Notes
Article history
The research reported in this issue of the journal was funded by PGfAR as project number RP-PG-0707-10162. The contractual start date was in December 2013. The final report began editorial review in May 2015 and was accepted for publication in September 2016. As the funder, the PGfAR programme agreed the research questions and study designs in advance with the investigators. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The PGfAR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Simon de Lusignan has received funding from Eli Lilly and GlaxoSmithKline (GSK) Biologicals. Funding has also been received from the Department of Health for the National Evaluation of Improving Access to Psychological Therapies (IAPT). Elspeth Guthrie reports grants from the National Institute for Health Research (NIHR) during the conduct of the study. Karina Lovell reports grants from NIHR, the National Institute of Mental Health (NIMH) and Arthritis Research UK during the conduct of the study. Chris Dickens reports grants from NIHR during the conduct of the study. Linda Davies reports grants from the NIHR, Medical Research Council, Economic and Social Research Council, Macmillan, Cancer Research UK, Arthritis Research UK and Central Manchester University Hospitals NHS Foundation Trust (CMFT) during the conduct of this study. Peter Salmon reports grants from Marie Curie Cancer Care, the Liverpool Institute of Health Inequalities Research, Merseycare NHS Trust, Royal Liverpool and Broadgreen Hospitals NHS Trust, the Economic and Social Research Council, and the Medical Research Council during the conduct of the study. Navneet Kapur reports other research funding from NIHR, the Department of Health and the Healthcare Quality Improvement Partnership during the conduct of the study. He was not in receipt of any industry funding. He was involved in National Institute for Health and Care Excellence guidelines and Department of Health (England) advisory groups on suicidal behaviour unrelated to the conduct of the current study.
Disclaimer
The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the National Institute for Health Research Public Health Research programme or the Department of Health.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2017. This work was produced by Guthrie et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Unscheduled care is defined as any unplanned contact with the health service by a person requiring or seeking help, care or advice. 1 It includes a wide range of service contacts from specialised hospital support, emergency hospital admissions (EHAs), attendances at emergency departments (EDs), attendances at minor injury units, unplanned primary care call-outs and, finally, self-care. 2 Unscheduled care also includes both urgent care, which refers to conditions that require assessment and treatment within 7 days, and emergency care, which needs assessment and intervention within 24 hours. 2
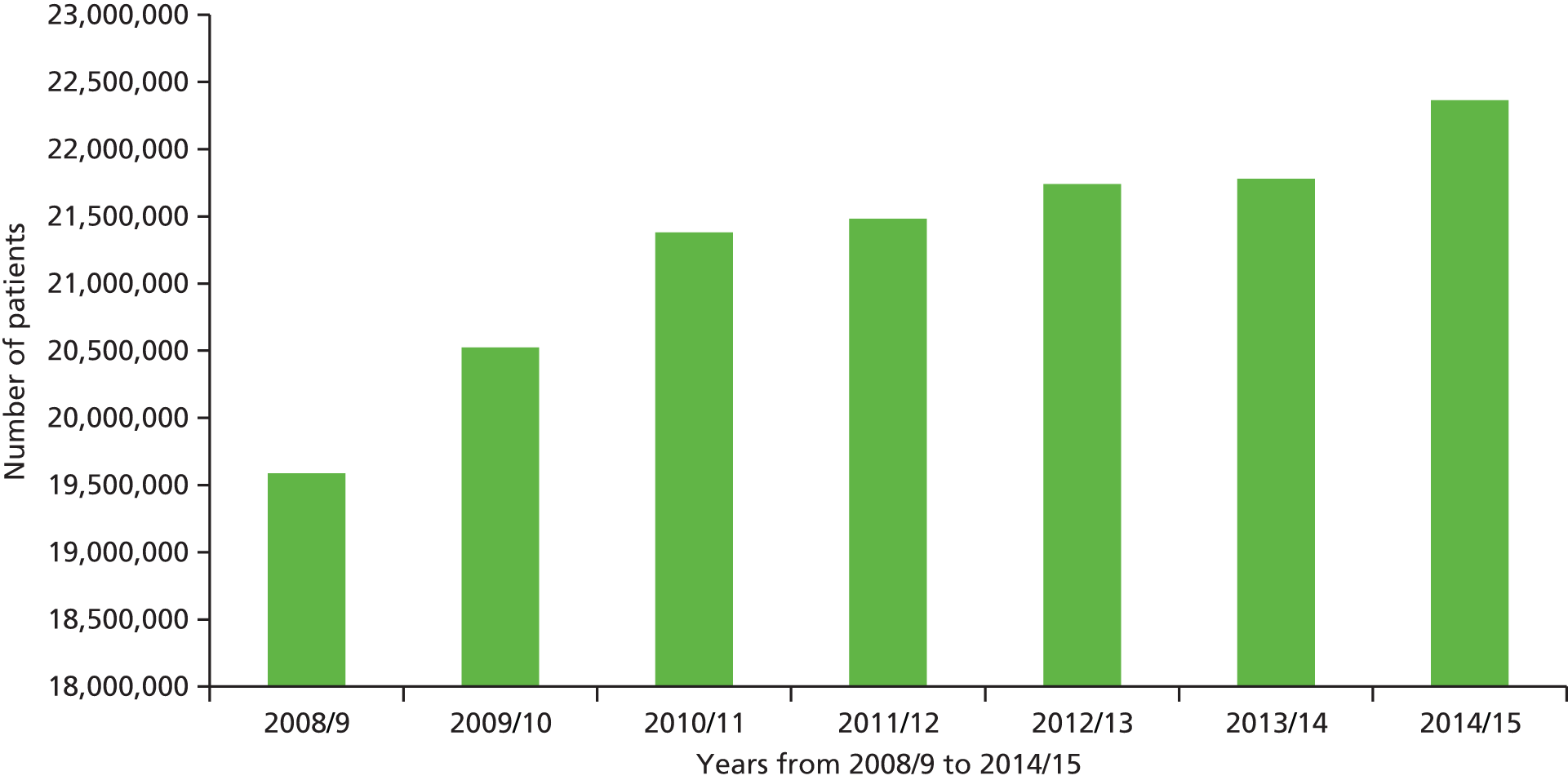
Use of urgent or unscheduled services is high. Sixteen per cent of the general population access unscheduled care over any 4-week period,3 and attendances at EDs in England have been increasing year on year (Figure 1). The total number of ED attendances in England has risen from 13.9 million in the year 1987–8 to 14.4 million in 1997–8, 19.1 million in 2007–8 and 22.4 million in 2013–14. 4
According to a report published by the Department of Health (DH) in 2013, in 2012–13, there were 5.3 million emergency admissions to hospital, costing approximately £12.5B. 5 In 2014–15, the total number of EHAs had risen to 5.5 million. 4 Reducing unscheduled care has become a DH priority. 6
The increase in EHAs has largely resulted from an increase in short-stay admissions of patients presenting to EDs. These ‘short-stay’ admissions (< 2 days) have increased by 124% over the last 15 years; in comparison, longer admissions have increased by 14%. 5
Avoiding unnecessary EHAs is a major concern for the NHS not only because of the costs associated with these admissions, but also because of the pressure and disruption caused to elective health care and to the people admitted. In a recent report from The King’s Fund, it was suggested that emergency admissions of people with long-term conditions (LTCs) that could have been managed in primary care cost the NHS £1.42B annually and that this could be reduced by 8–18% through investment in primary care- and community-based services. 7
It is estimated that 17.5 million adults in the UK have at least one LTC, by which is meant a chronic physical or mental health condition. 8,9 Over 70% of the health-care budget in England is spent on the care of people with LTCs,10 and many people live with multiple conditions. LTCs account for 50% of general practice consultations and 70% of inpatient bed-days per year in the UK. 11
During this programme of research, there was a growing awareness at a national level of the importance of improving the mental health of people with LTCs. 12 There was also a recognition of the need to better integrate physical and social health care, particularly for the elderly. 13–15 The national mental health strategy for England calls for patient-centred management together with joined-up personalised pathways and systems. The strategy states that interventions must be as efficient as possible at delivering outcomes that are effective, cost-effective and safe. 16 There is the expectation that better joined-up care will reduce costs, particularly the burden on emergency services. A recent report from The King’s Fund, entitled Bringing Together Physical and Mental Health: A New Frontier for Integrated Care, makes a strong case for change to the current ways in which services are organised. 17
There are major challenges to ensure that patients’ needs are met holistically, effectively and efficiently. Importantly, the evidence base underpinning many current recommendations is limited or patchy.
This programme of research addressed several of the key areas described above and its findings are both informative and timely for those involved in policy, practice and research. Instead of focusing on one LTC, the programme included four common exemplar LTCs. This allowed us to compare people with different LTCs but also to study the effects of multimorbidity on use of unscheduled care.
The use of unscheduled care is common in people with LTCs, such as asthma and chronic obstructive pulmonary disease. 18–20 It is argued that, in the case of certain long-term physical conditions, more effective management and treatment in primary care could reduce ED attendance and EHAs, and there has been, and remains, a drive from the DH to reduce urgent care and emergency readmissions to hospitals of those people with long-term physical conditions who could be managed in the community. 11
It is difficult to determine whether or not the use of unscheduled care by an individual is appropriate, as it depends on a value judgement made by a clinician at the point of contact, and the criteria for determining inappropriate contact vary considerably. 21 Although, undoubtedly, some use of unscheduled care is unjustified, it is recognised that, for many people, the use of unscheduled care is entirely appropriate and necessary at the time they access services. An important question, however, is whether or not such contacts could be prevented, in certain groups of patients, by improved management and treatment of their physical health problems, so that crises are avoided.
The provision of unscheduled care is complex, and the relationship between different components of unscheduled care and scheduled care is difficult to disentangle. For example, increased use of general practitioner (GP) out-of-hours (OOH) services, or seeing the same GP on a regular basis, may result in a reduced need to attend the ED. So an increase in one aspect of unscheduled care or scheduled care may result in a decrease in another aspect of unscheduled care. 22 With this in mind, many of the analyses in this programme focus on secondary care aspects of unscheduled care, namely ED attendance and EHAs. These are the two most costly aspects of unscheduled care and the two that government has identified as a priority area to reduce. 11 Where relevant, however, and where possible in the programme, we also comment on other kinds of unscheduled care use.
Several factors have been identified as being associated with increased unscheduled care use in people with LTCs. Higher levels of multimorbidity and greater illness severity are associated with higher rates of EHAs and readmission following discharge. 20,23–25
Organisational factors include proximity to EDs or other kinds of emergency provision, and the availability of scheduled services. 26 Like all health-care decisions, unscheduled care use occurs in a clinical, social and cultural context, which may differ in different countries and health-care settings. There has been relatively little work which has addressed the influence of family and providers of routine care, and the cultural norms which underlie people’s beliefs and their behaviours in relation to unscheduled care use.
Psychosocial factors have also been recognised as increasing the risk of unscheduled care use in people with physical LTCs. Depression and anxiety are associated with higher rates of unscheduled care use in people with a variety of different LTCs. 27–30 Poorer quality of life (QoL) and perceived control of illness are also associated with greater health-care utilisation and increased ED attendances for asthma and chronic obstructive pulmonary disease (COPD). 31–35 The reasons for these associations are unclear. Psychological factors may influence people’s decisions about when to use unscheduled care, or reduce their ability to cope in health emergencies, or may just be markers of greater morbidity.
There are major initiatives to improve the quality of care for people with LTCs11 and primary care is seen as the optimal context to deliver care. 36,37 One of the main drivers for the focus on primary care is to attempt to reduce the use of unscheduled secondary care use, by improved provision of scheduled or routine care.
In this context, the primary purpose of this programme of research was to ‘develop effective psychosocial strategies to reduce the need for unscheduled care in patients with LTCs’. The programme of work began in 2009 and its findings are therefore highly relevant to current health-care initiatives. The programme has become known as the ‘CHOICE’ programme, and this term will be used throughout this document in preference to its longer official title (Choosing Health Options in Chronic Care Emergencies). For the purposes of the programme, we focused on people with physical chronic health conditions, and the term ‘LTC’ will be used throughout this report to refer to physical long-term health conditions.
We chose to study four exemplar LTCs: asthma, COPD, coronary heart disease (CHD) and diabetes. We chose these conditions because (a) they are common; (b) they are all in the leading 15 discharge diagnoses of EDs;38 (c) they are associated with EHAs;39 (d) they are all recognised as ambulatory care-sensitive conditions for which effective care can prevent flare-ups and the need to use secondary care emergency services;40 and (e) GPs in England have a requirement under the Quality and Outcomes Framework (QOF)41 to review people with these conditions at least once per year. Therefore, all primary care practices in England keep electronic databases that enable people with these conditions to be identified.
In a recent European study, unscheduled care accounted for 56% of total health-care costs in adults with asthma,18 regardless of the severity of patients’ symptoms. In the USA, over 12 months, 8.3% of patients with asthma made at least one visit to the ED19 and 13% of COPD patients made six or more visits. 20 Over 300,000 people attend an ED in England and Wales each year because of chest pain. 42
The rate of EHAs for people with LTCs varies considerably across England, and is much higher in socially deprived areas than in the least deprived areas of the country, varying from 38 to 207 admissions per 1000 registered patients. 7 Differences are also apparent in the way people are assessed and treated in an emergency facility, with large variability in services that facilitate discharge. 43
Many of the GP practices participating in the programme are located in areas of high deprivation. Although it could be argued that the findings of the programme may not be representative of the most affluent areas of the UK, it is generally the more deprived and highly populated areas where there is high use of unscheduled care. 26
We chose to focus on psychosocial factors (e.g. depression, anxiety, life stress) that may contribute to unscheduled care use, as these factors (particularly mental health problems) are often hidden in clinical practice, under-reported, and under-researched. A recent systematic review, which examined features of primary care that impact on use of unscheduled care, identified several organisational factors and people-related factors of relevance. 26 Although social deprivation, social isolation, older age and having multiple conditions were identified as potential drivers, mental health did not feature in the studies included in the review.
The inclusion in this programme of four exemplar conditions enabled us to study not only each individual condition, but the effects of one or more of our exemplar conditions (i.e. comorbidity) on use of unscheduled care.
The focus for the programme was the potential relationship between psychosocial factors, LTCs and unscheduled care (primarily ED attendance and EHAs) and the possibility that a tailored psychosocial intervention may reduce the requirement to use unscheduled care in people with LTCs.
Six key factors underpinned the research programme.
High psychological morbidity associated with long-term conditions
Psychological morbidity is two to three times higher in people with LTCs than in those who do not have a LTC,44,45 and people with two or more LTCs are seven times more likely to have depression. 46
Each of the four conditions we chose to study is associated with high psychological morbidity. For example, rates of depression in patients with COPD are 2.5 times higher than in control subjects,47 and the presence of diabetes doubles the odds of comorbid depression compared with no diabetes. 48 There is similar evidence for high psychological morbidity in asthma and CHD. 49,50
Poor health outcomes associated with psychological symptoms
The presence of psychological symptoms in LTCs is associated with poor health outcomes in all four conditions. Depression has an adverse effect on QoL in asthma,49 COPD,51 diabetes52 and CHD. 53 Depression has been linked to adverse morbidity and mortality in CHD53,54 and COPD. 55 Those with CHD who are also depressed have higher rates of complications and are more likely to undergo invasive procedures. 56,57 Depression has also been linked to poorer self-care in asthma49 and in diabetes. 58 Recent work also suggests that depression in diabetes is associated with an increased risk of dementia. 59,60
Increased health expenditure associated with psychological morbidity
The presence of psychological symptoms is associated with increased health-care use and expenditure in LTCs, and those with depressive disorder are twice as likely to use EDs as those without depression. 61 Total health-care expenditure on patients with diabetes is 4.5 times higher for individuals with depression than for those without depression. 27
Perception of illness is associated with outcome
Knowledge about and perceptions of illness are critical factors for optimal medication adherence in patients with LTCs. 62
Treatment of depression improves outcome
In patients with diabetes, treatment of depression produces an improvement in symptoms at no additional overall cost, as savings are made by reductions from inpatient medical costs and other forms of medical care. 63 Improved self-care of diabetes also reduces health-care costs. 64 CHD patients who respond to treatment for depression have fewer cardiac events and associated costs. 65
Psychological morbidity is a known, but little researched, predictor of unscheduled care use
A variety of risk-modelling approaches have been utilised in the NHS to identify people at risk of using secondary forms of unscheduled care, predominantly EHAs. The most common models in use at the time the CHOICE programme began, such as the Patients at Risk of Re-Hospitalisation algorithm,66,67 employed data about prior health-care use, plus certain physical parameters, but did not include data on mental health. There is, however, preliminary evidence, from a small number of studies, that psychological factors are independent predictors of the use of unscheduled care in people with LTCs. 30,55,57,68
Aims and objectives
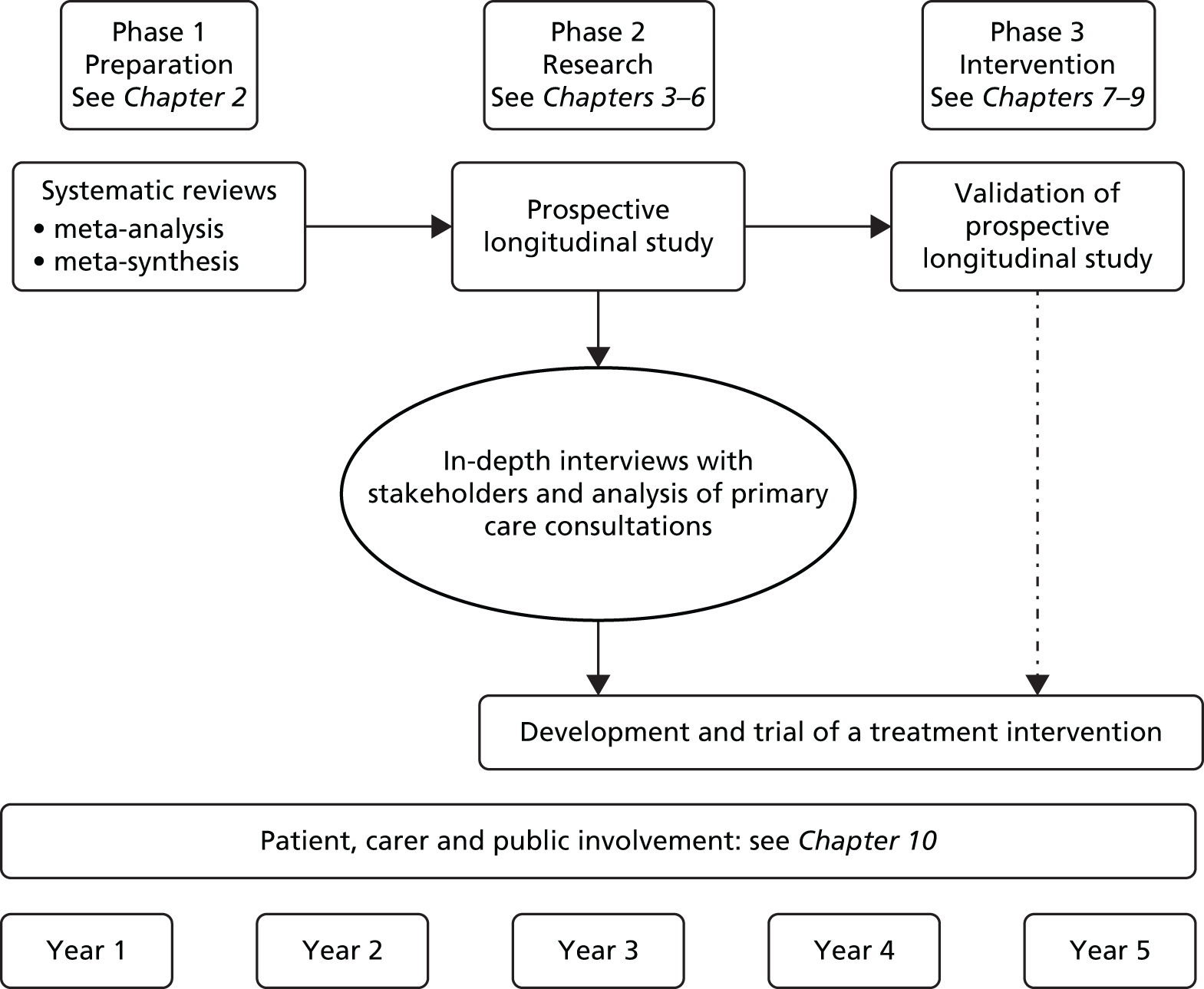
The programme was given the acronym CHOICE and was divided into three phases of work (Figure 2).
FIGURE 2.
The three phases of the CHOICE programme.
The overall aim was to better understand psychosocial drivers of unscheduled care in people with physical LTCs and develop an intervention to reduce unscheduled care.
Phase 1 involved understanding the problem in greater depth using systematic review methods to identify psychosocial factors that act as drivers for unscheduled care in LTCs, and to identify interventions or strategies to reduce the frequency of unscheduled care.
Phase 2 involved mapping the frequency and pattern of unscheduled care in patients with the four exemplar LTCs (asthma, CHD, COPD and diabetes) over a 12-month period, and the development of a red flag marker to identify patients at risk of becoming high users of unscheduled care.
Phase 3 involved developing and testing the validity and utility of the red flag marker using current NHS databases. We also developed and evaluated a low-intensity psychosocial intervention for use in primary care, with the intention of reducing use of unscheduled care in people with LTCs.
Qualitative methods and analyses were embedded within the programme to provide rich personalised accounts, which were used to:
-
triangulate findings with the quantitative methods
-
identify mechanisms accounting for the quantitative findings
-
evaluate the acceptability and inform the implementation of the intervention.
Our objectives were to:
-
Systematically synthesise the current evidence about psychosocial drivers of unscheduled care, and about interventions or strategies to reduce the frequency of unscheduled care use in patients with LTCs (phase 1).
-
Derive estimates of the frequency and pattern of unscheduled care use in patients with asthma, CHD, COPD and diabetes, as examples of common LTCs (phase 2).
-
Develop and validate a ‘red flag’ system that will identify patients with LTCs who are at risk of becoming frequent users of unscheduled care (phases 2 and 3).
-
Identify personal reasons for unscheduled care use, including barriers to access for routine care, patients’ motivations, expectations and decision-making processes, influences from families and relevant health-care workers, and factors in consultations with active case managers (ACMs) (phase 2). This objective was modified during the life of the programme as ACMs were phased out, so the focus of study was switched to routine health-care reviews in primary care for people with LTCs.
-
Develop and evaluate an intervention that will reduce/prevent unscheduled care use, while maintaining or improving patient benefit (phase 3).
-
Use statistical and health economic modelling to evaluate the costs and benefits associated with a treatment intervention (phase 3). This objective was modified over the life of the programme (there were unavoidable delays in phase 2). These and the findings from phases 1 and 2 meant that the planned decision analyses were unlikely to further inform the development of a red flag system and development of the intervention to reduce/prevent the use of unscheduled care. Accordingly, this objective was adapted to focus on statistical analysis of the costs of scheduled and unscheduled hospital care in phase 2 and exploratory sensitivity analyses of the trial data generated in phase 3.
Patient and public engagement and involvement
During the lifetime of the programme, we involved service users and user-led organisations in workshops/groups/stakeholder meetings to help us evaluate our findings, and to develop appropriate and practical interventions. Our service users were involved in all aspects of the programme and this work was led by our co-applicant, Mrs Jackie Macklin.
The work of the programme is described in this report in a sequential fashion, except for our patient and public engagement and involvement (PPE&I), which we have presented in a separate chapter (see Chapter 10), in order to capture the depth and quality of this important contribution that ran across all areas of our programme.
Terminology
We have used the term ‘unscheduled care’ to refer to all aspects of unplanned health-seeking, in both primary and secondary care. We use the term ‘ED attendance’ to refer to attendances at EDs and the term ‘EHA’ to refer to EHAs that do not included planned admissions to hospital. In this programme, the term ‘LTCs’ is used to refer to long-term physical conditions and does not include long-term mental health conditions. The term ‘scheduled care’ refers to all planned contacts with health care including GP appointments, reviews, outpatient appointments and planned hospital admissions.
Chapter 2 Evidence synthesis (phase 1)
Abstract
Background
Our objectives for evidence synthesis were to identify the psychosocial drivers of unscheduled care use in patients with LTCs; to identify existing evidence about psychosocial interventions to reduce unscheduled care use; and to understand patients’ reasons for using unscheduled care.
Method
We carried out systematic searches of prospective cohort studies, randomised controlled trials (RCTs) and qualitative studies in patients with asthma, diabetes, COPD and CHD, and conducted five systematic reviews.
Results
We found that depression predicts unscheduled care use in asthma, COPD, CHD and diabetes. Psychosocial interventions can reduce unscheduled care use in COPD and asthma by 32% and 21% respectively.
The value that patients with LTCs place on both unscheduled and routine health care depends on their previous health-care experiences and their personal circumstances. Patients report that unscheduled care is easily accessible, available and complements routine health care, but should only be used for pressing health-care needs. Patients do not talk about psychosocial factors as drivers for unscheduled care use.
Discussion
We conducted five systematic reviews that show that depression predicts unscheduled care use in LTCs; unscheduled care use can be reduced using psychosocial interventions in patients with COPD and asthma; and patients use unscheduled care when they feel they have a pressing health-care need. We were not able to control for the severity of LTCs in our quantitative analysis of the existing evidence; therefore, future work should aim to identify the relationship between depression, severity of LTC and unscheduled care use.
Overview
In this chapter we report the results from phase 1 of the CHOICE programme of research, which was principally involved with evidence synthesis. The aim was to systematically synthesise current evidence about psychosocial drivers of unscheduled care use and about interventions or strategies, which may reduce the frequency of unscheduled care use in patients with LTCs. As throughout the whole programme, we focused on four exemplar conditions: asthma, CHD, COPD and diabetes.
We carried out five major reviews: two focused on potential psychosocial drivers of use of unscheduled care and two focused on evidence concerning treatment interventions to reduce unscheduled care use. The final review synthesised evidence from studies that had used qualitative research methods to understand why people with LTCs use unscheduled care.
Psychosocial predictors of unscheduled health-care use
For the purpose of the first two reviews, we focused on the two most commonly identified psychosocial predictors of unscheduled care use: depression and anxiety. 55,68 Both are common in people with LTCs69,70 and are associated with poor health outcomes. 71,72 Evidence as to their role in the use of unscheduled care, however, is unclear.
Several studies have shown that depression increases ED attendances or EHAs in patients with LTCs. 63,73–75 However, other studies have found no significant impact of depression on unscheduled care use. 55 The relationship between anxiety and use of unscheduled care in patients with LTCs is also unclear. 68,76,77
Review 1 focused on the relationship between depression and use of unscheduled care, whereas review 2 focused on the role of anxiety and its effect on the use of unscheduled care. Both systematic reviews have been published and will be summarised in this chapter. 78,79 The findings from the review concerning the role of depression as a predictor of unscheduled care (i.e. Dickens et al. 78) are reproduced with the permission from Elsevier. 78
Aims of studies 1 and 2
The aim of the first review (study 1) was to conduct a systematic review of the literature with meta-analysis to determine if depression is a predictor of unscheduled care use in patients with any of four exemplar LTCs (asthma, CHD, COPD and diabetes).
The aim of the second review (study 2) was to conduct a systematic review of the literature with meta-analysis to identify if anxiety predicts the use of unscheduled care in patients with any of four exemplar LTCs (asthma, CHD, COPD and diabetes).
Method
The methods for both reviews are summarised below. Full descriptions of the methods are available in the published papers. 78,79
Studies were eligible for inclusion in these reviews if they met the following criteria:
-
employed a prospective cohort design
-
included adults with one or more of the following LTCs: asthma, CHD [myocardial infarction (MI), stable or unstable angina], COPD and diabetes
-
used a standardised measure of depression or anxiety at baseline
-
assessed the use of unscheduled health care prospectively, defined for the purposes of the reviews as urgent GP visits, attendance at EDs, urgent or EHAs.
Studies of children were not included. Search strategies (see Appendix 1) were developed and searches conducted in the following electronic databases: MEDLINE, EMBASE, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the British Nursing Index (BNI), PsycINFO and Cochrane database. Searches for both reviews were first conducted on 19 August 2008 and then updated on 22 June 2012 for depression studies and again on 1 April 2013 for anxiety studies. For eligible papers, reference lists were searched and citations of eligible papers were screened for relevance using the Social Sciences Citation Index.
The titles and abstracts of the identified papers were screened by one of three researchers [AB, Angee Khara (AK), CB] and the full text of studies that potentially met the inclusion criteria were then screened by two out of three researchers (AB, AK, CB). Any disagreements about eligible papers were discussed with another member of the team (CD, EG). Data extraction was completed by two out of three researchers (AB, AK, CB). Data were extracted on the characteristics of participants; measures of depression or anxiety used; methodological characteristics of the study; measure of unscheduled care use; and the strength of the association between depression or anxiety and unscheduled care use.
The methodological quality of the included studies was assessed using the Quality Assessment Tool for Quantitative Studies. 80,81
Statistical analysis
Odds ratios (ORs) and 95% confidence intervals (CIs) were extracted or calculated for each study in which number of subjects using unscheduled care with and without depression, or with and without anxiety, and the total number of subjects in each group were presented. Where data were presented in alternative formats (e.g. where studies presented results as continuous data, as p-values for comparisons across groups with group sizes, or as a correlation between depression or anxiety, and unscheduled health-care use), appropriate transformations were made using Comprehensive Meta-analysis software (version 2.2.048, 7 November 2008; Biostat, Englewood, NJ, USA). An OR of > 1 indicated that depression or anxiety was associated with increased use of unscheduled care. Where follow-up data were collected at multiple time points, data collected nearest to 1 year were used. Where studies included two measures of unscheduled care, ORs for each measure were averaged so that each independent study contributed a single effect to the meta-analysis. 81 ORs for depression and anxiety across independent studies were combined using the DerSimonian and Laird random-effects method. 82 Heterogeneity among studies was assessed using Cochrane’s Q and I2 statistics. 83,84 Publication bias was assessed using a funnel plot, Egger’s regression method, Peters’ regression method, and fail-safe N (i.e. the number of additional negative studies that would be required to make the results of our meta-analysis non-significant). 85–88 We used Duval and Tweedie’s trim and fill procedure to correct for the effects of studies missing due to publication bias. 89 Meta-analyses were performed using Comprehensive Meta-analysis software (version 2.2.048) and Stata (version 11; StataCorp LP, TX, USA).
Study 1: does depression predict the use of unscheduled care in patients with long-term conditions?
Results
Figure 3 shows the flow chart for the search strategy used in this review. Sixteen independent prospective studies were identified that had investigated whether or not depression predicted unscheduled care use in patients with either asthma, CHD, COPD or diabetes. 23,24,28–30,55,57,68,90–97 From the 16 studies, there were data for 8477 patients with LTCs (see Figure 3).
FIGURE 3.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 flow chart for longitudinal cohort studies of patients with LTCs (asthma, CHD, COPD and diabetes), which included a measure of depression at baseline and a measure of unscheduled care at follow-up. Reprinted from Journal of Psychosomatic Research, Vol. 73, Dickens C, Katon W, Blakemore A, Khara A, McGowan L, Tomenson B, et al. Does depression predict the use of urgent and unscheduled care by people with long term conditions? A systematic review with meta-analysis, pp. 334–42. © 2012, with permission from Elsevier. 78

Table 1 summarises the main characteristics of each of the included studies and is adapted from data in the published paper by Dickens et al. 78 There were eight studies of COPD,23,29,30,55,90,94–96 five of CHD,28,57,91–93 two of asthma24,68 and only one of diabetes. 98 There were no studies from the UK and only two were based in primary care. 68,98 Most studies involved following a cohort of hospital inpatients admitted for an acute episode of illness, or exacerbation of illness, after their discharge to determine readmission rates or ED visits. Depression was assessed in all of the studies using a self-rated measure, and in most as a categorical construct (case or non-case).
| First author and date | Condition of study | Sample size | Mean age | Males | Sample | Depression measure | Urgent health-care utilisation measure |
|---|---|---|---|---|---|---|---|
| Fan et al., 200755 | COPD | 603 | 66.5 years | 64.1% | 603/611 eligible patients with moderate to severe emphysema self-referred or were referred by a clinician at U.S. clinics to control arm of a lung surgery trial (NETT) | BDI (scores ≥ 10 vs. < 10) | Hospital records for COPD-related inpatient admissions and (ED visits for 1 year |
| Eisner et al., 200524 | Asthma | 756 | 59.9 years | 29.8% | All adults admitted to ITU with asthma plus sample of all patients hospitalised (without ITU) | CES-D (≥ 16 = depressed) | ED visits and hospitalisations recorded from hospital computerised records for 12 months |
| Schneider et al., 200868 | Asthma | 256 | 56.3 years | 38.3% | Consecutive patients with asthma consulting GPs, Germany | Validated German version of PHQ, from which derive DSM-IV depressive disorder | Patients’ self report of urgent hospitalisations and emergency hospital visits over 1 year |
| Ng et al., 200723 | COPD | 376 | 72.2 years | 85.1% | Eligible consecutive inpatients hospitalised for COPD exacerbation in Singapore | Chinese HADS (≥ 8 vs. < 8 on Depression Score) | Patient self-reported on urgent hospitalisation at 6 and 12 months after discharge |
| Almagro et al., 200630 | COPD | 141 | 72.0 years | 93% | Eligible, consecutive patients on inpatient ward for acute exacerbation of COPD over 7 months | Yesavage Depression Scale (continuous) | Clinical records checked for readmissions of 24 hours or more over 1 year |
| Gudmundsson et al., 200690 | COPD | 416 | 69.2 years | 48.8% | Patients hospitalised with COPD exacerbation | HADS depression score (continuous) | Self reported hospitalisations for acute exacerbations of COPD 1 year after discharge, checked via hospital records |
| Lauzon et al., 200357 | CHD | 550 | 60.0 years | 78.9% | Consecutive patients approached after admission in 10 Coronary Care Units | BDI (≥ 10 vs. < 10) | Hospital admissions recorded after 30 days, 6 months and 1 year by self report (postal questionnaire) and chart review |
| Frasure-Smith et al., 200091 | CHD | 848 | 59.3 years | 69.0% | Subjects were recruited to 2 separate studies – 1 prospective cohort study and 1 control arm of RCT. All patients were post MI | BDI (≥ 10 vs. < 10) | ED visits (all cause) and associated costs for 1 year post discharge for MI |
| Kurdyak et al., 200893 | CHD | 1941 | 64.0 years | 30.4% | MI in-patients from 53 hospitals across Ontario Canada | Depression questionnaire, based on Brief Carroll Depression Rating Scale (5 or more vs. < 5) | ED visits (all cause) |
| Xu et al., 200829 | COPD | 491 | 65.6 years | 68.8% | Patients with physician diagnosed COPD, attending 10 general hospitals in Beijing China | Mandarin Chinese HADS (≥ 8 vs. < 8 on Depression scale) | Medical interventions were monitored by a telephone-administered questionnaire over 12 months. Hospitalisations confirmed by chart review |
| Shiotani et al., 200292 | CHD | 1086 | 63.6 years | 80.4% | Eligible consecutive patients with AMI, directly admitted or transferred to 25 collaborating hospitals eligible | Zung Self-Rating Depression Scale. Non-depressed – scores < 40: depressed – scores ≥ 40 | Data on cardiac events 12 months following discharge from hospital records and telephone interviews with patients and family 12 months after baseline |
| Ciechanowski et al., 200098 | Diabetes | 350 | 61.3 years | 44% | Patients from a diabetes register of 2 primary care clinics | SCL-90-R – scores divided into tertiles (low/medium/high) – low vs. others used for meta analysis | ED visits for 6 months following questionnaire assessments collected using General Health Cooperative automated data |
| Ghanei et al., 200796 | COPD | 157 | 58.3 years | 63% | Patients attending chest clinic | HADS depression subscale used as continuous measure | Acute hospitalisation resulting from COPD exacerbation |
| Carneiro et al., 201095 | COPD | 45 | 68 years | 84.4% | Inpatients admitted due to exacerbation of COPD | BDI used as continuous scale | Hospitalisation resulting from exacerbation of COPD |
| Farkas et al., 201094 | COPD | 127 | 66 years | 79% | Hospital outpatients with COPD | CES-D scale used as continuous measure | Hospitalisation resulting from exacerbation of COPD |
| Pishgoo 201128 | CHD | 334 | 57.5 years | 67.8% | Cardiology outpatients with ≥ 50% stenosis in at least 1 major coronary artery | HADS depression scale – (Persian translated and validated | Al cause ED visits |
Table 2 summarises the results of each individual study, and includes univariate and multivariate analyses where they were reported. Eight of the 16 studies reported significant effects of depression on unscheduled care use (either EHAs or ED attendances)29,30,57,68,91–94 and two showed near-significant effects. 24,96
| Author and date | Univariate findings | Factors controlled | Multivariate findings |
|---|---|---|---|
| Fan et al., 200755 | Patients hospitalised or that had ED visit for COPD were slightly more likely to be depressed at baseline (29.9% vs. 24.8% in non-depressed, p = 0.16) | Adjusted for sex, COPD severity, previous admissions, comorbidity score | States BDI score > 10 not associated with hospitalisation in adjusted analyses |
| Eisner et al., 200524 | Depression did not predict ED visits (HR = 1.36, p = 0.12) Trend for depression to predict hospitalisation (HR = 1.34, p = 0.06) |
Age, sex, race, education and smoking | Depression did not predict ED visits (HR, 1.20; p = 0.36) Depression did not predict hospitalisation (HR, 1.34; p = 0.06) |
| Schneider et al., 200868 | Depression predicted hospitalisation (OR, 6.1, p = 0.011) but not emergency GP visits (OR = 1.7, p = 0.30) | Medication guideline adherence, smoking, age and sex | Depressive disorder predicted hospitalisation (p = 0.009) |
| Ng et al., 200723 | 61% depressed patients had ≥ 1 urgent hospitalisation 57.8% non-depressed had ≥ 1 urgent hospitalisation, p = 0.31 | Age, sex, FVC, previous admissions SGRQ | Depression did not predict hospitalisation [HR = 0.93 (95% CI 0.68 to 1.28)] |
| Almagro et al., 200630 | Readmitted patients showed higher baseline depression scores (5 vs. 3.7, p ≤ 0.05) than those who were not readmitted | Age, sex, FEV1, comorbidity, social support | Depression not independent predictor of readmission |
| Gudmundsson et al., 200690 | Hospitalised and non-hospitalised patients had similar baseline depression scores (5.6 vs. 5.4, p = 0.63) | Age, smoking status, FEV1 | Depression HR = 1.09 (95% CI 0.8 to 1.51) |
| Lauzon et al., 200357 | Patients depressed at baseline had higher rates of hospitalisation because of any cardiac complication (30.9% vs. 17.5%) | Age, previous MI, anterior MI, diabetes, hypertension, smoking, sex, previous angina | Readmission due to cardiac complications higher in depressed patients [HR = 1.4 (95% CI 1.05 to 1.86)] |
| Frasure-Smith et al., 200091 | Authors unable to confirm all hospitalisations were urgent Depressed patients had greater mean ED visits (1.3 vs. 0.9, p < 0.0001) |
– | Findings of multivariate analysis not presented for urgent health-care utilisation |
| Kurdyak et al., 200893 | Author unable to confirm that all hospital admissions were urgent Depressed patients had significantly more ED visits (mean = 1.7 vs. 1.3, p < 0.001) |
Age, sex, income, comorbidity, GRACE score, drugs at discharge, cardiac interventions and symptom burden | Adjusted risk for ED visits not presented. Approx adjusted risk for depression predicting ED visits read from figure = 1.1 |
| Xu et al., 200829 | More depressed patients were hospitalised for COPD exacerbations (29.5% vs. 19.8%) | Age, sex, marital, educational and employment status, smoking, FEV1, dyspnea, 6-minute walk, social support, self efficacy, comorbidities, hospital type, drug and O2 use, previous hospitalisations | Adjusted Incidence Rate Ratio for probable depression predicting hospitalisation = 1.72 (95% CI 1.04 to 2.85) |
| Shiotani et al., 200292 | Incidence of cardiac event-related readmission was significantly higher in depressed patients (7.8% vs. 4.3%, p = 0.018) | – | Multivariate results not presented for cardiac event-related readmissions |
| Ciechanowski et al., 200098 | ED visit costs-mean and SD Low depression group = 81 (375) Medium depression group = 128 (479) High depression group = 185 (548) |
– | Not reported for urgent health-care utilisation |
| Ghanei et al., 200796 | Rehospitalised patients had higher depression scores than those not rehospitalised (12 vs. 11, p = 0.039) | Monthly income and medical comorbidities | Depression is an independent predictor of urgent readmission (risk ratio = 0.31, p = 0.012) after controlling for monthly income and medical comorbidities |
| Carneiro et al., 201095 | Number of readmission correlated with depression score (p = 0.09) | – | Not reported |
| Farkas et al., 201094 | Depression score was higher for hospitalised compared to non-hospitalised [15 (SD = 11) vs. 11 (SD = 9)] | – | Depression not entered into the multivariate analyses |
| Pishgoo 201128 | Baseline depression score 5.7 among those with ED visits vs. 5.1 in those without, p = 0.22 | Sex, angina grade, anxiety and somatic comorbidity score contributed entered into final model | Sex, angina grade, anxiety and somatic comorbidity score contributed significantly to the final model. Depression wasn’t entered into the model as univariate findings were non-significant |
The results of the meta-analysis are shown in Figure 4. There is a combined OR for depression across all the studies of 1.49 (95% CI 1.35 to 1.64; p < 0.0005). Effects of individual studies were very homogeneous (Q = 13.2, I2 = 0.0, 95% CI 0 to 52; p = 0.58).
FIGURE 4.
Forest plot for associations between depression and subsequent use of unscheduled health care in patients with one of four exemplar LTCs (N = 16; OR 1.49, 95% CI 1.35 to 1.64; p < 0.001; Q = 13.2, I2 = 0.0%; 95% CI 0 to 52; p = 0.58). BDI, Beck Depression Inventory; CES-D, Centre for Epidemiological Studies Depression Scale; DRS, Depression Rating Scale; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition; GDS, Geriatric Depression Scale; HADS, Hospital Anxiety and Depression Scale; PHQ, Patient Health Questionnaire; Zung, Zung Self-Rating Depression Scale. Reprinted from Journal of Psychosomatic Research, Vol. 73, Dickens C, Katon W, Blakemore A, Khara A, McGowan L, Tomenson B, et al. Does depression predict the use of urgent and unscheduled care by people with long term conditions? A systematic review with meta-analysis, pp. 334–42. © 2012, with permission from Elsevier. 78
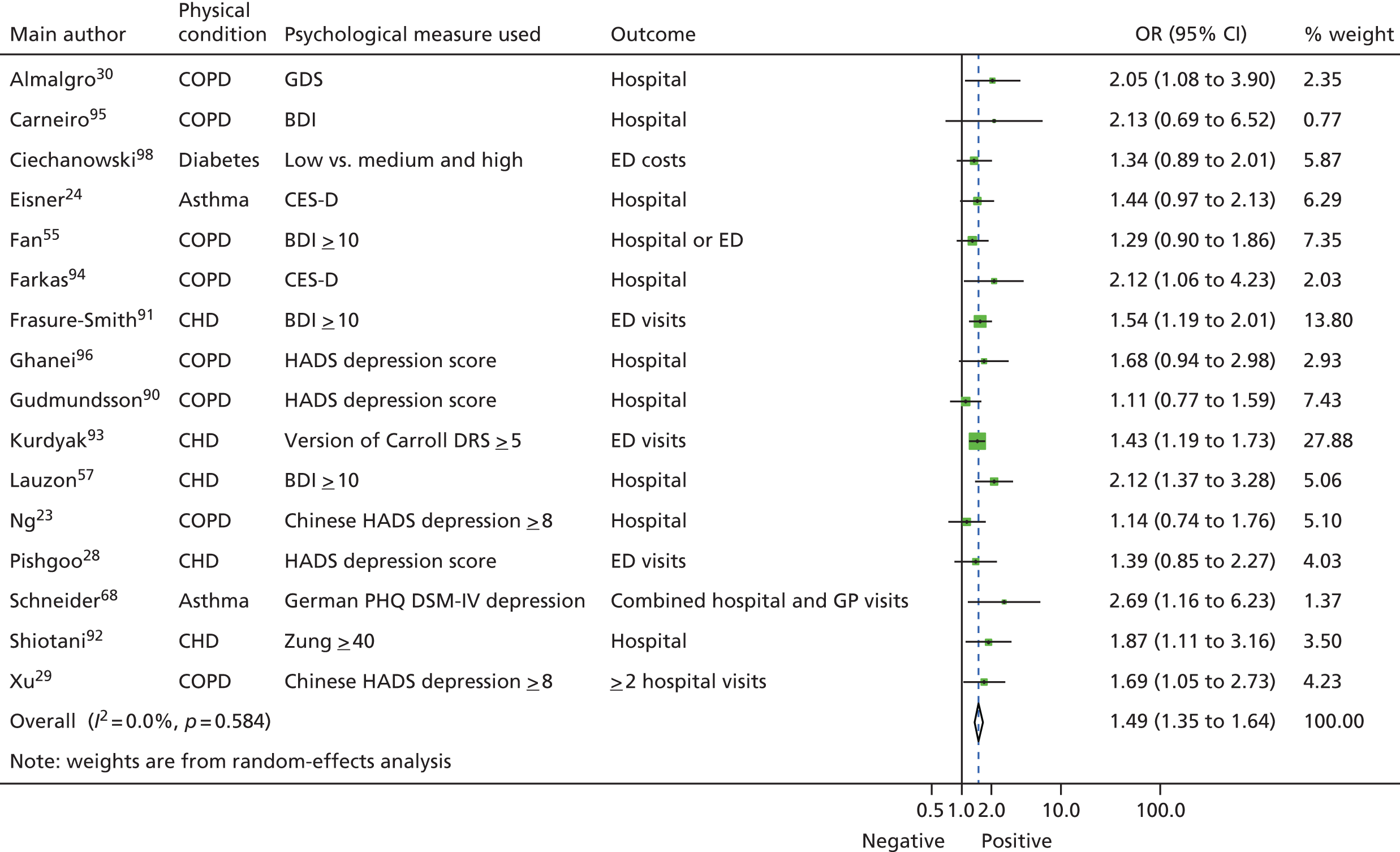
Effects varied with different types of unscheduled care. The combined OR for ED attendances was 1.45 (n = 4; 95% CI 1.26 to 1.66; p < 0.0005), for urgent hospitalisations it was 1.56 (n = 10; 95% CI 1.32 to 1.84; p < 0.0005), for combined urgent hospitalisations or ED visits it was 1.29 (n = 1; 95% CI 0.90 to 1.86; p = 0.17) and for combined urgent hospitalisation and urgent GP visits it was 2.69 (n = 1; 95% CI 1.16 to 6.23; p = 0.021). Comparison across groups using the analogue to analysis of variance (ANOVA) revealed that these differences across different types of unscheduled care were not statistically significant [Q = 2.9, degrees of freedom (df) = 3; p = 0.41].
Effects also varied across the different LTCs, but these were not statistically significant. Only eight of the studies included a measure of severity of illness. 23,28–30,55,57,90,93 In six of these,23,29,30,55,90,93 the independent effects of depression were not significant.
The funnel plot for depression studies appeared asymmetrical, with a relative absence of small negative studies (Figure 5). Egger’s regression method confirmed an association between log-OR and standard error of log-OR (Egger’s intercept = 1.98, 95% CI 0.22 to 3.74; p = 0.03). This suggested that there may be some publication bias in the papers included in our review.
FIGURE 5.
Funnel plot for studies of depression and subsequent use of unscheduled health care in patients with LTCs (Egger’s intercept = 1.98; 95% CI 0.22 to 3.74; p = 0.03). Reprinted from Journal of Psychosomatic Research, Vol. 73, Dickens C, Katon W, Blakemore A, Khara A, McGowan L, Tomenson B, et al. Does depression predict the use of urgent and unscheduled care by people with long term conditions? A systematic review with meta-analysis, pp. 334–42. © 2012, with permission from Elsevier. 78
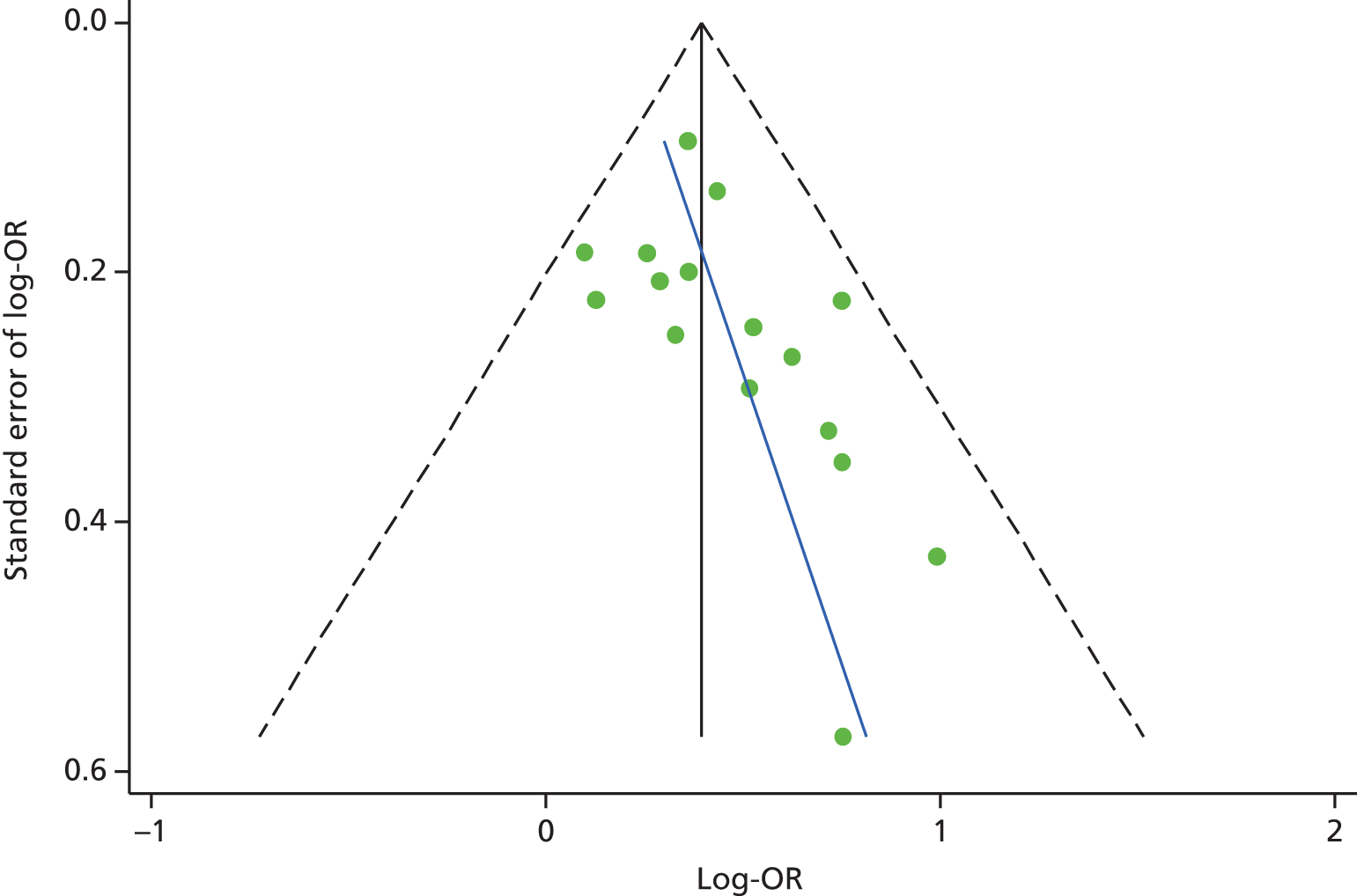
Table 3 shows the results of the quality review for studies that assessed the impact of depression on unscheduled care. Meta-analysis was repeated for the studies subgrouped according to methodological quality (strong, moderate or weak). There was no apparent association between study quality and effect size. There were two studies that were rated as methodologically strong,23,30 which, when combined, produced an OR of 1.46 (95% CI 0.82 to 2.58; p = 0.20). There were six studies that were rated as methodologically moderate, which resulted in a combined OR of 1.53 (95% CI 1.31 to 1.77; p < 0.0005). 24,29,55,57,68,93 Eight studies were rated as methodologically weak with a combined OR of 1.47 (95% CI 1.26 to 1.72; p < 0.0005). 28,90–92,94–96,98
| Author and date | Selection bias | Design | Confounding | Blinding | Data collection | Dropouts | Global rating | Discrepancy between reviewers | Reasons for discrepancy | Final rating |
|---|---|---|---|---|---|---|---|---|---|---|
| Almagro et al., 200630 | 2 | 2 | 1 | 2 | 1 | 1 | 1 | No | 1 | |
| Carneiro et al., 201095 | 3 | 2 | 3 | 2 | 1 | 1 | 3 | No | 3 | |
| Ciechanowski et al., 200098 | 2 | 2 | 3 | 2 | 1 | 3 | 2 | Yes | Oversight | 3 |
| Eisner et al., 200524 | 2 | 2 | 2 | 2 | 1 | 3 | 2 | No | 2 | |
| Fan et al., 200755 | 3 | 2 | 1 | 2 | 1 | 1 | 3 | Yes | Oversight, differences in interpretation | 2 |
| Farkas et al., 201094 | 3 | 2 | 3 | 2 | 1 | 3 | 3 | No | 3 | |
| Frasure-Smith et al., 200091 | 3 | 2 | 3 | 2 | 1 | 3 | 3 | No | 3 | |
| Ghanei et al., 200796 | 3 | 2 | 2 | 2 | 1 | 3 | 3 | Yes | Differences in interpretation | 3 |
| Gudmundsson et al., 200690 | 3 | 2 | 1 | 2 | 1 | 3 | 3 | No | 3 | |
| Kurdyak et al., 200893 | 3 | 2 | 1 | 2 | 1 | 2 | 2 | Yes | Differences in interpretation | 2 |
| Lauzon et al., 200357 | 3 | 2 | 1 | 2 | 1 | 1 | 2 | Yes | Oversight | 2 |
| Ng et al., 200723 | 2 | 2 | 1 | 2 | 1 | 2 | 1 | No | 1 | |
| Pishgoo 201128 | 3 | 2 | 3 | 2 | 1 | 1 | 3 | No | 3 | |
| Schneider et al., 200868 | 3 | 2 | 2 | 2 | 1 | 2 | 2 | No | 2 | |
| Shiotani et al., 200292 | 3 | 2 | 3 | 2 | 1 | 1 | 3 | Yes | Differences in interpretation | 3 |
| Xu et al., 200829 | 3 | 2 | 1 | 2 | 1 | 1 | 2 | No | 2 |
Conclusions
Our main conclusion from this systematic review was that depression was associated with a 49% increase in the odds of using subsequent unscheduled care among people with LTCs. The effects for depression were not significantly different across different types of unscheduled care (EHAs or ED attendances) or across different LTCs. However, only eight of studies included attempts to control for the severity of illness, and this appeared to be an important confounder. 23,28–30,55,57,90,93
Limitations
There are several limitations of this review of the relationship between depression and unscheduled care use. First, none of the studies that we identified had been carried out in the UK, so it is unclear how translatable the results are to the UK health-care system. The delivery and organisation of health care varies across countries and the same factors that drive attendance at an emergency room; for example, the factors that drive attendance in the USA may not be the same as those that drive health-care-seeking in the UK. Second, we were able to identify only one study on diabetes98 and two on asthma,24,68 which limits the generalisability of the results to these conditions and other LTCs. Third, only two of the studies were based in primary care,68,98 with most studies focusing on hospital populations, so the results may not be generalisable to patients in primary care with LTCs. Fourth, the methodological quality of the studies identified varied considerably, though this does not appear to have distorted our results. Fifth, we found evidence of a potential publication bias, which may have inflated the observed association between depression and unscheduled care. Finally, a wide variety of different measures of depression were utilised across the studies, as shown in Table 1. Although there is generally good agreement between measures of depression, there will have been some variability between the studies because of the different measures that were employed. Of note, only three of the studies used a depression measure specifically developed for use in populations with physical illness. 28,90,96
Study 2: does anxiety predict the use of unscheduled care in patients with long-term conditions?
Results
The results of the second systematic review we conducted will now be presented. Figure 6 shows the flow chart for this study. Eight independent studies with a prospective design and which included a measure of anxiety at baseline and a measure of unscheduled care at outcome were identified. 29,68,76,77,97,99–101 From the eight studies we identified, there were data for 28,823 participants. The details of the characteristics of each of the included studies can be found in the published paper by Blakeley et al. 79 and are summarised in Table 4.
FIGURE 6.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart for prospective longitudinal studies of patients with LTCs, which included a measure of anxiety at baseline and a measure of unscheduled care at follow-up. Adapted from Blakeley et al. 79

| Author, date, LTC and country of origin | Sample and size | Measure of | |
|---|---|---|---|
| Anxiety | Unscheduled care | ||
| Abrams et al., 2011;77 COPD, USA | Veterans with COPD exacerbation, acute or chronic bronchitis admitted to hospital (n = 26,591) | ICD-9 | 30-day readmission records |
| Schneider et al., 2008;68 asthma, Germany | Patients from 43 primary care practices (n = 256) | Validated German PHQ | Patient self-reported ED attendances and EHAs over 1 year |
| Greaves et al., 2002;97 asthma, UK | Community subjects with asthma, one group stable, one group who had suffered recent asthma attack (n = 74) | Seven-item panic fear scale of Asthma Symptom Checklist | Practice records ED attendance and hospital attendance over 1 year |
| Grace et al., 2004;99 CHD, Canada | Patients with unstable angina and MI admitted to CCUs (n = 913) | Phobic anxiety subscale of Middlesex Hospital Questionnaire Anxiety subscale of PRIME-MD |
Self-reported cardiac events over 6 months and 1 year |
| Kaptein, 1982;100 asthma, the Netherlands | Patients with acute, severe asthma hospitalised for condition (n = 40) | State–Trait Anxiety Inventory plus panic–fear personality scale | Rehospitalisation within 6-month period |
| Xu et al., 2008;29 COPD, China | COPD patients attending hospitals in Beijing (n = 491) | Mandarin HADS | EHAs confirmed by chart review over 1 year |
| Gudmundsson et al., 2006;90 COPD, Iceland | Patients hospitalised for COPD (n = 416) | HADS (cut-off point of ≥ 8) | Self-reported readmissions over 1 year |
| Coventry et al., 2011;76 COPD, UK | Patients admitted to hospital with COPD (n = 79) | HADS | Readmissions over 1 year |
Four of the studies involved patients with COPD,29,76,77,90 three involved patients with asthma68,97,100 and one involved patients with CHD. 99 There were no studies on diabetes. Two of the studies were based in the UK. 76,97
The main, multivariate results for each individual study are shown in Table 5. None of the eight studies included in this review found that anxiety had a significant effect on the use of unscheduled health care. One study found near significant effects for anxiety on unscheduled health-care use for patients with asthma (see Table 5). 68
| Author and date | Univariate findings | Factors controlled for | Multivariate findings |
|---|---|---|---|
| Abrams et al., 201177 | Patients with anxiety were not more significantly likely to be readmitted than those without anxiety [11.3% vs. 11.5% (NS)] | Smoking status | No significant difference in risk of admission regardless of smoking status. Smoking present (HR 1.22, 95% CI 1.04 to 1.44) and smoking absent (HR 1.22, 95% CI 1.03 to 1.43) |
| Schneider et al., 200868 | Panic disorder did not predict hospitalisation (OR 3.5, 95% CI 0.7 to 18.3; p = 0.145), but did predict emergency visits (OR 4.8, 95% CI 1.3 to 17.7; p = 0.019) | ||
| Greaves et al., 200297 | There was no main effect of panic (p > 0.05) | ||
| Grace et al., 200499 | Anxious patients (mean 1.11, SD 1.57) reported more visits to the ED than non-anxious (mean 0.83, SD 1.18) patients (t = –1.37; p = 0.17). However, this was NS | Age, family history of CVD, depression, Killip class, sex, family income, smoking status, diabetes and phobic anxiety | Age (OR 1.02, 95% CI 1.00 to 1.05; p = 0.05), family history of CVD (OR 1.63, 95% CI 1.04 to 2.54; p = 0.03), depression (OR 1.07, 95% CI 1.03 to 1.12; p ≤ 0.01) and PRIME-MD anxiety score at 6 months (OR 0.35, 95% CI 0.19 to 0.65; p < 0.01), were all significant predictors of self-reported recurrent cardiac events. All other factors NS |
| Kaptein, 1982100 | State and trait anxiety not associated with increased length of hospitalisations State anxiety not significantly associated with readmission; however, trait anxiety had slight effect (one-tailed t-test: t = 1.72; p = 0.048) |
||
| Xu et al., 200829 | Anxiety not associated with increased risk of EHA (p = 0.11); however, length of exacerbation in days was longer for patients with anxiety than for those without (p = 0.03) | Age, sex, smoking, marital status, education, employment, living situation, FEV1, dyspnoea score, 6-minute walk distance, social support, COPD-specific self-efficacy, significant comorbidities, hospital type, use of long-acting bronchodilator and inhaled corticosteroid, long-term oxygen therapy and past hospitalisation | Anxiety was not associated with hospitalisation: IRR 1.63 (95% CI 0.88 to 3.03) for HADS anxiety score of ≥ 11; or with length of hospitalisation for those readmitted: IRR 1.99 (95% CI 0.59 to 6.72) |
| Gudmundsson et al., 200690 | Anxiety had no significant effect on rehospitalisation (p = 0.61). No significant difference between HADS anxiety scores for those who were readmitted [mean 7.1 (SD 4.3)] and those who were not [mean 6.7 (SD 4.0); p = 0.28] | Age smoking status, FEV1, SGRQ | Significant association between the HADS anxiety score and the risk of readmission in patients with a low health status (HR 0.81, 95% CI 0.63 to 1.04). In the same group, anxiety (HADS score of ≥ 8) was related to increased risk of rehospitalisation (HR 0.43, 95% CI 0.25 to 0.74) |
| Coventry et al., 201176 | No significant difference between HADS anxiety scores for those who were readmitted (8.53 ± 4.2) and those who were not (9.47 ± 4.6; p = 0.407) | Age, race, sex, individual medical comorbidities and laboratory values | Depression (OR 1.300, 95% CI 1.06 to 1.60; p = 0.013), FEV1 score (OR 0.962, 95% CI 0.93 to 0.99; p = 0.021), and age (OR 1.092, 95% CI 1.01 to 1.18; p = 0.026) were the only significant predictors of readmission. Anxiety was insignificant |
Figure 7 shows a forest plot of the eight studies. 29,68,76,77,90,97,99,100 There was a combined OR for anxiety across all studies of 1.078 (95% CI 0.877 to 1.325; p = 0.476). Effects of individual studies showed a low level of heterogeneity (Q = 9.5, df = 7, I2 = 26.07%; p = 0.221).
FIGURE 7.
Forest plot for associations between anxiety and subsequent use of unscheduled health care. ASCL, Asthma Symptom Checklist; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition; HADS, Hospital Anxiety and Depression Scale; PHQ, Patient Health Questionnaire; PRIME-MD, Primary Care Evaluation of Mental Disorders screening questionnaire. From Blakeley et al. 79
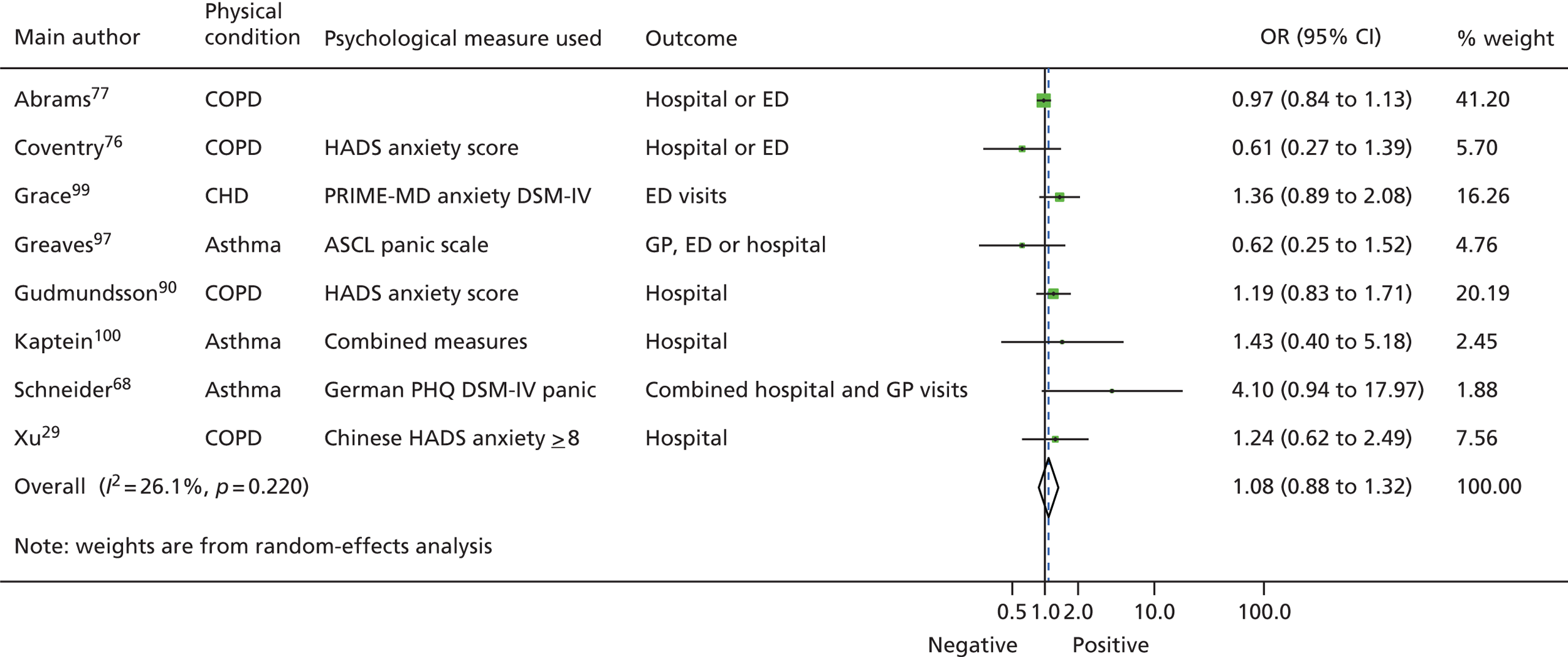
A sensitivity analysis was performed excluding the largest study, as this had the largest sample size (n = 26,591) of whom 97% were male. 77 The combined effect in random-effects meta-analysis for the studies excluding Abrams et al. 77 was 1.238 (95% CI 0.969 to 1.551; p = 0.087). Again, the effects of these studies showed low heterogeneity (Q = 5.116, df = 5, I2 = 2.27%; p = 0.402).
We investigated whether or not the effects of anxiety varied across the different types of unscheduled care used between studies and the different types of LTCs. The effect of anxiety varied across the different types of unscheduled health-care use and across the four LTCs, but none of the differences was significant.
Four studies conducted multivariate analysis that controlled for the severity of the LTC studied. 76,77,99,101 One out of four studies found that, when severity of the LTC was controlled for, anxiety was significantly related to hospital readmission rates for a subgroup of patients with COPD and poor health status. 101
Meta-analysis was repeated for the studies subgrouped according to methodological quality (strong, moderate or weak). Study quality did not change the results: anxiety had no significant impact on unscheduled care use across studies that were rated as methodologically strong (n = 2; OR 0.927, 95% CI 0.872 to 1.143; p = 0.978), moderate (n = 4; OR 1.243, 95% CI 0.650 to 2.375; p = 0.511) or weak (n = 2; OR 1.258, 95% CI 0.955 to 1.656; p = 0.102).
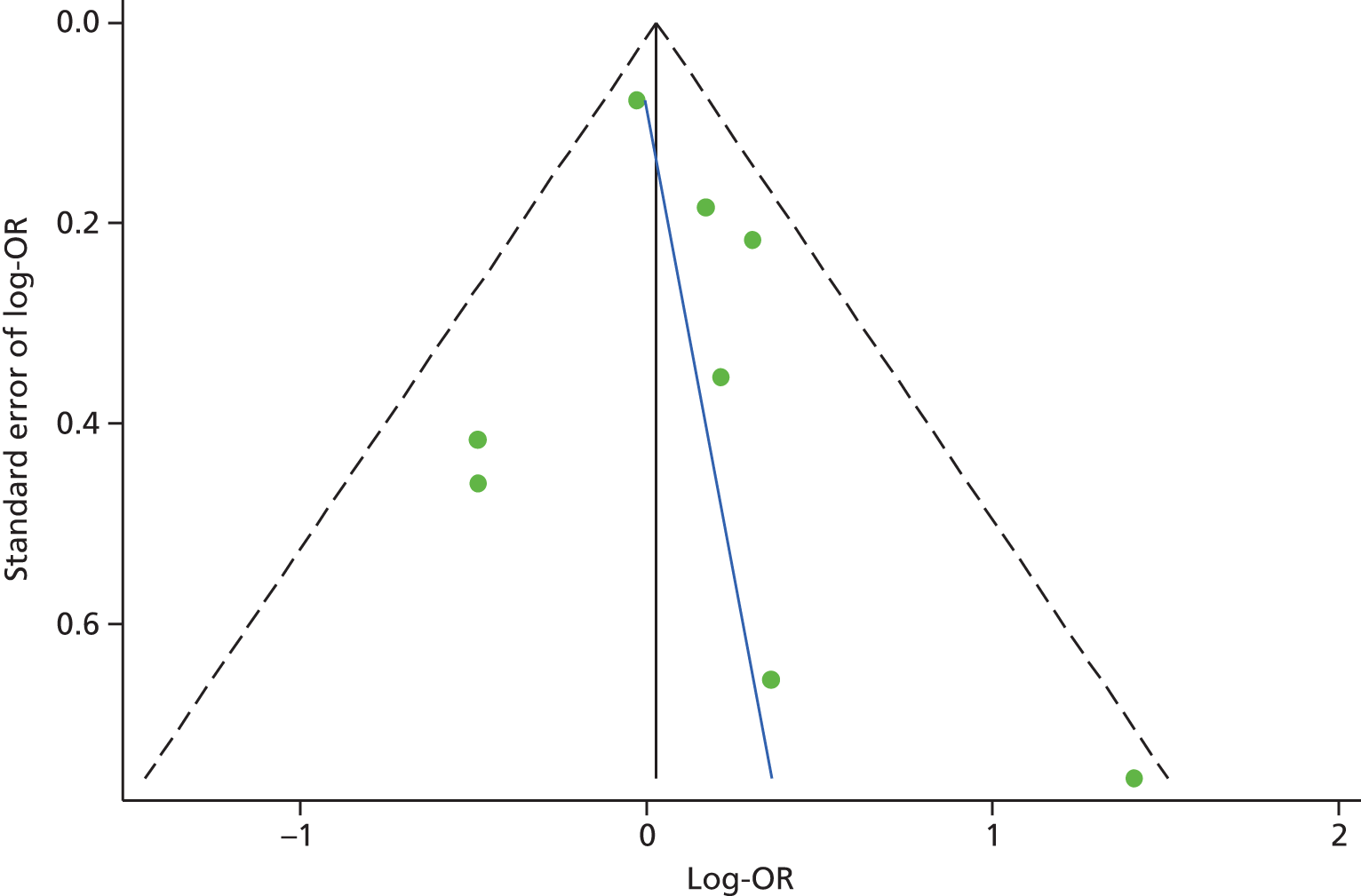
Publication bias was investigated using a funnel plot. The plot for anxiety studies did not appear to be asymmetrical, except for one small negative study (Figure 8). Egger’s regression method confirmed the lack of association between the loge-OR and standard error of loge-OR (Egger’s bias = 1.24, 95% CI –1.01 to 3.48; p = 0.23). The Duval and Tweedie trim and fill procedure created just one imputed study, giving a revised random-effects-combined OR for anxiety across all studies of 1.05 (95% CI 0.82 to 1.33; p = 0.69). The heterogeneity between studies was increased slightly, and was still significant (Q = 12.9, df = 8; p = 0.040).
FIGURE 8.
Funnel plot with pseudo-95% confidence limits for studies of anxiety and subsequent use of unscheduled health care (Egger’s test: bias = 1.24, 95% CI –1.01 to 3.48; p = 0.23). Adapted from Blakeley et al. 79
Conclusions
Our main conclusion from this systematic review was that anxiety does not appear to be associated with an increase in using unscheduled care in patients with LTCs. This is in contrast to our findings from the first systematic review, which showed a relationship between depression and use of unscheduled care.
The number of studies in the anxiety review was small and we did not focus specifically on panic or other forms of anxiety-related symptoms and disorders, which have been shown to be involved in care-seeking. 102,103 However, the findings for our review suggest anxiety does not appear to be an important psychosocial driver of unscheduled care in patients with asthma, CHD, COPD and diabetes.
Discussion for systematic reviews to determine whether depression or anxiety predicts unscheduled care use
The findings from our two first systematic reviews were puzzling. Why should depression, but not anxiety, be associated with unscheduled care use? Both are common in patients with LTCs and both are associated with adverse outcomes in LTCs. 10,69,104,105
One of the reasons for the difference we found between depression and anxiety may be methodological problems with the included studies, which are detailed below, and the relative paucity of studies that have examined the effects of anxiety at all. In addition, depression and anxiety often coexist, particularly in primary care,105 and it may be somewhat of an artificial distinction to separate them in the context of comorbid physical illness. However, as they have been analysed and reported separately in the included studies, it would not have been possible to combine them in a meaningful way for the purposes of the systematic reviews.
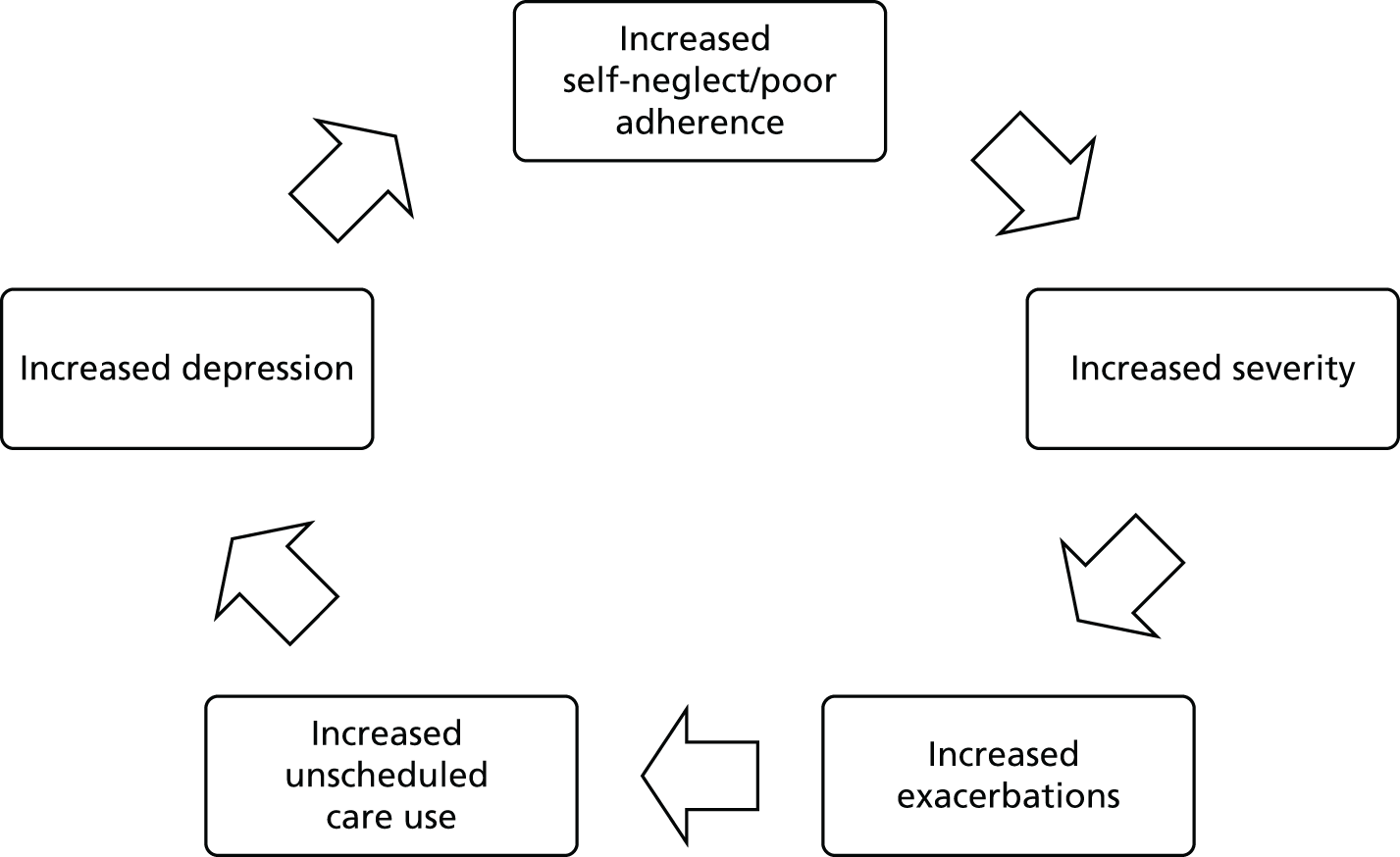
Our findings give support to a model of depression in relation to unscheduled care use in people with LTCs. Depression results in greater self-neglect106,107 and less adherence to routine treatment. 108 This in turn results in more symptoms, or more severe symptoms or exacerbations, which may increase the risk of unscheduled care.
This model is illustrated in Figure 9. A similar model has been put forward by Pooler and Beech in relation to COPD and increased hospital admissions,109 although that model also includes a role for anxiety, which is not supported by our findings.
FIGURE 9.
A model illustrating the potential mechanism whereby depression may affect unscheduled care use. Adapted from Pooler and Beech. 109
Anxiety is clearly associated with a variety of poor outcomes in people with LTCs, and is reported from the qualitative literature to be associated with acute exacerbations of illness. 110 It is possible that, once an acute exacerbation of illness is triggered, this is then associated with an increase in worry and anxiety, which heightens the focus on bodily symptoms, resulting in some cases, in fear of death and panic, which necessitates the need to seek immediate health care. Thus, anxiety may not be a long-term predictor of unscheduled care but may play an important role in the immediacy of treatment-seeking which would not be captured by studies employing a prospective longitudinal design, but may be captured by other methods (e.g. qualitative work). This potential mechanism is illustrated in Figure 10.
FIGURE 10.
A model illustrating the potential mechanism whereby depression and anxiety may affect unscheduled care use. Adapted from Pooler and Beech. 109

Limitations
By limiting the two systematic reviews to our four exemplar LTCs, our findings may not be generalisable to all people with LTCs. The studies in the two reviews were diverse and focused on different patient populations at different points in the care pathway, in different countries, with different health-care systems, and with different times to follow-up. In some countries, health care would not be free at the point of delivery, and this is likely to have an impact on health-care-seeking behaviour. There were very few UK studies, so it is difficult to determine whether or not the findings are relevant to the NHS.
We included only studies in which a prospective longitudinal cohort design had been employed and, particularly in relation to anxiety, there were very few published studies. We chose not to include cross-sectional studies because of the difficulty interpreting casual links with such designs; however, this limited the number of studies that were available to include.
Very few of the studies had been specifically designed to test the relationship between baseline depression or anxiety and unscheduled care, and in many cases the findings we used were the result of secondary analyses. There were also differences in the types of unscheduled care that were examined in the studies, including hospital readmission rates following discharge, acute admissions and ED attendances. The results of the systematic review focusing on depression were also potentially subject to publication bias, and only half the studies took account of the severity of patients’ physical symptoms.
Complex interventions that reduce unscheduled health-care use
The second aim of this section of the research programme was to systematically synthesise the evidence for existing complex interventions which have been used to reduce the frequency of unscheduled care use in patients with LTCs. Our initial search in 2008 identified that there were over 30 relevant studies for COPD and asthma, but only six studies that examined the effect of complex interventions on unscheduled care use in diabetes and four studies in CHD. We chose to focus on two of our four exemplar conditions [COPD (study 3) and asthma (study 4)], as there was a large number of studies, 32 and 33, respectively, for each condition, which had looked at the effect of complex interventions to reduce unscheduled care use.
Aims
The aim of our third review (study 3) was to identify the characteristics of complex interventions that reduce the unscheduled health-care use in adults with COPD. 111
The aim of our fourth review (study 4) was to identify the characteristics of complex interventions that reduce the unscheduled health-care use in adults with asthma. 112
Methods
The methods for studies 3 and 4 are summarised here. Full descriptions of the methods are available in the published papers. 111,112
Studies were eligible for inclusion in the reviews if they met the following criteria:
-
Included adults with COPD or asthma (aged ≥ 16 years).
-
Assessed the efficacy/effectiveness of a complex intervention. A complex intervention is defined as an intervention that involves multiple components and/or multiple professionals. The interventions can be delivered on an individual or a group basis, face to face, over the telephone, or on a computer. Interventions could include any of the following: education, rehabilitation, psychological therapy, social intervention (e.g. social support or support group), organisational intervention (e.g. collaborative care or case management) and drug trials that targeted a psychological problem (e.g. anxiety or depression).
-
Assessed unscheduled health-care use as an outcome. This could include ED visits, EHAs or unscheduled GP visits.
-
RCT design.
Search strategies (see Appendix 1) were developed and searches conducted in the following electronic databases: MEDLINE, EMBASE, CINAHL, BNI, PsycINFO and Cochrane databases. Searches for both reviews were first conducted on 19 August 2008 and then updated on 25 January 2013. Each eligible paper identified from searching the databases was also checked for relevant citations using the Social Science Citation Index to identify more papers.
We were not able to develop a search strategy that was sensitive and reliable enough to detect studies investigating unscheduled health care specifically. Therefore, searches were developed that more broadly identified studies that had measured all health-care use and further restriction to relevant papers that had looked at unscheduled care was specifically achieved by hand-searching. Additional papers were identified by screening reference lists and citations of eligible papers.
The titles and abstracts of all identified papers were screened by one of three researchers [AB, Angee Khara (AK), RA] and the full text of studies that potentially met the inclusion criteria were then screened by two out of three researchers (AB, AK, RA). Any disagreements about eligible papers were discussed with another member of the team (CD, EG).
Data extraction was completed by two out of three researchers (AB, AK, RA) using standardised electronic data extraction sheets that were developed by the team and modified after piloting the first five papers. Data were extracted on the characteristics of participants; the characteristics of the intervention; methodological characteristics of the study; and the effects of the intervention on the use of unscheduled health care.
The methodological quality of the included studies was evaluated using a component approach as recommended in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0113 to assess whether or not:
-
allocation sequence was adequately generated
-
treatment allocation was adequately concealed
-
knowledge of the allocated intervention was adequately prevented
-
incomplete outcome data were adequately dealt with
-
reports were free from selective outcome reporting
-
the study was free of other problems that may cause bias.
Each study was rated as low, medium or high risk for each of the components listed. Where the rating was not certain, studies were rated as unclear. 113
Statistical analysis
Odds ratios and 95% CIs were extracted or calculated for each study. An OR of < 1 indicated that the intervention reduced the use of unscheduled health care. 114 Where data were presented in alternative formats, appropriate transformations were made. 81 Where follow-up data were collected at multiple time points, data collected nearest to 1 year were used. Where studies included more than one measure of unscheduled care, ORs for each measure were averaged so that each independent study contributed a single effect to the meta-analysis. 81 For studies that included more than one intervention group, data for each intervention were entered as separate records and the sample size for the control group was halved for the comparison. ORs for interventions across independent studies were combined using the DerSimonian and Laird random-effects method. 82 Heterogeneity among studies was assessed using the Cochrane’s Q and I2 statistics. 83 Publication bias was assessed using a funnel plot and Egger’s regression method. 86
Differences in effect across the methodological characteristics of the trials were tested using the analogue to ANOVA for categorical variables and univariate meta-regression for continuous variables. 81 Random-effects multivariate meta-regression was used to identify which components of the interventions were independently associated with reductions in unscheduled health care. 115,116
Study 3: complex interventions that reduce unscheduled health-care use in chronic obstructive pulmonary disease
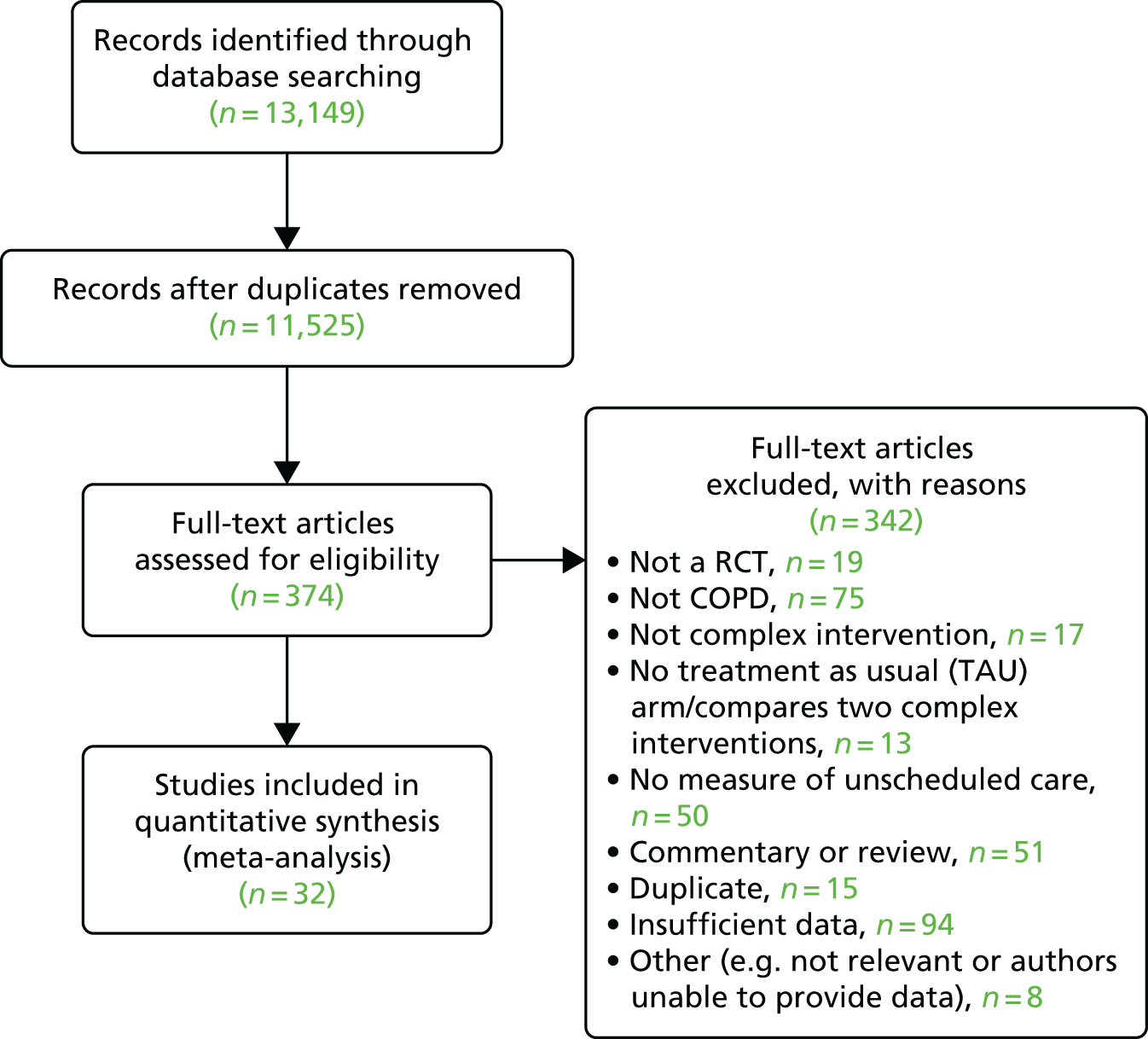
Results
The flow chart for this study is shown in Figure 11. Thirty-two independent studies were eligible for inclusion in this review, which included the comparison of 33 independent interventions. 114,117–148
FIGURE 11.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart for studies of the effectiveness of complex interventions in the reduction of unscheduled care use in COPD. Reprinted from Respiratory Medicine, Vol. 108, Dickens C, Katon W, Blakemore A, Khara A, Tomenson B, Woodcock A, et al. Complex interventions that reduce urgent care use in COPD: a systematic review with meta-regression, pp. 426–37. © 2014, with permission from Elsevier. 111
The details of the characteristics of each included study are summarised in Table 6. In the majority of studies, patients with COPD were recruited from secondary care settings, and either ED attendances and/or EHAs were the measure of unscheduled care use.
| Authors | Year of publication | Sample size | Where recruited | Assessment of urgent health-care use | Method of urgent health-care assessment | Duration of follow-up (months) |
|---|---|---|---|---|---|---|
| Bergner et al.119 | 1988 | 244 | Secondary | ED visits | Records | 12 |
| Bourbeau et al.118 | 2003 | 191 | Secondary | ED visits/urgent hospitalisation | Self-reported | 12 |
| Boxall et al.120 | 2005 | 46 | Combined | Urgent hospitalisations | Self-reported | 6 |
| Casas et al.114 | 2006 | 155 | Secondary | Urgent hospitalisations | Combined | 12 |
| Eaton et al.121 | 2009 | 97 | Secondary | Unscheduled emergency visits to primary or secondary care | Combined | 3 |
| Farrero et al.122 | 2001 | 94 | Primary | ED visits | Records | 12 |
| Gadoury et al.123 | 2005 | 191 | Secondary | ED visits/urgent hospitalisation | Records | 24 |
| Gallefoss and Bakke124 | 2000 | 71 | Secondary | Urgent hospitalisation | Combined | 12 |
| Güell et al.125 | 2000 | 60 | Secondary | Urgent hospitalisation | Unclear | 24 |
| Hermiz et al.126 | 2002 | 147 | Secondary | ED visits | Records | 3 |
| Hernandez et al.127 | 2003 | 209 | Secondary | ED visits | Combined | 2 |
| Jarab et al.128 | 2012 | 127 | Secondary | ED visits/urgent hospitalisation | Unclear | 6 |
| Khdour et al.129 | 2009 | 173 | Secondary | ED visits/urgent hospitalisation/urgent doctor visits | Combined | 12 |
| Ko et al.130 | 2011 | 60 | Secondary | ED visits/urgent hospitalisation | Combined | 12 |
| Koff et al.131 | 2009 | 40 | Secondary | ED visits/urgent hospitalisation | Combined | 3 |
| Lee et al.132 | 2002 | 89 | Secondary | ED visits | Unclear | 6 |
| Díaz Lobato et al.133 | 2005 | 40 | Secondary | Urgent ICU admission | Unclear | 1 |
| Man et al.148 | 2004 | 34 | Secondary | ED visits | Combined | 3 |
| Martin et al.134 | 2004 | 89 | Primary | Ambulance calls | Unclear | 12 |
| McGeoch et al.135 | 2006 | 154 | Primary | ED visits | Unclear | 12 |
| Rea et al.117 | 2004 | 135 | Combined | ED visits | Records | 12 |
| Rice et al.137 | 2010 | 554 | Primary | ED visits | Records | 12 |
| Ries et al.138 | 2003 | 137 | Secondary | ED visits | Self-reported | 12 |
| Seymour et al.139 | 2010 | 60 | Secondary | ED visits/urgent hospitalisation | Combined | 3 |
| Smith et al.140 | 1999 | 92 | Combined | ED visits | Records | 12 |
| Soler et al.141 | 2006 | 26 | Secondary | ED visits | Records | 12 |
| Sridhar et al.142 | 2008 | 104 | Secondary | Urgent doctor visits | Combined | 24 |
| Tougaard et al.143 | 1992 | 82 | Secondary | General practice emergency care | Records | 12 |
| Trappenburg et al.144 | 2011 | 216 | Combined | ED visits/urgent hospitalisation | Combined | 6 |
| Wakabayashi et al.145 | 2011 | 85 | Secondary | ED visits | Unclear | 12 |
| Wong et al.146 | 2005 | 60 | Secondary | ED visits | Self-reported | 3 |
| Wood-Baker et al.147 | 2006 | 112 | Primary | ED visits/urgent doctor visits | Self-reported | 12 |
Table 7 summarises the type of interventions that were employed in the studies included in the review of complex interventions in COPD, and the duration of the intervention.
| Study | Intervention | No. sessions | Who delivered the intervention? | Delivery method | Where delivered | Unidisciplinary or multidisciplinary | Intervention components |
|---|---|---|---|---|---|---|---|
| Bergner et al.119 | Respiratory home care vs. TAU and standard home care vs. TAU | Unclear | Non-mental health practitioner | Face to face | Home | Multidisciplinary | 12 |
| Bourbeau et al.118 | Education and case manager vs. TAU | 18 | Non-mental health practitioner | Combination | Home | Multidisciplinary | 1, 3, 4, 6, 10 |
| Boxall et al.120 | Home-based pulmonary rehabilitation vs. waiting list | 11 | Non-mental health practitioner | Face to face | Home | Multidisciplinary | 1, 3, 4 |
| Casas et al.114 | Integrated care vs. TAU | 1 comprehensive education session | Non-mental health practitioner | Face to face, telephone and online | Home, hospital, online | Multidisciplinary | 1, 12 |
| Eaton et al.121 | Early pulmonary rehabilitation | Unclear | Non-mental health practitioner | Face to face | Combination | Multidisciplinary | 1, 4, 6, 10, 12 |
| Farrero et al.122 | Hospital-based home care vs. TAU | 4.8 | Non-mental health practitioner | Combination | Combination | Multidisciplinary | 12 |
| Gadoury et al.123 | COPD self-management vs. TAU | 18 | Unclear | Combination | Home | Unclear | 1, 4, 12 |
| Gallefoss and Bakke124 | Self-management education vs. TAU | 4 | Non-mental health practitioner | Face to face | Unclear | Multidisciplinary | 1, 3, 6, 12 |
| Güell et al.125 | Rehabilitation vs. TAU | 31 | Non-mental health practitioner | Face to face | Hospital | Unclear | 1, 3, 4, 10 |
| Hermiz et al.126 | Home-based self-management education and support vs. TAU | 2 | Non-mental health practitioner | Face to face | Home | Multidisciplinary | 1, 12 |
| Hernandez et al.127 | Home hospitalisation vs. TAU | Unclear | Non-mental health practitioner | Combination | Home | Unclear | 1, 3, 6 |
| Jarab et al.128 | Pharmaceutical care programme with emphasis on self-management vs. TAU | Unclear | Non-mental health practitioner | Face to face | Unclear | Unidisciplinary | 1, 3 |
| Khdour et al.129 | Clinical pharmacy-led; disease and medicine; management programme vs. TAU | 1 initial education session, reinforcement at outpatient visit every 6 months plus telephone calls at 3 and 9 months | Non-mental health practitioner | Face to face and telephone | Clinic | Unidisciplinary | 1, 2, 3, 4, 6, 12 |
| Ko et al.130 | Outpatient pulmonary rehabilitation programme vs. TAU | 24 | Non-mental health practitioner | Face to face | Hospital | Unidisciplinary | 1, 3, 4 |
| Koff et al.131 | Proactive integrated care vs. TAU | Unclear | Non-mental health practitioner | Face to face and telephone | Clinic and home | Unidisciplinary | 1, 3, 12 |
| Lee et al.132 | Treatment guideline implementation vs. TAU | 9 | Non-mental health practitioner | Combination | Home | Unidisciplinary | 1, 2, 3, 6, 12 |
| Díaz Lobato et al.133 | Home hospital vs. conventional hospital | Unclear | Non-mental health practitioner | Face to face | Home | Multidisciplinary | 1, 12 |
| Man et al.148 | Community rehabilitation vs. TAU | 16 | Non-mental health practitioner | Face to face | Hospital | Multidisciplinary | 1, 4 |
| Martin et al.134 | Care plan vs. TAU | Unclear | Non-mental health practitioner | Face to face | Combination | Multidisciplinary | 1, 3, 12 |
| McGeoch et al.135 | Self-management action plan vs. TAU | 1 | Non-mental health practitioner | Combination | Home | Multidisciplinary | 1, 6, 12 |
| Rea et al.117 | Written disease management programme vs. TAU | Unclear | Non-mental health practitioner | Face to face | Combination | Multidisciplinary | 1, 6, 12 |
| Rice et al.137 | Disease management program vs. TAU | 1 | Mental health practitioner | Combination | Combination | Unidisciplinary | 1, 12 |
| Ries et al.138 | Self-management education and support vs. TAU | 60 | Non-mental health practitioner | Combination | Hospital | Unclear | 4, 12 |
| Seymour et al.139 | Post exacerbation pulmonary rehabilitation vs. TAU | 16 (2 × weekly for 8 weeks) | Non-mental health practitioner | Face to face | Hospital | Unidisciplinary | 1, 4 |
| Smith et al.140 | Home-based nursing intervention vs. TAU | 24 | Non-mental health practitioner | Face to face | Home | Multidisciplinary | 1, 3, 6, 12 |
| Soler et al.141 | Self-management education and scheduled clinic review vs. TAU | 12 | Non-mental health practitioner | Face to face | Hospital | Unidisciplinary | 1, 12 |
| Sridhar et al.142 | Rehabilitation and ongoing self-management support vs. TAU | 40 | Non-mental health practitioner | Combination | Combination | Unidisciplinary | 1, 4, 12 |
| Tougaard et al.143 | Self-management education vs. TAU | Unclear | Non-mental health practitioner | Face to face | Hospital | Multidisciplinary | 1, 3 |
| Trappenburg et al.144 | Self-management action plan vs. TAU | Unclear | Non-mental health practitioner | Face to face and telephone | Clinic and home | Unidisciplinary | 1, 2, 6, 12 |
| Wakabayashi et al.145 | Integrated care vs. TAU | Minimum of 6 | Non-mental health practitioner | Face to face | Unclear | Multidisciplinary | 1, 12 |
| Wong et al.146 | Nurse-led telephone follow-up vs. TAU | 4 | Non-mental health practitioner | Telephone | Home | Unidisciplinary | 1, 10 |
| Wood-Baker et al.147 | Written action plan vs. TAU | 1 | Non-mental health practitioner | Face to face | Hospital | Multidisciplinary | 6, 12 |
Table 7 shows the different types of complex interventions that were evaluated in the studies and the different components of each intervention. They were as follows: education (28 studies114,117,118,120,121,123–135,137,139–146,148), general discussion (three studies129,132,144), skills (13 studies118,120,124,125,127–132,134,140,143), exercise (11 studies118,120,121,123,125,129,130,138,139,142,148), behaviour therapy (zero studies), relapse prevention (11 studies117,118,121,124,127,129,132,135,140,144,147), problem-solving (zero studies), cognitive–behavioural therapy (CBT) (zero studies), increased social support (zero studies) and relaxation therapy (four studies118,121,125,146). The forest plot for the meta-analysis is shown in Figure 12. The plot shows that the overall combined effect across the 33 independent interventions was associated with a 32% reduction in the unscheduled care use (OR 0.68, 95% CI 0.57 to 0.80). There was a moderate degree of heterogeneity across the studies (Q = 51.1, df = 32, I2 = 37.4%; p < 0.0005).
FIGURE 12.
Forest plot for effect of complex interventions on unscheduled care use in COPD. Reprinted from Respiratory Medicine, Vol. 108, Dickens C, Katon W, Blakemore A, Khara A, Tomenson B, Woodcock A, et al. Complex interventions that reduce urgent care use in COPD: a systematic review with meta-regression, pp. 426–37. © 2014, with permission from Elsevier. 111

When study effects were grouped according to the components of their interventions, significant effects were seen for interventions that included general education (n = 28; OR 0.66, 95% CI 0.55 to 0.81; p < 0.0005), exercise (n = 11; OR 0.60, 95% CI 0.48 to 0.76; p < 0.0005) and relaxation therapy (n = 4; OR 0.48, 95% CI 0.33 to 0.70; p < 0.0005).
When studies were grouped according to their methodological characteristics there was no significant difference in the effect of interventions according to the age of the patient, sex, recruitment site, where treatment was delivered, who delivered treatment, how treatment was organised or the quality of the study.
The funnel plot, which is shown in the published paper,111 appeared asymmetrical with a relative absence of small studies in which interventions were associated with increased unscheduled care use, though Egger’s regression did not confirm a statistically significant association between log-OR and standard error of log-OR (Egger’s intercept = −0.79, 95% CI –1.87 to 0.29; p = 0.15).
Conclusions
The conclusion of this systematic review was that there is evidence that complex interventions have an independent effect on reducing unscheduled care for patients with COPD. Most of the interventions involved low-intensity-type treatments; however, relaxation therapy appeared to show promise.
Study 4: complex interventions that reduce unscheduled health-care use in asthma
The flow chart for this systematic review is shown in Figure 13. Thirty-three independent studies of asthma patients were eligible for inclusion in this review, which included a total of 39 comparisons of independent interventions. 149–181
FIGURE 13.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart for studies of complex interventions in asthma. Reproduced from Blakemore et al. 112
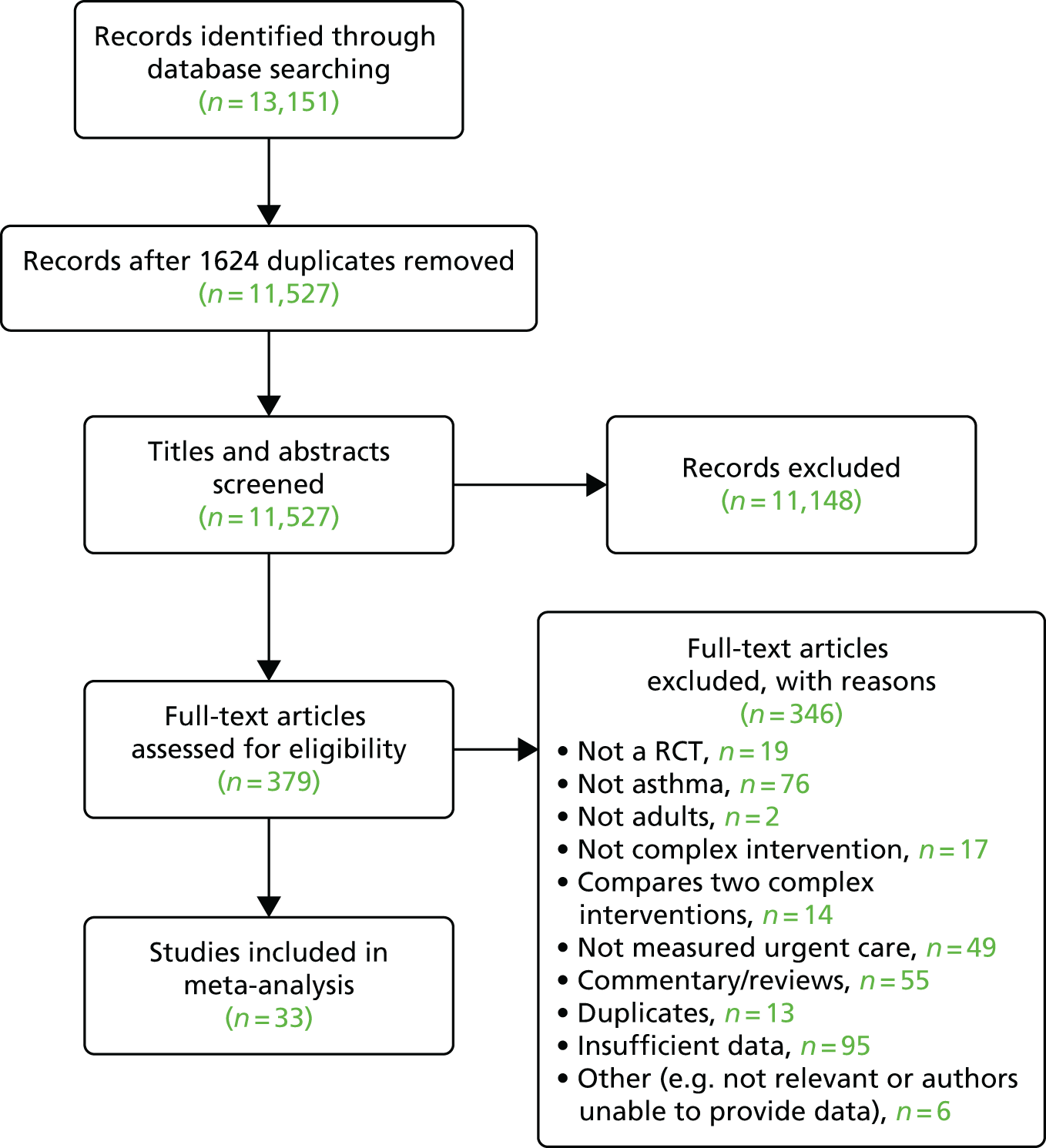
The details of the characteristics of each included study are summarised in Table 8 and further details are available in Blakemore et al. 112 Table 9 shows the characteristics of the different interventions employed in each study in the review.
| First author and year of publication | Sample size | Where recruited | Assessment of urgent health-care use | Duration of follow-up (months) |
|---|---|---|---|---|
| Aiolfi et al., 1995149 | 44 | Secondary | Hospitalisation; ED visits | 12 |
| Bolton et al., 1991150 | 241 | Secondary | Hospitalisation; ED visits | 12 |
| Brown et al., 2006151 | 111 | Secondary | Hospitalisation; ED visits; urgent doctor visits | 6 |
| Castro et al., 2003152 | 96 | Secondary | Hospitalisation; ED visits; direct and indirect health-care costs | 12 |
| Clark et al., 2007153 | 808 | Secondary | Hospitalisation; ED visits; unscheduled clinic visits | 12 |
| Coté et al., 1997179 | 188 | Secondary | Hospitalisations; ED visits | 12 |
| Donald et al., 2008155 | 71 | Secondary | Hospitalisation; ED visits; urgent doctor visits | 12 |
| Galbreath et al., 2008156 | 429 | Primary and secondary | Hospitalisations; ED visits; urgent doctor visits | 12 |
| Gallefoss et al., 1999157 | 78 | Secondary | Hospitalisations (n of days) | 12 |
| George et al., 1999158 | 77 | Secondary | Hospitalisations; ED visits | 6 |
| Huang et al., 2009159 | 173 | Secondary | ED visits; urgent health-care visit | 6 |
| Kauppinen et al., 1998160 | 162 | Secondary | ED visits (cost) | 12 |
| Kotses et al., 1995161 | 76 | Unclear | Hospitalisations; ED visits | 12 |
| Lahdensuo et al., 1996162 | 115 | Secondary | Hospitalisations; urgent ambulatory care visits | 12 |
| Levy et al., 2000163 | 211 | Secondary | ED visits | 6 |
| Milenkovic et al., 2007164 | 74 | Secondary | ED visits | 12 months plus follow-up survey at 60 months |
| Morice and Wrench, 2001180 | 80 | Secondary | ED visits | 18 |
| Osman et al., 2002165 | 280 | Secondary | Hospitalisations; ED visits; urgent calls to doctor | 12 |
| Patel et al., 2009166 | 52 | Unclear | Hospitalisations; ED visits | 12 |
| Perneger et al., 2002181 | 115 | Secondary | ED visits | 6 |
| Pilotto et al., 2004167 | 170 | Primary | Hospitalisations; ED visits | 9 |
| Pinnock et al., 2003168 | 278 | Primary | Hospitalisations; ED visits | 3 |
| Rasmussen et al., 2005169 | 253 | Unclear | Unscheduled visits to physician; ED visits; hospitalisation | 6 |
| Schermer et al., 2002170 | 193 | Primary | Hospitalisation; ED visits (cost) | 24 |
| Schott-Baer and Christensen 1999171 | 36 | Secondary | ED visits | 1.5 |
| Shelledy et al., 2009172 | 159 | Secondary | ED visits; ED costs | 6 |
| Sundberg et al., 2005173 | 97 | Secondary | Hospitalisations; urgent health-care visits | 12 |
| van der Meer et al., 2011174 | 200 | Primary | ED visits (cost) | 12 |
| Vojita et al., 1999175 | 121 | Unclear | ED visits | 6 |
| Wilson et al., 1993176 | 310 | Primary | Urgent health-care visits | 24 |
| Yilmaz and Akkaya, 2002177 | 52 | Secondary | Hospitalisations; ED visits | 36 |
| Yoon et al., 1993178 | 76 | Secondary | Hospitalisations; ED visits | 10 |
| First author and year of publication | Intervention | Control | Number of sessions | Where was the intervention delivered | Intervention component |
|---|---|---|---|---|---|
| Aiolfi et al., 1995149 | Education plus guidelines | TAU | 4 | Outpatient clinic | 1, 3, 6, 12 |
| Bolton et al., 1991150 | Education | TAU | 3 | Hospital | 1, 3, 6, 7, 10, 12 |
| Brown et al., 2006151 | Education | TAU | 2 | Clinic and home | 1, 10 |
| Castro et al., 2003152 | Collaborative care | TAU | 2 | Hospital and telephone/home | 1 |
| Clark et al., 2007153 | Education plus counselling | TAU | 5 | Telephone/home | 1, 7 |
| Coté et al., 1997179 | Education plus action plan | TAU | Unclear | Unclear | 1, 6, 12 |
| Coté et al., 1997179 | Education plus action plan based on asthma symptoms | TAU | 1, 3 | ||
| de Oliveira et al., 1999154 | Asthma education | TAU | 6 | Outpatient clinic | 1, 3, 6, 12 |
| Donald et al., 2008155 | Telephone support plus self-management education | Self-management education | 6 | Home | 12 |
| Galbreath et al., 2008156 | Disease management | TAU | 6/7 | Home | 1, 12 |
| Galbreath et al., 2008156 | Augmented disease management | TAU | 11 | 1, 3, 12 | |
| Gallefoss et al., 1999157 | Self-management education | TAU | 4 | Unclear | 1, 3, 6, 10, 12 |
| George et al., 1999158 | Education | TAU | Unclear | Hospital | 3, 6 |
| Huang et al., 2009159 | Individualised education plus peak flow monitoring | TAU | 24 | Unclear | 1, 6, 12 |
| Huang et al., 2009159 | Individualised education | TAU | As above | As above | As above |
| Kauppinen et al., 1998160 | Intensive self-management education | TAU | 5 | Clinic | 1 |
| Kotses et al., 1995161 | Self-management programme | Wait list | 7 | Unsure | 1, 2, 12 |
| Lahdensuo et al., 1996162 | Self-management education | TAU | 3 | Outpatient clinic | 1, 10, 12 |
| Levy et al., 2000163 | Self-management education | TAU | 3 | Clinic | 1, 3, 6, 12 |
| Milenkovic et al., 2007164 | Peak flow-based self-management | TAU | 3 | Outpatient clinic | 1, 3, 6, 12 |
| Morice and Wrench, 2001180 | Education | TAU | 2 or more | Hospital | 1, 2, 6, 11 |
| Osman et al., 2002165 | Self-management education | TAU | 2 | Hospital | 1, 2, 6 |
| Patel et al., 2009166 | Telephone questionnaire intervention | TAU | 2 | Telephone | 12 |
| Perneger et al., 2002181 | Self-management education | TAU | 3 | Hospital | 3, 6, 7, 10, 11, 12 |
| Pilotto et al., 2004167 | Self-management education plus scheduled follow-up | TAU | 3 | Outpatient clinic | 1, 3, 6, 12 |
| Pinnock et al., 2003168 | Telephone consultation | TAU | 1 | Telephone | 12 |
| Rasmussen et al., 2005169 | Internet-based asthma management | TAU | Unclear | Home | 6, 12 |
| Rasmussen et al., 2005169 | Specialist monitoring and management | TAU | Unclear | Outpatient clinic | 6, 12 |
| Schermer et al., 2002170 | Guided asthma self-management | TAU | 4 | Primary care clinic | 1, 3 |
| Schott-Baer and Christensen, 1999171 | Self-management training | TAU | 4 | Unclear | 1 |
| Shelledy et al., 2009172 | Nurse-led home disease management programme | TAU | 5 | Home | 1, 3 |
| Shelledy et al., 2009172 | Respiratory therapist led home disease management programme | TAU | 5 | Home | 1, 3, 6 |
| Sundberg et al., 2005173 | Supported computerised education | TAU | 4 | Outpatient clinic/telephone | 1 |
| van der Meer et al., 2011174 | Internet-based self management | TAU | Unclear | Home/internet | 1, 2, 6, 12 |
| Vojita et al., 1999175 | Nurse-led education programme | TAU | 4 | Home | 1, 6, 3 |
| Wilson et al., 1993176 | Group self-management education | TAU | 4 | Unclear | 1, 2, 3, 5, 6, 7, 9, 10, 12 |
| Wilson et al., 1993176 | Individual self-management education | TAU | 3–5 | Unclear | |
| Yilmaz and Akkaya, 2002177 | Asthma education | TAU | 6 | Outpatient clinic | 1, 3, 6, 12 |
| Yoon et al., 1993178 | Self-management education | TAU | 1 | Clinic | 1, 2, 3, 6, 12 |
Figure 14 shows the combined effect across the 39 independent studies. The results show that complex interventions were associated with a 21% reduction in the use of unscheduled care (OR 0.79, 95% CI 0.67 to 0.94). A moderate degree of heterogeneity was seen across the studies (Q = 58.1, df = 38, I2 = 34.6%; p = 0.020).
FIGURE 14.
Forest plot for effect of complex interventions on unscheduled health-care use in asthma. Reproduced from Blakemore et al. 112 TAU, treatment as usual; UC, unscheduled care.

When study effects for asthma were grouped according to the components of their interventions, significant effects were seen for general education (n = 32; OR 0.77, 95% CI 0.64 to 0.91; p = 0.003), skills training (n = 20; OR 0.64, 95% CI 0.48 to 0.86; p = 0.003) and relapse prevention (n = 23; OR 0.75, 95% CI 0.57 to 0.98; p = 0.04). No studies used relaxation or CBT.
When studies were grouped according to their methodological characteristics, there was no significant difference in the effect of interventions according to the age of patients, sex, duration of illness, recruitment site, how or where treatment was delivered, who delivered treatment, number of treatment sessions or whether or not there was a structured management plan or follow-up. Nor were the effect sizes significantly associated with the type of unscheduled care or quality of the study.
Publication bias was assessed using a funnel plot (Figure 15) and Egger’s regression method. The funnel plot appeared to be symmetrical. Egger’s regression method did not confirm statistically significant associations between log-OR and standard error of log-OR (Egger’s intercept = –0.37, 95% CI –1.21 to 0.48; p = 0.38).
FIGURE 15.
Funnel plot with pseudo-95% confidence limits for studies of complex interventions to reduce unscheduled health-care use in asthma. Reproduced from Blakemore et al. 112
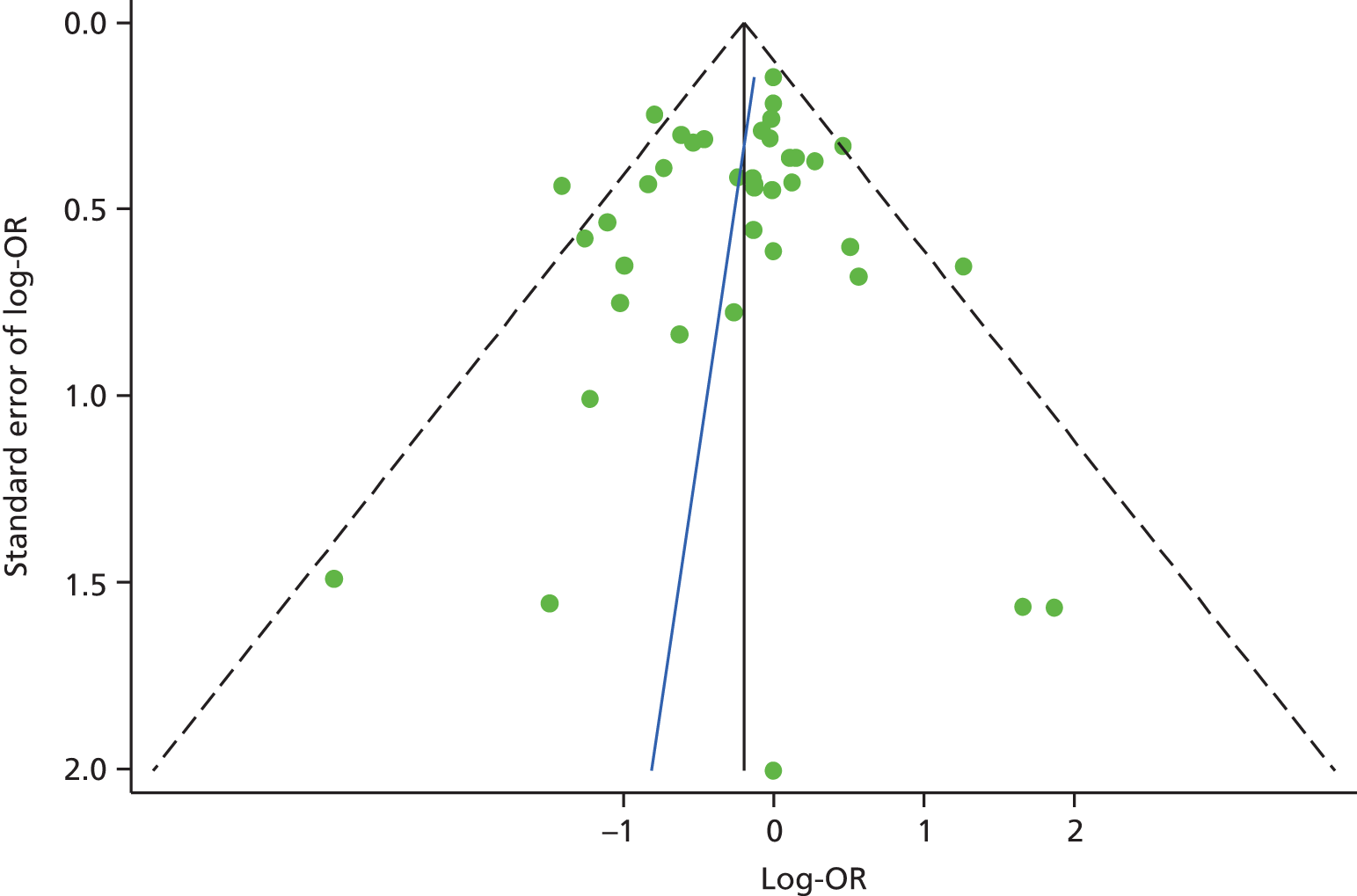
Conclusion
The conclusion of study 4 was that there is evidence that complex interventions have a small, independent, effect on reducing unscheduled care for patients with asthma. The results were similar to the previous review on COPD, with most of the interventions falling into the category of ‘very low-intensity treatments’ such as skills training.
Discussion
The findings from the systematic reviews on COPD and asthma were remarkably similar. Complex interventions have the potential to reduce unscheduled care, but the effect size is small. Very few of the interventions in the COPD review and none of the interventions in the asthma review included a specific psychological intervention and/or antidepressant therapy.
Our findings suggest that, if a psychosocial intervention is developed to reduce use of unscheduled care in people with LTCs, it should include elements of education, self-management and relaxation. There is a strong rationale for developing a psychosocial intervention, as depression predicts use of unscheduled care in people with LTCs (study 1), and the benefits of a psychosocial intervention have not been previously evaluated.
Limitations of systematic reviews 3 and 4 (complex interventions in chronic obstructive pulmonary disease and asthma)
The pooled effects across a wide range of different interventions must be interpreted with caution in both studies, as there was a moderate degree of heterogeneity in both the COPD and asthma review. Many of the studies included in both reviews were designed specifically to evaluate the effect not of the intervention in question on unscheduled care use, but rather the effect of the intervention on some aspect of patient well-being.
We focused on COPD and asthma, as these were the two conditions that our searches revealed had a relatively large number of published trials of relevance. There were far fewer relevant studies for diabetes or heart disease. Given the imbalance in the number of studies among our four exemplar conditions, we decided to focus on COPD and asthma, as there were over 60 published trials of complex interventions for these conditions. Our findings are therefore limited to COPD and asthma, and may not be generalisable to other LTCs.
Why do people use unscheduled care? A qualitative meta-synthesis
In the final part of our evidence synthesis, we carried out a review of qualitative studies which have examined reasons for unscheduled care use in patients with LTCs. Qualitative research is better able to identify personal and contextual reasons for using health care than quantitative research. Factors such as poor access to primary care and poor preventative care have been identified as being associated with higher risk of hospital admissions. 20,182–184 However, the decision-making process that patients with LTCs use to determine whether or not to use unscheduled care is unclear.
Aim
The aim of this study (study 5) was to systematically review and synthesise the available qualitative research about why patients with LTCs use unscheduled care.
Method
A full description of the method for study 5 is available in the published paper by Langer et al. 185 Below is a summary adapted from the published paper. 185
The following databases were included: MEDLINE, EMBASE, PsycINFO and CINAHL. The full search strategy is listed in Langer et al. ,185 but essentially we searched for papers that had used qualitative methods to investigate why people use unscheduled care. The searches were extended by hand-searching the references of included papers and by searching for key authors. Initial searches were conducted in 2009 and updated again in 2011.
From the initial searches we found that very few papers addressed the issue of unscheduled care use directly, and the majority of these focused on asthma. Therefore, we sought to identify research with potential relevance to unscheduled care use. This included reports of patients’ perceptions of health and health-care use generally; self-management; and clinical relationships from patient or practitioner perspectives.
Our search identified a total of 3055 papers. We initially screened the titles and/or abstracts of potentially eligible papers. Inclusion decisions were made by one researcher (SL) and reviewed by another (CH). Decisions about ambiguous papers were reached by discussion between at least two researchers.
Analysis plan
Data were recorded for three parallel, linked strands of analysis:
-
the empirical findings (meta-data analysis)
-
the research methods, including sampling, data collection and analysis, and the implications of these (meta-method analysis)
-
the conceptual starting point of the research together with theoretical inferences from the findings (meta-theory analysis).
The theoretical background of each study was not always clear. Therefore, in order to complete the meta-theory analysis, we examined not just explicit statements of theory, but also the language used in framing the research questions, handling the data and reporting the results. 186
An appraisal form was used to record information relevant to each strand of analysis for each paper. At least two researchers appraised each paper, reaching a joint perspective and consulting with the wider team where necessary. Any difficulties of interpretation were resolved by discussion within the team until consensus was reached. 186,187
A subteam of researchers on the CHOICE programme (SL, CC-G, PS, CH), met on a regular basis to discuss and synthesise the three stands of analysis to create an overarching reinterpretation of the primary research, rather than a narrative summary of the primary research findings. 187
The final meta-synthesis focused on empirical findings, but we used our analysis of methods and conceptual background to interpret and appraise these findings. We chose to use ‘lines-of-argument synthesis’,188 as although there were some areas of convergence in the empirical findings, the literature was very heterogeneous. Thus, the three parallel strands of analysis were drawn together in one overall synthesis.
Results
Forty-two papers were identified as eligible for inclusion in this review: Aroni et al. ,189 Becker et al. ,190 Cvetkovski et al. ,191 Donald et al. ,155 Douglass et al. ,192–194 Goeman et al. ,136,195 Griffiths et al. ,196 Hyland et al. ,197 Jones et al. ,198 Tumiel-Berhalter and Zayas,199 De Vito,200 Elkington et al. ,110 Hopley et al. ,201 Shipman et al. ,202 Wilson et al. ,203 Balcou-Debussche and Debussche,204 Brez et al. ,205 Broom,206 de-Graft,207 Heisler et al. ,208 Johnson et al. ,209 Lawton et al. ,210 McDowell et al. ,211 Perera et al. ,212 Mead et al. ,213 Paquet et al. ,214 Schoenberg and Peters,215 Cowie et al. ,216 Forsyth et al. ,217 Fried et al. ,218 Gately et al. ,219 Hsu-Hage,220 Jeon et al. ,221 Jerant et al. ,222 Kielmann et al. ,223 Levy et al. ,224 Malone,225 Townsend et al. 226 and Yang et al. 227 However, six of these papers reported different aspects of the data from a single sample, so this review reports on findings from 42 papers of 37 individual studies. The 42 papers were published between 1984 and 2011, and their country of origin and main findings are summarised in Table 10.
| First author, year of publication; country of origin and LTC | Method(s) | Finding(s) |
|---|---|---|
| Aroni et al., 2004;189 Australia, asthma | 62 interviews with ED attenders; 43 female and 19 male; aged 18–70 years | Main aim was to identify what constitutes an asthma attack. People defined asthma attacks as ‘major’ and ‘minor’, as determined by the degree of personal control they were able to exercise. A strongly unifying description of a severe attack was that it was ‘out of control’ |
| Balcou-Debussche and Debussche, 2009;204 France, COPD | Observation; 42 interviews | Hospitalisation still plays a major part in the management of uncontrolled type 2 diabetes and its complications. It offers respite and temporarily suspends the realities of daily life |
| Becker et al., 1993;190 USA, asthma | 95 adults studied over 15 months; 59 female and 36 male | Uncertainty about the quality and speed of care available in an ED and the appropriateness of when to seek it. Concerns created a push–pull dynamic, as patients struggled to weigh factors such as relief, autonomy, stigma and danger of death when deciding whether or not to use EDs |
| Brez et al., 2009;205 Canada, diabetes | Three focus groups involving 22 primary care physicians | Facilitators of good care included clear communication of a detailed, structured plan of care, ongoing access to specialist services for advice or re-referral, continuing education and mentoring. Barriers were gaps in knowledge and confidence related to diabetes treatment, excessive workload and competing time demands |
| Broom, 2003;206 Australia, type 2 diabetes | 119 interviews; 50% female; mean age 64 years | Half of the interviewed patients had a diagnosis resulting from circumstances that could be considered ‘discontinuous primary care’, for example diagnosis made during hospital admission for a different reason |
| Cowie et al., 2009;216 UK, multiple LTCs | 33 interviews | Across a range of LTCs, patients’ experiences of health care can be understood in terms of nuanced understandings of relational and management continuity. Continuity experiences, meanings and expectations, as well as barriers and facilitators, are influenced by the model of care rather than type of condition |
| Cvetkovski et al., 2009;191 Australia, asthma | 10 interviews | People with asthma were satisfied with their asthma management and the service provided by the health-care practitioners and described the involvement of family members and ambulance officers in their overall asthma management. The rural environment was an issue with regard to distance to the hospital during an emergency |
| de-Graft Aikins, 2005;207 Africa, diabetes | Observation; 67 interviews; focus groups | To minimise inappropriate healer shopping and maximise committed biomedical and regulated ethnomedical management for Ghanaians with diabetes, the greatest challenges lie in providing affordable pharmaceutical drugs, standardised ethnomedical drugs, recommended foods and psychosocial support |
| DeVito, 1990;200 USA, COPD | 96 interviews | During a non-acute phase of their illness, patients were asked to recall their feelings associated with sensations of shortness of breath during hospitalisations for the acute phase. Several themes were isolated that dominated the dyspnoeic experience, which were fear, helplessness, loss of vitality, preoccupation and legitimacy |
| Donald et al., 2008;155 Australia, asthma | Focus group with five inpatients | Patients delayed seeking medical attention until asthma symptoms were severe despite owning a peak expiratory flow meter, written plan or experience of a previous attack |
| Douglass et al., 2002;192 Australia, asthma | 63 interviews; ED attenders | Aim was to evaluate the usefulness of asthma plans. Patients viewed asthma plans positively and most had found them helpful |
| Douglass et al., 2004;193 Australia, asthma | 62 interviews; ED attenders | Patients made thoughtful choices on where they sought care according to their needs. Perceptions of doctors’ competence, listening to patients and time constraints were important influences on doctor–patient relationships |
| Douglass et al., 2005;194 Australia, asthma | 20 interviews; ED attenders | Patients who completed the Asthma 3+ Visit Plan had significant improvements in asthma-related QoL and asthma knowledge. Good relationship with the GP integral to the success of the plan |
| Elkington et al., 2004;110 UK, COPD | 25 interviews; carers of COPD patients who had died | Breathlessness led to patients being increasingly housebound. Anxiety and panic were common and associated with breathlessness. Depression was also reported |
| Forsyth et al., 1984;217 USA, chronically ill patients | Observation; 50 interviews | Hospitalisation expressed patient efforts to foster control and keep ahead of the disease |
| Fried et al., 1998;218 USA, CHF, COPD or pneumonia | 29 interviews; inpatients | Views of the home and hospital were shaped by patients’ social supports, self-reliance, religious beliefs and past illness experiences. Hospital seen as having better outcomes |
| Gately et al., 2007;219 UK, LTCs | 21 interviews, patient experts | A related RCT reported increased patient self-efficacy, but no reduction in health care. This was due to patients feeling they had established an appropriate level of health-care use |
| Goeman et al., 2002;195 Australia, asthma | 61 interviews; ED attenders (30 more than once) | 22 of the re-attenders had chronic severe asthma and the use of emergency care was deemed clinically appropriate for 18. Four of the presentations may have been avoidable if patient had been able to afford better medication. In one in three of the re-attenders, attendances were avoidable with reasons for re-presentation including poor asthma knowledge and costs |
| Goeman et al., 2004;136 Australia, asthma | 62 interviews; ED attenders | Patients make their own decisions regarding treatments, based on a cost–benefit analysis that weighs the beneficial effects of medications against their side effects, such as weight gain or risk of osteoporosis, the time and effort needed to acquire them and their financial costs |
| Griffiths et al., 2001;196 UK, asthma | 58 interviews with admitted and non-admitted patients (South Asian and white) | South Asian and white patients admitted to hospital coped differently with asthma. South Asians described less confidence in controlling their asthma, were unfamiliar with the concept of preventative medication, and often expressed less confidence in their GP. They managed asthma exacerbations with family advocacy, without systematic changes in prophylaxis and without systemic corticosteroids |
| Heisler et al., 2009;208 USA, diabetes | 40 interviews | Community health worker programmes that provide both one-on-one support and group self-management training sessions may be effective in promoting more effective diabetes care and patient–doctor relationships among Latino and African American adults with diabetes |
| Hopley et al., 2009;201 New Zealand, COPD | Nine interviews | Difficulties with transportation, physical access, communication and finances collectively added up to significant barriers to accessing specialist care for people in a rural area |
| Hsu-Hage, 2001;220 Australia, LTC unspecified | Seven focus groups; Chinese, Australian | Participants first try to self-medicate with over-the-counter medication before resorting to practitioners of traditional Chinese medicine alongside Western biomedical one |
| Hyland and Stahl, 2004;197 UK, asthma | One focus group | Patients wished for simpler regime with fewer drugs and most had concerns about their treatment. Patients and clinicians had different understanding of treatment and side effects |
| Jeon et al., 2009;221 Australia, chronic illness | 52 Interviews | Economic hardship requires households to make difficult decisions between care and basic living expenses |
| Jerant et al., 2005;222 USA, mixed LTCs | 10 focus groups with 54 patients | Barriers to active patient self-management are depression, difficulty exercising regularly, fatigue, poor communication with physicians, lack of familial support, pain and financial problems. Barriers to accessing self-management services are lack of awareness, physical symptoms, transportation and costs/health insurance |
| Johnson et al., 2006;209 UK, diabetes | 12 interviews; primary care | Study explored patient views of transfer of services into primary care. Patients welcomed transfer of services into primary care if it made care more accessible in terms of location and time. They were more positive about the move if a meaningful consultation had been conducted and the full range of diabetes care was available |
| Jones et al., 2008;198 UK, asthma | 75 interviews; 50 with inpatients, 25 with primary care patients | High users had often chaotic and stressful lives, but made little connections between psychosocial factors and asthma attacks, citing biomedical factors instead. Hospital was the preferred site of treatment for frequent attenders because it provided superior care compared with GPs. GPs’ approach to asthma care ranged from writing repeat prescriptions to taking a strong interest in asthma care. All expressed frustration at patients not attending routine reviews. Only some were aware of a relationship between psychosocial factors and asthma attacks |
| Kielmann et al., 2010;223 UK, respiratory conditions | 33 interviews | Patients were aware of the increasing focus on self-care, but felt that the term was incongruous as it described what they were already doing. Although many respondents appreciated increased clinical responsibility some felt ‘abandoned’ by professionals |
| Lawton et al., 2009;210 UK, diabetes | 20 interviews | Patients’ views about their current diabetes care were informed by their previous service contact. The devolvement of diabetes care/reviews to general practice was presented as a ‘mixed blessing’. Patients gained reassurance from their perception that receiving practice-based care/reviews signified that their diabetes was well controlled. However, they also expressed resentment that, by achieving good control, they received what they saw as inferior care and/or less frequent reviews to others with poorer control |
| Levy et al., 2004;224 USA, cardiovascular disease and diabetes | Focus groups | Multisource data yielded useful information for programme planning and a better understanding of the cultural differences and similarities between African Americans and Latinos |
| Malone, 1998;225 USA, LTC unspecified | Ethnographic fieldwork and interviews in two inner-city hospitals over a 12-month period | High use of emergency care should not be conceptualised as primarily a medical problem. Rather, EDs fulfil an important ‘almshouse’ function in that they attend to the high users’ medical needs as well as to their basic care and upkeep. Although heavy users often have multiple medical problems, especially substance abuse, mental health and chronic illness, high users themselves value the ED for largely non-medical reasons such as offering food, warmth, safety and companionship |
| McDowell et al., 2009;211 UK, diabetes | Eight focus groups with 35 people | Five main themes were identified:
|
| Mead et al., 2010;213 USA, heart disease | 33 focus groups | For disadvantaged populations, typical problems associated with self-management of a heart condition are aggravated by substantial obstacles to accessing care |
| Murray et al., 2009;228 UK, lung cancer and heart failure | Interviews over 3 months with 20 patients with each condition | Palliative care is largely directed towards cancer treatment, which means that those with non-malignant conditions are missing out because their prognosis is uncertain. Most people with heart failure do not understand the causes or prognosis of their disease and rarely discuss end of life issues with their professional carers. A dual approach combining continued active management while acknowledging and discussing the possibility of death with the patients is called for |
| Paquet et al., 2005;214 Canada, MI or angina | Focus group with 20 inpatients | Although the emphasis of cardiac rehabilitation programmes is on modifying health habits, patients wanted more help with managing stress. They also wanted more opportunity to socialise in support groups |
| Perera et al., 2007;212 Sri Lanka, diabetes | 49 interviews; focus groups | The need for frequent visits to clinics with appropriate facilities for diagnosis and management of diabetes, often far from rural communities, posed high costs. The need for frequent clinic visits posed repeated costs, which made it difficult for households to recover their economic status |
| Schoenberg and Peters, 2003;215 USA, CHD and LTCs | 40 interviews with older women | Factors identified as delaying help-seeking for CHD symptoms were not recognising symptoms as CHD; interactions between the women and their physicians were problematic; women feared their complaints were not serious enough; competing social demands; and structural barriers (e.g. transportation, lack of health insurance) |
| Shipman et al., 2009;202 UK, COPD | 16 interviews | Contact with health care was influenced by perceptions of ease of access, quality of relationship with GP and perceived severity of illness |
| Townsend et al., 2008;226 UK, people with four or more LTCs | 23 interviews (11 frequent consulters) | GP seen as the last resort. Frequent consulters saw GPs as allies. Frequent consulters reported severe and unpredictable symptoms over which they had little control, and used more alternative therapies and more over-the-counter medication. They feared intervention might interfere with the management of their conditions |
| Tumiel-Berhalter and Zayas, 2006;199 USA, asthma | Two workshops with Puerto Rican adults | Participants thought of asthma as deceptive and worrisome. They could identify household triggers and felt asthma had a limiting effect on their activities. They were also concerned about the side effects of asthma medication and frustrated by how it interfered with other forms of medication |
| Wilson et al., 2008;203 Canada, COPD | 12 interviews | All participants wanted to maintain their independence. This required considerable adaptation, as well as assistance from others. Ensuring and improving assistance is important to reduce exacerbations requiring hospitalisation |
| Yang et al., 2010;227 Australia, complex medical problems | 26 interviews | Patients’ decisions to attend GP follow-up after hospitalisation are influenced by a number of factors including understanding of the role of the GP; experiences of continuity of care; GP availability; presence of discharge instructions; access to transport; and level of social support |
In total, 14 studies were of patients with asthma,136,155,189–199,212 five were of patients with COPD,110,200–204 seven were of patients with diabetes,205–211 three were of patients with CHD213–215 and 11 were of patients with mixed/multiple LTCs. 216–219,221,222,224–228 The majority of studies were from the USA (n = 11190,199,200,208,213,215,217,218,222,224,225), Australia (n = 12136,155,189,191–195,206,220,221,227) or the UK (n = 12110,196,197,202,209–211,216,219,223,226,228). The most frequently studied types of unscheduled care were attendance at the ED and/or EHA. The studies included 2054 participants, comprising 1836 patients, 63 carers and 155 practitioners. The largest study sample size was 387213 and the smallest was a focus group of five people. 229 Twenty-five of the studies used interviews with users of services, eight used focus groups, five used focus groups and interviews, and four used observation plus interviews.
The decision to use unscheduled care
The starting point for most papers was that unscheduled care use is inherently problematic and it is a behaviour that needs to be reduced. Most papers also focused on patient factors rather than other sociocultural factors, which may influence care-seeking. A small number of papers examined cultural, political and economic factors. 196,207,215,221,224,225
The most consistent finding was that patients used unscheduled care in response to exacerbations of their illness. Unscheduled care use was seen to complement routine care and self-management. However, patients reported using unscheduled care when there was a pressing need and when they felt that they had no other option. In other words, they felt they had no other choice or alternative at the time they experienced an exacerbation in their illness. The decision to use unscheduled care was often made for the patients by others, such as family members.
Several papers reported that patients used unscheduled care because of intense and frightening illness exacerbations,136,189,190,193,196,202,229 and that any decision-making was outside their control because of the severity of physical symptoms (e.g. feeling unable to breathe or fearing death), and health-seeking was often initiated by health-care practitioners (HCPs), relatives or bystanders. 190,202,215,225,229
In one study of asthma there was an attempt to judge the appropriateness of care-seeking,136 and one-third of re-attenders at an ED department, with mainly mild to moderate asthma, were judged to have attended unnecessarily. However, this kind of approach was very unusual.
Value of different kinds of health care
A consistent theme was the high regard patients held for specialist expertise of the hospital in comparison with primary care or the community. 190,209–211,217,218,225,229 It was also reported that hospitalisation relieved family burden. 218
Previous experience of care
Patients’ beliefs about the kind of care they required (routine or unscheduled) were shaped by their previous experiences of care. Gradual accumulation of experience was punctuated by particularly pivotal episodes of care, which then heavily influenced decision-making. 189,190,229
Accessibility of care
Patients reported that unscheduled care use was easily accessible, but routine care could be difficult to access due to geography, travel infrastructure, costs of consultations, medication and health insurance. 201,212,215,222
Relationships with routine health-care practitioners
Frequent unscheduled care use was not linked to poor relationships with routine care practitioners. Routine care allowed for more personal and long-term relationships to develop between patients and health-care practitioners.
The emotional, social and cultural context
Very few papers explored how depression or anxiety may have affected people’s use of unscheduled care. One UK study of patients with asthma who were frequent attenders at EDs described people as having chaotic and stressful lives and making little or no connection between their asthma attacks and psychosocial factors. 198 Another UK study of patients with COPD reported that anxiety and panic were common and associated with breathlessness. 110 A US study, employing ethnographic fieldwork techniques and interviews, suggested that high use of emergency care should not be conceptualised as primarily a medical problem, but it should be seen in the context of EDs providing medical and non-medical care for the disadvantaged of society including those with substance misuse, mental health problems and chronic illness. 225
Conclusion
From a review of the qualitative literature we concluded that patients use unscheduled care because of a sense of pressing need, which is linked to their worsening physical health. Patients described having ‘no choice’ and having either exhausted all other alternatives or found that alternatives were not accessible, were unsuitable or did not exist. Patients’ previous experiences of health care shaped their future use. Emotional, social and cultural factors that could lead to use of unscheduled care remained unexplored in the existing literature.
Limitations
Very few papers directly addressed the question, ‘why do people with LTCs use unscheduled care?’, and those that did were mainly focused on asthma. This severely limits the findings of the review and its generalisability to other LTCs. We broadened the review to include papers that were potentially informative about unscheduled care use, even though it may not be the focus, but, again, the limits of this approach need to be recognised. The majority of papers focused on individual physical conditions, and there was a very limited coverage of multimorbidity, although approximately 45% of the 17.5 million adults in the UK with LTCs have comorbid conditions. 8 Many of the papers were not from the UK, and it is difficult to determine whether or not findings from other countries and cultures are relevant to the UK setting. There was very little attention given to the role of mental health issues in relation to care-seeking, which may reflect their relative unimportance or the need to target this area more explicitly in research in the future.
Overall discussion and conclusions of our evidence synthesis
The five studies that we carried out in this phase of the programme helped to clarify the existing evidence base. There was some evidence that depression, but not anxiety, is prospectively associated with an increased probability of using unscheduled care in patients with LTCs, but it is unclear from the work carried out to date whether or not this is mediated via the severity of physical illness.
As there is a bidirectional relationship between severity of physical illness and depression, our findings suggest that further work to fully elucidate the relationship between depression, severity of physical disease and unscheduled care use is required.
There were also very few studies that had been conducted in a primary care setting, yet this is the major health-care arena in which care of LTCs is provided in the UK. Further work based in primary care is essential to determine if depression or other psychosocial factors are important predictors of unscheduled care use in people with LTCs.
The results from the qualitative review suggested that patients make clear choices about when and what type of health care to use. Their main considerations are shaped by previous experience of health-care use, and the values they place on different kinds of health care, for example seeing hospitals as places of safety and expertise. Low mood or depression, or even anxiety, were cited in very few of the qualitative papers as being factors that people are aware may influence health-seeking at a time of crisis. This suggests that whatever role psychosocial factors play in increasing the likelihood of health-care-seeking behaviour; their influence is hidden or not uppermost in people’s minds when accounting for health-care use.
Chapter 3 A longitudinal prospective study to determine predictors of unscheduled care in patients with long-term conditions (phase 2)
Abstract
Background
Our main objective was to determine what factors independently predict use of unscheduled care in people with LTCs in primary care. Key factors we examined included physical comorbidity, severity of physical illness, demographic characteristics, prior use of unscheduled care, depression, life stress and distance to nearest ED.
Methods
We conducted a primary care-based prospective cohort study of patients aged ≥ 18 years, with at least one of four exemplar LTCs (asthma, CHD, COPD, diabetes). Patients were identified from QOF registers from 10 general practices in Manchester. Participants completed a baseline postal questionnaire and a follow-up questionnaire 1 year later. Two measures of outcome were used: EHAs recorded from the GP records and self-reported use of unscheduled care from questionnaire data.
Results
A postal questionnaire was mailed to 6884 patients; 1860 patients returned it completed (27.8%). A total of 1415 GP records were reviewed and 1203 patients completed the follow-up questionnaire.
Independent predictors of EHA were no partner, CHD, number of threatening experiences, emergency admission in baseline year and a Hospital Anxiety and Depression Scale (HADS) depression score of ≥ 8. Independent predictors of participant-reported use of unscheduled care were no partner, asthma, COPD, number of threatening experiences, self-reported use of unscheduled care in baseline year and a HADS depression score of ≥ 8. More severe depression was associated with a higher risk of use of unscheduled care.
Discussion
The most powerful predictor of prospective use of unscheduled care was previous use of unscheduled care, but psychosocial factors (having no partner, threatening experiences and depression) were also important. This suggests that a psychosocial intervention may be helpful in reducing use of unscheduled care in people with LTCs.
Overview
In this chapter, we report the findings from the main quantitative study of the second phase of the programme. This was a prospective longitudinal cohort study in primary care, which we carried out to identify factors that predict use of unscheduled care in patients with at least one of our four exemplar LTCs: asthma, CHD, COPD and diabetes. We were particularly interested in what role psychosocial factors play in the use of unscheduled care, as these have been relatively underinvestigated in comparison with physical factors. We had two major objectives:
-
to derive estimates of the frequency and pattern of unscheduled care use in patients with at least one of the four exemplar conditions we chose to study in this programme: asthma, CHD, COPD and diabetes
-
to develop predictors (i.e. a red flag system) in primary care to identify patients with LTCs who are at risk of becoming frequent users of unscheduled care, with a specific focus on potential psychosocial predictors.
Background
The focus for the CHOICE programme was to investigate the potential relationship between psychosocial factors, LTCs and unscheduled care, and to explore the possibility that a tailored psychosocial intervention may reduce the requirement to use unscheduled care in people with LTCs. We were therefore interested in potential psychosocial factors that may be risk factors for use of unscheduled care.
The evidence synthesis that we carried out in the first phase of the programme identified depression, but not anxiety, as being a potential predictor of use of unscheduled care in patients with LTCs. Depression is two to three times more common in people with a LTC than in the general population and is seven times more common in people with two or more LTCs. 69
Depression is particularly associated with poor QoL,69 and the presence of comorbid depression can have a greater effect on the functional status and QoL of people with LTCs than the level of severity of their physical illness. 230,231 Health-care utilisation and costs are substantially increased for patients with LTCs who are depressed in comparison with those who are not,111 and this effect persists after adjusting for medical severity. 232,233 It has been estimated that between 12% and 18% of all expenditure on LTCs in England is linked to poor mental health (mainly depression). 39
To identify potential risk factors for use of unscheduled care in patients with LTCs, we carried out a large longitudinal prospective study in primary care. We included key factors, identified from our own evidence synthesis, to measure at baseline, and we focused on patients with at least one of our four exemplar LTCs. The key factors included physical comorbidity, severity of physical illness, demographic characteristics, prior use of unscheduled care, distance to nearest ED, depression and life stress.
Specifically, we asked what factors independently predict use of unscheduled care in people with LTCs in primary care?
Method
The study was conducted in Manchester, which is a large city in the UK with a population of approximately 500,000 and a wider conurbation area population of 2.5 million. 234 According to the 2010 Index of Multiple Deprivation (IMD), Manchester is the fourth most deprived local place in England. 235 Unemployment throughout 2012–13 averaged 11.9%, which was above the national average, but lower than some of the country’s other comparably sized cities. The population density is approximately 4000 people per km2, which is below that of London, but similar to other urban conurbations in the UK including the West Midlands and Merseyside. Statistics from the 2011 census showed that 66.7% of the population of the city of Manchester were white, 17.1% Asian, 8.6% black, 4.7% were mixed race, 1.9% Arab and 1.2% of other ethnic heritage. Manchester is therefore fairly representative of a large UK urban population.
We conducted a primary care-based prospective cohort study of patients aged ≥ 18 years with LTCs. All patients with at least one of the four exemplar conditions (asthma, CHD, COPD and diabetes) were identified from QOF registers in 10 general practices in the city of Manchester. A variety of strategies were used to recruit practices, including direct invitation and presentations at local meetings. There were potentially 100 eligible practices within the Manchester area, and we targeted those with the highest combined prevalence rates for the four exemplar conditions; of these, 31 showed some interest in participation. We invited these practices to participate in a sequential fashion until we considered we had a sufficient baseline sample. In all, 21 practices were approached, of which 10 participated in the study and 11 declined. No incentives were given to practices to participate in the study, although they were provided with support costs to cover work they carried out in relation to the study.
All patients with at least one of the four exemplar LTCs were identified, and the lists were checked by practice GPs, who excluded patients who were receiving palliative care, were terminally ill or would not have capacity to participate in the study.
Eligible patients were sent a postal questionnaire between June 2010 and December 2010, with a further reminder questionnaire pack 3 weeks later. We used the following strategies to maximise response: a personalised letter addressed to the individual from their GP; an explanation which focused on the importance of both physical and mental health; coloured ink; stamped addressed envelopes; and an explanation that the research was funded by the NHS and conducted by the university, as opposed to a commercial organisation. We also offered translation services for participants who wished to complete the questionnaire in a language other than English.
Participants were asked whether or not they would give permission for review of their medical records for 1 year prior to the date of completion of the questionnaire and for the year of the prospective study period. This enabled the severity of patients’ physical health to be assessed, and the number of EHAs to be verified, for the year before the baseline questionnaire, and for the 12-month prospective period. The notes were reviewed by research staff and junior doctors who were gaining research experience on the project. Any queries by the research staff were checked by a medically qualified practitioner. Participants were also sent a follow-up questionnaire 12 months after the first one, when they were asked again about their use of unscheduled care in the previous 12 months, in order to establish their self-reported use of unscheduled care over the prospective year. The main results from this study have been published and full details are available. 236
Unscheduled care
We used two outcome measures of unscheduled care:
-
EHAs independently verified from GP records for those patients who gave consent for their notes to be reviewed [n = 1415 (total); 1411 for the pre-baseline year and 1398 for the prospective year]
-
participant self-reported use of unscheduled care obtained from the questionnaire, which included questions about the following: an emergency visit at home from the GP; use of GP OOH service; called an emergency ambulance; attended an ED/casualty department because of an emergency health problem; or attended some other department or agency for an emergency health problem (e.g. walk-in centre) (n = 1203).
We used participant self-reported data for unscheduled care contacts other than EHAs, as it was not possible to reliably access these kind of data from the GP records, or from other routine data sources, at the time of the study. Contacts with OOH services were not always recorded in the GP records and there was no definitive way of determining attendance at an ED.
Potential predictors of unscheduled care
Demographic details
These included age, sex, marital status, ethnicity, education level, current work status (including disability and retirement status) and distance from home to the nearest ED (calculated using each participant’s postal code).
Severity of long-term conditions
The severity of each of the four LTCs was rated using data obtained from the participant’s medical records and each condition was rated as being mild, moderate, severe or very severe.
Asthma severity was classified according to the intensity of the treatment that was required to achieve good control of asthma, as recommended by the Global Strategy for Asthma Management and Prevention. 237 CHD severity was classified using the 1994 New York Heart Association classification, which categorises patients based on how much they are limited during physical activity; the limitations are in regard to normal breathing and varying degrees in shortness of breath and/or angina pain. 238 COPD severity was classified using the forced expiratory volume in 1 second (FEV1) per cent predicted values for the patient’s age, height and sex, as recommended by the Global Initiative for Lung Disease (GOLD). 239 Diabetic severity was classified according to a proxy measure, glycated haemoglobin (HbA1c) levels, with 0–6.4% equivalent to mild, 6.5–7.4% to moderate, 7.5–8.4% to severe and ≥ 8.5% to very severe. We used the Diabetes Control and Complications Trial values as opposed to the more recent way of recording HbA1c in mmol/mol (International Federation of Clinical Chemistry units), as these were the values recorded in the GP records. HbA1c scores are associated with more severe disease and complications,240 and these data were more reliably recorded in the GP records than that for diabetic complications. If patients had more than one condition, the maximum severity and total severity scores (using 0 = none, 1 = mild, 2 = moderate, 3 = severe and 4 = very severe) were also derived for each patient.
Comorbidity of other long-term conditions
In addition to the four exemplar LTCs, participants were asked about their physical health using a checklist of other common medical conditions, which included cancer, stomach or bowel problems, high blood pressure and arthritis or joint problems. Respondents were also asked to add any condition that was not listed. We recorded the number of patients who would be classed as having multimorbidity (defined as at least two or two or more LTCs).
Depressive symptoms
The HADS was used to assess depression. It is a valid and reliable measure of anxiety and depression, which was developed for use in patients with physical health problems. 241 We focused on the depression subscale which has seven items with a maximum score of 21. The scale can either be continuously to measure the severity of depressive symptoms but cut-off points of 8 and 11 have been also used, with scores above these points identifying participants with ‘probable depression’. We used a cut-off point of 8 and considered that patients with scores of > 8 had ‘probable depression’, but we also divided the scale scores into quintiles to examine the individual effects of different severities of depression, as even mild, subthreshold depression has been shown influence disease outcome. 55
Recent stress
The List of Threatening Experiences Questionnaire measures the experience of threatening personal situations or events in the last 6 months. 242 The measure had 11 areas of enquiry after we had excluded serious illness to self. 242 These areas were serious illness to a close relative; death of a first-degree relative; death of a close family member; separation due to marital difficulties; broken off a steady relationship; problem with a neighbour; been unemployed for more than 1 month; been sacked from your job; a major financial crisis; problems with police; and had something valuable stolen. The total score of positive responses represents recent exposure to threatening experiences. We excluded an item related to stress caused by physical illness from the total, as it may have related to the patient’s chronic physical illness.
Ethics approval and guidelines for reporting
The study received ethics approval from the Northwest 8 Research Ethics Committee (REC) – GM East (reference number 09/H1013/80). All participants provided written informed consent. We followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines for reporting observational studies. 243,244
Statistical analysis
For all demographic variables, other LTCs reported by the participant and hospital admissions data, we present numbers and percentages for categorical variables for the whole group and for each of the four exemplar conditions. As there was considerable overlap in the four LTC groups with many patients having multiple diagnoses, patients with and without each of the four conditions were compared using Fisher’s exact tests followed by Bonferroni adjustments for multiple testing (four tests per variable).
Factors relating to emergency hospital admissions in the last 12 months
Our original intention was to explore the relationship between baseline potential predictors and frequent use of unscheduled care. However, very few patients used unscheduled care more than once or twice during the study period. We therefore focused on patients who had used any form of unscheduled care in comparison with those who had not, as opposed to ‘frequent use of unscheduled care’.
A detailed description of the statistical analysis is available in Guthrie et al. 236 In summary, we carried out a series of univariate analyses using appropriate statistical tests to compare patients who did and did not have an emergency admission to hospital in the year preceding the baseline questionnaire and for the 12-month prospective period.
Logistic regression was used to assess the relationship between baseline variables and EHAs in the prospective year. ORs and 95% CIs are presented for all baseline variables found to be significantly associated with emergency admission in the prospective year.
Factors related to use of unscheduled care (participant self-reported)
The logistic regression analysis was repeated to determine factors that contributed to participant reported use of unscheduled care in the prospective year. In these analyses, participant self-reported use of unscheduled care in the previous year was included as an independent variable instead of emergency admission in the previous year.
Variations on these logistic regression analyses were also carried out, replacing individual diagnoses by number of QOF diagnoses out of four, or the number of diagnoses out of eight (four QOF diagnoses plus four participant self-reported diagnoses), where stated.
In all the multivariate analyses, inverse probability sampling weights were used to adjust for the non-completion of baseline and/or follow-up questionnaires. These were calculated using the reciprocal of the probability of completion, based on age group, sex and GP practice, for baseline questionnaires (1860 returned out of 6692 eligible); for completion of follow-up questionnaires (1203 out of 1860), the probability of completion was calculated using baseline variables. Similarly, separate inverse probability sampling weights were used to adjust for lack of availability of GP record data. Internal cross-validation of the chosen models was conducted using leave-one-out/jackknife procedures, to avoid overfitting, which could result in overinflated p-values. If the p-values are largely similar to those calculated initially, then it may be concluded that there are no problems with overfitting. Multicollinearity was not a problem as the largest variance inflation factor was only 1.7. Analyses were carried out using Statistical Product and Service Solutions (SPSS) version 20 (IBM Corporation, Armonk, NY, USA), and Stata version 12 (StataCorp LP, College Station, TX, USA).
To examine the role of severity of depression, HADS depression scores were split into five quintile groups, which yielded five groups of approximately equal sample size, as follows: depression scores of 0–1, 2–4, 5–7, 8–10 and ≥ 11. Logistic regression was used to assess the association between the five depression quintile groups and EHA during the prospective study period, the latter was established via GP records. Unadjusted ORs are presented for each quintile group, with the lowest group as the reference group. The analyses were then repeated, adjusting for all relevant covariates.
Power calculation
This was based on the percentages of patients who had used unscheduled care, being 15% in the group without a risk factor, compared with 30% in the group with that risk factor (OR 2.43). The study would have 90% power to detect a difference at the 5% level with sample sizes of 400 and 100, respectively. The study aimed to get completed questionnaires from at least 500 patients for each LTC. This was achieved for all LTCs except for COPD (n = 449).
Results
Recruitment
The flow of participants into the study is shown in Figure 16. Baseline questionnaires were sent to 6682 participants, with 2553 responding (38.2%). Of those returned, there were 1860 usable questionnaires (27.8%). More women responded than men (28.6% vs. 25.6%; p = 0.007), and older patients responded more than younger patients (response rate was 36.0% for patients aged 70–79 years, decreasing to 9.9% for patients aged 18–29 years, but was 30.1% for patients aged ≥ 80 years; p < 0.001). The response rates ranged from 16.7% to 35.2% at the 10 different practices (p < 0.001). There were no significant differences on any variables between the patients who returned the questionnaire without prompting and those who returned it after receiving a reminder (n = 467; 25.1%). Eight out of the 10 practices were in the top 10% of the most deprived areas in England, with five in the top 5% and two in the top 1%. 235
FIGURE 16.
Prospective longitudinal study of patients with LTCs in primary care: flow of study participants for analyses using EHAs as main outcome. Adapted from Guthrie et al. 236
A total of 1488 patients (80%) provided consent for their medical records to be reviewed, and these were retrieved and examined for 1415 patients altogether, 1411 for the year before, and 1398 patients for the prospective year (four sets of notes were obtained for the prospective year but had been missed for the baseline year and were unavailable when researchers went back to them, which explains the discrepancy between 1415 and 1411 for the year before baseline). The disparity between the number of patients consented and notes being reviewed is largely accounted for by the death of one of the GPs working in a single-handed practice and we were unable to retrieve the notes as patients were subsequently registered in a number of practices across the city. This also accounts for the lower number of notes reviewed for the prospective year (1398 out of 1415).
Patients whose records were examined were significantly more likely to be male (p = 0.026) and to have reached a moderate educational standard [i.e. some ‘O’ Levels, General Certificates of Secondary Education (GCSEs), or higher] (p = 0.002) than patients whose records were not examined.
We achieved our predetermined sample size (n = 500) for all of the four LTCs except for COPD. Out of the 1860 patients who returned questionnaires, the QOF diagnoses from the GP databases were as follows: 590 had ischaemic heart disease, 708 had asthma, 617 had diabetes and 449 had COPD. There was considerable multimorbidity, with over 20% of patients having at least two of the four exemplar diagnoses, which explains why the number of patients with the four LTCs adds up to more than 1860. There were 963 females in the cohort (51.8%), the mean age was 62.3 years [standard deviation (SD) 15.4 years] and 81.6% identified themselves as white British. Approximately half of the participants were single, widowed, divorced or separated. Table 11 shows the basic demographic details for the cohort as a whole and for the four exemplar LTCs.
| Demographic variable | All patients (N = 1860), n (%) | Exemplar LTC | |||
|---|---|---|---|---|---|
| Asthma (N = 708), n (%) | CHD (N = 590), n (%) | COPD (N = 449), n (%) | Diabetes (N = 617), n (%) | ||
| Female | 963 (51.8) | 471 (66.5)a | 228 (38.6)a | 226 (50.3) | 268(43.4)a |
| Aged ≥ 65 years | 919 (49.4) | 233 (32.9)a | 396 (67.1)a | 276 (61.5)a | 323 (52.4) |
| Single | 349 (18.8) | 178 (25.1)a | 72 (12.2)a | 50 (11.1)a | 115 (18.6) |
| Widowed, separated or divorced | 598 (32.2) | 196 (27.7)a | 229 (38.8)a | 180 (40.1)a | 194 (31.4) |
| Poor educationb | 1110 (59.7) | 346 (48.9)a | 418 (70.8)a | 333 (74.2)a | 379 (61.4) |
| Not working due to ill health | 275 (14.8) | 136 (19.2)a | 70 (11.9) | 68 (15.1) | 86 (13.9) |
| Unemployed and seeking work | 50 (2.7) | 30 (4.2)a | 9 (1.5) | 6 (1.3) | 9 (1.5) |
Multimorbidity and severity
In addition to the four exemplar LTCs, patients self-reported a wide range of other comorbid medical conditions, including arthritis (43.3%, n = 805), hypertension (38.5%, n = 717), stomach/bowel problems (15.4%, n = 287) and cancer (4.4%, n = 81) (Table 12). There was considerable multimorbidity, with over 20% of patients having at least two of the four exemplar LTCs and 65.3% (n = 1214) having at least one of the exemplar LTCs plus another self-reported LTC. Overall, 71.7% (n = 1333) of patients had two or more conditions and would therefore be considered to have multimorbidity.
| Other self-reported physical condition | All patients (N = 1860), n (%) | Exemplar LTC | |||
|---|---|---|---|---|---|
| Asthma (N = 708), n (%) | CHD (N = 590), n (%) | COPD (N = 449), n (%) | Diabetes (N = 617), n (%) | ||
| Cancer | 81 (4.4) | 23 (3.2) | 33 (5.6) | 26 (5.8) | 26 (4.2) |
| Stomach/bowel problems | 287 (15.4) | 125 (17.7) | 95 (16.1) | 65 (14.5) | 93 (15.1) |
| High blood pressure | 717 (38.5) | 203 (28.7)a | 281 (47.6)a | 150 (33.4)a | 322 (52.2)a |
| Arthritis/joint problems | 805 (43.3) | 292 (41.2) | 312 (52.9)a | 215 (47.9) | 264 (42.8) |
| Infectious and parasitic diseases | 3 (0.2) | 1 (0.1) | 1 (0.2) | 0 | 1 (0.2) |
| Diseases of the blood and non-blood-forming organs | 30 (1.6) | 13 (1.8) | 7 (1.2) | 8 (1.8) | 11 (1.8) |
| Endocrine/metabolic | 34 (1.8) | 15 (2.1) | 6 (1.0) | 5 (1.1) | 13 (2.1) |
| Mental/behavioural | 45 (2.4) | 26 (3.7)a | 6 (1.0)a | 10 (2.2) | 10 (1.6) |
| Diseases of the nervous system | 23 (1.2) | 11 (1.6) | 4 (0.7) | 9 (2.0) | 4 (0.6) |
| Diseases of the eye and adnexa | 41 (2.2) | 13 (1.8) | 8 (1.4) | 11 (2.4) | 20 (3.2) |
| Diseases of the ear and mastoid process | 19 (1.0) | 6 (0.8) | 5 (0.8) | 6 (1.3) | 7 (1.1) |
| Circulatory system | 41 (2.2) | 11 (1.6) | 10 (1.7) | 13 (2.9) | 15 (2.4) |
| Respiratory system | 27 (1.5) | 11 (1.6) | 7 (1.2) | 9 (2.0) | 5 (0.8) |
| Digestive system | 23 (1.2) | 9 (1.3) | 5 (0.8) | 8 (1.8) | 12 (1.9) |
| Skin | 11 (0.6) | 8 (1.1) | 1 (0.2) | 3 (0.7) | 1 (0.2) |
| Musculoskeletal system and connective tissue | 78 (4.2) | 32 (4.5) | 22 (3.7) | 24 (5.3) | 22 (3.6) |
| Genitourinary system | 35 (1.9) | 11 (1.6) | 14 (2.4) | 3 (0.7) | 12 (1.9) |
| Other diseases | 26 (1.4) | 16 (2.3) | 5 (0.8) | 4 (0.9) | 8 (1.3) |
Of the 1415 patients whose GP records were examined, asthma severity information was available for 443 out of 523 (84.7%) patients with asthma: in 108 the disease was rated as mild, in 280 it was rated as moderate and in 55 it was rated as severe. CHD severity information was available for 278 out of 465 (59.8%) patients with CHD: in 174 the disease was rated as mild, in 64 it was rated as moderate and in 40 it was rated as severe. COPD severity information was available for 209 out of 355 (58.9%) patients with COPD: in 37 COPD was rated as mild, in 110 as moderate, in 54 as severe and in eight as very severe. Diabetes severity information was available for 406 out of 465 (87.3%) patients with diabetes, among whom it was rated as mild in 157, as moderate in 127, as severe in 77 and as very severe in 45.
Several patients had two or more of the exemplar LTCs and can therefore be included in more than one group. As there was considerable overlap in the four LTC groups, with many patients having multiple diagnoses, patients with and without each of the four conditions were compared using Fisher’s exact tests followed by Bonferroni adjustments for multiple testing (four tests per variable) (e.g. patients with asthma were compared with all patients who did not have asthma).
Depression and anxiety
A total of 1818 participants completed the HADS at baseline, of whom 39.6% scored ≥ 8 (95% CI 37.4% to 41.9%) and 20.7% scored ≥ 11 (95% CI 18.8% to 22.5%) for depression; and 48.4% scored ≥ 8 (95% CI 46.1% to 50.7%) and 29.6% scored ≥ 11 (95% CI 27.5% to 31.7%) for anxiety. A total of 33.2% of participants scored ≥ 8 on both subscales, and 15.1% scored ≥ 11 on both. The prevalence of a HADS depression score of ≥ 8 was 40.3% among participants with asthma, 42.8% among patients with CHD, 47.1% among those with COPD and 36.6% among those with diabetes; the prevalence was significantly higher among participants with COPD than among those with the other conditions. The prevalence of a HADS anxiety score of ≥ 8 was 54.2% among participants with asthma, 49.9% among patients with CHD, 52.0% among those with COPD and 41.6% among those with diabetes; the prevalence was significantly higher among participants with asthma and significantly lower among participants with diabetes than among participants with the other conditions.
Emergency hospital admissions during baseline and prospective study periods (recorded from data in general practitioner records)
Of the baseline cohort of 1411 patients whose GP records were reviewed, 221 (15.7%) had an emergency admission in the year prior to completing the questionnaire. For individual exemplar LTCs, the numbers of participants who had an emergency admission were as follows: asthma, 68 out of 520 (13.1%); CHD, 95 out of 465 (20.4%); COPD, 70 out of 354 (19.8%); and diabetes, 58 out of 465 (12.5%). Only 59 patients (4.0%) had more than one emergency admission in the year before completing the questionnaire. During the prospective follow-up period of 12 months, from 1398 GP records reviewed, 234 (16.7%) patients had at least one emergency admission to hospital. For individual exemplar LTCs, the numbers of participants were as follows: asthma, 62 out of 516 (12.0%); CHD, 102 out of 458 (22.3%); COPD, 77 out of 351 (21.9%); and diabetes, 86 out of 461 (18.7%). Only 72 patients (5.2%) had more than one emergency admission in the prospective year.
Univariate analyses showed that having an emergency admission in the year prior to completion of the questionnaire was associated with older age (p < 0.001); being widowed, separated or divorced (p = 0.005); a lower level of education (p < 0.001); a HADS depression score of ≥ 8 (p = 0.001); COPD (p = 0.018); CHD (p = 0.001); self-reported cancer (p = 0.021); stomach or bowel problems (p = 0.016); arthritis and/or joint problems (p = 0.022); and living closer to an ED (p < 0.001). Patients with diabetes (p = 0.024) or asthma (p = 0.048) were significantly less likely to have had an emergency admission than the rest of the patients in this study.
Having an EHA in the follow-up year was significantly associated with older age; being widowed, separated or divorced; a lower level of education; a HADS depression score of ≥ 8; COPD; CHD; self-reported arthritis and/or joint problems; more severe physical illness; experiencing a threatening life event; and an emergency admission to hospital in the previous year (Table 13). These variables were very similar to the variables that were associated with unscheduled care in the baseline year. There was a large but non-significant variation in the percentage of patients with an emergency admission across the 10 GP practices in the follow-up year, ranging from 3% to 27% (χ2 = 15.2, df = 9; p = 0.085) (data obtained from GP practice notes). This difference was not explained by social deprivation, as it was not significantly correlated with the IMD for each practice (Spearman’s r = 0.46; p = 0.18).
| Patient characteristic | Emergency admission | Comparisona | ||||
|---|---|---|---|---|---|---|
| Had (n = 234) | Did not have (n = 1164) | |||||
| n | % | n | % | χ2 | p-value | |
| Demographic variable | ||||||
| Female | 109 | 46.6 | 595 | 51.1 | 0.22 | |
| Marital status | ||||||
| Single | 37 | 16.0 | 219 | 19.2 | 12.9 | 0.002 |
| Married or cohabiting | 95 | 41.1 | 573 | 50.1 | ||
| Widowed, separated or divorced | 99 | 42.9 | 351 | 30.7 | ||
| Poor educationb | 150 | 64.1 | 657 | 56.4 | 0.035 | |
| Not working due to ill health | 40 | 17.1 | 162 | 13.9 | 0.22 | |
| HADS depression score of ≥ 8c | 113 | 48.9 | 415 | 36.4 | < 0.001 | |
| Medical condition | ||||||
| Asthma | 62 | 26.5 | 454 | 39.0 | < 0.001 | |
| CHD | 102 | 43.6 | 356 | 30.6 | < 0.001 | |
| COPD | 77 | 32.9 | 274 | 23.5 | 0.004 | |
| Diabetes | 86 | 36.8 | 375 | 32.2 | 0.20 | |
| Self-reported stomach/bowel problems | 47 | 20.1 | 177 | 15.2 | 0.078 | |
| Self-reported arthritis/joint problems | 129 | 55.1 | 473 | 40.6 | < 0.001 | |
| Severityd | ||||||
| Mild | 49 | 25.9 | 238 | 25.2 | ||
| Moderate | 68 | 36.0 | 449 | 47.6 | 12.9 | 0.005 |
| Severe | 45 | 23.8 | 180 | 19.1 | ||
| Very severe | 27 | 14.3 | 77 | 8.2 | ||
| Threatening life experiences (out of 11) | ||||||
| None | 92 | 39.3 | 534 | 45.9 | 6.5 | 0.039 |
| One | 54 | 23.1 | 289 | 24.8 | ||
| Two or more | 88 | 37.6 | 341 | 29.3 | ||
| Had an emergency admission in the previous year | 71 | 30.3 | 148 | 12.8 | < 0.001 | |
| Continuous variable | Mean | SD | Mean | SD | t-teste | p-value |
| Age (years) | 65.8 | 14.0 | 61.4 | 15.3 | 4.1 | < 0.001 |
| Distance to hospital (kilometres) | 2.60 | 1.26 | 2.74 | 1.26 | 1.6 | 0.11 |
In logistic regression, the baseline variables that were significantly independently associated with prospective EHAs were having no partner; CHD; reporting a threatening life experience; an EHA in the previous year; and a HADS depression score of ≥ 8 (Table 14).
| Possible risk factor | Model | ||
|---|---|---|---|
| OR | 95% CI | Siga | |
| No partner | 1.49 | 1.04 to 2.15 | 0.032 |
| CHD | 1.60 | 1.04 to 2.46 | 0.033 |
| For each threatening life experience | 1.16 | 1.04 to 1.29 | 0.008 |
| Had an emergency admission in the previous year | 3.41 | 1.98 to 5.86 | < 0.001 |
| HADS depression score of ≥ 8 | 1.58 | 1.04 to 2.40 | 0.031 |
When the logistic regression analysis was repeated using total number of the four exemplar LTCs instead of the four individual LTCs, the same risk factors were identified with very similar results as in Table 14, and the number of LTCs was significant (OR 1.38, 95% CI 1.02 to 1.88; p = 0.040). The ORs (and 95% CIs) for the other risk factors were an OR of 1.49 (95% CI 1.04 to 2.13) for lack of partner; an OR of 1.16 (95% CI 1.04 to 1.29) for number of threatening life experiences; an OR of 3.38 (95% CI 1.99 to 5.74) for having had an emergency admission in the previous year; and an OR of 1.69 (95% CI 1.14 to 2.49) for a HADS depression score of ≥ 8. When the analysis in Table 14 was repeated using the jackknife procedure, the variable of depression became just non-significant (p = 0.051), and the significance of the other variables remained unchanged.
Using the five significant independent variables from Table 14, a risk score was calculated for each patient, which ranged from 0 to 5. Fifteen out of 157 patients with none of the risk factors (9.6%) had an emergency admission in the prospective year, compared with 8.0% for any one risk, 17.9% for any two risks, 24.7% for any three risks, 28.6% for any four risks and 30.8% for all five risks. Having had a prior emergency admission to hospital was associated with the greatest risk of future admission to hospital (positive predictive value = 32.4%, sensitivity = 30.3%, specificity = 87.2%). The positive predictive value means that 32.4% of the patients who had an emergency admission in the previous year had another in the prospective year.
Participant-reported use of unscheduled care during baseline period
Out of the baseline cohort of 1860 patients, 664 (35.7%) reported having used some form of unscheduled care in the previous year. For individual exemplar LTCs, these numbers of participants were as follows: asthma, 267 out of 695 (38.4%); CHD, 221 out of 582 (38.0%); COPD, 195 out of 440 (44.3%); and, diabetes, 182 out of 614 (29.6%). Only 418 patients (22.5%) reported using unscheduled care more than once in the year before completing the questionnaire.
Univariate analyses showed that reporting use of unscheduled care in the year prior to completion of the questionnaire was associated with older age (p < 0.001); being widowed, separated or divorced (p = 0.004); a lower level of education (p < 0.001); a HADS depression score of ≥ 8 (p = 0.001); COPD (p = 0.022); CHD (p = 0.001); self-reported cancer (p = 0.021); stomach or bowel problems (p = 0.015); arthritis and/or joint problems (p = 0.021); and living closer to an ED (p < 0.001).
Participant-reported use of unscheduled care during 12-month follow-up period
Shortly before the 12-month follow-up questionnaires were mailed, the practices excluded a further 141 patients: 35 had died, 48 had left the practice and a further 58 were excluded for other reasons. Follow-up questionnaires were mailed to 1719 of the baseline cohort, of whom 1203 (70.4%) returned the completed questionnaire. Figure 17 shows the modified flow chart, which includes the responses to the 12-month follow-up questionnaire.
FIGURE 17.
Prospective longitudinal study of patients with LTCs in primary care. Flow of study participants, including eligibility and responses to the 12-month follow-up questionnaire.
Out of 1170 patients who completed the section on use of unscheduled care in the follow-up questionnaire, 455 (38.9%) reported having used unscheduled care in the prospective 12 months; 302 reported attending an ED; 176 reported attending a different emergency facility to an ED; 51 reported calling an ambulance; and 188 reported receiving an emergency GP home visit. For individual exemplar LTCs, these numbers of participants were as follows: asthma, 185 out of 430 (43.0%); CHD, 153 out of 385 (39.7%); COPD, 136 out of 270 (50.4%); and, diabetes, 149 out of 398 (37.4%). Only 310 patients (26.5%) reported having used unscheduled care more than once in the prospective 12 months.
Participant self-reported unscheduled care in the follow-up year was significantly associated with older age (p = 0.035); being widowed, separated or divorced (p < 0.001); a lower level of education (p = 0.010); a HADS depression score of ≥ 8 (p < 0.001); COPD (p < 0.001); asthma (p = 0.030); self-reported arthritis and/or joint problems (p < 0.001); self-reported stomach or bowel problems (p < 0.001); more severe physical illness (p = 0.021); experiencing a threatening life event (p < 0.001); and use of unscheduled care in the previous year (p < 0.001).
In logistic regression, the baseline variables which were significantly independently associated with self-reported prospective unscheduled care use were having no partner; asthma; COPD; reporting a threatening life experience; used unscheduled care in the year before baseline; and a HADS depression score of ≥ 8 (Table 15). Maximum severity of QOF diagnosis was not significant. The jackknife procedure found lack of a partner (p = 0.001), asthma (p = 0.015), COPD (p = 0.045), unscheduled care in the previous year (p < 0.001) and a HADS depression score of ≥ 8 (p = 0.008) to be significant.
| Possible risk factor | Model | ||
|---|---|---|---|
| OR | 95% CI | Siga | |
| No partner | 1.90 | 1.34 to 2.69 | < 0.001 |
| Asthma | 1.81 | 1.16 to 2.81 | 0.009 |
| COPD | 1.94 | 1.14 to 3.29 | 0.014 |
| Threatening life experiences (per each experience) | 1.16 | 1.00 to 1.34 | 0.043 |
| Used any unscheduled care in year before baseline (participant report) | 3.64 | 2.47 to 5.37 | < 0.001 |
| Maximum severity of QOF diagnoses | 1.26 | 0.97 to 1.62 | 0.080 |
| HADS depression score of ≥ 8 | 1.79 | 1.22 to 2.64 | 0.003 |
There was a significant variation between the 10 GP practices in the percentage of patients who self-reported using unscheduled care in the prospective year ranging from 26% to 62% (χ2 = 19.8, df = 9; p = 0.019). There was, however, no significant correlation between the IMD for each practice and percentage of patients who reported using unscheduled care (r = 0.16; p = 0.67).
The role of severity of depression
To determine whether or not a HADS cut-off score of ≥ 8 for depression was appropriate, we divided the HADS depression scores into quintiles. 55 Worsening depression scores were associated with an increased risk of an EHA with a baseline score of ≥ 11 on the HADS depression scale more than doubling the risk of requiring an EHA in the prospective year, after adjusting for all relevant covariates (Table 16).
| HADS depression score at baseline | Analysis | |||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | |||||
| OR | 95% CI | Sigb | OR | 95% CI | Sigb | |
| 0–1 | Reference group | Reference group | ||||
| 2–4 | 1.36 | 0.78 to 2.36 | 0.28 | 0.99 | 0.52 to 1.85 | 0.96 |
| 5–7 | 2.43 | 1.44 to 4.12 | 0.001 | 1.73 | 0.94 to 3.18 | 0.078 |
| 8–10 | 2.25 | 1.31 to 3.87 | 0.003 | 1.67 | 0.87 to 3.21 | 0.12 |
| ≥ 11 | 3.06 | 1.82 to 5.13 | < 0.001 | 2.42 | 1.12 to 5.23 | 0.025 |
The numbers of patients analysed in each quintile group of HADS depression score at baseline in Table 16 are as follows: score of 0–1, n = 237; score of 2–4, n = 320; score of 5–7, n = 286; score of 8–10, n = 251; score of ≥ 11 n = 277; and, total, n = 1371.
Table 17 shows a similar analysis using participant report of use of unscheduled care as the dependent variable. Worsening depression scores were associated with an increased risk of self-reported use of unscheduled care after adjusting for all relevant covariates. The numbers of patients analysed in each quintile group of HADS depression score at baseline in Table 17 are as follows: score of 0–1, n = 211; score of 2–4, n = 291; score of 5–7, n = 233; score of 8–10, n = 198; score of ≥ 11, n = 211 and total, n = 1144.
| HADS depression score at baseline | Analysis | |||||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | |||||
| OR | 95% CI | Sigb | OR | 95% CI | Sigb | |
| 0–1 | Reference group | Reference group | ||||
| 2–4 | 1.19 | 0.79 to 1.79 | 0.40 | 1.04 | 0.59 to 1.83 | 0.90 |
| 5–7 | 2.32 | 1.54 to 3.49 | < 0.001 | 2.16 | 1.20 to 3.91 | 0.011 |
| 8–10 | 2.78 | 1.82 to 4.23 | < 0.001 | 1.82 | 0.98 to 3.37 | 0.057 |
| ≥ 11 | 4.22 | 2.78 to 6.40 | < 0.001 | 3.58 | 1.88 to 6.82 | < 0.001 |
Discussion
In phase 2 of the programme, we conducted a large longitudinal prospective cohort study of patients with at least one of our four exemplar LTCs in primary care in the UK. In relation to our first main aim, which we stated at the beginning of this chapter, we were able to derive estimates of the frequency, and the pattern, of unscheduled care use in patients with our four exemplar LTCs.
The prospective study enabled us to collect data on patients over a 2-year period. During that time, we found that very few patients were actually ‘frequent users’ of unscheduled care, although many used it once or twice. Approximately 16% of patients in the cohort had an EHA in the baseline year, and the proportion was similar in the prospective year.
In relation to our second aim, we were not able to identify predictors of frequent use of unscheduled care, as so few patients were frequent users. Our findings suggest that the ED attendances and admissions to hospital of the participants in the study were most likely necessary and entirely justified, and prompted by an exacerbation in their physical condition.
In our analyses, we focused attention on those patients who used any unscheduled care during the relevant study time periods, in comparison with those who had not.
We used two measures of unscheduled care: EHAs recorded by contemporaneous data from GP records; and participant self-reported use of unscheduled care, via questionnaire data.
We found a similar pattern of results, for both objective data about EHAs from the GP records and participant self-reported data. The following factors were independent predictors of unscheduled care in both sets of analyses: use of unscheduled care in the previous year, depression, not having a partner and stressful life experiences. Other factors that were independent predictors in at least one of the analyses were having more than one of the four exemplar LTCs and severity of physical illness.
A prior history of use of unscheduled care in the year before completion of the questionnaire was by far the most powerful predictor of use of unscheduled care in the prospective study period (whether measured by EHAs or self-reported use of unscheduled care). This factor alone increased the risk of unscheduled care use by at least three and a half times.
We also found that baseline depression was a significant predictor of both prospective EHAs and participant self-reported use of unscheduled care. The ORs for depression that we found in the cohort study were of a very similar order to the results of the systematic review we carried out in the first phase of the programme. Depression was independently associated with both EHAs (OR 1.58, 95% CI 1.04 to 2.40; p = 0.031) and attendances at EDs (OR 1.79, 95% CI 1.22 to 2.64; p = 0.003). 236 More severe depression was associated with a greater than twofold increased risk of using unscheduled care (EHAs, OR 2.42, 95% CI 1.12 to 5.23; p = 0.025; and participant-reported use of unscheduled care, OR 3.58, 95% CI 1.88 to 6.82; p < 0.001). 236 To our knowledge, this is the only UK study, to date, which has shown that baseline depression is an independent predictor of future use of unscheduled care in people with LTCs, even when controlling for prior use of unscheduled care and severity of physical illness.
Over 70% of the participants in the prospective study had multimorbidity with at least two LTCs. We examined the effect of multimorbidity on use of unscheduled care by entering the number of exemplar conditions that each participant had (recorded by the QOF) into a separate analysis. Each additional LTC increased the risk of using unscheduled care by approximately 40%. This is consistent with other work in this area,26 but of note, depression remained an independent predictor in these separate analyses.
There is currently a debate whether or not there is benefit attached to routine case-finding for depression in primary care,245 and during the lifetime of this programme, case-finding for depression in certain LTCs has been withdrawn as a QOF target for primary care in England. There is insufficient evidence at present to support its reintroduction, but our findings suggest the value of case-finding for depression in particular high-risk groups has not been fully explored.
As we used the HADS in the cohort study, we also had the opportunity to investigate the role of anxiety, but not panic, as a predictor of unscheduled care in patients with LTCs. The analyses are not presented in this chapter, as they were not the main focus of our investigation. However, we found no strong evidence for anxiety acting as an independent predictor of unscheduled care. These findings, again, support the results of the systematic review we carried out in the first phase of the programme, which found no evidence of an association between anxiety and use of unscheduled care.
We found that lack of a partner and experiencing threatening life events were also risk factors for using unscheduled care. Although the ORs for life stress were relatively small, the ORs in each regression are for each additional item experienced. Hence, several threatening experiences in the same person would increase their risk of using unscheduled care quite substantially. Severity of illness was also included in one of the predictor models and is a well recognised risk factor for unscheduled care use. 20,23,30,57
The duration of our prospective follow-up period was 12 months, and beyond this period of time, the predictive power of factors appears to wane. 246 Even so, over 30% of our cohort reported using unscheduled care at least once during this 12-month period, and 17% had at least one EHA. This suggests that there may be a potential to reduce unscheduled care in this population if some of the contributing factors can be addressed.
The strengths of our study included the following: a large cohort of primary care patients; recruitment target achieved in three out of the four exemplar LTCs; an independent measurement of health care which employed scrutiny of GP records as well as a measure of participant self-report; use of a standardised instrument to measure depressive symptoms; and the independent assessment of severity of illness using GP records. A further strength was the inclusion of patients with several LTCs as opposed to focusing on a single disease, with most patients in the baseline cohort having multimorbidity. The follow-up data of the patients who were entered into the prospective study were good, with nearly 80% giving consent for their medical records to be checked. In addition, there was a good response to the follow-up questionnaire (over 70%).
The response rate to the baseline questionnaire was disappointingly low, despite efforts to maximise recruitment. Achieving good response rates to postal-based questionnaires is becoming a challenge and two other large-scale studies, which have been recently carried out, have reported similar low response rates to our own: the GP Patient Survey247 and a recent large primary care cohort study from Oxford that targeted patients with chronic physical disease. 248 In the area where we carried out our study, response rates to postal questionnaires have declined dramatically over the last 15 years, dropping by over 20%. 249
Our main concern with the low response rate to the baseline questionnaire was the potential for bias in our study sample. Participants in our study were more likely to be female and older than non-participants. They were also over-represented by people with a white British background and the study was carried out in a relatively deprived area of Manchester.
We attempted to adjust for differences in age and sex between responders and non-responders using inverse probability sampling weights based on age, sex and GP practice, which were the only variables available to us for the non-responders. We also compared the responses of patients who returned and completed the questionnaire spontaneously with the responses of those who returned the questionnaire after a reminder. The latter group could be argued to be more representative of non-responders, as they would not have responded without prompting. There was no difference between these two groups with any of the variables.
We also considered whether patients who participated in our study were either over- or under-represented in terms of depression. Approximately 25% of people in the study had a HADS depression score of ≥ 11 and approximately 40% had a HADS depression score of ≥ 8. If a score on the HADS depression subscale of ≥ 8 is broadly equivalent to subthreshold depression and a score of ≥ 11 is broadly equivalent to clinical depression, the prevalence of depression in our sample was commensurate with that expected for a population of patients with LTCs. 69
Our results cannot be generalised to all populations of patients with LTCs, but they suggest that, in a substantial proportion, depression is a potential important psychosocial determinant of use of unscheduled care, and could be a potential red flag in addition to other more recognised predictors of unscheduled care (e.g. previous use of unscheduled care). The results of our systematic review, conducted in phase 1, also suggested that depression should be considered as a strong candidate for inclusion as a red flag variable, and the similarity between the magnitude of the ORs for depression in the systematic review and the cohort study provides some support for the validity of the findings from the cohort study, despite the low response rate.
Our findings suggest a potential model that links physical and psychosocial factors in people with LTCs, increasing the risk of unscheduled care use through exacerbations of illness (Figure 18).
FIGURE 18.
A model that links together physical and psychosocial factors in people with LTCs as risk factors for the use of unscheduled care.

The model shows that the biggest risk factor for unscheduled care use is prior use of unscheduled care in the previous 12 months. Admission to hospital with an acute exacerbation of illness is linked both to severity of illness20,23,24,30,57,250 and to depression. 27,29,30,49,92,251 There is also a well-recognised bidirectional association between the severity of physical illness and depression. 252 Living alone is linked both to an increased risk of depression in people with LTCs253 and to worse outcome,254 and life stressors are also associated with an increased risk of depression in elderly people. 255 All the factors in the model have an independent influence on unscheduled care use, but there are also multiple interactions between different components of the model, which may worsen overall outcome. We also found that the number of LTCs increases the risk of unscheduled care use and this is represented by the boxes and arrows to the left of Figure 18. 256,257
We recognise that the relationship between physical illness and depression is more complex than represented by the model in Figure 18. The way in which depression and severity of illness interact with each other has been elegantly described by Katon in a detailed and thorough review of the relevant literature. 252 Katon’s conceptual model brings together genetic and childhood factors that predispose individuals to both physical and mental health problems later in life. Comorbid depression in physical illness affects the patient–physician relationship, the ability to seek comfort and support from others, and the ability to self-care. Depression is a major risk factor for non-compliance with medical treatment108 and, compared with non-depressed patients, depressed patients are three times more likely to be non-compliant with medical recommendations. Depression often involves a degree of hopelessness, impaired concentration and poor sleep, all of which may interfere with medication adherence as well as attendance at appointments. Problems with adherence to treatment may lead to exacerbations in physical illness, which then necessitate urgent treatment. In addition to effects on adherence, depression has also been linked to other forms of poor self-care in physical disease, which may include such behaviours as overeating, increased alcohol consumption, smoking, etc. 252 All these behaviours can have a deleterious effect on physical illness and increase the risk of exacerbations of illness in people with LTCs. Katon’s model will be discussed further in Chapter 11, when the overall findings of the programme are brought together.
Limitations
There were several limitations of the prospective study. The issue of low response rate has already been discussed above. There may have been recall bias of self-reported use of unscheduled care by the study participants. It was not feasible to send out follow-up questionnaires at either 3 months or 6 months, in the context of the overall programme. Limiting the follow-up to 12 months may have meant that some participants inaccurately recorded their contacts with unscheduled care.
Although great efforts were made to record severity using data recorded in the GP notes, there remain several methodological problems with the severity ratings we used. Each rating according to the specific LTC will be discussed in turn. Asthma severity was classified according to the Global Strategy for Asthma Management and Prevention. 237 This is based on the type of medication that is prescribed to control the disease. The rating does not take into account whether or not the patient adheres to the medication that is prescribed, or even collects the medication from the chemist. COPD was rated using the FEV1, as recommended by the GOLD criteria, as defined at the time of the study. 239 The GOLD criteria, however, have subsequently been updated and suggest that COPD severity should now be rated using a composite of COPD symptoms, plus FEV1 divided by forced vital capacity and risk of exacerbations. Although we recognise that the updated guidelines are an improvement on the ones that we used in the study, it would not have been possible to use the updated criteria had they been available at the time, as the kind of detailed information they require is not recorded routinely in GP records. CHD severity was based on how much patients were limited by their disease during physical activity. Such data, however, were not always recorded in the GP notes. HbA1c was used as a proxy for severity for diabetes because of difficulties in identifying reliable ways of recording severity from the data recorded in GP records. We recognise that HbA1c is more a marker of adherence to treatment than severity of disease, and this may have led to possible confounding in our results as, discussed earlier, adherence is influenced by depression. We were also unable to distinguish between type 1 or type 2 diabetes.
In the next chapter, we will report on the costs associated with use of unscheduled care.
Chapter 4 Economic analysis of the longitudinal prospective study of predictors of unscheduled care in patients with long-term conditions (phase 2)
Abstract
Background
People with LTCs account for 70% of all expenditure in the NHS. The costs of health care for people with LTCs who have comorbid mental health problems, in comparison with those who do not, are two to three times greater.
Methods
The detailed service use data collected in the Longitudinal Questionnaire Cohort Study (see Chapter 3) were costed using published national health- and social-care unit cost information. Chapter 3 describes the statistical methods used to analyse the data from the longitudinal cohort study. In line with these, descriptive statistics and multivariate regression models were used to explore the costs of events and total costs per year of the longitudinal cohort participants and the differences in costs between different groups of participants.
Results
The mean costs for unscheduled care accounted for roughly half of the total cost of health care during the period studied. The total costs for the group of patients as a whole were significantly higher for depressed patients than for non-depressed patients, for both scheduled and unscheduled care. The presence of depression at baseline, maximum severity of LTC, and the total costs in the year before baseline were statistically significant and associated with increased prospective unscheduled care costs and total costs.
Discussion
The results from the cost analysis support previous findings that depression increases the costs of both scheduled and unscheduled care for patients with LTCs, and may be an important independent predictor of future costs of unscheduled care, together with previous health-care costs and severity of illness.
Overview
In this chapter, we describe the economic analysis of the longitudinal cohort study that we described in the previous chapter (see Chapter 3).
The overall aim of the cost analyses was to explore the costs of unscheduled care in the exemplar conditions. Specific research questions to address this aim were:
-
What are the costs of scheduled and unscheduled health services used by people with the exemplar LTCs?
-
What is the relative contribution of unscheduled and other care to the total cost of health services used by people with the exemplar LTCs?
Background
People with LTCs account for 50% of all GP appointments, 64% of outpatient appointments and 70% of all inpatient bed-days. In total, around 70% of the total health and care spend in England (£7 out of every £10) is attributed to caring for people with LTCs. This means that 30% of the population account for 70% of the spend on health care. 258
All four of the exemplar conditions included in the CHOICE programme carry enormous economic health burdens. The total annual cost of all patients with CHD was £7.06B in 1999,259 the highest of all diseases in the UK for which comparable analyses have been done. 260,261 The UK has some of the highest prevalence rates for asthma in the world,262 with England, Wales and Scotland ranked among the top nine countries. The total annual cost of COPD to the NHS is estimated to be over £800M for direct health-care costs. 263,264 In the late 1990s, 24 million working days per year were lost because of COPD, with the cost of lost productivity being estimated at around £2.7B. 9 In a recent European study, unscheduled care accounted for 56% of total costs in adults with asthma,68 regardless of the severity of patients’ symptoms. In the USA, over 12 months, 8.3% of patients with asthma made at least one visit to the ED and 13% of COPD patients made six or more visits. 19 The costs of health care for patients with type 2 diabetes in the UK will rise by 25% over the next 30 years and the economic burden of the disease will rise by 40%. 265
Comorbid mental health problems in people with LTCs raise total health-care costs by at least 45% for each person, and it has been estimated that between 12% and 18% of all NHS expenditure on LTCs is linked to poor mental health and well-being. 38
Methods
The design and main methods of the longitudinal cohort study were described in Chapter 3. The following section focuses specifically on the cost analysis. Inverse probability survey weights, as described in Chapter 3, were also applied here.
In this analysis, we included data on both scheduled and unscheduled care. The items of service use included in the scheduled care costs were use of routine GP practice services at the surgery or at home (GP, nurse and other health-care workers, and telephone calls); hospital outpatient and day case visits; and routine or elective hospital admissions. The service use categories included in the unscheduled care costs included GP OOH service and OOH home visits, accident and emergency (A&E) services, including walk-in centres and minor injury facilities, non-routine hospital day case visits and inpatient admissions. The detailed service use data about whether or not a participant had used a service and the intensity or frequency of use were collected by participant questionnaire and GP case note review as part of the longitudinal prospective cohort study (see Chapter 3). Data were collected from GP records using a standardised data extraction form. The data collection form was pilot tested in two GP practices, for 72 patients’ records. The data collection form was revised to take account of any issues that arose. Data on scheduled and unscheduled care were also collected in the participant questionnaires at baseline and at the 12-month follow-up. Participants were asked to recall service use over the previous 12 months. The data from GP records were used as the primary source of data, supplemented by information from the participant questionnaire that reported service use not available from the GP notes. The data were recorded and costed per patient, to give a total cost per person in the study. This cost was used in all the analyses, with no attempt to attribute parts of the cost to any of the four exemplar conditions. These service use data were costed using published national health- and social-care unit costs. This was to facilitate the transferability of the results to different NHS settings. The two main sources of unit costs were the National Schedules of Reference Costs 2012–13 (NSRC) (hospital costs), which is published annually by the DH266 and The Unit Costs of Health and Social Care 2012, which is published annually by the Personal Social Services Research Unit. 250 The price year used was 2011–12, which was the latest available when the unit cost data were collected.
Unit costs and cost estimation
Hospital-based services
The NSRC266 was used to identify costs of hospital inpatient and outpatient services. It includes a range of different costs for each type of hospital service. For inpatient admissions, these include unit costs by level of severity for detailed diagnosis and associated health-care-relevant group (HRG), with and without complications. The costs of elective and non-elective admissions, by short and long stay, are included, as are the unit costs of excess bed-days. Accordingly, this source was searched by four reviewers [DN, EC, Sarah Parsons (SP) and Ming Quan (MQ)] to identify, and agree, the relevant national average unit costs of secondary care and tertiary care services. Any differences of opinion that could not be resolved by discussion, or cases about which there was a high level of uncertainty, were discussed with a fifth reviewer (LD).
The key criterion used to identify unit costs from the NSRC was that the description of the selected unit cost was the closest match to the items of service use captured in the longitudinal cohort database. Where it was not possible to identify a single unit cost for a hospital service (e.g. cost per day of admission, cost per outpatient admission) an average unit cost was estimated, weighted by the number of data submissions reported in the NSRC.
Additionally, the cohort database includes up to three reasons for a hospital admission. It was not always possible to identify the primary reason for admission. In participants with more than one reason, length of stay may be longer or costs higher than in the case of those with only one reason for admission. To adjust for this, the reason associated with the longest average length of stay was used. Any excess bed stay costs were calculated in relation to the main admission reason, as described in the next paragraph.
The unit costs of inpatient stays reported in the NSRC reflect the national average length of hospitalisation for that HRG. This may not reflect the average length of stay of hospital admissions for cohort participants. Accordingly, the recorded length of stay for an admission in the longitudinal cohort database was compared with the national average in the NSRC266 and the difference in length of stay was calculated (participant length of stay minus national average length of stay). The average cost per day includes the costs of procedures that may typically be used in the initial part of the inpatient admission. In addition, the intensity of care is likely to be higher at the start of the admission than at the end. Using the average cost per day to estimate the cost of an admission may then overestimate the total cost of the admission if the participant has a longer than average length of stay. To address this, we identified those admissions where the length of stay was more than 10% higher than the national average. These admissions were costed in two stages:
-
The NSRC HRG unit cost was used to cost the length of stay up to the national average. 266
-
The excess bed stay tariffs were used to cost the excess length of stay.
If the length of stay of an admission for a cohort participant was less than or equal to the national average, then the average unit cost per day was used to estimate the cost of the admission for that person. A similar approach was used to cost the use of community and primary health-care services using unit costs from The Unit Costs of Health and Social Care 2012 data. 250
Analysis
Chapter 3 describes the statistical methods used to analyse the data from the longitudinal cohort study. In line with these, descriptive statistics and multivariate regression models were used to explore the costs of events and total costs per year of the longitudinal cohort participants, and explore possible relationships with other factors. The analyses were exploratory, with no predefined hypotheses. The cost analyses were not designed to formally identify predictors to inform the red flag study or trial in the way that the statistical analyses reported in Chapter 3 were. Because of the high level of variance inherent in cost data, mean costs and 95% CIs are reported. The mean cost is important for policy-makers and health-care providers and funders. It is a measure that reflects the full variance in the sample, and when multiplied by the number of people studied gives a measure of total cost for the sample. The 95% CIs assume that the cost data are normally distributed. Typically, health-care cost data are skewed, with a large number of people having zero cost and a few having very high costs. In addition, costs are constrained to be zero or higher. These factors mean that the 95% CI may be biased. The impact of a skewed distribution of data on measures such as the 95% CI tends to reduce as the sample size increases. As the sample size for this study is relatively large, it is assumed that the 95% CI will give relevant information for the reader to assess the importance of the comparisons presented. Where relevant, p-values are also reported. All the cost analyses used inverse probability sampling weights (see Chapter 3) to adjust for differences in the characteristics of those who did not respond to the invitation to participate in the longitudinal cohort study, compared with those who did complete the study.
Results
Descriptive analysis
The costs of scheduled and unscheduled care are described in Tables 18–25 (mean; 95% CI) for all study participants with complete service use data. Most analyses describe costs for the prospective year, which is the year after participants completed the baseline questionnaire. The analyses for pre-baseline questionnaire year costs are reported in Appendix 2, Tables 57–63. This section focuses on the contribution of factors found to be associated with use of unscheduled care in Chapter 3. The contribution of multimorbidity to participant health-care costs was also considered. There was no evidence that the costs of participants with two or more QOF conditions differed from those of participants with one QOF condition. These analyses are reported in Appendix 2, Tables 64, 66, 68 and 70.
Table 18 shows the costs, for each condition, of both scheduled and unscheduled care. As people in the study may have had more than one condition, they can be represented in more than one group. Table 18 reports the cost per person for each condition according to whether or not they were recorded as having that condition. The ‘all conditions’ column presents the mean cost per person irrespective of which of the four conditions they had and does not represent the sum of the four conditions. The mean costs for unscheduled care were very similar to the cost of scheduled care for each of the four conditions, suggesting that it accounted for roughly half of the total costs of health care during the period studied. There was no evidence that the costs differed between the pre-baseline and prospective years (see Appendix 2, Tables 57–63). The 95% CIs for the costs by condition overlap, indicating that there are unlikely to be differences between them.
| Service use | All conditionsa (N = 1398) | Exemplar LTCa | |||
|---|---|---|---|---|---|
| CHD (N = 458) | Asthma (N = 516) | Diabetes (N = 461) | COPD (N = 351) | ||
| Scheduled service use costs (£, 2011–12) only, mean (95% CI) | 1291 (1172 to 1409) | 1468 (1264 to 1672) | 1121 (1004 to 1437) | 1473 (1291 to 1655) | 1294 (1091 to 1497) |
| Patients using service, n (%) | 1386 (99) | 455 (99) | 508 (98) | 459 (99) | 350 (99) |
| Unscheduled service use costs (£, 2011–12) only, mean (95% CI) | 1211 (647 to 1774) | 1458 (961 to 1955) | 1331 (53 to 2610) | 1156 (692 to 1620) | 1279 (788 to 1770) |
| Patients using service, n (%) | 491 (35) | 180 (39) | 177 (34) | 150 (33) | 140 (40) |
| Total scheduled and unscheduled service use costs (£, 2011–12), mean (95% CI) | 2501 (1915 to 3087) | 2925 (2346 to 3505) | 2552 (1237 to 3867) | 2629 (2098 to 3160) | 2573 (2026 to 3120) |
| Patients using service, n (%) | 1387 (99) | 455 (99) | 508 (98) | 459 (99) | 351 (100) |
Table 19 shows the costs of health care over the prospective year for patients who were and were not depressed at baseline (i.e. scored ≥ 8 on the HADS241), according to exemplar condition. There was a trend towards higher costs for participants with depression (HADS depression score of ≥ 8) than for those who were not depressed (HADS depression score of < 8). The total costs for the group as a whole were significantly higher for depressed patients than for non-depressed patients, for both scheduled and unscheduled care.
| Service use | All conditions (N = 1371)a,b | Exemplar LTCa | |||
|---|---|---|---|---|---|
| CHD (N = 451) | Asthma (N = 508) | Diabetes (N = 447) | COPD (N = 346) | ||
| Scheduled service use only | |||||
| No depression, mean cost (£, 2011–12) (95% CI) | 1124 (990 to 1258) | 1315 (1025 to 1605) | 957 (743 to 1172) | 1234 (1020 to 1448) | 1326 (1015 to 1637) |
| Patients using service, n/N (%) | 834/843 (99) | 266/268 (99) | 308/314 (98) | 276/278 (99) | 187/188 (99) |
| Depression,c mean cost (£, 2011–12) (95% CI) | 1551 (1320 to 1782) | 1690 (1403 to 1976) | 1668 (1183 to 2153) | 1828 (1485 to 2170) | 1256 (1010 to 1502) |
| Patients using service, n/N (%) | 525/528 (99) | 182/183 (99) | 192/194 (99) | 169/169 (100) | 158/158 (100) |
| p-value for difference (no depression vs. depression) | 0.002 | 0.072 | 0.009 | 0.004 | 0.729 |
| Unscheduled service use only | |||||
| No depression, mean cost (£, 2011–12) (95% CI) | 598 (417 to 779) | 840 (512 to 1167) | 360 (145 to 574) | 647 (372 to 922) | 813 (266 to 1361) |
| Patients using service, n/N (%) | 255/843 (30) | 101/268 (38) | 87/314 (28) | 79/278 (28) | 67/188 (36) |
| Depression,c mean cost (£, 2011–12) (95% CI) | 2197 (792 to 3603) | 2351 (1221 to 3481) | 2988d | 1951 (818 to 3084) | 1872 (1006 to 2737) |
| Patients using service, n/N (%) | 227/528 (43) | 77/183 (42) | 87/194 (45) | 67/169 (40) | 71/158 (45) |
| p-value for difference (no depression vs. depression) | 0.027 | 0.012 | 0.114 | 0.028 | 0.043 |
| Total scheduled and unscheduled service use | |||||
| No depression, mean cost (£, 2011–12) (95% CI) | 1722 (1490 to 1954) | 2155 (1696 to 2614) | 1317 (1018 to 1616) | 1881 (1501 to 2260) | 2139 (1547 to 2732) |
| Patients using service, n/N (%) | 835/843 (99) | 266/268 (99) | 308/314 (98) | 276/278 (99) | 188/188 (100) |
| Depression,c mean cost (£, 2011–12) (95% CI) | 3748 (2308 to 5188) | 4041 (2781 to 5301) | 4656 (1346 to 7966) | 3779 (2535 to 5024) | 3128 (2115 to 4140) |
| Patients using service, n/N (%) | 525/528 (99) | 182/183 (99) | 192/194 (99) | 169/169 (100) | 158/158 (100) |
| p-value for difference (no depression vs. depression) | 0.006 | 0.006 | 0.049 | 0.004 | 0.098 |
Table 20 presents the mean costs per person of unscheduled, scheduled and all care, by GP practice. There were statistically significant differences in the costs of scheduled care by GP practice, but not in the costs of unscheduled care. This analysis excludes one GP practice [practice identification number (ID) = 19], which had low numbers of patients with cost data because of problems with obtaining medical records after the lone GP died and patients were transferred to several different practices.
| GP practice ID (number of patients per practice) | Cost (£, 2011–12), mean (95% CI) | ||
|---|---|---|---|
| Scheduled care | Unscheduled care | Total scheduled and unscheduled care | |
| 1 (n = 252) | 1172 (1022 to 1321) | 872 (371 to 1373) | 2044 (1508 to 2580) |
| 3 (n = 192) | 1535 (1102 to 1969) | 1245 (554 to 1935) | 2780 (1763 to 3797) |
| 5 (n = 136) | 1012 (726 to 1299) | 3701 (–1927 to 9328) | 4713 (–959 to 10,386) |
| 6 (n = 369) | 1831 (1391 to 2272) | 1137 (654 to 1619) | 2968 (2143 to 3794) |
| 7 (n = 112) | 938 (475 to 1400) | 526 (72 to 980) | 1464 (809 to 2118) |
| 8 (n = 263) | 1278 (989 to 1567) | 1255 (533 to 1978) | 2533 (1693 to 3374) |
| 11 (n = 105) | 1436 (967 to 1905) | 580 (75 to 1085) | 2016 (1282 to 2751) |
| 14 (n = 112) | 1000 (692 to 1307) | 1062 (353 to 1772) | 2062 (407 to 2871) |
| 16 (n = 236) | 953 (798 to 1107) | 962 (9 to 1914) | 1914 (913 to 2915) |
| Range (of mean values) | 938–1535 | 526–3701 | 1464–4713 |
| p-value | 0.003 | 0.41 | 0.16 |
Table 21 gives a breakdown of the frequency and costs of unscheduled care used in the prospective year by type of service used and QOF condition, for the full sample. Table 22 reports the costs for the subgroup of people who used any unscheduled care in the prospective year.
| Service | All conditions (N = 1398) | Exemplar LTCa | |||
|---|---|---|---|---|---|
| CHD (N = 458) | Asthma (N = 516) | Diabetes (N = 461) | COPD (N = 351) | ||
| GP OOH services | |||||
| Number of times used, n (%) | |||||
| 0 | 1237 (88.5) | 406 (88.7) | 447 (86.6) | 408 (88.5) | 310 (88.3) |
| 1 | 123 (8.8) | 42 (9.2) | 53 (10.3) | 37 (8.0) | 32 (9.1) |
| 2 | 23 (1.7) | 6 (1.3) | 10 (1.9) | 8 (1.7) | 6 (1.7) |
| 3+ | 15 (1.1) | 4 (0.9) | 6 (1.2) | 8 (1.7) | 3 (0.8) |
| Mean cost (95% CI) | 11 (8 to 13) | 10 (6 to 14) | 12 (8 to 16) | 12 (7 to 18) | 11 (6 to 17) |
| ED visits | |||||
| Number of times used, n (%) | |||||
| 0 | 1114 (79.7) | 352 (76.9) | 408 (79.1) | 384 (83.3) | 266 (75.8) |
| 1 | 190 (13.6) | 74 (16.2) | 71 (13.8) | 51 (11.1) | 54 (15.4) |
| 2 | 54 (3.9) | 21 (4.6) | 19 (3.7) | 15 (3.3) | 19 (5.4) |
| 3+ | 40 (2.9) | 11 (2.4) | 18 (3.5) | 11 (2.4) | 12 (3.4) |
| Mean cost (95% CI) | 53 (42 to 63) | 51 (39 to 63) | 54 (37 to 70) | 36 (27 to 46) | 77 (45 to 108) |
| Emergency hospital inpatient admissions | |||||
| Number of times used, n (%) | |||||
| 0 | 1164 (83.3) | 356 (77.7) | 454 (88.0) | 375 (81.3) | 274 (78.1) |
| 1 | 162 (11.6) | 69 (15.1) | 40 (7.8) | 62 (13.5) | 45 (12.8) |
| 2 | 41 (2.9) | 20 (4.4) | 13 (2.5) | 12 (2.6) | 20 (5.7) |
| 3+ | 31 (2.2) | 13 (2.9) | 9 (1.8) | 12 (2.6) | 12 (3.4) |
| Mean costb (95% CI) | 1147 (586 to 1709) | 1397 (905 to 1889) | 1266 (–10 to 2541) | 1107 (647 to 1567) | 1191 (710 to 1673) |
| Service | All conditions | Exemplar LTCa | |||
|---|---|---|---|---|---|
| CHD | Asthma | Diabetes | COPD | ||
| GP OOH services | |||||
| Number of times used, n (%) | |||||
| 1 | 123 (76) | 42 (81) | 53 (77) | 37 (70) | 32 (78) |
| 2 | 23 (14) | 6 (12) | 10 (14) | 8 (15) | 6 (15) |
| 3+ | 15 (10) | 4 (7) | 6 (9) | 8 (15) | 3 (7) |
| Mean cost (95% CI) | 89 (78 to 101), n = 161 | 87, n = 52 | 88, n = 69 | 106, n = 53 | 92, n = 41 |
| ED visits | |||||
| Number of times used, n (%) | |||||
| 1 | 190 (56) | 74 (70) | 71 (56) | 51 (67) | 54 (64) |
| 2 | 54 (16) | 21 (20) | 19 (15) | 15 (19) | 19 (22) |
| 3+ | 40 (28) | 11 (10) | 18 (14) | 11 (14) | 12 (14) |
| Mean cost (95% CI) | 196, n = 341 | 174, n = 106 | 199 (155 to 243), n = 108 | 160 (132 to 188), n = 77 | 230, n = 85 |
| Emergency hospital inpatient admissions | |||||
| Number of times used, n (%) | |||||
| 1 | 162 (69) | 69 (68) | 40 (65) | 62 (72) | 45 (58) |
| 2 | 41 (18) | 20 (20) | 13 (21) | 12 (14) | 20 (26) |
| 3+ | 31 (13) | 13 (12) | 9 (14) | 12 (14) | 12 (16) |
| Mean costb (95% CI) | 7200 (4332 to 10,068), n = 234 | 6309 (4419 to 8199), n = 102 | 10,473, n = 62 | 5920 (3649 to 8191), n = 86 | 6297 (4236 to 8358), n = 77 |
The relative costs of non-elective hospital admissions were high and appeared to account for the main component of the costs of unscheduled care, when compared with the use of other unscheduled care services. Having had at least one emergency admission almost entirely explained the variance in unscheduled care cost (r2 = 0.998). Having used either of the other elements of unscheduled care, ED or GP OOH services, accounted for relatively little of the variance in total unscheduled care costs. However, this may in part be due to an artefact of the data. There was concern that GPs were consistently notified by the admitting hospital only about emergency inpatient admissions. Information about use of ED services and OOH services may be less consistently reported to GPs. Combined with issues of patient recall, this could mean that the costs of other unscheduled care are underestimated to a greater degree than the costs of emergency inpatient admissions.
The overall use of unscheduled care appeared to be related to higher costs of total care. Table 23 indicates that the average combined cost of scheduled and unscheduled care over the two study years was 5–10 times higher for those study participants who used any unscheduled care than for those who did not. Half of the participants who used unscheduled care in year 1 also used unscheduled care in year 2 (232/462). Notably, nearly half the participants used no unscheduled care in either year (48.4%; 674/1394).
| Year of unscheduled care | Unscheduled care use, mean total cost (£) (95% CI) | |||
|---|---|---|---|---|
| Timing | No unscheduled care use (n = 674) | |||
| Used in year 1 only (n = 230) | Used in year 2 only (n = 258) | Used in both years (n = 232) | ||
| Year 1 | 4113 (3049 to 5177) | 1075 (907 to 1242) | 5927 (3283 to 8572) | 838 (716 to 961) |
| Year 2 | 1153 (953 to 1353) | 4243 (3269 to 5216) | 6212 (3540 to 8884) | 841 (715 to 967) |
The next section describes the results of ordinary least squares multivariate regression analyses to explore the association between participants’ demographic and clinical characteristics, and the total costs of scheduled, unscheduled and all care over the prospective year of the study (Table 24). Table 24 summarises the key results. The full results of the multivariate analyses are presented in Appendix 2, Tables 63–70. This analysis used the participant characteristics that were included in the analysis of non-elective admissions reported in Chapter 3. The total costs during the baseline year were used as a covariate rather than whether or not the participant had a non-elective admission in that year.
| Baseline characteristic | Care cost (£), β-coefficient (95% CI) | ||
|---|---|---|---|
| Scheduled | Unscheduled | Total | |
| Age (per 10 years) | 121 (33 to 210)* | 39 (–236 to 314) | 160 (–125 to 446) |
| Not working because of ill health (vs. working or not working for other reasons) | 715 (146 to 1283)* | –447 (–1830 to 936) | 268 (–1286 to 1822) |
| Distance to nearest hospital (per mile) | –76 (–170 to 18) | 218 (12 to 423)* | 142 (–89 to 372) |
| Maximum LTC severity (vs. mild) | |||
| Moderate | 122 (–135 to 378) | 641 (–76 to, 1358) | 763 (33 to 1493)* |
| Severe/very severe | 552 (184 to 921)* | 1012 (291 to 1732)* | 1564 (751 to 2377)* |
| HADS depression score of ≥ 8 (vs. < 8) | 187 (–98 to 472) | 888 (144 to 1632)* | 1075 (283 to 1868)* |
| Total cost baseline year (per £1) | 0.08 (0.02 to 0.14)* | 0.58 (0.21 to 0.94)* | 0.65 (0.33 to 0.97)* |
| Constant | –95 (–712 to 522) | –2120 (–4059 to –181)* | –2215 (–4331 to –100)* |
The presence of depression at baseline, maximum severity of LTC, and the total costs in the year before baseline are significantly associated with increased prospective unscheduled care costs and total costs. The values reported in Table 24 are the estimated impact on cost of a unit (or category) change. For example, participants with a HADS depression score of ≥ 8 used, on average, additional unscheduled care services to the value of £888 compared with those with a score of < 8. For every additional mile between a participant’s home and the nearest hospital, unscheduled care services costing, on average £218, were used. The analysis was re-run using a number of exemplar conditions rather than the presence or absence of each of the exemplar conditions. This showed similar results in terms of the characteristics that were statistically associated with costs in the prospective year and the size of the effects.
A further analysis used a two-part regression model to explore possible associations between participant characteristics, and the use of unscheduled care and the cost of unscheduled care. The first part of the model uses a multivariate logistic regression to assess the likelihood (OR) of using unscheduled care by participant baseline characteristics. The second part uses a linear regression to estimate the impact of baseline characteristics on the cost of unscheduled care, given the participant used unscheduled care. The characteristics included in the analyses are the same as those used for the exploratory analysis of associations reported above. Only the statistically significant associations are reported in Table 25. Appendix 2, Table 70, reports the full results of the model.
| Baseline characteristic | Unscheduled care | |
|---|---|---|
| Association with use, coefficient (95% CI) | Cost (£), β-coefficient (95% CI) | |
| Number of threatening life experiences (per experience) | 0.16 (0.07 to 0.26) | –79 (–686 to 528) |
| Maximum LTC severity (vs. mild) | ||
| Severe/very severe | –0.20 (–0.65 to 0.25) | 2743 (826 to 4660) |
| HADS depression score of ≥ 8 | 0.35 (–0.02 to 0.73) | 3694 (46 to 7342) |
| Constant | –1.42 (–2.45 to –0.40) | 212 (–6110 to 5687) |
The results in Table 25 indicate that the number of threatening life experiences, maximum severity of LTC and presence of depression are likely to be associated with use of unscheduled care. Conditional on the use of unscheduled care, the maximum severity of LTC and presence of depression are likely to be associated with increased costs of unscheduled care. However, there is no evidence that this was so for the number of threatening life experiences.
Discussion
The total health-care costs for patients with LTCs in the longitudinal cohort study we conducted were similar to the national average cost per person. Health-care spending per person in the UK was £2268 in 2012. 234 The average costs for each condition in this study were also comparable to the most recent published estimates of health-care costs in the UK.
A focused search of the literature in October 2014 found a limited number of robust cost studies that provided robust and up-to-date data that are relevant to the UK health-care system. The results of these are compared with the findings from our longitudinal prospective cohort study below.
The total direct health-care costs of CHD for the UK were reported as £3859M in 2004 prices, in a study that used a top-down analysis of total health-care service use attributed to CHD. 260 Applying an annual prevalence of CHD of 3.5%261 to the population of the UK in 2004 (59,950,364),267 the average cost per person is approximately £1839 in 2004 prices. This equates to approximately £2821 per person if the costs are inflated to 2011 prices,250 which is similar to that found in this study.
The total direct health-care costs of diabetes for the UK were reported as £9777M in 2010–11, in a study that used a synthesis of literature review and total recorded health service use attributed to diabetes. 265 This total cost was based on an annual prevalence of diabetes of 3,818,545 people (adults and children with type 1 or type 2 diabetes) in 2010–11. 265 This indicates an average cost of £2560 per person with diabetes in 2010–11 prices. Inflating these to 2011–12 prices250 gives a cost of approximately £2790, which, again, is similar to that of year 2 costs found in this study.
A study using registry data estimated the average cost per person of severe refractory asthma to be in the range of £2912 to £4217, in 2011 prices. 268 The costs of non-severe asthma in a smaller sample of 80 people were estimated at £1670 to £2788. 268
The costs of COPD in this study are comparable to those found in a recent study using a similar methodology, in a sample of 58,589 participants with COPD. 269 This estimated the cost of COPD (excluding medications) at £1806 per person in the year prior to baseline and £2108 in the year following baseline (2011 unit costs). The costs ranged from £1523 for patients with no exacerbations to £3396 for patients with two or more moderate to severe exacerbations.
Overall, the descriptive and multivariate analyses indicated that the costs of unscheduled care are an important component of total care, and account for around 50% of annual costs for people with asthma, CHD, COPD or diabetes. Although the costs are similar between the conditions included in this study, there was a high level of variation within conditions. This is highlighted by the substantially lower costs of those participants who did not use any unscheduled care over the 2 years of the study (year 1: mean £838, 95% CI £716 to £961; year 2: mean £841, 95% CI £715 to £967), compared with those who used unscheduled care in both years (year 1: mean £5927, 95% CI £3283 to £8572; year 2: mean £6212, 95% CI £3540 to £8884).
The multivariate analyses indicated that the presence of depression (i.e. a HADS depression score of ≥ 8) and the LTC being rated as severe or very severe were associated with higher costs of scheduled and unscheduled care. The analyses included a broad range of participants’ demographic and clinical characteristics, most of which were not statistically associated with the use or costs of unscheduled care or the costs of scheduled care. This may indicate the need for further work to identify whether or not there are other factors that it would be useful to measure/include in future research.
The results reported in Chapter 3 and the current chapter emphasise the key role that depression plays in the use of unscheduled care in people with LTCs. The results from this cost analysis also indicate increased costs of scheduled care for patients with LTCs who suffer from depression. The severity of patients’ LTC was important in the cost analyses, as were total previous costs. These findings are similar to the activity-based analyses in Chapter 3, where previous use of health care was a very important predictor. Severity of physical condition just failed to reach significance in the analyses in Chapter 3, but is clearly an important contributor to overall cost of health care in people with LTCs.
There was great variability in terms of cost of health care for patients with LTCs between the practices, with costs, on average, £1000 more per annum per patient for some practices than others. This was also true for individual patients, as those who used unscheduled care in either year of the study had significantly higher costs than those who did not. This suggests that a ‘one size fits all’-type intervention may not be applicable and that interventions to reduce the use of unscheduled care may need to be tailored to the practice and the patient.
Limitations
The response rate to the baseline questionnaire in the cohort study was 27%, and all the cost analyses and the analyses reported in Chapter 3 need to be viewed in this context. It may be that the patients who consumed most health care in the practices in the cohort study were those who would be excluded from participation by their GPs, because they were too ill. The costs may therefore underestimate the actual costs of people with LTCs in the practices. However, all the cost analyses used inverse probability sampling weights to reduce the impact of this selection bias.
The costs of ambulance services to transport participants to and from unscheduled and scheduled care were not included in this study, which will underestimate the total costs of care. These data are not typically recorded on GP notes and it was not felt that asking for detailed data about the use of ambulance services in the patient health survey would provide consistent data. The costs of medications were also excluded from the study, which will again underestimate total costs. Information about the prescription and use of medications is complex to collect and analyse – it was beyond the resources available for this study to collect robust and consistent data on these items for costing and analysis.
The relative costs of non-elective hospital admissions were high and appeared to account for the main component of the costs of unscheduled care, when compared with the use of other unscheduled care services. However, this may in part be due to an artefact of the data. There was concern that GPs were only consistently notified by the admitting hospital about emergency inpatient admissions. Information about use of ED services and OOH services may be less consistently reported to GPs. Combined with issues of patient recall, this could mean that the costs of other unscheduled care are underestimated to a greater degree than the costs of emergency inpatient admissions.
Conclusions
The costs of CHD, diabetes, asthma and COPD estimated in this cohort study are within the range of other published studies in the UK.
Overall, the descriptive and multivariate analyses indicated that the costs of unscheduled care are an important component of total care, and account for around 50% of annual costs for people with asthma, CHD, COPD or diabetes.
The costs of participants who did not use any unscheduled care over the 2 years of the study were substantially lower than for participants who used unscheduled care in both years.
The results from Chapter 3 and this analysis of costs indicated that the presence of depression (i.e. HADS depression score of ≥ 8) and whether the LTC was rated as severe or very severe were associated with higher use and costs of scheduled and unscheduled care.
Chapter 5 Use of unscheduled care: perspectives of patients and health-care practitioners (phase 2)
Abstract
Background
UK health policy seeks to reduce unscheduled care use in people with LTCs. It is not known how people choose between available health-care options in times of health crisis. We conducted a qualitative study to improve understanding of why patients use unscheduled care.
Methods
Semistructured interviews, with a subset of the longitudinal cohort study participants, and interviews with HCPs across primary and secondary care were conducted. Interviews were audio-recorded with consent, transcribed verbatim and analysed using a framework approach.
Results
Twenty-nine HCPs and 50 patients were interviewed. HCPs typically did not view unscheduled care use as problematic, describing it as inevitable for patients with LTCs. HCPs’ views on how to reduce unscheduled care use were influenced by their role in relation to unscheduled care policy. Apart from ED doctors, HCPs described tackling unscheduled care use by promoting patient behaviour change. Patients described a reluctance to use unscheduled care and a preference to access routine care where they had established relationships with HCPs. Where patients reported using unscheduled care, this was described as in response to urgent need. Patients’ health-care choices and judgements of need were influenced by previous experiences of HCPs and services. The theoretical concepts of candidacy and recursivity help to understand patients’ help-seeking choices.
Discussion
Both HCPs and patients recognised the need for unscheduled care as a last resort in LTCs, with patients highlighting how previous experiences shaped their choices. In order to address unscheduled care use, HCPs need to be aware of the different values patients attach to routine and unscheduled care services.
Overview
In this chapter we describe a qualitative study that we undertook in parallel with the longitudinal cohort study described in Chapter 3. The main aim of this study was to improve our understanding of why patients with LTCs use unscheduled care. To do this, we carried out detailed interviews with HCPs who were involved in the care of patients with LTCs, and we also interviewed a subset of patients who were participants in the longitudinal cohort study. The work has been written up and published elsewhere. 270
Our main objective from the original programme application was to identify personal reasons for use of unscheduled care including barriers to access for routine care, patients’ motivations, expectations and decision-making processes, and influences from families and relevant health-care providers.
Background
The health-care practitioners’ perspective
General practitioners and other HCPs working in community settings are tasked with implementing policies, including reduction of unscheduled care use by patients with LTCs. 271 At the time of this programme of research, the UK primary care Quality and Productivity indicators (as part of the GP contract) provided financial incentives to general practices to implement strategies to reduce the use of unscheduled care. 271
A number of different HCPs are involved in the provision of unscheduled care for patients with LTCs, including GPs, practice nurses (PNs), district nurses (DNs) and ED clinicians (Table 26). More recently, other roles have been created in response to a policy shift towards case management for people with LTCs. 21,272 These include ACMs, with nurse or allied HCP backgrounds, introduced to case-manage patients with complex problems, with the explicit aims of reducing emergency admissions through improved routine care and self-management. 9,271 Similarly, specialist nurses (SNs) in both primary and secondary care are seen as having a key role in the management of people with LTCs and preventing unscheduled care use. 272,273 The introduction of the new General Medical Services contract in 2004 allowed GPs to opt out of the responsibility for 24-hour care, leading to OOH doctors making more management decisions for patients seen outside routine hours. 274
| HCP | Scheduled or unscheduled care | Care setting |
|---|---|---|
| GP | Both | Primary care |
| OOH GP | Unscheduled care | Primary care |
| PN | Scheduled care | Primary care |
| DN | Scheduled care | Community care |
| ED doctor | Unscheduled care | Hospital |
| ACM | Scheduled care | Community care |
| SN | Scheduled care | Hospital/community care |
Previous research has explored HCPs’ views on the appropriateness of patients’ use of unscheduled care, or their perceptions of why patients use unscheduled care. 196,210,225,275 However, it is not known what HCPs believe their roles and responsibilities to be in reducing unscheduled care use. As its first aim, this chapter reports the results of a qualitative study which explored HCPs’ perceptions of the reasons why patients with LTCs use unscheduled care, and the HCPs’ understanding of their own role in relation to reducing use of unscheduled care.
The patient perspective
Health policy implicitly adopts a ‘deficit’ model of patients, asserting that patients require education in order to make effective choices, but this assumption has not been based on clear evidence about how patients with LTCs choose from available health-care options in response to a health crisis. Our review of qualitative studies of health-care use in patients with LTCs, which we summarised in Chapter 2, found that patients’ use of unscheduled care was influenced by their previous experiences of health-care services, and reflected the values patients attributed to the different services. 185 This review suggests that, by focusing on patient education, policy may misinterpret how patients choose between health-care services. However, a limitation of this review was that few papers addressed unscheduled care use by patients directly. Moreover, none asked about instances where patients chose to avoid unscheduled care. As our second aim in this chapter, we elaborate on the processes by which patients with LTCs choose between available options for care in response to a health crisis, to inform the development of future policy and guidance on modifying unscheduled care use. Crucially, we explored with patients their instances of unscheduled care use and instances of avoiding unscheduled care use.
Aims
-
To explore HCPs’ perspectives and understanding of why patients with LTCs use unscheduled care, and their own role in reducing use of unscheduled care.
-
To explore patients’ choices in using or avoiding use of unscheduled care, and how they choose between different services.
Methods
Context
The study was set in north-west England, embedded within the CHOICE programme. Qualitative methods were used: semistructured interviews with patients and HCPs.
Research Ethics Committee approval
The study was approved by NHS REC 09/H1013/81 and local research and development.
Recruitment
Health-care practitioners
Health-care practitioners from different professions and varying responsibilities in unscheduled care were given the opportunity to participate in the study. All HCPs were identified through either purposive sampling or recruitment through local networks (Table 27 and Figure 19). Information sheets were given to prospective participants, and informed written consent was obtained prior to commencing interviews. Although some participants were recruited through local networks, interviewers only interviewed participants with whom they had no prior relationship.
| Profession | Recruitment method |
|---|---|
| GP | Purposive: identified from practices already involved in the CHOICE programme |
| PN | Purposive: identified from practices already involved in the CHOICE programme |
| OOH GP | Purposive: identified through local OOH provider |
| ED doctor | Purposive: identified from three local hospitals |
| ACM | Purposive: identified from contacting local networks |
| SN | Purposive: identified from local networks |
| DN | Purposive: identified from contacting local networks |
FIGURE 19.
The Consolidated Standards of Reporting Trials (CONSORT) flow diagram demonstrating sampling and recruitment of patients and practitioners.
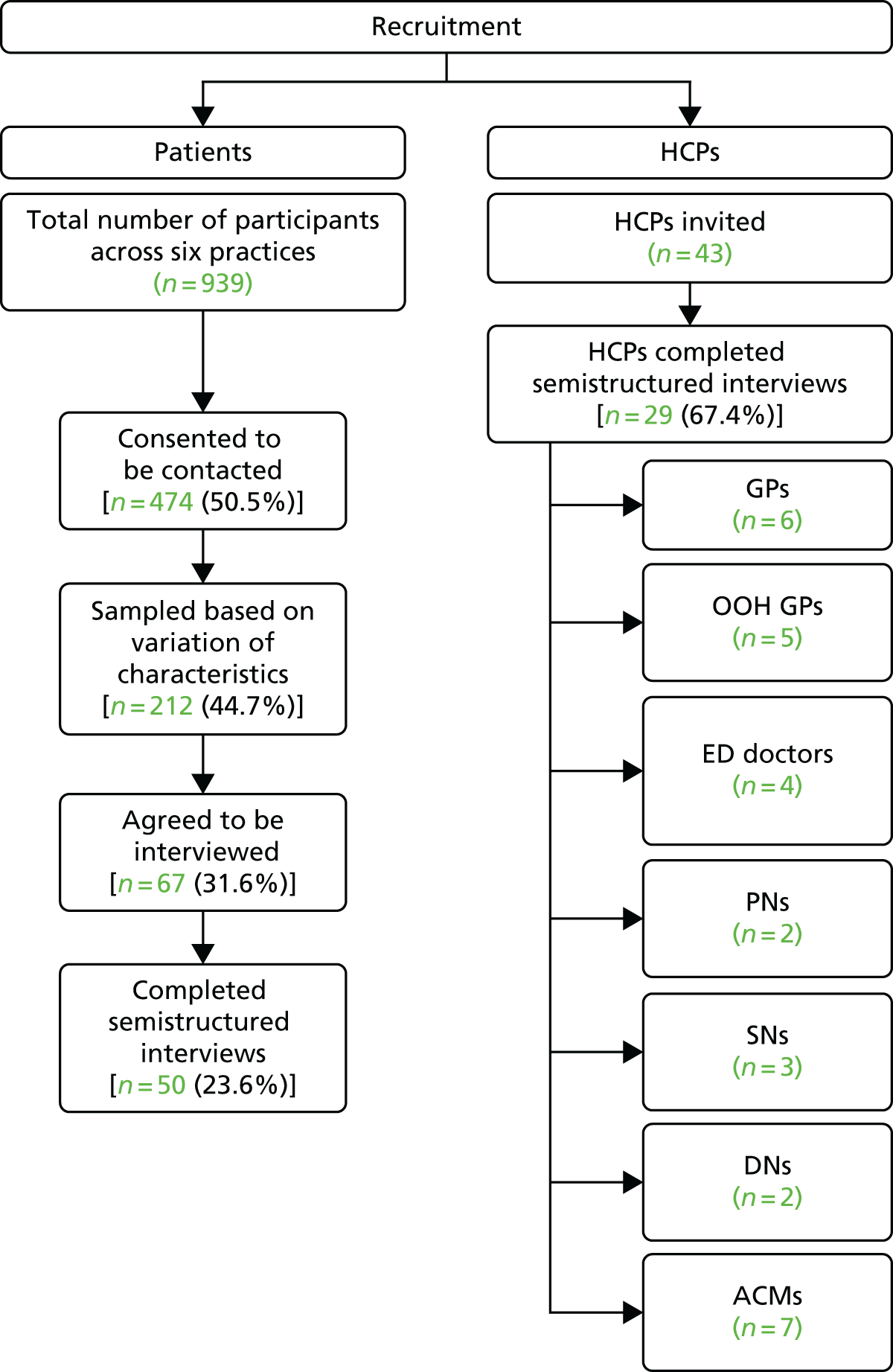
Patients
The sample of patients for this study was drawn from patients who had agreed to participate in the large, longitudinal cohort study to identify predictors of unscheduled care use, which was described in Chapter 3. The target patient population were patients aged ≥ 18 years with one or more of the four exemplar LTCs:
-
COPD
-
CHD
-
asthma
-
diabetes.
Six out of the 10 general practices involved in the cohort study were used for the qualitative study. Patients were identified using the QOF registers of general practices and invited to take part in the cohort study (see Chapter 3). The Consolidated Standards of Reporting Trials (CONSORT) flow diagram (see Figure 19) demonstrates that, of 939 patients across six different general practices who returned questionnaires for the cohort study, 474 (50%) consented to be contacted for interviews. From these consenting patients, the team strategically sampled 212 individuals on age, sex, type and number of LTCs, and different levels of self-reported use of routine primary care and unscheduled care use. The purpose was to achieve a variation of characteristics and to minimise sampling bias. Invitation packs including a patient information sheet (PIS), a reply slip, and a stamped addressed envelope were sent out to this sample of 212 individuals. A total of 67 participants agreed to be interviewed by returning a reply slip or calling the researchers directly; recruitment ceased when category saturation had been achieved, with the final sample comprising 50 patients. Informed written consent was obtained from all participants prior to commencing interviews. Patients were not known to the interviewers prior to the interview, and it was made clear on information sheets and in person what the study was about and that the interviewers were non-clinical and not involved in their health care in any way.
Data collection
Semistructured interviews with participants were conducted by the members of the research team (SL, CH and JD). The researchers were flexible to meet the demands and constraints of both the patients and HCPs, and conducted the interviews when and where it was most convenient for the participant. The HCP interviews were usually conducted at their place of work and lasted between 19 and 93 minutes (mean 44 minutes).
Both topic guides (see Appendix 3, Boxes 5 and 6) were developed from the literature and the results of the qualitative review (see Chapter 2) and through discussion in the research team (which included health-care practitioners). The interviewers familiarised themselves with guides prior to commencing interviews. HCPs were asked about their role in relation to patients with LTCs, their perceptions of reasons why patients use unscheduled care, and their role in managing patients’ use of unscheduled care.
For patient participants, interviews were conducted in each patient’s home and lasted between 30 and 90 minutes (mean 46 minutes). The interview began with a discussion of the patient’s health and social circumstances, then explored attitudes to, and expectations and specific experiences of, unscheduled care, primary care and other health-care and community services. During interviews, patients were guided to reflect on specific instances of using unscheduled care, the circumstances surrounding these and the factors that influenced their choice to use unscheduled care. In addition, patients were also asked to reflect on times when they decided not to use unscheduled care services, and on what influenced their decisions not to use unscheduled care services. As interviews progressed, the research team iteratively refined the topic guides through discussion of emerging themes and sharing field notes reflecting on the interviews.
All interviews were digitally audio-recorded and transcribed verbatim.
Analysis
Analysis for all interviews used the framework approach. 276 Through an inductive and iterative process, a thematic framework within each data set (patients, HCPs) was developed, and refined through constant comparison of data between and within accounts in that data set. Owing to the large sample size, each transcript was coded in depth in QSR-NVivo8 (QSR International, Warrington, UK), using this tool to develop, test and refine the thematic framework. Themes and transcripts were regularly discussed within a multidisciplinary research team, members of which had different professional backgrounds including primary care, psychology, social anthropology and psychiatry. Data collection continued until theoretical saturation was reached.
Results
Participant characteristics
Twenty-nine HCPs (Table 28) and 50 patients (Table 29) took part in a semistructured interview.
| Profession | Number of HCPs (% of total) |
|---|---|
| GP | 6 (21) |
| OOH GP | 5 (17) |
| ED doctor | 4 (14) |
| PN | 2 (7) |
| SN | 3 (10) |
| DN | 2 (7) |
| ACM | 7 (24) |
| Patient characteristics | Number of participants (% of total) |
|---|---|
| Sex | |
| Male | 26 (52) |
| Female | 24 (48) |
| LTC | |
| CHD | 5 (10) |
| Asthma | 10 (20) |
| COPD | 2 (4) |
| Diabetes | 9 (18) |
| More than one of above LTCs | 24 (48) |
| Ethnicity | |
| White British | 42 (84) |
| Black or black British-Caribbean | 3 (6) |
| Black or black British-African | 2 (4) |
| White Irish | 1 (2) |
| Other white background | 1 (2) |
| Mixed white and black Caribbean | 1 (2) |
| Use of unscheduled care in last 12 months (from self-reported on questionnaire) | |
| None | 15 (30) |
| One or two times | 23 (46) |
| Three times or more | 12 (24) |
Findings
Findings will be presented from HCPs’ and patients’ perspectives separately, and their implications discussed together. Illustrative data are given, with brief identifiers to help contextualise the data. For HCPs, the identifiers include a short acronym denoting professional status (see Table 28) and a number. For patients, this includes a number followed by a short demographic summary of the patient’s age, sex and LTC(s). Ellipses (. . .) signify omitted text. Square brackets denote explanatory text.
Health-care practitioners’ perspectives
Use of unscheduled care is understandable and necessary
All HCPs described unscheduled care use as an understandable and necessary part of care for patients with LTCs, and recognised exacerbations as a normal part of the disease process; for example, describing exacerbations as expected yet unpredictable:
People with chronic diseases it’s episodic isn’t it? So somebody might phone with an exacerbation of their COPD but the next one might be 8 months away and the next one 3 months after that.
HCP 10 GP OOH
General practitioners, PNs, and DNs discussed ways of managing exacerbations within primary care, but they recognised the limitations of their service, and the necessity of ED services for severe exacerbations:
He’d [COPD patient] been to hospital loads of times, but that’s not so much because he doesn’t know his disease I suspect, it’s ‘cause he’s just at the severe end and he’d end up at hospital anyway, but as I say, we started the treatment just to sort of, optimise the chances of not going in [hospital]. But there’s still a reasonable chance that he will go in.
HCP 17 GP OOH
Although some HCPs acknowledged a link between anxiety and breathlessness in a few individual patients with COPD, they attributed unscheduled care use overwhelmingly to clinical need and not to psychological or social factors. All ED doctors were explicit in describing unscheduled care use by patients with LTCs as legitimate:
The traditional definition of emergency care would be that it’s to provide care for any person who believes that they are suffering from an emergency condition, which requires either urgent investigation, or urgent treatment. So it’s defined by the patient.
HCP 11 ED doctor
They justified this view by referencing policies and protocols within emergency services that emphasise patient safety:
Our general policy, except with a few very minor exceptions, is that we do see everybody who comes and if you don’t do that, and turn them away, unless you turn them away to something that’s appropriate and timely, you can make mistakes.
HCP 23 ED doctor
General practitioners working in OOH services described this setting as inherently more risky than in-hours GP work because they lacked prior knowledge about individual patients. This affected their decision-making, as they could not draw on an understanding of a patient’s typical behaviour and they described prioritising patient safety:
I think that somebody who’s a diabetic or COPD or asthmatic who presents acutely (. . .) you don’t really have much information about them and you have got somebody who’s acutely unwell and oftentimes the safest thing to do will be to send them to hospital.
HCP 29 GP OOH
Unlike ED doctors and HCPs involved in providing routine primary care, ACMs and SNs were performance-managed against the policy aim to reduce unscheduled care use, and they discussed unscheduled care use as something that they were responsible for tackling. However, many described a tension between this policy aim and their beliefs about how best to support and manage patients. One ACM referred to the divergence between patient feedback, suggesting she was ‘doing a good job’, and perceived pressure to meet targets to reduce unscheduled care use:
You [ACM] felt you knew you were sort of doing a good job from the feedback that you got from the patient, but you never really felt like that when it came from your sort of performance. I mean you look at performance management, the difficulty with performance management is it doesn’t really capture, it doesn’t always capture good outcomes for patients. It captures good outcomes for, I suppose, finance. Well it can, it’s a difficult one because some of the, a lot of our stuff (. . .) is about prevention. Um it is difficult to capture prevention in the short term, isn’t it?
HCP 26 ACM
One SN reported spending time with patients to reduce unscheduled care use, but disclosed concerns that patients may become dependent on this service:
We do have a cohort of patients like that who will phone up purely for us to go out just to reassure them that they are fine (. . .) They will be phoning us on a Friday afternoon for that reassurance, ‘cos they know that we’re not here Saturday, Sunday. And a lot of that is anxiety-related, and maybe we perpetuate that because we go out and we say ‘Look, you’re fine, we’ve done all your obs [observations], you are fine. We have no concerns about you’. But if we didn’t do that, they would have gone to A&E and we might have stopped that visit over the weekend. So, I don’t know if we’re perpetuating it, or we’re avoiding other things happening, I don’t know.
HCP 13 SN, specialist in COPD
Other ACMs and SNs described the difficulty of managing patients with multiple problems in a highly complex health-care system, and suggested that avoiding unscheduled care was not always possible despite HCPs’ best efforts. An example of this complexity is illustrated below; this ACM described intensive case management being undermined by the OOH service’s response to exacerbations:
I see [patient with end-stage COPD] probably two, three times a week. I’ve done as much as I possibly can, the GP’s very good, we’ve had about probably three family conferences (. . .) the out-of-hours doctors who don’t really know her (. . .) go in, see her and think ‘Oh my God, this lady’s really poorly, this should be done, that should be done’, even though it’s an ongoing thing.
HCP 21 ACM
In summary, all HCPs framed patients’ unscheduled care use as understandable in terms of the clinical course of LTCs. However, reducing patients’ unscheduled care use was described as a problem by some HCPs, reflecting their role in relation to unscheduled care policy. At one extreme, ED doctors described their role as treating patients safely, with no pressure to reduce unscheduled care use. At the other extreme, ACMs described a conflict between needing to reduce unscheduled care use because of performance management targets and providing what they perceived to be good clinical management.
Approaches to reducing the use of unscheduled care
Health-care practitioners discussed different approaches to reducing unscheduled care use, again reflecting their roles within the health system. Three main approaches were ‘optimising the system’, ‘negotiating the system’ and ‘optimising the patient’.
GPs framed the problem of unscheduled care use in the context of the wider health-care system. They recognised that patients may need clinical care urgently, but discussed opportunities to reduce unscheduled care use by directing patients to other settings. They described potential system-level solutions, such as improved communication between primary and secondary care, in order to identify patients who were not using the system ‘correctly’:
Until I’m convinced that we’re getting all the information from casualty about who’s been, ‘cos although they seem to send us letters I’m not 100% sure that we’re getting all of them, until they make some clinical sense, because there’s absolute rubbish written on most of them and you don’t know which bit of bone they fractured or, really, was that serious or was it not. In fact the only way you can tell it was serious is they kept somebody in and then it’s probably not serious either.
HCP 7 GP
Similarly, this GP suggested improving triage mechanisms so that patients could be helped to understand which service to use for which problem:
Up until now I don’t think there’s been enough kind of triaging really (. . .) Some people are turning up [to ED] when in fact if they could just speak to somebody they might have had a different outcome.
HCP 12 GP
At the extreme, two GPs suggested fee-for-service as a system change that would discourage unscheduled care use:
At the end of the day it’s [ED and OOH services] free so why not [charge patients for attendance]? (. . .) Just makes you [the patient] think a little bit more about whether you really need some unscheduled care or not.
HCP 10 GP OOH
PNs, ACMs and SNs did not discuss trying to influence the way the wider health-care system worked. Frustration was common in their accounts, as they described a lack of clinical autonomy and professional power despite their responsibilities for addressing unscheduled care use. For example, they were not directly linked to the formal information flow about patients, to the extent that they might not know about patients in their care using unscheduled care, compromising their potential to respond:
No, often I don’t get to find out [if a patient has used unscheduled care] (. . .) The GPs would have a look at it [discharge summary], action it and then, you know, file it in the patient’s notes. (. . .) The GPs might ask them to come and see me for a review.
HCP 16 PN
This PN described advising patients about symptom control and about avoiding unscheduled care use, but she was not confident that this would be supported by other team members:
From my personal point of view I find it quite frustrating I suppose, in a way, that I’m probably about the only person within the practice that has a keen interest in respiratory disease. And sometimes it’s a bit of an uphill, an uphill battle. My barriers with clinicians sometimes are the patients are coming in being prescribed antibiotics, having numerous chest infections but they’re not then feeding them into me.
HCP 18 PN
To overcome their lack of formal influence, some PNs, ACMs and SNs described negotiating informal pathways. For example, one ACM used her informal links with secondary care to get information about patients:
You only know if the family ring up you know, the next day and say, oh by the way, they’ve gone in, or sometimes we’ll not know for days, that the patient has gone in (. . .) We just literally don’t know. It’s just by sheer luck (. . .) So, as soon as we find out, to improve communication, we go down, we go on to the wards, we speak to the staff, we write our details in their notes, please contact us, our phone number. Sometimes it works, other times it doesn’t.
HCP 21 ACM
A PN described making herself more accessible to patients so that they would contact her in a crisis rather than using unscheduled care services:
I just gave him [the patient] a bit of an open door, if he’d turn up at reception, which he’s done a couple of times, I need to see [PN] (. . .) Then often I’d squeeze him in and see him (. . .) I’ll make this appointment and come back and see me then but, in the meantime, you’ve got my phone number. And I speak to him on the phone.
HCP 16 PN
GPs, PNs, ACMs and SNs described changing individual patients’ behaviour as a key mechanism to reduce unscheduled care use, suggesting that they might teach patients to control their symptoms better. They described this approach as ‘self-management’:
Well of course you can make a difference, I see my role as instructing people, training them and trying to help them self-manage themselves, and part of self-management is when to seek advice, um, when they’re unwell (. . .) And, you know, I think if you can drill certain responses into people then, um, you know, eventually they will learn.
HCP 7 GP
Most HCPs who discussed optimising patients’ behaviour in this way described using educational strategies, predominantly giving information, rather than using behaviour change strategies:
Education, education, education. (. . .) Patient education for all um chronic diseases I think is so badly dealt with. (. . .) So these are why we manage you [to the patient], this is how we manage you, this is why we’re monitoring you. Um these are why we do your track, err your checks, your annual reviews, to check to your lung function to see if it’s deteriorating, to make sure you’re taking your medication properly because if you’re taking it properly, it’s gonna reduce the risk of infections.
HCP 16 PN
All GPs, PNs, ACMs and SNs discussed how their knowledge of individual patients was a way to understand where to focus efforts to optimise the patient, and thus reduce unscheduled care use. They described this knowledge as building over time as part of an ongoing HCP–patient relationship:
I think it’s just getting that they’re [the patient] confident in you and your ability and that you’ve got some sort of rapport going with a patient. And sometimes that does take quite a long time to build up that sort of rapport that you’ve got with a patient so that they trust you really, in a way. So to make changes it isn’t always easy but it’s something that you develop. And this is why I say nothing ever happens quickly, you’ve got to build that patient and get to know that patient and have that rapport.
HCP 18 PN
With PNs and GPs, this relationship could be sustained over time, but for ACMs, the time-limited nature of their interventions created an additional tension when working with patients:
So usually it’s [ACM service] about a twelve week period of interventions, so where people are referred to us, we go away and we work with them and just see what we can do to kind of improve the situation. (. . .) There’s a lot of people, even though you’re preventing hospital admissions and GP input, they phone you a lot, you know, and you can’t really discharge those patients.
HCP 4 ACM
Summary
-
HCPs do not generally see unscheduled care use as a problem, describing it as inevitable for patients with LTCs.
-
Attempting to reduce patients’ unscheduled care use was described as problematic by some HCPs, reflecting their role in relation to unscheduled care policy.
-
HCPs approached reducing unscheduled care use differently depending on their role. ED doctors did not see it as their role to reduce unscheduled care use. GPs described optimising the system. PNs, ACMs and SNs described negotiating the system. Apart from ED doctors, HCPs described attempting to optimise the patient through behaviour change.
Patient perspectives
Unscheduled care use framed as unavoidable
Patients consistently described reluctance to use unscheduled care services; this reluctance was expressed as a desire not to feel a ‘burden’ on services:
I’d prefer not to be a nuisance, you know, and I’ll phone them [hospital staff] up and take advice, but I’d sooner not go round and bother people.
P23, male, aged 53 years, with asthma
Hospital EDs were seen as a ‘last resort’, a service to be accessed only when other options were exhausted:
I kind of think that hospital is the last resort where you’d, where you’ve been through the doctor, or whatever and that’s where you end up when you’ve got to have something done that the GP can’t do.
P09, female, aged 62 years, with CHD and diabetes
Patients recognised that need for help had to be unequivocally serious to justify using unscheduled care. Consistent with this, patients who used unscheduled care described doing so as unavoidable, using language such as ‘had to’, ‘got to go’, ‘I just knew’ or ‘I needed it’. There was no evidence of deliberation or uncertainty:
It’s not something, it’s not something you think about. I just knew I needed an ambulance there and then, I needed it as soon as possible.
P10, male, aged 64 years, with CHD
Likewise, when patients talked about instances when they chose not to use unscheduled care, they explained that their need was insufficiently urgent to require it, choosing to wait and attend primary care instead:
If it’s something that I consider is minor (. . .) like with getting certain aches and pains [in] my tummy like I have been having or something connected with diabetes, I know I can get it sorted in the proper hours rather than out of hours, you know (. . .). Besides that, I think I’ve got in the back of my mind ‘I’m not getting everyone up for me to go to hospital (. . .) when I can sort it out tomorrow’ type of thing.
P33, male, aged 61 years, with CHD and diabetes
Previous experiences shaped future unscheduled care use
Patients described how previous experiences of health crises and of health-care services shaped their judgements about needing unscheduled care and their decisions about which unscheduled care service was most appropriate. The key aspects of previous experience were prior negotiation of urgency with family or friends, or with health-care practitioners in primary or specialist care; the technological expertise of different health-care services; and the accessibility of services.
Patients’ understanding of what constitutes urgent need (and thereby justifies unscheduled care use) was based on previous experiences of exacerbations and the responses of family and friends and health-care services at those times. These experiences then guided patients’ future choices of when to access unscheduled care and of which unscheduled care service to use.
Some patients talked about other people as the key decision-makers in their use of unscheduled care. These were often family or friends, but there were instances of health-care practitioners fulfilling this role:
I said ‘oh I’m not bad’. Anyhow I was going worse, obviously, and I couldn’t get my breath and you know, I tried to get up and I felt really ill. And um, [my nephew] said ‘I’m sorry [aunt], but I’m going to have to get an ambulance.
P25, female, aged 80 years, with diabetes and COPD
The GPs have said that to me, ‘don’t come, don’t come here [to the GP surgery]. If you’ve got something, if it’s your chest, go to hospital, because I’m just gonna send you to hospital anyway.
P02, male, aged 57 years, with CHD and asthma
In these circumstances, the patient was not making the judgement to use unscheduled care alone: this decision was sanctioned or made by another, trusted, decision-maker.
Judgements of urgency emerging from previous encounters with health-care providers were then applied in future instances of help-seeking. Box 1 illustrates how practitioners reinforced one patient’s concerns about his health. A specialist judged his initial choice of primary care to be inappropriate, and the patient inferred that he should access hospital emergency services in future. The care from health-care practitioners at hospital thus established a pattern that favoured future use of unscheduled care.
This patient described how, before knowing he had a heart condition, he experienced palpitations. He chose to attend primary care, and his GP referred him to hospital. During the time between the GP’s referral and the hospital appointment, he experienced pains between his shoulder blades and saw the GP again. The GP explained he might be having a heart attack. He was immediately directed to hospital, where he saw a cardiac surgeon. The surgeon insisted that he should have attended hospital earlier:
[The surgeon] was quite, you know, explicit, but he was being, he was being genuine about the way he felt. From all the angiogram and the tests I’d had, he couldn’t understand how I was, how the blood was getting through at all (. . .) so he said, ‘I, I’d have expected you to be dead by now.
The cardiologist and the staff at hospital reinforced the importance of attending as soon as possible, and, since this incident a decade ago (which resulted in a bypass), the patient felt that ‘as far as my heart’s concerned, there never is any hesitation any more’:
You realise that the support is there and you must use it to put your mind at rest because there’s nothing worse than something festering and you sit here and you worry about it and you think about it, when you know for a fact that the support’s there, so don’t hesitate, just [go to hospital], that’s what the people [at the hospital are there for].
An episode in the 6 months prior to interview illustrated this point. He experienced palpitations, which he described as ‘quite concerning. It wasn’t necessarily painful, but because of this pounding in my chest I, I was a bit concerned about it’. He called an ambulance immediately:
Because of the previous heart [problems], I know it was 10, 11 years ago, but, I get very anxious when things start to happen with my heart and I like to get it seen to straightaway.
Patients differentiated between routine primary care and unscheduled care services according to what they offered. Patients valued routine primary care as a source of personal relationships with practitioners:
I generally stick to one [GP] because he like gets to know your background and all your history and everything else, you know (. . .) but sometimes, like I said to you I just think what else can they do for me?
P27, female, aged 54 years, with asthma and COPD
Conversely, they valued unscheduled care services for their technological expertise, perceiving this to be unavailable in primary care:
They won’t do X-ray there [at the GP surgery], they won’t do, they’ll give you tablets. If I go to A&E they get everything there, everything to take blood, to take wee [urine], and then it’s sort me out there.
P07, female, aged 44 years, with diabetes
At times of urgent need, patients preferentially sought technological expertise. This often resulted in using EDs, but a few patients valued – and used – other services because of their perceived technological, and often disease-specific, expertise, as established in prior instances of help-seeking:
[If] you were getting really bad, um what do you think’s the first thing you would do?
Um I’d probably phone [diabetic nurse at hospital] (. . .) just because I know she knows how to advise me on the [insulin] pump (. . .) That’d probably be first point of call.
P11, female, aged 39 years, with diabetes
Previous experiences of services established this belief that routine primary care was not the best site for disease-specific care:
My GP is a wonderful GP, but he’s not geared to look after diabetics (. . .) The GP’s a general practitioner, he knows an awful lot about a lot of things, but the diabetic clinic are specialists for that disease.
P44, female, aged 54 years, with diabetes
Conversely, experience of services that were responsive and technologically capable informed future help-seeking, as illustrated by Box 2. This patient’s prior experience of a severe exacerbation, and the safety afforded by the hospital’s equipment and facilities, ensured his choice of this service in future.
Several years ago, this patient experienced a severe episode of asthma, where he was taken to the hospital and admitted for over a week. The experience of this severe episode meant that the patient saw his asthma as potentially ‘life-threatening’ and himself as being ‘given a second chance’ to look after himself. He praised the care in the hospital during this episode as being immediately responsive and without fault, and his experiences of hospital services since that episode had reinforced this praise. His belief in the hospital’s technological expertise even extended to being treated in the ED without being admitted:
I mean I’ve spent, on one or two occasions when, not for a long time, er, when I’ve had, er, felt an attack coming on, I’ve probably spent seven hours on a trolley in a cubicle. But I’m quite happy to do that because I know it’s not where you are, as regards being in a cubicle, it’s where you are as regards being in a hospital. You would still get the same treatment in the cubicle as you would on a ward.
He reflected that he would now rely on the ED of the hospital if he experienced another asthma exacerbation in the future:
If [the hospital staff] know you’re having any sort of attack or symptoms related to your asthma, they, they are good. I think they realise that it is asthma and it’s an attack coming on and they can get you in there quick. Whereas if you go to a doctor and he starts having, even though a doctor is qualified to know that it’s an asthma attack, they probably haven’t got the equipment and the facilities to, to bring you round if anything should happen very quickly. Where in hospital they’ve got everything there, they’ve got the ventilators, the drips, they’ve got everything, they can resuscitate you, if need be (. . .) I feel safe going in a hospital.
He contrasted his certainty that the hospital was equipped to look after him when he suffered from asthma exacerbations with his experience of primary care as lacking in the expertise to recognise and respond to asthma exacerbations as a potential emergency:
You seem to get rebuffed every time you go [to the general practice]. They [general practice] don’t seem to think that it [asthma] is a priority.
In recent years, several services similar to routine primary care have been established in the UK to meet increasing demand, including walk-in centres and OOH primary care providers. Patients only rarely talked about using these services. When patients did mention them, it was as less preferred and often ineffective alternatives that lacked both the technological expertise offered by hospitals and specialist clinics and the trusted relationships offered by routine primary care:
[Walk-in centre] don’t do nothing to you, you just walk in and they look at you and they say go to your doctor, everything like that.
P17, male, aged 77 years, with CHD and COPD
We go to A&E or I go and see my GP. It’s very rare I use the emergency doctor (. . .) Because, again, the emergency doctors, ‘cos they’re restricted to what they can do as well, a lot of them’ll say to you ‘Well, you know, go to A&E’, because I have a bad heart and I’m diabetic and everything else.
P45, female, aged 41 years, with CHD, diabetes and COPD
Patients experienced numerous barriers to unscheduled access to primary care at their general practices. Barriers described were mainly organisational, including limited opening hours, poor or delayed availability of named practitioners, gate-keeping practices by reception staff, and restrictive appointment systems:
Sometimes I don’t have the money to go up to see my doctors, and to see my doctor you have to be there at, like, 8 o’clock, half past eight because there’s a queue (. . .) It doesn’t open on 9 o’clock but there could be (. . .) 15 people stood outside waiting to go in to see [the doctor].
P40, male, aged 57 years, with COPD
Some patients, like P40, found travelling to primary care practices difficult because of a combination of ill health, inability to afford taxis and poor public transport. When patients talked about walk-in centres and OOH primary care providers, they were described as more accessible than routine primary care, as the barriers around appointment systems and travel tended to be reduced:
Very, very rare have I phoned up the doctor and been able to get in, you know what I mean, like, you know, to see my GP within 2 or 3 days. It’s nearly always next week, or the week after or whatever, so you need the err, you need the out-of-hours doctors really to help you out for them situations.
P24, male, aged 59 years, with asthma
Out-of-hours doctors who could perform home visits, and walk-in centres based in central locations with good transport links (in city centres or at hospitals) reduced the resources required for access:
The [out-of-hours service have] come out and seen me [at home].
P23, female, aged 53 years, with asthma
However, although some patients described these services as accessible, we saw above that patients thought them unable to meet their needs. The hospital ED, by contrast, was seen as both readily accessible and providing technological expertise:
[At the hospital ED] I always get seen to straightaway, no matter what (. . .) Once when I’m there, I know I’m alright, because I know they can pinpoint what it is and what’s doing it.
P02, male, aged 57 years, with CHD and asthma
The accessibility of a service therefore influenced patients’ use of health care both in the event of non-urgent need and in the event of urgent need. Routine primary care was seen as least accessible, requiring the most effort to use, whereas the hospital ED was the most accessible, with the additional benefit of readily available technological expertise.
Summary
-
Patients described a reluctance to use unscheduled care, preferring to wait until they could see routine providers with whom they had established relationships.
-
When patients reported using unscheduled care, they described feeling that they had no alternative in the face of urgent need.
-
Patients’ judgements that they needed unscheduled care, and their choice of unscheduled care provider, were influenced by previous experiences of health-care services and practitioners.
-
Family and carers play an important role in determining when patients access unscheduled care.
Discussion
Owing to the high financial costs of unscheduled care services and the increasing burden of LTCs, reducing unscheduled care use by patients with LTCs is a major policy priority across all levels of the UK health system and internationally. 270,277,278 This chapter reports the first study to explore the perceptions of a range of HCPs and patients about unscheduled care use in people with LTCs.
Across our sample of different HCPs involved in the provision, and reduction of unscheduled care use for patients with LTCs, unscheduled care use was viewed as a necessary component of care, with exacerbations recognised as inevitable in patients with LTCs. Thus, ED doctors did not see it as their role to reduce patients’ unscheduled care use. However, some HCPs saw unscheduled care use as a failure to meet policy targets, on which they were performance managed. Thus, GPs discussed the need to optimise the system to direct patients to more appropriate services, and PNs, ACMs and SNs, driven by policy targets to reduce unscheduled care use, described mitigating their relative lack of power and influence by negotiating the system. Most HCPs also described maximising their resources by using consultations with patients to attempt to enact behaviour change and reduce unscheduled care use. However, they only described information giving, and there is little evidence that this is effective in changing behaviour. Therefore, approaches to reducing unscheduled care use depended on the HCP’s role.
Consistent with HCPs’ views, patients viewed unscheduled care services as one of several health-care options available to them, complementing routine care, and using unscheduled care was not necessarily seen as a failure of self-management or routine care. 185 Patients drew on previous experiences of services and practitioners when choosing how to respond to illness exacerbations. The choice of unscheduled care compared with routine primary care was shaped by patients’ perceptions of urgency, which were in turn influenced by previous responses from health-care practitioners,26 and by involvement of friends or family. Choosing between different unscheduled care providers was also shaped by perceptions of those services, formed by previous experiences of their accessibility and technological expertise. These findings, from patients’ detailed accounts of specific instances when they decided to use or avoid unscheduled care, therefore confirm and expand on previous qualitative evidence from studies in which patients were asked about their use of unscheduled care in more general terms. 185
Two theoretical concepts from the health-care access literature, hitherto unapplied to the problem of unscheduled care use in people with LTCs, provide an interpretive framework for understanding how patients make choices between health-care services. 279,280 The first, ‘candidacy’, describes how access to health care is framed as often requiring work for patients to achieve, and eligibility to access care is continuously negotiated in patient–practitioner interactions. 279 Developed from interpretive synthesis of literature on access to health care in socioeconomically disadvantaged groups,279 the concept has been applied to health-care use in other vulnerable populations. 281,282 The second concept, ‘recursivity’, describes how future demand for services, and the process of help-seeking, is determined by a patient’s previous experiences. 280
The establishment of candidacy was evident in patients’ accounts of interactions with practitioners in both primary and secondary care services. Figure 17 describes a pivotal instance of health care in response to palpitations (perceived fast or irregular heart beat) wherein the specialist and hospital staff ratified the patient’s decision to use unscheduled care. Negotiations of candidacy were sometimes bypassed by family and friends who acted on behalf of patients. Patients were sensitive both to practitioners’ responses to a request for help, and to the responses of family and friends; both recursively shaped patients’ candidacy when making future health-care decisions, demonstrating that help-seeking is a social process involving more than just patients’ decisions.
Recursivity was seen in patient accounts of how they chose between health-care services, particularly in the choice to use unscheduled care. They framed these choices by drawing on previous experiences of help-seeking. Although patients described using unscheduled care as inevitable, their judgements of urgency and their understanding of why unscheduled care use was ‘inevitable’ were socially conditioned, arising out of previous encounters with health-care practitioners, family and friends, and particular services. Box 1 illustrates recursivity in how the judgement of urgency, and ultimately candidacy for accessing care, is established through previous encounters. Similarly, Box 2 illustrates how previous experience of particular qualities in a health-care service (in this case, easy accessibility and technological capability) ensures future reliance on that service for similar problems. That is, previous experiences of a service can build a foundation of trust, which strengthens patients’ confidence in choosing that service in future.
When considered together, the concepts of candidacy and recursivity highlight that the key determinants of patient choice of health care evolve over time and through social interactions, with future health-care use contingent on prior service responses to patients’ requests for care, and on previous experiences of the social process of care. 279,283 Patients rely on the knowledge they develop through previous experiences of services and practitioners to choose between services and to establish their candidacy for accessing services. The HCPs talked about changing individual patient behaviour as part of an ongoing relationship. This approach would fit well with the way patients make decisions, if the patient–practitioner relationship also addressed the wider health-care experiences of the patient. The HCPs highlighted difficulties with the lack of consistency across services in terms of responses to patients. In the case of the ACMs this lack of consistency undermined attempts to reduce unscheduled care. Patients make choices dependent on their knowledge of and confidence in the services they have used before, and judge their candidacy to use services based on previous responses. If services are inconsistent, this can make patient decisions more difficult and patients are likely to prioritise based on the most salient and positive service experiences.
Patients experienced barriers to using primary care in times of urgent need, and this recursively shaped future choices between services. ‘Permeability’ offers a way to conceptualise the impact of these barriers. 279 Highly permeable services require less work and fewer resources from patients who access them, for example EDs in the UK, which are open at all times. The permeability of emergency services is reinforced by ED doctors, who believe that their role is to see all patients regardless of need. A service that seems accessible may in fact be impermeable to particular patient groups. 281 For example, despite general practices being locally available, with designated systems for urgent access, patients in our study described that they were, in fact, impermeable because of factors such as receptionists’ gate-keeping, and travel cost or mobility problems. In our study, the combination of high permeability and technological expertise led most patients to choose the hospital ED in times of perceived urgent need.
In seeking to reduce unscheduled care use, health-care policy defines patients as in need of education to use services effectively, or suggests the need for reorganisation of health-care systems to reduce use of costly emergency care services, especially the ED. 284 This ‘deficit’ model also dominates previous research investigating unscheduled care use, with research focusing on characteristics of the patient20,278 or the health-care system20,285 that increase unscheduled care use. In this study, the GPs hinted at a deficit model of patients, suggesting changes to the health-care system such as triage and fee-for-service to force patients to use the system more appropriately. In contrast, this qualitative work demonstrates that patients understood the array of unscheduled care services available and were discriminating in their use of them, influenced primarily by previous experiences of services that recursively shaped their future health-care choices. It contributes to a growing body of research emphasising the social processes of help-seeking, and the expertise patients bring to decision-making around health-care use. 286,287
When patients with LTCs feel vulnerable in health crises, it is their previous experience of services that shapes their perception of candidacy and thus their choice of service to access, with patterns of under- or over-use of services becoming established recursively based on these responses. This is consistent with the ACMs and SNs who described making their systems permeable so that patients used them instead of unscheduled care. However, this does have implications for the short-term nature of their services and what happens once patients have learned about these services and are then excluded from using them following discharge.
Both HCPs and patients recognised the limitations of availability and accessibility of primary care and the need for unscheduled care as a last resort. As previous experiences of health-care practitioners and services have a recursive influence on patients’ health-care decisions, it may be that better management in primary care, utilising pre-existing practitioner–patient relationships to address health-care use, and improved access to primary care for patients with LTCs could reduce the frequency with which patients feel they need to draw on the last resort of unscheduled care.
There were several important strengths of the above study. The patient sample was large and heterogeneous, and patient interviews covered a broad range of health-care service use, asking about decisions to use or not use unscheduled care. The HCP interviews likewise included a broad range of practitioners from across the health-care system, and data collection and analysis were conducted by researchers of different professional backgrounds, which reduced the possibility of some form of professional bias.
Limitations
The study also had several limitations. It was undertaken in north-west England and patient participants had to opt into the study when they completed the baseline questionnaire from the cohort study. The participants were therefore a self-selected group. The participants’ recall of unscheduled care use may have been limited and there was a limited ethnic diversity in our sample. These limitations mean that caution should be applied when applying the findings to other geographical and cultural settings. The practitioner group was somewhat smaller than the patient group, and some professional groups within our sample were under-represented because of difficulties in recruitment (e.g. PNs and ED doctors). For those professional groups which are under-represented, it may be that there are other perspectives on unscheduled care use that have not been captured in this study and, therefore, further research may be required to test the findings of this research.
Conclusions
Health-care practitioners do not generally see unscheduled care use as a problem, describing it as inevitable for patients with LTCs. Patients similarly described feeling that they had no alternative but to seek unscheduled care in the face of perceived urgent need. Patients’ judgements that they needed unscheduled care, and their choice of unscheduled care provider, were influenced by previous experiences of health-care services and practitioners, in particular of the ED, valuing the ease of access and technical expertise available there. Although most HCPs saw it within their role to reduce unscheduled care use, the ED doctors we interviewed did not. They reported that attendance by patients with LTCs at EDs was legitimate. This is likely to reinforce help-seeking at EDs by this group of patients. Apart from ED doctors, HCPs described attempting to change the behaviour of the patient, but described limited strategies to do so. In addition, GPs described the need to optimise the system in which patients have to seek help, whereas PNs, ACMs and SNs described negotiating (and often bypassing) systems that constrain them and the care they perceive they can offer patients.
In all the interviews we conducted, there was very little consideration, by either HCPs or patients, of any psychosocial factors that may influence care-seeking. Although the quantitative data from our prospective cohort study identified depression, life stress and living alone as important independent predictors of unscheduled care use, neither patients themselves nor HCPs appear to be aware of such influences. Contacts with unscheduled care are framed almost entirely in terms of a physical context, both at the time the person decides to seek care and on reflection, after care has been sought.
Although, undoubtedly, physical health reasons are crucially important in decision-making about seeking unscheduled care, other, perhaps more subtle, contextual factors (e.g. the fact someone lives alone or is feeling very low) fail to be recognised. Even if HCPs have the opportunity to influence patients’ behaviour, the absence of any kind of dialogue or discourse about psychosocial factors, suggests problems of this nature will remain unchanged.
In the next chapter (Chapter 6), we explore this question further, by investigating what happens in consultations between HCPs and patients with LTCs to see how, if at all, psychosocial factors and planning for action during exacerbations of illness are addressed in routine primary care.
Chapter 6 The role of primary care consultations in people’s use of health care and in their management of long-term conditions over time (phase 2)
Abstract
Background
Primary care is central to LTC management, and routine reviews for LTCs are recommended as part of the QOF, which incentivises this activity. We aimed to explore how the QOF influenced the focus of consultations; explore how consultations may potentially influence unscheduled care; identify opportunities within reviews for behaviour change; and explore how psychosocial factors were addressed.
Methods
We audio-recorded primary care consultations for people with LTCs and used stimulated recall to encourage discussion of consultations during interviews with patients and HCPs. Using a longitudinal approach, we kept in contact with patients for 3 months, providing them with optional health-care logs for recording incidents in which they considered or did seek help, asking them to discuss their health on the telephone regularly, and to participate in a further interview after 3 months.
Results
Thirty-four patients agreed to participate. Twenty-nine consultations were audio-recorded; 10 HCP interviews, 27 initial and 22 follow-up patient interviews were completed. Reviews predominantly focused on the clinician agenda and achieving QOF targets, often to the detriment of other aspects of care. Discussion of unscheduled care or crisis management and behaviour change work tended to be absent, with patients positioned as passive recipients of care.
Discussion
This study suggests that routine reviews tend to have a narrow focus and fail to address patients’ needs holistically. Routine reviews present an opportunity to improve LTC management and influence patients’ health-care use, but this may require the adoption of a more holistic and patient-centred approach to consultations.
Overview
Patients with common LTCs are now routinely assessed on an annual basis in primary care as part of the QOF. In this chapter, we describe further work we carried out to explore what happens in these QOF reviews and in other consultations in primary care involving patients with LTCs. We were particularly interested in how the QOF reviews shaped the focus of consultations; how aspects of routine consultations may have a potential to influence use of unscheduled care; what opportunities there may be in routine reviews for behaviour change; and how any relevant psychosocial factors are addressed. More details of this work have been published. 288,289
Our main objective from the original programme application was to identify barriers to, and facilitators of, those aspects of HCP behaviour with the potential to reduce unscheduled care.
Background
The Chronic Care Model (URL: www.improvingchroniccare.org) and the NHS and Social Care Long Term Conditions Model (URL: http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/Healthcare/Longtermconditions/DH_4130652) highlight key system components that need to be addressed in order to deliver effective care for people with LTCs,9,290 including delivery system design, decision support, clinical information systems and self-management support. The QOF incentivises primary care professionals’ activity, financially rewarding the practice when evidence-based care in line with clinical quality indicators has been demonstrated,41 as well as providing a record to ensure payment to the practice against the quality indicators.
Two core activities in managing LTCs within primary care are the promotion of self-management behaviours, and enabling and supporting behavioural change. The chronic care model suggests that 70–80% of people with LTCs should be able to self-manage their conditions with support from health-care services. 291 Behavioural change with regard to risk factors such as smoking, alcohol use, diet and exercise are key targets for improving outcomes for those with LTCs, such as reducing disease-related complications, exacerbations and premature morbidity and mortality. Primary care consultations are crucial for enabling and supporting patients to become proactive managers of their own conditions.
In the qualitative literature review we carried out (see Chapter 2)185 and in our interview study of patient and practitioner perspectives (see Chapter 5), the importance of previous experiences of health care was highlighted in people’s accounts of using unscheduled care. This indicated that primary care practitioners may have a role in influencing patients’ choice of service in times of crisis. However, these data explored patients’ perspectives of decision-making retrospectively. Our intention in the study reported in this chapter was to investigate the role of primary care consultations in influencing patients’ health-care choices prospectively.
Aims
-
To explore what happens in routine consultations regarding LTCs within primary care, and how the QOF influenced the focus of these consultations.
-
To investigate health-care use as a topic in consultations and to explore how consultations might influence future health-care use.
-
To investigate behaviour change as a topic in routine consultations and identify any opportunities within reviews for behaviour change work.
-
To explore how psychosocial factors were addressed in routine consultations.
Methods
Context
The study was set in north-west England. Qualitative methods were used: audio-recordings of primary care consultations were made, and semistructured interviews using stimulated recall were carried out with patients and HCPs.
Research Ethics Committee approval
Ethics approval was received from North West 8 REC – GM East (reference number 10/H1013/74).
Recruitment
Primary care practices were invited to take part in the study by letter, e-mail and/or telephone. These practices included a mixture of those which took part in the cohort study and newly recruited practices from the north-west area. As before, researchers only conducted practitioner interviews or recruited patients from practices that they had no professional relationship with outside this research. Researchers attended participating practices on agreed dates and potentially relevant consultations were identified by the consulting practitioner (GP or PN). Relevant consultations were those that involved patients with one or more of the four exemplar conditions: asthma, CHD, COPD and diabetes. A range of consultations were sampled, including chronic disease reviews, when the patient was invited to attend by the practice, and patient-initiated appointments. Chronic disease reviews could be either disease specific (e.g. a COPD clinic) or encompass multiple chronic diseases. Although practices differed in how they arranged review appointments, review appointments were offered to all patients with one or more of the four conditions included in every practice that took part (Table 30). On arrival at the general practices, patients met reception staff, who provided participant information sheets about the study. A two-tiered approach to consent was adopted: patients gave initial written consent on the day of attendance to the audio-recording of their consultation, and were then given up to 7 days to consider further participation in a semistructured interview and to provide written consent to the retention of the recordings. Researchers were present in waiting rooms to take initial consent and answer any questions, but were not present in consultations. It was made clear during the initial discussion and afterwards that the study was interested in what happens in routine consultations for LTCs and that the interviewers were non-clinical and did not work for the primary care practice or have any involvement in the patient’s care. The primary care practitioner (either GP or PN) was responsible for ensuring the audio-recording of consultations for which patients gave permission.
| Practice | Review | |
|---|---|---|
| Type offered | Conducted by | |
| A | Specific disease clinic appointments for asthma/COPD, diabetes and CHD | PNs |
| B | Specific disease clinic appointments for asthma/COPD, diabetes and CHD | PNs; some reviews also handled by GPs |
| C | Mixed chronic disease review appointments | Initial tests by HCA or PN, review by GP |
| D | Specific disease clinic appointments for asthma/COPD, diabetes and CHD | Initial tests by HCA, review by PN |
| E | Specific disease clinic appointments for asthma/COPD, diabetes and CHD | PNs; some reviews also handled by GPs |
| F | Specific disease clinic appointments for asthma/COPD, diabetes, and CHD | PNs |
A purposive sampling approach was used, aiming for maximum variation across conditions, age and sex of patients, and type of health-care practitioner (GP or PN).
Data collection and analysis
Data collection
Patients and practitioners were interviewed separately after the recorded consultations, using a semistructured interview guide and stimulated recall; short snippets of the consultation were played back during the interview to prompt thoughts and reflections. Prior to the commencement of each initial interview, informed written consent was taken. For follow-up contacts and interviews, consent was checked and retaken to ensure that patients still felt informed and willing to participate in the study.
Topic guides for this study were developed within the research team, taking into account what had been learnt from the earlier portions of the programme. The interviews were framed around understanding the context of routine appointments and the structure of care for the patients, and the content of the specific consultations. The interviewers familiarised themselves with the topic guides prior to commencing interviews. Topic guides were refined over time through discussion within the research team of emerging themes and experiences of interviewing (see Appendix 3, Box 7).
Practitioners were interviewed once about all consultations they recorded, patients were interviewed once following their consultation and then invited to be followed up for a period of 3 months in order to gain insight into patients’ choices around health-care use over time. Follow-up with patients involved regular telephone contact and the option of completing health-care use diaries in between telephone contacts. Patients were then asked to participate in a final interview at the end of 3 months (Figure 20).
FIGURE 20.
Longitudinal, qualitative evaluation of primary care consultations between health-care practitioners and patients with a LTC.

Stimulated recall involved the researchers reviewing consultations prior to interviews, marking points in the consultations to play back to practitioners or patients. These points were identified using a topic guide developed by, and regularly reviewed and discussed within, the research team. These extracts were then played back during interviews and participants were asked to reflect on what was occurring at that point in the consultation, what they were thinking at that moment, their reflections on the topic being discussed or raised, and what had happened following on from that event. The focus was on gathering reflections on topics pertinent to health-care use (retrospective or prospective) and management of LTCs (e.g. medication advice, behavioural change issues, self-management).
During the final interviews with patients, at the end of 3 months, researchers used any patient-completed health-care use diaries and notes from telephone contacts to frame the interviews and focus in on specific decision-making activities around health-care use and management of health concerns during the follow-up period.
Analysis
All audio-recordings (consultations and interviews) were transcribed verbatim and anonymised. Data were analysed using an integrative framework approach, adapting the Ritchie and Spencer’s approach276 for the incorporation of multiple perspectives on the same event (consultation). Specifically, the research team analysed transcripts inductively, comparing data within, and between, the case. 291 For each case (identified as a single patient), short narrative summaries were constructed from the analysis, tracking themes for that patient from consultation to final interview, including discussion of that patient in the practitioner interview. Owing to the complexity of the analysis, with up to five different sources of data per patient (consultation, first patient interview, follow-up contacts, final patient interview and practitioner interview), a combination of Microsoft Word and Excel (Microsoft Corporation, Redmond, WA, USA) was used to handle the data. The research team was multidisciplinary, incorporating expertise in primary care, psychology and social anthropology. Analysis took place concurrently with data collection, with developing themes discussed within the team and incorporated into further recruitment and topic guides. Data collection ended when the team was satisfied that analytical saturation was reached, when the aim of characterising and understanding the experience of primary care consultations from multiple perspectives was achieved.
Results
Participant characteristics
Thirty-nine primary care practices were approached and six practices agreed to take part. Using publicly available QOF data, practices with high prevalence rates in at least one of the four LTCs compared with the median for England were identified and approached, with the aim of achieving a sample with a diverse socioeconomic and geographic spread within the region of recruitment. Practice recruitment ceased when a sufficiently large and diverse sample of practices had agreed to participate. From these six practices, five GPs (three senior partners and two GP registrars) and five PNs took part in audio-recorded consultations and interviews about these consultations (Table 31).
| Practice | Health-care practitioner | |
|---|---|---|
| ID | Role | |
| A | HCP1 | PN |
| B | HCP2 | PN/advanced nurse practitioner |
| C | HCP3 | GP (senior partner) |
| C | HCP4 | GP registrar |
| D | HCP5 | PN/specialist practitioner |
| D | HCP6 | GP (senior partner) |
| E | HCP7 | PN |
| E | HCP8 | GP (senior partner) |
| E | HCP9 | GP registrar |
| F | HCP10 | PN |
Twenty-nine consultations were audio-recorded by practitioners, with the number recorded per practitioner varying between 1 and 8 (mean 3). On five occasions, consultations were not recorded because of technical errors; in these instances, patients were still invited to take part in an interview about the consultation.
Sixty-five patients were approached within primary care practice waiting rooms, and 34 patients agreed to audio-recording of their consultations (Table 32). Patients were mainly white British (82.4%), male (65%) and aged from 34 to 87 years. Most patients had more than one LTC (73.5%; 29.4% had at least two of COPD, CHD, asthma and diabetes).
| Practice | Patient ID | Age (years) | Sex | Condition(s) |
|---|---|---|---|---|
| A | 1 | 70 | Male | COPD and cancer |
| A | 2 | 62 | Male | COPD and depression |
| A | 3 | 51 | Female | Asthma |
| B | 4 | 46 | Male | COPD |
| B | 5 | Not known | Female | Diabetes and COPD |
| B | 6 | 85 | Male | COPD, atrial fibrillation, dropped foot and balance problems |
| C | 7 | 51 | Male | Hypertension |
| C | 8 | Not known | Male | Diabetes |
| C | 9 | 82 | Male | Diabetes and asthma |
| C | 10 | 54 | Male | Diabetes and bowel problems |
| C | 11 | 47 | Female | Diabetes and cancer |
| C | 12 | 87 | Male | CHD, diabetes and depression |
| D | 13 | 65 | Female | Diabetes, COPD and angina |
| D | 14 | 60 | Male | Diabetes |
| D | 15 | Not known | Female | CHD and asthma |
| D | 16 | 50 | Female | Diabetes and nerve spasms |
| D | 17 | 76 | Female | CHD, cancer and high cholesterol |
| D | 18 | 69 | Female | COPD and arthritis |
| D | 19 | 74 | Male | CHD, asthma, COPD and meningioma |
| D | 20 | 50 | Male | CHD, depression and blindness |
| D | 21 | 43 | Male | Diabetes |
| D | 22 | 62 | Male | CHD, diabetes, hypertension and chronic kidney disease |
| D | 23 | 58 | Male | CHD, diabetes, cancer and piles |
| D | 24 | 57 | Female | Asthma, sarcoidosis and bronchiectasis |
| D | 25 | Not known | Female | Diabetes |
| E | 26 | 41 | Female | Asthma |
| E | 27 | 51 | Male | Asthma and hypertension |
| E | 28 | 73 | Male | CHD, diabetes and gout |
| E | 29 | 30s | Female | Asthma, depression and irritable bowel syndrome |
| E | 30 | 76 | Male | CHD, hypertension and arthritis, asbestosis |
| E | 31 | 76 | Female | Diabetes and arthritis |
| E | 32 | 67 | Male | Diabetes, hypertension and glaucoma |
| F | 33 | Not known | Male | Asthma and CHD |
| F | 34 | 67 | Male | CHD and hypertension |
There were 18 patients from whom consultation data, patient first interview, patient second interview and practitioner interview were all available – these constituted full cases (Figure 21). Of the remaining 16 patients, seven declined interviews but gave consent to retain their consultation; four had a recorded consultation and first interview, but declined participation in the second follow-up interview; and five took part in either one or two interviews, but lacked a recorded consultation. Retention during follow-up was high (81.5%), with 22 out of 27 patients who completed a first interview going on to complete the follow-up interview as well. Figure 21 details patient recruitment and retention throughout the study.
FIGURE 21.
Patient recruitment and retention. The figure is adapted from Hunter et al. 289 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

All of the participants who took part in the initial interview also took part in regular telephone calls, in which the researcher asked about their health status, health-care use and decision-making. Researchers kept a written record of each telephone call. If a participant described a problem with their health during these telephone calls, they were asked by the researcher to fill in a health-care log of the incident. These health-care logs asked participants to record their symptoms, when the incident occurred, where they were at the time and who they were with, and what they decided to do at the time. All but four of these participants then took part in a follow-up interview. Health-care logs and telephone calls were then analysed for content relating to health-care use and health-care decisions, and were used to inform the discussion in the follow-up interviews and the analysis of health-care use (Table 33).
| Source of data | Number of data |
|---|---|
| Number of patients | 34 |
| Consultations recorded | 29 |
| Patient baseline interviews | 27 |
| Health-care logs | 14 completed logs, from four patients |
| Telephone calls | Between 6 and 15 calls, with 22 patients |
| Patient follow-up interviews | 22 |
| Number of health-care practitioners | 10 |
| Health-care practitioner interviews | 10 |
Findings
The findings below first characterise the consultation data, then draw on the patients’ and practitioners’ perspectives on these consultations. The role of primary care consultations in informing health-care use and patient behaviour over time, and how patients and practitioners view this care, is examined in relation to three main areas. These are the QOF; the influence of primary care on health-care use; and behaviour change work in relation to LTCs. All three topics are areas of policy on the management of LTCs in primary care, and are therefore used to frame the analysis in terms of the key policy-identified tasks for primary care in relation to LTCs. Although one of the aims of this chapter was to explore how psychosocial factors were addressed in routine consultations, in our sample it was rare for psychological or social needs to be asked about or addressed. This is also discussed below. Illustrative data are given, with identifiers to contextualise the data. Ellipses (. . .) signify omitted text. Square brackets denote explanatory text.
Primary care consultations and the Quality and Outcomes Framework
Consultations as retrospective and Quality and Outcomes Framework focused
The main focus of the recorded consultations was on retrospective review of medications and test results, which was to be expected given that the consultations were for the most part initiated by the practices and their reviewing protocols.
The consultations were dominated by activities that could be characterised as QOF related, such as checking results and filling in QOF templates, even during consultations when patients brought up other illness-related needs. Many of the routine reviews for LTCs contained instances where patients indicated a need that was then not explored or addressed within the consultation (such as informational or emotional needs, or other biomedical needs not directly relevant to the LTC being reviewed). This occurred when patients brought up or highlighted an issue and the practitioner apparently missed the cue from the patient (or carer), or did not pick up on the importance of the issue, and instead focused on standard review activities, dictated by the computer template. Box 3 illustrates how one patient’s biomedical needs unrelated to the LTC being reviewed were apparently sidelined during a review appointment with his GP.
Male, aged 87 years, retired, married. Review consultation (diabetes)
Memory problems and urinary incontinence
ConsultationThe HCP3/GP opened the consultation with his agenda:
So there were two things really I need to discuss with you, one is your blood sugars, and we do a test which looks at the long-term control of your blood sugar, and it suggests that it probably could do with coming down a bit.
Right, well . . .
Now there are a couple of things you could do about that, one is the alcohol.
Knock it off?
You could try and decrease it.
Yeah.
And the other is these new tablets that I’d like you to try.
I’ll try them.
The other thing is, you could have a urine infection.
A who?
The HCP3/GP introduced the need for further blood tests as part of the QOF template for diabetes, and asked if the patient will also bring in urine samples. The GP twice brought up the need for patient to book these tests. Each time the patient at first appeared to show he understands (‘right’, ‘OK’, ‘oh I see’) before going off on topics unrelated to the consultation. The patient seemed overwhelmed by the requests:
Right, so you’re going to do these two urine tests and drop them back.
I will.
You’re going to do, arrange the blood tests.
Oh my god. This is very . . .
I’ll ring the front, I’ll ring the front [reception] and tell them what you have got to do.
The HCP3/GP recognised that multiple instructions are causing confusion for the patient, but did not address this, other than taking control of the organisation of the tests.
GP interviewThe HCP3/GP argued that the key purpose of the chronic disease review appointments in the practice is to ensure that patients are viewed holistically by the GP who can ensure that care is co-ordinated:
One of the things we wanted to do when we designed our chronic disease review, was to have a slightly less specialised clinic, but one that dealt holistically with people with a range of problems (. . .) I would count us as the general physician who directs the care to the various places.
Patient baseline interview
In contrast to the GP’s view of review appointments offering holistic care, the patient described having consultations with different doctors in the practice about different problems:
There’s [HCP3/GP], there’s [a second GP], and now I’ve got [a third GP, whom he saw for urinary incontinence]. Altogether, they can, they’ll probably pass it on, you know, ‘{The GP [new to practice] C/P12 saw for urinary incontinence}, you’ve just arrived here, will you go and see this mithering brain [C/P12]’, you see.
The patient describes himself as ‘this mithering brain’, a colloquial term meaning ‘bothersome’ or ‘complaining’. He suspects that some doctors may pass him onto new doctors in the practice because he has so many ailments.
Patient follow-up interviewThe patient and his wife described the number of different people involved in his care, and the confusion this caused:
Too many bloody pokers in the fire here.
We do not know where we are up to with [the care for his incontinence] really.
In this instance, a referral had been made by another GP in the practice for him to see a specialist about his incontinence. Following this referral, the patient and his wife were given contradictory information about the appointment. They had asked for clarification at the GP practice, but said no one had responded to them.
The patient’s presentation indicated possible memory problems, which the GP did not address while conducting the review, instead bypassing them to focus on the QOF template of blood and urine tests. Although the GP seemed aware that the patient was having difficulty understanding how to organise blood tests, he dealt with this confusion by directing the patient to the reception staff, rather than explore possible memory problems. The patient and his wife seemed superficially engaged in the process of review, but later described feeling that the review had added nothing to his care, which remained fragmented, with problems unaddressed. Fragmentation was observable in other aspects of the patient’s care beyond the review, despite the practitioner arguing for the review’s essential role in providing ‘holistic’ care.
Another example of how the QOF review seemed to take precedence over a patient’s needs is presented in Box 4.
Female, aged 41 years, British south Asian.
Attended for review consultation (asthma).
Patient recently moved back to the UK following breakdown of her marriage. She has two young children.
ConsultationThe patient had been invited to attend following an exacerbation of her asthma, which she, throughout the consultation, attributed to the stress of undergoing a divorce. On several occasions, the HCP7/PN disregarded the patient’s cues about stress, returning instead to the asthma template on the computer (in the example below, inhaler technique):
I’m so sorry [that I forgot to renew the inhaler], but like I say it’s just not like me. I had, the horrible thing I’m thinking about is, I had a Court date on [date] and I think, I’m wondering now, it’s not an excuse but . . .
No.
I lost track. Because I wrote it all down on my calendar, when to have my review is. So will I be able to get a prescription today, now?
Yes, we’ll give you a prescription today. I just want you to go through, have you got your inhaler? Do you carry it, your blue inhaler, with you?
No.
OK. So you should always, I mean you know, we’ve said this at the last one, that is your rescue therapy, so you’ve found out the hard way haven’t you really, of being [. . .] out shopping, struggling to get your breath, it’s very scary.
PN interview
When asked to describe E/P26’s needs, the HCP7/PN responded:
She’s quite an anxious lady, sometimes she has her own agenda on some things, or they take priority and she doesn’t always take care of her, you know, the problem. She sees that problem as something that happens at the time and then goes away and if things improve she forgets all about it until the next time.
When the nurse spoke about her role in medication use, it was in terms of encouraging adherence:
You’ve still got to get that message across that this is what you need to do and basically if you’re not complying and you’re not taking those inhalers well then the implications of that are you are going to get repeated exacerbations. You’re going to end up feeling horrible and panicking and like [E/P26] was, and that could be prevented as long as she complies with the medication.
HCP7/PN
Patient baseline interview
The patient felt her concerns were dismissed by the HCP7/PN:
I’m really disappointed in [HCP7/PN]. She’s very efficient and everything, but I didn’t feel that she had any empathy at all and the comment about, ‘Well you’ll remember for next time,’ it’s just stayed with me.
E/P26
Patient follow-up interview
By the time of the follow-up interview, the patient was more positive about her relationship with the PN, but has established limits on the types of problems she would raise with the PN:
I am building more of a relationship [with HCP7/PN], but again, if I’m brutally honest, I’m not that keen on seeing the nurse either. It’s just because I don’t really have any other option (. . .) it looks like we’re getting back on track because I had really deteriorated with my asthma care. So, yeah, I’m literally, the way I see the surgery is it’s for looking after my asthma, and it’s the nurse [for my asthma care] and that’s it.
E/P26
The PN seemed to use the patient’s fear of further exacerbations to encourage compliance with medication and the review process itself. The patient suggested that the PN’s agenda of ensuring compliance took prominence over the patient’s own explanations and concerns. As a result of this socialisation into what can and cannot be talked about in routine reviews, the patient’s expectations of the reviews were limited to ‘asthma care’ by the follow-up interview.
In all of the cases gathered in this study, there were only two cases which demonstrated a reorientation of the primary care consultation away from reviewing the QOF-driven clinical indicators for LTCs towards a more patient-centred model of care. These were an instance in a diabetes review when a GP responded to a patient’s evident distress about her recurrent cancer, and moved discussion away from diabetes-related issues as a result (C/P11); and an instance in a general chronic disease review when a GP focused on exploring a patient’s mood and coping strategies following a recent bereavement (E/P30). These consultations were reviews conducted by GPs, and involved discussion of a broad psychosocial agenda. In both cases, the patients expressed their appreciation of the GPs’ sensitivity during their interviews.
Patient perspectives on reviews
Understandably given the above, patients tended to characterise review appointments as focused on retrospective checking of their conditions, and they were aware of the practitioner-led nature of the appointment. Being ‘checked’ in this way was often experienced positively by patients, but this experience went together with a view that consultations were for practitioners to do things to patients, rather than for patients and practitioners to work collaboratively on the patient’s health:
[Primary care review]’s sort of a one-stop shop for a lot of things really. I get the blood tests done, I get my diabetic – they check your feet for the diabetic check up on your feet, and they do my blood pressure. And they usually, well, the last few times I’ve had my flu jab at the same time (. . .) it kind of covers everything.
C/P11, female, aged 47 years, with diabetes and cancer
I’ve gone [to the GPs] and they’ve checked me out and so on. And they immediately say, if it’s unclear come back and see me. There’s always that at the end [of the consultation].
A/P1, male, aged 70 years, with COPD and cancer
This experience of reviews as retrospective, focused on checking biomedical issues and practitioner led meant that patients did not typically view review appointments as the time or place to ask about issues beyond the illness currently under review. Although some patients did bring up pressing issues (such as C/P11, who expressed her distress over her recurrent cancer), this was rare. Notably, in one of the ‘outlier’ cases mentioned, the GP deliberately activated the review procedure in order to explore the patient’s mood, making this case different not only in the movement beyond QOF templates, but also in viewing the review process as an opportunity for engaging patients beyond their biomedical needs:
I followed him up proactively, I asked him to come back and we booked that appointment mutually, so I wanted to follow him up because I was partly concerned to get his atrial fibrillation and his angina sorted, but also I knew that the death of his wife was going to have a massive impact on him so I wanted to follow that up.
HCP8/GP
Mostly, patients saw their reviews as only about the particular LTC(s) included by the practitioner in the appointment, and about checking how they had been, and this perspective influenced what they considered discussing in consultations:
I think that’s what she does, smoking and asthma, I don’t know if she does anything else (. . .) Obviously I would only bring something up if I thought it was related to something she did (. . .) for the pain in my knee, I’m not going to say to her ‘well, I’ve got a pain in my knee’ because I don’t think she can help the pain in my knee sort of thing.
A/P3, female, aged 51 years, with asthma
Summary
-
The majority of consultations were focused on meeting the demands of the QOF, and were practitioner led.
-
Patients had unexplored and unmet needs in primary care review appointments for LTCs.
-
These unmet needs included informational and emotional needs, and also additional biomedical needs that were not directly relevant to the LTC under review.
-
This process of cues being missed and care being redirected onto the practitioner’s agenda socialised patients into what could or could not be raised in review appointments.
-
There were two instances where practitioners deviated from the QOF agenda, suggesting that certain circumstances (in these cases, the presence of major psychosocial issues) can take precedence over QOF work.
Primary care consultations and discussion of use of unscheduled care
Health-care use as a topic in consultations
During the recorded primary care consultations, explicit discussion of what to do in case of emergencies or other problems was rare. Out of 29 recorded consultations, only eight consultations contained any explicit mention of prospective help-seeking. In these consultations, the talk about help-seeking was led by the practitioner (seven out of the eight consultations were PN appointments), and the talk was directive (i.e. ‘do this in the event of X’). Out of the eight consultations where prospective help-seeking was mentioned, five were directions to come back to that specific practitioner if any problems arose with medications or if the patient had an exacerbation of a LTC. In the other three consultations, one was mixed (D/P13), as the nurse gave separate advice for COPD (come back to nurse with exacerbations) and for CHD [take glyceryl trinitrate (GTN) spray for angina, then if it does not work, call an ambulance]; another (D/P15) was the same advice about CHD (GTN spray, then if it does not work, call an ambulance); the third consultation (D/P16) involved the nurse giving advice to try a number of different sources for help if the participant’s blood sugars were worrying (try the nurse, the diabetes-related helpline, or the ED). This consultation was characterised by explicit reassurance and crisis management on the part of the nurse, as the participant displayed anxiety and recounted significant problems managing her blood sugars.
Use of unscheduled care during the study
There were five instances across 22 cases, during the 3-month study period, where index patients used unscheduled care and follow-up data were available. Out of these five instances, the reasons given for seeking unscheduled help were very similar to those found in the retrospective accounts of patients in the study described in Chapter 5. These were unscheduled care used due to the timing of the incident (in the middle of the night or at a weekend when GP appointments were unavailable) (for D/P20 and F/P34); unscheduled care used due to the referral by a GP, neighbour, or family member (for B/P6 and D/P20); and unscheduled care used because symptoms were unusually severe or unexplained by patient’s previous experiences (for D/P16, D/P24, and F/P34). For two patients (D/P20 and F/P34), more than one reason was given for using unscheduled care in relation to the same incident.
Several participants did experience health problems during the 3-month follow-up and reported that they chose to use GP services or pharmacists in routine hours or to self-manage the problem. Reasons given for this use again mirrored the findings from our qualitative study described in Chapter 5. Previous experience influenced their decision to manage at home or use primary care. If people had experienced the problem before, they reported feeling able to manage it at home or felt confident that the problem did not need emergency care as the primary care practitioner had indicated this on previous occasions. One participant used specialist diabetic services instead of primary care – this was due to a belief that the diabetic service would understand her problem best because they had more expertise with diabetes-related issues than primary care:
I always think that when you’ve got a specialist service it’s best to use a specialist service when it’s specific to something. If I felt just generally unwell then I’d go to the GP, but because my concern was my sugars I went to the diabetic nurses.
C/P11, female, aged 47 years, with diabetes and cancer
There were three participants who explicitly indicated that they did not feel confident about seeking help at their primary care practices, reporting that they felt that primary care did not really care about what was wrong (E/P26); that primary care did not seem able to solve their problem, having sought help in the past with the same issue (A/P2); or that they were having persistent difficulty controlling symptoms, despite frequent past use of primary care (D/P15). In all three cases, participants displayed some reluctance and hopelessness around the effectiveness of help-seeking. Two participants disclosed mental health issues during follow-up interviews [depression (B/P6) or panic attacks (D/P13)], and indicated that they were unlikely to seek help with these, as it was their responsibility to ‘talk myself round’ (D/P13) or ‘sort my head out myself’ (B/P6). They also talked about being reluctant to take more tablets, especially antidepressants, which they expected would be offered by the HCP.
With regard to answering the question of whether or not primary care consultations influenced how patients make decisions about unscheduled care use over time, it is difficult to judge as the patients in our sample were infrequent users of unscheduled care, and mostly did not experience worrying or upsetting incidents of health need during the 3-month follow-up period. Only four patients completed health-care logs during the period of follow-up, and these 14 incidents were mostly resolved with self-care (see Table 24). From the telephone calls and the logs, there is some evidence that patients chose to follow practitioner advice on using unscheduled care (as described above), and there is also evidence that patients chose to first employ self-care tactics if they had experience and knowledge of a symptom and had a means of dealing with it (e.g. rescue packs for COPD or GTN spray for angina). In the latter scenario, there was evidence in the recorded consultations of practitioners prescribing such medications and advising patients to use them in a crisis, so practitioners may be informing patients’ health-care choices through this mechanism.
Summary
-
Prospective health-care use was rarely mentioned or discussed as a topic in primary care consultations.
-
Use of unscheduled care during the 3-month follow-up period was not common.
-
Where patients described health-care use (unscheduled or scheduled) decisions during the follow-up period, these were described as influenced by past experiences of health care and patients’ perceptions of the acceptability and appropriateness of help-seeking for the experienced symptom.
-
Patients who disclosed concerns about mental health issues to the researchers said they would be unlikely to discuss these issues with health-care staff.
Primary care consultations and behaviour change
Behaviour change as a topic in consultations
Explicit talk about behaviour change or self-management was also uncommon in the primary care consultations. For the purposes of this analysis, a ‘behaviour change topic’ encompasses any health behaviour that could be targeted for change in order to improve patient health outcomes; this includes self-management strategies where the work would be done by the patient. All four core LTCs can benefit from addressing adverse health behaviours (such as smoking) or enhancing patient engagement with behaviours such as exercise and diet management. In 27 consultations focusing on the review of LTCs, the most frequently mentioned behaviour change topics were smoking (n = 10), medication use (n = 9) and diet (n = 9). Medication use was defined as talk around how and when to use currently prescribed medications, rather than talk around new prescriptions. Specific self-management programmes or strategies, as means to address behaviour change, were mentioned in only four consultations; these included pulmonary or coronary rehabilitation programmes (n = 2) and rescue packs of antibiotics and steroids for COPD (n = 2).
Behaviour change talk, when it was engaged in during primary care consultations, was characterised by deflection and diffidence on the part of practitioners. Patient cues about motivation were often not followed up by practitioners, and patients tended to remain unmotivated, uncertain or overwhelmed by the idea of behaviour change during the follow-up period. Looking closely at the types of talk around behaviour change revealed that practitioners’ most common strategies were to give directive advice (n = 11), to gather information about the behaviour in question (n = 11), or to give information about the behaviour and the need for change (n = 9). It was rare for the practitioner to explore patient motivation (n = 2), and often information was given but not discussed in any detail. Talk about behaviour change was typically practitioner led, with no shared discussion about the issues.
Diffidence was characterised by the practitioners suggesting or advising the need for behaviour change, but then withdrawing from further discussion. Examples of this communicative style in one GP’s consultations (HCP3/GP) were as follows: he typically used tentative language when bringing up behaviour change topics [such as ’(alcohol intake) could probably do with coming down a bit’] and negatively framed questions that closed down discussion [‘(smoking cessation) not something you want to consider at the moment?’]. When asked about this style in relation to one of his consultations, he offered the following reasoning:
If [the patient] wants to know more about [smoking cessation], that’s fine. If she says ‘I don’t want to deal with that now’, I’m not going to do any more about that, at that point (. . .) The success in smoking cessation is in people who want to stop.
HCP3/GP
Here, the practitioner decided to leave any further work or exploration of behaviour change to the patient to initiate, as behaviour change relies on patient engagement. This was a common line of argument among the practitioners’ accounts of behaviour change:
The driving factor, force, has got to be them [the patients].
HCP6/GP
[Behaviour change has] got to come from them. You can intimidate somebody into stopping, or frighten somebody into stopping smoking. Yeah, they will do it but they don’t sustain it, because they’ve been made to stop. They’ve got to want to do it.
HCP5/PN
Deflection was characterised by practitioners gathering information about a patient’s behaviour and then either deferring discussion to a later point or suggesting that another practitioner was better equipped to deal with the issue. An example of this style is given below.
In consultation with the GP, the patient (D/P23, male, aged 58 years, with CHD, diabetes and cancer) brought up smoking:
I started smoking again though.
Oh how many?
I’m back to where I was before (. . .) a big pack of 50 g [tobacco] will last me a week, about 9 days, 8 or 9 days.
So it’s a lot though isn’t it, I know you spoke to [health-care assistant], she mentioned to you, didn’t she, maybe did she?
What?
About [health-care assistant]’s talked to you about, did she talk to you about smoking or stopping smoking?
I did stop for 2 months (. . .) I restarted again.
But that’s [health-care assistant’s] area of expertise, helping people to stop.
I think I’m a lost cause, if I’ve started again after that business.
Yeah, and the other thing just to mention [GP starts talking about diabetes].
Here, the GP appeared reluctant to talk about smoking with the patient, despite the patient raising the issue. He deflected discussion by referring to the health-care assistant (HCA) repeatedly, giving the message that the GP was not the right person to tackle the issue, and not responding to or exploring the patient’s assertion that he was a ‘lost cause’.
Patients’ and practitioners’ views on behaviour change
Behaviour change was considered by patients to be their responsibility, with practitioners unable to help:
The only thing [HCP1/PN] can’t give me is the will power [to stop smoking] (. . .) you’re only going to do it when you think you’re ready, or feel ready to do it.
A/P3, female, aged 51 years, with asthma
Do you still think there’s nothing really that the practice can do?
No. I think it’s just a personal [trait], the way you are. It’s difficult to change isn’t it?
D/P23, male, aged 58 years, with CHD, diabetes and cancer
I am overweight because it’s my fault I’m overweight (. . .) [if I went to see another practitioner, as HCP6/GP suggested] I find that I would be wasting, I feel like I was wasting that person’s time, because I know what’s wrong, I know what I should do.
D/P21, male, aged 43 years, diabetes
The combination of a patient-held belief that they are responsible for any change, the experience of practitioners as diffident or deflecting when it comes to behaviour change topics, and the view of primary care reviews as focused on retrospective checking by the practitioner, create a situation where patients do not automatically consider primary care as the place to discuss behaviour change.
Practitioners presented themselves as limited in what they could do in primary care appointments around behaviour change. They presented information giving and support as the techniques available to them, and suggested that these techniques might influence patient motivation to change. However, practitioners did not seem confident in the efficacy of these techniques:
If you can get them to help them to see the relationship [between lifestyle choices and their illnesses], then I think you’re more likely hopefully for them to change their lifestyle.
HCP3/GP
I see my role specifically as, just passing on information that I know to hopefully help, that they will take on board to help manage their own symptoms.
HCP1/PN
General practitioners saw information giving as an essential aspect of behaviour change work, but often raised doubts over what information alone could achieve:
I could say, ‘Here’s an information leaflet or a little booklet’, that I did have on the shelf on the room, I know exactly where they are which says, ‘diet and diabetes’, I probably didn’t do it because I didn’t think it’d make any difference. I just, that kind of sense of despondency in some ways that giving him an information booklet I don’t think is what he probably needs to make those changes.
HCP6/GP
Practitioners cited a concern with protecting the patient–practitioner relationship as a reason why they might not engage with behaviour change work further in consultations. Although practitioners agreed that behaviour change was important, they argued that the potential cost of challenging patient behaviour outweighed the benefits – it risked damaging the relationship without resulting in behaviour change:
The thing is you do not need to emphasise to a smoker this is bad for you. You don’t need to go on and do this and do that – they know that. And quite often if you do that they will shut down on you and they won’t take it on board.
HCP10/PN
I don’t want [patients] to say I’m not going to see [the GP], because every time I go and see him, and we get this with people, he talks about smoking and I don’t want to stop. And then they don’t come back for other things, so you lose people if you are too evangelical, you lose people for other impacts you might have. So that’s the reason for not banging on about it once she said she’s not interested.
HCP3/GP
Summary
-
Similarly to prospective health-care use, behaviour change was rarely brought up in consultations.
-
Practitioners’ communication styles around behaviour change could be characterised as diffident or deflecting.
-
Both practitioners and patients positioned patients as the primary responsible individuals for behaviour change work, and it was left to the patient to raise or pursue the issue in practice.
-
Practitioners saw their main role as information giving, but described themselves as limited in what they can do.
-
Practitioners were also concerned with protecting the patient–practitioner relationship and would not pursue behaviour change work in case it damaged this relationship.
Discussion
In this study, primary care consultations for LTCs were characterised by a retrospective focus on meeting clinical targets, to the point at which issues such as prospective health-care use, behaviour change and talk of unmet patient needs was neglected. Except in two cases, psychological and social issues were sidelined in favour of completing the technical and biomedical aspects of review work. The patients were socialised into an expectation that primary care was for ‘checking’ their health and fulfilling the practitioner’s agenda, and that they themselves were responsible for instigating any lifestyle or dietary changes indicated by their LTCs. Patients were not clear about what practitioners could do for them, beyond provide medication, and, although patients appreciated receiving regular reviews, they tended not to see them as the place to bring up further needs.
Perhaps as a result of the focus on the QOF, practitioners often missed or did not follow-up cues around unmet needs, including informational, emotional and biomedical needs unrelated to the LTC under review. Having experienced consultations wherein their concerns were not picked up, or where the focus was primarily on checking biomedical test results and medications, patients tended to adapt their expectations accordingly, and became socialised into a particular way of participating in reviews. This socialisation is potentially damaging, as patients may delay bringing up serious issues, and it could also impact on how patients use primary care and unscheduled care, as patients may choose to use other services because of a mistaken belief that primary care cannot help them.
With regard to health-care use, it was unclear whether or not primary care reviews influenced patients’ decisions over time. This was in part due to the infrequency of unscheduled care use within the sample, but also in part due to the infrequency of health-care use or crisis management as a topic in the consultations. Practitioners rarely addressed prospective health-care use or crisis management with patients in review consultations, and when they did address it, it was usually in a prescriptive manner, giving advice on what to do but not discussing options with the patient. Despite this, there was evidence of patients using unscheduled care as a result of past practitioner advice, and also evidence of patients using self-care tactics that aligned with those which practitioners had recommended in consultations. The key features of unscheduled care decision-making, as seen in this study, mapped on to those seen in the previous qualitative study described in Chapter 5, with both prior experience of health care and the person’s perception of their own legitimacy to use unscheduled care in that instance being essential. From this perspective, this chapter offers further support for the candidacy and recursivity theories of the preceding chapter, and reinforces the notion of primary care practitioners as potential influences on this decision-making process by virtue of how they have responded to previous incidents of need experienced by the patients.
Behaviour change is a current UK priority for LTC management, and in discussing behaviour change in interviews, practitioners emphasised their belief in its importance and its centrality in LTC management. They described a role for themselves in providing information to patients about the importance of behaviour change. However, they also emphasised constraints on their ability (and, therefore, their responsibility) to effect behaviour change. Behaviour change work was constrained by a perceived lack of effective techniques to influence patient motivation, by the need to ensure a continued patient–practitioner relationship and, ultimately, by the patients themselves. 275,292–295 This included attempts to give directive information about when to seek help. The relegation of behaviour change work to the responsibility of the patients or other practitioners offered a way for practitioners to disengage with this work in their consultations.
The key finding in this study was that reviews were often focused on offering one form of care (evidence-based care as represented by the QOF) to the detriment of other, equally valuable aspects of care (a patient-centred approach which addressed patient-defined needs; behaviour change work essential to successful management of LTCs). The unintended, and concerning, consequence of this focus is that patients adapt their behaviour and their expectations accordingly, becoming more passive in reviews and less likely to raise problematic issues or engage in proactive work with the practitioner.
It is acknowledged that the practitioners in this study and in primary care in general are embedded in an increasingly busy and scrutinised context in which certain tasks are valued and counted (such as meeting biomedical targets),296 and others (such as less measurable behaviour change work) are not. 288 It may be that practitioners feel both ill-equipped to achieve behaviour change in patients and pressurised to achieve other targets, and the rhetoric of patient control and responsibility enables them to legitimately distance themselves from this work. 297 This may be why there was little discussion of health-care use and why patients felt disengaged from their GPs, as they offered only medication. Likewise, practitioners may be missing or not responding to patient cues about physical or emotional needs that fall outside the review consultation’s standard focus because they are under pressure to keep to time and achieve their targets. Depression and stressful life experiences are independent drivers of unscheduled care use, yet such factors are unlikely to be brought up by health-care staff, and patients are also reluctant to disclose or talk about such problems. Practitioners struggle to bring up or discuss depression within consultations where the agenda is driven by QOF and medication review, concerned that such discussions might derail this agenda or identify needs they do not have the skills or resources to manage. 298,299 Practitioners are likely to engage with psychosocial factors and behavioural change work only if the structures in primary care promote and value this work. 300,301
Addressing the tensions between patient-centred care and delivering evidence-based care might be achieved by longer consultation times,302 and greater continuity of care. 302 Careful consideration is also required of how practitioners and patients communicate, and how this influences the work that can be achieved by both parties around health outcomes; too much of a focus on an individual patient’s responsibility when it comes to behaviour change and managing LTCs can harm the patient–practitioner relationship and impede open discussion. 303 Health-care practitioners need to be aware that patients have to do more than biomedical work to manage their LTC, and require social resources in order to achieve LTC work, and consideration is needed of how practitioners can work with patients in these broader aspects of their lives, cognisant of multiple problems,37 and can support and encourage patients to bring their concerns to the consultation.
There are several strengths of this study. Its innovative design, involving repeated patient interviews over time, generated a rich understanding of patients’ attitudes, needs and experiences. The combination of multiple perspectives allowed for a richer and deeper analysis of the data than is usually possible in qualitative work. Stimulated recall helped situate discussions in specific consultations, thereby reducing recall bias, and the regular patient contact over time enabled participant–researcher relationships to form that encouraged greater openness about sensitive topics.
Conclusions
Routine reviews of patients with LTCs have become well established in primary care and represent a golden opportunity to provide high-quality, patient-centred care. Our findings from the current study suggest, however, that reviews have a narrow, ‘tick-box’ focus and fail to address people’s needs in a holistic fashion. Use of unscheduled care or crisis management is rarely discussed, so there is little opportunity to implement strategies that make overt use of unscheduled care. There is little space or time in consultations for behavioural change, which may improve the management of patients’ LTCs, and this kind of work is currently not valued as highly as ‘physical health’ procedures. Finally, emotional and social problems are regarded as peripheral to people’s physical health problems and, as such, are sidelined during routine reviews.
We also found examples of good practice, involving practitioners who engaged with their patients in a collaborative style, and were comfortable moving beyond the confines of ‘QOF’ to provide sensitive and personalised health care.
Limitations
There were several limitations regarding the study. The time frame for follow-up was kept short in order to reduce impact on patients; however, it may not have been long enough to fully explore episodes of unscheduled care use as these episodes were rare in the patients interviewed. Recordings of single consultations can only offer a snapshot of the care being given, and patients with multiple LTCs are likely to have input from multiple practitioners, which was not captured in this approach. As a result, it is possible that some of the cues or needs missed within the study were being addressed elsewhere. Only a small number of practitioners and practices were included in the study and, as the study was located in north-west England, the results may not be generalisable to other parts of the country.
Chapter 7 A replication study to determine predictors of unscheduled care in patients with long-term conditions using primary care electronic data sets (phase 3)
Abstract
Background
We conducted a replication study to test the validity of our findings from the longitudinal study in phase 2 of the CHOICE programme.
Methods
This was a medical records review study of adult patients with LTCs in primary care in two areas of north London, over a 12-month period. There were 8722 patients in the north-east data set and 14,700 patients in the north-west data set. Candidate predictor variables included prior use of unscheduled care, a diagnosis of any of the four LTCs, age, sex, depression, and a compound mental health variable termed ‘common mental health problem’ (CMHP). Outcome variables were EHAs and ED attendances.
Results
Prior use of unscheduled care was a strong predictor of future unscheduled care use, for both data sets. Other independent predictors were being aged ≥ 80 years and having a diagnosis of any of the four LTCs. Depression and CMHPs were not independent predictors of EHAs, but were independent predictors of attendances at EDs in both data sets.
Discussion
The results of the cohort study in phase 2 were partially replicated by this study. Prior use of unscheduled care was the most powerful predictor of future use of unscheduled care in both London data sets, as it had been in the Manchester cohort study. The size of the OR for each variable was very similar to those of the Manchester data, which suggests a degree of consistency across the two sites. Depression predicted ED attendance but not EHAs.
Introduction
In this chapter, we describe a replication study which we carried out to test the validity of our findings from the longitudinal cohort study conducted in phase 2 of the CHOICE programme. The findings from our longitudinal cohort study (see Chapter 3) showed that several potential red flag variables were associated with an increased risk of use of unscheduled care in primary care patients, with at least one of the four following LTCs: asthma, CHD, COPD or diabetes. These variables were prior use of unscheduled care; a diagnosis of any of the four LTCs; depression; lack of a partner; and threatening life experiences. The aim of this replication study was to determine whether or not our findings from the longitudinal study could be replicated using routinely collected NHS data.
The main objective, as described in the original grant application, was to ‘evaluate the feasibility and validity of establishing a red flag system to identify patients with LTCs who are at risk of becoming frequent users of unscheduled care’. As we had already established that ‘frequent use’ of unscheduled care was relatively rare in our primary care population of people with LTCs, we focused instead on prospective use of unscheduled care.
The collection and analysis of data were carried out by Professor Simon de Lusignan and his team at the University of Surrey. In the original grant application, we had specified that we would use a large data set in Salford to carry out the replication study. However, there were logistical problems in being able to access the data set during the time frame that was required for the CHOICE programme.
We looked therefore for viable alternatives and we chose to collaborate with the Surrey team for three reasons. First, they were able to access data in a different part of the country to Manchester, so if the findings of the cohort study were replicated, this would strengthen the generalisability of the results. Second, they had considerable expertise in the analysis of large primary care data sets. 304 Third, they had prior experience of extracting mental health variables from routine primary care data. 305
The main plan was to use routine primary care data of patients with at least one of our four exemplar conditions and collect data over a 3-year time period. This would then be divided into a ‘baseline’ period of 2 years and a ‘prospective’ period of 1 further year. Data collected over the baseline period would be used to predict use of unscheduled care over the prospective 12-month period. It was decided to increase the duration of the baseline period to better capture health-care activity and morbidity, but the follow-up period remained 12 months, as there is evidence that the predictive power of baseline factors appears to wane over a longer period of time. 246
Patients with asthma, CHD, COPD or diabetes were identified using the patient registers used for the QOF306 in GP practice information technology (IT) systems.
We worked with the Surrey team to identify variables that they would be able to extract from routine electronic GP data, which would map on to the predictor and outcome variables from the cohort study that we had undertaken in phase 2. Two variables, attendances at EDs and EHAs, were chosen for the main outcome (i.e. use of unscheduled care). Both these variables were also used to represent measures of previous use of unscheduled care during the baseline period. We chose these two variables because they were easily identifiable from NHS-linked data sets and were both a priority area for government.
Unfortunately, it was not possible from the data sets to identify people who did not have a partner. Although the Surrey team were able to identify some routine demographic data, such as age and sex, marital status was not available and there was no way to identify a proxy measure for either ‘living alone’ or ‘lack of partner’ from the electronic data. There was also no equivalent measure in the routine data sets for threatening life experiences, which we had used in the prospective longitudinal study.
The Surrey team, however, had previously developed a composite mental health variable which they termed CMHP,305 which, in addition to depression and anxiety codes, also included entries relating to stress in the GP notes (e.g. feeling stressed, stress-related problem, problems at work, home problems). Although this was not a direct indicator of ‘threatening experiences’ and CMHP also included all entries relating to depression and anxiety, we decided to carry out analyses using CMHP interchangeably with the depression variable to see if it performed better than depression, because of its inclusion of depression plus several indicators of life stress.
We also used the IMD,235 which could be mapped from patient postcodes, as another potential proxy measure for threatening experiences. The IMD measure is a composite of several factors, which overlap with some items on the threatening experiences scale. The IMD includes the following: income, employment, health deprivation and disability, education skills and training, barriers to housing and services, crime and living environment.
Read codes for depression in GP IT systems were used to construct a depression variable. The details are provided in Methods.
Methods
The data were collected from a large geographical area in north London. We conducted a medical records review study of adult patients with LTCs in primary care; the sources of information consisted of patient records from GP IT systems in primary care practices in this geographical area.
Our sample involved the total GP-registered population available at the time of this study (150,000+) from which we identified those records corresponding to people suffering from one or more of four specified QOF-defined LTCs, namely asthma, CHD, COPD and diabetes. There were 23,422 patients. Owing to regional differences in the way data are collected, we further subdivided the population into the geographical regions north-east and north-west London – there were 8722 patients in the north-east and 14,700 patients in the north-west data sets.
Data source
Routinely collected clinical data from GP information systems for primary care, and routinely collected data from secondary health care (outpatient clinics, day treatment units and hospital inpatient episodes), were used in this study. Primary and secondary health-care data were linked at the individual patient level by the information providers to remove the need for the research team to view and to process patient-identifiable information. This study used only health-care data for the adult population (i.e. those aged ≥ 18 years) between 2007/8 and 2013/14 for analysis. In line with Caldicott principles, only minimum data justified by the purposes of the study were extracted for the study.
The north-west London data were provided by the inner north-west London integrated care programme (ICP). The ICP was a joint provider programme that sought to improve the care of patients with diabetes and those aged ≥ 75 years through a range of support mechanisms, primarily risk stratification, individual care plans and multidisciplinary working across primary and secondary care. The identified issue that the ICP sought to address was of unco-ordinated patient management by multiple providers. The aims of the ICP were to reduce emergency admissions to hospital and improve the quality of care for patients in the target group. The programme was launched in July 2011, the interventions commenced in September 2011, and the programme ended in June 2014.
Data for the sample population were taken from the ICP data warehouse, which included data from all provider organisations engaged in the ICP, including 130 GP practices, three acute providers (Imperial College Healthcare NHS Trust, Chelsea and Westminster Hospital NHS Foundation Trust, and West Middlesex University Hospital NHS Trust), and the local mental health trust (West London Mental Health Trust). The total population size included in the data set was 1,146,819.
The north-east London data were provided by the NHS Waltham Forest Clinical Commissioning Group (CCG). A random sample of 12 GP practices from the CCG was selected by the information services provider (Business Intelligence Analyst at Barking and Dagenham, Havering and Redbridge CCGs) to achieve an adult population of 60,000 ‘active’ patient records at the time of data extraction (July 2014). This data set is more comprehensive in the sense that it captured all primary and secondary health care provided for adult patients, including out-of-area or tertiary referrals for which the CCG had a responsibility. Taken as a whole, Waltham Forest comprises built-up urban districts in the south with inner-city characteristics, and more affluent residential developments in the north.
Index dates
Each anonymised patient record consists of an index date, prior to which there are two full years of GP records, as well as a full year of follow-up records (post-index date). For the north-west London data from the ICP, the index date varies per practice according to the practice’s last successful data extract. The date is calculated from the last successful extract date to allow 2 years of observations before and 1 year of follow-up afterwards. For the north-east London data, the index date used was 28 January 2013, as the Hospital Episode Statistics data were complete up to 28 January 2014. The GP records provided information on emergency admissions to hospital and ED attendances.
We constructed indicator variables for the presence of each of the LTCs at index date, as well as emergency admissions and ED attendances in the pre-index and follow-up periods. We also constructed binary indicator variables for events in the pre-index date period: depression incident (none vs. at least one) and CMHP incidents (none vs. at least one). The codes that were used to construct the depression variable are shown in Table 34.
| Symptoms and signs | CTV3 codes to identify patients with depression | |
|---|---|---|
| Read code | Description | |
| History/symptoms | 1B1T% | Feeling stressed |
| 1B1U% | Symptoms of depression | |
| 1B17% | Depressed | |
| 1BT% | Depressed mood | |
| 1465% | History of depression | |
| Examination/signs | 212S% | Depression resolved |
| Diagnoses | E2B% | Depressive disorder NEC |
| E135% | Agitated depression | |
| E204% | Neurotic depression reactive type | |
| E291% | Prolonged depressive reaction | |
| E2003 | Anxiety with depression | |
| Eu3% | Mood: affective disorders | |
| Mental disorders | Eu412 | [X] Mixed anxiety and depressive disordera |
The variable common mental health problem was defined as having either depression or anxiety, stress or panic. The codes used to construct this variable included all the depression codes plus all the codes in Table 35.
| History, symptoms and diagnosis | CTV3 codes to identify patients with anxiety or stress | |
|---|---|---|
| Read code | Description | |
| History/symptoms | 1B1% | General nervous symptoms |
| 1B1L% | Stress-related problem | |
| 1B1T% | Feeling stressed | |
| 1B1V% | Co-occurring panic attack | |
| 13HT% | Home problems | |
| 13JM% | Problems at work | |
| Preventative procedures | 67J% | Stress counselling |
| Administration | 9ON% | Stress monitoring administration |
| Diagnoses | E200% | Anxiety states |
| E28% | Acute reaction to stress | |
| Eu4% | [X] Neurotic, stress-related and somatoform disordersa | |
| R00z% | [D] Other general symptomsb | |
| R2y2% | [D] Nervousnessb | |
| E2021 | Agoraphobia with panic attacks | |
We banded age into the groups: 18–65, 66–80 and > 80 years.
The IMD variables were mapped from full postcodes included in patient GP records using geographical information system methods. The IMD is a composite score based on the following deprivation measures (communities and local government 2010):235
-
income
-
employment
-
health deprivation and disability
-
education skills and training
-
barriers to housing and services
-
crime
-
living environment.
We treated IMD as a continuous variable, and in multivariate analyses, we analysed these data with and without IMD included as an independent variable.
Statistical methods
All analyses were performed for the north-east and north-west results separately. For each area, univariate analyses used the chi-squared test to determine the association between baseline factors (including emergency admissions in the pre-index 2 years) and emergency admissions in the post-index year. A Mann–Whitney U-test was used for the univariate analyses on IMD scores.
A series of multivariate logistic regression analyses was used to study the association between baseline variables (pre-index date) and either emergency admissions or ED attendance in the year following index date. In particular, we studied potential associations between depression incidence and the more broadly defined CMHP in the pre-index 2 years with subsequent emergency admissions and ED attendances. Other independent variables entered were age in three groups, sex, diagnosis of any of the four LTCs (asthma, CHD, COPD or diabetes), and either EHAs or ED attendance in the 2 years pre-index date. In each logistic regression analysis table presented all the independent variables were entered simultaneously in order to determine the effect of each variable while adjusting for the others. Multicollinearity was checked: the maximum of all variance inflation factor statistics was < 2.2.
All statistical analyses were carried out using RStudio (version 0.98.501; RStudio, Boston, MA, USA).
The potential issue of missing data bias was addressed by multiple imputation. The IMD data field from GP records had missing data (10 missing records from the north-east data set and 500 from the north-west data set); clearly the issue in the north-east data set was minimal. Nevertheless, we performed multiple imputation in both sets. Multiple imputation was implemented using the multiple imputation chained equations (MICE) 2.9 package for R (The R Foundation for Statistical Computing, Vienna, Austria). Using diagnostics from O’Cathain et al.,3 we achieved satisfactory convergence and modelling of conditional probability densities after five iterations of the MICE algorithm. Data were complete for all other measures, except for age (n = 2) in the north-west data set.
Approvals
Ethics approval was granted via a substantial amendment to our original approval for the longitudinal cohort study (REC 09/H1013/80 National Research Ethics Service Committee North West) and we obtained approval from the Confidentiality Advisory Group [CAG 2-07 (a)/2013].
Results
Data were obtained for 23,422 patients: 8722 patients in the north-east and 14,700 patients in the north-west.
Emergency hospital admissions during pre- and post-index date periods: north-east data
Of the north-east baseline cohort of 8722 patients, 1635 (18.7%) had at least one emergency admission to hospital in the 2-year pre-index date period, and 1033 (11.8%) patients had at least one emergency admission during the post-index period of 12 months. Three hundred and eighty-five patients (4.4%) had a depression incident at some time in the 2-year pre-index date period, and 588 (6.7%) had a CMHP.
On univariate analyses, older age, sex, each of the four LTCs, an emergency admission to hospital in the pre-index period and higher IMD scores were all significantly associated with having an EHA in the post-index year (Table 36). A CMHP was significantly associated with having an EHA in the post-index year, but depression was not.
| Baseline variable | Total number of patients (N = 8772) | Emergency admissions in the post-index year | Comparison | |
|---|---|---|---|---|
| Yes, n (%)a | No, n | |||
| Age group (years) | ||||
| 18–65 | 5737 | 489 (8.5) | 5248 | χ2 = 238.6 |
| 66–80 | 2147 | 329 (15.3) | 1818 | p < 0.001 |
| > 80 | 838 | 215 (25.7) | 623 | |
| Sex | ||||
| Female | 4100 | 519 (12.7) | 3581 | χ2 = 4.8 |
| Male | 4622 | 514 (11.1) | 4108 | p = 0.029 |
| Asthma | ||||
| Yes | 4670 | 468 (11.1) | 4202 | χ2 = 31.6 |
| No | 4052 | 565 (13.9) | 3487 | p < 0.001 |
| COPD | ||||
| Yes | 730 | 166 (22.7) | 564 | χ2 = 89.5 |
| No | 7992 | 867 (10.8) | 7125 | p < 0.001 |
| Diabetes | ||||
| Yes | 3517 | 508 (14.4) | 3009 | χ2 = 37.8 |
| No | 5205 | 525 (10.1) | 4680 | p < 0.001 |
| CHD | ||||
| Yes | 1471 | 329 (22.4) | 1142 | χ2 = 186.4 |
| No | 7251 | 704 (9.7) | 6547 | p < 0.001 |
| Emergency admission in pre-index 2-year period | ||||
| Yes | 1635 | 491 (30.0) | 1144 | χ2 = 635.3 |
| No | 7087 | 542 (7.6) | 6545 | p < 0.001 |
| Depression in pre-index 2-year period | ||||
| Yes | 385 | 58 (15.1) | 327 | χ2 = 3.7 |
| No | 8337 | 975 (11.7) | 7362 | p = 0.052 |
| CMHP in pre-index 2-year period | ||||
| Yes | 588 | 86 (14.6) | 502 | χ2 = 4.4 |
| No | 8134 | 947 (11.6) | 7187 | p = 0.036 |
| IMD | ||||
| Score | Median 35.8 (IQR 29.5–42.0) | Median 34.8 (IQR 27.9–40.2) | p < 0.001 | |
Emergency hospital admissions during pre- and post-index date periods: north-west data
Of the north-west baseline cohort of 14,700 patients, 2422 (16.4%) had at least one EHA in the 2-year pre-index period, and 1234 (8.4%) patients had at least one during the post-index period of 12 months. One thousand two hundred and twenty-four patients (8.3%) had depression and 2403 (16.3%) had a CMHP in the 2-year pre-index period.
On univariate analyses, older age, each of the four LTCs, having had an emergency admission to hospital in the pre-index 2 years, higher IMD scores, depression and a CMHP were all significantly associated with having an EHA in the post-index period, but sex was not (Table 37).
| Baseline variable | Total number of patients, N | Emergency admissions in the follow-up year | Comparison | |
|---|---|---|---|---|
| Yes, n (%)a | No, n | |||
| Age group (years) | ||||
| 18–65 | 8906 | 426 (4.8) | 8480 | χ2 = 526.9 |
| 66–80 | 4177 | 469 (11.2) | 3708 | p < 0.001 |
| > 80 | 1617 | 339 (21.0) | 1278 | |
| Sex | ||||
| Female | 6786 | 577 (8.5) | 6209 | χ2 = 0.2 |
| Male | 7912 | 657 (8.3) | 7255 | p = 0.69 |
| Asthma | ||||
| Yes | 7580 | 481 (6.3) | 7099 | χ2 = 84.9 |
| No | 7120 | 753 (10.6) | 6367 | p < 0.001 |
| COPD | ||||
| Yes | 1784 | 299 (16.8) | 1485 | χ2 = 183.5 |
| No | 12,916 | 935 (7.2) | 11,981 | p < 0.001 |
| Diabetes | ||||
| Yes | 5806 | 573 (9.9) | 5233 | χ2 = 26.8 |
| No | 8894 | 661 (7.4) | 8233 | p < 0.001 |
| CHD | ||||
| Yes | 1805 | 286 (15.8) | 1519 | χ2 = 147.4 |
| No | 12,895 | 948 (7.4) | 11,947 | p < 0.001 |
| Emergency admission in pre-index 2-year period | ||||
| Yes | 2422 | 592 (24.4) | 1830 | χ2 = 968.7 |
| No | 12,278 | 642 (5.2) | 11,636 | p < 0.001 |
| Depression in pre-index 2-year period | ||||
| Yes | 1224 | 131 (10.7) | 1093 | χ2 = 8.9 |
| No | 13,476 | 1103 (8.2) | 12,373 | p = 0.003 |
| CMHP in pre-index 2-year period | ||||
| Yes | 2403 | 247(10.3) | 2156 | χ2 = 13.0 |
| No | 12,297 | 987 (8.0) | 11,310 | p < 0.001 |
| IMD | ||||
| Score | Median 32.4 (IQR 21.9–44.7) | Median 30.5 (IQR 18.7–42.5) | p < 0.001 | |
Multivariate logistic regression analyses with emergency hospital admissions in the post-index period as the dependent variable
In the north-east, pre-index period variables that were significantly independently associated with post-index period EHAs were older age, female sex, each of the four LTCs and an emergency admission to hospital in the previous 2 years, but depression was not significant (Table 38).
| Baseline variable | OR | 95% CI | Significance |
|---|---|---|---|
| Depression | 1.17 | 0.86 to 1.59 | 0.33 |
| Age group | |||
| 18–65 (ref) | 1.0 | – | – |
| 66–80 | 1.27 | 1.07 to 1.50 | 0.006 |
| > 80 | 2.14 | 1.74 to 2.64 | < 0.001 |
| Male sex | 0.85 | 0.74 to 0.98 | 0.026 |
| Emergency admission in pre-index period | 4.01 | 3.47 to 4.63 | < 0.001 |
| Asthma | 1.49 | 1.25 to 1.77 | < 0.001 |
| COPD | 1.88 | 1.51 to 2.32 | < 0.001 |
| Diabetes | 1.84 | 1.56 to 2.18 | < 0.001 |
| CHD | 2.12 | 1.77 to 2.53 | < 0.001 |
In the second model, using CMHP instead of depression, the results were essentially unchanged. CMHP was not significant (OR 1.19, 95% CI 0.92 to 1.53; p = 0.19), when adjusted for the remaining covariates in the model.
Similar analyses of the north-west data set showed that older age, emergency admission to hospital during the pre-index period and each of the four LTCs, were all independent predictors of emergency admission to hospital in the post-index period. Neither depression (Table 39) nor CMHP (OR 1.09, 95% CI 0.93 to 1.27; p = 0.30) was significantly associated with emergency admission in the post-index year.
| Baseline variable | OR | 95% CI | Significance |
|---|---|---|---|
| Depression | 1.16 | 0.94 to 1.43 | 0.16 |
| Age group | |||
| 18–65 (ref) | 1.0 | – | – |
| 66–80 | 1.78 | 1.53 to 2.07 | < 0.001 |
| > 80 | 2.94 | 2.48 to 3.49 | < 0.001 |
| Male sex | 0.93 | 0.82 to 1.06 | 0.28 |
| Emergency admission in the pre-index period | 4.25 | 3.74 to 4.84 | < 0.001 |
| Asthma | 1.11 | 0.94 to 1.29 | 0.21 |
| COPD | 1.98 | 1.68 to 2.34 | < 0.001 |
| Diabetes | 1.52 | 1.30 to 1.77 | < 0.001 |
| CHD | 1.54 | 1.30 to 1.84 | < 0.001 |
These results were changed only marginally when IMD was also included as a covariate in each of these analyses. In the north-east, the OR for depression was 1.16 (95% CI 0.85 to 1.58), and for CMHP it was 1.19 (95% CI 0.92 to 1.53). In the north-west, the OR for depression was 1.16 (95% CI 0.94 to 1.42) and for CMHP it was 1.07 (95% CI 0.92 to 1.26).
Emergency hospital admission in the pre-index period was a significant predictor of EHA in the post-index period in all analyses, with an OR of 4.0 (95% CI 3.5 to 4.6) in the north-east (see Table 38) and of 4.3 (95% CI 3.7 to 4.8) in the north-west (see Table 39). Older age, COPD, diabetes and CHD were also independent predictors of post-index emergency admissions in all these logistic regression analyses. Sex and asthma were significant predictors in the north-east but not in the north-west.
Emergency department attendance in the pre- and post-index periods: univariate analyses
Of the north-east baseline cohort of 8722 patients, 2015 patients (23.1%) attended an ED in the post-index period. Depression and CMHP were both significantly positively associated with attendance at an ED in the post-index year: 119 of the 385 depressed patients (30.9%) and 1896 of the 8337 non-depressed patients (22.7%) attended an ED (χ2 = 13.4; p < 0.001), and 177 (30.1%) of patients with a CMHP attended an ED, compared with 1838 (22.6%) of those without a CMHP (χ2 = 17.0; p < 0.001).
Of the north-west baseline cohort of 14,700 patients, 2862 (19.5%) attended an ED in the post-index year. In univariate analyses, depression and CMHP were both significantly positively associated with attendance at an ED in the post-index year: 311 of the 1224 depressed patients (25.4%) and 2551 of the 13,476 non-depressed patients (18.9%) attended an ED (χ2 = 29.6; p < 0.001), and 612 (25.5%) of patients with a CMHP attended an ED, compared with 2250 (18.3%) of those without a CMHP (χ2 = 65.5; p < 0.001).
Multivariate logistic regression analyses with emergency department attendance as the dependent variable
In the north-east, the logistic regression analysis showed that the following variables were all significantly independently associated with attendance at the ED in the post-index period: age > 80 years; each of the four LTCs; an ED attendance in the pre-index 2-year period; and depression (Table 40).
| Baseline variable | OR | 95% CI | Significance |
|---|---|---|---|
| Depression | 1.32 | 1.05 to 1.67 | 0.019 |
| Age group (years) | |||
| 18–65 (ref) | 1.0 | – | – |
| 66–80 | 1.06 | 0.93 to 1.21 | 0.40 |
| > 80 | 1.61 | 1.35 to 1.92 | < 0.001 |
| Male sex | 0.91 | 0.82 to 1.01 | 0.072 |
| ED attendance in the pre-index period | 3.05 | 2.74 to 3.38 | < 0.001 |
| Asthma | 1.65 | 1.43 to 1.91 | < 0.001 |
| COPD | 1.70 | 1.42 to 2.03 | < 0.001 |
| Diabetes | 1.45 | 1.27 to 1.67 | < 0.001 |
| CHD | 1.83 | 1.58 to 2.14 | < 0.001 |
Similarly, in the CMHP analysis, age > 80 years, each of the four LTCs and an ED attendance in the previous 2 years were significant predictors of ED attendance in the post-index period, as was CMHP (OR 1.30, 95% CI 1.07 to 1.58; p = 0.008).
In the north-west, pre-index variables which were significantly independently associated with post-index ED attendance were age 66–80 years, age > 80 years, each of the four LTCs, an ED attendance in the pre-index 2 years and depression (Table 41).
| Baseline variable | OR | 95% CI | Significance |
|---|---|---|---|
| Depression | 1.20 | 1.04 to 1.38 | 0.015 |
| Age group (years) | |||
| 18–65 (ref) | 1.0 | – | – |
| 66–80 | 1.17 | 1.06 to 1.30 | 0.003 |
| > 80 | 1.56 | 1.37 to 1.78 | < 0.001 |
| Male sex | 0.92 | 0.84 to 1.00 | 0.057 |
| ED attendance in the pre-index period | 3.41 | 3.12 to 3.71 | < 0.001 |
| Asthma | 1.22 | 1.08 to 1.38 | 0.001 |
| COPD | 1.47 | 1.29 to 1.67 | < 0.001 |
| Diabetes | 1.30 | 1.15 to 1.47 | < 0.001 |
| CHD | 1.39 | 1.20 to 1.59 | < 0.001 |
Similarly, in the CMHP analysis, age 66–80 years, age > 80 years, each of the four LTCs and an ED attendance in the pre-index period were significant, as was CMHP (OR 1.25, 95% CI 1.12 to 1.39; p < 0.001).
When IMD score was also included in each of these analyses, the results were virtually unchanged. In the north-east, the OR for depression was 1.32 (95% CI 1.04 to 1.67; p = 0.021), and the OR for CMHP was 1.30 (95% CI 1.07 to 1.58; p = 0.008). In the north-west, the OR for depression was 1.19 (95% CI 1.03 to 1.37; p = 0.018) and the OR for CMHP was 1.24 (95% CI 1.11 to 1.38; p < 0.001).
Summary
The following variables were all consistent, independent predictors of unscheduled care (either EHA or attendances at an ED) during the post-index period, in both the north-east and north-west London data sets:
-
use of unscheduled care over the previous 2 years (either EHA or attendance at ED as appropriate)
-
age > 80 years
-
asthma
-
CHD
-
COPD
-
diabetes.
Depression and CMHP were independent predictors of ED attendances, but not of emergency admissions, in both the north-east and north-west data sets. None of these results was changed more than negligibly by the inclusion of IMD score as an additional covariate.
Discussion
We found that the results of the longitudinal cohort study in phase 2 were partially replicated by the analyses of the two separate electronic data sets from London. Prior use of unscheduled care was the most powerful predictor of future use of unscheduled care in both of the London data sets, as it had been in the Manchester cohort study. The magnitude of the relationship between prior and post use of unscheduled care was strikingly similar between the London and Manchester data, whether EHA, attendance at an ED or patient self-report was used as the main indicator of use of unscheduled care. Prior use of unscheduled care in patients with LTCs increased the likelihood of using unscheduled care in the following 12 months by three to four times. There may also be many other factors to account for differences between the Manchester and London data sets. It was not possible because of the anonymity of the data to drill down into the specifics of the reasons why people accessed unscheduled care or to take account of organisational differences in the delivery of health care (e.g. bed availability, OOH services, etc.).
All of the four LTCs, including CHD, were independent predictors of unscheduled care in the London data sets. The size of the ORs for each LTC were again very similar to those of the Manchester data, which suggests a degree of consistency across the London and Manchester data, despite the low response rate to the baseline questionnaire in the Manchester study.
Although depression was an independent predictor of both EHAs and patient self-reported use of other forms of unscheduled care in the Manchester cohort study, it was only an independent predictor of attendance at EDs in the London data sets, but not of EHAs. CMHP, which we used as a partial proxy for ‘stressors’, was also an independent predictor of ED attendances but not of emergency admissions.
Given the consistency across the Manchester and London data, an important question is why depression was not a predictor of emergency admissions in the London data sets, but it was in the Manchester cohort data. One possible explanation may be related to the low rates of depression recorded in the London GP data.
The prevalence of depression was lower in both the London data sets than in the Manchester data. In the north-east London data set, the prevalence of recorded depression over the baseline 2-year period was 4.4% and in the north-west London data set it was 8.8%. The equivalent Manchester rate, which used the HADS241 to detect depression via a questionnaire survey, was over 25%, and this figure is more consistent with that reported in populations of people with LTCs. 69 This suggests that depression was being underdetected in the London data sets, which will have considerably reduced power in the predictor analyses.
It is also possible that depression may have been a stronger predictor in the Manchester cohort study because it was assessed at the time of the baseline assessment, whereas in the London data sets it was recorded as having occurred at any time in the baseline 2-year period. Patients therefore may not have been depressed at the index point.
Case-finding for depression in people with diabetes and heart disease was ‘retired’ from the QOF targets in 2013 in England, but the London data we collected preceded this, so the very low rates cannot be explained by the removal of case-finding, as case-finding should have been in place over the whole duration of the data collection period that was used. It is also possible that GPs may have been recording depression differently to avoid QOF-related activity required by the diagnosis of depression. We have only anecdotal evidence of this but, if correct, it would have contributed to under-reporting.
As already discussed in Chapter 3, there is considerable uncertainty regarding the value of case-finding for depression in primary care. No trials have found that patients with LTCs who undergo case-finding have better outcomes than patients who do not when the same treatments are available to both groups. 307 The findings from our study also suggest that, even when case-finding is implemented in primary care, there are major problems with its execution and delivery.
This is supported by qualitative work which has shown that primary care professionals struggle to integrate case-finding for depression into a clinical review consultation for patients with LTCs, and may unintentionally bias the assessment towards a negative result. 308 There is also a tendency to normalise depression if comorbid with a LTC, and staff feel uncertain how to broach and discuss depression with patients. 297 This problem may be even greater in elderly people with LTCs,309 who are at the highest risk of using unscheduled care.
Although there was a great deal of consistency between the Manchester and London data sets, which support the value of screening for patients ‘at risk’ of using unscheduled care, not all the red flag psychosocial variables that we identified from the Manchester data can be reliably identified from routine GP databases. Certain variables, such as living alone, would be known to GPs and primary care staff, but are not recorded in a way that can be accessed currently by electronic systems. Even when key variables are recorded, such as depression, there is reason to believe that they are significantly under-recorded.
The uncertainty surrounding the value of depression as a predictor of unscheduled care was important to resolve, so we took the opportunity to examine this question further, with data collected from the feasibility trial, which will be described in full in Chapter 8, Feasibility trial.
Limitations
There were several limitations that need to be recognised in relation to the above linked data studies. First, the organisation of health care in the NHS in England is constantly changing, so the findings of any linked data set which involves the study of the use of unscheduled care will be of relevance for the organisation of services only at a specific point in time. Second, the data that are recorded in data-linked studies are clinically based and it is impossible to establish the reliability or validity of the data that are collected. Third, as the data reflect local service organisation, they may not be generalisable to other localities. Even in the two data sets we used from London, there were marked differences between the north-west and north-east data on many of the variables that we considered in terms of prevalence and frequency. Fourth, the composite mental health measure that we used included both depression and anxiety, which may have diluted the results.
Chapter 8 Feasibility trial (phase 3)
Abstract
Background
We developed a two-level intervention based on previous findings from the programme. At the level of the practice, we sought to increase awareness of risk factors associated with use of unscheduled care in people with LTCs, and improve mechanisms of response and referral. At the level of the patient, we developed a four-session intervention for patients with identified psychosocial problems, delivered by liaison health workers (LHWs).
Methods
An exploratory and feasibility cluster RCT involving GP practices in Manchester (three intervention and three control). The main outcome was use of unscheduled care for all patients with COPD in the trial practices. The trial was powered for effects at the level of the practice, but not for the targeted patient intervention. Mixed methods were used to assess the acceptability of the intervention at two levels and to determine key parameters for a future definitive study.
Results
The practice-level intervention was not successfully integrated into the practices and did not impact on use of unscheduled care. The targeted patient intervention was highly acceptable to patients with good recruitment and retention, excellent qualitative feedback and preliminary evidence of a reduction in depression and use of unscheduled care (ED attendances) for the subset of patients who received it.
Conclusions
Organisational change in primary care is difficult and challenging. A targeted patient intervention delivered by LHWs showed promise in terms of its acceptability to patients and in preliminary outcome data.
Overview
In this phase of the programme, our aim was to develop and evaluate an evidence-based, feasible, acceptable psychosocial intervention, which would have the potential to reduce/prevent unscheduled care, while maintaining or improving patient benefit.
To develop the intervention, we synthesised data from the two earlier phases of the programme, combining our evidence synthesis (see Chapter 2) with data from the longitudinal cohort study (see Chapter 3) and two qualitative studies (see Chapters 5 and 6). This earlier work informed both the need for a psychosocial intervention, and the form of the intervention, which was designed to impact at the level of the practice and the patient. We worked with all of our stakeholders to design both levels of the intervention. We took account of local and national changes in the organisation of NHS services, which may also impact on the future delivery of the intervention.
We then carried out an exploratory trial in which we sought to:
-
test the delivery of the intervention, and its adherence and acceptability
-
estimate key parameters for a definitive trial through examination of recruitment rates and a comparison of outcomes for patients in intervention practices compared with those in practices without the intervention.
As in our original grant application, the exploratory trial was powered to determine outcome at the level of the practice intervention. It was not powered to determine outcome for the targeted patient intervention. Elements of an exploratory study and a feasibility study were combined, to maximise grant resource use. We recognise the distinction between feasibility and pilot trials, and the risks of analysis of ‘outcome’ data from either pilot or feasibility studies. All outcome data are regarded as preliminary. Our main focus was on the qualitative evaluations of the acceptability of the intervention both at the level of the practice and the patient, and on determining key parameters to help with a future definitive study.
Developing the intervention
We held a series of meetings with the research team and a 1-day workshop with our stakeholders to draw together our plans for the intervention. We followed the Medical Research Council’s guidance for developing and evaluating complex interventions (URL: www.mrc.ac.uk/complexinterventionsguidance). 310 The first two phases of this approach involved defining the health outcome and its importance, and the target group.
The main results of our evidence synthesis, which brought together all the results from the systematic reviews in phase 1, together with the findings from the prospective longitudinal study and the qualitative work from phase 2, addressed specifically the health outcome in question (i.e. unscheduled care) and the potential target group, and are summarised below:
-
Prior use of unscheduled care, depression, social isolation and social stressors are all independent predictors of unscheduled care use in people with LTCs (evidence from systematic review phase 1 and longitudinal cohort study phase 2).
-
Previous use of unscheduled care is the most powerful predictor of future use of unscheduled care (evidence from longitudinal cohort study phase 2).
-
More severe depression is associated with a twofold increased risk of use of unscheduled care (evidence from longitudinal cohort study phase 2).
-
Threatening life experiences increase the risk of using unscheduled care in a summative fashion (evidence from the longitudinal cohort study phase 2).
-
People with LTCs use unscheduled care because they feel an urgent need to do so, based on their perception of their clinical need (evidence from qualitative review phase 1 and qualitative work phase 2).
-
People with LTCs regard using unscheduled care as the most appropriate way of seeking help when they feel they need to use it, and equate hospital-based services with safety, expertise and high levels of technology. They equate primary care with medication management and often cite barriers in relation to seeking urgent help in the community (evidence from qualitative review phase 1 and qualitative work phase 2).
-
Psychosocial factors, which influence the use of unscheduled care, are absent from patient or health-care workers’ narratives about why people seek urgent help (evidence from qualitative review phase 1 and qualitative review phase 2).
-
Approximately 33–47% of patients with LTCs in primary care report symptoms of depression if given a self–report questionnaire to complete, and patients with COPD report the highest rates of our four exemplar LTCs (evidence from longitudinal cohort study phase 2).
-
Mental health or social issues, which may impact on people’s physical condition, are rarely discussed during routine reviews in primary care for LTCs, and may even be actively blocked by health-care staff if raised by patients (evidence from qualitative work phase 2).
-
The use of unscheduled care as a topic is rarely discussed during routine reviews of patients with LTCs in primary care (evidence from qualitative work phase 2).
-
HCPs struggle to implement behavioural changes which may improve illness outcomes, and many appear to lack the skills to carry out this kind of work (evidence from qualitative work phase 2).
-
There is evidence that low-intensity complex interventions in COPD and asthma reduce the use of unscheduled care, with a low to modest effect size. Greater effects are seen in interventions that included specific psychological interventions. Low-intensity complex interventions included psychosocial interventions (such as psychoeducation, self-help management, or brief cognitive–behavioural approaches) that could be delivered by non-experts (evidence from systematic reviews phase 1).
-
There is great variability between general practices both in terms of the patterns of use of unscheduled care and the prevalence of psychosocial problems in patients with LTCs (evidence from longitudinal cohort study phase 2).
We used a causal modelling approach to develop a simple generic model linking behavioural and physical disease determinants in a causal way. Figure 18 in Chapter 3 illustrates the basic model we developed, which links together prior use of unscheduled care with severity of illness, depression, life stressors and living alone. The number of LTCs an individual suffers from is also factored into the model. The qualitative work that we carried out in relation to the prospective cohort study indicated that use of unscheduled care in people with LTCs was the result usually of an exacerbation of their LTC and contact with unscheduled care services was justified and appropriate. We also found no evidence that people with LTCs were frequent users of unscheduled care. To reduce unscheduled care use in people with LTCs, we would need to develop an intervention to reduce the risk of exacerbations of the LTC.
We had planned to carry out discrete choice experiments, with people with LTCs, to establish the preferences they held for differing types of care when they thought about using unscheduled care. However, the results of the qualitative work we carried out gave a strong steer that people with LTCs were not ‘making choices’ when they used unscheduled care and they felt an imperative to attend the ED or hospital. This view was strongly supported by our patient group recruited for the patient and public involvement (PPI) aspect of the programme. In view of this, we decided that carrying out discrete choice experiments would add little to the development of the intervention.
We determined that our final health outcome would be reduction in secondary unscheduled care use. Our target group would be people with a LTC at risk of use of unscheduled care by virtue of having at least one of our identified risk factors. The target behaviour would be improved mental health and physical health of patients with a LTC as measured by recognised instruments for assessing depression, anxiety and QoL.
In determining the nature of the intervention, we also considered the following: potential therapeutic benefit, simplicity, cost, acceptability to stakeholders, generalisability and sustainability.
The liaison health-care intervention
We designed the intervention to impact both at the level of the practice and the level of the patient. We chose to focus on patients with COPD, as, out of the four LTCs in our longitudinal cohort study (phase 2), COPD was associated with the highest rates of depression (approximately 47%) and high rates of use of unscheduled care. COPD had also become a priority area for our local primary care trust (PCT), and both participation of practices in the trial and the release of funding for the intervention were supported by the PCT and research sponsor. We sought to improve the overall health care of all patients with COPD via the practice-level intervention, and to provide specific additional psychosocial treatment for a subset of patients with identified psychosocial problems, via LHWs (see The individual intervention) attached to the practices.
Practice-level intervention
At the level of the practice, the intervention focused on ways in which the practice as a whole could improve the overall care of patients with COPD, improve methods of identifying at-risk patients, and develop appropriate referral pathways to the LHWs.
We sought to involve the practices in system change. The intervention was designed to improve practice professionals’ awareness of the red flag factors which increased the risk of using unscheduled care in people with LTCs, and to determine ways in which identification of high-risk patients with psychosocial problems could be improved and such patients could be referred to LHWs embedded at each intervention practice. In addition, we sought to improve communication about, and general management of, patients with COPD.
All practice staff were invited to participate in two half-day workshops. Our intention was not to ‘provide’ training for staff, but to facilitate staff themselves to identify current problem areas in their practice in the management of people with COPD and comorbid mental health problems, to discuss how care for people with COPD could be improved, and then to generate potential solutions for their own practice, utilising the LHWs.
Our reasons for this approach were drawn from the principles of normalisation process theory. 311,312 This approach focuses on the things people do when they implement a new or modified way of conceptualising, enacting or organising practice including changes and reorganisation of social relations and interactions, as opposed to their beliefs or intentions. There are four main constructs: coherence; cognitive participation; collective action; and reflexive monitoring. Coherence refers to the sense-making that people employ when working together to develop behaviour change. It includes understanding the value, benefits and importance of a new set of practices and each person understanding his or her specific tasks and responsibilities about the new set of practices. The aim of our workshops was to encourage reflection on the patient pathway in the practice, current management of people with COPD, including identification and management of comorbid mental health problems. Cognitive participation refers to the way people may need to organise or reorganise themselves in order to collectively contribute to the work involved in new practices. The workshops focused on how the LHW might be utilised in the practice. Collective action refers to the how people enact and implement a new set of practices, so we focused, in the workshops, on what changes would be needed in practice activity to offer improved care to people with COPD. Reflexive monitoring refers to what people do to appraise the effects of a new practice, and this was explored in qualitative interviews with practice staff following implementation of the CHOICE intervention.
There is good evidence that behavioural interventions which contribute to normative restructuring of practice, and modifying peer group norms and expectations through educational meetings or outreach work, for example, has the potential for benefit. 313,314
The intended approach of the workshop leaders was to engage participants in a rationale for change based on the evidence synthesis from the CHOICE programme, but predominantly to focus on what change or new set of practices were required to improve the identification of at-risk patients, and to integrate the LHWs into the practice, and what specifically practice staff needed to do to implement these new practices (i.e. what behaviour each person needed to effect for change to occur).
The workshops were run by two members of the CHOICE programme team (EG and CCG). The first workshop involved scene-setting and a presentation of the evidence synthesis from the first two phases of the programme. Practice staff then worked in multiprofessional small groups on three tasks. They were first asked to focus on a scenario where a patient with COPD had used unscheduled care, and to consider the following questions:
-
What happens at a practice level?
-
How does the practice find out someone has used unscheduled care?
-
Who in the practice would receive this information and what would they do with it?
-
What action, if any, would follow?
The practice staff were then asked to consider what aspects of care for patients with COPD currently worked well and where management could be improved. Finally, staff were asked about the psychosocial needs of patients with COPD, how they were detected and addressed and how management could be improved.
The workshop leaders gathered up all the responses generated by the small group work and developed a written summary of the workshop with the main findings, which was e-mailed to all staff who participated in the workshop.
The second workshop involved a recap and discussion of feedback from the first workshop, followed by further multiprofessional small-group work focusing on three key areas:
-
system changes within the practice that would improve care of patients with COPD, with a focus on simple, practical solutions that could be implemented
-
better detection and treatment of psychosocial issues
-
how best to integrate the LHWs into the practice, so that they could provide training and support for staff and individual treatment for patients.
The workshop concluded with a plan for improved care and collaborative working with the liaison mental health workers, which was individualised for each practice. It included the following: the LHWs would attend practice meetings; they would have access to rooms to see patients at the practice; they would enter clinical data on the patients they saw on practice electronic systems; and they would work closely with GPs and other staff to improve the detection and assessment of COPD patients with psychosocial problems.
The workshop leaders provided each practice with a written summary of the workshop with key points and a timetable regarding system changes and liaison mental health worker involvement.
The individual intervention
The individual intervention consisted of brief low-intensity cognitive–behavioural strategies aimed at decreasing depression and social isolation, coupled with social interventions to help with threatening life experiences. The treatment began with a patient-centred interview and development of a problem statement, followed by goals and implementation plans.
Our PPI members gave a strong steer that the intervention should be delivered by professionals who were seen as being part of the practice team and who were not identified as being part of routine mental health services. Our PPI members emphasised the importance of gaining the trust of the patient, and that staff delivering the intervention should be able to develop a personal relationship with the person they were seeing. They stressed the importance of ‘getting to know someone’ rather than getting to know a lot of medical or psychological facts about them. They strongly supported the integration of physical, mental and social aspects of care. The earlier qualitative work we conducted confirmed the published literature, which suggested that people with LTCs were very unlikely to identify and seek help for depression (if suffering from it), but would be ready to talk about problems and difficulties, in consultations that were less ‘QOF’ focused.
We decided to use the term ‘liaison health workers’ to represent a model of liaison psychiatry delivered in the primary care setting. The intention was that specially trained mental health workers would work alongside primary care teams to deliver treatment for depression and psychosocial problems for people with COPD. The LHWs would not be attached to traditional mental health services, but would be embedded in GP practices and receive weekly supervision from a hospital-based liaison psychiatry team (consultant and specialist registrars) to advise on management, prescribing and risk. The LHWs were based at a local mental health facility but visited practices on a regular basis and saw all patients at their own practice or at home. The LHWs also wore practice uniforms, so that patient participants would recognise that they were part of the practice team.
Two LHWs, who had a background training in mental health nursing and social work, were appointed. Training consisted of a 2-day training workshop plus 1 follow-up training day 3 weeks later, which was co-run by Karina Lovell and Elspeth Guthrie. The LHWs followed a cognitive–behavioural manual which had been specifically developed for working with people with chronic illness (principal author, KL) and was adapted by Karina Lovell and Elspeth Guthrie for use in people with COPD. The LHWs were able to test out their skills with a user-provided opportunity of live skills practice. One of the PPIs involved in the programme took a lead role in this part of the training. The LHWs also received training about the nature of COPD; routine investigations for COPD; common treatment approaches for COPD; and pharmacological treatment of depression in COPD.
Patient participants were offered an initial patient-centred assessment session with one of the LHWs. Their physical, mental and social care needs were discussed, and a problem statement was developed. The participant then worked collaboratively with the LHW to identify ways to improve their physical/mental health and ameliorate any major social stressors. The patient participant prioritised the problem areas with which he/she would like help.
The individual intervention used low-intensity cognitive–behavioural techniques including behavioural activation, cognitive restructuring, applied relaxation and problem-solving. It also included a review of medication and modification of any disease-impacting negative behaviours (e.g. smoking), with an additional emphasis on improving exercise engagement. The intervention was also underpinned by a focus on the therapeutic relationship with principles drawn from the conversational model of therapy. 315
The participant’s social stressors were explored and a range of help was provided in relation to debt, state benefit queries, housing problems, or other stressors. Local community resources were mapped so that participants could be linked up with social care services, leisure, social and educational opportunities.
The delivery mode was face to face, either in the participant’s home or at the GP practice dependine on participant preference. The individual intervention comprised four sessions. The first session was 45–60 minutes in duration. The follow-up sessions were of 30 minutes’ duration, with the possibility of four further telephone contacts if required. The duration of treatment was between 6 and 12 weeks. All sessions were digitally recorded if the participants gave consent. The LHWs received weekly supervision using the digital recordings to maintain adherence to the treatment manual protocol and to discuss any other clinical issues.
It was planned that the LHWs would work closely with practice staff, discussing cases and entering all relevant clinical data concerning treatment directly onto the practices’ information systems. The intention was to embed the LHWs into the practices so that they were seen as part of the practice team and not as an outside agency.
The LHWs also carried out regular risk assessments and discussed and advised about antidepressant medication, where indicated. All decisions regarding psychotropic medication were discussed first in supervision with a liaison psychiatrist.
The intervention bore similarities to low-intensity treatment offered by Improving Access to Psychological Therapies (IAPT) services, but differed in that (1) it was not badged as a separate mental health service; (2) patients could be seen in their homes if they wished or their local practice; (3) it included treatment with antidepressant medication, if appropriate, under the guidance of a liaison psychiatrist; (4) it included social interventions to address life stressors and (5) it also included a review of patient’s physical medications for COPD. Table 42 provides more details of the different components of the model and illustrates the combination of physical health, mental health and social interventions (adapted from Langer et al. 316).
| Type of activity | Example |
|---|---|
| Medication review and advice | Initiating or increasing antidepressant medication |
| Signposting of other services | Directing patients to health trainers, complementary practitioners, disability taxi services, ‘good neighbour schemes’, local support groups and charities |
| Cognitive–behavioural intervention | Treatment of low mood; linking of physical and mental health; management of anger, panic and sleep problems; relaxation training plus relaxation CD |
| Education and information | Explaining COPD; providing worksheets leaflets about COPD; information on coping strategies to support activities of daily living |
| Health behaviour advice | Smoking cessation, exercise and diet plans; accompanying patient in exercise; collaborative goal-setting |
| Empathic support | Listening to patient’s accounts and difficulties; providing ‘company’; talking informally about patient’s life; having tea with patient |
| Social interventions | Identifying state benefits to which patient may be entitled; helping complete applications for state benefits, a bus pass and a disability parking badge; supporting applications for volunteering and employment; writing to utility companies to have debts written off; application for grants for essential household items; identification of charities and applications to relevant charities for support regarding finances and debt |
| Providing a positive perspective | Encouraging patients; re-enforcing achievement with praise; suggesting new ways to think about problems and reframe positively |
| Practical support | Liaison with local services to effect practical adaptations in the home such as hand rails, fire alarms and chair supports; accompanying patients to local services or groups |
Testing the intervention
We carried out an exploratory cluster RCT to evaluate the acceptability of the intervention and to determine key parameters for a larger definitive study. In the following section, we describe the quantitative and qualitative methods we used to conduct and evaluate the cluster RCT. This is then followed by a focus on several specific objectives about feasibility issues and the acceptability of the intervention:
-
likely recruitment and retention rate to the trial groups at the level of the practice
-
feasibility and acceptability of data collection methods and measures
-
impact of the intervention at the level of the practice population
-
likely recruitment and retention of patients to the targeted patient intervention (intervention practices only)
-
acceptability of the targeted patient intervention
-
integration of the LHWs into the three intervention practices
-
impact of the targeted patient intervention.
Quantitative methods
The trial was a pragmatic, exploratory, two-arm practice-level cluster RCT evaluating outcomes and costs associated with the CHOICE programme intervention model. It combined elements of both a feasibility study and an exploratory study to maximise grant resource use. The general practice was the ‘cluster’ in the trial and we aimed to recruit six general practices as stipulated in our original application.
As a pragmatic trial of a clinical service embedded within a GP practice, the inclusion criteria were simple and broad with the specific intention of enhancing the external validity of the study without compromising the internal validity.
Eligibility
There were no specific eligibility criteria for practices regarding participation in the study, other than that they were in the footprint of the former Manchester PCT.
For patients, there were three levels of eligibility related to different methods of data collection and different kinds of intervention. As the main outcome measure for the practice-level intervention was based on pseudonymised health-care data, the eligibility for inclusion in this part of the study was that patients had to be aged ≥ 18 years and have a clinical diagnosis of COPD, identified from existing primary care systems, using the QOF register.
To complete the baseline and 12-month assessment questionnaires, which included a measure of QoL and a measure of depression and anxiety, patients had to be deemed well enough and able to give informed consent as judged by their GP. Exclusion criteria for the questionnaires included patients who were terminally ill or who had problems that would impair their ability to provide consent. All eligible patients with COPD were included at this level.
To be referred to the LHW-targeted patient intervention, the patient had to meet all of the above criteria and have at least one of the following risk factors: recent use of unscheduled care, depression, living alone or social stressors. Only patients at risk of unscheduled care use were included at this level.
Practice recruitment and randomisation
Practices were recruited in pairs according to size. The six practices recruited to the trial were randomised to receive intervention or control using random number tables after stratifying them into small (53 and 100 patients), medium (140 and 181 patients) and large (227 and 249 patients) in terms of expected numbers of COPD patients on the practice list (two practices of each size). In each pair of practices, one was assigned to intervention and one to control using a random number table. The statistician for the programme (BT) carried out the randomisation procedure. Three of the practices had previously participated in the cohort study in phase 2 of the programme.
The recruitment of practices was challenging because of the study being undertaken during a period of significant change in the NHS and a complete reorganisation of primary care commissioning. Delays in securing agreements regarding treatment costs, owing to the changes in various organisations, resulted in there being a small window of time to recruit practices. In addition, practices in the north of the city were unable to participate because of participation in a self-care project. The National Institute for Health Research (NIHR) was informed of these difficulties at the time. There was a clear 12-month period between the ending of the cohort study and the start of the trial in any of the practices that participated in both projects.
The practices provided age and sex information for every patient identified as having a QOF diagnosis of COPD on 1 February 2012. The presence of other LTCs, as recorded on the practice QOF electronic database, was also noted for each patient. There are a total of 16 possible LTCs in addition to COPD that are routinely recorded as part of the QOF: CHD, heart failure, stroke, hypertension, diabetes mellitus, epilepsy, hypothyroidism, cancer, mental health, asthma, dementia, depression, kidney disease, atrial fibrillation, obesity and learning difficulties.
Chronic obstructive pulmonary disease severity was classified using the FEV1 per cent predicted values for the patient’s age, height and sex as recommended by the GOLD. 239
Outcome of the intervention at the level of the practice
Use of unscheduled care
The main outcome measure involved data about use of unscheduled care over the 12-month period of the trial (1 February 2013 to 31 January 2014), which corresponds to the year when the LHWs were in post. These data were collected on all patients with COPD at the intervention and control practices. They were obtained on a pseudonymised basis via NHS Digital and linked to the rest of the data collected, using our own study ID numbers. These data included, therefore, data for patients who completed the questionnaire and/or LHW aspects of the study, but also patients who declined to complete the questionnaire and those for whom participation was deemed unsuitable by practice GPs.
These data enabled us to assess the impact of the intervention at the level of the practices on use of unscheduled care. This chapter focuses on two indicators of unscheduled care: EHAs and attendances at an ED. These data, along with the total length of stay of all emergency admissions for each patient, and the total cost of these, were collected from the CCG and the Data Service for Commissioners Regional Office (DSCRO) for the year prior to the intervention (1 February 2012 to 31 January 2013) to assess any baseline differences between the groups, and from NHS Digital for the following year. The cost-effectiveness analysis, which is reported in Chapter 9, estimated the total costs of scheduled and unscheduled care, using the cost data provided by the CCG/DSCRO and NHS Digital.
Anxiety, depression and quality of life
Each practice sent all eligible patients an invitation to participate in the study. Those who agreed completed a baseline assessment via postal questionnaire at the start of the trial, and a further assessment 12 months later at the end of the trial period.
The following data were collected: demographic information for each participant, which included age, sex and ethnicity; marital status; living alone or not; education level; and employment status.
Anxiety and depression were measured using the HADS. This is a well-recognised self-reported scale that measures the severity of symptoms of anxiety and depression. 241 There are seven items for depression and seven for anxiety. Each subscale has a maximum score of 21. Further details of the HADS are provided in Chapter 3.
Health status and associated utility values were measured using the EuroQol-5 Dimensions (EQ-5D). This is a widely used generic instrument for measuring health status (URL: www.euroqol.org/about-eq-5d.html). It comprises the EQ-5D index scale and the EQ-5D visual analogue scale (VAS). The EQ-5D VAS scale is a 20-cm VAS in which the respondent is asked to mark his or her own current state of health on a thermometer-like line calibrated from 0 to 100. The EQ-5D index scale has five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each dimension is divided into three degrees of severity, no problem, some problems or major problems, thus defining 243 possible health states, to which unconscious and dead have been added, for a total of 245 in all. 317 A single index score or utility weight can be produced using information from these five dimensions. The utility weights are an indication of the value or preferences for given health states. The utility values provide the quality-adjusted life-years (QALYs) for states less than full health.
Outcome of the targeted patient intervention
The subsample of patients in the intervention practices who were referred to and seen by the LHWs, completed the Patient Health Questionnaire-9 (PHQ-9)318 and the Generalised Anxiety Scale-7 (GAD-7)319 at the beginning and end of treatment. The PHQ-9 is an instrument for screening, diagnosing and monitoring the severity of depression and is widely used in general practice and IAPT services. PHQ-9 scores of 5, 10, 15 and 20 represent mild, moderate, moderately severe and severe depression, respectively. The GAD-7 is a seven-item, brief, self-reported measure for screening and measuring the severity of anxiety, which is widely used in general practice and IAPT services. Scores of 5, 10 and 15 on the GAD-7 are regarded as indications of mild, moderate and severe anxiety, respectively.
There was not an equivalent control group embedded within the control practices that could be used for comparison, as the absence of both the practice-level intervention and LHWs in the control practices meant similar referral pathways could not be identified.
Sample size
The data analysis is mainly descriptive and addresses the primary outcomes relating to the feasibility of conducting a future definitive RCT. We included a sample size calculation in the original grant application to help determine the future power and modelling for a definitive study. We updated this sample size calculation using data we collected from phase 2 of the programme. We used these data to power the trial at the level of the practice intervention but not at the level of the targeted patient intervention. In our longitudinal cohort study, 23% of COPD patients had an emergency inpatient admission in the prospective year. We determined that if the intervention was able to reduce this by 40% to 13.8%, then a two-group continuity-corrected chi-squared test with 0.10 two-sided significance level would have 90% power to detect the difference between intervention and control groups when the sample size in each group is 324 (nQuery Advisor, version 7; Statistical Solutions, Saugus, MA, USA).
We aimed to recruit 400 patients in each group, which would allow for modest levels of clustering within practices, although we did not find any in the longitudinal cohort study in which the intraclass correlation coefficient was zero.
This study involved 467 patients from the intervention practices and 483 from the control practices.
Statistical analysis
The main analyses were based on intention-to-treat principles. Data on use of unscheduled care were obtained for all patients in the study. For categorical variables, the numbers and percentages of patients in each group are presented, and were compared using the chi-squared test. Means and SDs are presented for continuous variables at baseline and follow-up, and were compared using t-tests. For follow-up on categorical measures (ED attendance and emergency admission), intervention effects were compared using logistic regression with the covariates age, sex, baseline score and intervention group, and for follow-up on continuous variables, regression was used. In both sets of analyses, robust standard errors were calculated specifying practices as the six clusters. Similar analyses were carried out to test for group differences on secondary outcome measures (HADS scores, EQ-5D VAS and EQ-5D utility scores).
Qualitative evaluation
We conducted a qualitative evaluation that was carried out in the intervention practices, with staff in those practices, and patients who had been offered, received, or declined the input of the LHWs. The main purpose of this work was to assess the acceptability of the intervention at the level of the practice and the patient.
Recruitment
We invited staff at the intervention practices to participate in interviews, seeking a range of staff in different roles, including GPs, PNs, HCAs and administrative staff. All participants received written information about the study and provided written consent for interview.
We also recruited patients who had been invited to see the LHW, using maximum variation sampling to recruit patients across the range of comorbidities and ages of those invited to see the LHW, and at a range of intervals from 1 to 22 weeks after the intervention ended. We made particularly strenuous efforts to recruit those who declined or withdrew from the intervention. For patients who completed the LHW intervention, the LHW provided a PIS describing the qualitative evaluation and a reply slip on which they could indicate interest in being interviewed and a pre-paid envelope. For patients who declined the intervention or withdrew from it, a member of the research team sent the information sheet and a reply slip. We then contacted patients whose replies were positive to arrange an interview.
Interviews and analysis
We conducted face-to-face interviews with practice staff in their practice (mean duration 30 minutes). The topic guide included their expectations and experience of the LHWs, effects on patients or the practice, and why patients were referred to the LHWs. We conducted semistructured, face-to-face interviews with patients (mean duration 47 minutes) either at their general practice or in their homes, according to participant preference. The topic guide included their health difficulties and psychosocial context; expectations of the LHW; experience of the LHW, including benefits or difficulties associated with the care from the LHW; comparison with other experienced treatments for mood difficulties; and reflections on the intervention since it ended.
The three female researchers conducting the interviews all had experience in qualitative interviewing and were supervised by senior members of the study team. They were independent of the intervention and clinical teams. Recruitment, both of patients and of staff, stopped when the analysis reached theoretical saturation.
Data were anonymised and transcribed verbatim. Analysis was inductive, taking a constant comparative approach. The researchers and key members of the study team read and reread the transcripts, and discussion among the whole team identified analytic categories that we documented in continually updated analysis notes. We paid particular attention to deviant cases that challenged our analysis. The ellipsis (. . .) signifies omitted text. Square brackets denote explanatory text. Illustrative quotations are labelled with the practice identifier (R, G or B) and patient identification number and for staff, the staff category.
Ethics approval was received from National Research Ethics Service Committee Northwest – Greater Manchester East (reference number 12/NW/0068).
Specific objectives
The feasibility outcomes are outlined in Table 43 together with the methods we used to evaluate each outcome.
| Feasibility | Evaluation |
|---|---|
| Recruitment and retention to the trial groups at the level of the practice | Numbers assessed for eligibility; numbers eligible; reasons for ineligibility; numbers who participated and did not participate; number of dropouts from intervention and control limbs |
| The feasibility and acceptability of data collection methods and measures | Number of missing items and follow-up rates. Ease of access to data from the CCG/DSCRO |
| Impact of the intervention at the level of the practice | Comparison of use of unscheduled care between intervention and control practices for the 12 months of the study |
| Recruitment and retention of patients to the targeted patient intervention | Number of patients eligible for the LHW intervention; number recruited to the intervention; and number who completed treatment |
| Acceptability of the targeted patient intervention | Qualitative process interviews with patients who complete and drop out of treatment |
| Integration of the LHWs into the three intervention practices | Qualitative process interviews with practice staff |
| Impact of the intervention at the level of the patient | Before-and-after change in the use of unscheduled care. Change in psychological measures of anxiety and depression before-and-after treatment |
Recruitment and retention to the trial groups at the level of the practice
This concerned the proportion of patients with COPD in the treatment and control practices who were deemed eligible by the GPs to participate in the overall evaluation at the beginning and end of the study. Essentially, it involved the numbers of patients who were eligible to participate in the postal assessment at baseline and at 12 months, and to complete the HADS and EQ-5D measures.
Feasibility and acceptability of data collection methods and measures
This concerned the ease of access to health-care utilisation data for patients in the intervention and control practices and the practicalities of requesting and obtaining data from the CCG/DSCRO. When we carried out the cohort study in phase 2 of the programme, we decided to use GP records and a participant self-reported measure to assess health-care use. It was, however, labour intensive to collect data using the GP records, and it would not be feasible to use this method for a definitive cluster trial. The establishment of NHS Digital during the lifetime of the programme meant that it became possible to request access to anonymised health-care utilisation data on all patients in the intervention and control practices in the study. Although we were directed to use the local DSCRO for time point 2 data, because NHS Digital was taking a review of their processes for handling release of data, we were able to demonstrate that the data were available and could be obtained.
Impact of the intervention at the level of the practice population
We examined three key outcomes to assess the impact of the intervention at the level of the practice. First, we compared the use of unscheduled care for all patients in the intervention and the control practices with COPD, for the year before the intervention and during the intervention year. We also compared the scores on the HADS (outcome two) and EQ-5D (outcome three) for all patients who agreed to participate in the study and who completed these measures at baseline and at the end of the intervention year.
Likely recruitment and retention of patients to the targeted patient intervention
This involved collecting data about the number of patients deemed suitable for the targeted patient intervention delivered by the LHWs. We were interested in the percentage of patients who could be recruited to the intervention and the number who completed an agreed number of sessions with an LHW.
Acceptability to patients of the targeted patient intervention
This was assessed by conducting qualitative interviews with patients who agreed to the LHW intervention, and then who either completed or dropped out of treatment. We also attempted to interview patients who declined the LHW intervention.
Integration of the liaison health workers into the practice
We conducted qualitative interviews with a range of practice staff at the intervention practices during the course of the 12-month intervention period to assess how well the LHWs were integrated into each of the three intervention practices.
Impact of the targeted patient intervention
We assessed this by comparing before-and-after scores for depression on the PHQ-9 and for anxiety on the GAD-7. We also compared data on use of unscheduled care for the baseline and intervention year for patients who saw the LHWs and patients in the intervention practices who did not see the LHWs.
Results
Recruitment and retention to the trial groups at the level of the practice
Baseline assessment
Six GP practices participated in the trial. It was agreed with the local PCT that involvement in the study would count towards the PCT Quality and Productivity Indicators for 2013. All practices were located in Manchester. Nine hundred and fifty patients with a QOF diagnosis of COPD were identified from the six practices (clusters) (ranging from 53 to 249 patients per practice). Of the 950 patients with COPD, 234 (142 in the intervention and 92 in the control practices) were excluded from the study by the practice GPs as they were considered to be ineligible (e.g. too ill to participate). A total of 325 (69.6%) intervention and 391(81.0%) control patients were sent a baseline questionnaire. This difference is significant (Fisher’s exact test p < 0.001). The individual practices’ percentages are 65.2%, 65.7% and 85.0% for the intervention practices and 81.9%, 84.0% and 66.0% for the control practices. Baseline assessments were mailed to the remaining 716 eligible patients, and these were returned by 397 people (55.4%). Of these, 32 were blank, indicating that the patient did not wish to take part in the study, nine were returned marked ‘not at this address’ and one was completed by the wrong person, leaving 355 (49.5%) valid baseline questionnaires, which were completed between November 2012 and February 2013 (Figure 22).
FIGURE 22.
Flow of participants through baseline and 12-month follow-up questionnaire components of the cluster RCT. C, control; I, intervention.
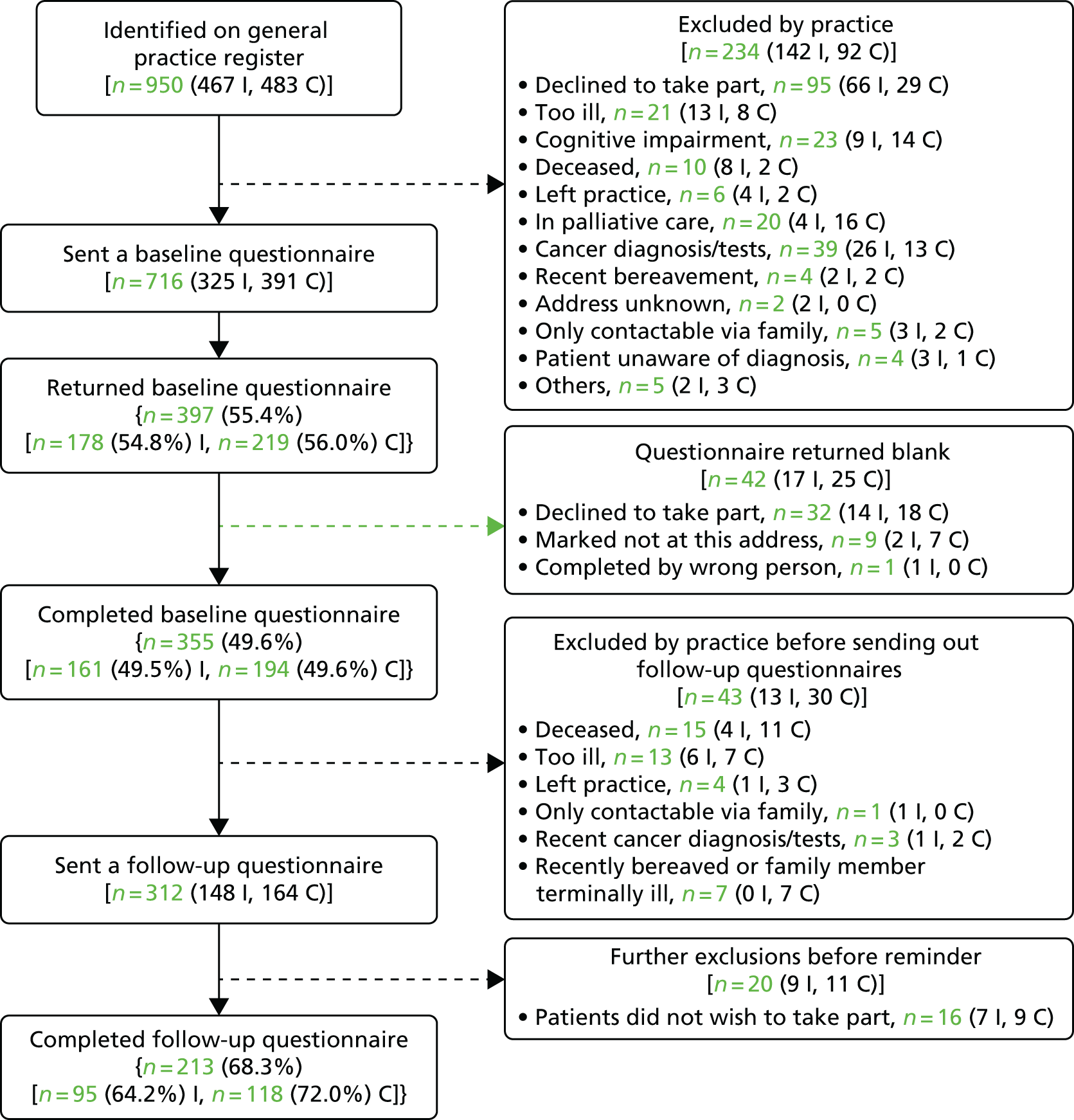
In Figure 22, every sample size n is followed by the sample sizes for the intervention and control groups separately in brackets. For example, identified on general practice registers n = 950 (467 I, 483 C) means 467 patients from the intervention practices and 483 patients from the control practices.
The 12-month assessment
Of the 355 patients who completed the baseline evaluation, 43 (n = 13 in intervention practices and n = 30 in control practices) were excluded by the practice GPs before 12-month assessments were mailed because they had died, left the practice or were too ill. The 12-month assessments were mailed to 312 eligible patients and were returned by 213 patients (68.3%; 95 in the intervention practices and 118 in control practices). These assessments were completed between October 2013 and January 2014, with a mean of 354 days between baseline and follow-up assessments.
The rates of completion of the 12-month assessments for patients in the intervention practices (64.1%) and those in control practices (72.0%) were not significantly different (p = 0.15; see Figure 22). However, the 12-month assessment completion rates were significantly lower for patients who had reported higher levels of depression and anxiety and poorer QoL at baseline.
There was large variability between completion rates of the baseline assessments at the different practices. Completion rates ranged from 36.5% to 65.7% at the six practices (χ2 = 14.8; p = 0.011), but the overall rates for intervention (49.5%) and control practices (49.6%) were similar. There was also large variability in terms of the age range of patients and completion rates. The highest rates were for patients aged 60–69 years (55.9%) and patients aged 70–79 years (53.1%). The lowest rates for completion were by patients aged < 50 years (33.3%). There was no significant difference between male (51.1%) and female (48.0%) completion rates (p = 0.41).
Differences between eligible and ineligible patients
There were significant differences between patients who were deemed eligible and ineligible by the practice GPs. Table 44 shows the use of unscheduled care in the year before the intervention for patients who took part in the various stages of the study, according to ED attendances and EHAs. Patients who were excluded by the practices from being sent questionnaires to complete were more likely to have attended an ED or had an EHA in the year prior to the study than those who were not excluded. However, of those patients who were sent a baseline questionnaire, there was no difference between those who returned a completed questionnaire and those who did not. This was also the case for the follow-up assessment at 12 months.
| Subgroup of patients | Attended an ED, n (%) | Had an emergency admission, n (%) |
|---|---|---|
| All patients (n = 950) | 323 (34.0) | 218 (22.9) |
| Excluded by practice (n = 234) | 107 (45.7) | 89 (38.0) |
| Sent baseline questionnaire (n = 716) | 216 (30.2) | 129 (18.0) |
| Significance for excluded vs. non-excluded | p < 0.001 | p < 0.001 |
| Declined (n = 361) | 112 (31.0) | 70 (19.4) |
| Returned a completed baseline questionnaire (n = 355) | 104 (29.3) | 59 (16.6) |
| Significance for completed vs. declined | p = 0.63 | p = 0.38 |
| Excluded by practice prior to 12-month assessment (n = 43) | 14 (32.6) | 7 (16.3) |
| Sent a 12-month assessment (n = 312) | 90 (28.8) | 52 (16.7) |
| Significance for excluded vs. sent | p = 0.60 | p = 1.0 |
| Did not complete 12-month assessment (n = 99) | 25 (25.3) | 14 (14.1) |
| Completed 12-month assessment (n = 213) | 65 (30.5) | 38 (17.8) |
| Significance for completed vs. not completed | p = 0.35 | p = 0.51 |
The feasibility and acceptability of data collection methods using NHS Digital data
The Associate Director for Primary Care Commissioning at NHS Manchester (now Manchester CCG) assisted the request for access to pseudonymised data relating to the use of unscheduled care of all patients registered on the COPD QOF registers at the six practices involved in the exploratory trial. A data request form was submitted to the Manchester PCT General Practice Information Group (GPIG) in early 2012 for the pseudonymised aggregated data relating to the use of unscheduled care of all COPD patients registered at the practices. Approval from the GPIG to authorise the sharing of these data was received in June 2012. Graham Hayler, Head of Information at NHS Manchester, provided oversight of the request and the Business Intelligence Manager at NHS Manchester supervised the data extraction.
A document summarising the data required was provided by the research team to NHS Manchester. This request listed the practices supporting the trial and the data required. Data were requested for the 12 months prior to the baseline questionnaire completion (time point 1) and 12 months after the baseline questionnaire completion (time point 2). A secure data transfer method was used to provide the NHS numbers of patients within the cohort at time point 1. These numbers were used as the unique identifier to extract the relevant data and were retained for the time point 2 data extraction. This was a fairly straightforward process when requesting time point 1 data; however, with the setting up of CCGs, challenges were experienced in acquiring the data at time point 2. A NHS Digital data request was completed, but due to the national ‘care.data programme’ issues, NHS Digital began a comprehensive review of their systems and processes which meant that the process for gaining permissions for accessing data was paused.
Owing to the potential impact on the timelines of the study we were required to approach the CCG to explain the issues being faced and asked if they could assist with obtaining the required data as they had processed the time point 1 data request. The CCG advised that the local office of NHS Digital (known as the DSCRO) would be able to process the request. These data were provided, in September 2013, by the local DSCRO regional office at the request of the CCG Head of Information. The original GPIG approval was used to reconfirm the correct permissions for this request.
The process followed to get the data demonstrates that the required data are available and can be obtained for research purposes. In future, it is expected that the data request would go through the central NHS Digital, which has streamlined its data request process. However, it is not clear what impact, if any, the ‘care.data programme’ will have on future requests.
Impact of the intervention at the level of the practice population: pseudonymised data
These analyses involve all patients with COPD at the six practices, whether they participated in the questionnaire components of the study or saw the LHWs in the intervention practices. Data were obtained from the practice lists and from NHS Digital.
There were significant differences between the intervention and control practices at baseline in demographic and disease data. Patients in the intervention practices were significantly older, more likely to be male, and their COPD was more severe than in the control practices. There was also more comorbidity in the intervention practices, with patients more likely to have a QOF diagnosis of stroke, hypertension, diabetes, cancer, mental health, asthma, dementia, depression or obesity than the patients in the control practices (Table 45).
| Data supplied by practices | Whole group (n = 950) | Intervention (n = 467) | Control (n = 483) | Comparisona | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | p-value | |
| Age group (years) | |||||||
| < 50 | 62 | 6.5 | 24 | 5.1 | 38 | 7.9 | χ2 = 14.6 |
| 50–59 | 176 | 18.5 | 81 | 17.3 | 95 | 19.7 | df = 4 |
| 60–69 | 285 | 30.0 | 143 | 30.6 | 142 | 29.4 | 0.006 |
| 70–79 | 254 | 26.7 | 114 | 24.4 | 140 | 29.0 | |
| ≥ 80 | 173 | 18.2 | 105 | 22.5 | 68 | 14.1 | |
| Female | 475 | 50.0 | 214 | 45.8 | 261 | 54.0 | 0.014 |
| QOF diagnoses | |||||||
| CHD | 153 | 16.1 | 82 | 17.6 | 71 | 14.7 | 0.25 |
| Heart failure | 60 | 6.3 | 34 | 7.3 | 26 | 5.4 | 0.23 |
| Stroke | 59 | 6.2 | 45 | 9.6 | 14 | 2.9 | < 0.001 |
| Hypertension | 453 | 47.7 | 239 | 51.2 | 214 | 44.3 | 0.038 |
| Diabetes mellitus | 177 | 18.6 | 102 | 21.8 | 75 | 15.5 | 0.015 |
| Epilepsy | 22 | 2.3 | 12 | 2.6 | 10 | 2.1 | 0.67 |
| Hypothyroidism | 64 | 6.7 | 37 | 7.9 | 27 | 5.6 | 0.16 |
| Cancer | 106 | 11.2 | 88 | 18.8 | 18 | 3.7 | < 0.001 |
| Mental health | 198 | 20.8 | 192 | 41.1 | 6 | 1.2 | < 0.001 |
| Asthma | 93 | 9.8 | 64 | 13.7 | 29 | 6.0 | < 0.001 |
| Dementia | 9 | 0.9 | 8 | 1.7 | 1 | 0.2 | 0.019 |
| Depression | 79 | 8.3 | 76 | 16.3 | 3 | 0.6 | < 0.001 |
| Kidney disease | 107 | 11.3 | 58 | 12.4 | 49 | 10.1 | 0.31 |
| Atrial fibrillation | 61 | 6.4 | 33 | 7.1 | 28 | 5.8 | 0.43 |
| Obesity | 6 | 0.6 | 6 | 1.3 | 0 | 0 | 0.014 |
| Learning difficulties | 2 | 0.2 | 1 | 0.2 | 1 | 0.2 | 1.0 |
| Severity of COPDb | |||||||
| Mild | 219 | 24.8 | 78 | 18.4 | 141 | 30.7 | χ2 = 21.1 |
| Moderate | 473 | 53.5 | 239 | 56.2 | 234 | 51.0 | df = 3 |
| Severe | 184 | 20.8 | 105 | 24.7 | 79 | 17.2 | < 0.001 |
| Very severe | 8 | 0.9 | 3 | 0.7 | 5 | 1.1 | |
In the year before baseline, 323 (34.0%) of the 950 patients with COPD at the intervention and control practices attended an ED, and 218 (22.9%) had an EHA (Table 46). Significantly more patients in the intervention practices than the controls attended an ED in the year before baseline (39.6% vs. 28.6%; p < 0.001) and had an EHA (27.0% vs. 19.0%; p = 0.004), but in the year of the intervention the two groups had similar attendance figures at an ED.
| Categorical data | Whole group (n = 950) | Intervention (n = 467) | Control (n = 483) | Comparisona | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | p-value | |
| ED attendance in the year before baseline | 323 | 34.0 | 185 | 39.6 | 138 | 28.6 | < 0.001 |
| ED attendance in the year after baseline | 244 | 25.7 | 123 | 26.3 | 121 | 25.1 | 0.66 |
| Emergency admission in the year before baseline | 218 | 22.9 | 126 | 27.0 | 92 | 19.0 | 0.004 |
| Emergency admission in the year after baseline | 254 | 26.7 | 129 | 27.6 | 125 | 25.9 | 0.56 |
There was a significant reduction in the proportion of patients who attended an ED in the intervention practices, but not in the control practices, for the year of the intervention compared with the baseline year. In the year prior to the study, 39.6% of patients in the intervention practices attended an ED, compared with 26.3% in the year of the intervention, a fall of 13.3% (McNemar’s test of change: p < 0.001). In contrast, in the control practices, there was little change, with 28.6% of patients attending an ED in the year prior to the intervention and 25.1% in the year of the intervention (McNemar’s test of change: p = 0.22)
In the intervention practices, the proportion of patients who had an EHA remained similar (27.0% in the year before intervention and 27.6% in the year after; p = 0.87), whereas the proportion increased in the control practices, from 19.0% to 25.9% (p = 0.008).
In the multivariate analyses, there was no significant difference between intervention and control groups on use of unscheduled care in the intervention year, after adjustment for age, sex, unscheduled care in the pre-baseline year and clustering of practices. In logistic regression analysis, the OR for intervention and ED attendance was 0.97 (95% CI 0.65 to 1.45; p = 0.89), and the OR for emergency admission was 1.01 (95% CI 0.66 to 1.53; p = 0.98). In multiple linear regression on the number of ED attendances, the regression coefficient for intervention was –0.06 (95% CI –0.29 to 0.16; p = 0.50), and in multiple linear regression on the number of emergency admissions the regression coefficient for the intervention was –0.05 (95% CI –0.30 to 0.19; p = 0.62).
Hospital Anxiety and Depression Scale and EuroQol-5 Dimensions utility scores
There were no major differences between the patients in the intervention practices and control practices in terms of demographic data, levels of anxiety and depression, and health status at the baseline assessment (Tables 47 and 48). Of 350 patients who completed the baseline HADS, 173 (49.4%) scored ≥ 8 on the depression subscale and 181 (51.7%) scored ≥ 8 on the anxiety subscale. At follow-up, 204 patients completed the HADS, of whom 86 (42.2%) scored ≥ 8 for depression and 95 (46.6%) scored ≥ 8 for anxiety. Neither change was significant (McNemar’s test of change: p = 0.72 for depression and p = 1.0 for anxiety).
| Baseline questionnaire data | Whole group (n = 355) | Intervention (n = 161) | Control (n = 194) | Comparison,a p-value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| White ethnic group | 337 | 94.9 | 153 | 95.0 | 184 | 94.8 | 1.0 |
| Marital statusb | |||||||
| Single | 56 | 16.0 | 25 | 16.0 | 31 | 16.1 | χ2 = 0.1 |
| Married or cohabiting | 169 | 48.4 | 74 | 47.4 | 95 | 49.2 | df = 2 |
| Widowed, separated or divorced | 124 | 35.5 | 57 | 36.5 | 67 | 34.7 | 0.93 |
| Living alone | 126 | 35.9 | 57 | 35.8 | 69 | 35.9 | 1.0 |
| Poor educationc | 266 | 74.9 | 113 | 70.2 | 153 | 78.9 | 0.066 |
| Not working due to ill health | 72 | 20.3 | 34 | 21.1 | 38 | 19.6 | 0.79 |
| Unemployed but seeking work | 7 | 2.0 | 2 | 1.2 | 5 | 2.6 | 0.46 |
| Mean | SD | Mean | SD | Mean | SD | Comparisond | |
| HADS scorese | |||||||
| Anxiety | 7.8 | 5.0 | 7.8 | 4.8 | 7.8 | 5.1 | t = 0.1; p = 0.92 |
| Depression | 7.7 | 4.9 | 7.9 | 4.9 | 7.6 | 4.9 | t = 0.6; p = 0.52 |
| Baseline questionnaire data | Whole group (n = 355) | Intervention (n = 161) | Control (n = 194) | Comparison,a p-value | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| EQ-5D | |||||||
| Mobilityb | |||||||
| No problems | 92 | 26.4 | 35 | 22.2 | 57 | 30.0 | χ2 = 4.8 |
| Some problems | 251 | 72.1 | 119 | 75.3 | 132 | 69.5 | df = 2 |
| Confined to bed | 5 | 1.4 | 4 | 2.5 | 1 | 0.5 | 0.089 |
| Self-carec | |||||||
| No problems | 220 | 65.3 | 92 | 61.3 | 128 | 68.4 | χ2 = 2.3 |
| Some problems | 107 | 31.8 | 52 | 34.7 | 55 | 29.4 | df = 2 |
| Unable to wash and dress | 10 | 3.0 | 6 | 4.0 | 4 | 2.1 | 0.31 |
| Usual activitiesb | |||||||
| No problems | 109 | 31.3 | 45 | 28.5 | 64 | 33.7 | χ2 = 4.2 |
| Some problems | 194 | 55.7 | 97 | 61.4 | 97 | 51.1 | df = 2 |
| Unable to perform usual activities | 45 | 12.9 | 16 | 10.1 | 29 | 15.3 | 0.13 |
| Pain/discomfortd | |||||||
| None | 80 | 23.3 | 26 | 16.8 | 54 | 28.6 | χ2 = 6.9 |
| Moderate | 218 | 63.4 | 108 | 69.7 | 110 | 58.2 | df = 2 |
| Extreme | 46 | 13.4 | 21 | 13.5 | 25 | 13.2 | 0.032 |
| Anxiety/depressione | |||||||
| None | 155 | 46.4 | 66 | 43.7 | 89 | 48.6 | χ2 = 2.3 |
| Moderate | 138 | 41.3 | 69 | 45.7 | 69 | 37.7 | df = 2 |
| Extreme | 41 | 12.3 | 16 | 10.6 | 25 | 13.7 | 0.31 |
| General health today compared with the last 12 monthsf | |||||||
| Better | 30 | 8.5 | 13 | 8.1 | 17 | 8.8 | χ2 = 1.9 |
| Much the same | 204 | 57.8 | 87 | 54.4 | 117 | 60.6 | df = 2 |
| Worse | 119 | 33.7 | 60 | 37.5 | 59 | 30.6 | 0.39 |
| Mean | SD | Mean | SD | Mean | SD | Comparisong | |
| EQ-5D utility scoreh | 0.54 | 0.34 | 0.52 | 0.33 | 0.55 | 0.35 | t = 0.9; p = 0.38 |
| EQ-5D VASi | 55.1 | 21.1 | 54.6 | 20.9 | 55.5 | 21.3 | t = 0.4; p = 0.71 |
There were no significant differences between the intervention and control groups at the 12-month assessment (Table 49).
| Follow-up questionnaire data (n = 213) | Intervention (n = 95) | Control (n = 118) | Comparisona | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| HADS scoresb | |||||
| Anxiety | 7.4 | 4.9 | 7.2 | 5.0 | t = 0.4; p = 0.72 |
| Depression | 7.5 | 4.6 | 6.9 | 4.6 | t = 1.0; p = 0.34 |
| EQ-5D | |||||
| EQ-5D utility scorec | 0.55 | 0.34 | 0.61 | 0.35 | t = 1.3; p = 0.20 |
| EQ-5D VASd | 59.1 | 20.6 | 57.2 | 21.3 | t = 0.6; p = 0.53 |
Recruitment and retention of patients to the targeted patient intervention
This next section focuses on the subset of patients in the intervention practices who were referred for treatment to the LHWs. Patients who were invited to participate in this part of the study were all considered to be at risk of using unscheduled care, as evidenced by having at least one of the following risk factors: depression, social stress, isolation and/or a recent episode of unscheduled care.
Out of the 467 patients in the intervention practices, 166 (35.5%) were referred by practice staff and contacted by the LHW (Figure 23).
FIGURE 23.
Flow of study participants for LHWs in three intervention practices.
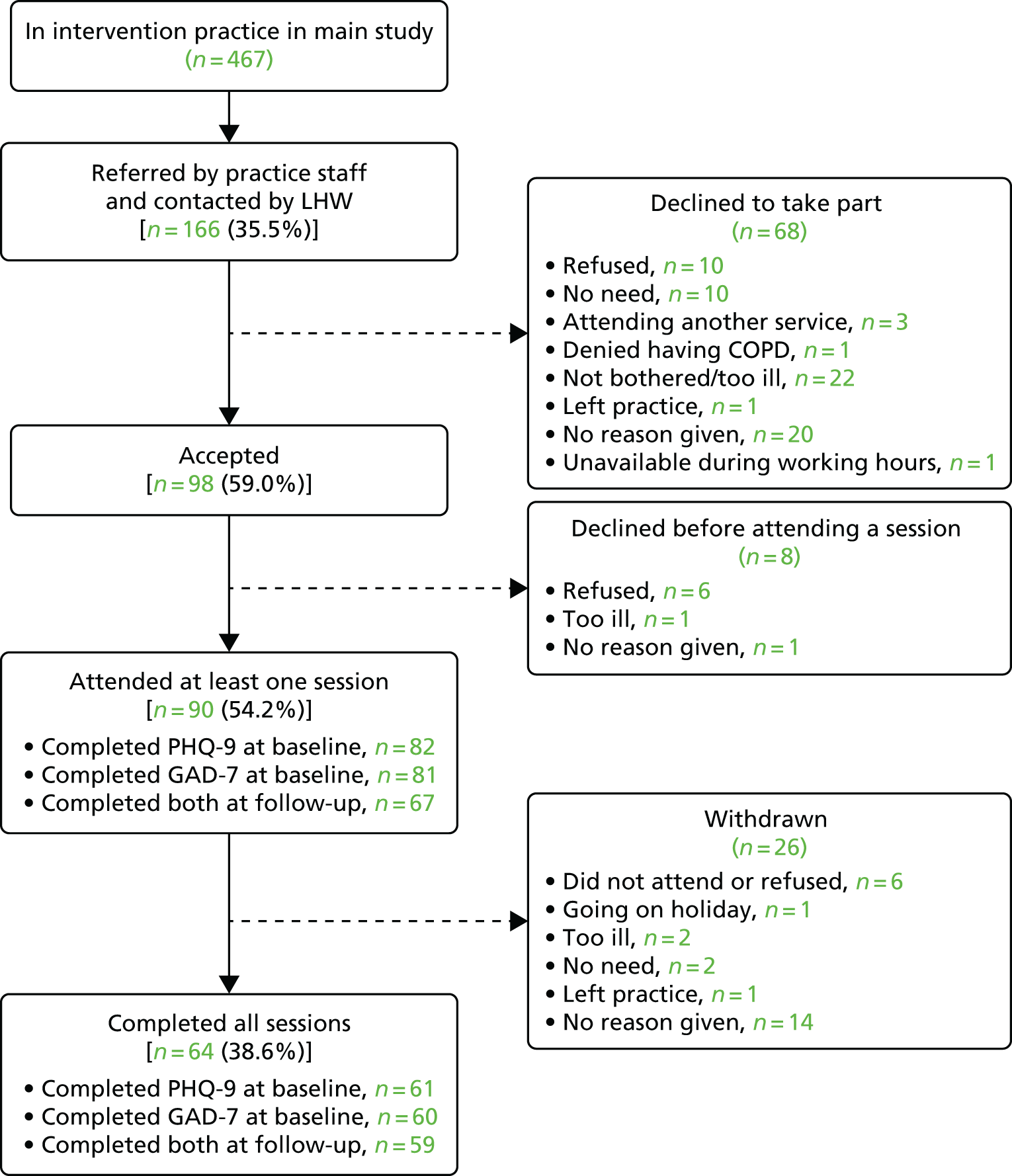
Sixty-eight patients declined to take part, of whom 10 refused, 10 said they had no need of help from the LHW, three were attending another service, one denied having COPD, 22 were not bothered or were too ill, one had left the practice, 20 did not give a reason and one was unavailable in working hours. This left 98 patients who accepted, of whom a further eight did not attend the first session. Of the 90 patients who started treatment, 64 (71%) completed an agreed number of sessions.
Acceptability of the intervention at the level of the patient
Of the patients who completed the intervention and indicated a willingness to be interviewed, we interviewed 26, ending recruitment when no new themes or categories were emerging from the data (category saturation). This assumes that the views of patients who were willing to be interviewed were representative of those who were not willing to be interviewed. The categories were checked for completeness across the sample. We sent the PIS and reply slip to 51 patients who declined the intervention, of whom one agreed and was interviewed. We invited 17 of the patients who withdrew before finishing the LHW intervention to take part in an interview, of whom two agreed and were interviewed. The 29 patients interviewed (16 female) had a mean age of 65 years (range 48–81 years). Mean PHQ-9 score for interviewed patients was 12.27; mean GAD-7 score was 9.50.
Almost all patients were positive about the LHWs. Comments were not merely ‘polite’, but described LHWs in effusive language (e.g. as my ‘angel in the darkness’, B198) or as having ‘done more than I even dreamt of’ (R195). Patients compared their LHW positively with other practitioners they had known. In particular, they described the LHWs as encompassing both physical and mental health problems, whereas GPs were limited to physical health, and counsellors or psychological therapists were confined to mental health. Patients described gaining a sense of responsibility for their lives in their relationship with LHWs that they did not recount with other practitioners {e.g. ‘[LHW] broke this stalemate’ (R195); ‘[LHW] made me understand’ (G89); and ’[LHW] made me think about what I needed to do’ (R70)}.
There was evidence that patients felt encouraged and motivated by the LHWs, and that this occurred in the context of a close personal relationship:320
Here’s somebody’s taking an actual personal interest in my health and you know . . . I would let myself down and her down if I hadn’t done everything I said I would do . . . I found it a bit more motivational . . . inspirational, and it wasn’y just a case of . . . ’Here’s a little booklet about keeping fit, read that’.
No patient was negative about their LHW as an individual. The only negative comments were those of a very few patients about what the LHWs could achieve. These were the patients who declined or withdrew from the intervention, and one who completed it. Each of these patients described having strong views before seeing the LHW that her involvement would be futile. One patient had previous experience of CBT, which she thought would define the LHW’s role and which she was sceptical about as ‘a bit of a waste of time’. Another explained that ‘I don’t want anybody to help . . . nobody can help, I am convinced of that’.
Acceptability of the intervention at the level of the practice and integration into the practice
Of 14 GPs, six PNs and HCAs, and four administrative staff whom we invited to be interviewed, five, four and four, respectively, agreed and were interviewed.
Practice staff were uniformly positive about the LHWs. They described them as an opportunity for more holistic care (e.g. ‘improving whole-person care’), or for encouraging self-management (e.g. ‘I wanted to get him [patient referred to LHW] to control his condition better, so to take the reins a little bit more’).
However, staff described these areas of care as marginal to normal practice, that is, as needing time that practitioners (GPs and PNs) could not provide because they prioritised biomedical care. As a PN explained, ‘We are very aware of their social needs and their psychological needs but, with the best will in the world, it is a time issue’. Practitioners referred very few patients to the LHWs (GPs referred, 8; PNs and HCAs referred, 20), and saw the LHWs, like other practice staff, as a separate component in an already fragmented primary care service; for example, a GP described how ‘[The LHWs]’d come back with all their bits and use the computers, but of course we’re all in our own little bubbles, aren’t we, our own little rooms, getting on with stuff’. Thus, the intervention was not integrated into routine practice from the perspectives of clinicians. In addition, administrative staff also saw the LHWs as self-sufficient and making few demands on the practice: ‘They just, like, fitted in, yeah. Yeah, they’re just there. Which I suppose is a compliment because they just, they do fit in and you know, they know their role that they’re doing . . . I don’t need to give loads of my time’.
Impact of the intervention at the level of the patient
For the 166 patients in the original cohort, there were no significant differences in age, sex, severity of COPD, or other QOF diagnoses, either in the year before baseline or during the follow-up year; or between the 98 patients who accepted the invitation to see the LHW and the remaining 68 who declined; or between the 90 who attended at least one session and the remaining 76; or between the 82 who completed the baseline PHQ-9 and the remaining 84 patients (Table 50). In addition, there were no significant differences in the reduction in the use of unscheduled care from the year before baseline to the follow-up year between those who agreed to see the LHW and those who did not agree; between those who attended at least one session with the LHW and those who did not; or between those who completed the baseline PHQ-9 and those who did not. Table 50 shows the use of unscheduled care in the year before the intervention for patients who were offered treatment with the LHWs, according to those who accepted and declined.
| Patients who accepted and declined to see the LHW | Attended an ED, n (%) | Had an emergency admission, n (%) |
|---|---|---|
| Declined to see a LHW (n = 76) | 23 (30.3%) | 17 (22.4%) |
| Attended at least one session with a LHW (n = 90) | 39 (43.3%) | 21 (23.3%) |
| Significance for attended vs. did not attend | p = 0.11 | p = 1.0 |
Use of unscheduled care
Of the 166 patients in the original study cohort (and, therefore, with CCG/DSCRO data on unscheduled care), 62 (37.3%) had attended an ED in the year before baseline, compared with only 34 (20.5%) in the follow-up year, which is a significant reduction (McNemar’s test of change: p < 0.001) whereas 38 (22.9%) had an emergency admission in the year before baseline, compared with 36 (21.7%) in the follow-up year, which is not a significant reduction (McNemar’s test of change: p = 0.88).
Patient Health Questionnaire-9 and Generalised Anxiety Scale-7 measures
Among the 67 (out of 166) patients who completed the PHQ-9 at both baseline and follow-up, the baseline mean score was 10.8 (SD 6.8) and the follow-up mean score was 8.1 (SD 6.6), which is a significant reduction (paired t = 4.0, effect size = 0.40; p < 0.001). At baseline, 34 (50.7%) of these patients scored ≥ 10, compared with 25 (37.3%) at follow-up, which is not quite a significant reduction (McNemar’s test of change: p = 0.064).
Among the 66 patients who completed the GAD-7 at both baseline and follow-up, the baseline mean score was 8.39 (SD 6.2) and the follow-up mean score was 6.94 (SD 6.2), which is a significant reduction (paired t = 2.9, effect size = 0.24; p = 0.005). At baseline, 28 (42.4%) of these patients scored ≥ 8, compared with 24 (36.4%) at follow-up, which is not a significant reduction (McNemar’s test of change: p = 0.39).
Discussion
We developed and evaluated a psychosocial intervention tailored for people with COPD who were at risk of using unscheduled care. We synthesised data from previous phases of the programme to design the intervention, and we conducted an exploratory study to test the intervention and the intervention protocol.
The study was set up as a feasibility and pilot study to assess the acceptability of the intervention, the ability to recruit and retain patients, and to collect outcome data to help inform the development and power of a definitive trial.
We designed the intervention to impact at two levels: at the level of the practice and at the level of the targeted patient intervention. Our evaluation therefore consisted of two levels: what impact the intervention had on the total practice population of patients with COPD and what impact the intervention have on the subset of patients who were treated by the LHWs.
Recruitment and retention to the trial groups at the level of the practice recruitment
Primary care trials are notoriously difficult, particularly those that involve a mental health component. Therefore, determining the potential recruitment rates and the most effective recruitment strategies is an important part of the preparatory work for a definitive study.
The practices themselves were recruited from a list of 26 practices drawn up by one of the commissioning managers and we also attended patch meetings to publicise the study. Recruitment was challenging because of the timing of the study and the large changes that were happening in primary care at the time.
Main outcome measure
We were able to obtain data for our main outcome measure (use of unscheduled care) on all eligible patients in the six practices because we used pseudonymised data.
Questionnaire data: quality of life and depression
Approximately 25% of patients with COPD were excluded by the practice GPs from participating in the baseline assessment part of the study and, of those who were eligible, approximately 50% returned a completed baseline assessment. The rates of recruitment for this aspect of the trial are very similar to those reported by a recent cluster RCT evaluating the cost-effectiveness of an integrated COPD training/management programme in primary care in the Netherlands. 321 The proportion of patients excluded by GPs in the Dutch study was 21% (24.6% in the present study), and the proportion of eligible patients who agreed to participate in the Dutch study was 48% (49.5% in the present study).
It was also important to establish the representativeness of our baseline sample. The range of severity of COPD (using the GOLD criteria) in our patient sample was similar to that in other studies on COPD conducted in primary care. 322 Baseline data from seven primary care databases reveal that the proportion of COPD patients with mild, moderate, severe and very severe disease is 20.7%, 53.3%, 21% and 5.8%, respectively. The comparable proportions in the current study were 24.8%, 53.5%, 20.8% and 0.9%, respectively. This suggests that our sample is representative of most patients with COPD in primary care, with the possible exception of patients with the most severe symptoms. It is important, however, to recognise that those patients who were excluded by the GPs as being too ill to participate had used more unscheduled care over the baseline period than those who were deemed eligible.
We obtained a response rate of 68% of patients who completed the 12-month assessment, which lies well within reported rates for RCTs of complex interventions for COPD in primary care. 135,323 Participants who completed the 12-month follow-up assessments differed on some key measures from those who did not. Of note, patients who reported more severe symptoms of depression and anxiety at baseline and poorer health status were less likely to complete the 12-month assessments than those patients with less severe symptoms, although their use of unscheduled care was similar.
Although we encountered large variability in the response rates for completion of assessments from the different practices in the study, there were similar overall response rates for intervention and control practices. Even in this small feasibility study with only six practices, the randomisation process evened out any ‘between-practice’ differences, which suggests that the variability between practices should not be a major problem in a large cluster randomised trial.
All the above factors, however, suggest that our HADS and EQ-5D data are subject to a degree of selection bias and are not representative of the patients with most severe COPD at the six practices in the study, or those who were the highest users of unscheduled care.
Feasibility and acceptability of data collection methods and measures
The process of obtaining the data from the CCG/DSCRO was relatively straightforward and for a larger trial such data could be obtained from NHS Digital. We used pseudonymised data from NHS Digital to obtain estimates of the effect of the intervention on our main outcome: use of hospital-based unscheduled care. Using this method, we were able to obtain all relevant data on all patients in the trial practices, whether or not they participated in the study or were deemed eligible or ineligible by the practice GPs. This meant that we were able to gauge the impact of the intervention on hospital-based unscheduled care at the practice level for all patients. This greatly enhances the external validity of the study, given the selection bias in patients who completed the anxiety and depression and health status measures. We did not include use of primary care unscheduled care.
Impact of the intervention at the level of the practice population
There was great variability between individual practices and between intervention and control practices at baseline on most of our measures. Intervention practices had significantly higher use of health care before the start of the intervention and greater levels of physical comorbidity, than control practices.
Using pseudonymised unscheduled care utilisation data from NHS Digital, we found preliminary evidence of greater reductions or smaller increases in unscheduled care in the intervention practices than in control practices in the univariate analyses. Attendance at an ED in the intervention practices fell from over 39% to 26% in the intervention year but the reduction was much lower (28.6% to 25.1%; raw data) in the control practices. The percentage of patients who required an EHA was much larger in the intervention than in the control practices for the baseline period, but similar in the intervention year. Given the greater use of unscheduled care in the intervention than in the control practices at baseline, it is difficult to be sure whether the lack of significant differences in the intervention year is simply caused by regression to the mean or the large variance in use and costs of unscheduled care. However, in our longitudinal cohort study (described in Chapter 3), the use of unscheduled care was remarkably stable between the baseline and follow-up year, with very similar rates of patients attending ED or requiring an EHA over the two time periods.
Statistical significance testing of baseline differences is not recommended in trials generally (CONSORT guidelines), and is of limited value in exploratory or feasibility studies because of limited power. 324,325 The outcome data we collected have provided valuable evidence regarding variability between practices and the potential impact of the intervention.
Nearly half of the patients who completed the baseline questionnaire scored ≥ 8 on the depression subscale of the HADS, which is consistent with the high levels of psychological morbidity reported in patients with COPD. 47 However, there was no evidence of any major impact on overall depression or anxiety symptoms for patients in the intervention practices. Nor was there evidence of any improvement in health status resulting from the intervention at the level of the practice.
The baseline EQ-5D scores of participants in the present study were much lower than those in the recent Dutch study. 321 The mean utility value for the EQ-5D was 0.74 (SD 0.26) in the Dutch study, whereas the baseline mean EQ-5D utility value in the present study was 0.54 (SD 0.34). The mean EQ-5D VAS for the Dutch study was 67.0 (SD 17.4), whereas it was 55.1 (SD 21.1) in the present study. This difference may be due to different utility weights that are applied to the same health state in different countries. However, a study that compared EQ-5D scores for over 1000 patients with COPD according to differing GOLD stages used the UK utility tariffs to estimate utility values for people from the UK, USA and France. The mean EQ-5D VAS scores for patients in GOLD stages 2, 3 and 4 were 68 (SD 16), 62 (SD 17) and 58 (SD 16), respectively. The corresponding mean utility scores were 0.79 (SD 0.20), 0.75 (SD 0.21) and 0.65 (SD 0.23), respectively. 326 Although there was variation between countries, with US utility scores on average 5% higher than UK scores, the findings still suggest that the patients in our study reported had lower levels of health than other UK samples of people with COPD.
Recruitment and retention of patients to the patient-targeted intervention
Our intention was not to recruit all patients with COPD to the LHW intervention, but to target at-risk patients, that is, those with depression or other psychosocial stressors.
Of the 35% of people with COPD in the intervention practices who were offered the LHW intervention, nearly 60% agreed to participate, and, of those who attended for a first session of treatment, 71% completed an agreed course of treatment. Other recent UK-based primary care studies involving low-intensity interventions for patients with physical health problems have reported participation rates of 20%. 327 Similar recruitment and retention rates in a definitive trial would confer acceptable external validity. 328
A recent meta-analytic review that examined retention and dropout rates among clients attending conventional psychological treatment reported that, averaging across studies using a random-effects model, the weighted dropout rate was 19.7% (95% CI 18.7% to 20.7%; 669 studies representing 83,834 clients). 329 The discontinuation rates from the present study are higher but comparable to rates reported in other trials on complex interventions in COPD. 330,331
Many patients with COPD are elderly, and conventional psychological services, such as IAPT, have struggled to recruit older people. The ability of the current study to recruit 60% of patients with identifiable psychosocial problems suggests the platform for delivering the psychosocial intervention was acceptable to patients. This aspect will be addressed in more detail in the next section.
Acceptability of the patient-targeted intervention
Almost all patients were enthusiastic about the intervention and described being motivated by the LHW to take responsibility for changing aspects of their lives. They contrasted the LHW intervention with help they had received previously from GPs, nurses or mental health services, in that it was holistic in encompassing both emotional and physical needs. Paradoxically, those who had experience of mental health practitioners found it easier to address emotional problems with the LHW because their care was not exclusively psychological.
A major limitation of the qualitative evaluation is that we could recruit very few patients who had declined the intervention or withdrawn from it. Nevertheless, patients’ accounts suggest that the LHW intervention has the potential to enhance motivation for self-care and to address psychosocial needs in patients with COPD.
Integration of the liaison health workers at the level of the practice
Practice staff were positive about the intervention, recognising that it addressed psychosocial needs in a holistic approach. However, clinical staff regarded those psychosocial needs as peripheral to the main priority of physical care. Therefore, they regarded the LHW intervention as marginal to the main work of the practice. Similarly, administrative staff valued the LHWs because they caused little additional administrative burden. From the perspective of practice staff, therefore, the LHWs were accepted, but not integrated, into the practices. We did not formally interview the LHWs (as there were only two, their anonymity could not be protected). Nevertheless, the practice staff perspective is consistent with previous findings that the compartmentalisation of UK primary care has prevented integration of interventions designed to address psychosocial needs. 299,332
The duration of the study was only 12 months, which meant there had to be a run-in period at the beginning, when the LHWs were being embedded in the practice, and a run-down period at the end, when no further patients could be recruited. This created a somewhat artificial dimension to the treatment and may have been an additional barrier to the integration of the LHWs within the practices.
Impact of the targeted patient intervention
It was not possible to identify similar patient pathways in the control practices, without the implementation of the practice-level workshop, so there was no direct equivalent group in the control practices for comparison. Our exploratory data for the impact of the LHW intervention are limited, therefore, to a before-and-after evaluation.
We found a significant reduction in the PHQ-9 scores in the patients treated by the LHWs, with a moderate effect size of 0.4. This is slightly higher than the effects reported by Coventry et al. 333 from comparative studies of psychosocial interventions to treat depression in COPD, so is of the order expected given the before-and-after analysis we used. We also included patients who were not depressed, but had other psychosocial problems, so some patients would have scored below threshold for depression at the start of their work with the LHWs. We also found a significant reduction in use of the ED in patients who were seen by the LHWs, but there was little effect on EHAs.
Limitations
There are several limitations, in addition to those already discussed, which need to be considered. First, we focused on people with COPD. We did this because we found very little evidence of any major differences between our four LTC groups in the previous work in the programme, and the feasibility trial dovetailed with local developments within our PCT, ensuring better recruitment of practices to the study. Had we not done this, we would not have been able to conduct the trial within the time frame of the programme. Although many of the patients in the trial with COPD had comorbid conditions, the generalisability of the trial findings to other LTCs may be limited.
We found very large differences in our baseline measures between groups and between the individual GP practices, which may have impacted on the overall outcome. In addition, patients who were considered by GPs too ill to complete the baseline questionnaire were disproportionately higher users of unscheduled care than those who were considered well enough to be sent a questionnaire. Thus, the very patient group we wish to target may be excluded from participating in a RCT. It may be, however, that this group of people may also be too unwell to receive or benefit from a psychosocial intervention.
The changes we tried to implement at the level of the practice were not successful and we were unable to embed the LHWs into the practice cultures. We may have been too ambitious in this aim, especially as the LHWs were only employed for 1 year, so practice staff knew the involvement of the LHWs would be short lived.
As the LHWs were employed only for 12 months, this also limited the number of patients that they were able to see. They had a run-in period of 3 months, before reaching a full caseload and then had to have a run-down period of 3 months before the end of the study, to make sure they finished with their clients in time. This dramatically reduced the number of patients that they were able to see over the 12-month period in comparison with the number they would have been able to see if they had been employed on an ongoing contract.
The six GP practices who agreed to participate in the study were, to a certain degree, self-selected as they were interested in participating in the research project and may not be representative of other inner-city practices.
Conclusions
We were able to conduct a small cluster RCT of a complex intervention in primary care with acceptable recruitment and retention rates. The intervention consisted of a workshop targeted at the level of the practice and also a targeted patient intervention for a subset of COPD patients with psychosocial problems. We used pseudonymised data from the CCGs to record use of hospital-based unscheduled care, which enabled us to collect data on all patients with COPD in the intervention and control practices. For an intervention that is targeted at the whole-practice population, the use of pseudonymised data enabled us to assess the impact of the intervention on all patients, including those who were excluded by GPs or declined to participate. This has clear advantages in determining the true impact of the intervention on unscheduled care, but creates difficulties in triangulating data with other outcomes, such as anxiety and depression and EQ-5D, which can only be collected on participants in the study.
Although there appeared to be favourable differences between the intervention and control groups in use of unscheduled care over the treatment period compared with the baseline period, the baseline differences between the two groups were very large, and the multivariate analyses showed no significant differences.
The LHW intervention was extremely well received by patients and we were able to demonstrate acceptable recruitment and retention rates and some evidence of improvement in depression over the course of treatment and a reduction in the use of the ED. The LHWs were not integrated into the practices and a more intensive training/workshop programme would probably be required for this to be the case.
We tried to integrate the LHWs into the practices as we believed this would improve referral rates and also convey ‘credibility’ to the patients we were hoping to recruit. Our findings suggest that it is possible to recruit a sufficient number of patients and engage them in treatment without the LHWs necessarily being part of the practices.
The cost-effectiveness analysis is reported separately (see Chapter 9), but suggests that the LHW intervention holds some promise as potentially providing a cost-effective intervention, whereas the practice-level intervention does not.
In the lifetime of the CHOICE study, there have been developments in integrated care for patients with severe and disabling physical disease. As we conducted the exploratory trial, practices across Manchester were beginning to develop integrated care teams. None of the intervention practices in our study was involved in this initiative, but there were many other potential factors that may have influenced use of unscheduled care during the duration of the study that were beyond the scope of the feasibility trial to take account of (e.g. changes in staff in the different GP practices, changes to local transport systems, changes to hospital admissions and discharge policies, changes to the organisation of ED services, etc.).
Any future definitive study will need to take account of the rapid changes to health care that have occurred and are continuing to develop in the NHS. Many integrated care teams are bringing together physical and social care for frail and elderly patients with comorbid physical health conditions. However, mental health is commonly omitted. The findings from this phase of the CHOICE programme suggest that psychosocial interventions can be warmly received and valued by patients with chronic physical disease.
Further analyses to examine the role of depression as a predictor of unscheduled care
This study was primarily designed as a cluster RCT, but it gave us the opportunity to compare routinely collected depression data from the QOF using primary care electronic systems with depression measured using the HADS241 in a population of primary care patients with COPD. Of 350 patients with COPD in the trial who completed the HADS at baseline, 173 (18.2%) suffered from depression (a score of ≥ 8 on the HADS), but only 7.7% of these patients were recorded as being depressed on the QOF depression database. There was also little agreement between the two ways of recording depression, with only 16 out of the 173 patients classified as having depression according to the HADS (9.2%) having a diagnosis of depression in GP IT systems.
Logistic regression showed a significant association between HADS depression score of ≥ 8 or more and prospective ED attendance (OR 2.7, 95% CI 1.5 to 4.7; p = 0.001) and prospective EHA (OR 2.6, 95% CI 1.5 to 4.4; p = 0.001), over the following 12 months, adjusting for age, sex, severity of COPD and previous use of unscheduled care. In the same data set, logistic regression found no significant association between QOF depression and either prospective ED attendance (OR 0.7, 95% CI 0.4 to 1.3; p = 0.23) or prospective emergency admission (OR 0.8, 95% CI 0.4 to 1.4; p = 0.36).
These findings support the evidence for depression being a powerful independent predictor of future use of unscheduled care in patients with a LTC, but suggest that current methods of case-finding for depression in primary care have low utility as potential red flag indicators for use of unscheduled care. Too few patients with depression are at present being identified using the current case-finding methods available, and, in fact, as case-finding for depression has been withdrawn, attempts at case-finding are likely to cease.
Chapter 9 The economic analysis of an intervention for people with long-term conditions to reduce unscheduled care
Abstract
Background
There are very few studies that have examined the cost-effectiveness of psychosocial interventions in patients with LTCs.
Methods
The health status and resource use data collected in the exploratory trial (see Chapter 8) were used to explore the potential cost-effectiveness of the intervention to reduce use of unscheduled care, from the perspective of the NHS. The time horizon for the economic analysis was the 1-year duration of scheduled follow-up.
Results
Overall, the analyses indicate that the intervention at the level of the practice is not cost-effective for all people with COPD. Exploratory sensitivity analyses indicate that the intervention may be associated with both lower costs and QALYs if only those people who attended at least one session with an LHW are included in the analysis.
Discussion
The data from the health economic analysis, combined with the feasibility and exploratory data from Chapter 8 and the qualitative analysis, strongly suggest that the intervention at the level of the practice was not successful, but there is preliminary evidence to support further evaluation of a targeted patient-level intervention, and sufficient data to inform the development of a definitive study.
Overview
This chapter reports the cost-effectiveness analysis of the cluster randomised feasibility study, which was described in Chapter 8.
The specific aims and objectives were to determine in patients with COPD:
-
What are the potential costs, health benefits and incremental cost-effectiveness ratios (ICERs) associated with a practice-level intervention to identify patients at high risk of using unscheduled care and/or reduce the use of unscheduled care?
-
What are the potential costs, health benefits and ICERs associated with a targeted psychosocial intervention for patients with psychosocial problems.
As the trial clearly indicated that the practice-level intervention was unlikely to reduce use of unscheduled care or be cost-effective, it was recognised that an economic decision model would not provide additional information to that generated by the exploratory analyses of the trial data. As such, the original objective to ‘use health economic modelling to evaluate the costs and benefits associated with a treatment intervention’ was replaced by the exploratory within-trial economic analyses.
Background
Our aim in this phase of the programme was to develop and evaluate an evidence-based, feasible, acceptable psychosocial intervention that would have the potential to reduce/prevent unscheduled care, while maintaining or improving patient benefit. The intervention, as described in Chapter 8, consisted of two elements:
-
a practice-level intervention to raise awareness of risk factors associated with use of unscheduled care in patients with LTCs, and to identify patients with psychosocial problems who could be referred for specific treatment
-
a targeted psychosocial intervention delivered to a subgroup of patients identified as having psychosocial needs.
This chapter reports the cost-effectiveness analysis that accompanied the exploratory cluster RCT (see Chapter 8) to evaluate the acceptability of our intervention and determine key parameters for a larger definitive study.
A small number of studies have evaluated the cost-effectiveness of interventions for depression in patients with LTCs. The TEAMcare study320 found that a collaborative care approach targeted at depressed patients with either poorly controlled diabetes or CHD, resulted in more depression-free days, and an estimated 0.335 (95% CI –0.18 to 0.85) additional QALYs, with lower mean outpatient costs. However, a recent systematic review highlighted the paucity of evidence of cost-effectiveness for the treatment of depression in diabetes. 333 Only four studies could be identified, all of which were US based. Two studies reported costs per QALY gained of US$267 to US$4317, whereas two studies reported the intervention dominated usual care with net savings of US$440 to US$612 and net gains in patient-free days or QALYs. 334
A recent trial which compared enhanced treatment of depression with usual care in patients with acute coronary syndrome reported improvements in depression and cost savings in the intervention group. 335 The higher costs of mental health care and higher use of psychotropic medications in the intervention group were offset by savings in hospitalisations for major adverse cardiac events and heart failure. The mean total health-care costs were over US$1000 lower in the intervention group than in the control group. 335
We are not aware of any RCTs that have evaluated the cost-effectiveness of a psychosocial intervention to reduce use of unscheduled care in people with LTCs.
Methods
The target population for the practice-level intervention was people with COPD registered at the intervention and control practices. The sample of participants used for the economic analysis comprises those patients who were initially considered eligible for the trial and completed baseline assessments. It was outside the scope of and beyond the resources available for this feasibility trial to collect patient outcomes for all the patients with COPD at the participating GP practices. It was not possible to assess the cost-effectiveness of the practice intervention using data for all patients in the cohort. Although costs of unscheduled care were available for all these participants, relevant outcome data were only available for those that completed the baseline and follow-up assessments. The measure used to assess the effectiveness of the intervention was use of unscheduled care. This is not a relevant measure for the economic evaluation as it is a measure of service use that is used to estimate the costs of unscheduled care and comprises part of the costs of the practice intervention.
The target population for the exploratory analyses of the targeted patient intervention was those patients with identified psychosocial needs. Four additional sensitivity analyses were used for this part of the economic evaluation work, using different subgroups of participants. These are described in more detail in Analysis.
The health status and resource use data collected in the feasibility study (see Chapter 8) were used to explore the potential cost-effectiveness of the intervention to reduce use of unscheduled care, from the perspective of the NHS. The time horizon for the economic analysis was the 1-year length of scheduled follow-up.
Cost and quality-adjusted life-year estimates
The service use data collected in this exploratory feasibility study were limited to the use of hospital-based scheduled and unscheduled care services, which will underestimate the total costs to the NHS. The implications of this for the potential cost-effectiveness of the intervention were explored in the sensitivity analyses (described in Analysis). As described in Chapter 8, data about use of services and costs of services were provided by NHS Digital for all participants in the trial. These included use of scheduled and unscheduled hospital inpatient, outpatient and ED care, which were the key items of service use identified in the longitudinal cohort (COPD, 81%). Data on the use of scheduled and unscheduled primary- and community-based services were not included in the analysis. Although this will underestimate the total costs of care, it was outside the scope of this feasibility trial to collect detailed information about these items.
The costs of the intervention were estimated as only the cost of LHWs employed. This excludes the costs of additional time spent by practice staff in identifying and referring participants. The cost per session of the intervention was estimated as:
The annual costs of the LHWs were estimated as the full salary and overhead costs of two band 6 staff. The number of eligible participants was estimated as the number of patients with COPD identified as potentially eligible for the trial. The number of LHW sessions was estimated from the number per participant planned for in the design of the trial. This approach will underestimate the cost per session if a low proportion of people with COPD are actually referred to the LHW and/or the planned number of sessions per participant is higher than actually used.
The measure of health benefit for the primary economic analysis was the QALY, estimated from the EQ-5D and associated utility tariffs completed at baseline and at the end of the scheduled follow-up. The EQ-5D is a validated generic health status measure, used in national health surveys in the UK and in clinical trials in mental health, covering five domains (mobility, self-care, usual activity, pain/distress and anxiety/depression). 317 The three-level version was used in the trial (no problems, some problems, severe problems/unable to do activity). The QALY and the EQ-5D are the measures recommended for economic evaluations by the National Institute for Health and Care Excellence (NICE). QALYs were estimated as:
where U = utility value and t = number of days between assessments.
Analysis
The data on service use were collected from NHS Digital. Accordingly, it was assumed there were no missing observations for service use or cost for those participants included in the economic evaluation. Missing data on EQ-5D utility scores were imputed using the MICE procedure, which is more robust against assumptions that data are missing not at random. The multiple imputation procedure included all the baseline covariates identified as predictors of costs and QALYs from the literature, analysis of the longitudinal cohort data, and the covariates used in the effectiveness analysis.
Net costs and QALYs associated with the intervention were estimated using linear regression adjusting for key covariates and clustering by GP practice.
The primary measure for the economic analysis was the ICER. Accordingly, no statistical tests of differences in mean costs or outcomes were conducted. The ICER was estimated as the:
The estimates of incremental costs and outcomes from the regression were bootstrapped to simulate 10,000 pairs of net cost and net outcomes of the therapy group for a cost-effectiveness acceptability analysis, as recommended by NICE for health technology appraisals. 11 These simulated data were used to estimate the probability that the LHW intervention is cost-effective compared with usual care.
This approach revalues effects or benefits in monetary terms. However, in the UK there is no universally agreed monetary value for the types of benefit measures used in cost-effectiveness analyses. An approach used in health care is to ask the question: what is the maximum amount decision-makers are willing to pay to gain one unit of benefit? The simulated net utility values were revalued using a range of maximum willingness-to-pay values from £1 to £30,000 to gain one unit of outcome, based on the range of willingness-to-pay values implied by NICE decisions. 336
The data for the cost-effectiveness acceptability curve were derived by first revaluing each of the 10,000 net outcome scores from the bootstrap simulation by a single willingness-to-pay threshold (WTPT). This is repeated for each WTPT. A net benefit (NB) statistic for each pair of simulated net costs and net outcomes for each WTPT can then be calculated as:
where O = net outcome score and C = net cost.
This calculation was repeated for each WTPT. Cost-effectiveness acceptability curves plotted the proportion of bootstrapped simulations where the NB of an intervention is greater than zero for each WTPT. 337–340
Sensitivity analyses explored the impact of changes in the estimates of the cost of the intervention and using alternative measures of health benefit.
The primary analysis included all participants who completed the baseline assessment and were considered to be at risk of using unscheduled care, to approximate a practice-level analysis. It was not possible to identify similar patient pathways in the control practices to directly compare the costs and QALYs of the targeted patient intervention in the intervention and control practices. The analyses in Chapter 8 indicate that the intervention was well received and that the LHW intervention may be effective when targeted at people with depression. Accordingly, further sensitivity analyses were used to explore whether or not the targeted patient intervention had the potential to be cost-effective in different subgroups of the study participants. This information could be used with that from the effectiveness analysis and qualitative data to inform whether or not future development and evaluation of the intervention may be needed. However, the feasibility study was not powered for subgroup analyses, with the result that each of the groups explored is small and selected. This means that the estimates of costs and QALYs from these sensitivity analyses are more uncertain than for the whole sample and need to be tested in further studies. The groups explored were:
-
All participants with depression at baseline (HADS depression score of ≥ 8: n = 83 in the intervention practices; n = 90 control practices). Includes costs of LHW screening and motivational intervention sessions. This compares all participants with depression whether or not those people with depression in the intervention practices were actually referred to the LHW.
-
All participants with depression at baseline in the control practices (n = 90) and only participants in the intervention practices who had depression and were also referred to the LHW (n = 40) (referred by practice staff or self-referral). This explores whether or not the LHW intervention has the potential to be cost-effective if participants with depression are actually referred to the LHW. This analysis includes costs of LHW intervention sessions only.
-
All participants with depression at baseline in the control practices (n = 90) and only participants who attended one or more sessions with the LHW (n = 23) in the intervention practices (referred by practice staff or self-referral). This analysis explores whether or not the LHW intervention has the potential to be cost-effective if all participants with depression are referred to the LHW and engage with the LHW at least once. This analysis includes costs of LHW motivational intervention sessions only.
-
All participants in control group (n = 194) whether or not they had depression and only participants in the intervention practices who attended one or more sessions with the LHW (n = 36). This analysis explores whether or not the intervention has the potential to be cost-effective if participants are referred to and engage with the LHW to address a risk of using unscheduled care.
Descriptive analysis, data manipulation and the main statistical analyses and estimation of NB statistics and cost-effectiveness acceptability analysis were conducted using Stata, version 13.0 (StataCorp LP, College Station, TX, USA).
Results
Practice-level intervention (n = 355)
Tables 51 and 52 report the descriptive analyses of health status, utility and costs and QALYs for those participants with complete baseline and follow-up data. These data were not adjusted for key baseline characteristics of participants. The data indicate some apparent differences in utility between the intervention and control groups at baseline, but the 95% CIs overlap, so this difference may not be significant. However, this may indicate some imbalance in health status at baseline. Accordingly, the EuroQol thermometer (EQ-5D VAS), a VAS of overall health, was used to control for the impact of any baseline difference in the regression analyses to estimate net QALYs.
| Item | Group, n (%) | |||
|---|---|---|---|---|
| Intervention (n = 79) | Control (n = 100) | |||
| Baseline | Follow-up | Baseline | Follow-up | |
| EQ-5D health status | ||||
| Mobility | ||||
| No problems | 19 (24) | 19 (24) | 40 (40) | 33 (33) |
| Self-care | ||||
| No problems | 53 (67) | 44 (56) | 76 (76) | 73 (73) |
| Usual activity | ||||
| No problems | 23 (29) | 20 (25) | 38 (38) | 33 (33) |
| Pain/discomfort | ||||
| No problems | 14 (18) | 15 (19) | 38 (38) | 37 (37) |
| Anxiety/depression | ||||
| No problems | 40 (51) | 40 (51) | 54 (54) | 53 (53) |
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | |
| EQ-5D utility index | 0.567 (0.501 to 0.633) | 0.541 (0.465 to 0.618) | 0.620 (0.554 to 0.685) | 0.614 (0.547 to 0.681) |
| Item | Group, n (%) | |||
|---|---|---|---|---|
| Intervention (n = 79) | Control (n = 100) | |||
| Baseline | Follow-up | Baseline | Follow-up | |
| Used a service | ||||
| Elective admissions | n.a. | 18 (23) | n.a. | 18 (18) |
| Emergency admissions | 25 (32) | 17 (22) | 32 (32) | 20 (20) |
| ED visits | 16 (20) | 17 (22) | 17 (17) | 21 (21) |
| Referred by practice staff and contacted to assess psychosocial need | 79 (100) | 0 | 0 | 0 |
| Identified by practice staff as having psychosocial need and were referred to LHW | 29 (37) | 0 | 0 | 0 |
| Had one or more LHW sessions, if referred to LHW intervention | 0 | 17 (59) | 0 | 0 |
| Number of LHW sessions, if had one or more sessions | 0 | 4 (95% CI 3 to 4) | 0 | 0 |
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | |
| Costs of services (£) | ||||
| Emergency admissions | 921 (397 to 1445) | 745 (335 to 1155) | 523 (243 to 804) | 615 (301 to 929) |
| ED visitsa | 54 (31 to 76) | 34 (18 to 51) | 42 (29 to 56) | 29 (16 to 43) |
| Total costs of unscheduled care | 975 (435 to 1515) | 780 (356 to 1203) | 566 (277 to 855) | 645 (320 to 970) |
| Costs of scheduled care (elective admissions only) | n.a. | 388 (60 to 616) | n.a. | 161 (62 to 251) |
| Intervention costb | 0 | 59 (49 to 69) | 0 | 0 |
| Total costs of scheduled and unscheduled care and intervention | n.a. | 1177 (657 to 1697) | n.a. | 806 (469 to 1142) |
The results in Table 52 indicate that there may be differences in the use and costs of unscheduled care between the intervention and control groups at baseline. Although a similar proportion of participants in the intervention and control groups used unscheduled care at baseline, the intervention appeared to have higher costs than the control group. The 95% CIs overlap, indicating that the difference may not be significant. However, this may indicate some imbalance in use and costs of care. Accordingly, the cost of unscheduled care prior to baseline was used in the regression analyses of differences in costs.
Table 53 reports the mean costs and 95% CIs for the complete case analysis and two levels of imputation. Again, these are not adjusted for any differences in baseline covariates. All the analyses include the costs of the LHWs and suggest that the intervention may be associated with higher costs and lower QALYS. However, the 95% CIs overlap, indicating that these differences may not be statistically significant.
| Participant sample | Group | |||||
|---|---|---|---|---|---|---|
| Intervention | Control | |||||
| n | Mean | 95% CI | n | Mean | 95% CI | |
| Complete case (costs and QALYs) | ||||||
| Days of follow-up | 76 | 347 | 339 to 354 | 97 | 359 | 354 to 365 |
| QALYs | 76 | 0.520 | 0.455 to 0.586 | 97 | 0.602 | 0.536 to 0.667 |
| Costs (£) | 79 | 1177 | 657 to 1697 | 100 | 806 | 469 to 1142 |
| Missing values imputed for full sample of patients eligible for follow-up (n = 312) | ||||||
| QALYs | 148 | 0.498 | 0.440 to 0.556 | 164 | 0.577 | 0.526 to 0.628 |
| Costs | 148 | 1713 | 1121 to 2305 | 164 | 1138 | 668 to 1608 |
| Missing values imputed for full sample of patients completing baseline questionnaire (n = 355) | ||||||
| QALYs | 161 | 0.495 | 0.447 to 0.544 | 194 | 0.559 | 0.513 to 0.604 |
| Costs | 161 | 2110 | 1293 to 2926 | 194 | 1516 | 1052 to 1980 |
Table 54 reports the net costs and net QALYs for the primary analyses. Both the unadjusted analysis and adjusted analysis are presented. Both of these analyses use the imputed QALY data for all patients who completed the baseline questionnaire. The adjusted analysis included the following baseline characteristics as covariates:
-
age
-
sex
-
ethnicity
-
level of education
-
marital status
-
baseline global health (to account for differences in utility values at baseline)
-
baseline costs of unscheduled care
-
whether or not unable to work due to health
-
diagnosis of CHD
-
maximum severity of COPD
-
HADS anxiety or depression score of ≥ 8
-
GP practice (cluster).
| Analysis | Net cost (£) (95% centiles) | Net QALY (95% centiles) | Probability cost-effective if decision-makers are | |
|---|---|---|---|---|
| Not willing to pay to gain one QALY | Willing to pay £20,000 to gain one QALY | |||
| Unadjusted for baseline characteristics | 594 (312 to 876) | –0.063 (–0.083 to –0.043) | 0.00 | 0.00 |
| Adjusted for baseline characteristics | 314 (19 to 609) | –0.046 (–0.060 to –0.032) | 0.02 | 0.00 |
Both the adjusted and unadjusted primary analyses indicate that the practice-level intervention, which includes the cost of the LHWs, is associated with additional costs and lower QALYS than the control group. The 95% CIs do not cross zero, indicating that the results may be statistically significant. These differences are illustrated diagrammatically in the scatterplot in Figure 24. There is no evidence that the holistic intervention (to embed LHWs into primary care practices, to facilitate identification, diagnosis and treatment of people with psychosocial needs) is cost-effective. This conclusion is supported by the evidence from the effectiveness analyses and qualitative assessments that suggests that there were problems with effectively embedding the intervention into the GP practices.
FIGURE 24.
Cost-effectiveness plane: distribution of bootstrapped net costs and QALY pairs (primary analysis, adjusted for baseline characteristics).
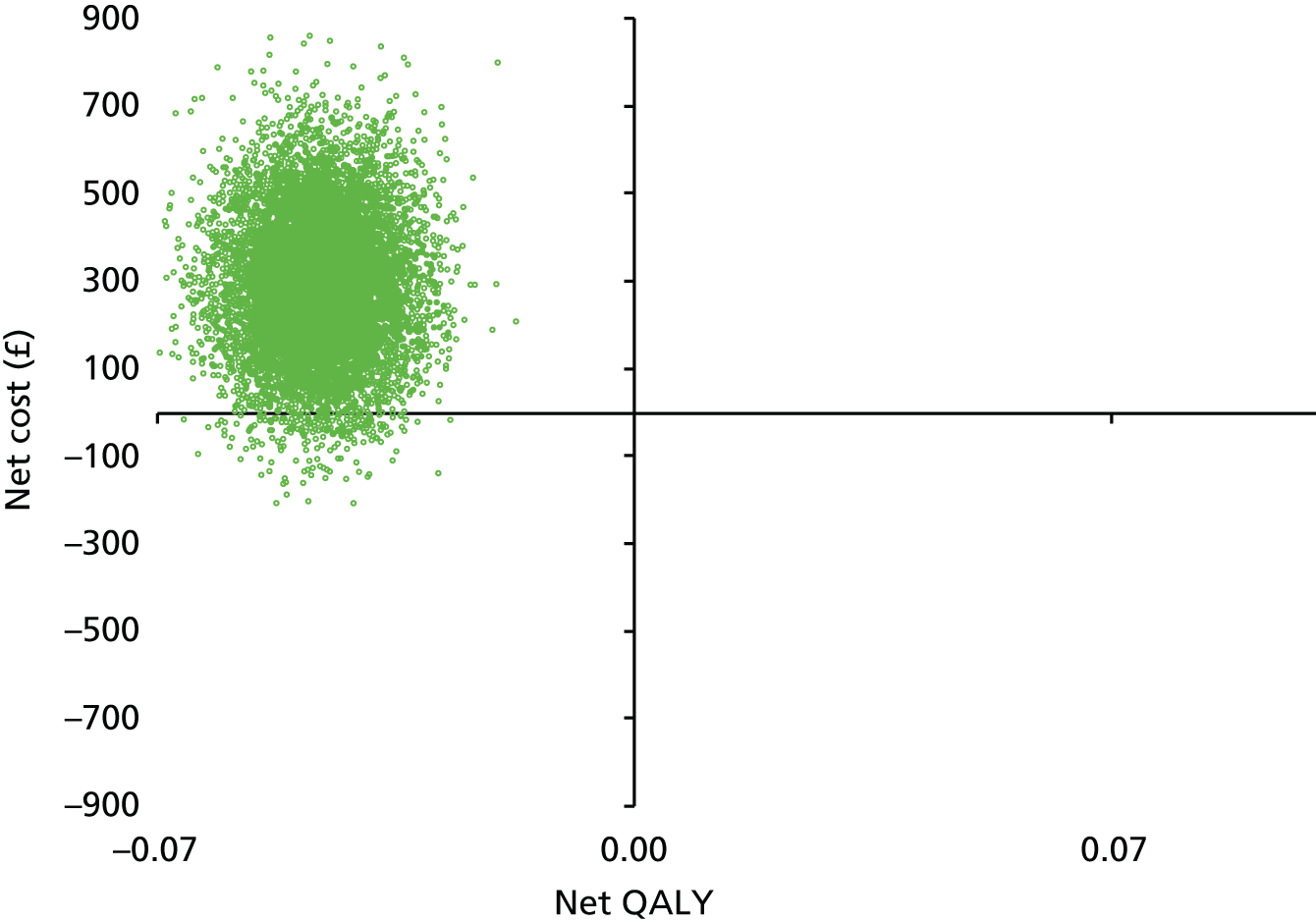
Targeted patient intervention
The sensitivity analyses that explored the cost-effectiveness of the intervention for different subgroups of the trial participants used the multiple imputation values for missing QALY values and were adjusted for baseline characteristics. The results are shown in Table 55. However, as the feasibility study was not powered for these analyses, the results are more uncertain and should be interpreted with caution. Additionally, these analyses are intended to be exploratory to help inform any future work to develop and evaluate the targeted intervention further.
| Analysis | Net cost (£) (95% centiles) | Net QALY (95% centiles) | Probability cost-effective if decision-makers are | |
|---|---|---|---|---|
| Not willing to pay to gain one QALY | Willing to pay £20,000 to gain one QALY | |||
| Subgroup analysis 1 | ||||
| Participants with depression at baseline (LHW intervention group, n = 83; control group, n = 90) | 86 (–258 to 430) | –0.009 (–0.031 to 0.012) | 0.35 | 0.17 |
| Subgroup analysis 2 | ||||
| Participants with depression at baseline in the control group (n = 90); participants with depression and referred to the LHW (n = 40) in the intervention group | 59 (–384 to 502) | –0.013 (–0.040 to 0.013) | 0.40 | 0.17 |
| Subgroup analysis 3 | ||||
| Participants with depression at baseline in the control group (n = 90); participants with depression and attended one or more sessions with the LHW (n = 23) in the intervention group | –567 (–993 to –142) | –0.005 (–0.037 to 0.028) | 0.99 | 0.85 |
| Subgroup analysis 4 | ||||
| All participants in control group (n = 194), participants who attended one or more sessions with the LHW (n = 36) in the intervention group | –487 (–765 to –210) | –0.04 (–0.06 to 0.02) | 0.99 | 0.09 |
Sensitivity analyses using subgroups 1 and 2 indicate a smaller net cost and lower QALY loss than the primary analyses. In addition, the 95th percentiles on the bootstrapped estimates cross zero for both net costs and QALYs, indicating that there may be no differences in costs and QALYs. This is illustrated in the scatterplots in Figures 25 and 26. The probability that the intervention is cost-effective is < 50% if decision-makers are not willing to pay to gain 1 QALY. This again suggests that the intervention is not cost-effective in these samples of participants.
FIGURE 25.
Cost-effectiveness plane: distribution of bootstrapped net costs and QALY pairs (sensitivity analysis subgroup 1, adjusted for baseline characteristics).
FIGURE 26.
Cost-effectiveness plane: distribution of bootstrapped net costs and QALY pairs (sensitivity analysis, subgroup 2, adjusted for baseline characteristics).
The sensitivity analysis using subgroup analysis 3 indicates a net saving and small QALY loss for the intervention, for participants with depression (HADS depression score of ≥ 8) who had one or more sessions with the LHW, compared with control group participants with depression. The 95 percentiles do not cross zero for the net costs, but do cross zero for the net QALYs, suggesting a possible difference in net costs but not QALYs, as illustrated in Figure 27. As decision-makers’ willingness to pay to gain a QALY increases, the probability the intervention is cost-effective decreases. This reflects the trend towards a net QALY loss in the intervention group. This is demonstrated in the cost-effectiveness acceptability curve in Figure 28 and applies to all of the primary and subgroup analyses.
FIGURE 27.
Cost-effectiveness plane: distribution of bootstrapped net costs and QALY pairs (sensitivity analysis, subgroup 3, adjusted for baseline characteristics).
FIGURE 28.
Cost-effectiveness plane: distribution of bootstrapped net costs and QALY pairs (sensitivity analysis, subgroup 4, adjusted for baseline characteristics).
Comparison of the distribution of baseline HADS depression scores and the mean scores suggests that there was no difference between the participants in the intervention group who had one or more LHW sessions and those in the control group. Accordingly, the sensitivity analysis using subgroup 4 includes all the participants in the control group, with or without depression and compares these to people in the intervention group who had one or more LHW sessions, with or without depression. This indicates a net saving in the cost of hospital-based scheduled and unscheduled care and a net loss in QALYs (Figure 29). If decision-makers value improvements in health and are willing to pay to gain a QALY increase, then the probability the LHW intervention is cost-effective for this group of participants decreases. If decision-makers are willing to pay £20,000 to gain one QALY, then the LHW intervention is unlikely to be cost-effective for people eligible for the intervention (see Table 55).
FIGURE 29.
Cost-effectiveness acceptability curves, primary and sensitivity analyses, adjusted for covariates, multiple imputation.
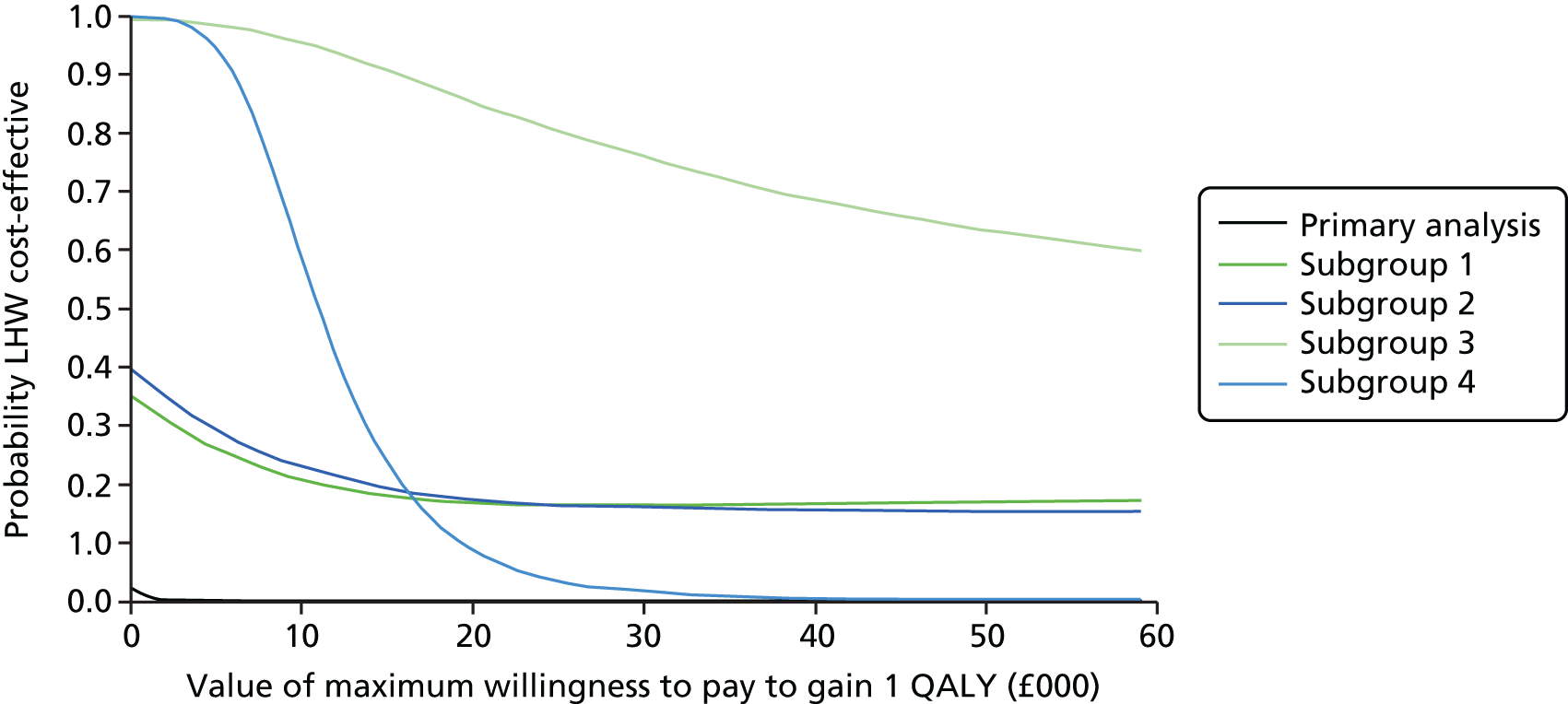
Discussion
Impact of the intervention at the level of the practice population
Based on the analysis of the 355 participants who completed a baseline assessment, the primary analysis indicates that the intervention at the level of the practice is unlikely to be cost-effective.
Impact of the targeted patient intervention
It was not possible to identify similar patient pathways in the control practices for a direct assessment of the potential cost-effectiveness of the targeted practice intervention. The analyses in Chapter 8 indicate that the intervention was well received and potentially effective. For the economic evaluation, different subgroups analyses were used to explore the potential of the targeted patient-level intervention to be cost-effective. If the intervention is targeted at people with depression who participate in the LHW treatment sessions, then it is associated with a net saving of costs, although there is also a trend towards a net QALY loss. This appears to be contrary to the results of economic evaluations of collaborative care interventions for people with comorbid depression and LTCs, but our analyses are preliminary and based on a small data set from essentially what was a feasibility study. A recent systematic review of collaborative care interventions in people with CHD and/or diabetes concluded that the evidence about the cost-effectiveness was uncertain and noted a number of issues with the design of the included studies that reduced the robustness of the results. 333 In addition, the four included studies were all conducted in a US setting, with different health-care systems to the UK. This may reduce the relevance of the evaluations to the UK setting. 333
The data from the cost-effectiveness analysis, combined with the feasibility and exploratory data and the qualitative analysis from the previous chapter, suggest that the intervention at the level of the practice may not be successful. But there is some preliminary evidence to inform further development and evaluation of the targeted patient-level intervention. However, the analyses are subject to a number of limitations and caveats that need to be accounted for when drawing any conclusions.
Limitations
As noted in Chapter 8, a number of issues mean that there may be selection bias in terms of the practices and participants who were recruited and who completed follow-up. These limited the extent to which the results of the economic evaluation can be generalised to the target population or to other settings. There was a high level of variability in patients’ characteristics at baseline that may mask important differences in service use and costs and QALYs, despite attempts to statistically control for baseline covariates. In addition to these limitations, there are some specific issues that limit the robustness of this economic evaluation.
The first is that the analyses include only hospital-based unscheduled and scheduled care costs. At baseline, only unscheduled hospital care costs were available. The costs of primary- and community-based services were excluded from the baseline and follow-up costs, although these may be affected by the practice or targeted patient-level interventions. It is not clear whether this would increase costs (e.g. by identifying health-care needs) or result in lower costs (e.g. by improving health and reducing use of services). If the use of these services differs between the control and intervention groups at follow-up, then the net costs (savings) associated with the intervention will be inaccurate. A comparison of the mean costs at baseline and follow-up indicates that the costs of hospital-based care increased slightly in the control group (mean increase of £79), but fell in the intervention group (mean reduction of –£195), although this difference may not be statistically significant (mean difference £274, 95% CI –£406 to £275). If there were a similar reduction in primary and community-based costs, this may have reduced the net costs associated with the intervention.
Additionally, the costs of additional time spent by practice staff to identify and refer patients to the LHW intervention were excluded. This will underestimate the costs of the intervention at the practice and targeted patient intervention level and overestimate the relative cost-effectiveness of the intervention. The costs of supervision and training were excluded, which will also have similar effects. However, the LHWs were only employed for 1 year, so the period included a 3-month run-in period to establish themselves in the practices and develop a case load and 3-month run-down period to complete and discharge all patients before the end of their contract. This limited the number of patients they could see during the 12 months, so will lead to an underestimate of their potential effect.
Eighty-eight per cent of the participants who completed the baseline questionnaire (n = 355) also completed the follow-up questionnaire (n = 312). There was a high level of missing observations in the baseline and follow-up utility values. This was attributable to incomplete ratings on one or more of the EQ-5D health status domains. In total, 179 people had complete EQ-5D ratings and utility values (57% of participants with complete follow-up and 50% of participants who completed baseline questionnaires). Multiple imputation was used to impute missing utility values, using the MICE procedure, which is more robust against assumptions that data are missing not at random. However, they may not be sufficient given the large number of missing data. If unobserved participant characteristics also have an impact on health status and utility values, the imputed data may also be subject to a greater level of uncertainty and imprecision. Again, it is not clear whether this would reduce or increase the likely cost-effectiveness of the LHW intervention.
The primary measure of health benefit was the QALY, estimated from the EQ-5D and associated utility tariffs completed at baseline and at the end of the scheduled follow-up. 341 The EQ-5D is a validated generic health status measure, used in national health surveys in the UK and in clinical trials in mental health. The QALY and the EQ-5D are the measures preferred by NICE for economic evaluations. 342 The QALY may not be sensitive to small but important changes in health. The EQ-5D domains have face validity in that COPD and exacerbations may be expected to affect mobility, self-care, usual activities and pain. The focus of the intervention on managing psychosocial stressors, including anxiety and depression, might also be expected to map onto the EQ-5D domains of self-care, usual activities, and anxiety and depression. A published literature review also supported the validity and reliability of EQ-5D in COPD. 343 Finally, there was a statistically significant association between the utility score and the HADS depression score, at baseline and at follow-up. In addition, there was no evidence that the practice-level intervention was associated with a statistically significant improvement in either depression or anxiety at follow-up.
Chapter 10 Patient and public engagement and involvement in the CHOICE programme
Abstract
This chapter illustrates the PPE&I aspects of the CHOICE programme. The intention from the pre-funding stage in 2006 was to ensure that PPE&I was an active part of the research programme.
The initial objective of our PPE&I activity was to recruit and support lay representatives to become embedded within the team and to represent the four LTCs (asthma, CHD, COPD and diabetes). The recruitment process was designed to be mutually beneficial and we actively encouraged lay representatives to integrate into the CHOICE programme team. We knew we had an excellent partnership working when the lay representatives became our ‘critical friends’, quick to challenge but equally quick to praise, helping build a true sense of belonging and working to secure continuity of the patient/public voice for the duration of the programme.
The PPE&I model was developed around four key themes: nurturing environment, innovation, quality and sustainability. We developed a wide range of accessible information and regular newsletters, all published on the CHOICE programme website. The engagement report, contributed to and presented at conferences by our lay representatives, led us to conduct an engagement impact assessment (EIA) as a 360-degree appraisal of our PPE&I work. We had helpful and constructive feedback that identified that we had conducted a successful PPE&I process. Our final evaluation identified the need for a robust induction process, ongoing for new members joining during the lifetime of grants, as a core consideration for future programmes.
Background
Patient and public engagement and involvement has become an important part of health- and social-care research. The NIHR has encouraged researchers to integrate PPE&I into research and to understand and assess what impact patient involvement has on research. INVOLVE is the national advisory body funded by the NIHR to promote and support greater PPI in the NHS. 344,345 They have said that PPI:
. . . helps to ensure that the entire research process is focused on what is important to people and is therefore more relevant and acceptable to the users of services. 346
INVOLVE define involvement and engagement as:344,345
-
involvement is where members of the public are actively involved in research projects and in research organisations
-
engagement is where information and knowledge about research is provided and disseminated.
The intention from the beginning of the CHOICE research programme including the pre-funding stage 8 years ago, was to ensure that PPE&I was actively built into each stage of the programme. It was important that we involve members of the public, not only to meet the requirements of the funder (NIHR), but also to ensure acceptability and to demonstrate accountability for the best use of public funds within this grant.
Our perspective on patient and public engagement and involvement
Within this section of the report we have endeavoured to describe the process for PPE&I used within this programme grant.
Within the CHOICE study, we adopted the INVOLVE definitions of PPE&I. 347 INVOLVE defines public involvement in research as research being carried out ‘with’ or ‘by’ members of the public rather than ‘to’, ‘about’ or ‘for’ them. Therefore, we worked to ensure that members of the public were actively involved in the CHOICE programme. We discussed, and agreed, with our PPI members that their title would be ‘lay representatives’, as the focus was to integrate the lay voice into the programme.
The CHOICE programme PPE&I was co-managed by the patient experience and diversity manager (JM) and the CHOICE programme manager (CA). We began by actively thinking about involvement in the programme at the grant application stage. We set out our intentions for the PPE&I work and a patient experience and diversity manager/expert patient was involved as a co-applicant on the grant. Together with the programme manager, she was asked to dedicate time to ensuring active engagement, and involvement of lay representatives was built into and nurtured within all phases of the programme.
At the pre-funding stage, a consultation exercise with users and user-led voluntary care organisations was carried out and we consulted with several organisations including Diabetes UK, the National Phobics Society and Asthma UK. This consultation helped to shape our thinking and ideas about the purpose and intentions for PPE&I and also what we wanted to achieve from PPE&I throughout the CHOICE programme, and it supported us to consider innovative approaches.
The initial objective of our PPE&I activity was to recruit and support lay representatives to become embedded within the CHOICE programme team. The aim of this was to ensure that their input was seen and experienced as a natural part of the programme. Lay representatives were recruited to represent the four LTCs of interest (asthma, CHD, COPD and diabetes). The recruitment process was designed to be mutually beneficial from the start. The settling in period for our lay representatives included several key stages: the sharing of information; listening to each other’s experiences; compiling remits; supporting those approached to become established into working as part of the multidisciplinary research team; and managing the expectations of all team members. We actively encouraged lay representatives to see themselves as part of the CHOICE programme team, helping them build up a true sense of engagement and working to secure continuity of the patient/public voice within the programme.
The PPE&I aims were to:
-
develop a robust PPE&I structure for the CHOICE programme
-
develop an informed patient group representing the four LTCs of asthma, CHD, COPD and diabetes
-
support and develop the skills and expertise of the lay representatives for involvement in the programme and to increase self-esteem and confidence
-
to ensure that all team members worked with, and learned from, the lay representatives.
The programme PPE&I model was developed around four key themes: nurturing environment, innovation, quality and sustainability.
Nurturing an active patient and public engagement and involvement environment
The ultimate objective of the PPE&I work was embedding involvement within key areas of CHOICE programme activity. Our intention was to achieve the best possible PPE&I practice we could. As the grant progressed, the area of PPE&I was evolving, both internally within the programme, and externally nationally, as INVOLVE and other groups were shaping a PPE&I agenda around consultation and active collaboration, and we set out to learn from these developments on an ongoing basis. We felt it was important to take a ‘temperature’ check at 6-monthly intervals to ensure lay representatives were given an opportunity to shape progress and share their opinions on how the involvement activity was progressing and what modifications, if any, to the involvement activity needed to be made.
Stakeholder events
As part of the programme we held biannual stakeholder events each year, where we reviewed progress and offered opportunities for capturing learning and actively gauging opinion on key project work. These events were used as conduits for gathering stakeholder opinions, especially focused around patient and public members.
We planned each event to support our lay representatives to be involved at each key stage of the programme. Each event required several planning meetings in order to determine and develop a specific theme for each event and to review the feedback received from previous events. There was a united, inclusive team approach to planning, developing workshop materials and presentations, and supporting attendees at each event.
Table 56 is designed to demonstrate our planning, delivery and outcome processes for each stakeholder event.
| Event | Event date | Aims and format | Key outcomes and learning |
|---|---|---|---|
| 1 | 29 January 2010 | The aim of the first stakeholder event was to inform stakeholders about the CHOICE programme and talk about ways in which we could effectively incorporate the ‘lay voice’ into the research programme |
|
| 2 | 11 February 2011 | After working with the lay representatives on individual parts of the programme, we ran a second stakeholder event. We had recruited more lay representatives in the previous year so we began by repeating our diamond exercise and reaffirming the phases of the programme. We aimed to build on stakeholder event 1 and also assist new attendees to better understand the programme and the expectations for involvement |
|
| 3 | 24 June 2011 | The third stakeholder event was designed to conclude the work of the systematic review, update on the quantitative and qualitative studies, and introduce phase 3, the red flag intervention We began the session by updating the attendees using the diamond exercise for the final time. This had worked well in creating a positive format to link the events and create a visual structure of the programme. After a brief overview, we discussed the top 10 points from our previous two sessions and explained how we hoped to address these in the sessions that day |
|
| 4 | 9 March 2012 | The fourth stakeholder event included presentations and active participation by the CHOICE programme lay representatives We used the theme of a growing plant and created a series of slides and posters to demonstrate the development of the programme, where each phase started, and how it continued to develop We started the session with an overview of the programme to bring attendees who were new to the CHOICE programme or had missed a stakeholder event up to date with the progress of each phase. We were able to demonstrate where previous phases were catalysts for future phases, where work overlapped, and also to identify the completion of part of a phase |
|
| 5 | 21 September 2012 | In preparation for this event, the team collated a booklet, which gave an overview of the achievements of the CHOICE programme so far and the projected planning for the next 2 years. This included an overview of previous events and updated profiles of the CHOICE programme lay representatives. The booklet was designed to be a reminder of the work the lay representatives had been involved in and an effective overview for some of our colleagues attending a stakeholder event for the first time |
|
| 6 | 12 April 2013 | The sixth stakeholder event was planned using specific feedback from the previous event As the CHOICE programme progressed there was an increasing amount of information, evidence, and evolving activities |
|
| 7 | 4 April 2014 | We approached the seventh stakeholder event with a different focus, as this was our last CHOICE programme stakeholder event and had to be special, a way to celebrate the work of our team and in particular our lay representatives, their work, their knowledge and experience and not least their commitment. It was lovely to see former members of the research team as well |
|
Before and during each stakeholder event, we worked to ensure that the CHOICE programme lay representatives felt at ease and informed about the objectives of the programme, and that research staff had an opportunity for face-to-face interactions with the lay representatives on an ongoing basis. The stakeholder events set the tone of involvement and nurtured an environment of mutual respect and understanding. Within stakeholder event 1, for example, we asked the lay representatives to tell us what they wanted to get from the programme and we highlighted how the involvement of the lay representatives was key to a successful outcome for the programme. We also used events to provide knowledge on the research methods used, such as systematic reviews, RCTs and qualitative interviews. These opportunities were used to inform lay representatives and also to provide informal training to allow them to acquire the skills to understand the research methods employed within the programme.
Our lay representatives were encouraged to comment on, robustly challenge and contribute to all aspects of the programme at stakeholder events. In particular, as people with lived experience of at least one of the four exemplar diseases we were focusing on, they each had their own unique views or experiences of depression or other emotional consequences of long-term illness. Within our group of lay representatives, there was a very wide range of differing views and opinions regarding the nature of depression/low mood in long-term illness. This resulted in lively debates and a cross-fertilisation of ideas at stakeholder events. Some lay representatives talked openly for the first time in a social situation about their own experiences of depression and how they had coped with it.
The CHOICE programme team members were encouraged to be available to support and accommodate the needs of lay representatives during, and between, events. We kept in touch by the methods of contact which the lay representatives specified as preferred. We learned to work together to write concisely, clearly and in a user-friendly style to ensure that information and research material was accessible and aided understanding of the progress being made. This material was posted on the CHOICE programme website and sent out as hard copies, where relevant. The CHOICE programme website allowed the evidence for the PPE&I activity to be captured in written and video form, and included summary reports of all the stakeholder events and activities (URL: http://choice.mhsc.nhs.uk/, accessed 31 December 2016; active during the study, but has now been taken down).
The research team regularly visited the support groups from which some of our lay representatives were drawn (e.g. Breatheasy Burnage, Diabetes Trafford and Heartline Middleton), which helped build mutual respect and understanding, allowing a platform for wider public engagement activities/interactions within the key groups. This supported our lay representatives to be able to refer to their groups’ activities at stakeholder events with the knowledge that other CHOICE programme team members understood the focus and remit of their groups.
Innovation
We wanted to push the boundaries of PPE&I involvement, setting aside time for both formal and informal interactions with lay representatives and members of the public. This was to ensure that any constraints to effective PPE&I were not used as excuses to limit the consultative, collaborative and inclusive approach to working on the programme. We used the website as a media tool to promote and share our progress. We posted published CHOICE programme papers on the website with easy to read versions, as developed by our PPE&I group.
The website told the story of the CHOICE programme, creating a simple but very effective overview of very complicated research processes. This material was designed for all stakeholders to be able to read and process easily, enabling their input to be timely and appropriate. Newsletters were used to tell the ongoing story of the programme as it evolved. Key programme grant activity, such as the development of the intervention, was carried out with the involvement of all of our lay representatives via the stakeholder events and meetings.
Our PPE&I group planned the stakeholder events, using a variety of different workshop formats and group tasks to promote involvement and open discussion. We developed innovative creative approaches, to encourage open dialogue with lay representatives and wider stakeholders, as we felt that formality could stifle open and candid discussions. The openness allowed for ongoing constructive feedback to shape the programme and build in an active feedback loop for lay members. The events also provided the opportunity for lay representatives to present their views and experiences of being involved in the programme. This two-way sharing of information enabled learning and also helped create a supportive environment.
A major focus of the PPE&I involvement was in helping the research team to develop a credible and effective intervention for the third phase of the programme. This involved both a training element for GP practices and a patient-centred treatment for psychosocial problems in people with chronic physical illness.
Individual lay representatives worked with the team to share specific expertise and knowledge to help shape the development and delivery of general practice training.
We carried out informal discussions about what the psychosocial intervention should look like and collected information on the lay perspective on issues around depression and anxiety. This information was fed directly back in to the intervention development group. At critical points throughout the programme the team produced posters, booklets, podcasts and a range of other evidence to share with the widest possible audience.
Our lay representatives were regarded as full team members and their personal biographies and photographs appeared alongside those of other team members in the body of our website and they were involved in the co-production and presentation of posters.
Quality
The quality of our involvement activity was very important and, therefore, we worked to evaluate this by requesting written and verbal feedback from our stakeholders on an ongoing basis. The lay representatives were involved in compiling an engagement report which told our PPE&I story over a 3-year period and retrospectively captured our PPE&I engagement activity (URL: www.invo.org.uk/resource-centre/library-resource/?id=638§i=involve, accessed 16 June 2017).
The ongoing feedback loop from lay representatives to the research team was important in ensuring quality; actively listening to our patient and public voice was an important part of our involvement activity. Participation in stakeholder events included joint presentations, hands on workshops to develop the research tools, ongoing discussions on shaping the intervention and, most importantly, presentations about the progress being made. Each stakeholder event began with an overview in lay language of the progress made within the different strands; CHOICE programme lay representatives shared their personal stories, allowing others to feel empowered to do the same. This allowed the quality and depth of the information being shared to be enhanced.
An experienced and confident lay representative was involved as an equal in the delivery of training for the LHWs who delivered the trial intervention (see Chapter 8). The prior training, and then the involvement of a lay representative in delivering the training for the LHWs, made them more sensitive to the needs of people with LTCs, allowing the delivery of the intervention to be modified to better support the patient. We were able to build the patient perspective/knowledge into the training, which was an important element in shaping the delivery of the intervention. An important part of involvement was seeing our lay representatives as colleagues who were able to provide perspectives on the research, which without their involvement could have been missed.
For example, the majority of the membership of the Programme Steering Committee (the committee which provided independent oversight of the programme) consisted of lay representatives, who were given an opportunity to challenge and hold to account the research team. This forum also allowed for consultation on key milestones within the programme, biannually. It enabled the lay representatives to scrutinise all aspects of the management of the programme, including financial expenditure.
Sustainability
For the duration of the 5-year programme grant, it was important to sustain the PPE&I element for the development of our outcomes, the intervention trial and the development of the red flag systems, and we wanted to ensure that our learning in involving PPE&I representatives was comprehensively captured in stakeholder reports, newsletters and engagement reports. We knew we had an excellent partnership working when the lay representatives became our ‘critical friends’, quick to challenge but equally quick to praise if appropriate. The constant supply of programme paperwork in an easy-read format or simply explained at meetings was appreciated by all including the researchers working on other studies in the programme.
The team developed a close working relationship with lay representatives as an integral part of the team. They learned easy-read skills, and ensured that presentations were simple and to the point for the events. Having the focus of enabling informed dialogue at the end of each event was a great driver. Following each stage of the programme and each event, our publications and summaries were produced on our website so that learning was captured and shared, enhancing and building capacity for engagement.
An important part of the CHOICE programme involvement work was to ensure that our lay representatives felt empowered to continue with their involvement with research. Some of the CHOICE programme lay representatives have been empowered to seek positions on new committees, which include the service user commissioning groups and PPE&I work on other NIHR-funded programmes of research.
Lay representatives were also supported in carrying out presentations. On 27 September 2013, the CHOICE programme team hosted a national conference on comorbidity in Manchester, which was attended by over 70 delegates, including 10 esteemed speakers. Two lay members delivered a poster presentation on lay involvement in the CHOICE programme and the CHOICE programme engagement report was presented as part of the conference. Lay representatives were also encouraged to attend external conferences with funding provided by the programme. These opportunities were designed to allow lay representatives to attend conferences of interest and report back to the CHOICE programme manager and the steering committee, and their experiences were reported in newsletter articles.
Assessing the impact of our lay representative involvement: the CHOICE programme engagement impact assessment
Managing non-direct research processes can raise a range of ‘grey’ areas, which can mean it is hard to track progress quantitatively or qualitatively without a formal structure. The CHOICE programme sought to undertake an EIA process, identifying two engagement impacts:
-
the impact of the involvement of lay representatives in the delivery, evaluation, and monitoring of the programme
-
to evaluate the additional significance engagement has embedded in the management, reporting, monitoring and evaluation of the CHOICE programme.
We explored EIAs used by other studies, as we wanted to capture the element of ‘belonging’ and ‘feeling part of’ the programme team. As a result, we developed our own assessment tool. The project was developed by the Patient Experience and Diversity lead (JM); the questionnaire was further developed by a member of the research team, and was distributed and analysed by the research team.
We developed a tool to assess the impact of the practical day-to-day working arrangements around lay representative involvement, recruitment, retention, support, how we engaged, ongoing communication, how we involved, travel and expenses.
The second part of the assessment was to evaluate the additional significance engagement has embedded in the management, reporting, monitoring and evaluation of the CHOICE programme. For the lay representatives, the process included answering questions to find out if we were to do it again, what should we do to do things better (i.e. what would give us 10 out of 10). For our research teams, we asked: what did you learn, did this make you change the way you were working, would you find this knowledge useful in the future, and how do you envisage using this knowledge? For our colleagues from partner organisations who were members of CHOICE programme committees, steering group meetings, etc., we asked how effective they thought the lay representatives were at sharing their opinions at meetings, did they think they were actively listened to? We wanted to identify if the CHOICE programme team could have been more effective in their support or use of the skills of the lay representatives.
The data from the questionnaire were analysed and an EIA report produced.
The EIA analysis demonstrated that the lay representatives had worked with the CHOICE programme as effectively as we had hoped. However, if we had spent more time with the lay representatives at the beginning and as new members joined over the first 2 years, we would have realised some of their additional skills earlier. We should have determined the capacity of their involvement and explained the intricacies of ethics approval and ethics processes throughout the lifetime of the programme. There was a particular point in question that caused one lay representative to feel ‘left out’: they felt they had been shut out of the process. We learned a lot from this occurrence and changed our practices: from this point onwards during the lifetime of the grant, we planned extra sessions for our lay representatives before-and-after meetings to ensure there were no surprises in the meetings, and that they fully understood the meeting they had just attended.
The EIA assured the CHOICE programme team that we had effectively involved the lay representatives as team members and identified key areas for induction for future research programmes. However, our main learning was from the few, but poignant, negative comments.
Our lay representatives were pleased with the report and felt it captured their contribution to the programme:
. . . this is a very important study and I am sure many people will look at the papers and reports in the future, when they do they will see we were a significant part of it, that makes me proud.
CHOICE programme lay representative
Within any programme of research, there will be challenges, and we have attempted to work through these to ensure that the lay voice was actively listened to and lay representatives felt they could be involved within the key decisions. Unfortunately, this was not possible on all occasions because of the timing of some decisions and, in hindsight, we would work to build in a more robust induction process to better manage expectations.
Conclusion
This chapter reports how we worked to provide supportive and nurturing structures for all of our lay representatives and wider PPE&I stakeholders in the CHOICE programme. We tapped into the public voice and used the lay representatives as sounding boards, influencers and shapers of key decisions on the programme.
We have provided examples of where active PPE&I has had an impact and aided the outcomes of the programme and has also helped the development of lay representatives in the area of research and PPE&I. Following the final stakeholder event, we were able to identify further development for our lay representatives. We identified PPE&I training for those who wished to further develop and hone their PPE&I skills, and several of our lay representatives expressed a desire to continue to be involved in research.
The work on PPE&I is a continuous and evolving process and what we have found is that it is very important to ensure that the work is meaningful, and that value for the use of public funds is demonstrated and acknowledged. We hope that the CHOICE programme has illustrated that an inclusive nurturing environment, where mutual respect and support are at the heart of the research, can enhance the roles of lay representatives and ensure patient-focused outcomes are an integral part of programme activity.
Chapter 11 Overall conclusion
Summary
The main findings of the research programme are drawn together linking the different components and placing them in the current context of health service research, policy and delivery of services.
The following areas are discussed: PPE&I; the link between depression and use of unscheduled care in people with LTCs; costs, unscheduled care and depression; the relevance of programme findings for risk modelling; difficulties in case-finding for depression in people with LTCs; LTCs and multimorbidity; reasons why people with LTCs use unscheduled care; the role of primary care consultations in modifying use of unscheduled care; effective interventions to reduce use of unscheduled care; and a rationale linking depression and increased risk of unscheduled care in people with LTCs.
The specific objectives for the programme are detailed, with the relevant actions taken by the research team and the outputs achieved.
Introduction
In this chapter, we draw together the main findings from the research programme, linking together the different components, and placing them in the current context of health service research, policy and delivery of services. We also review the main objectives of the programme and the degree to which they have been achieved.
Reducing use of unscheduled care was a priority for the NHS at the start of this programme and remains a key policy imperative. For example, during the lifetime of the programme, the NHS has introduced a number of performance indicators related to use of unscheduled care, including:
-
Reducing EHAs for conditions where effective community care and case management can help prevent the need for hospital admission. These are known as chronic ambulatory care-sensitive conditions. 16
-
Reducing Emergency admissions for acute conditions that should not usually require hospital admission. 5
-
Reducing emergency readmissions within 28 days of discharge from hospital. 11
However, the number of emergency admissions to hospitals and attendances at EDs remain a significant problem, at a time when NHS resources are under significant pressure (see Chapter 1).
Patient and public engagement and involvement
Our intention from the pre-funding stage in 2006 was to ensure PPE&I was actively built into each stage of the research programme. One of our co-applicants (JM) has lived experience of a LTC, and prior to the programme had worked both with the Royal College of Physicians and the Liaison Faculty of the Royal College of Psychiatrists on several initiatives in relation to LTCs.
Jackie Macklin was able to provide a strong lead throughout the programme to ensure excellent PPE&I activity. The initial objective of our PPE&I activity was to recruit and support lay representatives to become embedded within the team and to represent the four LTCs (asthma, CHD, COPD and diabetes). We approached well-known patient organisations, such as Diabetes UK and Heartline, and we actively encouraged lay representatives to integrate into the CHOICE programme team. We knew we had an excellent working partnership when the lay representatives became our ‘critical friends’, quick to challenge but equally quick to praise, helping build a true sense of belonging and working to secure continuity of the patient and public voice for the duration of the programme.
We did not actively set out to recruit people with LTCs who also had experience of mental health problems, but as the programme developed, several of our PPE&I group shared some of their own experiences of mental health problems, or those of their friends. This frank and open discussion enabled us to discuss more freely the issues of ‘hidden depression’ in patients with LTCs and how this might be tackled. One specific outcome of this involvement was that our PPIs gave us a strong steer that any kind of psychological treatment we developed should not be placed within mental health services, but within a GP practice. Another important point was that the psychological health worker should also be able to provide help with physical and social aspects of the person’s care. Both these things are in marked contrast to the current way psychological services are organised in primary care.
The PPE&I model was developed around four key themes: nurturing environment, innovation, quality and sustainability. We developed a wide range of accessible information and regular newsletters all published on the CHOICE programme website.
We met regularly with our PPE&I group, to develop the targeted patient intervention and one of our PPE&I members also became involved in the training of the LHWs. At stakeholder events, we presented cases (in an anonymised fashion) of people who were receiving treatment from the LHWs, so that our PPE&I group could discuss and continue to be involved in the intervention.
Towards the end of the programme, we carried out an EIA, obtaining 360° appraisal and feedback of our PPE&I work. We received helpful and constructive feedback (see Chapter 10), which confirmed the strong sense of involvement in the programme that our PPE&I representatives felt. Our final evaluation identified the need for a more robust induction process, which we will act on in relation to future research involvement. We continue to meet with our PPE&I group, and are keen to explore further ways of involving them in future research.
The link between depression and use of unscheduled care in people with long-term conditions
Our work in this programme suggests that depression is an important predictor of use of unscheduled care in people with LTCs. This finding was supported by evidence from work we conducted in:
-
phase 1: systematic reviews
-
phase 2: longitudinal prospective study
-
phase 3: replication study using electronic primary care data.
The systematic review on depression in phase 1 (see Chapter 2) showed that depression was associated with an approximate 50% increase in the use of unscheduled care (OR 1.49, 95% CI 1.35 to 1.64; p < 0.0005). 78
The longitudinal study we conducted in phase 2 of the study (see Chapter 3) involved 1860 patients in primary care with at least one of the four exemplar conditions. This also showed that depression was independently associated with both emergency admissions to hospital (OR 1.58, 95% CI 1.04 to 2.40; p = 0.031) and attendances at EDs (OR 1.79, 95% CI 1.22 to 2.64; p = 0.003). 251 This relationship was independent of other important factors including previous use of unscheduled care, and severity and comorbidity of physical illness. More severe depression was associated with a greater than twofold increased risk of using unscheduled care (ED admissions OR 2.42, 95% CI 1.12 to 5.23, p = 0.025; participant-reported use of unscheduled care OR 3.58, 95% CI 1.88 to 6.82, p < 0.001). 236
A study in phase 3 to test whether or not we could replicate the results of the longitudinal cohort study using prospective data in an alternative setting, used routine primary care databases from two large geographical areas in north London (see Chapter 7). Again, this showed an independent association between depression and prospective attendance at an ED (OR 1.32, 95% CI 1.05 to 1.67, p = 0.019, for the north-east area of London; and OR 1.20, 95% CI 1.04 to 1.38, p = 0.015, for the north-west area of London). We did not find an association between depression and EHAs in the replication study, but further analyses from the longitudinal prospective study in phase 2 and the exploratory trial we conducted in phase 3 suggested that this could be attributable to the underdetection and under-reporting of depression in primary care. The prevalence of recorded depression in the north-east area of London over a 2-year period was only 4.4%, and for the north-west area it was 8.8%. This contrasts with the prevalence rate of 33–47% for depression, as measured by a standardised questionnaire in our longitudinal cohort study in Manchester (see Chapter 3).
In the RCT in phase 3, the prevalence of depression in the trial participants using GP electronic data was 8.3%, whereas the prevalence of depression using a standardised measure was 18.2%. Taking the trial groups as a whole, depression when measured using a standardised measure was a significant independent risk factor for use of prospective care 1 year later, but not if recorded using the electronic practice data systems.
Taken together, these findings provide robust evidence that depression (when measured by a standardised questionnaire) is an important independent risk factor for use of unscheduled care in primary care patients with LTCs in the UK. Depression increases the risk of using unscheduled care in people with LTCs in primary care by roughly 50–80%, regardless of the severity of physical illness, multimorbidity, age, sex and prior use of emergency services. More severe depression increases the risk of using unscheduled care by more than twofold.
Costs, unscheduled care and depression
Approximately 70% of the entire NHS budget is spent on the treatment and care of people with LTCs. 38 Our findings suggest that the cost per person in primary care with LTCs in the UK is on a par with average costs per person in the overall population (see Chapter 4). However, we found that unscheduled care accounts for approximately half of all health-care costs of people with LTCs. Analysis of costs reported in other UK and European Union studies using similar designs indicates comparable costs for each of the exemplar conditions. 68,260,265,268 If the results from the longitudinal prospective study are generalisable to patients with other LTCs, and to other parts of the country, it suggests that nearly 35% of the NHS budget is spent on unscheduled care for people with LTCs.
We found that depression was associated with higher health-care costs, for both scheduled and unscheduled care. Depression was also an independent predictor of the overall costs of unscheduled care in patients in the longitudinal study in phase 2, together with severity of illness and health-care costs over the previous year of study (see Chapter 4).
Although there has been a great deal of interest in patients who are frequent attenders or high users of unscheduled care,74 the results of this programme suggest that, for people with LTCs, this is not a major problem. Frequent attendance or use of unscheduled care has been linked to mental health problems,74 but the population of people who are recognised as ‘frequent attenders’ may be different from the population of people with physical LTCs. Substance abuse appears to be associated with highly frequent ED use. 328 The qualitative work that we carried out on the programme suggested that people with LTCs do not make choices about using unscheduled care, but suffer a relapse and then feel an imperative to attend hospital urgently.
This led us to abandon our intended prior aim to evaluate patient choices concerning particular aspects of treatment and associated costs, as the assumption that people with LTCs make choices about using unscheduled care appeared to be at variance with our findings.
Relevance of programme findings for risk modelling
As discussed in the introduction (see Chapter 1), a variety of risk-modelling tools were being used in the NHS setting to improve the management of high-cost patients at the time the programme was initiated. Over the lifetime of the programme, the DH has moved away from recommending a specific risk model, preferring more local decision-making, but there remains a strong steer from government that local commissioners should utilise such tools in service planning and delivery. In spring 2013, NHS England announced a new Enhanced Service Specification to reward general practices for the identification and case management of patients identified as seriously ill or at risk of an emergency admission. 348 As part of this, GP practices need to undertake risk profiling and risk stratification of their registered patients on at least a quarterly basis.
Most predictive modelling approaches have used hospital data from Hospital Episode Statistics or users of secondary services in England. The most commonly used models in England (such as the Patients at Risk of Re-Hospitalisation algorithm)66 are based on hospital admissions data, with some use of ED and outpatient attendance data. More recently data from electronic GP medical records have been included, and new variants involving a variety of different data sets have been evaluated. 67
Most models, until more recently, did not include mental health data. A recent systematic review of risk prediction models to predict EHA in community-dwelling adults identified 27 unique risk prediction models,349 of which only 12 included data on mental illness,67,350–358 three included data on living alone356,359,360 and none included data about threatening life events. Of the 12 models that included data on mental illness, all were published during the lifetime of this programme.
When data on mental health are included in risk models, patients at high risk of hospital admission are noted to have high rates of mental illness (27–32%), in addition to extremely high rates of chronic physical disease (85–90%). 67 To a certain degree, this provides further verification for some of our findings from the longitudinal prospective study in phase 2, and suggests that accurate mental health data, particularly depression, should be included in current risk profiling models.
UK general practices have good levels of accuracy and completeness in recording clinical diagnoses and prescribed medications. 361 However, some of our findings question whether or not mental health problems are accurately detected and recorded. All models are limited by the scope and quality of data available. The quality of mental health data is one obvious area that could be improved.
Patients at high risk of using unscheduled care have other important characteristics related to care needs that are currently not captured by administrative data and primary care computerised medical record systems. For example, interviews with high-risk patients and their families document high levels of social isolation for many people, as well as insecure housing conditions. 362 The recent systematic review by Huntley et al. in 2014 identified social isolation and poor educational level as being specific drivers of unscheduled care. 26 Even risk profile tools designed to run off GP systems and be automatically populated by GP data are unable, at present, to include data about social conditions. 350
There are concerns about the role of risk profiling in primary care, particularly whether or not such an approach can help reduce unscheduled care. 246 There are three main reasons for this:
-
Risk profiling reliably identifies high-risk patients only over a relatively short period of time, as their use of unscheduled care naturally declines.
-
High-risk patients account for only a small percentage of the total unscheduled care use.
-
Interventions targeted at high-risk patients (e.g. case management) may increase rather than reduce costs, because of the additional attention that more intensive care brings (so-called supply-induced demand), although this may bring improvements in overall health.
The findings from this programme suggest that the performance of predictive models could be improved by the availability of detailed psychosocial data on individual patients. However, such data would have to be reliably recorded, and current data systems need to be strengthened to support improved systematic coding and recording of mental health and social needs in people with LTCs. 38 Further consideration needs to be given to the time frame in which they are used and the populations that are included in risk models, as well as the cost–benefits of such approaches.
Depression is invisible in people with long-term conditions
Although we found that depression is an independent predictor of unscheduled care, the literature suggests that there are barriers to discussing depression in consultations with people with LTCs. 270 Our evidence is supported by four studies in the programme: the systematic review of qualitative studies in phase 1 about reasons why people use unscheduled care (see Chapter 2); the qualitative study in phase 2, which explored people’s reasons for using unscheduled care (see Chapter 5); the second qualitative study in phase 2, which recorded interactions between patients and primary care staff during routine reviews (see Chapter 6); and the replication study in phase 3 using north London primary care data sets (see Chapter 7).
The systematic qualitative review in phase 1 included papers from Europe, Australia, North America and the rest of the world. There was no evidence of any discourse by patients or health-care workers involving depression in the context of using unscheduled care. The qualitative study in phase 2 investigated the reasons why people use unscheduled care in a subsample of participants from the longitudinal cohort study. Health-care workers from both primary care and the acute setting were also interviewed. Depression played no part in the lengthy interviews between patients and researchers about use of unscheduled care. In the second qualitative study in phase 2, which digitally recorded routine reviews between patients with LTCs and HCPs, there was some evidence that HCPs actively blocked discourse related to the person’s emotional state and diverted it back towards more mechanistic task-orientated aspects of the consultation (see Chapter 6).
The replication study we conducted in phase 3 using routinely collected electronic primary care data showed that depression was not being detected or recorded in people with LTCs (see Chapter 8). In one large area of London, the reported prevalence of depression over a full 2-year period was only 4%, whereas the expected rate would be five to six times higher than this over a 1-year period, and over 20 years would be even higher. 46
It seems that people with LTCs and HCPs do not see a connection between low mood and use of unscheduled care. On one level, this is understandable as people with LTCs do not usually present urgently because of a sudden change in their mood. However, their reasons why their health may have deteriorated, resulting in contact with emergency services, are only explored or considered in a physical domain or at a very superficial level. Psychological factors, including depression, which may greatly impact on people’s ability to self-care and adhere to medication regimes are not explored and may even be blocked by HCPs.
The findings from the longitudinal prospective study in phase 2 suggest that people with LTCs will report symptoms of depression if they are given a standardised measure to complete, which could then become the basis of a discussion or review of their mental health status and how this might impact on their physical health and vice versa. However, the Whooley questions346 and the PHQ-9318 have been available for use in primary care over the past several years, yet the depression disease register and the use of these tools were retired from the QOF (a pay-for-performance scheme for chronic disease management in primary care) for the 2014/15 season. The only indicator that remains in the QOF is the depression follow-up assessment. 347
Far from being a standardised process that encourages detection of depression in people with LTCs, primary care clinicians report feeling uncomfortable using the case-finding questions, and feel that they lack the necessary skills to provide support to people at the point of diagnosis. 308 In another study, health staff reported a disconnect between the management of physical and mental health, with subsequent under-reporting of depression and a bias towards false-negative results. In addition, there is evidence that patients may not be accepting of the management of approaches which encourage the holistic management of physical and mental health problems in primary care. 332 Even if depression is acknowledged, it is often normalised and conceptualised by staff as a common and understandable response to the losses and stresses associated with LTCs, mitigating against diagnosis and treatment. 308 Other issues linked to depression in long-term illness, such as a sense of loss, loneliness and other interpersonal problems, together with complex social issues, also need to be recognised and addressed. 362 Social isolation and problems in social relationships impact adversely on depression outcomes in primary care, unless they are addressed. 363
The findings from the CHOICE programme together with other work conducted during the lifetime of the programme about the barriers to managing depression in people with LTCs297,308 suggest the detection and treatment of depression in patients with LTCs is a major problem in primary care. Efforts to incentivise formalised case-finding for depression may have inadvertently shaped what should be sensitive and nuanced human interactions between HCPs and patients into mechanistic processes focused on ‘tick box’ completion.
Our findings should not support the overuse of antidepressant medication in primary care or the overmedicalisation of ‘unhappiness’. 364 Depression in the context of LTCs is complex and multifaceted, and is often linked to chronic interpersonal issues and social stressors. A decision to start antidepressant treatment for patients who saw the LHWs was made by a consultant liaison psychiatrist, who was able to take into consideration the severity and chronicity of the person’s symptoms, their age, multimorbidity and psychosocial issues. Even antidepressants of the same class, have widely differing profiles of drug-to-drug interactions and side effects. Familiarity of use of these drugs in older adults with a wide variety of physical health problems is essential.
Long-term conditions and multimorbidity
One of the major strengths of the programme was the focus on four common LTCs, as opposed to exclusively focusing on one condition, as has been the usual practice in research on LTCs. The four conditions are all included in models designed to predict use of unscheduled care67,350 and are associated with high morbidity and health-care costs (see Chapter 1).
The findings from the longitudinal cohort study we conducted in phase 2 of the programme (see Chapter 3) enabled us both to compare the individual conditions and the effects of multimorbidity (two or more LTCs) in relation to use of unscheduled care. Over 70% of the participants in this study had multimorbidity with at least two or more LTCs.
We found broadly similar rates of use of unscheduled care across the four conditions, and broadly similar rates of depression and QoL scores. There was no evidence from our data that any of the four conditions carries a much greater risk of use of unscheduled care than the others. Two out of the four were independent risk factors for use of unscheduled care in the longitudinal cohort study in phase 2 (CHD and COPD) and all four were independent predictors of both EHAs and ED attendances in the replication study we conducted in phase 3 using primary care electronic data sets (see Chapter 7). The magnitude of the effect for each condition and associated use of unscheduled care in that study was also similar across conditions.
The use of unscheduled care, the prevalence of depression and the QoL of patients varied considerably between practices, as did the cost data, which are described in Chapter 4. These differences are of a much larger magnitude than any differences between individual conditions in the practices themselves. Huntley et al. 26 have recently reviewed features of primary care that affect unscheduled care use, and cite patient demographic and socioeconomic factors, proximity to health-care provision, chronic disease and multimorbidity, together with organisational factors in primary care, such as better access to and continuity of care. All the participants in the cohort study had chronic disease and most had multimorbidity, so these factors were not responsible for the large differences between practices in the study. Further work is required to understand the reasons for the large variations we found between practices in both morbidity and service use.
We carried out two systematic reviews in the first phase of the programme to investigate the effects of complex interventions on use of unscheduled care in people with LTCs. One of the reviews focused on asthma112 and the other on COPD. 111 The similarity of the results from these two reviews (> 60 studies), coupled with published data on other LTCs,320,365–367 suggests that psychosocial interventions may have similar effects in LTCs, regardless of the individual nature of the condition itself. This may enable greater generalisation of research findings across studies, which have focused on different exemplar LTCs.
Not all LTCs are comparable, but current evidence-based guidelines have mostly been developed for people with single diseases. There is some evidence from the CHOICE programme study that at least four of the common LTCs appear to have more things in common than differences, in terms of use of care, QoL, depression and anxiety.
The results from the cohort study in phase 2 also confirmed the important role that multimorbidity plays in use of unscheduled care. Each additional LTC increased the risk of an EHA by approximately 38%. This effect was independent of a history of prior use of unscheduled care or any psychosocial factors, which were themselves independently associated with use of unscheduled care.
Multimorbidity itself is an important risk factor for depression, and there appears to be a dose–response relationship between the number of chronic physical problems that a person experiences and depressive symptoms. 368 A recent study has shown the prevalence of depression in a primary care cohort was 23% for people with one LTC; 27% for two LTCs; 30% for three LTCs; rising to 41% for five LTCs. 369 This emphasises, again, the bidirectional nature between depression and chronic physical health, both of which are independent risk factors for use of unscheduled care.
The problems associated with multimorbidity, particularly for frail and elderly patients, are of particular current concern to the health service. Physical treatment is complex and challenging, with risks of overmedication and polypharmacy. 370,371 The delivery of psychosocial treatments, however, may be less complicated, as our evidence suggests that the nature of the physical illness itself may not be that important. The basic psychological principles that underlie the treatment of depression in physical illness may be transferable to patients with more than one condition.
The role of primary care consultations in modifying use of unscheduled care
Routine reviews of patients with LTCs are well established in primary care and represent a golden opportunity to provide high-quality, patient-centred care, with the development of management plans that the patient can use in case of illness exacerbations.
We found in our second qualitative study in phase 2 (see Chapter 6) that explicit discussion of what to do in the case of an urgent exacerbation of someone’s illness may be uncommon in primary care consultations in people with LTCs. Recorded interviews between 29 patients with LTCs (24 with multimorbidity) (see Table 31) and primary care HCPs found that only eight contained explicit mention of prospective help-seeking. Out of the eight consultations, five were directions to merely come back to the specific practitioner if any problems arose. Discussion about behaviour change or self-management was also uncommon. Specific self-management programmes or strategies as means to address behaviour change were mentioned in only four consultations (two involved rehabilitation programmes and two involved rescue packs of antibiotics).
Although the QOF effectively directs resources into reviews of patients with LTCs, the reviews are too constrained and mechanistic with a focus on ‘box ticking’. Practitioners are diffident about tackling behaviour change, and may lack the skills and ability to carry out such work.
We also found examples of good practice, involving practitioners who engaged in a collaborative style and were comfortable moving beyond the confines of the QOF to provide sensitive and personalised health care.
Routine reviews of patients with LTCs do have the potential to become focal points of patient-centred health care, if not solely driven by the demands of the QOF. Physical health parameters can be reviewed, psychological issues and social problems flagged, and management plans (with action points regarding exacerbations in physical health) developed in a collaborative fashion. If these reviews are to be more useful in influencing patient behaviour, the role of QOF targets needs review, with a move away from rewarding achievement of biomedical ‘tick-box’ targets to incentivising more patient-centred care.
It is important to note that primary care provides GPs and practice staff with the opportunity to develop relationships with patients over a long period of time, and not all concerns can be discussed at every review. Our data, however, suggest that opportunities are being lost to engage patients holistically in all aspects of their care.
Reasons why people with long-term conditions use unscheduled care
The findings from the programme have provided an in-depth understanding of why people with LTCs use unscheduled care. Although it may seem obvious why people attend EDs or utilise other kinds of unscheduled care, each decision is underpinned by a process of individual decision-making, which can be influenced by a variety of different factors.
There were two studies in the programme that focused on this area. These were the systematic review of qualitative studies about the reasons why people use unscheduled care in phase 1 (see Chapter 2) and the first qualitative study in phase 2, as part of which we interviewed a subsample of people from the longitudinal cohort study about their reasons for using unscheduled care (see Chapter 5). The findings from the review and the study in phase 2 were remarkably similar.
We found that, although policy-makers view use of unscheduled care as a major problem area, people with LTCs view use of unscheduled care as a necessary component of care, regarding exacerbations in ill health as inevitable. The choice of using unscheduled care, as opposed to routine primary care services, was shaped by people’s perceptions of urgency, their prior experience of illness and help-seeking, and the influence of family and friends. People equated hospitals with being places of safety, medical expertise and technology, whereas they viewed general practices as places they went for non-acute problems, such as reviews or prescribing of medication.
Although, undoubtedly, physical health problems are crucially important in decision-making about seeking unscheduled care, other, more subtle, contextual factors fail to be recognised. As noted previously, the fact that someone may live alone, or is feeling very low or is struggling to cope with their illness, rarely emerges in the discourse around unscheduled care. Contacts with unscheduled care are framed almost entirely in terms of a physical context, both at the time the person decides to seek care and on reflection, after care has been sought. Often their attendance at ED or some other urgent point of access is reinforced by health-care providers.
Although people regard using unscheduled care as ‘inevitable’, these views are to a large extent socially conditioned, and are potentially amenable to change. However, this will require changing people’s experiences of health care, so that primary care services are more proactive and less passive. It will also require a broadening of the discourse around use of unscheduled care to include psychosocial or other relevant personal factors that shape use (e.g. proximity).
Effective interventions to reduce use of unscheduled care
Over the last several years, there has been growing interest in improving the management of care for people with LTCs and multimorbidity. A wide variety of different approaches have been employed, which include tools to improve self-management; technology that may improve health care; and different ways to integrate health and social care. An explicit aim of some approaches has been to reduce avoidable emergency admissions. However, there is little systematic evidence of what works in terms of community-based alternatives to hospital admissions. 372 The Nuffield Trust recently published findings of a 4-year evaluation of community-based care services across the NHS, outlining the nature of the interventions and their impact. 373 The report describes the complexities and challenges involved in organisational change, and highlights the length of time it takes to embed new ways of working before benefits can be realised. Over 30 different services were evaluated with nearly all failing to show any discernible impact on reductions in use of unscheduled care.
Although the results are disappointing, the report points to what may be an unrealistic timetable to achieve significant reductions in use of unscheduled care. There is also the possibility raised by Roland and Paddison371 that better integration of care may lead to improved but more costly care for patients with LTCs, through the mechanism of supplier-induced demand, and through exposing unmet need.
What can the findings from the CHOICE programme add to the current knowledge base around this rapidly developing and changing area of care for people with long-term conditions and multimorbidity?
The emphasis in most ICPs has been the integration of physical and social care, with far less of a focus on mental health or psychosocial issues.
The findings from three studies in the CHOICE programme are of relevance. These are the two systematic reviews from phase 1 which focused on studies of complex interventions in patients with either asthma or COPD and their effect on use of unscheduled care (see Chapter 2). The third study involves the findings from the exploratory and feasibility trial in phase 3 (see Chapters 8 and 9).
Both of the two systematic reviews we conducted in phase 1 to determine the effects of complex interventions on the use of unscheduled care included RCTs which had evaluated a complex intervention (such as self-management or psychoeducation) in patients with either COPD or asthma and included an outcome measure of use of unscheduled care. The findings were broadly similar from both reviews, in that complex interventions had a small but significantly positive impact on the reduction of unscheduled care of the order of approximately 20–30% (COPD: OR 0.68, 95% CI 0.57 to 0.80; asthma: OR 0.79, 95% CI 0.67 to 0.94). Although asthma can be a single morbidity, COPD is frequently associated with other physical comorbidities, so these findings may be generalisable to patients with more than one LTC.
There is mounting evidence that complex interventions can improve depression in people with LTCs although the effects are modest. 111,112,333,365,366 Treatment of psychosocial problems may provide an added dimension to the overall care of people with LTCs, which has been relatively neglected to date.
The exploratory and feasibility study that we conducted in the final phase of the programme enabled us to carry out a preliminary evaluation of the two-level intervention that we developed. The cluster RCT was powered to determine the effect of the practice-level intervention for all patients in the intervention practices on use of unscheduled care. It was not powered to determine the effect of the targeted patient intervention delivered by the LHWs for a subsample of patients within the practices with psychosocial needs.
The organisational change that we sought to effect within the three intervention practices was not achieved within the time frame of the study. Although the practices welcomed additional input from the LHWs, there was little evidence of their integration into the practice teams, or a change in organisation, or focus on unscheduled care after the practice workshops. We may have been too ambitious in our aims and unrealistic in the time and degree of resource required to bring about systematic change. 373
The primary outcome measure we used in the exploratory trial (use of hospital-based unscheduled care) may not be sufficiently broad to capture important changes in service use. The measure excludes use of primary care unscheduled care services and use of scheduled care. Moreover, use of one service may increase the use of another. For example, the cost analysis of the longitudinal cohort study indicated that participants used a range of scheduled and unscheduled care services. Use of GP OOH services had an impact on the total cost of care. Regression analysis indicated that, for every £1 increase in the cost of GP OOH services, the total cost of scheduled and unscheduled care increased by £2.88 (95% CI £2.67 to £4.10).
These factors may be particularly important in the context of high levels of variation in use of services between practices and between participants within practices, and the importance of prior use of services as a powerful determinant of future service use.
The longitudinal analysis also indicated that nearly 50% of participants did not use any unscheduled care over the 2 years of the study, which provides further evidence to support a targeted approach for patients at ‘higher risk’ of use of unscheduled care.
The targeted patient intervention consisted of a brief low-intensity treatment (predominantly behavioural activation) combined with social care interventions, plus antidepressant treatment if warranted. The LHWs who delivered the intervention received supervision from liaison psychiatrists on a weekly basis. The intervention had elements of the collaborative care model developed by Katon et al. ,365 plus active involvement from liaison psychiatrists similar to the recent model of treatment from the SMaRT Oncology-2 trial,366 which showed very positive effects for the treatment of depression in patients with cancer. It also builds on the intervention from the recent COllaborative INterventions for CIrculation and DEpression (COINCIDE) trial for the treatment of depression in patients with diabetes and CHD. 334
The intervention, however, was not solely focused on depression, and addressed physical health, mental health and social care. The experience and needs of people with LTCs are diverse and those at most risk of unscheduled care have complex varying social and health requirements. 374 Targeting depression in a vacuum without addressing other problems areas is unlikely to be successful for people with chronic health needs, and services for people with LTCs are beginning slowly to develop integrated medical, psychological and social care. 367
The LHW intervention showed great promise in terms of its acceptability to patients (see Chapter 8), and there was some limited evidence that it may have the potential to be cost-effective (see Chapter 9). Patients spoke very highly and positively about the treatment, and the recruitment and retention rates were good. There were significant reductions in depression and use of unscheduled care among the patients who received the intervention.
A model linking depression and increased risk of unscheduled care in people with long-term conditions
The findings from the CHOICE study add to a model linking LTCs with depression and an increased risk of unscheduled care, which has been discussed in Chapter 3.
The relationship between depression and LTCs, however, is very complex and bidirectional. Adverse health risk behaviours and psychobiological changes associated with depression increase the risk of chronic medical disorders, and biological changes and complications associated with chronic medical disorders may precipitate depressive episodes. 252
Figure 30 is adapted from a model developed by Katon to illustrate the bidirectional interaction between depression and chronic physical illness, and the importance of familial and childhood experience. 252 In this model, genetic vulnerability, childhood adversity and adverse life events predispose individuals to both an increased risk of depression and an increased risk of chronic physical diseases, the latter mediated by biological and behavioural risk factors (e.g. smoking, obesity, a sedentary lifestyle, autonomic nervous system changes). Once physical disease has developed, the associated symptom burden, and functional impairment, increases the risk of depression, which in turn has a deleterious effect on a range of key factors including the quality of the physician–patient relationship,375,376 self-care,377 medication adherence98 and adherence to other lifestyle behaviours, such as diet or an exercise plan. 108,378 This in turn leads to an increase in symptom burden and severity of the physical illness. Social isolation and life stressors increase the risk of depression,363 and may also affect self-care, increasing the likelihood of exacerbations in physical illness requiring use of unscheduled care. Multimorbidity is associated with both an increased likelihood of depression per each LTC368 and an increased likelihood of using unscheduled care. 226
FIGURE 30.
Bidirectional interaction between depression and LTCs and how this might lead to an increased risk of unscheduled care. Adapted from the model of depression and chronic medical disorders by Katon. 252
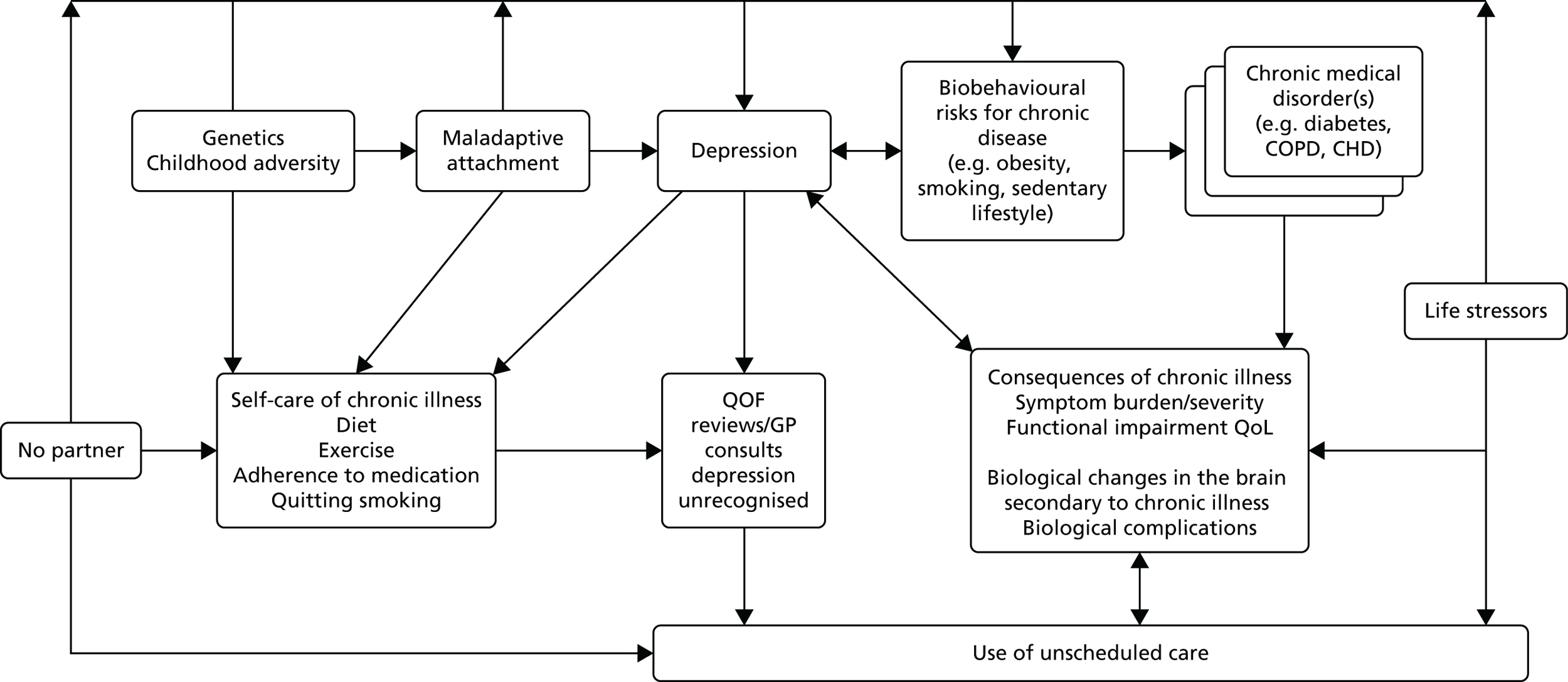
Opportunities during primary care reviews to identify depression and treat appropriately are frequently missed. 288 Once unscheduled care is accessed, reasons for its use are framed entirely within a physical context by patients and HCPs. 270 Patients’ experience of unscheduled care reinforces future use. 270 Contact is seen as inevitable (whether or not this is the case). 185 Underlying contributing psychosocial factors are not addressed, which further increase the likelihood of more unscheduled care use. 288
Limitations
There were several limitations in relation to this programme of work, which are summarised below and are discussed in more detail at the end of each chapter in the report.
One of our main intentions was to identify risk factors for frequent use of unscheduled care in people with LTCs. However, we found little evidence that people with LTCs were frequent users of unscheduled care, so we made a decision to switch focus to risk factors associated with any use of unscheduled care in people with LTCs. We also made a decision to focus on ED attendances and EHAs, as these were the most costly forms of unscheduled care use and a focus of government concern. Our findings are, therefore, in the main limited to these two areas, although we did included patient self-reported use of unscheduled care in the longitudinal prospective study in Chapter 3.
We chose to focus on four common LTCs (asthma, CHD, COPD and diabetes), which means that our findings may not be generalisable to all patients with LTCs. We also narrowed the focus of the systematic reviews in Chapter 2, regarding the evidence for the ability of complex interventions to reduce unscheduled care use, to COPD and asthma, which further limits the generalisability. The feasibility trial in Chapter 8 also focused on patients with COPD, although many people in the study had comorbid conditions.
The prospective longitudinal cohort study which we conducted in primary care to investigate the relationship between baseline factors and subsequent unscheduled care use had a low response rate (see Chapter 3), which again limits the generalisability of the findings.
The replication study with NHS routine data sets, which is discussed in Chapter 7, was limited in two ways. First, some of the psychosocial variables which we had identified as being predictors of routine care in the prospective study were not recorded routinely in GP data sets. Although we may not expect life stressors to be recorded on a routine basis, we were surprised that it not possible to identify people who lived alone or had no partner. Second, the prevalence of depression in the two areas of London which we use in the replication study was very low, which limited power.
Although many aspects of the feasibility study went well, we were unable to affect the behaviours of the practice staff, or embed the LHWs as part of the GP teams. We may have been too ambitious to try to do this and evaluate it over a relatively short space of time.
As we found no evidence of impact of our intervention at the level of the practice, it was not justified to conduct detailed health economic modelling, which was one of our original aims. We did carry out exploratory sensitivity analyses for the LHW intervention, but there are a number of issues that affect the robustness of these analyses, meaning that they should be treated with caution.
Our qualitative study which examined the reasons why patients chose unscheduled care was linked to the longitudinal prospective study and patient participants had to opt into the qualitative part. The participants were therefore a self-selected group, and their recall of use of unscheduled care may have been limited. Our second qualitative study was carried out in six GP practices in north-west England so the small number of practices and their limited geographical spread may again limit the generalisability of the findings.
Finally, in both the quantitative and qualitative aspects of the programme, people with LTCs from an ethnic background were under-represented.
Specific objectives and outputs of the programme
In the final part of this chapter, we review our original objectives together with the main outputs from the programme related to each specific objective.
Objective 1: systematically synthesise the current evidence about psychosocial drivers of unscheduled care and interventions or strategies to reduce the frequency of unscheduled care in patients with long-term conditions (phase 1)
Action
We carried out five systematic reviews to address our first objective. These were a systematic review:
-
of longitudinal cohort studies which had evaluated whether or not depression was associated with use of unscheduled care in people with at least one of the four exemplar conditions
-
of longitudinal cohort studies which had evaluated whether or not anxiety was associated with use of unscheduled care in people with at least one of the four exemplar conditions
-
to evaluate the effects of complex interventions on the use of unscheduled care in people with asthma
-
to evaluate the effects of complex interventions on the use of unscheduled care in people with COPD
-
of studies which had used qualitative methods to study reasons why people with LTCs use unscheduled care.
Output
Depression, but not anxiety, was a predictor of unscheduled care in people with LTCs. Complex interventions have a small but significant impact on reducing unscheduled care in people with LTCs. People with LTCs regard use of unscheduled care as inevitable. They equate hospitals with safety, expertise and high technology. Depression or other psychosocial issues do not feature in discourses about unscheduled care.
Objective 2: derive estimates of the frequency and pattern of unscheduled care in patients with asthma, coronary heart disease, chronic obstructive pulmonary disease and diabetes, as examples of common long-term conditions (phase 2)
Action
We carried out a longitudinal cohort study in primary care of 1860 patients with at least one of four exemplar conditions: asthma, CHD, COPD or diabetes. Over 70% of patients in the study had at least two LTCs.
Output
The frequency and pattern of unscheduled care use in patients with each of the four conditions was remarkably similar across conditions and relatively stable over a 2-year period. Approximately 16% of participants had an emergency admission to hospital during the baseline year and 17% during the follow-up year. Approximately 36% of participants reported having used some form of unscheduled care in the previous year, excluding admission to hospital, and 39% reported using unscheduled care in the follow-up year. Frequent use of unscheduled care was uncommon in the study population.
The following factors were independently associated with an increased risk of having an EHA or using some other form of unscheduled care in the 12-month follow-up period: prior use of unscheduled care in the previous 12 months, depression, having no partner, having threatening life experiences, COPD, CHD and a number of LTCs.
Objective 3: develop and validate a red flag system that will identify patients with long-term conditions who are at risk of becoming frequent users of unscheduled care (phases 2 and 3)
Action
We conducted a replication study using NHS routine data sets from a large geographical area in north London.
Output
We were unable to achieve our original aim as very few patients with LTCs in the main prospective study we undertook, were frequent users of unscheduled care, so it was not possible to identify a red flag for these patients. Instead we focused on people with LTCs who were at risk of using unscheduled care (EHAs or ED attendances). Of the candidate variables, prior use of unscheduled care, depression and each of the four LTCs were independent predictors of use of unscheduled care (either emergency admission to hospital or attendances at ED) in routine NHS electronic data sets. However, the prevalence of recorded depression in routine NHS data sets was very low, which limited its utility as a red flag variable. Nor were other psychosocial risk factors, ‘having no partner’ nor ‘threatening life experiences’, or comparable variables, recorded in GP data sets. These factors limited the development of a red flag system that could be embedded into routine GP data sets. The findings, however, suggest that predictive models which are currently being used in primary care should incorporate mental health and social data, and the quality of the mental health and social data that are recorded needs to be improved.
Objective 4: identify personal reasons for use of unscheduled care including barriers to access for routine care, patients’ motivations, expectations and decision-making processes, influences from families and relevant health-care workers, and factors in consultations with active case managers (phase 2)
Action
In a qualitative study, we carried out qualitative interviews with a subsample of participants from the longitudinal cohort study and HCPs from both primary care and secondary care settings. In a separate study, we digitally recorded routine primary care review consultations involving patients with at least one of the four exemplar conditions and primary care HCPs, and followed up patients over time to determine both the immediate and longer-term impact of the reviews.
Output
We found that patients’ decisions to use unscheduled care are based on perceived necessity, following a perceived exacerbation in their health, requiring the technical expertise of a hospital. However, patients drew on previous experiences of services and practitioners when choosing how to respond to illness exacerbations. The choice of using unscheduled care versus routine primary care was shaped by patients’ perceptions of urgency, which were in turn influenced by previous responses from health-care practitioners and by involvement of friends or family. ED doctors did not see it as their role to reduce patients’ use of unscheduled care, and patients reported several instances of their use of unscheduled care being reinforced by health-care staff. Choosing to attend ED services was shaped by people’s perceptions of it as a place of safety, clinical expertise and high technology.
In routine review consultations in primary care, there is little discussion of use of unscheduled care or joint planning of actions to follow in the event of an exacerbation in the person’s health. Discourse in relation to use of unscheduled care is confined entirely to the physical health domain, and other potential relevant psychosocial factors are not considered.
Objective 5: develop and evaluate an intervention which will reduce/prevent unscheduled care, while maintaining or improving patient benefit (phase 3)
Action
To develop the intervention, we synthesised data from phases 1 and 2 of the programme, combining our evidence synthesis with data from the longitudinal cohort study in Chapter 3 and the qualitative studies reported in Chapters 5 and 6.
We designed the intervention to impact both at the level of the practice and at the level of the patient. We chose to focus on patients with COPD as an exemplar group, as, out of all of the four LTCs, COPD had the highest rates of depression. COPD had also become a priority area for our local PCT.
Output
A two-level intervention with a practice-level intervention intended for the benefit of all patients with COPD, and a targeted patient intervention intended for patients with an identified psychosocial problem. At the level of the practice, the intervention focused on ways in which the practice as a whole could improve the overall care of patients with COPD and improve methods of identifying and referring at-risk patients. The targeted patient intervention consisted of brief low-intensity cognitive–behavioural strategies aimed at decreasing depression and social isolation, coupled with social interventions to help with social stressors. The treatment began with a patient-centred interview and development of a problem statement, followed by goal-setting and implementation plans. The intervention was delivered by LHWs with background training in mental health nursing and/or social work. Patients were offered four sessions of treatment plus an additional four telephone consultations if required. The LHWs received weekly supervision from liaison psychiatrists for advice on antidepressant treatment, if relevant.
Action
We conducted an exploratory cluster RCT involving six practices in Manchester, of which three were intervention practices and three control practices. Several key objectives were evaluated, including feasibility and acceptability of data collection methods and measures; the impact of the intervention at the level of the practice population; likely recruitment and retention of patients to the LHW intervention; acceptability of the LHW intervention; and implementation of the practice-level intervention. A process evaluation explored the acceptability of the intervention from the perspectives of patients and primary care clinicians and staff.
Output
We completed an exploratory and feasibility cluster randomised intervention trial in primary care to time with acceptable recruitment and retention rates. We used pseudonymised data from the CCGs to record use of hospital-based unscheduled care, which enabled us to collect data on all patients with COPD in the treatment and control practices. The study was powered for the effects of the intervention at the level of the practice, but not for targeted patient intervention delivered by the LHWs. There was no evidence that the practice-level intervention reduced unscheduled care.
The targeted patient intervention was well received by the subset of patients who received it, and we were able to demonstrate acceptable recruitment and retention rates for the treatment itself, with evidence of improvement in depression over the course of treatment and a reduction in the use of ED attendances. The process evaluation suggested that the LHW intervention was not embedded in routine care in the study practices.
Objective 6: use statistical and health economic modelling (statistical regression models) to evaluate patient choices concerning particular aspects of treatment, and estimate the costs and benefits associated with treatment intervention (phase 3)
Action
The reasons why patients used unscheduled care and the choices they made were extensively evaluated in the systematic reviews and qualitative components of phases 1 and 2 of the programme. In light of the results of phases 1 and 2, and the unavoidable delays to phase 2, we recognised that the planned decision analyses were unlikely to further inform the development of a red flag system and development of the intervention to reduce/prevent the use of unscheduled care. In phase 1, the systematic reviews were designed to identify associated costs and outcomes. However, the review did not identify any economic studies and information about the economic outcomes or costs was not reported in the studies included in the reviews. This meant there was little robust evidence relevant to the UK setting to inform the structure of the decision models or provide data with which to estimate costs and outcome variables for the model. Accordingly, we collected more detailed data about both scheduled and unscheduled service use in the longitudinal cohort study than originally anticipated. This meant that we were able to use the resources for the health economics component of the programme to focus on detailed costing and analysis of the longitudinal cohort data (phase 1).
We estimated detailed costs of the scheduled and unscheduled services used by the 1860 participants in the longitudinal cohort. Given the rich service use data collected within the longitudinal cohort study and the large number of complete data, statistical regression models (rather than decision-analytic models) were used to explore the costs for each of the exemplar conditions. These analyses included exploratory work to identify key covariates, cost drivers and sources of variations (e.g. between practices or between patient subgroups).
Output
The analyses indicated that the costs of unscheduled care are an important component of total care, and account for around 50% of annual costs for people with asthma, CHD, COPD or diabetes. Although the costs were similar between the conditions included in this study, there was a high level of variation within conditions. There was also great variability in terms of cost of health care for patients with LTCs between the practices, with costs on average £1000 more per annum per patient for some practices than others. A proportion of participants did not use any unscheduled care over the two years of the study and had substantially lower costs than those who used unscheduled care in one or both years.
This suggests a ‘one size fits all’-type intervention may not be applicable and that interventions to reduce the use of unscheduled care may need to be tailored to the practice and the patient.
We found that the presence of depression (i.e. HADS depression score of ≥ 8) and the LTC being rated as severe or very severe were associated with higher costs of scheduled and unscheduled care. The analyses included a broad range of participants’ demographic and clinical characteristics, most of which were not statistically associated with the use or costs of unscheduled care or the costs of scheduled care.
Action
The health status and resource use data collected in the trial were used to explore the potential cost-effectiveness of the intervention to reduce use of unscheduled care, from the perspective of the NHS. The time horizon for the economic analysis was the 1-year length of scheduled follow-up. Net costs and QALYs associated with the intervention were estimated using linear regression adjusting for key covariates and clustering by GP practice.
The primary measure for the economic analysis was the ICER. The estimates of incremental costs and outcomes from the regression were bootstrapped to estimate the probability that the targeted patient intervention is cost-effective compared with usual care. A series of one-way sensitivity analyses explored the impact of (1) changes in the estimates of the cost of the intervention; (2) using alternative measures of health benefit; and (3) implementing different levels of intervention for subgroups of the original sample. The within-trial analysis provided data with which to assess the likely cost-effectiveness of the practice-level intervention and the targeted patient intervention. Although the trial was a feasibility study, the trial indicated that the practice-level intervention may not reduce use of unscheduled care or be cost-effective. Combined with the reasons noted above, we recognised that an economic decision model would not provide additional information to that generated by the exploratory analyses of the trial data.
Output
Both the adjusted and unadjusted primary analyses indicated that the practice-level intervention, which includes the cost of the LHWs, is associated with additional costs and lower QALYs than the control group. There is no evidence that the practice-level intervention is cost-effective, even when the lowest feasible cost of the LHW intervention is used. This conclusion supports those of the effectiveness analyses and qualitative assessments, which suggest that there were problems with effectively embedding the intervention into the GP practices.
However, the sensitivity analyses indicated that the targeted patient intervention may be cost-effective. The relative cost-effectiveness of the intervention increases when only people with depression are included in the analysis. If the intervention is targeted at people with depression who participate in the LHW motivational sessions, then it is associated with a net saving, although there is a trend towards a net QALY loss.
Chapter 12 Implications for clinical practice and research
Implications for clinical practice
Our findings endorse recent policy initiatives to integrate the management of physical and mental health in people with LTCs. We suggest that HCPs who work routinely with people with LTCs are aware of factors that increase use of unscheduled care in the short term. These include recent prior use of unscheduled care; depression; comorbid other LTCs; and current social stressors. Risk models to identify people with LTCs at risk of using unscheduled care should consider including detailed psychological and social information.
Health-care practitioners should be aware of the different value that people attach to primary care and acute hospitals when they make choices about which kind of care to use.
Health-care practitioners need to actively enquire about low mood in people with LTCs and manage the patient appropriately if symptoms are detected.
Routine QOF reviews for people with LTCs are an opportunity to identify psychosocial problems, which may impact on use of unscheduled care.
Integrated care teams often combine physical and social care, but should also consider the inclusion of mental health care, with mental HCPs included as part of the integrated care team.
Older people with LTCs and mental health problems are open and receptive to a mental health intervention if this is viewed as being part of the care integral to their GP practice.
Services should keep up-to-date information concerning the availability of statutory and non-statutory services, self-help, and third-sector organisations in their local area, which may be of value to people with LTCs.
Research recommendations
In the context of ongoing service change, the programme raises a number of further research questions, summarised here in priority order:
-
Can routine primary care consultations for patients with LTCs become more patient centred to facilitate discussion of psychosocial issues that impact on health care, and can these consultations be used to implement behavioural change?
-
What are the costs and benefits of a targeted patient intervention to reduce unscheduled care and overall costs for people with LTCs and comorbid depression?
-
Does case-finding for depression in people with LTCs in primary care improve physical and mental health outcomes?
-
What is the mechanism whereby depression and other psychosocial factors affect use of unscheduled care in people with LTCs?
-
What is the most appropriate and sensitive primary outcome measure to accurately assess the impact of psychosocial interventions in people with LTCs and coexisting psychosocial problems?
Acknowledgements
This programme was successful because of the hard work and invaluable contributions made by many people, whose dedication and support ensured that public funds were well spent with a successful programme completed.
First of all a huge thank you to all of the patients that responded to the study questionnaires, agreed to be interviewed and participated in the intervention and thanks to all of the GP clinical staff who agreed to be interviewed within all phases of the programme.
A huge thank you to all of our lay representatives who supported the CHOICE research programme, they were critical friends positively influencing the design and execution of the programme:
-
Malcolom Nicholson, Breatheasy
-
Peter Swindles, Heartline
-
Fred Walton, Heartline
-
John Howe, Diabetes UK
-
Don McGeachin, Diabetes UK
-
Ruth Rudd, Breatheasy
-
Irene Blay, Asthma UK
-
Rosalind Nesbitt, service user, Manchester Mental Health and Social Care Trust
-
Glyn Tattan, service user, Manchester Mental Health and Social Care Trust.
Thank you to the research assistants who supported the programme with dedication, which ensured the smooth operation of the programme:
-
Rebecca Anderson, Research Assistant
-
Kimberley Keane, Senior Research Assistant
-
Angee Khara, Senior Research Assistant
-
Alexandra Stenhoff, Senior Research Assistant.
Thank you to Sarah Parsons and Ming Quan, both were summer intern students.
Finally, a big thank you to all of the practice leads and practice managers at the Manchester General Practices who supported the programme.
The programme received valuable support from CCG managers and analysts facilitated access to data.
Manchester CCGs:
-
Graham Hayler, Business Intelligence Lead
-
Dr Sally Huxham, Business Intelligence Manager (Manchester locality), Greater Manchester Commissioning Support Unit.
Greater Manchester Commissioning Support Unit:
-
Matrika Ramduny, DSCRO Lead
-
Jacqui Dorman, Business Intelligence Manager (Manchester)
-
Helen Kay, Senior Business Analyst (South Manchester), Business Intelligence Team.
South Manchester CCG:
-
Martyn Kent, Senior Commissioning Manager, Urgent Care
-
Nancy Ryalls, Commissioning Manager, Urgent Care
-
Deborah Greenham, Service Improvement Manager, Urgent Care.
The programme also received support from clinical and academic colleagues.
Thank you to the past and current chairperson of the Steering Committee: Dr John McBeth (Department of Primary Care and Health Sciences, Keele University) and Dr Richard Hopkins (Manchester Mental Health and Social Care Trust, Manchester Royal Infirmary and Manchester Academic Health Science Centre).
Thanks to the academic representative on the Steering Committee: Professor Peter Bower (Centre for Primary Care: Institute of Population Health, The University of Manchester and Manchester Academic Health Science Centre).
Thanks to the clinical staff member of the CHOICE programme Steering Committee: Dr Robert Baldwin (Manchester Mental Health and Social Care Trust, Manchester Royal Infirmary and Manchester Academic Health Science Centre).
Thanks to Professor Alicia O’Cathain (Health Services Research, School of Health and Related Research, The University of Sheffield) and Professor Jon Nicholl (Health Services Research, School of Health and Related Research, The University of Sheffield) who advised on the use of, and access to, emergency services.
A large number of specialist registrars in psychiatry also helped with note review and data collection. These include (with organisational affiliations at the time the work was completed): Dr Wakil Ahmed (Manchester Mental Health and Social Care Trust, Manchester Royal Infirmary and Manchester Academic Health Science Centre); Dr Julia Hose (Manchester Mental Health and Social Care Trust, Manchester Royal Infirmary and Manchester Academic Health Science Centre); Dr Andrew Nivison (Manchester Mental Health and Social Care Trust, Manchester Royal Infirmary and Manchester Academic Health Science Centre); Dr Mustafa Alanchkar (Manchester Mental Health and Social Care Trust, Manchester Royal Infirmary and Manchester Academic Health Science Centre); and Dr Sylvia Khan (Manchester Mental Health and Social Care Trust, Manchester Royal Infirmary and Manchester Academic Health Science Centre).
Two specialist registrars in psychiatry also provided supervision for the LHWs: Dr Amir Moghavemi (Manchester Mental Health and Social Care Trust, Manchester Royal Infirmary and Manchester Academic Health Science Centre) and Dr Joanne Cole (Manchester Mental Health and Social Care Trust, Manchester Royal Infirmary and Manchester Academic Health Science Centre).
Administrative, governance and sponsor support
The programme was supported by the research and development department of Manchester Mental Health and Social Care Trust (MMHSCT), including Mrs Sue Dobson who helped with the original grant application and provided ongoing support during the programme. The Chief Executive of MMHSCT, Mrs Michele Moran, and the current Medical Director, Dr JS Bamrah and former Medical Director Dr Sean Lennon, were supportive and helpful throughout the life of the programme.
Mrs Wendy Clarke ensured that the team were given the dedicated administrative support required within all phases.
The CHOICE programme would also like to thank the staff of the mental health and primary care clinical research networks, who helped with data collection.
Contributions of authors
Elspeth Guthrie (Professor of Psychological Medicine and Medical Psychotherapy) was the programme lead and chief investigator for all studies within the three phases of the programme.
Cara Afzal (Programme Manager) co-ordinated all phases and co-led the PPI aspects of the programme.
Claire Blakeley (Senior Research Assistant) worked on the quantitative studies in phases 2 and 3 of the programme.
Amy Blakemore (Senior Research Associate) worked on the quantitative studies in phases 1 and 2 of the programme and made substantial contributions to the systematic reviews and the collation of the final report.
Rachel Byford (Structured Query Language Developer) developed the codes for the testing and validity of the red flag markers.
Elizabeth Camacho (Senior Research Associate) supported the health economics work.
Tom Chan (Senior Research Fellow) co-ordinated the approvals for the validation study and supervised data collection and analysis on this part of the programme.
Carolyn Chew-Graham (Professor of General Practice) was the GP lead and co-lead for the qualitative studies in all phases of the programme, supervising the qualitative streams and making a substantial contribution to the development and execution of the intervention.
Linda Davies (Professor of Health Economics) led on the health economics components of the programme, within all phases.
Simon de Lusignan (Professor of Primary Care and Clinical Informatics) led the work on the validation study in phase 3 of the programme.
Chris Dickens (Professor of Psychological Medicine) led the systematic review work in phase 1 of the programme and contributed to the development of the intervention.
Jessica Drinkwater (Academic Clinical Fellow) worked on the qualitative studies within phases 1 and 2 of the programme.
Graham Dunn (Professor of Biomedical Statistics) supervised the statistical aspects of the programme.
Cheryl Hunter (Senior Research Associate) made a substantial contribution to the qualitative studies within all phases of the programme.
Mark Joy (Senior Lecturer in Data Modelling and Population Health) carried out the analysis regarding the validation study in phase 3.
Navneet Kapur (Professor of Psychiatry and Population Health) provided advice and guidance about all aspects of the programme and contributed to the development of the intervention.
Susanne Langer (Senior Research Associate) worked on the qualitative aspects of the programme, and made a contribution to the development of the intervention.
Karina Lovell (Professor Director of Research and Professor of Mental Health) had input across the programme and made a substantial contribution to the development and execution of the intervention.
Jackie Macklin (Patient Representative and co-applicant) co-led the PPI aspect of the programme.
Kevin Mackway-Jones (Consultant in Emergency Medicine) provided advice and guidance for all aspects of the programme.
Dionysios Ntais (Senior Research Associate) worked on the health economic analysis.
Peter Salmon (Professor of Clinical Psychology, Honorary Consultant Clinical Psychologist) co-led the qualitative studies in all phases of the programme, supervising the qualitative streams and making a substantial contribution to the development and execution of the intervention.
Barbara Tomenson (Senior Statistician) was the lead statistician for the whole programme leading on all aspects of data cleaning, analysis and reporting.
Jennifer Watson (Senior Research Associate) was involved in the quantitative studies in phases 1 and 2 of the programme, and led the GP note review data collection.
Publications
Dickens C, Katon W, Blakemore A, Khara A, McGowan L, Tomenson B, et al. Does depression predict the use of urgent and unscheduled care by people with long term conditions? A systematic review with meta-analysis. J Psychosom Res 2012;73:334–42.
Langer S, Chew-Graham C, Hunter C, Guthrie E, Salmon P. Why do patients with long term conditions use unscheduled care? A qualitative literature review. Health Soc Care Community 2012;21:339–51.
Chew-Graham C, Hunter C, Langer S, Stenhoff A, Drinkwater J, Guthrie E, et al. How is QOF shaping primary care review consultations: a longitudinal qualitative study. BMC Fam Pract 2013;14:103.
Dickens C, Katon W, Blakemore A, Khara A, Tomenson B, Woodcock A, et al. Complex interventions that reduce urgent care use in COPD: a systematic review with meta-regression. Respir Med 2013;108:426–37.
Drinkwater J, Salmon P, Langer S, Hunter C, Stenhoff A, Guthrie E, et al. Operationalising unscheduled care policy: a qualitative study of healthcare professionals’ perspectives. J Gen Pract 2013;63:e192–9.
Hunter C, Chew-Graham C, Langer S, Stenhoff A, Drinkwater J, Guthrie E. A qualitative study of patient choices in using emergency health care for long-term conditions: the importance of candidacy and recursivity. Patient Educ Couns 2013;93:335–41.
Blakeley C, Blakemore A, Hunter C, Guthrie E, Tomenson B, Dickens C. Does anxiety predict the use of urgent care by people with long term conditions? A systematic review with meta-analysis. J Psychosom Res 2014;77:232–9.
Blakemore A, Dickens C, Anderson R, Tomenson B, Woodcock A, Guthrie E. Complex interventions reduce care use in adults with asthma: systematic review with meta-regression. Respiratory Med 2014;109:147–56.
Hunter C, Chew-Graham C, Langer S, Drinkwater J, Stenhoff A, Guthrie E, et al. ‘I wouldn’t push that further because I don’t want to lose her’: a multiperspective qualitative study of behaviour change for long-term conditions in primary care. Health Expect 2015;18:1995–2010.
Guthrie E, Dickens C, Blakemore A, Watson J, Chew-Graham C, Lovell K, et al. Depression predicts future emergency hospital admissions in primary care patients with long-term physical illness. J Psychosom Res 2016;82:72–81.
Data sharing statement
Request for access to data should be addressed to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, CCF, NETSCC, PGfAR or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the PGfAR programme or the Department of Health.
References
- Commissioning a New Delivery Model for Unscheduled Care in London. London: NHS England; 2011.
- Hallaran F, Robertson-Steel I. A Guide to Good Practice: Unscheduled and Emergency Care Services. Cardiff: National Leadership and Innovation Agency for Healthcare; 2016.
- O’Cathain A, Knowles E, Munro J, Nicholl J. Exploring the effect of changes to service provision on the use of unscheduled care in England: population surveys. BMC Health Serv Res 2007;7. http://dx.doi.org/10.1186/1472-6963-7-61.
- NHS England . Annual A&Amp;E Activity and Emergency Admission Statistics, NHS and Independent Sector Organisations in England n.d. www.england.nhs.uk/statistics/statistical-work. /ae-waiting-times-and-activity/ (accessed 14 March 2016).
- Emergency Admissions to Hospital: Managing the Demand. London: The Stationery Office; 2013.
- Total Time Spent in Accident and Emergency. London: DH; 2011.
- Tian Y, Dixon A, Gao H. Emergency Hospital Admissions for Ambulatory Care-Sensitive Conditions: Identfying the Potential for Reductions. London: The King’s Fund; 2012.
- Chronic Disease Management – A Compendium of Information. London: DH; 2004.
- Supporting People with Long-Term Conditions: an NHS and Social Care Model to Support Local Innovation and Integration. London: DH; 2005.
- Ten Things You Need to Know About Long-Term Conditions. London: DH; 2011.
- Improving the Health and Well-Being of People with Long-Term Conditions. World Class Services for People with Long-Term Conditions: Information Tool for Commissioners. London: DH; 2010.
- Investing in Emotional and Psychological Wellbeing for Patients with Long-Term Conditions. London: NHS Confederation; 2012.
- A&E Performance and Activity Timeseries, Year to Date From First Publication. London: NHS England; 2013.
- A Call to Action: Achieving Parity of Esteem:Transformative Ideas for Commissioners. London: NHS England; 2014.
- Addicott R. Commissioning and Contracting for Integrated Care. London: The King’s Fund; 2014.
- No Health Without Mental Health: A Cross-Government Mental Health Outcomes Strategy for People of All Ages. London: DH; 2011.
- Naylor C, Das P, Ross S, Honeyman M, Thomson J, Gilburt H. Bringing Together Physical and Mental Health: A New Frontier for Integrated Care. London: The King’s Fund; 2016.
- Williams A, Lloyd A, Watson L, Rabe K. Cost of scheduled and unscheduled asthma management in seven European Union countries. Eur Respir Rev 2006;15:4-9. https://doi.org/10.1183/09059180.06.00009801.
- Magid DJ, Houry D, Ellis J, Lyons E, Rumsfeld JS. Health-related quality of life predicts emergency department utilization for patients with asthma. Ann Emerg Med 2004;43:551-7. http://dx.doi.org/10.1016/S0196064403012496.
- Tsai C, Grsiwold S, Clark S, Carmargo C. Factors associated with frequency of emergency department visits for chronic obstructive pulmonary disease exacerbation. J Gen Intern Med 2007;22:799-804. https://doi.org/10.1007/s11606-007-0191-7.
- Carret ML, Fassa AC, Domingues MR. Inappropriate use of emergency services: a systematic review of prevalence and associated factors. Cad Saude Publica 2009;25:7-28. https://doi.org/10.1590/S0102-311X2009000100002.
- Cowling TE, Cecil EV, Soljak MA, Lee JT, Millett C, Majeed A, et al. Access to primary care and visits to emergency departments in England: a cross-sectional, population-based study. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0066699.
- Ng T, Nitti M, Tan W, Cao Z, Ong K. Depressive symptoms and chornic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status and quality of life. Arch Intern Med 2007;67:60-7. https://doi.org/10.1001/archinte.167.1.60.
- Eisner MD, Katz PP, Lactao G, Iribarren C. Impact of depressive symptoms on adult asthma outcomes. Ann Allergy Asthma Immunol 2005;94:566-74. https://doi.org/10.1016/S1081-1206(10)61135-0.
- Gruneir A, Silver MJ, Rochon PA. Emergency department use by older adults: a literature review on trends, appropriateness, and consequences of unmet health care needs. Med Care Res Rev 2011;68:131-55. http://dx.doi.org/10.1177/1077558710379422.
- Huntley A, Lasserson D, Wye L, Morris R, Checkland K, England H, et al. Which features of primary care affect unscheduled secondary care use? A systematic review. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2013-004746.
- Egede LE, Zheng D, Simpson K. Comorbid depression is associated with increased health care use and expenditures in individuals with diabetes. Diabetes Care 2002;25:464-70. https://doi.org/10.2337/diacare.25.3.464.
- Pishgoo B. A novel prediction model for all cause emergency department visits in ischaemic heart disease. J Res Med Sci 2011;16:262-8.
- Xu W, Collet JP, Shapiro S, Lin Y, Yang T, Platt RW, et al. Independent effect of depression and anxiety on chronic obstructive pulmonary disease exacerbations and hospitalizations. Am J Respir Crit Care Med 2008;178:913-20. http://dx.doi.org/10.1164/rccm.200804-619OC.
- Almagro P, Barierio B, Ochoa de Echaguen A, Quintana S, Rodriguez Carballeira M, Heredia J, et al. Risk factors for hospital readmission in patients with chronic obstructive pulmonary disease. Respiration 2006;73:311-17. https://doi.org/10.1159/000088092.
- Nouwen A, Freeston MH, Labbé R, Boulet LP. Psychological factors associated with emergency room visits among asthmatic patients. Behav Modif 1999;23:217-33. https://doi.org/10.1177/0145445599232002.
- Adams RJ, Smith BJ, Ruffin RE. Factors associated with hospital admissions and repeat emergency department visits for adults with asthma. Thorax 2000;55:566-73. https://doi.org/10.1136/thorax.55.7.566.
- Cowie RL, Underwood MF, Revitt SG, Field SK. Predicting emergency department utilization in adults with asthma: a cohort study. J Asthma 2001;38:179-84. https://doi.org/10.1081/JAS-100000037.
- Dalcin PT, Piovesan DM, Kang S, Fernandes AK, Franciscatto E, Millan T, et al. Factors associated with emergency department visits due to acute asthma. Braz J Med Biol Res 2004;37:1331-8. https://doi.org/10.1590/S0100-879X2004000900007.
- Peters D, Chen C, Markson LE, Allen-Ramey FC, Vollmer WM. Using an asthma control questionnaire and administrative data to predict health-care utilization. Chest 2006;129:918-24. http://dx.doi.org/10.1378/chest.129.4.918.
- Bodenheimer T. The future of primary care: transforming practice. N Engl J Med 2008;359:2086-9. http://dx.doi.org/10.1056/NEJMp0805631.
- Barnett K, Mercer S, Norbury M, Wyatt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for healthcare, research, and medical education: a cross-sectional study. Lancet 2012;380:37-43. https://doi.org/10.1016/S0140-6736(12)60240-2.
- Naylor C, Parsonage M, McDaid D, Knapp M, Fossey M, Galea A. Long-Term Conditions and Mental Health: The Cost of Comorbidities. London: The King’s Fund and Centre for Mental Health; 2012.
- Damush TM, Smith DM, Perkins AJ, Dexter PR, Smith F. Risk factors for nonelective hospitalization in frail and older adult, inner-city outpatients. Gerontologist 2004;44:68-75. https://doi.org/10.1093/geront/44.1.68.
- Managing Variation in Emergency Admissions. London: NHS Institute for Innovation and Improvement; 2016.
- Investing in General Practice: The New General Medical Services Contract. London: DH; 2003.
- Goodacre S, Cross E, Arnold J, Angelini K, Capewell S, Nicholl J. The health care burden of acute chest pain. Heart 2005;91:229-30. http://dx.doi.org/10.1136/hrt.2003.027599.
- O’Cathain A, Knowles E, Turner J, Hirst E, Goodacre S, Nicholl J. Variation in avoidable emergency admissions: multiple case studies of emergency and urgent care systems. J Health Serv Res Policy 2016;21:5-14. http://dx.doi.org/10.1177/1355819615596543.
- Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with type 2 diabetes: a systematic review and meta-analysis. Diabet Med 2006;23:1165-73. http://dx.doi.org/10.1111/j.1464-5491.2006.01943.x.
- Spijkerman T, de Jonge P, van den Brink RH, Jansen JH, May JF, Crijns HJ, et al. Depression following myocardial infarction: first-ever versus ongoing and recurrent episodes. Gen Hosp Psychiatry 2005;27:411-17. http://dx.doi.org/10.1016/j.genhosppsych.2005.05.007.
- Depression With a Chronic Physical Health Problem [CG91]. London: NICE; 2009.
- Zhang MW, Ho RC, Cheung MW, Fu E, Mak A. Prevalence of depressive symptoms in patients with chronic obstructive pulmonary disease: a systematic review, meta-analysis and meta-regression. Gen Hosp Psychiatry 2011;33:217-23. http://dx.doi.org/10.1016/j.genhosppsych.2011.03.009.
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 2001;24:1069-78. https://doi.org/10.2337/diacare.24.6.1069.
- Opolski M, Wilson I. Asthma and depression: a pragmatic review of the literature and recommendations for future research. Clin Pract Epidemiol Ment Health 2005;1. http://dx.doi.org/10.1186/1745-0179-1-18.
- Carney RM, Freedland KE. Depression, mortality, and medical morbidity in patients with coronary heart disease. Biol Psychiatry 2003;54:241-7. https://doi.org/10.1016/S0006-3223(03)00111-2.
- Cleland JA, Lee AJ, Hall S. Associations of depression and anxiety with gender, age, health-related quality of life and symptoms in primary care COPD patients. Fam Pract 2007;24:217-23. http://dx.doi.org/10.1093/fampra/cmm009.
- Goldney RD, Phillips PJ, Fisher LJ, Wilson DH. Diabetes, depression, and quality of life: a population study. Diabetes Care 2004;27:1066-70. https://doi.org/10.2337/diacare.27.5.1066.
- Dickens CM, McGowan L, Percival C, Tomenson B, Cotter L, Heagerty A, et al. Contribution of depression and anxiety to impaired health-related quality of life following first myocardial infarction. Br J Psychiatry 2006;189:367-72. http://dx.doi.org/10.1192/bjp.bp.105.018234.
- Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med 2004;66:802-13. http://dx.doi.org/10.1097/01.psy.0000146332.53619.b2.
- Fan VS, Ramsey SD, Giardino ND, Make BJ, Emery CF, Diaz PT, et al. Sex, depression, and risk of hospitalization and mortality in chronic obstructive pulmonary disease. Arch Intern Med 2007;167:2345-53. http://dx.doi.org/10.1001/archinte.167.21.2345.
- van Melle JP, de Jonge P, Spijkerman T, Tijssen JG, Orme J, van Veldhuisen D, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med 2004;66:814-22. https://doi.org/10.1097/01.psy.0000146294.82810.9c.
- Lauzon C, Beck C, Huynh T, Dion D, Racine N, Carignan S, et al. Depression prognosis following hospital admission because of acute myocardial infarction. CMAJ 2003;168:547-52.
- Gonzalez JS, Safren SA, Cagliero E, Wexler DJ, Delahanty L, Wittenberg E, et al. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care 2007;30:2222-7. http://dx.doi.org/10.2337/dc07-0158.
- Katon WJ, Lin EH, Williams LH, Ciechanowski P, Heckbert SR, Ludman E, et al. Comorbid depression is associated with an increased risk of dementia diagnosis in patients with diabetes: a prospective cohort study. J Gen Intern Med 2010;25:423-9. http://dx.doi.org/10.1007/s11606-009-1248-6.
- Katon W, Lyles CR, Parker MM, Karter AJ, Huang ES, Whitmer RA. Association of depression with increased risk of dementia in patients with type 2 diabetes: the Diabetes and Ageing Study. Arch Gen Psychiatry 2012;69:410-17. http://dx.doi.org/10.1001/archgenpsychiatry.2011.154.
- Himelhoch S, Weller WE, Wu AW, Anderson GF, Cooper LA. Chronic medical illness, depression, and use of acute medical services among Medicare beneficiaries. Med Care 2004;42:512-21. https://doi.org/10.1097/01.mlr.0000127998.89246.ef.
- George J, Kong DC, Thoman R, Stewart K. Factors associated with medication nonadherence in patients with COPD. Chest 2005;128:3198-204. http://dx.doi.org/10.1378/chest.128.5.3198.
- Katon W, Unützer J, Fan MY, Williams JW, Schoenbaum M, Lin EH, et al. Cost-effectiveness and net benefit of enhanced treatment of depression for older adults with diabetes and depression. Diabetes Care 2006;29:265-70. https://doi.org/10.2337/diacare.29.02.06.dc05-1572.
- Fedder D, Chang R, Curry S, Nichols G. The effectiveness of a community health worker outreach programme on healthcare utilization of west Baltimore City Medicaid patients with diabetes, with or without hypertension. Ethn Dis 2003;13:22-7.
- Carney RM, Blumenthal JA, Freedland KE, Youngblood M, Veith RC, Burg MM, et al. Depression and late mortality after myocardial infarction in the Enhancing Recovery in Coronary Heart Disease (ENRICHD) study. Psychosom Med 2004;66:466-74. http://dx.doi.org/10.1097/01.psy.0000133362.75075.a6.
- Billings J, Dixon J, Mijanovich T, Wennberg D. Case finding for patients at risk of readmission to hospital: development of algorithm to identify high risk patients. BMJ 2006;333. http://dx.doi.org/10.1136/bmj.38870.657917.AE.
- Billings J, Georghiou T, Blunt I, Bardsley M. Choosing a model to predict hospital admission: an observational study of new variants of predictive models for case finding. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2013-003352.
- Schneider A, Löwe B, Meyer FJ, Biessecker K, Joos S, Szecsenyi J. Depression and panic disorder as predictors of health outcomes for patients with asthma in primary care. Respir Med 2008;102:359-66. http://dx.doi.org/10.1016/j.rmed.2007.10.016.
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 2007;370:851-8. http://dx.doi.org/10.1016/S0140-6736(07)61415-9.
- Stein MB, Cox BJ, Afifi TO, Belik SL, Sareen J. Does co-morbid depressive illness magnify the impact of chronic physical illness? A population-based perspective. Psychol Med 2006;36:587-96. http://dx.doi.org/10.1017/S0033291706007239.
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003;289:3095-105. http://dx.doi.org/10.1001/jama.289.23.3095.
- de Godoy DV, de Godoy RF. A randomized controlled trial of the effect of psychotherapy on anxiety and depression in chronic obstructive pulmonary disease. Arch Phys Med Rehabil 2003;84:1154-7. https://doi.org/10.1016/S0003-9993(03)00239-9.
- Keene J, Rodriguez J. Are mental health problems associated with use of accident and emergency and health-related harm?. Eur J Public Health 2007;17:387-93. http://dx.doi.org/10.1093/eurpub/ckl248.
- Williams ER, Guthrie E, Mackway-Jones K, James M, Tomenson B, Eastham J, et al. Psychiatric status, somatisation, and health care utilization of frequent attenders at the emergency department: a comparison with routine attenders. J Psychosom Res 2001;50:161-7. https://doi.org/10.1016/S0022-3999(00)00228-2.
- Mancuso CA, Peterson MG, Charlson ME. Effects of depressive symptoms on health-related quality of life in asthma patients. J Gen Intern Med 2000;15:301-10. https://doi.org/10.1046/j.1525-1497.2000.07006.x.
- Coventry PA, Gemmell I, Todd CJ. Psychosocial risk factors for hospital readmission in COPD patients on early discharge services: a cohort study. BMC Pulm Med 2011;11. http://dx.doi.org/10.1186/1471-2466-11-49.
- Abrams T, Vaughan-Sarrazin M, Vander Weg M. Acute exacerbations of chronic obstructive pulmonary disease and effect of exisiting psychiatric comorbidity on subsequent mortality. Psychosomatics 2011;52:441-9. https://doi.org/10.1016/j.psym.2011.03.005.
- Dickens C, Katon W, Blakemore A, Khara A, McGowan L, Tomenson B, et al. Does depression predict the use of urgent and unscheduled care by people with long term conditions? A systematic review with meta-analysis. J Psychosom Res 2012;73:334-42. http://dx.doi.org/10.1016/j.jpsychores.2012.08.018.
- Blakeley C, Blakemore A, Hunter C, Guthrie E, Tomenson B, Dickens C. Does anxiety predict the use of urgent care by people with long term conditions? A systematic review with meta-analysis. J Psychosom Res 2014;77:232-9. http://dx.doi.org/10.1016/j.jpsychores.2014.06.010.
- Quality Assessment Tool for Quantitative Studies. Ontario, ON: McMaster University; 2008.
- Lipsey M, Wilson D. Practical Meta-Analysis. London: Sage; 2001.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. https://doi.org/10.1016/0197-2456(86)90046-2.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. http://dx.doi.org/10.1136/bmj.327.7414.557.
- Moreno SG, Sutton AJ, Ades AE, Stanley TD, Abrams KR, Peters JL, et al. Assessment of regression-based methods to adjust for publication bias through a comprehensive simulation study. BMC Med Res Methodol 2009;9. http://dx.doi.org/10.1186/1471-2288-9-2.
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. https://doi.org/10.1136/bmj.315.7109.629.
- Egger M, Davey-Smith G, Altman D. Systematic Reviews in Health Care: Meta-Analysis in Context. London: BMJ Publishing; 2010.
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA 2006;295:676-80. http://dx.doi.org/10.1001/jama.295.6.676.
- Rosenthal R. Meta-Analytic Procedures for Social Research. London: Sage; 1991.
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455-63. https://doi.org/10.1111/j.0006-341X.2000.00455.x.
- Gudmundsson G, Gislason T, Lindberg E, Hallin R, Ulrik CS, Brøndum E, et al. Mortality in COPD patients discharged from hospital: the role of treatment and co-morbidity. Respir Res 2006;7. http://dx.doi.org/10.1186/1465-9921-7-109.
- Frasure-Smith N, Lespérance F, Gravel G, Masson A, Juneau M, Talajic M, et al. Depression and health-care costs during the first year following myocardial infarction. J Psychosom Res 2000;48:471-8. https://doi.org/10.1016/S0022-3999(99)00088-4.
- Shiotani I, Sato H, Kinjo K, Nakatani D, Mizuno H, Ohnishi Y, et al. Depressive symptoms predict 12-month prognosis in elderly patients with acute myocardial infarction. J Cardiovasc Risk 2002;9:153-60. https://doi.org/10.1177/174182670200900304.
- Kurdyak PA, Gnam WH, Goering P, Chong A, Alter DA. The relationship between depressive symptoms, health service consumption, and prognosis after acute myocardial infarction: a prospective cohort study. BMC Health Serv Res 2008;8. http://dx.doi.org/10.1186/1472-6963-8-200.
- Farkas J, Kosnik M, Flezar M, Suskovic S, Lainscak M. Self-rated health predicts acute exacerbations and hospitalizations in patients with COPD. Chest 2010;138:323-30. http://dx.doi.org/10.1378/chest.09-2459.
- Carneiro R, Sousa C, Pinto A, Almeida F, Oliveira JR, Rocha N. Risk factors for readmission after hospital discharge in chronic obstructive pulmonary disease. The role of quality of life indicators. Rev Port Pneumol 2010;16:759-77. https://doi.org/10.1016/S0873-2159(15)30070-2.
- Ghanei M, Aslani J, Azizabadi-Farahani M, Assari S, Saadat S. Logisitc regression model to predict chronic obstructive pulmonary disease exacerbation. Arch Med Sci 2007;3:360-6.
- Greaves CJ, Eiser C, Seamark D, Halpin DM. Attack context: an important mediator of the relationship between psychological status and asthma outcomes. Thorax 2002;57:217-21. https://doi.org/10.1136/thorax.57.3.217.
- Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med 2000;160:3278-85. https://doi.org/10.1001/archinte.160.21.3278.
- Grace S, Abbey S, Irvine J, Schnek Z, Stewart DE. Prospective examination of anxiety persistence and its relationship to cardiac symptoms and recurrent cardiac events. Psychother Psychosom 2004;73:344-52. https://doi.org/10.1159/000080387.
- Kaptein AA. Psychological correlates of length of hospitalization and rehospitalization in patients with acute, severe asthma. Soc Sci Med 1982;16:725-9. https://doi.org/10.1016/0277-9536(82)90463-4.
- Gudmundsson G, Gislason T, Janson C, Lindberg E, Hallin R, Ulrik CS, et al. Risk factors for rehospitalisation in COPD: role of health status, anxiety and depression. Eur Respir J 2005;26:414-19. http://dx.doi.org/10.1183/09031936.05.00078504.
- Fleury MJ, Ngui AN, Bamvita JM, Grenier G, Caron J. Predictors of healthcare service utilization for mental health reasons. Int J Environ Res Public Health 2014;11:10559-86. http://dx.doi.org/10.3390/ijerph111010559.
- Smits FT, Brouwer HJ, Zwinderman AH, Mohrs J, Schene AH, van Weert HC, et al. Why do they keep coming back? Psychosocial etiology of persisitence of frequent attendance in primary care: a prospective cohort study. J Psychosom Res 2014;77:492-503. https://doi.org/10.1016/j.jpsychores.2014.08.003.
- Creed F, Morgan R, Fiddler M, Marshall S, Guthrie E, House A. Depression and anxiety impair health-related quality of life and are associated with increased costs in general medical inpatients. Psychosomatics 2002;43:302-9. https://doi.org/10.1176/appi.psy.43.4.302.
- Das-Munshi J, Stewart R, Ismail K, Bebbington PE, Jenkins R, Prince MJ. Diabetes, common mental disorders, and disability: findings from the UK National Psychiatric Morbidity Survey. Psychosom Med 2007;69:543-50. http://dx.doi.org/10.1097/PSY.0b013e3180cc3062.
- Egede LE, Ellis C, Grubaugh AL. The effect of depression on self-care behaviours and quality of care in a national sample of adults with diabetes. Gen Hosp Psychiatry 2009;31:422-7. http://dx.doi.org/10.1016/j.genhosppsych.2009.06.007.
- Egede LE, Osborn CY. Role of motivation in the relationship between depression, self-care, and glycemic control in adults with type 2 diabetes. Diabetes Educ 2010;36:276-83. http://dx.doi.org/10.1177/0145721710361389.
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 2000;160:2101-7. https://doi.org/10.1001/archinte.160.14.2101.
- Pooler A, Beech R. Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. Int J Chron Obstruct Pulmon Dis 2014;9:315-30. http://dx.doi.org/10.2147/COPD.S53255.
- Elkington H, White P, Addington-Hall J, Higgs R, Pettinari C. The last year of life of COPD: a qualitative study of symptoms and services. Respir Med 2004;98:439-45. https://doi.org/10.1016/j.rmed.2003.11.006.
- Dickens C, Katon W, Blakemore A, Khara A, Tomenson B, Woodcock A, et al. Complex interventions that reduce urgent care use in COPD: a systematic review with meta-regression. Respir Med 2014;108:426-37. http://dx.doi.org/10.1016/j.rmed.2013.05.011.
- Blakemore A, Dickens C, Anderson R, Tomenson B, Woodcock A, Guthrie E. Complex interventions reduce use of urgent healthcare in adults with asthma: systematic review with meta-regression. Respir Med 2015;109:147-56. http://dx.doi.org/10.1016/j.rmed.2014.11.002.
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011] 2011. www.handbook.cochrane.org (accessed 4 April 2015).
- Casas A, Troosters T, Garcia-Aymerich J, Roca J, Hernández C, Alonso A, et al. Integrated care prevents hospitalisations for exacerbations in COPD patients. Eur Respir J 2006;28:123-30. http://dx.doi.org/10.1183/09031936.06.00063205.
- Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted?. Stat Med 2002;21:1559-73. http://dx.doi.org/10.1002/sim.1187.
- Berkey CS, Hoaglin DC, Mosteller F, Colditz GA. A random-effects regression model for meta-analysis. Stat Med 1995;14:395-411. https://doi.org/10.1002/sim.4780140406.
- Rea H, McAuley S, Stewart A, Lamont C, Roseman P, Didsbury P. A chronic disease management programme can reduce days in hospital for patients with chronic obstructive pulmonary disease. Intern Med J 2004;34:608-14. http://dx.doi.org/10.1111/j.1445-5994.2004.00672.x.
- Bourbeau J, Julien M, Maltais F, Rouleau M, Beaupré A, Bégin R, et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch Intern Med 2003;63:585-91. https://doi.org/10.1001/archinte.163.5.585.
- Bergner M, Hudson LD, Conrad DA, Patmont CM, McDonald GJ, Perrin EB, et al. The cost and efficacy of home care for patients with chronic lung disease. Med Care 1988;26:566-79. https://doi.org/10.1097/00005650-198806000-00005.
- Boxall AM, Barclay L, Sayers A, Caplan GA. Managing chronic obstructive pulmonary disease in the community. A randomized controlled trial of home-based pulmonary rehabilitation for elderly housebound patients. J Cardiopulm Rehabil 2005;25:378-85. https://doi.org/10.1097/00008483-200511000-00012.
- Eaton T, Young P, Fergusson W, Moodie L, Zeng I, O’Kane F, et al. Does early pulmonary rehabilitation reduce acute health-care utilization in COPD patients admitted with an exacerbation? A randomized controlled study. Respirology 2009;14:230-8. http://dx.doi.org/10.1111/j.1440-1843.2008.01418.x.
- Farrero E, Escarrabil J, Prats E, Maderal M, Manresa F. Impact of hospital-based home-care program on the management of COPD patients receiving long-term oxygen therapy. Chest 2001;119:364-9. https://doi.org/10.1378/chest.119.2.364.
- Gadoury MA, Schwartzman K, Rouleau M, Maltais F, Julien M, Beaupré A, et al. Self-management reduces both short- and long-term hospitalisation in COPD. Eur Respir J 2005;26:853-7. http://dx.doi.org/10.1183/09031936.05.00093204.
- Gallefoss F, Bakke PS. Impact of patient education and self-management on morbidity in asthmatics and patients with chronic obstructive pulmonary disease. Respir Med 2000;94:279-87. https://doi.org/10.1053/rmed.1999.0749.
- Güell R, Casan P, Belda J, Sangenis M, Morante F, Guyatt GH, et al. Long-term effects of outpatient rehabilitation of COPD: a randomized trial. Chest 2000;117:976-83. https://doi.org/10.1378/chest.117.4.976.
- Hermiz O, Comino E, Marks G, Daffurn K, Wilson S, Harris M. Randomised controlled trial of home based care of patients with chronic obstructive pulmonary disease. BMJ 2002;325. https://doi.org/10.1136/bmj.325.7370.938.
- Hernandez C, Casas A, Escarrabill J, Alonso J, Puig-Junoy J, Farrero E, et al. Home hospitalisation of exacerbated chronic obstructive pulmonary disease patients. Eur Respir J 2003;21:58-67. https://doi.org/10.1183/09031936.03.00015603.
- Jarab AS, Alqudah SG, Khdour M, Shamssain M, Mukattash TL. Impact of pharmaceutical care on health outcomes in patients with COPD. Int J Clin Pharm 2012;34:53-62. http://dx.doi.org/10.1007/s11096-011-9585-z.
- Khdour MR, Kidney JC, Smyth BM, McElnay JC. Clinical pharmacy-led disease and medicine management programme for patients with COPD. Br J Clin Pharmacol 2009;68:588-98. http://dx.doi.org/10.1111/j.1365-2125.2009.03493.x.
- Ko FW, Dai DL, Ngai J, Tung A, Ng S, Lai K, et al. Effect of early pulmonary rehabilitation on health care utilization and health status in patients hospitalized with acute exacerbations of COPD. Respirology 2011;16:617-24. http://dx.doi.org/10.1111/j.1440-1843.2010.01921.x.
- Koff PB, Jones RH, Cashman JM, Voelkel NF, Vandivier RW. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J 2009;33:1031-8. http://dx.doi.org/10.1183/09031936.00063108.
- Lee DT, Lee IF, Mackenzie AE, Ho RN. Effects of a care protocol on care outcomes in older nursing home patients with chronic obstructive pulmonary disease. J Am Geriatr Soc 2002;50:870-6. https://doi.org/10.1046/j.1532-5415.2002.50213.x.
- Díaz Lobato S, González Lorenzo F, Gómez Mendieta MA, Mayoralas Alises S, Martín Arechabala I, Villasante Fernández-Montes C. Evaluation of a home hospitalization program in patients with exacerbations of chronic obstructive pulmonary disease. Arch Bronconeumol 2005;41:5-10. https://doi.org/10.1016/s1579-2129(06)60387-x.
- Martin IR, McNamara D, Sutherland FR, Tilyard MW, Taylor DR. Care plans for acutely deteriorating COPD: a randomized controlled trial. Chron Respir Dis 2004;1:191-5. https://doi.org/10.1191/1479972304cd047oa.
- McGeoch GR, Willsman KJ, Dowson CA, Town GI, Frampton CM, McCartin FJ, et al. Self-management plans in the primary care of patients with chronic obstructive pulmonary disease. Respirology 2006;11:611-18. http://dx.doi.org/10.1111/j.1440-1843.2006.00892.x.
- Goeman DP, Aroni RA, Sawyer SM, Stewart K, Thien FC, Abramson MJ, et al. Back for more: a qualitative study of emergency department reattendance for asthma. Med J Aust 2004;180:113-17.
- Rice KL, Dewan N, Bloomfield HE, Grill J, Schult TM, Nelson DB, et al. Disease management program for chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med 2010;182:890-6. http://dx.doi.org/10.1164/rccm.200910-1579OC.
- Ries AL, Kaplan RM, Myers R, Prewitt LM. Maintenance after pulmonary rehabilitation in chronic lung disease: a randomized trial. Am J Respir Crit Care Med 2003;167:880-8. http://dx.doi.org/10.1164/rccm.200204-318OC.
- Seymour JM, Moore L, Jolley CJ, Ward K, Creasey J, Steier JS, et al. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax 2010;65:423-8. http://dx.doi.org/10.1136/thx.2009.124164.
- Smith BJ, Appleton SL, Bennett PW, Roberts GC, Del Fante P, Adams R, et al. The effect of a respiratory home nurse intervention in patients with chronic obstructive pulmonary disease (COPD). Aust N Z J Med 1999;29:718-25. https://doi.org/10.1111/j.1445-5994.1999.tb01621.x.
- Soler JJ, Martinez-Gracía MA, Román P, Orero R, Terrazas S, Martinez-Pechuán A. Effectiveness of a specific program for patients with chronic obstructive pulmonary disease and frequent exacerbations. Arch Bronconeumol 2006;42:501-8. https://doi.org/10.1016/S1579-2129(06)60576-4.
- Sridhar M, Taylor R, Dawson S, Roberts NJ, Partridge MR. A nurse led intermediate care package in patients who have been hospitalised with an acute exacerbation of chronic obstructive pulmonary disease. Thorax 2008;63:194-200. http://dx.doi.org/10.1136/thx.2007.077578.
- Tougaard L, Krone T, Sorknaes A, Ellegaard H. Economic benefits of teaching patients with chronic obstructive pulmonary disease about their illness. The PASTMA Group. Lancet 1992;339:1517-20. https://doi.org/10.1016/0140-6736(92)91274-C.
- Trappenburg JC, Monninkhof EM, Bourbeau J, Troosters T, Schrijvers AJ, Verheij TJ, et al. Effect of an action plan with ongoing support by a case manager on exacerbation-related outcome in patients with COPD: a multicentre randomised controlled trial. Thorax 2011;66:977-84. http://dx.doi.org/10.1136/thoraxjnl-2011-200071.
- Wakabayashi R, Motegi T, Yamada K, Ishii T, Jones RC, Hyland ME, et al. Efficient integrated education for older patients with chronic obstructive pulmonary disease using the Lung Information Needs Questionnaire. Geriatr Gerontol Int 2011;11:422-30. https://doi.org/10.1111/j.1447-0594.2011.00696.x.
- Wong KW, Wong FK, Chan MF. Effects of nurse-inititated telephone follow-up on self-efficacy among patients with chronic obstructive pulmonary disease. J Adv Nurs 2005;49:210-22. https://doi.org/10.1111/j.1365-2648.2004.03280.x.
- Wood-Baker R, McGlone S, Venn A, Walters EH. Written action plans in chronic obstructive pulmonary disease increase appropriate treatment for acute exacerbations. Respirology 2006;11:619-26. http://dx.doi.org/10.1111/j.1440-1843.2006.00902.x.
- Man WD, Polkey MI, Donaldson N, Gray BJ, Moxham J. Community pulmonary rehabilitation after hospitalisation for acute exacerbations of chronic obstructive pulmonay disease: randomised controlled study. BMJ 2004;329. https://doi.org/10.1136/bmj.38258.662720.3A.
- Aiolfi S, Confalonieri M, Scartabellati A, Patrini G, Ghio L, Mauri F, et al. International guidelines and educational experiences in an out-patient clinic for asthma. Monaldi Arch Chest Dis 1995;50:477-81.
- Bolton MB, Tilley BC, Kuder J, Reeves T, Schultz LR. The cost and effectiveness of an education program for adults who have asthma. J Gen Intern Med 1991;6:401-7. https://doi.org/10.1007/BF02598160.
- Brown MD, Reeves MJ, Meyerson K, Korzeniewski SJ. Randomized trial of a comprehensive asthma education program after an emergency department visit. Ann Allergy Asthma Immunol 2006;97:44-51. https://doi.org/10.1016/S1081-1206(10)61368-3.
- Castro M, Zimmermann NA, Crocker S, Bradley J, Leven C, Schechtman KB. Asthma intervention program prevents readmissions in high healthcare users. Am J Respir Crit Care Med 2003;168:1095-9. http://dx.doi.org/10.1164/rccm.200208-877OC.
- Clark NM, Gong ZM, Wang SJ, Lin X, Bria WF, Johnson TR. A randomized trial of a self-regulation intervention for women with asthma. Chest 2007;132:88-97. http://dx.doi.org/10.1378/chest.06-2539.
- de Oliveira MA, Faresin SM, Bruno VF, de Bittencourt AR, Fernandes AL. Evaluation of an educational programme for socially deprived asthma patients. Eur Respir J 1999;14:908-14. https://doi.org/10.1034/j.1399-3003.1999.14d30.x.
- Donald KJ, McBurney H, Teichtahl H, Irving L. A pilot study of telephone based asthma management. Aust Fam Physician 2008;37:170-3.
- Galbreath AD, Smith B, Wood PR, Inscore S, Forkner E, Vazquez M, et al. Assessing the value of disease management: impact of 2 disease management strategies in an underserved asthma population. Ann Allergy Asthma Immunol 2008;101:599-607. http://dx.doi.org/10.1016/S1081-1206(10)60222-0.
- Gallefoss F, Bakke PS, Kjaersgaard PK. Quality of life assessment after patient education in a randomized controlled study on asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;59:812-17. https://doi.org/10.1164/ajrccm.159.3.9804047.
- George MR, O’Dowd LC, Martin I, Lindell KO, Whitney F, Jones M, et al. A comprehensive educational program improves clinical outcome measures in inner-city patients with asthma. Arch Intern Med 1999;159:1710-16. https://doi.org/10.1001/archinte.159.15.1710.
- Huang TT, Li YT, Wang CH. Individualized programme to promote self-care among older adults with asthma: randomized controlled trial. J Adv Nurs 2009;65:348-58. http://dx.doi.org/10.1111/j.1365-2648.2008.04874.x.
- Kauppinen R, Sintonen H, Tukiainen H. One-year economic evaluation of intensive vs conventional patient education and supervision for self-management of new asthmatic patients. Respir Med 1998;92:300-7. https://doi.org/10.1016/S0954-6111(98)90113-5.
- Kotses H, Bernstein IL, Bernstein DI, Reynolds RV, Korbee L, Wigal JK, et al. A self-management program for adult asthma. Part I: development and evaluation. J Allergy Clin Immunol 1995;95:529-40. https://doi.org/10.1016/S0091-6749(95)70315-2.
- Lahdensuo A, Haahtela T, Herrala J, Kava T, Kiviranta K, Kuuisto P, et al. Randomised comparison of guided self management and traditional treatment of asthma over one year. BMJ 1996;312:748-52. https://doi.org/10.1136/bmj.312.7033.748.
- Levy ML, Robb M, Allen J, Doherty C, Bland JM, Winter RJ. A randomized controlled evaluation of specialist nurse education following accident and emergency department attendance for acute asthma. Respir Med 2000;94:900-8. https://doi.org/10.1053/rmed.2000.0861.
- Milenković BA, Stanković IJ, Ilić AM, Petrović VI. Peak expiratory flow-guided self-management treatment of asthma in Serbia. J Asthma 2007;44:699-704. http://dx.doi.org/10.1080/02770900701595543.
- Osman LM, Calder C, Godden DJ, Friend JA, McKenzie L, Legge JS, et al. A randomised trial of self-management planning for adult patients admitted to hospital with acute asthma. Thorax 2002;57:869-74. https://doi.org/10.1136/thorax.57.10.869.
- Patel RR, Saltoun CA, Grammer LC. Improving asthma care for the elderly: a randomized controlled trial using a simple telephone intervention. J Asthma 2009;46:30-5. http://dx.doi.org/10.1080/02770900802460563.
- Pilotto LS, Smith BJ, Heard AR, McElroy HJ, Weekley J, Bennett P. Trial of nurse-run asthma clinics based in general practice versus usual medical care. Respirology 2004;9:356-62. http://dx.doi.org/10.1111/j.1440-1843.2004.00589.x.
- Pinnock H, Bawden R, Proctor S, Wolfe S, Scullion J, Price D, et al. Accessibility, acceptability, and effectiveness in primary care of routine telephone review of asthma: pragmatic, randomised controlled trial. BMJ 2003;326:477-9. http://dx.doi.org/10.1136/bmj.326.7387.477.
- Rasmussen LM, Phanareth K, Nolte H, Backer V. Internet-based monitoring of asthma: a long-term, randomized clinical study of 300 asthmatic subjects. J Allergy Clin Immunol 2005;115:1137-42. http://dx.doi.org/10.1016/j.jaci.2005.03.030.
- Schermer TR, Thoonen BP, van den Boom G, Akkermans RP, Grol RP, Folgering HT, et al. Randomized controlled economic evaluation of asthma self-management in primary health care. Am J Respir Crit Care Med 2002;166:1062-72. http://dx.doi.org/10.1164/rccm.2105116.
- Schott-Baer D, Christensen M. A pilot program to increase self-care of adult asthma patients. Medsurg Nurs 1999;8:178-83.
- Shelledy DC, Legrand TS, Gardner DD, Peter JI. A randomized, controlled study to evaluate the role of an in-home asthma disease management porgram provided by respiratory therapists in improving outcomes and reducing the cost of care. J Asthma 2009;46:194-201. https://doi.org/10.1080/02770900802610068.
- Sundberg R, Tunsäter A, Palmqvist M, Ellbjär S, Löwhagen O, Torén K. A randomized controlled study of a computerized limited education program among young adults with asthma. Respir Med 2005;99:321-8. http://dx.doi.org/10.1016/j.rmed.2004.08.006.
- van der Meer V, van den Hout WB, Bakker MJ, Rabe KF, Sterk PJ, Assendelft WJ, et al. Cost-effectiveness of Internet-based self-management compared with usual care in asthma. PLOS ONE 2011;6. http://dx.doi.org/10.1371/journal.pone.0027108.
- Vojita CL, Amaya MA, Browngoehl K, Colburn KD, Vojta DD. A home-based asthma education program in managed Medicaid. J Clin Outcomes Manag 1999;6:30-4.
- Wilson SR, Scamagas P, German DF, Hughes GW, Lulla S, Coss S, et al. A controlled trial of two forms of self-management education for adults with asthma. Am J Med 1993;94:564-76. https://doi.org/10.1016/0002-9343(93)90206-5.
- Yilmaz A, Akkaya E. Evaluation of long-term efficacy of an asthma education programme in an out-patient clinic. Respir Med 2002;96:519-24. https://doi.org/10.1053/rmed.2001.1309.
- Yoon R, McKenzie DK, Bauman A, Miles DA. Controlled trial evaluation of an asthma education programme for adults. Thorax 1993;48:1110-16. https://doi.org/10.1136/thx.48.11.1110.
- Coté J, Cartier A, Robichaud P, Boutin H, Malo JL, Rouleau M, et al. Influence on asthma morbidity of asthma education programs based on self-management plans following treatment optimization. Am J Respir Crit Care Med 1997;155:1509-14. http://dx.doi.org/10.1164/ajrccm.155.5.9154850.
- Morice AH, Wrench C. The role of the asthma nurse in treatment compliance and self-management following hospital admission. Respir Med 2001;95:851-6. https://doi.org/10.1053/rmed.2001.1166.
- Perneger TV, Sudre P, Muntner P, Uldry C, Courteheuse C, Naef AF, et al. Effect of patient education on self-management skills and health status in patients with asthma: a randomized trial. Am J Med 2002;113:7-14. https://doi.org/10.1016/S0002-9343(02)01136-1.
- Bindman AB, Grumbach K, Osmond D, Komaromy M, Vranizan K, Lurie N, et al. Preventable hospitalizations and access to health care. JAMA 1995;274:305-11. https://doi.org/10.1001/jama.1995.03530040033037.
- Hanania NA, David-Wang A, Kesten S, Chapman KR. Factors associated with emergency department dependance of patients with asthma. Chest 1997;111:290-5. https://doi.org/10.1378/chest.111.2.290.
- Oster A, Bindman AB. Emergency department visits for ambulatory care sensitive conditions: insights into preventable hospitalizations. Med Care 2003;41:198-207. http://dx.doi.org/10.1097/01.MLR.0000045021.70297.9F.
- Langer S, Chew-Graham C, Hunter C, Guthrie EA, Salmon P. Why do patients with long-term conditions use unscheduled care? A qualitative literature review. Health Soc Care Community 2013;21:339-51. http://dx.doi.org/10.1111/j.1365-2524.2012.01093.x.
- Paterson B, Thorne S, Canam C, Jillings C. Meta-Study of Qualitative Health Research: A Practical Guide to Meta-Analysis and Meta-Synthesis. Thousand Oaks, CA: Sage; 2001.
- Thorne S, Jensen L, Kearney MH, Noblit G, Sandelowski M. Qualitative metasynthesis: reflections on methodological orientation and ideological agenda. Qual Health Res 2004;14:1342-65. http://dx.doi.org/10.1177/1049732304269888.
- Noblit GW, Hare RD. Meta-Ethnography: Synthesizing Qualitative Studies. London: Sage; 1988.
- Aroni R, Goeman D, Stewart K, Thien F, Sawyer S, Abramson M, et al. Enhancing validity: what counts as an asthma attack?. J Asthma 2004;41:729-37. https://doi.org/10.1081/JAS-200027980.
- Becker G, Janson-Bjerklie S, Benner P, Slobin K, Ferketich S. The dilemma of seeking urgent care: asthma episodes and emergency service use. Soc Sci Med 1993;37:305-13. https://doi.org/10.1016/0277-9536(93)90262-3.
- Cvetkovski B, Armour C, Bosnic-Anticevich S. Asthma management in rural New South Wales: perceptions of health care professionals and people with asthma. Aust J Rural Health 2009;17:195-200. http://dx.doi.org/10.1111/j.1440-1584.2009.01071.x.
- Douglass J, Aroni R, Goeman D, Stewart K, Sawyer S, Thien F, et al. A qualitative study of action plans for asthma. BMJ 2002;324:1003-5. https://doi.org/10.1136/bmj.324.7344.1003.
- Douglass J, Goeman D, Aroni R, Thien F, Abramson M, Stewart K, et al. Choosing to attend an asthma doctor: a qualitative study in adults attending emergency departments. Fam Pract 2004;21:166-72. https://doi.org/10.1093/fampra/cmh211.
- Douglass JA, Goeman DP, Yu EA, Abramson MJ. Asthma 3+ Visit Plan: a qualitative evaluation. Intern Med J 2005;35:457-62. http://dx.doi.org/10.1111/j.1445-5994.2005.00851.x.
- Goeman DP, Aroni RA, Stewart K, Sawyer SM, Thien FC, Abramson MJ, et al. Patients’ views of the burden of asthma: a qualitative study. Med J Aust 2002;177:295-9.
- Griffiths C, Kaur G, Gantley M, Feder G, Hillier S, Goddard J, et al. Influences on hospital admission for asthma in south Asian and white adults: qualitative interview study. BMJ 2001;323:962-6. https://doi.org/10.1136/bmj.323.7319.962.
- Hyland ME, Ståhl E. Asthma treatment needs: a comparison of patients’ and health care professionals’ perceptions. Clin Ther 2004;26:2141-52. http://dx.doi.org/10.1016/j.clinthera.2004.12.017.
- Jones IR, Ahmed N, Kelly M, Bothamley G, Rajakulasingam R, Victor C, et al. With an attack I associate it more with going into hospital: understandings of asthma and psychosocial stressors; are they related to use of services?. Soc Sci Med 2008;66:765-75. http://dx.doi.org/10.1016/j.socscimed.2007.09.012.
- Tumiel-Berhalter L, Zayas LE. Lay experiences and concerns with asthma in an urban Hispanic community. J Natl Med Assoc 2006;98:875-80.
- DeVito AJ. Dyspnea during hospitalizations for acute phase of illness as recalled by patients with chronic obstructive pulmonary disease. Heart Lung 1990;19:186-91.
- Hopley M, Horsburgh M, Peri K. Barriers to accessing specialist care for older people with chronic obstructive pulmonary disease in rural New Zealand. J Prim Health Care 2009;1:207-14.
- Shipman C, White S, Gysels M, White P. Access to care in advanced COPD: factors that influence contact with general practice services. Prim Care Respir J 2009;18:273-8. http://dx.doi.org/10.4104/pcrj.2009.00013.
- Wilson DM, Ross C, Goodridge D, Davis P, Landreville A, Roebuck K. The care needs of community-dwelling seniors suffering from advanced chronic obstructive pulmonary disease. Can J Aging 2008;27:347-57. http://dx.doi.org/10.3138/cja.27.4.347.
- Balcou-Debussche M, Debussche X. Hospitalization for type 2 diabetes: the effects of the suspension of reality on patients’ subsequent management of their condition. Qual Health Res 2009;19:1100-15. http://dx.doi.org/10.1177/1049732309341642.
- Brez S, Rowan M, Malcolm J, Izzi S, Maranger J, Liddy C, et al. Transition from specialist to primary diabetes care: a qualitative study of perspectives of primary care physicians. BMC Fam Pract 2009;10. http://dx.doi.org/10.1186/1471-2296-10-39.
- Broom DH. Familiarity breeds neglect? Unanticipated benefits of discontinuous primary care. Fam Pract 2003;20:503-7. https://doi.org/10.1093/fampra/cmg501.
- de-Graft Aikins A. Healer shopping in Africa: new evidence from rural-urban qualitative study of Ghanaian diabetes experiences. BMJ 2005;331. http://dx.doi.org/10.1136/bmj.331.7519.737.
- Heisler M, Spencer M, Forman J, Robinson C, Shultz C, Palmisano G, et al. Participants’ assessments of the effects of a community health worker intervention on their diabetes self-management and interactions with healthcare providers. Am J Prev Med 2009;37:270-9. http://dx.doi.org/10.1016/j.amepre.2009.08.016.
- Johnson M, Baird W, Goyder E. Understanding issues involved in the transfer of diabetes care to general practice: the patient perspective. Qual Prim Care 2006;14:247-52.
- Lawton J, Rankin D, Peel E, Douglas M. Patients’ perceptions and experiences of transitions in diabetes care: a longitudinal qualitative study. Health Expect 2009;12:138-48. http://dx.doi.org/10.1111/j.1369-7625.2009.00537.x.
- McDowell JR, McPhail K, Halyburton G, Brown M, Lindsay G. Perceptions of a service redesign by adults living with type 2 diabetes. J Adv Nurs 2009;65:1432-41. http://dx.doi.org/10.1111/j.1365-2648.2009.05003.x.
- Perera M, Gunatillele G, Bird P. Using an asthma control questionnaire and administrative data to predict health-care utilization. Int J Health Serv 2007;37:379-98. https://doi.org/10.2190/4635-18K0-2371-1843.
- Mead H, Andres E, Ramos C, Siegel B, Regenstein M. Barriers to effective self-management in cardiac patients: the patient’s experience. Patient Educ Couns 2010;79:69-76. http://dx.doi.org/10.1016/j.pec.2009.08.003.
- Pâquet M, Bolduc N, Xhignesse M, Vanasse A. Re-engineering cardiac rehabilitation programmes: considering the patient’s point of view. J Adv Nurs 2005;51:567-76. http://dx.doi.org/10.1111/j.1365-2648.2005.03544.x.
- Schoenberg NE, Peters JC, Drew EM. Unraveling the mysteries of timing: women’s perceptions about time to treatment for cardiac symptoms. Soc Sci Med 2003;56:271-84. https://doi.org/10.1016/S0277-9536(02)00026-6.
- Cowie L, Morgan M, White P, Gulliford M. Experience of continuity of care of patients with multiple long-term conditions in England. J Health Serv Res Policy 2009;14:82-7. http://dx.doi.org/10.1258/jhsrp.2009.008111.
- Forsyth GL, Delaney KD, Gresham ML. Vying for a winning position: management style of the chronically ill. Res Nurs Health 1984;7:181-8. https://doi.org/10.1002/nur.4770070306.
- Fried TR, van Doorn C, Tinetti ME, Drickamer MA. Older persons’ preferences for site of treatment in acute illness. J Gen Intern Med 1998;13:522-7. https://doi.org/10.1046/j.1525-1497.1998.00162.x.
- Gately C, Rogers A, Sanders C. Re-thinking the relationship between long-term condition self-management education and the utilisation of health services. Soc Sci Med 2007;65:934-45. http://dx.doi.org/10.1016/j.socscimed.2007.04.018.
- Hsu-Hage B, Tang KC, Li RJ, Thein F. A qualitative investigation into the use of health services among Melbourne Chinese. Aust J Prim Health 2001;7:38-44. https://doi.org/10.1071/PY01044.
- Jeon YH, Essue B, Jan S, Wells R, Whitworth JA. Economic hardship associated with managing chronic illness: a qualitative inquiry. BMC Health Serv Res 2009;9. http://dx.doi.org/10.1186/1472-6963-9-182.
- Jerant AF, von Friederichs-Fitzwater MM, Moore M. Patients’ perceived barriers to active self-management of chronic conditions. Patient Educ Couns 2005;57:300-7. http://dx.doi.org/10.1016/j.pec.2004.08.004.
- Kielmann T, Huby G, Powell A, Sheikh A, Price D, Williams S, et al. From support to boundary: a qualitative study of the border between self-care and professional care. Patient Educ Couns 2010;79:55-61. http://dx.doi.org/10.1016/j.pec.2009.07.015.
- Levy SR, Anderson EE, Issel LM, Willis MA, Dancy BL, Jacobson KM, et al. Using multilevel, multisource needs assessment data for planning community interventions. Health Promot Pract 2004;5:59-68. http://dx.doi.org/10.1177/1524839903257688.
- Malone RE. Whither the almshouse? Overutilization and the role of the emergency department. J Health Polit Policy Law 1998;23:795-832. http://doi.org/10.1215/03616878-23-5-795.
- Townsend A, Wyke S, Hunt K. Frequent consulting and multiple morbidity: a qualitative comparison of ‘high’ and ‘low’ consulters of GPs. Fam Pract 2008;25:168-75. http://dx.doi.org/10.1093/fampra/cmn017.
- Yang SC, Zwar N, Vagholkar S, Dennis S, Redmond H. Factors influencing general practice follow-up attendances of patients with complex medical problems after hospitalization. Fam Pract 2010;27:62-8. http://dx.doi.org/10.1093/fampra/cmp076.
- Murray SA, Kendall M, Carduff E, Worth A, Harris FM, Lloyd A, et al. Use of serial qualitative interviews to understand patients’ evolving experiences and needs. BMJ 2009;339. http://dx.doi.org/10.1136/bmj.b3702.
- Donald KJ, McBurney H, Browning C. Self management beliefs – attitudes and behaviour of adults with severe life threatening asthma requiring an admission to hospital. Aust Fam Physician 2005;34:197-200.
- Yohannes AM, Willgoss TG, Baldwin RC, Connolly MJ. Depression and anxiety in chronic heart failure and chronic obstructive pulmonary disease: prevalence, relevance, clinical implications and management principles. Int J Geriatr Psychiatry 2010;25:1209-21. http://dx.doi.org/10.1002/gps.2463.
- de Jonge P, Spijkerman TA, van den Brink RH, Ormel J. Depression after myocardial infarction is a risk factor for declining health related quality of life and increased disabililty and cardiac complaints at 12 months. Heart 2006;92:32-9. https://doi.org/10.1136/hrt.2004.059451.
- Welch CA, Czerwinski D, Ghimire B, Bertsimas D. Depression and costs of health care. Psychosomatics 2009;50:392-401. http://dx.doi.org/10.1176/appi.psy.50.4.392.
- Unützer J, Schoenbaum M, Katon WJ, Fan MY, Pincus HA, Hogan D, et al. Healthcare costs associated with depression in medically ill fee-for-service medicare participants. J Am Geriatr Soc 2009;57:506-10. http://dx.doi.org/10.1111/j.1532-5415.2008.02134.x.
- Expenditure on Healthcare in the UK, 2012. London: ONS; 2014.
- The English Indicies of Deprivation 2010. London: Department for Communities and Local Government; 2011.
- Guthrie EA, Dickens C, Blakemore A, Watson J, Chew-Graham C, Lovell K, et al. Depression predicts future emergency hospital admissions in primary care patients with chronic physical illness. J Psychosom Res 2016;82:54-61. http://dx.doi.org/10.1016/j.jpsychores.2014.10.002.
- Bousquet J. Global initiative for asthma (GINA) and its objectives. Clin Exp Allergy 2000;30:2-5. https://doi.org/10.1046/j.1365-2222.2000.00088.x.
- The Criteria Committee of the New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. Boston, MA: Little, Brown and Co; 1994.
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532-55. http://dx.doi.org/10.1164/rccm.200703-456SO.
- Young BA, Lin E, Von Korff M, Simon G, Ciechanowski P, Ludman EJ, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care 2008;14:15-23.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Brugha T, Bebbington P, Tennant C, Hurry J. The list of threatening experiences: a subset of 12 life event categories with considerable long-term contextual threat. Psychol Med 1985;15:189-94. https://doi.org/10.1017/S003329170002105X.
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344-9. http://dx.doi.org/10.1016/j.jclinepi.2007.11.008.
- Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology 2007;18:805-35. https://doi.org/10.1097/EDE.0b013e3181577511.
- Thombs BD, Roseman M, Coyne JC, de Jonge P, Delisle VC, Arthurs E, et al. Does evidence support the American Heart Association’s recommendation to screen patients for depression in cardiovascular care? An updated systematic review. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0052654.
- Roland M, Abel G. Reducing emergency admissions: are we on the right track?. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e6017.
- Campbell JL, Dickens A, Richards SH, Pound P, Greco M, Bower P. Capturing users’ experience of UK out-of-hours primary medical care: piloting and psychometric properties of the Out-of-hours Patient Questionnaire. Qual Saf Health Care 2007;16:462-8. http://dx.doi.org/10.1136/qshc.2006.020172.
- Peters M, Crocker H, Dummett S, Jenkinson C, Doll H, Fitzpatrick R. Change in health status in long-term conditions over a one year period: a cohort survey using patient-reported outcome measures. Health Qual Life Outcomes 2014;12. http://dx.doi.org/10.1186/s12955-014-0123-2.
- Hazell ML, Morris JA, Linehan MF, Frank PI, Frank TL. Factors influencing the response to postal questionnaire surveys about respiratory symptoms. Prim Care Respir J 2009;18:165-70. http://dx.doi.org/10.3132/pcrj.2009.00001.
- Curtis L. Unit Costs of Health and Social Care 2012. Kent: Personal Social Services Research Unit; 2013.
- Mikkelsen RL, Middelboe T, Pisinger C, Stage KB. Anxiety and depression in patients with chronic obstructive pulmonary disease (COPD). A review. Nord J Psychiatry 2004;58:65-70. http://dx.doi.org/10.1080/08039480310000824.
- Katon WJ. Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin Neurosci 2011;13:7-23.
- Frasure-Smith N, Lespérance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation 1995;91:999-1005. https://doi.org/10.1161/01.CIR.91.4.999.
- Crockett AJ, Cranston JM, Moss JR, Alpers JH. The impact of anxiety, depression and living alone in chronic obstructive pulmonary disease. Qual Life Res 2002;11:309-16. https://doi.org/10.1023/A:1015517606893.
- Kraaij V, Arensman E, Spinhoven P. Negative life events and depression in elderly persons: a meta-analysis. J Gerontol B Psychol Sci Soc Sci 2002;57:P87-94. https://doi.org/10.1093/geronb/57.1.P87.
- Glynn LG, Valderas JM, Healy P, Burke E, Newell J, Gillespie P, et al. The prevalence of multimorbidity in primary care and its effect on health care utilization and cost. Fam Pract 2011;28:516-23. http://dx.doi.org/10.1093/fampra/cmr013.
- Condelius A, Edberg AK, Jakobsson U, Hallberg IR. Hospital admissions among people 65+ related to multimorbidity, municipal and outpatient care. Arch Gerontol Geriatr 2008;46:41-55. http://dx.doi.org/10.1016/j.archger.2007.02.005.
- Long Term Conditions Compendium of Information. London: DH; 2012.
- Liu JL, Maniadakis N, Gray A, Rayner M. The economic burden of coronary heart disease in the UK. Heart 2002;88:597-603. https://doi.org/10.1136/heart.88.6.597.
- Luengo-Fernández R, Leal J, Gray A, Petersen S, Rayner M. Cost of cardiovascular diseases in the United Kingdom. Heart 2006;92:1384-9. http://dx.doi.org/10.1136/hrt.2005.072173.
- Coronary Heart Disease Statistics 2012. London: British Heart Foundation; 2012.
- Masoli M, Fabian D, Holt S, Beasley R. Global Initiative for Asthma (GINA) program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 2004;59:469-78. http://dx.doi.org/10.1111/j.1398-9995.2004.00526.x.
- Chronic Obstructive Pulmonary Disease: Costing Report. Implementing NICE Guidance. Clinical Guideline 101. London: NICE; 2011.
- van Manen JG, Bindels PJ, Dekker FW, IJzermans CJ, van der Zee JS, Schadé E. Risk of depression in patients with chronic obstructive pulmonary disease and its determinants. Thorax 2002;57:412-16. https://doi.org/10.1136/thorax.57.5.412.
- Hex N, Bartlett C, Wright D, Taylor M, Varley D. Estimating the current and future costs of type 1 and type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med 2012;29:855-62. https://doi.org/10.1111/j.1464-5491.2012.03698.x.
- National Schedules of Reference Costs 2012–2013. London: DH; 2013.
- Population Estimates for UK, England and Wales, Scotland and Northern Ireland, Mid-2001 to Mid-2010 Revised. London: ONS; 2013.
- O’Neill S, Sweeney J, Patterson CC, Menzies-Gow A, Niven R, Mansur AH, et al. The cost of treating severe refractory asthma in the UK: an economic analysis from the British Thoracic Society Difficult Asthma Registery. Thorax 2015;70:376-8. https://doi.org/10.1136/thoraxjnl-2013-204114.
- Punekar YS, Shukla A, Müllerova H. COPD management costs according to the frequency of COPD exacerbations in UK primary care. Int J Chron Obstruct Pulmon Dis 2014;9:65-73. http://dx.doi.org/10.2147/COPD.S54417.
- Hunter C, Chew-Graham C, Langer S, Stenhoff A, Drinkwater J, Guthrie E, et al. A qualitative study of patient choices in using emergency health care for long-term conditions: the importance of candidacy and recursivity. Patient Educ Couns 2013;93:335-41. http://dx.doi.org/10.1016/j.pec.2013.06.001.
- Challis D, Abell J, Reilly S, Hughes J, Berzins K, Brand C. Evaluating Active Case Managment in Greater Manchester. Manchester: Personal Social Services Research Unit; 2008.
- Wilson T, Buck D, Ham C. Rising to the challenge: will the NHS support people with long term conditions?. BMJ 2005;330:657-61. http://dx.doi.org/10.1136/bmj.330.7492.657.
- Our Health, Our Care, Our Say. London: DH; 2006.
- Investing in General Practice: The New Medical Services Contract. Leeds: NHS Employers; 2003.
- Chew-Graham CA, May CR, Roland MO. The harmful consequences of elevating the doctor–patient relationship to be a primary goal of the general practice consultation. Fam Pract 2004;21:229-31. https://doi.org/10.1093/fampra/cmh301.
- Ritchie J, Spencer L, Bryman A, Burgess R. Analysing Qualitative Data. Abingdon: Routledge; 1994.
- Wanless D, Forder J, Fernandez J, Poole T, Beesley L, Henwood M, et al. Securing Good Care for Older People: Taking a Long-Term View. London: The King’s Fund; 2006.
- Carret ML, Fassa AG, Kawachi I. Demand for emergency health service: factors associated with inappropriate use. BMC Health Serv Res 2007;7. http://dx.doi.org/10.1186/1472-6963-7-131.
- Dixon-Woods M, Cavers D, Agarwal S, Annandale E, Arthur A, Harvey J, et al. Conducting a critical interpretive synthesis of the literature on access to healthcare by vulnerable groups. BMC Med Res Methodol 2006;6. http://dx.doi.org/10.1186/1471-2288-6-35.
- Rogers A, Hassell K, Nicholaas G. Demanding Patients? Analysing the Use of Primary Care. Buckingham: Open University Press; 1999.
- Garrett CR, Gask LL, Hays R, Cherrington A, Bundy C, Dickens C, et al. Accessing primary health care: a meta-ethnography of the experiences of British South Asian patients with diabetes, coronary heart disease or a mental health problem. Chronic Illn 2012;8:135-55. http://dx.doi.org/10.1177/1742395312441631.
- Koehn S. Negotiating candidacy: ethnic minority seniors’ access to care. Aging Soc 2009;29:585-608. https://doi.org/10.1017/S0144686X08007952.
- Kovandžić M, Chew-Graham C, Reeve J, Edwards S, Peters S, Edge D, et al. Access to primary mental health care for hard-to-reach groups: from ‘silent suffering’ to ‘making it work’. Soc Sci Med 2011;72:763-72. http://dx.doi.org/10.1016/j.socscimed.2010.11.027.
- Byrne M, Murphy AW, Plunkett PK, McGee HM, Murray A, Bury G. Frequent attenders to an emergency department: a study of primary health care use, medical profile, and psychosocial characteristics. Ann Emerg Med 2003;41:309-18. http://dx.doi.org/10.1067/mem.2003.68.
- Penson R, Coleman P, Mason S, Nicholl J. Why do patients with minor or moderate conditions that could be managed in other settings attend the emergency department?. Emerg Med J 2012;29:487-91. http://dx.doi.org/10.1136/emj.2010.107276.
- Bristow K, Edwards S, Funnel E, Fisher L, Gask L, Dowrick C, et al. Help seeking and access to primary care for people from ‘hard-to-reach’ groups with common mental health problems. Int J Family Med 2011;2011. http://dx.doi.org/10.1155/2011/490634.
- Victoor A, Delnoij DM, Friele RD, Rademakers JJ. Determinants of patient choice of healthcare providers: a scoping review. BMC Health Serv Res 2012;12. http://dx.doi.org/10.1186/1472-6963-12-272.
- Chew-Graham CA, Hunter C, Langer S, Stenhoff A, Drinkwater J, Guthrie EA, et al. How QOF is shaping primary care review consultations: a longitudinal qualitative study. BMC Fam Pract 2013;14. http://dx.doi.org/10.1186/1471-2296-14-103.
- Hunter C, Chew-Graham CA, Langer S, Drinkwater J, Stenhoff A, Guthrie EA, et al. ‘I wouldn’t push that further because I don’t want to lose her’: a multiperspective qualitative study of behaviour change for long-term conditions in primary care. Health Expect 2015;18:1995-2010. http://dx.doi.org/10.1111/hex.12304.
- Wagner E, Groves T. Care for chronic disease: the efficacy of coordinated and patient centred care is established, but now is the time to test its effectiveness. BMJ 2002;325:913-14. https://doi.org/10.1136/bmj.325.7370.913.
- Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff 2001;20:64-78. https://doi.org/10.1377/hlthaff.20.6.64.
- Salmon P, Mendick N, Young B. Integrative qualitative communication analysis of consultation and pateint and practitioner perspectives: towards a theory of authentic caring and clinical relationships. Patient Educ Couns 2011;82:448-54. https://doi.org/10.1016/j.pec.2010.10.017.
- Blakeman T, Bower P, Reeves D, Chew-Graham C. Bringing self-management into clinical view: a qualitative study of long-term condition management in primary care consultations. Chronic Illn 2010;6:136-50. http://dx.doi.org/10.1177/1742395309358333.
- Taylor CA, Shaw RL, Dale J, French DP. Enhancing delivery of health behaviour change interventions in primary care: a meta-synthesis of views and experiences of primary care nurses. Patient Educ Couns 2011;85:315-22. http://dx.doi.org/10.1016/j.pec.2010.10.001.
- Swinglehurst D, Greenhalgh T, Roberts C. Computer templates in chronic disease management: ethnographic case study in general practice. BMJ Open 2012;2. http://dx.doi.org/10.1136/bmjopen-2012-001754.
- Salmon P, Hall GM. Patient empowerment and control: a psychological discourse in the service of medicine. Soc Sci Med 2003;57:1969-80. https://doi.org/10.1016/S0277-9536(03)00063-7.
- Coventry PA, Hays R, Dickens C, Bundy C, Garrett C, Cherrington A, et al. Talking about depression: a qualitative study of barriers to managing depression in people with long term conditions in primary care. BMC Fam Pract 2011;12. http://dx.doi.org/10.1186/1471-2296-12-10.
- Alderson SL, Russell AM, McLintock K, Potrata B, House A, Foy R. Incentivised case finding for depression in patients with chronic heart disease and diabetes in primary care: an ethnographic study. BMJ Open 2014;4. http://dx.doi.org/10.1136/bmjopen-2014-005146.
- Kennedy A, Bower P, Reeves D, Blakeman T, Bowen R, Chew-Graham C, et al. Implementation of self management support for long term conditions in routine primary care settings: cluster randomised controlled trial. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f2882.
- Salmon P, Peters S, Rogers A, Gask L, Clifford R, Iredale W, et al. Peering through the barriers in GPs’ explanations for declining to participate in research: the role of professional autonomy and the economy of time. Fam Pract 2007;24:269-75. http://dx.doi.org/10.1093/fampra/cmm015.
- Protheroe J, Blakeman T, Bower P, Chew-Graham C, Kennedy A. An intervention to promote patient participation and self-management in long term conditions: development and feasibility testing. BMC Health Serv Res 2010;10. http://dx.doi.org/10.1186/1472-6963-10-206.
- Kadam U. Redesigning the general practice consultation to improve care for patients with multimorbidity. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e6202.
- Thille P, Ward N, Russell G. Self-management support in primary care: enactments, disruptions, and conversational consequences. Soc Sci Med 2014;108:97-105. http://dx.doi.org/10.1016/j.socscimed.2014.02.041.
- de Lusignan S, van Weel C. The use of routinely collected computer data for research in primary care: opportunities and challenges. Fam Pract 2006;23:253-63. http://dx.doi.org/10.1093/fampra/cmi106.
- Parry G, Barkham M, Brazier J, Dent-Brown K, Hardy G, Kendrick T, et al. An Evaluation of a New Service Model: Improving Access to Psychological Therapies Demonstration Sites 2006–2009. Final Report. Southampton: NIHR Service Delivery and Organisation Programme; 2011.
- Quality and Outcomes Framework Guidance for GMS Contract 2011/2012. London: NHS Confederation (Employers) Company; 2011.
- Thombs BD, Coyne JC, Cuijpers P, de Jonge P, Gilbody S, Ioannidis JP, et al. Rethinking recommendations for screening for depression in primary care. CMAJ 2012;184:413-8. http://dx.doi.org/10.1503/cmaj.111035.
- Maxwell M, Harris F, Hibberd C, Donaghy E, Pratt R, Williams C, et al. A qualitative study of primary care professionals’ views of case finding for depression in patients with diabetes or coronary heart disease in the UK. BMC Fam Pract 2013;14. http://dx.doi.org/10.1186/1471-2296-14-46.
- Lamb J, Bower P, Rogers A, Dowrick C, Gask L. Access to mental health in primary care: a qualitative meta-synthesis of evidence from the experience of people from ‘hard to reach’ groups. Health 2012;16:76-104. http://dx.doi.org/10.1177/1363459311403945.
- Craig P, Dieppe P, Macintyre S, Mitchie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:979-83. https://doi.org/10.1136/bmj.a1655.
- May C, Finch T. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology 2009;43:535-54. https://doi.org/10.1177/0038038509103208.
- May C. Towards a general theory of implementation. Implement Sci 2013;8. http://dx.doi.org/10.1186/1748-5908-8-18.
- Brody AA, Galvin JE. A review of interprofessional dissemination and education interventions for recognizing and managing dementia. Gerontol Geriatr Educ 2013;34:225-56. http://dx.doi.org/10.1080/02701960.2013.801342.
- O’Brien MA, Rogers S, Jamtvedt G, Oxman AD, Odgaard-Jensen J, Kristoffersen DT, et al. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2007;17. https://doi.org/10.1002/14651858.cd000409.pub2.
- Hobson R. Forms of Feeling: Heart of Psychotherapy. London: Routledge; 1985.
- Langer S, Chew-Graham CA, Drinkwater J, Afzal C, Keane K, Hunter C, et al. A motivational intervention for patients with COPD in primary care: qualitative evaluation of a new practitioner role. BMC Fam Pract 2014;15. http://dx.doi.org/10.1186/1471-2296-15-164.
- Drummond MF, Schulper MJ, Claxton K, Torrance GW, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Kettering: Oxford Univeristy Press; 2005.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. https://doi.org/10.1046/j.1525-1497.2001.016009606.x.
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092-7. http://dx.doi.org/10.1001/archinte.166.10.1092.
- McGregor M, Lin EH, Katon WJ. TEAMcare: an integrated multicondition collaborative care program for chronic illnesses and depression. J Ambul Care Manage 2011;34:152-62. http://dx.doi.org/10.1097/JAC.0b013e31820ef6a4.
- Kruis AL, Boland MR, Schoonvelde CH, Assendelft WJ, Rutten-van Mölken MP, Gussekloo J, et al. RECODE: design and baseline results of a cluster randomized trial on cost-effectiveness of integrated COPD management in primary care. BMC Pulm Med 2013;13. http://dx.doi.org/10.1186/1471-2466-13-17.
- Kruis AL, Ställberg B, Jones RC, Tsiligianni IG, Lisspers K, van der Molen T, et al. Primary care COPD patients compared with large pharmaceutically-sponsored COPD studies: an UNLOCK validation study. PLOS ONE 2014;9. http://dx.doi.org/10.1371/journal.pone.0090145.
- Lamers F, Jonkers CC, Bosma H, Chavannes NH, Knottnerus JA, van Eijk JT. Improving quality of life in depressed COPD patients: effectiveness of a minimal psychological intervention. COPD 2010;7:315-22. http://dx.doi.org/10.3109/15412555.2010.510156.
- Arain M, Campbell MJ, Cooper CL, Lancaster GA. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med Res Methodol 2010;10. http://dx.doi.org/10.1186/1471-2288-10-67.
- Moher D, Hopewell S, Schulz K, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. https://doi.org/10.1136/bmj.c869.
- Rutten-van Mölken MP, Oostenbrink JB, Tashkin DP, Burkhart D, Monz BU. Does quality of life of COPD patients as measured by the generic EuroQol five-dimension questionnaire differentiate between COPD severity stages?. Chest 2006;130:1117-28. http://dx.doi.org/10.1378/chest.130.4.1117.
- Richards DA, Hill JJ, Gask L, Lovell K, Chew-Graham C, Bower P, et al. Clinical effectiveness of collaborative care for depression in UK primary care (CADET): cluster randomised controlled trial. BMJ 2013;347. https://doi.org/10.1136/bmj.f4913.
- Doupe MB, Palatnick W, Day S, Chateau D, Soodeen RA, Burchill C, et al. Frequent users of emergency departments: developing standard definitions and defining prominent risk factors. Ann Emerg Med 2012;60:24-32. http://dx.doi.org/10.1016/j.annemergmed.2011.11.036.
- Swift JK, Greenberg RP. Premature discontinuation in adult psychotherapy: a meta-analysis. J Consult Clin Psychol 2012;80:547-59. http://dx.doi.org/10.1037/a0028226.
- Kapella MC, Herdegen JJ, Perlis ML, Shaver JL, Larson JL, Law JA, et al. Cognitive behavioural therapy for insomnia comorbid with COPD is feasible with preliminary evidence of positive sleep and fatigue effects. Int J Chron Obstruct Pulmon Dis 2011;6:625-35. http://dx.doi.org/10.2147/COPD.S24858.
- Lolak S, Connors GL, Sheridan MJ, Wise TN. Effects of progressive muscle relaxation training on anxiety and depression in patients enrolled in an outpatient pulmonary rehabilitation program. Psychother Psychosom 2008;77:119-25. http://dx.doi.org/10.1159/000112889.
- Knowles SE, Chew-Graham C, Coupe N, Adeyemi I, Keyworth C, Thampy H, et al. Better together? a naturalistic qualitative study of inter-professional working in collaborative care for co-morbid depression and physical health problems. Implement Sci 2013;8. https://doi.org/10.1186/1748-5908-8-110.
- Coventry PA, Bower P, Keyworth C, Kenning C, Knopp J, Garrett C, et al. The effect of complex interventions on depression and anxiety in chronic obstructive pulmonary disease: systematic review and meta-analysis. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0060532.
- Coventry P, Lovell K, Dickens C, Bower P, Chew-Graham C, McElvenny D, et al. Integrated primary care for patients with mental and physical multimorbidity: cluster randomised controlled trial of collaborative care for patients with depression comorbid with diabetes or cardiovascular disease. BMJ 2015;350. http://dx.doi.org/10.1136/bmj.h638.
- Ladapo JA, Shaffer JA, Fang Y, Ye S, Davidson KW. Cost-effectiveness of enhanced depression care after acute coronary syndrome: results from the Coronary Psychosocial Evaluation Studies randomized controlled trial. Arch Intern Med 2012;172:1682-4. http://dx.doi.org/10.1001/archinternmed.2012.4448.
- Rawlins MD, Culyer AJ. National Institute for Clinical Excellence and its value judgments. BMJ 2004;329:224-7. http://dx.doi.org/10.1136/bmj.329.7459.224.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. https://doi.org/10.1002/hec.635.
- Sendi PP, Briggs AH. Affordability and cost-effectiveness: decision-making on the cost-effectiveness plane. Health Econ 2001;10:675-80. https://doi.org/10.1002/hec.639.
- Hoch JS, Briggs AH, Willan AR. Something old, something new, something borrowed, something blue: a framework for the marriage of health econometrics and cost-effectiveness analysis. Health Econ 2002;11:415-30. http://dx.doi.org/10.1002/hec.678.
- Briggs AH, O’Brien BJ. The death of cost-minimisation analysis?. Health Econ 2001;10:179-84. http://dx.doi.org/10.1002/hec.584.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. https://doi.org/10.1016/0168-8510(96)00822-6.
- Guide to Methods of Technology Appraisals. London: NICE; 2008.
- Pickard AS, Wilke C, Jung E, Patel S, Stavem K, Lee TA. Use of a preference-based measure of health (EQ-5D) in COPD and asthma. Respir Med 2008;102:519-36. http://dx.doi.org/10.1016/j.rmed.2007.11.016.
- What You Need to Know About Payment: An Introductory Guide for Members of the Public Who are Considering Active Involvement in NHS, Public Health or Social Care Research. Hampshire: INVOLVE; 2011.
- Briefing Notes for Researchers: Public Involvement in NHS, Public Health and Social Care Research. Eastleigh: INVOLVE; 2012.
- Whooley MA, Avins AL, Miranda J, Browner WS. Case-finding instruments for depression. Two questions are as good as many. J Gen Intern Med 1997;12:439-45. https://doi.org/10.1046/j.1525-1497.1997.00076.x.
- British Medical Association QOF Guidance According to Nation. QOF Guidance 2014–2015 (Seventh Revision). London: British Medical Association, NHS Employers and NHS England; 2014.
- Enhanced Service Specification: Risk Profiling and Care Management Scheme. London: NHS England; 2013.
- Wallace E, Stuart E, Vaughan N, Bennett K, Fahey T, Smith SM. Risk prediction models to predict emergency hospital admission in community-dwelling adults: a systematic review. Med Care 2014;52:751-65. http://dx.doi.org/10.1097/MLR.0000000000000171.
- Hippisley-Cox J, Coupland C. Predicting risk of emergency admission to hospital using primary care data: derivation and validation of QAdmissions score. BMJ Open 2013;3. http://dx.doi.org/10.1136/bmjopen-2013-003482.
- Baker A, Leak P, Ritchie LD, Lee AJ, Fielding S. Anticipatory care planning and integration: a primary care pilot study aimed at reducing unplanned hospitalisation. Br J Gen Pract 2012;62:e113-20. http://dx.doi.org/10.3399/bjgp12X625175.
- Scottish Patients at Risk of Readmission and Admission (SPARRA). Edinburgh: NHS National Services Scotland; 2011.
- Combined Predictive Model: Final Report. London: Department of Health; 2006.
- Gao J, Moran E, Li YF, Almenoff PL. Predicting potentially avoidable hospitalizations. Med Care 2014;52:164-71. http://dx.doi.org/10.1097/MLR.0000000000000041.
- Wang L, Porter B, Maynard C, Evans G, Bryson C, Sun H, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care 2013;51:368-73. http://dx.doi.org/10.1097/MLR.0b013e31827da95a.
- Falasca P, Berardo A, Di Tommaso F. Development and validation of predictive MoSaiCo (Modello Statistico Combinato) on emergency admissions: can it also identify patients at high risk of frailty?. Ann Ist Super Sanita 2011;47:220-8. http://dx.doi.org/10.4415/ANN_11_02_15.
- Louis DZ, Robeson M, Herrera K, Maio V, Gonnella JS, Richardson D, et al. Who is most likely to require a higher level of care: prediciting risk of hospitalisation. BMC Health Serv Res 2010;10. https://doi.org/10.1186/1472-6963-10-S2-A2.
- Lemke KW, Weiner JP, Clark JM. Development and validation of a model for predicting inpatient hospitalization. Med Care 2012;50:131-9. http://dx.doi.org/10.1097/MLR.0b013e3182353ceb.
- Walker L, Jamrozik K, Wingfield D. The Sherbrooke Questionnaire predicts use of emergency services. Age Ageing 2005;34:233-7. http://dx.doi.org/10.1093/ageing/afi020.
- Mazzaglia G, Roti L, Corsini G, Colombini A, Maciocco G, Marchionni N, et al. Screening of older community-dwelling people at risk for death and hospitalization: the Assistenza Socio-Sanitaria in Italia project. J Am Geriatr Soc 2007;55:1955-60. http://dx.doi.org/10.1111/j.1532-5415.2007.01446.x.
- Majeed A. Sources, uses, strengths and limitations of data collected in primary care in England. Health Stat Q 2004;21:5-14.
- Raven MC, Billings JC, Goldfrank LR, Manheimer ED, Gourevitch MN. Medicaid patients at high risk for frequent hospital admission: real-time identification and remediable risks. J Urban Health 2009;86:230-41. http://dx.doi.org/10.1007/s11524-008-9336-1.
- Davidson SK, Dowrick CF, Gunn JM. Impact of functional and structural social relationships on two year depression outcomes: a multivariate analysis. J Affect Disord 2016;193:274-81. http://dx.doi.org/10.1016/j.jad.2015.12.025.
- Dowrick C, Frances A. Medicalising unhappiness: new classification of depression risks more patients being put on drug treatment from which they will not benefit. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f7140.
- Katon WJ, Von Korff M, Lin EH, Simon G, Ludman E, Russo J, et al. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry 2004;61:1042-9. http://dx.doi.org/10.1001/archpsyc.61.10.1042.
- Sharpe M, Walker J, Holm Hansen C, Martin P, Symeonides S, Gourley C, et al. Integrated collaborative care for comorbid major depression in patients with cancer (SMaRT Oncology-2): a multicentre randomised controlled effectiveness trial. Lancet 2014;384:1099-108. http://dx.doi.org/10.1016/S0140-6736(14)61231-9.
- Doherty AM, Gayle C, Morgan-Jones R, Archer N, Lee L, Ismail K, et al. Improving quality of diabetes care by integrating psychological and social care for poorly controlled diabetes: 3 dimensions of care for diabetes. Int J Psychiatry Med 2016;51:3-15. https://doi.org/10.1177/0091217415621040.
- Smith DJ, Court H, McLean G, Martin D, Langan Martin J, Guthrie B, et al. Depression and multimorbidity: a cross-sectional study of 1,751,841 patients in primary care. J Clin Psychiatry 2014;75:1202-8. http://dx.doi.org/10.4088/JCP.14m09147.
- Gunn JM, Ayton DR, Densley K, Pallant JF, Chondros P, Herrman HE, et al. The association between chronic illness, multimorbidity and depressive symptoms in an Australian primary care cohort. Soc Psychiatry Psychiatr Epidemiol 2012;47:175-84. http://dx.doi.org/10.1007/s00127-010-0330-z.
- Hughes LD, McMurdo ME, Guthrie B. Guidelines for people not for diseases: the challenges of applying UK clinical guidelines to people with multimorbidity. Age Ageing 2013;42:62-9. http://dx.doi.org/10.1093/ageing/afs100.
- Roland M, Paddison C. Better management of patients with multimorbidity. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f2510.
- Purdy S. Avoiding Hospital Admissions: What Does the Research Evidence Say?. London: The King’s Fund; 2010.
- Bardsley M, Steventon A, Smith J, Dixon J. Evaluating Integrated and Community-Based Care: How Do We Know What Works?. London: Nuffield Trust; 2013.
- Simmonds RL, Tylee A, Walters P, Rose D. Patients’ perceptions of depression and coronary heart disease: a qualitative UPBEAT-UK study. BMC Fam Pract 2013;14. http://dx.doi.org/10.1186/1471-2296-14-38.
- Katon W, Von Korff M, Lin E, Walker E, Simon GE, Bush T, et al. Collaborative management to achieve treatment guidelines. Impact on depression in primary care. JAMA 1995;273:1026-31. https://doi.org/10.1001/jama.1995.03520370068039.
- Callahan EJ, Bertakis KD, Azari R, Robbins J, Helms LJ, Miller J. The influence of depression on physician-patient interaction in primary care. Fam Med 1996;28:346-51.
- Lin EH, Katon W, Von Korff M, Rutter C, Simon GE, Oliver M, et al. Relationship of depression and diabetes self-care, medication adherence, and preventative care. Diabetes Care 2004;27:2154-60. https://doi.org/10.2337/diacare.27.9.2154.
- Vangeli E, Bakhshi S, Baker A, Fisher A, Bucknor D, Mrowietz U, et al. A systematic review of factors associated with non-adherence to treatment for immune-mediated inflammatory diseases. Adv Ther 2015;32:983-1028. http://dx.doi.org/10.1007/s12325-015-0256-7.
Appendix 1 Systematic review search criteria
MEDLINE
Search strategy
-
Health Facilities/ut [Utilization]
-
Health Care Costs/
-
emergency service, hospital/ut [Utilization]
-
outpatient clinics, hospital/ut [Utilization]
-
psychiatric department, hospital/ut [Utilization]
-
Hospitals/ut [Utilization]
-
Health Services/ut [Utilization]
-
community health services/ut [Utilization]
-
community mental health services/ut [Utilization]
-
Emergency Medical Services/ut [Utilization]
-
emergency service, hospital/ut [Utilization]
-
Triage/ut [Utilization]
-
after-hours care/ut [Utilization]
-
"delivery of health care_ integrated"/ut [Utilization]
-
patient care team/ut [Utilization]
-
primary health care/ut [Utilization]
-
(unscheduled adj5 care).mp. [mp=title, original title, abstract, name of substance word, subject heading word]
-
(walk-in adj5 centre).mp.
-
(walk in adj5 centre).mp.
-
(walk-in adj5 centres).mp.
-
(walk in adj5 centres).mp.
-
(walk-in adj5 clinic).mp.
-
(walk in adj5 clinic).mp.
-
(walk-in adj5 clinics).mp.
-
(walk in adj5 clinics).mp.
-
(drop-in adj5 centre).mp.
-
(drop in adj5 centre).mp.
-
(drop-in adj5 centres).mp.
-
(drop in adj5 centres).mp.
-
(drop-in adj5 clinic).mp.
-
(drop in adj5 clinic).mp.
-
(drop-in adj5 clinics).mp.
-
(drop in adj5 clinics).mp.
-
(out-of-hours adj5 service).mp.
-
(out-of-hours adj5 services).mp.
-
((out of hours adj5 service) or (out of hours adj5 services)).mp.
-
Hospitalization/ut [Utilization]
-
hospitalization.mp.
-
hospitalisation.mp.
-
(emergency adj5 department).mp. [mp=title, original title, abstract, name of substance word, subject heading word]
-
(emergency adj5 departments).mp. [mp=title, original title, abstract, name of substance word, subject heading word]
-
(accident and emergency).mp. [mp=title, original title, abstract, name of substance word, subject heading word]
-
(healthcare adj5 utilisation).mp. [mp=title, original title, abstract, name of substance word, subject heading word]
-
(healthcare adj5 utilization).mp. [mp=title, original title, abstract, name of substance word, subject heading word]
-
(health care adj5 utilisation).mp.
-
(health care adj5 utilization).mp. [mp=title, original title, abstract, name of substance word, subject heading word]
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46
-
Asthma/ or asthma.mp.
-
Pulmonary Disease, Chronic Obstructive/ or chronic obstructive pulmonary disease.mp. or COPD.mp. or (COAD adj5 airways).mp.
-
Cardiovascular Diseases/ or cardiovascular diseases.mp. or cardiovascular disease.mp.
-
Diabetes Mellitus, Type 1/ or Diabetes.mp. or Diabetes Mellitus, Type 2/ or Diabetes Mellitus/
-
Diabetes Complications/
-
(Long-term conditions or long term conditions).mp.
-
(Long-term health problems or long term health problems).mp.
-
Chronic Disease/ or chronic disease.mp. or chronic diseases.mp.
-
(Chronic illness or chronic illnesses).mp.
-
(Chronic disease adj5 management).mp.
-
48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57
-
longitudinal studies/ or prospective studies/ or randomized controlled trials as topic/ or randomised controlled trials as topic/
-
47 and 58 and 59
Appropriate adaptations were made for the following databases: CINAHL; EMBASE search strategy; Evidence-Based Medicine Reviews; Cochrane Database of Systematic Reviews; Cochrane Central Register of Controlled Trials search strategy; PsycINFO search strategy; and BNI search strategy.
Appendix 2 Additional tables for Chapter 4: economic analysis of the longitudinal prospective study of predictors of unscheduled care in patients with long-term conditions
| Year of study | All conditionsa | Exemplar LTCa | |||
|---|---|---|---|---|---|
| CHD | Asthma | Diabetes | COPD | ||
| Year 1 | n = 1411 | n = 465 | n = 520 | n = 465 | n = 354 |
| Year 2 | n = 1398 | n = 458 | n = 516 | n = 461 | n = 351 |
| Scheduled service use costs (£, 2011–12) only, mean (95% CI) | |||||
| Year 1 | 1182 (1075 to 1288) | 1360 (1193 to 1528) | 1021 (887 to 1156) | 1226 (1094 to 1358) | 1342 (1031 to 1654) |
| Year 2 | 1291 (1172 to 1409) | 1468 (1264 to 1672) | 1121 (1004 to 1437) | 1473 (1291 to 1655) | 1294 (1091 to 1497) |
| Unscheduled service use costs (£, 2011–12) only, mean (95% CI) | |||||
| Year 1 | 1164 (616 to 1711) | 1332 (771 to 1893) | 1242 (49 to 2434) | 629 (388 to 871) | 961 (585 to 1336) |
| Year 2 | 1211 (647 to 1774) | 1458 (961 to 1955) | 1331 (53 to 2610) | 1156 (692 to 1620) | 1279 (788 to 1770) |
| Total scheduled and unscheduled service use costs (£, 2011–12), mean (95% CI) | |||||
| Year 1 | 2345 (1773 to 2918) | 2692 (2081 to 3303) | 2263 (1046 to 3480) | 1855 (1567 to 2143) | 2303 (1690 to 2916) |
| Year 2 | 2501 (1915 to 3087) | 2925 (2346 to 3505) | 2552 (1237 to 3867) | 2629 (2098 to 3160) | 2573 (2026 to 3120) |
| Year of study according to presence or absence of depression | All conditionsa | Exemplar LTCa | |||
|---|---|---|---|---|---|
| CHD | Asthma | Diabetes | COPD | ||
| Year 1 | n = 1383 | n = 457 | n = 512 | n = 451 | n = 349 |
| Year 2 | n = 1371 | n = 451 | n = 508 | n = 447 | n = 346 |
| Scheduled service use costs (£, 2011–12) only, mean (95% CI) | |||||
| Year 1 | |||||
| No depression | 990 (849 to 1132) | 1087 (928 to 1245) | 779 (629 to 930) | 1026 (883 to 1170) | 1214 (735 to 1693) |
| Depressionb | 1480 (1326 to 1634) | 1723 (1390 to 2057) | 1439 (1252 to 1626) | 1516 (1259 to 1772) | 1534 (1224 to 1844) |
| Year 2 | |||||
| No depression | 1124 (990 to 1258) | 1315 (1025 to 1605) | 957 (743 to 1172) | 1234 (1020 to 1448) | 1326 (1015 to 1637) |
| Depressionb | 1551 (1320 to 1782) | 1690 (1403 to 1976) | 1668 (1183 to 2153) | 1828 (1485 to 2170) | 1256 (1010 to 1502) |
| Unscheduled service use costs (£, 2011–12) only, mean (95% CI) | |||||
| Year 1 | |||||
| No depression | 709 (498 to 920) | 932 (594 to 1271) | 393 (169 to 616) | 596 (257 to 934) | 864 (307 to 1421) |
| Depressionb | 1927 (553 to 3302) | 1938 (674 to 3203) | 2758 (–374 to 5889) | 723 (365 to 1081) | 1101 (622 to 1579) |
| Year 2 | |||||
| No depression | 598 (417 to 779) | 840 (512 to 1167) | 360 (145 to 574) | 647 (372 to 922) | 813 (266 to 1361) |
| Depressionb | 2197 (792 to 3603) | 2351 (1221 to 3481) | 2988 (–271 to 6247) | 1951 (818 to 3084) | 1872 (1006 to 2737) |
| Total scheduled and unscheduled service use costs (£, 2011–12), mean (95% CI) | |||||
| Year 1 | |||||
| No depression | 1699 (1395 to 2004) | 2019 (1619 to 2420) | 1172 (886 to 1458) | 1622 (1240 to 2004) | 2078 (1113 to 3043) |
| Depressionb | 3407 (2026 to 4788) | 3662 (2323 to 5000) | 4197 (1077 to 7317) | 2239 (1770 to 2707) | 2635 (2054 to 3216) |
| Year 2 | |||||
| No depression | 1722 (1490 to 1954) | 2155 (1696 to 2614) | 1317 (1018 to 1616) | 1881 (1501 to 2260) | 2139 (1547 to 2732) |
| Depressionb | 3748 (2308 to 5188) | 4041 (2781 to 5301) | 4656 (1346 to 7966) | 3779 (2535 to 5024) | 3128 (2115 to 4140) |
| GP practice | Care cost (£, 2011–12), mean (95% CI) | |||||
|---|---|---|---|---|---|---|
| Scheduled care | Unscheduled care | Total scheduled and unscheduled care | ||||
| Year 1 | Year 2 | Year 1 | Year 2 | Year 1 | Year 2 | |
| 1 (n = 252) | 1173 (960 to 1385) | 1172 (1022 to 1321) | 726 (379 to 1073) | 872 (371 to 1373) | 1899 (1424 to 2373) | 2044 (1508 to 2580) |
| 3 (n = 192) | 1327 (993 to 1661) | 1535 (1102 to 1969) | 736 (385 to 1086) | 1245 (554 to 1935) | 2063 (1523 to 2602) | 2780 (1763 to 3797) |
| 5 (n = 136) | 993 (786 to 1200) | 1012 (726 to 1299) | 3362 (–2057 to 8782) | 3701 (–1927 to 9328) | 4356 (–1137 to 9849) | 4713 (–959 to 10,386) |
| 6 (n = 369) | 1418 (1215 to 1621) | 1831 (1391 to 2272) | 957 (669 to 1246) | 1137 (654 to 1619) | 2376 (1996 to 2756) | 2968 (2143 to 3794) |
| 7 (n = 112) | 718 (425 to 1011) | 938 (475 to 1400) | 456 (–258 to 1170) | 526 (72 to 980) | 1174 (382 to 1965) | 1464 (809 to 2118) |
| 8 (n = 263) | 1141 (904 to 1377) | 1278 (989 to 1567) | 784 (372 to 1195) | 1255 (533 to 1978) | 1924 (1405 to 2443) | 2533 (1693 to 3374) |
| 11 (n = 105) | 1256 (712 to 1800) | 1436 (967 to 1905) | 1199 (–9 to 2406) | 580 (75 to 1085) | 2455 (959 to 3950) | 2016 (1282 to 2751) |
| 14 (n = 112) | 1205 (844 to 1567) | 1000 (692 to 1307) | 2197 (959 to 3435) | 1062 (353 to 1772) | 3402 (694 to 4782) | 2062 (407 to 2871) |
| 16 (n = 236) | 1006 (776 to 1236) | 953 (798 to 1107) | 731 (220 to 1242) | 962 (9 to 1914) | 1737 (1171 to 2302) | 1914 (913 to 2915) |
| Range (of mean values) | 718–1418 | 938–1535 | 456–3362 | 526–3701 | 1174–4356 | 1464–4713 |
| p-value | 0.01a | 0.003a | 0.37 | 0.41 | 0.08 | 0.16 |
| Year and type of unscheduled care | All conditionsa | Exemplar LTCa | |||
|---|---|---|---|---|---|
| CHD | Asthma | Diabetes | COPD | ||
| Year 1 | n = 1411 | n = 465 | n = 520 | n = 465 | n = 354 |
| Year 2 | n = 1398 | n = 458 | n = 516 | n = 461 | n = 351 |
| GP OOH services costs (£, 2011–12), mean (95% CI) | |||||
| Year 1 | 10 (7 to 13) | 10 (4 to 17) | 13 (8 to 18) | 10 (4 to 16) | 10 (4 to 15) |
| Year 2 | 11 (8 to 13) | 10 (6 to 14) | 12 (8 to 16) | 12 (7 to 18) | 11 (6 to 17) |
| ED visits costs (£, 2011–12), mean (95% CI) | |||||
| Year 1 | 39 (29 to 49) | 27 (20 to 35) | 49 (28 to 70) | 20 (13 to 28) | 58 (36 to 81) |
| Year 2 | 53 (42 to 63) | 51 (39 to 63) | 54 (37 to 70) | 36 (27 to 46) | 77 (45 to 108) |
| Emergency hospital inpatient admissions costs (£, 2011–12), mean (95% CI) | |||||
| Year 1 | 1114 (570 to 1659) | 1294 (734 to 1854) | 1179 (–7 to 2366) | 599 (360 to 838) | 893 (524 to 1261) |
| Year 2 | 1147 (586 to 1709) | 1397 (905 to 1889) | 1266 (–10 to 2541) | 1107 (647 to 1567) | 1191 (710 to 1673) |
| Year and type of unscheduled care | All conditionsa,b | Exemplar LTCa,b | |||
|---|---|---|---|---|---|
| CHD | Asthma | Diabetes | COPD | ||
| GP OOH services costs (£, 2011–12), mean (95% CI); number of patients | |||||
| Year 1 | 90 (72 to 108); n = 139 | 97; n = 47 | 88 (66 to 110); n = 68 | 107; n = 40 | 73; n = 36 |
| Year 2 | 89 (78 to 101); n = 161 | 87; n = 52 | 88; n = 69 | 106; n = 53 | 92; n = 41 |
| ED visits costs (£, 2011–12), mean (95% CI); number of patients | |||||
| Year 1 | 164; n = 279 | 137; n = 88 | 158 (100 to 217); n = 123 | 144; n = 63 | 169; n = 95 |
| Year 2 | 196; n = 341 | 174; n = 123 | 199 (155 to 243); n = 126 | 160 (132 to 188); n = 96 | 230; n = 103 |
| Emergency hospital inpatient admissions costs (£, 2011–12), mean (95% CI); number of patients | |||||
| Year 1 | 7306 (4491 to 10,121); n = 221 | 6540; n = 95 | 8869; n = 68 | 4971 (3333 to 6608); n = 58 | 5795; n = 70 |
| Year 2 | 7200 (4332 to 10,068); n = 234 | 6309 (4419 to 8199); n = 102 | 10,473; n = 62 | 5920 (3649 to 8191); n = 86 | 6297 (4236 to 8358); n = 77 |
| Health-care contact | All conditions,a β-coefficient (95% CI)a | Exemplar LTC,a β-coefficient (95% CI) | |||
|---|---|---|---|---|---|
| CHD | Asthma | Diabetes | COPD | ||
| Year 1 | |||||
| n = 1411 | n = 465 | n = 520 | n = 465 | n = 354 | |
| GP surgery visit | 1.06 (1.00 to 1.12) | 1.05 (0.99 to 1.11) | 1.11 (1.03 to 1.18) | 1.17 (1.08 to 1.25) | 0.98 (0.88 to 1.07) |
| GP OOH services | 1.74 (1.16 to 2.33) | 1.43 (0.97 to 1.88) | 1.55 (1.20 to 1.89) | 1.29 (0.73 to 1.84) | 2.95 (0.03 to 5.88) |
| ED | 0.83 (0.67 to 0.98) | 0.37 (–0.03 to 0.78) | 0.89 (0.84 to 0.94) | 0.64 (0.40 to 0.88) | 0.95 (0.65 to 1.26) |
| Emergency admission | 1.01 (1.00 to 1.03) | 1.03 (1.01 to 1.05) | 1.00 (1.00 to 1.01) | 1.01 (1.00 to 1.02) | 1.02 (1.00 to 1.04) |
| Routine admission | 1.00 (0.98 to 1.01) | 0.99 (0.98 to 1.01) | 0.99 (0.98 to 1.00) | 1.00 (0.98 to 1.02) | 0.99 (0.96 to 1.02) |
| Hospital outpatient visit | 1.08 (1.04 to 1.12) | 1.09 (1.03 to 1.15) | 1.13 (1.05 to 1.21) | 1.01 (0.96 to 1.06) | 1.10 (1.01 to 1.19) |
| Year 2 | |||||
| n = 1398 | n = 458 | n = 516 | n = 461 | n = 351 | |
| GP surgery visit | 1.09 (1.02 to 1.17) | 1.08 (0.98 to 1.18) | 1.11 (1.00 to 1.21) | 1.24 (1.09 to 1.39) | 1.04 (0.95 to 1.13) |
| GP OOH services | 2.34 (1.52 to 3.15) | 1.33 (0.07 to 2.59) | 2.57 (1.34 to 3.79) | 1.55 (0.93 to 2.16) | 2.88 (1.67 to 4.10) |
| ED | 1.12 (0.85 to 1.40) | 1.16 (0.89 to 1.44) | 0.85 (0.71 to 0.98) | 1.06 (0.62 to 1.50) | 1.17 (0.87 to 1.48) |
| Emergency admission | 1.01 (1.00 to 1.02) | 1.01 (1.01 to 1.02) | 1.01 (0.99 to 1.02) | 1.01 (1.01 to 1.02) | 1.01 (1.00 to 1.02) |
| Routine admission | 1.00 (0.99 to 1.01) | 1.00 (0.99 to 1.01) | 1.00 (0.99 to 1.01) | 1.01 (0.99 to 1.03) | 1.01 (0.98 to 1.03) |
| Hospital outpatient visit | 1.13 (1.07 to 1.19) | 1.13 (1.06 to 1.20) | 1.15 (1.04 to 1.27) | 1.04 (0.98 to 1.11) | 1.12 (1.03 to 1.20) |
| Covariate | β-coefficient | Linearised standard error | t-test | p > t | 95% CI |
|---|---|---|---|---|---|
| Age (per 10 years) | 160 | 146 | 1.1 | 0.271 | –125 to 446 |
| Female | 64 | 267 | 0.24 | 0.810 | –459 to 587 |
| No partner | 255 | 278 | 0.92 | 0.360 | –291 to 800 |
| Not working due to ill health | 268 | 792 | 0.34 | 0.735 | –1286 to 1822 |
| Low level of education | –465 | 362 | –1.28 | 0.199 | –1175 to 245 |
| Number of threatening life experiences | –5 | 145 | –0.03 | 0.974 | –289 to 280 |
| Distance to nearest hospital | 142 | 117 | 1.21 | 0.228 | –89 to 372 |
| QOF condition reported | |||||
| CHD | 901 | 632 | 1.42 | 0.154 | –339 to 2141 |
| Asthma | 980 | 647 | 1.51 | 0.130 | –289 to 2249 |
| Diabetes | 942 | 613 | 1.54 | 0.124 | –260 to 2145 |
| COPD | –203 | 447 | –0.45 | 0.649 | –1081 to 674 |
| Maximum LTC severity (vs. mild) | |||||
| Moderate | 763 | 372 | 2.05 | 0.041 | 33 to 1493 |
| Severe/very severe | 1564 | 415 | 3.77 | 0.000 | 751 to 2377 |
| HADS depression score of ≥ 8 | 1075 | 404 | 2.66 | 0.008 | 283 to 1868 |
| Cancer | 82 | 675 | 0.12 | 0.903 | –1243 to 1407 |
| Stomach condition | –195 | 635 | –0.31 | 0.759 | –1441 to 1051 |
| High blood pressure | –596 | 373 | –1.6 | 0.110 | –1327 to 135 |
| Arthritis | 44 | 352 | 0.12 | 0.901 | –647 to 735 |
| Total cost year 1 | 0.65 | 0.16 | 4.01 | 0.000 | 0.33 to 0.97 |
| Constant | –2215 | 1078 | –2.05 | 0.040 | –4331 to –100 |
| Covariate | β-coefficient | Linearised standard error | t-test | p > t | 95% CI |
|---|---|---|---|---|---|
| Age (per 10 years) | 129 | 128 | 1.01 | 0.312 | –121 to 379 |
| Female | –10 | 274 | –0.04 | 0.972 | –546 to 527 |
| No partner | 277 | 284 | 0.98 | 0.329 | –280 to 834 |
| Not working due to ill health | 251 | 798 | 0.31 | 0.753 | –1315 to 1817 |
| Low level of education | –517 | 376 | –1.37 | 0.17 | –1254 to 221 |
| Number of threatening life experiences | –4 | 146 | –0.03 | 0.978 | –291 to 283 |
| Distance to nearest hospital | 148 | 120 | 1.24 | 0.216 | –87 to 383 |
| Two or more QOF conditions reported (vs. one) | 279 | 379 | 0.74 | 0.461 | –464 to 1023 |
| Maximum LTC severity (vs. mild) | |||||
| Moderate | 753 | 369 | 2.04 | 0.042 | 28 to 1477 |
| Severe/very severe | 1551 | 394 | 3.94 | 0 | 778 to 2324 |
| HADS depression score of ≥ 8 | 1030 | 398 | 2.59 | 0.01 | 250 to 1811 |
| Cancer | –18 | 704 | –0.03 | 0.98 | –1399 to 1363 |
| Stomach condition | –141 | 643 | –0.22 | 0.826 | –1402 to 1120 |
| High blood pressure | –442 | 346 | –1.28 | 0.201 | –1121 to 237 |
| Arthritis | 122 | 338 | 0.36 | 0.718 | –542 to 786 |
| Total cost year 1 | 0.65 | 0.17 | 3.94 | 0.000 | 0.33 to 0.98 |
| Constant | –1469 | 787 | –1.87 | 0.062 | –3013 to 74 |
| Covariate | β-coefficient | Linearised standard error | t-test | p > t | 95% CI |
|---|---|---|---|---|---|
| Age (per 10 years) | 121 | 45 | 2.69 | 0.007 | 33 to 210 |
| Female | 148 | 117 | 1.27 | 0.205 | –81 to 377 |
| No partner | –57 | 116 | –0.49 | 0.624 | –284 to 171 |
| Not working due to ill health | 715 | 290 | 2.47 | 0.014 | 146 to 1283 |
| Low level of education | –38 | 153 | –0.25 | 0.804 | –338 to 262 |
| Number of threatening life experiences | –46 | 63 | –0.72 | 0.469 | –169 to 78 |
| Distance to nearest hospital | –76 | 48 | –1.59 | 0.113 | –170 to 18 |
| QOF condition reported | |||||
| CHD | 149 | 152 | 0.98 | 0.327 | –149 to 447 |
| Asthma | 290 | 148 | 1.95 | 0.051 | –1 to 580 |
| Diabetes | 305 | 167 | 1.83 | 0.067 | –22 to 632 |
| COPD | –123 | 196 | –0.63 | 0.531 | –507 to 262 |
| Maximum LTC severity (vs. mild) | |||||
| Moderate | 122 | 131 | 0.93 | 0.351 | –135 to 378 |
| Severe/very severe | 552 | 188 | 2.94 | 0.003 | 184 to 921 |
| HADS depression score of ≥ 8 | 187 | 145 | 1.29 | 0.199 | –98 to 472 |
| Cancer | 538 | 372 | 1.44 | 0.149 | –193 to 1269 |
| Stomach condition | 570 | 298 | 1.91 | 0.056 | –15 to 1154 |
| High blood pressure | –167 | 154 | –1.09 | 0.277 | –469 to 134 |
| Arthritis | 184 | 145 | 1.27 | 0.203 | –100 to 468 |
| Total cost year 1 | 0.08 | 0.03 | 2.56 | 0.011 | 0.02 to 0.14 |
| Constant | –95 | 314 | –0.3 | 0.762 | –712 to 522 |
| Covariate | β-coefficient | Linearised standard error | t-test | p > t | 95% CI |
|---|---|---|---|---|---|
| Age (per 10 years) | 102 | 38 | 2.72 | 0.007 | 28 to 176 |
| Female | 150 | 113 | 1.32 | 0.187 | –72 to 372 |
| No partner | –50 | 114 | –0.44 | 0.662 | –274 to 174 |
| Not working due to ill health | 711 | 291 | 2.44 | 0.015 | 140 to 1281 |
| Low level of education | –66 | 153 | –0.43 | 0.664 | –366 to 233 |
| Number threatening life experiences | –46 | 63 | –0.73 | 0.463 | –169 to 77 |
| Distance to nearest hospital | –70 | 46 | –1.5 | 0.133 | –161 to 21 |
| Two or more QOF conditions reported (vs. one) | 153 | 161 | 0.95 | 0.343 | –163 to 469 |
| Maximum LTC severity (vs. mild) | |||||
| Moderate | 122 | 124 | 0.98 | 0.327 | –122 to 365 |
| Severe/very severe | 545 | 163 | 3.35 | 0.001 | 226 to 864 |
| HADS depression score of ≥ 8 | 161 | 139 | 1.16 | 0.248 | –112 to 434 |
| Cancer | 495 | 379 | 1.31 | 0.191 | –248 to 1237 |
| Stomach condition | 587 | 305 | 1.93 | 0.054 | –11 to 1186 |
| High blood pressure | –123 | 153 | –0.8 | 0.423 | –424 to 178 |
| Arthritis | 204 | 145 | 1.4 | 0.161 | –81 to 489 |
| Total cost year 1 | 0.08 | 0.03 | 2.62 | 0.009 | 0.02 to 0.13 |
| Constant | 45 | 282 | 0.16 | 0.872 | –508 to 598 |
| Covariate | β-coefficient | Linearised standard error | t-test | p > t | 95% CI |
|---|---|---|---|---|---|
| Age (per 10 years) | 39 | 140 | 0.28 | 0.781 | –236 to 314 |
| Female | –84 | 233 | –0.36 | 0.719 | –541 to 373 |
| No partner | 312 | 236 | 1.32 | 0.187 | –152 to 775 |
| Not working due to ill health | –447 | 705 | –0.63 | 0.526 | –1830 to 936 |
| Low level of education | –427 | 294 | –1.45 | 0.147 | –1004 to 150 |
| Number of threatening life experiences | 41 | 111 | 0.37 | 0.714 | –178 to 260 |
| Distance to nearest hospital | 218 | 105 | 2.08 | 0.038 | 12 to 423 |
| QOF condition reported | |||||
| CHD | 752 | 605 | 1.24 | 0.214 | –435 to 1938 |
| Asthma | 690 | 624 | 1.11 | 0.269 | –534 to 1915 |
| Diabetes | 637 | 575 | 1.11 | 0.268 | –490 to 1765 |
| COPD | –81 | 387 | –0.21 | 0.835 | –840 to 679 |
| Maximum LTC severity (vs. mild) | |||||
| Moderate | 641 | 365 | 1.75 | 0.08 | –76 to 1358 |
| Severe/very severe | 1012 | 367 | 2.76 | 0.006 | 291 to 1732 |
| HADS depression score of ≥ 8 | 888 | 379 | 2.34 | 0.019 | 144 to 1632 |
| Cancer | –456 | 510 | –0.89 | 0.371 | –1456 to 544 |
| Stomach condition | –765 | 480 | –1.59 | 0.111 | –1706 to 176 |
| High blood pressure | –429 | 312 | –1.38 | 0.169 | –1040 to 182 |
| Arthritis | –140 | 297 | –0.47 | 0.637 | –724 to 443 |
| Total cost year 1 | 0.58 | 0.19 | 3.08 | 0.002 | 0.21 to 0.94 |
| Constant | –2120 | 988 | –2.15 | 0.032 | –4059 to –181 |
| Covariate | β-coefficient | Linearised standard error | t-test | p > t | 95% CI |
|---|---|---|---|---|---|
| Age (per 10 years) | 27 | 128 | 0.21 | 0.834 | –224 to 278 |
| Female | –159 | 239 | –0.67 | 0.505 | –628 to 309 |
| No partner | 327 | 243 | 1.35 | 0.178 | –149 to 804 |
| Not working due to ill health | –459 | 709 | –0.65 | 0.517 | –1851 to 932 |
| Low level of education | –450 | 309 | –1.46 | 0.145 | –1056 to 156 |
| Number of threatening life experiences | 42 | 113 | 0.37 | 0.71 | –179 to 263 |
| Distance to nearest hospital | 218 | 108 | 2.03 | 0.043 | 7 to 429 |
| Two or more QOF conditions reported (vs. one) | 127 | 334 | 0.38 | 0.705 | –529 to 783 |
| Maximum LTC severity (vs. mild) | |||||
| Moderate | 631 | 361 | 1.75 | 0.081 | –78 to 1340 |
| Severe/very severe | 1006 | 348 | 2.89 | 0.004 | 323 to 1689 |
| HADS depression score of ≥ 8 | 870 | 372 | 2.34 | 0.02 | 140 to 1599 |
| Cancer | –513 | 526 | –0.97 | 0.33 | –1544 to 519 |
| Stomach condition | –728 | 473 | –1.54 | 0.124 | –1657 to 200 |
| High blood pressure | –319 | 271 | –1.18 | 0.239 | –851 to 213 |
| Arthritis | –82 | 277 | –0.3 | 0.767 | –626 to 462 |
| Total cost year 1 | 0.58 | 0.19 | 3.06 | 0.002 | 0.21 to 0.94 |
| Constant | –1515 | 679 | –2.23 | 0.026 | –2846 to –183 |
| Patient characteristic | β-coefficient | Linearised standard error | t-test | p > t | 95% CI |
|---|---|---|---|---|---|
| Logistic regression, OR | |||||
| Age (per 10 years) | 0.08 | 0.07 | 1.080 | 0.283 | –0.06 to 0.22 |
| Female | 0.16 | 0.17 | 0.930 | 0.353 | –0.18 to 0.50 |
| No partner | 0.24 | 0.16 | 1.460 | 0.145 | –0.08 to 0.56 |
| Not working due to ill health | 0.16 | 0.26 | 0.600 | 0.551 | –0.36 to 0.68 |
| Low level of education | –0.04 | 0.16 | –0.240 | 0.811 | –0.35 to 0.28 |
| Number of threatening life experiences (per experience) | 0.16 | 0.05 | 3.370 | 0.001 | 0.07 to 0.26 |
| Distance to nearest hospital (per mile) | –0.09 | 0.06 | –1.510 | 0.131 | –0.21 to 0.03 |
| QOF condition reported | |||||
| CHD | 0.25 | 0.18 | 1.390 | 0.165 | –0.10 to 0.60 |
| Asthma | 0.06 | 0.20 | 0.290 | 0.770 | –0.33 to 0.45 |
| Diabetes | –0.01 | 0.20 | –0.050 | 0.957 | –0.40 to 0.38 |
| COPD | 0.19 | 0.23 | 0.810 | 0.417 | –0.27 to 0.65 |
| Maximum LTC severity (vs. mild) | |||||
| Moderate | –0.05 | 0.19 | –0.230 | 0.817 | –0.43 to 0.34 |
| Severe/very severe | –0.20 | 0.23 | –0.880 | 0.379 | –0.65 to 0.25 |
| HADS depression score of ≥ 8 | 0.35 | 0.19 | 1.860 | 0.063 | –0.02 to 0.73 |
| Constant | –1.42 | 0.52 | –2.720 | 0.007 | –2.45 to –0.40 |
| Linear regression, β-coefficient | |||||
| Age (per 10 years) | –354 | 677 | –0.520 | 0.601 | –1682 to 974 |
| Female | 68 | 743 | 0.090 | 0.927 | –1391 to 1526 |
| Single | 2351 | 1614 | 1.460 | 0.146 | –816 to 5518 |
| Not working due to ill health | –3139 | 3247 | –0.970 | 0.334 | –9511 to 3233 |
| Low level of education | –942 | 796 | –1.180 | 0.237 | –2504 to 620 |
| Number of threatening life experiences (per experience) | –79 | 309 | –0.260 | 0.798 | –686 to 528 |
| Distance to nearest hospital (per mile) | 487 | 274 | 1.780 | 0.076 | –50 to 1023 |
| QOF condition reported | |||||
| CHD | 1799 | 1289 | 1.400 | 0.163 | –731 to 4328 |
| Asthma | 1109 | 1325 | 0.840 | 0.403 | –1490 to 3709 |
| Diabetes | 251 | 1350 | 0.190 | 0.853 | –2398 to 2900 |
| COPD | –945 | 1056 | –0.900 | 0.371 | –3017 to 1127 |
| Maximum LTC severity (vs. mild) | |||||
| Moderate | 1634 | 1496 | 1.090 | 0.275 | –1302 to 4570 |
| Severe/very severe | 2743 | 977 | 2.810 | 0.005 | 826 to 4660 |
| HADS depression score of ≥ 8 | 3694 | 1859 | 1.990 | 0.047 | 46 to 7342 |
| Constant | –212 | 3006 | –0.070 | 0.944 | –6110 to 5687 |
| Patient characteristic | β-coefficient | Linearised standard error | t-test | p > t | 95% CI |
|---|---|---|---|---|---|
| Logistic regression, OR | |||||
| Age (per 10 years) | 0.09 | 0.07 | 1.260 | 0.206 | –0.05 to 0.22 |
| Female | 0.14 | 0.17 | 0.870 | 0.384 | –0.18 to 0.47 |
| No partner | 0.24 | 0.16 | 1.460 | 0.145 | –0.08 to 0.56 |
| Not working due to ill health | 0.15 | 0.26 | 0.580 | 0.559 | –0.36 to 0.67 |
| Low level of education | –0.02 | 0.16 | –0.160 | 0.875 | –0.34 to 0.29 |
| Number of threatening life experiences (per experience) | 0.16 | 0.05 | 3.400 | 0.001 | 0.07 to 0.26 |
| Distance to nearest hospital (per mile) | –0.10 | 0.06 | –1.580 | 0.113 | –0.22 to 0.02 |
| Two or more QOF conditions reported (vs. one) | 0.19 | 0.20 | 0.950 | 0.340 | –0.20 to 0.59 |
| Maximum LTC severity (vs. mild) | |||||
| Moderate | –0.09 | 0.19 | –0.470 | 0.640 | –0.45 to 0.28 |
| Severe/very severe | –0.26 | 0.21 | –1.240 | 0.213 | –0.68 to 0.15 |
| HADS depression score of ≥ 8 | 0.37 | 0.19 | 1.940 | 0.052 | 0.00 to 0.75 |
| Constant | –1.54 | 0.50 | –3.110 | 0.002 | –2.52 to –0.57 |
| Linear regression, β-coefficient | |||||
| Age (per 10 years) | –382 | 682 | –0.560 | 0.575 | –1720 to 955 |
| Female | –166 | 749 | –0.220 | 0.825 | –1635 to 1304 |
| Single | 2525 | 1654 | 1.530 | 0.127 | –721 to 5770 |
| Not working due to ill health | –3109 | 3317 | –0.940 | 0.349 | –9618 to 3400 |
| Low level of education | –872 | 798 | –1.090 | 0.275 | –2438 to 693 |
| Number of threatening life experiences (per experience) | –66 | 320 | –0.210 | 0.837 | –694 to 562 |
| Distance to nearest hospital (per mile) | 439 | 263 | 1.670 | 0.096 | –78 to 955 |
| Two or more QOF conditions reported (vs. one) | –351 | 863 | –0.410 | 0.684 | –2045 to 1342 |
| Maximum LTC severity (vs. mild) | |||||
| Moderate | 1457 | 1418 | 1.030 | 0.304 | –1324 to 4238 |
| Severe/very severe | 2436 | 923 | 2.640 | 0.008 | 626 to 4247 |
| HADS depression score of ≥ 8 | 3607 | 1857 | 1.940 | 0.052 | –37 to 7250 |
| Constant | 1547 | 2989 | 0.520 | 0.605 | –4318 to 7412 |
Appendix 3 Interview topic guides
-
People involved in care.
-
Identifying primary HCP for condition(s).
-
Involvement of other HCPs and their roles.
-
Decision-making around different problems/exacerbations: past, present and future.
-
Self-management.
-
Involvement of others (family/friends, etc.).
-
-
Reviewing specific consultation (using stimulated recall where relevant).
-
Reason/s for attending.
-
Initiation and purpose of consultation.
-
Expectations and hopes regarding consultation.
-
Any unmet needs/expectations and any issues not brought up.
-
Involvement in consultation and decision-making.
-
Retrospective recall around content and value of consultation for self and practitioner.
-
Comparison with previous consultations.
-
Satisfaction or dissatisfaction with specific parts of consultation.
-
Examples of good/bad consultations.
-
Evaluation of consultation and any outcome/s from consultation.
-
-
Routine reviews.
-
Understanding of, and experience of, routine reviews.
-
Opinion/s on contribution of routine reviews to condition management.
-
Self-management.
-
Management of condition by health-care practitioners.
-
-
Unscheduled care.
-
Any recent use of unscheduled care.
-
What happened.
-
Any discussion of unscheduled care use with practitioners.
-
Any unmet needs or preferences around discussing unscheduled care use.
-
-
Review of health and health-care use in last 3 months (using telephone calls and health-care logs to guide discussion).
-
Discussion of any exacerbations or problems in last 3 months.
-
Comparing and contrasting different services.
-
Experiences of and satisfaction with health-care practitioners.
-
-
Routine reviews.
-
Understanding of, and experience of, routine reviews.
-
Opinion/s on contribution of routine reviews to condition management
-
– Self-management.
-
– Management of condition by health-care practitioners.
-
-
Unscheduled care.
-
Any recent use of unscheduled care.
-
– What happened.
-
– Any discussion of unscheduled care use with practitioners.
-
– Any unmet needs or preferences around discussing unscheduled care use.
-
The text is adapted from Hunter et al. 289 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
-
Management of LTCs within the practice.
-
Different roles within the practice.
-
Protocols around managing LTCs.
-
Goals of different types of LTC work.
-
-
Consultations (playing back snippets of consultations where relevant).
-
Type/s of consultation.
-
Purpose and value of consultations.
-
How consultations are organised.
-
Preparation for consultations.
-
Perspective on patient and practitioner expectations within specific consultations.
-
Perspective on patient and practitioner management of LTC/s, drawing on specific consultations.
-
Issues addressed in a specific consultation (and why).
-
Issues not addressed or difficult to address in a consultation (and why).
-
-
Unscheduled care (playing back snippets of consultations where relevant).
-
Discussion of unscheduled care in LTC consultations.
-
Role of primary care in reducing unscheduled care use.
-
The text is adapted from Hunter et al. 289 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Identify:
-
Context for consultation.
-
Focus of consultation.
-
Any additional issues brought up by patients in review appointments.
-
Outcome/s of consultation.
-
Discussion or mention of support at home (and by whom).
-
Discussion or mention of mood (and by whom).
-
Discussion or mention of self-management (and by whom).
-
Discussion or mention of exacerbations of condition/s (and by whom).
-
Discussion or mention of unscheduled care use (and by whom).
-
Any other issues arising that were not the primary/expected focus of the consultation.
From these notes, identify prompts for the interview.
-
Specific to this consultation.
-
About consultations more generally.
Identify time markers for sections of recording for stimulated recall.
The text is adapted from Hunter et al. 289 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Appendix 4 The CHOICE programme health survey
List of abbreviations
- A&E
- accident and emergency
- ACM
- active case manager
- ANOVA
- analysis of variance
- BNI
- British Nursing Index
- CBT
- cognitive–behavioural therapy
- CCG
- Clinical Commissioning Group
- CHD
- coronary heart disease
- CHOICE
- Choosing Health Options In Chronic Care Emergencies
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CMHP
- common mental health problem
- CONSORT
- Consolidated Standards of Reporting Trials
- COPD
- chronic obstructive pulmonary disease
- df
- degree of freedom
- DH
- Department of Health
- DN
- district nurse
- DSCRO
- Data Service for Commissioners Regional Office
- ED
- emergency department
- EHA
- emergency hospital admission
- EIA
- engagement impact assessment
- EQ-5D
- EuroQol-5 Dimensions
- FEV1
- forced expiratory volume in 1 second
- GAD-7
- Generalised Anxiety Scale-7
- GCSE
- General Certificate of Secondary Education
- GOLD
- Global Initiative for Lung Disease
- GP
- general practitioner
- GPIG
- General Practice Information Group
- GTN
- glyceryl trinitrate
- HADS
- Hospital Anxiety and Depression Scale
- HbA1c
- glycated haemoglobin
- HCA
- health-care assistant
- HCP
- health-care practitioner
- HRG
- health-care-relevant group
- IAPT
- Improving Access to Psychological Therapies
- ICER
- incremental cost-effectiveness ratio
- ICP
- integrated care programme
- ID
- identification number
- IMD
- Index of Multiple Deprivation
- IT
- information technology
- LHW
- liaison health worker
- LTC
- long-term condition
- MI
- myocardial infarction
- MICE
- multiple imputation chained equations
- NB
- net benefit
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NSRC
- National Schedules of Reference Costs 2012–13
- OOH
- out of hours
- OR
- odds ratio
- PCT
- primary care trust
- PHQ-9
- Patient Health Questionnaire-9
- PIS
- patient information sheet
- PN
- practice nurse
- PPE&I
- patient and public engagement and involvement
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- QOF
- Quality and Outcomes Framework
- QoL
- quality of life
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SD
- standard deviation
- SN
- specialist nurse
- VAS
- visual analogue scale
- WTPT
- willingness-to-pay threshold