rather than estimated. This removed four cases relating to F&V consumption, 12 for high-fat consumption and 11 for high-sugar consumption, thereby reducing the sample size to n = 99 for analyses of F&V consumption intentions, n = 91 for high-fat foods and n = 92 for high-sugar foods.
Results
Sample characteristics
Participants were aged from 20 to 45 years (mean age 33 years), and between 8 and 40 weeks pregnant (mean 27 weeks). The majority (n = 49; 48%) were in the second trimester (13–28 weeks) or third trimester (29 weeks to birth; n = 47; 46%), with seven participants (7%) in the first trimester (up to 12 weeks) of pregnancy. Most participants (n = 68; 66%) were nulliparous. Pre-pregnancy BMI was in the range 14.8–45.8 kg/m2 (mean 24.9 kg/m2). Twenty-two participants (21%) were overweight (BMI of ≥ 25.0 kg/m2 < 30.0 kg/m2) and a further 17 participants (17%) were obese (BMI of ≥ 30.0 kg/m2).
Participants were predominantly of white ethnic origin (n = 72; 70%). Eleven participants (11%) were of black or black British ethnicity, eight were Asian or Asian British (8%), nine were of mixed (n = 6; 6%) or other ethnicity (n = 3; 3%) and three participants did not indicate their ethnicity. Most had a university education (n = 74; 72%). Ten participants (10%) had an A-level education, 15 participants (15%) had National Vocational Qualifications or General Certificate of Secondary Education qualifications, and one participant had no formal qualifications (1%). Three participants (3%) did not report qualifications.
Descriptive statistics
Most participants intended to increase their F&V consumption (n = 66, 67%) or decrease their high sugar intake (n = 54, 57%) during their pregnancy, although fewer than half of the participants intended to reduce their fat consumption (n = 41, 45%). Only a minority felt that they ate too few F&V (n = 21, 21%), too much high-sugar food (n = 31, 34%) or too much high-fat food (n = 26%), with most participants viewing their current intake of these foods as ‘about right’.
Current behaviour and intentions were negatively correlated for each behaviour: participants who felt they ate ‘too little’ F&V had stronger intentions to increase F&V consumption (r = –0.26; p = 0.009) and those who felt they consumed ‘too much’ high-sugar or high-fat foods were more likely to want to decrease their intake (r = –0.41 and –0.42; p < 0.001, respectively).
Correlations were observed between high-fat and high-sugar consumption intentions (r = 0.69; p < 0.001) and between current intake of high-fat and high-sugar foods (r = 0.57; p < 0.001), indicating similar beliefs and behaviour surrounding these two foods. Weaker correlations were observed between F&V intentions and high-fat or high-sugar intentions (maximum r = 0.35; p < 0.001). Correlations between perceived adequacy of F&V consumption and of high-fat or high-sugar foods (maximum r = 0.22; p = 0.03) were moderate in size.
Participants tended to be aware of the potential health threat associated with unhealthy dietary consumption in pregnancy (mean score 4.25), although the perceived benefits of healthy eating for mother and baby were more modest (mean score 3.66; Table 1). Average barrier scores were consistently below the scale mid-point, implying that participants felt relatively unhindered in changing their diet, although participants tended to expect even fewer barriers to consuming more F&V (mean score 1.81) than to reducing high-fat (mean score 2.78) or high-sugar foods (mean score 2.85). Social approval from family and health-care professionals was seen as supportive for all three behaviours (mean score 3.91) and strong intergroup correlations for each behaviour reflected perceived agreement between family and health-care professionals in this respect.
| Variables | Food type | ||
|---|---|---|---|
| F&V (n = 99) | High-fat food (n = 91) | High-sugar food (n = 92) | |
| Perceived adequacy of current behaviour, n (%) | About right: 78 (78%) | About right: 67 (74%) | About right: 61 (66%) |
| Too few F&V: 21 (21%) | Too much high-fat food: 24 (26%) | Too much high-sugar food: 31 (34%) | |
| Intention to engage in health behaviour, n (%) | Do not intend to change: 33 (33%) | Do not intend to change: 50 (55%) | Do not intend to change: 38 (41%) |
| Intend to increase: 66 (67%) | Intend to decrease: 41 (45%) | Intend to decrease: 54 (59%) | |
| Threat (posed by inaction), mean (SD), median | 4.25 (0.78), 4.25 | 4.39 (0.53), 4.25 | 4.43 (0.55), 4.38 |
| Benefits of healthy behaviour for mother and baby, mean (SD), median | 3.66 (0.67), 3.80 | 3.67 (0.81), 3.80 | 3.65 (0.84), 3.60 |
| Barriers to healthy behaviour, mean (SD), median | 1.81 (0.82), 1.67 | 2.78 (0.72), 2.67 | 2.85 (1.02), 3.00 |
| Subjective norms (family, health professionals), mean (SD), median | 4.07 (0.89), 4.00 | 3.91 (0.96), 4.00 | 3.89 (0.98), 4.00 |
Predicting dietary consumption intentions
For each of the three food types, a model comprising demographic variables, current behaviour and psychological variables was significantly predictive of intentions to eat more healthily (minimum model χ2 = 25.37; p = 0.001).
As shown in Table 2, participants who perceived their current intake to be excessive were significantly more likely to intend to eat less high-fat foods [odds ratio (OR) 5.28; p = 0.02] and high-sugar foods (OR 5.65; p = 0.01) than those who believed their intake to be adequate. A tendency for participants who perceived that they ate ‘too few’ F&V to intend to increase their F&V intake (OR 3.36) was not statistically significant (p = 0.15).
| Variables | Intention to eat | |||||
|---|---|---|---|---|---|---|
| More F&V (n = 99) | Less high-fat foods (n = 91) | Less high-sugar foods (n = 92) | ||||
| OR (95% CI) | p-value for trend | OR (95% CI) | p-value for trend | OR (95% CI) | p-value for trend | |
| Current engagement in health behaviour | About right: 1.00 reference | 0.15 | About right: 1.00 reference | 0.02 | About right: 1.00 reference | 0.01 |
| Too few F&V: 3.36 (0.64 to 17.60) | Too much high-fat food: 5.28 (1.38 to 20.19) | Too much high-sugar food: 5.65 (1.50 to 21.22) | ||||
| Threat | Lower threat: 1.00 reference | 0.83 | Lower threat: 1.00 reference | 0.68 | Lower threat: 1.00 reference | 0.18 |
| Higher threat: 0.89 (0.31 to 2.55) | Higher threat: 1.27 (0.41 to 3.89) | Higher threat: 2.05 (0.72 to 5.89) | ||||
| Benefits for mother and baby | Lower benefits: 1.00 reference | 0.02 | Lower benefits: 1.00 reference | 0.003 | Lower benefits: 1.00 reference | 0.10 |
| Higher benefits: 6.59 (1.28 to 10.11) | Higher benefits: 5.70 (1.78 to 18.21) | Higher benefits: 2.66 (0.82 to 8.66) | ||||
| Barriers | Higher barriers: 1.00 reference | 0.27 | Higher barriers: 1.00 reference | 0.86 | Higher barriers: 1.00 reference | 0.21 |
| Lower barriers: 0.56 (0.20 to 1.57) | Lower barriers: 1.12 (0.33 to 3.80) | Lower barriers: 0.50 (0.17 to 1.49) | ||||
| Subjective norms | Lower norms: 1.00 reference | 0.22 | Lower norms: 1.00 reference | 0.29 | Lower norms: 1.00 reference | 0.21 |
| Higher norms: 1.98 (0.66 to 5.91) | Higher norms: 1.93 (0.57 to 6.57) | Higher norms: 2.24 (0.64 to 7.85) | ||||
| R2 (Cox and Shell) | 0.23 | 0.33 | 0.32 | |||
| R2 (Nagelkerke) | 0.31 | 0.45 | 0.43 | |||
| Model χ2 | 25.38*** | 36.84*** | 34.93*** | |||
There was no effect of threat on intentions for any of the three food types (maximum OR 2.05; p = 0.18). However, strong effects were found for perceived benefits of action for mother and baby, with participants who expected greater benefits being more likely to intend to eat more F&V (OR 3.59; p = 0.02) and fewer high-fat foods (OR 5.70, p = 0.003). A similar tendency was found for intention to eat fewer high-sugar foods (OR 2.66), although this was only marginally significant (p = 0.10). There were no effects of perceived barriers (minimum p = 0.21) or of subjective norms (minimum p = 0.21) on dietary intentions.
Discussion
A low-GI diet in pregnancy has the potential to improve obstetric outcome. 12,14 The design of diet-based interventions in pregnancy may be aided by identifying the psychological determinants of dietary choices because modifying these variables should translate into dietary change. 17 The present study examined appraisals of benefits and barriers, threat perceptions, evaluations of current behaviour and perceived social pressures (subjective norms) as potential influences on intentions to improve three aspects of diet linked to insulin resistance (increased F&V consumption, decreased high fat and high sugar consumption19) in a community sample of pregnant women. Perceived levels of current behaviour predicted intentions to eat fewer high-fat and high-sugar foods, with participants who felt that they ate too many high-sugar or high-fat foods being more likely to intend to reduce their consumption. Perceived benefits of action for mother and baby were associated with the intention to eat more F&V and less high-fat food, with participants who perceived greater benefits of adopting more healthy eating patterns being more likely to intend to do so. A similar association, which was only marginally significant, was observed between perceived benefits and intention to eat less high-sugar food. There were no associations between perceived barriers, threat perceptions and subjective norms and intentions.
Our analysis drew on variables derived from the HBM,20 which proposes that health behaviour arises from deliberation over the threat posed by health risks, as well as the benefits of, and barriers to, taking action recommended to minimise these threats. We observed consistently high levels of perceived threat among our sample and, perhaps as a result of minimal variation in threat levels, there was no association between threat and dietary intentions. As predicted by the HBM, perceived benefits for mother and baby were associated with healthy eating intentions, although, in contrast to theoretical predictions,16 perceived barriers had no impact. Although further evidence from larger samples is needed, these results suggest that, when making the cost–benefit analyses that underpin evaluations of health-related behaviours,28 pregnant women may weigh barriers to behaviour less heavily than do the general population. Perhaps this may be understood in the light of the immediacy with which health threats faced by pregnant women can be realised. Perceived health benefits of diet outside pregnancy may be typically distal and orientated towards the prevention of later morbidity or mortality, whereas barriers relate to immediate short-term obstacles (e.g. missing out on tasty foods, choosing less favoured options). By contrast, the benefits of healthy eating in pregnancy, such as the prevention of macrosomia and the reduced likelihood of delivery complications, are typically more proximal, becoming apparent at or shortly after birth. Consequently, these benefits may be easier to foresee or are perceived to be more real than the distal benefits associated with health behaviours outside pregnancy. To support this hypothesis, further exploration is needed of the health decision processes in pregnancy and other conditions when the health effects are more immediate than the decision processes in non-pregnant community samples. However, the stronger effects of perceived benefits than perceived barriers in our sample, coupled with raised threat perceptions, support the conceptualisation of pregnancy as a period of heightened responsiveness to potential health risks and a greater appreciation of the value of health-protective action. Pregnancy may therefore represent ‘an opportune time to initiate (behavior) change’.
Several behaviourally based pregnancy interventions have been described based on the provision of dietary ‘counselling’. 29–33 However, the term ‘counselling’ can refer to a variety of behaviour change techniques. 34 Although some interventions have focused primarily on identification of barriers to healthy eating,30,32 the results of the present study indicate that bolstering expectations of positive outcomes for both mother and child associated with adopting a healthier diet may have more impact. The under emphasis of the benefits of healthy diet in interventions tested to date may reflect an assumption that pregnant women are aware of the implications of gestational diet. 2 Changing dietary choices in pregnancy may require efforts to ensure that pregnant women consistently prioritise the benefits of healthy eating over beliefs that support consumption of unhealthy foods. No relationship was observed between perceived social approval from family or health-care professionals and healthy eating intentions. However, the high mean scores, coupled with the strong positive correlation between family and health-care professional expectations, suggest that our sample perceived strong and consistent approval for healthier dietary choices. The absence of an association between norms and intentions may therefore be the result of a lack of variation, although more work is needed to establish the generalisability of the normative beliefs of our sample.
The findings of the present study are limited in several respects. The survey was cross-sectional, and so we could not observe ‘prediction’ of intentions in a temporal sense or assess the effects of intentions on subsequent consumption of each of the three foods types. Although further longitudinal research is needed, there is considerable theoretical and empirical evidence to support the temporal relationships that we have inferred, as well as the assumption that modifying dietary intentions will change dietary behaviour. 35 The data were also based on self-report, which is susceptible to responses biased by participants’ motivations to portray themselves positively. 36 Retrospective self-reports may underestimate true pre-pregnancy weight especially in obese women,37 although objective weight data were not available to validate our BMI measure. Participants were not informed by us of weight gain or gestational diet recommendations or of the potential consequences of healthy and unhealthy eating in pregnancy, and the accuracy of their perceptions was not tested and cannot be estimated. Nonetheless, our findings suggest that modifying such perceptions may boost healthy eating intentions over the remainder of pregnancy. The representativeness of our sample is questionable; for example, participants were mostly white and well educated, and more than two-thirds were educated to university level. This may reflect a systematic participation bias within our pool of potential participants, although we cannot test this because no data were available from those who declined participation. However, our sample encompassed a wide distribution of participant BMI scores and gestational age. Most participants were in the second or third trimester of pregnancy, and more work is needed to explore beliefs and behaviour earlier in pregnancy because dietary changes achieved in early pregnancy and maintained over the remaining gestational period may be most beneficial. Our sample was also modest in size and, therefore, unable to identify small effects, neither could we reliably explore differences in the beliefs, intentions and behaviour of demographic subgroups of pregnant women. Further research, using larger samples, is needed into whether or not nutrition beliefs, intentions and behaviour differ systematically by, for example, parity, age, socioeconomic status or ethnicity. Such differences may have implications for the design and delivery of effective dietary interventions in pregnancy.
Notwithstanding these limitations, our results offer some insight into the health beliefs and dietary choices of pregnant women. Best practice for diet modification in pregnancy is likely to require the adoption of health promotion strategies to target the underlying psychological determinants of gestational diet.
Implications for intervention development
This study provided important insights into optimising delivery of the intervention, the results indicating that pregnant women are likely to respond to bolstering their expectations of positive outcomes for both mother and child through focus on a healthier diet, rather than an emphasis on overcoming perceived barriers to behavioural change.
To assess delivery style, mode and method of delivery and acceptability to recipients
Exploratory study: interviews with obese pregnant women to inform the development of a pilot randomised controlled trial intervention and protocol
This pre-trial exploratory interview study aimed to examine the sociocultural context within which women made decisions about pregnancy, diet and lifestyle, body image, health beliefs and their readiness to engage with the type of health interventions that the trial team was in the process of developing.
A total of 30 women were approached, and semistructured qualitative interviews were undertaken with 13 pregnant women with a BMI of ≥ 30 kg/m2. Maximum variation sampling was used, selecting by maternal age, family composition (e.g. lone parent, first baby) and sociodemographic characteristics (employment, education level, ethnicity). The interviews aimed to explore pregnant women’s existing beliefs about diet and activity in pregnancy, their receptiveness to dietary change, perceived economic and time implications, increased activity levels and other views on acceptability. We explored obese women’s preferred approaches to intervention delivery (e.g. individual or group contacts, inclusion of family members, frequency of contacts, setting for intervention delivery, etc.) and barriers and constraints to change, including the impact of family circumstances and need for childcare. We investigated what types of activity obese pregnant women perceive to be feasible and what they believe to be the key features of a healthy diet in pregnancy. The interview schedule is provided in Appendix 2. Interviews were taped, transcribed and analysed using a framework approach geared to producing policy- and practice-relevant findings. The interviews were undertaken at either Guy’s and St Thomas’ Hospital, London, or the Royal Victoria Infirmary, Newcastle. The qualitative studies were given ethics approval [Integrated Research Application System (IRAS) reference number 09/H0802/05].
Sample characteristics
The mean age of the women was 35 years (range 25–41 years). The mean gestational age at the time of the interview was 30 weeks (range 15–39 weeks). The mean BMI was 38 kg/m2 (range 32–58 kg/m2). Eight of the women were self-defined as white and five were black. Five women were multiparous and the others were primiparous. Eight women were employed at the time of the interview. Four women were managing pre-existing chronic health conditions.
Findings
Comparative thematic analysis of the interview data led to the identification of themes and subthemes that contributed to an overall category, ‘willingness to engage with diet and lifestyle changes during pregnancy’. This category included two first-order themes (meaning that they are drawn directly from interview text): ‘feeling ill, pregnancy symptoms, and complex obstetric histories’ and ‘experience of stigma’. Each had the potential to impact upon women’s engagement with the proposed RCT and willingness to engage in lifestyle change was also mediated (either facilitated or reduced) by the relative complexity of the women’s lives.
Theme 1: feeling ill, pregnancy symptoms and complex obstetric histories
Interviewees reported feeling ill during pregnancy or described experiencing pregnancy in the context of past pregnancy loss, including miscarriage and stillbirth. Four out of 10 of the interviewees were affected by GDM in their current pregnancies (note that women with known GDM were excluded from recruitment in phase 3, UPBEAT, but approximately one in four developed GDM, see Chapter 5). Common pregnancy-related symptoms, particularly nausea, tiredness and pelvic or back pain, also interfered substantially with women’s eating patterns and ability to undertake PA.
Some women interviewed had worked hard to address their weight and activity before pregnancy or as part of treatment for infertility. These activities involved notable cost and effort: some interviewees had attended diet groups such as Weight Watchers® (New York City, NY, USA) or Slimming World (Alfreton, UK) or bought diet plan foods; others paid to attend gyms or fitness classes or took up exercise activities with their partners. However, once pregnant, they were advised to stop dieting by commercial weight loss group leaders. Others were advised against exercise by clinicians, particularly after they had experienced past miscarriages:
I know that I was overweight before I was pregnant, but I was quite conscious of the fact and I was doing something about it, I was exercising loads, but then as soon as I fell pregnant I was like, I’m not doing any exercise, because I was scared in case anything happened.
White English ethnicity, aged 27 years, married, a BMI of 36 kg/m2 and interviewed at 29 weeks’ gestation
The tiredness and nausea of early pregnancy, combined with busy lives looking after other children, doing household chores and working, all decreased likelihood of additional physical exercise. Pelvic pain was a significant problem for 6 out of 10 interviewees, and others reported back pain. The combination of pain and illness symptoms as pregnancy progressed meant that many led increasingly sedentary lives:
I mean I was quite keen to like stay quite fit, and I go for long walks and stuff, just because I thought it’s better for me long term, but with this [pelvic] pain I’ve had I’ve been literally like, the physio who I see at the hospital, she’s just literally said, ‘You’ve got to treat it like a sprained ankle and rest it.’ And I’m saying, ‘Yeah but then you just sit, it’s not good for us just sitting all the time either.’ So that was kind of a goal, I think I wanted to like do more exercise but I haven’t been able to.
White English ethnicity, aged 27 years, married, a BMI of 36 kg/m2 and interviewed at 29 weeks’ gestation
At thirty-five I first got pregnant and I had . . . a miscarriage at 13 weeks. It was quite traumatic . . . And then I had three more [miscarriages], in the December I got pregnant again . . . I had a 7-week scan and there was no heart beat . . . then I lost it. So I thought to myself, well, it could be stress, maybe I should change my lifestyle . . . I didn’t feel that fit, I felt overweight . . . I actually stopped exercising because with my other pregnancies the doctor had said, ‘Don’t exercise, we don’t really know why you’re miscarrying’. And I really missed the exercise. I missed that feel-good factor. I’ve managed my expectations, I’m hoping to breastfeed successfully and the doctors have said that can help shift the weight. I’m definitely going to be eating an extremely healthy diet, in fact my mother would be proud of me.
White English ethnicity, aged 41 years, married, a BMI of 32 kg/m2 and interviewed at 39 weeks’ gestation
Do you have any specific goals for yourself during this pregnancy?
No. Just to get to the end of it.
White English ethnicity, aged 35 years, single, a BMI of 34 kg/m2 and interviewed at 38 weeks’ gestation
Interviewees rarely envisaged their pregnancies as ‘normal’ or healthy, and some had been informed by health professionals that the induction of labour at, or before, term was a possibility, either because a diagnosis of GDM meant they were being screened for elective induction to reduce complications of birth with a LGA baby or for other reasons, such as pre-eclampsia. However, this perspective was not universal: at least one interviewee who had GDM felt more positive about her physical health during pregnancy. She reported substantial weight loss prior to pregnancy, and felt confident that she was managing her diet and blood sugars well and could lose weight again following birth.
Theme 2: experience of stigma
Linked to the notion that their pregnancies were not experienced as healthy or ‘normal’, some interviewees recounted occasions when they felt stigmatised, singled out or labelled, with assumptions made about their intelligence and life skills on the basis of their body size. They felt that they did not receive the positive public reinforcement that other pregnant women experience as their ‘bumps’ were often assumed to be body fat. However, some interviewees challenged the stereotype of overweight women as lazy, uneducated consumers of fast food. Others felt happy with their size because, having struggled with being larger all their lives, they had come to terms with this, or because they had already lost substantial weight. Given the difficulties that openly discussing weight during pregnancy posed for women, the medical language used to discuss weight generated controversy. The term ‘obese’ was often problematic because it did not equate to women’s own views of their bodies. Some felt that even the term ‘BMI’, while perhaps more neutral than ‘overweight’ or ‘obese’, was still not useful, because failed to reflect their overall weight trajectories, especially if they had achieved pre-pregnancy weight loss:
I think the term ‘obese’ or ‘morbidly obese’ is absolutely horrendous. I think it’s really awful, even if you were a hundred stone or 12 stone, it’s a really, really awful word. Um . . . it’s just an awful kind of terminology. I just think if you’re overweight just say they’re overweight, you know, there’s no need to kind of say ‘morbidly’, it sounds like you’re some sort monstrous disgusting person type thing.
White English ethnicity, aged 27 years, married, a BMI of 36 kg/m2 and interviewed at 29 weeks’ gestation
Several interviewees recalled being told their BMI was raised or high, and that they found it painful and offensive to be described in this way (they described being ‘singled out’ or ‘pounced on’) and felt that health professionals addressed the subject in ways which were rude and insensitive. Other interviewees had not heard the term ‘BMI’, and said that their doctors or midwives had not mentioned their weight but focused instead on other issues, such as smoking or pregnancy complications.
Theme 3: willingness to engage with diet or lifestyle changes during pregnancy
Although interviews were undertaken with pregnant women who were known to have a BMI of 30 kg/m2 or above, and whose routine care during pregnancy included information about weight gain, diet and lifestyle from health professionals, most mentioned broad public health messages about diet (such as the need to eat ‘five-a-day’), rather than identifying implications of raised BMI for their individual pregnancy outcomes.
In the interviews, women volunteered information about their age, blood sugar control and smoking, but did not talk about whether or not altering their own behaviour might change pregnancy outcomes for themselves or their babies. Despite their awareness of broad public health dietary advice, women did not volunteer more specific information about fat or carbohydrate intake during pregnancy. Interviewees also questioned dietary advice during pregnancy, reasoning that health advice varies over time and should be taken with ‘a pinch of salt’, citing too much focus on food ‘scares’ and too little effort towards individualised education.
Many women felt they needed to suspend their attempts to manage their diet or exercise while they were pregnant; pregnancy was seen as time when weight gain was inevitable, dieting contraindicated and when metabolic demands and nausea altered food preference and appetite. Instead, their efforts were focused upon ‘getting through’ this pregnancy, with a view to addressing diet and exercise goals during the postnatal period:
I don’t have any problems with [pregnancy body changes] because when I had [my daughter] I lost all the weight I gained quite quickly, . . . so it’s not been a problem for me to worry about. And I’ve actually gained less weight now than I did when I was having her, so I haven’t really had any negative thoughts or worries about that. So that’s been good. [Laughs] That’s been good.
Black African ethnicity, aged 32 years, married, a BMI of 35 kg/m2 and interviewed at 36 weeks’ gestation
When the proposed pilot RCT diet and PA intervention was described to interviewees, responses were mixed. Most supported the idea of an educational approach to diet and cooking, but fewer thought that advice about PA would be valuable. The suggestion of a group-based intervention engendered a polarised response; about half welcomed this idea and thought they would benefit from sharing their experiences and social support. The remainder said they would not participate in a group intervention, either because this would pose difficulties in the contexts of their work commitments and family lives or because the intention to single women out on this basis was patronising and grew from a perceived assumption that they needed to be educated about issues that they understood only too well:
If [the health trainer (HT) group] was happening now, would that be something you’d be interested in?
Yes, I would. Because sometimes, especially about food, I don’t know what to eat and maybe they can give me ideas what I can do. Or maybe they can encourage me to go to the classes.
Black African ethnicity, aged 38 years, has partner, a BMI of 34 kg/m2 and interviewed at 15 weeks’ gestation
I would feel very belittled. [Laughs] I wouldn’t . . . no, I wouldn’t like that at all. Yes, fair enough, they could offer it. I know what’s healthy . . . well yes, I do know, even though I can understand some people say it and they don’t, but I do know! And, yes, I wouldn’t like someone telling me.
White English ethnicity, married, a BMI of 36 kg/m2 and interviewed at 27 weeks’ gestation
Me personally, no. Because of the fact of the family I’ve got. A new time mum it would be good for, somebody that can devote more time to it.
White English ethnicity, aged 41 years, lives with partner, a BMI of 37 kg/m2 and interviewed at 37 weeks’ gestation
Conclusions
The phase 1 exploratory study, undertaken with pregnant women with a BMI of ≥ 30 kg/m2 who matched criteria for inclusion in the proposed UPBEAT pilot RCT other than gestational age at recruitment and concurrent underlying health problems (see Implications for intervention development and limitations) but were not involved in the subsequent pilot RCT, suggested that willingness to change lifestyle was diminished by pregnancy illness and symptoms and by the demands placed upon women’s time by their family and work responsibilities. A lack of concrete knowledge about the effect of changing diet or increasing PA on pregnancy or birth complications also appeared to reduce the perceived value of the intervention. Previous experience of being stigmatised by peers or health professionals affected some women’s willingness to participate. Although most of these findings were also identified during the pilot trial process evaluation, the influence of stigma was less apparent. It is unclear whether or not this is because women who felt stigmatised were less likely to participate in the pilot RCT or whether modifications to the recruitment approach reduced the experience of stigma.
Implications for intervention development and limitations
Important information was provided by these interviews that contributed to the delivery of the intervention, notably the need to avoid stigmatisation of women with a higher than normal BMI and the care required to approach the subject. This became a topic of continued discussion and learning throughout the trial development and the trial itself. In addition, these interviews led us to appreciate that some pregnant women who are obese may suffer considerable physical discomfort, which is likely to impact upon their motivation to undertake PA.
There were several important limitations to this study. These included many of the interviews being carried out towards term rather than at the proposed time of recruitment, and that several of the women were already affected by health complications, including existing diagnosis of GDM. Relevance to women approached to join the trial at 15–18 weeks of pregnancy was therefore limited.
To produce a combined diet and activity intervention for use in obese pregnant women
An intervention was developed based on goal-setting and review, to be delivered over a period of 8 weeks from recruitment by study-specific health trainers (HTs). Full details are provided in Chapters 2 and 4.
-
Dietary intervention: a dietary intervention was developed by a postdoctoral nutritionist (Dr S Barr) and, principal investigator (PI), Professor T Sanders based on the intention of reducing insulin resistance and improving maternal glucose homeostasis; the dietary component of the intervention included a low dietary GI, reduced consumption of sugar-sweetened beverages and reduced saturated fat intake.
-
PA intervention: PA advice was developed by a postdoctoral researcher (Dr Kinnunen) and PI (Dr Ruth Bell) based on the available literature and the intention of improving maternal glucose homeostasis and on Royal College of Obstetricians and Gynaecologists guidelines. This component of the intervention was designed to encourage obese pregnant women to increase their daily activity incrementally over the period of delivery of the intervention and to maintain the achieved activity level as long as possible as their pregnancy progressed. Pedometers would be provided as a self-motivational tool and women would set individual step targets weekly.
-
Behavioural intervention: the behavioural theory on which to base the intervention was developed from control theory with elements from social cognitive theory. Strategies used included graded goals, behavioural goal-setting, monitoring behaviours, providing feedback regarding goal attainment, identification and problem-solving of barriers, enlisting social support and providing opportunities for social comparison. This approach also supported the building of self-efficacy. HTs delivered the intervention, following training that included the information gained during the development phase. Training of the HTs and midwifery staff who recruited the women continued during the 5-year programme.
To develop patient information leaflets, a provisional treatment manual and a training package to support intervention
The following were developed: patient information leaflets and consent forms, goal-setting and monitoring logbook, a PA ‘work-out’ digital versatile disc (DVD), a HT manual (standard operating procedures) (see Appendix 3) and a handbook for women in the intervention arm of the trial (see Appendix 4). All the trial literature was read, approved and amended with the help of obese women attending antenatal clinics at Guys and St Thomas’ NHS Foundation Trust, and approved by the King’s College London division of women’s health patient and public involvement group.
To assess feasibility, acceptability, validity and reliability of outcome measures
Physical Activity Measurement Study
The measurement of PA in a large number of subjects within a trial setting presents practical and financial issues. Accurate objective assessment would be the ideal, and at the time of study validation studies had shown that certain accelerometers could provide reasonably accurate objective measures. However, expense and collection and download of the accelerometer data were preclusive for the purposes of the main trial. A study was therefore designed to determine whether or not pedometers could be used instead of accelerometers in the pilot trial and the main RCT. This has been published as Kinnunen TI, Tennant PW, McParlin C, Poston L, Robson SC, Bell R. Agreement between pedometer and accelerometer in measuring physical activity in overweight and obese pregnant women. BMC Public Health 2011;11:501. 38 © Kinnunen et al. ; licensee BioMed Central Ltd 2011. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. For the purposes of this report minor formatting edits have been made to the original text.
Agreement between pedometers and accelerometers in measuring physical activity in overweight and obese pregnant women
Abstract
Background: Inexpensive, reliable objective methods are needed to measure PA in large-scale trials. This study compared the number of pedometer step counts with accelerometer data in pregnant women in free-living conditions to assess agreement between these measures.
Methods: Pregnant women (n = 58) with a BMI of ≥ 25 kg/m2 at a median of 13 weeks’ gestation wore a GT1M (ActiGraph, Pensacola, FL, USA) accelerometer and a CW701 Digi-Walker™ Pedometer (Yamax, Bridgnorth, UK) for four consecutive days. The Spearman rank correlation coefficients were determined between pedometer step counts and various accelerometer measures of PA. Total agreement between accelerometer and pedometer step counts was evaluated by determining the 95% limits of agreement estimated using a regression-based method. Agreement between the monitors in categorising participants as active or inactive was assessed by determining kappa.
Results: Pedometer step counts correlated moderately (r = 0.36–0.54) with most of the accelerometer measures of PA. Overall step counts recorded by the pedometer and the accelerometer were not significantly different (medians 5961 vs. 5687 steps/day; p = 0.37). However, the 95% limits of agreement ranged from –2690 to 2656 steps/day for the mean step count value (6026 steps/day) and changed substantially over the range of values. Agreement between the monitors in categorising participants to active and inactive varied from moderate to good depending on the criteria adopted.
Conclusions: Despite statistically significant correlations and similar median step counts, the overall agreement between the pedometer and accelerometer step counts was poor and varied with activity level. Pedometer and accelerometer steps cannot be used interchangeably in overweight and obese pregnant women.
Background
Current recommendations emphasise that regular moderate-intensity leisure-time PA during an uncomplicated pregnancy may have benefits such as reducing fatigue, back pain, stress and depression and improving glycaemic control but has no known harmful effects on the health of the mother or the fetus. 39,40 However, the available evidence is limited, and larger and better-quality trials are needed to define the potential role for PA promotion in preventing pregnancy complications such as GDM and pre-eclampsia. 41 Most previous studies assessing PA in relation to pregnancy outcome have used questionnaires or other self-reported measurements of PA, as these are cheap to administer in large-scale studies. 42 Although some of the questionnaires have been validated in pregnant women either against accelerometer,43–45 a portable activity monitor46 or pedometer and a PA logbook,47 their validity has usually been low or moderate, especially with regard to the low-intensity activity that is common among pregnant women. 48 These limitations are also observed for PA questionnaires in other populations. 49,50 Therefore, inexpensive, objective PA measurement methods are needed for large-scale studies to obtain more accurate information on PA levels during pregnancy. Accelerometers and pedometers are the most commonly used objective methods of assessing PA in epidemiological studies, and have been used in a number of previous studies of pregnant women. 43,44,48,51–54 Although accelerometers provide more detailed information on PA than pedometers, pedometers are much less expensive and, therefore, more economically feasible for larger studies. 55 It is unclear whether or not pedometers and accelerometers provide comparable estimates of PA in pregnant women. This issue was recently explored in two small studies (n = 30 in both cases) examining pregnant women in free-living conditions56 and on a treadmill. 57 Similar comparisons have also been reported in healthy adults58,59 infected with human immunodeficiency virus60 and older people61 in free-living conditions. These studies suggest that pedometer and accelerometer step counts are highly correlated, but large individual differences in step counts exist. Nevertheless, pedometer step counts for assessing overall PA were advocated in most of these studies. 56,59–61
This study was designed as a preliminary investigation to determine appropriate PA measurement methods for a large RCT (UPBEAT) of a lifestyle intervention in obese pregnant women. Overweight and obese women have a higher risk of several pregnancy complications and may benefit from increasing their PA levels during pregnancy. 62,63 The aim of this study was to compare pedometer step counts with several accelerometer-derived measures of PA in overweight and obese pregnant women in free-living conditions.
Methods
Study participants
Participants were overweight and obese pregnant women with a BMI of at least 25 kg/m2 based on self-reported height and measured weight at the first visit to antenatal care, usually before 12 weeks’ gestation. The exclusion criteria were a BMI of < 25 kg/m2, age < 16 years, multiple pregnancy, abnormal ultrasound scan result, complicated medical problems, inadequate language skills in English or inability to give written informed consent. A research midwife recruited the participants when they attended for their routine ultrasound scan at either 11–14 or ≥ 20 weeks’ gestation at the Royal Victoria Infirmary, Newcastle upon Tyne, UK, between July and December 2009. The participants were recruited in early pregnancy because information on the appropriate PA measurement methods for the intervention study starting in early pregnancy was needed.
A total of 286 women were eligible for the study and 93 (33%) agreed to participate. All participants signed a written informed consent for participation. Ethics approval for the study was obtained from St Thomas’s Hospital Research Ethics Committee, London, UK (National Research Ethics Service, Research Ethics Committee reference number 09/H0802/5).
Data collection
This study was a cross-sectional comparison of two objective PA measurement methods. The research midwife asked participants to wear an accelerometer and a pedometer for four consecutive days, including one weekend day. In adults, 3–5 days of monitoring by accelerometer usually provides a reliable estimate of PA. 64 The participants kept a diary to record when the monitors were put on and when they were taken off. Data on participants’ demographic details were collected using a short structured questionnaire. An appointment for returning the monitors was arranged after the 4-day period.
Accelerometer
The GT1M accelerometer used in this study was a small uniaxial monitor, which detects vertical accelerations over a user-specific time interval (epochs). 65 The former version of the ActiGraph accelerometer [Computer Science and Applications (CSA) 7164 model; ActiGraph, Pensacola, FL, USA] is one of the most extensively validated accelerometers and its activity counts correlate reasonably with double-labelled water-derived energy expenditure in non-pregnant populations. 66
Participants were asked to wear the accelerometer on the right hip during waking hours except while swimming or having a shower or bath. They were given the choice of belt or waistband attachment and information was recorded on which they found to be the most comfortable. Most participants (n = 40, 69%) wore the accelerometer using a belt, whereas 14 (24%) clipped it on to the waistband of their clothing; in four participants (7%) the status was unknown. A 60-second epoch length was used in this study. The raw data were processed using the MAHUffe program [Medical Research Council (MRC) Epidemiology Unit, University of Cambridge, Cambridge, UK] (www.mrc-epid.cam.ac.uk/physical-activity-downloads). Periods of at least 60 minutes with no counts and days with < 500 minutes of total valid recording time were excluded.
The following cut-off points were used to assess time spent at different intensity levels: sedentary < 100 counts per minute (cpm),67 light activity 100–1951 cpm, moderate activity 1952–5724 cpm and vigorous activity > 5724 cpm. 68 These cut-off points were originally developed for CSA Model 7164 accelerometer. Currently, there is no consensus on the best cut-off points to be used and these may vary in different populations. The Freedson cut-off points, derived from treadmill conditions, were selected because leisure time PA in our population mainly consisted of walking and because these cut-off points have been used in previous studies comparing pedometers to accelerometers. 56,58–60
Pedometer
The CW701 Digi-Walker™ pedometer was used to measure daily step counts. Yamax Digi-Walker models have been shown to be among the most accurate models in measuring step counts. 69,70 The participants were asked to wear this device during the same time period as the accelerometer. The participants clipped the pedometer either to the accelerometer belt or to the waistband of their clothing depending on how the accelerometer was attached.
Categorising participants as active or inactive
Three different criteria were used to categorise participants as active: (1) ≥ 30 minutes moderate to vigorous PA (MVPA)/day (for accelerometer data only, as this information could not be derived from the pedometer data), (2) ≥ 10,000 steps/day and (3) ≥ 8000 steps/day. The first criterion was based on current PA recommendations. The second criterion is a commonly used step target in health promotion and has been shown to be associated with health benefits. 71,72 The third criterion was chosen as there is some evidence to suggest that 8000 steps/day corresponds to 30 minutes of MVPA/day measured by accelerometer when using similar intensity cut-off points as in the present study. 58,59,72
Statistical analyses
All activity data were averaged over the valid days of recording. The majority of variables were not normally distributed and, therefore, non-parametric methods were employed for all analyses. Continuous variables were described using the median and interquartile range. Differences in background characteristics of included (n = 58) and excluded (n = 35) participants were tested using Mann–Whitney U-test for continuous variables and chi-squared test for categorised variables.
Agreement between the accelerometer and the pedometer was assessed in several ways. Absolute step count measurements were compared using the Wilcoxon signed-rank test. The relative agreement between pedometer-derived step counts and various accelerometer measures of PA was examined by determination of the Spearman rank correlation coefficient (r).
Total agreement between accelerometer-derived and pedometer-derived step counts was evaluated by determination of the 95% limits of agreement. The difference between both measures of step counts was plotted against the mean of both measures. As there was a statistically significant negative correlation between these variables, which was not resolved by transformation, the limits of agreement were estimated by a regression-based method. To test whether or not the limits of agreement varied by baseline BMI (25.0–29.9 kg/m2 vs. ≥ 30.0 kg/m2) or gestational age (11–14 vs. ≥ 20 weeks’ gestation), interactions terms were added to regression models, and absolute residuals were compared by Student’s t-tests.
The classification of participants according to whether or not they recorded a daily mean of at least 8000 or 10,000 steps/day was compared between pedometer and accelerometer by calculating Cohen’s kappa over 2 × 2 contingency tables. Kappa was also determined to assess the agreement between those reaching 8000 pedometer steps/day and those achieving 30 minutes MVPA, as measured by accelerometer. Kappa values of 0.81–1.00 were regarded as indicating almost perfect agreement, while values of 0.61–0.80 indicated good agreement, 0.41–0.60 moderate agreement, 0.21–0.40 fair agreement and 0.0–0.20 slight agreement. 73
Confidence intervals (CIs) and p-values for r were estimated by bootstrapping over 5000 iterations. A p-value of < 0.05 was considered statistically significant. The majority of statistical analyses were performed using SPSS version 17.0 for Windows (SPSS Inc., Chicago, IL, USA); however, bootstrapping methods used Stata® version 10.2 (StataCorp LP, College Station, TX, USA).
Results
Of the 93 women recruited, 32 (34%) had valid accelerometer data for fewer than three days, 17 (18%) had valid data for 3 days and 44 (47%) had valid data for 4 days. All valid days from women with valid data for at least 3 days (n = 61) were included in further analyses, excluding three women who did not have pedometer data. The final study sample consisted of 58 women (62% of those recruited). The excluded women (n = 35) were younger (median age 28 vs. 32 years; p = 0.018) and more often smokers during the previous year (46% vs. 13%; p = 0.002) than the included women, and fewer of them were highly educated (5% vs. 59%; p < 0.001), but gestational age, BMI, parity, ethnicity, marital status and employment status were similar to those of the included women. The background characteristics of the included women are described in Table 3. The median age was 32 years and the median BMI was 29.3 kg/m2 (range 25.3–46.2 kg/m2).
| Background characteristics | Participants |
|---|---|
| Continuous variables, median (interquartile range)a | |
| Age (years) | 32 (27–36) |
| Weeks’ gestation | 13 (12–20) |
| BMI (kg/m2) | 29.3 (27.5–33.8) |
| Categorised variables, number (%) | |
| Weeks’ gestation category | |
| 11–14 | 32 (55.2) |
| ≥ 20 | 26 (44.8) |
| BMI category | |
| 25.0–29.9 kg/m2 | 35 (60.3) |
| ≥ 30.0 kg/m2 | 22 (39.7) |
| Parity | |
| 0 | 27 (46.6) |
| 1 | 21 (36.2) |
| ≥ 2 | 10 (17.2) |
| Education (highest qualification)b | |
| GCSE or equivalent (at age ≥ 16 years) | 9 (17.0) |
| A-level or equivalent (at age ≥ 18 years) | 13 (24.5) |
| Degree or higher postgraduate qualification | 31 (58.5) |
| Ethnicity | |
| White | 48 (88.9) |
| Other | 6 (11.1) |
| Smoked during the last year | |
| Yes | 7 (12.7) |
| No | 48 (87.3) |
| Employed at the beginning of pregnancy | |
| Yes | 48 (84.2) |
| No | 9 (15.8) |
| Hours of employmentc | |
| Full time (≥ 37 hour/week) | 30 (63.8) |
| Part time (< 37 hour/week) | 17 (36.2) |
| Living with a partner/husband | |
| Yes | 55 (96.5) |
| No | 2 (3.5) |
Descriptive activity data
The median wear time of the accelerometer was 13 hours 40 minutes/day (Table 4). The women were sedentary for most of that time and total active time (median 4 hours 50 minutes/day) mainly comprised light-intensity activity. The median time spent in MVPA was 18 minutes/day.
| PA measure | Median (interquartile range) | Range |
|---|---|---|
| Accelerometer | ||
| Total included wear timeb | 821.8 (754.0–869.3) | 608.0–1111.0 |
| Sedentary timeb | 514.0 (464.3–583.1) | 255.7–849.8 |
| Total activity timeb | 290.9 (245.8–340.1) | 127.7–473.5 |
| Light activityb | 271.3 (218.8–315.4) | 99.3–429.3 |
| Moderate activity | 18.0 (11.4–29.1) | 5.3–70.0 |
| Vigorous activity | 0.0 (0.0–0.0) | 0.0–4.3 |
| Moderate or vigorous activity | 18.0 (11.7–30.1) | 5.3–70.0 |
| Total counts/day | 202,680 (166,951–248,348) | 92,131–429,497 |
| Average cpm | 256.3 (209.5–323.7) | 131.0–615.5 |
| Steps, counts/day | 5687 (4452–7086) | 1545–11,453 |
| Pedometer | ||
| Steps, counts/dayb | 5961 (3727–8510) | 267–12,833 |
Agreement between continuous pedometer and accelerometer measures of PA
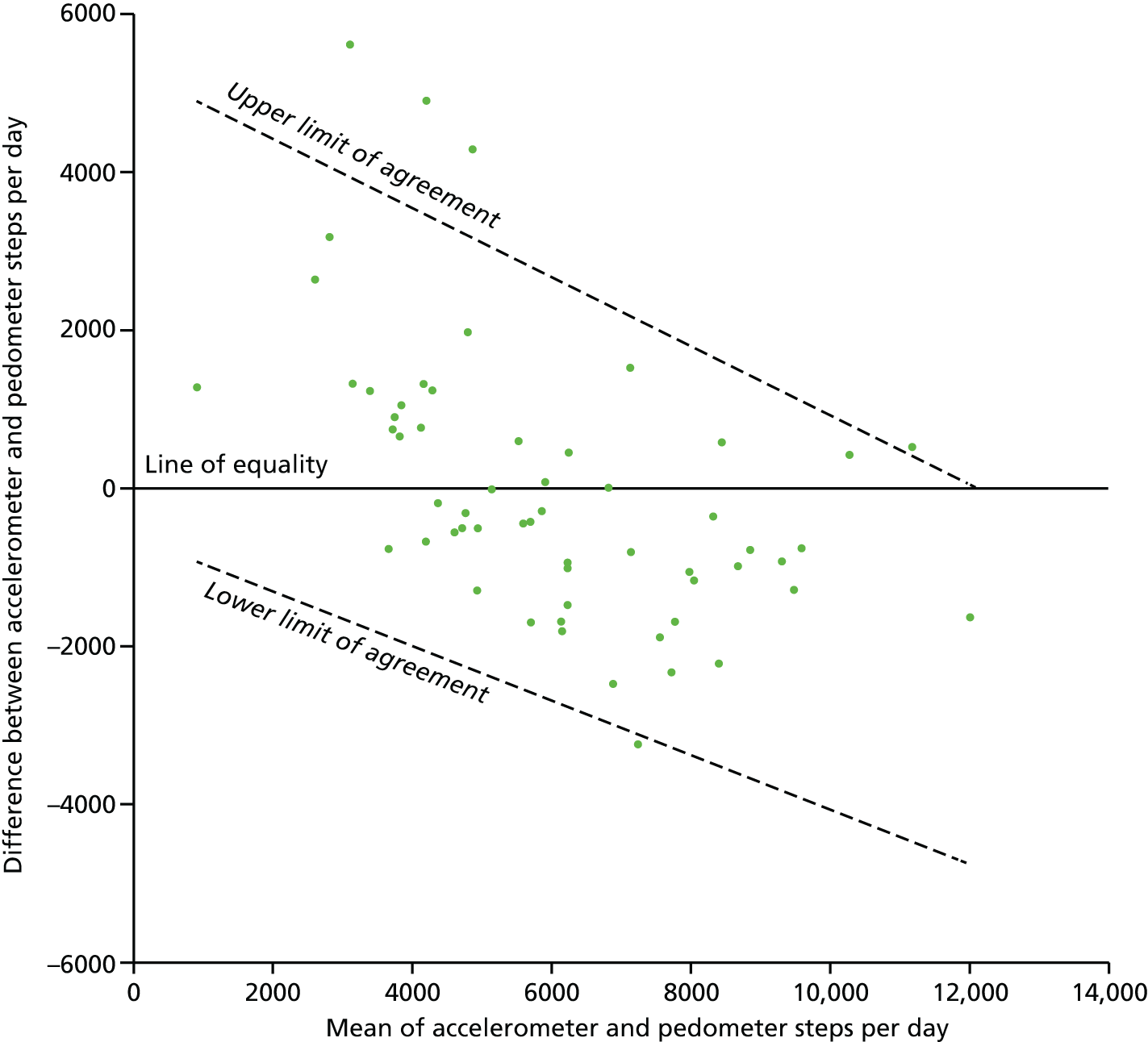
There was no significant difference between the overall step counts recorded by the pedometer and the accelerometer (median 5961 vs. 5687 steps/day, respectively; p = 0.37; see Table 4). Pedometer step counts were significantly correlated with all accelerometer measures of PA except for sedentary time (Table 5). The correlation was good for accelerometer step counts (r = 0.78) and moderate (r = 0.36 to 0.54) for all other measures of PA. Pedometer step counts are plotted against accelerometer step counts in Figure 1. Despite these statistically significant correlations, the 95% limits of agreement were very broad, ranging between –2690 and 2656 steps/day for the mean value (mean of accelerometer and pedometer steps/day = 6026) (Figure 2). The limits of agreement also varied substantially over the range of values, indicating a differential bias. At the lowest recorded step count (mean of accelerometer and pedometer steps/day = 906), the limits were –927 to 4897 steps/day (a range of 5824), indicating that the accelerometer was on average recording more steps/day than the pedometer. In contrast, at the highest step count value (mean of accelerometer and pedometer steps/day = 12,018) the limits were –4753 to 33 steps/day (a range of 4786) indicating that, while the level of random disagreement had decreased, the direction of bias had reversed, with the accelerometer recording less steps/day than the pedometer on average. BMI and gestational age did not modify the limits of agreement as there were no statistically significant differences in the slope of the regression line (p = 0.28 for BMI; p = 0.68 for gestational age) or in the absolute spread from the regression line (p = 0.64 for BMI; p = 0.35 for gestational age).
| Variable measured | Correlation coefficient | 95% CI | p-value |
|---|---|---|---|
| Sedentary time (minutes/day) | –0.30 | 0.51 to 0.05 | 0.023 |
| Total activity time (minutes/day) | 0.40 | 0.13 to 0.63 | 0.002 |
| Light activity (minutes/day) | 0.36 | 0.10 to 0.58 | 0.006 |
| Moderate or vigorous (minutes/day) | 0.47 | 0.18 to 0.69 | < 0.001 |
| Activity (minutes/day) | 0.51 | 0.24 to 0.72 | < 0.001 |
| Total counts/day | 0.54 | 0.28 to 0.74 | < 0.001 |
| Average | 0.78 | 0.59 to 0.90 | < 0.001 |
FIGURE 1.
Daily step counts as measured by accelerometers and pedometers. The figure also shows the line of equality and least-squares line.
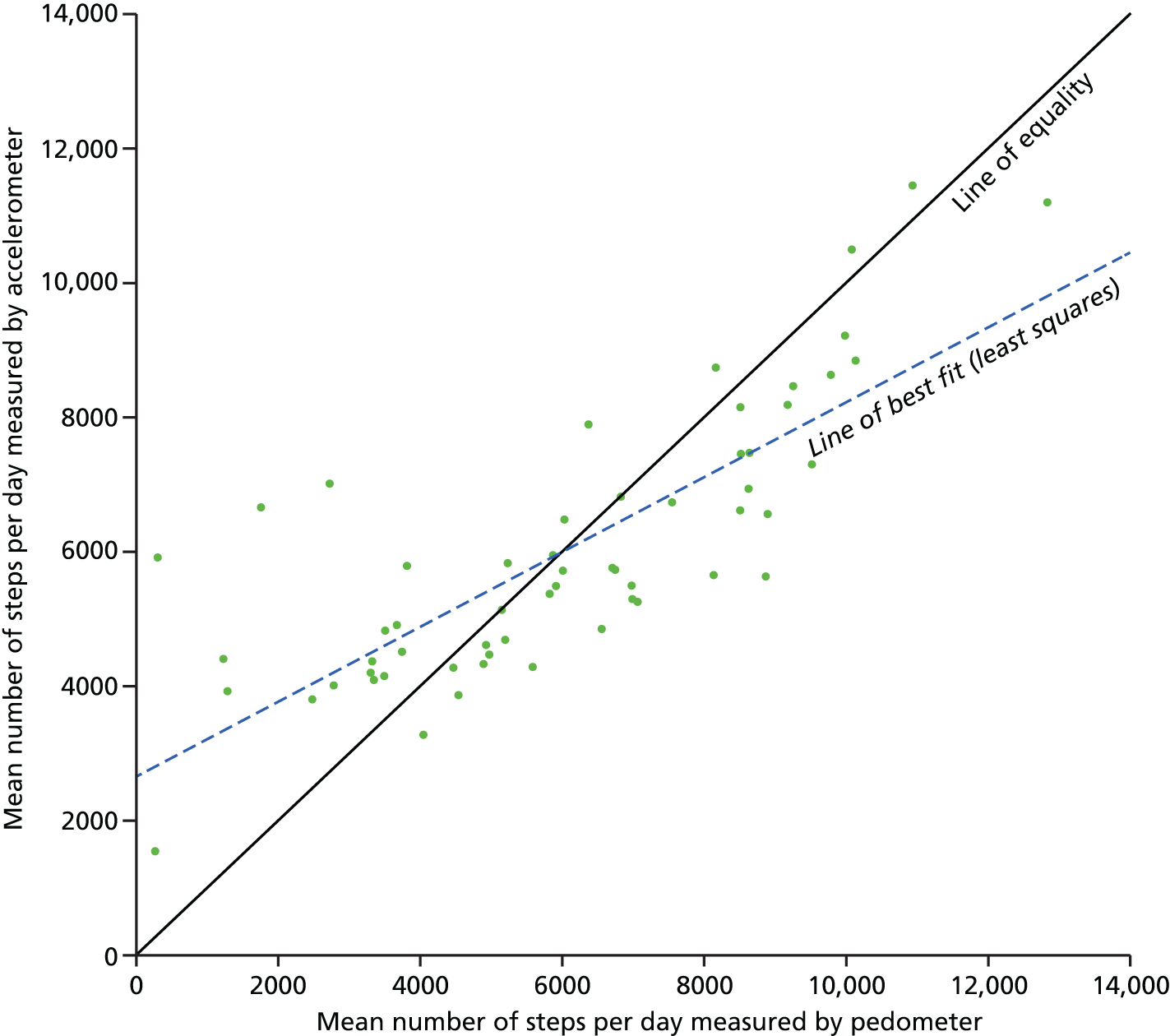
FIGURE 2.
Differences in step counts/day between accelerometers and pedometers, plotted against the mean of both (n = 58). The limits of agreement were calculated using a regression method previously described by Bland and Altman. 74
Agreement between categorised pedometer and accelerometer measures of physical activity
Based on the accelerometer data, 15 (26%) of these women recorded ≥ 30 minutes MVPA/day, 12 (19%) recorded ≥ 8000 steps/day and three (5%) recorded ≥ 10,000 steps/day. The pedometer data showed that 18 (29%) of the women recorded ≥ 8000 pedometer steps/day and four (7%) recorded ≥ 10,000 steps/day.
There was moderate agreement between those achieving ≥ 8000 pedometer steps/day and those achieving ≥ 30 minutes MVPA/day (kappa 0.45, 95% CI 0.24 to 0.67) (Table 6). Agreement between the pedometer and the accelerometer in categorising women to < 8000 or ≥ 8000 steps/day was good (kappa 0.63, 95% CI 0.43 to 0.83). Very few women achieved ≥ 10,000 steps/day with either of the monitors (n = 3 for accelerometers and n = 4 for pedometers), thus the agreement between these was artificially high (results not shown).
| Accelerometer dataa | Kappa (accelerometer data vs. ≥ 8000 pedometer steps counts/day)b | 95% CI |
|---|---|---|
| ≥ 30 minutes MVPA/day | 0.45 | 0.24 to 0.67 |
| ≥ 8000 steps/day | 0.63 | 0.43 to 0.83 |
Discussion
Large-scale trials are needed to assess the potential impact of increased PA on reducing pregnancy complications and these trials should ideally use objective measurement methods to measure changes in PA. 40,41,75 Pedometers would be a cost-effective measurement tool for large studies, provided that the simple step count measure is broadly comparable to the more specific accelerometer data. Our results show that, although there was a significant correlation between pedometer step counts and most accelerometer measures of PA and no difference in median step counts between the two devices, the 95% limits of agreement were very broad, especially among those participants who were less active. In addition, the direction of difference between the monitors appeared to reverse across the range of activity levels, suggesting a complicated pattern of disagreement. Agreement between the monitors in categorising participants as active and inactive varied from fair to good depending on the criteria adopted, being good when achievement of ≥ 8000 steps/day was used as the criterion. Pedometer step counts have been compared with accelerometer data in a number of previous reports,56,58–61 all of which used one of the ActiGraph/CSA/Manufacturing Technology Inc. accelerometer models and one of the Yamax pedometer models, as in the present study. These accelerometer models are not entirely comparable to each other in measuring steps and activity counts. 76,77 The study by Harrison et al. 56 included 30 overweight or obese pregnant women at 26–28 weeks’ gestation in Australia. The participants wore an accelerometer (GT1M) and a pedometer for 5–7 days and the accelerometer data processing rules were very similar to those used in our study. Despite a statistically significant correlation (r = 0.69, p < 0.01) between the step counts of each monitor, the mean difference was 505 steps/day and the limits of agreement were large (from –2491 to 3501 steps/day).
The other studies were not conducted in pregnant women and, generally, included subjects who were more active than our participants. However, the findings were essentially similar to those in the present study. Tudor-Locke et al. ,58 in a study of 60 adult volunteers in South Carolina, USA, observed a high correlation between accelerometer (CSA model 7164) and pedometer step counts (r = 0.86), but the accelerometer detected 1845 ± 2116 more steps/day, on average, than the pedometer and the limits of agreement were even broader (–2387 to 6077 steps/day) than reported in the present study. In a larger study of older adults in the UK (n = 121),61 Harris et al. reported that pedometer step counts were highly correlated to the accelerometer (GT1M) step counts (r = 0.86) and the mean step counts were similar. However, the limits of agreement were again large, around –3500 to 35,0081 steps/day. Ramirez-Marrero et al. 60 reported similar findings among 58 adults infected with human immunodeficiency virus in Puerto Rico. Although the limits of agreement were not calculated in that study, individual variation in differences in step counts seemed to be large. When comparing pedometer step counts with other accelerometer (ActiGraph model 7164) measures of PA, the correlations observed in the present study are generally similar, although weaker than in the previous studies. 58,60,61 Macfarlane et al. 59 observed, among 57 adult volunteers in Hong Kong, that the means for accelerometer (Manufacturing Technology Inc. model 7164) measures of PA increased with increasing pedometer step counts, but the CIs were broad.
Among the previous studies, Tudor-Locke et al. 58 were the only authors to conclude that agreement between pedometer step counts and accelerometer measures of PA was unacceptably low, despite others also reporting broad limits of agreement56,61 or CIs. 59 Future studies should pay more attention to correct interpretation of Bland–Altman plots and limits of agreement.
The present study confirms the findings of these studies in a sample of 58 overweight and obese pregnant women. Although there are currently no methods available to calculate 95% CI for the limits of agreement determined by a regression-based method, it is important to note that a larger sample size would not have affected the size of the limits of agreement. There are also no guidelines for acceptable 95% limits of agreement for step counts.
We propose that they should be no larger than ± 500 steps/day (i.e. a range of 1000 steps/day), which is likely to correspond to a maximum of a 10-minute difference in the duration of MVPA, such as brisk walking,71 and may therefore be of clinical and public health importance.
The difference between the accelerometer and the pedometer step counts was correlated to the mean of both measures in the present study, but not in the other studies. 56,58,61 In this study, the difference between the step counts was in the opposite direction for less active and more active women, that is the accelerometer detecting more steps among less active women and the pedometer detecting more steps among more active women. This discrepancy may be related to the general limitations of the monitors or differences in their sensitivity to detect PA. Pedometer accuracy is reported to be diminished at slow walking speeds, especially below 3 miles/hour,69,78 and both active and inactive participants undertook many episodes of low-intensity activity in the present study. This may also be the case with some accelerometers, although the GT1M model used in our study has been shown to have lower inter-monitor variability and lower sensitivity for low-intensity activity than the previous 7164 model. 76,77 On the other hand, the previous CSA model has also been reported to erroneously detect slightly more non-steps, for example when travelling by a motor vehicle. 79
The accuracy of the latest ActiGraph accelerometer model (GT3X, ActiGraph, Pensacola, FL, USA) and the Yamax Digi-Walker SW200 (Yamax, Bridgnorth, UK) was recently investigated in 30 pregnant women. 57 Both monitors underestimated the number of steps, especially at slow walking speeds, and even when the monitors were repositioned at a tilt angle, there was no correlation with the percentage of actual steps detected by either monitor. In contrast, Crouter et al. 69 suggested that the tilt angle reduced the accuracy of spring-levered pedometers in overweight or obese adults. The tilt angle may also reduce the accuracy of accelerometers in assessing vertical movement, which may happen more often among overweight and obese than normal weight people. 55 The tilt angle was not directly measured in the present study. However, BMI and gestational age did not significantly modify the results of the Bland–Altman plot, suggesting that the potential effect of the tilt angle on the results may have been the same regardless of BMI or gestational age.
We also assessed agreement between the monitors in categorising participants as active or inactive. Although agreement was relatively good (kappa 0.63) when using 8000 steps/day as the criterion for both monitors, agreement was lower (kappa 0.45) when comparing participants achieving 8000 pedometer steps/day with those achieving 30 minutes of MVPA/day measured by a accelerometer. Of the previous studies, Ramirez-Marrero et al. 60 reported fair agreement between 10,000 pedometer steps/day and 150 minutes of MVPA/week (kappa 0.25, p = 0.01). These discrepancies between pedometers and accelerometers in categorising participants into active and inactive may partly be because of the data processing rules, such as selection of the epoch length and intensity cut-off points to define MVPA for the accelerometer data.
Accelerometry should not be regarded as a gold standard to measure free-living PA nor necessarily as a more accurate method of measuring daily steps than with a pedometer. Although the previous version of the ActiGraph accelerometer was the only commercially available accelerometer66 that correlated reasonably with double-labelled water, most validation studies have been conducted in controlled environments. Validity is lower when applied to free-living settings. 55 Two armband accelerometers have also recently been shown to be highly correlated with double-labelled water in free-living conditions. 42 Both the accelerometer and the pedometer measure biomechanical body movement. Hence, validity of the monitors against energy expenditure should not be a major concern when assessing agreement between these devices.
This study had some limitations. First, although the participants were asked to record the times when they wore the monitors, we cannot be sure that both monitors were worn exactly for the same time. Some women had very low pedometer step counts but moderate accelerometer step counts, suggesting that the wearing time may have been different for each monitor or the pedometer may have been in a tilt angle. Therefore, studies in controlled conditions, such as that by Connolly et al. ,78 would be necessary to be certain that monitors were worn for exactly the same time. Second, almost all of our participants were in the first or second trimester of pregnancy and, therefore, we do not know whether or not the results can be generalised to the third trimester, when activity decreases and the abdominal circumference is much larger. On the other hand, these results may be generalised to non-pregnant overweight and obese women of similar age.
Third, participation was low (33%) and 34% of the participants were excluded because valid accelerometer data were available for fewer than 3 days. The activity levels of the participants were similar or slightly lower than those reported in pregnant women in other comparable studies. 42,44,54,80 The purpose of this study, however, was to compare methods of measurement, rather than to obtain representative estimates of PA levels in pregnancy.
Conclusions
Comparing median step counts or assessing correlation coefficient overestimates agreement between pedometer and accelerometer data. Examination of the 95% limits of agreement revealed a substantial lack of agreement between step counts measured by the two types of monitor. Pedometer step counts were not comparable to accelerometer data at an individual level in overweight and obese pregnant women. The choice of measurement method may depend on the target of the intervention. For example, accelerometers may be better at assessing changes in PA in trials that promote increases in moderate or vigorous PA or reduction in sedentary time, whereas spring-levered pedometers may be more appropriate for studies evaluating walking interventions in more active populations.
Implications for intervention development
As a result of this report, the decision was taken to use validated accelerometers for the pilot study to provide the most accurate, although not perfect, objective assessment of PA and that the pedometer would be supplied to all women in the intervention arm of both the pilot study and the main trial as a motivational aid only. Assessment in the pilot study would also include a self-reported validated questionnaire, and it was decided that self-reported questionnaire would provide the only practical measurement of PA for the main trial (phase 3).
Dietary assessment and analysis
A decision was made to calculate the dietary GI in obese pregnant women using the commercially available WISP version 3 (Tinuviel software, Llanfechell, Anglesey, UK) dietary analysis software. In order to assess diet most accurately, it was decided that dietary data in the pilot study would be obtained from a triple-pass 24-hour dietary recall at three time points in duplicate, performed at baseline and post randomisation [28/29(+1) weeks’ gestation and 36/37(+1) weeks’ gestation] (for details see Chapter 4). This dietary assessment methodology was undertaken in preference to a 4-day food diary, owing to recall bias and data quality issues reported with this method in similar population groups. A shortened food frequency questionnaire (FFQ) was also employed for assessment of long-term dietary habits, with particular reference to the dietary GI and for validation in the pilot study (for details see Chapter 4).
Method development for health-care cost assessment
This was undertaken by the Department of Health Economics, York University, York, UK. A within-trial cost–utility analysis was planned to estimate the cost-effectiveness of the health training (intervention) over and above routine care (control). Quality-adjusted life-years (QALYs) would be used to measure health outcomes and were derived from participant-completed EuroQol-5 Dimensions (EQ-5D) questionnaires. 81
Development of trial database
A secure, password-protected internet-based database was developed (MedSciNet Ltd, Stockholm, Sweden). This facilitated contemporaneous data collection and cleaning and enables 24-hour randomisation. The information collected includes maternal and paternal demographics; assessment of eligibility; consent; previous maternal, medical, family, social and obstetric history; maternal anthropometric measurements, maternal food questionnaires; maternal PA data (uploaded from accelerometer; pilot study only); lifestyle questionnaire; depression questionnaire; quality-of-life questionnaire; EQ-5D questionnaire; and maternal pregnancy outcome and neonatal outcome data. The database also included sample data storage and management fields.
Ethics approval
Ethics approval was granted for the entire programme (IRAS 09/H0802/5) and research and development approvals obtained from the trial centres. When necessary substantial amendments were requested and granted. The UPBEAT was accepted on the UK Clinical Research Network portfolio number 5305.
Chapter 2 The UK Pregnancies Better Eating and Activity trial: phase 2 – pilot trial
Following development of the intervention, detailed in this chapter and the protocol (see Chapter 4), the programme progressed to phase 2, a pilot trial designed to establish the efficacy of the intervention in changing dietary and PA behaviours and to evaluate all practical aspects of delivering the intervention.
This chapter has been published as Poston L, Briley AL, Barr S, Bell R, et al. Developing a complex intervention for diet and activity behaviour change in obese pregnant women (the UPBEAT trial); assessment of behavioural change and process evaluation in a pilot randomised controlled trial. BMC Pregnancy Childbirth 2013;13:148. 150 © Poston et al. ; licensee BioMed Central Ltd. 2013. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The text below includes minor additions and formatting changes to the original text.
Abstract
Background: Complex interventions in obese pregnant women should be theoretically based, feasible and shown to demonstrate expected behavioural change prior to inception of large RCTs. The aim was to determine if (1) a complex intervention in obese pregnant women leads to expected changes in diet and PA behaviours, and (2) to refine the intervention protocol through process evaluation of intervention fidelity.
Methods: We undertook a pilot RCT of a complex intervention in obese pregnant women, comparing routine antenatal care with an intervention to reduce dietary GL and saturated fat intake and increase PA. Subjects included 183 obese pregnant women (mean BMI 36.3 kg/m2). Diet was assessed by repeated triple-pass 24-hour dietary recall and PA by accelerometry and questionnaire at 16+0–18+6 and at 27+0–28+6 weeks’ gestation in women in control and intervention arms. Attitudes to behaviour change and quality of life were assessed and a process evaluation undertaken. The full RCT protocol was undertaken to assess feasibility.
Results: Compared with women in the control arm, women in the intervention arm had a significant reduction in dietary GL (33 points, 95% CI −47 to −20 points), (p < 0.001) and saturated fat intake (−1.6% energy, 95% CI −2.8% to −0.3%) at 28 weeks’ gestation. Objectively measured PA did not change. Physical discomfort and sustained barriers to PA were common at 28 weeks’ gestation. Process evaluation identified barriers to recruitment, group attendance and compliance, leading to modification of intervention delivery.
Conclusions: This pilot trial of a complex intervention in obese pregnant women suggests greater potential for change in dietary intake than for change in PA, and through process evaluation illustrates the considerable advantage of performing an exploratory trial of a complex intervention in obese pregnant women before undertaking a large RCT.
Trial registration: Trial registration number ISRCTN89971375.
Background
Obesity is prevalent in women of reproductive age in both high- and low- to middle-income countries. 82 Obese pregnant women have a heightened risk of adverse pregnancy outcomes,82 but at present there is no evidence-based intervention that can be introduced into clinical practice to improve pregnancy outcome in obese women. The majority of attempts to develop interventions have hitherto focused on limiting GWG in accordance with the US Institute of Medicine’s recommendations. 83 Recent meta-analyses of relevant studies in obese women show modest restriction of GWG without robust evidence for improved clinical outcome. 84,85 Limitations of the existing evidence include poor study design, small sample size, absence of a theoretical basis and, importantly, no a priori demonstration of the feasibility of the intervention in regard to changing the specific behaviours targeted. We have developed a theoretically based behavioural group intervention (diet and PA) for obese pregnant women with the primary aim of improving maternal glucose homeostasis. As maternal insulin resistance is integral to many complications of obese pregnancy, the dietary intervention focuses on lowering the dietary GI, previously shown to improve pregnancy outcome in women with GDM. 9,86 Recommendations were also made to lower saturated fat intake, as a diet high in saturated fats has been implicated in insulin resistance and GDM. 87 Increased PA can also improve metabolic control and reduce GDM risk in pregnant women. 88
Prior to embarking on a large RCT, and in accordance with UK Medical Research Council Guidance for development of a complex intervention,89 we first explored the theoretical basis for an intervention in obese pregnant women,2,3,85,90 leading to development of a novel intervention (phase 1). We now report on phase 2, an exploratory trial to determine whether or not this intervention achieved the changes in dietary and PA behaviours expected, and to undertake a process evaluation of every aspect of fidelity of the intervention and the protocol.
Participants
Potentially eligible participants attending clinics for general antenatal care were approached by research midwives in four UK study centres in urban settings providing a range of models of care. The contributing hospitals were (1) The Southern General Hospital and Princess Royal Maternity Hospital (Glasgow), (2) The Royal Victoria Infirmary (Newcastle), (3) Guy’s and St Thomas’ NHS Foundation Trust (London) and (4) King’s College Hospital Foundation Trust (London).
Methods
The protocol for the exploratory trial is shown in Figure 3. Verbal and printed information was provided to potential participants at a routine antenatal appointment in the first trimester of pregnancy and women were contacted > 24 hours later to ascertain willingness to participate. For those declining participation, consent to record basic demographic data and BMI was obtained. Those willing to participate were invited to return for their first study appointment in the early second trimester (> 15+0 to < 17+6 weeks’ gestation). This window of recruitment allowed adequate time for arrangement of the one-to-one session with the HT followed by the 8-week intervention programme prior to the oral glucose tolerance test (OGTT), carried out between 27+0 and 28+6 weeks’ gestation. Research midwives received a study-specific manual, attended at least one training session with the trial manager and continued feedback and training sessions for the study duration.
FIGURE 3.
Pilot study protocol. EPDS, Edinburgh Postnatal Depression Scale; OGTT, oral glucose tolerance test; RPAQ, Recent Physical Activity Questionnaire.

Inclusion criteria: a BMI of ≥ 30 kg/m2, singleton pregnancy and a gestational age of > 15+ and < 17+6 weeks.
Exclusion criteria: unable or unwilling to give written informed consent; gestation age of < 15+0 and > 17+6 weeks; pre-existing diabetes mellitus; pre-existing essential hypertension (treated); pre-existing renal disease; multiple pregnancy; systemic lupus erythematosus; antiphospholipid syndrome; sickle cell disease; thalassaemia; coeliac disease; currently prescribed metformin; thyroid disease; or current psychosis.
All data were entered onto a password-protected secure database (MedSciNet Ltd). Randomisation was performed online. The randomised treatment was allocated automatically, balanced by minimisation for maternal age, centre, ethnicity, parity and BMI. Data were analysed using Stata (version 11.2; StataCorp, College Station, TX, USA). All women randomised between 29 March 2010 and 13 May 2011 were included. Postcodes were matched to two national indices of deprivation: the Index of Multiple Deprivation91 for English addresses or the Scottish Index of Multiple Deprivation92 for addresses in Scotland.
Control arm
Following randomisation, women in the control arm (standard care) returned for data collection appointments with the study midwife at 27+0–28+6 and 34+0–36+6 weeks’ gestation, when possible coinciding with routine antenatal visits.
Intervention arm
Following randomisation, participants attended a one-to-one appointment with the HT and were invited to weekly group sessions for 8 consecutive weeks from approximately 19 weeks’ gestation. All women attended routine antenatal care appointments and received advice regarding diet and PA in accordance with local policies, which draw on the UK’s National Institute for Health and Care Excellence (NICE)’s guidelines. 93
Sample size
The primary outcome was change in dietary and PA behaviours at 28 weeks’ gestation (coinciding with the primary maternal outcome for the main RCT, GDM at 28 weeks’ gestation). No prior investigation in obese pregnant women was available to inform power at the planning stage. The sample size of 183 was determined by the predefined duration of phase 2, the exploratory phase. This number was adequate to enable power calculations for primary end points of the subsequent RCT, by providing estimates of the variance to within approximately 7% of the true value.
Ethics
Research Ethics Committee approval was obtained in all participating centres, UK IRAS reference number 09/H0802/5 (South East London Research Ethics Committee).
The intervention
The intervention was informed by psychological models of health behaviour including control theory94 and social cognitive theory. 95 Although no clear patterns between intervention characteristics and outcomes have been seen to date in lifestyle interventions in pregnancy, and few studies have described their theoretical basis,2,85 self-regulation techniques, drawn from control theory, suggest that behaviour change is facilitated by feedback about performance compared with prespecified goals. 94,96 This approach was utilised in this study by setting specific, measurable, achievable, relevant and time-specific (SMART) diet and activity goals, with behaviours recorded in a logbook. Identification of benefits of, and overcoming barriers to, behaviour change, and increasing self-efficacy were also included, and social support was facilitated through the group format. 95 As identified in social cognitive theory, the intervention aimed to build self-efficacy through mastery experiences (e.g. maximising the chance of success with SMART goals), vicarious experiences (encouraged through modelling in the group setting), and social persuasion (e.g. through enlisting social support). Following initial feedback from HTs regarding difficulties encountered by some women in attending sessions, for those women unable to attend, the session content was delivered by telephone or e-mail.
Dietary advice
Prespecified dietary outcomes were a change in the GI, GL [an indicator of carbohydrate quality (GI) and quantity consumed] and energy intake from saturated fatty acids (SFAs). The focus of the dietary advice to the intervention group was therefore on increased consumption of foods with a low dietary GI, including replacement of sugar-sweetened beverages with low-GI alternatives. Reduction in saturated fats and replacement with monounsaturated and polyunsaturated fat was also recommended. Exchange of foods was emphasised, for example a high-GI food for a low-GI food, rather than limiting energy intake.
Physical activity advice
Women in the intervention arm were encouraged to increase daily PA incrementally, setting goals of incremental step counts (monitored by pedometer) and maintaining the achieved PA level after the intervention period. Recommendations included an emphasis on walking at a moderate-intensity level. 39
Intervention delivery
The intervention was delivered by HTs. In the UK, HTs do not have prespecified health professional qualifications, but relevant experience [http://informationstrategy.dh.gov.uk/health-trainer-workforce (last accessed 10 March 2013), information now available at www.healthcareers.nhs.uk/explore-roles/public-health/health-trainer (last accessed 20 March 2017)]. All HTs received a comprehensive treatment manual, pre-study training (and within-study supervision) in behaviour modification and conducting group sessions (organised by Weight Concern; registered charity number 1059686). The sessions were held in a hospital setting in all but one centre, in which women attended a community children’s centre. At the initial one-to-one appointment women were provided with a participant handbook, reflecting the rationale and content of the HT sessions, a pedometer (Yamax SW200 Digi-Walker), a logbook for weekly SMART goals and related behaviours (steps, PA and diet) and a DVD of a specially devised pregnancy exercise regime. Potential benefits of attending group sessions were discussed. Each group session delivered a different element of the dietary and PA intervention. (Table 7). Goals from the previous week were reviewed and goals set for the following week. Discussion included barriers to behavioural change and ways these might be overcome.
| Session and main topics | Content |
|---|---|
| Introductory one-to-one session | |
| Introduction to the programme | Overview of programme structure, content and participant materials |
| Participant expectations of taking part and what they hope to gain | |
| Information regarding safe exercise in pregnancy | |
| Participants given pedometer and information on how to use it | |
| UPBEAT DVD and logbook given | |
| Session 1 | |
| Swapping soft drinks First steps to PA |
Introductions and group rules |
| Review of pedometer use | |
| Information on soft drinks | |
| Introduction to goal-setting | |
| Session 2 | |
| Reducing added sugar Increasing everyday activity |
Information on reducing added sugar |
| Information on SMART goal-setting | |
| Information on increasing everyday PA | |
| Session 3 | |
| Swapping bread Overcoming barriers to PA |
Problem-solving of barriers to being physically active |
| Information on choosing lower-GI bread | |
| Session 4 | |
| Swapping other starchy foods Benefits of PA |
Half-way review |
| Information on choosing lower-GI rice, potatoes and other starchy foods | |
| Information on benefits of PA | |
| Review of own PA level | |
| Discussion about the UPBEAT DVD | |
| Session 5 | |
| Swapping snacks Active leisure time |
Information on choosing healthier snacks |
| Cravings vs. hunger | |
| Discussion on leisure time PA and how to access services | |
| Session 6 | |
| Swapping breakfast cereals Local leisure services |
Discussion on experiences of accessing local leisure services |
| Information on choosing lower-GI breakfast cereals | |
| Discussion on maintaining changes made to behaviour | |
| Session 7 | |
| Choosing lower-fat dairy products Keeping active throughout the day |
Review of dietary and activity topics covered so far, any benefits participants have experienced and accessing support after the sessions |
| Discussion on keeping active throughout the day | |
| Information on choosing lower-fat dairy products | |
| Session 8 | |
| Choosing lower-fat meat and meat products PA in late pregnancy |
Information on choosing lower-fat meat and meat products, and non-meat alternatives |
| Information on PA in late pregnancy | |
| Maintaining behaviour changes | |
| Every session | |
| Review of goal attainment from previous week | |
| Review of self-monitoring (logbook) from previous week, including pedometer | |
| Setting of dietary and activity SMART goals for coming week | |
| After the sessions (until 36 weeks) | |
| Biweekly telephone support to discuss maintenance of behaviour changes and any further changes participants want to make | |
The following information was obtained from all participants (at visits indicated in Figure 3).
Attitudinal assessment questionnaire
The attitudinal assessment included questions relating to perceived benefits and barriers and confidence to carry out the dietary and PA behaviours. 27,97 The target behaviours were to consume lower-GI carbohydrates, to reduce saturated fat intake and to increase PA.
Health status and mental health
The EQ-5D98 was used to assess health status and the Edinburgh Postnatal Depression Scale (EPDS) to assess mental health. 99
Dietary assessment
Repeated, triple-pass 24-hour recall data obtained at baseline (randomisation) and at 28 weeks’ gestation were evaluated twice, 1 week apart, in both the intervention and control groups. The 24-hour dietary recall is a standard retrospective, interviewer-led dietary assessment methodology used to capture information on all food and drinks consumed in the preceding 24 hours. This is carried out in three stages (the triple pass): (1) recording a ‘quick’ list of foods eaten or drunk, (2) collecting more detailed information of these foods and (3) reviewing all items once more in order to clarify any ambiguities or omissions. A short FFQ, for later validation, was also completed.
Physical activity assessment
At the first and third appointments participants were asked to wear an ActiGraph accelerometer (either GT1M or GT3X set to uniaxial mode) for 7 consecutive days, removing it for washing, bathing, swimming and at night. PA was also assessed by questionnaire [via the Recent Physical Activity Questionnaire (RPAQ)].
Process evaluation
A process evaluation, following the framework of Steckler and Linnan,100 was undertaken. This explored (1) context (environmental, socioeconomic or political factors), (2) reach (the proportion of the intended target audience that participates and which subgroups, if any, do not participate), (3) dose delivered and dose received (the proportion of intended intervention received), (4) fidelity (if each component of the complex intervention was provided as intended) and (5) acceptability (if the intervention materials and advice were well-received by providers and participants).
Qualitative semistructured interviews were conducted to capture women’s experiences and perceptions of the trial and intervention. Women were recruited from each of the participating study sites using a maximum diversity sampling approach, following an informed consent procedure. Interviews took place between November 2010 and February 2011, and were either face to face (n = 17), mostly in hospital settings, or by telephone (n = 4). Control (n = 12) and intervention (n = 9) interviewees were asked about their involvement in the research and their experiences of the trial appointments, measurements, blood tests and accelerometry recordings. Women in the intervention arm were additionally asked about their perceptions of the different components of the intervention, and how these impacted upon their lives. The interviews were conducted by one researcher and took place during pregnancy after the intervention had been provided. In addition, HTs completed audio diaries (130 recordings) in which they reflected on the fidelity and feasibility of the intervention delivery. Attendance at sessions was recorded on the study database.
Clinical outcome data
Maternal primary outcome for the subsequent randomised controlled trial (diagnosis of gestational diabetes mellitus)
A blood sample for fasting glucose and insulin was taken after an overnight fast. For the OGTT, following a glucose load [410 ml of Lucozade (Suntory Holdings Ltd, Osaka, Japan) or 75 g of glucose in water], 1-hour and 2-hour samples were taken for glucose measurement. Diagnosis of GDM was confirmed by a fasting glucose level of ≥ 5.1 mmol/l and/or 1-hour glucose level of ≥ 10 mmol/l or 2-hour glucose level of ≥ 8.5 mmol/l, in accordance with the International Association of the Diabetes and Pregnancy Study Groups (IADPSG)’s guidelines. 6 Following GDM diagnosis, women were referred for routine GDM care in accordance with local criteria.
Neonatal primary outcome for the subsequent randomised controlled trial (large-for-gestational age delivery defined as ≥ 90th customised birthweight centile)
Customised birthweight centiles were calculated correcting for gestational age, maternal ethnicity, weight and height in early pregnancy, parity and infant sex. Weight adjustment for women with a BMI of ≥ 30 kg/m2 is based on a notional weight corresponding to a BMI of 29.9 kg/m2.
Outcome data also recorded (not reported)
These included maternal outcomes: diagnosis of GDM and pre-eclampsia, mode of delivery, blood loss at delivery, inpatient nights, detailed clinical and family history, health in current pregnancy, early pregnancy data (ultrasound scan, nuchal screening), blood pressure, routine blood results; neonatal outcomes: gestational age at delivery, birthweight, anthropometry and inpatient nights. Maternal urine and cord blood samples were also provided.
Data handling and statistical analysis
Health quality and attitudinal assessment questionnaires
The generic EQ-5D health-related quality-of-life instrument98 is reported as the proportion of women with problems on individual dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression). It is given as a summary index score calculated from preference values of different combinations of the dimensions elicited using the time trade-off method in a sample representative of non-institutionalised adults in England, Scotland and Wales (range −0.59 to 100, where −0.59 is severe problems on all dimensions);101,102 and also the visual analogue scale of health-related quality of life (range 0 to 100, where 0 is worst imaginable health state). The change between baseline and 28 weeks’ gestation in the percentage of women with any problem was assessed using McNemar’s test of changes. Attitudes to target behaviours (attitudinal assessment questionnaire) are based on the average of multiple responses on five-point scales (three responses for diet, 13 for PA) with five indicating the greatest perceived barrier, perceived benefit or level of confidence.
Assessment of deprivation
These scales for estimation of deprivation in England and Scotland91,92 use different reference populations to determine the actual indices of deprivation, and are therefore not directly comparable. For the purposes of this study, the most deprived quintile is presented separately for women in each population and compared with the remainder of the population (quintiles 1–4).
Dietary analysis
The quality of dietary data was checked within 1 week of entry. Dietary coding utilised McCance and Widdowson’s Composition of Foods (6th edition)103 food codes and nutrient composition was evaluated using WISP version 3 for GI and GL values. 104 Estimates using previously published methodology were made when GI values were not available. 105 Twenty-four-hour recall data obtained at baseline (randomisation) and at 28 weeks’ gestation were evaluated twice, 1 week apart, and then averaged. The validity of the short FFQ was assessed against the dietary recall data. Prespecified dietary outcomes were a change in GI, GL and energy intake from SFA. Total energy intake and the proportion of energy derived from macronutrients were assessed. GWG at 28 and 36 weeks’ gestation, a relevant secondary outcome of interest, is also reported.
ActiGraph analysis
An epoch length (time sampling interval) of 15 seconds was specified. Data were processed using the MAHuffe software package. Sedentary behaviour was defined as < 100 cpm, light activity as 100–1951 cpm, moderate-intensity activity as 1952–5725 cpm and vigorous activity as > 5725 cpm. 68 As time spent in vigorous activity was very low, minutes of moderate and vigorous physical activity (MVPA) were combined. Runs of zero counts lasting > 60 minutes were excluded, as these indicated monitor removal. A valid recording was defined as a day in which > 500 minutes of monitored on-time was recorded in 24 hours. 106 Data from participants recording ≥ 3 days of valid accelerometry data on 3 or more days were included in the analysis. The specified PA outcome was an increase in minutes per day of MVPA recorded by accelerometry.
Recent Physical Activity Questionnaire
The RPAQ was modified for the assessment of PA in the preceding 7 days. Estimates of minutes per day spent in light, moderate and vigorous activity in each of the domains were calculated. Sedentary activities were defined as those with a metabolic equivalent (MET) of < 1.5. Light activities were those of 1.5–3 METs. Moderate activities were those of 3–6 METs. Vigorous activities were those of 6 METs or greater. 107 MVPAs were combined to give one summary variable.
Process evaluation
Interviews were recorded and transcribed verbatim. Transcripts were anonymised and a unique identifier (ID) number was used to maintain confidentiality. Data were imported into a qualitative software analysis package (NVivo version 8, QSR International, Warrington, UK) and subject to comparative thematic analysis. 108 To enhance study validity and reliability, themes arising from the research were discussed, the data supporting these were reviewed by co-researchers, and data were compared between sites and with existing literature. By these methods, assumptions were tested and observations of differences and their relationship to the theoretical models underpinning the study were explored.
Statistical analysis
Analyses followed the intention-to-treat principle. Following Consolidated Standards of Reporting Trials (CONSORT) guidelines, risk ratios and risk differences were estimated by binary regression for yes/no outcomes. When measures were repeated at baseline and 28 weeks’ gestation, results of mean [standard deviation (SD)] or n (%) are presented separately at each time point. Randomised comparisons at 28 weeks were made using linear regression with robust standard errors, adjusting for the baseline value. For PA data, dummy variables were used when the baseline values were missing. Correlations between PA as assessed objectively (accelerometry) and when self-reported (RPAQ) were explored.
Results
Figure 4 provides a flow chart of participants through the study.
FIGURE 4.
The UPBEAT pilot study CONSORT diagram.

Participants
The mean first visit BMI was 36.3 kg/m2. More than half the women were white and the remainder were from black (38%) and minority ethnic communities. More than half (56%) already had at least one child. More than half of those from centres in England and over 40% in Scotland came from regions in the highest quintile of social deprivation (Table 8).
| Demographic variable | Trial arm | |
|---|---|---|
| Control (n = 89) | Intervention (n = 94) | |
| Age (years),a mean (SD) | 30.7 (4.9) | 30.4 (5.7) |
| Age categories (years), n (%) | ||
| 18–25 | 16 (18) | 22 (23) |
| 26–30 | 25 (28) | 27 (29) |
| 31–40 | 46 (52) | 42 (45) |
| ≥ 41 | 2 (2) | 3 (3) |
| Anthropometry, mean (SD) | ||
| Height (m) | 1.64 (0.07) | 1.64 (0.07) |
| Weight (kg) | 96.8 (16.2) | 97.8 (12.7) |
| BMI (kg/m2) | 36.1 (4.8) | 36.5 (4.7) |
| Ethnicity,a n (%) | ||
| White | 51 (57) | 52 (55) |
| Black | 32 (36) | 38 (40) |
| Asian | 1 (1) | 2 (2) |
| Other | 5 (6) | 2 (2) |
| Parity,a n (%) | ||
| 0 | 38 (43) | 63 (67) |
| 1 | 36 (40) | 25 (27) |
| ≥ 2 | 15 (17) | 6 (6) |
| Cigarette smoking, n (%) | ||
| Never | 61 (68) | 63 (67) |
| Ex-smoker | 22 (25) | 25 (27) |
| Current | 6 (7) | 6 (6) |
| Number of cigarettes, n (%) | ||
| 0 | 83 (93) | 83 (88) |
| 1–5 per day | 3 (3) | 3 (3) |
| 6–10 per day | 1 (1) | 6 (6) |
| 11–20 per day | 2 (2) | 2 (2) |
| Index of multiple deprivationb | ||
| England | ||
| n | 76 | 79 |
| Mean (SD) | 34 (12) | 36 (14) |
| Quintiles, n (%) | ||
| 1–4 (less deprived) | 35 (46) | 29 (37) |
| 5 (most deprived) | 41 (54) | 50 (63) |
| Scotland | ||
| n | 12 | 14 |
| Mean (SD) | 28 (11) | 30 (20) |
| Quintiles, n (%) | ||
| 1–4 (less deprived) | 7 (58) | 8 (57) |
| 5 (most deprived) | 5 (42) | 6 (43) |
| Living arrangements, n (%) | ||
| Single | 35 (39) | 50 (53) |
| With partner | 66 (74) | 69 (73) |
| With parent(s) | 7 (8) | 13 (14) |
| Without partner or parents | 17 (19) | 17 (18) |
| Accommodation, n (%) | ||
| Owned | 27 (30) | 21 (22) |
| Rented (private) | 26 (29) | 27 (29) |
| Rented (council owned) | 36 (40) | 46 (49) |
Diet and gestational weight gain
Table 9 shows the dietary intakes at baseline and at 28 weeks’ gestation. There were no differences between groups in energy intakes, GI, GL or other macronutrient at baseline. However, following the intervention, at 28 weeks’ gestation, total energy intake, dietary GL, GL (% energy), saturated fat (% energy) and total fat (% energy) were significantly lower, and fibre intake measured as non-starch polysaccharides was greater, in the intervention group than in the control arm. The proportion of energy derived from protein was higher in the intervention group, but absolute protein intake did not differ. There was a difference of 7 GI points between the intervention and control group, which achieved borderline statistical significance (p = 0.054).
| Dietary measure | Time point of measurement | Trial arm, mean (SD) | Difference (95% CI) | p-value | |
|---|---|---|---|---|---|
| Control (n = 89, 69) | Intervention (n = 94, 71) | ||||
| Total energy (MJ/day) | Baseline | 7.53 (2.21) | 7.26 (2.29) | −0.94 (−1.72 to −0.18) | 0.016 |
| 28 weeks’ gestation | 7.71 (2.30) | 6.75 (2.57) | |||
| Dietary GI (%) | Baseline | 58 (6) | 58 (5) | −7 (−15 to 0) | 0.054 |
| 28 weeks’ gestation | 60 (26) | 53 (13) | |||
| Dietary GL (g/day) | Baseline | 133 (48) | 129 (41) | −33 (−47 to −20) | < 0.001 |
| 28 weeks’ gestation | 146 (55) | 111 (39) | |||
| GL (% energy) | Baseline | 27.7 (5.3) | 28.5 (5.9) | −4.8 (−8.5 to −1.0) | 0.013 |
| 28 weeks’ gestation | 31.3 (13.3) | 26.6 (8.0) | |||
| Carbohydrate (% energy) | Baseline | 48.0 (8.4) | 48.9 (9.6) | 1.7 (−1.0 to 4.4) | 0.207 |
| 28 weeks’ gestation | 48.2 (8.0) | 50.0 (8.2) | |||
| Protein (% energy) | Baseline | 15.5 (3.6) | 16.0 (4.2) | 1.5 (0.1 to 2.8) | 0.034 |
| 28 weeks’ gestation | 15.5 (3.2) | 17.1 (4.9) | |||
| Protein (g) | Baseline | 69.3(25.3) | 68.5 (26.1) | −4.8 (−12.3 to 2.6) | 0.204 |
| 28 weeks’ gestation | 70.6 (24.0) | 66.5 (23.5) | |||
| Total fat (% energy) | Baseline | 36.0 (8.2) | 34.9 (9.3) | −3.2 (−5.6 to −0.8) | 0.010 |
| 28 weeks’ gestation | 35.9 (7.7) | 32.5 (7.4) | |||
| SFA (% energy) | Baseline | 12.7 (3.9) | 12.0 (4.3) | −1.6 (−2.8 to −0.3) | 0.015 |
| 28 weeks’ gestation | 12.9 (3.9) | 11.1 (3.8) | |||
| MUFA (% energy) | Baseline | 12.1 (4.1) | 11.4 (4.0) | −1.0 (−2.2 to 0.2) | 0.088 |
| 28 weeks’ gestation | 11.6 (4.0) | 10.4 (3.2) | |||
| PUFA (% energy) | Baseline | 6.4 (3.0) | 6.0 (3.1) | 0.13 (−0.8 to 1.1) | 0.774 |
| 28 weeks’ gestation | 5.9 (2.8) | 6.0 (2.7) | |||
| P to S ratio | Baseline | 0.56 (0.31) | 0.56 (0.40) | 0.13 (−0.01 to 0.28) | 0.075 |
| 28 weeks’ gestation | 0.51 (0.35) | 0.64 (0.52) | |||
| NSP (g) | Baseline | 11.2 (4.6) | 10.4 (4.6) | 1.77 (0.08 to 3.47) | 0.040 |
| 28 weeks’ gestation | 10.5 (4.2) | 12.0 (6.0) | |||
| (n = 88, 88; 75, 61) | (n = 94, 94; 80, 69) | ||||
| Maternal weight (kg) | Pre-pregnancy (estimated) | 95.75 (16.21) | 97.06 (12.62) | ||
| Baseline | 97.00 (16.21) | 98.31 (12.62) | |||
| 28 weeks’ gestation | 102.10 (16.71) | 101.44 (12.22) | –0.87 (–1.80 to 0.05) | 0.065 | |
| 34–36 weeks’ gestation | 105.06 (16.70) | 104.13 (12.82) | –0.54 (–2.09 to 1.01) | 0.494 | |
There was a marginal difference in GWG of –0.9 kg between the intervention and control group at 28 weeks’ gestation (p = 0.065).
Physical activity
There were no differences between the intervention and the control arms in objectively measured PA variables at baseline or at 28 weeks’ gestation, after adjustment for baseline activity. Self-reported moderate to vigorous PA at 28 weeks’ gestation was increased in the intervention group (mean difference 34 minutes/day; 95% CI 9 to 59 minutes/day), but this was not supported by the objective data. Women in the intervention group self-reported walking for leisure for 14 minutes/day more than those in the control group at 28 weeks’ gestation (95% CI 5 to 23 minutes; p = 0.003). Agreement between the RPAQ questionnaire and accelerometry was very poor; for example, correlation between MVPA in the two formats at baseline was r = 0.275 (95% CI 0.107 to 0.428) and at 28 weeks’ gestation was r = −0.069 (95% CI –0.296 to 0.165) (Table 10).
| PA measure | Time point of measurement | Trial arm, mean (SD) | Treatment effect | |
|---|---|---|---|---|
| Control | Intervention | |||
| By accelerometer | (n = 72, 39) | (n = 68, 36) | ||
| Sedentary | Baseline | 1172 (95) | 1165 (91) | 21 (−13 to 55) |
| 28 weeks’ gestation | 1175 (86) | 1197 (77) | ||
| Active | Baseline | 217 (65) | 225 (58) | −11 (−42 to 19) |
| 28 weeks’ gestation | 209 (82) | 194 (68) | ||
| Light | Baseline | 178 (54) | 184 (50) | −9 (−38 to 19) |
| 28 weeks’ gestation | 175 (81) | 161 (61) | ||
| MVPA | Baseline | 40 (20) | 42 (20) | −2 (−9 to 5) |
| 28 weeks’ gestation | 34 (18) | 33 (15) | ||
| By RPAQ questionnaire | (n = 80, 54) | (n = 79, 56) | ||
| Sedentary | Baseline | 1007 (207) | 1009 (187) | −50 (−115 to 16) |
| 28 weeks’ gestation | 1068 (177) | 1020 (226) | ||
| Active | Baseline | 408 (189) | 415 (180) | 45 (−16 to 106) |
| 28 weeks’ gestation | 367 (175) | 410 (219) | ||
| Light | Baseline | 354 (180) | 356 (164) | 11 (−46 to 68) |
| 28 weeks’ gestation | 333 (165) | 340 (204) | ||
| MVPA | Baseline | 54 (87) | 60 (99) | 34 (9 to 59) |
| 28 weeks’ gestation | 34 (52) | 70 (78) | ||
Attitudinal assessment of target behaviours
Benefits, barriers and confidence in making the target PA and dietary changes were unchanged in either the control and intervention groups from baseline to 28 weeks’ gestation (Table 11).
| Attitudes to target behaviours | Time point of measurement | Trial arm | Treatment effect | |
|---|---|---|---|---|
| Control | Intervention | |||
| Barriers, mean (SD) | ||||
| Diet | Baseline | 2.49 (0.58) | 2.37 (0.61) | −0.18 (−0.35 to 0.00) |
| 28 weeks’ gestation | 2.45 (0.58) | 2.14 (0.68) | ||
| PA | Baseline | 2.64 (0.55) | 2.48 (0.63) | −0.20 (−0.37 to −0.03) |
| 28 weeks’ gestation | 2.47 (0.50) | 2.20 (0.61) | ||
| Perceived benefits, mean (SD) | ||||
| Diet | Baseline | 3.75 (0.72) | 3.80 (0.64) | 0.13 (−0.10 to 0.36) |
| 28 weeks’ gestation | 3.79 (0.67) | 3.97 (0.80) | ||
| PA | Baseline | 3.94 (0.70) | 4.04 (0.54) | 0.17 (−0.04 to 0.38) |
| 28 weeks’ gestation | 3.84 (0.60) | 4.06 (0.69) | ||
| Confidence, mean (SD) | ||||
| Diet | Baseline | 3.78 (0.75) | 3.84 (0.64) | 0.11 (−0.15 to 0.37) |
| 28 weeks’ gestation | 3.71 (0.72) | 3.85 (0.81) | ||
| PA | Baseline | 3.76 (0.88) | 3.92 (0.81) | −0.05 (−0.40 to 0.30) |
| 28 weeks’ gestation | 3.77 (0.88) | 3.81 (1.06) | ||
| Quality of life (EQ-5D) | (n = 87; n = 75) | (n = 94; n = 80) | ||
| Numbers reporting problems | ||||
| Mobility, n (%) | Baseline | 10 (11%) | 11 (12%) | 4% (−10 to 18) |
| 28 weeks’ gestation | 21 (28%) | 25 (31%) | ||
| Change (all women) | 19% (11 to 27) | |||
| Self-care, n (%) | Baseline | 1 (1%) | 0 (0%) | −0.3% (−6 to 6) |
| 28 weeks’ gestation | 3 (4%) | 3 (4%) | ||
| Change (all women) | 4% (0 to 8) | |||
| Usual activities, n (%) | Baseline | 16 (18%) | 13 (14%) | −1% (−15 to 12) |
| 28 weeks’ gestation | 26 (34%) | 26 (33%) | ||
| Change (all women) | 16% (8 to 24) | |||
| Pain and discomfort, n (%) | Baseline | 38 (43%) | 34 (36%) | 10% (−1 to 22) |
| 28 weeks’ gestation | 45 (60%) | 54 (67%) | ||
| Change (all women) | 25% (17 to 34) | |||
| Anxiety and depression, n (%) | Baseline | 22 (25%) | 20 (21%) | 5% (−4 to 15) |
| 28 weeks’ gestation | 11 (15%) | 17 (21%) | ||
| Change (all women) | −6% (−14 to 1) | |||
| TTO score, mean (SD) | Baseline | 0.85 (0.18) | 0.88 (0.14) | −0.03 (−0.07 to 0.02) |
| 28 weeks’ gestation | 0.79 (0.24) | 0.79 (0.16) | ||
| Change (all women) | −0.08 (−0.10 to −0.06) | |||
| VAS score (0 to 100), mean (SD) | Baseline | 76 (20) | 76 (21) | 4 (−3 to 10) |
| 28 weeks’ gestation | 75 (21) | 78 (21) | ||
| Change (all women) | −2 (−6 to 2) | |||
| EPDS | ||||
| Total, mean score (SD) | Baseline | 7.1 (4.6) | 7.4 (4.5) | 0.1 (−1.1 to 1.3) |
| 28 weeks’ gestation | 6.9 (4.2) | 7.1 (5.2) | ||
| Total score of > 9, n (%) | Baseline | 25 (29) | 28 (30) | 1% (−9 to 11) |
| 28 weeks’ gestation | 17 (23) | 21 (26) | ||
| Total score of > 12, n (%) | Baseline | 9 (10) | 10 (11) | 7% (−1% to 16) |
| 28 weeks’ gestation | 6 (8) | 14 (18) | ||
Health Status, Mental Health Edinburgh Postnatal Depression Scale and EuroQol-5 Dimensions
There was no influence of the intervention on the numbers of women reporting problems in each of the EQ-5D domains, but, as a group, obese women experienced a significant increase in problems with mobility, self-care, usual activities and pain and discomfort from baseline to 28 weeks’ gestation. There was a 10% prevalence of probable depression at baseline and a 13% prevalence at 28 weeks (i.e. EPDS score of > 12), with no significant effect of the intervention on anxiety and depression at 28 weeks’ gestation (see Table 11).
Process evaluation
Context
This study coincided with the publication of new reports and guidance for obesity in pregnancy, with associated media coverage. 93,109 Most control group interviewees demonstrated awareness and reported taking steps to improve their diet or fitness (Box 1).
It [being part of the research] just makes you a bit more conscious of what you eat; even though you’re pregnant and you might crave for all these different things, you think what impact it will have on you afterwards when, obviously when your baby’s born and you’re trying to get off the weight and it just makes you think of what you put in your mouth really.
ID 3, control, aged 33 years, multiparous, a BMI of 44 kg/m2 and black African
[Being part of the research has] reminded me a lot more, because I’m overweight, it could be that it was on the radio yesterday as well, I’m thinking about it a lot more . . . Obviously as part of the control group I’m not supposed to necessarily do anything specific and change anything but obviously I’m trying anyway. I don’t think I’d take part [in the research] if I regarded being overweight as perfectly alright and perfectly fine and I wasn’t interested in fixing the situation.
ID 12, control, aged 35 years, multiparous, a BMI of 35 kg/m2 and white British
The midwife I’ve got through my GP [general practitioner] isn’t very friendly so I think it’s beneficial for me to come in and speak to [the research midwives] about the issues I have with the pregnancy and other stuff, because the one I have at my GP seems to be too busy to care, to listen to any of these things so it’s nice to come in and speak to them, it’s a bit reassuring I guess.
ID 1, control, aged 26 years, nulliparous, a BMI of 37 kg/m2 and black British
I’ve really enjoyed it (blood tests, measurements, questionnaires) because it’s really reassuring, because you do a lot of the stuff with the midwives and you feel like if there was something wrong I’d be told about it.
ID 4, intervention, aged 25 years, nulliparous, a BMI of 35 kg/m2 and white British
You feel more looked after. You feel like it’s more private care, more special than just the normal midwife care or the GP. Because they just want to get you in and out really quickly. Cos they have loads more people to see. But here you feel like you have the time to talk or say what you have to say. And ask any fears about anything, really, I think.
ID 17, intervention, aged 22 years, multiparous, a BMI of 37 kg/m2 and mixed ethnicity
Reach
The mean age of those approached who were eligible for recruitment but declined to participate (n = 473) was 29.9 years and their mean BMI was 35.39 kg/m2; 59.7% were white, and 32.8% were black and 43.0% were in the lowest quintile for the Index of Deprivation, indicating the most severe deprivation. The characteristics of participants providing semistructured interviews (n = 21) are shown in Table 12. Their demographic profile was similar to that of study participants (see Table 7). Overall, 29 out of 183 (15.8%) women were lost to follow-up (see Figure 4).
| Demographic variable | n | Interview sample | Pilot trial population (n = 183) |
|---|---|---|---|
| Control | 12 | ||
| Intervention | 9 | ||
| Ethnicity (%) | |||
| White | 8 | 38 | 56 |
| Black | 12 | 57 | 38 |
| Asian | 0 | 0 | 2 |
| Other | 1 | 5 | 4 |
| Age (years), mean (SD) | 29.6 (4.9) | 30.5 (5.4) | |
| Parity, % | |||
| Nulliparous | 9 | 43 | 44 |
| Multiparous | 12 | 57 | 56 |
| Gestational age at interview (weeks) | 29 | Not interviewed | |
| BMI (kg/m2) at recruitment, mean (SD) | 37.6 (4.6) | 35.6 (5.1) | |
Dose
Of the 94 women randomised to the intervention, 82 (88%) attended at least one group session, and 60 (64%) attended four or more. A total of 42 women (45%) received material from all eight sessions, six by full attendance (6%) and the remainder when partly/wholly covered by subsequent telephone contact. The mean number of sessions attended or partly/wholly covered was 6.1 (SD 2.6).
Fidelity
The intervention package (eight HT group sessions) was provided with good consistency at each study site. Goals were set at all group sessions, of which 88% were considered SMART by HTs according to their diaries. The maximum group size was 5 (mean 2).
Acceptability
Women in both arms of the trial found the research processes acceptable and felt supported by the study midwives. Women in the intervention group were generally willing, in principle, to attend the eight HT sessions, and most women who attended valued the group approach, citing opportunities to raise questions and discuss each other’s experiences. Some were surprised at the extent of the intervention, having expected a less intensive, more advice-based approach. Consistency of attendance at the sessions varied for different reasons including work commitments, school pick-up times or feeling too unwell or tired. Occasionally initial involvement waned when groups proved smaller than expected, although the HT input by telephone or e-mail was considered valuable. Some women found the information contained in the handbook new, whereas for others it was too basic. The pedometers and step goals were generally well received. Setting and reflecting on weekly goals was motivational for most but could also invoke feelings of guilt, or a sense of being observed and judged. Women reported having watched the DVD, but few used it regularly.
When interviewees were asked whether or not they had made any changes as a result of the intervention, most reported some degree of change, especially in relation to dietary intake. Reported changes in PA were more limited, particularly because of pelvic pain or tiredness as pregnancy progressed. Women often reported aspirations to increase exercise postnatally (Box 2 and Table 13).
I thought it was going to be healthy eating and exercising. I thought it was going to be like how they tell you in the news, that we have to eat better. Or what you hear media-wise. But it’s more in-depth and more suitable to how you are, basically. It’s more fitted to how you are. Instead of every thousand people. It’s just for you. It’s more suitable that way, I feel.
ID 17, intervention, aged 22 years, multiparous, a BMI of 37 kg/m2 and mixed ethnicity
The groups that I went to, there was only . . . two of us at maximum. I think there were supposed to be four.
ID 4, intervention, aged 25 years, nulliparous, a BMI of 35 kg/m2 and white British
Access to intervention
I couldn’t come to all the sessions, I think it started at 3 and school finishes at 3:15 so it’s a bit difficult to try and get [to the hospital] for that time.
ID 2, intervention, aged 27 years, multiparous, a BMI of 34 kg/m2 and black African
The only thing I was worrying about was being able to commit every week. But I think there was once that I couldn’t do it and [HT] e-mailed and she phoned so I didn’t miss out on anything.
ID 8, intervention, aged 29 years, a BMI of 43 kg/m2 and white British
I also found it quite hard saying ‘Oh, I need eight Thursday afternoons off work’, and I just felt like I was taking advantage of them by taking extra time off work.
ID 4, intervention, aged 25 years, nulliparous, a BMI of 35 kg/m2 and white British
Affect on mood
. . . I just felt quite . . . quite bad, and I felt that . . . that I wasn’t doing good . . . I wasn’t doing what was good for my baby . . . by not being healthy and fit and . . . and all of that, I felt like I was doing something wrong, so . . . I don’t know . . . you’re on a diet of guilt, you know, you should be eating this because otherwise you’re doing badly.
ID 4, intervention, aged 25 years, nulliparous, a BMI of 35 kg/m2 and white British
. . . because you’re on the [research] project, you feel good when you do something good. And when you do something bad, you feel bad. Because you feel like you’re letting yourself down.
ID 17, Intervention, aged 22 years, multiparous, a BMI of 37 kg/m2 and mixed ethnicity
Handbook and dietary change
Instead of the basmati rice, I’d had the normal long grain rice, and instead of mashed potato, you can have sweet potatoes, it’s just really silly things you didn’t know, you thought you were eating healthily and you weren’t, so changing that, swapping . . . The benefits are you’re definitely not gaining that much weight, which is a plus. All the women will like that bit of it, so it kept you going.
ID 13, intervention, aged 36 years, multiparous, a BMI of 32 kg/m2 and black African
I’ve always bought wholemeal bread but because we were encouraged to buy seedy bread, I am still buying it and I think I’ll continue to buy it.
ID 11, intervention, aged 35 years, multiparous, a BMI of 41 kg/m2 and black African
I don’t know how to say it, [the handbook] was more for people who didn’t really have . . . good knowledge with food, or cooking or eating well, do you know what I mean? It’s just like . . . I eat healthy . . . maybe too many desserts or cakes or sweets or whatever, but I do know how to eat healthy, but [the handbook] was more aimed at people who don’t know, who are just eating for the sake of eating.
ID 4, intervention, aged 25 years, nulliparous, a BMI of 35 kg/m2 and white British
Physical activity
The logbook was fantastic with the pedometer. It was so motivating. We were aiming for 10,000 steps a day so every day I was doing extra trying to get to that and that really motivated me and the family, all the kids were behind me.
ID 13, intervention, aged 36 years, multiparous, a BMI of 32 kg/m2 and black African
If I didn’t leave home at all [1 day], I would have like 1000 steps and I’m like ‘Oh my God, that’s really [low] . . .’ it sort of motivated me to try and do something about it the next day . . . if I hadn’t done this [research], I suppose I’d still be in that mindset, that, ‘Oh, I am pregnant, I’m not allowed to do anything’ whereas now, because of having looked at my step count, I am very aware that I have to stay active and when I don’t, it does bug me.
ID 11, intervention, aged 35 years, multiparous, a BMI of 41 kg/m2 and black African
I developed a condition called SPD [Symphysis Pubis Dysfunction], it was my pelvis which becomes really unbearable and very painful to walk . . . but obviously after this baby comes I am going to make a conscious effort to do a lot more. And obviously I’ll not have the problems with my pelvis as well which will be a great help.
ID 8, intervention, aged 29 years, nulliparous, a BMI of 43 kg/m2 and white English
I want to start on my diet after my baby’s born. More healthy cooking and stuff. And once or twice a week, swimming and stuff like that. It makes you feel positive about yourself to do more. So afterwards you feel, okay, if I can do this while I’m pregnant, I can do 100 times more when I’m not. So I think it’s a motivation thing. It makes you think about, basically, it makes you think about your health during your pregnancy. But it makes you think afterwards, as well, so if I can take this much care when I’m pregnant, I can do a lot more afterwards.
ID 17, intervention, aged 22 years, multiparous, a BMI of 37 kg/m2 and mixed ethnicity
| Outcome | Trial arm | Comparison | Treatment effect (95% CI) | p-value | |
|---|---|---|---|---|---|
| Control | Intervention | ||||
| Maternal | (n = 75) | (n = 79) | |||
| GDM | 24 (32%) | 22 (28%) | Risk difference | –4% (–19% to 13%) | 0.574 |
| Risk ratio | 0.87 (0.54 to 1.41) | ||||
| Neonatal | (n = 84) | (n = 86) | |||
| LGA | 7 (8%) | 7 (8%) | Risk difference | 0% (–8% to 8%) | 0.982 |
| Risk ratio | 0.99 (0.36 to 2.7) | ||||
| > 4 kg | 16 (19%) | 13 (15%) | Risk difference | –4% (–15% to 8%) | |
Maternal and neonatal outcomes
The primary maternal and neonatal outcomes for the subsequent RCT are shown in Table 13. There were no significant differences in GDM or LGA (≥ 90th customised centile) between control and intervention arms. There was also no significant difference in GWG between control and intervention arms (secondary outcome). The overall incidence of GDM, the primary outcome of the subsequent RCT (not powered for), in accordance with recent IADPSG criteria,6 was 30%, enabling calculation of the subsequent RCT sample size (1546 women) for the RCT, powered for a 25% reduction. As 38% of potentially eligible women took part in the pilot study, to achieve this sample size in the main RCT, approximately 4100 would need to be approached.
Discussion
This study describes a pragmatic and rigorously evaluated pilot study of a complex intervention for diet and activity behaviour change in obese pregnant women. The intervention was associated with a significant change in dietary behaviour. Process evaluation showed overall acceptability of the protocol, but led to several refinements to improve acceptability and fidelity.
In any lifestyle intervention of diet and PA, it is important that the pilot study design includes methods to assess the potential of the intervention to change these behaviours in the expected direction of effect. Few assessments of dietary intake in similar investigations of overweight or obese women have been attempted. 110–112 We used a 24-hour recall method to assess dietary intake and, while this may lead to under-reporting of energy intakes, the reported values are not dissimilar to those of non-pregnant women in the general UK population. 113 Both objectives of the dietary intervention, to bring about reductions in GL and the proportion of energy derived from SFAs, were achieved. This suggests that obese pregnant women are amenable to changing their diet in response to an intervention based on established theory, and that dietary advice, frequently delivered by health professionals, is likely to be successful in achieving dietary change in obese pregnant women, as previously implied. 111 The reduction in dietary GL achieved was similar (33% vs. 45%) to that reported in obese type 2 diabetic non-pregnant subjects, in whom improved glycaemic control was achieved. 114 Recently, a similar intervention in 759 pregnant women showed a lower change in GL (13%), which was associated with a reduction in GWG in women who had previously delivered a LGA infant. 115
The reduction in energy intake observed is consistent with other studies that have restricted the intake of fat from meat and dairy products and which has not been replaced by other sources of food energy. 116 The reduced GL may also have contributed through effects on satiety. 117 To our knowledge this is the first study demonstrating that expected changes in diet occur following delivery of an intervention to lower GL and saturated fat in obese pregnant women without GDM. Importantly, this occurred despite the focus being on reducing GL by lowering the intake of added sugars as well as advocating foods with a lower GI. Focusing on GI tends to modify the GL from starch, whereas the GI from sugar-sweetened beverages is less amenable to change. Consequently, dietary advice to decrease the intake of added sugar, particularly as sugar-sweetened beverages, is likely to have had an important impact on GL.
This study adds to the scant literature on the habitual diet of obese pregnant women. The macronutrient profile at randomisation was similar to that of women in the general population, with fibre (non-starch polysaccharides) intake below, and total sugars and saturated fat above, recommended UK guidelines. 118 The overall energy intake and macronutrient profile accords with one previous report in obese pregnant women. 111 Because of the time required to rigorously assess diet using the 24-hour recall method, which, according to the process evaluation, is likely to have influenced recruitment and compliance, a short FFQ (5–10 minutes) was evaluated for use in the subsequent RCT.
The few studies that have attempted to measure changes in PA in intervention trials in pregnancy have generally relied on self-report, and results have been equivocal. 111,119–122 Accelerometry, the standard method of objective assessment used previously in observational studies in pregnancy,121 has, to our knowledge, been employed in only one relevant RCT, the FitFor2 study,123 a supervised exercise intervention in 121 overweight and obese women. Consistent with the Fitfor2 study, we found no effect of the intervention on PA using the ActiGraph accelerometer, concurring with the reported absence of change in barriers to PA. The failure of accelerometry to mirror the increase in self-reported walking in the intervention group could reflect insufficient intensity of this activity, but also reporting bias49 that is common in the reporting of low-intensity activities, such as those frequently undertaken by pregnant women. 44 As reported elsewhere, compliance with accelerometry in pregnancy was an issue. 52,124
Nonetheless, 60% of obese pregnant women providing baseline accelerometry data met the current guidelines for PA in pregnancy (i.e. > 30 minutes of MVPA per day). A similar level of activity has been observed in pregnant women (all BMIs)52 and overweight and obese non-pregnant adults,125 but not previously among obese pregnant women. Levels of PA were similar to those we found previously among overweight and obese women,49 but substantially higher than those reported for non-pregnant women in the UK. 126 There is no consensus on change of MVPA over pregnancy. 51,124,127,128 In this study of obese women, both groups reduced the level of objectively measured MVPA as pregnancy progressed.
This assessment has highlighted a critical need to evaluate PA behaviour objectively. We may otherwise have erroneously concluded in the following RCT that increased PA does not affect clinically relevant outcomes. Despite showing no increase in PA, we have not recommended that the RCT focuses on diet only,84 but rather that women continue to be encouraged to adhere to PA recommended in clinical guidelines.
Although there were no changes in attitudinal outcomes, women were generally positive about the recommended dietary and PA behaviours despite perceived barriers to change. Attitudinal data relating to diet were comparable to those obtained in a population sample of pregnant women. 3 The intervention did not achieve any reduction in perceived barriers but, despite this, important dietary changes were achieved, which may imply low levels of self-efficacy. However, barriers to increasing PA appeared too great to overcome, possibly reflecting increased physical discomfort with gestation, as indicated by the EQ-5D questionnaire.
The relationship between mental health, diet and PA in obese pregnancies warrants further investigation in the RCT in view of the high prevalence of depressive symptoms (EPDS score of > 12). Another report129 has also found no effect on these symptoms of a complex behavioural intervention in obese women.
In terms of context, the process evaluation recruited women in urban hospitals serving regions with areas of high socioeconomic deprivation. Obesity rates are higher among women with lower socioeconomic status and fewer qualifications130 and among particular ethnic groups, particularly black African and black Caribbean. 131 It was important therefore to explore not only if recruitment was feasible, but also whether or not the intervention was acceptable to the women recruited. Prominent media coverage about obesity raised the possibility that women in the control group might proactively address diet and PA, and some interview data supported this, but the evaluation suggested that awareness through the media alone was not adequate to achieve sufficient behavioural change.
In relation to reach, just over one-third of eligible women agreed to participate. Similarly low recruitment rates are consistent with other intervention studies, particularly in populations with lower uptake of health care. In one previous relevant study of lifestyle advice in non-obese pregnant women, recruitment was slower than expected and low attendance at group exercise sessions and participant concern about burdensome data collection contributed to dropout. 132 However, perceived advantages to participation such as extra clinical tests and continuity of care from research midwives supported study uptake and continuation. Given the continued rise in obesity in the adult population in England,130 approximately one in five pregnant women would be eligible for inclusion. Recruitment of the numbers needed to be approached (4100) for the full trial is therefore unlikely to be affected by a shortage of eligible women.
Overall, the wide social and ethnic diversity among participants was similar in participants and those who declined, indicating that the intervention would be unlikely to increase health inequalities by attracting more educated and higher-income participants. 133 Importantly, although obese pregnant women, once recruited, were generally willing to attend group sessions, practicalities often interfered with regular attendance, thereby influencing dose.
However, the sessions did not appeal to all women. Some appreciated finding common issues with other group members, whereas others preferred one-to-one contact. Evidence for health improvement interventions in group settings is varied93 and this study adds to the recognition that a ‘one size fits all’ approach may not be effective,133 and that flexibility is key to retention. Fidelity was good with consistently high-level provision of SMART goals by HTs, which were viewed as a positive achievement, particularly since poor adherence to goal-setting has been associated with moderate attendance among pregnant women. 134 The high acceptability of the participant handbook and pedometer reinforced the theoretical approach,94 and women also responded well to motivational techniques, but physical issues presented barriers to PA. The information provided was valued, including increasing awareness of safe PA in pregnancy, and seen to have important educational benefit. Several components of the intervention therefore appeared beneficial and were well received by women. The intervention is relatively intensive and presents costs for providers, and while a full assessment of cost and benefit was not conducted in this pilot, steps taken during the preclinical development phase (using HTs rather than clinicians to deliver the intervention, adopting local group-based approach) helped keep the overall costs of the intervention low, recognising that, if beneficial, it should also be affordable to health providers and to women. The rationale for this choice of HTs to deliver the intervention lay principally in the lower cost of employment compared with trained health professionals, and from our experience in working with Weight Concern, a charity which has pioneered self-help programmes and self-support groups for the treatment of obesity, and which was employed in this programme to train the HTs. All HTs were employed on the basis of having received some training in behaviour change techniques relevant to the delivery of the UPBEAT intervention. Another major advantage is that the expertise required is attainable within the participant materials and HT manuals. This was deliberate, so that it could be delivered by anyone who has received the appropriate training (as delivered as part of the RCT and pilot). Indeed, Weight Concern has previously used a peer-learning approach to good effect; importantly, this enables service providers to adapt the delivery (regarding who is delivering) according to their local population and skill set.
There was also suggestion from the process evaluation that the intervention may extend to peers and family, and some women aspired towards better fitness following birth. This study has reinforced earlier reports suggesting that rapport between study staff and participants, interviews requiring short time commitment and participants’ perception of the study as informative are all important recruitment and retention factors. 135 Formal evaluation of the reasons for the high refusal rate was not permissible because of ethical constraints, but the time commitment was frequently commented upon by the recruitment staff, as well as lack of appreciation of the health consequences of obesity in pregnancy.
In summary, this study has emphasised the value of a pilot trial to assess expected behaviour change. Although seldom attempted by others, we have also highlighted the importance of process evaluation in a complex intervention of diet and PA for pregnant women. The pilot trial demonstrated reductions in GL and in the proportion of energy derived from saturated fat are achievable in obese pregnant women without GDM. The process evaluation identified that dietary advice and education were well-received, and confirmed that PA change is more problematic to achieve, although it remains important to consistently measure and support PA using technologies acceptable to women. The process evaluation also helped explain issues arising in relation to uptake, dose, fidelity and retention which informed the feasibility of the full trial.
As a consequence of this study, several modifications to improve compliance and fidelity have now been implemented in the protocol for the main trial. As well as process evaluation, HT feedback highlighted potential barriers to fidelity of the intervention and informed protocol modifications for the RCT. Flexibility has been increased regarding the timing and delivery of the sessions, and goal-setting can be undertaken by telephone or e-mail. It is recommended that women should receive at least five out of eight sessions. The two extra visits required for objective assessment of PA and accurate evaluation of diet have been omitted in all but two sites (as planned) and dietary assessment reduced to a validated FFQ. The RPAQ includes domestic and childcare activities considered appropriate for pregnant women,136 but following feedback has been replaced by the shorter and more relevant International Physical Activity Questionnaire (IPAQ). 137 Maintenance of dietary and PA behaviour change in the participant and her family is being formally evaluated at 6 months and at 3 years post partum. Although there was no significant influence of the intervention on objectively measured PA, and only a modest increase in self-reported MVPA, it was decided to continue to recommend PA, as this aligns with best practice as set out in clinical guidelines. To minimise loss to follow-up and attendance at these appointments, strategies that have been put in place include regular newsletters and sending greetings cards on special occasions such as the child’s birthday.
Conclusions
Assumptions should not be made that interventions in obese pregnant women necessarily change behaviour. We recommend that a pilot trial such as that described here, which has demonstrated evidence for expected change in behaviour, is a necessary prelude to any RCT of a complex intervention of diet and PA designed to improve pregnancy outcome in obese women. Without prior evidence for change in behaviour in the expected direction, pursuit of a large and costly trial would be futile. Similarly, we have demonstrated the value of early process evaluation, which can lead to important refinements in protocol to improve feasibility and compliance in the definitive trial.
Implications for proceeding to phase 3, the randomised controlled trial
As detailed in the discussion and conclusion section of the above report, there were many benefits gained from undertaking the pilot trial which led to us to change the delivery of the intervention, but not any of the principal elements of the intervention. Feasibility and general acceptability were established and, most importantly, the intervention led to changed dietary behaviours. PA behaviours that, as we found in the process evaluation, were less amenable to the women proved difficult to change, but the intervention was continued as PA is recommended nationally for all pregnant women. The study also enabled us to better define the power calculations for the main trial, based on the incidence of GDM in the women in the pilot study (see Chapter 4). The process evaluation provided some similar outcomes as structured interviews in phase 1, but several different outcomes, probably because the women in the pilot were more representative of the population to be recruited for the intervention. Importantly, it was recognised that recruitment rates would be lower than expected when the original application for funding was submitted, and that full recruitment to the main UPBEAT intervention would not be feasible within the time frame allotted. Following the pilot trial, an application for an extension to the trial funding was therefore submitted to the National Institute for Health Research (NIHR), and additional funding was provided to allow completion of recruitment.
Chapter 3 Pilot trial: additional analyses
The pilot trial provided the opportunity for additional analyses. In this chapter, two studies are reported which addressed hypotheses related to pregnancy outcomes in women with obesity. The first addressed the question of whether or not early second-trimester pregnancy measurements of clinical and biochemical markers could contribute to the development of a tool for early pregnancy risk assessment of GDM later in pregnancy, with the potential for targeted intervention. The second was a review of the relationships between PA and insulin sensitivity that also explored relationships between maternal PA and maternal and neonatal outcomes. Both studies used samples and data provided by the pilot trial.
Prediction of gestational diabetes mellitus in obese pregnant women from the UK Pregnancies Better Eating and Activity trial pilot trial
This study has been published in a shorter version as Maitland RA, Seed PT, Briley AL, Homsy M, Thomas S, Pasupathy D, Robson SC, Nelson SM, Sattar N, Poston L on behalf of the UPBEAT trial consortium. Prediction of gestational diabetes in obese pregnant women from the UK Pregnancies Better Eating and Activity (UPBEAT) pilot trial. Diabet Med 2014;31:963–70. © 2014 The Authors. Diabetic Medicine © 2014 Diabetes UK. Reproduced with permission. 138
Abstract
Aim: The aim was to examine the prediction of GDM in obese women using routine clinical measures and measurement of biomarkers related to insulin resistance in the early second trimester.
Methods: A total of 117 obese pregnant women participating in a pilot trial of a complex intervention of dietary advice and PA were studied. Blood samples were obtained at recruitment (15+0–17+6 weeks’ gestation) and demographic data, clinical history and anthropometric measures recorded. The biomarkers analysed were plasma lipids [high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides], high-sensitivity C-reactive protein, alanine transaminase, aspartate transaminase, ferritin, fructosamine, insulin, adiponectin, tissue plasminogen activator, interleukin 6, visfatin and leptin. Univariate and logistic regression analyses were performed to determine independent predictors and the area under the receiver operating curve was calculated for the model.
Results: Of the 106 participants included in the analysis, 29 (27.4%) developed GDM. Participants with GDM were older (p = 0.002), more often of a parity ≥ 2, had higher systolic (p = 0.02) and diastolic blood pressure (p = 0.02), and were more likely to be black (p = 0.009). Among the blood biomarkers measured, plasma adiponectin alone remained independently associated with GDM in adjusted models (p = 0.002). The area under the receiver operating curve for clinical factors alone (0.760) increased significantly [area under the curve = 0.834, χ2(1) = 4.00; p = 0.046] with the addition of adiponectin.
Conclusions: A combination of routinely measured clinical factors and adiponectin measured in the early second trimester in obese pregnant women may provide a useful approach to the prediction of GDM. Validation in a large prospective study is required to determine the usefulness of this algorithm in clinical practice (clinical trial registry number ISRCTN89971375).
Introduction
The prevalence of obesity in adults and children continues to rise. Obesity remains the sixth most important determinant of adverse health and reduced adult life expectancy globally. 139 In the UK, the incidence of obesity in women of reproductive age has almost doubled in the past 20 years;140 the most recent World Health Organization (WHO) Global InfoBase of obesity (BMI of ≥ 30 kg/m2) in UK women aged > 15 years (2010) reports an age-adjusted prevalence of obesity of 26.3% across all ethnic groups. 38
Maternal obesity carries a significant risk of adverse pregnancy outcomes, particularly GDM. Short- and long-term metabolic complications follow a continuous linear relationship with BMI,141 with the risk of developing GDM rising from two- to eightfold with increasing BMI category. 142 Not all obese women develop GDM, but this heterogeneity poses a burden on limited resources, with all women with a BMI of ≥ 30 kg/m2 currently managed as if at risk, often resulting in suboptimal management. Accurate and early identification of obese pregnant women who will subsequently develop GDM would enable early risk stratification, more appropriate use of health-care resources and the targeting of intervention strategies.
Currently, NICE, in the UK, recommends selected rather than universal GDM screening, according to risk factors that include obesity. Women who have previously delivered a macrosomic infant, who have had previous GDM or who have a first-degree relative with diabetes mellitus and high-risk ethnicity are also screened. A systematic review of screening for GDM undertaken for a health technology assessment reported low sensitivities (50–69%) and specificities (58–68%; eight studies) when traditional methods of risk factor screening were used. 143 Although there is at present no accepted early pregnancy intervention to improve clinical outcome in obese pregnant women,84 increased recognition of the problem has led to an international research effort to develop effective interventions. Several large-scale RCTs, including UPBEAT (registered as ISRCTN89971375), are investigating targeted dietary and PA interventions or metformin to improve glucose homeostasis and pregnancy outcome in overweight and obese women. 15,144
Research into the prediction of adverse outcomes in other pregnancy-related conditions, such as pre-eclampsia, has shown that a combination of clinical history and early pregnancy clinical measures, together with the addition of biomarkers measured in biological samples, may provide an effective strategy in early pregnancy risk assessment. 145 Several studies have adopted this approach to the prediction of GDM,146,147 but, to our knowledge, not previously in a population of obese women.
The aim of the present study was to undertake a preliminary investigation in obese pregnant women to determine whether or not the addition of biomarkers to routine clinical measurements further improves the prediction of GDM. For this purpose, we studied 117 women participating in a pilot trial for UPBEAT, and measured 16 biomarkers frequently implicated in the pathogenesis and prediction of GDM and/or type 2 diabetes mellitus and reflecting inflammatory pathways, markers of adipose tissue function, hepatic fat accumulation and vascular dysfunction. 146,148,149 As abnormal fatty acids (FAs) are associated with insulin resistance and fetal macrosomia and because significant differences were found in the dietary intake of SFAs and the ratio of polyunsaturated fatty acid to SFA, a detailed analysis of FAs was also undertaken. 150 Prediction models were then developed incorporating clinical and biomarker data.
Methods
UPBEAT is a multicentre RCT of a complex dietary and PA intervention aimed at improving glucose homeostasis in obese pregnant women. A pilot trial was undertaken in 183 women in four UK hospitals to evaluate changes in dietary and PA behaviours, trial all aspects of the protocol and undertake process evaluation. 150 Details of the intervention and protocol are available on the trial website (www.medscinet.net/upbeat/about.aspx).
NHS Research Ethics Committee approval was obtained by all contributing centres (UK IRAS reference number 09/H0802/5).
At recruitment (15+0–17+6 weeks’ gestation) and after informed consent had been obtained, information was collected on demographics, maternal history, maternal family and current pregnancy health. Randomisation to the intervention arm or the control arm (which consisted of standard antenatal care) was carried out at the second appointment, approximately 1 week later (7–10 days), between 16+0 and 18+6 weeks’ gestation, by a secure internet-based data management system (MedSciNet Ltd, www.medscinet.net/upbeat). The randomisation schedule was minimised according to ethnicity, parity (0 vs. ≥ 1), age and BMI (30–34.9 kg/m2 vs. 35–39.9 kg/m2 and > 40 kg/m2). Blood pressure was recorded using the Micro-life® BP3BT0-A automated blood pressure monitor (Micro-life, Widnau, Switzerland), which is validated for use in pregnancy. Maternal skinfold thickness (triceps, biceps, subscapular and suprailiac) was measured in triplicate with Harpenden skinfold callipers (validated for values ≤ 80 mm; Holtain Ltd, Felin-y-Gigfran, Crosswell, UK), in addition to the following circumferences: waist, mid-arm, thigh and hip. Skinfold thicknesses at four sites (triceps, biceps, suprailiac and subscapular) were summed. Blood samples were obtained from 117 participants in the three centres that had facilities for sample handling and storage. Serum and plasma was stored at –80 °C for future analysis.
At 28 weeks’ gestation, an OGTT was performed on all participants. Diagnosis of GDM following a 75-g 2-hour OGTT at 27+0–28+6 weeks was defined in accordance with the IADPSG’s criteria (fasting blood glucose concentration of ≥ 5.1 mmol/l or 1-hour glucose of ≥ 10.0 mmol/l or 2-hour glucose of ≥ 8.5 mmol/l). 6 If a diagnosis of GDM was made, women were referred for routine GDM care in accordance with local criteria.
Biochemical analyses
Plasma total cholesterol, HDL cholesterol triglycerides, alanine transaminase, aspartate transaminase, high-sensitivity C-reactive protein, fructosamine (c311, Roche Diagnostics, Burgess Hill, UK) and ferritin (Elecsys 2010, Roche Diagnostics, Burgess Hill, UK) were measured on clinically validated automated platforms using the manufacturers’ quality controls (QCs) and calibration materials. The coefficient of variation (CV) was < 6%. Plasma insulin was measured with an enzyme-linked immunosorbent assay (Mercodia, Uppsala, Sweden) that does not cross-react with proinsulin and the interassay’s CV was < 7%. Baseline plasma adiponectin, interleukin 6, leptin (R&D Systems, Abingdon, UK), tissue plasminogen activator (Stago, Theale, UK) and visfatin (Phoenix Peptide, Karlsruhe, Germany) were measured by enzyme-linked immunosorbent assay. These methods had an interassay CV of < 10%.
Plasma non-esterified fatty acids (NEFAs) and FAs were measured on samples obtained after fasting for 12 hours at 27+0–28+6 weeks’ gestation in the Division of Nutritional Sciences, King’s College London, London, UK.
Non-esterified fatty acids were measured on a clinically validated automated platform (Clinical Analyser ILab 650, Instrumentation Laboratories, Warrington, UK) using the Randox (FA115, Randox Laboratories, Montpellier, France) kit. QC was performed after each 60-sample batch at the upper and lower range of the assay, with the following CVs: QC1 target of 1.24 mmol/l (%CV = 0.95) and QC2 target of 0.58 mmol/l (%CV = 0.97). Plasma FAs were measured by gas–liquid chromatography. 151 Esterified and non-esterified fatty acid methyl esters were analysed using the one-step transesterification direct method. 152 Main FA peaks [C16:0, C18:0, C18:1(n-9), C18:2(n-6), C18:3(n-3), and C20:4(n-6)] were recognised by referring to standard retention times (Sigma-Aldrich Co. Ltd, Gillingham, UK). C20:5(n-3) and C22:6(n-3) were determined by cod liver oil fatty acid methyl esters standards (Sigma-Aldrich Co. Ltd, Gillingham, UK). The remaining FAs were measured by gas–liquid chromatography mass spectrophotometry. Each plasma FA concentration was determined as the area under the peak matched with the known standard. 153
All analyses were performed on previously unthawed ethylenediaminetetraacetic acid and serum samples. Samples were processed by technicians blinded to the identity of the samples.
Statistical methods
The analysis was exploratory with the aim of identifying potentially useful combinations of clinical and biochemical predictors154 of maternal GDM; therefore, potentially useful biomarkers were not excluded and no adjustment was made for multiple testing. Standard distributional checks (Box–Cox regression and normal distribution plots) were carried out, and separate decisions made on the appropriate transformation. Based on these findings, log-transformation was carried out for all biochemical variables. Differences between patient groups are reported as geometric means and ratios of geometric means, with 95% CIs.
The association of clinical indicators with GDM was established using linear or logistic regression as appropriate, with robust standard errors. Biochemical indicators were assessed as predictors of GDM, adjusting for significant clinical indicators. After univariate analysis, those variables which were identified as independent predictors of GDM were included in the model. The overall performance of the markers as predictors of GDM was assessed by comparison of receiver operating curve areas. Areas under receiver operator characteristic curves are compared using a non-parametric approach suggested by DeLong et al. 154 and implemented in Stata as the commands roccomp and rocgold. When necessary, composite predictors were derived using multiple logistic regression.
All data analysis was carried out using the statistical package Stata version 11.2.
Results
A total of 11 women were omitted from the analysis because of inadequate OGTT data. Of the remaining 106 women (53 in the control group and 53 in the intervention group), 29 were diagnosed with GDM (27.4%). The demographic and clinical characteristics of women who developed GDM compared with those who did not are summarised in Table 14. In general, women with GDM were older, more often of higher parity (≥ 2), had higher systolic and diastolic blood pressure and were more likely to be black than those without. BMI was not significantly different between the two groups, although skinfold thicknesses were greater in women who developed GDM; women who developed GDM had greater triceps thickness (37 vs. 31 mm; p = 0.004) and total sum of skinfold thicknesses (93 vs. 86 mm; p = 0.03). There was no evidence of interaction in terms of prediction of GDM by treatment group (p = 0.85).
| Maternal characteristic | GDM status | Comparison (95% CI) | p-value | |
|---|---|---|---|---|
| GDM (as measured via IADPSG’s guidelines) (n = 29) | No GDM (n = 77) | |||
| Age categories (years), median (interquartile range) | 34 (31–36) | 31 (26–34) | –3 (–5 to –1) | |
| 18–25, n (%) | 2 (6.9) | 17 (22.1) | – | 0.004 |
| 26–30, n (%) | 4 (13.8) | 20 (26.0) | 1.7 (0.3 to 10.5) | – |
| 31–40, n (%) | 10 (34.5) | 26 (33.8) | 3.3 (0.6 to 16.8) | – |
| 35+, n (%) | 13 (44.8) | 14 (18.2) | 7.9 (1.5 to 41.0) | – |
| Height (m), mean (SD) | 1.65 (0.08) | 1.65 (0.07) | 0.00 (–0.03 to 0.03) | 0.94 |
| Weight (kg), mean (SD) | 95.79 (12.38) | 97.98 (15.56) | –2.19 (–7.93 to 3.54) | 0.45 |
| BMI (kg/m2), mean (SD) | 35.27 (3.60) | 36.11 (4.95) | –0.84 (–2.57 to 0.89) | 0.34 |
| Circumferences (cm), mean (SD) | ||||
| Waist | 107.8 (7.4) | 107.6 (10.8) | 0.3 (–3.4 to 3.9) | 0.88 |
| Mid-arm | 37.8 (4.1) | 37.2 (4.0) | 0.6 (–1.1 to 2.4) | 0.48 |
| Hip | 120.5 (9.2) | 122.9 (11.8) | –2.4 (–6.7 to 1.9) | 0.27 |
| Thigh | 66.4 (9.0) | 69.4 (7.7) | –3.0 (–6.7 to 0.8) | 0.12 |
| Skinfolds (mm), mean (SD) | ||||
| Triceps | 37.4 (10.2) | 31.4 (7.4) | 6.0 (2.0 to 10.1) | 0.004 |
| Biceps | 28.0 (9.5) | 24.4 (7.5) | 3.6 (–0.3 to 7.5) | 0.07 |
| Subscapular | 36.0 (8.2) | 32.2 (9.2) | 3.7 (0.1 to 7.4) | 0.04 |
| Suprailiac | 29.9 (8.3) | 29.7 (8.3) | 0.2 (–3.4 to 3.7) | 0.92 |
| Total | 93.9 (16.5) | 86.1 (16.7) | 7.8 (0.7 to 14.9) | 0.03 |
| SBP (mmHg), mean (SD) | 123.3 (7.9) | 119.0 (8.7) | 4.3 (0.8 to 7.8) | 0.02 |
| DBP (mmHg), mean (SD) | 76.4 (7.5) | 72.5 (6.7) | 3.9 (0.8 to 7.0) | 0.02 |
| Ethnicity, n (%) | ||||
| White | 11 (37.9) | 53 (68.8) | – | – |
| Black | 16 (55.2) | 21 (27.3) | 3.3 (1.4 to 8.0) | 0.009 |
| Asian | 0 (0.0) | 1 (1.3) | – | 0.99 |
| Other | 2 (6.9) | 2 (2.6) | 2.8 (0.4 to 20.7) | 0.32 |
| Parity, n (%) | 0.03 | |||
| 0 | 9 (31) | 37 (48.1) | – | – |
| 1 | 10 (34.5) | 31 (40.3) | 1.33 (0.48 to 3.67) | – |
| ≥ 2 | 10 (34.5) | 9 (11.7) | 4.57 (1.43 to 14.55) | – |
| Previous GDM, n/N (%) | 1/29 (3.4) | 1/77 (1.3) | 2.71 (0.16 to 44.88) | 0.49 |
| Smoking, n (%) | ||||
| Never | 8/29 (27.6) | 33/77 (42.9) | 0.51 (0.20 to 1.29) | 0.15 |
| Current | 2/29 (6.9) | 5/77 (6.5) | 1.07 (0.20 to 5.83) | 0.94 |
| Number of cigarettes (< 8 weeks), n (%) | ||||
| 0 | 27 (93.1) | 66 (85.7) | – | – |
| 1–5 per day | 2 (6.9) | 2 (2.6) | – | – |
| 6–10 per day | 0 (0.0) | 5 (6.5) | – | – |
| 11–20 per day | 0 (0.0) | 4 (5.2) | – | – |
Table 15 shows the first trimester biomarkers for women who subsequently developed GDM and those who did not. Women with GDM had 34% lower plasma concentrations of adiponectin (95% CI –47% to –19%), adjusting for clinical predictors of age, parity ≥ 2, diastolic blood pressure and systolic blood pressure. There was a trend towards significance for fructosamine in the GDM group (p = 0.05), which attenuated to the null after adjustment (p = 0.82) (see Table 15).
| Biomarker | GDM status | Comparison (95% CI) | p-value | |
|---|---|---|---|---|
| GDMa (n = 29) | No GDMa (n = 77) | |||
| Fructosamine (µmol/l) | 200.87b (1.10) | 192.90 (1.09) | 1.00 (0.97 to 1.04) | 0.82 |
| ALT (U/l) | 21.41b (1.79) | 19.00 (1.57) | 1.12 (0.84 to 1.50) | 0.42 |
| AST (U/l) | 30.63b (1.53) | 25.07 (1.41) | 1.17 (0.96 to 1.43) | 0.11 |
| Ferritin (ng/ml) | 42.06b (2.27) | 39.48 (2.29) | 0.95 (0.64 to 1.41) | 0.79 |
| Adiponectin (µg/ml) | 4.97b (1.72) | 7.34 (1.76) | 0.66 (0.53 to 0.81) | < 0.001 |
| tPA (ng/ml) | 10.35b (1.49) | 9.00 (1.47) | 1.05 (0.86 to 1.28) | 0.64 |
| Interleukin 6 (pg/ml) | 1.01c (2.08) | 0.95d (2.54) | 0.91 (0.66 to 1.24) | 0.55 |
| Leptin (pg/ml) | 53.82b (1.49) | 59.36e (1.52) | 0.92 (0.76 to 1.13) | 0.44 |
| Visfatin (ng/ml) | 4.94b (1.40) | 5.28e (1.42) | 0.93 (0.77 to 1.12) | 0.42 |
| Insulin (mU/l) | 26.00 (2.99) | 20.20 (2.78) | 1.33 (0.80 to 2.21) | 0.27 |
| Cholesterol (mmol/l) | 5.31 (1.18) | 5.42 (1.21) | 1.01 (0.93 to 1.10) | 0.80 |
| Triglycerides (mmol/l) | 1.67 (1.42) | 1.53 (1.38) | 1.13 (0.96 to 1.32) | 0.13 |
| HDL (mmol/l) | 1.64 (1.32) | 1.71 (1.26) | 0.94 (0.82 to 1.08) | 0.39 |
| CRP (mg/l) | 9.18 (1.93) | 7.77 (2.30) | 1.28 (0.89 to 1.83) | 0.18 |
| VLDL (mmol/l) | 0.76 (1.42) | 0.71 (1.38) | 1.13 (0.97 to 1.32) | 0.12 |
| LDL (mmol/l) | 2.74 (1.39) | 2.93 (1.34) | 0.99 (0.86 to 1.14) | 0.86 |
| Cholesterol : HDL | 3.23 (1.31) | 3.17 (1.27) | 1.07 (0.95 to 1.21) | 0.27 |
| LDL : HDL | 1.67 (1.56) | 1.71 (1.45) | 1.05 (0.87 to 1.27) | 0.63 |
In a combined logistic regression model, including the biomarkers and clinical risk factors, the only consistent predictive variables were adiponectin (OR for a halving in adiponectin concentration 4.04, 95% CI 1.69 to 9.64; p = 0.002) and maternal age (OR per additional year 1.18, 95% CI 1.04 to 1.34; p = 0.01; Table 16). An area under the receiver operating curve of 0.760 (95% CI 0.645 to 0.875) for prediction of GDM was achieved with clinical predictors (age, parity, ethnicity and blood pressure) alone. The area under the receiver operating curve increased significantly to 0.834 [95% CI 0.742 to 0.927, χ2(1) = 4.00; p = 0.046] with the addition of adiponectin (Figure 5).
| Variable | OR | 95% CI | p-value |
|---|---|---|---|
| Log-adiponectin | 0.13 | 0.04 to 0.47 | 0.002 |
| Age (for each additional year) | 1.18 | 1.04 to 1.34 | 0.01 |
| Parity ≥ 2 | 2.09 | 0.50 to 8.73 | 0.31 |
| Black ethnicity | 1.35 | 0.42 to 4.33 | 0.62 |
| SBP | 1.04 | 0.95 to 1.13 | 0.41 |
| DBP | 1.08 | 0.98 to 1.19 | 0.15 |
FIGURE 5.
Receiver operating curve and summaries using the basic model (including age, parity, ethnicity, blood pressure), with the addition of adiponectin. AUC, area under the receiver operating curve.
Comparison of non-esterified fatty acids and fatty acid composition by gestational diabetes mellitus status
The plasma concentration of NEFAs was significantly higher in women with GDM (p = 0.037) and, although not significant, concentrations of the polyunsaturated fatty acid eicosapentaenoic acid (plasma 20:5n-3), an omega-3 fatty acid, were 25% higher in women with GDM (p = 0.28). Women without GDM had greater concentrations of the naturally occurring trans-vaccenic acid from the omega 7 group (C18:1n-7) (p = 0.01) and dihomo-γ-linolenic acid (C20:3n-6) (p = 0.016) (Table 17). Although these two FAs showed significant differences, they were not considered in the logistic regression mode for the development of a predictive algorithm, as it was considered that it would be impracticable to measure these FAs in a routine clinical setting.
| NEFA and FA composition (mmol/l) | GDM status | Comparison: difference in arithmetic means (95% CI) | p-value | |
|---|---|---|---|---|
| No GDMa (n = 75) | GDMa (n = 29) | |||
| NEFA | 0.30 (0.16) | 0.38 (0.19) | 0.08 (0.01 to 0.16) | 0.037 |
| Plasma 14:0 | 0.85 (0.26) | 0.87 (0.41) | 0.02 (–0.14 to 0.19) | 0.77 |
| Plasma 16:0 | 22.81 (1.69) | 23.66 (2.34) | 0.85 (–0.09 to 1.79) | 0.08 |
| Plasma 16:1 | 1.82 (0.66) | 1.72 (0.92) | –0.10 (–0.47 to 0.27) | 0.593 |
| Plasma 18:0 | 5.40 (0.46) | 5.50 (0.62) | 0.09 (–0.16 to 0.34) | 0.46 |
| Plasma 18:1n-9 | 22.59 (2.75) | 22.49 (2.79) | –0.10 (–1.30 to 1.10) | 0.87 |
| Plasma 18:1n-7 | 1.65 (0.25) | 1.52 (0.21) | –0.13 (–0.23 to –0.03) | 0.01 |
| Plasma 18:2n-6 | 26.22 (3.23) | 25.89 (4.62) | –0.33 (–2.18 to 1.51) | 0.72 |
| Plasma 18:3n-3 | 0.73 (0.20) | 0.65 (0.23) | –0.09 (–0.18 to 0.01) | 0.07 |
| Plasma 18:3n-6 | 0.23 (0.08) | 0.23 (0.07) | –0.01 (–0.04 to 0.02) | 0.64 |
| Plasma 20:3n-6 | 1.80 (0.41) | 1.61 (0.33) | –0.19 (–0.34 to –0.04) | 0.016 |
| Plasma 20:4n-6 | 6.66 (1.33) | 6.56 (1.11) | –0.10 (–0.61 to 0.41) | 0.69 |
| Plasma 20:5n-3 | 0.62 (0.73) | 0.77 (0.61) | 0.15 (–0.13 to 0.43) | 0.28 |
| Plasma 22:4n-6 | 0.24 (0.07) | 0.23 (0.06) | –0.00 (–0.03 to 0.02) | 0.94 |
| Plasma 22:5n-6 | 0.63 (0.19) | 0.63 (0.17) | –0.00 (–0.08 to 0.07) | 0.95 |
| Plasma 22:5n-3 | 0.36 (0.11) | 0.37 (0.08) | 0.01 (–0.03 to 0.04) | 0.77 |
| Plasma 22:6n-3 | 2.84 (0.77) | 3.00 (0.81) | 0.16 (–0.18 to 0.51) | 0.36 |
| Other plasma FAs | 4.54 (0.56) | 4.30 (0.71) | –0.24 (–0.53 to 0.05) | 0.10 |
Further sensitivity analysis was conducted with the addition of maternal anthropometry increasing the area under the receiver operating curve for clinical predictors alone to 0.796 [95% CI 0.692 to 0.898 (Table 18)]; however, in the fully adjusted model, only a low concentration of adiponectin remained independently predictive of GDM.
| Variable | OR | 95% CI | p-value |
|---|---|---|---|
| Log-adiponectin | 0.18 | 0.05 to 0.67 | 0.01 |
| Age (for each additional year) | 1.15 | 1.01 to 1.31 | 0.04 |
| Parity ≥ 2 | 3.38 | 0.75 to 15.24 | 0.11 |
| Black ethnicity | 0.80 | 0.21 to 3.05 | 0.74 |
| SBP | 1.0 | 0.91 to 1.11 | 0.93 |
| DBP | 1.09 | 0.98 to 1.21 | 0.10 |
| Triceps skinfold | 1.07 | 0.98 to 1.17 | 0.12 |
| Total skinfold | 1.01 | 0.96 to 1.05 | 0.82 |
Discussion
The present study highlights novel biochemical and clinical factors for the prediction of GDM in obese pregnant women and suggests that an algorithm based on simple clinical variables plus adiponectin concentration may provide a clinically useful method for the prediction of GDM in this population.
Four previous studies have identified a number of patient characteristics and biomarkers associated with the prediction of GDM. 146,149,155,156 These have been undertaken in populations of mixed risk, including non-white ethnicity,146,155,156 family history of diabetes mellitus,146,149,155,156 previous history of GDM,146,155,156 high pre-pregnancy BMI,146,149,156 older maternal age146,155,156 and differing parity. 149 Savvidou et al. 149 measured nine biomarkers in the first trimester and found that high concentrations of tissue plasminogen activator and low concentrations of HDLs increased the area under the receiver operating curve from 0.824 with clinical risk factors alone to 0.861 in a group of all comers, regardless of baseline BMI. The addition of adiponectin to prediction models for GDM has consistently increased the area under the receiver operating curve to values above those achieved with clinical measures alone. Further inclusion of adipokines and biomarkers has frequently demonstrated a modest, non-significant, increase in the area under the receiver operating curve. For example, in a case–control study of 400 women, those with GDM were reported to have higher levels of maternal serum visfatin and lower serum adiponectin concentrations at 11–13 weeks of gestation. The addition of adiponectin to the prediction model using clinical measures alone resulted in a significant change in the area under the receiver operating curve, whereas there was a non-significant increase after addition of visfatin [an area under the receiver operating curve of 0.828 (maternal characteristics alone), 0.854 (adiponectin) and 0.855 (adiponectin and visfatin)]. 146 Nanda et al. 156 measured three biomarkers and found that in the GDM group, compared with controls, adiponectin and sex hormone-binding globulin levels were lower. When screening for GDM by maternal characteristics alone, the detection rate was 61.6% (false-positive rate 20%), increasing to 74.1% with the addition of adiponectin and sex hormone-binding globulin. Alternative approaches to GDM risk assessment have included the measurement of biomarkers in the preconception period, with a recent report finding that maternal characteristics, fasting plasma glucose, glycosuria and preconception dyslipidaemia yielded an area under the receiver operating curve of 0.90 for the prediction of GDM;155 however, the varied diagnostic criteria for GDM used in previous studies limit comparisons with previous attempts to predict GDM. Importantly, no study has specifically addressed risk assessment in obese pregnant women, which has important implications for clinical practice given the recognition of obesity as the major risk factor for GDM, and the likelihood that the biomarker profile may be dissimilar to other risk groups in women with a high BMI.
The present results suggest that clinically useful prediction of GDM in obese pregnant women is achievable using a combination of clinical characteristics (older age, higher systolic and diastolic blood pressure, parity ≥ 2 and black ethnicity) combined with the plasma concentration of adiponectin. To reflect current clinical practice, routine clinical measurements recorded at antenatal visits were included. The inclusion of detailed maternal anthropometry (including skinfold thicknesses), which is undertaken in all women participating in UPBEAT, suggested a limited potential role for taking such measurements routinely as an aid to GDM prediction (see Table 18).
Adiponectin, an adipocyte-derived adipokine, is now recognised as being strongly associated with improved glucose metabolism and increasing insulin sensitivity, although the causality of this relationship remains debated. Irrespective of causal direction, adiponectin appears to provide a good ‘read-out’ of whole-body insulin sensitivity. In a recent meta-analysis of non-pregnant individuals, adiponectin was shown to be strongly predictive of type 2 diabetes mellitus, and inversely related to measures of insulin resistance and BMI. 157
The role of adiponectin in obese pregnant women may extend beyond usefulness as a biomarker. In the Hyperglycemia and Adverse Pregnancy Outcome study,158 serum concentrations of adiponectin decreased as glucose and maternal BMI increased and adiponectin was inversely associated with birthweight, neonatal skinfold thickness and total body fat (estimated using anthropometry), giving rise to the hypothesis that this cytokine may play a role in fetal growth regulation by modulation of placental nutrient transport in addition to maternal glucose homeostasis. Data in support of a placental origin of adiponectin remain equivocal, with evidence favouring maternal origin of adiponectin measured in the blood of pregnant women. 159 Maternal adiponectin has, therefore, the potential to be a ‘functional’ target for interventions in obese pregnant women whereby achievement of increased plasma concentrations could parallel a reduced risk of macrosomia. This may be a realistic target, as adiponectin has been shown to be modifiable by dietary intervention in non-pregnant populations. 160 Lifestyle interventions in pregnant women of differing pre-pregnancy BMI categories have been equivocal with regard to the effects on glucose metabolism and insulin resistance, although none has measured adiponectin concentration. 123,161 Following the completion of UPBEAT (1546 women), the influence of the intervention on plasma adiponectin concentration will therefore be explored.
To the best of our knowledge, there have been no previous studies of adiponectin concentration and GDM in an exclusively obese population, but the findings are consistent with other reports in women of all BMI categories with established disease or prior to the development of GDM. 156,162 A recent case–control study from Brazil of 79 and 129 women of mixed ethnicity with and without GDM, respectively, reported that GDM was associated with significantly lower serum concentrations of adiponectin in the third trimester (28–36 weeks) than in the control group (p = 0.0015). GDM and BMI both had an independent association with adiponectin concentration, with no significant interaction between the two factors (GDM, p = 0.04; BMI, p = 0.01; and interaction, p = 0.76; using a two-way analysis of variance test). 163 In contrast, although adiponectin concentration was significantly lower in women who developed GDM in our previous study in women of mixed risk, it did not contribute to the final model, which combined two factors (HDL cholesterol and tissue plasminogen activator antigen), both recognised to be related to adiponectin via linked hepatic/circulating triglyceride-mediated pathways. 148
Low serum adiponectin concentrations appear to be associated with ethnic groups known to have a higher risk of developing incident type 2 diabetes mellitus later in life. 164 In the present study, women of black ethnic origin had significantly lower plasma levels of adiponectin than non-black women, consistent with findings from previous work examining pregnant women of South Asian origin. 162 Although we acknowledge our diverse ethnic population is not representative of the UK population, having a large proportion of women of black ethnic origin (> 80%) has yielded further insights into the relationship between adiponectin concentration and ethnicity.
We also observed that adiponectin concentration was significantly related to current smoking status, a finding previously reported in a non-pregnant population in which the plasma adiponectin concentration increased in a stepwise fashion with never, past and current smokers. 165
The present study has some limitations. The sample size was small and the data obtained should be considered as a training data set for later validation in UPBEAT. Furthermore, fasting blood samples were not obtained at randomisation (15+0–17+6 weeks’ gestation), precluding the measurement of the fasting glucose or insulin concentration and assessment of HOMA2-IR (homeostatic model assessment of insulin resistance); however, as fasting is not mandatory for antenatal clinic visits, the present study was designed pragmatically, to be relevant to current clinical practice in obese women. In this high-risk group, it is possible that some women with GDM may have had undiagnosed type 2 diabetes mellitus; initial screening with fasting blood glucose or glycated haemoglobin (HbA1c) concentrations was not undertaken as IADPSG recommendations for universal testing for overt diabetes mellitus at the first antenatal visit have not been implemented. It is possible, therefore, that low early pregnancy adiponectin concentrations may have been influenced by type 2 diabetes mellitus in a small minority of women. Nevertheless, others have shown that low adiponectin concentrations are strongly predictive of GDM in women in whom type 2 diabetes mellitus was excluded antenatally. 166 The algorithm developed in the present study was based on the diagnosis of GDM by IADPSG’s criteria; it follows that it is potentially valid only for this method of diagnosis. As IADPSG’s guidelines are increasingly being adopted, for example by WHO, it would be appropriate to evaluate in studies of larger sample size their predictive potential for GDM diagnosis by other commonly used criteria.
In summary, we have demonstrated that the risk of developing GDM in obese pregnant women may be predicted in the early second trimester of pregnancy by using an algorithm which incorporates routine clinical variables as well as the biochemical marker, adiponectin. Our findings extend the work of previous studies and, collectively, the findings suggest that by additionally measuring adiponectin concentrations in women at high risk before routine clinical diagnosis of GDM, a potential therapeutic window for intervention could be created. As GDM is associated with a greater risk of incident type 2 diabetes mellitus and 10-year cardiovascular risk in mothers,167 as well as with maternal and neonatal pregnancy complications, successful intervention has the potential to improve both short- and long-term outcomes. We conclude that further large-scale studies of GDM prediction in obese pregnant women are warranted.
Relationships between Physical Activity and Pregnancy Outcomes in Obese Pregnant women from the UK Pregnancies Better Eating and Activity trial pilot trial
This is a shortened version of a longer review article published as Hayes L, Bell R, Robson S, Poston L on behalf of the UPBEAT Consortium. Association between Physical Activity in Obese Pregnant Women and Pregnancy Outcomes: The UPBEAT Pilot Study. Ann Nutr Metab 2014;64:239–46. 168 Reproduced by kind permission of S. Karger AG, Basel, Switzerland.
Abstract
Background: Obesity in pregnancy is associated with fetal macrosomia, a raised neonatal fat mass and an increased risk of obesity and poor metabolic health in childhood that persists into adulthood. The offspring of obese women are more likely to be obese than the offspring of lean women when they become pregnant themselves, perpetuating a cycle of obesity and its associated negative metabolic consequences. Increasing PA during pregnancy could improve insulin sensitivity and reduce the risk of maternal and offspring adverse outcomes. UPBEAT is a study of a complex intervention designed to improve pregnancy outcomes through dietary changes and PA. Data from the pilot trial of 183 women were available for analysis. The relationship between the time spent at different PA levels and maternal and infant pregnancy outcomes was examined.
Key messages: Strong evidence exists that PA improves insulin sensitivity in non-pregnant populations, and lifestyle interventions of proven effectiveness in non-pregnant populations have been developed. Women who are active in pregnancy demonstrate better glucose control and favourable pregnancy outcomes. There is a lack of effective interventions to support obese pregnant women to be physically active.
Conclusions: No difference was detected in objectively measured PA between women randomised to the intervention and the control arms of the UPBEAT pilot trial. Low-intensity PA was lower in early pregnancy in women who delivered macrosomic infants. Maternal sedentary time at 35–36 weeks’ gestation was positively associated and moderate-intensity PA was inversely associated with neonatal abdominal circumference. Maternal PA is associated with infant birthweight and abdominal circumference and is an appropriate target for intervention to improve infant outcomes. The challenge remains to develop an effective intervention to support obese pregnant women to be physically active.
Background
The prevalence of obesity in pregnant women is increasing. In the UK, maternal obesity doubled from 7.6% to 15.6% between 1989 and 2007. 140 This is of concern because of the well-established association between maternal obesity (typically defined as a BMI of ≥ 30 kg/m2 at the first antenatal appointment) and the risk of adverse pregnancy outcomes, including GDM, maternal hypertension, assisted delivery and macrosomia. 169 In addition to the adverse outcomes apparent at birth, it is becoming clear that offspring born to obese mothers are at an increased risk for obesity and its associated metabolic consequences in later life. A recent systematic review and meta-analysis of 20 published studies concluded that babies weighing > 4 kg at birth were more than twice as likely (OR 2.07, 95% CI 1.91 to 2.04) to be obese as adults as babies weighing ≤ 4 kg at birth. 170 In a birth cohort of 1400 individuals followed up at the age of 32 years, maternal pre-pregnancy BMI was associated with a worse cardiometabolic profile (higher BMI, waist circumference and blood pressure, and lower HDL cholesterol) in the adult offspring. 171
Interventions to reduce the impact of maternal obesity on adverse pregnancy outcomes have the potential to reap positive benefits not only for the offspring of the index pregnancy but also for subsequent generations born to these offspring. The relative importance of intrauterine exposure for an individual’s predisposition to obesity compared with the role of genetic factors and shared lifestyles during childhood is a subject of increasing interest. 172,173 It has been reported that the offspring of obese women already display metabolic disturbances at birth, supporting a role of the intrauterine environment in determining future metabolic health. An increasing maternal BMI is associated with higher levels of fat deposition in the abdomen and liver,174 insulin resistance and raised leptin levels172 at birth. An understanding of how the intrauterine environment exerts an impact on health that persists through childhood and into adulthood will help to identify appropriate targets for intervention.
Developmental overnutrition
The concept of developmental overnutrition has been proposed as one explanation for the association between maternal BMI and obesity and its sequelae in the offspring of obese mothers. 173 This hypothesis suggests that obese mothers who are often insulin resistant have raised levels of circulating glucose and other nutrients during pregnancy which result in fetal hyperinsulinaemia, stimulating excessive fetal growth. This sets these offspring on a lifetime course of acquisition of excess adipose tissue and poor metabolic health. 173
It has been hypothesised that overnutrition in utero, and particularly the influences of fetal hyperinsulinaemia and hyperleptinaemia, affects the development of the fetal hypothalamus and related neuroendocrine organs that play a critical role in appetite regulation. 175 This offers a potential mechanism by which maternal insulin resistance and associated raised circulating glucose impact on offspring health, independently of the environmental and genetic factors shared by the mother and offspring. Findings from animal models of maternal obesity support the hypothesis that exposure to raised levels of maternal glucose and insulin are associated with fetal hyperinsulinaemia, which could be implicated in programming of the development of obesity, perhaps because offspring exposed to raised insulin levels adapt to store rather than use energy. 176
Physical activity and insulin resistance
The role of PA in the prevention and treatment of diabetes mellitus in non-pregnant populations is well established. It has been suggested that PA helps to prevent diabetes mellitus and to improve glucose control in individuals with diabetes mellitus because it improves insulin sensitivity. 177 For example, in the European Relationship between Insulin Sensitivity and Cardiovascular risk study, in which PA was measured objectively in 800 individuals, a strong relationship between total PA and insulin sensitivity was found. 177
Lifestyle interventions that include a PA component are effective in reducing the incidence of diabetes mellitus in high-risk populations. For example, a systematic review of pharmacological and lifestyle interventions to reduce the incidence of type 2 diabetes mellitus in individuals with impaired glucose tolerance concluded that lifestyle interventions achieved a reduction in the risk of diabetes mellitus of 49%, compared with a reduction of 30% for pharmacological interventions. 178 When women were randomised to a weight loss intervention in which weight loss was achieved by either calorie restriction of 500 kcal/day or energy expenditure of 500 kcal/day through exercise for a 14-week period, a significant improvement in glucose disposal was achieved in the exercise group but not in the calorie restriction group. 179 Both groups achieved a similar weight loss (approximately 8 kg), suggesting that PA has a role in improving insulin sensitivity that is independent of weight loss.
Physical activity and glucose metabolism in pregnancy
A number of observational studies have reported an association between higher levels of PA in pre-pregnancy and early pregnancy and a lower prevalence of GDM. A meta-analysis that pooled data from eight studies, including pre-pregnancy PA data on 34,929 women and early pregnancy PA data on 4401 women, concluded that being in the highest pre-pregnancy PA category was associated with a greater than 50% reduction in risk of GDM, and for early pregnancy PA it was associated with a reduction of 25%. 88 An acute effect of PA (treadmill walking) on blood glucose concentrations in pregnancy was observed in 46 women in a study examining the impact of different intensities and durations of exercise on glucose concentration. 180 The study authors found an interaction between the estimated risk of GDM and the glucose response to exercise and concluded that walking for 25 minutes at a vigorous intensity (70% of the heart rate reserve) or for 35–40 minutes at a moderate intensity (30% of the heart rate reserve) achieved a substantial decrease (approximately 1.0 mmol/l) in the glucose concentration in women considered to be at a high risk of GDM (defined as a history of GDM/polycystic ovary syndrome, a family history of diabetes mellitus, overweight/obesity, a history of macrosomia or early weight gain in the current pregnancy).
Evidence is beginning to accumulate that improved insulin sensitivity, achieved through PA during pregnancy, could translate into favourable outcomes for the offspring. A recent observational study assessed PA in 30 pregnant women at 28–32 weeks’ gestation, using a combined heart rate monitor and accelerometer, and estimated insulin sensitivity using the Matsuda composite model. 25 The body composition of the offspring of these women was measured at 11–19 weeks post partum using air displacement plethysmography (PeaPod, COSMED, Rome, Italy). Infant fat-free mass was significantly related to both maternal insulin sensitivity and maternal PA. The authors of the study concluded that insulin sensitivity at 28–32 weeks’ gestation was related to a favourable body fat distribution in the offspring and that this relationship appeared to be influenced by maternal PA. 25 This finding, if confirmed in larger studies, provides justification for offering interventions to support pregnant women to be active and thereby improve the health of their offspring. Interventions to increase PA in obese women during pregnancy could improve insulin sensitivity and reduce the exposure of the developing fetus to excess glucose and insulin and thus help to reduce the burden of obesity and metabolic disturbances among the offspring.
Physical activity during pregnancy
Until relatively recently, pregnant women were discouraged from being physically active. As recently as 1985, the American College of Obstetricians and Gynecologists recommended that pregnant women ‘stringently limit the type, duration and intensity of their exercise to minimize both fetal and maternal risk’. Current guidance from the American College of Obstetricians and Gynecologists and other national bodies now recommends that all pregnant women, including those with a raised BMI, be encouraged to participate in regular moderate-intensity PA for 30 minutes on all or most days of the week. This change in guidance reflects accumulating evidence that PA during pregnancy confers benefits on both the mother and the offspring. In the Health Survey for England 2008, only 29% of (non-pregnant) women reported meeting this guideline, and objective measurement of PA revealed that as few as 4% of women actually met the guideline. 181 Most women, therefore, are insufficiently active at the beginning of pregnancy. Generally, PA declines with gestation. For example, a recent study found that the number of steps walked per day, assessed using a pedometer, fell by 1340 (equivalent to walking approximately 1000 m) between 12 and 28 weeks’ gestation in 97 women at high risk of developing GDM. 128 There is a need, therefore, for interventions to support women to be active during pregnancy.
The UK Pregnancies Better Eating and Activity trial
UPBEAT is an ongoing RCT of a combined PA and dietary intervention in > 1500 obese (BMI of ≥ 30 kg/m2) pregnant women which aims to improve glucose homeostasis and thereby prevent GDM (the primary outcome) (trial registration number ISRCTN89971375). A pilot RCT in 183 women to determine if the intervention led to changes in PA and diet was completed in 2011. 150 Research Ethics Committee approval was obtained for the trial (UK IRAS reference number 09/H082/5), and all participants provided written informed consent to participate in the trial. Details of how PA was measured have been reported previously. 150 Briefly, PA was assessed by an ActiGraph accelerometer worn for 7 consecutive days at baseline (16+0–18+6 weeks’ gestation), 27+0–28+6 weeks’ gestation and 35+0–36+6 weeks’ gestation and via a questionnaire (RPAQ). For accelerometry, a valid recording day was defined as > 500 minutes of monitored ‘on’ time in 24 hours, and women providing valid data on at least 3 days were included in the analysis. Recorded activity was categorised as sedentary (< 100 cpm), light physical activity (LPA; 100–1951 cpm) or moderate or vigorous activity (MVPA; ≥ 1952 cpm). For the RPAQ, self-reported time spent on activities with a MET of < 1.5 was categorised as sedentary. LPA was activity of 1.5–3.0 METs and MVPA as activity of > 3.0 METs. No difference in objectively measured PA between women in the intervention and control groups was detected at 28 or 36 weeks’ gestation, although women in the intervention group self-reported more minutes of MVPA than women in the control group at 28 weeks (mean difference 34 minutes/day; 95% CI 9 to 59 minutes/day). 150
As there was no significant difference between the intervention and control groups in objectively measured PA, the data from both arms of the pilot trial were combined to examine the relationship between PA and pregnancy outcomes in the analyses presented here. Forty-six women (29.9%) were diagnosed with GDM in the UPBEAT pilot trial, as assessed by an OGTT at 27+0–28+6 weeks’ gestation, using the IADPSG’s criteria for diagnosis. 6 Valid objective PA data were available for 37 of these women. No statistically significant differences in time spent at different PA levels were identified between women who developed GDM and those who did not. There was a trend towards a higher baseline sedentary time and lower LPA and MVPA time in women who developed GDM, but these differences were not statistically significant (see Table 14). Twenty-nine women gave birth to a macrosomic (> 4000 g) baby, with valid PA data available in 26 cases. LPA at baseline, but not MVPA, was significantly lower in women who delivered a macrosomic baby than in those who did not (158 minutes/day compared with 186 minutes/day; p = 0.014). The correlations between maternal PA and fasting and 2-hour post-75-g OGTT are presented in Table 19. A trend towards baseline sedentary time being weakly positively associated and LPA time being negatively associated with fasting and post-challenge glucose levels was observed, but the relationships did not reach statistical significance. LPA at 28 weeks’ gestation was negatively associated with fasting and 2-hour post-challenge glucose. The relationship between maternal PA and newborn infant abdominal circumference, as a proxy for abdominal adiposity, was also examined (Table 20). Maternal sedentary time at baseline was negatively associated with newborn infant abdominal circumference, but at 36 weeks’ gestation the relationship was positive. LPA and MVPA at 36 weeks’ gestation were negatively associated with newborn infant abdominal circumference.
| PA measure | GDM status | p-value | |
|---|---|---|---|
| GDM | No GDM | ||
| GDM | (n = 37) | (n = 103) | |
| Time of measurement | |||
| Baseline | |||
| Sedentary | 622 (148) | 581 (216) | 0.109 |
| LPA | 170 (53) | 184 (53) | 0.168 |
| MVPA | 38 (16) | 41 (21) | 0.354 |
| 28 weeks’ gestation | |||
| Sedentary | 591 (147) | 585 (103) | 0.860 |
| LPA | 155 (60) | 173 (73) | 0.336 |
| MVPA | 35 (19) | 33 (16) | 0.683 |
| 36 weeks’ gestationa | |||
| Sedentary | 563 (103) | 575 (96) | 0.698 |
| LPA | 145 (44) | 186 (59) | 0.024 |
| MVPA | 21 (9) | 29 (16) | 0.088 |
| Macrosomia | (n = 26) | (n = 114) | |
| Time of measurement | |||
| Baseline | |||
| Sedentary | 570 (90) | 598 (139) | 0.347 |
| LPA | 158 (43) | 186 (54) | 0.014 |
| MVPA | 42 (23) | 40 (19) | 0.741 |
| 28 weeks’ gestation | |||
| Sedentary | 570 (84) | 690 (122) | 0.606 |
| LPA | 143 (31) | 173 (76) | 0.202 |
| MVPA | 37 (23) | 33 (15) | 0.482 |
| 36 weeks’ gestationa | |||
| Sedentary | 555 (71) | 575 (103) | 0.587 |
| LPA | 153 (31) | 180 (61) | 0.197 |
| MVPA | 24 (13) | 28 (16) | 0.507 |
| Variable | Time point | ||
|---|---|---|---|
| Baseline (n = 61) | 28 weeks’ gestation (n = 43) | 36 weeks’ gestation (n = 34) | |
| Maternal fasting glucose | |||
| Sedentary | 0.162 | 0.090 | |
| LPA | –0.117 | –0.224a | |
| MVPA | –0.070 | –0.078 | |
| Maternal 2-hour glucose | |||
| Sedentary | 0.101 | 0.132 | |
| LPA | –0.169 | 0.225a | |
| MVPA | –0.046 | –0.114 | |
| Newborn infant abdominal circumference | |||
| Sedentary | –0.287a | –0.920 | 0.435a |
| LPA | –0.036 | 0.024 | –0.367a |
| MVPA | –0.101 | –0.011 | –0.466a |
Discussion
Maternal obesity is associated with an increased risk of obesity and an unfavourable cardiometabolic profile in the offspring, which persists into adulthood and, therefore, impacts future generations. Obese women are more insulin resistant than lean women during pregnancy and thus the offspring of obese women are exposed to higher levels of glucose and other nutrients in utero. Exposure to intrauterine overnutrition is associated with a higher birthweight, increased adiposity and indicators of poor metabolic health.
Physical activity during pregnancy has the potential to increase insulin sensitivity and improve the health of the offspring. Data presented here from the UPBEAT pilot trial and from other studies180,182,183 demonstrate that PA during pregnancy is associated with improved maternal glucose control and less macrosomia and adiposity in the offspring. 184 Specifically, in the UPBEAT pilot study, even LPA in obese women was associated with improved glucose tolerance and a lower infant abdominal circumference. It was of particular interest to note that more time spent on sedentary activities and less time spent on low- and moderate-intensity PA at 36 weeks’ gestation were related to an increasing infant abdominal circumference, whereas PA when measured at 28 weeks showed no such relationships. Should this observation be repeated in the larger cohort of the full RCT, in fully adjusted regression analyses, it could indicate that PA towards term may have specific benefits for reducing adiposity in the child.
Despite the existence of lifestyle interventions of proven effectiveness to increase PA and improve insulin sensitivity in non-pregnant populations, there is a paucity of interventions with demonstrated effectiveness for pregnant women. Many intervention studies targeting PA in pregnant women, including the UPBEAT pilot trial, have failed to impact PA levels.
Despite the difficulty of designing effective interventions to support obese pregnant women to be physically active on the basis of this and other studies, it is clearly premature to abandon efforts to do so. 185 In the context of the increasing problem of obesity internationally, the potential benefits that could be achieved if obese pregnant women become sufficiently active are substantial. A better understanding of the barriers to obese women engaging in PA during pregnancy is needed to inform the development of interventions. Process evaluation of the UPBEAT pilot trial revealed that the intervention was considered acceptable and well received by most women, although some reported that the lifestyle information they received was too basic and that attending group sessions was too time-consuming. 150 Refinement of the protocol for the delivery of the intervention in the UPBEAT has been made in view of these findings, although the intervention remains the same. Previous work has also found that pregnant women report a lack of time and childcare, as well as physical discomfort, as barriers to PA. 150 The intervention design needs to address these barriers by considering flexibility in the mode and location of intervention delivery, and in terms of facilitating engagement in appropriate types of activity.
In summary, it is increasingly acknowledged that efforts to prevent obesity and its associated poor health should begin in utero174 and should aim to improve maternal glucose control as this reduces the risk of obesity among offspring. 176 PA is an appropriate target for intervention to achieve this. The identification of methods by which obese pregnant women can best be supported to be sufficiently active remains a challenge.
Chapter 4 Trial protocol
Phase 1, the development phase, and phase 2, the pilot trial, led to completion of the protocol for phase 3, the RCT of a complex behavioural intervention in obese pregnant women to prevent GDM and LGA deliveries. This protocol has been published previously as Briley AL, Barr S, Badger S, Bell R, Croker H, Godfrey KM, Holmes B, Kinninen TI, Nelson SM, Oteng-Ntim O, Patel N, Robson SC, Sandall J, Sanders T, Sattar N, Wardle L, Poston L. A complex intervention to improve pregnancy outcome in obese women; the UPBEAT randomised controlled trial. BMC Pregnancy and Childbirth 2014;14:74. 186 © Briley et al. ; licensee BioMed Central Ltd. 2014. This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated. Minor additions and formatting changes have been made to the published text.
Abstract
Background: Despite the widespread recognition that obesity in pregnant women is associated with adverse outcomes for mother and child, no intervention has been proven to reduce the risk of these complications. The primary aim of this RCT is to assess, in obese pregnant women, whether a complex behavioural intervention, based on changing diet (to foods with a lower GI and lower saturated fat intake) and PA, will reduce the risk of GDM and delivery of a LGA infant. A secondary aim is to determine whether or not the intervention lowers the long-term risk of obesity in the offspring.
Methods/design: A multicentre RCT comparing a behavioural intervention designed to prevent GDM at 27+0–28+6 weeks’ gestation and LGA with standard antenatal care in obese pregnant women. Inclusion criteria: women with a BMI of ≥ 30 kg/m2 and a singleton pregnancy between 15+0 weeks and 18+6 weeks’ gestation.
Exclusion criteria: Predefined, pre-existing diseases and multiple pregnancy. Randomisation is online by a computer-generated program and is minimised by BMI category, maternal age, ethnicity, parity and centre.
Intervention: This is delivered by a HT over eight sessions. Based on control theory, with elements of social cognitive theory, the intervention is designed to improve maternal glucose homeostasis. Women randomised to the control arm receive standard antenatal care until delivery in accordance with local guidelines. All women have a 75-g OGTT at 27+0–28+6 weeks’ gestation.
Primary outcome: Maternal – diagnosis of GDM, in accordance with the IADPSG’s criteria; neonatal – LGA, defined as > 90th customised birthweight centile.
Sample size: 1546 women to provide 80% power to detect a 25% reduction in the incidence of GDM and a 30% reduction in the number of LGA infants.
Discussion: All aspects of this protocol have been evaluated in a pilot RCT, with subsequent optimisation of the intervention. The findings of this trial will inform whether or not lifestyle-mediated improvement of glucose homeostasis in obese pregnant women can minimise the risk of pregnancy complications.
Trial registration: Current Controlled Trials ISRCTN89971375.
Background
The rise in the global incidence of obesity has reached pandemic proportions. 187 In 2008, the WHO estimated that there were 1.5 billion individuals with a BMI of ≥ 25 kg/m2, including nearly 300 million obese women (BMI of ≥ 30 kg/m2). 82 The UK has seen a sharp increase in the proportion of obese women; as reported in a recent survey, approximately one in five women aged between 16 and 44 years is obese. 188 The UK Confidential Enquiry into Maternal and Child Health identified that overweight and obesity were, either directly or indirectly, the cause of over half of maternal deaths. 109 The adverse effects of obesity on reproductive health and childbearing are manifold. Obesity reduces fertility, and in pregnancy is associated with a heightened risk of GDM, hypertensive disorders of pregnancy including pre-eclampsia and failure to progress in labour. Caesarean section rates among obese women are high, and infants of obese mothers are at greater risk of congenital malformation, of being LGA at delivery (> 90th centile), macrosomia, shoulder dystocia and stillbirth. Following delivery, obese women are more likely to suffer a postpartum haemorrhage and have longer hospital stays than women with a normal BMI (18.5–24.9 kg/m2). 189,190 The effects of obesity may extend beyond health in pregnancy; increasing evidence suggests that the children of obese women or of those whose GWG was excessive may be at greater risk of obesity because of antenatal exposure to adverse metabolic influences in utero or in the early postnatal period. 9,191
In the UK, in contrast to the USA, women are no longer routinely weighed during pregnancy, except at their first antenatal appointment. The US Institute of Medicine’s guidelines for weight gain during pregnancy provide recommendations for women according to their pre-pregnancy BMI, which include that obese women should gain less weight in pregnancy (11–20 lb; 5–9 kg) than those with a lower pre-pregnancy BMI. 192 This advice is based on observational studies suggesting improved outcomes with lower weight gain. The UK NICE guidelines on weight management in pregnancy concluded that more evidence of improved outcomes from interventional studies is required before the US or similar guidelines for limitation of GWG are adopted. 93 Although review of the literature suggests that intervention studies designed to limit GWG may sometimes be effective in achieving a reduction in GWG, there is, at present, no evidence for improvement of pregnancy outcome among obese women. However, most studies, including those in overweight and obese pregnant women, have been small, have not been sufficiently powered for clinical outcomes and have suffered from design limitations. 84,193
The role of insulin resistance in obese pregnancies
An alternative approach to restricting GWG is to focus on the adverse clinical outcomes associated with obesity, and to develop interventions which are directly associated with known underlying mechanisms. A pre-pregnancy BMI of ≥ 30 kg/m2, irrespective of the amount of weight gained during pregnancy, is the most important independent determinant of the risk of caesarean section, delivery of a LGA infant and postpartum weight retention. 194 In addition, the evidence linking GWG with GDM, in contrast to the strong association with pre-pregnancy BMI, is relatively weak. 195 This is, at least in part, likely to be a reflection of the strong association between maternal fat mass and insulin resistance. 9 There is a physiological increase in insulin resistance during normal pregnancy and the obese pregnant woman is at greater risk of developing GDM. Maternal hyperglycaemia and, more recently, maternal hypertriglyceridaemia are strongly implicated in the development of fetal macrosomia. 196–199 Using the method of continuous blood glucose monitoring, Harmon et al. 198 have shown, as might be expected, that obese pregnant women have an exaggerated postprandial glucose response. As the magnitude of the postprandial response was directly implicated in increasing fetal adiposity and birthweight through fetal hyperinsulinaemia, a dietary intervention focusing on reducing postprandial hyperglycaemia by lowering the dietary GL could improve maternal glucose control, reduce the incidence of GDM and lower the incidence of delivery of LGA infants. Similarly, pre-eclampsia is associated with maternal insulin resistance, and improved glucose homeostasis might lower the risk of pre-eclampsia in obese women. 200
Improving glucose homeostasis in pregnancy
Specific dietary advice including intake of low-GI foods and reduction of dietary saturated fats, as well increased PA, could contribute to improved maternal glucose homeostasis. 87,201 In a study of 50 obese Danish women designed to limit GWG, Wolff et al. 202 found that an intense dietary regime (10 1-hour sessions with a dietitian) focusing on healthy eating resulted in a reduction in plasma insulin compared with women in the control arm of the study. Another study reported that a diet and exercise regime led to a reduction in GWG and a decrease in the incidence of GDM in 126 overweight and obese Australian women,110 but no difference in infant birthweight (3.5 vs. 3.4 kg). In non-obese women with mild GDM, in whom improved glucose homeostasis is achieved through a strict regime of dietary intervention and insulin treatment when required, a reduction in the risk of adverse pregnancy outcome is achievable, as shown in two RCTs. 4,203 Higher levels of PA in normoglycaemic pregnant women and those with GDM have also been shown to improve insulin sensitivity,9 but few data of adequate power are available for the obese pregnant population. A recent meta-analysis of eight prenatal PA intervention studies, however, showed that there is a lack of consistent evidence regarding the benefits of exercise combined or not combined with dietary advice for improving glucose tolerance in obese pregnant women, which was interpreted to reflect the limited power of current evidence and poor intervention compliance. 204
Systematic review of the literature
Louie et al. 7 conducted a systematic review of the influence of lowering dietary GI in pregnancies across all BMI categories. Of the eight studies included, two suggested that a low-GI diet can reduce the risk of LGA infants in healthy pregnancies, but one reported an increase in small-for-gestational-age infants. In the three studies in which pregnancies were complicated by GDM, the evidence supported the overall advantages of a low-GI diet. This review recommended that, until larger-scale intervention trials are completed, a low-GI diet should not replace the current dietary recommendations from government and health agencies, and that further research regarding the optimal time to start a low-GI diet for maximum protection against adverse pregnancy outcomes is warranted.
In a systematic review of nine randomised trials including 743 overweight and obese pregnant women, Dodd et al. 193 reported that there was no significant effect of interventions designed to limit GWG on weight gain or on delivery of a LGA infant. In a later systematic review of 13 randomised clinical trials of lifestyle interventions in overweight and obese pregnant women (n = 1228), we concluded that there was a modest influence on GWG (–2.21 kg; 95% CI –2.86 to –1.59 kg), but no significant effect on any relevant clinical outcome. 85 We have also reviewed dietary and PA interventions in normal BMI and obese pregnant women (n = 1656 women) for the purpose of limiting GWG; in a systematic review we assessed 12 trials. Overall, diet and PA change was effective in reducing GWG, but there was considerable heterogeneity in outcomes. 2 The analysis highlighted differences in sample characteristics and aspects of intervention design, content, delivery and evaluation which might explain variation in effectiveness. Furthermore, failure to evaluate changes in behaviour or its psychological determinants could have obscured identification of the processes by which weight change is effective, and limited the ability to discern active intervention ingredients. We concluded that interventions should be more systematically designed and built on insights from behavioural science.
More recently, Thangaratinam et al. ,84 in a meta-analysis of 44 clinical trials of lifestyle or dietary interventions or a combination of both during pregnancy across all BMI ranges, found a reduction in GWG (mean reduction 1.42 kg) with any intervention in comparison with the control. 84 PA alone was associated with a reduction in birthweight (mean difference –60 g, 95% CI –120 g to –10 g). Interventions based on diet were the most effective, being associated with reductions in maternal GWG (mean 3.84 kg, 95% CI 2.45 to 5.22 kg) and a modest improvement in obstetric outcomes. However, the combination of intervention methods did not result in a reduction in the incidence of LGA infants between the groups (relative risk 0.85, 95% CI 0.66 to 1.09). Among obese women, there was no evidence of an improvement in any clinical outcome. In an editorial to this review, we highlighted that there remains a paucity of information regarding intensity, duration and compliance of the interventions, all of which could account for the lack of efficacy, as well minimal evidence for any effect of the intervention on the targeted behaviours. If the intervention does not achieve a change in behaviour in the expected direction, it follows that there will be no influence on clinical outcomes. 185
The protocol presented here describes a complex behavioural intervention comprising dietary (low GI and reduced saturated fat intake9) and PA changes that we have developed with the aim of improving glycaemic control in obese pregnant women. The intervention is based on established control theory with elements of social cognitive theory. 94,95 The primary hypothesis being tested is that an antenatal intervention package of low-glycaemic dietary advice and reduced saturated fat intake combined with advice on increased PA will reduce the incidence of maternal GDM and delivery of LGA infants. A secondary hypothesis is that the intervention will reduce the risk of obesity in the child. Prior to undertaking a trial adequately powered to investigate clinical outcomes, we completed a pilot study (n = 183 women) to determine whether or not the intervention changed dietary and physical behaviours as expected. 150 This pilot study showed that diet but not PA (as objectively measured) changed with the intervention and that all aspects of the protocol were feasible. A process evaluation led to optimisation of intervention delivery. The trial steering committee recommended continuation with recruitment for the RCT, and it was decided that the PA aspect of the intervention should remain, as this follows standard guidelines for pregnant women. 39
Methods/design
Study design
Multicentre RCT. For participating centres see the UPBEAT website: www.medscinet.net/upbeat/.
Ethics approval
NHS Research Ethics Committee approval was obtained in all centres (UK IRAS, reference number 09/H0802/5).
Inclusion criteria
Women with a singleton pregnancy, 15+0–18+6 weeks’ gestation and a BMI of ≥ 30 kg/m2 at their first antenatal appointment.
Exclusion criteria
Women unable or unwilling to give informed consent; at < 15+0 weeks or at > 18+6 weeks’ gestation; essential hypertension requiring treatment either pre-pregnancy or in index pregnancy; (see Erratum below for omission in error of ‘pre-existing diabetes, type 1 or type 2’), pre-existing renal disease; systemic lupus erythematosus; antiphospholipid syndrome; sickle cell disease; thalassaemia; coeliac disease; thyroid disease; current psychosis; multiple pregnancy; or currently prescribed metformin.
The protocol for the study is shown in Figure 6.
FIGURE 6.
The UPBEAT protocol summary. ANC, antenatal clinic.
Trial entry
Eligible women are identified in antenatal clinics and from GP and midwives referral letters. Verbal and written information is given. Research midwives contact potential recruits, obtain verbal consent and arrange the first appointment. Care is taken to search the clinical records for those women screened in early pregnancy for pre-existing diabetes mellitus (UK recommendations are that all women with pre-GDM should be screened in early pregnancy). Women with a diagnosis of diabetes mellitus are excluded from the study (see Exclusion criteria). For those who decline to participate, permission is sought to collect minimal pregnancy outcome data.
15+0–18+6 weeks’ appointment: baseline and randomisation
At the first appointment, written informed consent is obtained. Baseline demographic information, medical and family history and current pregnancy information are collected. A short validated FFQ205 is completed to evaluate dietary GL, dietary GI, saturated fat and total sugar intake and other dietary variables. Women are weighed, their pulse and blood pressure are checked, anthropometric measurements obtained and blood and urine samples taken. Behavioural and psychological measures include the EQ-5D,98 the EPDS,99 the IPAQ206 and a binge-eating screening questionnaire. 207 Randomisation occurs at this visit via a secure internet-based data management system (MedSciNet Ltd), which is the repository for all trial data. The randomisation schedule is minimised according to ethnicity, parity (0 vs. ≥ 1), age, BMI (BMI 30–34 vs. 35–39.9 and > 40 kg/m2) and centre. Randomised women are allocated sequential study numbers, regardless of allocation to the intervention or standard care group.
Intervention
Women randomised to the intervention group attend a one-to-one interview with the HT, which includes discussion of the potential benefits of attending the weekly sessions. In the UK, HTs help people to change their behaviour to achieve personal choices and goals and, generally, do not have prespecified health professional qualifications, but do have relevant experience. All HTs in this trial receive study-specific training in all aspects of the intervention and ongoing support throughout the trial. Women in the intervention group receive a participant handbook, a DVD of an exercise regime safe for pregnancy, a pedometer and a logbook for recording weekly SMART goals and steps (as assessed by a pedometer). They are invited to attend eight sessions with the HT on a weekly basis, each lasting 1–1.5 hours. Women are encouraged to attend all sessions, but are strongly recommended to attend a minimum of five. When the sessions are not attended in person, the HT covers the session material by telephone or e-mail. Attendance and coverage of session material are documented in the study database. Following a review of the dietary and physical intervention, each session is designed to focus on different approaches in achieving the goals set. These include SMART goals, self-monitoring, and provision of feedback regarding goal attainment, identification and problem-solving of barriers, enlisting social support and providing opportunities for social comparison. At each session, a review of the previous week’s goals is undertaken.
The dietary intervention aims to promote a healthier pattern of eating, similar to that used in diabetes mellitus prevention studies, but does not aim to restrict energy intake. In order to decrease the GL, dietary advice includes replacing starchy foods with a medium/high GI by foods with a lower dietary GI, and restricting the consumption of sugar-sweetened beverages (including fruit juice) but not fruit. Participants are also given dietary advice to reduce SFA intake. No advice was given regarding GWG.
Advice regarding PA focuses on increasing the daily step count incrementally, and being more active in daily life. Pedometers are used for monitoring and motivation. The emphasis is on walking at a moderate intensity with additional options included, especially for those who are already engaging in some PA. This degree of activity accords with that recommended by the UK’s Royal College of Obstetricians and Gynaecologists. 39
Standard care
Women randomised to the standard care group attend routine antenatal care in accordance with local health-care provision. The UK recommendations state that all pregnant women, and particularly those with a BMI of ≥ 30 kg/m2, should be advised by a health professional at the earliest opportunity of the risks of obesity in pregnancy and be given advice about a healthy diet and safe levels of PA. Recommendations for referral to a registered dietitian are infrequently implemented. No specific advice is given about GWG and women are weighed only at their first antenatal visit. 93 Women should also be encouraged to lose weight after pregnancy.
27+0–28+6 weeks’ appointment
All women in both groups attend for an OGTT at 27+0–28+6 weeks’ gestation (fasting for a minimum of 10 hours, 75-g glucose load). At this visit weight and anthropometric measurements are taken, health in current pregnancy noted, additional blood and urine samples taken, and dietary FFQ, EQ-5D, EPDS, IPAQ and questionnaires about binge-eating completed. Early pregnancy data including blood pressure, blood chemistry and anomaly scan reports are entered from routine clinical records.
34+0–36+0 weeks’ appointment
Women in both arms of the study attend the research appointment at 34+0–36+0 weeks’ gestation. Current health in pregnancy is recorded, weight and anthropometric measurements taken, blood and urine samples collected and dietary FFQ, EQ-5D, EPDS, IPAQ and binge-eating questionnaires completed.
Unexpected adverse events are reported in accordance with good clinical practice guidance.
Pregnancy outcome data
Following delivery, information is collected from maternal medical records regarding health in late pregnancy, labour onset, mode of delivery, blood loss, antenatal and postnatal inpatient nights. When possible a cord blood sample is taken.
Neonatal and postnatal outcome data include Apgar scores, admission to special care baby unit and inpatient nights. To address the influence of the intervention on fetal growth and adiposity, neonatal anthropometry and length measurements are undertaken within 72 hours of birth.
Six months post partum
To determine whether or not the intervention has led to sustained change in maternal dietary and PA behaviours, diet is assessed by FFQ and PA by IPAQ. Maternal demographic data, health since pregnancy and smoking history are obtained. Maternal anthropometric measures are taken. EPDS, Three-Factor Eating Questionnaire-R18208 and binge-eating questionnaires are completed. To address safety and the influence of the intervention on the long-term health of the child, details regarding the child’s health from birth are obtained. If cord blood was not taken, and if the parents provide consent, a buccal cell sample is taken from the child’s mouth for deoxyribonucleic acid (DNA) extraction (Oragene, DNA Genotek, Ottawa, ON, Canada). To address the potential influence of the intervention on infant adiposity at 6 months and to obtain information on known determinants of childhood obesity, infant length and other anthropometric measures are taken. The mother provides information for an infant feeding and growth questionnaire209 and a validated questionnaire addressing appetite, the Baby Eating and Behaviour Questionnaire. 210 Information on activity using questions from the Infant Behaviour Questionnaire-Revised211 and sleep patterns are obtained212 and information on childcare (kindergarten, other carers) collected.
Paternal data
At any point during the pregnancy or at the 6-month postnatal appointment the father of the baby is asked to consent to taking part in the study to provide information that may influence the health of the child. A brief medical history, blood pressure and pulse are checked, anthropometric measurements are taken and a blood samples collected for the provision for DNA. In the absence of direct paternal measurement, women are asked to recall their partner’s height and weight and brief medical and smoking history.
Study end points
Primary maternal outcome
Gestational diabetes mellitus was diagnosed by OGTT at 27+0–28+6 weeks’ gestation in accordance with the criteria recommended by IADPSG (i.e. fasting blood glucose concentration of ≥ 5.1 mmol/l or 1-hour glucose of ≥ 10.0 mmol/l or 2-hour glucose of ≥ 8.5 mmol/l).
Primary neonatal outcome
A LGA infant is defined as an infant whose adjusted birthweight is > 90th centile for gestational age, adjusting for maternal height, corrected maternal weight, ethnicity, parity and sex of baby.
Secondary outcomes
Maternal
Pre-eclampsia, severe pre-eclampsia; mode of delivery: caesarean section (elective, emergency, pre labour, in labour), vaginal delivery, operative vaginal delivery; induction of labour; blood loss at delivery (> 1000 ml; > 2000 ml); inpatient nights (antenatal, postnatal); GWG, trimester-specific GWG; fasting plasma glucose, fasting plasma insulin, insulin resistance calculated by homeostatic model assessment 2 (Homeostatic Model Assessment 2 – Insulin Resistance, HOMA2-IR)213 at 28 weeks’ gestation; diagnosis of GDM by local criteria; referral to GDM antenatal service following OGTT; requirement for insulin or metformin during pregnancy; and fetal growth at 28 weeks. Health-related quality of life as assessed by EQ-5D. At 27+0–28+6 and 34+0–36+0 weeks’ gestation and 6 months post partum; mid-arm, neck, hip, thigh and wrist circumference and skinfold thickness (subscapular, triceps, biceps, suprailiac); plasma fructosamine, triglycerides, LDL, very low-density lipoprotein (VLDL) and HDL cholesterol, plasma insulin, C-reactive protein, other relevant epigenetic and metabolomic biomarkers, and urinary biomarkers; dietary measures including GL, saturated fat and total sugar intake; dietary feeding patterns; PA scores; measures of depression; and maternal smoking. At 6 months post partum, postnatal weight retention and existing maternal morbidity (diabetic status, hypertension, thromboembolism, low mood214).
Neonatal
Gestational age at delivery, delivery at < 37 weeks’ gestation, delivery at < 34 weeks’ gestation; birthweight > 4000 g; birthweight < 2500 g; birthweight > 95th, < 10th and< 5th customised birthweight centiles, distribution of birthweight, neonatal death, days in special care baby unit, total inpatient days, need for mechanical ventilation and duration, discharge home on oxygen, suspected and confirmed infection, evidence of intraventricular haemorrhage and other complications, (pulmonary haemorrhage, necrotising enterocolitis), retinopathy of prematurity, hypoglycaemia (blood glucose < 2.6 mmol/l), occipitofrontal head circumference, abdominal circumference, mid-arm circumference, chest circumference, crown–rump length and crown–heel length (neonatometer), triceps and subscapular skinfold thicknesses and estimated fat mass.
And other infrequent adverse outcomes, including shoulder dystocia were recorded as free text.
Key epigenetic and metabolomic biomarkers will be investigated using cord blood or whole blood (maternal and fetal) and their relation to specific outcomes.
Infant at 6 months
Duration of breastfeeding, choice of formula milk, weaning history (introduction of foods and frequency/timing of foods), a general measure of appetite, and four specific scales (enjoyment of food, food responsiveness, slowness in eating, satiety responsiveness); anthropometric measurements (occipitofrontal circumference, abdominal circumference, mid-arm circumference, chest circumference, crown–rump length and crown–heel length by infantometer, subscapular and triceps skinfold thicknesses and estimated fat mass); activity (total number of 14 standard milestones reached) and sleeping patterns (time spent sleeping morning, afternoon and night); health-care resource use (hospital admissions and medications); and frequency of use of kindergarten/mother’s help.
Subgroup analysis
Women who are treated for GDM; differences in diagnostic thresholds between centres will be accommodated by minimisation by centre. Other subgroups likely to be of interest include demographic and socioeconomic status (as assessed by the Index of Multiple Deprivation), ethnic groups, BMI categories, groups of different parity and smokers.
Interaction tests will be used to determine whether or not treatment is particularly effective in individual subgroups. Performance of subgroup analysis will be dependent on sufficient data. Because of the well-known risk of false positives, both main effects and interaction tests will be performed before considering results for subgroups.
Sample size
In the pilot RCT,150 30% of women in the standard care arm developed GDM in accordance with the IADPSG’s criteria. 208 A total of 1546 women (including allowance for 20% dropout) (773/arm) will be recruited to provide 80% power to detect a 25% reduction in the incidence of GDM. Considering LGA deliveries, for a 30% relative risk reduction from an estimated 17.2% of LGA to 12.0% in the intervention arm, 1546 women would give 80% power. 215,216
Analysis
To determine whether the trial participants are representative of the general population, relevant parameters available from electronic summary patient records will be compared between eligible women agreeing and declining to take part. Analyses will follow the intention-to-treat principle.
Following CONSORT guidelines, risk ratios and risk differences will be estimated by binary regression for yes/no outcomes. When measurements are repeated over time, results [mean (SD) or n (%)] will be presented separately at each time point. Randomised comparisons with 95% CIs will be made using linear regression with robust standard errors, adjusting for the baseline value when appropriate.
Multiple regression models will be used to address the influence of maternal exposures on neonatal and infant (6 months) body composition and the role of paternal factors.
Discussion
This RCT will determine whether a complex intervention addressing diet and PA will reduce the incidence of GDM and delivery of LGA infants in a population of obese pregnant women receiving antenatal care in the UK. The study will inform guidelines on the management of obesity in pregnancy and, if successful, is designed to be rapidly transferable to clinical practice. Determination of infant anthropometry at 6 months of age will assess whether or not the intervention in pregnancy can influence body composition of the infant. Further studies on childhood body composition at 3 years of age will also be undertaken.
Erratum
An erratum of this protocol has been published as follows:
Briley AL, Barr S, Badger S, Bell R, Croker H, Godfrey KM, et al. Erratum: a complex intervention to improve pregnancy outcome in obese women; the UPBEAT randomised controlled trial. BMC Pregnancy Childbirth 2015;15:111. http://dx.doi.org/10.1186/s12884-015-0540-1186
The paragraph describing exclusion criteria should read:
Exclusion criteria
Women unable or unwilling to give informed consent; < 15 + 0 weeks or > 18 + 6 weeks’ gestation; essential hypertension requiring treatment either pre pregnancy or in index pregnancy; pre-existing diabetes mellitus (type 1 or type 2); pre-existing renal disease; systemic lupus erythematosus; antiphospholipid syndrome; sickle cell disease; thalassaemia; coeliac disease; thyroid disease; current psychosis; multiple pregnancy; currently prescribed metformin.
Chapter 5 The UK Pregnancies Better Eating and Activity trial: phase 3 – randomised controlled trial
Following successful completion of phases 1 and 2, the programme continued with a RCT (phase 3) of a complex intervention designed to prevent GDM and LGA in pregnant women with obesity and their offspring.
Effect of a behavioural intervention in obese pregnant women (the UK Pregnancies Better Eating and Activity trial): a multicentre, randomised controlled trial
This study has been published previously as Poston L, Bell R, Croker H, Flynn AC, Godfrey KM, Goff L, et al. , on behalf of The UPBEAT Trial Consortium. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol 2015;3:767–77. 217 © Poston et al. Open Access article distributed under the terms of CC BY-NC-ND.
Summary
Background: Behavioural interventions might improve clinical outcomes in pregnant women who are obese. We aimed to investigate whether a complex intervention addressing diet and physical activity could reduce the incidence of gestational diabetes and large-for-gestational-age infants.
Methods: The UK Pregnancies Better Eating and Activity Trial (UPBEAT) is a randomised controlled trial done at antenatal clinics in eight hospitals in multi-ethnic, inner-city locations in the UK. We recruited pregnant women (15–18 weeks plus 6 days of gestation) older than 16 years who were obese (BMI ≥ 30 kg/m2). We randomly assigned participants to either a behavioural intervention or standard antenatal care with an internet-based, computer- generated, randomisation procedure, minimising by age, ethnic origin, centre, BMI, and parity. The intervention was delivered once a week through eight health trainer-led sessions. Primary outcomes were gestational diabetes (diagnosed with an oral glucose tolerance test and by criteria from the International Association of Diabetes in Pregnancy Study Groups) and large-for-gestational-age infants (≥ 90th customised birthweight centile). Analysis was by intention to treat. This trial is registered with Current Controlled Trials, ISCRTN89971375. Recruitment and pregnancy outcomes are complete but childhood follow-up is ongoing.
Findings: Between March 31, 2009, and June 2, 2014, we assessed 8820 women for eligibility and recruited 1555, with a mean BMI of 36.3 kg/m2 (SD 4.8). 772 were randomly assigned to standard antenatal care and 783 were allocated the behavioural intervention, of which 651 and 629 women, respectively, completed an oral glucose tolerance test. Gestational diabetes was reported in 172 (26%) women in the standard care group compared with 160 (25%) in the intervention group (risk ratio 0.96, 95% CI 0.79–1.16; p = 0.68). 61 (8%) of 751 babies in the standard care group were large for gestational age compared with 71 (9%) of 761 in the intervention group (1.15, 0.83–1.59; p = 0.40). Thus, the primary outcomes did not differ between groups, despite improvements in some maternal secondary outcomes in the intervention group, including reduced dietary glycaemic load, gestational weight gain, and maternal sum-of-skinfold thicknesses, and increased physical activity. Adverse events included neonatal death (two in the standard care group and three in the intervention group) and fetal death in utero (ten in the standard care group and six in the intervention group). No maternal deaths were reported. Incidence of miscarriage (2% in the standard care group vs. 2% in the intervention group), major obstetric haemorrhage (1% vs. 3%), and small-for-gestational-age infants (≤ 5th customised birthweight centile; 6% vs. 5%) did not differ between groups.
Interpretation: A behavioural intervention addressing diet and physical activity in women with obesity during pregnancy is not adequate to prevent gestational diabetes, or to reduce the incidence of large-for-gestational-age infants.
Funding: National Institute for Health Research, Guys and St Thomas’ Charity, Chief Scientist Office Scotland, Tommy’s Charity.
Introduction
In 2013, an estimated one in five women in the world aged 20 years or older was obese (BMI of ≥ 30 kg/m2). 218 Obesity in women was most widespread in high-income countries, with a prevalence of 25% in the UK and 34% in the USA. 218 Pregnant women with obesity are at risk of many complications, with insulin resistance and gestational diabetes being major concerns because they beget important adverse outcomes. These include stillbirth, large-for-gestational-age infants, and associated complications at birth. 9 Children born to women with gestational diabetes could themselves be at risk of metabolic disease in later life. 219
The increasing global problem of obesity in maternity care has led to national guideline recommendations for the development of interventions to improve pregnancy outcomes. 83,93 This advice stimulated many clinical trials, predominantly of behavioural interventions addressing diet and physical activity. However, most trials have been underpowered for clinical outcomes such as gestational diabetes, focusing instead on restriction of gestational weight gain. 84 Nonetheless, systematic reviews of these mostly small trials suggest the potential for prevention of gestational diabetes in women with obesity by behaviour change interventions in pregnancy. 85,220
Obesity is a risk factor for complications in pregnancy particularly gestational diabetes, large-for-gestational-age babies, and associated adverse outcomes. In a systematic review of 44 randomised controlled trials of behavioural interventions in pregnant women, irrespective of BMI, lifestyle interventions were shown to possibly improve clinical outcomes for both mother and baby. We and others have undertaken systematic reviews restricted to behavioural interventions in women with obesity, suggesting the potential for prevention of gestational diabetes. The contributing trials were mostly small scale and not powered for robust detection of differences in clinical outcomes. In the LIMIT trial of more than 2000 overweight and obese women, no reduction in gestational diabetes was recorded in individuals who took part in a lifestyle intervention, although gestational diabetes was not the primary endpoint of the trial.
Added value of this studyOur study compared a theory-based and intensive behavioural intervention with standard antenatal care for obese pregnant women from communities of ethnic diversity and high levels of socioeconomic deprivation. The intervention improved diet and physical activity, and modest reductions were noted in maternal weight gain and fat mass, but it had no effect on the incidence of gestational diabetes or large-for-gestational-age infants. Use of an oral glucose tolerance test and diagnosis of gestational diabetes with the stringent IADPSG diagnostic criteria (also used by WHO) was associated with a lower than anticipated incidence of large-for-gestational-age infants in the trial population.
InterpretationAn intervention addressing diet and physical activity in high-risk women with obesity does not prevent gestational diabetes or reduce the incidence of large-for-gestational-age infants. We recommend a shift in research focus towards improved screening for and treatment of gestational diabetes, in addition to renewed efforts towards effective public health measures that prevent obesity in women of reproductive age.
Here, we report the results of the UK Pregnancies Better Eating and Activity Trial (UPBEAT), a randomised controlled trial of a complex behavioural intervention addressing diet and physical activity versus standard antenatal care. The behavioural intervention was designed to prevent maternal gestational diabetes and reduce the incidence of large-for-gestational-age infants. By contrast with interventions tested in many previous small-scale studies,84 the intervention was more intensive in design. Findings of a pilot study have shown feasibility, acceptability, and efficacy of the intervention to change lifestyle behaviours. 150
Methods
Study design
We did this multicentre, randomised controlled trial at antenatal clinics in eight inner-city NHS Trust Hospitals in the UK – London (three centres), Bradford, Glasgow, Manchester, Newcastle, and Sunderland. The detailed study design and protocol have been published elsewhere. 186 A flow chart of the protocol is shown in the appendix (Figure 7). We did the study according to the UK’s National Institute for Health and Care Excellence (NICE) guidelines for diabetes in pregnancy, in which early pregnancy biochemistry screening for glucose intolerance and risk of gestational diabetes is not recommended. 221 The NHS research ethics committee approved the study protocol for all centres (UK integrated research application system, reference 09/H0802/5). The trial steering committee approved the protocol and the analysis plan and provided oversight of all aspects of the trial, including safety.
FIGURE 7.
Trial protocol summary. ANC; antenatal care.

Participants
We recruited women older than 16 years with a BMI of 30 kg/m2 or higher and a singleton pregnancy between 15 weeks and 18 weeks plus 6 days of gestation. We excluded individuals if they were unwilling or unable to give informed consent; if they had underlying disorders, including a pre-pregnancy diagnosis of essential hypertension, diabetes, renal disease, systemic lupus erythematosus, antiphospholipid syndrome, sickle-cell disease, thalassaemia, coeliac disease, thyroid disease, and current psychosis; or if they were currently being prescribed metformin. All participants provided written informed consent. For women who declined to participate, we recorded age, BMI, ethnic origin, socioeconomic status, and outcome data if permission was granted.
Randomisation and masking
We randomly allocated participants to either standard antenatal care or the behavioural intervention plus standard antenatal care. We used a computer-generated randomisation procedure via a password-protected website. Allocation to study groups was done by the centre’s UPBEAT trial midwife. We used minimisation, according to ethnic origin (black, white, Asian, other), parity (primiparous, multiparous), age (≤ 24, 25–29, 30–34, ≥ 35 years), BMI (30.0–34.9, 35.0–39.9, ≥ 40 kg/m2), and centre. In view of the nature of the intervention, participants and staff were aware of allocations.
Procedures
Within 1 week of randomisation, women in the intervention group attended an individual interview at their trial centre with a health trainer (a person with skills in assisting behavioural change, but not necessarily a health professional) who received coaching in all aspects of the intervention and ongoing support throughout the study period. 186 The intervention, which was informed by control theory and elements of social cognitive theory, consisted of eight further health trainer-led group or individual sessions of 1 h duration once a week for 8 weeks. 186 If a participant could not attend a session in person, the material was covered by telephone or e-mail, providing flexibility in intervention delivery. Every session addressed approaches to achieving SMART goals (i.e. specific, measurable, achievable, relevant, time-specific) and reviewed the previous week’s goals. Women assigned to the intervention received advice on self-monitoring, identification and problem-solving of barriers to behaviour change; enlisting social support; and providing opportunities for social comparison. We encouraged participants to attend all sessions and provided them with a handbook in which information was included about the intervention and the theory behind it, with recommended foods and recipes, and suggestions for physical activity. We also gave the women a DVD of an exercise regimen that was safe for pregnancy, a pedometer, and a log book for recording their weekly SMART goals. The intention of the intervention was to improve glucose tolerance through dietary and physical activity behaviour change. With the dietary intervention we aimed to promote a healthy pattern of eating but not necessarily to restrict energy intake. We tailored recommendations to the woman’s habitual diet and cultural preference, and suggested exchanging carbohydrate-rich foods with a medium-to-high glycaemic index for those with a lower glycaemic index to reduce the glycaemic load, and restricting dietary intake of saturated fat. With respect to advice on physical activity, we focused on incremental increases in walking from a pedometer- assessed baseline, tailored to pre-existing activities. The emphasis of the exercise intervention was on walking at a moderate intensity, with additional options included, particularly for women already engaging in some physical activity. Further details are available in the protocol. 186 Women in the intervention group continued with their routine antenatal care appointments.
Women who were allocated to the standard antenatal care group continued to attend routine antenatal appointments at their trial centre, according to local practice. Typically, women would attend nine appointments. Recommendations of UK guidelines are for women with obesity to be advised, at first contact with a health professional, and at no other time, about a healthy diet and the benefits of physical activity. 93,221 We did not provide any additional information, including any details of the nature of the intervention.
For diagnosis of gestational diabetes, we gave all participants an oral glucose tolerance test (75-g load) between 27 weeks and 28 weeks plus 6 days of gestation. We used diagnostic criteria recommended by the International Association of Diabetes and Pregnancy Study Groups (IADPSG) (i.e., fasting venous glucose of 5.1 mmol/l or higher, 1 h venous glucose of 10.0 mmol/l or higher, 2 h venous glucose of 8.5 mmol/l or higher, or a combination of these). 6 We used these criteria not only because of their increasing adoption globally (and by WHO) but also because of differences in routine diagnostic criteria used by trial centres. We referred women who were diagnosed with gestational diabetes for antenatal diabetic services, according to local practice at every centre.
To assess the efficacy of the behavioural intervention, we gathered maternal dietary data and physical activity scores, calculated gestational weight gain and took maternal anthropometric measurements. We used standard laboratory methods to measure biochemical outcomes between 27 weeks and 28 weeks plus 6 days of gestation.
We used a food frequency questionnaire186,222 to assess the diet of participants for the month before randomisation and for the month before the study visit at between 27 weeks and 28 weeks plus 6 days of gestation. We adapted this questionnaire from one used in the UK arm of the European Prospective Investigation into Cancer Study. 222 We used WISP 3.0 (Tinuviel Software, Llanfechell, Anglesey, UK) to calculate nutritional composition and glycaemic load per 100 g of food and beverage items. We excluded from the analysis data for participants who we estimated were under-reporting (≤ 4.5 MJ/day) and over-reporting. 223
We measured physical activity at randomisation and at the study visit between 27 weeks and 28 weeks plus 6 days of gestation. We used the International Physical Activity Questionnaire (IPAQ) and summarised data according to established methods. 186 We calculated physical activity (minutes/week) as metabolic equivalents (METs) – i.e., the ratio of energy expenditure for an activity to energy expenditure at rest – with the formula 8.0 × vigorous activity + 4.0 × moderate activity + 3.3 × light activity (walking).
At delivery of the infant, we measured and weighed the baby. We calculated customised birthweight centiles with Gestation Related Optimal Weight (GROW) software, version 6.7.5.1 (Gestation Network, Perinatal Institute, Birmingham, UK).
Outcomes
The primary maternal outcome was gestational diabetes. Pre-specified secondary outcomes included dietary measures, physical activity scores, gestational weight gain, maternal anthropometric measurements (mid-arm and thigh circumference and subscapular, triceps, biceps, and suprailiac skinfold thicknesses), and biochemical outcomes (maternal fasting plasma glucose, fasting plasma insulin, insulin resistance [calculated by homoeostatic model assessment, HOMA2-IR]213, fasting triglycerides, LDL cholesterol, HDL cholesterol, and VLDL cholesterol). We pre-specified several other secondary clinical maternal outcomes: pre-eclampsia (defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or both, on at least two occasions 4 h apart, with proteinuria ≥ 300 mg/24 h or spot urine protein to creatinine ratio ≥ 30 mg/mmol creatinine, or urine dipstick protein ≥ 2+); severe pre-eclampsia (defined as systolic blood pressure ≥ 170 mmHg, diastolic blood pressure ≥ 110 mmHg, or both, with proteinuria ≥ 500 mg/24 h or spot urine protein to creatinine ratio ≥ 50 mg/mmol creatinine, or urine dipstick protein ≥ 3+); mode of delivery (elective or emergency caesarean section, vaginal delivery, or operative vaginal delivery); induction of labour; blood loss at delivery (> 1000 ml or > 2000 ml); inpatient nights (antenatal and postnatal); referral to diabetic antenatal service after oral glucose tolerance test; and a requirement for insulin or metformin during pregnancy. Prespecified maternal secondary outcomes not reported here are listed in the Supplementary text below.
The primary neonatal outcome was delivery of a large-for-gestational-age infant, which we defined as the 90th or higher customised birthweight centile for gestational age, adjusting for maternal height and weight, ethnic origin, parity, and sex of the baby. We pre-specified several secondary neonatal outcomes: gestational age at delivery; delivery at less than 37 weeks and less than 34 weeks; birthweight; birthweight 4.0 kg or heavier, 2.5 kg or lighter, or 1.5 kg or lighter; customised birthweight centile (≥ 95th, ≤ 10th, and ≤ 5th); neonatal death; days in special care baby unit; total inpatient days; discharge home on oxygen; confirmed infection; retinopathy of prematurity; neonatal hypoglycaemia; intraventricular haemorrhage; need for mechanical ventilation and duration; necrotising enterocolitis; pulmonary haemorrhage, skinfold thicknesses and circumferences; and birthweight centiles as population centiles (≥ 90th, ≥ 95th, ≤ 10th, and ≤ 5th). Pre-specified neonatal secondary outcomes not reported here are listed in the Supplementary text.
Adverse events other than those prespecified as secondary outcomes included miscarriage, late termination of pregnancy, maternal accident, placental abruption, antenatal and postnatal sepsis, iatrogenic premature birth, intrauterine complications (fetal cardiac, renal, respiratory, and neurological), fetal death in utero, unspecified neonatal complications at birth, and confirmed neonatal sepsis.
Statistical analysis
We calculated that a sample size of 1546 women (allowing for 20% dropout) would provide at least 80% power to detect a clinically important 25% reduction in the incidence of gestational diabetes, from 30% (observed in the pilot study of 183 women)150 to 23%. From a review of published population birthweight centiles in obese UK women,16 1546 infants provided 80% power to detect a 30% relative risk reduction for large-for-gestational-age infants (17.2% to 12.0%). 215
Our analysis was by intention to treat. We expressed treatment effects for binary endpoints as risk ratios (relative risk) with 95% CIs, using binomial regression and adjusting for maternal BMI, ethnic origin, and parity (i.e. minimisation variables for intervention allocation). We calculated risk differences and did significance tests for both primary endpoints. For continuous measurements, we used linear regression with robust SEs, adjusting for baseline data or the variables used for minimisation. For physical activity data, we did median regression. For biochemical data, we did log transformations for normality, as appropriate. To check for the potential of a variable response to the intervention, we did subgroup analyses with interaction tests for BMI, ethnic origin, socioeconomic status, parity, and smoking. Moreover, to ascertain whether attendance at intervention sessions affected outcome, we did further interaction tests.
For the main analysis, we followed the missing-at-random assumption. Predictors of missingness, which we included to ensure an unbiased measure of treatment effect, were maternal BMI, ethnic origin and parity. To test the possibility of undetectable bias attributable to missing data, we did a series of analyses under different missing-not-at-random assumptions for the primary maternal and neonatal endpoints, with the Stata command rctmiss. We tested the assumptions that the odds of disease in participants with missing data were variously half or double that for women with complete data, in both study groups or in one group only. We did all analyses with Stata version 13.1.
This trial is registered with Current Controlled Trials, ISCRTN89971375.
Role of the funding source
The funders had no role in study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
From 31 March 2009 to 2 June 2014, 8820 pregnant women with a BMI of 30 kg/m2 or higher were assessed for inclusion. Of 8259 eligible individuals, 1555 (19%) gave informed consent to participate and were randomly allocated to either standard antenatal care (n = 772) or the behavioural intervention (n = 783; Figure 8). The mean BMI of participants was 36.3 kg/m2 (SD 4.8); three-quarters of women were in the two highest quintiles of the index of multiple deprivation (Table 21). Compared with 3711 individuals who declined to participate but agreed to use of routine data, participants were on average 10 months older and had a BMI that was 0.7 kg/m2 higher (Table 22).
FIGURE 8.
Trial profile.

| Standard care (n = 772) | Intervention (n = 783) | |
|---|---|---|
| Age (years) | 30.4 (5.6) | 30.5 (5.5) |
| BMI (kg/m2) | 36.3 (4.6) | 36.3 (5.0) |
| Ethnic origin | ||
| White | 483 (63%) | 490 (63%) |
| Black | 200 (26%) | 202 (26%) |
| Asian | 48 (6%) | 47 (6%) |
| Other | 41 (5%) | 44 (6%) |
| Parity | ||
| Primiparous | 338 (44%) | 336 (42%) |
| Multiparous | 434 (56%) | 447 (57%) |
| Current smoker | 60 (8%) | 48 (6%) |
| Previous history of gestational diabetes (multiparous only) | 13/434 (3%) | 19/447 (4%) |
| Family history of type 2 diabetes mellitus | 181/767 (24%) | 194/772 (25%) |
| Family history of gestational diabetes | 20/742 (3%) | 38/760 (5%) |
| Index of multiple deprivationa | ||
| 1 (least deprived fifth) | 36 (5%) | 29 (4%) |
| 2 | 44 (6%) | 59 (8%) |
| 3 | 84 (11%) | 93 (12%) |
| 4 | 289 (37%) | 245 (31%) |
| 5 (most deprived fifth) | 318 (41%) | 352 (45%) |
| Standard care | Intervention | Effect of intervention (95% CI) | p | ||
|---|---|---|---|---|---|
| Risk ratio (95% CI) | Mean difference (95% CI) | ||||
| Gestational diabetes | 172/651 (26%) | 160/629 (25%) | 0.96 (0.79 to 1.16) | –1.2% (–5.8 to 3.8)a | 0.68 |
| Fasting blood glucose (mmol/l) | 4.71 (0.6), n = 651 | 4.68 (0.6), n = 629 | –0.02 (–0.09 to 0.04) | 0.49 | |
| 1 h blood glucose (mmol/l) | 8.02 (2.1), n = 605 | 7.91 (2.1), n = 584 | –0.10 (–0.33 to 0.14) | 0.43 | |
| 2 h blood glucose (mmol/l) | 5.94 (1.5), n = 650 | 5.96 (1.5), n = 628 | 0.02 (–0.15 to 0.19) | 0.81 | |
| Treatment of gestational diabetesb | |||||
| Dietary advice | 69/146 (47%) | 62/127 (49%) | 1.03 (0.81 to 1.32) | 0.80 | |
| Metformin | 35/146 (24%) | 34/127 (27%) | 1.12 (0.74 to 1.68) | 0.60 | |
| Metformin and insulin | 16/146 (11%) | 14/127 (11%) | 1.01 (0.51 to 1.98) | 0.99 | |
| Insulin | 26/146 (18%) | 17/127 (13%) | 0.75 (0.43 to 1.32) | 0.32 | |
| All pre-eclampsia | 27/752 (4%) | 27/753 (4%) | 1.00 (0.59 to 1.69) | > 0.99 | |
| Severe pre-eclampsia | 10/752 (1.3%) | 6/753 (0.8%) | 1.64 (0.60 to 4.49) | 0.33 | |
| Labour and delivery | |||||
| Induction of labour | 275/757 (36%) | 251/765 (33%) | 0.90 (0.79 to 1.04) | 0.15 | |
| Unassisted vaginal | 399/757 (52%) | 400/765 (52%) | 0.99 (0.90 to 1.09) | 0.87 | |
| Operative vaginal | 84/757 (11%) | 94/765 (12%) | 1.11 (0.84 to 1.46) | 0.47 | |
| Caesarean section | 274/757 (36%) | 271/765 (35%) | 0.98 (0.86 to 1.12) | 0.75 | |
| Elective caesarean section | 136/757 (18%) | 160/765 (21%) | 1.16 (0.95 to 1.43) | 0.15 | |
| Emergency caesarean section | 138/757 (18%) | 111/765 (14%) | 0.80 (0.63 to 1.00) | 0.051 | |
| Postpartum haemorrhage | |||||
| ≥ 1000 ml | 91/747 (12%) | 109/755 (14%) | 1.19 (0.91 to 1.54) | 0.20 | |
| ≥ 2000 ml | 10/747 (1%) | 20/755 (3%) | 1.98 (0.93 to 4.20) | 0.075 | |
| Inpatient nights (n) | 2.3 (1.8), n = 691 | 2.4 (2), n = 691 | 0.14 (–0.06 to 0.34) | 0.16 | |
| Antenatal | 2.9 (2.5), n = 65 | 2.9 (3), n = 74 | –0.02 (–0.98 to 0.95) | 0.98 | |
| Postnatal | 2.2 (1.7), n = 685 | 2.3 (2), n = 684 | 0.08 (–0.09 to 0.25) | 0.37 | |
| Gestational weight gain (kg)c | |||||
| Total | 7.76 (4.6), n = 567 | 7.19 (4.6), n = 526 | –0.55 (–1.08 to –0.02) | 0.041 | |
| Before pregnancy to 27–28 weeks + 6 days | 5.40 (3.3), n = 664 | 4.97 (2.9), n = 637 | –0.42 (–0.75 to –0.09) | 0.013 | |
| Mid-arm circumference (cm) | |||||
| 15–18 weeks + 6 days | 36.8 (4.0), n = 766 | 36.7 (4.1), n = 775 | |||
| 27–28 weeks + 6 days | 36.9 (4.2), n = 663 | 36.6 (4.0), n = 634 | –0.19 (–0.39 to 0.01) | 0.063 | |
| 34–36 weeks + 0 days | 36.6 (4.1), n = 567 | 36.5 (3.9), n = 526 | –0.10 (–0.32 to 0.13) | 0.40 | |
| Thigh circumference (cm) | |||||
| 15–18 weeks + 6 days | 68.6 (6.5), n = 766 | 68.6 (6.8), n = 775 | |||
| 27–28 weeks + 6 days | 69.2 (6.8), n = 662 | 68.9 (6.6), n = 635 | –0.10 (–0.54 to 0.33) | 0.64 | |
| 34–36 weeks + 0 days | 69.3 (6.7), n = 566 | 68.9 (7.0), n = 526 | –0.48 (–1.01 to 0.05) | 0.078 | |
| Sum of skinfold thicknesses (mm)d | |||||
| 15–18 weeks + 6 days | 123 (27), n = 763 | 123 (29), n = 771 | |||
| 27–28 weeks + 6 days | 127 (26), n = 661 | 124 (27), n = 632 | –2.3 (–4.3 to –0.3) | 0.022 | |
| 34–36 weeks + 0 days | 25 (27), n = 561 | 122 (26), n = 520 | –3.2 (–5.6 to –0.8) | 0.0081 | |
| Plasma fasting insulin (mU/l) | 23.2 (2.4), n = 510 | 22.48 (2.3), n = 480 | 0.97 (0.87 to 1.08)e | 0.57 | |
| HOMA2-IR (units) | 3.04 (2.1), n = 496 | 2.99 (2.1), n = 471 | 0.98 (0.89 to 1.07)e | 0.60 | |
| Plasma triglycerides (mmol/l) | |||||
| 27–28 weeks + 6 days | 1.98 (1.41), n = 505 | 1.92 (1.40), n = 478 | 0.99 (0.96 to 1.02)e | 0.39 | |
| Plasma LDL cholesterol (mmol/l) | |||||
| 27–28 weeks + 6 days | 3.66 (1.31), n = 509 | 3.66 (1.35), n = 479 | 1.01 (0.99 to 1.04)e | 0.27 | |
| Plasma HDL cholesterol (mmol/l) | |||||
| 27–28 weeks + 6 days | 1.80 (1.29), n = 509 | 1.80 (1.28), n = 479 | 1.00 (0.98 to 1.02)e | 0.93 | |
| Plasma VLDL cholesterol (mmol/l)f | |||||
| 27–28 weeks + 6 days | 0.40 (1.41), n = 505 | 0.38 (1.40), n = 478 | 0.99 (0.96 to 1.02)e | 0.39 | |
On average, women who were assigned the intervention attended seven (SD 3) of eight health trainer-led sessions, including four in person, and a further three by telephone or e-mail. For sessions attended in person, 30% of women attended only one session, and 46% attended fewer than four. For sessions delivered by any method, 10% of women received only one session and 17% had fewer than four.
A total of 629 (80%) women in the intervention group and 651 (84%) in the standard care group had an oral glucose tolerance test and could be assessed for the primary maternal outcome. Demographic variables were similar between groups for women with primary outcome data (Table 23). The main reason for missing outcome data was that participants declined to attend further study visits (see Figure 8). 129 (16%) women in the intervention group failed to complete the oral glucose tolerance test compared with 92 (12%) in the standard care group (p = 0.02).
| Standard care | Intervention | Mean difference (95%CI) | p-value | |
|---|---|---|---|---|
| Nutrition | ||||
| Total energy (MJ/day) | ||||
| 15–18 weeks + 6 days | 7.8 (2.6) | 7.6 (2.5) | ||
| 27–28 weeks + 6 days | 7.5 (2.3) | 6.8 (1.9) | –0.70 (–0.96 to –0.45) | < 0.0001 |
| Glycaemic index | ||||
| 15–18 weeks + 6 days | 56.9 (4.1) | 56.8 (3.9) | ||
| 27–28 weeks + 6 days | 57.0 (3.9) | 54.3 (3.9) | –2.6 (–3.0 to –2.1) | < 0.0001 |
| Glycaemic load | ||||
| 15–18 weeks + 6 days | 141 (56) | 135 (51) | ||
| 27–28 weeks + 6 days | 133 (47) | 112 (38) | –21 (–26 to –16) | < 0.0001 |
| Carbohydrate (% energy) | ||||
| 15–18 weeks + 6 days | 49.4 (7.4) | 49.0 (7.4) | ||
| 27–28 weeks + 6 days | 48.6 (6.6) | 47.2 (7.2) | –1.4 (–2.2 to –0.58) | 0.0011 |
| Protein (% energy) | ||||
| 15–18 weeks + 6 days | 19.7 (4.4) | 20.1 (4.5) | ||
| 27–28 weeks + 6 days | 20.1 (4.0) | 22.3 (4.6) | 2.05 (1.5 to 2.5) | < 0.0001 |
| Total fat (% energy) | ||||
| 15–18 weeks + 6 days | 31.0 (5.5) | 31.0 (5.3) | ||
| 27–28 weeks + 6 days | 31.5 (5.1) | 30.5 (5.2) | –0.88 (–1.49 to –0.26) | 0.0011 |
| Saturated fat (g/day) | ||||
| 15–18 weeks + 6 days | 26.5 (11.5) | 25.4 (11.0) | ||
| 27–28 weeks + 6 days | 26.4 (10.9) | 22.0 (8.3) | –4.3 (–5.4 to –3.1) | < 0.0001 |
| Saturated fat (% energy) | ||||
| 15–18 weeks + 6 days | 12.7 (3.0) | 12.5 (2.9) | ||
| 27–28 weeks + 6 days | 13.1 (3.0) | 12.1 (2.8) | –0.85 (–1.2 to –0.51) | < 0.0001 |
| Fibre (g/day) | ||||
| 15–18 weeks + 6 days | 13.6 (6.0) | 13.1 (5.3) | ||
| 27–28 weeks + 6 days | 12.6 (5.3) | 13.4 (5.3) | 0.83 (0.17 to 1.48) | 0.013 |
| Physical activity | ||||
| MET (minutes per week) | ||||
| 15–18 weeks + 6 days | 1386 (660–3052) | 1386 (594–2982) | ||
| 27–28 weeks + 6 days | 1386 (639–3363) | 1836 (792–4158) | 295 (105 to 485)a | 0.0015 |
| Moderate or vigorous activity (minutes/week) | ||||
| 15–18 weeks + 6 days | 0.0 (0.0–180) | 0.0 (0–180) | ||
| 27–28 weeks + 6 days | 0.0 (0.0–240) | 30 (0.0–240) | 0.0 (–18 to 18) | > 0.99 |
| Walking (minutes/week) | ||||
| 15–18 weeks + 6 days | 280 (140–600) | 280 (140–540) | ||
| 27–28 weeks + 6 days | 300 (132–630) | 420 (180–840) | 77 (28 to 126) | 0.0018 |
The incidence of gestational diabetes was similar between groups (Table 24). Of women who had an oral glucose tolerance test, 10 women in the intervention group and eight in the standard care group had their test done outside the predefined period. A sensitivity analysis excluding all data obtained outside this period gave similar results to the main analysis [intervention 150 (25%) of 589 vs standard care 164 (27%) of 618; risk ratio 0.96, 95% CI 0.79–1.16; p = 0.67].
| Standard care | Intervention | Effect of intervention (95% confidence interval) | p | ||
|---|---|---|---|---|---|
| Risk ratio | Mean difference (95% CI) | ||||
| Large for gestational age (customised birth weight centiles) | |||||
| ≥ 90th | 61/751 (8%) | 71/761 (9%) | 1.15 (0.83–1.59) | 1.2% (–1.6 to 4.1)a | 0.40 |
| ≥ 95th | 32/751 (4%) | 39/761 (5%) | 1.20 (0.76–1.90) | 0.43 | |
| ≤ 10th | 76/751 (10%) | 95/761 (13%) | 1.24 (0.93–1.64) | 0.15 | |
| ≤ 5th | 43/751 (6%) | 36/761 (5%) | 0.83 (0.54–1.27) | 0.39 | |
| Population birthweight centiles | |||||
| ≥ 90th | 83/750 (11%) | 96/761 (13%) | 1.14 (0.87–1.50) | 0.35 | |
| ≥ 95th | 42/750 (6%) | 51/761 (7%) | 1.20 (0.81–1.78) | 0.37 | |
| ≤ 10th | 38/750 (5%) | 53/761 (7%) | 1.38 (0.92–2.06) | 0.12 | |
| ≤ 5th | 19/750 (3%) | 22/761 (3%) | 1.14 (0.62–2.09) | 0.67 | |
| Birthweight (kg) | 3450 (580), n = 751 | 3420 (580), n = 761 | –27 (–85 to 31) | 0.37 | |
| ≥ 4 | 105/751 (14%) | 105/761 (14%) | 0.99 (0.77–1.27) | 0.93 | |
| ≤ 2.5 | 36/751 (5%) | 31/761 (4%) | 0.85 (0.53–1.36) | 0.50 | |
| ≤ 1.5 | 9/751 (1%) | 7/761 (1%) | 0.77 (0.29–2.05) | 0.60 | |
| Gestational age at birth (weeks) | 39.5 (2.4), n = 751 | 39.5 (2.0), n = 761 | 0.02 (–0.2 to 0.2) | 0.89 | |
| Delivery ≤ 37 weeks | 48/751 (6%) | 45/761 (7%) | 0.93 (0.62–1.37) | 0.70 | |
| Delivery ≤ 34 weeks | 16/751 (2%) | 15/761 (2%) | 0.93 (0.46–1.86) | 0.83 | |
| Hospital admission | |||||
| Admission to neonatal unit | 57/751 (8%) | 65/761 (9%) | 1.13 (0.80–1.58) | 0.49 | |
| Time spent in neonatal unit, if admitted (days) | 16.8 (30.2), n = 52 | 11.6 (23.5), n = 61 | –0.26 (–9.65 to 9.13) | 0.96 | |
| Time spent in hospital after birth, if admitted (days) | 3.0 (9.0), n = 733 | 2.8 (7.3), n = 743 | –0.06 (–0.86 to 0.74) | 0.88 | |
| Neonatal death | 2/771 (< 1%) | 3/783 (< 1%) | 0.98 (0.14–6.97) | 0.99 | |
| Intraventricular haemorrhage grade 3–4 | 2/751 (< 1%) | 0/760 | |||
| Retinopathy of prematurity | 1/751 (< 1%) | 1/760 (< 1%) | 0.99 (0.06–15.7) | 0.99 | |
| Discharged home on oxygen | 4/751 (1%) | 2/760 (< 1%) | 0.49 (0.09–2.69) | 0.41 | |
| Neonatal hypoglycaemia | 12/751 (2%) | 27/760 (4%) | 2.22 (1.13–4.36) | 0.020 | |
| Confirmed infection | 14/751 (2%) | 7/760 (1%) | 0.49 (0.20–1.22) | 0.13 | |
| Congenital abnormalities | 6/751 (1%) | 5/760 (1%) | 0.82 (0.25–2.69) | 0.75 | |
| Mechanical ventilation | 21/751 (3%) | 19/760 (3%) | 0.89 (0.48–1.65) | 0.72 | |
| Duration of mechanical ventilation (h) | 500 (885), n = 20 | 330 (573), n = 16 | –170 (–667 to 327) | 0.49 | |
| Necrotising enterocolitis | 2/751 (< 1%) | 0/760 | |||
| Pulmonary haemorrhage | 2/751 (< 1%) | 1/760 (< 1%) | 0.49 (0.04–5.43) | 0.56 | |
Compared with women assigned standard antenatal care, glycaemic index was reduced in participants assigned the intervention, as was mean intake of total energy, carbohydrate, saturated fat, and total fat; protein and fibre intake was increased (Table 25). Physical activity was higher at 27–28 weeks plus 6 days of gestation in women in the intervention group versus the standard care group, which was attributable to more time spent walking (see Table 25).
| Standard care (n = 772) | Intervention (n = 783) | P a | |
|---|---|---|---|
| All miscarriage | 14 | 18 | 0.50 |
| Late termination of pregnancy | 3 | 1 | |
| Maternal accident | 1 | 0 | |
| Placental abruption | 0 | 1 | |
| Maternal antenatal sepsis | 1 | 0 | |
| Maternal postnatal sepsis | 1 | 0 | |
| Iatrogenic preterm birth | 2 | 2 | |
| Intrauterine complications (cardiac, neurological, renal, respiratory) | 2 | 3 | |
| Fetal death in utero | 10 | 6 | 0.30 |
| Unspecified neonatal complications at birth | 2 | 1 | |
| Neonatal sepsis | 1 | 0 |
Women in the intervention group had less gestational weight gain than did those in the standard care group at the time of the oral glucose tolerance test, and over the entire pregnancy (see Table 24). The sum of maternal skinfold thicknesses was also lower with the intervention at 27–28 weeks plus 6 days of gestation and at 34–36 weeks’ gestation (see Table 24). Mode of delivery, postpartum haemorrhage, or treatment of gestational diabetes did not differ between groups; likewise, no differences were noted between groups in fasting glucose, fasting insulin, or HOMA2-IR, or in any other biochemical variables measured at 27–28 weeks plus 6 days of gestation (see Table 24).
A total of 761 infants born to women allocated the intervention and 751 infants born to mothers in the standard care group had a known birthweight and could be assessed for the primary neonatal outcome (≥ 90th customised birthweight centile; Table 26). The incidence of large-for-gestational-age infants did not differ between groups. Similar results were recorded in a sensitivity analysis allowing for possible selective bias in missing data (odds ratio 0.95, 95% CI 0.72–1.25, assuming a halving of the odds of large-for-gestational-age infants in the intervention group with missing data).
| Declined randomisation (n = 3711) | Randomised (n = 1555) | Risk ratio (95% CI) | Mean difference (95% CI) | p-value | |
|---|---|---|---|---|---|
| Age (years) | 29.7 (5.7) | 30.5 (5.5) | 0.82 (0.49 to 1.15) | < 0.0001 | |
| Body mass index (kg/m2) (n = 3269; 1555) | 35.1 (4.5) | 35.7 (4.8) | 0.67 (0.38 to 0.96) | < 0.0001 | |
| Ethnicity (n = 3569, 1555) | |||||
| White | 2055 (58%) | 973 (63%) | 1.09 (1.04 to 1.14) | 0.001 | |
| Black | 1117 (31%) | 402 (26%) | 0.83 (0.75 to 0.91) | 0.0001 | |
| Asian | 212 (6%) | 95 (6%) | 1.03 (0.81 to 1.30) | 0.81 | |
| Other | 185 (5%) | 85 (5%) | 1.05 (0.82 to 1.35) | 0.68 | |
| Index of multiple deprivation (n = 3493; 1549)a | |||||
| 1 (Least deprived) | 115 (3%) | 65 (4%) | 1.27 (0.95 to 1.72) | 0.11 | |
| 2 | 186 (5%) | 103 (7%) | 1.25 (0.99 to 1.58) | 0.06 | |
| 3 | 377 (11%) | 177 (11%) | 1.06 (0.89 to 1.25) | 0.51 | |
| 4 | 1222 (35%) | 534 (35%) | 0.99 (0.91 to 1.07) | 0.73 | |
| 5 (Most deprived) | 1593 (46%) | 670 (44%) | 0.95 (0.89 to 1.01) | 0.13 | |
By population birthweight centiles (secondary outcome), 12% of infants were in the 90th centile or higher, and there was no difference between groups. Similarly, other neonatal secondary outcomes did not differ between groups, with the exception of neonatal hypoglycaemia, which was increased in the intervention group (see Table 26). As neonatal hypoglycaemia is treatable, it is not judged a severe adverse event. Neonatal anthropometric measures were evaluated in a subgroup of infants and did not differ between groups (Table 27).
| Standard care (n = 651) | Intervention (n = 629) | Risk ratio (95% CI) | Mean difference (95% CI) | |
|---|---|---|---|---|
| Age (years) | 30.7 (5.5) | 30.9 (5.3) | 0.26 (–0.33 to 0.86) | |
| Body mass index (kg/m2) | 36.3 (4.6) | 36.2 (4.9) | –0.13 (–0.65 to 0.40) | |
| Ethnicity | ||||
| White | 422 (65%) | 401 (64%) | 0.98 (0.91 to 1.07) | |
| Black | 156 (24%) | 155 (25%) | 1.03 (0.85 to 1.25) | |
| Asian | 41 (65) | 38 (6%) | 0.96 (0.63 to 1.47) | |
| Other | 32 (5%) | 35 (6%) | 1.13 (0.71 to 1.81) | |
| Current smoker | 50 (8%) | 36 (6%) | 0.75 (0.49 to 1.13) | |
| Previous history of gestational diabetes (multiparous only) | 10/362 (3%) | 15/351 (4%) | 1.55 (0.70 to 3.40) | |
| Family history of type 2 diabetes | 152/647 (24%) | 152/621 (25%) | 1.04 (0.86 to 1.27) | |
| Family history of gestational diabetes | 18/631 (3%) | 26/624 (4%) | 0.85 (0.51 to 1.40) | |
| Index of multiple deprivation (n = 650, 624)a | ||||
| 1 (Least deprived) | 32 (5%) | 26 (4%) | ||
| 2 | 42 (6%) | 47 (8%) | 1.17 (0.78 to 1.74) | |
| 3 | 74 (11%) | 75 (12%) | 1.06 (0.78 to 1.43) | |
| 4 | 243 (37%) | 200 (32%) | 0.86 (0.74 to 1.00) | |
| 5 (Most deprived) | 259 (40%) | 276 (44%) | 1.11 (0.98 to 1.26) | |
Table 28 shows adverse events that were not pre-specified as secondary outcomes. Adverse events did not differ between intervention and standard care groups.
| Standard care | Intervention | Mean difference | p-value | |
|---|---|---|---|---|
| Head circumference (cm) | N = 695; 34.7 (1.8) | N = 708; 34.7 (1.8); | –0.05 (–0.23 to 0.14) | 0.63 |
| Abdominal circumference (cm) | N = 311; 32.6 (2.1) | N = 285; 32.6 (2.5); | –0.01 (–0.39 to 0.36) | 0.95 |
| Crown rump (cm) | N = 177; 33.5 (2.2) | N = 155; 33.5 (2.0) | 0.02 (–0.44 to 0.48) | 0.93 |
| Triceps skinfold thickness (mm) | N = 268; 5.3 (1.6) | N = 249; 5.3 (1.4) | 0.05 (–0.21 to 0.31) | 0.72 |
| Subscapular skinfold thickness (mm) | N = 258; 5.6 (1.4) | N = 244; 5.7 (1.4) | 0.06 (–0.20 to 0.31) | 0.66 |
| Sum of skinfold thickness (mm) | N = 258; 10.9 (2.7) | N = 244; 11.0 (2.6) | 0.13 (–0.34 to 0.59) | 0.59 |
Interaction tests for prespecified maternal demographic variables (BMI, ethnic origin, socioeconomic status, parity, and smoking) did not differ between standard care and intervention groups for the primary maternal or neonatal outcomes (Tables 29 and 30). Furthermore, no differences were recorded in maternal and neonatal primary outcomes with respect to whether the intervention had been delivered mainly in person or by telephone or e-mail (maternal p = 0.39; neonatal p = 0.54), nor for women who attended more versus less than half the health trainer-led sessions (maternal p = 0.56; neonatal p = 0.59).
| Standard care N (%) | Intervention N (%) | Risk ratio (95% CI) | Wald test for interaction | ||||
|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | 30–34.9 | 294 | 62 (21.1) | 300 | 56 (18.7) | 0.89 (0.64 to 1.22) | 0.249 |
| 35–39.9 | 210 | 59 (28.1) | 180 | 60 (33.3) | 1.19 (0.88 to 1.60) | ||
| ≥ 40 | 109 | 34 (31.2) | 114 | 43 (37.7) | 0.83 (0.57 to 1.19) | ||
| Ethnicity | Asian | 13 | 37 (35.1) | 35 | 11 (31.4) | 0.89 (0.46 to 1.72) | 0.434 |
| Black | 148 | 41 (27.7) | 146 | 36 (24.7) | 0.89 (0.61 to 1.31) | ||
| Other | 33 | 13 (39.4) | 32 | 7 (21.9) | 1.80 (0.83 to 3.93) | ||
| White | 375 | 90 (24.0) | 401 | 103 (25.7) | 0.93 (0.73 to 1.19) | ||
| Index of multiple deprivation | 1 (Least deprived) | 31 | 4 (12.9) | 23 | 5 (21.7) | 1.68 (0.51 to 5.59) | 0.163 |
| 2 | 42 | 13 (31.0) | 43 | 4 (9.3) | 0.30 (0.11 to 0.85) | ||
| 3 | 69 | 20 (29.0) | 72 | 17 (23.6) | 0.81 (0.47 to 1.42) | ||
| 4 | 227 | 56 (24.7) | 183 | 48 (26.2) | 1.06 (0.76 to 1.48) | ||
| 5 (Most deprived) | 248 | 70 (28.2) | 263 | 76 (28.9) | 1.02 (0.78 to 1.35) | ||
| Parity | Nullip | 273 | 70 (25.6) | 260 | 61 (23.5) | 0.92 (0.68 to 1.23) | 0.680 |
| Multip | 345 | 94 (27.2) | 329 | 89 (27.1) | 0.99 (0.78 to 1.27) | ||
| Current smoking | Non-smoker | 570 | 149 (26.1) | 554 | 139 (25.1) | 0.96 (0.79 to 1.17) | 0.892 |
| Smoker | 48 | 15 (31.3) | 35 | 11 (31.4) | 1.01 (0.53 to 1.92) | ||
| Standard care n (%) | Intervention n (%) | Risk ratio (95% CI) | Wald test for interaction | ||||
|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | 30–34.9 | 367 | 24 (6.5) | 381 | 38 (10.0) | 1.53 (0.93 to 2.49) | 0.167 |
| 35–39.9 | 250 | 17 (6.8) | 238 | 18 (7.6) | 1.11 (0.59 to 2.11) | ||
| ≥ 40 | 134 | 20 (14.9) | 142 | 15 (10.6) | 0.71 (0.38 to 1.32) | ||
| Ethnicity | Asian | 47 | 5 (10.6) | 43 | 5 (11.6) | 1.09 (0.34 to 3.52) | 0.987 |
| Black | 193 | 16 (8.3) | 194 | 18 (9.3) | 1.12 (0.59 to 2.13) | ||
| Other | 40 | 3 (7.5) | 44 | 3 (6.8) | 0.91 (0.19 to 4.25) | ||
| White | 471 | 37 (7.9) | 480 | 45 (9.4) | 1.19 (0.79 to 1.81) | ||
| Index of multiple deprivation | 1 (Least deprived) | 33 | 2 (6.1) | 29 | 4 (13.8) | 2.28 (0.45 to 11.53) | 0.460 |
| 2 | 43 | 4 (9.3) | 57 | 7 (12.3) | 1.32 (0.41 to 4.22) | ||
| 3 | 84 | 5 (6.0) | 86 | 10 (11.6) | 1.95 (0.70 to 5.47) | ||
| 4 | 277 | 19 (6.9) | 241 | 21 (8.7) | 1.27 (0.70 to 2.31) | ||
| 5 (Most deprived) | 313 | 31 (9.9) | 343 | 28 (8.2) | 0.82 (0.51 to 1.34) | ||
| Parity | Nullip | 332 | 19 (5.7) | 325 | 29 (8.9) | 1.56 (0.89 to 2.72) | 0.169 |
| Multip | 419 | 42 (10.0) | 436 | 42 (9.6) | 0.96 (0.64 to 1.44) | ||
| Current smoking | Non-smoker | 693 | 56 (8.1) | 714 | 66 (9.2) | 1.14 (0.81 to 1.61) | 0.904 |
| Smoker | 58 | 5 (8.6) | 47 | 5 (10.6) | 1.23 (0.38 to 4.01) | ||
Discussion
Our findings suggest that a complex intervention addressing diet and physical activity in pregnant women with obesity is effective at improving diet quality and physical activity, reducing gestational weight gain, and decreasing surrogate measures of maternal body fatness. However, the intervention does not prevent the development of gestational diabetes nor change the incidence of large-for-gestational-age infants in this population. Neither was evidence noted of a benefit on other pregnancy outcomes, including pre-eclampsia, which is associated with raised BMI.
By contrast with previous systematic reviews and meta-analyses of studies on a smaller scale to ours,85,220 our null finding extends some observations. In particular, in two Danish studies of lifestyle interventions,224,225 more than 350 obese pregnant women in each study were screened with an oral glucose tolerance test. Although analysis was not by intention-to-treat, a reduction in the primary outcome of gestational weight gain of around 1.5 kg was recorded in both studies, but gestational diabetes was not decreased. In the Australian LIMIT randomised controlled trial in 2212 overweight and obese pregnant women,226 a lifestyle intervention less intense than ours (in terms of frequency and personal contact) had no effect on gestational diabetes (a secondary outcome). Furthermore, no difference was noted in the proportion of large-for- gestational-age infants (the primary outcome) or in gestational weight gain, but the proportion of babies 4 kg or heavier at birth was lower in the intervention group. 226 The inference from systematic reviews that pregnancy lifestyle interventions might be an effective means to prevent gestational diabetes in women with obesity seems to have been biased by small-scale studies and methodological limitations. 84
On average, seven of the eight intervention sessions were attended by women assigned to the intervention, whether in person or by telephone or e-mail. There was no indication that failure of adherence, mode of session delivery, ethnic origin, or socioeconomic status of the women affected the primary outcomes. Further planned analyses will ascertain whether coverage of sessions affected specific elements of dietary and physical activity behavioural change. Measurement of several biomarkers of glucose intolerance and insulin resistance, as well as the metabolome, at study entry and after the intervention will also establish whether early risk stratification can identify a subgroup of women in whom the intervention could show clinical benefit.
Our study was set in UK inner-city settings of ethnic diversity and high socioeconomic deprivation. Black women were the predominant minority ethnic subgroup (26%); individuals of this ethnic origin have a high risk of obesity in pregnancy in the UK,140 which, as elsewhere, is strongly related to socioeconomic deprivation. Similar to previous studies,224–226 large numbers of women had to be approached to meet our recruitment target, and the drop-out rate in our study for oral glucose tolerance testing was similar to previous studies. 224,225 The reluctance of pregnant women with obesity to take part in a complex behavioural intervention suggests that lifestyle interventions can improve healthy behaviours, but only in a subgroup of motivated individuals. Likewise, the 5% higher proportion of women who dropped out of the study from the intervention group than the standard care group, although a limitation, was not unexpected. Despite small numerical differences, participants were similar to individuals who declined participation with respect to demographic characteristics, suggesting generalisability of outcomes to populations of this demographic complexity. 220
The self-reported reduction in glycaemic load in the intervention group was larger than that noted in previous similar pregnancy intervention studies,115,226 a potential reflection of the intensity of our intervention, which included motivational interviewing every week for 8 weeks, goal-setting, and behavioural self-monitoring. 227 Together with reduced intake of saturated fat and total energy in the intervention group, these outcomes could be the reasons for the modest lowering of gestational weight gain and measures of fat mass noted in our study. Although we acknowledge the limitations of dietary assessment by self-report, the size of the improvement was similar to that recorded in the pilot trial,150 in which a more rigorous assessment method was used. Thus, we conclude that the behavioural intervention increases healthy dietary behaviours, but that the modest size of the effect is inadequate to reduce the risk of gestational diabetes or improve insulin sensitivity in women who are obese at the time of conception.
The incremental rise in physical activity achieved with the intervention was also inadequate to improve glucose tolerance. A minimum of 16 MET h/week of physical activity has been suggested to be needed to reduce the risk of gestational diabetes,228 which equates to 41 minutes/day of walking; this amount is well above the 12- to 13-minute increase (or < 1 mile) reported by women in our study, which was similar to the increase in physical activity reported in the LIMIT trial intervention group. 226 Again, we are aware of the limitations in accuracy of self-report; indeed, in the pilot trial, physical activity was assessed by accelerometry, and no increase in exercise levels was reported in the intervention group compared with women in the standard care group. 212 However, this method of objective assessment is recognised to be ineffective at measuring low-intensity activity that, as we report here, was increased by self-report.
Although not the primary maternal outcome of this study, the 0.55 kg lower gestational weight gain in the intervention group compared with the standard care group adds to growing evidence from other studies that a substantial reduction in gestational weight gain is unlikely to be achievable in women with obesity through interventions addressing diet, physical activity, or both. 224,225 The reduction achieved was less than that reported in a meta-analysis of previous studies (–2.41 kg),7 which could reflect our rigorous trial method (i.e., intention-to-treat analysis), the high mean BMI, ethnic diversity, and low socioeconomic status of the UPBEAT participants, or that gestational weight gain was not the main focus of this study.
Ongoing follow-up of mothers and their children in the UPBEAT study will ascertain whether the changes recorded in diet, physical activity, and maternal anthropometric measures are sustained or extended and can benefit maternal and child health in the longer term. Although gestational diabetes was not prevented, the behavioural intervention has the potential to reduce the risk of obesity and adverse metabolic risk in the child, because excessive gestational weight gain, high maternal fat mass, and increased glycaemic load are all associated independently with greater adiposity in the offspring, potentially through epigenetic pathways. 219,229,230
We had anticipated that 17% of babies in our study would be in the 90th centile or higher, whereas the recorded incidence was 9% and 12% by customised and population centiles, respectively. This incidence is well below the 16% reported in UK women with similar BMI (range 35.0–39.9 kg/m2),231 and roughly half of that noted in the LIMIT trial (20%), which included women who had a lower BMI. 226 Our use of IADPSG criteria for diagnosis of gestational diabetes could partly explain the low incidence of large-for-gestational-age babies in our study. To our knowledge, no previous study of women with obesity has diagnosed gestational diabetes with these criteria, and a quarter of women in both groups in our trial had gestational diabetes. Only 9% would have had a diagnosis of gestational diabetes had we used the previous WHO guidelines. 221 Diagnosis and treatment of more women with gestational diabetes in this study compared with current clinical practice in the UK could, therefore, account for the lower incidence of large-for-gestational-age infants to roughly population levels (10%). In line with this notion, a 50% reduction in large-for-gestational-age infants was reported after treatment of women with mild gestational diabetes5 that fell below conventional diagnostic thresholds but would have been treated by the new criteria. Women were treated according to local practice at trial centres, 83% receiving treatment after a diagnosis of gestational diabetes. Although local practice might have differed, randomisation was minimised to centre, and variable practice is unlikely to have affected primary trial outcomes. Indeed, had all women been treated, as recommended by the IADPSG, the incidence of large-for-gestational-age infants might have been reduced further. Universal testing of all participants in our study for gestational diabetes, independent of the diagnostic criteria, might have contributed to the difference between trial and population incidences of large-for-gestational-age infants because, despite NICE recommendations, universal testing of women with obesity is not adopted across the UK. 221
Several neonatal outcomes, including birthweight and inpatient days, were lower than UK outcomes for women with obesity,231 although caesarean section rates were similar, potentially a reflection of current management of women with a diagnosis of gestational diabetes. Participation in a clinical trial is in itself unlikely to be a cause of lower than anticipated incidence of large-for-gestational-age infants because no evidence for such an effect was noted in the LIMIT trial, in which the incidence of large-for-gestational-age infants was 20%. 226 Comparison of the incidence of large-for-gestational-age infants with eligible women who declined participation was precluded because those with available birthweight data had a significantly lower BMI than did the group as a whole.
Our study highlights the need for randomised controlled trials in women with obesity that do universal testing and formally compare IADPSG and older diagnostic criteria for gestational diabetes. In the UK, comparison should be made with the most recent NICE criteria, which do not align with IADPSG. 221
More infants born to mothers in the intervention group developed neonatal hypoglycaemia than did those in the standard care group, but statistical power for this outcome was low. This finding contrasts with that of a meta-analysis of smaller lifestyle intervention studies, which showed no effect. 82 Ten infants in the intervention group with hypoglycaemia were fed formula milk from birth, compared with two in the standard care group (37% vs. 16%; p = 0.04). Since early introduction of formula feeding has been associated with neonatal hypoglycaemia,232 this factor could be contributory. The rates of exclusive breastfeeding (p = 0.73) or formula feeding (p = 0.63) did not differ at neonatal discharge between the two study groups; therefore, this finding is likely to be attributable to chance.
The behavioural intervention we assessed in this study could provide a means to improve healthy behaviours in obese pregnant women. It offers an alternative to current UK NICE guidelines,93 which recommend general healthy eating and physical activity for pregnant women with obesity with little evidence for proven change in behaviours. The potential benefit of the intervention on post-pregnancy infant health and on maternal and infant long-term health needs further investigation, which is under way. Increasing the intensity and duration of the intervention, which is already greater than that adopted in previous studies,84,224,226,227 is likely to be impractical for most women with obesity.
The current focus on behavioural interventions to prevent gestational diabetes would seem to be misplaced. The intervention we assessed could be used as an evidence-based method to encourage healthy dietary and physical activity behaviours in women with obesity. However, efforts to prevent gestational diabetes should be diverted towards not only trials of targeted interventions, including pharmacotherapy but also establishing optimum diagnostic criteria for gestational diabetes to reduce risk of adverse outcomes. Importantly, renewed efforts are needed at the population level to prevent obesity in women of reproductive age.
Supplementary text
Pre-specified outcomes; as in published protocol
Prespecified secondary outcomes not included in the manuscript include:
Maternal
Trimester specific gestational weight gain, diagnosis of GDM by local criteria; fetal growth at 28 weeks, health related quality of life as assessed by EQ-5D. Neck, hip and wrist circumferences, plasma fructosamine, C-reactive protein and other relevant epigenetic and metabolomics biomarkers and urinary biomarkers, dietary feeding patterns and measures of depression. Data for these outcomes are available and will be included in preplanned further data analyses. Metabolomic and epigenetic biomarkers in blood also prespecified outcomes will be undertaken in future analyses for publication.
Neonatal
Estimated body fat and crown–heel length also included will be reported in detailed analyses of anthropometry. Metabolomic and epigenetic biomarkers in cord blood, also prespecified outcomes, will be undertaken in future analyses for publication.
Chapter 6 Economic evaluation methods and results
Overview
A within-trial cost–utility analysis was undertaken to estimate the cost-effectiveness of the health training (intervention) over and above routine care (control). Costs were estimated from the perspective of the NHS acute care provider. QALYs were used to measure health outcomes and were derived from participant-completed EQ-5D questionnaires. Participants were randomised to treatment groups between 15 and 18 weeks’ gestation and baseline data were collected at this point (see Chapter 4). Participants were followed up at 20 weeks’ gestation and 36 weeks’ gestation.
Methods
The aim of this economic evaluation is to examine the cost-effectiveness of an intensive complex intervention combining dietary and PA to the NHS health system compared with routine care over the time horizon of the trial.
Resource utilisation was recorded using trial management software (MedSciNet Ltd) that provided information on the intensity of resources required to deliver the intervention (health training), as well as consequences to health care of intervention (primarily observation of perinatal care).
The following items of resource use were assessed:
-
contacts to provide the health training intervention
-
antenatal admissions
-
cessation of pregnancy
-
postnatal admissions.
Consequences of the intervention were captured electronically for each participant and her infant during the period between randomisation and delivery, including the duration and intensity of antenatal, intrapartum and neonatal care. Data were also collected on the mode of birth: unassisted vaginal birth, instrumental vaginal birth or caesarean section.
Valuation of resource use
For standard NHS health care, UK unit costs are applied from national sources including NHS reference costs233 and Personal Social Services Research Unit’s Unit Costs of Health and Social Care 2014. 234
All costs were valued in pounds sterling, according to a price year representing the mid-point of the trial. Costs occurring in prior price years will be inflated using the Hospital and Community Health Services pay and prices index234 and costs from later price years will be discounted at a rate of 3.5%, as recommended by the NICE. 235 As the trial follow-up was less than 1 year post randomisation, no discounting was required.
Resource use profiles were constructed for each patient in the trial multiplying quantities of resource utilisation by the relevant unit costs to estimate cost profiles for women in the trial.
Outcome measures
The EQ-5D81 questionnaires were collected at baseline (15–18 weeks’ gestation), at 27–28 weeks’ gestation and at 34–36 weeks’ gestation in UPBEAT and form the primary cost-effectiveness end point following NICE guidance. 236 Responses were then converted into a utility score (a scale where death is equal to 0 and full health 1) using population-based valuation tariff237 and each participant was assigned their utility (U) for each time point (t). As intended time points varied, exact dates on which data were collected were used and the date of each time point is denoted as Dt. To enable cost–utility analysis to be undertaken, an area under the curve method was then used to estimate the QALY score over the available follow-up period,238 such that an individual QALYs can be expressed as:
Analysis
Participants received programme health training and fidelity to the programme was recorded during sessions. This information allows for an accurate examination of intervention uptake and the related potential variation in direct costs of intervention. Over the course of treatment, for each contact with the participants, the HT recorded information on whether or not the participant attended, whether or not dietary and PA were set and, in situations when participants did not attend face-to-face training, whether or not a follow-up contact (either by telephone or e-mail) successfully agreed at least one of the two goals. This information was used to estimate the expected cost of intervention.
As recommended by NICE in 2008, base case analysis was explored from a NHS and personal social services perspective, including direct health effects (QALYs) and costs (or cost savings) to the NHS. The base case analysis takes the form of an incremental cost-effectiveness analysis following the NICE guidance for health-care evaluations. 236 An incremental approach allows for meaningful comparisons between the treatments, as it compares the additional costs of one treatment with those of the other, as well as the additional benefits. The primary cost-effectiveness outcome of the study is the cost per QALY. The incremental cost-effectiveness ratio (ICER) calculates the mean cost of the intervention group over and above the control and divides by the mean difference in health benefits.
Equation 2 is used to calculate the ICER, where Δ represents change, C represents the costs, E represents the effects and subscripts I and C refer to the intervention and control, respectively:
All analyses are conducted on an intention-to-treat basis, including all randomised participants in the groups to which they were randomised. Analyses were conducted using Stata version 13. Results are reported as means and 95% CIs.
Economic data often demonstrate skewness creating difficulties for analysis using traditional parametric tests. In such cases the bootstrap method can be used to account for this expected skewness. Bootstrapping samples observations with replacement a fixed number of times in order to generate a new population of sample means with an approximate normal distribution. This allows for statistical analysis and the derivation of CIs. Bootstrapping will resample the data using 10,000 replications with Stata software.
To estimate the joint distributions of cost and QALYs, non-parametric bootstrapping was conducted on the observed data. 239 This non-parametric bootstrap resampling technique allows us to assess uncertainty in the ICER. 26 First, results of the bootstrapped cost and QALYs are presented on the cost-effectiveness plane.
To further evaluate the joint distributions of costs and benefits, a cost-effectiveness acceptability curve (CEAC) was generated. 240 The CEAC illustrates the probability that the health training intervention will be cost-effective as decision-makers’ willingness to pay increases. According to NICE, the willingness to pay for an additional QALY ranges between £20,000 and £30,000; the CEAC indicates the probability that antenatal behavioural intervention is within this range.
Results
Resource utilisation and costs
The trial protocol was based on the delivery of eight sessions of health training to participants. Figure 9 presents patterns of engagement with sessions and how successful trainers were at setting goals. The most common outcome is that participants received all eight sessions and, therefore, worked towards a series of eight training goals. The second most common pattern represents early withdrawal. Overall, the pattern suggests that the more face-to-face sessions received, the more likely that the participant would be to receive eight training goals via either face to face or follow-up contacts.
FIGURE 9.
Total number of face-to-face health training sessions attended by participants and the total number of goals set during the training programme.
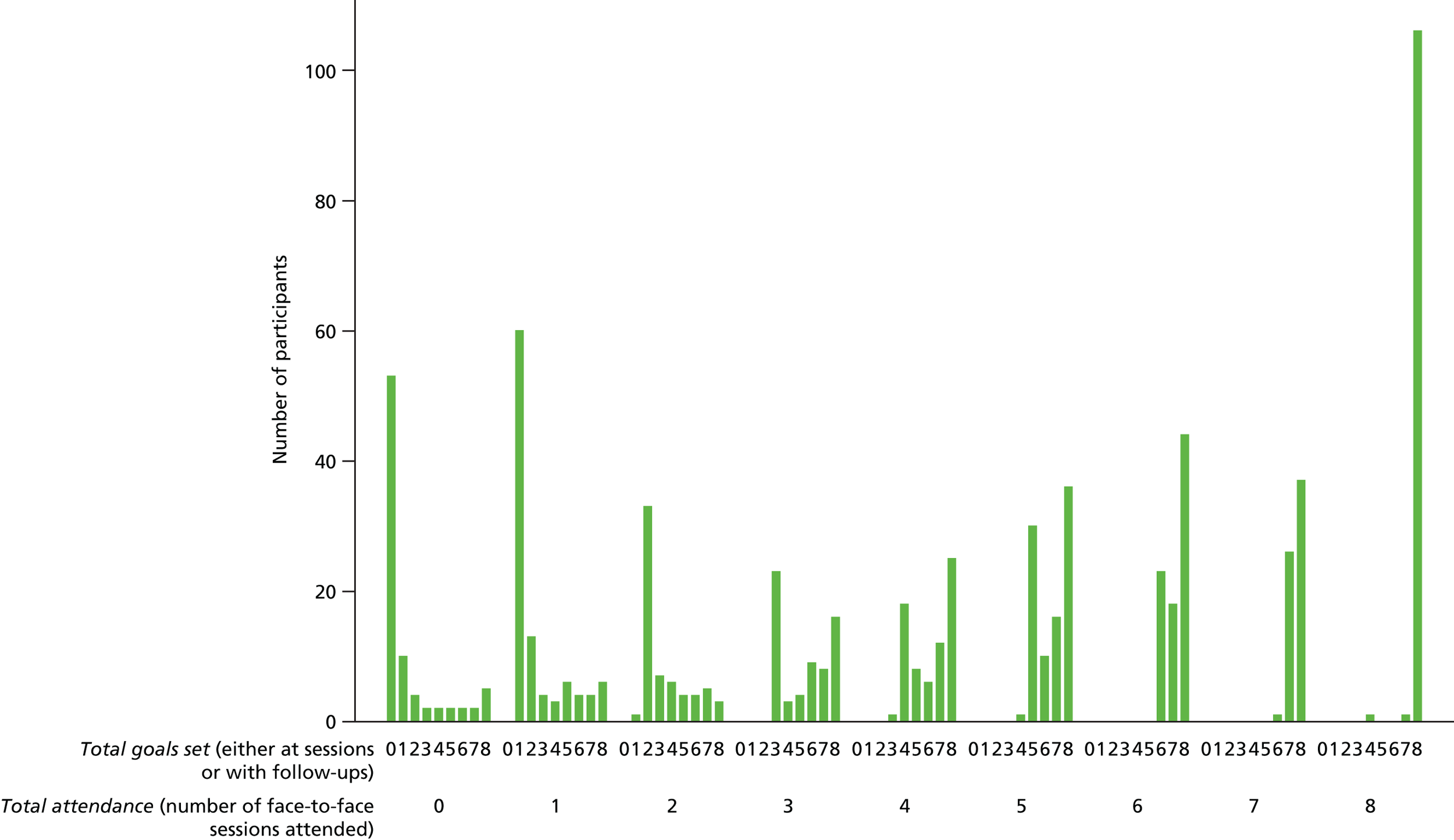
Table 31 presents resource utilisation (by study group) in four main categories. The first category presents average resources of the intervention (health training) and indicates the total number of patient contacts in which at least one goal was set. The second component examines antenatal admissions and type of admission (day case admission or overnight) and the related reason for admission. Third, resources related to pregnancy cessation present either mode of birth or rate of miscarriage or termination. Finally, postnatal admissions provide information on length of maternal admissions, rates of neonatal admission and neonatal length of stay. All resource use data are presented either for the full sample (all available information) or for complete cases (which informs the subsequent cost-effectiveness analysis).
| Resource category | Trial arm | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | |||||||||||
| Full sample | Complete case | Full sample | Complete case | |||||||||
| Mean | 95% CI | n | Mean | 95% CI | n | Mean | 95% CI | n | Mean | 95% CI | n | |
| Intervention (health training) | 5.37 | 5.2 to 5.54 | 728 | 6.220 | 6.01 to 6.44 | 510 | – | – | – | – | – | – |
| Antenatal admissions | ||||||||||||
| Asthma (per night) | 0.006 | 0.002 to 0.015 | 783 | 0.010 | 0.003 to 0.023 | 510 | 0.004 | 0.001 to 0.011 | 772 | 0.005 | 0.001 to 0.016 | 561 |
| Asthma (day case) | – | – | – | – | – | – | – | – | – | – | – | – |
| Hyperemesis/vaginal bleeding (per night) | 0.091 | 0.071 to 0.114 | 783 | 0.127 | 0.098 to 0.162 | 510 | 0.069 | 0.051 to 0.09 | 772 | 0.071 | 0.051 to 0.097 | 561 |
| Hyperemesis/vaginal bleeding (day case) | 0 | 0 to 0.005 | 783 | 0.000 | 0 to 0.0072331a | 510 | 0.009 | 0.004 to 0.019 | 772 | 0.012 | 0.005 to 0.026 | 561 |
| Other (per night) | 0.051 | 0.036 to 0.07 | 783 | 0.037 | 0.022 to 0.058 | 510 | 0.049 | 0.035 to 0.068 | 772 | 0.053 | 0.036 to 0.076 | 561 |
| Other (day case) | 0 | 0 to 0.005 | 783 | 0.000 | 0 to 0.0072331a | 510 | 0.003 | 0 to 0.009 | 772 | 0.002 | 0 to 0.01 | 561 |
| Other medical (per night) | 0.026 | 0.016 to 0.039 | 783 | 0.035 | 0.021 to 0.056 | 510 | 0.058 | 0.043 to 0.078 | 772 | 0.053 | 0.036 to 0.076 | 561 |
| Other medical (day case) | 0.001 | 0 to 0.007 | 783 | 0.002 | 0 to 0.011 | 510 | 0.000 | 0 to 0.005 | 772 | 0.000 | 0 to 0.0066a | 561 |
| Other obstetric (per night) | 0.089 | 0.07 to 0.113 | 783 | 0.127 | 0.098 to 0.162 | 510 | 0.049 | 0.035 to 0.068 | 772 | 0.059 | 0.04 to 0.083 | 561 |
| Other obstetric (day case) | 0.001 | 0 to 0.007 | 783 | 0.002 | 0 to 0.011 | 510 | 0.003 | 0 to 0.009 | 772 | 0.002 | 0 to 0.01 | 561 |
| Surgical (per night) | 0.004 | 0.001 to 0.011 | 783 | 0.006 | 0.001 to 0.017 | 510 | 0.003 | 0 to 0.009 | 772 | 0.002 | 0 to 0.01 | 561 |
| Surgical (day case) | – | – | – | – | – | – | – | – | – | – | – | – |
| Trauma (per night) | – | – | – | – | – | – | – | – | – | – | – | – |
| Trauma (day case) | 0.003 | 0 to 0.009 | 783 | 0.002 | 0 to 0.011 | 510 | 0.001 | 0 to 0.007 | 772 | 0.002 | 0 to 0.01 | 561 |
| Mode of pregnancy cessation | ||||||||||||
| Normal delivery with a CC score of 2+ | 0.006 | 0.002 to 0.015 | 783 | 0.004 | 0 to 0.014 | 510 | 0.009 | 0.004 to 0.019 | 772 | 0.004 | 0 to 0.013 | 561 |
| Normal delivery with a CC score of 1 | 0.066 | 0.05 to 0.087 | 783 | 0.065 | 0.045 to 0.091 | 510 | 0.063 | 0.047 to 0.084 | 772 | 0.068 | 0.048 to 0.093 | 561 |
| Normal delivery with a CC score of 0 | 0.253 | 0.219 to 0.291 | 783 | 0.243 | 0.202 to 0.29 | 510 | 0.245 | 0.211 to 0.282 | 772 | 0.239 | 0.2 to 0.283 | 561 |
| Normal delivery with induction, with a CC score of 2+ | 0.001 | 0 to 0.007 | 783 | 0.002 | 0 to 0.011 | 510 | 0.004 | 0.001 to 0.011 | 772 | 0.004 | 0 to 0.013 | 561 |
| Normal delivery with induction, with a CC score of 1 | 0.046 | 0.032 to 0.064 | 783 | 0.053 | 0.035 to 0.077 | 510 | 0.047 | 0.033 to 0.065 | 772 | 0.050 | 0.033 to 0.072 | 561 |
| Normal delivery with induction, with a CC score of 0 | 0.132 | 0.107 to 0.16 | 783 | 0.127 | 0.098 to 0.162 | 510 | 0.141 | 0.116 to 0.17 | 772 | 0.152 | 0.121 to 0.187 | 561 |
| Assisted delivery with a CC score of 2+ | 0.001 | 0 to 0.007 | 783 | 0.002 | 0 to 0.011 | 510 | 0.001 | 0 to 0.007 | 772 | 0.000 | 0 to 0.007a | 561 |
| Assisted delivery with a CC score of 1 | 0.028 | 0.018 to 0.043 | 783 | 0.031 | 0.018 to 0.051 | 510 | 0.013 | 0.006 to 0.024 | 772 | 0.016 | 0.007 to 0.03 | 561 |
| Assisted delivery with a CC score of 0 | 0.023 | 0.014 to 0.036 | 783 | 0.027 | 0.015 to 0.046 | 510 | 0.026 | 0.016 to 0.04 | 772 | 0.023 | 0.012 to 0.04 | 561 |
| Assisted delivery with induction, with a CC score of 2+ | 0.004 | 0.001 to 0.011 | 783 | 0.004 | 0 to 0.014 | 510 | 0.005 | 0.001 to 0.013 | 772 | 0.007 | 0.002 to 0.018 | 561 |
| Assisted delivery with induction, with a CC score of 1 | 0.036 | 0.024 to 0.052 | 783 | 0.049 | 0.032 to 0.072 | 510 | 0.030 | 0.019 to 0.045 | 772 | 0.034 | 0.02 to 0.053 | 561 |
| Assisted delivery with induction, with a CC score of 0 | 0.028 | 0.018 to 0.043 | 783 | 0.027 | 0.015 to 0.046 | 510 | 0.034 | 0.022 to 0.049 | 772 | 0.034 | 0.02 to 0.053 | 561 |
| Planned caesarean section, with a CC score of 4+ | 0.001 | 0 to 0.007 | 783 | 0.002 | 0 to 0.011 | 510 | 0.000 | 0 to 0.005a | 772 | 0.000 | 0 to 0.007a | 561 |
| Planned caesarean section, with a CC score of 2–3 | 0.005 | 0.001 to 0.013 | 783 | 0.000 | 0 to 0.007a | 510 | 0.008 | 0.003 to 0.017 | 772 | 0.000 | 0 to 0.007a | 561 |
| Planned caesarean section, with a CC score of 0–1 | 0.198 | 0.168 to 0.232 | 783 | 0.196 | 0.16 to 0.238 | 510 | 0.167 | 0.14 to 0.199 | 772 | 0.168 | 0.135 to 0.205 | 561 |
| Emergency caesarean section, with a CC score of 2–3 | 0.006 | 0.002 to 0.015 | 783 | 0.006 | 0.001 to 0.017 | 510 | 0.005 | 0.001 to 0.013 | 772 | 0.004 | 0 to 0.013 | 561 |
| Emergency caesarean section, with a CC score of 0–1 | 0.135 | 0.111 to 0.164 | 783 | 0.161 | 0.128 to 0.2 | 510 | 0.174 | 0.145 to 0.206 | 772 | 0.194 | 0.16 to 0.234 | 561 |
| Miscarriage < 23+6 weeks of gestation | 0.013 | 0.006 to 0.023 | 783 | 0.000 | 0 to 0.007a | 510 | 0.009 | 0.004 to 0.019 | 772 | 0.000 | 0 to 0.007a | 561 |
| Termination for anomaly < 23+6 weeks of gestation | 0.001 | 0 to 0.007 | 783 | 0.000 | 0 to 0.007a | 510 | 0.003 | 0 to 0.009 | 772 | 0.000 | 0 to 0.007a | 561 |
| Missing data about the end of pregnancy | 0.015 | 0.008 to 0.027 | 783 | 0.000 | 0 to 0.007a | 510 | 0.017 | 0.009 to 0.029 | 772 | 0.005 | 0.001 to 0.016 | 561 |
| Postnatal admissions | ||||||||||||
| Maternal length of stay (night) | 2.088 | 1.986 to 2.193 | 754 | 2.123 | 1.997 to 2.253 | 506 | 2.054 | 1.952 to 2.159 | 747 | 1.996 | 1.881 to 2.117 | 557 |
| Neonatal admission | 0.085 | 0.066 to 0.108 | 765 | 0.076 | 0.054 to 0.105 | 510 | 0.075 | 0.057 to 0.098 | 757 | 0.041 | 0.026 to 0.062 | 561 |
| Neonatal length of stay (night) | 2.833 | 2.713 to 2.957 | 743 | 2.235 | 2.106 to 2.369 | 503 | 3.041 | 2.916 to 3.17 | 734 | 2.096 | 1.977 to 2.22 | 552 |
Health training aimed to provide weekly 1.5-hour sessions for 8 weeks during the intervention period of the study. Participants received an average of 5.37 contacts, in which at least one goal was set, of which 4.10 contacts occurred face to face and 1.27 were follow-ups (telephone or e-mail).
The most commonly reported reason for an overnight prenatal hospital admission was for ‘hyperemesis/vaginal bleeding’. The most common day case was reported as related to trauma. Overall, the mean number of inpatient nights was 2.754 (SD 2.398) in the control group and 2.824 (SD 3.462) in the intervention group.
Pregnancy cessation was used in Healthcare Resource Groups (HRGs) categories; HRGs are commonly used to inform reimbursement of hospital trusts and as such form the subsequent basis for cost-effectiveness analysis. The most commonly recorded HRG mode of birth was normal delivery [comorbidities or complications (CC) score = 0] (intervention, 24.3%; control, 23.9%), followed by emergency caesarean section (CC score 0–1) (intervention, 16.1%; control, 19.4%). Rates of miscarriage (between 15 and 24 weeks) were low (intervention, 1.3%; control, 0.9%) and likewise observed terminations (between 15 and 24 weeks) were rare (intervention, 0.1%; control, 0.3%). Responses to EQ-5D questionnaires (required for complete case analysis) are missing in all cases of miscarriage and termination; therefore, the figure was drawn from the ‘full sample’. No data about the end of pregnancy were missing in the intervention group (complete case analysis); however, 0.5% of data were missing in the control group.
On average, maternal length of stay was similar to the national averages (intervention group, 2.123 days; control group, 1.996 days). Mean rates of neonatal admissions demonstrate some differences between groups (intervention 7.6% vs. control 4.1%); however, when compared in the full sample the difference diminishes (intervention 8.5% vs. control 7.5%), suggesting that this difference may be an artefact of sample attrition. Mean neonatal length of stay displays similarities between groups (intervention 2.235 nights vs. control 2.096 nights), although inspection of conditional means (i.e. mean given a neonatal admission) demonstrates interesting differences [intervention 12.951 nights (n = 61) vs. control 18.058 nights (n = 52)].
Table 32 presents unit cost data for each item of resource use. All unit costs are based on the NHS price year 2012–13 to represent the halfway time point of randomisation into UPBEAT. The HTs providing intervention were valued at the same rate as ‘clinical support worker (hospital)’. Specific costs were applied for all antenatal admissions. The numbers of CCs used to inform specific to modes of birth were based on either the presence of systemic comorbidities [pre-eclampsia, pre-existing hypertension, gestational hypertension, cardiovascular disease, pre-existing diabetes mellitus, GDM (previous history or currently diagnosed), genitourinary or general infection or existing mental health disorder] or labour complications [preterm labour (< 37 weeks’ gestation), long labour (i.e. second stage > 4 hours) or postpartum haemorrhage (blood loss of > 500 ml)]. Average bed stay post partum generally depends on mode of delivery; however, the cost for ‘non-elective inpatient – long-stay excess bed-days’ is applied when the maternal length of stay exceeds 2 days. Neonatal stay is incorporated into the cost of the mode of delivery; however, neonatal admissions are treated separately. In the absence of further information from the trial, all neonatal nights are assumed to be neonatal critical care, normal care.
| Resource category | Unit cost (£) | Price year | Reference |
|---|---|---|---|
| Health training | |||
| Clinical support worker (hospital) | 52 | 2012–13 | Personal Social Services Research Unit’s Unit Costs of Health and Social Care 2014, p. 249234 |
| Antenatal hospital admissions | |||
| Asthma (per night) | 709 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Asthma (day case) | 407 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Hyperemesis/vaginal bleeding (per night) | 659 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Hyperemesis/vaginal bleeding (day case) | 440 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Other (per night) | 480 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Other (day case) | 383 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Other medical (per night) | 791 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Other medical (day case) | 359 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Other obstetric (per night) | 804 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Other obstetric (day case) | 464 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Surgical (per night) | 1613 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Surgical (day case) | 229 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Trauma (per night) | 2537 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Trauma (day case) | 437 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Pregnancy cessation | |||
| Normal delivery with a CC score of 2+ | 1804 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Normal delivery with a CC score of 1 | 1529 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Normal delivery with a CC score of 0 | 1325 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Normal delivery with induction, with a CC score of 2+ | 2413 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Normal delivery with induction, with a CC score of 1 | 1987 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Normal delivery with induction, with a CC score of 0 | 1728 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Assisted delivery with a CC score of 2+ | 2407 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Assisted delivery with a CC score of 1 | 2063 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Assisted delivery with a CC score of 0 | 1802 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Assisted delivery with epidural or induction, with a CC score of 2+ | 2982 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Assisted delivery with epidural or induction, with a CC score of 1 | 2449 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Assisted delivery with epidural or induction, with a CC score of 0 | 2170 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Planned caesarean section, with a CC score of 4+ | 4161 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Planned caesarean section, with a CC score of 2–3 | 3288 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Planned caesarean section, with a CC score of 0–1 | 2684 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Emergency caesarean section, with a CC score of 4+ | 5302 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Emergency caesarean section, with a CC score of 2–3 | 4243 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Emergency caesarean section, with a CC score of 0–1 | 3414 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Miscarriage < 23+6 weeks of gestation | 1708 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Termination for anomaly < 23+6 weeks of gestation | 1282 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Postnatal hospital admissions | |||
| Non-elective inpatient – long-stay excess bed-days | 450 | 2012–13 | NHS Reference Costs 2012–13 233 |
| Neonatal critical care, normal care | 471 | 2012–13 | NHS Reference Costs 2012–13 233 |
Table 33 presents the total costs by study group, and subtotal costs (by item groups) and costs by specific item. Again, to aid consideration of the implications of missing data, the results are further subdivided into those drawn from the full sample and those available to inform the complete case cost-effectiveness analysis.
| Resource category | Trial arm | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | |||||||||||
| Full sample | Complete case | Full sample | Complete case | |||||||||
| Mean | 95% CI | n | Mean | 95% CI | n | Mean | 95% CI | n | Mean | 95% CI | n | |
| Intervention (health training) | 390 | 388 to 391 | 783 | 485 | 484 to 487 | 510 | – | – | – | – | – | – |
| Asthma (per night) | 5 | 4 to 5 | 783 | 7 | 7 to 7 | 510 | 3 | 3 to 3 | 772 | 4 | 4 to 4 | 561 |
| Asthma (day case) | – | – | – | – | – | – | – | – | – | – | – | – |
| Hyperemesis/vaginal bleeding (per night) | 60 | 59 to 60 | 783 | 84 | 83 to 85 | 510 | 45 | 45 to 46 | 772 | 47 | 46 to 48 | 561 |
| Hyperemesis/vaginal bleeding (day case) | 0 | 0 to 0.00a | 783 | 0 | 0 to 0.01a | 510 | 4 | 4 to 4 | 772 | 5 | 5 to 6 | 561 |
| Other (per night) | 25 | 24 to 25 | 783 | 18 | 18 to 18 | 510 | 24 | 23 to 24 | 772 | 26 | 25 to 26 | 561 |
| Other (day case) | 0 | 0 to 0.00a | 783 | 0 | 0 to 0.01a | 510 | 1 | 1 to 1 | 772 | 1 | 1 to 1 | 561 |
| Other medical (per night) | 20 | 20 to 21 | 783 | 28 | 27 to 28 | 510 | 46 | 46 to 47 | 772 | 42 | 42 to 43 | 561 |
| Other medical (day case) | 0 | 0 to 1 | 783 | 1 | 1 to 1 | 510 | 0 | 0 to 0.00a | 772 | 0 | 0 to 0.01a | 561 |
| Other obstetric (per night) | 72 | 71 to 72 | 783 | 102 | 102 to 103 | 510 | 40 | 39 to 40 | 772 | 47 | 47 to 48 | 561 |
| Other obstetric (day case) | 1 | 1 to 1 | 783 | 1 | 1 to 1 | 510 | 1 | 1 to 1 | 772 | 1 | 1 to 1 | 561 |
| Surgical (per night) | 6 | 6 to 6 | 783 | 9 | 9 to 10 | 510 | 4 | 4 to 4 | 772 | 3 | 3 to 3 | 561 |
| Surgical (day case) | – | – | – | – | – | – | – | – | – | – | – | – |
| Trauma (per night) | – | – | – | – | – | – | – | – | – | – | – | – |
| Trauma (day case) | 1 | 1 to 1 | 783 | 1 | 1 to 1 | 510 | 1 | 1 to 1 | 772 | 1 | 1 to 1 | 561 |
| Subtotal cost: antenatal admissions | 189 | 188 to 190 | 783 | 251 | 250 to 253 | 510 | 168 | 167 to 169 | 772 | 177 | 176 to 178 | 561 |
| Normal delivery with a CC score of 2+ | 12 | 11 to 12 | 783 | 7 | 7 to 7 | 510 | 16 | 16 to 17 | 772 | 6 | 6 to 7 | 561 |
| Normal delivery with a CC score of 1 | 102 | 101 to 102 | 783 | 99 | 98 to 100 | 510 | 97 | 96 to 98 | 772 | 104 | 103 to 104 | 561 |
| Normal delivery with a CC score of 0 | 335 | 334 to 336 | 783 | 322 | 320 to 324 | 510 | 324 | 323 to 326 | 772 | 316 | 315 to 318 | 561 |
| Normal delivery with induction, with a CC score of 2+ | 3 | 3 to 3 | 783 | 5 | 5 to 5 | 510 | 9 | 9 to 10 | 772 | 9 | 8 to 9 | 561 |
| Normal delivery with induction, with a CC score of 1 | 91 | 91 to 92 | 783 | 105 | 104 to 106 | 510 | 93 | 92 to 93 | 772 | 99 | 98 to 100 | 561 |
| Normal delivery with induction, with a CC score of 0 | 227 | 226 to 228 | 783 | 220 | 219 to 221 | 510 | 244 | 243 to 245 | 772 | 262 | 260 to 263 | 561 |
| Assisted delivery with a CC score of 2+ | 3 | 3 to 3 | 783 | 5 | 5 to 5 | 510 | 3 | 3 to 3 | 772 | 0 | 0 to 0.01a | 561 |
| Assisted delivery with a CC score of 1 | 58 | 57 to 58 | 783 | 65 | 64 to 65 | 510 | 27 | 26 to 27 | 772 | 33 | 33 to 34 | 561 |
| Assisted delivery with a CC score of 0 | 41 | 41 to 42 | 783 | 49 | 49 to 50 | 510 | 47 | 46 to 47 | 772 | 42 | 41 to 42 | 561 |
| Assisted delivery with epidural, with a CC score of 2+ | 11 | 11 to 12 | 783 | 12 | 11 to 12 | 510 | 15 | 15 to 16 | 772 | 21 | 21 to 22 | 561 |
| Assisted delivery with epidural, with a CC score of 1 | 88 | 87 to 88 | 783 | 120 | 119 to 121 | 510 | 73 | 72 to 74 | 772 | 83 | 82 to 84 | 561 |
| Assisted delivery with epidural, with a CC score of 0 | 61 | 60 to 62 | 783 | 60 | 59 to 60 | 510 | 73 | 72 to 74 | 772 | 73 | 73 to 74 | 561 |
| Planned caesarean section, with a CC score of 4+ | 5 | 5 to 5 | 783 | 8 | 8 to 8 | 510 | 0 | 0 to 0.00a | 772 | 0 | 0 to 0.01a | 561 |
| Planned caesarean section, with a CC score of 2–3 | 17 | 17 to 17 | 783 | 0 | 0 to 0.01a | 510 | 26 | 25 to 26 | 772 | 0 | 0 to 0.01a | 561 |
| Planned caesarean section, with a CC score of 0–1 | 531 | 530 to 533 | 783 | 526 | 524 to 528 | 510 | 449 | 447 to 450 | 772 | 450 | 448 to 452 | 561 |
| Emergency caesarean section, with a CC score of 2–3 | 27 | 27 to 27 | 783 | 25 | 25 to 25 | 510 | 22 | 22 to 22 | 772 | 15 | 15 to 15 | 561 |
| Emergency caesarean section, with a CC score of 0–1 | 462 | 461 to 464 | 783 | 549 | 547 to 551 | 510 | 593 | 591 to 594 | 772 | 663 | 661 to 665 | 561 |
| Miscarriage < 23+6 weeks of gestation | 22 | 21 to 22 | 783 | 0 | 0 to 0.01a | 510 | 15 | 15 to 16 | 772 | 0 | 0 to 0.01a | 561 |
| Termination for anomaly < 23+6 weeks of gestation | 2 | 2 to 2 | 783 | 0 | 0 to 0.01a | 510 | 3 | 3 to 3 | 772 | 0 | 0 to 0.01a | 561 |
| Subtotal cost: pregnancy cessation | 2098 | 2095 to 2102 | 783 | 2177 | 2173 to 2181 | 510 | 2129 | 2126 to 2132 | 772 | 2177 | 2173 to 2181 | 561 |
| Maternal admissions (more than two nights) | 497 | 496 to 499 | 783 | 536 | 534 to 538 | 510 | 460 | 459 to 462 | 772 | 432 | 430 to 433 | 561 |
| Neonatal critical care, normal care | 478 | 476 to 479 | 779 | 187 | 185 to 188 | 510 | 577 | 575 to 578 | 767 | 128 | 128 to 129 | 561 |
| Total costs | 3650 | 3646 to 3654 | 779 | 3636 | 3630 to 3641 | 510 | 3586 | 3581 to 3592 | 524 | 2913 | 2909 to 2918 | 561 |
The mean cost of health training was £485 based on complete cases (n = 510). This is lower than would have been expected based on the protocol description of the intended intervention (i.e. ‘eight group sessions with the HT on a weekly basis, each lasting 1.5 hours’ equating to £624). However, when compared with the intervention cost based on the full sample (£390) the complete case estimate is higher and may be attributed to several potential factors (e.g. consumer preferences, attrition during intervention due to the loss of the pregnancy, etc.).
Overall, aggregated costs of all antenatal admissions would seem significantly higher in the intervention group (intervention £251 vs. control £177), although again, compared with results from the full sample (intervention £189 vs. control £168), any difference may be related to underlying patterns of missing data.
As complete case data are contingent on the completion of the EQ-5D, the cost of pregnancy cessation (for complete case analysis) excludes costs related to pregnancy loss and is solely based on the HRG mode of birth. Over all the varying modes of birth, the averaged cost is found to be identical (intervention £2177 vs. control £2177).
Costs associated with longer stays (i.e. more than two nights) are slightly higher in the intervention group than in the control group (intervention £536 vs. control £432).
The cost related to neonatal stay was higher in the intervention group than in the control group (intervention £187 vs. control £128). This may initially seem counterintuitive when considering that the conditional mean length of stay was higher in the control group; however, the rate of neonatal admissions in the intervention group was almost double that in the control group (7.6% vs. 4.1%, respectively), meaning that over the entire observed sample costs were higher.
Outcomes
The EuroQol-5 Dimensions, three-level (EQ-5D-3L), scores at baseline, 20 weeks’ and 36 weeks’ gestation are presented in Table 34. Comparing the two groups, scores are displayed as similarities across time points and as an aggregated QALY.
| Intervention group | Baseline | Weeks’ gestation | QALY | |
|---|---|---|---|---|
| 20 | 36 | |||
| Intervention, mean (SD) | 0.883 (0.169) | 0.828 (0.185) | 0.76 (0.222) | 0.284 (0.054) |
| Control, mean (SD) | 0.868 (0.17) | 0.813 (0.19) | 0.76 (0.207) | 0.282 (0.056) |
Cost-effectiveness analysis
Table 35 presents the results of a seemingly unrelated regression. The model tests the joint distribution of the effect of treatment (health training) on the incremental total costs and QALY, whether or not the effects are significantly different from those in the control group and whether or not the cost and QALYs are significantly correlated.
| Variables | Total costs (NHS perspective, £) (95% CI) | QALY (95% CI) |
|---|---|---|
| Treatment | 722 (473 to 970)*** | 0.002 (–0.004 to 0.009) |
| Constant | 2191 (1804 to 2578)*** | 0.280 (0.267 to 0.290)*** |
The results suggest that health training results in a non-significant mean increase in QALYs of 0.002 (95% –0.004 to 0.009) and a significant increase in mean total costs of £722 (95% CI £473 to £970). The ICER is £331,630/QALY.
To further explore uncertainty surrounding the ICER, Figure 10 presents results of the non-parametric bootstrap (10,000 replications) on a cost-effectiveness plane.
FIGURE 10.
Cost-effectiveness plane (10,000 replications).
To illustrate implications of uncertainty within the context of health care decision-making, the results of the bootstrap analyis are further expressed on a CEAC (Figure 11). This suggests that, based on the available information from UPBEAT, the probability that health training would be below NICE’s stated upper threshold of willingness to pay (£30,000/QALY) is only 1%.
FIGURE 11.
Cost-effectiveness acceptability curve illustrating the probability that the intervention is cost-effective at increasing values of willingness to pay for an additional QALY.
Summary of within-trial cost-effectiveness findings
The programme of care was estimated to cost, on average, £485 more per participant (based solely on health training time) than routine care.
Participants allocated to the intervention group displayed a non-significant mean increase in QALYs of 0.002 (95% CI –0.004 to 0.009; p = 0.52).
Base case cost-effectiveness analysis found that the ICER was £331,630 per QALY. Accounting for uncertainty in the ICER on a CEAC suggests that the probability that antenatal behavioural intervention is below the £30,000/QALY willingness-to-pay threshold is 1%.
Based on total cost to the NHS provider and health gains (of the expectant mother), from randomisation up until 36 weeks’ gestation, UPBEAT provides no supporting evidence to suggest that the intervention represents value for money based on NICE benchmarks of cost-effectiveness.
Chapter 7 Conclusions
This study developed a complex behavioural intervention through a three-phase approach; the conclusions from the different phases and how each contributed to the final outcome are summarised below.
Phase 1: development phase
The programme commenced at a time of intense research activity in this field, a response to the growing clinical burden of obesity and excessive GWG among pregnant women in developed countries. One of the first activities was to review the current, expanding, literature in order to inform development of the intervention, particularly in regard to successful approaches to behavioural change in previous studies of healthy lifestyle interventions in pregnant women. We found a common issue to be the failure of prior studies to evaluate changes in behaviour or its psychological determinants, as well as the provision of inadequate detail of the intervention content. The conclusion that little could be learnt from previous studies in terms of effective approaches reinforced our intention to systematically develop an evidenced-based intervention based on known theory, and to pilot the intervention to evaluate all components and effects, particularly change in behaviour, acceptability and its psychological determinants. To provide new insight into means whereby we would target dietary behaviours, we asked 103 pregnant women, 38% of whom were overweight or obese, to complete a questionnaire to identify determinants of gestational dietary behaviour relating to the intended dietary components of the intervention. We were encouraged to find that women whose diet was the least healthy had the strongest intentions to make the proposed changes in their diet. Furthermore, and in contrast to the general population, we found no evidence of barriers to dietary change, and that perceived benefits for the health of the mother and baby were the most likely determinants of change. We also carried out semistructured qualitative interviews with obese pregnant women that identified significant barriers to change in PA, particularly in relation to physical discomfort. These also highlighted the already well-recognised issues around stigmatisation of obesity, and the overarching importance of care to avoid inadvertently offending women when approaching them to take part in the programme and throughout their involvement. Concern over the lack of any standardised method of objective assessment of PA led us to address optimal methods for assessment of PA in pregnant women. At the time, the most accurate validated devices were accelerometers worn on the waist, but the cost of these was prohibitive for a large trial. We therefore undertook a study of 93 women comparing the GT1M accelerometer, one of the most validated devices at the time, and the most accurate pedometer available (CW701 Digi-Walker™ Pedometer) to determine whether or not the pedometer could be an inexpensive option. This did not prove to be the case as the pedometer was insufficiently accurate in the sample of overweight and obese pregnant women. For the purposes of the pilot trial it was concluded that the accelerometer was the best option, and for the RCT we would need to revert to validated self-report questionnaires, with the pedometers serving only as motivational devices for women in the intervention arm.
On the basis of the studies in phase 1, and the relevant literature on diet and PA to target insulin resistance, we were well positioned to develop all components of the intervention, then evaluated in the pilot trial.
Phase 2: pilot trial
As recommended by the MRC, any complex intervention should include a feasibility study, and the NIHR understandably insisted that we show effectiveness of behaviour change before releasing funding for phase 3. Our experience confirmed the pilot trial to be an essential prelude to the main trial. As elaborated in Chapter 2, the most important conclusion drawn was that the intervention, delivered by HTs, led to a change in all the dietary targets of the intervention – a reduction in GL and a reduction in free sugar intake, and lower saturated fat intake. The reduction in GL achieved was similar to that which had been shown previously to improve glycaemic control in pregnant women with type 2 diabetes mellitus. Although evaluated by self-report, the method, triple-pass 24-hour recall, was rigorous but also time-consuming, and a shorter questionnaire was also used for the purposes of validation for the main trial. The objective measure of PA failed to show any increase, although women self-reported an increase in walking. Since pregnant women should be encouraged to adhere to the PA recommended in clinical guidelines, the PA element of the intervention was not dropped. Other conclusions drawn from elements of the process evaluation were that although most women found the intervention acceptable, some preferred one-to-one sessions with the HT and that the time of day of delivery of the intervention should be made more amenable to the daily lives of the women. We therefore accommodated these needs in revision of delivery of the intervention. Although we had expected that recruitment to the study would not be easy, we had underestimated the number of women who would refuse to take part. This had repercussions for funding but, recognising during the pilot study that the trial recruitment period would need extending, additional NIHR funding was secured ahead of need.
Phase 3: randomised controlled trial
This phase of the programme demonstrated that the UPBEAT intervention, a theoretically based intensive complex intervention combining dietary and PA advice designed to reduce insulin resistance and achieve changes in diet and PA in obese pregnant women and a modest reduction in GWG, was not associated with a reduction in GDM or the delivery rate of LGA infants.
The lack of effect of the intervention on the primary maternal and neonatal outcomes was disappointing but when viewed in association with the many other smaller trials84 and more recent large trials218 it is becoming clear that behavioural interventions in unselected obese pregnant women may be ineffective in improving clinically important outcomes. The health economic evaluation reinforced this conclusion. UPBEAT, being the largest study in the world of a behavioural intervention in obese pregnant women, may thus provide a turning point in research focus, with more emphasis being placed on preventing obesity/reducing the BMI of women of reproductive age. The negative effect of the intervention runs the risk, however, of diet and PA advice being sidelined. This is not our intention; the UPBEAT intervention, being successful in establishing improved diet in obese pregnant women, could be a component of a combined intervention or of a different approach. We know that women who have a diagnosis of GDM make rigorous changes to their diet which improve their glycaemic control and clinical outcomes. The lack of effective prevention of GDM in this study is likely to relate to an inadequate change of diet, as the effectiveness of a low-GI diet in the non-pregnant population to improve glycaemic control is frequently reported. Much effort, including that of the UPBEAT team, is now focused on early pregnancy risk assessment of obese women, to develop a screening test which will identify, with better accuracy than current risk assessment, which women will develop GDM in later pregnancy. This was the basis of the study reported in Chapter 3, in which we identified early second-trimester clinical and biochemical markers from pregnant women who had taken part in the pilot trial that showed good predictive potential for later development of GDM. This focus on risk assessment has now been extended to the whole trial population, with the addition of measurement of the metabolome. 241 On the basis that knowledge of diagnosis leads to effective dietary change, we hypothesise that women who are informed in the first weeks of pregnancy that they have a very high risk of GDM will be more likely to make clinically effective dietary change. In this setting the UPBEAT intervention might lead to improved outcomes or could be used in combination with a pharmacological intervention, such as metformin. In the light of the failure of this and other behavioural studies to prevent adverse outcomes in obese women,84,193 and because of the cost which would be incurred by introduction of an intervention in all obese pregnant women, we recommend that more focus should be placed on risk stratification in early pregnancy and delivery of interventions to those most at risk.
Another important outcome of this study arose from our decision to standardise GDM diagnosis and adopt the internationally accepted (IADPSG’s) criteria. These criteria are rigorous, as evidenced by the high rate (25%) of GDM in the trial population. Using these criteria, there were many fewer LGA infants than expected from population estimates, which may relate to the number of women who received treatment for GDM; this was more than twice that expected had the diagnosis been made on the basis of the previous WHO criteria. While not confirmatory, this contributes to the growing need to formally evaluate the IADPSG’s criteria in UK obese women, and to determine whether or not national adoption would reduce the burden of disease. Again, health economic benefit could potentially be improved by screening for diabetes mellitus using these criteria only among women designated as high risk in early pregnancy.
Changes in the diet of the mothers may have lasting effects on maternal health. Analyses of data from the mothers at 36 months and from the mothers and infants at 6 months post partum has shown, for the first time in any intervention study in obese women, continued improvement in diet and reduction in infant measures of body fat at 6 months. 242 The ongoing follow-up of the UPBEAT mothers and children will determine whether or not the improvements in maternal diet and PA are maintained, and whether or not the risk of obesity in the children is affected by improved health behaviours in the mother during and after pregnancy.
The strengths of this study included the phased design, in accordance with national (MRC) recommendations for complex interventions. The pilot study enabled very detailed assessment of diet and objective assessment of PA, at a level which would have been feasible in the setting of a large-scale randomised trial, and also determined the practical feasibility of every aspect of the trial. The delivery and content of the intervention were based on a sound evidence base drawn from the behavioural intervention and clinical literature with advice from a multidisciplinary team including obstetricians, a consultant midwife, a social scientist, psychologists and a psychiatrist. In relation to the population studied, our deliberate focus was on women from inner-city deprived populations who, worldwide, demonstrate a high prevalence of obesity and an associated burden to health-care resources.
Cost-effectiveness findings
Health economic analysis
Provision of the intervention was estimated to cost, per participant, an average of £485 more than routine care. Allocation to the intervention was associated with a non-significant increase in QALYs (0.002, 95% CI –0.004 to 0.009; p = 0.52). Base case cost-effectiveness analysis found that the ICER was £331,630 per QALY. Based on the total cost to the NHS provider and health gains from randomisation to 36 weeks’ gestation, UPBEAT provided no supporting evidence to suggest the intervention represented value for money based on NICE’s benchmarks for cost-effectiveness.
Limitations of the study
There were some limitations. In phase 1, the study of dietary behaviours by questionnaire was carried out mainly in women of higher socioeconomic status than the trial population, and the structured interviews included some women with underlying pathologies who were not included in the trial population. A common issue was the estimation of diet, which as in all large studies had to be by self-report. With the caveats of self-report in mind, in the pilot study, phase 2, we undertook a rigorous but time-consuming evaluation using triple-pass 24-hour recall. Data from the main trial confirmed similar changes using a more practical shorter method. Because of the expense and practical issues of using accelerometers and downloading data for objective measurement, PA was also by self-report. Another limitation was the number of women who had to be approached in order to recruit the trial sample in phase 3. Although those women showed no important demographic difference from the trial participants, this highlights reluctance of individuals who are obese to take part in intervention trials or indeed in established health-care lifestyle programmes. It also implies that the women who took part were the more motivated among the obese population to improve their health. Once recruited, compliance was good, suggesting, as identified by our process evaluation, that obese pregnant women will engage in a well-designed intervention.
Acknowledgements
Lucilla Poston and Keith M Godfrey are supported by the European Union’s Seventh Framework Programme (FP7/2007-2013), project EarlyNutrition under grant agreement number 289346.
We express our sincere thanks to all the staff in the UPBEAT consortium listed below and, particularly, the participants in the trial for their patience, time, interest and goodwill.
The UK Pregnancies Better Eating and Activity Trial consortium
Centres
King’s College London/Guy’s and St Thomas’ NHS Foundation Trust
Lucilla Poston, PI; Andrew Shennan, co-PI, centre clinical lead; Annette Briley, co-PI, trial manager; Claire Singh, senior research midwife; Paul Seed, trial statistician; Jane Sandall, co-PI; Thomas Sanders, co-PI; Nashita Patel, Doctor of Philosophy student; Angela Flynn, Doctor of Philosophy student; Shirlene Badger, postdoctoral fellow; Suzanne Barr, postdoctoral fellow; Bridget Holmes, postdoctoral fellow; Louise Goff, senior lecturer; Clare Hunt, research associate; Judy Filmer, research midwife; Jeni Fetherstone, research midwife; Laura Scholtz, research midwife; Hayley Tarft, research midwife; Anna Lucas, HT; Tsigerada Tekletdadik, HT; Deborah Ricketts, HT; Carolyn Gill, laboratory technician; Alex Seroge Ignatian, trial administrator; Catherine Boylen, research assistant; Funso Adegoke, research assistant; Elodie Lawley, research assistant; James Butler, research assistant; Rahat Maitland, Doctor of Medicine student; Matias Vieira, Doctor of Philosophy student; Dharmintra Pasupathy, senior lecturer; and Louise Goff, senior lecturer.
King’s College Hospital
Eugene Oteng-Ntim, co-PI, centre clinical lead; Nina Khazaezadeh, co-PI; Jill Demilew, consultant midwife; Sile O’Connor, research midwife; Yvonne Evans, research midwife; Susan O’Donnell, research midwife; Ari de la Llera, research midwife; Georgina Gutzwiller, HT; and Linda Hagg, HT.
Newcastle University/Newcastle NHS Foundation Trust
Stephen Robson, co-PI, centre clinical lead; Ruth Bell, co-PI; Louise Hayes, postdoctoral fellow; Tarja Kinnunen, postdoctoral fellow; Catherine McParlin, research midwife; Nicola Miller, research midwife; Alison Kimber, research midwife; Jill Riches, research midwife; Carly Allen, research midwife; Claire Boag, research midwife; Fiona Campbell, research midwife; Andrea Fenn, research midwife; Sarah Ritson, HT; Alison Rennie, HT; Robin Durkin, HT; Gayle Gills, HT; and Roger Carr, laboratory technician.
Glasgow University and Greater Clyde Health board
Scott Nelson, co-PI, centre clinical lead; Naveed Sattar, co-PI; Therese McSorley, research midwife; Hilary Alba, research midwife; Kirsteen Paterson, research midwife; Janet Johnston, research nurse; Suzanne Clements, research nurse; Maxine Fernon, research nurse; Savannah Bett, research nurse; Laura Rooney, research nurse; Sinead Miller, research nurse; Paul Welsh, postdoctoral fellow; and Lynn Cherry, laboratory technician.
Central Manchester Hospitals Foundation Trust
Melissa Whitworth, centre clinical lead; Natalie Patterson, research midwife; Sarah Lee, research midwife; Rachel Grimshaw, research midwife; Christine Hughes, HT; and Jay Brown, laboratory technician.
City Hospital Sunderland
Kim Hinshaw, centre clinical lead; Gillian Campbell, research midwife; and Joanne Knight, research midwife.
Bradford Royal Infirmary
Diane Farrar, centre clinical lead; Vicky Jones, research midwife; Gillian Butterfield, research midwife; Jennifer Syson, research midwife; Jennifer Eadle, research midwife; Dawn Wood, HT; and Merane Todd, HT.
St George’s NHS Trust, London
Asma Khalil, centre clinical lead; Deborah Brown, research midwife; Paola Fernandez, research midwife; Emma Cousins, laboratory support; and Melody Smith, laboratory support.
Others
University College London
Jane Wardle, co-PI; Helen Croker, postdoctoral fellow; Laura Broomfield, postdoctoral fellow; and Weight Concern (registered charity number 1059686).
University of Southampton
Keith Godfrey, co-PI; Sian Robinson, Professor of Nutritional Epidemiology; Sarah Canadine, postdoctoral fellow; and Lynne Greenwood, anthropometric trainer.
Trial Steering Committee
Catherine Nelson-Piercy, chairperson, Professor of Obstetric Medicine; Stephanie Amiel, Professor of Diabetic Medicine; Gail Goldberg, Senior Scientist in Nutrition; Daghni Rajasingham, Consultant Obstetrician; Penny Jackson, Diabetes Specialist Dietician; Sara Kenyon, Reader in Evidence Based Maternity Care; and Patrick Catalano, Professor of Maternal Fetal Medicine.
Contributions of authors
Lucilla Poston (Professor of Maternal and Fetal Health) was the lead applicant and chief investigator with overall responsibility for the programme grant and oversaw all aspects of writing the report.
Ruth Bell (Senior Lecturer in Public Health) was a co-applicant, was responsible for the design and supervision of the PA intervention and contributed to writing the report.
Annette L Briley (Consultant Midwife and Clinical Trial Co-ordinator) was a co-applicant, was responsible for the management of UPBEAT and contributed to the writing of the report.
Keith M Godfrey (Professor of Epidemiology and Human Development) was a co-applicant, contributed to the trial design, was responsible for the neonatal anthropometric measurement methods and contributed to writing the report.
Scott M Nelson (Professor of Obstetrics and Gynaecology) was clinical lead at the Glasgow trial centre and contributed to the design of the study and to the writing of the report.
Eugene Oteng-Ntim (Consultant Obstetrician) was lead obstetrician at the trial centre at King’s College Hospital and contributed to the design of the study and to the writing of the report.
Jane Sandall (Professor of Social Science and Women’s Health) was a co-applicant, was responsible for the process evaluation in the programme and contributed to the writing of the report.
Thomas AB Sanders (Professor of Nutrition and Dietetics) was a co-applicant, had overall responsibility for the nutritional intervention and dietary assessment and contributed to the writing of the report.
Naveed Sattar (Professor of Metabolic Medicine) had responsibility for laboratory measurements and contributed to the design of the study and the writing of the report.
Paul T Seed (Senior Lecturer in Medical Statistics) was responsible for the data analysis plan for the programme, was the trial statistician and contributed to the writing of the report.
Stephen C Robson (Professor of Fetal Medicine) was the clinical lead for the trial centre in Newcastle and contributed to the design of the study and to writing of the report.
Dominic Trépel (Research Fellow in Health Economics) was responsible for the health economics analysis and contributed to the writing of the report.
Jane Wardle (Professor of Clinical Psychology; Epidemiology & Public Health) was responsible for the psychological element of the intervention and contributed to the writing of the report (deceased October 2015).
Publications
Peer-reviewed papers arising from programme
Gardner B, Wardle J, Poston L, Croker H. Changing diet and physical activity to reduce gestational weight gain: a meta-analysis. Obes Rev 2011;12:e602–20. http://dx.doi.org/10.1111/j.1467-789X.2011.00884.x
Kinnunen TI, Tennant PW, McParlin C, Poston L, Robson SC, Bell R. Agreement between pedometer and accelerometer in measuring physical activity in overweight and obese pregnant women. BMC Public Health 2011;11:501. http://dx.doi.org/10.1186/1471-2458-11-501
Gardner B, Croker H, Barr S, Briley A, Poston L, Wardle J. Psychological predictors of dietary intentions in pregnancy. J Hum Nutr Diet 2012;25:345–53.
Oteng-Ntim E, Varma R, Croker H, Poston L, Doyle P. Lifestyle interventions for overweight and obese pregnant women to improve pregnancy outcome: systematic review and meta-analysis. BMC Med 2012;10:47. http://dx.doi.org/10.1186/1741-7015-10-47
Poston L, Briley AL, Barr S, Bell R, Croker H, Coxon K, et al. Developing a complex intervention for diet and activity behaviour change in obese pregnant women (the UPBEAT trial); assessment of behavioural change and process evaluation in a pilot randomised controlled trial. BMC Pregnancy Childbirth 2013;13:148.
Poston L. Gestational weight gain: influences on the long-term health of the child. Curr Opin Clin Nutr Metab Care 2012;15:252–7. http://dx.doi.org/10.1097/MCO.0b013e3283527cf2
Briley AL, Barr S, Badger S, Bell R, Croker H, Godfrey KM, et al. A complex intervention to improve pregnancy outcome in obese women; the UPBEAT randomised controlled trial. BMC Pregnancy Childbirth 2014;14:74.
Maitland RA, Seed PT, Briley AL, Homsy M, Thomas S, Pasupathy D, et al. Prediction of gestational diabetes in obese pregnant women from the UK Pregnancies Better Eating and Activity (UPBEAT) pilot trial. Diabet Med 2014;31:963–70. http://dx.doi.org/10.1111/dme.12482
Hayes L, Bell R, Robson S, Poston L, UPBEAT Consortium. Association between physical activity in obese pregnant women and pregnancy outcomes: the UPBEAT pilot study. Ann Nutr Metab 2014;64:239–46. http://dx.doi.org/10.1159/000365027
Hayes L, Mcparlin C, Kinnunen TI, Poston L, Robson SC, Bell R, on behalf of the UPBEAT Consortium. Change in level of physical activity during pregnancy in obese women: findings from the UPBEAT pilot trial. BMC Pregnancy Childbirth 2015;15:52.
Poston L, Bell R, Croker H, Flynn AC, Godfrey KM, Goff L, et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol 2015;3:767–77. http://dx.doi.org/10.1016/S2213-8587(15)00227-2
White SL, Lawlor DA, Briley AL, Godfrey KM, Nelson SM, Oteng-Ntim E, et al. Early antenatal prediction of gestational diabetes in obese women: development of prediction tools for targeted intervention. PLOS ONE 2016;11:e0167846.
Flynn AC, Seed PT, Patel N, Barr S, Bell R, Briley AL, et al. Dietary patterns in obese pregnant women; influence of a behavioral intervention of diet and physical activity in the UPBEAT randomized controlled trial. Int J Behav Nutr Phys Act 2016;13:124.
Conference proceedings
Sandall J, Hunt C, on behalf of the UPBEAT team. ‘Fat Groups, and All That Sort of Business, It Does Nothing for Me’: Gaining Insights into UPBEAT: the UK Better Eating and Activity Trial (UPBEAT). Annual Academic Meeting in Obstetrics and Gynaecology, Royal College of Obstetricians and Gynaecologists, London, UK, 18–19 November 2010.
Sandall J, Hunt C, Poston L, Briley A, Oteng-Ntim E, Khasaezadeh N, et al. Inside the ‘black box’: process evaluation of the upbeat pilot trial. Arch Dis Child Fetal Neonatal Ed 2011;96:Fa134.
Hunt C, Sandall J, Briley A, Robson S, Poston L, on behalf of the UPBEAT team. Inside the ‘Black Box’: Process Evaluation of the Upbeat Pilot Trial. Poster, Perinatal Medicine 2011, Harrogate, UK, 15–17 June 2011.
Osbourne K. Follow-up Study of Women Participating in UPBEAT. Master’s in Public Health. King’s College London, London, UK, 2013.
Hilton C. Factors Associated with Physical Activity in UPBEAT. Master’s in Research. King’s College London, London, UK, 2016.
Sandall J. Research Masterclass: Evaluation of Complex Interventions Drawing on Recent Process and Implementation Evaluation of the UPBEAT RCT. University of Technology, Sydney, NSW, Australia, 1 October 2014.
Sandall J, on behalf of UPBEAT trial group. A Multicentre Randomised Controlled Trial of a Behavioural Intervention in Obese Pregnant Women; the UPBEAT Study. 10th International Normal Birth Conference, Grange-Over-Sands, UK, 15–17 June 2015.
McParlin C, Kinnunen TI, Tennant PWG, Poston L, Bell R, Robson SC. Objective Measurement of Physical Activity in Overweight and Obese Pregnant Women. British Maternal & Fetal Medicine Society, Newcastle, UK, 10 June 2010.
Kinnunen TI, McParlin C, Tennant P, Robson S, Poston L, Bell R. Physical Activity in Overweight and Obese Pregnant Women Assessed by Two Objective Methods. 11th International Conference on Obesity, Stockholm, Sweden, 11–15 July 2010.
Barr S, Poston L, Briley A, Oteng-Ntim E, Holmes B, Kinnunen T, et al. Habitual Dietary Intake of Obese Pregnant Women in the UK. Nutrition Society, London, UK, 6 June 2011.
Poston L, Holmes B, Kinnunen T, Croker H, Bell R, Sanders T, et al. A Complex Intervention to Improve Outcome in Obese Pregnancies; the UPBEAT Study. British Maternal & Fetal Medicine Society, Harrogate, UK, 16 June 2011.
Sandall J, Hunt C, Poston L, Briley A, Oteng-Ntim E, Khasaezadeh N, et al. Inside the ‘Black Box’; Process Evaluation of the UPBEAT Pilot Trial. British Maternal & Fetal Medicine Society, Harrogate, UK, 16 June 2011.
Briley A, Holmes B, Kinnunen T, Croker H, Bell R, Sanders T, et al. Development of a Complex Intervention to Improve Outcome in Obese Pregnancies; the UPBEAT Protocol. 58th Annual Meeting, Society of Gynecologic Investigation, Miami, FL, USA, 16–19 March 2011.
Sandall J. What Happens When the Trial Finishes? Using Normalization Process Theory to Understand Implementation; Translating Research into Policy and Practice: Strategies and Challenges for Making Change Happen! Invited presentation, International Workshop on Trials of Complex Interventions, University of Technology Sydney, NSW, Australia, 17 February 2011.
Molyneaux E, Micali N, Poston L, Howard L. Depression, Obesity and Dietary Patterns During Pregnancy. Marcé Society, Paris, France, 3 October 2012.
Hayes L, Kinnunen T, Robson S, McParlin C, Poston L, Bell R. Factors Associated with Level of Physical Activity in Obese Pregnant Women Participating in the UPBEAT Pilot Trial. British Maternal & Fetal Medicine Society, Glasgow, UK, 19 April 2012.
Hayes L, Kinnunen TI, Robson S, Poston L, Bell R. Maternal Blood Glucose and Objectively Measured Physical Activity. Poster, European Diabetes Epidemiology Group Annual Meeting, Swansea, UK, 12–15 May 2012.
Hayes L, Kinnunen TI, Robson S, Poston L, Bell R. Relationship Between Objectively Measured Physical Activity and Maternal Blood Glucose. International Society for Behavioural Nutrition and Physical Activity Annual Meeting, Ghent, Belgium, 22–25 May 2013.
Maitland R, Sattar N, Seed PT, Briley AL, Thomas S, Pasupathy D, et al. Prediction of Gestational Diabetes in Obese Pregnant Women. Society for Gynecologic Investigation, Orlando, FL, USA, 20–23 March 2013.
Maitland R, Sattar N, Seed PT, Briley AL, Thomas S, Pasupathy D, et al. Prediction of Gestational Diabetes in Obese Pregnant Women. Diabetes UK, Manchester, UK, 13–15 March 2013.
Flynn A, Kader S, Poston L, Goff L. Improvements in Diet Quality in the UPBEAT study: A Lifestyle Intervention to Improve Outcomes in Obese Pregnancy. Physiology Society Meeting, Obesity: A Physiological Perspective, Newcastle, UK, 10–12 September 2014.
Poston L, Holmes B, Kinnunen T, Croker H, Bell R, Sanders T, et al. A Complex Intervention to Improve Outcome in Obese Pregnancies; the UPBEAT Study. Physiology Society Meeting, Obesity: A Physiological Perspective, Newcastle, UK, 10–12 September 2014.
Poston L, Holmes B, Kinnunen T, Croker H, Bell R, Sanders T, et al. A Complex Intervention to Improve Outcome in Obese Pregnancies; the UPBEAT Study. 17th World Congress of Basic and Clinical Pharmacology, Cape Town, South Africa, 13–18 July 2014.
Hayes L, Bell R, Robson S, Poston L, on behalf of the UPBEAT Consortium. Association Between Physical Activity in Obese Pregnant Women and Health of the Offspring. EU Early Nutrition Academy, The Power of Programming: International Conference on Developmental Origins of Adiposity and Long Term Health, Munich, Germany, 13–15 March 2014.
Poston L, Holmes B, Kinnunen T, Croker H, Bell R, Sanders T, et al. A Complex Intervention to Improve Outcome in Obese Pregnancies; the UPBEAT Study. EU Early Nutrition Academy. Power of Programming Meeting, Munich, Germany, 13–15 March 2014.
Hayes L. Association Between Physical Activity in Obese Pregnant Women and Health of the Offspring. Association for the Study of Obesity, Conference on Maternal Obesity, London, UK, 12 March 2014.
Hayes L, Robson S, Poston L, Bell R, on behalf of the UPBEAT Consortium. Changes in Physical Activity During Pregnancy and Relationship with Pregnancy Outcomes. European Diabetes Epidemiology Group Annual Meeting, Quartu Sant’Elena, Sardinia, 29 March–1 April 2014.
Schneeberger C, Flynn A, Barr S, Seed P, Inskip H, Poston L. Maternal Diet Patterns and Glycaemic Load in Obese Pregnant Women Taking Part in a Pilot Trial of a Lifestyle Intervention (the UPBEAT Trial). American Diabetes Association’s 74th Scientific Sessions, San Francisco, CA, USA, 13–17 June 2014.
Poston L, Holmes B, Kinnunen T, Croker H, Bell R, Sanders T, et al. A Complex Intervention to Improve Outcome in Obese Pregnancies; the UPBEAT Study. Keynote Speaker, 3rd National Diabetes in Pregnancy Conference, Gateshead, UK, 9 October 2014.
Poston L, Holmes B, Kinnunen T, Croker H, Bell R, Sanders T, et al. A Complex Intervention to Improve Outcome in Obese Pregnancies; the UPBEAT Study. American Diabetes Association’s 75th Scientific Sessions, Boston, MA, USA, 5–9 June 2015.
Ireland VF, Flynn A, Croker H, Goff LM. Observational Study of Eating Behaviours, BMI and Diet in Obese Pregnant Women. British Dietetic Association Research Symposium, Birmingham, UK, 2 December 2015.
Poston L. Obesity and Pregnancy. Hot Topic Conference World Obesity Confederation, invited speaker, London, UK, 30 October 2015.
Poston L, Holmes B, Kinnunen T, Croker H, Bell R, Sanders T, et al. The UPBEAT trial. 9th World Congress of Developmental Origins of Disease, Cape Town, South Africa, 8–11 November 2015.
Data sharing statement
The UPBEAT consortium agrees to data sharing subject to approval by the UPBEAT Scientific Advisory Committee and appropriate ethical and other regulatory approvals. Please contact the lead author for further information on available data or visit the study website www.medscinet.net/upbeat/ for general information.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health.
References
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. https://doi.org/10.1136/bmj.a1655.
- Gardner B, Wardle J, Poston L, Croker H. Changing diet and physical activity to reduce gestational weight gain: a meta-analysis. Obes Rev 2011;12:e602-20. http://dx.doi.org/10.1111/j.1467-789X.2011.00884.x.
- Gardner B, Croker H, Barr S, Briley A, Poston L, Wardle J. Psychological predictors of dietary intentions in pregnancy. J Hum Nutr Diet 2012;25:345-53. https://doi.org/10.1111/j.1365-277X.2012.01239.x.
- Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) trial group . Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005;352:2477-86. http://dx.doi.org/10.1056/NEJMoa042973.
- Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 2009;361:1339-48. http://dx.doi.org/10.1056/NEJMoa0902430.
- Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PM, Damm P, et al. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33:676-82. https://doi.org/10.2337/dc10-0719.
- Louie JC, Brand-Miller JC, Markovic TP, Ross GP, Moses RG. Glycemic index and pregnancy: a systematic literature review. J Nutr Metab 2010;2010. http://dx.doi.org/10.1155/2010/282464.
- Clapp JF. Effects of diet and exercise on insulin resistance during pregnancy. Metab Syndr Relat Disord 2006;4:84-90. http://dx.doi.org/10.1089/met.2006.4.84.
- Nelson SM, Matthews P, Poston L. Maternal metabolism and obesity: modifiable determinants of pregnancy outcome. Hum Reprod Update 2010;16:255-75. http://dx.doi.org/10.1093/humupd/dmp050.
- Brand-Miller J, McMillan-Price J, Steinbeck K, Caterson I. Dietary glycemic index: health implications. J Am Coll Nutr 2009;28:446S-9S. https://doi.org/10.1080/07315724.2009.10718110.
- Clapp JF. Diet, exercise, and feto-placental growth. Arch Gynecol Obstet 1997;260:101-8.
- Moses RG, Luebcke M, Davis WS, Coleman KJ, Tapsell LC, Petocz P, et al. Effect of a low-glycemic-index diet during pregnancy on obstetric outcomes. Am J Clin Nutr 2006;84:807-12.
- Scholl TO, Chen X, Khoo CS, Lenders C. The dietary glycemic index during pregnancy: influence on infant birth weight, fetal growth, and biomarkers of carbohydrate metabolism. Am J Epidemiol 2004;159:467-74. https://doi.org/10.1093/aje/kwh068.
- Rhodes ET, Pawlak DB, Takoudes TC, Ebbeling CB, Feldman HA, Lovesky MM, et al. Effects of a low-glycemic load diet in overweight and obese pregnant women: a pilot randomized controlled trial. Am J Clin Nutr 2010;92:1306-15. http://dx.doi.org/10.3945/ajcn.2010.30130.
- Walsh J, Mahony R, Foley M, Mc Auliffe F. A randomised control trial of low glycaemic index carbohydrate diet versus no dietary intervention in the prevention of recurrence of macrosomia. BMC Pregnancy Childbirth 2010;10. http://dx.doi.org/10.1186/1471-2393-10-16.
- Schwarzer R, Schwarzer R. Self-Efficacy: Thought Control of Action. Abingdon: Routledge; 1992.
- Bartholomew LK, Parcel GS, Kok G, Gottlieb NH. Intervention Mapping: Designing Theory and Evidence-based Health Promotion Programs. Boston, MA: McGraw-Hill; 2006.
- Skouteris H, Hartley-Clark L, McCabe M, Milgrom J, Kent B, Herring SJ, et al. Preventing excessive gestational weight gain: a systematic review of interventions. Obes Rev 2010;11:757-68. https://doi.org/10.1111/j.1467-789X.2010.00806.x.
- Phelan S. Pregnancy: a ‘teachable moment’ for weight control and obesity prevention. Am J Obstet Gynecol 2010;202:135.e1-8. http://dx.doi.org/10.1016/j.ajog.2009.06.008.
- Rosenstock IM. Historical origins of the health belief model. Health Educ Monogr 1974;2:328-35. https://doi.org/10.1177/109019817400200403.
- Abraham C, Sheeran P, Conner M, Norman P. Predicting Health Behaviour: Research and Practice with Social Cognition Models. London: Open University Press; 2005.
- Ajzen I, Fishbein M. Understanding Attitudes and Predicting Social Behaviour. Upper Saddle River, NJ: Prentice-Hall; 1975.
- The Pregnancy Book. London: Central Office of Information; 2009.
- Fowles ER, Fowles SL. Healthy eating during pregnancy: determinants and supportive strategies. J Community Health Nurs 2008;25:138-52. http://dx.doi.org/10.1080/07370010802221727.
- Olander EK, Atkinson L, Edmunds JK, French DP. The views of pre- and post-natal women and health professionals regarding gestational weight gain: an exploratory study. Sex Reprod Healthc 2011;2:43-8. https://doi.org/10.1016/j.srhc.2010.10.004.
- Trenkner LL, Rooney B, Viswanath K, Baxter J, Elmer P, Finnegan JR, et al. Development of a scale using nutrition attitudes for audience segmentation. Health Educ Res 1990;5:479-87. https://doi.org/10.1093/her/5.4.479.
- Tiedje LB, Kingry MJ, Stommel M. Patient attitudes concerning health behaviors during pregnancy: initial development of a questionnaire. Health Educ Q 1992;19:481-93. https://doi.org/10.1177/109019819201900411.
- Ajzen I. The theory of planned behavior. Organ Behav Hum Dec 1991;50:179-211. https://doi.org/10.1016/0749-5978(91)90020-T.
- Gray-Donald K, Robinson E, Collier A, David K, Renaud L, Rodrigues S. Intervening to reduce weight gain in pregnancy and gestational diabetes mellitus in Cree communities: an evaluation. CMAJ 2000;163:1247-51.
- Kinnunen TI, Pasanen M, Aittasalo M, Fogelholm M, Hilakivi-Clarke L, Weiderpass E, et al. Preventing excessive weight gain during pregnancy - a controlled trial in primary health care. Eur J Clin Nutr 2007;61:884-91. http://dx.doi.org/10.1038/sj.ejcn.1602602.
- Asbee SM, Jenkins TR, Butler JR, White J, Elliot M, Rutledge A. Preventing excessive weight gain during pregnancy through dietary and lifestyle counseling a randomized controlled trial. Obstet Gynecol 2009;113:305-11. https://doi.org/10.1097/AOG.0b013e318195baef.
- Shirazian T, Monteith S, Friedman F, Rebarber A. Lifestyle modification program decreases pregnancy weight gain in obese women. Am J Perinatol 2010;27:411-14. http://dx.doi.org/10.1055/s-0029-1243368.
- Luoto R, Kinnunen TI, Aittasalo M, Kolu P, Raitanen J, Ojala K, et al. Primary prevention of gestational diabetes mellitus and large-for-gestational-age newborns by lifestyle counseling: a cluster-randomized controlled trial. PLOS Med 2011;8. http://dx.doi.org/10.1371/journal.pmed.1001036.
- Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health 2011;26:1479-98. http://dx.doi.org/10.1080/08870446.2010.540664.
- Webb TL, Sheeran P. Does changing behavioral intentions engender behavior change? A meta-analysis of the experimental evidence. Psychol Bull 2006;132:249-68. http://dx.doi.org/10.1037/0033-2909.132.2.249.
- Paulhus DL, Paulhas DL. Personality Assessment Via Questionnaire. Berlin: Springer-Verlag; 1986.
- Yu SM, Nagey DA. Validity of self-reported pregravid weight. Ann Epidemiol 1992;2:715-21. https://doi.org/10.1016/1047-2797(92)90016-J.
- Kinnunen TI, Tennant PW, McParlin C, Poston L, Robson SC, Bell R. Agreement between pedometer and accelerometer in measuring physical activity in overweight and obese pregnant women. BMC Public Health 2011;11. http://dx.doi.org/10.1186/1471-2458-11-501.
- Exercise in Pregnancy. London: RCOG; 2006.
- Haskell WL, Nelson ME. Physical Activity Guidelines Committee Report. Washington, DC: US Department of Health and Human Services; 2008.
- Kramer MS, McDonald SW. Aerobic exercise for women during pregnancy. Cochrane Database Syst Rev 2006;3. https://doi.org/10.1002/14651858.cd000180.pub2.
- Chasan-Taber L, Evenson KR, Sternfeld B, Kengeri S. Assessment of recreational physical activity during pregnancy in epidemiologic studies of birthweight and length of gestation: methodologic aspects. Women Health 2007;45:85-107. http://dx.doi.org/10.1300/J013v45n04_05.
- Chasan-Taber L, Schmidt MD, Roberts DE, Hosmer D, Markenson G, Freedson PS. Development and validation of a pregnancy physical activity questionnaire. Med Sci Sport Exer 2004;36:1750-60. https://doi.org/10.1249/01.MSS.0000142303.49306.0D.
- Schmidt MD, Freedson PS, Pekow P, Roberts D, Sternfeld B, Chasan-Taber L. Validation of the Kaiser Physical Activity Survey in pregnant women. Med Sci Sports Exerc 2006;38:42-50. https://doi.org/10.1249/01.mss.0000181301.07516.d6.
- Evenson KR, Wen F. Measuring physical activity among pregnant women using a structured one-week recall questionnaire: evidence for validity and reliability. Int J Behav Nutr Phys Act 2010;7. http://dx.doi.org/10.1186/1479-5868-7-21.
- Haakstad LA, Gundersen I, Bø K. Self-reporting compared to motion monitor in the measurement of physical activity during pregnancy. Acta Obstet Gynecol Scand 2010;89:749-56. http://dx.doi.org/10.3109/00016349.2010.484482.
- Aittasalo M, Pasanen M, Fogelholm M, Ojala K. Validity and repeatability of a short pregnancy leisure time physical activity questionnaire. J Phys Act Health 2010;7:109-18. https://doi.org/10.1123/jpah.7.1.109.
- Poudevigne MS, O’Connor PJ. A review of physical activity patterns in pregnant women and their relationship to psychological health. Sports Med 2006;36:19-38. https://doi.org/10.2165/00007256-200636010-00003.
- Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act 2008;5. http://dx.doi.org/10.1186/1479-5868-5-56.
- Neilson HK, Robson PJ, Friedenreich CM, Csizmadi I. Estimating activity energy expenditure: how valid are physical activity questionnaires?. Am J Clin Nutr 2008;87:279-91.
- Stein AD, Rivera JM, Pivarnik JM. Measuring energy expenditure in habitually active and sedentary pregnant women. Med Sci Sports Exerc 2003;35:1441-6. http://dx.doi.org/10.1249/01.MSS.0000079107.04349.9A.
- Rousham EK, Clarke PE, Gross H. Significant changes in physical activity among pregnant women in the UK as assessed by accelerometry and self-reported activity. Eur J Clin Nutr 2006;60:393-400. http://dx.doi.org/10.1038/sj.ejcn.1602329.
- Oostdam N, van Poppel MN, Eekhoff EM, Wouters MG, van Mechelen W. Design of FitFor2 study: the effects of an exercise program on insulin sensitivity and plasma glucose levels in pregnant women at high risk for gestational diabetes. BMC Pregnancy Childbirth 2009;9. http://dx.doi.org/10.1186/1471-2393-9-1.
- Downs DS, LeMasurier GC, DiNallo JM. Baby steps: pedometer-determined and self-reported leisure-time exercise behaviors of pregnant women. J Phys Act Health 2009;6:63-72. https://doi.org/10.1123/jpah.6.1.63.
- Corder K, Brage S, Ekelund U. Accelerometers and pedometers: methodology and clinical application. Curr Opin Clin Nutr Metab Care 2007;10:597-603. https://doi.org/10.1097/MCO.0b013e328285d883.
- Harrison CL, Thompson RG, Teede HJ, Lombard CB. Measuring physical activity during pregnancy. Int J Behav Nutr Phys Act 2011;8. http://dx.doi.org/10.1186/1479-5868-8-19.
- Connolly CP, Coe DP, Kendrick JM, Bassett DRJ, Thompson DL. Accuracy of physical activity monitors in pregnant women. Med Sci Sport Exer 2010:1100-5. https://doi.org/10.1249/MSS.0b013e3182058883.
- Tudor-Locke C, Ainsworth BE, Thompson RW, Matthews CE. Comparison of pedometer and accelerometer measures of free-living physical activity. Med Sci Sport Exer 2002;34:2045-51. https://doi.org/10.1097/00005768-200212000-00027.
- Macfarlane DJ, Chan D, Chan KL, Ho EY, Lee CC. Using three objective criteria to examine pedometer guidelines for free-living individuals. Eur J Appl Physiol 2008;104:435-44. http://dx.doi.org/10.1007/s00421-008-0789-4.
- Ramírez-Marrero FA, Rivera-Brown AM, Nazario CM, Rodríguez-Orengo JF, Smit E, Smith BA. Self-reported physical activity in Hispanic adults living with HIV: comparison with accelerometer and pedometer. J Assoc Nurses AIDS Care 2008;19:283-94. http://dx.doi.org/10.1016/j.jana.2008.04.003.
- Harris TJ, Owen CG, Victor CR, Adams R, Ekelund U, Cook DG. A comparison of questionnaire, accelerometer, and pedometer: measures in older people. Med Sci Sports Exerc 2009;41:1392-402. http://dx.doi.org/10.1249/MSS.0b013e31819b3533.
- Siega-Riz AM, King JC. American Dietetic Association. Position of the American Dietetic Association and American Society for Nutrition: obesity, reproduction, and pregnancy outcomes. J Am Diet Assoc 2009;109:918-27. https://doi.org/10.1016/j.jada.2009.03.020.
- Weissgerber TL, Wolfe LA, Davies GA, Mottola MF. Exercise in the prevention and treatment of maternal-fetal disease: a review of the literature. Appl Physiol Nutr Metab 2006;31:661-74. http://dx.doi.org/10.1139/h06-060.
- Trost SG, McIver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc 2005;37:531-43. https://doi.org/10.1249/01.mss.0000185657.86065.98.
- Chen KY, Bassett DR. The technology of accelerometry-based activity monitors: current and future. Med Sci Sports Exerc 2005;37:490-50. https://doi.org/10.1249/01.mss.0000185571.49104.82.
- Plasqui G, Westerterp KR. Physical activity assessment with accelerometers: an evaluation against doubly labeled water. Obesity 2007;15:2371-9. https://doi.org/10.1038/oby.2007.281.
- Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381-95. http://dx.doi.org/10.1249/01.MSS.0000078924.61453.FB.
- Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc 1998;30:777-81. https://doi.org/10.1097/00005768-199805000-00021.
- Crouter SE, Schneider PL, Karabulut M, Bassett DR. Validity of 10 electronic pedometers for measuring steps, distance, and energy cost. Med Sci Sports Exerc 2003;35:1455-60. http://dx.doi.org/10.1249/01.MSS.0000078932.61440.A2.
- Schneider PL, Crouter SE, Lukajic O, Bassett DR. Accuracy and reliability of 10 pedometers for measuring steps over a 400-m walk. Med Sci Sports Exerc 2003;35:1779-84. http://dx.doi.org/10.1249/01.MSS.0000089342.96098.C4.
- Tudor-Locke C, Bassett DR. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med 2004;34:1-8. https://doi.org/10.2165/00007256-200434010-00001.
- Tudor-Locke C, Hatano Y, Pangrazi RP, Kang M. Revisiting ‘how many steps are enough?’. Med Sci Sports Exerc 2008;40:537-43. http://dx.doi.org/10.1249/MSS.0b013e31817c7133.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. https://doi.org/10.2307/2529310.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307-10. https://doi.org/10.1016/S0140-6736(86)90837-8.
- Schlussel M, Souza E, Reichenheim M, Kac G. Physical activity during pregnancy and maternal-child health outcomes: a systematic literature review. Cad Saude Publica 2008;24:531-44. https://doi.org/10.1590/S0102-311X2008001600006.
- Rothney MP, Apker GA, Song Y, Chen KY. Comparing the performance of three generations of ActiGraph accelerometers. J Appl Physiol 2008;105:1091-7. http://dx.doi.org/10.1152/japplphysiol.90641.2008.
- Kozey SL, Staudenmayer JW, Troiano RP, Freedson PS. Comparison of the ActiGraph 7164 and the ActiGraph GT1M during self-paced locomotion. Med Sci Sports Exerc 2010;42:971-6. http://dx.doi.org/10.1249/MSS.0b013e3181c29e90.
- Melanson EL, Knoll JR, Bell ML, Donahoo WT, Hill JO, Nysse LJ, et al. Commercially available pedometers: considerations for accurate step counting. Prev Med 2004;39:361-8. http://dx.doi.org/10.1016/j.ypmed.2004.01.032.
- Le Masurier GC, Tudor-Locke C. Comparison of pedometer and accelerometer accuracy under controlled conditions. Med Sci Sports Exerc 2003;35:867-71. http://dx.doi.org/10.1249/01.MSS.0000064996.63632.10.
- Mottola M, Giroux I, Gratton R, Hammond J, Hanley A, Harris S, et al. Nutrition and exercise prevents excess weight gain in overweight pregnant women. Med Sci Sports Exerc 2009:265-72. https://doi.org/10.1249/MSS.0b013e3181b5419a.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- World Health Organization . Obesity and Overweight Factsheet 311 2011. www.who.int/mediacentre/factsheets/fs311/en/ (accessed 9 January 2015).
- Rasmussen KM, Yaktine AL. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academies Press; 2009.
- Thangaratinam S, Rogozinska E, Jolly K, Glinkowski S, Roseboom T, Tomlinson JW, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e2088.
- Oteng-Ntim E, Varma R, Croker H, Poston L, Doyle P. Lifestyle interventions for overweight and obese pregnant women to improve pregnancy outcome: systematic review and meta-analysis. BMC Med 2012;10. http://dx.doi.org/10.1186/1741-7015-10-47.
- Louie JC, Brand-Miller JC, Moses RG. Carbohydrates, glycemic index, and pregnancy outcomes in gestational diabetes. Curr Diab Rep 2013;13:6-11. http://dx.doi.org/10.1007/s11892-012-0332-1.
- Bowers K, Tobias DK, Yeung E, Hu FB, Zhang C. A prospective study of prepregnancy dietary fat intake and risk of gestational diabetes. Am J Clin Nutr 2012;95:446-53. http://dx.doi.org/10.3945/ajcn.111.026294.
- Tobias DK, Zhang C, van Dam RM, Bowers K, Hu FB. Physical activity before and during pregnancy and risk of gestational diabetes mellitus: a meta-analysis. Diabetes Care 2011;34:223-9. http://dx.doi.org/10.2337/dc10-1368.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337. http://dx.doi.org/10.1136/bmj.a1655.
- Oteng-Ntim E, Pheasant H, Khazaezadeh N, Mohidden A, Bewley S, Wong J, et al. Developing a community-based maternal obesity intervention: a qualitative study of service providers’ views. BJOG 2010;117:1651-5. http://dx.doi.org/10.1111/j.1471-0528.2010.02730.x.
- Department for Communities and Local Government . English Indices of Deprivation 2010 2011. www.gov.uk/government/publications/english-indices-of-deprivation-2010 (accessed 16 July 2013).
- The Scottish Government . Scottish Indices of Multiple Deprivation 2009: General Report 2009. www.scotland.gov.uk/Publications/2009/10/28104046/0 (accessed 16 July 2013).
- Dietary Interventions and Physical Activity Interventions for Weight Management Before, During and After Pregnancy. London: National Institute for Health and Care Excellence; 2010.
- Carver C, Scheier M. Origins and functions of positive and negative affect: a control-process view. Psychological Review 1990;97:19-35. https://doi.org/10.1037/0033-295X.97.1.19.
- Bandura A. Health promotion by social cognitive means. Health Educ Behav 2004;31:143-64. http://dx.doi.org/10.1177/1090198104263660.
- Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health Psychol 2009;28:690-701. https://doi.org/10.1037/a0016136.
- Sechrist KR, Walker SN, Pender NJ. Development and psychometric evaluation of the exercise benefits/barriers scale. Res Nurs Health 1987;10:357-65. https://doi.org/10.1002/nur.4770100603.
- EuroQoL Group . EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry 1987;150:782-6. https://doi.org/10.1192/bjp.150.6.782.
- Steckler A, Linnan L. Process Evaluation for Public Health Interventions and Research. San Francisco, CA: John Wiley & Sons; 2002.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. https://doi.org/10.1097/00005650-199711000-00002.
- Dolan P, Gudex C, Kind P, Williams A. The time trade-off method: results from a general population study. Health Econ 1996;5:141-54. https://doi.org/10.1002/(SICI)1099-1050(199603)5:2<141::AID-HEC189>3.0.CO;2-N.
- McCance RA, Widdowson EM. McCance and Widdowson’s The Composition of Foods: Summary Edition. London, UK: Royal Society Of Chemistry; 2002.
- Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008;31:2281-3. http://dx.doi.org/10.2337/dc08-1239.
- Aston LM, Jackson D, Monsheimer S, Whybrow S, Handjieva-Darlenska T, Kreutzer M, et al. Developing a methodology for assigning glycaemic index values to foods consumed across Europe. Obes Rev 2010;11:92-100. http://dx.doi.org/10.1111/j.1467-789X.2009.00690.x.
- Matthew CE. Calibration of accelerometer output for adults. Med Sci Sports Exerc 2005;37:512-22. https://doi.org/10.1249/01.mss.0000185659.11982.3d.
- Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32:498-504. https://doi.org/10.1097/00005768-200009001-00009.
- Green J, Thorogood N. Qualitative Research Methods for Health Research. London: Sage Publications Ltd; 2009.
- McClure JH, Cooper GM, Clutton-Brock TH. behalf of the Centre for Maternal and Child Enquiries . Saving mothers’ lives: reviewing maternal deaths to make motherhood safer: 2006-8: a review. BJOG 2011:1-203. https://doi.org/10.1111/j.1471-0528.2010.02847.x.
- Quinlivan JA, Lam LT, Fisher J. A randomised trial of a four-step multidisciplinary approach to the antenatal care of obese pregnant women. Aust N Z J Obstet Gynaecol 2011;51:141-6. http://dx.doi.org/10.1111/j.1479-828X.2010.01268.x.
- Guelinckx I, Devlieger R, Mullie P, Vansant G. Effect of lifestyle intervention on dietary habits, physical activity, and gestational weight gain in obese pregnant women: a randomized controlled trial. Am J Clin Nutr 2010;91:373-80. http://dx.doi.org/10.3945/ajcn.2009.28166.
- Bogaerts A, Devlieger R, Nuyts E, Witters I, Gyselaers W, Van den Bergh B. Effects of lifestyle intervention in obese pregnant women on gestational weight gain and mental health: a randomized controlled trial. Int J Obes (Lond) 2012;37:814-21. https://doi.org/10.1038/ijo.2012.162.
- Whitton C, Nicholson SK, Roberts C, Prynne CJ, Pot GK, Olson A, et al. National Diet and Nutrition Survey: UK food consumption and nutrient intakes from the first year of the rolling programme and comparisons with previous surveys. Br J Nutr 2011;106:1899-914. http://dx.doi.org/10.1017/S0007114511002340.
- Fabricatore AN, Wadden TA, Ebbeling CB, Thomas JG, Stallings VA, Schwartz S, et al. Targeting dietary fat or glycemic load in the treatment of obesity and type 2 diabetes: a randomized controlled trial. Diabetes Res Clin Pract 2011;92:37-45. http://dx.doi.org/10.1016/j.diabres.2010.12.016.
- Walsh JM, McGowan CA, Mahony R, Foley ME, McAuliffe FM. Low glycaemic index diet in pregnancy to prevent macrosomia (ROLO study): randomised control trial. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e5605.
- Jebb SA, Lovegrove JA, Griffin BA, Frost GS, Moore CS, Chatfield MD, et al. Effect of changing the amount and type of fat and carbohydrate on insulin sensitivity and cardiovascular risk: the RISCK (Reading, Imperial, Surrey, Cambridge, and Kings) trial. Am J Clin Nutr 2010;92:748-58. http://dx.doi.org/10.3945/ajcn.2009.29096.
- Thomas DE, Elliott EJ, Baur L. Low glycaemic index or low glycaemic load diets for overweight and obesity. Cochrane Database Syst Rev 2007;3. https://doi.org/10.1002/14651858.cd005105.pub2.
- Dietary Reference Values for Food Energy and Nutrients for the United Kingdom. London: The Stationery Office; 1991.
- Hui A, Back L, Ludwig S, Gardiner P, Sevenhuysen G, Dean H, et al. Lifestyle intervention on diet and exercise reduced excessive gestational weight gain in pregnant women under a randomised controlled trial. Br J Obstet Gynaecol 2012;119:70-7. https://doi.org/10.1111/j.1471-0528.2011.03184.x.
- Callaway LK, Colditz PB, Byrne NM, Lingwood BE, Rowlands IJ, Foxcroft K, et al. BAMBINO Group . Prevention of gestational diabetes: feasibility issues for an exercise intervention in obese pregnant women. Diabetes Care 2010;33:1457-9. http://dx.doi.org/10.2337/dc09-2336.
- Evenson KR, Wen F. Prevalence and correlates of objectively measured physical activity and sedentary behavior among US pregnant women. Prev Med 2011;53:39-43. http://dx.doi.org/10.1016/j.ypmed.2011.04.014.
- Aittasalo M, Raitanen J, Kinnunen TI, Ojala K, Kolu P, Luoto R. Is intensive counseling in maternity care feasible and effective in promoting physical activity among women at risk for gestational diabetes? Secondary analysis of a cluster randomized NELLI study in Finland. Int J Behav Nutr Phys Act 2012;9. http://dx.doi.org/10.1186/1479-5868-9-104.
- Oostdam N, van Poppel MN, Wouters MG, Eekhoff EM, Bekedam DJ, Kuchenbecker WK, et al. No effect of the FitFor2 exercise programme on blood glucose, insulin sensitivity, and birthweight in pregnant women who were overweight and at risk for gestational diabetes: results of a randomised controlled trial. BJOG 2012;119:1098-107. http://dx.doi.org/10.1111/j.1471-0528.2012.03366.x.
- McParlin C, Robson SC, Tennant PW, Besson H, Rankin J, Adamson AJ, et al. Objectively measured physical activity during pregnancy: a study in obese and overweight women. BMC Pregnancy Childbirth 2010;10. http://dx.doi.org/10.1186/1471-2393-10-76.
- Evenson KR, Savitz DA, Huston SL. Leisure-time physical activity among pregnant women in the US. Paediatr Perinat Epidemiol 2004;18:400-7. http://dx.doi.org/10.1111/j.1365-3016.2004.00595.x.
- Townsend N, Bhatnagar P, Wickramasinghe K, Scarborough P, Foster C, Rayner M. Physical Activity Statistics 2012. London: British Heart Foundation; 2012.
- Lof M, Forsum E. Activity pattern and energy expenditure due to physical activity before and during pregnancy in healthy Swedish women. Br J Nutr 2006;95:296-302. https://doi.org/10.1079/BJN20051497.
- Harrison CL, Lombard CB, Teede HJ. Understanding health behaviours in a cohort of pregnant women at risk of gestational diabetes mellitus: an observational study. BJOG 2012;119:731-8. http://dx.doi.org/10.1111/j.1471-0528.2012.03296.x.
- Claesson IM, Josefsson A, Sydsjö G. Prevalence of anxiety and depressive symptoms among obese pregnant and postpartum women: an intervention study. BMC Public Health 2010;10. http://dx.doi.org/10.1186/1471-2458-10-766.
- Statistics on Obesity, Physical Activity and Diet, England. Leeds: NHS Digital; 2013.
- Gatineau M, Mathrani S. Obesity and Ethnicity. Oxford: National Obesity Observatory; 2011.
- Kinnunen TI, Aittasalo M, Koponen P, Ojala K, Mansikkamäki K, Weiderpass E, et al. Feasibility of a controlled trial aiming to prevent excessive pregnancy-related weight gain in primary health care. BMC Pregnancy Childbirth 2008;8. http://dx.doi.org/10.1186/1471-2393-8-37.
- Hoddinott P, Allan K, Avenell A, Britten J. Group interventions to improve health outcomes: a framework for their design and delivery. BMC Public Health 2010;10. http://dx.doi.org/10.1186/1471-2458-10-800.
- Broekhuizen K, Althuizen E, van Poppel MNM, Donker M, van Mechelen W. From theory to practice: intervention fidelity in a randomized controlled trial aiming to optimize weight development during pregnancy. Health Promot Pract 2012;13:816-25. https://doi.org/10.1177/1524839912447190.
- Barnett J, Aguilar S, Brittner M, Bonuck K. Recruiting and retaining low-income, multi-ethnic women into randomized controlled trials: successful strategies and staffing. Contemp Clin Trials 2012;33:925-32. http://dx.doi.org/10.1016/j.cct.2012.06.005.
- Borodulin K, Evenson KR, Herring AH. Physical activity patterns during pregnancy through postpartum. BMC Womens Health 2009;9. http://dx.doi.org/10.1186/1472-6874-9-32.
- Hallal PC, Victora CG. Reliability and validity of the International Physical Activity Questionnaire (IPAQ). Med Sci Sports Exerc 2004;36. https://doi.org/10.1249/01.MSS.0000117161.66394.07.
- Maitland RA, Seed PT, Briley AL, Homsy M, Thomas S, Pasupathy D, et al. behalf of the UPBEAT trial consortium . Prediction of gestational diabetes in obese pregnant women from the UK Pregnancies Better Eating and Activity (UPBEAT) pilot trial. Diabet Med 2014;31:963-70. http://dx.doi.org/10.1111/dme.12482.
- Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Comparative Risk Assessment Collaborating Group . Selected major risk factors and global and regional burden of disease. Lancet 2002;360:1347-60. https://doi.org/10.1016/S0140-6736(02)11403-6.
- Heslehurst N, Rankin J, Wilkinson JR, Summerbell CD. A nationally representative study of maternal obesity in England, UK: trends in incidence and demographic inequalities in 619 323 births, 1989-2007. Int J Obes 2010;34:420-8. http://dx.doi.org/10.1038/ijo.2009.250.
- Athukorala C, Rumbold AR, Willson KJ, Crowther CA. The risk of adverse pregnancy outcomes in women who are overweight or obese. BMC Pregnancy Childbirth 2010;10. http://dx.doi.org/10.1186/1471-2393-10-56.
- Chu SY, Callaghan WM, Kim SY, Schmid CH, Lau J, England LJ, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care 2007;30:2070-6. http://dx.doi.org/10.2337/dc06-2559a.
- Scott DA, Loveman E, McIntyre L, Waugh N. Screening for gestational diabetes: a systematic review and economic evaluation. Health Technol Assess 2002;6. https://doi.org/10.3310/hta6110.
- Dodd JM, Turnbull DA, McPhee AJ, Wittert G, Crowther CA, Robinson JS. Limiting weight gain in overweight and obese women during pregnancy to improve health outcomes: the LIMIT randomised controlled trial. BMC Pregnancy Childbirth 2011;11. http://dx.doi.org/10.1186/1471-2393-11-79.
- Akolekar R, Syngelaki A, Poon L, Wright D, Nicolaides KH. Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Fetal Diagn Ther 2013;33:8-15. http://dx.doi.org/10.1159/000341264.
- Ferreira AF, Rezende JC, Vaikousi E, Akolekar R, Nicolaides KH. Maternal serum visfatin at 11-13 weeks of gestation in gestational diabetes mellitus. Clin Chem 2011;57:609-13. http://dx.doi.org/10.1373/clinchem.2010.159806.
- Lacroix M, Battista MC, Doyon M, Ménard J, Ardilouze JL, Perron P, et al. Lower adiponectin levels at first trimester of pregnancy are associated with increased insulin resistance and higher risk of developing gestational diabetes mellitus. Diabetes Care 2013;36:1577-83. http://dx.doi.org/10.2337/dc12-1731.
- Sattar N, Wannamethee SG, Forouhi NG. Novel biochemical risk factors for type 2 diabetes: pathogenic insights or prediction possibilities?. Diabetologia 2008;51:926-40. http://dx.doi.org/10.1007/s00125-008-0954-7.
- Savvidou M, Nelson SM, Makgoba M, Messow CM, Sattar N, Nicolaides K. First-trimester prediction of gestational diabetes mellitus: examining the potential of combining maternal characteristics and laboratory measures. Diabetes 2010;59:3017-22. http://dx.doi.org/10.2337/db10-0688.
- Poston L, Briley AL, Barr S, Bell R, Croker H, Coxon K, et al. Developing a complex intervention for diet and activity behaviour change in obese pregnant women (the UPBEAT trial); assessment of behavioural change and process evaluation in a pilot randomised controlled trial. BMC Pregnancy Childbirth 2013;13. https://doi.org/10.1186/1471-2393-13-148.
- Bondia EM, Castellote AI, Lopez MC, Rivero M. Determination of plasma fatty acid composition in neonates by gas chromatography. J Chromatogr B, Biomed Appl 1994;658:369-74. https://doi.org/10.1016/0378-4347(94)00287-8.
- Lepage G, Roy CC. Improved recovery of fatty acid through direct transesterification without prior extraction or purification. J Lipid Res 1984;25:1391-6.
- Sun Q, Ma J, Campos H, Hankinson SE, Hu FB. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr 2007;86:74-81.
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837-45. https://doi.org/10.2307/2531595.
- Göbl CS, Bozkurt L, Rivic P, Schernthaner G, Weitgasser R, Pacini G, et al. A two-step screening algorithm including fasting plasma glucose measurement and a risk estimation model is an accurate strategy for detecting gestational diabetes mellitus. Diabetologia 2012;55:3173-81. https://doi.org/10.1007/s00125-012-2726-7.
- Nanda S, Savvidou M, Syngelaki A, Akolekar R, Nicolaides KH. Prediction of gestational diabetes mellitus by maternal factors and biomarkers at 11 to 13 weeks. Prenat Diagn 2011;31:135-41. http://dx.doi.org/10.1002/pd.2636.
- Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2009;302:179-88. http://dx.doi.org/10.1001/jama.2009.976.
- Lowe LP, Metzger BE, Lowe WL, Dyer AR, McDade TW, McIntyre HD. HAPO Study Cooperative Research Group . Inflammatory mediators and glucose in pregnancy: results from a subset of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. J Clin Endocrinol Metab 2010;95:5427-34. http://dx.doi.org/10.1210/jc.2010-1662.
- Aye IL, Powell TL, Jansson T. Review: Adiponectin – the missing link between maternal adiposity, placental transport and fetal growth?. Placenta 2013;34:S40-5. http://dx.doi.org/10.1016/j.placenta.2012.11.024.
- Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA 2003;289:1799-804. http://dx.doi.org/10.1001/jama.289.14.1799.
- Barakat R, Cordero Y, Coteron J, Luaces M, Montejo R. Exercise during pregnancy improves maternal glucose screen at 24-28 weeks: a randomised controlled trial. Br J Sports Med 2012;46:656-61. http://dx.doi.org/10.1136/bjsports-2011-090009.
- Retnakaran R, Hanley AJ, Raif N, Connelly PW, Sermer M, Zinman B. Reduced adiponectin concentration in women with gestational diabetes: a potential factor in progression to type 2 diabetes. Diabetes Care 2004;27:799-800. https://doi.org/10.2337/diacare.27.3.799.
- Gueuvoghlanian-Silva BY, Torloni MR, Mattar R, de Oliveira LS, Scomparini FB, Nakamura MU, et al. Profile of inflammatory mediators in gestational diabetes mellitus: phenotype and genotype. Am J Reprod Immunol 2012;67:241-50. http://dx.doi.org/10.1111/j.1600-0897.2011.01090.x.
- Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PA, et al. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet 2002;360:57-8. https://doi.org/10.1016/S0140-6736(02)09335-2.
- Miyazaki T, Shimada K, Mokuno H, Daida H. Adipocyte derived plasma protein, adiponectin, is associated with smoking status in patients with coronary artery disease. Heart 2003;89. https://doi.org/10.1136/heart.89.6.663.
- Hedderson MM, Darbinian J, Havel PJ, Quesenberry CP, Sridhar S, Ehrlich S, et al. Low prepregnancy adiponectin concentrations are associated with a marked increase in risk for development of gestational diabetes mellitus. Diabetes Care 2013;36:3930-7. http://dx.doi.org/10.2337/dc13-0389.
- Fraser A, Nelson SM, Macdonald-Wallis C, Cherry L, Butler E, Sattar N, et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation 2012;125:1367-80. http://dx.doi.org/10.1161/CIRCULATIONAHA.111.044784.
- Hayes L, Bell R, Robson S, Poston L. behalf of the UPBEAT Consortium . Association between Physical Activity in Obese Pregnant Women and Pregnancy Outcomes: The UPBEAT Pilot Study. Ann Nutr Metab 2014;64:239-46. http://dx.doi.org/10.1159/000365027.
- Dodd JM, Grivell RM, Nguyen AM, Chan A, Robinson JS. Maternal and perinatal health outcomes by body mass index category. Aust N Z J Obstet Gynaecol 2011;51:136-40. http://dx.doi.org/10.1111/j.1479-828X.2010.01272.x.
- Yu ZB, Han SP, Zhu GZ, Zhu C, Wang XJ, Cao XG, et al. Birth weight and subsequent risk of obesity: a systematic review and meta-analysis. Obes Rev 2011;12:525-42. http://dx.doi.org/10.1111/j.1467-789X.2011.00867.x.
- Hochner H, Friedlander Y, Calderon-Margalit R, Meiner V, Sagy Y, Avgil-Tsadok M, et al. Associations of maternal prepregnancy body mass index and gestational weight gain with adult offspring cardiometabolic risk factors: the Jerusalem Perinatal Family Follow-up Study. Circulation 2012;125:1381-9. http://dx.doi.org/10.1161/CIRCULATIONAHA.111.070060.
- Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 2009;32:1076-80. http://dx.doi.org/10.2337/dc08-2077.
- Lawlor DA. The Society for Social Medicine John Pemberton Lecture 2011. Developmental overnutrition – an old hypothesis with new importance?. Int J Epidemiol 2013;42:7-29. http://dx.doi.org/10.1093/ije/dys209.
- Modi N, Murgasova D, Ruager-Martin R, Thomas EL, Hyde MJ, Gale C, et al. The influence of maternal body mass index on infant adiposity and hepatic lipid content. Pediatr Res 2011;70:287-91. http://dx.doi.org/10.1038/pr.2011.512.
- Remmers F, Delemarre-van de Waal HA. Developmental programming of energy balance and its hypothalamic regulation. Endocr Rev 2011;32:272-311. http://dx.doi.org/10.1210/er.2009-0028.
- Chandler-Laney PC, Bush NC, Rouse DJ, Mancuso MS, Gower BA. Maternal glucose concentration during pregnancy predicts fat and lean mass of prepubertal offspring. Diabetes Care 2011;34:741-5. http://dx.doi.org/10.2337/dc10-1503.
- Balkau B, Mhamdi L, Oppert JM, Nolan J, Golay A, Porcellati F, et al. Physical activity and insulin sensitivity: the RISC study. Diabetes 2008;57:2613-18. http://dx.doi.org/10.2337/db07-1605.
- Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, Hsu RT, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ 2007;334. http://dx.doi.org/10.1136/bmj.39063.689375.55.
- Ross R, Janssen I, Dawson J, Kungl AM, Kuk JL, Wong SL, et al. Exercise-induced reduction in obesity and insulin resistance in women: a randomized controlled trial. Obes Res 2004;12:789-98. https://doi.org/10.1038/oby.2004.95.
- Ruchat SM, Davenport MH, Giroux I, Hillier M, Batada A, Sopper MM, et al. Effect of exercise intensity and duration on capillary glucose responses in pregnant women at low and high risk for gestational diabetes. Diabetes Metab Res Rev 2012;28:669-78. http://dx.doi.org/10.1002/dmrr.2324.
- Craig R, Mindall J, Hirani V. Health Survey for England 2008. Physical Activity and Fitness. Leeds: NHS Digital; 2008.
- Ong MJ, Guelfi KJ, Hunter T, Wallman KE, Fournier PA, Newnham JP. Supervised home-based exercise may attenuate the decline of glucose tolerance in obese pregnant women. Diabetes Metab 2009;35:418-21. http://dx.doi.org/10.1016/j.diabet.2009.04.008.
- Barakat R, Pelaez M, Montejo R, Luaces M, Zakynthinaki M. Exercise during pregnancy improves maternal health perception: a randomized controlled trial. Am J Obstet Gynecol 2011;204:402.e1-7. http://dx.doi.org/10.1016/j.ajog.2011.01.043.
- Pomeroy J, Renström F, Gradmark AM, Mogren I, Persson M, Bluck L, et al. Maternal physical activity and insulin action in pregnancy and their relationships with infant body composition. Diabetes Care 2013;36:267-9. http://dx.doi.org/10.2337/dc12-0885.
- Poston L, Chappell LC. How should women be advised on weight management in pregnancy?. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e2774.
- Briley AL, Barr S, Badger S, Bell R, Croker H, Godfrey KM, et al. A complex intervention to improve pregnancy outcome in obese women; the UPBEAT randomised controlled trial. BMC Pregnancy Childbirth 2014;14. https://doi.org/10.1186/1471-2393-14-74.
- Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet 2011;378:804-14. http://dx.doi.org/10.1016/S0140-6736(11)60813-1.
- Sutton R, Craig R, Mindell J. Health Survey for England 2011. Volume 1: Health, Social Care and Lifestyles. Leeds: NHS Digital; 2012.
- Heslehurst N, Simpson H, Ells LJ, Rankin J, Wilkinson J, Lang R, et al. The impact of maternal BMI status on pregnancy outcomes with immediate short-term obstetric resource implications: a meta-analysis. Obes Rev 2008;9:635-83. http://dx.doi.org/10.1111/j.1467-789X.2008.00511.x.
- Tsoi E, Shaikh H, Robinson S, Teoh TG. Obesity in pregnancy: a major healthcare issue. Postgrad Med J 2010;86:617-23. http://dx.doi.org/10.1136/pgmj.2010.098186.
- Poston L, Harthoorn LF, Van Der Beek EM. Contributors to the ILSI Europe Workshop . Obesity in pregnancy: implications for the mother and lifelong health of the child. A consensus statement. Pediatr Res 2011;69:175-80. http://dx.doi.org/10.1203/PDR.0b013e3182055ede.
- Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: The National Academies Press; 2009.
- Dodd JM, Grivell RM, Crowther CA, Robinson JS. Antenatal interventions for overweight or obese pregnant women: a systematic review of randomised trials. BJOG 2010;117:1316-26. http://dx.doi.org/10.1111/j.1471-0528.2010.02540.x.
- Nohr EA, Vaeth M, Baker JL, Sørensen TIa, Olsen J, Rasmussen KM. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. Am J Clin Nutr 2008;87:1750-9.
- Viswanathan M, Siega-Riz AM, Moos MK, Deierlein A, Mumford S, Knaack J, et al. Outcomes of maternal weight gain. Evid Rep Technol Assess 2008;168:1-223.
- Lowe LP, Metzger BE, Dyer AR, Lowe J, McCance DR, Lappin TR, et al. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations of maternal A1C and glucose with pregnancy outcomes. Diabetes Care 2012;35:574-80. http://dx.doi.org/10.2337/dc11-1687.
- Metzger BE, Contreras M, Sacks DA, Watson W, Dooley SL, Foderaro M, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991-2002. https://doi.org/10.1056/NEJMoa0707943.
- Harmon KA, Gerard L, Jensen DR, Kealey EH, Hernandez TL, Reece MS, et al. Continuous glucose profiles in obese and normal-weight pregnant women on a controlled diet: metabolic determinants of fetal growth. Diabetes Care 2011;34:2198-204. http://dx.doi.org/10.2337/dc11-0723.
- Vrijkotte TGM, Algera SJ, Brouwer IA, van Eijsden M, Twickler MB. Maternal triglyceride levels during early pregnancy are associated with birth weight and postnatal growth. J Pediatr 2011;159:736-42. https://doi.org/10.1016/j.jpeds.2011.05.001.
- Parretti E, Lapolla A, Dalfrà M, Pacini G, Mari A, Cioni R, et al. Preeclampsia in lean normotensive normotolerant pregnant women can be predicted by simple insulin sensitivity indexes. Hypertension 2006;47:449-53. http://dx.doi.org/10.1161/01.HYP.0000205122.47333.7f.
- Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. Am J Clin Nutr 2011;94:1975-9. http://dx.doi.org/10.3945/ajcn.110.001032.
- Wolff S, Legarth J, Vangsgaard K, Toubro S, Astrup A. A randomized trial of the effects of dietary counseling on gestational weight gain and glucose metabolism in obese pregnant women. Int J Obes 2008;32:495-501. http://dx.doi.org/10.1038/sj.ijo.0803710.
- Landon MB. Is there a benefit to the treatment of mild gestational diabetes mellitus?. Am J Obstet Gynecol 2010;202:649-53. http://dx.doi.org/10.1016/j.ajog.2010.02.006.
- Ruchat SM, Mottola MF. The important role of physical activity in the prevention and management of gestational diabetes mellitus. Diabetes Metab Res Rev 2013;29:334-46. https://doi.org/10.1002/dmrr.2402.
- Bingham SA, Gill C, Welch A, Cassidy A, Runswick SA, Oakes S, et al. Validation of dietary assessment methods in the UK arm of EPIC using weighed records, and 24-hour urinary nitrogen and potassium and serum vitamin C and carotenoids as biomarkers. Int J Epidemiol 1997;26:137-51. https://doi.org/10.1093/ije/26.suppl_1.S137.
- Ekelund U, Sepp H, Brage S, Becker W, Jakes R, Hennings M, et al. Criterion-related validity of the last 7-day, short form of the International Physical Activity Questionnaire in Swedish adults. Public Health Nutr 2006;9:258-65. https://doi.org/10.1079/PHN2005840.
- Stice E, Fisher M, Martinez E. Eating disorder diagnostic scale: additional evidence of reliability and validity. Psychol Assess 2004;16:60-71. http://dx.doi.org/10.1037/1040-3590.16.1.60.
- Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 1985;29:71-83. https://doi.org/10.1016/0022-3999(85)90010-8.
- Robinson S, Marriott L, Poole J, Crozier S, Borland S, Lawrence W, et al. Dietary patterns in infancy: the importance of maternal and family influences on feeding practice. Br J Nutr 2007;98:1029-37. http://dx.doi.org/10.1017/S0007114507750936.
- Llewellyn CH, van Jaarsveld CH, Johnson L, Carnell S, Wardle J. Development and factor structure of the Baby Eating Behaviour Questionnaire in the Gemini birth cohort. Appetite 2011;57:388-96. http://dx.doi.org/10.1016/j.appet.2011.05.324.
- Gartstein MA, Rothbart MK. Studying infant temperament via the Revised Infant Behavior Questionnaire. Infant Behav Dev 2003;26:64-86. https://doi.org/10.1016/S0163-6383(02)00169-8.
- Sadeh A. A brief screening questionnaire for infant sleep problems: validation and findings for an Internet sample. Pediatrics 2004;113:e570-7. https://doi.org/10.1542/peds.113.6.e570.
- Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487-95. https://doi.org/10.2337/diacare.27.6.1487.
- Gibson J, McKenzie-McHarg K, Shakespeare J, Price J, Gray R. A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatr Scand 2009;119:350-64. http://dx.doi.org/10.1111/j.1600-0447.2009.01363.x.
- Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW, et al. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord 2001;25:1175-82. http://dx.doi.org/10.1038/sj.ijo.0801670.
- Ehrenberg HM, Durnwald CP, Catalano P, Mercer BM. The influence of obesity and diabetes on the risk of cesarean delivery. Am J Obstet Gynecol 2004;191:969-74. https://doi.org/10.1016/j.ajog.2004.06.057.
- Poston L, Bell R, Croker H, Flynn AC, Godfrey KM, Goff L, et al. behalf of The UPBEAT Trial Consortium . Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol 2015;3:767-77. http://dx.doi.org/10.1016/S2213-8587(15)00227-2.
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766-81. http://dx.doi.org/10.1016/S0140-6736(14)60460-8.
- Ruchat SM, Houde AA, Voisin G, St-Pierre J, Perron P, Baillargeon JP, et al. Gestational diabetes mellitus epigenetically affects genes predominantly involved in metabolic diseases. Epigenetics 2013;8:935-43. http://dx.doi.org/10.4161/epi.25578.
- Rogozińska E, Chamillard M, Hitman GA, Khan KS, Thangaratinam S. Nutritional manipulation for the primary prevention of gestational diabetes mellitus: a meta-analysis of randomised studies. PLOS ONE 2015;10. http://dx.doi.org/10.1371/journal.pone.0115526.
- Diabetes in Pregnancy: Management of Diabetes and its Complications from Preconception to the Postnatal Period. London: NICE; 2015.
- Bingham SA, Welch AA, McTaggart A, Mulligan AA, Runswick SA, Luben R, et al. Nutritional methods in the European Prospective Investigation of Cancer in Norfolk. Public Health Nutr 2001;4:847-58. https://doi.org/10.1079/PHN2000102.
- Meltzer HM, Brantsaeter AL, Ydersbond TA, Alexander J, Haugen M. Methodological challenges when monitoring the diet of pregnant women in a large study: experiences from the Norwegian Mother and Child Cohort Study (MoBa). Matern Child Nutr 2008;4:14-27. http://dx.doi.org/10.1111/j.1740-8709.2007.00104.x.
- Vinter CA, Jensen DM, Ovesen P, Beck-Nielsen H, Jørgensen JS. The LiP (Lifestyle in Pregnancy) study: a randomized controlled trial of lifestyle intervention in 360 obese pregnant women. Diabetes Care 2011;34:2502-7. http://dx.doi.org/10.2337/dc11-1150.
- Renault KM, Nørgaard K, Nilas L, Carlsen EM, Cortes D, Pryds O, et al. The Treatment of Obese Pregnant Women (TOP) study: a randomized controlled trial of the effect of physical activity intervention assessed by pedometer with or without dietary intervention in obese pregnant women. Am J Obstet Gynecol 2014;210:134-43. https://doi.org/10.1016/j.ajog.2013.09.029.
- Dodd JM, Turnbull D, McPhee AJ, Deussen AR, Grivell RM, Yelland LN, et al. Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. BMJ 2014. https://doi.org/10.1097/01.ogx.0000451478.28533.cf.
- Hill B, Skouteris H, Fuller-Tyszkiewicz M. Interventions designed to limit gestational weight gain: a systematic review of theory and meta-analysis of intervention components. Obes Rev 2013;14:435-50. http://dx.doi.org/10.1111/obr.12022.
- Zavorsky GS, Longo LD. Exercise guidelines in pregnancy: new perspectives. Sports Med 2011;41:345-60. http://dx.doi.org/10.2165/11583930-000000000-00000.
- Robinson SM, Crozier SR, Harvey NC, Barton BD, Law CM, Godfrey KM, et al. Modifiable early-life risk factors for childhood adiposity and overweight: an analysis of their combined impact and potential for prevention. Am J Clin Nutr 2015;101:368-75. http://dx.doi.org/10.3945/ajcn.114.094268.
- Okubo H, Crozier SR, Harvey NC, Godfrey KM, Inskip HM, Cooper C, et al. Maternal dietary glycemic index and glycemic load in early pregnancy are associated with offspring adiposity in childhood: the Southampton Women’s Survey. Am J Clin Nutr 2014;100:676-83. http://dx.doi.org/10.3945/ajcn.114.084905.
- Maternal Obesity in the UK: Findings From a National Project. London: CMACE; 2010.
- Maayan-Metzger A, Schushan-Eisen I, Lubin D, Moran O, Kuint J, Mazkereth R. Delivery room breastfeeding for prevention of hypoglycaemia in infants of diabetic mothers. Fetal Pediatr Pathol 2014;33:23-8. http://dx.doi.org/10.3109/15513815.2013.842271.
- NHS Reference Costs 2012–13. London: DH; 2013.
- Curtis L. Unit Costs of Health and Social Care 2014. Canterbury: Personal Social Services Research Unit, University of Kent; 2014.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Earnshaw J, Lewis G. NICE Guide to the Methods of Technology Appraisal: pharmaceutical industry perspective. PharmacoEconomics 2008;26:725-7. https://doi.org/10.2165/00019053-200826090-00002.
- Dolan P, Roberts J. Modelling valuations for Eq-5d health states: an alternative model using differences in valuations. Med Care 2002;40:442-6. https://doi.org/10.1097/00005650-200205000-00009.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- O’Brien BJ, Briggs AH. Analysis of uncertainty in health care cost-effectiveness studies: an introduction to statistical issues and methods. Stat Methods Med Res 2002;11:455-68. https://doi.org/10.1191/0962280202sm304ra.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. https://doi.org/10.1002/hec.635.
- White SL, Lawlor DA, Briley AL, Godfrey KM, Nelson SM, Oteng-Ntim E, et al. Early antenatal prediction of gestational diabetes in obese women: development of prediction tools for targeted intervention. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0167846.
- Patel N, Godfrey KM, Pasupathy D, Levin J, Flynn AC, Hayes L, et al. Infant adiposity following a randomised controlled trial of a behavioural intervention in obese pregnancy [published online ahead of print 21 March 2017]. Int J Obes 2017. https://doi.org/10.1038/ijo.2017.44.
Appendix 1 Questionnaire phase 1
Appendix 2 Structured interview questionnaire phase 1
Appendix 3 Handbook for health trainers
Appendix 4 Participants handbook (intervention arm)
List of abbreviations
- BMI
- body mass index
- CC
- comorbidities or complications
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- cpm
- counts per minute
- CSA
- Computer Science and Applications
- CV
- coefficient of variation
- DNA
- deoxyribonucleic acid
- DVD
- digital versatile disc
- EPDS
- Edinburgh Postnatal Depression Scale
- EQ-5D
- EuroQol-5 Dimensions
- F&V
- fruit and vegetables
- FA
- fatty acid
- FFQ
- food frequency questionnaire
- GDM
- gestational diabetes mellitus
- GI
- glycaemic index
- GL
- glycaemic load
- GP
- general practitioner
- GWG
- gestational weight gain
- HBM
- health belief model
- HDL
- high-density lipoprotein
- HOMA2-IR
- Homeostatic Model Assessment 2 – Insulin Resistance
- HRG
- Healthcare Resource Group
- HT
- health trainer
- IADPSG
- International Association of the Diabetes and Pregnancy Study Groups
- ICER
- incremental cost-effectiveness ratio
- ID
- identifier
- IPAQ
- International Physical Activity Questionnaire
- IRAS
- Integrated Research Application System
- ISRCTN
- International Standard Randomised Controlled Trial Number
- LDL
- low-density lipoprotein
- LGA
- large-for-gestational-age
- LPA
- light physical activity
- MET
- metabolic equivalent
- MRC
- Medical Research Council
- MVPA
- moderate to vigorous physical activity
- NEFA
- non-esterified fatty acid
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- OGTT
- oral glucose tolerance test
- OR
- odds ratio
- PA
- physical activity
- PI
- principal investigator
- QALY
- quality-adjusted life-year
- QC
- quality control
- RCT
- randomised controlled trial
- RPAQ
- Recent Physical Activity Questionnaire
- SD
- standard deviation
- SFA
- saturated fatty acid
- SMART
- specific, measurable, achievable, relevant and time-specific
- UPBEAT
- UK Pregnancies Better Eating and Activity Trial
- VLDL
- very low-density lipoprotein
- WHO
- World Health Organization